User login
Cardiorespiratory fitness linked to cancer risk, mortality?
TOPLINE:
a large Swedish cohort study suggests.
METHODOLOGY:
- A prospective cohort study included 177,709 Swedish men (mean age, 42; mean body mass index, 26 kg/m2) who completed an occupational health profile assessment and were followed for a mean of 9.6 years.
- CRF was assessed by determining maximal oxygen consumption during an aerobic fitness test, known as a submaximal Åstrand cycle ergometer test.
- Participants reported physical activity habits, lifestyle, and perceived health.
- Data on prostate, colon, and lung cancer incidence and mortality were derived from national registers.
- Outcomes from three higher CRF groups (low, > 25-35; moderate, > 35-45; high, > 45 mL/min per kg) were compared with those from the very low CRF group (25 mL/min per kg or less). Models were adjusted for various factors, including age, BMI, education, dietary habits, comorbidity, and smoking.
TAKEAWAY:
- During follow-up, investigators identified 1,918 prostate, 499 colon, and 283 lung cancer cases as well as 141 prostate, 207 lung, and 152 colon cancer deaths.
- In the fully adjusted model, higher CRF levels were associated with a significantly lower risk for colon cancer (hazard ratio, 0.72 for moderate; HR, 0.63 for high).
- In this model, higher CRF was also associated with a lower risk of death from prostate cancer (HR, 0.67 for low; HR, 0.57 for moderate; HR, 0.29 for high).
- For lung cancer mortality, only high CRF was associated with a significantly lower risk of death (HR, 0.41).
- An association between CRF and lung cancer incidence (HR, 0.99) and death (HR, 0.99) was only evident among adults aged 60 and older.
IN PRACTICE:
“The clinical implications of these findings further emphasize the importance of CRF for possibly reducing cancer incidence and mortality,” the authors concluded. “It is important for the general public to understand that higher-intensity [physical activity] has greater effects on CRF and is likely to be more protective against the risk of developing and dying from certain cancers.”
SOURCE:
The study was led by Elin Ekblom-Bak, PhD, from the Swedish School of Sport and Health Sciences, Stockholm. It was published online in JAMA Network Open.
LIMITATIONS:
The study was limited by voluntary participation, inclusion of only employed individuals, and estimations of CRF via submaximal tests. Data on smoking status were not optimal and there was a small number of cancer cases and deaths.
DISCLOSURES:
Funding was provided by the Swedish Cancer Society. The authors have reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
a large Swedish cohort study suggests.
METHODOLOGY:
- A prospective cohort study included 177,709 Swedish men (mean age, 42; mean body mass index, 26 kg/m2) who completed an occupational health profile assessment and were followed for a mean of 9.6 years.
- CRF was assessed by determining maximal oxygen consumption during an aerobic fitness test, known as a submaximal Åstrand cycle ergometer test.
- Participants reported physical activity habits, lifestyle, and perceived health.
- Data on prostate, colon, and lung cancer incidence and mortality were derived from national registers.
- Outcomes from three higher CRF groups (low, > 25-35; moderate, > 35-45; high, > 45 mL/min per kg) were compared with those from the very low CRF group (25 mL/min per kg or less). Models were adjusted for various factors, including age, BMI, education, dietary habits, comorbidity, and smoking.
TAKEAWAY:
- During follow-up, investigators identified 1,918 prostate, 499 colon, and 283 lung cancer cases as well as 141 prostate, 207 lung, and 152 colon cancer deaths.
- In the fully adjusted model, higher CRF levels were associated with a significantly lower risk for colon cancer (hazard ratio, 0.72 for moderate; HR, 0.63 for high).
- In this model, higher CRF was also associated with a lower risk of death from prostate cancer (HR, 0.67 for low; HR, 0.57 for moderate; HR, 0.29 for high).
- For lung cancer mortality, only high CRF was associated with a significantly lower risk of death (HR, 0.41).
- An association between CRF and lung cancer incidence (HR, 0.99) and death (HR, 0.99) was only evident among adults aged 60 and older.
IN PRACTICE:
“The clinical implications of these findings further emphasize the importance of CRF for possibly reducing cancer incidence and mortality,” the authors concluded. “It is important for the general public to understand that higher-intensity [physical activity] has greater effects on CRF and is likely to be more protective against the risk of developing and dying from certain cancers.”
SOURCE:
The study was led by Elin Ekblom-Bak, PhD, from the Swedish School of Sport and Health Sciences, Stockholm. It was published online in JAMA Network Open.
LIMITATIONS:
The study was limited by voluntary participation, inclusion of only employed individuals, and estimations of CRF via submaximal tests. Data on smoking status were not optimal and there was a small number of cancer cases and deaths.
DISCLOSURES:
Funding was provided by the Swedish Cancer Society. The authors have reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
TOPLINE:
a large Swedish cohort study suggests.
METHODOLOGY:
- A prospective cohort study included 177,709 Swedish men (mean age, 42; mean body mass index, 26 kg/m2) who completed an occupational health profile assessment and were followed for a mean of 9.6 years.
- CRF was assessed by determining maximal oxygen consumption during an aerobic fitness test, known as a submaximal Åstrand cycle ergometer test.
- Participants reported physical activity habits, lifestyle, and perceived health.
- Data on prostate, colon, and lung cancer incidence and mortality were derived from national registers.
- Outcomes from three higher CRF groups (low, > 25-35; moderate, > 35-45; high, > 45 mL/min per kg) were compared with those from the very low CRF group (25 mL/min per kg or less). Models were adjusted for various factors, including age, BMI, education, dietary habits, comorbidity, and smoking.
TAKEAWAY:
- During follow-up, investigators identified 1,918 prostate, 499 colon, and 283 lung cancer cases as well as 141 prostate, 207 lung, and 152 colon cancer deaths.
- In the fully adjusted model, higher CRF levels were associated with a significantly lower risk for colon cancer (hazard ratio, 0.72 for moderate; HR, 0.63 for high).
- In this model, higher CRF was also associated with a lower risk of death from prostate cancer (HR, 0.67 for low; HR, 0.57 for moderate; HR, 0.29 for high).
- For lung cancer mortality, only high CRF was associated with a significantly lower risk of death (HR, 0.41).
- An association between CRF and lung cancer incidence (HR, 0.99) and death (HR, 0.99) was only evident among adults aged 60 and older.
IN PRACTICE:
“The clinical implications of these findings further emphasize the importance of CRF for possibly reducing cancer incidence and mortality,” the authors concluded. “It is important for the general public to understand that higher-intensity [physical activity] has greater effects on CRF and is likely to be more protective against the risk of developing and dying from certain cancers.”
SOURCE:
The study was led by Elin Ekblom-Bak, PhD, from the Swedish School of Sport and Health Sciences, Stockholm. It was published online in JAMA Network Open.
LIMITATIONS:
The study was limited by voluntary participation, inclusion of only employed individuals, and estimations of CRF via submaximal tests. Data on smoking status were not optimal and there was a small number of cancer cases and deaths.
DISCLOSURES:
Funding was provided by the Swedish Cancer Society. The authors have reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
New consensus on biomarkers for diagnosis of neurocognitive disorders
A new European consensus statement offers expert guidance on which biomarkers to use for patients presenting with cognitive complaints.
Led by Giovanni B. Frisoni, MD, laboratory of neuroimaging of aging, University of Geneva, and director of the memory clinic at Geneva University Hospital, the multidisciplinary task force set out to define a patient-centered diagnostic workflow for the rational and cost-effective use of biomarkers in memory clinics.
The new algorithm is part of a consensus statement presented at the Congress of the European Academy of Neurology 2023. An interim update was published in June in Alzheimer’s and Dementia.
Which biomarker?
Many biomarkers can aid diagnosis, said Dr. Frisoni; the challenge is choosing which biomarker to use for an individual patient.
A literature-based search, he said, yields a number of recommendations, but the vast majority of these are either disease based or biomarker based. The task force notes that “in vivo biomarkers enable early etiological diagnosis of neurocognitive disorders. While they have good analytical validity, their clinical validity and utility are uncertain.”
“When you have a patient in front of you, you don’ t know whether they have Alzheimer’s disease,” Dr. Frisoni said.
“You have a differential diagnosis to make, and you have a number of biomarkers – a number of weapons in your armamentarium – you have to choose. You can’t use all of them – we would like to, but we cannot.”
He added that trying to determine from the literature which biomarker is most appropriate given individual clinical conditions and all of the potential combinations is impossible.
“You will not find evidence of the comparative diagnostic value and the added diagnostic value” of one test vs, another, he noted.
“Is CSF [cerebrospinal fluid] better than amyloid PET in a particular clinical situation? What do I gain in terms of positive and negative predictive value in all the possible clinical conditions that I encounter in my clinical practice?”
Dr. Frisoni said the reality is that clinicians in memory clinics end up using biomarkers that are “based on clinical opportunities.”
For instance, “if you have a proficient nuclear medic, you use PET a lot.” In contrast, “if you have a proficient laboratory medic,” CSF markers will be favored – a situation that he said is “not ideal” and has resulted in large discrepancies in diagnostic approaches across Europe.
Harmonizing clinical practice
In a bid to harmonize clinical practice, 22 European experts from 11 European scientific societies and the executive director of Alzheimer Europe set out to develop a multidisciplinary consensus algorithm for the biomarker-based diagnosis of neurocognitive disorders in general, rather than specific neurocognitive disorders.
They used the Delphi method, in which a systematic literature review of the literature was followed by the drafting of a series of clinical statements by an executive board. These were then presented to the expert panel. If a majority consensus was reached on a given statement, it was considered closed. Questions for which there was no consensus were revised and presented to the panel again. The process was repeated until a consensus was reached.
A total of 56 statements underwent six rounds of discussion. A final online meeting led to the development of a diagnostic algorithm for patients who attend memory clinics for cognitive complaints.
The algorithm features three potential assessment waves. Wave 1 defines 11 clinical profiles that are based on the results of clinical and neuropsychological assessments, blood exams, brain imaging, and, in specific cases, electroencephalography. Wave 2 defines first-line biomarkers based on Wave 1 clinical profiles, and Wave 3 defines the second-line biomarker based on Wave 2 biomarker results.
When a patient’s clinical profile suggests Alzheimer’s disease and, in undefined cases, cerebrospinal fluid biomarkers are used first line. When CSF is inconclusive, 18-fluorodeoxyglucose positron emission tomography (FDG-PET) is used second line.
When the clinical profile suggests frontotemporal lobar degeneration or motor tauopathies, FDG-PET is first line and CSF biomarkers second line in atypical metabolic patter cases. When the clinical profile suggests Lewy body disease, dopamine transporter SPECT is first line and cardia I23I-metaiodobenzylguanidine scintigraphy is second line.
Dr. Frisoni noted that the panel strongly recommends performing biomarker tests for patients younger than 70. For those aged 70-85 years, biomarker testing is only recommended for patients with specific clinical features. For patients older than 85, biomarker testing is recommended only in “exceptional circumstances.”
Dr. Frisoni noted that the consensus document has a number of limitations.
“First of all, we could not capture all the theoretical possible combinations” of potential diagnosis and relevant biomarker tests. “There are so many that it’s virtually impossible.”
He also noted that the agreement among the panel for the use of some markers was “relatively low” at “barely 50%,” while for others, the agreement was approximately 70%.
The consensus document also does not explicitly address patients with “mixed pathologies,” which are common. In addition, it does not include emerging biomarkers, such as neurofilament light polypeptide levels, an indicator of axonal compromise.
“Last, but not least,” Dr. Frisoni said, the consensus document requires validation.
“This is a paper and pencil exercise. We, as self-appointed experts, can recommend ... whatever we want, but we must check whether what we write is applicable, feasible.”
In other words, it must be determined whether the “real patient journey” fits with the “ideal patient journey” set out in the consensus document.
This kind of validation, Dr. Frisoni said, is “usually not done for this type of exercise,” but “we want to do it in this case.”
Pros and cons
Bogdan Draganski, MD, consultant in neurology at the department of clinical neurosciences and director of the neuroimaging research laboratory, University Hospital of Lausanne (Switzerland), who cochaired the session, told this news organization that he was “swaying between two extremes” when considering the usefulness of the consensus document.
On one hand, the “reductionist approach” of breaking down a “complex issue into an algorithm” via the Delphi method risks introducing subjective bias.
He said machine learning and artificial intelligence could answer some of the questions posed by clinicians and, by extension, the statements included in the Delphi process by assessing the available data in a more objective manner.
On the other hand, Dr. Draganski said that reducing the options available to clinicians when making a differential diagnosis into the current algorithm is, pragmatically speaking, a “good approach.”
From this standpoint, the danger of using machine learning to answer clinical questions is that it “doesn’t take the responsibility” for the final decision, which means “we’re closing the loop of subjective decision-making for an individual doctor.”
He also applauded the idea of trying to provide more uniform patient assessment across Europe, although he believes “we have a long way to go” before it can deliver on the promise of personalized medicine.
Like Dr. Frisoni, Dr. Draganski noted the fact that patients with potential neurocognitive disorders often have multiple pathologies, which can include cardiovascular problems, depression, and cancer and that that could affect the choice of diagnostic biomarkers.
The second issue, he said, concerns implementation of the consensus document, which is a political decision that centers around “how politicians will define ‘uniformity’ and equal access to technological or nontechnological platforms.”
Achieving uniformity will require a pan-regional collaboration, he noted.
The task force was supported by unrestricted grants from F. Hoffmann-La Roche, Biogen International GmbH, Eisai Europe Limited, Life Molecular Imaging GmbH, and OM Pharma Suisse SA. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new European consensus statement offers expert guidance on which biomarkers to use for patients presenting with cognitive complaints.
Led by Giovanni B. Frisoni, MD, laboratory of neuroimaging of aging, University of Geneva, and director of the memory clinic at Geneva University Hospital, the multidisciplinary task force set out to define a patient-centered diagnostic workflow for the rational and cost-effective use of biomarkers in memory clinics.
The new algorithm is part of a consensus statement presented at the Congress of the European Academy of Neurology 2023. An interim update was published in June in Alzheimer’s and Dementia.
Which biomarker?
Many biomarkers can aid diagnosis, said Dr. Frisoni; the challenge is choosing which biomarker to use for an individual patient.
A literature-based search, he said, yields a number of recommendations, but the vast majority of these are either disease based or biomarker based. The task force notes that “in vivo biomarkers enable early etiological diagnosis of neurocognitive disorders. While they have good analytical validity, their clinical validity and utility are uncertain.”
“When you have a patient in front of you, you don’ t know whether they have Alzheimer’s disease,” Dr. Frisoni said.
“You have a differential diagnosis to make, and you have a number of biomarkers – a number of weapons in your armamentarium – you have to choose. You can’t use all of them – we would like to, but we cannot.”
He added that trying to determine from the literature which biomarker is most appropriate given individual clinical conditions and all of the potential combinations is impossible.
“You will not find evidence of the comparative diagnostic value and the added diagnostic value” of one test vs, another, he noted.
“Is CSF [cerebrospinal fluid] better than amyloid PET in a particular clinical situation? What do I gain in terms of positive and negative predictive value in all the possible clinical conditions that I encounter in my clinical practice?”
Dr. Frisoni said the reality is that clinicians in memory clinics end up using biomarkers that are “based on clinical opportunities.”
For instance, “if you have a proficient nuclear medic, you use PET a lot.” In contrast, “if you have a proficient laboratory medic,” CSF markers will be favored – a situation that he said is “not ideal” and has resulted in large discrepancies in diagnostic approaches across Europe.
Harmonizing clinical practice
In a bid to harmonize clinical practice, 22 European experts from 11 European scientific societies and the executive director of Alzheimer Europe set out to develop a multidisciplinary consensus algorithm for the biomarker-based diagnosis of neurocognitive disorders in general, rather than specific neurocognitive disorders.
They used the Delphi method, in which a systematic literature review of the literature was followed by the drafting of a series of clinical statements by an executive board. These were then presented to the expert panel. If a majority consensus was reached on a given statement, it was considered closed. Questions for which there was no consensus were revised and presented to the panel again. The process was repeated until a consensus was reached.
A total of 56 statements underwent six rounds of discussion. A final online meeting led to the development of a diagnostic algorithm for patients who attend memory clinics for cognitive complaints.
The algorithm features three potential assessment waves. Wave 1 defines 11 clinical profiles that are based on the results of clinical and neuropsychological assessments, blood exams, brain imaging, and, in specific cases, electroencephalography. Wave 2 defines first-line biomarkers based on Wave 1 clinical profiles, and Wave 3 defines the second-line biomarker based on Wave 2 biomarker results.
When a patient’s clinical profile suggests Alzheimer’s disease and, in undefined cases, cerebrospinal fluid biomarkers are used first line. When CSF is inconclusive, 18-fluorodeoxyglucose positron emission tomography (FDG-PET) is used second line.
When the clinical profile suggests frontotemporal lobar degeneration or motor tauopathies, FDG-PET is first line and CSF biomarkers second line in atypical metabolic patter cases. When the clinical profile suggests Lewy body disease, dopamine transporter SPECT is first line and cardia I23I-metaiodobenzylguanidine scintigraphy is second line.
Dr. Frisoni noted that the panel strongly recommends performing biomarker tests for patients younger than 70. For those aged 70-85 years, biomarker testing is only recommended for patients with specific clinical features. For patients older than 85, biomarker testing is recommended only in “exceptional circumstances.”
Dr. Frisoni noted that the consensus document has a number of limitations.
“First of all, we could not capture all the theoretical possible combinations” of potential diagnosis and relevant biomarker tests. “There are so many that it’s virtually impossible.”
He also noted that the agreement among the panel for the use of some markers was “relatively low” at “barely 50%,” while for others, the agreement was approximately 70%.
The consensus document also does not explicitly address patients with “mixed pathologies,” which are common. In addition, it does not include emerging biomarkers, such as neurofilament light polypeptide levels, an indicator of axonal compromise.
“Last, but not least,” Dr. Frisoni said, the consensus document requires validation.
“This is a paper and pencil exercise. We, as self-appointed experts, can recommend ... whatever we want, but we must check whether what we write is applicable, feasible.”
In other words, it must be determined whether the “real patient journey” fits with the “ideal patient journey” set out in the consensus document.
This kind of validation, Dr. Frisoni said, is “usually not done for this type of exercise,” but “we want to do it in this case.”
Pros and cons
Bogdan Draganski, MD, consultant in neurology at the department of clinical neurosciences and director of the neuroimaging research laboratory, University Hospital of Lausanne (Switzerland), who cochaired the session, told this news organization that he was “swaying between two extremes” when considering the usefulness of the consensus document.
On one hand, the “reductionist approach” of breaking down a “complex issue into an algorithm” via the Delphi method risks introducing subjective bias.
He said machine learning and artificial intelligence could answer some of the questions posed by clinicians and, by extension, the statements included in the Delphi process by assessing the available data in a more objective manner.
On the other hand, Dr. Draganski said that reducing the options available to clinicians when making a differential diagnosis into the current algorithm is, pragmatically speaking, a “good approach.”
From this standpoint, the danger of using machine learning to answer clinical questions is that it “doesn’t take the responsibility” for the final decision, which means “we’re closing the loop of subjective decision-making for an individual doctor.”
He also applauded the idea of trying to provide more uniform patient assessment across Europe, although he believes “we have a long way to go” before it can deliver on the promise of personalized medicine.
Like Dr. Frisoni, Dr. Draganski noted the fact that patients with potential neurocognitive disorders often have multiple pathologies, which can include cardiovascular problems, depression, and cancer and that that could affect the choice of diagnostic biomarkers.
The second issue, he said, concerns implementation of the consensus document, which is a political decision that centers around “how politicians will define ‘uniformity’ and equal access to technological or nontechnological platforms.”
Achieving uniformity will require a pan-regional collaboration, he noted.
The task force was supported by unrestricted grants from F. Hoffmann-La Roche, Biogen International GmbH, Eisai Europe Limited, Life Molecular Imaging GmbH, and OM Pharma Suisse SA. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new European consensus statement offers expert guidance on which biomarkers to use for patients presenting with cognitive complaints.
Led by Giovanni B. Frisoni, MD, laboratory of neuroimaging of aging, University of Geneva, and director of the memory clinic at Geneva University Hospital, the multidisciplinary task force set out to define a patient-centered diagnostic workflow for the rational and cost-effective use of biomarkers in memory clinics.
The new algorithm is part of a consensus statement presented at the Congress of the European Academy of Neurology 2023. An interim update was published in June in Alzheimer’s and Dementia.
Which biomarker?
Many biomarkers can aid diagnosis, said Dr. Frisoni; the challenge is choosing which biomarker to use for an individual patient.
A literature-based search, he said, yields a number of recommendations, but the vast majority of these are either disease based or biomarker based. The task force notes that “in vivo biomarkers enable early etiological diagnosis of neurocognitive disorders. While they have good analytical validity, their clinical validity and utility are uncertain.”
“When you have a patient in front of you, you don’ t know whether they have Alzheimer’s disease,” Dr. Frisoni said.
“You have a differential diagnosis to make, and you have a number of biomarkers – a number of weapons in your armamentarium – you have to choose. You can’t use all of them – we would like to, but we cannot.”
He added that trying to determine from the literature which biomarker is most appropriate given individual clinical conditions and all of the potential combinations is impossible.
