User login
PSA screening: The USPSTF got it right
In his book, How We Do Harm: A Doctor Breaks Ranks About Being Sick in America, Otis Brawley1 writes, “I believe that a man should know what we know, what we don’t know, and what we believe about prostate cancer. I have been concerned that many patients and physicians have confused what is believed with what is known.” I agree.
Common sense is what we believe. Does common sense trump science? Did the US Preventive Services Task Force (USPSTF) get it wrong? I don’t think so.
The USPSTF bases its recommendations on an explicit assessment of the science that informs us of the benefits and harms of a preventive service, and a judgment about the magnitude of net benefit.
So what do we know about the benefits of prostate cancer screening? When attempting to answer the question of whether an intervention is beneficial, there is a hierarchy of evidence, from most likely to be wrong to most likely to be right. Relying on our personal stories is the former; relying on well-conducted randomized trials is the latter.
In the multicenter Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial2 conducted in the United States, there was a nonsignificant increase in prostate-cancer mortality in the screening group, while the European Randomized Study of Screening for Prostate Cancer (ERSPC)3 trial showed a statistically significant absolute reduction of 0.10 prostate-cancer deaths per 1000 person-years after a median follow-up of 11 years. In the ERSPC trial, all-cause mortality was 19.1% in the screened group and 19.3% in the control group, a difference that was not significant. What we know is that after 10 years, even with aggressive treatment of 80% to 90% of screen-detected cancers, very few, if any, men will have lived longer because they were screened.
What don’t we know about the benefits? We don’t know whether following screened and nonscreened men for 15 or 20 years or longer will demonstrate a larger difference in mortality. Competing causes of mortality make it progressively less likely that men who are screened will actually live longer. The average age of death from prostate cancer is 80 years, and 70% of all deaths occur after age 75.4 Contrast those statistics to breast cancer, for which the average age of death is 68 years and 63% of all deaths occur before age 75.5
What do we believe about the benefits? Some certainly believe the trials must be wrong; common sense tells us that early detection and treatment must provide more benefit than what the evidence has shown. Common sense tells us that the decline in prostate cancer mortality over the past 2 decades must be due to screening, although the ERSPC results clearly show that neither the magnitude nor the timing of the decline can be attributed to screening.
What do we know—and not know—about the harms?
We know that much of the suffering from prostate cancer is a consequence of the diagnosis and management of the disease, rather than the disease itself. Complications of both diagnosis and treatment of prostate cancer are frequent and serious.6 We also know that many screen-detected cancers would never become apparent in a man’s lifetime without screening.
We don’t know the precise magnitude of overdiagnosis, although all estimates suggest it is substantial. In the ERSPC trial, 9.6% of the screened group received a prostate cancer diagnosis, vs 6.0% of the control group—a 60% increase in the rate of diagnosis. The recently published long-term results from the Prostate Cancer Prevention Trial7 are enlightening. Finasteride reduced the incidence of screen-detected cancers by 30%, with no impact on all-cause mortality at 18 years. If those screen-detected cancers had been a significant threat to health, then after 18 years we would have expected some mortality benefit from finasteride.
What do we believe about the harms of screening?
We believe that by being more conservative about who gets treated, we shift the balance of benefits and harms of screening. There is no question that reducing the burden of overdiagnosis and overtreatment would provide a welcome reduction in the harms. But can we do it?8
In the United States 90% of men found to have prostate cancer are treated (including about 75% of men with low-risk cancers).6 And although we hope to be able to reduce harms without changing benefits, we do not know what impact more conservative management of screen-detected cancers would have on the already small effect of screening on prostate cancer mortality.
So what is the balance of benefit and harms? Should we make that judgment on what we know, or on what we believe?
Science trumps common sense. For every 1000 men screened, at most, one will avoid a prostate cancer death at 10 years. But 30 to 40 will have erectile dysfunction, urinary incontinence, or both due to treatment, 2 men will experience a serious cardiovascular event, one will have a venous thromboembolic event, and one in 3000 screened will die from complications of surgical treatment.6
The USPSTF concluded that the benefits of PSA screening do not outweigh the harms, but acknowledged that shared decision making is still appropriate when a physician feels obliged to offer the test or a patient requests it.
What does shared decision making look like? Just offering screening and answering any questions is not good enough. We do an enormous disservice to our patients if we pretend that this is just a blood test and that we can decide later what to do with the information. Men will get biopsies and there will be complications. Cancer will be detected, and men will be treated, many unnecessarily.
We need to tell our patients that the likelihood of avoiding a prostate cancer death over 10 years as a result of regular PSA screening is at most very small, and that many more men will suffer the harms of unnecessary treatment than will benefit. A few will die prematurely as a result of the complications of treating a screen-detected cancer.
If, with this knowledge, a patient places a higher value on the possibility of avoiding a prostate cancer death than he does on the known harms of diagnosis and treatment, he can still decide to be screened. He has made an informed decision. However, routine screening for prostate cancer in the absence of a truly informed decision is unacceptable.
1. Brawley OW, Goldberg P. How We Do Harm: A Doctor Breaks Ranks About Being Sick in America. NY: St. Martin's Press; 2011.
2. Andriole GL, Crawford ED, Grub III RL, et al. Prostate cancer screening in the randomized prostate, lung, colorectal, and ovarian cancer screening trial. J Natl Cancer Inst. 2012;104:125-132.
3. Schröder FH, et al; ERSPC investigators. Prostate cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981-990.
4. SEER Stat Fact Sheets: Prostate. Surveillance, Epidemiology and End Results (SEER) Program Web site. 2012. Available at: http://seer.cancer.gov/statfacts/html/prost.html. Accessed August 28, 2013.
5. SEER Stat Fact Sheets: Breast. Surveillance, Epidemiology and End Results (SEER) Program Web site. 2012. Available at: http://seer.cancer.gov/statfacts/html/breast.html. Accessed August 28, 2013.
6. Chou R, Crosswell, JM, Dana T, et al. Screening for prostate cancer: A review of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2011;155:762-771.
7. Thompson IM Jr, Goodman PJ, Tangen CM, et al. Long-term survival of participants in the Prostate Cancer Prevention Trial. N Engl J Med. 2013;369:603-610.
8. Sartor AO. Surveillance for prostate cancer: are the proceduralists running amok? Oncology (Williston Park). 2013;27:523, 589.
In his book, How We Do Harm: A Doctor Breaks Ranks About Being Sick in America, Otis Brawley1 writes, “I believe that a man should know what we know, what we don’t know, and what we believe about prostate cancer. I have been concerned that many patients and physicians have confused what is believed with what is known.” I agree.
Common sense is what we believe. Does common sense trump science? Did the US Preventive Services Task Force (USPSTF) get it wrong? I don’t think so.
The USPSTF bases its recommendations on an explicit assessment of the science that informs us of the benefits and harms of a preventive service, and a judgment about the magnitude of net benefit.
So what do we know about the benefits of prostate cancer screening? When attempting to answer the question of whether an intervention is beneficial, there is a hierarchy of evidence, from most likely to be wrong to most likely to be right. Relying on our personal stories is the former; relying on well-conducted randomized trials is the latter.
In the multicenter Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial2 conducted in the United States, there was a nonsignificant increase in prostate-cancer mortality in the screening group, while the European Randomized Study of Screening for Prostate Cancer (ERSPC)3 trial showed a statistically significant absolute reduction of 0.10 prostate-cancer deaths per 1000 person-years after a median follow-up of 11 years. In the ERSPC trial, all-cause mortality was 19.1% in the screened group and 19.3% in the control group, a difference that was not significant. What we know is that after 10 years, even with aggressive treatment of 80% to 90% of screen-detected cancers, very few, if any, men will have lived longer because they were screened.
What don’t we know about the benefits? We don’t know whether following screened and nonscreened men for 15 or 20 years or longer will demonstrate a larger difference in mortality. Competing causes of mortality make it progressively less likely that men who are screened will actually live longer. The average age of death from prostate cancer is 80 years, and 70% of all deaths occur after age 75.4 Contrast those statistics to breast cancer, for which the average age of death is 68 years and 63% of all deaths occur before age 75.5
What do we believe about the benefits? Some certainly believe the trials must be wrong; common sense tells us that early detection and treatment must provide more benefit than what the evidence has shown. Common sense tells us that the decline in prostate cancer mortality over the past 2 decades must be due to screening, although the ERSPC results clearly show that neither the magnitude nor the timing of the decline can be attributed to screening.
What do we know—and not know—about the harms?
We know that much of the suffering from prostate cancer is a consequence of the diagnosis and management of the disease, rather than the disease itself. Complications of both diagnosis and treatment of prostate cancer are frequent and serious.6 We also know that many screen-detected cancers would never become apparent in a man’s lifetime without screening.
We don’t know the precise magnitude of overdiagnosis, although all estimates suggest it is substantial. In the ERSPC trial, 9.6% of the screened group received a prostate cancer diagnosis, vs 6.0% of the control group—a 60% increase in the rate of diagnosis. The recently published long-term results from the Prostate Cancer Prevention Trial7 are enlightening. Finasteride reduced the incidence of screen-detected cancers by 30%, with no impact on all-cause mortality at 18 years. If those screen-detected cancers had been a significant threat to health, then after 18 years we would have expected some mortality benefit from finasteride.
What do we believe about the harms of screening?
We believe that by being more conservative about who gets treated, we shift the balance of benefits and harms of screening. There is no question that reducing the burden of overdiagnosis and overtreatment would provide a welcome reduction in the harms. But can we do it?8
In the United States 90% of men found to have prostate cancer are treated (including about 75% of men with low-risk cancers).6 And although we hope to be able to reduce harms without changing benefits, we do not know what impact more conservative management of screen-detected cancers would have on the already small effect of screening on prostate cancer mortality.
So what is the balance of benefit and harms? Should we make that judgment on what we know, or on what we believe?
Science trumps common sense. For every 1000 men screened, at most, one will avoid a prostate cancer death at 10 years. But 30 to 40 will have erectile dysfunction, urinary incontinence, or both due to treatment, 2 men will experience a serious cardiovascular event, one will have a venous thromboembolic event, and one in 3000 screened will die from complications of surgical treatment.6
The USPSTF concluded that the benefits of PSA screening do not outweigh the harms, but acknowledged that shared decision making is still appropriate when a physician feels obliged to offer the test or a patient requests it.
What does shared decision making look like? Just offering screening and answering any questions is not good enough. We do an enormous disservice to our patients if we pretend that this is just a blood test and that we can decide later what to do with the information. Men will get biopsies and there will be complications. Cancer will be detected, and men will be treated, many unnecessarily.
We need to tell our patients that the likelihood of avoiding a prostate cancer death over 10 years as a result of regular PSA screening is at most very small, and that many more men will suffer the harms of unnecessary treatment than will benefit. A few will die prematurely as a result of the complications of treating a screen-detected cancer.
If, with this knowledge, a patient places a higher value on the possibility of avoiding a prostate cancer death than he does on the known harms of diagnosis and treatment, he can still decide to be screened. He has made an informed decision. However, routine screening for prostate cancer in the absence of a truly informed decision is unacceptable.
In his book, How We Do Harm: A Doctor Breaks Ranks About Being Sick in America, Otis Brawley1 writes, “I believe that a man should know what we know, what we don’t know, and what we believe about prostate cancer. I have been concerned that many patients and physicians have confused what is believed with what is known.” I agree.
Common sense is what we believe. Does common sense trump science? Did the US Preventive Services Task Force (USPSTF) get it wrong? I don’t think so.
The USPSTF bases its recommendations on an explicit assessment of the science that informs us of the benefits and harms of a preventive service, and a judgment about the magnitude of net benefit.
So what do we know about the benefits of prostate cancer screening? When attempting to answer the question of whether an intervention is beneficial, there is a hierarchy of evidence, from most likely to be wrong to most likely to be right. Relying on our personal stories is the former; relying on well-conducted randomized trials is the latter.
In the multicenter Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial2 conducted in the United States, there was a nonsignificant increase in prostate-cancer mortality in the screening group, while the European Randomized Study of Screening for Prostate Cancer (ERSPC)3 trial showed a statistically significant absolute reduction of 0.10 prostate-cancer deaths per 1000 person-years after a median follow-up of 11 years. In the ERSPC trial, all-cause mortality was 19.1% in the screened group and 19.3% in the control group, a difference that was not significant. What we know is that after 10 years, even with aggressive treatment of 80% to 90% of screen-detected cancers, very few, if any, men will have lived longer because they were screened.
What don’t we know about the benefits? We don’t know whether following screened and nonscreened men for 15 or 20 years or longer will demonstrate a larger difference in mortality. Competing causes of mortality make it progressively less likely that men who are screened will actually live longer. The average age of death from prostate cancer is 80 years, and 70% of all deaths occur after age 75.4 Contrast those statistics to breast cancer, for which the average age of death is 68 years and 63% of all deaths occur before age 75.5
What do we believe about the benefits? Some certainly believe the trials must be wrong; common sense tells us that early detection and treatment must provide more benefit than what the evidence has shown. Common sense tells us that the decline in prostate cancer mortality over the past 2 decades must be due to screening, although the ERSPC results clearly show that neither the magnitude nor the timing of the decline can be attributed to screening.
What do we know—and not know—about the harms?
We know that much of the suffering from prostate cancer is a consequence of the diagnosis and management of the disease, rather than the disease itself. Complications of both diagnosis and treatment of prostate cancer are frequent and serious.6 We also know that many screen-detected cancers would never become apparent in a man’s lifetime without screening.
We don’t know the precise magnitude of overdiagnosis, although all estimates suggest it is substantial. In the ERSPC trial, 9.6% of the screened group received a prostate cancer diagnosis, vs 6.0% of the control group—a 60% increase in the rate of diagnosis. The recently published long-term results from the Prostate Cancer Prevention Trial7 are enlightening. Finasteride reduced the incidence of screen-detected cancers by 30%, with no impact on all-cause mortality at 18 years. If those screen-detected cancers had been a significant threat to health, then after 18 years we would have expected some mortality benefit from finasteride.
What do we believe about the harms of screening?
We believe that by being more conservative about who gets treated, we shift the balance of benefits and harms of screening. There is no question that reducing the burden of overdiagnosis and overtreatment would provide a welcome reduction in the harms. But can we do it?8
In the United States 90% of men found to have prostate cancer are treated (including about 75% of men with low-risk cancers).6 And although we hope to be able to reduce harms without changing benefits, we do not know what impact more conservative management of screen-detected cancers would have on the already small effect of screening on prostate cancer mortality.
So what is the balance of benefit and harms? Should we make that judgment on what we know, or on what we believe?
Science trumps common sense. For every 1000 men screened, at most, one will avoid a prostate cancer death at 10 years. But 30 to 40 will have erectile dysfunction, urinary incontinence, or both due to treatment, 2 men will experience a serious cardiovascular event, one will have a venous thromboembolic event, and one in 3000 screened will die from complications of surgical treatment.6
The USPSTF concluded that the benefits of PSA screening do not outweigh the harms, but acknowledged that shared decision making is still appropriate when a physician feels obliged to offer the test or a patient requests it.
What does shared decision making look like? Just offering screening and answering any questions is not good enough. We do an enormous disservice to our patients if we pretend that this is just a blood test and that we can decide later what to do with the information. Men will get biopsies and there will be complications. Cancer will be detected, and men will be treated, many unnecessarily.
We need to tell our patients that the likelihood of avoiding a prostate cancer death over 10 years as a result of regular PSA screening is at most very small, and that many more men will suffer the harms of unnecessary treatment than will benefit. A few will die prematurely as a result of the complications of treating a screen-detected cancer.
If, with this knowledge, a patient places a higher value on the possibility of avoiding a prostate cancer death than he does on the known harms of diagnosis and treatment, he can still decide to be screened. He has made an informed decision. However, routine screening for prostate cancer in the absence of a truly informed decision is unacceptable.
1. Brawley OW, Goldberg P. How We Do Harm: A Doctor Breaks Ranks About Being Sick in America. NY: St. Martin's Press; 2011.
2. Andriole GL, Crawford ED, Grub III RL, et al. Prostate cancer screening in the randomized prostate, lung, colorectal, and ovarian cancer screening trial. J Natl Cancer Inst. 2012;104:125-132.
3. Schröder FH, et al; ERSPC investigators. Prostate cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981-990.
4. SEER Stat Fact Sheets: Prostate. Surveillance, Epidemiology and End Results (SEER) Program Web site. 2012. Available at: http://seer.cancer.gov/statfacts/html/prost.html. Accessed August 28, 2013.
5. SEER Stat Fact Sheets: Breast. Surveillance, Epidemiology and End Results (SEER) Program Web site. 2012. Available at: http://seer.cancer.gov/statfacts/html/breast.html. Accessed August 28, 2013.
6. Chou R, Crosswell, JM, Dana T, et al. Screening for prostate cancer: A review of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2011;155:762-771.
7. Thompson IM Jr, Goodman PJ, Tangen CM, et al. Long-term survival of participants in the Prostate Cancer Prevention Trial. N Engl J Med. 2013;369:603-610.
8. Sartor AO. Surveillance for prostate cancer: are the proceduralists running amok? Oncology (Williston Park). 2013;27:523, 589.
1. Brawley OW, Goldberg P. How We Do Harm: A Doctor Breaks Ranks About Being Sick in America. NY: St. Martin's Press; 2011.
2. Andriole GL, Crawford ED, Grub III RL, et al. Prostate cancer screening in the randomized prostate, lung, colorectal, and ovarian cancer screening trial. J Natl Cancer Inst. 2012;104:125-132.
3. Schröder FH, et al; ERSPC investigators. Prostate cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981-990.
4. SEER Stat Fact Sheets: Prostate. Surveillance, Epidemiology and End Results (SEER) Program Web site. 2012. Available at: http://seer.cancer.gov/statfacts/html/prost.html. Accessed August 28, 2013.
5. SEER Stat Fact Sheets: Breast. Surveillance, Epidemiology and End Results (SEER) Program Web site. 2012. Available at: http://seer.cancer.gov/statfacts/html/breast.html. Accessed August 28, 2013.
6. Chou R, Crosswell, JM, Dana T, et al. Screening for prostate cancer: A review of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2011;155:762-771.
7. Thompson IM Jr, Goodman PJ, Tangen CM, et al. Long-term survival of participants in the Prostate Cancer Prevention Trial. N Engl J Med. 2013;369:603-610.
8. Sartor AO. Surveillance for prostate cancer: are the proceduralists running amok? Oncology (Williston Park). 2013;27:523, 589.
Update on pelvic floor dysfunction: Focus on urinary incontinence
Urinary incontinence (UI) affects almost half of all women in the United States.1,2 Estimates suggest that the prevalence of UI gradually rises during young adult life, comes to a broad plateau in middle age, and then steadily increases from that plateau after age 65. Therefore, over the next 40 years, as the elderly population expands in size, the number of women affected by UI will significantly grow.3
For patients with UI, a multitude of therapeutic options are available. Which option is the best for your patient? In this article, we aim to answer that question by interpreting the results of four randomized trials, each of which directly compare two available treatment options. The first study examines patients with stress urinary incontinence (SUI), comparing the patients’ subjective improvement in urinary leakage and bladder function at 12 months after randomization to treatment with physiotherapy or midurethral sling surgery.
The three other trials examine patients with overactive bladder (OAB) and urgency urinary incontinence (UUI). Each trial directly compares the use of anticholinergic medications to an alternate treatment modality. Currently, anticholinergic medications and behavioral therapy are the recommended first-line therapies for OAB. Unfortunately, anticholinergic medications have poor patient compliance and significant systemic side effects.4 Caution should be used when considering anticholinergic medications in patients with impaired gastric emptying or a history of urinary retention. They also should be used with caution in elderly patients who are extremely frail. Additionally, clearance from an ophthalmologist must be obtained prior to starting anticholinergic medication in patients with narrow-angle glaucoma.5 Due to poor adherence and potential side effects, there is a growing effort to discover alternative treatment modalities that are safe and effective. Therefore, we chose to examine trials comparing: mirabegron versus tolterodine, percutaneous tibial nerve stimulation versus tolterodine, and onabotulinumtoxinA versus anticholingeric medications.
UI defined
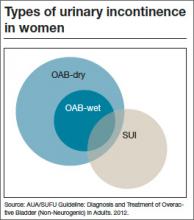
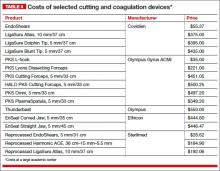
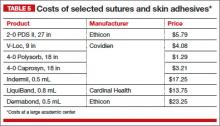
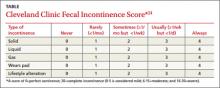
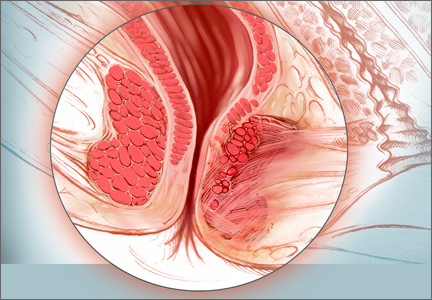
Before discussing treatment options, we want to clarify the main types of UI (FIGURE). UI is defined as the complaint of involuntary loss of urine. UI can be subdivided into SUI, OAB/UUI, or mixed urinary incontinence.6 While there are other less common genitourinary etiologies that can lead to UI, nongenitourinary etiologies are prevalent and can aggravate existing SUI or OAB (TABLE).
SUI is the complaint of involuntary loss of urine on effort or physical exertion (such as during sporting activities) or on sneezing or coughing. Often, SUI can be diagnosed by patient report alone and surgery can be considered in symptomatic patients who demonstrate cough leakage on physical examination and normal postvoid residual volumes.
UUI is the involuntary loss of urine associated with urgency; it often occurs in the setting of OAB, which is defined as the syndrome of urinary urgency, usually accompanied by frequency and nocturia, with or without UUI, in the absence of urinary tract infection or other obvious pathology (such as neurologic dysfunction, infection, or urologic neoplasm). OAB-dry is present when patients do not have leakage with urgency, but are bothered by urgency, frequency, and/or nocturia. OAB-wet occurs when a patient has urgencyincontinence.
The presence of both SUI and OAB/UUI is known as mixed urinary incontinence. Stress and urgency urinary symptoms often present together. In fact, 10% to 30% of women with stress symptoms are found to have bladder overactivity on subsequent evaluation.2,7 Therefore, it is important to take a good history and consider urodynamic evaluation to confirm the diagnosis of SUI prior to surgery in women with mixed stress and urge symptoms, a history of a previous surgery for incontinence, or when there is a poor correlation of physical examination findings to reported symptoms.
Is surgery a first-line option for patients with SUI?
Labrie J, Berghmans BL, Fischer K, et al. Surgery versus physiotherapy for stress urinary incontinence. NEJM. 2013;369(12):1124−1133.
Physiotherapy, including pelvic floor muscle training (“Kegel exercises”), is utilized as a first-line treatment option for women with SUI that carries minimal risk for the patient. Midurethral sling surgery is often recommended if an initial trial of conservative treatment fails.7 Up to 50% of women treated with pelvic floor physiotherapy will ultimately undergo surgery to treat their SUI.8
Related article: Does urodynamic testing before surgery for stress incontinence improve outcomes? G. Willy Davila, MD (Examining the Evidence, December 2012)
Details of the study
This was a randomized, multicenter trial of women aged 35 to 80 years with moderate to severe SUI. After excluding women with previous incontinence surgery and stage 2 or higher pelvic organ prolapse, 460 participants were randomly assigned to undergo either a midurethral sling surgery or physiotherapy (pelvic floor muscle training). The primary outcome was subjective improvement in urinary leakage and bladder function at 12 months, as measured by the Patient Global Impression of Improvement (PGI-I), a 7-point Likert scale ranging from “very much worse” to “very much better.”
In an intention-to-treat analysis, subjective improvement at 12 months was significantly higher in women randomized to midurethral sling surgery than in women randomized to physiotherapy (91% vs 64%, respectively).
Ten percent of patients had adverse events (AEs); all were related to surgery. The most common AEs were hematoma, vaginal epithelial perforation, and bladder perforation.
Notably, women had the option to cross over to the other treatment modality if they desired. In the physiotherapy group, 49% of women elected to cross over to surgery, while 11% of those who underwent midurethral sling surgery elected to cross over to physiotherapy during the 12-month follow-up period. When analyzing results by treatment received, the investigators found that the proportion of women who reported improvement was significantly lower among women who underwent physiotherapy only (32%), versus sling only (94%), or sling after physiotherapy (91%).
This randomized trial was well-designed and included a variety of treatment centers (university and general hospitals) with interventions performed by experienced surgeons (all of whom had performed at least 20 sling surgeries) and physiotherapists educated specifically in pelvic floor physiotherapy. The study population was limited to patients with moderate to severe SUI as defined by the Sandvik severity index.9 Therefore, these results may not be applicable to patients with milder symptoms, for whom physiotherapy has been recommended as first-line therapy with consideration of surgery if physiotherapy fails to sufficiently improve symptoms.7
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Women with moderate to severe SUI without significant prolapse or a history of prior incontinence surgery have significantly better outcomes at 12 months after undergoing midurethral sling surgery versus physiotherapy. Physiotherapy carries little to no risk of adverse effects. Women with moderate to severe SUI should be counseled regarding the risks and benefits of both physiotherapy and midurethral sling surgery as initial treatment options.
Because stress and urgency urinary symptoms often present together, it is important to consider urodynamic evaluation to confirm SUI prior to surgery in women with:
• mixed stress and urge symptoms
• a history of a previous surgery for incontinence, or
• poor correlation of physical examination findings to reported symptoms.
Safety and tolerability of mirabegron versus tolterodine for OAB
Chapple CR, Kaplan SA, Mitcheson D, et al. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a beta(3)-adrenoceptor agonist, in overactive bladder. Eur Urol. 2013;63(2):296−305.
In the bladder, beta3-receptors located within the detrusor smooth muscle facilitate urine storage by relaxing the detrusor, enabling the bladder to fill.10 The activation of beta3-receptors is thought to increase the bladder’s ability to store urine, with the goal of decreasing urgency, frequency, nocturia, and urgency incontinence. An alternative to anticholinergic medications, mirabegron is a beta3-agonist approved by the US Food and Drug Administration (FDA) in 2012 for clinical use in the treatment of OAB.
Details of the study
Chapple and colleagues aimed to assess the 12-month efficacy and safety of mirabegron in a randomized, double-blind active controlled trial. The primary outcome was incidence and severity of treatment-emergent adverse effects (TEAEs); the secondary outcome was the change in OAB symptoms from baseline to up to 12 months. Patients experiencing OAB symptoms for more than 3 months were eligible and were subsequently enrolled if they averaged 8 or more voids per day and 3 or more episodes of urgency with or without incontinence on a 3-day bladder diary. A total of 2,444 patients were randomly assigned in a 1:1:1 fashion to mirabegron 50 mg daily, mirabegron 100 mg daily, or tolterodine extended release (ER) 4 mg daily.
There was a similar incidence (60% to 63%) of TEAEs across all three groups. The most common TEAEs were hypertension (defined as average systolic blood pressure [BP] >140 mm Hg or average diastolic BP >90 mm Hg at two consecutive visits), UTI, headache, nasopharyngitis, and constipation. The adjusted mean changes in BP from baseline to final visit were less than 1 mm Hg for both systolic and diastolic BP for patients taking both doses of mirabegron, as well as for patients taking tolterodine. The incidence of dry mouth was higher in the tolterodine group than the mirabegron groups. Mirabegron 50 mg daily and 100 mg daily improved incontinence symptoms within 1 month of starting therapy; the degree of improvement was similar to that seen in the patients taking tolterodine ER 4 mg daily.
Related article: New overactive bladder treatment approved by the FDA (August 2012)
Some caveats
This study was well-designed to assess the safety and tolerability of mirabegron versus tolterodine. The doses utilized in the study were at or above the FDA-approved dosage of 25 mg to 50 mg daily for OAB treatment. Although investigators found mirabegron to be a safe alternative to anticholinergic medication, the study was not designed or powered to examine the efficacy of mirabegron versus tolterodine. No formal comparison of efficacy was made between mirabegron or tolterodine, or between the 50-mg and 100-mg doses of mirabegron. Moreover, 81% of participants had been treated with mirabegron in earlier Phase 3 studies, so most were not treatment naïve, limiting the applicability of results.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Mirabegron should be considered as a potential treatment option for patients who demonstrate poor tolerance of or response to anticholinergic medications; however, caution should be used in patients with severe uncontrolled high BP, end-stage kidney disease, or severe liver impairment.
Consider percutaneous tibial nerve stimulation over tolterodine for OAB in select patients
Peters KM, Macdiarmid SA, Wooldridge LS, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: Results from the overactive bladder innovative therapy trial. J Urol. 2009;182(3):1055−1061.
Neuromodulation utilizes electrical stimulation to improve bladder function and decrease OAB symptoms. First developed in the early 1980s by McGuire and colleagues, percutaneous tibial nerve stimulation (PTNS) was approved by the FDA in 2000 as Urgent PC and provides an outpatient, nonimplantable neuromodulation alternative to medication therapy for patients with OAB.11,12 By directly stimulating the posterior tibial nerve, PTNS works via the S3 sacral nerve plexus to alter the micturition reflex and improve bladder function.
Details of the study
Patients were eligible for the study if they demonstrated 8 or more voids per day on a 3-day bladder diary (whether or not they had a history of previous anticholinergic drug use). A total of 100 ambulatory adults with OAB symptoms were enrolled and randomly assigned to PTNS 30-minutes per week or tolterodine ER 4 mg daily.
At 12 weeks, both groups demonstrated a significant improvement in OAB measures as well as validated symptom severity and quality-of-life questionnaire scores. Subjective assessment of improvement in OAB symptoms was significantly greater in the PTNS group than in the tolterodine group (79.5% vs 54.8%, respectively; P = .01). However, mean reduction of voids for 24 hours was not significantly different between the two groups.
Both treatments were well tolerated, with only 15% to 16% of patients in both groups reporting mild to moderate side effects. The tolterodine group did have a significantly higher risk of dry mouth; however, the risk of constipation was not significantly different between the groups.
Study limitations
The authors performed an important multicenter, nonblinded, randomized, controlled trial, which was one of the first trials to directly compare two OAB therapies. The generalizability of the findings were limited, as the cohort included mostly patients with dry OAB who had no objective measures on UUI episodes. In addition, this trial had a limited observation period of only 12 weeks. Information regarding the effect of treatment after cessation of weekly PTNS therapy was not examined. Therefore, we are not able to determine whether repeat sessions provide adequate maintenance in the long term.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
PTNS 30 minutes daily is as effective as tolterodine ER 4 mg daily for 12 weeks in reducing OAB symptoms. PTNS is a safe alternative that should be considered in patients with OAB who poorly tolerate or have contraindications to medication therapy.
OnabotulinumtoxinA is an effective therapy for OAB
Visco AG, Brubaker L, Richter HE, et al. Anticholinergic therapy vs onabotulinumtoxinA for urgency urinary incontinence. NEJM. 2012;367(19):1803−1813.
The newest therapy for OAB is onabotulinumtoxinA, or Botox, which was FDA approved this year for the treatment of OAB in adults who cannot use or do not tolerate anticholinergic medications. Recommended doses are 100 U onabotulinumtoxinA in patients with idiopathic refractory OAB and 200 U onabotulinumtoxinA for patients with neurogenic OAB.
OnabotulinumtoxinA is a neurotoxin that blocks synaptic transmission at the neuromuscular junction to cause muscle paralysis and atrophy.13 Injecting onabotulinumtoxinA into the detrusor smooth muscle should relax the bladder and decrease sensations of urgency and frequency to achieve a longer duration of time for bladder filling and reduce the risk of urgency incontinence.
Effects of onabotulinumtoxinA appear to wear off over time, and patients may require repeat injections. Side effects of onabotulinumtoxinA therapy include an increased risk of UTI and the potential for urinary retention requiring intermittent self-catheterization.
Related article: Update on Pelvic Floor Dysfunction Autumn L. Edenfield, MD, and Cindy L. Amundsen, MD (October 2012)
Details of the study
The Anticholinergic Versus Botulinum Toxin Comparison (ABC) study was a multicenter, randomized, double-blind, double-placebo–controlled trial conducted in women without known neurologic disease with moderate to severe UUI (defined as >5 UUI episodes on a 3-day bladder diary). Women were randomly assigned to a single intradetrusor injection of 100 U onabotulinumtoxinA plus oral placebo or to a single intradetrusor injection of saline plus solifenacin 5 mg daily (with the option of dose escalation and then switching to trospium XR if no improvement was seen).
Of the 241 women included in the final analysis, approximately 70% in each group reported adequate control of symptoms at 6 months. Adequate control was defined as a response of “agree strongly” or “agree” to the statement: “This treatment has given me adequate control of my urinary leakage.” Women in the onabotulinumtoxinA group were significantly more likely than women in the anticholinergic medication group to report complete resolution of UUI at 6 months (27% vs 13%, P = .003). However, the mean reduction in episodes of UUI per day and the improvements in quality-of-life questionnaire scores were found to be similar. Interestingly, worse baseline UUI was associated with greater reduction in episodes of UUI for both therapies.
This was a rigorous and well-executed double-blind, double-placebo−controlled randomized trial. By utilizing broad inclusion criteria and enrolling patients both with and without previous exposure to anticholinergic medications, the generalizability of study findings are greatly improved. Because this study did not examine the effect or efficacy of repeat injections, these findings have limited applicability to patients undergoing multiple onabotulinumtoxinA injections.
When considering use in your patient population, keep the possible side effects in mind.There were important differences in the side effects experienced with each therapy. Specifically, while the anticholinergic group had a higher frequency of dry mouth (46% anticholinergic vs 31% onabotulinumtoxinA, P = .02), the onabotulinumtoxinA group demonstrated higher rates of incomplete bladder emptying requiring catheterization (peak of 5% at 2 months) and greater risk of UTI (33% onabotulinumtoxinA vs 13% anticholinergic, P <.001).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This study showed that, among women with UUI, anticholinergic medication and onabotulinumtoxinA are equally effective in reducing UUI episodes and improving quality of life. It is important to consider the side effect profile, determine the patient’s preferences, and weigh the risks and benefits of each therapy when deciding what is the best treatment for your individual patient.
We want to hear from you! Tell us what you think.
- Anger JT, Saigal CS, Litwin MS. The prevalence of urinary incontinence among community dwelling adult women: Results from the National Health and Nutrition Examination Survey. J Urol. 2006;175(2):601–604.
- Dooley Y, Kenton K, Cao G, et al. Urinary incontinence prevalence: Results from the National Health and Nutrition Examination Survey. J Urol. 2008;179(2):656–661.
- Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in U.S. Women: 2010 to 2050. Obstetr Gynecol. 2009;114(6):1278–1283.
- Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU Guideline. Americal Urological Association. http://www.auanet.org/common/pdf/education/clinical-guidance/Overactive-Bladder.pdf. Published 2012. Revised June 11, 2013. Accessed October 21, 2013.
- Yu YF, Nichol MB, Yu AP, Ahn J. Persistence and adherence of medications for chronic overactive bladder/urinary incontinence in the California Medicaid program. Value Health. 2005;8(4):495–505.
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
- ACOG Practice Bulletin No. 63: Urinary incontinence in women. American College of Obstetricians and Gynecologists. Obstetr Gynecol. 2005;105(6):1533–1545.
- Bo K, Kvarstein B, Nygaard I. Lower urinary tract symptoms and pelvic floor muscle exercise adherence after 15 years. Obstetr Gynecol. 2005;105(5 Pt 1):999–1005.
- Sandvik H, Hunskaar S, Seim A, Hermstad R, Vanvik A, Bratt H. Validation of a severity index in female urinary incontinence and its implementation in an epidemiological survey. J Epidemiol Community Health. 1993;47(6):497–499.
