User login
Do statins increase the risk of developing diabetes?
Yes. Statin therapy produces a small increase in the incidence of diabetes: one additional case per 255 patients taking statins over 4 years (strength of recommendation [SOR]: A, meta-analysis). Intensive statin therapy, compared with moderate therapy, produces an additional 2 cases of diabetes per 1000 patient years (SOR: B, meta-analysis with significant heterogeneity among trials).
EVIDENCE SUMMARY
A meta-analysis of 13 randomized, placebo or standard of care-controlled statin trials (113,148 patients, 81% without diabetes at enrollment, mean ages 55-76 years) found that statin therapy increased the incidence of diabetes by 9% over 4 years (odds ratio [OR]=1.09; 95% confidence interval [CI], 1.02-1.17), or one additional case per 255 patients.1 The increased risk was similar for lipophilic (pravastatin, rosuvastatin) and hydrophilic (atorvastatin, simvastatin, lovastatin) statins, although the analysis wasn’t adjusted for doses used.
In a meta-regression analysis, baseline body mass index or percentage change in low-density lipoprotein cholesterol didn’t appear to confer additional risk. The risk of diabetes with statins was generally higher in studies with older patients (data given graphically).
Higher statin doses mean higher risk
A meta-analysis of 5 placebo and standard-of-care randomized controlled trials (39,612 patients, 83% without diabetes at enrollment, mean age 58-64 years) found that the risk of diabetes was higher with higher-dose statins.2 Therapy with atorvastatin 80 mg or simvastatin 40 to 80 mg was defined as intensive. Treatment with simvastatin 20 to 40 mg, atorvastatin 10 mg, or pravastatin 40 mg was defined as moderate.
At a mean follow-up of 4.9 years, intensive statin therapy was associated with a higher risk of developing diabetes than moderate therapy (OR=1.12; 95% CI, 1.04-1.22) with 2 additional cases of diabetes per 1000 patient-years in the intensive therapy group. The authors noted significant heterogeneity between trials with regard to major cardiovascular events.
Similar results were found in a subsequent population-based cohort study of 471,250 nondiabetic patients older than 66 years who were newly prescribed a statin.3 The study authors used the incidence of new diabetes in patients taking pravastatin as the baseline, since it had been associated with reduced rates of diabetes in a large cardiovascular prevention trial.4 Without adjusting for dose, patients were at significantly higher risk of diabetes if prescribed atorvastatin (hazard ratio [HR]=1.22; 95% CI, 1.15-1.29), rosuvastatin (HR=1.18; 95% CI, 1.10-1.26), or simvastatin (HR=1.10; 95% CI, 1.04-1.17) compared with pravastatin. The risk with fluvastatin and lovastatin was similar to pravastatin.
A subanalysis that compared moderate- and high-dose statin therapy with low-dose therapy (atorvastatin <20 mg, rosuvastatin <10 mg, simvastatin <80 mg, or any dose of fluvastatin, lovastatin, or pravastatin) found a 22% increased risk of diabetes (HR=1.22; 95% CI, 1.19-1.26) for moderate-dose therapy (atorvastatin 20-79 mg, rosuvastatin 10-39 mg, or simvastatin >80 mg) and a 30% increased risk (HR=1.3; 95% CI, 1.2-1.4) for high-dose therapy (atorvastatin ≥80 mg or rosuvastatin ≥40 mg).
A cohort trial also shows increased diabetes risk
A smaller subsequent cohort trial based on data from Taiwan National Health Insurance records compared 8412 nondiabetic adult patients (mean age 63 years) taking statins with 33,648 age- and risk-matched controls not taking statins over a mean duration of 7.2 years.5 Statin use was associated with a 15% higher risk of developing diabetes (HR=1.15; 95% CI, 1.08-1.22).
RECOMMENDATIONS
The 2013 American College of Cardiology/American Heart Association guidelines for lipid-lowering therapy recommend that patients taking statins be screened for diabetes according to current screening recommendations.6 The guidelines advise encouraging patients who develop diabetes while on statin therapy to adhere to a heart-healthy dietary pattern, engage in physical activity, achieve and maintain a healthy body weight, cease tobacco use, and continue statin therapy to reduce the risk of cardiovascular events.
1. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
2. Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderatedose statin therapy: a meta-analysis. JAMA. 2011;305:2556-2564.
3. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population-based study. BMJ. 2013;346:f2610.
4. Freeman DJ, Morrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001;103:357-362.
5. Wang KL, Liu CJ, Chao TF, et al. Statins, risk of diabetes and implications on outcomes in the general population. J Am Coll Cardiol. 2012;60:1231-1238.
6. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1-S45.
Yes. Statin therapy produces a small increase in the incidence of diabetes: one additional case per 255 patients taking statins over 4 years (strength of recommendation [SOR]: A, meta-analysis). Intensive statin therapy, compared with moderate therapy, produces an additional 2 cases of diabetes per 1000 patient years (SOR: B, meta-analysis with significant heterogeneity among trials).
EVIDENCE SUMMARY
A meta-analysis of 13 randomized, placebo or standard of care-controlled statin trials (113,148 patients, 81% without diabetes at enrollment, mean ages 55-76 years) found that statin therapy increased the incidence of diabetes by 9% over 4 years (odds ratio [OR]=1.09; 95% confidence interval [CI], 1.02-1.17), or one additional case per 255 patients.1 The increased risk was similar for lipophilic (pravastatin, rosuvastatin) and hydrophilic (atorvastatin, simvastatin, lovastatin) statins, although the analysis wasn’t adjusted for doses used.
In a meta-regression analysis, baseline body mass index or percentage change in low-density lipoprotein cholesterol didn’t appear to confer additional risk. The risk of diabetes with statins was generally higher in studies with older patients (data given graphically).
Higher statin doses mean higher risk
A meta-analysis of 5 placebo and standard-of-care randomized controlled trials (39,612 patients, 83% without diabetes at enrollment, mean age 58-64 years) found that the risk of diabetes was higher with higher-dose statins.2 Therapy with atorvastatin 80 mg or simvastatin 40 to 80 mg was defined as intensive. Treatment with simvastatin 20 to 40 mg, atorvastatin 10 mg, or pravastatin 40 mg was defined as moderate.
At a mean follow-up of 4.9 years, intensive statin therapy was associated with a higher risk of developing diabetes than moderate therapy (OR=1.12; 95% CI, 1.04-1.22) with 2 additional cases of diabetes per 1000 patient-years in the intensive therapy group. The authors noted significant heterogeneity between trials with regard to major cardiovascular events.
Similar results were found in a subsequent population-based cohort study of 471,250 nondiabetic patients older than 66 years who were newly prescribed a statin.3 The study authors used the incidence of new diabetes in patients taking pravastatin as the baseline, since it had been associated with reduced rates of diabetes in a large cardiovascular prevention trial.4 Without adjusting for dose, patients were at significantly higher risk of diabetes if prescribed atorvastatin (hazard ratio [HR]=1.22; 95% CI, 1.15-1.29), rosuvastatin (HR=1.18; 95% CI, 1.10-1.26), or simvastatin (HR=1.10; 95% CI, 1.04-1.17) compared with pravastatin. The risk with fluvastatin and lovastatin was similar to pravastatin.
A subanalysis that compared moderate- and high-dose statin therapy with low-dose therapy (atorvastatin <20 mg, rosuvastatin <10 mg, simvastatin <80 mg, or any dose of fluvastatin, lovastatin, or pravastatin) found a 22% increased risk of diabetes (HR=1.22; 95% CI, 1.19-1.26) for moderate-dose therapy (atorvastatin 20-79 mg, rosuvastatin 10-39 mg, or simvastatin >80 mg) and a 30% increased risk (HR=1.3; 95% CI, 1.2-1.4) for high-dose therapy (atorvastatin ≥80 mg or rosuvastatin ≥40 mg).
A cohort trial also shows increased diabetes risk
A smaller subsequent cohort trial based on data from Taiwan National Health Insurance records compared 8412 nondiabetic adult patients (mean age 63 years) taking statins with 33,648 age- and risk-matched controls not taking statins over a mean duration of 7.2 years.5 Statin use was associated with a 15% higher risk of developing diabetes (HR=1.15; 95% CI, 1.08-1.22).
RECOMMENDATIONS
The 2013 American College of Cardiology/American Heart Association guidelines for lipid-lowering therapy recommend that patients taking statins be screened for diabetes according to current screening recommendations.6 The guidelines advise encouraging patients who develop diabetes while on statin therapy to adhere to a heart-healthy dietary pattern, engage in physical activity, achieve and maintain a healthy body weight, cease tobacco use, and continue statin therapy to reduce the risk of cardiovascular events.
Yes. Statin therapy produces a small increase in the incidence of diabetes: one additional case per 255 patients taking statins over 4 years (strength of recommendation [SOR]: A, meta-analysis). Intensive statin therapy, compared with moderate therapy, produces an additional 2 cases of diabetes per 1000 patient years (SOR: B, meta-analysis with significant heterogeneity among trials).
EVIDENCE SUMMARY
A meta-analysis of 13 randomized, placebo or standard of care-controlled statin trials (113,148 patients, 81% without diabetes at enrollment, mean ages 55-76 years) found that statin therapy increased the incidence of diabetes by 9% over 4 years (odds ratio [OR]=1.09; 95% confidence interval [CI], 1.02-1.17), or one additional case per 255 patients.1 The increased risk was similar for lipophilic (pravastatin, rosuvastatin) and hydrophilic (atorvastatin, simvastatin, lovastatin) statins, although the analysis wasn’t adjusted for doses used.
In a meta-regression analysis, baseline body mass index or percentage change in low-density lipoprotein cholesterol didn’t appear to confer additional risk. The risk of diabetes with statins was generally higher in studies with older patients (data given graphically).
Higher statin doses mean higher risk
A meta-analysis of 5 placebo and standard-of-care randomized controlled trials (39,612 patients, 83% without diabetes at enrollment, mean age 58-64 years) found that the risk of diabetes was higher with higher-dose statins.2 Therapy with atorvastatin 80 mg or simvastatin 40 to 80 mg was defined as intensive. Treatment with simvastatin 20 to 40 mg, atorvastatin 10 mg, or pravastatin 40 mg was defined as moderate.
At a mean follow-up of 4.9 years, intensive statin therapy was associated with a higher risk of developing diabetes than moderate therapy (OR=1.12; 95% CI, 1.04-1.22) with 2 additional cases of diabetes per 1000 patient-years in the intensive therapy group. The authors noted significant heterogeneity between trials with regard to major cardiovascular events.
Similar results were found in a subsequent population-based cohort study of 471,250 nondiabetic patients older than 66 years who were newly prescribed a statin.3 The study authors used the incidence of new diabetes in patients taking pravastatin as the baseline, since it had been associated with reduced rates of diabetes in a large cardiovascular prevention trial.4 Without adjusting for dose, patients were at significantly higher risk of diabetes if prescribed atorvastatin (hazard ratio [HR]=1.22; 95% CI, 1.15-1.29), rosuvastatin (HR=1.18; 95% CI, 1.10-1.26), or simvastatin (HR=1.10; 95% CI, 1.04-1.17) compared with pravastatin. The risk with fluvastatin and lovastatin was similar to pravastatin.
A subanalysis that compared moderate- and high-dose statin therapy with low-dose therapy (atorvastatin <20 mg, rosuvastatin <10 mg, simvastatin <80 mg, or any dose of fluvastatin, lovastatin, or pravastatin) found a 22% increased risk of diabetes (HR=1.22; 95% CI, 1.19-1.26) for moderate-dose therapy (atorvastatin 20-79 mg, rosuvastatin 10-39 mg, or simvastatin >80 mg) and a 30% increased risk (HR=1.3; 95% CI, 1.2-1.4) for high-dose therapy (atorvastatin ≥80 mg or rosuvastatin ≥40 mg).
A cohort trial also shows increased diabetes risk
A smaller subsequent cohort trial based on data from Taiwan National Health Insurance records compared 8412 nondiabetic adult patients (mean age 63 years) taking statins with 33,648 age- and risk-matched controls not taking statins over a mean duration of 7.2 years.5 Statin use was associated with a 15% higher risk of developing diabetes (HR=1.15; 95% CI, 1.08-1.22).
