User login
Study finds subcutaneous spesolimab reduces flares in patients with GPP
SINGAPORE – presented in a late-breaker session at the World Congress of Dermatology,
In the phase 2b study, patients who received the high-dose regimen (a 600-mg subcutaneous loading dose, then 300-mg SC every 4 weeks) of spesolimab experienced 84% fewer GPP fares over 48 weeks, compared with those on placebo, reported Bruce Strober, MD, PhD, Central Connecticut Dermatology, Cromwell, and clinical professor of dermatology, Yale University, New Haven, Conn. “Additionally, no flares occurred after week 4, and this, in turn, translated into improved patient outcomes.”
GPP is a rare, chronic, systemic neutrophilic skin disease. The resulting flares, characterized by painful pustules all over the body, can lead to sepsis, shock, and other life-threatening complications. “People who have it are considerably burdened by it, so targeted therapy of this disease is incredibly important because it leads to lessened morbidity and, importantly, mortality for these patients,” Dr. Strober said.
“It’s important not only to treat the flares but also to prevent them,” he noted.
The intravenous formulation of spesolimab (Spevigo) was approved for the treatment of GPP flares in adults by the Food and Drug Administration in September 2022. It is now authorized in nearly 40 countries, including Japan, China, and the European Union.
The phase 2 Effisayil 2 study presented at the meeting evaluated the subcutaneous formulation of spesolimab. Data on subcutaneous spesolimab has been submitted to the FDA, and has received breakthrough therapy designation, according to the manufacturer, Boehringer Ingelheim.
Flare prevention
In the study, 123 patients with GPP were randomly assigned 1:1:1:1 to one of four groups: high-dose spesolimab, medium-dose (600-mg SC loading dose, then 300-mg SC every 12 weeks), low-dose (300-mg SC loading dose, then 150-mg SC every 12 weeks), or placebo. In the event of a flare during the randomized treatment period, a patient was administered a single, 900-mg intravenous dose of spesolimab.
Nearly two-thirds of the participants were female and nearly two-thirds were Asian, with a mean age of about 39-43 years.
The mean numbers of GPP flares experienced annually by those in the low-, medium-, and high-dose spesolimab groups were 2.7, 1.9, and 2.4, respectively (2.4% in the placebo group). Fewer than a third had concurrent plaque psoriasis at baseline. Most (48.4%-63.3%) did not have an IL-36RN mutation.
Additionally, the Generalized Pustular Psoriasis Physician Global Assessment total score was 1 in 74.2%-93.5% of participants, and 0 in the remainder.
The primary study endpoint was the time to GPP flare by week 48. The risk of developing a flare among those on high-dose spesolimab was 84% lower, compared with that of those on placebo (hazard ratio, 0.16; 95% confidence interval, 0.05-0.54; P = .0005). No patients on the high dose had a flare after the 4th week of the study.
Similarly, for the secondary endpoint (occurrence of at least one GPP flare by week 48). Dr. Strober and his colleagues reported that high-dose spesolimab was superior to placebo with a risk difference of -39% (95% CI, –0.62 to –0.16; P = .0013). By contrast, the risk differences for the medium- and low-dose spesolimab arms were –0.23 (95% CI, –0.46 to 0.01) and -0.31 (95% CI, –0.54 to –0.08), respectively.
The safety profile of subcutaneous spesolimab across all three doses was similar to that of placebo, and there was no dose-dependent trend. Reported adverse events (AEs) were mild. There were five (5.4%) AEs leading to discontinuation of the drug in the medium- and high-dose groups, but none in the low-dose group. Overall, there were nine (9.7%) serious AEs reported in the spesolimab groups, and three (10%) in the high-dose group; no deaths occurred on any dose.
Participants most often reported injection-site erythema, reported in 13 (14%) of the patients on spesolimab versus 1 (3.3%) of those on placebo.
“Overall, the study demonstrates that subcutaneous spesolimab is effective at controlling GPP flares, especially at a high dose relative to placebo, and supports subcutaneous spesolimab for the therapy for GPP flare prevention,” Dr. Strober said at the meeting.
Targeting the IL-36 pathway
In a comment, Todd Schlesinger, MD, Clinical Research Center of the Carolinas, Charleston, S.C., who moderated the session, said: “It’s very exciting to be able to have a subcutaneous version of the medication.”
“I think the IL-36 is a great pathway,” he said, referring to the signaling pathway within the immune system that is central to the pathogenesis of GPP and several other autoinflammatory diseases.
However, Dr. Schlesinger said that he would have liked to have seen data on how many patients ended up treated with intravenous spesolimab.
He added that he would like future studies of subcutaneous spesolimab to examine the effect in different populations that vary by parameters such as weight, race, and disease severity. “Just seeing how somebody who’s flaring five times a year and you give them this medication and they’re now flaring once a year – that’s interesting data that we might like to know in the future.”
Other than for preventing GPP flares, spesolimab is being studied for treating other IL-36–mediated skin diseases, such as palmoplantar pustulosis.
The study was funded by Boehringer Ingelheim; both Dr. Strober and Dr. Schlesinger do research and consulting for BI, and receive funding from multiple other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
SINGAPORE – presented in a late-breaker session at the World Congress of Dermatology,
In the phase 2b study, patients who received the high-dose regimen (a 600-mg subcutaneous loading dose, then 300-mg SC every 4 weeks) of spesolimab experienced 84% fewer GPP fares over 48 weeks, compared with those on placebo, reported Bruce Strober, MD, PhD, Central Connecticut Dermatology, Cromwell, and clinical professor of dermatology, Yale University, New Haven, Conn. “Additionally, no flares occurred after week 4, and this, in turn, translated into improved patient outcomes.”
