User login
Enhancing Dermatology Education: Resident Presentation Opportunities
Dermatology residency is busy with 3 years of clinical duties, academic responsibilities, and administrative work. In addition, it is a time to maximize educational experiences in dermatology from didactics to hands-on learning. It also is a time to take advantage of special opportunities that are available to residents, including attending academic meetings and giving oral and/or poster presentations. Major dermatology conferences often have designated sessions for residents that provide an excellent chance for residents to share interesting cases or present their research. This article provides a review of selected presentation opportunities available to residents at the major academic dermatology meetings.
American Academy of Dermatology
The Annual Meeting of the American Academy of Dermatology (AAD) accepts abstracts for oral presentation from both residents and fellows for its “Residents and Fellows Symposium” and “Gross and Microscopic Symposium.” The “Residents and Fellows Symposium” is an opportunity to present either clinical or laboratory research in a 9-minute oral format. Up to 20 abstracts are chosen for presentation along with 4 alternate abstracts. Furthermore, awards are given to the top 3 abstracts in both the clinical and laboratory categories. Those accepted for the “Gross and Microscopic Symposium” give a 5-minute oral presentation of a case with interesting clinical and histopathological findings. Submission guidelines for these presentations are available on the AAD Web site (https://www.aad.org/symposium/am2016).
Residents and fellows also are eligible to submit abstracts for the AAD’s electronic poster exhibits and presentations. The posters are presented electronically and are displayed and/or are available to be viewed throughout the meeting. The abstracts are blind reviewed by the Poster Exhibits Task Force on a scale from 1 (unsatisfactory) to 10 (outstanding). Presenters with abstracts that receive a passing score (2.5 or higher) by judges are allowed to discuss their poster in a live 5-minute oral presentation.
The AAD’s Summer Academy Meeting, which also takes place annually, does not have separate resident-specific poster or oral presentation sessions; however, it does offer an electronic poster exhibit and presentation session.
Pediatric Dermatology
The Annual Meeting of the Society for Pediatric Dermatology (https://pedsderm.net/meetings/annual-meeting/) accepts abstract submissions for its “Cases of the Year” session as well as poster presentations. Residents, medical students, and fellows who are chosen for a “Cases of the Year” or poster presentation are eligible for a travel award that is available on a competitive basis. The American Academy of Pediatrics’ Section on Dermatology also offers an additional travel award for a resident or fellow who presents a case or poster at the Annual Meeting of the Society for Pediatric Dermatology.
American Society for Dermatologic Surgery
The American Society for Dermatologic Surgery has an Annual Meeting (https://www.asds.net/ annualmeeting/) that includes a competitive “Resident Oral Abstracts” session. If selected, residents give a 5-minute presentation and abstracts are published in the Annual Meeting program book.
American Society of Dermatopathology
The American Society of Dermatopathology Annual Meeting has several opportunities for residents and fellows to present abstracts (https://www.asdp.org/meetings-events/annual-meeting/52nd/call-for -abtracts/). Submissions to the “General Abstracts” category are selected for either oral or poster presentation. Ambitious dermatology or pathology residents may choose to submit their case report abstracts to the “Duel in Dermatopathology” competition, which includes an oral presentation and publication of abstracts in the meeting program book. Finally, the “Dermatopathology Fellows Abstract” category is a special category for dermatopathology fellows to present an oral or poster presentation. Any resident or fellow who is accepted for oral or poster presentations is eligible for a “Physician-in-Training Award” (except winners of the “Duel in Dermatopathology” competition), which are granted to the best oral and poster presentations.
Conclusion
Beyond dermatology residency, there are many opportunities for resident education through attendance at academic meetings as well as presentation of case reports and research. The major dermatology meetings often have specific sessions to give residents a chance to share their work or interesting cases. This guide may be helpful to residents who are hoping for such venues to enhance their education and even their curriculum vitae.
Dermatology residency is busy with 3 years of clinical duties, academic responsibilities, and administrative work. In addition, it is a time to maximize educational experiences in dermatology from didactics to hands-on learning. It also is a time to take advantage of special opportunities that are available to residents, including attending academic meetings and giving oral and/or poster presentations. Major dermatology conferences often have designated sessions for residents that provide an excellent chance for residents to share interesting cases or present their research. This article provides a review of selected presentation opportunities available to residents at the major academic dermatology meetings.
American Academy of Dermatology
The Annual Meeting of the American Academy of Dermatology (AAD) accepts abstracts for oral presentation from both residents and fellows for its “Residents and Fellows Symposium” and “Gross and Microscopic Symposium.” The “Residents and Fellows Symposium” is an opportunity to present either clinical or laboratory research in a 9-minute oral format. Up to 20 abstracts are chosen for presentation along with 4 alternate abstracts. Furthermore, awards are given to the top 3 abstracts in both the clinical and laboratory categories. Those accepted for the “Gross and Microscopic Symposium” give a 5-minute oral presentation of a case with interesting clinical and histopathological findings. Submission guidelines for these presentations are available on the AAD Web site (https://www.aad.org/symposium/am2016).
Residents and fellows also are eligible to submit abstracts for the AAD’s electronic poster exhibits and presentations. The posters are presented electronically and are displayed and/or are available to be viewed throughout the meeting. The abstracts are blind reviewed by the Poster Exhibits Task Force on a scale from 1 (unsatisfactory) to 10 (outstanding). Presenters with abstracts that receive a passing score (2.5 or higher) by judges are allowed to discuss their poster in a live 5-minute oral presentation.
The AAD’s Summer Academy Meeting, which also takes place annually, does not have separate resident-specific poster or oral presentation sessions; however, it does offer an electronic poster exhibit and presentation session.
Pediatric Dermatology
The Annual Meeting of the Society for Pediatric Dermatology (https://pedsderm.net/meetings/annual-meeting/) accepts abstract submissions for its “Cases of the Year” session as well as poster presentations. Residents, medical students, and fellows who are chosen for a “Cases of the Year” or poster presentation are eligible for a travel award that is available on a competitive basis. The American Academy of Pediatrics’ Section on Dermatology also offers an additional travel award for a resident or fellow who presents a case or poster at the Annual Meeting of the Society for Pediatric Dermatology.
American Society for Dermatologic Surgery
The American Society for Dermatologic Surgery has an Annual Meeting (https://www.asds.net/ annualmeeting/) that includes a competitive “Resident Oral Abstracts” session. If selected, residents give a 5-minute presentation and abstracts are published in the Annual Meeting program book.
American Society of Dermatopathology
The American Society of Dermatopathology Annual Meeting has several opportunities for residents and fellows to present abstracts (https://www.asdp.org/meetings-events/annual-meeting/52nd/call-for -abtracts/). Submissions to the “General Abstracts” category are selected for either oral or poster presentation. Ambitious dermatology or pathology residents may choose to submit their case report abstracts to the “Duel in Dermatopathology” competition, which includes an oral presentation and publication of abstracts in the meeting program book. Finally, the “Dermatopathology Fellows Abstract” category is a special category for dermatopathology fellows to present an oral or poster presentation. Any resident or fellow who is accepted for oral or poster presentations is eligible for a “Physician-in-Training Award” (except winners of the “Duel in Dermatopathology” competition), which are granted to the best oral and poster presentations.
Conclusion
Beyond dermatology residency, there are many opportunities for resident education through attendance at academic meetings as well as presentation of case reports and research. The major dermatology meetings often have specific sessions to give residents a chance to share their work or interesting cases. This guide may be helpful to residents who are hoping for such venues to enhance their education and even their curriculum vitae.
Dermatology residency is busy with 3 years of clinical duties, academic responsibilities, and administrative work. In addition, it is a time to maximize educational experiences in dermatology from didactics to hands-on learning. It also is a time to take advantage of special opportunities that are available to residents, including attending academic meetings and giving oral and/or poster presentations. Major dermatology conferences often have designated sessions for residents that provide an excellent chance for residents to share interesting cases or present their research. This article provides a review of selected presentation opportunities available to residents at the major academic dermatology meetings.
American Academy of Dermatology
The Annual Meeting of the American Academy of Dermatology (AAD) accepts abstracts for oral presentation from both residents and fellows for its “Residents and Fellows Symposium” and “Gross and Microscopic Symposium.” The “Residents and Fellows Symposium” is an opportunity to present either clinical or laboratory research in a 9-minute oral format. Up to 20 abstracts are chosen for presentation along with 4 alternate abstracts. Furthermore, awards are given to the top 3 abstracts in both the clinical and laboratory categories. Those accepted for the “Gross and Microscopic Symposium” give a 5-minute oral presentation of a case with interesting clinical and histopathological findings. Submission guidelines for these presentations are available on the AAD Web site (https://www.aad.org/symposium/am2016).
Residents and fellows also are eligible to submit abstracts for the AAD’s electronic poster exhibits and presentations. The posters are presented electronically and are displayed and/or are available to be viewed throughout the meeting. The abstracts are blind reviewed by the Poster Exhibits Task Force on a scale from 1 (unsatisfactory) to 10 (outstanding). Presenters with abstracts that receive a passing score (2.5 or higher) by judges are allowed to discuss their poster in a live 5-minute oral presentation.
The AAD’s Summer Academy Meeting, which also takes place annually, does not have separate resident-specific poster or oral presentation sessions; however, it does offer an electronic poster exhibit and presentation session.
Pediatric Dermatology
The Annual Meeting of the Society for Pediatric Dermatology (https://pedsderm.net/meetings/annual-meeting/) accepts abstract submissions for its “Cases of the Year” session as well as poster presentations. Residents, medical students, and fellows who are chosen for a “Cases of the Year” or poster presentation are eligible for a travel award that is available on a competitive basis. The American Academy of Pediatrics’ Section on Dermatology also offers an additional travel award for a resident or fellow who presents a case or poster at the Annual Meeting of the Society for Pediatric Dermatology.
American Society for Dermatologic Surgery
The American Society for Dermatologic Surgery has an Annual Meeting (https://www.asds.net/ annualmeeting/) that includes a competitive “Resident Oral Abstracts” session. If selected, residents give a 5-minute presentation and abstracts are published in the Annual Meeting program book.
American Society of Dermatopathology
The American Society of Dermatopathology Annual Meeting has several opportunities for residents and fellows to present abstracts (https://www.asdp.org/meetings-events/annual-meeting/52nd/call-for -abtracts/). Submissions to the “General Abstracts” category are selected for either oral or poster presentation. Ambitious dermatology or pathology residents may choose to submit their case report abstracts to the “Duel in Dermatopathology” competition, which includes an oral presentation and publication of abstracts in the meeting program book. Finally, the “Dermatopathology Fellows Abstract” category is a special category for dermatopathology fellows to present an oral or poster presentation. Any resident or fellow who is accepted for oral or poster presentations is eligible for a “Physician-in-Training Award” (except winners of the “Duel in Dermatopathology” competition), which are granted to the best oral and poster presentations.
Conclusion
Beyond dermatology residency, there are many opportunities for resident education through attendance at academic meetings as well as presentation of case reports and research. The major dermatology meetings often have specific sessions to give residents a chance to share their work or interesting cases. This guide may be helpful to residents who are hoping for such venues to enhance their education and even their curriculum vitae.
New atypical antipsychotic FDA approved for use in bipolar I and schizophrenia
The Food and Drug Administration on Sept. 17 approved cariprazine, an atypical antipsychotic, for the acute treatment of manic or mixed episodes in bipolar I disorder and schizophrenia in adults.
Results from three separate controlled trials in adults with manic or mixed episodes of bipolar I disorder showed cariprazine (Vraylar) was associated with improved total scores on the Young Mania Rating Scale (YMRS), compared with placebo. In three separate placebo-controlled trials in adults with schizophrenia, the study drug was associated with improvements in Positive and Negative Syndrome Scale (PANSS) total scores, compared with placebo. Cariprazine also demonstrated efficacy in the Clinical Global Impressions–Severity (CGI-S) rating scale, which was the secondary efficacy endpoint in the respective trials for each condition. In all, 2,700 persons were enrolled in the trials.
The recommended dose of cariprazine in adults with bipolar I is once daily at 3-6 mg per day. For schizophrenia in adults, 1.5-6 mg/day is the recommended dose.
Adverse reactions occurring in at least 5% of the study population and at a rate of twice that in the placebo groups were extrapyramidal symptoms, akathisia, dyspepsia, vomiting, somnolence, and restlessness in the bipolar group. In the group with schizophrenia, the most commonly reported adverse events were extrapyramidal symptoms and akathisia.
Cariprazine is a dopamine-2 and dopamine-3 receptor partial agonist, tending toward the D3 receptor. Although its mechanism of action in schizophrenia and bipolar I disorder is unknown, the drug’s codeveloper, Gedeon Richter said in a statement that cariprazine’s efficacy “could be mediated through a combination of partial agonist activity at central D2 and serotonin 5-HT1A receptors and antagonist activity at serotonin 5-HT2A receptors.” In the United States and Canada, the drug is licensed to Actavis, now Allergan. Vraylar is manufactured by Forest Laboratories.
Data indicating the drug’s ability to improve flat affect in schizophrenia were presented at this year’s annual congress of the European College of Neuropsychopharmacology in Amsterdam. According to Gedeon Richter and Allergan, cariprazine also is being investigated for the treatment of bipolar depression and as adjunctive treatment for major depressive disorder in adults.
On Twitter @whitneymcknight
*This article was updated 9/18/2015.
The Food and Drug Administration on Sept. 17 approved cariprazine, an atypical antipsychotic, for the acute treatment of manic or mixed episodes in bipolar I disorder and schizophrenia in adults.
Results from three separate controlled trials in adults with manic or mixed episodes of bipolar I disorder showed cariprazine (Vraylar) was associated with improved total scores on the Young Mania Rating Scale (YMRS), compared with placebo. In three separate placebo-controlled trials in adults with schizophrenia, the study drug was associated with improvements in Positive and Negative Syndrome Scale (PANSS) total scores, compared with placebo. Cariprazine also demonstrated efficacy in the Clinical Global Impressions–Severity (CGI-S) rating scale, which was the secondary efficacy endpoint in the respective trials for each condition. In all, 2,700 persons were enrolled in the trials.
The recommended dose of cariprazine in adults with bipolar I is once daily at 3-6 mg per day. For schizophrenia in adults, 1.5-6 mg/day is the recommended dose.
Adverse reactions occurring in at least 5% of the study population and at a rate of twice that in the placebo groups were extrapyramidal symptoms, akathisia, dyspepsia, vomiting, somnolence, and restlessness in the bipolar group. In the group with schizophrenia, the most commonly reported adverse events were extrapyramidal symptoms and akathisia.
Cariprazine is a dopamine-2 and dopamine-3 receptor partial agonist, tending toward the D3 receptor. Although its mechanism of action in schizophrenia and bipolar I disorder is unknown, the drug’s codeveloper, Gedeon Richter said in a statement that cariprazine’s efficacy “could be mediated through a combination of partial agonist activity at central D2 and serotonin 5-HT1A receptors and antagonist activity at serotonin 5-HT2A receptors.” In the United States and Canada, the drug is licensed to Actavis, now Allergan. Vraylar is manufactured by Forest Laboratories.
Data indicating the drug’s ability to improve flat affect in schizophrenia were presented at this year’s annual congress of the European College of Neuropsychopharmacology in Amsterdam. According to Gedeon Richter and Allergan, cariprazine also is being investigated for the treatment of bipolar depression and as adjunctive treatment for major depressive disorder in adults.
On Twitter @whitneymcknight
*This article was updated 9/18/2015.
The Food and Drug Administration on Sept. 17 approved cariprazine, an atypical antipsychotic, for the acute treatment of manic or mixed episodes in bipolar I disorder and schizophrenia in adults.
Results from three separate controlled trials in adults with manic or mixed episodes of bipolar I disorder showed cariprazine (Vraylar) was associated with improved total scores on the Young Mania Rating Scale (YMRS), compared with placebo. In three separate placebo-controlled trials in adults with schizophrenia, the study drug was associated with improvements in Positive and Negative Syndrome Scale (PANSS) total scores, compared with placebo. Cariprazine also demonstrated efficacy in the Clinical Global Impressions–Severity (CGI-S) rating scale, which was the secondary efficacy endpoint in the respective trials for each condition. In all, 2,700 persons were enrolled in the trials.
The recommended dose of cariprazine in adults with bipolar I is once daily at 3-6 mg per day. For schizophrenia in adults, 1.5-6 mg/day is the recommended dose.
Adverse reactions occurring in at least 5% of the study population and at a rate of twice that in the placebo groups were extrapyramidal symptoms, akathisia, dyspepsia, vomiting, somnolence, and restlessness in the bipolar group. In the group with schizophrenia, the most commonly reported adverse events were extrapyramidal symptoms and akathisia.
Cariprazine is a dopamine-2 and dopamine-3 receptor partial agonist, tending toward the D3 receptor. Although its mechanism of action in schizophrenia and bipolar I disorder is unknown, the drug’s codeveloper, Gedeon Richter said in a statement that cariprazine’s efficacy “could be mediated through a combination of partial agonist activity at central D2 and serotonin 5-HT1A receptors and antagonist activity at serotonin 5-HT2A receptors.” In the United States and Canada, the drug is licensed to Actavis, now Allergan. Vraylar is manufactured by Forest Laboratories.
Data indicating the drug’s ability to improve flat affect in schizophrenia were presented at this year’s annual congress of the European College of Neuropsychopharmacology in Amsterdam. According to Gedeon Richter and Allergan, cariprazine also is being investigated for the treatment of bipolar depression and as adjunctive treatment for major depressive disorder in adults.
On Twitter @whitneymcknight
*This article was updated 9/18/2015.
VIDEO: CDC urges flu shots for all eligible patients
WASHINGTON – While influenza vaccination rates have increased in recent years, work still needs to be done to achieve the Centers for Disease Control and Prevention’s goal of at least 70% vaccination.
“Vaccination is the single-most-important step people can take to protect themselves from influenza,” Dr. Tom Frieden, CDC director said at a press conference called by his agency and the National Foundation for Infectious Diseases (NFID). He urged people to get their influenza vaccination and make sure their children do as well.
The CDC estimates that 47% of U.S. residents aged 6 months or older received an influenza vaccination in the last flu season. The only age group that meets the federal 70% benchmark is the 6-23 months age group, with about 75% coverage. Children aged 2-4 years have a vaccination rate of 68%; adults aged 65 years and older have a vaccination rate of 67%; and 62% of children aged 5-12 years get vaccinated. The lowest vaccination rate is among adults aged 18-49 years, of whom only 40% get vaccinated.
Dr. Frieden was joined at the press event by Dr. William Schaffner, NFID medical director; Dr. Wendy Sue Swanson of Seattle Children’s Hospital; and Dr. Kathleen Neuzil, director of the Center for Vaccine Development at the University of Maryland, Baltimore.
In this interview, Dr. Neuzil discusses which strains of influenza are expected to be dominant in the coming flu season, whether to expect a strain mutation similar to what happened last season, the importance of getting children vaccinated, and pneumococcal vaccination for children and older adults.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – While influenza vaccination rates have increased in recent years, work still needs to be done to achieve the Centers for Disease Control and Prevention’s goal of at least 70% vaccination.
“Vaccination is the single-most-important step people can take to protect themselves from influenza,” Dr. Tom Frieden, CDC director said at a press conference called by his agency and the National Foundation for Infectious Diseases (NFID). He urged people to get their influenza vaccination and make sure their children do as well.
The CDC estimates that 47% of U.S. residents aged 6 months or older received an influenza vaccination in the last flu season. The only age group that meets the federal 70% benchmark is the 6-23 months age group, with about 75% coverage. Children aged 2-4 years have a vaccination rate of 68%; adults aged 65 years and older have a vaccination rate of 67%; and 62% of children aged 5-12 years get vaccinated. The lowest vaccination rate is among adults aged 18-49 years, of whom only 40% get vaccinated.
Dr. Frieden was joined at the press event by Dr. William Schaffner, NFID medical director; Dr. Wendy Sue Swanson of Seattle Children’s Hospital; and Dr. Kathleen Neuzil, director of the Center for Vaccine Development at the University of Maryland, Baltimore.
In this interview, Dr. Neuzil discusses which strains of influenza are expected to be dominant in the coming flu season, whether to expect a strain mutation similar to what happened last season, the importance of getting children vaccinated, and pneumococcal vaccination for children and older adults.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
WASHINGTON – While influenza vaccination rates have increased in recent years, work still needs to be done to achieve the Centers for Disease Control and Prevention’s goal of at least 70% vaccination.
“Vaccination is the single-most-important step people can take to protect themselves from influenza,” Dr. Tom Frieden, CDC director said at a press conference called by his agency and the National Foundation for Infectious Diseases (NFID). He urged people to get their influenza vaccination and make sure their children do as well.
The CDC estimates that 47% of U.S. residents aged 6 months or older received an influenza vaccination in the last flu season. The only age group that meets the federal 70% benchmark is the 6-23 months age group, with about 75% coverage. Children aged 2-4 years have a vaccination rate of 68%; adults aged 65 years and older have a vaccination rate of 67%; and 62% of children aged 5-12 years get vaccinated. The lowest vaccination rate is among adults aged 18-49 years, of whom only 40% get vaccinated.
Dr. Frieden was joined at the press event by Dr. William Schaffner, NFID medical director; Dr. Wendy Sue Swanson of Seattle Children’s Hospital; and Dr. Kathleen Neuzil, director of the Center for Vaccine Development at the University of Maryland, Baltimore.
In this interview, Dr. Neuzil discusses which strains of influenza are expected to be dominant in the coming flu season, whether to expect a strain mutation similar to what happened last season, the importance of getting children vaccinated, and pneumococcal vaccination for children and older adults.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT A CDC/NFID PRESS CONFERENCE
Using an Electronic Health Record-Based Registry to Improve Pediatric Sickle Cell Care
From the Department of Pediatrics, Boston University School of Medicine, Boston Medical Center, Boston, MA.
This article is the second in our Hemoglobinopathy Learning Collaborative series. See the related editorial by Oyeku et al in the February 2014 issue of JCOM. (—Ed.)
Abstract
- Objective: To describe the development and use of an electronic health record (EHR)–based sickle cell disease (SCD) registry for children with SCD to enhance case management and quality improvement (QI) efforts at an urban, academic, safety net institution.
- Methods: Using national guidelines and the literature, we created quality metrics for pediatric SCD that focused on vaccination delivery and use of transcranial Doppler screening and hydroxyurea. We revised EHR forms for SCD care and created an EHR-based SCD registry that permitted monthly and annual reporting on quality metrics.
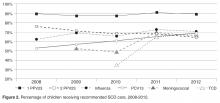
- Results: From 2008 to 2012, the percentage of children with SCD vaccinated for influenza increased from 52% to 65%, and for meningococcus from 53% to 70%. After licensure of PCV13 in 2010, the percentage of children vaccinated rose to 69% in 2012. Results for PPV23 were mixed: 87% to 91% received ≥1 dose, but the rate for receiving the second dose declined from 76% to 64%. Percentage of children screened annually with transcranial Doppler consistently ranged from 62% to 73% during the 5 years. QI initiatives in 2012–2013 led to increased influenza vaccination, from 65% to 83%, and increased hydroxyurea use, from 52% to 73%.
- Conclusion: In this study, a practical, replicable and feasible approach for improving the quality of SCD care combined the collaboration of a multidisciplinary team, an EHR-based disease registry, and QI initiatives. Additional work is needed to define and measure all elements of high-quality care for children with SCD and link process measures to clinical outcomes.
Sickle cell disease (SCD) is the most commonly inherited disorder in the United States, affecting approximately 100,000 individuals and 1 in 400 African American births [1,2]. The use of preventive strategies, such as immunizations [3], transcranial Doppler screening and transfusion protocols [4,5], and hydroxyurea therapy [6,7] has contributed to decreased morbidity and mortality among children with SCD [8,9]. However, a substantial gap exists between the care that children with SCD should receive and the care they actually receive [10–12]. An essential component of any effort that seeks to improve care is the ability to measure care processes and outcomes in a way that can drive quality improvement (QI) initiatives. Registries serve a vital role in quality improvement activities for many pediatric conditions, including inflammatory bowel disease [13] and cystic fibrosis [14]. However, there are no national or nationally representative registries currently available for children with SCD [15]. There is a pressing need for better information systems and tools that can be used in mainstream clinical settings to measure clinical performance with respect to quality indicators [16] if the goals of high quality care and better quality of life are to be achieved for children with SCD.
Electronic health records (EHRs) have been successfully used to improve the quality of care and enhance performance measurement in select institutions [17,18], and adoption of EHRs is growing. The 2009 American Recovery and Reinvestment Act allocated $20.8 billion in incentives to assist providers to adopt and “meaningfully use” EHRs [19,20]. As of 2011, 39% of office-based providers have implemented at least a basic EHR [21], up from 17% in 2008 [22]. The effective use of EHRs depends on collaboration between technical and medical experts so that functionality is achieved and clinical quality is appropriately measured. In addition, few EHRs contain specialized content for the care of persons with SCD.
While independent registries have been shown to be effective in improving care [13,14,23], they involve extra time and effort for data entry, can be difficult and expensive to maintain, and may not be feasible for many systems that care for SCD patients. In this paper, we describe the development and use of our EHR-based SCD registry for children with SCD, including our efforts to engage key technical and clinical experts to develop an EHR that is tailored to the outpatient workflow and data collection of quality measures and implement a fully functional system that collects data on quality measures to support case management and continuous QI.
Methods
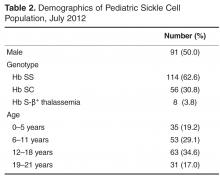
This study was conducted at Boston Medical Center, New England’s largest safety net hospital, which cares for 190 children with SCD ages 0 to 21 years. The outpatient EHR (Centricity, GE) has been in use since 2000 and is used for all aspects of outpatient care, including ordering of immunizations and tests, electronic prescription writing, and referrals to specialty care.
Outcome Measures
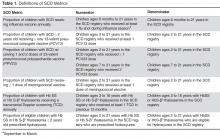
Vaccines: The Centers for Disease Control and Prevention (CDC) recommends vaccinating children with SCD [26] against influenza annually, given their susceptibility to the influenza virus [24,27]. The CDC also recommends the 23-valent pneumococcal polysaccharide vaccine (PPV23 2-dose series) and 13-valent pneumococcal conjugate vaccine (PCV13, per childhood routine vaccine schedule for young children and 1 catch-up dose for children previously vaccinated with PCV7), and meningococcal vaccine (2-dose series), given patients’ functional asplenic status [25,28].
Transcranial Doppler screening can identify children with hemoglobin (Hb) SS and Hb S-β0 thalassemia at higher risk of stroke, which may be prevented through hypertransfusion programs [4]. Screening is recommended annually for these children ages 2 to 16 years [25].
Hydroxyurea use among children with Hb SS and Hb S-β0 thalassemia is an established practice [29,30]. We consider hydroxyurea therapy for all children 2 years and older with Hb SS and Hb S-β0 thalassemia, given the recently published safety data from the Baby-HUG trial [7] and the benefits of hydroxyurea among children and adults with SCD [6,31–35].
EHR-based Registry
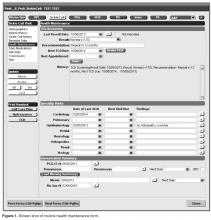
Our EHR-based SCD registry includes 3 key components: (1) forms to support detailed documentation at the point-of-care (ie, clinic visit); (2) a registry management form to allow the QI team to identify patients to be included or excluded from the registry; and (3) a central data warehouse to support quality measurement and improvement.
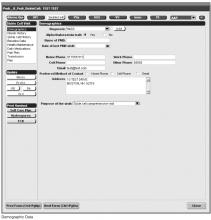
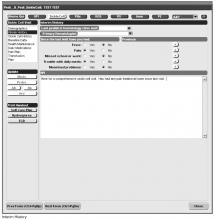
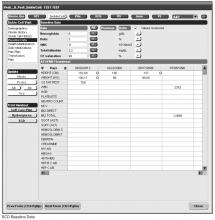
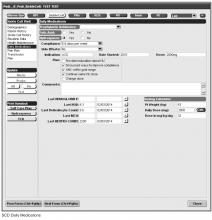
Documentation in the EHR is performed using a set of customized templates or “forms.” These forms allow documentation of care provision in a structured way. The discrete data elements are stored within the data warehousing system that supports the EHR. The SCD forms used in this project were a revised version of existing forms used by our pediatric hematologists for the past 6 years. The primary goal was to improve efficiency in a patient encounter and enhance data collection efforts. In particular, several changes were made to enhance data collection for quality measures included in the SCD registry. First, we collected genotype in a standardized way to better define subpopulations of SCD patients, as some of the care provided is dictated by genotype. We also expanded data capture for transcranial Doppler screening to include date of last screening to prompt scheduling. For hydroxyurea, the forms now capture if hydroxyurea has been prescribed, and if not, why (eg, declined, not indicated); adherence, current dose, and routine labs for monitoring are also listed to aid in clinical decision-making. Finally, the forms were revised to prominently display the subset of immunizations important to SCD (described above) to assess if the patient is current.
Within the new forms, we collected all data elements important to providing care to children with SCD. Several new items existed in other parts of the EHR and were automatically pulled into the forms, including laboratory results, medications and immunizations. Other new data elements required manual entry by providers based on EHR review, as they had previously not been documented, documented on an ad hoc basis, or found as free text within notes (eg, number of ED visits and hospitalizations in the past year). Initial completion of these forms took approximately 10 to 15 minutes per patient, as many of these data elements were not individually captured prior to this work; documentation for subsequent comprehensive visits required an additional 5 to 10 minutes per chart. Currently, the 3 pediatric hematologists regularly use the SCD forms for routine visits.
The registry management form was also created by the EHR design team. Although this form is separate from the SCD forms, it was readily accessible to the clinical team to quickly check whether patients should be included or excluded from the SCD registry. In this way, inactive patients could be removed and new patients could be included. This form was completed for all active pediatric patients with SCD as of February 2013 using data from a separately maintained clinical database. For patients who were new to the pediatric hematology practice between July 2012 and February 2013 (eg, infants born during this period, patients transferring care), we manually determined a registry start date in order to calculate accurate denominators for each measure. New patients were entered into the SCD registry by members of the care team on an ad hoc basis, and biannual searches of problem lists were planned to ensure the pediatric SCD registry was complete using the SCD-related ICD-9 codes 282.6, 282.41 and 282.4 to encompass all sickle hemoglobinopathies, including sickle cell thalassemia.
