User login
Smartwatches able to detect very early signs of Parkinson’s
new research shows.
An analysis of wearable motion-tracking data from UK Biobank participants showed a strong correlation between reduced daytime movement over 1 week and a clinical diagnosis of PD up to 7 years later.
“Smartwatch data is easily accessible and low cost. By using this type of data, we would potentially be able to identify individuals in the very early stages of Parkinson’s disease within the general population,” lead researcher Cynthia Sandor, PhD, from Cardiff (Wales) University, said in a statement.
“We have shown here that a single week of data captured can predict events up to 7 years in the future. With these results we could develop a valuable screening tool to aid in the early detection of Parkinson’s,” she added.
“This has implications both for research, in improving recruitment into clinical trials, and in clinical practice, in allowing patients to access treatments at an earlier stage, in future when such treatments become available,” said Dr. Sandor.
The study was published online in Nature Medicine.
Novel biomarker for PD
Using machine learning, the researchers analyzed accelerometry data from 103,712 UK Biobank participants who wore a medical-grade smartwatch for a 7-day period during 2013-2016.
At the time of or within 2 years after accelerometry data collection, 273 participants were diagnosed with PD. An additional 196 individuals received a new PD diagnosis more than 2 years after accelerometry data collection (the prodromal group).
The patients with prodromal symptoms of PD and those who were diagnosed with PD showed a significantly reduced daytime acceleration profile up to 7 years before diagnosis, compared with age- and sex-matched healthy control persons, the researchers found.
The reduction in acceleration both before and following diagnosis was unique to patients with PD, “suggesting this measure to be disease specific with potential for use in early identification of individuals likely to be diagnosed with PD,” they wrote.
Accelerometry data proved more accurate than other risk factors (lifestyle, genetics, blood chemistry) or recognized prodromal symptoms of PD in predicting whether an individual would develop PD.
“Our results suggest that accelerometry collected with wearable devices in the general population could be used to identify those at elevated risk for PD on an unprecedented scale and, importantly, individuals who will likely convert within the next few years can be included in studies for neuroprotective treatments,” the researchers conclude in their article.
High-quality research
In a statement from the U.K.-based nonprofit Science Media Centre, José López Barneo, MD, PhD, with the University of Seville (Spain), said this “good quality” study “fits well with current knowledge.”
Dr. Barneo noted that other investigators have also observed that slowness of movement is a characteristic feature of some people who subsequently develop PD.
But these studies involved preselected cohorts of persons at risk of developing PD, or they were carried out in a hospital that required healthcare staff to conduct the movement analysis. In contrast, the current study was conducted in a very large cohort from the general U.K. population.
Also weighing in, José Luis Lanciego, MD, PhD, with the University of Navarra (Spain), said the “main value of this study is that it has demonstrated that accelerometry measurements obtained using wearable devices (such as a smartwatch or other similar devices) are more useful than the assessment of any other potentially prodromal symptom in identifying which people in the [general] population are at increased risk of developing Parkinson’s disease in the future, as well as being able to estimate how many years it will take to start suffering from this neurodegenerative process.
“In these diseases, early diagnosis is to some extent questionable, as early diagnosis is of little use if neuroprotective treatment is not available,” Dr. Lanciego noted.
“However, it is of great importance for use in clinical trials aimed at evaluating the efficacy of new potentially neuroprotective treatments whose main objective is to slow down – and, ideally, even halt ― the clinical progression that typically characterizes Parkinson’s disease,” Dr. Lanciego added.
The study was funded by the UK Dementia Research Institute, the Welsh government, and Cardiff University. Dr. Sandor, Dr. Barneo, and Dr. Lanciego have no relevant disclosures.
A version of this article originally appeared on Medscape.com.
new research shows.
An analysis of wearable motion-tracking data from UK Biobank participants showed a strong correlation between reduced daytime movement over 1 week and a clinical diagnosis of PD up to 7 years later.
“Smartwatch data is easily accessible and low cost. By using this type of data, we would potentially be able to identify individuals in the very early stages of Parkinson’s disease within the general population,” lead researcher Cynthia Sandor, PhD, from Cardiff (Wales) University, said in a statement.
“We have shown here that a single week of data captured can predict events up to 7 years in the future. With these results we could develop a valuable screening tool to aid in the early detection of Parkinson’s,” she added.
“This has implications both for research, in improving recruitment into clinical trials, and in clinical practice, in allowing patients to access treatments at an earlier stage, in future when such treatments become available,” said Dr. Sandor.
The study was published online in Nature Medicine.
Novel biomarker for PD
Using machine learning, the researchers analyzed accelerometry data from 103,712 UK Biobank participants who wore a medical-grade smartwatch for a 7-day period during 2013-2016.
At the time of or within 2 years after accelerometry data collection, 273 participants were diagnosed with PD. An additional 196 individuals received a new PD diagnosis more than 2 years after accelerometry data collection (the prodromal group).
The patients with prodromal symptoms of PD and those who were diagnosed with PD showed a significantly reduced daytime acceleration profile up to 7 years before diagnosis, compared with age- and sex-matched healthy control persons, the researchers found.
The reduction in acceleration both before and following diagnosis was unique to patients with PD, “suggesting this measure to be disease specific with potential for use in early identification of individuals likely to be diagnosed with PD,” they wrote.
Accelerometry data proved more accurate than other risk factors (lifestyle, genetics, blood chemistry) or recognized prodromal symptoms of PD in predicting whether an individual would develop PD.
“Our results suggest that accelerometry collected with wearable devices in the general population could be used to identify those at elevated risk for PD on an unprecedented scale and, importantly, individuals who will likely convert within the next few years can be included in studies for neuroprotective treatments,” the researchers conclude in their article.
High-quality research
In a statement from the U.K.-based nonprofit Science Media Centre, José López Barneo, MD, PhD, with the University of Seville (Spain), said this “good quality” study “fits well with current knowledge.”
Dr. Barneo noted that other investigators have also observed that slowness of movement is a characteristic feature of some people who subsequently develop PD.
But these studies involved preselected cohorts of persons at risk of developing PD, or they were carried out in a hospital that required healthcare staff to conduct the movement analysis. In contrast, the current study was conducted in a very large cohort from the general U.K. population.
Also weighing in, José Luis Lanciego, MD, PhD, with the University of Navarra (Spain), said the “main value of this study is that it has demonstrated that accelerometry measurements obtained using wearable devices (such as a smartwatch or other similar devices) are more useful than the assessment of any other potentially prodromal symptom in identifying which people in the [general] population are at increased risk of developing Parkinson’s disease in the future, as well as being able to estimate how many years it will take to start suffering from this neurodegenerative process.
“In these diseases, early diagnosis is to some extent questionable, as early diagnosis is of little use if neuroprotective treatment is not available,” Dr. Lanciego noted.
“However, it is of great importance for use in clinical trials aimed at evaluating the efficacy of new potentially neuroprotective treatments whose main objective is to slow down – and, ideally, even halt ― the clinical progression that typically characterizes Parkinson’s disease,” Dr. Lanciego added.
The study was funded by the UK Dementia Research Institute, the Welsh government, and Cardiff University. Dr. Sandor, Dr. Barneo, and Dr. Lanciego have no relevant disclosures.
A version of this article originally appeared on Medscape.com.
new research shows.
An analysis of wearable motion-tracking data from UK Biobank participants showed a strong correlation between reduced daytime movement over 1 week and a clinical diagnosis of PD up to 7 years later.
“Smartwatch data is easily accessible and low cost. By using this type of data, we would potentially be able to identify individuals in the very early stages of Parkinson’s disease within the general population,” lead researcher Cynthia Sandor, PhD, from Cardiff (Wales) University, said in a statement.
“We have shown here that a single week of data captured can predict events up to 7 years in the future. With these results we could develop a valuable screening tool to aid in the early detection of Parkinson’s,” she added.
“This has implications both for research, in improving recruitment into clinical trials, and in clinical practice, in allowing patients to access treatments at an earlier stage, in future when such treatments become available,” said Dr. Sandor.
The study was published online in Nature Medicine.
Novel biomarker for PD
Using machine learning, the researchers analyzed accelerometry data from 103,712 UK Biobank participants who wore a medical-grade smartwatch for a 7-day period during 2013-2016.
At the time of or within 2 years after accelerometry data collection, 273 participants were diagnosed with PD. An additional 196 individuals received a new PD diagnosis more than 2 years after accelerometry data collection (the prodromal group).
The patients with prodromal symptoms of PD and those who were diagnosed with PD showed a significantly reduced daytime acceleration profile up to 7 years before diagnosis, compared with age- and sex-matched healthy control persons, the researchers found.
The reduction in acceleration both before and following diagnosis was unique to patients with PD, “suggesting this measure to be disease specific with potential for use in early identification of individuals likely to be diagnosed with PD,” they wrote.
Accelerometry data proved more accurate than other risk factors (lifestyle, genetics, blood chemistry) or recognized prodromal symptoms of PD in predicting whether an individual would develop PD.
“Our results suggest that accelerometry collected with wearable devices in the general population could be used to identify those at elevated risk for PD on an unprecedented scale and, importantly, individuals who will likely convert within the next few years can be included in studies for neuroprotective treatments,” the researchers conclude in their article.
High-quality research
In a statement from the U.K.-based nonprofit Science Media Centre, José López Barneo, MD, PhD, with the University of Seville (Spain), said this “good quality” study “fits well with current knowledge.”
Dr. Barneo noted that other investigators have also observed that slowness of movement is a characteristic feature of some people who subsequently develop PD.
But these studies involved preselected cohorts of persons at risk of developing PD, or they were carried out in a hospital that required healthcare staff to conduct the movement analysis. In contrast, the current study was conducted in a very large cohort from the general U.K. population.
Also weighing in, José Luis Lanciego, MD, PhD, with the University of Navarra (Spain), said the “main value of this study is that it has demonstrated that accelerometry measurements obtained using wearable devices (such as a smartwatch or other similar devices) are more useful than the assessment of any other potentially prodromal symptom in identifying which people in the [general] population are at increased risk of developing Parkinson’s disease in the future, as well as being able to estimate how many years it will take to start suffering from this neurodegenerative process.
“In these diseases, early diagnosis is to some extent questionable, as early diagnosis is of little use if neuroprotective treatment is not available,” Dr. Lanciego noted.
“However, it is of great importance for use in clinical trials aimed at evaluating the efficacy of new potentially neuroprotective treatments whose main objective is to slow down – and, ideally, even halt ― the clinical progression that typically characterizes Parkinson’s disease,” Dr. Lanciego added.
The study was funded by the UK Dementia Research Institute, the Welsh government, and Cardiff University. Dr. Sandor, Dr. Barneo, and Dr. Lanciego have no relevant disclosures.
A version of this article originally appeared on Medscape.com.
FROM NATURE MEDICINE
Does racial bias taint the Apgar score?
Experts say overhaul needed
In 1952, when Dr. Virginia Apgar developed her 10-point scale for assessing neonates’ health, the U.S. obstetrical anesthesiologst may not have foreseen it would one day become one of the commonest medical tests in the world.
Assigned even before the mother first holds her newborn, the score rapidly evaluates neonates with a score of 0-10, which leads to an algorithm of potential medical interventions. The scale evaluates heart rate, respiratory effort, muscle tone, reflex response, and skin coloring (typically described as blue body, pink body/blue limbs, or pink body).
“The Apgar is a very important tool used in millions of babies around the world in the very first minute after birth,” said Amos Grunebaum, MD, a professor of obstetrics and gynecology at Hofstra University, Hempstead, N.Y., and director of perinatal research at Northwell Lenox Hill Hospital in Manhattan.
But recently the venerable system has increasingly come under fire for colorism and racial bias, with some calling for an overhaul. That pressure is due to the 2 out of 10 points allotted to an overall “pink” skin tone, a measure that lowers the scores of non-White newborns and may expose them to unnecessary measures such as resuscitation, neonatal intensive care, and intubation.
“This is their first encounter with systemic racism,” said Dr. Grunebaum in an interview. “The score is prejudiced against Black babies because they can’t get perfect scores.”
Propagating ‘race-based medicine’
Concern about racial bias embedded in the Apgar score is not new, Dr. Grunebaum noted.
“Decades ago, when I was doing my training in Brooklyn, the nurses said that using skin color was ridiculous since Black and brown babies couldn’t be pink. And skin color looks different in different lighting. Dr. Apgar herself recognized the problem.”
Furthermore, men see color differently than women do, and some people are actually color-blind.“But nobody wanted to speak out,” Dr. Grunebaum said. “It was like the emperor’s new clothes scenario.”
In his view, embedding skin color scoring into basic data and health care decisions propagates race-based medicine. “It should not be used for White, Black, or brown babies,” he said.
Removing the skin color portion of the Apgar score – and its racial, colorist, and ethnic bias – will provide more accurate and equitable evaluation of newborn babies worldwide, Dr. Grunebaum said.
“I think there’s a pretty good argument to be made that the skin color measure should be eliminated,” agreed Sara E. Edwards, MD, an obstetrician-gynecologist at the University of Illinois Hospital in Chicago, who has also studied Apgar and racial bias in the clinical care of Black babies.
And such clinical bias may soon be illegal in the United States thanks to a proposed new antidiscrimination provision to the Affordable Care Act regarding the use of clinical algorithms in decision-making. The proposed section, § 92.210, states that a covered entity must not discriminate against any individual on the basis of race, color, national origin, sex, age, or disability through clinical algorithms used in decision-making. Hospitals may soon have to alter clinical algorithms in response.
Dr. Grunebaum’s research in the area of clinical racism includes a large 2022 cohort study of almost 10 million mothers and more than 8 million fathers using 2016-2019 natality data from the National Center for Health Statistics, and Division of Vital Statistics. This study found that Black newborns had a less than 50% chance of having a 5-minute Apgar score of 10, compared with White newborns. White babies, both non-Hispanic and Hispanic, had the highest proportion of perfect 10s.
But can the 2-point skin tone indicator be easily replaced? According to Dr. Grunebaum, substituting indicators such as oral mucosa color or oximetry readings are not satisfactory either. “For one thing, oximetry gives different readings in Black [people],” he said.
In her group’s Apgar research, Dr. Edwards found that care providers applied variable and inaccurate scores based on neonatal race – independently of clinical factors and umbilical-cord gas values.
“In Black neonates umbilical cord gases were not in agreement with lower Apgar scores,” she said. In her view, these inaccuracies point to the existence of colorism and racial bias among health care providers.
Bias ‘creeping in’ to neonatal care
Dr. Edwards’s research was prompted by anecdotal observations that Black babies generally had lower Apgar scores and were more frequently sent to the NICU. “Admission to the NICU can have a negative effect on maternal-child bonding and contribute to PTSD in mothers,” she said.
Her group looked at Apgar scores by race for the year 2019 in an academic hospital cohort of 977 neonates, of whom 56.5% were Black, while controlling for confounding clinical factors.
“Our anecdotal observations of how we score Black neonates were confirmed,” she said. Providers assigned Black babies significantly lower Apgar scores at 1 minute and 5 minutes (odds ratios, .63 and .64) when controlling for umbilical artery gases, gestational age, and maternal-fetal complications.
This difference was specifically associated with lower assigned color Apgar scores at 1 minute (odds ratio, .52). Moreover, full-term Black neonates were sent to neonatal intensive care at higher rates (odds ratio, 1.29) than non-Black neonates when controlling for all the above factors.
Providers applied inaccurate Apgar scores to Black neonates given that the umbilical cord gases were not in agreement with lower Apgar scores, suggesting that colorism and racial biases do exist among health care providers. “We saw bias creeping in because of subjective decisions about color,” Dr. Edwards said. But by the more objective measure of umbilical-cord gas, Black neonates did not have the abnormal values to support NICU admission. The mean umbilical artery pH was 7.259 for Black vs. 7.256 for non-Black neonates.
The solution may lie in switching to an 8 out of 8 score or looking at other indicators such as the eyes and the nail beds, she said. “Or there may be a way to score skin tone accurately when providers are appropriately trained to do so on neonates of all races, to recognize what a well-perfused skin color looks like in all babies.”
New scoring system needed
Interest in this issue continues. In 2022, a population study was conducted by Emma Gillette, MPH, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues in a cohort of almost 7 million singletons born in 2016-2017.
“We found that overall, Apgar scores were highly associated with mortality across the first year of life,” Ms. Gillette said in an interview. “But non-Hispanic Black infants were more likely to be assigned low Apgar scores compared to White infants, and the odds of death in the first year of life are not as strongly correlated with Apgar scores as in White infants.”
That finding was surprising. “Apgar scores are meant to be an indicator of newborn health and well-being and predictors of infant mortality, and therefore should not vary significantly by race or skin color,” she said. “So I think further study into the component scores of the Apgar score is warranted to try to tease out the reasons behind the differences we’re seeing.”
Ms. Gillette agreed that the skin coloring component of the variable could be inaccurate since variables related to skin color more generally are subjective and difficult to measure. What’s needed is a scoring system that performs equally well across racial groups.
In the meantime, some clinicians may be making practical accommodations. “I hate to tell you, but some people fake the skin score,” said Dr. Grunebaum. “I recently asked a doctor from Ethiopia how they handled it there, and he laughed and said they just automatically give skin color a 2. But faking it is not what you should have to do in medicine.”
Dr. Grunebaum, Dr. Edwards, and Ms. Gillette disclosed no relevant competing interests with respect to their comments.
Experts say overhaul needed
Experts say overhaul needed
In 1952, when Dr. Virginia Apgar developed her 10-point scale for assessing neonates’ health, the U.S. obstetrical anesthesiologst may not have foreseen it would one day become one of the commonest medical tests in the world.
Assigned even before the mother first holds her newborn, the score rapidly evaluates neonates with a score of 0-10, which leads to an algorithm of potential medical interventions. The scale evaluates heart rate, respiratory effort, muscle tone, reflex response, and skin coloring (typically described as blue body, pink body/blue limbs, or pink body).
“The Apgar is a very important tool used in millions of babies around the world in the very first minute after birth,” said Amos Grunebaum, MD, a professor of obstetrics and gynecology at Hofstra University, Hempstead, N.Y., and director of perinatal research at Northwell Lenox Hill Hospital in Manhattan.
But recently the venerable system has increasingly come under fire for colorism and racial bias, with some calling for an overhaul. That pressure is due to the 2 out of 10 points allotted to an overall “pink” skin tone, a measure that lowers the scores of non-White newborns and may expose them to unnecessary measures such as resuscitation, neonatal intensive care, and intubation.
“This is their first encounter with systemic racism,” said Dr. Grunebaum in an interview. “The score is prejudiced against Black babies because they can’t get perfect scores.”
Propagating ‘race-based medicine’
Concern about racial bias embedded in the Apgar score is not new, Dr. Grunebaum noted.
“Decades ago, when I was doing my training in Brooklyn, the nurses said that using skin color was ridiculous since Black and brown babies couldn’t be pink. And skin color looks different in different lighting. Dr. Apgar herself recognized the problem.”
Furthermore, men see color differently than women do, and some people are actually color-blind.“But nobody wanted to speak out,” Dr. Grunebaum said. “It was like the emperor’s new clothes scenario.”
In his view, embedding skin color scoring into basic data and health care decisions propagates race-based medicine. “It should not be used for White, Black, or brown babies,” he said.
Removing the skin color portion of the Apgar score – and its racial, colorist, and ethnic bias – will provide more accurate and equitable evaluation of newborn babies worldwide, Dr. Grunebaum said.
