User login
No laughing matter: Laughter is good psychiatric medicine
CASE REPORT: Laughter as therapy
Mrs. A is a 56-year-old married woman who has bipolar disorder. She has survived several suicide attempts. Her family history is positive for bipolar disorder and completed suicides.
After her most recent suicide attempt and a course of electroconvulsive therapy, Mrs. A recovered sufficiently to begin a spiritual journey that led her to practice a technique known as Laughter Yoga (Box) and, eventually, to become a Laughter Yoga instructor.
Mrs. A begins Laughter Yoga sessions by talking openly with students about her illness and the beneficial effects that laughter therapy has had on its course: She once had at least two major bipolar episodes a year, she explains, but has been in full remission for several years despite severe psychosocial stressors. In addition to practicing Laughter Yoga, Mrs. A is now maintained on a mood stabilizer that failed in the past to control her mood cycles.
Does laughter have a place in your practice?
It is said that laughter is good medicine—but is it good psychiatric medicine? Where might humor and laughter fit in the psychiatrist’s armamentarium? Is laughter physiologically beneficial to psychiatric patients? And are there adverse effects or contraindications to laughter in psychiatry? This article:
• reviews studies that have examined the anatomy, physiology, and psychology of humor and laughtera
• offers answers to the questions posed above (Table).
“Gelotology,” from the Greek “gelos,” laughter, is the science of laughter. The three components of humor and laughter are:
• the emotional component, which triggers emotions produced by a humorous situation
• the cognitive component, in which a person “gets it”
• the movement of facial, respiratory, and abdominal muscles.
Furthermore, tension and surprise are needed for laughter.
Theories about humor are varied
Philosophers since Plato have proposed theories of humor; modern theories of humor can be traced to Freud’s work.1 The psychoanalytic literature on humor focuses on the role of humor in sublimation of feelings of anger and hostility, while releasing affect in an economical way.
Erikson also wrote about the role of humor in a child’s developing superego, which helps resolve the conflict with maternal authority.2
In a comprehensive review of theories of humor, Krichtafovitch explains that cognitive theories address the role of incongruity and contrast in the induction of laughter, whereas social theories explore the roles of aggression, hostility, superiority, triumph, derision, and disparagement in humor and laughter. The effect of humor, Krichtafovitch explains, is to elevate the social status of the joker while the listener’s social status is lifted through his (her) ability to “get it.” Thus, humor plays a meaningful role in creating a bond between speaker and listener.3
The neuroanatomy of laughter
Here is some of what we have learned about mapping the brain to the basis of laughter:
• Consider a 16-year-old girl who underwent neurosurgery for intractable seizures. During surgery, various parts of the brain were stimulated to test for the focus of the seizures. She laughed every time the left frontal superior gyrus was stimulated. According to the report, she apparently laughed first, then made up a story that was funny to her.4
• Pseudobulbar affect—excessive, usually incongruent laughter, secondary to neurologic disease or traumatic brain injury—is an example of the biologic basis of laughter.
• Many functional brain imaging studies of laughter have been published.5 These studies show involvement of various regions of the brain in laughter, including the amygdala, hypothalamus, and temporal and cerebellar regions.
• Sex differences also have been noted in the neuroanatomy of laughter. Females activate the left prefrontal cortex more than males do, suggesting a greater degree of executive processing and language-based decoding. Females also exhibit greater activation of mesolimbic regions, including the nucleus accumbens, implying a greater reward network response.6
• Wild et al7 reported that separate cortical regions are responsible for the production of facial expressions that are emotionally driven (through laughter) and voluntary.
The physiology of laughter
Humans begin to laugh at approximately 4 months of age. Children laugh, on average, 400 times a day; adults do so an average of only 5 times a day.8 In addition:
• Tickling a baby induces her (him) to laugh, which, in turn, makes the parent laugh; a social bond develops during this playful exercise. This response is probably mediated by 5-HT1A receptors, which, when stimulated, induces the release of oxytocin, which facilitates social bonding.9
• Potent stimulation of 5-HT1A receptors through ingestion of 3,4-methylenedioxy-N-methylamphetamine (Ecstasy) leads to uncontrollable laughter and mirth.10
• Lower species are also known to enjoy humor. Mice emit a chirping sound when tickled, and laughter is contagious among monkeys.11
• Berk et al12,13 reported that, when 52 healthy men watched a funny video for 30 minutes, they had significantly higher activity of natural killer (NK) cells and higher levels of IgG, IgA, and IgM compared with men who watched an emotionally neutral documentary.
• Bennett et al14 showed that, in 33 healthy women, the harder the laughter, the higher the NK activity.
• Sugawara et al15 showed improved cardiovascular function in 17 healthy persons (age 23 to 42) who watched a 30-minute comedy video, compared with their cardiovascular function when they watched a documentary video of equal length.
• Svebak et al16 examined the effect of humor as measured by the Sense of Humor Survey on the survival rate of more then 53,000 adults in one county in Norway. They concluded that the higher the sense of humor score, the higher the odds ratio of surviving 7 years, compared with subjects who had a lower sense of humor.
Clinical studies of laughter
The Coping Humor Scale (CHS) and the Humor Response Scale (HRS) are the two most widely used tools to measure a person’s innate sense of humor (the CHS) and the ability to respond to a humorous situation (the HRS).17 Several studies about the effects of laughter on illness are notable:
• Laughter increased NK cell activity, lowered prorenin gene expression, and lowered the postprandial glucose level in 34 patients with diabetes, compared with 16 matched controls.18-21
• Clark et al studied the sense of humor of 150 patients with cardiac disease compared with 150 controls. They found that “people with heart disease responded less humorously to everyday life situations.” They generally laughed less, even in positive situations, and displayed more anger and hostility.22
• In his work on the salutatory effect of laughter on the experience of pain, Cousins described how he dealt with his painful arthritis by watching Marx Brothers movies23:
I made the joyous discovery that 10 minutes of genuine belly laughter had an anesthetic effect and would give me at least two hours of pain-free sleep… When the pain-killing effect of the laughter wore off, we would switch on the motion picture projector again and not infrequently, it would lead to another pain-free interval.
• Hearty laughter leads to pain relief, probably through the release of endorphins. Dunbar et al24 tested this hypothesis in a series of six experimental studies in the laboratory (watching videos) and in a naturalistic context (watching stage performances), using a change in pain threshold as an indirect measure of endorphin release. The results show that the pain threshold is significantly higher after laughter than in the control condition. This pain-tolerance effect is caused by the laughter itself, not simply because of a change in positive affect.
Laughter therapy for depression
Three studies have demonstrated the benefit of laughter therapy in depression:
• When Ko and Youn25 studied 48 geriatric depressed patients and 61 age-matched controls, they found a significantly lower Geriatric Depression Scale score and a better Pittsburgh Sleep Quality Index score in patients who had been exposed to four weekly laughter groups, compared with persons who had been exposed to a control group.
• Shahidi et al26 randomly assigned 60 community-dwelling female, geriatric, depressed patients to a laughter yoga group, an exercise group, and a control group. Laughter yoga and exercise were equally effective, and both were significantly superior to the control condition. The laughter yoga group scored significantly better than the other two groups on the Life Satisfaction Scale. The researchers concluded that, in addition to improved mood, patients who laugh experience increased life satisfaction.
• Fonzi et al27 summarized data on the neurophysiology of laughter and the effect of laughter on the hypothalamus-pituitary-adrenal axis. They noted that depression reduces the frequency of laughter and, inversely, laughter reduces the severity of depression. Laughter, they reported, also increases the connectivity of patients with people in their life, which further alleviates symptoms of depression.
Other therapeutic uses of laughter
Humor can strengthen the bond of the therapeutic relationship. Patients who laugh with their physicians are more likely to feel connected with them, follow their advice, and feel more satisfied with their encounter. One study found that primary care physicians who gave positive statements, spent more time with patients, and included humor or laughter during their visits lowered their risk of being sued for malpractice.28
Consider also the use of laughter in altering family dynamics in a therapeutic setting: Mr. and Mrs. B attend therapy in my practice to address a difficult situation with their adult children. One of them enables their children socially and financially; the other continually complains about this enabling. When the tension was high and the couple had reached an impasse during a visit, the therapist offered an anecdote from the 2006 motion picture Failure to Launch (in which a man lives in the security of his parents’ home even though he is in his 30s), that dissipated the hostility they had shown toward each other and toward their children. The couple was then able to proceed to conflict resolution.
Recommendations, caveats
If you are considering incorporating laughter into therapy, keep in mind that:
• you should ensure that the patient does not perceive humor as minimizing the seriousness of their problems
• humor can be a minefield if not used judiciously, or if used at all, around certain sensitive topics, such as race, ethnicity, religion, political affiliation, and sexual orientation
• the timing of humor is particularly essential for it to succeed in the context of a therapeutic relationship
• from a medical perspective, laughter in patients who are recovering from abdominal or other major surgery might compromise wound healing because of increased intra-abdominal pressure associated with laughing
• patients who have asthma, especially exercise-induced asthma, might be at risk of developing an acute asthmatic attack when they laugh very hard. Lebowitz et al29 demonstrated that laughter can have a negative effect on patients with chronic obstructive pulmonary disease.
It is advisable in some situations to avoid humor in psychotherapy, such as when the patient or family is hostile—because, as noted, they might perceive laughter and humor as an attempt to minimize the seriousness of their discontent.
Bottom Line
Humor and laughter are underutilized and underreported in therapy, in part because it is a nascent field of research. Laughter has social and physiologic benefits that can be used in the context of a therapeutic relationship to help patients with a variety of ailments, including depression, anxiety, and pain.
Related Resources
- Association for Applied and Therapeutic Humor. www.aath.org.
- Mora-Ripoll R. The therapeutic value of laughter in medicine. Altern Ther Health Med. 2010;16:56-64.
- Strean WB. Laughter prescription. Can Fam Physician. 2009;55:965-967.
Disclosure
Dr. Nasr reports no financial relationship with manufacturers of any products mentioned in this article or with manufacturers of competing products.
Acknowledgements
The author acknowledges the assistance of Francois E. Alouf, MD, for suggestions on topics to include in the article; John W. Crayton, MD, for reviewing the manuscript; and Burdette Wendt for assistance with the references.
1. Freud S, Strachey J, trans., ed. Jokes and their relation to the unconscious. New York, NY: W. W. Norton & Company; 1990.
2. Capps D. Mother, melancholia, and humor in Erik H. Erikson’s earliest writings. J Relig Health. 2008;47:415-432.
3. Krichtafovitch I. Humor theory. Parker, CO: Outskirts Press; 2006.
4. Fried I, Wilson CL, MacDonald KA, et al. Electric current stimulates laughter. Nature. 1998;12;391:650.
5. Bartolo A, Benuzzi F, Nocetti L, et al. Humor comprehension and appreciation: an FMRI study. J Cogn Neurosci. 2006;18:1789-1798.
6. Azim E, Mobbs D, Jo B, et al. Sex differences in brain activation elicited by humor. Proc Natl Acad Sci U S A. 2005;102:16496-16501.
7. Wild B, Rodden FA, Rapp A, et al. Humor and smiling: cortical regions selective for cognitive, affective, and volitional components. Neurology. 2006;66:887-893.
8. Freedman LW. Mosby’s complementary and alternative medicine. A research-based approach. St. Louis, MO: Mosby; 2004:24.
9. Lukas M, Toth I, Reber SO, et al. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:
2159-2168.
10. Thompson MR, Callaghan PD, Hunt GE, et al. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience. 2007;146:509-514.
11. Ross MD, Owren MJ, Zimmermann E. The evolution of laughter in great apes and humans. Commun Integr Biol. 2010;3(2):191-194.
12. Berk LS, Tan SA, Fry WF, et al. Neuroendocrine and stress hormone changes during mirthful laughter. Am J Med Sci. 1989;298:390-396.
13. Berk LS, Felten DL, Tan SA, et al. Modulation of neuroimmune parameters during the eustress of humor-associated mirthful laughter. Altern Ther Health Med. 2001; 7:62-72,74-76.
14. Bennett MP, Zeller JM, Rosenberg L, et al. The effect of mirthful laughter on stress and natural killer cell activity. Altern Ther Health Med. 2003;9:38-45.
15. Sugawara J, Tarumi T, Tanaka H. Effect of mirthful laughter on vascular function. Am J Cardiol. 2010;106:856-859.
16. Svebak S, Romundstad S, Holmen J. A 7-year prospective study of sense of humor and mortality in an adult county population: the HUNT-2 study. Int J Psychiatry Med. 2010;40:125-146.
17. Martin RA. The Situational Humor Response Questionnaire (SHRQ) and Coping Humor Scale (CHS): a decade of research findings. Humor: International Journal of Humor Research. 1996;9(3-4):251-272.
18. Hayashi T, Urayama O, Hori M, et al. Laughter modulates prorenin receptor gene expression in patients with type 2 diabetes. J Psychosom Res. 2007;62:703-706.
19. Hayashi T, Murakami K. The effects of laughter on post-prandial glucose levels and gene expression in type 2 diabetic patients. Life Sci. 2009;85:185-187.
20. Takahashi K, Iwase M, Yamashita K, et al. The elevation of natural killer cell activity induced by laughter in a crossover designed study. Int J Mol Med. 2001;8:645-650.
21. Nasir UM, Iwanaga S, Nabi AH, et al. Laughter therapy modulates the parameters of renin-angiotensin system in patients with type 2 diabetes. Int J Mol Med. 2005;16:1077-1081.
22. Clark A, Seidler A, Miller M. Inverse association between sense of humor and coronary heart disease. Int J Cardiol. 2001;80:87-88.
23. Cousins N. The anatomy of an illness as perceived by the patient: reflections on healing and regeneration. New York, NY: Norton; 1979:39.
24. Dunbar RI, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
25. Ko HJ, Youn CH. Effects of laughter therapy on depression, cognition and sleep among the community-dwelling elderly. Geriatr Gerontol Int. 2011;11:267-274.
26. Shahidi M, Mojtahed A, Modabbernia A, et al. Laughter yoga versus group exercise program in elderly depressed women: a randomized controlled trial. Int J Geriatr Psychiatry. 2011;26:322-327.
27. Fonzi L, Matteucci G, Bersani G. Laughter and depression: hypothesis of pathogenic and therapeutic correlation. Riv Psichiatr. 2010;45:1-6.
28. Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
29. Lebowitz KR, Suh S, Diaz PT, et al. Effects of humor and laughter on psychological functioning, quality of life, health status, and pulmonary functioning among patients with chronic obstructive pulmonary disease: a preliminary investigation. Heart Lung. 2011;40:310-319.
CASE REPORT: Laughter as therapy
Mrs. A is a 56-year-old married woman who has bipolar disorder. She has survived several suicide attempts. Her family history is positive for bipolar disorder and completed suicides.
After her most recent suicide attempt and a course of electroconvulsive therapy, Mrs. A recovered sufficiently to begin a spiritual journey that led her to practice a technique known as Laughter Yoga (Box) and, eventually, to become a Laughter Yoga instructor.
Mrs. A begins Laughter Yoga sessions by talking openly with students about her illness and the beneficial effects that laughter therapy has had on its course: She once had at least two major bipolar episodes a year, she explains, but has been in full remission for several years despite severe psychosocial stressors. In addition to practicing Laughter Yoga, Mrs. A is now maintained on a mood stabilizer that failed in the past to control her mood cycles.
Does laughter have a place in your practice?
It is said that laughter is good medicine—but is it good psychiatric medicine? Where might humor and laughter fit in the psychiatrist’s armamentarium? Is laughter physiologically beneficial to psychiatric patients? And are there adverse effects or contraindications to laughter in psychiatry? This article:
• reviews studies that have examined the anatomy, physiology, and psychology of humor and laughtera
• offers answers to the questions posed above (Table).
“Gelotology,” from the Greek “gelos,” laughter, is the science of laughter. The three components of humor and laughter are:
• the emotional component, which triggers emotions produced by a humorous situation
• the cognitive component, in which a person “gets it”
• the movement of facial, respiratory, and abdominal muscles.
Furthermore, tension and surprise are needed for laughter.
Theories about humor are varied
Philosophers since Plato have proposed theories of humor; modern theories of humor can be traced to Freud’s work.1 The psychoanalytic literature on humor focuses on the role of humor in sublimation of feelings of anger and hostility, while releasing affect in an economical way.
Erikson also wrote about the role of humor in a child’s developing superego, which helps resolve the conflict with maternal authority.2
In a comprehensive review of theories of humor, Krichtafovitch explains that cognitive theories address the role of incongruity and contrast in the induction of laughter, whereas social theories explore the roles of aggression, hostility, superiority, triumph, derision, and disparagement in humor and laughter. The effect of humor, Krichtafovitch explains, is to elevate the social status of the joker while the listener’s social status is lifted through his (her) ability to “get it.” Thus, humor plays a meaningful role in creating a bond between speaker and listener.3
The neuroanatomy of laughter
Here is some of what we have learned about mapping the brain to the basis of laughter:
• Consider a 16-year-old girl who underwent neurosurgery for intractable seizures. During surgery, various parts of the brain were stimulated to test for the focus of the seizures. She laughed every time the left frontal superior gyrus was stimulated. According to the report, she apparently laughed first, then made up a story that was funny to her.4
• Pseudobulbar affect—excessive, usually incongruent laughter, secondary to neurologic disease or traumatic brain injury—is an example of the biologic basis of laughter.
• Many functional brain imaging studies of laughter have been published.5 These studies show involvement of various regions of the brain in laughter, including the amygdala, hypothalamus, and temporal and cerebellar regions.
• Sex differences also have been noted in the neuroanatomy of laughter. Females activate the left prefrontal cortex more than males do, suggesting a greater degree of executive processing and language-based decoding. Females also exhibit greater activation of mesolimbic regions, including the nucleus accumbens, implying a greater reward network response.6
• Wild et al7 reported that separate cortical regions are responsible for the production of facial expressions that are emotionally driven (through laughter) and voluntary.
The physiology of laughter
Humans begin to laugh at approximately 4 months of age. Children laugh, on average, 400 times a day; adults do so an average of only 5 times a day.8 In addition:
• Tickling a baby induces her (him) to laugh, which, in turn, makes the parent laugh; a social bond develops during this playful exercise. This response is probably mediated by 5-HT1A receptors, which, when stimulated, induces the release of oxytocin, which facilitates social bonding.9
• Potent stimulation of 5-HT1A receptors through ingestion of 3,4-methylenedioxy-N-methylamphetamine (Ecstasy) leads to uncontrollable laughter and mirth.10
• Lower species are also known to enjoy humor. Mice emit a chirping sound when tickled, and laughter is contagious among monkeys.11
• Berk et al12,13 reported that, when 52 healthy men watched a funny video for 30 minutes, they had significantly higher activity of natural killer (NK) cells and higher levels of IgG, IgA, and IgM compared with men who watched an emotionally neutral documentary.
• Bennett et al14 showed that, in 33 healthy women, the harder the laughter, the higher the NK activity.
• Sugawara et al15 showed improved cardiovascular function in 17 healthy persons (age 23 to 42) who watched a 30-minute comedy video, compared with their cardiovascular function when they watched a documentary video of equal length.
• Svebak et al16 examined the effect of humor as measured by the Sense of Humor Survey on the survival rate of more then 53,000 adults in one county in Norway. They concluded that the higher the sense of humor score, the higher the odds ratio of surviving 7 years, compared with subjects who had a lower sense of humor.
Clinical studies of laughter
The Coping Humor Scale (CHS) and the Humor Response Scale (HRS) are the two most widely used tools to measure a person’s innate sense of humor (the CHS) and the ability to respond to a humorous situation (the HRS).17 Several studies about the effects of laughter on illness are notable:
• Laughter increased NK cell activity, lowered prorenin gene expression, and lowered the postprandial glucose level in 34 patients with diabetes, compared with 16 matched controls.18-21
• Clark et al studied the sense of humor of 150 patients with cardiac disease compared with 150 controls. They found that “people with heart disease responded less humorously to everyday life situations.” They generally laughed less, even in positive situations, and displayed more anger and hostility.22
• In his work on the salutatory effect of laughter on the experience of pain, Cousins described how he dealt with his painful arthritis by watching Marx Brothers movies23:
I made the joyous discovery that 10 minutes of genuine belly laughter had an anesthetic effect and would give me at least two hours of pain-free sleep… When the pain-killing effect of the laughter wore off, we would switch on the motion picture projector again and not infrequently, it would lead to another pain-free interval.
• Hearty laughter leads to pain relief, probably through the release of endorphins. Dunbar et al24 tested this hypothesis in a series of six experimental studies in the laboratory (watching videos) and in a naturalistic context (watching stage performances), using a change in pain threshold as an indirect measure of endorphin release. The results show that the pain threshold is significantly higher after laughter than in the control condition. This pain-tolerance effect is caused by the laughter itself, not simply because of a change in positive affect.
Laughter therapy for depression
Three studies have demonstrated the benefit of laughter therapy in depression:
• When Ko and Youn25 studied 48 geriatric depressed patients and 61 age-matched controls, they found a significantly lower Geriatric Depression Scale score and a better Pittsburgh Sleep Quality Index score in patients who had been exposed to four weekly laughter groups, compared with persons who had been exposed to a control group.
• Shahidi et al26 randomly assigned 60 community-dwelling female, geriatric, depressed patients to a laughter yoga group, an exercise group, and a control group. Laughter yoga and exercise were equally effective, and both were significantly superior to the control condition. The laughter yoga group scored significantly better than the other two groups on the Life Satisfaction Scale. The researchers concluded that, in addition to improved mood, patients who laugh experience increased life satisfaction.
• Fonzi et al27 summarized data on the neurophysiology of laughter and the effect of laughter on the hypothalamus-pituitary-adrenal axis. They noted that depression reduces the frequency of laughter and, inversely, laughter reduces the severity of depression. Laughter, they reported, also increases the connectivity of patients with people in their life, which further alleviates symptoms of depression.
Other therapeutic uses of laughter
Humor can strengthen the bond of the therapeutic relationship. Patients who laugh with their physicians are more likely to feel connected with them, follow their advice, and feel more satisfied with their encounter. One study found that primary care physicians who gave positive statements, spent more time with patients, and included humor or laughter during their visits lowered their risk of being sued for malpractice.28
Consider also the use of laughter in altering family dynamics in a therapeutic setting: Mr. and Mrs. B attend therapy in my practice to address a difficult situation with their adult children. One of them enables their children socially and financially; the other continually complains about this enabling. When the tension was high and the couple had reached an impasse during a visit, the therapist offered an anecdote from the 2006 motion picture Failure to Launch (in which a man lives in the security of his parents’ home even though he is in his 30s), that dissipated the hostility they had shown toward each other and toward their children. The couple was then able to proceed to conflict resolution.
Recommendations, caveats
If you are considering incorporating laughter into therapy, keep in mind that:
• you should ensure that the patient does not perceive humor as minimizing the seriousness of their problems
• humor can be a minefield if not used judiciously, or if used at all, around certain sensitive topics, such as race, ethnicity, religion, political affiliation, and sexual orientation
• the timing of humor is particularly essential for it to succeed in the context of a therapeutic relationship
• from a medical perspective, laughter in patients who are recovering from abdominal or other major surgery might compromise wound healing because of increased intra-abdominal pressure associated with laughing
• patients who have asthma, especially exercise-induced asthma, might be at risk of developing an acute asthmatic attack when they laugh very hard. Lebowitz et al29 demonstrated that laughter can have a negative effect on patients with chronic obstructive pulmonary disease.
It is advisable in some situations to avoid humor in psychotherapy, such as when the patient or family is hostile—because, as noted, they might perceive laughter and humor as an attempt to minimize the seriousness of their discontent.
Bottom Line
Humor and laughter are underutilized and underreported in therapy, in part because it is a nascent field of research. Laughter has social and physiologic benefits that can be used in the context of a therapeutic relationship to help patients with a variety of ailments, including depression, anxiety, and pain.
Related Resources
- Association for Applied and Therapeutic Humor. www.aath.org.
- Mora-Ripoll R. The therapeutic value of laughter in medicine. Altern Ther Health Med. 2010;16:56-64.
- Strean WB. Laughter prescription. Can Fam Physician. 2009;55:965-967.
Disclosure
Dr. Nasr reports no financial relationship with manufacturers of any products mentioned in this article or with manufacturers of competing products.
Acknowledgements
The author acknowledges the assistance of Francois E. Alouf, MD, for suggestions on topics to include in the article; John W. Crayton, MD, for reviewing the manuscript; and Burdette Wendt for assistance with the references.
CASE REPORT: Laughter as therapy
Mrs. A is a 56-year-old married woman who has bipolar disorder. She has survived several suicide attempts. Her family history is positive for bipolar disorder and completed suicides.
After her most recent suicide attempt and a course of electroconvulsive therapy, Mrs. A recovered sufficiently to begin a spiritual journey that led her to practice a technique known as Laughter Yoga (Box) and, eventually, to become a Laughter Yoga instructor.
Mrs. A begins Laughter Yoga sessions by talking openly with students about her illness and the beneficial effects that laughter therapy has had on its course: She once had at least two major bipolar episodes a year, she explains, but has been in full remission for several years despite severe psychosocial stressors. In addition to practicing Laughter Yoga, Mrs. A is now maintained on a mood stabilizer that failed in the past to control her mood cycles.
Does laughter have a place in your practice?
It is said that laughter is good medicine—but is it good psychiatric medicine? Where might humor and laughter fit in the psychiatrist’s armamentarium? Is laughter physiologically beneficial to psychiatric patients? And are there adverse effects or contraindications to laughter in psychiatry? This article:
• reviews studies that have examined the anatomy, physiology, and psychology of humor and laughtera
• offers answers to the questions posed above (Table).
“Gelotology,” from the Greek “gelos,” laughter, is the science of laughter. The three components of humor and laughter are:
• the emotional component, which triggers emotions produced by a humorous situation
• the cognitive component, in which a person “gets it”
• the movement of facial, respiratory, and abdominal muscles.
Furthermore, tension and surprise are needed for laughter.
Theories about humor are varied
Philosophers since Plato have proposed theories of humor; modern theories of humor can be traced to Freud’s work.1 The psychoanalytic literature on humor focuses on the role of humor in sublimation of feelings of anger and hostility, while releasing affect in an economical way.
Erikson also wrote about the role of humor in a child’s developing superego, which helps resolve the conflict with maternal authority.2
In a comprehensive review of theories of humor, Krichtafovitch explains that cognitive theories address the role of incongruity and contrast in the induction of laughter, whereas social theories explore the roles of aggression, hostility, superiority, triumph, derision, and disparagement in humor and laughter. The effect of humor, Krichtafovitch explains, is to elevate the social status of the joker while the listener’s social status is lifted through his (her) ability to “get it.” Thus, humor plays a meaningful role in creating a bond between speaker and listener.3
The neuroanatomy of laughter
Here is some of what we have learned about mapping the brain to the basis of laughter:
• Consider a 16-year-old girl who underwent neurosurgery for intractable seizures. During surgery, various parts of the brain were stimulated to test for the focus of the seizures. She laughed every time the left frontal superior gyrus was stimulated. According to the report, she apparently laughed first, then made up a story that was funny to her.4
• Pseudobulbar affect—excessive, usually incongruent laughter, secondary to neurologic disease or traumatic brain injury—is an example of the biologic basis of laughter.
• Many functional brain imaging studies of laughter have been published.5 These studies show involvement of various regions of the brain in laughter, including the amygdala, hypothalamus, and temporal and cerebellar regions.
• Sex differences also have been noted in the neuroanatomy of laughter. Females activate the left prefrontal cortex more than males do, suggesting a greater degree of executive processing and language-based decoding. Females also exhibit greater activation of mesolimbic regions, including the nucleus accumbens, implying a greater reward network response.6
• Wild et al7 reported that separate cortical regions are responsible for the production of facial expressions that are emotionally driven (through laughter) and voluntary.
The physiology of laughter
Humans begin to laugh at approximately 4 months of age. Children laugh, on average, 400 times a day; adults do so an average of only 5 times a day.8 In addition:
• Tickling a baby induces her (him) to laugh, which, in turn, makes the parent laugh; a social bond develops during this playful exercise. This response is probably mediated by 5-HT1A receptors, which, when stimulated, induces the release of oxytocin, which facilitates social bonding.9
• Potent stimulation of 5-HT1A receptors through ingestion of 3,4-methylenedioxy-N-methylamphetamine (Ecstasy) leads to uncontrollable laughter and mirth.10
• Lower species are also known to enjoy humor. Mice emit a chirping sound when tickled, and laughter is contagious among monkeys.11
• Berk et al12,13 reported that, when 52 healthy men watched a funny video for 30 minutes, they had significantly higher activity of natural killer (NK) cells and higher levels of IgG, IgA, and IgM compared with men who watched an emotionally neutral documentary.
• Bennett et al14 showed that, in 33 healthy women, the harder the laughter, the higher the NK activity.
• Sugawara et al15 showed improved cardiovascular function in 17 healthy persons (age 23 to 42) who watched a 30-minute comedy video, compared with their cardiovascular function when they watched a documentary video of equal length.
• Svebak et al16 examined the effect of humor as measured by the Sense of Humor Survey on the survival rate of more then 53,000 adults in one county in Norway. They concluded that the higher the sense of humor score, the higher the odds ratio of surviving 7 years, compared with subjects who had a lower sense of humor.
Clinical studies of laughter
The Coping Humor Scale (CHS) and the Humor Response Scale (HRS) are the two most widely used tools to measure a person’s innate sense of humor (the CHS) and the ability to respond to a humorous situation (the HRS).17 Several studies about the effects of laughter on illness are notable:
• Laughter increased NK cell activity, lowered prorenin gene expression, and lowered the postprandial glucose level in 34 patients with diabetes, compared with 16 matched controls.18-21
• Clark et al studied the sense of humor of 150 patients with cardiac disease compared with 150 controls. They found that “people with heart disease responded less humorously to everyday life situations.” They generally laughed less, even in positive situations, and displayed more anger and hostility.22
• In his work on the salutatory effect of laughter on the experience of pain, Cousins described how he dealt with his painful arthritis by watching Marx Brothers movies23:
I made the joyous discovery that 10 minutes of genuine belly laughter had an anesthetic effect and would give me at least two hours of pain-free sleep… When the pain-killing effect of the laughter wore off, we would switch on the motion picture projector again and not infrequently, it would lead to another pain-free interval.
• Hearty laughter leads to pain relief, probably through the release of endorphins. Dunbar et al24 tested this hypothesis in a series of six experimental studies in the laboratory (watching videos) and in a naturalistic context (watching stage performances), using a change in pain threshold as an indirect measure of endorphin release. The results show that the pain threshold is significantly higher after laughter than in the control condition. This pain-tolerance effect is caused by the laughter itself, not simply because of a change in positive affect.
Laughter therapy for depression
Three studies have demonstrated the benefit of laughter therapy in depression:
• When Ko and Youn25 studied 48 geriatric depressed patients and 61 age-matched controls, they found a significantly lower Geriatric Depression Scale score and a better Pittsburgh Sleep Quality Index score in patients who had been exposed to four weekly laughter groups, compared with persons who had been exposed to a control group.
• Shahidi et al26 randomly assigned 60 community-dwelling female, geriatric, depressed patients to a laughter yoga group, an exercise group, and a control group. Laughter yoga and exercise were equally effective, and both were significantly superior to the control condition. The laughter yoga group scored significantly better than the other two groups on the Life Satisfaction Scale. The researchers concluded that, in addition to improved mood, patients who laugh experience increased life satisfaction.
• Fonzi et al27 summarized data on the neurophysiology of laughter and the effect of laughter on the hypothalamus-pituitary-adrenal axis. They noted that depression reduces the frequency of laughter and, inversely, laughter reduces the severity of depression. Laughter, they reported, also increases the connectivity of patients with people in their life, which further alleviates symptoms of depression.
Other therapeutic uses of laughter
Humor can strengthen the bond of the therapeutic relationship. Patients who laugh with their physicians are more likely to feel connected with them, follow their advice, and feel more satisfied with their encounter. One study found that primary care physicians who gave positive statements, spent more time with patients, and included humor or laughter during their visits lowered their risk of being sued for malpractice.28
Consider also the use of laughter in altering family dynamics in a therapeutic setting: Mr. and Mrs. B attend therapy in my practice to address a difficult situation with their adult children. One of them enables their children socially and financially; the other continually complains about this enabling. When the tension was high and the couple had reached an impasse during a visit, the therapist offered an anecdote from the 2006 motion picture Failure to Launch (in which a man lives in the security of his parents’ home even though he is in his 30s), that dissipated the hostility they had shown toward each other and toward their children. The couple was then able to proceed to conflict resolution.
Recommendations, caveats
If you are considering incorporating laughter into therapy, keep in mind that:
• you should ensure that the patient does not perceive humor as minimizing the seriousness of their problems
• humor can be a minefield if not used judiciously, or if used at all, around certain sensitive topics, such as race, ethnicity, religion, political affiliation, and sexual orientation
• the timing of humor is particularly essential for it to succeed in the context of a therapeutic relationship
• from a medical perspective, laughter in patients who are recovering from abdominal or other major surgery might compromise wound healing because of increased intra-abdominal pressure associated with laughing
• patients who have asthma, especially exercise-induced asthma, might be at risk of developing an acute asthmatic attack when they laugh very hard. Lebowitz et al29 demonstrated that laughter can have a negative effect on patients with chronic obstructive pulmonary disease.
It is advisable in some situations to avoid humor in psychotherapy, such as when the patient or family is hostile—because, as noted, they might perceive laughter and humor as an attempt to minimize the seriousness of their discontent.
Bottom Line
Humor and laughter are underutilized and underreported in therapy, in part because it is a nascent field of research. Laughter has social and physiologic benefits that can be used in the context of a therapeutic relationship to help patients with a variety of ailments, including depression, anxiety, and pain.
Related Resources
- Association for Applied and Therapeutic Humor. www.aath.org.
- Mora-Ripoll R. The therapeutic value of laughter in medicine. Altern Ther Health Med. 2010;16:56-64.
- Strean WB. Laughter prescription. Can Fam Physician. 2009;55:965-967.
Disclosure
Dr. Nasr reports no financial relationship with manufacturers of any products mentioned in this article or with manufacturers of competing products.
Acknowledgements
The author acknowledges the assistance of Francois E. Alouf, MD, for suggestions on topics to include in the article; John W. Crayton, MD, for reviewing the manuscript; and Burdette Wendt for assistance with the references.
1. Freud S, Strachey J, trans., ed. Jokes and their relation to the unconscious. New York, NY: W. W. Norton & Company; 1990.
2. Capps D. Mother, melancholia, and humor in Erik H. Erikson’s earliest writings. J Relig Health. 2008;47:415-432.
3. Krichtafovitch I. Humor theory. Parker, CO: Outskirts Press; 2006.
4. Fried I, Wilson CL, MacDonald KA, et al. Electric current stimulates laughter. Nature. 1998;12;391:650.
5. Bartolo A, Benuzzi F, Nocetti L, et al. Humor comprehension and appreciation: an FMRI study. J Cogn Neurosci. 2006;18:1789-1798.
6. Azim E, Mobbs D, Jo B, et al. Sex differences in brain activation elicited by humor. Proc Natl Acad Sci U S A. 2005;102:16496-16501.
7. Wild B, Rodden FA, Rapp A, et al. Humor and smiling: cortical regions selective for cognitive, affective, and volitional components. Neurology. 2006;66:887-893.
8. Freedman LW. Mosby’s complementary and alternative medicine. A research-based approach. St. Louis, MO: Mosby; 2004:24.
9. Lukas M, Toth I, Reber SO, et al. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:
2159-2168.
10. Thompson MR, Callaghan PD, Hunt GE, et al. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience. 2007;146:509-514.
11. Ross MD, Owren MJ, Zimmermann E. The evolution of laughter in great apes and humans. Commun Integr Biol. 2010;3(2):191-194.
12. Berk LS, Tan SA, Fry WF, et al. Neuroendocrine and stress hormone changes during mirthful laughter. Am J Med Sci. 1989;298:390-396.
13. Berk LS, Felten DL, Tan SA, et al. Modulation of neuroimmune parameters during the eustress of humor-associated mirthful laughter. Altern Ther Health Med. 2001; 7:62-72,74-76.
14. Bennett MP, Zeller JM, Rosenberg L, et al. The effect of mirthful laughter on stress and natural killer cell activity. Altern Ther Health Med. 2003;9:38-45.
15. Sugawara J, Tarumi T, Tanaka H. Effect of mirthful laughter on vascular function. Am J Cardiol. 2010;106:856-859.
16. Svebak S, Romundstad S, Holmen J. A 7-year prospective study of sense of humor and mortality in an adult county population: the HUNT-2 study. Int J Psychiatry Med. 2010;40:125-146.
17. Martin RA. The Situational Humor Response Questionnaire (SHRQ) and Coping Humor Scale (CHS): a decade of research findings. Humor: International Journal of Humor Research. 1996;9(3-4):251-272.
18. Hayashi T, Urayama O, Hori M, et al. Laughter modulates prorenin receptor gene expression in patients with type 2 diabetes. J Psychosom Res. 2007;62:703-706.
19. Hayashi T, Murakami K. The effects of laughter on post-prandial glucose levels and gene expression in type 2 diabetic patients. Life Sci. 2009;85:185-187.
20. Takahashi K, Iwase M, Yamashita K, et al. The elevation of natural killer cell activity induced by laughter in a crossover designed study. Int J Mol Med. 2001;8:645-650.
21. Nasir UM, Iwanaga S, Nabi AH, et al. Laughter therapy modulates the parameters of renin-angiotensin system in patients with type 2 diabetes. Int J Mol Med. 2005;16:1077-1081.
22. Clark A, Seidler A, Miller M. Inverse association between sense of humor and coronary heart disease. Int J Cardiol. 2001;80:87-88.
23. Cousins N. The anatomy of an illness as perceived by the patient: reflections on healing and regeneration. New York, NY: Norton; 1979:39.
24. Dunbar RI, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
25. Ko HJ, Youn CH. Effects of laughter therapy on depression, cognition and sleep among the community-dwelling elderly. Geriatr Gerontol Int. 2011;11:267-274.
26. Shahidi M, Mojtahed A, Modabbernia A, et al. Laughter yoga versus group exercise program in elderly depressed women: a randomized controlled trial. Int J Geriatr Psychiatry. 2011;26:322-327.
27. Fonzi L, Matteucci G, Bersani G. Laughter and depression: hypothesis of pathogenic and therapeutic correlation. Riv Psichiatr. 2010;45:1-6.
28. Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
29. Lebowitz KR, Suh S, Diaz PT, et al. Effects of humor and laughter on psychological functioning, quality of life, health status, and pulmonary functioning among patients with chronic obstructive pulmonary disease: a preliminary investigation. Heart Lung. 2011;40:310-319.
1. Freud S, Strachey J, trans., ed. Jokes and their relation to the unconscious. New York, NY: W. W. Norton & Company; 1990.
2. Capps D. Mother, melancholia, and humor in Erik H. Erikson’s earliest writings. J Relig Health. 2008;47:415-432.
3. Krichtafovitch I. Humor theory. Parker, CO: Outskirts Press; 2006.
4. Fried I, Wilson CL, MacDonald KA, et al. Electric current stimulates laughter. Nature. 1998;12;391:650.
5. Bartolo A, Benuzzi F, Nocetti L, et al. Humor comprehension and appreciation: an FMRI study. J Cogn Neurosci. 2006;18:1789-1798.
6. Azim E, Mobbs D, Jo B, et al. Sex differences in brain activation elicited by humor. Proc Natl Acad Sci U S A. 2005;102:16496-16501.
7. Wild B, Rodden FA, Rapp A, et al. Humor and smiling: cortical regions selective for cognitive, affective, and volitional components. Neurology. 2006;66:887-893.
8. Freedman LW. Mosby’s complementary and alternative medicine. A research-based approach. St. Louis, MO: Mosby; 2004:24.
9. Lukas M, Toth I, Reber SO, et al. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:
2159-2168.
10. Thompson MR, Callaghan PD, Hunt GE, et al. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience. 2007;146:509-514.
11. Ross MD, Owren MJ, Zimmermann E. The evolution of laughter in great apes and humans. Commun Integr Biol. 2010;3(2):191-194.
12. Berk LS, Tan SA, Fry WF, et al. Neuroendocrine and stress hormone changes during mirthful laughter. Am J Med Sci. 1989;298:390-396.
13. Berk LS, Felten DL, Tan SA, et al. Modulation of neuroimmune parameters during the eustress of humor-associated mirthful laughter. Altern Ther Health Med. 2001; 7:62-72,74-76.
14. Bennett MP, Zeller JM, Rosenberg L, et al. The effect of mirthful laughter on stress and natural killer cell activity. Altern Ther Health Med. 2003;9:38-45.
15. Sugawara J, Tarumi T, Tanaka H. Effect of mirthful laughter on vascular function. Am J Cardiol. 2010;106:856-859.
16. Svebak S, Romundstad S, Holmen J. A 7-year prospective study of sense of humor and mortality in an adult county population: the HUNT-2 study. Int J Psychiatry Med. 2010;40:125-146.
17. Martin RA. The Situational Humor Response Questionnaire (SHRQ) and Coping Humor Scale (CHS): a decade of research findings. Humor: International Journal of Humor Research. 1996;9(3-4):251-272.
18. Hayashi T, Urayama O, Hori M, et al. Laughter modulates prorenin receptor gene expression in patients with type 2 diabetes. J Psychosom Res. 2007;62:703-706.
19. Hayashi T, Murakami K. The effects of laughter on post-prandial glucose levels and gene expression in type 2 diabetic patients. Life Sci. 2009;85:185-187.
20. Takahashi K, Iwase M, Yamashita K, et al. The elevation of natural killer cell activity induced by laughter in a crossover designed study. Int J Mol Med. 2001;8:645-650.
21. Nasir UM, Iwanaga S, Nabi AH, et al. Laughter therapy modulates the parameters of renin-angiotensin system in patients with type 2 diabetes. Int J Mol Med. 2005;16:1077-1081.
22. Clark A, Seidler A, Miller M. Inverse association between sense of humor and coronary heart disease. Int J Cardiol. 2001;80:87-88.
23. Cousins N. The anatomy of an illness as perceived by the patient: reflections on healing and regeneration. New York, NY: Norton; 1979:39.
24. Dunbar RI, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
25. Ko HJ, Youn CH. Effects of laughter therapy on depression, cognition and sleep among the community-dwelling elderly. Geriatr Gerontol Int. 2011;11:267-274.
26. Shahidi M, Mojtahed A, Modabbernia A, et al. Laughter yoga versus group exercise program in elderly depressed women: a randomized controlled trial. Int J Geriatr Psychiatry. 2011;26:322-327.
27. Fonzi L, Matteucci G, Bersani G. Laughter and depression: hypothesis of pathogenic and therapeutic correlation. Riv Psichiatr. 2010;45:1-6.
28. Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
29. Lebowitz KR, Suh S, Diaz PT, et al. Effects of humor and laughter on psychological functioning, quality of life, health status, and pulmonary functioning among patients with chronic obstructive pulmonary disease: a preliminary investigation. Heart Lung. 2011;40:310-319.
Incidental ovarian cysts: When to reassure, when to reassess, when to refer
Ovarian cysts, sometimes reported as ovarian masses or adnexal masses, are frequently found incidentally in women who have no symptoms. These cysts can be physiologic (having to do with ovulation) or neoplastic—either benign, borderline (having low malignant potential), or frankly malignant. Thus, these incidental lesions pose many diagnostic challenges to the clinician.
The vast majority of cysts are benign, but a few are malignant, and ovarian malignancies have a notoriously poor survival rate. The diagnosis can only be obtained surgically, as aspiration and biopsy are not definitive and may be harmful. Therefore, the clinician must try to balance the risks of surgery for what may be a benign lesion with the risk of delaying diagnosis of a malignancy.
In this article we provide an approach to evaluating these cysts, with guidance on when the patient can be reassured and when referral is needed.
THE DILEMMA OF OVARIAN CYSTS
Ovarian cysts are common
Premenopausal women can be expected to make at least a small cyst or follicle almost every month. The point prevalence for significant cysts has been reported to be almost 8% in premenopausal women.1
Surprisingly, the prevalence in postmenopausal women is as high as 14% to 18%, with a yearly incidence of 8%. From 30% to 54% of postmenopausal ovarian cysts persist for years.2,3
Little is known about the cause of most cysts
Little is known about the cause of most ovarian cysts. Functional or physiologic cysts are thought to be variations in the ovulatory process. They do not seem to be precursors to ovarian cancer.
Most benign neoplastic cysts are also not thought to be precancerous, with the possible exception of the mucinous kind.4 Ovarian cysts do not increase the risk of ovarian cancer later in life,3,9 and removing benign cysts has not been shown to decrease the risk of death from ovarian cancer.10
Most ovarian cysts and masses are benign
Simple ovarian cysts are much more likely to be benign than malignant. Complex and solid ovarian masses are also more likely to be benign, regardless of menopausal status, but more malignancies are found in this group.
With any kind of mass, the chances of malignancy increase with age. Children and adolescents are not discussed in this article; they should be referred to a specialist.
Ovarian cancer often has a poor prognosis
This “silent” cancer is most often discovered and treated when it has already spread, contributing to a reported 5-year survival rate of only 33% to 46%.11–13 Ideally, ovarian cancer would be found and removed while still confined to the ovary, when the 5-year survival rate is greater than 90%.
Unfortunately, there does not seem to be a precursor lesion for most ovarian cancers, and there is no good way of finding it in the stage 1 phase, so detecting this cancer before it spreads remains an elusive goal.11,14
Surgery is required to diagnose difficult cases
There is no perfect test for the preoperative assessment of a cystic ovarian mass. Every method has drawbacks (Table 1).15–18 Therefore, the National Institutes of Health estimates that 5% to 10% of women in the United States will undergo surgical exploration for an ovarian cyst in their lifetime. Only 13% to 21% of these cysts will be malignant.5
ASSESSING AN INCIDENTALLY DISCOVERED OVARIAN MASS
Certain factors in the history, physical examination, and blood work may suggest the cyst is either benign or malignant and may influence the subsequent assessment. However, in most cases, the best next step is to perform transvaginal ultrasonography, which we will discuss later in this paper.
History
Age is a major risk factor for ovarian cancer; the median age at diagnosis is 63 years.9 In the reproductive-age group, ovarian cysts are much more likely to be functional than neoplastic. Epithelial cancers are rare before the age of 40, but other cancer types such as borderline, germ cell, and sex cord stromal tumors may occur.19
In every age group a cyst is more likely to be benign than malignant, although, as noted above, the probability of malignancy increases with age.
Symptoms. Most ovarian cysts, benign or malignant, are asymptomatic and are found only incidentally.
The most commonly reported symptoms are pelvic or lower-abdominal pressure or pain. Acutely painful conditions include ovarian torsion, hemorrhage into the cyst, cyst rupture with or without intra-abdominal hemorrhage, ectopic pregnancy, and pelvic inflammatory disease with tubo-ovarian abscess.
Some patients who have ovarian cancer report vague symptoms such as urinary urgency or frequency, abdominal distention or bloating, and difficulty eating or early satiety.20 Although the positive predictive value of this symptom constellation is only about 1%, its usefulness increases if these symptoms arose recently (within the past year) and occur than 12 days a month.21
Family history of ovarian, breast, endometrial, or colon cancer is of particular interest. The greater the number of affected relatives and the closer the degree of relation, the greater the risk; in some cases the relative risk is 40 times greater.22 Breast-ovarian cancer syndromes, hereditary nonpolyposis colorectal cancer syndrome, and family cancer syndrome, as well as extremely high-risk pedigrees such as BRCA1, BRCA2, and Lynch syndrome, all place women at significantly higher risk. Daughters tend to develop cancer at a younger age than their affected mothers.
However, only 10% of ovarian cancers occur in patients who have a family history of it, leaving 90% as sporadic occurrences.
Other history. Factors protective against ovarian cancer include use of oral contraceptives at any time, tubal ligation, hysterectomy, having had children, breastfeeding, a low-fat diet, and possibly use of aspirin and acetaminophen.23,24
Risk factors for malignancy include advanced age; nulliparity; family history of ovarian or breast cancer; personal history of breast cancer; talc use; asbestos exposure; white ethnicity; pelvic irradiation; smoking; alcohol use; possibly the previous use of fertility drugs, estrogen, or androgen; history of mumps; urban location; early menarche; and late menopause.24
Physical examination
Vital signs. Fever can indicate an infectious process or torsion of the ovary. A sudden onset of low blood pressure or rapid pulse can indicate a hemorrhagic condition such as ectopic pregnancy or ruptured hemorrhagic cyst.
Bimanual pelvic examination is notoriously inaccurate for detecting and characterizing ovarian cysts. In one prospective study, examiners blinded to the reason for surgery evaluated women under anesthesia. The authors concluded that bimanual examination was of limited value even under the best circumstances.15 Pelvic examination can be even more difficult in patients who are obese, are virginal, have vaginal atrophy, or are in pain.
Useful information that can be obtained through the bimanual examination includes the exact location of pelvic tenderness, the relative firmness of an identified mass, and the existence of nodularity in the posterior cul-de-sac, suggesting advanced ovarian cancer.
Tumor markers
Cancer antigen 125 (CA125) is the most studied and widely used of the ovarian cancer tumor markers. When advanced epithelial ovarian cancer is associated with a markedly elevated level, the value correlates with tumor burden.25
Unfortunately, only about half of early-stage ovarian cancers and 75% to 80% of advanced ovarian cancers express this marker.26 Especially in premenopausal women, there are many pelvic conditions that can falsely elevate CA125. Therefore, its sensitivity and specificity for predicting ovarian cancer are suboptimal. Nevertheless, CA125 is often used to help stratify risk when assessing known ovarian cysts and masses.
The value considered abnormal in postmenopausal women is 35 U/mL or greater, while in premenopausal women the cutoff is less well defined. The lower the cutoff level is set, the more sensitive the test. Recent recommendations advise 50 U/mL or 67 U/mL, rather than the 200 U/mL recommended in the 2002 joint guidelines of the American Congress of Obstetricians and Gynecologists and the Society of Gynecologic Oncology.27,28
However, specificity is likely to be lower with these lower cutoff values. Conditions that can elevate CA125 levels include almost anything that irritates the peritoneum, including pregnancy, menstruation, fibroids, endometriosis, infection, and ovarian hyperstimulation, as well as medical conditions such as liver or renal disease, colitis, diverticulitis, congestive heart failure, diabetes, autoimmune diseases, and ascites.
Following serial CA125 levels may be more sensitive than trying to establish a single cutoff value.29 CA125 should not be used as a screening tool in average-risk women.26
OVA1. Several biomarker panels have been developed and evaluated for risk assessment in women with pelvic masses. OVA1, a proprietary panel of tests (Vermillion; Austin, TX) received US Food and Drug Administration approval in 2009. It includes CA125 and four other proteins, from which it calculates a probability score (high or low) using a proprietary formula.
In prospective studies, OVA1 was more sensitive than clinical assessment or CA125 alone.30 The higher sensitivity and negative predictive value were counterbalanced by a lower specificity and positive predictive value.31 Its cost ($650) is not always covered by insurance. OVA1 is not a screening tool.
EVALUATION WITH ULTRASONOGRAPHY
Ultrasonography is the imaging test of choice in assessing adnexal cysts and masses, and therefore it is the best next step after taking a history, performing a physical examination, and obtaining blood work.32 In cases in which an incidental ovarian mass is discovered on computed tomography (CT), further characterization by ultrasonography will likely yield helpful information.
Pelvic ultrasonography can be performed transabdominally or transvaginally. Vaginal ultrasonography gives the clearest images in most patients. Abdominal scanning is indicated for large masses, when vaginal access is difficult (as in virginal patients or those with vaginal atrophy) or when the mass is out of the focal length of the vaginal probe. A full bladder is usually required for the best transabdominal images.
