User login
Mother dies after cesarean delivery: $4.5M verdict
Mother dies after cesarean delivery: $4.5M verdict
A 31-year-old woman gave birth to her first child by cesarean delivery. Over the next 3 days she reported nausea, vomiting, severe abdominal pain, and had an elevated heart rate. On day 4, she was discharged from the hospital. She went to the ObGyn’s office the next day and was told, after several hours, to return to the hospital. There she was found to have sepsis and acute renal failure. A transfer to another hospital was attempted that night, but she died during transport.
Estate's Claim: The ObGyn should have responded to her reported symptoms prior to discharge by ordering tests. The ObGyn should have called an ambulance to transport her to the hospital from his office.
Defendants’ Defense: The hospital settled for an undisclosed amount before the trial. The ObGyn claimed that there was no negligence in the patient’s treatment.
Verdict: A $4.5 million North Carolina verdict was returned.
Brain-damaged child dies at age 2
A woman was admitted to the hospital in labor. Ninety minutes later a nonreassuring fetal heart-rate tracing was noted. Two hours after that, the ObGyn decided to perform an emergency cesarean delivery.
The child was depressed at birth and required resuscitation. She was transferred to another hospital’s neonatal intensive care unit (NICU), where she was found to have had a severe and catastrophic brain injury. The child died at 2 years of age.
Parent's Claim: An emergency cesarean delivery should have been performed as soon as the fetal heart-rate tracing was found to be nonreassuring. The ObGyn failed to respond to phone calls from the nurses to report fetal distress.
Physician's Defense: The delivery was performed in a timely manner. Brain damage was due to encephalopathy that occurred prior to labor.
Verdict: A Mississippi defense verdict was returned.
Who or what was at fault for ureter injury?
A 45-year-old woman underwent hysterectomy performed by her ObGyn. During surgery, the patient’s ureter was injured. Several additional operations were needed to repair the injury.
Patient's Claim: The patient was not fully informed of the extent of the surgery or possible complications. The ObGyn was negligent in injuring the ureter.
Defendants' Defense: Three months after surgery, the physician entered notes into the patient’s chart that indicated that the ureter injuries were due to a defective monopolar device that had been provided by the hospital. Informed consent included surgical options and complications.
The hospital argued that its equipment was not defective; other surgeons had used the device without any problems. The ObGyn had not used the device before; any injuries were due to his inexperience and negligence.
Verdict: A $2 million South Carolina verdict was returned against the ObGyn. The hospital received a defense verdict.
Did excessive force cause child’s C7 injury?
During delivery, shoulder dystocia was encountered. The child has nerve root avulsion at C7 with damage to adjacent nerve trunks at C5–C6. As a result of the brachial plexus injury, the patient underwent cable grafting and muscle surgeries, but he has permanent weakness and dysfunction in his left arm.
Parent's Claim: Excessive force was used to deliver the child during manipulations for shoulder dystocia.
Physician's Defense: The ObGyn denied using excessive traction. She claimed that she had never used upward traction during a shoulder dystocia presentation. Suprapubic pressure, McRoberts’ maneuver, and delivery of the posterior arm were used. The damage occurred prior to delivery of the head.
Verdict An Illinois defense verdict was returned.
Laparoscopic sheath and coils found at exploratory surgery
In april 2007, a woman underwent a sterilization procedure (Essure) after which she reported pelvic pain. In September 2007, she consented to right salpingo-oophorectomy plus appendectomy. The ObGyn performed the surgery using a robotic device. After surgery, the pathology report indicated that the resected organs were normal and functional.
The patient moved to another state. She continued to have pain and sought treatment with another physician. A computed tomography (CT) scan more than 3 years after robotic surgery revealed foreign objects in the patient’s body. One full Essure coil, a non-fired coil, a second partial coil, and a robotic laparoscopy sheath were surgically removed.
Patient's Claim: The ObGyn was negligent in the performance of the sterilization and robotic surgery procedures. The healthy ovary and fallopian tube should not have been removed and caused her to have surgical menopause.
Physician's defense: The right ovary appeared diseased. The Essure device dropped the coils. The robot malfunctioned during the salpingo-oophorectomy.
Verdict: A $110,513 Oregon verdict was returned, including $10,500 in medical expenses and $100,000 for pain and mental anguish.
Discrepancy in fundal height; child has CP
During her second pregnancy, a 37-year-old woman saw Dr. A, her ObGyn, for regular prenatal care. At 37 weeks’ gestation, the fundal height was not consistent with the fetus’ gestational age: the measurement was higher by 2 cm. No additional testing was scheduled.
At 39.5 weeks’ gestation, the mother reported decreased fetal movement. Because her regular ObGyn was on vacation, she was evaluated by another ObGyn (Dr. B). The fetal heart-rate monitor showed nonreactive results with minimal variability. Dr. B told the mother to drive herself to the emergency department (ED) for additional evaluation. At the hospital, when fetal heart-rate monitoring confirmed fetal distress, an emergency cesarean delivery was performed.
At birth, the baby was not breathing and resuscitation began. The infant was taken to a transitional care unit and then to the NICU, where he was intubated. Cord blood testing confirmed metabolic acidosis. The baby was later found to have dystonic cerebral palsy (CP). He is unable to speak, walk, eat, or care for himself, and he requires 24/7 nursing care.
Parents' Claim: Dr. A failed to order testing after the fundal height discrepancy was found. Testing could have led to an earlier delivery and avoided the injury. The pediatrician failed to ensure adequate oxygenation after delivery. The baby should have been transferred immediately to the NICU and intubated.
Physician's Defense: The fundal height discrepancy was explained by the baby’s position within the uterus. The pediatrician acted heroically to save the child’s life.
Verdict: A $3.5 million Massachusetts settlement was reached.
NT scans misread, not reported; child has Down syndrome
At 13 weeks’ gestation, a 38-year-old woman saw a maternal-fetal medicine (MFM) specialist, who interpreted a nuchal translucency (NT) scan as normal. At 20 weeks’ gestation, an ObGyn performed a second screening that indicated the fetus was at high risk for Down syndrome. However, no further testing was ordered.
At 26.5 weeks’ gestation, amniocentesis was performed after ultrasonography and an echocardiogram revealed fetal abnormalities. A diagnosis of Down syndrome was made at 29 weeks’ gestation, too late for termination of pregnancy.
Parent's Claim: The MFM specialist misread the first NT scan. The ObGyn did not inform the mother of the results of the second screening. Proper interpretation and reporting would have initiated further testing and determination that the baby had Down syndrome before the deadline for termination of pregnancy.
Defendants' Defense: The case was settled during trial.
Verdict: A $3 million New Jersey settlement was reached, including $2 million from the medical center where the second test was performed, $940,000 from the ObGyn, and $60,000 from the MFM specialist.
Uterine rupture, baby dies: $2.15M award
At 38 weeks’ gestation, a mother was admitted to a hospital for induction of labor due to pregnancy-induced hypertension. The fetus was estimated to be large for its gestational age. A uterine rupture occurred during labor. The baby was stillborn.
Parents' Claim: The uterine rupture was not immediately recognized. The ObGyn failed to come to the mother’s bedside until after the fetus had receded up the birth canal, which indicated that a rupture was occurring. The ObGyn ordered oxytocin instead of performing an immediate cesarean delivery. Eleven minutes later, the cesarean was ordered, but the baby had died.
Physician's Defense: There was no negligence; proper protocols were followed. A uterine rupture cannot be predicted.
Verdict: A $650,000 settlement was reached with the hospital before trial. Because the ObGyn was employed by a federally qualified clinic, the matter was filed in federal court. The Illinois court issued a bench decision awarding $1.5 million.
Migrated IUD causes years of pain
In september 2006, an ObGyn inserted an intrauterine device (IUD) in a patient. In February 2007, the patient had an ectopic pregnancy. The IUD was not found during dilation and curettage. The patient continued to report pain to the ObGyn. She sought treatment from another physician in November 2010 due to continuing pain. A CT scan revealed that the IUD had migrated to her abdomen. The IUD was surgically removed.
Patient's Claim: The ObGyn was unwilling to figure out why the patient had continuing pain, and told her to “just deal with it.” He should have found and removed the IUD after the ectopic pregnancy.
Physician's Defense: It was reasonable to assume that the IUD had been expelled, as 2 ultrasonographies performed after ectopic pregnancy revealed nothing. Since the IUD had not caused an abscess, infection, or inflammation, the patient suffered no injury.
Verdict: A Virginia defense verdict was returned.
Profoundly disabled child dies at age 5
A 17-year-old woman with a history of miscarriage received prenatal care from her ObGyn. A July due date was established by ultrasonography in January.
In May, the mother went to the ED with pelvic pain. She was treated for preterm labor and discharged 2 days later.
In early July, ultrasonography showed a fetus in cephalic position with a posterior-located placenta.
At a prenatal examination a week later, the patient reported vaginal discharge. Her ObGyn suspected premature rupture of membranes (PROM) and admitted her to the hospital. Oxytocin was used to induce labor. Intact membranes were artificially ruptured and an internal fetal heart-rate monitor was placed. The ObGyn recorded that the pregnancy was at term.
Hours later, the mother told the nurses that she thought the fetal monitor had become disconnected; the monitor’s placement was not confirmed. The mother was given a sedative. After a few hours, she awoke with intense pain and dizziness. She used her call button, but no one immediately responded.
When full cervical dilation was reached, the fetus was at –1, 0 station. When the fetus reached +1 station, delivery was attempted. The baby was delivered using vacuum extraction.
The child’s Apgar score was 0 at 1 minute of life. Resuscitation was started with intubation and mechanical ventilation. The child’s birth weight was 6.87 lb; arterial blood gas pH measured 6.9; and gestational age was estimated at 38 to 39 weeks.
An electro-encephalogram performed in the NICU suggested intraventricular hemorrhage. The child was found to have perinatal asphyxia, hypoxic ischemic encephalopathy, left parietal skull fracture and cephalohematoma, severe metabolic acidosis, suspected sepsis, transient oliguria, and seizure episodes. The baby was hospitalized for 3.5 months and then followed regularly.
The mother and child moved from Puerto Rico to New York City to obtain better medical care. The child was regularly hospitalized until she died at age 5.
Parent's Claim: There was a discrepancy in gestational age assessment. The nurses failed to monitor fetal heart-rate tracings at proper intervals, and they were unresponsive to the mother. Informed consent did not include vacuum extraction.
Defendants' Defense: The case was settled during trial.
Verdict: A $1.125 million Puerto Rico settlement was reached.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements, & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Mother dies after cesarean delivery: $4.5M verdict
A 31-year-old woman gave birth to her first child by cesarean delivery. Over the next 3 days she reported nausea, vomiting, severe abdominal pain, and had an elevated heart rate. On day 4, she was discharged from the hospital. She went to the ObGyn’s office the next day and was told, after several hours, to return to the hospital. There she was found to have sepsis and acute renal failure. A transfer to another hospital was attempted that night, but she died during transport.
Estate's Claim: The ObGyn should have responded to her reported symptoms prior to discharge by ordering tests. The ObGyn should have called an ambulance to transport her to the hospital from his office.
Defendants’ Defense: The hospital settled for an undisclosed amount before the trial. The ObGyn claimed that there was no negligence in the patient’s treatment.
Verdict: A $4.5 million North Carolina verdict was returned.
Brain-damaged child dies at age 2
A woman was admitted to the hospital in labor. Ninety minutes later a nonreassuring fetal heart-rate tracing was noted. Two hours after that, the ObGyn decided to perform an emergency cesarean delivery.
The child was depressed at birth and required resuscitation. She was transferred to another hospital’s neonatal intensive care unit (NICU), where she was found to have had a severe and catastrophic brain injury. The child died at 2 years of age.
Parent's Claim: An emergency cesarean delivery should have been performed as soon as the fetal heart-rate tracing was found to be nonreassuring. The ObGyn failed to respond to phone calls from the nurses to report fetal distress.
Physician's Defense: The delivery was performed in a timely manner. Brain damage was due to encephalopathy that occurred prior to labor.
Verdict: A Mississippi defense verdict was returned.
Who or what was at fault for ureter injury?
A 45-year-old woman underwent hysterectomy performed by her ObGyn. During surgery, the patient’s ureter was injured. Several additional operations were needed to repair the injury.
Patient's Claim: The patient was not fully informed of the extent of the surgery or possible complications. The ObGyn was negligent in injuring the ureter.
Defendants' Defense: Three months after surgery, the physician entered notes into the patient’s chart that indicated that the ureter injuries were due to a defective monopolar device that had been provided by the hospital. Informed consent included surgical options and complications.
The hospital argued that its equipment was not defective; other surgeons had used the device without any problems. The ObGyn had not used the device before; any injuries were due to his inexperience and negligence.
Verdict: A $2 million South Carolina verdict was returned against the ObGyn. The hospital received a defense verdict.
Did excessive force cause child’s C7 injury?
During delivery, shoulder dystocia was encountered. The child has nerve root avulsion at C7 with damage to adjacent nerve trunks at C5–C6. As a result of the brachial plexus injury, the patient underwent cable grafting and muscle surgeries, but he has permanent weakness and dysfunction in his left arm.
Parent's Claim: Excessive force was used to deliver the child during manipulations for shoulder dystocia.
Physician's Defense: The ObGyn denied using excessive traction. She claimed that she had never used upward traction during a shoulder dystocia presentation. Suprapubic pressure, McRoberts’ maneuver, and delivery of the posterior arm were used. The damage occurred prior to delivery of the head.
Verdict An Illinois defense verdict was returned.
Laparoscopic sheath and coils found at exploratory surgery
In april 2007, a woman underwent a sterilization procedure (Essure) after which she reported pelvic pain. In September 2007, she consented to right salpingo-oophorectomy plus appendectomy. The ObGyn performed the surgery using a robotic device. After surgery, the pathology report indicated that the resected organs were normal and functional.
The patient moved to another state. She continued to have pain and sought treatment with another physician. A computed tomography (CT) scan more than 3 years after robotic surgery revealed foreign objects in the patient’s body. One full Essure coil, a non-fired coil, a second partial coil, and a robotic laparoscopy sheath were surgically removed.
Patient's Claim: The ObGyn was negligent in the performance of the sterilization and robotic surgery procedures. The healthy ovary and fallopian tube should not have been removed and caused her to have surgical menopause.
Physician's defense: The right ovary appeared diseased. The Essure device dropped the coils. The robot malfunctioned during the salpingo-oophorectomy.
Verdict: A $110,513 Oregon verdict was returned, including $10,500 in medical expenses and $100,000 for pain and mental anguish.
Discrepancy in fundal height; child has CP
During her second pregnancy, a 37-year-old woman saw Dr. A, her ObGyn, for regular prenatal care. At 37 weeks’ gestation, the fundal height was not consistent with the fetus’ gestational age: the measurement was higher by 2 cm. No additional testing was scheduled.
At 39.5 weeks’ gestation, the mother reported decreased fetal movement. Because her regular ObGyn was on vacation, she was evaluated by another ObGyn (Dr. B). The fetal heart-rate monitor showed nonreactive results with minimal variability. Dr. B told the mother to drive herself to the emergency department (ED) for additional evaluation. At the hospital, when fetal heart-rate monitoring confirmed fetal distress, an emergency cesarean delivery was performed.
At birth, the baby was not breathing and resuscitation began. The infant was taken to a transitional care unit and then to the NICU, where he was intubated. Cord blood testing confirmed metabolic acidosis. The baby was later found to have dystonic cerebral palsy (CP). He is unable to speak, walk, eat, or care for himself, and he requires 24/7 nursing care.
Parents' Claim: Dr. A failed to order testing after the fundal height discrepancy was found. Testing could have led to an earlier delivery and avoided the injury. The pediatrician failed to ensure adequate oxygenation after delivery. The baby should have been transferred immediately to the NICU and intubated.
Physician's Defense: The fundal height discrepancy was explained by the baby’s position within the uterus. The pediatrician acted heroically to save the child’s life.
Verdict: A $3.5 million Massachusetts settlement was reached.
NT scans misread, not reported; child has Down syndrome
At 13 weeks’ gestation, a 38-year-old woman saw a maternal-fetal medicine (MFM) specialist, who interpreted a nuchal translucency (NT) scan as normal. At 20 weeks’ gestation, an ObGyn performed a second screening that indicated the fetus was at high risk for Down syndrome. However, no further testing was ordered.
At 26.5 weeks’ gestation, amniocentesis was performed after ultrasonography and an echocardiogram revealed fetal abnormalities. A diagnosis of Down syndrome was made at 29 weeks’ gestation, too late for termination of pregnancy.
Parent's Claim: The MFM specialist misread the first NT scan. The ObGyn did not inform the mother of the results of the second screening. Proper interpretation and reporting would have initiated further testing and determination that the baby had Down syndrome before the deadline for termination of pregnancy.
Defendants' Defense: The case was settled during trial.
Verdict: A $3 million New Jersey settlement was reached, including $2 million from the medical center where the second test was performed, $940,000 from the ObGyn, and $60,000 from the MFM specialist.
Uterine rupture, baby dies: $2.15M award
At 38 weeks’ gestation, a mother was admitted to a hospital for induction of labor due to pregnancy-induced hypertension. The fetus was estimated to be large for its gestational age. A uterine rupture occurred during labor. The baby was stillborn.
Parents' Claim: The uterine rupture was not immediately recognized. The ObGyn failed to come to the mother’s bedside until after the fetus had receded up the birth canal, which indicated that a rupture was occurring. The ObGyn ordered oxytocin instead of performing an immediate cesarean delivery. Eleven minutes later, the cesarean was ordered, but the baby had died.
Physician's Defense: There was no negligence; proper protocols were followed. A uterine rupture cannot be predicted.
Verdict: A $650,000 settlement was reached with the hospital before trial. Because the ObGyn was employed by a federally qualified clinic, the matter was filed in federal court. The Illinois court issued a bench decision awarding $1.5 million.
Migrated IUD causes years of pain
In september 2006, an ObGyn inserted an intrauterine device (IUD) in a patient. In February 2007, the patient had an ectopic pregnancy. The IUD was not found during dilation and curettage. The patient continued to report pain to the ObGyn. She sought treatment from another physician in November 2010 due to continuing pain. A CT scan revealed that the IUD had migrated to her abdomen. The IUD was surgically removed.
Patient's Claim: The ObGyn was unwilling to figure out why the patient had continuing pain, and told her to “just deal with it.” He should have found and removed the IUD after the ectopic pregnancy.
Physician's Defense: It was reasonable to assume that the IUD had been expelled, as 2 ultrasonographies performed after ectopic pregnancy revealed nothing. Since the IUD had not caused an abscess, infection, or inflammation, the patient suffered no injury.
Verdict: A Virginia defense verdict was returned.
Profoundly disabled child dies at age 5
A 17-year-old woman with a history of miscarriage received prenatal care from her ObGyn. A July due date was established by ultrasonography in January.
In May, the mother went to the ED with pelvic pain. She was treated for preterm labor and discharged 2 days later.
In early July, ultrasonography showed a fetus in cephalic position with a posterior-located placenta.
At a prenatal examination a week later, the patient reported vaginal discharge. Her ObGyn suspected premature rupture of membranes (PROM) and admitted her to the hospital. Oxytocin was used to induce labor. Intact membranes were artificially ruptured and an internal fetal heart-rate monitor was placed. The ObGyn recorded that the pregnancy was at term.
Hours later, the mother told the nurses that she thought the fetal monitor had become disconnected; the monitor’s placement was not confirmed. The mother was given a sedative. After a few hours, she awoke with intense pain and dizziness. She used her call button, but no one immediately responded.
When full cervical dilation was reached, the fetus was at –1, 0 station. When the fetus reached +1 station, delivery was attempted. The baby was delivered using vacuum extraction.
The child’s Apgar score was 0 at 1 minute of life. Resuscitation was started with intubation and mechanical ventilation. The child’s birth weight was 6.87 lb; arterial blood gas pH measured 6.9; and gestational age was estimated at 38 to 39 weeks.
An electro-encephalogram performed in the NICU suggested intraventricular hemorrhage. The child was found to have perinatal asphyxia, hypoxic ischemic encephalopathy, left parietal skull fracture and cephalohematoma, severe metabolic acidosis, suspected sepsis, transient oliguria, and seizure episodes. The baby was hospitalized for 3.5 months and then followed regularly.
The mother and child moved from Puerto Rico to New York City to obtain better medical care. The child was regularly hospitalized until she died at age 5.
Parent's Claim: There was a discrepancy in gestational age assessment. The nurses failed to monitor fetal heart-rate tracings at proper intervals, and they were unresponsive to the mother. Informed consent did not include vacuum extraction.
Defendants' Defense: The case was settled during trial.
Verdict: A $1.125 million Puerto Rico settlement was reached.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements, & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Mother dies after cesarean delivery: $4.5M verdict
A 31-year-old woman gave birth to her first child by cesarean delivery. Over the next 3 days she reported nausea, vomiting, severe abdominal pain, and had an elevated heart rate. On day 4, she was discharged from the hospital. She went to the ObGyn’s office the next day and was told, after several hours, to return to the hospital. There she was found to have sepsis and acute renal failure. A transfer to another hospital was attempted that night, but she died during transport.
Estate's Claim: The ObGyn should have responded to her reported symptoms prior to discharge by ordering tests. The ObGyn should have called an ambulance to transport her to the hospital from his office.
Defendants’ Defense: The hospital settled for an undisclosed amount before the trial. The ObGyn claimed that there was no negligence in the patient’s treatment.
Verdict: A $4.5 million North Carolina verdict was returned.
Brain-damaged child dies at age 2
A woman was admitted to the hospital in labor. Ninety minutes later a nonreassuring fetal heart-rate tracing was noted. Two hours after that, the ObGyn decided to perform an emergency cesarean delivery.
The child was depressed at birth and required resuscitation. She was transferred to another hospital’s neonatal intensive care unit (NICU), where she was found to have had a severe and catastrophic brain injury. The child died at 2 years of age.
Parent's Claim: An emergency cesarean delivery should have been performed as soon as the fetal heart-rate tracing was found to be nonreassuring. The ObGyn failed to respond to phone calls from the nurses to report fetal distress.
Physician's Defense: The delivery was performed in a timely manner. Brain damage was due to encephalopathy that occurred prior to labor.
Verdict: A Mississippi defense verdict was returned.
Who or what was at fault for ureter injury?
A 45-year-old woman underwent hysterectomy performed by her ObGyn. During surgery, the patient’s ureter was injured. Several additional operations were needed to repair the injury.
Patient's Claim: The patient was not fully informed of the extent of the surgery or possible complications. The ObGyn was negligent in injuring the ureter.
Defendants' Defense: Three months after surgery, the physician entered notes into the patient’s chart that indicated that the ureter injuries were due to a defective monopolar device that had been provided by the hospital. Informed consent included surgical options and complications.
The hospital argued that its equipment was not defective; other surgeons had used the device without any problems. The ObGyn had not used the device before; any injuries were due to his inexperience and negligence.
Verdict: A $2 million South Carolina verdict was returned against the ObGyn. The hospital received a defense verdict.
Did excessive force cause child’s C7 injury?
During delivery, shoulder dystocia was encountered. The child has nerve root avulsion at C7 with damage to adjacent nerve trunks at C5–C6. As a result of the brachial plexus injury, the patient underwent cable grafting and muscle surgeries, but he has permanent weakness and dysfunction in his left arm.
Parent's Claim: Excessive force was used to deliver the child during manipulations for shoulder dystocia.
Physician's Defense: The ObGyn denied using excessive traction. She claimed that she had never used upward traction during a shoulder dystocia presentation. Suprapubic pressure, McRoberts’ maneuver, and delivery of the posterior arm were used. The damage occurred prior to delivery of the head.
Verdict An Illinois defense verdict was returned.
Laparoscopic sheath and coils found at exploratory surgery
In april 2007, a woman underwent a sterilization procedure (Essure) after which she reported pelvic pain. In September 2007, she consented to right salpingo-oophorectomy plus appendectomy. The ObGyn performed the surgery using a robotic device. After surgery, the pathology report indicated that the resected organs were normal and functional.