GPP is a rare, chronic, systemic neutrophilic skin disease. The resulting flares, characterized by painful pustules all over the body, can lead to sepsis, shock, and other life-threatening complications. “People who have it are considerably burdened by it, so targeted therapy of this disease is incredibly important because it leads to lessened morbidity and, importantly, mortality for these patients,” Dr. Strober said.
“It’s important not only to treat the flares but also to prevent them,” he noted.
The intravenous formulation of spesolimab (Spevigo) was approved for the treatment of GPP flares in adults by the Food and Drug Administration in September 2022. It is now authorized in nearly 40 countries, including Japan, China, and the European Union.
The phase 2 Effisayil 2 study presented at the meeting evaluated the subcutaneous formulation of spesolimab. Data on subcutaneous spesolimab has been submitted to the FDA, and has received breakthrough therapy designation, according to the manufacturer, Boehringer Ingelheim.
Flare prevention
In the study, 123 patients with GPP were randomly assigned 1:1:1:1 to one of four groups: high-dose spesolimab, medium-dose (600-mg SC loading dose, then 300-mg SC every 12 weeks), low-dose (300-mg SC loading dose, then 150-mg SC every 12 weeks), or placebo. In the event of a flare during the randomized treatment period, a patient was administered a single, 900-mg intravenous dose of spesolimab.
Nearly two-thirds of the participants were female and nearly two-thirds were Asian, with a mean age of about 39-43 years.
The mean numbers of GPP flares experienced annually by those in the low-, medium-, and high-dose spesolimab groups were 2.7, 1.9, and 2.4, respectively (2.4% in the placebo group). Fewer than a third had concurrent plaque psoriasis at baseline. Most (48.4%-63.3%) did not have an IL-36RN mutation.
Additionally, the Generalized Pustular Psoriasis Physician Global Assessment total score was 1 in 74.2%-93.5% of participants, and 0 in the remainder.
The primary study endpoint was the time to GPP flare by week 48. The risk of developing a flare among those on high-dose spesolimab was 84% lower, compared with that of those on placebo (hazard ratio, 0.16; 95% confidence interval, 0.05-0.54; P = .0005). No patients on the high dose had a flare after the 4th week of the study.
Similarly, for the secondary endpoint (occurrence of at least one GPP flare by week 48). Dr. Strober and his colleagues reported that high-dose spesolimab was superior to placebo with a risk difference of -39% (95% CI, –0.62 to –0.16; P = .0013). By contrast, the risk differences for the medium- and low-dose spesolimab arms were –0.23 (95% CI, –0.46 to 0.01) and -0.31 (95% CI, –0.54 to –0.08), respectively.
The safety profile of subcutaneous spesolimab across all three doses was similar to that of placebo, and there was no dose-dependent trend. Reported adverse events (AEs) were mild. There were five (5.4%) AEs leading to discontinuation of the drug in the medium- and high-dose groups, but none in the low-dose group. Overall, there were nine (9.7%) serious AEs reported in the spesolimab groups, and three (10%) in the high-dose group; no deaths occurred on any dose.
Participants most often reported injection-site erythema, reported in 13 (14%) of the patients on spesolimab versus 1 (3.3%) of those on placebo.
“Overall, the study demonstrates that subcutaneous spesolimab is effective at controlling GPP flares, especially at a high dose relative to placebo, and supports subcutaneous spesolimab for the therapy for GPP flare prevention,” Dr. Strober said at the meeting.
Targeting the IL-36 pathway
In a comment, Todd Schlesinger, MD, Clinical Research Center of the Carolinas, Charleston, S.C., who moderated the session, said: “It’s very exciting to be able to have a subcutaneous version of the medication.”
“I think the IL-36 is a great pathway,” he said, referring to the signaling pathway within the immune system that is central to the pathogenesis of GPP and several other autoinflammatory diseases.
However, Dr. Schlesinger said that he would have liked to have seen data on how many patients ended up treated with intravenous spesolimab.
He added that he would like future studies of subcutaneous spesolimab to examine the effect in different populations that vary by parameters such as weight, race, and disease severity. “Just seeing how somebody who’s flaring five times a year and you give them this medication and they’re now flaring once a year – that’s interesting data that we might like to know in the future.”
Other than for preventing GPP flares, spesolimab is being studied for treating other IL-36–mediated skin diseases, such as palmoplantar pustulosis.
The study was funded by Boehringer Ingelheim; both Dr. Strober and Dr. Schlesinger do research and consulting for BI, and receive funding from multiple other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
SINGAPORE – presented in a late-breaker session at the World Congress of Dermatology,
In the phase 2b study, patients who received the high-dose regimen (a 600-mg subcutaneous loading dose, then 300-mg SC every 4 weeks) of spesolimab experienced 84% fewer GPP fares over 48 weeks, compared with those on placebo, reported Bruce Strober, MD, PhD, Central Connecticut Dermatology, Cromwell, and clinical professor of dermatology, Yale University, New Haven, Conn. “Additionally, no flares occurred after week 4, and this, in turn, translated into improved patient outcomes.”
GPP is a rare, chronic, systemic neutrophilic skin disease. The resulting flares, characterized by painful pustules all over the body, can lead to sepsis, shock, and other life-threatening complications. “People who have it are considerably burdened by it, so targeted therapy of this disease is incredibly important because it leads to lessened morbidity and, importantly, mortality for these patients,” Dr. Strober said.
“It’s important not only to treat the flares but also to prevent them,” he noted.
The intravenous formulation of spesolimab (Spevigo) was approved for the treatment of GPP flares in adults by the Food and Drug Administration in September 2022. It is now authorized in nearly 40 countries, including Japan, China, and the European Union.
