User login
Rising Medicare Spending for End-of-Life Care Brings Patients’ Wishes into Focus
A new report that shows ever-growing Medicare spending for chronically ill patients in the last two years of life can serve as a reminder for hospitalists to properly gauge patients’ wishes for end-of-life care, one of the authors says.
The brief from the Dartmouth Atlas Project [PDF] shows that from 2007 to 2010, average spending per patient in the last two years of life increased 15.2% to $69,947, and average spending in the last six months of life rose 13.4% to $36,392.
During the same three-year period, patients in their last six months of life were less likely to be hospitalized and logged more time in hospice care—21 days versus 18.3 days—reflecting the wishes of most patients to spend their last days in a homelike environment, the report notes. Accordingly, chronically ill Medicare patients were less likely to die in the hospital by the end of the study period.
David Goodman, MD, MS, co-principal investigator for Dartmouth Atlas of Health Care, says the growing use of hospice care and decreased hospitalization stays “aligns more closely with patients’ preferences.”
“The focus really needs to be on better diagnosis of patients’ preferences to reduce what has been well-documented as overutilization from the patient’s perspective,” Dr. Goodman says.
While costs and trends vary widely among regions and health-care systems, Dr. Goodman attributes the differences to local supplies of hospital beds and practice styles. For example, in regions with more beds, patients are more likely to spend time in the hospital near the end of life, he says. “There is definitely a national trend away from hospital care near the end of life,” he adds. “But that rate of change varies a lot from place to place. It’s helpful for hospitalists to understand where they fit on the spectrum.”
Visit our website for more information on end of life care.
A new report that shows ever-growing Medicare spending for chronically ill patients in the last two years of life can serve as a reminder for hospitalists to properly gauge patients’ wishes for end-of-life care, one of the authors says.
The brief from the Dartmouth Atlas Project [PDF] shows that from 2007 to 2010, average spending per patient in the last two years of life increased 15.2% to $69,947, and average spending in the last six months of life rose 13.4% to $36,392.
During the same three-year period, patients in their last six months of life were less likely to be hospitalized and logged more time in hospice care—21 days versus 18.3 days—reflecting the wishes of most patients to spend their last days in a homelike environment, the report notes. Accordingly, chronically ill Medicare patients were less likely to die in the hospital by the end of the study period.
David Goodman, MD, MS, co-principal investigator for Dartmouth Atlas of Health Care, says the growing use of hospice care and decreased hospitalization stays “aligns more closely with patients’ preferences.”
“The focus really needs to be on better diagnosis of patients’ preferences to reduce what has been well-documented as overutilization from the patient’s perspective,” Dr. Goodman says.
While costs and trends vary widely among regions and health-care systems, Dr. Goodman attributes the differences to local supplies of hospital beds and practice styles. For example, in regions with more beds, patients are more likely to spend time in the hospital near the end of life, he says. “There is definitely a national trend away from hospital care near the end of life,” he adds. “But that rate of change varies a lot from place to place. It’s helpful for hospitalists to understand where they fit on the spectrum.”
Visit our website for more information on end of life care.
A new report that shows ever-growing Medicare spending for chronically ill patients in the last two years of life can serve as a reminder for hospitalists to properly gauge patients’ wishes for end-of-life care, one of the authors says.
The brief from the Dartmouth Atlas Project [PDF] shows that from 2007 to 2010, average spending per patient in the last two years of life increased 15.2% to $69,947, and average spending in the last six months of life rose 13.4% to $36,392.
During the same three-year period, patients in their last six months of life were less likely to be hospitalized and logged more time in hospice care—21 days versus 18.3 days—reflecting the wishes of most patients to spend their last days in a homelike environment, the report notes. Accordingly, chronically ill Medicare patients were less likely to die in the hospital by the end of the study period.
David Goodman, MD, MS, co-principal investigator for Dartmouth Atlas of Health Care, says the growing use of hospice care and decreased hospitalization stays “aligns more closely with patients’ preferences.”
“The focus really needs to be on better diagnosis of patients’ preferences to reduce what has been well-documented as overutilization from the patient’s perspective,” Dr. Goodman says.
While costs and trends vary widely among regions and health-care systems, Dr. Goodman attributes the differences to local supplies of hospital beds and practice styles. For example, in regions with more beds, patients are more likely to spend time in the hospital near the end of life, he says. “There is definitely a national trend away from hospital care near the end of life,” he adds. “But that rate of change varies a lot from place to place. It’s helpful for hospitalists to understand where they fit on the spectrum.”
Visit our website for more information on end of life care.
3e Initiative releases multinational evidence-based gout recommendations
Identification of monosodium urate crystals, either in a joint fluid sample or in a tophi aspirate, should be performed for a definite diagnosis of gout, according to new multinational evidence-based recommendations on the diagnosis and management of the disease.
When identification of monosodium urate (MSU) crystals is not possible, the diagnosis can be supported by classical clinical features such as podagra, tophi, or rapid response to colchicine, or by characteristic imaging findings, Dr. Francisca Sivera of Hospital General Universitario de Elda (Spain) and her colleagues reported on behalf of the 2011 3e (Evidence, Expertise, Exchange) Initiative. The initiative is a multinational collaboration tasked with promoting evidence-based practice in rheumatology through the development of practical recommendations that address relevant clinical issues.
The MSU identification recommendation is one of 10 recommendations developed by 474 rheumatologists from 14 countries who participated in the 2011 3e Initiative. In keeping with 3e protocol, a panel of 78 experts representing the 14 countries developed 10 key clinical questions pertinent to the diagnosis and management of gout, each of which was investigated via extensive literature review. Recommendations for each of the questions were then formulated, debated, and voted on by Initiative participants, and each recommendation was graded based on the level of evidence.
In addition to diagnosis, the gout recommendations address comorbidity screening, acute gout treatment, lifestyle counseling, urate-lowering therapy, flare prophylaxis, the effect of comorbidities on drug selection, patient monitoring, tophi treatment, and management of asymptomatic hyperuricemia (Ann. Rheum. Dis. 2013 July 18 [doi: 10.1136/annrheumdis-2013-203325]).
Specifically, with a high level of agreement that ranged from a mean of 8.1 to 9.2 on a 1-10 scale, the 3e Initiative participants recommended:
• Assessing renal function and cardiovascular risk factors in patients with gout and/or hyperuricemia.
• Treating acute gout with low-dose colchicine, nonsteroidal anti-inflammatory drugs, and/or glucocorticoids, depending on comorbidities and the risk of adverse effects.
• Advising patients about health lifestyle choices.
• Using allopurinol first-line as urate-lowering therapy, and considering uricosurics as alternatives when necessary.
• Educating patients on the risk and management of flares – and using prophylaxis – when introducing urate-lowering therapy.
• Using allopurinol with close monitoring, and starting at a low dose with slow titration, in patients with mild to moderate renal impairment.
• Setting the treatment target for serum urate at below 0.36 mmol/L (6 mg/dL) with eventual absence of gout attacks and resolution of tophi.
• Treating tophi medically by achieving a sustained reduction in serum uric acid, preferably below 0.30 mmol/L (5 mg/dL) and reserving surgery for select cases, such as those involving nerve compression, mechanical impingement, or infection.
• Forgoing pharmacological treatment of asymptomatic hyperuricemia.
The ultimate goal of these recommendations, which were based on 1a-5 evidence levels, and which received recommendation grades ranging from B to D, is to improve patient care, Dr. Sivera said in an interview. The level of evidence and grade of recommendation were made according to the Oxford Centre for Evidence-Based Medicine levels of evidence.
Gout affects up to 2% of men in Western countries, and is associated with morbidity, disability, and poorer quality of life. Despite the availability of a number of guidelines and recommendations, management of the condition is often suboptimal, she said.
"Gout is a curable disease, but evidence shows that many patients are mismanaged with regard to both treatment and diagnosis. Even in a rheumatology clinic, only about a quarter of the patients have a diagnosis of gout established by MSU crystal identification, and in a U.K. study, only one in three patients with a diagnosis of gout were taking urate-lowering therapy," she said.
Research shows that when guidelines are implemented, they improve the quality of care. Educational outreach has an effect on implementation, she said, noting that "both dissemination and education – in both gout and in evidence-based medicine – are an integral part of the 3e Initiative, so these multinational recommendations have the potential to positively influence the standard of care."
The 3e recommendations follow those published in 2012 on behalf of the American College of Rheumatology, which centered on the treatment and prophylaxis of acute gout flares and the appropriate use of urate-lowering therapy.
"Some of the recommendations provided are similar, such as treating to a target serum uric acid level, and the ‘start low, go slow’ approach to allopurinol therapy. This highlights the general consensus on many aspects of the optimal standard of gout management," Dr. Sivera said.
Where the 3e recommendation and the ACR guidelines overlap, there is, indeed, general agreement, Dr. John FitzGerald of the University of California, Los Angeles, said in an interview.
Both processes benefited from Delphi consensus methodologies and systematic literature reviews to inform decision making. However, the two diverge with respect to other aspects of the methodology and presentation, he noted.
"The RAND/UCLA methodology used by ACR resulted in guidelines that were evidence based to be the most efficacious recommendations. As noted, the RAND/UCLA methodology excludes cost of therapy (as typically there are insufficient head-to-head therapeutic cost-efficacy studies on which to base recommendations). The ACR guidelines therefore leave it to the practitioner to use the efficacy-based recommendations, along with their clinical and practical knowledge, to then provide recommendations for specific patients. As an example, allopurinol and febuxostat have relatively similar efficacy but significant cost differences," said Dr. FitzGerald, who co-led the ACR guidelines development project.
"The 3e approach incorporates that next step in decision making to provide evidence-based and practical recommendations to the practitioner," he said.
The ACR effort addressed four specific domains of gout management: treatment of acute gouty attacks, management of urate-lowering therapy, management of chronic tophaceous gout, and prophylaxis of acute gouty attacks. Although the 3e effort focused on 10 specific, clinically relevant questions, it is valuable for other reasons as well, such as the inclusion of diagnosis as part of the recommendations, and the fact that asymptomatic hyperuricemia is addressed, he said, noting that neither of these was addressed by the ACR guidelines.
The 3e recommendations also address the use of benzbromarone, a uricosuric agent that is not available in the United States.
While the 3e effort lacks the extent of detail included in the ACR guidelines, such as the inclusion of specific information on allopurinol dosing, the 3e group is to be commended for the size of the effort, Dr. FitzGerald said, stressing the value of the input from nearly 500 rheumatologists from 14 countries.
Indeed, the extensive effort by "a large group of practicing rheumatologists from many different countries in Europe, South America, and Australasia resulted in the recommendations addressing those aspects [of gout diagnosis and management] that rheumatologists found most clinically relevant," Dr. Sivera said.
She and her colleagues concluded that "the high level of agreement with the final recommendations and the multinational participation increase their utility and will hopefully facilitate their dissemination and implementation worldwide."
The 3e Gout Program was sponsored by AbbVie. Dr. Sivera reported receiving fees from Menarini for preparing educational presentations, and other authors reported receiving lecture or consulting fees and/or research grants from many companies, including AbbVie. Dr. FitzGerald reported receiving honoraria and grant support from the ACR.
Identification of monosodium urate crystals, either in a joint fluid sample or in a tophi aspirate, should be performed for a definite diagnosis of gout, according to new multinational evidence-based recommendations on the diagnosis and management of the disease.
When identification of monosodium urate (MSU) crystals is not possible, the diagnosis can be supported by classical clinical features such as podagra, tophi, or rapid response to colchicine, or by characteristic imaging findings, Dr. Francisca Sivera of Hospital General Universitario de Elda (Spain) and her colleagues reported on behalf of the 2011 3e (Evidence, Expertise, Exchange) Initiative. The initiative is a multinational collaboration tasked with promoting evidence-based practice in rheumatology through the development of practical recommendations that address relevant clinical issues.
The MSU identification recommendation is one of 10 recommendations developed by 474 rheumatologists from 14 countries who participated in the 2011 3e Initiative. In keeping with 3e protocol, a panel of 78 experts representing the 14 countries developed 10 key clinical questions pertinent to the diagnosis and management of gout, each of which was investigated via extensive literature review. Recommendations for each of the questions were then formulated, debated, and voted on by Initiative participants, and each recommendation was graded based on the level of evidence.
In addition to diagnosis, the gout recommendations address comorbidity screening, acute gout treatment, lifestyle counseling, urate-lowering therapy, flare prophylaxis, the effect of comorbidities on drug selection, patient monitoring, tophi treatment, and management of asymptomatic hyperuricemia (Ann. Rheum. Dis. 2013 July 18 [doi: 10.1136/annrheumdis-2013-203325]).
Specifically, with a high level of agreement that ranged from a mean of 8.1 to 9.2 on a 1-10 scale, the 3e Initiative participants recommended:
• Assessing renal function and cardiovascular risk factors in patients with gout and/or hyperuricemia.
• Treating acute gout with low-dose colchicine, nonsteroidal anti-inflammatory drugs, and/or glucocorticoids, depending on comorbidities and the risk of adverse effects.
• Advising patients about health lifestyle choices.
• Using allopurinol first-line as urate-lowering therapy, and considering uricosurics as alternatives when necessary.
• Educating patients on the risk and management of flares – and using prophylaxis – when introducing urate-lowering therapy.
• Using allopurinol with close monitoring, and starting at a low dose with slow titration, in patients with mild to moderate renal impairment.
• Setting the treatment target for serum urate at below 0.36 mmol/L (6 mg/dL) with eventual absence of gout attacks and resolution of tophi.
• Treating tophi medically by achieving a sustained reduction in serum uric acid, preferably below 0.30 mmol/L (5 mg/dL) and reserving surgery for select cases, such as those involving nerve compression, mechanical impingement, or infection.
• Forgoing pharmacological treatment of asymptomatic hyperuricemia.
The ultimate goal of these recommendations, which were based on 1a-5 evidence levels, and which received recommendation grades ranging from B to D, is to improve patient care, Dr. Sivera said in an interview. The level of evidence and grade of recommendation were made according to the Oxford Centre for Evidence-Based Medicine levels of evidence.
Gout affects up to 2% of men in Western countries, and is associated with morbidity, disability, and poorer quality of life. Despite the availability of a number of guidelines and recommendations, management of the condition is often suboptimal, she said.
"Gout is a curable disease, but evidence shows that many patients are mismanaged with regard to both treatment and diagnosis. Even in a rheumatology clinic, only about a quarter of the patients have a diagnosis of gout established by MSU crystal identification, and in a U.K. study, only one in three patients with a diagnosis of gout were taking urate-lowering therapy," she said.
Research shows that when guidelines are implemented, they improve the quality of care. Educational outreach has an effect on implementation, she said, noting that "both dissemination and education – in both gout and in evidence-based medicine – are an integral part of the 3e Initiative, so these multinational recommendations have the potential to positively influence the standard of care."
The 3e recommendations follow those published in 2012 on behalf of the American College of Rheumatology, which centered on the treatment and prophylaxis of acute gout flares and the appropriate use of urate-lowering therapy.
"Some of the recommendations provided are similar, such as treating to a target serum uric acid level, and the ‘start low, go slow’ approach to allopurinol therapy. This highlights the general consensus on many aspects of the optimal standard of gout management," Dr. Sivera said.
Where the 3e recommendation and the ACR guidelines overlap, there is, indeed, general agreement, Dr. John FitzGerald of the University of California, Los Angeles, said in an interview.
Both processes benefited from Delphi consensus methodologies and systematic literature reviews to inform decision making. However, the two diverge with respect to other aspects of the methodology and presentation, he noted.
"The RAND/UCLA methodology used by ACR resulted in guidelines that were evidence based to be the most efficacious recommendations. As noted, the RAND/UCLA methodology excludes cost of therapy (as typically there are insufficient head-to-head therapeutic cost-efficacy studies on which to base recommendations). The ACR guidelines therefore leave it to the practitioner to use the efficacy-based recommendations, along with their clinical and practical knowledge, to then provide recommendations for specific patients. As an example, allopurinol and febuxostat have relatively similar efficacy but significant cost differences," said Dr. FitzGerald, who co-led the ACR guidelines development project.
"The 3e approach incorporates that next step in decision making to provide evidence-based and practical recommendations to the practitioner," he said.
The ACR effort addressed four specific domains of gout management: treatment of acute gouty attacks, management of urate-lowering therapy, management of chronic tophaceous gout, and prophylaxis of acute gouty attacks. Although the 3e effort focused on 10 specific, clinically relevant questions, it is valuable for other reasons as well, such as the inclusion of diagnosis as part of the recommendations, and the fact that asymptomatic hyperuricemia is addressed, he said, noting that neither of these was addressed by the ACR guidelines.
The 3e recommendations also address the use of benzbromarone, a uricosuric agent that is not available in the United States.
While the 3e effort lacks the extent of detail included in the ACR guidelines, such as the inclusion of specific information on allopurinol dosing, the 3e group is to be commended for the size of the effort, Dr. FitzGerald said, stressing the value of the input from nearly 500 rheumatologists from 14 countries.
Indeed, the extensive effort by "a large group of practicing rheumatologists from many different countries in Europe, South America, and Australasia resulted in the recommendations addressing those aspects [of gout diagnosis and management] that rheumatologists found most clinically relevant," Dr. Sivera said.
She and her colleagues concluded that "the high level of agreement with the final recommendations and the multinational participation increase their utility and will hopefully facilitate their dissemination and implementation worldwide."
The 3e Gout Program was sponsored by AbbVie. Dr. Sivera reported receiving fees from Menarini for preparing educational presentations, and other authors reported receiving lecture or consulting fees and/or research grants from many companies, including AbbVie. Dr. FitzGerald reported receiving honoraria and grant support from the ACR.
Identification of monosodium urate crystals, either in a joint fluid sample or in a tophi aspirate, should be performed for a definite diagnosis of gout, according to new multinational evidence-based recommendations on the diagnosis and management of the disease.
When identification of monosodium urate (MSU) crystals is not possible, the diagnosis can be supported by classical clinical features such as podagra, tophi, or rapid response to colchicine, or by characteristic imaging findings, Dr. Francisca Sivera of Hospital General Universitario de Elda (Spain) and her colleagues reported on behalf of the 2011 3e (Evidence, Expertise, Exchange) Initiative. The initiative is a multinational collaboration tasked with promoting evidence-based practice in rheumatology through the development of practical recommendations that address relevant clinical issues.
The MSU identification recommendation is one of 10 recommendations developed by 474 rheumatologists from 14 countries who participated in the 2011 3e Initiative. In keeping with 3e protocol, a panel of 78 experts representing the 14 countries developed 10 key clinical questions pertinent to the diagnosis and management of gout, each of which was investigated via extensive literature review. Recommendations for each of the questions were then formulated, debated, and voted on by Initiative participants, and each recommendation was graded based on the level of evidence.
In addition to diagnosis, the gout recommendations address comorbidity screening, acute gout treatment, lifestyle counseling, urate-lowering therapy, flare prophylaxis, the effect of comorbidities on drug selection, patient monitoring, tophi treatment, and management of asymptomatic hyperuricemia (Ann. Rheum. Dis. 2013 July 18 [doi: 10.1136/annrheumdis-2013-203325]).
Specifically, with a high level of agreement that ranged from a mean of 8.1 to 9.2 on a 1-10 scale, the 3e Initiative participants recommended:
• Assessing renal function and cardiovascular risk factors in patients with gout and/or hyperuricemia.
• Treating acute gout with low-dose colchicine, nonsteroidal anti-inflammatory drugs, and/or glucocorticoids, depending on comorbidities and the risk of adverse effects.
• Advising patients about health lifestyle choices.
• Using allopurinol first-line as urate-lowering therapy, and considering uricosurics as alternatives when necessary.
• Educating patients on the risk and management of flares – and using prophylaxis – when introducing urate-lowering therapy.
• Using allopurinol with close monitoring, and starting at a low dose with slow titration, in patients with mild to moderate renal impairment.
• Setting the treatment target for serum urate at below 0.36 mmol/L (6 mg/dL) with eventual absence of gout attacks and resolution of tophi.
• Treating tophi medically by achieving a sustained reduction in serum uric acid, preferably below 0.30 mmol/L (5 mg/dL) and reserving surgery for select cases, such as those involving nerve compression, mechanical impingement, or infection.
• Forgoing pharmacological treatment of asymptomatic hyperuricemia.
The ultimate goal of these recommendations, which were based on 1a-5 evidence levels, and which received recommendation grades ranging from B to D, is to improve patient care, Dr. Sivera said in an interview. The level of evidence and grade of recommendation were made according to the Oxford Centre for Evidence-Based Medicine levels of evidence.
Gout affects up to 2% of men in Western countries, and is associated with morbidity, disability, and poorer quality of life. Despite the availability of a number of guidelines and recommendations, management of the condition is often suboptimal, she said.
"Gout is a curable disease, but evidence shows that many patients are mismanaged with regard to both treatment and diagnosis. Even in a rheumatology clinic, only about a quarter of the patients have a diagnosis of gout established by MSU crystal identification, and in a U.K. study, only one in three patients with a diagnosis of gout were taking urate-lowering therapy," she said.
Research shows that when guidelines are implemented, they improve the quality of care. Educational outreach has an effect on implementation, she said, noting that "both dissemination and education – in both gout and in evidence-based medicine – are an integral part of the 3e Initiative, so these multinational recommendations have the potential to positively influence the standard of care."
The 3e recommendations follow those published in 2012 on behalf of the American College of Rheumatology, which centered on the treatment and prophylaxis of acute gout flares and the appropriate use of urate-lowering therapy.
"Some of the recommendations provided are similar, such as treating to a target serum uric acid level, and the ‘start low, go slow’ approach to allopurinol therapy. This highlights the general consensus on many aspects of the optimal standard of gout management," Dr. Sivera said.
Where the 3e recommendation and the ACR guidelines overlap, there is, indeed, general agreement, Dr. John FitzGerald of the University of California, Los Angeles, said in an interview.
Both processes benefited from Delphi consensus methodologies and systematic literature reviews to inform decision making. However, the two diverge with respect to other aspects of the methodology and presentation, he noted.
"The RAND/UCLA methodology used by ACR resulted in guidelines that were evidence based to be the most efficacious recommendations. As noted, the RAND/UCLA methodology excludes cost of therapy (as typically there are insufficient head-to-head therapeutic cost-efficacy studies on which to base recommendations). The ACR guidelines therefore leave it to the practitioner to use the efficacy-based recommendations, along with their clinical and practical knowledge, to then provide recommendations for specific patients. As an example, allopurinol and febuxostat have relatively similar efficacy but significant cost differences," said Dr. FitzGerald, who co-led the ACR guidelines development project.
"The 3e approach incorporates that next step in decision making to provide evidence-based and practical recommendations to the practitioner," he said.
The ACR effort addressed four specific domains of gout management: treatment of acute gouty attacks, management of urate-lowering therapy, management of chronic tophaceous gout, and prophylaxis of acute gouty attacks. Although the 3e effort focused on 10 specific, clinically relevant questions, it is valuable for other reasons as well, such as the inclusion of diagnosis as part of the recommendations, and the fact that asymptomatic hyperuricemia is addressed, he said, noting that neither of these was addressed by the ACR guidelines.
The 3e recommendations also address the use of benzbromarone, a uricosuric agent that is not available in the United States.
While the 3e effort lacks the extent of detail included in the ACR guidelines, such as the inclusion of specific information on allopurinol dosing, the 3e group is to be commended for the size of the effort, Dr. FitzGerald said, stressing the value of the input from nearly 500 rheumatologists from 14 countries.
Indeed, the extensive effort by "a large group of practicing rheumatologists from many different countries in Europe, South America, and Australasia resulted in the recommendations addressing those aspects [of gout diagnosis and management] that rheumatologists found most clinically relevant," Dr. Sivera said.
She and her colleagues concluded that "the high level of agreement with the final recommendations and the multinational participation increase their utility and will hopefully facilitate their dissemination and implementation worldwide."
The 3e Gout Program was sponsored by AbbVie. Dr. Sivera reported receiving fees from Menarini for preparing educational presentations, and other authors reported receiving lecture or consulting fees and/or research grants from many companies, including AbbVie. Dr. FitzGerald reported receiving honoraria and grant support from the ACR.
