User login
How do hydrochlorothiazide and chlorthalidone compare for treating hypertension?
Both medications reduce theincidence of cardiovascular events in patients with hypertension, but chlorthalidone may confer additional cardiovascular risk reduction (strength of recommendation [SOR]: B, conflicting network meta-analysis and cohort studies). (No head-to-head studies of hydrochlorothiazide [HCTZ] and chlorthalidone have been done.)
Serious hypokalemia and hyponatremia can occur with either medication; it is unclear if the rates of these adverse effects are the same at equivalent doses. Patients taking chlorthalidone are less likely to need a second antihypertensive medication but more likely to be nonadherent than patients taking HCTZ (SOR: B, cohort studies).
EVIDENCE SUMMARY
A network meta-analysis—designed to compare 2 interventions that haven’t been studied head-to-head—examined 9 trials that evaluated cardiovascular outcomes in 18,000 patients taking HCTZ and 60,000 patients taking chlorthalidone against outcomes for placebo or other antihypertensive agents.1 Daily doses ranged from 12.5 to 25 mg for HCTZ and 12.5 to 100 mg for chlorthalidone (although most patients taking chlorthalidone were on 12.5-25 mg).
In a drug-adjusted analysis using shared comparator medications, chlorthalidone proved superior to HCTZ in reducing the risk of both heart failure (relative risk [RR]=0.77; 95% confidence interval [CI], 0.61-0.98) and combined cardiovascular events—myocardial infarction (MI), stroke, a new diagnosis of coronary artery disease, and new-onset congestive heart failure (RR=0.79; 95% CI, 0.72-0.88).
After adjusting for achieved blood pressure, chlorthalidone was still associated with lower rates of cardiovascular events than HCTZ (RR=0.82; 95% CI, 0.70-0.97). Relative to HCTZ, the number needed to treat with chlorthalidone to prevent 1 additional cardiovascular event over 5 years was 27. Because network meta-analyses draw from a wider body of research than standard meta-analyses, they may be weakened by increased variability in study design and patient demographics.
But another study shows no significant difference in cardiovascular outcomes
A subsequent retrospective cohort study didn’t find a significant difference in cardiovascular outcomes between HCTZ and chlorthalidone. The study compared pooled cardiovascular outcomes (MI, heart failure, and stroke) in 10,400 patients recently started on chlorthalidone and 19,500 started on HCTZ.2 Initial doses were typically either 25 mg chlorthalidone (70% of patients on chlorthalidone) or 12.5 mg HCTZ (67% of patients on HCTZ). The median follow-up was about a year, but lasted as long as 5 years in some cases.
The 2 groups showed no significant difference in cardiovascular events (3.2 events per 100 person-years for chlorthalidone compared with 3.4 for HCTZ; adjusted hazard ratio [aHR]=0.93; 95% CI, 0.81-1.06).
Serious hypokalemia and hyponatremia are risks
Patients taking chlorthalidone were more likely to be hospitalized for hypokalemia (0.69 per 100 person-years vs 0.27 for HCTZ; aHR=3.1; 95% CI, 2.0-4.6; number needed to harm [NNH]=238 in 1 year) or hyponatremia (0.69 per 100 person-years vs 0.49 for HCTZ; aHR=1.7; 95% CI, 1.2-2.3; NNH=434 in 1 year).2 However, the all-cause hospitalization rates for the 2 drugs were the same (aHR=1.0; 95% CI, 0.93-1.07).
Lower systolic BP and serum potassium found with chlorthalidone
A smaller retrospective cohort analysis (6441 participants who received either chlorthalidone or HCTZ starting at 50 mg and stepped once to 100 mg) also assessed the difference in cardiovascular events between patients taking the 2 drugs.3 (Cardiovascular events were defined as pooled MIs, onset of angina or peripheral artery occlusive disease, or need for coronary artery bypass.) Although significant reductions in pooled events occurred in both groups over the 7-year study, these reductions were significantly lower in the chlorthalidone group than in the HCTZ group (aHR=0.79; 95% CI, 0.68-0.92).
Systolic blood pressures were statistically lower in the chlorthalidone group during Years 1 through 5 but not in Years 6 and 7 (difference 2-4 mm Hg). Serum potassium was also lower in patients taking chlorthalidone (3.8 mEq/L on chlorthalidone vs 4.0 mEq/L on HCTZ after 7 years; P<.05).
Chlorthalidone users more responsive, but less adherent than HCTZ users
A retrospective cohort study investigated medication tolerance in veterans who had recently started either HCTZ (120,000 patients) or chlorthalidone (2200 patients) and were followed for a year.4 Most received doses between 12.5 and 25 mg of active drug.
One primary outcome was “nonpersistence,” defined as failure to refill the medication after double the number of days as the initial prescription. The other was “insufficient response,” defined as the need to start another antihypertensive medication. Chlorthalidone users were less likely than HCTZ users to have an insufficient response (odds ratio [OR]=0.71; 95% CI, 0.63-0.80) but more likely to exhibit nonpersistence (OR=1.6; 95% CI, 1.5-1.8).
RECOMMENDATIONS
For primary hypertension, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommends diuretic monotherapy in patients older than 55 years who are poor candidates for calcium channel blockers.5 If a diuretic is to be initiated or changed, NICE recommends chlorthalidone (12.5-25 mg daily) or indapamide (1.5-2.5 mg daily) in preference to HCTZ. The guideline set forth in the eighth annual report of the United States Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure makes no distinction between chlorthalidone and HCTZ; it refers only to “thiazidetype diuretics.” Thiazide-type diuretics are listed as one option (along with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and calcium channel blockers) for initial monotherapy in nonblack patients.6
1. Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012;59:1110–1117.
2. Dhalla IA, Gomes T, Yao Z, et al. Chlorthalidone versus hydrochlorothiazide for the treatment of hypertension in older adults: a population-based cohort study. Ann Intern Med. 2013;158:447–455.
3. Dorsh MP, Gillespie BW, Erickson SR, et al. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57:689–694.
4. Lund BC, Ernst ME. The comparative effectiveness of hydrochlorothiazide and chlorthalidone in an observational cohort of veterans. J Clin Hypertension. 2012;14:623–629.
5. Hypertension: clinical management of primary hypertension in adults. (NICE Clinical Guideline 127). National Institute for Health and Care Excellence Web site. London, UK: National Institute for Health and Care Excellence; 2011. Available at: www.nice.org.UK/guidance/CG127. Accessed December 16, 2013.
6. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC8). JAMA. 2014;311:507-520.
Both medications reduce theincidence of cardiovascular events in patients with hypertension, but chlorthalidone may confer additional cardiovascular risk reduction (strength of recommendation [SOR]: B, conflicting network meta-analysis and cohort studies). (No head-to-head studies of hydrochlorothiazide [HCTZ] and chlorthalidone have been done.)
Serious hypokalemia and hyponatremia can occur with either medication; it is unclear if the rates of these adverse effects are the same at equivalent doses. Patients taking chlorthalidone are less likely to need a second antihypertensive medication but more likely to be nonadherent than patients taking HCTZ (SOR: B, cohort studies).
EVIDENCE SUMMARY
A network meta-analysis—designed to compare 2 interventions that haven’t been studied head-to-head—examined 9 trials that evaluated cardiovascular outcomes in 18,000 patients taking HCTZ and 60,000 patients taking chlorthalidone against outcomes for placebo or other antihypertensive agents.1 Daily doses ranged from 12.5 to 25 mg for HCTZ and 12.5 to 100 mg for chlorthalidone (although most patients taking chlorthalidone were on 12.5-25 mg).
In a drug-adjusted analysis using shared comparator medications, chlorthalidone proved superior to HCTZ in reducing the risk of both heart failure (relative risk [RR]=0.77; 95% confidence interval [CI], 0.61-0.98) and combined cardiovascular events—myocardial infarction (MI), stroke, a new diagnosis of coronary artery disease, and new-onset congestive heart failure (RR=0.79; 95% CI, 0.72-0.88).
After adjusting for achieved blood pressure, chlorthalidone was still associated with lower rates of cardiovascular events than HCTZ (RR=0.82; 95% CI, 0.70-0.97). Relative to HCTZ, the number needed to treat with chlorthalidone to prevent 1 additional cardiovascular event over 5 years was 27. Because network meta-analyses draw from a wider body of research than standard meta-analyses, they may be weakened by increased variability in study design and patient demographics.
But another study shows no significant difference in cardiovascular outcomes
A subsequent retrospective cohort study didn’t find a significant difference in cardiovascular outcomes between HCTZ and chlorthalidone. The study compared pooled cardiovascular outcomes (MI, heart failure, and stroke) in 10,400 patients recently started on chlorthalidone and 19,500 started on HCTZ.2 Initial doses were typically either 25 mg chlorthalidone (70% of patients on chlorthalidone) or 12.5 mg HCTZ (67% of patients on HCTZ). The median follow-up was about a year, but lasted as long as 5 years in some cases.
The 2 groups showed no significant difference in cardiovascular events (3.2 events per 100 person-years for chlorthalidone compared with 3.4 for HCTZ; adjusted hazard ratio [aHR]=0.93; 95% CI, 0.81-1.06).
Serious hypokalemia and hyponatremia are risks
Patients taking chlorthalidone were more likely to be hospitalized for hypokalemia (0.69 per 100 person-years vs 0.27 for HCTZ; aHR=3.1; 95% CI, 2.0-4.6; number needed to harm [NNH]=238 in 1 year) or hyponatremia (0.69 per 100 person-years vs 0.49 for HCTZ; aHR=1.7; 95% CI, 1.2-2.3; NNH=434 in 1 year).2 However, the all-cause hospitalization rates for the 2 drugs were the same (aHR=1.0; 95% CI, 0.93-1.07).
Lower systolic BP and serum potassium found with chlorthalidone
A smaller retrospective cohort analysis (6441 participants who received either chlorthalidone or HCTZ starting at 50 mg and stepped once to 100 mg) also assessed the difference in cardiovascular events between patients taking the 2 drugs.3 (Cardiovascular events were defined as pooled MIs, onset of angina or peripheral artery occlusive disease, or need for coronary artery bypass.) Although significant reductions in pooled events occurred in both groups over the 7-year study, these reductions were significantly lower in the chlorthalidone group than in the HCTZ group (aHR=0.79; 95% CI, 0.68-0.92).
Systolic blood pressures were statistically lower in the chlorthalidone group during Years 1 through 5 but not in Years 6 and 7 (difference 2-4 mm Hg). Serum potassium was also lower in patients taking chlorthalidone (3.8 mEq/L on chlorthalidone vs 4.0 mEq/L on HCTZ after 7 years; P<.05).
Chlorthalidone users more responsive, but less adherent than HCTZ users
A retrospective cohort study investigated medication tolerance in veterans who had recently started either HCTZ (120,000 patients) or chlorthalidone (2200 patients) and were followed for a year.4 Most received doses between 12.5 and 25 mg of active drug.
One primary outcome was “nonpersistence,” defined as failure to refill the medication after double the number of days as the initial prescription. The other was “insufficient response,” defined as the need to start another antihypertensive medication. Chlorthalidone users were less likely than HCTZ users to have an insufficient response (odds ratio [OR]=0.71; 95% CI, 0.63-0.80) but more likely to exhibit nonpersistence (OR=1.6; 95% CI, 1.5-1.8).
RECOMMENDATIONS
For primary hypertension, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommends diuretic monotherapy in patients older than 55 years who are poor candidates for calcium channel blockers.5 If a diuretic is to be initiated or changed, NICE recommends chlorthalidone (12.5-25 mg daily) or indapamide (1.5-2.5 mg daily) in preference to HCTZ. The guideline set forth in the eighth annual report of the United States Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure makes no distinction between chlorthalidone and HCTZ; it refers only to “thiazidetype diuretics.” Thiazide-type diuretics are listed as one option (along with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and calcium channel blockers) for initial monotherapy in nonblack patients.6
Both medications reduce theincidence of cardiovascular events in patients with hypertension, but chlorthalidone may confer additional cardiovascular risk reduction (strength of recommendation [SOR]: B, conflicting network meta-analysis and cohort studies). (No head-to-head studies of hydrochlorothiazide [HCTZ] and chlorthalidone have been done.)
Serious hypokalemia and hyponatremia can occur with either medication; it is unclear if the rates of these adverse effects are the same at equivalent doses. Patients taking chlorthalidone are less likely to need a second antihypertensive medication but more likely to be nonadherent than patients taking HCTZ (SOR: B, cohort studies).
EVIDENCE SUMMARY
A network meta-analysis—designed to compare 2 interventions that haven’t been studied head-to-head—examined 9 trials that evaluated cardiovascular outcomes in 18,000 patients taking HCTZ and 60,000 patients taking chlorthalidone against outcomes for placebo or other antihypertensive agents.1 Daily doses ranged from 12.5 to 25 mg for HCTZ and 12.5 to 100 mg for chlorthalidone (although most patients taking chlorthalidone were on 12.5-25 mg).
In a drug-adjusted analysis using shared comparator medications, chlorthalidone proved superior to HCTZ in reducing the risk of both heart failure (relative risk [RR]=0.77; 95% confidence interval [CI], 0.61-0.98) and combined cardiovascular events—myocardial infarction (MI), stroke, a new diagnosis of coronary artery disease, and new-onset congestive heart failure (RR=0.79; 95% CI, 0.72-0.88).
After adjusting for achieved blood pressure, chlorthalidone was still associated with lower rates of cardiovascular events than HCTZ (RR=0.82; 95% CI, 0.70-0.97). Relative to HCTZ, the number needed to treat with chlorthalidone to prevent 1 additional cardiovascular event over 5 years was 27. Because network meta-analyses draw from a wider body of research than standard meta-analyses, they may be weakened by increased variability in study design and patient demographics.
But another study shows no significant difference in cardiovascular outcomes
A subsequent retrospective cohort study didn’t find a significant difference in cardiovascular outcomes between HCTZ and chlorthalidone. The study compared pooled cardiovascular outcomes (MI, heart failure, and stroke) in 10,400 patients recently started on chlorthalidone and 19,500 started on HCTZ.2 Initial doses were typically either 25 mg chlorthalidone (70% of patients on chlorthalidone) or 12.5 mg HCTZ (67% of patients on HCTZ). The median follow-up was about a year, but lasted as long as 5 years in some cases.
The 2 groups showed no significant difference in cardiovascular events (3.2 events per 100 person-years for chlorthalidone compared with 3.4 for HCTZ; adjusted hazard ratio [aHR]=0.93; 95% CI, 0.81-1.06).
Serious hypokalemia and hyponatremia are risks
Patients taking chlorthalidone were more likely to be hospitalized for hypokalemia (0.69 per 100 person-years vs 0.27 for HCTZ; aHR=3.1; 95% CI, 2.0-4.6; number needed to harm [NNH]=238 in 1 year) or hyponatremia (0.69 per 100 person-years vs 0.49 for HCTZ; aHR=1.7; 95% CI, 1.2-2.3; NNH=434 in 1 year).2 However, the all-cause hospitalization rates for the 2 drugs were the same (aHR=1.0; 95% CI, 0.93-1.07).
Lower systolic BP and serum potassium found with chlorthalidone
A smaller retrospective cohort analysis (6441 participants who received either chlorthalidone or HCTZ starting at 50 mg and stepped once to 100 mg) also assessed the difference in cardiovascular events between patients taking the 2 drugs.3 (Cardiovascular events were defined as pooled MIs, onset of angina or peripheral artery occlusive disease, or need for coronary artery bypass.) Although significant reductions in pooled events occurred in both groups over the 7-year study, these reductions were significantly lower in the chlorthalidone group than in the HCTZ group (aHR=0.79; 95% CI, 0.68-0.92).
Systolic blood pressures were statistically lower in the chlorthalidone group during Years 1 through 5 but not in Years 6 and 7 (difference 2-4 mm Hg). Serum potassium was also lower in patients taking chlorthalidone (3.8 mEq/L on chlorthalidone vs 4.0 mEq/L on HCTZ after 7 years; P<.05).
Chlorthalidone users more responsive, but less adherent than HCTZ users
A retrospective cohort study investigated medication tolerance in veterans who had recently started either HCTZ (120,000 patients) or chlorthalidone (2200 patients) and were followed for a year.4 Most received doses between 12.5 and 25 mg of active drug.
One primary outcome was “nonpersistence,” defined as failure to refill the medication after double the number of days as the initial prescription. The other was “insufficient response,” defined as the need to start another antihypertensive medication. Chlorthalidone users were less likely than HCTZ users to have an insufficient response (odds ratio [OR]=0.71; 95% CI, 0.63-0.80) but more likely to exhibit nonpersistence (OR=1.6; 95% CI, 1.5-1.8).
RECOMMENDATIONS
For primary hypertension, the United Kingdom’s National Institute for Health and Care Excellence (NICE) recommends diuretic monotherapy in patients older than 55 years who are poor candidates for calcium channel blockers.5 If a diuretic is to be initiated or changed, NICE recommends chlorthalidone (12.5-25 mg daily) or indapamide (1.5-2.5 mg daily) in preference to HCTZ. The guideline set forth in the eighth annual report of the United States Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure makes no distinction between chlorthalidone and HCTZ; it refers only to “thiazidetype diuretics.” Thiazide-type diuretics are listed as one option (along with angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, and calcium channel blockers) for initial monotherapy in nonblack patients.6
1. Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012;59:1110–1117.
2. Dhalla IA, Gomes T, Yao Z, et al. Chlorthalidone versus hydrochlorothiazide for the treatment of hypertension in older adults: a population-based cohort study. Ann Intern Med. 2013;158:447–455.
3. Dorsh MP, Gillespie BW, Erickson SR, et al. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57:689–694.
4. Lund BC, Ernst ME. The comparative effectiveness of hydrochlorothiazide and chlorthalidone in an observational cohort of veterans. J Clin Hypertension. 2012;14:623–629.
5. Hypertension: clinical management of primary hypertension in adults. (NICE Clinical Guideline 127). National Institute for Health and Care Excellence Web site. London, UK: National Institute for Health and Care Excellence; 2011. Available at: www.nice.org.UK/guidance/CG127. Accessed December 16, 2013.
6. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC8). JAMA. 2014;311:507-520.
1. Roush GC, Holford TR, Guddati AK. Chlorthalidone compared with hydrochlorothiazide in reducing cardiovascular events: systematic review and network meta-analyses. Hypertension. 2012;59:1110–1117.
2. Dhalla IA, Gomes T, Yao Z, et al. Chlorthalidone versus hydrochlorothiazide for the treatment of hypertension in older adults: a population-based cohort study. Ann Intern Med. 2013;158:447–455.
3. Dorsh MP, Gillespie BW, Erickson SR, et al. Chlorthalidone reduces cardiovascular events compared with hydrochlorothiazide: a retrospective cohort analysis. Hypertension. 2011;57:689–694.
4. Lund BC, Ernst ME. The comparative effectiveness of hydrochlorothiazide and chlorthalidone in an observational cohort of veterans. J Clin Hypertension. 2012;14:623–629.
5. Hypertension: clinical management of primary hypertension in adults. (NICE Clinical Guideline 127). National Institute for Health and Care Excellence Web site. London, UK: National Institute for Health and Care Excellence; 2011. Available at: www.nice.org.UK/guidance/CG127. Accessed December 16, 2013.
6. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC8). JAMA. 2014;311:507-520.
Evidence-based answers from the Family Physicians Inquiries Network
Which prophylactic therapies best prevent gout attacks?
Allopurinol and febuxostat reduce the frequency of gout attacks equally after 8 weeks of treatment (strength of recommendation [SOR]: B, multiple randomized control trials [RCTs] with limitations).
Intravenous pegloticase decreases serum uric acid and gout attacks and improves quality of life (QOL) (SOR: A, 2 RCTs).
Colchicine reduces gout attacks when combined with probenecid or allopurinol at the start of urate-lowering therapy (SOR: B, 1 high-quality and 1 low-quality RCT).
EVIDENCE SUMMARY
A 28-week RCT compared the effects of placebo, allopurinol (300 mg/d), and febuxostat (80 mg, 120 mg, and 240 mg) on serum uric acid levels (sUA) and gout attacks in 1067 patients with gout and hyperuricemia (94% male, 78% white, 18 to 85 years of age with mean age ranging from 51 to 54 years ± 12 years in each group).1 Patients also received prophylaxis with either colchicine or naproxen during the first 8 weeks of the study.
During Weeks 1 through 8, investigators found no statistically significant differences in the percentage of patients requiring treatment for gout attacks between the febuxostat 80 mg, allopurinol, and placebo groups (28%, 23%, and 20%, respectively). During Weeks 8 through 28, no statistically significant differences in gout attack rates occurred between the allopurinol and febuxostat groups, although the study didn’t report specific attack rates for this period.
Both allopurinol and all doses of febuxostat reduced sUA to <6 mg/dL more effectively than placebo; more patients treated with febuxostat than allopurinol achieved a uric acid level of less than <6 mg/dL.
Another RCT of 762 mostly white, male patients (mean age 52 years) with gout and sUA >8 mg/dL—35% of whom had renal impairment, defined as creatinine clearance <80 mL/min/1.73m2—also concluded that febuxostat and allopurinol are equally effective in reducing gout attacks (incidence of gout flares during Weeks 9 to 52 was 64% with both febuxostat 80 mg and allopurinol 300 mg).2 The percentage of patients with sUA <6 mg/dL at the last 3 monthly visits was 53% in the febuxostat 80 mg group compared with 21% in the allopurinol 300 mg group (P<.001; number needed to treat [NNT]=4]).
One significant limitation of both RCTs was the fixed dose of allopurinol (300 mg/d). US Food and Drug Administration-approved dosing for allopurinol allows for titration to a maximum of 800 mg/d to achieve serum uric acid <6 mg/dL.
IV pegloticase decreases gout attacks after 3 months, improves quality of life
Pegloticase is an intravenously administered, recombinant form of uricase, the natural enzyme that converts uric acid to more soluble allantoin. Two RCTs compared pegloticase with placebo in a total of 212 patients with gout (mean age 54 to 59 years; 70% to 90% male) intolerant or refractory to allopurinol (defined as baseline sUA of ≥8 mg/dL and at least one of the following: ≥3 self-reported gout flares during the previous 18 months, ≥1 tophi, or gouty arthropathy.
These trials found that treatment with 8 mg of pegloticase every 2 weeks for 6 months initially increased gout flares during Months 1 to 3 (75% with pegloticase, 53% with placebo; P=.02; number needed to harm [NNH]=5) but then decreased the incidence of acute gout attacks during Months 4 to 6 (41% with pegloticase, 67% with placebo; P=.007; NNT=4).3 In addition, pegloticase resulted in statistically significant improvements in QOL measured at the final visit using the Health Assessment Questionnaire (HAQ) pain scale, the HAQ-Disability Index, and the 36-item Short Form Health Survey.
Colchicine plus probenecid or allopurinol reduces gout attacks
One small, low-quality RCT (N=38) found that colchicine 0.5 mg administered 3 times daily effectively prevented gout attacks when administered concomitantly with probenecid initiated to lower urate (gout attacks per month in colchicine and placebo-treated patients, respectively, were 0.19±0.05 and 0.48±0.12; P<.05).4
Another RCT that compared allopurinol with and without colchicine showed that coadministration of colchicine 0.6 mg twice daily reduced gout attacks: 33% of patients treated with colchicine experienced a gout flare compared with 77% of placebo-treated patients (P=.008; NNT=3 over 6 months).5
We identified no RCTs that evaluated the uricosuric agent probenecid and no studies that assessed the use of nonsteroidal anti-inflammatory drugs (NSAIDs) to prevent recurrent gout attacks.
RECOMMENDATIONS
The American College of Rheumatology (ACR) guidelines on managing gout recommend allopurinol or febuxostat as first-line pharmacologic urate-lowering therapy, with a goal of reducing sUA to <6 mg/dL. They recommend probenecid as an alternative if contraindications exist or the patient is intolerant to allopurinol and febuxostat.6 The guidelines note that allopurinol doses may exceed 300 mg/d, even in patients with chronic kidney disease.
The ACR recommends anti-inflammatory prophylaxis with colchicine or NSAIDs upon initiation of urate-lowering therapy. Anti-inflammatory prophylaxis should be continued as long as clinical evidence of continuing gout disease exists and until the sUA target has been acheived.7
1. Schumacher HR Jr, Becker MA, Wortmann RL, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008;59:1540-1548.
2. Becker MA, Schumacher HR Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450-2461.
3. Sundy JS, Baraf HSB, Yood RA, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA. 2011;306:711-720.
4. Paulus HE, Schlosstein LH, Godfrey RG, et al. Prophylactic colchicine therapy of intercritical gout: a placebo-controlled study of probenecid-treated patients. Arthritis Rheum. 1974;17:609-614.
5. Borstad GC, Bryant LR, Abel MP, et al. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol. 2004;31:2429-2432.
6. Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64:1431-1446.
7. Khanna D, Khanna PP, Fitzgerald JD, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64:1447-1461.
Allopurinol and febuxostat reduce the frequency of gout attacks equally after 8 weeks of treatment (strength of recommendation [SOR]: B, multiple randomized control trials [RCTs] with limitations).
Intravenous pegloticase decreases serum uric acid and gout attacks and improves quality of life (QOL) (SOR: A, 2 RCTs).
Colchicine reduces gout attacks when combined with probenecid or allopurinol at the start of urate-lowering therapy (SOR: B, 1 high-quality and 1 low-quality RCT).
EVIDENCE SUMMARY
A 28-week RCT compared the effects of placebo, allopurinol (300 mg/d), and febuxostat (80 mg, 120 mg, and 240 mg) on serum uric acid levels (sUA) and gout attacks in 1067 patients with gout and hyperuricemia (94% male, 78% white, 18 to 85 years of age with mean age ranging from 51 to 54 years ± 12 years in each group).1 Patients also received prophylaxis with either colchicine or naproxen during the first 8 weeks of the study.
During Weeks 1 through 8, investigators found no statistically significant differences in the percentage of patients requiring treatment for gout attacks between the febuxostat 80 mg, allopurinol, and placebo groups (28%, 23%, and 20%, respectively). During Weeks 8 through 28, no statistically significant differences in gout attack rates occurred between the allopurinol and febuxostat groups, although the study didn’t report specific attack rates for this period.
Both allopurinol and all doses of febuxostat reduced sUA to <6 mg/dL more effectively than placebo; more patients treated with febuxostat than allopurinol achieved a uric acid level of less than <6 mg/dL.
Another RCT of 762 mostly white, male patients (mean age 52 years) with gout and sUA >8 mg/dL—35% of whom had renal impairment, defined as creatinine clearance <80 mL/min/1.73m2—also concluded that febuxostat and allopurinol are equally effective in reducing gout attacks (incidence of gout flares during Weeks 9 to 52 was 64% with both febuxostat 80 mg and allopurinol 300 mg).2 The percentage of patients with sUA <6 mg/dL at the last 3 monthly visits was 53% in the febuxostat 80 mg group compared with 21% in the allopurinol 300 mg group (P<.001; number needed to treat [NNT]=4]).
One significant limitation of both RCTs was the fixed dose of allopurinol (300 mg/d). US Food and Drug Administration-approved dosing for allopurinol allows for titration to a maximum of 800 mg/d to achieve serum uric acid <6 mg/dL.
IV pegloticase decreases gout attacks after 3 months, improves quality of life
Pegloticase is an intravenously administered, recombinant form of uricase, the natural enzyme that converts uric acid to more soluble allantoin. Two RCTs compared pegloticase with placebo in a total of 212 patients with gout (mean age 54 to 59 years; 70% to 90% male) intolerant or refractory to allopurinol (defined as baseline sUA of ≥8 mg/dL and at least one of the following: ≥3 self-reported gout flares during the previous 18 months, ≥1 tophi, or gouty arthropathy.
These trials found that treatment with 8 mg of pegloticase every 2 weeks for 6 months initially increased gout flares during Months 1 to 3 (75% with pegloticase, 53% with placebo; P=.02; number needed to harm [NNH]=5) but then decreased the incidence of acute gout attacks during Months 4 to 6 (41% with pegloticase, 67% with placebo; P=.007; NNT=4).3 In addition, pegloticase resulted in statistically significant improvements in QOL measured at the final visit using the Health Assessment Questionnaire (HAQ) pain scale, the HAQ-Disability Index, and the 36-item Short Form Health Survey.