“You will not find evidence of the comparative diagnostic value and the added diagnostic value” of one test vs, another, he noted.
“Is CSF [cerebrospinal fluid] better than amyloid PET in a particular clinical situation? What do I gain in terms of positive and negative predictive value in all the possible clinical conditions that I encounter in my clinical practice?”
Dr. Frisoni said the reality is that clinicians in memory clinics end up using biomarkers that are “based on clinical opportunities.”
For instance, “if you have a proficient nuclear medic, you use PET a lot.” In contrast, “if you have a proficient laboratory medic,” CSF markers will be favored – a situation that he said is “not ideal” and has resulted in large discrepancies in diagnostic approaches across Europe.
Harmonizing clinical practice
In a bid to harmonize clinical practice, 22 European experts from 11 European scientific societies and the executive director of Alzheimer Europe set out to develop a multidisciplinary consensus algorithm for the biomarker-based diagnosis of neurocognitive disorders in general, rather than specific neurocognitive disorders.
They used the Delphi method, in which a systematic literature review of the literature was followed by the drafting of a series of clinical statements by an executive board. These were then presented to the expert panel. If a majority consensus was reached on a given statement, it was considered closed. Questions for which there was no consensus were revised and presented to the panel again. The process was repeated until a consensus was reached.
A total of 56 statements underwent six rounds of discussion. A final online meeting led to the development of a diagnostic algorithm for patients who attend memory clinics for cognitive complaints.
The algorithm features three potential assessment waves. Wave 1 defines 11 clinical profiles that are based on the results of clinical and neuropsychological assessments, blood exams, brain imaging, and, in specific cases, electroencephalography. Wave 2 defines first-line biomarkers based on Wave 1 clinical profiles, and Wave 3 defines the second-line biomarker based on Wave 2 biomarker results.
When a patient’s clinical profile suggests Alzheimer’s disease and, in undefined cases, cerebrospinal fluid biomarkers are used first line. When CSF is inconclusive, 18-fluorodeoxyglucose positron emission tomography (FDG-PET) is used second line.
When the clinical profile suggests frontotemporal lobar degeneration or motor tauopathies, FDG-PET is first line and CSF biomarkers second line in atypical metabolic patter cases. When the clinical profile suggests Lewy body disease, dopamine transporter SPECT is first line and cardia I23I-metaiodobenzylguanidine scintigraphy is second line.
Dr. Frisoni noted that the panel strongly recommends performing biomarker tests for patients younger than 70. For those aged 70-85 years, biomarker testing is only recommended for patients with specific clinical features. For patients older than 85, biomarker testing is recommended only in “exceptional circumstances.”
Dr. Frisoni noted that the consensus document has a number of limitations.
“First of all, we could not capture all the theoretical possible combinations” of potential diagnosis and relevant biomarker tests. “There are so many that it’s virtually impossible.”
He also noted that the agreement among the panel for the use of some markers was “relatively low” at “barely 50%,” while for others, the agreement was approximately 70%.
The consensus document also does not explicitly address patients with “mixed pathologies,” which are common. In addition, it does not include emerging biomarkers, such as neurofilament light polypeptide levels, an indicator of axonal compromise.
“Last, but not least,” Dr. Frisoni said, the consensus document requires validation.
“This is a paper and pencil exercise. We, as self-appointed experts, can recommend ... whatever we want, but we must check whether what we write is applicable, feasible.”
In other words, it must be determined whether the “real patient journey” fits with the “ideal patient journey” set out in the consensus document.
This kind of validation, Dr. Frisoni said, is “usually not done for this type of exercise,” but “we want to do it in this case.”
Pros and cons
Bogdan Draganski, MD, consultant in neurology at the department of clinical neurosciences and director of the neuroimaging research laboratory, University Hospital of Lausanne (Switzerland), who cochaired the session, told this news organization that he was “swaying between two extremes” when considering the usefulness of the consensus document.
On one hand, the “reductionist approach” of breaking down a “complex issue into an algorithm” via the Delphi method risks introducing subjective bias.
He said machine learning and artificial intelligence could answer some of the questions posed by clinicians and, by extension, the statements included in the Delphi process by assessing the available data in a more objective manner.
On the other hand, Dr. Draganski said that reducing the options available to clinicians when making a differential diagnosis into the current algorithm is, pragmatically speaking, a “good approach.”
From this standpoint, the danger of using machine learning to answer clinical questions is that it “doesn’t take the responsibility” for the final decision, which means “we’re closing the loop of subjective decision-making for an individual doctor.”
He also applauded the idea of trying to provide more uniform patient assessment across Europe, although he believes “we have a long way to go” before it can deliver on the promise of personalized medicine.
Like Dr. Frisoni, Dr. Draganski noted the fact that patients with potential neurocognitive disorders often have multiple pathologies, which can include cardiovascular problems, depression, and cancer and that that could affect the choice of diagnostic biomarkers.
The second issue, he said, concerns implementation of the consensus document, which is a political decision that centers around “how politicians will define ‘uniformity’ and equal access to technological or nontechnological platforms.”
Achieving uniformity will require a pan-regional collaboration, he noted.
The task force was supported by unrestricted grants from F. Hoffmann-La Roche, Biogen International GmbH, Eisai Europe Limited, Life Molecular Imaging GmbH, and OM Pharma Suisse SA. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In-office infusions at risk with new Medicare Part B reimbursement recommendation
The Medicare Payment Advisory Commission (MedPAC) is an independent agency to advise Congress on Medicare (MC) policy, much of which pertains to payment issues. The 17 commissioners meet publicly and issue two reports a year with their recommendations to Congress, who then decides whether to enact these recommendations or not.
One MedPAC recommendation in 2023 was quickly introduced in the House of Representatives in May and passed the Energy and Commerce Committee 49-0. That recommendation relates to “site neutrality” payments to MC providers. If passed by Congress, it would result in some “site-neutral” cuts to hospitals. That MedPAC recommendation was acted upon very quickly by Congress. Consequently, it is important to discuss the potential negative ramifications of other MedPAC recommendations released in June regarding reimbursement of Medicare Part B drugs and proactively educate Congress accordingly on those ramifications.
Medicare Part B drugs
Medicare Part B drugs are those administered by providers, unlike the Part D medications which are generally obtained through pharmacies. Presently, MC reimburses providers for the administered Part B medication based on the average sales price (ASP) plus 6%. However, with sequestration, that add-on amount is reduced to ASP plus 4.3%. It has long been touted by MedPAC and other policy makers that physicians choose to infuse higher-priced drugs in order to increase reimbursements. That has not been borne out when it comes to rheumatologists, and, in fact, a retired MedPAC commissioner even stated that premise did not hold true for rheumatologists.
Regardless, it continues to be suggested that MC should reduce its costs for Part B medications by reducing reimbursement to physicians. It should be noted that often the margins on the drugs are already quite thin, and at times the reimbursement amount, compared with the acquisition cost of the drug even leaves the physician “underwater.”
A few years ago, there was a proposed Part B demonstration project that essentially removed the +6% add-on and replaced it with a very low fixed amount that would have left most physicians “underwater” in their Part B drug acquisitions. This was vigorously opposed by physicians around the country, who let Congress know exactly how they felt. We have been told that the Coalition of State Rheumatology Organizations was one of the most vociferous organizations that helped in fighting back this proposal and resulting in its withdrawal.
MedPAC recommendations
That brings us back to MedPAC. In June, MedPAC released recommendations to Congress in an attempt to address the “high price of drugs” covered under MC Part B. Unfortunately, the recommendations do nothing to address the root cause of high drug prices, but once again attempt to balance MC expenditures on the backs of physicians. In this case, it is physicians who infuse Part B drugs in their office to chronically ill patients. In-office infusions have been shown to be the most cost-effective site of care, as well as being safer when compared with home infusion for a number of rheumatologic medications.
One of the MedPAC recommendations gives the Secretary of Health & Human Services the authority to establish a single ASP for drugs with “similar health effects.” The ambiguity of the phrase “similar health effects” should put us all on alert as to the significant unintended consequences that may result. For example, HHS could assign one ASP to all drugs that treat rheumatoid arthritis based on the lowest ASP of the group. This certainly would lead to a number of drugs being out of reach for MC beneficiaries if the artificial ASP of the medication is much lower than the actual acquisition cost of the drug, leaving physicians unable to acquire it. Yet, MedPAC states this recommendation would not affect access to care for MC beneficiaries.
Another recommendation would require HHS to reduce or eliminate the add-on percentage to the ASP for higher-priced drugs and/or put in an added fixed amount. This recommendation is clearly reminiscent of the old ill-conceived Part B demonstration project.
A fixed “add-on amount” might work if it is sufficient to cover the overhead of maintaining a provider’s infusion suite. But if practices are left underwater in their purchases of certain Part B drugs, there may be no choice but to stop offering those infusions to MC beneficiaries or – worst-case scenario – shut the door completely. Yet again, MedPAC stated that this recommendation would not result in a loss of access to these treatments for MC beneficiaries.
Loss of access?
Rheumatologists have gone to great lengths to continue offering care to MC patients in spite of the yearly cuts and threats of more cuts in the future to physician reimbursements. In addition, physicians have no annual inflationary update to their reimbursements. I am not sure how MedPAC concludes that continued cuts to physician fee schedules, along with a decrease in reimbursement for administered drugs, will not affect access to care for MC beneficiaries.
Finally, the timing on these recommendations is confusing, considering that implementation of the Inflation Reduction Act (IRA) has just begun. Next quarter, a number of Part B drugs will be subject to inflationary penalties; there will also be additional Part B biosimilars coming to market, resulting in lower ASPs. And don’t forget, the IRA just instituted an ASP plus 8% reimbursement for biosimilars in an attempt to get physicians to do something that the Centers for Medicare & Medicaid Services has asked them not to do. That is, choose a drug based on its reimbursement, not necessarily the one which is right for the patient.
Overall, with so many variables up in the air, now is not the time to create even more uncertainty for physicians and the Medicare patients that they take care of.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
The Medicare Payment Advisory Commission (MedPAC) is an independent agency to advise Congress on Medicare (MC) policy, much of which pertains to payment issues. The 17 commissioners meet publicly and issue two reports a year with their recommendations to Congress, who then decides whether to enact these recommendations or not.
One MedPAC recommendation in 2023 was quickly introduced in the House of Representatives in May and passed the Energy and Commerce Committee 49-0. That recommendation relates to “site neutrality” payments to MC providers. If passed by Congress, it would result in some “site-neutral” cuts to hospitals. That MedPAC recommendation was acted upon very quickly by Congress. Consequently, it is important to discuss the potential negative ramifications of other MedPAC recommendations released in June regarding reimbursement of Medicare Part B drugs and proactively educate Congress accordingly on those ramifications.
Medicare Part B drugs
Medicare Part B drugs are those administered by providers, unlike the Part D medications which are generally obtained through pharmacies. Presently, MC reimburses providers for the administered Part B medication based on the average sales price (ASP) plus 6%. However, with sequestration, that add-on amount is reduced to ASP plus 4.3%. It has long been touted by MedPAC and other policy makers that physicians choose to infuse higher-priced drugs in order to increase reimbursements. That has not been borne out when it comes to rheumatologists, and, in fact, a retired MedPAC commissioner even stated that premise did not hold true for rheumatologists.
Regardless, it continues to be suggested that MC should reduce its costs for Part B medications by reducing reimbursement to physicians. It should be noted that often the margins on the drugs are already quite thin, and at times the reimbursement amount, compared with the acquisition cost of the drug even leaves the physician “underwater.”
A few years ago, there was a proposed Part B demonstration project that essentially removed the +6% add-on and replaced it with a very low fixed amount that would have left most physicians “underwater” in their Part B drug acquisitions. This was vigorously opposed by physicians around the country, who let Congress know exactly how they felt. We have been told that the Coalition of State Rheumatology Organizations was one of the most vociferous organizations that helped in fighting back this proposal and resulting in its withdrawal.
MedPAC recommendations
That brings us back to MedPAC. In June, MedPAC released recommendations to Congress in an attempt to address the “high price of drugs” covered under MC Part B. Unfortunately, the recommendations do nothing to address the root cause of high drug prices, but once again attempt to balance MC expenditures on the backs of physicians. In this case, it is physicians who infuse Part B drugs in their office to chronically ill patients. In-office infusions have been shown to be the most cost-effective site of care, as well as being safer when compared with home infusion for a number of rheumatologic medications.
One of the MedPAC recommendations gives the Secretary of Health & Human Services the authority to establish a single ASP for drugs with “similar health effects.” The ambiguity of the phrase “similar health effects” should put us all on alert as to the significant unintended consequences that may result. For example, HHS could assign one ASP to all drugs that treat rheumatoid arthritis based on the lowest ASP of the group. This certainly would lead to a number of drugs being out of reach for MC beneficiaries if the artificial ASP of the medication is much lower than the actual acquisition cost of the drug, leaving physicians unable to acquire it. Yet, MedPAC states this recommendation would not affect access to care for MC beneficiaries.
Another recommendation would require HHS to reduce or eliminate the add-on percentage to the ASP for higher-priced drugs and/or put in an added fixed amount. This recommendation is clearly reminiscent of the old ill-conceived Part B demonstration project.
A fixed “add-on amount” might work if it is sufficient to cover the overhead of maintaining a provider’s infusion suite. But if practices are left underwater in their purchases of certain Part B drugs, there may be no choice but to stop offering those infusions to MC beneficiaries or – worst-case scenario – shut the door completely. Yet again, MedPAC stated that this recommendation would not result in a loss of access to these treatments for MC beneficiaries.
Loss of access?
Rheumatologists have gone to great lengths to continue offering care to MC patients in spite of the yearly cuts and threats of more cuts in the future to physician reimbursements. In addition, physicians have no annual inflationary update to their reimbursements. I am not sure how MedPAC concludes that continued cuts to physician fee schedules, along with a decrease in reimbursement for administered drugs, will not affect access to care for MC beneficiaries.
Finally, the timing on these recommendations is confusing, considering that implementation of the Inflation Reduction Act (IRA) has just begun. Next quarter, a number of Part B drugs will be subject to inflationary penalties; there will also be additional Part B biosimilars coming to market, resulting in lower ASPs. And don’t forget, the IRA just instituted an ASP plus 8% reimbursement for biosimilars in an attempt to get physicians to do something that the Centers for Medicare & Medicaid Services has asked them not to do. That is, choose a drug based on its reimbursement, not necessarily the one which is right for the patient.
Overall, with so many variables up in the air, now is not the time to create even more uncertainty for physicians and the Medicare patients that they take care of.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
The Medicare Payment Advisory Commission (MedPAC) is an independent agency to advise Congress on Medicare (MC) policy, much of which pertains to payment issues. The 17 commissioners meet publicly and issue two reports a year with their recommendations to Congress, who then decides whether to enact these recommendations or not.
One MedPAC recommendation in 2023 was quickly introduced in the House of Representatives in May and passed the Energy and Commerce Committee 49-0. That recommendation relates to “site neutrality” payments to MC providers. If passed by Congress, it would result in some “site-neutral” cuts to hospitals. That MedPAC recommendation was acted upon very quickly by Congress. Consequently, it is important to discuss the potential negative ramifications of other MedPAC recommendations released in June regarding reimbursement of Medicare Part B drugs and proactively educate Congress accordingly on those ramifications.
Medicare Part B drugs
Medicare Part B drugs are those administered by providers, unlike the Part D medications which are generally obtained through pharmacies. Presently, MC reimburses providers for the administered Part B medication based on the average sales price (ASP) plus 6%. However, with sequestration, that add-on amount is reduced to ASP plus 4.3%. It has long been touted by MedPAC and other policy makers that physicians choose to infuse higher-priced drugs in order to increase reimbursements. That has not been borne out when it comes to rheumatologists, and, in fact, a retired MedPAC commissioner even stated that premise did not hold true for rheumatologists.
Regardless, it continues to be suggested that MC should reduce its costs for Part B medications by reducing reimbursement to physicians. It should be noted that often the margins on the drugs are already quite thin, and at times the reimbursement amount, compared with the acquisition cost of the drug even leaves the physician “underwater.”
A few years ago, there was a proposed Part B demonstration project that essentially removed the +6% add-on and replaced it with a very low fixed amount that would have left most physicians “underwater” in their Part B drug acquisitions. This was vigorously opposed by physicians around the country, who let Congress know exactly how they felt. We have been told that the Coalition of State Rheumatology Organizations was one of the most vociferous organizations that helped in fighting back this proposal and resulting in its withdrawal.
MedPAC recommendations
That brings us back to MedPAC. In June, MedPAC released recommendations to Congress in an attempt to address the “high price of drugs” covered under MC Part B. Unfortunately, the recommendations do nothing to address the root cause of high drug prices, but once again attempt to balance MC expenditures on the backs of physicians. In this case, it is physicians who infuse Part B drugs in their office to chronically ill patients. In-office infusions have been shown to be the most cost-effective site of care, as well as being safer when compared with home infusion for a number of rheumatologic medications.
One of the MedPAC recommendations gives the Secretary of Health & Human Services the authority to establish a single ASP for drugs with “similar health effects.” The ambiguity of the phrase “similar health effects” should put us all on alert as to the significant unintended consequences that may result. For example, HHS could assign one ASP to all drugs that treat rheumatoid arthritis based on the lowest ASP of the group. This certainly would lead to a number of drugs being out of reach for MC beneficiaries if the artificial ASP of the medication is much lower than the actual acquisition cost of the drug, leaving physicians unable to acquire it. Yet, MedPAC states this recommendation would not affect access to care for MC beneficiaries.
Another recommendation would require HHS to reduce or eliminate the add-on percentage to the ASP for higher-priced drugs and/or put in an added fixed amount. This recommendation is clearly reminiscent of the old ill-conceived Part B demonstration project.
A fixed “add-on amount” might work if it is sufficient to cover the overhead of maintaining a provider’s infusion suite. But if practices are left underwater in their purchases of certain Part B drugs, there may be no choice but to stop offering those infusions to MC beneficiaries or – worst-case scenario – shut the door completely. Yet again, MedPAC stated that this recommendation would not result in a loss of access to these treatments for MC beneficiaries.
Loss of access?
Rheumatologists have gone to great lengths to continue offering care to MC patients in spite of the yearly cuts and threats of more cuts in the future to physician reimbursements. In addition, physicians have no annual inflationary update to their reimbursements. I am not sure how MedPAC concludes that continued cuts to physician fee schedules, along with a decrease in reimbursement for administered drugs, will not affect access to care for MC beneficiaries.
Finally, the timing on these recommendations is confusing, considering that implementation of the Inflation Reduction Act (IRA) has just begun. Next quarter, a number of Part B drugs will be subject to inflationary penalties; there will also be additional Part B biosimilars coming to market, resulting in lower ASPs. And don’t forget, the IRA just instituted an ASP plus 8% reimbursement for biosimilars in an attempt to get physicians to do something that the Centers for Medicare & Medicaid Services has asked them not to do. That is, choose a drug based on its reimbursement, not necessarily the one which is right for the patient.
Overall, with so many variables up in the air, now is not the time to create even more uncertainty for physicians and the Medicare patients that they take care of.
Dr. Feldman is a rheumatologist in private practice with The Rheumatology Group in New Orleans. She is the CSRO’s Vice President of Advocacy and Government Affairs and its immediate Past President, as well as past chair of the Alliance for Safe Biologic Medicines and a past member of the American College of Rheumatology insurance subcommittee. You can reach her at [email protected].
PCPs key to heart failure care after discharge
Madeline Sterling, MD, knew something was wrong when she heard her patient’s voice on the phone. The patient was breathing too fast and sounded fatigued. Like many people with heart failure, this patient had several comorbidities: diabetes, high blood pressure, and cancer, which was in remission.
The patient had been in and out of the hospital several times and was afraid of going back, but Dr. Sterling, a primary care physician, advised her that it was the safe thing to do.
During the woman’s stay, the inpatient cardiology team called Dr. Sterling to provide status updates and ask for input. When the patient was discharged, Dr. Sterling received information on what medicines had been changed and scheduled follow-up care within 10 days. Dr. Sterling, who’d cared for the woman for many years, called her family, her home health aide, and another caregiver to discuss the plan.
“When you know these patients really well, it’s helpful,” Dr. Sterling, a professor of medicine at Weill Cornell Medicine, New York, said. Primary care clinicians have “an appreciation for how all these conditions fit together, how the medicines fit together, and how to put that patient’s priorities at the front of the equation.”
Research has shown that follow-up care within 7-10 days after discharge, especially for patients with heart failure, can prevent hospital readmissions. Patients’ health can change rapidly following discharge: They may start retaining fluid or may not know how to maintain a low-sodium diet, or they might have trouble obtaining medication. Primary care clinicians spot these early warning signs in follow-up visits.
Heart failure affects more than 6 million adults in the United States, according to the Centers for Disease Control and Prevention. The condition is a common cause of hospital readmissions within 30 days of discharge, according to research published by the American Heart Association.
Patients with heart failure are particularly challenging to care for because of comorbidities.
“They’re a very, very sick group of patients that are very difficult to manage,” said Noah Moss, MD, an advanced heart failure and transplant cardiologist at Mount Sinai Hospital, New York.
But patients do not always receive the follow-up care they need, some studies have found.
Right drugs at the right time
Kelly Axsom, MD, a cardiologist at the Columbia University Medical Center, New York, and director of the centralized heart failure management program at the New York–Presbyterian Hospital System, called the primary care clinician the “captain of the ship,” ensuring that medications are reconciled and providing education about what to eat after discharge.