The value of the images obtained depends on the experience of the ultrasonographer and reader and on the equipment. Also, there is currently no widely used standard for reporting the findings33—descriptions are individualized, leading some authors to recommend that the clinician personally review the films to get the most accurate picture.19
Size
Size alone cannot be used to distinguish between benign and malignant lesions. Simple cysts up to 10 cm are most likely benign regardless of menopausal status.2,34 However, in a complex or solid mass, size correlates somewhat with the chance of malignancy, with notable exceptions, such as the famously large sizes of some solid fibromas or mucinous cystadenomas. Also, size may correlate with risk of other complications such as torsion or symptomatic rupture.
Complexity
Simple cysts have clear fluid, thin smooth walls, no loculations or septae, and enhanced through-transmission of echo waves.32,33
Complexity is described in terms of septations, wall thickness, internal echoes, and solid nodules. Increasing complexity does correlate with increased risk of malignancy.
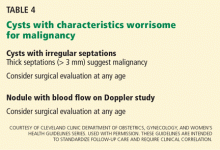
Worrisome findings
The most worrisome findings are:
- Solid areas that are not hyperechoic, especially when there is blood flow to them
- Thick septations, more than 2 or 3 mm wide, especially if there is blood flow within them
- Excrescences on the inner or outer aspect of a cystic area
- Ascites
- Other pelvic or omental masses.
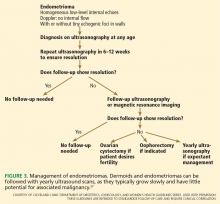
Benign conditions
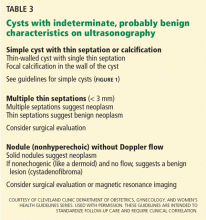
Several benign conditions have characteristic complex findings on ultrasonography (Table 2), whereas other findings can be indeterminate (Table 3) or worrisome for malignancy (Table 4).
Hemorrhagic corpus luteum cysts can be complex with an internal reticular pattern due to organizing clot and fibrin strands. A “ring of fire” vascular pattern is often seen around the cyst bed.
Dermoids (mature cystic teratomas) may have hyperechoic elements with acoustic shadowing and no internal Doppler flow. They can have a complex appearance due to fat, hair, and sebum within the cyst. Dermoid cysts have a pathognomonic appearance on CT with a clear fat-fluid level.
Endometriomas classically have a homogeneous “ground-glass” appearance or low-level echoes, without internal color Doppler flow, wall nodules, or other malignant features.
Fibroids may be pedunculated and may appear to be complex or solid adnexal masses.
Hydrosalpinges may present as tortuous tubular-shaped cystic masses. There may be incomplete septations or indentations seen on opposite sides (the “waist” sign).
Paratubal and paraovarian cysts are usually simple round cysts that can be demonstrated as separate from the ovary. Sometimes these appear complex as well.
Peritoneal inclusion cysts, also known as pseudocysts, are seen in patients with intra-abdominal adhesions. Often multiple septations are seen through clear fluid, with the cyst conforming to the shape of other pelvic structures.
Torsion of the ovary may occur with either benign or malignant masses. Torsion can be diagnosed when venous flow is absent on Doppler. The presence of flow, however, doesn’t rule out torsion, as torsion is often intermittent. The twisted ovary is most often enlarged and can have an edematous appearance. Although typically benign, these should be referred for urgent surgical treatment.
Vascularity
Doppler imaging is being extensively studied. The general principle is that malignant masses will be more vascular, with a high-volume, low-resistance pattern of flow. This can result in a pulsatility index of less than 1 or a resistive index of less than 0.4. In practice, however, there is significant overlap between high and low pulsatility indices and resistive indices in benign and malignant cysts. Low resistance can also be found in endometriomas, corpus luteum cysts, inflammatory masses, and vascular benign neoplasms. A normal (high) resistive index does not rule out malignancy.32,33
One Doppler finding that does seem to correlate with malignancy is the presence of any flow within a solid nodule or wall excrescence.
3D ultrasonography
As the use of 3D ultrasonography increases, studies are yielding different results as to its utility in describing ovarian masses. 3D ultrasonography may be useful in finding centrally located vessels so that Doppler can be applied.32
OTHER IMAGING
Although ultrasonography is the initial imaging study of choice in the evaluation of adnexal masses owing to its high sensitivity, availability, and low cost, studies have shown that up to 20% of adnexal masses can be reported as indeterminate by ultrasonography (Table 1).
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is emerging as a very valuable tool when ultrasonography is inconclusive or limited.35 Although MRI is very accurate (Table 1), it is not considered a first-line imaging test because it is more expensive, less available, and more inconvenient for the patient than ultrasonography.
MRI provides additional information on the composition of soft-tissue tumors. Usually, MRI is ordered with contrast, unless there are contraindications to it. The radiologist will evaluate morphologic features, signal intensity, and enhancement of solid areas. Techniques such as dynamic contrast-enhanced MRI (following the distribution of contrast material over time), in- and out-of-phase T1 imaging (looking for fat, such as in dermoids), and the newer diffusion-weighted imaging may further improve characterization.
In one study of MRI as second-line imaging, contrast-enhanced MRI contributed to a greater change in the probability of ovarian cancer than did CT, Doppler ultrasonography, or MRI without contrast.36 This may result in a reduction in unnecessary surgeries and in an increase in proper referrals in cases of suspected malignancy.
Computed tomography
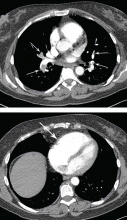
Disadvantages of CT include radiation exposure and poor discrimination of soft tissue. It can, however, differentiate fat or calcifications that may be found in dermoids. While CT is not often used to describe an ovarian lesion, it may be used preoperatively to stage an ovarian cancer or to look for a primary intra-abdominal cancer when an ovarian mass may represent metastasis.32
MANAGING AN INCIDENTAL OVARIAN CYST OR CYSTIC MASS
Combining information from the history, physical examination, imaging, and blood work to assign a level of risk of malignancy is not straightforward. The clinician must weigh several imperfect tests, each with its own sensitivity and specificity, against the background of the individual patient’s likelihood of malignancy. Whereas a 4-cm simple cyst in a premenopausal woman can be assigned to a low-risk category and a complex mass with flow to a solid component in a postmenopausal woman can be assigned to a high-risk category, many lesions are more difficult to assess.
Several systems have been proposed for analyzing data and standardizing risk assessment. There are a number of scoring systems based on ultrasonographic morphology and several mathematical regression models that include menopausal status and tumor markers. But each has drawbacks, and none is definitively superior to expert opinion.16,17,37,38
A 2012 systematic review and meta-analysis39 calculated sensitivity and specificity for several imaging tests, scoring systems, and blood tumor markers. Some results are presented in Table 1.
The management of an ovarian cyst depends on symptoms, likelihood of torsion or rupture, and the level of concern for malignancy. At the lower-risk end of the spectrum, reassurance or observation over time may be appropriate. A general gynecologist can evaluate indeterminate or symptomatic ovarian cysts. Patients with masses frankly suspicious for malignancy are best referred to a gynecologic oncologist.
Expectant management for low-risk lesions
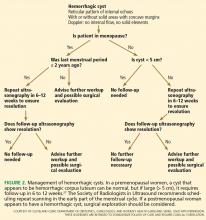
Low-risk lesions such as simple cysts, endometriomas, and dermoids have a less than 1% chance of malignancy. Most patients who have them require only reassurance or follow-up with serial ultrasonography. Oral contraceptives may prevent new cysts from forming. Aspiration is not recommended.
In 2010, the Society of Radiologists in Ultrasound issued a consensus statement regarding re-imaging of simple ovarian cysts.33
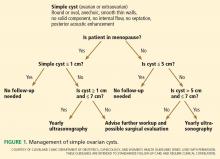
In premenopausal women, they recommend no further testing for cysts 5 cm or smaller, yearly follow-up for cysts larger than 5 cm and up to and including 7 cm, and MRI or surgical evaluation for cysts larger than 7 cm, as it is difficult to completely image a large cyst with ultrasonography.
In postmenopausal women, if the cyst is 1 cm in diameter or smaller, no further studies need to be done. For simple cysts larger than 1 cm and up to and including 7 cm, yearly re-imaging is recommended. And for cysts larger than 7 cm, MRI or surgery is indicated. The American College of Radiology recommends repeat ultrasonography and CA125 testing for cysts 3 cm and larger but doesn’t specify an interval.32
A cyst that is otherwise simple but has a single thin septation (< 3 mm) or a small calcification in the wall is almost always benign. Such a cyst should be followed as if it were a simple cyst, as indicated by patient age and cyst size.
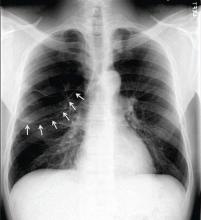
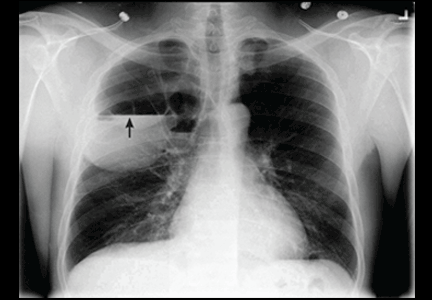
There are no official guidelines as to when to stop serial imaging,22,32 but a recent paper suggested one or two ultrasonographic examinations to confirm size and morphologic stability.19 Once a lesion has resolved, there is no need for further imaging (Figures 1–3).
Birth control pills for suppression of new cysts. Oral contraceptives do not hasten the resolution of ovarian cysts, according to a 2011 Cochrane review.40 Some practitioners will, nevertheless, prescribe them in an attempt to prevent new cysts from confusing the picture.
Aspiration is not recommended for either diagnosis or treatment. It can only be considered in patients at high risk who are not surgical candidates. Results of cytologic study of specimens obtained by fine-needle aspiration cannot reliably determine the presence or absence of malignancy.41 There is also a theoretical risk of spreading cancer from an early-stage lesion. A retrospective study has suggested that spillage of cyst contents during surgery in early ovarian cancer is associated with a worse prognosis.42
From a therapeutic point of view, studies have shown the same resolution rate at 6 months for aspirated cysts vs those followed expectantly.43 Another study found a recurrence rate of 25% within 1 year of aspiration.44
Referral for medium-risk or indeterminant-risk ovarian masses
Patients who have medium- or indeterminaterisk ovarian masses (Table 3) should be referred to a gynecologist. Further testing will help stratify the risk of malignancy. This can include tumor marker blood tests, MRI, or CT, the addition of Doppler or 3D ultrasonography, serial ultrasonography, or surgical exploration.
If repeat ultrasonography is chosen, the interval will likely be 6 to 12 weeks. Surgery may consist of removing only the cyst itself, or the whole ovary with or without the tube, or sometimes both ovaries. Purely diagnostic laparoscopy is rarely performed, as direct visualization of a lesion is rarely helpful. Frozen section should be employed, and the operating gynecologist should have oncologic backup, since the surgery is performed to rule out malignancy.
In the case of a benign-appearing cyst larger than 6 cm, thought must be given as to whether it is likely to rupture or twist. Rupture of a large cyst can lead to pain and in some cases to hemorrhage. Contents of a ruptured dermoid cyst can cause chemical peritonitis. Torsion of an ovary can result in loss of the ovary through compromised perfusion. A general gynecologist can decide with the patient whether preemptive surgery is indicated.
Operative evaluation for high-risk masses
Patients with high-risk ovarian masses (Table 4) are best referred to a gynecologic oncologist for operative evaluation. If features are seen that indicate malignancy, such as thick septations, solid areas with blood flow, ascites, or other pelvic masses, surgery is indicated. The surgical approach may be through laparoscopy or laparotomy.45 It should be noted that even in the face of worrisome features on ultrasonography, many masses turn out to be benign.
In 2011, the American Congress of Obstetricians and Gynecologists and the Society of Gynecologic Oncology issued new guidelines recommending oncologic referral of patients with high-risk masses. Elevated CA125, ascites, a nodular or fixed pelvic mass, or evidence of metastasis in postmenopausal women requires oncologic evaluation.26 For premenopausal women, a very elevated CA125, ascites, or metastasis requires referral (Table 4).26
Direct referral to a gynecologic oncologist is underutilized. A recent study found that fewer than half of primary care physicians said that they would refer a classic suspicious case directly to a subspecialist.46 It is estimated that only 33% of ovarian cancers are first operated on by a gynecologic oncologist.47
A 2011 Cochrane review confirmed a survival benefit for women with cancer who are operated on by gynecologic oncologists primarily, rather than by a general gynecologist and then referred.48 A gynecologic oncologist is most likely to perform proper staging and debulking at the time of initial diagnosis.49
Special situations require consultation
Ovarian cysts in pregnancy are most often benign,50 but malignancy is always a possibility. Functional cysts and dermoids are the most common. These may remain asymptomatic or may rupture or twist or cause difficulty with delivering the baby. Surgical intervention, if needed, should be performed in the second trimester if possible. A multidisciplinary approach and referral to a perinatologist and gynecologic oncologist are advised.
Symptomatic ovarian cysts that may need surgical intervention are the purview of the general gynecologist. If the risk of a surgical emergency is judged to be low, a symptomatic patient may be supported with pain medication and may be managed on an outpatient basis. Immediate surgical consultation is appropriate if the patient appears toxic or in shock. Depending on the clinical picture, there may be a ruptured tubo-ovarian abscess, ruptured ectopic pregnancy, ruptured hemorrhagic cyst, or ovarian torsion, any of which may need immediate surgical intervention.
If a symptomatic mass is highly suspicious for cancer, a gynecologic oncologist should be consulted directly.
WHEN TO REASSURE, REASSESS, REFER
Ovarian masses often pose diagnostic and management dilemmas. Reassurance can be offered to women with small simple cysts. Interval follow-up with ultrasonography is appropriate for cysts that are most likely to be benign. If malignancy is suspected based on ultrasonography, other imaging, blood testing, or expert opinion, referral to a surgical gynecologist or gynecologic oncologist is recommended. If malignancy is strongly suspected, direct referral to a gynecologic oncologist offers the best chance of survival if cancer is actually present.
Reassure
- When simple cysts are less than 1 cm in postmenopausal women
- When simple cysts are less than 5 cm in premenopausal patients.
Reassess
- With yearly ultrasonography in cases of very low risk
- With repeat ultrasonography in 6 to 12 weeks when the diagnosis is not clear but the cyst is likely benign.
Refer
- To a gynecologist for symptomatic cysts, cysts larger than 6 cm, and cysts that require ancillary testing
- To a gynecologic oncologist for findings worrisome for cancer, such as thick septations, solid areas with flow, ascites, evidence of metastasis, or high cancer antigen 125 levels.
- Borgfeldt C, Andolf E. Transvaginal sonographic ovarian findings in a random sample of women 25–40 years old. Ultrasound Obstet Gynecol 1999; 13:345–350.
- Modesitt SC, Pavlik EJ, Ueland FR, DePriest PD, Kryscio RJ, van Nagell JR. Risk of malignancy in unilocular ovarian cystic tumors less than 10 centimeters in diameter. Obstet Gynecol 2003; 102:594–599.
- Greenlee RT, Kessel B, Williams CR, et al. Prevalence, incidence, and natural history of simple ovarian cysts among women > 55 years old in a large cancer screening trial. Am J Obstet Gynecol 2010; 202:373.e1–373.e9.
- Jordan SJ, Green AC, Whiteman DC, Webb PM; Australian Ovarian Cancer Study Group. Risk factors for benign, borderline and invasive mucinous ovarian tumors: epidemiological evidence of a neoplastic continuum? Gynecol Oncol 2007; 107:223–230.
- NIH consensus conference. Ovarian cancer. Screening, treatment, and follow-up. NIH Consensus Development Panel on Ovarian Cancer. JAMA 1995; 273:491–497.
- The reduction in risk of ovarian cancer associated with oral-contraceptive use. The Cancer and Steroid Hormone Study of the Centers for Disease Control and the National Institute of Child Health and Human Development. N Engl J Med 1987; 316:650–655.
- Young RL, Snabes MC, Frank ML, Reilly M. A randomized, double-blind, placebo-controlled comparison of the impact of low-dose and triphasic oral contraceptives on follicular development. Am J Obstet Gynecol 1992; 167:678–682.
- Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol 2009; 113:1027–1037.
- Sharma A, Gentry-Maharaj A, Burnell M, et al; UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Assessing the malignant potential of ovarian inclusion cysts in postmenopausal women within the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a prospective cohort study. BJOG 2012; 119:207–219.
- Buys SS, Partridge E, Black A, et al; PLCO Project Team. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011; 305:2295–2303.
- Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med 2009; 361:170–177.
- National Cancer Institute. Surveillance Epidemiology and End Results (SEER). Cancer statistics on ovarian cancer. http://seer.cancer.gov/statfacts/html/ovary.html. Accessed May 9, 2013.
- American Cancer Society. Survival by ovarian cancer stage. www.cancer.org/Cancer/OvarianCancer/DetailedGuide/ovarian-cancer-survival-rates. Accessed May 9, 2013.
- Brown PO, Palmer C. The preclinical natural history of serous ovarian cancer: defining the target for early detection. PLoS Med 2009; 6:e1000114.
- Padilla LA, Radosevich DM, Milad MP. Limitations of the pelvic examination for evaluation of the female pelvic organs. Int J Gynaecol Obstet 2005; 88:84–88.
- Myers ER, Bastian LA, Havrilesky LJ, et al. Management of Adnexal Mass. Evidence Report/Technology Assessment No.130 (Prepared by the Duke Evidence-based Practice Center under Contract No. 290-02-0025.) AHRQ Publication No. 06-E004. Rockville, MD: Agency for Healthcare Research and Quality. February 2006.
- Ameye L, Timmerman D, Valentin L, et al. Clinically oriented three-step strategy for assessment of adnexal pathology. Ultrasound Obstet Gynecol 2012; 40:582–591.
- Covens AL, Dodge JE, Lacchetti C, et al; Gynecology Cancer Disease Site Group. Surgical management of a suspicious adnexal mass: a systematic review. Gynecol Oncol 2012; 126:149–156.
- Liu JH, Zanotti KM. Management of the adnexal mass. Obstet Gynecol 2011; 117:1413–1428.
- Goff BA, Mandel LS, Drescher CW, et al. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer 2007; 109:221–227.
- Rossing MA, Wicklund KG, Cushing-Haugen KL, Weiss NS. Predictive value of symptoms for early detection of ovarian cancer. J Natl Cancer Inst 2010; 102:222–229.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Management of adnexal masses. Obstet Gynecol 2007; 110:201–214.
- BestPractice BMJ Evidence Centre. Ovarian cysts-Diagnosis-History & examination—Risk factors. http://bestpractice.bmj.com/best-practice/monograph/660/diagnosis.html. Accessed June 2, 2013.
- American Cancer Society. Ovarian-cancer risk factors. www.cancer.org/Cancer/OvarianCancer/DetailedGuide/ovarian-cancer-survival-rates. Accessed May 9, 2013.
- Bast RC, Klug TL, St John E, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med 1983; 309:883–887.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 477: the role of the obstetrician-gynecologist in the early detection of epithelial ovarian cancer. Obstet Gynecol 2011; 117:742–746.
- Im SS, Gordon AN, Buttin BM, et al. Validation of referral guidelines for women with pelvic masses. Obstet Gynecol 2005; 105:35–41.
- Dearking AC, Aletti GD, McGree ME, Weaver AL, Sommerfield MK, Cliby WA. How relevant are ACOG and SGO guidelines for referral of adnexal mass? Obstet Gynecol 2007; 110:841–848.
- Skates SJ, Menon U, MacDonald N, et al. Calculation of the risk of ovarian cancer from serial CA-125 values for preclinical detection in postmenopausal women. J Clin Oncol 2003; 21(suppl 10):206s–210s.
- Ueland FR, Desimone CP, Seamon LG, et al. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet Gynecol 2011; 117:1289–1297.
- Ware Miller R, Smith A, DeSimone CP, et al. Performance of the American College of Obstetricians and Gynecologists’ ovarian tumor referral guidelines with a multivariate index assay. Obstet Gynecol 2011; 117:1298–1306.
- Lev-Toaff AS, Horrow MM, Andreotti RF, et al. Expert Panel on Women’s Imaging. ACR Appropriateness Criteria clinically suspected adnexal mass. Reston, VA: American College of Radiology (ACR), 2009. www.guidelines.gov/content.aspx?id=15780&search=adnexal+mass. Accessed May 9, 2013.
- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2010; 256:943–954.
- Alcázar JL, Castillo G, Jurado M, García GL. Is expectant management of sonographically benign adnexal cysts an option in selected asymptomatic premenopausal women? Hum Reprod 2005; 20:3231–3234.
- Medeiros LR, Freitas LB, Rosa DD, et al. Accuracy of magnetic resonance imaging in ovarian tumor: a systematic quantitative review. Am J Obstet Gynecol 2011; 204:67.e1–67.e10.
- Kinkel K, Lu Y, Mehdizade A, Pelte MF, Hricak H. Indeterminate ovarian mass at US: incremental value of second imaging test for characterization—meta-analysis and Bayesian analysis. Radiology 2005; 236:85–94.
- Timmerman D, Ameye L, Fischerova D, et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. BMJ 2010; 341:c6839.
- Valentin L, Ameye L, Savelli L, et al. Adnexal masses difficult to classify as benign or malignant using subjective assessment of gray-scale and Doppler ultrasound findings: logistic regression models do not help. Ultrasound Obstet Gynecol 2011; 38:456–465.
- Dodge JE, Covens AL, Lacchetti C, et al; Gynecology Cancer Disease Site Group. Preoperative identification of a suspicious adnexal mass: a systematic review and meta-analysis. Gynecol Oncol 2012; 126:157–166.
- Grimes DA, Jones LB, Lopez LM, Schulz KF. Oral contraceptives for functional ovarian cysts. Cochrane Database Syst Rev 2011; 9:CD006134.
- Higgins RV, Matkins JF, Marroum MC. Comparison of fine-needle aspiration cytologic findings of ovarian cysts with ovarian histologic findings. Am J Obstet Gynecol 1999; 180:550–553.
- Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet 2001; 357:176–182.
- Zanetta G, Lissoni A, Torri V, et al. Role of puncture and aspiration in expectant management of simple ovarian cysts: a randomised study. BMJ 1996; 313:1110–1113.
- Bonilla-Musoles F, Ballester MJ, Simon C, Serra V, Raga F. Is avoidance of surgery possible in patients with perimenopausal ovarian tumors using transvaginal ultrasound and duplex color Doppler sonography? J Ultrasound Med 1993; 12:33–39.
- Medeiros LR, Rosa DD, Bozzetti MC, et al. Laparoscopy versus laparotomy for FIGO Stage I ovarian cancer. Cochrane Database Syst Rev 2008; 4:CD005344.
- Goff BA, Miller JW, Matthews B, et al. Involvement of gynecologic oncologists in the treatment of patients with a suspicious ovarian mass. Obstet Gynecol 2011; 118:854–862.
- Earle CC, Schrag D, Neville BA, et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst 2006; 98:172–180.
- Giede KC, Kieser K, Dodge J, Rosen B. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol 2005; 99:447–461.
- Cress RD, Bauer K, O’Malley CD, et al. Surgical staging of early stage epithelial ovarian cancer: results from the CDC-NPCR ovarian patterns of care study. Gynecol Oncol 2011; 121:94–99.
- Horowitz NS. Management of adnexal masses in pregnancy. Clin Obstet Gynecol 2011; 54:519–527.
Ovarian cysts, sometimes reported as ovarian masses or adnexal masses, are frequently found incidentally in women who have no symptoms. These cysts can be physiologic (having to do with ovulation) or neoplastic—either benign, borderline (having low malignant potential), or frankly malignant. Thus, these incidental lesions pose many diagnostic challenges to the clinician.
The vast majority of cysts are benign, but a few are malignant, and ovarian malignancies have a notoriously poor survival rate. The diagnosis can only be obtained surgically, as aspiration and biopsy are not definitive and may be harmful. Therefore, the clinician must try to balance the risks of surgery for what may be a benign lesion with the risk of delaying diagnosis of a malignancy.
In this article we provide an approach to evaluating these cysts, with guidance on when the patient can be reassured and when referral is needed.
THE DILEMMA OF OVARIAN CYSTS
Ovarian cysts are common
Premenopausal women can be expected to make at least a small cyst or follicle almost every month. The point prevalence for significant cysts has been reported to be almost 8% in premenopausal women.1
Surprisingly, the prevalence in postmenopausal women is as high as 14% to 18%, with a yearly incidence of 8%. From 30% to 54% of postmenopausal ovarian cysts persist for years.2,3
Little is known about the cause of most cysts
Little is known about the cause of most ovarian cysts. Functional or physiologic cysts are thought to be variations in the ovulatory process. They do not seem to be precursors to ovarian cancer.
Most benign neoplastic cysts are also not thought to be precancerous, with the possible exception of the mucinous kind.4 Ovarian cysts do not increase the risk of ovarian cancer later in life,3,9 and removing benign cysts has not been shown to decrease the risk of death from ovarian cancer.10
Most ovarian cysts and masses are benign
Simple ovarian cysts are much more likely to be benign than malignant. Complex and solid ovarian masses are also more likely to be benign, regardless of menopausal status, but more malignancies are found in this group.
With any kind of mass, the chances of malignancy increase with age. Children and adolescents are not discussed in this article; they should be referred to a specialist.
Ovarian cancer often has a poor prognosis
This “silent” cancer is most often discovered and treated when it has already spread, contributing to a reported 5-year survival rate of only 33% to 46%.11–13 Ideally, ovarian cancer would be found and removed while still confined to the ovary, when the 5-year survival rate is greater than 90%.
Unfortunately, there does not seem to be a precursor lesion for most ovarian cancers, and there is no good way of finding it in the stage 1 phase, so detecting this cancer before it spreads remains an elusive goal.11,14
Surgery is required to diagnose difficult cases
There is no perfect test for the preoperative assessment of a cystic ovarian mass. Every method has drawbacks (Table 1).15–18 Therefore, the National Institutes of Health estimates that 5% to 10% of women in the United States will undergo surgical exploration for an ovarian cyst in their lifetime. Only 13% to 21% of these cysts will be malignant.5
ASSESSING AN INCIDENTALLY DISCOVERED OVARIAN MASS
Certain factors in the history, physical examination, and blood work may suggest the cyst is either benign or malignant and may influence the subsequent assessment. However, in most cases, the best next step is to perform transvaginal ultrasonography, which we will discuss later in this paper.
History
Age is a major risk factor for ovarian cancer; the median age at diagnosis is 63 years.9 In the reproductive-age group, ovarian cysts are much more likely to be functional than neoplastic. Epithelial cancers are rare before the age of 40, but other cancer types such as borderline, germ cell, and sex cord stromal tumors may occur.19
In every age group a cyst is more likely to be benign than malignant, although, as noted above, the probability of malignancy increases with age.
Symptoms. Most ovarian cysts, benign or malignant, are asymptomatic and are found only incidentally.
The most commonly reported symptoms are pelvic or lower-abdominal pressure or pain. Acutely painful conditions include ovarian torsion, hemorrhage into the cyst, cyst rupture with or without intra-abdominal hemorrhage, ectopic pregnancy, and pelvic inflammatory disease with tubo-ovarian abscess.
Some patients who have ovarian cancer report vague symptoms such as urinary urgency or frequency, abdominal distention or bloating, and difficulty eating or early satiety.20 Although the positive predictive value of this symptom constellation is only about 1%, its usefulness increases if these symptoms arose recently (within the past year) and occur than 12 days a month.21
Family history of ovarian, breast, endometrial, or colon cancer is of particular interest. The greater the number of affected relatives and the closer the degree of relation, the greater the risk; in some cases the relative risk is 40 times greater.22 Breast-ovarian cancer syndromes, hereditary nonpolyposis colorectal cancer syndrome, and family cancer syndrome, as well as extremely high-risk pedigrees such as BRCA1, BRCA2, and Lynch syndrome, all place women at significantly higher risk. Daughters tend to develop cancer at a younger age than their affected mothers.
However, only 10% of ovarian cancers occur in patients who have a family history of it, leaving 90% as sporadic occurrences.
Other history. Factors protective against ovarian cancer include use of oral contraceptives at any time, tubal ligation, hysterectomy, having had children, breastfeeding, a low-fat diet, and possibly use of aspirin and acetaminophen.23,24
Risk factors for malignancy include advanced age; nulliparity; family history of ovarian or breast cancer; personal history of breast cancer; talc use; asbestos exposure; white ethnicity; pelvic irradiation; smoking; alcohol use; possibly the previous use of fertility drugs, estrogen, or androgen; history of mumps; urban location; early menarche; and late menopause.24
Physical examination
Vital signs. Fever can indicate an infectious process or torsion of the ovary. A sudden onset of low blood pressure or rapid pulse can indicate a hemorrhagic condition such as ectopic pregnancy or ruptured hemorrhagic cyst.
Bimanual pelvic examination is notoriously inaccurate for detecting and characterizing ovarian cysts. In one prospective study, examiners blinded to the reason for surgery evaluated women under anesthesia. The authors concluded that bimanual examination was of limited value even under the best circumstances.15 Pelvic examination can be even more difficult in patients who are obese, are virginal, have vaginal atrophy, or are in pain.
Useful information that can be obtained through the bimanual examination includes the exact location of pelvic tenderness, the relative firmness of an identified mass, and the existence of nodularity in the posterior cul-de-sac, suggesting advanced ovarian cancer.
Tumor markers
Cancer antigen 125 (CA125) is the most studied and widely used of the ovarian cancer tumor markers. When advanced epithelial ovarian cancer is associated with a markedly elevated level, the value correlates with tumor burden.25
Unfortunately, only about half of early-stage ovarian cancers and 75% to 80% of advanced ovarian cancers express this marker.26 Especially in premenopausal women, there are many pelvic conditions that can falsely elevate CA125. Therefore, its sensitivity and specificity for predicting ovarian cancer are suboptimal. Nevertheless, CA125 is often used to help stratify risk when assessing known ovarian cysts and masses.
The value considered abnormal in postmenopausal women is 35 U/mL or greater, while in premenopausal women the cutoff is less well defined. The lower the cutoff level is set, the more sensitive the test. Recent recommendations advise 50 U/mL or 67 U/mL, rather than the 200 U/mL recommended in the 2002 joint guidelines of the American Congress of Obstetricians and Gynecologists and the Society of Gynecologic Oncology.27,28
However, specificity is likely to be lower with these lower cutoff values. Conditions that can elevate CA125 levels include almost anything that irritates the peritoneum, including pregnancy, menstruation, fibroids, endometriosis, infection, and ovarian hyperstimulation, as well as medical conditions such as liver or renal disease, colitis, diverticulitis, congestive heart failure, diabetes, autoimmune diseases, and ascites.
Following serial CA125 levels may be more sensitive than trying to establish a single cutoff value.29 CA125 should not be used as a screening tool in average-risk women.26
OVA1. Several biomarker panels have been developed and evaluated for risk assessment in women with pelvic masses. OVA1, a proprietary panel of tests (Vermillion; Austin, TX) received US Food and Drug Administration approval in 2009. It includes CA125 and four other proteins, from which it calculates a probability score (high or low) using a proprietary formula.
In prospective studies, OVA1 was more sensitive than clinical assessment or CA125 alone.30 The higher sensitivity and negative predictive value were counterbalanced by a lower specificity and positive predictive value.31 Its cost ($650) is not always covered by insurance. OVA1 is not a screening tool.
EVALUATION WITH ULTRASONOGRAPHY
Ultrasonography is the imaging test of choice in assessing adnexal cysts and masses, and therefore it is the best next step after taking a history, performing a physical examination, and obtaining blood work.32 In cases in which an incidental ovarian mass is discovered on computed tomography (CT), further characterization by ultrasonography will likely yield helpful information.
Pelvic ultrasonography can be performed transabdominally or transvaginally. Vaginal ultrasonography gives the clearest images in most patients. Abdominal scanning is indicated for large masses, when vaginal access is difficult (as in virginal patients or those with vaginal atrophy) or when the mass is out of the focal length of the vaginal probe. A full bladder is usually required for the best transabdominal images.
The value of the images obtained depends on the experience of the ultrasonographer and reader and on the equipment. Also, there is currently no widely used standard for reporting the findings33—descriptions are individualized, leading some authors to recommend that the clinician personally review the films to get the most accurate picture.19
Size
Size alone cannot be used to distinguish between benign and malignant lesions. Simple cysts up to 10 cm are most likely benign regardless of menopausal status.2,34 However, in a complex or solid mass, size correlates somewhat with the chance of malignancy, with notable exceptions, such as the famously large sizes of some solid fibromas or mucinous cystadenomas. Also, size may correlate with risk of other complications such as torsion or symptomatic rupture.
Complexity
Simple cysts have clear fluid, thin smooth walls, no loculations or septae, and enhanced through-transmission of echo waves.32,33
Complexity is described in terms of septations, wall thickness, internal echoes, and solid nodules. Increasing complexity does correlate with increased risk of malignancy.
Worrisome findings
The most worrisome findings are:
- Solid areas that are not hyperechoic, especially when there is blood flow to them
- Thick septations, more than 2 or 3 mm wide, especially if there is blood flow within them
- Excrescences on the inner or outer aspect of a cystic area
- Ascites
- Other pelvic or omental masses.
Benign conditions
Several benign conditions have characteristic complex findings on ultrasonography (Table 2), whereas other findings can be indeterminate (Table 3) or worrisome for malignancy (Table 4).
Hemorrhagic corpus luteum cysts can be complex with an internal reticular pattern due to organizing clot and fibrin strands. A “ring of fire” vascular pattern is often seen around the cyst bed.
Dermoids (mature cystic teratomas) may have hyperechoic elements with acoustic shadowing and no internal Doppler flow. They can have a complex appearance due to fat, hair, and sebum within the cyst. Dermoid cysts have a pathognomonic appearance on CT with a clear fat-fluid level.
Endometriomas classically have a homogeneous “ground-glass” appearance or low-level echoes, without internal color Doppler flow, wall nodules, or other malignant features.
Fibroids may be pedunculated and may appear to be complex or solid adnexal masses.
Hydrosalpinges may present as tortuous tubular-shaped cystic masses. There may be incomplete septations or indentations seen on opposite sides (the “waist” sign).
Paratubal and paraovarian cysts are usually simple round cysts that can be demonstrated as separate from the ovary. Sometimes these appear complex as well.
Peritoneal inclusion cysts, also known as pseudocysts, are seen in patients with intra-abdominal adhesions. Often multiple septations are seen through clear fluid, with the cyst conforming to the shape of other pelvic structures.
Torsion of the ovary may occur with either benign or malignant masses. Torsion can be diagnosed when venous flow is absent on Doppler. The presence of flow, however, doesn’t rule out torsion, as torsion is often intermittent. The twisted ovary is most often enlarged and can have an edematous appearance. Although typically benign, these should be referred for urgent surgical treatment.
Vascularity
Doppler imaging is being extensively studied. The general principle is that malignant masses will be more vascular, with a high-volume, low-resistance pattern of flow. This can result in a pulsatility index of less than 1 or a resistive index of less than 0.4. In practice, however, there is significant overlap between high and low pulsatility indices and resistive indices in benign and malignant cysts. Low resistance can also be found in endometriomas, corpus luteum cysts, inflammatory masses, and vascular benign neoplasms. A normal (high) resistive index does not rule out malignancy.32,33
One Doppler finding that does seem to correlate with malignancy is the presence of any flow within a solid nodule or wall excrescence.
3D ultrasonography
As the use of 3D ultrasonography increases, studies are yielding different results as to its utility in describing ovarian masses. 3D ultrasonography may be useful in finding centrally located vessels so that Doppler can be applied.32
OTHER IMAGING
Although ultrasonography is the initial imaging study of choice in the evaluation of adnexal masses owing to its high sensitivity, availability, and low cost, studies have shown that up to 20% of adnexal masses can be reported as indeterminate by ultrasonography (Table 1).
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is emerging as a very valuable tool when ultrasonography is inconclusive or limited.35 Although MRI is very accurate (Table 1), it is not considered a first-line imaging test because it is more expensive, less available, and more inconvenient for the patient than ultrasonography.
MRI provides additional information on the composition of soft-tissue tumors. Usually, MRI is ordered with contrast, unless there are contraindications to it. The radiologist will evaluate morphologic features, signal intensity, and enhancement of solid areas. Techniques such as dynamic contrast-enhanced MRI (following the distribution of contrast material over time), in- and out-of-phase T1 imaging (looking for fat, such as in dermoids), and the newer diffusion-weighted imaging may further improve characterization.
In one study of MRI as second-line imaging, contrast-enhanced MRI contributed to a greater change in the probability of ovarian cancer than did CT, Doppler ultrasonography, or MRI without contrast.36 This may result in a reduction in unnecessary surgeries and in an increase in proper referrals in cases of suspected malignancy.
Computed tomography
Disadvantages of CT include radiation exposure and poor discrimination of soft tissue. It can, however, differentiate fat or calcifications that may be found in dermoids. While CT is not often used to describe an ovarian lesion, it may be used preoperatively to stage an ovarian cancer or to look for a primary intra-abdominal cancer when an ovarian mass may represent metastasis.32
MANAGING AN INCIDENTAL OVARIAN CYST OR CYSTIC MASS
Combining information from the history, physical examination, imaging, and blood work to assign a level of risk of malignancy is not straightforward. The clinician must weigh several imperfect tests, each with its own sensitivity and specificity, against the background of the individual patient’s likelihood of malignancy. Whereas a 4-cm simple cyst in a premenopausal woman can be assigned to a low-risk category and a complex mass with flow to a solid component in a postmenopausal woman can be assigned to a high-risk category, many lesions are more difficult to assess.
Several systems have been proposed for analyzing data and standardizing risk assessment. There are a number of scoring systems based on ultrasonographic morphology and several mathematical regression models that include menopausal status and tumor markers. But each has drawbacks, and none is definitively superior to expert opinion.16,17,37,38
A 2012 systematic review and meta-analysis39 calculated sensitivity and specificity for several imaging tests, scoring systems, and blood tumor markers. Some results are presented in Table 1.
The management of an ovarian cyst depends on symptoms, likelihood of torsion or rupture, and the level of concern for malignancy. At the lower-risk end of the spectrum, reassurance or observation over time may be appropriate. A general gynecologist can evaluate indeterminate or symptomatic ovarian cysts. Patients with masses frankly suspicious for malignancy are best referred to a gynecologic oncologist.
Expectant management for low-risk lesions
Low-risk lesions such as simple cysts, endometriomas, and dermoids have a less than 1% chance of malignancy. Most patients who have them require only reassurance or follow-up with serial ultrasonography. Oral contraceptives may prevent new cysts from forming. Aspiration is not recommended.
In 2010, the Society of Radiologists in Ultrasound issued a consensus statement regarding re-imaging of simple ovarian cysts.33
In premenopausal women, they recommend no further testing for cysts 5 cm or smaller, yearly follow-up for cysts larger than 5 cm and up to and including 7 cm, and MRI or surgical evaluation for cysts larger than 7 cm, as it is difficult to completely image a large cyst with ultrasonography.
In postmenopausal women, if the cyst is 1 cm in diameter or smaller, no further studies need to be done. For simple cysts larger than 1 cm and up to and including 7 cm, yearly re-imaging is recommended. And for cysts larger than 7 cm, MRI or surgery is indicated. The American College of Radiology recommends repeat ultrasonography and CA125 testing for cysts 3 cm and larger but doesn’t specify an interval.32
A cyst that is otherwise simple but has a single thin septation (< 3 mm) or a small calcification in the wall is almost always benign. Such a cyst should be followed as if it were a simple cyst, as indicated by patient age and cyst size.
There are no official guidelines as to when to stop serial imaging,22,32 but a recent paper suggested one or two ultrasonographic examinations to confirm size and morphologic stability.19 Once a lesion has resolved, there is no need for further imaging (Figures 1–3).
Birth control pills for suppression of new cysts. Oral contraceptives do not hasten the resolution of ovarian cysts, according to a 2011 Cochrane review.40 Some practitioners will, nevertheless, prescribe them in an attempt to prevent new cysts from confusing the picture.
Aspiration is not recommended for either diagnosis or treatment. It can only be considered in patients at high risk who are not surgical candidates. Results of cytologic study of specimens obtained by fine-needle aspiration cannot reliably determine the presence or absence of malignancy.41 There is also a theoretical risk of spreading cancer from an early-stage lesion. A retrospective study has suggested that spillage of cyst contents during surgery in early ovarian cancer is associated with a worse prognosis.42
From a therapeutic point of view, studies have shown the same resolution rate at 6 months for aspirated cysts vs those followed expectantly.43 Another study found a recurrence rate of 25% within 1 year of aspiration.44
Referral for medium-risk or indeterminant-risk ovarian masses
Patients who have medium- or indeterminaterisk ovarian masses (Table 3) should be referred to a gynecologist. Further testing will help stratify the risk of malignancy. This can include tumor marker blood tests, MRI, or CT, the addition of Doppler or 3D ultrasonography, serial ultrasonography, or surgical exploration.
If repeat ultrasonography is chosen, the interval will likely be 6 to 12 weeks. Surgery may consist of removing only the cyst itself, or the whole ovary with or without the tube, or sometimes both ovaries. Purely diagnostic laparoscopy is rarely performed, as direct visualization of a lesion is rarely helpful. Frozen section should be employed, and the operating gynecologist should have oncologic backup, since the surgery is performed to rule out malignancy.
In the case of a benign-appearing cyst larger than 6 cm, thought must be given as to whether it is likely to rupture or twist. Rupture of a large cyst can lead to pain and in some cases to hemorrhage. Contents of a ruptured dermoid cyst can cause chemical peritonitis. Torsion of an ovary can result in loss of the ovary through compromised perfusion. A general gynecologist can decide with the patient whether preemptive surgery is indicated.
Operative evaluation for high-risk masses
Patients with high-risk ovarian masses (Table 4) are best referred to a gynecologic oncologist for operative evaluation. If features are seen that indicate malignancy, such as thick septations, solid areas with blood flow, ascites, or other pelvic masses, surgery is indicated. The surgical approach may be through laparoscopy or laparotomy.45 It should be noted that even in the face of worrisome features on ultrasonography, many masses turn out to be benign.
In 2011, the American Congress of Obstetricians and Gynecologists and the Society of Gynecologic Oncology issued new guidelines recommending oncologic referral of patients with high-risk masses. Elevated CA125, ascites, a nodular or fixed pelvic mass, or evidence of metastasis in postmenopausal women requires oncologic evaluation.26 For premenopausal women, a very elevated CA125, ascites, or metastasis requires referral (Table 4).26
Direct referral to a gynecologic oncologist is underutilized. A recent study found that fewer than half of primary care physicians said that they would refer a classic suspicious case directly to a subspecialist.46 It is estimated that only 33% of ovarian cancers are first operated on by a gynecologic oncologist.47
A 2011 Cochrane review confirmed a survival benefit for women with cancer who are operated on by gynecologic oncologists primarily, rather than by a general gynecologist and then referred.48 A gynecologic oncologist is most likely to perform proper staging and debulking at the time of initial diagnosis.49
Special situations require consultation
Ovarian cysts in pregnancy are most often benign,50 but malignancy is always a possibility. Functional cysts and dermoids are the most common. These may remain asymptomatic or may rupture or twist or cause difficulty with delivering the baby. Surgical intervention, if needed, should be performed in the second trimester if possible. A multidisciplinary approach and referral to a perinatologist and gynecologic oncologist are advised.
Symptomatic ovarian cysts that may need surgical intervention are the purview of the general gynecologist. If the risk of a surgical emergency is judged to be low, a symptomatic patient may be supported with pain medication and may be managed on an outpatient basis. Immediate surgical consultation is appropriate if the patient appears toxic or in shock. Depending on the clinical picture, there may be a ruptured tubo-ovarian abscess, ruptured ectopic pregnancy, ruptured hemorrhagic cyst, or ovarian torsion, any of which may need immediate surgical intervention.
If a symptomatic mass is highly suspicious for cancer, a gynecologic oncologist should be consulted directly.
WHEN TO REASSURE, REASSESS, REFER
Ovarian masses often pose diagnostic and management dilemmas. Reassurance can be offered to women with small simple cysts. Interval follow-up with ultrasonography is appropriate for cysts that are most likely to be benign. If malignancy is suspected based on ultrasonography, other imaging, blood testing, or expert opinion, referral to a surgical gynecologist or gynecologic oncologist is recommended. If malignancy is strongly suspected, direct referral to a gynecologic oncologist offers the best chance of survival if cancer is actually present.
Reassure
- When simple cysts are less than 1 cm in postmenopausal women
- When simple cysts are less than 5 cm in premenopausal patients.
Reassess
- With yearly ultrasonography in cases of very low risk
- With repeat ultrasonography in 6 to 12 weeks when the diagnosis is not clear but the cyst is likely benign.
Refer
- To a gynecologist for symptomatic cysts, cysts larger than 6 cm, and cysts that require ancillary testing
- To a gynecologic oncologist for findings worrisome for cancer, such as thick septations, solid areas with flow, ascites, evidence of metastasis, or high cancer antigen 125 levels.
Ovarian cysts, sometimes reported as ovarian masses or adnexal masses, are frequently found incidentally in women who have no symptoms. These cysts can be physiologic (having to do with ovulation) or neoplastic—either benign, borderline (having low malignant potential), or frankly malignant. Thus, these incidental lesions pose many diagnostic challenges to the clinician.
The vast majority of cysts are benign, but a few are malignant, and ovarian malignancies have a notoriously poor survival rate. The diagnosis can only be obtained surgically, as aspiration and biopsy are not definitive and may be harmful. Therefore, the clinician must try to balance the risks of surgery for what may be a benign lesion with the risk of delaying diagnosis of a malignancy.
In this article we provide an approach to evaluating these cysts, with guidance on when the patient can be reassured and when referral is needed.
THE DILEMMA OF OVARIAN CYSTS
Ovarian cysts are common
Premenopausal women can be expected to make at least a small cyst or follicle almost every month. The point prevalence for significant cysts has been reported to be almost 8% in premenopausal women.1
Surprisingly, the prevalence in postmenopausal women is as high as 14% to 18%, with a yearly incidence of 8%. From 30% to 54% of postmenopausal ovarian cysts persist for years.2,3
Little is known about the cause of most cysts
Little is known about the cause of most ovarian cysts. Functional or physiologic cysts are thought to be variations in the ovulatory process. They do not seem to be precursors to ovarian cancer.
Most benign neoplastic cysts are also not thought to be precancerous, with the possible exception of the mucinous kind.4 Ovarian cysts do not increase the risk of ovarian cancer later in life,3,9 and removing benign cysts has not been shown to decrease the risk of death from ovarian cancer.10
Most ovarian cysts and masses are benign
Simple ovarian cysts are much more likely to be benign than malignant. Complex and solid ovarian masses are also more likely to be benign, regardless of menopausal status, but more malignancies are found in this group.
With any kind of mass, the chances of malignancy increase with age. Children and adolescents are not discussed in this article; they should be referred to a specialist.
Ovarian cancer often has a poor prognosis
This “silent” cancer is most often discovered and treated when it has already spread, contributing to a reported 5-year survival rate of only 33% to 46%.11–13 Ideally, ovarian cancer would be found and removed while still confined to the ovary, when the 5-year survival rate is greater than 90%.
Unfortunately, there does not seem to be a precursor lesion for most ovarian cancers, and there is no good way of finding it in the stage 1 phase, so detecting this cancer before it spreads remains an elusive goal.11,14
Surgery is required to diagnose difficult cases
There is no perfect test for the preoperative assessment of a cystic ovarian mass. Every method has drawbacks (Table 1).15–18 Therefore, the National Institutes of Health estimates that 5% to 10% of women in the United States will undergo surgical exploration for an ovarian cyst in their lifetime. Only 13% to 21% of these cysts will be malignant.5
ASSESSING AN INCIDENTALLY DISCOVERED OVARIAN MASS
Certain factors in the history, physical examination, and blood work may suggest the cyst is either benign or malignant and may influence the subsequent assessment. However, in most cases, the best next step is to perform transvaginal ultrasonography, which we will discuss later in this paper.
History
Age is a major risk factor for ovarian cancer; the median age at diagnosis is 63 years.9 In the reproductive-age group, ovarian cysts are much more likely to be functional than neoplastic. Epithelial cancers are rare before the age of 40, but other cancer types such as borderline, germ cell, and sex cord stromal tumors may occur.19
In every age group a cyst is more likely to be benign than malignant, although, as noted above, the probability of malignancy increases with age.
Symptoms. Most ovarian cysts, benign or malignant, are asymptomatic and are found only incidentally.
The most commonly reported symptoms are pelvic or lower-abdominal pressure or pain. Acutely painful conditions include ovarian torsion, hemorrhage into the cyst, cyst rupture with or without intra-abdominal hemorrhage, ectopic pregnancy, and pelvic inflammatory disease with tubo-ovarian abscess.
Some patients who have ovarian cancer report vague symptoms such as urinary urgency or frequency, abdominal distention or bloating, and difficulty eating or early satiety.20 Although the positive predictive value of this symptom constellation is only about 1%, its usefulness increases if these symptoms arose recently (within the past year) and occur than 12 days a month.21
Family history of ovarian, breast, endometrial, or colon cancer is of particular interest. The greater the number of affected relatives and the closer the degree of relation, the greater the risk; in some cases the relative risk is 40 times greater.22 Breast-ovarian cancer syndromes, hereditary nonpolyposis colorectal cancer syndrome, and family cancer syndrome, as well as extremely high-risk pedigrees such as BRCA1, BRCA2, and Lynch syndrome, all place women at significantly higher risk. Daughters tend to develop cancer at a younger age than their affected mothers.
However, only 10% of ovarian cancers occur in patients who have a family history of it, leaving 90% as sporadic occurrences.
Other history. Factors protective against ovarian cancer include use of oral contraceptives at any time, tubal ligation, hysterectomy, having had children, breastfeeding, a low-fat diet, and possibly use of aspirin and acetaminophen.23,24
Risk factors for malignancy include advanced age; nulliparity; family history of ovarian or breast cancer; personal history of breast cancer; talc use; asbestos exposure; white ethnicity; pelvic irradiation; smoking; alcohol use; possibly the previous use of fertility drugs, estrogen, or androgen; history of mumps; urban location; early menarche; and late menopause.24
Physical examination
Vital signs. Fever can indicate an infectious process or torsion of the ovary. A sudden onset of low blood pressure or rapid pulse can indicate a hemorrhagic condition such as ectopic pregnancy or ruptured hemorrhagic cyst.
Bimanual pelvic examination is notoriously inaccurate for detecting and characterizing ovarian cysts. In one prospective study, examiners blinded to the reason for surgery evaluated women under anesthesia. The authors concluded that bimanual examination was of limited value even under the best circumstances.15 Pelvic examination can be even more difficult in patients who are obese, are virginal, have vaginal atrophy, or are in pain.
Useful information that can be obtained through the bimanual examination includes the exact location of pelvic tenderness, the relative firmness of an identified mass, and the existence of nodularity in the posterior cul-de-sac, suggesting advanced ovarian cancer.
Tumor markers
Cancer antigen 125 (CA125) is the most studied and widely used of the ovarian cancer tumor markers. When advanced epithelial ovarian cancer is associated with a markedly elevated level, the value correlates with tumor burden.25
Unfortunately, only about half of early-stage ovarian cancers and 75% to 80% of advanced ovarian cancers express this marker.26 Especially in premenopausal women, there are many pelvic conditions that can falsely elevate CA125. Therefore, its sensitivity and specificity for predicting ovarian cancer are suboptimal. Nevertheless, CA125 is often used to help stratify risk when assessing known ovarian cysts and masses.