RECOMMENDATIONS
The 2013 American College of Cardiology/American Heart Association guidelines for lipid-lowering therapy recommend that patients taking statins be screened for diabetes according to current screening recommendations.6 The guidelines advise encouraging patients who develop diabetes while on statin therapy to adhere to a heart-healthy dietary pattern, engage in physical activity, achieve and maintain a healthy body weight, cease tobacco use, and continue statin therapy to reduce the risk of cardiovascular events.
1. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
2. Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderatedose statin therapy: a meta-analysis. JAMA. 2011;305:2556-2564.
3. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population-based study. BMJ. 2013;346:f2610.
4. Freeman DJ, Morrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001;103:357-362.
5. Wang KL, Liu CJ, Chao TF, et al. Statins, risk of diabetes and implications on outcomes in the general population. J Am Coll Cardiol. 2012;60:1231-1238.
6. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1-S45.
1. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet. 2010;375:735-742.
2. Preiss D, Seshasai SR, Welsh P, et al. Risk of incident diabetes with intensive-dose compared with moderatedose statin therapy: a meta-analysis. JAMA. 2011;305:2556-2564.
3. Carter AA, Gomes T, Camacho X, et al. Risk of incident diabetes among patients treated with statins: population-based study. BMJ. 2013;346:f2610.
4. Freeman DJ, Morrie J, Sattar N, et al. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001;103:357-362.
5. Wang KL, Liu CJ, Chao TF, et al. Statins, risk of diabetes and implications on outcomes in the general population. J Am Coll Cardiol. 2012;60:1231-1238.
6. Stone NJ, Robinson JG, Lichtenstein AH, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1-S45.
Evidence-based answers from the Family Physicians Inquiries Network
Headache • fatigue • blurred vision • Dx?
THE CASE
One month after moving into her mother’s apartment, a 27-year-old woman sought care at our clinic for fatigue, headache, blurred vision, nausea, and morning vomiting. She had weakness and difficulty sleeping, but denied any fever, rashes, neck stiffness, recent travel, trauma, or tobacco or illicit drug use. She did, however, have a 6-year history of migraines. Her physical exam was normal. She was sent home with a prescription for tramadol 50 mg bid for her headaches.
The patient subsequently went to the emergency department 3 times for the same complaints; none of the treatments she received there (mostly acetaminophen with codeine) relieved her symptoms. Three weeks later she returned to our clinic. She was distressed that the symptoms hadn’t gone away, and noted that her family was now experiencing similar symptoms.
Her temperature was 98.1°F (36.7°C), blood pressure was 131/88 mm Hg, pulse was 85 beats/min, and respiratory rate was 18 breaths/min. Physical and neurologic exams were normal.
THE DIAGNOSIS
Although most of the patient’s lab test results were within normal ranges, her carboxyhemoglobin (COHb) level was 4.2%. COHb levels of >2% to 3% in nonsmokers or >9% to 10% in smokers suggest carbon monoxide (CO) poisoning.1,2 Based on this finding and our patient’s symptoms, we diagnosed unintentional CO poisoning. We recommended that she and her mother vacate the apartment and have it inspected.
DISCUSSION
CO is the leading cause of poisoning mortality in the United States, and causes half of all fatal poisonings worldwide.1,3,4 It is a colorless, odorless, and tasteless gas that is produced by the incomplete combustion of carbon-based products, such as coal or gas.5,6 Exposure can occur from car exhaust fumes, faulty room heaters, and other sources (TABLE 1).6 The incidence of CO poisoning is higher during the winter months and after natural disasters. Individuals who have a lowered oxygen capacity, such as older adults, pregnant women (and their fetuses), infants, and patients with anemia, cardiovascular disease, or cerebrovascular disease, are more susceptible to CO poisoning.5,6
COHb, a stable complex of CO that forms in red blood cells when CO is inhaled, impairs oxygen delivery and peripheral utilization, resulting in cellular hypoxia.1 Signs and symptoms of CO poisoning are nonspecific and require a high degree of clinical suspicion for early diagnosis and treatment. Although cherry-red lips, peripheral cyanosis, and retinal hemorrhages are often described as “classic” symptoms of CO poisoning, these are rarely seen.6 The most common symptoms are actually headache (90%), dizziness (82%), and weakness (53%).7 Other symptoms include nausea, vomiting, confusion, visual disturbances, loss of consciousness, angina, seizure, and fatigue.6,7 Symptoms of chronic CO poisoning may differ from those of acute poisoning and can include chronic fatigue, neuropathy, and memory deficit.8
The differential diagnosis for CO poisoning includes flu-like syndrome/influenza/other viral illnesses, migraine or tension headaches, depression, transient ischemic attack, encephalitis, coronary artery disease, gastroenteritis or food poisoning, seizures, and dysrhythmias.1,4 Lab testing for COHb can help narrow the diagnosis. CO poisoning can be classified as mild, moderate, or severe based on COHb levels and the patient’s signs and symptoms (TABLE 2).6 However, COHb level is a poor predictor of clinical presentation and should not be used to dictate management.2,7
Oxygen therapy is the recommended treatment
Early treatment with supplemental oxygen is recommended to reduce the length of time red blood cells are exposed to CO.1 A COHb level >25% is the criterion for hyperbaric oxygen therapy.1,3 Patients should receive treatment until their symptoms become less intense.
Delayed neuropsychiatric sequelae (DNS) can occur in up to one-third of patients with acute CO poisoning more than a month after apparent recovery.1,6,9 DNS symptoms include cognitive changes, emotional lability, visual disturbances, disorientation, depression, dementia, psychotic behavior, parkinsonism, amnesia, and incontinence.1,6,9 Approximately 50% to 75% of patients with DNS recover spontaneously within a year with symptomatic treatment.1,6,9
Our patient
After recommending that our patient (and her mother) leave the apartment and have it inspected, we later learned that the fire department was unable to determine the source of the CO. A CO detector was installed and our patient was advised to keep the windows in the apartment open to allow for adequate oxygen flow. One month later she returned to our clinic and reported that her symptoms resolved; serum COHb was negative upon repeat lab tests.
THE TAKEAWAY
Patients who present with headaches, dizziness and/or fatigue should be evaluated for CO poisoning. The patient’s environmental history should be reviewed carefully, especially because CO poisoning is more common during the winter months. Oxygen therapy is the mainstay of treatment. Up to one-third of patients with acute poisoning may develop delayed neuropsychiatric sequelae, including cognitive changes, emotional lability, visual disturbances, disorientation, and depression, that may resolve within one year.
1. Nikkanen H, Skolnik A. Diagnosis and management of carbon monoxide poisoning in the emergency department. Emerg Med Pract. 2011;13:1-14.
2. Hampson NB, Hauff NM. Carboxyhemoglobin levels in carbon monoxide poisoning: do they correlate with the clinical picture? Am J Emerg Med. 2008;26:665-669.
3. Kao LW, Nañagas KA. Toxicity associated with carbon monoxide. Clin Lab Med. 2006;26:99-125.
4. Varon J, Marik PE, Fromm RE Jr, et al. Carbon monoxide poisoning: a review for clinicians. J Emerg Med. 1999;17:87-93.
5. Harper A, Croft-Baker J. Carbon monoxide poisoning: undetected by both patients and their doctors. Age Ageing. 2004;33:105-109.
6. Smollin C, Olson K. Carbon monoxide poisoning (acute). BMJ Clin Evid. 2010;2010. pii:2103.
7. Wright J. Chronic and occult carbon monoxide poisoning: we don’t know what we’re missing. Emerg Med J. 2002;19:366-390.
8. Weaver LK. Clinical practice. Carbon monoxide poisoning. N Engl J Med. 2009;360:1217-1225.
9. Bhatia R, Chacko F, Lal V, et al. Reversible delayed neuropsychiatric syndrome following acute carbon monoxide exposure. Indian J Occup Environ Med. 2007;11:80-82.
THE CASE
One month after moving into her mother’s apartment, a 27-year-old woman sought care at our clinic for fatigue, headache, blurred vision, nausea, and morning vomiting. She had weakness and difficulty sleeping, but denied any fever, rashes, neck stiffness, recent travel, trauma, or tobacco or illicit drug use. She did, however, have a 6-year history of migraines. Her physical exam was normal. She was sent home with a prescription for tramadol 50 mg bid for her headaches.
The patient subsequently went to the emergency department 3 times for the same complaints; none of the treatments she received there (mostly acetaminophen with codeine) relieved her symptoms. Three weeks later she returned to our clinic. She was distressed that the symptoms hadn’t gone away, and noted that her family was now experiencing similar symptoms.
Her temperature was 98.1°F (36.7°C), blood pressure was 131/88 mm Hg, pulse was 85 beats/min, and respiratory rate was 18 breaths/min. Physical and neurologic exams were normal.
THE DIAGNOSIS
Although most of the patient’s lab test results were within normal ranges, her carboxyhemoglobin (COHb) level was 4.2%. COHb levels of >2% to 3% in nonsmokers or >9% to 10% in smokers suggest carbon monoxide (CO) poisoning.1,2 Based on this finding and our patient’s symptoms, we diagnosed unintentional CO poisoning. We recommended that she and her mother vacate the apartment and have it inspected.
DISCUSSION
CO is the leading cause of poisoning mortality in the United States, and causes half of all fatal poisonings worldwide.1,3,4 It is a colorless, odorless, and tasteless gas that is produced by the incomplete combustion of carbon-based products, such as coal or gas.5,6 Exposure can occur from car exhaust fumes, faulty room heaters, and other sources (TABLE 1).6 The incidence of CO poisoning is higher during the winter months and after natural disasters. Individuals who have a lowered oxygen capacity, such as older adults, pregnant women (and their fetuses), infants, and patients with anemia, cardiovascular disease, or cerebrovascular disease, are more susceptible to CO poisoning.5,6
COHb, a stable complex of CO that forms in red blood cells when CO is inhaled, impairs oxygen delivery and peripheral utilization, resulting in cellular hypoxia.1 Signs and symptoms of CO poisoning are nonspecific and require a high degree of clinical suspicion for early diagnosis and treatment. Although cherry-red lips, peripheral cyanosis, and retinal hemorrhages are often described as “classic” symptoms of CO poisoning, these are rarely seen.6 The most common symptoms are actually headache (90%), dizziness (82%), and weakness (53%).7 Other symptoms include nausea, vomiting, confusion, visual disturbances, loss of consciousness, angina, seizure, and fatigue.6,7 Symptoms of chronic CO poisoning may differ from those of acute poisoning and can include chronic fatigue, neuropathy, and memory deficit.8
The differential diagnosis for CO poisoning includes flu-like syndrome/influenza/other viral illnesses, migraine or tension headaches, depression, transient ischemic attack, encephalitis, coronary artery disease, gastroenteritis or food poisoning, seizures, and dysrhythmias.1,4 Lab testing for COHb can help narrow the diagnosis. CO poisoning can be classified as mild, moderate, or severe based on COHb levels and the patient’s signs and symptoms (TABLE 2).6 However, COHb level is a poor predictor of clinical presentation and should not be used to dictate management.2,7
Oxygen therapy is the recommended treatment
Early treatment with supplemental oxygen is recommended to reduce the length of time red blood cells are exposed to CO.1 A COHb level >25% is the criterion for hyperbaric oxygen therapy.1,3 Patients should receive treatment until their symptoms become less intense.
Delayed neuropsychiatric sequelae (DNS) can occur in up to one-third of patients with acute CO poisoning more than a month after apparent recovery.1,6,9 DNS symptoms include cognitive changes, emotional lability, visual disturbances, disorientation, depression, dementia, psychotic behavior, parkinsonism, amnesia, and incontinence.1,6,9 Approximately 50% to 75% of patients with DNS recover spontaneously within a year with symptomatic treatment.1,6,9
Our patient
After recommending that our patient (and her mother) leave the apartment and have it inspected, we later learned that the fire department was unable to determine the source of the CO. A CO detector was installed and our patient was advised to keep the windows in the apartment open to allow for adequate oxygen flow. One month later she returned to our clinic and reported that her symptoms resolved; serum COHb was negative upon repeat lab tests.
THE TAKEAWAY
Patients who present with headaches, dizziness and/or fatigue should be evaluated for CO poisoning. The patient’s environmental history should be reviewed carefully, especially because CO poisoning is more common during the winter months. Oxygen therapy is the mainstay of treatment. Up to one-third of patients with acute poisoning may develop delayed neuropsychiatric sequelae, including cognitive changes, emotional lability, visual disturbances, disorientation, and depression, that may resolve within one year.
