User login
PSA screening: The USPSTF got it wrong
Prostate cancer is an important disease. It is the second leading cause of cancer death in men who don’t smoke, and in many cases it is detectable early and curable. The rates of both diagnosis and death from prostate cancer in men are similar to the rates of breast cancer in women.1
The current practical screening test for prostate cancer is the prostate specific antigen (PSA). Making routine use of it, as we know, however, is controversial. The false positive rate for PSA testing is high, for example, in men with chronic prostatitis and benign prostatic hypertrophy.2 In addition, many prostate cancers are diagnosed that will never harm the patient. Treatment for prostate cancer may result in complications, such as incontinence and impotence. Because of these facts, the US Preventive Services Task Force (USPSTF) has recommended against routine screening.2
The PSA test itself never hurt anyone
It is just a lab value, a piece of information. What doctors do with the information is the issue. Physicians may cause more harm than good by being overly aggressive with elevated PSA levels and indolent or low-grade prostate cancer—and 75% of prostate cancer is considered indolent (Gleason score of 6 on biopsy).3 Patients with such a finding can be watched, using active surveillance. The majority will never need treatment.3
Common sense tells us we must screen for prostate cancer. Not doing so on the basis of evidence-based medicine is not a defense when advanced cancer is diagnosed and screening was not offered to the patient.4 Rather than using the data from past physician behavior and recommending against screening with PSA, the USPSTF should have criticized the response to PSA test results and recommended a better way. I see this change rapidly becoming current practice.
PSA testing saves lives
Since the early 1990s, when PSA testing became widespread, there has been a 40% decline in prostate cancer mortality.5 A randomized trial in 7 countries in Europe clearly showed a survival benefit from screening for prostate cancer.6 Clinical trials in the United States have been ambiguous.
Not screening for prostate cancer with PSA is unacceptable to many physicians and patients. Most physicians have seen preventable prostate cancer deaths. Two patients in my practice illustrate this point. The PSA of one of them—a 62-year-old man—went from 2.4 to 24 in 2 years. The PSA of another, age 56, went from 2.6 to 34 in one year. Both men had no symptoms, and their prostate cancer was found on routine screening. Both had a high Gleason score and locally invasive prostate cancer. Now, years after undergoing cancer treatment, both have undetectable PSA levels and full function. They think the USPSTF’s recommendation not to screen is evidence of the government’s attempt to save money, reinforcing the notion that the government cannot be trusted.
Patients are increasingly savvy
With all the controversy around prostate cancer screening and the adverse effects of treatment, patients are getting savvier. Shared decision making between doctor and patient is becoming the standard of care, and physicians can meet their professional obligations by offering screening and answering any questions the patient may have. I find that most men with low-grade disease are happy to avoid surgery and radiation if active surveillance is offered and explained.
The American Academy of Family Physicians adopted the recommendation of the USPSTF to advise against screening for prostate cancer.7 The American College of Physicians recommends that men ages 50 to 69 be given the opportunity for informed decision making before screening.8 The American Urological Association recently recommended that men ages 55 to 69 be offered screening, with a discussion about the risks and benefits9; and the American Cancer Society recommends screening starting at age 50, and earlier for high-risk men.10
Not satisfied that any of these organizations really knows what is best and aware that the data are confusing and evolving, I continue to follow my overall practice approach: Start routine cancer screening at age 50 in the general population and at age 40 for high-risk groups. This works for colon, breast, and prostate cancer, the big 3 that are common, sometimes fatal, and often curable with early detection.
Men in my practice are offered a PSA test starting at age 50, and every one to 2 years thereafter based on both patient preference and the results. Black men and those with a family history of prostate cancer before age 60 are offered screening starting at age 40. I suggest that screening be stopped at age 80, or earlier if the patient has a serious chronic illness with a life expectancy of less than 10 years.
Active surveillance for low-grade disease
What is done with elevated or rising PSA levels is most controversial, with lots of room for doing harm. Dramatic rises in PSA, like those of the patients I described earlier, are easy: Go right to biopsy and usually, treatment. Gleason 6 prostate cancer is likely to remain localized and indolent, and not threaten life. I work with urologists who are not aggressive and are willing to follow patients with PSA levels up to 10. Noninvasive options are available, such as fractionating the PSA (free and total) and imaging such as MRI. Genetic testing is available and can add to the evaluation of the patient’s risk.
Active surveillance has become a standard of care in monitoring patients with low-grade disease. The outcomes for survival with active surveillance are as good as radical prostatectomy.11 The goal is to be aggressive in treatment only with patients who have life-threatening disease. A collaboration among the patient, the primary care physician, and the urologist is crucial to optimizing patient outcomes.
Recommending against screening for prostate cancer is not tenable. The responsible approach is to continuously improve cancer detection and therapy to maximize good and minimize harm. This approach is available today.
1. Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300.
2. US Preventive Services Task Force. Screening for prostate cancer: Ann Int Med. 2008;149:185-191.
3. Klotz L. Active surveillance: current and future directions. Curr Opin Urol. 2013;23:237-238.
4. Merenstein D. A piece of my mind: Winners and losers. JAMA. 2004;291:15-16.
5. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;64:220-241.
6. Schroder FH, et al; ERSPC investigators. Screening and prostate cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
7. American Academy of Family Physicians Web site. Prostate cancer. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/prostate-cancer.html. Accessed October 16, 2013.
8. Qaseem A, Barry MJ, Denberg TD, et al. Screening for prostate cancer: a guideline statement from the clinical guidelines of the American College of Physicians. Ann Int Med. 2013;158:761-769.
9. Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. American Urological Association. 2013;1-28. Available at: http://www.auanet.org/common/pdf/education/clinical-guidance/Prostate-Cancer-Detection.pdf. Accessed October 16, 2013.
10. Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60:70-98.
11. Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203-213.
Prostate cancer is an important disease. It is the second leading cause of cancer death in men who don’t smoke, and in many cases it is detectable early and curable. The rates of both diagnosis and death from prostate cancer in men are similar to the rates of breast cancer in women.1
The current practical screening test for prostate cancer is the prostate specific antigen (PSA). Making routine use of it, as we know, however, is controversial. The false positive rate for PSA testing is high, for example, in men with chronic prostatitis and benign prostatic hypertrophy.2 In addition, many prostate cancers are diagnosed that will never harm the patient. Treatment for prostate cancer may result in complications, such as incontinence and impotence. Because of these facts, the US Preventive Services Task Force (USPSTF) has recommended against routine screening.2
The PSA test itself never hurt anyone
It is just a lab value, a piece of information. What doctors do with the information is the issue. Physicians may cause more harm than good by being overly aggressive with elevated PSA levels and indolent or low-grade prostate cancer—and 75% of prostate cancer is considered indolent (Gleason score of 6 on biopsy).3 Patients with such a finding can be watched, using active surveillance. The majority will never need treatment.3
Common sense tells us we must screen for prostate cancer. Not doing so on the basis of evidence-based medicine is not a defense when advanced cancer is diagnosed and screening was not offered to the patient.4 Rather than using the data from past physician behavior and recommending against screening with PSA, the USPSTF should have criticized the response to PSA test results and recommended a better way. I see this change rapidly becoming current practice.
PSA testing saves lives
Since the early 1990s, when PSA testing became widespread, there has been a 40% decline in prostate cancer mortality.5 A randomized trial in 7 countries in Europe clearly showed a survival benefit from screening for prostate cancer.6 Clinical trials in the United States have been ambiguous.
Not screening for prostate cancer with PSA is unacceptable to many physicians and patients. Most physicians have seen preventable prostate cancer deaths. Two patients in my practice illustrate this point. The PSA of one of them—a 62-year-old man—went from 2.4 to 24 in 2 years. The PSA of another, age 56, went from 2.6 to 34 in one year. Both men had no symptoms, and their prostate cancer was found on routine screening. Both had a high Gleason score and locally invasive prostate cancer. Now, years after undergoing cancer treatment, both have undetectable PSA levels and full function. They think the USPSTF’s recommendation not to screen is evidence of the government’s attempt to save money, reinforcing the notion that the government cannot be trusted.
Patients are increasingly savvy
With all the controversy around prostate cancer screening and the adverse effects of treatment, patients are getting savvier. Shared decision making between doctor and patient is becoming the standard of care, and physicians can meet their professional obligations by offering screening and answering any questions the patient may have. I find that most men with low-grade disease are happy to avoid surgery and radiation if active surveillance is offered and explained.
The American Academy of Family Physicians adopted the recommendation of the USPSTF to advise against screening for prostate cancer.7 The American College of Physicians recommends that men ages 50 to 69 be given the opportunity for informed decision making before screening.8 The American Urological Association recently recommended that men ages 55 to 69 be offered screening, with a discussion about the risks and benefits9; and the American Cancer Society recommends screening starting at age 50, and earlier for high-risk men.10
Not satisfied that any of these organizations really knows what is best and aware that the data are confusing and evolving, I continue to follow my overall practice approach: Start routine cancer screening at age 50 in the general population and at age 40 for high-risk groups. This works for colon, breast, and prostate cancer, the big 3 that are common, sometimes fatal, and often curable with early detection.
Men in my practice are offered a PSA test starting at age 50, and every one to 2 years thereafter based on both patient preference and the results. Black men and those with a family history of prostate cancer before age 60 are offered screening starting at age 40. I suggest that screening be stopped at age 80, or earlier if the patient has a serious chronic illness with a life expectancy of less than 10 years.
Active surveillance for low-grade disease
What is done with elevated or rising PSA levels is most controversial, with lots of room for doing harm. Dramatic rises in PSA, like those of the patients I described earlier, are easy: Go right to biopsy and usually, treatment. Gleason 6 prostate cancer is likely to remain localized and indolent, and not threaten life. I work with urologists who are not aggressive and are willing to follow patients with PSA levels up to 10. Noninvasive options are available, such as fractionating the PSA (free and total) and imaging such as MRI. Genetic testing is available and can add to the evaluation of the patient’s risk.
Active surveillance has become a standard of care in monitoring patients with low-grade disease. The outcomes for survival with active surveillance are as good as radical prostatectomy.11 The goal is to be aggressive in treatment only with patients who have life-threatening disease. A collaboration among the patient, the primary care physician, and the urologist is crucial to optimizing patient outcomes.
Recommending against screening for prostate cancer is not tenable. The responsible approach is to continuously improve cancer detection and therapy to maximize good and minimize harm. This approach is available today.
Prostate cancer is an important disease. It is the second leading cause of cancer death in men who don’t smoke, and in many cases it is detectable early and curable. The rates of both diagnosis and death from prostate cancer in men are similar to the rates of breast cancer in women.1
The current practical screening test for prostate cancer is the prostate specific antigen (PSA). Making routine use of it, as we know, however, is controversial. The false positive rate for PSA testing is high, for example, in men with chronic prostatitis and benign prostatic hypertrophy.2 In addition, many prostate cancers are diagnosed that will never harm the patient. Treatment for prostate cancer may result in complications, such as incontinence and impotence. Because of these facts, the US Preventive Services Task Force (USPSTF) has recommended against routine screening.2
The PSA test itself never hurt anyone
It is just a lab value, a piece of information. What doctors do with the information is the issue. Physicians may cause more harm than good by being overly aggressive with elevated PSA levels and indolent or low-grade prostate cancer—and 75% of prostate cancer is considered indolent (Gleason score of 6 on biopsy).3 Patients with such a finding can be watched, using active surveillance. The majority will never need treatment.3
Common sense tells us we must screen for prostate cancer. Not doing so on the basis of evidence-based medicine is not a defense when advanced cancer is diagnosed and screening was not offered to the patient.4 Rather than using the data from past physician behavior and recommending against screening with PSA, the USPSTF should have criticized the response to PSA test results and recommended a better way. I see this change rapidly becoming current practice.
PSA testing saves lives
Since the early 1990s, when PSA testing became widespread, there has been a 40% decline in prostate cancer mortality.5 A randomized trial in 7 countries in Europe clearly showed a survival benefit from screening for prostate cancer.6 Clinical trials in the United States have been ambiguous.
Not screening for prostate cancer with PSA is unacceptable to many physicians and patients. Most physicians have seen preventable prostate cancer deaths. Two patients in my practice illustrate this point. The PSA of one of them—a 62-year-old man—went from 2.4 to 24 in 2 years. The PSA of another, age 56, went from 2.6 to 34 in one year. Both men had no symptoms, and their prostate cancer was found on routine screening. Both had a high Gleason score and locally invasive prostate cancer. Now, years after undergoing cancer treatment, both have undetectable PSA levels and full function. They think the USPSTF’s recommendation not to screen is evidence of the government’s attempt to save money, reinforcing the notion that the government cannot be trusted.
Patients are increasingly savvy
With all the controversy around prostate cancer screening and the adverse effects of treatment, patients are getting savvier. Shared decision making between doctor and patient is becoming the standard of care, and physicians can meet their professional obligations by offering screening and answering any questions the patient may have. I find that most men with low-grade disease are happy to avoid surgery and radiation if active surveillance is offered and explained.
The American Academy of Family Physicians adopted the recommendation of the USPSTF to advise against screening for prostate cancer.7 The American College of Physicians recommends that men ages 50 to 69 be given the opportunity for informed decision making before screening.8 The American Urological Association recently recommended that men ages 55 to 69 be offered screening, with a discussion about the risks and benefits9; and the American Cancer Society recommends screening starting at age 50, and earlier for high-risk men.10
Not satisfied that any of these organizations really knows what is best and aware that the data are confusing and evolving, I continue to follow my overall practice approach: Start routine cancer screening at age 50 in the general population and at age 40 for high-risk groups. This works for colon, breast, and prostate cancer, the big 3 that are common, sometimes fatal, and often curable with early detection.
Men in my practice are offered a PSA test starting at age 50, and every one to 2 years thereafter based on both patient preference and the results. Black men and those with a family history of prostate cancer before age 60 are offered screening starting at age 40. I suggest that screening be stopped at age 80, or earlier if the patient has a serious chronic illness with a life expectancy of less than 10 years.
Active surveillance for low-grade disease
What is done with elevated or rising PSA levels is most controversial, with lots of room for doing harm. Dramatic rises in PSA, like those of the patients I described earlier, are easy: Go right to biopsy and usually, treatment. Gleason 6 prostate cancer is likely to remain localized and indolent, and not threaten life. I work with urologists who are not aggressive and are willing to follow patients with PSA levels up to 10. Noninvasive options are available, such as fractionating the PSA (free and total) and imaging such as MRI. Genetic testing is available and can add to the evaluation of the patient’s risk.
Active surveillance has become a standard of care in monitoring patients with low-grade disease. The outcomes for survival with active surveillance are as good as radical prostatectomy.11 The goal is to be aggressive in treatment only with patients who have life-threatening disease. A collaboration among the patient, the primary care physician, and the urologist is crucial to optimizing patient outcomes.
Recommending against screening for prostate cancer is not tenable. The responsible approach is to continuously improve cancer detection and therapy to maximize good and minimize harm. This approach is available today.
1. Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300.
2. US Preventive Services Task Force. Screening for prostate cancer: Ann Int Med. 2008;149:185-191.
3. Klotz L. Active surveillance: current and future directions. Curr Opin Urol. 2013;23:237-238.
4. Merenstein D. A piece of my mind: Winners and losers. JAMA. 2004;291:15-16.
5. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;64:220-241.
6. Schroder FH, et al; ERSPC investigators. Screening and prostate cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
7. American Academy of Family Physicians Web site. Prostate cancer. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/prostate-cancer.html. Accessed October 16, 2013.
