User login
Coating repels blood and bacteria
to form a blood clot
Credit: James Weaver
Researchers have developed a coating that can prevent blood and bacteria from adhering to the surface of medical devices.
The coating repelled blood from more than 20 medically relevant substrates, suppressed biofilm formation, and prevented coagulation in an animal model for at least 8 hours.
The researchers believe this technology could reduce the use of anticoagulants and help prevent thrombotic occlusion and biofouling of medical devices.
The idea for the coating evolved from a surface technology known as “slippery liquid-infused porous surfaces (SLIPS),” which was developed by Joanna Aizenberg, PhD, of Harvard University’s Wyss Institute for Biologically Inspired Engineering.
Inspired by the slippery surface of the carnivorous pitcher plant, which enables the plant to capture insects, SLIPS can repel nearly any material. The liquid layer on the surface provides a barrier to everything from ice to crude oil and blood.
“Traditional SLIPS uses porous, textured surface substrates to immobilize the liquid layer, whereas medical surfaces are mostly flat and smooth,” Dr Aizenberg said. “So we further adapted our approach by capitalizing on the natural roughness of chemically modified surfaces of medical devices.”
She and her colleagues described this work in Nature Biotechnology.
The researchers developed a super-repellent coating that can be adhered to existing, approved medical devices. In a 2-step surface-coating process, they chemically attached a monolayer of perfluorocarbon, which is similar to Teflon.
Then, they added a layer of liquid perfluorocarbon, which is widely used in medicine for applications such as liquid ventilation for infants with breathing challenges, blood substitution, and eye surgery. The team calls the tethered perfluorocarbon plus the liquid layer a “tethered-liquid perfluorocarbon surface (TLP)”.
TLP worked seamlessly when coated on more than 20 different medical surfaces, including plastics, glasses, and metals.
The researchers also implanted medical-grade tubing and catheters coated with TLP in large blood vessels in pigs, and the coating prevented blood from clotting for at least 8 hours without the use of anticoagulants.
TLP-treated medical tubing was stored for more than a year under normal temperature and humidity conditions and still prevented clot formation, repelling fibrin and platelets.
The TLP surface remained stable under the full range of clinically relevant physiological shear stresses, or rates of blood flow seen in catheters and central lines, all the way up to dialysis machines.
When the bacteria Pseudomonas aeruginosa were grown in TLP-coated medical tubing for more than 6 weeks, less than 1 in a billion bacteria were able to adhere. Central lines coated with TLP significantly reduced sepsis from central-line associated bloodstream infections.
Out of curiosity, the researchers even tested a TLP-coated surface with a gecko, whose footpads contain hair-like structures with tremendous adhesive strength. And the gecko was unable to hold on.
“We were wonderfully surprised by how well the TLP coating worked, particularly in vivo without heparin,” said Anna Waterhouse, PhD, of the Wyss Institute.
“Usually, the blood will start to clot within an hour in the extracorporeal circuit, so our experiments really demonstrate the clinical relevance of this new coating.” ![]()

to form a blood clot
Credit: James Weaver
Researchers have developed a coating that can prevent blood and bacteria from adhering to the surface of medical devices.
The coating repelled blood from more than 20 medically relevant substrates, suppressed biofilm formation, and prevented coagulation in an animal model for at least 8 hours.
The researchers believe this technology could reduce the use of anticoagulants and help prevent thrombotic occlusion and biofouling of medical devices.
The idea for the coating evolved from a surface technology known as “slippery liquid-infused porous surfaces (SLIPS),” which was developed by Joanna Aizenberg, PhD, of Harvard University’s Wyss Institute for Biologically Inspired Engineering.
Inspired by the slippery surface of the carnivorous pitcher plant, which enables the plant to capture insects, SLIPS can repel nearly any material. The liquid layer on the surface provides a barrier to everything from ice to crude oil and blood.
“Traditional SLIPS uses porous, textured surface substrates to immobilize the liquid layer, whereas medical surfaces are mostly flat and smooth,” Dr Aizenberg said. “So we further adapted our approach by capitalizing on the natural roughness of chemically modified surfaces of medical devices.”
She and her colleagues described this work in Nature Biotechnology.
The researchers developed a super-repellent coating that can be adhered to existing, approved medical devices. In a 2-step surface-coating process, they chemically attached a monolayer of perfluorocarbon, which is similar to Teflon.
Then, they added a layer of liquid perfluorocarbon, which is widely used in medicine for applications such as liquid ventilation for infants with breathing challenges, blood substitution, and eye surgery. The team calls the tethered perfluorocarbon plus the liquid layer a “tethered-liquid perfluorocarbon surface (TLP)”.
TLP worked seamlessly when coated on more than 20 different medical surfaces, including plastics, glasses, and metals.
The researchers also implanted medical-grade tubing and catheters coated with TLP in large blood vessels in pigs, and the coating prevented blood from clotting for at least 8 hours without the use of anticoagulants.
TLP-treated medical tubing was stored for more than a year under normal temperature and humidity conditions and still prevented clot formation, repelling fibrin and platelets.
The TLP surface remained stable under the full range of clinically relevant physiological shear stresses, or rates of blood flow seen in catheters and central lines, all the way up to dialysis machines.
When the bacteria Pseudomonas aeruginosa were grown in TLP-coated medical tubing for more than 6 weeks, less than 1 in a billion bacteria were able to adhere. Central lines coated with TLP significantly reduced sepsis from central-line associated bloodstream infections.
Out of curiosity, the researchers even tested a TLP-coated surface with a gecko, whose footpads contain hair-like structures with tremendous adhesive strength. And the gecko was unable to hold on.
“We were wonderfully surprised by how well the TLP coating worked, particularly in vivo without heparin,” said Anna Waterhouse, PhD, of the Wyss Institute.
“Usually, the blood will start to clot within an hour in the extracorporeal circuit, so our experiments really demonstrate the clinical relevance of this new coating.” ![]()

to form a blood clot
Credit: James Weaver
Researchers have developed a coating that can prevent blood and bacteria from adhering to the surface of medical devices.
The coating repelled blood from more than 20 medically relevant substrates, suppressed biofilm formation, and prevented coagulation in an animal model for at least 8 hours.
The researchers believe this technology could reduce the use of anticoagulants and help prevent thrombotic occlusion and biofouling of medical devices.
The idea for the coating evolved from a surface technology known as “slippery liquid-infused porous surfaces (SLIPS),” which was developed by Joanna Aizenberg, PhD, of Harvard University’s Wyss Institute for Biologically Inspired Engineering.
Inspired by the slippery surface of the carnivorous pitcher plant, which enables the plant to capture insects, SLIPS can repel nearly any material. The liquid layer on the surface provides a barrier to everything from ice to crude oil and blood.
“Traditional SLIPS uses porous, textured surface substrates to immobilize the liquid layer, whereas medical surfaces are mostly flat and smooth,” Dr Aizenberg said. “So we further adapted our approach by capitalizing on the natural roughness of chemically modified surfaces of medical devices.”
She and her colleagues described this work in Nature Biotechnology.
The researchers developed a super-repellent coating that can be adhered to existing, approved medical devices. In a 2-step surface-coating process, they chemically attached a monolayer of perfluorocarbon, which is similar to Teflon.
Then, they added a layer of liquid perfluorocarbon, which is widely used in medicine for applications such as liquid ventilation for infants with breathing challenges, blood substitution, and eye surgery. The team calls the tethered perfluorocarbon plus the liquid layer a “tethered-liquid perfluorocarbon surface (TLP)”.
TLP worked seamlessly when coated on more than 20 different medical surfaces, including plastics, glasses, and metals.
The researchers also implanted medical-grade tubing and catheters coated with TLP in large blood vessels in pigs, and the coating prevented blood from clotting for at least 8 hours without the use of anticoagulants.
TLP-treated medical tubing was stored for more than a year under normal temperature and humidity conditions and still prevented clot formation, repelling fibrin and platelets.
The TLP surface remained stable under the full range of clinically relevant physiological shear stresses, or rates of blood flow seen in catheters and central lines, all the way up to dialysis machines.
When the bacteria Pseudomonas aeruginosa were grown in TLP-coated medical tubing for more than 6 weeks, less than 1 in a billion bacteria were able to adhere. Central lines coated with TLP significantly reduced sepsis from central-line associated bloodstream infections.
Out of curiosity, the researchers even tested a TLP-coated surface with a gecko, whose footpads contain hair-like structures with tremendous adhesive strength. And the gecko was unable to hold on.
“We were wonderfully surprised by how well the TLP coating worked, particularly in vivo without heparin,” said Anna Waterhouse, PhD, of the Wyss Institute.
“Usually, the blood will start to clot within an hour in the extracorporeal circuit, so our experiments really demonstrate the clinical relevance of this new coating.” ![]()
System can detect sepsis early

Credit: CDC
An early warning and response system can predict a patient’s likelihood of developing sepsis, according to research published in the Journal of Hospital Medicine.
The system uses lab and vital-sign data in the electronic health record of hospital inpatients to identify those at risk for sepsis.
In a multi-hospital study, the system allowed for a marked increase in sepsis identification and care, transfer to the intensive care unit (ICU), and an indication of fewer deaths due to sepsis.
Craig A. Umscheid, MD, of Penn Medicine in Philadelphia, and his colleagues developed the system using 4575 patients admitted to the University of Pennsylvania Health System in October 2011.
The system monitored lab values and vital signs in real time. If a patient had 4 or more predefined abnormalities at any single time, an electronic communication was sent to the provider, nurse, and rapid response coordinator, who performed an immediate bedside patient evaluation.
The researchers validated the effectiveness of the system during a pre-implementation period from June to September 2012, when data on admitted patients was evaluated and alerts triggered in a database, but no notifications were sent to providers on the ground.
Outcomes in that control period were then compared to a post-implementation period from June to September 2013. The total number of patients included in both periods was 31,093.
In the pre- and post-implementation periods, 4% of patient visits triggered the alert. Analysis revealed that 90% of those patients received bedside evaluations by the care team within 30 minutes of the alert being issued.
The system resulted in a 2- to 3-fold increase in orders for tests that could help identify the presence of sepsis and a 1.5- to 2-fold increase in the administration of antibiotics and intravenous fluids.
The system prompted an increase of more than 50% in the proportion of patients quickly transferred to the ICU and a 50% increase in documentation of sepsis in the patients’ electronic health record.
There was a lower death rate from sepsis and an increase in the number of patients successfully discharged home in the post-implementation period. But these rates were not significantly different from those in the pre-implementation period.
“Our study is the first we’re aware of that was implemented throughout a multihospital health system,” Dr Umscheid said.
“Previous studies that have examined the impact of sepsis prediction tools at other institutions have only taken place on a limited number of inpatient wards. The varied patient populations, clinical staffing, practice models, and practice cultures across our health system increases the generalizability of our findings to other healthcare settings.”
Dr Umscheid also noted that the system could help triage patients for suitability of ICU transfer.
“By better identifying those with sepsis requiring advanced care,” he said, “the tool can help screen out patients not needing the inevitably limited number of ICU beds.” ![]()

Credit: CDC
An early warning and response system can predict a patient’s likelihood of developing sepsis, according to research published in the Journal of Hospital Medicine.
The system uses lab and vital-sign data in the electronic health record of hospital inpatients to identify those at risk for sepsis.
In a multi-hospital study, the system allowed for a marked increase in sepsis identification and care, transfer to the intensive care unit (ICU), and an indication of fewer deaths due to sepsis.
Craig A. Umscheid, MD, of Penn Medicine in Philadelphia, and his colleagues developed the system using 4575 patients admitted to the University of Pennsylvania Health System in October 2011.
The system monitored lab values and vital signs in real time. If a patient had 4 or more predefined abnormalities at any single time, an electronic communication was sent to the provider, nurse, and rapid response coordinator, who performed an immediate bedside patient evaluation.
The researchers validated the effectiveness of the system during a pre-implementation period from June to September 2012, when data on admitted patients was evaluated and alerts triggered in a database, but no notifications were sent to providers on the ground.
Outcomes in that control period were then compared to a post-implementation period from June to September 2013. The total number of patients included in both periods was 31,093.
In the pre- and post-implementation periods, 4% of patient visits triggered the alert. Analysis revealed that 90% of those patients received bedside evaluations by the care team within 30 minutes of the alert being issued.
The system resulted in a 2- to 3-fold increase in orders for tests that could help identify the presence of sepsis and a 1.5- to 2-fold increase in the administration of antibiotics and intravenous fluids.
The system prompted an increase of more than 50% in the proportion of patients quickly transferred to the ICU and a 50% increase in documentation of sepsis in the patients’ electronic health record.
There was a lower death rate from sepsis and an increase in the number of patients successfully discharged home in the post-implementation period. But these rates were not significantly different from those in the pre-implementation period.
“Our study is the first we’re aware of that was implemented throughout a multihospital health system,” Dr Umscheid said.
“Previous studies that have examined the impact of sepsis prediction tools at other institutions have only taken place on a limited number of inpatient wards. The varied patient populations, clinical staffing, practice models, and practice cultures across our health system increases the generalizability of our findings to other healthcare settings.”
Dr Umscheid also noted that the system could help triage patients for suitability of ICU transfer.
“By better identifying those with sepsis requiring advanced care,” he said, “the tool can help screen out patients not needing the inevitably limited number of ICU beds.” ![]()