The patient moved to another state. She continued to have pain and sought treatment with another physician. A computed tomography (CT) scan more than 3 years after robotic surgery revealed foreign objects in the patient’s body. One full Essure coil, a non-fired coil, a second partial coil, and a robotic laparoscopy sheath were surgically removed.
Patient's Claim: The ObGyn was negligent in the performance of the sterilization and robotic surgery procedures. The healthy ovary and fallopian tube should not have been removed and caused her to have surgical menopause.
Physician's defense: The right ovary appeared diseased. The Essure device dropped the coils. The robot malfunctioned during the salpingo-oophorectomy.
Verdict: A $110,513 Oregon verdict was returned, including $10,500 in medical expenses and $100,000 for pain and mental anguish.
Discrepancy in fundal height; child has CP
During her second pregnancy, a 37-year-old woman saw Dr. A, her ObGyn, for regular prenatal care. At 37 weeks’ gestation, the fundal height was not consistent with the fetus’ gestational age: the measurement was higher by 2 cm. No additional testing was scheduled.
At 39.5 weeks’ gestation, the mother reported decreased fetal movement. Because her regular ObGyn was on vacation, she was evaluated by another ObGyn (Dr. B). The fetal heart-rate monitor showed nonreactive results with minimal variability. Dr. B told the mother to drive herself to the emergency department (ED) for additional evaluation. At the hospital, when fetal heart-rate monitoring confirmed fetal distress, an emergency cesarean delivery was performed.
At birth, the baby was not breathing and resuscitation began. The infant was taken to a transitional care unit and then to the NICU, where he was intubated. Cord blood testing confirmed metabolic acidosis. The baby was later found to have dystonic cerebral palsy (CP). He is unable to speak, walk, eat, or care for himself, and he requires 24/7 nursing care.
Parents' Claim: Dr. A failed to order testing after the fundal height discrepancy was found. Testing could have led to an earlier delivery and avoided the injury. The pediatrician failed to ensure adequate oxygenation after delivery. The baby should have been transferred immediately to the NICU and intubated.
Physician's Defense: The fundal height discrepancy was explained by the baby’s position within the uterus. The pediatrician acted heroically to save the child’s life.
Verdict: A $3.5 million Massachusetts settlement was reached.
NT scans misread, not reported; child has Down syndrome
At 13 weeks’ gestation, a 38-year-old woman saw a maternal-fetal medicine (MFM) specialist, who interpreted a nuchal translucency (NT) scan as normal. At 20 weeks’ gestation, an ObGyn performed a second screening that indicated the fetus was at high risk for Down syndrome. However, no further testing was ordered.
At 26.5 weeks’ gestation, amniocentesis was performed after ultrasonography and an echocardiogram revealed fetal abnormalities. A diagnosis of Down syndrome was made at 29 weeks’ gestation, too late for termination of pregnancy.
Parent's Claim: The MFM specialist misread the first NT scan. The ObGyn did not inform the mother of the results of the second screening. Proper interpretation and reporting would have initiated further testing and determination that the baby had Down syndrome before the deadline for termination of pregnancy.
Defendants' Defense: The case was settled during trial.
Verdict: A $3 million New Jersey settlement was reached, including $2 million from the medical center where the second test was performed, $940,000 from the ObGyn, and $60,000 from the MFM specialist.
Uterine rupture, baby dies: $2.15M award
At 38 weeks’ gestation, a mother was admitted to a hospital for induction of labor due to pregnancy-induced hypertension. The fetus was estimated to be large for its gestational age. A uterine rupture occurred during labor. The baby was stillborn.
Parents' Claim: The uterine rupture was not immediately recognized. The ObGyn failed to come to the mother’s bedside until after the fetus had receded up the birth canal, which indicated that a rupture was occurring. The ObGyn ordered oxytocin instead of performing an immediate cesarean delivery. Eleven minutes later, the cesarean was ordered, but the baby had died.
Physician's Defense: There was no negligence; proper protocols were followed. A uterine rupture cannot be predicted.
Verdict: A $650,000 settlement was reached with the hospital before trial. Because the ObGyn was employed by a federally qualified clinic, the matter was filed in federal court. The Illinois court issued a bench decision awarding $1.5 million.
Migrated IUD causes years of pain
In september 2006, an ObGyn inserted an intrauterine device (IUD) in a patient. In February 2007, the patient had an ectopic pregnancy. The IUD was not found during dilation and curettage. The patient continued to report pain to the ObGyn. She sought treatment from another physician in November 2010 due to continuing pain. A CT scan revealed that the IUD had migrated to her abdomen. The IUD was surgically removed.
Patient's Claim: The ObGyn was unwilling to figure out why the patient had continuing pain, and told her to “just deal with it.” He should have found and removed the IUD after the ectopic pregnancy.
Physician's Defense: It was reasonable to assume that the IUD had been expelled, as 2 ultrasonographies performed after ectopic pregnancy revealed nothing. Since the IUD had not caused an abscess, infection, or inflammation, the patient suffered no injury.
Verdict: A Virginia defense verdict was returned.
Profoundly disabled child dies at age 5
A 17-year-old woman with a history of miscarriage received prenatal care from her ObGyn. A July due date was established by ultrasonography in January.
In May, the mother went to the ED with pelvic pain. She was treated for preterm labor and discharged 2 days later.
In early July, ultrasonography showed a fetus in cephalic position with a posterior-located placenta.
At a prenatal examination a week later, the patient reported vaginal discharge. Her ObGyn suspected premature rupture of membranes (PROM) and admitted her to the hospital. Oxytocin was used to induce labor. Intact membranes were artificially ruptured and an internal fetal heart-rate monitor was placed. The ObGyn recorded that the pregnancy was at term.
Hours later, the mother told the nurses that she thought the fetal monitor had become disconnected; the monitor’s placement was not confirmed. The mother was given a sedative. After a few hours, she awoke with intense pain and dizziness. She used her call button, but no one immediately responded.
When full cervical dilation was reached, the fetus was at –1, 0 station. When the fetus reached +1 station, delivery was attempted. The baby was delivered using vacuum extraction.
The child’s Apgar score was 0 at 1 minute of life. Resuscitation was started with intubation and mechanical ventilation. The child’s birth weight was 6.87 lb; arterial blood gas pH measured 6.9; and gestational age was estimated at 38 to 39 weeks.
An electro-encephalogram performed in the NICU suggested intraventricular hemorrhage. The child was found to have perinatal asphyxia, hypoxic ischemic encephalopathy, left parietal skull fracture and cephalohematoma, severe metabolic acidosis, suspected sepsis, transient oliguria, and seizure episodes. The baby was hospitalized for 3.5 months and then followed regularly.
The mother and child moved from Puerto Rico to New York City to obtain better medical care. The child was regularly hospitalized until she died at age 5.
Parent's Claim: There was a discrepancy in gestational age assessment. The nurses failed to monitor fetal heart-rate tracings at proper intervals, and they were unresponsive to the mother. Informed consent did not include vacuum extraction.
Defendants' Defense: The case was settled during trial.
Verdict: A $1.125 million Puerto Rico settlement was reached.
These cases were selected by the editors of OBG Management from Medical Malpractice Verdicts, Settlements, & Experts, with permission of the editor, Lewis Laska (www.verdictslaska.com). The information available to the editors about the cases presented here is sometimes incomplete. Moreover, the cases may or may not have merit. Nevertheless, these cases represent the types of clinical situations that typically result in litigation and are meant to illustrate nationwide variation in jury verdicts and awards.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
IN THIS ARTICLE
- Brain-damaged child dies at age 2
- Who or what was at fault for ureter injury?
- Did excessive force cause child’s C7 injury?
- Laparoscopic sheath and coils found at exploratory surgery
- Discrepancy in fundal height; child has CP
- NT scans misread, not reported; child has Down syndrome
- Uterine rupture, baby dies: $2.15M award
- Migrated IUD causes years of pain
- Profoundly disabled child dies at age 5
David Henry's JCSO podcast, March 2015
In his March podcast for The Journal of Community and Supportive Oncology, Dr David Henry discusses two original research articles that focus on women who have survived cancer, one that looks at the effects of ArginMax on sexual functioning and quality of life in female cancer survivors, and another that examines the need for decision and communication aids among breast cancer survivors. Also included in this month’s line-up of original research is a report on the cost of palliative external beam radiotherapy for bone metastases in patients with prostate cancer and a study of the impact of an electronic medical record intervention on the use of growth factor in patients with cancer. The regular Community Translations column features the recently approved combination therapy, palbociclib plus letrozole, for first-line treatment of postmenopausal women with estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer. Dr Henry rounds off the podcast with a discussion the Journal Club’s entry, which this month focuses on new approvals, genetic testing, and maintenance therapy in women with ovarian cancer.
In his March podcast for The Journal of Community and Supportive Oncology, Dr David Henry discusses two original research articles that focus on women who have survived cancer, one that looks at the effects of ArginMax on sexual functioning and quality of life in female cancer survivors, and another that examines the need for decision and communication aids among breast cancer survivors. Also included in this month’s line-up of original research is a report on the cost of palliative external beam radiotherapy for bone metastases in patients with prostate cancer and a study of the impact of an electronic medical record intervention on the use of growth factor in patients with cancer. The regular Community Translations column features the recently approved combination therapy, palbociclib plus letrozole, for first-line treatment of postmenopausal women with estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer. Dr Henry rounds off the podcast with a discussion the Journal Club’s entry, which this month focuses on new approvals, genetic testing, and maintenance therapy in women with ovarian cancer.
In his March podcast for The Journal of Community and Supportive Oncology, Dr David Henry discusses two original research articles that focus on women who have survived cancer, one that looks at the effects of ArginMax on sexual functioning and quality of life in female cancer survivors, and another that examines the need for decision and communication aids among breast cancer survivors. Also included in this month’s line-up of original research is a report on the cost of palliative external beam radiotherapy for bone metastases in patients with prostate cancer and a study of the impact of an electronic medical record intervention on the use of growth factor in patients with cancer. The regular Community Translations column features the recently approved combination therapy, palbociclib plus letrozole, for first-line treatment of postmenopausal women with estrogen receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer. Dr Henry rounds off the podcast with a discussion the Journal Club’s entry, which this month focuses on new approvals, genetic testing, and maintenance therapy in women with ovarian cancer.
Compounds could treat MLL leukemia

and Jolanta Grembecka, PhD
Photo courtesy of the
University of Michigan
Two small-molecule inhibitors can fight aggressive, acute leukemias by targeting a protein-protein interaction, according to preclinical research published in Cancer Cell.
The compounds, MI-463 and MI-503, work by inhibiting the interaction between menin and mixed-lineage leukemia (MLL) fusion proteins.
Menin binds to the N-terminal fragment of MLL retained in all MLL fusion proteins, and the fusion proteins require menin for leukemogenic activity.
That’s why Jolanta Grembecka, PhD, and Tomasz Cierpicki, PhD, both of the University of Michigan in Ann Arbor, have been working for several years to identify small-molecule inhibitors that would block the MLL-menin interaction.
“The MLL-menin interaction is a good drug target because it’s the primary driver in [MLL] leukemia,” Dr Grembecka said. “By blocking this interaction, it’s very likely to stop the cancer.”
With that in mind, Dr Grembecka and her colleagues tested 2 compounds they developed, MI-463 and MI-503, in cell lines and mice with MLL leukemia. The compounds blocked the MLL-menin interaction without affecting normal hematopoiesis.
The team also noted that both compounds demonstrated metabolic stability and favorable pharmacokinetic profiles.
“Against all odds, we decided to explore finding a way to block the MLL-menin interaction with small molecules,” Dr Cierpicki said. “From nothing, we have been able to identify and greatly improve a compound and show that it’s got valuable potential in blocking MLL fusion leukemia in animal models.”
In a separate study published in Nature Medicine, the researchers discovered that menin and MLL play a role in androgen receptor signaling, a key driver of prostate cancer.
The team found that MI-503 and MI-136, another inhibitor of the menin-MLL interaction, were both active against castration-resistant prostate cancer in vitro and in vivo.
The researchers said they will continue to investigate the role of MLL in castration-resistant prostate cancer. And they plan to further refine their inhibitors and put the compounds through more advanced preclinical testing in MLL leukemia. ![]()

and Jolanta Grembecka, PhD
Photo courtesy of the
University of Michigan
Two small-molecule inhibitors can fight aggressive, acute leukemias by targeting a protein-protein interaction, according to preclinical research published in Cancer Cell.
The compounds, MI-463 and MI-503, work by inhibiting the interaction between menin and mixed-lineage leukemia (MLL) fusion proteins.
Menin binds to the N-terminal fragment of MLL retained in all MLL fusion proteins, and the fusion proteins require menin for leukemogenic activity.
That’s why Jolanta Grembecka, PhD, and Tomasz Cierpicki, PhD, both of the University of Michigan in Ann Arbor, have been working for several years to identify small-molecule inhibitors that would block the MLL-menin interaction.
“The MLL-menin interaction is a good drug target because it’s the primary driver in [MLL] leukemia,” Dr Grembecka said. “By blocking this interaction, it’s very likely to stop the cancer.”
With that in mind, Dr Grembecka and her colleagues tested 2 compounds they developed, MI-463 and MI-503, in cell lines and mice with MLL leukemia. The compounds blocked the MLL-menin interaction without affecting normal hematopoiesis.
The team also noted that both compounds demonstrated metabolic stability and favorable pharmacokinetic profiles.
“Against all odds, we decided to explore finding a way to block the MLL-menin interaction with small molecules,” Dr Cierpicki said. “From nothing, we have been able to identify and greatly improve a compound and show that it’s got valuable potential in blocking MLL fusion leukemia in animal models.”
In a separate study published in Nature Medicine, the researchers discovered that menin and MLL play a role in androgen receptor signaling, a key driver of prostate cancer.
The team found that MI-503 and MI-136, another inhibitor of the menin-MLL interaction, were both active against castration-resistant prostate cancer in vitro and in vivo.
The researchers said they will continue to investigate the role of MLL in castration-resistant prostate cancer. And they plan to further refine their inhibitors and put the compounds through more advanced preclinical testing in MLL leukemia. ![]()

and Jolanta Grembecka, PhD
Photo courtesy of the
University of Michigan
Two small-molecule inhibitors can fight aggressive, acute leukemias by targeting a protein-protein interaction, according to preclinical research published in Cancer Cell.
The compounds, MI-463 and MI-503, work by inhibiting the interaction between menin and mixed-lineage leukemia (MLL) fusion proteins.
Menin binds to the N-terminal fragment of MLL retained in all MLL fusion proteins, and the fusion proteins require menin for leukemogenic activity.
That’s why Jolanta Grembecka, PhD, and Tomasz Cierpicki, PhD, both of the University of Michigan in Ann Arbor, have been working for several years to identify small-molecule inhibitors that would block the MLL-menin interaction.
“The MLL-menin interaction is a good drug target because it’s the primary driver in [MLL] leukemia,” Dr Grembecka said. “By blocking this interaction, it’s very likely to stop the cancer.”
With that in mind, Dr Grembecka and her colleagues tested 2 compounds they developed, MI-463 and MI-503, in cell lines and mice with MLL leukemia. The compounds blocked the MLL-menin interaction without affecting normal hematopoiesis.
The team also noted that both compounds demonstrated metabolic stability and favorable pharmacokinetic profiles.
“Against all odds, we decided to explore finding a way to block the MLL-menin interaction with small molecules,” Dr Cierpicki said. “From nothing, we have been able to identify and greatly improve a compound and show that it’s got valuable potential in blocking MLL fusion leukemia in animal models.”
In a separate study published in Nature Medicine, the researchers discovered that menin and MLL play a role in androgen receptor signaling, a key driver of prostate cancer.
The team found that MI-503 and MI-136, another inhibitor of the menin-MLL interaction, were both active against castration-resistant prostate cancer in vitro and in vivo.
The researchers said they will continue to investigate the role of MLL in castration-resistant prostate cancer. And they plan to further refine their inhibitors and put the compounds through more advanced preclinical testing in MLL leukemia. ![]()
Findings may aid development of antithrombotic drugs
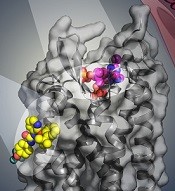
BPTU (yellow) bind to P2Y1R
Image courtesy of
Yekaterina Kadyshevskaya
Researchers say they have identified 2 disparate ligand-binding sites in the human P2Y1 receptor (P2Y1R), which plays a critical role in thrombosis.
Their research has provided a detailed molecular map of P2Y1R, a G protein-coupled receptor (GPCR), in complex with a nucleotide antagonist MRS2500 and a non-nucleotide antagonist BPTU.
The researchers believe their findings, published in Nature, could aid the development of new antithrombotic drugs.
Beili Wu, PhD, of the Shanghai Institute of Materia Medica in China, and her colleagues noted that the human purinergic receptors P2Y1R and P2Y12R play a major physiological role in adenosine 5′-diphosphate (ADP)-mediated platelet aggregation, an important component of thrombosis.
Although most of the available antithrombotic drugs act on P2Y12R, research has suggested that P2Y1R may be a promising antithrombotic drug target. In addition, P2Y1R inhibitors may offer a safety advantage over P2Y12R inhibitors by reducing the risk of bleeding. However, efforts to develop new drugs have been impeded by poor understanding of receptor-ligand interaction.
“The P2Y1R structures [we mapped] help us understand how this receptor and different types of experimental drugs interact at the molecular level and could enable further exploration to design new and safer antithrombotic drugs with reduced adverse effects,” Dr Wu said.
She and her colleagues found that the nucleotide ligand MRS2500 recognizes a binding site within the transmembrane bundle of P2Y1R. And it is different in shape and location from the nucleotide-binding site in P2Y12R.
“It is amazing to observe that 2 GPCRs recognize the same ligand in such different ways,” Dr Wu said. “The finding highlights the diversity of signal recognition mechanisms in GPCRs, and this is of great value to drug design for each receptor with high selectivity.”
The researchers also found that, instead of interacting within the transmembrane bundle, the non-nucleotide ligand BPTU binds to a pocket on the outer interface of P2Y1R embedded in the cell membrane.
This is the first structurally characterized, selective, and high-affinity GPCR ligand located entirely outside of the helical bundle, and it represents a new paradigm in ligand binding to alter signaling in GPCRs, according to the researchers.
The team believes this new understanding of the P2Y1R structure provides opportunities to broaden the scope of future GPCR drug discovery to target novel sites outside of the conventional GPCR ligand-binding pocket, which may improve drug selectivity and reduce side effects. ![]()

BPTU (yellow) bind to P2Y1R
Image courtesy of
Yekaterina Kadyshevskaya
Researchers say they have identified 2 disparate ligand-binding sites in the human P2Y1 receptor (P2Y1R), which plays a critical role in thrombosis.
Their research has provided a detailed molecular map of P2Y1R, a G protein-coupled receptor (GPCR), in complex with a nucleotide antagonist MRS2500 and a non-nucleotide antagonist BPTU.
The researchers believe their findings, published in Nature, could aid the development of new antithrombotic drugs.
Beili Wu, PhD, of the Shanghai Institute of Materia Medica in China, and her colleagues noted that the human purinergic receptors P2Y1R and P2Y12R play a major physiological role in adenosine 5′-diphosphate (ADP)-mediated platelet aggregation, an important component of thrombosis.
Although most of the available antithrombotic drugs act on P2Y12R, research has suggested that P2Y1R may be a promising antithrombotic drug target. In addition, P2Y1R inhibitors may offer a safety advantage over P2Y12R inhibitors by reducing the risk of bleeding. However, efforts to develop new drugs have been impeded by poor understanding of receptor-ligand interaction.
“The P2Y1R structures [we mapped] help us understand how this receptor and different types of experimental drugs interact at the molecular level and could enable further exploration to design new and safer antithrombotic drugs with reduced adverse effects,” Dr Wu said.
She and her colleagues found that the nucleotide ligand MRS2500 recognizes a binding site within the transmembrane bundle of P2Y1R. And it is different in shape and location from the nucleotide-binding site in P2Y12R.
“It is amazing to observe that 2 GPCRs recognize the same ligand in such different ways,” Dr Wu said. “The finding highlights the diversity of signal recognition mechanisms in GPCRs, and this is of great value to drug design for each receptor with high selectivity.”
The researchers also found that, instead of interacting within the transmembrane bundle, the non-nucleotide ligand BPTU binds to a pocket on the outer interface of P2Y1R embedded in the cell membrane.
This is the first structurally characterized, selective, and high-affinity GPCR ligand located entirely outside of the helical bundle, and it represents a new paradigm in ligand binding to alter signaling in GPCRs, according to the researchers.
The team believes this new understanding of the P2Y1R structure provides opportunities to broaden the scope of future GPCR drug discovery to target novel sites outside of the conventional GPCR ligand-binding pocket, which may improve drug selectivity and reduce side effects. ![]()

BPTU (yellow) bind to P2Y1R
Image courtesy of
Yekaterina Kadyshevskaya
Researchers say they have identified 2 disparate ligand-binding sites in the human P2Y1 receptor (P2Y1R), which plays a critical role in thrombosis.
Their research has provided a detailed molecular map of P2Y1R, a G protein-coupled receptor (GPCR), in complex with a nucleotide antagonist MRS2500 and a non-nucleotide antagonist BPTU.
The researchers believe their findings, published in Nature, could aid the development of new antithrombotic drugs.
Beili Wu, PhD, of the Shanghai Institute of Materia Medica in China, and her colleagues noted that the human purinergic receptors P2Y1R and P2Y12R play a major physiological role in adenosine 5′-diphosphate (ADP)-mediated platelet aggregation, an important component of thrombosis.
Although most of the available antithrombotic drugs act on P2Y12R, research has suggested that P2Y1R may be a promising antithrombotic drug target. In addition, P2Y1R inhibitors may offer a safety advantage over P2Y12R inhibitors by reducing the risk of bleeding. However, efforts to develop new drugs have been impeded by poor understanding of receptor-ligand interaction.
“The P2Y1R structures [we mapped] help us understand how this receptor and different types of experimental drugs interact at the molecular level and could enable further exploration to design new and safer antithrombotic drugs with reduced adverse effects,” Dr Wu said.
She and her colleagues found that the nucleotide ligand MRS2500 recognizes a binding site within the transmembrane bundle of P2Y1R. And it is different in shape and location from the nucleotide-binding site in P2Y12R.
“It is amazing to observe that 2 GPCRs recognize the same ligand in such different ways,” Dr Wu said. “The finding highlights the diversity of signal recognition mechanisms in GPCRs, and this is of great value to drug design for each receptor with high selectivity.”
The researchers also found that, instead of interacting within the transmembrane bundle, the non-nucleotide ligand BPTU binds to a pocket on the outer interface of P2Y1R embedded in the cell membrane.
This is the first structurally characterized, selective, and high-affinity GPCR ligand located entirely outside of the helical bundle, and it represents a new paradigm in ligand binding to alter signaling in GPCRs, according to the researchers.
The team believes this new understanding of the P2Y1R structure provides opportunities to broaden the scope of future GPCR drug discovery to target novel sites outside of the conventional GPCR ligand-binding pocket, which may improve drug selectivity and reduce side effects. ![]()
FDA strengthens warnings for anemia drug

Photo by Bill Branson
The US Food and Drug Administration (FDA) has strengthened an existing warning that serious, potentially fatal, allergic reactions can occur with the anemia drug Feraheme (ferumoxytol).
The FDA changed the drug’s prescribing information and approved a boxed warning detailing this risk.
The agency also added a new contraindication, which advises against the use of Feraheme in patients who have had an allergic reaction to any intravenous (IV) iron replacement product.
The FDA said it is continuing to monitor and evaluate the risk of serious allergic reactions with all IV iron products, and the agency will update the public as new information becomes available.
About Feraheme
Feraheme is an IV iron replacement product used to treat iron-deficiency anemia in patients with chronic kidney disease. Like other IV iron products, Feraheme may only be given where emergency personnel and equipment are immediately available to treat the potentially life-threatening allergic reactions that can occur with treatment.