FROM ANNALS OF THE RHEUMATIC DISEASES
EHR Use as a Measure of Care Intensity
Hospitals provide acute inpatient care all day and every day. Nonetheless, a number of studies have shown that weekend care may have lower quality than care delivered on weekdays. [1, 2, 3, 4, 5, 6, 7] Weekend care has been associated with increased mortality among patients suffering from a number of conditions [4, 5, 6, 7, 8, 9, 10] and in a number of clinical settings. [11, 12, 13, 14] Findings of poor outcomes on weekends are not universal, though exceptions occur typically in settings where services and clinical staffing are fairly constant throughout the week, such as intensive care units with on‐site intensivists. [15, 16, 17]
These differences in quality and outcomes suggest that there is a difference in intensity of care over the course of the week. Initiatives to address this important safety issue would be strengthened if a shared metric existed to describe the intensity of care provided at a given time of day or day of the week. However, few measures of global hospital intensity of care exist. The metrics that have been developed are limited by measuring only 1 aspect of care delivery, such as weekend nurse staffing, [18] by requiring extensive chart abstraction, [19] or through reliance on hospital expenses in an older payment model. [20] These measures notably predate contemporary hospital care delivery, which is increasingly dependent on the electronic health record (EHR). To our knowledge, no prior measure of hospital care intensity has been described using the EHR.
Recently, our medical center began an initiative to increase weekend services and staffing to create more uniform availability of care throughout the week. The purpose of this study was to develop a global measure of intensity of care using data derived from the EHR as part of the evaluation of this initiative. [21]
METHODS
We conducted a retrospective analysis of EHR activity for hospital inpatients between January 1, 2011 and December 31, 2011 at New York University Langone Medical Center (NYULMC). During that time period, nearly all inpatient clinical activities at NYULMC were performed and documented through the EHR, Sunrise Clinical Manager (Allscripts, Chicago, IL). These activities include clinical documentation, orders, medication administration, and results of diagnostic tests. Access to the EHR is through Citrix Xenapp Servers (Citrix Systems, Santa Clara, CA), which securely deliver applications from central servers to providers' local terminals.
The primary measure of EHR activity, which we refer to as EHR interactions, was defined as the accessing of a patient's electronic record by a clinician. A provider initiates an interaction by opening a patient's electronic record and concludes it by either opening the record of a different patient or logging out of the EHR. An EHR interaction thus captures a unit of direct patient care, such as documenting a clinical encounter, recording medication administration, or reviewing patient data. Most EHR systems routinely log such interactions to enable compliance departments to audit which users have accessed a patient chart.
The secondary measure of EHR activity was percent central processing unit (CPU), which represents the percent of total available server processing power being used by the Citrix servers at a given time. CPU utilization was averaged over an hour to determine the mean percentage of use for any given hour. As CPU data are not retained for more than 30 days at our institution, we examined CPU usage for the period July 1, 2012 to August 31, 2012.
We evaluated subgroups of EHR interactions by provider type: nurses, resident physicians, attending physicians, pharmacy staff, and all others. We also examined EHR interactions for 2 subgroups of patients: those admitted to the medicine service from the emergency room (ER) and those electively admitted to the surgical service. An elective hospitalization was defined as 1 in which the patient was neither admitted from the ER nor transferred from another hospital. These 2 subgroups were further refined to evaluate EHR interactions among groups of patients admitted during the day, night, weekday, and weeknight. Finally, we examined specific EHR orders that we considered reflective of advancing or altering care, including orders to increase the level of mobilization, to insert or remove a urinary catheter, or to initiate or discontinue antibiotics. As antibiotics are frequently initiated on the day of admission and stopped on the day of discharge, we excluded antibiotic orders that occurred on days of admission or discharge.
Statistical Analysis
EHR interactions were presented as hourly rates by dividing the number of patient chart accessions by the inpatient census for each hour. Hourly census was determined by summing the number of patients who were admitted prior to and discharged after the hour of interest. We calculated the arithmetic means of EHR interactions for each hour and day of the week in 2011. Analysis of variance was used to test differences in EHR interactions among days of the week, and t tests were used to test differences in EHR interactions between Saturday and Sunday, Tuesday and Wednesday, and weekend and weekdays. As a result of multiple comparisons in these analyses, we applied a Bonferroni correction.
EHR interaction rates were assigned to 1 of 3 periods based on a priori suspected peak and trough intensity: day (9:00 am to 4:59 pm ), morning/evening (7:00 am to 8:59 am and 5:00 pm to 7:59 pm ), and night (8:00 pm to 6:59 am ). Negative binomial regression models were used to determine the relative rate of weekday to weekend EHR interactions per patient for the 3 daily time periods. Similar models were developed for EHR interactions by provider types and for specific orders.
We calculated the correlation coefficient between total EHR interactions and occurrence of specific orders, EHR interactions by provider type, EHR interactions by patient subgroups, and CPU. A sensitivity analysis was performed in which EHR interactions were calculated as the number of patient chart accessions per number of daily discharges; this analysis considered the fact that discharges were likely to have high associated intensity but may be less common on weekends as compared to weekdays.
All analyses were performed using Stata 12 (StataCorp, College Station, TX). This study was approved by the institutional review board at NYU School of Medicine.
RESULTS
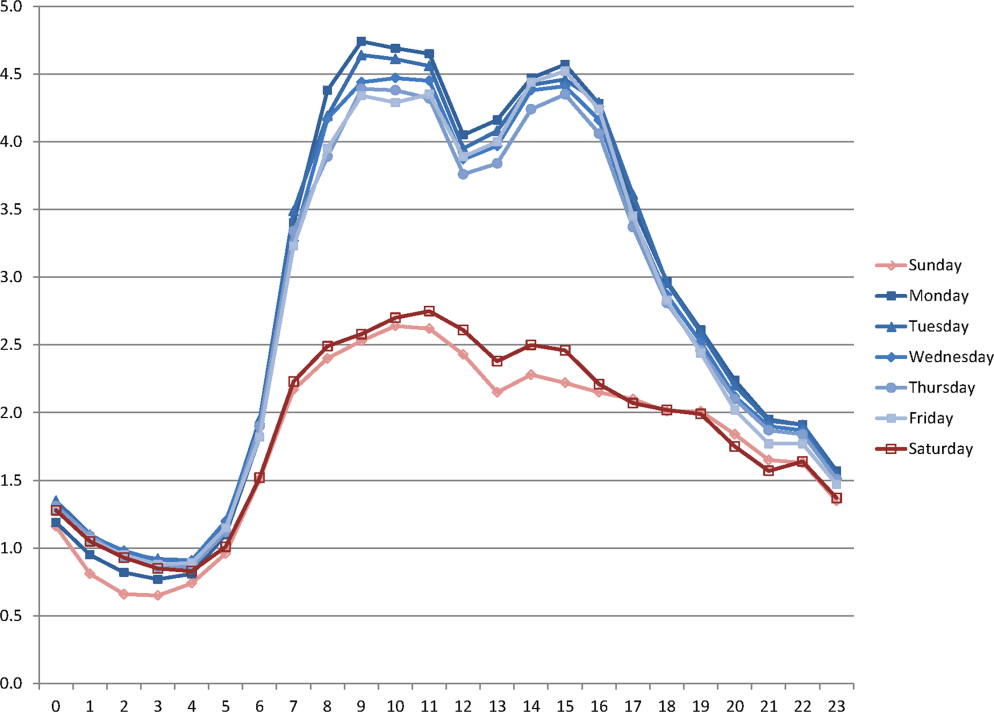
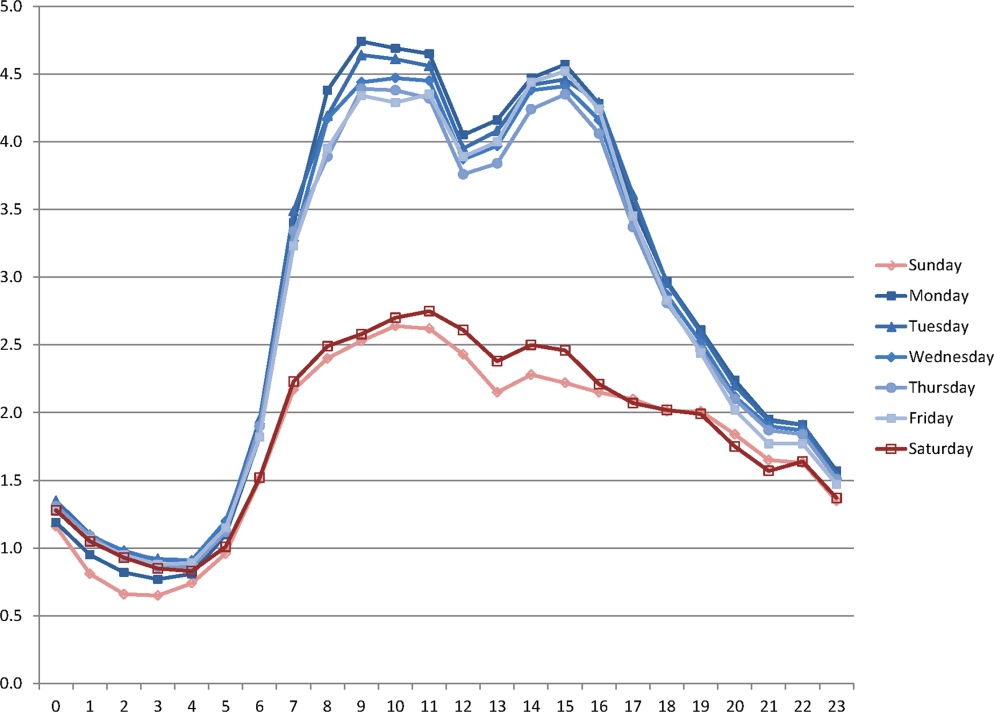
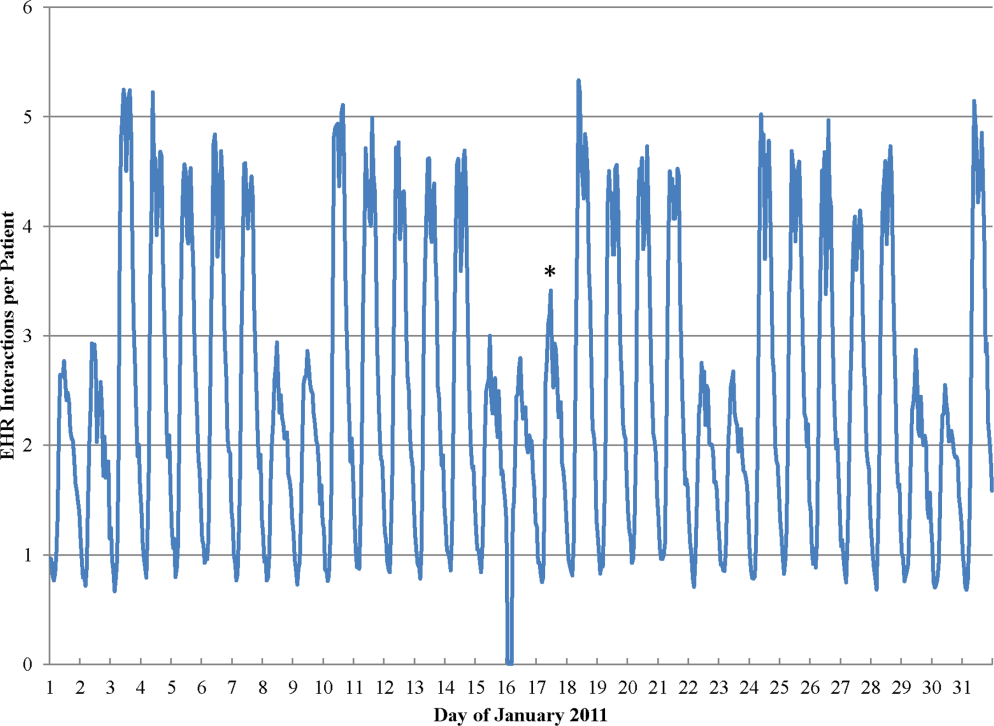
During the study period, the mean (standard deviation) number of EHR interactions per patient per hour was 2.49 (1.30). EHR interactions differed by hour and day of the week; the lowest number occurred in early morning on weekends, and the highest occurred around 11:00 am on weekdays (Figure 1 ). EHR interactions differed among days of the week at all times ( P <0.001), whereas EHR interactions were similar on most hours of Saturday and Sunday as well as Tuesday and Wednesday. At every hour, weekends had a lower number of EHR interactions in comparison to weekdays ( P <0.001). Weekdays showed a substantial increase in the number of EHR interactions between 8:00 am and 12:00 pm , followed by a slight decrease in activity between 12:00 pm and 2:00 pm , and another increase in activity from 2:00 pm to 5:00 pm (Figure 1 ). Weekend days demonstrated marked blunting of this midday peak in intensity. The tracking of an entire month of hospital EHR interactions produced a detailed graphic picture of hospital activity, clearly demarcating rounding hours, lunch hours, weekend days, hospital holidays, and other landmarks (Figure 2 ). The relative rates of census‐adjusted EHR interactions on weekdays versus weekends were 1.76 (95% confidence interval [CI]: 1.74‐1.77) for day, 1.52 (95% CI: 1.50‐1.55) for morning/evening, and 1.14 (95% CI: 1.12‐1.17) for night hours.
Nurses performed the largest number of EHR interactions (39.7%), followed by resident physicians (15.2%), attending physicians (10.2%), and pharmacists (7.6%). The remainder of EHR interactions were performed by other providers (27.4%), whose role in the majority of cases was undefined in the EHR (see Supporting Table 1 in the online version of this article). Daily variation in EHR interactions differed by provider type (Table 1 ). Nurses and resident physicians showed smaller differences in their EHR interactions between weekend and weekdays and among times of day when compared to attending physicians, pharmacy staff, and other staff. EHR interactions showed similar variations to the overall cohort for both medicine patients admitted from the ER and elective surgery patients (Table 1 ). Specific clinical orders designed to sample particularly meaningful interactions, including urinary catheter insertion and removal, patient mobilization orders, and new antibiotic orders, were moderately correlated with total EHR interactions (Table 2 ). In a sensitivity analysis, the comparison of weekday to weekend EHR interactions per daily discharge was similar to the primary analysis, with relative rates of 1.75 (95% CI: 1.73‐1.77), 1.54 (95% CI: 1.50‐1.57), and 1.15 (95% CI: 1.11‐1.18) for day, morning/evening, and night hours, respectively.
| Hour Group a | |||||||
|---|---|---|---|---|---|---|---|
| Daytime Hours | Morning/Evening Hours | Nighttime Hours | Correlation Coefficient to | ||||
| Rate Ratio | 95% CI | Rate Ratio | 95% CI | Rate Ratio | 95% CI | EHR Interactions | |
| |||||||
| All EHR interactions | 1.76 | 1.74‐1.77 | 1.52 | 1.50‐1.55 | 1.14 | 1.12‐1.17 | 1.00 |
| By provider subgroup | |||||||
| Nurses | 1.35 | 1.34‐1.37 | 1.21 | 1.20‐1.22 | 1.10 | 1.08‐1.12 | 0.92 |
| Residents | 1.38 | 1.36‐1.40 | 1.46 | 1.43‐1.49 | 1.14 | 1.11‐1.18 | 0.90 |
| Attending physicians | 1.70 | 1.67‐1.73 | 2.04 | 1.97‐2.10 | 1.32 | 1.26‐1.39 | 0.96 |
| Pharmacy staff | 1.64 | 1.61‐1.67 | 1.68 | 1.61‐1.75 | 1.19 | 1.15‐1.23 | 0.90 |
| All others | 2.75 | 2.70‐2.79 | 2.08 | 2.002.17 | 1.20 | 1.15‐1.25 | 0.97 |
| By admission subgroup | |||||||
| Medicine ER admissions | |||||||
| All | 1.64 | 1.62‐1.67 | 1.41 | 1.38‐1.44 | 1.07 | 1.04‐1.09 | 0.92 |
| Day admissions | 1.64 | 1.62‐1.67 | 1.41 | 1.38‐1.44 | 1.08 | 1.05‐1.11 | 0.92 |
| Night admissions | 1.65 | 1.62‐1.68 | 1.41 | 1.37‐1.45 | 1.03 | 1.001.05 | 0.88 |
| Weekday admissions | 1.76 | 1.73‐1.79 | 1.56 | 1.52‐1.59 | 1.28 | 1.23‐1.30 | 0.93 |
| Weekend admissions | 1.40 | 1.38‐1.42 | 1.14 | 1.11‐1.17 | 0.70 | 0.68‐0.72 | 0.88 |
| Surgery elective admissions | |||||||
| All | 1.63 | 1.61‐1.65 | 1.53 | 1.50‐1.56 | 1.25 | 1.21‐1.28 | 0.97 |
| Day admissions | 1.62 | 1.60‐1.64 | 1.54 | 1.51‐1.57 | 1.26 | 1.23‐1.30 | 0.96 |
| Night admissions | 1.65 | 1.62‐1.67 | 1.50 | 1.46‐1.54 | 1.22 | 1.18‐1.25 | 0.94 |
| Weekday admissions | 1.64 | 1.62‐1.66 | 1.55 | 1.52‐1.59 | 1.27 | 1.24‐1.31 | 0.96 |
| Weekend admissions | 1.60 | 1.56‐1.65 | 1.26 | 1.21‐1.31 | 0.89 | 0.85‐0.93 | 0.73 |
| Hour Group a | |||||||
|---|---|---|---|---|---|---|---|
| Daytime Hours | Morning/Evening Hours | Nighttime Hours | Correlation Coefficient to | ||||
| Rate Ratio | 95% CI | Rate Ratio | 95% CI | Rate Ratio | 95% CI | EHR Interactions | |
| |||||||
| Urinary catheter | 2.81 | 2.61‐3.02 | 3.17 | 2.85‐3.54 | 1.42 | 1.27‐1.58 | 0.66 |
| Mobilization | 2.51 | 2.39‐2.63 | 2.91 | 2.71‐3.12 | 1.42 | 1.34‐1.52 | 0.70 |
| Antibiotic initiation b | 1.33 | 1.24‐1.42 | 1.65 | 1.47‐1.85 | 1.13 | 1.01‐1.25 | 0.41 |
| Antibiotic discontinue c | 1.74 | 1.63‐1.85 | 1.68 | 1.50‐1.87 | 1.28 | 1.13‐1.45 | 0.61 |
As seen in Figure 3 , CPU usage was well correlated with EHR interactions ( r =0.90) and both metrics were lower on weekends as compared to weekdays. A few outliers of increased CPU usage on weekends were observed; these outliers all occurred on Sundays from 12:00 am to 2:00 am . The information technology (IT) department at our institution confirmed that these outliers coincided with a weekly virus scan performed at that time.
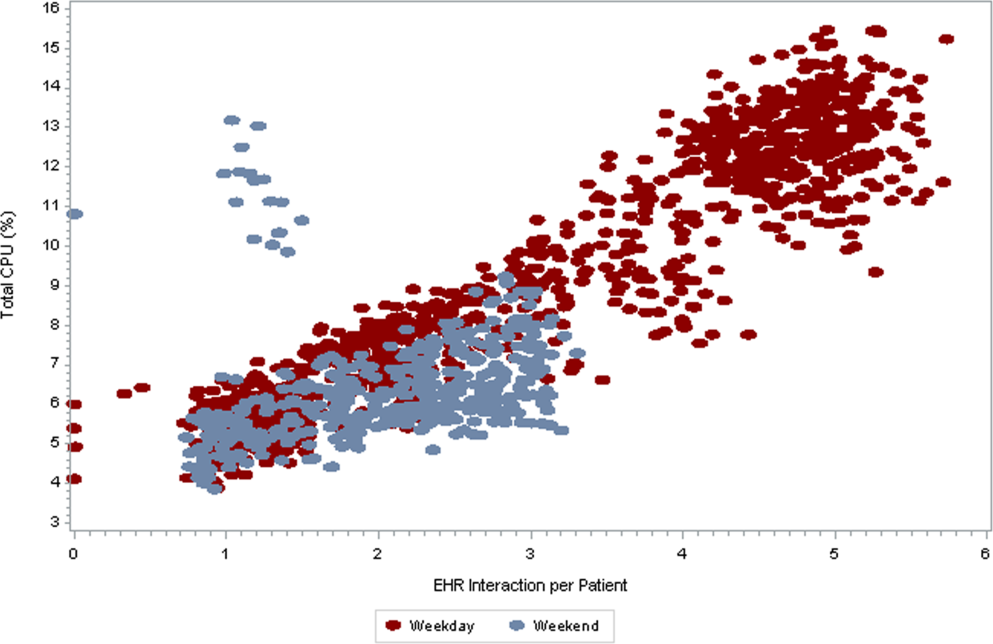
am
and 1:00
am
, which were determined to be outliers related to a weekly virus scan. The correlation coefficient is 0.90.
DISCUSSION
EHR interactions represents a new and accessible measure of hospital care intensity that may be used to track temporal variations in care that are likely to exist in all hospital systems. As the number of hospitals that process and document all clinical activities through the EHR continues to increase, [22, 23] such a measure has the potential to serve as a useful metric in efforts to raise nighttime and weekend care to the same quality as that during weekdays.
We found that EHR interactions differed markedly between days and nights and between weekdays and weekends. A number of prior studies have found temporal variations in clinical outcomes and care. [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14] Our study adds to this literature by defining a new measure of the overall intensity of the process of inpatient care. Using this measure, we demonstrate that the increase in midday clinical activity seen during the week is substantially blunted on weekends. This finding adds validity to the designation of both nights and weekends as off hours when examining temporal variations in clinical care. [2, 7]
Other subtle patterns of care delivery emerged throughout the day. For instance, we found a decrease in EHR interactions between 12:00 pm and 2:00 pm , likely representing a small lull in clinical activity during the lunchtime hour, whereas EHR interactions were higher in the night and evening hours than in the early morning. The clinical significance of such fluctuations remains undetermined.
Variation throughout the week differed by provider type. Nurses and resident physicians, who are scheduled around the clock, demonstrated the least fluctuation in EHR interaction intensity over time. Attending physicians and other staff showed the greatest variation in weekday versus weekend care.
We defined a second measure of care intensity based on EHR utilization, which we named CPU usage. The advantage of using CPU as a metric is that it easy to measure and track as it is routinely monitored by hospital IT departments as part of regular maintenance. CPU usage can therefore be easily implemented in any institution with an EHR, whereas measuring EHR interactions requires EHR data abstraction and manipulation. The disadvantage of CPU usage as a measure is that it includes nonclinical reporting and other functions and therefore represents a less specific measure of clinical activity. Nonetheless, at our institution, nearly all clinical activities are processed or documented through the EHR, which is maintained on central servers, and CPU usage was well correlated with EHR interactions. We therefore believe that CPU usage represents a useful indicator of hospital clinical activity at a given time.
Several limitations deserve mention. First, EHR interactions may not always reflect actual patient care or clinical documentation. Nonetheless, we also examined specific patient orders and found that variations in these process measures were similar to those in the EHR interaction measure. Second, our methods were developed and evaluated at a single institution and may not be generalizable. Third, increased intensity of care does not necessarily increase quality of care. For instance, laboratory tests are commonly obtained on a daily basis in the hospital, a practice that is costly and often unnecessary. [24] Weekends have been associated with fewer laboratory tests, which may be appropriate as compared to the more frequent testing observed on weekdays. [25] Finally, we do not ascertain whether variations in EHR interactions correlate with clinical outcomes, and believe this to be an area for further research.
In conclusion, EHR interactions represent a new global process measure of care intensity, which was demonstrated to vary over the course of a week. We intend to use this measure at our institution to track progress of an initiative to ensure a high standard of care throughout the course of the week. The extent to which temporal variations in EHR interactions or reductions in these variations are correlated with clinical outcomes deserves further study. We believe this measure, which can be adapted to other institutions, may have a valuable role to play in hospitals' efforts to eliminate excess morbidity and mortality associated with care delivered during nights and weekends.
- , , , et al. Impact of weekend admissions on quality of care and outcomes in patients with acute myeloid leukemia . Cancer. 2010 ; 116 : 3614 – 3620 .
- , , , et al. Impact of time of presentation on the care and outcomes of acute myocardial infarction . Circulation. 2008 ; 117 : 2502 – 2509 .
- , , , et al. Weekend hospital admission and discharge for heart failure: association with quality of care and clinical outcomes . Am Heart J. 2009 ; 158 : 451 – 458 .
- , , , . Hospital care for patients experiencing weekend vs weekday stroke: a comparison of quality and aggressiveness of care . Arch Neurol. 2010 ; 67 : 39 – 44 .