“I think there’s a pretty good argument to be made that the skin color measure should be eliminated,” agreed Sara E. Edwards, MD, an obstetrician-gynecologist at the University of Illinois Hospital in Chicago, who has also studied Apgar and racial bias in the clinical care of Black babies.
And such clinical bias may soon be illegal in the United States thanks to a proposed new antidiscrimination provision to the Affordable Care Act regarding the use of clinical algorithms in decision-making. The proposed section, § 92.210, states that a covered entity must not discriminate against any individual on the basis of race, color, national origin, sex, age, or disability through clinical algorithms used in decision-making. Hospitals may soon have to alter clinical algorithms in response.
Dr. Grunebaum’s research in the area of clinical racism includes a large 2022 cohort study of almost 10 million mothers and more than 8 million fathers using 2016-2019 natality data from the National Center for Health Statistics, and Division of Vital Statistics. This study found that Black newborns had a less than 50% chance of having a 5-minute Apgar score of 10, compared with White newborns. White babies, both non-Hispanic and Hispanic, had the highest proportion of perfect 10s.
But can the 2-point skin tone indicator be easily replaced? According to Dr. Grunebaum, substituting indicators such as oral mucosa color or oximetry readings are not satisfactory either. “For one thing, oximetry gives different readings in Black [people],” he said.
In her group’s Apgar research, Dr. Edwards found that care providers applied variable and inaccurate scores based on neonatal race – independently of clinical factors and umbilical-cord gas values.
“In Black neonates umbilical cord gases were not in agreement with lower Apgar scores,” she said. In her view, these inaccuracies point to the existence of colorism and racial bias among health care providers.
Bias ‘creeping in’ to neonatal care
Dr. Edwards’s research was prompted by anecdotal observations that Black babies generally had lower Apgar scores and were more frequently sent to the NICU. “Admission to the NICU can have a negative effect on maternal-child bonding and contribute to PTSD in mothers,” she said.
Her group looked at Apgar scores by race for the year 2019 in an academic hospital cohort of 977 neonates, of whom 56.5% were Black, while controlling for confounding clinical factors.
“Our anecdotal observations of how we score Black neonates were confirmed,” she said. Providers assigned Black babies significantly lower Apgar scores at 1 minute and 5 minutes (odds ratios, .63 and .64) when controlling for umbilical artery gases, gestational age, and maternal-fetal complications.
This difference was specifically associated with lower assigned color Apgar scores at 1 minute (odds ratio, .52). Moreover, full-term Black neonates were sent to neonatal intensive care at higher rates (odds ratio, 1.29) than non-Black neonates when controlling for all the above factors.
Providers applied inaccurate Apgar scores to Black neonates given that the umbilical cord gases were not in agreement with lower Apgar scores, suggesting that colorism and racial biases do exist among health care providers. “We saw bias creeping in because of subjective decisions about color,” Dr. Edwards said. But by the more objective measure of umbilical-cord gas, Black neonates did not have the abnormal values to support NICU admission. The mean umbilical artery pH was 7.259 for Black vs. 7.256 for non-Black neonates.
The solution may lie in switching to an 8 out of 8 score or looking at other indicators such as the eyes and the nail beds, she said. “Or there may be a way to score skin tone accurately when providers are appropriately trained to do so on neonates of all races, to recognize what a well-perfused skin color looks like in all babies.”
New scoring system needed
Interest in this issue continues. In 2022, a population study was conducted by Emma Gillette, MPH, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues in a cohort of almost 7 million singletons born in 2016-2017.
“We found that overall, Apgar scores were highly associated with mortality across the first year of life,” Ms. Gillette said in an interview. “But non-Hispanic Black infants were more likely to be assigned low Apgar scores compared to White infants, and the odds of death in the first year of life are not as strongly correlated with Apgar scores as in White infants.”
That finding was surprising. “Apgar scores are meant to be an indicator of newborn health and well-being and predictors of infant mortality, and therefore should not vary significantly by race or skin color,” she said. “So I think further study into the component scores of the Apgar score is warranted to try to tease out the reasons behind the differences we’re seeing.”
Ms. Gillette agreed that the skin coloring component of the variable could be inaccurate since variables related to skin color more generally are subjective and difficult to measure. What’s needed is a scoring system that performs equally well across racial groups.
In the meantime, some clinicians may be making practical accommodations. “I hate to tell you, but some people fake the skin score,” said Dr. Grunebaum. “I recently asked a doctor from Ethiopia how they handled it there, and he laughed and said they just automatically give skin color a 2. But faking it is not what you should have to do in medicine.”
Dr. Grunebaum, Dr. Edwards, and Ms. Gillette disclosed no relevant competing interests with respect to their comments.
In 1952, when Dr. Virginia Apgar developed her 10-point scale for assessing neonates’ health, the U.S. obstetrical anesthesiologst may not have foreseen it would one day become one of the commonest medical tests in the world.
Assigned even before the mother first holds her newborn, the score rapidly evaluates neonates with a score of 0-10, which leads to an algorithm of potential medical interventions. The scale evaluates heart rate, respiratory effort, muscle tone, reflex response, and skin coloring (typically described as blue body, pink body/blue limbs, or pink body).
“The Apgar is a very important tool used in millions of babies around the world in the very first minute after birth,” said Amos Grunebaum, MD, a professor of obstetrics and gynecology at Hofstra University, Hempstead, N.Y., and director of perinatal research at Northwell Lenox Hill Hospital in Manhattan.
But recently the venerable system has increasingly come under fire for colorism and racial bias, with some calling for an overhaul. That pressure is due to the 2 out of 10 points allotted to an overall “pink” skin tone, a measure that lowers the scores of non-White newborns and may expose them to unnecessary measures such as resuscitation, neonatal intensive care, and intubation.
“This is their first encounter with systemic racism,” said Dr. Grunebaum in an interview. “The score is prejudiced against Black babies because they can’t get perfect scores.”
Propagating ‘race-based medicine’
Concern about racial bias embedded in the Apgar score is not new, Dr. Grunebaum noted.
“Decades ago, when I was doing my training in Brooklyn, the nurses said that using skin color was ridiculous since Black and brown babies couldn’t be pink. And skin color looks different in different lighting. Dr. Apgar herself recognized the problem.”
Furthermore, men see color differently than women do, and some people are actually color-blind.“But nobody wanted to speak out,” Dr. Grunebaum said. “It was like the emperor’s new clothes scenario.”
In his view, embedding skin color scoring into basic data and health care decisions propagates race-based medicine. “It should not be used for White, Black, or brown babies,” he said.
Removing the skin color portion of the Apgar score – and its racial, colorist, and ethnic bias – will provide more accurate and equitable evaluation of newborn babies worldwide, Dr. Grunebaum said.
“I think there’s a pretty good argument to be made that the skin color measure should be eliminated,” agreed Sara E. Edwards, MD, an obstetrician-gynecologist at the University of Illinois Hospital in Chicago, who has also studied Apgar and racial bias in the clinical care of Black babies.
And such clinical bias may soon be illegal in the United States thanks to a proposed new antidiscrimination provision to the Affordable Care Act regarding the use of clinical algorithms in decision-making. The proposed section, § 92.210, states that a covered entity must not discriminate against any individual on the basis of race, color, national origin, sex, age, or disability through clinical algorithms used in decision-making. Hospitals may soon have to alter clinical algorithms in response.
Dr. Grunebaum’s research in the area of clinical racism includes a large 2022 cohort study of almost 10 million mothers and more than 8 million fathers using 2016-2019 natality data from the National Center for Health Statistics, and Division of Vital Statistics. This study found that Black newborns had a less than 50% chance of having a 5-minute Apgar score of 10, compared with White newborns. White babies, both non-Hispanic and Hispanic, had the highest proportion of perfect 10s.
But can the 2-point skin tone indicator be easily replaced? According to Dr. Grunebaum, substituting indicators such as oral mucosa color or oximetry readings are not satisfactory either. “For one thing, oximetry gives different readings in Black [people],” he said.
In her group’s Apgar research, Dr. Edwards found that care providers applied variable and inaccurate scores based on neonatal race – independently of clinical factors and umbilical-cord gas values.
“In Black neonates umbilical cord gases were not in agreement with lower Apgar scores,” she said. In her view, these inaccuracies point to the existence of colorism and racial bias among health care providers.
Bias ‘creeping in’ to neonatal care
Dr. Edwards’s research was prompted by anecdotal observations that Black babies generally had lower Apgar scores and were more frequently sent to the NICU. “Admission to the NICU can have a negative effect on maternal-child bonding and contribute to PTSD in mothers,” she said.
Her group looked at Apgar scores by race for the year 2019 in an academic hospital cohort of 977 neonates, of whom 56.5% were Black, while controlling for confounding clinical factors.
“Our anecdotal observations of how we score Black neonates were confirmed,” she said. Providers assigned Black babies significantly lower Apgar scores at 1 minute and 5 minutes (odds ratios, .63 and .64) when controlling for umbilical artery gases, gestational age, and maternal-fetal complications.
This difference was specifically associated with lower assigned color Apgar scores at 1 minute (odds ratio, .52). Moreover, full-term Black neonates were sent to neonatal intensive care at higher rates (odds ratio, 1.29) than non-Black neonates when controlling for all the above factors.
Providers applied inaccurate Apgar scores to Black neonates given that the umbilical cord gases were not in agreement with lower Apgar scores, suggesting that colorism and racial biases do exist among health care providers. “We saw bias creeping in because of subjective decisions about color,” Dr. Edwards said. But by the more objective measure of umbilical-cord gas, Black neonates did not have the abnormal values to support NICU admission. The mean umbilical artery pH was 7.259 for Black vs. 7.256 for non-Black neonates.
The solution may lie in switching to an 8 out of 8 score or looking at other indicators such as the eyes and the nail beds, she said. “Or there may be a way to score skin tone accurately when providers are appropriately trained to do so on neonates of all races, to recognize what a well-perfused skin color looks like in all babies.”
New scoring system needed
Interest in this issue continues. In 2022, a population study was conducted by Emma Gillette, MPH, of the Icahn School of Medicine at Mount Sinai, New York, and colleagues in a cohort of almost 7 million singletons born in 2016-2017.
“We found that overall, Apgar scores were highly associated with mortality across the first year of life,” Ms. Gillette said in an interview. “But non-Hispanic Black infants were more likely to be assigned low Apgar scores compared to White infants, and the odds of death in the first year of life are not as strongly correlated with Apgar scores as in White infants.”
That finding was surprising. “Apgar scores are meant to be an indicator of newborn health and well-being and predictors of infant mortality, and therefore should not vary significantly by race or skin color,” she said. “So I think further study into the component scores of the Apgar score is warranted to try to tease out the reasons behind the differences we’re seeing.”
Ms. Gillette agreed that the skin coloring component of the variable could be inaccurate since variables related to skin color more generally are subjective and difficult to measure. What’s needed is a scoring system that performs equally well across racial groups.
In the meantime, some clinicians may be making practical accommodations. “I hate to tell you, but some people fake the skin score,” said Dr. Grunebaum. “I recently asked a doctor from Ethiopia how they handled it there, and he laughed and said they just automatically give skin color a 2. But faking it is not what you should have to do in medicine.”
Dr. Grunebaum, Dr. Edwards, and Ms. Gillette disclosed no relevant competing interests with respect to their comments.
AMA supports APRN oversight by both medical and nursing boards
In a move that raises the stakes in doctors’ ongoing scope-creep battle against nonphysician providers, the American Medical Association’s legislative body voted recently to change its policy on the supervision of advanced practice registered nurses (APRNs). AMA’s House of Delegates called for state medical boards to regulate APRNs in addition to nursing boards.
The AMA has long claimed that nonphysician providers, such as nurse practitioners (NPs) and physician assistants (PAs), need greater oversight because expanded scope of practice for advanced practice practitioners threatens patient safety and undermines the physician-led team model.
APRNs have been touted as a solution to expand access to care and reduce disparities, especially in rural and underserved communities, and they have been promoted by organizations such as the National Academy of Medicine. But the AMA disputes that scope expansions are necessary to increase access to care.
The organization that represents the nation’s physicians said in a prepared statement that it opposes scope expansions because removing doctors from the care team results in higher costs to the patient and lower quality care.
Several nursing organizations swiftly criticized the policy recommendation, including the American Nurses Association, the American Association of Nurse Practitioners, and the National Council of State Boards of Nursing.
The policy shift would create more administrative burdens for APRNs and generate “a downstream effect that only hurts patients,” particularly those in underserved communities without timely access to care, ANA president Jennifer Mensik Kennedy, PhD, MBA, RN, NEA-BC, told this news organization.
“The licensing and regulation of APRNs have never required the oversight of state medical boards,” she said, adding that it should remain the obligation of nursing regulatory bodies.
Jon Fanning, MS, CAE, CNED, chief executive officer of the AANP, called the AMA proposal “flawed.”
“The restrictive involvement of the board of medicine directly contributes to health care access challenges, resulting in continued low health care rankings, geographic disparities in care, and unnecessary regulatory cost in these states,” he said in a press release.
Still, the AMA has vowed to #StopScopeCreep. Securing stricter practice guidelines was a central theme of the association’s recent annual meeting and a goal of its plan to strengthen the physician workforce. The organization invests heavily in advocacy and education efforts to defeat state bills seeking to extend APRN authority. To that end, the AMA Scope of Practice Partnership, a coalition of over 100 medical associations, has awarded members $3.5 million in grants to combat scope-expansion legislation.
The AMA and the American College of Radiology recently partnered to create advocacy materials, including handouts encouraging patients to ask questions such as: “Will a physician be reviewing my chart, lab results, x-rays, and other tests?”
The policy recommendation comes as concerns mount over the potential for significant physician shortages, fueled partly by older physicians’ retirements and doctors reducing hours or exiting the workforce due to pandemic fatigue and burnout.
While practice regulations vary by state, a new federal bill could change that by broadening the authority of APRNs under Medicare and Medicaid guidelines. Introduced in the U.S. House of Representatives in April and supported by the ANA, the Improving Care and Access to Nurses Act would allow APRNs to perform more procedures, including cardiac and pulmonary rehabilitation and certification of terminal illness for hospice, according to an ANA press release.
In the meantime, several state legislatures are considering bills that would expand APRN scope of practice. Utah is the latest to join a growing list of states – about half now – offering full practice authority to NPs.
Other states offer a reduced scope of practice for APRNs, typically requiring a collaborative agreement with a supervising physician. The remaining states enforce tighter regulations and physician oversight.
A recent Medscape survey found that most physicians report having a good rapport with NPs but many have mixed feelings about giving them expanded practice roles, with one-third saying it would harm patient care. Feelings were only slightly more favorable toward PAs. However, about 75% of patients were either neutral or supportive of independent practice for NPs and PAs.
A version of this article first appeared on Medscape.com.
In a move that raises the stakes in doctors’ ongoing scope-creep battle against nonphysician providers, the American Medical Association’s legislative body voted recently to change its policy on the supervision of advanced practice registered nurses (APRNs). AMA’s House of Delegates called for state medical boards to regulate APRNs in addition to nursing boards.
The AMA has long claimed that nonphysician providers, such as nurse practitioners (NPs) and physician assistants (PAs), need greater oversight because expanded scope of practice for advanced practice practitioners threatens patient safety and undermines the physician-led team model.
APRNs have been touted as a solution to expand access to care and reduce disparities, especially in rural and underserved communities, and they have been promoted by organizations such as the National Academy of Medicine. But the AMA disputes that scope expansions are necessary to increase access to care.
The organization that represents the nation’s physicians said in a prepared statement that it opposes scope expansions because removing doctors from the care team results in higher costs to the patient and lower quality care.
Several nursing organizations swiftly criticized the policy recommendation, including the American Nurses Association, the American Association of Nurse Practitioners, and the National Council of State Boards of Nursing.
The policy shift would create more administrative burdens for APRNs and generate “a downstream effect that only hurts patients,” particularly those in underserved communities without timely access to care, ANA president Jennifer Mensik Kennedy, PhD, MBA, RN, NEA-BC, told this news organization.
“The licensing and regulation of APRNs have never required the oversight of state medical boards,” she said, adding that it should remain the obligation of nursing regulatory bodies.
Jon Fanning, MS, CAE, CNED, chief executive officer of the AANP, called the AMA proposal “flawed.”
“The restrictive involvement of the board of medicine directly contributes to health care access challenges, resulting in continued low health care rankings, geographic disparities in care, and unnecessary regulatory cost in these states,” he said in a press release.
Still, the AMA has vowed to #StopScopeCreep. Securing stricter practice guidelines was a central theme of the association’s recent annual meeting and a goal of its plan to strengthen the physician workforce. The organization invests heavily in advocacy and education efforts to defeat state bills seeking to extend APRN authority. To that end, the AMA Scope of Practice Partnership, a coalition of over 100 medical associations, has awarded members $3.5 million in grants to combat scope-expansion legislation.
The AMA and the American College of Radiology recently partnered to create advocacy materials, including handouts encouraging patients to ask questions such as: “Will a physician be reviewing my chart, lab results, x-rays, and other tests?”
The policy recommendation comes as concerns mount over the potential for significant physician shortages, fueled partly by older physicians’ retirements and doctors reducing hours or exiting the workforce due to pandemic fatigue and burnout.
While practice regulations vary by state, a new federal bill could change that by broadening the authority of APRNs under Medicare and Medicaid guidelines. Introduced in the U.S. House of Representatives in April and supported by the ANA, the Improving Care and Access to Nurses Act would allow APRNs to perform more procedures, including cardiac and pulmonary rehabilitation and certification of terminal illness for hospice, according to an ANA press release.
In the meantime, several state legislatures are considering bills that would expand APRN scope of practice. Utah is the latest to join a growing list of states – about half now – offering full practice authority to NPs.
Other states offer a reduced scope of practice for APRNs, typically requiring a collaborative agreement with a supervising physician. The remaining states enforce tighter regulations and physician oversight.
A recent Medscape survey found that most physicians report having a good rapport with NPs but many have mixed feelings about giving them expanded practice roles, with one-third saying it would harm patient care. Feelings were only slightly more favorable toward PAs. However, about 75% of patients were either neutral or supportive of independent practice for NPs and PAs.
A version of this article first appeared on Medscape.com.
In a move that raises the stakes in doctors’ ongoing scope-creep battle against nonphysician providers, the American Medical Association’s legislative body voted recently to change its policy on the supervision of advanced practice registered nurses (APRNs). AMA’s House of Delegates called for state medical boards to regulate APRNs in addition to nursing boards.
The AMA has long claimed that nonphysician providers, such as nurse practitioners (NPs) and physician assistants (PAs), need greater oversight because expanded scope of practice for advanced practice practitioners threatens patient safety and undermines the physician-led team model.
APRNs have been touted as a solution to expand access to care and reduce disparities, especially in rural and underserved communities, and they have been promoted by organizations such as the National Academy of Medicine. But the AMA disputes that scope expansions are necessary to increase access to care.
The organization that represents the nation’s physicians said in a prepared statement that it opposes scope expansions because removing doctors from the care team results in higher costs to the patient and lower quality care.
Several nursing organizations swiftly criticized the policy recommendation, including the American Nurses Association, the American Association of Nurse Practitioners, and the National Council of State Boards of Nursing.
The policy shift would create more administrative burdens for APRNs and generate “a downstream effect that only hurts patients,” particularly those in underserved communities without timely access to care, ANA president Jennifer Mensik Kennedy, PhD, MBA, RN, NEA-BC, told this news organization.
“The licensing and regulation of APRNs have never required the oversight of state medical boards,” she said, adding that it should remain the obligation of nursing regulatory bodies.