The value considered abnormal in postmenopausal women is 35 U/mL or greater, while in premenopausal women the cutoff is less well defined. The lower the cutoff level is set, the more sensitive the test. Recent recommendations advise 50 U/mL or 67 U/mL, rather than the 200 U/mL recommended in the 2002 joint guidelines of the American Congress of Obstetricians and Gynecologists and the Society of Gynecologic Oncology.27,28
However, specificity is likely to be lower with these lower cutoff values. Conditions that can elevate CA125 levels include almost anything that irritates the peritoneum, including pregnancy, menstruation, fibroids, endometriosis, infection, and ovarian hyperstimulation, as well as medical conditions such as liver or renal disease, colitis, diverticulitis, congestive heart failure, diabetes, autoimmune diseases, and ascites.
Following serial CA125 levels may be more sensitive than trying to establish a single cutoff value.29 CA125 should not be used as a screening tool in average-risk women.26
OVA1. Several biomarker panels have been developed and evaluated for risk assessment in women with pelvic masses. OVA1, a proprietary panel of tests (Vermillion; Austin, TX) received US Food and Drug Administration approval in 2009. It includes CA125 and four other proteins, from which it calculates a probability score (high or low) using a proprietary formula.
In prospective studies, OVA1 was more sensitive than clinical assessment or CA125 alone.30 The higher sensitivity and negative predictive value were counterbalanced by a lower specificity and positive predictive value.31 Its cost ($650) is not always covered by insurance. OVA1 is not a screening tool.
EVALUATION WITH ULTRASONOGRAPHY
Ultrasonography is the imaging test of choice in assessing adnexal cysts and masses, and therefore it is the best next step after taking a history, performing a physical examination, and obtaining blood work.32 In cases in which an incidental ovarian mass is discovered on computed tomography (CT), further characterization by ultrasonography will likely yield helpful information.
Pelvic ultrasonography can be performed transabdominally or transvaginally. Vaginal ultrasonography gives the clearest images in most patients. Abdominal scanning is indicated for large masses, when vaginal access is difficult (as in virginal patients or those with vaginal atrophy) or when the mass is out of the focal length of the vaginal probe. A full bladder is usually required for the best transabdominal images.
The value of the images obtained depends on the experience of the ultrasonographer and reader and on the equipment. Also, there is currently no widely used standard for reporting the findings33—descriptions are individualized, leading some authors to recommend that the clinician personally review the films to get the most accurate picture.19
Size
Size alone cannot be used to distinguish between benign and malignant lesions. Simple cysts up to 10 cm are most likely benign regardless of menopausal status.2,34 However, in a complex or solid mass, size correlates somewhat with the chance of malignancy, with notable exceptions, such as the famously large sizes of some solid fibromas or mucinous cystadenomas. Also, size may correlate with risk of other complications such as torsion or symptomatic rupture.
Complexity
Simple cysts have clear fluid, thin smooth walls, no loculations or septae, and enhanced through-transmission of echo waves.32,33
Complexity is described in terms of septations, wall thickness, internal echoes, and solid nodules. Increasing complexity does correlate with increased risk of malignancy.
Worrisome findings
The most worrisome findings are:
- Solid areas that are not hyperechoic, especially when there is blood flow to them
- Thick septations, more than 2 or 3 mm wide, especially if there is blood flow within them
- Excrescences on the inner or outer aspect of a cystic area
- Ascites
- Other pelvic or omental masses.
Benign conditions
Several benign conditions have characteristic complex findings on ultrasonography (Table 2), whereas other findings can be indeterminate (Table 3) or worrisome for malignancy (Table 4).
Hemorrhagic corpus luteum cysts can be complex with an internal reticular pattern due to organizing clot and fibrin strands. A “ring of fire” vascular pattern is often seen around the cyst bed.
Dermoids (mature cystic teratomas) may have hyperechoic elements with acoustic shadowing and no internal Doppler flow. They can have a complex appearance due to fat, hair, and sebum within the cyst. Dermoid cysts have a pathognomonic appearance on CT with a clear fat-fluid level.
Endometriomas classically have a homogeneous “ground-glass” appearance or low-level echoes, without internal color Doppler flow, wall nodules, or other malignant features.
Fibroids may be pedunculated and may appear to be complex or solid adnexal masses.
Hydrosalpinges may present as tortuous tubular-shaped cystic masses. There may be incomplete septations or indentations seen on opposite sides (the “waist” sign).
Paratubal and paraovarian cysts are usually simple round cysts that can be demonstrated as separate from the ovary. Sometimes these appear complex as well.
Peritoneal inclusion cysts, also known as pseudocysts, are seen in patients with intra-abdominal adhesions. Often multiple septations are seen through clear fluid, with the cyst conforming to the shape of other pelvic structures.
Torsion of the ovary may occur with either benign or malignant masses. Torsion can be diagnosed when venous flow is absent on Doppler. The presence of flow, however, doesn’t rule out torsion, as torsion is often intermittent. The twisted ovary is most often enlarged and can have an edematous appearance. Although typically benign, these should be referred for urgent surgical treatment.
Vascularity
Doppler imaging is being extensively studied. The general principle is that malignant masses will be more vascular, with a high-volume, low-resistance pattern of flow. This can result in a pulsatility index of less than 1 or a resistive index of less than 0.4. In practice, however, there is significant overlap between high and low pulsatility indices and resistive indices in benign and malignant cysts. Low resistance can also be found in endometriomas, corpus luteum cysts, inflammatory masses, and vascular benign neoplasms. A normal (high) resistive index does not rule out malignancy.32,33
One Doppler finding that does seem to correlate with malignancy is the presence of any flow within a solid nodule or wall excrescence.
3D ultrasonography
As the use of 3D ultrasonography increases, studies are yielding different results as to its utility in describing ovarian masses. 3D ultrasonography may be useful in finding centrally located vessels so that Doppler can be applied.32
OTHER IMAGING
Although ultrasonography is the initial imaging study of choice in the evaluation of adnexal masses owing to its high sensitivity, availability, and low cost, studies have shown that up to 20% of adnexal masses can be reported as indeterminate by ultrasonography (Table 1).
Magnetic resonance imaging
Magnetic resonance imaging (MRI) is emerging as a very valuable tool when ultrasonography is inconclusive or limited.35 Although MRI is very accurate (Table 1), it is not considered a first-line imaging test because it is more expensive, less available, and more inconvenient for the patient than ultrasonography.
MRI provides additional information on the composition of soft-tissue tumors. Usually, MRI is ordered with contrast, unless there are contraindications to it. The radiologist will evaluate morphologic features, signal intensity, and enhancement of solid areas. Techniques such as dynamic contrast-enhanced MRI (following the distribution of contrast material over time), in- and out-of-phase T1 imaging (looking for fat, such as in dermoids), and the newer diffusion-weighted imaging may further improve characterization.
In one study of MRI as second-line imaging, contrast-enhanced MRI contributed to a greater change in the probability of ovarian cancer than did CT, Doppler ultrasonography, or MRI without contrast.36 This may result in a reduction in unnecessary surgeries and in an increase in proper referrals in cases of suspected malignancy.
Computed tomography
Disadvantages of CT include radiation exposure and poor discrimination of soft tissue. It can, however, differentiate fat or calcifications that may be found in dermoids. While CT is not often used to describe an ovarian lesion, it may be used preoperatively to stage an ovarian cancer or to look for a primary intra-abdominal cancer when an ovarian mass may represent metastasis.32
MANAGING AN INCIDENTAL OVARIAN CYST OR CYSTIC MASS
Combining information from the history, physical examination, imaging, and blood work to assign a level of risk of malignancy is not straightforward. The clinician must weigh several imperfect tests, each with its own sensitivity and specificity, against the background of the individual patient’s likelihood of malignancy. Whereas a 4-cm simple cyst in a premenopausal woman can be assigned to a low-risk category and a complex mass with flow to a solid component in a postmenopausal woman can be assigned to a high-risk category, many lesions are more difficult to assess.
Several systems have been proposed for analyzing data and standardizing risk assessment. There are a number of scoring systems based on ultrasonographic morphology and several mathematical regression models that include menopausal status and tumor markers. But each has drawbacks, and none is definitively superior to expert opinion.16,17,37,38
A 2012 systematic review and meta-analysis39 calculated sensitivity and specificity for several imaging tests, scoring systems, and blood tumor markers. Some results are presented in Table 1.
The management of an ovarian cyst depends on symptoms, likelihood of torsion or rupture, and the level of concern for malignancy. At the lower-risk end of the spectrum, reassurance or observation over time may be appropriate. A general gynecologist can evaluate indeterminate or symptomatic ovarian cysts. Patients with masses frankly suspicious for malignancy are best referred to a gynecologic oncologist.
Expectant management for low-risk lesions
Low-risk lesions such as simple cysts, endometriomas, and dermoids have a less than 1% chance of malignancy. Most patients who have them require only reassurance or follow-up with serial ultrasonography. Oral contraceptives may prevent new cysts from forming. Aspiration is not recommended.
In 2010, the Society of Radiologists in Ultrasound issued a consensus statement regarding re-imaging of simple ovarian cysts.33
In premenopausal women, they recommend no further testing for cysts 5 cm or smaller, yearly follow-up for cysts larger than 5 cm and up to and including 7 cm, and MRI or surgical evaluation for cysts larger than 7 cm, as it is difficult to completely image a large cyst with ultrasonography.
In postmenopausal women, if the cyst is 1 cm in diameter or smaller, no further studies need to be done. For simple cysts larger than 1 cm and up to and including 7 cm, yearly re-imaging is recommended. And for cysts larger than 7 cm, MRI or surgery is indicated. The American College of Radiology recommends repeat ultrasonography and CA125 testing for cysts 3 cm and larger but doesn’t specify an interval.32
A cyst that is otherwise simple but has a single thin septation (< 3 mm) or a small calcification in the wall is almost always benign. Such a cyst should be followed as if it were a simple cyst, as indicated by patient age and cyst size.
There are no official guidelines as to when to stop serial imaging,22,32 but a recent paper suggested one or two ultrasonographic examinations to confirm size and morphologic stability.19 Once a lesion has resolved, there is no need for further imaging (Figures 1–3).
Birth control pills for suppression of new cysts. Oral contraceptives do not hasten the resolution of ovarian cysts, according to a 2011 Cochrane review.40 Some practitioners will, nevertheless, prescribe them in an attempt to prevent new cysts from confusing the picture.
Aspiration is not recommended for either diagnosis or treatment. It can only be considered in patients at high risk who are not surgical candidates. Results of cytologic study of specimens obtained by fine-needle aspiration cannot reliably determine the presence or absence of malignancy.41 There is also a theoretical risk of spreading cancer from an early-stage lesion. A retrospective study has suggested that spillage of cyst contents during surgery in early ovarian cancer is associated with a worse prognosis.42
From a therapeutic point of view, studies have shown the same resolution rate at 6 months for aspirated cysts vs those followed expectantly.43 Another study found a recurrence rate of 25% within 1 year of aspiration.44
Referral for medium-risk or indeterminant-risk ovarian masses
Patients who have medium- or indeterminaterisk ovarian masses (Table 3) should be referred to a gynecologist. Further testing will help stratify the risk of malignancy. This can include tumor marker blood tests, MRI, or CT, the addition of Doppler or 3D ultrasonography, serial ultrasonography, or surgical exploration.
If repeat ultrasonography is chosen, the interval will likely be 6 to 12 weeks. Surgery may consist of removing only the cyst itself, or the whole ovary with or without the tube, or sometimes both ovaries. Purely diagnostic laparoscopy is rarely performed, as direct visualization of a lesion is rarely helpful. Frozen section should be employed, and the operating gynecologist should have oncologic backup, since the surgery is performed to rule out malignancy.
In the case of a benign-appearing cyst larger than 6 cm, thought must be given as to whether it is likely to rupture or twist. Rupture of a large cyst can lead to pain and in some cases to hemorrhage. Contents of a ruptured dermoid cyst can cause chemical peritonitis. Torsion of an ovary can result in loss of the ovary through compromised perfusion. A general gynecologist can decide with the patient whether preemptive surgery is indicated.
Operative evaluation for high-risk masses
Patients with high-risk ovarian masses (Table 4) are best referred to a gynecologic oncologist for operative evaluation. If features are seen that indicate malignancy, such as thick septations, solid areas with blood flow, ascites, or other pelvic masses, surgery is indicated. The surgical approach may be through laparoscopy or laparotomy.45 It should be noted that even in the face of worrisome features on ultrasonography, many masses turn out to be benign.
In 2011, the American Congress of Obstetricians and Gynecologists and the Society of Gynecologic Oncology issued new guidelines recommending oncologic referral of patients with high-risk masses. Elevated CA125, ascites, a nodular or fixed pelvic mass, or evidence of metastasis in postmenopausal women requires oncologic evaluation.26 For premenopausal women, a very elevated CA125, ascites, or metastasis requires referral (Table 4).26
Direct referral to a gynecologic oncologist is underutilized. A recent study found that fewer than half of primary care physicians said that they would refer a classic suspicious case directly to a subspecialist.46 It is estimated that only 33% of ovarian cancers are first operated on by a gynecologic oncologist.47
A 2011 Cochrane review confirmed a survival benefit for women with cancer who are operated on by gynecologic oncologists primarily, rather than by a general gynecologist and then referred.48 A gynecologic oncologist is most likely to perform proper staging and debulking at the time of initial diagnosis.49
Special situations require consultation
Ovarian cysts in pregnancy are most often benign,50 but malignancy is always a possibility. Functional cysts and dermoids are the most common. These may remain asymptomatic or may rupture or twist or cause difficulty with delivering the baby. Surgical intervention, if needed, should be performed in the second trimester if possible. A multidisciplinary approach and referral to a perinatologist and gynecologic oncologist are advised.
Symptomatic ovarian cysts that may need surgical intervention are the purview of the general gynecologist. If the risk of a surgical emergency is judged to be low, a symptomatic patient may be supported with pain medication and may be managed on an outpatient basis. Immediate surgical consultation is appropriate if the patient appears toxic or in shock. Depending on the clinical picture, there may be a ruptured tubo-ovarian abscess, ruptured ectopic pregnancy, ruptured hemorrhagic cyst, or ovarian torsion, any of which may need immediate surgical intervention.
If a symptomatic mass is highly suspicious for cancer, a gynecologic oncologist should be consulted directly.
WHEN TO REASSURE, REASSESS, REFER
Ovarian masses often pose diagnostic and management dilemmas. Reassurance can be offered to women with small simple cysts. Interval follow-up with ultrasonography is appropriate for cysts that are most likely to be benign. If malignancy is suspected based on ultrasonography, other imaging, blood testing, or expert opinion, referral to a surgical gynecologist or gynecologic oncologist is recommended. If malignancy is strongly suspected, direct referral to a gynecologic oncologist offers the best chance of survival if cancer is actually present.
Reassure
- When simple cysts are less than 1 cm in postmenopausal women
- When simple cysts are less than 5 cm in premenopausal patients.
Reassess
- With yearly ultrasonography in cases of very low risk
- With repeat ultrasonography in 6 to 12 weeks when the diagnosis is not clear but the cyst is likely benign.
Refer
- To a gynecologist for symptomatic cysts, cysts larger than 6 cm, and cysts that require ancillary testing
- To a gynecologic oncologist for findings worrisome for cancer, such as thick septations, solid areas with flow, ascites, evidence of metastasis, or high cancer antigen 125 levels.
- Borgfeldt C, Andolf E. Transvaginal sonographic ovarian findings in a random sample of women 25–40 years old. Ultrasound Obstet Gynecol 1999; 13:345–350.
- Modesitt SC, Pavlik EJ, Ueland FR, DePriest PD, Kryscio RJ, van Nagell JR. Risk of malignancy in unilocular ovarian cystic tumors less than 10 centimeters in diameter. Obstet Gynecol 2003; 102:594–599.
- Greenlee RT, Kessel B, Williams CR, et al. Prevalence, incidence, and natural history of simple ovarian cysts among women > 55 years old in a large cancer screening trial. Am J Obstet Gynecol 2010; 202:373.e1–373.e9.
- Jordan SJ, Green AC, Whiteman DC, Webb PM; Australian Ovarian Cancer Study Group. Risk factors for benign, borderline and invasive mucinous ovarian tumors: epidemiological evidence of a neoplastic continuum? Gynecol Oncol 2007; 107:223–230.
- NIH consensus conference. Ovarian cancer. Screening, treatment, and follow-up. NIH Consensus Development Panel on Ovarian Cancer. JAMA 1995; 273:491–497.
- The reduction in risk of ovarian cancer associated with oral-contraceptive use. The Cancer and Steroid Hormone Study of the Centers for Disease Control and the National Institute of Child Health and Human Development. N Engl J Med 1987; 316:650–655.
- Young RL, Snabes MC, Frank ML, Reilly M. A randomized, double-blind, placebo-controlled comparison of the impact of low-dose and triphasic oral contraceptives on follicular development. Am J Obstet Gynecol 1992; 167:678–682.
- Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol 2009; 113:1027–1037.
- Sharma A, Gentry-Maharaj A, Burnell M, et al; UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Assessing the malignant potential of ovarian inclusion cysts in postmenopausal women within the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a prospective cohort study. BJOG 2012; 119:207–219.
- Buys SS, Partridge E, Black A, et al; PLCO Project Team. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011; 305:2295–2303.
- Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med 2009; 361:170–177.
- National Cancer Institute. Surveillance Epidemiology and End Results (SEER). Cancer statistics on ovarian cancer. http://seer.cancer.gov/statfacts/html/ovary.html. Accessed May 9, 2013.
- American Cancer Society. Survival by ovarian cancer stage. www.cancer.org/Cancer/OvarianCancer/DetailedGuide/ovarian-cancer-survival-rates. Accessed May 9, 2013.
- Brown PO, Palmer C. The preclinical natural history of serous ovarian cancer: defining the target for early detection. PLoS Med 2009; 6:e1000114.
- Padilla LA, Radosevich DM, Milad MP. Limitations of the pelvic examination for evaluation of the female pelvic organs. Int J Gynaecol Obstet 2005; 88:84–88.
- Myers ER, Bastian LA, Havrilesky LJ, et al. Management of Adnexal Mass. Evidence Report/Technology Assessment No.130 (Prepared by the Duke Evidence-based Practice Center under Contract No. 290-02-0025.) AHRQ Publication No. 06-E004. Rockville, MD: Agency for Healthcare Research and Quality. February 2006.
- Ameye L, Timmerman D, Valentin L, et al. Clinically oriented three-step strategy for assessment of adnexal pathology. Ultrasound Obstet Gynecol 2012; 40:582–591.
- Covens AL, Dodge JE, Lacchetti C, et al; Gynecology Cancer Disease Site Group. Surgical management of a suspicious adnexal mass: a systematic review. Gynecol Oncol 2012; 126:149–156.
- Liu JH, Zanotti KM. Management of the adnexal mass. Obstet Gynecol 2011; 117:1413–1428.
- Goff BA, Mandel LS, Drescher CW, et al. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer 2007; 109:221–227.
- Rossing MA, Wicklund KG, Cushing-Haugen KL, Weiss NS. Predictive value of symptoms for early detection of ovarian cancer. J Natl Cancer Inst 2010; 102:222–229.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Management of adnexal masses. Obstet Gynecol 2007; 110:201–214.
- BestPractice BMJ Evidence Centre. Ovarian cysts-Diagnosis-History & examination—Risk factors. http://bestpractice.bmj.com/best-practice/monograph/660/diagnosis.html. Accessed June 2, 2013.
- American Cancer Society. Ovarian-cancer risk factors. www.cancer.org/Cancer/OvarianCancer/DetailedGuide/ovarian-cancer-survival-rates. Accessed May 9, 2013.
- Bast RC, Klug TL, St John E, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med 1983; 309:883–887.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 477: the role of the obstetrician-gynecologist in the early detection of epithelial ovarian cancer. Obstet Gynecol 2011; 117:742–746.
- Im SS, Gordon AN, Buttin BM, et al. Validation of referral guidelines for women with pelvic masses. Obstet Gynecol 2005; 105:35–41.
- Dearking AC, Aletti GD, McGree ME, Weaver AL, Sommerfield MK, Cliby WA. How relevant are ACOG and SGO guidelines for referral of adnexal mass? Obstet Gynecol 2007; 110:841–848.
- Skates SJ, Menon U, MacDonald N, et al. Calculation of the risk of ovarian cancer from serial CA-125 values for preclinical detection in postmenopausal women. J Clin Oncol 2003; 21(suppl 10):206s–210s.
- Ueland FR, Desimone CP, Seamon LG, et al. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet Gynecol 2011; 117:1289–1297.
- Ware Miller R, Smith A, DeSimone CP, et al. Performance of the American College of Obstetricians and Gynecologists’ ovarian tumor referral guidelines with a multivariate index assay. Obstet Gynecol 2011; 117:1298–1306.
- Lev-Toaff AS, Horrow MM, Andreotti RF, et al. Expert Panel on Women’s Imaging. ACR Appropriateness Criteria clinically suspected adnexal mass. Reston, VA: American College of Radiology (ACR), 2009. www.guidelines.gov/content.aspx?id=15780&search=adnexal+mass. Accessed May 9, 2013.
- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2010; 256:943–954.
- Alcázar JL, Castillo G, Jurado M, García GL. Is expectant management of sonographically benign adnexal cysts an option in selected asymptomatic premenopausal women? Hum Reprod 2005; 20:3231–3234.
- Medeiros LR, Freitas LB, Rosa DD, et al. Accuracy of magnetic resonance imaging in ovarian tumor: a systematic quantitative review. Am J Obstet Gynecol 2011; 204:67.e1–67.e10.
- Kinkel K, Lu Y, Mehdizade A, Pelte MF, Hricak H. Indeterminate ovarian mass at US: incremental value of second imaging test for characterization—meta-analysis and Bayesian analysis. Radiology 2005; 236:85–94.
- Timmerman D, Ameye L, Fischerova D, et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. BMJ 2010; 341:c6839.
- Valentin L, Ameye L, Savelli L, et al. Adnexal masses difficult to classify as benign or malignant using subjective assessment of gray-scale and Doppler ultrasound findings: logistic regression models do not help. Ultrasound Obstet Gynecol 2011; 38:456–465.
- Dodge JE, Covens AL, Lacchetti C, et al; Gynecology Cancer Disease Site Group. Preoperative identification of a suspicious adnexal mass: a systematic review and meta-analysis. Gynecol Oncol 2012; 126:157–166.
- Grimes DA, Jones LB, Lopez LM, Schulz KF. Oral contraceptives for functional ovarian cysts. Cochrane Database Syst Rev 2011; 9:CD006134.
- Higgins RV, Matkins JF, Marroum MC. Comparison of fine-needle aspiration cytologic findings of ovarian cysts with ovarian histologic findings. Am J Obstet Gynecol 1999; 180:550–553.
- Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet 2001; 357:176–182.
- Zanetta G, Lissoni A, Torri V, et al. Role of puncture and aspiration in expectant management of simple ovarian cysts: a randomised study. BMJ 1996; 313:1110–1113.
- Bonilla-Musoles F, Ballester MJ, Simon C, Serra V, Raga F. Is avoidance of surgery possible in patients with perimenopausal ovarian tumors using transvaginal ultrasound and duplex color Doppler sonography? J Ultrasound Med 1993; 12:33–39.
- Medeiros LR, Rosa DD, Bozzetti MC, et al. Laparoscopy versus laparotomy for FIGO Stage I ovarian cancer. Cochrane Database Syst Rev 2008; 4:CD005344.
- Goff BA, Miller JW, Matthews B, et al. Involvement of gynecologic oncologists in the treatment of patients with a suspicious ovarian mass. Obstet Gynecol 2011; 118:854–862.
- Earle CC, Schrag D, Neville BA, et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst 2006; 98:172–180.
- Giede KC, Kieser K, Dodge J, Rosen B. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol 2005; 99:447–461.
- Cress RD, Bauer K, O’Malley CD, et al. Surgical staging of early stage epithelial ovarian cancer: results from the CDC-NPCR ovarian patterns of care study. Gynecol Oncol 2011; 121:94–99.
- Horowitz NS. Management of adnexal masses in pregnancy. Clin Obstet Gynecol 2011; 54:519–527.
- Borgfeldt C, Andolf E. Transvaginal sonographic ovarian findings in a random sample of women 25–40 years old. Ultrasound Obstet Gynecol 1999; 13:345–350.
- Modesitt SC, Pavlik EJ, Ueland FR, DePriest PD, Kryscio RJ, van Nagell JR. Risk of malignancy in unilocular ovarian cystic tumors less than 10 centimeters in diameter. Obstet Gynecol 2003; 102:594–599.
- Greenlee RT, Kessel B, Williams CR, et al. Prevalence, incidence, and natural history of simple ovarian cysts among women > 55 years old in a large cancer screening trial. Am J Obstet Gynecol 2010; 202:373.e1–373.e9.
- Jordan SJ, Green AC, Whiteman DC, Webb PM; Australian Ovarian Cancer Study Group. Risk factors for benign, borderline and invasive mucinous ovarian tumors: epidemiological evidence of a neoplastic continuum? Gynecol Oncol 2007; 107:223–230.
- NIH consensus conference. Ovarian cancer. Screening, treatment, and follow-up. NIH Consensus Development Panel on Ovarian Cancer. JAMA 1995; 273:491–497.
- The reduction in risk of ovarian cancer associated with oral-contraceptive use. The Cancer and Steroid Hormone Study of the Centers for Disease Control and the National Institute of Child Health and Human Development. N Engl J Med 1987; 316:650–655.
- Young RL, Snabes MC, Frank ML, Reilly M. A randomized, double-blind, placebo-controlled comparison of the impact of low-dose and triphasic oral contraceptives on follicular development. Am J Obstet Gynecol 1992; 167:678–682.
- Parker WH, Broder MS, Chang E, et al. Ovarian conservation at the time of hysterectomy and long-term health outcomes in the Nurses’ Health Study. Obstet Gynecol 2009; 113:1027–1037.
- Sharma A, Gentry-Maharaj A, Burnell M, et al; UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS). Assessing the malignant potential of ovarian inclusion cysts in postmenopausal women within the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a prospective cohort study. BJOG 2012; 119:207–219.
- Buys SS, Partridge E, Black A, et al; PLCO Project Team. Effect of screening on ovarian cancer mortality: the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA 2011; 305:2295–2303.
- Clarke-Pearson DL. Clinical practice. Screening for ovarian cancer. N Engl J Med 2009; 361:170–177.
- National Cancer Institute. Surveillance Epidemiology and End Results (SEER). Cancer statistics on ovarian cancer. http://seer.cancer.gov/statfacts/html/ovary.html. Accessed May 9, 2013.
- American Cancer Society. Survival by ovarian cancer stage. www.cancer.org/Cancer/OvarianCancer/DetailedGuide/ovarian-cancer-survival-rates. Accessed May 9, 2013.
- Brown PO, Palmer C. The preclinical natural history of serous ovarian cancer: defining the target for early detection. PLoS Med 2009; 6:e1000114.
- Padilla LA, Radosevich DM, Milad MP. Limitations of the pelvic examination for evaluation of the female pelvic organs. Int J Gynaecol Obstet 2005; 88:84–88.
- Myers ER, Bastian LA, Havrilesky LJ, et al. Management of Adnexal Mass. Evidence Report/Technology Assessment No.130 (Prepared by the Duke Evidence-based Practice Center under Contract No. 290-02-0025.) AHRQ Publication No. 06-E004. Rockville, MD: Agency for Healthcare Research and Quality. February 2006.
- Ameye L, Timmerman D, Valentin L, et al. Clinically oriented three-step strategy for assessment of adnexal pathology. Ultrasound Obstet Gynecol 2012; 40:582–591.
- Covens AL, Dodge JE, Lacchetti C, et al; Gynecology Cancer Disease Site Group. Surgical management of a suspicious adnexal mass: a systematic review. Gynecol Oncol 2012; 126:149–156.
- Liu JH, Zanotti KM. Management of the adnexal mass. Obstet Gynecol 2011; 117:1413–1428.
- Goff BA, Mandel LS, Drescher CW, et al. Development of an ovarian cancer symptom index: possibilities for earlier detection. Cancer 2007; 109:221–227.
- Rossing MA, Wicklund KG, Cushing-Haugen KL, Weiss NS. Predictive value of symptoms for early detection of ovarian cancer. J Natl Cancer Inst 2010; 102:222–229.
- American College of Obstetricians and Gynecologists. ACOG Practice Bulletin. Management of adnexal masses. Obstet Gynecol 2007; 110:201–214.
- BestPractice BMJ Evidence Centre. Ovarian cysts-Diagnosis-History & examination—Risk factors. http://bestpractice.bmj.com/best-practice/monograph/660/diagnosis.html. Accessed June 2, 2013.
- American Cancer Society. Ovarian-cancer risk factors. www.cancer.org/Cancer/OvarianCancer/DetailedGuide/ovarian-cancer-survival-rates. Accessed May 9, 2013.
- Bast RC, Klug TL, St John E, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med 1983; 309:883–887.
- American College of Obstetricians and Gynecologists Committee on Gynecologic Practice. Committee Opinion No. 477: the role of the obstetrician-gynecologist in the early detection of epithelial ovarian cancer. Obstet Gynecol 2011; 117:742–746.
- Im SS, Gordon AN, Buttin BM, et al. Validation of referral guidelines for women with pelvic masses. Obstet Gynecol 2005; 105:35–41.
- Dearking AC, Aletti GD, McGree ME, Weaver AL, Sommerfield MK, Cliby WA. How relevant are ACOG and SGO guidelines for referral of adnexal mass? Obstet Gynecol 2007; 110:841–848.
- Skates SJ, Menon U, MacDonald N, et al. Calculation of the risk of ovarian cancer from serial CA-125 values for preclinical detection in postmenopausal women. J Clin Oncol 2003; 21(suppl 10):206s–210s.
- Ueland FR, Desimone CP, Seamon LG, et al. Effectiveness of a multivariate index assay in the preoperative assessment of ovarian tumors. Obstet Gynecol 2011; 117:1289–1297.
- Ware Miller R, Smith A, DeSimone CP, et al. Performance of the American College of Obstetricians and Gynecologists’ ovarian tumor referral guidelines with a multivariate index assay. Obstet Gynecol 2011; 117:1298–1306.
- Lev-Toaff AS, Horrow MM, Andreotti RF, et al. Expert Panel on Women’s Imaging. ACR Appropriateness Criteria clinically suspected adnexal mass. Reston, VA: American College of Radiology (ACR), 2009. www.guidelines.gov/content.aspx?id=15780&search=adnexal+mass. Accessed May 9, 2013.
- Levine D, Brown DL, Andreotti RF, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology 2010; 256:943–954.
- Alcázar JL, Castillo G, Jurado M, García GL. Is expectant management of sonographically benign adnexal cysts an option in selected asymptomatic premenopausal women? Hum Reprod 2005; 20:3231–3234.
- Medeiros LR, Freitas LB, Rosa DD, et al. Accuracy of magnetic resonance imaging in ovarian tumor: a systematic quantitative review. Am J Obstet Gynecol 2011; 204:67.e1–67.e10.
- Kinkel K, Lu Y, Mehdizade A, Pelte MF, Hricak H. Indeterminate ovarian mass at US: incremental value of second imaging test for characterization—meta-analysis and Bayesian analysis. Radiology 2005; 236:85–94.
- Timmerman D, Ameye L, Fischerova D, et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: prospective validation by IOTA group. BMJ 2010; 341:c6839.
- Valentin L, Ameye L, Savelli L, et al. Adnexal masses difficult to classify as benign or malignant using subjective assessment of gray-scale and Doppler ultrasound findings: logistic regression models do not help. Ultrasound Obstet Gynecol 2011; 38:456–465.
- Dodge JE, Covens AL, Lacchetti C, et al; Gynecology Cancer Disease Site Group. Preoperative identification of a suspicious adnexal mass: a systematic review and meta-analysis. Gynecol Oncol 2012; 126:157–166.
- Grimes DA, Jones LB, Lopez LM, Schulz KF. Oral contraceptives for functional ovarian cysts. Cochrane Database Syst Rev 2011; 9:CD006134.
- Higgins RV, Matkins JF, Marroum MC. Comparison of fine-needle aspiration cytologic findings of ovarian cysts with ovarian histologic findings. Am J Obstet Gynecol 1999; 180:550–553.
- Vergote I, De Brabanter J, Fyles A, et al. Prognostic importance of degree of differentiation and cyst rupture in stage I invasive epithelial ovarian carcinoma. Lancet 2001; 357:176–182.
- Zanetta G, Lissoni A, Torri V, et al. Role of puncture and aspiration in expectant management of simple ovarian cysts: a randomised study. BMJ 1996; 313:1110–1113.
- Bonilla-Musoles F, Ballester MJ, Simon C, Serra V, Raga F. Is avoidance of surgery possible in patients with perimenopausal ovarian tumors using transvaginal ultrasound and duplex color Doppler sonography? J Ultrasound Med 1993; 12:33–39.
- Medeiros LR, Rosa DD, Bozzetti MC, et al. Laparoscopy versus laparotomy for FIGO Stage I ovarian cancer. Cochrane Database Syst Rev 2008; 4:CD005344.
- Goff BA, Miller JW, Matthews B, et al. Involvement of gynecologic oncologists in the treatment of patients with a suspicious ovarian mass. Obstet Gynecol 2011; 118:854–862.
- Earle CC, Schrag D, Neville BA, et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst 2006; 98:172–180.
- Giede KC, Kieser K, Dodge J, Rosen B. Who should operate on patients with ovarian cancer? An evidence-based review. Gynecol Oncol 2005; 99:447–461.
- Cress RD, Bauer K, O’Malley CD, et al. Surgical staging of early stage epithelial ovarian cancer: results from the CDC-NPCR ovarian patterns of care study. Gynecol Oncol 2011; 121:94–99.
- Horowitz NS. Management of adnexal masses in pregnancy. Clin Obstet Gynecol 2011; 54:519–527.
KEY POINTS
- Incidentally discovered ovarian cysts are common and most are benign, but a minority can represent ovarian cancer, which is difficult to detect before it has spread and therefore often has a poor prognosis.
- Patients can be reassured if they are postmenopausal and have a simple cyst smaller than 1 cm or if they are premenopausal and have a simple cyst smaller than 5 cm.
- Reassess with yearly ultrasonography in very low-risk situations and with repeat ultrasonography in 6 to 12 weeks if the diagnosis is not clear but is likely benign.
- Refer to a gynecologist in cases of symptomatic cysts, cysts larger than 6 cm, and cysts that require ancillary testing.
- Refer to a gynecologic oncologist for findings worrisome for cancer such as thick septations, solid areas with flow, ascites, evidence of metastasis, or high cancer antigen 125 levels.
Lung air-fluid level in a smoker
A 49-year-old man was referred for evaluation of an abnormal chest radiograph. A 25-pack-year smoker, he had a history of chronic shortness of breath on exertion with occasional coughing and whitish sputum production. He also had a history of hypertension. He had not had hemoptysis, fever, chills, weight loss, or other symptoms, and he had not traveled recently.
On examination, he appeared comfortable. His breath sounds were decreased bilaterally; the rest of his physical examination was normal. His medical history, social history, and review of systems were otherwise unremarkable.
His white blood cell count was 9.4 × 109/L (reference range 4.5–11.0), with a normal differential. His hemoglobin concentration was 166 g/L (140–175).
Pulmonary function testing demonstrated moderate obstruction, with the following values:
- Forced expiratory volume in the first second of expiration/ forced vital capacity 0.65
- Forced expiratory volume in the first second of expiration 2.40 L (72% of predicted)
- Total lung capacity 7.11 L (92% of predicted)
- Diffusing capacity of lung for carbon monoxide 58% of predicted.
He underwent radiography (Figure 1) and computed tomography of the chest (Figure 2).
DIAGNOSIS: INFECTED EMPHYSEMATOUS BULLAE
The patient had infected emphysematous bullae.
The diagnosis can typically be made by the new development of an air-fluid level in a patient known to have preexisting emphysematous bullae.1 If previous images are not available, the presence of other bullae in a patient with established chronic obstructive pulmonary disease, a thin-walled cavity, and a disproportionate presentation with impressive radiographic findings along with a subtle clinical picture can support the diagnosis.2 In most reported cases, patients are not significantly symptomatic or ill.3 The differential diagnosis includes loculated parapneumonic pleural effusion,4 lung abscess,5 tuberculosis,6 and infected pneumatocele.
Since percutaneous aspiration of the bullae has been discouraged,2 the causative organism is often not identified. Also, the role of bronchoscopy in the diagnostic evaluation and treatment of infected emphysematous bullae appears to be limited.7
Our patient had minimal symptoms and did not appear ill; he had a relatively unremarkable physical examination, no leukocytosis, and negative blood and sputum cultures, suggesting a benign presentation. In addition, chest radiography a few months before this presentation showed multiple large emphysematous bullae (Figure 3). The current chest radiograph suggested multiple thin-walled cavitary lesions with an air-fluid level, which was confirmed on computed tomography.
TREATMENT OF INFECTED EMPHYSEMATOUS BULLAE
Currently, there is no established therapy for infected emphysematous bullae. Because the presentation is usually relatively benign in most case series, conservative treatment with a prolonged course of antibiotics alone seems to be the most appropriate initial course of action. A follow-up evaluation with chest imaging is recommended. On the other hand, in patients with worse symptoms, percutaneous aspiration of the bullae should be considered, as it may guide antibiotic therapy.8
We started our patient on clindamycin and scheduled him for follow-up chest imaging in 6 weeks.
- Burgener FA. Pulmonary cavitary and cystic lesions. In:Burgener FA, Kormano M, Pudas T, editors. Differential Diagnosis in Conventional Radiology. 3rd ed. New York, Thieme; 2008: chap.24.
- Chandra D, Soubra SH, Musher DM. A 57-year-old man with a fluid-containing lung cavity: infection of an emphysematous bulla with methicillin-resistant Staphylococcus aureus. Chest 2006; 130:1942–1946.
- Leatherman JW, McDonald FM, Niewohner DE. Fluid-containing bullae in the lung. South Med J 1985; 78:708–710.
- Sahn SA. Diagnosis and management of parapneumonic effusions and empyema. Clin Infect Dis 2007; 45:1480–1486.
- Hammond JM, Potgieter PD, Hanslo D, Scott H, Roditi D. The etiology and antimicrobial susceptibility patterns of microorganisms in acute community-acquired lung abscess. Chest 1995; 108:937–941.
- Woodring JH, Vandiviere HM, Fried AM, Dillon ML, Williams TD, Melvin IG. Update: the radiographic features of pulmonary tuberculosis. AJR Am J Roentgenol 1986; 146:497–506.
- Chandra D, Rose SR, Carter RB, Musher DM, Hamill RJ. Fluid-containing emphysematous bullae: a spectrum of illness. Eur Respir J 2008; 32:303–306.
- Henao-Martinez AF, Fernandez JF, Adams SG, Restrepo C. Lung bullae with air-fluid levels: what is the appropriate therapeutic approach? Respir Care 2012; 57:642–645.
A 49-year-old man was referred for evaluation of an abnormal chest radiograph. A 25-pack-year smoker, he had a history of chronic shortness of breath on exertion with occasional coughing and whitish sputum production. He also had a history of hypertension. He had not had hemoptysis, fever, chills, weight loss, or other symptoms, and he had not traveled recently.
On examination, he appeared comfortable. His breath sounds were decreased bilaterally; the rest of his physical examination was normal. His medical history, social history, and review of systems were otherwise unremarkable.
His white blood cell count was 9.4 × 109/L (reference range 4.5–11.0), with a normal differential. His hemoglobin concentration was 166 g/L (140–175).
Pulmonary function testing demonstrated moderate obstruction, with the following values:
- Forced expiratory volume in the first second of expiration/ forced vital capacity 0.65
- Forced expiratory volume in the first second of expiration 2.40 L (72% of predicted)
- Total lung capacity 7.11 L (92% of predicted)
- Diffusing capacity of lung for carbon monoxide 58% of predicted.
He underwent radiography (Figure 1) and computed tomography of the chest (Figure 2).
DIAGNOSIS: INFECTED EMPHYSEMATOUS BULLAE
The patient had infected emphysematous bullae.
The diagnosis can typically be made by the new development of an air-fluid level in a patient known to have preexisting emphysematous bullae.1 If previous images are not available, the presence of other bullae in a patient with established chronic obstructive pulmonary disease, a thin-walled cavity, and a disproportionate presentation with impressive radiographic findings along with a subtle clinical picture can support the diagnosis.2 In most reported cases, patients are not significantly symptomatic or ill.3 The differential diagnosis includes loculated parapneumonic pleural effusion,4 lung abscess,5 tuberculosis,6 and infected pneumatocele.
Since percutaneous aspiration of the bullae has been discouraged,2 the causative organism is often not identified. Also, the role of bronchoscopy in the diagnostic evaluation and treatment of infected emphysematous bullae appears to be limited.7
Our patient had minimal symptoms and did not appear ill; he had a relatively unremarkable physical examination, no leukocytosis, and negative blood and sputum cultures, suggesting a benign presentation. In addition, chest radiography a few months before this presentation showed multiple large emphysematous bullae (Figure 3). The current chest radiograph suggested multiple thin-walled cavitary lesions with an air-fluid level, which was confirmed on computed tomography.
TREATMENT OF INFECTED EMPHYSEMATOUS BULLAE
Currently, there is no established therapy for infected emphysematous bullae. Because the presentation is usually relatively benign in most case series, conservative treatment with a prolonged course of antibiotics alone seems to be the most appropriate initial course of action. A follow-up evaluation with chest imaging is recommended. On the other hand, in patients with worse symptoms, percutaneous aspiration of the bullae should be considered, as it may guide antibiotic therapy.8
We started our patient on clindamycin and scheduled him for follow-up chest imaging in 6 weeks.
A 49-year-old man was referred for evaluation of an abnormal chest radiograph. A 25-pack-year smoker, he had a history of chronic shortness of breath on exertion with occasional coughing and whitish sputum production. He also had a history of hypertension. He had not had hemoptysis, fever, chills, weight loss, or other symptoms, and he had not traveled recently.
On examination, he appeared comfortable. His breath sounds were decreased bilaterally; the rest of his physical examination was normal. His medical history, social history, and review of systems were otherwise unremarkable.
His white blood cell count was 9.4 × 109/L (reference range 4.5–11.0), with a normal differential. His hemoglobin concentration was 166 g/L (140–175).
Pulmonary function testing demonstrated moderate obstruction, with the following values:
- Forced expiratory volume in the first second of expiration/ forced vital capacity 0.65
- Forced expiratory volume in the first second of expiration 2.40 L (72% of predicted)
- Total lung capacity 7.11 L (92% of predicted)
- Diffusing capacity of lung for carbon monoxide 58% of predicted.
He underwent radiography (Figure 1) and computed tomography of the chest (Figure 2).
DIAGNOSIS: INFECTED EMPHYSEMATOUS BULLAE
The patient had infected emphysematous bullae.
The diagnosis can typically be made by the new development of an air-fluid level in a patient known to have preexisting emphysematous bullae.1 If previous images are not available, the presence of other bullae in a patient with established chronic obstructive pulmonary disease, a thin-walled cavity, and a disproportionate presentation with impressive radiographic findings along with a subtle clinical picture can support the diagnosis.2 In most reported cases, patients are not significantly symptomatic or ill.3 The differential diagnosis includes loculated parapneumonic pleural effusion,4 lung abscess,5 tuberculosis,6 and infected pneumatocele.
Since percutaneous aspiration of the bullae has been discouraged,2 the causative organism is often not identified. Also, the role of bronchoscopy in the diagnostic evaluation and treatment of infected emphysematous bullae appears to be limited.7
Our patient had minimal symptoms and did not appear ill; he had a relatively unremarkable physical examination, no leukocytosis, and negative blood and sputum cultures, suggesting a benign presentation. In addition, chest radiography a few months before this presentation showed multiple large emphysematous bullae (Figure 3). The current chest radiograph suggested multiple thin-walled cavitary lesions with an air-fluid level, which was confirmed on computed tomography.
TREATMENT OF INFECTED EMPHYSEMATOUS BULLAE
Currently, there is no established therapy for infected emphysematous bullae. Because the presentation is usually relatively benign in most case series, conservative treatment with a prolonged course of antibiotics alone seems to be the most appropriate initial course of action. A follow-up evaluation with chest imaging is recommended. On the other hand, in patients with worse symptoms, percutaneous aspiration of the bullae should be considered, as it may guide antibiotic therapy.8
We started our patient on clindamycin and scheduled him for follow-up chest imaging in 6 weeks.
- Burgener FA. Pulmonary cavitary and cystic lesions. In:Burgener FA, Kormano M, Pudas T, editors. Differential Diagnosis in Conventional Radiology. 3rd ed. New York, Thieme; 2008: chap.24.
- Chandra D, Soubra SH, Musher DM. A 57-year-old man with a fluid-containing lung cavity: infection of an emphysematous bulla with methicillin-resistant Staphylococcus aureus. Chest 2006; 130:1942–1946.
- Leatherman JW, McDonald FM, Niewohner DE. Fluid-containing bullae in the lung. South Med J 1985; 78:708–710.
- Sahn SA. Diagnosis and management of parapneumonic effusions and empyema. Clin Infect Dis 2007; 45:1480–1486.
- Hammond JM, Potgieter PD, Hanslo D, Scott H, Roditi D. The etiology and antimicrobial susceptibility patterns of microorganisms in acute community-acquired lung abscess. Chest 1995; 108:937–941.
- Woodring JH, Vandiviere HM, Fried AM, Dillon ML, Williams TD, Melvin IG. Update: the radiographic features of pulmonary tuberculosis. AJR Am J Roentgenol 1986; 146:497–506.
- Chandra D, Rose SR, Carter RB, Musher DM, Hamill RJ. Fluid-containing emphysematous bullae: a spectrum of illness. Eur Respir J 2008; 32:303–306.
- Henao-Martinez AF, Fernandez JF, Adams SG, Restrepo C. Lung bullae with air-fluid levels: what is the appropriate therapeutic approach? Respir Care 2012; 57:642–645.
- Burgener FA. Pulmonary cavitary and cystic lesions. In:Burgener FA, Kormano M, Pudas T, editors. Differential Diagnosis in Conventional Radiology. 3rd ed. New York, Thieme; 2008: chap.24.
- Chandra D, Soubra SH, Musher DM. A 57-year-old man with a fluid-containing lung cavity: infection of an emphysematous bulla with methicillin-resistant Staphylococcus aureus. Chest 2006; 130:1942–1946.
- Leatherman JW, McDonald FM, Niewohner DE. Fluid-containing bullae in the lung. South Med J 1985; 78:708–710.
- Sahn SA. Diagnosis and management of parapneumonic effusions and empyema. Clin Infect Dis 2007; 45:1480–1486.
- Hammond JM, Potgieter PD, Hanslo D, Scott H, Roditi D. The etiology and antimicrobial susceptibility patterns of microorganisms in acute community-acquired lung abscess. Chest 1995; 108:937–941.
- Woodring JH, Vandiviere HM, Fried AM, Dillon ML, Williams TD, Melvin IG. Update: the radiographic features of pulmonary tuberculosis. AJR Am J Roentgenol 1986; 146:497–506.
- Chandra D, Rose SR, Carter RB, Musher DM, Hamill RJ. Fluid-containing emphysematous bullae: a spectrum of illness. Eur Respir J 2008; 32:303–306.
- Henao-Martinez AF, Fernandez JF, Adams SG, Restrepo C. Lung bullae with air-fluid levels: what is the appropriate therapeutic approach? Respir Care 2012; 57:642–645.
A 51-year-old woman with dyspnea
A 51-year-old woman presents to the emergency department with dyspnea, which began 4 days ago. She reports no chest pain, palpitations, hemoptysis, fevers, chills, weight loss, recent travel, immobility, or surgery. One week ago she noticed cramping in her right calf, but that has since resolved.
Her history includes hypertension, hypothyroidism, and immune-mediated glomerulonephritis with proteinuria. She is premenopausal. She takes losartan and levothyroxine; she is not taking oral contraceptives or herbal supplements. She is up to date with her cancer screening and has had negative findings on colonoscopy and mammography within the past year.
She has never smoked and she does not drink alcohol or use illicit drugs. Her mother has a history of provoked deep vein thrombosis and colon cancer.
Her temperature is 36.2°C (97.2°F), heart rate 163 beats per minute, blood pressure 158/102 mm Hg, respiratory rate 40 breaths per minute, and oxygen saturation by pulse oximetry 80% while breathing room air, corrected to 94% with oxygen 6 L/min via nasal cannula.
On physical examination, she is sitting upright on a stretcher and appears uncomfortable and anxious. She is awake and able to communicate clearly. Examination of the head, ears, eyes, nose, and throat is unremarkable, with moist mucous membranes. Her lungs are clear to auscultation. Her heart beat is very rapid, with a regular rhythm and an accentuated P2 heart sound. A right parasternal heave can be palpated in addition to a rightwardly displaced point of maximal impulse. The abdomen is normal, with no tenderness or organomegaly. She has no pain, edema, or erythema in the legs or feet, and she has strong, symmetric pulses (2+) in all extremities. The neurologic examination is nonfocal.
Electrocardiography (ECG) done on arrival (Figure 1) reveals supraventricular tachycardia, a normal axis, and nonspecific ST-segment and T-wave abnormalities, findings commonly seen in pulmonary embolism.1,2 On the other hand, her ECG does not show some of the other signs of right ventricular strain due to pulmonary embolism such as atrial arrhythmias, complete right bundle branch block, or inferior Q-waves.3,4
In view of her ECG findings and her symptoms of dyspnea, calf pain, tachypnea, tachycardia, and a pronounced P2 heart sound, her physician concludes that she very likely has a pulmonary embolism1 and orders an intravenous infusion of unfractionated heparin to be started immediately.
TESTING FOR PULMONARY EMBOLISM
1. Which of the following would be the best initial diagnostic imaging study to perform in this patient, who has a high pretest probability of pulmonary embolism?
- Multidetector computed tomographic (CT) pulmonary angiography
- Transthoracic echocardiography
- Magnetic resonance imaging
- Lower-extremity duplex ultrasonography
- Pulmonary angiography
- Ventilation-perfusion scintigraphy
Multidetector CT angiography is rapid, noninvasive, and highly sensitive (83%–90%) and specific (96%) for pulmonary embolism.5,6 In patients such as ours who have a high pretest probability of having the disease, its positive predictive value is 96%.5 Therefore, it would be the initial diagnostic study to perform in our patient.
Although transthoracic echocardiography is noninvasive and can detect right ventricular strain in the setting of pulmonary embolism, it may miss half of all pulmonary emboli detected by angiography.7,8
When technically adequate images are obtained, the combination of magnetic resonance angiography and magnetic resonance venography is very sensitive (92%) and specific (96%) for pulmonary embolism.9 However, one-fourth of patients undergoing these studies may have technically inadequate results, so this is not the best choice for diagnosis.9
As our patient complained of recent cramping in the right calf, lower-extremity duplex ultrasonography would be a reasonable test to screen for acute deep vein thrombosis as the source of pulmonary embolism. However, given her worrisome vital signs and impending hemodynamic collapse, CT pulmonary angiography would be a better initial test as it may guide more aggressive therapy. Furthermore, even if ultrasonography showed no evidence of deep vein thrombosis, clinical suspicion for pulmonary embolism would remain high enough that therapeutic anticoagulation would be continued until further testing ruled out this diagnosis.
Pulmonary angiography is the gold-standard test for pulmonary embolism. However, it is time-consuming, expensive, and invasive and so is not usually done unless the diagnosis cannot be made with other imaging studies.
Ventilation-perfusion scintigraphy is an established and safe diagnostic test for pulmonary embolism. It is particularly helpful in patients who have renal dysfunction or contrast allergy. The sensitivity of a high-probability scan is 78%, while the specificity of a very-low-probability scan is 97%.10 However, this study is often nondiagnostic (in 26.5% of cases),10 and further imaging may be required.