THE CASE
One month after moving into her mother’s apartment, a 27-year-old woman sought care at our clinic for fatigue, headache, blurred vision, nausea, and morning vomiting. She had weakness and difficulty sleeping, but denied any fever, rashes, neck stiffness, recent travel, trauma, or tobacco or illicit drug use. She did, however, have a 6-year history of migraines. Her physical exam was normal. She was sent home with a prescription for tramadol 50 mg bid for her headaches.
The patient subsequently went to the emergency department 3 times for the same complaints; none of the treatments she received there (mostly acetaminophen with codeine) relieved her symptoms. Three weeks later she returned to our clinic. She was distressed that the symptoms hadn’t gone away, and noted that her family was now experiencing similar symptoms.
Her temperature was 98.1°F (36.7°C), blood pressure was 131/88 mm Hg, pulse was 85 beats/min, and respiratory rate was 18 breaths/min. Physical and neurologic exams were normal.
THE DIAGNOSIS
Although most of the patient’s lab test results were within normal ranges, her carboxyhemoglobin (COHb) level was 4.2%. COHb levels of >2% to 3% in nonsmokers or >9% to 10% in smokers suggest carbon monoxide (CO) poisoning.1,2 Based on this finding and our patient’s symptoms, we diagnosed unintentional CO poisoning. We recommended that she and her mother vacate the apartment and have it inspected.
DISCUSSION
CO is the leading cause of poisoning mortality in the United States, and causes half of all fatal poisonings worldwide.1,3,4 It is a colorless, odorless, and tasteless gas that is produced by the incomplete combustion of carbon-based products, such as coal or gas.5,6 Exposure can occur from car exhaust fumes, faulty room heaters, and other sources (TABLE 1).6 The incidence of CO poisoning is higher during the winter months and after natural disasters. Individuals who have a lowered oxygen capacity, such as older adults, pregnant women (and their fetuses), infants, and patients with anemia, cardiovascular disease, or cerebrovascular disease, are more susceptible to CO poisoning.5,6
COHb, a stable complex of CO that forms in red blood cells when CO is inhaled, impairs oxygen delivery and peripheral utilization, resulting in cellular hypoxia.1 Signs and symptoms of CO poisoning are nonspecific and require a high degree of clinical suspicion for early diagnosis and treatment. Although cherry-red lips, peripheral cyanosis, and retinal hemorrhages are often described as “classic” symptoms of CO poisoning, these are rarely seen.6 The most common symptoms are actually headache (90%), dizziness (82%), and weakness (53%).7 Other symptoms include nausea, vomiting, confusion, visual disturbances, loss of consciousness, angina, seizure, and fatigue.6,7 Symptoms of chronic CO poisoning may differ from those of acute poisoning and can include chronic fatigue, neuropathy, and memory deficit.8
The differential diagnosis for CO poisoning includes flu-like syndrome/influenza/other viral illnesses, migraine or tension headaches, depression, transient ischemic attack, encephalitis, coronary artery disease, gastroenteritis or food poisoning, seizures, and dysrhythmias.1,4 Lab testing for COHb can help narrow the diagnosis. CO poisoning can be classified as mild, moderate, or severe based on COHb levels and the patient’s signs and symptoms (TABLE 2).6 However, COHb level is a poor predictor of clinical presentation and should not be used to dictate management.2,7
Oxygen therapy is the recommended treatment
Early treatment with supplemental oxygen is recommended to reduce the length of time red blood cells are exposed to CO.1 A COHb level >25% is the criterion for hyperbaric oxygen therapy.1,3 Patients should receive treatment until their symptoms become less intense.
Delayed neuropsychiatric sequelae (DNS) can occur in up to one-third of patients with acute CO poisoning more than a month after apparent recovery.1,6,9 DNS symptoms include cognitive changes, emotional lability, visual disturbances, disorientation, depression, dementia, psychotic behavior, parkinsonism, amnesia, and incontinence.1,6,9 Approximately 50% to 75% of patients with DNS recover spontaneously within a year with symptomatic treatment.1,6,9
Our patient
After recommending that our patient (and her mother) leave the apartment and have it inspected, we later learned that the fire department was unable to determine the source of the CO. A CO detector was installed and our patient was advised to keep the windows in the apartment open to allow for adequate oxygen flow. One month later she returned to our clinic and reported that her symptoms resolved; serum COHb was negative upon repeat lab tests.
THE TAKEAWAY
Patients who present with headaches, dizziness and/or fatigue should be evaluated for CO poisoning. The patient’s environmental history should be reviewed carefully, especially because CO poisoning is more common during the winter months. Oxygen therapy is the mainstay of treatment. Up to one-third of patients with acute poisoning may develop delayed neuropsychiatric sequelae, including cognitive changes, emotional lability, visual disturbances, disorientation, and depression, that may resolve within one year.
1. Nikkanen H, Skolnik A. Diagnosis and management of carbon monoxide poisoning in the emergency department. Emerg Med Pract. 2011;13:1-14.
2. Hampson NB, Hauff NM. Carboxyhemoglobin levels in carbon monoxide poisoning: do they correlate with the clinical picture? Am J Emerg Med. 2008;26:665-669.
3. Kao LW, Nañagas KA. Toxicity associated with carbon monoxide. Clin Lab Med. 2006;26:99-125.
4. Varon J, Marik PE, Fromm RE Jr, et al. Carbon monoxide poisoning: a review for clinicians. J Emerg Med. 1999;17:87-93.
5. Harper A, Croft-Baker J. Carbon monoxide poisoning: undetected by both patients and their doctors. Age Ageing. 2004;33:105-109.
6. Smollin C, Olson K. Carbon monoxide poisoning (acute). BMJ Clin Evid. 2010;2010. pii:2103.
7. Wright J. Chronic and occult carbon monoxide poisoning: we don’t know what we’re missing. Emerg Med J. 2002;19:366-390.
8. Weaver LK. Clinical practice. Carbon monoxide poisoning. N Engl J Med. 2009;360:1217-1225.
9. Bhatia R, Chacko F, Lal V, et al. Reversible delayed neuropsychiatric syndrome following acute carbon monoxide exposure. Indian J Occup Environ Med. 2007;11:80-82.
1. Nikkanen H, Skolnik A. Diagnosis and management of carbon monoxide poisoning in the emergency department. Emerg Med Pract. 2011;13:1-14.
2. Hampson NB, Hauff NM. Carboxyhemoglobin levels in carbon monoxide poisoning: do they correlate with the clinical picture? Am J Emerg Med. 2008;26:665-669.
3. Kao LW, Nañagas KA. Toxicity associated with carbon monoxide. Clin Lab Med. 2006;26:99-125.
4. Varon J, Marik PE, Fromm RE Jr, et al. Carbon monoxide poisoning: a review for clinicians. J Emerg Med. 1999;17:87-93.
5. Harper A, Croft-Baker J. Carbon monoxide poisoning: undetected by both patients and their doctors. Age Ageing. 2004;33:105-109.
6. Smollin C, Olson K. Carbon monoxide poisoning (acute). BMJ Clin Evid. 2010;2010. pii:2103.
7. Wright J. Chronic and occult carbon monoxide poisoning: we don’t know what we’re missing. Emerg Med J. 2002;19:366-390.
8. Weaver LK. Clinical practice. Carbon monoxide poisoning. N Engl J Med. 2009;360:1217-1225.
9. Bhatia R, Chacko F, Lal V, et al. Reversible delayed neuropsychiatric syndrome following acute carbon monoxide exposure. Indian J Occup Environ Med. 2007;11:80-82.
CoreValve receives first TAVR valve-in-valve indication
The U.S. Food and Drug Administration on March 30 expanded its approved use of the CoreValve transcatheter aortic-valve replacement (TAVR) system to include patients who already have undergone aortic valve replacement and need a second valve replacement done as a valve-in-valve placement.
With this action, CoreValve became the first TAVR system to receive U.S. approval for valve-in-valve use. The CoreValve System received FDA approval for TAVR performed on native aortic valves in January 2014 in patients at “extreme risk,” and in June 2014 for those at “high risk,” for surgical aortic valve replacement.* Valve-in-valve TAVR is only feasible in patients with a failing bioprosthetic aortic valve: It is not an option for patients with a failing mechanical aortic valve.
“The CoreValve System offers a less-invasive treatment option for a significant number of patients with failed tissue aortic valves whose medical teams determine that the risks associated with repeat open-heart surgery are high or extremely high,” Dr. William H. Maisel, deputy center director for science and chief scientist in the FDA’s Center for Devices and Radiological Health, said in a written statement. “The approval is an important expansion of the authorized use of the transcatheter aortic valve replacement technology.”
The CoreValve, which is designed to sit in a supra-annular location 12 mm above the aortic valve annulus, is well suited for valve-in-valve replacement because the only portion of the CoreValve that actually fills the annular space and the ring of the existing valve is the CoreValve’s sealer. This results in a tight seal that produces less paravalvular leak than when the sealer sits in a native annulus that is often deformed with calcium, noted Dr. Michael J. Reardon, professor of cardiothoracic surgery at Methodist Hospital in Houston.
In addition, because the sealer exerts pressure on the old valve ring in the annulus instead of on myocardium, placing the CoreValve as a valve-in-valve produces much less conduction disruption and results in fewer patients who need a pacemaker following TAVR, he said.
The CoreValve as a valve-in-valve “works quite well, and is not hard to position,” said Dr. Reardon, who added that he has now performed several valve-in-valve TAVRs using the CoreValve.
Similar TAVR procedures are usually not possible using the balloon-expandable SAPIEN System because the SAPIEN valve is designed to sit directly in the annulus and, in most patients, the existing valve ring does not provide enough space to accommodate a SAPIEN valve.
Dr. Reardon anticipates that many U.S. patients now in their 80s with a failing bioprosthetic aortic valve will be interested in nonsurgical TAVR replacement. These patients often do not want conventional open-heart surgery, he said in an interview.
To evaluate the safety and efficacy of the CoreValve System for aortic valve-in-valve replacement, the FDA reviewed clinical data collected from a U.S. clinical trial with 143 patients, an agency representative said in the statement. In the clinical trial, the estimated rate of 30-day survival without major stroke was 96%, and 89% after 6 months. “This compares well to the corresponding rates reported previously for trial participants who received the same device to replace their own, native diseased or damaged aortic valve,” the agency’s statement said.
According to the agency, aortic valve-in-valve use of the CoreValve System should be limited to patients who need replacement of a failed tissue aortic valve but are at extreme or high risk of death or serious complications from traditional open-heart surgery. A decision as to whether the product and procedure are appropriate for a patient “should involve careful evaluation by the patient’s heart medical team, including a cardiologist and a cardiac surgeon.”
The FDA said that the CoreValve System should not be used in patients who have any infection, have a mechanical aortic heart valve, cannot tolerate anticoagulant drugs, or have sensitivity to titanium, nickel, or contrast media.
Dr. Maisel had no disclosures. Dr. Reardon has served as an advisor to Medtronic, the company that markets the CoreValve.
On Twitter @mitchelzoler
*Correction, 4/1/2015: An earlier version of this article misstated the device’s approval history.
The U.S. Food and Drug Administration on March 30 expanded its approved use of the CoreValve transcatheter aortic-valve replacement (TAVR) system to include patients who already have undergone aortic valve replacement and need a second valve replacement done as a valve-in-valve placement.
With this action, CoreValve became the first TAVR system to receive U.S. approval for valve-in-valve use. The CoreValve System received FDA approval for TAVR performed on native aortic valves in January 2014 in patients at “extreme risk,” and in June 2014 for those at “high risk,” for surgical aortic valve replacement.* Valve-in-valve TAVR is only feasible in patients with a failing bioprosthetic aortic valve: It is not an option for patients with a failing mechanical aortic valve.
“The CoreValve System offers a less-invasive treatment option for a significant number of patients with failed tissue aortic valves whose medical teams determine that the risks associated with repeat open-heart surgery are high or extremely high,” Dr. William H. Maisel, deputy center director for science and chief scientist in the FDA’s Center for Devices and Radiological Health, said in a written statement. “The approval is an important expansion of the authorized use of the transcatheter aortic valve replacement technology.”