8. Qaseem A, Barry MJ, Denberg TD, et al. Screening for prostate cancer: a guideline statement from the clinical guidelines of the American College of Physicians. Ann Int Med. 2013;158:761-769.
9. Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. American Urological Association. 2013;1-28. Available at: http://www.auanet.org/common/pdf/education/clinical-guidance/Prostate-Cancer-Detection.pdf. Accessed October 16, 2013.
10. Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60:70-98.
11. Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203-213.
1. Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277-300.
2. US Preventive Services Task Force. Screening for prostate cancer: Ann Int Med. 2008;149:185-191.
3. Klotz L. Active surveillance: current and future directions. Curr Opin Urol. 2013;23:237-238.
4. Merenstein D. A piece of my mind: Winners and losers. JAMA. 2004;291:15-16.
5. Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;64:220-241.
6. Schroder FH, et al; ERSPC investigators. Screening and prostate cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320-1328.
7. American Academy of Family Physicians Web site. Prostate cancer. Available at: http://www.aafp.org/patient-care/clinical-recommendations/all/prostate-cancer.html. Accessed October 16, 2013.
8. Qaseem A, Barry MJ, Denberg TD, et al. Screening for prostate cancer: a guideline statement from the clinical guidelines of the American College of Physicians. Ann Int Med. 2013;158:761-769.
9. Carter HB, Albertsen PC, Barry MJ, et al. Early detection of prostate cancer: AUA guideline. American Urological Association. 2013;1-28. Available at: http://www.auanet.org/common/pdf/education/clinical-guidance/Prostate-Cancer-Detection.pdf. Accessed October 16, 2013.
10. Wolf AM, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60:70-98.
11. Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203-213.
Construction Worker Falls From Scaffolding
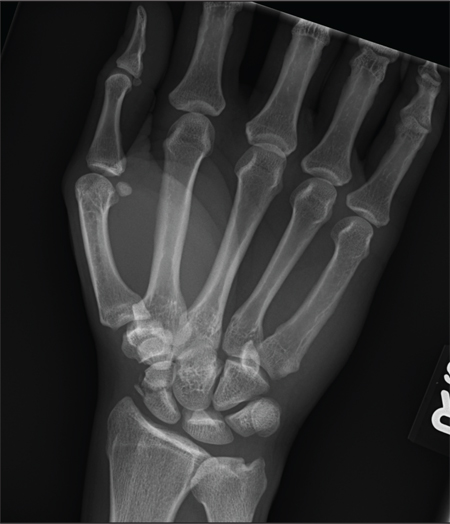
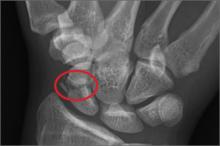
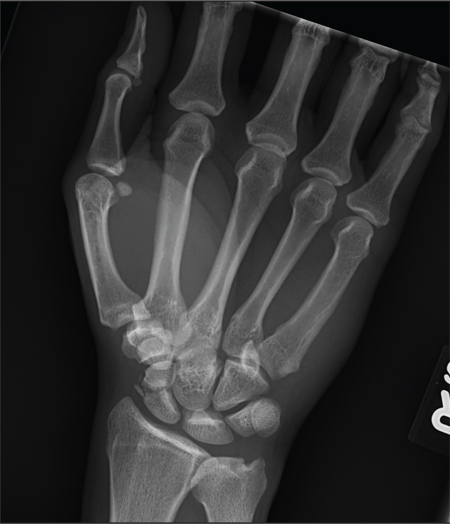
A 45-year-old construction worker is brought to your facility for evaluation following a fall. He was at a job site, standing on scaffolding approximately 20 feet above the ground, when he accidentally fell. He does not remember for sure, but he thinks he landed on his face. He did briefly lose consciousness. He is complaining of right-side facial pain and right wrist pain. His medical history is unremarkable. The physical exam reveals stable vital signs. The patient appears somewhat uncomfortable but is in no obvious distress. There is a moderate amount of periorbital soft-tissue swelling around his right eye, with moderate associated tenderness. Pupils are equal and react well bilaterally. Examination of the right wrist shows a moderate amount of soft-tissue swelling. The patient is unable to flex or extend his wrist due to pain. Good pulses and capillary refill of the nail beds are noted. There is also moderate tenderness along the base of the first metacarpal. Radiograph of the right wrist is shown. What is your impression?
Now Insured, Patient Wants to “Get Checked Out”
ANSWER
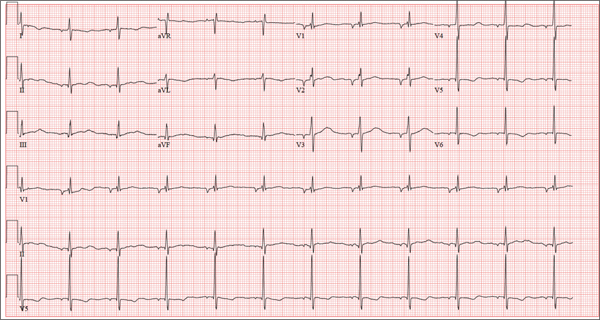
There are three findings on this ECG: unusual P waves consistent with a possible ectopic atrial rhythm, a prolonged QT interval, and T-wave abnormalities in the lateral leads.
Note that the P waves are negative in leads I and II, as well as in all chest leads. This is highly suggestive of an ectopic atrial rhythm originating low in the atria, conducting retrograde into the atria, and overriding the sinoatrial node. Limb lead reversal would result in negative P waves in lead I, but not in other leads.
A prolonged QT interval is determined by consulting any of the standard charts that correlate maximum heart rates with QT intervals and gender. In men, the QT interval is considered “prolonged” when it exceeds 440 ms, unless the heart rate is extremely slow.
Finally, T-wave inversions are present in the lateral leads (V5, V6). Although this may be an indication of lateral ischemia, there is no clinical correlation in this patient.
ANSWER
There are three findings on this ECG: unusual P waves consistent with a possible ectopic atrial rhythm, a prolonged QT interval, and T-wave abnormalities in the lateral leads.
Note that the P waves are negative in leads I and II, as well as in all chest leads. This is highly suggestive of an ectopic atrial rhythm originating low in the atria, conducting retrograde into the atria, and overriding the sinoatrial node. Limb lead reversal would result in negative P waves in lead I, but not in other leads.
A prolonged QT interval is determined by consulting any of the standard charts that correlate maximum heart rates with QT intervals and gender. In men, the QT interval is considered “prolonged” when it exceeds 440 ms, unless the heart rate is extremely slow.
Finally, T-wave inversions are present in the lateral leads (V5, V6). Although this may be an indication of lateral ischemia, there is no clinical correlation in this patient.
ANSWER
There are three findings on this ECG: unusual P waves consistent with a possible ectopic atrial rhythm, a prolonged QT interval, and T-wave abnormalities in the lateral leads.
Note that the P waves are negative in leads I and II, as well as in all chest leads. This is highly suggestive of an ectopic atrial rhythm originating low in the atria, conducting retrograde into the atria, and overriding the sinoatrial node. Limb lead reversal would result in negative P waves in lead I, but not in other leads.
A prolonged QT interval is determined by consulting any of the standard charts that correlate maximum heart rates with QT intervals and gender. In men, the QT interval is considered “prolonged” when it exceeds 440 ms, unless the heart rate is extremely slow.
Finally, T-wave inversions are present in the lateral leads (V5, V6). Although this may be an indication of lateral ischemia, there is no clinical correlation in this patient.
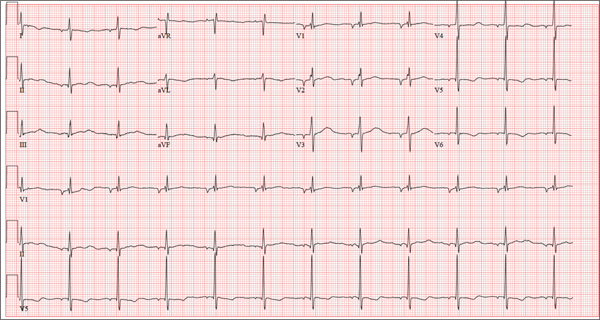
A 37-year-old man presents to your office to establish care. After being unemployed for two years, he recently obtained a position with a local manufacturing company and, as a result, has health benefits. He wants to “get checked out.” He has not seen a health care provider since having his tonsils removed at age 14. He says he is rarely ill, aside from an occasional cold. Besides the tonsillectomy, medical history is positive for a right clavicular fracture at age 6 and a left inguinal hernia repair at age 9. He had chickenpox and recalls that his immunizations were up to date until he graduated high school. His only medication is ibuprofen as needed for aches and pains. He has no known drug allergies. He uses two herbal supplements, fenugreek seed and horny goat weed, daily. He admits to recreational marijuana use. Family history is remarkable for coronary artery disease (father), diabetes (mother), and depression (sister). He consumes one six-pack of beer weekly and has smoked one pack of cigarettes per day for the past 23 years. He isn’t interested in quitting smoking. The patient is divorced, without children. He has been collecting unemployment since his last position was terminated due to budget constraints. A 20-point comprehensive review of systems is negative, with the exception of occasional palpitations and a productive morning smoker’s cough that quickly resolves. He states he’s “as healthy as a horse.” The physical exam reveals a thin, healthy-appearing middle-aged male. He is 72 in tall and weighs 167 lb. His blood pressure is 108/66 mm Hg; pulse, 70 beats/min and regular; respiratory rate, 14 breaths/min-1; and temperature, 98.4°F. The head, eyes, ears, nose, and throat (HEENT) exam is remarkable for poor dentition, with multiple caries readily visible. The tonsils are absent. Coarse expiratory crackles are present in both bases and clear with vigorous coughing. The abdominal exam is positive for a well-healed scar in the left inguinal crease. The remainder of the physical exam is normal. As part of a new patient visit, a chest x-ray and ECG are obtained. The ECG shows the following: a ventricular rate of 69 beats/min; PR interval, 178 ms; QRS duration, 90ms; QT/QTc interval, 442/473 ms; P axis, 231°; R axis, 84°; and T axis, 93°. What is your interpretation of this ECG?
A Purplish Rash on the Instep
ANSWER
The correct answer is lichen planus (choice “d”), an inflammatory condition marked by pathognomic histologic changes such as those described. Hardly on the tip of most primary care providers’ tongues, lichen planus is nonetheless quite commonly occuring.
Tinea pedis (choice “a”) certainly belongs in the differential, but it would be unusual in a woman of this age. The biopsy effectively ruled it out, as it did psoriasis (choice “b”) and contact dermatitis (choice “c”).
DISCUSSION
Lichen planus (LP) is the prototypical lichenoid interface dermatitis; an inflammatory infiltrate erases the normally well-defined dermoepidermal junction, replacing it with a jagged “sawtooth” band of lymphocytes. In the process, the cells it kills off (keratinocytes) collect at this interface and are incorporated as necrotic keratinocytes into the papillary dermis.
Classically, the recognition of LP is taught with “The Ps.” LP is said to be:
• Papular
• Planar
• Purple
• Polygonal
• Pruritic
• Plaquish
• Penile
• Puzzling
The word “puzzling” may sound nebulous, but it is actually quite useful for dermatology providers. It comes into play whenever a condition stumps us—as in, “What in the world is this?” This puzzlement causes us to at least consider LP.
LP is often papular, and these papules often have flat (planar) tops. The purple color is more striking in lighter-skinned patients. (Almost all inflammatory conditions are more difficult to diagnose in those with darker skin.) More typically, LP lesions are multi-angular or polygonal, often plaquish, and almost invariably pruritic.
LP is common on the legs and/or trunk, favoring the sacral area. It can affect the scalp (being in the differential for hair loss), can cause nail dystrophy, and is relatively common in the mouth, where it presents as a lacy, reticular, slightly erosive process, usually affecting the buccal mucosae. It may be found on the penis, where it is usually confined to the distal shaft and proximal coronal areas.
Like many otherwise benign conditions, LP can present with bullae. In anterior tibial areas, especially on darker-skinned persons, it can be remarkably hypertrophic.
The cause is usually unknown, although certain drugs—especially the antimalarials, gold salts, and penicillamine—have been known to cause LP-like eruptions. It’s been my observation that flares of LP are prompted by stress (certainly present in this patient’s case). LP may have a connection to hepatitis, although no convincing connection has been established.
TREATMENT
This patient’s condition was treated with topical class I corticosteroid ointment (halobetasol). For her, having a certain diagnosis was almost as important as successful treatment.
ANSWER
The correct answer is lichen planus (choice “d”), an inflammatory condition marked by pathognomic histologic changes such as those described. Hardly on the tip of most primary care providers’ tongues, lichen planus is nonetheless quite commonly occuring.
Tinea pedis (choice “a”) certainly belongs in the differential, but it would be unusual in a woman of this age. The biopsy effectively ruled it out, as it did psoriasis (choice “b”) and contact dermatitis (choice “c”).
DISCUSSION
Lichen planus (LP) is the prototypical lichenoid interface dermatitis; an inflammatory infiltrate erases the normally well-defined dermoepidermal junction, replacing it with a jagged “sawtooth” band of lymphocytes. In the process, the cells it kills off (keratinocytes) collect at this interface and are incorporated as necrotic keratinocytes into the papillary dermis.
Classically, the recognition of LP is taught with “The Ps.” LP is said to be:
• Papular
• Planar
• Purple
• Polygonal
• Pruritic
• Plaquish
• Penile
• Puzzling
The word “puzzling” may sound nebulous, but it is actually quite useful for dermatology providers. It comes into play whenever a condition stumps us—as in, “What in the world is this?” This puzzlement causes us to at least consider LP.
LP is often papular, and these papules often have flat (planar) tops. The purple color is more striking in lighter-skinned patients. (Almost all inflammatory conditions are more difficult to diagnose in those with darker skin.) More typically, LP lesions are multi-angular or polygonal, often plaquish, and almost invariably pruritic.
LP is common on the legs and/or trunk, favoring the sacral area. It can affect the scalp (being in the differential for hair loss), can cause nail dystrophy, and is relatively common in the mouth, where it presents as a lacy, reticular, slightly erosive process, usually affecting the buccal mucosae. It may be found on the penis, where it is usually confined to the distal shaft and proximal coronal areas.
Like many otherwise benign conditions, LP can present with bullae. In anterior tibial areas, especially on darker-skinned persons, it can be remarkably hypertrophic.
The cause is usually unknown, although certain drugs—especially the antimalarials, gold salts, and penicillamine—have been known to cause LP-like eruptions. It’s been my observation that flares of LP are prompted by stress (certainly present in this patient’s case). LP may have a connection to hepatitis, although no convincing connection has been established.
TREATMENT
This patient’s condition was treated with topical class I corticosteroid ointment (halobetasol). For her, having a certain diagnosis was almost as important as successful treatment.
ANSWER
The correct answer is lichen planus (choice “d”), an inflammatory condition marked by pathognomic histologic changes such as those described. Hardly on the tip of most primary care providers’ tongues, lichen planus is nonetheless quite commonly occuring.
Tinea pedis (choice “a”) certainly belongs in the differential, but it would be unusual in a woman of this age. The biopsy effectively ruled it out, as it did psoriasis (choice “b”) and contact dermatitis (choice “c”).
DISCUSSION
Lichen planus (LP) is the prototypical lichenoid interface dermatitis; an inflammatory infiltrate erases the normally well-defined dermoepidermal junction, replacing it with a jagged “sawtooth” band of lymphocytes. In the process, the cells it kills off (keratinocytes) collect at this interface and are incorporated as necrotic keratinocytes into the papillary dermis.