“It’s actually pretty complicated to go from being in the hospital to being at home,” Dr. Axsom said. “There are often many medication changes, there are lots of instructions that are told to you as a patient that are hard to remember.”
A patient’s weight might fluctuate in the days following discharge because the dose of diuretics might be too low or too high and need to be adjusted, according to Ishani Ganguli, MD, MPH, an assistant professor of medicine and a general internist in the Division of General Internal Medicine and Primary Care at Harvard Medical School and Brigham and Women’s Hospital, Boston.
K. Melissa Hayes, DNP, ANP-BC, CHFN, an assistant professor in the adult gerontology primary care program at the Vanderbilt University School of Nursing, Nashville, Tenn., recalled one patient who was given a months’ worth of medications following his discharge from the hospital.
“He was given expensive medications he couldn’t afford and not any refills or how to get those medications,” Dr. Hayes said.
Sometimes patients have no way to get to the pharmacy, or their pharmacy doesn’t have the medication they need, or their insurance doesn’t cover the drugs.
“The average patient is on at least six medications for heart failure, maybe even seven, and then that’s not including all their other medications,” Dr. Hayes said. “That can be a lot for people to keep up with.”
Dr. Hayes talks to her patients with heart failure about what drugs they have been prescribed and what medications they require more of, and she deprescribes any that are duplicative.
Helping patients understand why they are taking each drug encourages them to stick to the regimen. Diuretics, for example, can lead to frequent urination. If patients are unable to take regular bathroom breaks, they may be tempted to stop using the medication – a potentially catastrophic mistake.
“Often I have patients say, ‘Nobody ever explained it to me that way,’ ” Dr. Hayes said. “Someone can have a PhD but not understand their medications.”
Clinicians also can alert patients to commonly used medications that can worsen heart failure, such as diabetes drugs and over-the-counter medications such as ibuprofen.
Patients should be prescribed a combination of four recommended medications. But several studies have found that clinicians often fail to achieve the target doses for those medications. The use of guideline-directed medications reduces mortality and hospitalization rates, according to multiple clinical trials.
Eyes and ears on the patient
Once home, patients must stick to the right diet, weigh themselves every day, and monitor their blood pressure. But changing behaviors can be a struggle.
“Being seen quickly within a couple of days of discharge, you can catch things,” said Dr. Hayes, who has edited a book on managing patients with heart failure in primary care.
“It’s an opportunity to see how they’re doing at home, make sure they have their medications, make sure there’s been no misunderstanding or miscommunication about what they’re supposed to be doing at home,” says Marc Itskowitz, MD, a primary care physician affiliated with Allegheny General Hospital, Pittsburgh.
Ideally, a record that readily integrates information from wearables – such as blood pressure and weight – would make it easier to spot abnormalities, Dr. Itskowtiz said. “I think we’re still in the infancy of the electronic health record,” he said.
Ensuring that follow-up visits are as accessible as possible for patients is also important. Telehealth makes it easier for patients after they return home from the hospital, Dr. Itskowitz said.
More infrastructure
Another challenge of providing follow-up care for patients with heart failure is completing all the tasks a clinician must do within a 20-minute visit: an examination; education on the condition and medications; counseling on diet and exercise; coordination of medical equipment, such as a blood pressure cuff for home use; and making appointments with specialists.
“In the current system, additional support for primary care is needed so we can do all this,” Dr. Sterling said.
Staff at primary care clinics should be trained to answer calls from patients when they experience changes in their weight or are worried about other potential problems. “A lot of primary care practices are bare bones,” Dr. Hayes said, meaning they might not have the staff to field those calls. Educating patients as to when they should call their physician, especially after experiencing worsening symptoms, is also important.
Dr. Hayes suggests setting aside time in the schedule each week to see patients who have been recently discharged from the hospital. In the Cardiology and Vascular Clinic at Nashville General Hospital, Tenn., where she spends half a day each week, Dr. Hayes requests 30 minutes to see patients who have recently been discharged from hospital.
Even when the process goes smoothly, some patients will return to the hospital because of the progressive nature of heart failure, according to Dr. Hayes. Improving care following their hospitalization can keep these people from rapidly declining.
“Most patients with heart failure want to be taking care of the grandchildren or be able to enjoy family dinners together,” Dr. Axsom said. “I think anything we can do to help improve their quality of life is really important.”
Take-home
- See heart failure patients early after their discharge from hospital, ideally within 7-10 days.
- Make sure patients have access to the right medications at the right dosages and that they know why they’re taking them.
- Educate patients about the diet they should be following.
- Have a system to monitor patients’ symptoms and let them know when they should call.
A version of this article first appeared on Medscape.com.
Madeline Sterling, MD, knew something was wrong when she heard her patient’s voice on the phone. The patient was breathing too fast and sounded fatigued. Like many people with heart failure, this patient had several comorbidities: diabetes, high blood pressure, and cancer, which was in remission.
The patient had been in and out of the hospital several times and was afraid of going back, but Dr. Sterling, a primary care physician, advised her that it was the safe thing to do.
During the woman’s stay, the inpatient cardiology team called Dr. Sterling to provide status updates and ask for input. When the patient was discharged, Dr. Sterling received information on what medicines had been changed and scheduled follow-up care within 10 days. Dr. Sterling, who’d cared for the woman for many years, called her family, her home health aide, and another caregiver to discuss the plan.
“When you know these patients really well, it’s helpful,” Dr. Sterling, a professor of medicine at Weill Cornell Medicine, New York, said. Primary care clinicians have “an appreciation for how all these conditions fit together, how the medicines fit together, and how to put that patient’s priorities at the front of the equation.”
Research has shown that follow-up care within 7-10 days after discharge, especially for patients with heart failure, can prevent hospital readmissions. Patients’ health can change rapidly following discharge: They may start retaining fluid or may not know how to maintain a low-sodium diet, or they might have trouble obtaining medication. Primary care clinicians spot these early warning signs in follow-up visits.
Heart failure affects more than 6 million adults in the United States, according to the Centers for Disease Control and Prevention. The condition is a common cause of hospital readmissions within 30 days of discharge, according to research published by the American Heart Association.
Patients with heart failure are particularly challenging to care for because of comorbidities.
“They’re a very, very sick group of patients that are very difficult to manage,” said Noah Moss, MD, an advanced heart failure and transplant cardiologist at Mount Sinai Hospital, New York.
But patients do not always receive the follow-up care they need, some studies have found.
Right drugs at the right time
Kelly Axsom, MD, a cardiologist at the Columbia University Medical Center, New York, and director of the centralized heart failure management program at the New York–Presbyterian Hospital System, called the primary care clinician the “captain of the ship,” ensuring that medications are reconciled and providing education about what to eat after discharge.
“It’s actually pretty complicated to go from being in the hospital to being at home,” Dr. Axsom said. “There are often many medication changes, there are lots of instructions that are told to you as a patient that are hard to remember.”
A patient’s weight might fluctuate in the days following discharge because the dose of diuretics might be too low or too high and need to be adjusted, according to Ishani Ganguli, MD, MPH, an assistant professor of medicine and a general internist in the Division of General Internal Medicine and Primary Care at Harvard Medical School and Brigham and Women’s Hospital, Boston.
K. Melissa Hayes, DNP, ANP-BC, CHFN, an assistant professor in the adult gerontology primary care program at the Vanderbilt University School of Nursing, Nashville, Tenn., recalled one patient who was given a months’ worth of medications following his discharge from the hospital.
“He was given expensive medications he couldn’t afford and not any refills or how to get those medications,” Dr. Hayes said.
Sometimes patients have no way to get to the pharmacy, or their pharmacy doesn’t have the medication they need, or their insurance doesn’t cover the drugs.
“The average patient is on at least six medications for heart failure, maybe even seven, and then that’s not including all their other medications,” Dr. Hayes said. “That can be a lot for people to keep up with.”
Dr. Hayes talks to her patients with heart failure about what drugs they have been prescribed and what medications they require more of, and she deprescribes any that are duplicative.
Helping patients understand why they are taking each drug encourages them to stick to the regimen. Diuretics, for example, can lead to frequent urination. If patients are unable to take regular bathroom breaks, they may be tempted to stop using the medication – a potentially catastrophic mistake.
“Often I have patients say, ‘Nobody ever explained it to me that way,’ ” Dr. Hayes said. “Someone can have a PhD but not understand their medications.”
Clinicians also can alert patients to commonly used medications that can worsen heart failure, such as diabetes drugs and over-the-counter medications such as ibuprofen.
Patients should be prescribed a combination of four recommended medications. But several studies have found that clinicians often fail to achieve the target doses for those medications. The use of guideline-directed medications reduces mortality and hospitalization rates, according to multiple clinical trials.
Eyes and ears on the patient
Once home, patients must stick to the right diet, weigh themselves every day, and monitor their blood pressure. But changing behaviors can be a struggle.
“Being seen quickly within a couple of days of discharge, you can catch things,” said Dr. Hayes, who has edited a book on managing patients with heart failure in primary care.
“It’s an opportunity to see how they’re doing at home, make sure they have their medications, make sure there’s been no misunderstanding or miscommunication about what they’re supposed to be doing at home,” says Marc Itskowitz, MD, a primary care physician affiliated with Allegheny General Hospital, Pittsburgh.
Ideally, a record that readily integrates information from wearables – such as blood pressure and weight – would make it easier to spot abnormalities, Dr. Itskowtiz said. “I think we’re still in the infancy of the electronic health record,” he said.
Ensuring that follow-up visits are as accessible as possible for patients is also important. Telehealth makes it easier for patients after they return home from the hospital, Dr. Itskowitz said.
More infrastructure
Another challenge of providing follow-up care for patients with heart failure is completing all the tasks a clinician must do within a 20-minute visit: an examination; education on the condition and medications; counseling on diet and exercise; coordination of medical equipment, such as a blood pressure cuff for home use; and making appointments with specialists.
“In the current system, additional support for primary care is needed so we can do all this,” Dr. Sterling said.
Staff at primary care clinics should be trained to answer calls from patients when they experience changes in their weight or are worried about other potential problems. “A lot of primary care practices are bare bones,” Dr. Hayes said, meaning they might not have the staff to field those calls. Educating patients as to when they should call their physician, especially after experiencing worsening symptoms, is also important.
Dr. Hayes suggests setting aside time in the schedule each week to see patients who have been recently discharged from the hospital. In the Cardiology and Vascular Clinic at Nashville General Hospital, Tenn., where she spends half a day each week, Dr. Hayes requests 30 minutes to see patients who have recently been discharged from hospital.
Even when the process goes smoothly, some patients will return to the hospital because of the progressive nature of heart failure, according to Dr. Hayes. Improving care following their hospitalization can keep these people from rapidly declining.
“Most patients with heart failure want to be taking care of the grandchildren or be able to enjoy family dinners together,” Dr. Axsom said. “I think anything we can do to help improve their quality of life is really important.”
Take-home
- See heart failure patients early after their discharge from hospital, ideally within 7-10 days.
- Make sure patients have access to the right medications at the right dosages and that they know why they’re taking them.
- Educate patients about the diet they should be following.
- Have a system to monitor patients’ symptoms and let them know when they should call.
A version of this article first appeared on Medscape.com.
Madeline Sterling, MD, knew something was wrong when she heard her patient’s voice on the phone. The patient was breathing too fast and sounded fatigued. Like many people with heart failure, this patient had several comorbidities: diabetes, high blood pressure, and cancer, which was in remission.
The patient had been in and out of the hospital several times and was afraid of going back, but Dr. Sterling, a primary care physician, advised her that it was the safe thing to do.
During the woman’s stay, the inpatient cardiology team called Dr. Sterling to provide status updates and ask for input. When the patient was discharged, Dr. Sterling received information on what medicines had been changed and scheduled follow-up care within 10 days. Dr. Sterling, who’d cared for the woman for many years, called her family, her home health aide, and another caregiver to discuss the plan.
“When you know these patients really well, it’s helpful,” Dr. Sterling, a professor of medicine at Weill Cornell Medicine, New York, said. Primary care clinicians have “an appreciation for how all these conditions fit together, how the medicines fit together, and how to put that patient’s priorities at the front of the equation.”
Research has shown that follow-up care within 7-10 days after discharge, especially for patients with heart failure, can prevent hospital readmissions. Patients’ health can change rapidly following discharge: They may start retaining fluid or may not know how to maintain a low-sodium diet, or they might have trouble obtaining medication. Primary care clinicians spot these early warning signs in follow-up visits.
Heart failure affects more than 6 million adults in the United States, according to the Centers for Disease Control and Prevention. The condition is a common cause of hospital readmissions within 30 days of discharge, according to research published by the American Heart Association.
Patients with heart failure are particularly challenging to care for because of comorbidities.
“They’re a very, very sick group of patients that are very difficult to manage,” said Noah Moss, MD, an advanced heart failure and transplant cardiologist at Mount Sinai Hospital, New York.
But patients do not always receive the follow-up care they need, some studies have found.
Right drugs at the right time
Kelly Axsom, MD, a cardiologist at the Columbia University Medical Center, New York, and director of the centralized heart failure management program at the New York–Presbyterian Hospital System, called the primary care clinician the “captain of the ship,” ensuring that medications are reconciled and providing education about what to eat after discharge.
“It’s actually pretty complicated to go from being in the hospital to being at home,” Dr. Axsom said. “There are often many medication changes, there are lots of instructions that are told to you as a patient that are hard to remember.”
A patient’s weight might fluctuate in the days following discharge because the dose of diuretics might be too low or too high and need to be adjusted, according to Ishani Ganguli, MD, MPH, an assistant professor of medicine and a general internist in the Division of General Internal Medicine and Primary Care at Harvard Medical School and Brigham and Women’s Hospital, Boston.
K. Melissa Hayes, DNP, ANP-BC, CHFN, an assistant professor in the adult gerontology primary care program at the Vanderbilt University School of Nursing, Nashville, Tenn., recalled one patient who was given a months’ worth of medications following his discharge from the hospital.
“He was given expensive medications he couldn’t afford and not any refills or how to get those medications,” Dr. Hayes said.
Sometimes patients have no way to get to the pharmacy, or their pharmacy doesn’t have the medication they need, or their insurance doesn’t cover the drugs.
“The average patient is on at least six medications for heart failure, maybe even seven, and then that’s not including all their other medications,” Dr. Hayes said. “That can be a lot for people to keep up with.”
Dr. Hayes talks to her patients with heart failure about what drugs they have been prescribed and what medications they require more of, and she deprescribes any that are duplicative.
Helping patients understand why they are taking each drug encourages them to stick to the regimen. Diuretics, for example, can lead to frequent urination. If patients are unable to take regular bathroom breaks, they may be tempted to stop using the medication – a potentially catastrophic mistake.
“Often I have patients say, ‘Nobody ever explained it to me that way,’ ” Dr. Hayes said. “Someone can have a PhD but not understand their medications.”
Clinicians also can alert patients to commonly used medications that can worsen heart failure, such as diabetes drugs and over-the-counter medications such as ibuprofen.
Patients should be prescribed a combination of four recommended medications. But several studies have found that clinicians often fail to achieve the target doses for those medications. The use of guideline-directed medications reduces mortality and hospitalization rates, according to multiple clinical trials.
Eyes and ears on the patient
Once home, patients must stick to the right diet, weigh themselves every day, and monitor their blood pressure. But changing behaviors can be a struggle.
“Being seen quickly within a couple of days of discharge, you can catch things,” said Dr. Hayes, who has edited a book on managing patients with heart failure in primary care.
“It’s an opportunity to see how they’re doing at home, make sure they have their medications, make sure there’s been no misunderstanding or miscommunication about what they’re supposed to be doing at home,” says Marc Itskowitz, MD, a primary care physician affiliated with Allegheny General Hospital, Pittsburgh.
Ideally, a record that readily integrates information from wearables – such as blood pressure and weight – would make it easier to spot abnormalities, Dr. Itskowtiz said. “I think we’re still in the infancy of the electronic health record,” he said.
Ensuring that follow-up visits are as accessible as possible for patients is also important. Telehealth makes it easier for patients after they return home from the hospital, Dr. Itskowitz said.
More infrastructure
Another challenge of providing follow-up care for patients with heart failure is completing all the tasks a clinician must do within a 20-minute visit: an examination; education on the condition and medications; counseling on diet and exercise; coordination of medical equipment, such as a blood pressure cuff for home use; and making appointments with specialists.
“In the current system, additional support for primary care is needed so we can do all this,” Dr. Sterling said.
Staff at primary care clinics should be trained to answer calls from patients when they experience changes in their weight or are worried about other potential problems. “A lot of primary care practices are bare bones,” Dr. Hayes said, meaning they might not have the staff to field those calls. Educating patients as to when they should call their physician, especially after experiencing worsening symptoms, is also important.
Dr. Hayes suggests setting aside time in the schedule each week to see patients who have been recently discharged from the hospital. In the Cardiology and Vascular Clinic at Nashville General Hospital, Tenn., where she spends half a day each week, Dr. Hayes requests 30 minutes to see patients who have recently been discharged from hospital.
Even when the process goes smoothly, some patients will return to the hospital because of the progressive nature of heart failure, according to Dr. Hayes. Improving care following their hospitalization can keep these people from rapidly declining.
“Most patients with heart failure want to be taking care of the grandchildren or be able to enjoy family dinners together,” Dr. Axsom said. “I think anything we can do to help improve their quality of life is really important.”
Take-home
- See heart failure patients early after their discharge from hospital, ideally within 7-10 days.
- Make sure patients have access to the right medications at the right dosages and that they know why they’re taking them.
- Educate patients about the diet they should be following.
- Have a system to monitor patients’ symptoms and let them know when they should call.
A version of this article first appeared on Medscape.com.
New and transitioning gastroenterologists face burnout too
The field of gastroenterology can be challenging, both professionally and personally, leading to burnout, especially for new and transitioning gastroenterologists. Burnout is a state of emotional, physical, and mental exhaustion caused by prolonged or excessive stress.1 It is characterized by emotional fatigue, depersonalization, and a reduced sense of personal accomplishment.2,3 This condition can have severe consequences for physicians and their patients.
More than 50% of physicians report meeting the criteria for burnout, which is pervasive in all medical professions.3 Survey results of 7,288 U.S. physicians showed that burnout and dissatisfaction with work-life balance are significantly higher than among other working U.S. adults.3
The long and often irregular work hours expected of gastroenterologists significantly contribute to burnout within our field. The physically, intellectually, and technically demanding reality of managing complex patients and making high stakes decisions at all hours has far-reaching consequences.3 Most gastroenterologists work between 55 and 60 hours per week.4 This sharply contrasts the average 43-hour work week for full-time employees in the United States.5 Gastroenterologists may experience inaccurate perceptions of their commitment to patients, education, and their families based solely on time observed on each activity.4 Higher education and professional degrees usually protect against burnout.3 However, a degree in medicine (MD or DO) increases the burnout risk.3
New gastroenterologists are learning a wide range of intricate procedures and becoming proficient in diagnosing and managing gastrointestinal disorders. Extensive career demands often coincide with intense family-forming years, creating tension for a physician’s finite time and energy. The culture of medicine demanding “patients come first” while attempting to be fully human can sometimes feel irreconcilable, leading to feelings of inadequacy and anxiety.3 Gastroenterology training takes 3 years because of the complexity, danger, and need for thousands of procedures to gain proficiency and competence to recognize when complications occur. Oversight is ubiquitous during training, making this the ideal time to learn from mistakes and formulate lifelong habits of constant improvement. However, perfectionist tendencies and the Hippocratic Oath can create unrealistic self expectations.6 The risk of potential litigation, simply missing a diagnosis, or causing actual patient harm is never far from a proceduralist’s mind.
The diversity of gastroenterology requires high clinical knowledge, expertise, and emotional intelligence. Leading potentially intense end-of-life, cancer, fertility, and risk-factor discussions can be all-consuming. Keeping up with the latest research, treatments, and techniques in the field can be daunting. Furthermore, gastroenterologists spend many hours each day on electronic medical records. Constant re-documentation of interactions, seemingly endless prior authorizations, disability forms, referrals, and simply re-addressing patient and family concerns can feel low value. This uncompensated work also creates moral injury as it takes away from direct patient care.
Striking a work-life balance
New gastroenterologists are advised to find work-life balance. However, they are also plagued by the massive professional demands being constantly placed on them. The desire to find the mythical “balance” may create a mindset of significant sacrifices in their private lives as the only way to achieve professional successes.7 When gastroenterologists do not prioritize time for personal activities, including exercise, health checks, hobbies, rest, relaxation, family, and friends, they can get caught in a vicious cycle of continuing to feel poorly, resulting in overcompensating by working more in order to feel “accomplished.” The perfectionist pressure to maintain high productivity and patient satisfaction can also further contribute to burnout.