The CoreValve, which is designed to sit in a supra-annular location 12 mm above the aortic valve annulus, is well suited for valve-in-valve replacement because the only portion of the CoreValve that actually fills the annular space and the ring of the existing valve is the CoreValve’s sealer. This results in a tight seal that produces less paravalvular leak than when the sealer sits in a native annulus that is often deformed with calcium, noted Dr. Michael J. Reardon, professor of cardiothoracic surgery at Methodist Hospital in Houston.
In addition, because the sealer exerts pressure on the old valve ring in the annulus instead of on myocardium, placing the CoreValve as a valve-in-valve produces much less conduction disruption and results in fewer patients who need a pacemaker following TAVR, he said.
The CoreValve as a valve-in-valve “works quite well, and is not hard to position,” said Dr. Reardon, who added that he has now performed several valve-in-valve TAVRs using the CoreValve.
Similar TAVR procedures are usually not possible using the balloon-expandable SAPIEN System because the SAPIEN valve is designed to sit directly in the annulus and, in most patients, the existing valve ring does not provide enough space to accommodate a SAPIEN valve.
Dr. Reardon anticipates that many U.S. patients now in their 80s with a failing bioprosthetic aortic valve will be interested in nonsurgical TAVR replacement. These patients often do not want conventional open-heart surgery, he said in an interview.
To evaluate the safety and efficacy of the CoreValve System for aortic valve-in-valve replacement, the FDA reviewed clinical data collected from a U.S. clinical trial with 143 patients, an agency representative said in the statement. In the clinical trial, the estimated rate of 30-day survival without major stroke was 96%, and 89% after 6 months. “This compares well to the corresponding rates reported previously for trial participants who received the same device to replace their own, native diseased or damaged aortic valve,” the agency’s statement said.
According to the agency, aortic valve-in-valve use of the CoreValve System should be limited to patients who need replacement of a failed tissue aortic valve but are at extreme or high risk of death or serious complications from traditional open-heart surgery. A decision as to whether the product and procedure are appropriate for a patient “should involve careful evaluation by the patient’s heart medical team, including a cardiologist and a cardiac surgeon.”
The FDA said that the CoreValve System should not be used in patients who have any infection, have a mechanical aortic heart valve, cannot tolerate anticoagulant drugs, or have sensitivity to titanium, nickel, or contrast media.
Dr. Maisel had no disclosures. Dr. Reardon has served as an advisor to Medtronic, the company that markets the CoreValve.
On Twitter @mitchelzoler
*Correction, 4/1/2015: An earlier version of this article misstated the device’s approval history.
The U.S. Food and Drug Administration on March 30 expanded its approved use of the CoreValve transcatheter aortic-valve replacement (TAVR) system to include patients who already have undergone aortic valve replacement and need a second valve replacement done as a valve-in-valve placement.
With this action, CoreValve became the first TAVR system to receive U.S. approval for valve-in-valve use. The CoreValve System received FDA approval for TAVR performed on native aortic valves in January 2014 in patients at “extreme risk,” and in June 2014 for those at “high risk,” for surgical aortic valve replacement.* Valve-in-valve TAVR is only feasible in patients with a failing bioprosthetic aortic valve: It is not an option for patients with a failing mechanical aortic valve.
“The CoreValve System offers a less-invasive treatment option for a significant number of patients with failed tissue aortic valves whose medical teams determine that the risks associated with repeat open-heart surgery are high or extremely high,” Dr. William H. Maisel, deputy center director for science and chief scientist in the FDA’s Center for Devices and Radiological Health, said in a written statement. “The approval is an important expansion of the authorized use of the transcatheter aortic valve replacement technology.”
The CoreValve, which is designed to sit in a supra-annular location 12 mm above the aortic valve annulus, is well suited for valve-in-valve replacement because the only portion of the CoreValve that actually fills the annular space and the ring of the existing valve is the CoreValve’s sealer. This results in a tight seal that produces less paravalvular leak than when the sealer sits in a native annulus that is often deformed with calcium, noted Dr. Michael J. Reardon, professor of cardiothoracic surgery at Methodist Hospital in Houston.
In addition, because the sealer exerts pressure on the old valve ring in the annulus instead of on myocardium, placing the CoreValve as a valve-in-valve produces much less conduction disruption and results in fewer patients who need a pacemaker following TAVR, he said.
The CoreValve as a valve-in-valve “works quite well, and is not hard to position,” said Dr. Reardon, who added that he has now performed several valve-in-valve TAVRs using the CoreValve.
Similar TAVR procedures are usually not possible using the balloon-expandable SAPIEN System because the SAPIEN valve is designed to sit directly in the annulus and, in most patients, the existing valve ring does not provide enough space to accommodate a SAPIEN valve.
Dr. Reardon anticipates that many U.S. patients now in their 80s with a failing bioprosthetic aortic valve will be interested in nonsurgical TAVR replacement. These patients often do not want conventional open-heart surgery, he said in an interview.
To evaluate the safety and efficacy of the CoreValve System for aortic valve-in-valve replacement, the FDA reviewed clinical data collected from a U.S. clinical trial with 143 patients, an agency representative said in the statement. In the clinical trial, the estimated rate of 30-day survival without major stroke was 96%, and 89% after 6 months. “This compares well to the corresponding rates reported previously for trial participants who received the same device to replace their own, native diseased or damaged aortic valve,” the agency’s statement said.
According to the agency, aortic valve-in-valve use of the CoreValve System should be limited to patients who need replacement of a failed tissue aortic valve but are at extreme or high risk of death or serious complications from traditional open-heart surgery. A decision as to whether the product and procedure are appropriate for a patient “should involve careful evaluation by the patient’s heart medical team, including a cardiologist and a cardiac surgeon.”
The FDA said that the CoreValve System should not be used in patients who have any infection, have a mechanical aortic heart valve, cannot tolerate anticoagulant drugs, or have sensitivity to titanium, nickel, or contrast media.
Dr. Maisel had no disclosures. Dr. Reardon has served as an advisor to Medtronic, the company that markets the CoreValve.
On Twitter @mitchelzoler
*Correction, 4/1/2015: An earlier version of this article misstated the device’s approval history.
AUDIO: Psychiatry’s Darwinian moment
LAS VEGAS – A more-specific nomenclature of depression would help in the prescribing of better treatments or might prevent it altogether, according to Dr. Vladimir Maletic, a psychiatrist who spoke at this year’s Nevada Psychiatric Association’s annual Psychopharmacology update meeting.
But to do so requires psychiatry to find its own Darwinian moment, where the etiology of depression is made clear. In the conversation with Dr. Maletic, recorded at the meeting, hear his thoughts on the differences between existential and biological depression, how allowing patients to choose their treatment can double their response rate, and the lasting, negative effects on cognition post depression.
On Twitter @whitneymcknight
LAS VEGAS – A more-specific nomenclature of depression would help in the prescribing of better treatments or might prevent it altogether, according to Dr. Vladimir Maletic, a psychiatrist who spoke at this year’s Nevada Psychiatric Association’s annual Psychopharmacology update meeting.
But to do so requires psychiatry to find its own Darwinian moment, where the etiology of depression is made clear. In the conversation with Dr. Maletic, recorded at the meeting, hear his thoughts on the differences between existential and biological depression, how allowing patients to choose their treatment can double their response rate, and the lasting, negative effects on cognition post depression.
On Twitter @whitneymcknight
LAS VEGAS – A more-specific nomenclature of depression would help in the prescribing of better treatments or might prevent it altogether, according to Dr. Vladimir Maletic, a psychiatrist who spoke at this year’s Nevada Psychiatric Association’s annual Psychopharmacology update meeting.
But to do so requires psychiatry to find its own Darwinian moment, where the etiology of depression is made clear. In the conversation with Dr. Maletic, recorded at the meeting, hear his thoughts on the differences between existential and biological depression, how allowing patients to choose their treatment can double their response rate, and the lasting, negative effects on cognition post depression.
On Twitter @whitneymcknight
EXPERT ANALYSIS FROM THE NPA ANNUAL PSYCHOPHARMACOLOGY UPDATE
Pheochromocytoma
To the Editor: I read with avid interest the IM Board Review by Pagán et al on a man with pheochromocytoma.1 The article stated that the classic triad is headache, hypertension, and hyperglycemia. I found this to be incorrect. And Harrison’s Principles of Internal Medicine2 states that the classic triad is palpitations, headaches, and profuse sweating. I hope you will clarify this in the interest of the readers as it is a board review article.
- Pagán RJ, Kurklinsky AK, Chirila R. A 61-year-old man with fluctuating hypertension. Cleve Clin J Med 2014; 81:677–682.
- Neumann HH. Chapter 343. Pheochromocytoma. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson J, Loscalzo J, editors. Harrison’s Principles of Internal Medicine, 18th edition. New York, NY: McGraw-Hill, 2012. http://0-accessmedicine.mhmedical.com.library.ccf.org/content.aspx?bookid=331&Sectionid=40727148. Accessed March 12, 2015.
To the Editor: I read with avid interest the IM Board Review by Pagán et al on a man with pheochromocytoma.1 The article stated that the classic triad is headache, hypertension, and hyperglycemia. I found this to be incorrect. And Harrison’s Principles of Internal Medicine2 states that the classic triad is palpitations, headaches, and profuse sweating. I hope you will clarify this in the interest of the readers as it is a board review article.
To the Editor: I read with avid interest the IM Board Review by Pagán et al on a man with pheochromocytoma.1 The article stated that the classic triad is headache, hypertension, and hyperglycemia. I found this to be incorrect. And Harrison’s Principles of Internal Medicine2 states that the classic triad is palpitations, headaches, and profuse sweating. I hope you will clarify this in the interest of the readers as it is a board review article.
- Pagán RJ, Kurklinsky AK, Chirila R. A 61-year-old man with fluctuating hypertension. Cleve Clin J Med 2014; 81:677–682.
- Neumann HH. Chapter 343. Pheochromocytoma. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson J, Loscalzo J, editors. Harrison’s Principles of Internal Medicine, 18th edition. New York, NY: McGraw-Hill, 2012. http://0-accessmedicine.mhmedical.com.library.ccf.org/content.aspx?bookid=331&Sectionid=40727148. Accessed March 12, 2015.
- Pagán RJ, Kurklinsky AK, Chirila R. A 61-year-old man with fluctuating hypertension. Cleve Clin J Med 2014; 81:677–682.
- Neumann HH. Chapter 343. Pheochromocytoma. In: Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson J, Loscalzo J, editors. Harrison’s Principles of Internal Medicine, 18th edition. New York, NY: McGraw-Hill, 2012. http://0-accessmedicine.mhmedical.com.library.ccf.org/content.aspx?bookid=331&Sectionid=40727148. Accessed March 12, 2015.
What therapies alleviate symptoms of polycystic ovary syndrome?
Treatment of polycystic ovary syndrome (PCOS) in women not actively seeking to become pregnant is symptom-specific. Lifestyle modification (LSM) reduces body weight by 3.5 kg (strength of recommendation [SOR]: A, meta-analysis) and metformin reduces it by 3 kg (SOR B, cohort trial).
LSM may be better tolerated; adding metformin to LSM doesn’t lead to additional weight loss (SOR: B, randomized controlled trial [RCT]).
Spironolactone improves hirsutism scores by an absolute 8% to 22% (SOR: A, multiple RCTs); adding metformin to spironolactone improves Ferriman-Gallwey (FG) hirsutism scores an additional absolute 1.4% (SOR: B, RCT). Oral contraceptive pills (OCPs) are 12 times more likely to result in complete menstrual regularity than metformin (SOR: A, meta-analysis). Combining OCPs with metformin improves hirsutism scores by 8% over using an OCP alone (SOR: A, meta-analysis).
Statin medications don’t alter weight, hirsutism, or menstruation (SOR: B, small meta-analysis).
