User login
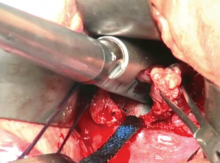
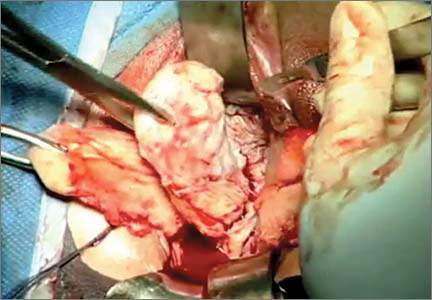
Complete excision most effective for BI-ALCL
![]()
Photo courtesy of the FDA
The optimal treatment approach for most women with breast implant-associated anaplastic large-cell lymphoma (BI-ALCL) is complete surgical excision of the implant and surrounding capsule, a new study suggests.
The study, published in the Journal of Clinical Oncology, represents the most comprehensive study of BI-ALCL to date, including 87 patients and 30 investigators from 14 institutions across 5 continents.
BI-ALCL is a rare T-cell lymphoma that forms in the scar tissue or in the fluid surrounding a breast implant. The disease manifests as a large fluid collection around the implant over a year after implantation, usually taking an average of 8 years to develop.
An estimated 10 million women worldwide have breast implants, and the annual incidence of BI-ALCL is estimated to be 0.1 to 0.3 per 100,000 women with breast implants.
“Although this disease is rare, it appears to be amenable to treatment, and, in the vast majority of patients, the outcome is very good,” said Mark Clemens, MD, of The University of Texas MD Anderson Cancer Center in Houston.
“The disease can be reliably diagnosed, and, when treated appropriately, it has a good prognosis.”
Still, the optimal management for BI-ALCL hasn’t been clear. So with this study, Dr Clemens and his colleagues sought to evaluate treatment efficacy on disease outcomes and determine the best treatment approach. The study expands on previous research published in the Journal of Clinical Oncology in 2014.
The researchers gathered detailed treatment and outcome information from a total of 87 BI-ALCL patients, including 37 whose information had not previously been published. A review of treatment approaches in relation to event-free survival and overall survival revealed that surgery was the optimal frontline therapy for BI-ALCL.
“We determined that complete surgical excision was essential for the management of this disease,” Dr Clemens said. “Patients did not do as well unless they were treated with full removal of the breast implant and complete excision of the capsule around the implant.”
Patients with complete surgical excision had a recurrence rate of 4% at 5 years, compared to 28% for patients who received radiation therapy and 32% for chemotherapy.
In addition, patients who underwent a complete surgical excision had better overall survival (P=0.022) and event-free survival (P=0.014) than patients who received a partial capsulectomy, systemic chemotherapy, or radiation.
“This lymphoma represents a different paradigm from systemic anaplastic large-cell lymphoma, in particular because of its strong association with breast implants,” said Roberto N. Miranda, MD, of The University of Texas MD Anderson Cancer Center.
“We have demonstrated that this is a predominantly localized disease where surgical excision has a primary role.”
The researchers emphasized that, despite the overall good prognosis, some rare cases of BI-ALCL exhibit more aggressive behavior with systemic dissemination. As a part of this study, the team is gathering tissue from these patients to assess underlying mechanisms for progression of disease.
Additional research is ongoing to optimize therapy for these cases through genetic profiling and defining the role of chemotherapy and radiation. The researchers are also studying animal models to further assess the role of breast implants in the pathogenesis of this lymphoma. ![]()
![]()
Photo courtesy of the FDA
The optimal treatment approach for most women with breast implant-associated anaplastic large-cell lymphoma (BI-ALCL) is complete surgical excision of the implant and surrounding capsule, a new study suggests.
The study, published in the Journal of Clinical Oncology, represents the most comprehensive study of BI-ALCL to date, including 87 patients and 30 investigators from 14 institutions across 5 continents.
BI-ALCL is a rare T-cell lymphoma that forms in the scar tissue or in the fluid surrounding a breast implant. The disease manifests as a large fluid collection around the implant over a year after implantation, usually taking an average of 8 years to develop.
An estimated 10 million women worldwide have breast implants, and the annual incidence of BI-ALCL is estimated to be 0.1 to 0.3 per 100,000 women with breast implants.
“Although this disease is rare, it appears to be amenable to treatment, and, in the vast majority of patients, the outcome is very good,” said Mark Clemens, MD, of The University of Texas MD Anderson Cancer Center in Houston.
“The disease can be reliably diagnosed, and, when treated appropriately, it has a good prognosis.”
Still, the optimal management for BI-ALCL hasn’t been clear. So with this study, Dr Clemens and his colleagues sought to evaluate treatment efficacy on disease outcomes and determine the best treatment approach. The study expands on previous research published in the Journal of Clinical Oncology in 2014.
The researchers gathered detailed treatment and outcome information from a total of 87 BI-ALCL patients, including 37 whose information had not previously been published. A review of treatment approaches in relation to event-free survival and overall survival revealed that surgery was the optimal frontline therapy for BI-ALCL.
“We determined that complete surgical excision was essential for the management of this disease,” Dr Clemens said. “Patients did not do as well unless they were treated with full removal of the breast implant and complete excision of the capsule around the implant.”
Patients with complete surgical excision had a recurrence rate of 4% at 5 years, compared to 28% for patients who received radiation therapy and 32% for chemotherapy.
In addition, patients who underwent a complete surgical excision had better overall survival (P=0.022) and event-free survival (P=0.014) than patients who received a partial capsulectomy, systemic chemotherapy, or radiation.
“This lymphoma represents a different paradigm from systemic anaplastic large-cell lymphoma, in particular because of its strong association with breast implants,” said Roberto N. Miranda, MD, of The University of Texas MD Anderson Cancer Center.
“We have demonstrated that this is a predominantly localized disease where surgical excision has a primary role.”
The researchers emphasized that, despite the overall good prognosis, some rare cases of BI-ALCL exhibit more aggressive behavior with systemic dissemination. As a part of this study, the team is gathering tissue from these patients to assess underlying mechanisms for progression of disease.
Additional research is ongoing to optimize therapy for these cases through genetic profiling and defining the role of chemotherapy and radiation. The researchers are also studying animal models to further assess the role of breast implants in the pathogenesis of this lymphoma. ![]()
![]()
Photo courtesy of the FDA
The optimal treatment approach for most women with breast implant-associated anaplastic large-cell lymphoma (BI-ALCL) is complete surgical excision of the implant and surrounding capsule, a new study suggests.
The study, published in the Journal of Clinical Oncology, represents the most comprehensive study of BI-ALCL to date, including 87 patients and 30 investigators from 14 institutions across 5 continents.
BI-ALCL is a rare T-cell lymphoma that forms in the scar tissue or in the fluid surrounding a breast implant. The disease manifests as a large fluid collection around the implant over a year after implantation, usually taking an average of 8 years to develop.
An estimated 10 million women worldwide have breast implants, and the annual incidence of BI-ALCL is estimated to be 0.1 to 0.3 per 100,000 women with breast implants.
“Although this disease is rare, it appears to be amenable to treatment, and, in the vast majority of patients, the outcome is very good,” said Mark Clemens, MD, of The University of Texas MD Anderson Cancer Center in Houston.
“The disease can be reliably diagnosed, and, when treated appropriately, it has a good prognosis.”
Still, the optimal management for BI-ALCL hasn’t been clear. So with this study, Dr Clemens and his colleagues sought to evaluate treatment efficacy on disease outcomes and determine the best treatment approach. The study expands on previous research published in the Journal of Clinical Oncology in 2014.
The researchers gathered detailed treatment and outcome information from a total of 87 BI-ALCL patients, including 37 whose information had not previously been published. A review of treatment approaches in relation to event-free survival and overall survival revealed that surgery was the optimal frontline therapy for BI-ALCL.
“We determined that complete surgical excision was essential for the management of this disease,” Dr Clemens said. “Patients did not do as well unless they were treated with full removal of the breast implant and complete excision of the capsule around the implant.”
Patients with complete surgical excision had a recurrence rate of 4% at 5 years, compared to 28% for patients who received radiation therapy and 32% for chemotherapy.
In addition, patients who underwent a complete surgical excision had better overall survival (P=0.022) and event-free survival (P=0.014) than patients who received a partial capsulectomy, systemic chemotherapy, or radiation.
“This lymphoma represents a different paradigm from systemic anaplastic large-cell lymphoma, in particular because of its strong association with breast implants,” said Roberto N. Miranda, MD, of The University of Texas MD Anderson Cancer Center.
“We have demonstrated that this is a predominantly localized disease where surgical excision has a primary role.”
The researchers emphasized that, despite the overall good prognosis, some rare cases of BI-ALCL exhibit more aggressive behavior with systemic dissemination. As a part of this study, the team is gathering tissue from these patients to assess underlying mechanisms for progression of disease.
Additional research is ongoing to optimize therapy for these cases through genetic profiling and defining the role of chemotherapy and radiation. The researchers are also studying animal models to further assess the role of breast implants in the pathogenesis of this lymphoma. ![]()
Team identifies potential biomarkers for AML therapy

Photo by Kristin Gladney
New research published in Cell Reports has revealed biomarkers that may help predict which acute myeloid leukemia (AML) patients will respond to treatment with PU-H71.
This drug targets a tumor-enriched form of the protein Hsp90 called teHSP90, which is critical to the growth of cancer cells.
However, previous preclinical experiments showed that PU-H71 only kills some AML cells.
“We observed that only a subset of leukemia patient samples were sensitive to the drug,” said study author Monica Guzman, PhD, of Weill Cornell Medical College in New York, New York.
“We wanted to be able to identify which patients with leukemia would respond to this drug.”
So Dr Guzman and her colleagues focused on groups of proteins that function within signaling networks in leukemia cells.
The researchers found that 2 of these networks, JAK-STAT and PI3K-AKT, were important for leukemia cells to function. These signaling pathways are critical for the survival of AML cells, and they, in turn, are dependent on teHsp90.
The team treated AML cells with PU-H71 and found that cells with greater JAK-STAT and PI3K-AKT activity were killed by the drug. Cells with less active signaling networks did not respond to PU-H71.
“Higher activation of these networks makes the leukemia cells more dependent on Hsp90,” Dr Guzman said. “Since PU-H71 targets teHsp90, leukemia samples with these features are good targets for treatment with the drug.”
The next step for Dr Guzman’s team is to test their findings in patients. Ideally, the JAK-STAT and PI3K-AKT signaling pathways will serve as biomarkers for identifying patients whose leukemias will be sensitive to PU-H71.
“We are working on a tool that will quickly and easily identify patients whose cancers will respond to PU-H71,” Dr Guzman said. “We are really looking forward to seeing this in leukemic patients and being able to offer them a new treatment.” ![]()

Photo by Kristin Gladney
New research published in Cell Reports has revealed biomarkers that may help predict which acute myeloid leukemia (AML) patients will respond to treatment with PU-H71.
This drug targets a tumor-enriched form of the protein Hsp90 called teHSP90, which is critical to the growth of cancer cells.
However, previous preclinical experiments showed that PU-H71 only kills some AML cells.
“We observed that only a subset of leukemia patient samples were sensitive to the drug,” said study author Monica Guzman, PhD, of Weill Cornell Medical College in New York, New York.
“We wanted to be able to identify which patients with leukemia would respond to this drug.”
So Dr Guzman and her colleagues focused on groups of proteins that function within signaling networks in leukemia cells.
The researchers found that 2 of these networks, JAK-STAT and PI3K-AKT, were important for leukemia cells to function. These signaling pathways are critical for the survival of AML cells, and they, in turn, are dependent on teHsp90.
The team treated AML cells with PU-H71 and found that cells with greater JAK-STAT and PI3K-AKT activity were killed by the drug. Cells with less active signaling networks did not respond to PU-H71.
“Higher activation of these networks makes the leukemia cells more dependent on Hsp90,” Dr Guzman said. “Since PU-H71 targets teHsp90, leukemia samples with these features are good targets for treatment with the drug.”
The next step for Dr Guzman’s team is to test their findings in patients. Ideally, the JAK-STAT and PI3K-AKT signaling pathways will serve as biomarkers for identifying patients whose leukemias will be sensitive to PU-H71.
“We are working on a tool that will quickly and easily identify patients whose cancers will respond to PU-H71,” Dr Guzman said. “We are really looking forward to seeing this in leukemic patients and being able to offer them a new treatment.” ![]()

Photo by Kristin Gladney
New research published in Cell Reports has revealed biomarkers that may help predict which acute myeloid leukemia (AML) patients will respond to treatment with PU-H71.
This drug targets a tumor-enriched form of the protein Hsp90 called teHSP90, which is critical to the growth of cancer cells.
However, previous preclinical experiments showed that PU-H71 only kills some AML cells.
“We observed that only a subset of leukemia patient samples were sensitive to the drug,” said study author Monica Guzman, PhD, of Weill Cornell Medical College in New York, New York.
“We wanted to be able to identify which patients with leukemia would respond to this drug.”
So Dr Guzman and her colleagues focused on groups of proteins that function within signaling networks in leukemia cells.
The researchers found that 2 of these networks, JAK-STAT and PI3K-AKT, were important for leukemia cells to function. These signaling pathways are critical for the survival of AML cells, and they, in turn, are dependent on teHsp90.
The team treated AML cells with PU-H71 and found that cells with greater JAK-STAT and PI3K-AKT activity were killed by the drug. Cells with less active signaling networks did not respond to PU-H71.
“Higher activation of these networks makes the leukemia cells more dependent on Hsp90,” Dr Guzman said. “Since PU-H71 targets teHsp90, leukemia samples with these features are good targets for treatment with the drug.”
The next step for Dr Guzman’s team is to test their findings in patients. Ideally, the JAK-STAT and PI3K-AKT signaling pathways will serve as biomarkers for identifying patients whose leukemias will be sensitive to PU-H71.
“We are working on a tool that will quickly and easily identify patients whose cancers will respond to PU-H71,” Dr Guzman said. “We are really looking forward to seeing this in leukemic patients and being able to offer them a new treatment.” ![]()
Catheter approved to treat DVT

thrombectomy catheter
Image courtesy of
Boston Scientific
The AngioJet ZelanteDVT thrombectomy catheter has been approved for commercialization in the US and Europe.
The device received the CE mark and approval from the US Food and Drug Administration to treat deep vein thrombosis (DVT) in large-diameter
upper and lower limb peripheral veins.
The ZelanteDVT catheter was designed to remove large venous clot burdens and facilitate rapid restoration of blood flow, potentially decreasing procedural time, relieving symptoms, and reducing late complications.
The ZelanteDVT Thrombectomy Set is intended for use with the AngioJet Ultra Console to break apart and remove thrombi, including DVT, from iliofemoral and lower extremity veins greater than or equal to 6.0 mm in diameter and upper extremity peripheral veins greater than or equal to 6.0 mm in diameter.
The set is also intended for use with the AngioJet Power Pulse technique for the controlled and selective infusion of physician-specified fluids, including thrombolytic agents, into the peripheral vascular system.
The ZelanteDVT catheter is a product of Boston Scientific. For more information on the device, visit the company’s website. ![]()

thrombectomy catheter
Image courtesy of
Boston Scientific
The AngioJet ZelanteDVT thrombectomy catheter has been approved for commercialization in the US and Europe.
The device received the CE mark and approval from the US Food and Drug Administration to treat deep vein thrombosis (DVT) in large-diameter
upper and lower limb peripheral veins.
The ZelanteDVT catheter was designed to remove large venous clot burdens and facilitate rapid restoration of blood flow, potentially decreasing procedural time, relieving symptoms, and reducing late complications.
The ZelanteDVT Thrombectomy Set is intended for use with the AngioJet Ultra Console to break apart and remove thrombi, including DVT, from iliofemoral and lower extremity veins greater than or equal to 6.0 mm in diameter and upper extremity peripheral veins greater than or equal to 6.0 mm in diameter.
The set is also intended for use with the AngioJet Power Pulse technique for the controlled and selective infusion of physician-specified fluids, including thrombolytic agents, into the peripheral vascular system.
The ZelanteDVT catheter is a product of Boston Scientific. For more information on the device, visit the company’s website. ![]()