Colchicine plus probenecid or allopurinol reduces gout attacks
One small, low-quality RCT (N=38) found that colchicine 0.5 mg administered 3 times daily effectively prevented gout attacks when administered concomitantly with probenecid initiated to lower urate (gout attacks per month in colchicine and placebo-treated patients, respectively, were 0.19±0.05 and 0.48±0.12; P<.05).4
Another RCT that compared allopurinol with and without colchicine showed that coadministration of colchicine 0.6 mg twice daily reduced gout attacks: 33% of patients treated with colchicine experienced a gout flare compared with 77% of placebo-treated patients (P=.008; NNT=3 over 6 months).5
We identified no RCTs that evaluated the uricosuric agent probenecid and no studies that assessed the use of nonsteroidal anti-inflammatory drugs (NSAIDs) to prevent recurrent gout attacks.
RECOMMENDATIONS
The American College of Rheumatology (ACR) guidelines on managing gout recommend allopurinol or febuxostat as first-line pharmacologic urate-lowering therapy, with a goal of reducing sUA to <6 mg/dL. They recommend probenecid as an alternative if contraindications exist or the patient is intolerant to allopurinol and febuxostat.6 The guidelines note that allopurinol doses may exceed 300 mg/d, even in patients with chronic kidney disease.
The ACR recommends anti-inflammatory prophylaxis with colchicine or NSAIDs upon initiation of urate-lowering therapy. Anti-inflammatory prophylaxis should be continued as long as clinical evidence of continuing gout disease exists and until the sUA target has been acheived.7
Allopurinol and febuxostat reduce the frequency of gout attacks equally after 8 weeks of treatment (strength of recommendation [SOR]: B, multiple randomized control trials [RCTs] with limitations).
Intravenous pegloticase decreases serum uric acid and gout attacks and improves quality of life (QOL) (SOR: A, 2 RCTs).
Colchicine reduces gout attacks when combined with probenecid or allopurinol at the start of urate-lowering therapy (SOR: B, 1 high-quality and 1 low-quality RCT).
EVIDENCE SUMMARY
A 28-week RCT compared the effects of placebo, allopurinol (300 mg/d), and febuxostat (80 mg, 120 mg, and 240 mg) on serum uric acid levels (sUA) and gout attacks in 1067 patients with gout and hyperuricemia (94% male, 78% white, 18 to 85 years of age with mean age ranging from 51 to 54 years ± 12 years in each group).1 Patients also received prophylaxis with either colchicine or naproxen during the first 8 weeks of the study.
During Weeks 1 through 8, investigators found no statistically significant differences in the percentage of patients requiring treatment for gout attacks between the febuxostat 80 mg, allopurinol, and placebo groups (28%, 23%, and 20%, respectively). During Weeks 8 through 28, no statistically significant differences in gout attack rates occurred between the allopurinol and febuxostat groups, although the study didn’t report specific attack rates for this period.
Both allopurinol and all doses of febuxostat reduced sUA to <6 mg/dL more effectively than placebo; more patients treated with febuxostat than allopurinol achieved a uric acid level of less than <6 mg/dL.
Another RCT of 762 mostly white, male patients (mean age 52 years) with gout and sUA >8 mg/dL—35% of whom had renal impairment, defined as creatinine clearance <80 mL/min/1.73m2—also concluded that febuxostat and allopurinol are equally effective in reducing gout attacks (incidence of gout flares during Weeks 9 to 52 was 64% with both febuxostat 80 mg and allopurinol 300 mg).2 The percentage of patients with sUA <6 mg/dL at the last 3 monthly visits was 53% in the febuxostat 80 mg group compared with 21% in the allopurinol 300 mg group (P<.001; number needed to treat [NNT]=4]).
One significant limitation of both RCTs was the fixed dose of allopurinol (300 mg/d). US Food and Drug Administration-approved dosing for allopurinol allows for titration to a maximum of 800 mg/d to achieve serum uric acid <6 mg/dL.
IV pegloticase decreases gout attacks after 3 months, improves quality of life
Pegloticase is an intravenously administered, recombinant form of uricase, the natural enzyme that converts uric acid to more soluble allantoin. Two RCTs compared pegloticase with placebo in a total of 212 patients with gout (mean age 54 to 59 years; 70% to 90% male) intolerant or refractory to allopurinol (defined as baseline sUA of ≥8 mg/dL and at least one of the following: ≥3 self-reported gout flares during the previous 18 months, ≥1 tophi, or gouty arthropathy.
These trials found that treatment with 8 mg of pegloticase every 2 weeks for 6 months initially increased gout flares during Months 1 to 3 (75% with pegloticase, 53% with placebo; P=.02; number needed to harm [NNH]=5) but then decreased the incidence of acute gout attacks during Months 4 to 6 (41% with pegloticase, 67% with placebo; P=.007; NNT=4).3 In addition, pegloticase resulted in statistically significant improvements in QOL measured at the final visit using the Health Assessment Questionnaire (HAQ) pain scale, the HAQ-Disability Index, and the 36-item Short Form Health Survey.
Colchicine plus probenecid or allopurinol reduces gout attacks
One small, low-quality RCT (N=38) found that colchicine 0.5 mg administered 3 times daily effectively prevented gout attacks when administered concomitantly with probenecid initiated to lower urate (gout attacks per month in colchicine and placebo-treated patients, respectively, were 0.19±0.05 and 0.48±0.12; P<.05).4
Another RCT that compared allopurinol with and without colchicine showed that coadministration of colchicine 0.6 mg twice daily reduced gout attacks: 33% of patients treated with colchicine experienced a gout flare compared with 77% of placebo-treated patients (P=.008; NNT=3 over 6 months).5
We identified no RCTs that evaluated the uricosuric agent probenecid and no studies that assessed the use of nonsteroidal anti-inflammatory drugs (NSAIDs) to prevent recurrent gout attacks.
RECOMMENDATIONS
The American College of Rheumatology (ACR) guidelines on managing gout recommend allopurinol or febuxostat as first-line pharmacologic urate-lowering therapy, with a goal of reducing sUA to <6 mg/dL. They recommend probenecid as an alternative if contraindications exist or the patient is intolerant to allopurinol and febuxostat.6 The guidelines note that allopurinol doses may exceed 300 mg/d, even in patients with chronic kidney disease.
The ACR recommends anti-inflammatory prophylaxis with colchicine or NSAIDs upon initiation of urate-lowering therapy. Anti-inflammatory prophylaxis should be continued as long as clinical evidence of continuing gout disease exists and until the sUA target has been acheived.7
1. Schumacher HR Jr, Becker MA, Wortmann RL, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008;59:1540-1548.
2. Becker MA, Schumacher HR Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450-2461.
3. Sundy JS, Baraf HSB, Yood RA, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA. 2011;306:711-720.
4. Paulus HE, Schlosstein LH, Godfrey RG, et al. Prophylactic colchicine therapy of intercritical gout: a placebo-controlled study of probenecid-treated patients. Arthritis Rheum. 1974;17:609-614.
5. Borstad GC, Bryant LR, Abel MP, et al. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol. 2004;31:2429-2432.
6. Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64:1431-1446.
7. Khanna D, Khanna PP, Fitzgerald JD, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64:1447-1461.
1. Schumacher HR Jr, Becker MA, Wortmann RL, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum. 2008;59:1540-1548.
2. Becker MA, Schumacher HR Jr, Wortmann RL, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med. 2005;353:2450-2461.
3. Sundy JS, Baraf HSB, Yood RA, et al. Efficacy and tolerability of pegloticase for the treatment of chronic gout in patients refractory to conventional treatment: two randomized controlled trials. JAMA. 2011;306:711-720.
4. Paulus HE, Schlosstein LH, Godfrey RG, et al. Prophylactic colchicine therapy of intercritical gout: a placebo-controlled study of probenecid-treated patients. Arthritis Rheum. 1974;17:609-614.
5. Borstad GC, Bryant LR, Abel MP, et al. Colchicine for prophylaxis of acute flares when initiating allopurinol for chronic gouty arthritis. J Rheumatol. 2004;31:2429-2432.
6. Khanna D, Fitzgerald JD, Khanna PP, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64:1431-1446.
7. Khanna D, Khanna PP, Fitzgerald JD, et al; American College of Rheumatology. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res (Hoboken). 2012;64:1447-1461.
Evidence-based answers from the Family Physicians Inquiries Network
ADHD medication is not working
Welcome to a new column designed to provide practical advice regarding issues related to child mental health. It will be a joint effort, featuring contributions from several child psychiatrists working at the University of Vermont and the Vermont Center for Children, Youth, and Families. While psychopharmacology will certainly be a part of many of the columns, all of us here feel strongly that medications should be only one part of a comprehensive family-oriented plan. We encourage you to submit questions that you would like us address in future issues to [email protected].
Case summary
A 10-year-old boy presents for a follow-up appointment. He was diagnosed by another pediatrician in the practice 2 months ago with attention-deficit/hyperactivity disorder (ADHD) and now returns to the office with continued symptoms and a complaint from the mother that medication "isn’t working." The boy was started on an extended-release preparation of methylphenidate at 18 mg to take each morning. The child is in the fifth grade and weighs 80 lb (36 kg). He lives with his mother and 8-year-old brother. The father is no longer involved in the patient’s life, which puts added stress on the mother. The diagnosis of ADHD was made by the pediatrician based upon the history, the child’s hyperactive and intrusive behavior in the office, and the results of a standardized rating scale that was completed by the mother, who now requests that the pediatrician "try something different."
Discussion
Many children and adolescents respond extremely well to ADHD medications. Some, however, do not, and the parental complaint that the "medication isn’t working" is a frequent expression heard in pediatrician offices across the country. It is also one of the primary reasons a family is referred to a child psychiatrist. In the course of performing hundreds of these consultations, I have found that there are several possibilities to consider before assuming the medication simply isn’t effective.
We will start with simpler problems and work our way toward more challenging reasons.
• The dose is too low. Methylphenidate often needs to be dosed over 1 mg/kg/day to be effective. If the patient reports minimal response to the medication while experiencing no side effects, an increase may certainly be reasonable.
• The medication is working but wearing off. Despite the advertisements of long-acting stimulants continuing their therapeutic effect for 10-12 hours, many children seem to lose the benefit of the medication much faster. Gathering some data from the school or asking the mother about weekend mornings compared with evenings can be useful. If indeed such a wear-off is found, adding a dose of an immediate-release stimulant in the early afternoon may help.
• Symptoms are being caused by something other than ADHD. Hyperactivity due to exposures such as lead may not change your management of the symptoms, but certainly could necessitate other types of intervention. Chronic sleep problems and inadequate nutrition, especially when it comes to breakfast, also should be queried and can lead to problems with concentration.
• There is psychiatric comorbidity. Unlike many differentials in other specialties, psychiatric differential diagnosis is often a matter of "and" rather than "or." Anxiety disorders, for example, can frequently masquerade as ADHD or be present in addition to ADHD. Oppositional behavior is also very commonly present with ADHD and suggests additional types of treatment.
• There is noncompliance. This problem can surface frequently in two ways. Older children may be responsible at home for taking their medications and forget or refuse to do so. I often ask, "Are you taking the medication every single day?" Diversion is also a potential problem from the parents or for an adolescent. Checking if the refills are occurring on time can provide a clue here, and some states have systems to check for duplicate prescriptions from multiple clinicians.
• Side effects are appearing as untreated ADHD. Sometimes medications are the problem, not the solution, and a failure to recognize this phenomenon can lead to unnecessary and sometimes harmful polypharmacy. Stimulants in some children can lead to increased agitation, anger outbursts, and impulsivity. Trying a medication holiday for several days can sometimes reveal the need to back off rather than add medications.
• Family is expecting improvement for non-ADHD symptoms. Asking what particular behaviors the family is hoping to improve can sometimes expose a situation in which parents expect change in non-ADHD domains. Unfortunately, there is no pill to make kids respect their parents more or want to do their homework. Being clear from the outset about what behaviors are and are not medication responsive can sometimes prevent this problem.
• There is substance abuse. In addition to the potential problem of abuse of the stimulants described previously, other substances such as cannabis can sabotage the benefits of medications.
• There is over-reliance on medications as the sole modality of treatment. ADHD is best treated using a wide range of strategies. Nonpharmacological interventions such as exercise, good nutrition and sleep, parent behavioral training, organizational help, regular reading, screen time reduction, and school supports are critical components of a comprehensive treatment approach.
• There is parental psychopathology. In our opinion, this area is one of the most frequently neglected aspects of child mental health treatment and can have huge implications. ADHD in particular is known to have very high heritability (similar to height). If a mother or father shares the condition, their struggles can frequently contribute to an environment that can exacerbate the child’s symptoms. A pattern in which the ADHD symptoms are more prominent at home compared with school is one clue to look in this direction. When addressing parental psychopathology, it can be important not to come off as blaming the parents for their child’s problems, but rather to convey how challenging dealing with ADHD can be as a parent and how they need to be functioning at their highest mental level as well.
Of course, sometimes the medication truly is not working, and it is time to try something else.
Dr. David C. Rettew is associate professor of psychiatry and pediatrics, director of the child and adolescent psychiatry fellowship, and director of the pediatric psychiatry clinic at the University of Vermont, Burlington.
Welcome to a new column designed to provide practical advice regarding issues related to child mental health. It will be a joint effort, featuring contributions from several child psychiatrists working at the University of Vermont and the Vermont Center for Children, Youth, and Families. While psychopharmacology will certainly be a part of many of the columns, all of us here feel strongly that medications should be only one part of a comprehensive family-oriented plan. We encourage you to submit questions that you would like us address in future issues to [email protected].
Case summary
A 10-year-old boy presents for a follow-up appointment. He was diagnosed by another pediatrician in the practice 2 months ago with attention-deficit/hyperactivity disorder (ADHD) and now returns to the office with continued symptoms and a complaint from the mother that medication "isn’t working." The boy was started on an extended-release preparation of methylphenidate at 18 mg to take each morning. The child is in the fifth grade and weighs 80 lb (36 kg). He lives with his mother and 8-year-old brother. The father is no longer involved in the patient’s life, which puts added stress on the mother. The diagnosis of ADHD was made by the pediatrician based upon the history, the child’s hyperactive and intrusive behavior in the office, and the results of a standardized rating scale that was completed by the mother, who now requests that the pediatrician "try something different."
Discussion
Many children and adolescents respond extremely well to ADHD medications. Some, however, do not, and the parental complaint that the "medication isn’t working" is a frequent expression heard in pediatrician offices across the country. It is also one of the primary reasons a family is referred to a child psychiatrist. In the course of performing hundreds of these consultations, I have found that there are several possibilities to consider before assuming the medication simply isn’t effective.
We will start with simpler problems and work our way toward more challenging reasons.
• The dose is too low. Methylphenidate often needs to be dosed over 1 mg/kg/day to be effective. If the patient reports minimal response to the medication while experiencing no side effects, an increase may certainly be reasonable.
• The medication is working but wearing off. Despite the advertisements of long-acting stimulants continuing their therapeutic effect for 10-12 hours, many children seem to lose the benefit of the medication much faster. Gathering some data from the school or asking the mother about weekend mornings compared with evenings can be useful. If indeed such a wear-off is found, adding a dose of an immediate-release stimulant in the early afternoon may help.
• Symptoms are being caused by something other than ADHD. Hyperactivity due to exposures such as lead may not change your management of the symptoms, but certainly could necessitate other types of intervention. Chronic sleep problems and inadequate nutrition, especially when it comes to breakfast, also should be queried and can lead to problems with concentration.
• There is psychiatric comorbidity. Unlike many differentials in other specialties, psychiatric differential diagnosis is often a matter of "and" rather than "or." Anxiety disorders, for example, can frequently masquerade as ADHD or be present in addition to ADHD. Oppositional behavior is also very commonly present with ADHD and suggests additional types of treatment.
• There is noncompliance. This problem can surface frequently in two ways. Older children may be responsible at home for taking their medications and forget or refuse to do so. I often ask, "Are you taking the medication every single day?" Diversion is also a potential problem from the parents or for an adolescent. Checking if the refills are occurring on time can provide a clue here, and some states have systems to check for duplicate prescriptions from multiple clinicians.
• Side effects are appearing as untreated ADHD. Sometimes medications are the problem, not the solution, and a failure to recognize this phenomenon can lead to unnecessary and sometimes harmful polypharmacy. Stimulants in some children can lead to increased agitation, anger outbursts, and impulsivity. Trying a medication holiday for several days can sometimes reveal the need to back off rather than add medications.
• Family is expecting improvement for non-ADHD symptoms. Asking what particular behaviors the family is hoping to improve can sometimes expose a situation in which parents expect change in non-ADHD domains. Unfortunately, there is no pill to make kids respect their parents more or want to do their homework. Being clear from the outset about what behaviors are and are not medication responsive can sometimes prevent this problem.
• There is substance abuse. In addition to the potential problem of abuse of the stimulants described previously, other substances such as cannabis can sabotage the benefits of medications.
• There is over-reliance on medications as the sole modality of treatment. ADHD is best treated using a wide range of strategies. Nonpharmacological interventions such as exercise, good nutrition and sleep, parent behavioral training, organizational help, regular reading, screen time reduction, and school supports are critical components of a comprehensive treatment approach.
• There is parental psychopathology. In our opinion, this area is one of the most frequently neglected aspects of child mental health treatment and can have huge implications. ADHD in particular is known to have very high heritability (similar to height). If a mother or father shares the condition, their struggles can frequently contribute to an environment that can exacerbate the child’s symptoms. A pattern in which the ADHD symptoms are more prominent at home compared with school is one clue to look in this direction. When addressing parental psychopathology, it can be important not to come off as blaming the parents for their child’s problems, but rather to convey how challenging dealing with ADHD can be as a parent and how they need to be functioning at their highest mental level as well.
Of course, sometimes the medication truly is not working, and it is time to try something else.
Dr. David C. Rettew is associate professor of psychiatry and pediatrics, director of the child and adolescent psychiatry fellowship, and director of the pediatric psychiatry clinic at the University of Vermont, Burlington.
Welcome to a new column designed to provide practical advice regarding issues related to child mental health. It will be a joint effort, featuring contributions from several child psychiatrists working at the University of Vermont and the Vermont Center for Children, Youth, and Families. While psychopharmacology will certainly be a part of many of the columns, all of us here feel strongly that medications should be only one part of a comprehensive family-oriented plan. We encourage you to submit questions that you would like us address in future issues to [email protected].
Case summary
A 10-year-old boy presents for a follow-up appointment. He was diagnosed by another pediatrician in the practice 2 months ago with attention-deficit/hyperactivity disorder (ADHD) and now returns to the office with continued symptoms and a complaint from the mother that medication "isn’t working." The boy was started on an extended-release preparation of methylphenidate at 18 mg to take each morning. The child is in the fifth grade and weighs 80 lb (36 kg). He lives with his mother and 8-year-old brother. The father is no longer involved in the patient’s life, which puts added stress on the mother. The diagnosis of ADHD was made by the pediatrician based upon the history, the child’s hyperactive and intrusive behavior in the office, and the results of a standardized rating scale that was completed by the mother, who now requests that the pediatrician "try something different."
Discussion
Many children and adolescents respond extremely well to ADHD medications. Some, however, do not, and the parental complaint that the "medication isn’t working" is a frequent expression heard in pediatrician offices across the country. It is also one of the primary reasons a family is referred to a child psychiatrist. In the course of performing hundreds of these consultations, I have found that there are several possibilities to consider before assuming the medication simply isn’t effective.
We will start with simpler problems and work our way toward more challenging reasons.
• The dose is too low. Methylphenidate often needs to be dosed over 1 mg/kg/day to be effective. If the patient reports minimal response to the medication while experiencing no side effects, an increase may certainly be reasonable.
• The medication is working but wearing off. Despite the advertisements of long-acting stimulants continuing their therapeutic effect for 10-12 hours, many children seem to lose the benefit of the medication much faster. Gathering some data from the school or asking the mother about weekend mornings compared with evenings can be useful. If indeed such a wear-off is found, adding a dose of an immediate-release stimulant in the early afternoon may help.
• Symptoms are being caused by something other than ADHD. Hyperactivity due to exposures such as lead may not change your management of the symptoms, but certainly could necessitate other types of intervention. Chronic sleep problems and inadequate nutrition, especially when it comes to breakfast, also should be queried and can lead to problems with concentration.
• There is psychiatric comorbidity. Unlike many differentials in other specialties, psychiatric differential diagnosis is often a matter of "and" rather than "or." Anxiety disorders, for example, can frequently masquerade as ADHD or be present in addition to ADHD. Oppositional behavior is also very commonly present with ADHD and suggests additional types of treatment.
• There is noncompliance. This problem can surface frequently in two ways. Older children may be responsible at home for taking their medications and forget or refuse to do so. I often ask, "Are you taking the medication every single day?" Diversion is also a potential problem from the parents or for an adolescent. Checking if the refills are occurring on time can provide a clue here, and some states have systems to check for duplicate prescriptions from multiple clinicians.
• Side effects are appearing as untreated ADHD. Sometimes medications are the problem, not the solution, and a failure to recognize this phenomenon can lead to unnecessary and sometimes harmful polypharmacy. Stimulants in some children can lead to increased agitation, anger outbursts, and impulsivity. Trying a medication holiday for several days can sometimes reveal the need to back off rather than add medications.
• Family is expecting improvement for non-ADHD symptoms. Asking what particular behaviors the family is hoping to improve can sometimes expose a situation in which parents expect change in non-ADHD domains. Unfortunately, there is no pill to make kids respect their parents more or want to do their homework. Being clear from the outset about what behaviors are and are not medication responsive can sometimes prevent this problem.
• There is substance abuse. In addition to the potential problem of abuse of the stimulants described previously, other substances such as cannabis can sabotage the benefits of medications.
• There is over-reliance on medications as the sole modality of treatment. ADHD is best treated using a wide range of strategies. Nonpharmacological interventions such as exercise, good nutrition and sleep, parent behavioral training, organizational help, regular reading, screen time reduction, and school supports are critical components of a comprehensive treatment approach.
• There is parental psychopathology. In our opinion, this area is one of the most frequently neglected aspects of child mental health treatment and can have huge implications. ADHD in particular is known to have very high heritability (similar to height). If a mother or father shares the condition, their struggles can frequently contribute to an environment that can exacerbate the child’s symptoms. A pattern in which the ADHD symptoms are more prominent at home compared with school is one clue to look in this direction. When addressing parental psychopathology, it can be important not to come off as blaming the parents for their child’s problems, but rather to convey how challenging dealing with ADHD can be as a parent and how they need to be functioning at their highest mental level as well.
Of course, sometimes the medication truly is not working, and it is time to try something else.
Dr. David C. Rettew is associate professor of psychiatry and pediatrics, director of the child and adolescent psychiatry fellowship, and director of the pediatric psychiatry clinic at the University of Vermont, Burlington.
How can we effectively treat stress urinary incontinence without drugs or surgery?
Pelvic floor muscle training (PFMT) and intravaginal electrical stimulation seem to be the best bets. PFMT increases urinary continence and improves symptoms of stress urinary incontinence (SUI) (strength of recommendation [SOR]: A, systematic review or randomized, controlled trials [RCTs]). PFMT also improves quality of life (QOL) (activity and psychological impact) (SOR: B, 1 RCT).
Intravaginal electrical stimulation increases urinary continence and improves SUI symptoms; percutaneous electrical stimulation also improves SUI symptoms and likely improves QOL measures (SOR: A, systematic review).
Magnetic stimulation doesn’t increase continence, has mixed effects on SUI symptoms, and produces no clinically meaningful improvement in QOL (SOR: B, heterogeneous RCTs with conflicting results). Vaginal cones don’t increase continence or QOL (SOR: B, 2 RCTs with methodologic flaws).
EVIDENCE SUMMARY
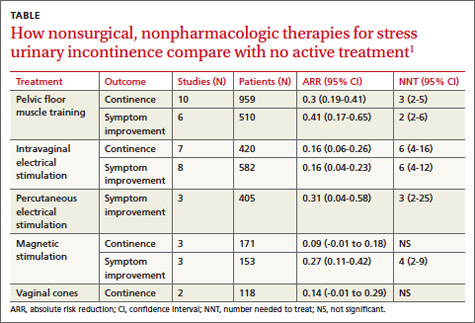
A systematic review by the Agency for Healthcare Research and Quality of adult female outpatients with SUI examined the effectiveness of PFMT, electrical stimulation, magnetic stimulation, and vaginal cones compared with no active treatment or sham treatment to produce continence (90% to 100% symptom reduction) or improve symptoms (at least 50% patient-reported symptom reduction).1 The TABLE summarizes the results.1 Investigators also assessed improvement in patient-reported QOL.
Pelvic floor muscle training improves continence, quality of life
A meta-analysis of 10 RCTs demonstrated that PFMT produced continence more often than placebo, and a meta-analysis of 6 RCTs found that PFMT improved SUI symptoms.1 PFMT regimens ranged in duration from 8 weeks to 6 months, including unsupervised treatment (8 to 12 repetitions, 3 to 10 times a day) and supervised treatment (as long as an hour, as often as 3 times a week).1
Both unsupervised and supervised PFMT produced similar results. One RCT evaluating QOL measures found that PFMT improved activity and reduced psychological impact (number needed to treat [NNT]=1; 95% confidence interval [CI], 1-2).1
Intravaginal electrical stimulation improves continence and symptoms
A meta-analysis of 7 RCTs found that intravaginal electrical stimulation increased continence compared with sham treatment.1 A meta-analysis of 8 RCTs found that intravaginal electrical stimulation also improved SUI symptoms.1 All of the trials used electrical stimulation at frequencies between 4 and 50 Hz for 15 to 20 minutes, 1 to 3 times daily for 4 to 15 weeks.
Percutaneous electrical stimulation improves symptoms
A meta-analysis of 3 RCTs found that percutaneous electrical stimulation improved SUI symptoms compared with no active treatment. Four RCTs found that electrical stimulation improved QOL, although a meta-analysis couldn’t be performed because of clinical heterogeneity.1
Magnetic stimulation produces conflicting results
A meta-analysis of 3 RCTs found that magnetic stimulation at frequencies of 10 to 18.5 Hz given over 1 to 8 weeks didn’t increase continence. A meta-analysis of an additional 3 RCTs concluded that magnetic stimulation improved continence, but the individual studies reported conflicting results and were heterogenous.1
Two RCTs evaluating QOL scores found conflicting results. One study found a mean difference of 3.9 points on the 100-point Incontinence Quality of Life Questionnaire (95% CI, 2.08-5.72; minimal clinically important difference rated 2-5 points).1
Vaginal cones are ineffective and not well-tolerated
Two RCTs found that vaginal cones didn’t improve continence or QOL compared with no treatment. Investigators reported high discontinuation rates and adverse effects with the cones, which weighed 20 to 70 g and were worn for 20 minutes a day for as long as 24 weeks.1
RECOMMENDATIONS
The National Institute for Health and Care Excellence recommends PFMT comprising at least 8 contractions 3 times daily for at least 3 months as first-line therapy for women with SUI.2 They don’t recommend electrical stimulation or intravaginal devices for women who can actively contract their pelvic floor muscles. The American College of Obstetricians and Gynecologists recommends PFMT as first-line therapy for women with SUI and states that PFMT is more effective than electrical stimulation or vaginal cones.3
1. Nonsurgical treatments for urinary incontinence in adult women: Diagnosis and comparative effectiveness. Executive summary. Agency for Healthcare Research and Quality Web site. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/169/1021/CER36_Urinary-Incontinence_execsumm.pdf. Accessed March 19, 2014.
2. Urinary Incontinence: The management of urinary incontinence in women. NICE Clinical Guideline 171. London: NICE; 2006. National Institute for Health and Care Excellence Web site. Available at: www.nice.org.uk/CG171. Accessed March 19, 2014.
3. American College of Obstetricians and Gynecologists. Urinary incontinence in women. Obstet Gynecol. 2005;105:1533-1545.
AHIP; stress urinary incontinence; SUI; pelvic floor muscle training; PFMT; intravaginal electrical stimulation
Pelvic floor muscle training (PFMT) and intravaginal electrical stimulation seem to be the best bets. PFMT increases urinary continence and improves symptoms of stress urinary incontinence (SUI) (strength of recommendation [SOR]: A, systematic review or randomized, controlled trials [RCTs]). PFMT also improves quality of life (QOL) (activity and psychological impact) (SOR: B, 1 RCT).