RESULTS OF CT ANGIOGRAPHY
Our patient undergoes CT angiography, which reveals multiple bilateral pulmonary emboli and right ventricular enlargement (Figure 2). Transthoracic echocardiography shows dilation of the right ventricle, with severely reduced systolic function, an underfilled and hyperdynamic left ventricle (ejection fraction 75%), and moderate tricuspid valve regurgitation. Her right ventricular systolic pressure is estimated to be 47 mm Hg.
Doppler ultrasonography of the legs reveals an occlusive thrombus within the right small saphenous vein that bulges and extends into the right popliteal vein. Also noted is a nonocclusive thrombus in the upper right popliteal vein that likely originated from the thrombus in the small saphenous vein.
Initial laboratory testing (Table 1) shows elevations of the cardiac enzymes troponin T and N-terminal pro-B-type natriuretic peptide (NT-pro-BNP).
ESTIMATING PROGNOSIS IN PULMONARY EMBOLISM
2. Which of the following laboratory results at presentation is independently associated with a worse outcome in patients with pulmonary embolism?
- Elevated NT-pro-BNP
- Hypercalcemia
- Thrombocytosis
- Hypernatremia
- Elevated procalcitonin
The Pulmonary Embolism Severity Index11 and the Simplified Pulmonary Embolism Severity Index12 (Table 2) are clinical calculators that help predict 30-day risk of death in patients with pulmonary embolism. Our patient’s Pulmonary Embolism Severity Index score is 60, indicating a very low risk, but her simplified severity index score is 2, indicating a high risk.
A shock index score (the heart rate divided by the systolic blood pressure) greater than 1 is also a sensitive measure of risk.13 (Our patient’s shock index score is 1.03.) Although the simplified version is more accurate,14 the shock index is also helpful when deciding whether patients with suspected pulmonary embolism should receive early fibrinolysis.15
In a large registry of patients with confirmed pulmonary embolism, risk factors for death were age greater than 70, cancer, clinical congestive heart failure, chronic obstructive pulmonary disease, systolic blood pressure lower than 90 mm Hg, respiratory rate less than 20 per minute, and right ventricular hypokinesis.16 Right ventricular dysfunction progressing to right ventricular failure and cardiogenic shock is the most common cause of death in patients with pulmonary embolism.16–18
Post hoc analysis has also shown that elevations of the biomarkers BNP, NT-pro-BNP, and cardiac troponins I and T are associated with a prolonged hospital course and a higher risk of death within 30 days.19 Interestingly, a recent retrospective analysis found hyponatremia to be an independent risk factor for death in the short term.20
Thrombocytopenia, not thrombocytosis, is associated with worse outcomes in patients with pulmonary embolism.16 Procalcitonin is elevated in bacterial pneumonia but is normal in pulmonary embolism and so may be helpful in differentiating between the two.21,22 Hypernatremia, hypercalcemia, and elevated procalcitonin have not been shown to be independently associated with worse outcomes in acute pulmonary embolism.
Thus, of the answer choices shown above, elevated NT-pro-BNP is the correct answer.
Classified as massive, submassive, or low-risk
Pulmonary embolism is often stratified as massive, submassive, or low-risk, reflecting the severity and the degree of cardiovascular collapse. The treatment depends on the classification.
Pulmonary embolism is classified as massive if the patient has a cardiac arrest or a systolic blood pressure lower than 90 mm Hg for more than 15 minutes.23 Nearly half of patients in this category die.24
Pulmonary embolism is submassive if the patient has systolic pressure greater than 90 mm Hg but has right ventricular dysfunction, as evidenced by physical examination, elevated cardiac biomarkers, electrocardiography, transthoracic echocardiography, or computed tomography. The death rate is as high as 15%.24
Pulmonary embolism in a normotensive patient with no right ventricular dysfunction is defined as low-risk.
Our patient so far
Our patient has bilateral pulmonary emboli, most likely originating from a deep vein thrombosis in her right lower leg. Her pulmonary embolism would be classified as submassive, as her systolic pressure is greater than 90 mm Hg and right ventricular dysfunction—significant right ventricular strain—was noted on both transthoracic echocardiography and computed tomography. Also, cardiac biomarkers are elevated, and the physical examination revealed a prominent P2 sound and right parasternal heave, also suggestive of right ventricular dysfunction.
Now, 6 hours have passed, and even though she has been receiving intravenous heparin during this time, her shock index remains greater than 1, indicating hemodynamic instability. Her pulse rate is still markedly high—over 160 bpm—and she still appears quite anxious and uncomfortable.
HOW SHOULD THIS PATIENT BE TREATED?
3. Which of the following is the most appropriate treatment for this patient?
- Start warfarin immediately while bridging with unfractionated heparin, low-molecular-weight heparin, or fondaparinux
- Start fibrinolysis with alteplase
- Give metoprolol intravenously to control her heart rate
- Start dabigatran immediately while bridging with unfractionated heparin
- Place an inferior vena cava filter
- Consult cardiothoracic surgery for emergency pulmonary embolectomy
All patients with confirmed pulmonary embolism and no contraindications to anticoagulation should begin treatment with low-molecular-weight heparin, unfractionated heparin, or fondaparinux.23 In addition, this therapy should be started empirically while the patient is still undergoing diagnostic testing if the pretest probability of pulmonary embolism is intermediate or high.23
Warfarin is indicated for all patients with pulmonary embolism who do not have contraindications to it (Table 3). If unfractionated heparin, low-molecular-weight heparin, or fondaparinux has not already been started, it should be started at the same time as warfarin and should be continued until the international normalized ratio (INR) is within the therapeutic range.
Fibrinolysis. Treatment with a fibrinolytic agent in addition to heparin results in faster improvement of right ventricular function and pulmonary perfusion than with heparin alone.25 It may also decrease the incidence of pulmonary hypertension secondary to chronic thromboembolic disease.26 It should be considered in patients with massive pulmonary embolism.23
Whether fibrinolysis is appropriate for all patients with submassive pulmonary embolism remains controversial. Currently, it is not recommended for minor right ventricular dysfunction or myocardial necrosis if the patient has no signs of overt clinical decline.23 The Pulmonary Embolism Thrombolysis trial27 is an ongoing prospective randomized comparison of tenecteplase in a single bolus plus heparin vs heparin alone in normotensive patients with submassive pulmonary embolism, such as our patient. This trial may elucidate the benefit of fibrinolytic therapy in patients with submassive pulmonary embolism.
Patients at low risk are generally treated with heparin and warfarin anticoagulation alone. Fibrinolysis is not recommended in these patients, as the risk of bleeding outweighs the potential benefits.23
Metoprolol may not be advisable for our patient, as her tachycardia is likely compensatory, and beta-blocker therapy could blunt this compensatory response, leading to inadequate systemic perfusion.
Dabigatran is an oral direct thrombin inhibitor that does not require laboratory monitoring. It is currently approved for the prevention of stroke in patients with atrial fibrillation. It has been shown to be as effective as warfarin in the treatment of acute venous thromboembolism28 and may be a viable option in the future, but as of this writing it has not yet been approved in the United States for this indication. Furthermore, dabigatran inhibits thrombin immediately, so continued heparin bridging would not be necessary.
An inferior vena cava filter may prevent recurrent pulmonary embolism for patients who have absolute contraindications to anticoagulation, most significantly in the short term, ie, in the first few weeks after placement. However, these devices have not yet been shown to improve long-term mortality rates.
Embolectomy, percutaneous or surgical, is also an option. For patients in whom thrombolytic therapy is not effective, “rescue” surgical embolectomy has been associated with better outcomes compared with secondary thrombolysis and so should be considered.29
Back to our patient
An intravenous infusion of alteplase is started, and the patient’s tachycardia improves. Her oxygen requirements normalize, and she is transferred to the general medical floor the next day. She receives subcutaneous dalteparin as a bridge therapy, and warfarin is titrated to a goal INR of 2.0 to 3.0. Because of the acute deep vein thrombosis in her right lower leg, she is instructed to wear knee-high fitted compression hose for primary prevention of postphlebitic syndrome.
HOW LONG TO TREAT? IS GENETIC TESTING INDICATED?
Patients with a first episode of unprovoked venous thromboembolism should receive oral anticoagulants for 6 months, while those with recurrent unprovoked venous thromboembolism require lifelong oral anticoagulation.23
Whether to test for inherited thrombophilia after a first episode of venous thromboembolism to guide the duration of anticoagulation is controversial.30 Indiscriminate testing has not been recommended in these patients,31 but the American College of Medical Genetics recommends genetic screening for factor V Leiden in patients who have an unprovoked incident of venous thromboembolism before age 50.32
No randomized controlled trial has assessed whether thrombophilia testing decreases the recurrence rate of venous thromboembolism.33 One uncontrolled study suggested that testing for inherited thrombophilias in patients with a first episode does not affect the risk of recurrence.34 Testing is costly and may cause psychological distress for patients and family members.
Our patient is discharged home on warfarin for 6 months with subsequent follow-up evaluation in the thrombophilia clinic.
WHEN SHOULD WARFARIN BE RESTARTED?
4. If our patient were to discontinue oral anticoagulation in 6 months, which of the following, if present 1 month afterwards, would be a reason to restart oral anticoagulation?
- Elevated serum cotinine
- Positive pregnancy test
- Elevated follicle-stimulating hormone and luteinizing hormone and low estradiol levels
- Elevated D-dimer
Cotinine is a nicotine metabolite, and serum levels are elevated in smokers. Smoking and pregnancy both increase the risk of venous thromboembolism. However, smoking or pregnancy alone would not be a reason to increase the duration of anticoagulation.
Warfarin is contraindicated in pregnancy because of its teratogenic effects, particularly in the first trimester. Follicle-stimulating hormone and luteinizing hormone levels increase in response to decreased estradiol at menopause. Postmenopausal women are not at increased risk of venous thromboembolism unless they are taking oral estrogen hormone replacement therapy.
An elevated D-dimer level 1 month after stopping of oral anticoagulation has been associated with a higher rate of recurrence of venous thromboembolism, which is reduced by a resumption of anticoagulation.35 Therefore, consideration should be given to resuming anticoagulation in patients who have an elevated D-dimer level.
TAKE-HOME POINTS
It is important to distinguish between massive, submassive, and low-risk pulmonary embolism, since each type has a different treatment and prognosis.
Fibrinolytic therapy is indicated in patients with massive pulmonary embolism when no contraindication is present, whereas it is not indicated in those with low-risk pulmonary embolism.
Management of submassive pulmonary embolism continues to be an area of considerable debate. Current American Heart Association guidelines recommend consideration of fibrinolysis initially in patients with submassive acute pulmonary embolism if there is concurrent clinical evidence of adverse prognosis—eg, new hemodynamic instability, worsening respiratory insufficiency, severe right ventricular dysfunction, or major myocardial necrosis.23 On the other hand, the American College of Chest Physicians recommends against initial systemic fibrinolysis in submassive acute pulmonary embolism based on biomarkers or findings on ECG, transthoracic echocardiography, or CT, recommending it only in therapeutically anticoagulated patients deemed to be at high risk of hypotension.36
Since the optimal treatment of submassive pulmonary embolism is still not known, it is important that clinicians remain up to date on the evidence and guidelines.
- Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med 2007; 120:871–879.
- Stein PD, Saltzman HA, Weg JG. Clinical characteristics of patients with acute pulmonary embolism. Am J Cardiol 1991; 68:1723–1724.
- Ferrari E, Imbert A, Chevalier T, Mihoubi A, Morand P, Baudouy M. The ECG in pulmonary embolism. Predictive value of negative T waves in precordial leads—80 case reports. Chest 1997; 111:537–543.
- Geibel A, Zehender M, Kasper W, Olschewski M, Klima C, Konstantinides SV. Prognostic value of the ECG on admission in patients with acute major pulmonary embolism. Eur Respir J 2005; 25:843–848.
- Perrier A, Roy PM, Sanchez O, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med 2005; 352:1760–1768.
- Qanadli SD, Hajjam ME, Mesurolle B, et al. Pulmonary embolism detection: prospective evaluation of dual-section helical CT versus selective pulmonary arteriography in 157 patients. Radiology 2000; 217:447–455.
- Miniati M, Monti S, Pratali L, et al. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med 2001; 110:528–535.
- Bova C, Greco F, Misuraca G, et al. Diagnostic utility of echocardiography in patients with suspected pulmonary embolism. Am J Emerg Med 2003; 21:180–183.
- Stein PD, Chenevert TL, Fowler SE, et al; PIOPED III (Prospective Investigation of Pulmonary Embolism Diagnosis III) Investigators. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III). Ann Intern Med 2010; 152:434–443,W142–W143.
- Sostman HD, Stein PD, Gottschalk A, Matta F, Hull R, Goodman L. Acute pulmonary embolism: sensitivity and specificity of ventilation-perfusion scintigraphy in PIOPED II study. Radiology 2008; 246:941–946.
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046.
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389.
- Otero R, Trujillo-Santos J, Cayuela A, et al; Registro Informatizado de la Enfermedad Tromboembólica (RIETE) Investigators. Haemodynamically unstable pulmonary embolism in the RIETE Registry: systolic blood pressure or shock index? Eur Respir J 2007; 30:1111–1116.
- Sam A, Sánchez D, Gómez V, et al. The shock index and the simplified PESI for identification of low-risk patients with acute pulmonary embolism. Eur Respir J 2011; 37:762–766.
- Kucher N, Luder CM, Dörnhöfer T, Windecker S, Meier B, Hess OM. Novel management strategy for patients with suspected pulmonary embolism. Eur Heart J 2003; 24:366–376.
- Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999; 353:1386–1389.
- ten Wolde M, Söhne M, Quak E, Mac Gillavry MR, Büller HR. Prognostic value of echocardiographically assessed right ventricular dysfunction in patients with pulmonary embolism. Arch Intern Med 2004; 164:1685–1689.
- Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J 2008; 29:1569–1577.
- Kucher N, Goldhaber SZ. Cardiac biomarkers for risk stratification of patients with acute pulmonary embolism. Circulation 2003; 108:2191–2194.
- Scherz N, Labarère J, Méan M, Ibrahim SA, Fine MJ, Aujesky D. Prognostic importance of hyponatremia in patients with acute pulmonary embolism. Am J Respir Crit Care Med 2010; 182:1178–1183.
- Delèvaux I, André M, Aumaître O, Bègue RJ, Colombier M, Piette JC. Procalcitonin measurement for differential diagnosis between pulmonary embolism and pneumonia. Crit Care Med 2003; 31:661.
- Köktürk N, Kanbay A, Bukan N, Ekim N. The value of serum procalcitonin in differential diagnosis of pulmonary embolism and community-acquired pneumonia. Clin Appl Thromb Hemost 2011; 17:519–525.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–582.
- Goldhaber SZ, Haire WD, Feldstein ML, et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet 1993; 341:507–511.
- Kline JA, Steuerwald MT, Marchick MR, Hernandez-Nino J, Rose GA. Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest 2009; 136:1202–1210.
- Steering Committee of PEITHO Investigators. Single-bolus tenecteplase plus heparin compared with heparin alone for normotensive patients with acute pulmonary embolism who have evidence of right ventricular dysfunction and myocardial injury: rationale and design of the Pulmonary Embolism Thrombolysis (PEITHO) trial. Am Heart J 2012; 163:33–38.e1.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Meneveau N, Séronde MF, Blonde MC, et al. Management of unsuccessful thrombolysis in acute massive pulmonary embolism. Chest 2006; 129:1043–1050.
- Ho WK, Hankey GJ, Eikelboom JW. Should adult patients be routinely tested for heritable thrombophilia after an episode of venous thromboembolism? Med J Aust 2011; 195:139–142.
- Baglin T, Gray E, Greaves M, et al; British Committee for Standards in Haematology. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010; 149:209–220.
- Grody WW, Griffin JH, Taylor AK, Korf BR, Heit JA; ACMG Factor V Leiden Working Group. American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med 2001; 3:139–148.
- Cohn D, Vansenne F, de Borgie C, Middeldorp S. Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev 2009; ( 1):CD007069.
- Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost 2008; 6:1474–1477.
- Palareti G, Cosmi B, Legnani C, et al; PROLONG Investigators. Ddimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006; 355:1780–1789.
- Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):7S–47S.
A 51-year-old woman presents to the emergency department with dyspnea, which began 4 days ago. She reports no chest pain, palpitations, hemoptysis, fevers, chills, weight loss, recent travel, immobility, or surgery. One week ago she noticed cramping in her right calf, but that has since resolved.
Her history includes hypertension, hypothyroidism, and immune-mediated glomerulonephritis with proteinuria. She is premenopausal. She takes losartan and levothyroxine; she is not taking oral contraceptives or herbal supplements. She is up to date with her cancer screening and has had negative findings on colonoscopy and mammography within the past year.
She has never smoked and she does not drink alcohol or use illicit drugs. Her mother has a history of provoked deep vein thrombosis and colon cancer.
Her temperature is 36.2°C (97.2°F), heart rate 163 beats per minute, blood pressure 158/102 mm Hg, respiratory rate 40 breaths per minute, and oxygen saturation by pulse oximetry 80% while breathing room air, corrected to 94% with oxygen 6 L/min via nasal cannula.
On physical examination, she is sitting upright on a stretcher and appears uncomfortable and anxious. She is awake and able to communicate clearly. Examination of the head, ears, eyes, nose, and throat is unremarkable, with moist mucous membranes. Her lungs are clear to auscultation. Her heart beat is very rapid, with a regular rhythm and an accentuated P2 heart sound. A right parasternal heave can be palpated in addition to a rightwardly displaced point of maximal impulse. The abdomen is normal, with no tenderness or organomegaly. She has no pain, edema, or erythema in the legs or feet, and she has strong, symmetric pulses (2+) in all extremities. The neurologic examination is nonfocal.
Electrocardiography (ECG) done on arrival (Figure 1) reveals supraventricular tachycardia, a normal axis, and nonspecific ST-segment and T-wave abnormalities, findings commonly seen in pulmonary embolism.1,2 On the other hand, her ECG does not show some of the other signs of right ventricular strain due to pulmonary embolism such as atrial arrhythmias, complete right bundle branch block, or inferior Q-waves.3,4
In view of her ECG findings and her symptoms of dyspnea, calf pain, tachypnea, tachycardia, and a pronounced P2 heart sound, her physician concludes that she very likely has a pulmonary embolism1 and orders an intravenous infusion of unfractionated heparin to be started immediately.
TESTING FOR PULMONARY EMBOLISM
1. Which of the following would be the best initial diagnostic imaging study to perform in this patient, who has a high pretest probability of pulmonary embolism?
- Multidetector computed tomographic (CT) pulmonary angiography
- Transthoracic echocardiography
- Magnetic resonance imaging
- Lower-extremity duplex ultrasonography
- Pulmonary angiography
- Ventilation-perfusion scintigraphy
Multidetector CT angiography is rapid, noninvasive, and highly sensitive (83%–90%) and specific (96%) for pulmonary embolism.5,6 In patients such as ours who have a high pretest probability of having the disease, its positive predictive value is 96%.5 Therefore, it would be the initial diagnostic study to perform in our patient.
Although transthoracic echocardiography is noninvasive and can detect right ventricular strain in the setting of pulmonary embolism, it may miss half of all pulmonary emboli detected by angiography.7,8
When technically adequate images are obtained, the combination of magnetic resonance angiography and magnetic resonance venography is very sensitive (92%) and specific (96%) for pulmonary embolism.9 However, one-fourth of patients undergoing these studies may have technically inadequate results, so this is not the best choice for diagnosis.9
As our patient complained of recent cramping in the right calf, lower-extremity duplex ultrasonography would be a reasonable test to screen for acute deep vein thrombosis as the source of pulmonary embolism. However, given her worrisome vital signs and impending hemodynamic collapse, CT pulmonary angiography would be a better initial test as it may guide more aggressive therapy. Furthermore, even if ultrasonography showed no evidence of deep vein thrombosis, clinical suspicion for pulmonary embolism would remain high enough that therapeutic anticoagulation would be continued until further testing ruled out this diagnosis.
Pulmonary angiography is the gold-standard test for pulmonary embolism. However, it is time-consuming, expensive, and invasive and so is not usually done unless the diagnosis cannot be made with other imaging studies.
Ventilation-perfusion scintigraphy is an established and safe diagnostic test for pulmonary embolism. It is particularly helpful in patients who have renal dysfunction or contrast allergy. The sensitivity of a high-probability scan is 78%, while the specificity of a very-low-probability scan is 97%.10 However, this study is often nondiagnostic (in 26.5% of cases),10 and further imaging may be required.
RESULTS OF CT ANGIOGRAPHY
Our patient undergoes CT angiography, which reveals multiple bilateral pulmonary emboli and right ventricular enlargement (Figure 2). Transthoracic echocardiography shows dilation of the right ventricle, with severely reduced systolic function, an underfilled and hyperdynamic left ventricle (ejection fraction 75%), and moderate tricuspid valve regurgitation. Her right ventricular systolic pressure is estimated to be 47 mm Hg.
Doppler ultrasonography of the legs reveals an occlusive thrombus within the right small saphenous vein that bulges and extends into the right popliteal vein. Also noted is a nonocclusive thrombus in the upper right popliteal vein that likely originated from the thrombus in the small saphenous vein.
Initial laboratory testing (Table 1) shows elevations of the cardiac enzymes troponin T and N-terminal pro-B-type natriuretic peptide (NT-pro-BNP).
ESTIMATING PROGNOSIS IN PULMONARY EMBOLISM
2. Which of the following laboratory results at presentation is independently associated with a worse outcome in patients with pulmonary embolism?
- Elevated NT-pro-BNP
- Hypercalcemia
- Thrombocytosis
- Hypernatremia
- Elevated procalcitonin
The Pulmonary Embolism Severity Index11 and the Simplified Pulmonary Embolism Severity Index12 (Table 2) are clinical calculators that help predict 30-day risk of death in patients with pulmonary embolism. Our patient’s Pulmonary Embolism Severity Index score is 60, indicating a very low risk, but her simplified severity index score is 2, indicating a high risk.
A shock index score (the heart rate divided by the systolic blood pressure) greater than 1 is also a sensitive measure of risk.13 (Our patient’s shock index score is 1.03.) Although the simplified version is more accurate,14 the shock index is also helpful when deciding whether patients with suspected pulmonary embolism should receive early fibrinolysis.15
In a large registry of patients with confirmed pulmonary embolism, risk factors for death were age greater than 70, cancer, clinical congestive heart failure, chronic obstructive pulmonary disease, systolic blood pressure lower than 90 mm Hg, respiratory rate less than 20 per minute, and right ventricular hypokinesis.16 Right ventricular dysfunction progressing to right ventricular failure and cardiogenic shock is the most common cause of death in patients with pulmonary embolism.16–18
Post hoc analysis has also shown that elevations of the biomarkers BNP, NT-pro-BNP, and cardiac troponins I and T are associated with a prolonged hospital course and a higher risk of death within 30 days.19 Interestingly, a recent retrospective analysis found hyponatremia to be an independent risk factor for death in the short term.20
Thrombocytopenia, not thrombocytosis, is associated with worse outcomes in patients with pulmonary embolism.16 Procalcitonin is elevated in bacterial pneumonia but is normal in pulmonary embolism and so may be helpful in differentiating between the two.21,22 Hypernatremia, hypercalcemia, and elevated procalcitonin have not been shown to be independently associated with worse outcomes in acute pulmonary embolism.
Thus, of the answer choices shown above, elevated NT-pro-BNP is the correct answer.
Classified as massive, submassive, or low-risk
Pulmonary embolism is often stratified as massive, submassive, or low-risk, reflecting the severity and the degree of cardiovascular collapse. The treatment depends on the classification.
Pulmonary embolism is classified as massive if the patient has a cardiac arrest or a systolic blood pressure lower than 90 mm Hg for more than 15 minutes.23 Nearly half of patients in this category die.24
Pulmonary embolism is submassive if the patient has systolic pressure greater than 90 mm Hg but has right ventricular dysfunction, as evidenced by physical examination, elevated cardiac biomarkers, electrocardiography, transthoracic echocardiography, or computed tomography. The death rate is as high as 15%.24
Pulmonary embolism in a normotensive patient with no right ventricular dysfunction is defined as low-risk.
Our patient so far
Our patient has bilateral pulmonary emboli, most likely originating from a deep vein thrombosis in her right lower leg. Her pulmonary embolism would be classified as submassive, as her systolic pressure is greater than 90 mm Hg and right ventricular dysfunction—significant right ventricular strain—was noted on both transthoracic echocardiography and computed tomography. Also, cardiac biomarkers are elevated, and the physical examination revealed a prominent P2 sound and right parasternal heave, also suggestive of right ventricular dysfunction.
Now, 6 hours have passed, and even though she has been receiving intravenous heparin during this time, her shock index remains greater than 1, indicating hemodynamic instability. Her pulse rate is still markedly high—over 160 bpm—and she still appears quite anxious and uncomfortable.
HOW SHOULD THIS PATIENT BE TREATED?
3. Which of the following is the most appropriate treatment for this patient?
- Start warfarin immediately while bridging with unfractionated heparin, low-molecular-weight heparin, or fondaparinux
- Start fibrinolysis with alteplase
- Give metoprolol intravenously to control her heart rate
- Start dabigatran immediately while bridging with unfractionated heparin
- Place an inferior vena cava filter
- Consult cardiothoracic surgery for emergency pulmonary embolectomy
All patients with confirmed pulmonary embolism and no contraindications to anticoagulation should begin treatment with low-molecular-weight heparin, unfractionated heparin, or fondaparinux.23 In addition, this therapy should be started empirically while the patient is still undergoing diagnostic testing if the pretest probability of pulmonary embolism is intermediate or high.23
Warfarin is indicated for all patients with pulmonary embolism who do not have contraindications to it (Table 3). If unfractionated heparin, low-molecular-weight heparin, or fondaparinux has not already been started, it should be started at the same time as warfarin and should be continued until the international normalized ratio (INR) is within the therapeutic range.
Fibrinolysis. Treatment with a fibrinolytic agent in addition to heparin results in faster improvement of right ventricular function and pulmonary perfusion than with heparin alone.25 It may also decrease the incidence of pulmonary hypertension secondary to chronic thromboembolic disease.26 It should be considered in patients with massive pulmonary embolism.23
Whether fibrinolysis is appropriate for all patients with submassive pulmonary embolism remains controversial. Currently, it is not recommended for minor right ventricular dysfunction or myocardial necrosis if the patient has no signs of overt clinical decline.23 The Pulmonary Embolism Thrombolysis trial27 is an ongoing prospective randomized comparison of tenecteplase in a single bolus plus heparin vs heparin alone in normotensive patients with submassive pulmonary embolism, such as our patient. This trial may elucidate the benefit of fibrinolytic therapy in patients with submassive pulmonary embolism.
Patients at low risk are generally treated with heparin and warfarin anticoagulation alone. Fibrinolysis is not recommended in these patients, as the risk of bleeding outweighs the potential benefits.23
Metoprolol may not be advisable for our patient, as her tachycardia is likely compensatory, and beta-blocker therapy could blunt this compensatory response, leading to inadequate systemic perfusion.
Dabigatran is an oral direct thrombin inhibitor that does not require laboratory monitoring. It is currently approved for the prevention of stroke in patients with atrial fibrillation. It has been shown to be as effective as warfarin in the treatment of acute venous thromboembolism28 and may be a viable option in the future, but as of this writing it has not yet been approved in the United States for this indication. Furthermore, dabigatran inhibits thrombin immediately, so continued heparin bridging would not be necessary.
An inferior vena cava filter may prevent recurrent pulmonary embolism for patients who have absolute contraindications to anticoagulation, most significantly in the short term, ie, in the first few weeks after placement. However, these devices have not yet been shown to improve long-term mortality rates.
Embolectomy, percutaneous or surgical, is also an option. For patients in whom thrombolytic therapy is not effective, “rescue” surgical embolectomy has been associated with better outcomes compared with secondary thrombolysis and so should be considered.29
Back to our patient
An intravenous infusion of alteplase is started, and the patient’s tachycardia improves. Her oxygen requirements normalize, and she is transferred to the general medical floor the next day. She receives subcutaneous dalteparin as a bridge therapy, and warfarin is titrated to a goal INR of 2.0 to 3.0. Because of the acute deep vein thrombosis in her right lower leg, she is instructed to wear knee-high fitted compression hose for primary prevention of postphlebitic syndrome.
HOW LONG TO TREAT? IS GENETIC TESTING INDICATED?
Patients with a first episode of unprovoked venous thromboembolism should receive oral anticoagulants for 6 months, while those with recurrent unprovoked venous thromboembolism require lifelong oral anticoagulation.23
Whether to test for inherited thrombophilia after a first episode of venous thromboembolism to guide the duration of anticoagulation is controversial.30 Indiscriminate testing has not been recommended in these patients,31 but the American College of Medical Genetics recommends genetic screening for factor V Leiden in patients who have an unprovoked incident of venous thromboembolism before age 50.32
No randomized controlled trial has assessed whether thrombophilia testing decreases the recurrence rate of venous thromboembolism.33 One uncontrolled study suggested that testing for inherited thrombophilias in patients with a first episode does not affect the risk of recurrence.34 Testing is costly and may cause psychological distress for patients and family members.
Our patient is discharged home on warfarin for 6 months with subsequent follow-up evaluation in the thrombophilia clinic.
WHEN SHOULD WARFARIN BE RESTARTED?
4. If our patient were to discontinue oral anticoagulation in 6 months, which of the following, if present 1 month afterwards, would be a reason to restart oral anticoagulation?
- Elevated serum cotinine
- Positive pregnancy test
- Elevated follicle-stimulating hormone and luteinizing hormone and low estradiol levels
- Elevated D-dimer
Cotinine is a nicotine metabolite, and serum levels are elevated in smokers. Smoking and pregnancy both increase the risk of venous thromboembolism. However, smoking or pregnancy alone would not be a reason to increase the duration of anticoagulation.
Warfarin is contraindicated in pregnancy because of its teratogenic effects, particularly in the first trimester. Follicle-stimulating hormone and luteinizing hormone levels increase in response to decreased estradiol at menopause. Postmenopausal women are not at increased risk of venous thromboembolism unless they are taking oral estrogen hormone replacement therapy.
An elevated D-dimer level 1 month after stopping of oral anticoagulation has been associated with a higher rate of recurrence of venous thromboembolism, which is reduced by a resumption of anticoagulation.35 Therefore, consideration should be given to resuming anticoagulation in patients who have an elevated D-dimer level.
TAKE-HOME POINTS
It is important to distinguish between massive, submassive, and low-risk pulmonary embolism, since each type has a different treatment and prognosis.
Fibrinolytic therapy is indicated in patients with massive pulmonary embolism when no contraindication is present, whereas it is not indicated in those with low-risk pulmonary embolism.
Management of submassive pulmonary embolism continues to be an area of considerable debate. Current American Heart Association guidelines recommend consideration of fibrinolysis initially in patients with submassive acute pulmonary embolism if there is concurrent clinical evidence of adverse prognosis—eg, new hemodynamic instability, worsening respiratory insufficiency, severe right ventricular dysfunction, or major myocardial necrosis.23 On the other hand, the American College of Chest Physicians recommends against initial systemic fibrinolysis in submassive acute pulmonary embolism based on biomarkers or findings on ECG, transthoracic echocardiography, or CT, recommending it only in therapeutically anticoagulated patients deemed to be at high risk of hypotension.36
Since the optimal treatment of submassive pulmonary embolism is still not known, it is important that clinicians remain up to date on the evidence and guidelines.
A 51-year-old woman presents to the emergency department with dyspnea, which began 4 days ago. She reports no chest pain, palpitations, hemoptysis, fevers, chills, weight loss, recent travel, immobility, or surgery. One week ago she noticed cramping in her right calf, but that has since resolved.
Her history includes hypertension, hypothyroidism, and immune-mediated glomerulonephritis with proteinuria. She is premenopausal. She takes losartan and levothyroxine; she is not taking oral contraceptives or herbal supplements. She is up to date with her cancer screening and has had negative findings on colonoscopy and mammography within the past year.
She has never smoked and she does not drink alcohol or use illicit drugs. Her mother has a history of provoked deep vein thrombosis and colon cancer.
Her temperature is 36.2°C (97.2°F), heart rate 163 beats per minute, blood pressure 158/102 mm Hg, respiratory rate 40 breaths per minute, and oxygen saturation by pulse oximetry 80% while breathing room air, corrected to 94% with oxygen 6 L/min via nasal cannula.
On physical examination, she is sitting upright on a stretcher and appears uncomfortable and anxious. She is awake and able to communicate clearly. Examination of the head, ears, eyes, nose, and throat is unremarkable, with moist mucous membranes. Her lungs are clear to auscultation. Her heart beat is very rapid, with a regular rhythm and an accentuated P2 heart sound. A right parasternal heave can be palpated in addition to a rightwardly displaced point of maximal impulse. The abdomen is normal, with no tenderness or organomegaly. She has no pain, edema, or erythema in the legs or feet, and she has strong, symmetric pulses (2+) in all extremities. The neurologic examination is nonfocal.
Electrocardiography (ECG) done on arrival (Figure 1) reveals supraventricular tachycardia, a normal axis, and nonspecific ST-segment and T-wave abnormalities, findings commonly seen in pulmonary embolism.1,2 On the other hand, her ECG does not show some of the other signs of right ventricular strain due to pulmonary embolism such as atrial arrhythmias, complete right bundle branch block, or inferior Q-waves.3,4
In view of her ECG findings and her symptoms of dyspnea, calf pain, tachypnea, tachycardia, and a pronounced P2 heart sound, her physician concludes that she very likely has a pulmonary embolism1 and orders an intravenous infusion of unfractionated heparin to be started immediately.
TESTING FOR PULMONARY EMBOLISM
1. Which of the following would be the best initial diagnostic imaging study to perform in this patient, who has a high pretest probability of pulmonary embolism?
- Multidetector computed tomographic (CT) pulmonary angiography
- Transthoracic echocardiography
- Magnetic resonance imaging
- Lower-extremity duplex ultrasonography
- Pulmonary angiography
- Ventilation-perfusion scintigraphy
Multidetector CT angiography is rapid, noninvasive, and highly sensitive (83%–90%) and specific (96%) for pulmonary embolism.5,6 In patients such as ours who have a high pretest probability of having the disease, its positive predictive value is 96%.5 Therefore, it would be the initial diagnostic study to perform in our patient.
Although transthoracic echocardiography is noninvasive and can detect right ventricular strain in the setting of pulmonary embolism, it may miss half of all pulmonary emboli detected by angiography.7,8
When technically adequate images are obtained, the combination of magnetic resonance angiography and magnetic resonance venography is very sensitive (92%) and specific (96%) for pulmonary embolism.9 However, one-fourth of patients undergoing these studies may have technically inadequate results, so this is not the best choice for diagnosis.9
As our patient complained of recent cramping in the right calf, lower-extremity duplex ultrasonography would be a reasonable test to screen for acute deep vein thrombosis as the source of pulmonary embolism. However, given her worrisome vital signs and impending hemodynamic collapse, CT pulmonary angiography would be a better initial test as it may guide more aggressive therapy. Furthermore, even if ultrasonography showed no evidence of deep vein thrombosis, clinical suspicion for pulmonary embolism would remain high enough that therapeutic anticoagulation would be continued until further testing ruled out this diagnosis.
Pulmonary angiography is the gold-standard test for pulmonary embolism. However, it is time-consuming, expensive, and invasive and so is not usually done unless the diagnosis cannot be made with other imaging studies.
Ventilation-perfusion scintigraphy is an established and safe diagnostic test for pulmonary embolism. It is particularly helpful in patients who have renal dysfunction or contrast allergy. The sensitivity of a high-probability scan is 78%, while the specificity of a very-low-probability scan is 97%.10 However, this study is often nondiagnostic (in 26.5% of cases),10 and further imaging may be required.
RESULTS OF CT ANGIOGRAPHY
Our patient undergoes CT angiography, which reveals multiple bilateral pulmonary emboli and right ventricular enlargement (Figure 2). Transthoracic echocardiography shows dilation of the right ventricle, with severely reduced systolic function, an underfilled and hyperdynamic left ventricle (ejection fraction 75%), and moderate tricuspid valve regurgitation. Her right ventricular systolic pressure is estimated to be 47 mm Hg.
Doppler ultrasonography of the legs reveals an occlusive thrombus within the right small saphenous vein that bulges and extends into the right popliteal vein. Also noted is a nonocclusive thrombus in the upper right popliteal vein that likely originated from the thrombus in the small saphenous vein.
Initial laboratory testing (Table 1) shows elevations of the cardiac enzymes troponin T and N-terminal pro-B-type natriuretic peptide (NT-pro-BNP).
ESTIMATING PROGNOSIS IN PULMONARY EMBOLISM
2. Which of the following laboratory results at presentation is independently associated with a worse outcome in patients with pulmonary embolism?
- Elevated NT-pro-BNP
- Hypercalcemia
- Thrombocytosis
- Hypernatremia
- Elevated procalcitonin
The Pulmonary Embolism Severity Index11 and the Simplified Pulmonary Embolism Severity Index12 (Table 2) are clinical calculators that help predict 30-day risk of death in patients with pulmonary embolism. Our patient’s Pulmonary Embolism Severity Index score is 60, indicating a very low risk, but her simplified severity index score is 2, indicating a high risk.
A shock index score (the heart rate divided by the systolic blood pressure) greater than 1 is also a sensitive measure of risk.13 (Our patient’s shock index score is 1.03.) Although the simplified version is more accurate,14 the shock index is also helpful when deciding whether patients with suspected pulmonary embolism should receive early fibrinolysis.15
In a large registry of patients with confirmed pulmonary embolism, risk factors for death were age greater than 70, cancer, clinical congestive heart failure, chronic obstructive pulmonary disease, systolic blood pressure lower than 90 mm Hg, respiratory rate less than 20 per minute, and right ventricular hypokinesis.16 Right ventricular dysfunction progressing to right ventricular failure and cardiogenic shock is the most common cause of death in patients with pulmonary embolism.16–18
Post hoc analysis has also shown that elevations of the biomarkers BNP, NT-pro-BNP, and cardiac troponins I and T are associated with a prolonged hospital course and a higher risk of death within 30 days.19 Interestingly, a recent retrospective analysis found hyponatremia to be an independent risk factor for death in the short term.20
Thrombocytopenia, not thrombocytosis, is associated with worse outcomes in patients with pulmonary embolism.16 Procalcitonin is elevated in bacterial pneumonia but is normal in pulmonary embolism and so may be helpful in differentiating between the two.21,22 Hypernatremia, hypercalcemia, and elevated procalcitonin have not been shown to be independently associated with worse outcomes in acute pulmonary embolism.
Thus, of the answer choices shown above, elevated NT-pro-BNP is the correct answer.
Classified as massive, submassive, or low-risk
Pulmonary embolism is often stratified as massive, submassive, or low-risk, reflecting the severity and the degree of cardiovascular collapse. The treatment depends on the classification.
Pulmonary embolism is classified as massive if the patient has a cardiac arrest or a systolic blood pressure lower than 90 mm Hg for more than 15 minutes.23 Nearly half of patients in this category die.24
Pulmonary embolism is submassive if the patient has systolic pressure greater than 90 mm Hg but has right ventricular dysfunction, as evidenced by physical examination, elevated cardiac biomarkers, electrocardiography, transthoracic echocardiography, or computed tomography. The death rate is as high as 15%.24
Pulmonary embolism in a normotensive patient with no right ventricular dysfunction is defined as low-risk.
Our patient so far
Our patient has bilateral pulmonary emboli, most likely originating from a deep vein thrombosis in her right lower leg. Her pulmonary embolism would be classified as submassive, as her systolic pressure is greater than 90 mm Hg and right ventricular dysfunction—significant right ventricular strain—was noted on both transthoracic echocardiography and computed tomography. Also, cardiac biomarkers are elevated, and the physical examination revealed a prominent P2 sound and right parasternal heave, also suggestive of right ventricular dysfunction.
Now, 6 hours have passed, and even though she has been receiving intravenous heparin during this time, her shock index remains greater than 1, indicating hemodynamic instability. Her pulse rate is still markedly high—over 160 bpm—and she still appears quite anxious and uncomfortable.
HOW SHOULD THIS PATIENT BE TREATED?
3. Which of the following is the most appropriate treatment for this patient?
- Start warfarin immediately while bridging with unfractionated heparin, low-molecular-weight heparin, or fondaparinux
- Start fibrinolysis with alteplase
- Give metoprolol intravenously to control her heart rate
- Start dabigatran immediately while bridging with unfractionated heparin
- Place an inferior vena cava filter
- Consult cardiothoracic surgery for emergency pulmonary embolectomy
All patients with confirmed pulmonary embolism and no contraindications to anticoagulation should begin treatment with low-molecular-weight heparin, unfractionated heparin, or fondaparinux.23 In addition, this therapy should be started empirically while the patient is still undergoing diagnostic testing if the pretest probability of pulmonary embolism is intermediate or high.23
Warfarin is indicated for all patients with pulmonary embolism who do not have contraindications to it (Table 3). If unfractionated heparin, low-molecular-weight heparin, or fondaparinux has not already been started, it should be started at the same time as warfarin and should be continued until the international normalized ratio (INR) is within the therapeutic range.
Fibrinolysis. Treatment with a fibrinolytic agent in addition to heparin results in faster improvement of right ventricular function and pulmonary perfusion than with heparin alone.25 It may also decrease the incidence of pulmonary hypertension secondary to chronic thromboembolic disease.26 It should be considered in patients with massive pulmonary embolism.23
Whether fibrinolysis is appropriate for all patients with submassive pulmonary embolism remains controversial. Currently, it is not recommended for minor right ventricular dysfunction or myocardial necrosis if the patient has no signs of overt clinical decline.23 The Pulmonary Embolism Thrombolysis trial27 is an ongoing prospective randomized comparison of tenecteplase in a single bolus plus heparin vs heparin alone in normotensive patients with submassive pulmonary embolism, such as our patient. This trial may elucidate the benefit of fibrinolytic therapy in patients with submassive pulmonary embolism.
Patients at low risk are generally treated with heparin and warfarin anticoagulation alone. Fibrinolysis is not recommended in these patients, as the risk of bleeding outweighs the potential benefits.23
Metoprolol may not be advisable for our patient, as her tachycardia is likely compensatory, and beta-blocker therapy could blunt this compensatory response, leading to inadequate systemic perfusion.
Dabigatran is an oral direct thrombin inhibitor that does not require laboratory monitoring. It is currently approved for the prevention of stroke in patients with atrial fibrillation. It has been shown to be as effective as warfarin in the treatment of acute venous thromboembolism28 and may be a viable option in the future, but as of this writing it has not yet been approved in the United States for this indication. Furthermore, dabigatran inhibits thrombin immediately, so continued heparin bridging would not be necessary.
An inferior vena cava filter may prevent recurrent pulmonary embolism for patients who have absolute contraindications to anticoagulation, most significantly in the short term, ie, in the first few weeks after placement. However, these devices have not yet been shown to improve long-term mortality rates.
Embolectomy, percutaneous or surgical, is also an option. For patients in whom thrombolytic therapy is not effective, “rescue” surgical embolectomy has been associated with better outcomes compared with secondary thrombolysis and so should be considered.29
Back to our patient
An intravenous infusion of alteplase is started, and the patient’s tachycardia improves. Her oxygen requirements normalize, and she is transferred to the general medical floor the next day. She receives subcutaneous dalteparin as a bridge therapy, and warfarin is titrated to a goal INR of 2.0 to 3.0. Because of the acute deep vein thrombosis in her right lower leg, she is instructed to wear knee-high fitted compression hose for primary prevention of postphlebitic syndrome.
HOW LONG TO TREAT? IS GENETIC TESTING INDICATED?
Patients with a first episode of unprovoked venous thromboembolism should receive oral anticoagulants for 6 months, while those with recurrent unprovoked venous thromboembolism require lifelong oral anticoagulation.23
Whether to test for inherited thrombophilia after a first episode of venous thromboembolism to guide the duration of anticoagulation is controversial.30 Indiscriminate testing has not been recommended in these patients,31 but the American College of Medical Genetics recommends genetic screening for factor V Leiden in patients who have an unprovoked incident of venous thromboembolism before age 50.32
No randomized controlled trial has assessed whether thrombophilia testing decreases the recurrence rate of venous thromboembolism.33 One uncontrolled study suggested that testing for inherited thrombophilias in patients with a first episode does not affect the risk of recurrence.34 Testing is costly and may cause psychological distress for patients and family members.
Our patient is discharged home on warfarin for 6 months with subsequent follow-up evaluation in the thrombophilia clinic.
WHEN SHOULD WARFARIN BE RESTARTED?
4. If our patient were to discontinue oral anticoagulation in 6 months, which of the following, if present 1 month afterwards, would be a reason to restart oral anticoagulation?
- Elevated serum cotinine
- Positive pregnancy test
- Elevated follicle-stimulating hormone and luteinizing hormone and low estradiol levels
- Elevated D-dimer
Cotinine is a nicotine metabolite, and serum levels are elevated in smokers. Smoking and pregnancy both increase the risk of venous thromboembolism. However, smoking or pregnancy alone would not be a reason to increase the duration of anticoagulation.
Warfarin is contraindicated in pregnancy because of its teratogenic effects, particularly in the first trimester. Follicle-stimulating hormone and luteinizing hormone levels increase in response to decreased estradiol at menopause. Postmenopausal women are not at increased risk of venous thromboembolism unless they are taking oral estrogen hormone replacement therapy.
An elevated D-dimer level 1 month after stopping of oral anticoagulation has been associated with a higher rate of recurrence of venous thromboembolism, which is reduced by a resumption of anticoagulation.35 Therefore, consideration should be given to resuming anticoagulation in patients who have an elevated D-dimer level.
TAKE-HOME POINTS
It is important to distinguish between massive, submassive, and low-risk pulmonary embolism, since each type has a different treatment and prognosis.
Fibrinolytic therapy is indicated in patients with massive pulmonary embolism when no contraindication is present, whereas it is not indicated in those with low-risk pulmonary embolism.
Management of submassive pulmonary embolism continues to be an area of considerable debate. Current American Heart Association guidelines recommend consideration of fibrinolysis initially in patients with submassive acute pulmonary embolism if there is concurrent clinical evidence of adverse prognosis—eg, new hemodynamic instability, worsening respiratory insufficiency, severe right ventricular dysfunction, or major myocardial necrosis.23 On the other hand, the American College of Chest Physicians recommends against initial systemic fibrinolysis in submassive acute pulmonary embolism based on biomarkers or findings on ECG, transthoracic echocardiography, or CT, recommending it only in therapeutically anticoagulated patients deemed to be at high risk of hypotension.36
Since the optimal treatment of submassive pulmonary embolism is still not known, it is important that clinicians remain up to date on the evidence and guidelines.
- Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med 2007; 120:871–879.
- Stein PD, Saltzman HA, Weg JG. Clinical characteristics of patients with acute pulmonary embolism. Am J Cardiol 1991; 68:1723–1724.
- Ferrari E, Imbert A, Chevalier T, Mihoubi A, Morand P, Baudouy M. The ECG in pulmonary embolism. Predictive value of negative T waves in precordial leads—80 case reports. Chest 1997; 111:537–543.
- Geibel A, Zehender M, Kasper W, Olschewski M, Klima C, Konstantinides SV. Prognostic value of the ECG on admission in patients with acute major pulmonary embolism. Eur Respir J 2005; 25:843–848.
- Perrier A, Roy PM, Sanchez O, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med 2005; 352:1760–1768.
- Qanadli SD, Hajjam ME, Mesurolle B, et al. Pulmonary embolism detection: prospective evaluation of dual-section helical CT versus selective pulmonary arteriography in 157 patients. Radiology 2000; 217:447–455.
- Miniati M, Monti S, Pratali L, et al. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med 2001; 110:528–535.
- Bova C, Greco F, Misuraca G, et al. Diagnostic utility of echocardiography in patients with suspected pulmonary embolism. Am J Emerg Med 2003; 21:180–183.
- Stein PD, Chenevert TL, Fowler SE, et al; PIOPED III (Prospective Investigation of Pulmonary Embolism Diagnosis III) Investigators. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III). Ann Intern Med 2010; 152:434–443,W142–W143.
- Sostman HD, Stein PD, Gottschalk A, Matta F, Hull R, Goodman L. Acute pulmonary embolism: sensitivity and specificity of ventilation-perfusion scintigraphy in PIOPED II study. Radiology 2008; 246:941–946.
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046.
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389.
- Otero R, Trujillo-Santos J, Cayuela A, et al; Registro Informatizado de la Enfermedad Tromboembólica (RIETE) Investigators. Haemodynamically unstable pulmonary embolism in the RIETE Registry: systolic blood pressure or shock index? Eur Respir J 2007; 30:1111–1116.
- Sam A, Sánchez D, Gómez V, et al. The shock index and the simplified PESI for identification of low-risk patients with acute pulmonary embolism. Eur Respir J 2011; 37:762–766.
- Kucher N, Luder CM, Dörnhöfer T, Windecker S, Meier B, Hess OM. Novel management strategy for patients with suspected pulmonary embolism. Eur Heart J 2003; 24:366–376.
- Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999; 353:1386–1389.
- ten Wolde M, Söhne M, Quak E, Mac Gillavry MR, Büller HR. Prognostic value of echocardiographically assessed right ventricular dysfunction in patients with pulmonary embolism. Arch Intern Med 2004; 164:1685–1689.
- Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J 2008; 29:1569–1577.
- Kucher N, Goldhaber SZ. Cardiac biomarkers for risk stratification of patients with acute pulmonary embolism. Circulation 2003; 108:2191–2194.
- Scherz N, Labarère J, Méan M, Ibrahim SA, Fine MJ, Aujesky D. Prognostic importance of hyponatremia in patients with acute pulmonary embolism. Am J Respir Crit Care Med 2010; 182:1178–1183.
- Delèvaux I, André M, Aumaître O, Bègue RJ, Colombier M, Piette JC. Procalcitonin measurement for differential diagnosis between pulmonary embolism and pneumonia. Crit Care Med 2003; 31:661.
- Köktürk N, Kanbay A, Bukan N, Ekim N. The value of serum procalcitonin in differential diagnosis of pulmonary embolism and community-acquired pneumonia. Clin Appl Thromb Hemost 2011; 17:519–525.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–582.
- Goldhaber SZ, Haire WD, Feldstein ML, et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet 1993; 341:507–511.
- Kline JA, Steuerwald MT, Marchick MR, Hernandez-Nino J, Rose GA. Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest 2009; 136:1202–1210.
- Steering Committee of PEITHO Investigators. Single-bolus tenecteplase plus heparin compared with heparin alone for normotensive patients with acute pulmonary embolism who have evidence of right ventricular dysfunction and myocardial injury: rationale and design of the Pulmonary Embolism Thrombolysis (PEITHO) trial. Am Heart J 2012; 163:33–38.e1.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Meneveau N, Séronde MF, Blonde MC, et al. Management of unsuccessful thrombolysis in acute massive pulmonary embolism. Chest 2006; 129:1043–1050.
- Ho WK, Hankey GJ, Eikelboom JW. Should adult patients be routinely tested for heritable thrombophilia after an episode of venous thromboembolism? Med J Aust 2011; 195:139–142.
- Baglin T, Gray E, Greaves M, et al; British Committee for Standards in Haematology. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010; 149:209–220.
- Grody WW, Griffin JH, Taylor AK, Korf BR, Heit JA; ACMG Factor V Leiden Working Group. American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med 2001; 3:139–148.
- Cohn D, Vansenne F, de Borgie C, Middeldorp S. Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev 2009; ( 1):CD007069.
- Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost 2008; 6:1474–1477.
- Palareti G, Cosmi B, Legnani C, et al; PROLONG Investigators. Ddimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006; 355:1780–1789.
- Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):7S–47S.
- Stein PD, Beemath A, Matta F, et al. Clinical characteristics of patients with acute pulmonary embolism: data from PIOPED II. Am J Med 2007; 120:871–879.