All IV iron products carry a risk of potentially life-threatening allergic reactions. At the time of Feraheme’s approval in 2009, this risk was described in the “Warnings and Precautions” section of the drug label.
Since then, serious reactions, including deaths, have occurred, despite the proper use of therapies to treat these reactions and emergency resuscitation measures.
Serious reactions reported
In the initial clinical trials of Feraheme, conducted predominantly in patients with chronic kidney disease, serious hypersensitivity reactions were reported in 0.2% (3/1726) of patients receiving Feraheme.
Other adverse reactions potentially associated with hypersensitivity (eg, pruritus, rash, urticaria, or wheezing) were reported in 3.7% (63/1726) of these patients.
In other trials that did not include patients with chronic kidney disease, moderate to severe hypersensitivity reactions, including anaphylaxis, were reported in 2.6% (26/1014) of patients treated with Feraheme.
Since the approval of Feraheme on June 30, 2009, cases of serious hypersensitivity reactions, including death, have occurred.
A search of the FDA Adverse Event Reporting System database revealed 79 cases of anaphylactic reactions associated with Feraheme administration, reported from the time of approval to June 30, 2014. Of the 79 cases, 18 were fatal, despite immediate medical intervention and emergency resuscitation attempts.
The 79 patients ranged in age from 19 to 96 years. In nearly half of all cases, the anaphylactic reactions occurred with the first dose of Feraheme. For approximately 75% (60/79) of the cases, the reaction began during the infusion or within 5 minutes after administration was complete.
Frequently reported symptoms included cardiac arrest, hypotension, dyspnea, nausea, vomiting, and flushing. Of the 79 patients, 43% (34/79) had a medical history of drug allergy, and 24% had a history of multiple drug allergies.
Recommendations for administering Feraheme
Initial symptoms of fatal and serious hypersensitivity reactions associated with Feraheme may include hypotension, syncope, unresponsiveness, and cardiac/cardiorespiratory arrest, with or without signs of rash.
All IV iron products carry a risk of anaphylaxis, so these products should be administered only in patients who require IV iron therapy.
Feraheme is only approved for use in adults with iron-deficiency anemia in the setting of chronic kidney disease. The drug is contraindicated in patients with a history of hypersensitivity to Feraheme or any other IV iron product.
Only administer Feraheme and other IV iron products when personnel and therapies are immediately available for the treatment of anaphylaxis and other hypersensitivity reactions.
Patients with a history of multiple drug allergies may have a greater risk of anaphylaxis with parenteral iron products. Carefully consider the potential risks and benefits before administering Feraheme to these patients.
Feraheme should only be administered as an IV infusion in 50-200 mL of 0.9% sodium chloride or 5% dextrose over a minimum period of 15 minutes following dilution. Do not administer Feraheme by undiluted IV injection.
Closely monitor patients for signs and symptoms of hypersensitivity reactions, including monitoring blood pressure and pulse during administration and for at least 30 minutes following each infusion of Feraheme.
Elderly patients 65 years of age and older with multiple or serious comorbidities who experience hypersensitivity reactions, hypotension, or both following administration of Feraheme may have more severe outcomes.
Advise patients to immediately report any signs and symptoms of hypersensitivity that may develop during and following Feraheme administration, such as respiratory distress, hypotension, dizziness or lightheadedness, edema, rash, or itching. Advise patients to seek immediate medical attention if these signs and symptoms occur.
Allow at least 30 minutes between administration of Feraheme and administration of other medications that could potentially cause serious hypersensitivity reactions, hypotension, or both, such as chemotherapeutic agents or monoclonal antibodies.
Report adverse events involving Feraheme to the FDA’s MedWatch Program. ![]()

Photo by Bill Branson
The US Food and Drug Administration (FDA) has strengthened an existing warning that serious, potentially fatal, allergic reactions can occur with the anemia drug Feraheme (ferumoxytol).
The FDA changed the drug’s prescribing information and approved a boxed warning detailing this risk.
The agency also added a new contraindication, which advises against the use of Feraheme in patients who have had an allergic reaction to any intravenous (IV) iron replacement product.
The FDA said it is continuing to monitor and evaluate the risk of serious allergic reactions with all IV iron products, and the agency will update the public as new information becomes available.
About Feraheme
Feraheme is an IV iron replacement product used to treat iron-deficiency anemia in patients with chronic kidney disease. Like other IV iron products, Feraheme may only be given where emergency personnel and equipment are immediately available to treat the potentially life-threatening allergic reactions that can occur with treatment.
All IV iron products carry a risk of potentially life-threatening allergic reactions. At the time of Feraheme’s approval in 2009, this risk was described in the “Warnings and Precautions” section of the drug label.
Since then, serious reactions, including deaths, have occurred, despite the proper use of therapies to treat these reactions and emergency resuscitation measures.
Serious reactions reported
In the initial clinical trials of Feraheme, conducted predominantly in patients with chronic kidney disease, serious hypersensitivity reactions were reported in 0.2% (3/1726) of patients receiving Feraheme.
Other adverse reactions potentially associated with hypersensitivity (eg, pruritus, rash, urticaria, or wheezing) were reported in 3.7% (63/1726) of these patients.
In other trials that did not include patients with chronic kidney disease, moderate to severe hypersensitivity reactions, including anaphylaxis, were reported in 2.6% (26/1014) of patients treated with Feraheme.
Since the approval of Feraheme on June 30, 2009, cases of serious hypersensitivity reactions, including death, have occurred.
A search of the FDA Adverse Event Reporting System database revealed 79 cases of anaphylactic reactions associated with Feraheme administration, reported from the time of approval to June 30, 2014. Of the 79 cases, 18 were fatal, despite immediate medical intervention and emergency resuscitation attempts.
The 79 patients ranged in age from 19 to 96 years. In nearly half of all cases, the anaphylactic reactions occurred with the first dose of Feraheme. For approximately 75% (60/79) of the cases, the reaction began during the infusion or within 5 minutes after administration was complete.
Frequently reported symptoms included cardiac arrest, hypotension, dyspnea, nausea, vomiting, and flushing. Of the 79 patients, 43% (34/79) had a medical history of drug allergy, and 24% had a history of multiple drug allergies.
Recommendations for administering Feraheme
Initial symptoms of fatal and serious hypersensitivity reactions associated with Feraheme may include hypotension, syncope, unresponsiveness, and cardiac/cardiorespiratory arrest, with or without signs of rash.
All IV iron products carry a risk of anaphylaxis, so these products should be administered only in patients who require IV iron therapy.
Feraheme is only approved for use in adults with iron-deficiency anemia in the setting of chronic kidney disease. The drug is contraindicated in patients with a history of hypersensitivity to Feraheme or any other IV iron product.
Only administer Feraheme and other IV iron products when personnel and therapies are immediately available for the treatment of anaphylaxis and other hypersensitivity reactions.
Patients with a history of multiple drug allergies may have a greater risk of anaphylaxis with parenteral iron products. Carefully consider the potential risks and benefits before administering Feraheme to these patients.
Feraheme should only be administered as an IV infusion in 50-200 mL of 0.9% sodium chloride or 5% dextrose over a minimum period of 15 minutes following dilution. Do not administer Feraheme by undiluted IV injection.
Closely monitor patients for signs and symptoms of hypersensitivity reactions, including monitoring blood pressure and pulse during administration and for at least 30 minutes following each infusion of Feraheme.
Elderly patients 65 years of age and older with multiple or serious comorbidities who experience hypersensitivity reactions, hypotension, or both following administration of Feraheme may have more severe outcomes.
Advise patients to immediately report any signs and symptoms of hypersensitivity that may develop during and following Feraheme administration, such as respiratory distress, hypotension, dizziness or lightheadedness, edema, rash, or itching. Advise patients to seek immediate medical attention if these signs and symptoms occur.
Allow at least 30 minutes between administration of Feraheme and administration of other medications that could potentially cause serious hypersensitivity reactions, hypotension, or both, such as chemotherapeutic agents or monoclonal antibodies.
Report adverse events involving Feraheme to the FDA’s MedWatch Program. ![]()

Photo by Bill Branson
The US Food and Drug Administration (FDA) has strengthened an existing warning that serious, potentially fatal, allergic reactions can occur with the anemia drug Feraheme (ferumoxytol).
The FDA changed the drug’s prescribing information and approved a boxed warning detailing this risk.
The agency also added a new contraindication, which advises against the use of Feraheme in patients who have had an allergic reaction to any intravenous (IV) iron replacement product.
The FDA said it is continuing to monitor and evaluate the risk of serious allergic reactions with all IV iron products, and the agency will update the public as new information becomes available.
About Feraheme
Feraheme is an IV iron replacement product used to treat iron-deficiency anemia in patients with chronic kidney disease. Like other IV iron products, Feraheme may only be given where emergency personnel and equipment are immediately available to treat the potentially life-threatening allergic reactions that can occur with treatment.
All IV iron products carry a risk of potentially life-threatening allergic reactions. At the time of Feraheme’s approval in 2009, this risk was described in the “Warnings and Precautions” section of the drug label.
Since then, serious reactions, including deaths, have occurred, despite the proper use of therapies to treat these reactions and emergency resuscitation measures.
Serious reactions reported
In the initial clinical trials of Feraheme, conducted predominantly in patients with chronic kidney disease, serious hypersensitivity reactions were reported in 0.2% (3/1726) of patients receiving Feraheme.
Other adverse reactions potentially associated with hypersensitivity (eg, pruritus, rash, urticaria, or wheezing) were reported in 3.7% (63/1726) of these patients.
In other trials that did not include patients with chronic kidney disease, moderate to severe hypersensitivity reactions, including anaphylaxis, were reported in 2.6% (26/1014) of patients treated with Feraheme.
Since the approval of Feraheme on June 30, 2009, cases of serious hypersensitivity reactions, including death, have occurred.
A search of the FDA Adverse Event Reporting System database revealed 79 cases of anaphylactic reactions associated with Feraheme administration, reported from the time of approval to June 30, 2014. Of the 79 cases, 18 were fatal, despite immediate medical intervention and emergency resuscitation attempts.
The 79 patients ranged in age from 19 to 96 years. In nearly half of all cases, the anaphylactic reactions occurred with the first dose of Feraheme. For approximately 75% (60/79) of the cases, the reaction began during the infusion or within 5 minutes after administration was complete.
Frequently reported symptoms included cardiac arrest, hypotension, dyspnea, nausea, vomiting, and flushing. Of the 79 patients, 43% (34/79) had a medical history of drug allergy, and 24% had a history of multiple drug allergies.
Recommendations for administering Feraheme
Initial symptoms of fatal and serious hypersensitivity reactions associated with Feraheme may include hypotension, syncope, unresponsiveness, and cardiac/cardiorespiratory arrest, with or without signs of rash.
All IV iron products carry a risk of anaphylaxis, so these products should be administered only in patients who require IV iron therapy.
Feraheme is only approved for use in adults with iron-deficiency anemia in the setting of chronic kidney disease. The drug is contraindicated in patients with a history of hypersensitivity to Feraheme or any other IV iron product.
Only administer Feraheme and other IV iron products when personnel and therapies are immediately available for the treatment of anaphylaxis and other hypersensitivity reactions.
Patients with a history of multiple drug allergies may have a greater risk of anaphylaxis with parenteral iron products. Carefully consider the potential risks and benefits before administering Feraheme to these patients.
Feraheme should only be administered as an IV infusion in 50-200 mL of 0.9% sodium chloride or 5% dextrose over a minimum period of 15 minutes following dilution. Do not administer Feraheme by undiluted IV injection.
Closely monitor patients for signs and symptoms of hypersensitivity reactions, including monitoring blood pressure and pulse during administration and for at least 30 minutes following each infusion of Feraheme.
Elderly patients 65 years of age and older with multiple or serious comorbidities who experience hypersensitivity reactions, hypotension, or both following administration of Feraheme may have more severe outcomes.
Advise patients to immediately report any signs and symptoms of hypersensitivity that may develop during and following Feraheme administration, such as respiratory distress, hypotension, dizziness or lightheadedness, edema, rash, or itching. Advise patients to seek immediate medical attention if these signs and symptoms occur.
Allow at least 30 minutes between administration of Feraheme and administration of other medications that could potentially cause serious hypersensitivity reactions, hypotension, or both, such as chemotherapeutic agents or monoclonal antibodies.
Report adverse events involving Feraheme to the FDA’s MedWatch Program. ![]()
Engineered protein overcomes radiation resistance in ALL

Photo courtesy of Dr Uckun
An engineered protein can enhance the effects of radiation and even overcome radiation resistance to treat B-precursor acute lymphoblastic leukemia (ALL), according to research published in EBioMedicine.
The protein, CD19L-sTRAIL, is a fusion of the CD19 ligand protein, which seeks out and binds to leukemia cells, with soluble TRAIL, a protein that can amplify the potency of radiation if it can be anchored on the membrane of leukemia cells.
Researchers found that CD19L-sTRAIL augmented the potency of radiation therapy even against the most aggressive and radiation-resistant forms of leukemia.
“Even very low doses of radiation were highly effective in mice challenged with aggressive human leukemia cells, when it was combined with [CD19L-sTRAIL],” said Fatih M. Uckun, MD, PhD, of The Saban Research Institute of Children’s Hospital Los Angeles in California.
“Due to its ability to selectively anchor to the surface of leukemia cells via its CD19L portion, CD19L-sTRAIL was 100,000-fold more potent than sTRAIL and consistently killed aggressive leukemia cells taken directly from children with ALL—not only in the test tube but also in mice.”
The researchers found that a combination of low-dose total body irradiation (TBI) and CD19L-sTRAIL greatly improved event-free survival (EFS) in mice that had received a typically fatal dose of cells from a patient with relapsed B-precursor ALL.
The median EFS for mice treated with CD19L-sTRAIL plus low-dose TBI was 72 days, which was significantly longer than the EFS for untreated control mice (17 days, P<0.0001), mice that received TBI alone (64 days, P=0.0014), mice that received CD19L-sTRAIL alone (20 days, P=0.0022), and mice that received a combination of vincristine, dexamethasone, and PEG-asparaginase (17 days, P=0.0033).
Dr Uckun and his colleagues noted that none of the mice that received CD19L-sTRAIL and TBI experienced a toxic death or signs of treatment-related toxicity.
The team also found that TBI plus CD19L-sTRAIL improved progression-free survival (PFS) in CD22ΔE12xBCR-ABL double transgenic mice with radiation-resistant, advanced stage, CD19+ murine B-precursor ALL with lymphomatous features.
The mean PFS was 24.0 ± 4.0 days for mice that received CD19L-sTRAIL plus TBI, which was significantly longer than the PFS for control mice (0 ± 0 days, P<0.0001), mice that received CD19L-sTRAIL alone (3.4 ± 0.9 days, P=0.0003), and mice that received TBI alone (9.0 ± 4.6 days, P=0.020).
Based on these results, Dr Uckun and his colleagues believe that incorporating CD19L-sTRAIL into the pre-transplant TBI regimens of patients with very high-risk B-precursor ALL could improve survival after hematopoietic stem cell transplant.
“We are hopeful that the knowledge gained from this study will open a new range of effective treatment opportunities for children with recurrent leukemia,” Dr Uckun said. ![]()

Photo courtesy of Dr Uckun
An engineered protein can enhance the effects of radiation and even overcome radiation resistance to treat B-precursor acute lymphoblastic leukemia (ALL), according to research published in EBioMedicine.
The protein, CD19L-sTRAIL, is a fusion of the CD19 ligand protein, which seeks out and binds to leukemia cells, with soluble TRAIL, a protein that can amplify the potency of radiation if it can be anchored on the membrane of leukemia cells.
Researchers found that CD19L-sTRAIL augmented the potency of radiation therapy even against the most aggressive and radiation-resistant forms of leukemia.
“Even very low doses of radiation were highly effective in mice challenged with aggressive human leukemia cells, when it was combined with [CD19L-sTRAIL],” said Fatih M. Uckun, MD, PhD, of The Saban Research Institute of Children’s Hospital Los Angeles in California.
“Due to its ability to selectively anchor to the surface of leukemia cells via its CD19L portion, CD19L-sTRAIL was 100,000-fold more potent than sTRAIL and consistently killed aggressive leukemia cells taken directly from children with ALL—not only in the test tube but also in mice.”
The researchers found that a combination of low-dose total body irradiation (TBI) and CD19L-sTRAIL greatly improved event-free survival (EFS) in mice that had received a typically fatal dose of cells from a patient with relapsed B-precursor ALL.
The median EFS for mice treated with CD19L-sTRAIL plus low-dose TBI was 72 days, which was significantly longer than the EFS for untreated control mice (17 days, P<0.0001), mice that received TBI alone (64 days, P=0.0014), mice that received CD19L-sTRAIL alone (20 days, P=0.0022), and mice that received a combination of vincristine, dexamethasone, and PEG-asparaginase (17 days, P=0.0033).
Dr Uckun and his colleagues noted that none of the mice that received CD19L-sTRAIL and TBI experienced a toxic death or signs of treatment-related toxicity.
The team also found that TBI plus CD19L-sTRAIL improved progression-free survival (PFS) in CD22ΔE12xBCR-ABL double transgenic mice with radiation-resistant, advanced stage, CD19+ murine B-precursor ALL with lymphomatous features.
The mean PFS was 24.0 ± 4.0 days for mice that received CD19L-sTRAIL plus TBI, which was significantly longer than the PFS for control mice (0 ± 0 days, P<0.0001), mice that received CD19L-sTRAIL alone (3.4 ± 0.9 days, P=0.0003), and mice that received TBI alone (9.0 ± 4.6 days, P=0.020).
Based on these results, Dr Uckun and his colleagues believe that incorporating CD19L-sTRAIL into the pre-transplant TBI regimens of patients with very high-risk B-precursor ALL could improve survival after hematopoietic stem cell transplant.
“We are hopeful that the knowledge gained from this study will open a new range of effective treatment opportunities for children with recurrent leukemia,” Dr Uckun said. ![]()

Photo courtesy of Dr Uckun
An engineered protein can enhance the effects of radiation and even overcome radiation resistance to treat B-precursor acute lymphoblastic leukemia (ALL), according to research published in EBioMedicine.
The protein, CD19L-sTRAIL, is a fusion of the CD19 ligand protein, which seeks out and binds to leukemia cells, with soluble TRAIL, a protein that can amplify the potency of radiation if it can be anchored on the membrane of leukemia cells.
Researchers found that CD19L-sTRAIL augmented the potency of radiation therapy even against the most aggressive and radiation-resistant forms of leukemia.
“Even very low doses of radiation were highly effective in mice challenged with aggressive human leukemia cells, when it was combined with [CD19L-sTRAIL],” said Fatih M. Uckun, MD, PhD, of The Saban Research Institute of Children’s Hospital Los Angeles in California.
“Due to its ability to selectively anchor to the surface of leukemia cells via its CD19L portion, CD19L-sTRAIL was 100,000-fold more potent than sTRAIL and consistently killed aggressive leukemia cells taken directly from children with ALL—not only in the test tube but also in mice.”
The researchers found that a combination of low-dose total body irradiation (TBI) and CD19L-sTRAIL greatly improved event-free survival (EFS) in mice that had received a typically fatal dose of cells from a patient with relapsed B-precursor ALL.
The median EFS for mice treated with CD19L-sTRAIL plus low-dose TBI was 72 days, which was significantly longer than the EFS for untreated control mice (17 days, P<0.0001), mice that received TBI alone (64 days, P=0.0014), mice that received CD19L-sTRAIL alone (20 days, P=0.0022), and mice that received a combination of vincristine, dexamethasone, and PEG-asparaginase (17 days, P=0.0033).
Dr Uckun and his colleagues noted that none of the mice that received CD19L-sTRAIL and TBI experienced a toxic death or signs of treatment-related toxicity.
The team also found that TBI plus CD19L-sTRAIL improved progression-free survival (PFS) in CD22ΔE12xBCR-ABL double transgenic mice with radiation-resistant, advanced stage, CD19+ murine B-precursor ALL with lymphomatous features.
The mean PFS was 24.0 ± 4.0 days for mice that received CD19L-sTRAIL plus TBI, which was significantly longer than the PFS for control mice (0 ± 0 days, P<0.0001), mice that received CD19L-sTRAIL alone (3.4 ± 0.9 days, P=0.0003), and mice that received TBI alone (9.0 ± 4.6 days, P=0.020).
Based on these results, Dr Uckun and his colleagues believe that incorporating CD19L-sTRAIL into the pre-transplant TBI regimens of patients with very high-risk B-precursor ALL could improve survival after hematopoietic stem cell transplant.
“We are hopeful that the knowledge gained from this study will open a new range of effective treatment opportunities for children with recurrent leukemia,” Dr Uckun said. ![]()
July Effect on Never Events
The simultaneous arrival of new residents, medical students, and faculty in July each year results in a complex transition period for hospitals. Medical centers strive to deliver high‐quality and efficient care while undergoing these cyclical changes, with over 100,000 interns/residents in the United States taking part in this changeover.[1] This period is hypothesized to hold an increased risk of adverse outcomes referred to as the July Effect.[1, 2, 3, 4, 5] Although studies have reported associated increases in mortality risk, decreases in efficiency, and an increase in undesirable events during this time, occurrences are still debated.[1, 3, 4, 5]
In 2008, the Centers for Medicare & Medicaid Services (CMS) published and instituted a nationwide series of never events. These events, narrowed to a list of hospital‐acquired complications (HACs), are characterized as iatrogenic adverse outcomes and deemed preventable and egregious. Medicare has subsequently withheld reimbursement for additional cost of treatment related to the events.[6, 7, 8] HACs include complications such as air embolism, retained foreign body, blood incompatibility, pressure ulcer, catheter‐associated urinary tract infection (UTI), vascular catheter‐associated infection, manifestations of poor glycemic control, falls/trauma, deep venous thrombosis or pulmonary embolism after total knee and hip replacements, surgical site infections after coronary artery bypass graft, and surgical site infections after certain orthopedic or bariatric surgeries. Prior studies have utilized HACs as a metric for quality of healthcare delivery in subspecialties such as cerebrovascular surgery, bowel surgery, and urology.[6, 9, 10]
Though the July effect has been assessed across multiple specialties and hospitals, no prior studies have evaluated this phenomenon on a national level and incorporated all hospital admission diagnoses. Through this study, we aim to provide insight into this relatively new quality metric when evaluating admissions made during the early months of the academic year. This study's primary aim is to evaluate the frequency of HAC occurrence across hospital discharges on a national level as a function of admission month after the initiation of the nonreimbursable nature of the CMS never events in 2008. Furthermore, the secondary aims of this study examine the impact of the July effect on inpatient length of stay (LOS) and charges. We hypothesized that July admission is associated with an increases in HAC occurrence, LOS, and inpatient charges.
METHODS
Data Source
An observational study was conducted using data extracted from the Nationwide Inpatient Sample (NIS) years 2008 to 2011. NIS is an annually compiled database maintained by the Agency for Healthcare Research and Quality and contains information on more than 8 million hospital admissions each year from more than 40 states and 1000 hospitals.[11] The database represents 20% of all US hospital discharges and contains a weighting system that allows for calculation of population estimates.[11]
Patient Sample
All patients who were admitted to a hospital from 2008 to 2011 were included in this study. NIS does not contain unique patient identifiers; thus, each discharge was treated as an independent event, even if it may have represented a repeat hospitalization by the same patient. Each hospitalization contained patient and hospital factors that were included as covariates for analysis. Patient factors such as race (white, black, Hispanic, Asian/Pacific Islander, Native American, other), payer information (Medicare, Medicaid, private insurance, no charge, self‐pay, other), and gender (male, female) were included as categorical variables. Other patient covariates of interest included age (recoded from a continuous to a categorical variable with the following groupings: <18, 19 to 30, 31 to 40, 41 to 50, 50 to 65, 66 to 80, and >80 years) and number of comorbidities (none, 1, 2 or more). The comorbidities variable was drawn from the NIS database and was derived directly from the Elixhauser comorbidity index that is often cited in other studies as a risk‐adjustment measure.[12] Hospital factors, such as bed size (small [<200], medium [201400], large [>400 beds]), teaching status (teaching, nonteaching), hospital region (Northeast, Midwest, South, West), and location (rural, urban) were included in the analysis as categorical variables. Variables with missing values were encoded as a missing category for all exposure variables.