Classically, the recognition of LP is taught with “The Ps.” LP is said to be:
• Papular
• Planar
• Purple
• Polygonal
• Pruritic
• Plaquish
• Penile
• Puzzling
The word “puzzling” may sound nebulous, but it is actually quite useful for dermatology providers. It comes into play whenever a condition stumps us—as in, “What in the world is this?” This puzzlement causes us to at least consider LP.
LP is often papular, and these papules often have flat (planar) tops. The purple color is more striking in lighter-skinned patients. (Almost all inflammatory conditions are more difficult to diagnose in those with darker skin.) More typically, LP lesions are multi-angular or polygonal, often plaquish, and almost invariably pruritic.
LP is common on the legs and/or trunk, favoring the sacral area. It can affect the scalp (being in the differential for hair loss), can cause nail dystrophy, and is relatively common in the mouth, where it presents as a lacy, reticular, slightly erosive process, usually affecting the buccal mucosae. It may be found on the penis, where it is usually confined to the distal shaft and proximal coronal areas.
Like many otherwise benign conditions, LP can present with bullae. In anterior tibial areas, especially on darker-skinned persons, it can be remarkably hypertrophic.
The cause is usually unknown, although certain drugs—especially the antimalarials, gold salts, and penicillamine—have been known to cause LP-like eruptions. It’s been my observation that flares of LP are prompted by stress (certainly present in this patient’s case). LP may have a connection to hepatitis, although no convincing connection has been established.
TREATMENT
This patient’s condition was treated with topical class I corticosteroid ointment (halobetasol). For her, having a certain diagnosis was almost as important as successful treatment.
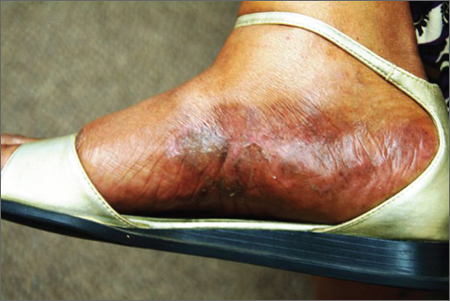
Several months ago, an itchy rash appeared on the foot of this 61-year-old African-American woman. She tried using a variety of OTC and prescription products, including tolnaftate cream, hydrocortisone 1% cream, and triamcinolone cream, but the rash persisted. She reports that the triamcinolone cream did relieve the itching for a few minutes after application, but the symptoms always returned. The rash has gradually grown. The patient, a retired schoolteacher, denies any other skin problems. She does have several relatively minor health problems, including hypertension (well controlled with metoprolol) and mild reactive airway disease (related to a 20-year history of smoking). The rash manifested around the time she was forced to retire in an unforeseen downsizing effort by her school district. As a result, she lost her health care coverage and could not afford private insurance for herself and her husband (neither of whom are old enough to qualify for Medicare). The rash, which covers a roughly 12 × 8–cm area, begins on her left instep and spills onto the lower ankle. It is quite dark, as would be expected in a person with type V skin, but there is a slightly purplish tinge to it. The surface of the affected area is a bit shiny and atrophic, and the margins are well defined and annular. There is almost no scaling, and the rest of the skin on her feet and legs is well within normal limits. A 4-mm punch biopsy is performed on the lesion. The pathology report shows obliteration of the dermoepidermal junction by a bandlike lymphocytic infiltrate in the papillary dermis, associated with vacuolar changes and the accumulation of necrotic keratinocytes in the basal layer.
Renal risk stratification with the new oral anticoagulants
To the Editor: I read with interest the review of the new oral anticoagulants by Fawole et al1 and agree with their comments on the prevention of bleeding and the importance of monitoring renal function in managing patients on the new classes of oral anticoagulants. However, no specifics were given on how to proceed. Thus, I recommend that renal risk stratification be done before and 1 week after starting these new drugs.
Originally, the US Food and Drug Administration approved dabigatran (Pradaxa) at a dose of 150 mg orally twice daily in patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2. This dosing corresponded to the estimated glomerular filtration rate (eGFR) in patients with stage 4 chronic kidney disease, but this dosing is contraindicated in other guidelines worldwide (Canada, Europe, the United Kingdom, Japan, Australia, and New Zealand).2 Not unexpectedly, 3,781 serious adverse effects were noted in the 2011 US postmarketing experience with dabigatran. These included death (542 cases), hemorrhage (2,367 cases), acute renal failure (291 cases), stroke (644 cases), and suspected liver failure (15 cases).3 Thirteen months after dabigatran’s approval in the United States, Boehringer Ingelheim changed the dosage and product guidelines.2–4 The new dosage4 is 75 mg twice daily for patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2.
Therefore, I suggest a nephrologic “way out”5 when using the new oral anticoagulants to avoid the problems with dabigatran noted above.
First, if these drugs are to be used in nonvalvular atrial fibrillation, risk factors should be determined using the CHADS2 or the CHADS2-VASc score. Special attention should be given to patients age 75 and older, women, and patients with a history of stroke, transient ischemic attack, or systemic embolism. All of these have been noted to be major risk factors.6,7
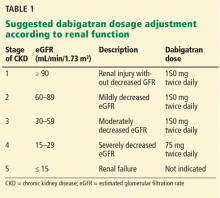
Second, renal risk stratification8 should be done using a comprehensive metabolic panel before and 1 week after starting new oral anticoagulants, or if there is a change in the patient’s clinical condition. Most US laboratories now provide an eGFR and the stage of chronic kidney disease.3,5 For example (Table 1), if dabigatran is used, one should follow current dosing guidelines for chronic kidney disease stages 1 through 3, ie, 150 mg twice daily. If stage 4 chronic kidney disease is detected (creatinine clearance 15–29 mL/min/1.73 m2), the updated recommended dosage is 75 mg twice daily. If stage 5 is noted (eGFR ≤ 15 mL/min/1.73 m2), dabigatran is not indicated. Similar steps can be done using current guidelines for the other new oral anticoagulants.
This simple renal risk stratification guideline should help avoid some of the problems noted in the dabigatran postmarketing experience, which were aggravated by the lack of approval of a 110-mg dose and by misleading advertising, claiming that no blood monitoring was required.2–5 Thus, the new oral anticoagulants should be a welcome addition to our armamentarium in patients who need them, and we hope to avoid the risks, morbidity, mortality, and expense of trying to reverse adverse effects.
- Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants, dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013, 80:443–451.
- Pazmiño PA. Dabigatran associated acute renal failure (DAARF). El Paso Physician 2011; 34:7–9.
- Pazmiño PA. Adverse effects of dabigatran (Letter). Ann Intern Med 2012; 157:916.
- Pradaxa (prescribing information). Ridgefield, CT. Boehringer Ingelheim Pharmaceuticals 2011.
- Pazmiño PA. Dabigatran: a nephrological way out. Am J Med 2013; 126;e21–e22.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. The Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- Reinecke H, Brand E, Mesters R, et al. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol 2009; 20:705–711.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic disease: evaluation, classification and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
To the Editor: I read with interest the review of the new oral anticoagulants by Fawole et al1 and agree with their comments on the prevention of bleeding and the importance of monitoring renal function in managing patients on the new classes of oral anticoagulants. However, no specifics were given on how to proceed. Thus, I recommend that renal risk stratification be done before and 1 week after starting these new drugs.
Originally, the US Food and Drug Administration approved dabigatran (Pradaxa) at a dose of 150 mg orally twice daily in patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2. This dosing corresponded to the estimated glomerular filtration rate (eGFR) in patients with stage 4 chronic kidney disease, but this dosing is contraindicated in other guidelines worldwide (Canada, Europe, the United Kingdom, Japan, Australia, and New Zealand).2 Not unexpectedly, 3,781 serious adverse effects were noted in the 2011 US postmarketing experience with dabigatran. These included death (542 cases), hemorrhage (2,367 cases), acute renal failure (291 cases), stroke (644 cases), and suspected liver failure (15 cases).3 Thirteen months after dabigatran’s approval in the United States, Boehringer Ingelheim changed the dosage and product guidelines.2–4 The new dosage4 is 75 mg twice daily for patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2.
Therefore, I suggest a nephrologic “way out”5 when using the new oral anticoagulants to avoid the problems with dabigatran noted above.
First, if these drugs are to be used in nonvalvular atrial fibrillation, risk factors should be determined using the CHADS2 or the CHADS2-VASc score. Special attention should be given to patients age 75 and older, women, and patients with a history of stroke, transient ischemic attack, or systemic embolism. All of these have been noted to be major risk factors.6,7
Second, renal risk stratification8 should be done using a comprehensive metabolic panel before and 1 week after starting new oral anticoagulants, or if there is a change in the patient’s clinical condition. Most US laboratories now provide an eGFR and the stage of chronic kidney disease.3,5 For example (Table 1), if dabigatran is used, one should follow current dosing guidelines for chronic kidney disease stages 1 through 3, ie, 150 mg twice daily. If stage 4 chronic kidney disease is detected (creatinine clearance 15–29 mL/min/1.73 m2), the updated recommended dosage is 75 mg twice daily. If stage 5 is noted (eGFR ≤ 15 mL/min/1.73 m2), dabigatran is not indicated. Similar steps can be done using current guidelines for the other new oral anticoagulants.
This simple renal risk stratification guideline should help avoid some of the problems noted in the dabigatran postmarketing experience, which were aggravated by the lack of approval of a 110-mg dose and by misleading advertising, claiming that no blood monitoring was required.2–5 Thus, the new oral anticoagulants should be a welcome addition to our armamentarium in patients who need them, and we hope to avoid the risks, morbidity, mortality, and expense of trying to reverse adverse effects.
To the Editor: I read with interest the review of the new oral anticoagulants by Fawole et al1 and agree with their comments on the prevention of bleeding and the importance of monitoring renal function in managing patients on the new classes of oral anticoagulants. However, no specifics were given on how to proceed. Thus, I recommend that renal risk stratification be done before and 1 week after starting these new drugs.
Originally, the US Food and Drug Administration approved dabigatran (Pradaxa) at a dose of 150 mg orally twice daily in patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2. This dosing corresponded to the estimated glomerular filtration rate (eGFR) in patients with stage 4 chronic kidney disease, but this dosing is contraindicated in other guidelines worldwide (Canada, Europe, the United Kingdom, Japan, Australia, and New Zealand).2 Not unexpectedly, 3,781 serious adverse effects were noted in the 2011 US postmarketing experience with dabigatran. These included death (542 cases), hemorrhage (2,367 cases), acute renal failure (291 cases), stroke (644 cases), and suspected liver failure (15 cases).3 Thirteen months after dabigatran’s approval in the United States, Boehringer Ingelheim changed the dosage and product guidelines.2–4 The new dosage4 is 75 mg twice daily for patients with a creatinine clearance of 15 to 30 mL/min/1.73 m2.
Therefore, I suggest a nephrologic “way out”5 when using the new oral anticoagulants to avoid the problems with dabigatran noted above.
First, if these drugs are to be used in nonvalvular atrial fibrillation, risk factors should be determined using the CHADS2 or the CHADS2-VASc score. Special attention should be given to patients age 75 and older, women, and patients with a history of stroke, transient ischemic attack, or systemic embolism. All of these have been noted to be major risk factors.6,7
Second, renal risk stratification8 should be done using a comprehensive metabolic panel before and 1 week after starting new oral anticoagulants, or if there is a change in the patient’s clinical condition. Most US laboratories now provide an eGFR and the stage of chronic kidney disease.3,5 For example (Table 1), if dabigatran is used, one should follow current dosing guidelines for chronic kidney disease stages 1 through 3, ie, 150 mg twice daily. If stage 4 chronic kidney disease is detected (creatinine clearance 15–29 mL/min/1.73 m2), the updated recommended dosage is 75 mg twice daily. If stage 5 is noted (eGFR ≤ 15 mL/min/1.73 m2), dabigatran is not indicated. Similar steps can be done using current guidelines for the other new oral anticoagulants.
This simple renal risk stratification guideline should help avoid some of the problems noted in the dabigatran postmarketing experience, which were aggravated by the lack of approval of a 110-mg dose and by misleading advertising, claiming that no blood monitoring was required.2–5 Thus, the new oral anticoagulants should be a welcome addition to our armamentarium in patients who need them, and we hope to avoid the risks, morbidity, mortality, and expense of trying to reverse adverse effects.
- Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants, dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013, 80:443–451.
- Pazmiño PA. Dabigatran associated acute renal failure (DAARF). El Paso Physician 2011; 34:7–9.
- Pazmiño PA. Adverse effects of dabigatran (Letter). Ann Intern Med 2012; 157:916.
- Pradaxa (prescribing information). Ridgefield, CT. Boehringer Ingelheim Pharmaceuticals 2011.
- Pazmiño PA. Dabigatran: a nephrological way out. Am J Med 2013; 126;e21–e22.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. The Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- Reinecke H, Brand E, Mesters R, et al. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol 2009; 20:705–711.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic disease: evaluation, classification and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
- Fawole A, Daw HA, Crowther MA. Practical management of bleeding due to the anticoagulants, dabigatran, rivaroxaban, and apixaban. Cleve Clin J Med 2013, 80:443–451.
- Pazmiño PA. Dabigatran associated acute renal failure (DAARF). El Paso Physician 2011; 34:7–9.
- Pazmiño PA. Adverse effects of dabigatran (Letter). Ann Intern Med 2012; 157:916.
- Pradaxa (prescribing information). Ridgefield, CT. Boehringer Ingelheim Pharmaceuticals 2011.
- Pazmiño PA. Dabigatran: a nephrological way out. Am J Med 2013; 126;e21–e22.
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach. The Euro Heart Survey on Atrial Fibrillation. Chest 2010; 137:263–272.
- Reinecke H, Brand E, Mesters R, et al. Dilemmas in the management of atrial fibrillation in chronic kidney disease. J Am Soc Nephrol 2009; 20:705–711.
- National Kidney Foundation. K/DOQI clinical practice guidelines for chronic disease: evaluation, classification and stratification. Am J Kidney Dis 2002; 39(suppl 1):S1–S266.
In reply: Renal risk stratification with the new oral anticoagulants
In Reply: We agree with the comments of Dr. Pazmiño regarding specifics of renal risk stratification in patients taking the new oral anticoagulants. In order to reduce the bleeding risks associated with these agents, they should be prescribed on the basis of the individual patient’s clinical characteristics. We did not discuss this since the focus of our article was management of bleeding that resulted from use of these drugs. We appreciate the recommendations of Dr. Pazmiño.
In Reply: We agree with the comments of Dr. Pazmiño regarding specifics of renal risk stratification in patients taking the new oral anticoagulants. In order to reduce the bleeding risks associated with these agents, they should be prescribed on the basis of the individual patient’s clinical characteristics. We did not discuss this since the focus of our article was management of bleeding that resulted from use of these drugs. We appreciate the recommendations of Dr. Pazmiño.
In Reply: We agree with the comments of Dr. Pazmiño regarding specifics of renal risk stratification in patients taking the new oral anticoagulants. In order to reduce the bleeding risks associated with these agents, they should be prescribed on the basis of the individual patient’s clinical characteristics. We did not discuss this since the focus of our article was management of bleeding that resulted from use of these drugs. We appreciate the recommendations of Dr. Pazmiño.
Not all joint pain is arthritis
To the Editor: I was somewhat confused by the Clinical Picture case in the May 2013 issue.1 The caption for Figure 1 stated that the MRI showed erosions and marrow edema, which were “asymmetrical compared with the other wrist, a finding highly suggestive of rheumatoid arthritis.” However, rheumatoid arthritis is generally considered to be symmetrical.2 Was this a typographical error, or did I miss a crucial concept somewhere?
- Kochhar GS, Rizk M, Patil DT. Not all joint pain is arthritis. Cleve Clin J Med 2013; 80:272–273.
