User login
CPAP for OSA: What is the verdict?
Obstructive sleep apnea (OSA) affects roughly 1 billion people worldwide, according to a report by the American Academy of Sleep Medicine. Severe OSA has been associated with an elevated risk of all-cause and cardiovascular-specific mortality. Studies support an association between OSA and a host of comorbidities, including hypertension, stroke, atrial fibrillation, mood disorders, and neurocognitive outcomes. Undiagnosed and untreated OSA also has major economic and societal costs, reducing workplace productivity and increasing one’s risk of accidents both on the job and while driving.
Positive airway pressure (PAP) is widely considered the most effective treatment for OSA. The majority of patients tolerate CPAP: real-world estimates using international big data show good adherence in over 70% of patients. Robust evidence shows that PAP reduces snoring, decreases daytime sleepiness, and improves quality of life in a dose-dependent manner. Economic analyses have also found CPAP to be cost-effective (Streatfeild, et al. Sleep. 2019;42[12]:zsz181).
But what do we know about the impact of PAP on health outcomes? Perhaps the best studied outcome is cardiovascular disease. Results of observational trials have suggested that CPAP adherence was associated with survival (Pepin JL et al. Chest. 2022;161[6]:1657). However, it has been speculated that these findings may have been driven, at least in part, by the “healthy user effect.” This phenomenon refers to the tendency for people who engage in one health-promoting behavior (eg, CPAP adherence) to engage in another as well (eg, eating well, exercising, taking prescribed medications). When we observe that patients who use CPAP live longer, we must ask ourselves whether perhaps their better outcomes resulted from healthy habits in general, as opposed to their CPAP usage per se.
Randomization eliminates the potential for the healthy user effect, by assigning patients to a certain intervention as opposed to simply observing whether they choose to use it. And herein lies one of the great disappointments for our field over the past decade: multiple large-scale randomized controlled trials have failed to demonstrate that CPAP reduces cardiovascular mortality, even in patients with pre-existing CAD. The first two of these were the SAVE (Sleep Apnea Cardiovascular Endpoints) (McEvoy R, et al. N Engl J Med. 2016;375[10]:919) and RICCADSA (Randomized Intervention with Continuous Positive Airway Pressure in CAD and OSA) (Peker Y, et al. Am J Respir Crit Care Med. 2016;194[5]:613) trials evaluating the effects of PAP on a composite endpoint that included cardiovascular death and nonfatal cardiovascular events. Both trials found no difference between PAP and control groups, leading to a conclusion that PAP did not prevent cardiovascular events in patients with moderate-to-severe OSA and established cardiovascular disease. The ISAAC study (Impact of Sleep Apnea syndrome in the evolution of Acute Coronary syndrome) also failed to show a benefit of CPAP for secondary prevention of cardiovascular events in patients with moderate to severe OSA.
These negative findings were echoed in a recent report by the Agency for Healthcare Research and Quality evaluating a variety of long-term health outcomes in obstructive sleep apnea. The authors stated that “RCTs do not provide evidence that CPAP prescription affects long-term, clinically important outcomes. Specifically, with low strength of evidence, RCTs do not demonstrate that CPAP affects all-cause mortality, various CV outcomes, clinically important changes in psychosocial measures, or other clinical events” (AHRQ, Project ID: SLPT0919, 12/1/2022).
What plausible explanations have been offered for these negative results? Perhaps trials were underpowered. Perhaps patients did not use PAP for a sufficient duration to achieve benefit (usage was under 3 hours in most studies). Perhaps the patients selected for these trials were at such low-risk of adverse outcomes in the first place that treating their OSA didn’t have much impact. Many trials have excluded sleepy patients due to ethical concerns about withholding treatment from this population. But this may have effectively excluded the patients most likely to benefit; in other studies, sleepy patients seem to experience the greatest cardiovascular risk reduction with CPAP. For example, a meta-analysis showed that CPAP is most strongly associated with blood pressure reduction in patients who are sleepy, compared with those with minimally symptomatic OSA (Bratton D, et al. Thorax. 2014;69[12]:1128). And, recent work suggests that even among non-sleepy patients, it might be possible to identify a subset who could benefit from CPAP. A recent analysis suggested that non-sleepy patients who exhibit a higher change in heart rate following a respiratory event may derive greater cardiovascular benefit from CPAP therapy (Azarbarzin, et al. Am J Respir Crit Care Med. 2022;206[6]:767).
Another, distinct reason for these negative results is that the AHI – our main metric for quantifying OSA severity for several decades – fails to capture the disorder’s heterogeneity. Identifying different phenotypes of OSA may enable more personalized approaches to prognostication as well as treatment. For example, one study identified four symptom clusters of OSA – patients with disturbed sleep, minimally symptomatic, excessively sleepy, and moderately sleepy – who may exhibit different responses to CPAP treatment. Further work is needed to discern whether these clusters reliably predict outcomes in a manner that can be useful clinically (Zinchuk A, et al. Sleep Med Rev. 2017;35:113).
So, what is the verdict for CPAP? Sleepy patients with even mild OSA warrant treatment, as is common practice, and these patients are more likely to adhere to therapy. Patients with other symptoms potentially related to untreated OSA should be offered treatment as well. But in asymptomatic patients, it is difficult to make a compelling case to start CPAP on the basis of the AHI alone. It is our hope that novel ways of classifying OSA severity and phenotype will allow better prediction of which patients will experience a protective effect from CPAP. For example, certain subsets of patients may realize greater benefits from CPAP, including those with a high hypoxic burden (Trzepizur W, et al. Am J Respir Crit Care Med. 2022;205[1]:108).
For now though, we can allow the evidence that has accumulated in recent years to guide our collaborative decision-making with patients about whether to try CPAP. Depending on how exuberantly we sang CPAP’s praises, we may need to temper our song – at least with regards to cardiovascular risk reduction. In the sleep world, patients are educated not only by sleep providers but also by respiratory therapists who help patients with initial CPAP setups. Consistent, evidence-based messaging by the entire health care team is key. We cannot say that “using CPAP prevents heart attacks” but rather “we’re still not quite sure.”
As in other areas of medicine, sleep medicine may see a shift in focus toward symptoms and patient-oriented outcomes as opposed to the presence of comorbidities. In fact, the recently revised International Classification of Sleep Disorders (ICSD-3-TR) released this year eliminated comorbidity criteria from the definition of Obstructive Sleep Apnea in adults. If adopted by Centers for Medicare & Medicaid Services and other insurers, patients with mild OSA by sleep testing (AHI≥5 but <15) who lack symptoms will no longer qualify for CPAP on the basis of having hypertension, a mood disorder, cognitive dysfunction, coronary artery disease, stroke, congestive heart failure, atrial fibrillation, or type 2 diabetes mellitus. How will this major revision impact the sleep medicine world? Practically speaking, it is likely that fewer patients who present without symptoms and are found to have only mild OSA will end up on PAP.
There will undoubtedly be frustration related to these greater restrictions on who qualifies for PAP. On the other hand, perhaps our energy is better focused on procuring PAP not for asymptomatic patients but rather promoting access and adherence in those who are symptomatic. Differential access to CPAP remains a major problem that very likely contributes to health disparities. In fact, a recent international committee acknowledged that the current CMS criteria for PAP coverage create disproportionate difficulties for non-white patients and those of low socioeconomic background to meet adherence criteria. Their specific recommendations to reduce this disparity in PAP access included eradication of requirements for repeat polysomnography and eliminating the 4-hour rule.
We are moving toward a more personalized approach to characterizing OSA, which eventually may allow for more nuanced, individualized counseling rather than a “one-size -called-CPAP-fits-all” approach. Until we are there, a patient-centered approach that elicits the presence of sleep-related symptoms and daytime impairment, as opposed to isolated comorbidities, provides the most compelling justification for CPAP.
Obstructive sleep apnea (OSA) affects roughly 1 billion people worldwide, according to a report by the American Academy of Sleep Medicine. Severe OSA has been associated with an elevated risk of all-cause and cardiovascular-specific mortality. Studies support an association between OSA and a host of comorbidities, including hypertension, stroke, atrial fibrillation, mood disorders, and neurocognitive outcomes. Undiagnosed and untreated OSA also has major economic and societal costs, reducing workplace productivity and increasing one’s risk of accidents both on the job and while driving.
Positive airway pressure (PAP) is widely considered the most effective treatment for OSA. The majority of patients tolerate CPAP: real-world estimates using international big data show good adherence in over 70% of patients. Robust evidence shows that PAP reduces snoring, decreases daytime sleepiness, and improves quality of life in a dose-dependent manner. Economic analyses have also found CPAP to be cost-effective (Streatfeild, et al. Sleep. 2019;42[12]:zsz181).
But what do we know about the impact of PAP on health outcomes? Perhaps the best studied outcome is cardiovascular disease. Results of observational trials have suggested that CPAP adherence was associated with survival (Pepin JL et al. Chest. 2022;161[6]:1657). However, it has been speculated that these findings may have been driven, at least in part, by the “healthy user effect.” This phenomenon refers to the tendency for people who engage in one health-promoting behavior (eg, CPAP adherence) to engage in another as well (eg, eating well, exercising, taking prescribed medications). When we observe that patients who use CPAP live longer, we must ask ourselves whether perhaps their better outcomes resulted from healthy habits in general, as opposed to their CPAP usage per se.
Randomization eliminates the potential for the healthy user effect, by assigning patients to a certain intervention as opposed to simply observing whether they choose to use it. And herein lies one of the great disappointments for our field over the past decade: multiple large-scale randomized controlled trials have failed to demonstrate that CPAP reduces cardiovascular mortality, even in patients with pre-existing CAD. The first two of these were the SAVE (Sleep Apnea Cardiovascular Endpoints) (McEvoy R, et al. N Engl J Med. 2016;375[10]:919) and RICCADSA (Randomized Intervention with Continuous Positive Airway Pressure in CAD and OSA) (Peker Y, et al. Am J Respir Crit Care Med. 2016;194[5]:613) trials evaluating the effects of PAP on a composite endpoint that included cardiovascular death and nonfatal cardiovascular events. Both trials found no difference between PAP and control groups, leading to a conclusion that PAP did not prevent cardiovascular events in patients with moderate-to-severe OSA and established cardiovascular disease. The ISAAC study (Impact of Sleep Apnea syndrome in the evolution of Acute Coronary syndrome) also failed to show a benefit of CPAP for secondary prevention of cardiovascular events in patients with moderate to severe OSA.
These negative findings were echoed in a recent report by the Agency for Healthcare Research and Quality evaluating a variety of long-term health outcomes in obstructive sleep apnea. The authors stated that “RCTs do not provide evidence that CPAP prescription affects long-term, clinically important outcomes. Specifically, with low strength of evidence, RCTs do not demonstrate that CPAP affects all-cause mortality, various CV outcomes, clinically important changes in psychosocial measures, or other clinical events” (AHRQ, Project ID: SLPT0919, 12/1/2022).
What plausible explanations have been offered for these negative results? Perhaps trials were underpowered. Perhaps patients did not use PAP for a sufficient duration to achieve benefit (usage was under 3 hours in most studies). Perhaps the patients selected for these trials were at such low-risk of adverse outcomes in the first place that treating their OSA didn’t have much impact. Many trials have excluded sleepy patients due to ethical concerns about withholding treatment from this population. But this may have effectively excluded the patients most likely to benefit; in other studies, sleepy patients seem to experience the greatest cardiovascular risk reduction with CPAP. For example, a meta-analysis showed that CPAP is most strongly associated with blood pressure reduction in patients who are sleepy, compared with those with minimally symptomatic OSA (Bratton D, et al. Thorax. 2014;69[12]:1128). And, recent work suggests that even among non-sleepy patients, it might be possible to identify a subset who could benefit from CPAP. A recent analysis suggested that non-sleepy patients who exhibit a higher change in heart rate following a respiratory event may derive greater cardiovascular benefit from CPAP therapy (Azarbarzin, et al. Am J Respir Crit Care Med. 2022;206[6]:767).
Another, distinct reason for these negative results is that the AHI – our main metric for quantifying OSA severity for several decades – fails to capture the disorder’s heterogeneity. Identifying different phenotypes of OSA may enable more personalized approaches to prognostication as well as treatment. For example, one study identified four symptom clusters of OSA – patients with disturbed sleep, minimally symptomatic, excessively sleepy, and moderately sleepy – who may exhibit different responses to CPAP treatment. Further work is needed to discern whether these clusters reliably predict outcomes in a manner that can be useful clinically (Zinchuk A, et al. Sleep Med Rev. 2017;35:113).
So, what is the verdict for CPAP? Sleepy patients with even mild OSA warrant treatment, as is common practice, and these patients are more likely to adhere to therapy. Patients with other symptoms potentially related to untreated OSA should be offered treatment as well. But in asymptomatic patients, it is difficult to make a compelling case to start CPAP on the basis of the AHI alone. It is our hope that novel ways of classifying OSA severity and phenotype will allow better prediction of which patients will experience a protective effect from CPAP. For example, certain subsets of patients may realize greater benefits from CPAP, including those with a high hypoxic burden (Trzepizur W, et al. Am J Respir Crit Care Med. 2022;205[1]:108).
For now though, we can allow the evidence that has accumulated in recent years to guide our collaborative decision-making with patients about whether to try CPAP. Depending on how exuberantly we sang CPAP’s praises, we may need to temper our song – at least with regards to cardiovascular risk reduction. In the sleep world, patients are educated not only by sleep providers but also by respiratory therapists who help patients with initial CPAP setups. Consistent, evidence-based messaging by the entire health care team is key. We cannot say that “using CPAP prevents heart attacks” but rather “we’re still not quite sure.”
As in other areas of medicine, sleep medicine may see a shift in focus toward symptoms and patient-oriented outcomes as opposed to the presence of comorbidities. In fact, the recently revised International Classification of Sleep Disorders (ICSD-3-TR) released this year eliminated comorbidity criteria from the definition of Obstructive Sleep Apnea in adults. If adopted by Centers for Medicare & Medicaid Services and other insurers, patients with mild OSA by sleep testing (AHI≥5 but <15) who lack symptoms will no longer qualify for CPAP on the basis of having hypertension, a mood disorder, cognitive dysfunction, coronary artery disease, stroke, congestive heart failure, atrial fibrillation, or type 2 diabetes mellitus. How will this major revision impact the sleep medicine world? Practically speaking, it is likely that fewer patients who present without symptoms and are found to have only mild OSA will end up on PAP.
There will undoubtedly be frustration related to these greater restrictions on who qualifies for PAP. On the other hand, perhaps our energy is better focused on procuring PAP not for asymptomatic patients but rather promoting access and adherence in those who are symptomatic. Differential access to CPAP remains a major problem that very likely contributes to health disparities. In fact, a recent international committee acknowledged that the current CMS criteria for PAP coverage create disproportionate difficulties for non-white patients and those of low socioeconomic background to meet adherence criteria. Their specific recommendations to reduce this disparity in PAP access included eradication of requirements for repeat polysomnography and eliminating the 4-hour rule.
We are moving toward a more personalized approach to characterizing OSA, which eventually may allow for more nuanced, individualized counseling rather than a “one-size -called-CPAP-fits-all” approach. Until we are there, a patient-centered approach that elicits the presence of sleep-related symptoms and daytime impairment, as opposed to isolated comorbidities, provides the most compelling justification for CPAP.
Obstructive sleep apnea (OSA) affects roughly 1 billion people worldwide, according to a report by the American Academy of Sleep Medicine. Severe OSA has been associated with an elevated risk of all-cause and cardiovascular-specific mortality. Studies support an association between OSA and a host of comorbidities, including hypertension, stroke, atrial fibrillation, mood disorders, and neurocognitive outcomes. Undiagnosed and untreated OSA also has major economic and societal costs, reducing workplace productivity and increasing one’s risk of accidents both on the job and while driving.
Positive airway pressure (PAP) is widely considered the most effective treatment for OSA. The majority of patients tolerate CPAP: real-world estimates using international big data show good adherence in over 70% of patients. Robust evidence shows that PAP reduces snoring, decreases daytime sleepiness, and improves quality of life in a dose-dependent manner. Economic analyses have also found CPAP to be cost-effective (Streatfeild, et al. Sleep. 2019;42[12]:zsz181).
But what do we know about the impact of PAP on health outcomes? Perhaps the best studied outcome is cardiovascular disease. Results of observational trials have suggested that CPAP adherence was associated with survival (Pepin JL et al. Chest. 2022;161[6]:1657). However, it has been speculated that these findings may have been driven, at least in part, by the “healthy user effect.” This phenomenon refers to the tendency for people who engage in one health-promoting behavior (eg, CPAP adherence) to engage in another as well (eg, eating well, exercising, taking prescribed medications). When we observe that patients who use CPAP live longer, we must ask ourselves whether perhaps their better outcomes resulted from healthy habits in general, as opposed to their CPAP usage per se.
Randomization eliminates the potential for the healthy user effect, by assigning patients to a certain intervention as opposed to simply observing whether they choose to use it. And herein lies one of the great disappointments for our field over the past decade: multiple large-scale randomized controlled trials have failed to demonstrate that CPAP reduces cardiovascular mortality, even in patients with pre-existing CAD. The first two of these were the SAVE (Sleep Apnea Cardiovascular Endpoints) (McEvoy R, et al. N Engl J Med. 2016;375[10]:919) and RICCADSA (Randomized Intervention with Continuous Positive Airway Pressure in CAD and OSA) (Peker Y, et al. Am J Respir Crit Care Med. 2016;194[5]:613) trials evaluating the effects of PAP on a composite endpoint that included cardiovascular death and nonfatal cardiovascular events. Both trials found no difference between PAP and control groups, leading to a conclusion that PAP did not prevent cardiovascular events in patients with moderate-to-severe OSA and established cardiovascular disease. The ISAAC study (Impact of Sleep Apnea syndrome in the evolution of Acute Coronary syndrome) also failed to show a benefit of CPAP for secondary prevention of cardiovascular events in patients with moderate to severe OSA.
These negative findings were echoed in a recent report by the Agency for Healthcare Research and Quality evaluating a variety of long-term health outcomes in obstructive sleep apnea. The authors stated that “RCTs do not provide evidence that CPAP prescription affects long-term, clinically important outcomes. Specifically, with low strength of evidence, RCTs do not demonstrate that CPAP affects all-cause mortality, various CV outcomes, clinically important changes in psychosocial measures, or other clinical events” (AHRQ, Project ID: SLPT0919, 12/1/2022).
What plausible explanations have been offered for these negative results? Perhaps trials were underpowered. Perhaps patients did not use PAP for a sufficient duration to achieve benefit (usage was under 3 hours in most studies). Perhaps the patients selected for these trials were at such low-risk of adverse outcomes in the first place that treating their OSA didn’t have much impact. Many trials have excluded sleepy patients due to ethical concerns about withholding treatment from this population. But this may have effectively excluded the patients most likely to benefit; in other studies, sleepy patients seem to experience the greatest cardiovascular risk reduction with CPAP. For example, a meta-analysis showed that CPAP is most strongly associated with blood pressure reduction in patients who are sleepy, compared with those with minimally symptomatic OSA (Bratton D, et al. Thorax. 2014;69[12]:1128). And, recent work suggests that even among non-sleepy patients, it might be possible to identify a subset who could benefit from CPAP. A recent analysis suggested that non-sleepy patients who exhibit a higher change in heart rate following a respiratory event may derive greater cardiovascular benefit from CPAP therapy (Azarbarzin, et al. Am J Respir Crit Care Med. 2022;206[6]:767).
Another, distinct reason for these negative results is that the AHI – our main metric for quantifying OSA severity for several decades – fails to capture the disorder’s heterogeneity. Identifying different phenotypes of OSA may enable more personalized approaches to prognostication as well as treatment. For example, one study identified four symptom clusters of OSA – patients with disturbed sleep, minimally symptomatic, excessively sleepy, and moderately sleepy – who may exhibit different responses to CPAP treatment. Further work is needed to discern whether these clusters reliably predict outcomes in a manner that can be useful clinically (Zinchuk A, et al. Sleep Med Rev. 2017;35:113).
So, what is the verdict for CPAP? Sleepy patients with even mild OSA warrant treatment, as is common practice, and these patients are more likely to adhere to therapy. Patients with other symptoms potentially related to untreated OSA should be offered treatment as well. But in asymptomatic patients, it is difficult to make a compelling case to start CPAP on the basis of the AHI alone. It is our hope that novel ways of classifying OSA severity and phenotype will allow better prediction of which patients will experience a protective effect from CPAP. For example, certain subsets of patients may realize greater benefits from CPAP, including those with a high hypoxic burden (Trzepizur W, et al. Am J Respir Crit Care Med. 2022;205[1]:108).
For now though, we can allow the evidence that has accumulated in recent years to guide our collaborative decision-making with patients about whether to try CPAP. Depending on how exuberantly we sang CPAP’s praises, we may need to temper our song – at least with regards to cardiovascular risk reduction. In the sleep world, patients are educated not only by sleep providers but also by respiratory therapists who help patients with initial CPAP setups. Consistent, evidence-based messaging by the entire health care team is key. We cannot say that “using CPAP prevents heart attacks” but rather “we’re still not quite sure.”
As in other areas of medicine, sleep medicine may see a shift in focus toward symptoms and patient-oriented outcomes as opposed to the presence of comorbidities. In fact, the recently revised International Classification of Sleep Disorders (ICSD-3-TR) released this year eliminated comorbidity criteria from the definition of Obstructive Sleep Apnea in adults. If adopted by Centers for Medicare & Medicaid Services and other insurers, patients with mild OSA by sleep testing (AHI≥5 but <15) who lack symptoms will no longer qualify for CPAP on the basis of having hypertension, a mood disorder, cognitive dysfunction, coronary artery disease, stroke, congestive heart failure, atrial fibrillation, or type 2 diabetes mellitus. How will this major revision impact the sleep medicine world? Practically speaking, it is likely that fewer patients who present without symptoms and are found to have only mild OSA will end up on PAP.