The phase 2 Effisayil 2 study presented at the meeting evaluated the subcutaneous formulation of spesolimab. Data on subcutaneous spesolimab has been submitted to the FDA, and has received breakthrough therapy designation, according to the manufacturer, Boehringer Ingelheim.
Flare prevention
In the study, 123 patients with GPP were randomly assigned 1:1:1:1 to one of four groups: high-dose spesolimab, medium-dose (600-mg SC loading dose, then 300-mg SC every 12 weeks), low-dose (300-mg SC loading dose, then 150-mg SC every 12 weeks), or placebo. In the event of a flare during the randomized treatment period, a patient was administered a single, 900-mg intravenous dose of spesolimab.
Nearly two-thirds of the participants were female and nearly two-thirds were Asian, with a mean age of about 39-43 years.
The mean numbers of GPP flares experienced annually by those in the low-, medium-, and high-dose spesolimab groups were 2.7, 1.9, and 2.4, respectively (2.4% in the placebo group). Fewer than a third had concurrent plaque psoriasis at baseline. Most (48.4%-63.3%) did not have an IL-36RN mutation.
Additionally, the Generalized Pustular Psoriasis Physician Global Assessment total score was 1 in 74.2%-93.5% of participants, and 0 in the remainder.
The primary study endpoint was the time to GPP flare by week 48. The risk of developing a flare among those on high-dose spesolimab was 84% lower, compared with that of those on placebo (hazard ratio, 0.16; 95% confidence interval, 0.05-0.54; P = .0005). No patients on the high dose had a flare after the 4th week of the study.
Similarly, for the secondary endpoint (occurrence of at least one GPP flare by week 48). Dr. Strober and his colleagues reported that high-dose spesolimab was superior to placebo with a risk difference of -39% (95% CI, –0.62 to –0.16; P = .0013). By contrast, the risk differences for the medium- and low-dose spesolimab arms were –0.23 (95% CI, –0.46 to 0.01) and -0.31 (95% CI, –0.54 to –0.08), respectively.
The safety profile of subcutaneous spesolimab across all three doses was similar to that of placebo, and there was no dose-dependent trend. Reported adverse events (AEs) were mild. There were five (5.4%) AEs leading to discontinuation of the drug in the medium- and high-dose groups, but none in the low-dose group. Overall, there were nine (9.7%) serious AEs reported in the spesolimab groups, and three (10%) in the high-dose group; no deaths occurred on any dose.
Participants most often reported injection-site erythema, reported in 13 (14%) of the patients on spesolimab versus 1 (3.3%) of those on placebo.
“Overall, the study demonstrates that subcutaneous spesolimab is effective at controlling GPP flares, especially at a high dose relative to placebo, and supports subcutaneous spesolimab for the therapy for GPP flare prevention,” Dr. Strober said at the meeting.
Targeting the IL-36 pathway
In a comment, Todd Schlesinger, MD, Clinical Research Center of the Carolinas, Charleston, S.C., who moderated the session, said: “It’s very exciting to be able to have a subcutaneous version of the medication.”
“I think the IL-36 is a great pathway,” he said, referring to the signaling pathway within the immune system that is central to the pathogenesis of GPP and several other autoinflammatory diseases.
However, Dr. Schlesinger said that he would have liked to have seen data on how many patients ended up treated with intravenous spesolimab.
He added that he would like future studies of subcutaneous spesolimab to examine the effect in different populations that vary by parameters such as weight, race, and disease severity. “Just seeing how somebody who’s flaring five times a year and you give them this medication and they’re now flaring once a year – that’s interesting data that we might like to know in the future.”
Other than for preventing GPP flares, spesolimab is being studied for treating other IL-36–mediated skin diseases, such as palmoplantar pustulosis.
The study was funded by Boehringer Ingelheim; both Dr. Strober and Dr. Schlesinger do research and consulting for BI, and receive funding from multiple other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
AT WCD 2023
A teenage girl refuses more cancer treatment; her father disagrees
This transcript has been edited for clarity.
Hi. I’m Art Caplan, PhD. I’m director of the division of medical ethics at the New York University Grossman School of Medicine.
Every once in a while at my school, I get referrals about interesting or difficult clinical cases where doctors would like some input or advice that they can consider in managing a patient. Sometimes those requests come from other hospitals to me. I’ve been doing that kind of ethics consulting, both as a member of various ethics committees and sometimes individually, when, for various reasons, doctors don’t want to go to the Ethics Committee as a first stop.
There was a very interesting case recently involving a young woman I’m going to call Tinslee. She was 17 years old and she suffered, sadly, from recurrent metastatic osteogenic sarcoma. She had bone cancer. It had first been diagnosed at the age of 9. She had received chemotherapy and been under that treatment for a while.
If osteosarcoma is treated before it spreads outside the area where it began, the 5-year survival rate for people like her is about 75%. If the cancer spreads outside of the bones and gets into surrounding tissues, organs, or – worse – into the lymph nodes and starts traveling around, the 5-year survival rate drops to about 60%. The two approaches are chemotherapy and amputation. That’s what we have to offer patients like Tinslee.
Initially, her chemotherapy worked. She went to school and enjoyed sports. She was a real fan of softball and tried to manage the team and be involved. At the time I learned about her, she was planning to go to college. Her love of softball remained, but given the recurrence of the cancer, she had no chance to pursue her athletic interests, not only as a player, but also as a manager or even as a coach for younger players. That was all off the table.