- Bukhari M, Lunt M, Harrison BJ, Scott DG, Symmons DP, Silman AJ. Erosions in inflammatory polyarthritis are symmetrical regardless of rheumatoid factor status: results from a primary care-based inception cohort of patients. Rheumatology 2002; 41:246–252.
To the Editor: I was somewhat confused by the Clinical Picture case in the May 2013 issue.1 The caption for Figure 1 stated that the MRI showed erosions and marrow edema, which were “asymmetrical compared with the other wrist, a finding highly suggestive of rheumatoid arthritis.” However, rheumatoid arthritis is generally considered to be symmetrical.2 Was this a typographical error, or did I miss a crucial concept somewhere?
To the Editor: I was somewhat confused by the Clinical Picture case in the May 2013 issue.1 The caption for Figure 1 stated that the MRI showed erosions and marrow edema, which were “asymmetrical compared with the other wrist, a finding highly suggestive of rheumatoid arthritis.” However, rheumatoid arthritis is generally considered to be symmetrical.2 Was this a typographical error, or did I miss a crucial concept somewhere?
- Kochhar GS, Rizk M, Patil DT. Not all joint pain is arthritis. Cleve Clin J Med 2013; 80:272–273.
- Bukhari M, Lunt M, Harrison BJ, Scott DG, Symmons DP, Silman AJ. Erosions in inflammatory polyarthritis are symmetrical regardless of rheumatoid factor status: results from a primary care-based inception cohort of patients. Rheumatology 2002; 41:246–252.
- Kochhar GS, Rizk M, Patil DT. Not all joint pain is arthritis. Cleve Clin J Med 2013; 80:272–273.
- Bukhari M, Lunt M, Harrison BJ, Scott DG, Symmons DP, Silman AJ. Erosions in inflammatory polyarthritis are symmetrical regardless of rheumatoid factor status: results from a primary care-based inception cohort of patients. Rheumatology 2002; 41:246–252.
In reply: Not all joint pain is arthritis
In Reply: We apologize for the confusion. We wanted to convey that, in that patient at that time, synovitis with erosions and edema indicating inflammation (greater on the right than on the left left) was suggestive of rheumatoid arthritis despite the asymmetry seen (findings greater in the right wrist than in the left). Given the patient’s clinical findings at that time and the above imaging findings, the initial diagnosis of rheumatoid arthritis was correct. But since the patient was not responding to therapy and since the abdominal pain was worsening, we probed further. Subsequently, the patient was diagnosed with Whipple disease. The fact that inflammatory arthritis can occur in other conditions that are not rheumatologic is a primary reason we found this case worth sharing.
In Reply: We apologize for the confusion. We wanted to convey that, in that patient at that time, synovitis with erosions and edema indicating inflammation (greater on the right than on the left left) was suggestive of rheumatoid arthritis despite the asymmetry seen (findings greater in the right wrist than in the left). Given the patient’s clinical findings at that time and the above imaging findings, the initial diagnosis of rheumatoid arthritis was correct. But since the patient was not responding to therapy and since the abdominal pain was worsening, we probed further. Subsequently, the patient was diagnosed with Whipple disease. The fact that inflammatory arthritis can occur in other conditions that are not rheumatologic is a primary reason we found this case worth sharing.
In Reply: We apologize for the confusion. We wanted to convey that, in that patient at that time, synovitis with erosions and edema indicating inflammation (greater on the right than on the left left) was suggestive of rheumatoid arthritis despite the asymmetry seen (findings greater in the right wrist than in the left). Given the patient’s clinical findings at that time and the above imaging findings, the initial diagnosis of rheumatoid arthritis was correct. But since the patient was not responding to therapy and since the abdominal pain was worsening, we probed further. Subsequently, the patient was diagnosed with Whipple disease. The fact that inflammatory arthritis can occur in other conditions that are not rheumatologic is a primary reason we found this case worth sharing.
Ponatinib sales and marketing suspended
After follow-up data from the phase 2 PACE trial revealed that ponatinib-treated patients experienced an increase in arterial and venous thrombotic events, the FDA decided to investigate the drug’s safety.
The agency placed current ponatinib trials on partial clinical hold and asked the drug’s makers, Ariad Pharmaceuticals, to end the phase 3 EPIC trial.
Now, the FDA has asked Ariad to temporarily suspend marketing and sales of ponatinib while the agency further evaluates the drug.
Ponatinib is approved in the US and the European Union to treat adults with chronic myeloid leukemia or Philadelphia chromosome-positive acute lymphoblastic leukemia that is resistant to or intolerant of other tyrosine kinase inhibitors.
Recommendations for ponatinib use
Until its safety evaluation is complete, the FDA is recommending that healthcare professionals reconsider the use of ponatinib.
For patients who are taking ponatinib but not responding, immediately discontinue their treatment and discuss alternative treatment options.
For patients who are currently taking ponatinib and responding, determine whether the potential benefits of the therapy outweigh the risks. If they do, these patients should be treated under a single-patient investigational new drug (IND) application or expanded access registry program while the FDA’s safety investigation continues.
Do not start treating new patients with ponatinib unless no other treatment options are available and all other available therapies have failed. Patients who meet these criteria can be considered for treatment under an IND or expanded access registry program.
For more information on obtaining access to treatment for your patient under an IND, please refer to the following website: Physician Request for an Individual Patient IND under Expanded Access for Non-emergency or Emergency Use.
Ponatinib safety data
Thus far, the FDA’s investigation of ponatinib has revealed an increased frequency of arterial and venous thrombotic events since the drug was approved in December 2012.
In clinical trials conducted before the drug’s approval, serious arterial thrombosis occurred in 8% of ponatinib-treated patients, and venous thromboembolism occurred in 3%. In the most recent clinical trial data, at least 20% of all participants treated with ponatinib have developed thrombosis or arteriosclerosis.
Serious adverse vascular events have occurred in about 24% of patients in the phase 2 trial of ponatinib (median treatment duration of 1.3 years) and about 48% of patients in the phase 1 trial (median treatment duration of 2.7 years).
These included fatal and life-threatening heart attack, stroke, loss of blood flow to the extremities resulting in tissue death, and severe narrowing of blood vessels in the extremities, heart, and brain requiring urgent surgical procedures to restore blood flow.
In the phase 2 trial, adverse events affecting the blood vessels that supply the heart, brain, and extremities were observed in 12%, 6%, and 8% of patients, respectively. Patients with and without cardiovascular risk factors, including patients in their 20s, have experienced these events.
Serious adverse reactions involving the eyes, which led to blindness or blurred vision, occurred in ponatinib-treated patients. High blood pressure occurred in 67% of patients treated with ponatinib in the trials. Heart failure, including fatalities, occurred in 8% of patients who received the drug.
In some patients, fatal and serious adverse events have occurred as early as 2 weeks after starting ponatinib therapy.
The phase 1 and 2 trials did not include a control group, so it is not possible to determine the relationship of these adverse events to ponatinib. However, the increasing rate and pattern of the events strongly suggests that many are drug-related, according to the FDA.
The agency said it cannot currently identify a dose level or exposure duration of ponatinib that is safe. Prior to the issues with adverse events, the recommended dose of ponatinib was a 45 mg tablet taken once daily.
The FDA said it plans to continue its investigation and will notify healthcare professionals and patients as more information becomes available. ![]()
After follow-up data from the phase 2 PACE trial revealed that ponatinib-treated patients experienced an increase in arterial and venous thrombotic events, the FDA decided to investigate the drug’s safety.
The agency placed current ponatinib trials on partial clinical hold and asked the drug’s makers, Ariad Pharmaceuticals, to end the phase 3 EPIC trial.
Now, the FDA has asked Ariad to temporarily suspend marketing and sales of ponatinib while the agency further evaluates the drug.
Ponatinib is approved in the US and the European Union to treat adults with chronic myeloid leukemia or Philadelphia chromosome-positive acute lymphoblastic leukemia that is resistant to or intolerant of other tyrosine kinase inhibitors.
Recommendations for ponatinib use
Until its safety evaluation is complete, the FDA is recommending that healthcare professionals reconsider the use of ponatinib.
For patients who are taking ponatinib but not responding, immediately discontinue their treatment and discuss alternative treatment options.
For patients who are currently taking ponatinib and responding, determine whether the potential benefits of the therapy outweigh the risks. If they do, these patients should be treated under a single-patient investigational new drug (IND) application or expanded access registry program while the FDA’s safety investigation continues.
Do not start treating new patients with ponatinib unless no other treatment options are available and all other available therapies have failed. Patients who meet these criteria can be considered for treatment under an IND or expanded access registry program.
For more information on obtaining access to treatment for your patient under an IND, please refer to the following website: Physician Request for an Individual Patient IND under Expanded Access for Non-emergency or Emergency Use.
Ponatinib safety data
Thus far, the FDA’s investigation of ponatinib has revealed an increased frequency of arterial and venous thrombotic events since the drug was approved in December 2012.
In clinical trials conducted before the drug’s approval, serious arterial thrombosis occurred in 8% of ponatinib-treated patients, and venous thromboembolism occurred in 3%. In the most recent clinical trial data, at least 20% of all participants treated with ponatinib have developed thrombosis or arteriosclerosis.
Serious adverse vascular events have occurred in about 24% of patients in the phase 2 trial of ponatinib (median treatment duration of 1.3 years) and about 48% of patients in the phase 1 trial (median treatment duration of 2.7 years).
These included fatal and life-threatening heart attack, stroke, loss of blood flow to the extremities resulting in tissue death, and severe narrowing of blood vessels in the extremities, heart, and brain requiring urgent surgical procedures to restore blood flow.
In the phase 2 trial, adverse events affecting the blood vessels that supply the heart, brain, and extremities were observed in 12%, 6%, and 8% of patients, respectively. Patients with and without cardiovascular risk factors, including patients in their 20s, have experienced these events.
Serious adverse reactions involving the eyes, which led to blindness or blurred vision, occurred in ponatinib-treated patients. High blood pressure occurred in 67% of patients treated with ponatinib in the trials. Heart failure, including fatalities, occurred in 8% of patients who received the drug.
In some patients, fatal and serious adverse events have occurred as early as 2 weeks after starting ponatinib therapy.
The phase 1 and 2 trials did not include a control group, so it is not possible to determine the relationship of these adverse events to ponatinib. However, the increasing rate and pattern of the events strongly suggests that many are drug-related, according to the FDA.
The agency said it cannot currently identify a dose level or exposure duration of ponatinib that is safe. Prior to the issues with adverse events, the recommended dose of ponatinib was a 45 mg tablet taken once daily.
The FDA said it plans to continue its investigation and will notify healthcare professionals and patients as more information becomes available. ![]()
After follow-up data from the phase 2 PACE trial revealed that ponatinib-treated patients experienced an increase in arterial and venous thrombotic events, the FDA decided to investigate the drug’s safety.
The agency placed current ponatinib trials on partial clinical hold and asked the drug’s makers, Ariad Pharmaceuticals, to end the phase 3 EPIC trial.
Now, the FDA has asked Ariad to temporarily suspend marketing and sales of ponatinib while the agency further evaluates the drug.
Ponatinib is approved in the US and the European Union to treat adults with chronic myeloid leukemia or Philadelphia chromosome-positive acute lymphoblastic leukemia that is resistant to or intolerant of other tyrosine kinase inhibitors.
Recommendations for ponatinib use
Until its safety evaluation is complete, the FDA is recommending that healthcare professionals reconsider the use of ponatinib.
For patients who are taking ponatinib but not responding, immediately discontinue their treatment and discuss alternative treatment options.
For patients who are currently taking ponatinib and responding, determine whether the potential benefits of the therapy outweigh the risks. If they do, these patients should be treated under a single-patient investigational new drug (IND) application or expanded access registry program while the FDA’s safety investigation continues.
Do not start treating new patients with ponatinib unless no other treatment options are available and all other available therapies have failed. Patients who meet these criteria can be considered for treatment under an IND or expanded access registry program.
For more information on obtaining access to treatment for your patient under an IND, please refer to the following website: Physician Request for an Individual Patient IND under Expanded Access for Non-emergency or Emergency Use.
Ponatinib safety data
Thus far, the FDA’s investigation of ponatinib has revealed an increased frequency of arterial and venous thrombotic events since the drug was approved in December 2012.
In clinical trials conducted before the drug’s approval, serious arterial thrombosis occurred in 8% of ponatinib-treated patients, and venous thromboembolism occurred in 3%. In the most recent clinical trial data, at least 20% of all participants treated with ponatinib have developed thrombosis or arteriosclerosis.
Serious adverse vascular events have occurred in about 24% of patients in the phase 2 trial of ponatinib (median treatment duration of 1.3 years) and about 48% of patients in the phase 1 trial (median treatment duration of 2.7 years).
These included fatal and life-threatening heart attack, stroke, loss of blood flow to the extremities resulting in tissue death, and severe narrowing of blood vessels in the extremities, heart, and brain requiring urgent surgical procedures to restore blood flow.
In the phase 2 trial, adverse events affecting the blood vessels that supply the heart, brain, and extremities were observed in 12%, 6%, and 8% of patients, respectively. Patients with and without cardiovascular risk factors, including patients in their 20s, have experienced these events.
Serious adverse reactions involving the eyes, which led to blindness or blurred vision, occurred in ponatinib-treated patients. High blood pressure occurred in 67% of patients treated with ponatinib in the trials. Heart failure, including fatalities, occurred in 8% of patients who received the drug.
In some patients, fatal and serious adverse events have occurred as early as 2 weeks after starting ponatinib therapy.
The phase 1 and 2 trials did not include a control group, so it is not possible to determine the relationship of these adverse events to ponatinib. However, the increasing rate and pattern of the events strongly suggests that many are drug-related, according to the FDA.
The agency said it cannot currently identify a dose level or exposure duration of ponatinib that is safe. Prior to the issues with adverse events, the recommended dose of ponatinib was a 45 mg tablet taken once daily.
The FDA said it plans to continue its investigation and will notify healthcare professionals and patients as more information becomes available. ![]()
Hospitalist‐Run Postdischarge Clinic
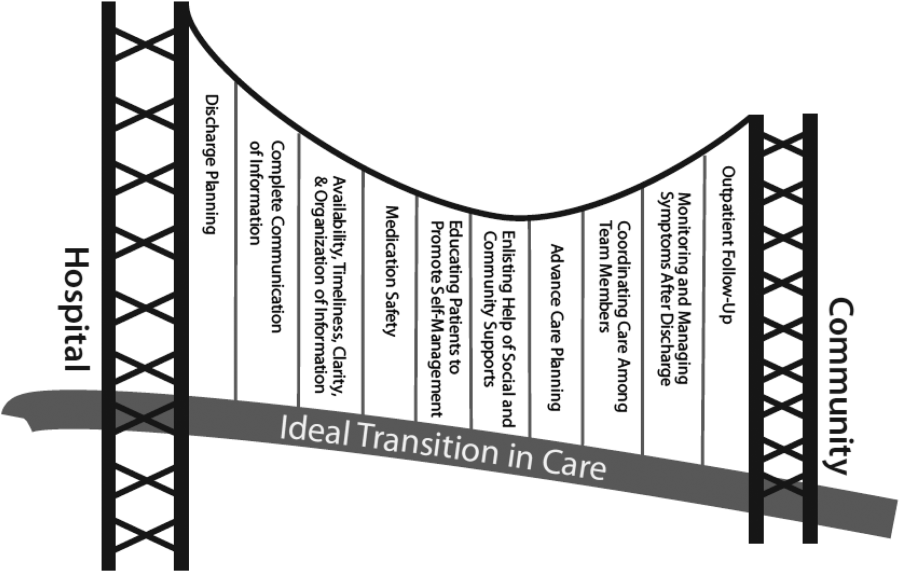
Currently, healthcare systems rarely provide ideal transitions of care for discharged patients,[1] resulting in fragmented care,[2, 3, 4, 5] significant patient uncertainty about how to manage at home,[6, 7] and frequent adverse events.[8, 9] These factors are so commonly experienced by discharged patients that they are recognizable as a postdischarge syndrome.[10]
One element important for reducing the postdischarge risk of adverse events is provision of adequate follow‐up.[11, 12] However, supplying this care is challenging in the modern era, and it will become progressively more difficult to achieve. In 2004, 50% of readmitted Medicare fee‐for‐service patients had no postdischarge visit within 30 days of their discharge,[9] likely due in part to difficulty arranging such care. Changes in insurance coverage and demographics are expected to result in more than 100 million newly insured patients by 2019, yet the primary‐care workforce is projected to begin shrinking by 2016.[13, 14] In the increasingly uncommon situation that a primary‐care clinician is available promptly after discharge, information transfer is often inadequate[4, 15, 16, 17] and can be exacerbated by the growing discontinuity between inpatient and outpatient care.[2, 3, 4] Efforts to increase the supply of primary‐care clinicians and thereby improve early access to postdischarge care are important for the future, but hospitals, particularly those penalized for high risk‐adjusted readmission rates, are seeking novel solutions now.