Jon Fanning, MS, CAE, CNED, chief executive officer of the AANP, called the AMA proposal “flawed.”
“The restrictive involvement of the board of medicine directly contributes to health care access challenges, resulting in continued low health care rankings, geographic disparities in care, and unnecessary regulatory cost in these states,” he said in a press release.
Still, the AMA has vowed to #StopScopeCreep. Securing stricter practice guidelines was a central theme of the association’s recent annual meeting and a goal of its plan to strengthen the physician workforce. The organization invests heavily in advocacy and education efforts to defeat state bills seeking to extend APRN authority. To that end, the AMA Scope of Practice Partnership, a coalition of over 100 medical associations, has awarded members $3.5 million in grants to combat scope-expansion legislation.
The AMA and the American College of Radiology recently partnered to create advocacy materials, including handouts encouraging patients to ask questions such as: “Will a physician be reviewing my chart, lab results, x-rays, and other tests?”
The policy recommendation comes as concerns mount over the potential for significant physician shortages, fueled partly by older physicians’ retirements and doctors reducing hours or exiting the workforce due to pandemic fatigue and burnout.
While practice regulations vary by state, a new federal bill could change that by broadening the authority of APRNs under Medicare and Medicaid guidelines. Introduced in the U.S. House of Representatives in April and supported by the ANA, the Improving Care and Access to Nurses Act would allow APRNs to perform more procedures, including cardiac and pulmonary rehabilitation and certification of terminal illness for hospice, according to an ANA press release.
In the meantime, several state legislatures are considering bills that would expand APRN scope of practice. Utah is the latest to join a growing list of states – about half now – offering full practice authority to NPs.
Other states offer a reduced scope of practice for APRNs, typically requiring a collaborative agreement with a supervising physician. The remaining states enforce tighter regulations and physician oversight.
A recent Medscape survey found that most physicians report having a good rapport with NPs but many have mixed feelings about giving them expanded practice roles, with one-third saying it would harm patient care. Feelings were only slightly more favorable toward PAs. However, about 75% of patients were either neutral or supportive of independent practice for NPs and PAs.
A version of this article first appeared on Medscape.com.
Nearly one in five in U.S. still hadn’t gotten COVID by end of 2022
, according to a new estimate.
The findings came from an analysis of blood donations. The Centers for Disease Control and Prevention analyzed donor blood from 143,000 people every 3 months during 2022, looking for the presence of COVID antibodies that meant a person had previously been infected with the virus. The prevalence of antibodies from previous infections steadily rose throughout the year. Antibodies from prior infection were found in 49% of donors as of Feb. 15, 2022, 59% of donors as of May 15, 2022, 70% of donors as of Aug. 15, 2022, and 78% of donors as of Nov. 15, 2022.
Donor blood also was analyzed for the presence of antibodies known to come from COVID vaccination. When the vaccine-induced and infection-induced antibody data were combined, the CDC estimated that 97% of people had antibodies as of the end of the 2022.
In the report, CDC authors explained that while the presence of antibodies is related to protection from infection and to less severe disease, the level of antibodies that a person has can vary. The authors said that no standards have yet been set that show a minimum level of antibodies needed to provide protection.
As of July 3, more than 1.1 million people had died in the United States from COVID-19, according to CDC data. Deaths for the first half of 2023 are down dramatically, compared with the first 3 years of the pandemic, with just 41,538 death certificates this year listing the virus as an underlying or contributing cause. About two in three COVID deaths this year occurred in a hospital or nursing home, and 89% of people who died from the virus this year have been age 65 or older.
A version of this article first appeared on WebMD.com.
, according to a new estimate.
The findings came from an analysis of blood donations. The Centers for Disease Control and Prevention analyzed donor blood from 143,000 people every 3 months during 2022, looking for the presence of COVID antibodies that meant a person had previously been infected with the virus. The prevalence of antibodies from previous infections steadily rose throughout the year. Antibodies from prior infection were found in 49% of donors as of Feb. 15, 2022, 59% of donors as of May 15, 2022, 70% of donors as of Aug. 15, 2022, and 78% of donors as of Nov. 15, 2022.
Donor blood also was analyzed for the presence of antibodies known to come from COVID vaccination. When the vaccine-induced and infection-induced antibody data were combined, the CDC estimated that 97% of people had antibodies as of the end of the 2022.
In the report, CDC authors explained that while the presence of antibodies is related to protection from infection and to less severe disease, the level of antibodies that a person has can vary. The authors said that no standards have yet been set that show a minimum level of antibodies needed to provide protection.
As of July 3, more than 1.1 million people had died in the United States from COVID-19, according to CDC data. Deaths for the first half of 2023 are down dramatically, compared with the first 3 years of the pandemic, with just 41,538 death certificates this year listing the virus as an underlying or contributing cause. About two in three COVID deaths this year occurred in a hospital or nursing home, and 89% of people who died from the virus this year have been age 65 or older.
A version of this article first appeared on WebMD.com.
, according to a new estimate.
The findings came from an analysis of blood donations. The Centers for Disease Control and Prevention analyzed donor blood from 143,000 people every 3 months during 2022, looking for the presence of COVID antibodies that meant a person had previously been infected with the virus. The prevalence of antibodies from previous infections steadily rose throughout the year. Antibodies from prior infection were found in 49% of donors as of Feb. 15, 2022, 59% of donors as of May 15, 2022, 70% of donors as of Aug. 15, 2022, and 78% of donors as of Nov. 15, 2022.
Donor blood also was analyzed for the presence of antibodies known to come from COVID vaccination. When the vaccine-induced and infection-induced antibody data were combined, the CDC estimated that 97% of people had antibodies as of the end of the 2022.
In the report, CDC authors explained that while the presence of antibodies is related to protection from infection and to less severe disease, the level of antibodies that a person has can vary. The authors said that no standards have yet been set that show a minimum level of antibodies needed to provide protection.
As of July 3, more than 1.1 million people had died in the United States from COVID-19, according to CDC data. Deaths for the first half of 2023 are down dramatically, compared with the first 3 years of the pandemic, with just 41,538 death certificates this year listing the virus as an underlying or contributing cause. About two in three COVID deaths this year occurred in a hospital or nursing home, and 89% of people who died from the virus this year have been age 65 or older.
A version of this article first appeared on WebMD.com.
The ‘psychological warfare’ of prior authorization
Shikha Jain, MD, felt the urgency of the moment.
It was 10:00 AM. A young patient had stepped into her Chicago cancer clinic. His face was red, and he was struggling to breathe.
The man had primary mediastinal B-cell lymphoma, a rare, aggressive form of non-Hodgkin lymphoma. Many cases involve large, fast‐growing masses that expand into the lungs and compress respiratory pathways, sometimes leaving patients breathless.
Dr. Jain rushed to his side and walked him from the clinic to an ICU bed at the hospital nearby.
“He was so sick,” recalled Dr. Jain, currently a tenured associate professor of medicine in the division of hematology and oncology at the University of Illinois Cancer Center, Chicago. “He needed chemotherapy immediately.”
The standard chemotherapy regimen at the time – R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) – required prior authorization.
Dr. Jain’s patient did not have days to wait, so Dr. Jain requested an expedited approval. The insurance company responded quickly, denying the request for treatment.
That evening, after hours on the phone trying to reverse the denial, Dr. Jain was able to arrange a peer-to-peer conversation with the insurer. She explained her patient’s pressing need for chemotherapy: He would die if he continued to wait.
But Dr. Jain’s argument did not move the reviewer. At that point, she had reached her limit.
“I asked for the gentleman’s full name. I told him he would be responsible for this 30-year-old man’s death, and my next call would be to CNN,” Dr. Jain told this news organization. “And that is how I got my patient’s chemotherapy approved.”
Her patient received the regimen that evening. He later went into remission.
This incident occurred almost a decade ago, but it has stayed with Dr. Jain. She knows that her persistence in that moment meant the difference between her patient’s life and death.
There was the denial for standard-of-care staging and surveillance imaging – dotatate PET/CT – for her patient with neuroendocrine cancer. “The specific insurance company simply doesn’t approve this imaging, despite being around for years,” she said.
There was the patient with metastatic colon cancer who needed third-line therapy. His insurer took more than a month to reverse its denial for a recently approved drug, and in that time, the man’s disease progressed. “He eventually succumbed to the cancer after receiving the drug, but it’s unclear if his life was cut short by the delay in care,” Dr. Jain said.
And there is the maze of insurance company phone calls and transfers. On one call, Dr. Jain recalled being transferred six times before being connected to the right department to discuss approving standard-of-care chemotherapy for a patient. After being denied approval, Dr. Jain was put on hold to speak with a manager, and the call was abruptly disconnected.
“I have wasted so many hours on prior authorization and have seen months and months of patient care delays,” Dr. Jain said. “It’s easy to see why people just give up.”
For Dr. Jain, prior authorization has begun to “feel like psychological warfare,” she said. “To have everything questioned by people who don’t understand the basics of oncology is demoralizing.”
The growing administrative – and emotional – burden of prior authorization is contributing to physician burnout.
According to Medscape’s ‘I Cry but No One Cares’: Physician Burnout & Depression Report 2023, more than half of oncologists reported feeling burned out this year – the highest percentage in 5 years. When asked what factors led to burnout, most doctors surveyed pointed to an overabundance of bureaucratic tasks, and specifically, “insurance companies telling me how to practice medicine and controlling what the patients can and can’t do.”
“Burnout is a real problem in medicine,” said Kelly Anderson, PhD, MPP, assistant professor in the department of clinical pharmacy, University of Colorado at Denver, Aurora. “While there are many factors that contribute to burnout, prior authorization is certainly one.”
In a 2022 survey from the American Medical Association, 88% of respondents reported that the burden associated with prior authorization requirements was “high or extremely high.”
Although insurers argue that prior authorization cuts down on unnecessary and expensive care, physicians in the AMA survey reported that this practice often leads to greater overall use of health care resources, including more emergency department and office visits.
“Insurers are confident that prior authorization is saving money overall, but there’s also no clear evidence of that,” Dr. Anderson noted. “Prior authorization may reduce spending without harming patients in some instances, but in others, it’s adding administrative burden, costs, and may be causing harm to patients.”
A version of this article originally appeared on Medscape.com.
Shikha Jain, MD, felt the urgency of the moment.
It was 10:00 AM. A young patient had stepped into her Chicago cancer clinic. His face was red, and he was struggling to breathe.
The man had primary mediastinal B-cell lymphoma, a rare, aggressive form of non-Hodgkin lymphoma. Many cases involve large, fast‐growing masses that expand into the lungs and compress respiratory pathways, sometimes leaving patients breathless.
Dr. Jain rushed to his side and walked him from the clinic to an ICU bed at the hospital nearby.
“He was so sick,” recalled Dr. Jain, currently a tenured associate professor of medicine in the division of hematology and oncology at the University of Illinois Cancer Center, Chicago. “He needed chemotherapy immediately.”
The standard chemotherapy regimen at the time – R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) – required prior authorization.
Dr. Jain’s patient did not have days to wait, so Dr. Jain requested an expedited approval. The insurance company responded quickly, denying the request for treatment.
That evening, after hours on the phone trying to reverse the denial, Dr. Jain was able to arrange a peer-to-peer conversation with the insurer. She explained her patient’s pressing need for chemotherapy: He would die if he continued to wait.
But Dr. Jain’s argument did not move the reviewer. At that point, she had reached her limit.
“I asked for the gentleman’s full name. I told him he would be responsible for this 30-year-old man’s death, and my next call would be to CNN,” Dr. Jain told this news organization. “And that is how I got my patient’s chemotherapy approved.”
Her patient received the regimen that evening. He later went into remission.
This incident occurred almost a decade ago, but it has stayed with Dr. Jain. She knows that her persistence in that moment meant the difference between her patient’s life and death.
There was the denial for standard-of-care staging and surveillance imaging – dotatate PET/CT – for her patient with neuroendocrine cancer. “The specific insurance company simply doesn’t approve this imaging, despite being around for years,” she said.
There was the patient with metastatic colon cancer who needed third-line therapy. His insurer took more than a month to reverse its denial for a recently approved drug, and in that time, the man’s disease progressed. “He eventually succumbed to the cancer after receiving the drug, but it’s unclear if his life was cut short by the delay in care,” Dr. Jain said.
And there is the maze of insurance company phone calls and transfers. On one call, Dr. Jain recalled being transferred six times before being connected to the right department to discuss approving standard-of-care chemotherapy for a patient. After being denied approval, Dr. Jain was put on hold to speak with a manager, and the call was abruptly disconnected.
“I have wasted so many hours on prior authorization and have seen months and months of patient care delays,” Dr. Jain said. “It’s easy to see why people just give up.”
For Dr. Jain, prior authorization has begun to “feel like psychological warfare,” she said. “To have everything questioned by people who don’t understand the basics of oncology is demoralizing.”
The growing administrative – and emotional – burden of prior authorization is contributing to physician burnout.
According to Medscape’s ‘I Cry but No One Cares’: Physician Burnout & Depression Report 2023, more than half of oncologists reported feeling burned out this year – the highest percentage in 5 years. When asked what factors led to burnout, most doctors surveyed pointed to an overabundance of bureaucratic tasks, and specifically, “insurance companies telling me how to practice medicine and controlling what the patients can and can’t do.”
“Burnout is a real problem in medicine,” said Kelly Anderson, PhD, MPP, assistant professor in the department of clinical pharmacy, University of Colorado at Denver, Aurora. “While there are many factors that contribute to burnout, prior authorization is certainly one.”
In a 2022 survey from the American Medical Association, 88% of respondents reported that the burden associated with prior authorization requirements was “high or extremely high.”
Although insurers argue that prior authorization cuts down on unnecessary and expensive care, physicians in the AMA survey reported that this practice often leads to greater overall use of health care resources, including more emergency department and office visits.
“Insurers are confident that prior authorization is saving money overall, but there’s also no clear evidence of that,” Dr. Anderson noted. “Prior authorization may reduce spending without harming patients in some instances, but in others, it’s adding administrative burden, costs, and may be causing harm to patients.”
A version of this article originally appeared on Medscape.com.
Shikha Jain, MD, felt the urgency of the moment.
It was 10:00 AM. A young patient had stepped into her Chicago cancer clinic. His face was red, and he was struggling to breathe.
The man had primary mediastinal B-cell lymphoma, a rare, aggressive form of non-Hodgkin lymphoma. Many cases involve large, fast‐growing masses that expand into the lungs and compress respiratory pathways, sometimes leaving patients breathless.
Dr. Jain rushed to his side and walked him from the clinic to an ICU bed at the hospital nearby.
“He was so sick,” recalled Dr. Jain, currently a tenured associate professor of medicine in the division of hematology and oncology at the University of Illinois Cancer Center, Chicago. “He needed chemotherapy immediately.”
The standard chemotherapy regimen at the time – R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone) – required prior authorization.
Dr. Jain’s patient did not have days to wait, so Dr. Jain requested an expedited approval. The insurance company responded quickly, denying the request for treatment.
That evening, after hours on the phone trying to reverse the denial, Dr. Jain was able to arrange a peer-to-peer conversation with the insurer. She explained her patient’s pressing need for chemotherapy: He would die if he continued to wait.
But Dr. Jain’s argument did not move the reviewer. At that point, she had reached her limit.
“I asked for the gentleman’s full name. I told him he would be responsible for this 30-year-old man’s death, and my next call would be to CNN,” Dr. Jain told this news organization. “And that is how I got my patient’s chemotherapy approved.”
Her patient received the regimen that evening. He later went into remission.
This incident occurred almost a decade ago, but it has stayed with Dr. Jain. She knows that her persistence in that moment meant the difference between her patient’s life and death.
There was the denial for standard-of-care staging and surveillance imaging – dotatate PET/CT – for her patient with neuroendocrine cancer. “The specific insurance company simply doesn’t approve this imaging, despite being around for years,” she said.
There was the patient with metastatic colon cancer who needed third-line therapy. His insurer took more than a month to reverse its denial for a recently approved drug, and in that time, the man’s disease progressed. “He eventually succumbed to the cancer after receiving the drug, but it’s unclear if his life was cut short by the delay in care,” Dr. Jain said.
And there is the maze of insurance company phone calls and transfers. On one call, Dr. Jain recalled being transferred six times before being connected to the right department to discuss approving standard-of-care chemotherapy for a patient. After being denied approval, Dr. Jain was put on hold to speak with a manager, and the call was abruptly disconnected.
“I have wasted so many hours on prior authorization and have seen months and months of patient care delays,” Dr. Jain said. “It’s easy to see why people just give up.”
For Dr. Jain, prior authorization has begun to “feel like psychological warfare,” she said. “To have everything questioned by people who don’t understand the basics of oncology is demoralizing.”
The growing administrative – and emotional – burden of prior authorization is contributing to physician burnout.
According to Medscape’s ‘I Cry but No One Cares’: Physician Burnout & Depression Report 2023, more than half of oncologists reported feeling burned out this year – the highest percentage in 5 years. When asked what factors led to burnout, most doctors surveyed pointed to an overabundance of bureaucratic tasks, and specifically, “insurance companies telling me how to practice medicine and controlling what the patients can and can’t do.”
“Burnout is a real problem in medicine,” said Kelly Anderson, PhD, MPP, assistant professor in the department of clinical pharmacy, University of Colorado at Denver, Aurora. “While there are many factors that contribute to burnout, prior authorization is certainly one.”
In a 2022 survey from the American Medical Association, 88% of respondents reported that the burden associated with prior authorization requirements was “high or extremely high.”
Although insurers argue that prior authorization cuts down on unnecessary and expensive care, physicians in the AMA survey reported that this practice often leads to greater overall use of health care resources, including more emergency department and office visits.
“Insurers are confident that prior authorization is saving money overall, but there’s also no clear evidence of that,” Dr. Anderson noted. “Prior authorization may reduce spending without harming patients in some instances, but in others, it’s adding administrative burden, costs, and may be causing harm to patients.”
A version of this article originally appeared on Medscape.com.
Parsing the split-decision victory for biologics in COPD
It’s tough to keep up with the proliferation of monoclonal antibodies. Seems every day I’m confronted by a patient who’s using a new drug with a name ending in “mab.” That drug blocks a cellular receptor I haven’t heard of that’s involved in a cascade of interactions I haven’t thought about since medical school. The resulting disruption reduces disease burden, typically at great expense to the medical system, the patient, or both. We’ve truly entered the era of precision medicine. It’s not enough to understand disease; you also must know its heterogeneous expression so that you can prescribe the ‘mab that targets the biology responsible for variants in behavior. All diseases are, in fact, syndromes. This isn’t a bad thing, but it’s a challenge.
A series of ‘mabs have been approved for treating type 2 high (TH2) or eosinophilic asthma. We refer to this group of ‘mabs generically as biologics. The group includes omalizumab, mepolizumab, dupilumab, benralizumab, reslizumab, and tezepelumab. While mechanism of action varies slightly across drugs, the biologics all target a specific arm of the immune system. Efficacy is linearly related to serum eosinophil count and there’s little clinically or pharmacologically to distinguish one from another. Of course, no head-to-head comparisons of efficacy are available and there’s no financial incentive for them to be performed.
Latest research
A new randomized controlled trial (RCT) of dupilumab for chronic obstructive pulmonary disease (COPD) adds to the aforementioned biologic knowledge base. Turns out it works as long as the patients are carefully selected. Researchers enrolled GOLD D (or E depending on which iteration of the GOLD Statement you use) patients on triple inhaler therapy (inhaled corticosteroids [ICS]/long-acting beta-agonist [LABA]/long-acting muscarinic antagonist [LAMA]) with two moderate exacerbations or one exacerbation requiring hospitalization in the past year. Blood eosinophil counts were > 300 cells/mcL and chronic bronchitis was present clinically. The primary and multiple secondary outcomes were improved with dupilumab.