- , , , , , . Weekend versus weekday admission and mortality from myocardial infarction . N Engl J Med. 2007 ; 356 : 1099 – 1109 .
- . Do hospitals provide lower quality care on weekends? Health Serv Res. 2007 ; 42 : 1589 – 1612 .
- , , , et al. Relationship between time of day, day of week, timeliness of reperfusion, and in‐hospital mortality for patients with acute ST‐segment elevation myocardial infarction . JAMA. 2005 ; 294 : 803 – 812 .
- , , , , , . Weekend versus weekday admission and mortality after acute pulmonary embolism . Circulation. 2009 ; 119 : 962 – 968 .
- , , , , , . Influence of weekend hospital admission on short‐term mortality after intracerebral hemorrhage . Stroke. 2009 ; 40 : 2387 – 2392 .
- , , , et al. Weekend hospital admission, acute kidney injury, and mortality . J Am Soc Nephrol. 2010 ; 21 : 845 – 851 .
- , . Mortality among patients admitted to hospitals on weekends as compared with weekdays . N Engl J Med. 2001 ; 345 : 663 – 668 .
- , , , et al. Mortality after noncardiac surgery: prediction from administrative versus clinical data . Med Care. 2005 ; 43 : 159 – 167 .
- , . Weekend birth and higher neonatal mortality: a problem of patient acuity or quality of care? J Obstet Gynecol Neonatal Nurs. 2003 ; 32 : 724 – 733 .
- , , , , , . Analysis of the mortality of patients admitted to internal medicine wards over the weekend . Am J Med Qual. 2010 ; 25 : 312 – 318 .
- , , , . Do weekends or evenings matter in a pediatric intensive care unit? Pediatr Crit Care Med. 2005 ; 6 : 523 – 530 .
- , , , , . Hospital mortality associated with day and time of admission to intensive care units . Intensive Care Medicine. 2004 ; 30 : 895 – 901 .
- , , . Weekend and weeknight admissions have the same outcome of weekday admissions to an intensive care unit with onsite intensivist coverage . Crit Care Med. 2006 ; 34 : 605 – 611 .
- , , , , . Measuring the intensity of nursing care: making use of the Belgian Nursing Minimum Data Set . Int J Nurs Stud. 2008 ; 45 : 1011 – 1021 .
- , , , , . The relationship between intensity and duration of medical services and outcomes for hospitalized patients . Med Care. 1979 ; 17 : 1088 – 1102 .
- . Differences in length of hospital stay for Medicaid and Blue Cross patients and the effect of intensity of services . Public Health Rep. 1979 ; 94 : 438 – 445 .
- . Health care's lost weekend. New York Times . October 3, 2010 . Section A, p. 27 .
- , , , . A progress report on electronic health records in U.S. hospitals . Health Aff (Millwood). 2010 ; 29 : 1951 – 1957 .
- US Department of Health 146 : 524 – 527 .
- , , . Temporal approach to hematological test usage in a major teaching hospital . Lab Hematol. 2003 ; 9 : 207 – 213 .
Hospitals provide acute inpatient care all day and every day. Nonetheless, a number of studies have shown that weekend care may have lower quality than care delivered on weekdays. [1, 2, 3, 4, 5, 6, 7] Weekend care has been associated with increased mortality among patients suffering from a number of conditions [4, 5, 6, 7, 8, 9, 10] and in a number of clinical settings. [11, 12, 13, 14] Findings of poor outcomes on weekends are not universal, though exceptions occur typically in settings where services and clinical staffing are fairly constant throughout the week, such as intensive care units with on‐site intensivists. [15, 16, 17]
These differences in quality and outcomes suggest that there is a difference in intensity of care over the course of the week. Initiatives to address this important safety issue would be strengthened if a shared metric existed to describe the intensity of care provided at a given time of day or day of the week. However, few measures of global hospital intensity of care exist. The metrics that have been developed are limited by measuring only 1 aspect of care delivery, such as weekend nurse staffing, [18] by requiring extensive chart abstraction, [19] or through reliance on hospital expenses in an older payment model. [20] These measures notably predate contemporary hospital care delivery, which is increasingly dependent on the electronic health record (EHR). To our knowledge, no prior measure of hospital care intensity has been described using the EHR.
Recently, our medical center began an initiative to increase weekend services and staffing to create more uniform availability of care throughout the week. The purpose of this study was to develop a global measure of intensity of care using data derived from the EHR as part of the evaluation of this initiative. [21]
METHODS
We conducted a retrospective analysis of EHR activity for hospital inpatients between January 1, 2011 and December 31, 2011 at New York University Langone Medical Center (NYULMC). During that time period, nearly all inpatient clinical activities at NYULMC were performed and documented through the EHR, Sunrise Clinical Manager (Allscripts, Chicago, IL). These activities include clinical documentation, orders, medication administration, and results of diagnostic tests. Access to the EHR is through Citrix Xenapp Servers (Citrix Systems, Santa Clara, CA), which securely deliver applications from central servers to providers' local terminals.
The primary measure of EHR activity, which we refer to as EHR interactions, was defined as the accessing of a patient's electronic record by a clinician. A provider initiates an interaction by opening a patient's electronic record and concludes it by either opening the record of a different patient or logging out of the EHR. An EHR interaction thus captures a unit of direct patient care, such as documenting a clinical encounter, recording medication administration, or reviewing patient data. Most EHR systems routinely log such interactions to enable compliance departments to audit which users have accessed a patient chart.
The secondary measure of EHR activity was percent central processing unit (CPU), which represents the percent of total available server processing power being used by the Citrix servers at a given time. CPU utilization was averaged over an hour to determine the mean percentage of use for any given hour. As CPU data are not retained for more than 30 days at our institution, we examined CPU usage for the period July 1, 2012 to August 31, 2012.
We evaluated subgroups of EHR interactions by provider type: nurses, resident physicians, attending physicians, pharmacy staff, and all others. We also examined EHR interactions for 2 subgroups of patients: those admitted to the medicine service from the emergency room (ER) and those electively admitted to the surgical service. An elective hospitalization was defined as 1 in which the patient was neither admitted from the ER nor transferred from another hospital. These 2 subgroups were further refined to evaluate EHR interactions among groups of patients admitted during the day, night, weekday, and weeknight. Finally, we examined specific EHR orders that we considered reflective of advancing or altering care, including orders to increase the level of mobilization, to insert or remove a urinary catheter, or to initiate or discontinue antibiotics. As antibiotics are frequently initiated on the day of admission and stopped on the day of discharge, we excluded antibiotic orders that occurred on days of admission or discharge.
Statistical Analysis
EHR interactions were presented as hourly rates by dividing the number of patient chart accessions by the inpatient census for each hour. Hourly census was determined by summing the number of patients who were admitted prior to and discharged after the hour of interest. We calculated the arithmetic means of EHR interactions for each hour and day of the week in 2011. Analysis of variance was used to test differences in EHR interactions among days of the week, and t tests were used to test differences in EHR interactions between Saturday and Sunday, Tuesday and Wednesday, and weekend and weekdays. As a result of multiple comparisons in these analyses, we applied a Bonferroni correction.
EHR interaction rates were assigned to 1 of 3 periods based on a priori suspected peak and trough intensity: day (9:00 am to 4:59 pm ), morning/evening (7:00 am to 8:59 am and 5:00 pm to 7:59 pm ), and night (8:00 pm to 6:59 am ). Negative binomial regression models were used to determine the relative rate of weekday to weekend EHR interactions per patient for the 3 daily time periods. Similar models were developed for EHR interactions by provider types and for specific orders.
We calculated the correlation coefficient between total EHR interactions and occurrence of specific orders, EHR interactions by provider type, EHR interactions by patient subgroups, and CPU. A sensitivity analysis was performed in which EHR interactions were calculated as the number of patient chart accessions per number of daily discharges; this analysis considered the fact that discharges were likely to have high associated intensity but may be less common on weekends as compared to weekdays.
All analyses were performed using Stata 12 (StataCorp, College Station, TX). This study was approved by the institutional review board at NYU School of Medicine.
RESULTS
During the study period, the mean (standard deviation) number of EHR interactions per patient per hour was 2.49 (1.30). EHR interactions differed by hour and day of the week; the lowest number occurred in early morning on weekends, and the highest occurred around 11:00 am on weekdays (Figure 1 ). EHR interactions differed among days of the week at all times ( P <0.001), whereas EHR interactions were similar on most hours of Saturday and Sunday as well as Tuesday and Wednesday. At every hour, weekends had a lower number of EHR interactions in comparison to weekdays ( P <0.001). Weekdays showed a substantial increase in the number of EHR interactions between 8:00 am and 12:00 pm , followed by a slight decrease in activity between 12:00 pm and 2:00 pm , and another increase in activity from 2:00 pm to 5:00 pm (Figure 1 ). Weekend days demonstrated marked blunting of this midday peak in intensity. The tracking of an entire month of hospital EHR interactions produced a detailed graphic picture of hospital activity, clearly demarcating rounding hours, lunch hours, weekend days, hospital holidays, and other landmarks (Figure 2 ). The relative rates of census‐adjusted EHR interactions on weekdays versus weekends were 1.76 (95% confidence interval [CI]: 1.74‐1.77) for day, 1.52 (95% CI: 1.50‐1.55) for morning/evening, and 1.14 (95% CI: 1.12‐1.17) for night hours.


Nurses performed the largest number of EHR interactions (39.7%), followed by resident physicians (15.2%), attending physicians (10.2%), and pharmacists (7.6%). The remainder of EHR interactions were performed by other providers (27.4%), whose role in the majority of cases was undefined in the EHR (see Supporting Table 1 in the online version of this article). Daily variation in EHR interactions differed by provider type (Table 1 ). Nurses and resident physicians showed smaller differences in their EHR interactions between weekend and weekdays and among times of day when compared to attending physicians, pharmacy staff, and other staff. EHR interactions showed similar variations to the overall cohort for both medicine patients admitted from the ER and elective surgery patients (Table 1 ). Specific clinical orders designed to sample particularly meaningful interactions, including urinary catheter insertion and removal, patient mobilization orders, and new antibiotic orders, were moderately correlated with total EHR interactions (Table 2 ). In a sensitivity analysis, the comparison of weekday to weekend EHR interactions per daily discharge was similar to the primary analysis, with relative rates of 1.75 (95% CI: 1.73‐1.77), 1.54 (95% CI: 1.50‐1.57), and 1.15 (95% CI: 1.11‐1.18) for day, morning/evening, and night hours, respectively.
| Hour Group a | |||||||
|---|---|---|---|---|---|---|---|
| Daytime Hours | Morning/Evening Hours | Nighttime Hours | Correlation Coefficient to | ||||
| Rate Ratio | 95% CI | Rate Ratio | 95% CI | Rate Ratio | 95% CI | EHR Interactions | |
| |||||||
| All EHR interactions | 1.76 | 1.74‐1.77 | 1.52 | 1.50‐1.55 | 1.14 | 1.12‐1.17 | 1.00 |
| By provider subgroup | |||||||
| Nurses | 1.35 | 1.34‐1.37 | 1.21 | 1.20‐1.22 | 1.10 | 1.08‐1.12 | 0.92 |
| Residents | 1.38 | 1.36‐1.40 | 1.46 | 1.43‐1.49 | 1.14 | 1.11‐1.18 | 0.90 |
| Attending physicians | 1.70 | 1.67‐1.73 | 2.04 | 1.97‐2.10 | 1.32 | 1.26‐1.39 | 0.96 |
| Pharmacy staff | 1.64 | 1.61‐1.67 | 1.68 | 1.61‐1.75 | 1.19 | 1.15‐1.23 | 0.90 |
| All others | 2.75 | 2.70‐2.79 | 2.08 | 2.002.17 | 1.20 | 1.15‐1.25 | 0.97 |
| By admission subgroup | |||||||
| Medicine ER admissions | |||||||
| All | 1.64 | 1.62‐1.67 | 1.41 | 1.38‐1.44 | 1.07 | 1.04‐1.09 | 0.92 |
| Day admissions | 1.64 | 1.62‐1.67 | 1.41 | 1.38‐1.44 | 1.08 | 1.05‐1.11 | 0.92 |
| Night admissions | 1.65 | 1.62‐1.68 | 1.41 | 1.37‐1.45 | 1.03 | 1.001.05 | 0.88 |
| Weekday admissions | 1.76 | 1.73‐1.79 | 1.56 | 1.52‐1.59 | 1.28 | 1.23‐1.30 | 0.93 |
| Weekend admissions | 1.40 | 1.38‐1.42 | 1.14 | 1.11‐1.17 | 0.70 | 0.68‐0.72 | 0.88 |
| Surgery elective admissions | |||||||
| All | 1.63 | 1.61‐1.65 | 1.53 | 1.50‐1.56 | 1.25 | 1.21‐1.28 | 0.97 |
| Day admissions | 1.62 | 1.60‐1.64 | 1.54 | 1.51‐1.57 | 1.26 | 1.23‐1.30 | 0.96 |
| Night admissions | 1.65 | 1.62‐1.67 | 1.50 | 1.46‐1.54 | 1.22 | 1.18‐1.25 | 0.94 |
| Weekday admissions | 1.64 | 1.62‐1.66 | 1.55 | 1.52‐1.59 | 1.27 | 1.24‐1.31 | 0.96 |
| Weekend admissions | 1.60 | 1.56‐1.65 | 1.26 | 1.21‐1.31 | 0.89 | 0.85‐0.93 | 0.73 |
| Hour Group a | |||||||
|---|---|---|---|---|---|---|---|
| Daytime Hours | Morning/Evening Hours | Nighttime Hours | Correlation Coefficient to | ||||
| Rate Ratio | 95% CI | Rate Ratio | 95% CI | Rate Ratio | 95% CI | EHR Interactions | |
| |||||||
| Urinary catheter | 2.81 | 2.61‐3.02 | 3.17 | 2.85‐3.54 | 1.42 | 1.27‐1.58 | 0.66 |
| Mobilization | 2.51 | 2.39‐2.63 | 2.91 | 2.71‐3.12 | 1.42 | 1.34‐1.52 | 0.70 |
| Antibiotic initiation b | 1.33 | 1.24‐1.42 | 1.65 | 1.47‐1.85 | 1.13 | 1.01‐1.25 | 0.41 |
| Antibiotic discontinue c | 1.74 | 1.63‐1.85 | 1.68 | 1.50‐1.87 | 1.28 | 1.13‐1.45 | 0.61 |
As seen in Figure 3 , CPU usage was well correlated with EHR interactions ( r =0.90) and both metrics were lower on weekends as compared to weekdays. A few outliers of increased CPU usage on weekends were observed; these outliers all occurred on Sundays from 12:00 am to 2:00 am . The information technology (IT) department at our institution confirmed that these outliers coincided with a weekly virus scan performed at that time.

am
and 1:00
am
, which were determined to be outliers related to a weekly virus scan. The correlation coefficient is 0.90.
DISCUSSION
EHR interactions represents a new and accessible measure of hospital care intensity that may be used to track temporal variations in care that are likely to exist in all hospital systems. As the number of hospitals that process and document all clinical activities through the EHR continues to increase, [22, 23] such a measure has the potential to serve as a useful metric in efforts to raise nighttime and weekend care to the same quality as that during weekdays.
We found that EHR interactions differed markedly between days and nights and between weekdays and weekends. A number of prior studies have found temporal variations in clinical outcomes and care. [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14] Our study adds to this literature by defining a new measure of the overall intensity of the process of inpatient care. Using this measure, we demonstrate that the increase in midday clinical activity seen during the week is substantially blunted on weekends. This finding adds validity to the designation of both nights and weekends as off hours when examining temporal variations in clinical care. [2, 7]
Other subtle patterns of care delivery emerged throughout the day. For instance, we found a decrease in EHR interactions between 12:00 pm and 2:00 pm , likely representing a small lull in clinical activity during the lunchtime hour, whereas EHR interactions were higher in the night and evening hours than in the early morning. The clinical significance of such fluctuations remains undetermined.
Variation throughout the week differed by provider type. Nurses and resident physicians, who are scheduled around the clock, demonstrated the least fluctuation in EHR interaction intensity over time. Attending physicians and other staff showed the greatest variation in weekday versus weekend care.
We defined a second measure of care intensity based on EHR utilization, which we named CPU usage. The advantage of using CPU as a metric is that it easy to measure and track as it is routinely monitored by hospital IT departments as part of regular maintenance. CPU usage can therefore be easily implemented in any institution with an EHR, whereas measuring EHR interactions requires EHR data abstraction and manipulation. The disadvantage of CPU usage as a measure is that it includes nonclinical reporting and other functions and therefore represents a less specific measure of clinical activity. Nonetheless, at our institution, nearly all clinical activities are processed or documented through the EHR, which is maintained on central servers, and CPU usage was well correlated with EHR interactions. We therefore believe that CPU usage represents a useful indicator of hospital clinical activity at a given time.
Several limitations deserve mention. First, EHR interactions may not always reflect actual patient care or clinical documentation. Nonetheless, we also examined specific patient orders and found that variations in these process measures were similar to those in the EHR interaction measure. Second, our methods were developed and evaluated at a single institution and may not be generalizable. Third, increased intensity of care does not necessarily increase quality of care. For instance, laboratory tests are commonly obtained on a daily basis in the hospital, a practice that is costly and often unnecessary. [24] Weekends have been associated with fewer laboratory tests, which may be appropriate as compared to the more frequent testing observed on weekdays. [25] Finally, we do not ascertain whether variations in EHR interactions correlate with clinical outcomes, and believe this to be an area for further research.
In conclusion, EHR interactions represent a new global process measure of care intensity, which was demonstrated to vary over the course of a week. We intend to use this measure at our institution to track progress of an initiative to ensure a high standard of care throughout the course of the week. The extent to which temporal variations in EHR interactions or reductions in these variations are correlated with clinical outcomes deserves further study. We believe this measure, which can be adapted to other institutions, may have a valuable role to play in hospitals' efforts to eliminate excess morbidity and mortality associated with care delivered during nights and weekends.
Hospitals provide acute inpatient care all day and every day. Nonetheless, a number of studies have shown that weekend care may have lower quality than care delivered on weekdays. [1, 2, 3, 4, 5, 6, 7] Weekend care has been associated with increased mortality among patients suffering from a number of conditions [4, 5, 6, 7, 8, 9, 10] and in a number of clinical settings. [11, 12, 13, 14] Findings of poor outcomes on weekends are not universal, though exceptions occur typically in settings where services and clinical staffing are fairly constant throughout the week, such as intensive care units with on‐site intensivists. [15, 16, 17]
These differences in quality and outcomes suggest that there is a difference in intensity of care over the course of the week. Initiatives to address this important safety issue would be strengthened if a shared metric existed to describe the intensity of care provided at a given time of day or day of the week. However, few measures of global hospital intensity of care exist. The metrics that have been developed are limited by measuring only 1 aspect of care delivery, such as weekend nurse staffing, [18] by requiring extensive chart abstraction, [19] or through reliance on hospital expenses in an older payment model. [20] These measures notably predate contemporary hospital care delivery, which is increasingly dependent on the electronic health record (EHR). To our knowledge, no prior measure of hospital care intensity has been described using the EHR.
Recently, our medical center began an initiative to increase weekend services and staffing to create more uniform availability of care throughout the week. The purpose of this study was to develop a global measure of intensity of care using data derived from the EHR as part of the evaluation of this initiative. [21]
METHODS
We conducted a retrospective analysis of EHR activity for hospital inpatients between January 1, 2011 and December 31, 2011 at New York University Langone Medical Center (NYULMC). During that time period, nearly all inpatient clinical activities at NYULMC were performed and documented through the EHR, Sunrise Clinical Manager (Allscripts, Chicago, IL). These activities include clinical documentation, orders, medication administration, and results of diagnostic tests. Access to the EHR is through Citrix Xenapp Servers (Citrix Systems, Santa Clara, CA), which securely deliver applications from central servers to providers' local terminals.
The primary measure of EHR activity, which we refer to as EHR interactions, was defined as the accessing of a patient's electronic record by a clinician. A provider initiates an interaction by opening a patient's electronic record and concludes it by either opening the record of a different patient or logging out of the EHR. An EHR interaction thus captures a unit of direct patient care, such as documenting a clinical encounter, recording medication administration, or reviewing patient data. Most EHR systems routinely log such interactions to enable compliance departments to audit which users have accessed a patient chart.
The secondary measure of EHR activity was percent central processing unit (CPU), which represents the percent of total available server processing power being used by the Citrix servers at a given time. CPU utilization was averaged over an hour to determine the mean percentage of use for any given hour. As CPU data are not retained for more than 30 days at our institution, we examined CPU usage for the period July 1, 2012 to August 31, 2012.
We evaluated subgroups of EHR interactions by provider type: nurses, resident physicians, attending physicians, pharmacy staff, and all others. We also examined EHR interactions for 2 subgroups of patients: those admitted to the medicine service from the emergency room (ER) and those electively admitted to the surgical service. An elective hospitalization was defined as 1 in which the patient was neither admitted from the ER nor transferred from another hospital. These 2 subgroups were further refined to evaluate EHR interactions among groups of patients admitted during the day, night, weekday, and weeknight. Finally, we examined specific EHR orders that we considered reflective of advancing or altering care, including orders to increase the level of mobilization, to insert or remove a urinary catheter, or to initiate or discontinue antibiotics. As antibiotics are frequently initiated on the day of admission and stopped on the day of discharge, we excluded antibiotic orders that occurred on days of admission or discharge.
Statistical Analysis
EHR interactions were presented as hourly rates by dividing the number of patient chart accessions by the inpatient census for each hour. Hourly census was determined by summing the number of patients who were admitted prior to and discharged after the hour of interest. We calculated the arithmetic means of EHR interactions for each hour and day of the week in 2011. Analysis of variance was used to test differences in EHR interactions among days of the week, and t tests were used to test differences in EHR interactions between Saturday and Sunday, Tuesday and Wednesday, and weekend and weekdays. As a result of multiple comparisons in these analyses, we applied a Bonferroni correction.
EHR interaction rates were assigned to 1 of 3 periods based on a priori suspected peak and trough intensity: day (9:00 am to 4:59 pm ), morning/evening (7:00 am to 8:59 am and 5:00 pm to 7:59 pm ), and night (8:00 pm to 6:59 am ). Negative binomial regression models were used to determine the relative rate of weekday to weekend EHR interactions per patient for the 3 daily time periods. Similar models were developed for EHR interactions by provider types and for specific orders.
We calculated the correlation coefficient between total EHR interactions and occurrence of specific orders, EHR interactions by provider type, EHR interactions by patient subgroups, and CPU. A sensitivity analysis was performed in which EHR interactions were calculated as the number of patient chart accessions per number of daily discharges; this analysis considered the fact that discharges were likely to have high associated intensity but may be less common on weekends as compared to weekdays.
All analyses were performed using Stata 12 (StataCorp, College Station, TX). This study was approved by the institutional review board at NYU School of Medicine.
RESULTS
During the study period, the mean (standard deviation) number of EHR interactions per patient per hour was 2.49 (1.30). EHR interactions differed by hour and day of the week; the lowest number occurred in early morning on weekends, and the highest occurred around 11:00 am on weekdays (Figure 1 ). EHR interactions differed among days of the week at all times ( P <0.001), whereas EHR interactions were similar on most hours of Saturday and Sunday as well as Tuesday and Wednesday. At every hour, weekends had a lower number of EHR interactions in comparison to weekdays ( P <0.001). Weekdays showed a substantial increase in the number of EHR interactions between 8:00 am and 12:00 pm , followed by a slight decrease in activity between 12:00 pm and 2:00 pm , and another increase in activity from 2:00 pm to 5:00 pm (Figure 1 ). Weekend days demonstrated marked blunting of this midday peak in intensity. The tracking of an entire month of hospital EHR interactions produced a detailed graphic picture of hospital activity, clearly demarcating rounding hours, lunch hours, weekend days, hospital holidays, and other landmarks (Figure 2 ). The relative rates of census‐adjusted EHR interactions on weekdays versus weekends were 1.76 (95% confidence interval [CI]: 1.74‐1.77) for day, 1.52 (95% CI: 1.50‐1.55) for morning/evening, and 1.14 (95% CI: 1.12‐1.17) for night hours.