Gastroenterology burnout can severely affect physicians’ health status, job performance, and patient satisfaction.9 It may erode professionalism, negatively influence the quality of care, increase the risk of medical errors, and promote early retirement.3 Burnout may also correlate with adverse personal consequences for physicians, such as broken relationships, problematic alcohol use, and suicidal ideation.3 Physician burnout and professional satisfaction have strategic importance to health care organizations.10 Less burned-out physicians have patient panels with higher adherence and satisfaction with medical care.10 With more physicians becoming employees, there are opportunities for accountability of organizational leadership.10 Interestingly, healthy well-being or burnout is contagious from leaders to their teams.10 A 2015 study by Shanafelt et al. found that at the work unit level, 11% and 47% of the variation in burnout and satisfaction, correlated with the leader’s relative scores.10
So, what can be done to prevent and treat burnout in new and transitioning gastroenterologists? The gastroenterologist may implement several strategies. It is essential for individuals to take responsibility for their well-being and to prioritize self-care by setting boundaries, practicing stress management techniques, and seeking support from colleagues and mental health professionals when needed. 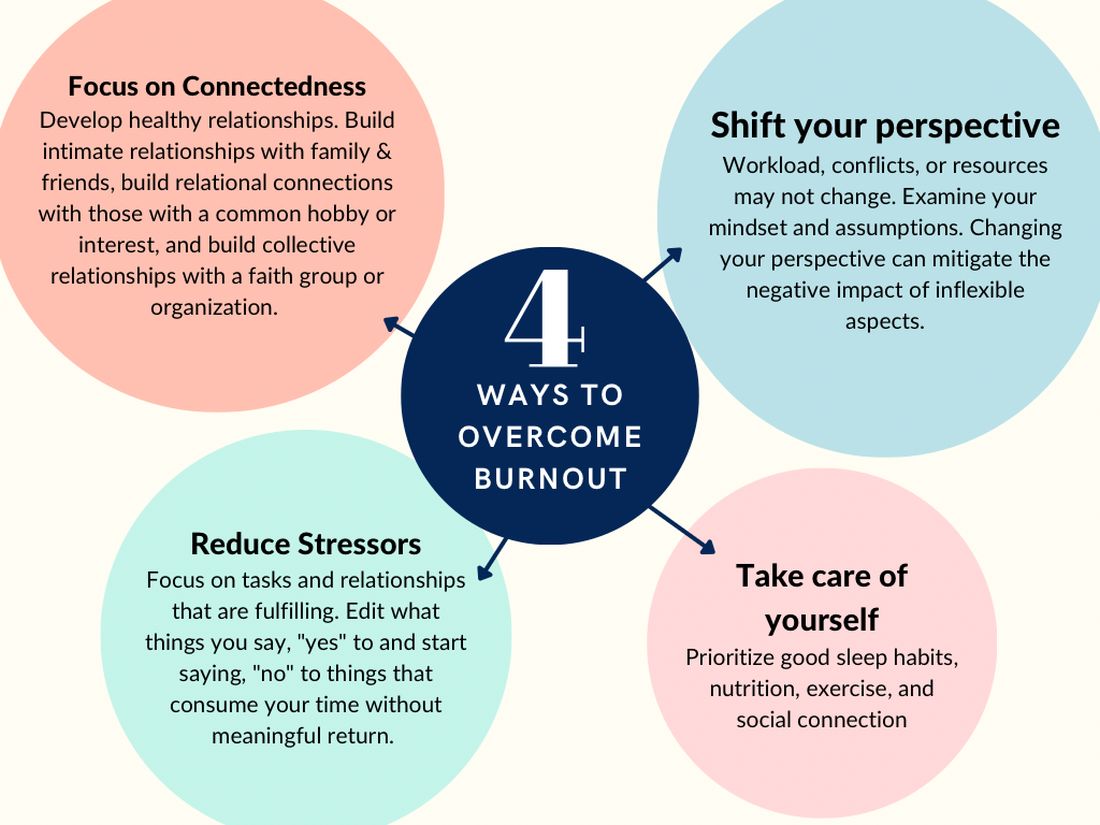
According to Dave et al. (2020), engagement in self-care practices such as mindfulness may offer advantages to gastroenterologists’ well-being and improved patient care.11
Burnout is not due to an individuals’ need for more resiliency. Instead, it developed from a systemic overwhelming of a health system near its breaking point. Recognizing that by 2033, there is a projected shortage of nearly 140,000 physicians in the United States, the U.S. Surgeon General, Dr. Vivek H. Murthy, issued a crisis advisory.12 This advisory highlights the urgent need to address the health worker burnout crisis nationwide that outlined “whole of society” efforts.12 Key components of the advisory on building a thriving health workforce included empowering health care workers, changing policies, reducing administrative burdens, prioritizing connections, and investing in our workforce.12
Provide access to mental health services
Institutions and practices would greatly benefit from providing access to mental health services, counseling, educational opportunities, potential mental health days, and mentorship programs. While the literature indicates that both individual-focused and structural or organizational strategies can result in clinically meaningful reductions in burnout among physicians, a meta-analysis revealed that corporate-led initiatives resulted in larger successes.12,13 Physicians who received support and resources from their institutions report lower levels of burnout and higher job satisfaction.2,3
New strategies to select and develop physician leaders who motivate, inspire, and effectively manage physicians may result in positive job satisfaction while decreasing employee burnout. Therefore, increased awareness of the importance of frontline leadership well-being and professional fulfillment of physicians working for a large health care organization is necessary.13 Robust and continual leadership training can ensure the entire team’s well-being, longevity, and success.13
Addressing the root causes of systemic burnout is imperative. Leadership could streamline administrative processes, optimize electronic medical records, delegate prior authorizations, and ensure staffing levels are appropriate to meet patient care demands. In a survey by Rao et al. (2017), the authors found that physicians who reported high levels of administrative burden and work overload were more likely to experience burnout.14
Institutions and practices should promote a culture of work-life balance by implementing flexible scheduling, promoting time off and vacation time, and encouraging regular exercise and healthy habits. The current compensation structure disincentivizes physicians from taking time away from patient care – this can be re-designed. Community and support mitigate burnout. Therefore, institutions and practices will benefit by intentionally providing opportunities for social connection and team building.
In reflection of the U.S. Surgeon General’s call for all of society to be part of the solution, we are pleased to see the Accreditation Council for Graduate Medical Education (ACGME) create mandatory 6 weeks of parental or caregiver leave for trainees.15 Continued positive pressure on overseeing agencies to minimize paperwork, preauthorizations, and non–value-added tasks to allow physicians to continue to provide medical services instead of documentation and auditing services would greatly positively impact all of health care. Therefore, communicating with legislators, policy makers, system leadership, and all health care societies to continue these improvements would be a wise use of time of resources.
In conclusion, burnout among new and transitioning gastroenterologists is a prevalent and concerning issue that can have severe consequences for both the individual and the health care system. Similar to the ergonomic considerations of being an endoscopist, A multifaceted approach to the well-being of all medical staff can help ensure the delivery of the highest quality patient care. By taking a proactive approach to preventing burnout, we can have a strong future for ourselves, our patients, and our profession.
Dr. Eboh is a gastroenterologist with Atrium Health, Charlotte, N.C.; Dr. Jaeger is with Baylor Scott & White Medical Center in Dallas. She is a gastroenterology fellow with Temple University Hospital, Philadelphia. Dr. Sears is clinical professor at Texas A&M University School of Medicine, and chief of gastroenterology at VA Central Texas Healthcare System. Dr. Sears owns GutGirlMD Consulting LLC, where she offers institutional and leadership coaching for physicians. Dr. Eboh on Instagram @Polyp.picker_EbohMD and on Twitter @PolypPicker_MD. Dr. Jaeger on Instagram @Doc.Tori.Fit and Twitter @DrToriJaeger. Dr. Sears is on Twitter @GutGirlMD.
References
1. Maslach C and Jackson S E. Maslach burnout inventory manual. Palo Alto, Calif: Consulting Psychologists Press, 1986.
2. Shanafelt TD et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015 Dec 12;90:1600-13.
3. Shanafelt TD et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012 Oct 8;172(18):1377-85.
4. Elta G. The challenges of being a female gastroenterologist. Gastroenterol Clin North Am. 2011 Jun;40(2):441-7.
5. Gallup. Work and Workplace. 2023.
6. Gawande A. When doctors make mistakes. The New Yorker. 1999 Feb 1.
7. Buscarini E et al. Burnout among gastroenterologists: How to manage and prevent it. United European Gastroenterol J. 2020 Aug;8(7):832-4.
8. West CP et al. Interventions to prevent and reduce physician burnout: A systematic review and meta-analysis. Lancet. 2016 Nov 5;388(10057):2272-81.
9. Adarkwah CC et al. Burnout and work satisfaction are differentially associated in gastroenterologists in Germany. F1000Res. 2022 Mar 30;11:368. doi: 10.12688/f1000research.110296.3. eCollection 2022.
10. Shanafelt TD et al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015 Apr;90(4):432-40.
11. Umakant D et al. Mindfulness in gastroenterology training and practice: A personal perspective. Clin Exp Gastroenterol. 2020 Nov 4;13:497-502.
12. Murthy VH. Addressing Health Worker Burnout: The U.S. Surgeon General’s Advisory on Building a Thriving Health Workforce. The U.S. Department of Health and Human Services: Office of the U.S. Surgeon General, 2022.
13. Panagioti M et al. Controlled interventions to reduce burnout in physicians: A systematic review and meta-analysis. JAMA Intern Med. 2017 Feb 1;177(2):195-205.
14. Rao SK et al. The impact of administrative burden on academic physicians: Results of a hospital-wide physician survey. Acad Med. 2017 Feb;92(2):237-43.
15. ACGME. ACME Institutional Requirements 2021.
The field of gastroenterology can be challenging, both professionally and personally, leading to burnout, especially for new and transitioning gastroenterologists. Burnout is a state of emotional, physical, and mental exhaustion caused by prolonged or excessive stress.1 It is characterized by emotional fatigue, depersonalization, and a reduced sense of personal accomplishment.2,3 This condition can have severe consequences for physicians and their patients.
More than 50% of physicians report meeting the criteria for burnout, which is pervasive in all medical professions.3 Survey results of 7,288 U.S. physicians showed that burnout and dissatisfaction with work-life balance are significantly higher than among other working U.S. adults.3
The long and often irregular work hours expected of gastroenterologists significantly contribute to burnout within our field. The physically, intellectually, and technically demanding reality of managing complex patients and making high stakes decisions at all hours has far-reaching consequences.3 Most gastroenterologists work between 55 and 60 hours per week.4 This sharply contrasts the average 43-hour work week for full-time employees in the United States.5 Gastroenterologists may experience inaccurate perceptions of their commitment to patients, education, and their families based solely on time observed on each activity.4 Higher education and professional degrees usually protect against burnout.3 However, a degree in medicine (MD or DO) increases the burnout risk.3
New gastroenterologists are learning a wide range of intricate procedures and becoming proficient in diagnosing and managing gastrointestinal disorders. Extensive career demands often coincide with intense family-forming years, creating tension for a physician’s finite time and energy. The culture of medicine demanding “patients come first” while attempting to be fully human can sometimes feel irreconcilable, leading to feelings of inadequacy and anxiety.3 Gastroenterology training takes 3 years because of the complexity, danger, and need for thousands of procedures to gain proficiency and competence to recognize when complications occur. Oversight is ubiquitous during training, making this the ideal time to learn from mistakes and formulate lifelong habits of constant improvement. However, perfectionist tendencies and the Hippocratic Oath can create unrealistic self expectations.6 The risk of potential litigation, simply missing a diagnosis, or causing actual patient harm is never far from a proceduralist’s mind.
The diversity of gastroenterology requires high clinical knowledge, expertise, and emotional intelligence. Leading potentially intense end-of-life, cancer, fertility, and risk-factor discussions can be all-consuming. Keeping up with the latest research, treatments, and techniques in the field can be daunting. Furthermore, gastroenterologists spend many hours each day on electronic medical records. Constant re-documentation of interactions, seemingly endless prior authorizations, disability forms, referrals, and simply re-addressing patient and family concerns can feel low value. This uncompensated work also creates moral injury as it takes away from direct patient care.
Striking a work-life balance
New gastroenterologists are advised to find work-life balance. However, they are also plagued by the massive professional demands being constantly placed on them. The desire to find the mythical “balance” may create a mindset of significant sacrifices in their private lives as the only way to achieve professional successes.7 When gastroenterologists do not prioritize time for personal activities, including exercise, health checks, hobbies, rest, relaxation, family, and friends, they can get caught in a vicious cycle of continuing to feel poorly, resulting in overcompensating by working more in order to feel “accomplished.” The perfectionist pressure to maintain high productivity and patient satisfaction can also further contribute to burnout.
Gastroenterology burnout can severely affect physicians’ health status, job performance, and patient satisfaction.9 It may erode professionalism, negatively influence the quality of care, increase the risk of medical errors, and promote early retirement.3 Burnout may also correlate with adverse personal consequences for physicians, such as broken relationships, problematic alcohol use, and suicidal ideation.3 Physician burnout and professional satisfaction have strategic importance to health care organizations.10 Less burned-out physicians have patient panels with higher adherence and satisfaction with medical care.10 With more physicians becoming employees, there are opportunities for accountability of organizational leadership.10 Interestingly, healthy well-being or burnout is contagious from leaders to their teams.10 A 2015 study by Shanafelt et al. found that at the work unit level, 11% and 47% of the variation in burnout and satisfaction, correlated with the leader’s relative scores.10
So, what can be done to prevent and treat burnout in new and transitioning gastroenterologists? The gastroenterologist may implement several strategies. It is essential for individuals to take responsibility for their well-being and to prioritize self-care by setting boundaries, practicing stress management techniques, and seeking support from colleagues and mental health professionals when needed. 
According to Dave et al. (2020), engagement in self-care practices such as mindfulness may offer advantages to gastroenterologists’ well-being and improved patient care.11
Burnout is not due to an individuals’ need for more resiliency. Instead, it developed from a systemic overwhelming of a health system near its breaking point. Recognizing that by 2033, there is a projected shortage of nearly 140,000 physicians in the United States, the U.S. Surgeon General, Dr. Vivek H. Murthy, issued a crisis advisory.12 This advisory highlights the urgent need to address the health worker burnout crisis nationwide that outlined “whole of society” efforts.12 Key components of the advisory on building a thriving health workforce included empowering health care workers, changing policies, reducing administrative burdens, prioritizing connections, and investing in our workforce.12
Provide access to mental health services
Institutions and practices would greatly benefit from providing access to mental health services, counseling, educational opportunities, potential mental health days, and mentorship programs. While the literature indicates that both individual-focused and structural or organizational strategies can result in clinically meaningful reductions in burnout among physicians, a meta-analysis revealed that corporate-led initiatives resulted in larger successes.12,13 Physicians who received support and resources from their institutions report lower levels of burnout and higher job satisfaction.2,3
New strategies to select and develop physician leaders who motivate, inspire, and effectively manage physicians may result in positive job satisfaction while decreasing employee burnout. Therefore, increased awareness of the importance of frontline leadership well-being and professional fulfillment of physicians working for a large health care organization is necessary.13 Robust and continual leadership training can ensure the entire team’s well-being, longevity, and success.13
Addressing the root causes of systemic burnout is imperative. Leadership could streamline administrative processes, optimize electronic medical records, delegate prior authorizations, and ensure staffing levels are appropriate to meet patient care demands. In a survey by Rao et al. (2017), the authors found that physicians who reported high levels of administrative burden and work overload were more likely to experience burnout.14
Institutions and practices should promote a culture of work-life balance by implementing flexible scheduling, promoting time off and vacation time, and encouraging regular exercise and healthy habits. The current compensation structure disincentivizes physicians from taking time away from patient care – this can be re-designed. Community and support mitigate burnout. Therefore, institutions and practices will benefit by intentionally providing opportunities for social connection and team building.
In reflection of the U.S. Surgeon General’s call for all of society to be part of the solution, we are pleased to see the Accreditation Council for Graduate Medical Education (ACGME) create mandatory 6 weeks of parental or caregiver leave for trainees.15 Continued positive pressure on overseeing agencies to minimize paperwork, preauthorizations, and non–value-added tasks to allow physicians to continue to provide medical services instead of documentation and auditing services would greatly positively impact all of health care. Therefore, communicating with legislators, policy makers, system leadership, and all health care societies to continue these improvements would be a wise use of time of resources.
In conclusion, burnout among new and transitioning gastroenterologists is a prevalent and concerning issue that can have severe consequences for both the individual and the health care system. Similar to the ergonomic considerations of being an endoscopist, A multifaceted approach to the well-being of all medical staff can help ensure the delivery of the highest quality patient care. By taking a proactive approach to preventing burnout, we can have a strong future for ourselves, our patients, and our profession.
Dr. Eboh is a gastroenterologist with Atrium Health, Charlotte, N.C.; Dr. Jaeger is with Baylor Scott & White Medical Center in Dallas. She is a gastroenterology fellow with Temple University Hospital, Philadelphia. Dr. Sears is clinical professor at Texas A&M University School of Medicine, and chief of gastroenterology at VA Central Texas Healthcare System. Dr. Sears owns GutGirlMD Consulting LLC, where she offers institutional and leadership coaching for physicians. Dr. Eboh on Instagram @Polyp.picker_EbohMD and on Twitter @PolypPicker_MD. Dr. Jaeger on Instagram @Doc.Tori.Fit and Twitter @DrToriJaeger. Dr. Sears is on Twitter @GutGirlMD.
References
1. Maslach C and Jackson S E. Maslach burnout inventory manual. Palo Alto, Calif: Consulting Psychologists Press, 1986.
2. Shanafelt TD et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015 Dec 12;90:1600-13.
3. Shanafelt TD et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012 Oct 8;172(18):1377-85.
4. Elta G. The challenges of being a female gastroenterologist. Gastroenterol Clin North Am. 2011 Jun;40(2):441-7.
5. Gallup. Work and Workplace. 2023.
6. Gawande A. When doctors make mistakes. The New Yorker. 1999 Feb 1.
7. Buscarini E et al. Burnout among gastroenterologists: How to manage and prevent it. United European Gastroenterol J. 2020 Aug;8(7):832-4.
8. West CP et al. Interventions to prevent and reduce physician burnout: A systematic review and meta-analysis. Lancet. 2016 Nov 5;388(10057):2272-81.
9. Adarkwah CC et al. Burnout and work satisfaction are differentially associated in gastroenterologists in Germany. F1000Res. 2022 Mar 30;11:368. doi: 10.12688/f1000research.110296.3. eCollection 2022.
10. Shanafelt TD et al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015 Apr;90(4):432-40.
11. Umakant D et al. Mindfulness in gastroenterology training and practice: A personal perspective. Clin Exp Gastroenterol. 2020 Nov 4;13:497-502.
12. Murthy VH. Addressing Health Worker Burnout: The U.S. Surgeon General’s Advisory on Building a Thriving Health Workforce. The U.S. Department of Health and Human Services: Office of the U.S. Surgeon General, 2022.
13. Panagioti M et al. Controlled interventions to reduce burnout in physicians: A systematic review and meta-analysis. JAMA Intern Med. 2017 Feb 1;177(2):195-205.
14. Rao SK et al. The impact of administrative burden on academic physicians: Results of a hospital-wide physician survey. Acad Med. 2017 Feb;92(2):237-43.
15. ACGME. ACME Institutional Requirements 2021.
The field of gastroenterology can be challenging, both professionally and personally, leading to burnout, especially for new and transitioning gastroenterologists. Burnout is a state of emotional, physical, and mental exhaustion caused by prolonged or excessive stress.1 It is characterized by emotional fatigue, depersonalization, and a reduced sense of personal accomplishment.2,3 This condition can have severe consequences for physicians and their patients.
More than 50% of physicians report meeting the criteria for burnout, which is pervasive in all medical professions.3 Survey results of 7,288 U.S. physicians showed that burnout and dissatisfaction with work-life balance are significantly higher than among other working U.S. adults.3
The long and often irregular work hours expected of gastroenterologists significantly contribute to burnout within our field. The physically, intellectually, and technically demanding reality of managing complex patients and making high stakes decisions at all hours has far-reaching consequences.3 Most gastroenterologists work between 55 and 60 hours per week.4 This sharply contrasts the average 43-hour work week for full-time employees in the United States.5 Gastroenterologists may experience inaccurate perceptions of their commitment to patients, education, and their families based solely on time observed on each activity.4 Higher education and professional degrees usually protect against burnout.3 However, a degree in medicine (MD or DO) increases the burnout risk.3
New gastroenterologists are learning a wide range of intricate procedures and becoming proficient in diagnosing and managing gastrointestinal disorders. Extensive career demands often coincide with intense family-forming years, creating tension for a physician’s finite time and energy. The culture of medicine demanding “patients come first” while attempting to be fully human can sometimes feel irreconcilable, leading to feelings of inadequacy and anxiety.3 Gastroenterology training takes 3 years because of the complexity, danger, and need for thousands of procedures to gain proficiency and competence to recognize when complications occur. Oversight is ubiquitous during training, making this the ideal time to learn from mistakes and formulate lifelong habits of constant improvement. However, perfectionist tendencies and the Hippocratic Oath can create unrealistic self expectations.6 The risk of potential litigation, simply missing a diagnosis, or causing actual patient harm is never far from a proceduralist’s mind.
The diversity of gastroenterology requires high clinical knowledge, expertise, and emotional intelligence. Leading potentially intense end-of-life, cancer, fertility, and risk-factor discussions can be all-consuming. Keeping up with the latest research, treatments, and techniques in the field can be daunting. Furthermore, gastroenterologists spend many hours each day on electronic medical records. Constant re-documentation of interactions, seemingly endless prior authorizations, disability forms, referrals, and simply re-addressing patient and family concerns can feel low value. This uncompensated work also creates moral injury as it takes away from direct patient care.