For this project, we were fortunate to have a well-established clinical data warehouse into which the medical center’s EHR data is copied nightly. In addition, the medical center already had multiple chronic disease registries and a framework for evaluating and sharing QI data. We were able to add SCD to this existing infrastructure, which was helpful since a secure and HIPAA-compliant location to post these patient-level reports had been previously identified.
We paid for 40 hours of technical staff time using grant funds to create reports using data collected in the EHR for patients who were actively in the SCD registry per the registry management form. Using these data, summary reports for our key SCD metrics were generated on both an annual and monthly basis. We tested and refined our key SCD metrics over a 4-month period to ensure that we had defined the numerators and denominators for each care process accurately. For example, children become eligible for influenza vaccine at 6 months of age, therefore, the eligible denominator would exclude infants < 6 months of age (Table 1). In addition, lists of patient names and phone numbers were automatically generated to identify those in need of care elements, facilitating both case management and continuous improvement for these measures, replacing the need for all external clinical databases.
Data Analysis
For children included in the SCD registry, we calculated the proportion who were appropriately vaccinated and received transcranial Doppler screening each year for the 5-year period 2008–2012. For the period July 2012–June 2013, we calculated the proportion of children with SCD in the registry who received influenza vaccine and children with Hb SS and Hb S-β0 thalassemia who were prescribed hydroxyurea.
This study was approved by the Boston University Medical Campus institutional review board.
Results
For influenza vaccination for the 2012–2013 season, only 49% of children were vaccinated as of November. This proportion increased after outreach
From July 2012 to June 2013, our rates of hydroxyurea use increased from 52% to 73% among eligible patients.
Discussion
In this paper we report on a practical approach for improving the quality of care for persons with SCD that combines the collaboration of a multidisciplinary team, the use of the EHR to create a disease registry, and QI initiatives. We identified where high-quality care is provided and where further attention is needed, and enhanced our case management capabilities with the generation of patient lists identifying those who are in need of care elements. We also used our registry to track care provision, achieving rates of influenza vaccination of 82% and hydroxyurea use to 73% as of June 2013. From these results, we have shown that our EHR can be used for registry management activities and provide real-time clinical data on the care that is provided, and can lead to improved performance on process measures important in the care for children with SCD.
After adjusting to the revised workflow required by the new SCD forms, the pediatric hematology team found them to be useful in tracking important clinical measures. They reported that the most important change was that all routine elements of SCD care, such as dates of last visits to pediatric subspecialists and receipt of recommended routine SCD care, were embedded into their note. This eliminated the need to search previous documents to find dates of the last cardiology visit or influenza immunizations and increased the likelihood that gaps in care would be addressed by the provider during the course of a clinic visit, thereby streamlining clinic workflow.
Healthy People 2020 recommend vaccination rates of 80% and 90% for influenza and PCV13 vaccines, respectively, in the general pediatric population [36]. We have met this goal for the influenza vaccine, but have room to improve for other recommended vaccines for children with SCD. Ultimately, our goal is to provide these vaccines to 100% of children with SCD at our institution. One barrier to achieving high vaccination rates is the lack of provider knowledge on the creation of catch-up vaccine schedules. A study of primary care providers showed that they frequently omitted vaccines when creating catch-up schedules, including the pneumococcal conjugate vaccine for healthy children [37]. Another hurdle is coordination of care between primary and specialty care, as these vaccines could be given in either setting. A recently published study found that only 20% of children with SCD had care coordination between primary and specialty care [38]. Promoting shared responsibility and information on the administration of vaccinations for children with SCD between primary and subspecialty care, and the development of state-wide immunization registries, may help alleviate these challenges.
In this study, our rates of hydroxyurea use among children with Hb SS and Hb S-β0 thalassemia are higher than in other reported studies [12]. We promote hydroxyurea use in this population of children based on the recently published safety data in infants and young children with Hb SS and Hb S-β0 thalassemia [7,32,39] and the significant benefits seen in adults, including improved survival [6,34,35,40]. Future efforts will include tracking outcomes, including the rates of acute chest syndrome and pain episodes, among children who are and are not taking hydroxyurea.
In this study, we found approximately 70% of eligible children were screened with transcranial Doppler each year from 2008–2012, which is higher than the 45% annual screening rate reported in the literature [10]. One reason our transcranial Doppler screening rates may be higher is that a technician is available to perform these tests on certain days that coincide with the pediatric hematology clinic, allowing patients and families to get this test and have a clinic visit on the same day. However, choosing a 12-month period for receipt of transcranial Doppler screening may be too conservative for centers who do not have such ready access to screening; reporting receipt of transcranial Doppler screening within a 15-month time period may be more appropriate and achievable.
Our study has several limitations. First, it was conducted in a single center with well-established electronic data systems, which are not available in many centers. Our hope is that this model can be replicated by others who seek to use EHR to improve the care of persons with SCD. Second, this work was performed in Massachusetts, a state with near-universal health care insurance coverage. As the Affordable Care Act is implemented nationally [41], other states may see improved performance on quality metrics as more people obtain health insurance. Third, although the EHR was designed to improve data capture for clinical care and quality initiatives, advanced clinical decision support systems were not incorporated due to the limitations of the EHR. The use of prompts for needed clinical care may further enhance performance on these measures. Fourth, this study is limited to children with SCD, who are traditionally monitored more closely than their adult counterparts. Efforts are currently underway to replicate these efforts with adults with SCD at our institution. Finally, the quality metrics in this study are process measures in the delivery of high quality SCD care. Future efforts will focus on linking outcomes to these measures, such as hydroxyurea use to reduce the frequency of acute chest syndrome and painful episodes.
Effective use of health information technology has proven challenging [42,43]. Although there are data that suggest that information technology has improved quality of care by increasing adherence to guidelines, enhancing disease surveillance, and decreasing medication errors, most of the high-quality literature to date comes from 4 research institutions [18]. We found that health IT can be effectively harnessed when end-users are engaged in the process of EHR design, there is a strong commitment to improve workflow and support documentation needs of end-users, the design of the EHR supports data collection for quality measures, and most importantly, there is close collaboration among those with overlapping technical, clinical, and health services research expertise.
There have been many calls for the creation of rare disease registries, as 6% to 8% of the population will develop one in their lifetime [44]. In 2010, the NIH’s Office of Rare Diseases Research funded 30 organizations with and without patient registries, and charged them with the creation of a common data collection template for rare diseases to be used internationally [45]. Common data collection elements for SCD, such as those used in our program, could be used in EHRs across US centers in an effort to improve the quality of care for these children. Although this work may be challenging for centers using large enterprise EHR systems, given the costs associated with modifications, once developed the content can often be shared easily with others using the same system. This would provide the opportunity to compare uniform data across institutions and facilitate learning nationally on ways to improve care. In addition, these efforts may serve as the beginnings of a national registry for pediatric SCD.
In conclusion, contemporary SCD care can lead to improved survival and quality of life, but only if the right care is delivered at the right time. In this study, we present our initial findings from the implementation of a population-based information system for children with SCD. Future efforts are needed to define and measure all elements of high quality care, and link improvements in the delivery of high quality care to outcomes for children and adults with SCD longitudinally.
Appendix. Additional Sickle Cell Disease Forms
Acknowledgments: We would like to thank David Botts for his tireless efforts in creating the sickle cell forms within our EHR. We would also like to thank Barry Zuckerman for his support of this project.
Corresponding author: Patricia Kavanagh, MD, Boston University School of Medicine/Boston Medical Center, 88 E Newton St, Vose Hall 3rd Fl, Boston, MA 02118.
Funding/support: This work was supported by the Health Resources and Services Administration Sickle Cell Disease and Newborn Screening Program, grant #U38MC22215. The authors have also actively participated in the Hemoglobinopathy Learning Collaborative, a quality improvement forum coordinated by HRSA and the National Initiative for Children’s Healthcare Quality.
Financial disclosures: None.
1. Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Preventive Med 2010;38(4 Suppl):S512–S521.
2. Steinberg MH. Management of sickle cell disease. N Engl J Med 1999;340:1021–30.
3. Adamkiewicz TV, Silk BJ, Howgate J, et al. Effectiveness of the 7-valent pneumococcal conjugate vaccine in children with sickle cell disease in the first decade of life. Pediatrics 2008;121:562–9.
4. Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial doppler ultrasonography. N Engl J Med 1998;339:5–1.
5. Adams RJ, Brambilla D, Optimizing Primary Stroke Prevention in Sickle Cell Anemia Trial I. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease.[see comment]. N Engl J Med 2005;353:2769–78.
6. Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med 1995;332:1317–22.
7. Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: A multicentre, randomised, controlled trial (baby hug). Lancet 2011;377:1663–72.
8. Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood 2010;115:3447–52.
9. Hamideh D, Alvarez O. Sickle cell disease related mortality in the united states (1999–2009). Pediatr Blood Cancer 2013;60:1482–6.
10. Raphael JL, Shetty PB, Liu H, et al. A critical assessment of transcranial doppler screening rates in a large pediatric sickle cell center: Opportunities to improve healthcare quality. Pediatr Blood Cancer 2008;51:647–51.
11. Sox CM, Cooper WO, Koepsell TD, et al. Provision of pneumococcal prophylaxis for publicly insured children with sickle cell disease. JAMA 2003;290:1057–61.
12. Oyeku SO, Driscoll MC, Cohen HW, et al. Parental and other factors associated with hydroxyurea use for pediatric sickle cell disease. Pediatr Blood Cancer 2013;60:653–58.
13. Crandall WV, Margolis PA, Kappelman MD, et al. Improved outcomes in a quality improvement collaborative for pediatric inflammatory bowel disease. Pediatrics 2012;129:e1030–e1041.
14. Schechter MS, Margolis P. Improving subspecialty healthcare: Lessons from cystic fibrosis. J Pediatr 2005;147:295–301.
15. Smith LA, Oyeku SO, Homer C, Zuckerman B. Sickle cell disease: A question of equity and quality. Pediatrics 2006;117:1763–70.
16. Wang CJ, Kavanagh PL, Little AA, et al. Quality-of-care indicators for children with sickle cell disease. Pediatrics 2011;128:484–93.
17. Jha AK, Perlin JB, Kizer KW, Dudley RA. Effect of the transformation of the veterans affairs health care system on the quality of care. N Engl J Med 2003;348:2218–27.
18. Chaudhry B, Wang J, Wu S, et al. Systematic review: Impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med 2006;144:742–52.
19. American recovery and reinvestment act of 2009. Obey D, Frank B, Gordon B, et al., trans. 111th Congress of the United States.
20. Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med 2010;363:501–4.
21. Electronic health record adoption by office-based providers. Office of National Coordinator for Health Information Technology. U.S. Department of Health and Human Services. Accessed 15 Jul 2013.
22. DesRoches CM, Campbell EG, Rao SR, et al. Electronic health records in ambulatory care — a national survey of physicians. N Engl J Med 2008;359:50–60.
23. Tricco AC, Ivers NM, Grimshaw JM, et al. Effectiveness of quality improvement strategies on the management of diabetes: A systematic review and meta-analysis. Lancet 379:2252–61.
24. Bundy DG, Strouse JJ, Casella JF, Miller MR. Burden of influenza-related hospitalizations among children with sickle cell disease. Pediatrics 2010;125:234–43.
25. National Heart Lung and Blood Institute. The management of sickle cell disease. NIH Pub No. 02-2117. Bethesda, MD: National Institutes of Health; 2002.
26. Centers for Disease Control and Prevention. Immunization schedules. Accessed 5 Jan 2013 at www.cdc.gov/vaccines/schedules/index.html.
27. Strouse JJ, Reller ME, Bundy DG, et al. Severe pandemic h1n1 and seasonal influenza in children and young adults with sickle cell disease. Blood 2010;116:3431–4.
28. Pilishvili T, Zell ER, Farley MM, et al. Risk factors for invasive pneumococcal disease in children in the era of conjugate vaccine use. Pediatrics 2010;126:e9–17.
29. Heeney MM, Ware RE. Hydroxyurea for children with sickle cell disease. Pediatr Clin North Am 008;55:483–501.
30. Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood 2010;115:5300–11.
31. Ferster A, Vermylen C, Cornu G, et al. Hydroxyurea for treatment of severe sickle cell anemia: a pediatric clinical trial. Blood 1996;88:1960–4.
32. Strouse JJ, Lanzkron S, Beach MC, et al. Hydroxyurea for sickle cell disease: a systematic review for efficacy and toxicity in children. Pediatrics 2008;122:1332–42.
33. Hankins JS, Ware RE, Rogers ZR, et al. Long-term hydroxyurea therapy for infants with sickle cell anemia: the husoft extension study. Blood 2005;106:2269–75.
34. Steinberg MH, McCarthy WF, Castro O, et al. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: a 17.5-year follow-up. Am J Hematol 2010;85:403–8.
35. Voskaridou E, Christoulas D, Bilalis A, et al. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: results of a 17-year, single-center trial (lashs). Blood 2010;115:2354–63.
36. Healthy people 2020. Immunization and infectious diseases. Accessed 3 Jun 2013 at www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicid=23.
37. Cohen NJ, Lauderdale DS, Shete PB, et al. Physician knowledge of catch-up regimens and contraindications for childhood immunizations. Pediatrics 2003;111:925–32.
38. Raphael JL, Rattler TL, Kowalkowski MA, et al. The medical home experience among children with sickle cell disease. Pediatr Blood Cancer 2013;60:275–80.
39. Strouse JJ, Heeney MM. Hydroxyurea for the treatment of sickle cell disease: efficacy, barriers, toxicity, and management in children. Pediatr Blood Cancer 2012;59:365–71.
40. Steinberg MH, Barton F, Castro O, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA 2003;289:1645–51.
41. Patient protection and affordable care act, US Pub. L. No. 111-148, §2702, 124 stat. 119, 318-319. 2010.
42. Harrison M, Koppel R, Bar-Lev S. Unintended consequences of information technologies in health care: an interactive sociotechnical analysis. J Am Med Inform Assoc 2007;14:542–9.
43. Haux R. Health information systems – past, present, future. Int J Med Informatics 2006;75:268–81.
44. Schieppati A, Henter J-I, Daina E, Aperia A. Why rare diseases are an important medical and social issue. Lancet 2008;371:2039–41.
45. Office of Rare Diseases Research National Institutes of Health. Rare diseases and related terms. Accessed 28 Jun 2013 at www.rarediseases.info.nih.gov/rarediseaselist.aspx.
From the Department of Pediatrics, Boston University School of Medicine, Boston Medical Center, Boston, MA.
This article is the second in our Hemoglobinopathy Learning Collaborative series. See the related editorial by Oyeku et al in the February 2014 issue of JCOM. (—Ed.)
Abstract
- Objective: To describe the development and use of an electronic health record (EHR)–based sickle cell disease (SCD) registry for children with SCD to enhance case management and quality improvement (QI) efforts at an urban, academic, safety net institution.
- Methods: Using national guidelines and the literature, we created quality metrics for pediatric SCD that focused on vaccination delivery and use of transcranial Doppler screening and hydroxyurea. We revised EHR forms for SCD care and created an EHR-based SCD registry that permitted monthly and annual reporting on quality metrics.
- Results: From 2008 to 2012, the percentage of children with SCD vaccinated for influenza increased from 52% to 65%, and for meningococcus from 53% to 70%. After licensure of PCV13 in 2010, the percentage of children vaccinated rose to 69% in 2012. Results for PPV23 were mixed: 87% to 91% received ≥1 dose, but the rate for receiving the second dose declined from 76% to 64%. Percentage of children screened annually with transcranial Doppler consistently ranged from 62% to 73% during the 5 years. QI initiatives in 2012–2013 led to increased influenza vaccination, from 65% to 83%, and increased hydroxyurea use, from 52% to 73%.
- Conclusion: In this study, a practical, replicable and feasible approach for improving the quality of SCD care combined the collaboration of a multidisciplinary team, an EHR-based disease registry, and QI initiatives. Additional work is needed to define and measure all elements of high-quality care for children with SCD and link process measures to clinical outcomes.
Sickle cell disease (SCD) is the most commonly inherited disorder in the United States, affecting approximately 100,000 individuals and 1 in 400 African American births [1,2]. The use of preventive strategies, such as immunizations [3], transcranial Doppler screening and transfusion protocols [4,5], and hydroxyurea therapy [6,7] has contributed to decreased morbidity and mortality among children with SCD [8,9]. However, a substantial gap exists between the care that children with SCD should receive and the care they actually receive [10–12]. An essential component of any effort that seeks to improve care is the ability to measure care processes and outcomes in a way that can drive quality improvement (QI) initiatives. Registries serve a vital role in quality improvement activities for many pediatric conditions, including inflammatory bowel disease [13] and cystic fibrosis [14]. However, there are no national or nationally representative registries currently available for children with SCD [15]. There is a pressing need for better information systems and tools that can be used in mainstream clinical settings to measure clinical performance with respect to quality indicators [16] if the goals of high quality care and better quality of life are to be achieved for children with SCD.
Electronic health records (EHRs) have been successfully used to improve the quality of care and enhance performance measurement in select institutions [17,18], and adoption of EHRs is growing. The 2009 American Recovery and Reinvestment Act allocated $20.8 billion in incentives to assist providers to adopt and “meaningfully use” EHRs [19,20]. As of 2011, 39% of office-based providers have implemented at least a basic EHR [21], up from 17% in 2008 [22]. The effective use of EHRs depends on collaboration between technical and medical experts so that functionality is achieved and clinical quality is appropriately measured. In addition, few EHRs contain specialized content for the care of persons with SCD.
While independent registries have been shown to be effective in improving care [13,14,23], they involve extra time and effort for data entry, can be difficult and expensive to maintain, and may not be feasible for many systems that care for SCD patients. In this paper, we describe the development and use of our EHR-based SCD registry for children with SCD, including our efforts to engage key technical and clinical experts to develop an EHR that is tailored to the outpatient workflow and data collection of quality measures and implement a fully functional system that collects data on quality measures to support case management and continuous QI.
Methods
This study was conducted at Boston Medical Center, New England’s largest safety net hospital, which cares for 190 children with SCD ages 0 to 21 years. The outpatient EHR (Centricity, GE) has been in use since 2000 and is used for all aspects of outpatient care, including ordering of immunizations and tests, electronic prescription writing, and referrals to specialty care.
Outcome Measures
Vaccines: The Centers for Disease Control and Prevention (CDC) recommends vaccinating children with SCD [26] against influenza annually, given their susceptibility to the influenza virus [24,27]. The CDC also recommends the 23-valent pneumococcal polysaccharide vaccine (PPV23 2-dose series) and 13-valent pneumococcal conjugate vaccine (PCV13, per childhood routine vaccine schedule for young children and 1 catch-up dose for children previously vaccinated with PCV7), and meningococcal vaccine (2-dose series), given patients’ functional asplenic status [25,28].
Transcranial Doppler screening can identify children with hemoglobin (Hb) SS and Hb S-β0 thalassemia at higher risk of stroke, which may be prevented through hypertransfusion programs [4]. Screening is recommended annually for these children ages 2 to 16 years [25].
Hydroxyurea use among children with Hb SS and Hb S-β0 thalassemia is an established practice [29,30]. We consider hydroxyurea therapy for all children 2 years and older with Hb SS and Hb S-β0 thalassemia, given the recently published safety data from the Baby-HUG trial [7] and the benefits of hydroxyurea among children and adults with SCD [6,31–35].
EHR-based Registry
Our EHR-based SCD registry includes 3 key components: (1) forms to support detailed documentation at the point-of-care (ie, clinic visit); (2) a registry management form to allow the QI team to identify patients to be included or excluded from the registry; and (3) a central data warehouse to support quality measurement and improvement.
Documentation in the EHR is performed using a set of customized templates or “forms.” These forms allow documentation of care provision in a structured way. The discrete data elements are stored within the data warehousing system that supports the EHR. The SCD forms used in this project were a revised version of existing forms used by our pediatric hematologists for the past 6 years. The primary goal was to improve efficiency in a patient encounter and enhance data collection efforts. In particular, several changes were made to enhance data collection for quality measures included in the SCD registry. First, we collected genotype in a standardized way to better define subpopulations of SCD patients, as some of the care provided is dictated by genotype. We also expanded data capture for transcranial Doppler screening to include date of last screening to prompt scheduling. For hydroxyurea, the forms now capture if hydroxyurea has been prescribed, and if not, why (eg, declined, not indicated); adherence, current dose, and routine labs for monitoring are also listed to aid in clinical decision-making. Finally, the forms were revised to prominently display the subset of immunizations important to SCD (described above) to assess if the patient is current.
Within the new forms, we collected all data elements important to providing care to children with SCD. Several new items existed in other parts of the EHR and were automatically pulled into the forms, including laboratory results, medications and immunizations. Other new data elements required manual entry by providers based on EHR review, as they had previously not been documented, documented on an ad hoc basis, or found as free text within notes (eg, number of ED visits and hospitalizations in the past year). Initial completion of these forms took approximately 10 to 15 minutes per patient, as many of these data elements were not individually captured prior to this work; documentation for subsequent comprehensive visits required an additional 5 to 10 minutes per chart. Currently, the 3 pediatric hematologists regularly use the SCD forms for routine visits.
The registry management form was also created by the EHR design team. Although this form is separate from the SCD forms, it was readily accessible to the clinical team to quickly check whether patients should be included or excluded from the SCD registry. In this way, inactive patients could be removed and new patients could be included. This form was completed for all active pediatric patients with SCD as of February 2013 using data from a separately maintained clinical database. For patients who were new to the pediatric hematology practice between July 2012 and February 2013 (eg, infants born during this period, patients transferring care), we manually determined a registry start date in order to calculate accurate denominators for each measure. New patients were entered into the SCD registry by members of the care team on an ad hoc basis, and biannual searches of problem lists were planned to ensure the pediatric SCD registry was complete using the SCD-related ICD-9 codes 282.6, 282.41 and 282.4 to encompass all sickle hemoglobinopathies, including sickle cell thalassemia.
For this project, we were fortunate to have a well-established clinical data warehouse into which the medical center’s EHR data is copied nightly. In addition, the medical center already had multiple chronic disease registries and a framework for evaluating and sharing QI data. We were able to add SCD to this existing infrastructure, which was helpful since a secure and HIPAA-compliant location to post these patient-level reports had been previously identified.
We paid for 40 hours of technical staff time using grant funds to create reports using data collected in the EHR for patients who were actively in the SCD registry per the registry management form. Using these data, summary reports for our key SCD metrics were generated on both an annual and monthly basis. We tested and refined our key SCD metrics over a 4-month period to ensure that we had defined the numerators and denominators for each care process accurately. For example, children become eligible for influenza vaccine at 6 months of age, therefore, the eligible denominator would exclude infants < 6 months of age (Table 1). In addition, lists of patient names and phone numbers were automatically generated to identify those in need of care elements, facilitating both case management and continuous improvement for these measures, replacing the need for all external clinical databases.
Data Analysis
For children included in the SCD registry, we calculated the proportion who were appropriately vaccinated and received transcranial Doppler screening each year for the 5-year period 2008–2012. For the period July 2012–June 2013, we calculated the proportion of children with SCD in the registry who received influenza vaccine and children with Hb SS and Hb S-β0 thalassemia who were prescribed hydroxyurea.
This study was approved by the Boston University Medical Campus institutional review board.
Results
For influenza vaccination for the 2012–2013 season, only 49% of children were vaccinated as of November. This proportion increased after outreach
From July 2012 to June 2013, our rates of hydroxyurea use increased from 52% to 73% among eligible patients.
Discussion
In this paper we report on a practical approach for improving the quality of care for persons with SCD that combines the collaboration of a multidisciplinary team, the use of the EHR to create a disease registry, and QI initiatives. We identified where high-quality care is provided and where further attention is needed, and enhanced our case management capabilities with the generation of patient lists identifying those who are in need of care elements. We also used our registry to track care provision, achieving rates of influenza vaccination of 82% and hydroxyurea use to 73% as of June 2013. From these results, we have shown that our EHR can be used for registry management activities and provide real-time clinical data on the care that is provided, and can lead to improved performance on process measures important in the care for children with SCD.
After adjusting to the revised workflow required by the new SCD forms, the pediatric hematology team found them to be useful in tracking important clinical measures. They reported that the most important change was that all routine elements of SCD care, such as dates of last visits to pediatric subspecialists and receipt of recommended routine SCD care, were embedded into their note. This eliminated the need to search previous documents to find dates of the last cardiology visit or influenza immunizations and increased the likelihood that gaps in care would be addressed by the provider during the course of a clinic visit, thereby streamlining clinic workflow.
Healthy People 2020 recommend vaccination rates of 80% and 90% for influenza and PCV13 vaccines, respectively, in the general pediatric population [36]. We have met this goal for the influenza vaccine, but have room to improve for other recommended vaccines for children with SCD. Ultimately, our goal is to provide these vaccines to 100% of children with SCD at our institution. One barrier to achieving high vaccination rates is the lack of provider knowledge on the creation of catch-up vaccine schedules. A study of primary care providers showed that they frequently omitted vaccines when creating catch-up schedules, including the pneumococcal conjugate vaccine for healthy children [37]. Another hurdle is coordination of care between primary and specialty care, as these vaccines could be given in either setting. A recently published study found that only 20% of children with SCD had care coordination between primary and specialty care [38]. Promoting shared responsibility and information on the administration of vaccinations for children with SCD between primary and subspecialty care, and the development of state-wide immunization registries, may help alleviate these challenges.
In this study, our rates of hydroxyurea use among children with Hb SS and Hb S-β0 thalassemia are higher than in other reported studies [12]. We promote hydroxyurea use in this population of children based on the recently published safety data in infants and young children with Hb SS and Hb S-β0 thalassemia [7,32,39] and the significant benefits seen in adults, including improved survival [6,34,35,40]. Future efforts will include tracking outcomes, including the rates of acute chest syndrome and pain episodes, among children who are and are not taking hydroxyurea.
In this study, we found approximately 70% of eligible children were screened with transcranial Doppler each year from 2008–2012, which is higher than the 45% annual screening rate reported in the literature [10]. One reason our transcranial Doppler screening rates may be higher is that a technician is available to perform these tests on certain days that coincide with the pediatric hematology clinic, allowing patients and families to get this test and have a clinic visit on the same day. However, choosing a 12-month period for receipt of transcranial Doppler screening may be too conservative for centers who do not have such ready access to screening; reporting receipt of transcranial Doppler screening within a 15-month time period may be more appropriate and achievable.
Our study has several limitations. First, it was conducted in a single center with well-established electronic data systems, which are not available in many centers. Our hope is that this model can be replicated by others who seek to use EHR to improve the care of persons with SCD. Second, this work was performed in Massachusetts, a state with near-universal health care insurance coverage. As the Affordable Care Act is implemented nationally [41], other states may see improved performance on quality metrics as more people obtain health insurance. Third, although the EHR was designed to improve data capture for clinical care and quality initiatives, advanced clinical decision support systems were not incorporated due to the limitations of the EHR. The use of prompts for needed clinical care may further enhance performance on these measures. Fourth, this study is limited to children with SCD, who are traditionally monitored more closely than their adult counterparts. Efforts are currently underway to replicate these efforts with adults with SCD at our institution. Finally, the quality metrics in this study are process measures in the delivery of high quality SCD care. Future efforts will focus on linking outcomes to these measures, such as hydroxyurea use to reduce the frequency of acute chest syndrome and painful episodes.
Effective use of health information technology has proven challenging [42,43]. Although there are data that suggest that information technology has improved quality of care by increasing adherence to guidelines, enhancing disease surveillance, and decreasing medication errors, most of the high-quality literature to date comes from 4 research institutions [18]. We found that health IT can be effectively harnessed when end-users are engaged in the process of EHR design, there is a strong commitment to improve workflow and support documentation needs of end-users, the design of the EHR supports data collection for quality measures, and most importantly, there is close collaboration among those with overlapping technical, clinical, and health services research expertise.
There have been many calls for the creation of rare disease registries, as 6% to 8% of the population will develop one in their lifetime [44]. In 2010, the NIH’s Office of Rare Diseases Research funded 30 organizations with and without patient registries, and charged them with the creation of a common data collection template for rare diseases to be used internationally [45]. Common data collection elements for SCD, such as those used in our program, could be used in EHRs across US centers in an effort to improve the quality of care for these children. Although this work may be challenging for centers using large enterprise EHR systems, given the costs associated with modifications, once developed the content can often be shared easily with others using the same system. This would provide the opportunity to compare uniform data across institutions and facilitate learning nationally on ways to improve care. In addition, these efforts may serve as the beginnings of a national registry for pediatric SCD.
In conclusion, contemporary SCD care can lead to improved survival and quality of life, but only if the right care is delivered at the right time. In this study, we present our initial findings from the implementation of a population-based information system for children with SCD. Future efforts are needed to define and measure all elements of high quality care, and link improvements in the delivery of high quality care to outcomes for children and adults with SCD longitudinally.
Appendix. Additional Sickle Cell Disease Forms
Acknowledgments: We would like to thank David Botts for his tireless efforts in creating the sickle cell forms within our EHR. We would also like to thank Barry Zuckerman for his support of this project.
Corresponding author: Patricia Kavanagh, MD, Boston University School of Medicine/Boston Medical Center, 88 E Newton St, Vose Hall 3rd Fl, Boston, MA 02118.
Funding/support: This work was supported by the Health Resources and Services Administration Sickle Cell Disease and Newborn Screening Program, grant #U38MC22215. The authors have also actively participated in the Hemoglobinopathy Learning Collaborative, a quality improvement forum coordinated by HRSA and the National Initiative for Children’s Healthcare Quality.
Financial disclosures: None.
From the Department of Pediatrics, Boston University School of Medicine, Boston Medical Center, Boston, MA.
This article is the second in our Hemoglobinopathy Learning Collaborative series. See the related editorial by Oyeku et al in the February 2014 issue of JCOM. (—Ed.)
Abstract
- Objective: To describe the development and use of an electronic health record (EHR)–based sickle cell disease (SCD) registry for children with SCD to enhance case management and quality improvement (QI) efforts at an urban, academic, safety net institution.