There will undoubtedly be frustration related to these greater restrictions on who qualifies for PAP. On the other hand, perhaps our energy is better focused on procuring PAP not for asymptomatic patients but rather promoting access and adherence in those who are symptomatic. Differential access to CPAP remains a major problem that very likely contributes to health disparities. In fact, a recent international committee acknowledged that the current CMS criteria for PAP coverage create disproportionate difficulties for non-white patients and those of low socioeconomic background to meet adherence criteria. Their specific recommendations to reduce this disparity in PAP access included eradication of requirements for repeat polysomnography and eliminating the 4-hour rule.
We are moving toward a more personalized approach to characterizing OSA, which eventually may allow for more nuanced, individualized counseling rather than a “one-size -called-CPAP-fits-all” approach. Until we are there, a patient-centered approach that elicits the presence of sleep-related symptoms and daytime impairment, as opposed to isolated comorbidities, provides the most compelling justification for CPAP.
Death anxiety in psychiatry and society: Facing our fears and embracing life
Our fear of death is exposed often in medicine. It is not uncommon to hear the last moments of a patient’s life described as a series of futile, sterile medical interventions that attempt to prolong that life in quasi-sadistic fashion. So much effort is placed in making sure that “everything necessary” is tried that less emphasis is made on providing a comfortable death.
It seems obvious that a profession dedicated to prolonging health would have difficulty confronting death. But it should also be natural for psychiatry to be the specialty able to integrate this discomfort within the medical psyche.
Yet, in training, we have noted much more time spent on the assessment of capacity in patients in order to refuse medical intervention than on time spent educating about the importance to die at the right time, as suggested by Friedrich Nietzsche.1 A psychiatry resident may graduate knowing dozens of questions to assess the ability of a family member to consider the risk, benefits, and alternatives of continued intubation in a comatose patient, but may feel very ill-equipped in discussing the meaning of a rightful life and a rightful death.
Death anxiety can also come outside the context of not having endured enough traumas or successes in one’s life, or not having lived life right. As poignantly described by Dostoevsky in his 1864 novella, “Notes from the Underground,” death anxiety can manifest as a result of the deterministic nature of life.2 Doing everything which is expected of us can feel like a betrayal of our one chance to have lived life authentically. This concept is also particularly familiar to physicians, who may have – in part – chosen their career path in response to a recommendation from their parents, rather than a more authentic feeling. Dostoevsky goads us to transgress, to act in a rebellious way, to truly feel alive. This can serve as a solution for death anxiety – if you are scared to die then live, live your fullest. Even if that means doing the unexpected or changing your path.
The fear of being forgotten after death can also drive many to pursue a legacy. Even a parent choosing to have children and teaching them values and belief systems is a way of leaving behind a mark on the world. For some, finding ways for being remembered after death – whether through fame, fortune, or having children, is a way of dealing with death anxiety.
The Mexican holiday “Dia de los Muertos,” or Day of the Dead, and the Japanese holiday “Obon” are examples from cultures where deceased ancestors are celebrated through rituals and offerings. Such cultures may relieve the anxiety of death by suggesting that one’s descendants will still care for the departed, and their legacy may remain.
Coping with death anxiety
For others, the road to recovery from death anxiety may take a completely different approach. Some may find comfort in the position that, to extinguish death anxiety, one should not live to the fullest but accept the tragic and mostly inconsequential aspects of life. The philosophical movement of “absurdism” addresses this perspective.
In our modern world, where we are so deeply attached to finding the cause and reason for things, absurdism reminds us that most of our lives and world do not have to make sense. While Albert Camus, arguably the most famous of the absurdist philosophers, encourages us to create meaning and transcend the tragedy and randomness of life,3 some patients can also find comfort in the idea that life is absurd, and thus one should not judge one’s own life and not fear own’s inevitable death.
Death anxiety can also be therapeutic. Especially in the existential tradition, one can enlist the fear of death for motivation. Many patients come to see us with a lack of motivation or drive. They feel paralyzed by their predicament and mental illness. As in the experiments of Martin Seligman, PhD, who shocked animals at random, a human exposed to repeated failure and abuse can get a sense of learned helplessness.4 Such patients can be very hard to reach, yet ultimately their despondence is no match for the reality that life will end. Reminding a patient that any day spent not feeling alive might as well be a metaphor for death is a challenging interpretation, but one that can lead to significant growth.
When considering the fear of death, psychiatry has generally taken the position that it is pathological, a form of anxiety. Psychiatry argues that one should strive to find fulfillment and joy in life. It thus may be a surprise to find that this is not a universally shared perspective.
In his 2010 book, author Thomas Ligotti argues on behalf of pessimistic and antinatalist views.5 Throughout the book he emphasizes the suffering that life can offer and argues against the endless pursuit of more life. To some psychiatrists, such arguments will be understood as insulting to our profession. Some may even interpret his texts as an argument in favor of ending one’s life.
However, psychiatrists must ask themselves “what are my answers to those arguments?” Mr. Ligotti’s book is a series of arguments against the idea that life will be pleasurable. Understanding those arguments and formulating a rebuttal would be an important process for any mental health provider. It is foolish to think that our patients do not have a rich and complicated relationship to death, and that none of our patients find death attractive in some ways. After all, accepting our fears as an important part of our body is a natural coping skill, which can also be taught.6
Part of the difficulty in discussing death and the fear of death may come from society’s resistance at having complicated conversations. It is not uncommon, currently, to include trigger warnings at the mention of discussions about death, even abstract ones. While we appreciate and encourage the articulation of feelings that a discussion about death may raise, we worry that such trigger warnings may be a form of censure that only makes society more resistant to talk about those important topics.
For another example of the avoidance of discussions about death, recall the “death panel” debates of 2009.7 When the U.S. government considered encouraging physicians to have discussions with their patients about end-of-life care, politicians and pundits decried that such discussions were “death panels,” and claimed they were an encouragement to patients to “cut [their] life short.” Such public projection of one’s anxiety about death has made it particularly difficult for psychiatry to make meaningful progress.
Acknowledging and addressing the fear
Death anxiety is such a common aspect of human life that most religions make some effort to address this fear. Many do so by offering a form of afterlife, often one described in idyllic fashion without anxiety.
Heaven, if one believes in it, is appealing for the person dreading death anxiety. Heaven is often described as being offered to those who have lived a rightful life, thus relieving the anxiety regarding the decisions one has made. Reincarnation can also be interpreted as another way of calming death anxiety, by promising a continual repetition of chances at getting life right. However, for many patients, religion doesn’t have the appeal that it once had.
Ultimately, the fear of death is a complex and multifaceted issue that can manifest in various ways. The medical profession, especially psychiatry, has a responsibility to address this fear in patients, but it also struggles with its own discomfort with the topic. The importance of providing a comfortable death is often overshadowed by the emphasis on prolonging life, which may manifest as a series of futile medical interventions.
The fear of death can be therapeutic and motivating, but it can also be pathological and lead to a lack of motivation or drive. The philosophical movements of absurdism and antinatalism offer alternative perspectives on death and life, and it is important for mental health providers to understand and engage with these views.
Yet acknowledging and addressing the fear of death is an important aspect of mental health care and a crucial part of the human experience.
Dr. Akkoor is a psychiatry resident at the University of California, San Diego. She is interested in immigrant mental health, ethics, consultation-liaison psychiatry, and medical education. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Badre and Dr. Akkoor have no conflicts of interest.
References
1. Nietzsche F. Thus Spoke Zarathustra. 1883-1892.
2. Dostoevsky F. Notes from the Underground. 1864.
3. Camus A. The Plague. 1947.
4. Seligman M. Helplessness: On depression, development, and death. 1975.
5. Ligotti T. The Conspiracy Against the Human Race. 2010.
6. Hayes SC. Behav Ther. 2016 Nov;47(6):869-85. doi: 10.1016/j.beth.2016.11.006.
7. Nyhan B. The Forum. 2010 April 27;8(1). doi: 10.2202/1540-8884.1354.
Our fear of death is exposed often in medicine. It is not uncommon to hear the last moments of a patient’s life described as a series of futile, sterile medical interventions that attempt to prolong that life in quasi-sadistic fashion. So much effort is placed in making sure that “everything necessary” is tried that less emphasis is made on providing a comfortable death.
It seems obvious that a profession dedicated to prolonging health would have difficulty confronting death. But it should also be natural for psychiatry to be the specialty able to integrate this discomfort within the medical psyche.
Yet, in training, we have noted much more time spent on the assessment of capacity in patients in order to refuse medical intervention than on time spent educating about the importance to die at the right time, as suggested by Friedrich Nietzsche.1 A psychiatry resident may graduate knowing dozens of questions to assess the ability of a family member to consider the risk, benefits, and alternatives of continued intubation in a comatose patient, but may feel very ill-equipped in discussing the meaning of a rightful life and a rightful death.
Death anxiety can also come outside the context of not having endured enough traumas or successes in one’s life, or not having lived life right. As poignantly described by Dostoevsky in his 1864 novella, “Notes from the Underground,” death anxiety can manifest as a result of the deterministic nature of life.2 Doing everything which is expected of us can feel like a betrayal of our one chance to have lived life authentically. This concept is also particularly familiar to physicians, who may have – in part – chosen their career path in response to a recommendation from their parents, rather than a more authentic feeling. Dostoevsky goads us to transgress, to act in a rebellious way, to truly feel alive. This can serve as a solution for death anxiety – if you are scared to die then live, live your fullest. Even if that means doing the unexpected or changing your path.
The fear of being forgotten after death can also drive many to pursue a legacy. Even a parent choosing to have children and teaching them values and belief systems is a way of leaving behind a mark on the world. For some, finding ways for being remembered after death – whether through fame, fortune, or having children, is a way of dealing with death anxiety.
The Mexican holiday “Dia de los Muertos,” or Day of the Dead, and the Japanese holiday “Obon” are examples from cultures where deceased ancestors are celebrated through rituals and offerings. Such cultures may relieve the anxiety of death by suggesting that one’s descendants will still care for the departed, and their legacy may remain.
Coping with death anxiety
For others, the road to recovery from death anxiety may take a completely different approach. Some may find comfort in the position that, to extinguish death anxiety, one should not live to the fullest but accept the tragic and mostly inconsequential aspects of life. The philosophical movement of “absurdism” addresses this perspective.
In our modern world, where we are so deeply attached to finding the cause and reason for things, absurdism reminds us that most of our lives and world do not have to make sense. While Albert Camus, arguably the most famous of the absurdist philosophers, encourages us to create meaning and transcend the tragedy and randomness of life,3 some patients can also find comfort in the idea that life is absurd, and thus one should not judge one’s own life and not fear own’s inevitable death.
Death anxiety can also be therapeutic. Especially in the existential tradition, one can enlist the fear of death for motivation. Many patients come to see us with a lack of motivation or drive. They feel paralyzed by their predicament and mental illness. As in the experiments of Martin Seligman, PhD, who shocked animals at random, a human exposed to repeated failure and abuse can get a sense of learned helplessness.4 Such patients can be very hard to reach, yet ultimately their despondence is no match for the reality that life will end. Reminding a patient that any day spent not feeling alive might as well be a metaphor for death is a challenging interpretation, but one that can lead to significant growth.
When considering the fear of death, psychiatry has generally taken the position that it is pathological, a form of anxiety. Psychiatry argues that one should strive to find fulfillment and joy in life. It thus may be a surprise to find that this is not a universally shared perspective.
In his 2010 book, author Thomas Ligotti argues on behalf of pessimistic and antinatalist views.5 Throughout the book he emphasizes the suffering that life can offer and argues against the endless pursuit of more life. To some psychiatrists, such arguments will be understood as insulting to our profession. Some may even interpret his texts as an argument in favor of ending one’s life.
However, psychiatrists must ask themselves “what are my answers to those arguments?” Mr. Ligotti’s book is a series of arguments against the idea that life will be pleasurable. Understanding those arguments and formulating a rebuttal would be an important process for any mental health provider. It is foolish to think that our patients do not have a rich and complicated relationship to death, and that none of our patients find death attractive in some ways. After all, accepting our fears as an important part of our body is a natural coping skill, which can also be taught.6
Part of the difficulty in discussing death and the fear of death may come from society’s resistance at having complicated conversations. It is not uncommon, currently, to include trigger warnings at the mention of discussions about death, even abstract ones. While we appreciate and encourage the articulation of feelings that a discussion about death may raise, we worry that such trigger warnings may be a form of censure that only makes society more resistant to talk about those important topics.
For another example of the avoidance of discussions about death, recall the “death panel” debates of 2009.7 When the U.S. government considered encouraging physicians to have discussions with their patients about end-of-life care, politicians and pundits decried that such discussions were “death panels,” and claimed they were an encouragement to patients to “cut [their] life short.” Such public projection of one’s anxiety about death has made it particularly difficult for psychiatry to make meaningful progress.
Acknowledging and addressing the fear
Death anxiety is such a common aspect of human life that most religions make some effort to address this fear. Many do so by offering a form of afterlife, often one described in idyllic fashion without anxiety.
Heaven, if one believes in it, is appealing for the person dreading death anxiety. Heaven is often described as being offered to those who have lived a rightful life, thus relieving the anxiety regarding the decisions one has made. Reincarnation can also be interpreted as another way of calming death anxiety, by promising a continual repetition of chances at getting life right. However, for many patients, religion doesn’t have the appeal that it once had.
Ultimately, the fear of death is a complex and multifaceted issue that can manifest in various ways. The medical profession, especially psychiatry, has a responsibility to address this fear in patients, but it also struggles with its own discomfort with the topic. The importance of providing a comfortable death is often overshadowed by the emphasis on prolonging life, which may manifest as a series of futile medical interventions.
The fear of death can be therapeutic and motivating, but it can also be pathological and lead to a lack of motivation or drive. The philosophical movements of absurdism and antinatalism offer alternative perspectives on death and life, and it is important for mental health providers to understand and engage with these views.
Yet acknowledging and addressing the fear of death is an important aspect of mental health care and a crucial part of the human experience.
Dr. Akkoor is a psychiatry resident at the University of California, San Diego. She is interested in immigrant mental health, ethics, consultation-liaison psychiatry, and medical education. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Badre and Dr. Akkoor have no conflicts of interest.
References
1. Nietzsche F. Thus Spoke Zarathustra. 1883-1892.
2. Dostoevsky F. Notes from the Underground. 1864.
3. Camus A. The Plague. 1947.
4. Seligman M. Helplessness: On depression, development, and death. 1975.
5. Ligotti T. The Conspiracy Against the Human Race. 2010.
6. Hayes SC. Behav Ther. 2016 Nov;47(6):869-85. doi: 10.1016/j.beth.2016.11.006.
7. Nyhan B. The Forum. 2010 April 27;8(1). doi: 10.2202/1540-8884.1354.
Our fear of death is exposed often in medicine. It is not uncommon to hear the last moments of a patient’s life described as a series of futile, sterile medical interventions that attempt to prolong that life in quasi-sadistic fashion. So much effort is placed in making sure that “everything necessary” is tried that less emphasis is made on providing a comfortable death.
It seems obvious that a profession dedicated to prolonging health would have difficulty confronting death. But it should also be natural for psychiatry to be the specialty able to integrate this discomfort within the medical psyche.
Yet, in training, we have noted much more time spent on the assessment of capacity in patients in order to refuse medical intervention than on time spent educating about the importance to die at the right time, as suggested by Friedrich Nietzsche.1 A psychiatry resident may graduate knowing dozens of questions to assess the ability of a family member to consider the risk, benefits, and alternatives of continued intubation in a comatose patient, but may feel very ill-equipped in discussing the meaning of a rightful life and a rightful death.
Death anxiety can also come outside the context of not having endured enough traumas or successes in one’s life, or not having lived life right. As poignantly described by Dostoevsky in his 1864 novella, “Notes from the Underground,” death anxiety can manifest as a result of the deterministic nature of life.2 Doing everything which is expected of us can feel like a betrayal of our one chance to have lived life authentically. This concept is also particularly familiar to physicians, who may have – in part – chosen their career path in response to a recommendation from their parents, rather than a more authentic feeling. Dostoevsky goads us to transgress, to act in a rebellious way, to truly feel alive. This can serve as a solution for death anxiety – if you are scared to die then live, live your fullest. Even if that means doing the unexpected or changing your path.
The fear of being forgotten after death can also drive many to pursue a legacy. Even a parent choosing to have children and teaching them values and belief systems is a way of leaving behind a mark on the world. For some, finding ways for being remembered after death – whether through fame, fortune, or having children, is a way of dealing with death anxiety.
The Mexican holiday “Dia de los Muertos,” or Day of the Dead, and the Japanese holiday “Obon” are examples from cultures where deceased ancestors are celebrated through rituals and offerings. Such cultures may relieve the anxiety of death by suggesting that one’s descendants will still care for the departed, and their legacy may remain.
Coping with death anxiety
For others, the road to recovery from death anxiety may take a completely different approach. Some may find comfort in the position that, to extinguish death anxiety, one should not live to the fullest but accept the tragic and mostly inconsequential aspects of life. The philosophical movement of “absurdism” addresses this perspective.
In our modern world, where we are so deeply attached to finding the cause and reason for things, absurdism reminds us that most of our lives and world do not have to make sense. While Albert Camus, arguably the most famous of the absurdist philosophers, encourages us to create meaning and transcend the tragedy and randomness of life,3 some patients can also find comfort in the idea that life is absurd, and thus one should not judge one’s own life and not fear own’s inevitable death.
Death anxiety can also be therapeutic. Especially in the existential tradition, one can enlist the fear of death for motivation. Many patients come to see us with a lack of motivation or drive. They feel paralyzed by their predicament and mental illness. As in the experiments of Martin Seligman, PhD, who shocked animals at random, a human exposed to repeated failure and abuse can get a sense of learned helplessness.4 Such patients can be very hard to reach, yet ultimately their despondence is no match for the reality that life will end. Reminding a patient that any day spent not feeling alive might as well be a metaphor for death is a challenging interpretation, but one that can lead to significant growth.
When considering the fear of death, psychiatry has generally taken the position that it is pathological, a form of anxiety. Psychiatry argues that one should strive to find fulfillment and joy in life. It thus may be a surprise to find that this is not a universally shared perspective.
In his 2010 book, author Thomas Ligotti argues on behalf of pessimistic and antinatalist views.5 Throughout the book he emphasizes the suffering that life can offer and argues against the endless pursuit of more life. To some psychiatrists, such arguments will be understood as insulting to our profession. Some may even interpret his texts as an argument in favor of ending one’s life.
However, psychiatrists must ask themselves “what are my answers to those arguments?” Mr. Ligotti’s book is a series of arguments against the idea that life will be pleasurable. Understanding those arguments and formulating a rebuttal would be an important process for any mental health provider. It is foolish to think that our patients do not have a rich and complicated relationship to death, and that none of our patients find death attractive in some ways. After all, accepting our fears as an important part of our body is a natural coping skill, which can also be taught.6
Part of the difficulty in discussing death and the fear of death may come from society’s resistance at having complicated conversations. It is not uncommon, currently, to include trigger warnings at the mention of discussions about death, even abstract ones. While we appreciate and encourage the articulation of feelings that a discussion about death may raise, we worry that such trigger warnings may be a form of censure that only makes society more resistant to talk about those important topics.
For another example of the avoidance of discussions about death, recall the “death panel” debates of 2009.7 When the U.S. government considered encouraging physicians to have discussions with their patients about end-of-life care, politicians and pundits decried that such discussions were “death panels,” and claimed they were an encouragement to patients to “cut [their] life short.” Such public projection of one’s anxiety about death has made it particularly difficult for psychiatry to make meaningful progress.
Acknowledging and addressing the fear
Death anxiety is such a common aspect of human life that most religions make some effort to address this fear. Many do so by offering a form of afterlife, often one described in idyllic fashion without anxiety.
Heaven, if one believes in it, is appealing for the person dreading death anxiety. Heaven is often described as being offered to those who have lived a rightful life, thus relieving the anxiety regarding the decisions one has made. Reincarnation can also be interpreted as another way of calming death anxiety, by promising a continual repetition of chances at getting life right. However, for many patients, religion doesn’t have the appeal that it once had.
Ultimately, the fear of death is a complex and multifaceted issue that can manifest in various ways. The medical profession, especially psychiatry, has a responsibility to address this fear in patients, but it also struggles with its own discomfort with the topic. The importance of providing a comfortable death is often overshadowed by the emphasis on prolonging life, which may manifest as a series of futile medical interventions.
The fear of death can be therapeutic and motivating, but it can also be pathological and lead to a lack of motivation or drive. The philosophical movements of absurdism and antinatalism offer alternative perspectives on death and life, and it is important for mental health providers to understand and engage with these views.
Yet acknowledging and addressing the fear of death is an important aspect of mental health care and a crucial part of the human experience.
Dr. Akkoor is a psychiatry resident at the University of California, San Diego. She is interested in immigrant mental health, ethics, consultation-liaison psychiatry, and medical education. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Badre and Dr. Akkoor have no conflicts of interest.
References
1. Nietzsche F. Thus Spoke Zarathustra. 1883-1892.
2. Dostoevsky F. Notes from the Underground. 1864.
3. Camus A. The Plague. 1947.
4. Seligman M. Helplessness: On depression, development, and death. 1975.
5. Ligotti T. The Conspiracy Against the Human Race. 2010.
6. Hayes SC. Behav Ther. 2016 Nov;47(6):869-85. doi: 10.1016/j.beth.2016.11.006.
7. Nyhan B. The Forum. 2010 April 27;8(1). doi: 10.2202/1540-8884.1354.
Schizophrenia up to three times more common than previously thought
, according to the first study to estimate the national prevalence of schizophrenia spectrum disorders.
This finding is “especially important,” given that people with schizophrenia spectrum disorders experience “high levels of disability that present significant challenges in all aspects of their life,” principal investigator Heather Ringeisen, PhD, with RTI International, a nonprofit research institute based on Research Triangle Park, N.C., said in a statement.
The results “highlight the need to improve systems of care and access to treatment for people with schizophrenia and other mental health disorders,” added co–principal investigator Mark J. Edlund, MD, PhD, also with RTI.