Credit: CDC
An early warning and response system can predict a patient’s likelihood of developing sepsis, according to research published in the Journal of Hospital Medicine.
The system uses lab and vital-sign data in the electronic health record of hospital inpatients to identify those at risk for sepsis.
In a multi-hospital study, the system allowed for a marked increase in sepsis identification and care, transfer to the intensive care unit (ICU), and an indication of fewer deaths due to sepsis.
Craig A. Umscheid, MD, of Penn Medicine in Philadelphia, and his colleagues developed the system using 4575 patients admitted to the University of Pennsylvania Health System in October 2011.
The system monitored lab values and vital signs in real time. If a patient had 4 or more predefined abnormalities at any single time, an electronic communication was sent to the provider, nurse, and rapid response coordinator, who performed an immediate bedside patient evaluation.
The researchers validated the effectiveness of the system during a pre-implementation period from June to September 2012, when data on admitted patients was evaluated and alerts triggered in a database, but no notifications were sent to providers on the ground.
Outcomes in that control period were then compared to a post-implementation period from June to September 2013. The total number of patients included in both periods was 31,093.
In the pre- and post-implementation periods, 4% of patient visits triggered the alert. Analysis revealed that 90% of those patients received bedside evaluations by the care team within 30 minutes of the alert being issued.
The system resulted in a 2- to 3-fold increase in orders for tests that could help identify the presence of sepsis and a 1.5- to 2-fold increase in the administration of antibiotics and intravenous fluids.
The system prompted an increase of more than 50% in the proportion of patients quickly transferred to the ICU and a 50% increase in documentation of sepsis in the patients’ electronic health record.
There was a lower death rate from sepsis and an increase in the number of patients successfully discharged home in the post-implementation period. But these rates were not significantly different from those in the pre-implementation period.
“Our study is the first we’re aware of that was implemented throughout a multihospital health system,” Dr Umscheid said.
“Previous studies that have examined the impact of sepsis prediction tools at other institutions have only taken place on a limited number of inpatient wards. The varied patient populations, clinical staffing, practice models, and practice cultures across our health system increases the generalizability of our findings to other healthcare settings.”
Dr Umscheid also noted that the system could help triage patients for suitability of ICU transfer.
“By better identifying those with sepsis requiring advanced care,” he said, “the tool can help screen out patients not needing the inevitably limited number of ICU beds.” ![]()
FDA approves drug for nausea, vomiting
The US Food and Drug Administration (FDA) has approved Akynzeo to treat nausea and vomiting in cancer patients undergoing chemotherapy. Akynzeo is a capsule consisting of two drugs, netupitant and palonosetron.
Palonosetron prevents nausea and vomiting in the acute phase—within the first 24 hours of chemotherapy initiation.
Netupitant prevents nausea and vomiting in the acute phase and the delayed phase—25 to 120 hours after chemotherapy began.
Akynzeo’s effectiveness was established in two clinical trials of 1720 cancer patients receiving chemotherapy. Patients were randomized to receive Akynzeo or oral palonosetron.
The trials were designed to measure whether the drugs prevented any vomiting episodes in the acute, delayed, and overall phases after the start of chemotherapy.
Most Akynzeo-treated patients did not experience any vomiting or require rescue medication for nausea during the acute (98.5%), delayed (90.4%), and overall phases (89.6%).
The same was true for patients who received palonosetron, although percentages were lower—89.7%, 80.1%, and 76.5%, respectively.
The second trial showed similar results.
Common side effects of Akynzeo in the clinical trials were headache, asthenia, fatigue, dyspepsia, and constipation.
Akynzeo is distributed and marketed by Eisai Inc., under license from Helsinn Healthcare S.A. ![]()
The US Food and Drug Administration (FDA) has approved Akynzeo to treat nausea and vomiting in cancer patients undergoing chemotherapy. Akynzeo is a capsule consisting of two drugs, netupitant and palonosetron.
Palonosetron prevents nausea and vomiting in the acute phase—within the first 24 hours of chemotherapy initiation.
Netupitant prevents nausea and vomiting in the acute phase and the delayed phase—25 to 120 hours after chemotherapy began.
Akynzeo’s effectiveness was established in two clinical trials of 1720 cancer patients receiving chemotherapy. Patients were randomized to receive Akynzeo or oral palonosetron.
The trials were designed to measure whether the drugs prevented any vomiting episodes in the acute, delayed, and overall phases after the start of chemotherapy.
Most Akynzeo-treated patients did not experience any vomiting or require rescue medication for nausea during the acute (98.5%), delayed (90.4%), and overall phases (89.6%).
The same was true for patients who received palonosetron, although percentages were lower—89.7%, 80.1%, and 76.5%, respectively.
The second trial showed similar results.
Common side effects of Akynzeo in the clinical trials were headache, asthenia, fatigue, dyspepsia, and constipation.
Akynzeo is distributed and marketed by Eisai Inc., under license from Helsinn Healthcare S.A. ![]()
The US Food and Drug Administration (FDA) has approved Akynzeo to treat nausea and vomiting in cancer patients undergoing chemotherapy. Akynzeo is a capsule consisting of two drugs, netupitant and palonosetron.
Palonosetron prevents nausea and vomiting in the acute phase—within the first 24 hours of chemotherapy initiation.
Netupitant prevents nausea and vomiting in the acute phase and the delayed phase—25 to 120 hours after chemotherapy began.
Akynzeo’s effectiveness was established in two clinical trials of 1720 cancer patients receiving chemotherapy. Patients were randomized to receive Akynzeo or oral palonosetron.
The trials were designed to measure whether the drugs prevented any vomiting episodes in the acute, delayed, and overall phases after the start of chemotherapy.
Most Akynzeo-treated patients did not experience any vomiting or require rescue medication for nausea during the acute (98.5%), delayed (90.4%), and overall phases (89.6%).
The same was true for patients who received palonosetron, although percentages were lower—89.7%, 80.1%, and 76.5%, respectively.
The second trial showed similar results.
Common side effects of Akynzeo in the clinical trials were headache, asthenia, fatigue, dyspepsia, and constipation.
Akynzeo is distributed and marketed by Eisai Inc., under license from Helsinn Healthcare S.A. ![]()
Product News: 04 2014
Keytruda
Merck & Co, Inc, announces US Food and Drug Administration approval of Keytruda (pembrolizumab) for the treatment of patients with unresectable or metastatic melanoma and disease progression following ipilimumab and, if BRAF V600–mutation positive, a BRAF inhibitor. Keytruda is the first anti-PD-1 (programmed death receptor-1) therapy in the United States and is approved as a breakthrough therapy based on tumor response rate and durability of response. It works by increasing the ability of the body’s immune system to fight advanced melanoma. It blocks the interaction between PD-1 and its ligands (PD-L1 and PD-L2) and may affect both tumor cells and healthy cells. For more information, visit www.keytruda.com.
Otezla
Celgene Corporation obtains US Food and Drug Administration approval for Otezla (apremilast), an oral, selective inhibitor of phosphodiesterase 4 for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy. Otezla is associated with an increase in adverse reactions of depression. Patients, their caregivers, and families should be advised of the need to be alert for the emergence or worsening of depression, suicidal thoughts, or other mood changes. Otezla also is approved for patients with active psoriatic arthritis. For more information, visit www.otezla.com.
Papaya Enzyme Cleanser
Revision Skincare introduces the Papaya Enzyme Cleanser, an energizing facial wash formulated with a unique extract derived from papayas to nourish skin with a multitude of vitamins and minerals. The Papaya Enzyme Cleanser, available exclusively through physicians, helps patients achieve naturally vibrant skin. For more information, visit www.revisionskincare.com.
Pigmentclar
La Roche-Posay Laboratoire Dermatologique launches Pigmentclar Intensive Dark Spot Correcting Serum and Pigmentclar SPF 30 Daily Dark Spot Correcting Moisturizer to target multiple stages of dark spot development, leaving patients with a brighter, more even skin tone. The products are formulated to correct dark spots that are underlying, visible, or recurrent. Phe-resorcinol helps reduce excess melanin at the early stages of production, lipohydroxy acid enables cell-by-cell exfoliation, and niacinamide reduces melanin transfer from one cell to another. Both products can be purchased over-the-counter at select retailers, physicians’ offices, and online. For more information, visit www.laroche-posay.us.
Regenacyn
Oculus Innovative Sciences, Inc, introduces Regenacyn Advanced Scar Management Hydrogel to improve the texture, color, softness, and overall appearance of scars. Formulated with Microcyn technology, Regenacyn is intended for the management of old and new hypertrophic and keloid scars of various sizes, shapes, and locations resulting from burns, general surgical procedures, and trauma wounds. Regenacyn is physician dispensed by plastic surgeons and obstetricians/gynecologists. For more information, visit www.oculusis.com/regenacyn-hydrogel.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Keytruda
Merck & Co, Inc, announces US Food and Drug Administration approval of Keytruda (pembrolizumab) for the treatment of patients with unresectable or metastatic melanoma and disease progression following ipilimumab and, if BRAF V600–mutation positive, a BRAF inhibitor. Keytruda is the first anti-PD-1 (programmed death receptor-1) therapy in the United States and is approved as a breakthrough therapy based on tumor response rate and durability of response. It works by increasing the ability of the body’s immune system to fight advanced melanoma. It blocks the interaction between PD-1 and its ligands (PD-L1 and PD-L2) and may affect both tumor cells and healthy cells. For more information, visit www.keytruda.com.
Otezla
Celgene Corporation obtains US Food and Drug Administration approval for Otezla (apremilast), an oral, selective inhibitor of phosphodiesterase 4 for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy. Otezla is associated with an increase in adverse reactions of depression. Patients, their caregivers, and families should be advised of the need to be alert for the emergence or worsening of depression, suicidal thoughts, or other mood changes. Otezla also is approved for patients with active psoriatic arthritis. For more information, visit www.otezla.com.
Papaya Enzyme Cleanser
Revision Skincare introduces the Papaya Enzyme Cleanser, an energizing facial wash formulated with a unique extract derived from papayas to nourish skin with a multitude of vitamins and minerals. The Papaya Enzyme Cleanser, available exclusively through physicians, helps patients achieve naturally vibrant skin. For more information, visit www.revisionskincare.com.
Pigmentclar
La Roche-Posay Laboratoire Dermatologique launches Pigmentclar Intensive Dark Spot Correcting Serum and Pigmentclar SPF 30 Daily Dark Spot Correcting Moisturizer to target multiple stages of dark spot development, leaving patients with a brighter, more even skin tone. The products are formulated to correct dark spots that are underlying, visible, or recurrent. Phe-resorcinol helps reduce excess melanin at the early stages of production, lipohydroxy acid enables cell-by-cell exfoliation, and niacinamide reduces melanin transfer from one cell to another. Both products can be purchased over-the-counter at select retailers, physicians’ offices, and online. For more information, visit www.laroche-posay.us.
Regenacyn
Oculus Innovative Sciences, Inc, introduces Regenacyn Advanced Scar Management Hydrogel to improve the texture, color, softness, and overall appearance of scars. Formulated with Microcyn technology, Regenacyn is intended for the management of old and new hypertrophic and keloid scars of various sizes, shapes, and locations resulting from burns, general surgical procedures, and trauma wounds. Regenacyn is physician dispensed by plastic surgeons and obstetricians/gynecologists. For more information, visit www.oculusis.com/regenacyn-hydrogel.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Keytruda
Merck & Co, Inc, announces US Food and Drug Administration approval of Keytruda (pembrolizumab) for the treatment of patients with unresectable or metastatic melanoma and disease progression following ipilimumab and, if BRAF V600–mutation positive, a BRAF inhibitor. Keytruda is the first anti-PD-1 (programmed death receptor-1) therapy in the United States and is approved as a breakthrough therapy based on tumor response rate and durability of response. It works by increasing the ability of the body’s immune system to fight advanced melanoma. It blocks the interaction between PD-1 and its ligands (PD-L1 and PD-L2) and may affect both tumor cells and healthy cells. For more information, visit www.keytruda.com.
Otezla
Celgene Corporation obtains US Food and Drug Administration approval for Otezla (apremilast), an oral, selective inhibitor of phosphodiesterase 4 for the treatment of adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy. Otezla is associated with an increase in adverse reactions of depression. Patients, their caregivers, and families should be advised of the need to be alert for the emergence or worsening of depression, suicidal thoughts, or other mood changes. Otezla also is approved for patients with active psoriatic arthritis. For more information, visit www.otezla.com.
Papaya Enzyme Cleanser
Revision Skincare introduces the Papaya Enzyme Cleanser, an energizing facial wash formulated with a unique extract derived from papayas to nourish skin with a multitude of vitamins and minerals. The Papaya Enzyme Cleanser, available exclusively through physicians, helps patients achieve naturally vibrant skin. For more information, visit www.revisionskincare.com.
Pigmentclar
La Roche-Posay Laboratoire Dermatologique launches Pigmentclar Intensive Dark Spot Correcting Serum and Pigmentclar SPF 30 Daily Dark Spot Correcting Moisturizer to target multiple stages of dark spot development, leaving patients with a brighter, more even skin tone. The products are formulated to correct dark spots that are underlying, visible, or recurrent. Phe-resorcinol helps reduce excess melanin at the early stages of production, lipohydroxy acid enables cell-by-cell exfoliation, and niacinamide reduces melanin transfer from one cell to another. Both products can be purchased over-the-counter at select retailers, physicians’ offices, and online. For more information, visit www.laroche-posay.us.
Regenacyn
Oculus Innovative Sciences, Inc, introduces Regenacyn Advanced Scar Management Hydrogel to improve the texture, color, softness, and overall appearance of scars. Formulated with Microcyn technology, Regenacyn is intended for the management of old and new hypertrophic and keloid scars of various sizes, shapes, and locations resulting from burns, general surgical procedures, and trauma wounds. Regenacyn is physician dispensed by plastic surgeons and obstetricians/gynecologists. For more information, visit www.oculusis.com/regenacyn-hydrogel.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Errata
Due to a submission error, an acknowledgment was missing from the July 2014 article “The Great Mimickers of Rosacea” (Cutis. 2014;94:39-45).
Acknowledgements—The authors thank Jennifer Rullan, MD, San Diego, California, and Jose Gonzalez-Chavez, MD, San Juan, Puerto Rico, for their assistance.
Also, the order of the authors was incorrect in the July 2014 e-only article “Lepromatous Leprosy Associated With Erythema Nodosum Leprosum” (Cutis. 2014;94:E19-E20). The correct order is:
Azeen Sadeghian, MD
These articles have been corrected online.
Due to a submission error, an acknowledgment was missing from the July 2014 article “The Great Mimickers of Rosacea” (Cutis. 2014;94:39-45).
Acknowledgements—The authors thank Jennifer Rullan, MD, San Diego, California, and Jose Gonzalez-Chavez, MD, San Juan, Puerto Rico, for their assistance.
Also, the order of the authors was incorrect in the July 2014 e-only article “Lepromatous Leprosy Associated With Erythema Nodosum Leprosum” (Cutis. 2014;94:E19-E20). The correct order is:
Azeen Sadeghian, MD
These articles have been corrected online.
Due to a submission error, an acknowledgment was missing from the July 2014 article “The Great Mimickers of Rosacea” (Cutis. 2014;94:39-45).
Acknowledgements—The authors thank Jennifer Rullan, MD, San Diego, California, and Jose Gonzalez-Chavez, MD, San Juan, Puerto Rico, for their assistance.
Also, the order of the authors was incorrect in the July 2014 e-only article “Lepromatous Leprosy Associated With Erythema Nodosum Leprosum” (Cutis. 2014;94:E19-E20). The correct order is:
Azeen Sadeghian, MD
These articles have been corrected online.
Allergic Contact Dermatitis to 2-Octyl Cyanoacrylate
Cyanoacrylates are widely used in adhesive products, with applications ranging from household products to nail and beauty salons and even dentistry. A topical skin adhesive containing 2-octyl cyanoacrylate was approved in 1998 for topical application for closure of skin edges of wounds from surgical incisions.1 Usually cyanoacrylates are not strong sensitizers, and despite their extensive use, there have been relatively few reports of associated allergic contact dermatitis (ACD).2-5 We report 4 cases of ACD to 2-octyl cyanoacrylate used in postsurgical wound closures as confirmed by patch tests.
Case Reports
Patient 1
A 33-year-old woman presented with an intensely pruritic peri-incisional rash on the lower back and right buttock of 1 week’s duration. The eruption started roughly 1 week following surgical implantation of a spinal cord stimulator for treatment of chronic back pain. Both incisions made during the implantation were closed with 2-octyl cyanoacrylate. The patient denied any prior exposure to topical skin adhesives or any history of contact dermatitis to nickel or other materials. The patient did not dress the wounds and did not apply topical agents to the area.
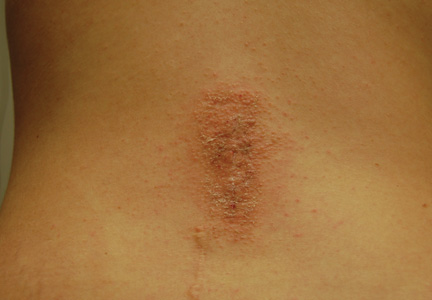
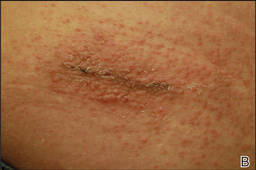
Physical examination revealed 6- to 8-cm linear surgical scars on the midline lumbar back and superior right buttock with surrounding excoriated erythematous papules coalescing into plaques consistent with acute eczematous dermatitis (Figure 1). Similar papules and plaques were scattered across the abdomen and chest. She was given triamcinolone acetonide ointment 0.1% twice daily and hydroxyzine pamoate 25 mg 3 times daily for itching. The surgical wounds healed within 2 weeks of presentation with postinflammatory hyperpigmentation surrounding the scars.