Nurses performed the largest number of EHR interactions (39.7%), followed by resident physicians (15.2%), attending physicians (10.2%), and pharmacists (7.6%). The remainder of EHR interactions were performed by other providers (27.4%), whose role in the majority of cases was undefined in the EHR (see Supporting Table 1 in the online version of this article). Daily variation in EHR interactions differed by provider type (Table 1 ). Nurses and resident physicians showed smaller differences in their EHR interactions between weekend and weekdays and among times of day when compared to attending physicians, pharmacy staff, and other staff. EHR interactions showed similar variations to the overall cohort for both medicine patients admitted from the ER and elective surgery patients (Table 1 ). Specific clinical orders designed to sample particularly meaningful interactions, including urinary catheter insertion and removal, patient mobilization orders, and new antibiotic orders, were moderately correlated with total EHR interactions (Table 2 ). In a sensitivity analysis, the comparison of weekday to weekend EHR interactions per daily discharge was similar to the primary analysis, with relative rates of 1.75 (95% CI: 1.73‐1.77), 1.54 (95% CI: 1.50‐1.57), and 1.15 (95% CI: 1.11‐1.18) for day, morning/evening, and night hours, respectively.
| Hour Group a | |||||||
|---|---|---|---|---|---|---|---|
| Daytime Hours | Morning/Evening Hours | Nighttime Hours | Correlation Coefficient to | ||||
| Rate Ratio | 95% CI | Rate Ratio | 95% CI | Rate Ratio | 95% CI | EHR Interactions | |
| |||||||
| All EHR interactions | 1.76 | 1.74‐1.77 | 1.52 | 1.50‐1.55 | 1.14 | 1.12‐1.17 | 1.00 |
| By provider subgroup | |||||||
| Nurses | 1.35 | 1.34‐1.37 | 1.21 | 1.20‐1.22 | 1.10 | 1.08‐1.12 | 0.92 |
| Residents | 1.38 | 1.36‐1.40 | 1.46 | 1.43‐1.49 | 1.14 | 1.11‐1.18 | 0.90 |
| Attending physicians | 1.70 | 1.67‐1.73 | 2.04 | 1.97‐2.10 | 1.32 | 1.26‐1.39 | 0.96 |
| Pharmacy staff | 1.64 | 1.61‐1.67 | 1.68 | 1.61‐1.75 | 1.19 | 1.15‐1.23 | 0.90 |
| All others | 2.75 | 2.70‐2.79 | 2.08 | 2.002.17 | 1.20 | 1.15‐1.25 | 0.97 |
| By admission subgroup | |||||||
| Medicine ER admissions | |||||||
| All | 1.64 | 1.62‐1.67 | 1.41 | 1.38‐1.44 | 1.07 | 1.04‐1.09 | 0.92 |
| Day admissions | 1.64 | 1.62‐1.67 | 1.41 | 1.38‐1.44 | 1.08 | 1.05‐1.11 | 0.92 |
| Night admissions | 1.65 | 1.62‐1.68 | 1.41 | 1.37‐1.45 | 1.03 | 1.001.05 | 0.88 |
| Weekday admissions | 1.76 | 1.73‐1.79 | 1.56 | 1.52‐1.59 | 1.28 | 1.23‐1.30 | 0.93 |
| Weekend admissions | 1.40 | 1.38‐1.42 | 1.14 | 1.11‐1.17 | 0.70 | 0.68‐0.72 | 0.88 |
| Surgery elective admissions | |||||||
| All | 1.63 | 1.61‐1.65 | 1.53 | 1.50‐1.56 | 1.25 | 1.21‐1.28 | 0.97 |
| Day admissions | 1.62 | 1.60‐1.64 | 1.54 | 1.51‐1.57 | 1.26 | 1.23‐1.30 | 0.96 |
| Night admissions | 1.65 | 1.62‐1.67 | 1.50 | 1.46‐1.54 | 1.22 | 1.18‐1.25 | 0.94 |
| Weekday admissions | 1.64 | 1.62‐1.66 | 1.55 | 1.52‐1.59 | 1.27 | 1.24‐1.31 | 0.96 |
| Weekend admissions | 1.60 | 1.56‐1.65 | 1.26 | 1.21‐1.31 | 0.89 | 0.85‐0.93 | 0.73 |
| Hour Group a | |||||||
|---|---|---|---|---|---|---|---|
| Daytime Hours | Morning/Evening Hours | Nighttime Hours | Correlation Coefficient to | ||||
| Rate Ratio | 95% CI | Rate Ratio | 95% CI | Rate Ratio | 95% CI | EHR Interactions | |
| |||||||
| Urinary catheter | 2.81 | 2.61‐3.02 | 3.17 | 2.85‐3.54 | 1.42 | 1.27‐1.58 | 0.66 |
| Mobilization | 2.51 | 2.39‐2.63 | 2.91 | 2.71‐3.12 | 1.42 | 1.34‐1.52 | 0.70 |
| Antibiotic initiation b | 1.33 | 1.24‐1.42 | 1.65 | 1.47‐1.85 | 1.13 | 1.01‐1.25 | 0.41 |
| Antibiotic discontinue c | 1.74 | 1.63‐1.85 | 1.68 | 1.50‐1.87 | 1.28 | 1.13‐1.45 | 0.61 |
As seen in Figure 3 , CPU usage was well correlated with EHR interactions ( r =0.90) and both metrics were lower on weekends as compared to weekdays. A few outliers of increased CPU usage on weekends were observed; these outliers all occurred on Sundays from 12:00 am to 2:00 am . The information technology (IT) department at our institution confirmed that these outliers coincided with a weekly virus scan performed at that time.

am
and 1:00
am
, which were determined to be outliers related to a weekly virus scan. The correlation coefficient is 0.90.
DISCUSSION
EHR interactions represents a new and accessible measure of hospital care intensity that may be used to track temporal variations in care that are likely to exist in all hospital systems. As the number of hospitals that process and document all clinical activities through the EHR continues to increase, [22, 23] such a measure has the potential to serve as a useful metric in efforts to raise nighttime and weekend care to the same quality as that during weekdays.
We found that EHR interactions differed markedly between days and nights and between weekdays and weekends. A number of prior studies have found temporal variations in clinical outcomes and care. [1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14] Our study adds to this literature by defining a new measure of the overall intensity of the process of inpatient care. Using this measure, we demonstrate that the increase in midday clinical activity seen during the week is substantially blunted on weekends. This finding adds validity to the designation of both nights and weekends as off hours when examining temporal variations in clinical care. [2, 7]
Other subtle patterns of care delivery emerged throughout the day. For instance, we found a decrease in EHR interactions between 12:00 pm and 2:00 pm , likely representing a small lull in clinical activity during the lunchtime hour, whereas EHR interactions were higher in the night and evening hours than in the early morning. The clinical significance of such fluctuations remains undetermined.
Variation throughout the week differed by provider type. Nurses and resident physicians, who are scheduled around the clock, demonstrated the least fluctuation in EHR interaction intensity over time. Attending physicians and other staff showed the greatest variation in weekday versus weekend care.
We defined a second measure of care intensity based on EHR utilization, which we named CPU usage. The advantage of using CPU as a metric is that it easy to measure and track as it is routinely monitored by hospital IT departments as part of regular maintenance. CPU usage can therefore be easily implemented in any institution with an EHR, whereas measuring EHR interactions requires EHR data abstraction and manipulation. The disadvantage of CPU usage as a measure is that it includes nonclinical reporting and other functions and therefore represents a less specific measure of clinical activity. Nonetheless, at our institution, nearly all clinical activities are processed or documented through the EHR, which is maintained on central servers, and CPU usage was well correlated with EHR interactions. We therefore believe that CPU usage represents a useful indicator of hospital clinical activity at a given time.
Several limitations deserve mention. First, EHR interactions may not always reflect actual patient care or clinical documentation. Nonetheless, we also examined specific patient orders and found that variations in these process measures were similar to those in the EHR interaction measure. Second, our methods were developed and evaluated at a single institution and may not be generalizable. Third, increased intensity of care does not necessarily increase quality of care. For instance, laboratory tests are commonly obtained on a daily basis in the hospital, a practice that is costly and often unnecessary. [24] Weekends have been associated with fewer laboratory tests, which may be appropriate as compared to the more frequent testing observed on weekdays. [25] Finally, we do not ascertain whether variations in EHR interactions correlate with clinical outcomes, and believe this to be an area for further research.
In conclusion, EHR interactions represent a new global process measure of care intensity, which was demonstrated to vary over the course of a week. We intend to use this measure at our institution to track progress of an initiative to ensure a high standard of care throughout the course of the week. The extent to which temporal variations in EHR interactions or reductions in these variations are correlated with clinical outcomes deserves further study. We believe this measure, which can be adapted to other institutions, may have a valuable role to play in hospitals' efforts to eliminate excess morbidity and mortality associated with care delivered during nights and weekends.
- , , , et al. Impact of weekend admissions on quality of care and outcomes in patients with acute myeloid leukemia . Cancer. 2010 ; 116 : 3614 – 3620 .
- , , , et al. Impact of time of presentation on the care and outcomes of acute myocardial infarction . Circulation. 2008 ; 117 : 2502 – 2509 .
- , , , et al. Weekend hospital admission and discharge for heart failure: association with quality of care and clinical outcomes . Am Heart J. 2009 ; 158 : 451 – 458 .
- , , , . Hospital care for patients experiencing weekend vs weekday stroke: a comparison of quality and aggressiveness of care . Arch Neurol. 2010 ; 67 : 39 – 44 .
- , , , , , . Weekend versus weekday admission and mortality from myocardial infarction . N Engl J Med. 2007 ; 356 : 1099 – 1109 .
- . Do hospitals provide lower quality care on weekends? Health Serv Res. 2007 ; 42 : 1589 – 1612 .
- , , , et al. Relationship between time of day, day of week, timeliness of reperfusion, and in‐hospital mortality for patients with acute ST‐segment elevation myocardial infarction . JAMA. 2005 ; 294 : 803 – 812 .
- , , , , , . Weekend versus weekday admission and mortality after acute pulmonary embolism . Circulation. 2009 ; 119 : 962 – 968 .
- , , , , , . Influence of weekend hospital admission on short‐term mortality after intracerebral hemorrhage . Stroke. 2009 ; 40 : 2387 – 2392 .
- , , , et al. Weekend hospital admission, acute kidney injury, and mortality . J Am Soc Nephrol. 2010 ; 21 : 845 – 851 .
- , . Mortality among patients admitted to hospitals on weekends as compared with weekdays . N Engl J Med. 2001 ; 345 : 663 – 668 .
- , , , et al. Mortality after noncardiac surgery: prediction from administrative versus clinical data . Med Care. 2005 ; 43 : 159 – 167 .
- , . Weekend birth and higher neonatal mortality: a problem of patient acuity or quality of care? J Obstet Gynecol Neonatal Nurs. 2003 ; 32 : 724 – 733 .
- , , , , , . Analysis of the mortality of patients admitted to internal medicine wards over the weekend . Am J Med Qual. 2010 ; 25 : 312 – 318 .
- , , , . Do weekends or evenings matter in a pediatric intensive care unit? Pediatr Crit Care Med. 2005 ; 6 : 523 – 530 .
- , , , , . Hospital mortality associated with day and time of admission to intensive care units . Intensive Care Medicine. 2004 ; 30 : 895 – 901 .
- , , . Weekend and weeknight admissions have the same outcome of weekday admissions to an intensive care unit with onsite intensivist coverage . Crit Care Med. 2006 ; 34 : 605 – 611 .
- , , , , . Measuring the intensity of nursing care: making use of the Belgian Nursing Minimum Data Set . Int J Nurs Stud. 2008 ; 45 : 1011 – 1021 .
- , , , , . The relationship between intensity and duration of medical services and outcomes for hospitalized patients . Med Care. 1979 ; 17 : 1088 – 1102 .
- . Differences in length of hospital stay for Medicaid and Blue Cross patients and the effect of intensity of services . Public Health Rep. 1979 ; 94 : 438 – 445 .
- . Health care's lost weekend. New York Times . October 3, 2010 . Section A, p. 27 .
- , , , . A progress report on electronic health records in U.S. hospitals . Health Aff (Millwood). 2010 ; 29 : 1951 – 1957 .
- US Department of Health 146 : 524 – 527 .
- , , . Temporal approach to hematological test usage in a major teaching hospital . Lab Hematol. 2003 ; 9 : 207 – 213 .
- , , , et al. Impact of weekend admissions on quality of care and outcomes in patients with acute myeloid leukemia . Cancer. 2010 ; 116 : 3614 – 3620 .
- , , , et al. Impact of time of presentation on the care and outcomes of acute myocardial infarction . Circulation. 2008 ; 117 : 2502 – 2509 .
- , , , et al. Weekend hospital admission and discharge for heart failure: association with quality of care and clinical outcomes . Am Heart J. 2009 ; 158 : 451 – 458 .
- , , , . Hospital care for patients experiencing weekend vs weekday stroke: a comparison of quality and aggressiveness of care . Arch Neurol. 2010 ; 67 : 39 – 44 .
- , , , , , . Weekend versus weekday admission and mortality from myocardial infarction . N Engl J Med. 2007 ; 356 : 1099 – 1109 .
- . Do hospitals provide lower quality care on weekends? Health Serv Res. 2007 ; 42 : 1589 – 1612 .
- , , , et al. Relationship between time of day, day of week, timeliness of reperfusion, and in‐hospital mortality for patients with acute ST‐segment elevation myocardial infarction . JAMA. 2005 ; 294 : 803 – 812 .
- , , , , , . Weekend versus weekday admission and mortality after acute pulmonary embolism . Circulation. 2009 ; 119 : 962 – 968 .
- , , , , , . Influence of weekend hospital admission on short‐term mortality after intracerebral hemorrhage . Stroke. 2009 ; 40 : 2387 – 2392 .
- , , , et al. Weekend hospital admission, acute kidney injury, and mortality . J Am Soc Nephrol. 2010 ; 21 : 845 – 851 .
- , . Mortality among patients admitted to hospitals on weekends as compared with weekdays . N Engl J Med. 2001 ; 345 : 663 – 668 .
- , , , et al. Mortality after noncardiac surgery: prediction from administrative versus clinical data . Med Care. 2005 ; 43 : 159 – 167 .
- , . Weekend birth and higher neonatal mortality: a problem of patient acuity or quality of care? J Obstet Gynecol Neonatal Nurs. 2003 ; 32 : 724 – 733 .
- , , , , , . Analysis of the mortality of patients admitted to internal medicine wards over the weekend . Am J Med Qual. 2010 ; 25 : 312 – 318 .
- , , , . Do weekends or evenings matter in a pediatric intensive care unit? Pediatr Crit Care Med. 2005 ; 6 : 523 – 530 .
- , , , , . Hospital mortality associated with day and time of admission to intensive care units . Intensive Care Medicine. 2004 ; 30 : 895 – 901 .
- , , . Weekend and weeknight admissions have the same outcome of weekday admissions to an intensive care unit with onsite intensivist coverage . Crit Care Med. 2006 ; 34 : 605 – 611 .
- , , , , . Measuring the intensity of nursing care: making use of the Belgian Nursing Minimum Data Set . Int J Nurs Stud. 2008 ; 45 : 1011 – 1021 .
- , , , , . The relationship between intensity and duration of medical services and outcomes for hospitalized patients . Med Care. 1979 ; 17 : 1088 – 1102 .
- . Differences in length of hospital stay for Medicaid and Blue Cross patients and the effect of intensity of services . Public Health Rep. 1979 ; 94 : 438 – 445 .
- . Health care's lost weekend. New York Times . October 3, 2010 . Section A, p. 27 .
- , , , . A progress report on electronic health records in U.S. hospitals . Health Aff (Millwood). 2010 ; 29 : 1951 – 1957 .
- US Department of Health 146 : 524 – 527 .
- , , . Temporal approach to hematological test usage in a major teaching hospital . Lab Hematol. 2003 ; 9 : 207 – 213 .
Copyright © 2013 Society of Hospital Medicine
[email protected]
Patients with Psychiatric Comorbidity
Mental illness is highly prevalent, with approximately 30% of the US population meeting criteria for at least 1 disorder.[1] In the medically ill population, psychiatric disease is even more common; a 2005 survey showed that half of all patients visiting primary care physicians met criteria for a mental disorder.[2] Conversely, those with serious mental illness suffer greater medical morbidity than the general population, with higher rates of obesity, diabetes, metabolic syndrome, cardiovascular disease, chronic obstructive pulmonary disease, human immunodeficiency virus, viral hepatitis, and tuberculosis.[3] When acute medical problems arise, those with mental illness endure longer hospitalizations; the presence of a psychiatric disturbance in the general medical setting has been shown to be a robust predictor of increased hospital length of stay.[4, 5]
Because of the strong correlation between medical and mental illness, hospitalists will care for patients with psychiatric disorders. Despite this, internists generally receive a paucity of formal training in the treatment of mental disturbances. One survey of university‐affiliated internal medicine residencies revealed that only 10% of programs offered any kind of modest curriculum in psychiatric education.[6] Regardless of this lack of preparation, hospitalists are called upon at each admission to make decisions that affect the psychiatric treatment of patients on psychotropic medication; namely, they must decide whether to continue or discontinue psychiatric medications. Many physicians reflexively discontinue a patient's chronic medications upon admission to the hospital; one study reported an adjusted odds ratio of between 1.18 and 1.86 for stopping a medication prescribed for a chronic condition.[7]
This review aims to assist the hospitalist in making an informed decision about the continuation of psychotropic medications in the medically ill patient. First, it examines the risks of stopping psychotropic medication, including psychiatric decompensation and discontinuation syndromes. It also explores the challenges of medication continuation in the context of changing pharmacokinetics and emerging side effects. Ultimately, physicians and patients must make collaborative decisions, weighing the risk of medication interactions against the potential adverse effects of psychiatric decompensation.
DISCONTINUATION
Decompensation of Mental Health
Approximately 10% to 15% of patients hospitalized for medical illness require reduction or discontinuation of psychotropic medications because they may be contributing to the clinical presentation.[4] The rate and method of drug discontinuation can affect the course of major psychiatric disorders.[8] A growing number of studies demonstrate high rates of relapse when medications are discontinued in patients suffering from mood disorders, schizophrenia, and anxiety disorders.[9] Abrupt cessation of psychotropics is especially dangerous, leading to a greater chance of destabilization than if medications are tapered. Episodes of active illness even appear to occur more frequently with sudden psychotropic cessation than they would in the natural course of untreated disease. This is true for several classes of psychotropics, including antidepressants, mood stabilizers, and antipsychotics. For example, in a study of pregnant women who suddenly stopped their psychotropic medication (both antidepressants and benzodiazepines), nearly one‐third experienced suicidal ideation.[10] Depression and suicidality have also been documented in bipolar patients who were abruptly taken off of lithium. More commonly, rapid lithium discontinuation in bipolar patients causes mania, with illness relapse as soon as 4 days after cessation.[10] Additionally, abrupt discontinuation of antipsychotics in patients with schizophrenia leads to early, and often severe, psychosis. One study found a relapse rate of 50% within 30 weeks of sudden oral neuroleptic cessation.[11] Furthermore, restarting medications, even at the previous effective dose, may not return the patient to their prior baseline.[12] Psychiatric decompensation in the hospitalized patient can worsen medical outcomes, with decreased adherence to treatment plans. In extreme circumstances, patients may be at risk of self‐harm or suicide.
DRUG‐SPECIFIC DISCONTINUATION SYNDROMES
Antidepressants
Discontinuation of medications presents additional problems, and sudden cessation of psychotropic medications can lead to uncomfortable or even dangerous symptoms. For example, the serotonin discontinuation syndrome has been well documented. Chronic use of serotonin re‐uptake inhibitors (generally greater than 6 to 8 weeks) leads to downregulation of postsynaptic serotonin receptors. When selective serotonin re‐uptake inhibitors (SSRIs) or serotonin‐norepinephrine re‐uptake inhibitors are abruptly stopped, the brain experiences a relative decline in serotonin. Symptoms include a flu‐like illness, nausea, imbalance, insomnia, sensory disturbances, and dysphoria. Onset may be within hours of missing a dose, but typically occurs within 3 days of medication discontinuation. The syndrome is more likely to occur with cessation of medications of shorter half‐life and less likely to occur with medications with a long half‐life, such as fluoxetine (Table 1).[13, 14] The symptoms can be ameliorated with a gradual tapering or reintroduction of the antidepressant.[15] Untreated symptoms resolve in 1 to 2 weeks. Although the syndrome in isolation is not life‐threatening, a number of the symptoms can complicate medical illness and muddle diagnosis of other diseases.[14, 16]
| Medication | Half‐Life (Hours) |
|---|---|
| |
| SSRIs | |
| Fluoxetine | 84144 |
| Paroxetine | 21 |
| Sertraline | 26 |
| Citalopram | 35 |
| Escitalopram | 2732 |
| Fluvoxamine | 15 |
| SNRIs | |
| Venlafaxine | 313 |
| Duloxetine | 1116 |
Older antidepressants, including the tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs), have serotonergic effects, and thus discontinuation may cause the symptoms described above. However, these agents also have effects on other neurotransmitters. The TCAs block muscarinic cholinergic receptors, leading to upregulation. Abrupt cessation can lead to cholinergic rebound, with parkinsonism and mania emerging. Multiple case reports document improvement in these symptoms with an anticholinergic agent, such as benztropine.[17, 18] MAOIs lead to changes in ‐2 adrenergic and dopaminergic receptors. Sudden discontinuation has been associated with agitation, delirium, and psychosis; 1 case report even documents catatonia associated with autonomic instability.[19]
In addition, sudden discontinuation of antidepressants (including the SSRIs) may provoke mania or hypomania in some patients, regardless of whether they have experienced previous spontaneous manic episodes.[8]
Neuroleptics
Data for an antipsychotic withdrawal syndrome are less convincing than those for serotonergic agents. However, certain symptoms have been associated with abrupt neuroleptic discontinuation. Most frequently, gastrointestinal distress and diaphoresis are described. Anxiety, agitation, and insomnia are also common. These symptoms are thought to be associated with cholinergic rebound, mediated by direct effects of neuroleptics on muscarinic receptors or indirectly through dopamine receptor blockade and the dopamine‐cholinergic balance. Symptoms may be more severe when antimuscarinic, antiparkinsonism drugs are simultaneously stopped. Some authors argue that the timing of symptom onset can differentiate antipsychotic withdrawal from illness relapse, with discontinuation syndrome occurring within the first 7 days of medication cessation.[20]
Additionally, abrupt cessation of antipsychotics may be associated with rapid‐onset psychosis. The data are strongest for clozapine discontinuation, where overall incidence is approximately 20%. This is hypothesized to be mediated by dopamine receptor upregulation and subsequent hypersensitivity to endogenous dopamine. The emerging psychosis is purportedly distinct from the underlying illness. Episodes have been described in patients on chronic metoclopramide who have no prior psychiatric history, as well as in patients with bipolar disorder without psychosis prior to neuroleptic discontinuation.[21]
Movement disorders may emerge during neuroleptic discontinuation. Both parkinsonism and dyskinesias have been described. In some patients, dyskinesias resolve within weeks of drug discontinuation; however, others experience permanent symptoms, termed covert dyskinesia.[22] In rare circumstances, dyskinesias may affect the respiratory muscles, causing distress. Several case reports of withdrawal‐emergent respiratory dyskinesia have been reported following risperidone cessation.[23, 24, 25] Additionally, several case reports have described catatonia occurring after abrupt discontinuation of clozapine. In all cases, symptoms promptly resolved with reinitiation of clozapine.[26]
Neuroleptic malignant syndrome (NMS) is a rare but potentially fatal complication of antipsychotic administration. Symptoms include fever, rigidity, autonomic instability, and mental status changes.[27] Though NMS is hypothesized to occur due to dopamine receptor blockade, rare cases of NMS have also been reported with abrupt cessation of neuroleptics. Of the 8 case reports in the literature, 1 resulted in death.[22, 28]
Mood Stabilizers
Though some of the atypical antipsychotics are used to treat bipolar disorder, mood stabilizers are the mainstay of pharmacotherapy. The most commonly used mood stabilizers are lithium, valproic acid, lamotrigine, topirimate, carbamezapine, and oxcarbazepine. When mood stabilizers are discontinued, patients are at risk of psychiatric relapse, as documented above. However, withdrawal symptoms have not been commonly documented upon abrupt discontinuation of lithium or the anticonvulsants used to treat bipolar disorder.[29, 30]
Benzodiazepines
Benzodiazepines are widely prescribed for insomnia and anxiety. Chronic legal use of benzodiazepines is approximately 2% in the general population.[31] Like ethanol, benzodiazepines bind nonselectively to the GABA‐A receptor, resulting in downregulation of GABA receptors and compensatory increased N‐methyl‐D‐aspartate transmission. Sudden discontinuation of benzodiazepines results in a syndrome that mirrors that of alcohol withdrawal. Symptoms range from mild (tremor, insomnia, and anxiety) to life‐threatening (seizures, delirium, and autonomic instability). Serious withdrawal is more likely with substances of shorter half‐life and with higher chronic doses. Onset often occurs between 2 and 10 days after discontinuation, depending on the half‐life of the benzodiazepine.[32] Other rare serious reactions have been documented following abrupt benzodiazepine cessation, including NMS and catatonia.[30]
CONTINUATION
Reflexive discontinuation of psychotropic medications can clearly lead to adverse outcomes. However, when hospitalists decide to continue a patient's psychotropic medications, they must also be cognizant of potential complications. Modifications may be necessary because of hepatic, renal, or cardiac disease. In addition, physicians need to be aware of drug‐drug interactions. Pharmacotherapy for medically ill elderly patients may require dose modifications to account for an increased lipophilic volume of distribution and a decreased rate of metabolism.[33] Finally, pregnancy can present additional challenges regarding dose modifications and teratogenicity.