Intravaginal electrical stimulation increases urinary continence and improves SUI symptoms; percutaneous electrical stimulation also improves SUI symptoms and likely improves QOL measures (SOR: A, systematic review).
Magnetic stimulation doesn’t increase continence, has mixed effects on SUI symptoms, and produces no clinically meaningful improvement in QOL (SOR: B, heterogeneous RCTs with conflicting results). Vaginal cones don’t increase continence or QOL (SOR: B, 2 RCTs with methodologic flaws).
EVIDENCE SUMMARY
A systematic review by the Agency for Healthcare Research and Quality of adult female outpatients with SUI examined the effectiveness of PFMT, electrical stimulation, magnetic stimulation, and vaginal cones compared with no active treatment or sham treatment to produce continence (90% to 100% symptom reduction) or improve symptoms (at least 50% patient-reported symptom reduction).1 The TABLE summarizes the results.1 Investigators also assessed improvement in patient-reported QOL.
Pelvic floor muscle training improves continence, quality of life
A meta-analysis of 10 RCTs demonstrated that PFMT produced continence more often than placebo, and a meta-analysis of 6 RCTs found that PFMT improved SUI symptoms.1 PFMT regimens ranged in duration from 8 weeks to 6 months, including unsupervised treatment (8 to 12 repetitions, 3 to 10 times a day) and supervised treatment (as long as an hour, as often as 3 times a week).1
Both unsupervised and supervised PFMT produced similar results. One RCT evaluating QOL measures found that PFMT improved activity and reduced psychological impact (number needed to treat [NNT]=1; 95% confidence interval [CI], 1-2).1
Intravaginal electrical stimulation improves continence and symptoms
A meta-analysis of 7 RCTs found that intravaginal electrical stimulation increased continence compared with sham treatment.1 A meta-analysis of 8 RCTs found that intravaginal electrical stimulation also improved SUI symptoms.1 All of the trials used electrical stimulation at frequencies between 4 and 50 Hz for 15 to 20 minutes, 1 to 3 times daily for 4 to 15 weeks.
Percutaneous electrical stimulation improves symptoms
A meta-analysis of 3 RCTs found that percutaneous electrical stimulation improved SUI symptoms compared with no active treatment. Four RCTs found that electrical stimulation improved QOL, although a meta-analysis couldn’t be performed because of clinical heterogeneity.1

Magnetic stimulation produces conflicting results
A meta-analysis of 3 RCTs found that magnetic stimulation at frequencies of 10 to 18.5 Hz given over 1 to 8 weeks didn’t increase continence. A meta-analysis of an additional 3 RCTs concluded that magnetic stimulation improved continence, but the individual studies reported conflicting results and were heterogenous.1
Two RCTs evaluating QOL scores found conflicting results. One study found a mean difference of 3.9 points on the 100-point Incontinence Quality of Life Questionnaire (95% CI, 2.08-5.72; minimal clinically important difference rated 2-5 points).1
Vaginal cones are ineffective and not well-tolerated
Two RCTs found that vaginal cones didn’t improve continence or QOL compared with no treatment. Investigators reported high discontinuation rates and adverse effects with the cones, which weighed 20 to 70 g and were worn for 20 minutes a day for as long as 24 weeks.1
RECOMMENDATIONS
The National Institute for Health and Care Excellence recommends PFMT comprising at least 8 contractions 3 times daily for at least 3 months as first-line therapy for women with SUI.2 They don’t recommend electrical stimulation or intravaginal devices for women who can actively contract their pelvic floor muscles. The American College of Obstetricians and Gynecologists recommends PFMT as first-line therapy for women with SUI and states that PFMT is more effective than electrical stimulation or vaginal cones.3
Pelvic floor muscle training (PFMT) and intravaginal electrical stimulation seem to be the best bets. PFMT increases urinary continence and improves symptoms of stress urinary incontinence (SUI) (strength of recommendation [SOR]: A, systematic review or randomized, controlled trials [RCTs]). PFMT also improves quality of life (QOL) (activity and psychological impact) (SOR: B, 1 RCT).
Intravaginal electrical stimulation increases urinary continence and improves SUI symptoms; percutaneous electrical stimulation also improves SUI symptoms and likely improves QOL measures (SOR: A, systematic review).
Magnetic stimulation doesn’t increase continence, has mixed effects on SUI symptoms, and produces no clinically meaningful improvement in QOL (SOR: B, heterogeneous RCTs with conflicting results). Vaginal cones don’t increase continence or QOL (SOR: B, 2 RCTs with methodologic flaws).
EVIDENCE SUMMARY
A systematic review by the Agency for Healthcare Research and Quality of adult female outpatients with SUI examined the effectiveness of PFMT, electrical stimulation, magnetic stimulation, and vaginal cones compared with no active treatment or sham treatment to produce continence (90% to 100% symptom reduction) or improve symptoms (at least 50% patient-reported symptom reduction).1 The TABLE summarizes the results.1 Investigators also assessed improvement in patient-reported QOL.
Pelvic floor muscle training improves continence, quality of life
A meta-analysis of 10 RCTs demonstrated that PFMT produced continence more often than placebo, and a meta-analysis of 6 RCTs found that PFMT improved SUI symptoms.1 PFMT regimens ranged in duration from 8 weeks to 6 months, including unsupervised treatment (8 to 12 repetitions, 3 to 10 times a day) and supervised treatment (as long as an hour, as often as 3 times a week).1
Both unsupervised and supervised PFMT produced similar results. One RCT evaluating QOL measures found that PFMT improved activity and reduced psychological impact (number needed to treat [NNT]=1; 95% confidence interval [CI], 1-2).1
Intravaginal electrical stimulation improves continence and symptoms
A meta-analysis of 7 RCTs found that intravaginal electrical stimulation increased continence compared with sham treatment.1 A meta-analysis of 8 RCTs found that intravaginal electrical stimulation also improved SUI symptoms.1 All of the trials used electrical stimulation at frequencies between 4 and 50 Hz for 15 to 20 minutes, 1 to 3 times daily for 4 to 15 weeks.
Percutaneous electrical stimulation improves symptoms
A meta-analysis of 3 RCTs found that percutaneous electrical stimulation improved SUI symptoms compared with no active treatment. Four RCTs found that electrical stimulation improved QOL, although a meta-analysis couldn’t be performed because of clinical heterogeneity.1

Magnetic stimulation produces conflicting results
A meta-analysis of 3 RCTs found that magnetic stimulation at frequencies of 10 to 18.5 Hz given over 1 to 8 weeks didn’t increase continence. A meta-analysis of an additional 3 RCTs concluded that magnetic stimulation improved continence, but the individual studies reported conflicting results and were heterogenous.1
Two RCTs evaluating QOL scores found conflicting results. One study found a mean difference of 3.9 points on the 100-point Incontinence Quality of Life Questionnaire (95% CI, 2.08-5.72; minimal clinically important difference rated 2-5 points).1
Vaginal cones are ineffective and not well-tolerated
Two RCTs found that vaginal cones didn’t improve continence or QOL compared with no treatment. Investigators reported high discontinuation rates and adverse effects with the cones, which weighed 20 to 70 g and were worn for 20 minutes a day for as long as 24 weeks.1
RECOMMENDATIONS
The National Institute for Health and Care Excellence recommends PFMT comprising at least 8 contractions 3 times daily for at least 3 months as first-line therapy for women with SUI.2 They don’t recommend electrical stimulation or intravaginal devices for women who can actively contract their pelvic floor muscles. The American College of Obstetricians and Gynecologists recommends PFMT as first-line therapy for women with SUI and states that PFMT is more effective than electrical stimulation or vaginal cones.3
1. Nonsurgical treatments for urinary incontinence in adult women: Diagnosis and comparative effectiveness. Executive summary. Agency for Healthcare Research and Quality Web site. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/169/1021/CER36_Urinary-Incontinence_execsumm.pdf. Accessed March 19, 2014.
2. Urinary Incontinence: The management of urinary incontinence in women. NICE Clinical Guideline 171. London: NICE; 2006. National Institute for Health and Care Excellence Web site. Available at: www.nice.org.uk/CG171. Accessed March 19, 2014.
3. American College of Obstetricians and Gynecologists. Urinary incontinence in women. Obstet Gynecol. 2005;105:1533-1545.
1. Nonsurgical treatments for urinary incontinence in adult women: Diagnosis and comparative effectiveness. Executive summary. Agency for Healthcare Research and Quality Web site. Available at: http://effectivehealthcare.ahrq.gov/ehc/products/169/1021/CER36_Urinary-Incontinence_execsumm.pdf. Accessed March 19, 2014.
2. Urinary Incontinence: The management of urinary incontinence in women. NICE Clinical Guideline 171. London: NICE; 2006. National Institute for Health and Care Excellence Web site. Available at: www.nice.org.uk/CG171. Accessed March 19, 2014.
3. American College of Obstetricians and Gynecologists. Urinary incontinence in women. Obstet Gynecol. 2005;105:1533-1545.
AHIP; stress urinary incontinence; SUI; pelvic floor muscle training; PFMT; intravaginal electrical stimulation
AHIP; stress urinary incontinence; SUI; pelvic floor muscle training; PFMT; intravaginal electrical stimulation
Evidence-based answers from the Family Physicians Inquiries Network
Pitfalls & pearls for 8 common lab tests
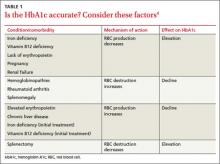
› When interpreting hemoglobin A1c (HbA1c) levels, assess for anemia and other comorbidities that can significantly affect the lifespan of red blood cells and skew HbA1c test results. B
› Order nonfasting lipid panels for patients for whom fasting laboratory tests are difficult to obtain, as they have good clinical utility in screening and initial treatment. A
› Avoid routine thyroid-stimulating hormone (TSH) testing in asymptomatic adults; when testing is indicated, start with TSH. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Laboratory mistakes are not defined as diagnostic errors, but they contribute significantly to the thousands of medical errors that occur every year.1 Part of the problem: While accurate interpretation of lab tests often depends on the use of statistical concepts we all learned in medical training, it is difficult to find the time to incorporate these principles into a busy practice.
Overuse of lab tests presents problems, as well. Because “normal ranges” for test results are based on statistical analysis, as many as 5% of patients in a standard distribution fall outside of the range.2 It is important to order only the tests you really need, as extra testing automatically means more false positive results.
This article was written with such pitfalls in mind. In the pages that follow, we focus on 8 types of tests family physicians rely on regularly—all cases in which test results are reliable only if comorbidities, pre- and post-test probabilities, and clinical context are carefully considered. To help you put these lab tests into the proper context, we’ve addressed a key question—and highlighted both pitfalls and pearls—about each.
1. Hemoglobin A1c: How does anemia affect it?
Hemoglobin A1c (HbA1c) can be measured in many ways, including high-performance liquid chromatography, boronate affinity, capillary electrophoresis, and immunoassay, all of which can provide equivalent values without significant variability.3,4 In interpreting these tests, however, it is important to understand the effect that anemia has on HbA1c.
It's important to order only the tests you really need, as extra testing automatically means more false positive results.
Two primary variables influencing HbA1c are the average glucose level and the average lifespan of red blood cells (RBCs). Normally, there is a direct correlation between average serum glucose and HbA1c.4 In patients with anemia, however, this relationship is less clear, and may be affected by erythropoiesis and RBC destruction.5 In iron deficiency anemia (IDA),6,7 hemoglobin production falls secondary to iron stores, resulting in microcytic cells with a longer lifespan and elevated HbA1c. In at least one study,5,7 HbA1c approached levels associated with diabetes (with increases as high as 1.5%) in nondiabetic patients, but resolved with treatment of IDA.
Increased destruction as well as increased production of RBCs lowers their lifespan, and in turn decreases HbA1c levels (TABLE 1).4 This can be seen in conditions such as splenomegaly and hemoglobinopathies. In patients with hemoglobinopathies, the percentage of hemoglobin A is significantly decreased, often to undetectable levels—thereby making HbA1c tests inaccurate. Hemoglobin electrophoresis and determination of glycation by capillary electrophoresis or high-performance liquid chromatography can be used instead, but neither is practical because of cost and limited availability.4,8,9
THE TAKEAWAY: When you evaluate HbA1c test results, it is crucial to assess the patient for anemia and other conditions or comorbidities that can significantly affect RBC lifespan and skew test results.2,4-6
2. D-dimer: When should you use it?
D-dimer is a fibrin degradation product that is increased when active clotting is present,10 and its assay—which has high sensitivity and low specificity—is widely used to screen for pulmonary embolism (PE) and deep vein thrombosis (DVT). While the minimal number of false negatives makes the D-dimer a good screening test, the higher rate of false positives makes it difficult to arrive at a definitive diagnosis. Appropriate use of the D-dimer assay is crucial to minimize the potential for adverse consequences, such as bleeding in patients who are subjected to unnecessary anticoagulation because of false positive results.
Further testing typically follows. A positive D-dimer test is commonly followed by a computed tomography (CT) scan of the chest or a ventilation/perfusion scan to establish a PE or DVT diagnosis. But this subsequent testing increases both the cost of health care and the patient’s radiation exposure. Use of these subsequent scans can be reduced by first considering the patient’s pretest probability for PE or DVT. The Wells’ Criteria (available at www.mdcalc.com/wells-criteriafor-pulmonary-embolism-pe/) and Geneva Score (Revised) (www.mdcalc.com/genevascore-revised-for-pulmonary-embolism/) can both be used for this purpose.10,11
Patients with high pretest probability should undergo immediate scanning, foregoing the D-dimer—which should be reserved for patients who have a low or moderate pretest probability but sufficient reason to suspect PE or DVT.10-12
The low specificity of the D-dimer assay poses another challenge to its effective use. There are many things that can increase D-dimer levels, such as age, cancer, prolonged immobility, autoimmune disease, inflammation, sickle cell disease, pregnancy, trauma, and surgery.13-15 All these factors must be taken into consideration prior to ordering this test.
In fact, one recent study found that using an age-adjusted D-dimer cutoff (patient’s age in years x 10 mcg/L)—rather than a conventional cutoff of 500 mcg/L—for patients older than 50 years reduces false positives without substantially increasing false negatives.16
Also of note: An anticoagulant can decrease D-dimer levels in plasma, so the test should not be used to rule out PE or DVT in patients who are undergoing anticoagulation.13,15
THE TAKEAWAY: In evaluating patients for PE or DVT, use the Wells’ Criteria or Geneva Score (Revised) to determine a patient’s pretest probability of disease. Use the D-dimer assay to safely rule out these conditions in patients with a low or intermediate pretest probability, but go directly to scans for those with a high pretest probability.
3. Lipid panels: How important is fasting?
Patients are often instructed to report for fasting lab studies, specifically for lipid profiles. Traditionally, this had been defined as an 8- to 12-hour period without food.17 In clinical practice, however, this is often misinterpreted by patients, who may be confused about the duration of the fast or unsure about whether to eat or drink immediately before the test.
Studies investigating the effect of meals on lab values have found that triglycerides are consistently elevated postprandially, to a maximum of 12 hours.18-21 The effect of the fasting state on total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol is more controversial; while some postprandial differences have been detected, the clinical relevance is equivocal.18-21
Nonfasting lipid values can offer useful information, particularly in patients who are unwilling or unable to return for fasting labs. The US Preventive Services Task Force (USPSTF) supports this practice.22 Because guidelines for evaluation and treatment are based on fasting lipids, however, fasting lab work should be used, whenever possible, for initiating treatment and monitoring patients with abnormal values. If nonfasting lipids are used, it is crucial to factor in the postprandial effects on triglycerides and the subsequent difficulty of assessing LDL cholesterol levels.
THE TAKEAWAY: The clinical relevance of postprandial vs fasting lipid levels is equivocal. Nonfasting lipid panels have reasonable clinical utility in screening and initial treatment, particularly in cases in which obtaining fasting lab values may be problematic.18,19
4. Mononucleoosis spot test: When should you use it?
The monospot test is a latex assay that causes hemagglutination of horse RBCs in the presence of heterophile antibodies characteristic of infectious mononucleosis.23 The antibodies develop within the first 7 days of onset of symptoms, but do not peak for 2 to 5 weeks.24 As a result, monospot testing yields a high incidence of false negatives during the first 2 weeks of active infection.25 False negatives are also common in patients younger than 14 years. Heterophile antibodies may be present for up to a year after active infection.24
Patients at increased risk for splenic rupture, such as athletes, pose considerable diagnostic difficulty.26 When there is strong clinical suspicion of mononucleosis despite a negative monospot test in such high-risk individuals, follow-up testing is recommended to differentiate it from other mononucleosis-like illnesses (TABLE 2).27 The optimal combination of Epstein-Barr virus (EBV) serologic testing consists of the antibody titration of 4 markers: immunoglobulins M (IgM) and G (IgG) to the viral capsid antigen, IgM to the early antigen, and antibody to Epstein-Barr nuclear antigen (EBNA).28 Acute phase reactants in the setting of an antibody to EBNA could indicate reactivation. A positive test does not exclude other medical causes, however, because up to 20% of patients have acute phase antibodies that persist for years.29
Appropriate diagnosis is important because of the significant morbidity associated with EBV. Risk of splenic injury is greatest between 4 and 21 days after onset of symptoms but persists at 7 weeks,26 so conservative therapy followed by monospot retesting one week later is a reasonable approach.
Mononucleosis or routine tonsillitis? It is important to note that there is no evidence that a positive monospot test will affect the management or outcome of routine tonsillitis, raising questions of the utility of the test in such cases. A better approach: Reserve testing for patients with additional findings—ie, splenomegaly—or whose symptoms have persisted ≥ 2 weeks.
THE TAKEAWAY: Wait at least 2 weeks to conduct monospot testing in patients with routine tonsillitis. If strong clinical suspicion exists, proceed with specific IgM and IgG serologic testing.24,25,27,28
5. Evaluating prescription drug levels: Which factors interfere?
Correct interpretation of lab tests conducted to measure prescription drug levels has major implications with regard to patient safety, particularly for medications with a narrow therapeutic index.
Most drug level tests measure the total concentration, which includes both bound and unbound (free) forms. The unbound forms are the active components of the drug; thus, for an accurate evaluation, it is important to be aware of factors that increase free drug concentration. Chief among them is low protein levels, or hypoalbuminemia.30
Risk factors for hypoalbuminemia include significant burns, advanced age, pregnancy, malnutrition, and human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS).30 HIV/AIDS is a particularly high risk because certain protease inhibitors are highly protein bound.
Drug protein binding is classified as low, moderate, or high. The main proteins involved in the process are albumin, alpha-1-acid glycoprotein, and lipoprotein. Medications that are highly protein bound (>80%) are the most affected by low protein levels: Problems can arise when drugs completely bind to all the available proteins and excess drug availability increases free drug levels.
Medications that are most likely to be affected by a high degree of protein binding include carbamazepine, cyclosporine, mycophenolic acid, phenytoin, protease inhibitors (with the exception of indinavir), tacrolimus, and valproic acid. It is important to consider free levels when you order medication assays for these drugs to avoid misinterpreting the serum levels as being too low-a scenario that raises the risk of drug toxicity and adverse outcomes.30,31
A study of 119 phenytoin samples from 70 patients found significantly higher free phenytoin levels in patients with lower albumin levels.32 Higher free phenytoin levels were also seen in older patients and in those with diminished renal function (creatinine clearance <25 mL/min).32 The degree of protein binding is affected by both the serum drug concentration and the albumin level, with saturable protein binding occurring at higher drug levels.33
Calculate phenytoin levels with this equation. To calculate corrected phenytoin levels in patients with low albumin levels, use the following formula, known as the Sheiner-Tozer equation:34
Concentration adjusted=concentration reported/([adjustment x serum albumin] + 0.1); adjustment=0.2 for creatinine clearance ≥20 or 0.1 for creatinine clearance <20.
Additional causes of misinterpreted drug levels. While hypoalbuminemia plays a major role in the misinterpretation of drug levels, other factors affect serum drug concentration, as well. These include drug-drug interactions, which can significantly increase the concentration of the medications involved, and the timing of the test with regard to medication administration. Digoxin levels, in particular, need to be drawn at least 6 to 8 hours after the last dose is taken to allow for appropriate drug distribution.35
THE TAKEAWAY: It is essential to consider free drug level monitoring in patients who either have hypoalbuminemia or have one or more risk factors for hypoalbuminemia to avoid falsely low estimation of drug levels.36,37
6 Liver function tests: Necessary for patients on statin therapy?
Since statins gained US Food and Drug Administration (FDA) approval, the drugs have been associated with increased liver function tests (LFTs). Indeed, there had been a long-standing belief, based on clinical trials, that by monitoring alanine aminotransferase (ALT) and maintaining it at <3 times the upper limit normal (ULN), hepatotoxicity could be avoided.38 In clinical practice, however, further ALT elevation is frequently allowed based on patient tolerability.
In February 2012, the FDA revised its safety data to reflect this practice.39 The FDA update confirmed that routine LFT monitoring is unnecessary for patients on statins—and that it is not very effective in identifying or preventing liver damage.
Overall, serious hepatotoxicity is very rare, with an incidence ≤2 per 1 million patient-years.39 The National Lipid Association Statin Safety Assessment Task Force recommends repeating LFTs that are 3 to 5 times the ULN within 6 months and continuing with the statin dose if the patient is asymptomatic.38
THE TAKEAWAY: Routine liver function monitoring is not necessary for patients on statins. A better approach: Obtain baseline ALT levels, and repeat the testing only as clinically indicated thereafter.38,39
7. Urine drug screens: Which factors affect their accuracy?
The gold standard for testing for drugs of abuse, urine drug screens (UDS) have good sensitivity and specificity, easy administration, and reasonable cost.40 UDS can detect various narcotics, such as morphine, oxycodone, ,and methadone, and identify other illicit drugs, although which drugs and metabolites are tested for is laboratory- and test-specific.
Cross-reactivity. There are 6 currently available immunoassays, all of which use competitive binding between the sample drug and a drug chemically labeled with an enzyme, radioisotope, or fluorophore. The sample drug and labeled drug compete for substrate binding sites on drug-specific antibodies.41,42 Similar to competitive binding for enzymatic reactions in the body, the substrate binding site can experience cross-reactivity—causing substances other than the drug in question to bind to the immunoglobulin, leading to a false positive result (TABLE 3).43 Other factors that can alter the results include the cutoff value of the test and the absorption, distribution, metabolism, and excretion of the drug.42 Thus, a confirmatory test of gas chromatography-mass spectrometry is recommended before making decisions based on the results of UDS.43-45
Routine screens for patients on chronic opioid therapy. Routine use of UDS in emergency departments is no longer recommended, based on evidence that the results are unlikely to have a significant effect on patient management.46 For patients on chronic opioid therapy, however, routine screening has proven helpful in detecting prescription opioid abuse, illicit drug use, and diversion. Up to 34% of patients on prescription opioids have been found to be using illicit drugs, as well.42
THE TAKEAWAY: Use UDS as a tool in managing patients on chronic opioid therapy, but before acting on results, assess for factors, such as the use of oral or topical medications and the cutoff value of the test, that may be associated with false positive or false negative results.43-45
8. Thyroid function testing: When should you test?
Thyroid-stimulating hormone (TSH) is the first-line test when investigating presumed hyper- or hypothyroidism.47,48 Third-generation chemiluminometric assays can reliably measure TSH concentrations <0.01 mU/L by using multiple antibodies to produce a sandwich-type effect on the molecule in question.49
TSH levels exhibit diurnal variation, however, and are affected by other medications, including steroids, opiates, and some antihistamines, among others, as well as comorbidities.47,48 Chronic and acute conditions unrelated to thyroid disease can cause transient changes in TSH concentrations, and have the potential to modify the binding capacity of plasma thyroid hormone binding proteins.48 Thus, TSH should be ordered for hospitalized patients only when clinical suspicion of a thyroid problem exists.48 The USPSTF recommends against routine TSH screening for asymptomatic adults.46
How to respond to abnormal results. For patients found to have abnormal TSH levels, free T4 (fT4) is the next test to order.47,49 An fT4 assay is a superior indicator of thyroid status because it is not affected by changes in iodothyronine-binding proteins, which influence total hormone measurements.49 The results will be elevated in hyperthyroidism and reduced in hypothyroidism.47
Triiodothyronine (T3) measures can be useful in diagnosing Graves’ disease, in which T3 toxicosis may be the initial symptom—or an indication of a relapse. Because T3 is often a peripheral product, however, nonthyroid illnesses and medications can cause artifactually abnormal results.49
Other thyroid-specific labs include thyroid ,antibodies such as antithyroid peroxidase, antithyroglobulin, and TSH receptor, both blocking and stimulating.49 Thyroglobulin is a precursor form of thyroid hormone and should be measured when factitious hyperthyroidism is suspected. Management of hyper- and hypothyroidism often is independent of etiology. Retesting TSH to assess treatment response should be postponed until ≥2 months after any change in medication or dosing.50
Thyroid studies can be very difficult to interpret. TSH should be the first test ordered. However, if TSH values do not match the clinical picture, fT4, T3, and other thyroid tests that are less affected by outside factors can be useful in identifying the cause.
THE TAKEAWAY: Routine TSH testing is not indicated for asymptomatic adults. When evaluating thyroid function is clinically indicated, TSH is the initial test of choice.47,48,51
CORRESPONDENCE
Joshua Tessier, DO, Iowa Lutheran Family Medicine Residency, 840 East University Avenue, Des Moines, IA 50316; [email protected]
1. Ottomano C. Errors in medicine and errors in laboratory medicine: what is the difference? Blood Transfus. 2010;8;79-81.
2. Wallach JB. Introduction to normal values (reference ranges). Interpretation of Diagnostic Tests. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:3-7.
3. Halwachs-Baumann G, Katzensteiner S, Schnedl W, et al. Comparative evaluation of three assay systems for automated determination of hemoglobin A1c. Clin Chem. 1997;43:511-517.
4. Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin A(1c) in the management of diabetes. J Diabetes. 2009;1:9-17.
5. Franco RS. The measurement and importance of red cell survival. Am J Hematol. 2009;84:109-114.
6. Tarim O, Küçükerdog˘an A, Gunay U, et al. Effects of iron deficiency anemia on hemoglobin A1c in type 1 diabetes mellitus. Pediatr Int. 1999;41:357-362.
7. Kim C, Bullard KM, Herman WH, et al. Association between iron deficiency and A1C levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999-2006. Diabetes Care. 2010;33:780-785.
8. Higgins T, Stewart D, Boehr E. Challenges in HbA1c analysis and reporting: an interesting case illustrating the many pitfalls. Clin Biochem. 2008;41:1104-1106.
9. Mongia SK, Little RR, Rohlfing CL, et al. Effects of hemoglobin C and S traits on the results of 14 commercial glycated hemoglobin assays. Am J Clin Pathol. 2008;130:136-140.
10. Brown MD, Rowe BH, Reeves MJ, et al. The accuracy of the enzyme-linked immunosorbent assay D-dimer test in the diagnosis of pulmonary embolism: a meta-analysis. Ann Emerg Med. 2002;40:133-144.
11. Squizzato A, Ageno W. What is the next step in D-dimer research? Education of physicians. Intern Emerg Med. 2006;1:165.
12. Kabrhel C, Mark Courtney D, Camargo CA Jr, et al. Potential impact of adjusting the threshold of the quantitative D-dimer based on pretest probability of acute pulmonary embolism. Acad Emerg Med. 2009;16:325-332.
13. Kabrhel C, Mark Courtney MD, Camargo CA Jr, et al. Factors associated with positive D-dimer results in patients evaluated for pulmonary embolism. Acad Emerg Med. 2010;17:589-597.
14. Berman AR. Pulmonary embolism in the elderly. Clin Geriatr Med. 2001;17:107-130.
15. Bruinstroop E, van de Ree MA, Huisman MV. The use of Ddimer in specific clinical conditions: a narrative review. Eur J Intern Med. 2009;20:441-446.
16. Schouten HJ, Geersing GI, Koek HL, et al. Diagnostic accuracy ,of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346:f2492.
17. Turgeon ML. Linne & Ringsrud’s Clinical Laboratory Science. 5th ed. Saint Louis, MO: Mosby; 2007:50.
18. Cohn JS,McNamara JR, Schaefer EJ. Lipoprotein cholesterol concentrations in the plasma of human subjects as measured in the fed and fasted states. Clin Chem. 1988;34:2456-2459.