thrombectomy catheter
Image courtesy of
Boston Scientific
The AngioJet ZelanteDVT thrombectomy catheter has been approved for commercialization in the US and Europe.
The device received the CE mark and approval from the US Food and Drug Administration to treat deep vein thrombosis (DVT) in large-diameter
upper and lower limb peripheral veins.
The ZelanteDVT catheter was designed to remove large venous clot burdens and facilitate rapid restoration of blood flow, potentially decreasing procedural time, relieving symptoms, and reducing late complications.
The ZelanteDVT Thrombectomy Set is intended for use with the AngioJet Ultra Console to break apart and remove thrombi, including DVT, from iliofemoral and lower extremity veins greater than or equal to 6.0 mm in diameter and upper extremity peripheral veins greater than or equal to 6.0 mm in diameter.
The set is also intended for use with the AngioJet Power Pulse technique for the controlled and selective infusion of physician-specified fluids, including thrombolytic agents, into the peripheral vascular system.
The ZelanteDVT catheter is a product of Boston Scientific. For more information on the device, visit the company’s website. ![]()
AHA: New spotlight on peripheral artery disease
ORLANDO – Peripheral artery disease constitutes “a health crisis that is largely unnoticed” by the public and all too often by physicians as well – but that’s all about to change, Dr. Mark A. Creager said in his presidential address at the American Heart Association scientific sessions.
In the coming months, look for rollout of major AHA initiatives on peripheral vascular disease. These programs grew out of a summit meeting of thought leaders in the field of vascular disease convened recently by the AHA in order to find ways to boost public awareness and improve the quality of care for patients with peripheral artery disease (PAD), venous thromboembolism, and aortic aneurysm.
PAD is all too often thought of as a disease of the legs, when in fact it is a clinical manifestation of systemic atherosclerosis, Dr. Creager noted. PAD affects on estimated 200 million people worldwide. In the United States alone, it accounts for $20 billion per year in health care costs. The mortality risk in affected patients is two- to fourfold greater than in individuals without PAD. Moreover, the risk of acute MI, stroke, or cardiovascular death among patients with PAD exceeds that of patients with established cerebrovascular disease.
“Clearly the unrecognized epidemic of vascular disease requires our attention,” observed Dr. Creager, professor of medicine and director of the Heart and Vascular Center at Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
Indeed, he made it clear that improved diagnosis and treatment of PAD will be a major AHA priority during his term as president. Noting that the annual rate of deaths due to cardiovascular disease and stroke has already dropped by 13.7% in the 5 years since the AHA set the ambitious goal of a 20% reduction by the year 2020, he emphasized that a critical element in getting the rest of the way there involves addressing peripheral vascular diseases more effectively.
Improved public awareness about PAD has to be a priority. One survey found that 75% of the public is unaware of PAD. It’s a condition which Dr. Creager and others have shown has its highest prevalence in Americans in the lowest income and educational strata, largely independent of traditional cardiovascular risk factors.
“Even physicians often fail to consider PAD, chalking up leg pain to age or arthritis. In one study, physicians missed the diagnosis of PAD half the time. And even when physicians diagnose PAD, they often don’t treat it adequately,” according to Dr. Creager.
The use of statins and antiplatelet therapy in patients with PAD each reduces the risk of MI, stroke, or cardiovascular death by about 25%. Yet when Dr. Creager and coworkers analyzed National Health and Nutrition Examination Survey data, they found only 19% of patients with PAD who didn’t have previously established coronary or cerebrovascular disease were on a statin, just 21% were on an ACE inhibitor or angiotensin receptor blocker, and 27% were on antiplatelet therapy (Circulation. 2011 Jul 5;124[1]:17-23).
Among patients with symptomatic PAD, studies have shown that supervised exercise training can double walking distance. That’s a major quality of life benefit, yet one that goes unconsidered if the diagnosis is missed. “It’s rarely instituted even with the diagnosis, largely because of the lack of reimbursement,” according to Dr. Creager.
He painted a picture of PAD as a field ripe with opportunities for improved outcomes.
“Our ability to diagnose and treat vascular diseases has never been greater. The field of vascular biology has virtually exploded in recent years,” he said. “I’d like to focus not only on the extent of this crisis, but also on the importance of using what we know to treat it and prevent it, and on the urgency of intensifying our research efforts to better understand it.”
In the diagnostic arena, optical coherence tomography, intravascular ultrasound, PET-CT, and contrast-enhanced MRI provide an unprecedented ability to image plaques and assess their vulnerability to rupture.
On the interventional front, innovative bioengineering has led to the development of drug-coated balloons and bioabsorbable vascular scaffolds with the potential to curb restenosis and preserve patency following endovascular treatment of critical lesions.
In terms of medical management, the novel, super-potent LDL cholesterol–lowering inhibitors of PCSK9 (proprotein convertase subtilisin-kexin type 9) will need to be studied in order to see if they have a special role in the treatment and/or prevention of PAD.
Roughly 2 decades ago, Dr. Creager and coinvestigators showed that endothelial function typically drops by 30% in our twenties and then in our thirties, and by 50% once we’re in our forties. A high research priority in PAD will be to learn how to more effectively prolong endothelial health and prevent vascular stiffness, he said.
He reported having no financial conflicts regarding his presentation.
ORLANDO – Peripheral artery disease constitutes “a health crisis that is largely unnoticed” by the public and all too often by physicians as well – but that’s all about to change, Dr. Mark A. Creager said in his presidential address at the American Heart Association scientific sessions.
In the coming months, look for rollout of major AHA initiatives on peripheral vascular disease. These programs grew out of a summit meeting of thought leaders in the field of vascular disease convened recently by the AHA in order to find ways to boost public awareness and improve the quality of care for patients with peripheral artery disease (PAD), venous thromboembolism, and aortic aneurysm.
PAD is all too often thought of as a disease of the legs, when in fact it is a clinical manifestation of systemic atherosclerosis, Dr. Creager noted. PAD affects on estimated 200 million people worldwide. In the United States alone, it accounts for $20 billion per year in health care costs. The mortality risk in affected patients is two- to fourfold greater than in individuals without PAD. Moreover, the risk of acute MI, stroke, or cardiovascular death among patients with PAD exceeds that of patients with established cerebrovascular disease.
“Clearly the unrecognized epidemic of vascular disease requires our attention,” observed Dr. Creager, professor of medicine and director of the Heart and Vascular Center at Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
Indeed, he made it clear that improved diagnosis and treatment of PAD will be a major AHA priority during his term as president. Noting that the annual rate of deaths due to cardiovascular disease and stroke has already dropped by 13.7% in the 5 years since the AHA set the ambitious goal of a 20% reduction by the year 2020, he emphasized that a critical element in getting the rest of the way there involves addressing peripheral vascular diseases more effectively.
Improved public awareness about PAD has to be a priority. One survey found that 75% of the public is unaware of PAD. It’s a condition which Dr. Creager and others have shown has its highest prevalence in Americans in the lowest income and educational strata, largely independent of traditional cardiovascular risk factors.
“Even physicians often fail to consider PAD, chalking up leg pain to age or arthritis. In one study, physicians missed the diagnosis of PAD half the time. And even when physicians diagnose PAD, they often don’t treat it adequately,” according to Dr. Creager.
The use of statins and antiplatelet therapy in patients with PAD each reduces the risk of MI, stroke, or cardiovascular death by about 25%. Yet when Dr. Creager and coworkers analyzed National Health and Nutrition Examination Survey data, they found only 19% of patients with PAD who didn’t have previously established coronary or cerebrovascular disease were on a statin, just 21% were on an ACE inhibitor or angiotensin receptor blocker, and 27% were on antiplatelet therapy (Circulation. 2011 Jul 5;124[1]:17-23).
Among patients with symptomatic PAD, studies have shown that supervised exercise training can double walking distance. That’s a major quality of life benefit, yet one that goes unconsidered if the diagnosis is missed. “It’s rarely instituted even with the diagnosis, largely because of the lack of reimbursement,” according to Dr. Creager.
He painted a picture of PAD as a field ripe with opportunities for improved outcomes.
“Our ability to diagnose and treat vascular diseases has never been greater. The field of vascular biology has virtually exploded in recent years,” he said. “I’d like to focus not only on the extent of this crisis, but also on the importance of using what we know to treat it and prevent it, and on the urgency of intensifying our research efforts to better understand it.”
In the diagnostic arena, optical coherence tomography, intravascular ultrasound, PET-CT, and contrast-enhanced MRI provide an unprecedented ability to image plaques and assess their vulnerability to rupture.
On the interventional front, innovative bioengineering has led to the development of drug-coated balloons and bioabsorbable vascular scaffolds with the potential to curb restenosis and preserve patency following endovascular treatment of critical lesions.
In terms of medical management, the novel, super-potent LDL cholesterol–lowering inhibitors of PCSK9 (proprotein convertase subtilisin-kexin type 9) will need to be studied in order to see if they have a special role in the treatment and/or prevention of PAD.
Roughly 2 decades ago, Dr. Creager and coinvestigators showed that endothelial function typically drops by 30% in our twenties and then in our thirties, and by 50% once we’re in our forties. A high research priority in PAD will be to learn how to more effectively prolong endothelial health and prevent vascular stiffness, he said.
He reported having no financial conflicts regarding his presentation.
ORLANDO – Peripheral artery disease constitutes “a health crisis that is largely unnoticed” by the public and all too often by physicians as well – but that’s all about to change, Dr. Mark A. Creager said in his presidential address at the American Heart Association scientific sessions.
In the coming months, look for rollout of major AHA initiatives on peripheral vascular disease. These programs grew out of a summit meeting of thought leaders in the field of vascular disease convened recently by the AHA in order to find ways to boost public awareness and improve the quality of care for patients with peripheral artery disease (PAD), venous thromboembolism, and aortic aneurysm.
PAD is all too often thought of as a disease of the legs, when in fact it is a clinical manifestation of systemic atherosclerosis, Dr. Creager noted. PAD affects on estimated 200 million people worldwide. In the United States alone, it accounts for $20 billion per year in health care costs. The mortality risk in affected patients is two- to fourfold greater than in individuals without PAD. Moreover, the risk of acute MI, stroke, or cardiovascular death among patients with PAD exceeds that of patients with established cerebrovascular disease.
“Clearly the unrecognized epidemic of vascular disease requires our attention,” observed Dr. Creager, professor of medicine and director of the Heart and Vascular Center at Dartmouth-Hitchcock Medical Center in Lebanon, N.H.
Indeed, he made it clear that improved diagnosis and treatment of PAD will be a major AHA priority during his term as president. Noting that the annual rate of deaths due to cardiovascular disease and stroke has already dropped by 13.7% in the 5 years since the AHA set the ambitious goal of a 20% reduction by the year 2020, he emphasized that a critical element in getting the rest of the way there involves addressing peripheral vascular diseases more effectively.
Improved public awareness about PAD has to be a priority. One survey found that 75% of the public is unaware of PAD. It’s a condition which Dr. Creager and others have shown has its highest prevalence in Americans in the lowest income and educational strata, largely independent of traditional cardiovascular risk factors.
“Even physicians often fail to consider PAD, chalking up leg pain to age or arthritis. In one study, physicians missed the diagnosis of PAD half the time. And even when physicians diagnose PAD, they often don’t treat it adequately,” according to Dr. Creager.
The use of statins and antiplatelet therapy in patients with PAD each reduces the risk of MI, stroke, or cardiovascular death by about 25%. Yet when Dr. Creager and coworkers analyzed National Health and Nutrition Examination Survey data, they found only 19% of patients with PAD who didn’t have previously established coronary or cerebrovascular disease were on a statin, just 21% were on an ACE inhibitor or angiotensin receptor blocker, and 27% were on antiplatelet therapy (Circulation. 2011 Jul 5;124[1]:17-23).
Among patients with symptomatic PAD, studies have shown that supervised exercise training can double walking distance. That’s a major quality of life benefit, yet one that goes unconsidered if the diagnosis is missed. “It’s rarely instituted even with the diagnosis, largely because of the lack of reimbursement,” according to Dr. Creager.
He painted a picture of PAD as a field ripe with opportunities for improved outcomes.
“Our ability to diagnose and treat vascular diseases has never been greater. The field of vascular biology has virtually exploded in recent years,” he said. “I’d like to focus not only on the extent of this crisis, but also on the importance of using what we know to treat it and prevent it, and on the urgency of intensifying our research efforts to better understand it.”
In the diagnostic arena, optical coherence tomography, intravascular ultrasound, PET-CT, and contrast-enhanced MRI provide an unprecedented ability to image plaques and assess their vulnerability to rupture.
On the interventional front, innovative bioengineering has led to the development of drug-coated balloons and bioabsorbable vascular scaffolds with the potential to curb restenosis and preserve patency following endovascular treatment of critical lesions.
In terms of medical management, the novel, super-potent LDL cholesterol–lowering inhibitors of PCSK9 (proprotein convertase subtilisin-kexin type 9) will need to be studied in order to see if they have a special role in the treatment and/or prevention of PAD.
Roughly 2 decades ago, Dr. Creager and coinvestigators showed that endothelial function typically drops by 30% in our twenties and then in our thirties, and by 50% once we’re in our forties. A high research priority in PAD will be to learn how to more effectively prolong endothelial health and prevent vascular stiffness, he said.
He reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM THE AHA SCIENTIFIC SESSIONS
Décolletage Rejuvenation With Cosmetic Injectables and Beyond

As more patients undergo facial rejuvenation procedures for a more youthful look, there is a growing demand for rejuvenation of the décolletage (neck and chest) to achieve a more natural and seamless transition from the skin of the face to the chest. The same modalities that are used on the face to treat skin rhytides, texture, and discoloration have been used successfully in the décolletage area.
Vanaman and Fabi (Plast Reconstr Surg. 2015;136[suppl 5]:276S-281S) recently reviewed the chest anatomy and discussed the safe and effective use of cosmetic injectables alone or in combination with other modalities to address rhytides of the décolletage. The relatively low density of skin pilosebaceous units on the chest allows for slower healing and thus makes the area more vulnerable to scarring with the use of more invasive resurfacing modalities (eg, deeper chemical peels, ablative lasers). The use of cosmetic injectables offers a safer treatment option of chest rhytides. Furthermore, proper candidate selection excludes patients with known sensitivity to cosmetic injectables or their components, history of keloid or hypertrophic scar formation, and active inflammation in the treatment area.
Poly-L-lactic acid (PLLA) is a biodegradable, biocompatible, semipermanent, synthetic soft tissue biostimulator that promotes neocollagenesis by fibroblasts over time (3–6 months). The manufacturer’s recommendation for PLLA reconstitution is at least 2 hours prior to injection with sterile water of no less than 5 mL dilution. Vanaman and Fabi reported usually diluting the day prior to injection with 16 mL total volume. This technique showed the greatest improvement in chest rhytides with no adverse events reported in a retrospective analysis. Poly-L-lactic acid should be injected in a retrograde linear fashion in the plane of the subcutaneous fat, with injection boundaries on the suprasternal notch superiorly, the midclavicular line laterally, and the fourth rib inferolaterally for rejuvenation of the décolletage.
Nodule formation is a well-known complication of PLLA injection, although pain, bruising, edema, pruritus, and hematomas are more commonly seen. The risk of nodule formation can be decreased using several techniques, including avoiding overcorrection and excessive use of product in each individual session, avoiding intradermal injection, diluting to more than 5 mL with reconstitution at least overnight, massaging the area posttreatment (in office by the clinician and at home by the patient), and scheduling treatment sessions at least 4 weeks apart. Usually, 3 to 4 treatments are required and the results can last 2 years or longer without touch-ups.
Nonanimal stabilized hyaluronic acid (NASHA) fillers also can be used to correct chest rhytides; however, using NASHA fillers requires more syringes and results typically last only 6 to 8 months, making it more cost effective to use 2 to 3 vials of PLLA. Moreover, in Vanaman and Fabi’s experience, PLLA is associated with fewer nodules, possibly due to the depth of injection of PLLA into the subcutaneous fat versus injection into the deep dermis with NASHA fillers. Vanaman and Fabi currently are investigating the use of calcium hydroxylapatite fillers alone or in combination with an energy-based modality (microfocused ultrasound) with visualization in the treatment of rhytides in the décolletage.
What’s the Issue?
The availability of many modalities to keep facial skin looking fresh and rejuvenated has led to an increased demand for products and procedures to rejuvenate the décolletage. It is important for dermatologists to acknowledge the more delicate nature of the décolletage versus the face. Less invasive modalities such as cosmetic injectables can be employed in a safe and effective manner to correct rhytides of the chest with proper techniques, products, and patient selection. For a more natural transition from the skin of the face to the décolletage, it also may be necessary to adopt a multimodal approach by using botulinum toxin and fillers, as well as going beyond correction of rhytides to address skin texture and discoloration with chemical peels and lasers. Have you seen an increased demand for procedures to rejuvenate the décolletage in your practice?

As more patients undergo facial rejuvenation procedures for a more youthful look, there is a growing demand for rejuvenation of the décolletage (neck and chest) to achieve a more natural and seamless transition from the skin of the face to the chest. The same modalities that are used on the face to treat skin rhytides, texture, and discoloration have been used successfully in the décolletage area.
Vanaman and Fabi (Plast Reconstr Surg. 2015;136[suppl 5]:276S-281S) recently reviewed the chest anatomy and discussed the safe and effective use of cosmetic injectables alone or in combination with other modalities to address rhytides of the décolletage. The relatively low density of skin pilosebaceous units on the chest allows for slower healing and thus makes the area more vulnerable to scarring with the use of more invasive resurfacing modalities (eg, deeper chemical peels, ablative lasers). The use of cosmetic injectables offers a safer treatment option of chest rhytides. Furthermore, proper candidate selection excludes patients with known sensitivity to cosmetic injectables or their components, history of keloid or hypertrophic scar formation, and active inflammation in the treatment area.
Poly-L-lactic acid (PLLA) is a biodegradable, biocompatible, semipermanent, synthetic soft tissue biostimulator that promotes neocollagenesis by fibroblasts over time (3–6 months). The manufacturer’s recommendation for PLLA reconstitution is at least 2 hours prior to injection with sterile water of no less than 5 mL dilution. Vanaman and Fabi reported usually diluting the day prior to injection with 16 mL total volume. This technique showed the greatest improvement in chest rhytides with no adverse events reported in a retrospective analysis. Poly-L-lactic acid should be injected in a retrograde linear fashion in the plane of the subcutaneous fat, with injection boundaries on the suprasternal notch superiorly, the midclavicular line laterally, and the fourth rib inferolaterally for rejuvenation of the décolletage.
Nodule formation is a well-known complication of PLLA injection, although pain, bruising, edema, pruritus, and hematomas are more commonly seen. The risk of nodule formation can be decreased using several techniques, including avoiding overcorrection and excessive use of product in each individual session, avoiding intradermal injection, diluting to more than 5 mL with reconstitution at least overnight, massaging the area posttreatment (in office by the clinician and at home by the patient), and scheduling treatment sessions at least 4 weeks apart. Usually, 3 to 4 treatments are required and the results can last 2 years or longer without touch-ups.
Nonanimal stabilized hyaluronic acid (NASHA) fillers also can be used to correct chest rhytides; however, using NASHA fillers requires more syringes and results typically last only 6 to 8 months, making it more cost effective to use 2 to 3 vials of PLLA. Moreover, in Vanaman and Fabi’s experience, PLLA is associated with fewer nodules, possibly due to the depth of injection of PLLA into the subcutaneous fat versus injection into the deep dermis with NASHA fillers. Vanaman and Fabi currently are investigating the use of calcium hydroxylapatite fillers alone or in combination with an energy-based modality (microfocused ultrasound) with visualization in the treatment of rhytides in the décolletage.
What’s the Issue?
The availability of many modalities to keep facial skin looking fresh and rejuvenated has led to an increased demand for products and procedures to rejuvenate the décolletage. It is important for dermatologists to acknowledge the more delicate nature of the décolletage versus the face. Less invasive modalities such as cosmetic injectables can be employed in a safe and effective manner to correct rhytides of the chest with proper techniques, products, and patient selection. For a more natural transition from the skin of the face to the décolletage, it also may be necessary to adopt a multimodal approach by using botulinum toxin and fillers, as well as going beyond correction of rhytides to address skin texture and discoloration with chemical peels and lasers. Have you seen an increased demand for procedures to rejuvenate the décolletage in your practice?