On the other hand, hospitalists must be aware that continuing a patient's psychotropic medication may not be the cause of new psychiatric symptoms. Drugs prescribed for medical disorders (eg, corticosteroids) often cause psychiatric symptoms. In addition, psychiatric symptoms may emerge at times of nonpsychotropic medication withdrawal or due to nonpsychotropic drug‐drug interactions.[4] Groups of medications commonly associated with psychiatric disturbances include analgesics, sedatives, anesthetics, anticonvulsants, and anticholinergics.
PHARMACOKINETICS: PSYCHOTROPIC TOXICITY
Medical illness alters the body's steady state, and renal or hepatic metabolism may be impaired when a patient requires hospitalization. Additionally, new medications may increase the effects of psychotropics, whether by intrinsic augmentation of effect or decreased psychotropic clearance. Ultimately, these changes can lead to psychotropic toxicity.
Specific toxicities merit discussion. First, serotonin syndrome is a potentially fatal condition. The majority of cases occur with synergistic serotonergic medication administration, though there are case reports of the syndrome occurring with addition of inhibitors of cytochrome p450 2D6 and/or 3A4 to SSRIs. A large number of medications from different classes have been indicated (Table 2).[34] Symptoms generally occur within 24 hours of medication administration and include mental status changes, autonomic instability, and neuromuscular hyperactivity. When serotonin syndrome is suspected, the offending agent should be discontinued immediately. There is no definitive treatment, though supportive care can be lifesaving.[34]
| Amphetamines and Derivatives | Antidepressants and Mood Stabilizers | Antimigraine Drugs | Analgesics | Antiemetics | Miscellaneous |
|---|---|---|---|---|---|
| |||||
| MDMA | Buspirone | Ergot alkaloids | Cyclobenzaprine | Metoclopramide | Cocaine |
| Dextroamphetamine | Carbamazepine | Triptans | Fentanyl | Ondansetron | Dextromethorphan |
| Methamphetamine | Lithium | Meperidine | Linezolid | ||
| Sibutramine | MAOIs | Tramadol | L‐tryptophan | ||
| SSRIs | 5‐hydroxytrytophan | ||||
| SNRIs | |||||
| Serotonin 2A receptor blockers (eg, trazodone) | |||||
| St. John's Wort | |||||
| TCAs | |||||
| Valproic Acid | |||||
In addition to serotonin syndrome, hypertensive emergency may occur due to drug interactions with MAOIs. MAOIs inhibit the enzyme monoamine oxidase, resulting in elevated levels of serotonin, histamine, and catecholamines in the blood. Coadministration of MAOIs and sympathomimetic agents (such as cough suppressants and analgesics) may dangerously increase adrenergic stimulation, elevating blood pressure to the point of end‐organ damage. Please see Table 3 for a full list of drugs indicated in MAOI‐associated hypertensive crisis.[35] To ensure safety, it is recommended that MAOIs be discontinued for 14 days prior to introducing medications with sympathomimetic properties, and vice versa. Because of its longer half‐life, a 5‐week washout period is recommended for fluoxetine.[35]
|
| Amphetamines |
| Analgesics: meperedine |
| Anesthetics |
| Antidepressants: buproprion, buspirone, other MAOIs, SSRIs, SNRIs, TCAs, |
| Mirtazapine |
| Cocaine |
| Dibenzazepine‐related agents: carbamezapine, cyclobenzaprine, perphenazine |
| Female sex steroids |
| Sympathomimetics: dopamine, epinephrine, levodopa, methyldopa, methylphenidate, norepinephrine, phenylalanine, reserpine, tyrosine, tryptophan |
| Other vasoconstrictors: pseudoephedrine, phenylephrine, phenylpropanolamine, ephedrine |
Lithium toxicity may result from changing patient pharmacokinetics. Lithium is almost entirely renally excreted, and acute kidney injury may precipitously raise serum levels. Within the renal collecting system, lithium is handled similarly to sodium, with 80% reabsorbed from the proximal tubule to the collecting duct. Thus, factors that decrease glomerular filtration rate (GFR) and increase proximal tubule absorption will increase serum lithium levels. For example, decreased effective arterial volume (due to dehydration, cirrhosis, nephrotic syndrome, or heart failure) may elevate lithium levels. Additionally, medications that decrease GFR may increase lithium reabsorption. These include nonsteroidal anti‐inflammatory drugs, angiotensin‐converting enzyme inhibitors, and thiazide diuretics. Because of lithium's narrow therapeutic index, small elevations in serum levels can lead to toxicity. Severity of intoxication correlates with serum concentration. Symptoms range from lethargy, weakness, tremor, ataxia, and gastrointestinal distress to coma, seizures, renal failure, and death. Toxicity is also associated with electrocardiograph (ECG) changes, including ST‐segment depression and T‐wave inversion in the lateral precordial leads. Sinus node dysfunction can also occur. Definitive treatment for lithium toxicity is hemodialysis.[36]
Though the therapeutic index is much wider for valproic acid than for lithium, valproate toxicity may also occur in the medically ill patient with previously stable serum levels. Valproic acid is highly protein‐bound at therapeutic levels, and is metabolized largely through hepatic glucuronidation. Initiation of medications that compete for protein‐binding sites, including aspirin, has led to valproate toxicity. Moreover, acute liver failure or addition of drugs that compete with hepatic microsomal enzymes may lead to decreased excretion of valproic acid. Poisoning may result in central nervous system (CNS) and respiratory depression, hypotension, cerebral edema, and pancreatitis. True hepatoxicity is rare, though hyperammonemia is widely documented. Thrombocytopenia is the most common hematologic abnormality associated with overdose. However, thrombocytopenia may also occur without complication in patients on stable therapeutic doses. Treatment is largely supportive, though hemoperfusion and hemodialysis may be used when serum levels are >300 g/mL, as only 35% of the drug is protein‐bound at that level. Naloxone has been shown in case reports to reverse valproic acid‐induced coma, and L‐carnitine has been increasingly recommended for hyperammonemia.[37, 38]
PHARMACOKINETICS: DRUG‐DRUG INTERACTIONS
As discussed above, the addition of a new medication can increase previously stable levels of psychotropic drugs, leading to toxicity. Conversely, mental health medications can alter the expected metabolism of a drug being used to treat acute medical illness. Many psychotropics are metabolized via the cytochrome p450 enzyme, particularly the SSRIs (Table 4).[39] A number of antimicrobial and antiarrythmic medications are also cleared via this route, leading to potential toxic or subtherapeutic levels when drug‐drug interactions occur.
| CYP 1A2 | CYP 2B6 | CYP 2C9 | CYP 2C19 | CYP 2D6 | CYP 3A4/3A5/3A7 |
|---|---|---|---|---|---|
| Clozapine | Bupropion | Amitriptyline | Phenytoin | Antidepressants | Alprazolam |
| Duloxetine | Methadone | Fluoxetine | Amitriptyline | Amitriptyline | Diazepam |
| Fluvoxamine | Phenytoin | Citalopram | Clomipramine | Midazolam | |
| Haloperidol | Clomipramine | Duloxetine | Aripiprazole | ||
| Imipramine | Diazepam | Desipramine | Buspirone | ||
| Olanzapine | Imipramine | Fluoxetine | Haldol | ||
| Ramelteon | Fluvoxamine | Quetiapine | |||
| Imipramine | Ziprasidone | ||||
| Nortriptyline | Zolpidem | ||||
| Paroxetine | Dextromethorphan | ||||
| Venlafaxine | |||||
| Antipsychotics | |||||
| Aripiprazole | |||||
| Clorpromazine | |||||
| Haldol | |||||
| Perphenazine | |||||
| Risperidone |
PSYCHOTROPIC ADVERSE EFFECTS
Psychotropic medications may also cause side effects that contribute to the clinical presentation, requiring ongoing monitoring, a dose reduction, or psychotropic discontinuation. Potential adverse effects that commonly impact psychopharmacologic management include anticholinergic side effects, cardiac effects, and sedation.
ANTICHOLINERGIC EFFECTS AND TOXICITY
Newly emerging anticholinergic effects may be particularly troubling. Dry mouth may cause swallowing difficulty and aspiration. Pupillary dilatation and dry eyes can increase risk of falls. Constipation may evolve into fecal impaction, and urinary retention can contribute to increased catheter use and infection. CNS effects are perhaps the most serious, ranging from drowsiness and memory impairment to frank delirium.[40]
Many psychotropic drugs are anti cholinergic. Among the antidepressants, the TCAs and paroxetine have the highest anticholinergic activity. Anticholinergic effects have also been reported with the low potency first generation neuroleptics and with the atypical antipsychotics olanzapine and clozapine. Additionally, medications used to treat the extrapyramidal symptoms associated with antipsychotics (such as benztropine and diphenhydramine) are strongly anticholinergic.[41] Patients without previous overt anticholinergic symptoms from these medications may experience adverse effects when hospitalized. Medical illness or new medications may alter psychotropic drug metabolism and elimination, leading to accumulation of their anticholinergic effects. Many medications used in the hospital also have intrinsic anticholinergic activity. These include some antiemetics, antispasmodics, antiarrhythmics, and histamine H2 receptor blockers. Elderly patients are particularly prone to anticholinergic effects due to age‐related deficits in cholinergic transmission.[40]
QTc PROLONGATION
QTc prolongation is a potentially lethal side effect of certain medications. Prolonged QTc increases the risk of cardiac mortality and sudden death, presumably related to onset of torsades de pointe. Certain antidepressants have consistently been associated with QTc prolongation, particularly the TCAs. In addition, the US Food and Drug Administration recently issued the recommendation that the SSRI citalopram not be used at doses >40 mg (and 20 mg in those with hepatic impairment or age >60 years) due to results of a randomized controlled trial that showed a dose‐response increase in QTc. Antipsychotic medications have also been shown to increase QTc, with the greatest evidence for thioridazine and the first‐generation, low‐potency neuroleptics. Haloperidol (particularly the intravenous formulation) has also been linked to both long QTc and torsades, though data may be confounded by the degree of medical illness of patients receiving the medication.[42] Of the atypicals, ziprasidone causes the greatest QTc prolongation.[43] However, a large retrospective cohort study showed similar risk increase of sudden cardiac death (2‐fold) among all antipsychotics (including typicals and atypicals) when examined individually.[44]
Additional risk factors for QTc prolongation abound in the hospitalized patient. These include many medical problems, including electrolyte abnormalities, heart conditions, renal and hepatic dysfunction, and CNS injury. Hospitalized patients are also exposed to the cumulative effects of medications that increase duration of QTc, such as class I and class III antiarrhythmics. Additionally, certain antimicrobials, including macrolide antibiotics and antifungals, have been associated with QTc prolongation via pharmacokinetic interactions.
Because of this, QTc should be measured upon admission in patients on stable doses of psychotropics that predispose to prolongation. Addition of other medications known to contribute to increased QTc should prompt further ECGs. Electrolytes, particularly potassium and magnesium, should be aggressively repleted. When QTc extends beyond 500 ms, consideration should be given to discontinuing or changing medications that can contribute to QTc prolongation (whether psychotropics or drugs used to treat acute medical problems).[42]
SEDATION
Sedation is another potential side effect of psychotropic medications. Although sedation is beneficial for agitation and anxiety, sedated patients may be unable to participate in treatment. They are also at greater risk of aspiration and falls.
Antipsychotics cause sedation through antagonism of 1 adrenergic and H1 histaminergic receptors. Effects are most pronounced with the low‐potency, first‐generation antipsychotics and the atypicals quetiapine and clozapine. Some antidepressants, including the TCAs and mirtazapine, also cause somnolence by histamine H1‐receptor antagonism. If a patient has been psychiatrically stable on a particular antipsychotic or antidepressant, but has psychotropic‐induced sedation that interferes with treatment, hospitalists may want to consider temporarily decreasing the dose.
Benzodiazepines induce sedation by increasing ‐aminobutyric acid‐ergic transmission. There is significant overlap between anxiolytic and sedating doses of benzodiazepines. The amount of sedation is related to dose, speed of absorption, and onset of CNS penetration. Thus, sedation may be minimized by using a lower dose or switching to an equivalent dose of a slower‐onset benzodiazepine.[45, 46]
CONCLUSION
The decision to continue or discontinue psychotropic medications is often challenging. It requires the hospitalist to carefully weigh the risks and benefits of the ongoing treatment versus discontinuation, while also considering the patient's preference whenever possible. Sudden cessation of psychotropics can lead to a number of unwanted complications, from mild withdrawal to life‐threatening autonomic instability and psychiatric decompensation. Yet psychotropic continuation can also lead to unwanted drug‐drug interactions and newly emergent side effects.
To improve patient safety and outcomes, hospitalists must take a cautious and well‐thought out approach to treating patients on psychotropic drugs. Reflexive cessation of home medications must be avoided. If hepatic or renal insufficiency develops, medication doses may need to be adjusted. When available, drug levels should guide dose adjustments. If side effects occur, the patient's medication list should be carefully reviewed for potential drug‐drug interactions. Maintaining mental health may mean substituting another drug for one that interferes with home psychotropic medications. Psychotropic doses may also be minimized to decrease side effects. When a psychotropic must be discontinued, tapering is recommended over an abrupt discontinuation, except in the case of an acute toxicity. Moreover, cross‐titration to another effective agent may prevent psychiatric decompensation.
Additionally, hospitalists should use all available resources when deciding on a patient's psychotropic regimen. Mobile devices and online resources can assist with pharmacokinetics. Pharmacists can help with more complex questions or potential drug substitutions. Consultation‐liaison psychiatrists can be a valuable resource in ensuring the safety and stability of a patient with psychiatric comorbidity within the medical environment. The consultant may assist by assessing a patient's current psychiatric state and recommending psychotropic medication changes when needed.
Disclosure
Nothing to report.
- , , , et al. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352(24):2515–2523.
- , , , , , . Mental disorders in primary care: prevalence and co‐morbidity among disorders. results from the functional illness in primary care (FIP) study. Psychol Med. 2005;35(8):1175–1184.
- , , , et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52–77.
- , , , et al. The Academy of Psychosomatic Medicine practice guidelines for psychiatric consultation in the general medical setting. The Academy of Psychosomatic Medicine. Psychosomatics. 1998;39(4):S8–S30.
- , , , , . Impact of psychiatric comorbidity on length of hospital stay for medical/surgical patients: a preliminary report. Am J Psychiatry. 1987;144(7):878–882.
- . Need for better psychiatric training for primary care providers. Acad Med. 1996;71(6):574–575.
- , , , et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306(8):840–847.
- . Discontinuing treatment for psychiatric disorders. J Psychiatry Neurosci. 2006;31(1):11–12.
- , , , , , . Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153(5):592–606.
- , , . Abrupt discontinuation of psychotropic drugs during pregnancy: fear of teratogenic risk and impact of counselling. J Psychiatry Neurosci. 2001;26(1):44–48.
- , , , , . Clinical risk following abrupt and gradual withdrawal of maintenance neuroleptic treatment. Arch Gen Psychiatry. 1997;54(1):49–55.
- . Potential adverse effects of discontinuing psychotropic drugs. Part 3: Antipsychotic, dopaminergic, and mood‐stabilizing drugs. J Psychosoc Nurs Ment Health Serv. 2010;48(8):11–14.
- , . Fluoxetine. In: Schatzberg AF, Nemeroff CB, eds. Textbook of Psychopharmacology. 2nd ed. Chapter 13. Washington, DC: American Psychiatric Press; 2004:235.
- , , , , . Antidepressant discontinuation syndrome. Am Fam Physician. 2006;74(3):449–456.
- , , , , . Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77–87.
- , , , . Selective serotonin reuptake inhibitor discontinuation syndrome: proposed diagnostic criteria. J Psychiatry Neurosci. 2000;25(3):255–261.
- . Antidepressant withdrawal syndromes: phenomenology and pathophysiology. Acta Psychiatr Scand. 1989;79(2):113–117.
- . Heterocyclic antidepressant, monoamine oxidase inhibitor and neuroleptic withdrawal phenomena. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14(2):137–161.
- . Monoamine oxidase inhibitor withdrawal phenomena: symptoms and pathophysiology. Acta Psychiatr Scand. 1988;78(1):1–7.
- , . Antipsychotic withdrawal symptoms: phenomenology and pathophysiology. Acta Psychiatr Scand. 1988;77(3):241–246.
- . Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal‐related relapse. Acta Psychiatr Scand. 2006;114(1):3–13.
- , . Neuroleptic malignant syndrome after neuroleptic discontinuation. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19(8):1323–1334.
- , . Withdrawal‐emergent dyskinesia in a patient on risperidone undergoing dosage reduction. Ann Clin Psychiatry. 1996;8(3):179–182.
- , , . Respiratory dyskinesia as discontinuation effect of risperidone. J Clin Psychopharmacol. 2005;25(6):609.
- , . Withdrawal‐emergent respiratory dyskinesia with risperidone treated with clozapine. J Neuropsychiatry Clin Neurosci. 2010;22(2):E24.
- , . Clozapine‐withdrawal catatonia. Psychosomatics. 2010;51(4):355–355.e2.
- , , . Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870–876.
- , , . Psychotropic discontinuation symptoms: a case of withdrawal neuroleptic malignant syndrome. Gen Hosp Psychiatry. 2006;28(6):541–543.
- , . Discontinuation symptoms and psychotropic drugs. Lancet. 2000;355(9210):1184.
- . Potential adverse effects of discontinuing psychotropic drugs. J Psychosoc Nurs Ment Health Serv. 2010;48(9):11–14.
- , , , . Pharmacological interventions for benzodiazepine mono‐dependence management in outpatient settings. Cochrane Database Syst Rev. 2006(3):CD005194.
- , . Substance abuse and withdrawal in the critical care setting. Crit Care Clin. 2008;24(4):767–788, viii.
- . Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67–76.
- , . Prevention, recognition, and management of serotonin syndrome. Am Fam Physician. 2010;81(9):1139–1142.
- . Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73(suppl 1):17–24.
- , . Lithium intoxication. J Am Soc Nephrol. 1999;10(3):666–674.
- . Valproic acid toxicity: overview and management. J Toxicol Clin Toxicol. 2002;40(6):789–801.
- , , . Lithium. In: Schatzberg AF, Nemeroff CB, eds. Textbook of Psychopharmacology. 2nd ed. Chapter 35. Washington, DC: American Psychiatric Press; 2004:547–549.
- , . 2010 guide to psychiatric drug interactions. Prim Psychiatry. 2009;16(12):45–74.
- , . Anticholinergic side‐effects of drugs in elderly people. J R Soc Med. 2000;93(9):457–462.
- , , . Measurement of anticholinergic effects of psychotropic drugs in humans. Pharmacopsychiatry. 2005;38(5):187–193.
- , , , , . QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics. 2013;54(1):1–13.
- , , , et al. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol. 2004;24(1):62–69.
- , , , , . Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225–235.
- , . Sedation, an unpleasant, undesirable and potentially dangerous side‐effect of many psychotropic drugs. Hum Psychopharmacol. 2004;19(2):135–139.
- , . Toxicology and overdose of atypical antipsychotics. J Emerg Med. 2012;43(5):906–913.
Mental illness is highly prevalent, with approximately 30% of the US population meeting criteria for at least 1 disorder.[1] In the medically ill population, psychiatric disease is even more common; a 2005 survey showed that half of all patients visiting primary care physicians met criteria for a mental disorder.[2] Conversely, those with serious mental illness suffer greater medical morbidity than the general population, with higher rates of obesity, diabetes, metabolic syndrome, cardiovascular disease, chronic obstructive pulmonary disease, human immunodeficiency virus, viral hepatitis, and tuberculosis.[3] When acute medical problems arise, those with mental illness endure longer hospitalizations; the presence of a psychiatric disturbance in the general medical setting has been shown to be a robust predictor of increased hospital length of stay.[4, 5]
Because of the strong correlation between medical and mental illness, hospitalists will care for patients with psychiatric disorders. Despite this, internists generally receive a paucity of formal training in the treatment of mental disturbances. One survey of university‐affiliated internal medicine residencies revealed that only 10% of programs offered any kind of modest curriculum in psychiatric education.[6] Regardless of this lack of preparation, hospitalists are called upon at each admission to make decisions that affect the psychiatric treatment of patients on psychotropic medication; namely, they must decide whether to continue or discontinue psychiatric medications. Many physicians reflexively discontinue a patient's chronic medications upon admission to the hospital; one study reported an adjusted odds ratio of between 1.18 and 1.86 for stopping a medication prescribed for a chronic condition.[7]
This review aims to assist the hospitalist in making an informed decision about the continuation of psychotropic medications in the medically ill patient. First, it examines the risks of stopping psychotropic medication, including psychiatric decompensation and discontinuation syndromes. It also explores the challenges of medication continuation in the context of changing pharmacokinetics and emerging side effects. Ultimately, physicians and patients must make collaborative decisions, weighing the risk of medication interactions against the potential adverse effects of psychiatric decompensation.
DISCONTINUATION
Decompensation of Mental Health
Approximately 10% to 15% of patients hospitalized for medical illness require reduction or discontinuation of psychotropic medications because they may be contributing to the clinical presentation.[4] The rate and method of drug discontinuation can affect the course of major psychiatric disorders.[8] A growing number of studies demonstrate high rates of relapse when medications are discontinued in patients suffering from mood disorders, schizophrenia, and anxiety disorders.[9] Abrupt cessation of psychotropics is especially dangerous, leading to a greater chance of destabilization than if medications are tapered. Episodes of active illness even appear to occur more frequently with sudden psychotropic cessation than they would in the natural course of untreated disease. This is true for several classes of psychotropics, including antidepressants, mood stabilizers, and antipsychotics. For example, in a study of pregnant women who suddenly stopped their psychotropic medication (both antidepressants and benzodiazepines), nearly one‐third experienced suicidal ideation.[10] Depression and suicidality have also been documented in bipolar patients who were abruptly taken off of lithium. More commonly, rapid lithium discontinuation in bipolar patients causes mania, with illness relapse as soon as 4 days after cessation.[10] Additionally, abrupt discontinuation of antipsychotics in patients with schizophrenia leads to early, and often severe, psychosis. One study found a relapse rate of 50% within 30 weeks of sudden oral neuroleptic cessation.[11] Furthermore, restarting medications, even at the previous effective dose, may not return the patient to their prior baseline.[12] Psychiatric decompensation in the hospitalized patient can worsen medical outcomes, with decreased adherence to treatment plans. In extreme circumstances, patients may be at risk of self‐harm or suicide.