This is welcome news. I’ve treated countless patients with severe COPD who have repeated exacerbations despite my efforts to prevent them. These patients are on ICS/LABA/LAMA and azithromycin or roflumilast, and occasionally both. While every COPD guideline known to man forbids using chronic oral corticosteroids (OCS), I’ve prescribed them repeatedly because the benefits to keeping a recalcitrant, exacerbating patient out of the hospital seem to outweigh OCS risks. It would be nice to have a better option. Although we were taught that they were immutably distinct in medical school, every first-year pulmonary fellow knows that asthma and COPD share more similarities than differences, so it makes sense that proven asthma therapies would work for some patients with COPD.
However, the dupilumab study must be placed in context. Past studies haven’t been as positive. In 2017, two separate RCTs found that mepolizumab reduced the annual rate of moderate to severe exacerbations (primary outcome) in one trial but not the other. Interpretation gets more complicated when broken down by intention to treat (ITT) vs. modified ITT and when secondary outcomes are considered. Sparing you those details, this trial does not instill confidence, leading the Food and Drug Administration to refuse approval for mepolizumab for COPD. A second RCT of benralizumab for COPD was published in 2019. Much less cognitive load was required to interpret this one; it was negative. FDA approval was not requested.
Looking through the trial designs for the three RCTs of biologics for COPD, I couldn’t find major differences that could explain the discordant results. Sample size and enrollment criteria were similar. As stated, I don’t believe that the biologic data in asthma allow for predicting efficacy in one eosinophilic patient vs. another and I assume the same would be true for COPD. All three trials found that eosinophils were eliminated, so responses were biologically equivalent.
Key takeaways
If trial design and pharmacology don’t account for the disparate outcomes, how do we explain them? More important, how do we translate these trials into clinical practice? I looked for a review or editorial by a scientist-clinician smarter than I so I could steal their ideas and express them as pedantic euphemisms here. I found it curious that I was unable to find one. A recent publication in the American Journal of Respiratory and Critical Care Medicine suggests that the answer lies within the complex lattice of eosinophil subtypes, but I’m unqualified to judge the veracity of this “phenotype within a phenotype” theory.
More trials in COPD are being done. We should have results on tezepelumab, that great savior that may cover noneosinophilic asthma phenotypes, within the next few years. Until then, we’re stuck defying guidelines with the anachronistic use of OCS for the COPD patient who exacerbates through ICS/LABA/LAMA, roflumilast, and azithromycin.
Dr. Holley is professor of medicine at Uniformed Services University in Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington. He reported receiving income from CHEST College, Metapharm, and WebMD.
A version of this article first appeared on Medscape.com.
It’s tough to keep up with the proliferation of monoclonal antibodies. Seems every day I’m confronted by a patient who’s using a new drug with a name ending in “mab.” That drug blocks a cellular receptor I haven’t heard of that’s involved in a cascade of interactions I haven’t thought about since medical school. The resulting disruption reduces disease burden, typically at great expense to the medical system, the patient, or both. We’ve truly entered the era of precision medicine. It’s not enough to understand disease; you also must know its heterogeneous expression so that you can prescribe the ‘mab that targets the biology responsible for variants in behavior. All diseases are, in fact, syndromes. This isn’t a bad thing, but it’s a challenge.
A series of ‘mabs have been approved for treating type 2 high (TH2) or eosinophilic asthma. We refer to this group of ‘mabs generically as biologics. The group includes omalizumab, mepolizumab, dupilumab, benralizumab, reslizumab, and tezepelumab. While mechanism of action varies slightly across drugs, the biologics all target a specific arm of the immune system. Efficacy is linearly related to serum eosinophil count and there’s little clinically or pharmacologically to distinguish one from another. Of course, no head-to-head comparisons of efficacy are available and there’s no financial incentive for them to be performed.
Latest research
A new randomized controlled trial (RCT) of dupilumab for chronic obstructive pulmonary disease (COPD) adds to the aforementioned biologic knowledge base. Turns out it works as long as the patients are carefully selected. Researchers enrolled GOLD D (or E depending on which iteration of the GOLD Statement you use) patients on triple inhaler therapy (inhaled corticosteroids [ICS]/long-acting beta-agonist [LABA]/long-acting muscarinic antagonist [LAMA]) with two moderate exacerbations or one exacerbation requiring hospitalization in the past year. Blood eosinophil counts were > 300 cells/mcL and chronic bronchitis was present clinically. The primary and multiple secondary outcomes were improved with dupilumab.
This is welcome news. I’ve treated countless patients with severe COPD who have repeated exacerbations despite my efforts to prevent them. These patients are on ICS/LABA/LAMA and azithromycin or roflumilast, and occasionally both. While every COPD guideline known to man forbids using chronic oral corticosteroids (OCS), I’ve prescribed them repeatedly because the benefits to keeping a recalcitrant, exacerbating patient out of the hospital seem to outweigh OCS risks. It would be nice to have a better option. Although we were taught that they were immutably distinct in medical school, every first-year pulmonary fellow knows that asthma and COPD share more similarities than differences, so it makes sense that proven asthma therapies would work for some patients with COPD.
However, the dupilumab study must be placed in context. Past studies haven’t been as positive. In 2017, two separate RCTs found that mepolizumab reduced the annual rate of moderate to severe exacerbations (primary outcome) in one trial but not the other. Interpretation gets more complicated when broken down by intention to treat (ITT) vs. modified ITT and when secondary outcomes are considered. Sparing you those details, this trial does not instill confidence, leading the Food and Drug Administration to refuse approval for mepolizumab for COPD. A second RCT of benralizumab for COPD was published in 2019. Much less cognitive load was required to interpret this one; it was negative. FDA approval was not requested.
Looking through the trial designs for the three RCTs of biologics for COPD, I couldn’t find major differences that could explain the discordant results. Sample size and enrollment criteria were similar. As stated, I don’t believe that the biologic data in asthma allow for predicting efficacy in one eosinophilic patient vs. another and I assume the same would be true for COPD. All three trials found that eosinophils were eliminated, so responses were biologically equivalent.
Key takeaways
If trial design and pharmacology don’t account for the disparate outcomes, how do we explain them? More important, how do we translate these trials into clinical practice? I looked for a review or editorial by a scientist-clinician smarter than I so I could steal their ideas and express them as pedantic euphemisms here. I found it curious that I was unable to find one. A recent publication in the American Journal of Respiratory and Critical Care Medicine suggests that the answer lies within the complex lattice of eosinophil subtypes, but I’m unqualified to judge the veracity of this “phenotype within a phenotype” theory.
More trials in COPD are being done. We should have results on tezepelumab, that great savior that may cover noneosinophilic asthma phenotypes, within the next few years. Until then, we’re stuck defying guidelines with the anachronistic use of OCS for the COPD patient who exacerbates through ICS/LABA/LAMA, roflumilast, and azithromycin.
Dr. Holley is professor of medicine at Uniformed Services University in Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington. He reported receiving income from CHEST College, Metapharm, and WebMD.
A version of this article first appeared on Medscape.com.
It’s tough to keep up with the proliferation of monoclonal antibodies. Seems every day I’m confronted by a patient who’s using a new drug with a name ending in “mab.” That drug blocks a cellular receptor I haven’t heard of that’s involved in a cascade of interactions I haven’t thought about since medical school. The resulting disruption reduces disease burden, typically at great expense to the medical system, the patient, or both. We’ve truly entered the era of precision medicine. It’s not enough to understand disease; you also must know its heterogeneous expression so that you can prescribe the ‘mab that targets the biology responsible for variants in behavior. All diseases are, in fact, syndromes. This isn’t a bad thing, but it’s a challenge.
A series of ‘mabs have been approved for treating type 2 high (TH2) or eosinophilic asthma. We refer to this group of ‘mabs generically as biologics. The group includes omalizumab, mepolizumab, dupilumab, benralizumab, reslizumab, and tezepelumab. While mechanism of action varies slightly across drugs, the biologics all target a specific arm of the immune system. Efficacy is linearly related to serum eosinophil count and there’s little clinically or pharmacologically to distinguish one from another. Of course, no head-to-head comparisons of efficacy are available and there’s no financial incentive for them to be performed.
Latest research
A new randomized controlled trial (RCT) of dupilumab for chronic obstructive pulmonary disease (COPD) adds to the aforementioned biologic knowledge base. Turns out it works as long as the patients are carefully selected. Researchers enrolled GOLD D (or E depending on which iteration of the GOLD Statement you use) patients on triple inhaler therapy (inhaled corticosteroids [ICS]/long-acting beta-agonist [LABA]/long-acting muscarinic antagonist [LAMA]) with two moderate exacerbations or one exacerbation requiring hospitalization in the past year. Blood eosinophil counts were > 300 cells/mcL and chronic bronchitis was present clinically. The primary and multiple secondary outcomes were improved with dupilumab.
This is welcome news. I’ve treated countless patients with severe COPD who have repeated exacerbations despite my efforts to prevent them. These patients are on ICS/LABA/LAMA and azithromycin or roflumilast, and occasionally both. While every COPD guideline known to man forbids using chronic oral corticosteroids (OCS), I’ve prescribed them repeatedly because the benefits to keeping a recalcitrant, exacerbating patient out of the hospital seem to outweigh OCS risks. It would be nice to have a better option. Although we were taught that they were immutably distinct in medical school, every first-year pulmonary fellow knows that asthma and COPD share more similarities than differences, so it makes sense that proven asthma therapies would work for some patients with COPD.
However, the dupilumab study must be placed in context. Past studies haven’t been as positive. In 2017, two separate RCTs found that mepolizumab reduced the annual rate of moderate to severe exacerbations (primary outcome) in one trial but not the other. Interpretation gets more complicated when broken down by intention to treat (ITT) vs. modified ITT and when secondary outcomes are considered. Sparing you those details, this trial does not instill confidence, leading the Food and Drug Administration to refuse approval for mepolizumab for COPD. A second RCT of benralizumab for COPD was published in 2019. Much less cognitive load was required to interpret this one; it was negative. FDA approval was not requested.
Looking through the trial designs for the three RCTs of biologics for COPD, I couldn’t find major differences that could explain the discordant results. Sample size and enrollment criteria were similar. As stated, I don’t believe that the biologic data in asthma allow for predicting efficacy in one eosinophilic patient vs. another and I assume the same would be true for COPD. All three trials found that eosinophils were eliminated, so responses were biologically equivalent.
Key takeaways
If trial design and pharmacology don’t account for the disparate outcomes, how do we explain them? More important, how do we translate these trials into clinical practice? I looked for a review or editorial by a scientist-clinician smarter than I so I could steal their ideas and express them as pedantic euphemisms here. I found it curious that I was unable to find one. A recent publication in the American Journal of Respiratory and Critical Care Medicine suggests that the answer lies within the complex lattice of eosinophil subtypes, but I’m unqualified to judge the veracity of this “phenotype within a phenotype” theory.
More trials in COPD are being done. We should have results on tezepelumab, that great savior that may cover noneosinophilic asthma phenotypes, within the next few years. Until then, we’re stuck defying guidelines with the anachronistic use of OCS for the COPD patient who exacerbates through ICS/LABA/LAMA, roflumilast, and azithromycin.
Dr. Holley is professor of medicine at Uniformed Services University in Bethesda, Md., and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington. He reported receiving income from CHEST College, Metapharm, and WebMD.
A version of this article first appeared on Medscape.com.
Coffee’s brain-boosting effect goes beyond caffeine
“There is a widespread anticipation that coffee boosts alertness and psychomotor performance. By gaining a deeper understanding of the mechanisms underlying this biological phenomenon, we pave the way for investigating the factors that can influence it and even exploring the potential advantages of those mechanisms,” study investigator Nuno Sousa, MD, PhD, with the University of Minho, Braga, Portugal, said in a statement.
The study was published online in Frontiers in Behavioral Neuroscience.
Caffeine can’t take all the credit
Certain compounds in coffee, including caffeine and chlorogenic acids, have well-documented psychoactive effects, but the psychological impact of coffee/caffeine consumption as a whole remains a matter of debate.
The researchers investigated the neurobiological impact of coffee drinking on brain connectivity using resting-state functional MRI (fMRI).
They recruited 47 generally healthy adults (mean age, 30 years; 31 women) who regularly drank a minimum of one cup of coffee per day. Participants refrained from eating or drinking caffeinated beverages for at least 3 hours prior to undergoing fMRI.
To tease out the specific impact of caffeinated coffee intake, 30 habitual coffee drinkers (mean age, 32 years; 27 women) were given hot water containing the same amount of caffeine, but they were not given coffee.
The investigators conducted two fMRI scans – one before, and one 30 minutes after drinking coffee or caffeine-infused water.
Both drinking coffee and drinking plain caffeine in water led to a decrease in functional connectivity of the brain’s default mode network, which is typically active during self-reflection in resting states.
This finding suggests that consuming either coffee or caffeine heightened individuals’ readiness to transition from a state of rest to engaging in task-related activities, the researchers noted.
However, drinking a cup of coffee also boosted connectivity in the higher visual network and the right executive control network, which are linked to working memory, cognitive control, and goal-directed behavior – something that did not occur from drinking caffeinated water.
“Put simply, individuals exhibited a heightened state of preparedness, being more responsive and attentive to external stimuli after drinking coffee,” said first author Maria Picó-Pérez, PhD, with the University of Minho.
Given that some of the effects of coffee also occurred with caffeine alone, it’s “plausible to assume that other caffeinated beverages may share similar effects,” she added.
Still, certain effects were specific to coffee drinking, “likely influenced by factors such as the distinct aroma and taste of coffee or the psychological expectations associated with consuming this particular beverage,” the researcher wrote.
The investigators report that the observations could provide a scientific foundation for the common belief that coffee increases alertness and cognitive functioning. Further research is needed to differentiate the effects of caffeine from the overall experience of drinking coffee.
A limitation of the study is the absence of a nondrinker control sample (to rule out the withdrawal effect) or an alternative group that consumed decaffeinated coffee (to rule out the placebo effect of coffee intake) – something that should be considered in future studies, the researchers noted.
The study was funded by the Institute for the Scientific Information on Coffee. The authors declared no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
“There is a widespread anticipation that coffee boosts alertness and psychomotor performance. By gaining a deeper understanding of the mechanisms underlying this biological phenomenon, we pave the way for investigating the factors that can influence it and even exploring the potential advantages of those mechanisms,” study investigator Nuno Sousa, MD, PhD, with the University of Minho, Braga, Portugal, said in a statement.
The study was published online in Frontiers in Behavioral Neuroscience.
Caffeine can’t take all the credit
Certain compounds in coffee, including caffeine and chlorogenic acids, have well-documented psychoactive effects, but the psychological impact of coffee/caffeine consumption as a whole remains a matter of debate.
The researchers investigated the neurobiological impact of coffee drinking on brain connectivity using resting-state functional MRI (fMRI).
They recruited 47 generally healthy adults (mean age, 30 years; 31 women) who regularly drank a minimum of one cup of coffee per day. Participants refrained from eating or drinking caffeinated beverages for at least 3 hours prior to undergoing fMRI.
To tease out the specific impact of caffeinated coffee intake, 30 habitual coffee drinkers (mean age, 32 years; 27 women) were given hot water containing the same amount of caffeine, but they were not given coffee.
The investigators conducted two fMRI scans – one before, and one 30 minutes after drinking coffee or caffeine-infused water.
Both drinking coffee and drinking plain caffeine in water led to a decrease in functional connectivity of the brain’s default mode network, which is typically active during self-reflection in resting states.
This finding suggests that consuming either coffee or caffeine heightened individuals’ readiness to transition from a state of rest to engaging in task-related activities, the researchers noted.
However, drinking a cup of coffee also boosted connectivity in the higher visual network and the right executive control network, which are linked to working memory, cognitive control, and goal-directed behavior – something that did not occur from drinking caffeinated water.
“Put simply, individuals exhibited a heightened state of preparedness, being more responsive and attentive to external stimuli after drinking coffee,” said first author Maria Picó-Pérez, PhD, with the University of Minho.
Given that some of the effects of coffee also occurred with caffeine alone, it’s “plausible to assume that other caffeinated beverages may share similar effects,” she added.
Still, certain effects were specific to coffee drinking, “likely influenced by factors such as the distinct aroma and taste of coffee or the psychological expectations associated with consuming this particular beverage,” the researcher wrote.
The investigators report that the observations could provide a scientific foundation for the common belief that coffee increases alertness and cognitive functioning. Further research is needed to differentiate the effects of caffeine from the overall experience of drinking coffee.
A limitation of the study is the absence of a nondrinker control sample (to rule out the withdrawal effect) or an alternative group that consumed decaffeinated coffee (to rule out the placebo effect of coffee intake) – something that should be considered in future studies, the researchers noted.
The study was funded by the Institute for the Scientific Information on Coffee. The authors declared no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
“There is a widespread anticipation that coffee boosts alertness and psychomotor performance. By gaining a deeper understanding of the mechanisms underlying this biological phenomenon, we pave the way for investigating the factors that can influence it and even exploring the potential advantages of those mechanisms,” study investigator Nuno Sousa, MD, PhD, with the University of Minho, Braga, Portugal, said in a statement.
The study was published online in Frontiers in Behavioral Neuroscience.
Caffeine can’t take all the credit
Certain compounds in coffee, including caffeine and chlorogenic acids, have well-documented psychoactive effects, but the psychological impact of coffee/caffeine consumption as a whole remains a matter of debate.
The researchers investigated the neurobiological impact of coffee drinking on brain connectivity using resting-state functional MRI (fMRI).
They recruited 47 generally healthy adults (mean age, 30 years; 31 women) who regularly drank a minimum of one cup of coffee per day. Participants refrained from eating or drinking caffeinated beverages for at least 3 hours prior to undergoing fMRI.
To tease out the specific impact of caffeinated coffee intake, 30 habitual coffee drinkers (mean age, 32 years; 27 women) were given hot water containing the same amount of caffeine, but they were not given coffee.
The investigators conducted two fMRI scans – one before, and one 30 minutes after drinking coffee or caffeine-infused water.
Both drinking coffee and drinking plain caffeine in water led to a decrease in functional connectivity of the brain’s default mode network, which is typically active during self-reflection in resting states.
This finding suggests that consuming either coffee or caffeine heightened individuals’ readiness to transition from a state of rest to engaging in task-related activities, the researchers noted.
However, drinking a cup of coffee also boosted connectivity in the higher visual network and the right executive control network, which are linked to working memory, cognitive control, and goal-directed behavior – something that did not occur from drinking caffeinated water.
“Put simply, individuals exhibited a heightened state of preparedness, being more responsive and attentive to external stimuli after drinking coffee,” said first author Maria Picó-Pérez, PhD, with the University of Minho.
Given that some of the effects of coffee also occurred with caffeine alone, it’s “plausible to assume that other caffeinated beverages may share similar effects,” she added.
Still, certain effects were specific to coffee drinking, “likely influenced by factors such as the distinct aroma and taste of coffee or the psychological expectations associated with consuming this particular beverage,” the researcher wrote.
The investigators report that the observations could provide a scientific foundation for the common belief that coffee increases alertness and cognitive functioning. Further research is needed to differentiate the effects of caffeine from the overall experience of drinking coffee.