Striking a work-life balance
New gastroenterologists are advised to find work-life balance. However, they are also plagued by the massive professional demands being constantly placed on them. The desire to find the mythical “balance” may create a mindset of significant sacrifices in their private lives as the only way to achieve professional successes.7 When gastroenterologists do not prioritize time for personal activities, including exercise, health checks, hobbies, rest, relaxation, family, and friends, they can get caught in a vicious cycle of continuing to feel poorly, resulting in overcompensating by working more in order to feel “accomplished.” The perfectionist pressure to maintain high productivity and patient satisfaction can also further contribute to burnout.
Gastroenterology burnout can severely affect physicians’ health status, job performance, and patient satisfaction.9 It may erode professionalism, negatively influence the quality of care, increase the risk of medical errors, and promote early retirement.3 Burnout may also correlate with adverse personal consequences for physicians, such as broken relationships, problematic alcohol use, and suicidal ideation.3 Physician burnout and professional satisfaction have strategic importance to health care organizations.10 Less burned-out physicians have patient panels with higher adherence and satisfaction with medical care.10 With more physicians becoming employees, there are opportunities for accountability of organizational leadership.10 Interestingly, healthy well-being or burnout is contagious from leaders to their teams.10 A 2015 study by Shanafelt et al. found that at the work unit level, 11% and 47% of the variation in burnout and satisfaction, correlated with the leader’s relative scores.10
So, what can be done to prevent and treat burnout in new and transitioning gastroenterologists? The gastroenterologist may implement several strategies. It is essential for individuals to take responsibility for their well-being and to prioritize self-care by setting boundaries, practicing stress management techniques, and seeking support from colleagues and mental health professionals when needed. 
According to Dave et al. (2020), engagement in self-care practices such as mindfulness may offer advantages to gastroenterologists’ well-being and improved patient care.11
Burnout is not due to an individuals’ need for more resiliency. Instead, it developed from a systemic overwhelming of a health system near its breaking point. Recognizing that by 2033, there is a projected shortage of nearly 140,000 physicians in the United States, the U.S. Surgeon General, Dr. Vivek H. Murthy, issued a crisis advisory.12 This advisory highlights the urgent need to address the health worker burnout crisis nationwide that outlined “whole of society” efforts.12 Key components of the advisory on building a thriving health workforce included empowering health care workers, changing policies, reducing administrative burdens, prioritizing connections, and investing in our workforce.12
Provide access to mental health services
Institutions and practices would greatly benefit from providing access to mental health services, counseling, educational opportunities, potential mental health days, and mentorship programs. While the literature indicates that both individual-focused and structural or organizational strategies can result in clinically meaningful reductions in burnout among physicians, a meta-analysis revealed that corporate-led initiatives resulted in larger successes.12,13 Physicians who received support and resources from their institutions report lower levels of burnout and higher job satisfaction.2,3
New strategies to select and develop physician leaders who motivate, inspire, and effectively manage physicians may result in positive job satisfaction while decreasing employee burnout. Therefore, increased awareness of the importance of frontline leadership well-being and professional fulfillment of physicians working for a large health care organization is necessary.13 Robust and continual leadership training can ensure the entire team’s well-being, longevity, and success.13
Addressing the root causes of systemic burnout is imperative. Leadership could streamline administrative processes, optimize electronic medical records, delegate prior authorizations, and ensure staffing levels are appropriate to meet patient care demands. In a survey by Rao et al. (2017), the authors found that physicians who reported high levels of administrative burden and work overload were more likely to experience burnout.14
Institutions and practices should promote a culture of work-life balance by implementing flexible scheduling, promoting time off and vacation time, and encouraging regular exercise and healthy habits. The current compensation structure disincentivizes physicians from taking time away from patient care – this can be re-designed. Community and support mitigate burnout. Therefore, institutions and practices will benefit by intentionally providing opportunities for social connection and team building.
In reflection of the U.S. Surgeon General’s call for all of society to be part of the solution, we are pleased to see the Accreditation Council for Graduate Medical Education (ACGME) create mandatory 6 weeks of parental or caregiver leave for trainees.15 Continued positive pressure on overseeing agencies to minimize paperwork, preauthorizations, and non–value-added tasks to allow physicians to continue to provide medical services instead of documentation and auditing services would greatly positively impact all of health care. Therefore, communicating with legislators, policy makers, system leadership, and all health care societies to continue these improvements would be a wise use of time of resources.
In conclusion, burnout among new and transitioning gastroenterologists is a prevalent and concerning issue that can have severe consequences for both the individual and the health care system. Similar to the ergonomic considerations of being an endoscopist, A multifaceted approach to the well-being of all medical staff can help ensure the delivery of the highest quality patient care. By taking a proactive approach to preventing burnout, we can have a strong future for ourselves, our patients, and our profession.
Dr. Eboh is a gastroenterologist with Atrium Health, Charlotte, N.C.; Dr. Jaeger is with Baylor Scott & White Medical Center in Dallas. She is a gastroenterology fellow with Temple University Hospital, Philadelphia. Dr. Sears is clinical professor at Texas A&M University School of Medicine, and chief of gastroenterology at VA Central Texas Healthcare System. Dr. Sears owns GutGirlMD Consulting LLC, where she offers institutional and leadership coaching for physicians. Dr. Eboh on Instagram @Polyp.picker_EbohMD and on Twitter @PolypPicker_MD. Dr. Jaeger on Instagram @Doc.Tori.Fit and Twitter @DrToriJaeger. Dr. Sears is on Twitter @GutGirlMD.
References
1. Maslach C and Jackson S E. Maslach burnout inventory manual. Palo Alto, Calif: Consulting Psychologists Press, 1986.
2. Shanafelt TD et al. Changes in burnout and satisfaction with work-life balance in physicians and the general US working population between 2011 and 2014. Mayo Clin Proc. 2015 Dec 12;90:1600-13.
3. Shanafelt TD et al. Burnout and satisfaction with work-life balance among US physicians relative to the general US population. Arch Intern Med. 2012 Oct 8;172(18):1377-85.
4. Elta G. The challenges of being a female gastroenterologist. Gastroenterol Clin North Am. 2011 Jun;40(2):441-7.
5. Gallup. Work and Workplace. 2023.
6. Gawande A. When doctors make mistakes. The New Yorker. 1999 Feb 1.
7. Buscarini E et al. Burnout among gastroenterologists: How to manage and prevent it. United European Gastroenterol J. 2020 Aug;8(7):832-4.
8. West CP et al. Interventions to prevent and reduce physician burnout: A systematic review and meta-analysis. Lancet. 2016 Nov 5;388(10057):2272-81.
9. Adarkwah CC et al. Burnout and work satisfaction are differentially associated in gastroenterologists in Germany. F1000Res. 2022 Mar 30;11:368. doi: 10.12688/f1000research.110296.3. eCollection 2022.
10. Shanafelt TD et al. Impact of organizational leadership on physician burnout and satisfaction. Mayo Clin Proc. 2015 Apr;90(4):432-40.
11. Umakant D et al. Mindfulness in gastroenterology training and practice: A personal perspective. Clin Exp Gastroenterol. 2020 Nov 4;13:497-502.
12. Murthy VH. Addressing Health Worker Burnout: The U.S. Surgeon General’s Advisory on Building a Thriving Health Workforce. The U.S. Department of Health and Human Services: Office of the U.S. Surgeon General, 2022.
13. Panagioti M et al. Controlled interventions to reduce burnout in physicians: A systematic review and meta-analysis. JAMA Intern Med. 2017 Feb 1;177(2):195-205.
14. Rao SK et al. The impact of administrative burden on academic physicians: Results of a hospital-wide physician survey. Acad Med. 2017 Feb;92(2):237-43.
15. ACGME. ACME Institutional Requirements 2021.
Developing training pathways in advanced endoscopic resection and third-space endoscopy in the U.S.
As a gastroenterology and hepatology fellow, choosing a career path was a daunting prospect. Despite the additional specialization, there seemed to be endless career options to consider. Did I want to join an academic, private, or hybrid practice? Should I subspecialize within the field? Was it important to incorporate research or teaching into my practice? What about opportunities to take on administrative or leadership roles?
Fellowship training at a large academic research institution provided me the opportunity to work with expert faculty in inflammatory bowel disease, esophageal disease, motility and functional gastrointestinal disease, pancreaticobiliary disease, and hepatology. I enjoyed seeing patients in each of these subspecialty clinics. But, by the end of my second year of GI fellowship, I still wasn’t sure what I wanted to do professionally.
A career in academic general gastroenterology seemed to be a good fit for my personality and goals. Rather than focusing on research, I chose to position myself as a clinician educator. I knew that having a subspecialty area of expertise would help improve my clinical practice and make me a more attractive candidate to academic centers. To help narrow my choice, I looked at the clinical enterprise at our institution and assessed where the unmet clinical needs were most acute. Simultaneously, I identified potential mentors to support and guide me through the transition from fellow to independent practitioner. I decided to focus on acquiring the skills to care for patients with anorectal diseases and lower-GI motility disorders, as this area met both of my criteria – excellent mentorship and an unmet clinical need. Under the guidance of Dr. Yolanda Scarlett, I spent my 3rd year in clinic learning to interpret anorectal manometry tests, defecograms, and sitz marker studies and treating patients with refractory constipation, fecal incontinence, and anal fissures.
With a plan to develop an expertise in anorectal diseases and low-GI motility disorders, I also wanted to focus on improving my endoscopic skills to graduate as well rounded a clinician as possible. To achieve this goal, I sought out a separate endoscopy mentor, Dr. Ian Grimm, the director of endoscopy at the University of North Carolina at Chapel Hill. Dr. Grimm, a classically trained advanced endoscopist performing endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP), had a burgeoning interest in endoscopic mucosal resection (EMR) and had just returned from a few months in Japan learning to perform endoscopic submucosal dissection (ESD) and peroral endoscopic myotomy (POEM).
When I began working with Dr. Grimm, I had not even heard the term third-space endoscopy and knew nothing about ESD or POEM. I spent as much time as possible watching and assisting Dr. Grimm with complex endoscopic mucosal resection (EMR) during the first few months of my 3rd year. Soon after my exposure to advanced endoscopic resection, it was clear that I wanted to learn and incorporate this into my clinical practice. I watched Dr. Grimm perform the first POEM at UNC in the fall of 2016 and by that time I was hooked on learning third-space endoscopy. I observed and assisted with as many EMR, ESD, and POEM cases as I could that year. In addition to the hands-on and cognitive training with Dr. Grimm, I attended national meetings and workshops focused on learning third-space endoscopy. In the spring of my 3rd year I was honored to be the first fellow to complete the Olympus master class in ESD – a 2-day hands-on training course sponsored by Olympus. By the end of that year, I was performing complex EMR with minimal assistance and had completed multiple ESDs and POEMs with cognitive supervision only.
After fellowship, I joined the UNC faculty as a general gastroenterologist with expertise in anorectal disease and lower-GI motility disorders. While I was comfortable performing complex EMR, I still needed additional training and supervision before I felt ready to independently perform ESD or POEM. With the gracious support and encouragement of our division chief, I continued third-space endoscopy training with Dr. Grimm during dedicated protected time 2 days each month. Over the ensuing 4 years, I transitioned to fully independent practice performing all types of advanced EMR and third-space endoscopy including complex EMR, ESD, endoscopic full-thickness resection (EFTR), submucosal tunnel endoscopic resection (STER), esophageal POEM, gastric POEM, and Zenker’s POEM.
As one of the first gastroenterologists in the United States to perform third-space endoscopy without any formal training in advanced pancreaticobiliary endoscopy, I believe learning advanced endoscopic resection and third-space endoscopy is best achieved through a training pathway separate from the conventional advanced endoscopy fellowship focused on teaching EUS and ERCP. Although there are transferable skills learned from EUS and ERCP to the techniques used in third-space endoscopy, there is nothing inherent to performing EUS or ERCP that enables one to learn how to perform an ESD or a POEM.
There is a robust training pathway to teach advanced pancreaticobiliary endoscopy, but no formal training pathway exists to teach third-space endoscopy in the United States. Historically, a small number of interested and motivated advanced pancreaticobiliary endoscopists sought out opportunities to learn third-space endoscopy after completion of their advanced endoscopy fellowship, in some cases many years after graduation. For these early adopters in the United States, the only training opportunities required travel to Japan or another Eastern country with arrangements made to observe and participate in third-space endoscopy cases with experts there. With increased recognition of the benefits of ESD and POEM over the past 5-10 years in the United States, there has been greater adoption of third-space endoscopy and with it, more training opportunities. Still, there are very few institutions with formalized training programs in advanced endoscopic resection and third-space endoscopy in the United States to date.
Proof that this model works
In Eastern countries such as Japan, training endoscopists to perform ESD and POEM has been successfully achieved through an apprenticeship model whereby an expert in third-space endoscopy closely supervises a trainee who gains greater autonomy with increasing experience and skill over time. My personal experience is proof that this model works. But, adopting such a model more widely in the United States may prove difficult. We lack a sufficient number of experienced third-space endoscopy operators and, given the challenges to appropriate reimbursement for third-space endoscopy in the United States, there is understandable resistance to accepting the prolonged training period necessary for technical mastery of this skill.
In part, a long training period is needed because of a relative paucity of appropriate target lesions for ESD and the rarity of achalasia in the United States. While there is consensus among experts regarding the benefits of ESD for resection of early gastric cancer (EGC), relatively few EGCs are found in the United States and indications for ESD outside resection of EGC are less well defined with less clear benefits over more widely performed piecemeal EMR. Despite these challenges, it is critical that we continue to develop dedicated training pathways to teach advanced endoscopic resection and third-space endoscopy in the United States. My practice has evolved considerably since completion of fellowship nearly 6 years ago, and I now focus almost exclusively on advanced endoscopic resection and third-space endoscopy. Recently, Dr. Grimm and I began an advanced endoscopic resection elective for the general GI fellows at UNC and we are excited to welcome our first advanced endoscopic resection and third-space endoscopy fellow to UNC this July.
While there are many possible avenues to expertise in advanced endoscopic resection, few will likely follow the same path that I have taken. Trainees who are interested in pursuing this subspecialty should seek out supportive mentors in a setting where there is already a robust case volume of esophageal motility disorders and endoscopic resections. Success requires the persistent motivation to seek out diverse opportunities for self-study, exposure to experts, data on developments in the field, and hands-on exposure to as many ex-vivo and in-vivo cases as possible.
Dr. Kroch is assistant professor of medicine in the division of gastroenterology and hepatology at the University of North Carolina at Chapel Hill. He disclosed having no conflicts of interest.
As a gastroenterology and hepatology fellow, choosing a career path was a daunting prospect. Despite the additional specialization, there seemed to be endless career options to consider. Did I want to join an academic, private, or hybrid practice? Should I subspecialize within the field? Was it important to incorporate research or teaching into my practice? What about opportunities to take on administrative or leadership roles?
Fellowship training at a large academic research institution provided me the opportunity to work with expert faculty in inflammatory bowel disease, esophageal disease, motility and functional gastrointestinal disease, pancreaticobiliary disease, and hepatology. I enjoyed seeing patients in each of these subspecialty clinics. But, by the end of my second year of GI fellowship, I still wasn’t sure what I wanted to do professionally.
A career in academic general gastroenterology seemed to be a good fit for my personality and goals. Rather than focusing on research, I chose to position myself as a clinician educator. I knew that having a subspecialty area of expertise would help improve my clinical practice and make me a more attractive candidate to academic centers. To help narrow my choice, I looked at the clinical enterprise at our institution and assessed where the unmet clinical needs were most acute. Simultaneously, I identified potential mentors to support and guide me through the transition from fellow to independent practitioner. I decided to focus on acquiring the skills to care for patients with anorectal diseases and lower-GI motility disorders, as this area met both of my criteria – excellent mentorship and an unmet clinical need. Under the guidance of Dr. Yolanda Scarlett, I spent my 3rd year in clinic learning to interpret anorectal manometry tests, defecograms, and sitz marker studies and treating patients with refractory constipation, fecal incontinence, and anal fissures.
With a plan to develop an expertise in anorectal diseases and low-GI motility disorders, I also wanted to focus on improving my endoscopic skills to graduate as well rounded a clinician as possible. To achieve this goal, I sought out a separate endoscopy mentor, Dr. Ian Grimm, the director of endoscopy at the University of North Carolina at Chapel Hill. Dr. Grimm, a classically trained advanced endoscopist performing endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP), had a burgeoning interest in endoscopic mucosal resection (EMR) and had just returned from a few months in Japan learning to perform endoscopic submucosal dissection (ESD) and peroral endoscopic myotomy (POEM).
When I began working with Dr. Grimm, I had not even heard the term third-space endoscopy and knew nothing about ESD or POEM. I spent as much time as possible watching and assisting Dr. Grimm with complex endoscopic mucosal resection (EMR) during the first few months of my 3rd year. Soon after my exposure to advanced endoscopic resection, it was clear that I wanted to learn and incorporate this into my clinical practice. I watched Dr. Grimm perform the first POEM at UNC in the fall of 2016 and by that time I was hooked on learning third-space endoscopy. I observed and assisted with as many EMR, ESD, and POEM cases as I could that year. In addition to the hands-on and cognitive training with Dr. Grimm, I attended national meetings and workshops focused on learning third-space endoscopy. In the spring of my 3rd year I was honored to be the first fellow to complete the Olympus master class in ESD – a 2-day hands-on training course sponsored by Olympus. By the end of that year, I was performing complex EMR with minimal assistance and had completed multiple ESDs and POEMs with cognitive supervision only.
After fellowship, I joined the UNC faculty as a general gastroenterologist with expertise in anorectal disease and lower-GI motility disorders. While I was comfortable performing complex EMR, I still needed additional training and supervision before I felt ready to independently perform ESD or POEM. With the gracious support and encouragement of our division chief, I continued third-space endoscopy training with Dr. Grimm during dedicated protected time 2 days each month. Over the ensuing 4 years, I transitioned to fully independent practice performing all types of advanced EMR and third-space endoscopy including complex EMR, ESD, endoscopic full-thickness resection (EFTR), submucosal tunnel endoscopic resection (STER), esophageal POEM, gastric POEM, and Zenker’s POEM.
As one of the first gastroenterologists in the United States to perform third-space endoscopy without any formal training in advanced pancreaticobiliary endoscopy, I believe learning advanced endoscopic resection and third-space endoscopy is best achieved through a training pathway separate from the conventional advanced endoscopy fellowship focused on teaching EUS and ERCP. Although there are transferable skills learned from EUS and ERCP to the techniques used in third-space endoscopy, there is nothing inherent to performing EUS or ERCP that enables one to learn how to perform an ESD or a POEM.
There is a robust training pathway to teach advanced pancreaticobiliary endoscopy, but no formal training pathway exists to teach third-space endoscopy in the United States. Historically, a small number of interested and motivated advanced pancreaticobiliary endoscopists sought out opportunities to learn third-space endoscopy after completion of their advanced endoscopy fellowship, in some cases many years after graduation. For these early adopters in the United States, the only training opportunities required travel to Japan or another Eastern country with arrangements made to observe and participate in third-space endoscopy cases with experts there. With increased recognition of the benefits of ESD and POEM over the past 5-10 years in the United States, there has been greater adoption of third-space endoscopy and with it, more training opportunities. Still, there are very few institutions with formalized training programs in advanced endoscopic resection and third-space endoscopy in the United States to date.
Proof that this model works
In Eastern countries such as Japan, training endoscopists to perform ESD and POEM has been successfully achieved through an apprenticeship model whereby an expert in third-space endoscopy closely supervises a trainee who gains greater autonomy with increasing experience and skill over time. My personal experience is proof that this model works. But, adopting such a model more widely in the United States may prove difficult. We lack a sufficient number of experienced third-space endoscopy operators and, given the challenges to appropriate reimbursement for third-space endoscopy in the United States, there is understandable resistance to accepting the prolonged training period necessary for technical mastery of this skill.
In part, a long training period is needed because of a relative paucity of appropriate target lesions for ESD and the rarity of achalasia in the United States. While there is consensus among experts regarding the benefits of ESD for resection of early gastric cancer (EGC), relatively few EGCs are found in the United States and indications for ESD outside resection of EGC are less well defined with less clear benefits over more widely performed piecemeal EMR. Despite these challenges, it is critical that we continue to develop dedicated training pathways to teach advanced endoscopic resection and third-space endoscopy in the United States. My practice has evolved considerably since completion of fellowship nearly 6 years ago, and I now focus almost exclusively on advanced endoscopic resection and third-space endoscopy. Recently, Dr. Grimm and I began an advanced endoscopic resection elective for the general GI fellows at UNC and we are excited to welcome our first advanced endoscopic resection and third-space endoscopy fellow to UNC this July.
While there are many possible avenues to expertise in advanced endoscopic resection, few will likely follow the same path that I have taken. Trainees who are interested in pursuing this subspecialty should seek out supportive mentors in a setting where there is already a robust case volume of esophageal motility disorders and endoscopic resections. Success requires the persistent motivation to seek out diverse opportunities for self-study, exposure to experts, data on developments in the field, and hands-on exposure to as many ex-vivo and in-vivo cases as possible.