One increasingly common innovation is to extend the role of inpatient providers (usually hospitalists) into the postdischarge period.[18] Preliminary evidence suggests improved continuity[19] and access[20] achieved by providing this care may decrease postdischarge adverse events,[19, 20, 21] though evidence is conflicting.[22]
As a closed, multilevel healthcare system, the Denver VA Medical Center is uniquely positioned to evaluate the influence of alternative postdischarge‐care strategies on subsequent adverse events. Discharged patients are seen in a well‐established hospitalist‐run postdischarge clinic (PDC), a robust urgent‐care system (UC), or by a large primary‐care provider (PCP) practice. The purpose of this study was to evaluate whether patients seen in a hospitalist‐run PDC have reduced adverse outcomes in the 30 days following hospital discharge compared with follow‐up with the patient's PCP or in an UC clinic.
METHODS
Patients
This was a retrospective cohort study of consecutive adult patients discharged from the general medical services of the Denver VA Medical Center after a nonelective admission between January 2005 and August 2012. This time range was chosen because all 3 clinics were fully operational during this period. The Denver VA Medical Center is an academically‐affiliated 128‐bed hospital that provides a full range of tertiary services. All medical patients, including intensive care unit (ICU) patients, are cared for on general medical teams by University of Colorado housestaff with hospitalists or subspecialty attendings. Patients who lived in the Denver metropolitan area, were discharged home, and who followed up with a PCP, UC clinic, or PDC within 30 days of discharge were included. Patients discharged to subacute facilities, hospice, or this tends to be capitalized as a special program at our VA were excluded. For patients with multiple admissions, only the first was included.
Clinics
Primary Care
Primary‐care clinics in the VA system are organized into Patient‐Aligned Care Teams (PACTs) and are available for appointments 5 days per week. Patients discharged from the medical service who have PCPs are called within 48 hours of discharge by PACT nurses to evaluate their postdischarge state. Primary‐care physicians could be resident housestaff or ambulatory attending physicians. Seventy‐two percent of patients seen at the Denver VA have an assigned PCP.
Urgent Care
The Office‐based Medical Team provides UC and short‐term regular appointments for recently discharged medical patients or patients who require frequent follow‐up (such as those that require serial paracenteses). It is a separate clinic from an emergency department (ED)‐based walk‐in clinic. It is also available 5 days per week; patients are seen by resident housestaff unfamiliar with the patient, and the clinic is staffed with an ambulatory attending physician. Patients are commonly seen multiple times in the same clinic, though usually with different providers.
Postdischarge Clinic
The hospitalist‐run PDC is scheduled 2 afternoons per week. Patients are always seen by housestaff and medical students from the team that cared for them as an inpatient, then staffed with a rotating hospitalist attending who may have been the supervising inpatient attending during the patient's inpatient stay. Thus, continuity is preserved with the housestaff team in all cases, although attending continuity is variable. This is added to the daily responsibility of the resident and hospitalist physicians who are providing care on the inpatient service at the time of the clinic. Capabilities of the clinic are similar to UC and PCP clinics. Patients are usually seen once postdischarge with referral to the PCP for further follow‐up; however, patients can be seen multiple times by the same provider team.
If a patient followed up with multiple clinics, the first clinic visited determined the group to which that patient was allocated for the purpose of analysis. If a patient was scheduled for clinic follow‐up but did not attend within 30 days of discharge, he or she was excluded. We did not collect data on visits outside of these 3 clinics, as pilot data demonstrated they accounted for nearly all (>90%) of posthospitalization follow‐up visits. During the study period, there were no guidelines for discharging physicians about which clinic to have the patient follow up in. The UC and PDC were known to have better early access to follow‐up appointments and thus tended to see patients requiring early follow‐up in the judgment of the discharging clinician.
Statistical Analysis
The VA's Computing and Informatics Infrastructure (VINCI) was used to collect predischarge patient data for descriptive and analytic purposes. Pertinent potential confounders included patient age, sex, marital status, comorbidities, number of prescribed medications on discharge, previous hospital admissions in the last year, ICU admission (as a dichotomous variable), ICU length of stay (LOS), and hospital LOS. Postdischarge variables included time to first follow‐up appointment and hospital LOS if readmitted.
The primary outcome was a composite of ED visits, hospital readmissions, and mortality in the 30 days following hospital discharge. These outcomes were captured in the VA system; we did not measure outside utilization. A power analysis indicated that the sample has >90% power to detect small differences (4%) in the composite outcome between types of outpatient care. We also evaluated the effect of different types of follow‐up on the 3 individual components of the primary outcome. To compare baseline categorical variables across 3 groups, 2 trend tests were used; analysis of variance (ANOVA) or Kruskal‐Wallis test was used for continuous variables in univariate analysis.
We then used propensity scoring to adjust for baseline differences between groups in an attempt to adjust for referral bias, using multivariate logistic regression to calculate a propensity score for each patient in 2‐way comparisons, and a single score for every patient in a multinomial comparison.[23] Our final propensity score incorporated age, number of hospital admissions in the past year, and Elixhauser comorbidity score,[24] with excellent overlap in propensity scores between groups. Although hospital LOS was different between groups, inclusion in the propensity score did not reduce this significant difference, and its inclusion in the propensity model decreased model fit. Limitations of the accessible data prevented high‐dimensional propensity scoring and limited the outcome of the propensity score to attendance at the clinic assigned, rather than referral to the clinic assigned. The propensity score, hospital LOS, time to the first outpatient visit, and group assignment (PDC, PCP, UC) were entered into a multivariate logistic regression model.
To find a subgroup who may benefit most from follow‐up in the PDC, we a priori identified patients with one of the 5 discharge diagnosis‐related groups (DRGs) most commonly associated with subsequent readmission[9] and examined outcomes between the 3 different kinds of follow‐up, restricted to patients discharged with one of these diagnoses. All analyses were conducted using SAS 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
9952 patients who met criteria were discharged during this time period; however, 48.9% did not follow up with one of these clinics within 30 days, leaving 5085 patients in our analysis. Of these, 538 followed up in PDC (10.6%), 1848 followed up with their PCP (36.3%), and 2699 followed up in UC (53.1%). Table 1 presents predischarge characteristics of these patients. Patients seen in PDC were older and had a more significant comorbidity burden.
| PDC, N=538 | UC, N=2699 | PCP, N=1848 | P Value | P Value After Propensity Adjustment | |
|---|---|---|---|---|---|
| |||||
| Age, years (SD) | 67.8 (12.6) | 67.1 (13.0) | 64.8 (13.0) | <0.01 | 0.86 |
| Male sex, % | 95.0 | 95.4 | 94.4 | 0.33 | |
| Marital status, % | |||||
| Divorced | 40.2 | 36.2 | 35.0 | 0.09 | |
| Married | 35.9 | 37.3 | 39.8 | 0.13 | |
| Never married | 12.3 | 13.7 | 14.3 | 0.48 | |
| LOS, days (SD) | 3.8 (3.6) | 5.0 (11.7) | 6.2 (10.8) | 0.04 | |
| Elixhauser score (SD) | 0.80 (1.1) | 0.69 (1.0) | 0.75 (1.0) | 0.02 | 0.06 |
| Admitted to ICU, % | 19.0 | 19.9 | 23.0 | 0.12 | |
| ICU LOS, days (SD) | 2.8 (4.4) | 2.8 (3.4) | 2.3 (1.5) | 0.15 | |
| Discharge medications, mean (SD) | 10.0 (6.7) | 10.4 (7.4) | 10.4 (8.2) | 0.37 | |
| Admissions per patient in prior year, mean (SD) | 0.18 (0.5) | 0.21 (0.6) | 0.23 (0.6) | 0.08 | 0.78 |
Patients seen in PDC had a mean 2.4‐day shorter LOS than those seen by their PCPs (PDC: 3.8 days, UC: 5.0 days, PCP: 6.2 days; P=0.04 for comparison). Neither the percentage of patients admitted to the ICU during their index hospitalization nor the ICU LOS was different between groups. Patients were seen earlier postdischarge in PDC than in other types of follow‐up (PDC: 5.0 days, UC: 9.4 days, PCP: 13.7 days; P<0.01 for comparison). In univariate analysis, there was no difference between groups in the composite 30‐day outcome (Table 2). Analysis of the individual components of the primary outcome revealed significant differences in readmission rates, with PDC having the highest rate.
| PDC | UC | PCP | P Value | P Value After Propensity Adjustment | |
|---|---|---|---|---|---|
| |||||
| Composite outcome, % | 19.9 | 18.3 | 17.5 | 0.42 | 0.30 |
| Hospital readmission | 13.0 | 11.1 | 9.4 | 0.03 | 0.03 |
| ED visit | 10.2 | 9.9 | 10.5 | 0.78 | 0.93 |
| Mortality | 1.1 | 0.7 | 0.7 | 0.58 | 0.65 |
| LOS if readmitted, days (SD) | 6.9 (18.1) | 4.9 (7.8) | 4.8 (6.5) | 0.28 | 0.23 |
| Time to first visit after discharge, days (SD) | 5.0 (3.0) | 9.4 (6.1) | 13.8 (8.5) | <0.01 | <0.01 |
Univariate analyses conducted on predischarge characteristics after multinomial propensity scoring revealed significant differences between groups no longer existed for the variables that were included in the propensity score (age, Elixhauser score, and inpatient stays prior to visit; Table 1).
In multivariate analysis comparing PDC to PCP follow‐up, there was no difference in the composite outcome after controlling for propensity score and time to outpatient visit (odds ratio [OR]: 1.07, 95% confidence interval [CI]: 0.81‐1.40). Similar results were obtained in comparing PDC with UC (OR: 1.05, 95% CI: 0.82‐1.34) and in multinomial logistic regression comparing PDC with other types of follow‐up (PDC vs PCP: OR: 1.01, 95% CI: 0.78‐1.31; PDC vs UC: OR: 0.99, 95% CI: 0.78‐1.26).
Restricting the multivariate analysis to those patients discharged with one of the 5 discharge DRGs most associated with readmission did not alter our findings regarding the primary outcome. We also found no change in the composite outcome or any subcomponent of the composite outcome when restricting the analysis to 7‐day outcomes or when excluding scheduled readmissions (which represented <5% of all readmission).
DISCUSSION
A hospitalist‐run postdischarge clinic did not reduce a composite of 30‐day postdischarge adverse outcomes in our study when compared with primary‐care or urgent‐care follow‐up. In fact, patients who followed up in PDC had a small increase in 30‐day readmissions. However, they also were sicker at baseline, considered higher risk by the discharging physician, were able to be seen significantly earlier, and had an associated 2.4‐day shorter hospital LOS than patients seen by their PCPs.
Our findings do not confirm those of prior research in this area, which indicated outpatient follow‐up by the same physician who was the treating inpatient physician was linked to lower mortality rates, hospital readmissions, and ED utilization.[19, 20, 21] In fact, in our study, there was a significant (albeit small) increase in 30‐day readmissions in patients seen in PDC. There are significant challenges to the generalizability and validity of these prior studies. In one study, inpatient care was provided by outpatient primary‐care doctors in Canada,[19] a payer and care model rare in the United States.[25] In a second, usual care was not specified, and it is likely the reduction in ED visits resulted from provision of follow‐up care of any kind compared with those who did not follow up after discharge.[20] In a third, the PDC was part of a larger bundle of postdischarge interventions, and it only reduced ED visits when compared with patients who did not have follow‐up; rates of ED visits were similar in a comparison with PCP follow‐up.[21]
There are several possible explanations for the lack of improvement in 30‐day adverse outcomes with a hospitalist‐run PDC. First, although early access to early postdischarge care was improved and evidence suggests this is important in reducing readmissions,[11, 12] in populations similar to that studied, more postdischarge care has also been linked to increased readmissions.[22] This may be due to more frequent re‐evaluation of fragile, chronically ill patients, presenting more options for readmission. Second, the intervention currently only addresses some components of the Ideal Transition of Care[1] (Figure 1) and may benefit from an enhanced visit structure using a multidisciplinary approach. Third, the intervention took place in the context of a robust primary‐care system with a universal electronic medical record; the effects of improved access and continuity may be magnified in a system without these advantages. Fourth, there was a low readmission rate overall, and it is unclear how many of these readmissions were preventable. Finally, it may be that although the initial postdischarge care was adequate, readmissions occurred after the first visit, suggesting subsequent care during the 30 days postdischarge could have been improved.
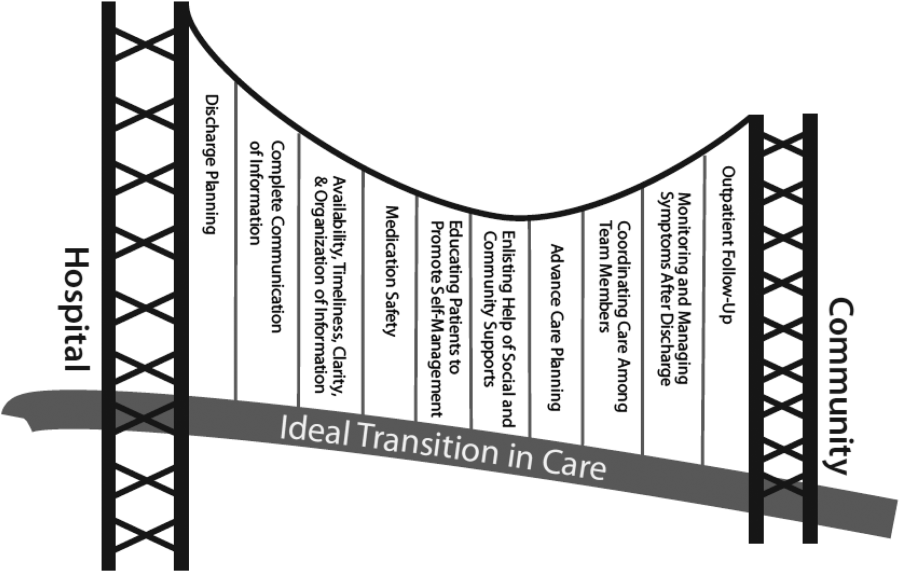
The most likely explanation for the substantially decreased LOS associated with follow‐up in PDC is that inpatient physicians who knew they could see their own patients early in the postdischarge process were more tolerant of uncertainty surrounding the patients' clinical course.