A limitation of the study is the absence of a nondrinker control sample (to rule out the withdrawal effect) or an alternative group that consumed decaffeinated coffee (to rule out the placebo effect of coffee intake) – something that should be considered in future studies, the researchers noted.
The study was funded by the Institute for the Scientific Information on Coffee. The authors declared no relevant conflicts of interest.
A version of this article originally appeared on Medscape.com.
FROM FRONTIERS IN BEHAVIORAL NEUROSCIENCE
Treating obesity: Will new drugs end the crisis?
This is the second in a three-part series on the obesity crisis. Part one tackles a complicated question – why does the obesity rate keep rising despite our efforts to stop it? – and can be found here. Part three shows how doctors and patients can make treatment better and can be found here.
In the mid-1980s, Louis J. Aronne, MD, strolled into a lab at Rockefeller University in New York where a colleague was breeding mice. “I will never forget what he showed me,” said Dr. Aronne, now the director of obesity research and treatment at Weill Cornell Medicine in New York. “He had a cage with 10 mice, one severely obese and the others normal weight. He took blood from one of the thin mice and gave it to the fat mouse.”
When Dr. Aronne returned 3 days later, that obese mouse had turned thin.
It was proof of something Dr. Aronne already suspected: Obesity had biological causes and wasn’t just a failure of willpower.
Years later, in 1994, that research led to the discovery of leptin, a hormone released from fat cells that’s involved in the regulation of body weight. It was a watershed moment in obesity research.
Since then, Dr. Aronne and others have worked to build the clinical field of obesity medicine, attempting to shift the public and medical view of obesity from a purely behavioral issue to a disease worthy of medical treatment.
All the while, the U.S. obesity rate soared.
Now, another watershed moment: We finally have highly effective obesity drugs. The hype is real, and so are the weight loss results.
“I’ve been saying for 30 years that when we find treatments that really work, people aren’t going to believe the results,” Dr. Aronne said. “It took longer than I expected, but it’s gratifying now to see.”
The big question
The emerging class of obesity medications known as GLP-1 agonists is indeed a game changer. The weight loss drug semaglutide (Ozempic, Wegovy) showed groundbreaking results, and studies suggest a parade of even more impressive drugs is on the way.
Yes, the drugs offer new hope to millions with obesity complications. But to truly turn the tide on our 42% obesity rate, much more work remains to be done, researchers said, including answering a big question:
How do these weight loss drugs work?
“We have new blockbuster drugs, and we don’t even know why they reduce body weight,” said Samuel Klein, MD, professor of medicine and nutritional science at Washington University in St. Louis. “It was by accident that this was discovered.”
Oops, we created a weight loss drug
Developed to treat diabetes, the GLP-1 drugs’ weight loss effects were a surprise. Now that those effects are confirmed, pharmaceutical companies and researchers are racing to figure out how these drugs work.
In the 1960s, scientists discovered the incretin effect – when you eat glucose (sugar), your body makes more insulin than it does if glucose is given intravenously. Glucose passes through the GI tract and the gut releases hormones that stimulate insulin secretion. It’s “essentially a feed-forward signal to your pancreas to tell it, ‘By the way, you need to be ready because there’s a bunch of glucose coming,’ ” said Randy Seeley, MD, director of the Michigan Nutrition Obesity Research Center, funded by the National Institutes of Health.
One of these hormones – or “incretins” – is GLP-1. In experiments, people with type 2 diabetes who were hooked up to GLP-1 saw their blood sugar go down.
“That led to the idea that if we could take this native hormone and make it last longer, we’d have a therapy for type 2 diabetes,” said Dr. Seeley. Thanks to a GLP-1-like compound in the saliva of the Gila monster, that idea became reality in the 2000s.
Along the way, a surprising side finding came to light: In early trials, diabetes patients on these drugs dropped weight.
Both Ozempic and Wegovy – brand names for semaglutide – are once-weekly injections (pill forms to treat obesity are on the way), but the latter is a higher dose.
“That dose results in about 40% of patients in the clinical trials achieving a 20% weight loss. We’ve just had nothing like that in terms of efficacy before,” said Dr. Seeley, who has worked with some of the drug companies (including Novo Nordisk, the maker of Ozempic and Wegovy, and Eli Lilly, maker of Mounjaro) that market the GLP-1s.
By contrast, semaglutide’s once-a-day predecessor liraglutide (Saxenda, made by Novo Nordisk) can lead to about 10% weight loss.
“And one of the ironies is, we don’t really know why,” Dr. Seeley said. “We don’t know why semaglutide is a better molecule for weight loss than liraglutide.”
Initially, scientists believed that the drugs, in addition to telling the pancreas to secrete more insulin, were also signaling the brain that you’re full. “Turns out that’s not really the way it works,” Dr. Seeley said. “GLP-1 made from your gut probably doesn’t get into your brain very much. But you make GLP-1 in your brain as well.”
For weight loss, it’s the brain’s GLP-1 system, not the gut’s, that the drugs are thought to hijack. But exactly which parts of the brain they affect and how is unknown. “That’s something lots of people are working on, including our own lab,” Dr. Seeley said. (Another surprise: The drugs may have potential as an anti-addiction treatment.)
The diabetes medication tirzepatide (Mounjaro), expected to be approved for weight loss as early as this year, is also a weekly injection, but it has a unique feature: It starts a response not just for GLP-1 but also for another incretin called GIP. Turns out, two is better than one: Trial participants on tirzepatide lost up to 22.5% of their body weight.
More of these hybrid drugs are on the way, Dr. Seeley said. In mid-stage clinical trials, the drug retatrutide, which targets three hormones, led to 24% weight loss. “The idea is the more bullets we can load into the gun, the more we can push the biology into a place where it’s easier to lose weight.”
Shifting from prevention to damage control
Less invasive and more scalable than surgery (only 1% of the eligible population gets bariatric surgery), the drugs offer doctors a safe, effective way to treat many patients with obesity. That’s cause for excitement, but concerns remain because they are expensive, costing about $800 to $1,300 per month out of pocket. Many health insurers, including Medicare, do not cover them for weight loss.
“You have this significant advance in obesity treatment, but very few will be able to access it,” said Gary Foster, PhD, adjunct professor of psychology in psychiatry at the University of Pennsylvania, Philadelphia, and chief scientific officer at WW (formerly Weight Watchers).
There is a push, including a proposed bill, to get Medicare to cover obesity medication. But given the expense of the drugs, the health economics do not support that move, according to an editorial in the New England Journal of Medicine. If Medicare were to cover obesity meds, the budget impact would likely be huge, potentially driving up premiums. If other payers followed suit, the impact could be felt across the U.S. health care system.
Other drawbacks include side effects – including nausea, diarrhea, stomach pain, and vomiting – that can be so bad that some patients can’t tolerate them.
And critically, the drugs do not deal with the root cause of the problem, said Robert Lustig, MD, an endocrinologist and pediatrician at the University of California, San Francisco, who has suggested that excess insulin is driving obesity. “No one has the disease that these drugs are treating. No one has GLP-1 deficiency. They’re bypassing the problem. They’re band-aiding the problem.”
Because the drugs work by mimicking starvation – they appear to curb hunger, so you eat less – people on them lose not just fat but also healthy lean mass, Dr. Lustig said.
Concerns about pancreatitis did not really bear out in postmarketing reports. (The drugs are still not recommended in people with pancreatitis or multiple endocrine neoplasia.) But predicting longer-term outcomes can be difficult, noted Dr. Lustig.
Then there are philosophical questions, said James Hill, PhD, director of the Nutrition Obesity Research Center at the University of Alabama at Birmingham.. “If you’re continuing to not exercise and eat not healthy foods and take a medication, is that success? Have we won when people are at a lower weight but not doing a healthy behavior?”
‘We can’t treat our way out of this’
The fact is, ending the obesity epidemic is a tall order, even for drugs as impressive as these.
“We can’t treat our way out of this,” said Jamy Ard, MD, codirector of Wake Forest Baptist Health Weight Management Center in Winston-Salem, N.C. “The treatments we have now are great, and there will be more coming. But we do need to figure out the prevention side of things.”
Dr. Seeley agreed but added that we can’t diet-and-exercise our way out either.
“There’s no switch to be flipped,” Dr. Seeley said. “If you told me we shouldn’t spend all this money on these drugs, we should spend it on prevention – great! What would we do?”
And prevention efforts won’t help the millions already living with health problems from obesity, Dr. Aronne said.
“Getting people to stop smoking prevents lung cancer. But stopping smoking doesn’t treat lung cancer,” Dr. Aronne said. “Once the physical changes occur in the lung that cause a tumor to grow, it’s too late. You have to think of obesity the same way.”
Dr. Seeley pointed out that “fearmongering” around the drugs highlights our lingering bias that obesity is a lifestyle issue that should not be medically treated.
“People say, ‘When you stop taking it, you’re going to gain the weight back,’ ” Dr. Seeley said. “There’s truth to that, but when you stop taking your hypertension medication, your blood pressure goes up. We don’t think of that as a [reason] for why you shouldn’t take your blood pressure medication. But that gets trumpeted into all these conversations about whether people [with obesity] should be treated at all.”
Like obesity, blood pressure was once thought to be a behavioral problem, Dr. Aronne said. But blood pressure meds prevent heart attacks and strokes. And it’s likely obesity meds will do the same.
One 55-year-old patient on the road to kidney failure lost weight on obesity medications, including semaglutide, Dr. Aronne said. Now, 6 years later, his kidney function is back to normal. “Normally, we think of kidney disease as irreversible,” Dr. Aronne said.
In that respect, these drugs should save money in the long run by virtue of heading off those health care costs, said Dr. Seeley, who imagines a future where obesity is not gone but better managed, like high blood pressure is now.
In the end, the drugs are another step toward what Dr. Aronne and many others have always pushed for: Treating obesity as a disease.
A version of this article originally appeared on WebMD.com.
This is the second in a three-part series on the obesity crisis. Part one tackles a complicated question – why does the obesity rate keep rising despite our efforts to stop it? – and can be found here. Part three shows how doctors and patients can make treatment better and can be found here.
In the mid-1980s, Louis J. Aronne, MD, strolled into a lab at Rockefeller University in New York where a colleague was breeding mice. “I will never forget what he showed me,” said Dr. Aronne, now the director of obesity research and treatment at Weill Cornell Medicine in New York. “He had a cage with 10 mice, one severely obese and the others normal weight. He took blood from one of the thin mice and gave it to the fat mouse.”
When Dr. Aronne returned 3 days later, that obese mouse had turned thin.
It was proof of something Dr. Aronne already suspected: Obesity had biological causes and wasn’t just a failure of willpower.
Years later, in 1994, that research led to the discovery of leptin, a hormone released from fat cells that’s involved in the regulation of body weight. It was a watershed moment in obesity research.
Since then, Dr. Aronne and others have worked to build the clinical field of obesity medicine, attempting to shift the public and medical view of obesity from a purely behavioral issue to a disease worthy of medical treatment.
All the while, the U.S. obesity rate soared.
Now, another watershed moment: We finally have highly effective obesity drugs. The hype is real, and so are the weight loss results.
“I’ve been saying for 30 years that when we find treatments that really work, people aren’t going to believe the results,” Dr. Aronne said. “It took longer than I expected, but it’s gratifying now to see.”
The big question
The emerging class of obesity medications known as GLP-1 agonists is indeed a game changer. The weight loss drug semaglutide (Ozempic, Wegovy) showed groundbreaking results, and studies suggest a parade of even more impressive drugs is on the way.
Yes, the drugs offer new hope to millions with obesity complications. But to truly turn the tide on our 42% obesity rate, much more work remains to be done, researchers said, including answering a big question:
How do these weight loss drugs work?
“We have new blockbuster drugs, and we don’t even know why they reduce body weight,” said Samuel Klein, MD, professor of medicine and nutritional science at Washington University in St. Louis. “It was by accident that this was discovered.”
Oops, we created a weight loss drug
Developed to treat diabetes, the GLP-1 drugs’ weight loss effects were a surprise. Now that those effects are confirmed, pharmaceutical companies and researchers are racing to figure out how these drugs work.
In the 1960s, scientists discovered the incretin effect – when you eat glucose (sugar), your body makes more insulin than it does if glucose is given intravenously. Glucose passes through the GI tract and the gut releases hormones that stimulate insulin secretion. It’s “essentially a feed-forward signal to your pancreas to tell it, ‘By the way, you need to be ready because there’s a bunch of glucose coming,’ ” said Randy Seeley, MD, director of the Michigan Nutrition Obesity Research Center, funded by the National Institutes of Health.
One of these hormones – or “incretins” – is GLP-1. In experiments, people with type 2 diabetes who were hooked up to GLP-1 saw their blood sugar go down.
“That led to the idea that if we could take this native hormone and make it last longer, we’d have a therapy for type 2 diabetes,” said Dr. Seeley. Thanks to a GLP-1-like compound in the saliva of the Gila monster, that idea became reality in the 2000s.
Along the way, a surprising side finding came to light: In early trials, diabetes patients on these drugs dropped weight.
Both Ozempic and Wegovy – brand names for semaglutide – are once-weekly injections (pill forms to treat obesity are on the way), but the latter is a higher dose.
“That dose results in about 40% of patients in the clinical trials achieving a 20% weight loss. We’ve just had nothing like that in terms of efficacy before,” said Dr. Seeley, who has worked with some of the drug companies (including Novo Nordisk, the maker of Ozempic and Wegovy, and Eli Lilly, maker of Mounjaro) that market the GLP-1s.
By contrast, semaglutide’s once-a-day predecessor liraglutide (Saxenda, made by Novo Nordisk) can lead to about 10% weight loss.
“And one of the ironies is, we don’t really know why,” Dr. Seeley said. “We don’t know why semaglutide is a better molecule for weight loss than liraglutide.”
Initially, scientists believed that the drugs, in addition to telling the pancreas to secrete more insulin, were also signaling the brain that you’re full. “Turns out that’s not really the way it works,” Dr. Seeley said. “GLP-1 made from your gut probably doesn’t get into your brain very much. But you make GLP-1 in your brain as well.”
For weight loss, it’s the brain’s GLP-1 system, not the gut’s, that the drugs are thought to hijack. But exactly which parts of the brain they affect and how is unknown. “That’s something lots of people are working on, including our own lab,” Dr. Seeley said. (Another surprise: The drugs may have potential as an anti-addiction treatment.)
The diabetes medication tirzepatide (Mounjaro), expected to be approved for weight loss as early as this year, is also a weekly injection, but it has a unique feature: It starts a response not just for GLP-1 but also for another incretin called GIP. Turns out, two is better than one: Trial participants on tirzepatide lost up to 22.5% of their body weight.
More of these hybrid drugs are on the way, Dr. Seeley said. In mid-stage clinical trials, the drug retatrutide, which targets three hormones, led to 24% weight loss. “The idea is the more bullets we can load into the gun, the more we can push the biology into a place where it’s easier to lose weight.”
Shifting from prevention to damage control
Less invasive and more scalable than surgery (only 1% of the eligible population gets bariatric surgery), the drugs offer doctors a safe, effective way to treat many patients with obesity. That’s cause for excitement, but concerns remain because they are expensive, costing about $800 to $1,300 per month out of pocket. Many health insurers, including Medicare, do not cover them for weight loss.
“You have this significant advance in obesity treatment, but very few will be able to access it,” said Gary Foster, PhD, adjunct professor of psychology in psychiatry at the University of Pennsylvania, Philadelphia, and chief scientific officer at WW (formerly Weight Watchers).
There is a push, including a proposed bill, to get Medicare to cover obesity medication. But given the expense of the drugs, the health economics do not support that move, according to an editorial in the New England Journal of Medicine. If Medicare were to cover obesity meds, the budget impact would likely be huge, potentially driving up premiums. If other payers followed suit, the impact could be felt across the U.S. health care system.
Other drawbacks include side effects – including nausea, diarrhea, stomach pain, and vomiting – that can be so bad that some patients can’t tolerate them.
And critically, the drugs do not deal with the root cause of the problem, said Robert Lustig, MD, an endocrinologist and pediatrician at the University of California, San Francisco, who has suggested that excess insulin is driving obesity. “No one has the disease that these drugs are treating. No one has GLP-1 deficiency. They’re bypassing the problem. They’re band-aiding the problem.”
Because the drugs work by mimicking starvation – they appear to curb hunger, so you eat less – people on them lose not just fat but also healthy lean mass, Dr. Lustig said.
Concerns about pancreatitis did not really bear out in postmarketing reports. (The drugs are still not recommended in people with pancreatitis or multiple endocrine neoplasia.) But predicting longer-term outcomes can be difficult, noted Dr. Lustig.
Then there are philosophical questions, said James Hill, PhD, director of the Nutrition Obesity Research Center at the University of Alabama at Birmingham.. “If you’re continuing to not exercise and eat not healthy foods and take a medication, is that success? Have we won when people are at a lower weight but not doing a healthy behavior?”
‘We can’t treat our way out of this’
The fact is, ending the obesity epidemic is a tall order, even for drugs as impressive as these.
“We can’t treat our way out of this,” said Jamy Ard, MD, codirector of Wake Forest Baptist Health Weight Management Center in Winston-Salem, N.C. “The treatments we have now are great, and there will be more coming. But we do need to figure out the prevention side of things.”
Dr. Seeley agreed but added that we can’t diet-and-exercise our way out either.
“There’s no switch to be flipped,” Dr. Seeley said. “If you told me we shouldn’t spend all this money on these drugs, we should spend it on prevention – great! What would we do?”
And prevention efforts won’t help the millions already living with health problems from obesity, Dr. Aronne said.
“Getting people to stop smoking prevents lung cancer. But stopping smoking doesn’t treat lung cancer,” Dr. Aronne said. “Once the physical changes occur in the lung that cause a tumor to grow, it’s too late. You have to think of obesity the same way.”
Dr. Seeley pointed out that “fearmongering” around the drugs highlights our lingering bias that obesity is a lifestyle issue that should not be medically treated.
“People say, ‘When you stop taking it, you’re going to gain the weight back,’ ” Dr. Seeley said. “There’s truth to that, but when you stop taking your hypertension medication, your blood pressure goes up. We don’t think of that as a [reason] for why you shouldn’t take your blood pressure medication. But that gets trumpeted into all these conversations about whether people [with obesity] should be treated at all.”
Like obesity, blood pressure was once thought to be a behavioral problem, Dr. Aronne said. But blood pressure meds prevent heart attacks and strokes. And it’s likely obesity meds will do the same.
One 55-year-old patient on the road to kidney failure lost weight on obesity medications, including semaglutide, Dr. Aronne said. Now, 6 years later, his kidney function is back to normal. “Normally, we think of kidney disease as irreversible,” Dr. Aronne said.
In that respect, these drugs should save money in the long run by virtue of heading off those health care costs, said Dr. Seeley, who imagines a future where obesity is not gone but better managed, like high blood pressure is now.
In the end, the drugs are another step toward what Dr. Aronne and many others have always pushed for: Treating obesity as a disease.
A version of this article originally appeared on WebMD.com.
This is the second in a three-part series on the obesity crisis. Part one tackles a complicated question – why does the obesity rate keep rising despite our efforts to stop it? – and can be found here. Part three shows how doctors and patients can make treatment better and can be found here.
In the mid-1980s, Louis J. Aronne, MD, strolled into a lab at Rockefeller University in New York where a colleague was breeding mice. “I will never forget what he showed me,” said Dr. Aronne, now the director of obesity research and treatment at Weill Cornell Medicine in New York. “He had a cage with 10 mice, one severely obese and the others normal weight. He took blood from one of the thin mice and gave it to the fat mouse.”