EVIDENCE SUMMARY
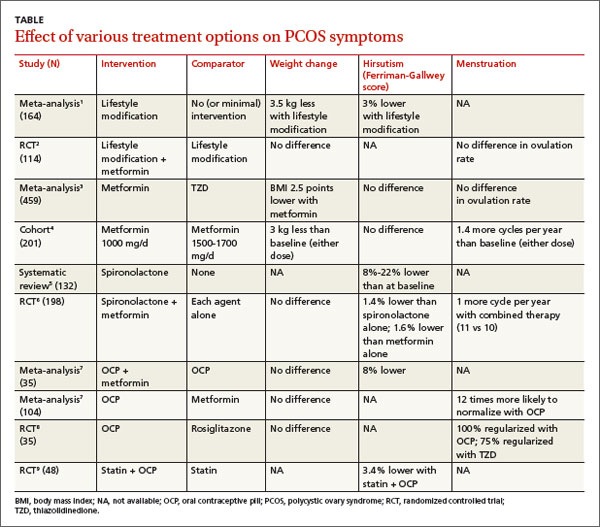
Women with PCOS who are not seeking pregnancy commonly have symptoms such as excessive weight, hirsutism, and menstrual irregularities. This review focuses on interventions to manage those symptoms. The TABLE summarizes the results of the interventions.
Lifestyle modification improves symptoms; no benefit to adding metformin
A Cochrane meta-analysis of 6 RCTs with 164 patients compared LSM (with diet and exercise) and no or minimal intervention. LSM reduced weight more than minimal intervention (mean difference [MD]=-3.5 kg; 95% confidence interval [CI], -4.5 to -2.0).1 It also improved hirsutism, assessed with the 36-point FG score, where a lower score corresponds to less hirsutism (MD=-1.2 points, 95% CI, -2.4 to -0.1). No data were available on menstrual regularity.
A double-blind RCT comparing LSM alone with LSM plus metformin in 114 patients with PCOS found no difference in mean weight reduction (-2- to -3 kg, data from graph), ovulation rate, or androgen levels at 6 months.2 Six patients dropped out of the LSM-with-metformin group, whereas no patients dropped out of the LSM-alone group.
Metformin decreases BMI more than thiazolidinediones
In a meta-analysis of 10 RCTs (459 patients) comparing the effects of metformin and thiazolidinediones (TZDs), metformin reduced body mass index (BMI) more than TZDs at 3 months (weighted mean difference [WMD]=-2.5 kg/m2; 95% CI, -3.3 to -.6) and 6 months (WMD=-0.70 kg/m2; 95% CI, -0.76 to -0.65).3
In a prospective cohort dose-comparison study, 201 women with PCOS received either metformin 1000 mg or 1500 to 1700 mg daily for 6 months. Patients were asked not to modify their diet or exercise routines. In both dosage groups, patients lost weight from baseline (-3 kg; P<.01), and the number of menstrual cycles increased (0.7 per 6 months; P<.001).4 No clear dose-response relationship was observed.
Spironolactone can significantly reduce hirsutism
A systematic review identified 4 studies (132 patients) of antiandrogen therapy for hirsutism in PCOS. The 3 studies that used the FG score as an outcome all showed significant reductions in hirsutism after 6 to 12 months of treatment with spironolactone.5
A 6-month RCT of 198 patients with PCOS compared outcomes for spironolactone (50 mg/d), metformin (1000 mg/d), or both. Combined therapy was marginally better than either agent alone for reducing the FG score (end score for combined therapy 9.1 vs 9.6 for spironolactone and 9.7 for metformin, an absolute difference for combined therapy vs spironolactone of -0.5 FG points or -1.4%; P<.05).6
OCPs normalize menstrual cycles and reduce hirsutism
A Cochrane review evaluating the effects of OCPs on patients with PCOS included 4 RCTs (104 patients) that compared OCPs with metformin (1500-2000 mg/d) and 2 RCTs (70 patients) that compared the combination of an OCP and metformin with the OCP alone. Use of an OCP was much more likely to normalize menstrual cycling than metformin alone (2 trials, N=35; odds ratio [OR]=12; 95% CI, 2.2-100). Combining an OCP with metformin resulted in slightly better FG scores than an OCP alone (1 trial, N=40; WMD=-2.8 points; 95% CI, -5.4 to -0.17).7 There was no difference in the final BMI between patients taking an OCP alone, metformin alone, or both.
An RCT of 35 patients compared the effect on insulin levels of an OCP with rosiglitazone 4 mg/d and also looked at menstrual cycling as a secondary outcome. The study found no difference in effect on insulin levels in the 2 groups. All patients taking the OCP reported regular menstrual cycles at the end of the study compared with 75% of the patients taking rosiglitazone (P=.7).8 The study was underpowered to find a difference, however.
Statins alone don’t affect hirsutism, menstruation, or BMI
A Cochrane review identified 4 RCTs (244 women, ages 18-39 years) that compared a statin alone with placebo, another agent, or another agent plus a statin.9 One RCT of 48 patients found that a statin combined with an OCP improved hirsutism compared with a statin alone. Two RCTs (85 patients) found that statins didn’t lead to resumption of regular menstrual cycles. Statins also didn’t alter BMI in 3 studies of 105 patients.
Trials report no adverse effects, but VTE may be a concern with OCPs
A meta-analysis evaluated the safety of metformin, OCPs, and antiandrogens in 22 clinical trials with 1335 patients, primarily PCOS patients. The trials reported no cases of lactic acidosis with metformin, no drug-induced liver injury with antiandrogens, and no venous thromboembolism (VTE) with OCPs. The meta-analysis authors noted, however, that in a cohort trial of 1.6 million Danish women followed for 15 years, OCPs were associated with a 2- to 3-fold increase in risk of VTE, with higher risks linked to higher ethinyl estradiol content.10
RECOMMENDATIONS
A 2009 practice bulletin from The American College of Obestetrics and Gynecology (ACOG) recommends OCPs, progestin, metformin, and TZDs for anovulation and amenorrhea in patients with PCOS. OCPs, antiandrogens, metformin, eflornithine, and mechanical hair removal are recommended for hirsutism. ACOG advocates LSM, insulin-sensitizing agents (such as metformin), and statins to prevent cardiovascular disease and diabetes.11
1. Moran LJ, Hutchison SK, Norman RJ, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011;(2):CD007506.
2. Ladson G, Dodson WC, Sweet SD, et al. The effects of metformin with lifestyle therapy in polycystic ovary syndrome: a randomized double-blind study. Fertil Steril. 2011;95:1059-1066.
3. Li XJ, Yu YX, Liu CQ, et al. Metformin vs thiazolidinediones for treatment of clinical, hormonal and metabolic characteristics of polycystic ovary syndrome: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:332-339.
4. Fulghesu AM, Romualdi D, Di Florio C, et al. Is there a doseresponse relationship of metformin treatment in patients with polycystic ovary syndrome? Results from a multicentric study. Hum Reprod. 2012; 27:3057-3066.
5. Christy NA, Franks AS, Cross LB. Spironolactone for hirsutism in polycystic ovary syndrome. Ann Pharmacother. 2005;39:1517-1521.
6. Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
7. Costello M, Shrestha B, Eden J, et al. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database Syst Rev. 2007;(1):CD005552.
8. Tfayli H, Ulnach JW, Lee S, et al. Drospirenone/ethinyl estradiol versus rosiglitazone treatment in overweight adolescents with polycystic ovary syndrome: comparison of metabolic, hormonal, and cardiovascular risk factors. J Clin Endocrinol Metab. 2011;96:1311-1319.
9. Raval AD, Hunter T, Stuckey B, et al. Statins for women with polycystic ovary syndrome not actively trying to conceive. Cochrane Database Syst Rev. 2011;(10):CD008565.
10. Domecq JP, Prutsky G, Mullan RJ, et al. Adverse effects of the common treatments for polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:4646-4654.
11. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 108: Polycystic ovary syndrome. Obstet Gynecol. 2009;114:936-949.
Treatment of polycystic ovary syndrome (PCOS) in women not actively seeking to become pregnant is symptom-specific. Lifestyle modification (LSM) reduces body weight by 3.5 kg (strength of recommendation [SOR]: A, meta-analysis) and metformin reduces it by 3 kg (SOR B, cohort trial).
LSM may be better tolerated; adding metformin to LSM doesn’t lead to additional weight loss (SOR: B, randomized controlled trial [RCT]).
Spironolactone improves hirsutism scores by an absolute 8% to 22% (SOR: A, multiple RCTs); adding metformin to spironolactone improves Ferriman-Gallwey (FG) hirsutism scores an additional absolute 1.4% (SOR: B, RCT). Oral contraceptive pills (OCPs) are 12 times more likely to result in complete menstrual regularity than metformin (SOR: A, meta-analysis). Combining OCPs with metformin improves hirsutism scores by 8% over using an OCP alone (SOR: A, meta-analysis).
Statin medications don’t alter weight, hirsutism, or menstruation (SOR: B, small meta-analysis).
EVIDENCE SUMMARY
Women with PCOS who are not seeking pregnancy commonly have symptoms such as excessive weight, hirsutism, and menstrual irregularities. This review focuses on interventions to manage those symptoms. The TABLE summarizes the results of the interventions.
Lifestyle modification improves symptoms; no benefit to adding metformin
A Cochrane meta-analysis of 6 RCTs with 164 patients compared LSM (with diet and exercise) and no or minimal intervention. LSM reduced weight more than minimal intervention (mean difference [MD]=-3.5 kg; 95% confidence interval [CI], -4.5 to -2.0).1 It also improved hirsutism, assessed with the 36-point FG score, where a lower score corresponds to less hirsutism (MD=-1.2 points, 95% CI, -2.4 to -0.1). No data were available on menstrual regularity.
A double-blind RCT comparing LSM alone with LSM plus metformin in 114 patients with PCOS found no difference in mean weight reduction (-2- to -3 kg, data from graph), ovulation rate, or androgen levels at 6 months.2 Six patients dropped out of the LSM-with-metformin group, whereas no patients dropped out of the LSM-alone group.

Metformin decreases BMI more than thiazolidinediones
In a meta-analysis of 10 RCTs (459 patients) comparing the effects of metformin and thiazolidinediones (TZDs), metformin reduced body mass index (BMI) more than TZDs at 3 months (weighted mean difference [WMD]=-2.5 kg/m2; 95% CI, -3.3 to -.6) and 6 months (WMD=-0.70 kg/m2; 95% CI, -0.76 to -0.65).3
In a prospective cohort dose-comparison study, 201 women with PCOS received either metformin 1000 mg or 1500 to 1700 mg daily for 6 months. Patients were asked not to modify their diet or exercise routines. In both dosage groups, patients lost weight from baseline (-3 kg; P<.01), and the number of menstrual cycles increased (0.7 per 6 months; P<.001).4 No clear dose-response relationship was observed.
Spironolactone can significantly reduce hirsutism
A systematic review identified 4 studies (132 patients) of antiandrogen therapy for hirsutism in PCOS. The 3 studies that used the FG score as an outcome all showed significant reductions in hirsutism after 6 to 12 months of treatment with spironolactone.5
A 6-month RCT of 198 patients with PCOS compared outcomes for spironolactone (50 mg/d), metformin (1000 mg/d), or both. Combined therapy was marginally better than either agent alone for reducing the FG score (end score for combined therapy 9.1 vs 9.6 for spironolactone and 9.7 for metformin, an absolute difference for combined therapy vs spironolactone of -0.5 FG points or -1.4%; P<.05).6
OCPs normalize menstrual cycles and reduce hirsutism
A Cochrane review evaluating the effects of OCPs on patients with PCOS included 4 RCTs (104 patients) that compared OCPs with metformin (1500-2000 mg/d) and 2 RCTs (70 patients) that compared the combination of an OCP and metformin with the OCP alone. Use of an OCP was much more likely to normalize menstrual cycling than metformin alone (2 trials, N=35; odds ratio [OR]=12; 95% CI, 2.2-100). Combining an OCP with metformin resulted in slightly better FG scores than an OCP alone (1 trial, N=40; WMD=-2.8 points; 95% CI, -5.4 to -0.17).7 There was no difference in the final BMI between patients taking an OCP alone, metformin alone, or both.
An RCT of 35 patients compared the effect on insulin levels of an OCP with rosiglitazone 4 mg/d and also looked at menstrual cycling as a secondary outcome. The study found no difference in effect on insulin levels in the 2 groups. All patients taking the OCP reported regular menstrual cycles at the end of the study compared with 75% of the patients taking rosiglitazone (P=.7).8 The study was underpowered to find a difference, however.