She’d been very compliant up until this time with her chemotherapy. When the recommendation came in that she undergo nonstandard chemotherapy because of the reoccurrence, with experimental drugs using an experimental protocol, she said to her family and the doctors that she didn’t want to do it. She would rather die. She couldn’t take any more chemotherapy and she certainly didn’t want to do it if it was experimental, with the outcomes of this intervention being uncertain.
Her mother said, “Her input matters. I want to listen to her.” Her mom wasn’t as adamant about doing it or not, but she really felt that Tinslee should be heard loudly because she felt she was mature enough or old enough, even though a minor, to really have a position about what it is to undergo chemotherapy.
Time matters in trying to control the spread, and the doctors were pushing for experimental intervention. I should add, by the way, that although it didn’t really drive the decision about whether to do it or not do it, experimental care like this is not covered by most insurance, and it wasn’t covered by their insurance, so they were facing a big bill if the experimental intervention was administered.
There was some money in a grant to cover some of it, but they were going to face some big financial costs. It never came up in my discussions with the doctors about what to do. I’m not sure whether it ever came up with the family’s discussion with the doctors about what to do, or even whether Tinslee was worrying and didn’t want her family to face a financial burden.
I suggested that we bring the family in. We did some counseling. We had a social worker and we brought in a pastor because these people were fairly religious. We talked about all scenarios, including accepting death, knowing that this disease was not likely to go into remission with the experimental effort; maybe it would, but the doctors were not optimistic.
We tried to talk about how much we should listen to what this young woman wanted. We knew there was the possibility of going to court and having a judge decide this, but in my experience, I do not like going to judges and courts because I know what they’re going to say. They almost always say “administer the intervention.” They don’t want to be in a position of saying don’t do something. They’re a little less willing to do that if something is experimental, but generally speaking, if you’re headed to court, it’s because you’ve decided that you want this to happen.
I felt, in all honesty, that this young woman should have some real respect of her position because the treatment was experimental. She is approaching the age of competency and consent, and she’s been through many interventions. She knows what’s involved. I think you really have to listen hard to what she’s saying.
By the way, after this case, I looked and there have been some surveys of residents in pediatrics. A large number of them said that they hadn’t received any training about what to do when mature minors refuse experimental treatments. The study I saw said that 30% had not undergone any training about this, so we certainly want to introduce that into the appropriate areas of medicine and talk about this with residents and fellows.
Long story short, we had the family meeting, we had another meeting with dad and mom and Tinslee, and the dad began to come around and he began to listen hard. Tinslee said what she wanted was to go to her prom. She wanted to get to her sister’s junior high school softball championship game. If you will, setting some smaller goals that seemed to make her very, very happy began to satisfy mom and dad and they could accept her refusal.
Ultimately, an agreement was reached that she would not undergo the experimental intervention. We agreed on a course of palliative care, recommended that as what the doctors follow, and they decided to do so. Sadly, Tinslee died. She died at home. She did make it to her prom.
I think the outcome, while difficult, sad, tragic, and a close call, was correct. Mature minors who have been through a rough life of interventions and know the price to pay – and for those who have recurrent disease and now face only experimental options – if they say no, that’s something we really have to listen to very hard.
Dr. Kaplan is director, division of medical ethics, New York University Langone Medical Center, New York. He reported a conflict of interest with Johnson & Johnson’s Panel for Compassionate Drug Use.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan, PhD. I’m director of the division of medical ethics at the New York University Grossman School of Medicine.
Every once in a while at my school, I get referrals about interesting or difficult clinical cases where doctors would like some input or advice that they can consider in managing a patient. Sometimes those requests come from other hospitals to me. I’ve been doing that kind of ethics consulting, both as a member of various ethics committees and sometimes individually, when, for various reasons, doctors don’t want to go to the Ethics Committee as a first stop.
There was a very interesting case recently involving a young woman I’m going to call Tinslee. She was 17 years old and she suffered, sadly, from recurrent metastatic osteogenic sarcoma. She had bone cancer. It had first been diagnosed at the age of 9. She had received chemotherapy and been under that treatment for a while.
If osteosarcoma is treated before it spreads outside the area where it began, the 5-year survival rate for people like her is about 75%. If the cancer spreads outside of the bones and gets into surrounding tissues, organs, or – worse – into the lymph nodes and starts traveling around, the 5-year survival rate drops to about 60%. The two approaches are chemotherapy and amputation. That’s what we have to offer patients like Tinslee.
Initially, her chemotherapy worked. She went to school and enjoyed sports. She was a real fan of softball and tried to manage the team and be involved. At the time I learned about her, she was planning to go to college. Her love of softball remained, but given the recurrence of the cancer, she had no chance to pursue her athletic interests, not only as a player, but also as a manager or even as a coach for younger players. That was all off the table.
She’d been very compliant up until this time with her chemotherapy. When the recommendation came in that she undergo nonstandard chemotherapy because of the reoccurrence, with experimental drugs using an experimental protocol, she said to her family and the doctors that she didn’t want to do it. She would rather die. She couldn’t take any more chemotherapy and she certainly didn’t want to do it if it was experimental, with the outcomes of this intervention being uncertain.
Her mother said, “Her input matters. I want to listen to her.” Her mom wasn’t as adamant about doing it or not, but she really felt that Tinslee should be heard loudly because she felt she was mature enough or old enough, even though a minor, to really have a position about what it is to undergo chemotherapy.
Time matters in trying to control the spread, and the doctors were pushing for experimental intervention. I should add, by the way, that although it didn’t really drive the decision about whether to do it or not do it, experimental care like this is not covered by most insurance, and it wasn’t covered by their insurance, so they were facing a big bill if the experimental intervention was administered.