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9(6):453–466.
- Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597.
- McGuire EJ, Zhang SC, Horwinski ER, Lytton B. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol. 1983;129(1):78–79.
- Schiavo G, Santucci A, Dasgupta BR, et al. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993;335(1):99–103.
Urinary incontinence (UI) affects almost half of all women in the United States.1,2 Estimates suggest that the prevalence of UI gradually rises during young adult life, comes to a broad plateau in middle age, and then steadily increases from that plateau after age 65. Therefore, over the next 40 years, as the elderly population expands in size, the number of women affected by UI will significantly grow.3
For patients with UI, a multitude of therapeutic options are available. Which option is the best for your patient? In this article, we aim to answer that question by interpreting the results of four randomized trials, each of which directly compare two available treatment options. The first study examines patients with stress urinary incontinence (SUI), comparing the patients’ subjective improvement in urinary leakage and bladder function at 12 months after randomization to treatment with physiotherapy or midurethral sling surgery.
The three other trials examine patients with overactive bladder (OAB) and urgency urinary incontinence (UUI). Each trial directly compares the use of anticholinergic medications to an alternate treatment modality. Currently, anticholinergic medications and behavioral therapy are the recommended first-line therapies for OAB. Unfortunately, anticholinergic medications have poor patient compliance and significant systemic side effects.4 Caution should be used when considering anticholinergic medications in patients with impaired gastric emptying or a history of urinary retention. They also should be used with caution in elderly patients who are extremely frail. Additionally, clearance from an ophthalmologist must be obtained prior to starting anticholinergic medication in patients with narrow-angle glaucoma.5 Due to poor adherence and potential side effects, there is a growing effort to discover alternative treatment modalities that are safe and effective. Therefore, we chose to examine trials comparing: mirabegron versus tolterodine, percutaneous tibial nerve stimulation versus tolterodine, and onabotulinumtoxinA versus anticholingeric medications.
UI defined
Before discussing treatment options, we want to clarify the main types of UI (FIGURE). UI is defined as the complaint of involuntary loss of urine. UI can be subdivided into SUI, OAB/UUI, or mixed urinary incontinence.6 While there are other less common genitourinary etiologies that can lead to UI, nongenitourinary etiologies are prevalent and can aggravate existing SUI or OAB (TABLE).
SUI is the complaint of involuntary loss of urine on effort or physical exertion (such as during sporting activities) or on sneezing or coughing. Often, SUI can be diagnosed by patient report alone and surgery can be considered in symptomatic patients who demonstrate cough leakage on physical examination and normal postvoid residual volumes.
UUI is the involuntary loss of urine associated with urgency; it often occurs in the setting of OAB, which is defined as the syndrome of urinary urgency, usually accompanied by frequency and nocturia, with or without UUI, in the absence of urinary tract infection or other obvious pathology (such as neurologic dysfunction, infection, or urologic neoplasm). OAB-dry is present when patients do not have leakage with urgency, but are bothered by urgency, frequency, and/or nocturia. OAB-wet occurs when a patient has urgencyincontinence.
The presence of both SUI and OAB/UUI is known as mixed urinary incontinence. Stress and urgency urinary symptoms often present together. In fact, 10% to 30% of women with stress symptoms are found to have bladder overactivity on subsequent evaluation.2,7 Therefore, it is important to take a good history and consider urodynamic evaluation to confirm the diagnosis of SUI prior to surgery in women with mixed stress and urge symptoms, a history of a previous surgery for incontinence, or when there is a poor correlation of physical examination findings to reported symptoms.
Is surgery a first-line option for patients with SUI?
Labrie J, Berghmans BL, Fischer K, et al. Surgery versus physiotherapy for stress urinary incontinence. NEJM. 2013;369(12):1124−1133.
Physiotherapy, including pelvic floor muscle training (“Kegel exercises”), is utilized as a first-line treatment option for women with SUI that carries minimal risk for the patient. Midurethral sling surgery is often recommended if an initial trial of conservative treatment fails.7 Up to 50% of women treated with pelvic floor physiotherapy will ultimately undergo surgery to treat their SUI.8
Related article: Does urodynamic testing before surgery for stress incontinence improve outcomes? G. Willy Davila, MD (Examining the Evidence, December 2012)
Details of the study
This was a randomized, multicenter trial of women aged 35 to 80 years with moderate to severe SUI. After excluding women with previous incontinence surgery and stage 2 or higher pelvic organ prolapse, 460 participants were randomly assigned to undergo either a midurethral sling surgery or physiotherapy (pelvic floor muscle training). The primary outcome was subjective improvement in urinary leakage and bladder function at 12 months, as measured by the Patient Global Impression of Improvement (PGI-I), a 7-point Likert scale ranging from “very much worse” to “very much better.”
In an intention-to-treat analysis, subjective improvement at 12 months was significantly higher in women randomized to midurethral sling surgery than in women randomized to physiotherapy (91% vs 64%, respectively).
Ten percent of patients had adverse events (AEs); all were related to surgery. The most common AEs were hematoma, vaginal epithelial perforation, and bladder perforation.
Notably, women had the option to cross over to the other treatment modality if they desired. In the physiotherapy group, 49% of women elected to cross over to surgery, while 11% of those who underwent midurethral sling surgery elected to cross over to physiotherapy during the 12-month follow-up period. When analyzing results by treatment received, the investigators found that the proportion of women who reported improvement was significantly lower among women who underwent physiotherapy only (32%), versus sling only (94%), or sling after physiotherapy (91%).
This randomized trial was well-designed and included a variety of treatment centers (university and general hospitals) with interventions performed by experienced surgeons (all of whom had performed at least 20 sling surgeries) and physiotherapists educated specifically in pelvic floor physiotherapy. The study population was limited to patients with moderate to severe SUI as defined by the Sandvik severity index.9 Therefore, these results may not be applicable to patients with milder symptoms, for whom physiotherapy has been recommended as first-line therapy with consideration of surgery if physiotherapy fails to sufficiently improve symptoms.7
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Women with moderate to severe SUI without significant prolapse or a history of prior incontinence surgery have significantly better outcomes at 12 months after undergoing midurethral sling surgery versus physiotherapy. Physiotherapy carries little to no risk of adverse effects. Women with moderate to severe SUI should be counseled regarding the risks and benefits of both physiotherapy and midurethral sling surgery as initial treatment options.
Because stress and urgency urinary symptoms often present together, it is important to consider urodynamic evaluation to confirm SUI prior to surgery in women with:
• mixed stress and urge symptoms
• a history of a previous surgery for incontinence, or
• poor correlation of physical examination findings to reported symptoms.
Safety and tolerability of mirabegron versus tolterodine for OAB
Chapple CR, Kaplan SA, Mitcheson D, et al. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a beta(3)-adrenoceptor agonist, in overactive bladder. Eur Urol. 2013;63(2):296−305.
In the bladder, beta3-receptors located within the detrusor smooth muscle facilitate urine storage by relaxing the detrusor, enabling the bladder to fill.10 The activation of beta3-receptors is thought to increase the bladder’s ability to store urine, with the goal of decreasing urgency, frequency, nocturia, and urgency incontinence. An alternative to anticholinergic medications, mirabegron is a beta3-agonist approved by the US Food and Drug Administration (FDA) in 2012 for clinical use in the treatment of OAB.
Details of the study
Chapple and colleagues aimed to assess the 12-month efficacy and safety of mirabegron in a randomized, double-blind active controlled trial. The primary outcome was incidence and severity of treatment-emergent adverse effects (TEAEs); the secondary outcome was the change in OAB symptoms from baseline to up to 12 months. Patients experiencing OAB symptoms for more than 3 months were eligible and were subsequently enrolled if they averaged 8 or more voids per day and 3 or more episodes of urgency with or without incontinence on a 3-day bladder diary. A total of 2,444 patients were randomly assigned in a 1:1:1 fashion to mirabegron 50 mg daily, mirabegron 100 mg daily, or tolterodine extended release (ER) 4 mg daily.
There was a similar incidence (60% to 63%) of TEAEs across all three groups. The most common TEAEs were hypertension (defined as average systolic blood pressure [BP] >140 mm Hg or average diastolic BP >90 mm Hg at two consecutive visits), UTI, headache, nasopharyngitis, and constipation. The adjusted mean changes in BP from baseline to final visit were less than 1 mm Hg for both systolic and diastolic BP for patients taking both doses of mirabegron, as well as for patients taking tolterodine. The incidence of dry mouth was higher in the tolterodine group than the mirabegron groups. Mirabegron 50 mg daily and 100 mg daily improved incontinence symptoms within 1 month of starting therapy; the degree of improvement was similar to that seen in the patients taking tolterodine ER 4 mg daily.
Related article: New overactive bladder treatment approved by the FDA (August 2012)
Some caveats
This study was well-designed to assess the safety and tolerability of mirabegron versus tolterodine. The doses utilized in the study were at or above the FDA-approved dosage of 25 mg to 50 mg daily for OAB treatment. Although investigators found mirabegron to be a safe alternative to anticholinergic medication, the study was not designed or powered to examine the efficacy of mirabegron versus tolterodine. No formal comparison of efficacy was made between mirabegron or tolterodine, or between the 50-mg and 100-mg doses of mirabegron. Moreover, 81% of participants had been treated with mirabegron in earlier Phase 3 studies, so most were not treatment naïve, limiting the applicability of results.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Mirabegron should be considered as a potential treatment option for patients who demonstrate poor tolerance of or response to anticholinergic medications; however, caution should be used in patients with severe uncontrolled high BP, end-stage kidney disease, or severe liver impairment.
Consider percutaneous tibial nerve stimulation over tolterodine for OAB in select patients
Peters KM, Macdiarmid SA, Wooldridge LS, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: Results from the overactive bladder innovative therapy trial. J Urol. 2009;182(3):1055−1061.
Neuromodulation utilizes electrical stimulation to improve bladder function and decrease OAB symptoms. First developed in the early 1980s by McGuire and colleagues, percutaneous tibial nerve stimulation (PTNS) was approved by the FDA in 2000 as Urgent PC and provides an outpatient, nonimplantable neuromodulation alternative to medication therapy for patients with OAB.11,12 By directly stimulating the posterior tibial nerve, PTNS works via the S3 sacral nerve plexus to alter the micturition reflex and improve bladder function.
Details of the study
Patients were eligible for the study if they demonstrated 8 or more voids per day on a 3-day bladder diary (whether or not they had a history of previous anticholinergic drug use). A total of 100 ambulatory adults with OAB symptoms were enrolled and randomly assigned to PTNS 30-minutes per week or tolterodine ER 4 mg daily.
At 12 weeks, both groups demonstrated a significant improvement in OAB measures as well as validated symptom severity and quality-of-life questionnaire scores. Subjective assessment of improvement in OAB symptoms was significantly greater in the PTNS group than in the tolterodine group (79.5% vs 54.8%, respectively; P = .01). However, mean reduction of voids for 24 hours was not significantly different between the two groups.
Both treatments were well tolerated, with only 15% to 16% of patients in both groups reporting mild to moderate side effects. The tolterodine group did have a significantly higher risk of dry mouth; however, the risk of constipation was not significantly different between the groups.
Study limitations
The authors performed an important multicenter, nonblinded, randomized, controlled trial, which was one of the first trials to directly compare two OAB therapies. The generalizability of the findings were limited, as the cohort included mostly patients with dry OAB who had no objective measures on UUI episodes. In addition, this trial had a limited observation period of only 12 weeks. Information regarding the effect of treatment after cessation of weekly PTNS therapy was not examined. Therefore, we are not able to determine whether repeat sessions provide adequate maintenance in the long term.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
PTNS 30 minutes daily is as effective as tolterodine ER 4 mg daily for 12 weeks in reducing OAB symptoms. PTNS is a safe alternative that should be considered in patients with OAB who poorly tolerate or have contraindications to medication therapy.
OnabotulinumtoxinA is an effective therapy for OAB
Visco AG, Brubaker L, Richter HE, et al. Anticholinergic therapy vs onabotulinumtoxinA for urgency urinary incontinence. NEJM. 2012;367(19):1803−1813.
The newest therapy for OAB is onabotulinumtoxinA, or Botox, which was FDA approved this year for the treatment of OAB in adults who cannot use or do not tolerate anticholinergic medications. Recommended doses are 100 U onabotulinumtoxinA in patients with idiopathic refractory OAB and 200 U onabotulinumtoxinA for patients with neurogenic OAB.
OnabotulinumtoxinA is a neurotoxin that blocks synaptic transmission at the neuromuscular junction to cause muscle paralysis and atrophy.13 Injecting onabotulinumtoxinA into the detrusor smooth muscle should relax the bladder and decrease sensations of urgency and frequency to achieve a longer duration of time for bladder filling and reduce the risk of urgency incontinence.
Effects of onabotulinumtoxinA appear to wear off over time, and patients may require repeat injections. Side effects of onabotulinumtoxinA therapy include an increased risk of UTI and the potential for urinary retention requiring intermittent self-catheterization.
Related article: Update on Pelvic Floor Dysfunction Autumn L. Edenfield, MD, and Cindy L. Amundsen, MD (October 2012)
Details of the study
The Anticholinergic Versus Botulinum Toxin Comparison (ABC) study was a multicenter, randomized, double-blind, double-placebo–controlled trial conducted in women without known neurologic disease with moderate to severe UUI (defined as >5 UUI episodes on a 3-day bladder diary). Women were randomly assigned to a single intradetrusor injection of 100 U onabotulinumtoxinA plus oral placebo or to a single intradetrusor injection of saline plus solifenacin 5 mg daily (with the option of dose escalation and then switching to trospium XR if no improvement was seen).
Of the 241 women included in the final analysis, approximately 70% in each group reported adequate control of symptoms at 6 months. Adequate control was defined as a response of “agree strongly” or “agree” to the statement: “This treatment has given me adequate control of my urinary leakage.” Women in the onabotulinumtoxinA group were significantly more likely than women in the anticholinergic medication group to report complete resolution of UUI at 6 months (27% vs 13%, P = .003). However, the mean reduction in episodes of UUI per day and the improvements in quality-of-life questionnaire scores were found to be similar. Interestingly, worse baseline UUI was associated with greater reduction in episodes of UUI for both therapies.
This was a rigorous and well-executed double-blind, double-placebo−controlled randomized trial. By utilizing broad inclusion criteria and enrolling patients both with and without previous exposure to anticholinergic medications, the generalizability of study findings are greatly improved. Because this study did not examine the effect or efficacy of repeat injections, these findings have limited applicability to patients undergoing multiple onabotulinumtoxinA injections.
When considering use in your patient population, keep the possible side effects in mind.There were important differences in the side effects experienced with each therapy. Specifically, while the anticholinergic group had a higher frequency of dry mouth (46% anticholinergic vs 31% onabotulinumtoxinA, P = .02), the onabotulinumtoxinA group demonstrated higher rates of incomplete bladder emptying requiring catheterization (peak of 5% at 2 months) and greater risk of UTI (33% onabotulinumtoxinA vs 13% anticholinergic, P <.001).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This study showed that, among women with UUI, anticholinergic medication and onabotulinumtoxinA are equally effective in reducing UUI episodes and improving quality of life. It is important to consider the side effect profile, determine the patient’s preferences, and weigh the risks and benefits of each therapy when deciding what is the best treatment for your individual patient.
We want to hear from you! Tell us what you think.
Urinary incontinence (UI) affects almost half of all women in the United States.1,2 Estimates suggest that the prevalence of UI gradually rises during young adult life, comes to a broad plateau in middle age, and then steadily increases from that plateau after age 65. Therefore, over the next 40 years, as the elderly population expands in size, the number of women affected by UI will significantly grow.3
For patients with UI, a multitude of therapeutic options are available. Which option is the best for your patient? In this article, we aim to answer that question by interpreting the results of four randomized trials, each of which directly compare two available treatment options. The first study examines patients with stress urinary incontinence (SUI), comparing the patients’ subjective improvement in urinary leakage and bladder function at 12 months after randomization to treatment with physiotherapy or midurethral sling surgery.
The three other trials examine patients with overactive bladder (OAB) and urgency urinary incontinence (UUI). Each trial directly compares the use of anticholinergic medications to an alternate treatment modality. Currently, anticholinergic medications and behavioral therapy are the recommended first-line therapies for OAB. Unfortunately, anticholinergic medications have poor patient compliance and significant systemic side effects.4 Caution should be used when considering anticholinergic medications in patients with impaired gastric emptying or a history of urinary retention. They also should be used with caution in elderly patients who are extremely frail. Additionally, clearance from an ophthalmologist must be obtained prior to starting anticholinergic medication in patients with narrow-angle glaucoma.5 Due to poor adherence and potential side effects, there is a growing effort to discover alternative treatment modalities that are safe and effective. Therefore, we chose to examine trials comparing: mirabegron versus tolterodine, percutaneous tibial nerve stimulation versus tolterodine, and onabotulinumtoxinA versus anticholingeric medications.
UI defined
Before discussing treatment options, we want to clarify the main types of UI (FIGURE). UI is defined as the complaint of involuntary loss of urine. UI can be subdivided into SUI, OAB/UUI, or mixed urinary incontinence.6 While there are other less common genitourinary etiologies that can lead to UI, nongenitourinary etiologies are prevalent and can aggravate existing SUI or OAB (TABLE).
SUI is the complaint of involuntary loss of urine on effort or physical exertion (such as during sporting activities) or on sneezing or coughing. Often, SUI can be diagnosed by patient report alone and surgery can be considered in symptomatic patients who demonstrate cough leakage on physical examination and normal postvoid residual volumes.
UUI is the involuntary loss of urine associated with urgency; it often occurs in the setting of OAB, which is defined as the syndrome of urinary urgency, usually accompanied by frequency and nocturia, with or without UUI, in the absence of urinary tract infection or other obvious pathology (such as neurologic dysfunction, infection, or urologic neoplasm). OAB-dry is present when patients do not have leakage with urgency, but are bothered by urgency, frequency, and/or nocturia. OAB-wet occurs when a patient has urgencyincontinence.
The presence of both SUI and OAB/UUI is known as mixed urinary incontinence. Stress and urgency urinary symptoms often present together. In fact, 10% to 30% of women with stress symptoms are found to have bladder overactivity on subsequent evaluation.2,7 Therefore, it is important to take a good history and consider urodynamic evaluation to confirm the diagnosis of SUI prior to surgery in women with mixed stress and urge symptoms, a history of a previous surgery for incontinence, or when there is a poor correlation of physical examination findings to reported symptoms.
Is surgery a first-line option for patients with SUI?
Labrie J, Berghmans BL, Fischer K, et al. Surgery versus physiotherapy for stress urinary incontinence. NEJM. 2013;369(12):1124−1133.
Physiotherapy, including pelvic floor muscle training (“Kegel exercises”), is utilized as a first-line treatment option for women with SUI that carries minimal risk for the patient. Midurethral sling surgery is often recommended if an initial trial of conservative treatment fails.7 Up to 50% of women treated with pelvic floor physiotherapy will ultimately undergo surgery to treat their SUI.8
Related article: Does urodynamic testing before surgery for stress incontinence improve outcomes? G. Willy Davila, MD (Examining the Evidence, December 2012)
Details of the study
This was a randomized, multicenter trial of women aged 35 to 80 years with moderate to severe SUI. After excluding women with previous incontinence surgery and stage 2 or higher pelvic organ prolapse, 460 participants were randomly assigned to undergo either a midurethral sling surgery or physiotherapy (pelvic floor muscle training). The primary outcome was subjective improvement in urinary leakage and bladder function at 12 months, as measured by the Patient Global Impression of Improvement (PGI-I), a 7-point Likert scale ranging from “very much worse” to “very much better.”
In an intention-to-treat analysis, subjective improvement at 12 months was significantly higher in women randomized to midurethral sling surgery than in women randomized to physiotherapy (91% vs 64%, respectively).
Ten percent of patients had adverse events (AEs); all were related to surgery. The most common AEs were hematoma, vaginal epithelial perforation, and bladder perforation.
Notably, women had the option to cross over to the other treatment modality if they desired. In the physiotherapy group, 49% of women elected to cross over to surgery, while 11% of those who underwent midurethral sling surgery elected to cross over to physiotherapy during the 12-month follow-up period. When analyzing results by treatment received, the investigators found that the proportion of women who reported improvement was significantly lower among women who underwent physiotherapy only (32%), versus sling only (94%), or sling after physiotherapy (91%).
This randomized trial was well-designed and included a variety of treatment centers (university and general hospitals) with interventions performed by experienced surgeons (all of whom had performed at least 20 sling surgeries) and physiotherapists educated specifically in pelvic floor physiotherapy. The study population was limited to patients with moderate to severe SUI as defined by the Sandvik severity index.9 Therefore, these results may not be applicable to patients with milder symptoms, for whom physiotherapy has been recommended as first-line therapy with consideration of surgery if physiotherapy fails to sufficiently improve symptoms.7
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Women with moderate to severe SUI without significant prolapse or a history of prior incontinence surgery have significantly better outcomes at 12 months after undergoing midurethral sling surgery versus physiotherapy. Physiotherapy carries little to no risk of adverse effects. Women with moderate to severe SUI should be counseled regarding the risks and benefits of both physiotherapy and midurethral sling surgery as initial treatment options.
Because stress and urgency urinary symptoms often present together, it is important to consider urodynamic evaluation to confirm SUI prior to surgery in women with:
• mixed stress and urge symptoms
• a history of a previous surgery for incontinence, or
• poor correlation of physical examination findings to reported symptoms.
Safety and tolerability of mirabegron versus tolterodine for OAB
Chapple CR, Kaplan SA, Mitcheson D, et al. Randomized double-blind, active-controlled phase 3 study to assess 12-month safety and efficacy of mirabegron, a beta(3)-adrenoceptor agonist, in overactive bladder. Eur Urol. 2013;63(2):296−305.
In the bladder, beta3-receptors located within the detrusor smooth muscle facilitate urine storage by relaxing the detrusor, enabling the bladder to fill.10 The activation of beta3-receptors is thought to increase the bladder’s ability to store urine, with the goal of decreasing urgency, frequency, nocturia, and urgency incontinence. An alternative to anticholinergic medications, mirabegron is a beta3-agonist approved by the US Food and Drug Administration (FDA) in 2012 for clinical use in the treatment of OAB.
Details of the study
Chapple and colleagues aimed to assess the 12-month efficacy and safety of mirabegron in a randomized, double-blind active controlled trial. The primary outcome was incidence and severity of treatment-emergent adverse effects (TEAEs); the secondary outcome was the change in OAB symptoms from baseline to up to 12 months. Patients experiencing OAB symptoms for more than 3 months were eligible and were subsequently enrolled if they averaged 8 or more voids per day and 3 or more episodes of urgency with or without incontinence on a 3-day bladder diary. A total of 2,444 patients were randomly assigned in a 1:1:1 fashion to mirabegron 50 mg daily, mirabegron 100 mg daily, or tolterodine extended release (ER) 4 mg daily.
There was a similar incidence (60% to 63%) of TEAEs across all three groups. The most common TEAEs were hypertension (defined as average systolic blood pressure [BP] >140 mm Hg or average diastolic BP >90 mm Hg at two consecutive visits), UTI, headache, nasopharyngitis, and constipation. The adjusted mean changes in BP from baseline to final visit were less than 1 mm Hg for both systolic and diastolic BP for patients taking both doses of mirabegron, as well as for patients taking tolterodine. The incidence of dry mouth was higher in the tolterodine group than the mirabegron groups. Mirabegron 50 mg daily and 100 mg daily improved incontinence symptoms within 1 month of starting therapy; the degree of improvement was similar to that seen in the patients taking tolterodine ER 4 mg daily.
Related article: New overactive bladder treatment approved by the FDA (August 2012)
Some caveats
This study was well-designed to assess the safety and tolerability of mirabegron versus tolterodine. The doses utilized in the study were at or above the FDA-approved dosage of 25 mg to 50 mg daily for OAB treatment. Although investigators found mirabegron to be a safe alternative to anticholinergic medication, the study was not designed or powered to examine the efficacy of mirabegron versus tolterodine. No formal comparison of efficacy was made between mirabegron or tolterodine, or between the 50-mg and 100-mg doses of mirabegron. Moreover, 81% of participants had been treated with mirabegron in earlier Phase 3 studies, so most were not treatment naïve, limiting the applicability of results.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
Mirabegron should be considered as a potential treatment option for patients who demonstrate poor tolerance of or response to anticholinergic medications; however, caution should be used in patients with severe uncontrolled high BP, end-stage kidney disease, or severe liver impairment.
Consider percutaneous tibial nerve stimulation over tolterodine for OAB in select patients
Peters KM, Macdiarmid SA, Wooldridge LS, et al. Randomized trial of percutaneous tibial nerve stimulation versus extended-release tolterodine: Results from the overactive bladder innovative therapy trial. J Urol. 2009;182(3):1055−1061.
Neuromodulation utilizes electrical stimulation to improve bladder function and decrease OAB symptoms. First developed in the early 1980s by McGuire and colleagues, percutaneous tibial nerve stimulation (PTNS) was approved by the FDA in 2000 as Urgent PC and provides an outpatient, nonimplantable neuromodulation alternative to medication therapy for patients with OAB.11,12 By directly stimulating the posterior tibial nerve, PTNS works via the S3 sacral nerve plexus to alter the micturition reflex and improve bladder function.
Details of the study
Patients were eligible for the study if they demonstrated 8 or more voids per day on a 3-day bladder diary (whether or not they had a history of previous anticholinergic drug use). A total of 100 ambulatory adults with OAB symptoms were enrolled and randomly assigned to PTNS 30-minutes per week or tolterodine ER 4 mg daily.
At 12 weeks, both groups demonstrated a significant improvement in OAB measures as well as validated symptom severity and quality-of-life questionnaire scores. Subjective assessment of improvement in OAB symptoms was significantly greater in the PTNS group than in the tolterodine group (79.5% vs 54.8%, respectively; P = .01). However, mean reduction of voids for 24 hours was not significantly different between the two groups.
Both treatments were well tolerated, with only 15% to 16% of patients in both groups reporting mild to moderate side effects. The tolterodine group did have a significantly higher risk of dry mouth; however, the risk of constipation was not significantly different between the groups.
Study limitations
The authors performed an important multicenter, nonblinded, randomized, controlled trial, which was one of the first trials to directly compare two OAB therapies. The generalizability of the findings were limited, as the cohort included mostly patients with dry OAB who had no objective measures on UUI episodes. In addition, this trial had a limited observation period of only 12 weeks. Information regarding the effect of treatment after cessation of weekly PTNS therapy was not examined. Therefore, we are not able to determine whether repeat sessions provide adequate maintenance in the long term.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
PTNS 30 minutes daily is as effective as tolterodine ER 4 mg daily for 12 weeks in reducing OAB symptoms. PTNS is a safe alternative that should be considered in patients with OAB who poorly tolerate or have contraindications to medication therapy.
OnabotulinumtoxinA is an effective therapy for OAB
Visco AG, Brubaker L, Richter HE, et al. Anticholinergic therapy vs onabotulinumtoxinA for urgency urinary incontinence. NEJM. 2012;367(19):1803−1813.
The newest therapy for OAB is onabotulinumtoxinA, or Botox, which was FDA approved this year for the treatment of OAB in adults who cannot use or do not tolerate anticholinergic medications. Recommended doses are 100 U onabotulinumtoxinA in patients with idiopathic refractory OAB and 200 U onabotulinumtoxinA for patients with neurogenic OAB.
OnabotulinumtoxinA is a neurotoxin that blocks synaptic transmission at the neuromuscular junction to cause muscle paralysis and atrophy.13 Injecting onabotulinumtoxinA into the detrusor smooth muscle should relax the bladder and decrease sensations of urgency and frequency to achieve a longer duration of time for bladder filling and reduce the risk of urgency incontinence.
Effects of onabotulinumtoxinA appear to wear off over time, and patients may require repeat injections. Side effects of onabotulinumtoxinA therapy include an increased risk of UTI and the potential for urinary retention requiring intermittent self-catheterization.
Related article: Update on Pelvic Floor Dysfunction Autumn L. Edenfield, MD, and Cindy L. Amundsen, MD (October 2012)
Details of the study
The Anticholinergic Versus Botulinum Toxin Comparison (ABC) study was a multicenter, randomized, double-blind, double-placebo–controlled trial conducted in women without known neurologic disease with moderate to severe UUI (defined as >5 UUI episodes on a 3-day bladder diary). Women were randomly assigned to a single intradetrusor injection of 100 U onabotulinumtoxinA plus oral placebo or to a single intradetrusor injection of saline plus solifenacin 5 mg daily (with the option of dose escalation and then switching to trospium XR if no improvement was seen).
Of the 241 women included in the final analysis, approximately 70% in each group reported adequate control of symptoms at 6 months. Adequate control was defined as a response of “agree strongly” or “agree” to the statement: “This treatment has given me adequate control of my urinary leakage.” Women in the onabotulinumtoxinA group were significantly more likely than women in the anticholinergic medication group to report complete resolution of UUI at 6 months (27% vs 13%, P = .003). However, the mean reduction in episodes of UUI per day and the improvements in quality-of-life questionnaire scores were found to be similar. Interestingly, worse baseline UUI was associated with greater reduction in episodes of UUI for both therapies.
This was a rigorous and well-executed double-blind, double-placebo−controlled randomized trial. By utilizing broad inclusion criteria and enrolling patients both with and without previous exposure to anticholinergic medications, the generalizability of study findings are greatly improved. Because this study did not examine the effect or efficacy of repeat injections, these findings have limited applicability to patients undergoing multiple onabotulinumtoxinA injections.
When considering use in your patient population, keep the possible side effects in mind.There were important differences in the side effects experienced with each therapy. Specifically, while the anticholinergic group had a higher frequency of dry mouth (46% anticholinergic vs 31% onabotulinumtoxinA, P = .02), the onabotulinumtoxinA group demonstrated higher rates of incomplete bladder emptying requiring catheterization (peak of 5% at 2 months) and greater risk of UTI (33% onabotulinumtoxinA vs 13% anticholinergic, P <.001).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This study showed that, among women with UUI, anticholinergic medication and onabotulinumtoxinA are equally effective in reducing UUI episodes and improving quality of life. It is important to consider the side effect profile, determine the patient’s preferences, and weigh the risks and benefits of each therapy when deciding what is the best treatment for your individual patient.
We want to hear from you! Tell us what you think.
- Anger JT, Saigal CS, Litwin MS. The prevalence of urinary incontinence among community dwelling adult women: Results from the National Health and Nutrition Examination Survey. J Urol. 2006;175(2):601–604.
- Dooley Y, Kenton K, Cao G, et al. Urinary incontinence prevalence: Results from the National Health and Nutrition Examination Survey. J Urol. 2008;179(2):656–661.
- Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in U.S. Women: 2010 to 2050. Obstetr Gynecol. 2009;114(6):1278–1283.
- Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU Guideline. Americal Urological Association. http://www.auanet.org/common/pdf/education/clinical-guidance/Overactive-Bladder.pdf. Published 2012. Revised June 11, 2013. Accessed October 21, 2013.
- Yu YF, Nichol MB, Yu AP, Ahn J. Persistence and adherence of medications for chronic overactive bladder/urinary incontinence in the California Medicaid program. Value Health. 2005;8(4):495–505.
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
- ACOG Practice Bulletin No. 63: Urinary incontinence in women. American College of Obstetricians and Gynecologists. Obstetr Gynecol. 2005;105(6):1533–1545.
- Bo K, Kvarstein B, Nygaard I. Lower urinary tract symptoms and pelvic floor muscle exercise adherence after 15 years. Obstetr Gynecol. 2005;105(5 Pt 1):999–1005.
- Sandvik H, Hunskaar S, Seim A, Hermstad R, Vanvik A, Bratt H. Validation of a severity index in female urinary incontinence and its implementation in an epidemiological survey. J Epidemiol Community Health. 1993;47(6):497–499.
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9(6):453–466.
- Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597.
- McGuire EJ, Zhang SC, Horwinski ER, Lytton B. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol. 1983;129(1):78–79.
- Schiavo G, Santucci A, Dasgupta BR, et al. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993;335(1):99–103.
- Anger JT, Saigal CS, Litwin MS. The prevalence of urinary incontinence among community dwelling adult women: Results from the National Health and Nutrition Examination Survey. J Urol. 2006;175(2):601–604.
- Dooley Y, Kenton K, Cao G, et al. Urinary incontinence prevalence: Results from the National Health and Nutrition Examination Survey. J Urol. 2008;179(2):656–661.
- Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in U.S. Women: 2010 to 2050. Obstetr Gynecol. 2009;114(6):1278–1283.
- Gormley EA, Lightner DJ, Burgio KL, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU Guideline. Americal Urological Association. http://www.auanet.org/common/pdf/education/clinical-guidance/Overactive-Bladder.pdf. Published 2012. Revised June 11, 2013. Accessed October 21, 2013.
- Yu YF, Nichol MB, Yu AP, Ahn J. Persistence and adherence of medications for chronic overactive bladder/urinary incontinence in the California Medicaid program. Value Health. 2005;8(4):495–505.
- Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Int Urogynecol J. 2010;21(1):5–26.
- ACOG Practice Bulletin No. 63: Urinary incontinence in women. American College of Obstetricians and Gynecologists. Obstetr Gynecol. 2005;105(6):1533–1545.
- Bo K, Kvarstein B, Nygaard I. Lower urinary tract symptoms and pelvic floor muscle exercise adherence after 15 years. Obstetr Gynecol. 2005;105(5 Pt 1):999–1005.
- Sandvik H, Hunskaar S, Seim A, Hermstad R, Vanvik A, Bratt H. Validation of a severity index in female urinary incontinence and its implementation in an epidemiological survey. J Epidemiol Community Health. 1993;47(6):497–499.
- Fowler CJ, Griffiths D, de Groat WC. The neural control of micturition. Nat Rev Neurosci. 2008;9(6):453–466.
- Levin PJ, Wu JM, Kawasaki A, Weidner AC, Amundsen CL. The efficacy of posterior tibial nerve stimulation for the treatment of overactive bladder in women: a systematic review. Int Urogynecol J. 2012;23(11):1591–1597.
- McGuire EJ, Zhang SC, Horwinski ER, Lytton B. Treatment of motor and sensory detrusor instability by electrical stimulation. J Urol. 1983;129(1):78–79.
- Schiavo G, Santucci A, Dasgupta BR, et al. Botulinum neurotoxins serotypes A and E cleave SNAP-25 at distinct COOH-terminal peptide bonds. FEBS Lett. 1993;335(1):99–103.
Cost-conscious choices for minimally invasive gynecologic surgery
CASE: COST-CONSCIOUS LAPAROSCOPIC HYSTERECTOMY
A 43-year-old woman undergoes laparoscopic hysterectomy for treatment of uterine fibroids and menorrhagia. Once she is prepped with ChloraPrep, a RUMI II uterine manipulator is placed. Laparoscopic ports include a Structural Balloon Trocar, a VersaStep Plus trocar, and two Versaport trocars. The surgeon uses an Olympus Thunderbeat device—a combination of ultrasonic and bipolar energy—to perform the majority of the procedure. The uterus is morcellated using the disposable Gynecare Morcellex, and the vaginal cuff is closed using a series of 2-0 PDS II sutures. The skin incisions are closed using Dermabond skin adhesive.
The total cost of the products used in this case: $1,705.60.
Could different product choices have reduced this figure?
As health-care costs continue to rise faster than inflation, with total health-care expenditures accounting for about 18% of the US gross domestic product, we face increasing pressure to take cost into account in the management of our patients.1 Not surprisingly, cost-effectiveness and outcome quality have become measures in many clinical trials that compare standard and alternative therapies. The field of women’s health—and, specifically, minimally invasive gynecologic surgery—is not immune to such comparisons.