DRUG‐SPECIFIC DISCONTINUATION SYNDROMES
Antidepressants
Discontinuation of medications presents additional problems, and sudden cessation of psychotropic medications can lead to uncomfortable or even dangerous symptoms. For example, the serotonin discontinuation syndrome has been well documented. Chronic use of serotonin re‐uptake inhibitors (generally greater than 6 to 8 weeks) leads to downregulation of postsynaptic serotonin receptors. When selective serotonin re‐uptake inhibitors (SSRIs) or serotonin‐norepinephrine re‐uptake inhibitors are abruptly stopped, the brain experiences a relative decline in serotonin. Symptoms include a flu‐like illness, nausea, imbalance, insomnia, sensory disturbances, and dysphoria. Onset may be within hours of missing a dose, but typically occurs within 3 days of medication discontinuation. The syndrome is more likely to occur with cessation of medications of shorter half‐life and less likely to occur with medications with a long half‐life, such as fluoxetine (Table 1).[13, 14] The symptoms can be ameliorated with a gradual tapering or reintroduction of the antidepressant.[15] Untreated symptoms resolve in 1 to 2 weeks. Although the syndrome in isolation is not life‐threatening, a number of the symptoms can complicate medical illness and muddle diagnosis of other diseases.[14, 16]
| Medication | Half‐Life (Hours) |
|---|---|
| |
| SSRIs | |
| Fluoxetine | 84144 |
| Paroxetine | 21 |
| Sertraline | 26 |
| Citalopram | 35 |
| Escitalopram | 2732 |
| Fluvoxamine | 15 |
| SNRIs | |
| Venlafaxine | 313 |
| Duloxetine | 1116 |
Older antidepressants, including the tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs), have serotonergic effects, and thus discontinuation may cause the symptoms described above. However, these agents also have effects on other neurotransmitters. The TCAs block muscarinic cholinergic receptors, leading to upregulation. Abrupt cessation can lead to cholinergic rebound, with parkinsonism and mania emerging. Multiple case reports document improvement in these symptoms with an anticholinergic agent, such as benztropine.[17, 18] MAOIs lead to changes in ‐2 adrenergic and dopaminergic receptors. Sudden discontinuation has been associated with agitation, delirium, and psychosis; 1 case report even documents catatonia associated with autonomic instability.[19]
In addition, sudden discontinuation of antidepressants (including the SSRIs) may provoke mania or hypomania in some patients, regardless of whether they have experienced previous spontaneous manic episodes.[8]
Neuroleptics
Data for an antipsychotic withdrawal syndrome are less convincing than those for serotonergic agents. However, certain symptoms have been associated with abrupt neuroleptic discontinuation. Most frequently, gastrointestinal distress and diaphoresis are described. Anxiety, agitation, and insomnia are also common. These symptoms are thought to be associated with cholinergic rebound, mediated by direct effects of neuroleptics on muscarinic receptors or indirectly through dopamine receptor blockade and the dopamine‐cholinergic balance. Symptoms may be more severe when antimuscarinic, antiparkinsonism drugs are simultaneously stopped. Some authors argue that the timing of symptom onset can differentiate antipsychotic withdrawal from illness relapse, with discontinuation syndrome occurring within the first 7 days of medication cessation.[20]
Additionally, abrupt cessation of antipsychotics may be associated with rapid‐onset psychosis. The data are strongest for clozapine discontinuation, where overall incidence is approximately 20%. This is hypothesized to be mediated by dopamine receptor upregulation and subsequent hypersensitivity to endogenous dopamine. The emerging psychosis is purportedly distinct from the underlying illness. Episodes have been described in patients on chronic metoclopramide who have no prior psychiatric history, as well as in patients with bipolar disorder without psychosis prior to neuroleptic discontinuation.[21]
Movement disorders may emerge during neuroleptic discontinuation. Both parkinsonism and dyskinesias have been described. In some patients, dyskinesias resolve within weeks of drug discontinuation; however, others experience permanent symptoms, termed covert dyskinesia.[22] In rare circumstances, dyskinesias may affect the respiratory muscles, causing distress. Several case reports of withdrawal‐emergent respiratory dyskinesia have been reported following risperidone cessation.[23, 24, 25] Additionally, several case reports have described catatonia occurring after abrupt discontinuation of clozapine. In all cases, symptoms promptly resolved with reinitiation of clozapine.[26]
Neuroleptic malignant syndrome (NMS) is a rare but potentially fatal complication of antipsychotic administration. Symptoms include fever, rigidity, autonomic instability, and mental status changes.[27] Though NMS is hypothesized to occur due to dopamine receptor blockade, rare cases of NMS have also been reported with abrupt cessation of neuroleptics. Of the 8 case reports in the literature, 1 resulted in death.[22, 28]
Mood Stabilizers
Though some of the atypical antipsychotics are used to treat bipolar disorder, mood stabilizers are the mainstay of pharmacotherapy. The most commonly used mood stabilizers are lithium, valproic acid, lamotrigine, topirimate, carbamezapine, and oxcarbazepine. When mood stabilizers are discontinued, patients are at risk of psychiatric relapse, as documented above. However, withdrawal symptoms have not been commonly documented upon abrupt discontinuation of lithium or the anticonvulsants used to treat bipolar disorder.[29, 30]
Benzodiazepines
Benzodiazepines are widely prescribed for insomnia and anxiety. Chronic legal use of benzodiazepines is approximately 2% in the general population.[31] Like ethanol, benzodiazepines bind nonselectively to the GABA‐A receptor, resulting in downregulation of GABA receptors and compensatory increased N‐methyl‐D‐aspartate transmission. Sudden discontinuation of benzodiazepines results in a syndrome that mirrors that of alcohol withdrawal. Symptoms range from mild (tremor, insomnia, and anxiety) to life‐threatening (seizures, delirium, and autonomic instability). Serious withdrawal is more likely with substances of shorter half‐life and with higher chronic doses. Onset often occurs between 2 and 10 days after discontinuation, depending on the half‐life of the benzodiazepine.[32] Other rare serious reactions have been documented following abrupt benzodiazepine cessation, including NMS and catatonia.[30]
CONTINUATION
Reflexive discontinuation of psychotropic medications can clearly lead to adverse outcomes. However, when hospitalists decide to continue a patient's psychotropic medications, they must also be cognizant of potential complications. Modifications may be necessary because of hepatic, renal, or cardiac disease. In addition, physicians need to be aware of drug‐drug interactions. Pharmacotherapy for medically ill elderly patients may require dose modifications to account for an increased lipophilic volume of distribution and a decreased rate of metabolism.[33] Finally, pregnancy can present additional challenges regarding dose modifications and teratogenicity.
On the other hand, hospitalists must be aware that continuing a patient's psychotropic medication may not be the cause of new psychiatric symptoms. Drugs prescribed for medical disorders (eg, corticosteroids) often cause psychiatric symptoms. In addition, psychiatric symptoms may emerge at times of nonpsychotropic medication withdrawal or due to nonpsychotropic drug‐drug interactions.[4] Groups of medications commonly associated with psychiatric disturbances include analgesics, sedatives, anesthetics, anticonvulsants, and anticholinergics.
PHARMACOKINETICS: PSYCHOTROPIC TOXICITY
Medical illness alters the body's steady state, and renal or hepatic metabolism may be impaired when a patient requires hospitalization. Additionally, new medications may increase the effects of psychotropics, whether by intrinsic augmentation of effect or decreased psychotropic clearance. Ultimately, these changes can lead to psychotropic toxicity.
Specific toxicities merit discussion. First, serotonin syndrome is a potentially fatal condition. The majority of cases occur with synergistic serotonergic medication administration, though there are case reports of the syndrome occurring with addition of inhibitors of cytochrome p450 2D6 and/or 3A4 to SSRIs. A large number of medications from different classes have been indicated (Table 2).[34] Symptoms generally occur within 24 hours of medication administration and include mental status changes, autonomic instability, and neuromuscular hyperactivity. When serotonin syndrome is suspected, the offending agent should be discontinued immediately. There is no definitive treatment, though supportive care can be lifesaving.[34]
| Amphetamines and Derivatives | Antidepressants and Mood Stabilizers | Antimigraine Drugs | Analgesics | Antiemetics | Miscellaneous |
|---|---|---|---|---|---|
| |||||
| MDMA | Buspirone | Ergot alkaloids | Cyclobenzaprine | Metoclopramide | Cocaine |
| Dextroamphetamine | Carbamazepine | Triptans | Fentanyl | Ondansetron | Dextromethorphan |
| Methamphetamine | Lithium | Meperidine | Linezolid | ||
| Sibutramine | MAOIs | Tramadol | L‐tryptophan | ||
| SSRIs | 5‐hydroxytrytophan | ||||
| SNRIs | |||||
| Serotonin 2A receptor blockers (eg, trazodone) | |||||
| St. John's Wort | |||||
| TCAs | |||||
| Valproic Acid | |||||
In addition to serotonin syndrome, hypertensive emergency may occur due to drug interactions with MAOIs. MAOIs inhibit the enzyme monoamine oxidase, resulting in elevated levels of serotonin, histamine, and catecholamines in the blood. Coadministration of MAOIs and sympathomimetic agents (such as cough suppressants and analgesics) may dangerously increase adrenergic stimulation, elevating blood pressure to the point of end‐organ damage. Please see Table 3 for a full list of drugs indicated in MAOI‐associated hypertensive crisis.[35] To ensure safety, it is recommended that MAOIs be discontinued for 14 days prior to introducing medications with sympathomimetic properties, and vice versa. Because of its longer half‐life, a 5‐week washout period is recommended for fluoxetine.[35]
|
| Amphetamines |
| Analgesics: meperedine |
| Anesthetics |
| Antidepressants: buproprion, buspirone, other MAOIs, SSRIs, SNRIs, TCAs, |
| Mirtazapine |
| Cocaine |
| Dibenzazepine‐related agents: carbamezapine, cyclobenzaprine, perphenazine |
| Female sex steroids |
| Sympathomimetics: dopamine, epinephrine, levodopa, methyldopa, methylphenidate, norepinephrine, phenylalanine, reserpine, tyrosine, tryptophan |
| Other vasoconstrictors: pseudoephedrine, phenylephrine, phenylpropanolamine, ephedrine |
Lithium toxicity may result from changing patient pharmacokinetics. Lithium is almost entirely renally excreted, and acute kidney injury may precipitously raise serum levels. Within the renal collecting system, lithium is handled similarly to sodium, with 80% reabsorbed from the proximal tubule to the collecting duct. Thus, factors that decrease glomerular filtration rate (GFR) and increase proximal tubule absorption will increase serum lithium levels. For example, decreased effective arterial volume (due to dehydration, cirrhosis, nephrotic syndrome, or heart failure) may elevate lithium levels. Additionally, medications that decrease GFR may increase lithium reabsorption. These include nonsteroidal anti‐inflammatory drugs, angiotensin‐converting enzyme inhibitors, and thiazide diuretics. Because of lithium's narrow therapeutic index, small elevations in serum levels can lead to toxicity. Severity of intoxication correlates with serum concentration. Symptoms range from lethargy, weakness, tremor, ataxia, and gastrointestinal distress to coma, seizures, renal failure, and death. Toxicity is also associated with electrocardiograph (ECG) changes, including ST‐segment depression and T‐wave inversion in the lateral precordial leads. Sinus node dysfunction can also occur. Definitive treatment for lithium toxicity is hemodialysis.[36]
Though the therapeutic index is much wider for valproic acid than for lithium, valproate toxicity may also occur in the medically ill patient with previously stable serum levels. Valproic acid is highly protein‐bound at therapeutic levels, and is metabolized largely through hepatic glucuronidation. Initiation of medications that compete for protein‐binding sites, including aspirin, has led to valproate toxicity. Moreover, acute liver failure or addition of drugs that compete with hepatic microsomal enzymes may lead to decreased excretion of valproic acid. Poisoning may result in central nervous system (CNS) and respiratory depression, hypotension, cerebral edema, and pancreatitis. True hepatoxicity is rare, though hyperammonemia is widely documented. Thrombocytopenia is the most common hematologic abnormality associated with overdose. However, thrombocytopenia may also occur without complication in patients on stable therapeutic doses. Treatment is largely supportive, though hemoperfusion and hemodialysis may be used when serum levels are >300 g/mL, as only 35% of the drug is protein‐bound at that level. Naloxone has been shown in case reports to reverse valproic acid‐induced coma, and L‐carnitine has been increasingly recommended for hyperammonemia.[37, 38]
PHARMACOKINETICS: DRUG‐DRUG INTERACTIONS
As discussed above, the addition of a new medication can increase previously stable levels of psychotropic drugs, leading to toxicity. Conversely, mental health medications can alter the expected metabolism of a drug being used to treat acute medical illness. Many psychotropics are metabolized via the cytochrome p450 enzyme, particularly the SSRIs (Table 4).[39] A number of antimicrobial and antiarrythmic medications are also cleared via this route, leading to potential toxic or subtherapeutic levels when drug‐drug interactions occur.
| CYP 1A2 | CYP 2B6 | CYP 2C9 | CYP 2C19 | CYP 2D6 | CYP 3A4/3A5/3A7 |
|---|---|---|---|---|---|
| Clozapine | Bupropion | Amitriptyline | Phenytoin | Antidepressants | Alprazolam |
| Duloxetine | Methadone | Fluoxetine | Amitriptyline | Amitriptyline | Diazepam |
| Fluvoxamine | Phenytoin | Citalopram | Clomipramine | Midazolam | |
| Haloperidol | Clomipramine | Duloxetine | Aripiprazole | ||
| Imipramine | Diazepam | Desipramine | Buspirone | ||
| Olanzapine | Imipramine | Fluoxetine | Haldol | ||
| Ramelteon | Fluvoxamine | Quetiapine | |||
| Imipramine | Ziprasidone | ||||
| Nortriptyline | Zolpidem | ||||
| Paroxetine | Dextromethorphan | ||||
| Venlafaxine | |||||
| Antipsychotics | |||||
| Aripiprazole | |||||
| Clorpromazine | |||||
| Haldol | |||||
| Perphenazine | |||||
| Risperidone |
PSYCHOTROPIC ADVERSE EFFECTS
Psychotropic medications may also cause side effects that contribute to the clinical presentation, requiring ongoing monitoring, a dose reduction, or psychotropic discontinuation. Potential adverse effects that commonly impact psychopharmacologic management include anticholinergic side effects, cardiac effects, and sedation.
ANTICHOLINERGIC EFFECTS AND TOXICITY
Newly emerging anticholinergic effects may be particularly troubling. Dry mouth may cause swallowing difficulty and aspiration. Pupillary dilatation and dry eyes can increase risk of falls. Constipation may evolve into fecal impaction, and urinary retention can contribute to increased catheter use and infection. CNS effects are perhaps the most serious, ranging from drowsiness and memory impairment to frank delirium.[40]
Many psychotropic drugs are anti cholinergic. Among the antidepressants, the TCAs and paroxetine have the highest anticholinergic activity. Anticholinergic effects have also been reported with the low potency first generation neuroleptics and with the atypical antipsychotics olanzapine and clozapine. Additionally, medications used to treat the extrapyramidal symptoms associated with antipsychotics (such as benztropine and diphenhydramine) are strongly anticholinergic.[41] Patients without previous overt anticholinergic symptoms from these medications may experience adverse effects when hospitalized. Medical illness or new medications may alter psychotropic drug metabolism and elimination, leading to accumulation of their anticholinergic effects. Many medications used in the hospital also have intrinsic anticholinergic activity. These include some antiemetics, antispasmodics, antiarrhythmics, and histamine H2 receptor blockers. Elderly patients are particularly prone to anticholinergic effects due to age‐related deficits in cholinergic transmission.[40]
QTc PROLONGATION
QTc prolongation is a potentially lethal side effect of certain medications. Prolonged QTc increases the risk of cardiac mortality and sudden death, presumably related to onset of torsades de pointe. Certain antidepressants have consistently been associated with QTc prolongation, particularly the TCAs. In addition, the US Food and Drug Administration recently issued the recommendation that the SSRI citalopram not be used at doses >40 mg (and 20 mg in those with hepatic impairment or age >60 years) due to results of a randomized controlled trial that showed a dose‐response increase in QTc. Antipsychotic medications have also been shown to increase QTc, with the greatest evidence for thioridazine and the first‐generation, low‐potency neuroleptics. Haloperidol (particularly the intravenous formulation) has also been linked to both long QTc and torsades, though data may be confounded by the degree of medical illness of patients receiving the medication.[42] Of the atypicals, ziprasidone causes the greatest QTc prolongation.[43] However, a large retrospective cohort study showed similar risk increase of sudden cardiac death (2‐fold) among all antipsychotics (including typicals and atypicals) when examined individually.[44]
Additional risk factors for QTc prolongation abound in the hospitalized patient. These include many medical problems, including electrolyte abnormalities, heart conditions, renal and hepatic dysfunction, and CNS injury. Hospitalized patients are also exposed to the cumulative effects of medications that increase duration of QTc, such as class I and class III antiarrhythmics. Additionally, certain antimicrobials, including macrolide antibiotics and antifungals, have been associated with QTc prolongation via pharmacokinetic interactions.
Because of this, QTc should be measured upon admission in patients on stable doses of psychotropics that predispose to prolongation. Addition of other medications known to contribute to increased QTc should prompt further ECGs. Electrolytes, particularly potassium and magnesium, should be aggressively repleted. When QTc extends beyond 500 ms, consideration should be given to discontinuing or changing medications that can contribute to QTc prolongation (whether psychotropics or drugs used to treat acute medical problems).[42]
SEDATION
Sedation is another potential side effect of psychotropic medications. Although sedation is beneficial for agitation and anxiety, sedated patients may be unable to participate in treatment. They are also at greater risk of aspiration and falls.
Antipsychotics cause sedation through antagonism of 1 adrenergic and H1 histaminergic receptors. Effects are most pronounced with the low‐potency, first‐generation antipsychotics and the atypicals quetiapine and clozapine. Some antidepressants, including the TCAs and mirtazapine, also cause somnolence by histamine H1‐receptor antagonism. If a patient has been psychiatrically stable on a particular antipsychotic or antidepressant, but has psychotropic‐induced sedation that interferes with treatment, hospitalists may want to consider temporarily decreasing the dose.
Benzodiazepines induce sedation by increasing ‐aminobutyric acid‐ergic transmission. There is significant overlap between anxiolytic and sedating doses of benzodiazepines. The amount of sedation is related to dose, speed of absorption, and onset of CNS penetration. Thus, sedation may be minimized by using a lower dose or switching to an equivalent dose of a slower‐onset benzodiazepine.[45, 46]
CONCLUSION
The decision to continue or discontinue psychotropic medications is often challenging. It requires the hospitalist to carefully weigh the risks and benefits of the ongoing treatment versus discontinuation, while also considering the patient's preference whenever possible. Sudden cessation of psychotropics can lead to a number of unwanted complications, from mild withdrawal to life‐threatening autonomic instability and psychiatric decompensation. Yet psychotropic continuation can also lead to unwanted drug‐drug interactions and newly emergent side effects.
To improve patient safety and outcomes, hospitalists must take a cautious and well‐thought out approach to treating patients on psychotropic drugs. Reflexive cessation of home medications must be avoided. If hepatic or renal insufficiency develops, medication doses may need to be adjusted. When available, drug levels should guide dose adjustments. If side effects occur, the patient's medication list should be carefully reviewed for potential drug‐drug interactions. Maintaining mental health may mean substituting another drug for one that interferes with home psychotropic medications. Psychotropic doses may also be minimized to decrease side effects. When a psychotropic must be discontinued, tapering is recommended over an abrupt discontinuation, except in the case of an acute toxicity. Moreover, cross‐titration to another effective agent may prevent psychiatric decompensation.
Additionally, hospitalists should use all available resources when deciding on a patient's psychotropic regimen. Mobile devices and online resources can assist with pharmacokinetics. Pharmacists can help with more complex questions or potential drug substitutions. Consultation‐liaison psychiatrists can be a valuable resource in ensuring the safety and stability of a patient with psychiatric comorbidity within the medical environment. The consultant may assist by assessing a patient's current psychiatric state and recommending psychotropic medication changes when needed.
Disclosure
Nothing to report.
Mental illness is highly prevalent, with approximately 30% of the US population meeting criteria for at least 1 disorder.[1] In the medically ill population, psychiatric disease is even more common; a 2005 survey showed that half of all patients visiting primary care physicians met criteria for a mental disorder.[2] Conversely, those with serious mental illness suffer greater medical morbidity than the general population, with higher rates of obesity, diabetes, metabolic syndrome, cardiovascular disease, chronic obstructive pulmonary disease, human immunodeficiency virus, viral hepatitis, and tuberculosis.[3] When acute medical problems arise, those with mental illness endure longer hospitalizations; the presence of a psychiatric disturbance in the general medical setting has been shown to be a robust predictor of increased hospital length of stay.[4, 5]
Because of the strong correlation between medical and mental illness, hospitalists will care for patients with psychiatric disorders. Despite this, internists generally receive a paucity of formal training in the treatment of mental disturbances. One survey of university‐affiliated internal medicine residencies revealed that only 10% of programs offered any kind of modest curriculum in psychiatric education.[6] Regardless of this lack of preparation, hospitalists are called upon at each admission to make decisions that affect the psychiatric treatment of patients on psychotropic medication; namely, they must decide whether to continue or discontinue psychiatric medications. Many physicians reflexively discontinue a patient's chronic medications upon admission to the hospital; one study reported an adjusted odds ratio of between 1.18 and 1.86 for stopping a medication prescribed for a chronic condition.[7]
This review aims to assist the hospitalist in making an informed decision about the continuation of psychotropic medications in the medically ill patient. First, it examines the risks of stopping psychotropic medication, including psychiatric decompensation and discontinuation syndromes. It also explores the challenges of medication continuation in the context of changing pharmacokinetics and emerging side effects. Ultimately, physicians and patients must make collaborative decisions, weighing the risk of medication interactions against the potential adverse effects of psychiatric decompensation.
DISCONTINUATION
Decompensation of Mental Health
Approximately 10% to 15% of patients hospitalized for medical illness require reduction or discontinuation of psychotropic medications because they may be contributing to the clinical presentation.[4] The rate and method of drug discontinuation can affect the course of major psychiatric disorders.[8] A growing number of studies demonstrate high rates of relapse when medications are discontinued in patients suffering from mood disorders, schizophrenia, and anxiety disorders.[9] Abrupt cessation of psychotropics is especially dangerous, leading to a greater chance of destabilization than if medications are tapered. Episodes of active illness even appear to occur more frequently with sudden psychotropic cessation than they would in the natural course of untreated disease. This is true for several classes of psychotropics, including antidepressants, mood stabilizers, and antipsychotics. For example, in a study of pregnant women who suddenly stopped their psychotropic medication (both antidepressants and benzodiazepines), nearly one‐third experienced suicidal ideation.[10] Depression and suicidality have also been documented in bipolar patients who were abruptly taken off of lithium. More commonly, rapid lithium discontinuation in bipolar patients causes mania, with illness relapse as soon as 4 days after cessation.[10] Additionally, abrupt discontinuation of antipsychotics in patients with schizophrenia leads to early, and often severe, psychosis. One study found a relapse rate of 50% within 30 weeks of sudden oral neuroleptic cessation.[11] Furthermore, restarting medications, even at the previous effective dose, may not return the patient to their prior baseline.[12] Psychiatric decompensation in the hospitalized patient can worsen medical outcomes, with decreased adherence to treatment plans. In extreme circumstances, patients may be at risk of self‐harm or suicide.