- Stein PD, Saltzman HA, Weg JG. Clinical characteristics of patients with acute pulmonary embolism. Am J Cardiol 1991; 68:1723–1724.
- Ferrari E, Imbert A, Chevalier T, Mihoubi A, Morand P, Baudouy M. The ECG in pulmonary embolism. Predictive value of negative T waves in precordial leads—80 case reports. Chest 1997; 111:537–543.
- Geibel A, Zehender M, Kasper W, Olschewski M, Klima C, Konstantinides SV. Prognostic value of the ECG on admission in patients with acute major pulmonary embolism. Eur Respir J 2005; 25:843–848.
- Perrier A, Roy PM, Sanchez O, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med 2005; 352:1760–1768.
- Qanadli SD, Hajjam ME, Mesurolle B, et al. Pulmonary embolism detection: prospective evaluation of dual-section helical CT versus selective pulmonary arteriography in 157 patients. Radiology 2000; 217:447–455.
- Miniati M, Monti S, Pratali L, et al. Value of transthoracic echocardiography in the diagnosis of pulmonary embolism: results of a prospective study in unselected patients. Am J Med 2001; 110:528–535.
- Bova C, Greco F, Misuraca G, et al. Diagnostic utility of echocardiography in patients with suspected pulmonary embolism. Am J Emerg Med 2003; 21:180–183.
- Stein PD, Chenevert TL, Fowler SE, et al; PIOPED III (Prospective Investigation of Pulmonary Embolism Diagnosis III) Investigators. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III). Ann Intern Med 2010; 152:434–443,W142–W143.
- Sostman HD, Stein PD, Gottschalk A, Matta F, Hull R, Goodman L. Acute pulmonary embolism: sensitivity and specificity of ventilation-perfusion scintigraphy in PIOPED II study. Radiology 2008; 246:941–946.
- Aujesky D, Obrosky DS, Stone RA, et al. Derivation and validation of a prognostic model for pulmonary embolism. Am J Respir Crit Care Med 2005; 172:1041–1046.
- Jiménez D, Aujesky D, Moores L, et al; RIETE Investigators. Simplification of the pulmonary embolism severity index for prognostication in patients with acute symptomatic pulmonary embolism. Arch Intern Med 2010; 170:1383–1389.
- Otero R, Trujillo-Santos J, Cayuela A, et al; Registro Informatizado de la Enfermedad Tromboembólica (RIETE) Investigators. Haemodynamically unstable pulmonary embolism in the RIETE Registry: systolic blood pressure or shock index? Eur Respir J 2007; 30:1111–1116.
- Sam A, Sánchez D, Gómez V, et al. The shock index and the simplified PESI for identification of low-risk patients with acute pulmonary embolism. Eur Respir J 2011; 37:762–766.
- Kucher N, Luder CM, Dörnhöfer T, Windecker S, Meier B, Hess OM. Novel management strategy for patients with suspected pulmonary embolism. Eur Heart J 2003; 24:366–376.
- Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet 1999; 353:1386–1389.
- ten Wolde M, Söhne M, Quak E, Mac Gillavry MR, Büller HR. Prognostic value of echocardiographically assessed right ventricular dysfunction in patients with pulmonary embolism. Arch Intern Med 2004; 164:1685–1689.
- Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J 2008; 29:1569–1577.
- Kucher N, Goldhaber SZ. Cardiac biomarkers for risk stratification of patients with acute pulmonary embolism. Circulation 2003; 108:2191–2194.
- Scherz N, Labarère J, Méan M, Ibrahim SA, Fine MJ, Aujesky D. Prognostic importance of hyponatremia in patients with acute pulmonary embolism. Am J Respir Crit Care Med 2010; 182:1178–1183.
- Delèvaux I, André M, Aumaître O, Bègue RJ, Colombier M, Piette JC. Procalcitonin measurement for differential diagnosis between pulmonary embolism and pneumonia. Crit Care Med 2003; 31:661.
- Köktürk N, Kanbay A, Bukan N, Ekim N. The value of serum procalcitonin in differential diagnosis of pulmonary embolism and community-acquired pneumonia. Clin Appl Thromb Hemost 2011; 17:519–525.
- Jaff MR, McMurtry MS, Archer SL, et al; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation 2011; 123:1788–1830.
- Kucher N, Rossi E, De Rosa M, Goldhaber SZ. Massive pulmonary embolism. Circulation 2006; 113:577–582.
- Goldhaber SZ, Haire WD, Feldstein ML, et al. Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion. Lancet 1993; 341:507–511.
- Kline JA, Steuerwald MT, Marchick MR, Hernandez-Nino J, Rose GA. Prospective evaluation of right ventricular function and functional status 6 months after acute submassive pulmonary embolism: frequency of persistent or subsequent elevation in estimated pulmonary artery pressure. Chest 2009; 136:1202–1210.
- Steering Committee of PEITHO Investigators. Single-bolus tenecteplase plus heparin compared with heparin alone for normotensive patients with acute pulmonary embolism who have evidence of right ventricular dysfunction and myocardial injury: rationale and design of the Pulmonary Embolism Thrombolysis (PEITHO) trial. Am Heart J 2012; 163:33–38.e1.
- Schulman S, Kearon C, Kakkar AK, et al; RE-COVER Study Group. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med 2009; 361:2342–2352.
- Meneveau N, Séronde MF, Blonde MC, et al. Management of unsuccessful thrombolysis in acute massive pulmonary embolism. Chest 2006; 129:1043–1050.
- Ho WK, Hankey GJ, Eikelboom JW. Should adult patients be routinely tested for heritable thrombophilia after an episode of venous thromboembolism? Med J Aust 2011; 195:139–142.
- Baglin T, Gray E, Greaves M, et al; British Committee for Standards in Haematology. Clinical guidelines for testing for heritable thrombophilia. Br J Haematol 2010; 149:209–220.
- Grody WW, Griffin JH, Taylor AK, Korf BR, Heit JA; ACMG Factor V Leiden Working Group. American College of Medical Genetics consensus statement on factor V Leiden mutation testing. Genet Med 2001; 3:139–148.
- Cohn D, Vansenne F, de Borgie C, Middeldorp S. Thrombophilia testing for prevention of recurrent venous thromboembolism. Cochrane Database Syst Rev 2009; ( 1):CD007069.
- Coppens M, Reijnders JH, Middeldorp S, Doggen CJ, Rosendaal FR. Testing for inherited thrombophilia does not reduce the recurrence of venous thrombosis. J Thromb Haemost 2008; 6:1474–1477.
- Palareti G, Cosmi B, Legnani C, et al; PROLONG Investigators. Ddimer testing to determine the duration of anticoagulation therapy. N Engl J Med 2006; 355:1780–1789.
- Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ; American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl):7S–47S.
Clinical applications of pharmacogenetics: Present and near future
“Change is the only constant.”
—Heraclitus (c 535–475 bce)
With the cost of health care rising and money to pay for it shrinking, there has never been a greater need to reduce waste.
Ineffective treatments and adverse drug effects account for much preventable morbidity and expense. New treatments, touted as more potent, are often introduced as replacements for traditional ones that are still effective in many patients, adding to costs and the potential for harm. For the pharmaceutical industry, the search for new “blockbuster” drugs seems to have hit a wall, at least in cardiovascular medicine.1 Advances often come at the cost of adverse effects, such as bleeding with triple antiplatelet therapy and diabetes with potent statin drugs.
The path to maximizing benefit and reducing harm now appears to lie in stratifying populations and appreciating patient individuality in response to treatment. For many decades we have known that patients vary widely in their response to drugs, owing to personal factors such as body surface area, age, environment, and genetics. And indeed, we treat our patients as individuals, for example by tailoring aminoglycoside dose to weight and renal function.
However, clinical trials typically give us an idea of the benefits only to the average patient. While subgroup analyses identify groups that may benefit more or less from treatment, the additional information they provide is not easily integrated into the clinical model of prescribing, in which one size fits all.
THE PROMISE OF PHARMACOGENETICS
The emerging field of pharmacogenetics promises to give clinicians the tools to make informed treatment decisions based on predictive genetic testing. This genetic testing aims to match treatment to an individual’s genetic profile. This often involves analyzing single-nucleotide polymorphisms in genes for enzymes that metabolize drugs, such as the cytochrome P450 enzymes, to predict efficacy or an adverse event with treatment.
Pharmacogenetics is playing an increasing role in clinical trials, particularly in the early stages of drug development, by helping to reduce the number of patients needed, prove efficacy, and identify subgroups in which alternative treatment can be targeted. At another level, a molecular understanding of disease is leading to truly targeted treatments based on genomics.
Over recent years, genetic testing has been increasingly used in clinical practice, thanks to a convergence of factors such as rapid, low-cost tests, a growing evidence base, and emerging interest among doctors and payers.
An advantage to using genetic testing as opposed to other types of laboratory testing, such as measuring the concentration of the drug in the blood during treatment, is that genetic tests can predict the response to treatment before the treatment is started. Moreover, with therapeutic drug monitoring after treatment has begun, there are sometimes no detectable measures of toxicity. For example, both carbamazepine and the antiviral drug abacavir can—fortunately only rarely—cause Stevens-Johnson syndrome. But before genetic markers were discovered, there was no method of estimating this risk apart from taking a family history.2,3 Considering the numbers of people involved, it was not feasible until recently to suggest genetic screening for patients starting on these drugs. However, the cost of genotyping and gene sequencing has been falling at a rate inversely faster than Moore’s law (an approximate annual doubling in computer power), and population genomics is becoming a reality.4
The US Food and Drug Administration (FDA) recognizes the current and future value of pharmacogenetics in drug safety and development. A number of approved pharmacogenetic biomarkers are listed on the FDA website (Table 1). Black box warnings have been mandated for a number of drugs on the basis of observational evidence.
The FDA also promotes rapid approval for novel drugs with pharmacogenetic “companion diagnostics.” A recent example of this was the approval of ivacaftor for cystic fibrosis patients who have the G551D mutation.5 Here, a molecular understanding of the condition led to the development of a targeted treatment. Although the cost of developing this drug was high, the path is now paved for similar advances. Oncologists are familiar with these advances with the emergence of new molecularly targeted treatments, eg, BRAF inhibitors in metastatic melanoma, imatinib in chronic myeloid leukemia, and gefitinib in non-small-cell lung cancer.
PHARMACOGENETICS IN CARDIOVASCULAR MEDICINE
Cardiovascular medicine also stands to benefit from rapid advances in pharmacogenetics.
While no treatment has been developed that targets the molecular basis of cardiovascular disease, a number of genomic biomarkers have emerged that identify patients at risk of adverse reactions or treatment failure. These include genetic tests to predict the maintenance dose and risk of bleeding with warfarin,6 the likelihood of myopathy and myositis with simvastatin,7 and the risk of recurrent thrombotic events with clopidogrel.8–10
Using pharmacogenetics in prescribing warfarin and its alternatives
The pharmacogenetics of warfarin has been extensively researched, but genotyping before prescribing this drug is not yet widely done.
In 2007, the FDA updated the labeling of warfarin to include information about the influence of two genes, VKORC1 and CYP2C9, on a patient’s response to this drug. In 2010 this was updated to add that testing for these genes could be used to predict the maintenance dose of the drug. Difficulties with algorithms used to integrate this into clinical practice have hindered adoption of this testing.
With the advent of new anticoagulants such as dabigatran, rivaroxaban, and apixaban, many have expected warfarin and its pharmacogenetics to become obsolete. However, the new agents cost considerably more. Further, they may not offer a very great advantage over warfarin: in the Randomized Evaluation of Long-term Anticoagulant Therapy (RE-LY) trial, the absolute risk reduction in intracranial hemorrhage with dabigatran vs warfarin was small.11 Therefore, dabigatran is probably not cost-effective in populations at low risk of bleeding.12 A cost-effectiveness analysis comparing warfarin with dabigatran in patients with uncomplicated atrial fibrillation has suggested that dabigatran is, however, cost-effective in patients at moderate risk.12
In the RE-LY trial, the international normalized ratios (INRs) of the patients in the warfarin group were in the therapeutic range only 64% of the time. The advantages of dabigatran over warfarin become less pronounced as warfarin control is tightened.13 Of note, pharmacogenetics and home monitoring of the INR have both been shown to lead to tighter control of the INR, with greater time within the therapeutic range.14,15
Moreover, genetic testing can help us reduce the number of bleeding events in patients taking warfarin.16 Patients who carry the CYP2C9*2 or CYP2C9*3 polymorphism metabolize S-warfarin slower and therefore have a threefold higher risk of hemorrhage after starting warfarin.17 We could speculate that patients carrying these variants may be better served by the newer anticoagulants, though this has not been tested in any clinical trial.
It is also worth appreciating that the conditions requiring anticoagulation, such as atrial fibrillation, also have a strong genetic basis. Variants in chromosomes 4q25, 1q21, and 16q22 have all been associated with atrial fibrillation.18 The risk of atrial fibrillation is five to six times higher in carriers of multiple variants within all of these loci.19 Genetic variants at 4q25 have been associated with the response to specific antiarrhythmic drug treatments,20 response to pulmonary vein isolation, 21,22 and direct-current cardioversion.23 One can imagine a future in which patients with palpitations, carrying multiple gene risk variants, will choose prolonged monitoring at home to confirm a diagnosis. They would then be provided with a personalized best management strategy, using their personal preferences, clinical data, and genetic profile to make a treatment decision.
Using pharmacogenetics in prescribing clopidogrel and its alternatives
The pharmacogenetics of clopidogrel is of particular interest, as it has the potential of establishing a rational basis for using newer antiplatelet drugs such as ticagrelor and prasugrel, which are considerably more expensive than generic clopidogrel.
Most of the people who do not respond to clopidogrel carry the common cytochrome P450 2C19 variants CYP2C19*2 or CYP2C19*3.9 These variants are present in particularly high frequency in Asians and African Americans, who often do not feature in large randomized trials.
Newer antiplatelet agents have failed to demonstrate consistent superiority to clopidogrel without a tradeoff of more bleeding. However, in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis in Myocardial Infarction 39 (TRITON TIMI-38),24,25 patients with the *2 variant receiving prasugrel had lower cardiovascular event rates than *2 carriers receiving clopidogrel.
Similarly, the patients who benefit the most from ticagrelor are carriers of the 2C19 nonresponder variants. In a large study, clopidogrel responders who did not carry either 2C19 nonresponder genetic variants or ABCB1 variants had cardiovascular outcomes similar to those of patients receiving ticagrelor.26
Clinicians have been cautious in prescribing potent antiplatelet agents to all patients because of the risk of bleeding. One could assume that by reserving newer agents for clopidogrel nonresponders, the bleeding risk could be minimized and overall benefit could be preserved with this strategy.
Cost may also be contained. The cost-effectiveness of such an approach with prasugrel has been tested with computer modeling and appears favorable.27 On the other hand, a similar yet limited analysis did not find genotype-driven use of ticagrelor to be cost-effective.28 This was mostly due to fewer deaths in patients receiving ticagrelor. However, the cost estimate for genotype-guided therapy was overestimated, as heterozygotes in the model were treated with ticagrelor instead of a high dose of clopidogrel.
It now appears that heterozygotes, ie, patients with one copy of the nonresponder variant, can achieve similar platelet inhibition with clopidogrel 225 mg daily as noncarriers on 75 mg daily.29 Since genotype-guided antiplatelet therapy has not been tested in a randomized outcomes trial, this tailored strategy has not been widely accepted.
THE FUTURE
The barriers to adoption of pharmacogenetics are considerable. Clinicians need to be educated about it, reimbursement needs to be worked out, and the pharmaceutical industry needs to get behind it. Nevertheless, the future of pharmacogenetics is extremely promising.
Research networks are forming to support the use of pharmacogenetics in clinical practice. The Pharmgkb (www.pharmgkb.org) database serves as a hub for educating clinicians and researchers as well as curating data for reference. Vanderbilt University is piloting the BioVu project, in which DNA and genotype data on patients are being stored and matched to the electronic clinical records.30 These projects not only provide clinically useful information on the current state of the art of pharmacogenetics, they also aid in disseminating new information about genotype-phenotype relationships.
Analytical software that uses “natural language processing” is being applied to clinician-generated notes to derive new observations and associations between genetic variants and clinical phenotypes. Integrating this information in real-time decision-support modules in the electronic health record provides a feedback loop for a rapid assimilation of new knowledge. Similar innovative decision-support modules are being established by Cleveland Clinic’s Center for Personalized Healthcare.31
The rise of ‘omic’ sciences
Pharmacogenetics and pharmacogenomics are part of a larger set of “omic” sciences. The suffix “-omics” implies a larger, more holistic view and is being applied to a number of fields—for example, the study of proteins (proteomics) and the study of metabolites (metabolomics). Profiling proteins and metabolites delivers a deluge of information on a patient that can be clustered, using pattern-recognition software, into population subgroups. Patterns of multiple proteins or metabolites are extracted from this spectral data to identify disease or response to treatment (pharmacometabolomics).
Metabolomics has been shown to predict the response to statins,32 diagnose myocardial infarction,33 and reclassify cardiovascular risk status.34 In addition, whereas traditional laboratory chemistry is reductionist, using single biomarkers for single-disease diagnosis, omic technologies hold the potential to reveal information on a number of possible health or disease states. The identification of “healthy” profiles using these technologies can potentially provide positive feedback to patients undertaking lifestyle changes and treatment.
The instrument costs for proteomic and metabolomic profiling are relatively high. However, the ongoing running costs are minimal, estimated at as low as less than $13 per test, as there are no expensive reagents.33 High-volume testing therefore becomes very cost-effective.
Although omic science appears futuristic, proteomics and metabolomics are already used in many clinical laboratories to rapidly identify bacteria. These methods have already revolutionized the way laboratories identify microbes, since they are automated, reduce workload, and give very fast results.
The cost of genetic testing is falling
Critics of pharmacogenetics claim that the predictive value of genetic testing is poor, that evidence is lacking, and that the cost is too high. In all new technologies, the first iteration is coarse, but performance improves with use. The first major barrier is adoption. Projects like BioVu are establishing the infrastructure for a feedback loop to iteratively improve upon the status quo and provide the evidence base clinicians demand.
The cost of genetic testing is falling rapidly, with whole-genome sequencing and annotation now costing less than $5,000. The cost of a pharmacogenetic test can be as low as $100 using low-cost nanotechnology, and the test needs to be performed only once in a patient’s lifetime.27
As other related molecular technologies such as proteomics and metabolomics become available and are integrated with genomics, the predictive ability of this science will improve.
AWAY FROM ONE-SIZE-FITS-ALL MEDICINE
Over the last decade there has been a trend away from “one size fits all” to customized “markets of one” in everything from consumer products to education to medicine. Mass customizing, also known as personalization, has been embraced by the internet community as a means to increase efficiency and reduce cost. This occurs by eliminating waste in redundant work or production of ineffective products.
Personalization on the Internet has been enabled through the use of informatics, mathematics, and supercomputing. The same tools that have personalized the delivery of consumer products are also being applied to the field of pharmacogenetics. Applied in an evidence-based fashion, these new technologies should profoundly improve patient care now and in the future.
- Topol EJ. Past the wall in cardiovascular R&D. Nat Rev Drug Discov 2009; 8:259.
- Mallal S, Phillips E, Carosi G, et al; PREDICT-1 Study Team. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med 2008; 358:568–579.
- Hung SI, Chung WH, Jee SH, et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics 2006; 16:297–306.
- Phimister EG, Feero WG, Guttmacher AE. Realizing genomic medicine. N Engl J Med 2012; 366:757–759.
- Van Goor F, Hadida S, Grootenhuis PD, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA 2009; 106:18825–18830.
- International Warfarin Pharmacogenetics Consortium; Klein TE, Altman RB, Eriksson N, et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med 2009; 360:753–764.
- SEARCH Collaborative Group; Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Simon T, Verstuyft C, Mary-Krause M, et al; French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009; 360:363–375.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362
- Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 2009; 373:309–317.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Shah SV, Gage BF. Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation 2011; 123:2562–2570.
- Wallentin L, Yusuf S, Ezekowitz MD, et al; RE-LY investigators. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet 2010; 376:975–983.
- Anderson JL, Horne BD, Stevens SM, et al; Couma-Gen Investigators. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation 2007; 116:2563–2570.
- Matchar DB, Jacobson A, Dolor R, et al; THINRS Executive Committee and Site Investigators. Effect of home testing of international normalized ratio on clinical events. N Engl J Med 2010; 363:1608–1620.
- Epstein RS, Moyer TP, Aubert RE, et al. Warfarin genotyping reduces hospitalization rates results from the MM-WES (Medco-Mayo Warfarin Effectiveness study). J Am Coll Cardiol 2010; 55:2804–2812.
- Sanderson S, Emery J, Higgins J. CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a HuGEnet systematic review and meta-analysis. Genet Med 2005; 7:97–104.
- Ellinor PT, Lunetta KL, Albert CM, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet 2012; 44:670–675.
- Lubitz SA, Sinner MF, Lunetta KL, et al. Independent susceptibility markers for atrial fibrillation on chromosome 4q25. Circulation 2010; 122:976–984.
- Parvez B, Vaglio J, Rowan S, et al. Symptomatic response to antiarrhythmic drug therapy is modulated by a common single nucleotide polymorphism in atrial fibrillation. J Am Coll Cardiol 2012; 60:539–545.
- Husser D, Adams V, Piorkowski C, Hindricks G, Bollmann A. Chromosome 4q25 variants and atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol 2010; 55:747–753.
- Benjamin Shoemaker M, Muhammad R, Parvez B, et al. Common atrial fibrillation risk alleles at 4q25 predict recurrence after catheter-based atrial fibrillation ablation.” Heart Rhythm 2012; Nov 23.pii: S1547-5271(12)01340–9. 10.1016/j.hrthm.2012.11.012. [Epub ahead of print]
- Parvez B, Benjamin Shoemaker M, Muhammad R, et al. Common genetic polymorphism at 4q25 locus predicts atrial fibrillation recurrence after successful cardioversion. Heart Rhythm 2013 Feb 18.pii: S1547-5271(13)00161–6. 10.1016/j.hrthm.2013.02.018. [Epub ahead of print]
- Mega JL, Close SL, Wiviott SD, et al. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet 2010; 376:1312–1319.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation 2009; 119:2553–2560.
- Wallentin L, James S, Storey RF, et al; PLATO investigators. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet 2010; 376:1320–1328.
- Guzauskas GF, Hughes DA, Bradley SM, Veenstra DL. A risk-benefit assessment of prasugrel, clopidogrel, and genotype-guided therapy in patients undergoing percutaneous coronary intervention. Clin Pharmacol Ther 2012; 91:829–837.
- Crespin DJ, Federspiel JJ, Biddle AK, Jonas DE, Rossi JS. Ticagrelor versus genotype-driven antiplatelet therapy for secondary prevention after acute coronary syndrome: a cost-effectiveness analysis. Value Health 2011; 14:483–491.
- Mega JL, Hochholzer W, Frelinger AL, et al. Dosing clopidogrel based on CYP2C19 genotype and the effect on platelet reactivity in patients with stable cardiovascular disease. JAMA 2011; 306:2221–2228.
- Xu H, Jiang M, Oetjens M, et al. Facilitating pharmacogenetic studies using electronic health records and natural-language processing: a case study of warfarin. J Am Med Inform Assoc 2011; 18:387–391.
- Teng K, Eng C, Hess CA, et al. Building an innovative model for personalized healthcare. Cleve Clin J Med 2012; 79( suppl 1):S1–S9.
- Kaddurah-Daouk R, Baillie RA, Zhu H, et al. Enteric microbiome metabolites correlate with response to simvastatin treatment. PLoS One 2011; 6:e25482.
- Bodi V, Sanchis J, Morales JM, et al. Metabolomic profile of human myocardial ischemia by nuclear magnetic resonance spectroscopy of peripheral blood serum: a translational study based on transient coronary occlusion models. J Am Coll Cardiol 2012; 59:1629–1641.
- Shah SH, Sun JL, Stevens RD, et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am Heart J 2012; 163:844–850.e1.
“Change is the only constant.”
—Heraclitus (c 535–475 bce)
With the cost of health care rising and money to pay for it shrinking, there has never been a greater need to reduce waste.
Ineffective treatments and adverse drug effects account for much preventable morbidity and expense. New treatments, touted as more potent, are often introduced as replacements for traditional ones that are still effective in many patients, adding to costs and the potential for harm. For the pharmaceutical industry, the search for new “blockbuster” drugs seems to have hit a wall, at least in cardiovascular medicine.1 Advances often come at the cost of adverse effects, such as bleeding with triple antiplatelet therapy and diabetes with potent statin drugs.
The path to maximizing benefit and reducing harm now appears to lie in stratifying populations and appreciating patient individuality in response to treatment. For many decades we have known that patients vary widely in their response to drugs, owing to personal factors such as body surface area, age, environment, and genetics. And indeed, we treat our patients as individuals, for example by tailoring aminoglycoside dose to weight and renal function.
However, clinical trials typically give us an idea of the benefits only to the average patient. While subgroup analyses identify groups that may benefit more or less from treatment, the additional information they provide is not easily integrated into the clinical model of prescribing, in which one size fits all.
THE PROMISE OF PHARMACOGENETICS
The emerging field of pharmacogenetics promises to give clinicians the tools to make informed treatment decisions based on predictive genetic testing. This genetic testing aims to match treatment to an individual’s genetic profile. This often involves analyzing single-nucleotide polymorphisms in genes for enzymes that metabolize drugs, such as the cytochrome P450 enzymes, to predict efficacy or an adverse event with treatment.
Pharmacogenetics is playing an increasing role in clinical trials, particularly in the early stages of drug development, by helping to reduce the number of patients needed, prove efficacy, and identify subgroups in which alternative treatment can be targeted. At another level, a molecular understanding of disease is leading to truly targeted treatments based on genomics.
Over recent years, genetic testing has been increasingly used in clinical practice, thanks to a convergence of factors such as rapid, low-cost tests, a growing evidence base, and emerging interest among doctors and payers.
An advantage to using genetic testing as opposed to other types of laboratory testing, such as measuring the concentration of the drug in the blood during treatment, is that genetic tests can predict the response to treatment before the treatment is started. Moreover, with therapeutic drug monitoring after treatment has begun, there are sometimes no detectable measures of toxicity. For example, both carbamazepine and the antiviral drug abacavir can—fortunately only rarely—cause Stevens-Johnson syndrome. But before genetic markers were discovered, there was no method of estimating this risk apart from taking a family history.2,3 Considering the numbers of people involved, it was not feasible until recently to suggest genetic screening for patients starting on these drugs. However, the cost of genotyping and gene sequencing has been falling at a rate inversely faster than Moore’s law (an approximate annual doubling in computer power), and population genomics is becoming a reality.4
The US Food and Drug Administration (FDA) recognizes the current and future value of pharmacogenetics in drug safety and development. A number of approved pharmacogenetic biomarkers are listed on the FDA website (Table 1). Black box warnings have been mandated for a number of drugs on the basis of observational evidence.
The FDA also promotes rapid approval for novel drugs with pharmacogenetic “companion diagnostics.” A recent example of this was the approval of ivacaftor for cystic fibrosis patients who have the G551D mutation.5 Here, a molecular understanding of the condition led to the development of a targeted treatment. Although the cost of developing this drug was high, the path is now paved for similar advances. Oncologists are familiar with these advances with the emergence of new molecularly targeted treatments, eg, BRAF inhibitors in metastatic melanoma, imatinib in chronic myeloid leukemia, and gefitinib in non-small-cell lung cancer.
PHARMACOGENETICS IN CARDIOVASCULAR MEDICINE
Cardiovascular medicine also stands to benefit from rapid advances in pharmacogenetics.
While no treatment has been developed that targets the molecular basis of cardiovascular disease, a number of genomic biomarkers have emerged that identify patients at risk of adverse reactions or treatment failure. These include genetic tests to predict the maintenance dose and risk of bleeding with warfarin,6 the likelihood of myopathy and myositis with simvastatin,7 and the risk of recurrent thrombotic events with clopidogrel.8–10
Using pharmacogenetics in prescribing warfarin and its alternatives
The pharmacogenetics of warfarin has been extensively researched, but genotyping before prescribing this drug is not yet widely done.
In 2007, the FDA updated the labeling of warfarin to include information about the influence of two genes, VKORC1 and CYP2C9, on a patient’s response to this drug. In 2010 this was updated to add that testing for these genes could be used to predict the maintenance dose of the drug. Difficulties with algorithms used to integrate this into clinical practice have hindered adoption of this testing.
With the advent of new anticoagulants such as dabigatran, rivaroxaban, and apixaban, many have expected warfarin and its pharmacogenetics to become obsolete. However, the new agents cost considerably more. Further, they may not offer a very great advantage over warfarin: in the Randomized Evaluation of Long-term Anticoagulant Therapy (RE-LY) trial, the absolute risk reduction in intracranial hemorrhage with dabigatran vs warfarin was small.11 Therefore, dabigatran is probably not cost-effective in populations at low risk of bleeding.12 A cost-effectiveness analysis comparing warfarin with dabigatran in patients with uncomplicated atrial fibrillation has suggested that dabigatran is, however, cost-effective in patients at moderate risk.12
In the RE-LY trial, the international normalized ratios (INRs) of the patients in the warfarin group were in the therapeutic range only 64% of the time. The advantages of dabigatran over warfarin become less pronounced as warfarin control is tightened.13 Of note, pharmacogenetics and home monitoring of the INR have both been shown to lead to tighter control of the INR, with greater time within the therapeutic range.14,15
Moreover, genetic testing can help us reduce the number of bleeding events in patients taking warfarin.16 Patients who carry the CYP2C9*2 or CYP2C9*3 polymorphism metabolize S-warfarin slower and therefore have a threefold higher risk of hemorrhage after starting warfarin.17 We could speculate that patients carrying these variants may be better served by the newer anticoagulants, though this has not been tested in any clinical trial.
It is also worth appreciating that the conditions requiring anticoagulation, such as atrial fibrillation, also have a strong genetic basis. Variants in chromosomes 4q25, 1q21, and 16q22 have all been associated with atrial fibrillation.18 The risk of atrial fibrillation is five to six times higher in carriers of multiple variants within all of these loci.19 Genetic variants at 4q25 have been associated with the response to specific antiarrhythmic drug treatments,20 response to pulmonary vein isolation, 21,22 and direct-current cardioversion.23 One can imagine a future in which patients with palpitations, carrying multiple gene risk variants, will choose prolonged monitoring at home to confirm a diagnosis. They would then be provided with a personalized best management strategy, using their personal preferences, clinical data, and genetic profile to make a treatment decision.
Using pharmacogenetics in prescribing clopidogrel and its alternatives
The pharmacogenetics of clopidogrel is of particular interest, as it has the potential of establishing a rational basis for using newer antiplatelet drugs such as ticagrelor and prasugrel, which are considerably more expensive than generic clopidogrel.
Most of the people who do not respond to clopidogrel carry the common cytochrome P450 2C19 variants CYP2C19*2 or CYP2C19*3.9 These variants are present in particularly high frequency in Asians and African Americans, who often do not feature in large randomized trials.
Newer antiplatelet agents have failed to demonstrate consistent superiority to clopidogrel without a tradeoff of more bleeding. However, in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis in Myocardial Infarction 39 (TRITON TIMI-38),24,25 patients with the *2 variant receiving prasugrel had lower cardiovascular event rates than *2 carriers receiving clopidogrel.
Similarly, the patients who benefit the most from ticagrelor are carriers of the 2C19 nonresponder variants. In a large study, clopidogrel responders who did not carry either 2C19 nonresponder genetic variants or ABCB1 variants had cardiovascular outcomes similar to those of patients receiving ticagrelor.26
Clinicians have been cautious in prescribing potent antiplatelet agents to all patients because of the risk of bleeding. One could assume that by reserving newer agents for clopidogrel nonresponders, the bleeding risk could be minimized and overall benefit could be preserved with this strategy.
Cost may also be contained. The cost-effectiveness of such an approach with prasugrel has been tested with computer modeling and appears favorable.27 On the other hand, a similar yet limited analysis did not find genotype-driven use of ticagrelor to be cost-effective.28 This was mostly due to fewer deaths in patients receiving ticagrelor. However, the cost estimate for genotype-guided therapy was overestimated, as heterozygotes in the model were treated with ticagrelor instead of a high dose of clopidogrel.
It now appears that heterozygotes, ie, patients with one copy of the nonresponder variant, can achieve similar platelet inhibition with clopidogrel 225 mg daily as noncarriers on 75 mg daily.29 Since genotype-guided antiplatelet therapy has not been tested in a randomized outcomes trial, this tailored strategy has not been widely accepted.
THE FUTURE
The barriers to adoption of pharmacogenetics are considerable. Clinicians need to be educated about it, reimbursement needs to be worked out, and the pharmaceutical industry needs to get behind it. Nevertheless, the future of pharmacogenetics is extremely promising.
Research networks are forming to support the use of pharmacogenetics in clinical practice. The Pharmgkb (www.pharmgkb.org) database serves as a hub for educating clinicians and researchers as well as curating data for reference. Vanderbilt University is piloting the BioVu project, in which DNA and genotype data on patients are being stored and matched to the electronic clinical records.30 These projects not only provide clinically useful information on the current state of the art of pharmacogenetics, they also aid in disseminating new information about genotype-phenotype relationships.
Analytical software that uses “natural language processing” is being applied to clinician-generated notes to derive new observations and associations between genetic variants and clinical phenotypes. Integrating this information in real-time decision-support modules in the electronic health record provides a feedback loop for a rapid assimilation of new knowledge. Similar innovative decision-support modules are being established by Cleveland Clinic’s Center for Personalized Healthcare.31
The rise of ‘omic’ sciences
Pharmacogenetics and pharmacogenomics are part of a larger set of “omic” sciences. The suffix “-omics” implies a larger, more holistic view and is being applied to a number of fields—for example, the study of proteins (proteomics) and the study of metabolites (metabolomics). Profiling proteins and metabolites delivers a deluge of information on a patient that can be clustered, using pattern-recognition software, into population subgroups. Patterns of multiple proteins or metabolites are extracted from this spectral data to identify disease or response to treatment (pharmacometabolomics).
Metabolomics has been shown to predict the response to statins,32 diagnose myocardial infarction,33 and reclassify cardiovascular risk status.34 In addition, whereas traditional laboratory chemistry is reductionist, using single biomarkers for single-disease diagnosis, omic technologies hold the potential to reveal information on a number of possible health or disease states. The identification of “healthy” profiles using these technologies can potentially provide positive feedback to patients undertaking lifestyle changes and treatment.
The instrument costs for proteomic and metabolomic profiling are relatively high. However, the ongoing running costs are minimal, estimated at as low as less than $13 per test, as there are no expensive reagents.33 High-volume testing therefore becomes very cost-effective.
Although omic science appears futuristic, proteomics and metabolomics are already used in many clinical laboratories to rapidly identify bacteria. These methods have already revolutionized the way laboratories identify microbes, since they are automated, reduce workload, and give very fast results.
The cost of genetic testing is falling
Critics of pharmacogenetics claim that the predictive value of genetic testing is poor, that evidence is lacking, and that the cost is too high. In all new technologies, the first iteration is coarse, but performance improves with use. The first major barrier is adoption. Projects like BioVu are establishing the infrastructure for a feedback loop to iteratively improve upon the status quo and provide the evidence base clinicians demand.
The cost of genetic testing is falling rapidly, with whole-genome sequencing and annotation now costing less than $5,000. The cost of a pharmacogenetic test can be as low as $100 using low-cost nanotechnology, and the test needs to be performed only once in a patient’s lifetime.27
As other related molecular technologies such as proteomics and metabolomics become available and are integrated with genomics, the predictive ability of this science will improve.
AWAY FROM ONE-SIZE-FITS-ALL MEDICINE
Over the last decade there has been a trend away from “one size fits all” to customized “markets of one” in everything from consumer products to education to medicine. Mass customizing, also known as personalization, has been embraced by the internet community as a means to increase efficiency and reduce cost. This occurs by eliminating waste in redundant work or production of ineffective products.
Personalization on the Internet has been enabled through the use of informatics, mathematics, and supercomputing. The same tools that have personalized the delivery of consumer products are also being applied to the field of pharmacogenetics. Applied in an evidence-based fashion, these new technologies should profoundly improve patient care now and in the future.
“Change is the only constant.”
—Heraclitus (c 535–475 bce)
With the cost of health care rising and money to pay for it shrinking, there has never been a greater need to reduce waste.
Ineffective treatments and adverse drug effects account for much preventable morbidity and expense. New treatments, touted as more potent, are often introduced as replacements for traditional ones that are still effective in many patients, adding to costs and the potential for harm. For the pharmaceutical industry, the search for new “blockbuster” drugs seems to have hit a wall, at least in cardiovascular medicine.1 Advances often come at the cost of adverse effects, such as bleeding with triple antiplatelet therapy and diabetes with potent statin drugs.
The path to maximizing benefit and reducing harm now appears to lie in stratifying populations and appreciating patient individuality in response to treatment. For many decades we have known that patients vary widely in their response to drugs, owing to personal factors such as body surface area, age, environment, and genetics. And indeed, we treat our patients as individuals, for example by tailoring aminoglycoside dose to weight and renal function.
However, clinical trials typically give us an idea of the benefits only to the average patient. While subgroup analyses identify groups that may benefit more or less from treatment, the additional information they provide is not easily integrated into the clinical model of prescribing, in which one size fits all.
THE PROMISE OF PHARMACOGENETICS
The emerging field of pharmacogenetics promises to give clinicians the tools to make informed treatment decisions based on predictive genetic testing. This genetic testing aims to match treatment to an individual’s genetic profile. This often involves analyzing single-nucleotide polymorphisms in genes for enzymes that metabolize drugs, such as the cytochrome P450 enzymes, to predict efficacy or an adverse event with treatment.
Pharmacogenetics is playing an increasing role in clinical trials, particularly in the early stages of drug development, by helping to reduce the number of patients needed, prove efficacy, and identify subgroups in which alternative treatment can be targeted. At another level, a molecular understanding of disease is leading to truly targeted treatments based on genomics.
Over recent years, genetic testing has been increasingly used in clinical practice, thanks to a convergence of factors such as rapid, low-cost tests, a growing evidence base, and emerging interest among doctors and payers.
An advantage to using genetic testing as opposed to other types of laboratory testing, such as measuring the concentration of the drug in the blood during treatment, is that genetic tests can predict the response to treatment before the treatment is started. Moreover, with therapeutic drug monitoring after treatment has begun, there are sometimes no detectable measures of toxicity. For example, both carbamazepine and the antiviral drug abacavir can—fortunately only rarely—cause Stevens-Johnson syndrome. But before genetic markers were discovered, there was no method of estimating this risk apart from taking a family history.2,3 Considering the numbers of people involved, it was not feasible until recently to suggest genetic screening for patients starting on these drugs. However, the cost of genotyping and gene sequencing has been falling at a rate inversely faster than Moore’s law (an approximate annual doubling in computer power), and population genomics is becoming a reality.4
The US Food and Drug Administration (FDA) recognizes the current and future value of pharmacogenetics in drug safety and development. A number of approved pharmacogenetic biomarkers are listed on the FDA website (Table 1). Black box warnings have been mandated for a number of drugs on the basis of observational evidence.
The FDA also promotes rapid approval for novel drugs with pharmacogenetic “companion diagnostics.” A recent example of this was the approval of ivacaftor for cystic fibrosis patients who have the G551D mutation.5 Here, a molecular understanding of the condition led to the development of a targeted treatment. Although the cost of developing this drug was high, the path is now paved for similar advances. Oncologists are familiar with these advances with the emergence of new molecularly targeted treatments, eg, BRAF inhibitors in metastatic melanoma, imatinib in chronic myeloid leukemia, and gefitinib in non-small-cell lung cancer.
PHARMACOGENETICS IN CARDIOVASCULAR MEDICINE
Cardiovascular medicine also stands to benefit from rapid advances in pharmacogenetics.
While no treatment has been developed that targets the molecular basis of cardiovascular disease, a number of genomic biomarkers have emerged that identify patients at risk of adverse reactions or treatment failure. These include genetic tests to predict the maintenance dose and risk of bleeding with warfarin,6 the likelihood of myopathy and myositis with simvastatin,7 and the risk of recurrent thrombotic events with clopidogrel.8–10
Using pharmacogenetics in prescribing warfarin and its alternatives
The pharmacogenetics of warfarin has been extensively researched, but genotyping before prescribing this drug is not yet widely done.
In 2007, the FDA updated the labeling of warfarin to include information about the influence of two genes, VKORC1 and CYP2C9, on a patient’s response to this drug. In 2010 this was updated to add that testing for these genes could be used to predict the maintenance dose of the drug. Difficulties with algorithms used to integrate this into clinical practice have hindered adoption of this testing.
With the advent of new anticoagulants such as dabigatran, rivaroxaban, and apixaban, many have expected warfarin and its pharmacogenetics to become obsolete. However, the new agents cost considerably more. Further, they may not offer a very great advantage over warfarin: in the Randomized Evaluation of Long-term Anticoagulant Therapy (RE-LY) trial, the absolute risk reduction in intracranial hemorrhage with dabigatran vs warfarin was small.11 Therefore, dabigatran is probably not cost-effective in populations at low risk of bleeding.12 A cost-effectiveness analysis comparing warfarin with dabigatran in patients with uncomplicated atrial fibrillation has suggested that dabigatran is, however, cost-effective in patients at moderate risk.12
In the RE-LY trial, the international normalized ratios (INRs) of the patients in the warfarin group were in the therapeutic range only 64% of the time. The advantages of dabigatran over warfarin become less pronounced as warfarin control is tightened.13 Of note, pharmacogenetics and home monitoring of the INR have both been shown to lead to tighter control of the INR, with greater time within the therapeutic range.14,15
Moreover, genetic testing can help us reduce the number of bleeding events in patients taking warfarin.16 Patients who carry the CYP2C9*2 or CYP2C9*3 polymorphism metabolize S-warfarin slower and therefore have a threefold higher risk of hemorrhage after starting warfarin.17 We could speculate that patients carrying these variants may be better served by the newer anticoagulants, though this has not been tested in any clinical trial.
It is also worth appreciating that the conditions requiring anticoagulation, such as atrial fibrillation, also have a strong genetic basis. Variants in chromosomes 4q25, 1q21, and 16q22 have all been associated with atrial fibrillation.18 The risk of atrial fibrillation is five to six times higher in carriers of multiple variants within all of these loci.19 Genetic variants at 4q25 have been associated with the response to specific antiarrhythmic drug treatments,20 response to pulmonary vein isolation, 21,22 and direct-current cardioversion.23 One can imagine a future in which patients with palpitations, carrying multiple gene risk variants, will choose prolonged monitoring at home to confirm a diagnosis. They would then be provided with a personalized best management strategy, using their personal preferences, clinical data, and genetic profile to make a treatment decision.
Using pharmacogenetics in prescribing clopidogrel and its alternatives
The pharmacogenetics of clopidogrel is of particular interest, as it has the potential of establishing a rational basis for using newer antiplatelet drugs such as ticagrelor and prasugrel, which are considerably more expensive than generic clopidogrel.
Most of the people who do not respond to clopidogrel carry the common cytochrome P450 2C19 variants CYP2C19*2 or CYP2C19*3.9 These variants are present in particularly high frequency in Asians and African Americans, who often do not feature in large randomized trials.
Newer antiplatelet agents have failed to demonstrate consistent superiority to clopidogrel without a tradeoff of more bleeding. However, in the Trial to Assess Improvement in Therapeutic Outcomes by Optimizing Platelet Inhibition With Prasugrel-Thrombolysis in Myocardial Infarction 39 (TRITON TIMI-38),24,25 patients with the *2 variant receiving prasugrel had lower cardiovascular event rates than *2 carriers receiving clopidogrel.
Similarly, the patients who benefit the most from ticagrelor are carriers of the 2C19 nonresponder variants. In a large study, clopidogrel responders who did not carry either 2C19 nonresponder genetic variants or ABCB1 variants had cardiovascular outcomes similar to those of patients receiving ticagrelor.26
Clinicians have been cautious in prescribing potent antiplatelet agents to all patients because of the risk of bleeding. One could assume that by reserving newer agents for clopidogrel nonresponders, the bleeding risk could be minimized and overall benefit could be preserved with this strategy.
Cost may also be contained. The cost-effectiveness of such an approach with prasugrel has been tested with computer modeling and appears favorable.27 On the other hand, a similar yet limited analysis did not find genotype-driven use of ticagrelor to be cost-effective.28 This was mostly due to fewer deaths in patients receiving ticagrelor. However, the cost estimate for genotype-guided therapy was overestimated, as heterozygotes in the model were treated with ticagrelor instead of a high dose of clopidogrel.
It now appears that heterozygotes, ie, patients with one copy of the nonresponder variant, can achieve similar platelet inhibition with clopidogrel 225 mg daily as noncarriers on 75 mg daily.29 Since genotype-guided antiplatelet therapy has not been tested in a randomized outcomes trial, this tailored strategy has not been widely accepted.
THE FUTURE
The barriers to adoption of pharmacogenetics are considerable. Clinicians need to be educated about it, reimbursement needs to be worked out, and the pharmaceutical industry needs to get behind it. Nevertheless, the future of pharmacogenetics is extremely promising.
Research networks are forming to support the use of pharmacogenetics in clinical practice. The Pharmgkb (www.pharmgkb.org) database serves as a hub for educating clinicians and researchers as well as curating data for reference. Vanderbilt University is piloting the BioVu project, in which DNA and genotype data on patients are being stored and matched to the electronic clinical records.30 These projects not only provide clinically useful information on the current state of the art of pharmacogenetics, they also aid in disseminating new information about genotype-phenotype relationships.
Analytical software that uses “natural language processing” is being applied to clinician-generated notes to derive new observations and associations between genetic variants and clinical phenotypes. Integrating this information in real-time decision-support modules in the electronic health record provides a feedback loop for a rapid assimilation of new knowledge. Similar innovative decision-support modules are being established by Cleveland Clinic’s Center for Personalized Healthcare.31
The rise of ‘omic’ sciences
Pharmacogenetics and pharmacogenomics are part of a larger set of “omic” sciences. The suffix “-omics” implies a larger, more holistic view and is being applied to a number of fields—for example, the study of proteins (proteomics) and the study of metabolites (metabolomics). Profiling proteins and metabolites delivers a deluge of information on a patient that can be clustered, using pattern-recognition software, into population subgroups. Patterns of multiple proteins or metabolites are extracted from this spectral data to identify disease or response to treatment (pharmacometabolomics).
Metabolomics has been shown to predict the response to statins,32 diagnose myocardial infarction,33 and reclassify cardiovascular risk status.34 In addition, whereas traditional laboratory chemistry is reductionist, using single biomarkers for single-disease diagnosis, omic technologies hold the potential to reveal information on a number of possible health or disease states. The identification of “healthy” profiles using these technologies can potentially provide positive feedback to patients undertaking lifestyle changes and treatment.
The instrument costs for proteomic and metabolomic profiling are relatively high. However, the ongoing running costs are minimal, estimated at as low as less than $13 per test, as there are no expensive reagents.33 High-volume testing therefore becomes very cost-effective.
Although omic science appears futuristic, proteomics and metabolomics are already used in many clinical laboratories to rapidly identify bacteria. These methods have already revolutionized the way laboratories identify microbes, since they are automated, reduce workload, and give very fast results.
The cost of genetic testing is falling
Critics of pharmacogenetics claim that the predictive value of genetic testing is poor, that evidence is lacking, and that the cost is too high. In all new technologies, the first iteration is coarse, but performance improves with use. The first major barrier is adoption. Projects like BioVu are establishing the infrastructure for a feedback loop to iteratively improve upon the status quo and provide the evidence base clinicians demand.
The cost of genetic testing is falling rapidly, with whole-genome sequencing and annotation now costing less than $5,000. The cost of a pharmacogenetic test can be as low as $100 using low-cost nanotechnology, and the test needs to be performed only once in a patient’s lifetime.27
As other related molecular technologies such as proteomics and metabolomics become available and are integrated with genomics, the predictive ability of this science will improve.
AWAY FROM ONE-SIZE-FITS-ALL MEDICINE
Over the last decade there has been a trend away from “one size fits all” to customized “markets of one” in everything from consumer products to education to medicine. Mass customizing, also known as personalization, has been embraced by the internet community as a means to increase efficiency and reduce cost. This occurs by eliminating waste in redundant work or production of ineffective products.
Personalization on the Internet has been enabled through the use of informatics, mathematics, and supercomputing. The same tools that have personalized the delivery of consumer products are also being applied to the field of pharmacogenetics. Applied in an evidence-based fashion, these new technologies should profoundly improve patient care now and in the future.
- Topol EJ. Past the wall in cardiovascular R&D. Nat Rev Drug Discov 2009; 8:259.
- Mallal S, Phillips E, Carosi G, et al; PREDICT-1 Study Team. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med 2008; 358:568–579.
- Hung SI, Chung WH, Jee SH, et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics 2006; 16:297–306.
- Phimister EG, Feero WG, Guttmacher AE. Realizing genomic medicine. N Engl J Med 2012; 366:757–759.
- Van Goor F, Hadida S, Grootenhuis PD, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA 2009; 106:18825–18830.
- International Warfarin Pharmacogenetics Consortium; Klein TE, Altman RB, Eriksson N, et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med 2009; 360:753–764.
- SEARCH Collaborative Group; Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Simon T, Verstuyft C, Mary-Krause M, et al; French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009; 360:363–375.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362
- Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 2009; 373:309–317.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Shah SV, Gage BF. Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation 2011; 123:2562–2570.
- Wallentin L, Yusuf S, Ezekowitz MD, et al; RE-LY investigators. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet 2010; 376:975–983.
- Anderson JL, Horne BD, Stevens SM, et al; Couma-Gen Investigators. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation 2007; 116:2563–2570.
- Matchar DB, Jacobson A, Dolor R, et al; THINRS Executive Committee and Site Investigators. Effect of home testing of international normalized ratio on clinical events. N Engl J Med 2010; 363:1608–1620.
- Epstein RS, Moyer TP, Aubert RE, et al. Warfarin genotyping reduces hospitalization rates results from the MM-WES (Medco-Mayo Warfarin Effectiveness study). J Am Coll Cardiol 2010; 55:2804–2812.
- Sanderson S, Emery J, Higgins J. CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a HuGEnet systematic review and meta-analysis. Genet Med 2005; 7:97–104.
- Ellinor PT, Lunetta KL, Albert CM, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet 2012; 44:670–675.
- Lubitz SA, Sinner MF, Lunetta KL, et al. Independent susceptibility markers for atrial fibrillation on chromosome 4q25. Circulation 2010; 122:976–984.
- Parvez B, Vaglio J, Rowan S, et al. Symptomatic response to antiarrhythmic drug therapy is modulated by a common single nucleotide polymorphism in atrial fibrillation. J Am Coll Cardiol 2012; 60:539–545.
- Husser D, Adams V, Piorkowski C, Hindricks G, Bollmann A. Chromosome 4q25 variants and atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol 2010; 55:747–753.
- Benjamin Shoemaker M, Muhammad R, Parvez B, et al. Common atrial fibrillation risk alleles at 4q25 predict recurrence after catheter-based atrial fibrillation ablation.” Heart Rhythm 2012; Nov 23.pii: S1547-5271(12)01340–9. 10.1016/j.hrthm.2012.11.012. [Epub ahead of print]
- Parvez B, Benjamin Shoemaker M, Muhammad R, et al. Common genetic polymorphism at 4q25 locus predicts atrial fibrillation recurrence after successful cardioversion. Heart Rhythm 2013 Feb 18.pii: S1547-5271(13)00161–6. 10.1016/j.hrthm.2013.02.018. [Epub ahead of print]
- Mega JL, Close SL, Wiviott SD, et al. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet 2010; 376:1312–1319.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation 2009; 119:2553–2560.