Outcomes
The primary outcome of interest was the probability of HAC occurrence. The frequency of HAC occurrence in July was compared to that of other months. HACs were defined using the International Classification of Diseases, Ninth Edition, Clinical Modification (ICD‐9‐CM) codes and verified through CMS literature and data.[13] Demographics of the patient and hospital variables, as well as the frequency of HACs were tabulated. Secondary outcomes included the likelihood of incurring higher inpatient charges and experiencing a prolonged LOS, defined as at or above the 90th percentile for both variables.
Statistical Analyses
Demographics were calculated using survey‐adjusted univariate frequency and means analysis. Multivariable logistic regressions were modeled using survey‐adjusted generalized estimating equations to assess the outcomes described above. Each model was adjusted for hospital (bed size, teaching status, hospital region, hospital location) and patient (race, payer information, gender, age, number of comorbidities) factors. The models assessing the prolonged LOS and higher inpatient charges outcomes were adjusted with the same patient and hospital factors, with the addition of HAC occurrence as a covariate. The main exposure of interest in this study was admission in the month of July. Admission month is included as a multilevel variable and recoded into a dichotomous variable.
Aside from hospital and patient covariates, multivariate analyses were also adjusted according to severity of admission. Admission severity was defined using three variables: All‐Patient Refined Disease‐Related Group (APR‐DRG), admission type, and admission source. 3M's APR‐DRG algorithm (3M Health Information Systems, Wallingford, CT) is a system of risk adjustment methods developed by 3M and based upon the existing DRG structure and used in a number of other NIS studies as a valid measure of admission severity.[14, 15, 16, 17] The algorithm divides patient admissions into 500 categories of similar clinical and resource utilization features. APR‐DRGs in the NIS are categorized into five classifications: no class specified, minor loss of function, moderate loss of function, major loss of function, and extreme loss of function. Additionally, admission type (emergency, urgent, elective, newborn, trauma center, other), and admission source (emergency department, another hospital, other health facility, court/law enforcement, routine) were coded in the NIS. Together, these three variables were utilized as covariates in all multivariable logistic regression models to adjust for the severity of injuries patients harbored prior to admission.
In addition to our primary analyses, we conducted a series of secondary analyses. We conducted survey‐adjusted multivariable logistic regression analysis with the primary predictor of interest being individual months, with July as a reference group and the outcome of HAC occurrence. We further analyzed our primary exposure of July versus non‐July admissions and stratified it by the presence of an operating room procedure as a surrogate measure of surgical versus nonsurgical admissions. We also analyzed LOS and total charges as continuous outcomes to elucidate the precise impact of HACs and July admission. Finally, to address the issue of missing values, we conducted a four‐step multiple imputation for complex data with categorical variables using the methods outlined by Berglund et al. [18] In doing so, we created five imputed datasets using a Markov Chain Monte Carol method, producing monotone missing data patterns for a four‐step procedure. We imputed the missing data using the monotone logistic facet of the multiple imputation model. We then used survey‐adjusted logistic regression to estimate odds ratios (ORs) for each of the imputed datasets. Finally, we combined the results from the five logistic regression models by fully incorporating the variance adjustment from both logistic regressions and multiple imputations (PROC MIANALYZE).[18] These analyses were similarly adjusted for with the same patient, hospital, and severity demographics adjusted for in the original model.
Statistical significance was achieved with a P value <0.05. All descriptive and logistic regression analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Overview Demographics
There were 143,019,381 inpatient admissions between 2008 and 2011 in the NIS. Overall, 4.7% (6,738,949) of all US hospital inpatient admissions had incurred at least 1 HAC (Table 1). Approximately 7.6% of inpatient admissions occurred in July, whereas 83.5% occurred in the months of August to June (8.9% of data is missing). July admits had a higher overall frequency of HAC occurrence compared to non‐July admissions (4.9% vs 4.7%). There were marginal differences between hospital and patient factors associated with admissions in July compared to those in other months. The majority of patients in both July and non‐July admissions were between 66 and 80 years old (Table 1).
| July Admission, n=12,003,545 | Non‐July Admission, n=131,015,837 | |||
|---|---|---|---|---|
| N | % | N | % | |
| ||||
| Patient demographic factors | ||||
| HAC occurrence | ||||
| HAC during admission | 594,000 | 4.9% | 6,145,000 | 4.7% |
| No HAC during admission | 11,410,000 | 95.1% | 124,871,000 | 95.3% |
| Race | ||||
| White | 6,783,000 | 56.5% | 74,222,000 | 56.7% |
| Black | 1,468,000 | 12.2% | 15,993,000 | 12.2% |
| Hispanic | 1,231,000 | 10.3% | 13,186,000 | 10.1% |
| API | 288,000 | 2.4% | 3,142,000 | 2.4% |
| Native American | 77,000 | 0.6% | 867,000 | 0.7% |
| Other | 360,000 | 3.0% | 3,931,000 | 3.0% |
| Missing | 1,798,000 | 15.0% | 19,675,000 | 15.0% |
| Payer information | ||||
| Medicare | 4,401,000 | 36.7% | 49,209,000 | 37.6% |
| Medicaid | 2,418,000 | 20.1% | 25,977,000 | 19.8% |
| Private insurance | 4,084,000 | 34.0% | 44,106,000 | 33.7% |
| Self‐pay | 636,000 | 5.3% | 6,693,000 | 5.1% |
| No charge | 43,000 | 0.4% | 445,000 | 0.3% |
| Other | 393,000 | 3.3% | 4,261,000 | 3.3% |
| Missing | 28,000 | 0.2% | 323,000 | 0.2% |
| Comorbidities | ||||
| No comorbidities | 3,957,000 | 33.0% | 42,249,000 | 32.2% |
| 1 | 2,104,000 | 17.5% | 23,209,000 | 17.7% |
| 2 or more | 5,943,000 | 49.5% | 65,557,000 | 50.0% |
| Age category | ||||
| 18 years | 1,965,000 | 16.4% | 21,702,000 | 16.6% |
| 1930 years | 1,482,000 | 12.3% | 15,385,000 | 11.7% |
| 3040 years | 1,156,000 | 9.6% | 12,091,000 | 9.2% |
| 4050 years | 1,196,000 | 10.0% | 12,737,000 | 9.7% |
| 5065 years | 2,323,000 | 19.4% | 25,458,000 | 19.4% |
| 6580 years | 2,345,000 | 19.5% | 26,218,000 | 20.0% |
| >80 years | 1,536,000 | 12.8% | 17,424,000 | 13.3% |
| Gender | ||||
| Female | 6,994,000 | 58.3% | 76,146,000 | 58.1% |
| Male | 4,984,000 | 41.5% | 54,571,000 | 41.7% |
| Missing | 26,000 | 0.2% | 300,000 | 0.2% |
| Hospital demographic factors | ||||
| Hospital region | ||||
| Northeast | 2,561,000 | 21.3% | 27,650,000 | 21.1% |
| Midwest | 3,007,000 | 25.1% | 32,799,000 | 25.0% |
| South | 3,878,000 | 32.3% | 42,696,000 | 32.6% |
| West | 2,557,000 | 21.3% | 27,872,000 | 21.3% |
| Hospital location | ||||
| Rural | 1,507,000 | 12.6% | 16,760,000 | 12.8% |
| Urban | 10,348,000 | 86.2% | 112,639,000 | 86.0% |
| Missing | 149,000 | 1.2% | 1,617,000 | 1.2% |
| Hospital teaching status | ||||
| Nonteaching | 6,129,000 | 51.1% | 67,447,000 | 51.5% |
| Teaching | 5,726,000 | 47.7% | 61,952,000 | 47.3% |
| Missing | 149,000 | 1.2% | 1,617,000 | 1.2% |
| Hospital bed size | ||||
| Small | 1,496,000 | 12.5% | 16,479,000 | 12.6% |
| Medium | 2,905,000 | 24.2% | 31,800,000 | 24.3% |
| Large | 7,453,000 | 62.1% | 81,120,000 | 61.9% |
| Missing | 149,000 | 1.2% | 1,617,000 | 1.2% |
| Admission severity factors | ||||
| Admission source | ||||
| Emergency department | 1,078,000 | 9.0% | 12,425,000 | 9.5% |
| Another hospital | 110,000 | 0.9% | 1,219,000 | 0.9% |
| Other health facility | 61,000 | 0.5% | 664,000 | 0.5% |
| Court/law enforcement | 3,000 | 0.0% | 35,000 | 0.0% |
| Routine | 1,545,000 | 12.9% | 16,529,000 | 12.6% |
| Missing | 9,205,000 | 76.7% | 100,144,000 | 76.4% |
| Admission type | ||||
| Emergency | 4,842,000 | 40.3% | 53,386,000 | 40.7% |
| Urgent | 1,985,000 | 16.5% | 21,747,000 | 16.6% |
| Elective | 2,570,000 | 21.4% | 28,276,000 | 21.6% |
| Newborn | 1,130,000 | 9.4% | 11,625,000 | 8.9% |
| Trauma | 57,000 | 0.5% | 508,000 | 0.4% |
| Other | 4,000 | 0.0% | 45,000 | 0.0% |
| Missing | 1,417,000 | 11.8% | 15,430,000 | 11.8% |
| All‐Patient Refined DRG, severity | ||||
| No class specified | 10,000 | 0.1% | 132,000 | 0.1% |
| Minor loss of function | 4,289,000 | 35.7% | 46,092,000 | 35.2% |
| Moderate loss of function | 4,313,000 | 35.9% | 47,150,000 | 36.0% |
| Major loss of function | 2,630,000 | 21.9% | 28,939,000 | 22.1% |
| Extreme loss of function | 762,000 | 6.3% | 8,704,000 | 6.6% |
The most commonly occurring HACs were falls (5,863,778), pressure ulcers (731,103), vascular catheter‐associated infections (364,204), and catheter UTIs (290,207). HAC frequency showed a marked increase from 2008 to 2011.
HAC Occurrence
Multivariate logistic regression demonstrated that the likelihood of having one or more HACs was 6% higher in July admits compared to non‐July admits, adjusting for patient and hospital covariates (OR=1.06, 95% confidence interval [CI]: 1.061.07, P<0.0001). However, admission during July was not the most significant predictor of an HAC occurrence (Table 2). Institutional factors, such as teaching hospitals (OR=1.22, 95% CI: 1.161.28, P<0.001 vs nonteaching hospitals) and large (OR=1.11, 95% CI: 1.061.17, P=0.0002 vs small bed‐size facilities) and medium‐sized facilities (OR=1.06, 95% CI: 1.001.13, P=0.0461 vs small bed‐size facilities) were the most powerful predictors of HAC occurrence during an inpatient hospitalization (Table 2). Additionally, in a separate subanalysis with the month of admission as the primary exposure of interest, we noted that each month except for August demonstrated statistically significant decreased odds of HAC occurrence when compared to July (see Supporting Table 1 in the online version of this article). As the adjusted HAC likelihood was not statistically different between August and July, an additional analysis was run with the primary exposure being July and August admission versus all other months of admission. These resulted in a finding of 7% increased likelihood of HAC occurrence among July and August admissions compared to all others (OR=1.07, 95% CI: 1.061.07, P<0.0001; see Supporting Table 2 in the online version of this article). Similarly, a multiple imputation model adjusting for missing values produced a very similar July effect estimate to the nonimputed model (OR=1.06, 95% CI: 1.031.09, P<0.01).
| OR | 95% CI | P Value | |
|---|---|---|---|
| |||
| Patient demographic factors | |||
| Admission time | |||
| July admit | 1.06 | 1.061.07 | <0.0001 |
| Non‐July admit | Reference | ||
| Race | |||
| White | Reference | ||
| Black | 0.78 | 0.750.80 | <0.0001 |
| Hispanic | 0.81 | 0.760.85 | <0.0001 |
| API | 0.75 | 0.710.80 | <0.0001 |
| Native American | 0.92 | 0.831.02 | 0.1256 |
| Other | 0.91 | 0.840.98 | <0.0001 |
| Payer information | |||
| Medicare | 1.00 | 0.971.02 | 0.7151 |
| Medicaid | 0.87 | 0.830.90 | <0.0001 |
| Private insurance | Reference | ||
| Selfpay | 1.27 | 1.201.33 | <0.0001 |
| No charge | 1.07 | 0.921.23 | 0.3871 |
| Other | 1.93 | 1.822.05 | <0.0001 |
| Comorbidities | |||
| No comorbidities | Reference | ||
| 1 | 0.84 | 0.820.86 | <0.0001 |
| 2 or more | 0.70 | 0.680.72 | <0.0001 |
| Age category | |||
| 18 years | 0.35 | 0.330.37 | <0.0001 |
| 1930 years | 0.33 | 0.320.35 | <0.0001 |
| 3040 years | 0.32 | 0.310.33 | <0.0001 |
| 4050 years | 0.36 | 0.350.37 | <0.0001 |
| 5065 years | 0.37 | 0.360.38 | <0.0001 |
| 6580 years | 0.45 | 0.450.46 | <0.0001 |
| >80 years | Reference | ||
| Gender | |||
| Female | 0.92 | 0.900.93 | <0.0001 |
| Male | Reference | ||
| Hospital demographic factors | |||
| Hospital region | |||
| Northeast | Reference | ||
| Midwest | 1.06 | 1.001.13 | 0.2563 |
| South | 1.11 | 1.061.22 | 0.0005 |
| West | 1.08 | 0.971.20 | 0.1431 |
| Hospital location | |||
| Rural | Reference | ||
| Urban | 1.01 | 0.961.06 | 0.7144 |
| Hospital teaching status | |||
| Nonteaching | Reference | ||
| Teaching | 1.22 | 1.161.28 | <0.0001 |
| Hospital bed size | |||
| Small | Reference | ||
| Medium | 1.06 | 1.001.13 | 0.0461 |
| Large | 1.11 | 1.061.17 | 0.0002 |
| Admission severity factors | |||
| Admission source | |||
| Emergency department | 1.63 | 1.481.80 | <0.0001 |
| Another hospital | 1.96 | 1.762.17 | <0.0001 |
| Other health facility | 1.62 | 1.302.03 | <0.0001 |
| Court/law enforcement | 1.37 | 1.011.85 | 0.0438 |
| Routine | Reference | ||
| Admission type | |||
| Emergency | 2.15 | 2.032.28 | <0.0001 |
| Urgent | 1.28 | 1.201.35 | <0.0001 |
| Elective | Reference | ||
| Newborn | 0.69 | 0.630.76 | <0.0001 |
| Trauma | >1000 | <0.001>1000 | 0.9962 |
| Other | 0.91 | 0.531.55 | 0.7183 |
| AllPatient Refined DRG, severity | |||
| No class specified | 0.73 | 0.620.85 | <0.0001 |
| Minor loss of function | Reference | ||
| Moderate loss of function | 1.14 | 1.121.16 | <0.0001 |
| Major loss of function | 1.61 | 1.571.66 | <0.0001 |
| Extreme loss of function | 4.65 | 4.504.80 | <0.0001 |
We utilized similar models adjusting for the same patient, hospital, and severity factors in teaching hospital population. Patients discharged from teaching hospitals were 7% more likely to incur an HAC during admission in July compared to those admitted in the other months (OR=1.07, 95% CI: 1.061.08, P<0.01).
Higher Inpatient Charges and Prolonged LOS
The presence of one or more HACs was a significant predictor for higher inpatient charges (Table 3; OR=1.81, 95% CI: 1.74‐1.87, P<0.0001), when adjusting for July admission, patient and hospital factors, and admission severity. HAC occurrence was also a significant predictor of prolonged LOS (Table 3; OR=1.45, 95% CI: 1.42‐1.48, P<0.0001). Mean inpatient charges and LOS in this sample were $33,662.00 and 4.6 days. Patients with at least 1 HAC had a mean inpatient charge of $61,457.00, whereas those with no HAC had a mean charge of $32,377.00. Furthermore, LOS was prolonged in patients with HACs versus those who did not have HACs during hospitalization (7.14 days vs 4.49 days). Our regression analyses indicated that HAC patients had 1.48 (P<0.0001) more days of LOS and $18,258.00 (P<0.0001) more in total charges.
| OR | 95% CI | P Value | |
|---|---|---|---|
| |||
| Higher inpatient costs | |||
| Admission time | |||
| July admit | 1.00 | 0.991.01 | 0.9693 |
| Non‐July admit | Reference | ||
| HAC occurrence | |||
| HAC occurrence | 1.81 | 1.741.87 | <0.0001 |
| No HAC occurrence | Reference | ||
| Prolonged LOS | |||
| Admission time | |||
| July admit | 0.98 | 0.980.99 | <0.0001 |
| Non‐July admit | Reference | ||
| HAC occurrence | |||
| HAC occurrence | 1.45 | 1.421.48 | <0.0001 |
| No HAC occurrence | Reference | ||
DISCUSSION
This study analyzes the relationship between admission month and the incidence of HACs in a national sample. This study is also among the first to examine preventable complications as a measure of inefficiencies and inexperience of new staff during staff turnover in the month of July. In our retrospective cohort study of more than 100 million admissions across 4 years, we found a 4.9% prevalence of HACs among July admits compared to 4.7% in the non‐July admission population. In multivariate analysis, July admissions were associated with a 6% increased likelihood of HACs. Such data are concordant with other studies that demonstrate a positive July effect on mortality and efficiency.[3, 19] Evaluation of a surgical cohort revealed an 18% increase in risk‐adjusted surgical morbidity and a 41% increase in risk‐adjusted surgical mortality during July and August, using the American College of Surgeons' National Surgical Quality Improvement Program.[20] Though several studies have noted worsened outcomes during the month of July, results have been mixed. Several studies in subspecialty populations or local databases suggest no clear increase in mortality or complication rate.[3, 4, 5, 21, 22, 23, 24, 25] This current study is the first to examine these relationships using HACs as a surrogate measure indicative of quality of care and safety of new staff in the month of July.
Although multiple investigations studying July admission use mortality as an outcome measure, evaluation of preventable hospital complications may actually be more reflective of the impact of new staff on care quality and safety.[20, 26, 27, 28, 29] Mortality rates can be significantly confounded by patient‐specific factors, such as disease severity and comorbidities, whereas iatrogenic adverse events, such as HACs, are postulated to be more reflective of errors in systems and processes within the healthcare delivery institution. For example, studies demonstrate that anesthetic procedures that do not result in mortality are often associated with significant increases in complications such as central and peripheral nerve injuries, inadequate oxygenation, perioperative vomiting/aspiration, and technical failures of tracheal tube placement.[1] It is therefore not surprising that studies show July admissions are associated with longer LOS and duration of procedure, in addition to increased hospital charges.[3]
We did attempt to adjust for the effect of disease severity on HACs by incorporating 3M's APR‐DRG system, admission source, and admission type into our multivariate analyses. After adjusting for disease severity in our multivariate analysis, July admission maintained a statistically significant association with increased HAC incidence.
In our secondary analyses, we noted that all months except for August experienced significantly decreased odds of HAC occurrence compared to July admissions with similar magnitudes of likelihood found when combining July and August admissions versus all others (see Supporting Tables 1 and 2 in the online version of this article). This spillover finding may indicate the learning curve of inexperienced and new hospital staff and also suggest that the July effect is not limited only to the month of July. However, because the magnitudes of the 2 models (Table 2; Supporting Table 2 in the online version of this article) are so similar, we continue to refer to this phenomena as the July effect, with the known implication that there is a continuance beyond July and into August.
It is of interest to note that when the analysis was subsetted to only teaching institutions, July admissions in the teaching hospital cohort showed significant increases in HAC likelihood compared to non‐July admissions. Although studies suggest that inexperienced residents contribute to patient complications, the increased rate of HACs in July admits may also be multifactorial.[30] It is likely that the need for new healthcare staff to gain experience, familiarity, and effective communication also influences the HAC rate. The impact of nursing and ancillary staff involvement in the prevention of HACs is crucial. Although the July effect was primarily focused on physician elements, the nursing and ancillary staff elements are more clearly noted when evaluating HACs as an outcome. Pilot studies including multidisciplinary hospital, nursing, and physician teams, involving a significant effort to streamline communication and established protocols, have resulted in drastic decreases in patient falls, the most common of the HAC occurrences.[31] The increased hires during this time period (as new physicians and nurses complete training in June) accompanied with the need to acclimate these groups to one another and to train them on established protocols may result in risks for HACs not previously noted when evaluating more standard outcome measures.
Separate studies have shown worsened outcomes for July surgical admissions in large databases, with results indicating longer operative times for July admissions, inpatient mortality, intraoperative complications, and postoperative morbidity in areas like cardiac or spinal surgeries.[20, 22, 26, 32, 33, 34] Similarly, other studies noted that medical admissions also demonstrated worse outcomes for July admissions, resulting in increased fatal medication errors, preventable complications, and worse documentation errors.[21, 28, 30, 35, 36] In the present study, July admissions demonstrated an increased likelihood of HACs when stratified by surgical and medical admissions, as seen in the current literature.
Our study also indicates that surgical patients are noted to have a 2% increase in HACs during July versus a 9% July increase in medical patients. To the authors' knowledge, this is one of the first studies to stratify a patient population by surgical and medical services to evaluate the effect of July admission on outcomes. Possible explanations are that surgical candidates are often medically optimized prior to elective procedures, requiring a stringent protocol to be executed prior to performing an operation on a patient. Thus, the surgical patients are inherently prescreened to be of better overall health to be deemed operable compared to the traditional medicine patient. Rich et al. ([36]) performed a comparison analysis of multiple services, noting that patients with internal medicine diagnoses demonstrated the expected July effect with declines in diagnostic and pharmaceutical changes throughout the year as an indicator of improved experience leading to decreased utilization.[36] However, in that same study, the authors noted no discernible July effect among surgical patients, possibly related to the difference in resource utilization emphasized in the medical versus surgical programs.[36]
The presence of one or more HACs was a significant predictor for higher inpatient charges, when adjusting for July admission and patient/hospital factors. HAC occurrence was also a significant predictor of prolonged LOS. This is in concordance with multiple prior studies noting the association of higher LOS with HAC occurrence.[37, 38, 39, 40, 41, 42] This further supports the elevated HAC‐associated burden predicted by the CMS when compiling specific HACs.[38, 39, 40, 41, 42] Further studies in the coming years may determine whether CMS HAC regulation translates to decreased inpatient admissions durations and cost reductions over time.
This study has several limitations largely associated with the use of a standardized national database. Coding of HACs depends on consistent and accurate reporting, with errors resulting in information bias. Estimates regarding ICD‐9‐CM coding in the NIS have been cited as approximately 80% accurate.[43] Furthermore, missing variables, though noted in results, and the heterogeneity of the study population, may influence the data. Unfortunately, the nature of the data collection throughout NIS practices is not uniform within states, which may explain why a percentage of data is missing. Because of this data structure, NIS does not have documentation of the month during which an HAC was noted for admissions spanning multiple months. In regard to the missing data, we attempted to account for this using a multiple imputation model that generated similar results to our original model with missing categories coded into it; with the only major difference being expectedly larger standard errors.[44] With yearly changes in CMS coding, the addition and familiarity with new codes may influence analysis over time. Of note, the HAC denoting pressure ulcer did not exist before 2009. We were also unable to use splines to incorporate a time‐series method, as the focus of our study was targeted to looking at the higher incidences of HACs associated with July admission and not a temporal trend prior to and after July, and also the limitations we had in number of time points required for a proper time series analysis.[45] Finally, HACs are only capable of evaluating inpatient events and omit events occurring after discharge.