- Methods: Using national guidelines and the literature, we created quality metrics for pediatric SCD that focused on vaccination delivery and use of transcranial Doppler screening and hydroxyurea. We revised EHR forms for SCD care and created an EHR-based SCD registry that permitted monthly and annual reporting on quality metrics.
- Results: From 2008 to 2012, the percentage of children with SCD vaccinated for influenza increased from 52% to 65%, and for meningococcus from 53% to 70%. After licensure of PCV13 in 2010, the percentage of children vaccinated rose to 69% in 2012. Results for PPV23 were mixed: 87% to 91% received ≥1 dose, but the rate for receiving the second dose declined from 76% to 64%. Percentage of children screened annually with transcranial Doppler consistently ranged from 62% to 73% during the 5 years. QI initiatives in 2012–2013 led to increased influenza vaccination, from 65% to 83%, and increased hydroxyurea use, from 52% to 73%.
- Conclusion: In this study, a practical, replicable and feasible approach for improving the quality of SCD care combined the collaboration of a multidisciplinary team, an EHR-based disease registry, and QI initiatives. Additional work is needed to define and measure all elements of high-quality care for children with SCD and link process measures to clinical outcomes.
Sickle cell disease (SCD) is the most commonly inherited disorder in the United States, affecting approximately 100,000 individuals and 1 in 400 African American births [1,2]. The use of preventive strategies, such as immunizations [3], transcranial Doppler screening and transfusion protocols [4,5], and hydroxyurea therapy [6,7] has contributed to decreased morbidity and mortality among children with SCD [8,9]. However, a substantial gap exists between the care that children with SCD should receive and the care they actually receive [10–12]. An essential component of any effort that seeks to improve care is the ability to measure care processes and outcomes in a way that can drive quality improvement (QI) initiatives. Registries serve a vital role in quality improvement activities for many pediatric conditions, including inflammatory bowel disease [13] and cystic fibrosis [14]. However, there are no national or nationally representative registries currently available for children with SCD [15]. There is a pressing need for better information systems and tools that can be used in mainstream clinical settings to measure clinical performance with respect to quality indicators [16] if the goals of high quality care and better quality of life are to be achieved for children with SCD.
Electronic health records (EHRs) have been successfully used to improve the quality of care and enhance performance measurement in select institutions [17,18], and adoption of EHRs is growing. The 2009 American Recovery and Reinvestment Act allocated $20.8 billion in incentives to assist providers to adopt and “meaningfully use” EHRs [19,20]. As of 2011, 39% of office-based providers have implemented at least a basic EHR [21], up from 17% in 2008 [22]. The effective use of EHRs depends on collaboration between technical and medical experts so that functionality is achieved and clinical quality is appropriately measured. In addition, few EHRs contain specialized content for the care of persons with SCD.
While independent registries have been shown to be effective in improving care [13,14,23], they involve extra time and effort for data entry, can be difficult and expensive to maintain, and may not be feasible for many systems that care for SCD patients. In this paper, we describe the development and use of our EHR-based SCD registry for children with SCD, including our efforts to engage key technical and clinical experts to develop an EHR that is tailored to the outpatient workflow and data collection of quality measures and implement a fully functional system that collects data on quality measures to support case management and continuous QI.
Methods
This study was conducted at Boston Medical Center, New England’s largest safety net hospital, which cares for 190 children with SCD ages 0 to 21 years. The outpatient EHR (Centricity, GE) has been in use since 2000 and is used for all aspects of outpatient care, including ordering of immunizations and tests, electronic prescription writing, and referrals to specialty care.
Outcome Measures
Vaccines: The Centers for Disease Control and Prevention (CDC) recommends vaccinating children with SCD [26] against influenza annually, given their susceptibility to the influenza virus [24,27]. The CDC also recommends the 23-valent pneumococcal polysaccharide vaccine (PPV23 2-dose series) and 13-valent pneumococcal conjugate vaccine (PCV13, per childhood routine vaccine schedule for young children and 1 catch-up dose for children previously vaccinated with PCV7), and meningococcal vaccine (2-dose series), given patients’ functional asplenic status [25,28].
Transcranial Doppler screening can identify children with hemoglobin (Hb) SS and Hb S-β0 thalassemia at higher risk of stroke, which may be prevented through hypertransfusion programs [4]. Screening is recommended annually for these children ages 2 to 16 years [25].
Hydroxyurea use among children with Hb SS and Hb S-β0 thalassemia is an established practice [29,30]. We consider hydroxyurea therapy for all children 2 years and older with Hb SS and Hb S-β0 thalassemia, given the recently published safety data from the Baby-HUG trial [7] and the benefits of hydroxyurea among children and adults with SCD [6,31–35].
EHR-based Registry
Our EHR-based SCD registry includes 3 key components: (1) forms to support detailed documentation at the point-of-care (ie, clinic visit); (2) a registry management form to allow the QI team to identify patients to be included or excluded from the registry; and (3) a central data warehouse to support quality measurement and improvement.
Documentation in the EHR is performed using a set of customized templates or “forms.” These forms allow documentation of care provision in a structured way. The discrete data elements are stored within the data warehousing system that supports the EHR. The SCD forms used in this project were a revised version of existing forms used by our pediatric hematologists for the past 6 years. The primary goal was to improve efficiency in a patient encounter and enhance data collection efforts. In particular, several changes were made to enhance data collection for quality measures included in the SCD registry. First, we collected genotype in a standardized way to better define subpopulations of SCD patients, as some of the care provided is dictated by genotype. We also expanded data capture for transcranial Doppler screening to include date of last screening to prompt scheduling. For hydroxyurea, the forms now capture if hydroxyurea has been prescribed, and if not, why (eg, declined, not indicated); adherence, current dose, and routine labs for monitoring are also listed to aid in clinical decision-making. Finally, the forms were revised to prominently display the subset of immunizations important to SCD (described above) to assess if the patient is current.
Within the new forms, we collected all data elements important to providing care to children with SCD. Several new items existed in other parts of the EHR and were automatically pulled into the forms, including laboratory results, medications and immunizations. Other new data elements required manual entry by providers based on EHR review, as they had previously not been documented, documented on an ad hoc basis, or found as free text within notes (eg, number of ED visits and hospitalizations in the past year). Initial completion of these forms took approximately 10 to 15 minutes per patient, as many of these data elements were not individually captured prior to this work; documentation for subsequent comprehensive visits required an additional 5 to 10 minutes per chart. Currently, the 3 pediatric hematologists regularly use the SCD forms for routine visits.
The registry management form was also created by the EHR design team. Although this form is separate from the SCD forms, it was readily accessible to the clinical team to quickly check whether patients should be included or excluded from the SCD registry. In this way, inactive patients could be removed and new patients could be included. This form was completed for all active pediatric patients with SCD as of February 2013 using data from a separately maintained clinical database. For patients who were new to the pediatric hematology practice between July 2012 and February 2013 (eg, infants born during this period, patients transferring care), we manually determined a registry start date in order to calculate accurate denominators for each measure. New patients were entered into the SCD registry by members of the care team on an ad hoc basis, and biannual searches of problem lists were planned to ensure the pediatric SCD registry was complete using the SCD-related ICD-9 codes 282.6, 282.41 and 282.4 to encompass all sickle hemoglobinopathies, including sickle cell thalassemia.
For this project, we were fortunate to have a well-established clinical data warehouse into which the medical center’s EHR data is copied nightly. In addition, the medical center already had multiple chronic disease registries and a framework for evaluating and sharing QI data. We were able to add SCD to this existing infrastructure, which was helpful since a secure and HIPAA-compliant location to post these patient-level reports had been previously identified.
We paid for 40 hours of technical staff time using grant funds to create reports using data collected in the EHR for patients who were actively in the SCD registry per the registry management form. Using these data, summary reports for our key SCD metrics were generated on both an annual and monthly basis. We tested and refined our key SCD metrics over a 4-month period to ensure that we had defined the numerators and denominators for each care process accurately. For example, children become eligible for influenza vaccine at 6 months of age, therefore, the eligible denominator would exclude infants < 6 months of age (Table 1). In addition, lists of patient names and phone numbers were automatically generated to identify those in need of care elements, facilitating both case management and continuous improvement for these measures, replacing the need for all external clinical databases.
Data Analysis
For children included in the SCD registry, we calculated the proportion who were appropriately vaccinated and received transcranial Doppler screening each year for the 5-year period 2008–2012. For the period July 2012–June 2013, we calculated the proportion of children with SCD in the registry who received influenza vaccine and children with Hb SS and Hb S-β0 thalassemia who were prescribed hydroxyurea.
This study was approved by the Boston University Medical Campus institutional review board.
Results
For influenza vaccination for the 2012–2013 season, only 49% of children were vaccinated as of November. This proportion increased after outreach
From July 2012 to June 2013, our rates of hydroxyurea use increased from 52% to 73% among eligible patients.
Discussion
In this paper we report on a practical approach for improving the quality of care for persons with SCD that combines the collaboration of a multidisciplinary team, the use of the EHR to create a disease registry, and QI initiatives. We identified where high-quality care is provided and where further attention is needed, and enhanced our case management capabilities with the generation of patient lists identifying those who are in need of care elements. We also used our registry to track care provision, achieving rates of influenza vaccination of 82% and hydroxyurea use to 73% as of June 2013. From these results, we have shown that our EHR can be used for registry management activities and provide real-time clinical data on the care that is provided, and can lead to improved performance on process measures important in the care for children with SCD.
After adjusting to the revised workflow required by the new SCD forms, the pediatric hematology team found them to be useful in tracking important clinical measures. They reported that the most important change was that all routine elements of SCD care, such as dates of last visits to pediatric subspecialists and receipt of recommended routine SCD care, were embedded into their note. This eliminated the need to search previous documents to find dates of the last cardiology visit or influenza immunizations and increased the likelihood that gaps in care would be addressed by the provider during the course of a clinic visit, thereby streamlining clinic workflow.
Healthy People 2020 recommend vaccination rates of 80% and 90% for influenza and PCV13 vaccines, respectively, in the general pediatric population [36]. We have met this goal for the influenza vaccine, but have room to improve for other recommended vaccines for children with SCD. Ultimately, our goal is to provide these vaccines to 100% of children with SCD at our institution. One barrier to achieving high vaccination rates is the lack of provider knowledge on the creation of catch-up vaccine schedules. A study of primary care providers showed that they frequently omitted vaccines when creating catch-up schedules, including the pneumococcal conjugate vaccine for healthy children [37]. Another hurdle is coordination of care between primary and specialty care, as these vaccines could be given in either setting. A recently published study found that only 20% of children with SCD had care coordination between primary and specialty care [38]. Promoting shared responsibility and information on the administration of vaccinations for children with SCD between primary and subspecialty care, and the development of state-wide immunization registries, may help alleviate these challenges.
In this study, our rates of hydroxyurea use among children with Hb SS and Hb S-β0 thalassemia are higher than in other reported studies [12]. We promote hydroxyurea use in this population of children based on the recently published safety data in infants and young children with Hb SS and Hb S-β0 thalassemia [7,32,39] and the significant benefits seen in adults, including improved survival [6,34,35,40]. Future efforts will include tracking outcomes, including the rates of acute chest syndrome and pain episodes, among children who are and are not taking hydroxyurea.
In this study, we found approximately 70% of eligible children were screened with transcranial Doppler each year from 2008–2012, which is higher than the 45% annual screening rate reported in the literature [10]. One reason our transcranial Doppler screening rates may be higher is that a technician is available to perform these tests on certain days that coincide with the pediatric hematology clinic, allowing patients and families to get this test and have a clinic visit on the same day. However, choosing a 12-month period for receipt of transcranial Doppler screening may be too conservative for centers who do not have such ready access to screening; reporting receipt of transcranial Doppler screening within a 15-month time period may be more appropriate and achievable.
Our study has several limitations. First, it was conducted in a single center with well-established electronic data systems, which are not available in many centers. Our hope is that this model can be replicated by others who seek to use EHR to improve the care of persons with SCD. Second, this work was performed in Massachusetts, a state with near-universal health care insurance coverage. As the Affordable Care Act is implemented nationally [41], other states may see improved performance on quality metrics as more people obtain health insurance. Third, although the EHR was designed to improve data capture for clinical care and quality initiatives, advanced clinical decision support systems were not incorporated due to the limitations of the EHR. The use of prompts for needed clinical care may further enhance performance on these measures. Fourth, this study is limited to children with SCD, who are traditionally monitored more closely than their adult counterparts. Efforts are currently underway to replicate these efforts with adults with SCD at our institution. Finally, the quality metrics in this study are process measures in the delivery of high quality SCD care. Future efforts will focus on linking outcomes to these measures, such as hydroxyurea use to reduce the frequency of acute chest syndrome and painful episodes.
Effective use of health information technology has proven challenging [42,43]. Although there are data that suggest that information technology has improved quality of care by increasing adherence to guidelines, enhancing disease surveillance, and decreasing medication errors, most of the high-quality literature to date comes from 4 research institutions [18]. We found that health IT can be effectively harnessed when end-users are engaged in the process of EHR design, there is a strong commitment to improve workflow and support documentation needs of end-users, the design of the EHR supports data collection for quality measures, and most importantly, there is close collaboration among those with overlapping technical, clinical, and health services research expertise.
There have been many calls for the creation of rare disease registries, as 6% to 8% of the population will develop one in their lifetime [44]. In 2010, the NIH’s Office of Rare Diseases Research funded 30 organizations with and without patient registries, and charged them with the creation of a common data collection template for rare diseases to be used internationally [45]. Common data collection elements for SCD, such as those used in our program, could be used in EHRs across US centers in an effort to improve the quality of care for these children. Although this work may be challenging for centers using large enterprise EHR systems, given the costs associated with modifications, once developed the content can often be shared easily with others using the same system. This would provide the opportunity to compare uniform data across institutions and facilitate learning nationally on ways to improve care. In addition, these efforts may serve as the beginnings of a national registry for pediatric SCD.
In conclusion, contemporary SCD care can lead to improved survival and quality of life, but only if the right care is delivered at the right time. In this study, we present our initial findings from the implementation of a population-based information system for children with SCD. Future efforts are needed to define and measure all elements of high quality care, and link improvements in the delivery of high quality care to outcomes for children and adults with SCD longitudinally.
Appendix. Additional Sickle Cell Disease Forms
Acknowledgments: We would like to thank David Botts for his tireless efforts in creating the sickle cell forms within our EHR. We would also like to thank Barry Zuckerman for his support of this project.
Corresponding author: Patricia Kavanagh, MD, Boston University School of Medicine/Boston Medical Center, 88 E Newton St, Vose Hall 3rd Fl, Boston, MA 02118.
Funding/support: This work was supported by the Health Resources and Services Administration Sickle Cell Disease and Newborn Screening Program, grant #U38MC22215. The authors have also actively participated in the Hemoglobinopathy Learning Collaborative, a quality improvement forum coordinated by HRSA and the National Initiative for Children’s Healthcare Quality.
Financial disclosures: None.
1. Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Preventive Med 2010;38(4 Suppl):S512–S521.
2. Steinberg MH. Management of sickle cell disease. N Engl J Med 1999;340:1021–30.
3. Adamkiewicz TV, Silk BJ, Howgate J, et al. Effectiveness of the 7-valent pneumococcal conjugate vaccine in children with sickle cell disease in the first decade of life. Pediatrics 2008;121:562–9.
4. Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial doppler ultrasonography. N Engl J Med 1998;339:5–1.
5. Adams RJ, Brambilla D, Optimizing Primary Stroke Prevention in Sickle Cell Anemia Trial I. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease.[see comment]. N Engl J Med 2005;353:2769–78.
6. Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med 1995;332:1317–22.
7. Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: A multicentre, randomised, controlled trial (baby hug). Lancet 2011;377:1663–72.
8. Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood 2010;115:3447–52.
9. Hamideh D, Alvarez O. Sickle cell disease related mortality in the united states (1999–2009). Pediatr Blood Cancer 2013;60:1482–6.
10. Raphael JL, Shetty PB, Liu H, et al. A critical assessment of transcranial doppler screening rates in a large pediatric sickle cell center: Opportunities to improve healthcare quality. Pediatr Blood Cancer 2008;51:647–51.
11. Sox CM, Cooper WO, Koepsell TD, et al. Provision of pneumococcal prophylaxis for publicly insured children with sickle cell disease. JAMA 2003;290:1057–61.
12. Oyeku SO, Driscoll MC, Cohen HW, et al. Parental and other factors associated with hydroxyurea use for pediatric sickle cell disease. Pediatr Blood Cancer 2013;60:653–58.
13. Crandall WV, Margolis PA, Kappelman MD, et al. Improved outcomes in a quality improvement collaborative for pediatric inflammatory bowel disease. Pediatrics 2012;129:e1030–e1041.
14. Schechter MS, Margolis P. Improving subspecialty healthcare: Lessons from cystic fibrosis. J Pediatr 2005;147:295–301.
15. Smith LA, Oyeku SO, Homer C, Zuckerman B. Sickle cell disease: A question of equity and quality. Pediatrics 2006;117:1763–70.
16. Wang CJ, Kavanagh PL, Little AA, et al. Quality-of-care indicators for children with sickle cell disease. Pediatrics 2011;128:484–93.
17. Jha AK, Perlin JB, Kizer KW, Dudley RA. Effect of the transformation of the veterans affairs health care system on the quality of care. N Engl J Med 2003;348:2218–27.
18. Chaudhry B, Wang J, Wu S, et al. Systematic review: Impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med 2006;144:742–52.
19. American recovery and reinvestment act of 2009. Obey D, Frank B, Gordon B, et al., trans. 111th Congress of the United States.
20. Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med 2010;363:501–4.
21. Electronic health record adoption by office-based providers. Office of National Coordinator for Health Information Technology. U.S. Department of Health and Human Services. Accessed 15 Jul 2013.
22. DesRoches CM, Campbell EG, Rao SR, et al. Electronic health records in ambulatory care — a national survey of physicians. N Engl J Med 2008;359:50–60.
23. Tricco AC, Ivers NM, Grimshaw JM, et al. Effectiveness of quality improvement strategies on the management of diabetes: A systematic review and meta-analysis. Lancet 379:2252–61.
24. Bundy DG, Strouse JJ, Casella JF, Miller MR. Burden of influenza-related hospitalizations among children with sickle cell disease. Pediatrics 2010;125:234–43.
25. National Heart Lung and Blood Institute. The management of sickle cell disease. NIH Pub No. 02-2117. Bethesda, MD: National Institutes of Health; 2002.
26. Centers for Disease Control and Prevention. Immunization schedules. Accessed 5 Jan 2013 at www.cdc.gov/vaccines/schedules/index.html.
27. Strouse JJ, Reller ME, Bundy DG, et al. Severe pandemic h1n1 and seasonal influenza in children and young adults with sickle cell disease. Blood 2010;116:3431–4.
28. Pilishvili T, Zell ER, Farley MM, et al. Risk factors for invasive pneumococcal disease in children in the era of conjugate vaccine use. Pediatrics 2010;126:e9–17.
29. Heeney MM, Ware RE. Hydroxyurea for children with sickle cell disease. Pediatr Clin North Am 008;55:483–501.
30. Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood 2010;115:5300–11.
31. Ferster A, Vermylen C, Cornu G, et al. Hydroxyurea for treatment of severe sickle cell anemia: a pediatric clinical trial. Blood 1996;88:1960–4.
32. Strouse JJ, Lanzkron S, Beach MC, et al. Hydroxyurea for sickle cell disease: a systematic review for efficacy and toxicity in children. Pediatrics 2008;122:1332–42.
33. Hankins JS, Ware RE, Rogers ZR, et al. Long-term hydroxyurea therapy for infants with sickle cell anemia: the husoft extension study. Blood 2005;106:2269–75.
34. Steinberg MH, McCarthy WF, Castro O, et al. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: a 17.5-year follow-up. Am J Hematol 2010;85:403–8.
35. Voskaridou E, Christoulas D, Bilalis A, et al. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: results of a 17-year, single-center trial (lashs). Blood 2010;115:2354–63.
36. Healthy people 2020. Immunization and infectious diseases. Accessed 3 Jun 2013 at www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicid=23.
37. Cohen NJ, Lauderdale DS, Shete PB, et al. Physician knowledge of catch-up regimens and contraindications for childhood immunizations. Pediatrics 2003;111:925–32.
38. Raphael JL, Rattler TL, Kowalkowski MA, et al. The medical home experience among children with sickle cell disease. Pediatr Blood Cancer 2013;60:275–80.
39. Strouse JJ, Heeney MM. Hydroxyurea for the treatment of sickle cell disease: efficacy, barriers, toxicity, and management in children. Pediatr Blood Cancer 2012;59:365–71.
40. Steinberg MH, Barton F, Castro O, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA 2003;289:1645–51.
41. Patient protection and affordable care act, US Pub. L. No. 111-148, §2702, 124 stat. 119, 318-319. 2010.
42. Harrison M, Koppel R, Bar-Lev S. Unintended consequences of information technologies in health care: an interactive sociotechnical analysis. J Am Med Inform Assoc 2007;14:542–9.
43. Haux R. Health information systems – past, present, future. Int J Med Informatics 2006;75:268–81.
44. Schieppati A, Henter J-I, Daina E, Aperia A. Why rare diseases are an important medical and social issue. Lancet 2008;371:2039–41.
45. Office of Rare Diseases Research National Institutes of Health. Rare diseases and related terms. Accessed 28 Jun 2013 at www.rarediseases.info.nih.gov/rarediseaselist.aspx.
1. Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Preventive Med 2010;38(4 Suppl):S512–S521.
2. Steinberg MH. Management of sickle cell disease. N Engl J Med 1999;340:1021–30.
3. Adamkiewicz TV, Silk BJ, Howgate J, et al. Effectiveness of the 7-valent pneumococcal conjugate vaccine in children with sickle cell disease in the first decade of life. Pediatrics 2008;121:562–9.
4. Adams RJ, McKie VC, Hsu L, et al. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial doppler ultrasonography. N Engl J Med 1998;339:5–1.
5. Adams RJ, Brambilla D, Optimizing Primary Stroke Prevention in Sickle Cell Anemia Trial I. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease.[see comment]. N Engl J Med 2005;353:2769–78.
6. Charache S, Terrin ML, Moore RD, et al. Effect of hydroxyurea on the frequency of painful crises in sickle cell anemia. N Engl J Med 1995;332:1317–22.
7. Wang WC, Ware RE, Miller ST, et al. Hydroxycarbamide in very young children with sickle-cell anaemia: A multicentre, randomised, controlled trial (baby hug). Lancet 2011;377:1663–72.
8. Quinn CT, Rogers ZR, McCavit TL, Buchanan GR. Improved survival of children and adolescents with sickle cell disease. Blood 2010;115:3447–52.
9. Hamideh D, Alvarez O. Sickle cell disease related mortality in the united states (1999–2009). Pediatr Blood Cancer 2013;60:1482–6.
10. Raphael JL, Shetty PB, Liu H, et al. A critical assessment of transcranial doppler screening rates in a large pediatric sickle cell center: Opportunities to improve healthcare quality. Pediatr Blood Cancer 2008;51:647–51.
11. Sox CM, Cooper WO, Koepsell TD, et al. Provision of pneumococcal prophylaxis for publicly insured children with sickle cell disease. JAMA 2003;290:1057–61.
12. Oyeku SO, Driscoll MC, Cohen HW, et al. Parental and other factors associated with hydroxyurea use for pediatric sickle cell disease. Pediatr Blood Cancer 2013;60:653–58.
13. Crandall WV, Margolis PA, Kappelman MD, et al. Improved outcomes in a quality improvement collaborative for pediatric inflammatory bowel disease. Pediatrics 2012;129:e1030–e1041.
14. Schechter MS, Margolis P. Improving subspecialty healthcare: Lessons from cystic fibrosis. J Pediatr 2005;147:295–301.
15. Smith LA, Oyeku SO, Homer C, Zuckerman B. Sickle cell disease: A question of equity and quality. Pediatrics 2006;117:1763–70.
16. Wang CJ, Kavanagh PL, Little AA, et al. Quality-of-care indicators for children with sickle cell disease. Pediatrics 2011;128:484–93.
17. Jha AK, Perlin JB, Kizer KW, Dudley RA. Effect of the transformation of the veterans affairs health care system on the quality of care. N Engl J Med 2003;348:2218–27.
18. Chaudhry B, Wang J, Wu S, et al. Systematic review: Impact of health information technology on quality, efficiency, and costs of medical care. Ann Intern Med 2006;144:742–52.
19. American recovery and reinvestment act of 2009. Obey D, Frank B, Gordon B, et al., trans. 111th Congress of the United States.
20. Blumenthal D, Tavenner M. The “meaningful use” regulation for electronic health records. N Engl J Med 2010;363:501–4.
21. Electronic health record adoption by office-based providers. Office of National Coordinator for Health Information Technology. U.S. Department of Health and Human Services. Accessed 15 Jul 2013.
22. DesRoches CM, Campbell EG, Rao SR, et al. Electronic health records in ambulatory care — a national survey of physicians. N Engl J Med 2008;359:50–60.
23. Tricco AC, Ivers NM, Grimshaw JM, et al. Effectiveness of quality improvement strategies on the management of diabetes: A systematic review and meta-analysis. Lancet 379:2252–61.
24. Bundy DG, Strouse JJ, Casella JF, Miller MR. Burden of influenza-related hospitalizations among children with sickle cell disease. Pediatrics 2010;125:234–43.
25. National Heart Lung and Blood Institute. The management of sickle cell disease. NIH Pub No. 02-2117. Bethesda, MD: National Institutes of Health; 2002.
26. Centers for Disease Control and Prevention. Immunization schedules. Accessed 5 Jan 2013 at www.cdc.gov/vaccines/schedules/index.html.
27. Strouse JJ, Reller ME, Bundy DG, et al. Severe pandemic h1n1 and seasonal influenza in children and young adults with sickle cell disease. Blood 2010;116:3431–4.
28. Pilishvili T, Zell ER, Farley MM, et al. Risk factors for invasive pneumococcal disease in children in the era of conjugate vaccine use. Pediatrics 2010;126:e9–17.
29. Heeney MM, Ware RE. Hydroxyurea for children with sickle cell disease. Pediatr Clin North Am 008;55:483–501.
30. Ware RE. How I use hydroxyurea to treat young patients with sickle cell anemia. Blood 2010;115:5300–11.
31. Ferster A, Vermylen C, Cornu G, et al. Hydroxyurea for treatment of severe sickle cell anemia: a pediatric clinical trial. Blood 1996;88:1960–4.
32. Strouse JJ, Lanzkron S, Beach MC, et al. Hydroxyurea for sickle cell disease: a systematic review for efficacy and toxicity in children. Pediatrics 2008;122:1332–42.
33. Hankins JS, Ware RE, Rogers ZR, et al. Long-term hydroxyurea therapy for infants with sickle cell anemia: the husoft extension study. Blood 2005;106:2269–75.
34. Steinberg MH, McCarthy WF, Castro O, et al. The risks and benefits of long-term use of hydroxyurea in sickle cell anemia: a 17.5-year follow-up. Am J Hematol 2010;85:403–8.
35. Voskaridou E, Christoulas D, Bilalis A, et al. The effect of prolonged administration of hydroxyurea on morbidity and mortality in adult patients with sickle cell syndromes: results of a 17-year, single-center trial (lashs). Blood 2010;115:2354–63.
36. Healthy people 2020. Immunization and infectious diseases. Accessed 3 Jun 2013 at www.healthypeople.gov/2020/topicsobjectives2020/objectiveslist.aspx?topicid=23.
37. Cohen NJ, Lauderdale DS, Shete PB, et al. Physician knowledge of catch-up regimens and contraindications for childhood immunizations. Pediatrics 2003;111:925–32.
38. Raphael JL, Rattler TL, Kowalkowski MA, et al. The medical home experience among children with sickle cell disease. Pediatr Blood Cancer 2013;60:275–80.
39. Strouse JJ, Heeney MM. Hydroxyurea for the treatment of sickle cell disease: efficacy, barriers, toxicity, and management in children. Pediatr Blood Cancer 2012;59:365–71.
40. Steinberg MH, Barton F, Castro O, et al. Effect of hydroxyurea on mortality and morbidity in adult sickle cell anemia: risks and benefits up to 9 years of treatment. JAMA 2003;289:1645–51.
41. Patient protection and affordable care act, US Pub. L. No. 111-148, §2702, 124 stat. 119, 318-319. 2010.
42. Harrison M, Koppel R, Bar-Lev S. Unintended consequences of information technologies in health care: an interactive sociotechnical analysis. J Am Med Inform Assoc 2007;14:542–9.
43. Haux R. Health information systems – past, present, future. Int J Med Informatics 2006;75:268–81.
44. Schieppati A, Henter J-I, Daina E, Aperia A. Why rare diseases are an important medical and social issue. Lancet 2008;371:2039–41.
45. Office of Rare Diseases Research National Institutes of Health. Rare diseases and related terms. Accessed 28 Jun 2013 at www.rarediseases.info.nih.gov/rarediseaselist.aspx.
Evidence-based Management of Newly Diagnosed Chronic Lymphocytic Leukemia
From the Division of Hematology, Ohio State University, Columbus, OH.
Abstract
- Objective: To describe the diagnosis and initial management of chronic lymphocytic leukemia (CLL), including first-line treatment options.
- Methods: Case presentation and review of the literature.
- Results: Most CLL patients demonstrate a chronic, relapsing and remitting course with intervals of months to years between treatments. Recent advances in genetic and molecular markers for risk stratification of CLL significantly impact how clinicians determine prognosis and predict response to treatment for patients with newly diagnosed disease. This information, along with patient factors such as age and health status, should be considered when formulating an initial treatment strategy. Combinations of chemotherapy and immunotherapy offer the longest progression-free survival and overall survival benefit yet reported. For elderly patients or those with significant comorbidities who may not tolerate standard chemoimmunotherapy, less intensive but still effective therapies now exist. Patients with the highest risk disease, such as those with deletions of chromosome 17p, respond poorly to conventional treatment and should be referred to experienced centers where investigational therapies and allogeneic stem cell transplantation are available.
- Conclusion: Both disease characteristics and patient factors should guide the selection among the various effective therapies for CLL. While chemoimmunotherapy is the most effective treatment developed to date, its use may become less prevalent as newer agents are incorporated into initial and relapse treatment algorithms.