- Wallentin L, James S, Storey RF, et al; PLATO investigators. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet 2010; 376:1320–1328.
- Guzauskas GF, Hughes DA, Bradley SM, Veenstra DL. A risk-benefit assessment of prasugrel, clopidogrel, and genotype-guided therapy in patients undergoing percutaneous coronary intervention. Clin Pharmacol Ther 2012; 91:829–837.
- Crespin DJ, Federspiel JJ, Biddle AK, Jonas DE, Rossi JS. Ticagrelor versus genotype-driven antiplatelet therapy for secondary prevention after acute coronary syndrome: a cost-effectiveness analysis. Value Health 2011; 14:483–491.
- Mega JL, Hochholzer W, Frelinger AL, et al. Dosing clopidogrel based on CYP2C19 genotype and the effect on platelet reactivity in patients with stable cardiovascular disease. JAMA 2011; 306:2221–2228.
- Xu H, Jiang M, Oetjens M, et al. Facilitating pharmacogenetic studies using electronic health records and natural-language processing: a case study of warfarin. J Am Med Inform Assoc 2011; 18:387–391.
- Teng K, Eng C, Hess CA, et al. Building an innovative model for personalized healthcare. Cleve Clin J Med 2012; 79( suppl 1):S1–S9.
- Kaddurah-Daouk R, Baillie RA, Zhu H, et al. Enteric microbiome metabolites correlate with response to simvastatin treatment. PLoS One 2011; 6:e25482.
- Bodi V, Sanchis J, Morales JM, et al. Metabolomic profile of human myocardial ischemia by nuclear magnetic resonance spectroscopy of peripheral blood serum: a translational study based on transient coronary occlusion models. J Am Coll Cardiol 2012; 59:1629–1641.
- Shah SH, Sun JL, Stevens RD, et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am Heart J 2012; 163:844–850.e1.
- Topol EJ. Past the wall in cardiovascular R&D. Nat Rev Drug Discov 2009; 8:259.
- Mallal S, Phillips E, Carosi G, et al; PREDICT-1 Study Team. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med 2008; 358:568–579.
- Hung SI, Chung WH, Jee SH, et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics 2006; 16:297–306.
- Phimister EG, Feero WG, Guttmacher AE. Realizing genomic medicine. N Engl J Med 2012; 366:757–759.
- Van Goor F, Hadida S, Grootenhuis PD, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA 2009; 106:18825–18830.
- International Warfarin Pharmacogenetics Consortium; Klein TE, Altman RB, Eriksson N, et al. Estimation of the warfarin dose with clinical and pharmacogenetic data. N Engl J Med 2009; 360:753–764.
- SEARCH Collaborative Group; Link E, Parish S, Armitage J, et al. SLCO1B1 variants and statin-induced myopathy—a genomewide study. N Engl J Med 2008; 359:789–799.
- Simon T, Verstuyft C, Mary-Krause M, et al; French Registry of Acute ST-Elevation and Non-ST-Elevation Myocardial Infarction (FAST-MI) Investigators. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med 2009; 360:363–375.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome p-450 polymorphisms and response to clopidogrel. N Engl J Med 2009; 360:354–362
- Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet 2009; 373:309–317.
- Connolly SJ, Ezekowitz MD, Yusuf S, et al; RE-LY Steering Committee and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med 2009; 361:1139–1151.
- Shah SV, Gage BF. Cost-effectiveness of dabigatran for stroke prophylaxis in atrial fibrillation. Circulation 2011; 123:2562–2570.
- Wallentin L, Yusuf S, Ezekowitz MD, et al; RE-LY investigators. Efficacy and safety of dabigatran compared with warfarin at different levels of international normalised ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet 2010; 376:975–983.
- Anderson JL, Horne BD, Stevens SM, et al; Couma-Gen Investigators. Randomized trial of genotype-guided versus standard warfarin dosing in patients initiating oral anticoagulation. Circulation 2007; 116:2563–2570.
- Matchar DB, Jacobson A, Dolor R, et al; THINRS Executive Committee and Site Investigators. Effect of home testing of international normalized ratio on clinical events. N Engl J Med 2010; 363:1608–1620.
- Epstein RS, Moyer TP, Aubert RE, et al. Warfarin genotyping reduces hospitalization rates results from the MM-WES (Medco-Mayo Warfarin Effectiveness study). J Am Coll Cardiol 2010; 55:2804–2812.
- Sanderson S, Emery J, Higgins J. CYP2C9 gene variants, drug dose, and bleeding risk in warfarin-treated patients: a HuGEnet systematic review and meta-analysis. Genet Med 2005; 7:97–104.
- Ellinor PT, Lunetta KL, Albert CM, et al. Meta-analysis identifies six new susceptibility loci for atrial fibrillation. Nat Genet 2012; 44:670–675.
- Lubitz SA, Sinner MF, Lunetta KL, et al. Independent susceptibility markers for atrial fibrillation on chromosome 4q25. Circulation 2010; 122:976–984.
- Parvez B, Vaglio J, Rowan S, et al. Symptomatic response to antiarrhythmic drug therapy is modulated by a common single nucleotide polymorphism in atrial fibrillation. J Am Coll Cardiol 2012; 60:539–545.
- Husser D, Adams V, Piorkowski C, Hindricks G, Bollmann A. Chromosome 4q25 variants and atrial fibrillation recurrence after catheter ablation. J Am Coll Cardiol 2010; 55:747–753.
- Benjamin Shoemaker M, Muhammad R, Parvez B, et al. Common atrial fibrillation risk alleles at 4q25 predict recurrence after catheter-based atrial fibrillation ablation.” Heart Rhythm 2012; Nov 23.pii: S1547-5271(12)01340–9. 10.1016/j.hrthm.2012.11.012. [Epub ahead of print]
- Parvez B, Benjamin Shoemaker M, Muhammad R, et al. Common genetic polymorphism at 4q25 locus predicts atrial fibrillation recurrence after successful cardioversion. Heart Rhythm 2013 Feb 18.pii: S1547-5271(13)00161–6. 10.1016/j.hrthm.2013.02.018. [Epub ahead of print]
- Mega JL, Close SL, Wiviott SD, et al. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet 2010; 376:1312–1319.
- Mega JL, Close SL, Wiviott SD, et al. Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation 2009; 119:2553–2560.
- Wallentin L, James S, Storey RF, et al; PLATO investigators. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet 2010; 376:1320–1328.
- Guzauskas GF, Hughes DA, Bradley SM, Veenstra DL. A risk-benefit assessment of prasugrel, clopidogrel, and genotype-guided therapy in patients undergoing percutaneous coronary intervention. Clin Pharmacol Ther 2012; 91:829–837.
- Crespin DJ, Federspiel JJ, Biddle AK, Jonas DE, Rossi JS. Ticagrelor versus genotype-driven antiplatelet therapy for secondary prevention after acute coronary syndrome: a cost-effectiveness analysis. Value Health 2011; 14:483–491.
- Mega JL, Hochholzer W, Frelinger AL, et al. Dosing clopidogrel based on CYP2C19 genotype and the effect on platelet reactivity in patients with stable cardiovascular disease. JAMA 2011; 306:2221–2228.
- Xu H, Jiang M, Oetjens M, et al. Facilitating pharmacogenetic studies using electronic health records and natural-language processing: a case study of warfarin. J Am Med Inform Assoc 2011; 18:387–391.
- Teng K, Eng C, Hess CA, et al. Building an innovative model for personalized healthcare. Cleve Clin J Med 2012; 79( suppl 1):S1–S9.
- Kaddurah-Daouk R, Baillie RA, Zhu H, et al. Enteric microbiome metabolites correlate with response to simvastatin treatment. PLoS One 2011; 6:e25482.
- Bodi V, Sanchis J, Morales JM, et al. Metabolomic profile of human myocardial ischemia by nuclear magnetic resonance spectroscopy of peripheral blood serum: a translational study based on transient coronary occlusion models. J Am Coll Cardiol 2012; 59:1629–1641.
- Shah SH, Sun JL, Stevens RD, et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am Heart J 2012; 163:844–850.e1.
Should we use pharmacogenetic testing when prescribing warfarin?
The answer is not clear. There is evidence in favor of pharmacogenetic testing, but not yet enough to strongly recommend it. However, we do believe that physicians should consider it when starting patients on warfarin therapy.
WARFARIN HAS A NARROW THERAPEUTIC WINDOW
Although newer drugs are available, warfarin is still the most commonly used oral anticoagulant for preventing and treating thromboembolism.1 It is highly effective but has a narrow therapeutic window and wide interindividual variability in dosage requirements, which poses challenges to achieving adequate anticoagulation.1–3 Inappropriate dosing contributes to a high rate of bleeding events and emergency room visits.4
Warfarin is monitored using the prothrombin time. Because the prothrombin time varies depending on the assay used, the standardized value called the international normalized ratio (INR) is more commonly used.
Clinical factors such as age, body size, and drug interactions affect warfarin dosage requirements and are important to consider,5 even though they account for only 15% to 20% of the variability in warfarin dose.6
Genetic factors also affect warfarin dosage requirements. The combination of genetic and clinical factors accounts for up to 47% of the dose variability.7
GENES THAT AFFECT WARFARIN
Several genes are known to influence warfarin’s pharmacokinetics and pharmacodynamics. Of these, the two most clinically relevant and well studied are CYP2C9 (which codes for cytochrome P450 2C9) and VKORC1 (which codes for vitamin K epoxide reductase).7 These genes are polymorphic, with some variants producing less-active enzymes that allow warfarin to be more active. Therefore, patients who carry these variants need lower doses of this drug (see below).
CYP2C9 variants
The CYP2C9 gene has several variants. Of these, CYP2C9*2 and CYP2C9*3 are associated with the lowest enzyme activity.
Patients with either of these variants require significantly lower warfarin doses to reach therapeutic levels than those with the wild-type gene (ie, CYP2C9*1). CYP2C9*2 reduces warfarin clearance by 40%, and the CYP2C9*3 variant reduces it by 75%.7 Having a *2 or *3 allele increases the risk of bleeding during warfarin therapy and the time needed to achieve a stable dose.8 Other variants associated with lower warfarin dose requirements are *5, *6, and *11.
The prevalence of these variants is significantly higher in people of European ancestry (roughly one-third) than in Asian people and African Americans,7 although no one has recommended not testing in these low-prevalence populations. Limdi et al9 reported that by including the *5, *6, and *11 variants in genetic testing (in addition to *2 and *3), they could identify more African Americans (9.7%) who carried at least one of these abnormal variants than reported previously. Differences among ethnic groups need to be taken into account when interpreting pharmacogenetic studies.
VKORC1 variants
Patients also need lower doses of warfarin if they carry the VKORC1 −1639G>A variant, and they spend more time with an INR above the therapeutic range and have higher overall INR values. However, having this variant does not appear to increase the risk of bleeding.
The −1639G>A variant is the most common variant of VKORC1. Rarer ones have also been described, but most commercially available tests do not detect them.
Racial differences exist in the prevalence rates of the various VKORC1 polymorphisms, with the most sensitive (low-dose) genotype predominating in Asians and the least sensitive (high-dose) genotype predominating in African Americans. Over 50% of people of European ancestry carry the intermediate-sensitivity genotype (typical dose).7
CURRENT RECOMMENDATIONS FOR OR AGAINST TESTING
FDA labeling
In 2007, the US Food and Drug Administration (FDA) required that the warfarin package insert carry information about initial dosing based on CYP2C9 and VKORC1 testing. This recommendation was revised in 2010 to include a table to help clinicians select an initial warfarin dose if CYP2C9 and VKORC1 genotype information is available. However, the FDA does not require pharmacogenetic testing, leaving the decision to the discretion of the clinician.7
American College of Chest Physicians
The American College of Chest Physicians recommends against routine pharmacogenetic testing (grade 1B) because of a lack of evidence that it improves clinical end points or that it is cost-effective.5
WHAT EVIDENCE SUPORTS GENETIC TESTING TO GUIDE WARFARIN THERAPY?
To date, no large randomized, controlled trial has been published that looked at clinical outcomes with warfarin dosing based on pharmacogenetic testing. However, several smaller studies have suggested it is beneficial.
One trial found that when dosing was informed by pharmacogenetic testing, patients had significantly more time in the therapeutic range, a lower percentage of INRs greater than 4 or less than 1.5, and fewer serious adverse events (death, myocardial infarction, stroke, thromboembolism, and clinically significant bleeding events).10 Patients whose dosage was determined using pharmacogenetic algorithms as opposed to traditional clinical algorithms maintained a therapeutic INR more consistently.11
In addition, compared with historical controls, patients whose physician used pharmacogenetic testing to guide warfarin dosing had a rate of hospitalization 31% lower and a rate of hospitalization specifically for bleeding or thromboembolism 28% lower during 6 months of follow-up.12,13
Several studies have attempted to assess the cost-effectiveness and utility of pharmacogenetic testing in warfarin therapy. As yet, the results have been inconclusive.14 Larger prospective trials are under way and are estimated to be completed in late 2013.15 These include:
- COAG (Clarification of Optimal Anticoagulation Through Genetics)
- GIFT (Genetics Informatics Trial of Warfarin to Prevent Venous Thrombosis)
- EU-PACT (European Pharmacogenetics of Anticoagulant Therapy-Warfarin).
We hope these studies will provide greater clarity on the clinical utility and cost-effectiveness of pharmacogenetic testing to guide warfarin dosing.
HOW SHOULD GENETIC INFORMATION BE USED TO GUIDE OR ALTER THERAPY?
Algorithms are available for estimating initial and maintenance warfarin doses based on genetic information (CYP2C9 and VKORC1), race or ethnicity, age, sex, body mass index, smoking status, and other medications taken. In addition, models incorporating the INR on day 4 and days 6 to 11 have been developed for dose refinement.15 The algorithms explain 30% to 60% of the variability of the data, with lower values for African Americans.7
A well-developed dosing model that includes traditional clinical factors and patient genetic status is publicly available online at www.warfarindosing.org.4
CPIC: A leader in applied pharmacogenetics
In late 2009, PharmGKB joined forces with the Pharmacogenomics Research Network of the National Institutes of Health to form the Clinical Pharmacogenetics Implementation Consortium (CPIC). This organization issues guidelines that are written by expert clinicians and scientists and then are peer-reviewed, published in leading journals, and simultaneously posted to the PharmGKB website along with supplemental information and updates.
CPIC’s goal is to review the current evidence and to address barriers to the adoption of pharmacogenetic testing into clinical practice. Its guidelines do not advise when or which pharmacogenetic tests should be ordered. Rather, they provide guidance on interpreting and applying such testing, should the test results be available.7
CPIC has guidelines on CYP2C9 and VKORC1 genotypes and warfarin dosing.8 If a patient’s genetic information is available, CPIC strongly recommends the use of pharmacogenetic algorithm-based dosing. If such an algorithm is not accessible, use of a genotype dosing table is recommended.8
Monitoring is still needed
Many factors can affect an individual’s response to warfarin above and beyond the above-noted clinical and genetic traits. These include diet, concomitant medications (both prescription and over-the-counter and herbal), and disease state. There may also be additional genetic polymorphisms not yet identified in various racial and ethnic groups that may affect dosing requirements. And as with all medications, patient compliance and dosing errors have a large potential to affect individual response. Therefore, clinicians should still be diligent about clinical monitoring.15
Most useful for initial dose
As with most pharmacogenetic information, the greatest benefit can be achieved when this information is used to guide the initial dose, although there is also some effect noted when this information is known and acted upon into the 2nd week of treatment.8
Patients on long-term warfarin treatment with stable doses and those unable to achieve stable dosing because of variable adherence or dietary vitamin K intake are less likely to benefit from genetic testing.
There are no published guidelines on the utility of pharmacogenetic testing if a patient is already on a stable dose of warfarin or has a known historical stable dose. There are also no published guidelines on changing the frequency of monitoring based on known genotype.
In children, the data are sparse at this time regarding the utility of pharmacogenetically informed dosing.
HOW DOES ONE ORDER TESTING, AND WHAT IS THE COST?
The FDA has approved four warfarin pharmacogenetic test kits. To be used in clinical decision-making, these tests must be done in a laboratory certified by the Clinical Laboratory Improvement Amendments (CLIA) program.
Testing typically costs a few hundred dollars and may take days for results to be returned if not available on site.15 At Cleveland Clinic, CYP2C9 and VKORC1 testing can be run in-house at a cost of about $700. Generally, many third-party payers do not reimburse for testing without a prior-approval process.
TO TEST OR NOT TO TEST
Pharmacogenetic testing is available and may help optimize warfarin dosing early in treatment, as well as help maintain therapeutic INRs more consistently. There is preliminary evidence that using this information to guide dosing improves clinical outcomes. Several large trials are under way to address additional questions of clinical utility, with results expected in the next year. There are also readily available decision-support tools to guide therapeutic dosing, and when pharmacogenetic test results are available, utilization of a warfarin dosing algorithm is recommended.
The largest barrier remaining appears to be cost (relative to perceived benefit), and until larger trials of clinical utility and cost-effectiveness are completed and analyzed, hurdles exist to obtaining coverage for such testing.
If it is readily available (and can be paid for by insurance companies or out-of-pocket) and test results can be obtained within 24 to 48 hours or before prescribing, pharmacogenetic testing can be a valuable tool to guide and manage warfarin dosing. Particularly for patients who want to be as proactive as possible, warfarin pharmacogenetic testing offers the ability to participate in this decision-making and to potentially reduce their risk of adverse drug events. And in view of the evidence and FDA recommendations, we propose that the discussion with our patients is not whether we should consider pharmacogenetic testing, but that we have considered pharmacogenetic testing, and why we have decided for or against it.
- Jacobs LG. Warfarin pharmacology, clinical management, and evaluation of hemorrhagic risk for the elderly. Clin Geriatr Med 2006; 22:17–32,vii–viii.
- Rieder MJ, Reiner AP, Gage BF, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med 2005; 352:2285–2293.
- Higashi MK, Veenstra DL, Kondo LM, et al. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA 2002; 287:1690–1698.
- Shehab N, Sperling LS, Kegler SR, Budnitz DS. National estimates of emergency department visits for hemorrhage-related adverse events from clopidogrel plus aspirin and from warfarin. Arch Intern Med 2010; 170:1926–1933.
- Holbrook A, Schulman S, Witt DM, et al; American College of Chest Physicians. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e152S–e184S.
- Gage BF, Eby C, Johnson JA, et al. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin Pharmacol Ther 2008; 84:326–331.
- Cavallari LH, Shin J, Perera MA. Role of pharmacogenomics in the management of traditional and novel oral anticoagulants. Pharmacotherapy 2011; 31:1192–1207.
- Johnson JA, Gong L, Whirl-Carillo M, et al; Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin Pharmacol Ther 2011; 90:625–629.
- Limdi NA, McGwin G, Goldstein JA, et al. Influence of CYP2C9 and VKORC1 1173C/T genotype on the risk of hemorrhagic complications in African-American and European-American patients on warfarin. Clin Pharmacol Ther 2008; 83:312–321.
- Anderson JL, Horne BD, Stevens SM, et al. A randomized and clinical effectiveness trial comparing two pharmacogenetic algorithms and standard care for individualizing warfarin dosing (CoumaGen-II). Circulation 2012; 125:1997–2005.
- Yip VL, Pirmohamed M. Expanding role of pharmacogenomics in the management of cardiovascular disorders. Am J Cardiovasc Drugs 2013; 12 Apr; Epub ahead of print.
- Epstein RS, Moyer TP, Aubert RE, et al. Warfarin genotyping reduces hospitalization rates: results from the MM-WES (Medco-Mayo Warfarin Effectiveness Study). J Am Coll Cardiol 2010; 55:2804–2812.
- Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. N Engl J Med 2011; 364:1144–1153.
- Kitzmiller JP, Groen DK, Phelps MA, Sadee W. Pharmacogenomic testing: relevance in medical practice: why drugs work in some patients but not in others. Cleve Clin J Med 2011; 78:243–257.
- Carlquist JF, Anderson JL. Using pharmacogenetics in real time to guide warfarin initiation: a clinician update. Circulation 2011; 124:2554–2559.
The answer is not clear. There is evidence in favor of pharmacogenetic testing, but not yet enough to strongly recommend it. However, we do believe that physicians should consider it when starting patients on warfarin therapy.
WARFARIN HAS A NARROW THERAPEUTIC WINDOW
Although newer drugs are available, warfarin is still the most commonly used oral anticoagulant for preventing and treating thromboembolism.1 It is highly effective but has a narrow therapeutic window and wide interindividual variability in dosage requirements, which poses challenges to achieving adequate anticoagulation.1–3 Inappropriate dosing contributes to a high rate of bleeding events and emergency room visits.4
Warfarin is monitored using the prothrombin time. Because the prothrombin time varies depending on the assay used, the standardized value called the international normalized ratio (INR) is more commonly used.
Clinical factors such as age, body size, and drug interactions affect warfarin dosage requirements and are important to consider,5 even though they account for only 15% to 20% of the variability in warfarin dose.6
Genetic factors also affect warfarin dosage requirements. The combination of genetic and clinical factors accounts for up to 47% of the dose variability.7
GENES THAT AFFECT WARFARIN
Several genes are known to influence warfarin’s pharmacokinetics and pharmacodynamics. Of these, the two most clinically relevant and well studied are CYP2C9 (which codes for cytochrome P450 2C9) and VKORC1 (which codes for vitamin K epoxide reductase).7 These genes are polymorphic, with some variants producing less-active enzymes that allow warfarin to be more active. Therefore, patients who carry these variants need lower doses of this drug (see below).
CYP2C9 variants
The CYP2C9 gene has several variants. Of these, CYP2C9*2 and CYP2C9*3 are associated with the lowest enzyme activity.
Patients with either of these variants require significantly lower warfarin doses to reach therapeutic levels than those with the wild-type gene (ie, CYP2C9*1). CYP2C9*2 reduces warfarin clearance by 40%, and the CYP2C9*3 variant reduces it by 75%.7 Having a *2 or *3 allele increases the risk of bleeding during warfarin therapy and the time needed to achieve a stable dose.8 Other variants associated with lower warfarin dose requirements are *5, *6, and *11.
The prevalence of these variants is significantly higher in people of European ancestry (roughly one-third) than in Asian people and African Americans,7 although no one has recommended not testing in these low-prevalence populations. Limdi et al9 reported that by including the *5, *6, and *11 variants in genetic testing (in addition to *2 and *3), they could identify more African Americans (9.7%) who carried at least one of these abnormal variants than reported previously. Differences among ethnic groups need to be taken into account when interpreting pharmacogenetic studies.
VKORC1 variants
Patients also need lower doses of warfarin if they carry the VKORC1 −1639G>A variant, and they spend more time with an INR above the therapeutic range and have higher overall INR values. However, having this variant does not appear to increase the risk of bleeding.
The −1639G>A variant is the most common variant of VKORC1. Rarer ones have also been described, but most commercially available tests do not detect them.
Racial differences exist in the prevalence rates of the various VKORC1 polymorphisms, with the most sensitive (low-dose) genotype predominating in Asians and the least sensitive (high-dose) genotype predominating in African Americans. Over 50% of people of European ancestry carry the intermediate-sensitivity genotype (typical dose).7
CURRENT RECOMMENDATIONS FOR OR AGAINST TESTING
FDA labeling
In 2007, the US Food and Drug Administration (FDA) required that the warfarin package insert carry information about initial dosing based on CYP2C9 and VKORC1 testing. This recommendation was revised in 2010 to include a table to help clinicians select an initial warfarin dose if CYP2C9 and VKORC1 genotype information is available. However, the FDA does not require pharmacogenetic testing, leaving the decision to the discretion of the clinician.7
American College of Chest Physicians
The American College of Chest Physicians recommends against routine pharmacogenetic testing (grade 1B) because of a lack of evidence that it improves clinical end points or that it is cost-effective.5
WHAT EVIDENCE SUPORTS GENETIC TESTING TO GUIDE WARFARIN THERAPY?
To date, no large randomized, controlled trial has been published that looked at clinical outcomes with warfarin dosing based on pharmacogenetic testing. However, several smaller studies have suggested it is beneficial.
One trial found that when dosing was informed by pharmacogenetic testing, patients had significantly more time in the therapeutic range, a lower percentage of INRs greater than 4 or less than 1.5, and fewer serious adverse events (death, myocardial infarction, stroke, thromboembolism, and clinically significant bleeding events).10 Patients whose dosage was determined using pharmacogenetic algorithms as opposed to traditional clinical algorithms maintained a therapeutic INR more consistently.11
In addition, compared with historical controls, patients whose physician used pharmacogenetic testing to guide warfarin dosing had a rate of hospitalization 31% lower and a rate of hospitalization specifically for bleeding or thromboembolism 28% lower during 6 months of follow-up.12,13
Several studies have attempted to assess the cost-effectiveness and utility of pharmacogenetic testing in warfarin therapy. As yet, the results have been inconclusive.14 Larger prospective trials are under way and are estimated to be completed in late 2013.15 These include:
- COAG (Clarification of Optimal Anticoagulation Through Genetics)
- GIFT (Genetics Informatics Trial of Warfarin to Prevent Venous Thrombosis)
- EU-PACT (European Pharmacogenetics of Anticoagulant Therapy-Warfarin).
We hope these studies will provide greater clarity on the clinical utility and cost-effectiveness of pharmacogenetic testing to guide warfarin dosing.
HOW SHOULD GENETIC INFORMATION BE USED TO GUIDE OR ALTER THERAPY?
Algorithms are available for estimating initial and maintenance warfarin doses based on genetic information (CYP2C9 and VKORC1), race or ethnicity, age, sex, body mass index, smoking status, and other medications taken. In addition, models incorporating the INR on day 4 and days 6 to 11 have been developed for dose refinement.15 The algorithms explain 30% to 60% of the variability of the data, with lower values for African Americans.7
A well-developed dosing model that includes traditional clinical factors and patient genetic status is publicly available online at www.warfarindosing.org.4
CPIC: A leader in applied pharmacogenetics
In late 2009, PharmGKB joined forces with the Pharmacogenomics Research Network of the National Institutes of Health to form the Clinical Pharmacogenetics Implementation Consortium (CPIC). This organization issues guidelines that are written by expert clinicians and scientists and then are peer-reviewed, published in leading journals, and simultaneously posted to the PharmGKB website along with supplemental information and updates.
CPIC’s goal is to review the current evidence and to address barriers to the adoption of pharmacogenetic testing into clinical practice. Its guidelines do not advise when or which pharmacogenetic tests should be ordered. Rather, they provide guidance on interpreting and applying such testing, should the test results be available.7
CPIC has guidelines on CYP2C9 and VKORC1 genotypes and warfarin dosing.8 If a patient’s genetic information is available, CPIC strongly recommends the use of pharmacogenetic algorithm-based dosing. If such an algorithm is not accessible, use of a genotype dosing table is recommended.8
Monitoring is still needed
Many factors can affect an individual’s response to warfarin above and beyond the above-noted clinical and genetic traits. These include diet, concomitant medications (both prescription and over-the-counter and herbal), and disease state. There may also be additional genetic polymorphisms not yet identified in various racial and ethnic groups that may affect dosing requirements. And as with all medications, patient compliance and dosing errors have a large potential to affect individual response. Therefore, clinicians should still be diligent about clinical monitoring.15
Most useful for initial dose
As with most pharmacogenetic information, the greatest benefit can be achieved when this information is used to guide the initial dose, although there is also some effect noted when this information is known and acted upon into the 2nd week of treatment.8
Patients on long-term warfarin treatment with stable doses and those unable to achieve stable dosing because of variable adherence or dietary vitamin K intake are less likely to benefit from genetic testing.
There are no published guidelines on the utility of pharmacogenetic testing if a patient is already on a stable dose of warfarin or has a known historical stable dose. There are also no published guidelines on changing the frequency of monitoring based on known genotype.
In children, the data are sparse at this time regarding the utility of pharmacogenetically informed dosing.
HOW DOES ONE ORDER TESTING, AND WHAT IS THE COST?
The FDA has approved four warfarin pharmacogenetic test kits. To be used in clinical decision-making, these tests must be done in a laboratory certified by the Clinical Laboratory Improvement Amendments (CLIA) program.
Testing typically costs a few hundred dollars and may take days for results to be returned if not available on site.15 At Cleveland Clinic, CYP2C9 and VKORC1 testing can be run in-house at a cost of about $700. Generally, many third-party payers do not reimburse for testing without a prior-approval process.
TO TEST OR NOT TO TEST
Pharmacogenetic testing is available and may help optimize warfarin dosing early in treatment, as well as help maintain therapeutic INRs more consistently. There is preliminary evidence that using this information to guide dosing improves clinical outcomes. Several large trials are under way to address additional questions of clinical utility, with results expected in the next year. There are also readily available decision-support tools to guide therapeutic dosing, and when pharmacogenetic test results are available, utilization of a warfarin dosing algorithm is recommended.
The largest barrier remaining appears to be cost (relative to perceived benefit), and until larger trials of clinical utility and cost-effectiveness are completed and analyzed, hurdles exist to obtaining coverage for such testing.
If it is readily available (and can be paid for by insurance companies or out-of-pocket) and test results can be obtained within 24 to 48 hours or before prescribing, pharmacogenetic testing can be a valuable tool to guide and manage warfarin dosing. Particularly for patients who want to be as proactive as possible, warfarin pharmacogenetic testing offers the ability to participate in this decision-making and to potentially reduce their risk of adverse drug events. And in view of the evidence and FDA recommendations, we propose that the discussion with our patients is not whether we should consider pharmacogenetic testing, but that we have considered pharmacogenetic testing, and why we have decided for or against it.
The answer is not clear. There is evidence in favor of pharmacogenetic testing, but not yet enough to strongly recommend it. However, we do believe that physicians should consider it when starting patients on warfarin therapy.
WARFARIN HAS A NARROW THERAPEUTIC WINDOW
Although newer drugs are available, warfarin is still the most commonly used oral anticoagulant for preventing and treating thromboembolism.1 It is highly effective but has a narrow therapeutic window and wide interindividual variability in dosage requirements, which poses challenges to achieving adequate anticoagulation.1–3 Inappropriate dosing contributes to a high rate of bleeding events and emergency room visits.4
Warfarin is monitored using the prothrombin time. Because the prothrombin time varies depending on the assay used, the standardized value called the international normalized ratio (INR) is more commonly used.
Clinical factors such as age, body size, and drug interactions affect warfarin dosage requirements and are important to consider,5 even though they account for only 15% to 20% of the variability in warfarin dose.6
Genetic factors also affect warfarin dosage requirements. The combination of genetic and clinical factors accounts for up to 47% of the dose variability.7
GENES THAT AFFECT WARFARIN
Several genes are known to influence warfarin’s pharmacokinetics and pharmacodynamics. Of these, the two most clinically relevant and well studied are CYP2C9 (which codes for cytochrome P450 2C9) and VKORC1 (which codes for vitamin K epoxide reductase).7 These genes are polymorphic, with some variants producing less-active enzymes that allow warfarin to be more active. Therefore, patients who carry these variants need lower doses of this drug (see below).
CYP2C9 variants
The CYP2C9 gene has several variants. Of these, CYP2C9*2 and CYP2C9*3 are associated with the lowest enzyme activity.
Patients with either of these variants require significantly lower warfarin doses to reach therapeutic levels than those with the wild-type gene (ie, CYP2C9*1). CYP2C9*2 reduces warfarin clearance by 40%, and the CYP2C9*3 variant reduces it by 75%.7 Having a *2 or *3 allele increases the risk of bleeding during warfarin therapy and the time needed to achieve a stable dose.8 Other variants associated with lower warfarin dose requirements are *5, *6, and *11.
The prevalence of these variants is significantly higher in people of European ancestry (roughly one-third) than in Asian people and African Americans,7 although no one has recommended not testing in these low-prevalence populations. Limdi et al9 reported that by including the *5, *6, and *11 variants in genetic testing (in addition to *2 and *3), they could identify more African Americans (9.7%) who carried at least one of these abnormal variants than reported previously. Differences among ethnic groups need to be taken into account when interpreting pharmacogenetic studies.
VKORC1 variants
Patients also need lower doses of warfarin if they carry the VKORC1 −1639G>A variant, and they spend more time with an INR above the therapeutic range and have higher overall INR values. However, having this variant does not appear to increase the risk of bleeding.
The −1639G>A variant is the most common variant of VKORC1. Rarer ones have also been described, but most commercially available tests do not detect them.
Racial differences exist in the prevalence rates of the various VKORC1 polymorphisms, with the most sensitive (low-dose) genotype predominating in Asians and the least sensitive (high-dose) genotype predominating in African Americans. Over 50% of people of European ancestry carry the intermediate-sensitivity genotype (typical dose).7
CURRENT RECOMMENDATIONS FOR OR AGAINST TESTING
FDA labeling
In 2007, the US Food and Drug Administration (FDA) required that the warfarin package insert carry information about initial dosing based on CYP2C9 and VKORC1 testing. This recommendation was revised in 2010 to include a table to help clinicians select an initial warfarin dose if CYP2C9 and VKORC1 genotype information is available. However, the FDA does not require pharmacogenetic testing, leaving the decision to the discretion of the clinician.7
American College of Chest Physicians
The American College of Chest Physicians recommends against routine pharmacogenetic testing (grade 1B) because of a lack of evidence that it improves clinical end points or that it is cost-effective.5
WHAT EVIDENCE SUPORTS GENETIC TESTING TO GUIDE WARFARIN THERAPY?
To date, no large randomized, controlled trial has been published that looked at clinical outcomes with warfarin dosing based on pharmacogenetic testing. However, several smaller studies have suggested it is beneficial.
One trial found that when dosing was informed by pharmacogenetic testing, patients had significantly more time in the therapeutic range, a lower percentage of INRs greater than 4 or less than 1.5, and fewer serious adverse events (death, myocardial infarction, stroke, thromboembolism, and clinically significant bleeding events).10 Patients whose dosage was determined using pharmacogenetic algorithms as opposed to traditional clinical algorithms maintained a therapeutic INR more consistently.11
In addition, compared with historical controls, patients whose physician used pharmacogenetic testing to guide warfarin dosing had a rate of hospitalization 31% lower and a rate of hospitalization specifically for bleeding or thromboembolism 28% lower during 6 months of follow-up.12,13
Several studies have attempted to assess the cost-effectiveness and utility of pharmacogenetic testing in warfarin therapy. As yet, the results have been inconclusive.14 Larger prospective trials are under way and are estimated to be completed in late 2013.15 These include:
- COAG (Clarification of Optimal Anticoagulation Through Genetics)
- GIFT (Genetics Informatics Trial of Warfarin to Prevent Venous Thrombosis)
- EU-PACT (European Pharmacogenetics of Anticoagulant Therapy-Warfarin).
We hope these studies will provide greater clarity on the clinical utility and cost-effectiveness of pharmacogenetic testing to guide warfarin dosing.
HOW SHOULD GENETIC INFORMATION BE USED TO GUIDE OR ALTER THERAPY?
Algorithms are available for estimating initial and maintenance warfarin doses based on genetic information (CYP2C9 and VKORC1), race or ethnicity, age, sex, body mass index, smoking status, and other medications taken. In addition, models incorporating the INR on day 4 and days 6 to 11 have been developed for dose refinement.15 The algorithms explain 30% to 60% of the variability of the data, with lower values for African Americans.7
A well-developed dosing model that includes traditional clinical factors and patient genetic status is publicly available online at www.warfarindosing.org.4
CPIC: A leader in applied pharmacogenetics
In late 2009, PharmGKB joined forces with the Pharmacogenomics Research Network of the National Institutes of Health to form the Clinical Pharmacogenetics Implementation Consortium (CPIC). This organization issues guidelines that are written by expert clinicians and scientists and then are peer-reviewed, published in leading journals, and simultaneously posted to the PharmGKB website along with supplemental information and updates.
CPIC’s goal is to review the current evidence and to address barriers to the adoption of pharmacogenetic testing into clinical practice. Its guidelines do not advise when or which pharmacogenetic tests should be ordered. Rather, they provide guidance on interpreting and applying such testing, should the test results be available.7
CPIC has guidelines on CYP2C9 and VKORC1 genotypes and warfarin dosing.8 If a patient’s genetic information is available, CPIC strongly recommends the use of pharmacogenetic algorithm-based dosing. If such an algorithm is not accessible, use of a genotype dosing table is recommended.8
Monitoring is still needed
Many factors can affect an individual’s response to warfarin above and beyond the above-noted clinical and genetic traits. These include diet, concomitant medications (both prescription and over-the-counter and herbal), and disease state. There may also be additional genetic polymorphisms not yet identified in various racial and ethnic groups that may affect dosing requirements. And as with all medications, patient compliance and dosing errors have a large potential to affect individual response. Therefore, clinicians should still be diligent about clinical monitoring.15
Most useful for initial dose
As with most pharmacogenetic information, the greatest benefit can be achieved when this information is used to guide the initial dose, although there is also some effect noted when this information is known and acted upon into the 2nd week of treatment.8
Patients on long-term warfarin treatment with stable doses and those unable to achieve stable dosing because of variable adherence or dietary vitamin K intake are less likely to benefit from genetic testing.
There are no published guidelines on the utility of pharmacogenetic testing if a patient is already on a stable dose of warfarin or has a known historical stable dose. There are also no published guidelines on changing the frequency of monitoring based on known genotype.
In children, the data are sparse at this time regarding the utility of pharmacogenetically informed dosing.
HOW DOES ONE ORDER TESTING, AND WHAT IS THE COST?
The FDA has approved four warfarin pharmacogenetic test kits. To be used in clinical decision-making, these tests must be done in a laboratory certified by the Clinical Laboratory Improvement Amendments (CLIA) program.
Testing typically costs a few hundred dollars and may take days for results to be returned if not available on site.15 At Cleveland Clinic, CYP2C9 and VKORC1 testing can be run in-house at a cost of about $700. Generally, many third-party payers do not reimburse for testing without a prior-approval process.
TO TEST OR NOT TO TEST
Pharmacogenetic testing is available and may help optimize warfarin dosing early in treatment, as well as help maintain therapeutic INRs more consistently. There is preliminary evidence that using this information to guide dosing improves clinical outcomes. Several large trials are under way to address additional questions of clinical utility, with results expected in the next year. There are also readily available decision-support tools to guide therapeutic dosing, and when pharmacogenetic test results are available, utilization of a warfarin dosing algorithm is recommended.
The largest barrier remaining appears to be cost (relative to perceived benefit), and until larger trials of clinical utility and cost-effectiveness are completed and analyzed, hurdles exist to obtaining coverage for such testing.
If it is readily available (and can be paid for by insurance companies or out-of-pocket) and test results can be obtained within 24 to 48 hours or before prescribing, pharmacogenetic testing can be a valuable tool to guide and manage warfarin dosing. Particularly for patients who want to be as proactive as possible, warfarin pharmacogenetic testing offers the ability to participate in this decision-making and to potentially reduce their risk of adverse drug events. And in view of the evidence and FDA recommendations, we propose that the discussion with our patients is not whether we should consider pharmacogenetic testing, but that we have considered pharmacogenetic testing, and why we have decided for or against it.
- Jacobs LG. Warfarin pharmacology, clinical management, and evaluation of hemorrhagic risk for the elderly. Clin Geriatr Med 2006; 22:17–32,vii–viii.
- Rieder MJ, Reiner AP, Gage BF, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med 2005; 352:2285–2293.
- Higashi MK, Veenstra DL, Kondo LM, et al. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA 2002; 287:1690–1698.
- Shehab N, Sperling LS, Kegler SR, Budnitz DS. National estimates of emergency department visits for hemorrhage-related adverse events from clopidogrel plus aspirin and from warfarin. Arch Intern Med 2010; 170:1926–1933.
- Holbrook A, Schulman S, Witt DM, et al; American College of Chest Physicians. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e152S–e184S.
- Gage BF, Eby C, Johnson JA, et al. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin Pharmacol Ther 2008; 84:326–331.
- Cavallari LH, Shin J, Perera MA. Role of pharmacogenomics in the management of traditional and novel oral anticoagulants. Pharmacotherapy 2011; 31:1192–1207.
- Johnson JA, Gong L, Whirl-Carillo M, et al; Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin Pharmacol Ther 2011; 90:625–629.
- Limdi NA, McGwin G, Goldstein JA, et al. Influence of CYP2C9 and VKORC1 1173C/T genotype on the risk of hemorrhagic complications in African-American and European-American patients on warfarin. Clin Pharmacol Ther 2008; 83:312–321.
- Anderson JL, Horne BD, Stevens SM, et al. A randomized and clinical effectiveness trial comparing two pharmacogenetic algorithms and standard care for individualizing warfarin dosing (CoumaGen-II). Circulation 2012; 125:1997–2005.
- Yip VL, Pirmohamed M. Expanding role of pharmacogenomics in the management of cardiovascular disorders. Am J Cardiovasc Drugs 2013; 12 Apr; Epub ahead of print.
- Epstein RS, Moyer TP, Aubert RE, et al. Warfarin genotyping reduces hospitalization rates: results from the MM-WES (Medco-Mayo Warfarin Effectiveness Study). J Am Coll Cardiol 2010; 55:2804–2812.
- Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. N Engl J Med 2011; 364:1144–1153.
- Kitzmiller JP, Groen DK, Phelps MA, Sadee W. Pharmacogenomic testing: relevance in medical practice: why drugs work in some patients but not in others. Cleve Clin J Med 2011; 78:243–257.
- Carlquist JF, Anderson JL. Using pharmacogenetics in real time to guide warfarin initiation: a clinician update. Circulation 2011; 124:2554–2559.
- Jacobs LG. Warfarin pharmacology, clinical management, and evaluation of hemorrhagic risk for the elderly. Clin Geriatr Med 2006; 22:17–32,vii–viii.
- Rieder MJ, Reiner AP, Gage BF, et al. Effect of VKORC1 haplotypes on transcriptional regulation and warfarin dose. N Engl J Med 2005; 352:2285–2293.
- Higashi MK, Veenstra DL, Kondo LM, et al. Association between CYP2C9 genetic variants and anticoagulation-related outcomes during warfarin therapy. JAMA 2002; 287:1690–1698.
- Shehab N, Sperling LS, Kegler SR, Budnitz DS. National estimates of emergency department visits for hemorrhage-related adverse events from clopidogrel plus aspirin and from warfarin. Arch Intern Med 2010; 170:1926–1933.
- Holbrook A, Schulman S, Witt DM, et al; American College of Chest Physicians. Evidence-based management of anticoagulant therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 2012; 141(suppl 2):e152S–e184S.
- Gage BF, Eby C, Johnson JA, et al. Use of pharmacogenetic and clinical factors to predict the therapeutic dose of warfarin. Clin Pharmacol Ther 2008; 84:326–331.
- Cavallari LH, Shin J, Perera MA. Role of pharmacogenomics in the management of traditional and novel oral anticoagulants. Pharmacotherapy 2011; 31:1192–1207.
- Johnson JA, Gong L, Whirl-Carillo M, et al; Clinical Pharmacogenetics Implementation Consortium. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin Pharmacol Ther 2011; 90:625–629.
- Limdi NA, McGwin G, Goldstein JA, et al. Influence of CYP2C9 and VKORC1 1173C/T genotype on the risk of hemorrhagic complications in African-American and European-American patients on warfarin. Clin Pharmacol Ther 2008; 83:312–321.
- Anderson JL, Horne BD, Stevens SM, et al. A randomized and clinical effectiveness trial comparing two pharmacogenetic algorithms and standard care for individualizing warfarin dosing (CoumaGen-II). Circulation 2012; 125:1997–2005.
- Yip VL, Pirmohamed M. Expanding role of pharmacogenomics in the management of cardiovascular disorders. Am J Cardiovasc Drugs 2013; 12 Apr; Epub ahead of print.
- Epstein RS, Moyer TP, Aubert RE, et al. Warfarin genotyping reduces hospitalization rates: results from the MM-WES (Medco-Mayo Warfarin Effectiveness Study). J Am Coll Cardiol 2010; 55:2804–2812.
- Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. N Engl J Med 2011; 364:1144–1153.
- Kitzmiller JP, Groen DK, Phelps MA, Sadee W. Pharmacogenomic testing: relevance in medical practice: why drugs work in some patients but not in others. Cleve Clin J Med 2011; 78:243–257.
- Carlquist JF, Anderson JL. Using pharmacogenetics in real time to guide warfarin initiation: a clinician update. Circulation 2011; 124:2554–2559.
The pipe and the plug: Is unblocking arteries enough?
It seems anachronistic that we still debate how best to fix the plumbing of clogged arteries. Our understanding of the pathogenesis of acute coronary syndromes has evolved in leaps and bounds since the first attempts at coronary revascularization. And yet, as Aggarwal et al discuss in their analysis of the FREEDOM trial,1 practical and technical questions about how best to open coronary blockages remain clinically relevant, even as we develop strategies to reverse the atherosclerotic processes that created those blockages.
Many acute coronary events arise not from coronary stenoses but from unstable, vulnerable plaques, which may be a distance away from the stable stenoses and thus undetectable. These unstable plaques, embedded within the remodeled arterial wall and without a protective fibrous cap, may rupture and cause an acute thrombotic occlusion. Statins, aspirin, and perhaps anti-inflammatory drugs (now including colchicine) decrease acute coronary events, likely by interfering with the chain of events initiated by plaque rupture.
So why should coronary artery bypass grafting (CABG) be superior to drug-eluting stents (with antiplatelet therapy) in some diabetic patients, as the FREEDOM trial1 found?
Stenting and balloon dilation repair discrete areas of critical narrowing presumed to be contributing to downstream myocardial ischemia. But areas of vulnerable, non-calcified plaque (with outward remodeling of the vessel wall but generally preserved lumen integrity) may be geographically separated from the identified stenosis and thus be left untreated by stenting. On the other hand, CABG may circumvent “silent” areas of nascent vulnerable plaque that, if left in place, might later rupture and cause acute syndromes or death.
This explanation is clearly hypothetical and one of many possibilities. But paying attention to the new biology of the atherosclerotic process should lead us all to be more aggressive in using treatments shown to reduce the progression of coronary artery disease and the occurrence of acute coronary syndromes. This is especially true in patients with diabetes who are known to have diffuse coronary involvement. So even as we more fully recognize the value of CABG in these patients, perhaps if we intervene earlier—with statins, hypertension control, improved diet, smoking cessation, prevention of chronic kidney disease, antiplatelet therapy, and anti-inflammatory therapy—we will not need it.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
It seems anachronistic that we still debate how best to fix the plumbing of clogged arteries. Our understanding of the pathogenesis of acute coronary syndromes has evolved in leaps and bounds since the first attempts at coronary revascularization. And yet, as Aggarwal et al discuss in their analysis of the FREEDOM trial,1 practical and technical questions about how best to open coronary blockages remain clinically relevant, even as we develop strategies to reverse the atherosclerotic processes that created those blockages.
Many acute coronary events arise not from coronary stenoses but from unstable, vulnerable plaques, which may be a distance away from the stable stenoses and thus undetectable. These unstable plaques, embedded within the remodeled arterial wall and without a protective fibrous cap, may rupture and cause an acute thrombotic occlusion. Statins, aspirin, and perhaps anti-inflammatory drugs (now including colchicine) decrease acute coronary events, likely by interfering with the chain of events initiated by plaque rupture.
So why should coronary artery bypass grafting (CABG) be superior to drug-eluting stents (with antiplatelet therapy) in some diabetic patients, as the FREEDOM trial1 found?
Stenting and balloon dilation repair discrete areas of critical narrowing presumed to be contributing to downstream myocardial ischemia. But areas of vulnerable, non-calcified plaque (with outward remodeling of the vessel wall but generally preserved lumen integrity) may be geographically separated from the identified stenosis and thus be left untreated by stenting. On the other hand, CABG may circumvent “silent” areas of nascent vulnerable plaque that, if left in place, might later rupture and cause acute syndromes or death.
This explanation is clearly hypothetical and one of many possibilities. But paying attention to the new biology of the atherosclerotic process should lead us all to be more aggressive in using treatments shown to reduce the progression of coronary artery disease and the occurrence of acute coronary syndromes. This is especially true in patients with diabetes who are known to have diffuse coronary involvement. So even as we more fully recognize the value of CABG in these patients, perhaps if we intervene earlier—with statins, hypertension control, improved diet, smoking cessation, prevention of chronic kidney disease, antiplatelet therapy, and anti-inflammatory therapy—we will not need it.
It seems anachronistic that we still debate how best to fix the plumbing of clogged arteries. Our understanding of the pathogenesis of acute coronary syndromes has evolved in leaps and bounds since the first attempts at coronary revascularization. And yet, as Aggarwal et al discuss in their analysis of the FREEDOM trial,1 practical and technical questions about how best to open coronary blockages remain clinically relevant, even as we develop strategies to reverse the atherosclerotic processes that created those blockages.
Many acute coronary events arise not from coronary stenoses but from unstable, vulnerable plaques, which may be a distance away from the stable stenoses and thus undetectable. These unstable plaques, embedded within the remodeled arterial wall and without a protective fibrous cap, may rupture and cause an acute thrombotic occlusion. Statins, aspirin, and perhaps anti-inflammatory drugs (now including colchicine) decrease acute coronary events, likely by interfering with the chain of events initiated by plaque rupture.
So why should coronary artery bypass grafting (CABG) be superior to drug-eluting stents (with antiplatelet therapy) in some diabetic patients, as the FREEDOM trial1 found?
Stenting and balloon dilation repair discrete areas of critical narrowing presumed to be contributing to downstream myocardial ischemia. But areas of vulnerable, non-calcified plaque (with outward remodeling of the vessel wall but generally preserved lumen integrity) may be geographically separated from the identified stenosis and thus be left untreated by stenting. On the other hand, CABG may circumvent “silent” areas of nascent vulnerable plaque that, if left in place, might later rupture and cause acute syndromes or death.
This explanation is clearly hypothetical and one of many possibilities. But paying attention to the new biology of the atherosclerotic process should lead us all to be more aggressive in using treatments shown to reduce the progression of coronary artery disease and the occurrence of acute coronary syndromes. This is especially true in patients with diabetes who are known to have diffuse coronary involvement. So even as we more fully recognize the value of CABG in these patients, perhaps if we intervene earlier—with statins, hypertension control, improved diet, smoking cessation, prevention of chronic kidney disease, antiplatelet therapy, and anti-inflammatory therapy—we will not need it.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
The FREEDOM trial: In appropriate patients with diabetes and multivessel coronary artery disease, CABG beats PCI
Many patients with diabetes mellitus develop complex, accelerated, multifocal coronary artery disease. Moreover, if they undergo revascularization with either coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI), their risk of morbidity and death afterward is higher than in those without diabetes.1,2
Over the last 2 decades, CABG and PCI have advanced significantly, as have antithrombotic therapy and drug therapies to modify cardiovascular risk factors such as hyperlipidemia, hypertension, and diabetes.
Several earlier studies showed CABG to be more beneficial than PCI in diabetic patients with multivessel coronary artery disease.3–5 However, the topic has been controversial, and a substantial proportion of these patients continue to undergo PCI rather than CABG.
There are two main reasons for the continued use of PCI in this population. First, PCI is evolving, with new adjuvant drugs and drugeluting stents. Many cardiologists believe that earlier trials, which did not use contemporary PCI techniques, are outdated and that current, state-of-the-art PCI may be equivalent to—if not superior to—CABG.
Second, PCI is often performed on an ad hoc basis immediately after diagnostic angiography, leaving little time for discussion with the patient about alternative treatments. In this scenario, patients are inclined to undergo PCI immediately, while they are already on the table in the catheterization suite, rather than CABG at a later date.6
In addition, although the current joint guide-lines of the American College of Cardiology and the American Heart Association state that CABG is preferable to PCI for patients with diabetes and multivessel coronary artery disease, they give it only a level IIa recommendation.7
The much-anticipated Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial8 was designed to settle the CABG-vs-PCI debate, thereby leading to a stronger guideline recommendation for the preferred revascularization strategy in this patient population.