| 
|
| Figure 1. Surgical scars with surrounding excoriated erythematous papules coalescing into plaques on the midline lumbar back (A) and superior right buttock (B). | |
Six weeks later she underwent patch testing to confirm the diagnosis. She was screened using the North American Contact Dermatitis Group standard 65-allergen series and a miscellaneous tray including hardware obtained from the spinal cord stimulator device manufacturer. A use test to 2-octyl cyanoacrylate also was performed. At 96 hours, true positives included cinnamic aldehyde (1+), nickel (1+), bacitracin (1+), fragrance mix (2+), disperse blue dyes 106 and 124 (2+), and 2-octyl cyanoacrylate (3+)(1+=weak positive; 2+=strong positive; 3+=extreme reaction). There was no response to any components of the device. The pattern of dermatitis and positive patch-test results strongly supported the diagnosis of ACD to 2-octyl cyanoacrylate.
Patients 2, 3, and 4
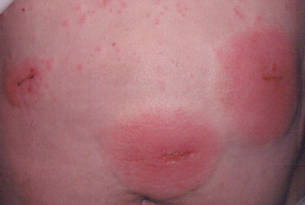
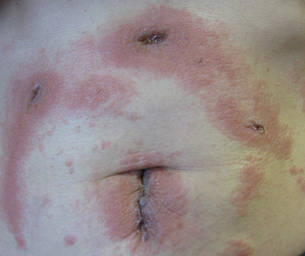
Three patients—a 65-year-old woman, a 35-year-old woman, and a 44-year-old woman—presented to us with eczematous dermatitis at laparoscopic portal sites that were closed with 2-octyl cyanoacrylate (Figures 2 and 3). They presented approximately 1 week following laparoscopic Nissen fundoplication, laparoscopic left hepatectomy, and laparoscopic cholecystectomy, respectively. None of these 3 patients had been using any topical medications. All of them had a positive reaction (2+) to 2-octyl cyanoacrylate on use testing. Interestingly, use tests for 2 other cyanoacrylates containing 2-butyl cyanoacrylate were negative in 2 patients.
|
| Figure 2. Acute eczematous plaques at wound closures. |
|
| Figure 3. Coalescing acute eczematous plaques focused at wound closures. |
Although patient 1 reported no prior exposure to 2-octyl cyanoacrylate, these 3 additional patients reported prior exposure with no reaction. Other possible contact allergens associated with wound closure included iodine, topical antibiotics, and dressing tape.
Comment
Contact allergies to acrylates are not uncommon. In a series of 275 patients, Kanerva et al6 found that 17.5% of patients had an allergic reaction to at least 1 acrylate or methacrylate. In the same series, no allergic reactions to cyanoacrylates were noted.6 The role of methacrylates in the development of occupational ACD and irritant dermatitis has been well characterized among dentists, orthopedic surgeons, beauticians, and industrial workers who are commonly exposed to these agents.7-12 Partially because of their longer carbon chains, cyanoacrylates have reduced toxicity and improved bonding strength as well as flexibility. Given their availability and the ease and speed of their use, skin adhesives have become widely used in the closure of surgical wounds.13-16
Postoperative contact dermatitis is problematic, as patients are exposed to many potential allergens during surgery. In our clinical practice, the most common allergens causing ACD associated with surgery are iodine, topical antibiotics (ie, bacitracin, neomycin), tape adhesives, suture materials, and less commonly surgical hardware. Although they are rarely reported, contact allergies to skin adhesives such as cyanoacrylates are of particular importance because they may complicate surgical wounds, leading to dehiscence, infection, and scarring, among other complications. In our patients, there were no adverse outcomes in wound healing with the exception of postinflammatory hyperpigmentation.
Under ideal conditions, 2-octyl cyanoacrylate generally is not a strong sensitizer; however, application to open wounds or thinner skin such as the eyelids may permit exposure of antigen-presenting cells to cyanoacrylate monomers, thereby initiating sensitization. Postsurgical occlusive dressings, which often are left in place for 7 to 14 days, also may contribute to sensitization. The role of the degradation of skin adhesive products in the development of contact dermatitis is unknown.
Management of ACD from skin adhesives should involve the immediate removal of any remaining adhesive. One manufacturer recommends removal of the product using acetone or petroleum jelly.1 In our experience, rubbing the adhesive with 2×2-in gauze pads or using forceps have been successful methods for removal. The use of petroleum jelly prior to rubbing with gauze also can aid in removal of the adhesive. Warm water soaks and soap also may be helpful but are not expected to immediately loosen the bond. A mid-potency steroid ointment such as triamcinolone may be effective in treating dermatitis, though the use of higher-potency steroids such as clobetasol may be needed for severe reactions.1,2
As members of the cyano group, cyanoacrylates are highly reactive molecules that polymerize and rapidly bind to the stratum corneum when they come in contact with traces of water. During polymerization, the individual constituents or monomer cyanoacrylate molecules are joined into a polymer chain, which should be trapped by keratinocytes and not reach immunomodulators2,10; however, as postulated during the first report of contact dermatitis, an arid environment could delay polymerization and increase the risk of sensitization.2 The first report was made in Las Vegas, Nevada,2 and our cases presented in San Antonio, Texas.
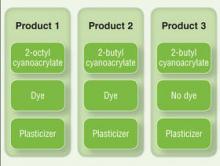
There currently are 2 main cutaneous adhesives containing cyanoacrylate on the market, including 2-octyl cyanoacrylate and 2-butyl cyanoacrylate. These products are known by various trade names and differ primarily in the length of the carbon chain in the cyanoacrylate. A dye is added to allow better visibility of the glue during application, and a plasticizer increases viscosity and accelerates polymerization. The 2 most widely used products contain the same dye (D&C Violet No. 2) and similar but proprietary plasticizers.
Although plasticizers and dyes may be potential contact allergens, we postulated that the cyanoacrylate was the responsible sensitizer in our cases. Because the individual ingredients were not readily available for use testing, we devised a logical method to attempt to determine the specific component of the skin adhesive that was responsible for contact sensitization (Figure 4). Patients 3 and 4 in our series were tested using this method and were found to be sensitive to the product containing 2-octyl cyanoacrylate but not the products containing 2-butyl cyanoacrylate.
Conclusion
Given the many advantages of cyanoacrylates, it is likely that their use in skin adhesive products will continue to increase. Our 4 patients may represent a rise in the incidence of ACD associated with increased use of skin adhesives, but it is important to look critically at this agent when patients present with postoperative pruritus in the absence of topical bacitracin or neomycin use and surgical dressing irritation. By using the technique we described, it is possible to identify the component responsible for the reaction; however, in the future, the exact mechanisms of sensitization and the specific components should be further elucidated by researchers working in conjunction with the manufacturers. Use testing on abraded skin and/or under occlusive dressings more closely mimics the initial exposure and may have a role in determining true allergy.
1. Dermabond Advanced [package insert]. San Lorenzo, PR: Ethicon, LLC; 2013.
2. Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octyl cyanoacrylate. Arch Dermatol. 2008;144:814-815.
3. Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
4. El-Dars LD, Chaudhury W, Hughes TM, et al. Allergic contact dermatitis to Dermabond after orthopaedic joint replacement. Contact Dermatitis. 2010;62:315-317.
5. Howard BK, Hudkins ML. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
6. Kanerva L, Jolanki R, Estlander T. 10 years of patch testing with the (meth)acrylate series. Contact Dermatitis. 1997;37:255-258.
7. Belsito DV. Contact dermatitis to ethyl-cyanoacrylate-containing glue. Contact Dermatitis. 1987;17:234-236.
8. Leggat PA, Kedjarune U, Smith DR. Toxicity of cyanoacrylate adhesives and their occupational impacts for dental staff. Ind Health. 2004;42:207-211.
9. Conde-Salazar L, Rojo S, Guimaraens D. Occupational allergic contact dermatitis from cyanoacrylate. Am J Contact Dermat. 1998;9:188-189.
10. Aalto-Korte K, Alanko K, Kuuliala O, et al. Occupational methacrylate and acrylate allergy from glues. Contact Dermatitis. 2008;58:340-346.
11. Tomb RR, Lepoittevin JP, Durepaire F, et al. Ectopic contact dermatitis from ethyl cyanoacrylate instant adhesives. Contact Dermatitis. 1993;28:206-208.
12. Dragu A, Unglaub F, Schwarz S, et al. Foreign body reaction after usage of tissue adhesives for skin closure: a case report and review of the literature. Arch Orthop Trauma Surg. 2009;129:167-169.
13. Eaglstein WH, Sullivan T. Cyanoacrylates for skin closure. Dermatol Clin. 2005;23:193-198.
14. Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med. 2008;26:490-496.
15. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004;187:238-248.
16. Calnan CD. Cyanoacrylate dermatitis. Contact Dermatitis. 1979;5:165-167.
Cyanoacrylates are widely used in adhesive products, with applications ranging from household products to nail and beauty salons and even dentistry. A topical skin adhesive containing 2-octyl cyanoacrylate was approved in 1998 for topical application for closure of skin edges of wounds from surgical incisions.1 Usually cyanoacrylates are not strong sensitizers, and despite their extensive use, there have been relatively few reports of associated allergic contact dermatitis (ACD).2-5 We report 4 cases of ACD to 2-octyl cyanoacrylate used in postsurgical wound closures as confirmed by patch tests.
Case Reports
Patient 1
A 33-year-old woman presented with an intensely pruritic peri-incisional rash on the lower back and right buttock of 1 week’s duration. The eruption started roughly 1 week following surgical implantation of a spinal cord stimulator for treatment of chronic back pain. Both incisions made during the implantation were closed with 2-octyl cyanoacrylate. The patient denied any prior exposure to topical skin adhesives or any history of contact dermatitis to nickel or other materials. The patient did not dress the wounds and did not apply topical agents to the area.
Physical examination revealed 6- to 8-cm linear surgical scars on the midline lumbar back and superior right buttock with surrounding excoriated erythematous papules coalescing into plaques consistent with acute eczematous dermatitis (Figure 1). Similar papules and plaques were scattered across the abdomen and chest. She was given triamcinolone acetonide ointment 0.1% twice daily and hydroxyzine pamoate 25 mg 3 times daily for itching. The surgical wounds healed within 2 weeks of presentation with postinflammatory hyperpigmentation surrounding the scars.

| 
|
| Figure 1. Surgical scars with surrounding excoriated erythematous papules coalescing into plaques on the midline lumbar back (A) and superior right buttock (B). | |
Six weeks later she underwent patch testing to confirm the diagnosis. She was screened using the North American Contact Dermatitis Group standard 65-allergen series and a miscellaneous tray including hardware obtained from the spinal cord stimulator device manufacturer. A use test to 2-octyl cyanoacrylate also was performed. At 96 hours, true positives included cinnamic aldehyde (1+), nickel (1+), bacitracin (1+), fragrance mix (2+), disperse blue dyes 106 and 124 (2+), and 2-octyl cyanoacrylate (3+)(1+=weak positive; 2+=strong positive; 3+=extreme reaction). There was no response to any components of the device. The pattern of dermatitis and positive patch-test results strongly supported the diagnosis of ACD to 2-octyl cyanoacrylate.
Patients 2, 3, and 4
Three patients—a 65-year-old woman, a 35-year-old woman, and a 44-year-old woman—presented to us with eczematous dermatitis at laparoscopic portal sites that were closed with 2-octyl cyanoacrylate (Figures 2 and 3). They presented approximately 1 week following laparoscopic Nissen fundoplication, laparoscopic left hepatectomy, and laparoscopic cholecystectomy, respectively. None of these 3 patients had been using any topical medications. All of them had a positive reaction (2+) to 2-octyl cyanoacrylate on use testing. Interestingly, use tests for 2 other cyanoacrylates containing 2-butyl cyanoacrylate were negative in 2 patients.

|
| Figure 2. Acute eczematous plaques at wound closures. |

|
| Figure 3. Coalescing acute eczematous plaques focused at wound closures. |
Although patient 1 reported no prior exposure to 2-octyl cyanoacrylate, these 3 additional patients reported prior exposure with no reaction. Other possible contact allergens associated with wound closure included iodine, topical antibiotics, and dressing tape.
Comment
Contact allergies to acrylates are not uncommon. In a series of 275 patients, Kanerva et al6 found that 17.5% of patients had an allergic reaction to at least 1 acrylate or methacrylate. In the same series, no allergic reactions to cyanoacrylates were noted.6 The role of methacrylates in the development of occupational ACD and irritant dermatitis has been well characterized among dentists, orthopedic surgeons, beauticians, and industrial workers who are commonly exposed to these agents.7-12 Partially because of their longer carbon chains, cyanoacrylates have reduced toxicity and improved bonding strength as well as flexibility. Given their availability and the ease and speed of their use, skin adhesives have become widely used in the closure of surgical wounds.13-16
Postoperative contact dermatitis is problematic, as patients are exposed to many potential allergens during surgery. In our clinical practice, the most common allergens causing ACD associated with surgery are iodine, topical antibiotics (ie, bacitracin, neomycin), tape adhesives, suture materials, and less commonly surgical hardware. Although they are rarely reported, contact allergies to skin adhesives such as cyanoacrylates are of particular importance because they may complicate surgical wounds, leading to dehiscence, infection, and scarring, among other complications. In our patients, there were no adverse outcomes in wound healing with the exception of postinflammatory hyperpigmentation.
Under ideal conditions, 2-octyl cyanoacrylate generally is not a strong sensitizer; however, application to open wounds or thinner skin such as the eyelids may permit exposure of antigen-presenting cells to cyanoacrylate monomers, thereby initiating sensitization. Postsurgical occlusive dressings, which often are left in place for 7 to 14 days, also may contribute to sensitization. The role of the degradation of skin adhesive products in the development of contact dermatitis is unknown.
Management of ACD from skin adhesives should involve the immediate removal of any remaining adhesive. One manufacturer recommends removal of the product using acetone or petroleum jelly.1 In our experience, rubbing the adhesive with 2×2-in gauze pads or using forceps have been successful methods for removal. The use of petroleum jelly prior to rubbing with gauze also can aid in removal of the adhesive. Warm water soaks and soap also may be helpful but are not expected to immediately loosen the bond. A mid-potency steroid ointment such as triamcinolone may be effective in treating dermatitis, though the use of higher-potency steroids such as clobetasol may be needed for severe reactions.1,2
As members of the cyano group, cyanoacrylates are highly reactive molecules that polymerize and rapidly bind to the stratum corneum when they come in contact with traces of water. During polymerization, the individual constituents or monomer cyanoacrylate molecules are joined into a polymer chain, which should be trapped by keratinocytes and not reach immunomodulators2,10; however, as postulated during the first report of contact dermatitis, an arid environment could delay polymerization and increase the risk of sensitization.2 The first report was made in Las Vegas, Nevada,2 and our cases presented in San Antonio, Texas.
There currently are 2 main cutaneous adhesives containing cyanoacrylate on the market, including 2-octyl cyanoacrylate and 2-butyl cyanoacrylate. These products are known by various trade names and differ primarily in the length of the carbon chain in the cyanoacrylate. A dye is added to allow better visibility of the glue during application, and a plasticizer increases viscosity and accelerates polymerization. The 2 most widely used products contain the same dye (D&C Violet No. 2) and similar but proprietary plasticizers.
Although plasticizers and dyes may be potential contact allergens, we postulated that the cyanoacrylate was the responsible sensitizer in our cases. Because the individual ingredients were not readily available for use testing, we devised a logical method to attempt to determine the specific component of the skin adhesive that was responsible for contact sensitization (Figure 4). Patients 3 and 4 in our series were tested using this method and were found to be sensitive to the product containing 2-octyl cyanoacrylate but not the products containing 2-butyl cyanoacrylate.
Conclusion
Given the many advantages of cyanoacrylates, it is likely that their use in skin adhesive products will continue to increase. Our 4 patients may represent a rise in the incidence of ACD associated with increased use of skin adhesives, but it is important to look critically at this agent when patients present with postoperative pruritus in the absence of topical bacitracin or neomycin use and surgical dressing irritation. By using the technique we described, it is possible to identify the component responsible for the reaction; however, in the future, the exact mechanisms of sensitization and the specific components should be further elucidated by researchers working in conjunction with the manufacturers. Use testing on abraded skin and/or under occlusive dressings more closely mimics the initial exposure and may have a role in determining true allergy.
Cyanoacrylates are widely used in adhesive products, with applications ranging from household products to nail and beauty salons and even dentistry. A topical skin adhesive containing 2-octyl cyanoacrylate was approved in 1998 for topical application for closure of skin edges of wounds from surgical incisions.1 Usually cyanoacrylates are not strong sensitizers, and despite their extensive use, there have been relatively few reports of associated allergic contact dermatitis (ACD).2-5 We report 4 cases of ACD to 2-octyl cyanoacrylate used in postsurgical wound closures as confirmed by patch tests.
Case Reports
Patient 1
A 33-year-old woman presented with an intensely pruritic peri-incisional rash on the lower back and right buttock of 1 week’s duration. The eruption started roughly 1 week following surgical implantation of a spinal cord stimulator for treatment of chronic back pain. Both incisions made during the implantation were closed with 2-octyl cyanoacrylate. The patient denied any prior exposure to topical skin adhesives or any history of contact dermatitis to nickel or other materials. The patient did not dress the wounds and did not apply topical agents to the area.
Physical examination revealed 6- to 8-cm linear surgical scars on the midline lumbar back and superior right buttock with surrounding excoriated erythematous papules coalescing into plaques consistent with acute eczematous dermatitis (Figure 1). Similar papules and plaques were scattered across the abdomen and chest. She was given triamcinolone acetonide ointment 0.1% twice daily and hydroxyzine pamoate 25 mg 3 times daily for itching. The surgical wounds healed within 2 weeks of presentation with postinflammatory hyperpigmentation surrounding the scars.

| 
|
| Figure 1. Surgical scars with surrounding excoriated erythematous papules coalescing into plaques on the midline lumbar back (A) and superior right buttock (B). | |
Six weeks later she underwent patch testing to confirm the diagnosis. She was screened using the North American Contact Dermatitis Group standard 65-allergen series and a miscellaneous tray including hardware obtained from the spinal cord stimulator device manufacturer. A use test to 2-octyl cyanoacrylate also was performed. At 96 hours, true positives included cinnamic aldehyde (1+), nickel (1+), bacitracin (1+), fragrance mix (2+), disperse blue dyes 106 and 124 (2+), and 2-octyl cyanoacrylate (3+)(1+=weak positive; 2+=strong positive; 3+=extreme reaction). There was no response to any components of the device. The pattern of dermatitis and positive patch-test results strongly supported the diagnosis of ACD to 2-octyl cyanoacrylate.
Patients 2, 3, and 4
Three patients—a 65-year-old woman, a 35-year-old woman, and a 44-year-old woman—presented to us with eczematous dermatitis at laparoscopic portal sites that were closed with 2-octyl cyanoacrylate (Figures 2 and 3). They presented approximately 1 week following laparoscopic Nissen fundoplication, laparoscopic left hepatectomy, and laparoscopic cholecystectomy, respectively. None of these 3 patients had been using any topical medications. All of them had a positive reaction (2+) to 2-octyl cyanoacrylate on use testing. Interestingly, use tests for 2 other cyanoacrylates containing 2-butyl cyanoacrylate were negative in 2 patients.