The study also found that prevalence rates of many other nonpsychotic disorders were generally within an expected range in light of findings from prior research – with three exceptions.
Rates of major depressive disorder (MDD), generalized anxiety disorder (GAD), and obsessive-compulsive disorder (OCD) were higher than reported in past nationally representative samples.
The new data come from the Mental and Substance Use Disorder Prevalence Study (MDPS), a pilot program funded by the Substance Abuse and Mental Health Services Administration (SAMHSA).
A nationally representative sample of 5,679 adults aged 18-65 residing in U.S. households, prisons, homeless shelters, and state psychiatric hospitals were interviewed, virtually or in person, between October 2020 and October 2022.
The research team used a population-based version of the Structured Clinical Interview of the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; SCID-5) for mental health and substance use disorder diagnostic assessment.
Among the key findings in the report:
- Nearly 2% of adults (about 3.7 million) had a lifetime history of schizophrenia spectrum disorders, which include schizophrenia, schizoaffective disorder, and schizophreniform disorder.
- Roughly 2.5 million adults (1.2%) met diagnostic criteria for a schizophrenia spectrum disorder in the past year.
- The two most common mental disorders among adults were MDD (15.5%, or about 31.4 million) and GAD (10.0%, or about 20.2 million).
- Approximately 8.2 million adults (4.1%) had past-year posttraumatic stress disorder, about 5.0 million (2.5%) had OCD, and roughly 3.1 million (1.5%) had bipolar I disorder.
- Alcohol use disorder (AUD) was the most common substance use disorder among adults aged 18-65; roughly 13.4 million adults (6.7%) met criteria for AUD in the past year.
- About 7.7 million adults (3.8%) had cannabis use disorder, about 3.2 million (1.6%) had stimulant use disorder, and about 1 million (0.5%) had opioid use disorder.
Multiple comorbidities
The data also show that one in four adults had at least one mental health disorder in the past year, most commonly MDD and GAD.
About 11% of adults met the criteria for at least one substance use disorder, with AUD and cannabis use disorder the most common.
In addition, an estimated 11 million adults aged 18-65 had both a mental health disorder and a substance use disorder in the past year.
Encouragingly, the findings suggest that more individuals are seeking and accessing treatment compared with previous studies, the authors noted; 61% of adults with a mental health disorder reported having at least one visit with a treatment provider in the past year.
However, considerable treatment gaps still exist for the most common mental health disorders, they reported. Within the past year, more than 40% of adults with MDD and more than 30% of those with GAD did not receive any treatment services.
The full report is available online.
A version of this article originally appeared on Medscape.com.
, according to the first study to estimate the national prevalence of schizophrenia spectrum disorders.
This finding is “especially important,” given that people with schizophrenia spectrum disorders experience “high levels of disability that present significant challenges in all aspects of their life,” principal investigator Heather Ringeisen, PhD, with RTI International, a nonprofit research institute based on Research Triangle Park, N.C., said in a statement.
The results “highlight the need to improve systems of care and access to treatment for people with schizophrenia and other mental health disorders,” added co–principal investigator Mark J. Edlund, MD, PhD, also with RTI.
The study also found that prevalence rates of many other nonpsychotic disorders were generally within an expected range in light of findings from prior research – with three exceptions.
Rates of major depressive disorder (MDD), generalized anxiety disorder (GAD), and obsessive-compulsive disorder (OCD) were higher than reported in past nationally representative samples.
The new data come from the Mental and Substance Use Disorder Prevalence Study (MDPS), a pilot program funded by the Substance Abuse and Mental Health Services Administration (SAMHSA).
A nationally representative sample of 5,679 adults aged 18-65 residing in U.S. households, prisons, homeless shelters, and state psychiatric hospitals were interviewed, virtually or in person, between October 2020 and October 2022.
The research team used a population-based version of the Structured Clinical Interview of the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; SCID-5) for mental health and substance use disorder diagnostic assessment.
Among the key findings in the report:
- Nearly 2% of adults (about 3.7 million) had a lifetime history of schizophrenia spectrum disorders, which include schizophrenia, schizoaffective disorder, and schizophreniform disorder.
- Roughly 2.5 million adults (1.2%) met diagnostic criteria for a schizophrenia spectrum disorder in the past year.
- The two most common mental disorders among adults were MDD (15.5%, or about 31.4 million) and GAD (10.0%, or about 20.2 million).
- Approximately 8.2 million adults (4.1%) had past-year posttraumatic stress disorder, about 5.0 million (2.5%) had OCD, and roughly 3.1 million (1.5%) had bipolar I disorder.
- Alcohol use disorder (AUD) was the most common substance use disorder among adults aged 18-65; roughly 13.4 million adults (6.7%) met criteria for AUD in the past year.
- About 7.7 million adults (3.8%) had cannabis use disorder, about 3.2 million (1.6%) had stimulant use disorder, and about 1 million (0.5%) had opioid use disorder.
Multiple comorbidities
The data also show that one in four adults had at least one mental health disorder in the past year, most commonly MDD and GAD.
About 11% of adults met the criteria for at least one substance use disorder, with AUD and cannabis use disorder the most common.
In addition, an estimated 11 million adults aged 18-65 had both a mental health disorder and a substance use disorder in the past year.
Encouragingly, the findings suggest that more individuals are seeking and accessing treatment compared with previous studies, the authors noted; 61% of adults with a mental health disorder reported having at least one visit with a treatment provider in the past year.
However, considerable treatment gaps still exist for the most common mental health disorders, they reported. Within the past year, more than 40% of adults with MDD and more than 30% of those with GAD did not receive any treatment services.
The full report is available online.
A version of this article originally appeared on Medscape.com.
, according to the first study to estimate the national prevalence of schizophrenia spectrum disorders.
This finding is “especially important,” given that people with schizophrenia spectrum disorders experience “high levels of disability that present significant challenges in all aspects of their life,” principal investigator Heather Ringeisen, PhD, with RTI International, a nonprofit research institute based on Research Triangle Park, N.C., said in a statement.
The results “highlight the need to improve systems of care and access to treatment for people with schizophrenia and other mental health disorders,” added co–principal investigator Mark J. Edlund, MD, PhD, also with RTI.
The study also found that prevalence rates of many other nonpsychotic disorders were generally within an expected range in light of findings from prior research – with three exceptions.
Rates of major depressive disorder (MDD), generalized anxiety disorder (GAD), and obsessive-compulsive disorder (OCD) were higher than reported in past nationally representative samples.
The new data come from the Mental and Substance Use Disorder Prevalence Study (MDPS), a pilot program funded by the Substance Abuse and Mental Health Services Administration (SAMHSA).
A nationally representative sample of 5,679 adults aged 18-65 residing in U.S. households, prisons, homeless shelters, and state psychiatric hospitals were interviewed, virtually or in person, between October 2020 and October 2022.
The research team used a population-based version of the Structured Clinical Interview of the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5; SCID-5) for mental health and substance use disorder diagnostic assessment.
Among the key findings in the report:
- Nearly 2% of adults (about 3.7 million) had a lifetime history of schizophrenia spectrum disorders, which include schizophrenia, schizoaffective disorder, and schizophreniform disorder.
- Roughly 2.5 million adults (1.2%) met diagnostic criteria for a schizophrenia spectrum disorder in the past year.
- The two most common mental disorders among adults were MDD (15.5%, or about 31.4 million) and GAD (10.0%, or about 20.2 million).
- Approximately 8.2 million adults (4.1%) had past-year posttraumatic stress disorder, about 5.0 million (2.5%) had OCD, and roughly 3.1 million (1.5%) had bipolar I disorder.
- Alcohol use disorder (AUD) was the most common substance use disorder among adults aged 18-65; roughly 13.4 million adults (6.7%) met criteria for AUD in the past year.
- About 7.7 million adults (3.8%) had cannabis use disorder, about 3.2 million (1.6%) had stimulant use disorder, and about 1 million (0.5%) had opioid use disorder.
Multiple comorbidities
The data also show that one in four adults had at least one mental health disorder in the past year, most commonly MDD and GAD.
About 11% of adults met the criteria for at least one substance use disorder, with AUD and cannabis use disorder the most common.
In addition, an estimated 11 million adults aged 18-65 had both a mental health disorder and a substance use disorder in the past year.
Encouragingly, the findings suggest that more individuals are seeking and accessing treatment compared with previous studies, the authors noted; 61% of adults with a mental health disorder reported having at least one visit with a treatment provider in the past year.
However, considerable treatment gaps still exist for the most common mental health disorders, they reported. Within the past year, more than 40% of adults with MDD and more than 30% of those with GAD did not receive any treatment services.
The full report is available online.
A version of this article originally appeared on Medscape.com.
Lupus flares linked to gut bacteria overgrowth
Flares of systemic lupus erythematosus (SLE), particularly those involving severe kidney disease, were associated with growth spikes of the gut bacteria Ruminococcus blautia gnavus in a small, 4-year observational study that also demonstrated an underlying, inherent instability in the gut microbiome of patients with SLE.
Of 16 patients with SLE studied during the provision of routine care and monitoring, 5 had R. gnavus blooms that were “strikingly concordant with periods of raised disease activity,” Gregg J. Silverman, MD, of NYU Grossman School of Medicine, New York, and coinvestigators reported in Annals of the Rheumatic Diseases.
Four of the five patients with flare-associated R. gnavus blooms had lupus nephritis (LN); the other had a flare involving inflammation in multiple joints. The four patients with concurrent LN and spikes in R. gnavus also represented almost half of patients who had LN disease flares (four of nine) during the study period. The nine patients in the study with renal involvement, and the four with concurrent R. gnavus spikes and flares, represented different races and ethnicities.
The findings build upon research published by the NYU group several years ago showing that patients with SLE had more R. gnavus in the gut than similar patients without the disease, and that flares closely tracked major increases in R. gnavus growth. Evidence of R. gnavus expansions in patients with SLE now comes from several cohorts in the United States as well as cohorts in Europe and China, the researchers noted in their new paper.
An underlying, unstable microbiome
The new study at NYU took a “deeper dive” than previous research, looking at individuals over a longer period of time, Dr. Silverman, the study’s senior investigator and associate director of rheumatology at NYU Langone Health, said in an interview. Blood and a total of 44 stool samples from the 16 patients were analyzed, as were a total of 72 stool samples from 22 healthy control volunteers.
Importantly, he said, the gut microbiome in patients with SLE was found to be inherently unstable over time, compared with the microbiota communities of the controls. “There was an instability, a shifting dynamic composition of the microbiome [in patients with lupus]. ... Healthy individuals had more of a balance, with small changes over time” and a stable, low abundance of R. gnavus, Dr. Silverman said.
Transient expansions of several pathogenic species occurred in some of the patients with lupus (and not in controls), but blooms of R. gnavus were the most common. The researchers said in their paper that they “speculate that susceptibility for specific clinical features during R. gnavus blooms reflect in part differences in genetic susceptibility of the patient.”
Patients on cytotoxic agents or antibiotics were excluded from the study, but the study was not designed to disentangle the potential influence of diet or prior antibiotic exposure, they noted. Larger studies are needed that are better controlled and that include more frequent assessments, Dr. Silverman added.
A sure association and probable cause
“There seems to be a special connection [of R. gnavus] to lupus nephritis, which is an important, major subset of disease,” said Martin Kriegel, MD, PhD, chief or rheumatology and clinical immunology at the University of Munster (Germany). Dr. Kriegel also researches the gut microbiome in lupus and was asked to comment on the new findings from NYU.
The “difficult question is, is the bug driving the flare [as the NYU paper proposes], or is it the lupus nephritis that leads to overgrowth?” he said, noting that it “is well known that kidney disease, whether from lupus or other causes, creates disturbances in the microbiome.”
It’s “likely the case” that the pathobiome – with R. gnavus being an important pathobiont – helps to drive flares, he said. The new research shows only an association, but studies done in mice – including prior research by Dr. Silverman – support a mechanistic link, said Dr. Kriegel, also adjunct associate professor of immunobiology and of medicine at Yale University, New Haven, Conn.
Investigators in the microbiome space are moving toward more strain-level analysis – “not only measuring what organisms are there, but culturing them and sequencing them,” Dr. Kriegel noted, and the new study does just this.
The R. gnavus strains isolated during lupus flares were distinguishable from strains found in healthy people – and from strains found by other researchers in patients with inflammatory bowel disease – by their common expression of a novel type of cell membrane–associated lipoglycan. The lipoglycans were recognized by specific serum IgG2 antibodies that were detected concurrently with R. gnavus blooms and lupus flares, Dr. Silverman and his colleagues reported.
Dr. Silverman and Dr. Kriegel both study the paradigm of a gut-barrier breach, whereby pathogenic bacteria cause intestinal permeability, allowing bacterial leakages that trigger inflammation and immune responses. “We think that in lupus and other rheumatic diseases like rheumatoid arthritis, a leaky gut barrier is an important mechanism, even though these patients don’t have gastrointestinal symptoms,” said Dr. Kriegel, who has studied the role of another potentially pathogenic bacteria, Enterococcus gallinarum, in SLE.
Strengthening the gut barrier may be a plausible, general approach to reducing the severity of diseases like SLE and RA until more personalized approaches targeting individuals’ microbiome are developed, he noted.
Future treatments involving antibacterial agents, probiotics or dietary regimens that prevent imbalances in the gut microbiome would be “benign,” compared with currently utilized immunosuppressive medications, Dr. Silverman said.
The NYU study was funded in part by grants from the National Institutes of Health and the Lupus Research Alliance. Dr. Silverman disclosed that NYU has filed a patent application for an antibody test to detect serum antibodies to the lipoglycan made by some strains of R. gnavus. Dr. Kriegel disclosed that he holds a patent at Yale related to the Enterococcus bacteria he studies, and that he consults for Roche, Enterome, and Eligo Biosciences.
Flares of systemic lupus erythematosus (SLE), particularly those involving severe kidney disease, were associated with growth spikes of the gut bacteria Ruminococcus blautia gnavus in a small, 4-year observational study that also demonstrated an underlying, inherent instability in the gut microbiome of patients with SLE.
Of 16 patients with SLE studied during the provision of routine care and monitoring, 5 had R. gnavus blooms that were “strikingly concordant with periods of raised disease activity,” Gregg J. Silverman, MD, of NYU Grossman School of Medicine, New York, and coinvestigators reported in Annals of the Rheumatic Diseases.
Four of the five patients with flare-associated R. gnavus blooms had lupus nephritis (LN); the other had a flare involving inflammation in multiple joints. The four patients with concurrent LN and spikes in R. gnavus also represented almost half of patients who had LN disease flares (four of nine) during the study period. The nine patients in the study with renal involvement, and the four with concurrent R. gnavus spikes and flares, represented different races and ethnicities.
The findings build upon research published by the NYU group several years ago showing that patients with SLE had more R. gnavus in the gut than similar patients without the disease, and that flares closely tracked major increases in R. gnavus growth. Evidence of R. gnavus expansions in patients with SLE now comes from several cohorts in the United States as well as cohorts in Europe and China, the researchers noted in their new paper.
An underlying, unstable microbiome
The new study at NYU took a “deeper dive” than previous research, looking at individuals over a longer period of time, Dr. Silverman, the study’s senior investigator and associate director of rheumatology at NYU Langone Health, said in an interview. Blood and a total of 44 stool samples from the 16 patients were analyzed, as were a total of 72 stool samples from 22 healthy control volunteers.
Importantly, he said, the gut microbiome in patients with SLE was found to be inherently unstable over time, compared with the microbiota communities of the controls. “There was an instability, a shifting dynamic composition of the microbiome [in patients with lupus]. ... Healthy individuals had more of a balance, with small changes over time” and a stable, low abundance of R. gnavus, Dr. Silverman said.
Transient expansions of several pathogenic species occurred in some of the patients with lupus (and not in controls), but blooms of R. gnavus were the most common. The researchers said in their paper that they “speculate that susceptibility for specific clinical features during R. gnavus blooms reflect in part differences in genetic susceptibility of the patient.”
Patients on cytotoxic agents or antibiotics were excluded from the study, but the study was not designed to disentangle the potential influence of diet or prior antibiotic exposure, they noted. Larger studies are needed that are better controlled and that include more frequent assessments, Dr. Silverman added.
A sure association and probable cause
“There seems to be a special connection [of R. gnavus] to lupus nephritis, which is an important, major subset of disease,” said Martin Kriegel, MD, PhD, chief or rheumatology and clinical immunology at the University of Munster (Germany). Dr. Kriegel also researches the gut microbiome in lupus and was asked to comment on the new findings from NYU.
The “difficult question is, is the bug driving the flare [as the NYU paper proposes], or is it the lupus nephritis that leads to overgrowth?” he said, noting that it “is well known that kidney disease, whether from lupus or other causes, creates disturbances in the microbiome.”
It’s “likely the case” that the pathobiome – with R. gnavus being an important pathobiont – helps to drive flares, he said. The new research shows only an association, but studies done in mice – including prior research by Dr. Silverman – support a mechanistic link, said Dr. Kriegel, also adjunct associate professor of immunobiology and of medicine at Yale University, New Haven, Conn.
Investigators in the microbiome space are moving toward more strain-level analysis – “not only measuring what organisms are there, but culturing them and sequencing them,” Dr. Kriegel noted, and the new study does just this.
The R. gnavus strains isolated during lupus flares were distinguishable from strains found in healthy people – and from strains found by other researchers in patients with inflammatory bowel disease – by their common expression of a novel type of cell membrane–associated lipoglycan. The lipoglycans were recognized by specific serum IgG2 antibodies that were detected concurrently with R. gnavus blooms and lupus flares, Dr. Silverman and his colleagues reported.
Dr. Silverman and Dr. Kriegel both study the paradigm of a gut-barrier breach, whereby pathogenic bacteria cause intestinal permeability, allowing bacterial leakages that trigger inflammation and immune responses. “We think that in lupus and other rheumatic diseases like rheumatoid arthritis, a leaky gut barrier is an important mechanism, even though these patients don’t have gastrointestinal symptoms,” said Dr. Kriegel, who has studied the role of another potentially pathogenic bacteria, Enterococcus gallinarum, in SLE.
Strengthening the gut barrier may be a plausible, general approach to reducing the severity of diseases like SLE and RA until more personalized approaches targeting individuals’ microbiome are developed, he noted.
Future treatments involving antibacterial agents, probiotics or dietary regimens that prevent imbalances in the gut microbiome would be “benign,” compared with currently utilized immunosuppressive medications, Dr. Silverman said.
The NYU study was funded in part by grants from the National Institutes of Health and the Lupus Research Alliance. Dr. Silverman disclosed that NYU has filed a patent application for an antibody test to detect serum antibodies to the lipoglycan made by some strains of R. gnavus. Dr. Kriegel disclosed that he holds a patent at Yale related to the Enterococcus bacteria he studies, and that he consults for Roche, Enterome, and Eligo Biosciences.
Flares of systemic lupus erythematosus (SLE), particularly those involving severe kidney disease, were associated with growth spikes of the gut bacteria Ruminococcus blautia gnavus in a small, 4-year observational study that also demonstrated an underlying, inherent instability in the gut microbiome of patients with SLE.
Of 16 patients with SLE studied during the provision of routine care and monitoring, 5 had R. gnavus blooms that were “strikingly concordant with periods of raised disease activity,” Gregg J. Silverman, MD, of NYU Grossman School of Medicine, New York, and coinvestigators reported in Annals of the Rheumatic Diseases.
Four of the five patients with flare-associated R. gnavus blooms had lupus nephritis (LN); the other had a flare involving inflammation in multiple joints. The four patients with concurrent LN and spikes in R. gnavus also represented almost half of patients who had LN disease flares (four of nine) during the study period. The nine patients in the study with renal involvement, and the four with concurrent R. gnavus spikes and flares, represented different races and ethnicities.
The findings build upon research published by the NYU group several years ago showing that patients with SLE had more R. gnavus in the gut than similar patients without the disease, and that flares closely tracked major increases in R. gnavus growth. Evidence of R. gnavus expansions in patients with SLE now comes from several cohorts in the United States as well as cohorts in Europe and China, the researchers noted in their new paper.
An underlying, unstable microbiome
The new study at NYU took a “deeper dive” than previous research, looking at individuals over a longer period of time, Dr. Silverman, the study’s senior investigator and associate director of rheumatology at NYU Langone Health, said in an interview. Blood and a total of 44 stool samples from the 16 patients were analyzed, as were a total of 72 stool samples from 22 healthy control volunteers.
Importantly, he said, the gut microbiome in patients with SLE was found to be inherently unstable over time, compared with the microbiota communities of the controls. “There was an instability, a shifting dynamic composition of the microbiome [in patients with lupus]. ... Healthy individuals had more of a balance, with small changes over time” and a stable, low abundance of R. gnavus, Dr. Silverman said.
Transient expansions of several pathogenic species occurred in some of the patients with lupus (and not in controls), but blooms of R. gnavus were the most common. The researchers said in their paper that they “speculate that susceptibility for specific clinical features during R. gnavus blooms reflect in part differences in genetic susceptibility of the patient.”
Patients on cytotoxic agents or antibiotics were excluded from the study, but the study was not designed to disentangle the potential influence of diet or prior antibiotic exposure, they noted. Larger studies are needed that are better controlled and that include more frequent assessments, Dr. Silverman added.
A sure association and probable cause
“There seems to be a special connection [of R. gnavus] to lupus nephritis, which is an important, major subset of disease,” said Martin Kriegel, MD, PhD, chief or rheumatology and clinical immunology at the University of Munster (Germany). Dr. Kriegel also researches the gut microbiome in lupus and was asked to comment on the new findings from NYU.
The “difficult question is, is the bug driving the flare [as the NYU paper proposes], or is it the lupus nephritis that leads to overgrowth?” he said, noting that it “is well known that kidney disease, whether from lupus or other causes, creates disturbances in the microbiome.”
It’s “likely the case” that the pathobiome – with R. gnavus being an important pathobiont – helps to drive flares, he said. The new research shows only an association, but studies done in mice – including prior research by Dr. Silverman – support a mechanistic link, said Dr. Kriegel, also adjunct associate professor of immunobiology and of medicine at Yale University, New Haven, Conn.