19. Watts GF, Cohn JS. Whither the lipid profile: feast, famine, or no free lunch? Clin Chem. 2011;57:363-365.
20. Mora S, Rifai N, Buring JE, et al. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993-1001.
21. Nordestgaard BG, Langsted A, Freiberg JJ. Nonfasting hyperlipidemia and cardiovascular disease. Curr Drug Targets. 2009;10:328-335.
22. Screening for lipid disorders in adults: US Preventive Services Task Force Recommendation statement. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf08/lipid/lipidrs.htm. Accessed March 13, 2014.
23. Wolf DM, Friedrichs I, Toma AG. Lymphocyte-white blood cell count ratio: a quickly available screening tool to differentiate acute purulent tonsillitis from glandular fever. Arch Otolaryngol Head Neck Surg. 2007;133:61-64.
24. McCormack R, O’Shea T. The uptake and use of the Monospot test in patients with tonsillitis. Ir Med J. 2009;102:226-228.
25. Ebell MH. Epstein-Barr virus infectious mononucleosis. Am Fam Physician. 2004;70:1279-1287.
26. Waninger KN, Harcke HT. Determination of safe return to play for athletes recovering from infectious mononucleosis: a review of the literature. Clin J Sport Med. 2005;15:410-416.
27. Hurt C, Tammaro D. Diagnostic evaluation of mononucleosislike illnesses. Am J Med. 2007;120:911.e1-911.e8.
28. Vouloumanou EK, Rafailidis PI, Falagas ME. Current diagnosis and management of infectious mononucleosis. Curr Opin Hematol. 2012;19:14-20.
29. Epstein-Barr virus and infectious mononucleosis. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/epstein-barr/laboratory-testing.html. Updated January 7, 2014. Accessed March 12, 2014.
30. Dasgupta A. Clinical utility of free drug monitoring. Clin Chem Lab Med. 2002;40:986-993.
31. Dasgupta A. Usefulness of monitoring free (unbound) concentrations of therapeutic drugs in patient management. Clin Chim Acta. 2007;377:1-13.
32. Iwamoto T, Kagawa Y, Naito Y, et al. Clinical evaluation of plasma free phenytoin measurement and factors influencing its protein binding. Biopharm Drug Dispos. 2006;27:77-84.
33. Hermida J, Tutor JC. A theoretical method for normalizing total serum valproic acid concentration in hypoalbuminemic patients. J Pharmacol Sci. 2005;97:489-493.
34. Wolf GK, McClain CD, Zurakowski D, et al. Total phenytoin concentrations do not accurately predict free phenytoin concentrations in critically ill children. Pediatr Crit Care Med. 2006;7:434-439; quiz 440.
35. Lanoxin (digoxin) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2011.
36. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421.
37. De Backer G, Ambrosini E, Borch-Johnsen K, et al; Third Joint Force of European and other Societies on Cardiovascular Disease and Prevention in Clinical Practice. European guidelines on cardiovascular disease and prevention in clinical practice. Atherosclerosis. 2003;171:145-155.
38. McKenney JM, Davidson MH, Jacobson TA, et al. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
39. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Updated July 3, 2012. Accessed May 17, 2013.
40. Eskridge KD, Guthrie SK. Clinical issues associated with urine testing of substances of abuse. Pharmacotherapy. 1997;17:497-510.
41. Herring C, Muzyk AJ, Johnston C. Interferences with urine drug screens. J Pharm Pract. 2001;24:102-108.
42. Jaffee WB, Trucco E, Teter C, et al. Focus on alcohol & drug abuse: ensuring validity in urine drug testing. Psychiatr Serv. 2008;59:140-142.
43. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67:1344-1350.
44. Moeller KE, Lee KC, Kissack JC. Urine drug screening: Practical guide for clinicians. Mayo Clin Proc. 2008;83:66-76.
45. Christo PJ, Manchikanti L, Ruan X, et al. Urine drug testing in chronic pain. Pain Physician. 2011;14:123-143.
46. Tenenbein M. Do you really need that emergency drug screen? Clin Toxicol (Phila). 2009;47:286-291.
47. Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: an update. Am Fam Physician. 2012;86:244-251.
48. UK guidelines for the use of thyroid function tests. British Thyroid Association Web site. Available at: http://www.british-thyroid-association.org/info-for-patients/Docs/TFT_guideline_final_version_July_2006.pdf. Accessed March 11, 2014.
49. Volpé, R. Rational use of thyroid function tests. Crit Rev Clin Lab Sci. 1997;34:405-438.
50. Graber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
51. Helfand M; US Preventive Services Task Force. Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2004;140:128-141.
› When interpreting hemoglobin A1c (HbA1c) levels, assess for anemia and other comorbidities that can significantly affect the lifespan of red blood cells and skew HbA1c test results. B
› Order nonfasting lipid panels for patients for whom fasting laboratory tests are difficult to obtain, as they have good clinical utility in screening and initial treatment. A
› Avoid routine thyroid-stimulating hormone (TSH) testing in asymptomatic adults; when testing is indicated, start with TSH. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Laboratory mistakes are not defined as diagnostic errors, but they contribute significantly to the thousands of medical errors that occur every year.1 Part of the problem: While accurate interpretation of lab tests often depends on the use of statistical concepts we all learned in medical training, it is difficult to find the time to incorporate these principles into a busy practice.
Overuse of lab tests presents problems, as well. Because “normal ranges” for test results are based on statistical analysis, as many as 5% of patients in a standard distribution fall outside of the range.2 It is important to order only the tests you really need, as extra testing automatically means more false positive results.
This article was written with such pitfalls in mind. In the pages that follow, we focus on 8 types of tests family physicians rely on regularly—all cases in which test results are reliable only if comorbidities, pre- and post-test probabilities, and clinical context are carefully considered. To help you put these lab tests into the proper context, we’ve addressed a key question—and highlighted both pitfalls and pearls—about each.
1. Hemoglobin A1c: How does anemia affect it?
Hemoglobin A1c (HbA1c) can be measured in many ways, including high-performance liquid chromatography, boronate affinity, capillary electrophoresis, and immunoassay, all of which can provide equivalent values without significant variability.3,4 In interpreting these tests, however, it is important to understand the effect that anemia has on HbA1c.
It's important to order only the tests you really need, as extra testing automatically means more false positive results.
Two primary variables influencing HbA1c are the average glucose level and the average lifespan of red blood cells (RBCs). Normally, there is a direct correlation between average serum glucose and HbA1c.4 In patients with anemia, however, this relationship is less clear, and may be affected by erythropoiesis and RBC destruction.5 In iron deficiency anemia (IDA),6,7 hemoglobin production falls secondary to iron stores, resulting in microcytic cells with a longer lifespan and elevated HbA1c. In at least one study,5,7 HbA1c approached levels associated with diabetes (with increases as high as 1.5%) in nondiabetic patients, but resolved with treatment of IDA.
Increased destruction as well as increased production of RBCs lowers their lifespan, and in turn decreases HbA1c levels (TABLE 1).4 This can be seen in conditions such as splenomegaly and hemoglobinopathies. In patients with hemoglobinopathies, the percentage of hemoglobin A is significantly decreased, often to undetectable levels—thereby making HbA1c tests inaccurate. Hemoglobin electrophoresis and determination of glycation by capillary electrophoresis or high-performance liquid chromatography can be used instead, but neither is practical because of cost and limited availability.4,8,9
THE TAKEAWAY: When you evaluate HbA1c test results, it is crucial to assess the patient for anemia and other conditions or comorbidities that can significantly affect RBC lifespan and skew test results.2,4-6
2. D-dimer: When should you use it?
D-dimer is a fibrin degradation product that is increased when active clotting is present,10 and its assay—which has high sensitivity and low specificity—is widely used to screen for pulmonary embolism (PE) and deep vein thrombosis (DVT). While the minimal number of false negatives makes the D-dimer a good screening test, the higher rate of false positives makes it difficult to arrive at a definitive diagnosis. Appropriate use of the D-dimer assay is crucial to minimize the potential for adverse consequences, such as bleeding in patients who are subjected to unnecessary anticoagulation because of false positive results.
Further testing typically follows. A positive D-dimer test is commonly followed by a computed tomography (CT) scan of the chest or a ventilation/perfusion scan to establish a PE or DVT diagnosis. But this subsequent testing increases both the cost of health care and the patient’s radiation exposure. Use of these subsequent scans can be reduced by first considering the patient’s pretest probability for PE or DVT. The Wells’ Criteria (available at www.mdcalc.com/wells-criteriafor-pulmonary-embolism-pe/) and Geneva Score (Revised) (www.mdcalc.com/genevascore-revised-for-pulmonary-embolism/) can both be used for this purpose.10,11
Patients with high pretest probability should undergo immediate scanning, foregoing the D-dimer—which should be reserved for patients who have a low or moderate pretest probability but sufficient reason to suspect PE or DVT.10-12
The low specificity of the D-dimer assay poses another challenge to its effective use. There are many things that can increase D-dimer levels, such as age, cancer, prolonged immobility, autoimmune disease, inflammation, sickle cell disease, pregnancy, trauma, and surgery.13-15 All these factors must be taken into consideration prior to ordering this test.
In fact, one recent study found that using an age-adjusted D-dimer cutoff (patient’s age in years x 10 mcg/L)—rather than a conventional cutoff of 500 mcg/L—for patients older than 50 years reduces false positives without substantially increasing false negatives.16
Also of note: An anticoagulant can decrease D-dimer levels in plasma, so the test should not be used to rule out PE or DVT in patients who are undergoing anticoagulation.13,15
THE TAKEAWAY: In evaluating patients for PE or DVT, use the Wells’ Criteria or Geneva Score (Revised) to determine a patient’s pretest probability of disease. Use the D-dimer assay to safely rule out these conditions in patients with a low or intermediate pretest probability, but go directly to scans for those with a high pretest probability.
3. Lipid panels: How important is fasting?
Patients are often instructed to report for fasting lab studies, specifically for lipid profiles. Traditionally, this had been defined as an 8- to 12-hour period without food.17 In clinical practice, however, this is often misinterpreted by patients, who may be confused about the duration of the fast or unsure about whether to eat or drink immediately before the test.
Studies investigating the effect of meals on lab values have found that triglycerides are consistently elevated postprandially, to a maximum of 12 hours.18-21 The effect of the fasting state on total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol is more controversial; while some postprandial differences have been detected, the clinical relevance is equivocal.18-21
Nonfasting lipid values can offer useful information, particularly in patients who are unwilling or unable to return for fasting labs. The US Preventive Services Task Force (USPSTF) supports this practice.22 Because guidelines for evaluation and treatment are based on fasting lipids, however, fasting lab work should be used, whenever possible, for initiating treatment and monitoring patients with abnormal values. If nonfasting lipids are used, it is crucial to factor in the postprandial effects on triglycerides and the subsequent difficulty of assessing LDL cholesterol levels.
THE TAKEAWAY: The clinical relevance of postprandial vs fasting lipid levels is equivocal. Nonfasting lipid panels have reasonable clinical utility in screening and initial treatment, particularly in cases in which obtaining fasting lab values may be problematic.18,19
4. Mononucleoosis spot test: When should you use it?
The monospot test is a latex assay that causes hemagglutination of horse RBCs in the presence of heterophile antibodies characteristic of infectious mononucleosis.23 The antibodies develop within the first 7 days of onset of symptoms, but do not peak for 2 to 5 weeks.24 As a result, monospot testing yields a high incidence of false negatives during the first 2 weeks of active infection.25 False negatives are also common in patients younger than 14 years. Heterophile antibodies may be present for up to a year after active infection.24
Patients at increased risk for splenic rupture, such as athletes, pose considerable diagnostic difficulty.26 When there is strong clinical suspicion of mononucleosis despite a negative monospot test in such high-risk individuals, follow-up testing is recommended to differentiate it from other mononucleosis-like illnesses (TABLE 2).27 The optimal combination of Epstein-Barr virus (EBV) serologic testing consists of the antibody titration of 4 markers: immunoglobulins M (IgM) and G (IgG) to the viral capsid antigen, IgM to the early antigen, and antibody to Epstein-Barr nuclear antigen (EBNA).28 Acute phase reactants in the setting of an antibody to EBNA could indicate reactivation. A positive test does not exclude other medical causes, however, because up to 20% of patients have acute phase antibodies that persist for years.29
Appropriate diagnosis is important because of the significant morbidity associated with EBV. Risk of splenic injury is greatest between 4 and 21 days after onset of symptoms but persists at 7 weeks,26 so conservative therapy followed by monospot retesting one week later is a reasonable approach.
Mononucleosis or routine tonsillitis? It is important to note that there is no evidence that a positive monospot test will affect the management or outcome of routine tonsillitis, raising questions of the utility of the test in such cases. A better approach: Reserve testing for patients with additional findings—ie, splenomegaly—or whose symptoms have persisted ≥ 2 weeks.
THE TAKEAWAY: Wait at least 2 weeks to conduct monospot testing in patients with routine tonsillitis. If strong clinical suspicion exists, proceed with specific IgM and IgG serologic testing.24,25,27,28
5. Evaluating prescription drug levels: Which factors interfere?
Correct interpretation of lab tests conducted to measure prescription drug levels has major implications with regard to patient safety, particularly for medications with a narrow therapeutic index.
Most drug level tests measure the total concentration, which includes both bound and unbound (free) forms. The unbound forms are the active components of the drug; thus, for an accurate evaluation, it is important to be aware of factors that increase free drug concentration. Chief among them is low protein levels, or hypoalbuminemia.30
Risk factors for hypoalbuminemia include significant burns, advanced age, pregnancy, malnutrition, and human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS).30 HIV/AIDS is a particularly high risk because certain protease inhibitors are highly protein bound.
Drug protein binding is classified as low, moderate, or high. The main proteins involved in the process are albumin, alpha-1-acid glycoprotein, and lipoprotein. Medications that are highly protein bound (>80%) are the most affected by low protein levels: Problems can arise when drugs completely bind to all the available proteins and excess drug availability increases free drug levels.
Medications that are most likely to be affected by a high degree of protein binding include carbamazepine, cyclosporine, mycophenolic acid, phenytoin, protease inhibitors (with the exception of indinavir), tacrolimus, and valproic acid. It is important to consider free levels when you order medication assays for these drugs to avoid misinterpreting the serum levels as being too low-a scenario that raises the risk of drug toxicity and adverse outcomes.30,31
A study of 119 phenytoin samples from 70 patients found significantly higher free phenytoin levels in patients with lower albumin levels.32 Higher free phenytoin levels were also seen in older patients and in those with diminished renal function (creatinine clearance <25 mL/min).32 The degree of protein binding is affected by both the serum drug concentration and the albumin level, with saturable protein binding occurring at higher drug levels.33
Calculate phenytoin levels with this equation. To calculate corrected phenytoin levels in patients with low albumin levels, use the following formula, known as the Sheiner-Tozer equation:34
Concentration adjusted=concentration reported/([adjustment x serum albumin] + 0.1); adjustment=0.2 for creatinine clearance ≥20 or 0.1 for creatinine clearance <20.
Additional causes of misinterpreted drug levels. While hypoalbuminemia plays a major role in the misinterpretation of drug levels, other factors affect serum drug concentration, as well. These include drug-drug interactions, which can significantly increase the concentration of the medications involved, and the timing of the test with regard to medication administration. Digoxin levels, in particular, need to be drawn at least 6 to 8 hours after the last dose is taken to allow for appropriate drug distribution.35
THE TAKEAWAY: It is essential to consider free drug level monitoring in patients who either have hypoalbuminemia or have one or more risk factors for hypoalbuminemia to avoid falsely low estimation of drug levels.36,37
6 Liver function tests: Necessary for patients on statin therapy?
Since statins gained US Food and Drug Administration (FDA) approval, the drugs have been associated with increased liver function tests (LFTs). Indeed, there had been a long-standing belief, based on clinical trials, that by monitoring alanine aminotransferase (ALT) and maintaining it at <3 times the upper limit normal (ULN), hepatotoxicity could be avoided.38 In clinical practice, however, further ALT elevation is frequently allowed based on patient tolerability.
In February 2012, the FDA revised its safety data to reflect this practice.39 The FDA update confirmed that routine LFT monitoring is unnecessary for patients on statins—and that it is not very effective in identifying or preventing liver damage.
Overall, serious hepatotoxicity is very rare, with an incidence ≤2 per 1 million patient-years.39 The National Lipid Association Statin Safety Assessment Task Force recommends repeating LFTs that are 3 to 5 times the ULN within 6 months and continuing with the statin dose if the patient is asymptomatic.38
THE TAKEAWAY: Routine liver function monitoring is not necessary for patients on statins. A better approach: Obtain baseline ALT levels, and repeat the testing only as clinically indicated thereafter.38,39
7. Urine drug screens: Which factors affect their accuracy?
The gold standard for testing for drugs of abuse, urine drug screens (UDS) have good sensitivity and specificity, easy administration, and reasonable cost.40 UDS can detect various narcotics, such as morphine, oxycodone, ,and methadone, and identify other illicit drugs, although which drugs and metabolites are tested for is laboratory- and test-specific.
Cross-reactivity. There are 6 currently available immunoassays, all of which use competitive binding between the sample drug and a drug chemically labeled with an enzyme, radioisotope, or fluorophore. The sample drug and labeled drug compete for substrate binding sites on drug-specific antibodies.41,42 Similar to competitive binding for enzymatic reactions in the body, the substrate binding site can experience cross-reactivity—causing substances other than the drug in question to bind to the immunoglobulin, leading to a false positive result (TABLE 3).43 Other factors that can alter the results include the cutoff value of the test and the absorption, distribution, metabolism, and excretion of the drug.42 Thus, a confirmatory test of gas chromatography-mass spectrometry is recommended before making decisions based on the results of UDS.43-45
Routine screens for patients on chronic opioid therapy. Routine use of UDS in emergency departments is no longer recommended, based on evidence that the results are unlikely to have a significant effect on patient management.46 For patients on chronic opioid therapy, however, routine screening has proven helpful in detecting prescription opioid abuse, illicit drug use, and diversion. Up to 34% of patients on prescription opioids have been found to be using illicit drugs, as well.42
THE TAKEAWAY: Use UDS as a tool in managing patients on chronic opioid therapy, but before acting on results, assess for factors, such as the use of oral or topical medications and the cutoff value of the test, that may be associated with false positive or false negative results.43-45
8. Thyroid function testing: When should you test?
Thyroid-stimulating hormone (TSH) is the first-line test when investigating presumed hyper- or hypothyroidism.47,48 Third-generation chemiluminometric assays can reliably measure TSH concentrations <0.01 mU/L by using multiple antibodies to produce a sandwich-type effect on the molecule in question.49
TSH levels exhibit diurnal variation, however, and are affected by other medications, including steroids, opiates, and some antihistamines, among others, as well as comorbidities.47,48 Chronic and acute conditions unrelated to thyroid disease can cause transient changes in TSH concentrations, and have the potential to modify the binding capacity of plasma thyroid hormone binding proteins.48 Thus, TSH should be ordered for hospitalized patients only when clinical suspicion of a thyroid problem exists.48 The USPSTF recommends against routine TSH screening for asymptomatic adults.46
How to respond to abnormal results. For patients found to have abnormal TSH levels, free T4 (fT4) is the next test to order.47,49 An fT4 assay is a superior indicator of thyroid status because it is not affected by changes in iodothyronine-binding proteins, which influence total hormone measurements.49 The results will be elevated in hyperthyroidism and reduced in hypothyroidism.47
Triiodothyronine (T3) measures can be useful in diagnosing Graves’ disease, in which T3 toxicosis may be the initial symptom—or an indication of a relapse. Because T3 is often a peripheral product, however, nonthyroid illnesses and medications can cause artifactually abnormal results.49
Other thyroid-specific labs include thyroid ,antibodies such as antithyroid peroxidase, antithyroglobulin, and TSH receptor, both blocking and stimulating.49 Thyroglobulin is a precursor form of thyroid hormone and should be measured when factitious hyperthyroidism is suspected. Management of hyper- and hypothyroidism often is independent of etiology. Retesting TSH to assess treatment response should be postponed until ≥2 months after any change in medication or dosing.50
Thyroid studies can be very difficult to interpret. TSH should be the first test ordered. However, if TSH values do not match the clinical picture, fT4, T3, and other thyroid tests that are less affected by outside factors can be useful in identifying the cause.
THE TAKEAWAY: Routine TSH testing is not indicated for asymptomatic adults. When evaluating thyroid function is clinically indicated, TSH is the initial test of choice.47,48,51
CORRESPONDENCE
Joshua Tessier, DO, Iowa Lutheran Family Medicine Residency, 840 East University Avenue, Des Moines, IA 50316; [email protected]
› When interpreting hemoglobin A1c (HbA1c) levels, assess for anemia and other comorbidities that can significantly affect the lifespan of red blood cells and skew HbA1c test results. B
› Order nonfasting lipid panels for patients for whom fasting laboratory tests are difficult to obtain, as they have good clinical utility in screening and initial treatment. A
› Avoid routine thyroid-stimulating hormone (TSH) testing in asymptomatic adults; when testing is indicated, start with TSH. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Laboratory mistakes are not defined as diagnostic errors, but they contribute significantly to the thousands of medical errors that occur every year.1 Part of the problem: While accurate interpretation of lab tests often depends on the use of statistical concepts we all learned in medical training, it is difficult to find the time to incorporate these principles into a busy practice.
Overuse of lab tests presents problems, as well. Because “normal ranges” for test results are based on statistical analysis, as many as 5% of patients in a standard distribution fall outside of the range.2 It is important to order only the tests you really need, as extra testing automatically means more false positive results.
This article was written with such pitfalls in mind. In the pages that follow, we focus on 8 types of tests family physicians rely on regularly—all cases in which test results are reliable only if comorbidities, pre- and post-test probabilities, and clinical context are carefully considered. To help you put these lab tests into the proper context, we’ve addressed a key question—and highlighted both pitfalls and pearls—about each.
1. Hemoglobin A1c: How does anemia affect it?
Hemoglobin A1c (HbA1c) can be measured in many ways, including high-performance liquid chromatography, boronate affinity, capillary electrophoresis, and immunoassay, all of which can provide equivalent values without significant variability.3,4 In interpreting these tests, however, it is important to understand the effect that anemia has on HbA1c.
It's important to order only the tests you really need, as extra testing automatically means more false positive results.
Two primary variables influencing HbA1c are the average glucose level and the average lifespan of red blood cells (RBCs). Normally, there is a direct correlation between average serum glucose and HbA1c.4 In patients with anemia, however, this relationship is less clear, and may be affected by erythropoiesis and RBC destruction.5 In iron deficiency anemia (IDA),6,7 hemoglobin production falls secondary to iron stores, resulting in microcytic cells with a longer lifespan and elevated HbA1c. In at least one study,5,7 HbA1c approached levels associated with diabetes (with increases as high as 1.5%) in nondiabetic patients, but resolved with treatment of IDA.
Increased destruction as well as increased production of RBCs lowers their lifespan, and in turn decreases HbA1c levels (TABLE 1).4 This can be seen in conditions such as splenomegaly and hemoglobinopathies. In patients with hemoglobinopathies, the percentage of hemoglobin A is significantly decreased, often to undetectable levels—thereby making HbA1c tests inaccurate. Hemoglobin electrophoresis and determination of glycation by capillary electrophoresis or high-performance liquid chromatography can be used instead, but neither is practical because of cost and limited availability.4,8,9
THE TAKEAWAY: When you evaluate HbA1c test results, it is crucial to assess the patient for anemia and other conditions or comorbidities that can significantly affect RBC lifespan and skew test results.2,4-6
2. D-dimer: When should you use it?
D-dimer is a fibrin degradation product that is increased when active clotting is present,10 and its assay—which has high sensitivity and low specificity—is widely used to screen for pulmonary embolism (PE) and deep vein thrombosis (DVT). While the minimal number of false negatives makes the D-dimer a good screening test, the higher rate of false positives makes it difficult to arrive at a definitive diagnosis. Appropriate use of the D-dimer assay is crucial to minimize the potential for adverse consequences, such as bleeding in patients who are subjected to unnecessary anticoagulation because of false positive results.
Further testing typically follows. A positive D-dimer test is commonly followed by a computed tomography (CT) scan of the chest or a ventilation/perfusion scan to establish a PE or DVT diagnosis. But this subsequent testing increases both the cost of health care and the patient’s radiation exposure. Use of these subsequent scans can be reduced by first considering the patient’s pretest probability for PE or DVT. The Wells’ Criteria (available at www.mdcalc.com/wells-criteriafor-pulmonary-embolism-pe/) and Geneva Score (Revised) (www.mdcalc.com/genevascore-revised-for-pulmonary-embolism/) can both be used for this purpose.10,11
Patients with high pretest probability should undergo immediate scanning, foregoing the D-dimer—which should be reserved for patients who have a low or moderate pretest probability but sufficient reason to suspect PE or DVT.10-12
The low specificity of the D-dimer assay poses another challenge to its effective use. There are many things that can increase D-dimer levels, such as age, cancer, prolonged immobility, autoimmune disease, inflammation, sickle cell disease, pregnancy, trauma, and surgery.13-15 All these factors must be taken into consideration prior to ordering this test.
In fact, one recent study found that using an age-adjusted D-dimer cutoff (patient’s age in years x 10 mcg/L)—rather than a conventional cutoff of 500 mcg/L—for patients older than 50 years reduces false positives without substantially increasing false negatives.16
Also of note: An anticoagulant can decrease D-dimer levels in plasma, so the test should not be used to rule out PE or DVT in patients who are undergoing anticoagulation.13,15
THE TAKEAWAY: In evaluating patients for PE or DVT, use the Wells’ Criteria or Geneva Score (Revised) to determine a patient’s pretest probability of disease. Use the D-dimer assay to safely rule out these conditions in patients with a low or intermediate pretest probability, but go directly to scans for those with a high pretest probability.
3. Lipid panels: How important is fasting?
Patients are often instructed to report for fasting lab studies, specifically for lipid profiles. Traditionally, this had been defined as an 8- to 12-hour period without food.17 In clinical practice, however, this is often misinterpreted by patients, who may be confused about the duration of the fast or unsure about whether to eat or drink immediately before the test.
Studies investigating the effect of meals on lab values have found that triglycerides are consistently elevated postprandially, to a maximum of 12 hours.18-21 The effect of the fasting state on total cholesterol, low-density lipoprotein (LDL) cholesterol, and high-density lipoprotein (HDL) cholesterol is more controversial; while some postprandial differences have been detected, the clinical relevance is equivocal.18-21
Nonfasting lipid values can offer useful information, particularly in patients who are unwilling or unable to return for fasting labs. The US Preventive Services Task Force (USPSTF) supports this practice.22 Because guidelines for evaluation and treatment are based on fasting lipids, however, fasting lab work should be used, whenever possible, for initiating treatment and monitoring patients with abnormal values. If nonfasting lipids are used, it is crucial to factor in the postprandial effects on triglycerides and the subsequent difficulty of assessing LDL cholesterol levels.
THE TAKEAWAY: The clinical relevance of postprandial vs fasting lipid levels is equivocal. Nonfasting lipid panels have reasonable clinical utility in screening and initial treatment, particularly in cases in which obtaining fasting lab values may be problematic.18,19
4. Mononucleoosis spot test: When should you use it?
The monospot test is a latex assay that causes hemagglutination of horse RBCs in the presence of heterophile antibodies characteristic of infectious mononucleosis.23 The antibodies develop within the first 7 days of onset of symptoms, but do not peak for 2 to 5 weeks.24 As a result, monospot testing yields a high incidence of false negatives during the first 2 weeks of active infection.25 False negatives are also common in patients younger than 14 years. Heterophile antibodies may be present for up to a year after active infection.24
Patients at increased risk for splenic rupture, such as athletes, pose considerable diagnostic difficulty.26 When there is strong clinical suspicion of mononucleosis despite a negative monospot test in such high-risk individuals, follow-up testing is recommended to differentiate it from other mononucleosis-like illnesses (TABLE 2).27 The optimal combination of Epstein-Barr virus (EBV) serologic testing consists of the antibody titration of 4 markers: immunoglobulins M (IgM) and G (IgG) to the viral capsid antigen, IgM to the early antigen, and antibody to Epstein-Barr nuclear antigen (EBNA).28 Acute phase reactants in the setting of an antibody to EBNA could indicate reactivation. A positive test does not exclude other medical causes, however, because up to 20% of patients have acute phase antibodies that persist for years.29
Appropriate diagnosis is important because of the significant morbidity associated with EBV. Risk of splenic injury is greatest between 4 and 21 days after onset of symptoms but persists at 7 weeks,26 so conservative therapy followed by monospot retesting one week later is a reasonable approach.