DRUG‐SPECIFIC DISCONTINUATION SYNDROMES
Antidepressants
Discontinuation of medications presents additional problems, and sudden cessation of psychotropic medications can lead to uncomfortable or even dangerous symptoms. For example, the serotonin discontinuation syndrome has been well documented. Chronic use of serotonin re‐uptake inhibitors (generally greater than 6 to 8 weeks) leads to downregulation of postsynaptic serotonin receptors. When selective serotonin re‐uptake inhibitors (SSRIs) or serotonin‐norepinephrine re‐uptake inhibitors are abruptly stopped, the brain experiences a relative decline in serotonin. Symptoms include a flu‐like illness, nausea, imbalance, insomnia, sensory disturbances, and dysphoria. Onset may be within hours of missing a dose, but typically occurs within 3 days of medication discontinuation. The syndrome is more likely to occur with cessation of medications of shorter half‐life and less likely to occur with medications with a long half‐life, such as fluoxetine (Table 1).[13, 14] The symptoms can be ameliorated with a gradual tapering or reintroduction of the antidepressant.[15] Untreated symptoms resolve in 1 to 2 weeks. Although the syndrome in isolation is not life‐threatening, a number of the symptoms can complicate medical illness and muddle diagnosis of other diseases.[14, 16]
| Medication | Half‐Life (Hours) |
|---|---|
| |
| SSRIs | |
| Fluoxetine | 84144 |
| Paroxetine | 21 |
| Sertraline | 26 |
| Citalopram | 35 |
| Escitalopram | 2732 |
| Fluvoxamine | 15 |
| SNRIs | |
| Venlafaxine | 313 |
| Duloxetine | 1116 |
Older antidepressants, including the tricyclic antidepressants (TCAs) and monoamine oxidase inhibitors (MAOIs), have serotonergic effects, and thus discontinuation may cause the symptoms described above. However, these agents also have effects on other neurotransmitters. The TCAs block muscarinic cholinergic receptors, leading to upregulation. Abrupt cessation can lead to cholinergic rebound, with parkinsonism and mania emerging. Multiple case reports document improvement in these symptoms with an anticholinergic agent, such as benztropine.[17, 18] MAOIs lead to changes in ‐2 adrenergic and dopaminergic receptors. Sudden discontinuation has been associated with agitation, delirium, and psychosis; 1 case report even documents catatonia associated with autonomic instability.[19]
In addition, sudden discontinuation of antidepressants (including the SSRIs) may provoke mania or hypomania in some patients, regardless of whether they have experienced previous spontaneous manic episodes.[8]
Neuroleptics
Data for an antipsychotic withdrawal syndrome are less convincing than those for serotonergic agents. However, certain symptoms have been associated with abrupt neuroleptic discontinuation. Most frequently, gastrointestinal distress and diaphoresis are described. Anxiety, agitation, and insomnia are also common. These symptoms are thought to be associated with cholinergic rebound, mediated by direct effects of neuroleptics on muscarinic receptors or indirectly through dopamine receptor blockade and the dopamine‐cholinergic balance. Symptoms may be more severe when antimuscarinic, antiparkinsonism drugs are simultaneously stopped. Some authors argue that the timing of symptom onset can differentiate antipsychotic withdrawal from illness relapse, with discontinuation syndrome occurring within the first 7 days of medication cessation.[20]
Additionally, abrupt cessation of antipsychotics may be associated with rapid‐onset psychosis. The data are strongest for clozapine discontinuation, where overall incidence is approximately 20%. This is hypothesized to be mediated by dopamine receptor upregulation and subsequent hypersensitivity to endogenous dopamine. The emerging psychosis is purportedly distinct from the underlying illness. Episodes have been described in patients on chronic metoclopramide who have no prior psychiatric history, as well as in patients with bipolar disorder without psychosis prior to neuroleptic discontinuation.[21]
Movement disorders may emerge during neuroleptic discontinuation. Both parkinsonism and dyskinesias have been described. In some patients, dyskinesias resolve within weeks of drug discontinuation; however, others experience permanent symptoms, termed covert dyskinesia.[22] In rare circumstances, dyskinesias may affect the respiratory muscles, causing distress. Several case reports of withdrawal‐emergent respiratory dyskinesia have been reported following risperidone cessation.[23, 24, 25] Additionally, several case reports have described catatonia occurring after abrupt discontinuation of clozapine. In all cases, symptoms promptly resolved with reinitiation of clozapine.[26]
Neuroleptic malignant syndrome (NMS) is a rare but potentially fatal complication of antipsychotic administration. Symptoms include fever, rigidity, autonomic instability, and mental status changes.[27] Though NMS is hypothesized to occur due to dopamine receptor blockade, rare cases of NMS have also been reported with abrupt cessation of neuroleptics. Of the 8 case reports in the literature, 1 resulted in death.[22, 28]
Mood Stabilizers
Though some of the atypical antipsychotics are used to treat bipolar disorder, mood stabilizers are the mainstay of pharmacotherapy. The most commonly used mood stabilizers are lithium, valproic acid, lamotrigine, topirimate, carbamezapine, and oxcarbazepine. When mood stabilizers are discontinued, patients are at risk of psychiatric relapse, as documented above. However, withdrawal symptoms have not been commonly documented upon abrupt discontinuation of lithium or the anticonvulsants used to treat bipolar disorder.[29, 30]
Benzodiazepines
Benzodiazepines are widely prescribed for insomnia and anxiety. Chronic legal use of benzodiazepines is approximately 2% in the general population.[31] Like ethanol, benzodiazepines bind nonselectively to the GABA‐A receptor, resulting in downregulation of GABA receptors and compensatory increased N‐methyl‐D‐aspartate transmission. Sudden discontinuation of benzodiazepines results in a syndrome that mirrors that of alcohol withdrawal. Symptoms range from mild (tremor, insomnia, and anxiety) to life‐threatening (seizures, delirium, and autonomic instability). Serious withdrawal is more likely with substances of shorter half‐life and with higher chronic doses. Onset often occurs between 2 and 10 days after discontinuation, depending on the half‐life of the benzodiazepine.[32] Other rare serious reactions have been documented following abrupt benzodiazepine cessation, including NMS and catatonia.[30]
CONTINUATION
Reflexive discontinuation of psychotropic medications can clearly lead to adverse outcomes. However, when hospitalists decide to continue a patient's psychotropic medications, they must also be cognizant of potential complications. Modifications may be necessary because of hepatic, renal, or cardiac disease. In addition, physicians need to be aware of drug‐drug interactions. Pharmacotherapy for medically ill elderly patients may require dose modifications to account for an increased lipophilic volume of distribution and a decreased rate of metabolism.[33] Finally, pregnancy can present additional challenges regarding dose modifications and teratogenicity.
On the other hand, hospitalists must be aware that continuing a patient's psychotropic medication may not be the cause of new psychiatric symptoms. Drugs prescribed for medical disorders (eg, corticosteroids) often cause psychiatric symptoms. In addition, psychiatric symptoms may emerge at times of nonpsychotropic medication withdrawal or due to nonpsychotropic drug‐drug interactions.[4] Groups of medications commonly associated with psychiatric disturbances include analgesics, sedatives, anesthetics, anticonvulsants, and anticholinergics.
PHARMACOKINETICS: PSYCHOTROPIC TOXICITY
Medical illness alters the body's steady state, and renal or hepatic metabolism may be impaired when a patient requires hospitalization. Additionally, new medications may increase the effects of psychotropics, whether by intrinsic augmentation of effect or decreased psychotropic clearance. Ultimately, these changes can lead to psychotropic toxicity.
Specific toxicities merit discussion. First, serotonin syndrome is a potentially fatal condition. The majority of cases occur with synergistic serotonergic medication administration, though there are case reports of the syndrome occurring with addition of inhibitors of cytochrome p450 2D6 and/or 3A4 to SSRIs. A large number of medications from different classes have been indicated (Table 2).[34] Symptoms generally occur within 24 hours of medication administration and include mental status changes, autonomic instability, and neuromuscular hyperactivity. When serotonin syndrome is suspected, the offending agent should be discontinued immediately. There is no definitive treatment, though supportive care can be lifesaving.[34]
| Amphetamines and Derivatives | Antidepressants and Mood Stabilizers | Antimigraine Drugs | Analgesics | Antiemetics | Miscellaneous |
|---|---|---|---|---|---|
| |||||
| MDMA | Buspirone | Ergot alkaloids | Cyclobenzaprine | Metoclopramide | Cocaine |
| Dextroamphetamine | Carbamazepine | Triptans | Fentanyl | Ondansetron | Dextromethorphan |
| Methamphetamine | Lithium | Meperidine | Linezolid | ||
| Sibutramine | MAOIs | Tramadol | L‐tryptophan | ||
| SSRIs | 5‐hydroxytrytophan | ||||
| SNRIs | |||||
| Serotonin 2A receptor blockers (eg, trazodone) | |||||
| St. John's Wort | |||||
| TCAs | |||||
| Valproic Acid | |||||
In addition to serotonin syndrome, hypertensive emergency may occur due to drug interactions with MAOIs. MAOIs inhibit the enzyme monoamine oxidase, resulting in elevated levels of serotonin, histamine, and catecholamines in the blood. Coadministration of MAOIs and sympathomimetic agents (such as cough suppressants and analgesics) may dangerously increase adrenergic stimulation, elevating blood pressure to the point of end‐organ damage. Please see Table 3 for a full list of drugs indicated in MAOI‐associated hypertensive crisis.[35] To ensure safety, it is recommended that MAOIs be discontinued for 14 days prior to introducing medications with sympathomimetic properties, and vice versa. Because of its longer half‐life, a 5‐week washout period is recommended for fluoxetine.[35]
|
| Amphetamines |
| Analgesics: meperedine |
| Anesthetics |
| Antidepressants: buproprion, buspirone, other MAOIs, SSRIs, SNRIs, TCAs, |
| Mirtazapine |
| Cocaine |
| Dibenzazepine‐related agents: carbamezapine, cyclobenzaprine, perphenazine |
| Female sex steroids |
| Sympathomimetics: dopamine, epinephrine, levodopa, methyldopa, methylphenidate, norepinephrine, phenylalanine, reserpine, tyrosine, tryptophan |
| Other vasoconstrictors: pseudoephedrine, phenylephrine, phenylpropanolamine, ephedrine |
Lithium toxicity may result from changing patient pharmacokinetics. Lithium is almost entirely renally excreted, and acute kidney injury may precipitously raise serum levels. Within the renal collecting system, lithium is handled similarly to sodium, with 80% reabsorbed from the proximal tubule to the collecting duct. Thus, factors that decrease glomerular filtration rate (GFR) and increase proximal tubule absorption will increase serum lithium levels. For example, decreased effective arterial volume (due to dehydration, cirrhosis, nephrotic syndrome, or heart failure) may elevate lithium levels. Additionally, medications that decrease GFR may increase lithium reabsorption. These include nonsteroidal anti‐inflammatory drugs, angiotensin‐converting enzyme inhibitors, and thiazide diuretics. Because of lithium's narrow therapeutic index, small elevations in serum levels can lead to toxicity. Severity of intoxication correlates with serum concentration. Symptoms range from lethargy, weakness, tremor, ataxia, and gastrointestinal distress to coma, seizures, renal failure, and death. Toxicity is also associated with electrocardiograph (ECG) changes, including ST‐segment depression and T‐wave inversion in the lateral precordial leads. Sinus node dysfunction can also occur. Definitive treatment for lithium toxicity is hemodialysis.[36]
Though the therapeutic index is much wider for valproic acid than for lithium, valproate toxicity may also occur in the medically ill patient with previously stable serum levels. Valproic acid is highly protein‐bound at therapeutic levels, and is metabolized largely through hepatic glucuronidation. Initiation of medications that compete for protein‐binding sites, including aspirin, has led to valproate toxicity. Moreover, acute liver failure or addition of drugs that compete with hepatic microsomal enzymes may lead to decreased excretion of valproic acid. Poisoning may result in central nervous system (CNS) and respiratory depression, hypotension, cerebral edema, and pancreatitis. True hepatoxicity is rare, though hyperammonemia is widely documented. Thrombocytopenia is the most common hematologic abnormality associated with overdose. However, thrombocytopenia may also occur without complication in patients on stable therapeutic doses. Treatment is largely supportive, though hemoperfusion and hemodialysis may be used when serum levels are >300 g/mL, as only 35% of the drug is protein‐bound at that level. Naloxone has been shown in case reports to reverse valproic acid‐induced coma, and L‐carnitine has been increasingly recommended for hyperammonemia.[37, 38]
PHARMACOKINETICS: DRUG‐DRUG INTERACTIONS
As discussed above, the addition of a new medication can increase previously stable levels of psychotropic drugs, leading to toxicity. Conversely, mental health medications can alter the expected metabolism of a drug being used to treat acute medical illness. Many psychotropics are metabolized via the cytochrome p450 enzyme, particularly the SSRIs (Table 4).[39] A number of antimicrobial and antiarrythmic medications are also cleared via this route, leading to potential toxic or subtherapeutic levels when drug‐drug interactions occur.
| CYP 1A2 | CYP 2B6 | CYP 2C9 | CYP 2C19 | CYP 2D6 | CYP 3A4/3A5/3A7 |
|---|---|---|---|---|---|
| Clozapine | Bupropion | Amitriptyline | Phenytoin | Antidepressants | Alprazolam |
| Duloxetine | Methadone | Fluoxetine | Amitriptyline | Amitriptyline | Diazepam |
| Fluvoxamine | Phenytoin | Citalopram | Clomipramine | Midazolam | |
| Haloperidol | Clomipramine | Duloxetine | Aripiprazole | ||
| Imipramine | Diazepam | Desipramine | Buspirone | ||
| Olanzapine | Imipramine | Fluoxetine | Haldol | ||
| Ramelteon | Fluvoxamine | Quetiapine | |||
| Imipramine | Ziprasidone | ||||
| Nortriptyline | Zolpidem | ||||
| Paroxetine | Dextromethorphan | ||||
| Venlafaxine | |||||
| Antipsychotics | |||||
| Aripiprazole | |||||
| Clorpromazine | |||||
| Haldol | |||||
| Perphenazine | |||||
| Risperidone |
PSYCHOTROPIC ADVERSE EFFECTS
Psychotropic medications may also cause side effects that contribute to the clinical presentation, requiring ongoing monitoring, a dose reduction, or psychotropic discontinuation. Potential adverse effects that commonly impact psychopharmacologic management include anticholinergic side effects, cardiac effects, and sedation.
ANTICHOLINERGIC EFFECTS AND TOXICITY
Newly emerging anticholinergic effects may be particularly troubling. Dry mouth may cause swallowing difficulty and aspiration. Pupillary dilatation and dry eyes can increase risk of falls. Constipation may evolve into fecal impaction, and urinary retention can contribute to increased catheter use and infection. CNS effects are perhaps the most serious, ranging from drowsiness and memory impairment to frank delirium.[40]
Many psychotropic drugs are anti cholinergic. Among the antidepressants, the TCAs and paroxetine have the highest anticholinergic activity. Anticholinergic effects have also been reported with the low potency first generation neuroleptics and with the atypical antipsychotics olanzapine and clozapine. Additionally, medications used to treat the extrapyramidal symptoms associated with antipsychotics (such as benztropine and diphenhydramine) are strongly anticholinergic.[41] Patients without previous overt anticholinergic symptoms from these medications may experience adverse effects when hospitalized. Medical illness or new medications may alter psychotropic drug metabolism and elimination, leading to accumulation of their anticholinergic effects. Many medications used in the hospital also have intrinsic anticholinergic activity. These include some antiemetics, antispasmodics, antiarrhythmics, and histamine H2 receptor blockers. Elderly patients are particularly prone to anticholinergic effects due to age‐related deficits in cholinergic transmission.[40]
QTc PROLONGATION
QTc prolongation is a potentially lethal side effect of certain medications. Prolonged QTc increases the risk of cardiac mortality and sudden death, presumably related to onset of torsades de pointe. Certain antidepressants have consistently been associated with QTc prolongation, particularly the TCAs. In addition, the US Food and Drug Administration recently issued the recommendation that the SSRI citalopram not be used at doses >40 mg (and 20 mg in those with hepatic impairment or age >60 years) due to results of a randomized controlled trial that showed a dose‐response increase in QTc. Antipsychotic medications have also been shown to increase QTc, with the greatest evidence for thioridazine and the first‐generation, low‐potency neuroleptics. Haloperidol (particularly the intravenous formulation) has also been linked to both long QTc and torsades, though data may be confounded by the degree of medical illness of patients receiving the medication.[42] Of the atypicals, ziprasidone causes the greatest QTc prolongation.[43] However, a large retrospective cohort study showed similar risk increase of sudden cardiac death (2‐fold) among all antipsychotics (including typicals and atypicals) when examined individually.[44]
Additional risk factors for QTc prolongation abound in the hospitalized patient. These include many medical problems, including electrolyte abnormalities, heart conditions, renal and hepatic dysfunction, and CNS injury. Hospitalized patients are also exposed to the cumulative effects of medications that increase duration of QTc, such as class I and class III antiarrhythmics. Additionally, certain antimicrobials, including macrolide antibiotics and antifungals, have been associated with QTc prolongation via pharmacokinetic interactions.
Because of this, QTc should be measured upon admission in patients on stable doses of psychotropics that predispose to prolongation. Addition of other medications known to contribute to increased QTc should prompt further ECGs. Electrolytes, particularly potassium and magnesium, should be aggressively repleted. When QTc extends beyond 500 ms, consideration should be given to discontinuing or changing medications that can contribute to QTc prolongation (whether psychotropics or drugs used to treat acute medical problems).[42]
SEDATION
Sedation is another potential side effect of psychotropic medications. Although sedation is beneficial for agitation and anxiety, sedated patients may be unable to participate in treatment. They are also at greater risk of aspiration and falls.
Antipsychotics cause sedation through antagonism of 1 adrenergic and H1 histaminergic receptors. Effects are most pronounced with the low‐potency, first‐generation antipsychotics and the atypicals quetiapine and clozapine. Some antidepressants, including the TCAs and mirtazapine, also cause somnolence by histamine H1‐receptor antagonism. If a patient has been psychiatrically stable on a particular antipsychotic or antidepressant, but has psychotropic‐induced sedation that interferes with treatment, hospitalists may want to consider temporarily decreasing the dose.
Benzodiazepines induce sedation by increasing ‐aminobutyric acid‐ergic transmission. There is significant overlap between anxiolytic and sedating doses of benzodiazepines. The amount of sedation is related to dose, speed of absorption, and onset of CNS penetration. Thus, sedation may be minimized by using a lower dose or switching to an equivalent dose of a slower‐onset benzodiazepine.[45, 46]
CONCLUSION
The decision to continue or discontinue psychotropic medications is often challenging. It requires the hospitalist to carefully weigh the risks and benefits of the ongoing treatment versus discontinuation, while also considering the patient's preference whenever possible. Sudden cessation of psychotropics can lead to a number of unwanted complications, from mild withdrawal to life‐threatening autonomic instability and psychiatric decompensation. Yet psychotropic continuation can also lead to unwanted drug‐drug interactions and newly emergent side effects.
To improve patient safety and outcomes, hospitalists must take a cautious and well‐thought out approach to treating patients on psychotropic drugs. Reflexive cessation of home medications must be avoided. If hepatic or renal insufficiency develops, medication doses may need to be adjusted. When available, drug levels should guide dose adjustments. If side effects occur, the patient's medication list should be carefully reviewed for potential drug‐drug interactions. Maintaining mental health may mean substituting another drug for one that interferes with home psychotropic medications. Psychotropic doses may also be minimized to decrease side effects. When a psychotropic must be discontinued, tapering is recommended over an abrupt discontinuation, except in the case of an acute toxicity. Moreover, cross‐titration to another effective agent may prevent psychiatric decompensation.
Additionally, hospitalists should use all available resources when deciding on a patient's psychotropic regimen. Mobile devices and online resources can assist with pharmacokinetics. Pharmacists can help with more complex questions or potential drug substitutions. Consultation‐liaison psychiatrists can be a valuable resource in ensuring the safety and stability of a patient with psychiatric comorbidity within the medical environment. The consultant may assist by assessing a patient's current psychiatric state and recommending psychotropic medication changes when needed.
Disclosure
Nothing to report.
- , , , et al. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352(24):2515–2523.
- , , , , , . Mental disorders in primary care: prevalence and co‐morbidity among disorders. results from the functional illness in primary care (FIP) study. Psychol Med. 2005;35(8):1175–1184.
- , , , et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52–77.
- , , , et al. The Academy of Psychosomatic Medicine practice guidelines for psychiatric consultation in the general medical setting. The Academy of Psychosomatic Medicine. Psychosomatics. 1998;39(4):S8–S30.
- , , , , . Impact of psychiatric comorbidity on length of hospital stay for medical/surgical patients: a preliminary report. Am J Psychiatry. 1987;144(7):878–882.
- . Need for better psychiatric training for primary care providers. Acad Med. 1996;71(6):574–575.
- , , , et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306(8):840–847.
- . Discontinuing treatment for psychiatric disorders. J Psychiatry Neurosci. 2006;31(1):11–12.
- , , , , , . Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153(5):592–606.
- , , . Abrupt discontinuation of psychotropic drugs during pregnancy: fear of teratogenic risk and impact of counselling. J Psychiatry Neurosci. 2001;26(1):44–48.
- , , , , . Clinical risk following abrupt and gradual withdrawal of maintenance neuroleptic treatment. Arch Gen Psychiatry. 1997;54(1):49–55.
- . Potential adverse effects of discontinuing psychotropic drugs. Part 3: Antipsychotic, dopaminergic, and mood‐stabilizing drugs. J Psychosoc Nurs Ment Health Serv. 2010;48(8):11–14.
- , . Fluoxetine. In: Schatzberg AF, Nemeroff CB, eds. Textbook of Psychopharmacology. 2nd ed. Chapter 13. Washington, DC: American Psychiatric Press; 2004:235.
- , , , , . Antidepressant discontinuation syndrome. Am Fam Physician. 2006;74(3):449–456.
- , , , , . Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77–87.
- , , , . Selective serotonin reuptake inhibitor discontinuation syndrome: proposed diagnostic criteria. J Psychiatry Neurosci. 2000;25(3):255–261.
- . Antidepressant withdrawal syndromes: phenomenology and pathophysiology. Acta Psychiatr Scand. 1989;79(2):113–117.
- . Heterocyclic antidepressant, monoamine oxidase inhibitor and neuroleptic withdrawal phenomena. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14(2):137–161.
- . Monoamine oxidase inhibitor withdrawal phenomena: symptoms and pathophysiology. Acta Psychiatr Scand. 1988;78(1):1–7.
- , . Antipsychotic withdrawal symptoms: phenomenology and pathophysiology. Acta Psychiatr Scand. 1988;77(3):241–246.
- . Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal‐related relapse. Acta Psychiatr Scand. 2006;114(1):3–13.
- , . Neuroleptic malignant syndrome after neuroleptic discontinuation. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19(8):1323–1334.
- , . Withdrawal‐emergent dyskinesia in a patient on risperidone undergoing dosage reduction. Ann Clin Psychiatry. 1996;8(3):179–182.
- , , . Respiratory dyskinesia as discontinuation effect of risperidone. J Clin Psychopharmacol. 2005;25(6):609.
- , . Withdrawal‐emergent respiratory dyskinesia with risperidone treated with clozapine. J Neuropsychiatry Clin Neurosci. 2010;22(2):E24.
- , . Clozapine‐withdrawal catatonia. Psychosomatics. 2010;51(4):355–355.e2.
- , , . Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870–876.
- , , . Psychotropic discontinuation symptoms: a case of withdrawal neuroleptic malignant syndrome. Gen Hosp Psychiatry. 2006;28(6):541–543.
- , . Discontinuation symptoms and psychotropic drugs. Lancet. 2000;355(9210):1184.
- . Potential adverse effects of discontinuing psychotropic drugs. J Psychosoc Nurs Ment Health Serv. 2010;48(9):11–14.
- , , , . Pharmacological interventions for benzodiazepine mono‐dependence management in outpatient settings. Cochrane Database Syst Rev. 2006(3):CD005194.
- , . Substance abuse and withdrawal in the critical care setting. Crit Care Clin. 2008;24(4):767–788, viii.
- . Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67–76.
- , . Prevention, recognition, and management of serotonin syndrome. Am Fam Physician. 2010;81(9):1139–1142.
- . Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73(suppl 1):17–24.
- , . Lithium intoxication. J Am Soc Nephrol. 1999;10(3):666–674.
- . Valproic acid toxicity: overview and management. J Toxicol Clin Toxicol. 2002;40(6):789–801.
- , , . Lithium. In: Schatzberg AF, Nemeroff CB, eds. Textbook of Psychopharmacology. 2nd ed. Chapter 35. Washington, DC: American Psychiatric Press; 2004:547–549.
- , . 2010 guide to psychiatric drug interactions. Prim Psychiatry. 2009;16(12):45–74.
- , . Anticholinergic side‐effects of drugs in elderly people. J R Soc Med. 2000;93(9):457–462.
- , , . Measurement of anticholinergic effects of psychotropic drugs in humans. Pharmacopsychiatry. 2005;38(5):187–193.
- , , , , . QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics. 2013;54(1):1–13.
- , , , et al. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol. 2004;24(1):62–69.
- , , , , . Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225–235.
- , . Sedation, an unpleasant, undesirable and potentially dangerous side‐effect of many psychotropic drugs. Hum Psychopharmacol. 2004;19(2):135–139.
- , . Toxicology and overdose of atypical antipsychotics. J Emerg Med. 2012;43(5):906–913.
- , , , et al. Prevalence and treatment of mental disorders, 1990 to 2003. N Engl J Med. 2005;352(24):2515–2523.
- , , , , , . Mental disorders in primary care: prevalence and co‐morbidity among disorders. results from the functional illness in primary care (FIP) study. Psychol Med. 2005;35(8):1175–1184.