Chronic lymphocytic leukemia (CLL) is a chronic malignancy of B-lymphocytes demonstrating a heterogeneous clinical course ranging from indolent to more rapidly progressive. The chief clinical feature is an elevated peripheral blood lymphocyte count, and patients can demonstrate lymphadenopathy, splenomegaly, hepatomegaly, constitutional symptoms, and in late stages bone marrow failure. It is the most common leukemia among adults in the Western world, accounting for between 22% to 30% of new leukemia diagnoses worldwide [1]. Recent incidence rates in the United States are 3.83 cases per 100,000 person-years [2]. The incidence of CLL increases with age, and most new cases are diagnosed in persons 65 years of age or older [1,2]. As reported 5-year survival rates are between 68% and 81% with a median survival of 10 years in some series, the prevalence is significantly higher than the incidence [3]. However, this may even be an underestimate of the population burden of disease, as many cases are not reported to tumor registries [4].
Many patients with CLL are asymptomatic and do not require treatment until years after diagnosis. In these cases a watch and wait approach is taken. The typical natural history of CLL is characterized by periods of effective treatment when required, followed by treatment-free intervals of several years in many cases. However, this can be misleading, as the clinical course for any individual patient is highly variable. Development of cytogenetic and molecular testing has allowed for identification of patients with a higher risk of progression and lower response rates to traditional cytotoxic treatments [5]. For example, depending on chromosomal abnormalities present, median survival can vary from 32 to 133 months [3].
The assessment of underlying disease risk thus provides important information when considering a treatment approach and should be routinely performed for newly diagnosed patients. While the development of highly effective chemoimmunotherapy has allowed most groups of CLL patients to live for many years, some groups do not enjoy the same survival. Recent advances in CLL treatment seek to abrogate such adverse risk factors, thereby improving the survival for all patients with CLL. Given the expected survival of years for most CLL patients, frontline treatment planning must be done in the context of a long-term treatment strategy keeping the risk for late toxicities, such as secondary malignancies, in mind.
Case Study
Initial Presentation
A 50-year-old man is referred for evaluation of cervical lymphadenopathy that had progressed over the prior 6 months. He denies associated symptoms of fatigue, fevers, night sweats, or unintentional weight loss but does report early satiety. On examination there are multiple mobile, enlarged cervical lymph nodes bilaterally. Axillary lymph nodes are likewise enlarged. The liver edge is not palpable, but the spleen is palpable below the belt line. Complete blood count reveals a white blood cell count of 196,000 with 97% lymphocytes. Hemoglobin is 11.0 g/dL and platelet count is 122,000/dL. He recalls being told 3 years previously that his white blood cell count was 48,000 during an emergency department visit for cellulitis.
• How is CLL diagnosed and staged?
CLL is often suspected when patients present with an elevated lymphocyte count. Presenting symptoms of CLL commonly include lymphadenopathy, an enlarged spleen, and constitutional or “B” symptoms such as fatigue, unintentional weight loss, or drenching night sweats. However, only 25% of patients are symptomatic at diagnosis [1]. Many patients with CLL are now diagnosed after a routine blood test, long before the disease is clinically apparent.
The diagnosis of CLL can be made from the peripheral blood and does not require a bone marrow biopsy. According to 2008 guidelines from the International Workshop on Chronic Lymphocytic Leukemia (IWCLL), diagnosis requires at least 5000/uL clonal B-lymphocytes in the peripheral blood. The clonality must be confirmed by immunophenotyping. At time of diagnosis the peripheral blood smear should be examined for the characteristic cells: small mature lymphocytes with a narrow rim of cytoplasm and dense nuclei consisting of clumped chromatin. Larger, atypical cells can be present as long as they do not exceed 55% of the total number of lymphocytes [6].
The immunophenotype of CLL includes aberrant expression of CD5 and a T-cell antigen, along with the characteristic B-cell antigens CD19, CD20, and CD23. The leukemic clone may be either kappa or lambda light chain restricted. Expression of surface immunoglobulin, CD20, and CD79a is typically low compared to that of normal B cells, although there can be some variability in the immunophenotype [6].
Care should be taken to exclude other malignancies with a similar morphology. Leukemic phase mantle cell lymphoma, other low grade lymphomas, and hairy cell leukemia are commonly mistaken for CLL. Immunophenotyping and cytogenetics are usually sufficient to differentiate these. Testing for a balanced translocation involving chromosomes 11 and 14 to exclude mantle cell lymphoma can be helpful, as both CLL and mantle cell lymphoma can appear morphologically similar and share immunophenotypic features (CD5+/CD19+).
Case Continued
The patient’s peripheral blood is drawn for routine immunophenotyping as well as cytogenetic and molecular testing. When he returns to discuss the results 10 days later, he learns that peripheral blood immunophenotyping demonstrates a dim kappa restricted monoclonal population of B-cells that expressed CD19, CD20(dim), CD23, CD38, CD5, and CD43. The lymphocytes are negative for CD10, FMC7, and CD79b, consistent with a CLL immunophenotype. This patient fulfills diagnostic criteria for CLL and has Rai stage II or intermediate-risk disease. Interphase cytogenetic studies of the peripheral blood demonstrate deletions of chromosomes 11q22.3 and 13q14.3. The immunoglobulin heavy chain gene (IGHV) is unmutated.
• How can a CLL patient’s disease risk be characterized?
Historically, staging at diagnosis, pattern of bone marrow infiltration, and response to therapy were used to gauge prognosis. In more recent years, cytogenetic and molecular testing methods have been developed to augment risk stratification. Testing of prognostic significance that influences clinical management includes IGHV mutational status and interphase cytogenetics using FISH [3,12–14]. Expression of ZAP-70 and CD38 are both independent predictors of poorer prognosis in CLL but are not recommended for routine clinical use. Standardized methodology for the measurement of Zap-70 in particular limits the utility of that test in routine clinical practice [15]. Performed at diagnosis, a time when many patients are asymptomatic, cytogenetic testing with FISH and IGHV mutational analysis can predict time to first treatment and increasingly identify high-risk patients for whom investigational early intervention approaches may be considered [16]. While cytogenetic testing has utility at time of diagnosis, it should be considered necessary prior to deciding on the first-line treatment.
Cytogenetics are also important in predicting response to therapy. For instance, patients with deletion(11q) disease have improved survival when treated with regimens containing an alkylating agent [18]. Deletion(17p) patients respond poorly to traditional cytotoxic agents, and treatments with alternate mechanisms of action should be used [5,19]. The gene for tumor suppressor protein TP53 is encoded in this region of chromosome 17, thus treatment with agents that act independent of pathways involving TP53 are preferred [20].
In addition to cytogenetic testing, quantization of somatic mutations in the gene encoding the variable region of the immune globulin heavy chain gene (IGHV) can help define disease-specific risk. When greater than 98% sequence homology is seen, the gene is considered IGHV unmutated. Patients with an unmutated IGHV have worse overall survival. In one study of Rai stage 0 CLL patients, those with an unmutated IGHV had a survival of only 95 months, compared with 293 months in the mutated group [12].
• When should CLL be treated?
CLL is not curable with current standard therapies, and starting treatment at time of diagnosis for early stage, asymptomatic, CLL patients does not improve overall survival and adds treatment-related toxicities [21,22]. Consequently, the decision to treat is based on treating or preventing complications from the disease, and observation is recommended for most asymptomatic, early-stage patients [6]. Because median survival in CLL is often measured in years, deferring treatment can limit both the short- and long-term complications of therapy, especially the significant risk of secondary malignancies associated with some therapies [23]. However, deferring treatment can significantly impact both a patient’s emotional well-being and quality of life, which should be kept in mind when first discussing the rationale for observation with asymptomatic patients [24].
causes.
For patients with anemia, neutropenia, or thrombocytopenia that is autoimmune in nature, treatment should typically begin with corticosteroids, as it would for non-CLL associated cases of autoimmune cytopenias. If steroids are not effective, second-line treatments appropriate for the situation are generally employed, including intravenous immunoglobulin, cyclosporine, azathioprine, and splenectomy. Rituximab has also been shown to be effective in steroid-refractory cases of autoimmune hemolytic anemia associated with CLL [26]. Only if cytopenias are refractory to appropriate second-line therapy should CLL-directed treatments be considered, assuming there are no other indications to treat the underlying CLL [6]. Bone marrow biopsy can be helpful in differentiating autoimmune cytopenias from marrow failure due to CLL infiltration.
• What treatments are most appropriate for young, fit patients?
For younger patients who are in good general health, the standard treatment choice is combination chemoimmunotherapy. While single agent therapies can effectively palliate symptoms in most cases, they do not offer a survival benefit. Treatment with chemoimmunotherapy, consisting of cytotoxic chemotherapy given in combination with an anti-CD20 monoclonal antibody (generally rituximab), results in high response rates and conveys an advantage with respect to both progression-free survival (PFS) and overall survival (OS). Several chemoimmunotherapy regimens are commonly used.
As compared to fludarabine alone, frontline therapy with the combination of rituximab and fludarabine (FR) results in both a higher overall response rate (84% compared with 63% with fludarabine alone) and more complete responses (38% compared with 20% with fludarabine alone). The probability of PFS at 2 years is also better with FR: 67% compared to 45% with single agent fludarabine [28,29]. Neutropenia is more common with the combination regimen but does not appear to increase the rate of infection. Rituximab infusion reactions are commonly observed, so a stepped-up dosing schedule was developed to decrease their incidence and severity.
Fludarabine, cyclophosphamide, and rituximab (FCR) is another highly effective regimen. This combination has similar efficacy to FR with a 90% to 95% overall response rate (ORR) and 44% to 70% complete response (CR) rate [19,30]. Long-term results with this regimen are favorable; 6-year OS of 77% and median time to progression of 80 months have been reported in a follow-up study [31]. However, hematologic toxicity, including severe neutropenia, is common, and many patients are unable to complete all planned therapy [19]. The addition of cyclophosphamide does appear to be especially important for patients with a deletion(11q). Several clinical trials have consistently found that measures of response and survival are improved for deletion(11q) patients receiving an alkylating agent in addition to a nucleoside analogue [18,32,33]. Outcomes in patients with deletion(17p) disease remain poor after FCR; this subset demonstrates the shortest PFS at only 11.5 months [19].
A more recently developed chemoimmunotherapy option for younger, fit patients is bendamustine and rituximab (BR). Bendamustine has structural similarities to both alkylating agents and purine analogues, and is significantly more efficacious than chlorambucil as a single agent [34]. The combination is generally well tolerated, and a phase 2 trial of the combination reported an overall response rate (ORR) of 88.0% [32]. Notably, when the results were examined by genetic risk group, the regimen remained effective for deletion(11q) patients, who achieved overall and CR rates of 90% and 40%, respectively. Unfortunately, only 37.5% of deletion(17p) patients responded, and no patients achieved a CR [32].
The risk for therapy-related neoplasms should be taken into account when selecting initial therapy given the expected long-term survival of most CLL patients. About 8 out of 300 FCR-treated patients developed a therapy-related neoplasm in one study [31]. Treatment with FR, which does not include an alkylating agent, does not appear to have the same risk. In a study reporting long-term follow-up on 104 patients treated with FR, none developed a therapy related neoplasm [35]. Risks associated with bendamustine have not been well characterized but appear to be lower than FC. While inclusion of an alkylating agent is important for deletion(11q) patients, it is not clear if other patients similarly benefit, thus meriting the potentially increased risk for second cancers.
Fortunately, the choice among these similarly effective regimens will soon be based on high-quality, comparative data. FCR and BR have now been directly compared as a first-line treatment in the German CLL Study Group CLL10 trial. At interim analysis, both regimens had the same ORR and 2-year OS. However, CRs were less common in the BR group (38.1% versus 47.4% with FCR) and PFS was likewise inferior. Expectedly, the FCR group experienced more myelotoxicity and infections. The rate of severe neutropenia with FCR was higher at 81.7% compared to only 56.8% with BR [36]. This may be an important consideration when selecting a regimen for individual patients. Baseline renal function may influence choice as well. The active metabolite of fludarabine is eliminated through the kidneys and patients with decreased renal function have been excluded from clinical trials of FCR [19,37]. The phase 2 study of BR included patients with impaired renal function and 35% of participants had a creatinine clearance of less than 70 mL/min. It is notable that increased toxicity was seen in this subset, including higher rates of myelosuppression and infection [32]. As few direct comparisons have been done, the choice between effective first-line chemoimmunotherapy regimens can be difficult. The final results of the CLL 10 trial, as well as the now completed CALGB 10404 trial comparing FCR to FR, will provide new evidence regarding the relative risks and benefits of these regimens, particularly for patients without high-risk chromosomal abnormalities.
• What treatments are most effective for patients with deletion(17p) CLL?
As noted above, deletion(17p) CLL responds poorly to standard treatments. This relative lack of durable response to chemoimmunotherapy appears attributable to loss of function of the tumor suppressor protein TP53 which is encoded in the affected area [20,32,38]. In vivo evidence suggests that fludarabine works through a TP53-dependent mechanism, which likely explains the poor results obtained when deletion(17p) patients are treated with fludarabine-based combinations [38]. Patients harboring deletion(17p) or TP53 mutations should thus be referred for participation in clinical trials or allogeneic stem cell transplantation [17,27].
If initial treatment of a patient with deletion(17p) begins outside of a clinical trial, it should ideally be comprised of agents that have a TP53-independent mechanism of action [20]. Alemtuzumab, a humanized monoclonal antibody against the CD52 antigen expressed on the surface of normal and malignant B- and T-lymphocytes, demonstrated ORR of 33% to 50% in studies of patients with relapsed and refractory CLL [39–42]. A retrospective analysis found that similar outcomes were seen in those who had a TP53 mutation or deletion(17p). A subsequent study of previously untreated CLL patients randomized to treatment with 12 weeks of alemtuzumab or chlorambucil found that alemtuzumab-treated deletion(17p) patients had an ORR of 64% and median PFS of 10.7 months [43]. Alemtuzumab is therefore a rational choice for first-line therapy in this population. Hematologic toxicity is frequent, however, and all patients must receive prophylaxis against and monitoring for reactivation of CMV infection [43]. Infusion reactions are common but may be reduced by subcutaneous administration without apparent loss of efficacy [42,44]. While alemtuzumab is no longer marketed in the United States for the indication of CLL, it is available free of charge from the manufacturer [45].
High-dose methylprednisolone with rituximab (HDMP-R) has also been successfully used as both salvage and first-line therapy in this group. As salvage therapy, responses were seen in greater than 90% of patients, including over 50% of deletion(17p) patients [46-48]. In treatment-naïve CLL, the ORR was 96% [49], although data for patients with deletion 17p is limited in the frontline setting. Myelotoxicity attributable to the regimen is modest, but good antimicrobial prophylaxis is warranted, as well as close monitoring for hyperglycemia in at-risk patients.
• How is treatment modified for older or less fit patients?
For patients older than 70, or those who have significant comorbidities, effective therapies are still available. As most new diagnoses of CLL are made in patients older than 65, age is but one important factor determining an individual patient’s ability to tolerate treatment. The German CLL Study Group has usefully classified elderly patients into 3 treatment groups based on fitness and goals of care. The first group of medically fit patients with a normal life expectancy, sometimes referred to as the “go go” group, generally tolerate standard chemoimmunotherapy. A second group of older patients with significant life-limiting comorbid conditions—the so-called “no go” patients —should be offered best supportive care rather than CLL-directed treatment. A third group of “slow go” patients falls in between these two; these patients have comorbidities with variable life expectancy and will likely tolerate and benefit from CLL-directed therapy [50].
While some older patients can safely receive chemoimmunotherapy at standard doses and schedules, FCR can prove intolerable for even the medically fit elderly. Because inferior outcomes have been reported among patients older than 70 [30,31], a reduced-dose FCR regimen (FCR-lite) has been studied. Doses of fludarabine and cyclophosphamide were reduced by 20% and 40% respectively and dosing frequency of rituximab was increased. The CR rate was favorable at 77%, the rate of severe neutropenia was reduced to only 13%, and most patients completed all planned therapy [51]. Alternatively, the combination of pentostatin, cyclophosphamide, and rituximab (PCR) has also been successfully used in older patients. The overall and CR rates, 91% and 63% respectively, were durable at 26 months of follow-up. Importantly, there was no statistically significant difference in response or toxicity among the 28% of patients older than 70 [52,53].
For less fit patients, chlorambucil remains a reasonable option. Chlorambucil, a well-tolerated oral alkylating agent, has been used as a frontline therapy in CLL for decades. Chlorambucil has demonstrated consistent response rates in at least 4 clinical trials and is an appropriate option for patients who cannot tolerate more intensive therapy [54]. When a multicenter phase III trial compared it directly to fludarabine in patients over 65, the PFS and OS were no different despite favorable response rates in fludarabine-treated patients [55]. The effectiveness of single-agent chlorambucil can be improved, and the tolerability maintained, with the addition of a CD20-directed monoclonal antibody [56]. Obinutuzumab, a glycolengineered type II antibody against CD20, has recently been shown to improve treatment efficacy when used in combination with chlorambucil [57]. The CLL11 trial randomized patients with comorbid conditions to 1 of 3 treatments: single-agent chlorambucil, chlorambucil with rituximab (R-Clb), or chlorambucil with obinutuzumab (G-Clb). Both chemoimmunotherapy combinations outperformed chlorambucil alone, but the inclusion of obinutuzumab was associated with higher CR rates and longer PFS than rituximab, although infusion reactions and neutropenia were more common in the obinutuzumab arm [57]. Based on this result, the US Food and Drug Administration has now approved obinutuzumab for use in combination with chlorambucil as frontline therapy. While regulatory approval is without restriction with respect to patient age or fitness, a chlorambucil backbone remains most appropriate for older patients and/or those with significant comorbidities.
• What therapies are currently under development?
Numerous targeted treatments and novel immunotherapies are under active investigation in CLL. With greater specificity for CLL, these emerging agents offer the possibility of more effective yet less toxic treatments that will undoubtedly change the landscape for future CLL therapy. These agents are currently most studied as salvage therapies, and given their targeted mechanism of action can be highly effective in relapsed and refractory patients who frequently harbor poor risk cytogenetic abnormalities such as deletion(17p). Data for these agents as initial treatment is limited. Ongoing clinical trials employing these newer agents will need to be reported before these drugs can be recommended as frontline therapies.
Frontline experience with the oral immunomodulatory agent lenalidomide is more extensive. Lenalidomide offers convenient daily dosing and a favorable toxicity profile. When given on a continuous dosing schedule to patients who were 65 years old or older, the ORR was 65%, and 88% of patients were still alive at 2 years’ follow-up. The quality of response continued to improve beyond 18 months of treatment. Neutropenia, the most common severe toxicity, complicated about a third of cycles. Tumor flare attributable to immune activation was also seen, but in most cases was low-grade and did not require intervention [58,59]. While life-threatening tumor lysis syndrome and tumor flare have been seen with lenalidomide in CLL, such concerns are largely abrogated by a lower starting dose and careful intrapatient dose titration [60]. Lenalidomide has also been combined with rituximab and yielded promising results. Sixty-nine treatment-naïve patients were treated with escalating doses of lenalidomide along with rituximab infusions starting at the end of cycle 1 in a phase 2 study. They achieved an 88% ORR with 16% CRs. Toxicities were generally manageable, but patients over 65 were less likely to reach higher doses of lenalidomide or complete all planned treatment cycles [61]. Unfortunately, the FDA recently halted accrual to a phase 3 frontline clinical trial comparing lenalidomide to chlorambucil due to excess mortality in the lenalidomide arm among patients over the age of 80 [62]. More detailed outcomes from that study should be forthcoming.
Perhaps the most remarkable recent advance in CLL medicine, however, is the advent of orally bioavailable small molecule inhibitors of the B-cell receptor (BCR) signaling pathway. BCR signaling plays a vitally important role in supporting the growth and survival of malignant B-cells, activating a number of downstream kinases (Syk, Btk, PI3K, among others) which are potential therapeutic targets. Proof of principle for this approach was demonstrated with the Syk inhibitor fostamatinib in a phase 1/2 trial enrolling patients with B-cell non-Hodgkin lymphoma and CLL. CLL/SLL patients had the highest response rates of any subgroup in that study, with 6 out of 11 patients responding [63]. In a subsequent phase 1b study of the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib, durable partial remissions were reported in more than 70% of multiply relapsed and refractory patients, including genetically high-risk patients [64–66]. Ibrutinib appears safer and better tolerated than traditional chemoimmunotherapy in the relapsed setting; consequently, it is now being studied as a first-line therapy both alone and in combination with other agents [67]. Other BCR signaling agents under study, such as the phosphatidylinositol 3-kinase inhibitor idelalisib, demonstrate similar safety and high response rates across both genetic risk and patient age groups [68].
New targeted drugs are not limited to the BCR signaling pathway. ABT-199 inhibits B-cell leukemia/lymphoma 2 (BCL-2), which is an anti-apoptotic protein in the cell death pathway, and has demonstrated remarkable clinical efficacy in relapsed and refractory CLL patients [69]. As more experience is gained with these targeted agents, it is expected that they will be rapidly incorporated into frontline therapies. However, these agents are just now being studied in comparison to standard initial treatments, such as FCR, and it is not yet clear they will offer an advantage over current chemoimmunotherapy in this setting [70–72]. Since these single agents typically do not induce complete remissions, and require indefinite therapy to maintain response, optimal combination therapies are under intensive investigation.
Case Conclusion
The patient and his physician elect to begin treatment owing to symptomatic cervical lymphadenopathy and massive splenomegaly. Given the presence of a deletion(11q) abnormality, but hoping to limit the risk for both short- and long-term toxicities, this younger, fit patient is treated with 6 cycles of bendamustine and rituximab. At the conclusion of treatment, neither the cervical lymph nodes nor spleen remain palpable. His blood counts have also normalized, with a white blood cell count of 4700 with 8.1% lymphocyotes, hemoglobin of 14.3 gm/dL, and platelets of 151,000/dL.
Summary
CLL follows a chronic course requiring treatment at variable intervals. Both genetic risk features and patient factors should be considered when determining initial therapy. Cytogenetic and molecular testing can characterize the likelihood of treatment success, information useful for treatment planning. Chemoimmunotherapy is highly effective for most patients, including patients with deletion(11q) CLL, where the inclusion of an alkylating agent in frontline therapy alters the natural history of disease. However, patients with deletion(17p) and or TP53-mutated disease respond poorly to standard treatment and should be considered for investigational therapies [73]. Novel approaches to CLL therapy, most notably immunotherapies and BCR-targeted agents, hold the promise to further improve outcomes, particularly for the highest risk patients and those elderly and/or infirm patients who tolerate chemotherapy poorly. Frontline therapy should rapidly evolve as emerging agents enter advanced phase investigation.
Corresponding author: Jeffrey Jones, MD, MPH, Div. of Hematology, Ohio State University, A350B Starling Loving Hall, 320 West 10th Ave., Columbus, OH 43210, [email protected].
Financial disclosures: Dr. Jones disclosed that he is on the advisory boards and has received research support from Genentech, Pharmacyclics, and Gilead.
Author contributions: conception and design, KAR, JAJ; analysis and interpretation of data, KAR, JAJ; drafting of article, KAR, JAJ; critical revision of the article, KAR, JAJ.
1. Redaelli A, Laskin BL, Stephens JM, et al. The clinical and epidemiological burden of chronic lymphocytic leukaemia. Eur J Cancer Care (Engl) 2004;13:279–87.
2. Dores GM, Anderson WF, Curtis RE, et al. Chronic lymphocytic leukaemia and small lymphocytic lymphoma: overview of the descriptive epidemiology. Br J Haematol 2007;139:809–19.
3. Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med 2000;343:1910–6.
4. Zent CS, Kyasa MJ, Evans R, Schichman SA. Chronic lymphocytic leukemia incidence is substantially higher than estimated from tumor registry data. Cancer 2001;92:1325–30.
5. Byrd JC, Gribben JG, Peterson BL, et al. Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: justification for risk-adapted therapy. J Clin Oncol 2006;24:437–43.
6. Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008;111:5446–56.
7. Rawstron AC, Bennett FL, O'Connor SJ, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med 2008;359:575–83.
8. Rai KR, Sawitsky A, Cronkite EP, et al. Clinical staging of chronic lymphocytic leukemia. Blood 1975;46:219–34.
9. Binet JL, Auquier A, Dighiero G, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer 1981;48:198–206.
10. Binet JL, Lepoprier M, Dighiero G, et al. A clinical staging system for chronic lymphocytic leukemia: prognostic significance. Cancer 1977:40:855–64.
11. Eichhorst BF, Fischer K, Fink AM, et al. Limited clinical relevance of imaging techniques in the follow-up of patients with advanced chronic lymphocytic leukemia: results of a meta-analysis. Blood 2011;117:1817–21.
12. Hamblin TJ, Davis Z, Gardiner A, et al. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood 1999;94:1848–54.
13. Crespo M, Bosch F, Villamor N, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med 2003;348:
1764–75.
14. Hamblin TJ, Orchard JA, Ibbotson RE, et al. CD38 expression and immunoglobulin variable region mutations are independent prognostic variables in chronic lymphocytic leukemia, but CD38 expression may vary during the course of the disease. Blood 2002;99:1023–9.
15. Rassenti LZ, Kipps TJ. Clinical utility of assessing ZAP-70 and CD38 in chronic lymphocytic leukemia. Cytometry B Clin Cytom 2006;70:209–13.
16. Wierda WG, O'Brien S, Wang X, et al. Multivariable model for time to first treatment in patients with chronic lymphocytic leukemia. J Clin Oncol 2011;29:4088–95.
17. Schetelig J, van Biezen A, Brand R, et al. Allogeneic hematopoietic stem-cell transplantation for chronic lymphocytic leukemia with 17p deletion: a retrospective European Group for Blood and Marrow Transplantation analysis. J Clin Oncol 2008;26:5094–100.
18. Ding W, Ferrajoli A. Evidence-based mini-review: the role of alkylating agents in the initial treatment of chronic lymphocytic leukemia patients with the 11q deletion. Hematology Am Soc Hematol Educ Program 2010;2010:90–2.
19. Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010;376:1164–74.
20. Badoux XC, Keating MJ, Wierda WG.What is the best frontline therapy for patients with CLL and 17p deletion? Curr Hematol Malig Rep 2011;6:36–46.
21. Dighiero G, Maloum K, Desablens B, et al. Chlorambucil in indolent chronic lymphocytic leukemia. French Cooperative Group on Chronic Lymphocytic Leukemia. N Engl J Med 1998;338:1506–14.
22. Chemotherapeutic options in chronic lymphocytic leukemia: a meta-analysis of the randomized trials. CLL Trialists' Collaborative Group. J Natl Cancer Inst 1999;91:861–8.
23. Morton LM, Curtis RE, Linet MS, et al. Second malignancy risks after non-Hodgkin's lymphoma and chronic lymphocytic leukemia: differences by lymphoma subtype. J Clin Oncol 2010;28:4935–44.
24. Shanafelt TD, Bowen D, Venkat C, et al. Quality of life in chronic lymphocytic leukemia: an international survey of 1482 patients. Br J Haematol 2007;139:255–64.
25. Baer MR, Stein RS, Dessypris EN. Chronic lymphocytic leukemia with hyperleukocytosis. The hyperviscosity syndrome. Cancer 1985;56:2865–9.
26. Gupta N, Kavuru S, Patel D, et al. Rituximab-based chemotherapy for steroid-refractory autoimmune hemolytic anemia of chronic lymphocytic leukemia. Leukemia 2002;16:2092–5.
27. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: non-hodgkin's lymphomas. Version 2.2013. Available at http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf.
28. Byrd JC, Rai K, Peterson BL, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood 2005;105:49–53.
29. Byrd JC, Peterson BL, Morrison VA, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712). Blood 2003;101:6–14.
30. Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol 2005;23:4079–88.
31. Tam CS, O'Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood 2008;112:975–80.
32. Fischer K, Cramer P, Busch R, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol 2012;30:3209–16.
33. Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet 2007;370:230–9.
34. Knauf WU, Lissichkov T, Aldaoud A, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol 2009;27:4378–84.
35. Woyach JA, Ruppert AS, Heerema NA, et al. Chemoimmunotherapy with fludarabine and rituximab produces extended overall survival and progression-free survival in chronic lymphocytic leukemia: long-term follow-up of CALGB study 9712. J Clin Oncol 2011;29:1349–55.
36. Fink AM, et al., Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximabversus bendamustine and rituximabin previously untreated and physically fit patientswith advanced chronic lymphocytic leukemia: results of a planned interim analysis of the CLL10 Trial, an international, randomized study of the German CLL Study Group (GCLLSG). Blood 2013;122:526.
37. Gandhi V, Plunkett W. Cellular and clinical pharmacology of fludarabine. Clin Pharmacokinet 2002;41:93–103.
38. Rosenwald A, Chuang EY, Davis RE, et al. Fludarabine treatment of patients with chronic lymphocytic leukemia induces a p53-dependent gene expression response. Blood 2004;104:1428–34.
39. Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood 2002;99:3554–61.
40. Lozanski G, Heerema NA, Flinn IW, et al. Alemtuzumab is an effective therapy for chronic lymphocytic leukemia with p53 mutations and deletions. Blood 2004;103:3278–81.
41. Osuji NC, Del Giudice I, Matutes E, et al, The efficacy of alemtuzumab for refractory chronic lymphocytic leukemia in relation to cytogenetic abnormalities of p53. Haematologica 2005;90:1435–6.
42. Stilgenbauer S, Zenz T, Winkler D, et al. Subcutaneous alemtuzumab in fludarabine-refractory chronic lymphocytic leukemia: clinical results and prognostic marker analyses from the CLL2H study of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol 2009;27:3994–4001.
43. Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol 2007;25:5616–23.
44. Lundin J, Kimby E, Björkholm M, et al. Phase II trial of subcutaneous anti-CD52 monoclonal antibody alemtuzumab (Campath-1H) as first-line treatment for patients with B-cell chronic lymphocytic leukemia (B-CLL). Blood 2002;100:768–73.