As more patients undergo facial rejuvenation procedures for a more youthful look, there is a growing demand for rejuvenation of the décolletage (neck and chest) to achieve a more natural and seamless transition from the skin of the face to the chest. The same modalities that are used on the face to treat skin rhytides, texture, and discoloration have been used successfully in the décolletage area.
Vanaman and Fabi (Plast Reconstr Surg. 2015;136[suppl 5]:276S-281S) recently reviewed the chest anatomy and discussed the safe and effective use of cosmetic injectables alone or in combination with other modalities to address rhytides of the décolletage. The relatively low density of skin pilosebaceous units on the chest allows for slower healing and thus makes the area more vulnerable to scarring with the use of more invasive resurfacing modalities (eg, deeper chemical peels, ablative lasers). The use of cosmetic injectables offers a safer treatment option of chest rhytides. Furthermore, proper candidate selection excludes patients with known sensitivity to cosmetic injectables or their components, history of keloid or hypertrophic scar formation, and active inflammation in the treatment area.
Poly-L-lactic acid (PLLA) is a biodegradable, biocompatible, semipermanent, synthetic soft tissue biostimulator that promotes neocollagenesis by fibroblasts over time (3–6 months). The manufacturer’s recommendation for PLLA reconstitution is at least 2 hours prior to injection with sterile water of no less than 5 mL dilution. Vanaman and Fabi reported usually diluting the day prior to injection with 16 mL total volume. This technique showed the greatest improvement in chest rhytides with no adverse events reported in a retrospective analysis. Poly-L-lactic acid should be injected in a retrograde linear fashion in the plane of the subcutaneous fat, with injection boundaries on the suprasternal notch superiorly, the midclavicular line laterally, and the fourth rib inferolaterally for rejuvenation of the décolletage.
Nodule formation is a well-known complication of PLLA injection, although pain, bruising, edema, pruritus, and hematomas are more commonly seen. The risk of nodule formation can be decreased using several techniques, including avoiding overcorrection and excessive use of product in each individual session, avoiding intradermal injection, diluting to more than 5 mL with reconstitution at least overnight, massaging the area posttreatment (in office by the clinician and at home by the patient), and scheduling treatment sessions at least 4 weeks apart. Usually, 3 to 4 treatments are required and the results can last 2 years or longer without touch-ups.
Nonanimal stabilized hyaluronic acid (NASHA) fillers also can be used to correct chest rhytides; however, using NASHA fillers requires more syringes and results typically last only 6 to 8 months, making it more cost effective to use 2 to 3 vials of PLLA. Moreover, in Vanaman and Fabi’s experience, PLLA is associated with fewer nodules, possibly due to the depth of injection of PLLA into the subcutaneous fat versus injection into the deep dermis with NASHA fillers. Vanaman and Fabi currently are investigating the use of calcium hydroxylapatite fillers alone or in combination with an energy-based modality (microfocused ultrasound) with visualization in the treatment of rhytides in the décolletage.
What’s the Issue?
The availability of many modalities to keep facial skin looking fresh and rejuvenated has led to an increased demand for products and procedures to rejuvenate the décolletage. It is important for dermatologists to acknowledge the more delicate nature of the décolletage versus the face. Less invasive modalities such as cosmetic injectables can be employed in a safe and effective manner to correct rhytides of the chest with proper techniques, products, and patient selection. For a more natural transition from the skin of the face to the décolletage, it also may be necessary to adopt a multimodal approach by using botulinum toxin and fillers, as well as going beyond correction of rhytides to address skin texture and discoloration with chemical peels and lasers. Have you seen an increased demand for procedures to rejuvenate the décolletage in your practice?
Lixisenatide not cardioprotective in type 2 diabetes
Adding lixisenatide to usual care failed to prevent major cardiovascular events in an industry-sponsored clinical trial involving patients with type 2 diabetes who had a recent acute coronary syndrome, according to a report published online Dec. 3 in the New England Journal of Medicine.
Lixisenatide, a GLP-1-receptor agonist, is a glucose-lowering agent that inhibits glucagon secretion, prompts insulin production in response to hyperglycemia, and slows gastric emptying. In preliminary studies, lixisenatide showed some cardioprotective effects in myocardial ischemia and heart failure. To assess whether the drug would benefit diabetes patients at high CV risk, investigators conducted a randomized double-blind trial comparing lixisenatide with placebo in 6,068 patients who had type 2 diabetes and who had experienced acute coronary syndrome (ACS) during the preceding 6 months.
In addition to receiving usual diabetes care provided by their treating physicians, these patients (mean age, 60 years) were randomly assigned to self-administer once-daily subcutaneous injections of lixisenatide (n = 3,034) or a matching placebo (n = 3,034) and were followed for a mean of 25 months at 49 medical centers worldwide, said Dr. Marc A. Pfeffer of the cardiovascular division, Brigham and Women’s Hospital, and Dzau Professor of Medicine a Harvard Medical School, Boston.
The primary endpoint of the study – a composite of death from CV causes, nonfatal MI, nonfatal stroke, or hospitalization for unstable angina – occurred in 13.4% of patients receiving lixisenatide and 13.2% of those receiving placebo, a nonsignificant difference. There were no differences between the two study groups in any of the individual components of this composite endpoint (N Engl J Med. 2015 Dec. 3. doi:10.1056/NEJMoa1509225).
Sensitivity analyses and post hoc analyses of several subgroups of patients yielded similar results. When hospitalization for heart failure and coronary revascularization procedures were added to the primary endpoint, lixisenatide still provided no cardioprotective effect compared with placebo. Mortality from any cause was not significantly different between the two study groups, at 7.0% with lixisenatide and 7.4% with placebo.
Adverse effects leading to withdrawal from the study were more common with lixisenatide (11.4%) than placebo (7.2%). In particular, treatment discontinuation due to nausea and vomiting occurred in 3.0% of patients taking active treatment, compared with 0.4% of those taking placebo.
Sanofi, maker of lixisenatide, funded the study. Dr. Pfeffer reported receiving grants and personal fees from Sanofi and 20 other drug companies; his associates reported ties to numerous industry sources.
Adding lixisenatide to usual care failed to prevent major cardiovascular events in an industry-sponsored clinical trial involving patients with type 2 diabetes who had a recent acute coronary syndrome, according to a report published online Dec. 3 in the New England Journal of Medicine.
Lixisenatide, a GLP-1-receptor agonist, is a glucose-lowering agent that inhibits glucagon secretion, prompts insulin production in response to hyperglycemia, and slows gastric emptying. In preliminary studies, lixisenatide showed some cardioprotective effects in myocardial ischemia and heart failure. To assess whether the drug would benefit diabetes patients at high CV risk, investigators conducted a randomized double-blind trial comparing lixisenatide with placebo in 6,068 patients who had type 2 diabetes and who had experienced acute coronary syndrome (ACS) during the preceding 6 months.
In addition to receiving usual diabetes care provided by their treating physicians, these patients (mean age, 60 years) were randomly assigned to self-administer once-daily subcutaneous injections of lixisenatide (n = 3,034) or a matching placebo (n = 3,034) and were followed for a mean of 25 months at 49 medical centers worldwide, said Dr. Marc A. Pfeffer of the cardiovascular division, Brigham and Women’s Hospital, and Dzau Professor of Medicine a Harvard Medical School, Boston.
The primary endpoint of the study – a composite of death from CV causes, nonfatal MI, nonfatal stroke, or hospitalization for unstable angina – occurred in 13.4% of patients receiving lixisenatide and 13.2% of those receiving placebo, a nonsignificant difference. There were no differences between the two study groups in any of the individual components of this composite endpoint (N Engl J Med. 2015 Dec. 3. doi:10.1056/NEJMoa1509225).
Sensitivity analyses and post hoc analyses of several subgroups of patients yielded similar results. When hospitalization for heart failure and coronary revascularization procedures were added to the primary endpoint, lixisenatide still provided no cardioprotective effect compared with placebo. Mortality from any cause was not significantly different between the two study groups, at 7.0% with lixisenatide and 7.4% with placebo.
Adverse effects leading to withdrawal from the study were more common with lixisenatide (11.4%) than placebo (7.2%). In particular, treatment discontinuation due to nausea and vomiting occurred in 3.0% of patients taking active treatment, compared with 0.4% of those taking placebo.
Sanofi, maker of lixisenatide, funded the study. Dr. Pfeffer reported receiving grants and personal fees from Sanofi and 20 other drug companies; his associates reported ties to numerous industry sources.
Adding lixisenatide to usual care failed to prevent major cardiovascular events in an industry-sponsored clinical trial involving patients with type 2 diabetes who had a recent acute coronary syndrome, according to a report published online Dec. 3 in the New England Journal of Medicine.
Lixisenatide, a GLP-1-receptor agonist, is a glucose-lowering agent that inhibits glucagon secretion, prompts insulin production in response to hyperglycemia, and slows gastric emptying. In preliminary studies, lixisenatide showed some cardioprotective effects in myocardial ischemia and heart failure. To assess whether the drug would benefit diabetes patients at high CV risk, investigators conducted a randomized double-blind trial comparing lixisenatide with placebo in 6,068 patients who had type 2 diabetes and who had experienced acute coronary syndrome (ACS) during the preceding 6 months.
In addition to receiving usual diabetes care provided by their treating physicians, these patients (mean age, 60 years) were randomly assigned to self-administer once-daily subcutaneous injections of lixisenatide (n = 3,034) or a matching placebo (n = 3,034) and were followed for a mean of 25 months at 49 medical centers worldwide, said Dr. Marc A. Pfeffer of the cardiovascular division, Brigham and Women’s Hospital, and Dzau Professor of Medicine a Harvard Medical School, Boston.
The primary endpoint of the study – a composite of death from CV causes, nonfatal MI, nonfatal stroke, or hospitalization for unstable angina – occurred in 13.4% of patients receiving lixisenatide and 13.2% of those receiving placebo, a nonsignificant difference. There were no differences between the two study groups in any of the individual components of this composite endpoint (N Engl J Med. 2015 Dec. 3. doi:10.1056/NEJMoa1509225).
Sensitivity analyses and post hoc analyses of several subgroups of patients yielded similar results. When hospitalization for heart failure and coronary revascularization procedures were added to the primary endpoint, lixisenatide still provided no cardioprotective effect compared with placebo. Mortality from any cause was not significantly different between the two study groups, at 7.0% with lixisenatide and 7.4% with placebo.
Adverse effects leading to withdrawal from the study were more common with lixisenatide (11.4%) than placebo (7.2%). In particular, treatment discontinuation due to nausea and vomiting occurred in 3.0% of patients taking active treatment, compared with 0.4% of those taking placebo.
Sanofi, maker of lixisenatide, funded the study. Dr. Pfeffer reported receiving grants and personal fees from Sanofi and 20 other drug companies; his associates reported ties to numerous industry sources.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Key clinical point: Adding lixisenatide to usual care failed to prevent major cardiovascular events in patients with type 2 diabetes who had a recent ACS.
Major finding: Death from CV causes, nonfatal MI, nonfatal stroke, or hospitalization for unstable angina occurred in 13.4% of participants receiving lixisenatide and 13.2% of those receiving placebo.
Data source: An international randomized double-blind placebo-controlled trial involving 6,068 patients followed for a median of 2 years.
Disclosures: Sanofi, maker of lixisenatide, funded the study. Dr. Pfeffer reported receiving grants and personal fees from Sanofi and 20 other drug companies; his associates reported ties to numerous industry sources.
Pediatric Dermatology Consult - November 2015
Tinea versicolor
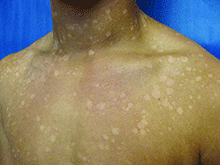
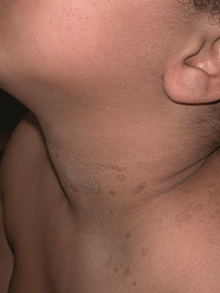
Tinea versicolor – also called pityriasis versicolor – is a benign superficial fungal skin infection caused by Malassezia. It presents as well-demarcated, oval, finely scaling macules, patches, or thin plaques, which can be hypopigmented, hyperpigmented, or erythematous.1,2,3 . The name, tinea versicolor, highlights the variability in the color of lesions.4
Scale may be minimal, but becomes more noticeable when lesions are scraped, which is called the “evoked scale sign.”5 The lesions may be asymptomatic or slightly pruritic.1 Lesions range in size from several millimeters to several centimeters and may coalesce.6 They are most commonly found on the chest, back, upper arms, and neck,2,7 but in children the face may be affected.1,3,8 Hypopigmented lesions may be most noticeable during the summer when the surrounding uninvolved skin darkens with sun exposure.9 Tinea versicolor is not contagious, but pigmentary changes may cause cosmetic concerns, and the condition may persist for years if not treated.2,4
Malassezia is a dimorphic fungus that is part of the normal skin flora in its yeast form, but if Malassezia converts to its hyphal form, it is able penetrate the stratum corneum and cause the tinea versicolor rash.1,10 The reason for the conversion from yeast to hyphal form is not fully understood.11Malassezia is lipophilic, so it thrives when sebum production is high, which is why tinea versicolor most commonly develops in adolescence or young adulthood, although it may be seen in younger children and older adults.12 Genetic predisposition, warm and humid environments, oily skin, use of oily creams, use of corticosteroids, hyperhidrosis, physical activity, malnutrition, immunosuppression, and exposure to sunlight increase susceptibility.1,13,14
Tinea versicolor is most commonly caused by Malassezia globosa and Malassezia furfur.13,15,16 Hypopigmentation may be caused by Malassezia’s production of azelaic acid, which inhibits the dopa-tyrosinase reaction that is part of melanin synthesis.15,17,18 Hyperpigmentation may result from inflammation.15,18 The evoked scale sign results from the production of keratinase, which disrupts the stratum corneum.5
Tinea versicolor often can be diagnosed by its characteristic clinical appearance and may fluoresce golden under a Wood’s ultraviolet lamp.19 Diagnosis can be confirmed by microscopic examination of skin scrapings treated with potassium hydroxide (KOH), which will have a “spaghetti and meatball” appearance, with the hyphae resembling spaghetti and spores resembling meatballs.1 For young children, removing scale with transparent tape can be a good alternative to scraping skin with a blade.2,19
Differential diagnosis
Postinflammatory pigment changes, both hypo and hyper, usually lack scale, may be anywhere on the body, and should have the same distribution as some original inflammation.
Pityriasis alba presents with hypopigmented patches, typically on the face, and has a more subtle “blotchy” appearance, without discrete oval patches. Pityriasis rosea may appear similar to tinea versicolor with erythema and scale, but it typically begins with a single, large herald patch, and scale is primarily at the outer border of the lesions.1
Tinea corporis (“ringworm”), which is caused by a dermatophyte, is more distinctly ring shaped with a scaly, vesicular, papular, or pustular border and there is often a clear center that may not scale when scraped.5,9 It is much more commonly localized, except in immunosuppressed patients or if mistreated with topical corticosteroids. Vitiligo lesions are completely depigmented, rather than just hypopigmented, and lack scale.1 Psoriasis scale is thicker and is visible without any provocation.
Treatment
First-line treatments for tinea versicolor include ketoconazole shampoo, selenium sulfide lotion or shampoo, and zinc pyrithione shampoo, which are left on for 5-10 minutes before rinsing.1,20 Any of these treatments is a fine first choice, as all are effective, and there are no robust data establishing the superiority of any single treatment.20 The typical treatment duration is 1-4 weeks.1 Longer treatment durations yield better cure rates.20 Ketoconazole and selenium sulfide also are available in foam formulations.11 Shampoo and foam formulations have the benefit of easily covering a large affected area.
Alternatively, terbinafine cream can be applied twice daily for a week or ketoconazole cream can be applied twice daily for 1-4 weeks.1,21 It is advisable to treat the whole trunk, neck, arms, and legs down to the knees, even if only a small area is involved.14,22 Antifungal treatments are well tolerated, with skin irritation and contact allergy being the most common adverse effects.1 Selenium sulfide has a strong odor.1
Hypopigmentation and hyperpigmentation can persist for months after the active infection has resolved and do not necessarily indicate a treatment failure.2,20 However, because Malassezia is a part of the normal skin flora, recurrence is common, occurring in 60%-80% of patients within 2 years.14 Recurrence or persistence of an active infection can be proven by a positive KOH scrape test. If a first treatment fails, a different first-line topical medication should be tried.1 Referral to a dermatologist is recommended if the eruption is unresponsive to two treatments.1
Oral antifungals such as itraconazole, fluconazole, and pramiconazole are effective for tinea versicolor, but have more adverse effects than topicals and interact with other medications because of their inhibition of the cytochrome P450 system, so they are used only for refractory or widespread disease.1,11 In 2013, the Food and Drug Administration issued a black box warning against oral ketoconazole due its ability to cause life-threatening hepatotoxicity and adrenal insufficiency1,23,24; it should not be used to treat tinea versicolor. Topical ketoconazole is safe and remains a first-line treatment for tinea versicolor, as discussed above.24 Oral terbinafine is not effective for tinea versicolor despite its efficacy as a topical treatment.11
Patients with recurrent tinea versicolor can try preventive therapy with ketoconazole shampoo, zinc pyrithione shampoo, or selenium sulfide lotion or shampoo one to four times per month.1 Oral antifungals also are effective for prevention of recurrence, but should be used only if the condition is refractory to topical prophylaxis.20,25 It is important to remember that hypopigmentation and hyperpigmentation may persist for months after resolution of active infection; absence of hyphae on skin scraping prepared with KOH confirms absence of active disease.15,16
References
- BMJ. 2015;350:h1394. doi:10.1136/bmj.h1394.
- Lancet. 2004 Sep 25-Oct 1;364(9440):1173-82.
- Pediatr Dermatol. 1991;8(1):9-12.
- J Eur Acad Dermatol Venereol. 2002;16(1):19-33.
- Arch Dermatol. 2009;145(9):1078.
- Vitiligo and other disorders of pigmentation, in: “Dermatology,” Vol 1. 3rd ed. (Philadelphia: Elsevier Saunders, 2012, pp.1041-2.)
- Am J Clin Dermatol. 2000 Mar-Apr;1(2):75-80.
- Mycoses. 1995;38(5-6):227-8.
- “Skin Disorders Due to Fungi,” in: Hurwitz Clinical Pediatric Dermatology 4th ed. (Philadelphia: Saunders, 2011, pp. 396-403).
- Infect Control Hosp Epidemiol. 2002 Apr;23(4):212-6.
- Expert Opin Pharmacother. 2014 Aug;15(12):1707-13.
- Clin Microbiol Rev. 2002 Jan;15(1):21-57.
- Clin Dermatol. 2010 Mar 4;28(2):185-9.
- J Am Acad Dermatol. 1994 Sep;31(3 Pt 2):S18-20.
- Int J Dermatol. 2014 Feb;53(2):137-41
- Mycopathologia. 2006 Dec;162(6):373-6.
- J Invest Dermatol. 1978 Sep;71(3):205-8.
- Int J Dermatol. 1992 Apr;31(4):253-6.
- Pediatr Dermatol. 2000 Jan-Feb;17(1):68-9.
- Arch Dermatol. 2010 Oct;146(10):1132-40.
- Dermatology (Basel). 1997;194(1):22-4. doi:10.1159/000246179.
- Red Book Plus: 2009 Report of the Committee on Infectious Disease.
- http://www.fda.gov/Drugs/DrugSafety/ucm362415.htm
- J Cutan Med Surg. 2015 Jul-Aug;19(4):352-7.
- Arch Dermatol. 2002 Jan;138(1):69-73.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Ms. Haddock is a medical student at the University of California, San Diego, and a research associate at the hospital. Dr. Eichenfield and Ms. Haddock said they have no relevant financial disclosures.
Email to [email protected].
Tinea versicolor
Tinea versicolor – also called pityriasis versicolor – is a benign superficial fungal skin infection caused by Malassezia. It presents as well-demarcated, oval, finely scaling macules, patches, or thin plaques, which can be hypopigmented, hyperpigmented, or erythematous.1,2,3 . The name, tinea versicolor, highlights the variability in the color of lesions.4
Scale may be minimal, but becomes more noticeable when lesions are scraped, which is called the “evoked scale sign.”5 The lesions may be asymptomatic or slightly pruritic.1 Lesions range in size from several millimeters to several centimeters and may coalesce.6 They are most commonly found on the chest, back, upper arms, and neck,2,7 but in children the face may be affected.1,3,8 Hypopigmented lesions may be most noticeable during the summer when the surrounding uninvolved skin darkens with sun exposure.9 Tinea versicolor is not contagious, but pigmentary changes may cause cosmetic concerns, and the condition may persist for years if not treated.2,4
Malassezia is a dimorphic fungus that is part of the normal skin flora in its yeast form, but if Malassezia converts to its hyphal form, it is able penetrate the stratum corneum and cause the tinea versicolor rash.1,10 The reason for the conversion from yeast to hyphal form is not fully understood.11Malassezia is lipophilic, so it thrives when sebum production is high, which is why tinea versicolor most commonly develops in adolescence or young adulthood, although it may be seen in younger children and older adults.12 Genetic predisposition, warm and humid environments, oily skin, use of oily creams, use of corticosteroids, hyperhidrosis, physical activity, malnutrition, immunosuppression, and exposure to sunlight increase susceptibility.1,13,14
Tinea versicolor is most commonly caused by Malassezia globosa and Malassezia furfur.13,15,16 Hypopigmentation may be caused by Malassezia’s production of azelaic acid, which inhibits the dopa-tyrosinase reaction that is part of melanin synthesis.15,17,18 Hyperpigmentation may result from inflammation.15,18 The evoked scale sign results from the production of keratinase, which disrupts the stratum corneum.5
Tinea versicolor often can be diagnosed by its characteristic clinical appearance and may fluoresce golden under a Wood’s ultraviolet lamp.19 Diagnosis can be confirmed by microscopic examination of skin scrapings treated with potassium hydroxide (KOH), which will have a “spaghetti and meatball” appearance, with the hyphae resembling spaghetti and spores resembling meatballs.1 For young children, removing scale with transparent tape can be a good alternative to scraping skin with a blade.2,19
Differential diagnosis
Postinflammatory pigment changes, both hypo and hyper, usually lack scale, may be anywhere on the body, and should have the same distribution as some original inflammation.
Pityriasis alba presents with hypopigmented patches, typically on the face, and has a more subtle “blotchy” appearance, without discrete oval patches. Pityriasis rosea may appear similar to tinea versicolor with erythema and scale, but it typically begins with a single, large herald patch, and scale is primarily at the outer border of the lesions.1
Tinea corporis (“ringworm”), which is caused by a dermatophyte, is more distinctly ring shaped with a scaly, vesicular, papular, or pustular border and there is often a clear center that may not scale when scraped.5,9 It is much more commonly localized, except in immunosuppressed patients or if mistreated with topical corticosteroids. Vitiligo lesions are completely depigmented, rather than just hypopigmented, and lack scale.1 Psoriasis scale is thicker and is visible without any provocation.
Treatment
First-line treatments for tinea versicolor include ketoconazole shampoo, selenium sulfide lotion or shampoo, and zinc pyrithione shampoo, which are left on for 5-10 minutes before rinsing.1,20 Any of these treatments is a fine first choice, as all are effective, and there are no robust data establishing the superiority of any single treatment.20 The typical treatment duration is 1-4 weeks.1 Longer treatment durations yield better cure rates.20 Ketoconazole and selenium sulfide also are available in foam formulations.11 Shampoo and foam formulations have the benefit of easily covering a large affected area.
Alternatively, terbinafine cream can be applied twice daily for a week or ketoconazole cream can be applied twice daily for 1-4 weeks.1,21 It is advisable to treat the whole trunk, neck, arms, and legs down to the knees, even if only a small area is involved.14,22 Antifungal treatments are well tolerated, with skin irritation and contact allergy being the most common adverse effects.1 Selenium sulfide has a strong odor.1
Hypopigmentation and hyperpigmentation can persist for months after the active infection has resolved and do not necessarily indicate a treatment failure.2,20 However, because Malassezia is a part of the normal skin flora, recurrence is common, occurring in 60%-80% of patients within 2 years.14 Recurrence or persistence of an active infection can be proven by a positive KOH scrape test. If a first treatment fails, a different first-line topical medication should be tried.1 Referral to a dermatologist is recommended if the eruption is unresponsive to two treatments.1
Oral antifungals such as itraconazole, fluconazole, and pramiconazole are effective for tinea versicolor, but have more adverse effects than topicals and interact with other medications because of their inhibition of the cytochrome P450 system, so they are used only for refractory or widespread disease.1,11 In 2013, the Food and Drug Administration issued a black box warning against oral ketoconazole due its ability to cause life-threatening hepatotoxicity and adrenal insufficiency1,23,24; it should not be used to treat tinea versicolor. Topical ketoconazole is safe and remains a first-line treatment for tinea versicolor, as discussed above.24 Oral terbinafine is not effective for tinea versicolor despite its efficacy as a topical treatment.11
Patients with recurrent tinea versicolor can try preventive therapy with ketoconazole shampoo, zinc pyrithione shampoo, or selenium sulfide lotion or shampoo one to four times per month.1 Oral antifungals also are effective for prevention of recurrence, but should be used only if the condition is refractory to topical prophylaxis.20,25 It is important to remember that hypopigmentation and hyperpigmentation may persist for months after resolution of active infection; absence of hyphae on skin scraping prepared with KOH confirms absence of active disease.15,16
References
- BMJ. 2015;350:h1394. doi:10.1136/bmj.h1394.
- Lancet. 2004 Sep 25-Oct 1;364(9440):1173-82.
- Pediatr Dermatol. 1991;8(1):9-12.
- J Eur Acad Dermatol Venereol. 2002;16(1):19-33.
- Arch Dermatol. 2009;145(9):1078.
- Vitiligo and other disorders of pigmentation, in: “Dermatology,” Vol 1. 3rd ed. (Philadelphia: Elsevier Saunders, 2012, pp.1041-2.)
- Am J Clin Dermatol. 2000 Mar-Apr;1(2):75-80.
- Mycoses. 1995;38(5-6):227-8.
- “Skin Disorders Due to Fungi,” in: Hurwitz Clinical Pediatric Dermatology 4th ed. (Philadelphia: Saunders, 2011, pp. 396-403).
- Infect Control Hosp Epidemiol. 2002 Apr;23(4):212-6.
- Expert Opin Pharmacother. 2014 Aug;15(12):1707-13.
- Clin Microbiol Rev. 2002 Jan;15(1):21-57.