- , , , et al. Physical illness in patients with severe mental disorders. I. Prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52–77.
- , , , et al. The Academy of Psychosomatic Medicine practice guidelines for psychiatric consultation in the general medical setting. The Academy of Psychosomatic Medicine. Psychosomatics. 1998;39(4):S8–S30.
- , , , , . Impact of psychiatric comorbidity on length of hospital stay for medical/surgical patients: a preliminary report. Am J Psychiatry. 1987;144(7):878–882.
- . Need for better psychiatric training for primary care providers. Acad Med. 1996;71(6):574–575.
- , , , et al. Association of ICU or hospital admission with unintentional discontinuation of medications for chronic diseases. JAMA. 2011;306(8):840–847.
- . Discontinuing treatment for psychiatric disorders. J Psychiatry Neurosci. 2006;31(1):11–12.
- , , , , , . Pharmacologic management of psychiatric illness during pregnancy: dilemmas and guidelines. Am J Psychiatry. 1996;153(5):592–606.
- , , . Abrupt discontinuation of psychotropic drugs during pregnancy: fear of teratogenic risk and impact of counselling. J Psychiatry Neurosci. 2001;26(1):44–48.
- , , , , . Clinical risk following abrupt and gradual withdrawal of maintenance neuroleptic treatment. Arch Gen Psychiatry. 1997;54(1):49–55.
- . Potential adverse effects of discontinuing psychotropic drugs. Part 3: Antipsychotic, dopaminergic, and mood‐stabilizing drugs. J Psychosoc Nurs Ment Health Serv. 2010;48(8):11–14.
- , . Fluoxetine. In: Schatzberg AF, Nemeroff CB, eds. Textbook of Psychopharmacology. 2nd ed. Chapter 13. Washington, DC: American Psychiatric Press; 2004:235.
- , , , , . Antidepressant discontinuation syndrome. Am Fam Physician. 2006;74(3):449–456.
- , , , , . Selective serotonin reuptake inhibitor discontinuation syndrome: a randomized clinical trial. Biol Psychiatry. 1998;44(2):77–87.
- , , , . Selective serotonin reuptake inhibitor discontinuation syndrome: proposed diagnostic criteria. J Psychiatry Neurosci. 2000;25(3):255–261.
- . Antidepressant withdrawal syndromes: phenomenology and pathophysiology. Acta Psychiatr Scand. 1989;79(2):113–117.
- . Heterocyclic antidepressant, monoamine oxidase inhibitor and neuroleptic withdrawal phenomena. Prog Neuropsychopharmacol Biol Psychiatry. 1990;14(2):137–161.
- . Monoamine oxidase inhibitor withdrawal phenomena: symptoms and pathophysiology. Acta Psychiatr Scand. 1988;78(1):1–7.
- , . Antipsychotic withdrawal symptoms: phenomenology and pathophysiology. Acta Psychiatr Scand. 1988;77(3):241–246.
- . Does antipsychotic withdrawal provoke psychosis? Review of the literature on rapid onset psychosis (supersensitivity psychosis) and withdrawal‐related relapse. Acta Psychiatr Scand. 2006;114(1):3–13.
- , . Neuroleptic malignant syndrome after neuroleptic discontinuation. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19(8):1323–1334.
- , . Withdrawal‐emergent dyskinesia in a patient on risperidone undergoing dosage reduction. Ann Clin Psychiatry. 1996;8(3):179–182.
- , , . Respiratory dyskinesia as discontinuation effect of risperidone. J Clin Psychopharmacol. 2005;25(6):609.
- , . Withdrawal‐emergent respiratory dyskinesia with risperidone treated with clozapine. J Neuropsychiatry Clin Neurosci. 2010;22(2):E24.
- , . Clozapine‐withdrawal catatonia. Psychosomatics. 2010;51(4):355–355.e2.
- , , . Neuroleptic malignant syndrome. Am J Psychiatry. 2007;164(6):870–876.
- , , . Psychotropic discontinuation symptoms: a case of withdrawal neuroleptic malignant syndrome. Gen Hosp Psychiatry. 2006;28(6):541–543.
- , . Discontinuation symptoms and psychotropic drugs. Lancet. 2000;355(9210):1184.
- . Potential adverse effects of discontinuing psychotropic drugs. J Psychosoc Nurs Ment Health Serv. 2010;48(9):11–14.
- , , , . Pharmacological interventions for benzodiazepine mono‐dependence management in outpatient settings. Cochrane Database Syst Rev. 2006(3):CD005194.
- , . Substance abuse and withdrawal in the critical care setting. Crit Care Clin. 2008;24(4):767–788, viii.
- . Pharmacokinetics and drug metabolism in the elderly. Drug Metab Rev. 2009;41(2):67–76.
- , . Prevention, recognition, and management of serotonin syndrome. Am Fam Physician. 2010;81(9):1139–1142.
- . Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73(suppl 1):17–24.
- , . Lithium intoxication. J Am Soc Nephrol. 1999;10(3):666–674.
- . Valproic acid toxicity: overview and management. J Toxicol Clin Toxicol. 2002;40(6):789–801.
- , , . Lithium. In: Schatzberg AF, Nemeroff CB, eds. Textbook of Psychopharmacology. 2nd ed. Chapter 35. Washington, DC: American Psychiatric Press; 2004:547–549.
- , . 2010 guide to psychiatric drug interactions. Prim Psychiatry. 2009;16(12):45–74.
- , . Anticholinergic side‐effects of drugs in elderly people. J R Soc Med. 2000;93(9):457–462.
- , , . Measurement of anticholinergic effects of psychotropic drugs in humans. Pharmacopsychiatry. 2005;38(5):187–193.
- , , , , . QTc prolongation, torsades de pointes, and psychotropic medications. Psychosomatics. 2013;54(1):1–13.
- , , , et al. A randomized evaluation of the effects of six antipsychotic agents on QTc, in the absence and presence of metabolic inhibition. J Clin Psychopharmacol. 2004;24(1):62–69.
- , , , , . Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225–235.
- , . Sedation, an unpleasant, undesirable and potentially dangerous side‐effect of many psychotropic drugs. Hum Psychopharmacol. 2004;19(2):135–139.
- , . Toxicology and overdose of atypical antipsychotics. J Emerg Med. 2012;43(5):906–913.
Woman With Hip Pain After Car Accident
ANSWER
The radiograph demonstrates evidence of contrast material within the bladder. There is evidence of fixation of an old subcapital femoral neck fracture on the left.
There is an acute, mildly displaced right intertrochanteric fracture of the right hip. The orthopedic service was consulted, and plans were established to subsequently fix this fracture surgically.
ANSWER
The radiograph demonstrates evidence of contrast material within the bladder. There is evidence of fixation of an old subcapital femoral neck fracture on the left.
There is an acute, mildly displaced right intertrochanteric fracture of the right hip. The orthopedic service was consulted, and plans were established to subsequently fix this fracture surgically.
ANSWER
The radiograph demonstrates evidence of contrast material within the bladder. There is evidence of fixation of an old subcapital femoral neck fracture on the left.
There is an acute, mildly displaced right intertrochanteric fracture of the right hip. The orthopedic service was consulted, and plans were established to subsequently fix this fracture surgically.
A 55-year-old woman is transferred to your facility with injuries sustained in a motor vehicle collision. She was an unrestrained front-seat passenger in a vehicle that rear-ended another vehicle. There was no airbag deployment, and the patient believes she struck her face on the windshield. At the outside facility, it was determined that she had a cervical fracture and facial fractures. Upon arrival at your facility, she is complaining of bilateral hip pain as well. Her medical history is significant for coronary artery disease, several myocardial infarctions, hypertension, and stroke. She has a pacemaker. Six months ago, she had an open reduction internal fixation of her left hip for a fracture she sustained in a fall. Primary survey reveals a female who is uncomfortable but alert and oriented. Vital signs are normal. She has some facial swelling and bruising. Her heart and lungs are clear; abdomen is benign. She is able to move her upper extremities with-out any problems. She has limited movement of her lower extremities due to pain in her pelvis. She is able to move both feet and toes, and distal pulses and sensation are intact. No obvious leg shortening is noted. A portable radiograph of the pelvis is obtained. What is your impression?
Child hit by car
ANSWER
The chest radiograph demonstrates no acute abnormalities within the lungs, ribs, or chest. Of note, there are two radiodensities consistent with teeth, which are presumed to be in the patient’s stomach (most likely secondary to being swallowed following trauma to his face). Upon reexamination, it is noted that the child’s two front incisors are missing, with minimally bleeding sockets. Other than reassurance, no specific intervention was required.
ANSWER
The chest radiograph demonstrates no acute abnormalities within the lungs, ribs, or chest. Of note, there are two radiodensities consistent with teeth, which are presumed to be in the patient’s stomach (most likely secondary to being swallowed following trauma to his face). Upon reexamination, it is noted that the child’s two front incisors are missing, with minimally bleeding sockets. Other than reassurance, no specific intervention was required.
ANSWER
The chest radiograph demonstrates no acute abnormalities within the lungs, ribs, or chest. Of note, there are two radiodensities consistent with teeth, which are presumed to be in the patient’s stomach (most likely secondary to being swallowed following trauma to his face). Upon reexamination, it is noted that the child’s two front incisors are missing, with minimally bleeding sockets. Other than reassurance, no specific intervention was required.
A 6-year-old boy is brought to your facility by ambulance after being hit by a car. The child was apparently riding his bike when a slow-moving vehicle turned onto the street and accidentally bumped him, knocking him to the ground. He was not wearing a helmet. The child is crying but somewhat consolable. His medical history is unremarkable. On initial assessment, he is awake, crying, and moving all of his extremities spontaneously. His vital signs include a temperature of 36.3°C; blood pressure, 149/72 mm Hg; pulse, 110 beats/min; and respiratory rate, 22 breaths/min. Physical examination reveals several abrasions to his face, nose, and lips. Otherwise, he is normocephalic. His pupils are equal and react appropriately. Heart and lung sounds are clear, and the abdomen appears benign. You order some preliminary labwork and CT of the head. In addition, a portable chest radiograph is obtained (shown). What is your impression?
Is Active Patient a “Picture of Health”?
ANSWER
The correct interpretation includes marked sinus bradycardia, a right bundle branch block, and T-wave abnormalities in the lateral leads.
Sinus bradycardia is evidenced by a sinus rate less than 60 beats/min and may be considered “marked” if the rate is less than 50 beats/min.
A right bundle branch block is indicated by a QRS duration ≥ 120 ms, a terminal broad S wave in lead I, and the presence of an RSR’ pattern in lead V1.
Small or nonexistent T waves in leads V5 and V6 are suggestive of lateral ischemia but are not diagnostic in this individual.
His marked bradycardia was attributed to his exceptional athleticism and the fact that the ECG was taken “at rest.” It was not of concern, nor did it require treatment.
ANSWER
The correct interpretation includes marked sinus bradycardia, a right bundle branch block, and T-wave abnormalities in the lateral leads.
Sinus bradycardia is evidenced by a sinus rate less than 60 beats/min and may be considered “marked” if the rate is less than 50 beats/min.
A right bundle branch block is indicated by a QRS duration ≥ 120 ms, a terminal broad S wave in lead I, and the presence of an RSR’ pattern in lead V1.
Small or nonexistent T waves in leads V5 and V6 are suggestive of lateral ischemia but are not diagnostic in this individual.
His marked bradycardia was attributed to his exceptional athleticism and the fact that the ECG was taken “at rest.” It was not of concern, nor did it require treatment.
ANSWER
The correct interpretation includes marked sinus bradycardia, a right bundle branch block, and T-wave abnormalities in the lateral leads.
Sinus bradycardia is evidenced by a sinus rate less than 60 beats/min and may be considered “marked” if the rate is less than 50 beats/min.
A right bundle branch block is indicated by a QRS duration ≥ 120 ms, a terminal broad S wave in lead I, and the presence of an RSR’ pattern in lead V1.
Small or nonexistent T waves in leads V5 and V6 are suggestive of lateral ischemia but are not diagnostic in this individual.
His marked bradycardia was attributed to his exceptional athleticism and the fact that the ECG was taken “at rest.” It was not of concern, nor did it require treatment.
A 62-year-old man presents for a preoperative history and physical exam prior to surgical repair of an injury to his right anterior cruciate ligament (ACL). He reports that he has been healthy all his life and has never had an injury or illness requiring hospitalization. He is an accountant at a local financial institution and has a very active lifestyle, which includes competitive cycling, running, and skiing. He recently completed his fourth triathlon and was training for his sixth marathon until his injury occurred. One week ago, he was skiing moguls on a black diamond course when he fell and tumbled about 20 feet before stopping. His right ski binding did not release from the boot. He felt his right knee “pop” and knew immediately that he had sustained a serious injury. When he tried to stand, he was unable to bear weight on his right leg. The ski patrol transported him off the slope via basket. He was then taken to a local hospital by a colleague. Physical exam and MRI confirmed an avulsion of the ACL. He has been convalescing at home (having delayed his surgery in order to catch up on paper-work for work) and is scheduled for surgery in two days’ time. Medical history is unremarkable. Aside from the usual childhood illnesses (eg, ear infections, chicken pox, mumps), he has been very healthy and attributes this to a strict diet and rigorous exercise. Social history reveals that he is married to an attorney and has no children. He has never smoked or taken recreational drugs, and he consumes approximately one bottle of wine per week. His current medications include naproxen as needed for pain, a daily aspirin, fish oil, a multivitamin, and omeprazole on rare occasions. The review of systems is remarkable only for occasional gastroesophageal re-flux, which is exacerbated by spicy dishes containing curry. Physical exam reveals a thin, athletic male in no acute distress. His weight is 168 lb, and his height is 74”. Vital signs include a blood pressure of 104/62 mm Hg; pulse, 50 beats/min; respiratory rate, 14 breaths/min-1; and temperature, 98°F. Examination of the head, neck, lungs, heart, abdomen, skin, and nervous system yields normal findings. Lachman, pivot shift, and anterior drawer maneuvers of the right knee are all positive. A routine ECG is performed that reveals the following: a ventricular rate of 49 beats/min; PR interval, 176 ms; QRS dura-tion, 120 ms; QT/QTc interval, 430/388 ms; P axis, 14°; R axis, 38°; and T axis, 103°. What is your interpretation of this ECG?
Clear Cell Acanthoma
Test your knowledge on clear cell acanthoma with MD-IQ: the medical intelligence quiz. Click here to answer 5 questions.
A Compulsion to Scratch, But Is There an Itch?
ANSWER
The correct answer is neurotic excoriations (choice “d”), a chronic condition thought to be a psychologic process with dermatologic manifestations, consciously created by repetitive scratching and rubbing. Focally, it can manifest with skin alterations very similar to lichen simplex chronicus (choice “a”; also known as neurodermatitis), but the latter refers to very limited, localized processes and does not involve the psychiatric overlay seen with neurotic excoriations.
Patients with dermatitis artefacta (choice “b”; formerly called factitial dermatitis) consciously create their lesions for secondary gain, often using sharp objects such as nail files, kitchen utensils, or even shards of broken glass. Dermatitis artefacta lesions, which are relatively sparse and bizarre in appearance, can also be created by the application of caustic chemicals, or even by injection of foreign substances.
The differential rightly includes any number of skin conditions such as bullous pemphigoid (choice “c”). However, this was effectively ruled out by the biopsy and also by the morphology and extended chronicity of the patient’s complaint.
DISCUSSION
Neurotic excoriations (NE) are usually created by unconscious picking, scratching, or rubbing. There may be a precipitating minor skin pathology (eg, insect bite, folliculitis or acne), but it can develop independent of any such process. Its origins can often be tied to upsetting life events, such as divorce, death, or early dementia.
More history taking from this patient and her family revealed that her skin problems began after her husband died in an accident, after which, according to her children, “she has never been the same.” Her picking accelerated when she moved to an assisted living setting.
Because patients create neurotic excoriations, their lesions have the quality of an “outside job,” with clean linear erosions, crusts, and scars that can be hypopigmented or hyperpigmented, depending on the patient’s skin type. Similar in size and shape, the lesions tend to be bilaterally and symmetrically distributed and confined to areas within easy reach, such as the extensor surfaces of the arms and the upper part of the back.
The vast majority of NE patients are adult women, though it is also seen in children as a manifestation of comorbid psychopathology or other psychosocial stressor.
TREATMENT
As one might expect, treatment of NE is difficult, particularly since many patients find it impossible to accept the role their mental state plays in the creation and perpetuation of their condition. In the best of all possible scenarios, the patient would be seen and followed by a psychiatrist, who would probably prescribe psychoactive medication.
Failing that—or even in addition to that treatment—one could, at a minimum, find ways to distract the patient, trim her nails as much as possible, and/or place barriers between the offending nails and the skin in question.
Topical medications, such as steroid creams, are of very limited usefulness, as are oral antibiotics and antihistamines.
ANSWER
The correct answer is neurotic excoriations (choice “d”), a chronic condition thought to be a psychologic process with dermatologic manifestations, consciously created by repetitive scratching and rubbing. Focally, it can manifest with skin alterations very similar to lichen simplex chronicus (choice “a”; also known as neurodermatitis), but the latter refers to very limited, localized processes and does not involve the psychiatric overlay seen with neurotic excoriations.
Patients with dermatitis artefacta (choice “b”; formerly called factitial dermatitis) consciously create their lesions for secondary gain, often using sharp objects such as nail files, kitchen utensils, or even shards of broken glass. Dermatitis artefacta lesions, which are relatively sparse and bizarre in appearance, can also be created by the application of caustic chemicals, or even by injection of foreign substances.
The differential rightly includes any number of skin conditions such as bullous pemphigoid (choice “c”). However, this was effectively ruled out by the biopsy and also by the morphology and extended chronicity of the patient’s complaint.
DISCUSSION
Neurotic excoriations (NE) are usually created by unconscious picking, scratching, or rubbing. There may be a precipitating minor skin pathology (eg, insect bite, folliculitis or acne), but it can develop independent of any such process. Its origins can often be tied to upsetting life events, such as divorce, death, or early dementia.
More history taking from this patient and her family revealed that her skin problems began after her husband died in an accident, after which, according to her children, “she has never been the same.” Her picking accelerated when she moved to an assisted living setting.
Because patients create neurotic excoriations, their lesions have the quality of an “outside job,” with clean linear erosions, crusts, and scars that can be hypopigmented or hyperpigmented, depending on the patient’s skin type. Similar in size and shape, the lesions tend to be bilaterally and symmetrically distributed and confined to areas within easy reach, such as the extensor surfaces of the arms and the upper part of the back.
The vast majority of NE patients are adult women, though it is also seen in children as a manifestation of comorbid psychopathology or other psychosocial stressor.
TREATMENT
As one might expect, treatment of NE is difficult, particularly since many patients find it impossible to accept the role their mental state plays in the creation and perpetuation of their condition. In the best of all possible scenarios, the patient would be seen and followed by a psychiatrist, who would probably prescribe psychoactive medication.
Failing that—or even in addition to that treatment—one could, at a minimum, find ways to distract the patient, trim her nails as much as possible, and/or place barriers between the offending nails and the skin in question.
Topical medications, such as steroid creams, are of very limited usefulness, as are oral antibiotics and antihistamines.
ANSWER
The correct answer is neurotic excoriations (choice “d”), a chronic condition thought to be a psychologic process with dermatologic manifestations, consciously created by repetitive scratching and rubbing. Focally, it can manifest with skin alterations very similar to lichen simplex chronicus (choice “a”; also known as neurodermatitis), but the latter refers to very limited, localized processes and does not involve the psychiatric overlay seen with neurotic excoriations.
Patients with dermatitis artefacta (choice “b”; formerly called factitial dermatitis) consciously create their lesions for secondary gain, often using sharp objects such as nail files, kitchen utensils, or even shards of broken glass. Dermatitis artefacta lesions, which are relatively sparse and bizarre in appearance, can also be created by the application of caustic chemicals, or even by injection of foreign substances.
The differential rightly includes any number of skin conditions such as bullous pemphigoid (choice “c”). However, this was effectively ruled out by the biopsy and also by the morphology and extended chronicity of the patient’s complaint.
DISCUSSION
Neurotic excoriations (NE) are usually created by unconscious picking, scratching, or rubbing. There may be a precipitating minor skin pathology (eg, insect bite, folliculitis or acne), but it can develop independent of any such process. Its origins can often be tied to upsetting life events, such as divorce, death, or early dementia.
More history taking from this patient and her family revealed that her skin problems began after her husband died in an accident, after which, according to her children, “she has never been the same.” Her picking accelerated when she moved to an assisted living setting.
Because patients create neurotic excoriations, their lesions have the quality of an “outside job,” with clean linear erosions, crusts, and scars that can be hypopigmented or hyperpigmented, depending on the patient’s skin type. Similar in size and shape, the lesions tend to be bilaterally and symmetrically distributed and confined to areas within easy reach, such as the extensor surfaces of the arms and the upper part of the back.
The vast majority of NE patients are adult women, though it is also seen in children as a manifestation of comorbid psychopathology or other psychosocial stressor.
TREATMENT
As one might expect, treatment of NE is difficult, particularly since many patients find it impossible to accept the role their mental state plays in the creation and perpetuation of their condition. In the best of all possible scenarios, the patient would be seen and followed by a psychiatrist, who would probably prescribe psychoactive medication.
Failing that—or even in addition to that treatment—one could, at a minimum, find ways to distract the patient, trim her nails as much as possible, and/or place barriers between the offending nails and the skin in question.
Topical medications, such as steroid creams, are of very limited usefulness, as are oral antibiotics and antihistamines.
At her daughters’ insistence, this 69-year-old woman requests referral to dermatology for a skin condition that has been present for at least 20 years. During that time, she has seen many medical providers (including dermatologists) and has tried many different treatments (eg, creams, oral antibiotics, oral steroids, and antihistamines). While some of these helped a bit, most did not help at all—nor did the constant nagging at the patient by family and caregivers. Nonetheless, her daughters feel strongly that their mother is perpetuating the problem with her “scratching and picking.” They have observed that when she is able to leave her arms alone, the improvement in her skin is dramatic. For example, years ago, she broke her wrist and was placed in a cast for six weeks; when it was removed, the affected arm was completely clear (except for multiple old scars). Everyone, including the patient, was ecstatic—but a week later, the lesions returned. The extensor aspects of both arms and hands are covered with linear excoriations, scars, and scabs, with focal hyperpigmentation in many of the excoriated areas. Overall, the skin in these areas is re-markably thickened and focally shiny. Her skin elsewhere—such as her palms and the volar aspects of her arms—is relatively clear. Throughout the examination, the patient’s hands never stop rubbing and scratching her arms, even as she weakly denies doing so. “Whatever happens, I’m not going to see a shrink,” she says. Clearly, a biopsy is in order, with a sample taken from a typical section of her forearm. The results show minimal changes but demonstrate hyperkeratosis. Blood work, including a complete metabolic profile and complete blood count, fail to show any evidence of systemic disease.
Foot Pain Following a Car Crash
The radiograph demonstrates an acute fracture of the second, third, and fourth distal metatarsals. The third and fourth are mildly impacted.
In addition, there is a deformity noted within the medial cuneiform, strongly suggestive of a fracture. This was later confirmed by CT. Orthopedic consultation was obtained.
The radiograph demonstrates an acute fracture of the second, third, and fourth distal metatarsals. The third and fourth are mildly impacted.
In addition, there is a deformity noted within the medial cuneiform, strongly suggestive of a fracture. This was later confirmed by CT. Orthopedic consultation was obtained.
The radiograph demonstrates an acute fracture of the second, third, and fourth distal metatarsals. The third and fourth are mildly impacted.
In addition, there is a deformity noted within the medial cuneiform, strongly suggestive of a fracture. This was later confirmed by CT. Orthopedic consultation was obtained.
Following a motor vehicle collision, a 60-year-old woman is brought in by emergency medical transport. She was a restrained driver in a vehicle that went out of control, hit a tree, and ended up in a ditch. There was a prolonged extrication time (> 30 minutes) due to extensive damage to the front of the vehicle. On arrival, the patient is awake and alert, complaining primarily of pain in her left hip and right foot. Her medical history is unremarkable. She has an initial Glasgow Coma Scale score of 15. Her vital signs are: blood pressure, 154/100 mm Hg; pulse, 108 beats/min; respiratory rate, 16 breaths/min; and O2 saturation, 100% on room air. Primary survey is otherwise unremarkable. A series of radiographs are ordered; that of the right foot is shown. What is your impression?