45. Genzyme. US Campath Distribution Program. Cambridge, MA: Genzyme. Available at http://www.campath.com/.
46. Thornton PD, Matutes E, Bosanquet AG, et al. High dose methylprednisolone can induce remissions in CLL patients with p53 abnormalities. Ann Hematol 2003;82:759–65.
47. Bowen DA, Call TG, Jenkins GD, et al. Methylprednisolone-rituximab is an effective salvage therapy for patients with relapsed chronic lymphocytic leukemia including those with unfavorable cytogenetic features. Leuk Lymphoma 2007;48:2412–7.
48. Castro JE, Sandoval-Sus JD, Bole J, et al. Rituximab in combination with high-dose methylprednisolone for the treatment of fludarabine refractory high-risk chronic lymphocytic leukemia. Leukemia 2008;22:2048–53.
49. Castro JE, James DF, Sandoval-Sus JD, et al. Rituximab in combination with high-dose methylprednisolone for the treatment of chronic lymphocytic leukemia. Leukemia 2009;23:1779–89.
50. Eichhorst B, Goede V, Hallek M. Treatment of elderly patients with chronic lymphocytic leukemia. Leuk Lymphoma 2009;50:171–8.
51. Foon KA, Boyiadzis M, Land SR, et al. Chemoimmunotherapy with low-dose fludarabine and cyclophosphamide and high dose rituximab in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol 2009;27:498–503.
52. Kay NE, Geyer SM, Call TG, et al. Combination chemoimmunotherapy with pentostatin, cyclophosphamide, and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B chronic lymphocytic leukemia. Blood 2007;109:405–11.
53. Shanafelt TD, Lin T, Geyer SM, et al. Pentostatin, cyclophosphamide, and rituximab regimen in older patients with chronic lymphocytic leukemia. Cancer 2007;109:2291–8.
54. Catovsky D, Else M, Richards S. Chlorambucil--still not bad: a reappraisal. Clin Lymphoma Myeloma Leuk 2011;11 Suppl 1:S2–6.
55. Eichhorst BF, Busch R, Stilgenbauer S, et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood 2009;114:3382–91.
56. Laurenti L, Vannata B, Innocenti I, et al. Chlorambucil plus rituximab as front-line therapy in elderly/unfit patients affected by b-cell chronic lymphocytic leukemia: results of a single-centre experience. Mediterr J Hematol Infect Dis 2013;5:e2013031.
57. Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014 Jan 8. [Epub ahead of print].
58. Badoux XC, Keating MJ, Wen S, et al. Lenalidomide as initial therapy of elderly patients with chronic lymphocytic leukemia. Blood 2011;118:3489–98.
59. Strati P, Keating MJ, Wierda WG, et al. Lenalidomide induces long-lasting responses in elderly patients with chronic lymphocytic leukemia. Blood 2013;122:734–7.
60. Moutouh-de Parseval LA, Weiss L, DeLap RJ, et al. Tumor lysis syndrome/tumor flare reaction in lenalidomide-treated chronic lymphocytic leukemia. J Clin Oncol 2007;25:5047.
61. James DF, Brown JR, Werner L, et al. Lenalidomide and rituximab for the initial treatment of patients with chronic lymphocytic leukemia (CLL): a multicenter study of the CLL Research Consortium. ASH Annual Meeting Abstracts 2011;118:291.
62. US Food and Drug Administration. FDA halts clinical trial of drug Revlimid (lenalidomide) for chronic lymphocytic leukemia due to safety concerns. Available at http://www.fda.gov/Drugs/DrugSafety/ucm361444.htm.
63. Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 2010;115:2578–85.
64. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013;369:32–42.
65. Farooqui M, Aue G, Valdez J, et al. Single agent ibrutinib (PCI-32765) achieves equally good and durable responses in chronic lymphocytic leukemia (CLL) patients with and without deletion 17p. Blood 2013;122:673.
66. Byrd JC, Furman RR, Coutre S, et al. The Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib (PCI-32765) monotherapy demonstrates long-term safety and durability of response in chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) patients in an open-label extension study. Blood 2013;122:4163.
67. Brown JR, Barrientos JC, Barr PM, et al. Ibrutinib in combination with bendamustine and rituximab is active and tolerable in patients with relapsed/refractory CLL/SLL: final results of a phase 1b study. ASH Annual Meeting Abstracts 2013.
68. O'Brien SM, Lamanna N, Kipps TJ, et al. A phase II study of the selective phosphatidylinositol 3-kinase delta (PI3K{delta}) inhibitor idelalisib (GS-1101) in combination with rituximab (R) in treatment-naive patients (pts) ≥ 65 years with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). J Clin Oncol 2013;31(15 Suppl); Abstract 7005.
69. Seymour JF, Davids MS, Pagel JM, et al. Bcl-2 Inhibitor ABT-199 (GDC-0199) monotherapy shows anti-tumor activity including complete remissions in high-risk relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL). Blood 2013;122:872.
70. Rituxumab and bendamustine hydrochloride, rituxumab and ibrutunib, or ibrutinib alone in treating older patients with previously untreated chronic lymphocytic leukemia. Available at http://clinicaltrials.gov/ct2/show/NCT01886872?term=ibrutinib+cll&rank=8.
71. A multicenter, open-label, phase 3 study of the bruton's tyrosine kinase inhibitor pci-32765 versus chlorambucil in patients 65 years of older with treatment-naive chronic lymphocytic leukemia or small lymphocytic lymphoma (RESONATE-2) Available at http://clinicaltrials.gov/ct2/show/NCT01722487?term=ibrutinib+cll&rank=12.
72. Ibrutinib and rituximab compared with fludarabine phosphate, cyclophosphamide, and rituxumab in treating patients with untreated chronic lymphocytic leukemia. Available at http://clinicaltrials.gov/ct2/show/NCT02048813?term=ibrutinib+cll&rank=2.
73. Strati P, Keating MJ, O'Brien SM, et al. Outcomes of first-line treatment for chronic lymphocytic leukemia (CLL) with 17p deletion. J Clin Oncol 2013;31(15 suppl): Abstract 7102.
From the Division of Hematology, Ohio State University, Columbus, OH.
Abstract
- Objective: To describe the diagnosis and initial management of chronic lymphocytic leukemia (CLL), including first-line treatment options.
- Methods: Case presentation and review of the literature.
- Results: Most CLL patients demonstrate a chronic, relapsing and remitting course with intervals of months to years between treatments. Recent advances in genetic and molecular markers for risk stratification of CLL significantly impact how clinicians determine prognosis and predict response to treatment for patients with newly diagnosed disease. This information, along with patient factors such as age and health status, should be considered when formulating an initial treatment strategy. Combinations of chemotherapy and immunotherapy offer the longest progression-free survival and overall survival benefit yet reported. For elderly patients or those with significant comorbidities who may not tolerate standard chemoimmunotherapy, less intensive but still effective therapies now exist. Patients with the highest risk disease, such as those with deletions of chromosome 17p, respond poorly to conventional treatment and should be referred to experienced centers where investigational therapies and allogeneic stem cell transplantation are available.
- Conclusion: Both disease characteristics and patient factors should guide the selection among the various effective therapies for CLL. While chemoimmunotherapy is the most effective treatment developed to date, its use may become less prevalent as newer agents are incorporated into initial and relapse treatment algorithms.
Chronic lymphocytic leukemia (CLL) is a chronic malignancy of B-lymphocytes demonstrating a heterogeneous clinical course ranging from indolent to more rapidly progressive. The chief clinical feature is an elevated peripheral blood lymphocyte count, and patients can demonstrate lymphadenopathy, splenomegaly, hepatomegaly, constitutional symptoms, and in late stages bone marrow failure. It is the most common leukemia among adults in the Western world, accounting for between 22% to 30% of new leukemia diagnoses worldwide [1]. Recent incidence rates in the United States are 3.83 cases per 100,000 person-years [2]. The incidence of CLL increases with age, and most new cases are diagnosed in persons 65 years of age or older [1,2]. As reported 5-year survival rates are between 68% and 81% with a median survival of 10 years in some series, the prevalence is significantly higher than the incidence [3]. However, this may even be an underestimate of the population burden of disease, as many cases are not reported to tumor registries [4].
Many patients with CLL are asymptomatic and do not require treatment until years after diagnosis. In these cases a watch and wait approach is taken. The typical natural history of CLL is characterized by periods of effective treatment when required, followed by treatment-free intervals of several years in many cases. However, this can be misleading, as the clinical course for any individual patient is highly variable. Development of cytogenetic and molecular testing has allowed for identification of patients with a higher risk of progression and lower response rates to traditional cytotoxic treatments [5]. For example, depending on chromosomal abnormalities present, median survival can vary from 32 to 133 months [3].
The assessment of underlying disease risk thus provides important information when considering a treatment approach and should be routinely performed for newly diagnosed patients. While the development of highly effective chemoimmunotherapy has allowed most groups of CLL patients to live for many years, some groups do not enjoy the same survival. Recent advances in CLL treatment seek to abrogate such adverse risk factors, thereby improving the survival for all patients with CLL. Given the expected survival of years for most CLL patients, frontline treatment planning must be done in the context of a long-term treatment strategy keeping the risk for late toxicities, such as secondary malignancies, in mind.
Case Study
Initial Presentation
A 50-year-old man is referred for evaluation of cervical lymphadenopathy that had progressed over the prior 6 months. He denies associated symptoms of fatigue, fevers, night sweats, or unintentional weight loss but does report early satiety. On examination there are multiple mobile, enlarged cervical lymph nodes bilaterally. Axillary lymph nodes are likewise enlarged. The liver edge is not palpable, but the spleen is palpable below the belt line. Complete blood count reveals a white blood cell count of 196,000 with 97% lymphocytes. Hemoglobin is 11.0 g/dL and platelet count is 122,000/dL. He recalls being told 3 years previously that his white blood cell count was 48,000 during an emergency department visit for cellulitis.
• How is CLL diagnosed and staged?
CLL is often suspected when patients present with an elevated lymphocyte count. Presenting symptoms of CLL commonly include lymphadenopathy, an enlarged spleen, and constitutional or “B” symptoms such as fatigue, unintentional weight loss, or drenching night sweats. However, only 25% of patients are symptomatic at diagnosis [1]. Many patients with CLL are now diagnosed after a routine blood test, long before the disease is clinically apparent.
The diagnosis of CLL can be made from the peripheral blood and does not require a bone marrow biopsy. According to 2008 guidelines from the International Workshop on Chronic Lymphocytic Leukemia (IWCLL), diagnosis requires at least 5000/uL clonal B-lymphocytes in the peripheral blood. The clonality must be confirmed by immunophenotyping. At time of diagnosis the peripheral blood smear should be examined for the characteristic cells: small mature lymphocytes with a narrow rim of cytoplasm and dense nuclei consisting of clumped chromatin. Larger, atypical cells can be present as long as they do not exceed 55% of the total number of lymphocytes [6].
The immunophenotype of CLL includes aberrant expression of CD5 and a T-cell antigen, along with the characteristic B-cell antigens CD19, CD20, and CD23. The leukemic clone may be either kappa or lambda light chain restricted. Expression of surface immunoglobulin, CD20, and CD79a is typically low compared to that of normal B cells, although there can be some variability in the immunophenotype [6].
Care should be taken to exclude other malignancies with a similar morphology. Leukemic phase mantle cell lymphoma, other low grade lymphomas, and hairy cell leukemia are commonly mistaken for CLL. Immunophenotyping and cytogenetics are usually sufficient to differentiate these. Testing for a balanced translocation involving chromosomes 11 and 14 to exclude mantle cell lymphoma can be helpful, as both CLL and mantle cell lymphoma can appear morphologically similar and share immunophenotypic features (CD5+/CD19+).
Case Continued
The patient’s peripheral blood is drawn for routine immunophenotyping as well as cytogenetic and molecular testing. When he returns to discuss the results 10 days later, he learns that peripheral blood immunophenotyping demonstrates a dim kappa restricted monoclonal population of B-cells that expressed CD19, CD20(dim), CD23, CD38, CD5, and CD43. The lymphocytes are negative for CD10, FMC7, and CD79b, consistent with a CLL immunophenotype. This patient fulfills diagnostic criteria for CLL and has Rai stage II or intermediate-risk disease. Interphase cytogenetic studies of the peripheral blood demonstrate deletions of chromosomes 11q22.3 and 13q14.3. The immunoglobulin heavy chain gene (IGHV) is unmutated.
• How can a CLL patient’s disease risk be characterized?
Historically, staging at diagnosis, pattern of bone marrow infiltration, and response to therapy were used to gauge prognosis. In more recent years, cytogenetic and molecular testing methods have been developed to augment risk stratification. Testing of prognostic significance that influences clinical management includes IGHV mutational status and interphase cytogenetics using FISH [3,12–14]. Expression of ZAP-70 and CD38 are both independent predictors of poorer prognosis in CLL but are not recommended for routine clinical use. Standardized methodology for the measurement of Zap-70 in particular limits the utility of that test in routine clinical practice [15]. Performed at diagnosis, a time when many patients are asymptomatic, cytogenetic testing with FISH and IGHV mutational analysis can predict time to first treatment and increasingly identify high-risk patients for whom investigational early intervention approaches may be considered [16]. While cytogenetic testing has utility at time of diagnosis, it should be considered necessary prior to deciding on the first-line treatment.
Cytogenetics are also important in predicting response to therapy. For instance, patients with deletion(11q) disease have improved survival when treated with regimens containing an alkylating agent [18]. Deletion(17p) patients respond poorly to traditional cytotoxic agents, and treatments with alternate mechanisms of action should be used [5,19]. The gene for tumor suppressor protein TP53 is encoded in this region of chromosome 17, thus treatment with agents that act independent of pathways involving TP53 are preferred [20].
In addition to cytogenetic testing, quantization of somatic mutations in the gene encoding the variable region of the immune globulin heavy chain gene (IGHV) can help define disease-specific risk. When greater than 98% sequence homology is seen, the gene is considered IGHV unmutated. Patients with an unmutated IGHV have worse overall survival. In one study of Rai stage 0 CLL patients, those with an unmutated IGHV had a survival of only 95 months, compared with 293 months in the mutated group [12].
• When should CLL be treated?
CLL is not curable with current standard therapies, and starting treatment at time of diagnosis for early stage, asymptomatic, CLL patients does not improve overall survival and adds treatment-related toxicities [21,22]. Consequently, the decision to treat is based on treating or preventing complications from the disease, and observation is recommended for most asymptomatic, early-stage patients [6]. Because median survival in CLL is often measured in years, deferring treatment can limit both the short- and long-term complications of therapy, especially the significant risk of secondary malignancies associated with some therapies [23]. However, deferring treatment can significantly impact both a patient’s emotional well-being and quality of life, which should be kept in mind when first discussing the rationale for observation with asymptomatic patients [24].
causes.
For patients with anemia, neutropenia, or thrombocytopenia that is autoimmune in nature, treatment should typically begin with corticosteroids, as it would for non-CLL associated cases of autoimmune cytopenias. If steroids are not effective, second-line treatments appropriate for the situation are generally employed, including intravenous immunoglobulin, cyclosporine, azathioprine, and splenectomy. Rituximab has also been shown to be effective in steroid-refractory cases of autoimmune hemolytic anemia associated with CLL [26]. Only if cytopenias are refractory to appropriate second-line therapy should CLL-directed treatments be considered, assuming there are no other indications to treat the underlying CLL [6]. Bone marrow biopsy can be helpful in differentiating autoimmune cytopenias from marrow failure due to CLL infiltration.
• What treatments are most appropriate for young, fit patients?
For younger patients who are in good general health, the standard treatment choice is combination chemoimmunotherapy. While single agent therapies can effectively palliate symptoms in most cases, they do not offer a survival benefit. Treatment with chemoimmunotherapy, consisting of cytotoxic chemotherapy given in combination with an anti-CD20 monoclonal antibody (generally rituximab), results in high response rates and conveys an advantage with respect to both progression-free survival (PFS) and overall survival (OS). Several chemoimmunotherapy regimens are commonly used.
As compared to fludarabine alone, frontline therapy with the combination of rituximab and fludarabine (FR) results in both a higher overall response rate (84% compared with 63% with fludarabine alone) and more complete responses (38% compared with 20% with fludarabine alone). The probability of PFS at 2 years is also better with FR: 67% compared to 45% with single agent fludarabine [28,29]. Neutropenia is more common with the combination regimen but does not appear to increase the rate of infection. Rituximab infusion reactions are commonly observed, so a stepped-up dosing schedule was developed to decrease their incidence and severity.
Fludarabine, cyclophosphamide, and rituximab (FCR) is another highly effective regimen. This combination has similar efficacy to FR with a 90% to 95% overall response rate (ORR) and 44% to 70% complete response (CR) rate [19,30]. Long-term results with this regimen are favorable; 6-year OS of 77% and median time to progression of 80 months have been reported in a follow-up study [31]. However, hematologic toxicity, including severe neutropenia, is common, and many patients are unable to complete all planned therapy [19]. The addition of cyclophosphamide does appear to be especially important for patients with a deletion(11q). Several clinical trials have consistently found that measures of response and survival are improved for deletion(11q) patients receiving an alkylating agent in addition to a nucleoside analogue [18,32,33]. Outcomes in patients with deletion(17p) disease remain poor after FCR; this subset demonstrates the shortest PFS at only 11.5 months [19].
A more recently developed chemoimmunotherapy option for younger, fit patients is bendamustine and rituximab (BR). Bendamustine has structural similarities to both alkylating agents and purine analogues, and is significantly more efficacious than chlorambucil as a single agent [34]. The combination is generally well tolerated, and a phase 2 trial of the combination reported an overall response rate (ORR) of 88.0% [32]. Notably, when the results were examined by genetic risk group, the regimen remained effective for deletion(11q) patients, who achieved overall and CR rates of 90% and 40%, respectively. Unfortunately, only 37.5% of deletion(17p) patients responded, and no patients achieved a CR [32].
The risk for therapy-related neoplasms should be taken into account when selecting initial therapy given the expected long-term survival of most CLL patients. About 8 out of 300 FCR-treated patients developed a therapy-related neoplasm in one study [31]. Treatment with FR, which does not include an alkylating agent, does not appear to have the same risk. In a study reporting long-term follow-up on 104 patients treated with FR, none developed a therapy related neoplasm [35]. Risks associated with bendamustine have not been well characterized but appear to be lower than FC. While inclusion of an alkylating agent is important for deletion(11q) patients, it is not clear if other patients similarly benefit, thus meriting the potentially increased risk for second cancers.
Fortunately, the choice among these similarly effective regimens will soon be based on high-quality, comparative data. FCR and BR have now been directly compared as a first-line treatment in the German CLL Study Group CLL10 trial. At interim analysis, both regimens had the same ORR and 2-year OS. However, CRs were less common in the BR group (38.1% versus 47.4% with FCR) and PFS was likewise inferior. Expectedly, the FCR group experienced more myelotoxicity and infections. The rate of severe neutropenia with FCR was higher at 81.7% compared to only 56.8% with BR [36]. This may be an important consideration when selecting a regimen for individual patients. Baseline renal function may influence choice as well. The active metabolite of fludarabine is eliminated through the kidneys and patients with decreased renal function have been excluded from clinical trials of FCR [19,37]. The phase 2 study of BR included patients with impaired renal function and 35% of participants had a creatinine clearance of less than 70 mL/min. It is notable that increased toxicity was seen in this subset, including higher rates of myelosuppression and infection [32]. As few direct comparisons have been done, the choice between effective first-line chemoimmunotherapy regimens can be difficult. The final results of the CLL 10 trial, as well as the now completed CALGB 10404 trial comparing FCR to FR, will provide new evidence regarding the relative risks and benefits of these regimens, particularly for patients without high-risk chromosomal abnormalities.
• What treatments are most effective for patients with deletion(17p) CLL?
As noted above, deletion(17p) CLL responds poorly to standard treatments. This relative lack of durable response to chemoimmunotherapy appears attributable to loss of function of the tumor suppressor protein TP53 which is encoded in the affected area [20,32,38]. In vivo evidence suggests that fludarabine works through a TP53-dependent mechanism, which likely explains the poor results obtained when deletion(17p) patients are treated with fludarabine-based combinations [38]. Patients harboring deletion(17p) or TP53 mutations should thus be referred for participation in clinical trials or allogeneic stem cell transplantation [17,27].
If initial treatment of a patient with deletion(17p) begins outside of a clinical trial, it should ideally be comprised of agents that have a TP53-independent mechanism of action [20]. Alemtuzumab, a humanized monoclonal antibody against the CD52 antigen expressed on the surface of normal and malignant B- and T-lymphocytes, demonstrated ORR of 33% to 50% in studies of patients with relapsed and refractory CLL [39–42]. A retrospective analysis found that similar outcomes were seen in those who had a TP53 mutation or deletion(17p). A subsequent study of previously untreated CLL patients randomized to treatment with 12 weeks of alemtuzumab or chlorambucil found that alemtuzumab-treated deletion(17p) patients had an ORR of 64% and median PFS of 10.7 months [43]. Alemtuzumab is therefore a rational choice for first-line therapy in this population. Hematologic toxicity is frequent, however, and all patients must receive prophylaxis against and monitoring for reactivation of CMV infection [43]. Infusion reactions are common but may be reduced by subcutaneous administration without apparent loss of efficacy [42,44]. While alemtuzumab is no longer marketed in the United States for the indication of CLL, it is available free of charge from the manufacturer [45].
High-dose methylprednisolone with rituximab (HDMP-R) has also been successfully used as both salvage and first-line therapy in this group. As salvage therapy, responses were seen in greater than 90% of patients, including over 50% of deletion(17p) patients [46-48]. In treatment-naïve CLL, the ORR was 96% [49], although data for patients with deletion 17p is limited in the frontline setting. Myelotoxicity attributable to the regimen is modest, but good antimicrobial prophylaxis is warranted, as well as close monitoring for hyperglycemia in at-risk patients.
• How is treatment modified for older or less fit patients?
For patients older than 70, or those who have significant comorbidities, effective therapies are still available. As most new diagnoses of CLL are made in patients older than 65, age is but one important factor determining an individual patient’s ability to tolerate treatment. The German CLL Study Group has usefully classified elderly patients into 3 treatment groups based on fitness and goals of care. The first group of medically fit patients with a normal life expectancy, sometimes referred to as the “go go” group, generally tolerate standard chemoimmunotherapy. A second group of older patients with significant life-limiting comorbid conditions—the so-called “no go” patients —should be offered best supportive care rather than CLL-directed treatment. A third group of “slow go” patients falls in between these two; these patients have comorbidities with variable life expectancy and will likely tolerate and benefit from CLL-directed therapy [50].
While some older patients can safely receive chemoimmunotherapy at standard doses and schedules, FCR can prove intolerable for even the medically fit elderly. Because inferior outcomes have been reported among patients older than 70 [30,31], a reduced-dose FCR regimen (FCR-lite) has been studied. Doses of fludarabine and cyclophosphamide were reduced by 20% and 40% respectively and dosing frequency of rituximab was increased. The CR rate was favorable at 77%, the rate of severe neutropenia was reduced to only 13%, and most patients completed all planned therapy [51]. Alternatively, the combination of pentostatin, cyclophosphamide, and rituximab (PCR) has also been successfully used in older patients. The overall and CR rates, 91% and 63% respectively, were durable at 26 months of follow-up. Importantly, there was no statistically significant difference in response or toxicity among the 28% of patients older than 70 [52,53].
For less fit patients, chlorambucil remains a reasonable option. Chlorambucil, a well-tolerated oral alkylating agent, has been used as a frontline therapy in CLL for decades. Chlorambucil has demonstrated consistent response rates in at least 4 clinical trials and is an appropriate option for patients who cannot tolerate more intensive therapy [54]. When a multicenter phase III trial compared it directly to fludarabine in patients over 65, the PFS and OS were no different despite favorable response rates in fludarabine-treated patients [55]. The effectiveness of single-agent chlorambucil can be improved, and the tolerability maintained, with the addition of a CD20-directed monoclonal antibody [56]. Obinutuzumab, a glycolengineered type II antibody against CD20, has recently been shown to improve treatment efficacy when used in combination with chlorambucil [57]. The CLL11 trial randomized patients with comorbid conditions to 1 of 3 treatments: single-agent chlorambucil, chlorambucil with rituximab (R-Clb), or chlorambucil with obinutuzumab (G-Clb). Both chemoimmunotherapy combinations outperformed chlorambucil alone, but the inclusion of obinutuzumab was associated with higher CR rates and longer PFS than rituximab, although infusion reactions and neutropenia were more common in the obinutuzumab arm [57]. Based on this result, the US Food and Drug Administration has now approved obinutuzumab for use in combination with chlorambucil as frontline therapy. While regulatory approval is without restriction with respect to patient age or fitness, a chlorambucil backbone remains most appropriate for older patients and/or those with significant comorbidities.
• What therapies are currently under development?
Numerous targeted treatments and novel immunotherapies are under active investigation in CLL. With greater specificity for CLL, these emerging agents offer the possibility of more effective yet less toxic treatments that will undoubtedly change the landscape for future CLL therapy. These agents are currently most studied as salvage therapies, and given their targeted mechanism of action can be highly effective in relapsed and refractory patients who frequently harbor poor risk cytogenetic abnormalities such as deletion(17p). Data for these agents as initial treatment is limited. Ongoing clinical trials employing these newer agents will need to be reported before these drugs can be recommended as frontline therapies.
Frontline experience with the oral immunomodulatory agent lenalidomide is more extensive. Lenalidomide offers convenient daily dosing and a favorable toxicity profile. When given on a continuous dosing schedule to patients who were 65 years old or older, the ORR was 65%, and 88% of patients were still alive at 2 years’ follow-up. The quality of response continued to improve beyond 18 months of treatment. Neutropenia, the most common severe toxicity, complicated about a third of cycles. Tumor flare attributable to immune activation was also seen, but in most cases was low-grade and did not require intervention [58,59]. While life-threatening tumor lysis syndrome and tumor flare have been seen with lenalidomide in CLL, such concerns are largely abrogated by a lower starting dose and careful intrapatient dose titration [60]. Lenalidomide has also been combined with rituximab and yielded promising results. Sixty-nine treatment-naïve patients were treated with escalating doses of lenalidomide along with rituximab infusions starting at the end of cycle 1 in a phase 2 study. They achieved an 88% ORR with 16% CRs. Toxicities were generally manageable, but patients over 65 were less likely to reach higher doses of lenalidomide or complete all planned treatment cycles [61]. Unfortunately, the FDA recently halted accrual to a phase 3 frontline clinical trial comparing lenalidomide to chlorambucil due to excess mortality in the lenalidomide arm among patients over the age of 80 [62]. More detailed outcomes from that study should be forthcoming.
Perhaps the most remarkable recent advance in CLL medicine, however, is the advent of orally bioavailable small molecule inhibitors of the B-cell receptor (BCR) signaling pathway. BCR signaling plays a vitally important role in supporting the growth and survival of malignant B-cells, activating a number of downstream kinases (Syk, Btk, PI3K, among others) which are potential therapeutic targets. Proof of principle for this approach was demonstrated with the Syk inhibitor fostamatinib in a phase 1/2 trial enrolling patients with B-cell non-Hodgkin lymphoma and CLL. CLL/SLL patients had the highest response rates of any subgroup in that study, with 6 out of 11 patients responding [63]. In a subsequent phase 1b study of the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib, durable partial remissions were reported in more than 70% of multiply relapsed and refractory patients, including genetically high-risk patients [64–66]. Ibrutinib appears safer and better tolerated than traditional chemoimmunotherapy in the relapsed setting; consequently, it is now being studied as a first-line therapy both alone and in combination with other agents [67]. Other BCR signaling agents under study, such as the phosphatidylinositol 3-kinase inhibitor idelalisib, demonstrate similar safety and high response rates across both genetic risk and patient age groups [68].
New targeted drugs are not limited to the BCR signaling pathway. ABT-199 inhibits B-cell leukemia/lymphoma 2 (BCL-2), which is an anti-apoptotic protein in the cell death pathway, and has demonstrated remarkable clinical efficacy in relapsed and refractory CLL patients [69]. As more experience is gained with these targeted agents, it is expected that they will be rapidly incorporated into frontline therapies. However, these agents are just now being studied in comparison to standard initial treatments, such as FCR, and it is not yet clear they will offer an advantage over current chemoimmunotherapy in this setting [70–72]. Since these single agents typically do not induce complete remissions, and require indefinite therapy to maintain response, optimal combination therapies are under intensive investigation.
Case Conclusion
The patient and his physician elect to begin treatment owing to symptomatic cervical lymphadenopathy and massive splenomegaly. Given the presence of a deletion(11q) abnormality, but hoping to limit the risk for both short- and long-term toxicities, this younger, fit patient is treated with 6 cycles of bendamustine and rituximab. At the conclusion of treatment, neither the cervical lymph nodes nor spleen remain palpable. His blood counts have also normalized, with a white blood cell count of 4700 with 8.1% lymphocyotes, hemoglobin of 14.3 gm/dL, and platelets of 151,000/dL.
Summary
CLL follows a chronic course requiring treatment at variable intervals. Both genetic risk features and patient factors should be considered when determining initial therapy. Cytogenetic and molecular testing can characterize the likelihood of treatment success, information useful for treatment planning. Chemoimmunotherapy is highly effective for most patients, including patients with deletion(11q) CLL, where the inclusion of an alkylating agent in frontline therapy alters the natural history of disease. However, patients with deletion(17p) and or TP53-mutated disease respond poorly to standard treatment and should be considered for investigational therapies [73]. Novel approaches to CLL therapy, most notably immunotherapies and BCR-targeted agents, hold the promise to further improve outcomes, particularly for the highest risk patients and those elderly and/or infirm patients who tolerate chemotherapy poorly. Frontline therapy should rapidly evolve as emerging agents enter advanced phase investigation.
Corresponding author: Jeffrey Jones, MD, MPH, Div. of Hematology, Ohio State University, A350B Starling Loving Hall, 320 West 10th Ave., Columbus, OH 43210, [email protected].
Financial disclosures: Dr. Jones disclosed that he is on the advisory boards and has received research support from Genentech, Pharmacyclics, and Gilead.
Author contributions: conception and design, KAR, JAJ; analysis and interpretation of data, KAR, JAJ; drafting of article, KAR, JAJ; critical revision of the article, KAR, JAJ.