Mononucleosis or routine tonsillitis? It is important to note that there is no evidence that a positive monospot test will affect the management or outcome of routine tonsillitis, raising questions of the utility of the test in such cases. A better approach: Reserve testing for patients with additional findings—ie, splenomegaly—or whose symptoms have persisted ≥ 2 weeks.
THE TAKEAWAY: Wait at least 2 weeks to conduct monospot testing in patients with routine tonsillitis. If strong clinical suspicion exists, proceed with specific IgM and IgG serologic testing.24,25,27,28
5. Evaluating prescription drug levels: Which factors interfere?
Correct interpretation of lab tests conducted to measure prescription drug levels has major implications with regard to patient safety, particularly for medications with a narrow therapeutic index.
Most drug level tests measure the total concentration, which includes both bound and unbound (free) forms. The unbound forms are the active components of the drug; thus, for an accurate evaluation, it is important to be aware of factors that increase free drug concentration. Chief among them is low protein levels, or hypoalbuminemia.30
Risk factors for hypoalbuminemia include significant burns, advanced age, pregnancy, malnutrition, and human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS).30 HIV/AIDS is a particularly high risk because certain protease inhibitors are highly protein bound.
Drug protein binding is classified as low, moderate, or high. The main proteins involved in the process are albumin, alpha-1-acid glycoprotein, and lipoprotein. Medications that are highly protein bound (>80%) are the most affected by low protein levels: Problems can arise when drugs completely bind to all the available proteins and excess drug availability increases free drug levels.
Medications that are most likely to be affected by a high degree of protein binding include carbamazepine, cyclosporine, mycophenolic acid, phenytoin, protease inhibitors (with the exception of indinavir), tacrolimus, and valproic acid. It is important to consider free levels when you order medication assays for these drugs to avoid misinterpreting the serum levels as being too low-a scenario that raises the risk of drug toxicity and adverse outcomes.30,31
A study of 119 phenytoin samples from 70 patients found significantly higher free phenytoin levels in patients with lower albumin levels.32 Higher free phenytoin levels were also seen in older patients and in those with diminished renal function (creatinine clearance <25 mL/min).32 The degree of protein binding is affected by both the serum drug concentration and the albumin level, with saturable protein binding occurring at higher drug levels.33
Calculate phenytoin levels with this equation. To calculate corrected phenytoin levels in patients with low albumin levels, use the following formula, known as the Sheiner-Tozer equation:34
Concentration adjusted=concentration reported/([adjustment x serum albumin] + 0.1); adjustment=0.2 for creatinine clearance ≥20 or 0.1 for creatinine clearance <20.
Additional causes of misinterpreted drug levels. While hypoalbuminemia plays a major role in the misinterpretation of drug levels, other factors affect serum drug concentration, as well. These include drug-drug interactions, which can significantly increase the concentration of the medications involved, and the timing of the test with regard to medication administration. Digoxin levels, in particular, need to be drawn at least 6 to 8 hours after the last dose is taken to allow for appropriate drug distribution.35
THE TAKEAWAY: It is essential to consider free drug level monitoring in patients who either have hypoalbuminemia or have one or more risk factors for hypoalbuminemia to avoid falsely low estimation of drug levels.36,37
6 Liver function tests: Necessary for patients on statin therapy?
Since statins gained US Food and Drug Administration (FDA) approval, the drugs have been associated with increased liver function tests (LFTs). Indeed, there had been a long-standing belief, based on clinical trials, that by monitoring alanine aminotransferase (ALT) and maintaining it at <3 times the upper limit normal (ULN), hepatotoxicity could be avoided.38 In clinical practice, however, further ALT elevation is frequently allowed based on patient tolerability.
In February 2012, the FDA revised its safety data to reflect this practice.39 The FDA update confirmed that routine LFT monitoring is unnecessary for patients on statins—and that it is not very effective in identifying or preventing liver damage.
Overall, serious hepatotoxicity is very rare, with an incidence ≤2 per 1 million patient-years.39 The National Lipid Association Statin Safety Assessment Task Force recommends repeating LFTs that are 3 to 5 times the ULN within 6 months and continuing with the statin dose if the patient is asymptomatic.38
THE TAKEAWAY: Routine liver function monitoring is not necessary for patients on statins. A better approach: Obtain baseline ALT levels, and repeat the testing only as clinically indicated thereafter.38,39
7. Urine drug screens: Which factors affect their accuracy?
The gold standard for testing for drugs of abuse, urine drug screens (UDS) have good sensitivity and specificity, easy administration, and reasonable cost.40 UDS can detect various narcotics, such as morphine, oxycodone, ,and methadone, and identify other illicit drugs, although which drugs and metabolites are tested for is laboratory- and test-specific.
Cross-reactivity. There are 6 currently available immunoassays, all of which use competitive binding between the sample drug and a drug chemically labeled with an enzyme, radioisotope, or fluorophore. The sample drug and labeled drug compete for substrate binding sites on drug-specific antibodies.41,42 Similar to competitive binding for enzymatic reactions in the body, the substrate binding site can experience cross-reactivity—causing substances other than the drug in question to bind to the immunoglobulin, leading to a false positive result (TABLE 3).43 Other factors that can alter the results include the cutoff value of the test and the absorption, distribution, metabolism, and excretion of the drug.42 Thus, a confirmatory test of gas chromatography-mass spectrometry is recommended before making decisions based on the results of UDS.43-45
Routine screens for patients on chronic opioid therapy. Routine use of UDS in emergency departments is no longer recommended, based on evidence that the results are unlikely to have a significant effect on patient management.46 For patients on chronic opioid therapy, however, routine screening has proven helpful in detecting prescription opioid abuse, illicit drug use, and diversion. Up to 34% of patients on prescription opioids have been found to be using illicit drugs, as well.42
THE TAKEAWAY: Use UDS as a tool in managing patients on chronic opioid therapy, but before acting on results, assess for factors, such as the use of oral or topical medications and the cutoff value of the test, that may be associated with false positive or false negative results.43-45
8. Thyroid function testing: When should you test?
Thyroid-stimulating hormone (TSH) is the first-line test when investigating presumed hyper- or hypothyroidism.47,48 Third-generation chemiluminometric assays can reliably measure TSH concentrations <0.01 mU/L by using multiple antibodies to produce a sandwich-type effect on the molecule in question.49
TSH levels exhibit diurnal variation, however, and are affected by other medications, including steroids, opiates, and some antihistamines, among others, as well as comorbidities.47,48 Chronic and acute conditions unrelated to thyroid disease can cause transient changes in TSH concentrations, and have the potential to modify the binding capacity of plasma thyroid hormone binding proteins.48 Thus, TSH should be ordered for hospitalized patients only when clinical suspicion of a thyroid problem exists.48 The USPSTF recommends against routine TSH screening for asymptomatic adults.46
How to respond to abnormal results. For patients found to have abnormal TSH levels, free T4 (fT4) is the next test to order.47,49 An fT4 assay is a superior indicator of thyroid status because it is not affected by changes in iodothyronine-binding proteins, which influence total hormone measurements.49 The results will be elevated in hyperthyroidism and reduced in hypothyroidism.47
Triiodothyronine (T3) measures can be useful in diagnosing Graves’ disease, in which T3 toxicosis may be the initial symptom—or an indication of a relapse. Because T3 is often a peripheral product, however, nonthyroid illnesses and medications can cause artifactually abnormal results.49
Other thyroid-specific labs include thyroid ,antibodies such as antithyroid peroxidase, antithyroglobulin, and TSH receptor, both blocking and stimulating.49 Thyroglobulin is a precursor form of thyroid hormone and should be measured when factitious hyperthyroidism is suspected. Management of hyper- and hypothyroidism often is independent of etiology. Retesting TSH to assess treatment response should be postponed until ≥2 months after any change in medication or dosing.50
Thyroid studies can be very difficult to interpret. TSH should be the first test ordered. However, if TSH values do not match the clinical picture, fT4, T3, and other thyroid tests that are less affected by outside factors can be useful in identifying the cause.
THE TAKEAWAY: Routine TSH testing is not indicated for asymptomatic adults. When evaluating thyroid function is clinically indicated, TSH is the initial test of choice.47,48,51
CORRESPONDENCE
Joshua Tessier, DO, Iowa Lutheran Family Medicine Residency, 840 East University Avenue, Des Moines, IA 50316; [email protected]
1. Ottomano C. Errors in medicine and errors in laboratory medicine: what is the difference? Blood Transfus. 2010;8;79-81.
2. Wallach JB. Introduction to normal values (reference ranges). Interpretation of Diagnostic Tests. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:3-7.
3. Halwachs-Baumann G, Katzensteiner S, Schnedl W, et al. Comparative evaluation of three assay systems for automated determination of hemoglobin A1c. Clin Chem. 1997;43:511-517.
4. Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin A(1c) in the management of diabetes. J Diabetes. 2009;1:9-17.
5. Franco RS. The measurement and importance of red cell survival. Am J Hematol. 2009;84:109-114.
6. Tarim O, Küçükerdog˘an A, Gunay U, et al. Effects of iron deficiency anemia on hemoglobin A1c in type 1 diabetes mellitus. Pediatr Int. 1999;41:357-362.
7. Kim C, Bullard KM, Herman WH, et al. Association between iron deficiency and A1C levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999-2006. Diabetes Care. 2010;33:780-785.
8. Higgins T, Stewart D, Boehr E. Challenges in HbA1c analysis and reporting: an interesting case illustrating the many pitfalls. Clin Biochem. 2008;41:1104-1106.
9. Mongia SK, Little RR, Rohlfing CL, et al. Effects of hemoglobin C and S traits on the results of 14 commercial glycated hemoglobin assays. Am J Clin Pathol. 2008;130:136-140.
10. Brown MD, Rowe BH, Reeves MJ, et al. The accuracy of the enzyme-linked immunosorbent assay D-dimer test in the diagnosis of pulmonary embolism: a meta-analysis. Ann Emerg Med. 2002;40:133-144.
11. Squizzato A, Ageno W. What is the next step in D-dimer research? Education of physicians. Intern Emerg Med. 2006;1:165.
12. Kabrhel C, Mark Courtney D, Camargo CA Jr, et al. Potential impact of adjusting the threshold of the quantitative D-dimer based on pretest probability of acute pulmonary embolism. Acad Emerg Med. 2009;16:325-332.
13. Kabrhel C, Mark Courtney MD, Camargo CA Jr, et al. Factors associated with positive D-dimer results in patients evaluated for pulmonary embolism. Acad Emerg Med. 2010;17:589-597.
14. Berman AR. Pulmonary embolism in the elderly. Clin Geriatr Med. 2001;17:107-130.
15. Bruinstroop E, van de Ree MA, Huisman MV. The use of Ddimer in specific clinical conditions: a narrative review. Eur J Intern Med. 2009;20:441-446.
16. Schouten HJ, Geersing GI, Koek HL, et al. Diagnostic accuracy ,of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346:f2492.
17. Turgeon ML. Linne & Ringsrud’s Clinical Laboratory Science. 5th ed. Saint Louis, MO: Mosby; 2007:50.
18. Cohn JS,McNamara JR, Schaefer EJ. Lipoprotein cholesterol concentrations in the plasma of human subjects as measured in the fed and fasted states. Clin Chem. 1988;34:2456-2459.
19. Watts GF, Cohn JS. Whither the lipid profile: feast, famine, or no free lunch? Clin Chem. 2011;57:363-365.
20. Mora S, Rifai N, Buring JE, et al. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993-1001.
21. Nordestgaard BG, Langsted A, Freiberg JJ. Nonfasting hyperlipidemia and cardiovascular disease. Curr Drug Targets. 2009;10:328-335.
22. Screening for lipid disorders in adults: US Preventive Services Task Force Recommendation statement. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf08/lipid/lipidrs.htm. Accessed March 13, 2014.
23. Wolf DM, Friedrichs I, Toma AG. Lymphocyte-white blood cell count ratio: a quickly available screening tool to differentiate acute purulent tonsillitis from glandular fever. Arch Otolaryngol Head Neck Surg. 2007;133:61-64.
24. McCormack R, O’Shea T. The uptake and use of the Monospot test in patients with tonsillitis. Ir Med J. 2009;102:226-228.
25. Ebell MH. Epstein-Barr virus infectious mononucleosis. Am Fam Physician. 2004;70:1279-1287.
26. Waninger KN, Harcke HT. Determination of safe return to play for athletes recovering from infectious mononucleosis: a review of the literature. Clin J Sport Med. 2005;15:410-416.
27. Hurt C, Tammaro D. Diagnostic evaluation of mononucleosislike illnesses. Am J Med. 2007;120:911.e1-911.e8.
28. Vouloumanou EK, Rafailidis PI, Falagas ME. Current diagnosis and management of infectious mononucleosis. Curr Opin Hematol. 2012;19:14-20.
29. Epstein-Barr virus and infectious mononucleosis. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/epstein-barr/laboratory-testing.html. Updated January 7, 2014. Accessed March 12, 2014.
30. Dasgupta A. Clinical utility of free drug monitoring. Clin Chem Lab Med. 2002;40:986-993.
31. Dasgupta A. Usefulness of monitoring free (unbound) concentrations of therapeutic drugs in patient management. Clin Chim Acta. 2007;377:1-13.
32. Iwamoto T, Kagawa Y, Naito Y, et al. Clinical evaluation of plasma free phenytoin measurement and factors influencing its protein binding. Biopharm Drug Dispos. 2006;27:77-84.
33. Hermida J, Tutor JC. A theoretical method for normalizing total serum valproic acid concentration in hypoalbuminemic patients. J Pharmacol Sci. 2005;97:489-493.
34. Wolf GK, McClain CD, Zurakowski D, et al. Total phenytoin concentrations do not accurately predict free phenytoin concentrations in critically ill children. Pediatr Crit Care Med. 2006;7:434-439; quiz 440.
35. Lanoxin (digoxin) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2011.
36. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421.
37. De Backer G, Ambrosini E, Borch-Johnsen K, et al; Third Joint Force of European and other Societies on Cardiovascular Disease and Prevention in Clinical Practice. European guidelines on cardiovascular disease and prevention in clinical practice. Atherosclerosis. 2003;171:145-155.
38. McKenney JM, Davidson MH, Jacobson TA, et al. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
39. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Updated July 3, 2012. Accessed May 17, 2013.
40. Eskridge KD, Guthrie SK. Clinical issues associated with urine testing of substances of abuse. Pharmacotherapy. 1997;17:497-510.
41. Herring C, Muzyk AJ, Johnston C. Interferences with urine drug screens. J Pharm Pract. 2001;24:102-108.
42. Jaffee WB, Trucco E, Teter C, et al. Focus on alcohol & drug abuse: ensuring validity in urine drug testing. Psychiatr Serv. 2008;59:140-142.
43. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67:1344-1350.
44. Moeller KE, Lee KC, Kissack JC. Urine drug screening: Practical guide for clinicians. Mayo Clin Proc. 2008;83:66-76.
45. Christo PJ, Manchikanti L, Ruan X, et al. Urine drug testing in chronic pain. Pain Physician. 2011;14:123-143.
46. Tenenbein M. Do you really need that emergency drug screen? Clin Toxicol (Phila). 2009;47:286-291.
47. Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: an update. Am Fam Physician. 2012;86:244-251.
48. UK guidelines for the use of thyroid function tests. British Thyroid Association Web site. Available at: http://www.british-thyroid-association.org/info-for-patients/Docs/TFT_guideline_final_version_July_2006.pdf. Accessed March 11, 2014.
49. Volpé, R. Rational use of thyroid function tests. Crit Rev Clin Lab Sci. 1997;34:405-438.
50. Graber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
51. Helfand M; US Preventive Services Task Force. Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2004;140:128-141.
1. Ottomano C. Errors in medicine and errors in laboratory medicine: what is the difference? Blood Transfus. 2010;8;79-81.
2. Wallach JB. Introduction to normal values (reference ranges). Interpretation of Diagnostic Tests. 8th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:3-7.
3. Halwachs-Baumann G, Katzensteiner S, Schnedl W, et al. Comparative evaluation of three assay systems for automated determination of hemoglobin A1c. Clin Chem. 1997;43:511-517.
4. Gallagher EJ, Le Roith D, Bloomgarden Z. Review of hemoglobin A(1c) in the management of diabetes. J Diabetes. 2009;1:9-17.
5. Franco RS. The measurement and importance of red cell survival. Am J Hematol. 2009;84:109-114.
6. Tarim O, Küçükerdog˘an A, Gunay U, et al. Effects of iron deficiency anemia on hemoglobin A1c in type 1 diabetes mellitus. Pediatr Int. 1999;41:357-362.
7. Kim C, Bullard KM, Herman WH, et al. Association between iron deficiency and A1C levels among adults without diabetes in the National Health and Nutrition Examination Survey, 1999-2006. Diabetes Care. 2010;33:780-785.
8. Higgins T, Stewart D, Boehr E. Challenges in HbA1c analysis and reporting: an interesting case illustrating the many pitfalls. Clin Biochem. 2008;41:1104-1106.
9. Mongia SK, Little RR, Rohlfing CL, et al. Effects of hemoglobin C and S traits on the results of 14 commercial glycated hemoglobin assays. Am J Clin Pathol. 2008;130:136-140.
10. Brown MD, Rowe BH, Reeves MJ, et al. The accuracy of the enzyme-linked immunosorbent assay D-dimer test in the diagnosis of pulmonary embolism: a meta-analysis. Ann Emerg Med. 2002;40:133-144.
11. Squizzato A, Ageno W. What is the next step in D-dimer research? Education of physicians. Intern Emerg Med. 2006;1:165.
12. Kabrhel C, Mark Courtney D, Camargo CA Jr, et al. Potential impact of adjusting the threshold of the quantitative D-dimer based on pretest probability of acute pulmonary embolism. Acad Emerg Med. 2009;16:325-332.
13. Kabrhel C, Mark Courtney MD, Camargo CA Jr, et al. Factors associated with positive D-dimer results in patients evaluated for pulmonary embolism. Acad Emerg Med. 2010;17:589-597.
14. Berman AR. Pulmonary embolism in the elderly. Clin Geriatr Med. 2001;17:107-130.
15. Bruinstroop E, van de Ree MA, Huisman MV. The use of Ddimer in specific clinical conditions: a narrative review. Eur J Intern Med. 2009;20:441-446.
16. Schouten HJ, Geersing GI, Koek HL, et al. Diagnostic accuracy ,of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346:f2492.
17. Turgeon ML. Linne & Ringsrud’s Clinical Laboratory Science. 5th ed. Saint Louis, MO: Mosby; 2007:50.
18. Cohn JS,McNamara JR, Schaefer EJ. Lipoprotein cholesterol concentrations in the plasma of human subjects as measured in the fed and fasted states. Clin Chem. 1988;34:2456-2459.
19. Watts GF, Cohn JS. Whither the lipid profile: feast, famine, or no free lunch? Clin Chem. 2011;57:363-365.
20. Mora S, Rifai N, Buring JE, et al. Fasting compared with nonfasting lipids and apolipoproteins for predicting incident cardiovascular events. Circulation. 2008;118:993-1001.
21. Nordestgaard BG, Langsted A, Freiberg JJ. Nonfasting hyperlipidemia and cardiovascular disease. Curr Drug Targets. 2009;10:328-335.
22. Screening for lipid disorders in adults: US Preventive Services Task Force Recommendation statement. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf08/lipid/lipidrs.htm. Accessed March 13, 2014.
23. Wolf DM, Friedrichs I, Toma AG. Lymphocyte-white blood cell count ratio: a quickly available screening tool to differentiate acute purulent tonsillitis from glandular fever. Arch Otolaryngol Head Neck Surg. 2007;133:61-64.
24. McCormack R, O’Shea T. The uptake and use of the Monospot test in patients with tonsillitis. Ir Med J. 2009;102:226-228.
25. Ebell MH. Epstein-Barr virus infectious mononucleosis. Am Fam Physician. 2004;70:1279-1287.
26. Waninger KN, Harcke HT. Determination of safe return to play for athletes recovering from infectious mononucleosis: a review of the literature. Clin J Sport Med. 2005;15:410-416.
27. Hurt C, Tammaro D. Diagnostic evaluation of mononucleosislike illnesses. Am J Med. 2007;120:911.e1-911.e8.
28. Vouloumanou EK, Rafailidis PI, Falagas ME. Current diagnosis and management of infectious mononucleosis. Curr Opin Hematol. 2012;19:14-20.
29. Epstein-Barr virus and infectious mononucleosis. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/epstein-barr/laboratory-testing.html. Updated January 7, 2014. Accessed March 12, 2014.
30. Dasgupta A. Clinical utility of free drug monitoring. Clin Chem Lab Med. 2002;40:986-993.
31. Dasgupta A. Usefulness of monitoring free (unbound) concentrations of therapeutic drugs in patient management. Clin Chim Acta. 2007;377:1-13.
32. Iwamoto T, Kagawa Y, Naito Y, et al. Clinical evaluation of plasma free phenytoin measurement and factors influencing its protein binding. Biopharm Drug Dispos. 2006;27:77-84.
33. Hermida J, Tutor JC. A theoretical method for normalizing total serum valproic acid concentration in hypoalbuminemic patients. J Pharmacol Sci. 2005;97:489-493.
34. Wolf GK, McClain CD, Zurakowski D, et al. Total phenytoin concentrations do not accurately predict free phenytoin concentrations in critically ill children. Pediatr Crit Care Med. 2006;7:434-439; quiz 440.
35. Lanoxin (digoxin) [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2011.
36. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421.
37. De Backer G, Ambrosini E, Borch-Johnsen K, et al; Third Joint Force of European and other Societies on Cardiovascular Disease and Prevention in Clinical Practice. European guidelines on cardiovascular disease and prevention in clinical practice. Atherosclerosis. 2003;171:145-155.
38. McKenney JM, Davidson MH, Jacobson TA, et al. Final conclusions and recommendations of the National Lipid Association Statin Safety Assessment Task Force. Am J Cardiol. 2006;97:89C-94C.
39. FDA drug safety communication: important safety label changes to cholesterol-lowering statin drugs. US Food and Drug Administration Web site. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm293101.htm. Updated July 3, 2012. Accessed May 17, 2013.
40. Eskridge KD, Guthrie SK. Clinical issues associated with urine testing of substances of abuse. Pharmacotherapy. 1997;17:497-510.
41. Herring C, Muzyk AJ, Johnston C. Interferences with urine drug screens. J Pharm Pract. 2001;24:102-108.
42. Jaffee WB, Trucco E, Teter C, et al. Focus on alcohol & drug abuse: ensuring validity in urine drug testing. Psychiatr Serv. 2008;59:140-142.
43. Brahm NC, Yeager LL, Fox MD, et al. Commonly prescribed medications and potential false-positive urine drug screens. Am J Health Syst Pharm. 2010;67:1344-1350.
44. Moeller KE, Lee KC, Kissack JC. Urine drug screening: Practical guide for clinicians. Mayo Clin Proc. 2008;83:66-76.
45. Christo PJ, Manchikanti L, Ruan X, et al. Urine drug testing in chronic pain. Pain Physician. 2011;14:123-143.
46. Tenenbein M. Do you really need that emergency drug screen? Clin Toxicol (Phila). 2009;47:286-291.
47. Gaitonde DY, Rowley KD, Sweeney LB. Hypothyroidism: an update. Am Fam Physician. 2012;86:244-251.
48. UK guidelines for the use of thyroid function tests. British Thyroid Association Web site. Available at: http://www.british-thyroid-association.org/info-for-patients/Docs/TFT_guideline_final_version_July_2006.pdf. Accessed March 11, 2014.
49. Volpé, R. Rational use of thyroid function tests. Crit Rev Clin Lab Sci. 1997;34:405-438.
50. Graber JR, Cobin RH, Gharib H, et al; American Association of Clinical Endocrinologists and American Thyroid Association Taskforce on Hypothyroidism in Adults. Clinical practice guidelines for hypothyroidism in adults: cosponsored by the American Association of Clinical Endocrinologists and the American Thyroid Association. Endocr Pract. 2012;18:988-1028.
51. Helfand M; US Preventive Services Task Force. Screening for subclinical thyroid dysfunction in nonpregnant adults: a summary of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2004;140:128-141.
How STAT3 blocks an antitumor mechanism

Credit: Ed Uthman
Researchers say they’ve discovered how the protein STAT3 interferes with an antitumor mechanism in cells, thereby promoting the growth of lymphoma and other cancers.
The group made their discovery using the Epstein-Barr virus (EBV) as a tool to investigate cancer development.
“Our findings add to the short list of known mechanisms by which a key cellular antitumor barrier is breached by STAT3 prior to cancer development,” said Sumita Bhaduri-McIntosh, MD, PhD, of Stony Brook University School of Medicine in New York.
“Because STAT3 interferes with this innate antitumor mechanism in cells, the opposite occurs when blood cells are infected in the lab with the cancer-causing virus EBV, and the cells continue to divide—a necessary step in cancer development.”
Dr Bhaduri-McIntosh and her colleagues described their research in PNAS.
The team explained that STAT3 inhibits a cancer-suppressing cellular activity called the DNA damage response (DDR). Normally, this response pauses cell division, allowing for the repair of damaged DNA.
But this study showed that EBV not only causes DNA damage when it infects and replicates in cells; it also activates and increases STAT3 expression. This starts a chain reaction that leads to an “un-pause” in cell division, thereby promoting cell proliferation. This, in combination with other pro-proliferative effects of the virus, can lead to cancers.
The researchers found that DDR does detect replication stress-associated DNA damage resulting from EBV infection. But signaling downstream of ATR proteins is impaired by STAT3. And this leads to relaxation of the intra-S phase checkpoint of the cell cycle.
STAT3 interrupts signaling from ATR to the protein Chk1 by promoting the loss of Claspin, a protein that assists ATR to phosphorylate Chk1. The loss of Claspin, which facilitates cell proliferation, is mediated by caspase 7.
Previous research suggested that STAT3 and Chk1 are potential targets for cancer therapies. Dr Bhaduri-McIntosh’s team said their results provide new insight into anticancer drug development because they reveal a mechanistic link between these 2 proteins.
Dr Bhaduri-McIntosh emphasized that, because STAT3 is involved in most cancers, her group’s findings could potentially impact the prevention or treatment of several types of cancer—something her lab is investigating. ![]()

Credit: Ed Uthman
Researchers say they’ve discovered how the protein STAT3 interferes with an antitumor mechanism in cells, thereby promoting the growth of lymphoma and other cancers.
The group made their discovery using the Epstein-Barr virus (EBV) as a tool to investigate cancer development.
“Our findings add to the short list of known mechanisms by which a key cellular antitumor barrier is breached by STAT3 prior to cancer development,” said Sumita Bhaduri-McIntosh, MD, PhD, of Stony Brook University School of Medicine in New York.
“Because STAT3 interferes with this innate antitumor mechanism in cells, the opposite occurs when blood cells are infected in the lab with the cancer-causing virus EBV, and the cells continue to divide—a necessary step in cancer development.”
Dr Bhaduri-McIntosh and her colleagues described their research in PNAS.
The team explained that STAT3 inhibits a cancer-suppressing cellular activity called the DNA damage response (DDR). Normally, this response pauses cell division, allowing for the repair of damaged DNA.
But this study showed that EBV not only causes DNA damage when it infects and replicates in cells; it also activates and increases STAT3 expression. This starts a chain reaction that leads to an “un-pause” in cell division, thereby promoting cell proliferation. This, in combination with other pro-proliferative effects of the virus, can lead to cancers.
The researchers found that DDR does detect replication stress-associated DNA damage resulting from EBV infection. But signaling downstream of ATR proteins is impaired by STAT3. And this leads to relaxation of the intra-S phase checkpoint of the cell cycle.
STAT3 interrupts signaling from ATR to the protein Chk1 by promoting the loss of Claspin, a protein that assists ATR to phosphorylate Chk1. The loss of Claspin, which facilitates cell proliferation, is mediated by caspase 7.
Previous research suggested that STAT3 and Chk1 are potential targets for cancer therapies. Dr Bhaduri-McIntosh’s team said their results provide new insight into anticancer drug development because they reveal a mechanistic link between these 2 proteins.