Overall, conventional laparoscopic gynecologic procedures tend to have lower costs than laparotomy, due to shorter hospital stays, faster recovery, and fewer complications.2–4 What is not fully appreciated is how the choice of laparoscopic instrumentation and associated products affects surgical costs. In this article, we review these costs with the goal of raising awareness among minimally invasive gynecologic surgeons.
In the sections that follow, we highlight several aspects of laparoscopic gynecologic surgery that may affect your selection of instruments and products, describing the difference in cost as well as some unique characteristics of the products. Please note that our comparison focuses solely on cost, not ease of utility, effectiveness, surgical technique, risk of complications, or any other assessment. We’d also like to point out that numerous other instruments and devices are commercially available besides those listed in this article.
A few variables to keep in mind
Even when taking cost into consideration, tailor the selection of instruments and supplies to your capabilities and comfort, as well as characteristics particular to the patient and the planned procedure. Also keep in mind that your institution may have arrangements with companies that supply minimally invasive instruments, and such arrangements may limit your options to some degree. Be aware that reprocessed ports and instruments are now available at a reduced cost.
We believe it is crucial for surgeons to be cognizant of all products potentially available to them prior to attending a surgical case.
Related article: Update on Technology Barbara S. Levy, MD (September 2013)
Skin preparation and other preoperative considerations
Multiple preoperative skin preparations are available (TABLE 1). Traditionally, a povidone-iodine topical antiseptic such as Betadine has been used for skin and vaginal preparation prior to gynecologic surgery. Hibaclens and ChloraPrep are different combinations of chlorhexidine gluconate and isopropyl alcohol that act as broad-spectrum antiseptics. ChloraPrep is applied with a wand-like applicator and contains a much higher concentration of isopropyl alcohol than Hibaclens (70% vs 4%), rendering it more flammable. It also requires more drying time before the case is started. Clear and tinted ChloraPrep formulations are available.
It makes good sense to have instruments and devices readily available in the operating room (OR) at the start of a case, to avoid unnecessary surgical delays, but we recommend that you refrain from opening these tools until they are required intraoperatively. It is possible that the case will require conversion to laparotomy or that, after direct visualization of the pathology, different ports or instruments will be required.
Uterine manipulators
Cannulation of the cervical canal allows for uterine manipulation, increasing intraoperative traction and exposure and visualization of the adnexae and peritoneal surfaces.
The Hulka and Pelosi reusable uterine manipulators are fairly standard and easy to apply. Specialized, single-use manipulators also are available, including the VCare uterine manipulator/elevator, which consists of two opposing cups. One cup (available in four sizes, from small to extra-large) fits around the cervix and defines the site for colpotomy, and the other cup helps maintain pneumoperitoneum once a colpotomy is created.
The Zinnanti Uterine Manipulator Injector (ZUMI) is a rigid, curved shaft with an intrauterine balloon to help prevent expulsion. It also has an integrated injection channel to allow for intraoperative chromotubation.
The RUMI System fits individual patient anatomy with various tip lengths and colpotomy cup sizes (TABLE 2).
Related article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (August 2013)
Entry style and ports
The peritoneal cavity can be entered using either a closed (Veress needle) or open (Hasson) technique.5 Closed entry may allow for quicker access to the peritoneal cavity. A recent Cochrane review of 28 randomized, controlled trials, including 4,860 patients undergoing laparoscopy, compared outcomes between laparoscopic entry techniques.6 It found no difference in major vascular or visceral injury between closed and open techniques at the umbilicus. However, open entry was associated with greater successful entry into the peritoneal cavity, as well as less extraperitoneal insufflation and a lower omental injury rate, compared with closed entry.6
Left-upper-quadrant entry is another option when adhesions are anticipated or abnormal anatomy is encountered at the umbilicus.
In general, complications related to laparoscopic entry are quite rare in gynecologic surgery, ranging from 0.18% to 0.5%.7,8 A minimally invasive surgeon may prefer one entry technique over the other, but should be able to perform both methods competently and recognize when a particular technique is warranted.
Choosing a port
Laparoscopic ports usually range from 5 mm to 12 mm and can be fixed or variable in size. The primary port, usually placed through the umbilicus, can be a standard, blunt, 10-mm (Hasson) port, or it can be specialized to ease entry of the port or stabilize the port once it is introduced through the skin incision.
Optical trocars have a transparent tip that allows the surgeon to visualize the abdominal-wall entry layer by layer using a 0° laparoscope, usually after pneumoperitoneum is created with a Veress needle. Other specialized ports include those that have balloons or foam collars, or both, to secure the port without traditional stay sutures on the fascia and minimize leakage of pneumoperitoneum.
Accessory ports
When choosing an accessory port type and size, it is important to anticipate what instruments and devices, such as an Endo Catch bag, suture, needle, or morcellator, will need to pass through it. Also know whether 5-mm and 10-mm laparoscopes are available, and anticipate whether a second port with insufflation capabilities will be required.
Related article: Update on Minimally Invasive Surgery Amy Garcia, MD (April 2011)
Pediport trocars are user-friendly 5-mm bladed ports that deploy a mushroom-shaped stabilizer to prevent dislodgement. A Versaport bladed trocar has a spring-loaded entry shield, which slides over to protect the blade once the peritoneal cavity is entered.
VersaStep bladeless trocars are introduced after a Step insufflation needle has been inserted. These trocars create a smaller fascial defect than conventional bladed trocars for an equivalent cannula size (TABLE 3).
Cutting and coagulating
Both monopolar and bipolar electrosurgical techniques are commonly employed in gynecologic laparoscopy. A wide variety of disposable and reusable instruments are available for monopolar energy, such as scissors, a hook, and a spatula.
Bipolar devices also can be disposable or reusable. Although bipolar electrosurgery minimizes injury to surrounding tissues by containing the current within the jaws of the forceps, it cannot cut or seal large vessels. As a result, several advanced bipolar devices with sealing and transecting capabilities have emerged (LigaSure, ENSEAL). Ultrasonic devices also can coagulate and cut at lower temperatures by converting electrical energy to mechanical energy (TABLE 4).
Sutures
Aspects of minimally invasive gynecologic surgery that require the use of suture include, but are not limited to, closure of the vaginal cuff, oophoropexy, and reapproximation of the ovarian cortex after cystectomy. Syntheticand delayed absorbable sutures, such as PDS II, are used frequently.
Recently, a new class of suture emerged and continues to gain popularity: barbed suture. This type of suture anchors to the tissue without the need for intra- or extracorporeal knots (TABLE 5).
Related article: Ins and outs of straight-stick laparoscopic myomectomy James Robinson, MD, MS, and Gaby Moawad, MD (September 2012)
Tissue removal
Adnexae and pathologic tissue, such as dermoid cysts, can be removed intact from the peritoneal cavity using an Endo Catch Single-Use Specimen Pouch, a polyurethane sac. Careful use, with placement of the ovary with the cyst into the pouch prior to cystectomy, can prevent spillage beyond the bag.
Large uteri that cannot be extracted through a colpotomy can be morcellated into smaller pieces to ease removal through a small laparoscopic incision or the colpotomy. Both reusable and disposable morcellator hand pieces are available (TABLE 6).
Skin closure
Final subcuticular closure can be accomplished using sutures or skin adhesive. Sutures may be synthetic, absorbable monofilament (eg, Caprosyn), or synthetic, absorbable, braided multifilament (eg, PolySorb).
Related article: 10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery Baha M. Sibai, MD (March 2012)
Skin adhesives close incisions quickly, avoid inflammation related to foreign bodies, and ease patient concerns that sometimes arise when absorbable suture persists postoperatively (TABLE 5).
Take-home points
As health-care third-party payers and hospitals continue to evaluate surgeons individually and compare procedures between surgeons, reimbursements may be stratified, favoring physicians who demonstrate both quality outcomes and cost-containment.
There are many ways a minimally invasive surgeon can implement cost-conscious choices that have little to no impact on the quality of the outcome.
Surgeons who are familiar with the instruments and models available for use at their institution are better prepared to make wise cost-conscious decisions.
Cost is not the only indicator of value, however. The surgeon must know how to apply tools correctly and be familiar with their limitations, and should choose instruments and products for their safety and ease of use. More often than not, a surgeon’s training and personal experience define—and sometimes restrict—the choice of devices.
CASE: SAME OUTCOME, LOWER COST
The patient undergoes laparoscopic hysterectomy for treatment of uterine fibroids and menorrhagia. However, in this scenario, the surgeon makes the following product choices: The patient is prepped with Betadine, and a reusable Pelosi uterine manipulator is placed. Laparoscopic ports include a Kii Balloon Blunt Tip, a reprocessed VersaStep Plus trocar, and two Pediport trocars. The surgeon uses the PKS Lyons Dissecting Forceps and reprocessed EndoShears to perform the hysterectomy, incorporating the Karl Storz Rotocut G1 Morcellator, a reusable system with single-use blades, to morcellate the uterus. The vaginal cuff is closed using V-Loc barbed suture, and skin incisions are closed with 4-0 Polysorb, a polyglycolic acid, absorbable suture.
The cost of these products: $1,005.68, or roughly $700 less than the set-up described at the beginning of this article (TABLE 7).
Acknowledgment
The authors thank Susan Wykoop, BSN, RN, and Anna Emery, MSN, RN, for their assistance in gathering specific cost-related information.
We want to hear from you! Tell us what you think.
- Health expenditure, total (% of GDP). The World Bank. http://data.worldbank.org/indicator/SH.XPD.TOTL.ZS. Accessed October 17, 2013.
- Vilos GA, Alshimmiri MM. Cost-benefit analysis of laparoscopic versus laparotomy salpingo-oophorectomy for benign tubo-ovarian disease. J Am Assoc Gynecol Laparosc. 1995;2(3):299–303.
- Gray DT, Thorburn J, Lundorff P, et al. A cost-effectiveness study of a randomized trial of laparoscopy versus laparotomy for ectopic pregnancy. Lancet. 1995;345(8958):1139–1143.
- Chapron C, Fauconnier A, Goffinet F, et al. Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynaecologic pathology. Results of a meta-analysis. Hum Reprod. 2002;17(5):1334–1342.
- Hasson HM. A modified instrument and method for laparoscopy. Am J Obstet Gynecol. 1971;110(6):886–887.
- Ahmad G, O’Flynn H, Duffy JM, Phillips K, Watson A. Laparoscopic entry techniques. Cochrane Database Syst Rev. 2012;2:CD006583.
- Hasson HM, Rotman C, Rana N, Kumari NA. Open laparoscopy: 29-year experience. Obstet Gynecol. 2000;96(5 Pt 1):763–766.
- Schafer M, Lauper M, Krahenbuhl L. Trocar and Veress needle injuries during laparoscopy. Surg Endosc. 2001;15(3):275–280.
CASE: COST-CONSCIOUS LAPAROSCOPIC HYSTERECTOMY
A 43-year-old woman undergoes laparoscopic hysterectomy for treatment of uterine fibroids and menorrhagia. Once she is prepped with ChloraPrep, a RUMI II uterine manipulator is placed. Laparoscopic ports include a Structural Balloon Trocar, a VersaStep Plus trocar, and two Versaport trocars. The surgeon uses an Olympus Thunderbeat device—a combination of ultrasonic and bipolar energy—to perform the majority of the procedure. The uterus is morcellated using the disposable Gynecare Morcellex, and the vaginal cuff is closed using a series of 2-0 PDS II sutures. The skin incisions are closed using Dermabond skin adhesive.
The total cost of the products used in this case: $1,705.60.
Could different product choices have reduced this figure?
As health-care costs continue to rise faster than inflation, with total health-care expenditures accounting for about 18% of the US gross domestic product, we face increasing pressure to take cost into account in the management of our patients.1 Not surprisingly, cost-effectiveness and outcome quality have become measures in many clinical trials that compare standard and alternative therapies. The field of women’s health—and, specifically, minimally invasive gynecologic surgery—is not immune to such comparisons.
Overall, conventional laparoscopic gynecologic procedures tend to have lower costs than laparotomy, due to shorter hospital stays, faster recovery, and fewer complications.2–4 What is not fully appreciated is how the choice of laparoscopic instrumentation and associated products affects surgical costs. In this article, we review these costs with the goal of raising awareness among minimally invasive gynecologic surgeons.
In the sections that follow, we highlight several aspects of laparoscopic gynecologic surgery that may affect your selection of instruments and products, describing the difference in cost as well as some unique characteristics of the products. Please note that our comparison focuses solely on cost, not ease of utility, effectiveness, surgical technique, risk of complications, or any other assessment. We’d also like to point out that numerous other instruments and devices are commercially available besides those listed in this article.
A few variables to keep in mind
Even when taking cost into consideration, tailor the selection of instruments and supplies to your capabilities and comfort, as well as characteristics particular to the patient and the planned procedure. Also keep in mind that your institution may have arrangements with companies that supply minimally invasive instruments, and such arrangements may limit your options to some degree. Be aware that reprocessed ports and instruments are now available at a reduced cost.
We believe it is crucial for surgeons to be cognizant of all products potentially available to them prior to attending a surgical case.
Related article: Update on Technology Barbara S. Levy, MD (September 2013)
Skin preparation and other preoperative considerations
Multiple preoperative skin preparations are available (TABLE 1). Traditionally, a povidone-iodine topical antiseptic such as Betadine has been used for skin and vaginal preparation prior to gynecologic surgery. Hibaclens and ChloraPrep are different combinations of chlorhexidine gluconate and isopropyl alcohol that act as broad-spectrum antiseptics. ChloraPrep is applied with a wand-like applicator and contains a much higher concentration of isopropyl alcohol than Hibaclens (70% vs 4%), rendering it more flammable. It also requires more drying time before the case is started. Clear and tinted ChloraPrep formulations are available.
It makes good sense to have instruments and devices readily available in the operating room (OR) at the start of a case, to avoid unnecessary surgical delays, but we recommend that you refrain from opening these tools until they are required intraoperatively. It is possible that the case will require conversion to laparotomy or that, after direct visualization of the pathology, different ports or instruments will be required.
Uterine manipulators
Cannulation of the cervical canal allows for uterine manipulation, increasing intraoperative traction and exposure and visualization of the adnexae and peritoneal surfaces.
The Hulka and Pelosi reusable uterine manipulators are fairly standard and easy to apply. Specialized, single-use manipulators also are available, including the VCare uterine manipulator/elevator, which consists of two opposing cups. One cup (available in four sizes, from small to extra-large) fits around the cervix and defines the site for colpotomy, and the other cup helps maintain pneumoperitoneum once a colpotomy is created.
The Zinnanti Uterine Manipulator Injector (ZUMI) is a rigid, curved shaft with an intrauterine balloon to help prevent expulsion. It also has an integrated injection channel to allow for intraoperative chromotubation.
The RUMI System fits individual patient anatomy with various tip lengths and colpotomy cup sizes (TABLE 2).
Related article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (August 2013)
Entry style and ports
The peritoneal cavity can be entered using either a closed (Veress needle) or open (Hasson) technique.5 Closed entry may allow for quicker access to the peritoneal cavity. A recent Cochrane review of 28 randomized, controlled trials, including 4,860 patients undergoing laparoscopy, compared outcomes between laparoscopic entry techniques.6 It found no difference in major vascular or visceral injury between closed and open techniques at the umbilicus. However, open entry was associated with greater successful entry into the peritoneal cavity, as well as less extraperitoneal insufflation and a lower omental injury rate, compared with closed entry.6
Left-upper-quadrant entry is another option when adhesions are anticipated or abnormal anatomy is encountered at the umbilicus.
In general, complications related to laparoscopic entry are quite rare in gynecologic surgery, ranging from 0.18% to 0.5%.7,8 A minimally invasive surgeon may prefer one entry technique over the other, but should be able to perform both methods competently and recognize when a particular technique is warranted.
Choosing a port
Laparoscopic ports usually range from 5 mm to 12 mm and can be fixed or variable in size. The primary port, usually placed through the umbilicus, can be a standard, blunt, 10-mm (Hasson) port, or it can be specialized to ease entry of the port or stabilize the port once it is introduced through the skin incision.
Optical trocars have a transparent tip that allows the surgeon to visualize the abdominal-wall entry layer by layer using a 0° laparoscope, usually after pneumoperitoneum is created with a Veress needle. Other specialized ports include those that have balloons or foam collars, or both, to secure the port without traditional stay sutures on the fascia and minimize leakage of pneumoperitoneum.
Accessory ports
When choosing an accessory port type and size, it is important to anticipate what instruments and devices, such as an Endo Catch bag, suture, needle, or morcellator, will need to pass through it. Also know whether 5-mm and 10-mm laparoscopes are available, and anticipate whether a second port with insufflation capabilities will be required.
Related article: Update on Minimally Invasive Surgery Amy Garcia, MD (April 2011)
Pediport trocars are user-friendly 5-mm bladed ports that deploy a mushroom-shaped stabilizer to prevent dislodgement. A Versaport bladed trocar has a spring-loaded entry shield, which slides over to protect the blade once the peritoneal cavity is entered.
VersaStep bladeless trocars are introduced after a Step insufflation needle has been inserted. These trocars create a smaller fascial defect than conventional bladed trocars for an equivalent cannula size (TABLE 3).
Cutting and coagulating
Both monopolar and bipolar electrosurgical techniques are commonly employed in gynecologic laparoscopy. A wide variety of disposable and reusable instruments are available for monopolar energy, such as scissors, a hook, and a spatula.
Bipolar devices also can be disposable or reusable. Although bipolar electrosurgery minimizes injury to surrounding tissues by containing the current within the jaws of the forceps, it cannot cut or seal large vessels. As a result, several advanced bipolar devices with sealing and transecting capabilities have emerged (LigaSure, ENSEAL). Ultrasonic devices also can coagulate and cut at lower temperatures by converting electrical energy to mechanical energy (TABLE 4).
Sutures
Aspects of minimally invasive gynecologic surgery that require the use of suture include, but are not limited to, closure of the vaginal cuff, oophoropexy, and reapproximation of the ovarian cortex after cystectomy. Syntheticand delayed absorbable sutures, such as PDS II, are used frequently.
Recently, a new class of suture emerged and continues to gain popularity: barbed suture. This type of suture anchors to the tissue without the need for intra- or extracorporeal knots (TABLE 5).
Related article: Ins and outs of straight-stick laparoscopic myomectomy James Robinson, MD, MS, and Gaby Moawad, MD (September 2012)
Tissue removal
Adnexae and pathologic tissue, such as dermoid cysts, can be removed intact from the peritoneal cavity using an Endo Catch Single-Use Specimen Pouch, a polyurethane sac. Careful use, with placement of the ovary with the cyst into the pouch prior to cystectomy, can prevent spillage beyond the bag.
Large uteri that cannot be extracted through a colpotomy can be morcellated into smaller pieces to ease removal through a small laparoscopic incision or the colpotomy. Both reusable and disposable morcellator hand pieces are available (TABLE 6).
Skin closure
Final subcuticular closure can be accomplished using sutures or skin adhesive. Sutures may be synthetic, absorbable monofilament (eg, Caprosyn), or synthetic, absorbable, braided multifilament (eg, PolySorb).
Related article: 10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery Baha M. Sibai, MD (March 2012)
Skin adhesives close incisions quickly, avoid inflammation related to foreign bodies, and ease patient concerns that sometimes arise when absorbable suture persists postoperatively (TABLE 5).
Take-home points
As health-care third-party payers and hospitals continue to evaluate surgeons individually and compare procedures between surgeons, reimbursements may be stratified, favoring physicians who demonstrate both quality outcomes and cost-containment.
There are many ways a minimally invasive surgeon can implement cost-conscious choices that have little to no impact on the quality of the outcome.
Surgeons who are familiar with the instruments and models available for use at their institution are better prepared to make wise cost-conscious decisions.
Cost is not the only indicator of value, however. The surgeon must know how to apply tools correctly and be familiar with their limitations, and should choose instruments and products for their safety and ease of use. More often than not, a surgeon’s training and personal experience define—and sometimes restrict—the choice of devices.
CASE: SAME OUTCOME, LOWER COST
The patient undergoes laparoscopic hysterectomy for treatment of uterine fibroids and menorrhagia. However, in this scenario, the surgeon makes the following product choices: The patient is prepped with Betadine, and a reusable Pelosi uterine manipulator is placed. Laparoscopic ports include a Kii Balloon Blunt Tip, a reprocessed VersaStep Plus trocar, and two Pediport trocars. The surgeon uses the PKS Lyons Dissecting Forceps and reprocessed EndoShears to perform the hysterectomy, incorporating the Karl Storz Rotocut G1 Morcellator, a reusable system with single-use blades, to morcellate the uterus. The vaginal cuff is closed using V-Loc barbed suture, and skin incisions are closed with 4-0 Polysorb, a polyglycolic acid, absorbable suture.
The cost of these products: $1,005.68, or roughly $700 less than the set-up described at the beginning of this article (TABLE 7).
Acknowledgment
The authors thank Susan Wykoop, BSN, RN, and Anna Emery, MSN, RN, for their assistance in gathering specific cost-related information.
We want to hear from you! Tell us what you think.
CASE: COST-CONSCIOUS LAPAROSCOPIC HYSTERECTOMY
A 43-year-old woman undergoes laparoscopic hysterectomy for treatment of uterine fibroids and menorrhagia. Once she is prepped with ChloraPrep, a RUMI II uterine manipulator is placed. Laparoscopic ports include a Structural Balloon Trocar, a VersaStep Plus trocar, and two Versaport trocars. The surgeon uses an Olympus Thunderbeat device—a combination of ultrasonic and bipolar energy—to perform the majority of the procedure. The uterus is morcellated using the disposable Gynecare Morcellex, and the vaginal cuff is closed using a series of 2-0 PDS II sutures. The skin incisions are closed using Dermabond skin adhesive.
The total cost of the products used in this case: $1,705.60.
Could different product choices have reduced this figure?
As health-care costs continue to rise faster than inflation, with total health-care expenditures accounting for about 18% of the US gross domestic product, we face increasing pressure to take cost into account in the management of our patients.1 Not surprisingly, cost-effectiveness and outcome quality have become measures in many clinical trials that compare standard and alternative therapies. The field of women’s health—and, specifically, minimally invasive gynecologic surgery—is not immune to such comparisons.
Overall, conventional laparoscopic gynecologic procedures tend to have lower costs than laparotomy, due to shorter hospital stays, faster recovery, and fewer complications.2–4 What is not fully appreciated is how the choice of laparoscopic instrumentation and associated products affects surgical costs. In this article, we review these costs with the goal of raising awareness among minimally invasive gynecologic surgeons.
In the sections that follow, we highlight several aspects of laparoscopic gynecologic surgery that may affect your selection of instruments and products, describing the difference in cost as well as some unique characteristics of the products. Please note that our comparison focuses solely on cost, not ease of utility, effectiveness, surgical technique, risk of complications, or any other assessment. We’d also like to point out that numerous other instruments and devices are commercially available besides those listed in this article.
A few variables to keep in mind
Even when taking cost into consideration, tailor the selection of instruments and supplies to your capabilities and comfort, as well as characteristics particular to the patient and the planned procedure. Also keep in mind that your institution may have arrangements with companies that supply minimally invasive instruments, and such arrangements may limit your options to some degree. Be aware that reprocessed ports and instruments are now available at a reduced cost.
We believe it is crucial for surgeons to be cognizant of all products potentially available to them prior to attending a surgical case.
Related article: Update on Technology Barbara S. Levy, MD (September 2013)
Skin preparation and other preoperative considerations
Multiple preoperative skin preparations are available (TABLE 1). Traditionally, a povidone-iodine topical antiseptic such as Betadine has been used for skin and vaginal preparation prior to gynecologic surgery. Hibaclens and ChloraPrep are different combinations of chlorhexidine gluconate and isopropyl alcohol that act as broad-spectrum antiseptics. ChloraPrep is applied with a wand-like applicator and contains a much higher concentration of isopropyl alcohol than Hibaclens (70% vs 4%), rendering it more flammable. It also requires more drying time before the case is started. Clear and tinted ChloraPrep formulations are available.
It makes good sense to have instruments and devices readily available in the operating room (OR) at the start of a case, to avoid unnecessary surgical delays, but we recommend that you refrain from opening these tools until they are required intraoperatively. It is possible that the case will require conversion to laparotomy or that, after direct visualization of the pathology, different ports or instruments will be required.
Uterine manipulators
Cannulation of the cervical canal allows for uterine manipulation, increasing intraoperative traction and exposure and visualization of the adnexae and peritoneal surfaces.
The Hulka and Pelosi reusable uterine manipulators are fairly standard and easy to apply. Specialized, single-use manipulators also are available, including the VCare uterine manipulator/elevator, which consists of two opposing cups. One cup (available in four sizes, from small to extra-large) fits around the cervix and defines the site for colpotomy, and the other cup helps maintain pneumoperitoneum once a colpotomy is created.
The Zinnanti Uterine Manipulator Injector (ZUMI) is a rigid, curved shaft with an intrauterine balloon to help prevent expulsion. It also has an integrated injection channel to allow for intraoperative chromotubation.
The RUMI System fits individual patient anatomy with various tip lengths and colpotomy cup sizes (TABLE 2).
Related article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (August 2013)
Entry style and ports
The peritoneal cavity can be entered using either a closed (Veress needle) or open (Hasson) technique.5 Closed entry may allow for quicker access to the peritoneal cavity. A recent Cochrane review of 28 randomized, controlled trials, including 4,860 patients undergoing laparoscopy, compared outcomes between laparoscopic entry techniques.6 It found no difference in major vascular or visceral injury between closed and open techniques at the umbilicus. However, open entry was associated with greater successful entry into the peritoneal cavity, as well as less extraperitoneal insufflation and a lower omental injury rate, compared with closed entry.6
Left-upper-quadrant entry is another option when adhesions are anticipated or abnormal anatomy is encountered at the umbilicus.
In general, complications related to laparoscopic entry are quite rare in gynecologic surgery, ranging from 0.18% to 0.5%.7,8 A minimally invasive surgeon may prefer one entry technique over the other, but should be able to perform both methods competently and recognize when a particular technique is warranted.
Choosing a port
Laparoscopic ports usually range from 5 mm to 12 mm and can be fixed or variable in size. The primary port, usually placed through the umbilicus, can be a standard, blunt, 10-mm (Hasson) port, or it can be specialized to ease entry of the port or stabilize the port once it is introduced through the skin incision.
Optical trocars have a transparent tip that allows the surgeon to visualize the abdominal-wall entry layer by layer using a 0° laparoscope, usually after pneumoperitoneum is created with a Veress needle. Other specialized ports include those that have balloons or foam collars, or both, to secure the port without traditional stay sutures on the fascia and minimize leakage of pneumoperitoneum.
Accessory ports
When choosing an accessory port type and size, it is important to anticipate what instruments and devices, such as an Endo Catch bag, suture, needle, or morcellator, will need to pass through it. Also know whether 5-mm and 10-mm laparoscopes are available, and anticipate whether a second port with insufflation capabilities will be required.
Related article: Update on Minimally Invasive Surgery Amy Garcia, MD (April 2011)
Pediport trocars are user-friendly 5-mm bladed ports that deploy a mushroom-shaped stabilizer to prevent dislodgement. A Versaport bladed trocar has a spring-loaded entry shield, which slides over to protect the blade once the peritoneal cavity is entered.
VersaStep bladeless trocars are introduced after a Step insufflation needle has been inserted. These trocars create a smaller fascial defect than conventional bladed trocars for an equivalent cannula size (TABLE 3).
Cutting and coagulating
Both monopolar and bipolar electrosurgical techniques are commonly employed in gynecologic laparoscopy. A wide variety of disposable and reusable instruments are available for monopolar energy, such as scissors, a hook, and a spatula.
Bipolar devices also can be disposable or reusable. Although bipolar electrosurgery minimizes injury to surrounding tissues by containing the current within the jaws of the forceps, it cannot cut or seal large vessels. As a result, several advanced bipolar devices with sealing and transecting capabilities have emerged (LigaSure, ENSEAL). Ultrasonic devices also can coagulate and cut at lower temperatures by converting electrical energy to mechanical energy (TABLE 4).
Sutures
Aspects of minimally invasive gynecologic surgery that require the use of suture include, but are not limited to, closure of the vaginal cuff, oophoropexy, and reapproximation of the ovarian cortex after cystectomy. Syntheticand delayed absorbable sutures, such as PDS II, are used frequently.
Recently, a new class of suture emerged and continues to gain popularity: barbed suture. This type of suture anchors to the tissue without the need for intra- or extracorporeal knots (TABLE 5).
Related article: Ins and outs of straight-stick laparoscopic myomectomy James Robinson, MD, MS, and Gaby Moawad, MD (September 2012)
Tissue removal
Adnexae and pathologic tissue, such as dermoid cysts, can be removed intact from the peritoneal cavity using an Endo Catch Single-Use Specimen Pouch, a polyurethane sac. Careful use, with placement of the ovary with the cyst into the pouch prior to cystectomy, can prevent spillage beyond the bag.
Large uteri that cannot be extracted through a colpotomy can be morcellated into smaller pieces to ease removal through a small laparoscopic incision or the colpotomy. Both reusable and disposable morcellator hand pieces are available (TABLE 6).
Skin closure
Final subcuticular closure can be accomplished using sutures or skin adhesive. Sutures may be synthetic, absorbable monofilament (eg, Caprosyn), or synthetic, absorbable, braided multifilament (eg, PolySorb).
Related article: 10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery Baha M. Sibai, MD (March 2012)
Skin adhesives close incisions quickly, avoid inflammation related to foreign bodies, and ease patient concerns that sometimes arise when absorbable suture persists postoperatively (TABLE 5).
Take-home points
As health-care third-party payers and hospitals continue to evaluate surgeons individually and compare procedures between surgeons, reimbursements may be stratified, favoring physicians who demonstrate both quality outcomes and cost-containment.
There are many ways a minimally invasive surgeon can implement cost-conscious choices that have little to no impact on the quality of the outcome.
Surgeons who are familiar with the instruments and models available for use at their institution are better prepared to make wise cost-conscious decisions.
Cost is not the only indicator of value, however. The surgeon must know how to apply tools correctly and be familiar with their limitations, and should choose instruments and products for their safety and ease of use. More often than not, a surgeon’s training and personal experience define—and sometimes restrict—the choice of devices.
CASE: SAME OUTCOME, LOWER COST
The patient undergoes laparoscopic hysterectomy for treatment of uterine fibroids and menorrhagia. However, in this scenario, the surgeon makes the following product choices: The patient is prepped with Betadine, and a reusable Pelosi uterine manipulator is placed. Laparoscopic ports include a Kii Balloon Blunt Tip, a reprocessed VersaStep Plus trocar, and two Pediport trocars. The surgeon uses the PKS Lyons Dissecting Forceps and reprocessed EndoShears to perform the hysterectomy, incorporating the Karl Storz Rotocut G1 Morcellator, a reusable system with single-use blades, to morcellate the uterus. The vaginal cuff is closed using V-Loc barbed suture, and skin incisions are closed with 4-0 Polysorb, a polyglycolic acid, absorbable suture.
The cost of these products: $1,005.68, or roughly $700 less than the set-up described at the beginning of this article (TABLE 7).
Acknowledgment
The authors thank Susan Wykoop, BSN, RN, and Anna Emery, MSN, RN, for their assistance in gathering specific cost-related information.
We want to hear from you! Tell us what you think.
- Health expenditure, total (% of GDP). The World Bank. http://data.worldbank.org/indicator/SH.XPD.TOTL.ZS. Accessed October 17, 2013.
- Vilos GA, Alshimmiri MM. Cost-benefit analysis of laparoscopic versus laparotomy salpingo-oophorectomy for benign tubo-ovarian disease. J Am Assoc Gynecol Laparosc. 1995;2(3):299–303.
- Gray DT, Thorburn J, Lundorff P, et al. A cost-effectiveness study of a randomized trial of laparoscopy versus laparotomy for ectopic pregnancy. Lancet. 1995;345(8958):1139–1143.
- Chapron C, Fauconnier A, Goffinet F, et al. Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynaecologic pathology. Results of a meta-analysis. Hum Reprod. 2002;17(5):1334–1342.
- Hasson HM. A modified instrument and method for laparoscopy. Am J Obstet Gynecol. 1971;110(6):886–887.
- Ahmad G, O’Flynn H, Duffy JM, Phillips K, Watson A. Laparoscopic entry techniques. Cochrane Database Syst Rev. 2012;2:CD006583.
- Hasson HM, Rotman C, Rana N, Kumari NA. Open laparoscopy: 29-year experience. Obstet Gynecol. 2000;96(5 Pt 1):763–766.
- Schafer M, Lauper M, Krahenbuhl L. Trocar and Veress needle injuries during laparoscopy. Surg Endosc. 2001;15(3):275–280.
- Health expenditure, total (% of GDP). The World Bank. http://data.worldbank.org/indicator/SH.XPD.TOTL.ZS. Accessed October 17, 2013.
- Vilos GA, Alshimmiri MM. Cost-benefit analysis of laparoscopic versus laparotomy salpingo-oophorectomy for benign tubo-ovarian disease. J Am Assoc Gynecol Laparosc. 1995;2(3):299–303.
- Gray DT, Thorburn J, Lundorff P, et al. A cost-effectiveness study of a randomized trial of laparoscopy versus laparotomy for ectopic pregnancy. Lancet. 1995;345(8958):1139–1143.
- Chapron C, Fauconnier A, Goffinet F, et al. Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynaecologic pathology. Results of a meta-analysis. Hum Reprod. 2002;17(5):1334–1342.
- Hasson HM. A modified instrument and method for laparoscopy. Am J Obstet Gynecol. 1971;110(6):886–887.
- Ahmad G, O’Flynn H, Duffy JM, Phillips K, Watson A. Laparoscopic entry techniques. Cochrane Database Syst Rev. 2012;2:CD006583.
- Hasson HM, Rotman C, Rana N, Kumari NA. Open laparoscopy: 29-year experience. Obstet Gynecol. 2000;96(5 Pt 1):763–766.
- Schafer M, Lauper M, Krahenbuhl L. Trocar and Veress needle injuries during laparoscopy. Surg Endosc. 2001;15(3):275–280.
For uterine-sparing fibroid treatment, consider laparoscopic ultrasound-guided radiofrequency ablation
CASE: WOMAN WITH HEAVY BLEEDING HOPES TO AVOID HYSTERECTOMY
L.W. is a 44-year-old woman (G2P2) with a 2-year history of menorrhagia and severe dysmenorrhea but no intermenstrual spotting or bleeding. She reports that she has tried to control her symptoms using hormonal methods, without success.
Examination reveals that she has an irregular, nontender uterus 8 weeks in size. Transvaginal ultrasonography shows two deep, prominent, intramural fibroids. The first is 2 cm by 3 cm in size in the left lateral uterus, adjacent to the endometrial stripe. The second fibroid is 3 cm by 4 cm in the fundal region. Sonohysterography reveals no intracavitary fibroids, although the left lateral myoma has distorted the endometrial cavity.
The patient is seeking removal of her fibroids but would like to preserve her uterus, if at all possible.
What options would you offer her?
Uterine fibroids are common in premenopausal women, affecting 70% to 80% of the population, especially women of African descent.1 Although the majority of women with fibroids are asymptomatic, approximately 30% report pelvic pressure, dyspareunia, dysmenorrhea, or abnormal uterine bleeding.2
Our patients are increasingly aware of uterine-sparing treatments for symptomatic fibroids.3,4 Women seek conservative procedures to avoid the risks and extended recovery times commonly associated with major surgery. Current laparoscopic techniques for removal of uterine fibroids can be complex and require advanced surgical skills. Deep intramural fibroids may not always be visible or readily accessible, making laparoscopic removal a more invasive and challenging approach for many gynecologic surgeons.
A significant recent advancement in minimally invasive gynecologic treatment of fibroids is the Acessa System (Halt Medical, Brentwood, California). This laparoscopic system involves the outpatient use of ultrasound-guided radiofrequency volumetric thermal ablation, or RFVTA.