- Clin Dermatol. 2010 Mar 4;28(2):185-9.
- J Am Acad Dermatol. 1994 Sep;31(3 Pt 2):S18-20.
- Int J Dermatol. 2014 Feb;53(2):137-41
- Mycopathologia. 2006 Dec;162(6):373-6.
- J Invest Dermatol. 1978 Sep;71(3):205-8.
- Int J Dermatol. 1992 Apr;31(4):253-6.
- Pediatr Dermatol. 2000 Jan-Feb;17(1):68-9.
- Arch Dermatol. 2010 Oct;146(10):1132-40.
- Dermatology (Basel). 1997;194(1):22-4. doi:10.1159/000246179.
- Red Book Plus: 2009 Report of the Committee on Infectious Disease.
- http://www.fda.gov/Drugs/DrugSafety/ucm362415.htm
- J Cutan Med Surg. 2015 Jul-Aug;19(4):352-7.
- Arch Dermatol. 2002 Jan;138(1):69-73.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Ms. Haddock is a medical student at the University of California, San Diego, and a research associate at the hospital. Dr. Eichenfield and Ms. Haddock said they have no relevant financial disclosures.
Email to [email protected].
Tinea versicolor
Tinea versicolor – also called pityriasis versicolor – is a benign superficial fungal skin infection caused by Malassezia. It presents as well-demarcated, oval, finely scaling macules, patches, or thin plaques, which can be hypopigmented, hyperpigmented, or erythematous.1,2,3 . The name, tinea versicolor, highlights the variability in the color of lesions.4
Scale may be minimal, but becomes more noticeable when lesions are scraped, which is called the “evoked scale sign.”5 The lesions may be asymptomatic or slightly pruritic.1 Lesions range in size from several millimeters to several centimeters and may coalesce.6 They are most commonly found on the chest, back, upper arms, and neck,2,7 but in children the face may be affected.1,3,8 Hypopigmented lesions may be most noticeable during the summer when the surrounding uninvolved skin darkens with sun exposure.9 Tinea versicolor is not contagious, but pigmentary changes may cause cosmetic concerns, and the condition may persist for years if not treated.2,4
Malassezia is a dimorphic fungus that is part of the normal skin flora in its yeast form, but if Malassezia converts to its hyphal form, it is able penetrate the stratum corneum and cause the tinea versicolor rash.1,10 The reason for the conversion from yeast to hyphal form is not fully understood.11Malassezia is lipophilic, so it thrives when sebum production is high, which is why tinea versicolor most commonly develops in adolescence or young adulthood, although it may be seen in younger children and older adults.12 Genetic predisposition, warm and humid environments, oily skin, use of oily creams, use of corticosteroids, hyperhidrosis, physical activity, malnutrition, immunosuppression, and exposure to sunlight increase susceptibility.1,13,14
Tinea versicolor is most commonly caused by Malassezia globosa and Malassezia furfur.13,15,16 Hypopigmentation may be caused by Malassezia’s production of azelaic acid, which inhibits the dopa-tyrosinase reaction that is part of melanin synthesis.15,17,18 Hyperpigmentation may result from inflammation.15,18 The evoked scale sign results from the production of keratinase, which disrupts the stratum corneum.5
Tinea versicolor often can be diagnosed by its characteristic clinical appearance and may fluoresce golden under a Wood’s ultraviolet lamp.19 Diagnosis can be confirmed by microscopic examination of skin scrapings treated with potassium hydroxide (KOH), which will have a “spaghetti and meatball” appearance, with the hyphae resembling spaghetti and spores resembling meatballs.1 For young children, removing scale with transparent tape can be a good alternative to scraping skin with a blade.2,19
Differential diagnosis
Postinflammatory pigment changes, both hypo and hyper, usually lack scale, may be anywhere on the body, and should have the same distribution as some original inflammation.
Pityriasis alba presents with hypopigmented patches, typically on the face, and has a more subtle “blotchy” appearance, without discrete oval patches. Pityriasis rosea may appear similar to tinea versicolor with erythema and scale, but it typically begins with a single, large herald patch, and scale is primarily at the outer border of the lesions.1
Tinea corporis (“ringworm”), which is caused by a dermatophyte, is more distinctly ring shaped with a scaly, vesicular, papular, or pustular border and there is often a clear center that may not scale when scraped.5,9 It is much more commonly localized, except in immunosuppressed patients or if mistreated with topical corticosteroids. Vitiligo lesions are completely depigmented, rather than just hypopigmented, and lack scale.1 Psoriasis scale is thicker and is visible without any provocation.
Treatment
First-line treatments for tinea versicolor include ketoconazole shampoo, selenium sulfide lotion or shampoo, and zinc pyrithione shampoo, which are left on for 5-10 minutes before rinsing.1,20 Any of these treatments is a fine first choice, as all are effective, and there are no robust data establishing the superiority of any single treatment.20 The typical treatment duration is 1-4 weeks.1 Longer treatment durations yield better cure rates.20 Ketoconazole and selenium sulfide also are available in foam formulations.11 Shampoo and foam formulations have the benefit of easily covering a large affected area.
Alternatively, terbinafine cream can be applied twice daily for a week or ketoconazole cream can be applied twice daily for 1-4 weeks.1,21 It is advisable to treat the whole trunk, neck, arms, and legs down to the knees, even if only a small area is involved.14,22 Antifungal treatments are well tolerated, with skin irritation and contact allergy being the most common adverse effects.1 Selenium sulfide has a strong odor.1
Hypopigmentation and hyperpigmentation can persist for months after the active infection has resolved and do not necessarily indicate a treatment failure.2,20 However, because Malassezia is a part of the normal skin flora, recurrence is common, occurring in 60%-80% of patients within 2 years.14 Recurrence or persistence of an active infection can be proven by a positive KOH scrape test. If a first treatment fails, a different first-line topical medication should be tried.1 Referral to a dermatologist is recommended if the eruption is unresponsive to two treatments.1
Oral antifungals such as itraconazole, fluconazole, and pramiconazole are effective for tinea versicolor, but have more adverse effects than topicals and interact with other medications because of their inhibition of the cytochrome P450 system, so they are used only for refractory or widespread disease.1,11 In 2013, the Food and Drug Administration issued a black box warning against oral ketoconazole due its ability to cause life-threatening hepatotoxicity and adrenal insufficiency1,23,24; it should not be used to treat tinea versicolor. Topical ketoconazole is safe and remains a first-line treatment for tinea versicolor, as discussed above.24 Oral terbinafine is not effective for tinea versicolor despite its efficacy as a topical treatment.11
Patients with recurrent tinea versicolor can try preventive therapy with ketoconazole shampoo, zinc pyrithione shampoo, or selenium sulfide lotion or shampoo one to four times per month.1 Oral antifungals also are effective for prevention of recurrence, but should be used only if the condition is refractory to topical prophylaxis.20,25 It is important to remember that hypopigmentation and hyperpigmentation may persist for months after resolution of active infection; absence of hyphae on skin scraping prepared with KOH confirms absence of active disease.15,16
References
- BMJ. 2015;350:h1394. doi:10.1136/bmj.h1394.
- Lancet. 2004 Sep 25-Oct 1;364(9440):1173-82.
- Pediatr Dermatol. 1991;8(1):9-12.
- J Eur Acad Dermatol Venereol. 2002;16(1):19-33.
- Arch Dermatol. 2009;145(9):1078.
- Vitiligo and other disorders of pigmentation, in: “Dermatology,” Vol 1. 3rd ed. (Philadelphia: Elsevier Saunders, 2012, pp.1041-2.)
- Am J Clin Dermatol. 2000 Mar-Apr;1(2):75-80.
- Mycoses. 1995;38(5-6):227-8.
- “Skin Disorders Due to Fungi,” in: Hurwitz Clinical Pediatric Dermatology 4th ed. (Philadelphia: Saunders, 2011, pp. 396-403).
- Infect Control Hosp Epidemiol. 2002 Apr;23(4):212-6.
- Expert Opin Pharmacother. 2014 Aug;15(12):1707-13.
- Clin Microbiol Rev. 2002 Jan;15(1):21-57.
- Clin Dermatol. 2010 Mar 4;28(2):185-9.
- J Am Acad Dermatol. 1994 Sep;31(3 Pt 2):S18-20.
- Int J Dermatol. 2014 Feb;53(2):137-41
- Mycopathologia. 2006 Dec;162(6):373-6.
- J Invest Dermatol. 1978 Sep;71(3):205-8.
- Int J Dermatol. 1992 Apr;31(4):253-6.
- Pediatr Dermatol. 2000 Jan-Feb;17(1):68-9.
- Arch Dermatol. 2010 Oct;146(10):1132-40.
- Dermatology (Basel). 1997;194(1):22-4. doi:10.1159/000246179.
- Red Book Plus: 2009 Report of the Committee on Infectious Disease.
- http://www.fda.gov/Drugs/DrugSafety/ucm362415.htm
- J Cutan Med Surg. 2015 Jul-Aug;19(4):352-7.
- Arch Dermatol. 2002 Jan;138(1):69-73.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Ms. Haddock is a medical student at the University of California, San Diego, and a research associate at the hospital. Dr. Eichenfield and Ms. Haddock said they have no relevant financial disclosures.
Email to [email protected].
A 16-year-old male youth presents for evaluation of a worsening rash of pale spots on his chest, shoulders, neck, and back. He first noticed spots on his chest a month ago during his summer break from high school. The spots do not itch or cause any discomfort, but they have spread to his neck, back, and shoulders, and his friends on the basketball team have begun making comments about them after practice. He has no other history of skin disease, and he is otherwise healthy, with no recent illness. There is a family history of vitiligo in his father and paternal uncle. On exam, the patient is a healthy male with Fitzpatrick type V brownish skin. He has scattered hypopigmented patches ranging in size from 1 mm to 3 mm on his chest, shoulders, neck, and back. The lesions are slightly erythematous, and some of them overlap. He has a few small hypopigmented patches on his cheeks. His lesions do not appear scaly initially, but a fine scale becomes visible when the lesions are scraped with the edge of a glass slide. He has a few comedones on his forehead consistent with mild acne.
Factors Associated with Missed Dermatology Appointments
To the Editor:
Missed appointments are a major issue in every discipline of medicine1 and can be detrimental for dermatologists,2,3 whose clinics often have long wait times for referred patients and can lose up to $200 for each missed appointment.4 The purpose of this study was to quantify the rate of missed appointments at an academic dermatology clinic and identify factors associated with patient nonattendance.
After approval by an institutional review board, appointment data was collected from the electronic medical record at the dermatology clinic at Wake Forest Baptist Health, Winston-Salem, North Carolina, for the period from May 1, 2013, to April 30, 2014. Variables that were evaluated included age, race, sex, primary language, employment status, zip code, appointment time, insurance coverage, scheduled provider, patient status (new vs returning), and the nature of the visit (cosmetic vs noncosmetic visits and procedural vs nonprocedural visits). Zip codes served as a representation of distance traveled and were stratified into 4 concentric zones: zone 1 represented the region corresponding to the clinic’s zip code; zone 2 represented regions with zip codes adjacent to zone 1; and the remaining zones were determined by regions with zip codes adjacent to the prior zone. Primary language spoken was categorized as English or non-English. Insurance coverage was categorized as private, Medicaid, Medicare, self-pay, and other. Using stepwise selection, both a univariate model and a multivariable logistic regression model were created (variable inclusion, P≤.10; variable exclusion, P>.05). Of the 28,772 appointments scheduled during the study period, 5584 (19.4%) were missed. Univariate and multivariable analyses of the factors associated with missed appointments are shown in Table 1.
A telephone survey also was conducted to evaluate patient-reported factors associated with missed dermatology appointments. A list of patients who missed appointments during the period from January 1, 2014, to April 30, 2014, was extracted and every fourth patient was called within 6 weeks of the appointment to minimize recall bias. Patients were excluded from the study if they could not be reached after 3 attempts. Of the 799 patients contacted, 300 (38%) responded to the survey; 98 (12%) had phone numbers on record that were incorrect or were no longer in service; and 401 (50%) could not be reached after 3 attempts. The results of the telephone survey are provided in Table 2.
The demographic data suggested that characteristics associated with higher rates of missed appointments tended to reflect physical or financial barriers, such as dependency on others for transportation (eg, pediatric patients), longer distance traveled to the clinic, and lack of insurance coverage; however, only 4% and 8% of the survey respondents reported that they missed their appointment due to financial reasons or that they were unable to obtain transportation, respectively. Of the patients surveyed, 35% cited that the reason they missed their appointment was that they forgot about the appointment; additionally, 24% of respondents reported that they had not been reminded of the appointment.
Although physicians cannot directly address physical or financial barriers to attendance, we can introduce more effective methods of communication for patient reminders. Of the 799 patients who were called for the telephone survey, 12.3% had phone numbers on record that were either incorrect or no longer in service. As these patients’ phone numbers were listed in the electronic medical record for contact purposes, they likely did not receive telephone calls reminding them about their appointments. Although it was not formally evaluated in this study, many respondents expressed that they had other preferred methods of receiving appointment reminders (eg, e-mail, text message) than those that are currently considered commonplace (ie, telephone calls, voicemails).
This study was limited in that the appointment data came from a single academic dermatology clinic. There also were limitations in the data set for subgroup analysis; for example, to appropriately assess socioeconomic barriers to attendance of dermatology appointments, it would be valuable to stratify income within established factors of socioeconomic barriers (eg, race, employment status) to avoid research bias. Although many variables assessed were statistically significant (P<.05), the odds ratios often were close to 1, suggesting that they may not be clinically or practically relevant.
By identifying factors associated with missed dermatology appointments, interventions can be instituted to target high-risk groups and alter patient reminder protocols. If possible, identifying patients’ preferred contact methods (eg, telephone call, text message, etc) and verifying contact information may be cost-effective ways to reduce missed appointments in dermatology offices.
1. George A, Rubin G. Non-attendance in general practice: a systematic review and its implications for access to primary health care. Fam Pract. 2003;20:178-184.
2. Canizares MJ, Penneys NS. The incidence of nonattendance at an urgent care dermatology clinic. J Am Acad Dermatol. 2002;46:457-459.
3. Cronin PR, DeCoste L, Kimball AB. A multivariate analysis of dermatology missed appointment predictors. JAMA Dermatol. 2013;149:1435-1437.
4. Perez FD, Xie J, Sin A, et al. Characteristics and direct costs of academic pediatric subspecialty outpatient no-show events. J Healthc Qual. 2014;36:32-42.
To the Editor:
Missed appointments are a major issue in every discipline of medicine1 and can be detrimental for dermatologists,2,3 whose clinics often have long wait times for referred patients and can lose up to $200 for each missed appointment.4 The purpose of this study was to quantify the rate of missed appointments at an academic dermatology clinic and identify factors associated with patient nonattendance.
After approval by an institutional review board, appointment data was collected from the electronic medical record at the dermatology clinic at Wake Forest Baptist Health, Winston-Salem, North Carolina, for the period from May 1, 2013, to April 30, 2014. Variables that were evaluated included age, race, sex, primary language, employment status, zip code, appointment time, insurance coverage, scheduled provider, patient status (new vs returning), and the nature of the visit (cosmetic vs noncosmetic visits and procedural vs nonprocedural visits). Zip codes served as a representation of distance traveled and were stratified into 4 concentric zones: zone 1 represented the region corresponding to the clinic’s zip code; zone 2 represented regions with zip codes adjacent to zone 1; and the remaining zones were determined by regions with zip codes adjacent to the prior zone. Primary language spoken was categorized as English or non-English. Insurance coverage was categorized as private, Medicaid, Medicare, self-pay, and other. Using stepwise selection, both a univariate model and a multivariable logistic regression model were created (variable inclusion, P≤.10; variable exclusion, P>.05). Of the 28,772 appointments scheduled during the study period, 5584 (19.4%) were missed. Univariate and multivariable analyses of the factors associated with missed appointments are shown in Table 1.
A telephone survey also was conducted to evaluate patient-reported factors associated with missed dermatology appointments. A list of patients who missed appointments during the period from January 1, 2014, to April 30, 2014, was extracted and every fourth patient was called within 6 weeks of the appointment to minimize recall bias. Patients were excluded from the study if they could not be reached after 3 attempts. Of the 799 patients contacted, 300 (38%) responded to the survey; 98 (12%) had phone numbers on record that were incorrect or were no longer in service; and 401 (50%) could not be reached after 3 attempts. The results of the telephone survey are provided in Table 2.
The demographic data suggested that characteristics associated with higher rates of missed appointments tended to reflect physical or financial barriers, such as dependency on others for transportation (eg, pediatric patients), longer distance traveled to the clinic, and lack of insurance coverage; however, only 4% and 8% of the survey respondents reported that they missed their appointment due to financial reasons or that they were unable to obtain transportation, respectively. Of the patients surveyed, 35% cited that the reason they missed their appointment was that they forgot about the appointment; additionally, 24% of respondents reported that they had not been reminded of the appointment.
Although physicians cannot directly address physical or financial barriers to attendance, we can introduce more effective methods of communication for patient reminders. Of the 799 patients who were called for the telephone survey, 12.3% had phone numbers on record that were either incorrect or no longer in service. As these patients’ phone numbers were listed in the electronic medical record for contact purposes, they likely did not receive telephone calls reminding them about their appointments. Although it was not formally evaluated in this study, many respondents expressed that they had other preferred methods of receiving appointment reminders (eg, e-mail, text message) than those that are currently considered commonplace (ie, telephone calls, voicemails).
This study was limited in that the appointment data came from a single academic dermatology clinic. There also were limitations in the data set for subgroup analysis; for example, to appropriately assess socioeconomic barriers to attendance of dermatology appointments, it would be valuable to stratify income within established factors of socioeconomic barriers (eg, race, employment status) to avoid research bias. Although many variables assessed were statistically significant (P<.05), the odds ratios often were close to 1, suggesting that they may not be clinically or practically relevant.
By identifying factors associated with missed dermatology appointments, interventions can be instituted to target high-risk groups and alter patient reminder protocols. If possible, identifying patients’ preferred contact methods (eg, telephone call, text message, etc) and verifying contact information may be cost-effective ways to reduce missed appointments in dermatology offices.
To the Editor:
Missed appointments are a major issue in every discipline of medicine1 and can be detrimental for dermatologists,2,3 whose clinics often have long wait times for referred patients and can lose up to $200 for each missed appointment.4 The purpose of this study was to quantify the rate of missed appointments at an academic dermatology clinic and identify factors associated with patient nonattendance.
After approval by an institutional review board, appointment data was collected from the electronic medical record at the dermatology clinic at Wake Forest Baptist Health, Winston-Salem, North Carolina, for the period from May 1, 2013, to April 30, 2014. Variables that were evaluated included age, race, sex, primary language, employment status, zip code, appointment time, insurance coverage, scheduled provider, patient status (new vs returning), and the nature of the visit (cosmetic vs noncosmetic visits and procedural vs nonprocedural visits). Zip codes served as a representation of distance traveled and were stratified into 4 concentric zones: zone 1 represented the region corresponding to the clinic’s zip code; zone 2 represented regions with zip codes adjacent to zone 1; and the remaining zones were determined by regions with zip codes adjacent to the prior zone. Primary language spoken was categorized as English or non-English. Insurance coverage was categorized as private, Medicaid, Medicare, self-pay, and other. Using stepwise selection, both a univariate model and a multivariable logistic regression model were created (variable inclusion, P≤.10; variable exclusion, P>.05). Of the 28,772 appointments scheduled during the study period, 5584 (19.4%) were missed. Univariate and multivariable analyses of the factors associated with missed appointments are shown in Table 1.
A telephone survey also was conducted to evaluate patient-reported factors associated with missed dermatology appointments. A list of patients who missed appointments during the period from January 1, 2014, to April 30, 2014, was extracted and every fourth patient was called within 6 weeks of the appointment to minimize recall bias. Patients were excluded from the study if they could not be reached after 3 attempts. Of the 799 patients contacted, 300 (38%) responded to the survey; 98 (12%) had phone numbers on record that were incorrect or were no longer in service; and 401 (50%) could not be reached after 3 attempts. The results of the telephone survey are provided in Table 2.
The demographic data suggested that characteristics associated with higher rates of missed appointments tended to reflect physical or financial barriers, such as dependency on others for transportation (eg, pediatric patients), longer distance traveled to the clinic, and lack of insurance coverage; however, only 4% and 8% of the survey respondents reported that they missed their appointment due to financial reasons or that they were unable to obtain transportation, respectively. Of the patients surveyed, 35% cited that the reason they missed their appointment was that they forgot about the appointment; additionally, 24% of respondents reported that they had not been reminded of the appointment.
Although physicians cannot directly address physical or financial barriers to attendance, we can introduce more effective methods of communication for patient reminders. Of the 799 patients who were called for the telephone survey, 12.3% had phone numbers on record that were either incorrect or no longer in service. As these patients’ phone numbers were listed in the electronic medical record for contact purposes, they likely did not receive telephone calls reminding them about their appointments. Although it was not formally evaluated in this study, many respondents expressed that they had other preferred methods of receiving appointment reminders (eg, e-mail, text message) than those that are currently considered commonplace (ie, telephone calls, voicemails).
This study was limited in that the appointment data came from a single academic dermatology clinic. There also were limitations in the data set for subgroup analysis; for example, to appropriately assess socioeconomic barriers to attendance of dermatology appointments, it would be valuable to stratify income within established factors of socioeconomic barriers (eg, race, employment status) to avoid research bias. Although many variables assessed were statistically significant (P<.05), the odds ratios often were close to 1, suggesting that they may not be clinically or practically relevant.
By identifying factors associated with missed dermatology appointments, interventions can be instituted to target high-risk groups and alter patient reminder protocols. If possible, identifying patients’ preferred contact methods (eg, telephone call, text message, etc) and verifying contact information may be cost-effective ways to reduce missed appointments in dermatology offices.
1. George A, Rubin G. Non-attendance in general practice: a systematic review and its implications for access to primary health care. Fam Pract. 2003;20:178-184.
2. Canizares MJ, Penneys NS. The incidence of nonattendance at an urgent care dermatology clinic. J Am Acad Dermatol. 2002;46:457-459.
3. Cronin PR, DeCoste L, Kimball AB. A multivariate analysis of dermatology missed appointment predictors. JAMA Dermatol. 2013;149:1435-1437.
4. Perez FD, Xie J, Sin A, et al. Characteristics and direct costs of academic pediatric subspecialty outpatient no-show events. J Healthc Qual. 2014;36:32-42.
1. George A, Rubin G. Non-attendance in general practice: a systematic review and its implications for access to primary health care. Fam Pract. 2003;20:178-184.
2. Canizares MJ, Penneys NS. The incidence of nonattendance at an urgent care dermatology clinic. J Am Acad Dermatol. 2002;46:457-459.
3. Cronin PR, DeCoste L, Kimball AB. A multivariate analysis of dermatology missed appointment predictors. JAMA Dermatol. 2013;149:1435-1437.
4. Perez FD, Xie J, Sin A, et al. Characteristics and direct costs of academic pediatric subspecialty outpatient no-show events. J Healthc Qual. 2014;36:32-42.
ExCITE: Minimally invasive tissue extraction made simple with simulation
In November 2014, following concerns regarding the use of electromechanical, or power, morcellation, we published a surgical technique called the extracorporeal C-incision tissue extraction, or ExCITE, technique, as an alternative to contained tissue extraction during minimally invasive gynecologic procedures such as myomectomy and hysterectomy.1 This technique was developed to create a simple, reproducible, and minimally invasive approach to tissue extraction without the need for power morcellation. ExCITE is trainee-friendly and teachable.
In this article, we will review the steps for successful execution of the ExCITE technique. In addition, we will describe how to create your own cost-effective simulation model for teaching, learning, and practicing this technique with a few simple materials found in any craft or grocery store. Simulation is essential. It helps to troubleshoot issues that may arise in an actual case and allows for learning and practicing of surgical techniques to improve the learning curve and efficiency in the operating room (OR).
The model described here is viewable in the video, “The ExCITE technique, Part 2: Simulation made simple.” It is archived in Arnold Advincula’s Surgical Techniques Video Channel, which also is accessible through the “multimedia” section of this Web site.
ExCITE operative technique
“Traditional” intracorporeal tissue extraction techniques use power morcellation without specimen containment. The specimen is grasped with a tenaculum and pulled through the device. The specimen is essentially peeled like an apple and results in long strips of tissue with both a “cut” and “noncut” or intact surface due to the way the blade incises the tissue (FIGURE 1). When performing extracorporeal tissue extraction, we are replicating essentially the same mechanism of tissue removal. With ExCITE, however, the specimen is contained, there is no power morcellator, and tissue extraction is performed manually (FIGURE 2).