Dr. Kroch is assistant professor of medicine in the division of gastroenterology and hepatology at the University of North Carolina at Chapel Hill. He disclosed having no conflicts of interest.
As a gastroenterology and hepatology fellow, choosing a career path was a daunting prospect. Despite the additional specialization, there seemed to be endless career options to consider. Did I want to join an academic, private, or hybrid practice? Should I subspecialize within the field? Was it important to incorporate research or teaching into my practice? What about opportunities to take on administrative or leadership roles?
Fellowship training at a large academic research institution provided me the opportunity to work with expert faculty in inflammatory bowel disease, esophageal disease, motility and functional gastrointestinal disease, pancreaticobiliary disease, and hepatology. I enjoyed seeing patients in each of these subspecialty clinics. But, by the end of my second year of GI fellowship, I still wasn’t sure what I wanted to do professionally.
A career in academic general gastroenterology seemed to be a good fit for my personality and goals. Rather than focusing on research, I chose to position myself as a clinician educator. I knew that having a subspecialty area of expertise would help improve my clinical practice and make me a more attractive candidate to academic centers. To help narrow my choice, I looked at the clinical enterprise at our institution and assessed where the unmet clinical needs were most acute. Simultaneously, I identified potential mentors to support and guide me through the transition from fellow to independent practitioner. I decided to focus on acquiring the skills to care for patients with anorectal diseases and lower-GI motility disorders, as this area met both of my criteria – excellent mentorship and an unmet clinical need. Under the guidance of Dr. Yolanda Scarlett, I spent my 3rd year in clinic learning to interpret anorectal manometry tests, defecograms, and sitz marker studies and treating patients with refractory constipation, fecal incontinence, and anal fissures.
With a plan to develop an expertise in anorectal diseases and low-GI motility disorders, I also wanted to focus on improving my endoscopic skills to graduate as well rounded a clinician as possible. To achieve this goal, I sought out a separate endoscopy mentor, Dr. Ian Grimm, the director of endoscopy at the University of North Carolina at Chapel Hill. Dr. Grimm, a classically trained advanced endoscopist performing endoscopic ultrasound (EUS) and endoscopic retrograde cholangiopancreatography (ERCP), had a burgeoning interest in endoscopic mucosal resection (EMR) and had just returned from a few months in Japan learning to perform endoscopic submucosal dissection (ESD) and peroral endoscopic myotomy (POEM).
When I began working with Dr. Grimm, I had not even heard the term third-space endoscopy and knew nothing about ESD or POEM. I spent as much time as possible watching and assisting Dr. Grimm with complex endoscopic mucosal resection (EMR) during the first few months of my 3rd year. Soon after my exposure to advanced endoscopic resection, it was clear that I wanted to learn and incorporate this into my clinical practice. I watched Dr. Grimm perform the first POEM at UNC in the fall of 2016 and by that time I was hooked on learning third-space endoscopy. I observed and assisted with as many EMR, ESD, and POEM cases as I could that year. In addition to the hands-on and cognitive training with Dr. Grimm, I attended national meetings and workshops focused on learning third-space endoscopy. In the spring of my 3rd year I was honored to be the first fellow to complete the Olympus master class in ESD – a 2-day hands-on training course sponsored by Olympus. By the end of that year, I was performing complex EMR with minimal assistance and had completed multiple ESDs and POEMs with cognitive supervision only.
After fellowship, I joined the UNC faculty as a general gastroenterologist with expertise in anorectal disease and lower-GI motility disorders. While I was comfortable performing complex EMR, I still needed additional training and supervision before I felt ready to independently perform ESD or POEM. With the gracious support and encouragement of our division chief, I continued third-space endoscopy training with Dr. Grimm during dedicated protected time 2 days each month. Over the ensuing 4 years, I transitioned to fully independent practice performing all types of advanced EMR and third-space endoscopy including complex EMR, ESD, endoscopic full-thickness resection (EFTR), submucosal tunnel endoscopic resection (STER), esophageal POEM, gastric POEM, and Zenker’s POEM.
As one of the first gastroenterologists in the United States to perform third-space endoscopy without any formal training in advanced pancreaticobiliary endoscopy, I believe learning advanced endoscopic resection and third-space endoscopy is best achieved through a training pathway separate from the conventional advanced endoscopy fellowship focused on teaching EUS and ERCP. Although there are transferable skills learned from EUS and ERCP to the techniques used in third-space endoscopy, there is nothing inherent to performing EUS or ERCP that enables one to learn how to perform an ESD or a POEM.
There is a robust training pathway to teach advanced pancreaticobiliary endoscopy, but no formal training pathway exists to teach third-space endoscopy in the United States. Historically, a small number of interested and motivated advanced pancreaticobiliary endoscopists sought out opportunities to learn third-space endoscopy after completion of their advanced endoscopy fellowship, in some cases many years after graduation. For these early adopters in the United States, the only training opportunities required travel to Japan or another Eastern country with arrangements made to observe and participate in third-space endoscopy cases with experts there. With increased recognition of the benefits of ESD and POEM over the past 5-10 years in the United States, there has been greater adoption of third-space endoscopy and with it, more training opportunities. Still, there are very few institutions with formalized training programs in advanced endoscopic resection and third-space endoscopy in the United States to date.
Proof that this model works
In Eastern countries such as Japan, training endoscopists to perform ESD and POEM has been successfully achieved through an apprenticeship model whereby an expert in third-space endoscopy closely supervises a trainee who gains greater autonomy with increasing experience and skill over time. My personal experience is proof that this model works. But, adopting such a model more widely in the United States may prove difficult. We lack a sufficient number of experienced third-space endoscopy operators and, given the challenges to appropriate reimbursement for third-space endoscopy in the United States, there is understandable resistance to accepting the prolonged training period necessary for technical mastery of this skill.
In part, a long training period is needed because of a relative paucity of appropriate target lesions for ESD and the rarity of achalasia in the United States. While there is consensus among experts regarding the benefits of ESD for resection of early gastric cancer (EGC), relatively few EGCs are found in the United States and indications for ESD outside resection of EGC are less well defined with less clear benefits over more widely performed piecemeal EMR. Despite these challenges, it is critical that we continue to develop dedicated training pathways to teach advanced endoscopic resection and third-space endoscopy in the United States. My practice has evolved considerably since completion of fellowship nearly 6 years ago, and I now focus almost exclusively on advanced endoscopic resection and third-space endoscopy. Recently, Dr. Grimm and I began an advanced endoscopic resection elective for the general GI fellows at UNC and we are excited to welcome our first advanced endoscopic resection and third-space endoscopy fellow to UNC this July.
While there are many possible avenues to expertise in advanced endoscopic resection, few will likely follow the same path that I have taken. Trainees who are interested in pursuing this subspecialty should seek out supportive mentors in a setting where there is already a robust case volume of esophageal motility disorders and endoscopic resections. Success requires the persistent motivation to seek out diverse opportunities for self-study, exposure to experts, data on developments in the field, and hands-on exposure to as many ex-vivo and in-vivo cases as possible.
Dr. Kroch is assistant professor of medicine in the division of gastroenterology and hepatology at the University of North Carolina at Chapel Hill. He disclosed having no conflicts of interest.
Geriatric care principles should apply to ICUs as well
Baseball legend Leroy “Satchel” Paige famously said that “age is a question of mind over matter: If you don’t mind, it doesn’t matter.”
But even the strongest and most supple minds can’t avoid the effects of advanced age and accompanying physical frailty, and for community-dwelling elderly with pulmonary diseases frailty is a predictor of both hospitalization and death, investigators have found.
For example, among 1,188 community-dwelling older adults enrolled in the Toledo (Spain) Study for Healthy Aging, declining pulmonary function measured by forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) was associated with increased risk for frailty and hospitalization, and a more than twofold greater risk for death in participants both with and without respiratory diseases. These findings were reported by Walter Sepulveda-Loyola, PT, MSC, PhD, from the Faculty of Health and Social Sciences at Universidad de Las Americas in Santiago, Chile, and colleagues in the journal Heart & Lung.
Similarly, results of a meta-analysis performed by investigators at Jiangsu (China) University showed that among 13,203 patients with chronic obstructive pulmonary disease (COPD), frailty was associated with a more than 2.6-fold relative increase in risk for death from any cause, and “prefrailty,” an intermediate state between frailty and “robustness,” was associated with a 48% relative increase in all-cause mortality. Frailty was also associated with a 2.2-fold risk for COPD exacerbations of any severity, the authors reported in JAMDA: The Journal of Post-Acute and Long-Term Care Medicine.
The good (old) USA
In June 2023 the U.S. Census Bureau announced that the median age of the U.S. population is now 38.9 years, and according to a 2016 Census Bureau report funded by the National Institutes of Health, “America’s 65-and-over population is projected to nearly double over the next three decades, from 48 million to 88 million by 2050.”
With the graying of the U.S. population the burden on pulmonary and critical care experts will almost inevitably increase, as evidenced by research from Julien Cobert, MD, from the University of California, San Francisco, and colleagues.
The investigators looked at trends over time in older adults admitted to ICUs from 1988 through 2015 using data from the Health and Retirement Study (HRS), a nationally representative, longitudinal study of older adults. They found that rates of preexisting frailty, disability, and multimorbidity increased over the study period.
“Our findings suggest a growing prevalence of geriatric conditions among older adults admitted to the ICU, suggesting a pressing need to integrate geriatric principles into critical care medicine. Further research could examine if early interventions emphasizing physical, cognitive, mental health, delirium prevention, advance care planning, and rehabilitation individualized to critically ill elderly patients with preexisting geriatric conditions could improve ICU outcomes and post-ICU recovery,” they wrote in a study published in the journal CHEST.
In an editorial accompanying the study by Dr. Cobert and colleagues, Nathan E. Brummel, MD, from The Ohio State University College of Medicine and Davis Heart and Lung Research Institute in Columbus, said “the finding that nearly 30% of overall HRS participants were admitted to the ICU provides novel data about the extent to which older Americans are affected by critical illness. Because the number of older Americans is projected to continue to increase for the next 30 years or more, these data make clear the ongoing importance of aging-focused research and clinical care.”
Dr. Brummel also noted that older adults who are admitted to the ICU today are at greater risk for poor outcomes than those admitted in prior years, as evidenced by the increased prevalence of disability, frailty, and multimorbidity.
“Moreover, because the average age of those admitted to the ICU only changed by 1 year during the study, these data show that increases in vulnerability are not simply due to chronological age, and they suggest that to identify those with greater baseline vulnerability, screening for geriatric syndromes at ICU admission may be warranted,” he wrote.
Geriatric principles in the ICU
“I think what’s most important is that we think about patients from a geriatric principles standpoint, not just when they’re admitted to the hospital but especially when they’re admitted to the ICU,” Dr. Cobert said in an interview.
“The first step is ensuring that we’re asking questions about their underlying comorbidities, especially around frailty, hearing, vision loss, falls, multimorbidities, polypharmacy – things that are primarily done on the outpatient side in geriatric clinics, but things that we should probably be a little bit more cognizant of, given that we’re starting to see higher rates of patients coming in with these issues,” he said.
Critical care specialists need to take a more holistic approach and try to understand as best they can each patients’ goals and then determine whether the ICU staff are acting in concordance with those goals, he emphasized.
For example, ICU clinicians should try to understand whether patients were losing function or having mobility difficulties before hospital and ICU admission, and what they hope to retain when or if they are discharged. ICU staff can then try as much as reasonably possible to minimize interventions that could contribute to impairment after discharge.
Frailty and COPD in the ICU
There are special considerations for frail elderly with obstructive airway disease, Dr. Cobert noted.
Patients with advanced COPD, for example, are likely to be on home oxygen.
“Home oxygen is a big deal,” he said. “It can definitely help with functioning and there’s potentially a mortality benefit in certain populations. But that said, it’s a flammable object that they have to carry around and lug with them all the time. It contributes to falls, it’s tethering, it’s life-limiting in many ways.”
In addition, many patients with COPD have multiple re-hospitalizations, and for clinicians the challenge is “understanding what their goals are, what their motivations are, especially when they live with dyspnea, with advanced lung disease. Is intubation within their goals of care? Has their functional status been declining over time? Are there things that we can optimize holistically and globally as their COPD advances over time?”
Another important component of critical care for the frail elderly is consideration of patients’ palliative care needs and what their symptoms and symptom burdens were like prior to hospitalizations.
“The ICU experience and the critical illness experience may serve as an inflexion point – more likely a downward inflection point – whereby their needs increase, their symptoms can worsen, and their health, especially their global health, worsens. Their preexisting geriatric conditions might be a moving target after another hit and another traumatic stressor like the ICU setting,” Dr. Cobert said.
The study by Dr. Cobert and colleagues was supported by the National Institute on Aging. Dr. Cobert had no reported conflicts of interest.
Baseball legend Leroy “Satchel” Paige famously said that “age is a question of mind over matter: If you don’t mind, it doesn’t matter.”
But even the strongest and most supple minds can’t avoid the effects of advanced age and accompanying physical frailty, and for community-dwelling elderly with pulmonary diseases frailty is a predictor of both hospitalization and death, investigators have found.
For example, among 1,188 community-dwelling older adults enrolled in the Toledo (Spain) Study for Healthy Aging, declining pulmonary function measured by forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) was associated with increased risk for frailty and hospitalization, and a more than twofold greater risk for death in participants both with and without respiratory diseases. These findings were reported by Walter Sepulveda-Loyola, PT, MSC, PhD, from the Faculty of Health and Social Sciences at Universidad de Las Americas in Santiago, Chile, and colleagues in the journal Heart & Lung.
Similarly, results of a meta-analysis performed by investigators at Jiangsu (China) University showed that among 13,203 patients with chronic obstructive pulmonary disease (COPD), frailty was associated with a more than 2.6-fold relative increase in risk for death from any cause, and “prefrailty,” an intermediate state between frailty and “robustness,” was associated with a 48% relative increase in all-cause mortality. Frailty was also associated with a 2.2-fold risk for COPD exacerbations of any severity, the authors reported in JAMDA: The Journal of Post-Acute and Long-Term Care Medicine.
The good (old) USA
In June 2023 the U.S. Census Bureau announced that the median age of the U.S. population is now 38.9 years, and according to a 2016 Census Bureau report funded by the National Institutes of Health, “America’s 65-and-over population is projected to nearly double over the next three decades, from 48 million to 88 million by 2050.”
With the graying of the U.S. population the burden on pulmonary and critical care experts will almost inevitably increase, as evidenced by research from Julien Cobert, MD, from the University of California, San Francisco, and colleagues.
The investigators looked at trends over time in older adults admitted to ICUs from 1988 through 2015 using data from the Health and Retirement Study (HRS), a nationally representative, longitudinal study of older adults. They found that rates of preexisting frailty, disability, and multimorbidity increased over the study period.
“Our findings suggest a growing prevalence of geriatric conditions among older adults admitted to the ICU, suggesting a pressing need to integrate geriatric principles into critical care medicine. Further research could examine if early interventions emphasizing physical, cognitive, mental health, delirium prevention, advance care planning, and rehabilitation individualized to critically ill elderly patients with preexisting geriatric conditions could improve ICU outcomes and post-ICU recovery,” they wrote in a study published in the journal CHEST.
In an editorial accompanying the study by Dr. Cobert and colleagues, Nathan E. Brummel, MD, from The Ohio State University College of Medicine and Davis Heart and Lung Research Institute in Columbus, said “the finding that nearly 30% of overall HRS participants were admitted to the ICU provides novel data about the extent to which older Americans are affected by critical illness. Because the number of older Americans is projected to continue to increase for the next 30 years or more, these data make clear the ongoing importance of aging-focused research and clinical care.”
Dr. Brummel also noted that older adults who are admitted to the ICU today are at greater risk for poor outcomes than those admitted in prior years, as evidenced by the increased prevalence of disability, frailty, and multimorbidity.
“Moreover, because the average age of those admitted to the ICU only changed by 1 year during the study, these data show that increases in vulnerability are not simply due to chronological age, and they suggest that to identify those with greater baseline vulnerability, screening for geriatric syndromes at ICU admission may be warranted,” he wrote.
Geriatric principles in the ICU
“I think what’s most important is that we think about patients from a geriatric principles standpoint, not just when they’re admitted to the hospital but especially when they’re admitted to the ICU,” Dr. Cobert said in an interview.
“The first step is ensuring that we’re asking questions about their underlying comorbidities, especially around frailty, hearing, vision loss, falls, multimorbidities, polypharmacy – things that are primarily done on the outpatient side in geriatric clinics, but things that we should probably be a little bit more cognizant of, given that we’re starting to see higher rates of patients coming in with these issues,” he said.
Critical care specialists need to take a more holistic approach and try to understand as best they can each patients’ goals and then determine whether the ICU staff are acting in concordance with those goals, he emphasized.
For example, ICU clinicians should try to understand whether patients were losing function or having mobility difficulties before hospital and ICU admission, and what they hope to retain when or if they are discharged. ICU staff can then try as much as reasonably possible to minimize interventions that could contribute to impairment after discharge.
Frailty and COPD in the ICU
There are special considerations for frail elderly with obstructive airway disease, Dr. Cobert noted.
Patients with advanced COPD, for example, are likely to be on home oxygen.
“Home oxygen is a big deal,” he said. “It can definitely help with functioning and there’s potentially a mortality benefit in certain populations. But that said, it’s a flammable object that they have to carry around and lug with them all the time. It contributes to falls, it’s tethering, it’s life-limiting in many ways.”
In addition, many patients with COPD have multiple re-hospitalizations, and for clinicians the challenge is “understanding what their goals are, what their motivations are, especially when they live with dyspnea, with advanced lung disease. Is intubation within their goals of care? Has their functional status been declining over time? Are there things that we can optimize holistically and globally as their COPD advances over time?”
Another important component of critical care for the frail elderly is consideration of patients’ palliative care needs and what their symptoms and symptom burdens were like prior to hospitalizations.
“The ICU experience and the critical illness experience may serve as an inflexion point – more likely a downward inflection point – whereby their needs increase, their symptoms can worsen, and their health, especially their global health, worsens. Their preexisting geriatric conditions might be a moving target after another hit and another traumatic stressor like the ICU setting,” Dr. Cobert said.
The study by Dr. Cobert and colleagues was supported by the National Institute on Aging. Dr. Cobert had no reported conflicts of interest.
Baseball legend Leroy “Satchel” Paige famously said that “age is a question of mind over matter: If you don’t mind, it doesn’t matter.”
But even the strongest and most supple minds can’t avoid the effects of advanced age and accompanying physical frailty, and for community-dwelling elderly with pulmonary diseases frailty is a predictor of both hospitalization and death, investigators have found.
For example, among 1,188 community-dwelling older adults enrolled in the Toledo (Spain) Study for Healthy Aging, declining pulmonary function measured by forced expiratory volume in 1 second (FEV1) and forced vital capacity (FVC) was associated with increased risk for frailty and hospitalization, and a more than twofold greater risk for death in participants both with and without respiratory diseases. These findings were reported by Walter Sepulveda-Loyola, PT, MSC, PhD, from the Faculty of Health and Social Sciences at Universidad de Las Americas in Santiago, Chile, and colleagues in the journal Heart & Lung.
Similarly, results of a meta-analysis performed by investigators at Jiangsu (China) University showed that among 13,203 patients with chronic obstructive pulmonary disease (COPD), frailty was associated with a more than 2.6-fold relative increase in risk for death from any cause, and “prefrailty,” an intermediate state between frailty and “robustness,” was associated with a 48% relative increase in all-cause mortality. Frailty was also associated with a 2.2-fold risk for COPD exacerbations of any severity, the authors reported in JAMDA: The Journal of Post-Acute and Long-Term Care Medicine.
The good (old) USA
In June 2023 the U.S. Census Bureau announced that the median age of the U.S. population is now 38.9 years, and according to a 2016 Census Bureau report funded by the National Institutes of Health, “America’s 65-and-over population is projected to nearly double over the next three decades, from 48 million to 88 million by 2050.”
With the graying of the U.S. population the burden on pulmonary and critical care experts will almost inevitably increase, as evidenced by research from Julien Cobert, MD, from the University of California, San Francisco, and colleagues.
The investigators looked at trends over time in older adults admitted to ICUs from 1988 through 2015 using data from the Health and Retirement Study (HRS), a nationally representative, longitudinal study of older adults. They found that rates of preexisting frailty, disability, and multimorbidity increased over the study period.
“Our findings suggest a growing prevalence of geriatric conditions among older adults admitted to the ICU, suggesting a pressing need to integrate geriatric principles into critical care medicine. Further research could examine if early interventions emphasizing physical, cognitive, mental health, delirium prevention, advance care planning, and rehabilitation individualized to critically ill elderly patients with preexisting geriatric conditions could improve ICU outcomes and post-ICU recovery,” they wrote in a study published in the journal CHEST.
In an editorial accompanying the study by Dr. Cobert and colleagues, Nathan E. Brummel, MD, from The Ohio State University College of Medicine and Davis Heart and Lung Research Institute in Columbus, said “the finding that nearly 30% of overall HRS participants were admitted to the ICU provides novel data about the extent to which older Americans are affected by critical illness. Because the number of older Americans is projected to continue to increase for the next 30 years or more, these data make clear the ongoing importance of aging-focused research and clinical care.”
Dr. Brummel also noted that older adults who are admitted to the ICU today are at greater risk for poor outcomes than those admitted in prior years, as evidenced by the increased prevalence of disability, frailty, and multimorbidity.