In this article, I outline the development of this option and step you through its technique. I also review the outcomes data that have been published to date.
How the procedure evolved
In the 1980s, Donnez and Nisolle developed a method of laparoscopic Nd:YAG laser treatment of uterine fibroids, commonly referred to as myolysis.5 Later, Goldfarb developed a laparoscopic bipolar needle technique that coagulated and occluded blood vessels at the periphery of the fibroid.6 However, myolysis led to the formation of significant adhesions to the small bowel or omentum, or both, and was abandoned by the surgical community.
Other fibroid treatments have been developed, such as laparoscopic myomectomy, uterine artery ligation, uterine artery embolization (alone or as adjuvant treatment), and high-intensity focused ultrasound. Although these procedures have made a significant contribution to the minimally invasive treatment of fibroids, the need for reintervention can be substantial, as high as 29% at 1 to 10 years of follow-up in some reports.7–17
Related article: Ins and outs of straight-stick myomectomy James Robinson, MD, MD, and Gaby Moawad, MD (September 2012)
Radiofrequency needle ablation has been used successfully in the treatment and destruction of liver and kidney tumors for years. Lee envisioned use of the technology to treat uterine fibroids. He developed a technique using a retractable multiarray radiofrequency needle (Starburst XL, RITA Medical Systems, Fremont, California) that is inserted directly into the fibroid. In 2002, he reported the first use of intraperitoneal ultrasound for needle guidance in the laparoscopic ablation of 197 myomas in 52 symptomatic women who had declined hysterectomy and myomectomy.18 He found that a significant proportion of women experienced resolution of their symptoms, including heavy menstrual bleeding, dysmenorrhea, dyspareunia, and pelvic pressure, by 3 months, with continued improvement at 12 months.
Because he was unhappy with aspects of the RITA needle that prevented accurate and consistent ablation of fibroids, he developed other devices that would lead to RFVTA and the Acessa System. This system allows the gynecologic surgeon to target and treat deep intramural fibroids that may not be readily visible via conventional laparoscopy.
Between the time of Lee’s first report and his subsequent refinement of the device and procedure, several international fibroid radiofrequency ablation procedures emerged, including those of Bergamini, Milic, Ghezzi, and Carrafiello.19–22 These investigators published results in small cohorts and described significant improvement in patient-reported Uterine Fibroid Symptom and Quality-of-Life (UFS-QOL) scores as well as a reduction in myoma volume.23 Their studies provided evidence that RFA is a safe and effective uterine-sparing treatment of symptomatic uterine fibroids for selected patients.
Related article: Update on Minimally Invasive Surgery Amy Garcia, MD (April 2011)
How it works
RFVTA uses laparoscopy and laparoscopic ultrasound to guide placement of a needle electrode with a deployable array. Earlier international studies of radiofrequency ablation18–22 and ongoing studies of RFVTA23–26 have demonstrated the procedure’s therapeutic potential. Acessa was recently cleared (November 2012) by the US Food and Drug Administration (FDA).
RFVTA technique, step by step
1. Begin with a standard 5-mm laparoscopic infraumbilical port for the camera and video laparoscope (See VIDEO).
2. Place a 12-mm port in the midline, suprapubically at the level of the uterus, for insertion of the laparoscopic ultrasound probe. Now it is possible to map the uterus and plan an approach to destroy the fibroids.
3. Once you have determined your approach, insert the handpiece containing the radiofrequency needle through the abdominal wall under laparoscopic visualization.
4. Place the needle into the targeted fibroid using both laparoscopic and ultrasound guidance. Depending on the size of the fibroid, the needle array can be deployed to the maximum diameter necessary to effect destruction.
5. Engage the radiofrequency generator (FIGURE 1), utilizing the timing function for optimal destruction of the fibroid, based on the appropriate algorithm. The fibroid then will be ablated and destroyed without damage to the surrounding healthy myometrium.
The Acessa radiofrequency generator and accessories are designed to deliver monopolar radiofrequency energy to tissue through the handheld electrical probe. The procedure is monitored by real-time feedback to and from the generator via each of the thermocouples at the tips of the seven-needle array (FIGURE 1). In addition, laparoscopic ultrasound-guided visualization permits monitoring of needle placement within the fibroid capsule (FIGURES 2A and 2B) and confirmation of hemostasis upon removal of the probe tip (FIGURE 2C).
RFVTA treats a wide range of fibroids
The Acessa System is used to treat fibroids of any location and type, with the exception of pedunculated or Type 0 myomas, in which case laparoscopic or hysteroscopic myomectomy is recommended. Calcified fibroids pose no challenges. A single radiofrequency needle generally is used to treat multiple fibroids, with ablation zones ranging in diameter from 1.0 to 6.7 cm. Large, irregularly shaped fibroids may require multiple ablation zones during the same procedure.
Final steps
6. Once the treatment is complete, as confirmed by informatics transmitted by the generator, retract the needle array, set the generator to coagulation mode to coagulate the needle track during withdrawal of the probe, and confirm hemostasis (FIGURE 2C).
7. After completion of all fibroid destruction, remove the disposable radiofrequency probe and close the laparoscopic and ultrasound port sites using standard procedures.
The patient can be discharged the same day and generally is able to return to normal activities within 3 to 5 days.
Postoperative analgesia usually consists solely of nonsteroidal anti-inflammatory agents.
A look at outcomes data
Garza and colleagues published the first feasibility report in 2011.24 They described the results of laparoscopic, ultrasound-guided RFVTA for the treatment of symptomatic uterine fibroids in Mexico using an Acessa predecessor. The 31 women enrolled in the study desired uterine preservation. Of these participants, 19 had completed 12 months of follow-up by the time the report was submitted for publication. Their responses to the UFS-QOL questionnaire indicated that their symptoms had decreased significantly in severity from baseline to 12 months, and their quality-of-life scores also improved significantly (P <.001). Mean uterine volume also declined significantly.
Robles and colleagues set out to confirm these findings using the investigational Halt Ablation System. They conducted a prospective, single-center, open-label clinical trial in Guatemala.25 They found dramatic improvement in symptom severity and health-related quality of life from baseline to 12 months. Mean uterine volume also decreased by 23.6% during this period. In addition, the severity and duration of menses declined.
Acessa is currently not approved for women seeking future childbearing. However, several successful pregnancies have been reported following the procedure.24,26–28 Theoretically, because it causes less damage to and disruption of healthy myometrium, Acessa may prove to be preferable to myomectomy. Head-to-head studies to evaluate this premise are ongoing.
Latest findings confirm widespread improvement of symptoms
The most recent and detailed reports of safety and efficacy of laparoscopic, ultrasound-guided RFVTA at 12 and 24 months were published by teams led by Chudnoff26 and Guido27 earlier this year. These investigations involved 135 women who reported moderate to severe heavy menstrual bleeding (160 mL–500 mL by alkaline hematin analysis). Clinically significant reduction in menstrual blood loss was achieved in 67.7% of participants at 12 months of follow-up.29
Related article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (August 2013)
Galen and colleagues performed a retrospective analysis of these same patients, demonstrating that RFVTA of intramural fibroids without submucosal components leads to a clinically and statistically significant reduction in menstrual blood loss.30
Patients generally began these trials with high symptom-severity scores and low health-related quality of life, yet all achieved significant improvement over time, as their UFS-QOL scores indicate (TABLE). The majority of patients (96%) were treated on an outpatient basis. As measured by contrast-enhanced magnetic resonance imaging, total mean uterine volume decreased by 24.3% at 12 months, and total mean fibroid volume decreased by 45.1% during the same interval. Ninety-four percent of the women reported that the treatment was effective in eliminating their symptoms at 12 months, and 98% said they would recommend the procedure to a friend having the same health problems.
Complication rates were low
Complications included a postoperative pelvic abscess and four other minor events. The latter were treated at the time of RFVTA or resolved without additional procedures. The reintervention rate was 0.7% (1/135 patients).26
Guido and colleagues reported 24-month outcomes for the 124 women entering the second year of the Halt study and affirmed the findings of Chudnoff’s team.27 The 112 patient-reported UFS-QOL scores at 24 months were virtually identical to those reported at 12 months. Six surgical reinterventions (6 of 124 patients, or 4.8%) for fibroid-related bleeding were reported between 12 and 24 months.
280 women have been treated so far
As of September 2013, 280 women have been treated under five international study protocols using the Halt Acessa System, and approximately two-thirds have been followed for more than 12 months, with excellent results. Two of the five clinical trials are under way in Germany and Canada:
- The Laparoscopic Uterine-Sparing Techniques Outcomes and Reinterventions (LUSTOR) trial in Germany is a prospective, randomized, single-center study comparing outcomes of RFVTA and laparoscopic myomectomy. Fifty women were enrolled and treated and will be followed for as long as 60 months.
- The Treatment Results of Uterine-Sparing Technologies (TRUST) trial is a prospective, multicenter, randomized study in Canada that compares direct and indirect costs associated with RFVTA, laparoscopic and abdominal myomectomy, and uterine artery embolization. Some US sites also will participate. As many as 200 women will be treated and followed for as long as 60 months.
RFVTA offers several benefits
Although hysterectomy provides a definitive “cure” to fibroid-related symptoms, it is not preferred by many patients and generally is more costly, with an increased risk of complications, a longer hospital stay, longer recovery (6–8 weeks), longer disability and more time off from work, and potential long-term sequelae.
The Acessa procedure not only is an outpatient, minimally invasive, uterine-sparing fibroid treatment option, it permits access to and ablation of almost all fibroids (with the exception of penduculated or Type 0 myomas). The data to date suggest that RFVTA provides many benefits, including a decline in symptom severity and improved health-related quality of life, as well as good cosmesis, quick recovery, and a rapid return to full activity.
There is a learning curve for RFVTA with Acessa—as with any new procedure. If physicians already perform ultrasound imaging, the learning process is shorter. Physicians interested in Acessa and furthering their education and training in laparoscopic RFVTA should consult the manufacturer’s professional education division through the company Web site at http://www.haltmedical.com.
CASE: RESOLVED
After she is counseled about the various uterine-sparing options for treatment, L.W. chooses to undergo laparoscopic, ultrasound-guided RFVTA using the Acessa System. The procedure is performed successfully, with the two fibroids destroyed using the appropriate algorithms, which took into account the size of the fibroids and the deployed needle array. Two additional 1-cm fibroids were found during the procedure and ablated. The patient was discharged home shortly after the procedure, with a nonsteroidal anti-inflammatory drug given for pain relief.
We want to hear from you! Tell us what you think.
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107.
- Katz VL, Lentz G, Lobo RA, Gershenson D, eds. Comprehensive Gynecology. 5th ed. Philadelphia, PA: Elsevier Mosby Saunders; 2007.
- Rosen P. The endangered uterus. More Magazine Web site. http://www.more.com/health/wellness/endangered-uterus. December 2008/January 2009. Accessed October 16, 2013.
- Tulandi T, Levy BS. So You’re Having a Hysterectomy? Hoboken, NJ: John Wiley & Sons; 2004.
- Donnez J, Squifflet J, Polet R, Nisolle M. Laparoscopic myolysis. Human Reprod Update. 2000;6(6):609–613.
- Goldfarb HA. Bipolar laparoscopic needles for myoma coagulation. J Am Assoc Gynecol Laparosc. 1995;2(2):175–179.
- Hanafi M. Predictors of leiomyoma recurrence after myomectomy. Obstet Gynecol. 2005;105(4):877–881.
- Goodwin SC, Spies JB, Worthington-Kirsch R, et al; Fibroid Registry for Outcomes Data (FIBROID) Registry Steering Committee and Core Site Investigators. Uterine artery embolization for treatment of leiomyomata: long-term outcomes from the FIBROID Registry. Obstet Gynecol. 2008;111(1):22–33.
- Yoo EH, Lee PI, Huh C-Y, et al. Predictors of leiomyoma recurrence after laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(6):690–697.
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356(4):360–370.
- Huang JY, Kafy S, Dugas A, Valenti D, Tulandi T. Failure of uterine fibroid embolization. Fertil Steril. 2006;85(1):30–35.
- Kroncke TJ, Gauruder-Burmester A, Scheurig C, et al. Transarterial embolization for uterine fibroids: clinical success rate and results of magnetic resonance imaging [in German]. Rofo. 2005;177(1):89–98.
- Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31(1):73–85.
- Munoz JL, Jimenez JS, Hernandez C, et al. Hysteroscopic myomectomy: our experience and review. JSLS. 2003;7(1):39–48.
- Reed SD, Newton KM, Thompson LB, McCrummen BA, Warolin AK. The incidence of repeat uterine surgery following myomectomy. J Womens Health (Larchmt). 2006;15(9):1046–1052.
- Spies JB, Bruno J, Czeyda-Pommersheim F, et al. Long-term outcome of uterine artery embolization of leiomyomata. Obstet Gynecol. 2005;106(5 Pt 1):933–939.
- Lee BB. Radiofrequency ablation of uterine leiomyomata: a new minimally invasive hysterectomy alternative. Obstet Gynecol. 2002;99(4Suppl):9S.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192(3):768–773.
- Milic A, Asch MR, Hawrylyshyn PA, et al. Laparoscopic ultrasound-guided radiofrequency ablation of uterine fibroids. Cardiovasc Intervent Radiol. 2006;29(4):694–698.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21(11):2081–2085.
- Carrafiello G, Recaldini C, Fontana F, et al. Ultrasound-guided radiofrequency thermal ablation of uterine fibroids: medium-term follow-up. Cardiovasc Intervent Radiol. 2010;33(1):113–119.
- Spies JB, Coyne K, Guaou Guaou N, et al. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomymata. Obstet Gynecol. 2002;99(2):290–300.
- Garza Leal JG, Hernandez Leon I, Castillo Saenz L, Lee BB. Laparoscopic ultrasound-guided radiofrequency volumetric thermal ablation of symptomatic uterine leiomyomas: feasibility study using the Halt 2000 ablation system. J Minim Invasive Gynecol. 2011;18(3):364–371.
- Robles R, Aguirre VA, Argueta AI, Guerrero MR. Laparoscopic radiofrequency volumetric ablation of uterine myomas with 12 months of follow-up. Int J Gynecol Obstet. 2013;120(1):65–69.
- Chudnoff SG, Berman JM, Levine DJ, Harris M, Guido RS, Banks E. Outpatient procedure for the treatment and relief of symptomatic uterine myomas. Obstet Gynecol. 2013;121(5):1075–1082.
- Guido RS, Macer JA, Abbott K, Falls JL, Tilley IB, Chudnoff SG. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Quality Life Outcomes. 2013;11(139):1–8.
- Berman JM, Puscheck EE, Diamond MP. Full-term vaginal live birth after laparoscopic radiofrequency ablation of a large, symptomatic intramural fibroid. J Reprod Med. 2012;57:159–163.
- Galen DI, Isaacson KB, Lee BB. Does menstrual bleeding decrease after ablation of intramural myomas? A retrospective study [published online ahead of print September 6, 2013]. J Minim Invasive Gynecol. doi: 10.1016/j.jmig.2013.05.007.
- Lukes AS, Muse K, Richter HE, Moore KA, Patrick DL. Estimating a meaningful reduction in menstrual blood loss for women with heavy bleeding. Curr Med Res Opinion. 2010;26(11):2673–2678.
CASE: WOMAN WITH HEAVY BLEEDING HOPES TO AVOID HYSTERECTOMY
L.W. is a 44-year-old woman (G2P2) with a 2-year history of menorrhagia and severe dysmenorrhea but no intermenstrual spotting or bleeding. She reports that she has tried to control her symptoms using hormonal methods, without success.
Examination reveals that she has an irregular, nontender uterus 8 weeks in size. Transvaginal ultrasonography shows two deep, prominent, intramural fibroids. The first is 2 cm by 3 cm in size in the left lateral uterus, adjacent to the endometrial stripe. The second fibroid is 3 cm by 4 cm in the fundal region. Sonohysterography reveals no intracavitary fibroids, although the left lateral myoma has distorted the endometrial cavity.
The patient is seeking removal of her fibroids but would like to preserve her uterus, if at all possible.
What options would you offer her?
Uterine fibroids are common in premenopausal women, affecting 70% to 80% of the population, especially women of African descent.1 Although the majority of women with fibroids are asymptomatic, approximately 30% report pelvic pressure, dyspareunia, dysmenorrhea, or abnormal uterine bleeding.2
Our patients are increasingly aware of uterine-sparing treatments for symptomatic fibroids.3,4 Women seek conservative procedures to avoid the risks and extended recovery times commonly associated with major surgery. Current laparoscopic techniques for removal of uterine fibroids can be complex and require advanced surgical skills. Deep intramural fibroids may not always be visible or readily accessible, making laparoscopic removal a more invasive and challenging approach for many gynecologic surgeons.
A significant recent advancement in minimally invasive gynecologic treatment of fibroids is the Acessa System (Halt Medical, Brentwood, California). This laparoscopic system involves the outpatient use of ultrasound-guided radiofrequency volumetric thermal ablation, or RFVTA.
In this article, I outline the development of this option and step you through its technique. I also review the outcomes data that have been published to date.
How the procedure evolved
In the 1980s, Donnez and Nisolle developed a method of laparoscopic Nd:YAG laser treatment of uterine fibroids, commonly referred to as myolysis.5 Later, Goldfarb developed a laparoscopic bipolar needle technique that coagulated and occluded blood vessels at the periphery of the fibroid.6 However, myolysis led to the formation of significant adhesions to the small bowel or omentum, or both, and was abandoned by the surgical community.
Other fibroid treatments have been developed, such as laparoscopic myomectomy, uterine artery ligation, uterine artery embolization (alone or as adjuvant treatment), and high-intensity focused ultrasound. Although these procedures have made a significant contribution to the minimally invasive treatment of fibroids, the need for reintervention can be substantial, as high as 29% at 1 to 10 years of follow-up in some reports.7–17
Related article: Ins and outs of straight-stick myomectomy James Robinson, MD, MD, and Gaby Moawad, MD (September 2012)
Radiofrequency needle ablation has been used successfully in the treatment and destruction of liver and kidney tumors for years. Lee envisioned use of the technology to treat uterine fibroids. He developed a technique using a retractable multiarray radiofrequency needle (Starburst XL, RITA Medical Systems, Fremont, California) that is inserted directly into the fibroid. In 2002, he reported the first use of intraperitoneal ultrasound for needle guidance in the laparoscopic ablation of 197 myomas in 52 symptomatic women who had declined hysterectomy and myomectomy.18 He found that a significant proportion of women experienced resolution of their symptoms, including heavy menstrual bleeding, dysmenorrhea, dyspareunia, and pelvic pressure, by 3 months, with continued improvement at 12 months.
Because he was unhappy with aspects of the RITA needle that prevented accurate and consistent ablation of fibroids, he developed other devices that would lead to RFVTA and the Acessa System. This system allows the gynecologic surgeon to target and treat deep intramural fibroids that may not be readily visible via conventional laparoscopy.
Between the time of Lee’s first report and his subsequent refinement of the device and procedure, several international fibroid radiofrequency ablation procedures emerged, including those of Bergamini, Milic, Ghezzi, and Carrafiello.19–22 These investigators published results in small cohorts and described significant improvement in patient-reported Uterine Fibroid Symptom and Quality-of-Life (UFS-QOL) scores as well as a reduction in myoma volume.23 Their studies provided evidence that RFA is a safe and effective uterine-sparing treatment of symptomatic uterine fibroids for selected patients.
Related article: Update on Minimally Invasive Surgery Amy Garcia, MD (April 2011)
How it works
RFVTA uses laparoscopy and laparoscopic ultrasound to guide placement of a needle electrode with a deployable array. Earlier international studies of radiofrequency ablation18–22 and ongoing studies of RFVTA23–26 have demonstrated the procedure’s therapeutic potential. Acessa was recently cleared (November 2012) by the US Food and Drug Administration (FDA).
RFVTA technique, step by step
1. Begin with a standard 5-mm laparoscopic infraumbilical port for the camera and video laparoscope (See VIDEO).
2. Place a 12-mm port in the midline, suprapubically at the level of the uterus, for insertion of the laparoscopic ultrasound probe. Now it is possible to map the uterus and plan an approach to destroy the fibroids.
3. Once you have determined your approach, insert the handpiece containing the radiofrequency needle through the abdominal wall under laparoscopic visualization.
4. Place the needle into the targeted fibroid using both laparoscopic and ultrasound guidance. Depending on the size of the fibroid, the needle array can be deployed to the maximum diameter necessary to effect destruction.
5. Engage the radiofrequency generator (FIGURE 1), utilizing the timing function for optimal destruction of the fibroid, based on the appropriate algorithm. The fibroid then will be ablated and destroyed without damage to the surrounding healthy myometrium.
The Acessa radiofrequency generator and accessories are designed to deliver monopolar radiofrequency energy to tissue through the handheld electrical probe. The procedure is monitored by real-time feedback to and from the generator via each of the thermocouples at the tips of the seven-needle array (FIGURE 1). In addition, laparoscopic ultrasound-guided visualization permits monitoring of needle placement within the fibroid capsule (FIGURES 2A and 2B) and confirmation of hemostasis upon removal of the probe tip (FIGURE 2C).
RFVTA treats a wide range of fibroids
The Acessa System is used to treat fibroids of any location and type, with the exception of pedunculated or Type 0 myomas, in which case laparoscopic or hysteroscopic myomectomy is recommended. Calcified fibroids pose no challenges. A single radiofrequency needle generally is used to treat multiple fibroids, with ablation zones ranging in diameter from 1.0 to 6.7 cm. Large, irregularly shaped fibroids may require multiple ablation zones during the same procedure.
Final steps
6. Once the treatment is complete, as confirmed by informatics transmitted by the generator, retract the needle array, set the generator to coagulation mode to coagulate the needle track during withdrawal of the probe, and confirm hemostasis (FIGURE 2C).
7. After completion of all fibroid destruction, remove the disposable radiofrequency probe and close the laparoscopic and ultrasound port sites using standard procedures.
The patient can be discharged the same day and generally is able to return to normal activities within 3 to 5 days.
Postoperative analgesia usually consists solely of nonsteroidal anti-inflammatory agents.
A look at outcomes data
Garza and colleagues published the first feasibility report in 2011.24 They described the results of laparoscopic, ultrasound-guided RFVTA for the treatment of symptomatic uterine fibroids in Mexico using an Acessa predecessor. The 31 women enrolled in the study desired uterine preservation. Of these participants, 19 had completed 12 months of follow-up by the time the report was submitted for publication. Their responses to the UFS-QOL questionnaire indicated that their symptoms had decreased significantly in severity from baseline to 12 months, and their quality-of-life scores also improved significantly (P <.001). Mean uterine volume also declined significantly.
Robles and colleagues set out to confirm these findings using the investigational Halt Ablation System. They conducted a prospective, single-center, open-label clinical trial in Guatemala.25 They found dramatic improvement in symptom severity and health-related quality of life from baseline to 12 months. Mean uterine volume also decreased by 23.6% during this period. In addition, the severity and duration of menses declined.
Acessa is currently not approved for women seeking future childbearing. However, several successful pregnancies have been reported following the procedure.24,26–28 Theoretically, because it causes less damage to and disruption of healthy myometrium, Acessa may prove to be preferable to myomectomy. Head-to-head studies to evaluate this premise are ongoing.
Latest findings confirm widespread improvement of symptoms
The most recent and detailed reports of safety and efficacy of laparoscopic, ultrasound-guided RFVTA at 12 and 24 months were published by teams led by Chudnoff26 and Guido27 earlier this year. These investigations involved 135 women who reported moderate to severe heavy menstrual bleeding (160 mL–500 mL by alkaline hematin analysis). Clinically significant reduction in menstrual blood loss was achieved in 67.7% of participants at 12 months of follow-up.29
Related article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (August 2013)
Galen and colleagues performed a retrospective analysis of these same patients, demonstrating that RFVTA of intramural fibroids without submucosal components leads to a clinically and statistically significant reduction in menstrual blood loss.30
Patients generally began these trials with high symptom-severity scores and low health-related quality of life, yet all achieved significant improvement over time, as their UFS-QOL scores indicate (TABLE). The majority of patients (96%) were treated on an outpatient basis. As measured by contrast-enhanced magnetic resonance imaging, total mean uterine volume decreased by 24.3% at 12 months, and total mean fibroid volume decreased by 45.1% during the same interval. Ninety-four percent of the women reported that the treatment was effective in eliminating their symptoms at 12 months, and 98% said they would recommend the procedure to a friend having the same health problems.
Complication rates were low
Complications included a postoperative pelvic abscess and four other minor events. The latter were treated at the time of RFVTA or resolved without additional procedures. The reintervention rate was 0.7% (1/135 patients).26
Guido and colleagues reported 24-month outcomes for the 124 women entering the second year of the Halt study and affirmed the findings of Chudnoff’s team.27 The 112 patient-reported UFS-QOL scores at 24 months were virtually identical to those reported at 12 months. Six surgical reinterventions (6 of 124 patients, or 4.8%) for fibroid-related bleeding were reported between 12 and 24 months.
280 women have been treated so far
As of September 2013, 280 women have been treated under five international study protocols using the Halt Acessa System, and approximately two-thirds have been followed for more than 12 months, with excellent results. Two of the five clinical trials are under way in Germany and Canada:
- The Laparoscopic Uterine-Sparing Techniques Outcomes and Reinterventions (LUSTOR) trial in Germany is a prospective, randomized, single-center study comparing outcomes of RFVTA and laparoscopic myomectomy. Fifty women were enrolled and treated and will be followed for as long as 60 months.
- The Treatment Results of Uterine-Sparing Technologies (TRUST) trial is a prospective, multicenter, randomized study in Canada that compares direct and indirect costs associated with RFVTA, laparoscopic and abdominal myomectomy, and uterine artery embolization. Some US sites also will participate. As many as 200 women will be treated and followed for as long as 60 months.
RFVTA offers several benefits
Although hysterectomy provides a definitive “cure” to fibroid-related symptoms, it is not preferred by many patients and generally is more costly, with an increased risk of complications, a longer hospital stay, longer recovery (6–8 weeks), longer disability and more time off from work, and potential long-term sequelae.
The Acessa procedure not only is an outpatient, minimally invasive, uterine-sparing fibroid treatment option, it permits access to and ablation of almost all fibroids (with the exception of penduculated or Type 0 myomas). The data to date suggest that RFVTA provides many benefits, including a decline in symptom severity and improved health-related quality of life, as well as good cosmesis, quick recovery, and a rapid return to full activity.
There is a learning curve for RFVTA with Acessa—as with any new procedure. If physicians already perform ultrasound imaging, the learning process is shorter. Physicians interested in Acessa and furthering their education and training in laparoscopic RFVTA should consult the manufacturer’s professional education division through the company Web site at http://www.haltmedical.com.
CASE: RESOLVED
After she is counseled about the various uterine-sparing options for treatment, L.W. chooses to undergo laparoscopic, ultrasound-guided RFVTA using the Acessa System. The procedure is performed successfully, with the two fibroids destroyed using the appropriate algorithms, which took into account the size of the fibroids and the deployed needle array. Two additional 1-cm fibroids were found during the procedure and ablated. The patient was discharged home shortly after the procedure, with a nonsteroidal anti-inflammatory drug given for pain relief.
We want to hear from you! Tell us what you think.
CASE: WOMAN WITH HEAVY BLEEDING HOPES TO AVOID HYSTERECTOMY
L.W. is a 44-year-old woman (G2P2) with a 2-year history of menorrhagia and severe dysmenorrhea but no intermenstrual spotting or bleeding. She reports that she has tried to control her symptoms using hormonal methods, without success.
Examination reveals that she has an irregular, nontender uterus 8 weeks in size. Transvaginal ultrasonography shows two deep, prominent, intramural fibroids. The first is 2 cm by 3 cm in size in the left lateral uterus, adjacent to the endometrial stripe. The second fibroid is 3 cm by 4 cm in the fundal region. Sonohysterography reveals no intracavitary fibroids, although the left lateral myoma has distorted the endometrial cavity.
The patient is seeking removal of her fibroids but would like to preserve her uterus, if at all possible.
What options would you offer her?
Uterine fibroids are common in premenopausal women, affecting 70% to 80% of the population, especially women of African descent.1 Although the majority of women with fibroids are asymptomatic, approximately 30% report pelvic pressure, dyspareunia, dysmenorrhea, or abnormal uterine bleeding.2
Our patients are increasingly aware of uterine-sparing treatments for symptomatic fibroids.3,4 Women seek conservative procedures to avoid the risks and extended recovery times commonly associated with major surgery. Current laparoscopic techniques for removal of uterine fibroids can be complex and require advanced surgical skills. Deep intramural fibroids may not always be visible or readily accessible, making laparoscopic removal a more invasive and challenging approach for many gynecologic surgeons.
A significant recent advancement in minimally invasive gynecologic treatment of fibroids is the Acessa System (Halt Medical, Brentwood, California). This laparoscopic system involves the outpatient use of ultrasound-guided radiofrequency volumetric thermal ablation, or RFVTA.
In this article, I outline the development of this option and step you through its technique. I also review the outcomes data that have been published to date.
How the procedure evolved
In the 1980s, Donnez and Nisolle developed a method of laparoscopic Nd:YAG laser treatment of uterine fibroids, commonly referred to as myolysis.5 Later, Goldfarb developed a laparoscopic bipolar needle technique that coagulated and occluded blood vessels at the periphery of the fibroid.6 However, myolysis led to the formation of significant adhesions to the small bowel or omentum, or both, and was abandoned by the surgical community.
Other fibroid treatments have been developed, such as laparoscopic myomectomy, uterine artery ligation, uterine artery embolization (alone or as adjuvant treatment), and high-intensity focused ultrasound. Although these procedures have made a significant contribution to the minimally invasive treatment of fibroids, the need for reintervention can be substantial, as high as 29% at 1 to 10 years of follow-up in some reports.7–17
Related article: Ins and outs of straight-stick myomectomy James Robinson, MD, MD, and Gaby Moawad, MD (September 2012)
Radiofrequency needle ablation has been used successfully in the treatment and destruction of liver and kidney tumors for years. Lee envisioned use of the technology to treat uterine fibroids. He developed a technique using a retractable multiarray radiofrequency needle (Starburst XL, RITA Medical Systems, Fremont, California) that is inserted directly into the fibroid. In 2002, he reported the first use of intraperitoneal ultrasound for needle guidance in the laparoscopic ablation of 197 myomas in 52 symptomatic women who had declined hysterectomy and myomectomy.18 He found that a significant proportion of women experienced resolution of their symptoms, including heavy menstrual bleeding, dysmenorrhea, dyspareunia, and pelvic pressure, by 3 months, with continued improvement at 12 months.
Because he was unhappy with aspects of the RITA needle that prevented accurate and consistent ablation of fibroids, he developed other devices that would lead to RFVTA and the Acessa System. This system allows the gynecologic surgeon to target and treat deep intramural fibroids that may not be readily visible via conventional laparoscopy.
Between the time of Lee’s first report and his subsequent refinement of the device and procedure, several international fibroid radiofrequency ablation procedures emerged, including those of Bergamini, Milic, Ghezzi, and Carrafiello.19–22 These investigators published results in small cohorts and described significant improvement in patient-reported Uterine Fibroid Symptom and Quality-of-Life (UFS-QOL) scores as well as a reduction in myoma volume.23 Their studies provided evidence that RFA is a safe and effective uterine-sparing treatment of symptomatic uterine fibroids for selected patients.
Related article: Update on Minimally Invasive Surgery Amy Garcia, MD (April 2011)
How it works
RFVTA uses laparoscopy and laparoscopic ultrasound to guide placement of a needle electrode with a deployable array. Earlier international studies of radiofrequency ablation18–22 and ongoing studies of RFVTA23–26 have demonstrated the procedure’s therapeutic potential. Acessa was recently cleared (November 2012) by the US Food and Drug Administration (FDA).
RFVTA technique, step by step
1. Begin with a standard 5-mm laparoscopic infraumbilical port for the camera and video laparoscope (See VIDEO).
2. Place a 12-mm port in the midline, suprapubically at the level of the uterus, for insertion of the laparoscopic ultrasound probe. Now it is possible to map the uterus and plan an approach to destroy the fibroids.
3. Once you have determined your approach, insert the handpiece containing the radiofrequency needle through the abdominal wall under laparoscopic visualization.
4. Place the needle into the targeted fibroid using both laparoscopic and ultrasound guidance. Depending on the size of the fibroid, the needle array can be deployed to the maximum diameter necessary to effect destruction.
5. Engage the radiofrequency generator (FIGURE 1), utilizing the timing function for optimal destruction of the fibroid, based on the appropriate algorithm. The fibroid then will be ablated and destroyed without damage to the surrounding healthy myometrium.
The Acessa radiofrequency generator and accessories are designed to deliver monopolar radiofrequency energy to tissue through the handheld electrical probe. The procedure is monitored by real-time feedback to and from the generator via each of the thermocouples at the tips of the seven-needle array (FIGURE 1). In addition, laparoscopic ultrasound-guided visualization permits monitoring of needle placement within the fibroid capsule (FIGURES 2A and 2B) and confirmation of hemostasis upon removal of the probe tip (FIGURE 2C).
RFVTA treats a wide range of fibroids
The Acessa System is used to treat fibroids of any location and type, with the exception of pedunculated or Type 0 myomas, in which case laparoscopic or hysteroscopic myomectomy is recommended. Calcified fibroids pose no challenges. A single radiofrequency needle generally is used to treat multiple fibroids, with ablation zones ranging in diameter from 1.0 to 6.7 cm. Large, irregularly shaped fibroids may require multiple ablation zones during the same procedure.
Final steps
6. Once the treatment is complete, as confirmed by informatics transmitted by the generator, retract the needle array, set the generator to coagulation mode to coagulate the needle track during withdrawal of the probe, and confirm hemostasis (FIGURE 2C).
7. After completion of all fibroid destruction, remove the disposable radiofrequency probe and close the laparoscopic and ultrasound port sites using standard procedures.
The patient can be discharged the same day and generally is able to return to normal activities within 3 to 5 days.
Postoperative analgesia usually consists solely of nonsteroidal anti-inflammatory agents.
A look at outcomes data
Garza and colleagues published the first feasibility report in 2011.24 They described the results of laparoscopic, ultrasound-guided RFVTA for the treatment of symptomatic uterine fibroids in Mexico using an Acessa predecessor. The 31 women enrolled in the study desired uterine preservation. Of these participants, 19 had completed 12 months of follow-up by the time the report was submitted for publication. Their responses to the UFS-QOL questionnaire indicated that their symptoms had decreased significantly in severity from baseline to 12 months, and their quality-of-life scores also improved significantly (P <.001). Mean uterine volume also declined significantly.
Robles and colleagues set out to confirm these findings using the investigational Halt Ablation System. They conducted a prospective, single-center, open-label clinical trial in Guatemala.25 They found dramatic improvement in symptom severity and health-related quality of life from baseline to 12 months. Mean uterine volume also decreased by 23.6% during this period. In addition, the severity and duration of menses declined.
Acessa is currently not approved for women seeking future childbearing. However, several successful pregnancies have been reported following the procedure.24,26–28 Theoretically, because it causes less damage to and disruption of healthy myometrium, Acessa may prove to be preferable to myomectomy. Head-to-head studies to evaluate this premise are ongoing.