CONCLUSIONS
These data reveal an increase in HAC frequency during the month of July in a large national sample of patients. Recognition of a noted statistical trend in July may direct necessary attention to a time associated with increased occurrence of preventable iatrogenic adverse events. The HACs represent potential breakdowns in organizational structure distinct from traditional measures of safety, such as mortality and specialty‐specific morbidity. New guidelines dedicated to improving HACs during this time may help to decrease prevalence in both teaching and nonteaching hospitals.
Disclosure
Nothing to report.
- , , , , . Rate of undesirable events at beginning of academic year: retrospective cohort study. BMJ. 2009;339:b3974.
- , . Medical schools in the United States, 2007–2008. JAMA. 2008;300(10):1221–1227.
- , , , , , . “July effect”: impact of the academic year‐end changeover on patient outcomes: a systematic review. Ann Intern Med. 2011;155(5):309–315.
- , , , . Influence of house‐staff experience on teaching‐hospital mortality: the “July phenomenon” revisited. J Hosp Med. 2011;6(7):389–394.
- , , . Impact of admission month and hospital teaching status on outcomes in subarachnoid hemorrhage: evidence against the July effect. J Neurosurg. 2012;116(1):157–163.
- , , , . Analysis of Centers for Medicaid and Medicare Services ‘never events’ in elderly patients undergoing bowel operations. Am Surg. 2010;76(8):841–845.
- . Ending extra payment for “never events”—stronger incentives for patients' safety. N Engl J Med. 2009;360(23):2388–2390.
- . Nonpayment for performance? Medicare's new reimbursement rule. N Engl J Med. 2007;357(16):1573–1575.
- , , , et al. The impact of patient age and comorbidities on the occurrence of “never events” in cerebrovascular surgery: an analysis of the Nationwide Inpatient Sample. J Neurosurg. 2014;121(3):580–586.
- , , , , . “Never events”: Centers for Medicare and Medicaid Services complications after radical cystectomy. Urology. 2013;81(3):527–532.
- Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. Available at: http://www.ahrq.gov/data/hcup/index.html. Accessed June 2013.
- Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. HCUP frequently asked questions. Available at: www.hcup‐us.ahrq.gov/tech_assist/faq.jsp. Accessed January 2015.
- US Department of Health and Human Services. Centers for Medicare 290(14):1868–1874.
- , , , , , . Differences in resource utilization between patients with diabetes receiving glycemia‐targeted specialized nutrition vs standard nutrition formulas in U.S. hospitals. JPEN J Parenter Enteral Nutr. 2014;38(2 suppl):86S–91S.
- , , . The cost of asthma in the emergency department and hospital. Am J Respir Crit Care Med. 1999;160(1):211–215.
- , , , , , . Joint Commission primary stroke centers utilize more rt‐PA in the Nationwide Inpatient Sample. J Am Heart Assoc. 2013;2(2):e000071.
- . An introduction to multiple imputation of complex sample data using SAS 9.2. Paper presented at: SAS Global Forum Proceedings, April 11–14, 2010; Seattle, WA.
- . Measuring surgical quality in Maryland: a model. Health Aff. 1988;7(1):62–78.
- , , , et al. Seasonal variation in surgical outcomes as measured by the American College of Surgeons‐National Surgical Quality Improvement Program (ACS‐NSQIP). Ann Surg. 2007;246(3):456–462; discussion 463–465.
- , , , et al. Complications and death at the start of the new academic year: is there a July phenomenon? J Trauma. 2010;68(1):19–22.
- , , , et al. The July effect: impact of the beginning of the academic cycle on cardiac surgical outcomes in a cohort of 70,616 patients. Ann Thorac Surg. 2009;88(1):70–75.
- , , , . Nationwide data confirms absence of 'July phenomenon' in obstetrics: it's safe to deliver in July. J Perinatol. 2007;27(2):73–76.
- , . Is there a July phenomenon? The effect of July admission on intensive care mortality and length of stay in teaching hospitals. J Gen Intern Med. 2003;18(8):639–645.
- , , , , , . The “July phenomenon” for neurosurgical mortality and complications in teaching hospitals: an analysis of more than 850,000 neurosurgical patients in the nationwide inpatient sample database, 1998 to 2008. Neurosurgery. 2012;71(3):562–571; discussion 571.
- , , . Hip fracture outcome: is there a “July effect.” Am J Orthop. 2009;38(12):606–611.
- , , . Impact of cardiothoracic resident turnover on mortality after cardiac surgery: a dynamic human factor. Ann Thorac Surg. 2008;86(1):123–131.
- , . A July spike in fatal medication errors: a possible effect of new medical residents. J Gen Intern Med. 2010;25(8):774–779.
- . Human factors engineering and patient safety. Qual Saf Health Care. 2002;11(4):352–354.
- , . Ordering errors by first‐year residents: evidence of learning from mistakes. Mo Med. 2004;101(2):128–131.
- , , , . Hourly rounding and patient falls: What factors boost success? Nursing. 2015;45(2):25–30.
- , , , . The effect of July admission on inpatient outcomes following spinal surgery: clinical article. J Neurosurg Spine. 2013;18(3):280–288.
- , , , et al. The July effect and cardiac surgery: the effect of the beginning of the academic cycle on outcomes. Am J Surg. 2008;196(5):720–725.
- , , , , , . “July Effect” in elective spine surgery: analysis of the American College of Surgeons National Surgical Quality Improvement Program Database. Spine. 2014;39(7):603–611.
- . The July phenomenon revisited: are hospital complications associated with new house staff? Am J Med Qual. 1995;10(1):14–17.
- , , , . Specialty differences in the 'July Phenomenon' for Twin Cities teaching hospitals. Med Care. 1993;31(1):73–83.
- , , , , , . Potential financial impact of restriction in “never event” and periprocedural hospital‐acquired condition reimbursement at a tertiary neurosurgical center: a single‐institution prospective study. J neurosurg. 2010;112(2):249–256.
- , , , , . Pressure ulcers, hospital complications, and disease severity: impact on hospital costs and length of stay. Adv Wound Care. 1999;12(1):22–30.
- , , , . Association between cardiac and noncardiac complications in patients undergoing noncardiac surgery: outcomes and effects on length of stay. Am J Med. 2003;115(7):515–520.
- , , , , . Effect of occurrence of infection‐related never events on length of stay and hospital charges in patients undergoing radical neck dissection for head and neck cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(2):147–158.
- , , , , . Ethnic disparities in stroke: epidemiology, acute care, and postacute outcomes. Stroke. 2005;36(2):374–386.
- , , , , . The impact of surgical‐site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol. 2002;23(4):183–189.
- , , , et al. Systematic review of discharge coding accuracy. J Public Health (Oxf). 2012;34(1):138–148.
- , . Assessing bias associated with missing data from joint Canada/US survey of health: an application. Biometrics Section JSM. 2008:3394–3401.
- , , , . Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309.
The simultaneous arrival of new residents, medical students, and faculty in July each year results in a complex transition period for hospitals. Medical centers strive to deliver high‐quality and efficient care while undergoing these cyclical changes, with over 100,000 interns/residents in the United States taking part in this changeover.[1] This period is hypothesized to hold an increased risk of adverse outcomes referred to as the July Effect.[1, 2, 3, 4, 5] Although studies have reported associated increases in mortality risk, decreases in efficiency, and an increase in undesirable events during this time, occurrences are still debated.[1, 3, 4, 5]
In 2008, the Centers for Medicare & Medicaid Services (CMS) published and instituted a nationwide series of never events. These events, narrowed to a list of hospital‐acquired complications (HACs), are characterized as iatrogenic adverse outcomes and deemed preventable and egregious. Medicare has subsequently withheld reimbursement for additional cost of treatment related to the events.[6, 7, 8] HACs include complications such as air embolism, retained foreign body, blood incompatibility, pressure ulcer, catheter‐associated urinary tract infection (UTI), vascular catheter‐associated infection, manifestations of poor glycemic control, falls/trauma, deep venous thrombosis or pulmonary embolism after total knee and hip replacements, surgical site infections after coronary artery bypass graft, and surgical site infections after certain orthopedic or bariatric surgeries. Prior studies have utilized HACs as a metric for quality of healthcare delivery in subspecialties such as cerebrovascular surgery, bowel surgery, and urology.[6, 9, 10]
Though the July effect has been assessed across multiple specialties and hospitals, no prior studies have evaluated this phenomenon on a national level and incorporated all hospital admission diagnoses. Through this study, we aim to provide insight into this relatively new quality metric when evaluating admissions made during the early months of the academic year. This study's primary aim is to evaluate the frequency of HAC occurrence across hospital discharges on a national level as a function of admission month after the initiation of the nonreimbursable nature of the CMS never events in 2008. Furthermore, the secondary aims of this study examine the impact of the July effect on inpatient length of stay (LOS) and charges. We hypothesized that July admission is associated with an increases in HAC occurrence, LOS, and inpatient charges.
METHODS
Data Source
An observational study was conducted using data extracted from the Nationwide Inpatient Sample (NIS) years 2008 to 2011. NIS is an annually compiled database maintained by the Agency for Healthcare Research and Quality and contains information on more than 8 million hospital admissions each year from more than 40 states and 1000 hospitals.[11] The database represents 20% of all US hospital discharges and contains a weighting system that allows for calculation of population estimates.[11]
Patient Sample
All patients who were admitted to a hospital from 2008 to 2011 were included in this study. NIS does not contain unique patient identifiers; thus, each discharge was treated as an independent event, even if it may have represented a repeat hospitalization by the same patient. Each hospitalization contained patient and hospital factors that were included as covariates for analysis. Patient factors such as race (white, black, Hispanic, Asian/Pacific Islander, Native American, other), payer information (Medicare, Medicaid, private insurance, no charge, self‐pay, other), and gender (male, female) were included as categorical variables. Other patient covariates of interest included age (recoded from a continuous to a categorical variable with the following groupings: <18, 19 to 30, 31 to 40, 41 to 50, 50 to 65, 66 to 80, and >80 years) and number of comorbidities (none, 1, 2 or more). The comorbidities variable was drawn from the NIS database and was derived directly from the Elixhauser comorbidity index that is often cited in other studies as a risk‐adjustment measure.[12] Hospital factors, such as bed size (small [<200], medium [201400], large [>400 beds]), teaching status (teaching, nonteaching), hospital region (Northeast, Midwest, South, West), and location (rural, urban) were included in the analysis as categorical variables. Variables with missing values were encoded as a missing category for all exposure variables.
Outcomes
The primary outcome of interest was the probability of HAC occurrence. The frequency of HAC occurrence in July was compared to that of other months. HACs were defined using the International Classification of Diseases, Ninth Edition, Clinical Modification (ICD‐9‐CM) codes and verified through CMS literature and data.[13] Demographics of the patient and hospital variables, as well as the frequency of HACs were tabulated. Secondary outcomes included the likelihood of incurring higher inpatient charges and experiencing a prolonged LOS, defined as at or above the 90th percentile for both variables.
Statistical Analyses
Demographics were calculated using survey‐adjusted univariate frequency and means analysis. Multivariable logistic regressions were modeled using survey‐adjusted generalized estimating equations to assess the outcomes described above. Each model was adjusted for hospital (bed size, teaching status, hospital region, hospital location) and patient (race, payer information, gender, age, number of comorbidities) factors. The models assessing the prolonged LOS and higher inpatient charges outcomes were adjusted with the same patient and hospital factors, with the addition of HAC occurrence as a covariate. The main exposure of interest in this study was admission in the month of July. Admission month is included as a multilevel variable and recoded into a dichotomous variable.
Aside from hospital and patient covariates, multivariate analyses were also adjusted according to severity of admission. Admission severity was defined using three variables: All‐Patient Refined Disease‐Related Group (APR‐DRG), admission type, and admission source. 3M's APR‐DRG algorithm (3M Health Information Systems, Wallingford, CT) is a system of risk adjustment methods developed by 3M and based upon the existing DRG structure and used in a number of other NIS studies as a valid measure of admission severity.[14, 15, 16, 17] The algorithm divides patient admissions into 500 categories of similar clinical and resource utilization features. APR‐DRGs in the NIS are categorized into five classifications: no class specified, minor loss of function, moderate loss of function, major loss of function, and extreme loss of function. Additionally, admission type (emergency, urgent, elective, newborn, trauma center, other), and admission source (emergency department, another hospital, other health facility, court/law enforcement, routine) were coded in the NIS. Together, these three variables were utilized as covariates in all multivariable logistic regression models to adjust for the severity of injuries patients harbored prior to admission.
In addition to our primary analyses, we conducted a series of secondary analyses. We conducted survey‐adjusted multivariable logistic regression analysis with the primary predictor of interest being individual months, with July as a reference group and the outcome of HAC occurrence. We further analyzed our primary exposure of July versus non‐July admissions and stratified it by the presence of an operating room procedure as a surrogate measure of surgical versus nonsurgical admissions. We also analyzed LOS and total charges as continuous outcomes to elucidate the precise impact of HACs and July admission. Finally, to address the issue of missing values, we conducted a four‐step multiple imputation for complex data with categorical variables using the methods outlined by Berglund et al. [18] In doing so, we created five imputed datasets using a Markov Chain Monte Carol method, producing monotone missing data patterns for a four‐step procedure. We imputed the missing data using the monotone logistic facet of the multiple imputation model. We then used survey‐adjusted logistic regression to estimate odds ratios (ORs) for each of the imputed datasets. Finally, we combined the results from the five logistic regression models by fully incorporating the variance adjustment from both logistic regressions and multiple imputations (PROC MIANALYZE).[18] These analyses were similarly adjusted for with the same patient, hospital, and severity demographics adjusted for in the original model.
Statistical significance was achieved with a P value <0.05. All descriptive and logistic regression analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Overview Demographics
There were 143,019,381 inpatient admissions between 2008 and 2011 in the NIS. Overall, 4.7% (6,738,949) of all US hospital inpatient admissions had incurred at least 1 HAC (Table 1). Approximately 7.6% of inpatient admissions occurred in July, whereas 83.5% occurred in the months of August to June (8.9% of data is missing). July admits had a higher overall frequency of HAC occurrence compared to non‐July admissions (4.9% vs 4.7%). There were marginal differences between hospital and patient factors associated with admissions in July compared to those in other months. The majority of patients in both July and non‐July admissions were between 66 and 80 years old (Table 1).
| July Admission, n=12,003,545 | Non‐July Admission, n=131,015,837 | |||
|---|---|---|---|---|
| N | % | N | % | |
| ||||
| Patient demographic factors | ||||
| HAC occurrence | ||||
| HAC during admission | 594,000 | 4.9% | 6,145,000 | 4.7% |
| No HAC during admission | 11,410,000 | 95.1% | 124,871,000 | 95.3% |
| Race | ||||
| White | 6,783,000 | 56.5% | 74,222,000 | 56.7% |
| Black | 1,468,000 | 12.2% | 15,993,000 | 12.2% |
| Hispanic | 1,231,000 | 10.3% | 13,186,000 | 10.1% |
| API | 288,000 | 2.4% | 3,142,000 | 2.4% |
| Native American | 77,000 | 0.6% | 867,000 | 0.7% |
| Other | 360,000 | 3.0% | 3,931,000 | 3.0% |
| Missing | 1,798,000 | 15.0% | 19,675,000 | 15.0% |
| Payer information | ||||
| Medicare | 4,401,000 | 36.7% | 49,209,000 | 37.6% |
| Medicaid | 2,418,000 | 20.1% | 25,977,000 | 19.8% |
| Private insurance | 4,084,000 | 34.0% | 44,106,000 | 33.7% |
| Self‐pay | 636,000 | 5.3% | 6,693,000 | 5.1% |
| No charge | 43,000 | 0.4% | 445,000 | 0.3% |
| Other | 393,000 | 3.3% | 4,261,000 | 3.3% |
| Missing | 28,000 | 0.2% | 323,000 | 0.2% |
| Comorbidities | ||||
| No comorbidities | 3,957,000 | 33.0% | 42,249,000 | 32.2% |
| 1 | 2,104,000 | 17.5% | 23,209,000 | 17.7% |
| 2 or more | 5,943,000 | 49.5% | 65,557,000 | 50.0% |
| Age category | ||||
| 18 years | 1,965,000 | 16.4% | 21,702,000 | 16.6% |
| 1930 years | 1,482,000 | 12.3% | 15,385,000 | 11.7% |
| 3040 years | 1,156,000 | 9.6% | 12,091,000 | 9.2% |
| 4050 years | 1,196,000 | 10.0% | 12,737,000 | 9.7% |
| 5065 years | 2,323,000 | 19.4% | 25,458,000 | 19.4% |
| 6580 years | 2,345,000 | 19.5% | 26,218,000 | 20.0% |
| >80 years | 1,536,000 | 12.8% | 17,424,000 | 13.3% |
| Gender | ||||
| Female | 6,994,000 | 58.3% | 76,146,000 | 58.1% |
| Male | 4,984,000 | 41.5% | 54,571,000 | 41.7% |
| Missing | 26,000 | 0.2% | 300,000 | 0.2% |
| Hospital demographic factors | ||||
| Hospital region | ||||
| Northeast | 2,561,000 | 21.3% | 27,650,000 | 21.1% |
| Midwest | 3,007,000 | 25.1% | 32,799,000 | 25.0% |
| South | 3,878,000 | 32.3% | 42,696,000 | 32.6% |
| West | 2,557,000 | 21.3% | 27,872,000 | 21.3% |
| Hospital location | ||||
| Rural | 1,507,000 | 12.6% | 16,760,000 | 12.8% |
| Urban | 10,348,000 | 86.2% | 112,639,000 | 86.0% |
| Missing | 149,000 | 1.2% | 1,617,000 | 1.2% |
| Hospital teaching status | ||||
| Nonteaching | 6,129,000 | 51.1% | 67,447,000 | 51.5% |
| Teaching | 5,726,000 | 47.7% | 61,952,000 | 47.3% |
| Missing | 149,000 | 1.2% | 1,617,000 | 1.2% |
| Hospital bed size | ||||
| Small | 1,496,000 | 12.5% | 16,479,000 | 12.6% |
| Medium | 2,905,000 | 24.2% | 31,800,000 | 24.3% |
| Large | 7,453,000 | 62.1% | 81,120,000 | 61.9% |
| Missing | 149,000 | 1.2% | 1,617,000 | 1.2% |
| Admission severity factors | ||||
| Admission source | ||||
| Emergency department | 1,078,000 | 9.0% | 12,425,000 | 9.5% |
| Another hospital | 110,000 | 0.9% | 1,219,000 | 0.9% |
| Other health facility | 61,000 | 0.5% | 664,000 | 0.5% |
| Court/law enforcement | 3,000 | 0.0% | 35,000 | 0.0% |
| Routine | 1,545,000 | 12.9% | 16,529,000 | 12.6% |
| Missing | 9,205,000 | 76.7% | 100,144,000 | 76.4% |
| Admission type | ||||
| Emergency | 4,842,000 | 40.3% | 53,386,000 | 40.7% |
| Urgent | 1,985,000 | 16.5% | 21,747,000 | 16.6% |
| Elective | 2,570,000 | 21.4% | 28,276,000 | 21.6% |
| Newborn | 1,130,000 | 9.4% | 11,625,000 | 8.9% |
| Trauma | 57,000 | 0.5% | 508,000 | 0.4% |
| Other | 4,000 | 0.0% | 45,000 | 0.0% |
| Missing | 1,417,000 | 11.8% | 15,430,000 | 11.8% |
| All‐Patient Refined DRG, severity | ||||
| No class specified | 10,000 | 0.1% | 132,000 | 0.1% |
| Minor loss of function | 4,289,000 | 35.7% | 46,092,000 | 35.2% |
| Moderate loss of function | 4,313,000 | 35.9% | 47,150,000 | 36.0% |
| Major loss of function | 2,630,000 | 21.9% | 28,939,000 | 22.1% |
| Extreme loss of function | 762,000 | 6.3% | 8,704,000 | 6.6% |
The most commonly occurring HACs were falls (5,863,778), pressure ulcers (731,103), vascular catheter‐associated infections (364,204), and catheter UTIs (290,207). HAC frequency showed a marked increase from 2008 to 2011.
HAC Occurrence
Multivariate logistic regression demonstrated that the likelihood of having one or more HACs was 6% higher in July admits compared to non‐July admits, adjusting for patient and hospital covariates (OR=1.06, 95% confidence interval [CI]: 1.061.07, P<0.0001). However, admission during July was not the most significant predictor of an HAC occurrence (Table 2). Institutional factors, such as teaching hospitals (OR=1.22, 95% CI: 1.161.28, P<0.001 vs nonteaching hospitals) and large (OR=1.11, 95% CI: 1.061.17, P=0.0002 vs small bed‐size facilities) and medium‐sized facilities (OR=1.06, 95% CI: 1.001.13, P=0.0461 vs small bed‐size facilities) were the most powerful predictors of HAC occurrence during an inpatient hospitalization (Table 2). Additionally, in a separate subanalysis with the month of admission as the primary exposure of interest, we noted that each month except for August demonstrated statistically significant decreased odds of HAC occurrence when compared to July (see Supporting Table 1 in the online version of this article). As the adjusted HAC likelihood was not statistically different between August and July, an additional analysis was run with the primary exposure being July and August admission versus all other months of admission. These resulted in a finding of 7% increased likelihood of HAC occurrence among July and August admissions compared to all others (OR=1.07, 95% CI: 1.061.07, P<0.0001; see Supporting Table 2 in the online version of this article). Similarly, a multiple imputation model adjusting for missing values produced a very similar July effect estimate to the nonimputed model (OR=1.06, 95% CI: 1.031.09, P<0.01).
| OR | 95% CI | P Value | |
|---|---|---|---|
| |||
| Patient demographic factors | |||
| Admission time | |||
| July admit | 1.06 | 1.061.07 | <0.0001 |
| Non‐July admit | Reference | ||
| Race | |||
| White | Reference | ||
| Black | 0.78 | 0.750.80 | <0.0001 |
| Hispanic | 0.81 | 0.760.85 | <0.0001 |
| API | 0.75 | 0.710.80 | <0.0001 |
| Native American | 0.92 | 0.831.02 | 0.1256 |
| Other | 0.91 | 0.840.98 | <0.0001 |
| Payer information | |||
| Medicare | 1.00 | 0.971.02 | 0.7151 |
| Medicaid | 0.87 | 0.830.90 | <0.0001 |
| Private insurance | Reference | ||
| Selfpay | 1.27 | 1.201.33 | <0.0001 |
| No charge | 1.07 | 0.921.23 | 0.3871 |
| Other | 1.93 | 1.822.05 | <0.0001 |
| Comorbidities | |||
| No comorbidities | Reference | ||
| 1 | 0.84 | 0.820.86 | <0.0001 |
| 2 or more | 0.70 | 0.680.72 | <0.0001 |
| Age category | |||
| 18 years | 0.35 | 0.330.37 | <0.0001 |
| 1930 years | 0.33 | 0.320.35 | <0.0001 |
| 3040 years | 0.32 | 0.310.33 | <0.0001 |
| 4050 years | 0.36 | 0.350.37 | <0.0001 |
| 5065 years | 0.37 | 0.360.38 | <0.0001 |
| 6580 years | 0.45 | 0.450.46 | <0.0001 |
| >80 years | Reference | ||
| Gender | |||
| Female | 0.92 | 0.900.93 | <0.0001 |
| Male | Reference | ||
| Hospital demographic factors | |||
| Hospital region | |||
| Northeast | Reference | ||
| Midwest | 1.06 | 1.001.13 | 0.2563 |
| South | 1.11 | 1.061.22 | 0.0005 |
| West | 1.08 | 0.971.20 | 0.1431 |
| Hospital location | |||
| Rural | Reference | ||
| Urban | 1.01 | 0.961.06 | 0.7144 |
| Hospital teaching status | |||
| Nonteaching | Reference | ||
| Teaching | 1.22 | 1.161.28 | <0.0001 |
| Hospital bed size | |||
| Small | Reference | ||
| Medium | 1.06 | 1.001.13 | 0.0461 |
| Large | 1.11 | 1.061.17 | 0.0002 |
| Admission severity factors | |||
| Admission source | |||
| Emergency department | 1.63 | 1.481.80 | <0.0001 |
| Another hospital | 1.96 | 1.762.17 | <0.0001 |
| Other health facility | 1.62 | 1.302.03 | <0.0001 |
| Court/law enforcement | 1.37 | 1.011.85 | 0.0438 |
| Routine | Reference | ||
| Admission type | |||
| Emergency | 2.15 | 2.032.28 | <0.0001 |
| Urgent | 1.28 | 1.201.35 | <0.0001 |
| Elective | Reference | ||
| Newborn | 0.69 | 0.630.76 | <0.0001 |
| Trauma | >1000 | <0.001>1000 | 0.9962 |
| Other | 0.91 | 0.531.55 | 0.7183 |
| AllPatient Refined DRG, severity | |||
| No class specified | 0.73 | 0.620.85 | <0.0001 |
| Minor loss of function | Reference | ||
| Moderate loss of function | 1.14 | 1.121.16 | <0.0001 |
| Major loss of function | 1.61 | 1.571.66 | <0.0001 |
| Extreme loss of function | 4.65 | 4.504.80 | <0.0001 |
We utilized similar models adjusting for the same patient, hospital, and severity factors in teaching hospital population. Patients discharged from teaching hospitals were 7% more likely to incur an HAC during admission in July compared to those admitted in the other months (OR=1.07, 95% CI: 1.061.08, P<0.01).