Investigators in the microbiome space are moving toward more strain-level analysis – “not only measuring what organisms are there, but culturing them and sequencing them,” Dr. Kriegel noted, and the new study does just this.
The R. gnavus strains isolated during lupus flares were distinguishable from strains found in healthy people – and from strains found by other researchers in patients with inflammatory bowel disease – by their common expression of a novel type of cell membrane–associated lipoglycan. The lipoglycans were recognized by specific serum IgG2 antibodies that were detected concurrently with R. gnavus blooms and lupus flares, Dr. Silverman and his colleagues reported.
Dr. Silverman and Dr. Kriegel both study the paradigm of a gut-barrier breach, whereby pathogenic bacteria cause intestinal permeability, allowing bacterial leakages that trigger inflammation and immune responses. “We think that in lupus and other rheumatic diseases like rheumatoid arthritis, a leaky gut barrier is an important mechanism, even though these patients don’t have gastrointestinal symptoms,” said Dr. Kriegel, who has studied the role of another potentially pathogenic bacteria, Enterococcus gallinarum, in SLE.
Strengthening the gut barrier may be a plausible, general approach to reducing the severity of diseases like SLE and RA until more personalized approaches targeting individuals’ microbiome are developed, he noted.
Future treatments involving antibacterial agents, probiotics or dietary regimens that prevent imbalances in the gut microbiome would be “benign,” compared with currently utilized immunosuppressive medications, Dr. Silverman said.
The NYU study was funded in part by grants from the National Institutes of Health and the Lupus Research Alliance. Dr. Silverman disclosed that NYU has filed a patent application for an antibody test to detect serum antibodies to the lipoglycan made by some strains of R. gnavus. Dr. Kriegel disclosed that he holds a patent at Yale related to the Enterococcus bacteria he studies, and that he consults for Roche, Enterome, and Eligo Biosciences.
FROM ANNALS OF THE RHEUMATIC DISEASES
Circulating Tumor DNA Testing and Liquid Biopsy: The Future for Precision Medicine and Guided Targeted Therapy for Breast Cancer?
The current standard for breast cancer screening (for non–high-risk patients) is an annual or semiannual mammogram for women aged 40 and older.1 However, mammography-based screening can give false-positive or false-negative results. This can lead to excessive use of invasive tissue biopsies and unnecessary exposure to ionizing radiation—which can also become expensive and time-consuming for patients.2
Both normal and cancerous cells shed cell-free DNA (cfDNA) into the blood circulation.3 Circulating tumor DNA (ctDNA) are fragments of DNA derived from tumor cells that circulate in the blood together with cfDNA. The ctDNA originates directly from a tumor or from circulating tumor cells (and carries information from the tumor cell genome), whereas cfDNA enters the bloodstream after apoptosis or necrosis and carries genome-wide DNA information. The amount of ctDNA in the blood has been shown to be elevated in patients with cancer.3 Different cancers release varying levels of ctDNA; the amount of ctDNA released depends on the number of tumor cells that are in senescence vs undergoing apoptosis.4
The possibility of incorporating this biomarker obtained from a “liquid biopsy” is currently being studied and will hopefully become a standard of care for breast cancer screening and monitoring. The liquid biopsy detects ctDNA that has been released into the bloodstream from tumor regions and helps identify intratumoral heterogeneity and clonal evolution.5 Additionally, sequencing tumor DNA has opened new possibilities for precision oncology.6 Detection of somatic gene mutations, amplifications, and gene fusions helps to deliver targeted therapies.6 Analysis of potential somatic mutations in ctDNA, in combination with cfDNA levels, can help capture clinically relevant information beyond single genetic alterations and tumor fraction, potentially improving the accuracy of early detection and screening for breast cancer.
Recent advances in ctDNA testing technology have made it more accurate and reliable. ctDNA testing has several benefits, including early detection of cancer (detecting ctDNA at low levels)7; monitoring of tumor dynamics, therapeutic response, and residual disease8; as well as analysis of the evolution of genetic or epigenetic alterations characterizing the tumor.9 Its noninvasiveness, rapidity, and low cost allow for longitudinal monitoring of cancer in real time, potentially capturing tumor heterogeneity.10,11
The liquid biopsy potentially can give more options for therapeutic monitoring for breast cancer and may mirror clinically relevant genetic alterations that occur in all tumor tissues. Liquid biopsy offers many advantages. It allows for the detection of minimal residual disease and micrometastatic disease that may be difficult to detect with a traditional tissue biopsy.12 Liquid biopsy detects ctDNA that has been released into the bloodstream from multiple tumor regions and allows the possibility of identifying intratumoral heterogeneity and clonal evolution.5 It can also detect small quantitative variations within the blood, enabling real-time surveillance.
The liquid biopsy can offer earlier and easier access to some tumor-based genetic information at any given timepoint and can replace a tumor tissue biopsy in some cases, helping to avoid delays and complications of a solid tumor invasive biopsy procedure. This is especially relevant in the metastatic setting, in which ctDNA might be the only available genetic material from tumors.13 Tissue biopsy can only provide a static and spatially limited view of the disease at the time of sampling; ctDNA analysis could potentially reflect the genetic alterations that occur in all metastatic breast cancer sites over time.14,15 Furthermore, machine learning of multi-gene signatures, obtained from ctDNA, can possibly identify complex biological features, including measures of tumor proliferation and estrogen receptor signaling, similar to direct tumor tissue DNA or RNA profiling.16
ctDNA testing is currently being studied to monitor patients who have been diagnosed with breast cancer. Small retrospective studies have shown that detection of ctDNA in plasma, after patients have completed therapy for early-stage breast cancer, is associated with a very high risk of relapse.17
Ongoing studies are examining the tailoring of adjuvant treatment based on ctDNA. If these trials are successful, certain aspects of adjuvant treatment could be lessened, or omitted, for patients who have undetectable ctDNA or intensified for patients who have detectable ctDNA after definitive treatments. This could personalize treatment specifically to the patient.
The detection and persistence of ctDNA in the middle of neoadjuvant systemic therapy may have the potential to negatively predict response to treatment and identify patients who will not achieve pathologic complete response. This may have the potential to aid in clinical decision-making for treatment escalation in these nonresponders.18
Despite these distinct characteristics, the low levels of ctDNA found in early-stage disease, along with the lack of ctDNA shedding from some tumors, can further complicate or impede detection of recurrence in early-stage breast cancer. Testing is further complicated by hematologic genetic alterations.5 The limitation of ctDNA approaches is that these techniques only detect known mutations in certain genes, so patients without these mutations could be overlooked, limiting the application of this technology.19
Overall, ctDNA testing represents a promising area of research for the diagnosis, treatment, and monitoring of breast cancer. While more research is needed to fully understand its potential, the advances in this technology are certainly exciting and could lead to significant improvements in patient outcomes. It is hopeful that in the near future, ctDNA testing from liquid biopsy could become a standard of care in breast cancer screening, ultimately helping clinicians to personalize treatment therapies and improve patient outcomes when treating patients with breast cancer.
1. Oeffinger KC, Fontham ETH, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599-1614.
2. Zubor P, Kubatka P, Kajo K, et al. Why the gold standard approach by mammography demands extension by multiomics? Application of liquid biopsy miRNA profiles to breast cancer disease management. Int J Mol Sci. 2019;20(12):E2878.
3. Thierry AR, El Messaoudi S, Gahan PB, Anker P, Stroun M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016;35(3):347-376.
4. Rostami A, Lambie M, Yu CW, Stambolic V, Waldron JN, Bratman SV. Senescence, necrosis, and apoptosis govern circulating cell-free DNA release kinetics. Cell Rep. 2020;31(13):107830.
5. De Rubis G, Rajeev Krishnan S, Bebawy M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis. Trends Pharmacol Sci. 2019;40(3):172-186.
6. Mateo J, Chakravarty D, Dienstmann R, et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol. 2018;29:1895-1902.
7. Wang J, Han X, Sun Y. DNA methylation signatures in circulating cell-free DNA as biomarkers for the early detection of cancer. Sci China Life Sci. 2017;60(4):356-362.
8. Dawson S-J, Tsui DWY, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368(13):1199-1209.
9. Diaz Jr LA, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579-586.
10. Oxnard GR, Paweletz CP, Kuang Y, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res. 2014;20(6):1698-1705.
11. Jamal-Hanjani M, Wilson GA, Horswell S, et al. Detection of ubiquitous and heterogeneous mutations in cell-free DNA from patients with early-stage non-small-cell lung cancer. Ann Oncol. 2016;27(5):862-867.
12. Fiala C, Diamandis EP. Utility of circulating tumor DNA in cancer diagnostics with
13. Xia Y, Fan C, Hoadley KA, Parker JS, Perou CM. Genetic determinants of the molecular portraits of epithelial cancers. Nat Commun. 2019;10(1):5666.
14. Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17(4):223-238.
15. Boldrin E, Nardo G, Zulato E, et al. Detection of loss of heterozygosity in cfDNA of advanced EGFR- or KRAS-mutated non-small-cell lung cancer patients. Int J Mol Sci. 2019;21(1):66.
16. Prat A, Brasó-Maristany F, Martínez-Sáez O, et al. Circulating tumor DNA reveals complex biological features with clinical relevance in metastatic breast cancer. Nat Commun. 2023;14(1):1157.
17. Coombes RC, Page K, Salari R, et al. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res. 2019;25(14):4255-4263.
18. Zhou Q, Gampenrieder SP, Frantal S, et al. Persistence of ctDNA in patients with breast cancer during neoadjuvant treatment is a significant predictor of poor tumor response. Clin Cancer Res. 2022;28(4):697-707.
19. Lin C, Liu X, Zheng B, Ke R, Tzeng C-M. Liquid biopsy, ctDNA diagnosis through NGS. Life (Basel). 2021;11(9):890.
The current standard for breast cancer screening (for non–high-risk patients) is an annual or semiannual mammogram for women aged 40 and older.1 However, mammography-based screening can give false-positive or false-negative results. This can lead to excessive use of invasive tissue biopsies and unnecessary exposure to ionizing radiation—which can also become expensive and time-consuming for patients.2
Both normal and cancerous cells shed cell-free DNA (cfDNA) into the blood circulation.3 Circulating tumor DNA (ctDNA) are fragments of DNA derived from tumor cells that circulate in the blood together with cfDNA. The ctDNA originates directly from a tumor or from circulating tumor cells (and carries information from the tumor cell genome), whereas cfDNA enters the bloodstream after apoptosis or necrosis and carries genome-wide DNA information. The amount of ctDNA in the blood has been shown to be elevated in patients with cancer.3 Different cancers release varying levels of ctDNA; the amount of ctDNA released depends on the number of tumor cells that are in senescence vs undergoing apoptosis.4
The possibility of incorporating this biomarker obtained from a “liquid biopsy” is currently being studied and will hopefully become a standard of care for breast cancer screening and monitoring. The liquid biopsy detects ctDNA that has been released into the bloodstream from tumor regions and helps identify intratumoral heterogeneity and clonal evolution.5 Additionally, sequencing tumor DNA has opened new possibilities for precision oncology.6 Detection of somatic gene mutations, amplifications, and gene fusions helps to deliver targeted therapies.6 Analysis of potential somatic mutations in ctDNA, in combination with cfDNA levels, can help capture clinically relevant information beyond single genetic alterations and tumor fraction, potentially improving the accuracy of early detection and screening for breast cancer.
Recent advances in ctDNA testing technology have made it more accurate and reliable. ctDNA testing has several benefits, including early detection of cancer (detecting ctDNA at low levels)7; monitoring of tumor dynamics, therapeutic response, and residual disease8; as well as analysis of the evolution of genetic or epigenetic alterations characterizing the tumor.9 Its noninvasiveness, rapidity, and low cost allow for longitudinal monitoring of cancer in real time, potentially capturing tumor heterogeneity.10,11
The liquid biopsy potentially can give more options for therapeutic monitoring for breast cancer and may mirror clinically relevant genetic alterations that occur in all tumor tissues. Liquid biopsy offers many advantages. It allows for the detection of minimal residual disease and micrometastatic disease that may be difficult to detect with a traditional tissue biopsy.12 Liquid biopsy detects ctDNA that has been released into the bloodstream from multiple tumor regions and allows the possibility of identifying intratumoral heterogeneity and clonal evolution.5 It can also detect small quantitative variations within the blood, enabling real-time surveillance.
The liquid biopsy can offer earlier and easier access to some tumor-based genetic information at any given timepoint and can replace a tumor tissue biopsy in some cases, helping to avoid delays and complications of a solid tumor invasive biopsy procedure. This is especially relevant in the metastatic setting, in which ctDNA might be the only available genetic material from tumors.13 Tissue biopsy can only provide a static and spatially limited view of the disease at the time of sampling; ctDNA analysis could potentially reflect the genetic alterations that occur in all metastatic breast cancer sites over time.14,15 Furthermore, machine learning of multi-gene signatures, obtained from ctDNA, can possibly identify complex biological features, including measures of tumor proliferation and estrogen receptor signaling, similar to direct tumor tissue DNA or RNA profiling.16
ctDNA testing is currently being studied to monitor patients who have been diagnosed with breast cancer. Small retrospective studies have shown that detection of ctDNA in plasma, after patients have completed therapy for early-stage breast cancer, is associated with a very high risk of relapse.17
Ongoing studies are examining the tailoring of adjuvant treatment based on ctDNA. If these trials are successful, certain aspects of adjuvant treatment could be lessened, or omitted, for patients who have undetectable ctDNA or intensified for patients who have detectable ctDNA after definitive treatments. This could personalize treatment specifically to the patient.
The detection and persistence of ctDNA in the middle of neoadjuvant systemic therapy may have the potential to negatively predict response to treatment and identify patients who will not achieve pathologic complete response. This may have the potential to aid in clinical decision-making for treatment escalation in these nonresponders.18
Despite these distinct characteristics, the low levels of ctDNA found in early-stage disease, along with the lack of ctDNA shedding from some tumors, can further complicate or impede detection of recurrence in early-stage breast cancer. Testing is further complicated by hematologic genetic alterations.5 The limitation of ctDNA approaches is that these techniques only detect known mutations in certain genes, so patients without these mutations could be overlooked, limiting the application of this technology.19
Overall, ctDNA testing represents a promising area of research for the diagnosis, treatment, and monitoring of breast cancer. While more research is needed to fully understand its potential, the advances in this technology are certainly exciting and could lead to significant improvements in patient outcomes. It is hopeful that in the near future, ctDNA testing from liquid biopsy could become a standard of care in breast cancer screening, ultimately helping clinicians to personalize treatment therapies and improve patient outcomes when treating patients with breast cancer.
The current standard for breast cancer screening (for non–high-risk patients) is an annual or semiannual mammogram for women aged 40 and older.1 However, mammography-based screening can give false-positive or false-negative results. This can lead to excessive use of invasive tissue biopsies and unnecessary exposure to ionizing radiation—which can also become expensive and time-consuming for patients.2
Both normal and cancerous cells shed cell-free DNA (cfDNA) into the blood circulation.3 Circulating tumor DNA (ctDNA) are fragments of DNA derived from tumor cells that circulate in the blood together with cfDNA. The ctDNA originates directly from a tumor or from circulating tumor cells (and carries information from the tumor cell genome), whereas cfDNA enters the bloodstream after apoptosis or necrosis and carries genome-wide DNA information. The amount of ctDNA in the blood has been shown to be elevated in patients with cancer.3 Different cancers release varying levels of ctDNA; the amount of ctDNA released depends on the number of tumor cells that are in senescence vs undergoing apoptosis.4
The possibility of incorporating this biomarker obtained from a “liquid biopsy” is currently being studied and will hopefully become a standard of care for breast cancer screening and monitoring. The liquid biopsy detects ctDNA that has been released into the bloodstream from tumor regions and helps identify intratumoral heterogeneity and clonal evolution.5 Additionally, sequencing tumor DNA has opened new possibilities for precision oncology.6 Detection of somatic gene mutations, amplifications, and gene fusions helps to deliver targeted therapies.6 Analysis of potential somatic mutations in ctDNA, in combination with cfDNA levels, can help capture clinically relevant information beyond single genetic alterations and tumor fraction, potentially improving the accuracy of early detection and screening for breast cancer.
Recent advances in ctDNA testing technology have made it more accurate and reliable. ctDNA testing has several benefits, including early detection of cancer (detecting ctDNA at low levels)7; monitoring of tumor dynamics, therapeutic response, and residual disease8; as well as analysis of the evolution of genetic or epigenetic alterations characterizing the tumor.9 Its noninvasiveness, rapidity, and low cost allow for longitudinal monitoring of cancer in real time, potentially capturing tumor heterogeneity.10,11
The liquid biopsy potentially can give more options for therapeutic monitoring for breast cancer and may mirror clinically relevant genetic alterations that occur in all tumor tissues. Liquid biopsy offers many advantages. It allows for the detection of minimal residual disease and micrometastatic disease that may be difficult to detect with a traditional tissue biopsy.12 Liquid biopsy detects ctDNA that has been released into the bloodstream from multiple tumor regions and allows the possibility of identifying intratumoral heterogeneity and clonal evolution.5 It can also detect small quantitative variations within the blood, enabling real-time surveillance.
The liquid biopsy can offer earlier and easier access to some tumor-based genetic information at any given timepoint and can replace a tumor tissue biopsy in some cases, helping to avoid delays and complications of a solid tumor invasive biopsy procedure. This is especially relevant in the metastatic setting, in which ctDNA might be the only available genetic material from tumors.13 Tissue biopsy can only provide a static and spatially limited view of the disease at the time of sampling; ctDNA analysis could potentially reflect the genetic alterations that occur in all metastatic breast cancer sites over time.14,15 Furthermore, machine learning of multi-gene signatures, obtained from ctDNA, can possibly identify complex biological features, including measures of tumor proliferation and estrogen receptor signaling, similar to direct tumor tissue DNA or RNA profiling.16
ctDNA testing is currently being studied to monitor patients who have been diagnosed with breast cancer. Small retrospective studies have shown that detection of ctDNA in plasma, after patients have completed therapy for early-stage breast cancer, is associated with a very high risk of relapse.17
Ongoing studies are examining the tailoring of adjuvant treatment based on ctDNA. If these trials are successful, certain aspects of adjuvant treatment could be lessened, or omitted, for patients who have undetectable ctDNA or intensified for patients who have detectable ctDNA after definitive treatments. This could personalize treatment specifically to the patient.
The detection and persistence of ctDNA in the middle of neoadjuvant systemic therapy may have the potential to negatively predict response to treatment and identify patients who will not achieve pathologic complete response. This may have the potential to aid in clinical decision-making for treatment escalation in these nonresponders.18
Despite these distinct characteristics, the low levels of ctDNA found in early-stage disease, along with the lack of ctDNA shedding from some tumors, can further complicate or impede detection of recurrence in early-stage breast cancer. Testing is further complicated by hematologic genetic alterations.5 The limitation of ctDNA approaches is that these techniques only detect known mutations in certain genes, so patients without these mutations could be overlooked, limiting the application of this technology.19
Overall, ctDNA testing represents a promising area of research for the diagnosis, treatment, and monitoring of breast cancer. While more research is needed to fully understand its potential, the advances in this technology are certainly exciting and could lead to significant improvements in patient outcomes. It is hopeful that in the near future, ctDNA testing from liquid biopsy could become a standard of care in breast cancer screening, ultimately helping clinicians to personalize treatment therapies and improve patient outcomes when treating patients with breast cancer.
1. Oeffinger KC, Fontham ETH, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599-1614.
2. Zubor P, Kubatka P, Kajo K, et al. Why the gold standard approach by mammography demands extension by multiomics? Application of liquid biopsy miRNA profiles to breast cancer disease management. Int J Mol Sci. 2019;20(12):E2878.
3. Thierry AR, El Messaoudi S, Gahan PB, Anker P, Stroun M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016;35(3):347-376.
4. Rostami A, Lambie M, Yu CW, Stambolic V, Waldron JN, Bratman SV. Senescence, necrosis, and apoptosis govern circulating cell-free DNA release kinetics. Cell Rep. 2020;31(13):107830.
5. De Rubis G, Rajeev Krishnan S, Bebawy M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis. Trends Pharmacol Sci. 2019;40(3):172-186.
6. Mateo J, Chakravarty D, Dienstmann R, et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol. 2018;29:1895-1902.
7. Wang J, Han X, Sun Y. DNA methylation signatures in circulating cell-free DNA as biomarkers for the early detection of cancer. Sci China Life Sci. 2017;60(4):356-362.
8. Dawson S-J, Tsui DWY, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368(13):1199-1209.
9. Diaz Jr LA, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579-586.
10. Oxnard GR, Paweletz CP, Kuang Y, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res. 2014;20(6):1698-1705.
11. Jamal-Hanjani M, Wilson GA, Horswell S, et al. Detection of ubiquitous and heterogeneous mutations in cell-free DNA from patients with early-stage non-small-cell lung cancer. Ann Oncol. 2016;27(5):862-867.
12. Fiala C, Diamandis EP. Utility of circulating tumor DNA in cancer diagnostics with
13. Xia Y, Fan C, Hoadley KA, Parker JS, Perou CM. Genetic determinants of the molecular portraits of epithelial cancers. Nat Commun. 2019;10(1):5666.
14. Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17(4):223-238.
15. Boldrin E, Nardo G, Zulato E, et al. Detection of loss of heterozygosity in cfDNA of advanced EGFR- or KRAS-mutated non-small-cell lung cancer patients. Int J Mol Sci. 2019;21(1):66.
16. Prat A, Brasó-Maristany F, Martínez-Sáez O, et al. Circulating tumor DNA reveals complex biological features with clinical relevance in metastatic breast cancer. Nat Commun. 2023;14(1):1157.
17. Coombes RC, Page K, Salari R, et al. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res. 2019;25(14):4255-4263.
18. Zhou Q, Gampenrieder SP, Frantal S, et al. Persistence of ctDNA in patients with breast cancer during neoadjuvant treatment is a significant predictor of poor tumor response. Clin Cancer Res. 2022;28(4):697-707.