Latest findings confirm widespread improvement of symptoms
The most recent and detailed reports of safety and efficacy of laparoscopic, ultrasound-guided RFVTA at 12 and 24 months were published by teams led by Chudnoff26 and Guido27 earlier this year. These investigations involved 135 women who reported moderate to severe heavy menstrual bleeding (160 mL–500 mL by alkaline hematin analysis). Clinically significant reduction in menstrual blood loss was achieved in 67.7% of participants at 12 months of follow-up.29
Related article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (August 2013)
Galen and colleagues performed a retrospective analysis of these same patients, demonstrating that RFVTA of intramural fibroids without submucosal components leads to a clinically and statistically significant reduction in menstrual blood loss.30
Patients generally began these trials with high symptom-severity scores and low health-related quality of life, yet all achieved significant improvement over time, as their UFS-QOL scores indicate (TABLE). The majority of patients (96%) were treated on an outpatient basis. As measured by contrast-enhanced magnetic resonance imaging, total mean uterine volume decreased by 24.3% at 12 months, and total mean fibroid volume decreased by 45.1% during the same interval. Ninety-four percent of the women reported that the treatment was effective in eliminating their symptoms at 12 months, and 98% said they would recommend the procedure to a friend having the same health problems.
Complication rates were low
Complications included a postoperative pelvic abscess and four other minor events. The latter were treated at the time of RFVTA or resolved without additional procedures. The reintervention rate was 0.7% (1/135 patients).26
Guido and colleagues reported 24-month outcomes for the 124 women entering the second year of the Halt study and affirmed the findings of Chudnoff’s team.27 The 112 patient-reported UFS-QOL scores at 24 months were virtually identical to those reported at 12 months. Six surgical reinterventions (6 of 124 patients, or 4.8%) for fibroid-related bleeding were reported between 12 and 24 months.
280 women have been treated so far
As of September 2013, 280 women have been treated under five international study protocols using the Halt Acessa System, and approximately two-thirds have been followed for more than 12 months, with excellent results. Two of the five clinical trials are under way in Germany and Canada:
- The Laparoscopic Uterine-Sparing Techniques Outcomes and Reinterventions (LUSTOR) trial in Germany is a prospective, randomized, single-center study comparing outcomes of RFVTA and laparoscopic myomectomy. Fifty women were enrolled and treated and will be followed for as long as 60 months.
- The Treatment Results of Uterine-Sparing Technologies (TRUST) trial is a prospective, multicenter, randomized study in Canada that compares direct and indirect costs associated with RFVTA, laparoscopic and abdominal myomectomy, and uterine artery embolization. Some US sites also will participate. As many as 200 women will be treated and followed for as long as 60 months.
RFVTA offers several benefits
Although hysterectomy provides a definitive “cure” to fibroid-related symptoms, it is not preferred by many patients and generally is more costly, with an increased risk of complications, a longer hospital stay, longer recovery (6–8 weeks), longer disability and more time off from work, and potential long-term sequelae.
The Acessa procedure not only is an outpatient, minimally invasive, uterine-sparing fibroid treatment option, it permits access to and ablation of almost all fibroids (with the exception of penduculated or Type 0 myomas). The data to date suggest that RFVTA provides many benefits, including a decline in symptom severity and improved health-related quality of life, as well as good cosmesis, quick recovery, and a rapid return to full activity.
There is a learning curve for RFVTA with Acessa—as with any new procedure. If physicians already perform ultrasound imaging, the learning process is shorter. Physicians interested in Acessa and furthering their education and training in laparoscopic RFVTA should consult the manufacturer’s professional education division through the company Web site at http://www.haltmedical.com.
CASE: RESOLVED
After she is counseled about the various uterine-sparing options for treatment, L.W. chooses to undergo laparoscopic, ultrasound-guided RFVTA using the Acessa System. The procedure is performed successfully, with the two fibroids destroyed using the appropriate algorithms, which took into account the size of the fibroids and the deployed needle array. Two additional 1-cm fibroids were found during the procedure and ablated. The patient was discharged home shortly after the procedure, with a nonsteroidal anti-inflammatory drug given for pain relief.
We want to hear from you! Tell us what you think.
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107.
- Katz VL, Lentz G, Lobo RA, Gershenson D, eds. Comprehensive Gynecology. 5th ed. Philadelphia, PA: Elsevier Mosby Saunders; 2007.
- Rosen P. The endangered uterus. More Magazine Web site. http://www.more.com/health/wellness/endangered-uterus. December 2008/January 2009. Accessed October 16, 2013.
- Tulandi T, Levy BS. So You’re Having a Hysterectomy? Hoboken, NJ: John Wiley & Sons; 2004.
- Donnez J, Squifflet J, Polet R, Nisolle M. Laparoscopic myolysis. Human Reprod Update. 2000;6(6):609–613.
- Goldfarb HA. Bipolar laparoscopic needles for myoma coagulation. J Am Assoc Gynecol Laparosc. 1995;2(2):175–179.
- Hanafi M. Predictors of leiomyoma recurrence after myomectomy. Obstet Gynecol. 2005;105(4):877–881.
- Goodwin SC, Spies JB, Worthington-Kirsch R, et al; Fibroid Registry for Outcomes Data (FIBROID) Registry Steering Committee and Core Site Investigators. Uterine artery embolization for treatment of leiomyomata: long-term outcomes from the FIBROID Registry. Obstet Gynecol. 2008;111(1):22–33.
- Yoo EH, Lee PI, Huh C-Y, et al. Predictors of leiomyoma recurrence after laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(6):690–697.
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356(4):360–370.
- Huang JY, Kafy S, Dugas A, Valenti D, Tulandi T. Failure of uterine fibroid embolization. Fertil Steril. 2006;85(1):30–35.
- Kroncke TJ, Gauruder-Burmester A, Scheurig C, et al. Transarterial embolization for uterine fibroids: clinical success rate and results of magnetic resonance imaging [in German]. Rofo. 2005;177(1):89–98.
- Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31(1):73–85.
- Munoz JL, Jimenez JS, Hernandez C, et al. Hysteroscopic myomectomy: our experience and review. JSLS. 2003;7(1):39–48.
- Reed SD, Newton KM, Thompson LB, McCrummen BA, Warolin AK. The incidence of repeat uterine surgery following myomectomy. J Womens Health (Larchmt). 2006;15(9):1046–1052.
- Spies JB, Bruno J, Czeyda-Pommersheim F, et al. Long-term outcome of uterine artery embolization of leiomyomata. Obstet Gynecol. 2005;106(5 Pt 1):933–939.
- Lee BB. Radiofrequency ablation of uterine leiomyomata: a new minimally invasive hysterectomy alternative. Obstet Gynecol. 2002;99(4Suppl):9S.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192(3):768–773.
- Milic A, Asch MR, Hawrylyshyn PA, et al. Laparoscopic ultrasound-guided radiofrequency ablation of uterine fibroids. Cardiovasc Intervent Radiol. 2006;29(4):694–698.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21(11):2081–2085.
- Carrafiello G, Recaldini C, Fontana F, et al. Ultrasound-guided radiofrequency thermal ablation of uterine fibroids: medium-term follow-up. Cardiovasc Intervent Radiol. 2010;33(1):113–119.
- Spies JB, Coyne K, Guaou Guaou N, et al. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomymata. Obstet Gynecol. 2002;99(2):290–300.
- Garza Leal JG, Hernandez Leon I, Castillo Saenz L, Lee BB. Laparoscopic ultrasound-guided radiofrequency volumetric thermal ablation of symptomatic uterine leiomyomas: feasibility study using the Halt 2000 ablation system. J Minim Invasive Gynecol. 2011;18(3):364–371.
- Robles R, Aguirre VA, Argueta AI, Guerrero MR. Laparoscopic radiofrequency volumetric ablation of uterine myomas with 12 months of follow-up. Int J Gynecol Obstet. 2013;120(1):65–69.
- Chudnoff SG, Berman JM, Levine DJ, Harris M, Guido RS, Banks E. Outpatient procedure for the treatment and relief of symptomatic uterine myomas. Obstet Gynecol. 2013;121(5):1075–1082.
- Guido RS, Macer JA, Abbott K, Falls JL, Tilley IB, Chudnoff SG. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Quality Life Outcomes. 2013;11(139):1–8.
- Berman JM, Puscheck EE, Diamond MP. Full-term vaginal live birth after laparoscopic radiofrequency ablation of a large, symptomatic intramural fibroid. J Reprod Med. 2012;57:159–163.
- Galen DI, Isaacson KB, Lee BB. Does menstrual bleeding decrease after ablation of intramural myomas? A retrospective study [published online ahead of print September 6, 2013]. J Minim Invasive Gynecol. doi: 10.1016/j.jmig.2013.05.007.
- Lukes AS, Muse K, Richter HE, Moore KA, Patrick DL. Estimating a meaningful reduction in menstrual blood loss for women with heavy bleeding. Curr Med Res Opinion. 2010;26(11):2673–2678.
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107.
- Katz VL, Lentz G, Lobo RA, Gershenson D, eds. Comprehensive Gynecology. 5th ed. Philadelphia, PA: Elsevier Mosby Saunders; 2007.
- Rosen P. The endangered uterus. More Magazine Web site. http://www.more.com/health/wellness/endangered-uterus. December 2008/January 2009. Accessed October 16, 2013.
- Tulandi T, Levy BS. So You’re Having a Hysterectomy? Hoboken, NJ: John Wiley & Sons; 2004.
- Donnez J, Squifflet J, Polet R, Nisolle M. Laparoscopic myolysis. Human Reprod Update. 2000;6(6):609–613.
- Goldfarb HA. Bipolar laparoscopic needles for myoma coagulation. J Am Assoc Gynecol Laparosc. 1995;2(2):175–179.
- Hanafi M. Predictors of leiomyoma recurrence after myomectomy. Obstet Gynecol. 2005;105(4):877–881.
- Goodwin SC, Spies JB, Worthington-Kirsch R, et al; Fibroid Registry for Outcomes Data (FIBROID) Registry Steering Committee and Core Site Investigators. Uterine artery embolization for treatment of leiomyomata: long-term outcomes from the FIBROID Registry. Obstet Gynecol. 2008;111(1):22–33.
- Yoo EH, Lee PI, Huh C-Y, et al. Predictors of leiomyoma recurrence after laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(6):690–697.
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356(4):360–370.
- Huang JY, Kafy S, Dugas A, Valenti D, Tulandi T. Failure of uterine fibroid embolization. Fertil Steril. 2006;85(1):30–35.
- Kroncke TJ, Gauruder-Burmester A, Scheurig C, et al. Transarterial embolization for uterine fibroids: clinical success rate and results of magnetic resonance imaging [in German]. Rofo. 2005;177(1):89–98.
- Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31(1):73–85.
- Munoz JL, Jimenez JS, Hernandez C, et al. Hysteroscopic myomectomy: our experience and review. JSLS. 2003;7(1):39–48.
- Reed SD, Newton KM, Thompson LB, McCrummen BA, Warolin AK. The incidence of repeat uterine surgery following myomectomy. J Womens Health (Larchmt). 2006;15(9):1046–1052.
- Spies JB, Bruno J, Czeyda-Pommersheim F, et al. Long-term outcome of uterine artery embolization of leiomyomata. Obstet Gynecol. 2005;106(5 Pt 1):933–939.
- Lee BB. Radiofrequency ablation of uterine leiomyomata: a new minimally invasive hysterectomy alternative. Obstet Gynecol. 2002;99(4Suppl):9S.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192(3):768–773.
- Milic A, Asch MR, Hawrylyshyn PA, et al. Laparoscopic ultrasound-guided radiofrequency ablation of uterine fibroids. Cardiovasc Intervent Radiol. 2006;29(4):694–698.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21(11):2081–2085.
- Carrafiello G, Recaldini C, Fontana F, et al. Ultrasound-guided radiofrequency thermal ablation of uterine fibroids: medium-term follow-up. Cardiovasc Intervent Radiol. 2010;33(1):113–119.
- Spies JB, Coyne K, Guaou Guaou N, et al. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomymata. Obstet Gynecol. 2002;99(2):290–300.
- Garza Leal JG, Hernandez Leon I, Castillo Saenz L, Lee BB. Laparoscopic ultrasound-guided radiofrequency volumetric thermal ablation of symptomatic uterine leiomyomas: feasibility study using the Halt 2000 ablation system. J Minim Invasive Gynecol. 2011;18(3):364–371.
- Robles R, Aguirre VA, Argueta AI, Guerrero MR. Laparoscopic radiofrequency volumetric ablation of uterine myomas with 12 months of follow-up. Int J Gynecol Obstet. 2013;120(1):65–69.
- Chudnoff SG, Berman JM, Levine DJ, Harris M, Guido RS, Banks E. Outpatient procedure for the treatment and relief of symptomatic uterine myomas. Obstet Gynecol. 2013;121(5):1075–1082.
- Guido RS, Macer JA, Abbott K, Falls JL, Tilley IB, Chudnoff SG. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Quality Life Outcomes. 2013;11(139):1–8.
- Berman JM, Puscheck EE, Diamond MP. Full-term vaginal live birth after laparoscopic radiofrequency ablation of a large, symptomatic intramural fibroid. J Reprod Med. 2012;57:159–163.
- Galen DI, Isaacson KB, Lee BB. Does menstrual bleeding decrease after ablation of intramural myomas? A retrospective study [published online ahead of print September 6, 2013]. J Minim Invasive Gynecol. doi: 10.1016/j.jmig.2013.05.007.
- Lukes AS, Muse K, Richter HE, Moore KA, Patrick DL. Estimating a meaningful reduction in menstrual blood loss for women with heavy bleeding. Curr Med Res Opinion. 2010;26(11):2673–2678.
Delayed Dx leads to blindness
Delayed Dx leads to blindness
A WOMAN WITH DISABLING RHEUMATOID ARTHRITIS visited her long-time internist with pulmonary symptoms. Shortly thereafter the 59-year-old patient was diagnosed with lung cancer with a moderate prognosis and underwent surgery.
The following month, the woman complained of jaw pain to her internist. She also reported an “achy” temple to the nurse who saw her initially. The internist surmised that the cause of the pain might be an allergic reaction to dye used in a CT scan the patient had undergone because the patient said the pain had begun immediately after the scan. She was treated with methylprednisolone and the symptoms improved temporarily.
Within a few weeks, the patient complained of vision problems in her left eye. An ophthalmologist to whom she was referred thought the cause might be metastasis of the lung cancer. After an MRI of the optic area, a neuroradiologist reported to the ophthalmologist that the findings were consistent with metastatic cancer.
Before the patient could keep a follow-up appointment with the ophthalmologist, she lost all vision in her left eye. When she called the internist’s office for the results of the MRI, she told the person who answered the phone about the vision loss. Her call wasn’t returned.
The patient also told the ophthalmologist’s office about her loss of vision when she received a call to remind her of her follow-up appointment. The person she spoke to claimed the patient was offered an appointment that same day with another doctor, but declined it.
On the day before the follow-up appointment, the patient lost all sight in her right eye, as well. She received emergency treatment with corticosteroids the next day, but her vision didn’t return, leaving her completely blind. A temporal artery biopsy confirmed giant cell arteritis.
PLAINTIFF'S CLAIM The patient had classic symptoms of giant cell arteritis when she saw both the internist and ophthalmologist.
THE DEFENSE No negligence occurred because the patient had additional medical conditions; the patient didn’t describe her symptoms effectively and was negligent in failing to seek emergency medical care when she lost vision in her left eye.
VERDICT $1.4 million Washington settlement.
COMMENT This is a tough case with plenty of blame to go around, but it provides a good reminder to think of temporal arteritis whenever an older patient complains of jaw pain. Sedimentation rate measurements are cheap.
Lack of vigilance ends badly
SHORTNESS OF BREATH, FATIGUE, AND DIARRHEA prompted a 36-year-old man with diabetes and hypothyroidism to consult his primary care physician. The doctor prescribed levofloxacin and told the patient to return in 4 weeks.
Three days later, the man went back to the physician, reporting weakness, diarrhea, and a fever of 103°F. The physician diagnosed bronchitis and prescribed extended-release amoxicillin tablets. Two days later, the patient went to the emergency department; a chest radiograph showed advanced bilateral pneumonia. He died about 2 weeks later.
PLAINTIFF'S CLAIM The physician was negligent in failing to order a radiograph, admit the patient to the hospital, and prescribe proper medication.
THE DEFENSE No information about the defense is available.
VERDICT $1 million New Jersey settlement.
COMMENT Shortness of breath, fatigue, and diarrhea in a 36-year-old patient with diabetes sounds potentially serious to me. Presumably the physician diagnosed pneumonia on the initial exam, and one cannot fault him for that diagnosis or the treatment he prescribed. But return in 4 weeks? No way. Such patients require close follow-up and escalation of evaluation and treatment if they’re not doing well.
Delayed Dx leads to blindness
A WOMAN WITH DISABLING RHEUMATOID ARTHRITIS visited her long-time internist with pulmonary symptoms. Shortly thereafter the 59-year-old patient was diagnosed with lung cancer with a moderate prognosis and underwent surgery.
The following month, the woman complained of jaw pain to her internist. She also reported an “achy” temple to the nurse who saw her initially. The internist surmised that the cause of the pain might be an allergic reaction to dye used in a CT scan the patient had undergone because the patient said the pain had begun immediately after the scan. She was treated with methylprednisolone and the symptoms improved temporarily.
Within a few weeks, the patient complained of vision problems in her left eye. An ophthalmologist to whom she was referred thought the cause might be metastasis of the lung cancer. After an MRI of the optic area, a neuroradiologist reported to the ophthalmologist that the findings were consistent with metastatic cancer.
Before the patient could keep a follow-up appointment with the ophthalmologist, she lost all vision in her left eye. When she called the internist’s office for the results of the MRI, she told the person who answered the phone about the vision loss. Her call wasn’t returned.
The patient also told the ophthalmologist’s office about her loss of vision when she received a call to remind her of her follow-up appointment. The person she spoke to claimed the patient was offered an appointment that same day with another doctor, but declined it.
On the day before the follow-up appointment, the patient lost all sight in her right eye, as well. She received emergency treatment with corticosteroids the next day, but her vision didn’t return, leaving her completely blind. A temporal artery biopsy confirmed giant cell arteritis.
PLAINTIFF'S CLAIM The patient had classic symptoms of giant cell arteritis when she saw both the internist and ophthalmologist.
THE DEFENSE No negligence occurred because the patient had additional medical conditions; the patient didn’t describe her symptoms effectively and was negligent in failing to seek emergency medical care when she lost vision in her left eye.
VERDICT $1.4 million Washington settlement.
COMMENT This is a tough case with plenty of blame to go around, but it provides a good reminder to think of temporal arteritis whenever an older patient complains of jaw pain. Sedimentation rate measurements are cheap.
Lack of vigilance ends badly
SHORTNESS OF BREATH, FATIGUE, AND DIARRHEA prompted a 36-year-old man with diabetes and hypothyroidism to consult his primary care physician. The doctor prescribed levofloxacin and told the patient to return in 4 weeks.
Three days later, the man went back to the physician, reporting weakness, diarrhea, and a fever of 103°F. The physician diagnosed bronchitis and prescribed extended-release amoxicillin tablets. Two days later, the patient went to the emergency department; a chest radiograph showed advanced bilateral pneumonia. He died about 2 weeks later.
PLAINTIFF'S CLAIM The physician was negligent in failing to order a radiograph, admit the patient to the hospital, and prescribe proper medication.
THE DEFENSE No information about the defense is available.
VERDICT $1 million New Jersey settlement.
COMMENT Shortness of breath, fatigue, and diarrhea in a 36-year-old patient with diabetes sounds potentially serious to me. Presumably the physician diagnosed pneumonia on the initial exam, and one cannot fault him for that diagnosis or the treatment he prescribed. But return in 4 weeks? No way. Such patients require close follow-up and escalation of evaluation and treatment if they’re not doing well.
Delayed Dx leads to blindness
A WOMAN WITH DISABLING RHEUMATOID ARTHRITIS visited her long-time internist with pulmonary symptoms. Shortly thereafter the 59-year-old patient was diagnosed with lung cancer with a moderate prognosis and underwent surgery.
The following month, the woman complained of jaw pain to her internist. She also reported an “achy” temple to the nurse who saw her initially. The internist surmised that the cause of the pain might be an allergic reaction to dye used in a CT scan the patient had undergone because the patient said the pain had begun immediately after the scan. She was treated with methylprednisolone and the symptoms improved temporarily.
Within a few weeks, the patient complained of vision problems in her left eye. An ophthalmologist to whom she was referred thought the cause might be metastasis of the lung cancer. After an MRI of the optic area, a neuroradiologist reported to the ophthalmologist that the findings were consistent with metastatic cancer.
Before the patient could keep a follow-up appointment with the ophthalmologist, she lost all vision in her left eye. When she called the internist’s office for the results of the MRI, she told the person who answered the phone about the vision loss. Her call wasn’t returned.
The patient also told the ophthalmologist’s office about her loss of vision when she received a call to remind her of her follow-up appointment. The person she spoke to claimed the patient was offered an appointment that same day with another doctor, but declined it.
On the day before the follow-up appointment, the patient lost all sight in her right eye, as well. She received emergency treatment with corticosteroids the next day, but her vision didn’t return, leaving her completely blind. A temporal artery biopsy confirmed giant cell arteritis.
PLAINTIFF'S CLAIM The patient had classic symptoms of giant cell arteritis when she saw both the internist and ophthalmologist.
THE DEFENSE No negligence occurred because the patient had additional medical conditions; the patient didn’t describe her symptoms effectively and was negligent in failing to seek emergency medical care when she lost vision in her left eye.
VERDICT $1.4 million Washington settlement.
COMMENT This is a tough case with plenty of blame to go around, but it provides a good reminder to think of temporal arteritis whenever an older patient complains of jaw pain. Sedimentation rate measurements are cheap.
Lack of vigilance ends badly
SHORTNESS OF BREATH, FATIGUE, AND DIARRHEA prompted a 36-year-old man with diabetes and hypothyroidism to consult his primary care physician. The doctor prescribed levofloxacin and told the patient to return in 4 weeks.
Three days later, the man went back to the physician, reporting weakness, diarrhea, and a fever of 103°F. The physician diagnosed bronchitis and prescribed extended-release amoxicillin tablets. Two days later, the patient went to the emergency department; a chest radiograph showed advanced bilateral pneumonia. He died about 2 weeks later.
PLAINTIFF'S CLAIM The physician was negligent in failing to order a radiograph, admit the patient to the hospital, and prescribe proper medication.
THE DEFENSE No information about the defense is available.
VERDICT $1 million New Jersey settlement.
COMMENT Shortness of breath, fatigue, and diarrhea in a 36-year-old patient with diabetes sounds potentially serious to me. Presumably the physician diagnosed pneumonia on the initial exam, and one cannot fault him for that diagnosis or the treatment he prescribed. But return in 4 weeks? No way. Such patients require close follow-up and escalation of evaluation and treatment if they’re not doing well.
An incidental finding
A 37-year-old Caucasian man sought care at our clinic for the pruritic patches on his trunk and extremities that had developed 3 days earlier. The patient said that the lesions started on his right arm but had spread to his left arm, posterior legs, and trunk. He reported that the trunk lesions had resolved, but the extremity lesions persisted. He’d had no specific contact exposures.
Upon further questioning, the patient indicated that he had noted the pigmented patch for at least 4 years, but was not sure how long the nodular area had been there. He thought it was a birthmark. He grew up spending a lot of time at the beach in the sun and recalled at least one blistering sunburn on his back.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Melanoma
The patient underwent elliptical excisional biopsy of the primary lesion after no palpable lymphadenopathy was noted. The lateral and deep margins were negative for melanoma; the mitotic rate was <1/mm2. Aggregates of lymphocytes were associated with the lesion, but did not infiltrate it. There was no tumor regression or ulceration of the lesion. The Breslow depth was 1.25 mm.
A histopathologic evaluation revealed a superficial spreading melanoma (inferior lesion in the FIGURE) and a nodular lesion (the superior reddish-black lesion in the FIGURE). (For more on these and other forms of melanoma, see “The 4 main types of melanoma”1-4 see below.) It was unclear from the patient’s history whether this represented 2 types of melanoma (superficial spreading and nodular) in the same field of skin or the development of a nodular component in a superficial spreading lesion. Important clinical information was also missing, including the evolution of the lesions and how quickly the nodule had grown.
Who’s affected most? More than 45,000 cases of melanoma occurred in 45 states and the District of Columbia annually between 2004 and 2006, according to a 2011 report from the Centers for Disease Control and Prevention.5 White, non-Hispanics have a far higher incidence of melanoma than any other race or ethnicity.6 Women are more likely than men to be diagnosed with melanoma early in life, while men are twice as likely as women to be diagnosed after age 60.6 The etiology for the malignant transformation of melanocytes has not been fully clarified, but it is likely multifactorial, including genetic susceptibility and ultraviolet (UV) radiation damage.4
The ABCDE mnemonic is widely taught to aid in the detection of melanomas: A = asymmetry of the lesion; B = border irregularities; C = color variegation; D = diameter >6 mm; and E = evolution. The evolution of the lesion has been shown in some studies to be the most specific finding for detecting melanomas.4
Regional lymph nodes should also be carefully examined for evidence of clinical spread prior to biopsy of a suspicious lesion. This is important because biopsy may cause regional lymphadenopathy, which could confound later examinations and staging of the disease.1
The differential: Is it a worrisome lesion—or not?
The differential diagnosis of melanoma includes both benign and malignant lesions. Malignant and potentially malignant diagnoses to consider include pigmented basal cell carcinoma, pigmented squamous cell carcinoma, carcinoma metastatic to the skin, and dysplastic nevi.2,3
Benign diagnoses to consider include pigmented seborrheic keratoses, lentigo, pyogenic granuloma, Kaposi sarcoma, cherry angioma, subungual traumatic hematoma, dermatofibroma, and nevi (including blue nevi).2,3
The biopsy is paramount
A diagnosis is established based on the microscopic evaluation of suspicious lesions. Studies suggest that one-third to one-half of melanomas arise from existing nevi, with the remainder developing from previously normal-appearing skin.1,2 Patients with increased numbers of either common or dysplastic nevi are at increased risk of melanoma compared with the general population.4 Historical clues include changes in a lesion’s size, color, or symmetry; new growths; personal or family history of melanoma; and bleeding.
A biopsy for histopathologic evaluation is mandatory when a lesion is suspicious for melanoma. Dermoscopy, which involves a device that magnifies skin lesions, may reveal highly specific dermoscopic features for melanoma that can help in determining the need for biopsy.4
The preferred method for biopsy is complete elliptical excision with 2- to 3-mm margins of normal skin.2,3 However, a deep shave biopsy may also be appropriate, depending upon the clinical situation and physician experience with the technique.4
A deep shave biopsy is less time consuming than elliptical excision, making it easier to perform at the time the lesion is first evaluated. Deep shave biopsy may provide several benefits, including reducing the amount of normal tissue that is removed (especially if pathology is benign) as well as, the cost, scarring, and likelihood of wound infections. Deep shave biopsy also can avoid the need for a second elliptical excision.7,8
Determine margins, proceed with surgical excision
Early surgical excision is the primary treatment for malignant melanoma. After the diagnosis is confirmed by initial biopsy, the depth of the lesion dictates the surgical recommendations. Recommended surgical margins based on depth are: 5 mm with a layer of subcutaneous fat for melanoma in situ, 1 cm down to the fascia for lesions with a Breslow depth ≤2 mm, and 2 cm down to the fascia for lesions with a depth >2 mm.1,4
The surgical treatment of lentigo maligna melanoma can be challenging due to indistinct borders and large size. Mohs micrographic surgery can be helpful to fully remove the lesion with sparing of healthy surrounding tissue.4 When surgical excision of large lentigo maligna is technically difficult, radiation therapy is another option.3
Subungual melanoma may necessitate amputation or grafting of the digit. Mohs micrographic surgery can be useful in these situations for tissue sparing.2 (To learn more, see “When to consider Mohs surgery,” J Fam Pract. 2013;558-564.)
Is a sentinel lymph node biopsy needed?
Sentinel lymph node biopsy is often recommended for melanomas >1 mm in depth.2-4 It provides guidance on who may benefit from regional lymphadenectomy and adjuvant immunotherapy.1,3
Adjuvant therapy for patients without evidence of distant metastases can be considered in patients with positive nodes or node-negative melanoma that is 4 mm thick or Clark Level IV or V. Adjuvant high-dose interferon alpha-2b is the most commonly used agent in these situations.2 Some studies suggest an increase in median overall survival of up to 11 months with high-dose interferon as compared to no treatment.3 Limitations include toxicity from these high-dose regimens.3 Treatment with interferon does not represent a cure; rather, it should be considered a palliative intervention with marginal benefit.1
When there are distant metastases…
Once distant metastases are identified, the goal of therapy should be palliative care as this condition is generally incurable. Chemotherapy, radiation, and excision of solitary metastases are all interventions that have traditionally been employed.1 The primary site for metastasis is the skin, but all organs are potential sites of spread. Central nervous system metastasis is the most common cause of the death.2
Novel therapies include inhibition of BRAF, an enzyme of the mitogen-activated protein kinase pathway (MAPK), and blocking of cytotoxic T lymphocyte-associated antigen-4 (CTLA-4).
BRAF-enzyme inhibitor. Mutated BRAF contributes to uncontrolled cell growth and resistance to programmed cell death (apoptosis).9,10 In August 2011, vemurafenib (Zelboraf ), a BRAF-enzyme inhibitor, was approved by the US Food and Drug Administration (FDA) for the treatment of late-stage melanoma. It works only in patients with the BRAFV600E mutation, which is found in approximately 60% of melanomas.11,12 One phase 1 study showed partial to complete regression in 80% of patients treated, but this regression lasted only 2 to 18 months.13
Anti-CTLA-4 monoclonal antibody. Cytotoxic T-lymphocytes can recognize and potentially destroy cancer cells.14 However, CTLA-4, which is expressed on the surface of cytotoxic T-lymphocytes, has a suppressive effect on the T-lymphocyte response after interaction with the antigen-presenting cell. Researchers deduced that blocking CTLA-4 would allow the immune system to remain responsive to abnormal antigens, including those from melanoma.9
Ipilimumab (Yervoy), an anti-CTLA-4 monoclonal antibody, was approved by the FDA in March 2011 for the treatment of late-stage melanoma.11 Partial and complete responses have been shown in trials of ipilimumab as monotherapy and in combination with vaccines, chemotherapy, and interleukin-2, but these responses have not been sustained.9
Although these novel therapies represent significant advancements in the understanding of the pathogenesis of melanoma, the short duration of efficacy highlights the cancer’s ability to develop resistance to these treatments. This ability to adapt suggests that melanoma harbors multiple oncogenes and several pathways for carcinogenesis. Combination targeted therapies may be required to improve clinical results; research is ongoing.9 Toxicities associated with these medications are also a limiting factor.
What about vaccines? Vaccine studies are also ongoing. Although some have shown promising results, no clearly effective therapy has been produced to date.2
Factors that affect prognosis
The prognosis for melanoma is related to tumor thickness, presence or absence of melanoma in regional lymph nodes, and extent of metastases.1-4 The TNM classification system takes these factors into account in staging melanoma.4 Based on this staging, a 5- and 10-year survival estimate can be discussed with the patient.
Survival estimates based on depth of invasion alone are also used. As an example, one study cited a 5-year survival rate of 95% for a tumor thickness <.75 mm; 85% (.75-1.4 mm); 66% (1.5-3.9 mm); and 46% (≥4 mm).1
Other variables that affect prognosis include lymphocytic infiltrate (more brisk and tumor infiltrating is better prognostically), mitotic rate (less is better; >6/mm2 is worse), ulceration (worse prognostically), and regression of the tumor.2 Regression will appear as areas of depigmentation in a previously completely pigmented lesion; it is associated with a poorer prognosis.1
Follow-up with patients is key
Regular skin, lymph node, and general follow-up exams are recommended to detect metastatic disease or new primary lesions. It has been estimated that approximately 5% of patients with a history of melanoma will develop a new primary lesion.3 Lab and imaging studies should be used when prompted by clinical findings.2
Some protocols recommend routine use of labs, including lactate dehydrogenase, complete blood count, and chemistries, as well as imaging such as chest x-ray, positron emission tomography (PET), or computed tomography (CT) based on the stage of the disease.4 No evidence has shown that routine laboratory or imaging studies affect prognosis.2
Close surveillance for my patient
My patient underwent re-excision of the tumor site with wide margins. Sentinel lymph nodes excised from the bilateral axilla were negative for melanoma. He was seen by colleagues in the oncology department, and his lab work and chest x-ray were normal. PET/CT revealed no evidence of fluorodeoxyglucose avid metastatic disease.
Based on staging, my patient’s 5-year survival was estimated at 81% and his 10-year survival at 67%. No further oncology follow-up was planned and the patient was instructed to be seen by a dermatologist for close clinical surveillance.
CORRESPONDENCE
Suresh K. Menon, MD, Hahn Medical Practices, 5078 Williamsport Pike, Martinsburg, WV 25404; [email protected]
1. Fleischer AB, Feldman SR, Clayton E, et al. Melanoma. In: Hefta J, Noujaim SR, Edmonson KG, eds. 20 Common Problems in Dermatology. 1st ed. New York, NY: McGraw-Hill; 2000:201-217.
2. James WD, Berger TG, Elston DM. Melanoma (malignant melanoma). In: James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin: Clinical Dermatology. 10th ed. Philadelphia, Pa: Saunders Elsevier; 2006:694-699.
3. Marks JG, Miller JJ. Malignant melanoma. In: Marks JG, Miller JJ, Lookingbill DP, eds. Principles of Dermatology. 4th ed. Philadelphia, Pa: Saunders Elsevier; 2006:78-81.
4. Shenenberger DW. Cutaneous malignant melanoma: a primary care perspective. Am Fam Physician. 2012;85:161-168.
5. Melanoma surveillance in the United States. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/cancer/skin/what_cdc_is_doing/melanoma_supplement.htm. Accessed October 11, 2013.
6. Bleyer A, O’Leary M, Barr R, et al, eds. Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975-2000. Bethesda, MD: National Cancer Institute, NIH Pub. No. 06-5767; 2006.
7. Usatine RP, Pfenninger JL, Stulberg DL, et al. Choosing the biopsy type. In: Usatine RP, Pfenninger, JL, Stulberg DL, et al, eds. Dermatologic and Cosmetic Procedures in Office Practice. 1st ed. Philadelphia, Pa: Elsevier; 2011: 75.
8. Usatine RP, Pfenninger JL, Stulberg DL, et al. The shave biopsy. In: Usatine RP, Pfenninger, JL, Stulberg DL, et al, eds. Dermatologic and Cosmetic Procedures in Office Practice. 1st ed. Philadelphia, Pa: Elsevier; 2011: 88.
9. Weber, JS. A New Era Approaches: Anti-CTLA-4 Monoclonal Antibodies for the Treatment of Malignant Melanoma. Medscape Education Web site. Available at: http://www.medscape.org/viewprogram/17800. Accessed July 7, 2012.
10. Shao Y, Aplin AE. Akt3-mediated resistance to apoptosis in B-RAF-targeted melanoma cells. Cancer Res. 2010;70:6670-6681.
11. Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435.
12. Halaban R, Zhang W, Bacchiocchi A, et al. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 2010;23:190-200.
13. Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809-819.
14. Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366:2517-2519.
A 37-year-old Caucasian man sought care at our clinic for the pruritic patches on his trunk and extremities that had developed 3 days earlier. The patient said that the lesions started on his right arm but had spread to his left arm, posterior legs, and trunk. He reported that the trunk lesions had resolved, but the extremity lesions persisted. He’d had no specific contact exposures.
Upon further questioning, the patient indicated that he had noted the pigmented patch for at least 4 years, but was not sure how long the nodular area had been there. He thought it was a birthmark. He grew up spending a lot of time at the beach in the sun and recalled at least one blistering sunburn on his back.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Melanoma
The patient underwent elliptical excisional biopsy of the primary lesion after no palpable lymphadenopathy was noted. The lateral and deep margins were negative for melanoma; the mitotic rate was <1/mm2. Aggregates of lymphocytes were associated with the lesion, but did not infiltrate it. There was no tumor regression or ulceration of the lesion. The Breslow depth was 1.25 mm.