From the Division of Hematology, Ohio State University, Columbus, OH.
Abstract
- Objective: To describe the diagnosis and initial management of chronic lymphocytic leukemia (CLL), including first-line treatment options.
- Methods: Case presentation and review of the literature.
- Results: Most CLL patients demonstrate a chronic, relapsing and remitting course with intervals of months to years between treatments. Recent advances in genetic and molecular markers for risk stratification of CLL significantly impact how clinicians determine prognosis and predict response to treatment for patients with newly diagnosed disease. This information, along with patient factors such as age and health status, should be considered when formulating an initial treatment strategy. Combinations of chemotherapy and immunotherapy offer the longest progression-free survival and overall survival benefit yet reported. For elderly patients or those with significant comorbidities who may not tolerate standard chemoimmunotherapy, less intensive but still effective therapies now exist. Patients with the highest risk disease, such as those with deletions of chromosome 17p, respond poorly to conventional treatment and should be referred to experienced centers where investigational therapies and allogeneic stem cell transplantation are available.
- Conclusion: Both disease characteristics and patient factors should guide the selection among the various effective therapies for CLL. While chemoimmunotherapy is the most effective treatment developed to date, its use may become less prevalent as newer agents are incorporated into initial and relapse treatment algorithms.
Chronic lymphocytic leukemia (CLL) is a chronic malignancy of B-lymphocytes demonstrating a heterogeneous clinical course ranging from indolent to more rapidly progressive. The chief clinical feature is an elevated peripheral blood lymphocyte count, and patients can demonstrate lymphadenopathy, splenomegaly, hepatomegaly, constitutional symptoms, and in late stages bone marrow failure. It is the most common leukemia among adults in the Western world, accounting for between 22% to 30% of new leukemia diagnoses worldwide [1]. Recent incidence rates in the United States are 3.83 cases per 100,000 person-years [2]. The incidence of CLL increases with age, and most new cases are diagnosed in persons 65 years of age or older [1,2]. As reported 5-year survival rates are between 68% and 81% with a median survival of 10 years in some series, the prevalence is significantly higher than the incidence [3]. However, this may even be an underestimate of the population burden of disease, as many cases are not reported to tumor registries [4].
Many patients with CLL are asymptomatic and do not require treatment until years after diagnosis. In these cases a watch and wait approach is taken. The typical natural history of CLL is characterized by periods of effective treatment when required, followed by treatment-free intervals of several years in many cases. However, this can be misleading, as the clinical course for any individual patient is highly variable. Development of cytogenetic and molecular testing has allowed for identification of patients with a higher risk of progression and lower response rates to traditional cytotoxic treatments [5]. For example, depending on chromosomal abnormalities present, median survival can vary from 32 to 133 months [3].
The assessment of underlying disease risk thus provides important information when considering a treatment approach and should be routinely performed for newly diagnosed patients. While the development of highly effective chemoimmunotherapy has allowed most groups of CLL patients to live for many years, some groups do not enjoy the same survival. Recent advances in CLL treatment seek to abrogate such adverse risk factors, thereby improving the survival for all patients with CLL. Given the expected survival of years for most CLL patients, frontline treatment planning must be done in the context of a long-term treatment strategy keeping the risk for late toxicities, such as secondary malignancies, in mind.
Case Study
Initial Presentation
A 50-year-old man is referred for evaluation of cervical lymphadenopathy that had progressed over the prior 6 months. He denies associated symptoms of fatigue, fevers, night sweats, or unintentional weight loss but does report early satiety. On examination there are multiple mobile, enlarged cervical lymph nodes bilaterally. Axillary lymph nodes are likewise enlarged. The liver edge is not palpable, but the spleen is palpable below the belt line. Complete blood count reveals a white blood cell count of 196,000 with 97% lymphocytes. Hemoglobin is 11.0 g/dL and platelet count is 122,000/dL. He recalls being told 3 years previously that his white blood cell count was 48,000 during an emergency department visit for cellulitis.
• How is CLL diagnosed and staged?
CLL is often suspected when patients present with an elevated lymphocyte count. Presenting symptoms of CLL commonly include lymphadenopathy, an enlarged spleen, and constitutional or “B” symptoms such as fatigue, unintentional weight loss, or drenching night sweats. However, only 25% of patients are symptomatic at diagnosis [1]. Many patients with CLL are now diagnosed after a routine blood test, long before the disease is clinically apparent.
The diagnosis of CLL can be made from the peripheral blood and does not require a bone marrow biopsy. According to 2008 guidelines from the International Workshop on Chronic Lymphocytic Leukemia (IWCLL), diagnosis requires at least 5000/uL clonal B-lymphocytes in the peripheral blood. The clonality must be confirmed by immunophenotyping. At time of diagnosis the peripheral blood smear should be examined for the characteristic cells: small mature lymphocytes with a narrow rim of cytoplasm and dense nuclei consisting of clumped chromatin. Larger, atypical cells can be present as long as they do not exceed 55% of the total number of lymphocytes [6].
The immunophenotype of CLL includes aberrant expression of CD5 and a T-cell antigen, along with the characteristic B-cell antigens CD19, CD20, and CD23. The leukemic clone may be either kappa or lambda light chain restricted. Expression of surface immunoglobulin, CD20, and CD79a is typically low compared to that of normal B cells, although there can be some variability in the immunophenotype [6].
Care should be taken to exclude other malignancies with a similar morphology. Leukemic phase mantle cell lymphoma, other low grade lymphomas, and hairy cell leukemia are commonly mistaken for CLL. Immunophenotyping and cytogenetics are usually sufficient to differentiate these. Testing for a balanced translocation involving chromosomes 11 and 14 to exclude mantle cell lymphoma can be helpful, as both CLL and mantle cell lymphoma can appear morphologically similar and share immunophenotypic features (CD5+/CD19+).
Case Continued
The patient’s peripheral blood is drawn for routine immunophenotyping as well as cytogenetic and molecular testing. When he returns to discuss the results 10 days later, he learns that peripheral blood immunophenotyping demonstrates a dim kappa restricted monoclonal population of B-cells that expressed CD19, CD20(dim), CD23, CD38, CD5, and CD43. The lymphocytes are negative for CD10, FMC7, and CD79b, consistent with a CLL immunophenotype. This patient fulfills diagnostic criteria for CLL and has Rai stage II or intermediate-risk disease. Interphase cytogenetic studies of the peripheral blood demonstrate deletions of chromosomes 11q22.3 and 13q14.3. The immunoglobulin heavy chain gene (IGHV) is unmutated.
• How can a CLL patient’s disease risk be characterized?
Historically, staging at diagnosis, pattern of bone marrow infiltration, and response to therapy were used to gauge prognosis. In more recent years, cytogenetic and molecular testing methods have been developed to augment risk stratification. Testing of prognostic significance that influences clinical management includes IGHV mutational status and interphase cytogenetics using FISH [3,12–14]. Expression of ZAP-70 and CD38 are both independent predictors of poorer prognosis in CLL but are not recommended for routine clinical use. Standardized methodology for the measurement of Zap-70 in particular limits the utility of that test in routine clinical practice [15]. Performed at diagnosis, a time when many patients are asymptomatic, cytogenetic testing with FISH and IGHV mutational analysis can predict time to first treatment and increasingly identify high-risk patients for whom investigational early intervention approaches may be considered [16]. While cytogenetic testing has utility at time of diagnosis, it should be considered necessary prior to deciding on the first-line treatment.
Cytogenetics are also important in predicting response to therapy. For instance, patients with deletion(11q) disease have improved survival when treated with regimens containing an alkylating agent [18]. Deletion(17p) patients respond poorly to traditional cytotoxic agents, and treatments with alternate mechanisms of action should be used [5,19]. The gene for tumor suppressor protein TP53 is encoded in this region of chromosome 17, thus treatment with agents that act independent of pathways involving TP53 are preferred [20].
In addition to cytogenetic testing, quantization of somatic mutations in the gene encoding the variable region of the immune globulin heavy chain gene (IGHV) can help define disease-specific risk. When greater than 98% sequence homology is seen, the gene is considered IGHV unmutated. Patients with an unmutated IGHV have worse overall survival. In one study of Rai stage 0 CLL patients, those with an unmutated IGHV had a survival of only 95 months, compared with 293 months in the mutated group [12].
• When should CLL be treated?
CLL is not curable with current standard therapies, and starting treatment at time of diagnosis for early stage, asymptomatic, CLL patients does not improve overall survival and adds treatment-related toxicities [21,22]. Consequently, the decision to treat is based on treating or preventing complications from the disease, and observation is recommended for most asymptomatic, early-stage patients [6]. Because median survival in CLL is often measured in years, deferring treatment can limit both the short- and long-term complications of therapy, especially the significant risk of secondary malignancies associated with some therapies [23]. However, deferring treatment can significantly impact both a patient’s emotional well-being and quality of life, which should be kept in mind when first discussing the rationale for observation with asymptomatic patients [24].
causes.
For patients with anemia, neutropenia, or thrombocytopenia that is autoimmune in nature, treatment should typically begin with corticosteroids, as it would for non-CLL associated cases of autoimmune cytopenias. If steroids are not effective, second-line treatments appropriate for the situation are generally employed, including intravenous immunoglobulin, cyclosporine, azathioprine, and splenectomy. Rituximab has also been shown to be effective in steroid-refractory cases of autoimmune hemolytic anemia associated with CLL [26]. Only if cytopenias are refractory to appropriate second-line therapy should CLL-directed treatments be considered, assuming there are no other indications to treat the underlying CLL [6]. Bone marrow biopsy can be helpful in differentiating autoimmune cytopenias from marrow failure due to CLL infiltration.
• What treatments are most appropriate for young, fit patients?
For younger patients who are in good general health, the standard treatment choice is combination chemoimmunotherapy. While single agent therapies can effectively palliate symptoms in most cases, they do not offer a survival benefit. Treatment with chemoimmunotherapy, consisting of cytotoxic chemotherapy given in combination with an anti-CD20 monoclonal antibody (generally rituximab), results in high response rates and conveys an advantage with respect to both progression-free survival (PFS) and overall survival (OS). Several chemoimmunotherapy regimens are commonly used.
As compared to fludarabine alone, frontline therapy with the combination of rituximab and fludarabine (FR) results in both a higher overall response rate (84% compared with 63% with fludarabine alone) and more complete responses (38% compared with 20% with fludarabine alone). The probability of PFS at 2 years is also better with FR: 67% compared to 45% with single agent fludarabine [28,29]. Neutropenia is more common with the combination regimen but does not appear to increase the rate of infection. Rituximab infusion reactions are commonly observed, so a stepped-up dosing schedule was developed to decrease their incidence and severity.
Fludarabine, cyclophosphamide, and rituximab (FCR) is another highly effective regimen. This combination has similar efficacy to FR with a 90% to 95% overall response rate (ORR) and 44% to 70% complete response (CR) rate [19,30]. Long-term results with this regimen are favorable; 6-year OS of 77% and median time to progression of 80 months have been reported in a follow-up study [31]. However, hematologic toxicity, including severe neutropenia, is common, and many patients are unable to complete all planned therapy [19]. The addition of cyclophosphamide does appear to be especially important for patients with a deletion(11q). Several clinical trials have consistently found that measures of response and survival are improved for deletion(11q) patients receiving an alkylating agent in addition to a nucleoside analogue [18,32,33]. Outcomes in patients with deletion(17p) disease remain poor after FCR; this subset demonstrates the shortest PFS at only 11.5 months [19].
A more recently developed chemoimmunotherapy option for younger, fit patients is bendamustine and rituximab (BR). Bendamustine has structural similarities to both alkylating agents and purine analogues, and is significantly more efficacious than chlorambucil as a single agent [34]. The combination is generally well tolerated, and a phase 2 trial of the combination reported an overall response rate (ORR) of 88.0% [32]. Notably, when the results were examined by genetic risk group, the regimen remained effective for deletion(11q) patients, who achieved overall and CR rates of 90% and 40%, respectively. Unfortunately, only 37.5% of deletion(17p) patients responded, and no patients achieved a CR [32].
The risk for therapy-related neoplasms should be taken into account when selecting initial therapy given the expected long-term survival of most CLL patients. About 8 out of 300 FCR-treated patients developed a therapy-related neoplasm in one study [31]. Treatment with FR, which does not include an alkylating agent, does not appear to have the same risk. In a study reporting long-term follow-up on 104 patients treated with FR, none developed a therapy related neoplasm [35]. Risks associated with bendamustine have not been well characterized but appear to be lower than FC. While inclusion of an alkylating agent is important for deletion(11q) patients, it is not clear if other patients similarly benefit, thus meriting the potentially increased risk for second cancers.
Fortunately, the choice among these similarly effective regimens will soon be based on high-quality, comparative data. FCR and BR have now been directly compared as a first-line treatment in the German CLL Study Group CLL10 trial. At interim analysis, both regimens had the same ORR and 2-year OS. However, CRs were less common in the BR group (38.1% versus 47.4% with FCR) and PFS was likewise inferior. Expectedly, the FCR group experienced more myelotoxicity and infections. The rate of severe neutropenia with FCR was higher at 81.7% compared to only 56.8% with BR [36]. This may be an important consideration when selecting a regimen for individual patients. Baseline renal function may influence choice as well. The active metabolite of fludarabine is eliminated through the kidneys and patients with decreased renal function have been excluded from clinical trials of FCR [19,37]. The phase 2 study of BR included patients with impaired renal function and 35% of participants had a creatinine clearance of less than 70 mL/min. It is notable that increased toxicity was seen in this subset, including higher rates of myelosuppression and infection [32]. As few direct comparisons have been done, the choice between effective first-line chemoimmunotherapy regimens can be difficult. The final results of the CLL 10 trial, as well as the now completed CALGB 10404 trial comparing FCR to FR, will provide new evidence regarding the relative risks and benefits of these regimens, particularly for patients without high-risk chromosomal abnormalities.
• What treatments are most effective for patients with deletion(17p) CLL?
As noted above, deletion(17p) CLL responds poorly to standard treatments. This relative lack of durable response to chemoimmunotherapy appears attributable to loss of function of the tumor suppressor protein TP53 which is encoded in the affected area [20,32,38]. In vivo evidence suggests that fludarabine works through a TP53-dependent mechanism, which likely explains the poor results obtained when deletion(17p) patients are treated with fludarabine-based combinations [38]. Patients harboring deletion(17p) or TP53 mutations should thus be referred for participation in clinical trials or allogeneic stem cell transplantation [17,27].
If initial treatment of a patient with deletion(17p) begins outside of a clinical trial, it should ideally be comprised of agents that have a TP53-independent mechanism of action [20]. Alemtuzumab, a humanized monoclonal antibody against the CD52 antigen expressed on the surface of normal and malignant B- and T-lymphocytes, demonstrated ORR of 33% to 50% in studies of patients with relapsed and refractory CLL [39–42]. A retrospective analysis found that similar outcomes were seen in those who had a TP53 mutation or deletion(17p). A subsequent study of previously untreated CLL patients randomized to treatment with 12 weeks of alemtuzumab or chlorambucil found that alemtuzumab-treated deletion(17p) patients had an ORR of 64% and median PFS of 10.7 months [43]. Alemtuzumab is therefore a rational choice for first-line therapy in this population. Hematologic toxicity is frequent, however, and all patients must receive prophylaxis against and monitoring for reactivation of CMV infection [43]. Infusion reactions are common but may be reduced by subcutaneous administration without apparent loss of efficacy [42,44]. While alemtuzumab is no longer marketed in the United States for the indication of CLL, it is available free of charge from the manufacturer [45].
High-dose methylprednisolone with rituximab (HDMP-R) has also been successfully used as both salvage and first-line therapy in this group. As salvage therapy, responses were seen in greater than 90% of patients, including over 50% of deletion(17p) patients [46-48]. In treatment-naïve CLL, the ORR was 96% [49], although data for patients with deletion 17p is limited in the frontline setting. Myelotoxicity attributable to the regimen is modest, but good antimicrobial prophylaxis is warranted, as well as close monitoring for hyperglycemia in at-risk patients.
• How is treatment modified for older or less fit patients?
For patients older than 70, or those who have significant comorbidities, effective therapies are still available. As most new diagnoses of CLL are made in patients older than 65, age is but one important factor determining an individual patient’s ability to tolerate treatment. The German CLL Study Group has usefully classified elderly patients into 3 treatment groups based on fitness and goals of care. The first group of medically fit patients with a normal life expectancy, sometimes referred to as the “go go” group, generally tolerate standard chemoimmunotherapy. A second group of older patients with significant life-limiting comorbid conditions—the so-called “no go” patients —should be offered best supportive care rather than CLL-directed treatment. A third group of “slow go” patients falls in between these two; these patients have comorbidities with variable life expectancy and will likely tolerate and benefit from CLL-directed therapy [50].
While some older patients can safely receive chemoimmunotherapy at standard doses and schedules, FCR can prove intolerable for even the medically fit elderly. Because inferior outcomes have been reported among patients older than 70 [30,31], a reduced-dose FCR regimen (FCR-lite) has been studied. Doses of fludarabine and cyclophosphamide were reduced by 20% and 40% respectively and dosing frequency of rituximab was increased. The CR rate was favorable at 77%, the rate of severe neutropenia was reduced to only 13%, and most patients completed all planned therapy [51]. Alternatively, the combination of pentostatin, cyclophosphamide, and rituximab (PCR) has also been successfully used in older patients. The overall and CR rates, 91% and 63% respectively, were durable at 26 months of follow-up. Importantly, there was no statistically significant difference in response or toxicity among the 28% of patients older than 70 [52,53].
For less fit patients, chlorambucil remains a reasonable option. Chlorambucil, a well-tolerated oral alkylating agent, has been used as a frontline therapy in CLL for decades. Chlorambucil has demonstrated consistent response rates in at least 4 clinical trials and is an appropriate option for patients who cannot tolerate more intensive therapy [54]. When a multicenter phase III trial compared it directly to fludarabine in patients over 65, the PFS and OS were no different despite favorable response rates in fludarabine-treated patients [55]. The effectiveness of single-agent chlorambucil can be improved, and the tolerability maintained, with the addition of a CD20-directed monoclonal antibody [56]. Obinutuzumab, a glycolengineered type II antibody against CD20, has recently been shown to improve treatment efficacy when used in combination with chlorambucil [57]. The CLL11 trial randomized patients with comorbid conditions to 1 of 3 treatments: single-agent chlorambucil, chlorambucil with rituximab (R-Clb), or chlorambucil with obinutuzumab (G-Clb). Both chemoimmunotherapy combinations outperformed chlorambucil alone, but the inclusion of obinutuzumab was associated with higher CR rates and longer PFS than rituximab, although infusion reactions and neutropenia were more common in the obinutuzumab arm [57]. Based on this result, the US Food and Drug Administration has now approved obinutuzumab for use in combination with chlorambucil as frontline therapy. While regulatory approval is without restriction with respect to patient age or fitness, a chlorambucil backbone remains most appropriate for older patients and/or those with significant comorbidities.
• What therapies are currently under development?
Numerous targeted treatments and novel immunotherapies are under active investigation in CLL. With greater specificity for CLL, these emerging agents offer the possibility of more effective yet less toxic treatments that will undoubtedly change the landscape for future CLL therapy. These agents are currently most studied as salvage therapies, and given their targeted mechanism of action can be highly effective in relapsed and refractory patients who frequently harbor poor risk cytogenetic abnormalities such as deletion(17p). Data for these agents as initial treatment is limited. Ongoing clinical trials employing these newer agents will need to be reported before these drugs can be recommended as frontline therapies.
Frontline experience with the oral immunomodulatory agent lenalidomide is more extensive. Lenalidomide offers convenient daily dosing and a favorable toxicity profile. When given on a continuous dosing schedule to patients who were 65 years old or older, the ORR was 65%, and 88% of patients were still alive at 2 years’ follow-up. The quality of response continued to improve beyond 18 months of treatment. Neutropenia, the most common severe toxicity, complicated about a third of cycles. Tumor flare attributable to immune activation was also seen, but in most cases was low-grade and did not require intervention [58,59]. While life-threatening tumor lysis syndrome and tumor flare have been seen with lenalidomide in CLL, such concerns are largely abrogated by a lower starting dose and careful intrapatient dose titration [60]. Lenalidomide has also been combined with rituximab and yielded promising results. Sixty-nine treatment-naïve patients were treated with escalating doses of lenalidomide along with rituximab infusions starting at the end of cycle 1 in a phase 2 study. They achieved an 88% ORR with 16% CRs. Toxicities were generally manageable, but patients over 65 were less likely to reach higher doses of lenalidomide or complete all planned treatment cycles [61]. Unfortunately, the FDA recently halted accrual to a phase 3 frontline clinical trial comparing lenalidomide to chlorambucil due to excess mortality in the lenalidomide arm among patients over the age of 80 [62]. More detailed outcomes from that study should be forthcoming.
Perhaps the most remarkable recent advance in CLL medicine, however, is the advent of orally bioavailable small molecule inhibitors of the B-cell receptor (BCR) signaling pathway. BCR signaling plays a vitally important role in supporting the growth and survival of malignant B-cells, activating a number of downstream kinases (Syk, Btk, PI3K, among others) which are potential therapeutic targets. Proof of principle for this approach was demonstrated with the Syk inhibitor fostamatinib in a phase 1/2 trial enrolling patients with B-cell non-Hodgkin lymphoma and CLL. CLL/SLL patients had the highest response rates of any subgroup in that study, with 6 out of 11 patients responding [63]. In a subsequent phase 1b study of the Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib, durable partial remissions were reported in more than 70% of multiply relapsed and refractory patients, including genetically high-risk patients [64–66]. Ibrutinib appears safer and better tolerated than traditional chemoimmunotherapy in the relapsed setting; consequently, it is now being studied as a first-line therapy both alone and in combination with other agents [67]. Other BCR signaling agents under study, such as the phosphatidylinositol 3-kinase inhibitor idelalisib, demonstrate similar safety and high response rates across both genetic risk and patient age groups [68].
New targeted drugs are not limited to the BCR signaling pathway. ABT-199 inhibits B-cell leukemia/lymphoma 2 (BCL-2), which is an anti-apoptotic protein in the cell death pathway, and has demonstrated remarkable clinical efficacy in relapsed and refractory CLL patients [69]. As more experience is gained with these targeted agents, it is expected that they will be rapidly incorporated into frontline therapies. However, these agents are just now being studied in comparison to standard initial treatments, such as FCR, and it is not yet clear they will offer an advantage over current chemoimmunotherapy in this setting [70–72]. Since these single agents typically do not induce complete remissions, and require indefinite therapy to maintain response, optimal combination therapies are under intensive investigation.
Case Conclusion
The patient and his physician elect to begin treatment owing to symptomatic cervical lymphadenopathy and massive splenomegaly. Given the presence of a deletion(11q) abnormality, but hoping to limit the risk for both short- and long-term toxicities, this younger, fit patient is treated with 6 cycles of bendamustine and rituximab. At the conclusion of treatment, neither the cervical lymph nodes nor spleen remain palpable. His blood counts have also normalized, with a white blood cell count of 4700 with 8.1% lymphocyotes, hemoglobin of 14.3 gm/dL, and platelets of 151,000/dL.
Summary
CLL follows a chronic course requiring treatment at variable intervals. Both genetic risk features and patient factors should be considered when determining initial therapy. Cytogenetic and molecular testing can characterize the likelihood of treatment success, information useful for treatment planning. Chemoimmunotherapy is highly effective for most patients, including patients with deletion(11q) CLL, where the inclusion of an alkylating agent in frontline therapy alters the natural history of disease. However, patients with deletion(17p) and or TP53-mutated disease respond poorly to standard treatment and should be considered for investigational therapies [73]. Novel approaches to CLL therapy, most notably immunotherapies and BCR-targeted agents, hold the promise to further improve outcomes, particularly for the highest risk patients and those elderly and/or infirm patients who tolerate chemotherapy poorly. Frontline therapy should rapidly evolve as emerging agents enter advanced phase investigation.
Corresponding author: Jeffrey Jones, MD, MPH, Div. of Hematology, Ohio State University, A350B Starling Loving Hall, 320 West 10th Ave., Columbus, OH 43210, [email protected].
Financial disclosures: Dr. Jones disclosed that he is on the advisory boards and has received research support from Genentech, Pharmacyclics, and Gilead.
Author contributions: conception and design, KAR, JAJ; analysis and interpretation of data, KAR, JAJ; drafting of article, KAR, JAJ; critical revision of the article, KAR, JAJ.
1. Redaelli A, Laskin BL, Stephens JM, et al. The clinical and epidemiological burden of chronic lymphocytic leukaemia. Eur J Cancer Care (Engl) 2004;13:279–87.
2. Dores GM, Anderson WF, Curtis RE, et al. Chronic lymphocytic leukaemia and small lymphocytic lymphoma: overview of the descriptive epidemiology. Br J Haematol 2007;139:809–19.
3. Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med 2000;343:1910–6.
4. Zent CS, Kyasa MJ, Evans R, Schichman SA. Chronic lymphocytic leukemia incidence is substantially higher than estimated from tumor registry data. Cancer 2001;92:1325–30.
5. Byrd JC, Gribben JG, Peterson BL, et al. Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: justification for risk-adapted therapy. J Clin Oncol 2006;24:437–43.
6. Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008;111:5446–56.
7. Rawstron AC, Bennett FL, O'Connor SJ, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med 2008;359:575–83.
8. Rai KR, Sawitsky A, Cronkite EP, et al. Clinical staging of chronic lymphocytic leukemia. Blood 1975;46:219–34.
9. Binet JL, Auquier A, Dighiero G, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer 1981;48:198–206.
10. Binet JL, Lepoprier M, Dighiero G, et al. A clinical staging system for chronic lymphocytic leukemia: prognostic significance. Cancer 1977:40:855–64.
11. Eichhorst BF, Fischer K, Fink AM, et al. Limited clinical relevance of imaging techniques in the follow-up of patients with advanced chronic lymphocytic leukemia: results of a meta-analysis. Blood 2011;117:1817–21.
12. Hamblin TJ, Davis Z, Gardiner A, et al. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood 1999;94:1848–54.
13. Crespo M, Bosch F, Villamor N, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med 2003;348:
1764–75.
14. Hamblin TJ, Orchard JA, Ibbotson RE, et al. CD38 expression and immunoglobulin variable region mutations are independent prognostic variables in chronic lymphocytic leukemia, but CD38 expression may vary during the course of the disease. Blood 2002;99:1023–9.
15. Rassenti LZ, Kipps TJ. Clinical utility of assessing ZAP-70 and CD38 in chronic lymphocytic leukemia. Cytometry B Clin Cytom 2006;70:209–13.
16. Wierda WG, O'Brien S, Wang X, et al. Multivariable model for time to first treatment in patients with chronic lymphocytic leukemia. J Clin Oncol 2011;29:4088–95.
17. Schetelig J, van Biezen A, Brand R, et al. Allogeneic hematopoietic stem-cell transplantation for chronic lymphocytic leukemia with 17p deletion: a retrospective European Group for Blood and Marrow Transplantation analysis. J Clin Oncol 2008;26:5094–100.
18. Ding W, Ferrajoli A. Evidence-based mini-review: the role of alkylating agents in the initial treatment of chronic lymphocytic leukemia patients with the 11q deletion. Hematology Am Soc Hematol Educ Program 2010;2010:90–2.
19. Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010;376:1164–74.
20. Badoux XC, Keating MJ, Wierda WG.What is the best frontline therapy for patients with CLL and 17p deletion? Curr Hematol Malig Rep 2011;6:36–46.
21. Dighiero G, Maloum K, Desablens B, et al. Chlorambucil in indolent chronic lymphocytic leukemia. French Cooperative Group on Chronic Lymphocytic Leukemia. N Engl J Med 1998;338:1506–14.
22. Chemotherapeutic options in chronic lymphocytic leukemia: a meta-analysis of the randomized trials. CLL Trialists' Collaborative Group. J Natl Cancer Inst 1999;91:861–8.
23. Morton LM, Curtis RE, Linet MS, et al. Second malignancy risks after non-Hodgkin's lymphoma and chronic lymphocytic leukemia: differences by lymphoma subtype. J Clin Oncol 2010;28:4935–44.
24. Shanafelt TD, Bowen D, Venkat C, et al. Quality of life in chronic lymphocytic leukemia: an international survey of 1482 patients. Br J Haematol 2007;139:255–64.
25. Baer MR, Stein RS, Dessypris EN. Chronic lymphocytic leukemia with hyperleukocytosis. The hyperviscosity syndrome. Cancer 1985;56:2865–9.
26. Gupta N, Kavuru S, Patel D, et al. Rituximab-based chemotherapy for steroid-refractory autoimmune hemolytic anemia of chronic lymphocytic leukemia. Leukemia 2002;16:2092–5.
27. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: non-hodgkin's lymphomas. Version 2.2013. Available at http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf.
28. Byrd JC, Rai K, Peterson BL, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood 2005;105:49–53.
29. Byrd JC, Peterson BL, Morrison VA, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712). Blood 2003;101:6–14.
30. Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol 2005;23:4079–88.
31. Tam CS, O'Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood 2008;112:975–80.
32. Fischer K, Cramer P, Busch R, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol 2012;30:3209–16.
33. Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet 2007;370:230–9.
34. Knauf WU, Lissichkov T, Aldaoud A, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol 2009;27:4378–84.
35. Woyach JA, Ruppert AS, Heerema NA, et al. Chemoimmunotherapy with fludarabine and rituximab produces extended overall survival and progression-free survival in chronic lymphocytic leukemia: long-term follow-up of CALGB study 9712. J Clin Oncol 2011;29:1349–55.
36. Fink AM, et al., Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximabversus bendamustine and rituximabin previously untreated and physically fit patientswith advanced chronic lymphocytic leukemia: results of a planned interim analysis of the CLL10 Trial, an international, randomized study of the German CLL Study Group (GCLLSG). Blood 2013;122:526.
37. Gandhi V, Plunkett W. Cellular and clinical pharmacology of fludarabine. Clin Pharmacokinet 2002;41:93–103.
38. Rosenwald A, Chuang EY, Davis RE, et al. Fludarabine treatment of patients with chronic lymphocytic leukemia induces a p53-dependent gene expression response. Blood 2004;104:1428–34.
39. Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood 2002;99:3554–61.
40. Lozanski G, Heerema NA, Flinn IW, et al. Alemtuzumab is an effective therapy for chronic lymphocytic leukemia with p53 mutations and deletions. Blood 2004;103:3278–81.
41. Osuji NC, Del Giudice I, Matutes E, et al, The efficacy of alemtuzumab for refractory chronic lymphocytic leukemia in relation to cytogenetic abnormalities of p53. Haematologica 2005;90:1435–6.
42. Stilgenbauer S, Zenz T, Winkler D, et al. Subcutaneous alemtuzumab in fludarabine-refractory chronic lymphocytic leukemia: clinical results and prognostic marker analyses from the CLL2H study of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol 2009;27:3994–4001.
43. Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol 2007;25:5616–23.
44. Lundin J, Kimby E, Björkholm M, et al. Phase II trial of subcutaneous anti-CD52 monoclonal antibody alemtuzumab (Campath-1H) as first-line treatment for patients with B-cell chronic lymphocytic leukemia (B-CLL). Blood 2002;100:768–73.
45. Genzyme. US Campath Distribution Program. Cambridge, MA: Genzyme. Available at http://www.campath.com/.
46. Thornton PD, Matutes E, Bosanquet AG, et al. High dose methylprednisolone can induce remissions in CLL patients with p53 abnormalities. Ann Hematol 2003;82:759–65.
47. Bowen DA, Call TG, Jenkins GD, et al. Methylprednisolone-rituximab is an effective salvage therapy for patients with relapsed chronic lymphocytic leukemia including those with unfavorable cytogenetic features. Leuk Lymphoma 2007;48:2412–7.
48. Castro JE, Sandoval-Sus JD, Bole J, et al. Rituximab in combination with high-dose methylprednisolone for the treatment of fludarabine refractory high-risk chronic lymphocytic leukemia. Leukemia 2008;22:2048–53.
49. Castro JE, James DF, Sandoval-Sus JD, et al. Rituximab in combination with high-dose methylprednisolone for the treatment of chronic lymphocytic leukemia. Leukemia 2009;23:1779–89.
50. Eichhorst B, Goede V, Hallek M. Treatment of elderly patients with chronic lymphocytic leukemia. Leuk Lymphoma 2009;50:171–8.
51. Foon KA, Boyiadzis M, Land SR, et al. Chemoimmunotherapy with low-dose fludarabine and cyclophosphamide and high dose rituximab in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol 2009;27:498–503.
52. Kay NE, Geyer SM, Call TG, et al. Combination chemoimmunotherapy with pentostatin, cyclophosphamide, and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B chronic lymphocytic leukemia. Blood 2007;109:405–11.
53. Shanafelt TD, Lin T, Geyer SM, et al. Pentostatin, cyclophosphamide, and rituximab regimen in older patients with chronic lymphocytic leukemia. Cancer 2007;109:2291–8.
54. Catovsky D, Else M, Richards S. Chlorambucil--still not bad: a reappraisal. Clin Lymphoma Myeloma Leuk 2011;11 Suppl 1:S2–6.
55. Eichhorst BF, Busch R, Stilgenbauer S, et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood 2009;114:3382–91.
56. Laurenti L, Vannata B, Innocenti I, et al. Chlorambucil plus rituximab as front-line therapy in elderly/unfit patients affected by b-cell chronic lymphocytic leukemia: results of a single-centre experience. Mediterr J Hematol Infect Dis 2013;5:e2013031.
57. Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014 Jan 8. [Epub ahead of print].
58. Badoux XC, Keating MJ, Wen S, et al. Lenalidomide as initial therapy of elderly patients with chronic lymphocytic leukemia. Blood 2011;118:3489–98.
59. Strati P, Keating MJ, Wierda WG, et al. Lenalidomide induces long-lasting responses in elderly patients with chronic lymphocytic leukemia. Blood 2013;122:734–7.
60. Moutouh-de Parseval LA, Weiss L, DeLap RJ, et al. Tumor lysis syndrome/tumor flare reaction in lenalidomide-treated chronic lymphocytic leukemia. J Clin Oncol 2007;25:5047.
61. James DF, Brown JR, Werner L, et al. Lenalidomide and rituximab for the initial treatment of patients with chronic lymphocytic leukemia (CLL): a multicenter study of the CLL Research Consortium. ASH Annual Meeting Abstracts 2011;118:291.
62. US Food and Drug Administration. FDA halts clinical trial of drug Revlimid (lenalidomide) for chronic lymphocytic leukemia due to safety concerns. Available at http://www.fda.gov/Drugs/DrugSafety/ucm361444.htm.
63. Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 2010;115:2578–85.
64. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013;369:32–42.
65. Farooqui M, Aue G, Valdez J, et al. Single agent ibrutinib (PCI-32765) achieves equally good and durable responses in chronic lymphocytic leukemia (CLL) patients with and without deletion 17p. Blood 2013;122:673.
66. Byrd JC, Furman RR, Coutre S, et al. The Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib (PCI-32765) monotherapy demonstrates long-term safety and durability of response in chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) patients in an open-label extension study. Blood 2013;122:4163.
67. Brown JR, Barrientos JC, Barr PM, et al. Ibrutinib in combination with bendamustine and rituximab is active and tolerable in patients with relapsed/refractory CLL/SLL: final results of a phase 1b study. ASH Annual Meeting Abstracts 2013.
68. O'Brien SM, Lamanna N, Kipps TJ, et al. A phase II study of the selective phosphatidylinositol 3-kinase delta (PI3K{delta}) inhibitor idelalisib (GS-1101) in combination with rituximab (R) in treatment-naive patients (pts) ≥ 65 years with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). J Clin Oncol 2013;31(15 Suppl); Abstract 7005.
69. Seymour JF, Davids MS, Pagel JM, et al. Bcl-2 Inhibitor ABT-199 (GDC-0199) monotherapy shows anti-tumor activity including complete remissions in high-risk relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL). Blood 2013;122:872.
70. Rituxumab and bendamustine hydrochloride, rituxumab and ibrutunib, or ibrutinib alone in treating older patients with previously untreated chronic lymphocytic leukemia. Available at http://clinicaltrials.gov/ct2/show/NCT01886872?term=ibrutinib+cll&rank=8.
71. A multicenter, open-label, phase 3 study of the bruton's tyrosine kinase inhibitor pci-32765 versus chlorambucil in patients 65 years of older with treatment-naive chronic lymphocytic leukemia or small lymphocytic lymphoma (RESONATE-2) Available at http://clinicaltrials.gov/ct2/show/NCT01722487?term=ibrutinib+cll&rank=12.
72. Ibrutinib and rituximab compared with fludarabine phosphate, cyclophosphamide, and rituxumab in treating patients with untreated chronic lymphocytic leukemia. Available at http://clinicaltrials.gov/ct2/show/NCT02048813?term=ibrutinib+cll&rank=2.
73. Strati P, Keating MJ, O'Brien SM, et al. Outcomes of first-line treatment for chronic lymphocytic leukemia (CLL) with 17p deletion. J Clin Oncol 2013;31(15 suppl): Abstract 7102.
1. Redaelli A, Laskin BL, Stephens JM, et al. The clinical and epidemiological burden of chronic lymphocytic leukaemia. Eur J Cancer Care (Engl) 2004;13:279–87.
2. Dores GM, Anderson WF, Curtis RE, et al. Chronic lymphocytic leukaemia and small lymphocytic lymphoma: overview of the descriptive epidemiology. Br J Haematol 2007;139:809–19.
3. Döhner H, Stilgenbauer S, Benner A, et al. Genomic aberrations and survival in chronic lymphocytic leukemia. N Engl J Med 2000;343:1910–6.
4. Zent CS, Kyasa MJ, Evans R, Schichman SA. Chronic lymphocytic leukemia incidence is substantially higher than estimated from tumor registry data. Cancer 2001;92:1325–30.
5. Byrd JC, Gribben JG, Peterson BL, et al. Select high-risk genetic features predict earlier progression following chemoimmunotherapy with fludarabine and rituximab in chronic lymphocytic leukemia: justification for risk-adapted therapy. J Clin Oncol 2006;24:437–43.
6. Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008;111:5446–56.
7. Rawstron AC, Bennett FL, O'Connor SJ, et al. Monoclonal B-cell lymphocytosis and chronic lymphocytic leukemia. N Engl J Med 2008;359:575–83.
8. Rai KR, Sawitsky A, Cronkite EP, et al. Clinical staging of chronic lymphocytic leukemia. Blood 1975;46:219–34.
9. Binet JL, Auquier A, Dighiero G, et al. A new prognostic classification of chronic lymphocytic leukemia derived from a multivariate survival analysis. Cancer 1981;48:198–206.
10. Binet JL, Lepoprier M, Dighiero G, et al. A clinical staging system for chronic lymphocytic leukemia: prognostic significance. Cancer 1977:40:855–64.
11. Eichhorst BF, Fischer K, Fink AM, et al. Limited clinical relevance of imaging techniques in the follow-up of patients with advanced chronic lymphocytic leukemia: results of a meta-analysis. Blood 2011;117:1817–21.
12. Hamblin TJ, Davis Z, Gardiner A, et al. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood 1999;94:1848–54.
13. Crespo M, Bosch F, Villamor N, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med 2003;348:
1764–75.
14. Hamblin TJ, Orchard JA, Ibbotson RE, et al. CD38 expression and immunoglobulin variable region mutations are independent prognostic variables in chronic lymphocytic leukemia, but CD38 expression may vary during the course of the disease. Blood 2002;99:1023–9.
15. Rassenti LZ, Kipps TJ. Clinical utility of assessing ZAP-70 and CD38 in chronic lymphocytic leukemia. Cytometry B Clin Cytom 2006;70:209–13.
16. Wierda WG, O'Brien S, Wang X, et al. Multivariable model for time to first treatment in patients with chronic lymphocytic leukemia. J Clin Oncol 2011;29:4088–95.
17. Schetelig J, van Biezen A, Brand R, et al. Allogeneic hematopoietic stem-cell transplantation for chronic lymphocytic leukemia with 17p deletion: a retrospective European Group for Blood and Marrow Transplantation analysis. J Clin Oncol 2008;26:5094–100.
18. Ding W, Ferrajoli A. Evidence-based mini-review: the role of alkylating agents in the initial treatment of chronic lymphocytic leukemia patients with the 11q deletion. Hematology Am Soc Hematol Educ Program 2010;2010:90–2.
19. Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet 2010;376:1164–74.
20. Badoux XC, Keating MJ, Wierda WG.What is the best frontline therapy for patients with CLL and 17p deletion? Curr Hematol Malig Rep 2011;6:36–46.
21. Dighiero G, Maloum K, Desablens B, et al. Chlorambucil in indolent chronic lymphocytic leukemia. French Cooperative Group on Chronic Lymphocytic Leukemia. N Engl J Med 1998;338:1506–14.
22. Chemotherapeutic options in chronic lymphocytic leukemia: a meta-analysis of the randomized trials. CLL Trialists' Collaborative Group. J Natl Cancer Inst 1999;91:861–8.
23. Morton LM, Curtis RE, Linet MS, et al. Second malignancy risks after non-Hodgkin's lymphoma and chronic lymphocytic leukemia: differences by lymphoma subtype. J Clin Oncol 2010;28:4935–44.
24. Shanafelt TD, Bowen D, Venkat C, et al. Quality of life in chronic lymphocytic leukemia: an international survey of 1482 patients. Br J Haematol 2007;139:255–64.
25. Baer MR, Stein RS, Dessypris EN. Chronic lymphocytic leukemia with hyperleukocytosis. The hyperviscosity syndrome. Cancer 1985;56:2865–9.
26. Gupta N, Kavuru S, Patel D, et al. Rituximab-based chemotherapy for steroid-refractory autoimmune hemolytic anemia of chronic lymphocytic leukemia. Leukemia 2002;16:2092–5.
27. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: non-hodgkin's lymphomas. Version 2.2013. Available at http://www.nccn.org/professionals/physician_gls/pdf/nhl.pdf.
28. Byrd JC, Rai K, Peterson BL, et al. Addition of rituximab to fludarabine may prolong progression-free survival and overall survival in patients with previously untreated chronic lymphocytic leukemia: an updated retrospective comparative analysis of CALGB 9712 and CALGB 9011. Blood 2005;105:49–53.
29. Byrd JC, Peterson BL, Morrison VA, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712). Blood 2003;101:6–14.
30. Keating MJ, O'Brien S, Albitar M, et al. Early results of a chemoimmunotherapy regimen of fludarabine, cyclophosphamide, and rituximab as initial therapy for chronic lymphocytic leukemia. J Clin Oncol 2005;23:4079–88.
31. Tam CS, O'Brien S, Wierda W, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood 2008;112:975–80.
32. Fischer K, Cramer P, Busch R, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol 2012;30:3209–16.
33. Catovsky D, Richards S, Matutes E, et al. Assessment of fludarabine plus cyclophosphamide for patients with chronic lymphocytic leukaemia (the LRF CLL4 Trial): a randomised controlled trial. Lancet 2007;370:230–9.
34. Knauf WU, Lissichkov T, Aldaoud A, et al. Phase III randomized study of bendamustine compared with chlorambucil in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol 2009;27:4378–84.
35. Woyach JA, Ruppert AS, Heerema NA, et al. Chemoimmunotherapy with fludarabine and rituximab produces extended overall survival and progression-free survival in chronic lymphocytic leukemia: long-term follow-up of CALGB study 9712. J Clin Oncol 2011;29:1349–55.
36. Fink AM, et al., Chemoimmunotherapy with fludarabine, cyclophosphamide, and rituximabversus bendamustine and rituximabin previously untreated and physically fit patientswith advanced chronic lymphocytic leukemia: results of a planned interim analysis of the CLL10 Trial, an international, randomized study of the German CLL Study Group (GCLLSG). Blood 2013;122:526.
37. Gandhi V, Plunkett W. Cellular and clinical pharmacology of fludarabine. Clin Pharmacokinet 2002;41:93–103.
38. Rosenwald A, Chuang EY, Davis RE, et al. Fludarabine treatment of patients with chronic lymphocytic leukemia induces a p53-dependent gene expression response. Blood 2004;104:1428–34.
39. Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: results of a large international study. Blood 2002;99:3554–61.
40. Lozanski G, Heerema NA, Flinn IW, et al. Alemtuzumab is an effective therapy for chronic lymphocytic leukemia with p53 mutations and deletions. Blood 2004;103:3278–81.
41. Osuji NC, Del Giudice I, Matutes E, et al, The efficacy of alemtuzumab for refractory chronic lymphocytic leukemia in relation to cytogenetic abnormalities of p53. Haematologica 2005;90:1435–6.
42. Stilgenbauer S, Zenz T, Winkler D, et al. Subcutaneous alemtuzumab in fludarabine-refractory chronic lymphocytic leukemia: clinical results and prognostic marker analyses from the CLL2H study of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol 2009;27:3994–4001.
43. Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol 2007;25:5616–23.
44. Lundin J, Kimby E, Björkholm M, et al. Phase II trial of subcutaneous anti-CD52 monoclonal antibody alemtuzumab (Campath-1H) as first-line treatment for patients with B-cell chronic lymphocytic leukemia (B-CLL). Blood 2002;100:768–73.
45. Genzyme. US Campath Distribution Program. Cambridge, MA: Genzyme. Available at http://www.campath.com/.
46. Thornton PD, Matutes E, Bosanquet AG, et al. High dose methylprednisolone can induce remissions in CLL patients with p53 abnormalities. Ann Hematol 2003;82:759–65.
47. Bowen DA, Call TG, Jenkins GD, et al. Methylprednisolone-rituximab is an effective salvage therapy for patients with relapsed chronic lymphocytic leukemia including those with unfavorable cytogenetic features. Leuk Lymphoma 2007;48:2412–7.
48. Castro JE, Sandoval-Sus JD, Bole J, et al. Rituximab in combination with high-dose methylprednisolone for the treatment of fludarabine refractory high-risk chronic lymphocytic leukemia. Leukemia 2008;22:2048–53.
49. Castro JE, James DF, Sandoval-Sus JD, et al. Rituximab in combination with high-dose methylprednisolone for the treatment of chronic lymphocytic leukemia. Leukemia 2009;23:1779–89.
50. Eichhorst B, Goede V, Hallek M. Treatment of elderly patients with chronic lymphocytic leukemia. Leuk Lymphoma 2009;50:171–8.
51. Foon KA, Boyiadzis M, Land SR, et al. Chemoimmunotherapy with low-dose fludarabine and cyclophosphamide and high dose rituximab in previously untreated patients with chronic lymphocytic leukemia. J Clin Oncol 2009;27:498–503.
52. Kay NE, Geyer SM, Call TG, et al. Combination chemoimmunotherapy with pentostatin, cyclophosphamide, and rituximab shows significant clinical activity with low accompanying toxicity in previously untreated B chronic lymphocytic leukemia. Blood 2007;109:405–11.
53. Shanafelt TD, Lin T, Geyer SM, et al. Pentostatin, cyclophosphamide, and rituximab regimen in older patients with chronic lymphocytic leukemia. Cancer 2007;109:2291–8.
54. Catovsky D, Else M, Richards S. Chlorambucil--still not bad: a reappraisal. Clin Lymphoma Myeloma Leuk 2011;11 Suppl 1:S2–6.
55. Eichhorst BF, Busch R, Stilgenbauer S, et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood 2009;114:3382–91.
56. Laurenti L, Vannata B, Innocenti I, et al. Chlorambucil plus rituximab as front-line therapy in elderly/unfit patients affected by b-cell chronic lymphocytic leukemia: results of a single-centre experience. Mediterr J Hematol Infect Dis 2013;5:e2013031.
57. Goede V, Fischer K, Busch R, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med 2014 Jan 8. [Epub ahead of print].
58. Badoux XC, Keating MJ, Wen S, et al. Lenalidomide as initial therapy of elderly patients with chronic lymphocytic leukemia. Blood 2011;118:3489–98.
59. Strati P, Keating MJ, Wierda WG, et al. Lenalidomide induces long-lasting responses in elderly patients with chronic lymphocytic leukemia. Blood 2013;122:734–7.
60. Moutouh-de Parseval LA, Weiss L, DeLap RJ, et al. Tumor lysis syndrome/tumor flare reaction in lenalidomide-treated chronic lymphocytic leukemia. J Clin Oncol 2007;25:5047.
61. James DF, Brown JR, Werner L, et al. Lenalidomide and rituximab for the initial treatment of patients with chronic lymphocytic leukemia (CLL): a multicenter study of the CLL Research Consortium. ASH Annual Meeting Abstracts 2011;118:291.
62. US Food and Drug Administration. FDA halts clinical trial of drug Revlimid (lenalidomide) for chronic lymphocytic leukemia due to safety concerns. Available at http://www.fda.gov/Drugs/DrugSafety/ucm361444.htm.
63. Friedberg JW, Sharman J, Sweetenham J, et al. Inhibition of Syk with fostamatinib disodium has significant clinical activity in non-Hodgkin lymphoma and chronic lymphocytic leukemia. Blood 2010;115:2578–85.
64. Byrd JC, Furman RR, Coutre SE, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013;369:32–42.
65. Farooqui M, Aue G, Valdez J, et al. Single agent ibrutinib (PCI-32765) achieves equally good and durable responses in chronic lymphocytic leukemia (CLL) patients with and without deletion 17p. Blood 2013;122:673.
66. Byrd JC, Furman RR, Coutre S, et al. The Bruton’s tyrosine kinase (BTK) inhibitor ibrutinib (PCI-32765) monotherapy demonstrates long-term safety and durability of response in chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL) patients in an open-label extension study. Blood 2013;122:4163.
67. Brown JR, Barrientos JC, Barr PM, et al. Ibrutinib in combination with bendamustine and rituximab is active and tolerable in patients with relapsed/refractory CLL/SLL: final results of a phase 1b study. ASH Annual Meeting Abstracts 2013.
68. O'Brien SM, Lamanna N, Kipps TJ, et al. A phase II study of the selective phosphatidylinositol 3-kinase delta (PI3K{delta}) inhibitor idelalisib (GS-1101) in combination with rituximab (R) in treatment-naive patients (pts) ≥ 65 years with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). J Clin Oncol 2013;31(15 Suppl); Abstract 7005.
69. Seymour JF, Davids MS, Pagel JM, et al. Bcl-2 Inhibitor ABT-199 (GDC-0199) monotherapy shows anti-tumor activity including complete remissions in high-risk relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL). Blood 2013;122:872.
70. Rituxumab and bendamustine hydrochloride, rituxumab and ibrutunib, or ibrutinib alone in treating older patients with previously untreated chronic lymphocytic leukemia. Available at http://clinicaltrials.gov/ct2/show/NCT01886872?term=ibrutinib+cll&rank=8.
71. A multicenter, open-label, phase 3 study of the bruton's tyrosine kinase inhibitor pci-32765 versus chlorambucil in patients 65 years of older with treatment-naive chronic lymphocytic leukemia or small lymphocytic lymphoma (RESONATE-2) Available at http://clinicaltrials.gov/ct2/show/NCT01722487?term=ibrutinib+cll&rank=12.
72. Ibrutinib and rituximab compared with fludarabine phosphate, cyclophosphamide, and rituxumab in treating patients with untreated chronic lymphocytic leukemia. Available at http://clinicaltrials.gov/ct2/show/NCT02048813?term=ibrutinib+cll&rank=2.
73. Strati P, Keating MJ, O'Brien SM, et al. Outcomes of first-line treatment for chronic lymphocytic leukemia (CLL) with 17p deletion. J Clin Oncol 2013;31(15 suppl): Abstract 7102.
Mutation testing aids CML treatment decisions
Patients with Ph+ CML-CP (Philadelphia chromosome–positive chronic myeloid leukemia, chronic phase) who fail to achieve and maintain treatment response at key milestones should be considered for mutation screening, based on data from the DASISION trial.
Patients with mutations had poor outcomes and high rates of treatment discontinuation in an extended 4-year minimum follow-up of patients in the trial; 14 of 17 dasatinib-treated patients and 14 of 18 imatinib-treated patients with mutations discontinued treatment. The primary reason for treatment discontinuation was protocol-defined disease progression (dasatinib, n = 11; imatinib, n = 8); patients with mutations accounted for 61% of discontinuations on dasatinib (n = 11/18) and 42% on imatinib (n = 8/19).
“With the introduction of generic imatinib into the market in 2016, choosing the most appropriate second-line tyrosine-kinase inhibitor for patients, based on factors such as mutation status, will become increasingly important,” Dr. Tim Hughes of the South Australian Health and Medical Research Institute in Adelaide and his colleagues wrote. Having the option to choose the most suitable second-line therapy may ensure improved outcomes and decreased health care costs.
In the DASISION (Dasatinib vs. Imatinib Study in Treatment-Naive CML-CP) trial, all participants had newly diagnosed Ph+ CML-CP; they were treated with dasatinib (n = 259) or imatinib (n = 260) and followed for a minimum of 3 years (Leukemia. 2015 Sep;29[9]:1832-8). Dr. Hughes and his colleagues conducted a retrospective study of the patients who were potentially at a higher risk for developing mutations. This included patients on treatment who had at least one clinically relevant event – no confirmed complete cytogenetic response (cCCyR) within 12 months, no major molecular response (MMR) within 12 months; a fivefold increase in BCR-ABL1 transcript levels with loss of MMR; loss of CCyR – and/or who discontinued treatment for any reason.
Screening identified only a small number of patients with mutations (dasatinib, n = 17; imatinib, n = 18). Those on dasatinib had a narrower spectrum of mutations (4 sites for dasatinib vs. 12 sites for imatinib), fewer phosphate-binding loop mutations (1 mutation for dasatinib vs, 9 mutations for imatinib), and fewer multiple mutations (1 patient on dasatinib vs. 6 patients on imatinib).
However, patients on dasatinib had a greater occurrence of T315I mutations (11 patients on dasatinib vs. 0 patients on imatinib). The researchers hypothesized that this finding resulted from differences in competitive advantage between mutant clones. For example, P-loop mutations Y253F, E255K were found to have higher transformation potency and proliferation rates, compared with T315I, even in the absence of BCR-ABL1 inhibitors. If one assumes that imatinib has lower activity than dasatinib against these mutations, then mutant clones with select P-loop mutations might expand more rapidly than clones with the T315I mutation when exposed to imatinib.
Consistent with this idea, T315I is less common than all P-loop mutations in CML-CP patients with imatinib resistance. In addition, dasatinib suppresses P-loop mutations to a greater extent than does T315I; therefore, T315I may be able to develop during dasatinib treatment with relatively little competition from rapidly proliferating clones.
“Dasatinib, nilotinib, bosutinib, and ponatinib have enabled many patients, including those with mutations, to overcome imatinib resistance; however, each lack[s] efficacy against a small number of different leukemic clones, and all except ponatinib lack efficacy against T315I,” the researchers wrote.
The study was sponsored by Bristol-Myers Squibb. Dr. Hughes reported receiving honoraria and research funding from ARIAD, the maker of ponatinib; Bristol-Myers Squibb, the maker of dasatinib; and Novartis, the maker of imatinib.
Patients with Ph+ CML-CP (Philadelphia chromosome–positive chronic myeloid leukemia, chronic phase) who fail to achieve and maintain treatment response at key milestones should be considered for mutation screening, based on data from the DASISION trial.
Patients with mutations had poor outcomes and high rates of treatment discontinuation in an extended 4-year minimum follow-up of patients in the trial; 14 of 17 dasatinib-treated patients and 14 of 18 imatinib-treated patients with mutations discontinued treatment. The primary reason for treatment discontinuation was protocol-defined disease progression (dasatinib, n = 11; imatinib, n = 8); patients with mutations accounted for 61% of discontinuations on dasatinib (n = 11/18) and 42% on imatinib (n = 8/19).
“With the introduction of generic imatinib into the market in 2016, choosing the most appropriate second-line tyrosine-kinase inhibitor for patients, based on factors such as mutation status, will become increasingly important,” Dr. Tim Hughes of the South Australian Health and Medical Research Institute in Adelaide and his colleagues wrote. Having the option to choose the most suitable second-line therapy may ensure improved outcomes and decreased health care costs.
In the DASISION (Dasatinib vs. Imatinib Study in Treatment-Naive CML-CP) trial, all participants had newly diagnosed Ph+ CML-CP; they were treated with dasatinib (n = 259) or imatinib (n = 260) and followed for a minimum of 3 years (Leukemia. 2015 Sep;29[9]:1832-8). Dr. Hughes and his colleagues conducted a retrospective study of the patients who were potentially at a higher risk for developing mutations. This included patients on treatment who had at least one clinically relevant event – no confirmed complete cytogenetic response (cCCyR) within 12 months, no major molecular response (MMR) within 12 months; a fivefold increase in BCR-ABL1 transcript levels with loss of MMR; loss of CCyR – and/or who discontinued treatment for any reason.
Screening identified only a small number of patients with mutations (dasatinib, n = 17; imatinib, n = 18). Those on dasatinib had a narrower spectrum of mutations (4 sites for dasatinib vs. 12 sites for imatinib), fewer phosphate-binding loop mutations (1 mutation for dasatinib vs, 9 mutations for imatinib), and fewer multiple mutations (1 patient on dasatinib vs. 6 patients on imatinib).
However, patients on dasatinib had a greater occurrence of T315I mutations (11 patients on dasatinib vs. 0 patients on imatinib). The researchers hypothesized that this finding resulted from differences in competitive advantage between mutant clones. For example, P-loop mutations Y253F, E255K were found to have higher transformation potency and proliferation rates, compared with T315I, even in the absence of BCR-ABL1 inhibitors. If one assumes that imatinib has lower activity than dasatinib against these mutations, then mutant clones with select P-loop mutations might expand more rapidly than clones with the T315I mutation when exposed to imatinib.
Consistent with this idea, T315I is less common than all P-loop mutations in CML-CP patients with imatinib resistance. In addition, dasatinib suppresses P-loop mutations to a greater extent than does T315I; therefore, T315I may be able to develop during dasatinib treatment with relatively little competition from rapidly proliferating clones.
“Dasatinib, nilotinib, bosutinib, and ponatinib have enabled many patients, including those with mutations, to overcome imatinib resistance; however, each lack[s] efficacy against a small number of different leukemic clones, and all except ponatinib lack efficacy against T315I,” the researchers wrote.
The study was sponsored by Bristol-Myers Squibb. Dr. Hughes reported receiving honoraria and research funding from ARIAD, the maker of ponatinib; Bristol-Myers Squibb, the maker of dasatinib; and Novartis, the maker of imatinib.
Patients with Ph+ CML-CP (Philadelphia chromosome–positive chronic myeloid leukemia, chronic phase) who fail to achieve and maintain treatment response at key milestones should be considered for mutation screening, based on data from the DASISION trial.
Patients with mutations had poor outcomes and high rates of treatment discontinuation in an extended 4-year minimum follow-up of patients in the trial; 14 of 17 dasatinib-treated patients and 14 of 18 imatinib-treated patients with mutations discontinued treatment. The primary reason for treatment discontinuation was protocol-defined disease progression (dasatinib, n = 11; imatinib, n = 8); patients with mutations accounted for 61% of discontinuations on dasatinib (n = 11/18) and 42% on imatinib (n = 8/19).
“With the introduction of generic imatinib into the market in 2016, choosing the most appropriate second-line tyrosine-kinase inhibitor for patients, based on factors such as mutation status, will become increasingly important,” Dr. Tim Hughes of the South Australian Health and Medical Research Institute in Adelaide and his colleagues wrote. Having the option to choose the most suitable second-line therapy may ensure improved outcomes and decreased health care costs.
In the DASISION (Dasatinib vs. Imatinib Study in Treatment-Naive CML-CP) trial, all participants had newly diagnosed Ph+ CML-CP; they were treated with dasatinib (n = 259) or imatinib (n = 260) and followed for a minimum of 3 years (Leukemia. 2015 Sep;29[9]:1832-8). Dr. Hughes and his colleagues conducted a retrospective study of the patients who were potentially at a higher risk for developing mutations. This included patients on treatment who had at least one clinically relevant event – no confirmed complete cytogenetic response (cCCyR) within 12 months, no major molecular response (MMR) within 12 months; a fivefold increase in BCR-ABL1 transcript levels with loss of MMR; loss of CCyR – and/or who discontinued treatment for any reason.