Higher Inpatient Charges and Prolonged LOS
The presence of one or more HACs was a significant predictor for higher inpatient charges (Table 3; OR=1.81, 95% CI: 1.74‐1.87, P<0.0001), when adjusting for July admission, patient and hospital factors, and admission severity. HAC occurrence was also a significant predictor of prolonged LOS (Table 3; OR=1.45, 95% CI: 1.42‐1.48, P<0.0001). Mean inpatient charges and LOS in this sample were $33,662.00 and 4.6 days. Patients with at least 1 HAC had a mean inpatient charge of $61,457.00, whereas those with no HAC had a mean charge of $32,377.00. Furthermore, LOS was prolonged in patients with HACs versus those who did not have HACs during hospitalization (7.14 days vs 4.49 days). Our regression analyses indicated that HAC patients had 1.48 (P<0.0001) more days of LOS and $18,258.00 (P<0.0001) more in total charges.
| OR | 95% CI | P Value | |
|---|---|---|---|
| |||
| Higher inpatient costs | |||
| Admission time | |||
| July admit | 1.00 | 0.991.01 | 0.9693 |
| Non‐July admit | Reference | ||
| HAC occurrence | |||
| HAC occurrence | 1.81 | 1.741.87 | <0.0001 |
| No HAC occurrence | Reference | ||
| Prolonged LOS | |||
| Admission time | |||
| July admit | 0.98 | 0.980.99 | <0.0001 |
| Non‐July admit | Reference | ||
| HAC occurrence | |||
| HAC occurrence | 1.45 | 1.421.48 | <0.0001 |
| No HAC occurrence | Reference | ||
DISCUSSION
This study analyzes the relationship between admission month and the incidence of HACs in a national sample. This study is also among the first to examine preventable complications as a measure of inefficiencies and inexperience of new staff during staff turnover in the month of July. In our retrospective cohort study of more than 100 million admissions across 4 years, we found a 4.9% prevalence of HACs among July admits compared to 4.7% in the non‐July admission population. In multivariate analysis, July admissions were associated with a 6% increased likelihood of HACs. Such data are concordant with other studies that demonstrate a positive July effect on mortality and efficiency.[3, 19] Evaluation of a surgical cohort revealed an 18% increase in risk‐adjusted surgical morbidity and a 41% increase in risk‐adjusted surgical mortality during July and August, using the American College of Surgeons' National Surgical Quality Improvement Program.[20] Though several studies have noted worsened outcomes during the month of July, results have been mixed. Several studies in subspecialty populations or local databases suggest no clear increase in mortality or complication rate.[3, 4, 5, 21, 22, 23, 24, 25] This current study is the first to examine these relationships using HACs as a surrogate measure indicative of quality of care and safety of new staff in the month of July.
Although multiple investigations studying July admission use mortality as an outcome measure, evaluation of preventable hospital complications may actually be more reflective of the impact of new staff on care quality and safety.[20, 26, 27, 28, 29] Mortality rates can be significantly confounded by patient‐specific factors, such as disease severity and comorbidities, whereas iatrogenic adverse events, such as HACs, are postulated to be more reflective of errors in systems and processes within the healthcare delivery institution. For example, studies demonstrate that anesthetic procedures that do not result in mortality are often associated with significant increases in complications such as central and peripheral nerve injuries, inadequate oxygenation, perioperative vomiting/aspiration, and technical failures of tracheal tube placement.[1] It is therefore not surprising that studies show July admissions are associated with longer LOS and duration of procedure, in addition to increased hospital charges.[3]
We did attempt to adjust for the effect of disease severity on HACs by incorporating 3M's APR‐DRG system, admission source, and admission type into our multivariate analyses. After adjusting for disease severity in our multivariate analysis, July admission maintained a statistically significant association with increased HAC incidence.
In our secondary analyses, we noted that all months except for August experienced significantly decreased odds of HAC occurrence compared to July admissions with similar magnitudes of likelihood found when combining July and August admissions versus all others (see Supporting Tables 1 and 2 in the online version of this article). This spillover finding may indicate the learning curve of inexperienced and new hospital staff and also suggest that the July effect is not limited only to the month of July. However, because the magnitudes of the 2 models (Table 2; Supporting Table 2 in the online version of this article) are so similar, we continue to refer to this phenomena as the July effect, with the known implication that there is a continuance beyond July and into August.
It is of interest to note that when the analysis was subsetted to only teaching institutions, July admissions in the teaching hospital cohort showed significant increases in HAC likelihood compared to non‐July admissions. Although studies suggest that inexperienced residents contribute to patient complications, the increased rate of HACs in July admits may also be multifactorial.[30] It is likely that the need for new healthcare staff to gain experience, familiarity, and effective communication also influences the HAC rate. The impact of nursing and ancillary staff involvement in the prevention of HACs is crucial. Although the July effect was primarily focused on physician elements, the nursing and ancillary staff elements are more clearly noted when evaluating HACs as an outcome. Pilot studies including multidisciplinary hospital, nursing, and physician teams, involving a significant effort to streamline communication and established protocols, have resulted in drastic decreases in patient falls, the most common of the HAC occurrences.[31] The increased hires during this time period (as new physicians and nurses complete training in June) accompanied with the need to acclimate these groups to one another and to train them on established protocols may result in risks for HACs not previously noted when evaluating more standard outcome measures.
Separate studies have shown worsened outcomes for July surgical admissions in large databases, with results indicating longer operative times for July admissions, inpatient mortality, intraoperative complications, and postoperative morbidity in areas like cardiac or spinal surgeries.[20, 22, 26, 32, 33, 34] Similarly, other studies noted that medical admissions also demonstrated worse outcomes for July admissions, resulting in increased fatal medication errors, preventable complications, and worse documentation errors.[21, 28, 30, 35, 36] In the present study, July admissions demonstrated an increased likelihood of HACs when stratified by surgical and medical admissions, as seen in the current literature.
Our study also indicates that surgical patients are noted to have a 2% increase in HACs during July versus a 9% July increase in medical patients. To the authors' knowledge, this is one of the first studies to stratify a patient population by surgical and medical services to evaluate the effect of July admission on outcomes. Possible explanations are that surgical candidates are often medically optimized prior to elective procedures, requiring a stringent protocol to be executed prior to performing an operation on a patient. Thus, the surgical patients are inherently prescreened to be of better overall health to be deemed operable compared to the traditional medicine patient. Rich et al. ([36]) performed a comparison analysis of multiple services, noting that patients with internal medicine diagnoses demonstrated the expected July effect with declines in diagnostic and pharmaceutical changes throughout the year as an indicator of improved experience leading to decreased utilization.[36] However, in that same study, the authors noted no discernible July effect among surgical patients, possibly related to the difference in resource utilization emphasized in the medical versus surgical programs.[36]
The presence of one or more HACs was a significant predictor for higher inpatient charges, when adjusting for July admission and patient/hospital factors. HAC occurrence was also a significant predictor of prolonged LOS. This is in concordance with multiple prior studies noting the association of higher LOS with HAC occurrence.[37, 38, 39, 40, 41, 42] This further supports the elevated HAC‐associated burden predicted by the CMS when compiling specific HACs.[38, 39, 40, 41, 42] Further studies in the coming years may determine whether CMS HAC regulation translates to decreased inpatient admissions durations and cost reductions over time.
This study has several limitations largely associated with the use of a standardized national database. Coding of HACs depends on consistent and accurate reporting, with errors resulting in information bias. Estimates regarding ICD‐9‐CM coding in the NIS have been cited as approximately 80% accurate.[43] Furthermore, missing variables, though noted in results, and the heterogeneity of the study population, may influence the data. Unfortunately, the nature of the data collection throughout NIS practices is not uniform within states, which may explain why a percentage of data is missing. Because of this data structure, NIS does not have documentation of the month during which an HAC was noted for admissions spanning multiple months. In regard to the missing data, we attempted to account for this using a multiple imputation model that generated similar results to our original model with missing categories coded into it; with the only major difference being expectedly larger standard errors.[44] With yearly changes in CMS coding, the addition and familiarity with new codes may influence analysis over time. Of note, the HAC denoting pressure ulcer did not exist before 2009. We were also unable to use splines to incorporate a time‐series method, as the focus of our study was targeted to looking at the higher incidences of HACs associated with July admission and not a temporal trend prior to and after July, and also the limitations we had in number of time points required for a proper time series analysis.[45] Finally, HACs are only capable of evaluating inpatient events and omit events occurring after discharge.
CONCLUSIONS
These data reveal an increase in HAC frequency during the month of July in a large national sample of patients. Recognition of a noted statistical trend in July may direct necessary attention to a time associated with increased occurrence of preventable iatrogenic adverse events. The HACs represent potential breakdowns in organizational structure distinct from traditional measures of safety, such as mortality and specialty‐specific morbidity. New guidelines dedicated to improving HACs during this time may help to decrease prevalence in both teaching and nonteaching hospitals.
Disclosure
Nothing to report.
The simultaneous arrival of new residents, medical students, and faculty in July each year results in a complex transition period for hospitals. Medical centers strive to deliver high‐quality and efficient care while undergoing these cyclical changes, with over 100,000 interns/residents in the United States taking part in this changeover.[1] This period is hypothesized to hold an increased risk of adverse outcomes referred to as the July Effect.[1, 2, 3, 4, 5] Although studies have reported associated increases in mortality risk, decreases in efficiency, and an increase in undesirable events during this time, occurrences are still debated.[1, 3, 4, 5]
In 2008, the Centers for Medicare & Medicaid Services (CMS) published and instituted a nationwide series of never events. These events, narrowed to a list of hospital‐acquired complications (HACs), are characterized as iatrogenic adverse outcomes and deemed preventable and egregious. Medicare has subsequently withheld reimbursement for additional cost of treatment related to the events.[6, 7, 8] HACs include complications such as air embolism, retained foreign body, blood incompatibility, pressure ulcer, catheter‐associated urinary tract infection (UTI), vascular catheter‐associated infection, manifestations of poor glycemic control, falls/trauma, deep venous thrombosis or pulmonary embolism after total knee and hip replacements, surgical site infections after coronary artery bypass graft, and surgical site infections after certain orthopedic or bariatric surgeries. Prior studies have utilized HACs as a metric for quality of healthcare delivery in subspecialties such as cerebrovascular surgery, bowel surgery, and urology.[6, 9, 10]
Though the July effect has been assessed across multiple specialties and hospitals, no prior studies have evaluated this phenomenon on a national level and incorporated all hospital admission diagnoses. Through this study, we aim to provide insight into this relatively new quality metric when evaluating admissions made during the early months of the academic year. This study's primary aim is to evaluate the frequency of HAC occurrence across hospital discharges on a national level as a function of admission month after the initiation of the nonreimbursable nature of the CMS never events in 2008. Furthermore, the secondary aims of this study examine the impact of the July effect on inpatient length of stay (LOS) and charges. We hypothesized that July admission is associated with an increases in HAC occurrence, LOS, and inpatient charges.
METHODS
Data Source
An observational study was conducted using data extracted from the Nationwide Inpatient Sample (NIS) years 2008 to 2011. NIS is an annually compiled database maintained by the Agency for Healthcare Research and Quality and contains information on more than 8 million hospital admissions each year from more than 40 states and 1000 hospitals.[11] The database represents 20% of all US hospital discharges and contains a weighting system that allows for calculation of population estimates.[11]
Patient Sample
All patients who were admitted to a hospital from 2008 to 2011 were included in this study. NIS does not contain unique patient identifiers; thus, each discharge was treated as an independent event, even if it may have represented a repeat hospitalization by the same patient. Each hospitalization contained patient and hospital factors that were included as covariates for analysis. Patient factors such as race (white, black, Hispanic, Asian/Pacific Islander, Native American, other), payer information (Medicare, Medicaid, private insurance, no charge, self‐pay, other), and gender (male, female) were included as categorical variables. Other patient covariates of interest included age (recoded from a continuous to a categorical variable with the following groupings: <18, 19 to 30, 31 to 40, 41 to 50, 50 to 65, 66 to 80, and >80 years) and number of comorbidities (none, 1, 2 or more). The comorbidities variable was drawn from the NIS database and was derived directly from the Elixhauser comorbidity index that is often cited in other studies as a risk‐adjustment measure.[12] Hospital factors, such as bed size (small [<200], medium [201400], large [>400 beds]), teaching status (teaching, nonteaching), hospital region (Northeast, Midwest, South, West), and location (rural, urban) were included in the analysis as categorical variables. Variables with missing values were encoded as a missing category for all exposure variables.
Outcomes
The primary outcome of interest was the probability of HAC occurrence. The frequency of HAC occurrence in July was compared to that of other months. HACs were defined using the International Classification of Diseases, Ninth Edition, Clinical Modification (ICD‐9‐CM) codes and verified through CMS literature and data.[13] Demographics of the patient and hospital variables, as well as the frequency of HACs were tabulated. Secondary outcomes included the likelihood of incurring higher inpatient charges and experiencing a prolonged LOS, defined as at or above the 90th percentile for both variables.
Statistical Analyses
Demographics were calculated using survey‐adjusted univariate frequency and means analysis. Multivariable logistic regressions were modeled using survey‐adjusted generalized estimating equations to assess the outcomes described above. Each model was adjusted for hospital (bed size, teaching status, hospital region, hospital location) and patient (race, payer information, gender, age, number of comorbidities) factors. The models assessing the prolonged LOS and higher inpatient charges outcomes were adjusted with the same patient and hospital factors, with the addition of HAC occurrence as a covariate. The main exposure of interest in this study was admission in the month of July. Admission month is included as a multilevel variable and recoded into a dichotomous variable.
Aside from hospital and patient covariates, multivariate analyses were also adjusted according to severity of admission. Admission severity was defined using three variables: All‐Patient Refined Disease‐Related Group (APR‐DRG), admission type, and admission source. 3M's APR‐DRG algorithm (3M Health Information Systems, Wallingford, CT) is a system of risk adjustment methods developed by 3M and based upon the existing DRG structure and used in a number of other NIS studies as a valid measure of admission severity.[14, 15, 16, 17] The algorithm divides patient admissions into 500 categories of similar clinical and resource utilization features. APR‐DRGs in the NIS are categorized into five classifications: no class specified, minor loss of function, moderate loss of function, major loss of function, and extreme loss of function. Additionally, admission type (emergency, urgent, elective, newborn, trauma center, other), and admission source (emergency department, another hospital, other health facility, court/law enforcement, routine) were coded in the NIS. Together, these three variables were utilized as covariates in all multivariable logistic regression models to adjust for the severity of injuries patients harbored prior to admission.
In addition to our primary analyses, we conducted a series of secondary analyses. We conducted survey‐adjusted multivariable logistic regression analysis with the primary predictor of interest being individual months, with July as a reference group and the outcome of HAC occurrence. We further analyzed our primary exposure of July versus non‐July admissions and stratified it by the presence of an operating room procedure as a surrogate measure of surgical versus nonsurgical admissions. We also analyzed LOS and total charges as continuous outcomes to elucidate the precise impact of HACs and July admission. Finally, to address the issue of missing values, we conducted a four‐step multiple imputation for complex data with categorical variables using the methods outlined by Berglund et al. [18] In doing so, we created five imputed datasets using a Markov Chain Monte Carol method, producing monotone missing data patterns for a four‐step procedure. We imputed the missing data using the monotone logistic facet of the multiple imputation model. We then used survey‐adjusted logistic regression to estimate odds ratios (ORs) for each of the imputed datasets. Finally, we combined the results from the five logistic regression models by fully incorporating the variance adjustment from both logistic regressions and multiple imputations (PROC MIANALYZE).[18] These analyses were similarly adjusted for with the same patient, hospital, and severity demographics adjusted for in the original model.
Statistical significance was achieved with a P value <0.05. All descriptive and logistic regression analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
RESULTS
Overview Demographics
There were 143,019,381 inpatient admissions between 2008 and 2011 in the NIS. Overall, 4.7% (6,738,949) of all US hospital inpatient admissions had incurred at least 1 HAC (Table 1). Approximately 7.6% of inpatient admissions occurred in July, whereas 83.5% occurred in the months of August to June (8.9% of data is missing). July admits had a higher overall frequency of HAC occurrence compared to non‐July admissions (4.9% vs 4.7%). There were marginal differences between hospital and patient factors associated with admissions in July compared to those in other months. The majority of patients in both July and non‐July admissions were between 66 and 80 years old (Table 1).
| July Admission, n=12,003,545 | Non‐July Admission, n=131,015,837 | |||
|---|---|---|---|---|
| N | % | N | % | |
| ||||
| Patient demographic factors | ||||
| HAC occurrence | ||||
| HAC during admission | 594,000 | 4.9% | 6,145,000 | 4.7% |
| No HAC during admission | 11,410,000 | 95.1% | 124,871,000 | 95.3% |
| Race | ||||
| White | 6,783,000 | 56.5% | 74,222,000 | 56.7% |
| Black | 1,468,000 | 12.2% | 15,993,000 | 12.2% |
| Hispanic | 1,231,000 | 10.3% | 13,186,000 | 10.1% |
| API | 288,000 | 2.4% | 3,142,000 | 2.4% |
| Native American | 77,000 | 0.6% | 867,000 | 0.7% |
| Other | 360,000 | 3.0% | 3,931,000 | 3.0% |
| Missing | 1,798,000 | 15.0% | 19,675,000 | 15.0% |
| Payer information | ||||
| Medicare | 4,401,000 | 36.7% | 49,209,000 | 37.6% |
| Medicaid | 2,418,000 | 20.1% | 25,977,000 | 19.8% |
| Private insurance | 4,084,000 | 34.0% | 44,106,000 | 33.7% |
| Self‐pay | 636,000 | 5.3% | 6,693,000 | 5.1% |
| No charge | 43,000 | 0.4% | 445,000 | 0.3% |
| Other | 393,000 | 3.3% | 4,261,000 | 3.3% |
| Missing | 28,000 | 0.2% | 323,000 | 0.2% |
| Comorbidities | ||||
| No comorbidities | 3,957,000 | 33.0% | 42,249,000 | 32.2% |
| 1 | 2,104,000 | 17.5% | 23,209,000 | 17.7% |
| 2 or more | 5,943,000 | 49.5% | 65,557,000 | 50.0% |
| Age category | ||||
| 18 years | 1,965,000 | 16.4% | 21,702,000 | 16.6% |
| 1930 years | 1,482,000 | 12.3% | 15,385,000 | 11.7% |
| 3040 years | 1,156,000 | 9.6% | 12,091,000 | 9.2% |
| 4050 years | 1,196,000 | 10.0% | 12,737,000 | 9.7% |
| 5065 years | 2,323,000 | 19.4% | 25,458,000 | 19.4% |
| 6580 years | 2,345,000 | 19.5% | 26,218,000 | 20.0% |
| >80 years | 1,536,000 | 12.8% | 17,424,000 | 13.3% |
| Gender | ||||
| Female | 6,994,000 | 58.3% | 76,146,000 | 58.1% |
| Male | 4,984,000 | 41.5% | 54,571,000 | 41.7% |
| Missing | 26,000 | 0.2% | 300,000 | 0.2% |
| Hospital demographic factors | ||||
| Hospital region | ||||
| Northeast | 2,561,000 | 21.3% | 27,650,000 | 21.1% |
| Midwest | 3,007,000 | 25.1% | 32,799,000 | 25.0% |
| South | 3,878,000 | 32.3% | 42,696,000 | 32.6% |
| West | 2,557,000 | 21.3% | 27,872,000 | 21.3% |
| Hospital location | ||||
| Rural | 1,507,000 | 12.6% | 16,760,000 | 12.8% |
| Urban | 10,348,000 | 86.2% | 112,639,000 | 86.0% |
| Missing | 149,000 | 1.2% | 1,617,000 | 1.2% |
| Hospital teaching status | ||||
| Nonteaching | 6,129,000 | 51.1% | 67,447,000 | 51.5% |
| Teaching | 5,726,000 | 47.7% | 61,952,000 | 47.3% |
| Missing | 149,000 | 1.2% | 1,617,000 | 1.2% |
| Hospital bed size | ||||
| Small | 1,496,000 | 12.5% | 16,479,000 | 12.6% |
| Medium | 2,905,000 | 24.2% | 31,800,000 | 24.3% |
| Large | 7,453,000 | 62.1% | 81,120,000 | 61.9% |
| Missing | 149,000 | 1.2% | 1,617,000 | 1.2% |
| Admission severity factors | ||||
| Admission source | ||||
| Emergency department | 1,078,000 | 9.0% | 12,425,000 | 9.5% |
| Another hospital | 110,000 | 0.9% | 1,219,000 | 0.9% |
| Other health facility | 61,000 | 0.5% | 664,000 | 0.5% |
| Court/law enforcement | 3,000 | 0.0% | 35,000 | 0.0% |
| Routine | 1,545,000 | 12.9% | 16,529,000 | 12.6% |
| Missing | 9,205,000 | 76.7% | 100,144,000 | 76.4% |
| Admission type | ||||
| Emergency | 4,842,000 | 40.3% | 53,386,000 | 40.7% |
| Urgent | 1,985,000 | 16.5% | 21,747,000 | 16.6% |
| Elective | 2,570,000 | 21.4% | 28,276,000 | 21.6% |
| Newborn | 1,130,000 | 9.4% | 11,625,000 | 8.9% |
| Trauma | 57,000 | 0.5% | 508,000 | 0.4% |
| Other | 4,000 | 0.0% | 45,000 | 0.0% |
| Missing | 1,417,000 | 11.8% | 15,430,000 | 11.8% |
| All‐Patient Refined DRG, severity | ||||
| No class specified | 10,000 | 0.1% | 132,000 | 0.1% |
| Minor loss of function | 4,289,000 | 35.7% | 46,092,000 | 35.2% |
| Moderate loss of function | 4,313,000 | 35.9% | 47,150,000 | 36.0% |
| Major loss of function | 2,630,000 | 21.9% | 28,939,000 | 22.1% |
| Extreme loss of function | 762,000 | 6.3% | 8,704,000 | 6.6% |
The most commonly occurring HACs were falls (5,863,778), pressure ulcers (731,103), vascular catheter‐associated infections (364,204), and catheter UTIs (290,207). HAC frequency showed a marked increase from 2008 to 2011.