There was some money in a grant to cover some of it, but they were going to face some big financial costs. It never came up in my discussions with the doctors about what to do. I’m not sure whether it ever came up with the family’s discussion with the doctors about what to do, or even whether Tinslee was worrying and didn’t want her family to face a financial burden.
I suggested that we bring the family in. We did some counseling. We had a social worker and we brought in a pastor because these people were fairly religious. We talked about all scenarios, including accepting death, knowing that this disease was not likely to go into remission with the experimental effort; maybe it would, but the doctors were not optimistic.
We tried to talk about how much we should listen to what this young woman wanted. We knew there was the possibility of going to court and having a judge decide this, but in my experience, I do not like going to judges and courts because I know what they’re going to say. They almost always say “administer the intervention.” They don’t want to be in a position of saying don’t do something. They’re a little less willing to do that if something is experimental, but generally speaking, if you’re headed to court, it’s because you’ve decided that you want this to happen.
I felt, in all honesty, that this young woman should have some real respect of her position because the treatment was experimental. She is approaching the age of competency and consent, and she’s been through many interventions. She knows what’s involved. I think you really have to listen hard to what she’s saying.
By the way, after this case, I looked and there have been some surveys of residents in pediatrics. A large number of them said that they hadn’t received any training about what to do when mature minors refuse experimental treatments. The study I saw said that 30% had not undergone any training about this, so we certainly want to introduce that into the appropriate areas of medicine and talk about this with residents and fellows.
Long story short, we had the family meeting, we had another meeting with dad and mom and Tinslee, and the dad began to come around and he began to listen hard. Tinslee said what she wanted was to go to her prom. She wanted to get to her sister’s junior high school softball championship game. If you will, setting some smaller goals that seemed to make her very, very happy began to satisfy mom and dad and they could accept her refusal.
Ultimately, an agreement was reached that she would not undergo the experimental intervention. We agreed on a course of palliative care, recommended that as what the doctors follow, and they decided to do so. Sadly, Tinslee died. She died at home. She did make it to her prom.
I think the outcome, while difficult, sad, tragic, and a close call, was correct. Mature minors who have been through a rough life of interventions and know the price to pay – and for those who have recurrent disease and now face only experimental options – if they say no, that’s something we really have to listen to very hard.
Dr. Kaplan is director, division of medical ethics, New York University Langone Medical Center, New York. He reported a conflict of interest with Johnson & Johnson’s Panel for Compassionate Drug Use.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan, PhD. I’m director of the division of medical ethics at the New York University Grossman School of Medicine.
Every once in a while at my school, I get referrals about interesting or difficult clinical cases where doctors would like some input or advice that they can consider in managing a patient. Sometimes those requests come from other hospitals to me. I’ve been doing that kind of ethics consulting, both as a member of various ethics committees and sometimes individually, when, for various reasons, doctors don’t want to go to the Ethics Committee as a first stop.
There was a very interesting case recently involving a young woman I’m going to call Tinslee. She was 17 years old and she suffered, sadly, from recurrent metastatic osteogenic sarcoma. She had bone cancer. It had first been diagnosed at the age of 9. She had received chemotherapy and been under that treatment for a while.
If osteosarcoma is treated before it spreads outside the area where it began, the 5-year survival rate for people like her is about 75%. If the cancer spreads outside of the bones and gets into surrounding tissues, organs, or – worse – into the lymph nodes and starts traveling around, the 5-year survival rate drops to about 60%. The two approaches are chemotherapy and amputation. That’s what we have to offer patients like Tinslee.
Initially, her chemotherapy worked. She went to school and enjoyed sports. She was a real fan of softball and tried to manage the team and be involved. At the time I learned about her, she was planning to go to college. Her love of softball remained, but given the recurrence of the cancer, she had no chance to pursue her athletic interests, not only as a player, but also as a manager or even as a coach for younger players. That was all off the table.
She’d been very compliant up until this time with her chemotherapy. When the recommendation came in that she undergo nonstandard chemotherapy because of the reoccurrence, with experimental drugs using an experimental protocol, she said to her family and the doctors that she didn’t want to do it. She would rather die. She couldn’t take any more chemotherapy and she certainly didn’t want to do it if it was experimental, with the outcomes of this intervention being uncertain.
Her mother said, “Her input matters. I want to listen to her.” Her mom wasn’t as adamant about doing it or not, but she really felt that Tinslee should be heard loudly because she felt she was mature enough or old enough, even though a minor, to really have a position about what it is to undergo chemotherapy.
Time matters in trying to control the spread, and the doctors were pushing for experimental intervention. I should add, by the way, that although it didn’t really drive the decision about whether to do it or not do it, experimental care like this is not covered by most insurance, and it wasn’t covered by their insurance, so they were facing a big bill if the experimental intervention was administered.
There was some money in a grant to cover some of it, but they were going to face some big financial costs. It never came up in my discussions with the doctors about what to do. I’m not sure whether it ever came up with the family’s discussion with the doctors about what to do, or even whether Tinslee was worrying and didn’t want her family to face a financial burden.
I suggested that we bring the family in. We did some counseling. We had a social worker and we brought in a pastor because these people were fairly religious. We talked about all scenarios, including accepting death, knowing that this disease was not likely to go into remission with the experimental effort; maybe it would, but the doctors were not optimistic.
We tried to talk about how much we should listen to what this young woman wanted. We knew there was the possibility of going to court and having a judge decide this, but in my experience, I do not like going to judges and courts because I know what they’re going to say. They almost always say “administer the intervention.” They don’t want to be in a position of saying don’t do something. They’re a little less willing to do that if something is experimental, but generally speaking, if you’re headed to court, it’s because you’ve decided that you want this to happen.