“Moreover, because the average age of those admitted to the ICU only changed by 1 year during the study, these data show that increases in vulnerability are not simply due to chronological age, and they suggest that to identify those with greater baseline vulnerability, screening for geriatric syndromes at ICU admission may be warranted,” he wrote.
Geriatric principles in the ICU
“I think what’s most important is that we think about patients from a geriatric principles standpoint, not just when they’re admitted to the hospital but especially when they’re admitted to the ICU,” Dr. Cobert said in an interview.
“The first step is ensuring that we’re asking questions about their underlying comorbidities, especially around frailty, hearing, vision loss, falls, multimorbidities, polypharmacy – things that are primarily done on the outpatient side in geriatric clinics, but things that we should probably be a little bit more cognizant of, given that we’re starting to see higher rates of patients coming in with these issues,” he said.
Critical care specialists need to take a more holistic approach and try to understand as best they can each patients’ goals and then determine whether the ICU staff are acting in concordance with those goals, he emphasized.
For example, ICU clinicians should try to understand whether patients were losing function or having mobility difficulties before hospital and ICU admission, and what they hope to retain when or if they are discharged. ICU staff can then try as much as reasonably possible to minimize interventions that could contribute to impairment after discharge.
Frailty and COPD in the ICU
There are special considerations for frail elderly with obstructive airway disease, Dr. Cobert noted.
Patients with advanced COPD, for example, are likely to be on home oxygen.
“Home oxygen is a big deal,” he said. “It can definitely help with functioning and there’s potentially a mortality benefit in certain populations. But that said, it’s a flammable object that they have to carry around and lug with them all the time. It contributes to falls, it’s tethering, it’s life-limiting in many ways.”
In addition, many patients with COPD have multiple re-hospitalizations, and for clinicians the challenge is “understanding what their goals are, what their motivations are, especially when they live with dyspnea, with advanced lung disease. Is intubation within their goals of care? Has their functional status been declining over time? Are there things that we can optimize holistically and globally as their COPD advances over time?”
Another important component of critical care for the frail elderly is consideration of patients’ palliative care needs and what their symptoms and symptom burdens were like prior to hospitalizations.
“The ICU experience and the critical illness experience may serve as an inflexion point – more likely a downward inflection point – whereby their needs increase, their symptoms can worsen, and their health, especially their global health, worsens. Their preexisting geriatric conditions might be a moving target after another hit and another traumatic stressor like the ICU setting,” Dr. Cobert said.
The study by Dr. Cobert and colleagues was supported by the National Institute on Aging. Dr. Cobert had no reported conflicts of interest.
Long COVID ‘brain fog’ confounds doctors, but new research offers hope
Kate Whitley was petrified of COVID-19 from the beginning of the pandemic because she has Hashimoto disease, an autoimmune disorder that she knew put her at high risk for complications.
She was right to be worried. Two months after contracting the infection in September 2022, the 42-year-old Nashville resident was diagnosed with long COVID. For Ms. Whitley, the resulting brain fog has been the most challenging factor. She is the owner of a successful paper goods store, and she can’t remember basic aspects of her job. She can’t tolerate loud noises and gets so distracted that she has trouble remembering what she was doing.
Ms. Whitley doesn’t like the term “brain fog” because it doesn’t begin to describe the dramatic disruption to her life over the past 7 months.
Brain fog is among the most common symptoms of long COVID, and also one of the most poorly understood. A reported 46% of those diagnosed with long COVID complain of brain fog or a loss of memory. Many clinicians agree that the term is vague and often doesn’t truly represent the condition. That, in turn, makes it harder for doctors to diagnose and treat it. There are no standard tests for it, nor are there guidelines for symptom management or treatment.
“There’s a lot of imprecision in the term because it might mean different things to different patients,” said James C. Jackson, PsyD, a neuropsychiatrist at Vanderbilt University, Nashville, Tenn., and author of a new book, “Clearing the Fog: From Surviving to Thriving With Long COVID – A Practical Guide.”
Dr. Jackson, who began treating Ms. Whitley in February 2023, said that it makes more sense to call brain fog a brain impairment or an acquired brain injury (ABI) because it doesn’t occur gradually. COVID damages the brain and causes injury. For those with long COVID who were previously in the intensive care unit and may have undergone ventilation, hypoxic brain injury may result from the lack of oxygen to the brain.
Even among those with milder cases of acute COVID, there’s some evidence that persistent neuroinflammation in the brain caused by an activated immune system may also cause damage.
In both cases, the results can be debilitating. Ms. Whitley also has dysautonomia – a disorder of the autonomic nervous system that can cause dizziness, sweating, and headaches along with fatigue and heart palpitations.
She said that she’s so forgetful that when she sees people socially, she’s nervous of what she’ll say. “I feel like I’m constantly sticking my foot in my mouth because I can’t remember details of other people’s lives,” she said.
Although brain disorders such as Alzheimer’s disease and other forms of dementia are marked by a slow decline, ABI occurs more suddenly and may include a loss of executive function and attention.
“With a brain injury, you’re doing fine, and then some event happens (in this case COVID), and immediately after that, your cognitive function is different,” said Dr. Jackson.
Additionally, ABI is an actual diagnosis, whereas brain fog is not.
“With a brain injury, there’s a treatment pathway for cognitive rehabilitation,” said Dr. Jackson.
Treatments may include speech, cognitive, and occupational therapy as well as meeting with a neuropsychiatrist for treatment of the mental and behavioral disorders that may result. Dr. Jackson said that while many patients aren’t functioning cognitively or physically at 100%, they can make enough strides that they don’t have to give up things such as driving and, in some cases, their jobs.
Other experts agree that long COVID may damage the brain. An April 2022 study published in the journal Nature found strong evidence that SARS-CoV-2 infection may cause brain-related abnormalities, for example, a reduction in gray matter in certain parts of the brain, including the prefrontal cortex, hypothalamus, and amygdala.
Additionally, white matter, which is found deeper in the brain and is responsible for the exchange of information between different parts of the brain, may also be at risk of damage as a result of the virus, according to a November 2022 study published in the journal SN Comprehensive Clinical Medicine.
Calling it a “fog” makes it easier for clinicians and the general public to dismiss its severity, said Tyler Reed Bell, PhD, a researcher who specializes in viruses that cause brain injury. He is a fellow in the department of psychiatry at the University of California, San Diego. Brain fog can make driving and returning to work especially dangerous. Because of difficulty focusing, patients are much more likely to make mistakes that cause accidents.
“The COVID virus is very invasive to the brain,” Dr. Bell said.
Others contend this may be a rush to judgment. Karla L. Thompson, PhD, lead neuropsychologist at the University of North Carolina at Chapel Hill’s COVID Recovery Clinic, agrees that in more serious cases of COVID that cause a lack of oxygen to the brain, it’s reasonable to call it a brain injury. But brain fog can also be associated with other long COVID symptoms, not just damage to the brain.
Chronic fatigue and poor sleep are both commonly reported symptoms of long COVID that negatively affect brain function, she said. Sleep disturbances, cardiac problems, dysautonomia, and emotional distress could also affect the way the brain functions post COVID. Finding the right treatment requires identifying all the factors contributing to cognitive impairment.
Part of the problem in treating long COVID brain fog is that diagnostic technology is not sensitive enough to detect inflammation that could be causing damage.
Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, said her team is working on identifying biomarkers that could detect brain inflammation in a way similar to the manner researchers have identified biomarkers to help diagnose chronic fatigue syndrome. Additionally, a new study published last month in JAMA for the first time clearly defined 12 symptoms of long COVID, and brain fog was listed among them. All of this contributes to the development of clear diagnostic criteria.
“It will make a big difference once we have some consistency among clinicians in diagnosing the condition,” said Dr. McComsey.
Ms. Whitley is thankful for the treatment that she’s received thus far. She’s seeing a cognitive rehabilitation therapist, who assesses her memory, cognition, and attention span and gives her tools to break up simple tasks, such as driving, so that they don’t feel overwhelming. She’s back behind the wheel and back to work.
But perhaps most importantly, Ms. Whitley joined a support group, led by Dr. Jackson, that includes other people experiencing the same symptoms she is. When she was at her darkest, they understood.
“Talking to other survivors has been the only solace in all this,” Ms. Whitley said. “Together, we grieve all that’s been lost.”
A version of this article first appeared on Medscape.com.
Kate Whitley was petrified of COVID-19 from the beginning of the pandemic because she has Hashimoto disease, an autoimmune disorder that she knew put her at high risk for complications.
She was right to be worried. Two months after contracting the infection in September 2022, the 42-year-old Nashville resident was diagnosed with long COVID. For Ms. Whitley, the resulting brain fog has been the most challenging factor. She is the owner of a successful paper goods store, and she can’t remember basic aspects of her job. She can’t tolerate loud noises and gets so distracted that she has trouble remembering what she was doing.
Ms. Whitley doesn’t like the term “brain fog” because it doesn’t begin to describe the dramatic disruption to her life over the past 7 months.
Brain fog is among the most common symptoms of long COVID, and also one of the most poorly understood. A reported 46% of those diagnosed with long COVID complain of brain fog or a loss of memory. Many clinicians agree that the term is vague and often doesn’t truly represent the condition. That, in turn, makes it harder for doctors to diagnose and treat it. There are no standard tests for it, nor are there guidelines for symptom management or treatment.
“There’s a lot of imprecision in the term because it might mean different things to different patients,” said James C. Jackson, PsyD, a neuropsychiatrist at Vanderbilt University, Nashville, Tenn., and author of a new book, “Clearing the Fog: From Surviving to Thriving With Long COVID – A Practical Guide.”
Dr. Jackson, who began treating Ms. Whitley in February 2023, said that it makes more sense to call brain fog a brain impairment or an acquired brain injury (ABI) because it doesn’t occur gradually. COVID damages the brain and causes injury. For those with long COVID who were previously in the intensive care unit and may have undergone ventilation, hypoxic brain injury may result from the lack of oxygen to the brain.
Even among those with milder cases of acute COVID, there’s some evidence that persistent neuroinflammation in the brain caused by an activated immune system may also cause damage.
In both cases, the results can be debilitating. Ms. Whitley also has dysautonomia – a disorder of the autonomic nervous system that can cause dizziness, sweating, and headaches along with fatigue and heart palpitations.
She said that she’s so forgetful that when she sees people socially, she’s nervous of what she’ll say. “I feel like I’m constantly sticking my foot in my mouth because I can’t remember details of other people’s lives,” she said.
Although brain disorders such as Alzheimer’s disease and other forms of dementia are marked by a slow decline, ABI occurs more suddenly and may include a loss of executive function and attention.
“With a brain injury, you’re doing fine, and then some event happens (in this case COVID), and immediately after that, your cognitive function is different,” said Dr. Jackson.
Additionally, ABI is an actual diagnosis, whereas brain fog is not.
“With a brain injury, there’s a treatment pathway for cognitive rehabilitation,” said Dr. Jackson.
Treatments may include speech, cognitive, and occupational therapy as well as meeting with a neuropsychiatrist for treatment of the mental and behavioral disorders that may result. Dr. Jackson said that while many patients aren’t functioning cognitively or physically at 100%, they can make enough strides that they don’t have to give up things such as driving and, in some cases, their jobs.
Other experts agree that long COVID may damage the brain. An April 2022 study published in the journal Nature found strong evidence that SARS-CoV-2 infection may cause brain-related abnormalities, for example, a reduction in gray matter in certain parts of the brain, including the prefrontal cortex, hypothalamus, and amygdala.
Additionally, white matter, which is found deeper in the brain and is responsible for the exchange of information between different parts of the brain, may also be at risk of damage as a result of the virus, according to a November 2022 study published in the journal SN Comprehensive Clinical Medicine.
Calling it a “fog” makes it easier for clinicians and the general public to dismiss its severity, said Tyler Reed Bell, PhD, a researcher who specializes in viruses that cause brain injury. He is a fellow in the department of psychiatry at the University of California, San Diego. Brain fog can make driving and returning to work especially dangerous. Because of difficulty focusing, patients are much more likely to make mistakes that cause accidents.
“The COVID virus is very invasive to the brain,” Dr. Bell said.
Others contend this may be a rush to judgment. Karla L. Thompson, PhD, lead neuropsychologist at the University of North Carolina at Chapel Hill’s COVID Recovery Clinic, agrees that in more serious cases of COVID that cause a lack of oxygen to the brain, it’s reasonable to call it a brain injury. But brain fog can also be associated with other long COVID symptoms, not just damage to the brain.
Chronic fatigue and poor sleep are both commonly reported symptoms of long COVID that negatively affect brain function, she said. Sleep disturbances, cardiac problems, dysautonomia, and emotional distress could also affect the way the brain functions post COVID. Finding the right treatment requires identifying all the factors contributing to cognitive impairment.
Part of the problem in treating long COVID brain fog is that diagnostic technology is not sensitive enough to detect inflammation that could be causing damage.
Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, said her team is working on identifying biomarkers that could detect brain inflammation in a way similar to the manner researchers have identified biomarkers to help diagnose chronic fatigue syndrome. Additionally, a new study published last month in JAMA for the first time clearly defined 12 symptoms of long COVID, and brain fog was listed among them. All of this contributes to the development of clear diagnostic criteria.
“It will make a big difference once we have some consistency among clinicians in diagnosing the condition,” said Dr. McComsey.
Ms. Whitley is thankful for the treatment that she’s received thus far. She’s seeing a cognitive rehabilitation therapist, who assesses her memory, cognition, and attention span and gives her tools to break up simple tasks, such as driving, so that they don’t feel overwhelming. She’s back behind the wheel and back to work.
But perhaps most importantly, Ms. Whitley joined a support group, led by Dr. Jackson, that includes other people experiencing the same symptoms she is. When she was at her darkest, they understood.
“Talking to other survivors has been the only solace in all this,” Ms. Whitley said. “Together, we grieve all that’s been lost.”
A version of this article first appeared on Medscape.com.
Kate Whitley was petrified of COVID-19 from the beginning of the pandemic because she has Hashimoto disease, an autoimmune disorder that she knew put her at high risk for complications.
She was right to be worried. Two months after contracting the infection in September 2022, the 42-year-old Nashville resident was diagnosed with long COVID. For Ms. Whitley, the resulting brain fog has been the most challenging factor. She is the owner of a successful paper goods store, and she can’t remember basic aspects of her job. She can’t tolerate loud noises and gets so distracted that she has trouble remembering what she was doing.
Ms. Whitley doesn’t like the term “brain fog” because it doesn’t begin to describe the dramatic disruption to her life over the past 7 months.
Brain fog is among the most common symptoms of long COVID, and also one of the most poorly understood. A reported 46% of those diagnosed with long COVID complain of brain fog or a loss of memory. Many clinicians agree that the term is vague and often doesn’t truly represent the condition. That, in turn, makes it harder for doctors to diagnose and treat it. There are no standard tests for it, nor are there guidelines for symptom management or treatment.
“There’s a lot of imprecision in the term because it might mean different things to different patients,” said James C. Jackson, PsyD, a neuropsychiatrist at Vanderbilt University, Nashville, Tenn., and author of a new book, “Clearing the Fog: From Surviving to Thriving With Long COVID – A Practical Guide.”
Dr. Jackson, who began treating Ms. Whitley in February 2023, said that it makes more sense to call brain fog a brain impairment or an acquired brain injury (ABI) because it doesn’t occur gradually. COVID damages the brain and causes injury. For those with long COVID who were previously in the intensive care unit and may have undergone ventilation, hypoxic brain injury may result from the lack of oxygen to the brain.
Even among those with milder cases of acute COVID, there’s some evidence that persistent neuroinflammation in the brain caused by an activated immune system may also cause damage.
In both cases, the results can be debilitating. Ms. Whitley also has dysautonomia – a disorder of the autonomic nervous system that can cause dizziness, sweating, and headaches along with fatigue and heart palpitations.
She said that she’s so forgetful that when she sees people socially, she’s nervous of what she’ll say. “I feel like I’m constantly sticking my foot in my mouth because I can’t remember details of other people’s lives,” she said.
Although brain disorders such as Alzheimer’s disease and other forms of dementia are marked by a slow decline, ABI occurs more suddenly and may include a loss of executive function and attention.
“With a brain injury, you’re doing fine, and then some event happens (in this case COVID), and immediately after that, your cognitive function is different,” said Dr. Jackson.
Additionally, ABI is an actual diagnosis, whereas brain fog is not.
“With a brain injury, there’s a treatment pathway for cognitive rehabilitation,” said Dr. Jackson.
Treatments may include speech, cognitive, and occupational therapy as well as meeting with a neuropsychiatrist for treatment of the mental and behavioral disorders that may result. Dr. Jackson said that while many patients aren’t functioning cognitively or physically at 100%, they can make enough strides that they don’t have to give up things such as driving and, in some cases, their jobs.
Other experts agree that long COVID may damage the brain. An April 2022 study published in the journal Nature found strong evidence that SARS-CoV-2 infection may cause brain-related abnormalities, for example, a reduction in gray matter in certain parts of the brain, including the prefrontal cortex, hypothalamus, and amygdala.
Additionally, white matter, which is found deeper in the brain and is responsible for the exchange of information between different parts of the brain, may also be at risk of damage as a result of the virus, according to a November 2022 study published in the journal SN Comprehensive Clinical Medicine.
Calling it a “fog” makes it easier for clinicians and the general public to dismiss its severity, said Tyler Reed Bell, PhD, a researcher who specializes in viruses that cause brain injury. He is a fellow in the department of psychiatry at the University of California, San Diego. Brain fog can make driving and returning to work especially dangerous. Because of difficulty focusing, patients are much more likely to make mistakes that cause accidents.
“The COVID virus is very invasive to the brain,” Dr. Bell said.
Others contend this may be a rush to judgment. Karla L. Thompson, PhD, lead neuropsychologist at the University of North Carolina at Chapel Hill’s COVID Recovery Clinic, agrees that in more serious cases of COVID that cause a lack of oxygen to the brain, it’s reasonable to call it a brain injury. But brain fog can also be associated with other long COVID symptoms, not just damage to the brain.
Chronic fatigue and poor sleep are both commonly reported symptoms of long COVID that negatively affect brain function, she said. Sleep disturbances, cardiac problems, dysautonomia, and emotional distress could also affect the way the brain functions post COVID. Finding the right treatment requires identifying all the factors contributing to cognitive impairment.
Part of the problem in treating long COVID brain fog is that diagnostic technology is not sensitive enough to detect inflammation that could be causing damage.
Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, said her team is working on identifying biomarkers that could detect brain inflammation in a way similar to the manner researchers have identified biomarkers to help diagnose chronic fatigue syndrome. Additionally, a new study published last month in JAMA for the first time clearly defined 12 symptoms of long COVID, and brain fog was listed among them. All of this contributes to the development of clear diagnostic criteria.
“It will make a big difference once we have some consistency among clinicians in diagnosing the condition,” said Dr. McComsey.
Ms. Whitley is thankful for the treatment that she’s received thus far. She’s seeing a cognitive rehabilitation therapist, who assesses her memory, cognition, and attention span and gives her tools to break up simple tasks, such as driving, so that they don’t feel overwhelming. She’s back behind the wheel and back to work.
But perhaps most importantly, Ms. Whitley joined a support group, led by Dr. Jackson, that includes other people experiencing the same symptoms she is. When she was at her darkest, they understood.
“Talking to other survivors has been the only solace in all this,” Ms. Whitley said. “Together, we grieve all that’s been lost.”
A version of this article first appeared on Medscape.com.
Finding the optimal fluid strategies for sepsis
The document offers guidance on the four forms of fluid use; assessing whether intravenous fluid administration is indicated; and fluid therapy goals, timing, type, and other clinical parameters. The recommendations are based on a literature search that included 28 randomized clinical trials, 7 secondary analyses of RCTs, 20 observational studies, 5 systematic reviews or meta-analyses, 1 scoping review, 1 practice guideline, and 14 references from a reference review.
“Our review highlights that crystalloids should remain the standard of care for most critically ill patients, especially during early resuscitation,” Fernando G. Zampieri, MD, PhD, assistant adjunct professor of critical care medicine at the University of Alberta and Alberta Health Services, both in Edmonton, said in an interview. “In particular, starches should not be used in critically ill patients. Balanced solutions might be better for most patients, except for patients with traumatic brain injury, where 0.9% saline is recommended.”
The review was published online in JAMA.
Four therapeutic phases
Approximately 20%-30% of patients admitted to an intensive care unit have sepsis, and fluid therapy is a key component of their treatment. Although intravenous fluid can increase cardiac output and blood pressure, maintain or increase intravascular fluid volume, and deliver medications, too much fluid or the wrong type of fluid may cause harm.
“Deciding which type of fluid is the best for a patient [with sepsis] can be challenging,” said Dr. Zampieri.
Fluid therapy can be conceptualized as encompassing four overlapping phases from early illness through resolution of sepsis, according to the review. These phases include resuscitation (rapidly administering fluid to restore perfusion), optimization (assessing risks and benefits of additional fluids to treat shock and ensure organ perfusion), stabilization (using fluid therapy only when there is a signal of fluid responsiveness), and evacuation (eliminating excess fluid accumulated during treatment).