19. Lin C, Liu X, Zheng B, Ke R, Tzeng C-M. Liquid biopsy, ctDNA diagnosis through NGS. Life (Basel). 2021;11(9):890.
1. Oeffinger KC, Fontham ETH, Etzioni R, et al. Breast cancer screening for women at average risk: 2015 guideline update from the American Cancer Society. JAMA. 2015;314(15):1599-1614.
2. Zubor P, Kubatka P, Kajo K, et al. Why the gold standard approach by mammography demands extension by multiomics? Application of liquid biopsy miRNA profiles to breast cancer disease management. Int J Mol Sci. 2019;20(12):E2878.
3. Thierry AR, El Messaoudi S, Gahan PB, Anker P, Stroun M. Origins, structures, and functions of circulating DNA in oncology. Cancer Metastasis Rev. 2016;35(3):347-376.
4. Rostami A, Lambie M, Yu CW, Stambolic V, Waldron JN, Bratman SV. Senescence, necrosis, and apoptosis govern circulating cell-free DNA release kinetics. Cell Rep. 2020;31(13):107830.
5. De Rubis G, Rajeev Krishnan S, Bebawy M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis. Trends Pharmacol Sci. 2019;40(3):172-186.
6. Mateo J, Chakravarty D, Dienstmann R, et al. A framework to rank genomic alterations as targets for cancer precision medicine: the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann Oncol. 2018;29:1895-1902.
7. Wang J, Han X, Sun Y. DNA methylation signatures in circulating cell-free DNA as biomarkers for the early detection of cancer. Sci China Life Sci. 2017;60(4):356-362.
8. Dawson S-J, Tsui DWY, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368(13):1199-1209.
9. Diaz Jr LA, Bardelli A. Liquid biopsies: genotyping circulating tumor DNA. J Clin Oncol. 2014;32(6):579-586.
10. Oxnard GR, Paweletz CP, Kuang Y, et al. Noninvasive detection of response and resistance in EGFR-mutant lung cancer using quantitative next-generation genotyping of cell-free plasma DNA. Clin Cancer Res. 2014;20(6):1698-1705.
11. Jamal-Hanjani M, Wilson GA, Horswell S, et al. Detection of ubiquitous and heterogeneous mutations in cell-free DNA from patients with early-stage non-small-cell lung cancer. Ann Oncol. 2016;27(5):862-867.
12. Fiala C, Diamandis EP. Utility of circulating tumor DNA in cancer diagnostics with
13. Xia Y, Fan C, Hoadley KA, Parker JS, Perou CM. Genetic determinants of the molecular portraits of epithelial cancers. Nat Commun. 2019;10(1):5666.
14. Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17(4):223-238.
15. Boldrin E, Nardo G, Zulato E, et al. Detection of loss of heterozygosity in cfDNA of advanced EGFR- or KRAS-mutated non-small-cell lung cancer patients. Int J Mol Sci. 2019;21(1):66.
16. Prat A, Brasó-Maristany F, Martínez-Sáez O, et al. Circulating tumor DNA reveals complex biological features with clinical relevance in metastatic breast cancer. Nat Commun. 2023;14(1):1157.
17. Coombes RC, Page K, Salari R, et al. Personalized detection of circulating tumor DNA antedates breast cancer metastatic recurrence. Clin Cancer Res. 2019;25(14):4255-4263.
18. Zhou Q, Gampenrieder SP, Frantal S, et al. Persistence of ctDNA in patients with breast cancer during neoadjuvant treatment is a significant predictor of poor tumor response. Clin Cancer Res. 2022;28(4):697-707.
19. Lin C, Liu X, Zheng B, Ke R, Tzeng C-M. Liquid biopsy, ctDNA diagnosis through NGS. Life (Basel). 2021;11(9):890.
Anti-obesity medications: Breakthroughs and limitations
Obesity is a major health problem in the United States. The Centers for Disease Control and Prevention (CDC) defines the problem as weight that is higher than what is healthy for a given height, with quantitative definitions of overweight and obesity as body mass indices (BMIs) of 25 to 29.9 kg/m2 and ≥ 30 kg/m2, respectively.1 The prevalence of obesity among adults in 2017 ̶ 2018 was reported by the CDC to be 42.4%.2 Among women, the reported prevalence of obesity was lowest among Asian individuals (17.2%) and greatest among non-Hispanic Black individuals (56.9%), with White (39.8%) and Hispanic individuals (43.7%) having rates in between.2 In a meta-analysis of prospective studies that included 4 million people who were never smokers and had no chronic disease at baseline, age- and sex-adjusted mortality rates were studied over a median of 14 years of follow-up.3 Compared with those with a BMI of 20 to 25 kg/m2, people with a BMI of 30 to 34.9 kg/m2 or a BMI of 35 to 39.9 kg/m2 had increased risks of death of 46% and 94%, respectively, demonstrating that obesity increases this risk.3
The increased risk of death associated with obesity is caused by obesity-related diseases that cause early mortality, including diabetes mellitus (DM), dyslipidemia, hypertension, coronary heart disease, heart failure, atrial fibrillation, stroke, and venous thromboembolic events.4 Obesity is also associated with an increased risk of many cancers, including cancer of the endometrium, kidney, esophagus, stomach, colon, rectum, gallbladder, pancreas, liver, and breast.5 With regard to gynecologic disease, obesity is associated with an increased risk of fibroids and heavy menstrual bleeding.6 For pregnant patients, obesity is associated with increased risks of7:
- miscarriage and stillbirth
- preeclampsia and gestational hypertension
- gestational diabetes
- severe maternal morbidity
- postterm pregnancy
- venous thromboembolism
- endometritis.
For obese patients, weight loss can normalize blood pressure, reduce the risk of cardiovascular events, decrease the risk of cancer, and cure type 2 DM.8
Bariatric surgery: The gold standard treatment for reliable and sustained weight loss
All patients with obesity should be counseled to reduce caloric intake and increase physical activity. Dietary counseling provided by a nutritionist may help reinforce advice given by a provider. However, lifestyle interventions are associated with modest weight loss (<5% of bodyweight; FIGURE
In the Swedish Obese Subjects study, involving 2,010 people, following bariatric surgery the mean decrease in bodyweight was 23% at 2 years, with a slow increase in weight thereafter, resulting in a sustained mean weight loss of 18% at 10 years.8 In this study, people in the diet and exercise control group had no change in bodyweight over 10 years of follow-up.8 Not all eligible obese patients want to undergo bariatric surgery because it is an arduous sequential process involving 6 months of intensive preoperative preparation, bariatric surgery, recovery, and intensive postoperative follow-up. The perioperative mortality rate is 0.03% to 0.2%.10 Following bariatric surgery, additional operations may be necessary for more than 10% of patients.10 With recent breakthroughs in the medication management of obesity, patients who do not want bariatric surgery can achieve reliable weight loss of greater than 10% of body weight with glucagon-like peptide -1 (GLP-1) agonists.
GLP-1 agonist analogues: Practice-changing breakthrough in medication treatment
GLP-1, a 30 amino acid peptide, is produced by intestinal enteroendocrine cells and neurons in the medulla and hypothalamus.11 GLP-1 reduces hunger cravings and causes satiety, reducing daily food intake.12 GLP-1 also enhances the secretion of insulin, making GLP-1 agonists an effective treatment for type 2 DM. In humans and experimental animals, the administration of exogenous GLP-1 agonists decreases hunger cravings and causes satiety, reducing food intake, resulting in weight loss.12 The synthetic GLP-1 agonists, liraglutide (Saxenda) and semaglutide (Wegovy) are approved by the US Food and Drug Administration (FDA) as anti-obesity medications.
Native GLP-1 has a short circulating half-life of approximately 2 minutes. The synthetic GLP-1 agonist medications liraglutide and semaglutide are modified to significantly increase their half-life. Liraglutide is a modified version of GLP-1 with a palmitic acid side chain and an amino acid spacer resulting in reduced degradation and a 15-hour half-life, necessitating daily administration. Semaglutide has a steric acid diacid at Lys26, a large synthetic spacer, a modification of amino acid 8 with the addition of α-aminobutyric acid and a 165-hour half-life, permitting weekly administration.13 For weight loss, liraglutide and semaglultide are administered by subcutaneous injection. Tirzepatide (Mounjaro) is a novel GLP-1 agonist. It is also a gastric inhibitory peptide, is FDA approved to treat type 2 DM, and is awaiting FDA approval as a weight loss medication.Tirzepatide causes substantial weight loss, similar to the effect of semaglutide.14
Semaglutide and weight loss
Semaglutide is approved by the FDA for chronic weight management as an adjunct to a reduced-calorie diet and increased physical activity in adults with a BMI ≥ 30 kg/m2 or ≥ 27 kg/m2 in the presence of a weight-related comorbidity. It is also FDA approved to treat type 2 DM.
In a weight loss trial, 1,961 overweight and obese patients with a mean BMI of 38 kg/m2, were randomly assigned to semaglutide or placebo treatment for 68 weeks. All the participants were following a regimen that included a calorie-reduced diet and increased physical activity. The mean changes in body weight for the patients in the semaglutide and placebo treatment groups were -14.9% and -2.4%, respectively. The treatment difference was -12.4% (95% confidence interval [CI], -13.4% to -11.5%; P <.001). In this study, compared with placebo, semaglutide treatment resulted in a greater decrease in waist circumference, -5.3 in versus -1.6 in.15 A network meta-analysis of the efficacy of weight loss medicines indicates that semaglutide is the most effective medication currently FDA approved for weight loss, reliably producing substantial weight loss (FIGURE).9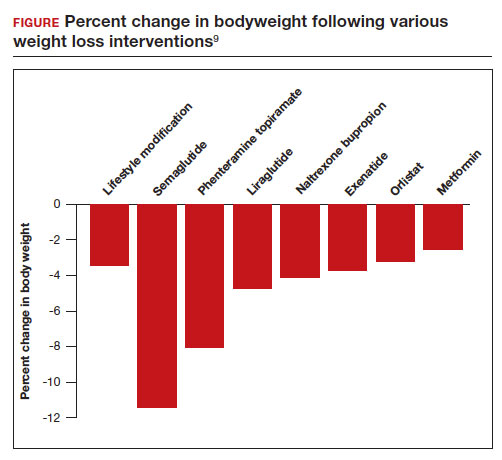
In one randomized clinical trial, investigators directly compared the efficacy of semaglutide and liraglutide in achieving weight loss. In this trial, 338 patients were assigned randomly to treatment with semaglutide 2.4 mg weekly subcutaneous injection, liraglutide 3.0 mg daily subcutaneous injection, or placebo. All the participants were following a regimen that included a calorie-reduced diet and increased physical activity.16 After 68 weeks of treatment, the mean weight changes were -15.8%, -6.4%, and -1.9% in the semaglutide, liraglutide, and placebo groups, respectively. The difference between the semaglutide and liraglutide groups was -9.4% (95% CI, -12% to -6.8%; P <.001).16
Continue to: Semaglutide dose-escalation and contraindications...
Semaglutide dose-escalation and contraindications
For weight loss, the target dose of semaglutide is 2.4 mg once weekly subcutaneous injection achieved by sequential dose escalation. To give patients time to adjust to adverse effects caused by the medication, a standardized dose-escalation regimen is recommended. The FDA-approved escalation regimen for semaglutide treatment begins with a weekly subcutaneous dose of 0.25 mg for 4 weeks, followed by an increase in the weekly dosage every 4 weeks: 0.5 mg, 1.0 mg, 1.7 mg, and 2.4 mg.17 To support the dose-escalation process there are 5 unique autoinjectors that deliver the appropriate dose for the current step.
Semaglutide is contraindicated if the patient has an allergy to the medication or if there is a personal or family history of medullary thyroid cancer.17 In animal toxicology studies, semaglutide at clinically relevant dosing was associated with an increased risk of developing medullary thyroid cancer. Patients with a personal history of multiple endocrine neoplasia syndrome type 2, (medullary thyroid cancer, pheochromocytoma, and primary hyperparathyroidism) should not take semaglutide. Semaglutide may cause fetal harm and the FDA recommends discontinuing semaglutide at least 2 months before pregnancy.17 According to the FDA, the safety of semaglutide during breastfeeding has not been established. In Canada, breastfeeding is a contraindication to semaglutide treatment.18
Limitations of medication treatment of obesity
There are important limitations to semaglutide treatment of obesity, including:
- weight gain after stopping treatment
- limited medical insurance supportfor an expensive medication treatment
- bothersome adverse effects.
Weight gain posttreatment. After stopping medication treatment of obesity, weight gain occurs in most patients. However, patients may remain below baseline weight for a long time after stopping medication therapy. In one trial of 803 patients, after 20 weeks of semaglutide treatment (16-week dose-escalation phase, followed by 4 weeks on a weekly dose of 2.4 mg), the participants were randomized to 48 additional weeks of semaglutide or placebo.19 All the participants were following a regimen that included a calorie-reduced diet and increased physical activity. At the initial 20 weeks of treatment time point the mean weight change was -10.6%. Over the following 48 weeks, the patients treated with semaglutidehad an additional mean weight change of -7.9%, while the mean weight change for the placebo group was +6.9%.
Medical insurance coverage. A major barrier to semaglutide treatment of obesity is the medication’s cost. At the website GoodRx (https://www.goodrx.com/), the estimated price for a 1-month supply of semaglutide (Wegovy) is $1,350.20 By contrast, a 1-month supply of phentermine-topiramate (Qsymia) is approximately $205. Currently, many medical insurance plans do not cover the cost of semaglutide treatment for weight loss. Patent protection for liraglutide may expire in the next few years, permitting the marketing of a lower-cost generic formulation, increasing the availability of the medication. However, as noted above, compared with liraglutide, semaglutide treatment results in much greater weight loss.
The most common adverse effects associated with semaglutide treatment are nausea, vomiting, diarrhea, and constipation. In one randomized clinical trial involving 1,961 patients, the frequency of adverse effects reported by patients taking semaglutide incrementally above the frequency of the same adverse effect reported by patients on placebo was: nausea (27%), vomiting (18%), diarrhea (16%), constipation (14%), dyspepsia (7%), and abdominal pain (5%).15 In this study, treatment was discontinued due to adverse effects in 7% and 3% of the patients in the semaglutide and placebo groups, respectively. Experts believe that adverse effects can be minimized by increasing the dose slowly and decreasing the dose if adverse effects are bothersome to the patient.
Measuring the benefits of semaglutide weight loss
Overweight and obesity are prevalent problems with many adverse consequences, including an increased risk of death. In population studies, weight loss following bariatric surgery is associated with a substantial reduction in mortality, cancer, and heart disease compared with conventional therapy.21 Over the next few years, the effect of semaglutide-induced weight loss on the rate of cancer and heart disease should become clear. If semaglutide treatment of obesity is associated with a reduction in cancer and heart disease, it would be a truly breakthrough medication. ●
- Defining adult and overweight obesity. Centers for Disease Control and Prevention website. https://www.cdc.gov/obesity/basics/adult-defining.html. Accessed June 19, 2023.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCH Data Brief. 2020;360. https://www.cdc.gov/nchs/data /databriefs/db360-h.pdf. Accessed June 19, 2023.
- The Global BMI Mortality Collaboration. Bodymass index and all-cause mortality: individual- participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776-786.
- Grover SA, Kaouache M, Rempel P, et al. Years of life lost and health life-years lost from diabetes and cardiovascular disease in the overweight and obese people: a modelling study. Lancet Diabetes Endocrinol. 2015;3:114-122.
- Lega IC, Lipscombe LL. Review: diabetes, obesity and cancer—pathophysiology and clinical implications. Endocr Rev. 2020;41:bnz014.
- Venkatesh SS, Ferreira T, Benonisdottir S, et al. Obesity and risk of female reproductive conditions: a mendelian randomization study. PLoS Med. 19:e1003679.
- Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and longterm adverse consequences for mother and child. BMJ. 2017;356:j1.
- Sjorstrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234.
- Shi Q, Wang Y, Hao Q, et al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomized controlled trials. Lancet. 2022;399:259-269.
- Arterburn DE, Telem DA, Kushner RF, et al. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324:879-887.
- Brierly DI, Holt MK, Singh A, et al. Central and peripheral GLP-1 systems are involved in the control of eating behavior by linking food intake and satiety. Nat Metab. 2021;3:258-273.
- Friedrichsen M, Breitschaft A, Tadayon S, et al. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating and gastric emptying in adults with obesity. Diabetes Obes Metab. 2021;23:754-762.
- Gotfredsen CF, Molck AM, Thorup I, et al. The human GLP-1 analogs liraglutide and semaglutide: absence of histopathological effects on the pancreas in nonhuman primates. Diabetes. 2014;63:2486-2497.
- Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503-515.
- Wilding JPH, Batterham RL, Calanna S, et al. Once weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989-1000.
- Rubino DM, Greenway FL, Khalid U, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes. JAMA. 2022;327:138-150.
- Wegovy [package insert]. Bagsvaerd, Denmark: Novo Nordisk; 2021.
- Wegovy Product Monograph. Mississauga, Ontario: Novo Nordisk Canada Inc; June 30, 2022. https://pdf.hres.ca/dpd_pm/00066484.PDF
- Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity. JAMA. 2021;325: 1414-1425.
- GoodRx website. https://www.goodrx.com/. Accessed June 19, 2023.
- Wiggins T, Guidozzi N, Welbourn R, et al. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: a systematic review and metaanalysis. PLoS Med. 2020;17:e1003206.
Obesity is a major health problem in the United States. The Centers for Disease Control and Prevention (CDC) defines the problem as weight that is higher than what is healthy for a given height, with quantitative definitions of overweight and obesity as body mass indices (BMIs) of 25 to 29.9 kg/m2 and ≥ 30 kg/m2, respectively.1 The prevalence of obesity among adults in 2017 ̶ 2018 was reported by the CDC to be 42.4%.2 Among women, the reported prevalence of obesity was lowest among Asian individuals (17.2%) and greatest among non-Hispanic Black individuals (56.9%), with White (39.8%) and Hispanic individuals (43.7%) having rates in between.2 In a meta-analysis of prospective studies that included 4 million people who were never smokers and had no chronic disease at baseline, age- and sex-adjusted mortality rates were studied over a median of 14 years of follow-up.3 Compared with those with a BMI of 20 to 25 kg/m2, people with a BMI of 30 to 34.9 kg/m2 or a BMI of 35 to 39.9 kg/m2 had increased risks of death of 46% and 94%, respectively, demonstrating that obesity increases this risk.3
The increased risk of death associated with obesity is caused by obesity-related diseases that cause early mortality, including diabetes mellitus (DM), dyslipidemia, hypertension, coronary heart disease, heart failure, atrial fibrillation, stroke, and venous thromboembolic events.4 Obesity is also associated with an increased risk of many cancers, including cancer of the endometrium, kidney, esophagus, stomach, colon, rectum, gallbladder, pancreas, liver, and breast.5 With regard to gynecologic disease, obesity is associated with an increased risk of fibroids and heavy menstrual bleeding.6 For pregnant patients, obesity is associated with increased risks of7:
- miscarriage and stillbirth
- preeclampsia and gestational hypertension
- gestational diabetes
- severe maternal morbidity
- postterm pregnancy
- venous thromboembolism
- endometritis.
For obese patients, weight loss can normalize blood pressure, reduce the risk of cardiovascular events, decrease the risk of cancer, and cure type 2 DM.8
Bariatric surgery: The gold standard treatment for reliable and sustained weight loss
All patients with obesity should be counseled to reduce caloric intake and increase physical activity. Dietary counseling provided by a nutritionist may help reinforce advice given by a provider. However, lifestyle interventions are associated with modest weight loss (<5% of bodyweight; FIGURE
In the Swedish Obese Subjects study, involving 2,010 people, following bariatric surgery the mean decrease in bodyweight was 23% at 2 years, with a slow increase in weight thereafter, resulting in a sustained mean weight loss of 18% at 10 years.8 In this study, people in the diet and exercise control group had no change in bodyweight over 10 years of follow-up.8 Not all eligible obese patients want to undergo bariatric surgery because it is an arduous sequential process involving 6 months of intensive preoperative preparation, bariatric surgery, recovery, and intensive postoperative follow-up. The perioperative mortality rate is 0.03% to 0.2%.10 Following bariatric surgery, additional operations may be necessary for more than 10% of patients.10 With recent breakthroughs in the medication management of obesity, patients who do not want bariatric surgery can achieve reliable weight loss of greater than 10% of body weight with glucagon-like peptide -1 (GLP-1) agonists.

GLP-1 agonist analogues: Practice-changing breakthrough in medication treatment
GLP-1, a 30 amino acid peptide, is produced by intestinal enteroendocrine cells and neurons in the medulla and hypothalamus.11 GLP-1 reduces hunger cravings and causes satiety, reducing daily food intake.12 GLP-1 also enhances the secretion of insulin, making GLP-1 agonists an effective treatment for type 2 DM. In humans and experimental animals, the administration of exogenous GLP-1 agonists decreases hunger cravings and causes satiety, reducing food intake, resulting in weight loss.12 The synthetic GLP-1 agonists, liraglutide (Saxenda) and semaglutide (Wegovy) are approved by the US Food and Drug Administration (FDA) as anti-obesity medications.
Native GLP-1 has a short circulating half-life of approximately 2 minutes. The synthetic GLP-1 agonist medications liraglutide and semaglutide are modified to significantly increase their half-life. Liraglutide is a modified version of GLP-1 with a palmitic acid side chain and an amino acid spacer resulting in reduced degradation and a 15-hour half-life, necessitating daily administration. Semaglutide has a steric acid diacid at Lys26, a large synthetic spacer, a modification of amino acid 8 with the addition of α-aminobutyric acid and a 165-hour half-life, permitting weekly administration.13 For weight loss, liraglutide and semaglultide are administered by subcutaneous injection. Tirzepatide (Mounjaro) is a novel GLP-1 agonist. It is also a gastric inhibitory peptide, is FDA approved to treat type 2 DM, and is awaiting FDA approval as a weight loss medication.Tirzepatide causes substantial weight loss, similar to the effect of semaglutide.14
Semaglutide and weight loss
Semaglutide is approved by the FDA for chronic weight management as an adjunct to a reduced-calorie diet and increased physical activity in adults with a BMI ≥ 30 kg/m2 or ≥ 27 kg/m2 in the presence of a weight-related comorbidity. It is also FDA approved to treat type 2 DM.