A histopathologic evaluation revealed a superficial spreading melanoma (inferior lesion in the FIGURE) and a nodular lesion (the superior reddish-black lesion in the FIGURE). (For more on these and other forms of melanoma, see “The 4 main types of melanoma”1-4 see below.) It was unclear from the patient’s history whether this represented 2 types of melanoma (superficial spreading and nodular) in the same field of skin or the development of a nodular component in a superficial spreading lesion. Important clinical information was also missing, including the evolution of the lesions and how quickly the nodule had grown.
Who’s affected most? More than 45,000 cases of melanoma occurred in 45 states and the District of Columbia annually between 2004 and 2006, according to a 2011 report from the Centers for Disease Control and Prevention.5 White, non-Hispanics have a far higher incidence of melanoma than any other race or ethnicity.6 Women are more likely than men to be diagnosed with melanoma early in life, while men are twice as likely as women to be diagnosed after age 60.6 The etiology for the malignant transformation of melanocytes has not been fully clarified, but it is likely multifactorial, including genetic susceptibility and ultraviolet (UV) radiation damage.4
The ABCDE mnemonic is widely taught to aid in the detection of melanomas: A = asymmetry of the lesion; B = border irregularities; C = color variegation; D = diameter >6 mm; and E = evolution. The evolution of the lesion has been shown in some studies to be the most specific finding for detecting melanomas.4
Regional lymph nodes should also be carefully examined for evidence of clinical spread prior to biopsy of a suspicious lesion. This is important because biopsy may cause regional lymphadenopathy, which could confound later examinations and staging of the disease.1
The differential: Is it a worrisome lesion—or not?
The differential diagnosis of melanoma includes both benign and malignant lesions. Malignant and potentially malignant diagnoses to consider include pigmented basal cell carcinoma, pigmented squamous cell carcinoma, carcinoma metastatic to the skin, and dysplastic nevi.2,3
Benign diagnoses to consider include pigmented seborrheic keratoses, lentigo, pyogenic granuloma, Kaposi sarcoma, cherry angioma, subungual traumatic hematoma, dermatofibroma, and nevi (including blue nevi).2,3
The biopsy is paramount
A diagnosis is established based on the microscopic evaluation of suspicious lesions. Studies suggest that one-third to one-half of melanomas arise from existing nevi, with the remainder developing from previously normal-appearing skin.1,2 Patients with increased numbers of either common or dysplastic nevi are at increased risk of melanoma compared with the general population.4 Historical clues include changes in a lesion’s size, color, or symmetry; new growths; personal or family history of melanoma; and bleeding.
A biopsy for histopathologic evaluation is mandatory when a lesion is suspicious for melanoma. Dermoscopy, which involves a device that magnifies skin lesions, may reveal highly specific dermoscopic features for melanoma that can help in determining the need for biopsy.4
The preferred method for biopsy is complete elliptical excision with 2- to 3-mm margins of normal skin.2,3 However, a deep shave biopsy may also be appropriate, depending upon the clinical situation and physician experience with the technique.4
A deep shave biopsy is less time consuming than elliptical excision, making it easier to perform at the time the lesion is first evaluated. Deep shave biopsy may provide several benefits, including reducing the amount of normal tissue that is removed (especially if pathology is benign) as well as, the cost, scarring, and likelihood of wound infections. Deep shave biopsy also can avoid the need for a second elliptical excision.7,8
Determine margins, proceed with surgical excision
Early surgical excision is the primary treatment for malignant melanoma. After the diagnosis is confirmed by initial biopsy, the depth of the lesion dictates the surgical recommendations. Recommended surgical margins based on depth are: 5 mm with a layer of subcutaneous fat for melanoma in situ, 1 cm down to the fascia for lesions with a Breslow depth ≤2 mm, and 2 cm down to the fascia for lesions with a depth >2 mm.1,4
The surgical treatment of lentigo maligna melanoma can be challenging due to indistinct borders and large size. Mohs micrographic surgery can be helpful to fully remove the lesion with sparing of healthy surrounding tissue.4 When surgical excision of large lentigo maligna is technically difficult, radiation therapy is another option.3
Subungual melanoma may necessitate amputation or grafting of the digit. Mohs micrographic surgery can be useful in these situations for tissue sparing.2 (To learn more, see “When to consider Mohs surgery,” J Fam Pract. 2013;558-564.)
Is a sentinel lymph node biopsy needed?
Sentinel lymph node biopsy is often recommended for melanomas >1 mm in depth.2-4 It provides guidance on who may benefit from regional lymphadenectomy and adjuvant immunotherapy.1,3
Adjuvant therapy for patients without evidence of distant metastases can be considered in patients with positive nodes or node-negative melanoma that is 4 mm thick or Clark Level IV or V. Adjuvant high-dose interferon alpha-2b is the most commonly used agent in these situations.2 Some studies suggest an increase in median overall survival of up to 11 months with high-dose interferon as compared to no treatment.3 Limitations include toxicity from these high-dose regimens.3 Treatment with interferon does not represent a cure; rather, it should be considered a palliative intervention with marginal benefit.1
When there are distant metastases…
Once distant metastases are identified, the goal of therapy should be palliative care as this condition is generally incurable. Chemotherapy, radiation, and excision of solitary metastases are all interventions that have traditionally been employed.1 The primary site for metastasis is the skin, but all organs are potential sites of spread. Central nervous system metastasis is the most common cause of the death.2
Novel therapies include inhibition of BRAF, an enzyme of the mitogen-activated protein kinase pathway (MAPK), and blocking of cytotoxic T lymphocyte-associated antigen-4 (CTLA-4).
BRAF-enzyme inhibitor. Mutated BRAF contributes to uncontrolled cell growth and resistance to programmed cell death (apoptosis).9,10 In August 2011, vemurafenib (Zelboraf ), a BRAF-enzyme inhibitor, was approved by the US Food and Drug Administration (FDA) for the treatment of late-stage melanoma. It works only in patients with the BRAFV600E mutation, which is found in approximately 60% of melanomas.11,12 One phase 1 study showed partial to complete regression in 80% of patients treated, but this regression lasted only 2 to 18 months.13
Anti-CTLA-4 monoclonal antibody. Cytotoxic T-lymphocytes can recognize and potentially destroy cancer cells.14 However, CTLA-4, which is expressed on the surface of cytotoxic T-lymphocytes, has a suppressive effect on the T-lymphocyte response after interaction with the antigen-presenting cell. Researchers deduced that blocking CTLA-4 would allow the immune system to remain responsive to abnormal antigens, including those from melanoma.9
Ipilimumab (Yervoy), an anti-CTLA-4 monoclonal antibody, was approved by the FDA in March 2011 for the treatment of late-stage melanoma.11 Partial and complete responses have been shown in trials of ipilimumab as monotherapy and in combination with vaccines, chemotherapy, and interleukin-2, but these responses have not been sustained.9
Although these novel therapies represent significant advancements in the understanding of the pathogenesis of melanoma, the short duration of efficacy highlights the cancer’s ability to develop resistance to these treatments. This ability to adapt suggests that melanoma harbors multiple oncogenes and several pathways for carcinogenesis. Combination targeted therapies may be required to improve clinical results; research is ongoing.9 Toxicities associated with these medications are also a limiting factor.
What about vaccines? Vaccine studies are also ongoing. Although some have shown promising results, no clearly effective therapy has been produced to date.2
Factors that affect prognosis
The prognosis for melanoma is related to tumor thickness, presence or absence of melanoma in regional lymph nodes, and extent of metastases.1-4 The TNM classification system takes these factors into account in staging melanoma.4 Based on this staging, a 5- and 10-year survival estimate can be discussed with the patient.
Survival estimates based on depth of invasion alone are also used. As an example, one study cited a 5-year survival rate of 95% for a tumor thickness <.75 mm; 85% (.75-1.4 mm); 66% (1.5-3.9 mm); and 46% (≥4 mm).1
Other variables that affect prognosis include lymphocytic infiltrate (more brisk and tumor infiltrating is better prognostically), mitotic rate (less is better; >6/mm2 is worse), ulceration (worse prognostically), and regression of the tumor.2 Regression will appear as areas of depigmentation in a previously completely pigmented lesion; it is associated with a poorer prognosis.1
Follow-up with patients is key
Regular skin, lymph node, and general follow-up exams are recommended to detect metastatic disease or new primary lesions. It has been estimated that approximately 5% of patients with a history of melanoma will develop a new primary lesion.3 Lab and imaging studies should be used when prompted by clinical findings.2
Some protocols recommend routine use of labs, including lactate dehydrogenase, complete blood count, and chemistries, as well as imaging such as chest x-ray, positron emission tomography (PET), or computed tomography (CT) based on the stage of the disease.4 No evidence has shown that routine laboratory or imaging studies affect prognosis.2
Close surveillance for my patient
My patient underwent re-excision of the tumor site with wide margins. Sentinel lymph nodes excised from the bilateral axilla were negative for melanoma. He was seen by colleagues in the oncology department, and his lab work and chest x-ray were normal. PET/CT revealed no evidence of fluorodeoxyglucose avid metastatic disease.
Based on staging, my patient’s 5-year survival was estimated at 81% and his 10-year survival at 67%. No further oncology follow-up was planned and the patient was instructed to be seen by a dermatologist for close clinical surveillance.
CORRESPONDENCE
Suresh K. Menon, MD, Hahn Medical Practices, 5078 Williamsport Pike, Martinsburg, WV 25404; [email protected]
A 37-year-old Caucasian man sought care at our clinic for the pruritic patches on his trunk and extremities that had developed 3 days earlier. The patient said that the lesions started on his right arm but had spread to his left arm, posterior legs, and trunk. He reported that the trunk lesions had resolved, but the extremity lesions persisted. He’d had no specific contact exposures.
Upon further questioning, the patient indicated that he had noted the pigmented patch for at least 4 years, but was not sure how long the nodular area had been there. He thought it was a birthmark. He grew up spending a lot of time at the beach in the sun and recalled at least one blistering sunburn on his back.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Melanoma
The patient underwent elliptical excisional biopsy of the primary lesion after no palpable lymphadenopathy was noted. The lateral and deep margins were negative for melanoma; the mitotic rate was <1/mm2. Aggregates of lymphocytes were associated with the lesion, but did not infiltrate it. There was no tumor regression or ulceration of the lesion. The Breslow depth was 1.25 mm.
A histopathologic evaluation revealed a superficial spreading melanoma (inferior lesion in the FIGURE) and a nodular lesion (the superior reddish-black lesion in the FIGURE). (For more on these and other forms of melanoma, see “The 4 main types of melanoma”1-4 see below.) It was unclear from the patient’s history whether this represented 2 types of melanoma (superficial spreading and nodular) in the same field of skin or the development of a nodular component in a superficial spreading lesion. Important clinical information was also missing, including the evolution of the lesions and how quickly the nodule had grown.
Who’s affected most? More than 45,000 cases of melanoma occurred in 45 states and the District of Columbia annually between 2004 and 2006, according to a 2011 report from the Centers for Disease Control and Prevention.5 White, non-Hispanics have a far higher incidence of melanoma than any other race or ethnicity.6 Women are more likely than men to be diagnosed with melanoma early in life, while men are twice as likely as women to be diagnosed after age 60.6 The etiology for the malignant transformation of melanocytes has not been fully clarified, but it is likely multifactorial, including genetic susceptibility and ultraviolet (UV) radiation damage.4
The ABCDE mnemonic is widely taught to aid in the detection of melanomas: A = asymmetry of the lesion; B = border irregularities; C = color variegation; D = diameter >6 mm; and E = evolution. The evolution of the lesion has been shown in some studies to be the most specific finding for detecting melanomas.4
Regional lymph nodes should also be carefully examined for evidence of clinical spread prior to biopsy of a suspicious lesion. This is important because biopsy may cause regional lymphadenopathy, which could confound later examinations and staging of the disease.1
The differential: Is it a worrisome lesion—or not?
The differential diagnosis of melanoma includes both benign and malignant lesions. Malignant and potentially malignant diagnoses to consider include pigmented basal cell carcinoma, pigmented squamous cell carcinoma, carcinoma metastatic to the skin, and dysplastic nevi.2,3
Benign diagnoses to consider include pigmented seborrheic keratoses, lentigo, pyogenic granuloma, Kaposi sarcoma, cherry angioma, subungual traumatic hematoma, dermatofibroma, and nevi (including blue nevi).2,3
The biopsy is paramount
A diagnosis is established based on the microscopic evaluation of suspicious lesions. Studies suggest that one-third to one-half of melanomas arise from existing nevi, with the remainder developing from previously normal-appearing skin.1,2 Patients with increased numbers of either common or dysplastic nevi are at increased risk of melanoma compared with the general population.4 Historical clues include changes in a lesion’s size, color, or symmetry; new growths; personal or family history of melanoma; and bleeding.
A biopsy for histopathologic evaluation is mandatory when a lesion is suspicious for melanoma. Dermoscopy, which involves a device that magnifies skin lesions, may reveal highly specific dermoscopic features for melanoma that can help in determining the need for biopsy.4
The preferred method for biopsy is complete elliptical excision with 2- to 3-mm margins of normal skin.2,3 However, a deep shave biopsy may also be appropriate, depending upon the clinical situation and physician experience with the technique.4
A deep shave biopsy is less time consuming than elliptical excision, making it easier to perform at the time the lesion is first evaluated. Deep shave biopsy may provide several benefits, including reducing the amount of normal tissue that is removed (especially if pathology is benign) as well as, the cost, scarring, and likelihood of wound infections. Deep shave biopsy also can avoid the need for a second elliptical excision.7,8
Determine margins, proceed with surgical excision
Early surgical excision is the primary treatment for malignant melanoma. After the diagnosis is confirmed by initial biopsy, the depth of the lesion dictates the surgical recommendations. Recommended surgical margins based on depth are: 5 mm with a layer of subcutaneous fat for melanoma in situ, 1 cm down to the fascia for lesions with a Breslow depth ≤2 mm, and 2 cm down to the fascia for lesions with a depth >2 mm.1,4
The surgical treatment of lentigo maligna melanoma can be challenging due to indistinct borders and large size. Mohs micrographic surgery can be helpful to fully remove the lesion with sparing of healthy surrounding tissue.4 When surgical excision of large lentigo maligna is technically difficult, radiation therapy is another option.3
Subungual melanoma may necessitate amputation or grafting of the digit. Mohs micrographic surgery can be useful in these situations for tissue sparing.2 (To learn more, see “When to consider Mohs surgery,” J Fam Pract. 2013;558-564.)
Is a sentinel lymph node biopsy needed?
Sentinel lymph node biopsy is often recommended for melanomas >1 mm in depth.2-4 It provides guidance on who may benefit from regional lymphadenectomy and adjuvant immunotherapy.1,3
Adjuvant therapy for patients without evidence of distant metastases can be considered in patients with positive nodes or node-negative melanoma that is 4 mm thick or Clark Level IV or V. Adjuvant high-dose interferon alpha-2b is the most commonly used agent in these situations.2 Some studies suggest an increase in median overall survival of up to 11 months with high-dose interferon as compared to no treatment.3 Limitations include toxicity from these high-dose regimens.3 Treatment with interferon does not represent a cure; rather, it should be considered a palliative intervention with marginal benefit.1
When there are distant metastases…
Once distant metastases are identified, the goal of therapy should be palliative care as this condition is generally incurable. Chemotherapy, radiation, and excision of solitary metastases are all interventions that have traditionally been employed.1 The primary site for metastasis is the skin, but all organs are potential sites of spread. Central nervous system metastasis is the most common cause of the death.2
Novel therapies include inhibition of BRAF, an enzyme of the mitogen-activated protein kinase pathway (MAPK), and blocking of cytotoxic T lymphocyte-associated antigen-4 (CTLA-4).
BRAF-enzyme inhibitor. Mutated BRAF contributes to uncontrolled cell growth and resistance to programmed cell death (apoptosis).9,10 In August 2011, vemurafenib (Zelboraf ), a BRAF-enzyme inhibitor, was approved by the US Food and Drug Administration (FDA) for the treatment of late-stage melanoma. It works only in patients with the BRAFV600E mutation, which is found in approximately 60% of melanomas.11,12 One phase 1 study showed partial to complete regression in 80% of patients treated, but this regression lasted only 2 to 18 months.13
Anti-CTLA-4 monoclonal antibody. Cytotoxic T-lymphocytes can recognize and potentially destroy cancer cells.14 However, CTLA-4, which is expressed on the surface of cytotoxic T-lymphocytes, has a suppressive effect on the T-lymphocyte response after interaction with the antigen-presenting cell. Researchers deduced that blocking CTLA-4 would allow the immune system to remain responsive to abnormal antigens, including those from melanoma.9
Ipilimumab (Yervoy), an anti-CTLA-4 monoclonal antibody, was approved by the FDA in March 2011 for the treatment of late-stage melanoma.11 Partial and complete responses have been shown in trials of ipilimumab as monotherapy and in combination with vaccines, chemotherapy, and interleukin-2, but these responses have not been sustained.9
Although these novel therapies represent significant advancements in the understanding of the pathogenesis of melanoma, the short duration of efficacy highlights the cancer’s ability to develop resistance to these treatments. This ability to adapt suggests that melanoma harbors multiple oncogenes and several pathways for carcinogenesis. Combination targeted therapies may be required to improve clinical results; research is ongoing.9 Toxicities associated with these medications are also a limiting factor.
What about vaccines? Vaccine studies are also ongoing. Although some have shown promising results, no clearly effective therapy has been produced to date.2
Factors that affect prognosis
The prognosis for melanoma is related to tumor thickness, presence or absence of melanoma in regional lymph nodes, and extent of metastases.1-4 The TNM classification system takes these factors into account in staging melanoma.4 Based on this staging, a 5- and 10-year survival estimate can be discussed with the patient.
Survival estimates based on depth of invasion alone are also used. As an example, one study cited a 5-year survival rate of 95% for a tumor thickness <.75 mm; 85% (.75-1.4 mm); 66% (1.5-3.9 mm); and 46% (≥4 mm).1
Other variables that affect prognosis include lymphocytic infiltrate (more brisk and tumor infiltrating is better prognostically), mitotic rate (less is better; >6/mm2 is worse), ulceration (worse prognostically), and regression of the tumor.2 Regression will appear as areas of depigmentation in a previously completely pigmented lesion; it is associated with a poorer prognosis.1
Follow-up with patients is key
Regular skin, lymph node, and general follow-up exams are recommended to detect metastatic disease or new primary lesions. It has been estimated that approximately 5% of patients with a history of melanoma will develop a new primary lesion.3 Lab and imaging studies should be used when prompted by clinical findings.2
Some protocols recommend routine use of labs, including lactate dehydrogenase, complete blood count, and chemistries, as well as imaging such as chest x-ray, positron emission tomography (PET), or computed tomography (CT) based on the stage of the disease.4 No evidence has shown that routine laboratory or imaging studies affect prognosis.2
Close surveillance for my patient
My patient underwent re-excision of the tumor site with wide margins. Sentinel lymph nodes excised from the bilateral axilla were negative for melanoma. He was seen by colleagues in the oncology department, and his lab work and chest x-ray were normal. PET/CT revealed no evidence of fluorodeoxyglucose avid metastatic disease.
Based on staging, my patient’s 5-year survival was estimated at 81% and his 10-year survival at 67%. No further oncology follow-up was planned and the patient was instructed to be seen by a dermatologist for close clinical surveillance.
CORRESPONDENCE
Suresh K. Menon, MD, Hahn Medical Practices, 5078 Williamsport Pike, Martinsburg, WV 25404; [email protected]
1. Fleischer AB, Feldman SR, Clayton E, et al. Melanoma. In: Hefta J, Noujaim SR, Edmonson KG, eds. 20 Common Problems in Dermatology. 1st ed. New York, NY: McGraw-Hill; 2000:201-217.
2. James WD, Berger TG, Elston DM. Melanoma (malignant melanoma). In: James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin: Clinical Dermatology. 10th ed. Philadelphia, Pa: Saunders Elsevier; 2006:694-699.
3. Marks JG, Miller JJ. Malignant melanoma. In: Marks JG, Miller JJ, Lookingbill DP, eds. Principles of Dermatology. 4th ed. Philadelphia, Pa: Saunders Elsevier; 2006:78-81.
4. Shenenberger DW. Cutaneous malignant melanoma: a primary care perspective. Am Fam Physician. 2012;85:161-168.
5. Melanoma surveillance in the United States. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/cancer/skin/what_cdc_is_doing/melanoma_supplement.htm. Accessed October 11, 2013.
6. Bleyer A, O’Leary M, Barr R, et al, eds. Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975-2000. Bethesda, MD: National Cancer Institute, NIH Pub. No. 06-5767; 2006.
7. Usatine RP, Pfenninger JL, Stulberg DL, et al. Choosing the biopsy type. In: Usatine RP, Pfenninger, JL, Stulberg DL, et al, eds. Dermatologic and Cosmetic Procedures in Office Practice. 1st ed. Philadelphia, Pa: Elsevier; 2011: 75.
8. Usatine RP, Pfenninger JL, Stulberg DL, et al. The shave biopsy. In: Usatine RP, Pfenninger, JL, Stulberg DL, et al, eds. Dermatologic and Cosmetic Procedures in Office Practice. 1st ed. Philadelphia, Pa: Elsevier; 2011: 88.
9. Weber, JS. A New Era Approaches: Anti-CTLA-4 Monoclonal Antibodies for the Treatment of Malignant Melanoma. Medscape Education Web site. Available at: http://www.medscape.org/viewprogram/17800. Accessed July 7, 2012.
10. Shao Y, Aplin AE. Akt3-mediated resistance to apoptosis in B-RAF-targeted melanoma cells. Cancer Res. 2010;70:6670-6681.
11. Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435.
12. Halaban R, Zhang W, Bacchiocchi A, et al. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 2010;23:190-200.
13. Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809-819.
14. Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366:2517-2519.
1. Fleischer AB, Feldman SR, Clayton E, et al. Melanoma. In: Hefta J, Noujaim SR, Edmonson KG, eds. 20 Common Problems in Dermatology. 1st ed. New York, NY: McGraw-Hill; 2000:201-217.
2. James WD, Berger TG, Elston DM. Melanoma (malignant melanoma). In: James WD, Berger TG, Elston DM, eds. Andrews’ Diseases of the Skin: Clinical Dermatology. 10th ed. Philadelphia, Pa: Saunders Elsevier; 2006:694-699.
3. Marks JG, Miller JJ. Malignant melanoma. In: Marks JG, Miller JJ, Lookingbill DP, eds. Principles of Dermatology. 4th ed. Philadelphia, Pa: Saunders Elsevier; 2006:78-81.
4. Shenenberger DW. Cutaneous malignant melanoma: a primary care perspective. Am Fam Physician. 2012;85:161-168.
5. Melanoma surveillance in the United States. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/cancer/skin/what_cdc_is_doing/melanoma_supplement.htm. Accessed October 11, 2013.
6. Bleyer A, O’Leary M, Barr R, et al, eds. Cancer Epidemiology in Older Adolescents and Young Adults 15 to 29 Years of Age, Including SEER Incidence and Survival: 1975-2000. Bethesda, MD: National Cancer Institute, NIH Pub. No. 06-5767; 2006.
7. Usatine RP, Pfenninger JL, Stulberg DL, et al. Choosing the biopsy type. In: Usatine RP, Pfenninger, JL, Stulberg DL, et al, eds. Dermatologic and Cosmetic Procedures in Office Practice. 1st ed. Philadelphia, Pa: Elsevier; 2011: 75.
8. Usatine RP, Pfenninger JL, Stulberg DL, et al. The shave biopsy. In: Usatine RP, Pfenninger, JL, Stulberg DL, et al, eds. Dermatologic and Cosmetic Procedures in Office Practice. 1st ed. Philadelphia, Pa: Elsevier; 2011: 88.
9. Weber, JS. A New Era Approaches: Anti-CTLA-4 Monoclonal Antibodies for the Treatment of Malignant Melanoma. Medscape Education Web site. Available at: http://www.medscape.org/viewprogram/17800. Accessed July 7, 2012.
10. Shao Y, Aplin AE. Akt3-mediated resistance to apoptosis in B-RAF-targeted melanoma cells. Cancer Res. 2010;70:6670-6681.
11. Hatzivassiliou G, Song K, Yen I, et al. RAF inhibitors prime wild-type RAF to activate the MAPK pathway and enhance growth. Nature. 2010;464:431-435.
12. Halaban R, Zhang W, Bacchiocchi A, et al. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 2010;23:190-200.
13. Flaherty KT, Puzanov I, Kim KB, et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N Engl J Med. 2010;363:809-819.
14. Ribas A. Tumor immunotherapy directed at PD-1. N Engl J Med. 2012;366:2517-2519.
Author response: Venous ulcer treatment
My coauthors and I appreciate the comments of Drs. Mayer and Hansen. Regarding the cost effectiveness and utility of the Unna boot, we would point out that the focus of our Clinical Inquiry was on the initial management of venous stasis ulcers and how best to promote healing.
Little data exist on Unna boot application alone. But it is likely that many of the compression therapy modality studies in the Cochrane meta-analysis included in our review featured Unna boot dressings as part of some form of multilayer compression therapy being evaluated.1
As Dr. Hansen observes, compression, the standard compression classes, and the minimal benefits of low pressure levels provided by the classic TED hose and OTC support hose should have been addressed. This information was not included in our review due to space limitations. This subject deserves a dedicated article, as there is a great deal of confusion about terminology and types of dressings.
Both the 2009 Cochrane meta-analysis1 and a 2012 update2 found that adding a component of elastic compression therapy results in faster ulcer healing compared with inelastic compression therapy alone. Venous ulcers treated with 4-layer bandages heal faster, on average, than those treated with short stretch bandages, and regular use of compression stockings lowers the risk of recurrence.3 Correcting underlying venous incompetency issues is certainly a consideration, particularly for ulcers that initially heal but later recur.
Mark Andrews, MD
Winston-Salem, NC
1. O’Meara S, Cullum NA, Nelson EA. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2009;(1):CD000265.
2. O’Meara S, Cullum N, Nelson EA, et al. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2012;(11):CD000265.
3. Mayberry JC, Moneta GL, Taylor LM Jr, et al. Fifteen-year results of ambulatory compression therapy for chronic venous ulcers. Surgery. 1991;109:575-581.
My coauthors and I appreciate the comments of Drs. Mayer and Hansen. Regarding the cost effectiveness and utility of the Unna boot, we would point out that the focus of our Clinical Inquiry was on the initial management of venous stasis ulcers and how best to promote healing.
Little data exist on Unna boot application alone. But it is likely that many of the compression therapy modality studies in the Cochrane meta-analysis included in our review featured Unna boot dressings as part of some form of multilayer compression therapy being evaluated.1
As Dr. Hansen observes, compression, the standard compression classes, and the minimal benefits of low pressure levels provided by the classic TED hose and OTC support hose should have been addressed. This information was not included in our review due to space limitations. This subject deserves a dedicated article, as there is a great deal of confusion about terminology and types of dressings.
Both the 2009 Cochrane meta-analysis1 and a 2012 update2 found that adding a component of elastic compression therapy results in faster ulcer healing compared with inelastic compression therapy alone. Venous ulcers treated with 4-layer bandages heal faster, on average, than those treated with short stretch bandages, and regular use of compression stockings lowers the risk of recurrence.3 Correcting underlying venous incompetency issues is certainly a consideration, particularly for ulcers that initially heal but later recur.
Mark Andrews, MD
Winston-Salem, NC
My coauthors and I appreciate the comments of Drs. Mayer and Hansen. Regarding the cost effectiveness and utility of the Unna boot, we would point out that the focus of our Clinical Inquiry was on the initial management of venous stasis ulcers and how best to promote healing.
Little data exist on Unna boot application alone. But it is likely that many of the compression therapy modality studies in the Cochrane meta-analysis included in our review featured Unna boot dressings as part of some form of multilayer compression therapy being evaluated.1
As Dr. Hansen observes, compression, the standard compression classes, and the minimal benefits of low pressure levels provided by the classic TED hose and OTC support hose should have been addressed. This information was not included in our review due to space limitations. This subject deserves a dedicated article, as there is a great deal of confusion about terminology and types of dressings.
Both the 2009 Cochrane meta-analysis1 and a 2012 update2 found that adding a component of elastic compression therapy results in faster ulcer healing compared with inelastic compression therapy alone. Venous ulcers treated with 4-layer bandages heal faster, on average, than those treated with short stretch bandages, and regular use of compression stockings lowers the risk of recurrence.3 Correcting underlying venous incompetency issues is certainly a consideration, particularly for ulcers that initially heal but later recur.
Mark Andrews, MD
Winston-Salem, NC
1. O’Meara S, Cullum NA, Nelson EA. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2009;(1):CD000265.
2. O’Meara S, Cullum N, Nelson EA, et al. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2012;(11):CD000265.
3. Mayberry JC, Moneta GL, Taylor LM Jr, et al. Fifteen-year results of ambulatory compression therapy for chronic venous ulcers. Surgery. 1991;109:575-581.
1. O’Meara S, Cullum NA, Nelson EA. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2009;(1):CD000265.
2. O’Meara S, Cullum N, Nelson EA, et al. Compression for venous leg ulcers. Cochrane Database Syst Rev. 2012;(11):CD000265.
3. Mayberry JC, Moneta GL, Taylor LM Jr, et al. Fifteen-year results of ambulatory compression therapy for chronic venous ulcers. Surgery. 1991;109:575-581.
Readers weigh in on venous ulcer treatment
In “What is the best initial treatment for venous stasis ulcers?” (Clinical Inquiries. J Fam Pract. 2013;62:433-434), Dr. Poynter et al seemed to say that various kinds of compression stockings are suitable. What about going back to the Unna boot?
I have had great success treating venous stasis ulcers with the Unna boot. This calamine-infused gauze dressing, which can easily be applied in a primary care setting, has been replaced by wound treatments that are much more expensive—and may be less effective. Cost matters, and all things being equal, it’s time to promote the Unna boot once more.
Charles Mayer, MD, MPH
Seattle, Wash
As a family physician certified by the American Board of Venous and Lymphatic Disease, I would like to point out a couple of things.
First, while the authors discussed compression, they did not define what that meant. This is important, as many physicians prescribe TED hose for venous stasis ulcers, although it does not provide enough compression.
Secondly, fixing the underlying problem should be considered part of the initial treatment. Studies show that a combination of compression and intervention is the best way to ensure long-term healing of venous stasis ulcers.1,2 I would recommend that such patients be referred to a phlebologist for evaluation. Compression alone has a poor long-term outcome for the healing of ulceration.
Lornell E. Hansen II, MD, FAAFP
Sioux Falls, SD
1. Barwell JR, Davies CE, Deacon J, et al. Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study). Lancet. 2004;363:1854-1859.
2. Gohel MS, Barwell JR, Taylor M, et al. Long term results of compression therapy alone versus compressson lus surgery in chronic venous ulceration (ESCHAR): randomised controlled trial. BMJ. 2007;335:83.
In “What is the best initial treatment for venous stasis ulcers?” (Clinical Inquiries. J Fam Pract. 2013;62:433-434), Dr. Poynter et al seemed to say that various kinds of compression stockings are suitable. What about going back to the Unna boot?
I have had great success treating venous stasis ulcers with the Unna boot. This calamine-infused gauze dressing, which can easily be applied in a primary care setting, has been replaced by wound treatments that are much more expensive—and may be less effective. Cost matters, and all things being equal, it’s time to promote the Unna boot once more.
Charles Mayer, MD, MPH
Seattle, Wash
As a family physician certified by the American Board of Venous and Lymphatic Disease, I would like to point out a couple of things.
First, while the authors discussed compression, they did not define what that meant. This is important, as many physicians prescribe TED hose for venous stasis ulcers, although it does not provide enough compression.
Secondly, fixing the underlying problem should be considered part of the initial treatment. Studies show that a combination of compression and intervention is the best way to ensure long-term healing of venous stasis ulcers.1,2 I would recommend that such patients be referred to a phlebologist for evaluation. Compression alone has a poor long-term outcome for the healing of ulceration.
Lornell E. Hansen II, MD, FAAFP
Sioux Falls, SD
In “What is the best initial treatment for venous stasis ulcers?” (Clinical Inquiries. J Fam Pract. 2013;62:433-434), Dr. Poynter et al seemed to say that various kinds of compression stockings are suitable. What about going back to the Unna boot?
I have had great success treating venous stasis ulcers with the Unna boot. This calamine-infused gauze dressing, which can easily be applied in a primary care setting, has been replaced by wound treatments that are much more expensive—and may be less effective. Cost matters, and all things being equal, it’s time to promote the Unna boot once more.
Charles Mayer, MD, MPH
Seattle, Wash
As a family physician certified by the American Board of Venous and Lymphatic Disease, I would like to point out a couple of things.
First, while the authors discussed compression, they did not define what that meant. This is important, as many physicians prescribe TED hose for venous stasis ulcers, although it does not provide enough compression.
Secondly, fixing the underlying problem should be considered part of the initial treatment. Studies show that a combination of compression and intervention is the best way to ensure long-term healing of venous stasis ulcers.1,2 I would recommend that such patients be referred to a phlebologist for evaluation. Compression alone has a poor long-term outcome for the healing of ulceration.
Lornell E. Hansen II, MD, FAAFP
Sioux Falls, SD
1. Barwell JR, Davies CE, Deacon J, et al. Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study). Lancet. 2004;363:1854-1859.
2. Gohel MS, Barwell JR, Taylor M, et al. Long term results of compression therapy alone versus compressson lus surgery in chronic venous ulceration (ESCHAR): randomised controlled trial. BMJ. 2007;335:83.
1. Barwell JR, Davies CE, Deacon J, et al. Comparison of surgery and compression with compression alone in chronic venous ulceration (ESCHAR study). Lancet. 2004;363:1854-1859.
2. Gohel MS, Barwell JR, Taylor M, et al. Long term results of compression therapy alone versus compressson lus surgery in chronic venous ulceration (ESCHAR): randomised controlled trial. BMJ. 2007;335:83.
How do antidepressants affect sexual function?
Patients treated with selective serotonin reuptake inhibitors (SSRIs) and the serotonin/norepinephrine reuptake inhibitor (SNRI) venlafaxine have significantly higher rates of overall sexual dysfunction—including desire, arousal, and orgasm—than patients treated with placebo (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] with heterogeneous results). Patients treated with bupropion, a norepinephrine-dopamine reuptake inhibitor (NDRI), have rates of overall sexual dysfunction comparable to placebo (SOR: B, RCTs with heterogeneous results).
EVIDENCE SUMMARY
In a meta-analysis of 31 studies with 10,130 patients, researchers reported that the total rate of sexual dysfunction (SD) associated with selective serotonin reuptake inhibitors (SSRIs) was significantly higher than the placebo rate of 14.2% (TABLE).1 The SSRIs citalopram, fluoxetine, paroxetine, and sertraline as well as the SNRI venlafaxine, had significantly greater rates (70%-80%) of reported total sexual dysfunction, including desire, arousal, and orgasm, than placebo.
Bupropion has sexual dysfunction rates comparable to placebo
Other SSRIs (fluvoxamine, escitalopram), the tricyclic antidepressant imipramine, and the SNRI duloxetine also had total SD rates significantly greater than placebo. However, the rates of dysfunction with these agents are often lower than the dysfunction rates of SSRIs such as sertraline and citalopram, and thus, may be viewed as falling into an intermediate risk category. The total SD rates for the NDRI bupropion were comparable to the placebo rate.1
With few exceptions, all drugs associated with overall SD were associated with significant dysfunction affecting the sexual components of desire, arousal, and orgasm. The results of this meta-analysis should be interpreted with some degree of caution because methods of assessing SD varied within individual studies.