HAC Occurrence
Multivariate logistic regression demonstrated that the likelihood of having one or more HACs was 6% higher in July admits compared to non‐July admits, adjusting for patient and hospital covariates (OR=1.06, 95% confidence interval [CI]: 1.061.07, P<0.0001). However, admission during July was not the most significant predictor of an HAC occurrence (Table 2). Institutional factors, such as teaching hospitals (OR=1.22, 95% CI: 1.161.28, P<0.001 vs nonteaching hospitals) and large (OR=1.11, 95% CI: 1.061.17, P=0.0002 vs small bed‐size facilities) and medium‐sized facilities (OR=1.06, 95% CI: 1.001.13, P=0.0461 vs small bed‐size facilities) were the most powerful predictors of HAC occurrence during an inpatient hospitalization (Table 2). Additionally, in a separate subanalysis with the month of admission as the primary exposure of interest, we noted that each month except for August demonstrated statistically significant decreased odds of HAC occurrence when compared to July (see Supporting Table 1 in the online version of this article). As the adjusted HAC likelihood was not statistically different between August and July, an additional analysis was run with the primary exposure being July and August admission versus all other months of admission. These resulted in a finding of 7% increased likelihood of HAC occurrence among July and August admissions compared to all others (OR=1.07, 95% CI: 1.061.07, P<0.0001; see Supporting Table 2 in the online version of this article). Similarly, a multiple imputation model adjusting for missing values produced a very similar July effect estimate to the nonimputed model (OR=1.06, 95% CI: 1.031.09, P<0.01).
| OR | 95% CI | P Value | |
|---|---|---|---|
| |||
| Patient demographic factors | |||
| Admission time | |||
| July admit | 1.06 | 1.061.07 | <0.0001 |
| Non‐July admit | Reference | ||
| Race | |||
| White | Reference | ||
| Black | 0.78 | 0.750.80 | <0.0001 |
| Hispanic | 0.81 | 0.760.85 | <0.0001 |
| API | 0.75 | 0.710.80 | <0.0001 |
| Native American | 0.92 | 0.831.02 | 0.1256 |
| Other | 0.91 | 0.840.98 | <0.0001 |
| Payer information | |||
| Medicare | 1.00 | 0.971.02 | 0.7151 |
| Medicaid | 0.87 | 0.830.90 | <0.0001 |
| Private insurance | Reference | ||
| Selfpay | 1.27 | 1.201.33 | <0.0001 |
| No charge | 1.07 | 0.921.23 | 0.3871 |
| Other | 1.93 | 1.822.05 | <0.0001 |
| Comorbidities | |||
| No comorbidities | Reference | ||
| 1 | 0.84 | 0.820.86 | <0.0001 |
| 2 or more | 0.70 | 0.680.72 | <0.0001 |
| Age category | |||
| 18 years | 0.35 | 0.330.37 | <0.0001 |
| 1930 years | 0.33 | 0.320.35 | <0.0001 |
| 3040 years | 0.32 | 0.310.33 | <0.0001 |
| 4050 years | 0.36 | 0.350.37 | <0.0001 |
| 5065 years | 0.37 | 0.360.38 | <0.0001 |
| 6580 years | 0.45 | 0.450.46 | <0.0001 |
| >80 years | Reference | ||
| Gender | |||
| Female | 0.92 | 0.900.93 | <0.0001 |
| Male | Reference | ||
| Hospital demographic factors | |||
| Hospital region | |||
| Northeast | Reference | ||
| Midwest | 1.06 | 1.001.13 | 0.2563 |
| South | 1.11 | 1.061.22 | 0.0005 |
| West | 1.08 | 0.971.20 | 0.1431 |
| Hospital location | |||
| Rural | Reference | ||
| Urban | 1.01 | 0.961.06 | 0.7144 |
| Hospital teaching status | |||
| Nonteaching | Reference | ||
| Teaching | 1.22 | 1.161.28 | <0.0001 |
| Hospital bed size | |||
| Small | Reference | ||
| Medium | 1.06 | 1.001.13 | 0.0461 |
| Large | 1.11 | 1.061.17 | 0.0002 |
| Admission severity factors | |||
| Admission source | |||
| Emergency department | 1.63 | 1.481.80 | <0.0001 |
| Another hospital | 1.96 | 1.762.17 | <0.0001 |
| Other health facility | 1.62 | 1.302.03 | <0.0001 |
| Court/law enforcement | 1.37 | 1.011.85 | 0.0438 |
| Routine | Reference | ||
| Admission type | |||
| Emergency | 2.15 | 2.032.28 | <0.0001 |
| Urgent | 1.28 | 1.201.35 | <0.0001 |
| Elective | Reference | ||
| Newborn | 0.69 | 0.630.76 | <0.0001 |
| Trauma | >1000 | <0.001>1000 | 0.9962 |
| Other | 0.91 | 0.531.55 | 0.7183 |
| AllPatient Refined DRG, severity | |||
| No class specified | 0.73 | 0.620.85 | <0.0001 |
| Minor loss of function | Reference | ||
| Moderate loss of function | 1.14 | 1.121.16 | <0.0001 |
| Major loss of function | 1.61 | 1.571.66 | <0.0001 |
| Extreme loss of function | 4.65 | 4.504.80 | <0.0001 |
We utilized similar models adjusting for the same patient, hospital, and severity factors in teaching hospital population. Patients discharged from teaching hospitals were 7% more likely to incur an HAC during admission in July compared to those admitted in the other months (OR=1.07, 95% CI: 1.061.08, P<0.01).
Higher Inpatient Charges and Prolonged LOS
The presence of one or more HACs was a significant predictor for higher inpatient charges (Table 3; OR=1.81, 95% CI: 1.74‐1.87, P<0.0001), when adjusting for July admission, patient and hospital factors, and admission severity. HAC occurrence was also a significant predictor of prolonged LOS (Table 3; OR=1.45, 95% CI: 1.42‐1.48, P<0.0001). Mean inpatient charges and LOS in this sample were $33,662.00 and 4.6 days. Patients with at least 1 HAC had a mean inpatient charge of $61,457.00, whereas those with no HAC had a mean charge of $32,377.00. Furthermore, LOS was prolonged in patients with HACs versus those who did not have HACs during hospitalization (7.14 days vs 4.49 days). Our regression analyses indicated that HAC patients had 1.48 (P<0.0001) more days of LOS and $18,258.00 (P<0.0001) more in total charges.
| OR | 95% CI | P Value | |
|---|---|---|---|
| |||
| Higher inpatient costs | |||
| Admission time | |||
| July admit | 1.00 | 0.991.01 | 0.9693 |
| Non‐July admit | Reference | ||
| HAC occurrence | |||
| HAC occurrence | 1.81 | 1.741.87 | <0.0001 |
| No HAC occurrence | Reference | ||
| Prolonged LOS | |||
| Admission time | |||
| July admit | 0.98 | 0.980.99 | <0.0001 |
| Non‐July admit | Reference | ||
| HAC occurrence | |||
| HAC occurrence | 1.45 | 1.421.48 | <0.0001 |
| No HAC occurrence | Reference | ||
DISCUSSION
This study analyzes the relationship between admission month and the incidence of HACs in a national sample. This study is also among the first to examine preventable complications as a measure of inefficiencies and inexperience of new staff during staff turnover in the month of July. In our retrospective cohort study of more than 100 million admissions across 4 years, we found a 4.9% prevalence of HACs among July admits compared to 4.7% in the non‐July admission population. In multivariate analysis, July admissions were associated with a 6% increased likelihood of HACs. Such data are concordant with other studies that demonstrate a positive July effect on mortality and efficiency.[3, 19] Evaluation of a surgical cohort revealed an 18% increase in risk‐adjusted surgical morbidity and a 41% increase in risk‐adjusted surgical mortality during July and August, using the American College of Surgeons' National Surgical Quality Improvement Program.[20] Though several studies have noted worsened outcomes during the month of July, results have been mixed. Several studies in subspecialty populations or local databases suggest no clear increase in mortality or complication rate.[3, 4, 5, 21, 22, 23, 24, 25] This current study is the first to examine these relationships using HACs as a surrogate measure indicative of quality of care and safety of new staff in the month of July.
Although multiple investigations studying July admission use mortality as an outcome measure, evaluation of preventable hospital complications may actually be more reflective of the impact of new staff on care quality and safety.[20, 26, 27, 28, 29] Mortality rates can be significantly confounded by patient‐specific factors, such as disease severity and comorbidities, whereas iatrogenic adverse events, such as HACs, are postulated to be more reflective of errors in systems and processes within the healthcare delivery institution. For example, studies demonstrate that anesthetic procedures that do not result in mortality are often associated with significant increases in complications such as central and peripheral nerve injuries, inadequate oxygenation, perioperative vomiting/aspiration, and technical failures of tracheal tube placement.[1] It is therefore not surprising that studies show July admissions are associated with longer LOS and duration of procedure, in addition to increased hospital charges.[3]
We did attempt to adjust for the effect of disease severity on HACs by incorporating 3M's APR‐DRG system, admission source, and admission type into our multivariate analyses. After adjusting for disease severity in our multivariate analysis, July admission maintained a statistically significant association with increased HAC incidence.
In our secondary analyses, we noted that all months except for August experienced significantly decreased odds of HAC occurrence compared to July admissions with similar magnitudes of likelihood found when combining July and August admissions versus all others (see Supporting Tables 1 and 2 in the online version of this article). This spillover finding may indicate the learning curve of inexperienced and new hospital staff and also suggest that the July effect is not limited only to the month of July. However, because the magnitudes of the 2 models (Table 2; Supporting Table 2 in the online version of this article) are so similar, we continue to refer to this phenomena as the July effect, with the known implication that there is a continuance beyond July and into August.
It is of interest to note that when the analysis was subsetted to only teaching institutions, July admissions in the teaching hospital cohort showed significant increases in HAC likelihood compared to non‐July admissions. Although studies suggest that inexperienced residents contribute to patient complications, the increased rate of HACs in July admits may also be multifactorial.[30] It is likely that the need for new healthcare staff to gain experience, familiarity, and effective communication also influences the HAC rate. The impact of nursing and ancillary staff involvement in the prevention of HACs is crucial. Although the July effect was primarily focused on physician elements, the nursing and ancillary staff elements are more clearly noted when evaluating HACs as an outcome. Pilot studies including multidisciplinary hospital, nursing, and physician teams, involving a significant effort to streamline communication and established protocols, have resulted in drastic decreases in patient falls, the most common of the HAC occurrences.[31] The increased hires during this time period (as new physicians and nurses complete training in June) accompanied with the need to acclimate these groups to one another and to train them on established protocols may result in risks for HACs not previously noted when evaluating more standard outcome measures.
Separate studies have shown worsened outcomes for July surgical admissions in large databases, with results indicating longer operative times for July admissions, inpatient mortality, intraoperative complications, and postoperative morbidity in areas like cardiac or spinal surgeries.[20, 22, 26, 32, 33, 34] Similarly, other studies noted that medical admissions also demonstrated worse outcomes for July admissions, resulting in increased fatal medication errors, preventable complications, and worse documentation errors.[21, 28, 30, 35, 36] In the present study, July admissions demonstrated an increased likelihood of HACs when stratified by surgical and medical admissions, as seen in the current literature.
Our study also indicates that surgical patients are noted to have a 2% increase in HACs during July versus a 9% July increase in medical patients. To the authors' knowledge, this is one of the first studies to stratify a patient population by surgical and medical services to evaluate the effect of July admission on outcomes. Possible explanations are that surgical candidates are often medically optimized prior to elective procedures, requiring a stringent protocol to be executed prior to performing an operation on a patient. Thus, the surgical patients are inherently prescreened to be of better overall health to be deemed operable compared to the traditional medicine patient. Rich et al. ([36]) performed a comparison analysis of multiple services, noting that patients with internal medicine diagnoses demonstrated the expected July effect with declines in diagnostic and pharmaceutical changes throughout the year as an indicator of improved experience leading to decreased utilization.[36] However, in that same study, the authors noted no discernible July effect among surgical patients, possibly related to the difference in resource utilization emphasized in the medical versus surgical programs.[36]
The presence of one or more HACs was a significant predictor for higher inpatient charges, when adjusting for July admission and patient/hospital factors. HAC occurrence was also a significant predictor of prolonged LOS. This is in concordance with multiple prior studies noting the association of higher LOS with HAC occurrence.[37, 38, 39, 40, 41, 42] This further supports the elevated HAC‐associated burden predicted by the CMS when compiling specific HACs.[38, 39, 40, 41, 42] Further studies in the coming years may determine whether CMS HAC regulation translates to decreased inpatient admissions durations and cost reductions over time.
This study has several limitations largely associated with the use of a standardized national database. Coding of HACs depends on consistent and accurate reporting, with errors resulting in information bias. Estimates regarding ICD‐9‐CM coding in the NIS have been cited as approximately 80% accurate.[43] Furthermore, missing variables, though noted in results, and the heterogeneity of the study population, may influence the data. Unfortunately, the nature of the data collection throughout NIS practices is not uniform within states, which may explain why a percentage of data is missing. Because of this data structure, NIS does not have documentation of the month during which an HAC was noted for admissions spanning multiple months. In regard to the missing data, we attempted to account for this using a multiple imputation model that generated similar results to our original model with missing categories coded into it; with the only major difference being expectedly larger standard errors.[44] With yearly changes in CMS coding, the addition and familiarity with new codes may influence analysis over time. Of note, the HAC denoting pressure ulcer did not exist before 2009. We were also unable to use splines to incorporate a time‐series method, as the focus of our study was targeted to looking at the higher incidences of HACs associated with July admission and not a temporal trend prior to and after July, and also the limitations we had in number of time points required for a proper time series analysis.[45] Finally, HACs are only capable of evaluating inpatient events and omit events occurring after discharge.
CONCLUSIONS
These data reveal an increase in HAC frequency during the month of July in a large national sample of patients. Recognition of a noted statistical trend in July may direct necessary attention to a time associated with increased occurrence of preventable iatrogenic adverse events. The HACs represent potential breakdowns in organizational structure distinct from traditional measures of safety, such as mortality and specialty‐specific morbidity. New guidelines dedicated to improving HACs during this time may help to decrease prevalence in both teaching and nonteaching hospitals.
Disclosure
Nothing to report.
- , , , , . Rate of undesirable events at beginning of academic year: retrospective cohort study. BMJ. 2009;339:b3974.
- , . Medical schools in the United States, 2007–2008. JAMA. 2008;300(10):1221–1227.
- , , , , , . “July effect”: impact of the academic year‐end changeover on patient outcomes: a systematic review. Ann Intern Med. 2011;155(5):309–315.
- , , , . Influence of house‐staff experience on teaching‐hospital mortality: the “July phenomenon” revisited. J Hosp Med. 2011;6(7):389–394.
- , , . Impact of admission month and hospital teaching status on outcomes in subarachnoid hemorrhage: evidence against the July effect. J Neurosurg. 2012;116(1):157–163.
- , , , . Analysis of Centers for Medicaid and Medicare Services ‘never events’ in elderly patients undergoing bowel operations. Am Surg. 2010;76(8):841–845.
- . Ending extra payment for “never events”—stronger incentives for patients' safety. N Engl J Med. 2009;360(23):2388–2390.
- . Nonpayment for performance? Medicare's new reimbursement rule. N Engl J Med. 2007;357(16):1573–1575.
- , , , et al. The impact of patient age and comorbidities on the occurrence of “never events” in cerebrovascular surgery: an analysis of the Nationwide Inpatient Sample. J Neurosurg. 2014;121(3):580–586.
- , , , , . “Never events”: Centers for Medicare and Medicaid Services complications after radical cystectomy. Urology. 2013;81(3):527–532.
- Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. Available at: http://www.ahrq.gov/data/hcup/index.html. Accessed June 2013.
- Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. HCUP frequently asked questions. Available at: www.hcup‐us.ahrq.gov/tech_assist/faq.jsp. Accessed January 2015.
- US Department of Health and Human Services. Centers for Medicare 290(14):1868–1874.
- , , , , , . Differences in resource utilization between patients with diabetes receiving glycemia‐targeted specialized nutrition vs standard nutrition formulas in U.S. hospitals. JPEN J Parenter Enteral Nutr. 2014;38(2 suppl):86S–91S.
- , , . The cost of asthma in the emergency department and hospital. Am J Respir Crit Care Med. 1999;160(1):211–215.
- , , , , , . Joint Commission primary stroke centers utilize more rt‐PA in the Nationwide Inpatient Sample. J Am Heart Assoc. 2013;2(2):e000071.
- . An introduction to multiple imputation of complex sample data using SAS 9.2. Paper presented at: SAS Global Forum Proceedings, April 11–14, 2010; Seattle, WA.
- . Measuring surgical quality in Maryland: a model. Health Aff. 1988;7(1):62–78.
- , , , et al. Seasonal variation in surgical outcomes as measured by the American College of Surgeons‐National Surgical Quality Improvement Program (ACS‐NSQIP). Ann Surg. 2007;246(3):456–462; discussion 463–465.
- , , , et al. Complications and death at the start of the new academic year: is there a July phenomenon? J Trauma. 2010;68(1):19–22.
- , , , et al. The July effect: impact of the beginning of the academic cycle on cardiac surgical outcomes in a cohort of 70,616 patients. Ann Thorac Surg. 2009;88(1):70–75.
- , , , . Nationwide data confirms absence of 'July phenomenon' in obstetrics: it's safe to deliver in July. J Perinatol. 2007;27(2):73–76.
- , . Is there a July phenomenon? The effect of July admission on intensive care mortality and length of stay in teaching hospitals. J Gen Intern Med. 2003;18(8):639–645.
- , , , , , . The “July phenomenon” for neurosurgical mortality and complications in teaching hospitals: an analysis of more than 850,000 neurosurgical patients in the nationwide inpatient sample database, 1998 to 2008. Neurosurgery. 2012;71(3):562–571; discussion 571.
- , , . Hip fracture outcome: is there a “July effect.” Am J Orthop. 2009;38(12):606–611.
- , , . Impact of cardiothoracic resident turnover on mortality after cardiac surgery: a dynamic human factor. Ann Thorac Surg. 2008;86(1):123–131.
- , . A July spike in fatal medication errors: a possible effect of new medical residents. J Gen Intern Med. 2010;25(8):774–779.
- . Human factors engineering and patient safety. Qual Saf Health Care. 2002;11(4):352–354.
- , . Ordering errors by first‐year residents: evidence of learning from mistakes. Mo Med. 2004;101(2):128–131.
- , , , . Hourly rounding and patient falls: What factors boost success? Nursing. 2015;45(2):25–30.
- , , , . The effect of July admission on inpatient outcomes following spinal surgery: clinical article. J Neurosurg Spine. 2013;18(3):280–288.
- , , , et al. The July effect and cardiac surgery: the effect of the beginning of the academic cycle on outcomes. Am J Surg. 2008;196(5):720–725.
- , , , , , . “July Effect” in elective spine surgery: analysis of the American College of Surgeons National Surgical Quality Improvement Program Database. Spine. 2014;39(7):603–611.
- . The July phenomenon revisited: are hospital complications associated with new house staff? Am J Med Qual. 1995;10(1):14–17.
- , , , . Specialty differences in the 'July Phenomenon' for Twin Cities teaching hospitals. Med Care. 1993;31(1):73–83.
- , , , , , . Potential financial impact of restriction in “never event” and periprocedural hospital‐acquired condition reimbursement at a tertiary neurosurgical center: a single‐institution prospective study. J neurosurg. 2010;112(2):249–256.
- , , , , . Pressure ulcers, hospital complications, and disease severity: impact on hospital costs and length of stay. Adv Wound Care. 1999;12(1):22–30.
- , , , . Association between cardiac and noncardiac complications in patients undergoing noncardiac surgery: outcomes and effects on length of stay. Am J Med. 2003;115(7):515–520.
- , , , , . Effect of occurrence of infection‐related never events on length of stay and hospital charges in patients undergoing radical neck dissection for head and neck cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(2):147–158.
- , , , , . Ethnic disparities in stroke: epidemiology, acute care, and postacute outcomes. Stroke. 2005;36(2):374–386.
- , , , , . The impact of surgical‐site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol. 2002;23(4):183–189.
- , , , et al. Systematic review of discharge coding accuracy. J Public Health (Oxf). 2012;34(1):138–148.
- , . Assessing bias associated with missing data from joint Canada/US survey of health: an application. Biometrics Section JSM. 2008:3394–3401.
- , , , . Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309.
- , , , , . Rate of undesirable events at beginning of academic year: retrospective cohort study. BMJ. 2009;339:b3974.
- , . Medical schools in the United States, 2007–2008. JAMA. 2008;300(10):1221–1227.
- , , , , , . “July effect”: impact of the academic year‐end changeover on patient outcomes: a systematic review. Ann Intern Med. 2011;155(5):309–315.
- , , , . Influence of house‐staff experience on teaching‐hospital mortality: the “July phenomenon” revisited. J Hosp Med. 2011;6(7):389–394.
- , , . Impact of admission month and hospital teaching status on outcomes in subarachnoid hemorrhage: evidence against the July effect. J Neurosurg. 2012;116(1):157–163.
- , , , . Analysis of Centers for Medicaid and Medicare Services ‘never events’ in elderly patients undergoing bowel operations. Am Surg. 2010;76(8):841–845.
- . Ending extra payment for “never events”—stronger incentives for patients' safety. N Engl J Med. 2009;360(23):2388–2390.
- . Nonpayment for performance? Medicare's new reimbursement rule. N Engl J Med. 2007;357(16):1573–1575.
- , , , et al. The impact of patient age and comorbidities on the occurrence of “never events” in cerebrovascular surgery: an analysis of the Nationwide Inpatient Sample. J Neurosurg. 2014;121(3):580–586.
- , , , , . “Never events”: Centers for Medicare and Medicaid Services complications after radical cystectomy. Urology. 2013;81(3):527–532.
- Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. Available at: http://www.ahrq.gov/data/hcup/index.html. Accessed June 2013.
- Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. HCUP frequently asked questions. Available at: www.hcup‐us.ahrq.gov/tech_assist/faq.jsp. Accessed January 2015.
- US Department of Health and Human Services. Centers for Medicare 290(14):1868–1874.
- , , , , , . Differences in resource utilization between patients with diabetes receiving glycemia‐targeted specialized nutrition vs standard nutrition formulas in U.S. hospitals. JPEN J Parenter Enteral Nutr. 2014;38(2 suppl):86S–91S.
- , , . The cost of asthma in the emergency department and hospital. Am J Respir Crit Care Med. 1999;160(1):211–215.
- , , , , , . Joint Commission primary stroke centers utilize more rt‐PA in the Nationwide Inpatient Sample. J Am Heart Assoc. 2013;2(2):e000071.
- . An introduction to multiple imputation of complex sample data using SAS 9.2. Paper presented at: SAS Global Forum Proceedings, April 11–14, 2010; Seattle, WA.
- . Measuring surgical quality in Maryland: a model. Health Aff. 1988;7(1):62–78.
- , , , et al. Seasonal variation in surgical outcomes as measured by the American College of Surgeons‐National Surgical Quality Improvement Program (ACS‐NSQIP). Ann Surg. 2007;246(3):456–462; discussion 463–465.
- , , , et al. Complications and death at the start of the new academic year: is there a July phenomenon? J Trauma. 2010;68(1):19–22.
- , , , et al. The July effect: impact of the beginning of the academic cycle on cardiac surgical outcomes in a cohort of 70,616 patients. Ann Thorac Surg. 2009;88(1):70–75.
- , , , . Nationwide data confirms absence of 'July phenomenon' in obstetrics: it's safe to deliver in July. J Perinatol. 2007;27(2):73–76.
- , . Is there a July phenomenon? The effect of July admission on intensive care mortality and length of stay in teaching hospitals. J Gen Intern Med. 2003;18(8):639–645.
- , , , , , . The “July phenomenon” for neurosurgical mortality and complications in teaching hospitals: an analysis of more than 850,000 neurosurgical patients in the nationwide inpatient sample database, 1998 to 2008. Neurosurgery. 2012;71(3):562–571; discussion 571.
- , , . Hip fracture outcome: is there a “July effect.” Am J Orthop. 2009;38(12):606–611.
- , , . Impact of cardiothoracic resident turnover on mortality after cardiac surgery: a dynamic human factor. Ann Thorac Surg. 2008;86(1):123–131.
- , . A July spike in fatal medication errors: a possible effect of new medical residents. J Gen Intern Med. 2010;25(8):774–779.