For example, a frequent clinical conundrum for hospitalists is when to discharge patients improving on diuretic therapy for a heart failure exacerbation or antibiotics for cellulitis. Provided a PDC, these hospitalists may choose to discharge a patient still actively being treated, because they may feel they have access to early follow‐up to change course if needed as well as the ability to see the patient themselves, allowing precise evaluation of the change in their condition. Without this clinic, the hospitalist may wonder when postdischarge follow‐up will occur. They may be more hesitant to discharge a patient who has not fully completed treatment for fear he or she will still appear decompensated to the postdischarge provider (though greatly improved from admission), or will not have timely‐enough follow‐up to change treatment if the condition worsens.
Our finding that the LOS was still shorter when comparing PDC with UC suggests continuity may be a significant component of this effect. It seems unlikely that patients following up in PDC had less complex hospitalizations given similar ICU exposure and LOS, as well as older age and larger baseline comorbidity burden.
The LOS seen in patients who followed up in PDC was lower than Medicare rates[26] but similar to reported rates at other VA acute‐care hospitals.[27] It is consistent with prior findings that hospitalist care reduces LOS,[26] though the magnitude in our study was much larger than that in prior reports. Prior studies have suggested this decreased LOS is linked to increased adverse postdischarge outcomes, such as ED visits and readmissions, as well as increased costs and decreased discharges to home.[28] The PDC was not associated with increased postdischarge adverse events measured, though a formal cost analysis and analysis of other postdischarge outcomes, such as placement in a skilled nursing or rehabilitation facility after return home, could be assessed in future work.
The findings of our study should be interpreted in the context of the study design. Our study was retrospective, observational, and single‐center. There may have been additional baseline differences between groups predisposing to bias we did not capture in the propensity score. For example, we could not measure rates of attendance at the different clinics and cannot rule out that outcomes associated with PDC were also associated with increased attendance rates. However, none of the clinics had mechanisms in place to improve follow‐up rates; patients referred to PDC were those considered highest risk for readmission and were sicker at baseline, making it very unlikely that they were predisposed to attend clinic more frequently and/or to have better outcomes; and even if PDC improved follow‐up rates, this would be a significant contribution given the limitations of primary‐care access. Our propensity score could not perfectly mimic randomization to a treatment assignment, but rather to treatment received, because of this limitation.
We did not ascertain ED visits or readmissions outside the VA system; it is possible these differentially affected one group more than another, though this seems unlikely. Our patient population was representative of veteran populations elsewhere who are at high risk of adverse postdischarge outcomes, but our findings may not be generalizable to younger, more ethnically diverse populations or to women.
CONCLUSIONS
Provision of postdischarge care by hospitalists may reduce LOS without increasing postdischarge adverse events. Further work is required to evaluate the role of hospitalist‐run PDCs in healthcare systems with more limited postdischarge access to care, to formally evaluate the costs associated with extending hospitalists to the outpatient setting, and to prospectively evaluate the role of a PDC compared with other kinds of hospital follow‐up.
Acknowledgments
The authors thank Melver Anderson, MD, for editorial assistance with the manuscript.
Disclosures: Dr. Burke had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. Dr. Burke was supported by grant funding from the Colorado Research Enhancement Award Program to Improve Care Coordination for Veterans. Initial results of this study were presented at the Society of General Internal Medicine National Meeting in Denver, Colorado, April 24, 2013.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109.
- , , , , , . Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults. JAMA. 2009;301(16):1671–1680.
- , , , , . Trends in inpatient continuity of care for a cohort of Medicare patients 1996–2006. J Hosp Med. 2011;6(8):438–444.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , . Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449–1465.
- , , , , . Patient experiences of transitioning from hospital to home: an ethnographic quality improvement project. J Hosp Med. 2012;7(5):382–387.
- , , , et al. Understanding and execution of discharge instructions. Am J Med Qual. 2013;28(5):383–391.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- . Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102.
- , , , et al. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716–1722.
- , , . Post‐hospitalization transitions: examining the effects of timing of primary care provider follow‐up. J Hosp Med. 2010;5(7):392–397.
- , , . Will generalist physician supply meet demands of an increasing and aging population? Health Aff (Millwood). 2008;27(3):w232–w241.
- Association of American Medical Colleges. The Impact of Health Care Reform on the Future Supply and Demand for Physicians: Updated Projections Through 2025. Available at: http://www.aamc.org/download/158076/data/updated_projections_through_2025.pdf. Published June 2010. Accessed May 1, 2012.
- , , , . Effect of discharge summary availability during post‐discharge visits on hospital readmission. J Gen Intern Med. 2002;17(3):186–192.
- , , , et al. Comprehensive quality of discharge summaries at an academic medical center. J Hosp Med. 2013;8(8):436–443.
- , , , et al. Association of communication between hospital‐based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381–386.
- . Is a post‐discharge clinic in your hospital's future? Available at: http://www.the‐hospitalist.org/details/article/1409011/Is_a_Post‐Discharge_Clinic_in_Your_Hospitals_Future.html. Published December 2011. Accessed May 1, 2013.
- , , , . Continuity of care and patient outcomes after hospital discharge. J Gen Intern Med. 2004;19(6):624–631.
- , , , . Effects of a postdischarge clinic on housestaff satisfaction and utilization of hospital services. J Gen Intern Med. 1996;11(3):179–181.
- , , , , , . Integrated postdischarge transitional care in a hospitalist system to improve discharge outcome: an experimental study. BMC Med. 2011;9:96.
- , , . Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med. 1996;334(22):1441–1447.
- , . An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J. 2011;32(14):1704–1708.
- , , . Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004;42(4):355–360.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . Association of hospitalist care with medical utilization after discharge: evidence of cost shift from a cohort study. Ann Intern Med. 2011;155(3):152–159.
- , , , et al. Associations between reduced hospital length of stay and 30‐day readmission rate and mortality: 14‐year experience in 129 Veterans Affairs hospitals. Ann Intern Med. 2012;157(12):837–845.
- , , , . Variation in length of stay and outcomes among hospitalized patients attributable to hospitals and hospitalists. J Gen Intern Med. 2013;28(3):370–376.
Currently, healthcare systems rarely provide ideal transitions of care for discharged patients,[1] resulting in fragmented care,[2, 3, 4, 5] significant patient uncertainty about how to manage at home,[6, 7] and frequent adverse events.[8, 9] These factors are so commonly experienced by discharged patients that they are recognizable as a postdischarge syndrome.[10]
One element important for reducing the postdischarge risk of adverse events is provision of adequate follow‐up.[11, 12] However, supplying this care is challenging in the modern era, and it will become progressively more difficult to achieve. In 2004, 50% of readmitted Medicare fee‐for‐service patients had no postdischarge visit within 30 days of their discharge,[9] likely due in part to difficulty arranging such care. Changes in insurance coverage and demographics are expected to result in more than 100 million newly insured patients by 2019, yet the primary‐care workforce is projected to begin shrinking by 2016.[13, 14] In the increasingly uncommon situation that a primary‐care clinician is available promptly after discharge, information transfer is often inadequate[4, 15, 16, 17] and can be exacerbated by the growing discontinuity between inpatient and outpatient care.[2, 3, 4] Efforts to increase the supply of primary‐care clinicians and thereby improve early access to postdischarge care are important for the future, but hospitals, particularly those penalized for high risk‐adjusted readmission rates, are seeking novel solutions now.
One increasingly common innovation is to extend the role of inpatient providers (usually hospitalists) into the postdischarge period.[18] Preliminary evidence suggests improved continuity[19] and access[20] achieved by providing this care may decrease postdischarge adverse events,[19, 20, 21] though evidence is conflicting.[22]
As a closed, multilevel healthcare system, the Denver VA Medical Center is uniquely positioned to evaluate the influence of alternative postdischarge‐care strategies on subsequent adverse events. Discharged patients are seen in a well‐established hospitalist‐run postdischarge clinic (PDC), a robust urgent‐care system (UC), or by a large primary‐care provider (PCP) practice. The purpose of this study was to evaluate whether patients seen in a hospitalist‐run PDC have reduced adverse outcomes in the 30 days following hospital discharge compared with follow‐up with the patient's PCP or in an UC clinic.
METHODS
Patients
This was a retrospective cohort study of consecutive adult patients discharged from the general medical services of the Denver VA Medical Center after a nonelective admission between January 2005 and August 2012. This time range was chosen because all 3 clinics were fully operational during this period. The Denver VA Medical Center is an academically‐affiliated 128‐bed hospital that provides a full range of tertiary services. All medical patients, including intensive care unit (ICU) patients, are cared for on general medical teams by University of Colorado housestaff with hospitalists or subspecialty attendings. Patients who lived in the Denver metropolitan area, were discharged home, and who followed up with a PCP, UC clinic, or PDC within 30 days of discharge were included. Patients discharged to subacute facilities, hospice, or this tends to be capitalized as a special program at our VA were excluded. For patients with multiple admissions, only the first was included.
Clinics
Primary Care
Primary‐care clinics in the VA system are organized into Patient‐Aligned Care Teams (PACTs) and are available for appointments 5 days per week. Patients discharged from the medical service who have PCPs are called within 48 hours of discharge by PACT nurses to evaluate their postdischarge state. Primary‐care physicians could be resident housestaff or ambulatory attending physicians. Seventy‐two percent of patients seen at the Denver VA have an assigned PCP.
Urgent Care
The Office‐based Medical Team provides UC and short‐term regular appointments for recently discharged medical patients or patients who require frequent follow‐up (such as those that require serial paracenteses). It is a separate clinic from an emergency department (ED)‐based walk‐in clinic. It is also available 5 days per week; patients are seen by resident housestaff unfamiliar with the patient, and the clinic is staffed with an ambulatory attending physician. Patients are commonly seen multiple times in the same clinic, though usually with different providers.
Postdischarge Clinic
The hospitalist‐run PDC is scheduled 2 afternoons per week. Patients are always seen by housestaff and medical students from the team that cared for them as an inpatient, then staffed with a rotating hospitalist attending who may have been the supervising inpatient attending during the patient's inpatient stay. Thus, continuity is preserved with the housestaff team in all cases, although attending continuity is variable. This is added to the daily responsibility of the resident and hospitalist physicians who are providing care on the inpatient service at the time of the clinic. Capabilities of the clinic are similar to UC and PCP clinics. Patients are usually seen once postdischarge with referral to the PCP for further follow‐up; however, patients can be seen multiple times by the same provider team.
If a patient followed up with multiple clinics, the first clinic visited determined the group to which that patient was allocated for the purpose of analysis. If a patient was scheduled for clinic follow‐up but did not attend within 30 days of discharge, he or she was excluded. We did not collect data on visits outside of these 3 clinics, as pilot data demonstrated they accounted for nearly all (>90%) of posthospitalization follow‐up visits. During the study period, there were no guidelines for discharging physicians about which clinic to have the patient follow up in. The UC and PDC were known to have better early access to follow‐up appointments and thus tended to see patients requiring early follow‐up in the judgment of the discharging clinician.
Statistical Analysis
The VA's Computing and Informatics Infrastructure (VINCI) was used to collect predischarge patient data for descriptive and analytic purposes. Pertinent potential confounders included patient age, sex, marital status, comorbidities, number of prescribed medications on discharge, previous hospital admissions in the last year, ICU admission (as a dichotomous variable), ICU length of stay (LOS), and hospital LOS. Postdischarge variables included time to first follow‐up appointment and hospital LOS if readmitted.
The primary outcome was a composite of ED visits, hospital readmissions, and mortality in the 30 days following hospital discharge. These outcomes were captured in the VA system; we did not measure outside utilization. A power analysis indicated that the sample has >90% power to detect small differences (4%) in the composite outcome between types of outpatient care. We also evaluated the effect of different types of follow‐up on the 3 individual components of the primary outcome. To compare baseline categorical variables across 3 groups, 2 trend tests were used; analysis of variance (ANOVA) or Kruskal‐Wallis test was used for continuous variables in univariate analysis.
We then used propensity scoring to adjust for baseline differences between groups in an attempt to adjust for referral bias, using multivariate logistic regression to calculate a propensity score for each patient in 2‐way comparisons, and a single score for every patient in a multinomial comparison.[23] Our final propensity score incorporated age, number of hospital admissions in the past year, and Elixhauser comorbidity score,[24] with excellent overlap in propensity scores between groups. Although hospital LOS was different between groups, inclusion in the propensity score did not reduce this significant difference, and its inclusion in the propensity model decreased model fit. Limitations of the accessible data prevented high‐dimensional propensity scoring and limited the outcome of the propensity score to attendance at the clinic assigned, rather than referral to the clinic assigned. The propensity score, hospital LOS, time to the first outpatient visit, and group assignment (PDC, PCP, UC) were entered into a multivariate logistic regression model.
To find a subgroup who may benefit most from follow‐up in the PDC, we a priori identified patients with one of the 5 discharge diagnosis‐related groups (DRGs) most commonly associated with subsequent readmission[9] and examined outcomes between the 3 different kinds of follow‐up, restricted to patients discharged with one of these diagnoses. All analyses were conducted using SAS 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
9952 patients who met criteria were discharged during this time period; however, 48.9% did not follow up with one of these clinics within 30 days, leaving 5085 patients in our analysis. Of these, 538 followed up in PDC (10.6%), 1848 followed up with their PCP (36.3%), and 2699 followed up in UC (53.1%). Table 1 presents predischarge characteristics of these patients. Patients seen in PDC were older and had a more significant comorbidity burden.
| PDC, N=538 | UC, N=2699 | PCP, N=1848 | P Value | P Value After Propensity Adjustment | |
|---|---|---|---|---|---|
| |||||
| Age, years (SD) | 67.8 (12.6) | 67.1 (13.0) | 64.8 (13.0) | <0.01 | 0.86 |
| Male sex, % | 95.0 | 95.4 | 94.4 | 0.33 | |
| Marital status, % | |||||
| Divorced | 40.2 | 36.2 | 35.0 | 0.09 | |
| Married | 35.9 | 37.3 | 39.8 | 0.13 | |
| Never married | 12.3 | 13.7 | 14.3 | 0.48 | |
| LOS, days (SD) | 3.8 (3.6) | 5.0 (11.7) | 6.2 (10.8) | 0.04 | |
| Elixhauser score (SD) | 0.80 (1.1) | 0.69 (1.0) | 0.75 (1.0) | 0.02 | 0.06 |
| Admitted to ICU, % | 19.0 | 19.9 | 23.0 | 0.12 | |
| ICU LOS, days (SD) | 2.8 (4.4) | 2.8 (3.4) | 2.3 (1.5) | 0.15 | |
| Discharge medications, mean (SD) | 10.0 (6.7) | 10.4 (7.4) | 10.4 (8.2) | 0.37 | |
| Admissions per patient in prior year, mean (SD) | 0.18 (0.5) | 0.21 (0.6) | 0.23 (0.6) | 0.08 | 0.78 |
Patients seen in PDC had a mean 2.4‐day shorter LOS than those seen by their PCPs (PDC: 3.8 days, UC: 5.0 days, PCP: 6.2 days; P=0.04 for comparison). Neither the percentage of patients admitted to the ICU during their index hospitalization nor the ICU LOS was different between groups. Patients were seen earlier postdischarge in PDC than in other types of follow‐up (PDC: 5.0 days, UC: 9.4 days, PCP: 13.7 days; P<0.01 for comparison). In univariate analysis, there was no difference between groups in the composite 30‐day outcome (Table 2). Analysis of the individual components of the primary outcome revealed significant differences in readmission rates, with PDC having the highest rate.
| PDC | UC | PCP | P Value | P Value After Propensity Adjustment | |
|---|---|---|---|---|---|
| |||||
| Composite outcome, % | 19.9 | 18.3 | 17.5 | 0.42 | 0.30 |
| Hospital readmission | 13.0 | 11.1 | 9.4 | 0.03 | 0.03 |
| ED visit | 10.2 | 9.9 | 10.5 | 0.78 | 0.93 |
| Mortality | 1.1 | 0.7 | 0.7 | 0.58 | 0.65 |
| LOS if readmitted, days (SD) | 6.9 (18.1) | 4.9 (7.8) | 4.8 (6.5) | 0.28 | 0.23 |
| Time to first visit after discharge, days (SD) | 5.0 (3.0) | 9.4 (6.1) | 13.8 (8.5) | <0.01 | <0.01 |
Univariate analyses conducted on predischarge characteristics after multinomial propensity scoring revealed significant differences between groups no longer existed for the variables that were included in the propensity score (age, Elixhauser score, and inpatient stays prior to visit; Table 1).