AHRQ weighs in
An Agency for Healthcare Research and Quality (AHRQ) review of antidepressants found that paroxetine, citalopram, and venlafaxine, when compared with other antidepressants (fluoxetine, fluvoxamine, nefazodone, sertraline), generally were associated with more reports of SD, specifically complaints of erectile dysfunction in men and decreased vaginal lubrication in women. 2 The number needed to treat one additional person with general sexual functioning satisfaction was 6 (95% CI, 4-9) with buproprion.2
RECOMMENDATIONS
The American College of Physicians’ clinical practice guidelines suggest that although SD is likely underreported, the NDRI bupropion has consistently shown lower rates of associated dysfunction than the SSRIs fluoxetine and sertraline.3 Conversely, the SSRI paroxetine has shown higher rates of adverse sexual events than other SSRIs, such as fluoxetine and fluvoxamine, and the serotonin reuptake inhibitor/antagonist nefazodone.3
1. Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol. 2009;29:259-266.
2. Garlehner G, Hansen R, Thieda P, et al. Comparative Effectiveness of Second-Generation Antidepressants in the Pharmacologic Treatment of Adult Depression: Comparative Effectiveness Review Number 7. Rockville, MD; Agency for Healthcare Research and Quality; 2007. Available at: www.effectivehealthcare.ahrq.gov/ehc/products/7/59/Antidepressants_Final_Report.pdf. Accessed: March 5, 2012.
3. Qaseem A, Snow V, Denberg TD, et al; Clinical Efficacy Assessment Subcomittee of Physicians. Using second-generation antidepressants to treat depressive disorders: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149:725-733.
Patients treated with selective serotonin reuptake inhibitors (SSRIs) and the serotonin/norepinephrine reuptake inhibitor (SNRI) venlafaxine have significantly higher rates of overall sexual dysfunction—including desire, arousal, and orgasm—than patients treated with placebo (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] with heterogeneous results). Patients treated with bupropion, a norepinephrine-dopamine reuptake inhibitor (NDRI), have rates of overall sexual dysfunction comparable to placebo (SOR: B, RCTs with heterogeneous results).
EVIDENCE SUMMARY
In a meta-analysis of 31 studies with 10,130 patients, researchers reported that the total rate of sexual dysfunction (SD) associated with selective serotonin reuptake inhibitors (SSRIs) was significantly higher than the placebo rate of 14.2% (TABLE).1 The SSRIs citalopram, fluoxetine, paroxetine, and sertraline as well as the SNRI venlafaxine, had significantly greater rates (70%-80%) of reported total sexual dysfunction, including desire, arousal, and orgasm, than placebo.
Bupropion has sexual dysfunction rates comparable to placebo
Other SSRIs (fluvoxamine, escitalopram), the tricyclic antidepressant imipramine, and the SNRI duloxetine also had total SD rates significantly greater than placebo. However, the rates of dysfunction with these agents are often lower than the dysfunction rates of SSRIs such as sertraline and citalopram, and thus, may be viewed as falling into an intermediate risk category. The total SD rates for the NDRI bupropion were comparable to the placebo rate.1
With few exceptions, all drugs associated with overall SD were associated with significant dysfunction affecting the sexual components of desire, arousal, and orgasm. The results of this meta-analysis should be interpreted with some degree of caution because methods of assessing SD varied within individual studies.
AHRQ weighs in
An Agency for Healthcare Research and Quality (AHRQ) review of antidepressants found that paroxetine, citalopram, and venlafaxine, when compared with other antidepressants (fluoxetine, fluvoxamine, nefazodone, sertraline), generally were associated with more reports of SD, specifically complaints of erectile dysfunction in men and decreased vaginal lubrication in women. 2 The number needed to treat one additional person with general sexual functioning satisfaction was 6 (95% CI, 4-9) with buproprion.2
RECOMMENDATIONS
The American College of Physicians’ clinical practice guidelines suggest that although SD is likely underreported, the NDRI bupropion has consistently shown lower rates of associated dysfunction than the SSRIs fluoxetine and sertraline.3 Conversely, the SSRI paroxetine has shown higher rates of adverse sexual events than other SSRIs, such as fluoxetine and fluvoxamine, and the serotonin reuptake inhibitor/antagonist nefazodone.3
Patients treated with selective serotonin reuptake inhibitors (SSRIs) and the serotonin/norepinephrine reuptake inhibitor (SNRI) venlafaxine have significantly higher rates of overall sexual dysfunction—including desire, arousal, and orgasm—than patients treated with placebo (strength of recommendation [SOR]: B, randomized controlled trials [RCTs] with heterogeneous results). Patients treated with bupropion, a norepinephrine-dopamine reuptake inhibitor (NDRI), have rates of overall sexual dysfunction comparable to placebo (SOR: B, RCTs with heterogeneous results).
EVIDENCE SUMMARY
In a meta-analysis of 31 studies with 10,130 patients, researchers reported that the total rate of sexual dysfunction (SD) associated with selective serotonin reuptake inhibitors (SSRIs) was significantly higher than the placebo rate of 14.2% (TABLE).1 The SSRIs citalopram, fluoxetine, paroxetine, and sertraline as well as the SNRI venlafaxine, had significantly greater rates (70%-80%) of reported total sexual dysfunction, including desire, arousal, and orgasm, than placebo.
Bupropion has sexual dysfunction rates comparable to placebo
Other SSRIs (fluvoxamine, escitalopram), the tricyclic antidepressant imipramine, and the SNRI duloxetine also had total SD rates significantly greater than placebo. However, the rates of dysfunction with these agents are often lower than the dysfunction rates of SSRIs such as sertraline and citalopram, and thus, may be viewed as falling into an intermediate risk category. The total SD rates for the NDRI bupropion were comparable to the placebo rate.1
With few exceptions, all drugs associated with overall SD were associated with significant dysfunction affecting the sexual components of desire, arousal, and orgasm. The results of this meta-analysis should be interpreted with some degree of caution because methods of assessing SD varied within individual studies.
AHRQ weighs in
An Agency for Healthcare Research and Quality (AHRQ) review of antidepressants found that paroxetine, citalopram, and venlafaxine, when compared with other antidepressants (fluoxetine, fluvoxamine, nefazodone, sertraline), generally were associated with more reports of SD, specifically complaints of erectile dysfunction in men and decreased vaginal lubrication in women. 2 The number needed to treat one additional person with general sexual functioning satisfaction was 6 (95% CI, 4-9) with buproprion.2
RECOMMENDATIONS
The American College of Physicians’ clinical practice guidelines suggest that although SD is likely underreported, the NDRI bupropion has consistently shown lower rates of associated dysfunction than the SSRIs fluoxetine and sertraline.3 Conversely, the SSRI paroxetine has shown higher rates of adverse sexual events than other SSRIs, such as fluoxetine and fluvoxamine, and the serotonin reuptake inhibitor/antagonist nefazodone.3
1. Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol. 2009;29:259-266.
2. Garlehner G, Hansen R, Thieda P, et al. Comparative Effectiveness of Second-Generation Antidepressants in the Pharmacologic Treatment of Adult Depression: Comparative Effectiveness Review Number 7. Rockville, MD; Agency for Healthcare Research and Quality; 2007. Available at: www.effectivehealthcare.ahrq.gov/ehc/products/7/59/Antidepressants_Final_Report.pdf. Accessed: March 5, 2012.
3. Qaseem A, Snow V, Denberg TD, et al; Clinical Efficacy Assessment Subcomittee of Physicians. Using second-generation antidepressants to treat depressive disorders: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149:725-733.
1. Serretti A, Chiesa A. Treatment-emergent sexual dysfunction related to antidepressants: a meta-analysis. J Clin Psychopharmacol. 2009;29:259-266.
2. Garlehner G, Hansen R, Thieda P, et al. Comparative Effectiveness of Second-Generation Antidepressants in the Pharmacologic Treatment of Adult Depression: Comparative Effectiveness Review Number 7. Rockville, MD; Agency for Healthcare Research and Quality; 2007. Available at: www.effectivehealthcare.ahrq.gov/ehc/products/7/59/Antidepressants_Final_Report.pdf. Accessed: March 5, 2012.
3. Qaseem A, Snow V, Denberg TD, et al; Clinical Efficacy Assessment Subcomittee of Physicians. Using second-generation antidepressants to treat depressive disorders: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149:725-733.
Evidence-based answers from the Family Physicians Inquiries Network
Fecal incontinence: Help for patients who suffer silently
› Consider adding a question about fecal incontinence—a condition often unreported by patients and undetected by physicians—to your medical intake form. C
› Use bowel diaries and fecal incontinence grading systems, as needed, to better understand the extent of the problem and assess the effects of treatment. C
› Consider sacral nerve stimulation, the first-line surgical treatment for fecal incontinence, for those who fail to respond to medical therapies. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Estimates suggest that about 18 million adults in the United States suffer from fecal incontinence.1 But because the condition often goes unreported by patients and undetected by physicians, the actual prevalence is not known—and may be considerably higher.
What is known is that fecal incontinence carries a substantial socioeconomic burden. The average annual per patient cost is estimated at $4110.2 But fecal incontinence also exacts a heavy personal toll, and is one of the main reasons elderly individuals are placed in nursing homes.3
But it’s not just the elderly who are affected. A recent study of women ages 45 years and older found that nearly one in 5 had an episode of fecal incontinence at least once a year, and for nearly half, the frequency was once a month or more.4 Less than 3 in 10 reported their symptoms to a clinician, but those who did were most likely to have confided in their primary care physician.5
Fortunately, recent developments—most notably, sacral nerve stimulation, a minimally invasive surgical technique with a high success rate—have changed the outlook for patients with fecal incontinence. Here’s what you need to know to help patients who suffer from this embarrassing condition achieve optimal outcomes.
Risk factors and key causes
Maintaining fecal continence involves a complex series of events, both voluntary and involuntary. Problems at various levels—stool consistency, anatomic and neurologic abnormalities, and psychological problems among them—can disrupt the process.
Those at high risk for fecal incontinence, in addition to the elderly, include patients who are mentally ill and institutionalized, individuals with neurologic disorders, patients who have had anorectal surgery, and women who have had vaginal deliveries.6-8 Obstetric and operative injuries account for most cases of fecal incontinence.9-10
Sphincter defects including attenuation and scarring (shown here), are commonly caused by obstetric and operative injuries.
Risks of vaginal delivery
As many as 25% of women report some degree of fecal incontinence—although often confined to loss of control of flatus—3 months after giving birth.11 Stool incontinence is more frequent among women who sustained third- or fourth-degree perineal tears. Obstetrical risk factors include first vaginal birth, median episiotomy, forceps delivery, vacuum-assisted delivery, and a prolonged second stage of labor.
Asymptomatic sphincter defects. Studies in which women underwent endosonographic examination of the sphincter complex both before and after vaginal delivery have found sphincter defects in anywhere from 7% to 41% of new mothers.12-14 It is important to note, however, that as many as 70% of those with defects detected by sonogram were asymptomatic.15 (Despite the risk of sphincter injury during vaginal delivery and evidence suggesting that the risk of fecal incontinence increases with additional deliveries after a previous perineal tear, prophylactic cesarean section is not recommended.)
Fistula surgery and postop incontinence
Fistula surgery is the primary cause of postoperative incontinence, typically resulting from inadvertent injury to either the internal or external sphincter muscle.16 Other relatively common causes of fecal incontinence are rectal prolapse, trauma, irradiation, neurologic and demyelinating disorders such as multiple sclerosis, neoplasms, stroke, infection (eg, of a perineal wound), and diabetes.17 As diagnostic modalities have improved, much of what was previously termed idiopathic incontinence has been found to have identifiable underlying pathology, such as pudendal and inferior hypogastric neuropathies.18-20
Identifying fecal incontinence starts with a single question
As already noted, most patients with symptoms of bowel leakage do not voluntarily mention it to their physician. Many are likely to acknowledge the problem, however, if they’re specifically asked. While little has been written about how best to screen for fecal incontinence, simply adding it to the checklist on your medical intake form may be a good starting point.
Follow up with a targeted history and physical
When a patient checks fecal incontinence on a form or broaches the subject, it is important to question him or her about medical conditions that may be related. These include urinary incontinence, prolapsing tissue, diabetes, and a history of radiation, as well as childbirth. A medication history is also needed, as certain drugs—including some antacids and laxatives—have been implicated in fecal incontinence.21
Physical assessment should include a general neurologic exam as well as a perineal exam, to look for prolapsing tissue and evidence of scars from prior surgery or obstetrical trauma. Check the anocutaneous reflex by stroking the perianal skin. Absence of the anal wink in a younger patient is likely associated with nerve damage; in an older patient, it may simply indicate muscle weakness. Perform a digital rectal exam to assess for normal resting tone and augmentation with squeeze, regardless of the patient’s age.
Use tools to assess the severity
Anal incontinence can be broadly characterized as complete or partial. Numerous other systems have been proposed for classifying severity, the simplest of which has the following 4 components:
A: Continent of solid, liquid, and flatus (complete continence)
B: Continent of solid and liquid, but not flatus
C: Continent of solid, but not liquid or flatus
D: Continued fecal leakage (complete incontinence).22
Although this classification system may be helpful, it yields little information about the significance of the problem from the patient’s perspective.23 Thus, scales that take into account both the frequency of incontinence episodes and the extent of both the mental and physical impact are used more frequently.
One of the most widely used scales is the Cleveland Clinic Fecal Incontinence Score (TABLE),24 which quantifies both the frequency and type of incontinence and scores the level of severity. Fecal incontinence quality of life scales are available, as well, and include questions about the impact on the patient’s lifestyle, coping behavior, mood, and level of embarrassment.25
Even without a quality of life scale, a couple of targeted questions—(eg, Are you ever afraid to go out? Do you worry about others smelling stool on you?)—will give you an idea of how great an impact fecal incontinence is having on your patient’s life. Asking patients to keep bowel diaries can also be helpful in assessing the extent of the problem and the effect of treatment.
Next steps: Start with modifiable risks
While there are numerous diagnostic tests for fecal incontinence (more about these in a bit), none is necessary for initial treatment, which starts with modifiable risks. Chief among them is smoking.
Smoking cessation. Nicotine is believed to have a direct effect on colonic transit and rectal compliance.26 Thus, smoking is associated with an increased risk for fecal incontinence, independent of chronic cough or chronic obstructive pulmonary disease. Patients should be advised to quit smoking and referred to a smoking cessation program.
Dietary fiber. Diet may be a factor in fecal incontinence, as well. Ask patients to record everything they eat, and advise those with a low intake of dietary fiber to eat more fruits, vegetables, whole grains, and other high-fiber food. Recommend that they avoid caffeine and alcohol, as well.
Some medications may also affect stool form and frequency, and precipitate fecal incontinence. Common offenders, in addition to laxatives and antacids, include antibiotics, proton pump inhibitors, and senna-based colon cleansers.27 Consider a switch to another drug class. A trial with a drug thought to improve bowel continence is recommended, as well.
Prescribe pharmacologic treatment
Kaolin, pectin, bulking agents, bismuth salts, anticholinergics, opium derivatives, diphenoxylate/atropine, and loperamide have all been used to treat fecal incontinence, with variable success. Loperamide, the drug most extensively studied for this purpose, has been found to increase resting anal pressure and improve anal sphincter function and continence by acting directly on the circular and longitudinal muscles of the bowel.28
Amitriptyline has also been used empirically, with some success. It is believed to work by decreasing the frequency and amplitude of rectal motor complexes.29 Clonidine in the form of a transdermal patch has been shown to increase the number of problem-free days and overall quality of life for patients with fecal incontinence.30
Consider biofeedback
Biofeedback training is often the next step after pharmacologic treatment. It has been investigated for the treatment of fecal incontinence, and many patients—particularly if they are highly motivated—have reported improvement.31 Therapy generally has 3 components: exercising the external sphincter complex, training in the discrimination of rectal sensations, and developing synchrony of the internal and external sphincter responses during rectal distension.
The goal is for the patient to learn to contract the sphincter in response to small amounts of rectal distension.
But a significant time commitment on the part of the patient and sophisticated apparatus are necessary to carry out such therapy, and only a few randomized controlled trials (RCTs) have evaluated the effect. The largest RCT had 4 arms: a standard care group; standard care plus instruction on sphincter exercises; standard care with sphincter exercises and biofeedback; and standard care with sphincter exercises, biofeedback, and training at home.32
All 4 groups had similar improvement in symptoms, raising questions about the therapeutic value of biofeedback.32 Long-term studies have found that 60% to 80% of patients will continue to have episodes of incontinence after undergoing biofeedback. A Cochrane review of RCTs concluded that there is not enough evidence to judge whether sphincter exercises and biofeedback are effective in reducing fecal incontinence.33
Still no relief? Order tests and consider surgery
For patients with fecal incontinence refractory to conservative management, more sophisticated diagnostic studies can provide invaluable information for guiding further treatment.
Endoanal ultrasound is considered the gold standard diagnostic test for fecal incontinence. It is superior to electromyography in terms of availability, patient tolerance, and ability to assess the internal anal sphincter, except in cases in which nerve injury is suspected.34
Other tests sometimes used to pinpoint the cause of fecal incontinence include an enema challenge (which can differentiate between liquid and solid incontinence) and anal manometry (which can quantify anal sphincter tone). Defecography (which makes it possible to visualize the rectal emptying process) can be helpful if a diagnosis of rectal prolapse is being considered.
Magnetic resonance imaging is among the most costly diagnostic studies associated with fecal incontinence. But it is the only modality that can depict the morphology of the external sphincter and the presence of muscle atrophy—providing information that has been shown to significantly improve the likelihood of successful sphincter repair.35
A wider range of surgical options
When medical therapy and biofeedback fail to produce adequate results, referral to a colorectal surgeon is appropriate. (Although conservative management is frequently unsuccessful, health plans typically require that they be attempted before surgical intervention is considered.)
Sphincteroplasty, or anterior anal sphincter repair, addresses the most common cause of fecal incontinence—and is still a common surgical procedure.36 Sphincteroplasty generally has good to excellent results, providing there is sufficient muscle mass for a successful repair.37,38
The procedure involves dissecting the sphincter complex from the surrounding anoderm, then overlapping the edges of the sphincter muscle and suturing them together. Continence has been reported nearly 80% of the time, although a longer duration of fecal incontinence and incontinence secondary to operative injury of the sphincter are risk factors for poorer outcomes.39,40
Recent studies have called into question the durability of anterior sphincter repair. A systematic review of 16 studies reporting short- and long-term outcomes for more than 900 patients found that all but one of the studies showed a decline over time in the number of patients who were happy with the outcome.39
Sacral nerve stimulation is first-line surgical treatment
Sacral nerve stimulation (SNS) is the most promising development in the treatment of fecal incontinence. In the last decade, SNS has become the first-line surgical treatment for patients for whom medical and behavioral therapy are unsuccessful.40
A minimally invasive procedure that involves an implantable device, SNS is always preceded by an effectiveness trial in which a finder needle is percutaneously inserted into the third sacral foramen. Stimulation should result in immediate contraction of the pelvic floor and external sphincter and plantar flexion of the big toe.
The next step is the insertion of a temporary stimulator lead, which remains in place for a 2- to 3-week test of low-frequency stimulation. If significant reduction in the number of incontinence episodes during the trial period occurs, the device is inserted (See “Sacral nerve stimulation: A case study” above).
Improvement in fecal continence has been reported to be as high as 100% in some cases, with up to 75% of patients achieving complete continence.41 While the mechanism involved remains unclear, multiple studies have confirmed its effectiveness.42,43
Posterior tibial nerve stimulation is another recent development, in which a small, thin lead is placed at the posterior tibial nerve, then connected to a temporary stimulator. Less data are available for this treatment, but a recent review summarized the findings of 8 published studies and found success rates ranging from 30% to 83%.44
The Secca procedure—a relatively new therapy that delivers radiofrequency energy to the anal sphincter—is another option, believed to work by reducing compliance of the sphincter complex and the level of tolerable rectal distension.45 Procedures using injectable bulking materials and fat grafting around the sphincter complex have demonstrated some promise, as well.46
A number of other surgical modalities are available, and often effective under certain circumstances. Among them are rotational and free muscle transfers, used only in cases in which the bulk of the sphincter complex has been destroyed.47,48 Implantable anal sphincters (made from human muscle and nerve cells) are occasionally used, as well, but frequently need to be removed because of infection.49-51
Regardless of the type of treatment they receive, patients often do not achieve total continence. Anyone who continues to have occasional episodes of fecal incontinence or leakage should be advised to wear incontinence pads, as needed.
Consider colostomy when incontinence is severe
For patients with fecal incontinence severe enough to be disabling—often as a result of irradiation—colostomy remains a tried and true treatment. The rectum can either be left intact or a proctectomy performed in concert with ostomy creation. Most studies evaluating colostomy for the treatment of incontinence have found that it significantly improves the quality of life and that most patients say they would choose to undergo the procedure again.52
1. Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512-517.
2. Xu X, Menees SB, Zochowski MK, et al. Economic cost of fecal incontinence. Dis Colon Rectum. 2012;55:586-598.
3. Grover M, Busby-Whitehead J, Palmer MH, et al. Survey of geriatricians on the impact of fecal incontinence on nursing home referral. J Am Geriatr Soc. 2010;58:1058-1062.
4. Brown HW, Wexner SD, Segall MM, et al. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int Clin Pract. 2012;66:1101–1108.
5. Brown HW, Wexner SD, Segall MM, et al. Quality of life impact in women with accidental bowel leakage. Int Clin Pract. 2012;66:1109–1116.
6. Townsend MK, Matthews CA, Whitehead WE, et al. Risk factors for fecal incontinence in older women. Am J Gastroenterol. 2013;108:113-119.
7. Sundquist JC. Long-term outcome after obstetric injury: a retrospective study. Acta Obstet Gynecol Scand. 2012 Jun;91:715-718.
8. Planting A, Phang PT, Raval MJ, et al. Transanal endoscopic microsurgery: impact on fecal incontinence and quality of life. Can J Surg. 2013;56:243-248.
9. Ctercteko GC, Fazio VW, Jagelman DG, et al. Anal sphincter repair: a report of 60 cases and review of the literature. Aust N Z J Surg. 1988;58:703–710.
10. Keighley MRB, Fielding JWL. Management of faecal incontinence and results of surgical treatment. Br J Surg. 1983;70: 463–468.
11. Eason E, Labrecque M, Marcoux S, et al. Anal incontinence after childbirth. CMAJ. 2002;166:326–330.
12. Rieger N, Schloithe A, Saccone G, et al. A prospective study of analsphincter injury due to childbirth. Scand J Gastroenterol. 1998;33:950–955.
13. Zetterstrom J, Mellgren A, Jensen LL, et al. Effect of delivery on anal sphinctermorphology and function. Dis Colon Rectum. 1999;42:1253–1260.
14. Varma A, Gunn J, Gardiner A, et al. Obstetric anal sphincter injury: prospective evaluation of incidence. Dis Colon Rectum. 1999;42:1537–1543.
15. Oberwalder M, Connor J, Wexner SD. Meta-analysis to determine the incidence of obstetric anal sphincter damage. Br J Surg. 2003;90:1333–1337.
16. Lindsey I, Jones OM, Smilgin-Humphreys MM, et al. Patterns of fecal incontinence after anal surgery. Dis Colon Rectum. 2004;47:1643–1649.
17. National Digestive Diseases Information Clearinghouse. Fecal
incontinence. Available at: http://digestive.niddk.nih.gov/ddiseases/pubs/fecalincontinence. Accessed October 20, 2013.
18. Roig JV, Villoslada C, Lledo S, et al. Prevalence of pudendal neuropathy in fecal incontinence. Results of a prospective study. Dis Colon Rectum. 1995;38:952–958.
19. Swash M, Gray A, Lubowski DZ, et al. Ultrastructural changes in internal sphincter in neurogenic incontinence. Gut. 1988;29:1692–1698.
20. Rogers J, Henry MM, Misiewicz JJ. Combined sensory and motor deficit in primary fecal incontinence. Gut. 1988;29:5–9.
21. Medline Plus Web site. Bowel incontinence. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/003135.htm. Accessed October 20, 2013.
22. Browning GP, Parks AG. Post anal repair for neuropathic fecal incontinence: correlation of clinical result and anal canal pressures. Br J Surg. 1983;70:101–104.
23. Baxter NN, Rothenberger DA, Lowry AC. Measuring fecal incontinence. Dis Colon Rectum. 2003;46:1591–1605.
24. Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77–97.
25. American Society of Colon & Rectal Surgeons Web site. Fecal incontinence quality of life scale. Available at: http://www.fascrs.org/physicians/Fecal_Incontinence_Quality_of_Life_Scale/. Accessed October 20, 2013.
26. Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factor for late onset fecal incontinence: a population based case-control study in women. Gastroenterology. 2010;139:1559-1566.
27. MedlinePlus Web site. Drug-induced diarrhea. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/000293.htm. Accessed October 21, 2013.
28. Hallgren T, Fasth S, Delbro DS, et al. Loperamide improves anal sphincter function and continence after restorative proctocolectomy. Dig Dis Sci. 1994;39:2612-2618.
29. Santoro GA, Eitan BZ, Pryde A, et al. Open study of low-dose amitriptyline in the treatment of patients with idiopathic fecal incontinence. Dis Colon Rectum. 2000;43:1676-1681.
30. Bharucha AE, Seide BM, Zinsmeister AR, et al. The effects of clonodine on symptoms and anorectal sensoriomotor function in women with faecal incontinence. Aliment Pharmacol Ther. 2010;32:681-688.
31. Engel BT, Nikoomnesh P, Schuster MM. Operant conditioning of rectosphincteric responses in the treatment of fecal incontinence. N Engl J Med. 1974;290:646-649.
32. Norton C, Chelvanayagam S, Wilson-Barnett J, et al. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenterology. 2003;125:1320–1329.
33. Norton C, Cody JD, Hosker G. Biofeedback and/or sphincter exercises for the treatment of fecal incontinence in adults. Cochrane Database Syst Rev. 2006;(3):CD002111.
34. Sultan AH, Nicholls RJ, Kamm MA, et al. Anal endosonography and correlation with in vitro and in vivo anatomy. Br J Surg. 1993; 80:508–511.
35. Briel JW, Stoker J, Rociu E, et al. External anal sphincter atrophy on endoanal MRI adversely affects continence after sphincteroplasty. Br J Surg. 1999;86:1322–1327.
36. Goetz LH, Lowry AC. Overlapping sphincteroplasty: is it the standard of care? Clin Colon Rectal Surg. 2005;18:22-31.
37. El-Gazzazz G, Zutshi M, Hannaway C, et al. Overlapping sphincter repair: does age matter? Dis Colon Rectum. 2012;55:256-261.
38. Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum. 2012;55:482-490.
39. Lehto K, Hyoty M, Collin P, et al. Seven-year follow-up after anterior sphincter reconstruction for faecal incontinence. Int J Colorectal Dis. 2013;5:653-658.
40. George AT, Kalmar K, Panarese A, et al. Long-term outcomes of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2012;55:302-306.
41. Jarrett MED, Mowatt G, Glazener CMA, et al. Systematic review of sacral nerve stimulation for faecal incontinence and constipation. Br J Surg. 2004;91:1559–1569.
42. Melenhorst J, Koch SM, Uludag O, et al. Is a morphologically intact anal sphincter necessary for success with sacral nerve modulation in patients with faecal incontinence? Colorectal Dis. 2008;10:257-262.
43. Dudding TC, Pares D, Vaizey CJ, et al. Predictive factors for successful sacral nerve stimulation in the treatment of faecal incontinence: a 10-year cohort analysis. Colorectal Dis. 2008;10:294-256.
44. Findlay JM, Mawell-Armstrong C. Posterior tibial nerve stimulation and faecal incontinence: a review. Int J Colorectal Dis. 2011;26:265-273.
45. Feretis C, Benakis P, Dailianas A, et al. Implantation of microballoons in the management of fecal incontinence. Dis Colon Rectum. 2001;44:1605–1609.
46. Kenefick NJ, Vaizey CJ, Malouf AJ, et al. Injectable silicone biomaterial for faecal incontinence due to internal anal sphincter dysfunction. Gut. 2002;55:225–228.
47. Konsten J, Baeten CG, Spaans F, et al. Follow-up of anal dynamic graciloplasty for fecal continence. World J Surg. 1993;17:404–409.
48. Baeten C, Spaans F, Fluks A. An implanted neuromuscular stimulator for fecal continence following previously implanted gracilis muscle: report of a case. Dis Colon Rectum. 1988;31:134–137.
49. Wong MT, Meurette G, Stangherlin P, et al. The magnetic anal sphincter versus the artificial bowel sphincter: a comparison of 2 treatments for fecal incontinence. Dis Colon Rectum. 2011;54:773-779.
50. Parker SC, Spencer MP, Madoff RD, et al. Artificial bowel sphincter: long-term experience at a single institution. Dis Colon Rectum 2003;46:722–729.
51. Takahashi T, Garcia-Osogobio S, Valdovinos MA, et al. Extended two-year results of radio-frequency energy delivery for the treatment of fecal incontinence (Secca procedure). Dis Colon Rectum. 2003;46:711–715.
52. Norton C, Burch J, Kamm MA. Patient’s views of a colostomy for fecal incontinence. Dis Colon Rectum. 2005;48:1062.
› Consider adding a question about fecal incontinence—a condition often unreported by patients and undetected by physicians—to your medical intake form. C
› Use bowel diaries and fecal incontinence grading systems, as needed, to better understand the extent of the problem and assess the effects of treatment. C
› Consider sacral nerve stimulation, the first-line surgical treatment for fecal incontinence, for those who fail to respond to medical therapies. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Estimates suggest that about 18 million adults in the United States suffer from fecal incontinence.1 But because the condition often goes unreported by patients and undetected by physicians, the actual prevalence is not known—and may be considerably higher.
What is known is that fecal incontinence carries a substantial socioeconomic burden. The average annual per patient cost is estimated at $4110.2 But fecal incontinence also exacts a heavy personal toll, and is one of the main reasons elderly individuals are placed in nursing homes.3
But it’s not just the elderly who are affected. A recent study of women ages 45 years and older found that nearly one in 5 had an episode of fecal incontinence at least once a year, and for nearly half, the frequency was once a month or more.4 Less than 3 in 10 reported their symptoms to a clinician, but those who did were most likely to have confided in their primary care physician.5
Fortunately, recent developments—most notably, sacral nerve stimulation, a minimally invasive surgical technique with a high success rate—have changed the outlook for patients with fecal incontinence. Here’s what you need to know to help patients who suffer from this embarrassing condition achieve optimal outcomes.
Risk factors and key causes
Maintaining fecal continence involves a complex series of events, both voluntary and involuntary. Problems at various levels—stool consistency, anatomic and neurologic abnormalities, and psychological problems among them—can disrupt the process.
Those at high risk for fecal incontinence, in addition to the elderly, include patients who are mentally ill and institutionalized, individuals with neurologic disorders, patients who have had anorectal surgery, and women who have had vaginal deliveries.6-8 Obstetric and operative injuries account for most cases of fecal incontinence.9-10
Sphincter defects including attenuation and scarring (shown here), are commonly caused by obstetric and operative injuries.
Risks of vaginal delivery
As many as 25% of women report some degree of fecal incontinence—although often confined to loss of control of flatus—3 months after giving birth.11 Stool incontinence is more frequent among women who sustained third- or fourth-degree perineal tears. Obstetrical risk factors include first vaginal birth, median episiotomy, forceps delivery, vacuum-assisted delivery, and a prolonged second stage of labor.
Asymptomatic sphincter defects. Studies in which women underwent endosonographic examination of the sphincter complex both before and after vaginal delivery have found sphincter defects in anywhere from 7% to 41% of new mothers.12-14 It is important to note, however, that as many as 70% of those with defects detected by sonogram were asymptomatic.15 (Despite the risk of sphincter injury during vaginal delivery and evidence suggesting that the risk of fecal incontinence increases with additional deliveries after a previous perineal tear, prophylactic cesarean section is not recommended.)
Fistula surgery and postop incontinence
Fistula surgery is the primary cause of postoperative incontinence, typically resulting from inadvertent injury to either the internal or external sphincter muscle.16 Other relatively common causes of fecal incontinence are rectal prolapse, trauma, irradiation, neurologic and demyelinating disorders such as multiple sclerosis, neoplasms, stroke, infection (eg, of a perineal wound), and diabetes.17 As diagnostic modalities have improved, much of what was previously termed idiopathic incontinence has been found to have identifiable underlying pathology, such as pudendal and inferior hypogastric neuropathies.18-20
Identifying fecal incontinence starts with a single question
As already noted, most patients with symptoms of bowel leakage do not voluntarily mention it to their physician. Many are likely to acknowledge the problem, however, if they’re specifically asked. While little has been written about how best to screen for fecal incontinence, simply adding it to the checklist on your medical intake form may be a good starting point.
Follow up with a targeted history and physical
When a patient checks fecal incontinence on a form or broaches the subject, it is important to question him or her about medical conditions that may be related. These include urinary incontinence, prolapsing tissue, diabetes, and a history of radiation, as well as childbirth. A medication history is also needed, as certain drugs—including some antacids and laxatives—have been implicated in fecal incontinence.21
Physical assessment should include a general neurologic exam as well as a perineal exam, to look for prolapsing tissue and evidence of scars from prior surgery or obstetrical trauma. Check the anocutaneous reflex by stroking the perianal skin. Absence of the anal wink in a younger patient is likely associated with nerve damage; in an older patient, it may simply indicate muscle weakness. Perform a digital rectal exam to assess for normal resting tone and augmentation with squeeze, regardless of the patient’s age.
Use tools to assess the severity
Anal incontinence can be broadly characterized as complete or partial. Numerous other systems have been proposed for classifying severity, the simplest of which has the following 4 components:
A: Continent of solid, liquid, and flatus (complete continence)
B: Continent of solid and liquid, but not flatus
C: Continent of solid, but not liquid or flatus
D: Continued fecal leakage (complete incontinence).22
Although this classification system may be helpful, it yields little information about the significance of the problem from the patient’s perspective.23 Thus, scales that take into account both the frequency of incontinence episodes and the extent of both the mental and physical impact are used more frequently.
One of the most widely used scales is the Cleveland Clinic Fecal Incontinence Score (TABLE),24 which quantifies both the frequency and type of incontinence and scores the level of severity. Fecal incontinence quality of life scales are available, as well, and include questions about the impact on the patient’s lifestyle, coping behavior, mood, and level of embarrassment.25
Even without a quality of life scale, a couple of targeted questions—(eg, Are you ever afraid to go out? Do you worry about others smelling stool on you?)—will give you an idea of how great an impact fecal incontinence is having on your patient’s life. Asking patients to keep bowel diaries can also be helpful in assessing the extent of the problem and the effect of treatment.
Next steps: Start with modifiable risks
While there are numerous diagnostic tests for fecal incontinence (more about these in a bit), none is necessary for initial treatment, which starts with modifiable risks. Chief among them is smoking.
Smoking cessation. Nicotine is believed to have a direct effect on colonic transit and rectal compliance.26 Thus, smoking is associated with an increased risk for fecal incontinence, independent of chronic cough or chronic obstructive pulmonary disease. Patients should be advised to quit smoking and referred to a smoking cessation program.
Dietary fiber. Diet may be a factor in fecal incontinence, as well. Ask patients to record everything they eat, and advise those with a low intake of dietary fiber to eat more fruits, vegetables, whole grains, and other high-fiber food. Recommend that they avoid caffeine and alcohol, as well.
Some medications may also affect stool form and frequency, and precipitate fecal incontinence. Common offenders, in addition to laxatives and antacids, include antibiotics, proton pump inhibitors, and senna-based colon cleansers.27 Consider a switch to another drug class. A trial with a drug thought to improve bowel continence is recommended, as well.