Statins alone don’t affect hirsutism, menstruation, or BMI
A Cochrane review identified 4 RCTs (244 women, ages 18-39 years) that compared a statin alone with placebo, another agent, or another agent plus a statin.9 One RCT of 48 patients found that a statin combined with an OCP improved hirsutism compared with a statin alone. Two RCTs (85 patients) found that statins didn’t lead to resumption of regular menstrual cycles. Statins also didn’t alter BMI in 3 studies of 105 patients.
Trials report no adverse effects, but VTE may be a concern with OCPs
A meta-analysis evaluated the safety of metformin, OCPs, and antiandrogens in 22 clinical trials with 1335 patients, primarily PCOS patients. The trials reported no cases of lactic acidosis with metformin, no drug-induced liver injury with antiandrogens, and no venous thromboembolism (VTE) with OCPs. The meta-analysis authors noted, however, that in a cohort trial of 1.6 million Danish women followed for 15 years, OCPs were associated with a 2- to 3-fold increase in risk of VTE, with higher risks linked to higher ethinyl estradiol content.10
RECOMMENDATIONS
A 2009 practice bulletin from The American College of Obestetrics and Gynecology (ACOG) recommends OCPs, progestin, metformin, and TZDs for anovulation and amenorrhea in patients with PCOS. OCPs, antiandrogens, metformin, eflornithine, and mechanical hair removal are recommended for hirsutism. ACOG advocates LSM, insulin-sensitizing agents (such as metformin), and statins to prevent cardiovascular disease and diabetes.11
Treatment of polycystic ovary syndrome (PCOS) in women not actively seeking to become pregnant is symptom-specific. Lifestyle modification (LSM) reduces body weight by 3.5 kg (strength of recommendation [SOR]: A, meta-analysis) and metformin reduces it by 3 kg (SOR B, cohort trial).
LSM may be better tolerated; adding metformin to LSM doesn’t lead to additional weight loss (SOR: B, randomized controlled trial [RCT]).
Spironolactone improves hirsutism scores by an absolute 8% to 22% (SOR: A, multiple RCTs); adding metformin to spironolactone improves Ferriman-Gallwey (FG) hirsutism scores an additional absolute 1.4% (SOR: B, RCT). Oral contraceptive pills (OCPs) are 12 times more likely to result in complete menstrual regularity than metformin (SOR: A, meta-analysis). Combining OCPs with metformin improves hirsutism scores by 8% over using an OCP alone (SOR: A, meta-analysis).
Statin medications don’t alter weight, hirsutism, or menstruation (SOR: B, small meta-analysis).
EVIDENCE SUMMARY
Women with PCOS who are not seeking pregnancy commonly have symptoms such as excessive weight, hirsutism, and menstrual irregularities. This review focuses on interventions to manage those symptoms. The TABLE summarizes the results of the interventions.
Lifestyle modification improves symptoms; no benefit to adding metformin
A Cochrane meta-analysis of 6 RCTs with 164 patients compared LSM (with diet and exercise) and no or minimal intervention. LSM reduced weight more than minimal intervention (mean difference [MD]=-3.5 kg; 95% confidence interval [CI], -4.5 to -2.0).1 It also improved hirsutism, assessed with the 36-point FG score, where a lower score corresponds to less hirsutism (MD=-1.2 points, 95% CI, -2.4 to -0.1). No data were available on menstrual regularity.
A double-blind RCT comparing LSM alone with LSM plus metformin in 114 patients with PCOS found no difference in mean weight reduction (-2- to -3 kg, data from graph), ovulation rate, or androgen levels at 6 months.2 Six patients dropped out of the LSM-with-metformin group, whereas no patients dropped out of the LSM-alone group.

Metformin decreases BMI more than thiazolidinediones
In a meta-analysis of 10 RCTs (459 patients) comparing the effects of metformin and thiazolidinediones (TZDs), metformin reduced body mass index (BMI) more than TZDs at 3 months (weighted mean difference [WMD]=-2.5 kg/m2; 95% CI, -3.3 to -.6) and 6 months (WMD=-0.70 kg/m2; 95% CI, -0.76 to -0.65).3
In a prospective cohort dose-comparison study, 201 women with PCOS received either metformin 1000 mg or 1500 to 1700 mg daily for 6 months. Patients were asked not to modify their diet or exercise routines. In both dosage groups, patients lost weight from baseline (-3 kg; P<.01), and the number of menstrual cycles increased (0.7 per 6 months; P<.001).4 No clear dose-response relationship was observed.
Spironolactone can significantly reduce hirsutism
A systematic review identified 4 studies (132 patients) of antiandrogen therapy for hirsutism in PCOS. The 3 studies that used the FG score as an outcome all showed significant reductions in hirsutism after 6 to 12 months of treatment with spironolactone.5
A 6-month RCT of 198 patients with PCOS compared outcomes for spironolactone (50 mg/d), metformin (1000 mg/d), or both. Combined therapy was marginally better than either agent alone for reducing the FG score (end score for combined therapy 9.1 vs 9.6 for spironolactone and 9.7 for metformin, an absolute difference for combined therapy vs spironolactone of -0.5 FG points or -1.4%; P<.05).6
OCPs normalize menstrual cycles and reduce hirsutism
A Cochrane review evaluating the effects of OCPs on patients with PCOS included 4 RCTs (104 patients) that compared OCPs with metformin (1500-2000 mg/d) and 2 RCTs (70 patients) that compared the combination of an OCP and metformin with the OCP alone. Use of an OCP was much more likely to normalize menstrual cycling than metformin alone (2 trials, N=35; odds ratio [OR]=12; 95% CI, 2.2-100). Combining an OCP with metformin resulted in slightly better FG scores than an OCP alone (1 trial, N=40; WMD=-2.8 points; 95% CI, -5.4 to -0.17).7 There was no difference in the final BMI between patients taking an OCP alone, metformin alone, or both.
An RCT of 35 patients compared the effect on insulin levels of an OCP with rosiglitazone 4 mg/d and also looked at menstrual cycling as a secondary outcome. The study found no difference in effect on insulin levels in the 2 groups. All patients taking the OCP reported regular menstrual cycles at the end of the study compared with 75% of the patients taking rosiglitazone (P=.7).8 The study was underpowered to find a difference, however.
Statins alone don’t affect hirsutism, menstruation, or BMI
A Cochrane review identified 4 RCTs (244 women, ages 18-39 years) that compared a statin alone with placebo, another agent, or another agent plus a statin.9 One RCT of 48 patients found that a statin combined with an OCP improved hirsutism compared with a statin alone. Two RCTs (85 patients) found that statins didn’t lead to resumption of regular menstrual cycles. Statins also didn’t alter BMI in 3 studies of 105 patients.
Trials report no adverse effects, but VTE may be a concern with OCPs
A meta-analysis evaluated the safety of metformin, OCPs, and antiandrogens in 22 clinical trials with 1335 patients, primarily PCOS patients. The trials reported no cases of lactic acidosis with metformin, no drug-induced liver injury with antiandrogens, and no venous thromboembolism (VTE) with OCPs. The meta-analysis authors noted, however, that in a cohort trial of 1.6 million Danish women followed for 15 years, OCPs were associated with a 2- to 3-fold increase in risk of VTE, with higher risks linked to higher ethinyl estradiol content.10
RECOMMENDATIONS
A 2009 practice bulletin from The American College of Obestetrics and Gynecology (ACOG) recommends OCPs, progestin, metformin, and TZDs for anovulation and amenorrhea in patients with PCOS. OCPs, antiandrogens, metformin, eflornithine, and mechanical hair removal are recommended for hirsutism. ACOG advocates LSM, insulin-sensitizing agents (such as metformin), and statins to prevent cardiovascular disease and diabetes.11
1. Moran LJ, Hutchison SK, Norman RJ, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011;(2):CD007506.
2. Ladson G, Dodson WC, Sweet SD, et al. The effects of metformin with lifestyle therapy in polycystic ovary syndrome: a randomized double-blind study. Fertil Steril. 2011;95:1059-1066.
3. Li XJ, Yu YX, Liu CQ, et al. Metformin vs thiazolidinediones for treatment of clinical, hormonal and metabolic characteristics of polycystic ovary syndrome: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:332-339.
4. Fulghesu AM, Romualdi D, Di Florio C, et al. Is there a doseresponse relationship of metformin treatment in patients with polycystic ovary syndrome? Results from a multicentric study. Hum Reprod. 2012; 27:3057-3066.
5. Christy NA, Franks AS, Cross LB. Spironolactone for hirsutism in polycystic ovary syndrome. Ann Pharmacother. 2005;39:1517-1521.
6. Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
7. Costello M, Shrestha B, Eden J, et al. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database Syst Rev. 2007;(1):CD005552.
8. Tfayli H, Ulnach JW, Lee S, et al. Drospirenone/ethinyl estradiol versus rosiglitazone treatment in overweight adolescents with polycystic ovary syndrome: comparison of metabolic, hormonal, and cardiovascular risk factors. J Clin Endocrinol Metab. 2011;96:1311-1319.
9. Raval AD, Hunter T, Stuckey B, et al. Statins for women with polycystic ovary syndrome not actively trying to conceive. Cochrane Database Syst Rev. 2011;(10):CD008565.
10. Domecq JP, Prutsky G, Mullan RJ, et al. Adverse effects of the common treatments for polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:4646-4654.
11. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 108: Polycystic ovary syndrome. Obstet Gynecol. 2009;114:936-949.
1. Moran LJ, Hutchison SK, Norman RJ, et al. Lifestyle changes in women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2011;(2):CD007506.
2. Ladson G, Dodson WC, Sweet SD, et al. The effects of metformin with lifestyle therapy in polycystic ovary syndrome: a randomized double-blind study. Fertil Steril. 2011;95:1059-1066.
3. Li XJ, Yu YX, Liu CQ, et al. Metformin vs thiazolidinediones for treatment of clinical, hormonal and metabolic characteristics of polycystic ovary syndrome: a meta-analysis. Clin Endocrinol (Oxf). 2011;74:332-339.
4. Fulghesu AM, Romualdi D, Di Florio C, et al. Is there a doseresponse relationship of metformin treatment in patients with polycystic ovary syndrome? Results from a multicentric study. Hum Reprod. 2012; 27:3057-3066.
5. Christy NA, Franks AS, Cross LB. Spironolactone for hirsutism in polycystic ovary syndrome. Ann Pharmacother. 2005;39:1517-1521.
6. Ganie MA, Khurana ML, Nisar S, et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): a six-month, open-label randomized study. J Clin Endocrinol Metab. 2013;98:3599-3607.
7. Costello M, Shrestha B, Eden J, et al. Insulin-sensitising drugs versus the combined oral contraceptive pill for hirsutism, acne and risk of diabetes, cardiovascular disease, and endometrial cancer in polycystic ovary syndrome. Cochrane Database Syst Rev. 2007;(1):CD005552.
8. Tfayli H, Ulnach JW, Lee S, et al. Drospirenone/ethinyl estradiol versus rosiglitazone treatment in overweight adolescents with polycystic ovary syndrome: comparison of metabolic, hormonal, and cardiovascular risk factors. J Clin Endocrinol Metab. 2011;96:1311-1319.
9. Raval AD, Hunter T, Stuckey B, et al. Statins for women with polycystic ovary syndrome not actively trying to conceive. Cochrane Database Syst Rev. 2011;(10):CD008565.
10. Domecq JP, Prutsky G, Mullan RJ, et al. Adverse effects of the common treatments for polycystic ovary syndrome: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2013;98:4646-4654.
11. ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 108: Polycystic ovary syndrome. Obstet Gynecol. 2009;114:936-949.
Evidence-based answers from the Family Physicians Inquiries Network
In reply: Pheochromocytoma
In Reply: Thanks to Dr. Belur for his observation. He is correct in that the classic triad includes headaches, palpitations, and diaphoresis, although hypertension and hyperglycemia have been described in the literature as frequently occurring, and the clinical presentation can be extremely variable.
In Reply: Thanks to Dr. Belur for his observation. He is correct in that the classic triad includes headaches, palpitations, and diaphoresis, although hypertension and hyperglycemia have been described in the literature as frequently occurring, and the clinical presentation can be extremely variable.
In Reply: Thanks to Dr. Belur for his observation. He is correct in that the classic triad includes headaches, palpitations, and diaphoresis, although hypertension and hyperglycemia have been described in the literature as frequently occurring, and the clinical presentation can be extremely variable.