Dr Bhaduri-McIntosh emphasized that, because STAT3 is involved in most cancers, her group’s findings could potentially impact the prevention or treatment of several types of cancer—something her lab is investigating. ![]()

Credit: Ed Uthman
Researchers say they’ve discovered how the protein STAT3 interferes with an antitumor mechanism in cells, thereby promoting the growth of lymphoma and other cancers.
The group made their discovery using the Epstein-Barr virus (EBV) as a tool to investigate cancer development.
“Our findings add to the short list of known mechanisms by which a key cellular antitumor barrier is breached by STAT3 prior to cancer development,” said Sumita Bhaduri-McIntosh, MD, PhD, of Stony Brook University School of Medicine in New York.
“Because STAT3 interferes with this innate antitumor mechanism in cells, the opposite occurs when blood cells are infected in the lab with the cancer-causing virus EBV, and the cells continue to divide—a necessary step in cancer development.”
Dr Bhaduri-McIntosh and her colleagues described their research in PNAS.
The team explained that STAT3 inhibits a cancer-suppressing cellular activity called the DNA damage response (DDR). Normally, this response pauses cell division, allowing for the repair of damaged DNA.
But this study showed that EBV not only causes DNA damage when it infects and replicates in cells; it also activates and increases STAT3 expression. This starts a chain reaction that leads to an “un-pause” in cell division, thereby promoting cell proliferation. This, in combination with other pro-proliferative effects of the virus, can lead to cancers.
The researchers found that DDR does detect replication stress-associated DNA damage resulting from EBV infection. But signaling downstream of ATR proteins is impaired by STAT3. And this leads to relaxation of the intra-S phase checkpoint of the cell cycle.
STAT3 interrupts signaling from ATR to the protein Chk1 by promoting the loss of Claspin, a protein that assists ATR to phosphorylate Chk1. The loss of Claspin, which facilitates cell proliferation, is mediated by caspase 7.
Previous research suggested that STAT3 and Chk1 are potential targets for cancer therapies. Dr Bhaduri-McIntosh’s team said their results provide new insight into anticancer drug development because they reveal a mechanistic link between these 2 proteins.
Dr Bhaduri-McIntosh emphasized that, because STAT3 is involved in most cancers, her group’s findings could potentially impact the prevention or treatment of several types of cancer—something her lab is investigating. ![]()
Mediterranean diet tied to decreased platelets, WBCs

part of a Mediterranean diet
In a large study, individuals who strictly followed a Mediterranean diet had lower levels of platelets and white blood cells (WBCs) than those who deviated from the diet.
And the lower cell counts were associated with lower levels of inflammation.
The research also suggested the diet as a whole, and not just certain components, was responsible for these markers of improved health.
Marialaura Bonaccio, PhD, of the IRCCS Istituto Neurologico Mediterraneo NEUROMED in Italy, and her colleagues reported these findings in Blood.
The team noted that the Mediterranean diet—which is characterized by a wide consumption of plant foods, cereals, legumes, fish, and olive oil, as well as moderate wine consumption—has long been hailed as a heart-healthy eating plan. And previous research suggested the diet can reduce inflammation.
But the connection between the diet and levels of platelets and WBCs, 2 specific inflammatory markers in the body, has remained unclear.
“We undertook this study to understand the correlation between consuming a Mediterranean diet and specific health markers, including platelet levels and white blood cell counts, which can more specifically explain the diet’s benefits in reducing the long-term risk of cerebral and heart disease or other chronic conditions,” Dr Bonaccio said.
To do this, she and her colleagues analyzed the eating habits of 14,586 healthy Italian men and women aged 35 and older. At baseline, all subjects were healthy.
The researchers measured total platelet and WBC counts and grouped participants according to their levels (low, normal, or high), based on age- and gender-specific cut-offs.
Participants with high platelet levels were younger and had a greater incidence of high cholesterol and increased levels of common inflammation marker C-reactive protein when compared to subjects in the normal or low-platelet categories.
Individuals in the high-WBC category were mainly younger, male, and smokers. They had a higher body-mass index and higher levels of C-reactive protein and blood glucose than subjects in the other groups. They also showed higher prevalence of high blood pressure and high cholesterol.
The researchers determined participants’ adherence to a Mediterranean using 2 dietary scoring systems, the Mediterranean diet score or the Italian Mediterranean Index, which helped to accurately determine intake levels and portion sizes.
Results of these analyses revealed that adherence to the Mediterranean diet was directly related to lower levels of platelets and WBCs (P<0.0001 and P=0.008, respectively), which was correlated with lower levels of inflammation.
When compared with participants who did not follow the eating plan as closely, subjects who strictly followed the diet were less likely to belong to the group with the highest platelet counts (odds ratio=0.50) and more likely to belong to the group with the lowest WBC counts (odds ratio=1.41).
“Because the study included healthy participants, the lower levels of platelets and white blood cells in those who were more strictly consuming a Mediterranean diet indicate that this eating plan could account for substantial changes within normal ranges of variability,” Dr Bonaccio said.
“This is an important finding that has implications for how these anti-inflammatory markers are tracked among the general population.”
The researchers also evaluated the role of specific components of the diet to help clarify the observed correlation, including food antioxidant content and fiber intake, both of which have previously been connected to cardiovascular benefits.
These components only partially accounted for the link between the diet and WBC count. And they did not fully explain the correlation to platelet levels.
“An important finding of this study is that it indicates that the Mediterranean diet as a whole, and not just a few specific ingredients, is likely responsible for the beneficial health outcomes among the healthy population and should be encouraged as part of healthy eating habits,” Dr Bonaccio said.
“Building on these important findings, we continue to study this population to determine if the dietary habits may have an influence on cardiovascular disease-related mortality.” ![]()

part of a Mediterranean diet
In a large study, individuals who strictly followed a Mediterranean diet had lower levels of platelets and white blood cells (WBCs) than those who deviated from the diet.
And the lower cell counts were associated with lower levels of inflammation.
The research also suggested the diet as a whole, and not just certain components, was responsible for these markers of improved health.
Marialaura Bonaccio, PhD, of the IRCCS Istituto Neurologico Mediterraneo NEUROMED in Italy, and her colleagues reported these findings in Blood.
The team noted that the Mediterranean diet—which is characterized by a wide consumption of plant foods, cereals, legumes, fish, and olive oil, as well as moderate wine consumption—has long been hailed as a heart-healthy eating plan. And previous research suggested the diet can reduce inflammation.
But the connection between the diet and levels of platelets and WBCs, 2 specific inflammatory markers in the body, has remained unclear.
“We undertook this study to understand the correlation between consuming a Mediterranean diet and specific health markers, including platelet levels and white blood cell counts, which can more specifically explain the diet’s benefits in reducing the long-term risk of cerebral and heart disease or other chronic conditions,” Dr Bonaccio said.
To do this, she and her colleagues analyzed the eating habits of 14,586 healthy Italian men and women aged 35 and older. At baseline, all subjects were healthy.
The researchers measured total platelet and WBC counts and grouped participants according to their levels (low, normal, or high), based on age- and gender-specific cut-offs.
Participants with high platelet levels were younger and had a greater incidence of high cholesterol and increased levels of common inflammation marker C-reactive protein when compared to subjects in the normal or low-platelet categories.
Individuals in the high-WBC category were mainly younger, male, and smokers. They had a higher body-mass index and higher levels of C-reactive protein and blood glucose than subjects in the other groups. They also showed higher prevalence of high blood pressure and high cholesterol.
The researchers determined participants’ adherence to a Mediterranean using 2 dietary scoring systems, the Mediterranean diet score or the Italian Mediterranean Index, which helped to accurately determine intake levels and portion sizes.
Results of these analyses revealed that adherence to the Mediterranean diet was directly related to lower levels of platelets and WBCs (P<0.0001 and P=0.008, respectively), which was correlated with lower levels of inflammation.
When compared with participants who did not follow the eating plan as closely, subjects who strictly followed the diet were less likely to belong to the group with the highest platelet counts (odds ratio=0.50) and more likely to belong to the group with the lowest WBC counts (odds ratio=1.41).
“Because the study included healthy participants, the lower levels of platelets and white blood cells in those who were more strictly consuming a Mediterranean diet indicate that this eating plan could account for substantial changes within normal ranges of variability,” Dr Bonaccio said.
“This is an important finding that has implications for how these anti-inflammatory markers are tracked among the general population.”
The researchers also evaluated the role of specific components of the diet to help clarify the observed correlation, including food antioxidant content and fiber intake, both of which have previously been connected to cardiovascular benefits.
These components only partially accounted for the link between the diet and WBC count. And they did not fully explain the correlation to platelet levels.
“An important finding of this study is that it indicates that the Mediterranean diet as a whole, and not just a few specific ingredients, is likely responsible for the beneficial health outcomes among the healthy population and should be encouraged as part of healthy eating habits,” Dr Bonaccio said.
“Building on these important findings, we continue to study this population to determine if the dietary habits may have an influence on cardiovascular disease-related mortality.” ![]()

part of a Mediterranean diet
In a large study, individuals who strictly followed a Mediterranean diet had lower levels of platelets and white blood cells (WBCs) than those who deviated from the diet.
And the lower cell counts were associated with lower levels of inflammation.
The research also suggested the diet as a whole, and not just certain components, was responsible for these markers of improved health.
Marialaura Bonaccio, PhD, of the IRCCS Istituto Neurologico Mediterraneo NEUROMED in Italy, and her colleagues reported these findings in Blood.
The team noted that the Mediterranean diet—which is characterized by a wide consumption of plant foods, cereals, legumes, fish, and olive oil, as well as moderate wine consumption—has long been hailed as a heart-healthy eating plan. And previous research suggested the diet can reduce inflammation.
But the connection between the diet and levels of platelets and WBCs, 2 specific inflammatory markers in the body, has remained unclear.
“We undertook this study to understand the correlation between consuming a Mediterranean diet and specific health markers, including platelet levels and white blood cell counts, which can more specifically explain the diet’s benefits in reducing the long-term risk of cerebral and heart disease or other chronic conditions,” Dr Bonaccio said.
To do this, she and her colleagues analyzed the eating habits of 14,586 healthy Italian men and women aged 35 and older. At baseline, all subjects were healthy.
The researchers measured total platelet and WBC counts and grouped participants according to their levels (low, normal, or high), based on age- and gender-specific cut-offs.
Participants with high platelet levels were younger and had a greater incidence of high cholesterol and increased levels of common inflammation marker C-reactive protein when compared to subjects in the normal or low-platelet categories.
Individuals in the high-WBC category were mainly younger, male, and smokers. They had a higher body-mass index and higher levels of C-reactive protein and blood glucose than subjects in the other groups. They also showed higher prevalence of high blood pressure and high cholesterol.
The researchers determined participants’ adherence to a Mediterranean using 2 dietary scoring systems, the Mediterranean diet score or the Italian Mediterranean Index, which helped to accurately determine intake levels and portion sizes.
Results of these analyses revealed that adherence to the Mediterranean diet was directly related to lower levels of platelets and WBCs (P<0.0001 and P=0.008, respectively), which was correlated with lower levels of inflammation.
When compared with participants who did not follow the eating plan as closely, subjects who strictly followed the diet were less likely to belong to the group with the highest platelet counts (odds ratio=0.50) and more likely to belong to the group with the lowest WBC counts (odds ratio=1.41).
“Because the study included healthy participants, the lower levels of platelets and white blood cells in those who were more strictly consuming a Mediterranean diet indicate that this eating plan could account for substantial changes within normal ranges of variability,” Dr Bonaccio said.
“This is an important finding that has implications for how these anti-inflammatory markers are tracked among the general population.”
The researchers also evaluated the role of specific components of the diet to help clarify the observed correlation, including food antioxidant content and fiber intake, both of which have previously been connected to cardiovascular benefits.
These components only partially accounted for the link between the diet and WBC count. And they did not fully explain the correlation to platelet levels.
“An important finding of this study is that it indicates that the Mediterranean diet as a whole, and not just a few specific ingredients, is likely responsible for the beneficial health outcomes among the healthy population and should be encouraged as part of healthy eating habits,” Dr Bonaccio said.
“Building on these important findings, we continue to study this population to determine if the dietary habits may have an influence on cardiovascular disease-related mortality.” ![]()
FDA working to alleviate saline shortage

In an attempt to alleviate the shortage of saline (0.9% sodium chloride injection) in the US, the Food and Drug Administration (FDA) is allowing saline products to be imported from Norway.
The company importing the products is Fresenius Kabi USA. The FDA inspected the company’s Norway manufacturing facility and found the site meets FDA standards.
So Fresenius Kabi has begun importing Sodium Chloride 0.9% Freeflex Injection Solution for Intravenous Infusion.
The European product contains the same active ingredient in the same concentration as the 0.9% sodium chloride injection products approved in the US.
For a complete list of all the Fresenius Kabi saline products, as well as a list of differences between the European and US prescribing information, see the “Dear Healthcare Professional” letter posted on the FDA website.
The FDA is asking that healthcare professionals contact Fresenius Kabi USA directly to obtain saline products. The company’s customer service number is 1-888-386-1300.
The FDA concedes that, while the shipments from Norway will help, they will not resolve the saline shortage. However, the agency says it is working closely with manufacturers to meet the needs for saline across the US. ![]()

In an attempt to alleviate the shortage of saline (0.9% sodium chloride injection) in the US, the Food and Drug Administration (FDA) is allowing saline products to be imported from Norway.
The company importing the products is Fresenius Kabi USA. The FDA inspected the company’s Norway manufacturing facility and found the site meets FDA standards.
So Fresenius Kabi has begun importing Sodium Chloride 0.9% Freeflex Injection Solution for Intravenous Infusion.
The European product contains the same active ingredient in the same concentration as the 0.9% sodium chloride injection products approved in the US.
For a complete list of all the Fresenius Kabi saline products, as well as a list of differences between the European and US prescribing information, see the “Dear Healthcare Professional” letter posted on the FDA website.
The FDA is asking that healthcare professionals contact Fresenius Kabi USA directly to obtain saline products. The company’s customer service number is 1-888-386-1300.
The FDA concedes that, while the shipments from Norway will help, they will not resolve the saline shortage. However, the agency says it is working closely with manufacturers to meet the needs for saline across the US. ![]()

In an attempt to alleviate the shortage of saline (0.9% sodium chloride injection) in the US, the Food and Drug Administration (FDA) is allowing saline products to be imported from Norway.
The company importing the products is Fresenius Kabi USA. The FDA inspected the company’s Norway manufacturing facility and found the site meets FDA standards.
So Fresenius Kabi has begun importing Sodium Chloride 0.9% Freeflex Injection Solution for Intravenous Infusion.
The European product contains the same active ingredient in the same concentration as the 0.9% sodium chloride injection products approved in the US.
For a complete list of all the Fresenius Kabi saline products, as well as a list of differences between the European and US prescribing information, see the “Dear Healthcare Professional” letter posted on the FDA website.
The FDA is asking that healthcare professionals contact Fresenius Kabi USA directly to obtain saline products. The company’s customer service number is 1-888-386-1300.
The FDA concedes that, while the shipments from Norway will help, they will not resolve the saline shortage. However, the agency says it is working closely with manufacturers to meet the needs for saline across the US. ![]()
Cangrelor bests clopidogrel for stent thrombosis

Credit: Andre E.X. Brown
WASHINGTON, DC—A new analysis suggests cangrelor can reduce the risk of stent thrombosis in patients undergoing percutaneous coronary intervention (PCI), when compared to clopidogrel.
In fact, cangrelor was an independent predictor of freedom from stent thrombosis at 30 days after PCI.
These results, obtained by analyzing patients from the CHAMPION PHOENIX trial, were presented at the American College of Cardiology’s 63rd Annual Scientific Session & Expo (presentation 2105-290).
CHAMPION PHOENIX was a prospective, double-blind trial that included 11,145 patients. They were randomized to receive intravenous cangrelor or oral clopidogrel at the time of PCI.
In a previous analysis of the trial data, researchers found that cangrelor reduced the overall odds of complications from stenting procedures—including death, myocardial infarction, ischemia-driven revascularization, and stent thrombosis—compared to clopidogrel. However, cangrelor also increased the risk of major and minor bleeding.
In the new analysis, an independent core laboratory blinded to the treatment performed angiographic analysis in 10,939 of the patients. The researchers defined stent thrombosis as the occurrence of intraprocedural stent thrombosis (IPST) or ARC-defined stent thrombosis (definite or probable).
Stent thrombosis occurred in 120 patients (1.1%) at 48 hours after PCI and in 175 patients (1.6%) at 30 days. The occurrence of stent thrombosis at 48 hours and 30 days was associated with a marked increase in 30-day mortality, with odds ratios (ORs) of 15.3 (P<0.001) and 55.2 (P<0.001), respectively.
IPST, ARC acute stent thrombosis (≤ 24 hrs), and ARC subacute stent thrombosis (1-30 days) occurred in 89 (0.8%), 32 (0.3%), and 60 (0.5%) patients, respectively.
Each type of stent thrombosis was associated with an increase in 30-day mortality. The ORs were 17.4 for IPST (P<0.001), 43.3 for ARC acute stent thrombosis (P<0.001), and 189.1 for ARC subacute stent thrombosis (P<0.001).
“Regardless of the exact type of stent thrombosis, it remains associated with a high rate of death,” said investigator Deepak L. Bhatt, MD, MPH, a professor at Harvard Medical School and co-chair of the CHAMPION program.
However, patients who received cangrelor were less likely than those treated with clopidogrel to develop stent thrombosis, both at 48 hours and at 30 days.
At 48 hours, stent thrombosis had occurred in 0.8% and 1.4% of patients, respectively (P=0.01). And at 30 days, stent thrombosis had occurred in 1.3% and 1.9%, respectively (P=0.01).
Cangrelor appeared to be more effective than clopidogrel at preventing all types of stent thrombosis, although the difference was only statistically significant for IPST.
IPST occurred in 0.6% and 1.0% of patients, respectively (P=0.04). Acute ARC stent thrombosis occurred in 0.2% and 0.4%, respectively (P=0.8). And subacute ARC stent thrombosis occurred in 0.5% and 0.6%, respectively (P=0.60).
Multivariable analysis suggested the use of cangrelor was as an independent predictor of freedom from stent thrombosis at 30 days.
The CHAMPION PHOENIX trial was funded by The Medicines Company, makers of cangrelor. ![]()

Credit: Andre E.X. Brown
WASHINGTON, DC—A new analysis suggests cangrelor can reduce the risk of stent thrombosis in patients undergoing percutaneous coronary intervention (PCI), when compared to clopidogrel.
In fact, cangrelor was an independent predictor of freedom from stent thrombosis at 30 days after PCI.
These results, obtained by analyzing patients from the CHAMPION PHOENIX trial, were presented at the American College of Cardiology’s 63rd Annual Scientific Session & Expo (presentation 2105-290).
CHAMPION PHOENIX was a prospective, double-blind trial that included 11,145 patients. They were randomized to receive intravenous cangrelor or oral clopidogrel at the time of PCI.
In a previous analysis of the trial data, researchers found that cangrelor reduced the overall odds of complications from stenting procedures—including death, myocardial infarction, ischemia-driven revascularization, and stent thrombosis—compared to clopidogrel. However, cangrelor also increased the risk of major and minor bleeding.
In the new analysis, an independent core laboratory blinded to the treatment performed angiographic analysis in 10,939 of the patients. The researchers defined stent thrombosis as the occurrence of intraprocedural stent thrombosis (IPST) or ARC-defined stent thrombosis (definite or probable).
Stent thrombosis occurred in 120 patients (1.1%) at 48 hours after PCI and in 175 patients (1.6%) at 30 days. The occurrence of stent thrombosis at 48 hours and 30 days was associated with a marked increase in 30-day mortality, with odds ratios (ORs) of 15.3 (P<0.001) and 55.2 (P<0.001), respectively.
IPST, ARC acute stent thrombosis (≤ 24 hrs), and ARC subacute stent thrombosis (1-30 days) occurred in 89 (0.8%), 32 (0.3%), and 60 (0.5%) patients, respectively.
Each type of stent thrombosis was associated with an increase in 30-day mortality. The ORs were 17.4 for IPST (P<0.001), 43.3 for ARC acute stent thrombosis (P<0.001), and 189.1 for ARC subacute stent thrombosis (P<0.001).
“Regardless of the exact type of stent thrombosis, it remains associated with a high rate of death,” said investigator Deepak L. Bhatt, MD, MPH, a professor at Harvard Medical School and co-chair of the CHAMPION program.
However, patients who received cangrelor were less likely than those treated with clopidogrel to develop stent thrombosis, both at 48 hours and at 30 days.
At 48 hours, stent thrombosis had occurred in 0.8% and 1.4% of patients, respectively (P=0.01). And at 30 days, stent thrombosis had occurred in 1.3% and 1.9%, respectively (P=0.01).
Cangrelor appeared to be more effective than clopidogrel at preventing all types of stent thrombosis, although the difference was only statistically significant for IPST.
IPST occurred in 0.6% and 1.0% of patients, respectively (P=0.04). Acute ARC stent thrombosis occurred in 0.2% and 0.4%, respectively (P=0.8). And subacute ARC stent thrombosis occurred in 0.5% and 0.6%, respectively (P=0.60).
Multivariable analysis suggested the use of cangrelor was as an independent predictor of freedom from stent thrombosis at 30 days.
The CHAMPION PHOENIX trial was funded by The Medicines Company, makers of cangrelor. ![]()

Credit: Andre E.X. Brown
WASHINGTON, DC—A new analysis suggests cangrelor can reduce the risk of stent thrombosis in patients undergoing percutaneous coronary intervention (PCI), when compared to clopidogrel.
In fact, cangrelor was an independent predictor of freedom from stent thrombosis at 30 days after PCI.
These results, obtained by analyzing patients from the CHAMPION PHOENIX trial, were presented at the American College of Cardiology’s 63rd Annual Scientific Session & Expo (presentation 2105-290).
CHAMPION PHOENIX was a prospective, double-blind trial that included 11,145 patients. They were randomized to receive intravenous cangrelor or oral clopidogrel at the time of PCI.
In a previous analysis of the trial data, researchers found that cangrelor reduced the overall odds of complications from stenting procedures—including death, myocardial infarction, ischemia-driven revascularization, and stent thrombosis—compared to clopidogrel. However, cangrelor also increased the risk of major and minor bleeding.
In the new analysis, an independent core laboratory blinded to the treatment performed angiographic analysis in 10,939 of the patients. The researchers defined stent thrombosis as the occurrence of intraprocedural stent thrombosis (IPST) or ARC-defined stent thrombosis (definite or probable).
Stent thrombosis occurred in 120 patients (1.1%) at 48 hours after PCI and in 175 patients (1.6%) at 30 days. The occurrence of stent thrombosis at 48 hours and 30 days was associated with a marked increase in 30-day mortality, with odds ratios (ORs) of 15.3 (P<0.001) and 55.2 (P<0.001), respectively.
IPST, ARC acute stent thrombosis (≤ 24 hrs), and ARC subacute stent thrombosis (1-30 days) occurred in 89 (0.8%), 32 (0.3%), and 60 (0.5%) patients, respectively.
Each type of stent thrombosis was associated with an increase in 30-day mortality. The ORs were 17.4 for IPST (P<0.001), 43.3 for ARC acute stent thrombosis (P<0.001), and 189.1 for ARC subacute stent thrombosis (P<0.001).
“Regardless of the exact type of stent thrombosis, it remains associated with a high rate of death,” said investigator Deepak L. Bhatt, MD, MPH, a professor at Harvard Medical School and co-chair of the CHAMPION program.
However, patients who received cangrelor were less likely than those treated with clopidogrel to develop stent thrombosis, both at 48 hours and at 30 days.
At 48 hours, stent thrombosis had occurred in 0.8% and 1.4% of patients, respectively (P=0.01). And at 30 days, stent thrombosis had occurred in 1.3% and 1.9%, respectively (P=0.01).
Cangrelor appeared to be more effective than clopidogrel at preventing all types of stent thrombosis, although the difference was only statistically significant for IPST.
IPST occurred in 0.6% and 1.0% of patients, respectively (P=0.04). Acute ARC stent thrombosis occurred in 0.2% and 0.4%, respectively (P=0.8). And subacute ARC stent thrombosis occurred in 0.5% and 0.6%, respectively (P=0.60).
Multivariable analysis suggested the use of cangrelor was as an independent predictor of freedom from stent thrombosis at 30 days.
The CHAMPION PHOENIX trial was funded by The Medicines Company, makers of cangrelor. ![]()
Inpatient Trainee Clinical Exposures
The clinical learning model in medical education, specifically in the third and fourth years of medical school and in residency and fellowship training, is driven by direct patient‐care experiences and complemented by mentorship and supervision provided by experienced physicians.[1] Despite the emphasis on experiential learning in medical school and graduate training, the ability of educators to quantify the clinical experiences of learners has been limited. Case logs, often self‐reported, are frequently required during educational rotations to attempt to measure clinical experience.[2] Logs have been utilized to document diagnoses, demographics, disease severity, procedures, and chief complaints.[3, 4, 5, 6] Unfortunately, self‐reported logs are vulnerable to delayed updates, misreported data, and unreliable data validation.[7, 8] Automated data collection has been shown to be more reliable than self‐reported logs.[8, 9]
The enhanced data mining methods now available allow educators to appraise learners' exposures during patient‐care interactions beyond just the diagnosis or chief complaint (eg, how many electrocardiograms do our learners evaluate during a cardiology rotation, how often do our learners gain experience prescribing a specific class of antibiotics, how many of the patients seen by our learners are diabetic). For example, a learner's interaction with a patient during an inpatient admission for community‐acquired pneumonia, at minimum, would include assessing of past medical history, reviewing outpatient medications and allergies, evaluating tests completed (chest x‐ray, complete blood count, blood cultures), prescribing antibiotics, and monitoring comorbidities. The lack of knowledge regarding the frequency and context of these exposures is a key gap in our understanding of the clinical experience of inpatient trainees. Additionally, there are no data on clinical exposures specific to team‐based inpatient learning. When a rotation is team‐based, the educational experience is not limited to the learner's assigned patients, and this arrangement allows for educational exposures from patients who are not the learner's primary assignments through experiences gained during team rounds, cross‐coverage assessments, and informal discussions of patient care.
In this study, we quantify the clinical exposures of learners on an acting internship (AI) rotation in internal medicine by utilizing the Veterans Affairs (VA) electronic medical records (EMR) as collected through the VA Veterans Integrated Service Network 10 Clinical Data Warehouse (CDW). The AI or subinternship is a medical school clinical rotation typically completed in the fourth year, where the learning experience is expected to mirror a 1‐month rotation of a first‐year resident.[10] The AI has historically been defined as an experiential curriculum, during which students assume many of the responsibilities and activities that they will manage as graduate medical trainees.[10, 11] The exposures of AI learners include primary diagnoses encountered, problem lists evaluated at the time of admission, medications prescribed, laboratory tests ordered, and radiologic imaging evaluated. We additionally explored the exposures of the AI learner's team to assess the experiences available through team‐based care.
METHODS
This study was completed at the Louis Stokes Veterans Affairs Medical Center (LSVAMC) in Cleveland, Ohio, which is an academic affiliate of the Case Western Reserve University School of Medicine. The study was approved by the LSVAMC institutional review board.
At the LSVAMC, the AI rotation in internal medicine is a 4‐week inpatient rotation for fourth‐year medical students, in which the student is assigned to an inpatient medical team consisting of an attending physician, a senior resident, and a combination of first‐year residents and acting interns. Compared to a first‐year resident, the acting intern is assigned approximately half of the number of admissions. The teams rounds as a group at least once per day. Acting interns are permitted to place orders and write notes in the EMR; all orders require a cosignature by a resident or attending physician to be released.
We identified students who rotated through the LSVAMC for an AI in internal medicine rotation from July 2008 to November 2011 from rotation records. Using the CDW, we queried student names and their rotation dates and analyzed the results using a Structured Query Language Query Analyzer. Each student's patient encounters during the rotation were identified. A patient encounter was defined as a patient for whom the student wrote at least 1 note titled either Medicine Admission Note or Medicine Inpatient Progress Note, on any of the dates during their AI rotation. We then counted the total number of notes written by each student during their rotation. A patient identifier is associated with each note. The number of distinct patient identifiers was also tallied to establish the total number of patients seen during the rotation by the individual student as the primary caregiver.