In multivariate analysis comparing PDC to PCP follow‐up, there was no difference in the composite outcome after controlling for propensity score and time to outpatient visit (odds ratio [OR]: 1.07, 95% confidence interval [CI]: 0.81‐1.40). Similar results were obtained in comparing PDC with UC (OR: 1.05, 95% CI: 0.82‐1.34) and in multinomial logistic regression comparing PDC with other types of follow‐up (PDC vs PCP: OR: 1.01, 95% CI: 0.78‐1.31; PDC vs UC: OR: 0.99, 95% CI: 0.78‐1.26).
Restricting the multivariate analysis to those patients discharged with one of the 5 discharge DRGs most associated with readmission did not alter our findings regarding the primary outcome. We also found no change in the composite outcome or any subcomponent of the composite outcome when restricting the analysis to 7‐day outcomes or when excluding scheduled readmissions (which represented <5% of all readmission).
DISCUSSION
A hospitalist‐run postdischarge clinic did not reduce a composite of 30‐day postdischarge adverse outcomes in our study when compared with primary‐care or urgent‐care follow‐up. In fact, patients who followed up in PDC had a small increase in 30‐day readmissions. However, they also were sicker at baseline, considered higher risk by the discharging physician, were able to be seen significantly earlier, and had an associated 2.4‐day shorter hospital LOS than patients seen by their PCPs.
Our findings do not confirm those of prior research in this area, which indicated outpatient follow‐up by the same physician who was the treating inpatient physician was linked to lower mortality rates, hospital readmissions, and ED utilization.[19, 20, 21] In fact, in our study, there was a significant (albeit small) increase in 30‐day readmissions in patients seen in PDC. There are significant challenges to the generalizability and validity of these prior studies. In one study, inpatient care was provided by outpatient primary‐care doctors in Canada,[19] a payer and care model rare in the United States.[25] In a second, usual care was not specified, and it is likely the reduction in ED visits resulted from provision of follow‐up care of any kind compared with those who did not follow up after discharge.[20] In a third, the PDC was part of a larger bundle of postdischarge interventions, and it only reduced ED visits when compared with patients who did not have follow‐up; rates of ED visits were similar in a comparison with PCP follow‐up.[21]
There are several possible explanations for the lack of improvement in 30‐day adverse outcomes with a hospitalist‐run PDC. First, although early access to early postdischarge care was improved and evidence suggests this is important in reducing readmissions,[11, 12] in populations similar to that studied, more postdischarge care has also been linked to increased readmissions.[22] This may be due to more frequent re‐evaluation of fragile, chronically ill patients, presenting more options for readmission. Second, the intervention currently only addresses some components of the Ideal Transition of Care[1] (Figure 1) and may benefit from an enhanced visit structure using a multidisciplinary approach. Third, the intervention took place in the context of a robust primary‐care system with a universal electronic medical record; the effects of improved access and continuity may be magnified in a system without these advantages. Fourth, there was a low readmission rate overall, and it is unclear how many of these readmissions were preventable. Finally, it may be that although the initial postdischarge care was adequate, readmissions occurred after the first visit, suggesting subsequent care during the 30 days postdischarge could have been improved.

The most likely explanation for the substantially decreased LOS associated with follow‐up in PDC is that inpatient physicians who knew they could see their own patients early in the postdischarge process were more tolerant of uncertainty surrounding the patients' clinical course.
For example, a frequent clinical conundrum for hospitalists is when to discharge patients improving on diuretic therapy for a heart failure exacerbation or antibiotics for cellulitis. Provided a PDC, these hospitalists may choose to discharge a patient still actively being treated, because they may feel they have access to early follow‐up to change course if needed as well as the ability to see the patient themselves, allowing precise evaluation of the change in their condition. Without this clinic, the hospitalist may wonder when postdischarge follow‐up will occur. They may be more hesitant to discharge a patient who has not fully completed treatment for fear he or she will still appear decompensated to the postdischarge provider (though greatly improved from admission), or will not have timely‐enough follow‐up to change treatment if the condition worsens.
Our finding that the LOS was still shorter when comparing PDC with UC suggests continuity may be a significant component of this effect. It seems unlikely that patients following up in PDC had less complex hospitalizations given similar ICU exposure and LOS, as well as older age and larger baseline comorbidity burden.
The LOS seen in patients who followed up in PDC was lower than Medicare rates[26] but similar to reported rates at other VA acute‐care hospitals.[27] It is consistent with prior findings that hospitalist care reduces LOS,[26] though the magnitude in our study was much larger than that in prior reports. Prior studies have suggested this decreased LOS is linked to increased adverse postdischarge outcomes, such as ED visits and readmissions, as well as increased costs and decreased discharges to home.[28] The PDC was not associated with increased postdischarge adverse events measured, though a formal cost analysis and analysis of other postdischarge outcomes, such as placement in a skilled nursing or rehabilitation facility after return home, could be assessed in future work.
The findings of our study should be interpreted in the context of the study design. Our study was retrospective, observational, and single‐center. There may have been additional baseline differences between groups predisposing to bias we did not capture in the propensity score. For example, we could not measure rates of attendance at the different clinics and cannot rule out that outcomes associated with PDC were also associated with increased attendance rates. However, none of the clinics had mechanisms in place to improve follow‐up rates; patients referred to PDC were those considered highest risk for readmission and were sicker at baseline, making it very unlikely that they were predisposed to attend clinic more frequently and/or to have better outcomes; and even if PDC improved follow‐up rates, this would be a significant contribution given the limitations of primary‐care access. Our propensity score could not perfectly mimic randomization to a treatment assignment, but rather to treatment received, because of this limitation.
We did not ascertain ED visits or readmissions outside the VA system; it is possible these differentially affected one group more than another, though this seems unlikely. Our patient population was representative of veteran populations elsewhere who are at high risk of adverse postdischarge outcomes, but our findings may not be generalizable to younger, more ethnically diverse populations or to women.
CONCLUSIONS
Provision of postdischarge care by hospitalists may reduce LOS without increasing postdischarge adverse events. Further work is required to evaluate the role of hospitalist‐run PDCs in healthcare systems with more limited postdischarge access to care, to formally evaluate the costs associated with extending hospitalists to the outpatient setting, and to prospectively evaluate the role of a PDC compared with other kinds of hospital follow‐up.
Acknowledgments
The authors thank Melver Anderson, MD, for editorial assistance with the manuscript.
Disclosures: Dr. Burke had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. Dr. Burke was supported by grant funding from the Colorado Research Enhancement Award Program to Improve Care Coordination for Veterans. Initial results of this study were presented at the Society of General Internal Medicine National Meeting in Denver, Colorado, April 24, 2013.
Currently, healthcare systems rarely provide ideal transitions of care for discharged patients,[1] resulting in fragmented care,[2, 3, 4, 5] significant patient uncertainty about how to manage at home,[6, 7] and frequent adverse events.[8, 9] These factors are so commonly experienced by discharged patients that they are recognizable as a postdischarge syndrome.[10]
One element important for reducing the postdischarge risk of adverse events is provision of adequate follow‐up.[11, 12] However, supplying this care is challenging in the modern era, and it will become progressively more difficult to achieve. In 2004, 50% of readmitted Medicare fee‐for‐service patients had no postdischarge visit within 30 days of their discharge,[9] likely due in part to difficulty arranging such care. Changes in insurance coverage and demographics are expected to result in more than 100 million newly insured patients by 2019, yet the primary‐care workforce is projected to begin shrinking by 2016.[13, 14] In the increasingly uncommon situation that a primary‐care clinician is available promptly after discharge, information transfer is often inadequate[4, 15, 16, 17] and can be exacerbated by the growing discontinuity between inpatient and outpatient care.[2, 3, 4] Efforts to increase the supply of primary‐care clinicians and thereby improve early access to postdischarge care are important for the future, but hospitals, particularly those penalized for high risk‐adjusted readmission rates, are seeking novel solutions now.
One increasingly common innovation is to extend the role of inpatient providers (usually hospitalists) into the postdischarge period.[18] Preliminary evidence suggests improved continuity[19] and access[20] achieved by providing this care may decrease postdischarge adverse events,[19, 20, 21] though evidence is conflicting.[22]
As a closed, multilevel healthcare system, the Denver VA Medical Center is uniquely positioned to evaluate the influence of alternative postdischarge‐care strategies on subsequent adverse events. Discharged patients are seen in a well‐established hospitalist‐run postdischarge clinic (PDC), a robust urgent‐care system (UC), or by a large primary‐care provider (PCP) practice. The purpose of this study was to evaluate whether patients seen in a hospitalist‐run PDC have reduced adverse outcomes in the 30 days following hospital discharge compared with follow‐up with the patient's PCP or in an UC clinic.
METHODS
Patients
This was a retrospective cohort study of consecutive adult patients discharged from the general medical services of the Denver VA Medical Center after a nonelective admission between January 2005 and August 2012. This time range was chosen because all 3 clinics were fully operational during this period. The Denver VA Medical Center is an academically‐affiliated 128‐bed hospital that provides a full range of tertiary services. All medical patients, including intensive care unit (ICU) patients, are cared for on general medical teams by University of Colorado housestaff with hospitalists or subspecialty attendings. Patients who lived in the Denver metropolitan area, were discharged home, and who followed up with a PCP, UC clinic, or PDC within 30 days of discharge were included. Patients discharged to subacute facilities, hospice, or this tends to be capitalized as a special program at our VA were excluded. For patients with multiple admissions, only the first was included.
Clinics
Primary Care
Primary‐care clinics in the VA system are organized into Patient‐Aligned Care Teams (PACTs) and are available for appointments 5 days per week. Patients discharged from the medical service who have PCPs are called within 48 hours of discharge by PACT nurses to evaluate their postdischarge state. Primary‐care physicians could be resident housestaff or ambulatory attending physicians. Seventy‐two percent of patients seen at the Denver VA have an assigned PCP.
Urgent Care
The Office‐based Medical Team provides UC and short‐term regular appointments for recently discharged medical patients or patients who require frequent follow‐up (such as those that require serial paracenteses). It is a separate clinic from an emergency department (ED)‐based walk‐in clinic. It is also available 5 days per week; patients are seen by resident housestaff unfamiliar with the patient, and the clinic is staffed with an ambulatory attending physician. Patients are commonly seen multiple times in the same clinic, though usually with different providers.
Postdischarge Clinic
The hospitalist‐run PDC is scheduled 2 afternoons per week. Patients are always seen by housestaff and medical students from the team that cared for them as an inpatient, then staffed with a rotating hospitalist attending who may have been the supervising inpatient attending during the patient's inpatient stay. Thus, continuity is preserved with the housestaff team in all cases, although attending continuity is variable. This is added to the daily responsibility of the resident and hospitalist physicians who are providing care on the inpatient service at the time of the clinic. Capabilities of the clinic are similar to UC and PCP clinics. Patients are usually seen once postdischarge with referral to the PCP for further follow‐up; however, patients can be seen multiple times by the same provider team.
If a patient followed up with multiple clinics, the first clinic visited determined the group to which that patient was allocated for the purpose of analysis. If a patient was scheduled for clinic follow‐up but did not attend within 30 days of discharge, he or she was excluded. We did not collect data on visits outside of these 3 clinics, as pilot data demonstrated they accounted for nearly all (>90%) of posthospitalization follow‐up visits. During the study period, there were no guidelines for discharging physicians about which clinic to have the patient follow up in. The UC and PDC were known to have better early access to follow‐up appointments and thus tended to see patients requiring early follow‐up in the judgment of the discharging clinician.
Statistical Analysis
The VA's Computing and Informatics Infrastructure (VINCI) was used to collect predischarge patient data for descriptive and analytic purposes. Pertinent potential confounders included patient age, sex, marital status, comorbidities, number of prescribed medications on discharge, previous hospital admissions in the last year, ICU admission (as a dichotomous variable), ICU length of stay (LOS), and hospital LOS. Postdischarge variables included time to first follow‐up appointment and hospital LOS if readmitted.
The primary outcome was a composite of ED visits, hospital readmissions, and mortality in the 30 days following hospital discharge. These outcomes were captured in the VA system; we did not measure outside utilization. A power analysis indicated that the sample has >90% power to detect small differences (4%) in the composite outcome between types of outpatient care. We also evaluated the effect of different types of follow‐up on the 3 individual components of the primary outcome. To compare baseline categorical variables across 3 groups, 2 trend tests were used; analysis of variance (ANOVA) or Kruskal‐Wallis test was used for continuous variables in univariate analysis.
We then used propensity scoring to adjust for baseline differences between groups in an attempt to adjust for referral bias, using multivariate logistic regression to calculate a propensity score for each patient in 2‐way comparisons, and a single score for every patient in a multinomial comparison.[23] Our final propensity score incorporated age, number of hospital admissions in the past year, and Elixhauser comorbidity score,[24] with excellent overlap in propensity scores between groups. Although hospital LOS was different between groups, inclusion in the propensity score did not reduce this significant difference, and its inclusion in the propensity model decreased model fit. Limitations of the accessible data prevented high‐dimensional propensity scoring and limited the outcome of the propensity score to attendance at the clinic assigned, rather than referral to the clinic assigned. The propensity score, hospital LOS, time to the first outpatient visit, and group assignment (PDC, PCP, UC) were entered into a multivariate logistic regression model.
To find a subgroup who may benefit most from follow‐up in the PDC, we a priori identified patients with one of the 5 discharge diagnosis‐related groups (DRGs) most commonly associated with subsequent readmission[9] and examined outcomes between the 3 different kinds of follow‐up, restricted to patients discharged with one of these diagnoses. All analyses were conducted using SAS 9.3 (SAS Institute, Inc., Cary, NC).