The ExCITE technique can be broken down into 5 major steps:
1. specimen retrieval and containment
2. self-retaining retractor placement
3. creation of the C-incision
4. tissue extraction
5. fascial closure.
1. Specimen retrieval and containment
First, place the specimen in an endoscopic specimen retrieval bag. Extend the incision at the umbilicus, to approximately 2.5 to 3.5 cm (roughly 2 good fingerbreadths), and exteriorize the bag at the level of the umbilicus.
2. Self-retaining retractor placement
Next, place a small disposable self-retaining retractor, (we prefer the extra-small Alexis-O) inside the bag, which helps keep the bag in position at the umbilicus (FIGURE 3).

Tip. When inserting the retractor, push it in all the way until the entire bottom ring is inside of the bag. This allows for the retractor ring to deploy. Allow some space between the specimen and the opening of the bag when placing the retractor. Do not pull the bag too tightly against the anterior abdominal wall as this may prevent the retractor ring from deploying fully and make the specimen extraction step more difficult.
3. Creation of C-incision
Grasp the specimen with a penetrating clamp (such as a tenaculum, Lahey, or towel clamp) and pull the specimen flush against the incision and retractor. Use a #11 or #10 blade scalpel to create a reverse “C-incision,” with the clamp in your nondominant hand and the scalpel starting the C-incision from your nondominant side moving toward your dominant side. (The curve of the “C” faces your dominant side.)
Tip. It is important to make your C-incision wide enough to get an adequate sized specimen strip through the umbilicus but not too wide (ie, too flush with the retractor), as this will decrease your workspace and increase the risk of cutting the retractor or the bag. It is helpful to hold the scalpel like a pencil and use a sawing-like motion rather than trying to advance the scalpel through the tissue in one motion.
4. Tissue extraction
Re-grasp the tissue flap, or “nub,” created by the C-incision with the penetrating clamp. While maintaining tension on the specimen, continue cutting with a sawing-like motion, using a reverse C coring technique, keeping one surface completely intact. (Generally this is the surface facing your nondominant side.) When cutting, the tissue becomes a strip, similar in appearance to when using a power morcellator. In fact, the technique is very similar to peeling an apple all the way around while trying to keep the skin of the fruit intact.
Tip. Try to angle the scalpel slightly when cutting the tissue, especially at the curve of the C. In other words, keep the tip of the scalpel toward the center of the strip and the handle away from the center, angled closer to the abdominal wall. When achieving an adequate strip of tissue, often the specimen will start rolling (similar to power morcellation). If this occurs, “go with the roll” by modifying the C-coring incision to a half C and incising along the top part of the C repeatedly until the specimen stops rolling. At that point, complete the C. Be sure to re-grasp near the specimen base as you continue the procedure and as the strip gets longer to prevent premature breakage of the strip and for ease of maintaining tension.
5. Fascial closure
After the specimen is completely extracted, remove the self-retaining retractor and specimen bag. Close the fascia at the umbilical incision. We prefer to close the fascia with an 0-polysorb (absorbable) suture in a running fashion, but you may consider an interrupted closure or use delayed absorbable sutures such as polyglyconate/polydioxanone (maxon/PDS).
Tip. To facilitate removal of the self-retaining retractor, pull on the specimen retrieval bag at one apex in order to collapse the retractor ring inside the bag. This allows removal of the bag and retractor simultaneously.
Keys to success
- Perfect the cutting technique; it is imperative to achieve tissue removal in long strip-like pieces for efficiency. Achieving the “saw cut” is like connecting the dots on a piece of paper with a pencil, where you try not to lift up the pencil (or the scalpel in this case). Rock the tissue back and forth with your nondominant hand and pull the specimen flush to the incision. This helps expose maximal surface area so you can continue to cut tissue pieces that are as large as possible. When rocking, move your dominant (cutting) and nondominant (holding the specimen with the tenaculum) hands in opposite directions.
- Ensure that the appropriate amount of tissue is cut when performing the C-incision. If the tissue strip is too thick, it becomes hard to see and incise the tissue, especially as you come around the back curve of the C. Limited visualization will increase your risk of cutting the retractor or the bag. If the cut is too thick, angle the scalpel in to make the tissue strip thinner (ie, make a “V-like” incision into the noncut surface). If the tissue strip becomes small, do the opposite; instead of cutting at a diagonal toward the noncut surface, aim out from your last incision (“V-out”). You should re-grasp below the narrowed area of the strip in this case before continuing to cut to prevent premature breakage of the strip.
- Maintain traction on the specimen. Keep it flush against the abdominal wall and the opening of the self-retaining rectractor. Use your finger to help “roll” the specimen when continuing the C-incision, if necessary. Maintaining traction will help avoid the need to use your finger.
- If you cannot remove the tissue fully intact, reorient or resect, and move forward. When the tissue is not easily extractable, try to roll the specimen by pushing near or behind the junction of the cut surface and the specimen. This helps reorient the specimen and exposes more smooth, noncut surfaces so coring can continue. The strip of tissue may need to be completely incised at times. If this occurs, drop the specimen back into the bag, find a smoother surface, re-grasp, and begin the C-incision again.
To view ExCITE performed in real-time during removal of an 8-cm, 130-g fibroid after a robot-assisted laparoscopic myomectomy, access the video “The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique” at obgmanagement.com, found in Arnold Advincula’s Surgical Techniques Video Channel.
Building the ExCITE simulation model
Creation of the ExCITE simulation model can be broken down into 4 simple steps: creating the self-retaining retractor, building the torso, preparing the specimen, and simulating the ExCITE technique.
Supplies
To complete all 4 steps, you will need several materials, all of which are easily accessible and easy to prepare for simulation (FIGURE 4).

- 1 beef tongue (2−3 lb)
- 1 pantyhose
- 2 silicone rings (4−5 cm in diameter, such as those used as wrist bracelets for cancer awareness)
- 1-gallon resealable (Ziploc) plastic bags
- 8x12 cardboard/corrugated box (or plastic storage box)
- duct or masking tape
- instruments:
– #11-blade (or your preference) scalpel
– penetrating clamps (tenaculum, Lahey, or towel clamps)
Note that beef tongue, given its muscular texture, closely mimics uterine tissue and therefore is used to represent the fibroid or uterus during simulation. Sometimes, a piece of beef tongue can be marbleized, or fatty, in which case it can simulate a degenerated fibroid. Beef tongue usually comes in one large piece, which could be suitable for up to 4 surgical exercises. The cost of a single tongue is approximately $20 to $30, so it averages about $5 to $7 per exercise/surgical trainee.
1. Create the self-retaining retractor
Supplies: pantyhose, 2 silicone rings
A self-retaining retractor is tubular and made up of a thin plastic material that has a pliable ring on either end. The pantyhose is used to simulate the tubular plastic material, and the silicone bracelets serve as the ring ends of the retractor. The retractor should be large enough so that it does not slip through the incision.
First, cut off the toe end of the pantyhose. Measure and cut a pantyhose strip to approximately 38 cm (15 in). Place one end of the pantyhose through the center of one of the silicone bracelets and wrap it around the edges of the bracelet. Make it as even as possible all the way around the ring. Roll the pantyhose over the bracelet twice more to secure it. Repeat these steps for the other end of the pantyhose to create the simulated self-retaining retractor (FIGURE 5).

2. Build the torso
Supplies: cardboard (ie, office paper box) or plastic box, scissors, duct tape
Place the cardboard box upside down and cut a hole (approximately 2−3 cm wide) at the center of the box top (technically the bottom of the box) to simulate the umbilical incision. Cut another opening on either side of the box (large enough to fit a hand so that the specimen can be inserted inside the box). When performing the ExCITE technique, a constant upward traction is required. In order to keep the box from lifting off the table, tape the box to the table with masking or duct tape. Alternatively, place weights in the bottom of the inside of the box.
3. Prepare the specimen
Supplies: beef tongue, resealable plastic bag
To simulate the contained fibroid or uterus, slice the beef tongue into 3 to 4 pieces (approximately 1-lb pieces) and place one piece of beef tongue inside the resealable plastic bag. Using the side opening in the box, place the bag with the specimen inside the box, and pull the bag through the “umbilical incision” hole, just as you would in a real case. When exteriorizing the bag, ensure some slack so the simulated self-retaining retractor can be placed inside the bag with the ring rolled over it (FIGURE 6).


|
4. ExCITE technique simulation: Grasp, cut, extract
Supplies: #11-blade scalpel, penetrating clamps (tenaculum, Lahey, or towel clamps).
After exteriorizing the bag, place the self-retaining retractor inside the bag and roll the silicone ring until the retractor is flush with the anterior abdominal wall. Grab the specimen (beef tongue) inside the bag. Perform the ExCITE technique using the beef tongue and the simulated model to fully remove the specimen (FIGURE 7).