I felt, in all honesty, that this young woman should have some real respect of her position because the treatment was experimental. She is approaching the age of competency and consent, and she’s been through many interventions. She knows what’s involved. I think you really have to listen hard to what she’s saying.
By the way, after this case, I looked and there have been some surveys of residents in pediatrics. A large number of them said that they hadn’t received any training about what to do when mature minors refuse experimental treatments. The study I saw said that 30% had not undergone any training about this, so we certainly want to introduce that into the appropriate areas of medicine and talk about this with residents and fellows.
Long story short, we had the family meeting, we had another meeting with dad and mom and Tinslee, and the dad began to come around and he began to listen hard. Tinslee said what she wanted was to go to her prom. She wanted to get to her sister’s junior high school softball championship game. If you will, setting some smaller goals that seemed to make her very, very happy began to satisfy mom and dad and they could accept her refusal.
Ultimately, an agreement was reached that she would not undergo the experimental intervention. We agreed on a course of palliative care, recommended that as what the doctors follow, and they decided to do so. Sadly, Tinslee died. She died at home. She did make it to her prom.
I think the outcome, while difficult, sad, tragic, and a close call, was correct. Mature minors who have been through a rough life of interventions and know the price to pay – and for those who have recurrent disease and now face only experimental options – if they say no, that’s something we really have to listen to very hard.
Dr. Kaplan is director, division of medical ethics, New York University Langone Medical Center, New York. He reported a conflict of interest with Johnson & Johnson’s Panel for Compassionate Drug Use.
A version of this article first appeared on Medscape.com.
Can a biodegradable brain implant deliver lifesaving cancer meds?
It’s the latest advance in a rapidly growing field using ultrasound – high-frequency sound waves undetectable to humans – to fight cancer and other diseases.
The problem addressed by the researchers is the blood-brain barrier, a nearly impenetrable blood vessel lining that keeps harmful molecules from passing into the brain from the blood. But this lining can also block chemo drugs from reaching cancer cells.
So the scientists implanted 1-cm2 devices into the skulls of mice, directly behind the tumor site. The implants generate ultrasound waves, loosening the barrier and allowing the drugs to reach the tumor. The sound waves leave healthy tissue undamaged.
“You inject the drug into the body and turn on the ultrasound at the same time. You’re going to hit precisely at the tumor area every single time you use it,” said lead study author Thanh Nguyen, PhD, an associate professor of mechanical engineering at the University of Connecticut, Storrs.
The drug used in the study was paclitaxel, which normally struggles to get through the blood-brain barrier. The tumors shrank, and the mice doubled their lifetime, compared with untreated mice. The mice showed no bad health effects 6 months later.
Breaking through the blood-brain barrier
The biodegradable implant is made of glycine, an amino acid that’s also strongly piezoelectric, meaning it vibrates when subjected to an electrical current. To make it, researchers cultivated glycine crystals, shattered them into pieces, and finally used a process called electrospinning, which applies a high electrical voltage to the nanocrystals.
Voltage flows to the implant via an external device. The resulting ultrasound causes the tightly adhered cells of the blood-brain barrier to vibrate, stretching them out and creating space for pores to form.
“That allows in very tiny particles, including chemo drugs,” said Dr. Nguyen.
His earlier biodegradable implant broke apart from the force, but the new glycine implant is more flexible, stable, and highly piezoelectric. It could be implanted after a patient has surgery to remove a brain tumor, to continue treating residual cancer cells. The implant dissolves harmlessly in the body over time, and doctors can control its lifespan.
A new wave of uses for ultrasound
Dr. Nguyen’s study builds on similar efforts, including a recent clinical trial of a nonbiodegradable implant for treating brain tumors. Ultrasound can focus energy on precise targets in the body.
It’s like “using a magnifying glass to focus multiple beams of light on a point and burn a hole in a leaf,” said Neal Kassell, MD, founder and chairman of the Focused Ultrasound Foundation. This approach spares adjacent normal tissue.
Doctors now understand more than 30 ways that ultrasound interacts with tissue – from destroying abnormal tissue to delivering drugs more effectively to stimulating an immune response. A decade ago, only five such interactions were known.
This opens the door for treating “a wide spectrum of medical disorders,” from neurodegenerative diseases like Alzheimer’s and Parkinson’s to difficult-to-treat cancers of the prostate and pancreas, and even addiction, said Dr. Kassell.
Dr. Kassell envisions using focused ultrasound to treat brain tumors as an alternative (or complement) to surgery, chemotherapy, immunotherapy, or radiation therapy. In the meantime, implants have helped show “the effectiveness of opening the blood-brain barrier.”
Dr. Nguyen’s team plans on testing the safety and efficacy of their implant in pigs next. Eventually, Dr. Nguyen hopes to develop a patch with an array of implants to target different areas of the brain.
One study coauthor is cofounder of PiezoBioMembrane and SingleTimeMicroneedles. The other study authors reported no conflicts of interest.
A version of this article originally appeared on WebMD.com.
It’s the latest advance in a rapidly growing field using ultrasound – high-frequency sound waves undetectable to humans – to fight cancer and other diseases.
The problem addressed by the researchers is the blood-brain barrier, a nearly impenetrable blood vessel lining that keeps harmful molecules from passing into the brain from the blood. But this lining can also block chemo drugs from reaching cancer cells.
So the scientists implanted 1-cm2 devices into the skulls of mice, directly behind the tumor site. The implants generate ultrasound waves, loosening the barrier and allowing the drugs to reach the tumor. The sound waves leave healthy tissue undamaged.