When Dr. Aronne returned 3 days later, that obese mouse had turned thin.
It was proof of something Dr. Aronne already suspected: Obesity had biological causes and wasn’t just a failure of willpower.
Years later, in 1994, that research led to the discovery of leptin, a hormone released from fat cells that’s involved in the regulation of body weight. It was a watershed moment in obesity research.
Since then, Dr. Aronne and others have worked to build the clinical field of obesity medicine, attempting to shift the public and medical view of obesity from a purely behavioral issue to a disease worthy of medical treatment.
All the while, the U.S. obesity rate soared.
Now, another watershed moment: We finally have highly effective obesity drugs. The hype is real, and so are the weight loss results.
“I’ve been saying for 30 years that when we find treatments that really work, people aren’t going to believe the results,” Dr. Aronne said. “It took longer than I expected, but it’s gratifying now to see.”
The big question
The emerging class of obesity medications known as GLP-1 agonists is indeed a game changer. The weight loss drug semaglutide (Ozempic, Wegovy) showed groundbreaking results, and studies suggest a parade of even more impressive drugs is on the way.
Yes, the drugs offer new hope to millions with obesity complications. But to truly turn the tide on our 42% obesity rate, much more work remains to be done, researchers said, including answering a big question:
How do these weight loss drugs work?
“We have new blockbuster drugs, and we don’t even know why they reduce body weight,” said Samuel Klein, MD, professor of medicine and nutritional science at Washington University in St. Louis. “It was by accident that this was discovered.”
Oops, we created a weight loss drug
Developed to treat diabetes, the GLP-1 drugs’ weight loss effects were a surprise. Now that those effects are confirmed, pharmaceutical companies and researchers are racing to figure out how these drugs work.
In the 1960s, scientists discovered the incretin effect – when you eat glucose (sugar), your body makes more insulin than it does if glucose is given intravenously. Glucose passes through the GI tract and the gut releases hormones that stimulate insulin secretion. It’s “essentially a feed-forward signal to your pancreas to tell it, ‘By the way, you need to be ready because there’s a bunch of glucose coming,’ ” said Randy Seeley, MD, director of the Michigan Nutrition Obesity Research Center, funded by the National Institutes of Health.
One of these hormones – or “incretins” – is GLP-1. In experiments, people with type 2 diabetes who were hooked up to GLP-1 saw their blood sugar go down.
“That led to the idea that if we could take this native hormone and make it last longer, we’d have a therapy for type 2 diabetes,” said Dr. Seeley. Thanks to a GLP-1-like compound in the saliva of the Gila monster, that idea became reality in the 2000s.
Along the way, a surprising side finding came to light: In early trials, diabetes patients on these drugs dropped weight.
Both Ozempic and Wegovy – brand names for semaglutide – are once-weekly injections (pill forms to treat obesity are on the way), but the latter is a higher dose.
“That dose results in about 40% of patients in the clinical trials achieving a 20% weight loss. We’ve just had nothing like that in terms of efficacy before,” said Dr. Seeley, who has worked with some of the drug companies (including Novo Nordisk, the maker of Ozempic and Wegovy, and Eli Lilly, maker of Mounjaro) that market the GLP-1s.
By contrast, semaglutide’s once-a-day predecessor liraglutide (Saxenda, made by Novo Nordisk) can lead to about 10% weight loss.
“And one of the ironies is, we don’t really know why,” Dr. Seeley said. “We don’t know why semaglutide is a better molecule for weight loss than liraglutide.”
Initially, scientists believed that the drugs, in addition to telling the pancreas to secrete more insulin, were also signaling the brain that you’re full. “Turns out that’s not really the way it works,” Dr. Seeley said. “GLP-1 made from your gut probably doesn’t get into your brain very much. But you make GLP-1 in your brain as well.”
For weight loss, it’s the brain’s GLP-1 system, not the gut’s, that the drugs are thought to hijack. But exactly which parts of the brain they affect and how is unknown. “That’s something lots of people are working on, including our own lab,” Dr. Seeley said. (Another surprise: The drugs may have potential as an anti-addiction treatment.)
The diabetes medication tirzepatide (Mounjaro), expected to be approved for weight loss as early as this year, is also a weekly injection, but it has a unique feature: It starts a response not just for GLP-1 but also for another incretin called GIP. Turns out, two is better than one: Trial participants on tirzepatide lost up to 22.5% of their body weight.
More of these hybrid drugs are on the way, Dr. Seeley said. In mid-stage clinical trials, the drug retatrutide, which targets three hormones, led to 24% weight loss. “The idea is the more bullets we can load into the gun, the more we can push the biology into a place where it’s easier to lose weight.”
Shifting from prevention to damage control
Less invasive and more scalable than surgery (only 1% of the eligible population gets bariatric surgery), the drugs offer doctors a safe, effective way to treat many patients with obesity. That’s cause for excitement, but concerns remain because they are expensive, costing about $800 to $1,300 per month out of pocket. Many health insurers, including Medicare, do not cover them for weight loss.
“You have this significant advance in obesity treatment, but very few will be able to access it,” said Gary Foster, PhD, adjunct professor of psychology in psychiatry at the University of Pennsylvania, Philadelphia, and chief scientific officer at WW (formerly Weight Watchers).
There is a push, including a proposed bill, to get Medicare to cover obesity medication. But given the expense of the drugs, the health economics do not support that move, according to an editorial in the New England Journal of Medicine. If Medicare were to cover obesity meds, the budget impact would likely be huge, potentially driving up premiums. If other payers followed suit, the impact could be felt across the U.S. health care system.
Other drawbacks include side effects – including nausea, diarrhea, stomach pain, and vomiting – that can be so bad that some patients can’t tolerate them.
And critically, the drugs do not deal with the root cause of the problem, said Robert Lustig, MD, an endocrinologist and pediatrician at the University of California, San Francisco, who has suggested that excess insulin is driving obesity. “No one has the disease that these drugs are treating. No one has GLP-1 deficiency. They’re bypassing the problem. They’re band-aiding the problem.”
Because the drugs work by mimicking starvation – they appear to curb hunger, so you eat less – people on them lose not just fat but also healthy lean mass, Dr. Lustig said.
Concerns about pancreatitis did not really bear out in postmarketing reports. (The drugs are still not recommended in people with pancreatitis or multiple endocrine neoplasia.) But predicting longer-term outcomes can be difficult, noted Dr. Lustig.
Then there are philosophical questions, said James Hill, PhD, director of the Nutrition Obesity Research Center at the University of Alabama at Birmingham.. “If you’re continuing to not exercise and eat not healthy foods and take a medication, is that success? Have we won when people are at a lower weight but not doing a healthy behavior?”
‘We can’t treat our way out of this’
The fact is, ending the obesity epidemic is a tall order, even for drugs as impressive as these.
“We can’t treat our way out of this,” said Jamy Ard, MD, codirector of Wake Forest Baptist Health Weight Management Center in Winston-Salem, N.C. “The treatments we have now are great, and there will be more coming. But we do need to figure out the prevention side of things.”
Dr. Seeley agreed but added that we can’t diet-and-exercise our way out either.
“There’s no switch to be flipped,” Dr. Seeley said. “If you told me we shouldn’t spend all this money on these drugs, we should spend it on prevention – great! What would we do?”
And prevention efforts won’t help the millions already living with health problems from obesity, Dr. Aronne said.
“Getting people to stop smoking prevents lung cancer. But stopping smoking doesn’t treat lung cancer,” Dr. Aronne said. “Once the physical changes occur in the lung that cause a tumor to grow, it’s too late. You have to think of obesity the same way.”
Dr. Seeley pointed out that “fearmongering” around the drugs highlights our lingering bias that obesity is a lifestyle issue that should not be medically treated.
“People say, ‘When you stop taking it, you’re going to gain the weight back,’ ” Dr. Seeley said. “There’s truth to that, but when you stop taking your hypertension medication, your blood pressure goes up. We don’t think of that as a [reason] for why you shouldn’t take your blood pressure medication. But that gets trumpeted into all these conversations about whether people [with obesity] should be treated at all.”
Like obesity, blood pressure was once thought to be a behavioral problem, Dr. Aronne said. But blood pressure meds prevent heart attacks and strokes. And it’s likely obesity meds will do the same.
One 55-year-old patient on the road to kidney failure lost weight on obesity medications, including semaglutide, Dr. Aronne said. Now, 6 years later, his kidney function is back to normal. “Normally, we think of kidney disease as irreversible,” Dr. Aronne said.
In that respect, these drugs should save money in the long run by virtue of heading off those health care costs, said Dr. Seeley, who imagines a future where obesity is not gone but better managed, like high blood pressure is now.
In the end, the drugs are another step toward what Dr. Aronne and many others have always pushed for: Treating obesity as a disease.
A version of this article originally appeared on WebMD.com.
Racial Disparities in Hidradenitis Suppurativa–Related Pain: A Cross-sectional Analysis
Hidradenitis suppurativa (HS), a chronic inflammatory disease that is characterized by tender inflamed nodules of the skin and subcutaneous tissue, disproportionately affects postpubertal females as well as Black/African American individuals. The nodules can rupture, form sinus tracts, and scar. 1 Hidradenitis suppurativa has been associated with cardiovascular disease, type 2 diabetes mellitus, polycystic ovary syndrome, depression, suicide, and substance use disorders. Because of the symptom burden and associated conditions, HS can be a painful and distressing disease that substantially impairs the quality of life for individuals with this condition. 2
Pain is a commonly reported symptom in HS that often goes untreated. Furthermore, HS-related pain is complex due to the involvement of different pain types that require various treatment modalities.3 According to Savage et al,4 recognizing whether HS-related pain is acute, chronic, neuropathic, or nociceptive is vital in establishing a framework for an effective pain management scheme. Currently, such established multimodal pain management strategies in dermatology do not exist. In 2021, dermatology-specific pain management strategies proposed the use of a multimodal regimen to address the multifaceted nature of HS-related pain.4 However, these strategies failed to recognize the systemic racial and ethnic biases in the US health care system that undermine pain management care for minority groups.5,6 One approach to combatting racial disparities in pain management is determining average pain levels across racial groups.7 This study sought to compare HS-related pain scores by racial groups. Furthermore, we assessed differences in perception of patients’ respective pain management regimens by race. We hypothesized that the average HS-related pain intensities and pain management would differ between self-reported racial groups.
Methods
This cross-sectional study took place over 5 months (August through December 2021). A survey was emailed to 2198 adult patients with HS in the University of Alabama Health System. The survey consisted of demographic and general questions about a patient’s HS. Pain scores were captured using the numeric rating scale (NRS), a measurement tool for pain intensity on a scale from 0 to 10. 8 Age at diagnosis, gender, education level, household income, total body areas affected by HS, disease severity (categorized as mild, moderate, and severe), comorbidities including mood disorders, tobacco use, and HS and HS-related pain medication regimens also were collected. Additionally, participants were asked about their level of agreement with the following statements: “I am satisfied with how my pain related to HS is being managed by my doctors” and “My pain related to HS is under control.” The level of agreement was measured using a 5-point Likert scale, with responses ranging from strongly disagree to strongly agree. All data included in the analysis were self-reported. The study received institutional review board approval for the University of Alabama at Birmingham.
Statistical Analysis—Descriptive statistics were used to assess statistical differences in patient characteristics of Black/African American participants compared to other participants, including White, Asian, and Hispanic/Latino participants. Thirteen participants were excluded from the final analysis: 2 participants were missing data, and 11 biracial participants were excluded due to overlapping White and Black/African American races that may have confounded the analysis. Categorical variables were reported as frequencies and percentages, and χ2 and Fisher exact tests, when necessary, were used to test for statistically significant differences. Continuous variables were summarized with means and standard deviations, and a t test was used for statistically significant differences.
Logistic regression was performed to assess the relationship between race and pain after adjusting for confounding variables such as obesity, current tobacco use, self-reported HS severity, and the presence of comorbidities. A total of 204 patient records were included in the analysis, of which 70 (34.3%) had a pain score of 8 or higher, which indicates very severe pain intensity levels on the NRS,8 and were selected as a cut point based on the distribution of responses. For this cross-sectional cohort, our approach was to compare characteristics of those classified with a top score of 8 or higher (n=70) vs a top score of 0 to 7 (n=134)(cases vs noncases). Statistical analyses were performed using JMP Pro 16 (JMP Statistical Discovery LLC) at an α=.05 significance level; logistic regression was performed using SPSS Statistics (IBM). For the logistic regression, we grouped patient race into 2 categories: Black/African American and Other, which included White, Asian, and Hispanic/Latino participants.
Crude and adjusted multivariable logistic regression analyses were used to calculate prevalence odds ratios with 95% confidence intervals. Covariate inclusion in the multivariable logistic regression was based on a priori hypothesis/knowledge and was meant to estimate the independent effect of race after adjustment for income, HS severity, and history of prescription pain medication use. Other variables, including tobacco use, obesity, mood disorders, and current HS treatments, were all individually tested in the multivariate analysis and did not significantly impact the odds ratio for high pain. Statistical adjustment slightly decreased (19%) the magnitude between crude and adjusted prevalence odds ratios for the association between Black/African American race and high pain score.
Results
Survey Demographics —The final analysis included 204 survey respondents. Most respondents were Black/African American (58.82%), and nearly all were female (89.71%)(Table 1). The mean age (SD) of respondents was 37.37 (11.29) years (range, 19-70 years). Many respondents reported having completed some college (36.27%) or receiving a bachelor’s degree (19.12%). Of patients who were not Black/African American, 10.71% had higher than a master’s degree, whereas no Black/African American patients held a degree higher than a master’s ( P = .0052). Additionally, more Black/African American respondents (35.83%) reported an annual household income level of less than $25,000 compared with respondents who were not Black/African American (19.05%, P = .0001). Most respondents rated the severity of their HS as moderate or severe (46.57% and 41.67%, respectively), and there was no significant difference in reported severity of HS between racial groups ( P = .5395).


Pain Scores—As documented in the Methods, respondents were asked to rate their HS-related pain intensity from 0 to 10 using the NRS. The average pain score (SD)—the level of pain intensity over the prior month—was 6.39 (2.56)(range, 0–10). The mean pain score (SD) at the time of the survey was 3.61 (2.98)(range, 0–10)(Table 1). These data revealed that Black/African American patients had a significantly higher average pain score (SD) than patients with HS who were not Black/African American (7.08 [2.49] and 5.40 [2.35], respectively; P<.0001). After adjustment with multivariable logistical regression, Black/African American patients had 4-fold increased odds for very severe levels of pain (score of ≥8) compared with patients who were not Black/African American.
Pain Management—Although pain scores were higher for Black/African American patients with HS, there was no significant difference in the perception of pain control between racial groups (P=.0761). Additionally, we found low income (adjusted prevalence odds ratio [POR], 0.22; 95% CI, 0.05-0.91), a history of prescription pain medication use (adjusted POR, 2.25; 95% CI, 1.13-4.51), and HS severity (adjusted POR, 4.40; 95% CI, 1.11-17.36) all to be independent risk factors contributing to higher pain scores in patients with HS (Table 2). Lastly, we noted current or reported history of pain medication use was significantly correlated with higher pain scores (P=.0280 and P=.0213, respectively).
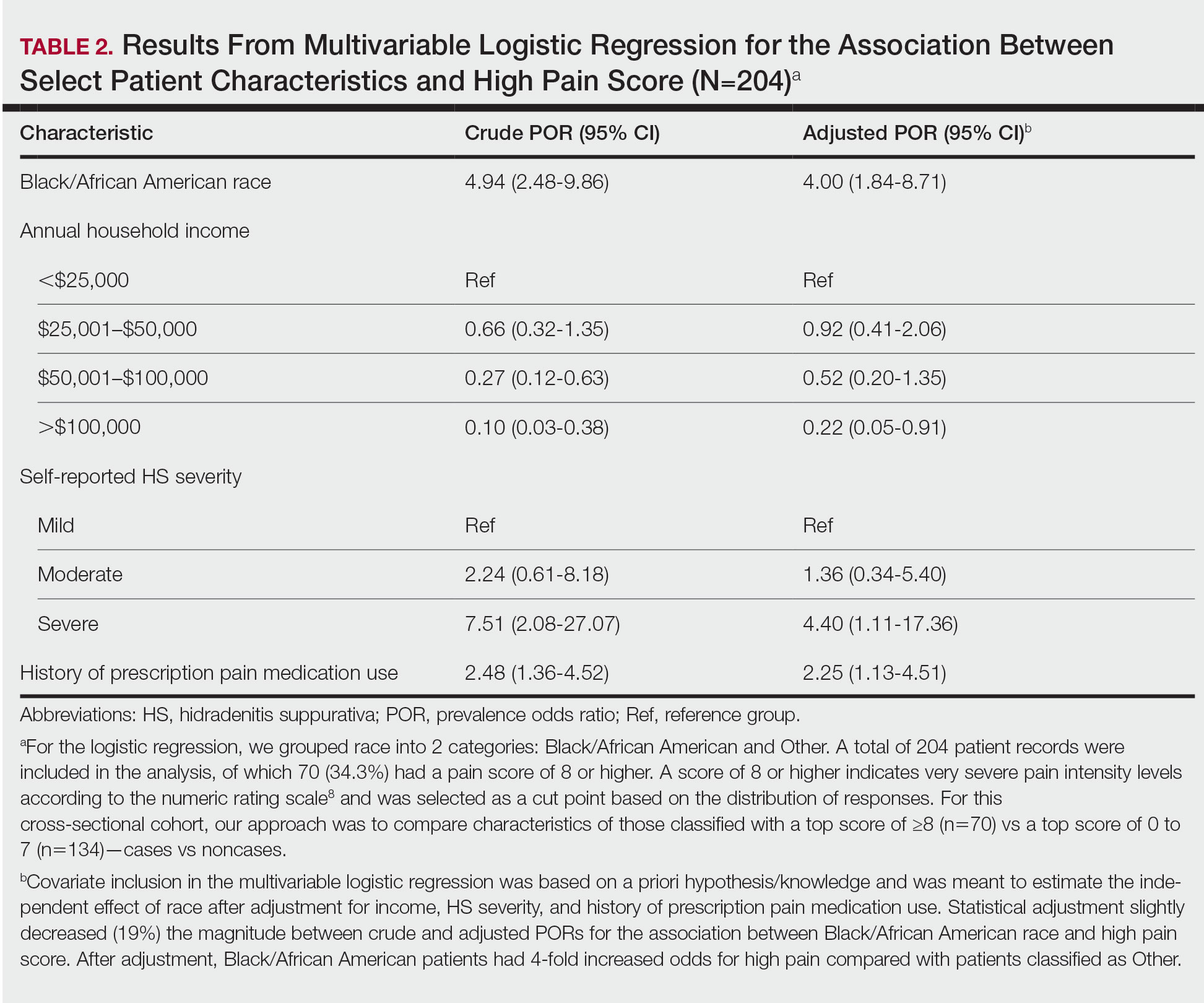
Satisfaction With Pain Management—The level of satisfaction with physician management of HS-related pain was significantly different between Black/African American patients and those who were not Black/African American (P=.0129). Of those who identified as Black/African American, 26.7% (n=32) strongly disagreed with the statement, “I am satisfied with how my pain related to HS is being managed by my doctors,” whereas only 15.5% (n=13) of patients who were not Black/African American strongly disagreed.