Ready, set, simulate
There are many advantages to being able to teach and practice the ExCITE technique outside of the OR. Simulation helps the surgeon to better understand the nuances of tissue extraction in a risk-free environment, and it can improve efficiency in the OR. Building the simulation model as we have described is simple, quick, and inexpensive. We hope that this technique will add to your surgical armamentarium so that you can continue to provide your patients minimally invasive gynecologic surgical options. We recommend that you view both of our videos related to the ExCITE technique and its simulation model at obgmanagement.com, and soon you will be ready to teach or practice the ExCITE technique.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
1. Truong MD, Advincula AP. The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique. OBG Manag. 2014;26(11):56.
In November 2014, following concerns regarding the use of electromechanical, or power, morcellation, we published a surgical technique called the extracorporeal C-incision tissue extraction, or ExCITE, technique, as an alternative to contained tissue extraction during minimally invasive gynecologic procedures such as myomectomy and hysterectomy.1 This technique was developed to create a simple, reproducible, and minimally invasive approach to tissue extraction without the need for power morcellation. ExCITE is trainee-friendly and teachable.
In this article, we will review the steps for successful execution of the ExCITE technique. In addition, we will describe how to create your own cost-effective simulation model for teaching, learning, and practicing this technique with a few simple materials found in any craft or grocery store. Simulation is essential. It helps to troubleshoot issues that may arise in an actual case and allows for learning and practicing of surgical techniques to improve the learning curve and efficiency in the operating room (OR).
The model described here is viewable in the video, “The ExCITE technique, Part 2: Simulation made simple.” It is archived in Arnold Advincula’s Surgical Techniques Video Channel, which also is accessible through the “multimedia” section of this Web site.
ExCITE operative technique
“Traditional” intracorporeal tissue extraction techniques use power morcellation without specimen containment. The specimen is grasped with a tenaculum and pulled through the device. The specimen is essentially peeled like an apple and results in long strips of tissue with both a “cut” and “noncut” or intact surface due to the way the blade incises the tissue (FIGURE 1). When performing extracorporeal tissue extraction, we are replicating essentially the same mechanism of tissue removal. With ExCITE, however, the specimen is contained, there is no power morcellator, and tissue extraction is performed manually (FIGURE 2).


The ExCITE technique can be broken down into 5 major steps:
1. specimen retrieval and containment
2. self-retaining retractor placement
3. creation of the C-incision
4. tissue extraction
5. fascial closure.
1. Specimen retrieval and containment
First, place the specimen in an endoscopic specimen retrieval bag. Extend the incision at the umbilicus, to approximately 2.5 to 3.5 cm (roughly 2 good fingerbreadths), and exteriorize the bag at the level of the umbilicus.
2. Self-retaining retractor placement
Next, place a small disposable self-retaining retractor, (we prefer the extra-small Alexis-O) inside the bag, which helps keep the bag in position at the umbilicus (FIGURE 3).

Tip. When inserting the retractor, push it in all the way until the entire bottom ring is inside of the bag. This allows for the retractor ring to deploy. Allow some space between the specimen and the opening of the bag when placing the retractor. Do not pull the bag too tightly against the anterior abdominal wall as this may prevent the retractor ring from deploying fully and make the specimen extraction step more difficult.
3. Creation of C-incision
Grasp the specimen with a penetrating clamp (such as a tenaculum, Lahey, or towel clamp) and pull the specimen flush against the incision and retractor. Use a #11 or #10 blade scalpel to create a reverse “C-incision,” with the clamp in your nondominant hand and the scalpel starting the C-incision from your nondominant side moving toward your dominant side. (The curve of the “C” faces your dominant side.)
Tip. It is important to make your C-incision wide enough to get an adequate sized specimen strip through the umbilicus but not too wide (ie, too flush with the retractor), as this will decrease your workspace and increase the risk of cutting the retractor or the bag. It is helpful to hold the scalpel like a pencil and use a sawing-like motion rather than trying to advance the scalpel through the tissue in one motion.
4. Tissue extraction
Re-grasp the tissue flap, or “nub,” created by the C-incision with the penetrating clamp. While maintaining tension on the specimen, continue cutting with a sawing-like motion, using a reverse C coring technique, keeping one surface completely intact. (Generally this is the surface facing your nondominant side.) When cutting, the tissue becomes a strip, similar in appearance to when using a power morcellator. In fact, the technique is very similar to peeling an apple all the way around while trying to keep the skin of the fruit intact.
Tip. Try to angle the scalpel slightly when cutting the tissue, especially at the curve of the C. In other words, keep the tip of the scalpel toward the center of the strip and the handle away from the center, angled closer to the abdominal wall. When achieving an adequate strip of tissue, often the specimen will start rolling (similar to power morcellation). If this occurs, “go with the roll” by modifying the C-coring incision to a half C and incising along the top part of the C repeatedly until the specimen stops rolling. At that point, complete the C. Be sure to re-grasp near the specimen base as you continue the procedure and as the strip gets longer to prevent premature breakage of the strip and for ease of maintaining tension.
5. Fascial closure
After the specimen is completely extracted, remove the self-retaining retractor and specimen bag. Close the fascia at the umbilical incision. We prefer to close the fascia with an 0-polysorb (absorbable) suture in a running fashion, but you may consider an interrupted closure or use delayed absorbable sutures such as polyglyconate/polydioxanone (maxon/PDS).
Tip. To facilitate removal of the self-retaining retractor, pull on the specimen retrieval bag at one apex in order to collapse the retractor ring inside the bag. This allows removal of the bag and retractor simultaneously.
Keys to success
- Perfect the cutting technique; it is imperative to achieve tissue removal in long strip-like pieces for efficiency. Achieving the “saw cut” is like connecting the dots on a piece of paper with a pencil, where you try not to lift up the pencil (or the scalpel in this case). Rock the tissue back and forth with your nondominant hand and pull the specimen flush to the incision. This helps expose maximal surface area so you can continue to cut tissue pieces that are as large as possible. When rocking, move your dominant (cutting) and nondominant (holding the specimen with the tenaculum) hands in opposite directions.
- Ensure that the appropriate amount of tissue is cut when performing the C-incision. If the tissue strip is too thick, it becomes hard to see and incise the tissue, especially as you come around the back curve of the C. Limited visualization will increase your risk of cutting the retractor or the bag. If the cut is too thick, angle the scalpel in to make the tissue strip thinner (ie, make a “V-like” incision into the noncut surface). If the tissue strip becomes small, do the opposite; instead of cutting at a diagonal toward the noncut surface, aim out from your last incision (“V-out”). You should re-grasp below the narrowed area of the strip in this case before continuing to cut to prevent premature breakage of the strip.
- Maintain traction on the specimen. Keep it flush against the abdominal wall and the opening of the self-retaining rectractor. Use your finger to help “roll” the specimen when continuing the C-incision, if necessary. Maintaining traction will help avoid the need to use your finger.
- If you cannot remove the tissue fully intact, reorient or resect, and move forward. When the tissue is not easily extractable, try to roll the specimen by pushing near or behind the junction of the cut surface and the specimen. This helps reorient the specimen and exposes more smooth, noncut surfaces so coring can continue. The strip of tissue may need to be completely incised at times. If this occurs, drop the specimen back into the bag, find a smoother surface, re-grasp, and begin the C-incision again.
To view ExCITE performed in real-time during removal of an 8-cm, 130-g fibroid after a robot-assisted laparoscopic myomectomy, access the video “The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique” at obgmanagement.com, found in Arnold Advincula’s Surgical Techniques Video Channel.
Building the ExCITE simulation model
Creation of the ExCITE simulation model can be broken down into 4 simple steps: creating the self-retaining retractor, building the torso, preparing the specimen, and simulating the ExCITE technique.
Supplies
To complete all 4 steps, you will need several materials, all of which are easily accessible and easy to prepare for simulation (FIGURE 4).

- 1 beef tongue (2−3 lb)
- 1 pantyhose
- 2 silicone rings (4−5 cm in diameter, such as those used as wrist bracelets for cancer awareness)
- 1-gallon resealable (Ziploc) plastic bags
- 8x12 cardboard/corrugated box (or plastic storage box)
- duct or masking tape
- instruments:
– #11-blade (or your preference) scalpel
– penetrating clamps (tenaculum, Lahey, or towel clamps)
Note that beef tongue, given its muscular texture, closely mimics uterine tissue and therefore is used to represent the fibroid or uterus during simulation. Sometimes, a piece of beef tongue can be marbleized, or fatty, in which case it can simulate a degenerated fibroid. Beef tongue usually comes in one large piece, which could be suitable for up to 4 surgical exercises. The cost of a single tongue is approximately $20 to $30, so it averages about $5 to $7 per exercise/surgical trainee.
1. Create the self-retaining retractor
Supplies: pantyhose, 2 silicone rings
A self-retaining retractor is tubular and made up of a thin plastic material that has a pliable ring on either end. The pantyhose is used to simulate the tubular plastic material, and the silicone bracelets serve as the ring ends of the retractor. The retractor should be large enough so that it does not slip through the incision.
First, cut off the toe end of the pantyhose. Measure and cut a pantyhose strip to approximately 38 cm (15 in). Place one end of the pantyhose through the center of one of the silicone bracelets and wrap it around the edges of the bracelet. Make it as even as possible all the way around the ring. Roll the pantyhose over the bracelet twice more to secure it. Repeat these steps for the other end of the pantyhose to create the simulated self-retaining retractor (FIGURE 5).

2. Build the torso
Supplies: cardboard (ie, office paper box) or plastic box, scissors, duct tape
Place the cardboard box upside down and cut a hole (approximately 2−3 cm wide) at the center of the box top (technically the bottom of the box) to simulate the umbilical incision. Cut another opening on either side of the box (large enough to fit a hand so that the specimen can be inserted inside the box). When performing the ExCITE technique, a constant upward traction is required. In order to keep the box from lifting off the table, tape the box to the table with masking or duct tape. Alternatively, place weights in the bottom of the inside of the box.
3. Prepare the specimen
Supplies: beef tongue, resealable plastic bag
To simulate the contained fibroid or uterus, slice the beef tongue into 3 to 4 pieces (approximately 1-lb pieces) and place one piece of beef tongue inside the resealable plastic bag. Using the side opening in the box, place the bag with the specimen inside the box, and pull the bag through the “umbilical incision” hole, just as you would in a real case. When exteriorizing the bag, ensure some slack so the simulated self-retaining retractor can be placed inside the bag with the ring rolled over it (FIGURE 6).


|
4. ExCITE technique simulation: Grasp, cut, extract
Supplies: #11-blade scalpel, penetrating clamps (tenaculum, Lahey, or towel clamps).
After exteriorizing the bag, place the self-retaining retractor inside the bag and roll the silicone ring until the retractor is flush with the anterior abdominal wall. Grab the specimen (beef tongue) inside the bag. Perform the ExCITE technique using the beef tongue and the simulated model to fully remove the specimen (FIGURE 7).

Ready, set, simulate
There are many advantages to being able to teach and practice the ExCITE technique outside of the OR. Simulation helps the surgeon to better understand the nuances of tissue extraction in a risk-free environment, and it can improve efficiency in the OR. Building the simulation model as we have described is simple, quick, and inexpensive. We hope that this technique will add to your surgical armamentarium so that you can continue to provide your patients minimally invasive gynecologic surgical options. We recommend that you view both of our videos related to the ExCITE technique and its simulation model at obgmanagement.com, and soon you will be ready to teach or practice the ExCITE technique.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In November 2014, following concerns regarding the use of electromechanical, or power, morcellation, we published a surgical technique called the extracorporeal C-incision tissue extraction, or ExCITE, technique, as an alternative to contained tissue extraction during minimally invasive gynecologic procedures such as myomectomy and hysterectomy.1 This technique was developed to create a simple, reproducible, and minimally invasive approach to tissue extraction without the need for power morcellation. ExCITE is trainee-friendly and teachable.
In this article, we will review the steps for successful execution of the ExCITE technique. In addition, we will describe how to create your own cost-effective simulation model for teaching, learning, and practicing this technique with a few simple materials found in any craft or grocery store. Simulation is essential. It helps to troubleshoot issues that may arise in an actual case and allows for learning and practicing of surgical techniques to improve the learning curve and efficiency in the operating room (OR).
The model described here is viewable in the video, “The ExCITE technique, Part 2: Simulation made simple.” It is archived in Arnold Advincula’s Surgical Techniques Video Channel, which also is accessible through the “multimedia” section of this Web site.
ExCITE operative technique
“Traditional” intracorporeal tissue extraction techniques use power morcellation without specimen containment. The specimen is grasped with a tenaculum and pulled through the device. The specimen is essentially peeled like an apple and results in long strips of tissue with both a “cut” and “noncut” or intact surface due to the way the blade incises the tissue (FIGURE 1). When performing extracorporeal tissue extraction, we are replicating essentially the same mechanism of tissue removal. With ExCITE, however, the specimen is contained, there is no power morcellator, and tissue extraction is performed manually (FIGURE 2).


The ExCITE technique can be broken down into 5 major steps:
1. specimen retrieval and containment
2. self-retaining retractor placement
3. creation of the C-incision
4. tissue extraction
5. fascial closure.
1. Specimen retrieval and containment
First, place the specimen in an endoscopic specimen retrieval bag. Extend the incision at the umbilicus, to approximately 2.5 to 3.5 cm (roughly 2 good fingerbreadths), and exteriorize the bag at the level of the umbilicus.
2. Self-retaining retractor placement
Next, place a small disposable self-retaining retractor, (we prefer the extra-small Alexis-O) inside the bag, which helps keep the bag in position at the umbilicus (FIGURE 3).

Tip. When inserting the retractor, push it in all the way until the entire bottom ring is inside of the bag. This allows for the retractor ring to deploy. Allow some space between the specimen and the opening of the bag when placing the retractor. Do not pull the bag too tightly against the anterior abdominal wall as this may prevent the retractor ring from deploying fully and make the specimen extraction step more difficult.
3. Creation of C-incision
Grasp the specimen with a penetrating clamp (such as a tenaculum, Lahey, or towel clamp) and pull the specimen flush against the incision and retractor. Use a #11 or #10 blade scalpel to create a reverse “C-incision,” with the clamp in your nondominant hand and the scalpel starting the C-incision from your nondominant side moving toward your dominant side. (The curve of the “C” faces your dominant side.)
Tip. It is important to make your C-incision wide enough to get an adequate sized specimen strip through the umbilicus but not too wide (ie, too flush with the retractor), as this will decrease your workspace and increase the risk of cutting the retractor or the bag. It is helpful to hold the scalpel like a pencil and use a sawing-like motion rather than trying to advance the scalpel through the tissue in one motion.
4. Tissue extraction
Re-grasp the tissue flap, or “nub,” created by the C-incision with the penetrating clamp. While maintaining tension on the specimen, continue cutting with a sawing-like motion, using a reverse C coring technique, keeping one surface completely intact. (Generally this is the surface facing your nondominant side.) When cutting, the tissue becomes a strip, similar in appearance to when using a power morcellator. In fact, the technique is very similar to peeling an apple all the way around while trying to keep the skin of the fruit intact.
Tip. Try to angle the scalpel slightly when cutting the tissue, especially at the curve of the C. In other words, keep the tip of the scalpel toward the center of the strip and the handle away from the center, angled closer to the abdominal wall. When achieving an adequate strip of tissue, often the specimen will start rolling (similar to power morcellation). If this occurs, “go with the roll” by modifying the C-coring incision to a half C and incising along the top part of the C repeatedly until the specimen stops rolling. At that point, complete the C. Be sure to re-grasp near the specimen base as you continue the procedure and as the strip gets longer to prevent premature breakage of the strip and for ease of maintaining tension.
5. Fascial closure
After the specimen is completely extracted, remove the self-retaining retractor and specimen bag. Close the fascia at the umbilical incision. We prefer to close the fascia with an 0-polysorb (absorbable) suture in a running fashion, but you may consider an interrupted closure or use delayed absorbable sutures such as polyglyconate/polydioxanone (maxon/PDS).
Tip. To facilitate removal of the self-retaining retractor, pull on the specimen retrieval bag at one apex in order to collapse the retractor ring inside the bag. This allows removal of the bag and retractor simultaneously.
Keys to success
- Perfect the cutting technique; it is imperative to achieve tissue removal in long strip-like pieces for efficiency. Achieving the “saw cut” is like connecting the dots on a piece of paper with a pencil, where you try not to lift up the pencil (or the scalpel in this case). Rock the tissue back and forth with your nondominant hand and pull the specimen flush to the incision. This helps expose maximal surface area so you can continue to cut tissue pieces that are as large as possible. When rocking, move your dominant (cutting) and nondominant (holding the specimen with the tenaculum) hands in opposite directions.
- Ensure that the appropriate amount of tissue is cut when performing the C-incision. If the tissue strip is too thick, it becomes hard to see and incise the tissue, especially as you come around the back curve of the C. Limited visualization will increase your risk of cutting the retractor or the bag. If the cut is too thick, angle the scalpel in to make the tissue strip thinner (ie, make a “V-like” incision into the noncut surface). If the tissue strip becomes small, do the opposite; instead of cutting at a diagonal toward the noncut surface, aim out from your last incision (“V-out”). You should re-grasp below the narrowed area of the strip in this case before continuing to cut to prevent premature breakage of the strip.
- Maintain traction on the specimen. Keep it flush against the abdominal wall and the opening of the self-retaining rectractor. Use your finger to help “roll” the specimen when continuing the C-incision, if necessary. Maintaining traction will help avoid the need to use your finger.
- If you cannot remove the tissue fully intact, reorient or resect, and move forward. When the tissue is not easily extractable, try to roll the specimen by pushing near or behind the junction of the cut surface and the specimen. This helps reorient the specimen and exposes more smooth, noncut surfaces so coring can continue. The strip of tissue may need to be completely incised at times. If this occurs, drop the specimen back into the bag, find a smoother surface, re-grasp, and begin the C-incision again.
To view ExCITE performed in real-time during removal of an 8-cm, 130-g fibroid after a robot-assisted laparoscopic myomectomy, access the video “The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique” at obgmanagement.com, found in Arnold Advincula’s Surgical Techniques Video Channel.
Building the ExCITE simulation model
Creation of the ExCITE simulation model can be broken down into 4 simple steps: creating the self-retaining retractor, building the torso, preparing the specimen, and simulating the ExCITE technique.
Supplies
To complete all 4 steps, you will need several materials, all of which are easily accessible and easy to prepare for simulation (FIGURE 4).

- 1 beef tongue (2−3 lb)
- 1 pantyhose
- 2 silicone rings (4−5 cm in diameter, such as those used as wrist bracelets for cancer awareness)
- 1-gallon resealable (Ziploc) plastic bags
- 8x12 cardboard/corrugated box (or plastic storage box)
- duct or masking tape
- instruments:
– #11-blade (or your preference) scalpel
– penetrating clamps (tenaculum, Lahey, or towel clamps)
Note that beef tongue, given its muscular texture, closely mimics uterine tissue and therefore is used to represent the fibroid or uterus during simulation. Sometimes, a piece of beef tongue can be marbleized, or fatty, in which case it can simulate a degenerated fibroid. Beef tongue usually comes in one large piece, which could be suitable for up to 4 surgical exercises. The cost of a single tongue is approximately $20 to $30, so it averages about $5 to $7 per exercise/surgical trainee.
1. Create the self-retaining retractor
Supplies: pantyhose, 2 silicone rings
A self-retaining retractor is tubular and made up of a thin plastic material that has a pliable ring on either end. The pantyhose is used to simulate the tubular plastic material, and the silicone bracelets serve as the ring ends of the retractor. The retractor should be large enough so that it does not slip through the incision.
First, cut off the toe end of the pantyhose. Measure and cut a pantyhose strip to approximately 38 cm (15 in). Place one end of the pantyhose through the center of one of the silicone bracelets and wrap it around the edges of the bracelet. Make it as even as possible all the way around the ring. Roll the pantyhose over the bracelet twice more to secure it. Repeat these steps for the other end of the pantyhose to create the simulated self-retaining retractor (FIGURE 5).