WHY ARE DIABETIC PATIENTS DIFFERENT?
Diabetes mellitus is a major risk factor for premature and aggressive coronary artery disease. Several mechanisms have been proposed to explain this association.
Diabetic patients have higher concentrations of several inflammatory proteins than those without diabetes, including C-reactive protein, tumor necrosis factor, and platelet-derived soluble CD40 ligand. They also have higher levels of adhesion molecules such as vascular cell adhesion molecule-1 and intercellular adhesion molecule.9,10 In addition, when blood sugar levels are high, platelets express more glycoprotein IIb/IIIa receptors and are therefore more prone to aggregate.11
These prothrombotic and proinflammatory cytokines, in conjunction with endothelial dysfunction and metabolic disorders such as hyperglycemia, hyperlipidemia, obesity, insulin resistance, and oxidative stress, lead to accelerated atherosclerosis in patients with diabetes.12 Also, because diabetes is a systemic disease, the atherosclerotic process is diffuse, and many patients with diabetes have left main coronary artery lesions and diffuse multivessel coronary artery disease.13,14
Although the short-term outcomes of revascularization by any means are comparable in patients with and without diabetes, diabetic patients have lower long-term survival rates and higher rates of myocardial infarction and need for repeat procedures.15 Diabetic patients who undergo PCI have a high rate of stent thrombosis and restenosis.16,17 Similarly, those undergoing CABG have higher rates of postoperative infection and renal and neurologic complications.18,19
BEFORE THE FREEDOM TRIAL
The question of CABG vs PCI has plagued physicians ever since PCI came to the forefront in the 1980s. Before stents were widely used, PCI with balloon angioplasty was known to be comparable to CABG for single-vessel disease, but whether it was beneficial in patients with multivessel disease or left main disease was not entirely evident. Randomized clinical trials were launched to answer the question.
Studies of balloon angioplasty vs CABG
The BARI trial (Bypass Angioplasty Revascularization Investigation),5,20 published in 1996, compared PCI (using balloon angioplasty without a stent) and CABG in patients with multivessel coronary artery disease (Table 120–29).
Between 1988 and 1991, the trial randomly assigned 1,829 patients with multivessel disease to receive either PCI or CABG and compared their long-term outcomes. Although there was no difference in mortality rates between the two groups overall, the diabetic subgroup had a significantly better survival rate with CABG than with PCI, which was sustained over a follow-up period of 10 years.5
BARI had a significant clinical impact at the time and led to a clinical alert by the National Heart, Lung, and Blood Institute recommending CABG over PCI for patients with diabetes. However, not everyone accepted the results, because they were based on a small number of patients (n = 353) in a retrospectively determined subgroup. Further, the BARI trial was conducted before the advent of coronary stents, which were later shown to improve outcomes after PCI. Also, optimal medical therapy after revascularization was not specified in the protocol, which likely affected outcomes.
EAST (Emory Angioplasty Versus Surgery Trial)21 and CABRI (Coronary Angioplasty Versus Bypass Revascularization Investigation) 22 were similar randomized trials comparing angioplasty and CABG in patients with multivessel coronary artery disease. These showed better outcomes after CABG in patients with diabetes. However, lack of statistical significance because of small sample sizes limited their clinical impact.
Studies of PCI with bare-metal stents vs CABG
The ARTS trial (Arterial Revascularization Therapy Study) compared PCI (with bare-metal stents) and CABG in 1,205 patients with multivessel coronary artery disease.23 The mortality rate did not differ significantly between two treatment groups overall or in the diabetic subgroup. However, the repeat revascularization rate was higher with PCI than with CABG.
The SoS trial (Stenting or Surgery)24 had similar results.
The ERACI II trial (Argentine Randomized Study: Coronary Angioplasty With Stenting Versus Coronary Bypass Surgery in Multi-Vessel Disease)25 found no difference in mortality rates at 5 years with CABG vs PCI.
These trials were criticized, as none of them routinely used glycoprotein IIb/IIIa inhibitors with PCI, which by then had been shown to reduce mortality rates.30 However, these trials made it clear that restenosis requiring repeat revascularization was a major disadvantage of PCI with bare-metal stents compared with CABG in patients with diabetes. Drug-eluting stents, which significantly reduced the rates of in-stent restenosis and target-lesion revascularization, were expected to overcome this major disadvantage.
Studies of PCI with drug-eluting stents vs CABG
ARTS II was the first trial to compare PCI with drug-eluting stents vs CABG. This was a nonrandomized single-arm study of 607 patients (including 159 with diabetes) who were treated with drug-eluting stents; the outcomes were compared with the CABG group from the earlier ARTS trial.31
At 3 years, in the diabetic subgroup, the rates of death, myocardial infarction, and stroke were not significantly different between treatments, although a trend favored PCI. However, this comparison was limited by selection bias, as ARTS II was a nonrandomized trial in which operators chose patients for drug-eluting stents in an attempt to match already known outcomes from the CABG cohort of ARTS.
SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) was the first randomized trial comparing PCI with drug-eluting stents (in this trial, paclitaxel-eluting) vs CABG in patients with three-vessel or left main coronary artery disease.26,27 Subgroup analysis in patients with diabetes mellitus revealed a higher rate of major adverse cardiac and cerebrovascular events (death, myocardial infarction, stroke, or repeat revascularization) in the PCI group than in the CABG patients, largely driven by higher rates of repeat revascularization after PCI.32,33 SYNTAX was not designed to assess significant differences in rates of death.
The CARDIa trial (Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and multivessel coronary artery disease to PCI (about one-third with bare-metal stents and two-thirds with drug-eluting stents) or CABG. Rates of major adverse cardiac and cerebrovascular events were higher in the PCI group, again largely driven by higher rates of repeat revascularization.4 CARDIa was stopped early because of a lack of enrollment and could not provide sufficient evidence to endorse one strategy over the other.
VA-CARDS (Veteran Affairs Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and proximal left anterior descending artery or multivessel coronary artery disease to receive PCI with drug-eluting stents or CABG.28 Although the rate of death was lower with CABG than with PCI at 2 years, the trial was underpowered and was terminated at 25% of the initial intended patient enrollment. In addition, only 9% of diabetic patients screened were angiographically eligible for the study.29
Registry data. Analysis of a large data set from the National Cardiovascular Disease Registry and the Society of Thoracic Surgeons revealed a survival advantage of CABG over PCI for a follow-up period of 5 years.34 However, this was a nonrandomized study, so its conclusions were not definitive.
THE FREEDOM TRIAL
Given the limitations of the trials described above, the National Heart, Lung, and Blood Institute sponsored the FREEDOM trial—an appropriately powered, randomized comparison of PCI (with drug-eluting stents) and CABG (using arterial grafting) in patients with diabetes and multivessel coronary artery disease using contemporary techniques and concomitant optimal medical therapy.8
FREEDOM study design
The FREEDOM trial enrolled 1,900 patients with diabetes and angiographically confirmed multivessel coronary artery disease (83% with three-vessel disease) with stenosis of more than 70% in two or more major epicardial vessels involving at least two separate coronary-artery territories. The main exclusion criteria were severe left main coronary artery stenosis (≥ 50% stenosis), class III or IV congestive heart failure, and previous CABG or valve surgery. For CABG surgery, arterial revascularization was encouraged.
Dual antiplatelet therapy was recommended for at least 12 months in patients receiving a drug-eluting stent, and optimal medical management for diabetes, hypertension, and hyperlipidemia was strongly advocated.
Between April 2005 and April 2010, 32,966 patients were screened, of whom 3,309 were eligible for the trial and 1,900 consented and were randomized (953 to the PCI group and 947 to the CABG group). The patients were followed for a minimum of 2 years and had a median follow-up time of 3.8 years. Outcomes were measured with an intention-to-treat analysis.
Study results
Patients. The groups were comparable with regard to baseline demographics and cardiac risk factors.
The mean age was 63; 29% of the patients were women, and 83% had three-vessel coronary artery disease. The mean hemoglobin A1c was 7.8%, and the mean ejection fraction was 66%. The mean SYNTAX score, which defines the anatomic complexity of lesions, was 26 (≤ 22 is mild, 23–32 is intermediate, and ≥ 33 is high). The mean EURO score, which defines surgical risk, was 2.7 (a score ≥ 5 being associated with a lower rate of survival).
The primary composite outcome (death, nonfatal myocardial infarction, or nonfatal stroke) occurred less frequently in the CABG group than in the PCI group (Table 2). CABG was also associated with significantly lower rates of death from any cause and of myocardial infarction. Importantly, survival curves comparing the two groups diverged at 2-year follow-up. In contrast to other outcomes assessed, stroke occurred more often in the CABG group. The 5-year rates in the CABG group vs the PCI group were:
- Primary outcome—18.7% vs 26.6%, P = .005
- Death from any cause—10.9% vs 16.3%, P = .049
- Myocardial infarction—6% vs 13.9%, P < .0001
- Stroke—5.2% vs 2.4%, P = .03.
The secondary outcome (death, nonfatal myocardial infarction, nonfatal stroke, or repeat revascularization at 30 days or 12 months) had occurred significantly more often in the PCI group than in the CABG group at 1 year (16.8% vs 11.8%, P = .004), with most of the difference attributable to a higher repeat revascularization rate in the PCI group (12.6% vs 4.8%, P < .001).
Subgroup analysis. CABG was superior to PCI across all prespecified subgroups, covering the complexity of the coronary artery disease. Event rates with CABG vs PCI, by tertiles of the SYNTAX score:
- SYNTAX scores ≤ 22: 17.2% vs 23.2%
- SYNTAX scores 23–32: 17.7% vs 27.2%
- SYNTAX scores ≥ 33: 22.8% vs 30.6%.
Cost-effectiveness. Although up-front costs were higher with CABG, at $34,467 for the index hospitalization vs $25,845 for PCI (P < .001), when the in-trial results were extended to a lifetime horizon, CABG had an incremental cost-effectiveness ratio of $8,132 per quality-adjusted life-year gained vs PCI.35 Traditionally, therapies are considered costeffective if the incremental cost-effectiveness ratio is less than $50,000 per quality-adjusted life-year gained.
WHY MAY CABG BE SUPERIOR IN DIABETIC PATIENTS?
The major advantage of CABG over PCI is the ability to achieve complete revascularization. Diabetic patients with coronary artery disease tend to have diffuse, multifocal disease with several stenotic lesions in multiple coronary arteries. While stents only treat the focal area of most significant occlusion, CABG may bypass all proximal vulnerable plaques that could potentially develop into culprit lesions over time, truly bypassing the diseased segments (Figure 1).
In addition, heavy calcification may not allow optimal stenting in these patients.
Use of multiple stents increases the risk of restenosis, which could lead to a higher incidence of myocardial infarction and need for repeat revascularization. This was evident in the FREEDOM trial, in which the mean number of stents per patient was 4.2. Also, some lesions need to be left untreated because of the complexity involved.
The major improvement in outcomes after CABG has resulted from using arterial conduits such as the internal mammary artery rather than the saphenous vein.36 The patency rates of internal mammary artery grafts exceed 80% over 10 years.37 Internal mammary artery grafting was done in 94% of patients receiving CABG in the FREEDOM trial.
WHAT DOES THIS MEAN?
FREEDOM was a landmark trial that confirmed that CABG provides significant benefit compared with contemporary PCI with drug-eluting stents in patients with diabetes mellitus and multivessel coronary artery disease. It was a large multicenter trial that was adequately powered, unlike most of the earlier trials of this topic.
Unlike previous trials in which the benefit of CABG was driven by reduction in repeat revascularizations alone, FREEDOM showed lower incidence rates of all-cause mortality and myocardial infarction with CABG than with PCI. CABG was better regardless of SYNTAX score, number of diseased vessels, ejection fraction, race, or sex of the patient, indicating that it leads to superior outcomes across a wide spectrum of patients.
An argument that cardiologists often cite when recommending PCI is that it can save money due to lower length of index hospital stay and lower procedure costs of with PCI than with CABG. However, in FREEDOM, CABG also appeared to be highly cost-effective.
FREEDOM had limitations
While FREEDOM provided robust data proving the superiority of CABG, the study had several limitations.
Although there was an overall survival benefit with CABG compared with PCI, the difference in incidence of cardiovascular deaths (which accounted for 64% of all deaths) was not statistically significant.
The trial included only patients who were eligible for both PCI and CABG. Hence, the results may not be generalizable to all diabetic patients with multivessel coronary artery disease—indeed, only 10% of those screened were considered eligible for the trial. However, it is likely that several patients screened in the FREEDOM trial may not have been eligible for PCI or CABG at the time of screening, since the revascularization decision was made by a multidisciplinary team and a more appropriate decision (either CABG or PCI) was then made.
Other factors limiting the general applicability of the results were low numbers of female patients (28.6%), black patients (6.3%), patients with an ejection fraction of 40% or less (2.5%), and patients with a low SYNTAX score (35%).
There were several unexplained observations as well. The difference in events between the treatment groups was much higher in North America than in other regions. The number of coronary lesions in the CABG group was high (mean = 5.74), but the average numbers of grafts used was only 2.9, and data were not provided regarding use of sequential grafting. Similarly, an average of only 3.5 of the six stenotic lesions per patient in the PCI group were revascularized; whether this was the result of procedural limitations with PCI was not entirely clear.
In addition, while the investigators mention that an average patient received four stents, a surprising finding was that the mean total length of the stents used was only 26 mm. This appears too small, as the usual length of one drug-eluting stent is about 20 to 30 mm.
Since only high-volume centers with good outcome data were included in the trial, the results may lack validity for patients undergoing revascularization at low-volume community centers.
It remains to be seen if the benefits of CABG will be sustained over 10 years and longer, when saphenous vein grafts tend to fail and require repeat revascularization, commonly performed with PCI. Previous data suggest that the longer the follow-up, the better the results with CABG. However, long-term results (> 10 years) in studies comparing drugeluting stents and CABG are not available.
Despite limitations, FREEDOM may change clinical practice
Despite these limitations, the FREEDOM trial has the potential to change clinical practice and strengthen current recommendations for CABG in these patients.
The trial underscored the importance of a multidisciplinary heart team approach in managing patients with complex coronary artery disease, similar to that being used in patients with severe aortic stenosis since transcatheter aortic valve replacement became available.
It should also bring an end to the practice of ad hoc PCI, especially in patients with diabetes and multivessel coronary artery disease. It is now imperative that physicians discuss current evidence for therapeutic options with the patients and their families before performing diagnostic angiography rather than immediately afterward, to give the patients ample time to make an informed decision. This is important, as most patients are likely to choose PCI in the same setting over CABG unless there is extensive discussion about the risks and benefits of both strategies done in an unbiased manner before angiography.
The fear of open heart surgery, a longer hospital stay, and a higher risk of stroke with CABG may lead some patients to choose PCI instead. In addition, factors that may preclude CABG in otherwise-eligible patients include anatomic considerations (diffuse distal vessel disease, poor conduits), individual factors (frailty, poor renal function, poor pulmonary function, patient preference), and local expertise.
Nevertheless, the patient should be presented with current evidence, and discussions regarding the optimal procedure should be held with a heart team, which should include an interventional cardiologist, a cardiothoracic surgeon, and a noninvasive cardiologist to facilitate an unbiased decision.
Regardless of the strategy chosen, the importance of compliance with optimal medical therapy (statins, antiplatelet agents, diabetes treatment) should be continuously emphasized to the patient.
WHAT DOES THE FUTURE HOLD?
Despite unequivocal evidence that CABG is superior to PCI in eligible patients with diabetes mellitus in the current era, PCI technologies continue to evolve rapidly. Newer second-generation drug-eluting stents have shown lower rates of restenosis38,39 and may shorten the duration of post-PCI dual-antiplatelet therapy, a nuisance that has negatively affected outcomes with drug-eluting stents (because of problems of cost, poor compliance, and increased bleeding risk).
At the same time, CABG has also improved, with more extensive use of complete arterial conduits and use of an off-pump bypass technique that in theory poses a lower risk of stroke, although this has not yet been shown in a randomized trial.40
Alternative approaches are being investigated. One of them is a hybrid procedure in which minimally invasive off-pump arterial grafting is combined with drug-eluting stents, which may reduce the risk of stroke and speed postoperative recovery.
- Flaherty JD, Davidson CJ. Diabetes and coronary revascularization. JAMA 2005; 293:1501–1508.
- Nicholls SJ, Tuzcu EM, Kalidindi S, et al. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol 2008; 52:255–262.
- Mack MJ, Banning AP, Serruys PW, et al. Bypass versus drug-eluting stents at three years in SYNTAX patients with diabetes mellitus or metabolic syndrome. Ann Thorac Surg 2011; 92:2140–2146.
- Kapur A, Hall RJ, Malik IS, et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients. 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol 2010; 55:432–440.
- The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol 2007; 49:1600–1606.
- Hlatky MA. Compelling evidence for coronary-bypass surgery in patients with diabetes. N Engl J Med 2012; 367:2437–2438.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124:2574–2609.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Moreno PR, Murcia AM, Palacios IF, et al. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation 2000; 102:2180–2184.
- Bluher M, Unger R, Rassoul F, et al. Relation between glycaemic control, hyperinsulinaemia and plasma concentrations of soluble adhesion molecules in patients with impaired glucose tolerance or type II diabetes. Diabetologia 2002; 45:210–216.
- Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 2003; 108:1527–1532.
- Biondi-Zoccai GG, Abbate A, Liuzzo G, Biasucci LM. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol 2003; 41:1071–1077.
- Waller BF, Palumbo PJ, Lie JT, Roberts WC. Status of the coronary arteries at necropsy in diabetes mellitus with onset after age 30 years. Analysis of 229 diabetic patients with and without clinical evidence of coronary heart disease and comparison to 183 control subjects. Am J Med 1980; 69:498–506.
- Morrish NJ, Stevens LK, Head J, et al. A prospective study of mortality among middle-aged diabetic patients (the London Cohort of the WHO Multinational Study of Vascular Disease in Diabetics) I: causes and death rates. Diabetologia 1990; 33:538–541.
- Laskey WK, Selzer F, Vlachos HA, et al. Comparison of in-hospital and one-year outcomes in patients with and without diabetes mellitus undergoing percutaneous catheter intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol 2002; 90:1062–1067.
- Mathew V, Gersh BJ, Williams BA, et al. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation 2004; 109:476–480.
- Glaser R, Selzer F, Faxon DP, et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation 2005; 111:143–149.
- Morricone L, Ranucci M, Denti S, et al. Diabetes and complications after cardiac surgery: comparison with a non-diabetic population. Acta Diabetologica 1999; 36:77–84.
- Hogue CW, Murphy SF, Schechtman KB, Davila-Roman VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation 1999; 100:642–647.
- The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med 1996; 335:217–225.
- King SB, Kosinski AS, Guyton RA, Lembo NJ, Weintraub WS. Eightyear mortality in the Emory Angioplasty versus Surgery Trial (East). J Am Coll Cardiol 2000; 35:1116–1121.
- Kurbaan AS, Bowker TJ, Ilsley CD, Sigwart U, Rickards AF; CABRI Investigators (Coronary Angioplasty versus Bypass Revascularization Investigation). Difference in the mortality of the CABRI diabetic and nondiabetic populations and its relation to coronary artery disease and the revascularization mode. Am J Cardiol 2001; 87:947–950.
- Serruys PW, Ong AT, van Herwerden LA, et al. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol 2005; 46:575–581.
- Booth J, Clayton T, Pepper J, et al. Randomized, controlled trial of coronary artery bypass surgery versus percutaneous coronary intervention in patients with multivessel coronary artery disease: six-year follow-up from the Stent or Surgery Trial (SoS). Circulation 2008; 118:381–388.
- Rodriguez AE, Baldi J, Fernandez Pereira C, et al. Five-year follow-up of the Argentine randomized trial of coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple vessel disease (ERACI II). J Am Coll Cardiol 2005; 46:582–588.
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009; 360:961–972.
- Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013; 381:629–638.
- Kamalesh M, Sharp TG, Tang XC, et al. Percutaneous coronary intervention versus coronary bypass surgery in United States veterans with diabetes. J Am Coll Cardiol 2013; 61:808–816.
- Ellis SG. Coronary revascularization for patients with diabetes: updated data favor coronary artery bypass grafting. J Am Coll Cardiol 2013; 61:817–819.
- Bhatt DL, Marso SP, Lincoff AM, et al. Abciximab reduces mortality in diabetics following percutaneous coronary intervention. J Am Coll Cardiol 2000; 35:922–928.
- Serruys PW, Ong AT, Morice MC, et al. Arterial Revascularisation Therapies Study Part II - Sirolimus-eluting stents for the treatment of patients with multivessel de novo coronary artery lesions. EuroIntervention 2005; 1:147–156.
- Kappetein AP, Head SJ, Morice MC, et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg 2013; 43:1006–1013.
- Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075.
- Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med 2012; 366:1467–1476.
- Magnuson EA, Farkouh ME, Fuster V, et al; FREEDOM Trial Investigators. Cost-effectiveness of percutaneous coronary intervention with drug eluting stents versus bypass surgery for patients with diabetes and multivessel coronary artery disease: results from the FREEDOM trial. Circulation 2013; 127:820–831.
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986; 314:1–6.
- Tector AJ, Schmahl TM, Janson B, et al. The internal mammary artery graft. Its longevity after coronary bypass. JAMA 1981; 246:2181–2183.
- Stone GW, Rizvi A, Newman W, et al. Everolimus-eluting versus paclitax-eleluting stents in coronary artery disease. N Engl J Med 2010; 362:1663–1674.
- Serruys PW, Silber S, Garg S, et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med 2010; 363:136–146.
- Lamy A, Devereaux PJ, Prabhakaran D, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med 2012; 366:1489–1497.
Many patients with diabetes mellitus develop complex, accelerated, multifocal coronary artery disease. Moreover, if they undergo revascularization with either coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI), their risk of morbidity and death afterward is higher than in those without diabetes.1,2
Over the last 2 decades, CABG and PCI have advanced significantly, as have antithrombotic therapy and drug therapies to modify cardiovascular risk factors such as hyperlipidemia, hypertension, and diabetes.
Several earlier studies showed CABG to be more beneficial than PCI in diabetic patients with multivessel coronary artery disease.3–5 However, the topic has been controversial, and a substantial proportion of these patients continue to undergo PCI rather than CABG.
There are two main reasons for the continued use of PCI in this population. First, PCI is evolving, with new adjuvant drugs and drugeluting stents. Many cardiologists believe that earlier trials, which did not use contemporary PCI techniques, are outdated and that current, state-of-the-art PCI may be equivalent to—if not superior to—CABG.
Second, PCI is often performed on an ad hoc basis immediately after diagnostic angiography, leaving little time for discussion with the patient about alternative treatments. In this scenario, patients are inclined to undergo PCI immediately, while they are already on the table in the catheterization suite, rather than CABG at a later date.6
In addition, although the current joint guide-lines of the American College of Cardiology and the American Heart Association state that CABG is preferable to PCI for patients with diabetes and multivessel coronary artery disease, they give it only a level IIa recommendation.7
The much-anticipated Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial8 was designed to settle the CABG-vs-PCI debate, thereby leading to a stronger guideline recommendation for the preferred revascularization strategy in this patient population.
WHY ARE DIABETIC PATIENTS DIFFERENT?
Diabetes mellitus is a major risk factor for premature and aggressive coronary artery disease. Several mechanisms have been proposed to explain this association.
Diabetic patients have higher concentrations of several inflammatory proteins than those without diabetes, including C-reactive protein, tumor necrosis factor, and platelet-derived soluble CD40 ligand. They also have higher levels of adhesion molecules such as vascular cell adhesion molecule-1 and intercellular adhesion molecule.9,10 In addition, when blood sugar levels are high, platelets express more glycoprotein IIb/IIIa receptors and are therefore more prone to aggregate.11
These prothrombotic and proinflammatory cytokines, in conjunction with endothelial dysfunction and metabolic disorders such as hyperglycemia, hyperlipidemia, obesity, insulin resistance, and oxidative stress, lead to accelerated atherosclerosis in patients with diabetes.12 Also, because diabetes is a systemic disease, the atherosclerotic process is diffuse, and many patients with diabetes have left main coronary artery lesions and diffuse multivessel coronary artery disease.13,14
Although the short-term outcomes of revascularization by any means are comparable in patients with and without diabetes, diabetic patients have lower long-term survival rates and higher rates of myocardial infarction and need for repeat procedures.15 Diabetic patients who undergo PCI have a high rate of stent thrombosis and restenosis.16,17 Similarly, those undergoing CABG have higher rates of postoperative infection and renal and neurologic complications.18,19
BEFORE THE FREEDOM TRIAL
The question of CABG vs PCI has plagued physicians ever since PCI came to the forefront in the 1980s. Before stents were widely used, PCI with balloon angioplasty was known to be comparable to CABG for single-vessel disease, but whether it was beneficial in patients with multivessel disease or left main disease was not entirely evident. Randomized clinical trials were launched to answer the question.
Studies of balloon angioplasty vs CABG
The BARI trial (Bypass Angioplasty Revascularization Investigation),5,20 published in 1996, compared PCI (using balloon angioplasty without a stent) and CABG in patients with multivessel coronary artery disease (Table 120–29).
Between 1988 and 1991, the trial randomly assigned 1,829 patients with multivessel disease to receive either PCI or CABG and compared their long-term outcomes. Although there was no difference in mortality rates between the two groups overall, the diabetic subgroup had a significantly better survival rate with CABG than with PCI, which was sustained over a follow-up period of 10 years.5
BARI had a significant clinical impact at the time and led to a clinical alert by the National Heart, Lung, and Blood Institute recommending CABG over PCI for patients with diabetes. However, not everyone accepted the results, because they were based on a small number of patients (n = 353) in a retrospectively determined subgroup. Further, the BARI trial was conducted before the advent of coronary stents, which were later shown to improve outcomes after PCI. Also, optimal medical therapy after revascularization was not specified in the protocol, which likely affected outcomes.
EAST (Emory Angioplasty Versus Surgery Trial)21 and CABRI (Coronary Angioplasty Versus Bypass Revascularization Investigation) 22 were similar randomized trials comparing angioplasty and CABG in patients with multivessel coronary artery disease. These showed better outcomes after CABG in patients with diabetes. However, lack of statistical significance because of small sample sizes limited their clinical impact.
Studies of PCI with bare-metal stents vs CABG
The ARTS trial (Arterial Revascularization Therapy Study) compared PCI (with bare-metal stents) and CABG in 1,205 patients with multivessel coronary artery disease.23 The mortality rate did not differ significantly between two treatment groups overall or in the diabetic subgroup. However, the repeat revascularization rate was higher with PCI than with CABG.
The SoS trial (Stenting or Surgery)24 had similar results.
The ERACI II trial (Argentine Randomized Study: Coronary Angioplasty With Stenting Versus Coronary Bypass Surgery in Multi-Vessel Disease)25 found no difference in mortality rates at 5 years with CABG vs PCI.
These trials were criticized, as none of them routinely used glycoprotein IIb/IIIa inhibitors with PCI, which by then had been shown to reduce mortality rates.30 However, these trials made it clear that restenosis requiring repeat revascularization was a major disadvantage of PCI with bare-metal stents compared with CABG in patients with diabetes. Drug-eluting stents, which significantly reduced the rates of in-stent restenosis and target-lesion revascularization, were expected to overcome this major disadvantage.
Studies of PCI with drug-eluting stents vs CABG
ARTS II was the first trial to compare PCI with drug-eluting stents vs CABG. This was a nonrandomized single-arm study of 607 patients (including 159 with diabetes) who were treated with drug-eluting stents; the outcomes were compared with the CABG group from the earlier ARTS trial.31
At 3 years, in the diabetic subgroup, the rates of death, myocardial infarction, and stroke were not significantly different between treatments, although a trend favored PCI. However, this comparison was limited by selection bias, as ARTS II was a nonrandomized trial in which operators chose patients for drug-eluting stents in an attempt to match already known outcomes from the CABG cohort of ARTS.
SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) was the first randomized trial comparing PCI with drug-eluting stents (in this trial, paclitaxel-eluting) vs CABG in patients with three-vessel or left main coronary artery disease.26,27 Subgroup analysis in patients with diabetes mellitus revealed a higher rate of major adverse cardiac and cerebrovascular events (death, myocardial infarction, stroke, or repeat revascularization) in the PCI group than in the CABG patients, largely driven by higher rates of repeat revascularization after PCI.32,33 SYNTAX was not designed to assess significant differences in rates of death.
The CARDIa trial (Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and multivessel coronary artery disease to PCI (about one-third with bare-metal stents and two-thirds with drug-eluting stents) or CABG. Rates of major adverse cardiac and cerebrovascular events were higher in the PCI group, again largely driven by higher rates of repeat revascularization.4 CARDIa was stopped early because of a lack of enrollment and could not provide sufficient evidence to endorse one strategy over the other.
VA-CARDS (Veteran Affairs Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and proximal left anterior descending artery or multivessel coronary artery disease to receive PCI with drug-eluting stents or CABG.28 Although the rate of death was lower with CABG than with PCI at 2 years, the trial was underpowered and was terminated at 25% of the initial intended patient enrollment. In addition, only 9% of diabetic patients screened were angiographically eligible for the study.29
Registry data. Analysis of a large data set from the National Cardiovascular Disease Registry and the Society of Thoracic Surgeons revealed a survival advantage of CABG over PCI for a follow-up period of 5 years.34 However, this was a nonrandomized study, so its conclusions were not definitive.
THE FREEDOM TRIAL
Given the limitations of the trials described above, the National Heart, Lung, and Blood Institute sponsored the FREEDOM trial—an appropriately powered, randomized comparison of PCI (with drug-eluting stents) and CABG (using arterial grafting) in patients with diabetes and multivessel coronary artery disease using contemporary techniques and concomitant optimal medical therapy.8
FREEDOM study design
The FREEDOM trial enrolled 1,900 patients with diabetes and angiographically confirmed multivessel coronary artery disease (83% with three-vessel disease) with stenosis of more than 70% in two or more major epicardial vessels involving at least two separate coronary-artery territories. The main exclusion criteria were severe left main coronary artery stenosis (≥ 50% stenosis), class III or IV congestive heart failure, and previous CABG or valve surgery. For CABG surgery, arterial revascularization was encouraged.
Dual antiplatelet therapy was recommended for at least 12 months in patients receiving a drug-eluting stent, and optimal medical management for diabetes, hypertension, and hyperlipidemia was strongly advocated.
Between April 2005 and April 2010, 32,966 patients were screened, of whom 3,309 were eligible for the trial and 1,900 consented and were randomized (953 to the PCI group and 947 to the CABG group). The patients were followed for a minimum of 2 years and had a median follow-up time of 3.8 years. Outcomes were measured with an intention-to-treat analysis.
Study results
Patients. The groups were comparable with regard to baseline demographics and cardiac risk factors.
The mean age was 63; 29% of the patients were women, and 83% had three-vessel coronary artery disease. The mean hemoglobin A1c was 7.8%, and the mean ejection fraction was 66%. The mean SYNTAX score, which defines the anatomic complexity of lesions, was 26 (≤ 22 is mild, 23–32 is intermediate, and ≥ 33 is high). The mean EURO score, which defines surgical risk, was 2.7 (a score ≥ 5 being associated with a lower rate of survival).
The primary composite outcome (death, nonfatal myocardial infarction, or nonfatal stroke) occurred less frequently in the CABG group than in the PCI group (Table 2). CABG was also associated with significantly lower rates of death from any cause and of myocardial infarction. Importantly, survival curves comparing the two groups diverged at 2-year follow-up. In contrast to other outcomes assessed, stroke occurred more often in the CABG group. The 5-year rates in the CABG group vs the PCI group were:
- Primary outcome—18.7% vs 26.6%, P = .005
- Death from any cause—10.9% vs 16.3%, P = .049
- Myocardial infarction—6% vs 13.9%, P < .0001
- Stroke—5.2% vs 2.4%, P = .03.
The secondary outcome (death, nonfatal myocardial infarction, nonfatal stroke, or repeat revascularization at 30 days or 12 months) had occurred significantly more often in the PCI group than in the CABG group at 1 year (16.8% vs 11.8%, P = .004), with most of the difference attributable to a higher repeat revascularization rate in the PCI group (12.6% vs 4.8%, P < .001).
Subgroup analysis. CABG was superior to PCI across all prespecified subgroups, covering the complexity of the coronary artery disease. Event rates with CABG vs PCI, by tertiles of the SYNTAX score:
- SYNTAX scores ≤ 22: 17.2% vs 23.2%
- SYNTAX scores 23–32: 17.7% vs 27.2%
- SYNTAX scores ≥ 33: 22.8% vs 30.6%.
Cost-effectiveness. Although up-front costs were higher with CABG, at $34,467 for the index hospitalization vs $25,845 for PCI (P < .001), when the in-trial results were extended to a lifetime horizon, CABG had an incremental cost-effectiveness ratio of $8,132 per quality-adjusted life-year gained vs PCI.35 Traditionally, therapies are considered costeffective if the incremental cost-effectiveness ratio is less than $50,000 per quality-adjusted life-year gained.
WHY MAY CABG BE SUPERIOR IN DIABETIC PATIENTS?
The major advantage of CABG over PCI is the ability to achieve complete revascularization. Diabetic patients with coronary artery disease tend to have diffuse, multifocal disease with several stenotic lesions in multiple coronary arteries. While stents only treat the focal area of most significant occlusion, CABG may bypass all proximal vulnerable plaques that could potentially develop into culprit lesions over time, truly bypassing the diseased segments (Figure 1).
In addition, heavy calcification may not allow optimal stenting in these patients.
Use of multiple stents increases the risk of restenosis, which could lead to a higher incidence of myocardial infarction and need for repeat revascularization. This was evident in the FREEDOM trial, in which the mean number of stents per patient was 4.2. Also, some lesions need to be left untreated because of the complexity involved.
The major improvement in outcomes after CABG has resulted from using arterial conduits such as the internal mammary artery rather than the saphenous vein.36 The patency rates of internal mammary artery grafts exceed 80% over 10 years.37 Internal mammary artery grafting was done in 94% of patients receiving CABG in the FREEDOM trial.
WHAT DOES THIS MEAN?
FREEDOM was a landmark trial that confirmed that CABG provides significant benefit compared with contemporary PCI with drug-eluting stents in patients with diabetes mellitus and multivessel coronary artery disease. It was a large multicenter trial that was adequately powered, unlike most of the earlier trials of this topic.
Unlike previous trials in which the benefit of CABG was driven by reduction in repeat revascularizations alone, FREEDOM showed lower incidence rates of all-cause mortality and myocardial infarction with CABG than with PCI. CABG was better regardless of SYNTAX score, number of diseased vessels, ejection fraction, race, or sex of the patient, indicating that it leads to superior outcomes across a wide spectrum of patients.
An argument that cardiologists often cite when recommending PCI is that it can save money due to lower length of index hospital stay and lower procedure costs of with PCI than with CABG. However, in FREEDOM, CABG also appeared to be highly cost-effective.
FREEDOM had limitations
While FREEDOM provided robust data proving the superiority of CABG, the study had several limitations.
Although there was an overall survival benefit with CABG compared with PCI, the difference in incidence of cardiovascular deaths (which accounted for 64% of all deaths) was not statistically significant.
The trial included only patients who were eligible for both PCI and CABG. Hence, the results may not be generalizable to all diabetic patients with multivessel coronary artery disease—indeed, only 10% of those screened were considered eligible for the trial. However, it is likely that several patients screened in the FREEDOM trial may not have been eligible for PCI or CABG at the time of screening, since the revascularization decision was made by a multidisciplinary team and a more appropriate decision (either CABG or PCI) was then made.
Other factors limiting the general applicability of the results were low numbers of female patients (28.6%), black patients (6.3%), patients with an ejection fraction of 40% or less (2.5%), and patients with a low SYNTAX score (35%).
There were several unexplained observations as well. The difference in events between the treatment groups was much higher in North America than in other regions. The number of coronary lesions in the CABG group was high (mean = 5.74), but the average numbers of grafts used was only 2.9, and data were not provided regarding use of sequential grafting. Similarly, an average of only 3.5 of the six stenotic lesions per patient in the PCI group were revascularized; whether this was the result of procedural limitations with PCI was not entirely clear.
In addition, while the investigators mention that an average patient received four stents, a surprising finding was that the mean total length of the stents used was only 26 mm. This appears too small, as the usual length of one drug-eluting stent is about 20 to 30 mm.
Since only high-volume centers with good outcome data were included in the trial, the results may lack validity for patients undergoing revascularization at low-volume community centers.
It remains to be seen if the benefits of CABG will be sustained over 10 years and longer, when saphenous vein grafts tend to fail and require repeat revascularization, commonly performed with PCI. Previous data suggest that the longer the follow-up, the better the results with CABG. However, long-term results (> 10 years) in studies comparing drugeluting stents and CABG are not available.
Despite limitations, FREEDOM may change clinical practice
Despite these limitations, the FREEDOM trial has the potential to change clinical practice and strengthen current recommendations for CABG in these patients.
The trial underscored the importance of a multidisciplinary heart team approach in managing patients with complex coronary artery disease, similar to that being used in patients with severe aortic stenosis since transcatheter aortic valve replacement became available.
It should also bring an end to the practice of ad hoc PCI, especially in patients with diabetes and multivessel coronary artery disease. It is now imperative that physicians discuss current evidence for therapeutic options with the patients and their families before performing diagnostic angiography rather than immediately afterward, to give the patients ample time to make an informed decision. This is important, as most patients are likely to choose PCI in the same setting over CABG unless there is extensive discussion about the risks and benefits of both strategies done in an unbiased manner before angiography.
The fear of open heart surgery, a longer hospital stay, and a higher risk of stroke with CABG may lead some patients to choose PCI instead. In addition, factors that may preclude CABG in otherwise-eligible patients include anatomic considerations (diffuse distal vessel disease, poor conduits), individual factors (frailty, poor renal function, poor pulmonary function, patient preference), and local expertise.
Nevertheless, the patient should be presented with current evidence, and discussions regarding the optimal procedure should be held with a heart team, which should include an interventional cardiologist, a cardiothoracic surgeon, and a noninvasive cardiologist to facilitate an unbiased decision.
Regardless of the strategy chosen, the importance of compliance with optimal medical therapy (statins, antiplatelet agents, diabetes treatment) should be continuously emphasized to the patient.
WHAT DOES THE FUTURE HOLD?
Despite unequivocal evidence that CABG is superior to PCI in eligible patients with diabetes mellitus in the current era, PCI technologies continue to evolve rapidly. Newer second-generation drug-eluting stents have shown lower rates of restenosis38,39 and may shorten the duration of post-PCI dual-antiplatelet therapy, a nuisance that has negatively affected outcomes with drug-eluting stents (because of problems of cost, poor compliance, and increased bleeding risk).
At the same time, CABG has also improved, with more extensive use of complete arterial conduits and use of an off-pump bypass technique that in theory poses a lower risk of stroke, although this has not yet been shown in a randomized trial.40
Alternative approaches are being investigated. One of them is a hybrid procedure in which minimally invasive off-pump arterial grafting is combined with drug-eluting stents, which may reduce the risk of stroke and speed postoperative recovery.
Many patients with diabetes mellitus develop complex, accelerated, multifocal coronary artery disease. Moreover, if they undergo revascularization with either coronary artery bypass grafting (CABG) or percutaneous coronary intervention (PCI), their risk of morbidity and death afterward is higher than in those without diabetes.1,2
Over the last 2 decades, CABG and PCI have advanced significantly, as have antithrombotic therapy and drug therapies to modify cardiovascular risk factors such as hyperlipidemia, hypertension, and diabetes.
Several earlier studies showed CABG to be more beneficial than PCI in diabetic patients with multivessel coronary artery disease.3–5 However, the topic has been controversial, and a substantial proportion of these patients continue to undergo PCI rather than CABG.
There are two main reasons for the continued use of PCI in this population. First, PCI is evolving, with new adjuvant drugs and drugeluting stents. Many cardiologists believe that earlier trials, which did not use contemporary PCI techniques, are outdated and that current, state-of-the-art PCI may be equivalent to—if not superior to—CABG.
Second, PCI is often performed on an ad hoc basis immediately after diagnostic angiography, leaving little time for discussion with the patient about alternative treatments. In this scenario, patients are inclined to undergo PCI immediately, while they are already on the table in the catheterization suite, rather than CABG at a later date.6
In addition, although the current joint guide-lines of the American College of Cardiology and the American Heart Association state that CABG is preferable to PCI for patients with diabetes and multivessel coronary artery disease, they give it only a level IIa recommendation.7
The much-anticipated Future Revascularization Evaluation in Patients With Diabetes Mellitus: Optimal Management of Multivessel Disease (FREEDOM) trial8 was designed to settle the CABG-vs-PCI debate, thereby leading to a stronger guideline recommendation for the preferred revascularization strategy in this patient population.
WHY ARE DIABETIC PATIENTS DIFFERENT?
Diabetes mellitus is a major risk factor for premature and aggressive coronary artery disease. Several mechanisms have been proposed to explain this association.
Diabetic patients have higher concentrations of several inflammatory proteins than those without diabetes, including C-reactive protein, tumor necrosis factor, and platelet-derived soluble CD40 ligand. They also have higher levels of adhesion molecules such as vascular cell adhesion molecule-1 and intercellular adhesion molecule.9,10 In addition, when blood sugar levels are high, platelets express more glycoprotein IIb/IIIa receptors and are therefore more prone to aggregate.11
These prothrombotic and proinflammatory cytokines, in conjunction with endothelial dysfunction and metabolic disorders such as hyperglycemia, hyperlipidemia, obesity, insulin resistance, and oxidative stress, lead to accelerated atherosclerosis in patients with diabetes.12 Also, because diabetes is a systemic disease, the atherosclerotic process is diffuse, and many patients with diabetes have left main coronary artery lesions and diffuse multivessel coronary artery disease.13,14
Although the short-term outcomes of revascularization by any means are comparable in patients with and without diabetes, diabetic patients have lower long-term survival rates and higher rates of myocardial infarction and need for repeat procedures.15 Diabetic patients who undergo PCI have a high rate of stent thrombosis and restenosis.16,17 Similarly, those undergoing CABG have higher rates of postoperative infection and renal and neurologic complications.18,19
BEFORE THE FREEDOM TRIAL
The question of CABG vs PCI has plagued physicians ever since PCI came to the forefront in the 1980s. Before stents were widely used, PCI with balloon angioplasty was known to be comparable to CABG for single-vessel disease, but whether it was beneficial in patients with multivessel disease or left main disease was not entirely evident. Randomized clinical trials were launched to answer the question.
Studies of balloon angioplasty vs CABG
The BARI trial (Bypass Angioplasty Revascularization Investigation),5,20 published in 1996, compared PCI (using balloon angioplasty without a stent) and CABG in patients with multivessel coronary artery disease (Table 120–29).
Between 1988 and 1991, the trial randomly assigned 1,829 patients with multivessel disease to receive either PCI or CABG and compared their long-term outcomes. Although there was no difference in mortality rates between the two groups overall, the diabetic subgroup had a significantly better survival rate with CABG than with PCI, which was sustained over a follow-up period of 10 years.5
BARI had a significant clinical impact at the time and led to a clinical alert by the National Heart, Lung, and Blood Institute recommending CABG over PCI for patients with diabetes. However, not everyone accepted the results, because they were based on a small number of patients (n = 353) in a retrospectively determined subgroup. Further, the BARI trial was conducted before the advent of coronary stents, which were later shown to improve outcomes after PCI. Also, optimal medical therapy after revascularization was not specified in the protocol, which likely affected outcomes.
EAST (Emory Angioplasty Versus Surgery Trial)21 and CABRI (Coronary Angioplasty Versus Bypass Revascularization Investigation) 22 were similar randomized trials comparing angioplasty and CABG in patients with multivessel coronary artery disease. These showed better outcomes after CABG in patients with diabetes. However, lack of statistical significance because of small sample sizes limited their clinical impact.
Studies of PCI with bare-metal stents vs CABG
The ARTS trial (Arterial Revascularization Therapy Study) compared PCI (with bare-metal stents) and CABG in 1,205 patients with multivessel coronary artery disease.23 The mortality rate did not differ significantly between two treatment groups overall or in the diabetic subgroup. However, the repeat revascularization rate was higher with PCI than with CABG.
The SoS trial (Stenting or Surgery)24 had similar results.
The ERACI II trial (Argentine Randomized Study: Coronary Angioplasty With Stenting Versus Coronary Bypass Surgery in Multi-Vessel Disease)25 found no difference in mortality rates at 5 years with CABG vs PCI.
These trials were criticized, as none of them routinely used glycoprotein IIb/IIIa inhibitors with PCI, which by then had been shown to reduce mortality rates.30 However, these trials made it clear that restenosis requiring repeat revascularization was a major disadvantage of PCI with bare-metal stents compared with CABG in patients with diabetes. Drug-eluting stents, which significantly reduced the rates of in-stent restenosis and target-lesion revascularization, were expected to overcome this major disadvantage.
Studies of PCI with drug-eluting stents vs CABG
ARTS II was the first trial to compare PCI with drug-eluting stents vs CABG. This was a nonrandomized single-arm study of 607 patients (including 159 with diabetes) who were treated with drug-eluting stents; the outcomes were compared with the CABG group from the earlier ARTS trial.31
At 3 years, in the diabetic subgroup, the rates of death, myocardial infarction, and stroke were not significantly different between treatments, although a trend favored PCI. However, this comparison was limited by selection bias, as ARTS II was a nonrandomized trial in which operators chose patients for drug-eluting stents in an attempt to match already known outcomes from the CABG cohort of ARTS.
SYNTAX (Synergy Between PCI With Taxus and Cardiac Surgery) was the first randomized trial comparing PCI with drug-eluting stents (in this trial, paclitaxel-eluting) vs CABG in patients with three-vessel or left main coronary artery disease.26,27 Subgroup analysis in patients with diabetes mellitus revealed a higher rate of major adverse cardiac and cerebrovascular events (death, myocardial infarction, stroke, or repeat revascularization) in the PCI group than in the CABG patients, largely driven by higher rates of repeat revascularization after PCI.32,33 SYNTAX was not designed to assess significant differences in rates of death.
The CARDIa trial (Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and multivessel coronary artery disease to PCI (about one-third with bare-metal stents and two-thirds with drug-eluting stents) or CABG. Rates of major adverse cardiac and cerebrovascular events were higher in the PCI group, again largely driven by higher rates of repeat revascularization.4 CARDIa was stopped early because of a lack of enrollment and could not provide sufficient evidence to endorse one strategy over the other.
VA-CARDS (Veteran Affairs Coronary Artery Revascularization in Diabetes) randomized patients with diabetes and proximal left anterior descending artery or multivessel coronary artery disease to receive PCI with drug-eluting stents or CABG.28 Although the rate of death was lower with CABG than with PCI at 2 years, the trial was underpowered and was terminated at 25% of the initial intended patient enrollment. In addition, only 9% of diabetic patients screened were angiographically eligible for the study.29
Registry data. Analysis of a large data set from the National Cardiovascular Disease Registry and the Society of Thoracic Surgeons revealed a survival advantage of CABG over PCI for a follow-up period of 5 years.34 However, this was a nonrandomized study, so its conclusions were not definitive.
THE FREEDOM TRIAL
Given the limitations of the trials described above, the National Heart, Lung, and Blood Institute sponsored the FREEDOM trial—an appropriately powered, randomized comparison of PCI (with drug-eluting stents) and CABG (using arterial grafting) in patients with diabetes and multivessel coronary artery disease using contemporary techniques and concomitant optimal medical therapy.8
FREEDOM study design
The FREEDOM trial enrolled 1,900 patients with diabetes and angiographically confirmed multivessel coronary artery disease (83% with three-vessel disease) with stenosis of more than 70% in two or more major epicardial vessels involving at least two separate coronary-artery territories. The main exclusion criteria were severe left main coronary artery stenosis (≥ 50% stenosis), class III or IV congestive heart failure, and previous CABG or valve surgery. For CABG surgery, arterial revascularization was encouraged.
Dual antiplatelet therapy was recommended for at least 12 months in patients receiving a drug-eluting stent, and optimal medical management for diabetes, hypertension, and hyperlipidemia was strongly advocated.
Between April 2005 and April 2010, 32,966 patients were screened, of whom 3,309 were eligible for the trial and 1,900 consented and were randomized (953 to the PCI group and 947 to the CABG group). The patients were followed for a minimum of 2 years and had a median follow-up time of 3.8 years. Outcomes were measured with an intention-to-treat analysis.
Study results
Patients. The groups were comparable with regard to baseline demographics and cardiac risk factors.
The mean age was 63; 29% of the patients were women, and 83% had three-vessel coronary artery disease. The mean hemoglobin A1c was 7.8%, and the mean ejection fraction was 66%. The mean SYNTAX score, which defines the anatomic complexity of lesions, was 26 (≤ 22 is mild, 23–32 is intermediate, and ≥ 33 is high). The mean EURO score, which defines surgical risk, was 2.7 (a score ≥ 5 being associated with a lower rate of survival).
The primary composite outcome (death, nonfatal myocardial infarction, or nonfatal stroke) occurred less frequently in the CABG group than in the PCI group (Table 2). CABG was also associated with significantly lower rates of death from any cause and of myocardial infarction. Importantly, survival curves comparing the two groups diverged at 2-year follow-up. In contrast to other outcomes assessed, stroke occurred more often in the CABG group. The 5-year rates in the CABG group vs the PCI group were:
- Primary outcome—18.7% vs 26.6%, P = .005
- Death from any cause—10.9% vs 16.3%, P = .049
- Myocardial infarction—6% vs 13.9%, P < .0001
- Stroke—5.2% vs 2.4%, P = .03.
The secondary outcome (death, nonfatal myocardial infarction, nonfatal stroke, or repeat revascularization at 30 days or 12 months) had occurred significantly more often in the PCI group than in the CABG group at 1 year (16.8% vs 11.8%, P = .004), with most of the difference attributable to a higher repeat revascularization rate in the PCI group (12.6% vs 4.8%, P < .001).
Subgroup analysis. CABG was superior to PCI across all prespecified subgroups, covering the complexity of the coronary artery disease. Event rates with CABG vs PCI, by tertiles of the SYNTAX score:
- SYNTAX scores ≤ 22: 17.2% vs 23.2%
- SYNTAX scores 23–32: 17.7% vs 27.2%
- SYNTAX scores ≥ 33: 22.8% vs 30.6%.
Cost-effectiveness. Although up-front costs were higher with CABG, at $34,467 for the index hospitalization vs $25,845 for PCI (P < .001), when the in-trial results were extended to a lifetime horizon, CABG had an incremental cost-effectiveness ratio of $8,132 per quality-adjusted life-year gained vs PCI.35 Traditionally, therapies are considered costeffective if the incremental cost-effectiveness ratio is less than $50,000 per quality-adjusted life-year gained.
WHY MAY CABG BE SUPERIOR IN DIABETIC PATIENTS?
The major advantage of CABG over PCI is the ability to achieve complete revascularization. Diabetic patients with coronary artery disease tend to have diffuse, multifocal disease with several stenotic lesions in multiple coronary arteries. While stents only treat the focal area of most significant occlusion, CABG may bypass all proximal vulnerable plaques that could potentially develop into culprit lesions over time, truly bypassing the diseased segments (Figure 1).
In addition, heavy calcification may not allow optimal stenting in these patients.
Use of multiple stents increases the risk of restenosis, which could lead to a higher incidence of myocardial infarction and need for repeat revascularization. This was evident in the FREEDOM trial, in which the mean number of stents per patient was 4.2. Also, some lesions need to be left untreated because of the complexity involved.