Comment
There is no cure for HS, and a large focus of treatment is pain management. Because racial disparities in the treatment of chronic pain will affect those with HS, we conducted a cross-sectional analysis of pain and pain management among HS patients. We found that Black/African American patients with HS have higher average pain scores than those who are not Black/African American and were 4 times more likely to experience very severe pain. Prior studies have established that patients with HS often report higher pain levels than patients with other chronic inflammatory skin conditions, 7,8 and our study identified racial disparities in HS-related pain management.
Measuring pain is challenging because of its multidimensional and subjective nature, making it essential to consider underlying causes and patients’ emotional responses to pain.9 By adjusting for confounding factors that may influence pain, such as mood disorders, disease severity, comorbidities, and medication use, we were able to gain better insight into fundamental differences in average pain intensity levels among racial groups and assess what factors may be contributing to a patient’s pain perception. Our study determined that lower income levels, higher HS disease severity, and a history of prescription pain medication use were all independent risk factors for high pain. Of note, obesity, tobacco use, and mood disorders such as anxiety and depression did not significantly differ between racial groups or increase the odds of high pain between racial groups identified.
With low income being an independent risk factor for high pain, we must consider the social determinants of health and how they may influence the pain experience in HS. We speculate that low income may be associated with other social determinants of health for the patients assessed in this study, such as lack of social and community support or limited health care access that contribute to worse health outcomes.10,11 In addition, low income contributes to limited access to medical care or treatments12; without access to effective HS management, lower-income patients may be at risk for higher disease severity and thus higher pain levels. However, economic stability is only a part of the whole picture; therefore, assessing the other social determinants of health in patients with HS may lead to better health outcomes and quality of life.
Another identified risk factor for high pain was a reported history of prescription pain medication use. This finding suggests that patients with moderate to severe pain likely have required stronger analgesic medications in the past. We further speculate that high pain levels in patients who have received prescription pain medications indicate either undertreatment, mistreatment, or recalcitrant pain. More research is needed to assess the relationship between HS-related pain intensity, analgesic medications, and providers who manage HS-related pain.
We also found that Black/African American patients with HS had a significantly higher dissatisfaction with their physician’s management of their pain, which could be attributable to several factors, including biological differences in medication metabolism (in which the patient has medication-resistant HS), undertreatment of pain, and/or poor doctor-patient relations. These reasons coincide with other diseases where health disparities are found.13-15 Recognizing these factors will be key to dismantling the disparities in HS that are noted within this study. The limitations of this work include the cross-sectional study design and its inability to evaluate causal factors of high pain levels across racial groups, the NRS lack of insight on pain chronicity or pain experience,7 the lack of provider or institution perspectives, and self-reported data. Additionally, only patients with email access were included, which may have excluded vulnerable populations with more pain associated with their HS.
Our findings highlight an area for further investigation to assess why these racial differences exist in HS-related pain. The results also emphasize the need for research evaluating whether systemic or health care provider biases contribute to racial differences in HS-related pain management.
Acknowledgment — Dr. Weir was supported by the Predoctoral Clinical/Translational Research Program (TL1), a National Institutes of Health Ruth L. Kirschstein National Research Service Award (NRSA), through the University of Alabama at Birmingham (UAB) Center for Clinical and Translational Science (CCTS).
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764. doi:10.1001/jamadermatol.2017.0201
- Nguyen TV, Damiani G, Orenstein LAV, et al. Hidradenitis suppurativa: an update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J Eur Acad Dermatol Venereol. 2021;35:50-61. doi:10.1111/jdv.16677
- Krajewski PK, Matusiak Ł, von Stebut E, et al. Pain in hidradenitis suppurativa: a cross-sectional study of 1,795 patients. Acta Derm Venereol. 2021;101:adv00364. doi:10.2340/00015555-3724
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Morales ME, Yong RJ. Racial and ethnic disparities in the treatment of chronic pain. Pain Med. 2021;22:75-90. doi:10.1093/pm/pnaa427
- US Department of Health and Human Services. 2019 National Healthcare Quality and Disparities Report. December 2020. Accessed June 21, 2023. https://www.ahrq.gov/sites/default/files/wysiwyg/research/findings/nhqrdr/2019qdr.pdf
- Hoffman KM, Trawalter S, Axt JR, et al. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A. 2016;113:4296-4301. doi:10.1073/pnas.1516047113
- Patel ZS, Hoffman LK, Buse DC, et al. Pain, psychological comorbidities, disability, and impaired quality of life in hidradenitis suppurativa. Curr Pain Headache Rep. 2017;21:49. doi:10.1007/s11916-017-0647-3. Published correction appears in Curr Pain Headache Rep. 2017;21:52.
- McDowell I. Pain measurements. In: Measuring Health: A Guide to Rating Scales and Questionnaires. Oxford University Press; 2006:477-478.
- Singh GK, Daus GP, Allender M, et al. Social determinants of health in the United States: addressing major health inequality trends for the nation, 1935-2016. Int J MCH AIDS. 2017;6:139-164. doi:10.21106/ijma.236
- Sulley S, Bayssie M. Social determinants of health: an evaluation of risk factors associated with inpatient presentations in the United States. Cureus. 2021;13:E13287. doi:10.7759/cureus.13287
- Lazar M, Davenport L. Barriers to health care access for low income families: a review of literature. J Community Health Nurs. 2018;35:28-37. doi:10.1080/07370016.2018.1404832
- Ghoshal M, Shapiro H, Todd K, et al. Chronic noncancer pain management and systemic racism: time to move toward equal care standards.J Pain Res. 2020;13:2825-2836. doi:10.214/JPR.S287314
- Cintron A, Morrison RS. Pain and ethnicity in the United States: a systematic review. J Palliat Med. 2006;9:1454-1473. doi:10.1089/jpm.2006.9.1454
- Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003;4:277-294. doi:10.1046/j.1526-4637.2003.03034.x. Published correction appears in Pain Med. 2005;6:99.
Hidradenitis suppurativa (HS), a chronic inflammatory disease that is characterized by tender inflamed nodules of the skin and subcutaneous tissue, disproportionately affects postpubertal females as well as Black/African American individuals. The nodules can rupture, form sinus tracts, and scar. 1 Hidradenitis suppurativa has been associated with cardiovascular disease, type 2 diabetes mellitus, polycystic ovary syndrome, depression, suicide, and substance use disorders. Because of the symptom burden and associated conditions, HS can be a painful and distressing disease that substantially impairs the quality of life for individuals with this condition. 2
Pain is a commonly reported symptom in HS that often goes untreated. Furthermore, HS-related pain is complex due to the involvement of different pain types that require various treatment modalities.3 According to Savage et al,4 recognizing whether HS-related pain is acute, chronic, neuropathic, or nociceptive is vital in establishing a framework for an effective pain management scheme. Currently, such established multimodal pain management strategies in dermatology do not exist. In 2021, dermatology-specific pain management strategies proposed the use of a multimodal regimen to address the multifaceted nature of HS-related pain.4 However, these strategies failed to recognize the systemic racial and ethnic biases in the US health care system that undermine pain management care for minority groups.5,6 One approach to combatting racial disparities in pain management is determining average pain levels across racial groups.7 This study sought to compare HS-related pain scores by racial groups. Furthermore, we assessed differences in perception of patients’ respective pain management regimens by race. We hypothesized that the average HS-related pain intensities and pain management would differ between self-reported racial groups.
Methods
This cross-sectional study took place over 5 months (August through December 2021). A survey was emailed to 2198 adult patients with HS in the University of Alabama Health System. The survey consisted of demographic and general questions about a patient’s HS. Pain scores were captured using the numeric rating scale (NRS), a measurement tool for pain intensity on a scale from 0 to 10. 8 Age at diagnosis, gender, education level, household income, total body areas affected by HS, disease severity (categorized as mild, moderate, and severe), comorbidities including mood disorders, tobacco use, and HS and HS-related pain medication regimens also were collected. Additionally, participants were asked about their level of agreement with the following statements: “I am satisfied with how my pain related to HS is being managed by my doctors” and “My pain related to HS is under control.” The level of agreement was measured using a 5-point Likert scale, with responses ranging from strongly disagree to strongly agree. All data included in the analysis were self-reported. The study received institutional review board approval for the University of Alabama at Birmingham.
Statistical Analysis—Descriptive statistics were used to assess statistical differences in patient characteristics of Black/African American participants compared to other participants, including White, Asian, and Hispanic/Latino participants. Thirteen participants were excluded from the final analysis: 2 participants were missing data, and 11 biracial participants were excluded due to overlapping White and Black/African American races that may have confounded the analysis. Categorical variables were reported as frequencies and percentages, and χ2 and Fisher exact tests, when necessary, were used to test for statistically significant differences. Continuous variables were summarized with means and standard deviations, and a t test was used for statistically significant differences.
Logistic regression was performed to assess the relationship between race and pain after adjusting for confounding variables such as obesity, current tobacco use, self-reported HS severity, and the presence of comorbidities. A total of 204 patient records were included in the analysis, of which 70 (34.3%) had a pain score of 8 or higher, which indicates very severe pain intensity levels on the NRS,8 and were selected as a cut point based on the distribution of responses. For this cross-sectional cohort, our approach was to compare characteristics of those classified with a top score of 8 or higher (n=70) vs a top score of 0 to 7 (n=134)(cases vs noncases). Statistical analyses were performed using JMP Pro 16 (JMP Statistical Discovery LLC) at an α=.05 significance level; logistic regression was performed using SPSS Statistics (IBM). For the logistic regression, we grouped patient race into 2 categories: Black/African American and Other, which included White, Asian, and Hispanic/Latino participants.
Crude and adjusted multivariable logistic regression analyses were used to calculate prevalence odds ratios with 95% confidence intervals. Covariate inclusion in the multivariable logistic regression was based on a priori hypothesis/knowledge and was meant to estimate the independent effect of race after adjustment for income, HS severity, and history of prescription pain medication use. Other variables, including tobacco use, obesity, mood disorders, and current HS treatments, were all individually tested in the multivariate analysis and did not significantly impact the odds ratio for high pain. Statistical adjustment slightly decreased (19%) the magnitude between crude and adjusted prevalence odds ratios for the association between Black/African American race and high pain score.
Results
Survey Demographics —The final analysis included 204 survey respondents. Most respondents were Black/African American (58.82%), and nearly all were female (89.71%)(Table 1). The mean age (SD) of respondents was 37.37 (11.29) years (range, 19-70 years). Many respondents reported having completed some college (36.27%) or receiving a bachelor’s degree (19.12%). Of patients who were not Black/African American, 10.71% had higher than a master’s degree, whereas no Black/African American patients held a degree higher than a master’s ( P = .0052). Additionally, more Black/African American respondents (35.83%) reported an annual household income level of less than $25,000 compared with respondents who were not Black/African American (19.05%, P = .0001). Most respondents rated the severity of their HS as moderate or severe (46.57% and 41.67%, respectively), and there was no significant difference in reported severity of HS between racial groups ( P = .5395).


Pain Scores—As documented in the Methods, respondents were asked to rate their HS-related pain intensity from 0 to 10 using the NRS. The average pain score (SD)—the level of pain intensity over the prior month—was 6.39 (2.56)(range, 0–10). The mean pain score (SD) at the time of the survey was 3.61 (2.98)(range, 0–10)(Table 1). These data revealed that Black/African American patients had a significantly higher average pain score (SD) than patients with HS who were not Black/African American (7.08 [2.49] and 5.40 [2.35], respectively; P<.0001). After adjustment with multivariable logistical regression, Black/African American patients had 4-fold increased odds for very severe levels of pain (score of ≥8) compared with patients who were not Black/African American.
Pain Management—Although pain scores were higher for Black/African American patients with HS, there was no significant difference in the perception of pain control between racial groups (P=.0761). Additionally, we found low income (adjusted prevalence odds ratio [POR], 0.22; 95% CI, 0.05-0.91), a history of prescription pain medication use (adjusted POR, 2.25; 95% CI, 1.13-4.51), and HS severity (adjusted POR, 4.40; 95% CI, 1.11-17.36) all to be independent risk factors contributing to higher pain scores in patients with HS (Table 2). Lastly, we noted current or reported history of pain medication use was significantly correlated with higher pain scores (P=.0280 and P=.0213, respectively).

Satisfaction With Pain Management—The level of satisfaction with physician management of HS-related pain was significantly different between Black/African American patients and those who were not Black/African American (P=.0129). Of those who identified as Black/African American, 26.7% (n=32) strongly disagreed with the statement, “I am satisfied with how my pain related to HS is being managed by my doctors,” whereas only 15.5% (n=13) of patients who were not Black/African American strongly disagreed.
Comment
There is no cure for HS, and a large focus of treatment is pain management. Because racial disparities in the treatment of chronic pain will affect those with HS, we conducted a cross-sectional analysis of pain and pain management among HS patients. We found that Black/African American patients with HS have higher average pain scores than those who are not Black/African American and were 4 times more likely to experience very severe pain. Prior studies have established that patients with HS often report higher pain levels than patients with other chronic inflammatory skin conditions, 7,8 and our study identified racial disparities in HS-related pain management.
Measuring pain is challenging because of its multidimensional and subjective nature, making it essential to consider underlying causes and patients’ emotional responses to pain.9 By adjusting for confounding factors that may influence pain, such as mood disorders, disease severity, comorbidities, and medication use, we were able to gain better insight into fundamental differences in average pain intensity levels among racial groups and assess what factors may be contributing to a patient’s pain perception. Our study determined that lower income levels, higher HS disease severity, and a history of prescription pain medication use were all independent risk factors for high pain. Of note, obesity, tobacco use, and mood disorders such as anxiety and depression did not significantly differ between racial groups or increase the odds of high pain between racial groups identified.
With low income being an independent risk factor for high pain, we must consider the social determinants of health and how they may influence the pain experience in HS. We speculate that low income may be associated with other social determinants of health for the patients assessed in this study, such as lack of social and community support or limited health care access that contribute to worse health outcomes.10,11 In addition, low income contributes to limited access to medical care or treatments12; without access to effective HS management, lower-income patients may be at risk for higher disease severity and thus higher pain levels. However, economic stability is only a part of the whole picture; therefore, assessing the other social determinants of health in patients with HS may lead to better health outcomes and quality of life.
Another identified risk factor for high pain was a reported history of prescription pain medication use. This finding suggests that patients with moderate to severe pain likely have required stronger analgesic medications in the past. We further speculate that high pain levels in patients who have received prescription pain medications indicate either undertreatment, mistreatment, or recalcitrant pain. More research is needed to assess the relationship between HS-related pain intensity, analgesic medications, and providers who manage HS-related pain.
We also found that Black/African American patients with HS had a significantly higher dissatisfaction with their physician’s management of their pain, which could be attributable to several factors, including biological differences in medication metabolism (in which the patient has medication-resistant HS), undertreatment of pain, and/or poor doctor-patient relations. These reasons coincide with other diseases where health disparities are found.13-15 Recognizing these factors will be key to dismantling the disparities in HS that are noted within this study. The limitations of this work include the cross-sectional study design and its inability to evaluate causal factors of high pain levels across racial groups, the NRS lack of insight on pain chronicity or pain experience,7 the lack of provider or institution perspectives, and self-reported data. Additionally, only patients with email access were included, which may have excluded vulnerable populations with more pain associated with their HS.
Our findings highlight an area for further investigation to assess why these racial differences exist in HS-related pain. The results also emphasize the need for research evaluating whether systemic or health care provider biases contribute to racial differences in HS-related pain management.
Acknowledgment — Dr. Weir was supported by the Predoctoral Clinical/Translational Research Program (TL1), a National Institutes of Health Ruth L. Kirschstein National Research Service Award (NRSA), through the University of Alabama at Birmingham (UAB) Center for Clinical and Translational Science (CCTS).
Hidradenitis suppurativa (HS), a chronic inflammatory disease that is characterized by tender inflamed nodules of the skin and subcutaneous tissue, disproportionately affects postpubertal females as well as Black/African American individuals. The nodules can rupture, form sinus tracts, and scar. 1 Hidradenitis suppurativa has been associated with cardiovascular disease, type 2 diabetes mellitus, polycystic ovary syndrome, depression, suicide, and substance use disorders. Because of the symptom burden and associated conditions, HS can be a painful and distressing disease that substantially impairs the quality of life for individuals with this condition. 2
Pain is a commonly reported symptom in HS that often goes untreated. Furthermore, HS-related pain is complex due to the involvement of different pain types that require various treatment modalities.3 According to Savage et al,4 recognizing whether HS-related pain is acute, chronic, neuropathic, or nociceptive is vital in establishing a framework for an effective pain management scheme. Currently, such established multimodal pain management strategies in dermatology do not exist. In 2021, dermatology-specific pain management strategies proposed the use of a multimodal regimen to address the multifaceted nature of HS-related pain.4 However, these strategies failed to recognize the systemic racial and ethnic biases in the US health care system that undermine pain management care for minority groups.5,6 One approach to combatting racial disparities in pain management is determining average pain levels across racial groups.7 This study sought to compare HS-related pain scores by racial groups. Furthermore, we assessed differences in perception of patients’ respective pain management regimens by race. We hypothesized that the average HS-related pain intensities and pain management would differ between self-reported racial groups.
Methods
This cross-sectional study took place over 5 months (August through December 2021). A survey was emailed to 2198 adult patients with HS in the University of Alabama Health System. The survey consisted of demographic and general questions about a patient’s HS. Pain scores were captured using the numeric rating scale (NRS), a measurement tool for pain intensity on a scale from 0 to 10. 8 Age at diagnosis, gender, education level, household income, total body areas affected by HS, disease severity (categorized as mild, moderate, and severe), comorbidities including mood disorders, tobacco use, and HS and HS-related pain medication regimens also were collected. Additionally, participants were asked about their level of agreement with the following statements: “I am satisfied with how my pain related to HS is being managed by my doctors” and “My pain related to HS is under control.” The level of agreement was measured using a 5-point Likert scale, with responses ranging from strongly disagree to strongly agree. All data included in the analysis were self-reported. The study received institutional review board approval for the University of Alabama at Birmingham.
Statistical Analysis—Descriptive statistics were used to assess statistical differences in patient characteristics of Black/African American participants compared to other participants, including White, Asian, and Hispanic/Latino participants. Thirteen participants were excluded from the final analysis: 2 participants were missing data, and 11 biracial participants were excluded due to overlapping White and Black/African American races that may have confounded the analysis. Categorical variables were reported as frequencies and percentages, and χ2 and Fisher exact tests, when necessary, were used to test for statistically significant differences. Continuous variables were summarized with means and standard deviations, and a t test was used for statistically significant differences.
Logistic regression was performed to assess the relationship between race and pain after adjusting for confounding variables such as obesity, current tobacco use, self-reported HS severity, and the presence of comorbidities. A total of 204 patient records were included in the analysis, of which 70 (34.3%) had a pain score of 8 or higher, which indicates very severe pain intensity levels on the NRS,8 and were selected as a cut point based on the distribution of responses. For this cross-sectional cohort, our approach was to compare characteristics of those classified with a top score of 8 or higher (n=70) vs a top score of 0 to 7 (n=134)(cases vs noncases). Statistical analyses were performed using JMP Pro 16 (JMP Statistical Discovery LLC) at an α=.05 significance level; logistic regression was performed using SPSS Statistics (IBM). For the logistic regression, we grouped patient race into 2 categories: Black/African American and Other, which included White, Asian, and Hispanic/Latino participants.