|
| Figure 2. Acute eczematous plaques at wound closures. |

|
| Figure 3. Coalescing acute eczematous plaques focused at wound closures. |
Although patient 1 reported no prior exposure to 2-octyl cyanoacrylate, these 3 additional patients reported prior exposure with no reaction. Other possible contact allergens associated with wound closure included iodine, topical antibiotics, and dressing tape.
Comment
Contact allergies to acrylates are not uncommon. In a series of 275 patients, Kanerva et al6 found that 17.5% of patients had an allergic reaction to at least 1 acrylate or methacrylate. In the same series, no allergic reactions to cyanoacrylates were noted.6 The role of methacrylates in the development of occupational ACD and irritant dermatitis has been well characterized among dentists, orthopedic surgeons, beauticians, and industrial workers who are commonly exposed to these agents.7-12 Partially because of their longer carbon chains, cyanoacrylates have reduced toxicity and improved bonding strength as well as flexibility. Given their availability and the ease and speed of their use, skin adhesives have become widely used in the closure of surgical wounds.13-16
Postoperative contact dermatitis is problematic, as patients are exposed to many potential allergens during surgery. In our clinical practice, the most common allergens causing ACD associated with surgery are iodine, topical antibiotics (ie, bacitracin, neomycin), tape adhesives, suture materials, and less commonly surgical hardware. Although they are rarely reported, contact allergies to skin adhesives such as cyanoacrylates are of particular importance because they may complicate surgical wounds, leading to dehiscence, infection, and scarring, among other complications. In our patients, there were no adverse outcomes in wound healing with the exception of postinflammatory hyperpigmentation.
Under ideal conditions, 2-octyl cyanoacrylate generally is not a strong sensitizer; however, application to open wounds or thinner skin such as the eyelids may permit exposure of antigen-presenting cells to cyanoacrylate monomers, thereby initiating sensitization. Postsurgical occlusive dressings, which often are left in place for 7 to 14 days, also may contribute to sensitization. The role of the degradation of skin adhesive products in the development of contact dermatitis is unknown.
Management of ACD from skin adhesives should involve the immediate removal of any remaining adhesive. One manufacturer recommends removal of the product using acetone or petroleum jelly.1 In our experience, rubbing the adhesive with 2×2-in gauze pads or using forceps have been successful methods for removal. The use of petroleum jelly prior to rubbing with gauze also can aid in removal of the adhesive. Warm water soaks and soap also may be helpful but are not expected to immediately loosen the bond. A mid-potency steroid ointment such as triamcinolone may be effective in treating dermatitis, though the use of higher-potency steroids such as clobetasol may be needed for severe reactions.1,2
As members of the cyano group, cyanoacrylates are highly reactive molecules that polymerize and rapidly bind to the stratum corneum when they come in contact with traces of water. During polymerization, the individual constituents or monomer cyanoacrylate molecules are joined into a polymer chain, which should be trapped by keratinocytes and not reach immunomodulators2,10; however, as postulated during the first report of contact dermatitis, an arid environment could delay polymerization and increase the risk of sensitization.2 The first report was made in Las Vegas, Nevada,2 and our cases presented in San Antonio, Texas.
There currently are 2 main cutaneous adhesives containing cyanoacrylate on the market, including 2-octyl cyanoacrylate and 2-butyl cyanoacrylate. These products are known by various trade names and differ primarily in the length of the carbon chain in the cyanoacrylate. A dye is added to allow better visibility of the glue during application, and a plasticizer increases viscosity and accelerates polymerization. The 2 most widely used products contain the same dye (D&C Violet No. 2) and similar but proprietary plasticizers.
Although plasticizers and dyes may be potential contact allergens, we postulated that the cyanoacrylate was the responsible sensitizer in our cases. Because the individual ingredients were not readily available for use testing, we devised a logical method to attempt to determine the specific component of the skin adhesive that was responsible for contact sensitization (Figure 4). Patients 3 and 4 in our series were tested using this method and were found to be sensitive to the product containing 2-octyl cyanoacrylate but not the products containing 2-butyl cyanoacrylate.
Conclusion
Given the many advantages of cyanoacrylates, it is likely that their use in skin adhesive products will continue to increase. Our 4 patients may represent a rise in the incidence of ACD associated with increased use of skin adhesives, but it is important to look critically at this agent when patients present with postoperative pruritus in the absence of topical bacitracin or neomycin use and surgical dressing irritation. By using the technique we described, it is possible to identify the component responsible for the reaction; however, in the future, the exact mechanisms of sensitization and the specific components should be further elucidated by researchers working in conjunction with the manufacturers. Use testing on abraded skin and/or under occlusive dressings more closely mimics the initial exposure and may have a role in determining true allergy.
1. Dermabond Advanced [package insert]. San Lorenzo, PR: Ethicon, LLC; 2013.
2. Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octyl cyanoacrylate. Arch Dermatol. 2008;144:814-815.
3. Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
4. El-Dars LD, Chaudhury W, Hughes TM, et al. Allergic contact dermatitis to Dermabond after orthopaedic joint replacement. Contact Dermatitis. 2010;62:315-317.
5. Howard BK, Hudkins ML. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
6. Kanerva L, Jolanki R, Estlander T. 10 years of patch testing with the (meth)acrylate series. Contact Dermatitis. 1997;37:255-258.
7. Belsito DV. Contact dermatitis to ethyl-cyanoacrylate-containing glue. Contact Dermatitis. 1987;17:234-236.
8. Leggat PA, Kedjarune U, Smith DR. Toxicity of cyanoacrylate adhesives and their occupational impacts for dental staff. Ind Health. 2004;42:207-211.
9. Conde-Salazar L, Rojo S, Guimaraens D. Occupational allergic contact dermatitis from cyanoacrylate. Am J Contact Dermat. 1998;9:188-189.
10. Aalto-Korte K, Alanko K, Kuuliala O, et al. Occupational methacrylate and acrylate allergy from glues. Contact Dermatitis. 2008;58:340-346.
11. Tomb RR, Lepoittevin JP, Durepaire F, et al. Ectopic contact dermatitis from ethyl cyanoacrylate instant adhesives. Contact Dermatitis. 1993;28:206-208.
12. Dragu A, Unglaub F, Schwarz S, et al. Foreign body reaction after usage of tissue adhesives for skin closure: a case report and review of the literature. Arch Orthop Trauma Surg. 2009;129:167-169.
13. Eaglstein WH, Sullivan T. Cyanoacrylates for skin closure. Dermatol Clin. 2005;23:193-198.
14. Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med. 2008;26:490-496.
15. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004;187:238-248.
16. Calnan CD. Cyanoacrylate dermatitis. Contact Dermatitis. 1979;5:165-167.
1. Dermabond Advanced [package insert]. San Lorenzo, PR: Ethicon, LLC; 2013.
2. Hivnor CM, Hudkins ML. Allergic contact dermatitis after postsurgical repair with 2-octyl cyanoacrylate. Arch Dermatol. 2008;144:814-815.
3. Perry AW, Sosin M. Severe allergic reaction to Dermabond. Aesthet Surg J. 2009;29:314-316.
4. El-Dars LD, Chaudhury W, Hughes TM, et al. Allergic contact dermatitis to Dermabond after orthopaedic joint replacement. Contact Dermatitis. 2010;62:315-317.
5. Howard BK, Hudkins ML. Contact dermatitis from Dermabond. Plast Reconstr Surg. 2010;125:E252-E253.
6. Kanerva L, Jolanki R, Estlander T. 10 years of patch testing with the (meth)acrylate series. Contact Dermatitis. 1997;37:255-258.
7. Belsito DV. Contact dermatitis to ethyl-cyanoacrylate-containing glue. Contact Dermatitis. 1987;17:234-236.
8. Leggat PA, Kedjarune U, Smith DR. Toxicity of cyanoacrylate adhesives and their occupational impacts for dental staff. Ind Health. 2004;42:207-211.
9. Conde-Salazar L, Rojo S, Guimaraens D. Occupational allergic contact dermatitis from cyanoacrylate. Am J Contact Dermat. 1998;9:188-189.
10. Aalto-Korte K, Alanko K, Kuuliala O, et al. Occupational methacrylate and acrylate allergy from glues. Contact Dermatitis. 2008;58:340-346.
11. Tomb RR, Lepoittevin JP, Durepaire F, et al. Ectopic contact dermatitis from ethyl cyanoacrylate instant adhesives. Contact Dermatitis. 1993;28:206-208.
12. Dragu A, Unglaub F, Schwarz S, et al. Foreign body reaction after usage of tissue adhesives for skin closure: a case report and review of the literature. Arch Orthop Trauma Surg. 2009;129:167-169.
13. Eaglstein WH, Sullivan T. Cyanoacrylates for skin closure. Dermatol Clin. 2005;23:193-198.
14. Singer AJ, Quinn JV, Hollander JE. The cyanoacrylate topical skin adhesives. Am J Emerg Med. 2008;26:490-496.
15. Singer AJ, Thode HC Jr. A review of the literature on octylcyanoacrylate tissue adhesive. Am J Surg. 2004;187:238-248.
16. Calnan CD. Cyanoacrylate dermatitis. Contact Dermatitis. 1979;5:165-167.
Practice Points
- It is important for physicians to recognize that skin adhesives are a potential source of allergic contact dermatitis (ACD) in a postsurgical setting.
- There are 3 primary components of skin adhesives that are potential contactants, including a cyanoacrylate, a plasticizer, and a dye.
- Treatment of ACD to skin adhesives is straightforward, including removal of any remaining adhesive and applying topical steroids.
First drug-coated angioplasty balloon approved for PAD
A drug-coated angioplasty balloon catheter has been approved for treating peripheral artery disease, the first such device approved for this use, the Food and Drug Administration announced on October 10.
The device is the Lutonix 035 Drug Coated Balloon Percutaneous Transluminal Angioplasty Catheter (Lutonix DCB), manufactured by Lutonix; its outer surface is coated with paclitaxel, “which may help to prevent” restenosis after the angioplasty procedure, according to the FDA statement announcing the approval. “The clinical data show that Lutonix DCB may be more effective than traditional balloon angioplasty at helping to prevent further blockage in the artery,” Dr. William Maisel, deputy director for science and chief scientist in the FDA’s Center for Devices and Radiological Health, said in the statement.
Approval was based on the results of three clinical trials and nonclinical testing:
• A randomized, multicenter study of 101 people in Europe, which found that after 6 months, no further treatment for PAD was needed in almost 72% of the patients treated with Lutonix DCB, compared with almost 50% of those treated with conventional balloon angioplasty.
• A single-blind, multicenter, randomized study of 476 people in the United States and Europe, which found that 65% of those randomized to treatment with Lutonix DCB had no restenosis at 12 months, compared with roughly 53% of those randomized to treatment with conventional balloon angioplasty.
• A single-arm, ongoing study that is further evaluating safety and effectiveness in 657 people treated with the device in the United States and Europe, which, at the time of approval, “show that there have been no unanticipated device- or drug-related adverse events,” the FDA said.
These studies also indicated that the safety of Lutonix DCB was comparable to conventional balloon angioplasty. The most common major adverse events included additional intervention, pain as a result of poor blood flow, narrowing of arteries that were not treated, chest pain, and abnormal growth of tissue.
Contraindications include women who are breastfeeding, pregnant, or plan to become pregnant; and men who plan to father children.
The company is required by the FDA to conduct two postapproval studies, the ongoing 5-year study of 657 patients, and a randomized, single-blind, multicenter study that will evaluate safety and effectiveness of the device in women in the United States, “due to differences in observed outcomes in this group as compared to outcomes for the general study population,” according to the FDA.
The device was reviewed at an FDA advisory panel meeting in June.
A drug-coated angioplasty balloon catheter has been approved for treating peripheral artery disease, the first such device approved for this use, the Food and Drug Administration announced on October 10.
The device is the Lutonix 035 Drug Coated Balloon Percutaneous Transluminal Angioplasty Catheter (Lutonix DCB), manufactured by Lutonix; its outer surface is coated with paclitaxel, “which may help to prevent” restenosis after the angioplasty procedure, according to the FDA statement announcing the approval. “The clinical data show that Lutonix DCB may be more effective than traditional balloon angioplasty at helping to prevent further blockage in the artery,” Dr. William Maisel, deputy director for science and chief scientist in the FDA’s Center for Devices and Radiological Health, said in the statement.
Approval was based on the results of three clinical trials and nonclinical testing:
• A randomized, multicenter study of 101 people in Europe, which found that after 6 months, no further treatment for PAD was needed in almost 72% of the patients treated with Lutonix DCB, compared with almost 50% of those treated with conventional balloon angioplasty.
• A single-blind, multicenter, randomized study of 476 people in the United States and Europe, which found that 65% of those randomized to treatment with Lutonix DCB had no restenosis at 12 months, compared with roughly 53% of those randomized to treatment with conventional balloon angioplasty.
• A single-arm, ongoing study that is further evaluating safety and effectiveness in 657 people treated with the device in the United States and Europe, which, at the time of approval, “show that there have been no unanticipated device- or drug-related adverse events,” the FDA said.
These studies also indicated that the safety of Lutonix DCB was comparable to conventional balloon angioplasty. The most common major adverse events included additional intervention, pain as a result of poor blood flow, narrowing of arteries that were not treated, chest pain, and abnormal growth of tissue.
Contraindications include women who are breastfeeding, pregnant, or plan to become pregnant; and men who plan to father children.
The company is required by the FDA to conduct two postapproval studies, the ongoing 5-year study of 657 patients, and a randomized, single-blind, multicenter study that will evaluate safety and effectiveness of the device in women in the United States, “due to differences in observed outcomes in this group as compared to outcomes for the general study population,” according to the FDA.
The device was reviewed at an FDA advisory panel meeting in June.
A drug-coated angioplasty balloon catheter has been approved for treating peripheral artery disease, the first such device approved for this use, the Food and Drug Administration announced on October 10.
The device is the Lutonix 035 Drug Coated Balloon Percutaneous Transluminal Angioplasty Catheter (Lutonix DCB), manufactured by Lutonix; its outer surface is coated with paclitaxel, “which may help to prevent” restenosis after the angioplasty procedure, according to the FDA statement announcing the approval. “The clinical data show that Lutonix DCB may be more effective than traditional balloon angioplasty at helping to prevent further blockage in the artery,” Dr. William Maisel, deputy director for science and chief scientist in the FDA’s Center for Devices and Radiological Health, said in the statement.
Approval was based on the results of three clinical trials and nonclinical testing:
• A randomized, multicenter study of 101 people in Europe, which found that after 6 months, no further treatment for PAD was needed in almost 72% of the patients treated with Lutonix DCB, compared with almost 50% of those treated with conventional balloon angioplasty.
• A single-blind, multicenter, randomized study of 476 people in the United States and Europe, which found that 65% of those randomized to treatment with Lutonix DCB had no restenosis at 12 months, compared with roughly 53% of those randomized to treatment with conventional balloon angioplasty.
• A single-arm, ongoing study that is further evaluating safety and effectiveness in 657 people treated with the device in the United States and Europe, which, at the time of approval, “show that there have been no unanticipated device- or drug-related adverse events,” the FDA said.
These studies also indicated that the safety of Lutonix DCB was comparable to conventional balloon angioplasty. The most common major adverse events included additional intervention, pain as a result of poor blood flow, narrowing of arteries that were not treated, chest pain, and abnormal growth of tissue.
Contraindications include women who are breastfeeding, pregnant, or plan to become pregnant; and men who plan to father children.
The company is required by the FDA to conduct two postapproval studies, the ongoing 5-year study of 657 patients, and a randomized, single-blind, multicenter study that will evaluate safety and effectiveness of the device in women in the United States, “due to differences in observed outcomes in this group as compared to outcomes for the general study population,” according to the FDA.
The device was reviewed at an FDA advisory panel meeting in June.
Topical Therapy for Acne in Women: Is There a Role for Clindamycin Phosphate–Benzoyl Peroxide Gel?
The management of acne vulgaris (AV) in women has been the subject of considerable attention over the last few years. It has become increasingly recognized that a greater number of patient encounters in dermatology offices involve women with AV who are beyond their adolescent years. Overall, it is estimated that up to approximately 22% of women in the United States are affected by AV, with approximately half of women in their 20s and one-third of women in their 30s reporting some degree of AV.1-4 Among women, the disease shows no predilection for certain skin types or ethnicities, can start during the preteenaged or adolescent years, can persist or recur in adulthood (persistent acne, 75%), or can start in adulthood (late-onset acne, 25%) in females with minimal or no history of AV occurring earlier in life.3,5-7 In the subpopulation of adult women, AV occurs at a time when many expect to be far beyond this “teenage affliction.” Women who are affected commonly express feeling embarrassed and frustrated.5-8
Most of the emphasis in the literature and in presentations at dermatology meetings regarding the management of AV in adult women has focused on excluding underlying disorders that cause excess androgens (eg, polycystic ovary syndrome, congenital adrenal hyperplasia, tumors, exogenous sources) as well as the use of systemic therapies such as oral contraceptives (OCs) and spironolactone.5-7,9,10 Little attention has been given to the selection of topical therapies in this patient population, especially with regard to evidence from clinical studies. To date, results from published study analyses using topical agents specifically for adult females with facial AV have only included adapalene gel 0.3% applied once daily and dapsone gel 5% applied twice daily.11-13 Both agents have been evaluated in subset analysis comparisons of outcomes in women aged 18 years and older versus adolescents aged 12 to 17 years based on data from 12-week phase 3 pivotal trials.14-16
Are there clinically relevant differences between AV in adult versus adolescent females?
Although much has been written about AV in women, epidemiology, demographics, assessment of clinical presentation, and correlation of clinical presentation with excess androgens have not been emphasized,1-3,5-10,17 likely due to marketing campaigns that emphasize AV as a disorder that predominantly affects teenagers as well as the focus on optimal use of oral spironolactone and/or OCs in the management of AV in the adult female population. Attention to spironolactone use is important because it is not approved by the US Food and Drug Administration for the treatment of AV. Spironolactone carries certain black-box warnings that may not be clinically relevant in all patients but still require attention. It also is associated with risks if taken during pregnancy, and it is a potassium-sparing diuretic with potential for hyperkalemia, especially in patients with reduced renal function or those who are taking potassium supplements or certain other medications.6,9,11,17 Use of OCs to treat AV also is not without potential risks, with specific warnings and relative contraindications reported, especially in relation to increased risks for cardiac complications, stroke, and thromboembolism.6,9,11,12 Because adult females are in a different stage of life than teenagers, there are defined psychosocial and medical considerations in managing AV in these patients compared with adolescents.5-8,17 Importantly for both clinicians and patients, addressing these differences and considerations can have a major impact on whether or not women with AV experience successful treatment outcomes.1-3,5,6,8,10,13 Skin color and ethnicity also can affect the psychosocial and physical factors that influence the overall management of adult female patients with AV, including selection of therapies and handling long-term visible sequelae that occur in some AV patients, such as dyschromia (eg, persistent or postinflammatory erythema or hyperpigmentation) and acne scarring.5-8,13,17-20