“You inject the drug into the body and turn on the ultrasound at the same time. You’re going to hit precisely at the tumor area every single time you use it,” said lead study author Thanh Nguyen, PhD, an associate professor of mechanical engineering at the University of Connecticut, Storrs.
The drug used in the study was paclitaxel, which normally struggles to get through the blood-brain barrier. The tumors shrank, and the mice doubled their lifetime, compared with untreated mice. The mice showed no bad health effects 6 months later.
Breaking through the blood-brain barrier
The biodegradable implant is made of glycine, an amino acid that’s also strongly piezoelectric, meaning it vibrates when subjected to an electrical current. To make it, researchers cultivated glycine crystals, shattered them into pieces, and finally used a process called electrospinning, which applies a high electrical voltage to the nanocrystals.
Voltage flows to the implant via an external device. The resulting ultrasound causes the tightly adhered cells of the blood-brain barrier to vibrate, stretching them out and creating space for pores to form.
“That allows in very tiny particles, including chemo drugs,” said Dr. Nguyen.
His earlier biodegradable implant broke apart from the force, but the new glycine implant is more flexible, stable, and highly piezoelectric. It could be implanted after a patient has surgery to remove a brain tumor, to continue treating residual cancer cells. The implant dissolves harmlessly in the body over time, and doctors can control its lifespan.
A new wave of uses for ultrasound
Dr. Nguyen’s study builds on similar efforts, including a recent clinical trial of a nonbiodegradable implant for treating brain tumors. Ultrasound can focus energy on precise targets in the body.
It’s like “using a magnifying glass to focus multiple beams of light on a point and burn a hole in a leaf,” said Neal Kassell, MD, founder and chairman of the Focused Ultrasound Foundation. This approach spares adjacent normal tissue.
Doctors now understand more than 30 ways that ultrasound interacts with tissue – from destroying abnormal tissue to delivering drugs more effectively to stimulating an immune response. A decade ago, only five such interactions were known.
This opens the door for treating “a wide spectrum of medical disorders,” from neurodegenerative diseases like Alzheimer’s and Parkinson’s to difficult-to-treat cancers of the prostate and pancreas, and even addiction, said Dr. Kassell.
Dr. Kassell envisions using focused ultrasound to treat brain tumors as an alternative (or complement) to surgery, chemotherapy, immunotherapy, or radiation therapy. In the meantime, implants have helped show “the effectiveness of opening the blood-brain barrier.”
Dr. Nguyen’s team plans on testing the safety and efficacy of their implant in pigs next. Eventually, Dr. Nguyen hopes to develop a patch with an array of implants to target different areas of the brain.
One study coauthor is cofounder of PiezoBioMembrane and SingleTimeMicroneedles. The other study authors reported no conflicts of interest.
A version of this article originally appeared on WebMD.com.
It’s the latest advance in a rapidly growing field using ultrasound – high-frequency sound waves undetectable to humans – to fight cancer and other diseases.
The problem addressed by the researchers is the blood-brain barrier, a nearly impenetrable blood vessel lining that keeps harmful molecules from passing into the brain from the blood. But this lining can also block chemo drugs from reaching cancer cells.
So the scientists implanted 1-cm2 devices into the skulls of mice, directly behind the tumor site. The implants generate ultrasound waves, loosening the barrier and allowing the drugs to reach the tumor. The sound waves leave healthy tissue undamaged.
“You inject the drug into the body and turn on the ultrasound at the same time. You’re going to hit precisely at the tumor area every single time you use it,” said lead study author Thanh Nguyen, PhD, an associate professor of mechanical engineering at the University of Connecticut, Storrs.
The drug used in the study was paclitaxel, which normally struggles to get through the blood-brain barrier. The tumors shrank, and the mice doubled their lifetime, compared with untreated mice. The mice showed no bad health effects 6 months later.
Breaking through the blood-brain barrier
The biodegradable implant is made of glycine, an amino acid that’s also strongly piezoelectric, meaning it vibrates when subjected to an electrical current. To make it, researchers cultivated glycine crystals, shattered them into pieces, and finally used a process called electrospinning, which applies a high electrical voltage to the nanocrystals.
Voltage flows to the implant via an external device. The resulting ultrasound causes the tightly adhered cells of the blood-brain barrier to vibrate, stretching them out and creating space for pores to form.
“That allows in very tiny particles, including chemo drugs,” said Dr. Nguyen.
His earlier biodegradable implant broke apart from the force, but the new glycine implant is more flexible, stable, and highly piezoelectric. It could be implanted after a patient has surgery to remove a brain tumor, to continue treating residual cancer cells. The implant dissolves harmlessly in the body over time, and doctors can control its lifespan.
A new wave of uses for ultrasound
Dr. Nguyen’s study builds on similar efforts, including a recent clinical trial of a nonbiodegradable implant for treating brain tumors. Ultrasound can focus energy on precise targets in the body.
It’s like “using a magnifying glass to focus multiple beams of light on a point and burn a hole in a leaf,” said Neal Kassell, MD, founder and chairman of the Focused Ultrasound Foundation. This approach spares adjacent normal tissue.
Doctors now understand more than 30 ways that ultrasound interacts with tissue – from destroying abnormal tissue to delivering drugs more effectively to stimulating an immune response. A decade ago, only five such interactions were known.
This opens the door for treating “a wide spectrum of medical disorders,” from neurodegenerative diseases like Alzheimer’s and Parkinson’s to difficult-to-treat cancers of the prostate and pancreas, and even addiction, said Dr. Kassell.
Dr. Kassell envisions using focused ultrasound to treat brain tumors as an alternative (or complement) to surgery, chemotherapy, immunotherapy, or radiation therapy. In the meantime, implants have helped show “the effectiveness of opening the blood-brain barrier.”