Pulmonary tuberculosis
To the Editor: The article by Dr. Catherine Curley,1 “Rule out pulmonary tuberculosis: clinical and radiographic clues for the internist,” was very well written, but we would like to point out two facts regarding the diagnosis of pulmonary tuberculosis, especially in high-prevalence countries like India, that might make the article more informative.
First, it has been shown conclusively that good-quality microscopy of two consecutive sputum specimens identifies the majority (95%–98%) of smear-positive tuberculosis patients. The World Health Organization (WHO) therefore revised its policy on case detection by microscopy2 in 2007 to recommend a reduction in the number of specimens examined, from three to two in settings with appropriate external quality assurance and documented good-quality microscopy. This approach greatly reduces the workload of laboratories, a considerable advantage in countries with a high proportion of smear-negative tuberculosis patients because of human immunodeficiency virus (HIV), extrapulmonary disease, or both.
Moreover, in 2011, the WHO recommended in a policy statement that countries that have implemented the current WHO policy for two-specimen case-finding consider switching to same-day diagnosis, especially in settings where patients are likely to default from the diagnostic pathway.3
Second, regarding the interferon-gamma-release assay, the 2011 WHO policy stated that there are not only insufficient data and low-quality evidence on the performance of this assay in low- and middle-income countries, typically those with a high tuberculosis and HIV burden, but also that the interferon-gamma-release assay and the tuberculin skin test cannot accurately predict the risk of infected individuals developing active tuberculosis. Moreover, neither the assay nor the skin test should be used for the diagnosis of active tuberculosis disease. The interferon-gamma-release assay is more costly and technically complex than the skin test. Given comparable performance but the increased cost, replacing the skin test with the interferon-gamma-release assay is not recommended as a public health intervention in resource-constrained settings.4 The majority of tuberculosis cases (on average 85.8%) were detected with the first sputum specimen. With the second sputum specimen, the average incremental yield was 11.9%, while the incremental yield of the third specimen, when the first two specimens were negative, was 3.1%.5
- Curley CA. Rule out pulmonary tuberculosis: clinical and radiographic clues for the internist. Cleve Clin J Med 2015; 82:32–38.
- World Health Organization. TB diagnostics and laboratory strengthening—WHO policy. Reduction of number of smears for the diagnosis of pulmonary TB, 2007. www.who.int/tb/laboratory/policy_diagnosis_pulmonary_tb/en/. Accessed March 12, 2015.
- World Health Organization. Same-day diagnosis of tuberculosis by microscopy. WHO policy statement. www.who.int/tb/publications/2011/tb_microscopy_9789241501606/en/. Accessed March 12, 2015.
- World Health Organization. Use of tuberculosis interferon-gamma release assays (IGRAs) in low- and middle income countries. Policy statement. http://apps.who.int/iris/bitstream/10665/44759/1/9789241502672_eng.pdf?ua=1. Accessed March 12, 2015.
- Mase S, Ramsay A, Ng N, et al. Yield of serial sputum specimen examinations in the diagnosis of pulmonary tuberculosis: a systematic review. Int J Tuberc Lung Dis 2007; 11:485–495.
To the Editor: The article by Dr. Catherine Curley,1 “Rule out pulmonary tuberculosis: clinical and radiographic clues for the internist,” was very well written, but we would like to point out two facts regarding the diagnosis of pulmonary tuberculosis, especially in high-prevalence countries like India, that might make the article more informative.
First, it has been shown conclusively that good-quality microscopy of two consecutive sputum specimens identifies the majority (95%–98%) of smear-positive tuberculosis patients. The World Health Organization (WHO) therefore revised its policy on case detection by microscopy2 in 2007 to recommend a reduction in the number of specimens examined, from three to two in settings with appropriate external quality assurance and documented good-quality microscopy. This approach greatly reduces the workload of laboratories, a considerable advantage in countries with a high proportion of smear-negative tuberculosis patients because of human immunodeficiency virus (HIV), extrapulmonary disease, or both.
Moreover, in 2011, the WHO recommended in a policy statement that countries that have implemented the current WHO policy for two-specimen case-finding consider switching to same-day diagnosis, especially in settings where patients are likely to default from the diagnostic pathway.3
Second, regarding the interferon-gamma-release assay, the 2011 WHO policy stated that there are not only insufficient data and low-quality evidence on the performance of this assay in low- and middle-income countries, typically those with a high tuberculosis and HIV burden, but also that the interferon-gamma-release assay and the tuberculin skin test cannot accurately predict the risk of infected individuals developing active tuberculosis. Moreover, neither the assay nor the skin test should be used for the diagnosis of active tuberculosis disease. The interferon-gamma-release assay is more costly and technically complex than the skin test. Given comparable performance but the increased cost, replacing the skin test with the interferon-gamma-release assay is not recommended as a public health intervention in resource-constrained settings.4 The majority of tuberculosis cases (on average 85.8%) were detected with the first sputum specimen. With the second sputum specimen, the average incremental yield was 11.9%, while the incremental yield of the third specimen, when the first two specimens were negative, was 3.1%.5
To the Editor: The article by Dr. Catherine Curley,1 “Rule out pulmonary tuberculosis: clinical and radiographic clues for the internist,” was very well written, but we would like to point out two facts regarding the diagnosis of pulmonary tuberculosis, especially in high-prevalence countries like India, that might make the article more informative.
First, it has been shown conclusively that good-quality microscopy of two consecutive sputum specimens identifies the majority (95%–98%) of smear-positive tuberculosis patients. The World Health Organization (WHO) therefore revised its policy on case detection by microscopy2 in 2007 to recommend a reduction in the number of specimens examined, from three to two in settings with appropriate external quality assurance and documented good-quality microscopy. This approach greatly reduces the workload of laboratories, a considerable advantage in countries with a high proportion of smear-negative tuberculosis patients because of human immunodeficiency virus (HIV), extrapulmonary disease, or both.
Moreover, in 2011, the WHO recommended in a policy statement that countries that have implemented the current WHO policy for two-specimen case-finding consider switching to same-day diagnosis, especially in settings where patients are likely to default from the diagnostic pathway.3
Second, regarding the interferon-gamma-release assay, the 2011 WHO policy stated that there are not only insufficient data and low-quality evidence on the performance of this assay in low- and middle-income countries, typically those with a high tuberculosis and HIV burden, but also that the interferon-gamma-release assay and the tuberculin skin test cannot accurately predict the risk of infected individuals developing active tuberculosis. Moreover, neither the assay nor the skin test should be used for the diagnosis of active tuberculosis disease. The interferon-gamma-release assay is more costly and technically complex than the skin test. Given comparable performance but the increased cost, replacing the skin test with the interferon-gamma-release assay is not recommended as a public health intervention in resource-constrained settings.4 The majority of tuberculosis cases (on average 85.8%) were detected with the first sputum specimen. With the second sputum specimen, the average incremental yield was 11.9%, while the incremental yield of the third specimen, when the first two specimens were negative, was 3.1%.5
- Curley CA. Rule out pulmonary tuberculosis: clinical and radiographic clues for the internist. Cleve Clin J Med 2015; 82:32–38.
- World Health Organization. TB diagnostics and laboratory strengthening—WHO policy. Reduction of number of smears for the diagnosis of pulmonary TB, 2007. www.who.int/tb/laboratory/policy_diagnosis_pulmonary_tb/en/. Accessed March 12, 2015.
- World Health Organization. Same-day diagnosis of tuberculosis by microscopy. WHO policy statement. www.who.int/tb/publications/2011/tb_microscopy_9789241501606/en/. Accessed March 12, 2015.
- World Health Organization. Use of tuberculosis interferon-gamma release assays (IGRAs) in low- and middle income countries. Policy statement. http://apps.who.int/iris/bitstream/10665/44759/1/9789241502672_eng.pdf?ua=1. Accessed March 12, 2015.
- Mase S, Ramsay A, Ng N, et al. Yield of serial sputum specimen examinations in the diagnosis of pulmonary tuberculosis: a systematic review. Int J Tuberc Lung Dis 2007; 11:485–495.
- Curley CA. Rule out pulmonary tuberculosis: clinical and radiographic clues for the internist. Cleve Clin J Med 2015; 82:32–38.
- World Health Organization. TB diagnostics and laboratory strengthening—WHO policy. Reduction of number of smears for the diagnosis of pulmonary TB, 2007. www.who.int/tb/laboratory/policy_diagnosis_pulmonary_tb/en/. Accessed March 12, 2015.
- World Health Organization. Same-day diagnosis of tuberculosis by microscopy. WHO policy statement. www.who.int/tb/publications/2011/tb_microscopy_9789241501606/en/. Accessed March 12, 2015.
- World Health Organization. Use of tuberculosis interferon-gamma release assays (IGRAs) in low- and middle income countries. Policy statement. http://apps.who.int/iris/bitstream/10665/44759/1/9789241502672_eng.pdf?ua=1. Accessed March 12, 2015.
- Mase S, Ramsay A, Ng N, et al. Yield of serial sputum specimen examinations in the diagnosis of pulmonary tuberculosis: a systematic review. Int J Tuberc Lung Dis 2007; 11:485–495.
In reply: Pulmonary tuberculosis
In Reply: Thank you for your interesting and appropriate comments. The workup and testing of patients with suspected tuberculosis is clearly different in countries with a higher prevalence of tuberculosis than in countries with a lower prevalence. I appreciate that both the purified protein derivative and the interferon-gamma-release assay have very limited utility in the evaluation for active tuberculosis when there is a very high background prevalence of latent tuberculosis infection. In low-prevalence countries like the United States, tuberculosis is often considered in the differential diagnosis even when other infections or lung cancer is more likely. The tests for latent tuberculosis are considered quite important in the workup of active tuberculosis in this setting.
In Reply: Thank you for your interesting and appropriate comments. The workup and testing of patients with suspected tuberculosis is clearly different in countries with a higher prevalence of tuberculosis than in countries with a lower prevalence. I appreciate that both the purified protein derivative and the interferon-gamma-release assay have very limited utility in the evaluation for active tuberculosis when there is a very high background prevalence of latent tuberculosis infection. In low-prevalence countries like the United States, tuberculosis is often considered in the differential diagnosis even when other infections or lung cancer is more likely. The tests for latent tuberculosis are considered quite important in the workup of active tuberculosis in this setting.
In Reply: Thank you for your interesting and appropriate comments. The workup and testing of patients with suspected tuberculosis is clearly different in countries with a higher prevalence of tuberculosis than in countries with a lower prevalence. I appreciate that both the purified protein derivative and the interferon-gamma-release assay have very limited utility in the evaluation for active tuberculosis when there is a very high background prevalence of latent tuberculosis infection. In low-prevalence countries like the United States, tuberculosis is often considered in the differential diagnosis even when other infections or lung cancer is more likely. The tests for latent tuberculosis are considered quite important in the workup of active tuberculosis in this setting.
Does primary nocturnal enuresis affect childrens’ self-esteem?
Yes. Children with primary nocturnal enuresis often, but not always, score about 10% lower on standardized rating scales for self-esteem, or scores for symptoms similar to low self-esteem (sadness, anxiety, social fears, distress) than children without enuresis (strength of recommendation [SOR]: B, systematic review of cohort and case-control studies with some heterogenous results).
Enuretic children 8 to 9 years of age are less likely to have lower self-esteem than older children, ages 10 to 12 years (SOR: B, case-control study).
Successful treatment of primary nocturnal enuresis improves self-esteem ratings, probably to normal (SOR: B, randomized, controlled trial, prospective cohort, and case-control studies).
EVIDENCE SUMMARY
A systematic review including 4 case-control and 3 cohort studies of the impact of nocturnal enuresis on children and young people found that bedwetting was often, but not always, associated with lower self-esteem scores (or scores for symptoms similar to lower self-esteem) on standardized questionnaires.1 The studies defined self-esteem in various ways and used a variety of questionnaires to measure it, so direct comparisons weren’t possible.
The first case-control study in the review found that enuretic older children (10-12 years) and girls had lower self-esteem scores than younger children (8-9 years) and boys. The second case-control study reported lower self-esteem scores on only 1 of 3 assessment instruments.
The third case-control study, which compared self-esteem scores in enuretic children with scores for children who had asthma and heart disease, found that enuresis was associated with the lowest self-esteem. The final case-control study reported that young adolescents with enuresis were more likely to suffer “angry distress.”