Psychosocial Considerations
With regard to psychosocial, emotional, and attitudinal considerations in women with AV, common findings include concern or frustration regarding the presence of AV beyond adolescence; anxiety; symptoms of depression; decreased self-confidence; increased self-consciousness, especially during public interactions or intimate situations; and interference with steady concentration at work or school.5,6,8,13 Long-term complications of AV, such as dyschromia and acne scarring, are more likely to be encountered in adult patients, especially if they had AV as a teenager, with women reporting that they remain conscious of these adverse sequelae.8 It is estimated that approximately three-fourths of women with AV also had AV as teenagers; therefore, most of them have already used many over-the-counter and prescription therapies and are likely to want treatments that are newer, well-tolerated, safe, and known to be effective in adult women.8,16,17 Convenience and simplicity are vital components of treatment selection and regimen design, as many women with AV frequently face time constraints in their daily routines due to family, social, employment, and home-related demands and responsibilities.6-8,17
Medical Considerations
It is apparent from reports in the literature as well as from clinical experience that some women with AV present with a U-shaped pattern of involvement on the face,5-7,10,13,17 which refers to the presence of predominantly inflammatory papules (many of them deep) and some nodules on the lower face, jawline, and anterolateral neck region, with comedones often sparse or absent.5-7 It often is perceived and may be true that women who present with this pattern of distribution are more androgen sensitive despite having normal serum androgen levels or in some cases exhibit detectable excess androgens (eg, in the setting of polycystic ovary syndrome) and may be more likely to respond to hormonal therapies (eg, spironolactone, OCs) than those with mixed facial AV (ie, multiple comedonal and inflammatory acne lesions, not limited to a U-shaped pattern, similar to adolescent AV), but data are limited to support differentiation between the U-shaped pattern group and the conventional mixed facial AV group.5-7,17 Adult and adolescent females in both groups sometimes report perimenstrual flares and frequent persistent papular AV that tends to concentrate on the perioral and chin area.
It is also important to consider that the current literature suggests approximately three-fourths of women with AV report that they also had AV as a teenager, with many indicating the same clinical pattern of AV and approximately one-third reporting AV that is more severe in adulthood than adolescence.5-8,17 The available literature on topical and oral therapies used to treat AV in both adolescent and adult females predominantly focuses on inclusion of both inflammatory and noninflammatory (comedonal) facial AV lesions, does not specifically address or include the U-shaped pattern of AV in adult women for inclusion in studies that evaluate efficacy in this subgroup, and does not include AV involving the neck region and below the jawline margin as part of any study protocols and/or discussions about therapy.5-7,9-12,17,21-26 Involvement of the neck and lower jawline is common in women presenting with the U-shaped pattern of AV, and available studies only evaluate AV involving the face and do not include AV lesions present below the jawline margin. As a result, there is a considerable need for well-designed studies with laboratory assessments to include or exclude underlying detectable excess androgens and to assess the efficacy, tolerability, and safety of specific therapeutic agents both alone and in combination in adult women who present with a U-shaped pattern of AV.17
Other medical considerations that can influence treatment selection and are more likely to be present in adult versus adolescent females include underlying chronic medical disorders; concomitant medications that may interact with other oral agents; potential for pregnancy; age, particularly when prescribing OCs; and the potential desire to stop taking OCs if already used over a prolonged period.6,7
Age-Related Differentiation of Female Subgroups With AV
The age-based dividing line that defines AV in adults versus adolescent females has been described in the literature; however, the basis for published definitions of female subgroups with AV is not well-supported by strong scientific evidence.1-3,5-7,17 The conventional dividing line that was originally selected to define adult females with AV was 25 years of age or older; persistent acne is present both during adolescence and at or after 25 years of age, while late-onset acne is described as AV that first presents at 25 years of age or older.3,5-7
More recently, a range of 18 years or older has been used to classify adult female AV and a range of 12 to 17 years for adolescent female AV in subset analyses that evaluated treatment outcomes in both patient populations from phase 3 pivotal trials completed with adapalene gel 0.3% applied once daily and dapsone gel 5% applied twice daily.14-16 These subanalyses included participants with facial AV that was predominantly moderate in severity, mandated specific lesion count ranges for both comedonal and inflammatory lesions, and included only facial AV that was above the mandibular (jawline) margin.15,16,21,26 Therefore, patients with AV presenting in a U-shaped pattern with involvement below the jawline and on the neck were not included in these study analyses, as these patients were excluded from the phase 3 trials on which the analyses were based. The outcomes of these analyses apply to treatment in women who present with both inflammatory and noninflammatory facial AV lesions, which supports the observation that AV in this patient population is not always predominantly inflammatory and does not always present in a U-shaped distribution.14-16 In fact, a U-shaped pattern of distribution appears to be less common in women with AV than a mixed inflammatory and comedonal distribution that involves the face more diffusely, though more data are needed from well-designed and large-scale epidemiologic and demographic studies.5,14,17
Are there data available on the use of benzoyl peroxide with or without a topical antibiotic in women with AV?
There is a conspicuous absence of prospective clinical trials and retrospective analyses evaluating the specific use of individual AV therapies in adult females, with a particular lack of studies with topical agents (eg, benzoyl peroxide [BP]).14 Subset analyses have been completed for adapalene gel 0.3% and dapsone gel 5%.15,16 Additionally, an age-based subset analysis in females with facial AV also has been completed with clindamycin phosphate (CP) 1.2%–BP 2.5% gel once daily, with data presented but not yet fully published.14
Two identical phase 3, double-blind, randomized, 12-week, 4-arm trials compared treatment outcomes in groups treated with an aqueous-based combination gel formulation containing BP 2.5% and CP 1.2% (n=797), active monad gels (BP [n=809] or CP [n=812]), or vehicle gel (n=395), all applied once daily in patients with facial AV.22 Participants were 12 years or older (mean age range, 19.1–19.6 years; age range, 12.1–70.2 years), were of either gender (approximately 50% split in each study arm), and presented with moderate (approximately 80% of participants) or severe AV (approximately 20% of participants) at baseline. The entry criteria for lesion types and number of lesions were 17 to 40 inflammatory lesions (ie, papules, pustules, <2 nodules)(range of mean number of lesions, 25.8–26.4) and 20 to 100 noninflammatory lesions (ie, closed comedones, open comedones)(range of mean number of lesions, 44.0–47.4). Participant demographics included white (73.9%–77.5%), black/African American (16.1%–20.4%), and Asian (2.1%–3.3%), with the remaining participants distributed among a variety of other ethnic groups such as Native Hawaiian/Native Pacific Islander and Native American Indian/Native Alaskan (collectively <5% in each study arm). Therefore, approximately 1 of every 4 patients had skin of color, which provided good diversity of patients considering the large study size (N=2813). Data analysis included dichotomization of participants by severity rating (moderate or severe based on evaluator global severity score) and skin phototype (Fitzpatrick skin types I–III or IV–VI).22
The pooled results from both studies completed at 68 investigative sites demonstrated that CP 1.2%–BP 2.5% gel was superior in efficacy to each individual monad and to the vehicle in inflammatory, noninflammatory, and total lesion reductions as early as week 4 (P<.001) and at week 12, which was the study end point (P<.001), with superiority also demonstrated in achieving treatment success (defined as a >2 grade improvement according to the evaluator global severity score) compared to the 3 other study arms (P<.001).22 Subject assessments also were consistent with outcomes noted by the investigators. Cutaneous tolerability was favorable and comparable in all 4 study arms with less than 1% of participants discontinuing treatment due to adverse events.22
A subset analysis of the data from the phase 3 pivotal trials with CP 1.2%–BP 2.5% gel was completed to compare reductions in both inflammatory and noninflammatory lesions in female participants who were younger than 25 years and 25 years of age or older in all 4 study arms. This information has been presented14,17 but has not been previously published. Based on the overall results reported in the phase 3 studies, there were no differentiations in skin tolerability or safety based on participant age, gender, or skin type.22 The subanalysis included a total of 1080 females who were younger than 25 years and 395 females who were 25 years of age or older. The lesion reduction outcomes of this subanalysis are presented in the Table. Statistical analyses of the results among these age groups in the 4 study arms were not completed because the objective was to determine if there were any major or obvious differences in reduction of AV lesions based on the conventional dividing line of 25 years of age in adult women as compared to adolescent females treated with CP 1.2%–BP 2.5% gel. In addition, the large difference in numbers of female participants between the 2 age groups (>25 years of age, n=395; <25 years of age, n=1080) at least partially confounds both statistical and observational analysis. Among the women who were 25 years of age or older who were included in the subanalysis, 67.0% and 25.8% were between the ages of 25 to 35 years and 36 to 45 years, respectively. Based on the outcomes reported in the phase 3 trials and in this subgroup analysis, CP 1.2%–BP 2.5% gel applied once daily over a 12-week period appeared overall to be comparably effective in females regardless of age and with no apparent adverse events regarding differences in skin tolerability or safety.14,22 One observation that was noted was the possible trend of greater reduction in both lesion types in women older than 35 years versus younger females with the use of the combination gel or BP alone; however, the number of female participants who were older than 35 years of age was substantially less (n=102) than those who were 35 years of age or younger (n=1345), thus precluding support for any definitive conclusions about this possible trend.22
How can CP 1.2%–BP 2.5% gel be incorporated into a treatment regimen for women with facial AV?
The incorporation of CP 1.2%–BP 2.5% gel into a treatment regimen for women with facial AV is similar to the general use of BP-containing formulations in the overall management of AV.9,14,27,28 Because women with AV commonly present with facial inflammatory lesions and many also with facial comedones, CP 1.2%–BP 2.5% gel is best used once daily in the morning in combination with a topical retinoid in the evening,9,27 which can be achieved with use of CP 1.2%–BP 2.5% gel in the morning and a topical retinoid (ie, tretinoin, adapalene, tazarotene) in the evening or CP 1.2%–tretinoin 0.025% gel in the evening. It is important to note that cutaneous irritation may be more likely if neck lesions are present; the potential for bleaching of colored fabric by BP also is a practical concern.28 In addition, CP 1.2%–BP 2.5% gel may also be used in combination with topical dapsone, but both products should be applied separately at different times of the day to avoid temporary orange discoloration of the skin, which appears to be an uncommon side effect but remains a possibility based on the product information for dapsone gel 5% with regard to its concomitant use with BP.29,30
1. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health. 2012;21:223-230.
2. Zeichner J. Evaluating and treating the adult female patient with acne. J Drugs Dermatol. 2013;12:1416-1427.
3. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41:577-580.
4. Collier CN, Harper J, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
5. Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
6. Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
7. Kim GK, Michaels BB. Post-adolescent acne in women: more common and more clinical considerations. J Drugs Dermatol. 2012;11:708-713.
8. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
9. Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(suppl 1):1-37.
10. Thiboutot DM. Endocrinological evaluation and hormonal therapy for women with difficult acne. J Eur Acad Dermatol Venereol. 2001;15(suppl 3):57-61.
11. Sawaya ME, Samani N. Antiandrogens and androgen receptors. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Saunders-Elsevier; 2012:361-374.
12. Harper JC. Should dermatologists prescribe hormonal contraceptives for acne? Dermatol Ther. 2009;22:452-457.
13. Preneau S, Dreno B. Female acne: a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
14. Del Rosso JQ, Zeichner JA. What’s new in the medicine cabinet?: a panoramic review of clinically relevant information for the busy dermatologist. J Clin Aesthet Dermatol. 2014;7:26-30.
15. Berson D, Alexis A. Adapalene 0.3% for the treatment of acne in women. J Clin Aesthet Dermatol. 2013;6:32-35.
16. Del Rosso JQ, Kircik L, Gallagher C. Facing up to adult women with acne vulgaris: an analysis of pivotal trial data on dapsone 5% gel in the adult female population. Poster presented at: Fall Clinical Dermatology; October 2013; Las Vegas, NV.
17. Del Rosso JQ. Management of acne with oral spironolactone. Presented at: American Academy of Dermatology Summer Meeting; August 2013; Boston, MA.
18. Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
19. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
20. Perkins AC, Cheng CE, Hillebrand GG, et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
21. Draelos ZD, Carter E, Maloney JM, et al. Two randomized studies demonstrate the efficacy and safety of dapsone gel, 5% for the treatment of acne vulgaris [published online ahead of print January 17, 2007]. J Am Acad Dermatol. 2007;56:439.e1-439.e10.
22. Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
23. Schlessinger J, Menter A, Gold M, et al. Clinical safety and efficacy studies of a novel formulation combining
1.2% clindamycin phosphate and 0.025% tretinoin for the treatment of acne vulgaris. J Drugs Dermatol. 2007;6:607-615.
24. Fleischer AB Jr, Dinehart S, Stough D, et al. Safety and efficacy of a new extended-release formulation of minocycline. Cutis. 2006;78(suppl 4):21-31.
25. Gollnick HP, Draelos Z, Glenn MJ, et al. Adapalene-benzoyl peroxide, a unique fixed-dose combination topical gel for the treatment of acne vulgaris: a transatlantic, randomized, double-blind, controlled study in 1670 patients. Br J Dermatol. 2009;161:1180-1189.
26. Thiboutot D, Arsonnaud S, Soto P. Efficacy and tolerability of adapalene 0.3% gel compared to tazarotene 0.1% gel in the treatment of acne vulgaris. J Drugs Dermatol. 2008;7(suppl 6):3-10.
27. Zeichner JA. Optimizing topical combination therapy for the treatment of acne vulgaris. J Drugs Dermatol. 2012;11:313-317.
28. Tanghetti EA, Popp KF. A current review of topical benzoyl peroxide: new perspectives on formulation and utilization. Dermatol Clin. 2009;27:17-24.
29. Fleischer AB, Shalita A, Eichenfield LF. Dapsone gel 5% in combination with adapalene gel 0.1%, benzoyl peroxide gel 4% or moisturizer for the treatment of acne vulgaris: a 12-week, randomized, double-blind study. J Drugs Dermatol. 2010;9:33-40.
30. Aczone (dapsone gel 5%) [package insert]. Irvine, CA: Allergan, Inc; 2013.
The management of acne vulgaris (AV) in women has been the subject of considerable attention over the last few years. It has become increasingly recognized that a greater number of patient encounters in dermatology offices involve women with AV who are beyond their adolescent years. Overall, it is estimated that up to approximately 22% of women in the United States are affected by AV, with approximately half of women in their 20s and one-third of women in their 30s reporting some degree of AV.1-4 Among women, the disease shows no predilection for certain skin types or ethnicities, can start during the preteenaged or adolescent years, can persist or recur in adulthood (persistent acne, 75%), or can start in adulthood (late-onset acne, 25%) in females with minimal or no history of AV occurring earlier in life.3,5-7 In the subpopulation of adult women, AV occurs at a time when many expect to be far beyond this “teenage affliction.” Women who are affected commonly express feeling embarrassed and frustrated.5-8
Most of the emphasis in the literature and in presentations at dermatology meetings regarding the management of AV in adult women has focused on excluding underlying disorders that cause excess androgens (eg, polycystic ovary syndrome, congenital adrenal hyperplasia, tumors, exogenous sources) as well as the use of systemic therapies such as oral contraceptives (OCs) and spironolactone.5-7,9,10 Little attention has been given to the selection of topical therapies in this patient population, especially with regard to evidence from clinical studies. To date, results from published study analyses using topical agents specifically for adult females with facial AV have only included adapalene gel 0.3% applied once daily and dapsone gel 5% applied twice daily.11-13 Both agents have been evaluated in subset analysis comparisons of outcomes in women aged 18 years and older versus adolescents aged 12 to 17 years based on data from 12-week phase 3 pivotal trials.14-16
Are there clinically relevant differences between AV in adult versus adolescent females?
Although much has been written about AV in women, epidemiology, demographics, assessment of clinical presentation, and correlation of clinical presentation with excess androgens have not been emphasized,1-3,5-10,17 likely due to marketing campaigns that emphasize AV as a disorder that predominantly affects teenagers as well as the focus on optimal use of oral spironolactone and/or OCs in the management of AV in the adult female population. Attention to spironolactone use is important because it is not approved by the US Food and Drug Administration for the treatment of AV. Spironolactone carries certain black-box warnings that may not be clinically relevant in all patients but still require attention. It also is associated with risks if taken during pregnancy, and it is a potassium-sparing diuretic with potential for hyperkalemia, especially in patients with reduced renal function or those who are taking potassium supplements or certain other medications.6,9,11,17 Use of OCs to treat AV also is not without potential risks, with specific warnings and relative contraindications reported, especially in relation to increased risks for cardiac complications, stroke, and thromboembolism.6,9,11,12 Because adult females are in a different stage of life than teenagers, there are defined psychosocial and medical considerations in managing AV in these patients compared with adolescents.5-8,17 Importantly for both clinicians and patients, addressing these differences and considerations can have a major impact on whether or not women with AV experience successful treatment outcomes.1-3,5,6,8,10,13 Skin color and ethnicity also can affect the psychosocial and physical factors that influence the overall management of adult female patients with AV, including selection of therapies and handling long-term visible sequelae that occur in some AV patients, such as dyschromia (eg, persistent or postinflammatory erythema or hyperpigmentation) and acne scarring.5-8,13,17-20
Psychosocial Considerations
With regard to psychosocial, emotional, and attitudinal considerations in women with AV, common findings include concern or frustration regarding the presence of AV beyond adolescence; anxiety; symptoms of depression; decreased self-confidence; increased self-consciousness, especially during public interactions or intimate situations; and interference with steady concentration at work or school.5,6,8,13 Long-term complications of AV, such as dyschromia and acne scarring, are more likely to be encountered in adult patients, especially if they had AV as a teenager, with women reporting that they remain conscious of these adverse sequelae.8 It is estimated that approximately three-fourths of women with AV also had AV as teenagers; therefore, most of them have already used many over-the-counter and prescription therapies and are likely to want treatments that are newer, well-tolerated, safe, and known to be effective in adult women.8,16,17 Convenience and simplicity are vital components of treatment selection and regimen design, as many women with AV frequently face time constraints in their daily routines due to family, social, employment, and home-related demands and responsibilities.6-8,17