We associated each patient encounter with an inpatient admission profile that included patient admission and discharge dates, International Classification of Diseases, 9th Revision (ICD‐9) diagnosis codes, and admitting specialty. Primary diagnosis codes were queried for each admission and were counted for individual students and in aggregate. We tallied both the individual student and aggregate patient medications prescribed during the dates of admission and ordered to a patient location consistent with an acute medical ward (therefore excluding orders placed if a patient was transferred to an intensive care unit). Similar queries were completed for laboratory and radiological testing.
The VA EMR keeps an active problem list on each patient, and items are associated with an ICD‐9 code. To assemble the active problems available for evaluation by the student on the day of a patient's admission, we queried all problem list items added prior to, but not discontinued before, the day of admission. We then tallied the results for every patient seen by each individual student and in aggregate.
To assess the team exposures for each AI student, we queried all discharge summaries cosigned by the student's attending during the dates of the student's rotation. We assumed the student's team members wrote these discharge summaries. After excluding the student's patients, the resultant list represented the team patient exposures for each student. This list was also queried for the number of patients seen, primary diagnoses, medications, problems, labs, and radiology. The number of team admissions counted included all patients who spent at least 1 day on the team while the student was rotating. All other team exposure counts completed included only patients who were both admitted and discharged within the dates of the student's rotation.
RESULTS
An AI rotation is 4 weeks in duration. Students competed a total of 128 rotations from July 30, 2008 through November 21, 2011. We included all rotations during this time period in the analysis. Tables 1, 2, 3, 4, 5 report results in 4 categories. The Student category tallies the total number of specific exposures (diagnoses, problems, medications, lab values, or radiology tests) for all patients primarily assigned to a student. The Team category tallies the total number of exposures for all patients assigned to other members of the student's inpatient team. The Primary % category identifies the percentage of students who had at least 1 assigned patient with the evaluated clinical exposure. The All Patients % category identifies the percentage of students who had at least 1 student‐assigned patient or at least 1 team‐assigned patient with the evaluated clinical exposure.
| Diagnosis | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| Obstructive chronic bronchitis, with acute exacerbation | 102 | 241 | 57% | 91% |
| Pneumonia, organism unspecified | 91 | 228 | 49% | 91% |
| Acute renal failure, unspecified | 73 | 170 | 46% | 83% |
| Urinary tract infection, site not specified | 69 | 149 | 43% | 87% |
| Congestive heart failure, unspecified | 65 | 114 | 41% | 68% |
| Alcohol withdrawal | 46 | 101 | 26% | 61% |
| Alcoholic cirrhosis of liver | 28 | 98 | 16% | 57% |
| Cellulitis and abscess of leg, except foot | 26 | 61 | 18% | 45% |
| Acute pancreatitis | 23 | 51 | 16% | 43% |
| Intestinal infection due to Clostridium difficile | 22 | 30 | 17% | 33% |
| Malignant neoplasm of bronchus and lung, unspecified | 22 | 38 | 16% | 35% |
| Acute on chronic diastolic heart failure | 22 | 45 | 16% | 39% |
| Encounter for antineoplastic chemotherapy | 21 | 96 | 15% | 48% |
| Dehydration | 19 | 78 | 13% | 46% |
| Anemia, unspecified | 19 | 36 | 13% | 30% |
| Pneumonitis due to inhalation of food or vomitus | 19 | 25 | 13% | 24% |
| Syncope and collapse | 16 | 38 | 13% | 39% |
| Other pulmonary embolism and infarction | 15 | 41 | 12% | 26% |
| Unspecified pleural effusion | 15 | 37 | 10% | 34% |
| Acute respiratory failure | 15 | 42 | 11% | 35% |
| Problem | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| Hypertension | 1,665 | 3,280 | 100% | 100% |
| Tobacco use disorder | 1,350 | 2,759 | 100% | 100% |
| Unknown cause morbidity/mortality | 1,154 | 2,370 | 100% | 100% |
| Hyperlipidemia | 1,036 | 2,044 | 99% | 100% |
| Diabetes mellitus 2 without complication | 865 | 1,709 | 100% | 100% |
| Chronic airway obstruction | 600 | 1,132 | 100% | 100% |
| Esophageal reflux | 583 | 1,131 | 99% | 100% |
| Depressive disorder | 510 | 1,005 | 100% | 100% |
| Dermatophytosis of nail | 498 | 939 | 98% | 100% |
| Alcohol dependence | 441 | 966 | 97% | 100% |
| Chronic ischemic heart disease | 385 | 758 | 95% | 100% |
| Osteoarthritis | 383 | 791 | 96% | 100% |
| Lumbago | 357 | 692 | 97% | 100% |
| Current useanticoagulation | 342 | 629 | 94% | 100% |
| Anemia | 337 | 674 | 97% | 100% |
| Inhibited sex excitement | 317 | 610 | 91% | 100% |
| Congestive heart failure | 294 | 551 | 91% | 100% |
| Peripheral vascular disease | 288 | 529 | 88% | 99% |
| Sensorineural hearing loss | 280 | 535 | 88% | 99% |
| Post‐traumatic stress disorder | 274 | 528 | 91% | 100% |
| Pure hypercholesterolemia | 262 | 521 | 88% | 100% |
| Coronary atherosclerosis | 259 | 396 | 87% | 95% |
| Obesity | 246 | 509 | 89% | 99% |
| Atrial fibrillation | 236 | 469 | 85% | 100% |
| Gout | 216 | 389 | 85% | 100% |
| Medication | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| Omeprazole | 1,372 | 2,981 | 99% | 100% |
| Heparin | 1,067 | 2,271 | 95% | 96% |
| Sodium chloride 0.9% | 925 | 2,036 | 99% | 100% |
| Aspirin | 844 | 1,782 | 98% | 100% |
| Potassium chloride | 707 | 1,387 | 99% | 100% |
| Metoprolol tartrate | 693 | 1,318 | 98% | 100% |
| Insulin regular | 692 | 1,518 | 99% | 100% |
| Acetaminophen | 669 | 1,351 | 98% | 100% |
| Simvastatin | 648 | 1,408 | 99% | 100% |
| Lisinopril | 582 | 1,309 | 98% | 100% |
| Furosemide | 577 | 1,186 | 98% | 100% |
| Docusate sodium | 541 | 1,127 | 98% | 100% |
| Vancomycin | 531 | 977 | 98% | 100% |
| Multivitamin | 478 | 1,074 | 96% | 100% |
| Piperacillin/tazobactam | 470 | 781 | 98% | 100% |
| Selected examples | ||||
| Prednisone | 305 | 613 | 93% | 100% |
| Insulin glargine | 244 | 492 | 81% | 98% |
| Spironolactone | 167 | 380 | 73% | 98% |
| Digoxin | 68 | 125 | 40% | 77% |
| Meropenem | 16 | 21 | 11% | 24% |
| Lab Test | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| ||||
| Fingerstick glucose | 12,869 | 24,946 | 100% | 100% |
| Renal panel (serum sodium) | 7,728 | 14,504 | 100% | 100% |
| Complete blood count (blood hematocrit) | 7,372 | 14,188 | 100% | 100% |
| International normalized ratio | 3,725 | 6,259 | 100% | 100% |
| Liver function tests (serum SGOT) | 1,570 | 3,180 | 99% | 100% |
| Urinalysis (urine nitrite) | 789 | 1,537 | 100% | 100% |
| Arterial blood gas (arterial blood pH) | 767 | 704 | 78% | 99% |
| Hemoglobin A1C | 485 | 1,177 | 96% | 100% |
| Fractional excretion of sodium (urine creatinine) | 336 | 677 | 85% | 99% |
| Lactic acid | 195 | 314 | 65% | 96% |
| Ferritin | 193 | 413 | 74% | 99% |
| Thyroid‐stimulating hormone | 184 | 391 | 55% | 64% |
| Lipase | 157 | 317 | 58% | 91% |
| Hepatitis C antibody | 139 | 327 | 70% | 98% |
| Haptoglobin | 101 | 208 | 46% | 83% |
| B‐type natriuretic peptide | 98 | 212 | 48% | 87% |
| Cortisol | 70 | 119 | 34% | 60% |
| Rapid plasma reagin | 70 | 173 | 44% | 82% |
| Urine legionella antigen | 70 | 126 | 38% | 64% |
| D‐dimer | 59 | 111 | 34% | 72% |
| Digoxin | 45 | 69 | 18% | 39% |
| Paracentesis labs (peritoneal fluid total protein) | 34 | 47 | 16% | 34% |
| Thoracentesis labs (pleural fluid WBC count) | 33 | 42 | 20% | 38% |
| C‐reactive protein | 30 | 65 | 17% | 34% |
| Lumbar puncture labs (cerebrospinal fluid WBC count) | 22 | 57 | 11% | 27% |
| Arthrocentesis (synovial fluid WBC count) | 14 | 23 | 9% | 23% |
| Radiology Test | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| ||||
| Chest,2 views,PA and lateral | 938 | 1,955 | 100% | 100% |
| Chest portable | 414 | 751 | 96% | 100% |
| CT head without contrast | 235 | 499 | 82% | 100% |
| CT abdomen with contrast | 218 | 365 | 59% | 71% |
| CT pelvis with contrast | 213 | 364 | 59% | 70% |
| CT chest with contrast | 163 | 351 | 75% | 99% |
| Ultrasound kidney, bilateral | 119 | 208 | 61% | 92% |
| Abdomen 1 view | 107 | 220 | 59% | 93% |
| Ultrasound liver | 100 | 183 | 48% | 82% |
| Modified barium swallow | 93 | 130 | 53% | 82% |
| PET scan | 93 | 181 | 49% | 79% |
| Selected examples | ||||
| Acute abdomen series | 85 | 177 | 48% | 81% |
| CT chest, PE protocol | 67 | 126 | 37% | 73% |
| MRI brain with andwithout contrast | 56 | 109 | 34% | 66% |
| Chest decubitus | 51 | 76 | 34% | 60% |
| Portable KUBfor Dobhoff placement | 42 | 62 | 30% | 48% |
| Ventilation/perfusion lung scan | 15 | 25 | 12% | 27% |
| Ultrasound thyroid | 8 | 16 | 5% | 17% |
Distinct Patients and Progress Notes
The mean number of progress notes written by a student was 67.2 (standard deviation [SD] 16.3). The mean number of distinct patients evaluated by a student during a rotation was 18.4 (SD 4.2). The mean number of team admissions per student rotation was 46.7 (SD 9.6) distinct patients.
Primary Diagnoses
A total of 2213 primary diagnoses were documented on patients assigned to students on AI rotations. A total of 5323 primary diagnoses were documented on patients assigned to other members of the team during the students' rotations. Therefore, the mean number of primary diagnoses seen by a student during a rotation was 58.9 (17.3 primary diagnoses for student‐assigned patients and 41.6 primary diagnoses for team patients). The students and teams encountered similar diagnoses (Table 1).
Problem List
Students and teams evaluated a total of 40,015 and 78,643 past medical problems, respectively. The mean number of problems seen by a student during a rotation was 927 (313 student, 614 team). Table 2 reports the most frequent problems assigned to primary student admissions. Students and teams evaluated similar problems. Hepatitis C (196 student, 410 team) was the only team problem that was in the team top 25 but not in the student top 25.
Medications
A total of 38,149 medications were prescribed to the students' primary patients. A total of 77,738 medications were prescribed to patients assigned to the rest of the team. The mean number of medication exposures for a student during a rotation was 905 (298 student, 607 team). The most frequently prescribed medications were similar between student and the team (Table 3). Team medications that were in the top 25 but not in the student top 25 included: hydralazine (300 student, 629 team), prednisone (305 student, 613 team), and oxycodone/acetaminophen (286 student, 608 team).
Labs
All laboratory tests with reported results were tallied. For common laboratory panels, single lab values (eg, serum hematocrit for a complete blood count) were selected as proxies to count the number of studies completed and evaluated. Table 4 shows a cross‐section of laboratory tests evaluated during AI rotations.
Radiology
A total of 6197 radiology tests were completed on patients assigned to students, whereas 11,761 radiology tests were completed on patients assigned to other team members. The mean number of radiology exposures for a student was 140 (48 student, 92 team). The most frequently seen radiology tests were similar between student and the team (Table 5).
DISCUSSION
As medical educators, we assume that the clinical training years allow learners to develop essential skills through their varied clinical experiences. Through exposure to direct patient care, to medical decision‐making scenarios, and to senior physician management practices, trainees build the knowledge base for independent practice. To ensure there is sufficient clinical exposure, data on what trainees are encountering may prove beneficial.
In this novel study, we quantified what learners encounter during a 1‐month team‐based inpatient rotation at a large teaching hospital. We effectively measured a number of aspects of internal medicine inpatient training that have been difficult to quantify in the past. The ability to extract learner‐specific data is becoming increasingly available in academic teaching hospitals. For example, VA medical centers have available a daily updated national data warehouse. The other steps necessary for using learner‐specific data include an understanding of the local inpatient processhow tests are ordered, what note titles are used by traineesas well as someone able to build the queries necessary for data extraction. Once built, data extraction should be able to continue as an automated process and used in real time by medical educators.
Our method of data collection has limitations. The orders placed on a learner's primary patients may not have been placed by the learner. For example, orders may have been placed by an overnight resident cross‐covering the learner's patients. We assumed that learners evaluated the results of all tests (or medication changes) that occurred at any time during their rotation, including cross‐cover periods or days off. In addition, our method for evaluating team exposure underestimates the number of team patients calculated for each learner by limiting the query only to patients whose hospital stay was completed before the student left the inpatient service. It is also difficult to know the how many of the exposures are realized by the learner. Differences in learner attention, contrasts in rounding styles, and varying presentation methods will affect the number of exposures truly attained by the learner. Finally, not all clinical exposures can be evaluated through review of an EMR. Clinical experiences, such as care coordination, patient education, and family counseling, cannot be easily extracted.
Data mining EMRs can enhance clinical medical education. Although our data collection was completed retrospectively, we could easily provide learner‐specific data in real time to ward attendings, chief residents, and program directors. This information could direct the development of teaching tools and individualization of curricula. Perhaps, even more importantly, it would also allow educators to define curricular gaps. Whether these gaps are due to the particular patient demographics of a medical center, the practice patterns and strengths of a particular institution, or career interests of a trainee, these gaps may skew the patient‐care experiences encountered by individual trainees. We can use these data to identify differences in clinical experience and then develop opportunities for learnersclinical, didactic, or simulatedto address deficiencies and provide well‐rounded clinical experiences.
Further investigation to better understand the relationship between direct patient‐care experience and clinical skill acquisition is needed. This information could help guide the development of standards on the number of exposures we expect our learners to have with different diagnostic or treatment modalities prior to independent practice. Using learner data to better understand the clinical experiences of our medical trainees, we can hopefully develop more precise and focused curricula to ensure we produce competent graduates.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Louis Stokes Cleveland VA Medical Center. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
- Accreditation Council for Graduate Medical Education. Program requirements for graduate medical education in internal medicine. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/2013-PR-FAQ-PIF/140_internal_medicine_07012013.pdf. Originally accessed December 18, 2012.
- , , . Residents make their lists and program directors check them twice: reviewing case logs. J Grad Med Educ. 2012;34:257–260.
- , , , et al. Quantifying internal medicine resident clinical experience using resident‐selected primary diagnosis codes. J Hosp Med. 2011;6(7):395–400.
- , , , et al. Documenting and comparing medical students' clinical experiences. JAMA. 2001;286:1035–1040.
- , , , , . Use of an electronic medical record to profile the continuity clinic experiences of primary care residents. Acad Med. 2005;80:390–394.
- , , . Using a Web‐based system to monitor practice profiles in primary care residency training. Can Fam Physician. 2011;57:1030–1037.
- , , . An automated electronic case log: using electronic information systems to assess training in emergency medicine. Acad Emergency Med. 2006;13:733–739.
- , , , , , . The design and implementation of an automated system for logging clinical experiences using an anesthesia information management system. Anesth Analg. 2011;112(2):422–429.
- , , , . Validation of an electronic system for recording medical student patient encounters. AMIA Annu Symp Proc. 2008;2008:510–514.
- . The structure and content of the medical subinternship: a national survey. J Gen Intern Med. 2001;16:550–553.
- , . Education for practice: the role of practical experience in undergraduate and general clinical training. Med Educ. 1989;23:189–195.
The clinical learning model in medical education, specifically in the third and fourth years of medical school and in residency and fellowship training, is driven by direct patient‐care experiences and complemented by mentorship and supervision provided by experienced physicians.[1] Despite the emphasis on experiential learning in medical school and graduate training, the ability of educators to quantify the clinical experiences of learners has been limited. Case logs, often self‐reported, are frequently required during educational rotations to attempt to measure clinical experience.[2] Logs have been utilized to document diagnoses, demographics, disease severity, procedures, and chief complaints.[3, 4, 5, 6] Unfortunately, self‐reported logs are vulnerable to delayed updates, misreported data, and unreliable data validation.[7, 8] Automated data collection has been shown to be more reliable than self‐reported logs.[8, 9]
The enhanced data mining methods now available allow educators to appraise learners' exposures during patient‐care interactions beyond just the diagnosis or chief complaint (eg, how many electrocardiograms do our learners evaluate during a cardiology rotation, how often do our learners gain experience prescribing a specific class of antibiotics, how many of the patients seen by our learners are diabetic). For example, a learner's interaction with a patient during an inpatient admission for community‐acquired pneumonia, at minimum, would include assessing of past medical history, reviewing outpatient medications and allergies, evaluating tests completed (chest x‐ray, complete blood count, blood cultures), prescribing antibiotics, and monitoring comorbidities. The lack of knowledge regarding the frequency and context of these exposures is a key gap in our understanding of the clinical experience of inpatient trainees. Additionally, there are no data on clinical exposures specific to team‐based inpatient learning. When a rotation is team‐based, the educational experience is not limited to the learner's assigned patients, and this arrangement allows for educational exposures from patients who are not the learner's primary assignments through experiences gained during team rounds, cross‐coverage assessments, and informal discussions of patient care.
In this study, we quantify the clinical exposures of learners on an acting internship (AI) rotation in internal medicine by utilizing the Veterans Affairs (VA) electronic medical records (EMR) as collected through the VA Veterans Integrated Service Network 10 Clinical Data Warehouse (CDW). The AI or subinternship is a medical school clinical rotation typically completed in the fourth year, where the learning experience is expected to mirror a 1‐month rotation of a first‐year resident.[10] The AI has historically been defined as an experiential curriculum, during which students assume many of the responsibilities and activities that they will manage as graduate medical trainees.[10, 11] The exposures of AI learners include primary diagnoses encountered, problem lists evaluated at the time of admission, medications prescribed, laboratory tests ordered, and radiologic imaging evaluated. We additionally explored the exposures of the AI learner's team to assess the experiences available through team‐based care.
METHODS
This study was completed at the Louis Stokes Veterans Affairs Medical Center (LSVAMC) in Cleveland, Ohio, which is an academic affiliate of the Case Western Reserve University School of Medicine. The study was approved by the LSVAMC institutional review board.
At the LSVAMC, the AI rotation in internal medicine is a 4‐week inpatient rotation for fourth‐year medical students, in which the student is assigned to an inpatient medical team consisting of an attending physician, a senior resident, and a combination of first‐year residents and acting interns. Compared to a first‐year resident, the acting intern is assigned approximately half of the number of admissions. The teams rounds as a group at least once per day. Acting interns are permitted to place orders and write notes in the EMR; all orders require a cosignature by a resident or attending physician to be released.
We identified students who rotated through the LSVAMC for an AI in internal medicine rotation from July 2008 to November 2011 from rotation records. Using the CDW, we queried student names and their rotation dates and analyzed the results using a Structured Query Language Query Analyzer. Each student's patient encounters during the rotation were identified. A patient encounter was defined as a patient for whom the student wrote at least 1 note titled either Medicine Admission Note or Medicine Inpatient Progress Note, on any of the dates during their AI rotation. We then counted the total number of notes written by each student during their rotation. A patient identifier is associated with each note. The number of distinct patient identifiers was also tallied to establish the total number of patients seen during the rotation by the individual student as the primary caregiver.
We associated each patient encounter with an inpatient admission profile that included patient admission and discharge dates, International Classification of Diseases, 9th Revision (ICD‐9) diagnosis codes, and admitting specialty. Primary diagnosis codes were queried for each admission and were counted for individual students and in aggregate. We tallied both the individual student and aggregate patient medications prescribed during the dates of admission and ordered to a patient location consistent with an acute medical ward (therefore excluding orders placed if a patient was transferred to an intensive care unit). Similar queries were completed for laboratory and radiological testing.
The VA EMR keeps an active problem list on each patient, and items are associated with an ICD‐9 code. To assemble the active problems available for evaluation by the student on the day of a patient's admission, we queried all problem list items added prior to, but not discontinued before, the day of admission. We then tallied the results for every patient seen by each individual student and in aggregate.
To assess the team exposures for each AI student, we queried all discharge summaries cosigned by the student's attending during the dates of the student's rotation. We assumed the student's team members wrote these discharge summaries. After excluding the student's patients, the resultant list represented the team patient exposures for each student. This list was also queried for the number of patients seen, primary diagnoses, medications, problems, labs, and radiology. The number of team admissions counted included all patients who spent at least 1 day on the team while the student was rotating. All other team exposure counts completed included only patients who were both admitted and discharged within the dates of the student's rotation.
RESULTS
An AI rotation is 4 weeks in duration. Students competed a total of 128 rotations from July 30, 2008 through November 21, 2011. We included all rotations during this time period in the analysis. Tables 1, 2, 3, 4, 5 report results in 4 categories. The Student category tallies the total number of specific exposures (diagnoses, problems, medications, lab values, or radiology tests) for all patients primarily assigned to a student. The Team category tallies the total number of exposures for all patients assigned to other members of the student's inpatient team. The Primary % category identifies the percentage of students who had at least 1 assigned patient with the evaluated clinical exposure. The All Patients % category identifies the percentage of students who had at least 1 student‐assigned patient or at least 1 team‐assigned patient with the evaluated clinical exposure.
| Diagnosis | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| Obstructive chronic bronchitis, with acute exacerbation | 102 | 241 | 57% | 91% |
| Pneumonia, organism unspecified | 91 | 228 | 49% | 91% |
| Acute renal failure, unspecified | 73 | 170 | 46% | 83% |
| Urinary tract infection, site not specified | 69 | 149 | 43% | 87% |
| Congestive heart failure, unspecified | 65 | 114 | 41% | 68% |
| Alcohol withdrawal | 46 | 101 | 26% | 61% |
| Alcoholic cirrhosis of liver | 28 | 98 | 16% | 57% |
| Cellulitis and abscess of leg, except foot | 26 | 61 | 18% | 45% |
| Acute pancreatitis | 23 | 51 | 16% | 43% |
| Intestinal infection due to Clostridium difficile | 22 | 30 | 17% | 33% |
| Malignant neoplasm of bronchus and lung, unspecified | 22 | 38 | 16% | 35% |
| Acute on chronic diastolic heart failure | 22 | 45 | 16% | 39% |
| Encounter for antineoplastic chemotherapy | 21 | 96 | 15% | 48% |
| Dehydration | 19 | 78 | 13% | 46% |
| Anemia, unspecified | 19 | 36 | 13% | 30% |
| Pneumonitis due to inhalation of food or vomitus | 19 | 25 | 13% | 24% |
| Syncope and collapse | 16 | 38 | 13% | 39% |
| Other pulmonary embolism and infarction | 15 | 41 | 12% | 26% |
| Unspecified pleural effusion | 15 | 37 | 10% | 34% |
| Acute respiratory failure | 15 | 42 | 11% | 35% |
| Problem | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| Hypertension | 1,665 | 3,280 | 100% | 100% |
| Tobacco use disorder | 1,350 | 2,759 | 100% | 100% |
| Unknown cause morbidity/mortality | 1,154 | 2,370 | 100% | 100% |
| Hyperlipidemia | 1,036 | 2,044 | 99% | 100% |
| Diabetes mellitus 2 without complication | 865 | 1,709 | 100% | 100% |
| Chronic airway obstruction | 600 | 1,132 | 100% | 100% |
| Esophageal reflux | 583 | 1,131 | 99% | 100% |
| Depressive disorder | 510 | 1,005 | 100% | 100% |
| Dermatophytosis of nail | 498 | 939 | 98% | 100% |
| Alcohol dependence | 441 | 966 | 97% | 100% |
| Chronic ischemic heart disease | 385 | 758 | 95% | 100% |
| Osteoarthritis | 383 | 791 | 96% | 100% |
| Lumbago | 357 | 692 | 97% | 100% |
| Current useanticoagulation | 342 | 629 | 94% | 100% |
| Anemia | 337 | 674 | 97% | 100% |
| Inhibited sex excitement | 317 | 610 | 91% | 100% |
| Congestive heart failure | 294 | 551 | 91% | 100% |
| Peripheral vascular disease | 288 | 529 | 88% | 99% |
| Sensorineural hearing loss | 280 | 535 | 88% | 99% |
| Post‐traumatic stress disorder | 274 | 528 | 91% | 100% |
| Pure hypercholesterolemia | 262 | 521 | 88% | 100% |
| Coronary atherosclerosis | 259 | 396 | 87% | 95% |
| Obesity | 246 | 509 | 89% | 99% |
| Atrial fibrillation | 236 | 469 | 85% | 100% |
| Gout | 216 | 389 | 85% | 100% |
| Medication | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| Omeprazole | 1,372 | 2,981 | 99% | 100% |
| Heparin | 1,067 | 2,271 | 95% | 96% |
| Sodium chloride 0.9% | 925 | 2,036 | 99% | 100% |
| Aspirin | 844 | 1,782 | 98% | 100% |
| Potassium chloride | 707 | 1,387 | 99% | 100% |
| Metoprolol tartrate | 693 | 1,318 | 98% | 100% |
| Insulin regular | 692 | 1,518 | 99% | 100% |
| Acetaminophen | 669 | 1,351 | 98% | 100% |
| Simvastatin | 648 | 1,408 | 99% | 100% |
| Lisinopril | 582 | 1,309 | 98% | 100% |
| Furosemide | 577 | 1,186 | 98% | 100% |
| Docusate sodium | 541 | 1,127 | 98% | 100% |
| Vancomycin | 531 | 977 | 98% | 100% |
| Multivitamin | 478 | 1,074 | 96% | 100% |
| Piperacillin/tazobactam | 470 | 781 | 98% | 100% |
| Selected examples | ||||
| Prednisone | 305 | 613 | 93% | 100% |
| Insulin glargine | 244 | 492 | 81% | 98% |
| Spironolactone | 167 | 380 | 73% | 98% |
| Digoxin | 68 | 125 | 40% | 77% |
| Meropenem | 16 | 21 | 11% | 24% |
| Lab Test | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| ||||
| Fingerstick glucose | 12,869 | 24,946 | 100% | 100% |
| Renal panel (serum sodium) | 7,728 | 14,504 | 100% | 100% |
| Complete blood count (blood hematocrit) | 7,372 | 14,188 | 100% | 100% |
| International normalized ratio | 3,725 | 6,259 | 100% | 100% |
| Liver function tests (serum SGOT) | 1,570 | 3,180 | 99% | 100% |
| Urinalysis (urine nitrite) | 789 | 1,537 | 100% | 100% |
| Arterial blood gas (arterial blood pH) | 767 | 704 | 78% | 99% |
| Hemoglobin A1C | 485 | 1,177 | 96% | 100% |
| Fractional excretion of sodium (urine creatinine) | 336 | 677 | 85% | 99% |
| Lactic acid | 195 | 314 | 65% | 96% |
| Ferritin | 193 | 413 | 74% | 99% |
| Thyroid‐stimulating hormone | 184 | 391 | 55% | 64% |
| Lipase | 157 | 317 | 58% | 91% |
| Hepatitis C antibody | 139 | 327 | 70% | 98% |
| Haptoglobin | 101 | 208 | 46% | 83% |
| B‐type natriuretic peptide | 98 | 212 | 48% | 87% |
| Cortisol | 70 | 119 | 34% | 60% |
| Rapid plasma reagin | 70 | 173 | 44% | 82% |
| Urine legionella antigen | 70 | 126 | 38% | 64% |
| D‐dimer | 59 | 111 | 34% | 72% |
| Digoxin | 45 | 69 | 18% | 39% |
| Paracentesis labs (peritoneal fluid total protein) | 34 | 47 | 16% | 34% |
| Thoracentesis labs (pleural fluid WBC count) | 33 | 42 | 20% | 38% |
| C‐reactive protein | 30 | 65 | 17% | 34% |
| Lumbar puncture labs (cerebrospinal fluid WBC count) | 22 | 57 | 11% | 27% |
| Arthrocentesis (synovial fluid WBC count) | 14 | 23 | 9% | 23% |
| Radiology Test | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| ||||
| Chest,2 views,PA and lateral | 938 | 1,955 | 100% | 100% |
| Chest portable | 414 | 751 | 96% | 100% |
| CT head without contrast | 235 | 499 | 82% | 100% |
| CT abdomen with contrast | 218 | 365 | 59% | 71% |
| CT pelvis with contrast | 213 | 364 | 59% | 70% |
| CT chest with contrast | 163 | 351 | 75% | 99% |
| Ultrasound kidney, bilateral | 119 | 208 | 61% | 92% |
| Abdomen 1 view | 107 | 220 | 59% | 93% |
| Ultrasound liver | 100 | 183 | 48% | 82% |
| Modified barium swallow | 93 | 130 | 53% | 82% |
| PET scan | 93 | 181 | 49% | 79% |
| Selected examples | ||||
| Acute abdomen series | 85 | 177 | 48% | 81% |
| CT chest, PE protocol | 67 | 126 | 37% | 73% |
| MRI brain with andwithout contrast | 56 | 109 | 34% | 66% |
| Chest decubitus | 51 | 76 | 34% | 60% |
| Portable KUBfor Dobhoff placement | 42 | 62 | 30% | 48% |
| Ventilation/perfusion lung scan | 15 | 25 | 12% | 27% |
| Ultrasound thyroid | 8 | 16 | 5% | 17% |
Distinct Patients and Progress Notes
The mean number of progress notes written by a student was 67.2 (standard deviation [SD] 16.3). The mean number of distinct patients evaluated by a student during a rotation was 18.4 (SD 4.2). The mean number of team admissions per student rotation was 46.7 (SD 9.6) distinct patients.