- . Human factors engineering and patient safety. Qual Saf Health Care. 2002;11(4):352–354.
- , . Ordering errors by first‐year residents: evidence of learning from mistakes. Mo Med. 2004;101(2):128–131.
- , , , . Hourly rounding and patient falls: What factors boost success? Nursing. 2015;45(2):25–30.
- , , , . The effect of July admission on inpatient outcomes following spinal surgery: clinical article. J Neurosurg Spine. 2013;18(3):280–288.
- , , , et al. The July effect and cardiac surgery: the effect of the beginning of the academic cycle on outcomes. Am J Surg. 2008;196(5):720–725.
- , , , , , . “July Effect” in elective spine surgery: analysis of the American College of Surgeons National Surgical Quality Improvement Program Database. Spine. 2014;39(7):603–611.
- . The July phenomenon revisited: are hospital complications associated with new house staff? Am J Med Qual. 1995;10(1):14–17.
- , , , . Specialty differences in the 'July Phenomenon' for Twin Cities teaching hospitals. Med Care. 1993;31(1):73–83.
- , , , , , . Potential financial impact of restriction in “never event” and periprocedural hospital‐acquired condition reimbursement at a tertiary neurosurgical center: a single‐institution prospective study. J neurosurg. 2010;112(2):249–256.
- , , , , . Pressure ulcers, hospital complications, and disease severity: impact on hospital costs and length of stay. Adv Wound Care. 1999;12(1):22–30.
- , , , . Association between cardiac and noncardiac complications in patients undergoing noncardiac surgery: outcomes and effects on length of stay. Am J Med. 2003;115(7):515–520.
- , , , , . Effect of occurrence of infection‐related never events on length of stay and hospital charges in patients undergoing radical neck dissection for head and neck cancer. Oral Surg Oral Med Oral Pathol Oral Radiol. 2013;116(2):147–158.
- , , , , . Ethnic disparities in stroke: epidemiology, acute care, and postacute outcomes. Stroke. 2005;36(2):374–386.
- , , , , . The impact of surgical‐site infections following orthopedic surgery at a community hospital and a university hospital: adverse quality of life, excess length of stay, and extra cost. Infect Control Hosp Epidemiol. 2002;23(4):183–189.
- , , , et al. Systematic review of discharge coding accuracy. J Public Health (Oxf). 2012;34(1):138–148.
- , . Assessing bias associated with missing data from joint Canada/US survey of health: an application. Biometrics Section JSM. 2008:3394–3401.
- , , , . Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27(4):299–309.
© 2015 Society of Hospital Medicine
Letter to the Editor
We read with great interest the study by Butcher and colleagues[1] on resident perceptions of rapid response teams (RRTs) with regard to education and autonomy. We found it interesting to note that one‐third of residents felt the nurse should always notify the primary resident when calling an RRT. Nursing literature demonstrates that ambivalence exists on when to notify the physician,[2] thus suggesting nurse‐physician interactions are still suboptimal and an area for future improvement. Given the focus on interprofessional training and practice by both the Accreditation Council of Graduate Medical Education and Liaison Committee on Medical Education,[3, 4] RRTs provide a perfect opportunity to improve interprofessional training and practice through better physician‐nurse collaboration.
Interestingly, the future of RRT activation can also be streamlined to avoid nurse‐physician conflicts about who should be notified. For example, the technology exists for automated alerts in the electronic medical record to trigger when a patient decompensates,[5] thereby activating an RRT. One can imagine this technology circumvents the physician and nurse when initiating the RRT. Given the potential uses of such technology, future studies regarding physician autonomy with automatic triggering of an RRT will be equally valuable.
- , , , The effect of a rapid response team on resident perceptions of education and autonomy. J Hosp Med. 2015;10(1):8–12.
- , , , , Qualitative exploration of nurses' decisions to activate rapid response teams. J Clin Nurs. 2013;22(19‐20):2876–2882.
- , , , et al.; Internal Medicine Milestone Group. The Internal Medicine Milestone Project. The Accreditation Council for Graduate Medical Education and The American Board of Internal Medicine. Available at: https://www.acgme.org/acgmeweb/Portals/0/PDFs/Milestones/InternalMedicineMilestones.pdf. Accessed February 12, 2015.
- Liaison Committee on Medical Education. 2013 summary of new and revised LCME accreditation standards and annotations. Available at: http://www.lcme.org/2013‐new‐and_revised‐standards‐summary.pdf. Accessed February 12, 2015.
- , , , , , Derivation of a cardiac arrest prediction model using ward vital signs. Crit Care Med. 2012;40(7):2102–2108.
We read with great interest the study by Butcher and colleagues[1] on resident perceptions of rapid response teams (RRTs) with regard to education and autonomy. We found it interesting to note that one‐third of residents felt the nurse should always notify the primary resident when calling an RRT. Nursing literature demonstrates that ambivalence exists on when to notify the physician,[2] thus suggesting nurse‐physician interactions are still suboptimal and an area for future improvement. Given the focus on interprofessional training and practice by both the Accreditation Council of Graduate Medical Education and Liaison Committee on Medical Education,[3, 4] RRTs provide a perfect opportunity to improve interprofessional training and practice through better physician‐nurse collaboration.
Interestingly, the future of RRT activation can also be streamlined to avoid nurse‐physician conflicts about who should be notified. For example, the technology exists for automated alerts in the electronic medical record to trigger when a patient decompensates,[5] thereby activating an RRT. One can imagine this technology circumvents the physician and nurse when initiating the RRT. Given the potential uses of such technology, future studies regarding physician autonomy with automatic triggering of an RRT will be equally valuable.
We read with great interest the study by Butcher and colleagues[1] on resident perceptions of rapid response teams (RRTs) with regard to education and autonomy. We found it interesting to note that one‐third of residents felt the nurse should always notify the primary resident when calling an RRT. Nursing literature demonstrates that ambivalence exists on when to notify the physician,[2] thus suggesting nurse‐physician interactions are still suboptimal and an area for future improvement. Given the focus on interprofessional training and practice by both the Accreditation Council of Graduate Medical Education and Liaison Committee on Medical Education,[3, 4] RRTs provide a perfect opportunity to improve interprofessional training and practice through better physician‐nurse collaboration.
Interestingly, the future of RRT activation can also be streamlined to avoid nurse‐physician conflicts about who should be notified. For example, the technology exists for automated alerts in the electronic medical record to trigger when a patient decompensates,[5] thereby activating an RRT. One can imagine this technology circumvents the physician and nurse when initiating the RRT. Given the potential uses of such technology, future studies regarding physician autonomy with automatic triggering of an RRT will be equally valuable.
- , , , The effect of a rapid response team on resident perceptions of education and autonomy. J Hosp Med. 2015;10(1):8–12.
- , , , , Qualitative exploration of nurses' decisions to activate rapid response teams. J Clin Nurs. 2013;22(19‐20):2876–2882.
- , , , et al.; Internal Medicine Milestone Group. The Internal Medicine Milestone Project. The Accreditation Council for Graduate Medical Education and The American Board of Internal Medicine. Available at: https://www.acgme.org/acgmeweb/Portals/0/PDFs/Milestones/InternalMedicineMilestones.pdf. Accessed February 12, 2015.
- Liaison Committee on Medical Education. 2013 summary of new and revised LCME accreditation standards and annotations. Available at: http://www.lcme.org/2013‐new‐and_revised‐standards‐summary.pdf. Accessed February 12, 2015.
- , , , , , Derivation of a cardiac arrest prediction model using ward vital signs. Crit Care Med. 2012;40(7):2102–2108.
- , , , The effect of a rapid response team on resident perceptions of education and autonomy. J Hosp Med. 2015;10(1):8–12.
- , , , , Qualitative exploration of nurses' decisions to activate rapid response teams. J Clin Nurs. 2013;22(19‐20):2876–2882.
- , , , et al.; Internal Medicine Milestone Group. The Internal Medicine Milestone Project. The Accreditation Council for Graduate Medical Education and The American Board of Internal Medicine. Available at: https://www.acgme.org/acgmeweb/Portals/0/PDFs/Milestones/InternalMedicineMilestones.pdf. Accessed February 12, 2015.
- Liaison Committee on Medical Education. 2013 summary of new and revised LCME accreditation standards and annotations. Available at: http://www.lcme.org/2013‐new‐and_revised‐standards‐summary.pdf. Accessed February 12, 2015.
- , , , , , Derivation of a cardiac arrest prediction model using ward vital signs. Crit Care Med. 2012;40(7):2102–2108.
A vaginoscopic approach to diagnostic hysteroscopy
In this video, diffuse complex endometrial hyperplasia is identified using a vaginoscopic approach with a 1.9-mm diagnostic rigid hysteroscope. The posterior fornix of the vagina is filled with saline until the cervix is elevated with the fluid and the cervical os is identified. The cervical canal is entered and gentle rotational movement and hydrodistension allows the canal to be traversed into the uterine cavity.
Video provided by Amy L. Garcia, MD

Read Dr. Garcia’s “Update on minimally invasive gynecology” (April 2015)
In this video, diffuse complex endometrial hyperplasia is identified using a vaginoscopic approach with a 1.9-mm diagnostic rigid hysteroscope. The posterior fornix of the vagina is filled with saline until the cervix is elevated with the fluid and the cervical os is identified. The cervical canal is entered and gentle rotational movement and hydrodistension allows the canal to be traversed into the uterine cavity.
Video provided by Amy L. Garcia, MD

Read Dr. Garcia’s “Update on minimally invasive gynecology” (April 2015)
In this video, diffuse complex endometrial hyperplasia is identified using a vaginoscopic approach with a 1.9-mm diagnostic rigid hysteroscope. The posterior fornix of the vagina is filled with saline until the cervix is elevated with the fluid and the cervical os is identified. The cervical canal is entered and gentle rotational movement and hydrodistension allows the canal to be traversed into the uterine cavity.
Video provided by Amy L. Garcia, MD

Read Dr. Garcia’s “Update on minimally invasive gynecology” (April 2015)
Consent to treat minors: a major complexity
The relationship between parents and pediatricians is unique. More than any other field of medicine, there is a level of trust that develops because of the consistent and ongoing interaction for several years. But as the child grows older and enters the adolescent years, the relationship shifts from catering to the desires of the parent to the needs of the child.
When a strong relationship is established, the transition of trust is usually easy, and parents are very comfortable and welcoming of an independent relationship between the physician and the child. But there are many issues that come up in adolescence that may be very difficult for a child to discuss with the parent despite having a good relationship, putting the physician directly in the middle.
The issue of minor consent is complex, and because it differs from state to state, it becomes even more complex. Of course, the best approach is to have a conversation with the parent to determine their views on various issues and ask for consent to address them should their child present to you for treatment, but new patients present, and time to establish a relationship is not always possible. The American Academy of Pediatrics statement on treatment of adolescents requires that every attempt is made to encourage inclusion of the parent in any decision making.
Understanding the laws that govern the state in which you practice is imperative. The state policies and laws can be found at www.Guttmacher.org. Although there has not been a physician held liable for nonnegligent care given to a minor who gave consent, it is important for parents to understand what their child can consent to or against. It also is important for the physician to be explicitly clear as to what their limitations are by law.
A minor status is defined by age under 18 years of age. An emancipated minor is someone who attained legal adulthood because of marriage, military service, or living separately from parents and managing one’s financial affairs (Understanding Legal Aspects of Care, in “Adolescent Health Care: A Practical Guide,” 5th ed [Philadelphia Lippincott Williams & Wilkins, 2008]). These laws are very clear and do not usually cause much confusion. Where the situation becomes very grey is in the case of the mature minor. This category is recognized in some states as an exception to the rules requiring parental consent for medical care (Int. J. Gynaecol. Obstet. 1998;63:295-300). The mature minor is defined as being at least 14 years old, having the ability to understand risk and benefits, and having the ability to provide informed consent. But this requires a subjective assessment of the adolescent, which could be argued by the parent.
Minors can consent to contraceptive services in most states. In 1977, the Supreme Court ruled that the right to privacy protects a minor’s access to nonprescriptive contraception, and although prescribed contraception is not included, it is generally considered to be included (Med. Clin. North Am, 1990; 74:1097-112). It is important to note that a pharmacist under the Pharmacist Conscience Clause, in some states, can refuse to fill the prescription without parental consent at their discretion (Arch. Pediatr. Adolesc. Med. 2003;157:361-5). Although this not a common issue, it may present a larger issue if the patient requested confidentiality.
Diagnosis and treatment of sexually transmitted disease also can be done with the consent of a minor, but the age of the patient, usually greater than 14 years, is required in most states. A careful assessment must be done for abuse regardless of whether the minor admitted to consensual sex or not. The laws regarding statutory rape are clearly defined state to state and may present a larger problem if disputed by the parent.
Elective abortion is always a topic of debate. States require at least one parent to consent when a minor is seeking an abortion, but a minor also can seek a judicial bypass, which is a request from a minor to not have parental consent for an abortion if they believe that notification will bring harm to the minor. Conversely, an adolescent also can refuse to consent to an abortion that the parent requests.
Immunizations also can be given with the consent of the minor, but extra precaution should be given to documentation of clear explanation of risk and benefits. Despite there being no federal law requiring parental consent, some states do require it, and it is prudent to obtain it.
Parents don’t often realize the limitations of their ability to prevent or demand treatment. So although the abortion itself falls outside the scope of care of a pediatrician, educating parents on the laws can help them navigate the situation better. Parents also may request drug, sexually transmitted infection, or pregnancy testing without the knowledge of the minor. Whether it is done is left to the discretion of the physician but the AAP advises that this only be done as a rare exception (Pediatrics 2007;119:627-30).
Now a larger consideration for physicians is financial liability. Parents are not obligated to pay for treatment and procedures for which they did not consent. The financial responsibility falls on the minor who requested it. Obviously, this could be costly for the facility, and therefore a decision has to be made to either disrupt continuity of care and refer to an outside facility or absorb the cost. This can be a challenging decision. Disclosing to the minor that payment sent through the insurance might unintentionally breach the confidentiality of the treatment is also an important consideration if the minor’s desire is to keep the parent uninformed.
The issue of consent to treatment when it comes to minors is multifaceted. Maintaining the trust of the parent and gaining the trust of the adolescent is tricky when the lines of communication between them are limited. Establishing early a relationship of trust with the parent to advise and treat the child appropriately in the event he or she does present with complex issues will settle many of the issues. More importantly, as pediatricians our goal is to establish a relationship with the adolescent so that he or she knows where to go to get good sound advice and treatment to ensure good health and prevent avoidable consequences.
Dr. Pearce is a pediatrician in Frankfort, Ill. E-mail her at [email protected]. Scan this QR code to view similar columns or go to pediatricnews.com.
The relationship between parents and pediatricians is unique. More than any other field of medicine, there is a level of trust that develops because of the consistent and ongoing interaction for several years. But as the child grows older and enters the adolescent years, the relationship shifts from catering to the desires of the parent to the needs of the child.
When a strong relationship is established, the transition of trust is usually easy, and parents are very comfortable and welcoming of an independent relationship between the physician and the child. But there are many issues that come up in adolescence that may be very difficult for a child to discuss with the parent despite having a good relationship, putting the physician directly in the middle.
The issue of minor consent is complex, and because it differs from state to state, it becomes even more complex. Of course, the best approach is to have a conversation with the parent to determine their views on various issues and ask for consent to address them should their child present to you for treatment, but new patients present, and time to establish a relationship is not always possible. The American Academy of Pediatrics statement on treatment of adolescents requires that every attempt is made to encourage inclusion of the parent in any decision making.
Understanding the laws that govern the state in which you practice is imperative. The state policies and laws can be found at www.Guttmacher.org. Although there has not been a physician held liable for nonnegligent care given to a minor who gave consent, it is important for parents to understand what their child can consent to or against. It also is important for the physician to be explicitly clear as to what their limitations are by law.
A minor status is defined by age under 18 years of age. An emancipated minor is someone who attained legal adulthood because of marriage, military service, or living separately from parents and managing one’s financial affairs (Understanding Legal Aspects of Care, in “Adolescent Health Care: A Practical Guide,” 5th ed [Philadelphia Lippincott Williams & Wilkins, 2008]). These laws are very clear and do not usually cause much confusion. Where the situation becomes very grey is in the case of the mature minor. This category is recognized in some states as an exception to the rules requiring parental consent for medical care (Int. J. Gynaecol. Obstet. 1998;63:295-300). The mature minor is defined as being at least 14 years old, having the ability to understand risk and benefits, and having the ability to provide informed consent. But this requires a subjective assessment of the adolescent, which could be argued by the parent.
Minors can consent to contraceptive services in most states. In 1977, the Supreme Court ruled that the right to privacy protects a minor’s access to nonprescriptive contraception, and although prescribed contraception is not included, it is generally considered to be included (Med. Clin. North Am, 1990; 74:1097-112). It is important to note that a pharmacist under the Pharmacist Conscience Clause, in some states, can refuse to fill the prescription without parental consent at their discretion (Arch. Pediatr. Adolesc. Med. 2003;157:361-5). Although this not a common issue, it may present a larger issue if the patient requested confidentiality.
Diagnosis and treatment of sexually transmitted disease also can be done with the consent of a minor, but the age of the patient, usually greater than 14 years, is required in most states. A careful assessment must be done for abuse regardless of whether the minor admitted to consensual sex or not. The laws regarding statutory rape are clearly defined state to state and may present a larger problem if disputed by the parent.
Elective abortion is always a topic of debate. States require at least one parent to consent when a minor is seeking an abortion, but a minor also can seek a judicial bypass, which is a request from a minor to not have parental consent for an abortion if they believe that notification will bring harm to the minor. Conversely, an adolescent also can refuse to consent to an abortion that the parent requests.
Immunizations also can be given with the consent of the minor, but extra precaution should be given to documentation of clear explanation of risk and benefits. Despite there being no federal law requiring parental consent, some states do require it, and it is prudent to obtain it.
Parents don’t often realize the limitations of their ability to prevent or demand treatment. So although the abortion itself falls outside the scope of care of a pediatrician, educating parents on the laws can help them navigate the situation better. Parents also may request drug, sexually transmitted infection, or pregnancy testing without the knowledge of the minor. Whether it is done is left to the discretion of the physician but the AAP advises that this only be done as a rare exception (Pediatrics 2007;119:627-30).
Now a larger consideration for physicians is financial liability. Parents are not obligated to pay for treatment and procedures for which they did not consent. The financial responsibility falls on the minor who requested it. Obviously, this could be costly for the facility, and therefore a decision has to be made to either disrupt continuity of care and refer to an outside facility or absorb the cost. This can be a challenging decision. Disclosing to the minor that payment sent through the insurance might unintentionally breach the confidentiality of the treatment is also an important consideration if the minor’s desire is to keep the parent uninformed.
The issue of consent to treatment when it comes to minors is multifaceted. Maintaining the trust of the parent and gaining the trust of the adolescent is tricky when the lines of communication between them are limited. Establishing early a relationship of trust with the parent to advise and treat the child appropriately in the event he or she does present with complex issues will settle many of the issues. More importantly, as pediatricians our goal is to establish a relationship with the adolescent so that he or she knows where to go to get good sound advice and treatment to ensure good health and prevent avoidable consequences.
Dr. Pearce is a pediatrician in Frankfort, Ill. E-mail her at [email protected]. Scan this QR code to view similar columns or go to pediatricnews.com.
The relationship between parents and pediatricians is unique. More than any other field of medicine, there is a level of trust that develops because of the consistent and ongoing interaction for several years. But as the child grows older and enters the adolescent years, the relationship shifts from catering to the desires of the parent to the needs of the child.
When a strong relationship is established, the transition of trust is usually easy, and parents are very comfortable and welcoming of an independent relationship between the physician and the child. But there are many issues that come up in adolescence that may be very difficult for a child to discuss with the parent despite having a good relationship, putting the physician directly in the middle.
The issue of minor consent is complex, and because it differs from state to state, it becomes even more complex. Of course, the best approach is to have a conversation with the parent to determine their views on various issues and ask for consent to address them should their child present to you for treatment, but new patients present, and time to establish a relationship is not always possible. The American Academy of Pediatrics statement on treatment of adolescents requires that every attempt is made to encourage inclusion of the parent in any decision making.
Understanding the laws that govern the state in which you practice is imperative. The state policies and laws can be found at www.Guttmacher.org. Although there has not been a physician held liable for nonnegligent care given to a minor who gave consent, it is important for parents to understand what their child can consent to or against. It also is important for the physician to be explicitly clear as to what their limitations are by law.
A minor status is defined by age under 18 years of age. An emancipated minor is someone who attained legal adulthood because of marriage, military service, or living separately from parents and managing one’s financial affairs (Understanding Legal Aspects of Care, in “Adolescent Health Care: A Practical Guide,” 5th ed [Philadelphia Lippincott Williams & Wilkins, 2008]). These laws are very clear and do not usually cause much confusion. Where the situation becomes very grey is in the case of the mature minor. This category is recognized in some states as an exception to the rules requiring parental consent for medical care (Int. J. Gynaecol. Obstet. 1998;63:295-300). The mature minor is defined as being at least 14 years old, having the ability to understand risk and benefits, and having the ability to provide informed consent. But this requires a subjective assessment of the adolescent, which could be argued by the parent.
Minors can consent to contraceptive services in most states. In 1977, the Supreme Court ruled that the right to privacy protects a minor’s access to nonprescriptive contraception, and although prescribed contraception is not included, it is generally considered to be included (Med. Clin. North Am, 1990; 74:1097-112). It is important to note that a pharmacist under the Pharmacist Conscience Clause, in some states, can refuse to fill the prescription without parental consent at their discretion (Arch. Pediatr. Adolesc. Med. 2003;157:361-5). Although this not a common issue, it may present a larger issue if the patient requested confidentiality.
Diagnosis and treatment of sexually transmitted disease also can be done with the consent of a minor, but the age of the patient, usually greater than 14 years, is required in most states. A careful assessment must be done for abuse regardless of whether the minor admitted to consensual sex or not. The laws regarding statutory rape are clearly defined state to state and may present a larger problem if disputed by the parent.
Elective abortion is always a topic of debate. States require at least one parent to consent when a minor is seeking an abortion, but a minor also can seek a judicial bypass, which is a request from a minor to not have parental consent for an abortion if they believe that notification will bring harm to the minor. Conversely, an adolescent also can refuse to consent to an abortion that the parent requests.
Immunizations also can be given with the consent of the minor, but extra precaution should be given to documentation of clear explanation of risk and benefits. Despite there being no federal law requiring parental consent, some states do require it, and it is prudent to obtain it.
Parents don’t often realize the limitations of their ability to prevent or demand treatment. So although the abortion itself falls outside the scope of care of a pediatrician, educating parents on the laws can help them navigate the situation better. Parents also may request drug, sexually transmitted infection, or pregnancy testing without the knowledge of the minor. Whether it is done is left to the discretion of the physician but the AAP advises that this only be done as a rare exception (Pediatrics 2007;119:627-30).
Now a larger consideration for physicians is financial liability. Parents are not obligated to pay for treatment and procedures for which they did not consent. The financial responsibility falls on the minor who requested it. Obviously, this could be costly for the facility, and therefore a decision has to be made to either disrupt continuity of care and refer to an outside facility or absorb the cost. This can be a challenging decision. Disclosing to the minor that payment sent through the insurance might unintentionally breach the confidentiality of the treatment is also an important consideration if the minor’s desire is to keep the parent uninformed.
The issue of consent to treatment when it comes to minors is multifaceted. Maintaining the trust of the parent and gaining the trust of the adolescent is tricky when the lines of communication between them are limited. Establishing early a relationship of trust with the parent to advise and treat the child appropriately in the event he or she does present with complex issues will settle many of the issues. More importantly, as pediatricians our goal is to establish a relationship with the adolescent so that he or she knows where to go to get good sound advice and treatment to ensure good health and prevent avoidable consequences.
Dr. Pearce is a pediatrician in Frankfort, Ill. E-mail her at [email protected]. Scan this QR code to view similar columns or go to pediatricnews.com.