Medical Considerations
It is apparent from reports in the literature as well as from clinical experience that some women with AV present with a U-shaped pattern of involvement on the face,5-7,10,13,17 which refers to the presence of predominantly inflammatory papules (many of them deep) and some nodules on the lower face, jawline, and anterolateral neck region, with comedones often sparse or absent.5-7 It often is perceived and may be true that women who present with this pattern of distribution are more androgen sensitive despite having normal serum androgen levels or in some cases exhibit detectable excess androgens (eg, in the setting of polycystic ovary syndrome) and may be more likely to respond to hormonal therapies (eg, spironolactone, OCs) than those with mixed facial AV (ie, multiple comedonal and inflammatory acne lesions, not limited to a U-shaped pattern, similar to adolescent AV), but data are limited to support differentiation between the U-shaped pattern group and the conventional mixed facial AV group.5-7,17 Adult and adolescent females in both groups sometimes report perimenstrual flares and frequent persistent papular AV that tends to concentrate on the perioral and chin area.
It is also important to consider that the current literature suggests approximately three-fourths of women with AV report that they also had AV as a teenager, with many indicating the same clinical pattern of AV and approximately one-third reporting AV that is more severe in adulthood than adolescence.5-8,17 The available literature on topical and oral therapies used to treat AV in both adolescent and adult females predominantly focuses on inclusion of both inflammatory and noninflammatory (comedonal) facial AV lesions, does not specifically address or include the U-shaped pattern of AV in adult women for inclusion in studies that evaluate efficacy in this subgroup, and does not include AV involving the neck region and below the jawline margin as part of any study protocols and/or discussions about therapy.5-7,9-12,17,21-26 Involvement of the neck and lower jawline is common in women presenting with the U-shaped pattern of AV, and available studies only evaluate AV involving the face and do not include AV lesions present below the jawline margin. As a result, there is a considerable need for well-designed studies with laboratory assessments to include or exclude underlying detectable excess androgens and to assess the efficacy, tolerability, and safety of specific therapeutic agents both alone and in combination in adult women who present with a U-shaped pattern of AV.17
Other medical considerations that can influence treatment selection and are more likely to be present in adult versus adolescent females include underlying chronic medical disorders; concomitant medications that may interact with other oral agents; potential for pregnancy; age, particularly when prescribing OCs; and the potential desire to stop taking OCs if already used over a prolonged period.6,7
Age-Related Differentiation of Female Subgroups With AV
The age-based dividing line that defines AV in adults versus adolescent females has been described in the literature; however, the basis for published definitions of female subgroups with AV is not well-supported by strong scientific evidence.1-3,5-7,17 The conventional dividing line that was originally selected to define adult females with AV was 25 years of age or older; persistent acne is present both during adolescence and at or after 25 years of age, while late-onset acne is described as AV that first presents at 25 years of age or older.3,5-7
More recently, a range of 18 years or older has been used to classify adult female AV and a range of 12 to 17 years for adolescent female AV in subset analyses that evaluated treatment outcomes in both patient populations from phase 3 pivotal trials completed with adapalene gel 0.3% applied once daily and dapsone gel 5% applied twice daily.14-16 These subanalyses included participants with facial AV that was predominantly moderate in severity, mandated specific lesion count ranges for both comedonal and inflammatory lesions, and included only facial AV that was above the mandibular (jawline) margin.15,16,21,26 Therefore, patients with AV presenting in a U-shaped pattern with involvement below the jawline and on the neck were not included in these study analyses, as these patients were excluded from the phase 3 trials on which the analyses were based. The outcomes of these analyses apply to treatment in women who present with both inflammatory and noninflammatory facial AV lesions, which supports the observation that AV in this patient population is not always predominantly inflammatory and does not always present in a U-shaped distribution.14-16 In fact, a U-shaped pattern of distribution appears to be less common in women with AV than a mixed inflammatory and comedonal distribution that involves the face more diffusely, though more data are needed from well-designed and large-scale epidemiologic and demographic studies.5,14,17
Are there data available on the use of benzoyl peroxide with or without a topical antibiotic in women with AV?
There is a conspicuous absence of prospective clinical trials and retrospective analyses evaluating the specific use of individual AV therapies in adult females, with a particular lack of studies with topical agents (eg, benzoyl peroxide [BP]).14 Subset analyses have been completed for adapalene gel 0.3% and dapsone gel 5%.15,16 Additionally, an age-based subset analysis in females with facial AV also has been completed with clindamycin phosphate (CP) 1.2%–BP 2.5% gel once daily, with data presented but not yet fully published.14
Two identical phase 3, double-blind, randomized, 12-week, 4-arm trials compared treatment outcomes in groups treated with an aqueous-based combination gel formulation containing BP 2.5% and CP 1.2% (n=797), active monad gels (BP [n=809] or CP [n=812]), or vehicle gel (n=395), all applied once daily in patients with facial AV.22 Participants were 12 years or older (mean age range, 19.1–19.6 years; age range, 12.1–70.2 years), were of either gender (approximately 50% split in each study arm), and presented with moderate (approximately 80% of participants) or severe AV (approximately 20% of participants) at baseline. The entry criteria for lesion types and number of lesions were 17 to 40 inflammatory lesions (ie, papules, pustules, <2 nodules)(range of mean number of lesions, 25.8–26.4) and 20 to 100 noninflammatory lesions (ie, closed comedones, open comedones)(range of mean number of lesions, 44.0–47.4). Participant demographics included white (73.9%–77.5%), black/African American (16.1%–20.4%), and Asian (2.1%–3.3%), with the remaining participants distributed among a variety of other ethnic groups such as Native Hawaiian/Native Pacific Islander and Native American Indian/Native Alaskan (collectively <5% in each study arm). Therefore, approximately 1 of every 4 patients had skin of color, which provided good diversity of patients considering the large study size (N=2813). Data analysis included dichotomization of participants by severity rating (moderate or severe based on evaluator global severity score) and skin phototype (Fitzpatrick skin types I–III or IV–VI).22
The pooled results from both studies completed at 68 investigative sites demonstrated that CP 1.2%–BP 2.5% gel was superior in efficacy to each individual monad and to the vehicle in inflammatory, noninflammatory, and total lesion reductions as early as week 4 (P<.001) and at week 12, which was the study end point (P<.001), with superiority also demonstrated in achieving treatment success (defined as a >2 grade improvement according to the evaluator global severity score) compared to the 3 other study arms (P<.001).22 Subject assessments also were consistent with outcomes noted by the investigators. Cutaneous tolerability was favorable and comparable in all 4 study arms with less than 1% of participants discontinuing treatment due to adverse events.22
A subset analysis of the data from the phase 3 pivotal trials with CP 1.2%–BP 2.5% gel was completed to compare reductions in both inflammatory and noninflammatory lesions in female participants who were younger than 25 years and 25 years of age or older in all 4 study arms. This information has been presented14,17 but has not been previously published. Based on the overall results reported in the phase 3 studies, there were no differentiations in skin tolerability or safety based on participant age, gender, or skin type.22 The subanalysis included a total of 1080 females who were younger than 25 years and 395 females who were 25 years of age or older. The lesion reduction outcomes of this subanalysis are presented in the Table. Statistical analyses of the results among these age groups in the 4 study arms were not completed because the objective was to determine if there were any major or obvious differences in reduction of AV lesions based on the conventional dividing line of 25 years of age in adult women as compared to adolescent females treated with CP 1.2%–BP 2.5% gel. In addition, the large difference in numbers of female participants between the 2 age groups (>25 years of age, n=395; <25 years of age, n=1080) at least partially confounds both statistical and observational analysis. Among the women who were 25 years of age or older who were included in the subanalysis, 67.0% and 25.8% were between the ages of 25 to 35 years and 36 to 45 years, respectively. Based on the outcomes reported in the phase 3 trials and in this subgroup analysis, CP 1.2%–BP 2.5% gel applied once daily over a 12-week period appeared overall to be comparably effective in females regardless of age and with no apparent adverse events regarding differences in skin tolerability or safety.14,22 One observation that was noted was the possible trend of greater reduction in both lesion types in women older than 35 years versus younger females with the use of the combination gel or BP alone; however, the number of female participants who were older than 35 years of age was substantially less (n=102) than those who were 35 years of age or younger (n=1345), thus precluding support for any definitive conclusions about this possible trend.22
How can CP 1.2%–BP 2.5% gel be incorporated into a treatment regimen for women with facial AV?
The incorporation of CP 1.2%–BP 2.5% gel into a treatment regimen for women with facial AV is similar to the general use of BP-containing formulations in the overall management of AV.9,14,27,28 Because women with AV commonly present with facial inflammatory lesions and many also with facial comedones, CP 1.2%–BP 2.5% gel is best used once daily in the morning in combination with a topical retinoid in the evening,9,27 which can be achieved with use of CP 1.2%–BP 2.5% gel in the morning and a topical retinoid (ie, tretinoin, adapalene, tazarotene) in the evening or CP 1.2%–tretinoin 0.025% gel in the evening. It is important to note that cutaneous irritation may be more likely if neck lesions are present; the potential for bleaching of colored fabric by BP also is a practical concern.28 In addition, CP 1.2%–BP 2.5% gel may also be used in combination with topical dapsone, but both products should be applied separately at different times of the day to avoid temporary orange discoloration of the skin, which appears to be an uncommon side effect but remains a possibility based on the product information for dapsone gel 5% with regard to its concomitant use with BP.29,30
The management of acne vulgaris (AV) in women has been the subject of considerable attention over the last few years. It has become increasingly recognized that a greater number of patient encounters in dermatology offices involve women with AV who are beyond their adolescent years. Overall, it is estimated that up to approximately 22% of women in the United States are affected by AV, with approximately half of women in their 20s and one-third of women in their 30s reporting some degree of AV.1-4 Among women, the disease shows no predilection for certain skin types or ethnicities, can start during the preteenaged or adolescent years, can persist or recur in adulthood (persistent acne, 75%), or can start in adulthood (late-onset acne, 25%) in females with minimal or no history of AV occurring earlier in life.3,5-7 In the subpopulation of adult women, AV occurs at a time when many expect to be far beyond this “teenage affliction.” Women who are affected commonly express feeling embarrassed and frustrated.5-8
Most of the emphasis in the literature and in presentations at dermatology meetings regarding the management of AV in adult women has focused on excluding underlying disorders that cause excess androgens (eg, polycystic ovary syndrome, congenital adrenal hyperplasia, tumors, exogenous sources) as well as the use of systemic therapies such as oral contraceptives (OCs) and spironolactone.5-7,9,10 Little attention has been given to the selection of topical therapies in this patient population, especially with regard to evidence from clinical studies. To date, results from published study analyses using topical agents specifically for adult females with facial AV have only included adapalene gel 0.3% applied once daily and dapsone gel 5% applied twice daily.11-13 Both agents have been evaluated in subset analysis comparisons of outcomes in women aged 18 years and older versus adolescents aged 12 to 17 years based on data from 12-week phase 3 pivotal trials.14-16
Are there clinically relevant differences between AV in adult versus adolescent females?
Although much has been written about AV in women, epidemiology, demographics, assessment of clinical presentation, and correlation of clinical presentation with excess androgens have not been emphasized,1-3,5-10,17 likely due to marketing campaigns that emphasize AV as a disorder that predominantly affects teenagers as well as the focus on optimal use of oral spironolactone and/or OCs in the management of AV in the adult female population. Attention to spironolactone use is important because it is not approved by the US Food and Drug Administration for the treatment of AV. Spironolactone carries certain black-box warnings that may not be clinically relevant in all patients but still require attention. It also is associated with risks if taken during pregnancy, and it is a potassium-sparing diuretic with potential for hyperkalemia, especially in patients with reduced renal function or those who are taking potassium supplements or certain other medications.6,9,11,17 Use of OCs to treat AV also is not without potential risks, with specific warnings and relative contraindications reported, especially in relation to increased risks for cardiac complications, stroke, and thromboembolism.6,9,11,12 Because adult females are in a different stage of life than teenagers, there are defined psychosocial and medical considerations in managing AV in these patients compared with adolescents.5-8,17 Importantly for both clinicians and patients, addressing these differences and considerations can have a major impact on whether or not women with AV experience successful treatment outcomes.1-3,5,6,8,10,13 Skin color and ethnicity also can affect the psychosocial and physical factors that influence the overall management of adult female patients with AV, including selection of therapies and handling long-term visible sequelae that occur in some AV patients, such as dyschromia (eg, persistent or postinflammatory erythema or hyperpigmentation) and acne scarring.5-8,13,17-20
Psychosocial Considerations
With regard to psychosocial, emotional, and attitudinal considerations in women with AV, common findings include concern or frustration regarding the presence of AV beyond adolescence; anxiety; symptoms of depression; decreased self-confidence; increased self-consciousness, especially during public interactions or intimate situations; and interference with steady concentration at work or school.5,6,8,13 Long-term complications of AV, such as dyschromia and acne scarring, are more likely to be encountered in adult patients, especially if they had AV as a teenager, with women reporting that they remain conscious of these adverse sequelae.8 It is estimated that approximately three-fourths of women with AV also had AV as teenagers; therefore, most of them have already used many over-the-counter and prescription therapies and are likely to want treatments that are newer, well-tolerated, safe, and known to be effective in adult women.8,16,17 Convenience and simplicity are vital components of treatment selection and regimen design, as many women with AV frequently face time constraints in their daily routines due to family, social, employment, and home-related demands and responsibilities.6-8,17
Medical Considerations
It is apparent from reports in the literature as well as from clinical experience that some women with AV present with a U-shaped pattern of involvement on the face,5-7,10,13,17 which refers to the presence of predominantly inflammatory papules (many of them deep) and some nodules on the lower face, jawline, and anterolateral neck region, with comedones often sparse or absent.5-7 It often is perceived and may be true that women who present with this pattern of distribution are more androgen sensitive despite having normal serum androgen levels or in some cases exhibit detectable excess androgens (eg, in the setting of polycystic ovary syndrome) and may be more likely to respond to hormonal therapies (eg, spironolactone, OCs) than those with mixed facial AV (ie, multiple comedonal and inflammatory acne lesions, not limited to a U-shaped pattern, similar to adolescent AV), but data are limited to support differentiation between the U-shaped pattern group and the conventional mixed facial AV group.5-7,17 Adult and adolescent females in both groups sometimes report perimenstrual flares and frequent persistent papular AV that tends to concentrate on the perioral and chin area.
It is also important to consider that the current literature suggests approximately three-fourths of women with AV report that they also had AV as a teenager, with many indicating the same clinical pattern of AV and approximately one-third reporting AV that is more severe in adulthood than adolescence.5-8,17 The available literature on topical and oral therapies used to treat AV in both adolescent and adult females predominantly focuses on inclusion of both inflammatory and noninflammatory (comedonal) facial AV lesions, does not specifically address or include the U-shaped pattern of AV in adult women for inclusion in studies that evaluate efficacy in this subgroup, and does not include AV involving the neck region and below the jawline margin as part of any study protocols and/or discussions about therapy.5-7,9-12,17,21-26 Involvement of the neck and lower jawline is common in women presenting with the U-shaped pattern of AV, and available studies only evaluate AV involving the face and do not include AV lesions present below the jawline margin. As a result, there is a considerable need for well-designed studies with laboratory assessments to include or exclude underlying detectable excess androgens and to assess the efficacy, tolerability, and safety of specific therapeutic agents both alone and in combination in adult women who present with a U-shaped pattern of AV.17
Other medical considerations that can influence treatment selection and are more likely to be present in adult versus adolescent females include underlying chronic medical disorders; concomitant medications that may interact with other oral agents; potential for pregnancy; age, particularly when prescribing OCs; and the potential desire to stop taking OCs if already used over a prolonged period.6,7
Age-Related Differentiation of Female Subgroups With AV
The age-based dividing line that defines AV in adults versus adolescent females has been described in the literature; however, the basis for published definitions of female subgroups with AV is not well-supported by strong scientific evidence.1-3,5-7,17 The conventional dividing line that was originally selected to define adult females with AV was 25 years of age or older; persistent acne is present both during adolescence and at or after 25 years of age, while late-onset acne is described as AV that first presents at 25 years of age or older.3,5-7
More recently, a range of 18 years or older has been used to classify adult female AV and a range of 12 to 17 years for adolescent female AV in subset analyses that evaluated treatment outcomes in both patient populations from phase 3 pivotal trials completed with adapalene gel 0.3% applied once daily and dapsone gel 5% applied twice daily.14-16 These subanalyses included participants with facial AV that was predominantly moderate in severity, mandated specific lesion count ranges for both comedonal and inflammatory lesions, and included only facial AV that was above the mandibular (jawline) margin.15,16,21,26 Therefore, patients with AV presenting in a U-shaped pattern with involvement below the jawline and on the neck were not included in these study analyses, as these patients were excluded from the phase 3 trials on which the analyses were based. The outcomes of these analyses apply to treatment in women who present with both inflammatory and noninflammatory facial AV lesions, which supports the observation that AV in this patient population is not always predominantly inflammatory and does not always present in a U-shaped distribution.14-16 In fact, a U-shaped pattern of distribution appears to be less common in women with AV than a mixed inflammatory and comedonal distribution that involves the face more diffusely, though more data are needed from well-designed and large-scale epidemiologic and demographic studies.5,14,17