The first cohort study in the systematic review found a significantly higher incidence of sadness, anxiety, and social fears in children with enuresis than in children without and reported that 65% were “not happy” about having enuresis.
In the second cohort study, children with more severe enuresis, and girls, had significantly worse self-esteem scores than children with mild enuresis or boys (actual scores and some statistics not supplied), although these findings weren’t replicated on the second standardized scale that the investigators used.
The third cohort study reported that 37% of approximately 800 children with enuresis rated it “really difficult,” on a 4-point Likert scale.
How enuresis treatment affects self-esteem
The same systematic review, plus 2 additional studies, demonstrated that successful treatment of enuresis improves self-esteem scores, likely to normal.1-3 A randomized controlled trial found that treatment improved self-esteem scores by about 5%; children with the greatest treatment success showed the largest improvement (no statistics supplied).2
In a prospective cohort study, treated children demonstrated about a 30% improvement in scores measuring anxiety, depression, and internal distress.3 A case-control study in the systematic review also found about a 30% improvement in self-esteem scores among successfully treated children (both boys and girls) and a return to nonenuretic norms.1 Scores for unsuccessfully treated children didn’t improve.
RECOMMENDATIONS
A guideline on the management of bedwetting from the National Institute for Health and Clinical Excellence (now called the National Institute for Health and Care Excellence) says that enuresis can have a deep impact on a child’s behavior and emotional well-being and that treatment has a positive effect on self-esteem.4
The Evidence-Based Medicine guidelines for enuresis in a child5 say that enuresis as such does not indicate a psychological disturbance and that psychotherapy may be useful when enuresis is associated with significant problems of self-esteem or behavior.
The American Academy of Child and Adolescent Psychiatry practice parameter for children with enuresis states that the psychological consequences of enuresis must be recognized and addressed with sensitivity during evaluation and management.6
1. National Clinical Guideline Centre (UK). Impact of bedwetting on children and young people and their families. In: Nocturnal Enuresis: The Management of Bedwetting in Children and Young People. London, UK: Royal College of Physicians; 2010. Available at: www.ncbi.nlm.nih.gov/books/NBK62729/. Accessed January 24, 2014.
2. Moffatt ME, Kato C, Pless IB. Improvements in self-concept after treatment of nocturnal enuresis: randomized controlled trial. J Pediatr. 1987;110:647-652.
3. HiraSing RA, van Leerdam FJ, Bolk-Bennink LF, et al. Effect of dry bed training on behavioural problems in enuretic children. Acta Paediatr. 2002; 91:960-964.
4. Nunes VD, O’Flynn N, Evans J, et al; Guideline Development Group. Management of bedwetting in children and young people: summary of NICE guidance. BMJ. 2010;341:c5399.
5. Enuresis in a child. Evidence-Based Medicine Guidelines. Essential Evidence Plus [online database]. Available at: www.essentialevidenceplus.com/content/ebmg_ebm/633. Accessed January 24, 2014.
6. Fritz G, Rockney R; American Academy of Child and Adolescent Psychiatry Work Group on Quality Issues. Summary of the practice parameter for the assessment and treatment of children and adolescents with enuresis. J Am Acad Child Adolesc Psychiatry. 2004;43:123-125.
Yes. Children with primary nocturnal enuresis often, but not always, score about 10% lower on standardized rating scales for self-esteem, or scores for symptoms similar to low self-esteem (sadness, anxiety, social fears, distress) than children without enuresis (strength of recommendation [SOR]: B, systematic review of cohort and case-control studies with some heterogenous results).
Enuretic children 8 to 9 years of age are less likely to have lower self-esteem than older children, ages 10 to 12 years (SOR: B, case-control study).
Successful treatment of primary nocturnal enuresis improves self-esteem ratings, probably to normal (SOR: B, randomized, controlled trial, prospective cohort, and case-control studies).
EVIDENCE SUMMARY
A systematic review including 4 case-control and 3 cohort studies of the impact of nocturnal enuresis on children and young people found that bedwetting was often, but not always, associated with lower self-esteem scores (or scores for symptoms similar to lower self-esteem) on standardized questionnaires.1 The studies defined self-esteem in various ways and used a variety of questionnaires to measure it, so direct comparisons weren’t possible.
The first case-control study in the review found that enuretic older children (10-12 years) and girls had lower self-esteem scores than younger children (8-9 years) and boys. The second case-control study reported lower self-esteem scores on only 1 of 3 assessment instruments.
The third case-control study, which compared self-esteem scores in enuretic children with scores for children who had asthma and heart disease, found that enuresis was associated with the lowest self-esteem. The final case-control study reported that young adolescents with enuresis were more likely to suffer “angry distress.”
The first cohort study in the systematic review found a significantly higher incidence of sadness, anxiety, and social fears in children with enuresis than in children without and reported that 65% were “not happy” about having enuresis.
In the second cohort study, children with more severe enuresis, and girls, had significantly worse self-esteem scores than children with mild enuresis or boys (actual scores and some statistics not supplied), although these findings weren’t replicated on the second standardized scale that the investigators used.
The third cohort study reported that 37% of approximately 800 children with enuresis rated it “really difficult,” on a 4-point Likert scale.
How enuresis treatment affects self-esteem
The same systematic review, plus 2 additional studies, demonstrated that successful treatment of enuresis improves self-esteem scores, likely to normal.1-3 A randomized controlled trial found that treatment improved self-esteem scores by about 5%; children with the greatest treatment success showed the largest improvement (no statistics supplied).2
In a prospective cohort study, treated children demonstrated about a 30% improvement in scores measuring anxiety, depression, and internal distress.3 A case-control study in the systematic review also found about a 30% improvement in self-esteem scores among successfully treated children (both boys and girls) and a return to nonenuretic norms.1 Scores for unsuccessfully treated children didn’t improve.
RECOMMENDATIONS
A guideline on the management of bedwetting from the National Institute for Health and Clinical Excellence (now called the National Institute for Health and Care Excellence) says that enuresis can have a deep impact on a child’s behavior and emotional well-being and that treatment has a positive effect on self-esteem.4
The Evidence-Based Medicine guidelines for enuresis in a child5 say that enuresis as such does not indicate a psychological disturbance and that psychotherapy may be useful when enuresis is associated with significant problems of self-esteem or behavior.
The American Academy of Child and Adolescent Psychiatry practice parameter for children with enuresis states that the psychological consequences of enuresis must be recognized and addressed with sensitivity during evaluation and management.6
Yes. Children with primary nocturnal enuresis often, but not always, score about 10% lower on standardized rating scales for self-esteem, or scores for symptoms similar to low self-esteem (sadness, anxiety, social fears, distress) than children without enuresis (strength of recommendation [SOR]: B, systematic review of cohort and case-control studies with some heterogenous results).
Enuretic children 8 to 9 years of age are less likely to have lower self-esteem than older children, ages 10 to 12 years (SOR: B, case-control study).
Successful treatment of primary nocturnal enuresis improves self-esteem ratings, probably to normal (SOR: B, randomized, controlled trial, prospective cohort, and case-control studies).
EVIDENCE SUMMARY
A systematic review including 4 case-control and 3 cohort studies of the impact of nocturnal enuresis on children and young people found that bedwetting was often, but not always, associated with lower self-esteem scores (or scores for symptoms similar to lower self-esteem) on standardized questionnaires.1 The studies defined self-esteem in various ways and used a variety of questionnaires to measure it, so direct comparisons weren’t possible.
The first case-control study in the review found that enuretic older children (10-12 years) and girls had lower self-esteem scores than younger children (8-9 years) and boys. The second case-control study reported lower self-esteem scores on only 1 of 3 assessment instruments.
The third case-control study, which compared self-esteem scores in enuretic children with scores for children who had asthma and heart disease, found that enuresis was associated with the lowest self-esteem. The final case-control study reported that young adolescents with enuresis were more likely to suffer “angry distress.”
The first cohort study in the systematic review found a significantly higher incidence of sadness, anxiety, and social fears in children with enuresis than in children without and reported that 65% were “not happy” about having enuresis.
In the second cohort study, children with more severe enuresis, and girls, had significantly worse self-esteem scores than children with mild enuresis or boys (actual scores and some statistics not supplied), although these findings weren’t replicated on the second standardized scale that the investigators used.
The third cohort study reported that 37% of approximately 800 children with enuresis rated it “really difficult,” on a 4-point Likert scale.
How enuresis treatment affects self-esteem
The same systematic review, plus 2 additional studies, demonstrated that successful treatment of enuresis improves self-esteem scores, likely to normal.1-3 A randomized controlled trial found that treatment improved self-esteem scores by about 5%; children with the greatest treatment success showed the largest improvement (no statistics supplied).2
In a prospective cohort study, treated children demonstrated about a 30% improvement in scores measuring anxiety, depression, and internal distress.3 A case-control study in the systematic review also found about a 30% improvement in self-esteem scores among successfully treated children (both boys and girls) and a return to nonenuretic norms.1 Scores for unsuccessfully treated children didn’t improve.
RECOMMENDATIONS
A guideline on the management of bedwetting from the National Institute for Health and Clinical Excellence (now called the National Institute for Health and Care Excellence) says that enuresis can have a deep impact on a child’s behavior and emotional well-being and that treatment has a positive effect on self-esteem.4
The Evidence-Based Medicine guidelines for enuresis in a child5 say that enuresis as such does not indicate a psychological disturbance and that psychotherapy may be useful when enuresis is associated with significant problems of self-esteem or behavior.
The American Academy of Child and Adolescent Psychiatry practice parameter for children with enuresis states that the psychological consequences of enuresis must be recognized and addressed with sensitivity during evaluation and management.6
1. National Clinical Guideline Centre (UK). Impact of bedwetting on children and young people and their families. In: Nocturnal Enuresis: The Management of Bedwetting in Children and Young People. London, UK: Royal College of Physicians; 2010. Available at: www.ncbi.nlm.nih.gov/books/NBK62729/. Accessed January 24, 2014.
2. Moffatt ME, Kato C, Pless IB. Improvements in self-concept after treatment of nocturnal enuresis: randomized controlled trial. J Pediatr. 1987;110:647-652.
3. HiraSing RA, van Leerdam FJ, Bolk-Bennink LF, et al. Effect of dry bed training on behavioural problems in enuretic children. Acta Paediatr. 2002; 91:960-964.
4. Nunes VD, O’Flynn N, Evans J, et al; Guideline Development Group. Management of bedwetting in children and young people: summary of NICE guidance. BMJ. 2010;341:c5399.
5. Enuresis in a child. Evidence-Based Medicine Guidelines. Essential Evidence Plus [online database]. Available at: www.essentialevidenceplus.com/content/ebmg_ebm/633. Accessed January 24, 2014.
6. Fritz G, Rockney R; American Academy of Child and Adolescent Psychiatry Work Group on Quality Issues. Summary of the practice parameter for the assessment and treatment of children and adolescents with enuresis. J Am Acad Child Adolesc Psychiatry. 2004;43:123-125.
1. National Clinical Guideline Centre (UK). Impact of bedwetting on children and young people and their families. In: Nocturnal Enuresis: The Management of Bedwetting in Children and Young People. London, UK: Royal College of Physicians; 2010. Available at: www.ncbi.nlm.nih.gov/books/NBK62729/. Accessed January 24, 2014.
2. Moffatt ME, Kato C, Pless IB. Improvements in self-concept after treatment of nocturnal enuresis: randomized controlled trial. J Pediatr. 1987;110:647-652.
3. HiraSing RA, van Leerdam FJ, Bolk-Bennink LF, et al. Effect of dry bed training on behavioural problems in enuretic children. Acta Paediatr. 2002; 91:960-964.
4. Nunes VD, O’Flynn N, Evans J, et al; Guideline Development Group. Management of bedwetting in children and young people: summary of NICE guidance. BMJ. 2010;341:c5399.
5. Enuresis in a child. Evidence-Based Medicine Guidelines. Essential Evidence Plus [online database]. Available at: www.essentialevidenceplus.com/content/ebmg_ebm/633. Accessed January 24, 2014.
6. Fritz G, Rockney R; American Academy of Child and Adolescent Psychiatry Work Group on Quality Issues. Summary of the practice parameter for the assessment and treatment of children and adolescents with enuresis. J Am Acad Child Adolesc Psychiatry. 2004;43:123-125.
Evidence-based answers from the Family Physicians Inquiries Network