Prescribe pharmacologic treatment
Kaolin, pectin, bulking agents, bismuth salts, anticholinergics, opium derivatives, diphenoxylate/atropine, and loperamide have all been used to treat fecal incontinence, with variable success. Loperamide, the drug most extensively studied for this purpose, has been found to increase resting anal pressure and improve anal sphincter function and continence by acting directly on the circular and longitudinal muscles of the bowel.28
Amitriptyline has also been used empirically, with some success. It is believed to work by decreasing the frequency and amplitude of rectal motor complexes.29 Clonidine in the form of a transdermal patch has been shown to increase the number of problem-free days and overall quality of life for patients with fecal incontinence.30
Consider biofeedback
Biofeedback training is often the next step after pharmacologic treatment. It has been investigated for the treatment of fecal incontinence, and many patients—particularly if they are highly motivated—have reported improvement.31 Therapy generally has 3 components: exercising the external sphincter complex, training in the discrimination of rectal sensations, and developing synchrony of the internal and external sphincter responses during rectal distension.
The goal is for the patient to learn to contract the sphincter in response to small amounts of rectal distension.
But a significant time commitment on the part of the patient and sophisticated apparatus are necessary to carry out such therapy, and only a few randomized controlled trials (RCTs) have evaluated the effect. The largest RCT had 4 arms: a standard care group; standard care plus instruction on sphincter exercises; standard care with sphincter exercises and biofeedback; and standard care with sphincter exercises, biofeedback, and training at home.32
All 4 groups had similar improvement in symptoms, raising questions about the therapeutic value of biofeedback.32 Long-term studies have found that 60% to 80% of patients will continue to have episodes of incontinence after undergoing biofeedback. A Cochrane review of RCTs concluded that there is not enough evidence to judge whether sphincter exercises and biofeedback are effective in reducing fecal incontinence.33
Still no relief? Order tests and consider surgery
For patients with fecal incontinence refractory to conservative management, more sophisticated diagnostic studies can provide invaluable information for guiding further treatment.
Endoanal ultrasound is considered the gold standard diagnostic test for fecal incontinence. It is superior to electromyography in terms of availability, patient tolerance, and ability to assess the internal anal sphincter, except in cases in which nerve injury is suspected.34
Other tests sometimes used to pinpoint the cause of fecal incontinence include an enema challenge (which can differentiate between liquid and solid incontinence) and anal manometry (which can quantify anal sphincter tone). Defecography (which makes it possible to visualize the rectal emptying process) can be helpful if a diagnosis of rectal prolapse is being considered.
Magnetic resonance imaging is among the most costly diagnostic studies associated with fecal incontinence. But it is the only modality that can depict the morphology of the external sphincter and the presence of muscle atrophy—providing information that has been shown to significantly improve the likelihood of successful sphincter repair.35
A wider range of surgical options
When medical therapy and biofeedback fail to produce adequate results, referral to a colorectal surgeon is appropriate. (Although conservative management is frequently unsuccessful, health plans typically require that they be attempted before surgical intervention is considered.)
Sphincteroplasty, or anterior anal sphincter repair, addresses the most common cause of fecal incontinence—and is still a common surgical procedure.36 Sphincteroplasty generally has good to excellent results, providing there is sufficient muscle mass for a successful repair.37,38
The procedure involves dissecting the sphincter complex from the surrounding anoderm, then overlapping the edges of the sphincter muscle and suturing them together. Continence has been reported nearly 80% of the time, although a longer duration of fecal incontinence and incontinence secondary to operative injury of the sphincter are risk factors for poorer outcomes.39,40
Recent studies have called into question the durability of anterior sphincter repair. A systematic review of 16 studies reporting short- and long-term outcomes for more than 900 patients found that all but one of the studies showed a decline over time in the number of patients who were happy with the outcome.39
Sacral nerve stimulation is first-line surgical treatment
Sacral nerve stimulation (SNS) is the most promising development in the treatment of fecal incontinence. In the last decade, SNS has become the first-line surgical treatment for patients for whom medical and behavioral therapy are unsuccessful.40
A minimally invasive procedure that involves an implantable device, SNS is always preceded by an effectiveness trial in which a finder needle is percutaneously inserted into the third sacral foramen. Stimulation should result in immediate contraction of the pelvic floor and external sphincter and plantar flexion of the big toe.
The next step is the insertion of a temporary stimulator lead, which remains in place for a 2- to 3-week test of low-frequency stimulation. If significant reduction in the number of incontinence episodes during the trial period occurs, the device is inserted (See “Sacral nerve stimulation: A case study” above).
Improvement in fecal continence has been reported to be as high as 100% in some cases, with up to 75% of patients achieving complete continence.41 While the mechanism involved remains unclear, multiple studies have confirmed its effectiveness.42,43
Posterior tibial nerve stimulation is another recent development, in which a small, thin lead is placed at the posterior tibial nerve, then connected to a temporary stimulator. Less data are available for this treatment, but a recent review summarized the findings of 8 published studies and found success rates ranging from 30% to 83%.44
The Secca procedure—a relatively new therapy that delivers radiofrequency energy to the anal sphincter—is another option, believed to work by reducing compliance of the sphincter complex and the level of tolerable rectal distension.45 Procedures using injectable bulking materials and fat grafting around the sphincter complex have demonstrated some promise, as well.46
A number of other surgical modalities are available, and often effective under certain circumstances. Among them are rotational and free muscle transfers, used only in cases in which the bulk of the sphincter complex has been destroyed.47,48 Implantable anal sphincters (made from human muscle and nerve cells) are occasionally used, as well, but frequently need to be removed because of infection.49-51
Regardless of the type of treatment they receive, patients often do not achieve total continence. Anyone who continues to have occasional episodes of fecal incontinence or leakage should be advised to wear incontinence pads, as needed.
Consider colostomy when incontinence is severe
For patients with fecal incontinence severe enough to be disabling—often as a result of irradiation—colostomy remains a tried and true treatment. The rectum can either be left intact or a proctectomy performed in concert with ostomy creation. Most studies evaluating colostomy for the treatment of incontinence have found that it significantly improves the quality of life and that most patients say they would choose to undergo the procedure again.52
› Consider adding a question about fecal incontinence—a condition often unreported by patients and undetected by physicians—to your medical intake form. C
› Use bowel diaries and fecal incontinence grading systems, as needed, to better understand the extent of the problem and assess the effects of treatment. C
› Consider sacral nerve stimulation, the first-line surgical treatment for fecal incontinence, for those who fail to respond to medical therapies. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Estimates suggest that about 18 million adults in the United States suffer from fecal incontinence.1 But because the condition often goes unreported by patients and undetected by physicians, the actual prevalence is not known—and may be considerably higher.
What is known is that fecal incontinence carries a substantial socioeconomic burden. The average annual per patient cost is estimated at $4110.2 But fecal incontinence also exacts a heavy personal toll, and is one of the main reasons elderly individuals are placed in nursing homes.3
But it’s not just the elderly who are affected. A recent study of women ages 45 years and older found that nearly one in 5 had an episode of fecal incontinence at least once a year, and for nearly half, the frequency was once a month or more.4 Less than 3 in 10 reported their symptoms to a clinician, but those who did were most likely to have confided in their primary care physician.5
Fortunately, recent developments—most notably, sacral nerve stimulation, a minimally invasive surgical technique with a high success rate—have changed the outlook for patients with fecal incontinence. Here’s what you need to know to help patients who suffer from this embarrassing condition achieve optimal outcomes.
Risk factors and key causes
Maintaining fecal continence involves a complex series of events, both voluntary and involuntary. Problems at various levels—stool consistency, anatomic and neurologic abnormalities, and psychological problems among them—can disrupt the process.
Those at high risk for fecal incontinence, in addition to the elderly, include patients who are mentally ill and institutionalized, individuals with neurologic disorders, patients who have had anorectal surgery, and women who have had vaginal deliveries.6-8 Obstetric and operative injuries account for most cases of fecal incontinence.9-10
Sphincter defects including attenuation and scarring (shown here), are commonly caused by obstetric and operative injuries.
Risks of vaginal delivery
As many as 25% of women report some degree of fecal incontinence—although often confined to loss of control of flatus—3 months after giving birth.11 Stool incontinence is more frequent among women who sustained third- or fourth-degree perineal tears. Obstetrical risk factors include first vaginal birth, median episiotomy, forceps delivery, vacuum-assisted delivery, and a prolonged second stage of labor.
Asymptomatic sphincter defects. Studies in which women underwent endosonographic examination of the sphincter complex both before and after vaginal delivery have found sphincter defects in anywhere from 7% to 41% of new mothers.12-14 It is important to note, however, that as many as 70% of those with defects detected by sonogram were asymptomatic.15 (Despite the risk of sphincter injury during vaginal delivery and evidence suggesting that the risk of fecal incontinence increases with additional deliveries after a previous perineal tear, prophylactic cesarean section is not recommended.)
Fistula surgery and postop incontinence
Fistula surgery is the primary cause of postoperative incontinence, typically resulting from inadvertent injury to either the internal or external sphincter muscle.16 Other relatively common causes of fecal incontinence are rectal prolapse, trauma, irradiation, neurologic and demyelinating disorders such as multiple sclerosis, neoplasms, stroke, infection (eg, of a perineal wound), and diabetes.17 As diagnostic modalities have improved, much of what was previously termed idiopathic incontinence has been found to have identifiable underlying pathology, such as pudendal and inferior hypogastric neuropathies.18-20
Identifying fecal incontinence starts with a single question
As already noted, most patients with symptoms of bowel leakage do not voluntarily mention it to their physician. Many are likely to acknowledge the problem, however, if they’re specifically asked. While little has been written about how best to screen for fecal incontinence, simply adding it to the checklist on your medical intake form may be a good starting point.
Follow up with a targeted history and physical
When a patient checks fecal incontinence on a form or broaches the subject, it is important to question him or her about medical conditions that may be related. These include urinary incontinence, prolapsing tissue, diabetes, and a history of radiation, as well as childbirth. A medication history is also needed, as certain drugs—including some antacids and laxatives—have been implicated in fecal incontinence.21
Physical assessment should include a general neurologic exam as well as a perineal exam, to look for prolapsing tissue and evidence of scars from prior surgery or obstetrical trauma. Check the anocutaneous reflex by stroking the perianal skin. Absence of the anal wink in a younger patient is likely associated with nerve damage; in an older patient, it may simply indicate muscle weakness. Perform a digital rectal exam to assess for normal resting tone and augmentation with squeeze, regardless of the patient’s age.
Use tools to assess the severity
Anal incontinence can be broadly characterized as complete or partial. Numerous other systems have been proposed for classifying severity, the simplest of which has the following 4 components:
A: Continent of solid, liquid, and flatus (complete continence)
B: Continent of solid and liquid, but not flatus
C: Continent of solid, but not liquid or flatus
D: Continued fecal leakage (complete incontinence).22
Although this classification system may be helpful, it yields little information about the significance of the problem from the patient’s perspective.23 Thus, scales that take into account both the frequency of incontinence episodes and the extent of both the mental and physical impact are used more frequently.
One of the most widely used scales is the Cleveland Clinic Fecal Incontinence Score (TABLE),24 which quantifies both the frequency and type of incontinence and scores the level of severity. Fecal incontinence quality of life scales are available, as well, and include questions about the impact on the patient’s lifestyle, coping behavior, mood, and level of embarrassment.25
Even without a quality of life scale, a couple of targeted questions—(eg, Are you ever afraid to go out? Do you worry about others smelling stool on you?)—will give you an idea of how great an impact fecal incontinence is having on your patient’s life. Asking patients to keep bowel diaries can also be helpful in assessing the extent of the problem and the effect of treatment.
Next steps: Start with modifiable risks
While there are numerous diagnostic tests for fecal incontinence (more about these in a bit), none is necessary for initial treatment, which starts with modifiable risks. Chief among them is smoking.
Smoking cessation. Nicotine is believed to have a direct effect on colonic transit and rectal compliance.26 Thus, smoking is associated with an increased risk for fecal incontinence, independent of chronic cough or chronic obstructive pulmonary disease. Patients should be advised to quit smoking and referred to a smoking cessation program.
Dietary fiber. Diet may be a factor in fecal incontinence, as well. Ask patients to record everything they eat, and advise those with a low intake of dietary fiber to eat more fruits, vegetables, whole grains, and other high-fiber food. Recommend that they avoid caffeine and alcohol, as well.
Some medications may also affect stool form and frequency, and precipitate fecal incontinence. Common offenders, in addition to laxatives and antacids, include antibiotics, proton pump inhibitors, and senna-based colon cleansers.27 Consider a switch to another drug class. A trial with a drug thought to improve bowel continence is recommended, as well.
Prescribe pharmacologic treatment
Kaolin, pectin, bulking agents, bismuth salts, anticholinergics, opium derivatives, diphenoxylate/atropine, and loperamide have all been used to treat fecal incontinence, with variable success. Loperamide, the drug most extensively studied for this purpose, has been found to increase resting anal pressure and improve anal sphincter function and continence by acting directly on the circular and longitudinal muscles of the bowel.28
Amitriptyline has also been used empirically, with some success. It is believed to work by decreasing the frequency and amplitude of rectal motor complexes.29 Clonidine in the form of a transdermal patch has been shown to increase the number of problem-free days and overall quality of life for patients with fecal incontinence.30
Consider biofeedback
Biofeedback training is often the next step after pharmacologic treatment. It has been investigated for the treatment of fecal incontinence, and many patients—particularly if they are highly motivated—have reported improvement.31 Therapy generally has 3 components: exercising the external sphincter complex, training in the discrimination of rectal sensations, and developing synchrony of the internal and external sphincter responses during rectal distension.
The goal is for the patient to learn to contract the sphincter in response to small amounts of rectal distension.
But a significant time commitment on the part of the patient and sophisticated apparatus are necessary to carry out such therapy, and only a few randomized controlled trials (RCTs) have evaluated the effect. The largest RCT had 4 arms: a standard care group; standard care plus instruction on sphincter exercises; standard care with sphincter exercises and biofeedback; and standard care with sphincter exercises, biofeedback, and training at home.32
All 4 groups had similar improvement in symptoms, raising questions about the therapeutic value of biofeedback.32 Long-term studies have found that 60% to 80% of patients will continue to have episodes of incontinence after undergoing biofeedback. A Cochrane review of RCTs concluded that there is not enough evidence to judge whether sphincter exercises and biofeedback are effective in reducing fecal incontinence.33
Still no relief? Order tests and consider surgery
For patients with fecal incontinence refractory to conservative management, more sophisticated diagnostic studies can provide invaluable information for guiding further treatment.
Endoanal ultrasound is considered the gold standard diagnostic test for fecal incontinence. It is superior to electromyography in terms of availability, patient tolerance, and ability to assess the internal anal sphincter, except in cases in which nerve injury is suspected.34
Other tests sometimes used to pinpoint the cause of fecal incontinence include an enema challenge (which can differentiate between liquid and solid incontinence) and anal manometry (which can quantify anal sphincter tone). Defecography (which makes it possible to visualize the rectal emptying process) can be helpful if a diagnosis of rectal prolapse is being considered.
Magnetic resonance imaging is among the most costly diagnostic studies associated with fecal incontinence. But it is the only modality that can depict the morphology of the external sphincter and the presence of muscle atrophy—providing information that has been shown to significantly improve the likelihood of successful sphincter repair.35
A wider range of surgical options
When medical therapy and biofeedback fail to produce adequate results, referral to a colorectal surgeon is appropriate. (Although conservative management is frequently unsuccessful, health plans typically require that they be attempted before surgical intervention is considered.)
Sphincteroplasty, or anterior anal sphincter repair, addresses the most common cause of fecal incontinence—and is still a common surgical procedure.36 Sphincteroplasty generally has good to excellent results, providing there is sufficient muscle mass for a successful repair.37,38
The procedure involves dissecting the sphincter complex from the surrounding anoderm, then overlapping the edges of the sphincter muscle and suturing them together. Continence has been reported nearly 80% of the time, although a longer duration of fecal incontinence and incontinence secondary to operative injury of the sphincter are risk factors for poorer outcomes.39,40
Recent studies have called into question the durability of anterior sphincter repair. A systematic review of 16 studies reporting short- and long-term outcomes for more than 900 patients found that all but one of the studies showed a decline over time in the number of patients who were happy with the outcome.39
Sacral nerve stimulation is first-line surgical treatment
Sacral nerve stimulation (SNS) is the most promising development in the treatment of fecal incontinence. In the last decade, SNS has become the first-line surgical treatment for patients for whom medical and behavioral therapy are unsuccessful.40
A minimally invasive procedure that involves an implantable device, SNS is always preceded by an effectiveness trial in which a finder needle is percutaneously inserted into the third sacral foramen. Stimulation should result in immediate contraction of the pelvic floor and external sphincter and plantar flexion of the big toe.
The next step is the insertion of a temporary stimulator lead, which remains in place for a 2- to 3-week test of low-frequency stimulation. If significant reduction in the number of incontinence episodes during the trial period occurs, the device is inserted (See “Sacral nerve stimulation: A case study” above).
Improvement in fecal continence has been reported to be as high as 100% in some cases, with up to 75% of patients achieving complete continence.41 While the mechanism involved remains unclear, multiple studies have confirmed its effectiveness.42,43
Posterior tibial nerve stimulation is another recent development, in which a small, thin lead is placed at the posterior tibial nerve, then connected to a temporary stimulator. Less data are available for this treatment, but a recent review summarized the findings of 8 published studies and found success rates ranging from 30% to 83%.44
The Secca procedure—a relatively new therapy that delivers radiofrequency energy to the anal sphincter—is another option, believed to work by reducing compliance of the sphincter complex and the level of tolerable rectal distension.45 Procedures using injectable bulking materials and fat grafting around the sphincter complex have demonstrated some promise, as well.46
A number of other surgical modalities are available, and often effective under certain circumstances. Among them are rotational and free muscle transfers, used only in cases in which the bulk of the sphincter complex has been destroyed.47,48 Implantable anal sphincters (made from human muscle and nerve cells) are occasionally used, as well, but frequently need to be removed because of infection.49-51
Regardless of the type of treatment they receive, patients often do not achieve total continence. Anyone who continues to have occasional episodes of fecal incontinence or leakage should be advised to wear incontinence pads, as needed.
Consider colostomy when incontinence is severe
For patients with fecal incontinence severe enough to be disabling—often as a result of irradiation—colostomy remains a tried and true treatment. The rectum can either be left intact or a proctectomy performed in concert with ostomy creation. Most studies evaluating colostomy for the treatment of incontinence have found that it significantly improves the quality of life and that most patients say they would choose to undergo the procedure again.52
1. Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512-517.
2. Xu X, Menees SB, Zochowski MK, et al. Economic cost of fecal incontinence. Dis Colon Rectum. 2012;55:586-598.
3. Grover M, Busby-Whitehead J, Palmer MH, et al. Survey of geriatricians on the impact of fecal incontinence on nursing home referral. J Am Geriatr Soc. 2010;58:1058-1062.
4. Brown HW, Wexner SD, Segall MM, et al. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int Clin Pract. 2012;66:1101–1108.
5. Brown HW, Wexner SD, Segall MM, et al. Quality of life impact in women with accidental bowel leakage. Int Clin Pract. 2012;66:1109–1116.
6. Townsend MK, Matthews CA, Whitehead WE, et al. Risk factors for fecal incontinence in older women. Am J Gastroenterol. 2013;108:113-119.
7. Sundquist JC. Long-term outcome after obstetric injury: a retrospective study. Acta Obstet Gynecol Scand. 2012 Jun;91:715-718.
8. Planting A, Phang PT, Raval MJ, et al. Transanal endoscopic microsurgery: impact on fecal incontinence and quality of life. Can J Surg. 2013;56:243-248.
9. Ctercteko GC, Fazio VW, Jagelman DG, et al. Anal sphincter repair: a report of 60 cases and review of the literature. Aust N Z J Surg. 1988;58:703–710.
10. Keighley MRB, Fielding JWL. Management of faecal incontinence and results of surgical treatment. Br J Surg. 1983;70: 463–468.
11. Eason E, Labrecque M, Marcoux S, et al. Anal incontinence after childbirth. CMAJ. 2002;166:326–330.
12. Rieger N, Schloithe A, Saccone G, et al. A prospective study of analsphincter injury due to childbirth. Scand J Gastroenterol. 1998;33:950–955.
13. Zetterstrom J, Mellgren A, Jensen LL, et al. Effect of delivery on anal sphinctermorphology and function. Dis Colon Rectum. 1999;42:1253–1260.
14. Varma A, Gunn J, Gardiner A, et al. Obstetric anal sphincter injury: prospective evaluation of incidence. Dis Colon Rectum. 1999;42:1537–1543.
15. Oberwalder M, Connor J, Wexner SD. Meta-analysis to determine the incidence of obstetric anal sphincter damage. Br J Surg. 2003;90:1333–1337.
16. Lindsey I, Jones OM, Smilgin-Humphreys MM, et al. Patterns of fecal incontinence after anal surgery. Dis Colon Rectum. 2004;47:1643–1649.
17. National Digestive Diseases Information Clearinghouse. Fecal
incontinence. Available at: http://digestive.niddk.nih.gov/ddiseases/pubs/fecalincontinence. Accessed October 20, 2013.
18. Roig JV, Villoslada C, Lledo S, et al. Prevalence of pudendal neuropathy in fecal incontinence. Results of a prospective study. Dis Colon Rectum. 1995;38:952–958.
19. Swash M, Gray A, Lubowski DZ, et al. Ultrastructural changes in internal sphincter in neurogenic incontinence. Gut. 1988;29:1692–1698.
20. Rogers J, Henry MM, Misiewicz JJ. Combined sensory and motor deficit in primary fecal incontinence. Gut. 1988;29:5–9.
21. Medline Plus Web site. Bowel incontinence. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/003135.htm. Accessed October 20, 2013.
22. Browning GP, Parks AG. Post anal repair for neuropathic fecal incontinence: correlation of clinical result and anal canal pressures. Br J Surg. 1983;70:101–104.
23. Baxter NN, Rothenberger DA, Lowry AC. Measuring fecal incontinence. Dis Colon Rectum. 2003;46:1591–1605.
24. Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77–97.
25. American Society of Colon & Rectal Surgeons Web site. Fecal incontinence quality of life scale. Available at: http://www.fascrs.org/physicians/Fecal_Incontinence_Quality_of_Life_Scale/. Accessed October 20, 2013.
26. Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factor for late onset fecal incontinence: a population based case-control study in women. Gastroenterology. 2010;139:1559-1566.
27. MedlinePlus Web site. Drug-induced diarrhea. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/000293.htm. Accessed October 21, 2013.
28. Hallgren T, Fasth S, Delbro DS, et al. Loperamide improves anal sphincter function and continence after restorative proctocolectomy. Dig Dis Sci. 1994;39:2612-2618.
29. Santoro GA, Eitan BZ, Pryde A, et al. Open study of low-dose amitriptyline in the treatment of patients with idiopathic fecal incontinence. Dis Colon Rectum. 2000;43:1676-1681.
30. Bharucha AE, Seide BM, Zinsmeister AR, et al. The effects of clonodine on symptoms and anorectal sensoriomotor function in women with faecal incontinence. Aliment Pharmacol Ther. 2010;32:681-688.
31. Engel BT, Nikoomnesh P, Schuster MM. Operant conditioning of rectosphincteric responses in the treatment of fecal incontinence. N Engl J Med. 1974;290:646-649.
32. Norton C, Chelvanayagam S, Wilson-Barnett J, et al. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenterology. 2003;125:1320–1329.
33. Norton C, Cody JD, Hosker G. Biofeedback and/or sphincter exercises for the treatment of fecal incontinence in adults. Cochrane Database Syst Rev. 2006;(3):CD002111.
34. Sultan AH, Nicholls RJ, Kamm MA, et al. Anal endosonography and correlation with in vitro and in vivo anatomy. Br J Surg. 1993; 80:508–511.
35. Briel JW, Stoker J, Rociu E, et al. External anal sphincter atrophy on endoanal MRI adversely affects continence after sphincteroplasty. Br J Surg. 1999;86:1322–1327.
36. Goetz LH, Lowry AC. Overlapping sphincteroplasty: is it the standard of care? Clin Colon Rectal Surg. 2005;18:22-31.
37. El-Gazzazz G, Zutshi M, Hannaway C, et al. Overlapping sphincter repair: does age matter? Dis Colon Rectum. 2012;55:256-261.
38. Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum. 2012;55:482-490.
39. Lehto K, Hyoty M, Collin P, et al. Seven-year follow-up after anterior sphincter reconstruction for faecal incontinence. Int J Colorectal Dis. 2013;5:653-658.
40. George AT, Kalmar K, Panarese A, et al. Long-term outcomes of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2012;55:302-306.
41. Jarrett MED, Mowatt G, Glazener CMA, et al. Systematic review of sacral nerve stimulation for faecal incontinence and constipation. Br J Surg. 2004;91:1559–1569.
42. Melenhorst J, Koch SM, Uludag O, et al. Is a morphologically intact anal sphincter necessary for success with sacral nerve modulation in patients with faecal incontinence? Colorectal Dis. 2008;10:257-262.
43. Dudding TC, Pares D, Vaizey CJ, et al. Predictive factors for successful sacral nerve stimulation in the treatment of faecal incontinence: a 10-year cohort analysis. Colorectal Dis. 2008;10:294-256.
44. Findlay JM, Mawell-Armstrong C. Posterior tibial nerve stimulation and faecal incontinence: a review. Int J Colorectal Dis. 2011;26:265-273.
45. Feretis C, Benakis P, Dailianas A, et al. Implantation of microballoons in the management of fecal incontinence. Dis Colon Rectum. 2001;44:1605–1609.
46. Kenefick NJ, Vaizey CJ, Malouf AJ, et al. Injectable silicone biomaterial for faecal incontinence due to internal anal sphincter dysfunction. Gut. 2002;55:225–228.
47. Konsten J, Baeten CG, Spaans F, et al. Follow-up of anal dynamic graciloplasty for fecal continence. World J Surg. 1993;17:404–409.
48. Baeten C, Spaans F, Fluks A. An implanted neuromuscular stimulator for fecal continence following previously implanted gracilis muscle: report of a case. Dis Colon Rectum. 1988;31:134–137.
49. Wong MT, Meurette G, Stangherlin P, et al. The magnetic anal sphincter versus the artificial bowel sphincter: a comparison of 2 treatments for fecal incontinence. Dis Colon Rectum. 2011;54:773-779.
50. Parker SC, Spencer MP, Madoff RD, et al. Artificial bowel sphincter: long-term experience at a single institution. Dis Colon Rectum 2003;46:722–729.
51. Takahashi T, Garcia-Osogobio S, Valdovinos MA, et al. Extended two-year results of radio-frequency energy delivery for the treatment of fecal incontinence (Secca procedure). Dis Colon Rectum. 2003;46:711–715.
52. Norton C, Burch J, Kamm MA. Patient’s views of a colostomy for fecal incontinence. Dis Colon Rectum. 2005;48:1062.
1. Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adults: epidemiology and risk factors. Gastroenterology. 2009;137:512-517.
2. Xu X, Menees SB, Zochowski MK, et al. Economic cost of fecal incontinence. Dis Colon Rectum. 2012;55:586-598.
3. Grover M, Busby-Whitehead J, Palmer MH, et al. Survey of geriatricians on the impact of fecal incontinence on nursing home referral. J Am Geriatr Soc. 2010;58:1058-1062.
4. Brown HW, Wexner SD, Segall MM, et al. Accidental bowel leakage in the mature women’s health study: prevalence and predictors. Int Clin Pract. 2012;66:1101–1108.
5. Brown HW, Wexner SD, Segall MM, et al. Quality of life impact in women with accidental bowel leakage. Int Clin Pract. 2012;66:1109–1116.
6. Townsend MK, Matthews CA, Whitehead WE, et al. Risk factors for fecal incontinence in older women. Am J Gastroenterol. 2013;108:113-119.
7. Sundquist JC. Long-term outcome after obstetric injury: a retrospective study. Acta Obstet Gynecol Scand. 2012 Jun;91:715-718.
8. Planting A, Phang PT, Raval MJ, et al. Transanal endoscopic microsurgery: impact on fecal incontinence and quality of life. Can J Surg. 2013;56:243-248.
9. Ctercteko GC, Fazio VW, Jagelman DG, et al. Anal sphincter repair: a report of 60 cases and review of the literature. Aust N Z J Surg. 1988;58:703–710.
10. Keighley MRB, Fielding JWL. Management of faecal incontinence and results of surgical treatment. Br J Surg. 1983;70: 463–468.
11. Eason E, Labrecque M, Marcoux S, et al. Anal incontinence after childbirth. CMAJ. 2002;166:326–330.
12. Rieger N, Schloithe A, Saccone G, et al. A prospective study of analsphincter injury due to childbirth. Scand J Gastroenterol. 1998;33:950–955.
13. Zetterstrom J, Mellgren A, Jensen LL, et al. Effect of delivery on anal sphinctermorphology and function. Dis Colon Rectum. 1999;42:1253–1260.
14. Varma A, Gunn J, Gardiner A, et al. Obstetric anal sphincter injury: prospective evaluation of incidence. Dis Colon Rectum. 1999;42:1537–1543.
15. Oberwalder M, Connor J, Wexner SD. Meta-analysis to determine the incidence of obstetric anal sphincter damage. Br J Surg. 2003;90:1333–1337.
16. Lindsey I, Jones OM, Smilgin-Humphreys MM, et al. Patterns of fecal incontinence after anal surgery. Dis Colon Rectum. 2004;47:1643–1649.
17. National Digestive Diseases Information Clearinghouse. Fecal
incontinence. Available at: http://digestive.niddk.nih.gov/ddiseases/pubs/fecalincontinence. Accessed October 20, 2013.
18. Roig JV, Villoslada C, Lledo S, et al. Prevalence of pudendal neuropathy in fecal incontinence. Results of a prospective study. Dis Colon Rectum. 1995;38:952–958.
19. Swash M, Gray A, Lubowski DZ, et al. Ultrastructural changes in internal sphincter in neurogenic incontinence. Gut. 1988;29:1692–1698.
20. Rogers J, Henry MM, Misiewicz JJ. Combined sensory and motor deficit in primary fecal incontinence. Gut. 1988;29:5–9.
21. Medline Plus Web site. Bowel incontinence. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/003135.htm. Accessed October 20, 2013.
22. Browning GP, Parks AG. Post anal repair for neuropathic fecal incontinence: correlation of clinical result and anal canal pressures. Br J Surg. 1983;70:101–104.
23. Baxter NN, Rothenberger DA, Lowry AC. Measuring fecal incontinence. Dis Colon Rectum. 2003;46:1591–1605.
24. Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36:77–97.
25. American Society of Colon & Rectal Surgeons Web site. Fecal incontinence quality of life scale. Available at: http://www.fascrs.org/physicians/Fecal_Incontinence_Quality_of_Life_Scale/. Accessed October 20, 2013.
26. Bharucha AE, Zinsmeister AR, Schleck CD, et al. Bowel disturbances are the most important risk factor for late onset fecal incontinence: a population based case-control study in women. Gastroenterology. 2010;139:1559-1566.
27. MedlinePlus Web site. Drug-induced diarrhea. Available at: http://www.nlm.nih.gov/medlineplus/ency/article/000293.htm. Accessed October 21, 2013.
28. Hallgren T, Fasth S, Delbro DS, et al. Loperamide improves anal sphincter function and continence after restorative proctocolectomy. Dig Dis Sci. 1994;39:2612-2618.
29. Santoro GA, Eitan BZ, Pryde A, et al. Open study of low-dose amitriptyline in the treatment of patients with idiopathic fecal incontinence. Dis Colon Rectum. 2000;43:1676-1681.
30. Bharucha AE, Seide BM, Zinsmeister AR, et al. The effects of clonodine on symptoms and anorectal sensoriomotor function in women with faecal incontinence. Aliment Pharmacol Ther. 2010;32:681-688.
31. Engel BT, Nikoomnesh P, Schuster MM. Operant conditioning of rectosphincteric responses in the treatment of fecal incontinence. N Engl J Med. 1974;290:646-649.
32. Norton C, Chelvanayagam S, Wilson-Barnett J, et al. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenterology. 2003;125:1320–1329.
33. Norton C, Cody JD, Hosker G. Biofeedback and/or sphincter exercises for the treatment of fecal incontinence in adults. Cochrane Database Syst Rev. 2006;(3):CD002111.
34. Sultan AH, Nicholls RJ, Kamm MA, et al. Anal endosonography and correlation with in vitro and in vivo anatomy. Br J Surg. 1993; 80:508–511.
35. Briel JW, Stoker J, Rociu E, et al. External anal sphincter atrophy on endoanal MRI adversely affects continence after sphincteroplasty. Br J Surg. 1999;86:1322–1327.
36. Goetz LH, Lowry AC. Overlapping sphincteroplasty: is it the standard of care? Clin Colon Rectal Surg. 2005;18:22-31.
37. El-Gazzazz G, Zutshi M, Hannaway C, et al. Overlapping sphincter repair: does age matter? Dis Colon Rectum. 2012;55:256-261.
38. Glasgow SC, Lowry AC. Long-term outcomes of anal sphincter repair for fecal incontinence: a systematic review. Dis Colon Rectum. 2012;55:482-490.
39. Lehto K, Hyoty M, Collin P, et al. Seven-year follow-up after anterior sphincter reconstruction for faecal incontinence. Int J Colorectal Dis. 2013;5:653-658.
40. George AT, Kalmar K, Panarese A, et al. Long-term outcomes of sacral nerve stimulation for fecal incontinence. Dis Colon Rectum. 2012;55:302-306.
41. Jarrett MED, Mowatt G, Glazener CMA, et al. Systematic review of sacral nerve stimulation for faecal incontinence and constipation. Br J Surg. 2004;91:1559–1569.
42. Melenhorst J, Koch SM, Uludag O, et al. Is a morphologically intact anal sphincter necessary for success with sacral nerve modulation in patients with faecal incontinence? Colorectal Dis. 2008;10:257-262.
43. Dudding TC, Pares D, Vaizey CJ, et al. Predictive factors for successful sacral nerve stimulation in the treatment of faecal incontinence: a 10-year cohort analysis. Colorectal Dis. 2008;10:294-256.
44. Findlay JM, Mawell-Armstrong C. Posterior tibial nerve stimulation and faecal incontinence: a review. Int J Colorectal Dis. 2011;26:265-273.
45. Feretis C, Benakis P, Dailianas A, et al. Implantation of microballoons in the management of fecal incontinence. Dis Colon Rectum. 2001;44:1605–1609.
46. Kenefick NJ, Vaizey CJ, Malouf AJ, et al. Injectable silicone biomaterial for faecal incontinence due to internal anal sphincter dysfunction. Gut. 2002;55:225–228.
47. Konsten J, Baeten CG, Spaans F, et al. Follow-up of anal dynamic graciloplasty for fecal continence. World J Surg. 1993;17:404–409.
48. Baeten C, Spaans F, Fluks A. An implanted neuromuscular stimulator for fecal continence following previously implanted gracilis muscle: report of a case. Dis Colon Rectum. 1988;31:134–137.
49. Wong MT, Meurette G, Stangherlin P, et al. The magnetic anal sphincter versus the artificial bowel sphincter: a comparison of 2 treatments for fecal incontinence. Dis Colon Rectum. 2011;54:773-779.
50. Parker SC, Spencer MP, Madoff RD, et al. Artificial bowel sphincter: long-term experience at a single institution. Dis Colon Rectum 2003;46:722–729.
51. Takahashi T, Garcia-Osogobio S, Valdovinos MA, et al. Extended two-year results of radio-frequency energy delivery for the treatment of fecal incontinence (Secca procedure). Dis Colon Rectum. 2003;46:711–715.
52. Norton C, Burch J, Kamm MA. Patient’s views of a colostomy for fecal incontinence. Dis Colon Rectum. 2005;48:1062.