RESULTS
9952 patients who met criteria were discharged during this time period; however, 48.9% did not follow up with one of these clinics within 30 days, leaving 5085 patients in our analysis. Of these, 538 followed up in PDC (10.6%), 1848 followed up with their PCP (36.3%), and 2699 followed up in UC (53.1%). Table 1 presents predischarge characteristics of these patients. Patients seen in PDC were older and had a more significant comorbidity burden.
| PDC, N=538 | UC, N=2699 | PCP, N=1848 | P Value | P Value After Propensity Adjustment | |
|---|---|---|---|---|---|
| |||||
| Age, years (SD) | 67.8 (12.6) | 67.1 (13.0) | 64.8 (13.0) | <0.01 | 0.86 |
| Male sex, % | 95.0 | 95.4 | 94.4 | 0.33 | |
| Marital status, % | |||||
| Divorced | 40.2 | 36.2 | 35.0 | 0.09 | |
| Married | 35.9 | 37.3 | 39.8 | 0.13 | |
| Never married | 12.3 | 13.7 | 14.3 | 0.48 | |
| LOS, days (SD) | 3.8 (3.6) | 5.0 (11.7) | 6.2 (10.8) | 0.04 | |
| Elixhauser score (SD) | 0.80 (1.1) | 0.69 (1.0) | 0.75 (1.0) | 0.02 | 0.06 |
| Admitted to ICU, % | 19.0 | 19.9 | 23.0 | 0.12 | |
| ICU LOS, days (SD) | 2.8 (4.4) | 2.8 (3.4) | 2.3 (1.5) | 0.15 | |
| Discharge medications, mean (SD) | 10.0 (6.7) | 10.4 (7.4) | 10.4 (8.2) | 0.37 | |
| Admissions per patient in prior year, mean (SD) | 0.18 (0.5) | 0.21 (0.6) | 0.23 (0.6) | 0.08 | 0.78 |
Patients seen in PDC had a mean 2.4‐day shorter LOS than those seen by their PCPs (PDC: 3.8 days, UC: 5.0 days, PCP: 6.2 days; P=0.04 for comparison). Neither the percentage of patients admitted to the ICU during their index hospitalization nor the ICU LOS was different between groups. Patients were seen earlier postdischarge in PDC than in other types of follow‐up (PDC: 5.0 days, UC: 9.4 days, PCP: 13.7 days; P<0.01 for comparison). In univariate analysis, there was no difference between groups in the composite 30‐day outcome (Table 2). Analysis of the individual components of the primary outcome revealed significant differences in readmission rates, with PDC having the highest rate.
| PDC | UC | PCP | P Value | P Value After Propensity Adjustment | |
|---|---|---|---|---|---|
| |||||
| Composite outcome, % | 19.9 | 18.3 | 17.5 | 0.42 | 0.30 |
| Hospital readmission | 13.0 | 11.1 | 9.4 | 0.03 | 0.03 |
| ED visit | 10.2 | 9.9 | 10.5 | 0.78 | 0.93 |
| Mortality | 1.1 | 0.7 | 0.7 | 0.58 | 0.65 |
| LOS if readmitted, days (SD) | 6.9 (18.1) | 4.9 (7.8) | 4.8 (6.5) | 0.28 | 0.23 |
| Time to first visit after discharge, days (SD) | 5.0 (3.0) | 9.4 (6.1) | 13.8 (8.5) | <0.01 | <0.01 |
Univariate analyses conducted on predischarge characteristics after multinomial propensity scoring revealed significant differences between groups no longer existed for the variables that were included in the propensity score (age, Elixhauser score, and inpatient stays prior to visit; Table 1).
In multivariate analysis comparing PDC to PCP follow‐up, there was no difference in the composite outcome after controlling for propensity score and time to outpatient visit (odds ratio [OR]: 1.07, 95% confidence interval [CI]: 0.81‐1.40). Similar results were obtained in comparing PDC with UC (OR: 1.05, 95% CI: 0.82‐1.34) and in multinomial logistic regression comparing PDC with other types of follow‐up (PDC vs PCP: OR: 1.01, 95% CI: 0.78‐1.31; PDC vs UC: OR: 0.99, 95% CI: 0.78‐1.26).
Restricting the multivariate analysis to those patients discharged with one of the 5 discharge DRGs most associated with readmission did not alter our findings regarding the primary outcome. We also found no change in the composite outcome or any subcomponent of the composite outcome when restricting the analysis to 7‐day outcomes or when excluding scheduled readmissions (which represented <5% of all readmission).
DISCUSSION
A hospitalist‐run postdischarge clinic did not reduce a composite of 30‐day postdischarge adverse outcomes in our study when compared with primary‐care or urgent‐care follow‐up. In fact, patients who followed up in PDC had a small increase in 30‐day readmissions. However, they also were sicker at baseline, considered higher risk by the discharging physician, were able to be seen significantly earlier, and had an associated 2.4‐day shorter hospital LOS than patients seen by their PCPs.
Our findings do not confirm those of prior research in this area, which indicated outpatient follow‐up by the same physician who was the treating inpatient physician was linked to lower mortality rates, hospital readmissions, and ED utilization.[19, 20, 21] In fact, in our study, there was a significant (albeit small) increase in 30‐day readmissions in patients seen in PDC. There are significant challenges to the generalizability and validity of these prior studies. In one study, inpatient care was provided by outpatient primary‐care doctors in Canada,[19] a payer and care model rare in the United States.[25] In a second, usual care was not specified, and it is likely the reduction in ED visits resulted from provision of follow‐up care of any kind compared with those who did not follow up after discharge.[20] In a third, the PDC was part of a larger bundle of postdischarge interventions, and it only reduced ED visits when compared with patients who did not have follow‐up; rates of ED visits were similar in a comparison with PCP follow‐up.[21]
There are several possible explanations for the lack of improvement in 30‐day adverse outcomes with a hospitalist‐run PDC. First, although early access to early postdischarge care was improved and evidence suggests this is important in reducing readmissions,[11, 12] in populations similar to that studied, more postdischarge care has also been linked to increased readmissions.[22] This may be due to more frequent re‐evaluation of fragile, chronically ill patients, presenting more options for readmission. Second, the intervention currently only addresses some components of the Ideal Transition of Care[1] (Figure 1) and may benefit from an enhanced visit structure using a multidisciplinary approach. Third, the intervention took place in the context of a robust primary‐care system with a universal electronic medical record; the effects of improved access and continuity may be magnified in a system without these advantages. Fourth, there was a low readmission rate overall, and it is unclear how many of these readmissions were preventable. Finally, it may be that although the initial postdischarge care was adequate, readmissions occurred after the first visit, suggesting subsequent care during the 30 days postdischarge could have been improved.

The most likely explanation for the substantially decreased LOS associated with follow‐up in PDC is that inpatient physicians who knew they could see their own patients early in the postdischarge process were more tolerant of uncertainty surrounding the patients' clinical course.
For example, a frequent clinical conundrum for hospitalists is when to discharge patients improving on diuretic therapy for a heart failure exacerbation or antibiotics for cellulitis. Provided a PDC, these hospitalists may choose to discharge a patient still actively being treated, because they may feel they have access to early follow‐up to change course if needed as well as the ability to see the patient themselves, allowing precise evaluation of the change in their condition. Without this clinic, the hospitalist may wonder when postdischarge follow‐up will occur. They may be more hesitant to discharge a patient who has not fully completed treatment for fear he or she will still appear decompensated to the postdischarge provider (though greatly improved from admission), or will not have timely‐enough follow‐up to change treatment if the condition worsens.
Our finding that the LOS was still shorter when comparing PDC with UC suggests continuity may be a significant component of this effect. It seems unlikely that patients following up in PDC had less complex hospitalizations given similar ICU exposure and LOS, as well as older age and larger baseline comorbidity burden.
The LOS seen in patients who followed up in PDC was lower than Medicare rates[26] but similar to reported rates at other VA acute‐care hospitals.[27] It is consistent with prior findings that hospitalist care reduces LOS,[26] though the magnitude in our study was much larger than that in prior reports. Prior studies have suggested this decreased LOS is linked to increased adverse postdischarge outcomes, such as ED visits and readmissions, as well as increased costs and decreased discharges to home.[28] The PDC was not associated with increased postdischarge adverse events measured, though a formal cost analysis and analysis of other postdischarge outcomes, such as placement in a skilled nursing or rehabilitation facility after return home, could be assessed in future work.
The findings of our study should be interpreted in the context of the study design. Our study was retrospective, observational, and single‐center. There may have been additional baseline differences between groups predisposing to bias we did not capture in the propensity score. For example, we could not measure rates of attendance at the different clinics and cannot rule out that outcomes associated with PDC were also associated with increased attendance rates. However, none of the clinics had mechanisms in place to improve follow‐up rates; patients referred to PDC were those considered highest risk for readmission and were sicker at baseline, making it very unlikely that they were predisposed to attend clinic more frequently and/or to have better outcomes; and even if PDC improved follow‐up rates, this would be a significant contribution given the limitations of primary‐care access. Our propensity score could not perfectly mimic randomization to a treatment assignment, but rather to treatment received, because of this limitation.
We did not ascertain ED visits or readmissions outside the VA system; it is possible these differentially affected one group more than another, though this seems unlikely. Our patient population was representative of veteran populations elsewhere who are at high risk of adverse postdischarge outcomes, but our findings may not be generalizable to younger, more ethnically diverse populations or to women.
CONCLUSIONS
Provision of postdischarge care by hospitalists may reduce LOS without increasing postdischarge adverse events. Further work is required to evaluate the role of hospitalist‐run PDCs in healthcare systems with more limited postdischarge access to care, to formally evaluate the costs associated with extending hospitalists to the outpatient setting, and to prospectively evaluate the role of a PDC compared with other kinds of hospital follow‐up.
Acknowledgments
The authors thank Melver Anderson, MD, for editorial assistance with the manuscript.
Disclosures: Dr. Burke had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. Dr. Burke was supported by grant funding from the Colorado Research Enhancement Award Program to Improve Care Coordination for Veterans. Initial results of this study were presented at the Society of General Internal Medicine National Meeting in Denver, Colorado, April 24, 2013.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109.
- , , , , , . Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults. JAMA. 2009;301(16):1671–1680.
- , , , , . Trends in inpatient continuity of care for a cohort of Medicare patients 1996–2006. J Hosp Med. 2011;6(8):438–444.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , . Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449–1465.
- , , , , . Patient experiences of transitioning from hospital to home: an ethnographic quality improvement project. J Hosp Med. 2012;7(5):382–387.
- , , , et al. Understanding and execution of discharge instructions. Am J Med Qual. 2013;28(5):383–391.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- . Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102.
- , , , et al. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716–1722.
- , , . Post‐hospitalization transitions: examining the effects of timing of primary care provider follow‐up. J Hosp Med. 2010;5(7):392–397.
- , , . Will generalist physician supply meet demands of an increasing and aging population? Health Aff (Millwood). 2008;27(3):w232–w241.
- Association of American Medical Colleges. The Impact of Health Care Reform on the Future Supply and Demand for Physicians: Updated Projections Through 2025. Available at: http://www.aamc.org/download/158076/data/updated_projections_through_2025.pdf. Published June 2010. Accessed May 1, 2012.
- , , , . Effect of discharge summary availability during post‐discharge visits on hospital readmission. J Gen Intern Med. 2002;17(3):186–192.
- , , , et al. Comprehensive quality of discharge summaries at an academic medical center. J Hosp Med. 2013;8(8):436–443.
- , , , et al. Association of communication between hospital‐based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381–386.
- . Is a post‐discharge clinic in your hospital's future? Available at: http://www.the‐hospitalist.org/details/article/1409011/Is_a_Post‐Discharge_Clinic_in_Your_Hospitals_Future.html. Published December 2011. Accessed May 1, 2013.
- , , , . Continuity of care and patient outcomes after hospital discharge. J Gen Intern Med. 2004;19(6):624–631.
- , , , . Effects of a postdischarge clinic on housestaff satisfaction and utilization of hospital services. J Gen Intern Med. 1996;11(3):179–181.
- , , , , , . Integrated postdischarge transitional care in a hospitalist system to improve discharge outcome: an experimental study. BMC Med. 2011;9:96.
- , , . Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med. 1996;334(22):1441–1447.
- , . An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J. 2011;32(14):1704–1708.
- , , . Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004;42(4):355–360.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . Association of hospitalist care with medical utilization after discharge: evidence of cost shift from a cohort study. Ann Intern Med. 2011;155(3):152–159.
- , , , et al. Associations between reduced hospital length of stay and 30‐day readmission rate and mortality: 14‐year experience in 129 Veterans Affairs hospitals. Ann Intern Med. 2012;157(12):837–845.
- , , , . Variation in length of stay and outcomes among hospitalized patients attributable to hospitals and hospitalists. J Gen Intern Med. 2013;28(3):370–376.
- , , , . Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109.
- , , , , , . Continuity of outpatient and inpatient care by primary care physicians for hospitalized older adults. JAMA. 2009;301(16):1671–1680.
- , , , , . Trends in inpatient continuity of care for a cohort of Medicare patients 1996–2006. J Hosp Med. 2011;6(8):438–444.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , . Posthospital care transitions: patterns, complications, and risk identification. Health Serv Res. 2004;39(5):1449–1465.
- , , , , . Patient experiences of transitioning from hospital to home: an ethnographic quality improvement project. J Hosp Med. 2012;7(5):382–387.
- , , , et al. Understanding and execution of discharge instructions. Am J Med Qual. 2013;28(5):383–391.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , . Rehospitalizations among patients in the Medicare fee‐for‐service program. N Engl J Med. 2009;360(14):1418–1428.
- . Post‐hospital syndrome—an acquired, transient condition of generalized risk. N Engl J Med. 2013;368(2):100–102.
- , , , et al. Relationship between early physician follow‐up and 30‐day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303(17):1716–1722.
- , , . Post‐hospitalization transitions: examining the effects of timing of primary care provider follow‐up. J Hosp Med. 2010;5(7):392–397.
- , , . Will generalist physician supply meet demands of an increasing and aging population? Health Aff (Millwood). 2008;27(3):w232–w241.
- Association of American Medical Colleges. The Impact of Health Care Reform on the Future Supply and Demand for Physicians: Updated Projections Through 2025. Available at: http://www.aamc.org/download/158076/data/updated_projections_through_2025.pdf. Published June 2010. Accessed May 1, 2012.
- , , , . Effect of discharge summary availability during post‐discharge visits on hospital readmission. J Gen Intern Med. 2002;17(3):186–192.
- , , , et al. Comprehensive quality of discharge summaries at an academic medical center. J Hosp Med. 2013;8(8):436–443.
- , , , et al. Association of communication between hospital‐based physicians and primary care providers with patient outcomes. J Gen Intern Med. 2009;24(3):381–386.
- . Is a post‐discharge clinic in your hospital's future? Available at: http://www.the‐hospitalist.org/details/article/1409011/Is_a_Post‐Discharge_Clinic_in_Your_Hospitals_Future.html. Published December 2011. Accessed May 1, 2013.
- , , , . Continuity of care and patient outcomes after hospital discharge. J Gen Intern Med. 2004;19(6):624–631.
- , , , . Effects of a postdischarge clinic on housestaff satisfaction and utilization of hospital services. J Gen Intern Med. 1996;11(3):179–181.
- , , , , , . Integrated postdischarge transitional care in a hospitalist system to improve discharge outcome: an experimental study. BMC Med. 2011;9:96.
- , , . Does increased access to primary care reduce hospital readmissions? Veterans Affairs Cooperative Study Group on Primary Care and Hospital Readmission. N Engl J Med. 1996;334(22):1441–1447.
- , . An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J. 2011;32(14):1704–1708.
- , , . Comparison of the Elixhauser and Charlson/Deyo methods of comorbidity measurement in administrative data. Med Care. 2004;42(4):355–360.
- , , , . Growth in the care of older patients by hospitalists in the United States. N Engl J Med. 2009;360(11):1102–1112.
- , . Association of hospitalist care with medical utilization after discharge: evidence of cost shift from a cohort study. Ann Intern Med. 2011;155(3):152–159.
- , , , et al. Associations between reduced hospital length of stay and 30‐day readmission rate and mortality: 14‐year experience in 129 Veterans Affairs hospitals. Ann Intern Med. 2012;157(12):837–845.
- , , , . Variation in length of stay and outcomes among hospitalized patients attributable to hospitals and hospitalists. J Gen Intern Med. 2013;28(3):370–376.
© 2013 Society of Hospital Medicine