Primary Diagnoses
A total of 2213 primary diagnoses were documented on patients assigned to students on AI rotations. A total of 5323 primary diagnoses were documented on patients assigned to other members of the team during the students' rotations. Therefore, the mean number of primary diagnoses seen by a student during a rotation was 58.9 (17.3 primary diagnoses for student‐assigned patients and 41.6 primary diagnoses for team patients). The students and teams encountered similar diagnoses (Table 1).
Problem List
Students and teams evaluated a total of 40,015 and 78,643 past medical problems, respectively. The mean number of problems seen by a student during a rotation was 927 (313 student, 614 team). Table 2 reports the most frequent problems assigned to primary student admissions. Students and teams evaluated similar problems. Hepatitis C (196 student, 410 team) was the only team problem that was in the team top 25 but not in the student top 25.
Medications
A total of 38,149 medications were prescribed to the students' primary patients. A total of 77,738 medications were prescribed to patients assigned to the rest of the team. The mean number of medication exposures for a student during a rotation was 905 (298 student, 607 team). The most frequently prescribed medications were similar between student and the team (Table 3). Team medications that were in the top 25 but not in the student top 25 included: hydralazine (300 student, 629 team), prednisone (305 student, 613 team), and oxycodone/acetaminophen (286 student, 608 team).
Labs
All laboratory tests with reported results were tallied. For common laboratory panels, single lab values (eg, serum hematocrit for a complete blood count) were selected as proxies to count the number of studies completed and evaluated. Table 4 shows a cross‐section of laboratory tests evaluated during AI rotations.
Radiology
A total of 6197 radiology tests were completed on patients assigned to students, whereas 11,761 radiology tests were completed on patients assigned to other team members. The mean number of radiology exposures for a student was 140 (48 student, 92 team). The most frequently seen radiology tests were similar between student and the team (Table 5).
DISCUSSION
As medical educators, we assume that the clinical training years allow learners to develop essential skills through their varied clinical experiences. Through exposure to direct patient care, to medical decision‐making scenarios, and to senior physician management practices, trainees build the knowledge base for independent practice. To ensure there is sufficient clinical exposure, data on what trainees are encountering may prove beneficial.
In this novel study, we quantified what learners encounter during a 1‐month team‐based inpatient rotation at a large teaching hospital. We effectively measured a number of aspects of internal medicine inpatient training that have been difficult to quantify in the past. The ability to extract learner‐specific data is becoming increasingly available in academic teaching hospitals. For example, VA medical centers have available a daily updated national data warehouse. The other steps necessary for using learner‐specific data include an understanding of the local inpatient processhow tests are ordered, what note titles are used by traineesas well as someone able to build the queries necessary for data extraction. Once built, data extraction should be able to continue as an automated process and used in real time by medical educators.
Our method of data collection has limitations. The orders placed on a learner's primary patients may not have been placed by the learner. For example, orders may have been placed by an overnight resident cross‐covering the learner's patients. We assumed that learners evaluated the results of all tests (or medication changes) that occurred at any time during their rotation, including cross‐cover periods or days off. In addition, our method for evaluating team exposure underestimates the number of team patients calculated for each learner by limiting the query only to patients whose hospital stay was completed before the student left the inpatient service. It is also difficult to know the how many of the exposures are realized by the learner. Differences in learner attention, contrasts in rounding styles, and varying presentation methods will affect the number of exposures truly attained by the learner. Finally, not all clinical exposures can be evaluated through review of an EMR. Clinical experiences, such as care coordination, patient education, and family counseling, cannot be easily extracted.
Data mining EMRs can enhance clinical medical education. Although our data collection was completed retrospectively, we could easily provide learner‐specific data in real time to ward attendings, chief residents, and program directors. This information could direct the development of teaching tools and individualization of curricula. Perhaps, even more importantly, it would also allow educators to define curricular gaps. Whether these gaps are due to the particular patient demographics of a medical center, the practice patterns and strengths of a particular institution, or career interests of a trainee, these gaps may skew the patient‐care experiences encountered by individual trainees. We can use these data to identify differences in clinical experience and then develop opportunities for learnersclinical, didactic, or simulatedto address deficiencies and provide well‐rounded clinical experiences.
Further investigation to better understand the relationship between direct patient‐care experience and clinical skill acquisition is needed. This information could help guide the development of standards on the number of exposures we expect our learners to have with different diagnostic or treatment modalities prior to independent practice. Using learner data to better understand the clinical experiences of our medical trainees, we can hopefully develop more precise and focused curricula to ensure we produce competent graduates.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Louis Stokes Cleveland VA Medical Center. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
The clinical learning model in medical education, specifically in the third and fourth years of medical school and in residency and fellowship training, is driven by direct patient‐care experiences and complemented by mentorship and supervision provided by experienced physicians.[1] Despite the emphasis on experiential learning in medical school and graduate training, the ability of educators to quantify the clinical experiences of learners has been limited. Case logs, often self‐reported, are frequently required during educational rotations to attempt to measure clinical experience.[2] Logs have been utilized to document diagnoses, demographics, disease severity, procedures, and chief complaints.[3, 4, 5, 6] Unfortunately, self‐reported logs are vulnerable to delayed updates, misreported data, and unreliable data validation.[7, 8] Automated data collection has been shown to be more reliable than self‐reported logs.[8, 9]
The enhanced data mining methods now available allow educators to appraise learners' exposures during patient‐care interactions beyond just the diagnosis or chief complaint (eg, how many electrocardiograms do our learners evaluate during a cardiology rotation, how often do our learners gain experience prescribing a specific class of antibiotics, how many of the patients seen by our learners are diabetic). For example, a learner's interaction with a patient during an inpatient admission for community‐acquired pneumonia, at minimum, would include assessing of past medical history, reviewing outpatient medications and allergies, evaluating tests completed (chest x‐ray, complete blood count, blood cultures), prescribing antibiotics, and monitoring comorbidities. The lack of knowledge regarding the frequency and context of these exposures is a key gap in our understanding of the clinical experience of inpatient trainees. Additionally, there are no data on clinical exposures specific to team‐based inpatient learning. When a rotation is team‐based, the educational experience is not limited to the learner's assigned patients, and this arrangement allows for educational exposures from patients who are not the learner's primary assignments through experiences gained during team rounds, cross‐coverage assessments, and informal discussions of patient care.
In this study, we quantify the clinical exposures of learners on an acting internship (AI) rotation in internal medicine by utilizing the Veterans Affairs (VA) electronic medical records (EMR) as collected through the VA Veterans Integrated Service Network 10 Clinical Data Warehouse (CDW). The AI or subinternship is a medical school clinical rotation typically completed in the fourth year, where the learning experience is expected to mirror a 1‐month rotation of a first‐year resident.[10] The AI has historically been defined as an experiential curriculum, during which students assume many of the responsibilities and activities that they will manage as graduate medical trainees.[10, 11] The exposures of AI learners include primary diagnoses encountered, problem lists evaluated at the time of admission, medications prescribed, laboratory tests ordered, and radiologic imaging evaluated. We additionally explored the exposures of the AI learner's team to assess the experiences available through team‐based care.
METHODS
This study was completed at the Louis Stokes Veterans Affairs Medical Center (LSVAMC) in Cleveland, Ohio, which is an academic affiliate of the Case Western Reserve University School of Medicine. The study was approved by the LSVAMC institutional review board.
At the LSVAMC, the AI rotation in internal medicine is a 4‐week inpatient rotation for fourth‐year medical students, in which the student is assigned to an inpatient medical team consisting of an attending physician, a senior resident, and a combination of first‐year residents and acting interns. Compared to a first‐year resident, the acting intern is assigned approximately half of the number of admissions. The teams rounds as a group at least once per day. Acting interns are permitted to place orders and write notes in the EMR; all orders require a cosignature by a resident or attending physician to be released.
We identified students who rotated through the LSVAMC for an AI in internal medicine rotation from July 2008 to November 2011 from rotation records. Using the CDW, we queried student names and their rotation dates and analyzed the results using a Structured Query Language Query Analyzer. Each student's patient encounters during the rotation were identified. A patient encounter was defined as a patient for whom the student wrote at least 1 note titled either Medicine Admission Note or Medicine Inpatient Progress Note, on any of the dates during their AI rotation. We then counted the total number of notes written by each student during their rotation. A patient identifier is associated with each note. The number of distinct patient identifiers was also tallied to establish the total number of patients seen during the rotation by the individual student as the primary caregiver.
We associated each patient encounter with an inpatient admission profile that included patient admission and discharge dates, International Classification of Diseases, 9th Revision (ICD‐9) diagnosis codes, and admitting specialty. Primary diagnosis codes were queried for each admission and were counted for individual students and in aggregate. We tallied both the individual student and aggregate patient medications prescribed during the dates of admission and ordered to a patient location consistent with an acute medical ward (therefore excluding orders placed if a patient was transferred to an intensive care unit). Similar queries were completed for laboratory and radiological testing.
The VA EMR keeps an active problem list on each patient, and items are associated with an ICD‐9 code. To assemble the active problems available for evaluation by the student on the day of a patient's admission, we queried all problem list items added prior to, but not discontinued before, the day of admission. We then tallied the results for every patient seen by each individual student and in aggregate.
To assess the team exposures for each AI student, we queried all discharge summaries cosigned by the student's attending during the dates of the student's rotation. We assumed the student's team members wrote these discharge summaries. After excluding the student's patients, the resultant list represented the team patient exposures for each student. This list was also queried for the number of patients seen, primary diagnoses, medications, problems, labs, and radiology. The number of team admissions counted included all patients who spent at least 1 day on the team while the student was rotating. All other team exposure counts completed included only patients who were both admitted and discharged within the dates of the student's rotation.
RESULTS
An AI rotation is 4 weeks in duration. Students competed a total of 128 rotations from July 30, 2008 through November 21, 2011. We included all rotations during this time period in the analysis. Tables 1, 2, 3, 4, 5 report results in 4 categories. The Student category tallies the total number of specific exposures (diagnoses, problems, medications, lab values, or radiology tests) for all patients primarily assigned to a student. The Team category tallies the total number of exposures for all patients assigned to other members of the student's inpatient team. The Primary % category identifies the percentage of students who had at least 1 assigned patient with the evaluated clinical exposure. The All Patients % category identifies the percentage of students who had at least 1 student‐assigned patient or at least 1 team‐assigned patient with the evaluated clinical exposure.
| Diagnosis | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| Obstructive chronic bronchitis, with acute exacerbation | 102 | 241 | 57% | 91% |
| Pneumonia, organism unspecified | 91 | 228 | 49% | 91% |
| Acute renal failure, unspecified | 73 | 170 | 46% | 83% |
| Urinary tract infection, site not specified | 69 | 149 | 43% | 87% |
| Congestive heart failure, unspecified | 65 | 114 | 41% | 68% |
| Alcohol withdrawal | 46 | 101 | 26% | 61% |
| Alcoholic cirrhosis of liver | 28 | 98 | 16% | 57% |
| Cellulitis and abscess of leg, except foot | 26 | 61 | 18% | 45% |
| Acute pancreatitis | 23 | 51 | 16% | 43% |
| Intestinal infection due to Clostridium difficile | 22 | 30 | 17% | 33% |
| Malignant neoplasm of bronchus and lung, unspecified | 22 | 38 | 16% | 35% |
| Acute on chronic diastolic heart failure | 22 | 45 | 16% | 39% |
| Encounter for antineoplastic chemotherapy | 21 | 96 | 15% | 48% |
| Dehydration | 19 | 78 | 13% | 46% |
| Anemia, unspecified | 19 | 36 | 13% | 30% |
| Pneumonitis due to inhalation of food or vomitus | 19 | 25 | 13% | 24% |
| Syncope and collapse | 16 | 38 | 13% | 39% |
| Other pulmonary embolism and infarction | 15 | 41 | 12% | 26% |
| Unspecified pleural effusion | 15 | 37 | 10% | 34% |
| Acute respiratory failure | 15 | 42 | 11% | 35% |
| Problem | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| Hypertension | 1,665 | 3,280 | 100% | 100% |
| Tobacco use disorder | 1,350 | 2,759 | 100% | 100% |
| Unknown cause morbidity/mortality | 1,154 | 2,370 | 100% | 100% |
| Hyperlipidemia | 1,036 | 2,044 | 99% | 100% |
| Diabetes mellitus 2 without complication | 865 | 1,709 | 100% | 100% |
| Chronic airway obstruction | 600 | 1,132 | 100% | 100% |
| Esophageal reflux | 583 | 1,131 | 99% | 100% |
| Depressive disorder | 510 | 1,005 | 100% | 100% |
| Dermatophytosis of nail | 498 | 939 | 98% | 100% |
| Alcohol dependence | 441 | 966 | 97% | 100% |
| Chronic ischemic heart disease | 385 | 758 | 95% | 100% |
| Osteoarthritis | 383 | 791 | 96% | 100% |
| Lumbago | 357 | 692 | 97% | 100% |
| Current useanticoagulation | 342 | 629 | 94% | 100% |
| Anemia | 337 | 674 | 97% | 100% |
| Inhibited sex excitement | 317 | 610 | 91% | 100% |
| Congestive heart failure | 294 | 551 | 91% | 100% |
| Peripheral vascular disease | 288 | 529 | 88% | 99% |
| Sensorineural hearing loss | 280 | 535 | 88% | 99% |
| Post‐traumatic stress disorder | 274 | 528 | 91% | 100% |
| Pure hypercholesterolemia | 262 | 521 | 88% | 100% |
| Coronary atherosclerosis | 259 | 396 | 87% | 95% |
| Obesity | 246 | 509 | 89% | 99% |
| Atrial fibrillation | 236 | 469 | 85% | 100% |
| Gout | 216 | 389 | 85% | 100% |
| Medication | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| Omeprazole | 1,372 | 2,981 | 99% | 100% |
| Heparin | 1,067 | 2,271 | 95% | 96% |
| Sodium chloride 0.9% | 925 | 2,036 | 99% | 100% |
| Aspirin | 844 | 1,782 | 98% | 100% |
| Potassium chloride | 707 | 1,387 | 99% | 100% |
| Metoprolol tartrate | 693 | 1,318 | 98% | 100% |
| Insulin regular | 692 | 1,518 | 99% | 100% |
| Acetaminophen | 669 | 1,351 | 98% | 100% |
| Simvastatin | 648 | 1,408 | 99% | 100% |
| Lisinopril | 582 | 1,309 | 98% | 100% |
| Furosemide | 577 | 1,186 | 98% | 100% |
| Docusate sodium | 541 | 1,127 | 98% | 100% |
| Vancomycin | 531 | 977 | 98% | 100% |
| Multivitamin | 478 | 1,074 | 96% | 100% |
| Piperacillin/tazobactam | 470 | 781 | 98% | 100% |
| Selected examples | ||||
| Prednisone | 305 | 613 | 93% | 100% |
| Insulin glargine | 244 | 492 | 81% | 98% |
| Spironolactone | 167 | 380 | 73% | 98% |
| Digoxin | 68 | 125 | 40% | 77% |
| Meropenem | 16 | 21 | 11% | 24% |
| Lab Test | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| ||||
| Fingerstick glucose | 12,869 | 24,946 | 100% | 100% |
| Renal panel (serum sodium) | 7,728 | 14,504 | 100% | 100% |
| Complete blood count (blood hematocrit) | 7,372 | 14,188 | 100% | 100% |
| International normalized ratio | 3,725 | 6,259 | 100% | 100% |
| Liver function tests (serum SGOT) | 1,570 | 3,180 | 99% | 100% |
| Urinalysis (urine nitrite) | 789 | 1,537 | 100% | 100% |
| Arterial blood gas (arterial blood pH) | 767 | 704 | 78% | 99% |
| Hemoglobin A1C | 485 | 1,177 | 96% | 100% |
| Fractional excretion of sodium (urine creatinine) | 336 | 677 | 85% | 99% |
| Lactic acid | 195 | 314 | 65% | 96% |
| Ferritin | 193 | 413 | 74% | 99% |
| Thyroid‐stimulating hormone | 184 | 391 | 55% | 64% |
| Lipase | 157 | 317 | 58% | 91% |
| Hepatitis C antibody | 139 | 327 | 70% | 98% |
| Haptoglobin | 101 | 208 | 46% | 83% |
| B‐type natriuretic peptide | 98 | 212 | 48% | 87% |
| Cortisol | 70 | 119 | 34% | 60% |
| Rapid plasma reagin | 70 | 173 | 44% | 82% |
| Urine legionella antigen | 70 | 126 | 38% | 64% |
| D‐dimer | 59 | 111 | 34% | 72% |
| Digoxin | 45 | 69 | 18% | 39% |
| Paracentesis labs (peritoneal fluid total protein) | 34 | 47 | 16% | 34% |
| Thoracentesis labs (pleural fluid WBC count) | 33 | 42 | 20% | 38% |
| C‐reactive protein | 30 | 65 | 17% | 34% |
| Lumbar puncture labs (cerebrospinal fluid WBC count) | 22 | 57 | 11% | 27% |
| Arthrocentesis (synovial fluid WBC count) | 14 | 23 | 9% | 23% |
| Radiology Test | Student | Team | Primary% | All Patients % |
|---|---|---|---|---|
| ||||
| Chest,2 views,PA and lateral | 938 | 1,955 | 100% | 100% |
| Chest portable | 414 | 751 | 96% | 100% |
| CT head without contrast | 235 | 499 | 82% | 100% |
| CT abdomen with contrast | 218 | 365 | 59% | 71% |
| CT pelvis with contrast | 213 | 364 | 59% | 70% |
| CT chest with contrast | 163 | 351 | 75% | 99% |
| Ultrasound kidney, bilateral | 119 | 208 | 61% | 92% |
| Abdomen 1 view | 107 | 220 | 59% | 93% |
| Ultrasound liver | 100 | 183 | 48% | 82% |
| Modified barium swallow | 93 | 130 | 53% | 82% |
| PET scan | 93 | 181 | 49% | 79% |
| Selected examples | ||||
| Acute abdomen series | 85 | 177 | 48% | 81% |
| CT chest, PE protocol | 67 | 126 | 37% | 73% |
| MRI brain with andwithout contrast | 56 | 109 | 34% | 66% |
| Chest decubitus | 51 | 76 | 34% | 60% |
| Portable KUBfor Dobhoff placement | 42 | 62 | 30% | 48% |
| Ventilation/perfusion lung scan | 15 | 25 | 12% | 27% |
| Ultrasound thyroid | 8 | 16 | 5% | 17% |
Distinct Patients and Progress Notes
The mean number of progress notes written by a student was 67.2 (standard deviation [SD] 16.3). The mean number of distinct patients evaluated by a student during a rotation was 18.4 (SD 4.2). The mean number of team admissions per student rotation was 46.7 (SD 9.6) distinct patients.
Primary Diagnoses
A total of 2213 primary diagnoses were documented on patients assigned to students on AI rotations. A total of 5323 primary diagnoses were documented on patients assigned to other members of the team during the students' rotations. Therefore, the mean number of primary diagnoses seen by a student during a rotation was 58.9 (17.3 primary diagnoses for student‐assigned patients and 41.6 primary diagnoses for team patients). The students and teams encountered similar diagnoses (Table 1).
Problem List
Students and teams evaluated a total of 40,015 and 78,643 past medical problems, respectively. The mean number of problems seen by a student during a rotation was 927 (313 student, 614 team). Table 2 reports the most frequent problems assigned to primary student admissions. Students and teams evaluated similar problems. Hepatitis C (196 student, 410 team) was the only team problem that was in the team top 25 but not in the student top 25.
Medications
A total of 38,149 medications were prescribed to the students' primary patients. A total of 77,738 medications were prescribed to patients assigned to the rest of the team. The mean number of medication exposures for a student during a rotation was 905 (298 student, 607 team). The most frequently prescribed medications were similar between student and the team (Table 3). Team medications that were in the top 25 but not in the student top 25 included: hydralazine (300 student, 629 team), prednisone (305 student, 613 team), and oxycodone/acetaminophen (286 student, 608 team).
Labs
All laboratory tests with reported results were tallied. For common laboratory panels, single lab values (eg, serum hematocrit for a complete blood count) were selected as proxies to count the number of studies completed and evaluated. Table 4 shows a cross‐section of laboratory tests evaluated during AI rotations.
Radiology
A total of 6197 radiology tests were completed on patients assigned to students, whereas 11,761 radiology tests were completed on patients assigned to other team members. The mean number of radiology exposures for a student was 140 (48 student, 92 team). The most frequently seen radiology tests were similar between student and the team (Table 5).
DISCUSSION
As medical educators, we assume that the clinical training years allow learners to develop essential skills through their varied clinical experiences. Through exposure to direct patient care, to medical decision‐making scenarios, and to senior physician management practices, trainees build the knowledge base for independent practice. To ensure there is sufficient clinical exposure, data on what trainees are encountering may prove beneficial.
In this novel study, we quantified what learners encounter during a 1‐month team‐based inpatient rotation at a large teaching hospital. We effectively measured a number of aspects of internal medicine inpatient training that have been difficult to quantify in the past. The ability to extract learner‐specific data is becoming increasingly available in academic teaching hospitals. For example, VA medical centers have available a daily updated national data warehouse. The other steps necessary for using learner‐specific data include an understanding of the local inpatient processhow tests are ordered, what note titles are used by traineesas well as someone able to build the queries necessary for data extraction. Once built, data extraction should be able to continue as an automated process and used in real time by medical educators.
Our method of data collection has limitations. The orders placed on a learner's primary patients may not have been placed by the learner. For example, orders may have been placed by an overnight resident cross‐covering the learner's patients. We assumed that learners evaluated the results of all tests (or medication changes) that occurred at any time during their rotation, including cross‐cover periods or days off. In addition, our method for evaluating team exposure underestimates the number of team patients calculated for each learner by limiting the query only to patients whose hospital stay was completed before the student left the inpatient service. It is also difficult to know the how many of the exposures are realized by the learner. Differences in learner attention, contrasts in rounding styles, and varying presentation methods will affect the number of exposures truly attained by the learner. Finally, not all clinical exposures can be evaluated through review of an EMR. Clinical experiences, such as care coordination, patient education, and family counseling, cannot be easily extracted.
Data mining EMRs can enhance clinical medical education. Although our data collection was completed retrospectively, we could easily provide learner‐specific data in real time to ward attendings, chief residents, and program directors. This information could direct the development of teaching tools and individualization of curricula. Perhaps, even more importantly, it would also allow educators to define curricular gaps. Whether these gaps are due to the particular patient demographics of a medical center, the practice patterns and strengths of a particular institution, or career interests of a trainee, these gaps may skew the patient‐care experiences encountered by individual trainees. We can use these data to identify differences in clinical experience and then develop opportunities for learnersclinical, didactic, or simulatedto address deficiencies and provide well‐rounded clinical experiences.
Further investigation to better understand the relationship between direct patient‐care experience and clinical skill acquisition is needed. This information could help guide the development of standards on the number of exposures we expect our learners to have with different diagnostic or treatment modalities prior to independent practice. Using learner data to better understand the clinical experiences of our medical trainees, we can hopefully develop more precise and focused curricula to ensure we produce competent graduates.
Acknowledgments
This material is the result of work supported with resources and the use of facilities at the Louis Stokes Cleveland VA Medical Center. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
- Accreditation Council for Graduate Medical Education. Program requirements for graduate medical education in internal medicine. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/2013-PR-FAQ-PIF/140_internal_medicine_07012013.pdf. Originally accessed December 18, 2012.
- , , . Residents make their lists and program directors check them twice: reviewing case logs. J Grad Med Educ. 2012;34:257–260.
- , , , et al. Quantifying internal medicine resident clinical experience using resident‐selected primary diagnosis codes. J Hosp Med. 2011;6(7):395–400.
- , , , et al. Documenting and comparing medical students' clinical experiences. JAMA. 2001;286:1035–1040.
- , , , , . Use of an electronic medical record to profile the continuity clinic experiences of primary care residents. Acad Med. 2005;80:390–394.
- , , . Using a Web‐based system to monitor practice profiles in primary care residency training. Can Fam Physician. 2011;57:1030–1037.
- , , . An automated electronic case log: using electronic information systems to assess training in emergency medicine. Acad Emergency Med. 2006;13:733–739.
- , , , , , . The design and implementation of an automated system for logging clinical experiences using an anesthesia information management system. Anesth Analg. 2011;112(2):422–429.
- , , , . Validation of an electronic system for recording medical student patient encounters. AMIA Annu Symp Proc. 2008;2008:510–514.
- . The structure and content of the medical subinternship: a national survey. J Gen Intern Med. 2001;16:550–553.
- , . Education for practice: the role of practical experience in undergraduate and general clinical training. Med Educ. 1989;23:189–195.
- Accreditation Council for Graduate Medical Education. Program requirements for graduate medical education in internal medicine. Available at: http://www.acgme.org/acgmeweb/Portals/0/PFAssets/2013-PR-FAQ-PIF/140_internal_medicine_07012013.pdf. Originally accessed December 18, 2012.
- , , . Residents make their lists and program directors check them twice: reviewing case logs. J Grad Med Educ. 2012;34:257–260.
- , , , et al. Quantifying internal medicine resident clinical experience using resident‐selected primary diagnosis codes. J Hosp Med. 2011;6(7):395–400.
- , , , et al. Documenting and comparing medical students' clinical experiences. JAMA. 2001;286:1035–1040.
- , , , , . Use of an electronic medical record to profile the continuity clinic experiences of primary care residents. Acad Med. 2005;80:390–394.
- , , . Using a Web‐based system to monitor practice profiles in primary care residency training. Can Fam Physician. 2011;57:1030–1037.
- , , . An automated electronic case log: using electronic information systems to assess training in emergency medicine. Acad Emergency Med. 2006;13:733–739.
- , , , , , . The design and implementation of an automated system for logging clinical experiences using an anesthesia information management system. Anesth Analg. 2011;112(2):422–429.
- , , , . Validation of an electronic system for recording medical student patient encounters. AMIA Annu Symp Proc. 2008;2008:510–514.
- . The structure and content of the medical subinternship: a national survey. J Gen Intern Med. 2001;16:550–553.
- , . Education for practice: the role of practical experience in undergraduate and general clinical training. Med Educ. 1989;23:189–195.
© 2014 Society of Hospital Medicine