The review described the studies that underpin its key recommendations for management in these phases. Three RCTs included 3,723 patients with sepsis who received 1-2 L of fluid. They found that goal-directed therapy with administration of fluid boluses to attain a central venous pressure of 8-12 mm Hg, vasopressors to attain a mean arterial blood pressure of 65-90 mm Hg, and red blood cell transfusions or inotropes to attain a central venous oxygen saturation of at least 70% did not decrease mortality, compared with unstructured clinical care (24.9% vs. 25.4%, P = .68).
One RCT with 1,563 patients with sepsis and hypotension who received 1 L of fluid found that favoring vasopressor treatment did not improve mortality, compared with further fluid administration (14.0% vs. 14.9%, P = .61).
In another RCT, among 1,554 patients with septic shock who were treated in the ICU with at least 1 L of fluid, restricting fluid administration in the absence of severe hypoperfusion did not reduce mortality, compared with more liberal fluid administration (42.3% vs. 42.1%, P = .96).
An RCT of 1,000 patients with acute respiratory distress during the evacuation phase found that limiting fluid administration and giving diuretics improved the number of days alive without mechanical ventilation, compared with fluid treatment to attain higher intracardiac pressure (14.6 vs. 12.1 days, P < .001).
This study also found that hydroxyethyl starch significantly increased the incidence of kidney replacement therapy, compared with saline (7.0% vs. 5.8%, P = .04), Ringer lactate, or Ringer acetate.
Ultrasonography lacks validation
The authors summarized the key concerns about fluid therapy. Fluid therapy should be initiated for patients with evidence of sepsis-induced hypoperfusion who are likely to have increased cardiac output with fluid administration. Fluid administration should be discontinued when evidence of hypoperfusion resolves, the patient no longer responds to fluid, or the patient shows evidence of fluid overload.
Balanced solutions should be selected over 0.9% saline for fluid therapy, according to the review. Hydroxyethyl starches should not be used.
Fluid removal should be considered after the resuscitation and optimization phases and when a patient has stabilized, the authors wrote. Diuretics are first-line therapy to facilitate fluid elimination.
Kidney replacement therapy may be considered for patients with severe acute kidney injury who have complications from fluid overload and are unresponsive to diuretic therapy.
“The use of ultrasonography as a bedside tool to guide fluid resuscitation is promising but lacks validation in robust randomized controlled trials,” said Dr. Zampieri. “Point-of-care ultrasound may be useful to assess causes of shock and [helping to exclude] a life-threatening diagnosis at presentation, such as cardiac tamponade.”
Pending the emergence of further evidence, the authors suggest that clinicians prescribe fluids judiciously, preferably at aliquots followed by frequent reassessment. “Defining a resuscitation target (such as capillary refill time or lactate, among others) and performing fluid challenges to correct them while no overt signs of fluid overload (such as pulmonary edema) occur is a common practice that is also sustained by clinical research,” said Dr. Zampieri.
He added that the review’s recommendations are based on research conducted mainly in high-income settings, and that generalizability will depend on factors such as local standards of care and resource availability.
“Our review provides an overall guidance, but caution is warranted before extrapolating the suggestion to every possible clinical scenario,” he concluded.
Fluids as drugs
Commenting on the review, Hernando Gomez, MD, MPH, an associate professor of critical care medicine at the University of Pittsburgh, said: “I agree with the conclusions and commend the authors for this very practical revision of the literature.” Dr. Gomez was not involved in the review.
“I would like to stress the point, however, that although fluids can be harmful, particularly when not indicated and when used in excess, fluid resuscitation in patients with sepsis who have evidence of hypoperfusion is paramount,” he said.
“The association between fluid accumulation and poor outcomes is truly a Goldilocks problem, often described in the literature as a ‘U’ shape, where too little fluid (i.e., a very restrictive strategy) or too much fluid (i.e., use in excess and in discordance with the patient’s needs) can be harmful,” said Dr. Gomez.
Furthermore, every strategy to assess fluid responsiveness has limitations. “It is key that clinicians resist the temptation to dismiss these limitations, because decisions made on flawed data are as dangerous as not assessing fluid responsiveness in the first place,” he said.
Based on the evidence, clinicians should “think of fluids as a drug and carefully assess risks and benefits before deciding to administer fluids to their patients,” Dr. Gomez added. It is also important to separate the question “Does my patient need fluids?” from the question “Is my patient fluid responsive?”
“These are two different questions that often get conflated,” Dr. Gomez said. “If a bolus of fluid given to a patient who needs fluids and is fluid-responsive does not improve tissue perfusion, then fluids should not be given.”
No funding was reported for the review. Dr. Zampieri reported receiving fluids and logistics from Baxter Hospitalar during the conduct of the BaSICS trial, personal fees from Bactiguard for statistical consulting and from Baxter for participating in an advisory board, grants from Ionis Pharmaceuticals outside the submitted work, and serving as lead investigator of the BaSICS trial. Dr. Gomez reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The document offers guidance on the four forms of fluid use; assessing whether intravenous fluid administration is indicated; and fluid therapy goals, timing, type, and other clinical parameters. The recommendations are based on a literature search that included 28 randomized clinical trials, 7 secondary analyses of RCTs, 20 observational studies, 5 systematic reviews or meta-analyses, 1 scoping review, 1 practice guideline, and 14 references from a reference review.
“Our review highlights that crystalloids should remain the standard of care for most critically ill patients, especially during early resuscitation,” Fernando G. Zampieri, MD, PhD, assistant adjunct professor of critical care medicine at the University of Alberta and Alberta Health Services, both in Edmonton, said in an interview. “In particular, starches should not be used in critically ill patients. Balanced solutions might be better for most patients, except for patients with traumatic brain injury, where 0.9% saline is recommended.”
The review was published online in JAMA.
Four therapeutic phases
Approximately 20%-30% of patients admitted to an intensive care unit have sepsis, and fluid therapy is a key component of their treatment. Although intravenous fluid can increase cardiac output and blood pressure, maintain or increase intravascular fluid volume, and deliver medications, too much fluid or the wrong type of fluid may cause harm.
“Deciding which type of fluid is the best for a patient [with sepsis] can be challenging,” said Dr. Zampieri.
Fluid therapy can be conceptualized as encompassing four overlapping phases from early illness through resolution of sepsis, according to the review. These phases include resuscitation (rapidly administering fluid to restore perfusion), optimization (assessing risks and benefits of additional fluids to treat shock and ensure organ perfusion), stabilization (using fluid therapy only when there is a signal of fluid responsiveness), and evacuation (eliminating excess fluid accumulated during treatment).
The review described the studies that underpin its key recommendations for management in these phases. Three RCTs included 3,723 patients with sepsis who received 1-2 L of fluid. They found that goal-directed therapy with administration of fluid boluses to attain a central venous pressure of 8-12 mm Hg, vasopressors to attain a mean arterial blood pressure of 65-90 mm Hg, and red blood cell transfusions or inotropes to attain a central venous oxygen saturation of at least 70% did not decrease mortality, compared with unstructured clinical care (24.9% vs. 25.4%, P = .68).
One RCT with 1,563 patients with sepsis and hypotension who received 1 L of fluid found that favoring vasopressor treatment did not improve mortality, compared with further fluid administration (14.0% vs. 14.9%, P = .61).
In another RCT, among 1,554 patients with septic shock who were treated in the ICU with at least 1 L of fluid, restricting fluid administration in the absence of severe hypoperfusion did not reduce mortality, compared with more liberal fluid administration (42.3% vs. 42.1%, P = .96).
An RCT of 1,000 patients with acute respiratory distress during the evacuation phase found that limiting fluid administration and giving diuretics improved the number of days alive without mechanical ventilation, compared with fluid treatment to attain higher intracardiac pressure (14.6 vs. 12.1 days, P < .001).
This study also found that hydroxyethyl starch significantly increased the incidence of kidney replacement therapy, compared with saline (7.0% vs. 5.8%, P = .04), Ringer lactate, or Ringer acetate.
Ultrasonography lacks validation
The authors summarized the key concerns about fluid therapy. Fluid therapy should be initiated for patients with evidence of sepsis-induced hypoperfusion who are likely to have increased cardiac output with fluid administration. Fluid administration should be discontinued when evidence of hypoperfusion resolves, the patient no longer responds to fluid, or the patient shows evidence of fluid overload.
Balanced solutions should be selected over 0.9% saline for fluid therapy, according to the review. Hydroxyethyl starches should not be used.
Fluid removal should be considered after the resuscitation and optimization phases and when a patient has stabilized, the authors wrote. Diuretics are first-line therapy to facilitate fluid elimination.
Kidney replacement therapy may be considered for patients with severe acute kidney injury who have complications from fluid overload and are unresponsive to diuretic therapy.
“The use of ultrasonography as a bedside tool to guide fluid resuscitation is promising but lacks validation in robust randomized controlled trials,” said Dr. Zampieri. “Point-of-care ultrasound may be useful to assess causes of shock and [helping to exclude] a life-threatening diagnosis at presentation, such as cardiac tamponade.”
Pending the emergence of further evidence, the authors suggest that clinicians prescribe fluids judiciously, preferably at aliquots followed by frequent reassessment. “Defining a resuscitation target (such as capillary refill time or lactate, among others) and performing fluid challenges to correct them while no overt signs of fluid overload (such as pulmonary edema) occur is a common practice that is also sustained by clinical research,” said Dr. Zampieri.
He added that the review’s recommendations are based on research conducted mainly in high-income settings, and that generalizability will depend on factors such as local standards of care and resource availability.
“Our review provides an overall guidance, but caution is warranted before extrapolating the suggestion to every possible clinical scenario,” he concluded.
Fluids as drugs
Commenting on the review, Hernando Gomez, MD, MPH, an associate professor of critical care medicine at the University of Pittsburgh, said: “I agree with the conclusions and commend the authors for this very practical revision of the literature.” Dr. Gomez was not involved in the review.
“I would like to stress the point, however, that although fluids can be harmful, particularly when not indicated and when used in excess, fluid resuscitation in patients with sepsis who have evidence of hypoperfusion is paramount,” he said.
“The association between fluid accumulation and poor outcomes is truly a Goldilocks problem, often described in the literature as a ‘U’ shape, where too little fluid (i.e., a very restrictive strategy) or too much fluid (i.e., use in excess and in discordance with the patient’s needs) can be harmful,” said Dr. Gomez.
Furthermore, every strategy to assess fluid responsiveness has limitations. “It is key that clinicians resist the temptation to dismiss these limitations, because decisions made on flawed data are as dangerous as not assessing fluid responsiveness in the first place,” he said.
Based on the evidence, clinicians should “think of fluids as a drug and carefully assess risks and benefits before deciding to administer fluids to their patients,” Dr. Gomez added. It is also important to separate the question “Does my patient need fluids?” from the question “Is my patient fluid responsive?”
“These are two different questions that often get conflated,” Dr. Gomez said. “If a bolus of fluid given to a patient who needs fluids and is fluid-responsive does not improve tissue perfusion, then fluids should not be given.”
No funding was reported for the review. Dr. Zampieri reported receiving fluids and logistics from Baxter Hospitalar during the conduct of the BaSICS trial, personal fees from Bactiguard for statistical consulting and from Baxter for participating in an advisory board, grants from Ionis Pharmaceuticals outside the submitted work, and serving as lead investigator of the BaSICS trial. Dr. Gomez reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The document offers guidance on the four forms of fluid use; assessing whether intravenous fluid administration is indicated; and fluid therapy goals, timing, type, and other clinical parameters. The recommendations are based on a literature search that included 28 randomized clinical trials, 7 secondary analyses of RCTs, 20 observational studies, 5 systematic reviews or meta-analyses, 1 scoping review, 1 practice guideline, and 14 references from a reference review.
“Our review highlights that crystalloids should remain the standard of care for most critically ill patients, especially during early resuscitation,” Fernando G. Zampieri, MD, PhD, assistant adjunct professor of critical care medicine at the University of Alberta and Alberta Health Services, both in Edmonton, said in an interview. “In particular, starches should not be used in critically ill patients. Balanced solutions might be better for most patients, except for patients with traumatic brain injury, where 0.9% saline is recommended.”
The review was published online in JAMA.
Four therapeutic phases
Approximately 20%-30% of patients admitted to an intensive care unit have sepsis, and fluid therapy is a key component of their treatment. Although intravenous fluid can increase cardiac output and blood pressure, maintain or increase intravascular fluid volume, and deliver medications, too much fluid or the wrong type of fluid may cause harm.
“Deciding which type of fluid is the best for a patient [with sepsis] can be challenging,” said Dr. Zampieri.
Fluid therapy can be conceptualized as encompassing four overlapping phases from early illness through resolution of sepsis, according to the review. These phases include resuscitation (rapidly administering fluid to restore perfusion), optimization (assessing risks and benefits of additional fluids to treat shock and ensure organ perfusion), stabilization (using fluid therapy only when there is a signal of fluid responsiveness), and evacuation (eliminating excess fluid accumulated during treatment).
The review described the studies that underpin its key recommendations for management in these phases. Three RCTs included 3,723 patients with sepsis who received 1-2 L of fluid. They found that goal-directed therapy with administration of fluid boluses to attain a central venous pressure of 8-12 mm Hg, vasopressors to attain a mean arterial blood pressure of 65-90 mm Hg, and red blood cell transfusions or inotropes to attain a central venous oxygen saturation of at least 70% did not decrease mortality, compared with unstructured clinical care (24.9% vs. 25.4%, P = .68).
One RCT with 1,563 patients with sepsis and hypotension who received 1 L of fluid found that favoring vasopressor treatment did not improve mortality, compared with further fluid administration (14.0% vs. 14.9%, P = .61).
In another RCT, among 1,554 patients with septic shock who were treated in the ICU with at least 1 L of fluid, restricting fluid administration in the absence of severe hypoperfusion did not reduce mortality, compared with more liberal fluid administration (42.3% vs. 42.1%, P = .96).
An RCT of 1,000 patients with acute respiratory distress during the evacuation phase found that limiting fluid administration and giving diuretics improved the number of days alive without mechanical ventilation, compared with fluid treatment to attain higher intracardiac pressure (14.6 vs. 12.1 days, P < .001).
This study also found that hydroxyethyl starch significantly increased the incidence of kidney replacement therapy, compared with saline (7.0% vs. 5.8%, P = .04), Ringer lactate, or Ringer acetate.
Ultrasonography lacks validation
The authors summarized the key concerns about fluid therapy. Fluid therapy should be initiated for patients with evidence of sepsis-induced hypoperfusion who are likely to have increased cardiac output with fluid administration. Fluid administration should be discontinued when evidence of hypoperfusion resolves, the patient no longer responds to fluid, or the patient shows evidence of fluid overload.
Balanced solutions should be selected over 0.9% saline for fluid therapy, according to the review. Hydroxyethyl starches should not be used.
Fluid removal should be considered after the resuscitation and optimization phases and when a patient has stabilized, the authors wrote. Diuretics are first-line therapy to facilitate fluid elimination.
Kidney replacement therapy may be considered for patients with severe acute kidney injury who have complications from fluid overload and are unresponsive to diuretic therapy.
“The use of ultrasonography as a bedside tool to guide fluid resuscitation is promising but lacks validation in robust randomized controlled trials,” said Dr. Zampieri. “Point-of-care ultrasound may be useful to assess causes of shock and [helping to exclude] a life-threatening diagnosis at presentation, such as cardiac tamponade.”
Pending the emergence of further evidence, the authors suggest that clinicians prescribe fluids judiciously, preferably at aliquots followed by frequent reassessment. “Defining a resuscitation target (such as capillary refill time or lactate, among others) and performing fluid challenges to correct them while no overt signs of fluid overload (such as pulmonary edema) occur is a common practice that is also sustained by clinical research,” said Dr. Zampieri.
He added that the review’s recommendations are based on research conducted mainly in high-income settings, and that generalizability will depend on factors such as local standards of care and resource availability.
“Our review provides an overall guidance, but caution is warranted before extrapolating the suggestion to every possible clinical scenario,” he concluded.
Fluids as drugs
Commenting on the review, Hernando Gomez, MD, MPH, an associate professor of critical care medicine at the University of Pittsburgh, said: “I agree with the conclusions and commend the authors for this very practical revision of the literature.” Dr. Gomez was not involved in the review.
“I would like to stress the point, however, that although fluids can be harmful, particularly when not indicated and when used in excess, fluid resuscitation in patients with sepsis who have evidence of hypoperfusion is paramount,” he said.
“The association between fluid accumulation and poor outcomes is truly a Goldilocks problem, often described in the literature as a ‘U’ shape, where too little fluid (i.e., a very restrictive strategy) or too much fluid (i.e., use in excess and in discordance with the patient’s needs) can be harmful,” said Dr. Gomez.
Furthermore, every strategy to assess fluid responsiveness has limitations. “It is key that clinicians resist the temptation to dismiss these limitations, because decisions made on flawed data are as dangerous as not assessing fluid responsiveness in the first place,” he said.
Based on the evidence, clinicians should “think of fluids as a drug and carefully assess risks and benefits before deciding to administer fluids to their patients,” Dr. Gomez added. It is also important to separate the question “Does my patient need fluids?” from the question “Is my patient fluid responsive?”
“These are two different questions that often get conflated,” Dr. Gomez said. “If a bolus of fluid given to a patient who needs fluids and is fluid-responsive does not improve tissue perfusion, then fluids should not be given.”
No funding was reported for the review. Dr. Zampieri reported receiving fluids and logistics from Baxter Hospitalar during the conduct of the BaSICS trial, personal fees from Bactiguard for statistical consulting and from Baxter for participating in an advisory board, grants from Ionis Pharmaceuticals outside the submitted work, and serving as lead investigator of the BaSICS trial. Dr. Gomez reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA
Ticks use static electricity to latch onto hosts: Study
It turns out that some people really are tick magnets.
Researchers have discovered that ticks can defy gravity in their quest to latch onto people and animals. The key is static electricity, just like when someone rubs a balloon and things stick to it.
The study was published in the journal Current Biology. In the first phase of the research, scientists exposed ticks to furry rabbit feet and to acrylic surfaces that each had electrostatic charges.
“Ticks were readily attracted across air gaps of up to several millimeters or centimeters onto these statically charged surfaces,” the authors wrote.
In a second part of the study, the researchers created computer models simulating the electrostatic charges that exist in environments where both ticks and mammals are found. In one simulation, the researchers observed that the body parts of a cow with the most electric charge were the nose, tail, and legs, which are the body parts most likely to be encountered by a tick. They also found that the vegetation near the animal had a strong electric field that is just a few millimeters wide.
In a final phase of the study, the researchers conducted laboratory experiments in which they re-created the electric field conditions from the computer model and successfully lifted some ticks across an air gap, although some ticks did not make the full leap if they were observed to be resisting.
The authors noted that their findings could be applied to developing new tick prevention strategies, such as designing clothing that resists electrostatic charges or spraying livestock.
The study authors reported that they had no conflicts of interest.
A version of this article first appeared on WebMD.com.
It turns out that some people really are tick magnets.
Researchers have discovered that ticks can defy gravity in their quest to latch onto people and animals. The key is static electricity, just like when someone rubs a balloon and things stick to it.
The study was published in the journal Current Biology. In the first phase of the research, scientists exposed ticks to furry rabbit feet and to acrylic surfaces that each had electrostatic charges.
“Ticks were readily attracted across air gaps of up to several millimeters or centimeters onto these statically charged surfaces,” the authors wrote.
In a second part of the study, the researchers created computer models simulating the electrostatic charges that exist in environments where both ticks and mammals are found. In one simulation, the researchers observed that the body parts of a cow with the most electric charge were the nose, tail, and legs, which are the body parts most likely to be encountered by a tick. They also found that the vegetation near the animal had a strong electric field that is just a few millimeters wide.
In a final phase of the study, the researchers conducted laboratory experiments in which they re-created the electric field conditions from the computer model and successfully lifted some ticks across an air gap, although some ticks did not make the full leap if they were observed to be resisting.
The authors noted that their findings could be applied to developing new tick prevention strategies, such as designing clothing that resists electrostatic charges or spraying livestock.
The study authors reported that they had no conflicts of interest.
A version of this article first appeared on WebMD.com.
It turns out that some people really are tick magnets.
Researchers have discovered that ticks can defy gravity in their quest to latch onto people and animals. The key is static electricity, just like when someone rubs a balloon and things stick to it.
The study was published in the journal Current Biology. In the first phase of the research, scientists exposed ticks to furry rabbit feet and to acrylic surfaces that each had electrostatic charges.
“Ticks were readily attracted across air gaps of up to several millimeters or centimeters onto these statically charged surfaces,” the authors wrote.
In a second part of the study, the researchers created computer models simulating the electrostatic charges that exist in environments where both ticks and mammals are found. In one simulation, the researchers observed that the body parts of a cow with the most electric charge were the nose, tail, and legs, which are the body parts most likely to be encountered by a tick. They also found that the vegetation near the animal had a strong electric field that is just a few millimeters wide.
In a final phase of the study, the researchers conducted laboratory experiments in which they re-created the electric field conditions from the computer model and successfully lifted some ticks across an air gap, although some ticks did not make the full leap if they were observed to be resisting.
The authors noted that their findings could be applied to developing new tick prevention strategies, such as designing clothing that resists electrostatic charges or spraying livestock.
The study authors reported that they had no conflicts of interest.
A version of this article first appeared on WebMD.com.
FROM CURRENT BIOLOGY