In a weight loss trial, 1,961 overweight and obese patients with a mean BMI of 38 kg/m2, were randomly assigned to semaglutide or placebo treatment for 68 weeks. All the participants were following a regimen that included a calorie-reduced diet and increased physical activity. The mean changes in body weight for the patients in the semaglutide and placebo treatment groups were -14.9% and -2.4%, respectively. The treatment difference was -12.4% (95% confidence interval [CI], -13.4% to -11.5%; P <.001). In this study, compared with placebo, semaglutide treatment resulted in a greater decrease in waist circumference, -5.3 in versus -1.6 in.15 A network meta-analysis of the efficacy of weight loss medicines indicates that semaglutide is the most effective medication currently FDA approved for weight loss, reliably producing substantial weight loss (FIGURE).9
In one randomized clinical trial, investigators directly compared the efficacy of semaglutide and liraglutide in achieving weight loss. In this trial, 338 patients were assigned randomly to treatment with semaglutide 2.4 mg weekly subcutaneous injection, liraglutide 3.0 mg daily subcutaneous injection, or placebo. All the participants were following a regimen that included a calorie-reduced diet and increased physical activity.16 After 68 weeks of treatment, the mean weight changes were -15.8%, -6.4%, and -1.9% in the semaglutide, liraglutide, and placebo groups, respectively. The difference between the semaglutide and liraglutide groups was -9.4% (95% CI, -12% to -6.8%; P <.001).16
Continue to: Semaglutide dose-escalation and contraindications...
Semaglutide dose-escalation and contraindications
For weight loss, the target dose of semaglutide is 2.4 mg once weekly subcutaneous injection achieved by sequential dose escalation. To give patients time to adjust to adverse effects caused by the medication, a standardized dose-escalation regimen is recommended. The FDA-approved escalation regimen for semaglutide treatment begins with a weekly subcutaneous dose of 0.25 mg for 4 weeks, followed by an increase in the weekly dosage every 4 weeks: 0.5 mg, 1.0 mg, 1.7 mg, and 2.4 mg.17 To support the dose-escalation process there are 5 unique autoinjectors that deliver the appropriate dose for the current step.
Semaglutide is contraindicated if the patient has an allergy to the medication or if there is a personal or family history of medullary thyroid cancer.17 In animal toxicology studies, semaglutide at clinically relevant dosing was associated with an increased risk of developing medullary thyroid cancer. Patients with a personal history of multiple endocrine neoplasia syndrome type 2, (medullary thyroid cancer, pheochromocytoma, and primary hyperparathyroidism) should not take semaglutide. Semaglutide may cause fetal harm and the FDA recommends discontinuing semaglutide at least 2 months before pregnancy.17 According to the FDA, the safety of semaglutide during breastfeeding has not been established. In Canada, breastfeeding is a contraindication to semaglutide treatment.18
Limitations of medication treatment of obesity
There are important limitations to semaglutide treatment of obesity, including:
- weight gain after stopping treatment
- limited medical insurance supportfor an expensive medication treatment
- bothersome adverse effects.
Weight gain posttreatment. After stopping medication treatment of obesity, weight gain occurs in most patients. However, patients may remain below baseline weight for a long time after stopping medication therapy. In one trial of 803 patients, after 20 weeks of semaglutide treatment (16-week dose-escalation phase, followed by 4 weeks on a weekly dose of 2.4 mg), the participants were randomized to 48 additional weeks of semaglutide or placebo.19 All the participants were following a regimen that included a calorie-reduced diet and increased physical activity. At the initial 20 weeks of treatment time point the mean weight change was -10.6%. Over the following 48 weeks, the patients treated with semaglutidehad an additional mean weight change of -7.9%, while the mean weight change for the placebo group was +6.9%.
Medical insurance coverage. A major barrier to semaglutide treatment of obesity is the medication’s cost. At the website GoodRx (https://www.goodrx.com/), the estimated price for a 1-month supply of semaglutide (Wegovy) is $1,350.20 By contrast, a 1-month supply of phentermine-topiramate (Qsymia) is approximately $205. Currently, many medical insurance plans do not cover the cost of semaglutide treatment for weight loss. Patent protection for liraglutide may expire in the next few years, permitting the marketing of a lower-cost generic formulation, increasing the availability of the medication. However, as noted above, compared with liraglutide, semaglutide treatment results in much greater weight loss.
The most common adverse effects associated with semaglutide treatment are nausea, vomiting, diarrhea, and constipation. In one randomized clinical trial involving 1,961 patients, the frequency of adverse effects reported by patients taking semaglutide incrementally above the frequency of the same adverse effect reported by patients on placebo was: nausea (27%), vomiting (18%), diarrhea (16%), constipation (14%), dyspepsia (7%), and abdominal pain (5%).15 In this study, treatment was discontinued due to adverse effects in 7% and 3% of the patients in the semaglutide and placebo groups, respectively. Experts believe that adverse effects can be minimized by increasing the dose slowly and decreasing the dose if adverse effects are bothersome to the patient.
Measuring the benefits of semaglutide weight loss
Overweight and obesity are prevalent problems with many adverse consequences, including an increased risk of death. In population studies, weight loss following bariatric surgery is associated with a substantial reduction in mortality, cancer, and heart disease compared with conventional therapy.21 Over the next few years, the effect of semaglutide-induced weight loss on the rate of cancer and heart disease should become clear. If semaglutide treatment of obesity is associated with a reduction in cancer and heart disease, it would be a truly breakthrough medication. ●
Obesity is a major health problem in the United States. The Centers for Disease Control and Prevention (CDC) defines the problem as weight that is higher than what is healthy for a given height, with quantitative definitions of overweight and obesity as body mass indices (BMIs) of 25 to 29.9 kg/m2 and ≥ 30 kg/m2, respectively.1 The prevalence of obesity among adults in 2017 ̶ 2018 was reported by the CDC to be 42.4%.2 Among women, the reported prevalence of obesity was lowest among Asian individuals (17.2%) and greatest among non-Hispanic Black individuals (56.9%), with White (39.8%) and Hispanic individuals (43.7%) having rates in between.2 In a meta-analysis of prospective studies that included 4 million people who were never smokers and had no chronic disease at baseline, age- and sex-adjusted mortality rates were studied over a median of 14 years of follow-up.3 Compared with those with a BMI of 20 to 25 kg/m2, people with a BMI of 30 to 34.9 kg/m2 or a BMI of 35 to 39.9 kg/m2 had increased risks of death of 46% and 94%, respectively, demonstrating that obesity increases this risk.3
The increased risk of death associated with obesity is caused by obesity-related diseases that cause early mortality, including diabetes mellitus (DM), dyslipidemia, hypertension, coronary heart disease, heart failure, atrial fibrillation, stroke, and venous thromboembolic events.4 Obesity is also associated with an increased risk of many cancers, including cancer of the endometrium, kidney, esophagus, stomach, colon, rectum, gallbladder, pancreas, liver, and breast.5 With regard to gynecologic disease, obesity is associated with an increased risk of fibroids and heavy menstrual bleeding.6 For pregnant patients, obesity is associated with increased risks of7:
- miscarriage and stillbirth
- preeclampsia and gestational hypertension
- gestational diabetes
- severe maternal morbidity
- postterm pregnancy
- venous thromboembolism
- endometritis.
For obese patients, weight loss can normalize blood pressure, reduce the risk of cardiovascular events, decrease the risk of cancer, and cure type 2 DM.8
Bariatric surgery: The gold standard treatment for reliable and sustained weight loss
All patients with obesity should be counseled to reduce caloric intake and increase physical activity. Dietary counseling provided by a nutritionist may help reinforce advice given by a provider. However, lifestyle interventions are associated with modest weight loss (<5% of bodyweight; FIGURE
In the Swedish Obese Subjects study, involving 2,010 people, following bariatric surgery the mean decrease in bodyweight was 23% at 2 years, with a slow increase in weight thereafter, resulting in a sustained mean weight loss of 18% at 10 years.8 In this study, people in the diet and exercise control group had no change in bodyweight over 10 years of follow-up.8 Not all eligible obese patients want to undergo bariatric surgery because it is an arduous sequential process involving 6 months of intensive preoperative preparation, bariatric surgery, recovery, and intensive postoperative follow-up. The perioperative mortality rate is 0.03% to 0.2%.10 Following bariatric surgery, additional operations may be necessary for more than 10% of patients.10 With recent breakthroughs in the medication management of obesity, patients who do not want bariatric surgery can achieve reliable weight loss of greater than 10% of body weight with glucagon-like peptide -1 (GLP-1) agonists.

GLP-1 agonist analogues: Practice-changing breakthrough in medication treatment
GLP-1, a 30 amino acid peptide, is produced by intestinal enteroendocrine cells and neurons in the medulla and hypothalamus.11 GLP-1 reduces hunger cravings and causes satiety, reducing daily food intake.12 GLP-1 also enhances the secretion of insulin, making GLP-1 agonists an effective treatment for type 2 DM. In humans and experimental animals, the administration of exogenous GLP-1 agonists decreases hunger cravings and causes satiety, reducing food intake, resulting in weight loss.12 The synthetic GLP-1 agonists, liraglutide (Saxenda) and semaglutide (Wegovy) are approved by the US Food and Drug Administration (FDA) as anti-obesity medications.
Native GLP-1 has a short circulating half-life of approximately 2 minutes. The synthetic GLP-1 agonist medications liraglutide and semaglutide are modified to significantly increase their half-life. Liraglutide is a modified version of GLP-1 with a palmitic acid side chain and an amino acid spacer resulting in reduced degradation and a 15-hour half-life, necessitating daily administration. Semaglutide has a steric acid diacid at Lys26, a large synthetic spacer, a modification of amino acid 8 with the addition of α-aminobutyric acid and a 165-hour half-life, permitting weekly administration.13 For weight loss, liraglutide and semaglultide are administered by subcutaneous injection. Tirzepatide (Mounjaro) is a novel GLP-1 agonist. It is also a gastric inhibitory peptide, is FDA approved to treat type 2 DM, and is awaiting FDA approval as a weight loss medication.Tirzepatide causes substantial weight loss, similar to the effect of semaglutide.14
Semaglutide and weight loss
Semaglutide is approved by the FDA for chronic weight management as an adjunct to a reduced-calorie diet and increased physical activity in adults with a BMI ≥ 30 kg/m2 or ≥ 27 kg/m2 in the presence of a weight-related comorbidity. It is also FDA approved to treat type 2 DM.
In a weight loss trial, 1,961 overweight and obese patients with a mean BMI of 38 kg/m2, were randomly assigned to semaglutide or placebo treatment for 68 weeks. All the participants were following a regimen that included a calorie-reduced diet and increased physical activity. The mean changes in body weight for the patients in the semaglutide and placebo treatment groups were -14.9% and -2.4%, respectively. The treatment difference was -12.4% (95% confidence interval [CI], -13.4% to -11.5%; P <.001). In this study, compared with placebo, semaglutide treatment resulted in a greater decrease in waist circumference, -5.3 in versus -1.6 in.15 A network meta-analysis of the efficacy of weight loss medicines indicates that semaglutide is the most effective medication currently FDA approved for weight loss, reliably producing substantial weight loss (FIGURE).9
In one randomized clinical trial, investigators directly compared the efficacy of semaglutide and liraglutide in achieving weight loss. In this trial, 338 patients were assigned randomly to treatment with semaglutide 2.4 mg weekly subcutaneous injection, liraglutide 3.0 mg daily subcutaneous injection, or placebo. All the participants were following a regimen that included a calorie-reduced diet and increased physical activity.16 After 68 weeks of treatment, the mean weight changes were -15.8%, -6.4%, and -1.9% in the semaglutide, liraglutide, and placebo groups, respectively. The difference between the semaglutide and liraglutide groups was -9.4% (95% CI, -12% to -6.8%; P <.001).16
Continue to: Semaglutide dose-escalation and contraindications...
Semaglutide dose-escalation and contraindications
For weight loss, the target dose of semaglutide is 2.4 mg once weekly subcutaneous injection achieved by sequential dose escalation. To give patients time to adjust to adverse effects caused by the medication, a standardized dose-escalation regimen is recommended. The FDA-approved escalation regimen for semaglutide treatment begins with a weekly subcutaneous dose of 0.25 mg for 4 weeks, followed by an increase in the weekly dosage every 4 weeks: 0.5 mg, 1.0 mg, 1.7 mg, and 2.4 mg.17 To support the dose-escalation process there are 5 unique autoinjectors that deliver the appropriate dose for the current step.
Semaglutide is contraindicated if the patient has an allergy to the medication or if there is a personal or family history of medullary thyroid cancer.17 In animal toxicology studies, semaglutide at clinically relevant dosing was associated with an increased risk of developing medullary thyroid cancer. Patients with a personal history of multiple endocrine neoplasia syndrome type 2, (medullary thyroid cancer, pheochromocytoma, and primary hyperparathyroidism) should not take semaglutide. Semaglutide may cause fetal harm and the FDA recommends discontinuing semaglutide at least 2 months before pregnancy.17 According to the FDA, the safety of semaglutide during breastfeeding has not been established. In Canada, breastfeeding is a contraindication to semaglutide treatment.18
Limitations of medication treatment of obesity
There are important limitations to semaglutide treatment of obesity, including:
- weight gain after stopping treatment
- limited medical insurance supportfor an expensive medication treatment
- bothersome adverse effects.
Weight gain posttreatment. After stopping medication treatment of obesity, weight gain occurs in most patients. However, patients may remain below baseline weight for a long time after stopping medication therapy. In one trial of 803 patients, after 20 weeks of semaglutide treatment (16-week dose-escalation phase, followed by 4 weeks on a weekly dose of 2.4 mg), the participants were randomized to 48 additional weeks of semaglutide or placebo.19 All the participants were following a regimen that included a calorie-reduced diet and increased physical activity. At the initial 20 weeks of treatment time point the mean weight change was -10.6%. Over the following 48 weeks, the patients treated with semaglutidehad an additional mean weight change of -7.9%, while the mean weight change for the placebo group was +6.9%.
Medical insurance coverage. A major barrier to semaglutide treatment of obesity is the medication’s cost. At the website GoodRx (https://www.goodrx.com/), the estimated price for a 1-month supply of semaglutide (Wegovy) is $1,350.20 By contrast, a 1-month supply of phentermine-topiramate (Qsymia) is approximately $205. Currently, many medical insurance plans do not cover the cost of semaglutide treatment for weight loss. Patent protection for liraglutide may expire in the next few years, permitting the marketing of a lower-cost generic formulation, increasing the availability of the medication. However, as noted above, compared with liraglutide, semaglutide treatment results in much greater weight loss.
The most common adverse effects associated with semaglutide treatment are nausea, vomiting, diarrhea, and constipation. In one randomized clinical trial involving 1,961 patients, the frequency of adverse effects reported by patients taking semaglutide incrementally above the frequency of the same adverse effect reported by patients on placebo was: nausea (27%), vomiting (18%), diarrhea (16%), constipation (14%), dyspepsia (7%), and abdominal pain (5%).15 In this study, treatment was discontinued due to adverse effects in 7% and 3% of the patients in the semaglutide and placebo groups, respectively. Experts believe that adverse effects can be minimized by increasing the dose slowly and decreasing the dose if adverse effects are bothersome to the patient.
Measuring the benefits of semaglutide weight loss
Overweight and obesity are prevalent problems with many adverse consequences, including an increased risk of death. In population studies, weight loss following bariatric surgery is associated with a substantial reduction in mortality, cancer, and heart disease compared with conventional therapy.21 Over the next few years, the effect of semaglutide-induced weight loss on the rate of cancer and heart disease should become clear. If semaglutide treatment of obesity is associated with a reduction in cancer and heart disease, it would be a truly breakthrough medication. ●
- Defining adult and overweight obesity. Centers for Disease Control and Prevention website. https://www.cdc.gov/obesity/basics/adult-defining.html. Accessed June 19, 2023.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCH Data Brief. 2020;360. https://www.cdc.gov/nchs/data /databriefs/db360-h.pdf. Accessed June 19, 2023.
- The Global BMI Mortality Collaboration. Bodymass index and all-cause mortality: individual- participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776-786.
- Grover SA, Kaouache M, Rempel P, et al. Years of life lost and health life-years lost from diabetes and cardiovascular disease in the overweight and obese people: a modelling study. Lancet Diabetes Endocrinol. 2015;3:114-122.
- Lega IC, Lipscombe LL. Review: diabetes, obesity and cancer—pathophysiology and clinical implications. Endocr Rev. 2020;41:bnz014.
- Venkatesh SS, Ferreira T, Benonisdottir S, et al. Obesity and risk of female reproductive conditions: a mendelian randomization study. PLoS Med. 19:e1003679.
- Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and longterm adverse consequences for mother and child. BMJ. 2017;356:j1.
- Sjorstrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234.
- Shi Q, Wang Y, Hao Q, et al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomized controlled trials. Lancet. 2022;399:259-269.
- Arterburn DE, Telem DA, Kushner RF, et al. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324:879-887.
- Brierly DI, Holt MK, Singh A, et al. Central and peripheral GLP-1 systems are involved in the control of eating behavior by linking food intake and satiety. Nat Metab. 2021;3:258-273.
- Friedrichsen M, Breitschaft A, Tadayon S, et al. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating and gastric emptying in adults with obesity. Diabetes Obes Metab. 2021;23:754-762.
- Gotfredsen CF, Molck AM, Thorup I, et al. The human GLP-1 analogs liraglutide and semaglutide: absence of histopathological effects on the pancreas in nonhuman primates. Diabetes. 2014;63:2486-2497.
- Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503-515.
- Wilding JPH, Batterham RL, Calanna S, et al. Once weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989-1000.
- Rubino DM, Greenway FL, Khalid U, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes. JAMA. 2022;327:138-150.
- Wegovy [package insert]. Bagsvaerd, Denmark: Novo Nordisk; 2021.
- Wegovy Product Monograph. Mississauga, Ontario: Novo Nordisk Canada Inc; June 30, 2022. https://pdf.hres.ca/dpd_pm/00066484.PDF
- Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity. JAMA. 2021;325: 1414-1425.
- GoodRx website. https://www.goodrx.com/. Accessed June 19, 2023.
- Wiggins T, Guidozzi N, Welbourn R, et al. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: a systematic review and metaanalysis. PLoS Med. 2020;17:e1003206.
- Defining adult and overweight obesity. Centers for Disease Control and Prevention website. https://www.cdc.gov/obesity/basics/adult-defining.html. Accessed June 19, 2023.
- Hales CM, Carroll MD, Fryar CD, et al. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCH Data Brief. 2020;360. https://www.cdc.gov/nchs/data /databriefs/db360-h.pdf. Accessed June 19, 2023.
- The Global BMI Mortality Collaboration. Bodymass index and all-cause mortality: individual- participant-data meta-analysis of 239 prospective studies in four continents. Lancet. 2016;388:776-786.
- Grover SA, Kaouache M, Rempel P, et al. Years of life lost and health life-years lost from diabetes and cardiovascular disease in the overweight and obese people: a modelling study. Lancet Diabetes Endocrinol. 2015;3:114-122.
- Lega IC, Lipscombe LL. Review: diabetes, obesity and cancer—pathophysiology and clinical implications. Endocr Rev. 2020;41:bnz014.
- Venkatesh SS, Ferreira T, Benonisdottir S, et al. Obesity and risk of female reproductive conditions: a mendelian randomization study. PLoS Med. 19:e1003679.
- Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and longterm adverse consequences for mother and child. BMJ. 2017;356:j1.
- Sjorstrom L. Review of the key results from the Swedish Obese Subjects (SOS) trial—a prospective controlled intervention study of bariatric surgery. J Intern Med. 2013;273:219-234.
- Shi Q, Wang Y, Hao Q, et al. Pharmacotherapy for adults with overweight and obesity: a systematic review and network meta-analysis of randomized controlled trials. Lancet. 2022;399:259-269.
- Arterburn DE, Telem DA, Kushner RF, et al. Benefits and risks of bariatric surgery in adults: a review. JAMA. 2020;324:879-887.
- Brierly DI, Holt MK, Singh A, et al. Central and peripheral GLP-1 systems are involved in the control of eating behavior by linking food intake and satiety. Nat Metab. 2021;3:258-273.
- Friedrichsen M, Breitschaft A, Tadayon S, et al. The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating and gastric emptying in adults with obesity. Diabetes Obes Metab. 2021;23:754-762.
- Gotfredsen CF, Molck AM, Thorup I, et al. The human GLP-1 analogs liraglutide and semaglutide: absence of histopathological effects on the pancreas in nonhuman primates. Diabetes. 2014;63:2486-2497.
- Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385:503-515.
- Wilding JPH, Batterham RL, Calanna S, et al. Once weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384:989-1000.
- Rubino DM, Greenway FL, Khalid U, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes. JAMA. 2022;327:138-150.
- Wegovy [package insert]. Bagsvaerd, Denmark: Novo Nordisk; 2021.
- Wegovy Product Monograph. Mississauga, Ontario: Novo Nordisk Canada Inc; June 30, 2022. https://pdf.hres.ca/dpd_pm/00066484.PDF
- Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity. JAMA. 2021;325: 1414-1425.
- GoodRx website. https://www.goodrx.com/. Accessed June 19, 2023.
- Wiggins T, Guidozzi N, Welbourn R, et al. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: a systematic review and metaanalysis. PLoS Med. 2020;17:e1003206.
To what extent do growth abnormalities increase the risk of stillbirth near term in pregnancies complicated by diabetes?