Are there data available on the use of benzoyl peroxide with or without a topical antibiotic in women with AV?
There is a conspicuous absence of prospective clinical trials and retrospective analyses evaluating the specific use of individual AV therapies in adult females, with a particular lack of studies with topical agents (eg, benzoyl peroxide [BP]).14 Subset analyses have been completed for adapalene gel 0.3% and dapsone gel 5%.15,16 Additionally, an age-based subset analysis in females with facial AV also has been completed with clindamycin phosphate (CP) 1.2%–BP 2.5% gel once daily, with data presented but not yet fully published.14
Two identical phase 3, double-blind, randomized, 12-week, 4-arm trials compared treatment outcomes in groups treated with an aqueous-based combination gel formulation containing BP 2.5% and CP 1.2% (n=797), active monad gels (BP [n=809] or CP [n=812]), or vehicle gel (n=395), all applied once daily in patients with facial AV.22 Participants were 12 years or older (mean age range, 19.1–19.6 years; age range, 12.1–70.2 years), were of either gender (approximately 50% split in each study arm), and presented with moderate (approximately 80% of participants) or severe AV (approximately 20% of participants) at baseline. The entry criteria for lesion types and number of lesions were 17 to 40 inflammatory lesions (ie, papules, pustules, <2 nodules)(range of mean number of lesions, 25.8–26.4) and 20 to 100 noninflammatory lesions (ie, closed comedones, open comedones)(range of mean number of lesions, 44.0–47.4). Participant demographics included white (73.9%–77.5%), black/African American (16.1%–20.4%), and Asian (2.1%–3.3%), with the remaining participants distributed among a variety of other ethnic groups such as Native Hawaiian/Native Pacific Islander and Native American Indian/Native Alaskan (collectively <5% in each study arm). Therefore, approximately 1 of every 4 patients had skin of color, which provided good diversity of patients considering the large study size (N=2813). Data analysis included dichotomization of participants by severity rating (moderate or severe based on evaluator global severity score) and skin phototype (Fitzpatrick skin types I–III or IV–VI).22
The pooled results from both studies completed at 68 investigative sites demonstrated that CP 1.2%–BP 2.5% gel was superior in efficacy to each individual monad and to the vehicle in inflammatory, noninflammatory, and total lesion reductions as early as week 4 (P<.001) and at week 12, which was the study end point (P<.001), with superiority also demonstrated in achieving treatment success (defined as a >2 grade improvement according to the evaluator global severity score) compared to the 3 other study arms (P<.001).22 Subject assessments also were consistent with outcomes noted by the investigators. Cutaneous tolerability was favorable and comparable in all 4 study arms with less than 1% of participants discontinuing treatment due to adverse events.22
A subset analysis of the data from the phase 3 pivotal trials with CP 1.2%–BP 2.5% gel was completed to compare reductions in both inflammatory and noninflammatory lesions in female participants who were younger than 25 years and 25 years of age or older in all 4 study arms. This information has been presented14,17 but has not been previously published. Based on the overall results reported in the phase 3 studies, there were no differentiations in skin tolerability or safety based on participant age, gender, or skin type.22 The subanalysis included a total of 1080 females who were younger than 25 years and 395 females who were 25 years of age or older. The lesion reduction outcomes of this subanalysis are presented in the Table. Statistical analyses of the results among these age groups in the 4 study arms were not completed because the objective was to determine if there were any major or obvious differences in reduction of AV lesions based on the conventional dividing line of 25 years of age in adult women as compared to adolescent females treated with CP 1.2%–BP 2.5% gel. In addition, the large difference in numbers of female participants between the 2 age groups (>25 years of age, n=395; <25 years of age, n=1080) at least partially confounds both statistical and observational analysis. Among the women who were 25 years of age or older who were included in the subanalysis, 67.0% and 25.8% were between the ages of 25 to 35 years and 36 to 45 years, respectively. Based on the outcomes reported in the phase 3 trials and in this subgroup analysis, CP 1.2%–BP 2.5% gel applied once daily over a 12-week period appeared overall to be comparably effective in females regardless of age and with no apparent adverse events regarding differences in skin tolerability or safety.14,22 One observation that was noted was the possible trend of greater reduction in both lesion types in women older than 35 years versus younger females with the use of the combination gel or BP alone; however, the number of female participants who were older than 35 years of age was substantially less (n=102) than those who were 35 years of age or younger (n=1345), thus precluding support for any definitive conclusions about this possible trend.22
How can CP 1.2%–BP 2.5% gel be incorporated into a treatment regimen for women with facial AV?
The incorporation of CP 1.2%–BP 2.5% gel into a treatment regimen for women with facial AV is similar to the general use of BP-containing formulations in the overall management of AV.9,14,27,28 Because women with AV commonly present with facial inflammatory lesions and many also with facial comedones, CP 1.2%–BP 2.5% gel is best used once daily in the morning in combination with a topical retinoid in the evening,9,27 which can be achieved with use of CP 1.2%–BP 2.5% gel in the morning and a topical retinoid (ie, tretinoin, adapalene, tazarotene) in the evening or CP 1.2%–tretinoin 0.025% gel in the evening. It is important to note that cutaneous irritation may be more likely if neck lesions are present; the potential for bleaching of colored fabric by BP also is a practical concern.28 In addition, CP 1.2%–BP 2.5% gel may also be used in combination with topical dapsone, but both products should be applied separately at different times of the day to avoid temporary orange discoloration of the skin, which appears to be an uncommon side effect but remains a possibility based on the product information for dapsone gel 5% with regard to its concomitant use with BP.29,30
1. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health. 2012;21:223-230.
2. Zeichner J. Evaluating and treating the adult female patient with acne. J Drugs Dermatol. 2013;12:1416-1427.
3. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41:577-580.
4. Collier CN, Harper J, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
5. Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
6. Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
7. Kim GK, Michaels BB. Post-adolescent acne in women: more common and more clinical considerations. J Drugs Dermatol. 2012;11:708-713.
8. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
9. Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(suppl 1):1-37.
10. Thiboutot DM. Endocrinological evaluation and hormonal therapy for women with difficult acne. J Eur Acad Dermatol Venereol. 2001;15(suppl 3):57-61.
11. Sawaya ME, Samani N. Antiandrogens and androgen receptors. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Saunders-Elsevier; 2012:361-374.
12. Harper JC. Should dermatologists prescribe hormonal contraceptives for acne? Dermatol Ther. 2009;22:452-457.
13. Preneau S, Dreno B. Female acne: a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
14. Del Rosso JQ, Zeichner JA. What’s new in the medicine cabinet?: a panoramic review of clinically relevant information for the busy dermatologist. J Clin Aesthet Dermatol. 2014;7:26-30.
15. Berson D, Alexis A. Adapalene 0.3% for the treatment of acne in women. J Clin Aesthet Dermatol. 2013;6:32-35.
16. Del Rosso JQ, Kircik L, Gallagher C. Facing up to adult women with acne vulgaris: an analysis of pivotal trial data on dapsone 5% gel in the adult female population. Poster presented at: Fall Clinical Dermatology; October 2013; Las Vegas, NV.
17. Del Rosso JQ. Management of acne with oral spironolactone. Presented at: American Academy of Dermatology Summer Meeting; August 2013; Boston, MA.
18. Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
19. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
20. Perkins AC, Cheng CE, Hillebrand GG, et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
21. Draelos ZD, Carter E, Maloney JM, et al. Two randomized studies demonstrate the efficacy and safety of dapsone gel, 5% for the treatment of acne vulgaris [published online ahead of print January 17, 2007]. J Am Acad Dermatol. 2007;56:439.e1-439.e10.
22. Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
23. Schlessinger J, Menter A, Gold M, et al. Clinical safety and efficacy studies of a novel formulation combining
1.2% clindamycin phosphate and 0.025% tretinoin for the treatment of acne vulgaris. J Drugs Dermatol. 2007;6:607-615.
24. Fleischer AB Jr, Dinehart S, Stough D, et al. Safety and efficacy of a new extended-release formulation of minocycline. Cutis. 2006;78(suppl 4):21-31.
25. Gollnick HP, Draelos Z, Glenn MJ, et al. Adapalene-benzoyl peroxide, a unique fixed-dose combination topical gel for the treatment of acne vulgaris: a transatlantic, randomized, double-blind, controlled study in 1670 patients. Br J Dermatol. 2009;161:1180-1189.
26. Thiboutot D, Arsonnaud S, Soto P. Efficacy and tolerability of adapalene 0.3% gel compared to tazarotene 0.1% gel in the treatment of acne vulgaris. J Drugs Dermatol. 2008;7(suppl 6):3-10.
27. Zeichner JA. Optimizing topical combination therapy for the treatment of acne vulgaris. J Drugs Dermatol. 2012;11:313-317.
28. Tanghetti EA, Popp KF. A current review of topical benzoyl peroxide: new perspectives on formulation and utilization. Dermatol Clin. 2009;27:17-24.
29. Fleischer AB, Shalita A, Eichenfield LF. Dapsone gel 5% in combination with adapalene gel 0.1%, benzoyl peroxide gel 4% or moisturizer for the treatment of acne vulgaris: a 12-week, randomized, double-blind study. J Drugs Dermatol. 2010;9:33-40.
30. Aczone (dapsone gel 5%) [package insert]. Irvine, CA: Allergan, Inc; 2013.
1. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health. 2012;21:223-230.
2. Zeichner J. Evaluating and treating the adult female patient with acne. J Drugs Dermatol. 2013;12:1416-1427.
3. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41:577-580.
4. Collier CN, Harper J, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
5. Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
6. Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
7. Kim GK, Michaels BB. Post-adolescent acne in women: more common and more clinical considerations. J Drugs Dermatol. 2012;11:708-713.
8. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
9. Gollnick H, Cunliffe W, Berson D, et al. Management of acne: a report from a Global Alliance to Improve Outcomes in Acne. J Am Acad Dermatol. 2003;49(suppl 1):1-37.
10. Thiboutot DM. Endocrinological evaluation and hormonal therapy for women with difficult acne. J Eur Acad Dermatol Venereol. 2001;15(suppl 3):57-61.
11. Sawaya ME, Samani N. Antiandrogens and androgen receptors. In: Wolverton SE, ed. Comprehensive Dermatologic Drug Therapy. 3rd ed. Philadelphia, PA: Saunders-Elsevier; 2012:361-374.
12. Harper JC. Should dermatologists prescribe hormonal contraceptives for acne? Dermatol Ther. 2009;22:452-457.
13. Preneau S, Dreno B. Female acne: a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
14. Del Rosso JQ, Zeichner JA. What’s new in the medicine cabinet?: a panoramic review of clinically relevant information for the busy dermatologist. J Clin Aesthet Dermatol. 2014;7:26-30.
15. Berson D, Alexis A. Adapalene 0.3% for the treatment of acne in women. J Clin Aesthet Dermatol. 2013;6:32-35.
16. Del Rosso JQ, Kircik L, Gallagher C. Facing up to adult women with acne vulgaris: an analysis of pivotal trial data on dapsone 5% gel in the adult female population. Poster presented at: Fall Clinical Dermatology; October 2013; Las Vegas, NV.
17. Del Rosso JQ. Management of acne with oral spironolactone. Presented at: American Academy of Dermatology Summer Meeting; August 2013; Boston, MA.
18. Davis EC, Callender VD. A review of acne in ethnic skin: pathogenesis, clinical manifestations, and management strategies. J Clin Aesthet Dermatol. 2010;3:24-38.
19. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
20. Perkins AC, Cheng CE, Hillebrand GG, et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25:1054-1060.
21. Draelos ZD, Carter E, Maloney JM, et al. Two randomized studies demonstrate the efficacy and safety of dapsone gel, 5% for the treatment of acne vulgaris [published online ahead of print January 17, 2007]. J Am Acad Dermatol. 2007;56:439.e1-439.e10.
22. Thiboutot D, Zaenglein A, Weiss J, et al. An aqueous gel fixed combination of clindamycin phosphate 1.2% and benzoyl peroxide 2.5% for the once-daily treatment of moderate to severe acne vulgaris: assessment of efficacy and safety in 2813 patients. J Am Acad Dermatol. 2008;59:792-800.
23. Schlessinger J, Menter A, Gold M, et al. Clinical safety and efficacy studies of a novel formulation combining
1.2% clindamycin phosphate and 0.025% tretinoin for the treatment of acne vulgaris. J Drugs Dermatol. 2007;6:607-615.
24. Fleischer AB Jr, Dinehart S, Stough D, et al. Safety and efficacy of a new extended-release formulation of minocycline. Cutis. 2006;78(suppl 4):21-31.
25. Gollnick HP, Draelos Z, Glenn MJ, et al. Adapalene-benzoyl peroxide, a unique fixed-dose combination topical gel for the treatment of acne vulgaris: a transatlantic, randomized, double-blind, controlled study in 1670 patients. Br J Dermatol. 2009;161:1180-1189.
26. Thiboutot D, Arsonnaud S, Soto P. Efficacy and tolerability of adapalene 0.3% gel compared to tazarotene 0.1% gel in the treatment of acne vulgaris. J Drugs Dermatol. 2008;7(suppl 6):3-10.
27. Zeichner JA. Optimizing topical combination therapy for the treatment of acne vulgaris. J Drugs Dermatol. 2012;11:313-317.
28. Tanghetti EA, Popp KF. A current review of topical benzoyl peroxide: new perspectives on formulation and utilization. Dermatol Clin. 2009;27:17-24.
29. Fleischer AB, Shalita A, Eichenfield LF. Dapsone gel 5% in combination with adapalene gel 0.1%, benzoyl peroxide gel 4% or moisturizer for the treatment of acne vulgaris: a 12-week, randomized, double-blind study. J Drugs Dermatol. 2010;9:33-40.
30. Aczone (dapsone gel 5%) [package insert]. Irvine, CA: Allergan, Inc; 2013.
Practice Points
- Adult women with acne vulgaris (AV) can present with a U-shaped pattern of predominantly inflammatory papules, pustules, and nodules involving the lower face, jawline region, and anterior and lateral neck; however, many women present with mixed comedonal and inflammatory acne involving the face more diffusely with a presentation similar to what is typically seen in adolescent AV.
- Topical therapy for adult women with acne has been evaluated in subanalyses of data from phase 3 studies with good efficacy and favorable tolerability shown with adapalene gel 0.3% once daily, dapsone gel 5% twice daily, and clindamycin phosphate 1.2%–benzoyl peroxide (BP) 2.5% gel once daily. The latter agent requires cautious use below the jawline margin, as BP can bleach colored fabric of clothing such as shirts, blouses, and sweaters. These topical agents may be used in combination based on the clinical situation, including with systemic therapies for AV.
Lipidized Dermatofibroma
Lipidized dermatofibromas most commonly are found on the ankles, which has led some authors to refer to these lesions as ankle-type fibrous histiocytomas.1 Compared to ordinary dermatofibromas, patients with lipidized dermatofibromas tend to be older, most commonly presenting in the fifth or sixth decades of life, and are predominantly male. Lipidized dermatofibromas typically present as well-circumscribed solitary nodules in the dermis. Characteristic features include numerous xanthomatous cells dissected by distinctive hyalinized wiry collagen fibers (Figures 1 and 2).1 Xanthomatous cells can be round, polygonal, or stellate in shape. These characteristic features in combination with others of dermatofibromas (eg, epidermal acanthosis [Figure 1]) fulfill the criteria for diagnosis of a lipidized dermatofibroma. Additionally, lipidized dermatofibromas tend to be larger than ordinary dermatofibromas, which typically are less than 2 cm in diameter.1
|
Figure 1. Lipidized dermatofibromas are characterized by classic epidermal features of dermatofibromas, such as acanthosis, along with numerous foam cells and extensive stromal hyalinization (H&E, original magnification ×1.5). |
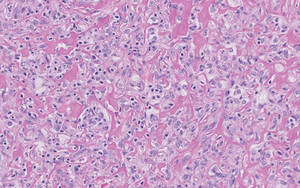
|
Figure 2. Higher-power view of a lipidized dermatofibroma shows the characteristic irregular dissection of hyalinized wiry collagen fibers between the xanthomatous cells (H&E, original magnification ×20). |
Eruptive xanthomas are characterized by a lacelike infiltrate of extravascular lipid deposits between collagen bundles (Figure 3).2 Granular cell tumors are composed of sheets and/or nests of large cells with abundant eosinophilic cytoplasm and may be confused with lipidized dermatofibromas, as they also may induce overlying pseudoepitheliomatous hyperplasia3; however, on closer examination of the cells, the cytoplasm is found to be granular (Figure 4), which contrasts the finely vacuolated cytoplasm of xanthomatous cells found in lipidized dermatofibromas. Giant lysosomal granules (eg, pustulo-ovoid bodies of Milian) are present in some cases.2 Of note, an unusual variant of dermatofibroma exists that features prominent granular cells.4
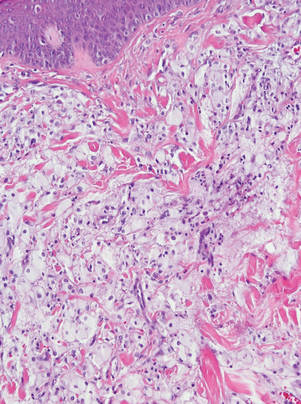
|
Figure 3. Lacelike deposition of extravascular lipid deposits is seen infiltrating between collagen bundles in an eruptive xanthoma (H&E, original magnification ×20). |
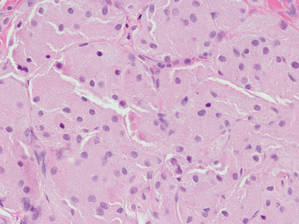
|
Figure 4. An abundant eosinophilic, finely granular cytoplasm is characteristic of granular cell tumor (H&E, original magnification ×40). |
Tuberous xanthomas most commonly occur around the pressure areas, such as the knees, elbows, and buttocks. Foam cells are a main feature of tuberous xanthomas and are arranged in large aggregates throughout the dermis.2 Tuberous xanthomas lack Touton giant cells or inflammatory cells. Older lesions tend to develop substantial fibrosis (Figure 5). Although foam cells can be present in older lesions, they are never as conspicuous as those found in other xanthomas.
Xanthogranulomas commonly occur on the head and neck. Findings noted on low magnification include a well-circumscribed exophytic nodule and an epidermal collarette, which help to easily distinguish xanthogranulomas from lipidized dermatofibromas. Additionally, the presence of a more prominent inflammatory infiltrate, which often includes eosinophils, as well as multinucleated Touton giant cells (Figure 6) and histiocytes with more eosinophilic and less xanthomatous cytoplasm can help distinguish between the lesions.1,5 Notably, Touton giant cells also can be seen in lipidized dermatofibromas,1 but the presence of unique features such as distinctive stromal hyalinization are clues to the correct diagnosis of a lipidized dermatofibroma.
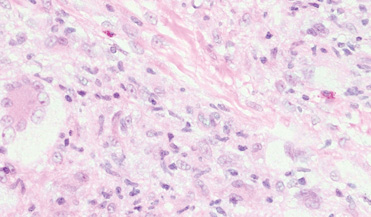
- Iwata J, Fletcher CD. Lipidized fibrous histiocytoma: clinicopathologic analysis of 22 cases. Am J Dermatopathol. 2000;22:126-134.
- Weedon D. Weedon’s Skin Pathology. 3rd ed. Edinburgh, Scotland: Elsevier Health Sciences; 2009.
- Elston DM, Ferringer T. Dermatopathology. Philadelphia, PA: Saunders Elsevier; 2009.
- Yogesh TL, Sowmya SV. Granules in granular cell lesions of the head and neck: a review. ISRN Pathol. 2011;2011:10.
- Fujita Y, Tsunemi Y, Kadono T, et al. Lipidized fibrous histiocytoma on the left condyle of the tibia. Int J Dermatol. 2011;50:634-636.
Lipidized dermatofibromas most commonly are found on the ankles, which has led some authors to refer to these lesions as ankle-type fibrous histiocytomas.1 Compared to ordinary dermatofibromas, patients with lipidized dermatofibromas tend to be older, most commonly presenting in the fifth or sixth decades of life, and are predominantly male. Lipidized dermatofibromas typically present as well-circumscribed solitary nodules in the dermis. Characteristic features include numerous xanthomatous cells dissected by distinctive hyalinized wiry collagen fibers (Figures 1 and 2).1 Xanthomatous cells can be round, polygonal, or stellate in shape. These characteristic features in combination with others of dermatofibromas (eg, epidermal acanthosis [Figure 1]) fulfill the criteria for diagnosis of a lipidized dermatofibroma. Additionally, lipidized dermatofibromas tend to be larger than ordinary dermatofibromas, which typically are less than 2 cm in diameter.1