Screening identified only a small number of patients with mutations (dasatinib, n = 17; imatinib, n = 18). Those on dasatinib had a narrower spectrum of mutations (4 sites for dasatinib vs. 12 sites for imatinib), fewer phosphate-binding loop mutations (1 mutation for dasatinib vs, 9 mutations for imatinib), and fewer multiple mutations (1 patient on dasatinib vs. 6 patients on imatinib).
However, patients on dasatinib had a greater occurrence of T315I mutations (11 patients on dasatinib vs. 0 patients on imatinib). The researchers hypothesized that this finding resulted from differences in competitive advantage between mutant clones. For example, P-loop mutations Y253F, E255K were found to have higher transformation potency and proliferation rates, compared with T315I, even in the absence of BCR-ABL1 inhibitors. If one assumes that imatinib has lower activity than dasatinib against these mutations, then mutant clones with select P-loop mutations might expand more rapidly than clones with the T315I mutation when exposed to imatinib.
Consistent with this idea, T315I is less common than all P-loop mutations in CML-CP patients with imatinib resistance. In addition, dasatinib suppresses P-loop mutations to a greater extent than does T315I; therefore, T315I may be able to develop during dasatinib treatment with relatively little competition from rapidly proliferating clones.
“Dasatinib, nilotinib, bosutinib, and ponatinib have enabled many patients, including those with mutations, to overcome imatinib resistance; however, each lack[s] efficacy against a small number of different leukemic clones, and all except ponatinib lack efficacy against T315I,” the researchers wrote.
The study was sponsored by Bristol-Myers Squibb. Dr. Hughes reported receiving honoraria and research funding from ARIAD, the maker of ponatinib; Bristol-Myers Squibb, the maker of dasatinib; and Novartis, the maker of imatinib.
FROM LEUKEMIA
Key clinical point:Mutation testing may aid treatment selection in patients with chronic myeloid leukemia (CML) when selecting an alternative therapy because of treatment failure.
Major finding: Patients with mutations accounted for 61% of discontinuations on dasatinib (n = 11/18) and 42% on imatinib (n = 8/19).
Data source: A retrospective analysis of the DASISION trial results of 259 patients treated with dasatinib and 260 treated with imatinib.
Disclosures: The study was sponsored by Bristol-Myers Squibb. Dr. Hughes reported receiving honoraria and research funding from ARIAD, the maker of ponatinib; Bristol-Myers Squibb, the maker of dasatinib; and Novartis, the maker of imatinib.
Uptick in health care workers getting flu shots in 2014-2015 season
Overall, 77.3% of health care personnel reported receiving an influenza vaccination during the 2014-2015 season, with the highest vaccination coverage was reported in work sites with employer requirements for vaccination, according to an investigation published in MMWR (2015 Sep 18;64[36]:993-9).
Vaccination data came from an opt-in Internet panel survey conducted by Abt Associates for the Centers for Disease Control and Prevention, and included questions on demographic characteristics, occupation, work setting, self-reported influenza vaccination, and employer vaccination policies. Results from 1,914 survey responses were analyzed. The overall health care personnel (HCP) influenza vaccination coverage estimate for the 2014-2015 season was 77.3%, compared with 75.2% for the 2013-2014 season. When compared with the 2013-2014 season, coverage in 2014-2015 was higher among pharmacists (95.3% vs. 85.7%), assistants/aides (64.4% vs. 57.7%), and nonclinical personnel (75.2% vs. 68.6%). Coverage among other clinical personnel decreased from 87.4% in 2013-2014 to 81.3% in 2014-2015, while other categories experienced little change between the two time periods.
The team of researchers, led by Carla L. Black, Ph.D., of the National Center for Immunization and Respiratory Diseases, CDC, noted that among HCP whose employers did not require vaccination, vaccination coverage among those who worked in locations where their employer made vaccination available on-site at no cost for more than 1 day was 83.9%, compared with coverage of 73.6% among those who worked in locations where their employer made vaccination available at no cost for 1 day only, and 59.5% among those who worked in locations where their employer did not provide influenza vaccination on-site at no cost but actively promoted vaccination through other mechanisms.
“These findings support recommendations for a comprehensive strategy that includes easy access to vaccination at no cost on multiple days, along with promotion of vaccination, to increase HCP influenza vaccination coverage,” the authors wrote.
Read the full report here: http://www.cdc.gov/mmwr/.
Overall, 77.3% of health care personnel reported receiving an influenza vaccination during the 2014-2015 season, with the highest vaccination coverage was reported in work sites with employer requirements for vaccination, according to an investigation published in MMWR (2015 Sep 18;64[36]:993-9).
Vaccination data came from an opt-in Internet panel survey conducted by Abt Associates for the Centers for Disease Control and Prevention, and included questions on demographic characteristics, occupation, work setting, self-reported influenza vaccination, and employer vaccination policies. Results from 1,914 survey responses were analyzed. The overall health care personnel (HCP) influenza vaccination coverage estimate for the 2014-2015 season was 77.3%, compared with 75.2% for the 2013-2014 season. When compared with the 2013-2014 season, coverage in 2014-2015 was higher among pharmacists (95.3% vs. 85.7%), assistants/aides (64.4% vs. 57.7%), and nonclinical personnel (75.2% vs. 68.6%). Coverage among other clinical personnel decreased from 87.4% in 2013-2014 to 81.3% in 2014-2015, while other categories experienced little change between the two time periods.
The team of researchers, led by Carla L. Black, Ph.D., of the National Center for Immunization and Respiratory Diseases, CDC, noted that among HCP whose employers did not require vaccination, vaccination coverage among those who worked in locations where their employer made vaccination available on-site at no cost for more than 1 day was 83.9%, compared with coverage of 73.6% among those who worked in locations where their employer made vaccination available at no cost for 1 day only, and 59.5% among those who worked in locations where their employer did not provide influenza vaccination on-site at no cost but actively promoted vaccination through other mechanisms.
“These findings support recommendations for a comprehensive strategy that includes easy access to vaccination at no cost on multiple days, along with promotion of vaccination, to increase HCP influenza vaccination coverage,” the authors wrote.
Read the full report here: http://www.cdc.gov/mmwr/.
Overall, 77.3% of health care personnel reported receiving an influenza vaccination during the 2014-2015 season, with the highest vaccination coverage was reported in work sites with employer requirements for vaccination, according to an investigation published in MMWR (2015 Sep 18;64[36]:993-9).
Vaccination data came from an opt-in Internet panel survey conducted by Abt Associates for the Centers for Disease Control and Prevention, and included questions on demographic characteristics, occupation, work setting, self-reported influenza vaccination, and employer vaccination policies. Results from 1,914 survey responses were analyzed. The overall health care personnel (HCP) influenza vaccination coverage estimate for the 2014-2015 season was 77.3%, compared with 75.2% for the 2013-2014 season. When compared with the 2013-2014 season, coverage in 2014-2015 was higher among pharmacists (95.3% vs. 85.7%), assistants/aides (64.4% vs. 57.7%), and nonclinical personnel (75.2% vs. 68.6%). Coverage among other clinical personnel decreased from 87.4% in 2013-2014 to 81.3% in 2014-2015, while other categories experienced little change between the two time periods.
The team of researchers, led by Carla L. Black, Ph.D., of the National Center for Immunization and Respiratory Diseases, CDC, noted that among HCP whose employers did not require vaccination, vaccination coverage among those who worked in locations where their employer made vaccination available on-site at no cost for more than 1 day was 83.9%, compared with coverage of 73.6% among those who worked in locations where their employer made vaccination available at no cost for 1 day only, and 59.5% among those who worked in locations where their employer did not provide influenza vaccination on-site at no cost but actively promoted vaccination through other mechanisms.
“These findings support recommendations for a comprehensive strategy that includes easy access to vaccination at no cost on multiple days, along with promotion of vaccination, to increase HCP influenza vaccination coverage,” the authors wrote.
Read the full report here: http://www.cdc.gov/mmwr/.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Prion-like transmission of neurodegenerative pathology stirs concern
Recent developments regarding the nature of transmissible pathologic proteins in a variety of common neurodegenerative diseases have started to cause clinicians and public health experts to wonder if there is cause for concern.
A group from the National Hospital for Neurology & Neurosurgery at Queen Square, London, recently published a study presenting evidence that Alzheimer’s disease may have transmissible properties similar to traditionally regarded prion diseases such as Creutzfeldt-Jakob disease (Nature. 2015 Sep 10;525:247-50. doi:10.1038/nature15369). This conclusion was based upon the observation of cerebral and vascular amyloid-beta deposition in several patients who had received cadaveric growth hormone extracts ranging from 19 to 39 years earlier and who developed iatrogenic Creutzfeldt-Jakob disease (CJD). Amyloid deposition was also identified in the pituitary glands of patients with amyloid-beta pathology also felt to be iatrogenically transmitted.
This is not the first time, however, that analogies have been drawn between neurodegenerative diseases and prion-related diseases. Perhaps inspired by the evident connectivity of affected upper and lower motor neuronal populations in amyotrophic lateral sclerosis, previous investigators have sought cell-to-cell transmission of neurodegenerative pathology and have demonstrated this for amyloid as well as tau species in Alzheimer’s disease, and for synuclein species in Parkinson’s disease.
A recent article by Dr. Stanley Prusiner of the University of California, San Francisco, and his colleagues presented evidence that brain extracts from patients with multiple system atrophy (MSA) transmitted the characteristic MSA alpha-synuclein aggregates in the brains of transgenic mice as well as in cultured human embryonic kidney cells (Proc Natl Acad Sci U S A. 2015 Aug 31. doi:10.1073/pnas.1514475112).
An emerging theme from these recent and prior studies is that neurodegenerative diseases behave a lot like prion diseases, and although they follow a much slower time course, may spread through synaptic pathways as well as between people through prion-like mechanisms. CJD, while transmissible, is not “contagious,” and public messaging should draw this distinction in regard to neurodegenerative diseases. Nonetheless, further research is urgently needed given the implications of this work. Dr. Prusiner and his associates concluded, for example, that deep brain stimulation electrodes and related equipment should not be reused from Parkinson’s disease patients for fear of spreading the synucleinopathy from one person to another. Should there be restrictions with regard to any form of tissue transplantation or blood transfusion from patients with Parkinson’s disease or Alzheimer’s disease? Questions such as these need further clarification, and given the prevalence of these procedures, such research should be highly prioritized.
Dr. Caselli is professor of neurology at the Mayo Clinic, Scottsdale, Ariz. He also serves there as associate director and clinical core director of the Alzheimer’s Disease Center.
Recent developments regarding the nature of transmissible pathologic proteins in a variety of common neurodegenerative diseases have started to cause clinicians and public health experts to wonder if there is cause for concern.
A group from the National Hospital for Neurology & Neurosurgery at Queen Square, London, recently published a study presenting evidence that Alzheimer’s disease may have transmissible properties similar to traditionally regarded prion diseases such as Creutzfeldt-Jakob disease (Nature. 2015 Sep 10;525:247-50. doi:10.1038/nature15369). This conclusion was based upon the observation of cerebral and vascular amyloid-beta deposition in several patients who had received cadaveric growth hormone extracts ranging from 19 to 39 years earlier and who developed iatrogenic Creutzfeldt-Jakob disease (CJD). Amyloid deposition was also identified in the pituitary glands of patients with amyloid-beta pathology also felt to be iatrogenically transmitted.
This is not the first time, however, that analogies have been drawn between neurodegenerative diseases and prion-related diseases. Perhaps inspired by the evident connectivity of affected upper and lower motor neuronal populations in amyotrophic lateral sclerosis, previous investigators have sought cell-to-cell transmission of neurodegenerative pathology and have demonstrated this for amyloid as well as tau species in Alzheimer’s disease, and for synuclein species in Parkinson’s disease.
A recent article by Dr. Stanley Prusiner of the University of California, San Francisco, and his colleagues presented evidence that brain extracts from patients with multiple system atrophy (MSA) transmitted the characteristic MSA alpha-synuclein aggregates in the brains of transgenic mice as well as in cultured human embryonic kidney cells (Proc Natl Acad Sci U S A. 2015 Aug 31. doi:10.1073/pnas.1514475112).
An emerging theme from these recent and prior studies is that neurodegenerative diseases behave a lot like prion diseases, and although they follow a much slower time course, may spread through synaptic pathways as well as between people through prion-like mechanisms. CJD, while transmissible, is not “contagious,” and public messaging should draw this distinction in regard to neurodegenerative diseases. Nonetheless, further research is urgently needed given the implications of this work. Dr. Prusiner and his associates concluded, for example, that deep brain stimulation electrodes and related equipment should not be reused from Parkinson’s disease patients for fear of spreading the synucleinopathy from one person to another. Should there be restrictions with regard to any form of tissue transplantation or blood transfusion from patients with Parkinson’s disease or Alzheimer’s disease? Questions such as these need further clarification, and given the prevalence of these procedures, such research should be highly prioritized.
Dr. Caselli is professor of neurology at the Mayo Clinic, Scottsdale, Ariz. He also serves there as associate director and clinical core director of the Alzheimer’s Disease Center.
Recent developments regarding the nature of transmissible pathologic proteins in a variety of common neurodegenerative diseases have started to cause clinicians and public health experts to wonder if there is cause for concern.
A group from the National Hospital for Neurology & Neurosurgery at Queen Square, London, recently published a study presenting evidence that Alzheimer’s disease may have transmissible properties similar to traditionally regarded prion diseases such as Creutzfeldt-Jakob disease (Nature. 2015 Sep 10;525:247-50. doi:10.1038/nature15369). This conclusion was based upon the observation of cerebral and vascular amyloid-beta deposition in several patients who had received cadaveric growth hormone extracts ranging from 19 to 39 years earlier and who developed iatrogenic Creutzfeldt-Jakob disease (CJD). Amyloid deposition was also identified in the pituitary glands of patients with amyloid-beta pathology also felt to be iatrogenically transmitted.
This is not the first time, however, that analogies have been drawn between neurodegenerative diseases and prion-related diseases. Perhaps inspired by the evident connectivity of affected upper and lower motor neuronal populations in amyotrophic lateral sclerosis, previous investigators have sought cell-to-cell transmission of neurodegenerative pathology and have demonstrated this for amyloid as well as tau species in Alzheimer’s disease, and for synuclein species in Parkinson’s disease.
A recent article by Dr. Stanley Prusiner of the University of California, San Francisco, and his colleagues presented evidence that brain extracts from patients with multiple system atrophy (MSA) transmitted the characteristic MSA alpha-synuclein aggregates in the brains of transgenic mice as well as in cultured human embryonic kidney cells (Proc Natl Acad Sci U S A. 2015 Aug 31. doi:10.1073/pnas.1514475112).
An emerging theme from these recent and prior studies is that neurodegenerative diseases behave a lot like prion diseases, and although they follow a much slower time course, may spread through synaptic pathways as well as between people through prion-like mechanisms. CJD, while transmissible, is not “contagious,” and public messaging should draw this distinction in regard to neurodegenerative diseases. Nonetheless, further research is urgently needed given the implications of this work. Dr. Prusiner and his associates concluded, for example, that deep brain stimulation electrodes and related equipment should not be reused from Parkinson’s disease patients for fear of spreading the synucleinopathy from one person to another. Should there be restrictions with regard to any form of tissue transplantation or blood transfusion from patients with Parkinson’s disease or Alzheimer’s disease? Questions such as these need further clarification, and given the prevalence of these procedures, such research should be highly prioritized.
Dr. Caselli is professor of neurology at the Mayo Clinic, Scottsdale, Ariz. He also serves there as associate director and clinical core director of the Alzheimer’s Disease Center.
Executive attention deficits often persist in euthymic bipolar disorder
Euthymic bipolar disorder patients have impaired executive control (greater interference), reduced vigilance, slower overall reaction times, and poorer accuracy scores compared with healthy controls, a recent research article shows.
Lead author Andrea Marotta, Ph.D., of the University of Rome, and colleagues compared a sample of euthymic bipolar disorder patients and age-matched healthy controls, and had both groups complete the Attention Network Test for Interactions and Vigilance (ANTI-V), a neurocognitive test that assesses the efficiency of attentional networks and measures orienting, executive and alerting networks, and vigilance (tonic alerting). Although the bipolar disorder patients exhibited normal phasic alerting and orienting, they performed worse on alerting and executive control, the authors found.
“Our results show that deficits in executive attention and sustained attention often persist in [bipolar disorder] patients even after complete remission of affective symptoms, thus suggesting that cognitive enhancing treatments programmed to improve these deficits could contribute to improve their functional recovery,” they wrote.
Read the full article in Psychiatry Research (2015 Sept 30;229[1-2]:490-6. doi: 10.1016/jpsychres.2015.06.026).
Euthymic bipolar disorder patients have impaired executive control (greater interference), reduced vigilance, slower overall reaction times, and poorer accuracy scores compared with healthy controls, a recent research article shows.
Lead author Andrea Marotta, Ph.D., of the University of Rome, and colleagues compared a sample of euthymic bipolar disorder patients and age-matched healthy controls, and had both groups complete the Attention Network Test for Interactions and Vigilance (ANTI-V), a neurocognitive test that assesses the efficiency of attentional networks and measures orienting, executive and alerting networks, and vigilance (tonic alerting). Although the bipolar disorder patients exhibited normal phasic alerting and orienting, they performed worse on alerting and executive control, the authors found.
“Our results show that deficits in executive attention and sustained attention often persist in [bipolar disorder] patients even after complete remission of affective symptoms, thus suggesting that cognitive enhancing treatments programmed to improve these deficits could contribute to improve their functional recovery,” they wrote.
Read the full article in Psychiatry Research (2015 Sept 30;229[1-2]:490-6. doi: 10.1016/jpsychres.2015.06.026).
Euthymic bipolar disorder patients have impaired executive control (greater interference), reduced vigilance, slower overall reaction times, and poorer accuracy scores compared with healthy controls, a recent research article shows.
Lead author Andrea Marotta, Ph.D., of the University of Rome, and colleagues compared a sample of euthymic bipolar disorder patients and age-matched healthy controls, and had both groups complete the Attention Network Test for Interactions and Vigilance (ANTI-V), a neurocognitive test that assesses the efficiency of attentional networks and measures orienting, executive and alerting networks, and vigilance (tonic alerting). Although the bipolar disorder patients exhibited normal phasic alerting and orienting, they performed worse on alerting and executive control, the authors found.
“Our results show that deficits in executive attention and sustained attention often persist in [bipolar disorder] patients even after complete remission of affective symptoms, thus suggesting that cognitive enhancing treatments programmed to improve these deficits could contribute to improve their functional recovery,” they wrote.
Read the full article in Psychiatry Research (2015 Sept 30;229[1-2]:490-6. doi: 10.1016/jpsychres.2015.06.026).
FROM PSYCHIATRY RESEARCH
Do you answer patient e-mails?
Recently I received a lengthy e-mail from a very worried woman. She claimed to be an established patient in my office, which I had no way of confirming because she did not sign her message. She asked many questions about sexually transmitted diseases and how they might affect her and a new boyfriend.
I was undecided on how to reply – or even whether to reply at all – so I queried several dozen dermatology colleagues around the country, as well as a few physician friends and acquaintances in other specialties.
Responses varied all over the map – from “I never answer patient e-mails,” to “What harm could it do, she’s better off getting correct answers from you than incorrect answers from some ‘advocacy’ web site” – and everything in between.
Clearly, this is a controversial issue which will only get more controversial in the future, so I decided to look at what has been published on the subject.
It turns out that as early as 1998, a group of investigators asked this same question and designed a study to address it. (Eysenbach and Diepgen: “Responses to unsolicited patient e-mail requests for medical advice on the World Wide Web. JAMA. 1998;280[15]:1333-5). Posing as a fictitious patient, they sent e-mails to random dermatologists describing an acute dermatological problem, tallied the responses they received, and followed up with a questionnaire to responders and nonresponders alike.
As with my informal survey, the authors found what they termed “a striking lack of consensus” on how to deal with this situation: Fifty percent responded to the fictitious patient’s e-mail. Of those, 31% refused to give advice without seeing the patient, but 59% offered a diagnosis, and a third of that group went on to provide specific advice about therapy. In response to the questionnaire, 28% said that they tended not to answer any patient e-mails, 24% said they usually replied with a standard message, and 24% said they answer each request individually. The authors concluded that “standards for physician response to unsolicited patient e-mail are needed.”
Indeed; but my own survey suggests that, 17 years later, there is still nothing resembling a consensus on this issue. In the interim, several groups, including the American Medical Informatics Association, Medem, and the AMA have proposed guidelines; but none have been generally accepted.
Until such time as that happens, it seems advisable for each individual practice to take the time to adopt its own guidelines. For ideas, take a look at the examples I’ve listed, plus any others you can find. When you’re done, consider running your list past your lawyer to make sure you haven’t forgotten anything, and that there are no peculiar requirements in your state.
Your guidelines may be very simple (if you decide never to answer any queries) or very complex, depending on your situation and personal philosophy. But all guidelines should cover such issues of authentication of patient correspondents, informed consent of those patients, licensing jurisdiction (if you receive e-mails from states in which you are not licensed), and above all, confidentiality.
Contrary to popular belief, the Health Insurance Portability and Accountability Act (HIPAA) does not prohibit such communication, nor require that it be encrypted. The HIPAA website says, “Patients may initiate communications with a provider using e-mail. If this situation occurs, the health care provider can assume (unless the patient has explicitly stated otherwise) that e-mail communications are acceptable to the individual.”
Still, if the lack of encryption and other privacy safeguards makes you (or your patients) uncomfortable, encryption software can be added to your practice’s e-mail system. Enli (www.enli.net), Sigaba (www.sigaba.com), Tumbleweed (www.axway.com), Zix (www.zixcorp.com), and many other vendors sell encryption packages. (As always, I have no financial interest in any product or enterprise mentioned in this column.)
But rather than simply encrypting your e-mail, consider adopting web-based messaging. Patients enter your web site and send a message using an electronic template that you design. You (or a designated staffer) will be notified by regular e-mail when messages are received, and you can post a reply on a page that can be accessed only by the patient. Besides enhancing privacy and security, you can state your guidelines in plain English to preclude any misunderstanding of what you will and will not address online.
Web-based messaging services can be freestanding or incorporated into existing secure websites. Medfusion (www.medfusion.net), and RelayHealth (www.relayhealth.com) are among the leading vendors of secure messaging services.
As for the e-mail query which triggered all this: I responded, but I told the patient I could not provide specific answers to such personal questions over the Internet, particularly when they were asked anonymously; but I would be happy to address her concerns in person, in my office.
And now, I’m writing my guidelines.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Recently I received a lengthy e-mail from a very worried woman. She claimed to be an established patient in my office, which I had no way of confirming because she did not sign her message. She asked many questions about sexually transmitted diseases and how they might affect her and a new boyfriend.
I was undecided on how to reply – or even whether to reply at all – so I queried several dozen dermatology colleagues around the country, as well as a few physician friends and acquaintances in other specialties.
Responses varied all over the map – from “I never answer patient e-mails,” to “What harm could it do, she’s better off getting correct answers from you than incorrect answers from some ‘advocacy’ web site” – and everything in between.
Clearly, this is a controversial issue which will only get more controversial in the future, so I decided to look at what has been published on the subject.
It turns out that as early as 1998, a group of investigators asked this same question and designed a study to address it. (Eysenbach and Diepgen: “Responses to unsolicited patient e-mail requests for medical advice on the World Wide Web. JAMA. 1998;280[15]:1333-5). Posing as a fictitious patient, they sent e-mails to random dermatologists describing an acute dermatological problem, tallied the responses they received, and followed up with a questionnaire to responders and nonresponders alike.
As with my informal survey, the authors found what they termed “a striking lack of consensus” on how to deal with this situation: Fifty percent responded to the fictitious patient’s e-mail. Of those, 31% refused to give advice without seeing the patient, but 59% offered a diagnosis, and a third of that group went on to provide specific advice about therapy. In response to the questionnaire, 28% said that they tended not to answer any patient e-mails, 24% said they usually replied with a standard message, and 24% said they answer each request individually. The authors concluded that “standards for physician response to unsolicited patient e-mail are needed.”
Indeed; but my own survey suggests that, 17 years later, there is still nothing resembling a consensus on this issue. In the interim, several groups, including the American Medical Informatics Association, Medem, and the AMA have proposed guidelines; but none have been generally accepted.
Until such time as that happens, it seems advisable for each individual practice to take the time to adopt its own guidelines. For ideas, take a look at the examples I’ve listed, plus any others you can find. When you’re done, consider running your list past your lawyer to make sure you haven’t forgotten anything, and that there are no peculiar requirements in your state.
Your guidelines may be very simple (if you decide never to answer any queries) or very complex, depending on your situation and personal philosophy. But all guidelines should cover such issues of authentication of patient correspondents, informed consent of those patients, licensing jurisdiction (if you receive e-mails from states in which you are not licensed), and above all, confidentiality.
Contrary to popular belief, the Health Insurance Portability and Accountability Act (HIPAA) does not prohibit such communication, nor require that it be encrypted. The HIPAA website says, “Patients may initiate communications with a provider using e-mail. If this situation occurs, the health care provider can assume (unless the patient has explicitly stated otherwise) that e-mail communications are acceptable to the individual.”
Still, if the lack of encryption and other privacy safeguards makes you (or your patients) uncomfortable, encryption software can be added to your practice’s e-mail system. Enli (www.enli.net), Sigaba (www.sigaba.com), Tumbleweed (www.axway.com), Zix (www.zixcorp.com), and many other vendors sell encryption packages. (As always, I have no financial interest in any product or enterprise mentioned in this column.)
But rather than simply encrypting your e-mail, consider adopting web-based messaging. Patients enter your web site and send a message using an electronic template that you design. You (or a designated staffer) will be notified by regular e-mail when messages are received, and you can post a reply on a page that can be accessed only by the patient. Besides enhancing privacy and security, you can state your guidelines in plain English to preclude any misunderstanding of what you will and will not address online.
Web-based messaging services can be freestanding or incorporated into existing secure websites. Medfusion (www.medfusion.net), and RelayHealth (www.relayhealth.com) are among the leading vendors of secure messaging services.
As for the e-mail query which triggered all this: I responded, but I told the patient I could not provide specific answers to such personal questions over the Internet, particularly when they were asked anonymously; but I would be happy to address her concerns in person, in my office.
And now, I’m writing my guidelines.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Recently I received a lengthy e-mail from a very worried woman. She claimed to be an established patient in my office, which I had no way of confirming because she did not sign her message. She asked many questions about sexually transmitted diseases and how they might affect her and a new boyfriend.
I was undecided on how to reply – or even whether to reply at all – so I queried several dozen dermatology colleagues around the country, as well as a few physician friends and acquaintances in other specialties.
Responses varied all over the map – from “I never answer patient e-mails,” to “What harm could it do, she’s better off getting correct answers from you than incorrect answers from some ‘advocacy’ web site” – and everything in between.
Clearly, this is a controversial issue which will only get more controversial in the future, so I decided to look at what has been published on the subject.
It turns out that as early as 1998, a group of investigators asked this same question and designed a study to address it. (Eysenbach and Diepgen: “Responses to unsolicited patient e-mail requests for medical advice on the World Wide Web. JAMA. 1998;280[15]:1333-5). Posing as a fictitious patient, they sent e-mails to random dermatologists describing an acute dermatological problem, tallied the responses they received, and followed up with a questionnaire to responders and nonresponders alike.
As with my informal survey, the authors found what they termed “a striking lack of consensus” on how to deal with this situation: Fifty percent responded to the fictitious patient’s e-mail. Of those, 31% refused to give advice without seeing the patient, but 59% offered a diagnosis, and a third of that group went on to provide specific advice about therapy. In response to the questionnaire, 28% said that they tended not to answer any patient e-mails, 24% said they usually replied with a standard message, and 24% said they answer each request individually. The authors concluded that “standards for physician response to unsolicited patient e-mail are needed.”
Indeed; but my own survey suggests that, 17 years later, there is still nothing resembling a consensus on this issue. In the interim, several groups, including the American Medical Informatics Association, Medem, and the AMA have proposed guidelines; but none have been generally accepted.
Until such time as that happens, it seems advisable for each individual practice to take the time to adopt its own guidelines. For ideas, take a look at the examples I’ve listed, plus any others you can find. When you’re done, consider running your list past your lawyer to make sure you haven’t forgotten anything, and that there are no peculiar requirements in your state.
Your guidelines may be very simple (if you decide never to answer any queries) or very complex, depending on your situation and personal philosophy. But all guidelines should cover such issues of authentication of patient correspondents, informed consent of those patients, licensing jurisdiction (if you receive e-mails from states in which you are not licensed), and above all, confidentiality.
Contrary to popular belief, the Health Insurance Portability and Accountability Act (HIPAA) does not prohibit such communication, nor require that it be encrypted. The HIPAA website says, “Patients may initiate communications with a provider using e-mail. If this situation occurs, the health care provider can assume (unless the patient has explicitly stated otherwise) that e-mail communications are acceptable to the individual.”
Still, if the lack of encryption and other privacy safeguards makes you (or your patients) uncomfortable, encryption software can be added to your practice’s e-mail system. Enli (www.enli.net), Sigaba (www.sigaba.com), Tumbleweed (www.axway.com), Zix (www.zixcorp.com), and many other vendors sell encryption packages. (As always, I have no financial interest in any product or enterprise mentioned in this column.)
But rather than simply encrypting your e-mail, consider adopting web-based messaging. Patients enter your web site and send a message using an electronic template that you design. You (or a designated staffer) will be notified by regular e-mail when messages are received, and you can post a reply on a page that can be accessed only by the patient. Besides enhancing privacy and security, you can state your guidelines in plain English to preclude any misunderstanding of what you will and will not address online.
Web-based messaging services can be freestanding or incorporated into existing secure websites. Medfusion (www.medfusion.net), and RelayHealth (www.relayhealth.com) are among the leading vendors of secure messaging services.
As for the e-mail query which triggered all this: I responded, but I told the patient I could not provide specific answers to such personal questions over the Internet, particularly when they were asked anonymously; but I would be happy to address her concerns in person, in my office.
And now, I’m writing my guidelines.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].