The major improvement in outcomes after CABG has resulted from using arterial conduits such as the internal mammary artery rather than the saphenous vein.36 The patency rates of internal mammary artery grafts exceed 80% over 10 years.37 Internal mammary artery grafting was done in 94% of patients receiving CABG in the FREEDOM trial.
WHAT DOES THIS MEAN?
FREEDOM was a landmark trial that confirmed that CABG provides significant benefit compared with contemporary PCI with drug-eluting stents in patients with diabetes mellitus and multivessel coronary artery disease. It was a large multicenter trial that was adequately powered, unlike most of the earlier trials of this topic.
Unlike previous trials in which the benefit of CABG was driven by reduction in repeat revascularizations alone, FREEDOM showed lower incidence rates of all-cause mortality and myocardial infarction with CABG than with PCI. CABG was better regardless of SYNTAX score, number of diseased vessels, ejection fraction, race, or sex of the patient, indicating that it leads to superior outcomes across a wide spectrum of patients.
An argument that cardiologists often cite when recommending PCI is that it can save money due to lower length of index hospital stay and lower procedure costs of with PCI than with CABG. However, in FREEDOM, CABG also appeared to be highly cost-effective.
FREEDOM had limitations
While FREEDOM provided robust data proving the superiority of CABG, the study had several limitations.
Although there was an overall survival benefit with CABG compared with PCI, the difference in incidence of cardiovascular deaths (which accounted for 64% of all deaths) was not statistically significant.
The trial included only patients who were eligible for both PCI and CABG. Hence, the results may not be generalizable to all diabetic patients with multivessel coronary artery disease—indeed, only 10% of those screened were considered eligible for the trial. However, it is likely that several patients screened in the FREEDOM trial may not have been eligible for PCI or CABG at the time of screening, since the revascularization decision was made by a multidisciplinary team and a more appropriate decision (either CABG or PCI) was then made.
Other factors limiting the general applicability of the results were low numbers of female patients (28.6%), black patients (6.3%), patients with an ejection fraction of 40% or less (2.5%), and patients with a low SYNTAX score (35%).
There were several unexplained observations as well. The difference in events between the treatment groups was much higher in North America than in other regions. The number of coronary lesions in the CABG group was high (mean = 5.74), but the average numbers of grafts used was only 2.9, and data were not provided regarding use of sequential grafting. Similarly, an average of only 3.5 of the six stenotic lesions per patient in the PCI group were revascularized; whether this was the result of procedural limitations with PCI was not entirely clear.
In addition, while the investigators mention that an average patient received four stents, a surprising finding was that the mean total length of the stents used was only 26 mm. This appears too small, as the usual length of one drug-eluting stent is about 20 to 30 mm.
Since only high-volume centers with good outcome data were included in the trial, the results may lack validity for patients undergoing revascularization at low-volume community centers.
It remains to be seen if the benefits of CABG will be sustained over 10 years and longer, when saphenous vein grafts tend to fail and require repeat revascularization, commonly performed with PCI. Previous data suggest that the longer the follow-up, the better the results with CABG. However, long-term results (> 10 years) in studies comparing drugeluting stents and CABG are not available.
Despite limitations, FREEDOM may change clinical practice
Despite these limitations, the FREEDOM trial has the potential to change clinical practice and strengthen current recommendations for CABG in these patients.
The trial underscored the importance of a multidisciplinary heart team approach in managing patients with complex coronary artery disease, similar to that being used in patients with severe aortic stenosis since transcatheter aortic valve replacement became available.
It should also bring an end to the practice of ad hoc PCI, especially in patients with diabetes and multivessel coronary artery disease. It is now imperative that physicians discuss current evidence for therapeutic options with the patients and their families before performing diagnostic angiography rather than immediately afterward, to give the patients ample time to make an informed decision. This is important, as most patients are likely to choose PCI in the same setting over CABG unless there is extensive discussion about the risks and benefits of both strategies done in an unbiased manner before angiography.
The fear of open heart surgery, a longer hospital stay, and a higher risk of stroke with CABG may lead some patients to choose PCI instead. In addition, factors that may preclude CABG in otherwise-eligible patients include anatomic considerations (diffuse distal vessel disease, poor conduits), individual factors (frailty, poor renal function, poor pulmonary function, patient preference), and local expertise.
Nevertheless, the patient should be presented with current evidence, and discussions regarding the optimal procedure should be held with a heart team, which should include an interventional cardiologist, a cardiothoracic surgeon, and a noninvasive cardiologist to facilitate an unbiased decision.
Regardless of the strategy chosen, the importance of compliance with optimal medical therapy (statins, antiplatelet agents, diabetes treatment) should be continuously emphasized to the patient.
WHAT DOES THE FUTURE HOLD?
Despite unequivocal evidence that CABG is superior to PCI in eligible patients with diabetes mellitus in the current era, PCI technologies continue to evolve rapidly. Newer second-generation drug-eluting stents have shown lower rates of restenosis38,39 and may shorten the duration of post-PCI dual-antiplatelet therapy, a nuisance that has negatively affected outcomes with drug-eluting stents (because of problems of cost, poor compliance, and increased bleeding risk).
At the same time, CABG has also improved, with more extensive use of complete arterial conduits and use of an off-pump bypass technique that in theory poses a lower risk of stroke, although this has not yet been shown in a randomized trial.40
Alternative approaches are being investigated. One of them is a hybrid procedure in which minimally invasive off-pump arterial grafting is combined with drug-eluting stents, which may reduce the risk of stroke and speed postoperative recovery.
- Flaherty JD, Davidson CJ. Diabetes and coronary revascularization. JAMA 2005; 293:1501–1508.
- Nicholls SJ, Tuzcu EM, Kalidindi S, et al. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol 2008; 52:255–262.
- Mack MJ, Banning AP, Serruys PW, et al. Bypass versus drug-eluting stents at three years in SYNTAX patients with diabetes mellitus or metabolic syndrome. Ann Thorac Surg 2011; 92:2140–2146.
- Kapur A, Hall RJ, Malik IS, et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients. 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol 2010; 55:432–440.
- The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol 2007; 49:1600–1606.
- Hlatky MA. Compelling evidence for coronary-bypass surgery in patients with diabetes. N Engl J Med 2012; 367:2437–2438.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124:2574–2609.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Moreno PR, Murcia AM, Palacios IF, et al. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation 2000; 102:2180–2184.
- Bluher M, Unger R, Rassoul F, et al. Relation between glycaemic control, hyperinsulinaemia and plasma concentrations of soluble adhesion molecules in patients with impaired glucose tolerance or type II diabetes. Diabetologia 2002; 45:210–216.
- Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 2003; 108:1527–1532.
- Biondi-Zoccai GG, Abbate A, Liuzzo G, Biasucci LM. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol 2003; 41:1071–1077.
- Waller BF, Palumbo PJ, Lie JT, Roberts WC. Status of the coronary arteries at necropsy in diabetes mellitus with onset after age 30 years. Analysis of 229 diabetic patients with and without clinical evidence of coronary heart disease and comparison to 183 control subjects. Am J Med 1980; 69:498–506.
- Morrish NJ, Stevens LK, Head J, et al. A prospective study of mortality among middle-aged diabetic patients (the London Cohort of the WHO Multinational Study of Vascular Disease in Diabetics) I: causes and death rates. Diabetologia 1990; 33:538–541.
- Laskey WK, Selzer F, Vlachos HA, et al. Comparison of in-hospital and one-year outcomes in patients with and without diabetes mellitus undergoing percutaneous catheter intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol 2002; 90:1062–1067.
- Mathew V, Gersh BJ, Williams BA, et al. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation 2004; 109:476–480.
- Glaser R, Selzer F, Faxon DP, et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation 2005; 111:143–149.
- Morricone L, Ranucci M, Denti S, et al. Diabetes and complications after cardiac surgery: comparison with a non-diabetic population. Acta Diabetologica 1999; 36:77–84.
- Hogue CW, Murphy SF, Schechtman KB, Davila-Roman VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation 1999; 100:642–647.
- The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med 1996; 335:217–225.
- King SB, Kosinski AS, Guyton RA, Lembo NJ, Weintraub WS. Eightyear mortality in the Emory Angioplasty versus Surgery Trial (East). J Am Coll Cardiol 2000; 35:1116–1121.
- Kurbaan AS, Bowker TJ, Ilsley CD, Sigwart U, Rickards AF; CABRI Investigators (Coronary Angioplasty versus Bypass Revascularization Investigation). Difference in the mortality of the CABRI diabetic and nondiabetic populations and its relation to coronary artery disease and the revascularization mode. Am J Cardiol 2001; 87:947–950.
- Serruys PW, Ong AT, van Herwerden LA, et al. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol 2005; 46:575–581.
- Booth J, Clayton T, Pepper J, et al. Randomized, controlled trial of coronary artery bypass surgery versus percutaneous coronary intervention in patients with multivessel coronary artery disease: six-year follow-up from the Stent or Surgery Trial (SoS). Circulation 2008; 118:381–388.
- Rodriguez AE, Baldi J, Fernandez Pereira C, et al. Five-year follow-up of the Argentine randomized trial of coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple vessel disease (ERACI II). J Am Coll Cardiol 2005; 46:582–588.
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009; 360:961–972.
- Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013; 381:629–638.
- Kamalesh M, Sharp TG, Tang XC, et al. Percutaneous coronary intervention versus coronary bypass surgery in United States veterans with diabetes. J Am Coll Cardiol 2013; 61:808–816.
- Ellis SG. Coronary revascularization for patients with diabetes: updated data favor coronary artery bypass grafting. J Am Coll Cardiol 2013; 61:817–819.
- Bhatt DL, Marso SP, Lincoff AM, et al. Abciximab reduces mortality in diabetics following percutaneous coronary intervention. J Am Coll Cardiol 2000; 35:922–928.
- Serruys PW, Ong AT, Morice MC, et al. Arterial Revascularisation Therapies Study Part II - Sirolimus-eluting stents for the treatment of patients with multivessel de novo coronary artery lesions. EuroIntervention 2005; 1:147–156.
- Kappetein AP, Head SJ, Morice MC, et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg 2013; 43:1006–1013.
- Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075.
- Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med 2012; 366:1467–1476.
- Magnuson EA, Farkouh ME, Fuster V, et al; FREEDOM Trial Investigators. Cost-effectiveness of percutaneous coronary intervention with drug eluting stents versus bypass surgery for patients with diabetes and multivessel coronary artery disease: results from the FREEDOM trial. Circulation 2013; 127:820–831.
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986; 314:1–6.
- Tector AJ, Schmahl TM, Janson B, et al. The internal mammary artery graft. Its longevity after coronary bypass. JAMA 1981; 246:2181–2183.
- Stone GW, Rizvi A, Newman W, et al. Everolimus-eluting versus paclitax-eleluting stents in coronary artery disease. N Engl J Med 2010; 362:1663–1674.
- Serruys PW, Silber S, Garg S, et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med 2010; 363:136–146.
- Lamy A, Devereaux PJ, Prabhakaran D, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med 2012; 366:1489–1497.
- Flaherty JD, Davidson CJ. Diabetes and coronary revascularization. JAMA 2005; 293:1501–1508.
- Nicholls SJ, Tuzcu EM, Kalidindi S, et al. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol 2008; 52:255–262.
- Mack MJ, Banning AP, Serruys PW, et al. Bypass versus drug-eluting stents at three years in SYNTAX patients with diabetes mellitus or metabolic syndrome. Ann Thorac Surg 2011; 92:2140–2146.
- Kapur A, Hall RJ, Malik IS, et al. Randomized comparison of percutaneous coronary intervention with coronary artery bypass grafting in diabetic patients. 1-year results of the CARDia (Coronary Artery Revascularization in Diabetes) trial. J Am Coll Cardiol 2010; 55:432–440.
- The final 10-year follow-up results from the BARI randomized trial. J Am Coll Cardiol 2007; 49:1600–1606.
- Hlatky MA. Compelling evidence for coronary-bypass surgery in patients with diabetes. N Engl J Med 2012; 367:2437–2438.
- Levine GN, Bates ER, Blankenship JC, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2011; 124:2574–2609.
- Farkouh ME, Domanski M, Sleeper LA, et al. Strategies for multivessel revascularization in patients with diabetes. N Engl J Med 2012; 367:2375–2384.
- Moreno PR, Murcia AM, Palacios IF, et al. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation 2000; 102:2180–2184.
- Bluher M, Unger R, Rassoul F, et al. Relation between glycaemic control, hyperinsulinaemia and plasma concentrations of soluble adhesion molecules in patients with impaired glucose tolerance or type II diabetes. Diabetologia 2002; 45:210–216.
- Creager MA, Luscher TF, Cosentino F, Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Circulation 2003; 108:1527–1532.
- Biondi-Zoccai GG, Abbate A, Liuzzo G, Biasucci LM. Atherothrombosis, inflammation, and diabetes. J Am Coll Cardiol 2003; 41:1071–1077.
- Waller BF, Palumbo PJ, Lie JT, Roberts WC. Status of the coronary arteries at necropsy in diabetes mellitus with onset after age 30 years. Analysis of 229 diabetic patients with and without clinical evidence of coronary heart disease and comparison to 183 control subjects. Am J Med 1980; 69:498–506.
- Morrish NJ, Stevens LK, Head J, et al. A prospective study of mortality among middle-aged diabetic patients (the London Cohort of the WHO Multinational Study of Vascular Disease in Diabetics) I: causes and death rates. Diabetologia 1990; 33:538–541.
- Laskey WK, Selzer F, Vlachos HA, et al. Comparison of in-hospital and one-year outcomes in patients with and without diabetes mellitus undergoing percutaneous catheter intervention (from the National Heart, Lung, and Blood Institute Dynamic Registry). Am J Cardiol 2002; 90:1062–1067.
- Mathew V, Gersh BJ, Williams BA, et al. Outcomes in patients with diabetes mellitus undergoing percutaneous coronary intervention in the current era: a report from the Prevention of REStenosis with Tranilast and its Outcomes (PRESTO) trial. Circulation 2004; 109:476–480.
- Glaser R, Selzer F, Faxon DP, et al. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation 2005; 111:143–149.
- Morricone L, Ranucci M, Denti S, et al. Diabetes and complications after cardiac surgery: comparison with a non-diabetic population. Acta Diabetologica 1999; 36:77–84.
- Hogue CW, Murphy SF, Schechtman KB, Davila-Roman VG. Risk factors for early or delayed stroke after cardiac surgery. Circulation 1999; 100:642–647.
- The Bypass Angioplasty Revascularization Investigation (BARI) Investigators. Comparison of coronary bypass surgery with angioplasty in patients with multivessel disease. N Engl J Med 1996; 335:217–225.
- King SB, Kosinski AS, Guyton RA, Lembo NJ, Weintraub WS. Eightyear mortality in the Emory Angioplasty versus Surgery Trial (East). J Am Coll Cardiol 2000; 35:1116–1121.
- Kurbaan AS, Bowker TJ, Ilsley CD, Sigwart U, Rickards AF; CABRI Investigators (Coronary Angioplasty versus Bypass Revascularization Investigation). Difference in the mortality of the CABRI diabetic and nondiabetic populations and its relation to coronary artery disease and the revascularization mode. Am J Cardiol 2001; 87:947–950.
- Serruys PW, Ong AT, van Herwerden LA, et al. Five-year outcomes after coronary stenting versus bypass surgery for the treatment of multivessel disease: the final analysis of the Arterial Revascularization Therapies Study (ARTS) randomized trial. J Am Coll Cardiol 2005; 46:575–581.
- Booth J, Clayton T, Pepper J, et al. Randomized, controlled trial of coronary artery bypass surgery versus percutaneous coronary intervention in patients with multivessel coronary artery disease: six-year follow-up from the Stent or Surgery Trial (SoS). Circulation 2008; 118:381–388.
- Rodriguez AE, Baldi J, Fernandez Pereira C, et al. Five-year follow-up of the Argentine randomized trial of coronary angioplasty with stenting versus coronary bypass surgery in patients with multiple vessel disease (ERACI II). J Am Coll Cardiol 2005; 46:582–588.
- Serruys PW, Morice MC, Kappetein AP, et al. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 2009; 360:961–972.
- Mohr FW, Morice MC, Kappetein AP, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical SYNTAX trial. Lancet 2013; 381:629–638.
- Kamalesh M, Sharp TG, Tang XC, et al. Percutaneous coronary intervention versus coronary bypass surgery in United States veterans with diabetes. J Am Coll Cardiol 2013; 61:808–816.
- Ellis SG. Coronary revascularization for patients with diabetes: updated data favor coronary artery bypass grafting. J Am Coll Cardiol 2013; 61:817–819.
- Bhatt DL, Marso SP, Lincoff AM, et al. Abciximab reduces mortality in diabetics following percutaneous coronary intervention. J Am Coll Cardiol 2000; 35:922–928.
- Serruys PW, Ong AT, Morice MC, et al. Arterial Revascularisation Therapies Study Part II - Sirolimus-eluting stents for the treatment of patients with multivessel de novo coronary artery lesions. EuroIntervention 2005; 1:147–156.
- Kappetein AP, Head SJ, Morice MC, et al. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur J Cardiothorac Surg 2013; 43:1006–1013.
- Banning AP, Westaby S, Morice MC, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease: comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol 2010; 55:1067–1075.
- Weintraub WS, Grau-Sepulveda MV, Weiss JM, et al. Comparative effectiveness of revascularization strategies. N Engl J Med 2012; 366:1467–1476.
- Magnuson EA, Farkouh ME, Fuster V, et al; FREEDOM Trial Investigators. Cost-effectiveness of percutaneous coronary intervention with drug eluting stents versus bypass surgery for patients with diabetes and multivessel coronary artery disease: results from the FREEDOM trial. Circulation 2013; 127:820–831.
- Loop FD, Lytle BW, Cosgrove DM, et al. Influence of the internal-mammary-artery graft on 10-year survival and other cardiac events. N Engl J Med 1986; 314:1–6.
- Tector AJ, Schmahl TM, Janson B, et al. The internal mammary artery graft. Its longevity after coronary bypass. JAMA 1981; 246:2181–2183.
- Stone GW, Rizvi A, Newman W, et al. Everolimus-eluting versus paclitax-eleluting stents in coronary artery disease. N Engl J Med 2010; 362:1663–1674.
- Serruys PW, Silber S, Garg S, et al. Comparison of zotarolimus-eluting and everolimus-eluting coronary stents. N Engl J Med 2010; 363:136–146.
- Lamy A, Devereaux PJ, Prabhakaran D, et al. Off-pump or on-pump coronary-artery bypass grafting at 30 days. N Engl J Med 2012; 366:1489–1497.
KEY POINTS
- Patients with diabetes have a higher prevalence of multivessel coronary artery disease and often have complex, diffuse lesions.
- Bypass surgery is the preferred method of revascularization in appropriately selected patients with diabetes and multivessel coronary artery disease.
- In the FREEDOM trial, only about 10% of the screened patients were eligible for the study, limiting its generalizability; however, this is comparable to exclusion rates in previous large randomized trials.
- When choosing a revascularization method, the physician team needs to discuss the options with the patient before performing diagnostic angiography. The team should include a cardiac surgeon and a cardiologist.
Would you recognize this unusual cause of acute back pain?
› Order an emergent magnetic resonance imaging scan to rule out epidural hematoma for patients with severe focal acute back pain that presents with limb dysesthesia, thoracic radiculopathy, or an unusual clinical course. A
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case-series
CASE A 34-year-old, otherwise healthy man presented at our emergency department (ED) complaining of severe, acute,high-thoracic back pain. He had left-sided chest pain radiating to his left arm and nonspecific paresthesias that did not track along a specific dermatome. The pain started 2 days earlier while the patient was lifting a heavy object at work. He was initially seen by his family physician, who diagnosed acute back pain, prescribed a COX-2 selective nonsteroidal anti-inflammatory agent, and advised him to stay home from work. This treatment did not improve the patient’s pain, which became more severe shortly before his arrival in our ED.
On physical examination, we found the patient in general good health, with no evidence of distress. His blood pressure was 128/78 mm Hg, his respiratory rate was 16 breaths/min,and his oral temperature was 36.5°C (97.7°F). An electrocardiogram(EKG) showed a normal sinus rhythm with no evidence of ischemia. Troponin I was 0 ng/mL, and complete blood count,C-reactive protein, and erythrocyte sedimentation rate were within normal range. After observation, the patient was discharged for ambulatory follow-up, with a diagnosis of acute back pain and nonischemic chest pain.
Two days later, the patient returned to the ED. He said that the pain had not gotten better, and he reported the gradual development of numbness in both legs, along with urinary retention. Re-examination revealed an ASIA D paraplegia at T4,with 650 mL postvoid residual urine volume. (ASIA D paraplegia means no sensory loss below the level of the injury and a motorfunction score above 3/5, which indicates active joint movement is possible against gravity but not against resistance.)
An emergent magnetic resonance imaging (MRI) scan was ordered; it revealed a large left-sided epidural mass spanning the length of T4 and T5 vertebrae with severe cord displacement and compression (FIGURE 1). On a T1-weighted MRI, the mass appeared hyperintense only on the periphery,indicating the presence of extracellular methemoglobin—a radiographic manifestation of early subacute hemorrhage.1
Surgery. We started the patient on IV dexamethasone and performed an urgent T3-T5 laminectomy, which revealed a large brown epidural mass adhering to the duramater (FIGURE 2). We removed the mass and sent it to pathology, which confirmed an epidural hematoma.
The patient’s postoperative course was uneventful, and he was discharged from the hospital with complete motor strength in both legs and complete urinary control. The patient rapidly regained all sensory function, and we re-examined him one month postop in our outpatient clinic. He was intact neurologically and completely free of pain and dysesthesia. A follow-up MRI 3 months postop revealed no residual compression or signal abnormalities within the spinal cord.
Diagnosis remains a challenge
Spinal epidural hematoma (SEH) is an uncommon entity, with an estimated incidence of 0.1/100,000 per year.2 Because there are no studies that look at a large series of patients in the literature, our knowledge of the disorder is limited to case reports and small case series.
In most reports, SEH is linked to predisposing factors such as an underlying coagulopathy or anticoagulant therapy, epidural venous malformation, spinal trauma, inflammatory spine disease, pregnancy or neoplastic encroachment, and bleeding into the spinal canal.3 Spontaneous spinal epidural hematoma (SSEH) with no recognizable underlying predisposing factor is even rarer,4 and the paucity of published data makes it difficult to estimate the true incidence.
In the largest study to date, Zhong etal5 found 7 of 30 cases of SSEH were not associated with bleeding risk factors, making the calculated incidence approximately 0.023/100,000 per year. However, it is impossible to estimate how many cases remain undetected because neurological loss does not occur, and the pain is labeled a nonspecific backache that resolves spontaneously.
The typical patient will present with nothing more than severe midline spinal pain located anywhere from the head to the buttocks. Patients with acute onset of axial back pain with no distinguishing signs or symptoms (“red flags”) are seen daily in every practice. Commonly accepted guidelines from the American College of Physicians (ACP) and the American Pain Society (APS) direct the physician away from MRI scanning that can confirm the diagnosis.6
As our patient’s case illustrates, acute pain in the axial spine is a very common complaint, and usually has a benign etiology. Compression of one or more of the exiting thoracic nerve roots can cause thoracic radiculopathy, which might confound the diagnosis even further and lead to an unnecessary cardiothoracic work-up.4 That was the case for our patient. Only when neurological loss is evident will the need for an MRI become clear, and the diagnosis became apparent.
Definitive treatment is elusive
Several authors advocate emergent surgical decompression and evacuation of the hematoma, because the neurological outcome could be catastrophic,7 resulting in complete quadri/paraplegia. This approach is equivocal, however, as spontaneous resolution of neurological symptoms has been described in many cases.1,7-11 Therefore, the decision whether to operate largely depends on the individual’s surgical risk factors and clinical course. In a high-risk patient who is improving neurologically, watchful waiting may be prudent, while in neurologically deteriorating low-risk cases little is lost with immediate surgical decompression.12
The prognosis of SSEH is also difficult to estimate. Several reports confirm that cervical and cervicothoracic hematomas carry a worse prognosis than thoracolumbar and lumbosacral hematomas.3,5 A rapid onset of neurological deterioration (less than12 hours) also heralds graver consequences, as does spinal cord edema on initial MRI.5
Two windows of opportunity
Two time periods stand out as possible course-changing opportunities for clinicians.The first occurs when the patient initially complains of an acute backache. At this point, ACP/APS guidelines direct us to look for red flags that focus attention on the more troubling etiologies for backache.6 Unfortunately, SSEH has no specific red flag, and itis misleading to suggest that such an esoteric diagnosis should routinely be considered.
A better approach would be to look for the unusual: very sharp, acute-onset, highly localized back pain that does not respond well to analgesics (TABLE [developed by NR]),along with any dysesthesia or unusual radicular complaint. A positive l’hermitte sign—anelectrical sensation that runs down the back and into the limbs—on physical examination might also warrant placing SSEH in the differential diagnosis.
The second time when swiftness might be crucial is from diagnosis to surgery. In a neurologically deteriorating patient, time is of utmost importance, and taking action at this juncture can produce a marked difference in the final outcome.3
CORRESPONDENCE
Nimrod Rahamimov, MD, Department of Orthopedics Band Spine Surgery, Western Galilee Hospital, PO Box 21, Naharia 22100, Israel; [email protected]
- Lipton ML. Totally Accessible MRI: A User’s Guide to Principles,Technology, and Applications. New York, NY: Springer; 2008.
- Taniguchi LU, Pahl FH, Lúcio JE, et al. Complete motor recoveryafter acute paraparesis caused by spontaneous spinal epiduralhematoma: case report. BMC Emerg Med. 2011;11:10.
- Binder DK, Sonne DC, Lawton MT. Spinal epidural hematoma.Neurosurg Q. 2004;14:51-59.
- Tsen AR, Burrows AM, Dumont TM, et al. Spinal epidural hematomamasquerading as atypical chest pain. Am J Emerg Med.2011;29:1236.e1-e3.
- Zhong W, Chen H, You C, et al. Spontaneous spinal epidural hematoma. J Clin Neurosci. 2011;18:1490-1494.
- Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of lowback pain: a joint clinical practice guideline from the AmericanCollege of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
- Groen RJ. Non-operative treatment of spontaneous spinal epidural hematomas: a review of the literature and a comparison with operative cases. Acta Neurochir (Wien). 2004;146:103-110.
- Subbiah M, Avadhani A, Shetty AP, et al. Acute spontaneouscervical epidural hematoma with neurological deficit after lowmolecular-weight heparin therapy: role of conservative management.Spine J. 2010;10:e11-e15.
- Jang JW, Lee JK, Seo BR, et al. Spontaneous resolution of atraumatic cervicothoracic epidural hematoma presentingwith transient paraplegia: a case report. Spine (Phila Pa 1976). 2010;35:E564-E567.
- Sirin S, Arslan E, Yasar S, et al. Is spontaneous spinal epidural hematoma in elderly patients an emergency surgical case?Turk Neurosurg. 2010;20:557-560.
- Jang JW, Lee JK, Seo BR, et al. Spontaneous resolution of tetraparesis because of postoperative cervical epidural hematoma.Spine J. 2010;10:e1-e5.
- Fleager K, Lee A, Cheng I, et al. Massive spontaneous epidural hematoma in a high-level swimmer: a case report. J Bone Joint Surg Am. 2010;92:2843-2846.
› Order an emergent magnetic resonance imaging scan to rule out epidural hematoma for patients with severe focal acute back pain that presents with limb dysesthesia, thoracic radiculopathy, or an unusual clinical course. A
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case-series
CASE A 34-year-old, otherwise healthy man presented at our emergency department (ED) complaining of severe, acute,high-thoracic back pain. He had left-sided chest pain radiating to his left arm and nonspecific paresthesias that did not track along a specific dermatome. The pain started 2 days earlier while the patient was lifting a heavy object at work. He was initially seen by his family physician, who diagnosed acute back pain, prescribed a COX-2 selective nonsteroidal anti-inflammatory agent, and advised him to stay home from work. This treatment did not improve the patient’s pain, which became more severe shortly before his arrival in our ED.
On physical examination, we found the patient in general good health, with no evidence of distress. His blood pressure was 128/78 mm Hg, his respiratory rate was 16 breaths/min,and his oral temperature was 36.5°C (97.7°F). An electrocardiogram(EKG) showed a normal sinus rhythm with no evidence of ischemia. Troponin I was 0 ng/mL, and complete blood count,C-reactive protein, and erythrocyte sedimentation rate were within normal range. After observation, the patient was discharged for ambulatory follow-up, with a diagnosis of acute back pain and nonischemic chest pain.
Two days later, the patient returned to the ED. He said that the pain had not gotten better, and he reported the gradual development of numbness in both legs, along with urinary retention. Re-examination revealed an ASIA D paraplegia at T4,with 650 mL postvoid residual urine volume. (ASIA D paraplegia means no sensory loss below the level of the injury and a motorfunction score above 3/5, which indicates active joint movement is possible against gravity but not against resistance.)
An emergent magnetic resonance imaging (MRI) scan was ordered; it revealed a large left-sided epidural mass spanning the length of T4 and T5 vertebrae with severe cord displacement and compression (FIGURE 1). On a T1-weighted MRI, the mass appeared hyperintense only on the periphery,indicating the presence of extracellular methemoglobin—a radiographic manifestation of early subacute hemorrhage.1
Surgery. We started the patient on IV dexamethasone and performed an urgent T3-T5 laminectomy, which revealed a large brown epidural mass adhering to the duramater (FIGURE 2). We removed the mass and sent it to pathology, which confirmed an epidural hematoma.
The patient’s postoperative course was uneventful, and he was discharged from the hospital with complete motor strength in both legs and complete urinary control. The patient rapidly regained all sensory function, and we re-examined him one month postop in our outpatient clinic. He was intact neurologically and completely free of pain and dysesthesia. A follow-up MRI 3 months postop revealed no residual compression or signal abnormalities within the spinal cord.
Diagnosis remains a challenge
Spinal epidural hematoma (SEH) is an uncommon entity, with an estimated incidence of 0.1/100,000 per year.2 Because there are no studies that look at a large series of patients in the literature, our knowledge of the disorder is limited to case reports and small case series.
In most reports, SEH is linked to predisposing factors such as an underlying coagulopathy or anticoagulant therapy, epidural venous malformation, spinal trauma, inflammatory spine disease, pregnancy or neoplastic encroachment, and bleeding into the spinal canal.3 Spontaneous spinal epidural hematoma (SSEH) with no recognizable underlying predisposing factor is even rarer,4 and the paucity of published data makes it difficult to estimate the true incidence.
In the largest study to date, Zhong etal5 found 7 of 30 cases of SSEH were not associated with bleeding risk factors, making the calculated incidence approximately 0.023/100,000 per year. However, it is impossible to estimate how many cases remain undetected because neurological loss does not occur, and the pain is labeled a nonspecific backache that resolves spontaneously.
The typical patient will present with nothing more than severe midline spinal pain located anywhere from the head to the buttocks. Patients with acute onset of axial back pain with no distinguishing signs or symptoms (“red flags”) are seen daily in every practice. Commonly accepted guidelines from the American College of Physicians (ACP) and the American Pain Society (APS) direct the physician away from MRI scanning that can confirm the diagnosis.6
As our patient’s case illustrates, acute pain in the axial spine is a very common complaint, and usually has a benign etiology. Compression of one or more of the exiting thoracic nerve roots can cause thoracic radiculopathy, which might confound the diagnosis even further and lead to an unnecessary cardiothoracic work-up.4 That was the case for our patient. Only when neurological loss is evident will the need for an MRI become clear, and the diagnosis became apparent.
Definitive treatment is elusive
Several authors advocate emergent surgical decompression and evacuation of the hematoma, because the neurological outcome could be catastrophic,7 resulting in complete quadri/paraplegia. This approach is equivocal, however, as spontaneous resolution of neurological symptoms has been described in many cases.1,7-11 Therefore, the decision whether to operate largely depends on the individual’s surgical risk factors and clinical course. In a high-risk patient who is improving neurologically, watchful waiting may be prudent, while in neurologically deteriorating low-risk cases little is lost with immediate surgical decompression.12
The prognosis of SSEH is also difficult to estimate. Several reports confirm that cervical and cervicothoracic hematomas carry a worse prognosis than thoracolumbar and lumbosacral hematomas.3,5 A rapid onset of neurological deterioration (less than12 hours) also heralds graver consequences, as does spinal cord edema on initial MRI.5
Two windows of opportunity
Two time periods stand out as possible course-changing opportunities for clinicians.The first occurs when the patient initially complains of an acute backache. At this point, ACP/APS guidelines direct us to look for red flags that focus attention on the more troubling etiologies for backache.6 Unfortunately, SSEH has no specific red flag, and itis misleading to suggest that such an esoteric diagnosis should routinely be considered.
A better approach would be to look for the unusual: very sharp, acute-onset, highly localized back pain that does not respond well to analgesics (TABLE [developed by NR]),along with any dysesthesia or unusual radicular complaint. A positive l’hermitte sign—anelectrical sensation that runs down the back and into the limbs—on physical examination might also warrant placing SSEH in the differential diagnosis.
The second time when swiftness might be crucial is from diagnosis to surgery. In a neurologically deteriorating patient, time is of utmost importance, and taking action at this juncture can produce a marked difference in the final outcome.3
CORRESPONDENCE
Nimrod Rahamimov, MD, Department of Orthopedics Band Spine Surgery, Western Galilee Hospital, PO Box 21, Naharia 22100, Israel; [email protected]
› Order an emergent magnetic resonance imaging scan to rule out epidural hematoma for patients with severe focal acute back pain that presents with limb dysesthesia, thoracic radiculopathy, or an unusual clinical course. A
Strength of recommendation (SOR)
A. Good-quality patient-oriented evidence
B. Inconsistent or limited-quality patient-oriented evidence
C. Consensus, usual practice, opinion, disease-oriented evidence, case-series
CASE A 34-year-old, otherwise healthy man presented at our emergency department (ED) complaining of severe, acute,high-thoracic back pain. He had left-sided chest pain radiating to his left arm and nonspecific paresthesias that did not track along a specific dermatome. The pain started 2 days earlier while the patient was lifting a heavy object at work. He was initially seen by his family physician, who diagnosed acute back pain, prescribed a COX-2 selective nonsteroidal anti-inflammatory agent, and advised him to stay home from work. This treatment did not improve the patient’s pain, which became more severe shortly before his arrival in our ED.
On physical examination, we found the patient in general good health, with no evidence of distress. His blood pressure was 128/78 mm Hg, his respiratory rate was 16 breaths/min,and his oral temperature was 36.5°C (97.7°F). An electrocardiogram(EKG) showed a normal sinus rhythm with no evidence of ischemia. Troponin I was 0 ng/mL, and complete blood count,C-reactive protein, and erythrocyte sedimentation rate were within normal range. After observation, the patient was discharged for ambulatory follow-up, with a diagnosis of acute back pain and nonischemic chest pain.
Two days later, the patient returned to the ED. He said that the pain had not gotten better, and he reported the gradual development of numbness in both legs, along with urinary retention. Re-examination revealed an ASIA D paraplegia at T4,with 650 mL postvoid residual urine volume. (ASIA D paraplegia means no sensory loss below the level of the injury and a motorfunction score above 3/5, which indicates active joint movement is possible against gravity but not against resistance.)
An emergent magnetic resonance imaging (MRI) scan was ordered; it revealed a large left-sided epidural mass spanning the length of T4 and T5 vertebrae with severe cord displacement and compression (FIGURE 1). On a T1-weighted MRI, the mass appeared hyperintense only on the periphery,indicating the presence of extracellular methemoglobin—a radiographic manifestation of early subacute hemorrhage.1
Surgery. We started the patient on IV dexamethasone and performed an urgent T3-T5 laminectomy, which revealed a large brown epidural mass adhering to the duramater (FIGURE 2). We removed the mass and sent it to pathology, which confirmed an epidural hematoma.
The patient’s postoperative course was uneventful, and he was discharged from the hospital with complete motor strength in both legs and complete urinary control. The patient rapidly regained all sensory function, and we re-examined him one month postop in our outpatient clinic. He was intact neurologically and completely free of pain and dysesthesia. A follow-up MRI 3 months postop revealed no residual compression or signal abnormalities within the spinal cord.
Diagnosis remains a challenge
Spinal epidural hematoma (SEH) is an uncommon entity, with an estimated incidence of 0.1/100,000 per year.2 Because there are no studies that look at a large series of patients in the literature, our knowledge of the disorder is limited to case reports and small case series.
In most reports, SEH is linked to predisposing factors such as an underlying coagulopathy or anticoagulant therapy, epidural venous malformation, spinal trauma, inflammatory spine disease, pregnancy or neoplastic encroachment, and bleeding into the spinal canal.3 Spontaneous spinal epidural hematoma (SSEH) with no recognizable underlying predisposing factor is even rarer,4 and the paucity of published data makes it difficult to estimate the true incidence.
In the largest study to date, Zhong etal5 found 7 of 30 cases of SSEH were not associated with bleeding risk factors, making the calculated incidence approximately 0.023/100,000 per year. However, it is impossible to estimate how many cases remain undetected because neurological loss does not occur, and the pain is labeled a nonspecific backache that resolves spontaneously.
The typical patient will present with nothing more than severe midline spinal pain located anywhere from the head to the buttocks. Patients with acute onset of axial back pain with no distinguishing signs or symptoms (“red flags”) are seen daily in every practice. Commonly accepted guidelines from the American College of Physicians (ACP) and the American Pain Society (APS) direct the physician away from MRI scanning that can confirm the diagnosis.6
As our patient’s case illustrates, acute pain in the axial spine is a very common complaint, and usually has a benign etiology. Compression of one or more of the exiting thoracic nerve roots can cause thoracic radiculopathy, which might confound the diagnosis even further and lead to an unnecessary cardiothoracic work-up.4 That was the case for our patient. Only when neurological loss is evident will the need for an MRI become clear, and the diagnosis became apparent.
Definitive treatment is elusive
Several authors advocate emergent surgical decompression and evacuation of the hematoma, because the neurological outcome could be catastrophic,7 resulting in complete quadri/paraplegia. This approach is equivocal, however, as spontaneous resolution of neurological symptoms has been described in many cases.1,7-11 Therefore, the decision whether to operate largely depends on the individual’s surgical risk factors and clinical course. In a high-risk patient who is improving neurologically, watchful waiting may be prudent, while in neurologically deteriorating low-risk cases little is lost with immediate surgical decompression.12
The prognosis of SSEH is also difficult to estimate. Several reports confirm that cervical and cervicothoracic hematomas carry a worse prognosis than thoracolumbar and lumbosacral hematomas.3,5 A rapid onset of neurological deterioration (less than12 hours) also heralds graver consequences, as does spinal cord edema on initial MRI.5
Two windows of opportunity
Two time periods stand out as possible course-changing opportunities for clinicians.The first occurs when the patient initially complains of an acute backache. At this point, ACP/APS guidelines direct us to look for red flags that focus attention on the more troubling etiologies for backache.6 Unfortunately, SSEH has no specific red flag, and itis misleading to suggest that such an esoteric diagnosis should routinely be considered.
A better approach would be to look for the unusual: very sharp, acute-onset, highly localized back pain that does not respond well to analgesics (TABLE [developed by NR]),along with any dysesthesia or unusual radicular complaint. A positive l’hermitte sign—anelectrical sensation that runs down the back and into the limbs—on physical examination might also warrant placing SSEH in the differential diagnosis.
The second time when swiftness might be crucial is from diagnosis to surgery. In a neurologically deteriorating patient, time is of utmost importance, and taking action at this juncture can produce a marked difference in the final outcome.3
CORRESPONDENCE
Nimrod Rahamimov, MD, Department of Orthopedics Band Spine Surgery, Western Galilee Hospital, PO Box 21, Naharia 22100, Israel; [email protected]
- Lipton ML. Totally Accessible MRI: A User’s Guide to Principles,Technology, and Applications. New York, NY: Springer; 2008.
- Taniguchi LU, Pahl FH, Lúcio JE, et al. Complete motor recoveryafter acute paraparesis caused by spontaneous spinal epiduralhematoma: case report. BMC Emerg Med. 2011;11:10.
- Binder DK, Sonne DC, Lawton MT. Spinal epidural hematoma.Neurosurg Q. 2004;14:51-59.
- Tsen AR, Burrows AM, Dumont TM, et al. Spinal epidural hematomamasquerading as atypical chest pain. Am J Emerg Med.2011;29:1236.e1-e3.
- Zhong W, Chen H, You C, et al. Spontaneous spinal epidural hematoma. J Clin Neurosci. 2011;18:1490-1494.
- Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of lowback pain: a joint clinical practice guideline from the AmericanCollege of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
- Groen RJ. Non-operative treatment of spontaneous spinal epidural hematomas: a review of the literature and a comparison with operative cases. Acta Neurochir (Wien). 2004;146:103-110.
- Subbiah M, Avadhani A, Shetty AP, et al. Acute spontaneouscervical epidural hematoma with neurological deficit after lowmolecular-weight heparin therapy: role of conservative management.Spine J. 2010;10:e11-e15.
- Jang JW, Lee JK, Seo BR, et al. Spontaneous resolution of atraumatic cervicothoracic epidural hematoma presentingwith transient paraplegia: a case report. Spine (Phila Pa 1976). 2010;35:E564-E567.
- Sirin S, Arslan E, Yasar S, et al. Is spontaneous spinal epidural hematoma in elderly patients an emergency surgical case?Turk Neurosurg. 2010;20:557-560.
- Jang JW, Lee JK, Seo BR, et al. Spontaneous resolution of tetraparesis because of postoperative cervical epidural hematoma.Spine J. 2010;10:e1-e5.
- Fleager K, Lee A, Cheng I, et al. Massive spontaneous epidural hematoma in a high-level swimmer: a case report. J Bone Joint Surg Am. 2010;92:2843-2846.
- Lipton ML. Totally Accessible MRI: A User’s Guide to Principles,Technology, and Applications. New York, NY: Springer; 2008.
- Taniguchi LU, Pahl FH, Lúcio JE, et al. Complete motor recoveryafter acute paraparesis caused by spontaneous spinal epiduralhematoma: case report. BMC Emerg Med. 2011;11:10.
- Binder DK, Sonne DC, Lawton MT. Spinal epidural hematoma.Neurosurg Q. 2004;14:51-59.
- Tsen AR, Burrows AM, Dumont TM, et al. Spinal epidural hematomamasquerading as atypical chest pain. Am J Emerg Med.2011;29:1236.e1-e3.
- Zhong W, Chen H, You C, et al. Spontaneous spinal epidural hematoma. J Clin Neurosci. 2011;18:1490-1494.
- Chou R, Qaseem A, Snow V, et al. Diagnosis and treatment of lowback pain: a joint clinical practice guideline from the AmericanCollege of Physicians and the American Pain Society. Ann Intern Med. 2007;147:478-491.
- Groen RJ. Non-operative treatment of spontaneous spinal epidural hematomas: a review of the literature and a comparison with operative cases. Acta Neurochir (Wien). 2004;146:103-110.
- Subbiah M, Avadhani A, Shetty AP, et al. Acute spontaneouscervical epidural hematoma with neurological deficit after lowmolecular-weight heparin therapy: role of conservative management.Spine J. 2010;10:e11-e15.
- Jang JW, Lee JK, Seo BR, et al. Spontaneous resolution of atraumatic cervicothoracic epidural hematoma presentingwith transient paraplegia: a case report. Spine (Phila Pa 1976). 2010;35:E564-E567.
- Sirin S, Arslan E, Yasar S, et al. Is spontaneous spinal epidural hematoma in elderly patients an emergency surgical case?Turk Neurosurg. 2010;20:557-560.
- Jang JW, Lee JK, Seo BR, et al. Spontaneous resolution of tetraparesis because of postoperative cervical epidural hematoma.Spine J. 2010;10:e1-e5.
- Fleager K, Lee A, Cheng I, et al. Massive spontaneous epidural hematoma in a high-level swimmer: a case report. J Bone Joint Surg Am. 2010;92:2843-2846.
Hospitals Report 30-Day Readmissions Dip with SHM’s Project BOOST
Initial outcomes data for SHM's Project BOOST show a reduction in 30-day hospital readmission rates to 12.7% from 14.7% among a select group of 11 participating hospitals.
Results were reported online July 22 in the Journal of Hospital Medicine. “Participation in Project BOOST appeared to be associated with a decrease in readmission rates,” the study authors cautiously observe, although two accompanying editorials label the results as "limited" and "disappointing."
The research compares outcomes data for clinical acute-care units at 11 of 30 hospitals in the first two BOOST cohorts, started in 2008 and 2009, with clinically matched non-BOOST control units for all-patient, 30-day readmissions. Each BOOST site adopted two or more of the recommended interventions from the program’s toolkit for improving care transitions, with the support of an expert mentor. Reporting clinical outcomes was voluntary and uncompensated, and 19 of the hospitals in the initial cohorts did not share their data—cited as a serious limitation by authors of the editorials.
"You can look at our study on a couple of different levels," says lead author and BOOST lead analyst Luke Hansen, MD, MHS, of the division of hospital medicine at Northwestern University Feinberg School of Medicine in Chicago. "One is the sites that gave us data, and in that group there were statistically significant results. But I think the more important question is: Will that be enough? What is the magnitude of the effect, and is it enough to give hospitals the impetus they need to prevent avoidable readmissions?"
Listen to more of our interview with Dr. Luke Hansen, Project BOOST's lead analyst.
Project BOOST is one of SHM's national QI programs aimed at improving care transitions through such strategies as readmission risk assessments, medication reconciliation, patient coaching, and post-discharge follow-up calls. For hospitals and HM groups searching for solutions to the 30-day readmission dilemma—and thereby avoid Medicare reimbursement penalties, clear answers remain elusive.
"BOOST is one of a number of care transitions improvement methodologies that have been applied to the problem of readmissions, each of which has evidence to support their effectiveness in their initial settings, but has proven difficult to translate to other sites," JHM Editor-in-Chief Andrew D. Auerbach, MD, MPH, SFHM, a professor of medicine in residence at the University of California at San Francisco's division of hospital medicine, and co-authors note in an accompanying editorial.
Dr. Auerbach notes in his editorial that research problems limited the study's robustness but that "the authors provide the necessary start down the road towards a fuller understanding of real-world efforts to reduce readmissions."
In the other editorial, Ashish K. Jha, MD, MPH, of the Harvard School of Public Health, Health Policy, and Management suggests that readmissions ultimately may be the wrong target. A better goal, Dr. Jha says, is to improve transitions of care and demonstrate better processes in achieving those results—even though that might not significantly alter readmission rates. "We need to get clearer on what we’re trying to achieve," he adds.
Visit our website for more information on hospital readmission studies.
Initial outcomes data for SHM's Project BOOST show a reduction in 30-day hospital readmission rates to 12.7% from 14.7% among a select group of 11 participating hospitals.
Results were reported online July 22 in the Journal of Hospital Medicine. “Participation in Project BOOST appeared to be associated with a decrease in readmission rates,” the study authors cautiously observe, although two accompanying editorials label the results as "limited" and "disappointing."
The research compares outcomes data for clinical acute-care units at 11 of 30 hospitals in the first two BOOST cohorts, started in 2008 and 2009, with clinically matched non-BOOST control units for all-patient, 30-day readmissions. Each BOOST site adopted two or more of the recommended interventions from the program’s toolkit for improving care transitions, with the support of an expert mentor. Reporting clinical outcomes was voluntary and uncompensated, and 19 of the hospitals in the initial cohorts did not share their data—cited as a serious limitation by authors of the editorials.
"You can look at our study on a couple of different levels," says lead author and BOOST lead analyst Luke Hansen, MD, MHS, of the division of hospital medicine at Northwestern University Feinberg School of Medicine in Chicago. "One is the sites that gave us data, and in that group there were statistically significant results. But I think the more important question is: Will that be enough? What is the magnitude of the effect, and is it enough to give hospitals the impetus they need to prevent avoidable readmissions?"
Listen to more of our interview with Dr. Luke Hansen, Project BOOST's lead analyst.
Project BOOST is one of SHM's national QI programs aimed at improving care transitions through such strategies as readmission risk assessments, medication reconciliation, patient coaching, and post-discharge follow-up calls. For hospitals and HM groups searching for solutions to the 30-day readmission dilemma—and thereby avoid Medicare reimbursement penalties, clear answers remain elusive.
"BOOST is one of a number of care transitions improvement methodologies that have been applied to the problem of readmissions, each of which has evidence to support their effectiveness in their initial settings, but has proven difficult to translate to other sites," JHM Editor-in-Chief Andrew D. Auerbach, MD, MPH, SFHM, a professor of medicine in residence at the University of California at San Francisco's division of hospital medicine, and co-authors note in an accompanying editorial.
Dr. Auerbach notes in his editorial that research problems limited the study's robustness but that "the authors provide the necessary start down the road towards a fuller understanding of real-world efforts to reduce readmissions."
In the other editorial, Ashish K. Jha, MD, MPH, of the Harvard School of Public Health, Health Policy, and Management suggests that readmissions ultimately may be the wrong target. A better goal, Dr. Jha says, is to improve transitions of care and demonstrate better processes in achieving those results—even though that might not significantly alter readmission rates. "We need to get clearer on what we’re trying to achieve," he adds.
Visit our website for more information on hospital readmission studies.
Initial outcomes data for SHM's Project BOOST show a reduction in 30-day hospital readmission rates to 12.7% from 14.7% among a select group of 11 participating hospitals.
Results were reported online July 22 in the Journal of Hospital Medicine. “Participation in Project BOOST appeared to be associated with a decrease in readmission rates,” the study authors cautiously observe, although two accompanying editorials label the results as "limited" and "disappointing."
The research compares outcomes data for clinical acute-care units at 11 of 30 hospitals in the first two BOOST cohorts, started in 2008 and 2009, with clinically matched non-BOOST control units for all-patient, 30-day readmissions. Each BOOST site adopted two or more of the recommended interventions from the program’s toolkit for improving care transitions, with the support of an expert mentor. Reporting clinical outcomes was voluntary and uncompensated, and 19 of the hospitals in the initial cohorts did not share their data—cited as a serious limitation by authors of the editorials.
"You can look at our study on a couple of different levels," says lead author and BOOST lead analyst Luke Hansen, MD, MHS, of the division of hospital medicine at Northwestern University Feinberg School of Medicine in Chicago. "One is the sites that gave us data, and in that group there were statistically significant results. But I think the more important question is: Will that be enough? What is the magnitude of the effect, and is it enough to give hospitals the impetus they need to prevent avoidable readmissions?"
Listen to more of our interview with Dr. Luke Hansen, Project BOOST's lead analyst.
Project BOOST is one of SHM's national QI programs aimed at improving care transitions through such strategies as readmission risk assessments, medication reconciliation, patient coaching, and post-discharge follow-up calls. For hospitals and HM groups searching for solutions to the 30-day readmission dilemma—and thereby avoid Medicare reimbursement penalties, clear answers remain elusive.
"BOOST is one of a number of care transitions improvement methodologies that have been applied to the problem of readmissions, each of which has evidence to support their effectiveness in their initial settings, but has proven difficult to translate to other sites," JHM Editor-in-Chief Andrew D. Auerbach, MD, MPH, SFHM, a professor of medicine in residence at the University of California at San Francisco's division of hospital medicine, and co-authors note in an accompanying editorial.
Dr. Auerbach notes in his editorial that research problems limited the study's robustness but that "the authors provide the necessary start down the road towards a fuller understanding of real-world efforts to reduce readmissions."
In the other editorial, Ashish K. Jha, MD, MPH, of the Harvard School of Public Health, Health Policy, and Management suggests that readmissions ultimately may be the wrong target. A better goal, Dr. Jha says, is to improve transitions of care and demonstrate better processes in achieving those results—even though that might not significantly alter readmission rates. "We need to get clearer on what we’re trying to achieve," he adds.
Visit our website for more information on hospital readmission studies.