McElwee ER, Oliver EA, McFarling K, et al. Risk of stillbirth in pregnancies complicated by diabetes, stratified by fetal growth. Obstet Gynecol. 2023;141:801-809. doi:10.1097/AOG.0000000000005102.
EXPERT COMMENTARY
Stillbirth is defined as intrauterine demise at or beyond 20 weeks’ gestation. Pregestational DM and GDM significantly increase the risk of stillbirth. Both fetal growth restriction and macrosomia are common complications of pregnancies affected by diabetes, and they further increase the risk of stillbirth. While maternal variables such as glycemic control and medication requirement are currently used to assess the risks of expectant management and inform delivery timing, abnormal fetal growth is not.
Investigators sought to evaluate the stillbirth rates per week of expectant management during the late third trimester stratified by birth weight (as a surrogate for fetal growth) in pregnancies complicated by PG-DM or GDM.
Details of the study
McElwee and colleagues used the US National Vital Statistics System to identify nonanomalous singleton pregnancies complicated by PG-DM or GDM from 2014 to 2017.1 Pregnancies were stratified by birth weight and categorized as being LGA (birth weight > 90th percentile for gestational age), SGA (birth weight < 10th percentile for gestational age), or AGA. Stillbirths were identified from 34 0/7 through 39 6/7 weeks of gestation, and conditional stillbirth rates per 10,000 pregnancies were calculated for each week of gestation.
Results. Among 834,631 pregnancies complicated by PG-DM (13.1%) or GDM (86.9%), there were 3,033 stillbirths, of which 61% were in pregnancies with PG-DM. Stillbirth rates increased with advancing gestational age for both PG-DM and GDM regardless of birth weight. In pregnancies with PG-DM, fetuses that were LGA or SGA had a higher relative risk of stillbirth compared with their AGA counterparts at each gestational age. This stillbirth risk was highest in pregnancies with PG-DM that were LGA. At 39 weeks, the stillbirth rate in this population was 96.9/10,000 ongoing pregnancies and was 5 times higher than pregnancies with PG-DM that were AGA. When the GDM-related AGA group was selected as the referent (as the lowest-risk comparison group), pregnancies with PG-DM that were LGA had a 21-times higher relative risk of stillbirth at 37 and 38 weeks of gestation.
Study strengths and limitations
Decisions on the optimal timing of delivery seek to strike a balance between the increased neonatal morbidity with delivery before 39 weeks’ gestation and the increased risk of stillbirth with expectant management. In pregnancies complicated by diabetes, current guidelines from the American College of Obstetricians and Gynecologists recommend consideration of maternal variables, such as medication requirement, glycemic control, and vascular sequelae, to inform decisions on delivery timing, as these factors have been postulated to influence the risk of stillbirth with pregnancy prolongation.2 These recommendations are based largely on expert opinion and retrospective data.
The question of how fetal growth abnormalities factor into this complicated decision making is also an area of low-quality evidence despite studies that demonstrate that both SGA and LGA fetuses in pregnancies complicated by diabetes are at increased risk of stillbirth.3
The large population-based study design by McElwee and colleagues allowed the investigators to examine a rare event (stillbirth) with multiple stratification levels and sufficient statistical power and to contribute to this literature.
Significant limitations, however, must be considered before generalizing these results. The data were restricted to variables available on birth and death certificates, and more granular information—such as the type of DM, level of glycemic control, frequency of antenatal testing, and stillbirth work-up—could not be assessed. Ultrasonographic estimations of fetal weight also were not included. Birth weight data were used as a proxy, although we know that these variables do not always correlate well given the limited accuracy of ultrasonography in assessing projected birth weight, particularly later in pregnancy. The authors also did not control for highly prevalent variables (for example, hypertension, obesity) that are likely associated with abnormal fetal growth and stillbirth in these populations. ●
The present study demonstrates that both SGA and LGA are significant risk factors for stillbirth in pregnancies with either PG-DM or GDM in the late preterm and early term periods, and this risk should be considered when making decisions on appropriate timing of delivery. The conditional stillbirth rate was highest in pregnancies with PG-DM with LGA fetuses, and this risk increased with each week of expectant management. This population may benefit the most from critical assessment of the risk of stillbirth with ongoing pregnancy. Notably, the quality of evidence is not sufficient to universally alter delivery timing guidelines in this population. We recommend individual assessment of each clinical scenario when making these decisions.
NIGEL MADDEN, MD; MICHELLE A. KOMINIAREK, MD, MS
- McElwee ER, Oliver EA, McFarling K, et al. Risk of stillbirth in pregnancies complicated by diabetes, stratified by fetal growth. Obstet Gynecol. 2023;141:801-809. doi:10.1097 /AOG.0000000000005102
- ACOG Committee Opinion No. 764. Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151-e155. doi:10.1097/AOG.0000000000003083
- Starikov R, Dudley D, Reddy UM. Stillbirth in the pregnancy complicated by diabetes. Curr Diab Rep. 2015;15:11. doi:10.1007/s11892-015-0580-y
McElwee ER, Oliver EA, McFarling K, et al. Risk of stillbirth in pregnancies complicated by diabetes, stratified by fetal growth. Obstet Gynecol. 2023;141:801-809. doi:10.1097/AOG.0000000000005102.
EXPERT COMMENTARY
Stillbirth is defined as intrauterine demise at or beyond 20 weeks’ gestation. Pregestational DM and GDM significantly increase the risk of stillbirth. Both fetal growth restriction and macrosomia are common complications of pregnancies affected by diabetes, and they further increase the risk of stillbirth. While maternal variables such as glycemic control and medication requirement are currently used to assess the risks of expectant management and inform delivery timing, abnormal fetal growth is not.
Investigators sought to evaluate the stillbirth rates per week of expectant management during the late third trimester stratified by birth weight (as a surrogate for fetal growth) in pregnancies complicated by PG-DM or GDM.
Details of the study
McElwee and colleagues used the US National Vital Statistics System to identify nonanomalous singleton pregnancies complicated by PG-DM or GDM from 2014 to 2017.1 Pregnancies were stratified by birth weight and categorized as being LGA (birth weight > 90th percentile for gestational age), SGA (birth weight < 10th percentile for gestational age), or AGA. Stillbirths were identified from 34 0/7 through 39 6/7 weeks of gestation, and conditional stillbirth rates per 10,000 pregnancies were calculated for each week of gestation.
Results. Among 834,631 pregnancies complicated by PG-DM (13.1%) or GDM (86.9%), there were 3,033 stillbirths, of which 61% were in pregnancies with PG-DM. Stillbirth rates increased with advancing gestational age for both PG-DM and GDM regardless of birth weight. In pregnancies with PG-DM, fetuses that were LGA or SGA had a higher relative risk of stillbirth compared with their AGA counterparts at each gestational age. This stillbirth risk was highest in pregnancies with PG-DM that were LGA. At 39 weeks, the stillbirth rate in this population was 96.9/10,000 ongoing pregnancies and was 5 times higher than pregnancies with PG-DM that were AGA. When the GDM-related AGA group was selected as the referent (as the lowest-risk comparison group), pregnancies with PG-DM that were LGA had a 21-times higher relative risk of stillbirth at 37 and 38 weeks of gestation.
Study strengths and limitations
Decisions on the optimal timing of delivery seek to strike a balance between the increased neonatal morbidity with delivery before 39 weeks’ gestation and the increased risk of stillbirth with expectant management. In pregnancies complicated by diabetes, current guidelines from the American College of Obstetricians and Gynecologists recommend consideration of maternal variables, such as medication requirement, glycemic control, and vascular sequelae, to inform decisions on delivery timing, as these factors have been postulated to influence the risk of stillbirth with pregnancy prolongation.2 These recommendations are based largely on expert opinion and retrospective data.
The question of how fetal growth abnormalities factor into this complicated decision making is also an area of low-quality evidence despite studies that demonstrate that both SGA and LGA fetuses in pregnancies complicated by diabetes are at increased risk of stillbirth.3
The large population-based study design by McElwee and colleagues allowed the investigators to examine a rare event (stillbirth) with multiple stratification levels and sufficient statistical power and to contribute to this literature.
Significant limitations, however, must be considered before generalizing these results. The data were restricted to variables available on birth and death certificates, and more granular information—such as the type of DM, level of glycemic control, frequency of antenatal testing, and stillbirth work-up—could not be assessed. Ultrasonographic estimations of fetal weight also were not included. Birth weight data were used as a proxy, although we know that these variables do not always correlate well given the limited accuracy of ultrasonography in assessing projected birth weight, particularly later in pregnancy. The authors also did not control for highly prevalent variables (for example, hypertension, obesity) that are likely associated with abnormal fetal growth and stillbirth in these populations. ●
The present study demonstrates that both SGA and LGA are significant risk factors for stillbirth in pregnancies with either PG-DM or GDM in the late preterm and early term periods, and this risk should be considered when making decisions on appropriate timing of delivery. The conditional stillbirth rate was highest in pregnancies with PG-DM with LGA fetuses, and this risk increased with each week of expectant management. This population may benefit the most from critical assessment of the risk of stillbirth with ongoing pregnancy. Notably, the quality of evidence is not sufficient to universally alter delivery timing guidelines in this population. We recommend individual assessment of each clinical scenario when making these decisions.
NIGEL MADDEN, MD; MICHELLE A. KOMINIAREK, MD, MS
McElwee ER, Oliver EA, McFarling K, et al. Risk of stillbirth in pregnancies complicated by diabetes, stratified by fetal growth. Obstet Gynecol. 2023;141:801-809. doi:10.1097/AOG.0000000000005102.
EXPERT COMMENTARY
Stillbirth is defined as intrauterine demise at or beyond 20 weeks’ gestation. Pregestational DM and GDM significantly increase the risk of stillbirth. Both fetal growth restriction and macrosomia are common complications of pregnancies affected by diabetes, and they further increase the risk of stillbirth. While maternal variables such as glycemic control and medication requirement are currently used to assess the risks of expectant management and inform delivery timing, abnormal fetal growth is not.
Investigators sought to evaluate the stillbirth rates per week of expectant management during the late third trimester stratified by birth weight (as a surrogate for fetal growth) in pregnancies complicated by PG-DM or GDM.
Details of the study
McElwee and colleagues used the US National Vital Statistics System to identify nonanomalous singleton pregnancies complicated by PG-DM or GDM from 2014 to 2017.1 Pregnancies were stratified by birth weight and categorized as being LGA (birth weight > 90th percentile for gestational age), SGA (birth weight < 10th percentile for gestational age), or AGA. Stillbirths were identified from 34 0/7 through 39 6/7 weeks of gestation, and conditional stillbirth rates per 10,000 pregnancies were calculated for each week of gestation.
Results. Among 834,631 pregnancies complicated by PG-DM (13.1%) or GDM (86.9%), there were 3,033 stillbirths, of which 61% were in pregnancies with PG-DM. Stillbirth rates increased with advancing gestational age for both PG-DM and GDM regardless of birth weight. In pregnancies with PG-DM, fetuses that were LGA or SGA had a higher relative risk of stillbirth compared with their AGA counterparts at each gestational age. This stillbirth risk was highest in pregnancies with PG-DM that were LGA. At 39 weeks, the stillbirth rate in this population was 96.9/10,000 ongoing pregnancies and was 5 times higher than pregnancies with PG-DM that were AGA. When the GDM-related AGA group was selected as the referent (as the lowest-risk comparison group), pregnancies with PG-DM that were LGA had a 21-times higher relative risk of stillbirth at 37 and 38 weeks of gestation.
Study strengths and limitations
Decisions on the optimal timing of delivery seek to strike a balance between the increased neonatal morbidity with delivery before 39 weeks’ gestation and the increased risk of stillbirth with expectant management. In pregnancies complicated by diabetes, current guidelines from the American College of Obstetricians and Gynecologists recommend consideration of maternal variables, such as medication requirement, glycemic control, and vascular sequelae, to inform decisions on delivery timing, as these factors have been postulated to influence the risk of stillbirth with pregnancy prolongation.2 These recommendations are based largely on expert opinion and retrospective data.
The question of how fetal growth abnormalities factor into this complicated decision making is also an area of low-quality evidence despite studies that demonstrate that both SGA and LGA fetuses in pregnancies complicated by diabetes are at increased risk of stillbirth.3
The large population-based study design by McElwee and colleagues allowed the investigators to examine a rare event (stillbirth) with multiple stratification levels and sufficient statistical power and to contribute to this literature.
Significant limitations, however, must be considered before generalizing these results. The data were restricted to variables available on birth and death certificates, and more granular information—such as the type of DM, level of glycemic control, frequency of antenatal testing, and stillbirth work-up—could not be assessed. Ultrasonographic estimations of fetal weight also were not included. Birth weight data were used as a proxy, although we know that these variables do not always correlate well given the limited accuracy of ultrasonography in assessing projected birth weight, particularly later in pregnancy. The authors also did not control for highly prevalent variables (for example, hypertension, obesity) that are likely associated with abnormal fetal growth and stillbirth in these populations. ●
The present study demonstrates that both SGA and LGA are significant risk factors for stillbirth in pregnancies with either PG-DM or GDM in the late preterm and early term periods, and this risk should be considered when making decisions on appropriate timing of delivery. The conditional stillbirth rate was highest in pregnancies with PG-DM with LGA fetuses, and this risk increased with each week of expectant management. This population may benefit the most from critical assessment of the risk of stillbirth with ongoing pregnancy. Notably, the quality of evidence is not sufficient to universally alter delivery timing guidelines in this population. We recommend individual assessment of each clinical scenario when making these decisions.
NIGEL MADDEN, MD; MICHELLE A. KOMINIAREK, MD, MS
- McElwee ER, Oliver EA, McFarling K, et al. Risk of stillbirth in pregnancies complicated by diabetes, stratified by fetal growth. Obstet Gynecol. 2023;141:801-809. doi:10.1097 /AOG.0000000000005102
- ACOG Committee Opinion No. 764. Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151-e155. doi:10.1097/AOG.0000000000003083
- Starikov R, Dudley D, Reddy UM. Stillbirth in the pregnancy complicated by diabetes. Curr Diab Rep. 2015;15:11. doi:10.1007/s11892-015-0580-y
- McElwee ER, Oliver EA, McFarling K, et al. Risk of stillbirth in pregnancies complicated by diabetes, stratified by fetal growth. Obstet Gynecol. 2023;141:801-809. doi:10.1097 /AOG.0000000000005102
- ACOG Committee Opinion No. 764. Medically indicated late-preterm and early-term deliveries. Obstet Gynecol. 2019;133:e151-e155. doi:10.1097/AOG.0000000000003083
- Starikov R, Dudley D, Reddy UM. Stillbirth in the pregnancy complicated by diabetes. Curr Diab Rep. 2015;15:11. doi:10.1007/s11892-015-0580-y
Men and women react differently to acute stress
Topline
Methodology
- The study included 80 healthy participants, mean age 24 years.
- Half the subjects immersed their nondominant hand (including the wrist) in ice water for up to 3 minutes; the other half, which served as the control group, immersed their hand in warm water for 3 minutes.
- Participants were asked to deliberately downregulate emotional responses to high-intensity negative pictures.
- Participants regularly provided saliva samples to check cortisol levels and were monitored for cardiovascular activity.
- Researchers assessed pupil dilation, which along with subject ratings of their affective state served as emotion regulation (ER) outcome measures.
Takeaway
- In men, stress rapidly improved the ability to downregulate emotional arousal via distraction that was fully mediated by cortisol.
- In women, sympathetic nervous system (SNS) reactivity was linked to decreased regulatory performances.
- Direct stress effects on ER were smaller than expected.
In practice
The study contributes to a “better understanding of the neuroendocrinological mechanisms of stress effects on ER that may help to develop adequate preventive and curative interventions of stress- and emotion-related disorders,” the researchers write.
Source
The study was conducted by Katja Langer, Valerie Jentsch, and Oliver Wolf from the Department of Cognitive Psychology, Ruhr University Bochum (Germany). It was published in the May 2023 issue of Psychoneuroendocrinology.
Limitations
The results have some inconsistencies. The ER paradigm is somewhat artificial and not fully comparable with emotional trigger and regulatory requirements in everyday life. The study did not directly assess levels of catecholamines such as adrenaline and noradrenaline.
Disclosures
The study received support from the German Research Foundation (DFG). The authors have no reported conflicts of interest.
A version of this article originally appeared on Medscape.com.
Topline
Methodology
- The study included 80 healthy participants, mean age 24 years.
- Half the subjects immersed their nondominant hand (including the wrist) in ice water for up to 3 minutes; the other half, which served as the control group, immersed their hand in warm water for 3 minutes.
- Participants were asked to deliberately downregulate emotional responses to high-intensity negative pictures.
- Participants regularly provided saliva samples to check cortisol levels and were monitored for cardiovascular activity.
- Researchers assessed pupil dilation, which along with subject ratings of their affective state served as emotion regulation (ER) outcome measures.
Takeaway
- In men, stress rapidly improved the ability to downregulate emotional arousal via distraction that was fully mediated by cortisol.
- In women, sympathetic nervous system (SNS) reactivity was linked to decreased regulatory performances.
- Direct stress effects on ER were smaller than expected.
In practice
The study contributes to a “better understanding of the neuroendocrinological mechanisms of stress effects on ER that may help to develop adequate preventive and curative interventions of stress- and emotion-related disorders,” the researchers write.
Source
The study was conducted by Katja Langer, Valerie Jentsch, and Oliver Wolf from the Department of Cognitive Psychology, Ruhr University Bochum (Germany). It was published in the May 2023 issue of Psychoneuroendocrinology.
Limitations
The results have some inconsistencies. The ER paradigm is somewhat artificial and not fully comparable with emotional trigger and regulatory requirements in everyday life. The study did not directly assess levels of catecholamines such as adrenaline and noradrenaline.
Disclosures
The study received support from the German Research Foundation (DFG). The authors have no reported conflicts of interest.
A version of this article originally appeared on Medscape.com.
Topline
Methodology
- The study included 80 healthy participants, mean age 24 years.
- Half the subjects immersed their nondominant hand (including the wrist) in ice water for up to 3 minutes; the other half, which served as the control group, immersed their hand in warm water for 3 minutes.
- Participants were asked to deliberately downregulate emotional responses to high-intensity negative pictures.
- Participants regularly provided saliva samples to check cortisol levels and were monitored for cardiovascular activity.
- Researchers assessed pupil dilation, which along with subject ratings of their affective state served as emotion regulation (ER) outcome measures.
Takeaway
- In men, stress rapidly improved the ability to downregulate emotional arousal via distraction that was fully mediated by cortisol.
- In women, sympathetic nervous system (SNS) reactivity was linked to decreased regulatory performances.
- Direct stress effects on ER were smaller than expected.
In practice
The study contributes to a “better understanding of the neuroendocrinological mechanisms of stress effects on ER that may help to develop adequate preventive and curative interventions of stress- and emotion-related disorders,” the researchers write.
Source
The study was conducted by Katja Langer, Valerie Jentsch, and Oliver Wolf from the Department of Cognitive Psychology, Ruhr University Bochum (Germany). It was published in the May 2023 issue of Psychoneuroendocrinology.
Limitations
The results have some inconsistencies. The ER paradigm is somewhat artificial and not fully comparable with emotional trigger and regulatory requirements in everyday life. The study did not directly assess levels of catecholamines such as adrenaline and noradrenaline.
Disclosures
The study received support from the German Research Foundation (DFG). The authors have no reported conflicts of interest.
A version of this article originally appeared on Medscape.com.
30 days in, UHC offers little guidance on advance notification
It’s been just over 1 month since UnitedHealthcare (UHC) launched its advance notification program requiring providers to record nonscreening colonoscopy and other gastroenterology procedures to be eligible for its 2024 Gold Card program.
The program, which will begin next year, may eliminate prior authorization requirements for providers who successfully complete the advance notification program this year. However, there is no guarantee that providers who complete the advance notification program will be enrolled in the Gold Card program, which means they would have to seek prior authorization for nonscreening procedures, according to the American Gastroenterological Association.
While UHC has provided some information about how advance notification works, there are many unanswered questions, said Barbara H. Jung, MD,AGAF, AGA president.
“UnitedHealthcare’s haphazard approach to rolling out a policy that will ultimately control patient access to critical, often lifesaving medical procedures are the opposite of what should be our common goal of expeditious access to essential care,” she said in a written statement.
The advance notification program was announced on June 1 when UHC said it was dropping its controversial prior authorization program, which was due to go into effect that day.
AGA is concerned that UHC’s advance notification program is merely a delay tactic because prior authorization may be required next year for providers who are not accepted into the Gold Card program. Providers who are not accepted into the program may face delays in administering procedures due to the need for prior authorizations. Thousands of endoscopies and colonoscopies could potentially be disrupted in the first month alone due to canceled procedures because of new prior authorization requirements, they said.
UHC has been trying to rein in health care costs by first considering prior authorizations for most gastrointestinal (GI) endoscopic procedures, except for screening colonoscopy, but ultimately adopting advance notification. Providers, UHC has said, don’t always follow evidence-based medicine treatment recommendations or they overutilize procedures. Their goal, according to a summary document it issued outlining changes to advance notification and prior authorization requirements, is “better care, improved health outcomes, and lower costs.”
“Clinical studies demonstrate overutilization of these procedures and lack of adherence to specialty society–endorsed guidelines and recommendations. Up to one-third of upper GI procedures and almost half of nonscreening colonoscopies performed for common clinical conditions are not consistent with clinical guidelines,” UHC stated in an FAQ. “A UHC review of upper endoscopy and lower endoscopy procedures performed in 2022 revealed two- to fivefold practice-level variation in the use of both procedure types, even after adjusting for member characteristics including age and comorbidities.”
However, according to a statement from the AGA, it has not seen utilization data specific to UHC: “It is clear that UHC does not currently have any data indicating significant overutilization of critical colonoscopy and endoscopy procedures and therefore no justification to impose burdensome barriers like prior authorization.” AGA also pointed to research showing there is an unmet need for colonoscopies in the United States, which suggests there is an underutilization of this crucial procedure.