Crude and adjusted multivariable logistic regression analyses were used to calculate prevalence odds ratios with 95% confidence intervals. Covariate inclusion in the multivariable logistic regression was based on a priori hypothesis/knowledge and was meant to estimate the independent effect of race after adjustment for income, HS severity, and history of prescription pain medication use. Other variables, including tobacco use, obesity, mood disorders, and current HS treatments, were all individually tested in the multivariate analysis and did not significantly impact the odds ratio for high pain. Statistical adjustment slightly decreased (19%) the magnitude between crude and adjusted prevalence odds ratios for the association between Black/African American race and high pain score.
Results
Survey Demographics —The final analysis included 204 survey respondents. Most respondents were Black/African American (58.82%), and nearly all were female (89.71%)(Table 1). The mean age (SD) of respondents was 37.37 (11.29) years (range, 19-70 years). Many respondents reported having completed some college (36.27%) or receiving a bachelor’s degree (19.12%). Of patients who were not Black/African American, 10.71% had higher than a master’s degree, whereas no Black/African American patients held a degree higher than a master’s ( P = .0052). Additionally, more Black/African American respondents (35.83%) reported an annual household income level of less than $25,000 compared with respondents who were not Black/African American (19.05%, P = .0001). Most respondents rated the severity of their HS as moderate or severe (46.57% and 41.67%, respectively), and there was no significant difference in reported severity of HS between racial groups ( P = .5395).


Pain Scores—As documented in the Methods, respondents were asked to rate their HS-related pain intensity from 0 to 10 using the NRS. The average pain score (SD)—the level of pain intensity over the prior month—was 6.39 (2.56)(range, 0–10). The mean pain score (SD) at the time of the survey was 3.61 (2.98)(range, 0–10)(Table 1). These data revealed that Black/African American patients had a significantly higher average pain score (SD) than patients with HS who were not Black/African American (7.08 [2.49] and 5.40 [2.35], respectively; P<.0001). After adjustment with multivariable logistical regression, Black/African American patients had 4-fold increased odds for very severe levels of pain (score of ≥8) compared with patients who were not Black/African American.
Pain Management—Although pain scores were higher for Black/African American patients with HS, there was no significant difference in the perception of pain control between racial groups (P=.0761). Additionally, we found low income (adjusted prevalence odds ratio [POR], 0.22; 95% CI, 0.05-0.91), a history of prescription pain medication use (adjusted POR, 2.25; 95% CI, 1.13-4.51), and HS severity (adjusted POR, 4.40; 95% CI, 1.11-17.36) all to be independent risk factors contributing to higher pain scores in patients with HS (Table 2). Lastly, we noted current or reported history of pain medication use was significantly correlated with higher pain scores (P=.0280 and P=.0213, respectively).

Satisfaction With Pain Management—The level of satisfaction with physician management of HS-related pain was significantly different between Black/African American patients and those who were not Black/African American (P=.0129). Of those who identified as Black/African American, 26.7% (n=32) strongly disagreed with the statement, “I am satisfied with how my pain related to HS is being managed by my doctors,” whereas only 15.5% (n=13) of patients who were not Black/African American strongly disagreed.
Comment
There is no cure for HS, and a large focus of treatment is pain management. Because racial disparities in the treatment of chronic pain will affect those with HS, we conducted a cross-sectional analysis of pain and pain management among HS patients. We found that Black/African American patients with HS have higher average pain scores than those who are not Black/African American and were 4 times more likely to experience very severe pain. Prior studies have established that patients with HS often report higher pain levels than patients with other chronic inflammatory skin conditions, 7,8 and our study identified racial disparities in HS-related pain management.
Measuring pain is challenging because of its multidimensional and subjective nature, making it essential to consider underlying causes and patients’ emotional responses to pain.9 By adjusting for confounding factors that may influence pain, such as mood disorders, disease severity, comorbidities, and medication use, we were able to gain better insight into fundamental differences in average pain intensity levels among racial groups and assess what factors may be contributing to a patient’s pain perception. Our study determined that lower income levels, higher HS disease severity, and a history of prescription pain medication use were all independent risk factors for high pain. Of note, obesity, tobacco use, and mood disorders such as anxiety and depression did not significantly differ between racial groups or increase the odds of high pain between racial groups identified.
With low income being an independent risk factor for high pain, we must consider the social determinants of health and how they may influence the pain experience in HS. We speculate that low income may be associated with other social determinants of health for the patients assessed in this study, such as lack of social and community support or limited health care access that contribute to worse health outcomes.10,11 In addition, low income contributes to limited access to medical care or treatments12; without access to effective HS management, lower-income patients may be at risk for higher disease severity and thus higher pain levels. However, economic stability is only a part of the whole picture; therefore, assessing the other social determinants of health in patients with HS may lead to better health outcomes and quality of life.
Another identified risk factor for high pain was a reported history of prescription pain medication use. This finding suggests that patients with moderate to severe pain likely have required stronger analgesic medications in the past. We further speculate that high pain levels in patients who have received prescription pain medications indicate either undertreatment, mistreatment, or recalcitrant pain. More research is needed to assess the relationship between HS-related pain intensity, analgesic medications, and providers who manage HS-related pain.
We also found that Black/African American patients with HS had a significantly higher dissatisfaction with their physician’s management of their pain, which could be attributable to several factors, including biological differences in medication metabolism (in which the patient has medication-resistant HS), undertreatment of pain, and/or poor doctor-patient relations. These reasons coincide with other diseases where health disparities are found.13-15 Recognizing these factors will be key to dismantling the disparities in HS that are noted within this study. The limitations of this work include the cross-sectional study design and its inability to evaluate causal factors of high pain levels across racial groups, the NRS lack of insight on pain chronicity or pain experience,7 the lack of provider or institution perspectives, and self-reported data. Additionally, only patients with email access were included, which may have excluded vulnerable populations with more pain associated with their HS.
Our findings highlight an area for further investigation to assess why these racial differences exist in HS-related pain. The results also emphasize the need for research evaluating whether systemic or health care provider biases contribute to racial differences in HS-related pain management.
Acknowledgment — Dr. Weir was supported by the Predoctoral Clinical/Translational Research Program (TL1), a National Institutes of Health Ruth L. Kirschstein National Research Service Award (NRSA), through the University of Alabama at Birmingham (UAB) Center for Clinical and Translational Science (CCTS).
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764. doi:10.1001/jamadermatol.2017.0201
- Nguyen TV, Damiani G, Orenstein LAV, et al. Hidradenitis suppurativa: an update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J Eur Acad Dermatol Venereol. 2021;35:50-61. doi:10.1111/jdv.16677
- Krajewski PK, Matusiak Ł, von Stebut E, et al. Pain in hidradenitis suppurativa: a cross-sectional study of 1,795 patients. Acta Derm Venereol. 2021;101:adv00364. doi:10.2340/00015555-3724
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Morales ME, Yong RJ. Racial and ethnic disparities in the treatment of chronic pain. Pain Med. 2021;22:75-90. doi:10.1093/pm/pnaa427
- US Department of Health and Human Services. 2019 National Healthcare Quality and Disparities Report. December 2020. Accessed June 21, 2023. https://www.ahrq.gov/sites/default/files/wysiwyg/research/findings/nhqrdr/2019qdr.pdf
- Hoffman KM, Trawalter S, Axt JR, et al. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A. 2016;113:4296-4301. doi:10.1073/pnas.1516047113
- Patel ZS, Hoffman LK, Buse DC, et al. Pain, psychological comorbidities, disability, and impaired quality of life in hidradenitis suppurativa. Curr Pain Headache Rep. 2017;21:49. doi:10.1007/s11916-017-0647-3. Published correction appears in Curr Pain Headache Rep. 2017;21:52.
- McDowell I. Pain measurements. In: Measuring Health: A Guide to Rating Scales and Questionnaires. Oxford University Press; 2006:477-478.
- Singh GK, Daus GP, Allender M, et al. Social determinants of health in the United States: addressing major health inequality trends for the nation, 1935-2016. Int J MCH AIDS. 2017;6:139-164. doi:10.21106/ijma.236
- Sulley S, Bayssie M. Social determinants of health: an evaluation of risk factors associated with inpatient presentations in the United States. Cureus. 2021;13:E13287. doi:10.7759/cureus.13287
- Lazar M, Davenport L. Barriers to health care access for low income families: a review of literature. J Community Health Nurs. 2018;35:28-37. doi:10.1080/07370016.2018.1404832
- Ghoshal M, Shapiro H, Todd K, et al. Chronic noncancer pain management and systemic racism: time to move toward equal care standards.J Pain Res. 2020;13:2825-2836. doi:10.214/JPR.S287314
- Cintron A, Morrison RS. Pain and ethnicity in the United States: a systematic review. J Palliat Med. 2006;9:1454-1473. doi:10.1089/jpm.2006.9.1454
- Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003;4:277-294. doi:10.1046/j.1526-4637.2003.03034.x. Published correction appears in Pain Med. 2005;6:99.
- Garg A, Kirby JS, Lavian J, et al. Sex- and age-adjusted population analysis of prevalence estimates for hidradenitis suppurativa in the United States. JAMA Dermatol. 2017;153:760-764. doi:10.1001/jamadermatol.2017.0201
- Nguyen TV, Damiani G, Orenstein LAV, et al. Hidradenitis suppurativa: an update on epidemiology, phenotypes, diagnosis, pathogenesis, comorbidities and quality of life. J Eur Acad Dermatol Venereol. 2021;35:50-61. doi:10.1111/jdv.16677
- Krajewski PK, Matusiak Ł, von Stebut E, et al. Pain in hidradenitis suppurativa: a cross-sectional study of 1,795 patients. Acta Derm Venereol. 2021;101:adv00364. doi:10.2340/00015555-3724
- Savage KT, Singh V, Patel ZS, et al. Pain management in hidradenitis suppurativa and a proposed treatment algorithm. J Am Acad Dermatol. 2021;85:187-199. doi:10.1016/j.jaad.2020.09.039
- Morales ME, Yong RJ. Racial and ethnic disparities in the treatment of chronic pain. Pain Med. 2021;22:75-90. doi:10.1093/pm/pnaa427
- US Department of Health and Human Services. 2019 National Healthcare Quality and Disparities Report. December 2020. Accessed June 21, 2023. https://www.ahrq.gov/sites/default/files/wysiwyg/research/findings/nhqrdr/2019qdr.pdf
- Hoffman KM, Trawalter S, Axt JR, et al. Racial bias in pain assessment and treatment recommendations, and false beliefs about biological differences between blacks and whites. Proc Natl Acad Sci U S A. 2016;113:4296-4301. doi:10.1073/pnas.1516047113
- Patel ZS, Hoffman LK, Buse DC, et al. Pain, psychological comorbidities, disability, and impaired quality of life in hidradenitis suppurativa. Curr Pain Headache Rep. 2017;21:49. doi:10.1007/s11916-017-0647-3. Published correction appears in Curr Pain Headache Rep. 2017;21:52.
- McDowell I. Pain measurements. In: Measuring Health: A Guide to Rating Scales and Questionnaires. Oxford University Press; 2006:477-478.
- Singh GK, Daus GP, Allender M, et al. Social determinants of health in the United States: addressing major health inequality trends for the nation, 1935-2016. Int J MCH AIDS. 2017;6:139-164. doi:10.21106/ijma.236
- Sulley S, Bayssie M. Social determinants of health: an evaluation of risk factors associated with inpatient presentations in the United States. Cureus. 2021;13:E13287. doi:10.7759/cureus.13287
- Lazar M, Davenport L. Barriers to health care access for low income families: a review of literature. J Community Health Nurs. 2018;35:28-37. doi:10.1080/07370016.2018.1404832
- Ghoshal M, Shapiro H, Todd K, et al. Chronic noncancer pain management and systemic racism: time to move toward equal care standards.J Pain Res. 2020;13:2825-2836. doi:10.214/JPR.S287314
- Cintron A, Morrison RS. Pain and ethnicity in the United States: a systematic review. J Palliat Med. 2006;9:1454-1473. doi:10.1089/jpm.2006.9.1454
- Green CR, Anderson KO, Baker TA, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003;4:277-294. doi:10.1046/j.1526-4637.2003.03034.x. Published correction appears in Pain Med. 2005;6:99.
Practice Points
- Racial disparities exist in the management of hidradenitis suppurativa (HS)–related pain.
- Black/African American patients with HS are 4 times more likely to experience very severe pain than patients of other races or ethnicities.
- Lower income levels, higher HS disease severity, and a history of prescription pain medication use are all independent risk factors for very severe pain in patients with HS.
Higher alcohol consumption linked to early-onset CRC
TOPLINE:
Higher levels of alcohol consumption appear to increase an individual’s risk of early-onset colorectal cancer (CRC), particularly distal colon and rectal cancers, according to a population-based study from South Korea.
METHODOLOGY:
- The investigators retrospectively compared average daily alcohol consumption with early-onset CRC risk among nearly 5.7 million adults younger than 50 years, using data from the Korean National Health Insurance Service.
- Alcohol consumption levels were defined as nondrinker, light (< 10 g/day or < 0.7 U.S. drinks/day), moderate (10-30 g/day for men, 10-20 g/day for women), and heavy (≥ 30 g/day or ≥ 2.1 drinks/day for men, ≥ 20 g/day or ≥ 1.4 drinks/day for women).
- The primary outcome was incidence of early-onset CRC diagnosed before age 50. Models were adjusted for age, sex, smoking status, exercise, and income, as well as for comorbidities.
TAKEAWAY:
- Overall, 8,314 incident early-onset CRC cases occurred during the mean follow-up period of 7.4 years.
- Compared with light drinking, moderate and heavy drinking were associated with a significantly elevated risk of early-onset CRC (adjusted hazard ratio, 1.09 and 1.20, respectively); by sex, significant associations were found only among men.
- Among men, heavy drinking vs. light drinking was associated with a 26% increased risk of distal colon cancer, a 17% higher risk of rectal cancer, and a 29% higher risk of unspecified colon cancer (but not proximal colon cancer).
- Among women, moderate drinking was associated with a 47% increased risk of distal colon cancer. Among nondrinkers, there was a 14% reduced risk of rectal cancer, compared with light drinkers.
IN PRACTICE:
“This population-based study provides evidence that higher levels of alcohol consumption may increase the risk of early-onset CRC,” the investigators concluded. “[E]ffective interventions are required to discourage alcohol consumption among young people and to tailor CRC screening approaches for high-risk individuals.”
SOURCE:
The study was led by researchers at Seoul National University, South Korea. It was published online in the Journal of Clinical Oncology.
LIMITATIONS:
Study limitations include self-reported alcohol consumption. Data were missing for a higher number of male participants and younger participants, and there was a potential problem related to multiple comparisons and confounders. Only Korean individuals were included in the study, so larger studies involving various races are needed.
DISCLOSURES:
Funding was provided by grants from the Korea Health Technology R&D Project and the Ministry of Health and Welfare, Republic of Korea. No potential conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
Higher levels of alcohol consumption appear to increase an individual’s risk of early-onset colorectal cancer (CRC), particularly distal colon and rectal cancers, according to a population-based study from South Korea.
METHODOLOGY:
- The investigators retrospectively compared average daily alcohol consumption with early-onset CRC risk among nearly 5.7 million adults younger than 50 years, using data from the Korean National Health Insurance Service.
- Alcohol consumption levels were defined as nondrinker, light (< 10 g/day or < 0.7 U.S. drinks/day), moderate (10-30 g/day for men, 10-20 g/day for women), and heavy (≥ 30 g/day or ≥ 2.1 drinks/day for men, ≥ 20 g/day or ≥ 1.4 drinks/day for women).
- The primary outcome was incidence of early-onset CRC diagnosed before age 50. Models were adjusted for age, sex, smoking status, exercise, and income, as well as for comorbidities.
TAKEAWAY:
- Overall, 8,314 incident early-onset CRC cases occurred during the mean follow-up period of 7.4 years.
- Compared with light drinking, moderate and heavy drinking were associated with a significantly elevated risk of early-onset CRC (adjusted hazard ratio, 1.09 and 1.20, respectively); by sex, significant associations were found only among men.
- Among men, heavy drinking vs. light drinking was associated with a 26% increased risk of distal colon cancer, a 17% higher risk of rectal cancer, and a 29% higher risk of unspecified colon cancer (but not proximal colon cancer).
- Among women, moderate drinking was associated with a 47% increased risk of distal colon cancer. Among nondrinkers, there was a 14% reduced risk of rectal cancer, compared with light drinkers.
IN PRACTICE:
“This population-based study provides evidence that higher levels of alcohol consumption may increase the risk of early-onset CRC,” the investigators concluded. “[E]ffective interventions are required to discourage alcohol consumption among young people and to tailor CRC screening approaches for high-risk individuals.”
SOURCE:
The study was led by researchers at Seoul National University, South Korea. It was published online in the Journal of Clinical Oncology.
LIMITATIONS:
Study limitations include self-reported alcohol consumption. Data were missing for a higher number of male participants and younger participants, and there was a potential problem related to multiple comparisons and confounders. Only Korean individuals were included in the study, so larger studies involving various races are needed.
DISCLOSURES:
Funding was provided by grants from the Korea Health Technology R&D Project and the Ministry of Health and Welfare, Republic of Korea. No potential conflicts of interest were reported.
A version of this article first appeared on Medscape.com.
TOPLINE:
Higher levels of alcohol consumption appear to increase an individual’s risk of early-onset colorectal cancer (CRC), particularly distal colon and rectal cancers, according to a population-based study from South Korea.
METHODOLOGY:
- The investigators retrospectively compared average daily alcohol consumption with early-onset CRC risk among nearly 5.7 million adults younger than 50 years, using data from the Korean National Health Insurance Service.
- Alcohol consumption levels were defined as nondrinker, light (< 10 g/day or < 0.7 U.S. drinks/day), moderate (10-30 g/day for men, 10-20 g/day for women), and heavy (≥ 30 g/day or ≥ 2.1 drinks/day for men, ≥ 20 g/day or ≥ 1.4 drinks/day for women).
- The primary outcome was incidence of early-onset CRC diagnosed before age 50. Models were adjusted for age, sex, smoking status, exercise, and income, as well as for comorbidities.
TAKEAWAY:
- Overall, 8,314 incident early-onset CRC cases occurred during the mean follow-up period of 7.4 years.
- Compared with light drinking, moderate and heavy drinking were associated with a significantly elevated risk of early-onset CRC (adjusted hazard ratio, 1.09 and 1.20, respectively); by sex, significant associations were found only among men.
- Among men, heavy drinking vs. light drinking was associated with a 26% increased risk of distal colon cancer, a 17% higher risk of rectal cancer, and a 29% higher risk of unspecified colon cancer (but not proximal colon cancer).
- Among women, moderate drinking was associated with a 47% increased risk of distal colon cancer. Among nondrinkers, there was a 14% reduced risk of rectal cancer, compared with light drinkers.
IN PRACTICE:
“This population-based study provides evidence that higher levels of alcohol consumption may increase the risk of early-onset CRC,” the investigators concluded. “[E]ffective interventions are required to discourage alcohol consumption among young people and to tailor CRC screening approaches for high-risk individuals.”
SOURCE:
The study was led by researchers at Seoul National University, South Korea. It was published online in the Journal of Clinical Oncology.
LIMITATIONS:
Study limitations include self-reported alcohol consumption. Data were missing for a higher number of male participants and younger participants, and there was a potential problem related to multiple comparisons and confounders. Only Korean individuals were included in the study, so larger studies involving various races are needed.
DISCLOSURES:
Funding was provided by grants from the Korea Health Technology R&D Project and the Ministry of Health and Welfare, Republic of Korea. No potential conflicts of interest were reported.
A version of this article first appeared on Medscape.com.