2. Build the torso
Supplies: cardboard (ie, office paper box) or plastic box, scissors, duct tape
Place the cardboard box upside down and cut a hole (approximately 2−3 cm wide) at the center of the box top (technically the bottom of the box) to simulate the umbilical incision. Cut another opening on either side of the box (large enough to fit a hand so that the specimen can be inserted inside the box). When performing the ExCITE technique, a constant upward traction is required. In order to keep the box from lifting off the table, tape the box to the table with masking or duct tape. Alternatively, place weights in the bottom of the inside of the box.
3. Prepare the specimen
Supplies: beef tongue, resealable plastic bag
To simulate the contained fibroid or uterus, slice the beef tongue into 3 to 4 pieces (approximately 1-lb pieces) and place one piece of beef tongue inside the resealable plastic bag. Using the side opening in the box, place the bag with the specimen inside the box, and pull the bag through the “umbilical incision” hole, just as you would in a real case. When exteriorizing the bag, ensure some slack so the simulated self-retaining retractor can be placed inside the bag with the ring rolled over it (FIGURE 6).


|
4. ExCITE technique simulation: Grasp, cut, extract
Supplies: #11-blade scalpel, penetrating clamps (tenaculum, Lahey, or towel clamps).
After exteriorizing the bag, place the self-retaining retractor inside the bag and roll the silicone ring until the retractor is flush with the anterior abdominal wall. Grab the specimen (beef tongue) inside the bag. Perform the ExCITE technique using the beef tongue and the simulated model to fully remove the specimen (FIGURE 7).