“AGA has expressed its willingness to work collaboratively with UnitedHealthcare to address any concerns and educate physicians, but communication and transparency with the insurer are nearly nonexistent. Instead, the GI community is confronted with a nebulous concept called advance notification, which is not conducive to seamless patient care. Ultimately, it appears advance notification will form the basis of prior authorization, which we know can delay, disrupt, and deny timely care,” Dr. Jung said.
How advance notification works
Beginning June 1, providers have been asked to provide advance notification for nonscreening GI endoscopy procedures that include: esophagogastroduodenoscopy, capsule endoscopy, diagnostic colonoscopy and surveillance colonoscopy. The notification can be made by phone (866-889-8054) or through a UHC online portal at UHCprovider.com.
The AGA has said that some GI practices have found the portal to be confusing and it lacks a standard software application raising concerns for high error rates.
Advance notification applies to patients who have UHC commercial plans, including UnitedHealthcare, UnitedHealthcare Plan of the River Valley, Neighborhood Health Partnership, UnitedHealthcare Level Funded, and UnitedHealthcare Oxford Health Plans in all states, except Rhode Island, Kentucky, and New Mexico.
Providers who opt out of participating in advance notification will not be eligible to participate in the Gold Card program in 2024. The program will essentially allow providers to order most GI endoscopy procedures, except for screening colonoscopy, without prior authorization. However, UHC has not released any information about how it will implement its planned Gold Card prior authorization program or how many providers will be accepted into the program.
UHC has assured providers it will not issue medical necessity denials through this process, but it may ask providers to participate in a “comprehensive peer-to-peer discussion with a board-certified gastroenterologist around clinical guidelines.”
The fear for practices is that advance notification will be an onerous process adding burdensome paperwork that practices are not equipped to manage. UHC is the largest health insurer in the country representing 46% of the total market.
Lawrence Kim, MD, AGAF, vice president of AGA and a gastroenterologist practicing in Denver said that each physician in his practice does over 1,000 procedures annually and 25% of their patients carry UHC.
“We are currently completing 30-40 notifications a day, requiring two staff members to comply with this program. UHC is not asking for any clinical information, just procedure and diagnosis codes, and in some cases site of service. Therefore, the advance notification program as it stands will not provide UHC with any additional information beyond what they already have through claims data. This highlights the strain these requirements are putting on providers and practices for repetitive data,” he said.
For more details about UHC’s advance notification program, UHC has prepared this FAQ. To learn more about AGA’s advocacy, visit www.gastro.org/UHC.
It’s been just over 1 month since UnitedHealthcare (UHC) launched its advance notification program requiring providers to record nonscreening colonoscopy and other gastroenterology procedures to be eligible for its 2024 Gold Card program.
The program, which will begin next year, may eliminate prior authorization requirements for providers who successfully complete the advance notification program this year. However, there is no guarantee that providers who complete the advance notification program will be enrolled in the Gold Card program, which means they would have to seek prior authorization for nonscreening procedures, according to the American Gastroenterological Association.
While UHC has provided some information about how advance notification works, there are many unanswered questions, said Barbara H. Jung, MD,AGAF, AGA president.
“UnitedHealthcare’s haphazard approach to rolling out a policy that will ultimately control patient access to critical, often lifesaving medical procedures are the opposite of what should be our common goal of expeditious access to essential care,” she said in a written statement.
The advance notification program was announced on June 1 when UHC said it was dropping its controversial prior authorization program, which was due to go into effect that day.
AGA is concerned that UHC’s advance notification program is merely a delay tactic because prior authorization may be required next year for providers who are not accepted into the Gold Card program. Providers who are not accepted into the program may face delays in administering procedures due to the need for prior authorizations. Thousands of endoscopies and colonoscopies could potentially be disrupted in the first month alone due to canceled procedures because of new prior authorization requirements, they said.
UHC has been trying to rein in health care costs by first considering prior authorizations for most gastrointestinal (GI) endoscopic procedures, except for screening colonoscopy, but ultimately adopting advance notification. Providers, UHC has said, don’t always follow evidence-based medicine treatment recommendations or they overutilize procedures. Their goal, according to a summary document it issued outlining changes to advance notification and prior authorization requirements, is “better care, improved health outcomes, and lower costs.”
“Clinical studies demonstrate overutilization of these procedures and lack of adherence to specialty society–endorsed guidelines and recommendations. Up to one-third of upper GI procedures and almost half of nonscreening colonoscopies performed for common clinical conditions are not consistent with clinical guidelines,” UHC stated in an FAQ. “A UHC review of upper endoscopy and lower endoscopy procedures performed in 2022 revealed two- to fivefold practice-level variation in the use of both procedure types, even after adjusting for member characteristics including age and comorbidities.”
However, according to a statement from the AGA, it has not seen utilization data specific to UHC: “It is clear that UHC does not currently have any data indicating significant overutilization of critical colonoscopy and endoscopy procedures and therefore no justification to impose burdensome barriers like prior authorization.” AGA also pointed to research showing there is an unmet need for colonoscopies in the United States, which suggests there is an underutilization of this crucial procedure.
“AGA has expressed its willingness to work collaboratively with UnitedHealthcare to address any concerns and educate physicians, but communication and transparency with the insurer are nearly nonexistent. Instead, the GI community is confronted with a nebulous concept called advance notification, which is not conducive to seamless patient care. Ultimately, it appears advance notification will form the basis of prior authorization, which we know can delay, disrupt, and deny timely care,” Dr. Jung said.
How advance notification works
Beginning June 1, providers have been asked to provide advance notification for nonscreening GI endoscopy procedures that include: esophagogastroduodenoscopy, capsule endoscopy, diagnostic colonoscopy and surveillance colonoscopy. The notification can be made by phone (866-889-8054) or through a UHC online portal at UHCprovider.com.
The AGA has said that some GI practices have found the portal to be confusing and it lacks a standard software application raising concerns for high error rates.
Advance notification applies to patients who have UHC commercial plans, including UnitedHealthcare, UnitedHealthcare Plan of the River Valley, Neighborhood Health Partnership, UnitedHealthcare Level Funded, and UnitedHealthcare Oxford Health Plans in all states, except Rhode Island, Kentucky, and New Mexico.
Providers who opt out of participating in advance notification will not be eligible to participate in the Gold Card program in 2024. The program will essentially allow providers to order most GI endoscopy procedures, except for screening colonoscopy, without prior authorization. However, UHC has not released any information about how it will implement its planned Gold Card prior authorization program or how many providers will be accepted into the program.
UHC has assured providers it will not issue medical necessity denials through this process, but it may ask providers to participate in a “comprehensive peer-to-peer discussion with a board-certified gastroenterologist around clinical guidelines.”
The fear for practices is that advance notification will be an onerous process adding burdensome paperwork that practices are not equipped to manage. UHC is the largest health insurer in the country representing 46% of the total market.
Lawrence Kim, MD, AGAF, vice president of AGA and a gastroenterologist practicing in Denver said that each physician in his practice does over 1,000 procedures annually and 25% of their patients carry UHC.
“We are currently completing 30-40 notifications a day, requiring two staff members to comply with this program. UHC is not asking for any clinical information, just procedure and diagnosis codes, and in some cases site of service. Therefore, the advance notification program as it stands will not provide UHC with any additional information beyond what they already have through claims data. This highlights the strain these requirements are putting on providers and practices for repetitive data,” he said.
For more details about UHC’s advance notification program, UHC has prepared this FAQ. To learn more about AGA’s advocacy, visit www.gastro.org/UHC.
It’s been just over 1 month since UnitedHealthcare (UHC) launched its advance notification program requiring providers to record nonscreening colonoscopy and other gastroenterology procedures to be eligible for its 2024 Gold Card program.
The program, which will begin next year, may eliminate prior authorization requirements for providers who successfully complete the advance notification program this year. However, there is no guarantee that providers who complete the advance notification program will be enrolled in the Gold Card program, which means they would have to seek prior authorization for nonscreening procedures, according to the American Gastroenterological Association.
While UHC has provided some information about how advance notification works, there are many unanswered questions, said Barbara H. Jung, MD,AGAF, AGA president.
“UnitedHealthcare’s haphazard approach to rolling out a policy that will ultimately control patient access to critical, often lifesaving medical procedures are the opposite of what should be our common goal of expeditious access to essential care,” she said in a written statement.
The advance notification program was announced on June 1 when UHC said it was dropping its controversial prior authorization program, which was due to go into effect that day.
AGA is concerned that UHC’s advance notification program is merely a delay tactic because prior authorization may be required next year for providers who are not accepted into the Gold Card program. Providers who are not accepted into the program may face delays in administering procedures due to the need for prior authorizations. Thousands of endoscopies and colonoscopies could potentially be disrupted in the first month alone due to canceled procedures because of new prior authorization requirements, they said.
UHC has been trying to rein in health care costs by first considering prior authorizations for most gastrointestinal (GI) endoscopic procedures, except for screening colonoscopy, but ultimately adopting advance notification. Providers, UHC has said, don’t always follow evidence-based medicine treatment recommendations or they overutilize procedures. Their goal, according to a summary document it issued outlining changes to advance notification and prior authorization requirements, is “better care, improved health outcomes, and lower costs.”
“Clinical studies demonstrate overutilization of these procedures and lack of adherence to specialty society–endorsed guidelines and recommendations. Up to one-third of upper GI procedures and almost half of nonscreening colonoscopies performed for common clinical conditions are not consistent with clinical guidelines,” UHC stated in an FAQ. “A UHC review of upper endoscopy and lower endoscopy procedures performed in 2022 revealed two- to fivefold practice-level variation in the use of both procedure types, even after adjusting for member characteristics including age and comorbidities.”
However, according to a statement from the AGA, it has not seen utilization data specific to UHC: “It is clear that UHC does not currently have any data indicating significant overutilization of critical colonoscopy and endoscopy procedures and therefore no justification to impose burdensome barriers like prior authorization.” AGA also pointed to research showing there is an unmet need for colonoscopies in the United States, which suggests there is an underutilization of this crucial procedure.
“AGA has expressed its willingness to work collaboratively with UnitedHealthcare to address any concerns and educate physicians, but communication and transparency with the insurer are nearly nonexistent. Instead, the GI community is confronted with a nebulous concept called advance notification, which is not conducive to seamless patient care. Ultimately, it appears advance notification will form the basis of prior authorization, which we know can delay, disrupt, and deny timely care,” Dr. Jung said.
How advance notification works
Beginning June 1, providers have been asked to provide advance notification for nonscreening GI endoscopy procedures that include: esophagogastroduodenoscopy, capsule endoscopy, diagnostic colonoscopy and surveillance colonoscopy. The notification can be made by phone (866-889-8054) or through a UHC online portal at UHCprovider.com.
The AGA has said that some GI practices have found the portal to be confusing and it lacks a standard software application raising concerns for high error rates.
Advance notification applies to patients who have UHC commercial plans, including UnitedHealthcare, UnitedHealthcare Plan of the River Valley, Neighborhood Health Partnership, UnitedHealthcare Level Funded, and UnitedHealthcare Oxford Health Plans in all states, except Rhode Island, Kentucky, and New Mexico.
Providers who opt out of participating in advance notification will not be eligible to participate in the Gold Card program in 2024. The program will essentially allow providers to order most GI endoscopy procedures, except for screening colonoscopy, without prior authorization. However, UHC has not released any information about how it will implement its planned Gold Card prior authorization program or how many providers will be accepted into the program.
UHC has assured providers it will not issue medical necessity denials through this process, but it may ask providers to participate in a “comprehensive peer-to-peer discussion with a board-certified gastroenterologist around clinical guidelines.”
The fear for practices is that advance notification will be an onerous process adding burdensome paperwork that practices are not equipped to manage. UHC is the largest health insurer in the country representing 46% of the total market.
Lawrence Kim, MD, AGAF, vice president of AGA and a gastroenterologist practicing in Denver said that each physician in his practice does over 1,000 procedures annually and 25% of their patients carry UHC.
“We are currently completing 30-40 notifications a day, requiring two staff members to comply with this program. UHC is not asking for any clinical information, just procedure and diagnosis codes, and in some cases site of service. Therefore, the advance notification program as it stands will not provide UHC with any additional information beyond what they already have through claims data. This highlights the strain these requirements are putting on providers and practices for repetitive data,” he said.
For more details about UHC’s advance notification program, UHC has prepared this FAQ. To learn more about AGA’s advocacy, visit www.gastro.org/UHC.
FDA adds safety-related information to its dermal filler webpage
On July 6, the .
Along with a list of common reactions such as bruising, redness, swelling, and pain, the webpage now includes language to inform the public and health care providers about reports of delayed-onset inflammation that have been reported to occur near the dermal filler treatment site following viral or bacterial illnesses or infections, vaccinations, or dental procedures. According to an FDA spokesperson, the update is based on several sources of information, including postmarketing data from adverse event–reporting databases, such as the Manufacturer and User Facility Device Experience (MAUDE) for devices and the Vaccine Adverse Event Reporting System (VAERS) for vaccines, published literature, and recommendations from federal agencies and professional societies.
“More specifically, the site was updated to include certain risks of using dermal fillers such as swelling and bruising as well as some less common risks such as inflammation – swelling or redness near the dermal filler injection site – following viral or bacterial illnesses or infections, vaccinations, or dental procedures,” the spokesperson said.
The announcement about the update also states that “typically, the reported inflammation is responsive to treatment or resolves on its own.”
Other less common risks from dermal filler use listed on the website include bumps in or under the skin (nodules or granulomas) that may need to be treated with injections, oral antibiotics, or surgical removal; infection; open or draining wounds; a sore at the injection site; allergic reactions; or necrosis.
Meanwhile, rare risks from dermal filler use that have been reported to the FDA include severe allergic reactions (anaphylactic shock) that require immediate emergency medical assistance; migration (movement of filler material from the site of injection); leakage or rupture of the filler material at the injection site or through the skin (which may result from a tissue reaction or an infection); the formation of permanent hard nodules; and injury to the blood supply after an unintentional injection into a blood vessel, resulting in necrosis, vision abnormalities (including blindness), or stroke.
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment about the FDA update on dermal fillers, said that the agency “is doing its job by making consumers aware of all possible complications [common and uncommon], as it does when it creates a package insert for a medication. Fortunately, however, comprehensive reviews published in the peer-reviewed dermatology literature show delayed inflammation to be a very rare event. So, while it is important for dermatologists during informed consent – prior to filler – to discuss that redness and/or nodules after infection/vaccinations, etc. are possible, it is important to add that based on the data, they are also highly unlikely.”
Sue Ellen Cox, MD, a dermatologist who practices in Chapel Hill, N.C., said that she was glad to see separate sections of recommendations geared to patients and health care providers. For example, the website recommends that patients seek a physician in the field of dermatology or plastic surgery to perform procedures that use dermal fillers. “These are not procedures to be done in an unsupervised spa setting,” said Dr. Cox, a past president of the American Society for Dermatologic Surgery and one of the task force authors of recommendations on preventing and treating adverse events of injectable fillers.
“It also makes the point of using products that are acquired from FDA-approved manufacturers, not products sold online or bootlegged from other countries. Finally, it goes into detail about the importance of in-depth knowledge of anatomy, which is crucial for safe injections and reviews potential complications such as intravascular events and hypersensitivity reactions. The administering physician should have extensive knowledge regarding how to treat any potential problems that arise.”
Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies. Dr. Cox disclosed that she has been a clinical investigator for many injectable companies including AbbVie, Galderma, Revance, and Chroma.
Health care professionals, patients, and others can report adverse events related to dermal fillers and other medical devices to the FDA at 800-FDA-1088 or on the MAUDE website.
On July 6, the .
Along with a list of common reactions such as bruising, redness, swelling, and pain, the webpage now includes language to inform the public and health care providers about reports of delayed-onset inflammation that have been reported to occur near the dermal filler treatment site following viral or bacterial illnesses or infections, vaccinations, or dental procedures. According to an FDA spokesperson, the update is based on several sources of information, including postmarketing data from adverse event–reporting databases, such as the Manufacturer and User Facility Device Experience (MAUDE) for devices and the Vaccine Adverse Event Reporting System (VAERS) for vaccines, published literature, and recommendations from federal agencies and professional societies.
“More specifically, the site was updated to include certain risks of using dermal fillers such as swelling and bruising as well as some less common risks such as inflammation – swelling or redness near the dermal filler injection site – following viral or bacterial illnesses or infections, vaccinations, or dental procedures,” the spokesperson said.
The announcement about the update also states that “typically, the reported inflammation is responsive to treatment or resolves on its own.”
Other less common risks from dermal filler use listed on the website include bumps in or under the skin (nodules or granulomas) that may need to be treated with injections, oral antibiotics, or surgical removal; infection; open or draining wounds; a sore at the injection site; allergic reactions; or necrosis.
Meanwhile, rare risks from dermal filler use that have been reported to the FDA include severe allergic reactions (anaphylactic shock) that require immediate emergency medical assistance; migration (movement of filler material from the site of injection); leakage or rupture of the filler material at the injection site or through the skin (which may result from a tissue reaction or an infection); the formation of permanent hard nodules; and injury to the blood supply after an unintentional injection into a blood vessel, resulting in necrosis, vision abnormalities (including blindness), or stroke.
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment about the FDA update on dermal fillers, said that the agency “is doing its job by making consumers aware of all possible complications [common and uncommon], as it does when it creates a package insert for a medication. Fortunately, however, comprehensive reviews published in the peer-reviewed dermatology literature show delayed inflammation to be a very rare event. So, while it is important for dermatologists during informed consent – prior to filler – to discuss that redness and/or nodules after infection/vaccinations, etc. are possible, it is important to add that based on the data, they are also highly unlikely.”
Sue Ellen Cox, MD, a dermatologist who practices in Chapel Hill, N.C., said that she was glad to see separate sections of recommendations geared to patients and health care providers. For example, the website recommends that patients seek a physician in the field of dermatology or plastic surgery to perform procedures that use dermal fillers. “These are not procedures to be done in an unsupervised spa setting,” said Dr. Cox, a past president of the American Society for Dermatologic Surgery and one of the task force authors of recommendations on preventing and treating adverse events of injectable fillers.
“It also makes the point of using products that are acquired from FDA-approved manufacturers, not products sold online or bootlegged from other countries. Finally, it goes into detail about the importance of in-depth knowledge of anatomy, which is crucial for safe injections and reviews potential complications such as intravascular events and hypersensitivity reactions. The administering physician should have extensive knowledge regarding how to treat any potential problems that arise.”
Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies. Dr. Cox disclosed that she has been a clinical investigator for many injectable companies including AbbVie, Galderma, Revance, and Chroma.
Health care professionals, patients, and others can report adverse events related to dermal fillers and other medical devices to the FDA at 800-FDA-1088 or on the MAUDE website.
On July 6, the .
Along with a list of common reactions such as bruising, redness, swelling, and pain, the webpage now includes language to inform the public and health care providers about reports of delayed-onset inflammation that have been reported to occur near the dermal filler treatment site following viral or bacterial illnesses or infections, vaccinations, or dental procedures. According to an FDA spokesperson, the update is based on several sources of information, including postmarketing data from adverse event–reporting databases, such as the Manufacturer and User Facility Device Experience (MAUDE) for devices and the Vaccine Adverse Event Reporting System (VAERS) for vaccines, published literature, and recommendations from federal agencies and professional societies.
“More specifically, the site was updated to include certain risks of using dermal fillers such as swelling and bruising as well as some less common risks such as inflammation – swelling or redness near the dermal filler injection site – following viral or bacterial illnesses or infections, vaccinations, or dental procedures,” the spokesperson said.
The announcement about the update also states that “typically, the reported inflammation is responsive to treatment or resolves on its own.”
Other less common risks from dermal filler use listed on the website include bumps in or under the skin (nodules or granulomas) that may need to be treated with injections, oral antibiotics, or surgical removal; infection; open or draining wounds; a sore at the injection site; allergic reactions; or necrosis.
Meanwhile, rare risks from dermal filler use that have been reported to the FDA include severe allergic reactions (anaphylactic shock) that require immediate emergency medical assistance; migration (movement of filler material from the site of injection); leakage or rupture of the filler material at the injection site or through the skin (which may result from a tissue reaction or an infection); the formation of permanent hard nodules; and injury to the blood supply after an unintentional injection into a blood vessel, resulting in necrosis, vision abnormalities (including blindness), or stroke.
Lawrence J. Green, MD, of the department of dermatology at George Washington University, Washington, who was asked to comment about the FDA update on dermal fillers, said that the agency “is doing its job by making consumers aware of all possible complications [common and uncommon], as it does when it creates a package insert for a medication. Fortunately, however, comprehensive reviews published in the peer-reviewed dermatology literature show delayed inflammation to be a very rare event. So, while it is important for dermatologists during informed consent – prior to filler – to discuss that redness and/or nodules after infection/vaccinations, etc. are possible, it is important to add that based on the data, they are also highly unlikely.”
Sue Ellen Cox, MD, a dermatologist who practices in Chapel Hill, N.C., said that she was glad to see separate sections of recommendations geared to patients and health care providers. For example, the website recommends that patients seek a physician in the field of dermatology or plastic surgery to perform procedures that use dermal fillers. “These are not procedures to be done in an unsupervised spa setting,” said Dr. Cox, a past president of the American Society for Dermatologic Surgery and one of the task force authors of recommendations on preventing and treating adverse events of injectable fillers.
“It also makes the point of using products that are acquired from FDA-approved manufacturers, not products sold online or bootlegged from other countries. Finally, it goes into detail about the importance of in-depth knowledge of anatomy, which is crucial for safe injections and reviews potential complications such as intravascular events and hypersensitivity reactions. The administering physician should have extensive knowledge regarding how to treat any potential problems that arise.”
Dr. Green disclosed that he is a speaker, consultant, or investigator for many pharmaceutical companies. Dr. Cox disclosed that she has been a clinical investigator for many injectable companies including AbbVie, Galderma, Revance, and Chroma.
Health care professionals, patients, and others can report adverse events related to dermal fillers and other medical devices to the FDA at 800-FDA-1088 or on the MAUDE website.