|
Figure 1. Lipidized dermatofibromas are characterized by classic epidermal features of dermatofibromas, such as acanthosis, along with numerous foam cells and extensive stromal hyalinization (H&E, original magnification ×1.5). |

|
Figure 2. Higher-power view of a lipidized dermatofibroma shows the characteristic irregular dissection of hyalinized wiry collagen fibers between the xanthomatous cells (H&E, original magnification ×20). |
Eruptive xanthomas are characterized by a lacelike infiltrate of extravascular lipid deposits between collagen bundles (Figure 3).2 Granular cell tumors are composed of sheets and/or nests of large cells with abundant eosinophilic cytoplasm and may be confused with lipidized dermatofibromas, as they also may induce overlying pseudoepitheliomatous hyperplasia3; however, on closer examination of the cells, the cytoplasm is found to be granular (Figure 4), which contrasts the finely vacuolated cytoplasm of xanthomatous cells found in lipidized dermatofibromas. Giant lysosomal granules (eg, pustulo-ovoid bodies of Milian) are present in some cases.2 Of note, an unusual variant of dermatofibroma exists that features prominent granular cells.4

|
Figure 3. Lacelike deposition of extravascular lipid deposits is seen infiltrating between collagen bundles in an eruptive xanthoma (H&E, original magnification ×20). |

|
Figure 4. An abundant eosinophilic, finely granular cytoplasm is characteristic of granular cell tumor (H&E, original magnification ×40). |
Tuberous xanthomas most commonly occur around the pressure areas, such as the knees, elbows, and buttocks. Foam cells are a main feature of tuberous xanthomas and are arranged in large aggregates throughout the dermis.2 Tuberous xanthomas lack Touton giant cells or inflammatory cells. Older lesions tend to develop substantial fibrosis (Figure 5). Although foam cells can be present in older lesions, they are never as conspicuous as those found in other xanthomas.

Xanthogranulomas commonly occur on the head and neck. Findings noted on low magnification include a well-circumscribed exophytic nodule and an epidermal collarette, which help to easily distinguish xanthogranulomas from lipidized dermatofibromas. Additionally, the presence of a more prominent inflammatory infiltrate, which often includes eosinophils, as well as multinucleated Touton giant cells (Figure 6) and histiocytes with more eosinophilic and less xanthomatous cytoplasm can help distinguish between the lesions.1,5 Notably, Touton giant cells also can be seen in lipidized dermatofibromas,1 but the presence of unique features such as distinctive stromal hyalinization are clues to the correct diagnosis of a lipidized dermatofibroma.

Lipidized dermatofibromas most commonly are found on the ankles, which has led some authors to refer to these lesions as ankle-type fibrous histiocytomas.1 Compared to ordinary dermatofibromas, patients with lipidized dermatofibromas tend to be older, most commonly presenting in the fifth or sixth decades of life, and are predominantly male. Lipidized dermatofibromas typically present as well-circumscribed solitary nodules in the dermis. Characteristic features include numerous xanthomatous cells dissected by distinctive hyalinized wiry collagen fibers (Figures 1 and 2).1 Xanthomatous cells can be round, polygonal, or stellate in shape. These characteristic features in combination with others of dermatofibromas (eg, epidermal acanthosis [Figure 1]) fulfill the criteria for diagnosis of a lipidized dermatofibroma. Additionally, lipidized dermatofibromas tend to be larger than ordinary dermatofibromas, which typically are less than 2 cm in diameter.1

|
Figure 1. Lipidized dermatofibromas are characterized by classic epidermal features of dermatofibromas, such as acanthosis, along with numerous foam cells and extensive stromal hyalinization (H&E, original magnification ×1.5). |

|
Figure 2. Higher-power view of a lipidized dermatofibroma shows the characteristic irregular dissection of hyalinized wiry collagen fibers between the xanthomatous cells (H&E, original magnification ×20). |
Eruptive xanthomas are characterized by a lacelike infiltrate of extravascular lipid deposits between collagen bundles (Figure 3).2 Granular cell tumors are composed of sheets and/or nests of large cells with abundant eosinophilic cytoplasm and may be confused with lipidized dermatofibromas, as they also may induce overlying pseudoepitheliomatous hyperplasia3; however, on closer examination of the cells, the cytoplasm is found to be granular (Figure 4), which contrasts the finely vacuolated cytoplasm of xanthomatous cells found in lipidized dermatofibromas. Giant lysosomal granules (eg, pustulo-ovoid bodies of Milian) are present in some cases.2 Of note, an unusual variant of dermatofibroma exists that features prominent granular cells.4

|
Figure 3. Lacelike deposition of extravascular lipid deposits is seen infiltrating between collagen bundles in an eruptive xanthoma (H&E, original magnification ×20). |

|
Figure 4. An abundant eosinophilic, finely granular cytoplasm is characteristic of granular cell tumor (H&E, original magnification ×40). |
Tuberous xanthomas most commonly occur around the pressure areas, such as the knees, elbows, and buttocks. Foam cells are a main feature of tuberous xanthomas and are arranged in large aggregates throughout the dermis.2 Tuberous xanthomas lack Touton giant cells or inflammatory cells. Older lesions tend to develop substantial fibrosis (Figure 5). Although foam cells can be present in older lesions, they are never as conspicuous as those found in other xanthomas.

Xanthogranulomas commonly occur on the head and neck. Findings noted on low magnification include a well-circumscribed exophytic nodule and an epidermal collarette, which help to easily distinguish xanthogranulomas from lipidized dermatofibromas. Additionally, the presence of a more prominent inflammatory infiltrate, which often includes eosinophils, as well as multinucleated Touton giant cells (Figure 6) and histiocytes with more eosinophilic and less xanthomatous cytoplasm can help distinguish between the lesions.1,5 Notably, Touton giant cells also can be seen in lipidized dermatofibromas,1 but the presence of unique features such as distinctive stromal hyalinization are clues to the correct diagnosis of a lipidized dermatofibroma.

- Iwata J, Fletcher CD. Lipidized fibrous histiocytoma: clinicopathologic analysis of 22 cases. Am J Dermatopathol. 2000;22:126-134.
- Weedon D. Weedon’s Skin Pathology. 3rd ed. Edinburgh, Scotland: Elsevier Health Sciences; 2009.
- Elston DM, Ferringer T. Dermatopathology. Philadelphia, PA: Saunders Elsevier; 2009.
- Yogesh TL, Sowmya SV. Granules in granular cell lesions of the head and neck: a review. ISRN Pathol. 2011;2011:10.
- Fujita Y, Tsunemi Y, Kadono T, et al. Lipidized fibrous histiocytoma on the left condyle of the tibia. Int J Dermatol. 2011;50:634-636.
- Iwata J, Fletcher CD. Lipidized fibrous histiocytoma: clinicopathologic analysis of 22 cases. Am J Dermatopathol. 2000;22:126-134.
- Weedon D. Weedon’s Skin Pathology. 3rd ed. Edinburgh, Scotland: Elsevier Health Sciences; 2009.
- Elston DM, Ferringer T. Dermatopathology. Philadelphia, PA: Saunders Elsevier; 2009.
- Yogesh TL, Sowmya SV. Granules in granular cell lesions of the head and neck: a review. ISRN Pathol. 2011;2011:10.
- Fujita Y, Tsunemi Y, Kadono T, et al. Lipidized fibrous histiocytoma on the left condyle of the tibia. Int J Dermatol. 2011;50:634-636.
MULTIMEDIA: Guide to being a good intern
The first year of residency can be overwhelming. Just ask Dr. Anneliese Beaubrun, an intern at the University of Central Florida College of Medicine’s internal medicine residency program in Orlando.
You’re suddenly responsible for patients, and you definitely don’t want to be the one asking what you think is the wrong question, she says.
But the professors and program directors have a different view.
In a video interview, Dr. Abdo Asmar, associate director of the internal medicine residency program at UCF, shares his advice to first-year residents. Chief resident Olga Karasik, too, looks back at her first year and shares what she’s learned.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
And there’s more.
In her blog, Wellness Rounds, Dr. Mary L. Brandt, professor of surgery, pediatrics and medical ethics and interim senior associate dean of Medical Education at Baylor College of Medicine, Houston, takes a holistic approach to medical school and residency. Her posts cover a wide range of topics, from the type of shoes to wear at the hospital, to advice on preparing for residency interviews.
Being a good intern is among the popular posts on her blog, and in a phone interview she shared some of her top tips and advice for first-year residents.
What are your tips and advice for interns? Leave us a comment.
On Twitter @naseemmiller
The first year of residency can be overwhelming. Just ask Dr. Anneliese Beaubrun, an intern at the University of Central Florida College of Medicine’s internal medicine residency program in Orlando.
You’re suddenly responsible for patients, and you definitely don’t want to be the one asking what you think is the wrong question, she says.
But the professors and program directors have a different view.
In a video interview, Dr. Abdo Asmar, associate director of the internal medicine residency program at UCF, shares his advice to first-year residents. Chief resident Olga Karasik, too, looks back at her first year and shares what she’s learned.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
And there’s more.
In her blog, Wellness Rounds, Dr. Mary L. Brandt, professor of surgery, pediatrics and medical ethics and interim senior associate dean of Medical Education at Baylor College of Medicine, Houston, takes a holistic approach to medical school and residency. Her posts cover a wide range of topics, from the type of shoes to wear at the hospital, to advice on preparing for residency interviews.
Being a good intern is among the popular posts on her blog, and in a phone interview she shared some of her top tips and advice for first-year residents.
What are your tips and advice for interns? Leave us a comment.
On Twitter @naseemmiller
The first year of residency can be overwhelming. Just ask Dr. Anneliese Beaubrun, an intern at the University of Central Florida College of Medicine’s internal medicine residency program in Orlando.
You’re suddenly responsible for patients, and you definitely don’t want to be the one asking what you think is the wrong question, she says.
But the professors and program directors have a different view.
In a video interview, Dr. Abdo Asmar, associate director of the internal medicine residency program at UCF, shares his advice to first-year residents. Chief resident Olga Karasik, too, looks back at her first year and shares what she’s learned.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
And there’s more.
In her blog, Wellness Rounds, Dr. Mary L. Brandt, professor of surgery, pediatrics and medical ethics and interim senior associate dean of Medical Education at Baylor College of Medicine, Houston, takes a holistic approach to medical school and residency. Her posts cover a wide range of topics, from the type of shoes to wear at the hospital, to advice on preparing for residency interviews.
Being a good intern is among the popular posts on her blog, and in a phone interview she shared some of her top tips and advice for first-year residents.
What are your tips and advice for interns? Leave us a comment.
On Twitter @naseemmiller