Ready, set, simulate
There are many advantages to being able to teach and practice the ExCITE technique outside of the OR. Simulation helps the surgeon to better understand the nuances of tissue extraction in a risk-free environment, and it can improve efficiency in the OR. Building the simulation model as we have described is simple, quick, and inexpensive. We hope that this technique will add to your surgical armamentarium so that you can continue to provide your patients minimally invasive gynecologic surgical options. We recommend that you view both of our videos related to the ExCITE technique and its simulation model at obgmanagement.com, and soon you will be ready to teach or practice the ExCITE technique.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Reference
1. Truong MD, Advincula AP. The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique. OBG Manag. 2014;26(11):56.
Reference
1. Truong MD, Advincula AP. The Extracorporeal C-Incision Tissue Extraction (ExCITE) technique. OBG Manag. 2014;26(11):56.
In this Article
- 5 steps to execute ExCITE
- Keys to technique success
- Building the simulation model
Managing complications at the time of vaginal hysterectomy
Careful attention to technique at the time of vaginal hysterectomy is vital. Equally important is prior consideration of potential complications and the best ways to address them. Four trouble spots include:
- uterine tissue extraction (Although this is not a complication of vaginal hysterectomy, tissue extraction aids in debulking and removal of a large uterus.)
- protection of the ureters (It is important to palpate these structures before placing cardinal pedicle clamps, to protect ureteral integrity.)
- repair of inadvertent cystotomy
- control of bleeding in the setting of adnexectomy.
I focus on optimal approaches to these 4 scenarios in this article.
For a review of vaginal hysterectomy technique, see “Vaginal hysterectomy with basic instrumentation,” by Barbara S. Levy, MD, which appeared in the October 2015 issue of OBG Management. For salpingectomy and salpingo-oophorectomy technique, see my article entitled “Salpingectomy after vaginal hysterectomy: Technique, tips, and pearls,” which appeared in the November issue of this journal.
Both articles are available in the archive at obgmanagement.com and, like this one, are based on the AAGL-produced Online Master Class on Vaginal Hysterectomy, a Web-based program cosponsored by the American College of Obstetricians and Gynecologists and the Society of Gynecologic Surgeons. That program is available at https://www.aagl.org/vaghystwebinar/.
A step toward success: Begin morcellation by splitting the uterus
Manual morcellation to reduce uterine size and ease transvaginal removal is a useful technique to know. Five aspects of manual morcellation warrant emphasis:
1. Anterior and posterior entry into the cul-de-sacs is essential before attempting morcellation.
2. The blood supply on both sides of the uterus must be controlled.
3. During resection, take care to cut only tissue that can be visualized. Avoid resection beyond what you can easily see.
4. Once morcellation is completed, always go back and check the pedicles for hemostasis. During morcellation, these pedicles tend to get stretched, and bleeding may arise that wasn’t present originally.
5. Morcellation should be performed only after malignancy has been ruled out—it is a technique intended for benign uteri only.
By bivalving the uterus it is possible to follow the endocervical canal up into the uterine cavity (FIGURE 1). Our technique at the Mayo Clinic is to place tenacula at the 3 and 9 o’clock positions prior to bivalving. A small amount of bleeding may occur because of collateral blood supply from the gonadal pedicles, but it should be minimal, as the uterine vessels have been secured.
FIGURE 1 Bivalve the uterus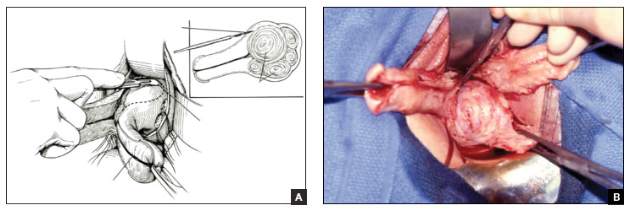
|
To begin morcellation, split the uterus down the midline, with tenacula placed at the 3- and 9-o’clock positions, then follow the endocervical canal into the uterine cavity (A). Use a knife blade to take portions of myomas and other tissue to debulk the uterus (B). |
Proceed with morcellation once the uterus is bivalved. Use a Jacobs tenaculum to grasp the serosal portion of the uterus. Apply downward traction with your nondominant hand, and use the knife blade to resect portions of the uterus so that it can be debulked.
When a large myoma is encountered during morcellation, it often is possible to “finger-fracture” some of the filmy adhesions holding it in place, or to follow the pseudo-capsule of the fibroid in order to shell it out. In many cases, fibroids can be removed intact using these methods. If intact removal is not possible, debulk the fibroid by taking individual “bites.”
Tip. When the uterus is greatly enlarged, grasp it with a tenaculum so that it does not retract when you incise it. When large myomas are anticipated, keep an extra tenaculum on hand, as well as extra knife blades, as blades dull quickly when used to cut through calcified tissue. Continue to apply traction with your nondominant hand to allow each piece of tissue to be more readily developed (FIGURE 2).
Tip. When managing the round-ligament complex on each side, stay between the round ligaments (your “goal posts”) to avoid getting too lateral. Keep the cervix intact for orientation purposes. Focus on diminishing the bulk of the uterus so that you can get around the utero-ovarian pedicles.
To control the utero-ovarian pedicle on the patient’s right side, place a finger underneath it, with traction applied. Place a Heaney clamp from the top down. Repeat this action on the patient’s left side, but place the Heaney clamp from the bottom up.
Manual morcellation of tissue is useful in small uteri that are tough to access, but the procedure is very helpful in large uteri in order to remove them transvaginally.
Protect the ureters: Palpate them before clamping the pedicles
Palpating the ureters at the time of hysterectomy can protect their integrity during the procedure. The following technique has been used at the Mayo Clinic for many years and allows for location of the ureter so a cardinal pedicle clamp can be placed without injury.
Enter the anterior cul-de-sac so that you can insert a finger and palpate the ureter before you place the cardinal pedicle clamp on each side. Place Deaver retractors at the 12 o’clock and 2- to 3-o’clock positions. Insert your nondominant index finger into the anterior cul-de-sac and palpate the ureter against the Deaver clamp in the 2- to 3-o’clock position (FIGURE 3). (The ureter can be felt between your index finger and the Deaver retractor.) The ureter will have the most descent in a uterus that has some prolapse, compared with a nonprolapsed uterus.
Tip. One common error is mistaking the edge of the vaginal cuff for the ureter. Be certain that you insert your finger deeply into the cul-de-sac so that it is the ureter you feel and not the cuff edge.
Successful cystotomy repair technique
Inadvertent cystotomy is a common fear for surgeons at the time of vaginal hysterectomy. I prefer to empty the bladder before beginning the hysterectomy because it reduces the target zone that a distended bladder presents. Some surgeons prefer to maintain a bit of fluid in the bladder so that, if they cut into the bladder, a small urine stream results. The approach is a matter of preference.
Cystotomy is most common during anterior dissection. If it occurs, recognize it and mark the defect with suture. Do not attempt to repair the hole at this point, but opt to finish the hysterectomy.
Cystoscopy is an important element of cystotomy repair. Once the hysterectomy is completed, look inside the bladder and determine where the defect is in relationship to the ureteral orifices. Typically, it will be beyond the interureteric ridge, along the posterior portion of the bladder, usually in the midline.
As critical as the repair itself is management of bladder drainage afterward. If you repair the hole thoroughly and drain the bladder adequately for 14 days, the defect should heal fully.
Technique for entry into anterior cul-de-sac
One way to avert bladder injury is to enter the anterior cul-de-sac very carefully. Begin by ensuring that the bladder is empty and placing a Deaver retractor at the 12 o’clock position. Also place tenacula anteriorly and posteriorly to help direct traction. This will allow good visualization of the bladder reflection.
Tip. One common mistake is making the incision too low or too near the cervix, which makes dissection more difficult and increases the likelihood that you will enter the wrong plane. Be sure you know where the bladder is, and make an adequate incision that is not too distal. Otherwise, dissection will be harder to carry out.
I prefer to make one clean incision with the knife, rather than multiple incisions, because multiple cuts increase the likelihood that you will inadvertently injure the wrong tissue. Use good traction and countertraction, and hug the uterus. Work low on the uterus, but not in the uterus. If you cut into muscle, you will get more bleeding and may end up digging a hole.
After you make the incision, put your finger through it to help develop that space further. You can confirm entry into the peritoneum by noting the characteristic slippery feel of the peritoneal lining. After you insert a Deaver retractor anteriorly, reinsert your finger and mobilize the area further. Then you can easily reach in and tent the peritoneum to cut it.
Technique for cystotomy repair
Two-layer closure is a minimum. On occasion, a third layer may be beneficial. Begin with running closure of the first layer using 2-0 chromic suture—a good suture choice in the urinary tract. This suture is inflammatory, which will help seal the wound, but it also dissolves quickly, preventing stone formation.
Use through-and-through closure on the first layer, followed by a second imbricating layer. If desired, use the peritoneum as a third layer.
Horizontal repair is typical, although vertical closure may be necessary if the defect is near a ureteral orifice and horizontal closure might compromise that side. That decision must be made intraoperatively.
When vertical repair is necessary, begin your repair just above the defect, placing the suture through and through. The hole should be visible. There is no need to be extramucosal in needle placement. Simply get a good bite of the tissue and run the repair down the bladder wall.
Next, stop and apply traction to the repair to check for any small defects that may have been overlooked. By placing a little traction on that first suture tag, any such defects will become apparent. Then go back and close them in a secondary imbricating layer.
After 2-layer closure, fill the bladder retrograde. I prefer to use a couple of drops of methylene blue in normal saline and place a clean white piece of packing material beneath the wound. If the packing material remains unstained by blue, the repair is watertight.
Incorporate the peritoneum as another layer of repair of the defect. I imbricate 2 layers in the bladder. Then, if necessary, I use that peritoneum as an additional layer (FIGURE 4).
Strategies to control bleeding at adnexectomy
Be vigilant for bleeding when removing the tubes and/or ovaries. At salpingectomy, be extremely gentle with the mesosalpinx because it can be avulsed easily off of surrounding tissue. If bleeding occurs, oversewing, or even ovary removal, could end up being the only options.
Good visualization is essential during vaginal procedures. Retractors, lighted suction irrigators, a headlamp, good overhead lighting, and appropriate instrumentation are critical for success.
Heaney clamp technique for vaginal oophorectomy
Begin by placing an Allis clamp on the utero-ovarian pedicle. Then clamp the ovary and tube with a second Allis clamp. Next, insert a Heaney clamp through the small window between the cardinal pedicle and the utero-ovarian pedicle (FIGURE 5). Clamp the tissue and place a free tie around it.
Because this is a major vascular pedicle, doubly ligate it. As you tie the first suture, have an assistant flash the clamp open and closed, then excise the specimen. There is no need to worry about losing the pedicle because it already has been ligated once. Next, stick-tie it, placing the needle distal to the free tie to avoid piercing the gonadal vessels beyond.
The technique is standard. Be gentle, and ensure good hemostasis when finished.
Tip. In my experience, any bleeding runs down from the pedicle rather than out toward me. So be sure to look down and below the pedicle to ensure hemostasis.
Additional pearls
- When performing vaginal hysterectomy, the ovaries are almost always removable transvaginally. There is no need to begin the case laparoscopically to remove the tubes and/or ovaries and then perform the hysterectomy vaginally.
- Deaver retractors offer good exposure; visualization is critical.
- Make sure the tissue is dry before you cut the last suture.
- If you prefer to use a laparoscopic stapler to secure the pedicles, proceed as before: Place an Allis clamp on the pedicle. Place a second clamp on the ovary and tube. Now you can insert the stapler into the created window, as with the Heaney clamp (FIGURE 6).
- Use a 60-mm stapler to cut the pedicle in one try. If using a 45-mm device, the stapler may need to be fired twice. This makes the procedure more expensive and risks more bleeding.
- When closing the stapler jaws, avoid clamping small bowel or packing material. Ensure stapler tip visibility well before firing.
The round ligament technique
When transecting the round ligament, it is critical to stay just beneath it to avoid bleeding and venturing into the mesosalpinx. Gently hug the tissue inferior to the round ligament and let it retract (FIGURE 7). This will allow isolation of the gonadal vessels nicely, especially if an adnexal mass is present. Then isolate the specimen and remove it, stick-tying the pedicle afterward to secure it.
When tying the pedicle, place the suture around the distal aspect to ensure that the back of the pedicle is enclosed, and do not lose it when you release the clamp. A slightly different technique is to use an endoloop to cross the gonadal vessels and control them. Use a suction irrigator and good lighting to get good exposure.
Next, place the clamp, making sure you don’t inadvertently grasp the packing material. Visualize both tips of the clamp before incising. Trim the specimen flush with the clamp. Then you can thread an endoloop over the top of the clamp. This is an inexpensive technique that allows a higher reach into the pelvic cavity. Finally, cinch down the endoloop to control the vessels.
When performing bilateral salpingo-oophorectomy, a long, fine clamp, such as the M.D. Anderson clamp, can help you reach up to control the gonadal vessels in the event that you lose your initial grip on those vessels (FIGURE 8).
Be prepared
Have a plan in place to manage any complications that arise during surgery. Just as obstetricians plan ahead to prepare for shoulder dystocia and other emergencies, gynecologic surgeons must prepare for surgical complications. Tissue extraction strategies can aid in the debulking and removal of large uteri, and the proper tools, lighting, and assistance are critical to success.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Careful attention to technique at the time of vaginal hysterectomy is vital. Equally important is prior consideration of potential complications and the best ways to address them. Four trouble spots include:
- uterine tissue extraction (Although this is not a complication of vaginal hysterectomy, tissue extraction aids in debulking and removal of a large uterus.)
- protection of the ureters (It is important to palpate these structures before placing cardinal pedicle clamps, to protect ureteral integrity.)
- repair of inadvertent cystotomy
- control of bleeding in the setting of adnexectomy.
I focus on optimal approaches to these 4 scenarios in this article.
For a review of vaginal hysterectomy technique, see “Vaginal hysterectomy with basic instrumentation,” by Barbara S. Levy, MD, which appeared in the October 2015 issue of OBG Management. For salpingectomy and salpingo-oophorectomy technique, see my article entitled “Salpingectomy after vaginal hysterectomy: Technique, tips, and pearls,” which appeared in the November issue of this journal.
Both articles are available in the archive at obgmanagement.com and, like this one, are based on the AAGL-produced Online Master Class on Vaginal Hysterectomy, a Web-based program cosponsored by the American College of Obstetricians and Gynecologists and the Society of Gynecologic Surgeons. That program is available at https://www.aagl.org/vaghystwebinar/.
A step toward success: Begin morcellation by splitting the uterus
Manual morcellation to reduce uterine size and ease transvaginal removal is a useful technique to know. Five aspects of manual morcellation warrant emphasis:
1. Anterior and posterior entry into the cul-de-sacs is essential before attempting morcellation.
2. The blood supply on both sides of the uterus must be controlled.
3. During resection, take care to cut only tissue that can be visualized. Avoid resection beyond what you can easily see.
4. Once morcellation is completed, always go back and check the pedicles for hemostasis. During morcellation, these pedicles tend to get stretched, and bleeding may arise that wasn’t present originally.
5. Morcellation should be performed only after malignancy has been ruled out—it is a technique intended for benign uteri only.
By bivalving the uterus it is possible to follow the endocervical canal up into the uterine cavity (FIGURE 1). Our technique at the Mayo Clinic is to place tenacula at the 3 and 9 o’clock positions prior to bivalving. A small amount of bleeding may occur because of collateral blood supply from the gonadal pedicles, but it should be minimal, as the uterine vessels have been secured.
FIGURE 1 Bivalve the uterus
|
To begin morcellation, split the uterus down the midline, with tenacula placed at the 3- and 9-o’clock positions, then follow the endocervical canal into the uterine cavity (A). Use a knife blade to take portions of myomas and other tissue to debulk the uterus (B). |
Proceed with morcellation once the uterus is bivalved. Use a Jacobs tenaculum to grasp the serosal portion of the uterus. Apply downward traction with your nondominant hand, and use the knife blade to resect portions of the uterus so that it can be debulked.
When a large myoma is encountered during morcellation, it often is possible to “finger-fracture” some of the filmy adhesions holding it in place, or to follow the pseudo-capsule of the fibroid in order to shell it out. In many cases, fibroids can be removed intact using these methods. If intact removal is not possible, debulk the fibroid by taking individual “bites.”
Tip. When the uterus is greatly enlarged, grasp it with a tenaculum so that it does not retract when you incise it. When large myomas are anticipated, keep an extra tenaculum on hand, as well as extra knife blades, as blades dull quickly when used to cut through calcified tissue. Continue to apply traction with your nondominant hand to allow each piece of tissue to be more readily developed (FIGURE 2).
Tip. When managing the round-ligament complex on each side, stay between the round ligaments (your “goal posts”) to avoid getting too lateral. Keep the cervix intact for orientation purposes. Focus on diminishing the bulk of the uterus so that you can get around the utero-ovarian pedicles.
To control the utero-ovarian pedicle on the patient’s right side, place a finger underneath it, with traction applied. Place a Heaney clamp from the top down. Repeat this action on the patient’s left side, but place the Heaney clamp from the bottom up.
Manual morcellation of tissue is useful in small uteri that are tough to access, but the procedure is very helpful in large uteri in order to remove them transvaginally.
Protect the ureters: Palpate them before clamping the pedicles
Palpating the ureters at the time of hysterectomy can protect their integrity during the procedure. The following technique has been used at the Mayo Clinic for many years and allows for location of the ureter so a cardinal pedicle clamp can be placed without injury.
Enter the anterior cul-de-sac so that you can insert a finger and palpate the ureter before you place the cardinal pedicle clamp on each side. Place Deaver retractors at the 12 o’clock and 2- to 3-o’clock positions. Insert your nondominant index finger into the anterior cul-de-sac and palpate the ureter against the Deaver clamp in the 2- to 3-o’clock position (FIGURE 3). (The ureter can be felt between your index finger and the Deaver retractor.) The ureter will have the most descent in a uterus that has some prolapse, compared with a nonprolapsed uterus.
Tip. One common error is mistaking the edge of the vaginal cuff for the ureter. Be certain that you insert your finger deeply into the cul-de-sac so that it is the ureter you feel and not the cuff edge.
Successful cystotomy repair technique
Inadvertent cystotomy is a common fear for surgeons at the time of vaginal hysterectomy. I prefer to empty the bladder before beginning the hysterectomy because it reduces the target zone that a distended bladder presents. Some surgeons prefer to maintain a bit of fluid in the bladder so that, if they cut into the bladder, a small urine stream results. The approach is a matter of preference.
Cystotomy is most common during anterior dissection. If it occurs, recognize it and mark the defect with suture. Do not attempt to repair the hole at this point, but opt to finish the hysterectomy.
Cystoscopy is an important element of cystotomy repair. Once the hysterectomy is completed, look inside the bladder and determine where the defect is in relationship to the ureteral orifices. Typically, it will be beyond the interureteric ridge, along the posterior portion of the bladder, usually in the midline.
As critical as the repair itself is management of bladder drainage afterward. If you repair the hole thoroughly and drain the bladder adequately for 14 days, the defect should heal fully.
Technique for entry into anterior cul-de-sac
One way to avert bladder injury is to enter the anterior cul-de-sac very carefully. Begin by ensuring that the bladder is empty and placing a Deaver retractor at the 12 o’clock position. Also place tenacula anteriorly and posteriorly to help direct traction. This will allow good visualization of the bladder reflection.
Tip. One common mistake is making the incision too low or too near the cervix, which makes dissection more difficult and increases the likelihood that you will enter the wrong plane. Be sure you know where the bladder is, and make an adequate incision that is not too distal. Otherwise, dissection will be harder to carry out.
I prefer to make one clean incision with the knife, rather than multiple incisions, because multiple cuts increase the likelihood that you will inadvertently injure the wrong tissue. Use good traction and countertraction, and hug the uterus. Work low on the uterus, but not in the uterus. If you cut into muscle, you will get more bleeding and may end up digging a hole.
After you make the incision, put your finger through it to help develop that space further. You can confirm entry into the peritoneum by noting the characteristic slippery feel of the peritoneal lining. After you insert a Deaver retractor anteriorly, reinsert your finger and mobilize the area further. Then you can easily reach in and tent the peritoneum to cut it.
Technique for cystotomy repair
Two-layer closure is a minimum. On occasion, a third layer may be beneficial. Begin with running closure of the first layer using 2-0 chromic suture—a good suture choice in the urinary tract. This suture is inflammatory, which will help seal the wound, but it also dissolves quickly, preventing stone formation.
Use through-and-through closure on the first layer, followed by a second imbricating layer. If desired, use the peritoneum as a third layer.
Horizontal repair is typical, although vertical closure may be necessary if the defect is near a ureteral orifice and horizontal closure might compromise that side. That decision must be made intraoperatively.
When vertical repair is necessary, begin your repair just above the defect, placing the suture through and through. The hole should be visible. There is no need to be extramucosal in needle placement. Simply get a good bite of the tissue and run the repair down the bladder wall.
Next, stop and apply traction to the repair to check for any small defects that may have been overlooked. By placing a little traction on that first suture tag, any such defects will become apparent. Then go back and close them in a secondary imbricating layer.
After 2-layer closure, fill the bladder retrograde. I prefer to use a couple of drops of methylene blue in normal saline and place a clean white piece of packing material beneath the wound. If the packing material remains unstained by blue, the repair is watertight.
Incorporate the peritoneum as another layer of repair of the defect. I imbricate 2 layers in the bladder. Then, if necessary, I use that peritoneum as an additional layer (FIGURE 4).
Strategies to control bleeding at adnexectomy
Be vigilant for bleeding when removing the tubes and/or ovaries. At salpingectomy, be extremely gentle with the mesosalpinx because it can be avulsed easily off of surrounding tissue. If bleeding occurs, oversewing, or even ovary removal, could end up being the only options.
Good visualization is essential during vaginal procedures. Retractors, lighted suction irrigators, a headlamp, good overhead lighting, and appropriate instrumentation are critical for success.
Heaney clamp technique for vaginal oophorectomy
Begin by placing an Allis clamp on the utero-ovarian pedicle. Then clamp the ovary and tube with a second Allis clamp. Next, insert a Heaney clamp through the small window between the cardinal pedicle and the utero-ovarian pedicle (FIGURE 5). Clamp the tissue and place a free tie around it.
Because this is a major vascular pedicle, doubly ligate it. As you tie the first suture, have an assistant flash the clamp open and closed, then excise the specimen. There is no need to worry about losing the pedicle because it already has been ligated once. Next, stick-tie it, placing the needle distal to the free tie to avoid piercing the gonadal vessels beyond.
The technique is standard. Be gentle, and ensure good hemostasis when finished.
Tip. In my experience, any bleeding runs down from the pedicle rather than out toward me. So be sure to look down and below the pedicle to ensure hemostasis.
Additional pearls
- When performing vaginal hysterectomy, the ovaries are almost always removable transvaginally. There is no need to begin the case laparoscopically to remove the tubes and/or ovaries and then perform the hysterectomy vaginally.
- Deaver retractors offer good exposure; visualization is critical.
- Make sure the tissue is dry before you cut the last suture.
- If you prefer to use a laparoscopic stapler to secure the pedicles, proceed as before: Place an Allis clamp on the pedicle. Place a second clamp on the ovary and tube. Now you can insert the stapler into the created window, as with the Heaney clamp (FIGURE 6).
- Use a 60-mm stapler to cut the pedicle in one try. If using a 45-mm device, the stapler may need to be fired twice. This makes the procedure more expensive and risks more bleeding.
- When closing the stapler jaws, avoid clamping small bowel or packing material. Ensure stapler tip visibility well before firing.
The round ligament technique
When transecting the round ligament, it is critical to stay just beneath it to avoid bleeding and venturing into the mesosalpinx. Gently hug the tissue inferior to the round ligament and let it retract (FIGURE 7). This will allow isolation of the gonadal vessels nicely, especially if an adnexal mass is present. Then isolate the specimen and remove it, stick-tying the pedicle afterward to secure it.
When tying the pedicle, place the suture around the distal aspect to ensure that the back of the pedicle is enclosed, and do not lose it when you release the clamp. A slightly different technique is to use an endoloop to cross the gonadal vessels and control them. Use a suction irrigator and good lighting to get good exposure.
Next, place the clamp, making sure you don’t inadvertently grasp the packing material. Visualize both tips of the clamp before incising. Trim the specimen flush with the clamp. Then you can thread an endoloop over the top of the clamp. This is an inexpensive technique that allows a higher reach into the pelvic cavity. Finally, cinch down the endoloop to control the vessels.
When performing bilateral salpingo-oophorectomy, a long, fine clamp, such as the M.D. Anderson clamp, can help you reach up to control the gonadal vessels in the event that you lose your initial grip on those vessels (FIGURE 8).
Be prepared
Have a plan in place to manage any complications that arise during surgery. Just as obstetricians plan ahead to prepare for shoulder dystocia and other emergencies, gynecologic surgeons must prepare for surgical complications. Tissue extraction strategies can aid in the debulking and removal of large uteri, and the proper tools, lighting, and assistance are critical to success.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Careful attention to technique at the time of vaginal hysterectomy is vital. Equally important is prior consideration of potential complications and the best ways to address them. Four trouble spots include:
- uterine tissue extraction (Although this is not a complication of vaginal hysterectomy, tissue extraction aids in debulking and removal of a large uterus.)
- protection of the ureters (It is important to palpate these structures before placing cardinal pedicle clamps, to protect ureteral integrity.)
- repair of inadvertent cystotomy
- control of bleeding in the setting of adnexectomy.
I focus on optimal approaches to these 4 scenarios in this article.
For a review of vaginal hysterectomy technique, see “Vaginal hysterectomy with basic instrumentation,” by Barbara S. Levy, MD, which appeared in the October 2015 issue of OBG Management. For salpingectomy and salpingo-oophorectomy technique, see my article entitled “Salpingectomy after vaginal hysterectomy: Technique, tips, and pearls,” which appeared in the November issue of this journal.
Both articles are available in the archive at obgmanagement.com and, like this one, are based on the AAGL-produced Online Master Class on Vaginal Hysterectomy, a Web-based program cosponsored by the American College of Obstetricians and Gynecologists and the Society of Gynecologic Surgeons. That program is available at https://www.aagl.org/vaghystwebinar/.
A step toward success: Begin morcellation by splitting the uterus
Manual morcellation to reduce uterine size and ease transvaginal removal is a useful technique to know. Five aspects of manual morcellation warrant emphasis:
1. Anterior and posterior entry into the cul-de-sacs is essential before attempting morcellation.
2. The blood supply on both sides of the uterus must be controlled.
3. During resection, take care to cut only tissue that can be visualized. Avoid resection beyond what you can easily see.
4. Once morcellation is completed, always go back and check the pedicles for hemostasis. During morcellation, these pedicles tend to get stretched, and bleeding may arise that wasn’t present originally.
5. Morcellation should be performed only after malignancy has been ruled out—it is a technique intended for benign uteri only.
By bivalving the uterus it is possible to follow the endocervical canal up into the uterine cavity (FIGURE 1). Our technique at the Mayo Clinic is to place tenacula at the 3 and 9 o’clock positions prior to bivalving. A small amount of bleeding may occur because of collateral blood supply from the gonadal pedicles, but it should be minimal, as the uterine vessels have been secured.
FIGURE 1 Bivalve the uterus
|
To begin morcellation, split the uterus down the midline, with tenacula placed at the 3- and 9-o’clock positions, then follow the endocervical canal into the uterine cavity (A). Use a knife blade to take portions of myomas and other tissue to debulk the uterus (B). |
Proceed with morcellation once the uterus is bivalved. Use a Jacobs tenaculum to grasp the serosal portion of the uterus. Apply downward traction with your nondominant hand, and use the knife blade to resect portions of the uterus so that it can be debulked.
When a large myoma is encountered during morcellation, it often is possible to “finger-fracture” some of the filmy adhesions holding it in place, or to follow the pseudo-capsule of the fibroid in order to shell it out. In many cases, fibroids can be removed intact using these methods. If intact removal is not possible, debulk the fibroid by taking individual “bites.”
Tip. When the uterus is greatly enlarged, grasp it with a tenaculum so that it does not retract when you incise it. When large myomas are anticipated, keep an extra tenaculum on hand, as well as extra knife blades, as blades dull quickly when used to cut through calcified tissue. Continue to apply traction with your nondominant hand to allow each piece of tissue to be more readily developed (FIGURE 2).
Tip. When managing the round-ligament complex on each side, stay between the round ligaments (your “goal posts”) to avoid getting too lateral. Keep the cervix intact for orientation purposes. Focus on diminishing the bulk of the uterus so that you can get around the utero-ovarian pedicles.
To control the utero-ovarian pedicle on the patient’s right side, place a finger underneath it, with traction applied. Place a Heaney clamp from the top down. Repeat this action on the patient’s left side, but place the Heaney clamp from the bottom up.
Manual morcellation of tissue is useful in small uteri that are tough to access, but the procedure is very helpful in large uteri in order to remove them transvaginally.
Protect the ureters: Palpate them before clamping the pedicles
Palpating the ureters at the time of hysterectomy can protect their integrity during the procedure. The following technique has been used at the Mayo Clinic for many years and allows for location of the ureter so a cardinal pedicle clamp can be placed without injury.
Enter the anterior cul-de-sac so that you can insert a finger and palpate the ureter before you place the cardinal pedicle clamp on each side. Place Deaver retractors at the 12 o’clock and 2- to 3-o’clock positions. Insert your nondominant index finger into the anterior cul-de-sac and palpate the ureter against the Deaver clamp in the 2- to 3-o’clock position (FIGURE 3). (The ureter can be felt between your index finger and the Deaver retractor.) The ureter will have the most descent in a uterus that has some prolapse, compared with a nonprolapsed uterus.
Tip. One common error is mistaking the edge of the vaginal cuff for the ureter. Be certain that you insert your finger deeply into the cul-de-sac so that it is the ureter you feel and not the cuff edge.
Successful cystotomy repair technique
Inadvertent cystotomy is a common fear for surgeons at the time of vaginal hysterectomy. I prefer to empty the bladder before beginning the hysterectomy because it reduces the target zone that a distended bladder presents. Some surgeons prefer to maintain a bit of fluid in the bladder so that, if they cut into the bladder, a small urine stream results. The approach is a matter of preference.
Cystotomy is most common during anterior dissection. If it occurs, recognize it and mark the defect with suture. Do not attempt to repair the hole at this point, but opt to finish the hysterectomy.
Cystoscopy is an important element of cystotomy repair. Once the hysterectomy is completed, look inside the bladder and determine where the defect is in relationship to the ureteral orifices. Typically, it will be beyond the interureteric ridge, along the posterior portion of the bladder, usually in the midline.
As critical as the repair itself is management of bladder drainage afterward. If you repair the hole thoroughly and drain the bladder adequately for 14 days, the defect should heal fully.
Technique for entry into anterior cul-de-sac
One way to avert bladder injury is to enter the anterior cul-de-sac very carefully. Begin by ensuring that the bladder is empty and placing a Deaver retractor at the 12 o’clock position. Also place tenacula anteriorly and posteriorly to help direct traction. This will allow good visualization of the bladder reflection.
Tip. One common mistake is making the incision too low or too near the cervix, which makes dissection more difficult and increases the likelihood that you will enter the wrong plane. Be sure you know where the bladder is, and make an adequate incision that is not too distal. Otherwise, dissection will be harder to carry out.
I prefer to make one clean incision with the knife, rather than multiple incisions, because multiple cuts increase the likelihood that you will inadvertently injure the wrong tissue. Use good traction and countertraction, and hug the uterus. Work low on the uterus, but not in the uterus. If you cut into muscle, you will get more bleeding and may end up digging a hole.
After you make the incision, put your finger through it to help develop that space further. You can confirm entry into the peritoneum by noting the characteristic slippery feel of the peritoneal lining. After you insert a Deaver retractor anteriorly, reinsert your finger and mobilize the area further. Then you can easily reach in and tent the peritoneum to cut it.
Technique for cystotomy repair
Two-layer closure is a minimum. On occasion, a third layer may be beneficial. Begin with running closure of the first layer using 2-0 chromic suture—a good suture choice in the urinary tract. This suture is inflammatory, which will help seal the wound, but it also dissolves quickly, preventing stone formation.
Use through-and-through closure on the first layer, followed by a second imbricating layer. If desired, use the peritoneum as a third layer.
Horizontal repair is typical, although vertical closure may be necessary if the defect is near a ureteral orifice and horizontal closure might compromise that side. That decision must be made intraoperatively.
When vertical repair is necessary, begin your repair just above the defect, placing the suture through and through. The hole should be visible. There is no need to be extramucosal in needle placement. Simply get a good bite of the tissue and run the repair down the bladder wall.
Next, stop and apply traction to the repair to check for any small defects that may have been overlooked. By placing a little traction on that first suture tag, any such defects will become apparent. Then go back and close them in a secondary imbricating layer.
After 2-layer closure, fill the bladder retrograde. I prefer to use a couple of drops of methylene blue in normal saline and place a clean white piece of packing material beneath the wound. If the packing material remains unstained by blue, the repair is watertight.
Incorporate the peritoneum as another layer of repair of the defect. I imbricate 2 layers in the bladder. Then, if necessary, I use that peritoneum as an additional layer (FIGURE 4).
Strategies to control bleeding at adnexectomy
Be vigilant for bleeding when removing the tubes and/or ovaries. At salpingectomy, be extremely gentle with the mesosalpinx because it can be avulsed easily off of surrounding tissue. If bleeding occurs, oversewing, or even ovary removal, could end up being the only options.
Good visualization is essential during vaginal procedures. Retractors, lighted suction irrigators, a headlamp, good overhead lighting, and appropriate instrumentation are critical for success.
Heaney clamp technique for vaginal oophorectomy
Begin by placing an Allis clamp on the utero-ovarian pedicle. Then clamp the ovary and tube with a second Allis clamp. Next, insert a Heaney clamp through the small window between the cardinal pedicle and the utero-ovarian pedicle (FIGURE 5). Clamp the tissue and place a free tie around it.
Because this is a major vascular pedicle, doubly ligate it. As you tie the first suture, have an assistant flash the clamp open and closed, then excise the specimen. There is no need to worry about losing the pedicle because it already has been ligated once. Next, stick-tie it, placing the needle distal to the free tie to avoid piercing the gonadal vessels beyond.
The technique is standard. Be gentle, and ensure good hemostasis when finished.
Tip. In my experience, any bleeding runs down from the pedicle rather than out toward me. So be sure to look down and below the pedicle to ensure hemostasis.
Additional pearls
- When performing vaginal hysterectomy, the ovaries are almost always removable transvaginally. There is no need to begin the case laparoscopically to remove the tubes and/or ovaries and then perform the hysterectomy vaginally.
- Deaver retractors offer good exposure; visualization is critical.
- Make sure the tissue is dry before you cut the last suture.
- If you prefer to use a laparoscopic stapler to secure the pedicles, proceed as before: Place an Allis clamp on the pedicle. Place a second clamp on the ovary and tube. Now you can insert the stapler into the created window, as with the Heaney clamp (FIGURE 6).
- Use a 60-mm stapler to cut the pedicle in one try. If using a 45-mm device, the stapler may need to be fired twice. This makes the procedure more expensive and risks more bleeding.
- When closing the stapler jaws, avoid clamping small bowel or packing material. Ensure stapler tip visibility well before firing.
The round ligament technique
When transecting the round ligament, it is critical to stay just beneath it to avoid bleeding and venturing into the mesosalpinx. Gently hug the tissue inferior to the round ligament and let it retract (FIGURE 7). This will allow isolation of the gonadal vessels nicely, especially if an adnexal mass is present. Then isolate the specimen and remove it, stick-tying the pedicle afterward to secure it.
When tying the pedicle, place the suture around the distal aspect to ensure that the back of the pedicle is enclosed, and do not lose it when you release the clamp. A slightly different technique is to use an endoloop to cross the gonadal vessels and control them. Use a suction irrigator and good lighting to get good exposure.
Next, place the clamp, making sure you don’t inadvertently grasp the packing material. Visualize both tips of the clamp before incising. Trim the specimen flush with the clamp. Then you can thread an endoloop over the top of the clamp. This is an inexpensive technique that allows a higher reach into the pelvic cavity. Finally, cinch down the endoloop to control the vessels.
When performing bilateral salpingo-oophorectomy, a long, fine clamp, such as the M.D. Anderson clamp, can help you reach up to control the gonadal vessels in the event that you lose your initial grip on those vessels (FIGURE 8).
Be prepared
Have a plan in place to manage any complications that arise during surgery. Just as obstetricians plan ahead to prepare for shoulder dystocia and other emergencies, gynecologic surgeons must prepare for surgical complications. Tissue extraction strategies can aid in the debulking and removal of large uteri, and the proper tools, lighting, and assistance are critical to success.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this Article
- Ensuring ureter protection
- Cystotomy repair
- Bleeding control strategies
This article is based on the AAGL-produced and ACOG/SGS cosponsored Online Master Class on Vaginal Hysterectomy