Dr. Nguyen’s team plans on testing the safety and efficacy of their implant in pigs next. Eventually, Dr. Nguyen hopes to develop a patch with an array of implants to target different areas of the brain.
One study coauthor is cofounder of PiezoBioMembrane and SingleTimeMicroneedles. The other study authors reported no conflicts of interest.
A version of this article originally appeared on WebMD.com.
FROM SCIENCE ADVANCES
The surprising occupations with higher-than-expected ovarian cancer rates
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
Basically, all cancers are caused by a mix of genetic and environmental factors, with some cancers driven more strongly by one or the other. When it comes to ovarian cancer, which kills more than 13,000 women per year in the United States, genetic factors like the BRCA gene mutations are well described.
Other risk factors, like early menarche and nulliparity, are difficult to modify. The only slam-dunk environmental toxin to be linked to ovarian cancer is asbestos. Still, the vast majority of women who develop ovarian cancer do not have a known high-risk gene or asbestos exposure, so other triggers may be out there. How do we find them? The answer may just be good old-fashioned epidemiology.
That’s just what researchers, led by Anita Koushik at the University of Montreal, did in a new study appearing in the journal Occupational and Environmental Medicine.
They identified 497 women in Montreal who had recently been diagnosed with ovarian cancer. They then matched those women to 897 women without ovarian cancer, based on age and address. (This approach would not work well in the United States, as diagnosis of ovarian cancer might depend on access to medical care, which is not universal here. In Canada, however, it’s safer to assume that anyone who could have gotten ovarian cancer in Montreal would have been detected.)
Cases and controls identified, the researchers took a detailed occupational history for each participant: every job they ever worked, and when, and for how long. Each occupation was mapped to a standardized set of industries and, interestingly, to a set of environmental exposures ranging from cosmetic talc to cooking fumes to cotton dust, in what is known as a job-exposure matrix. Of course, they also collected data on other ovarian cancer risk factors.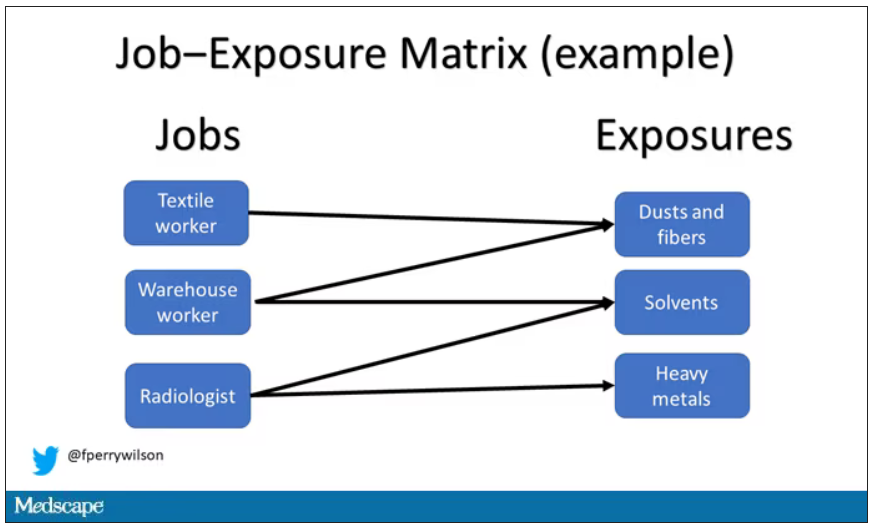
After that, it’s a simple matter of looking at the rate of ovarian cancer by occupation and occupation-associated exposures, accounting for differences in things like pregnancy rates.
A brief aside here. I was at dinner with my wife the other night and telling her about this study, and I asked, “What do you think the occupation with the highest rate of ovarian cancer is?” And without missing a beat, she said: “Hairdressers.” Which blew my mind because of how random that was, but she was also – as usual – 100% correct.
Hairdressers, at least those who had been in the industry for more than 10 years, had a threefold higher risk for ovarian cancer than matched controls who had never been hairdressers.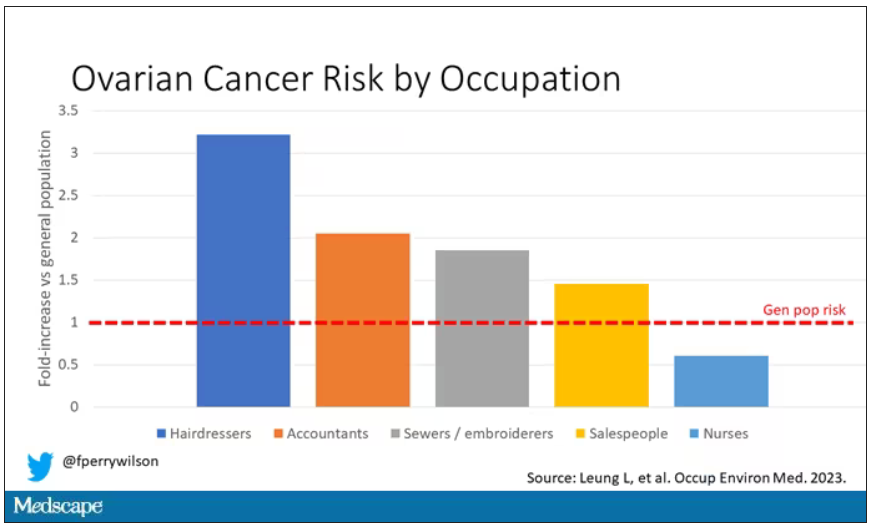
Of course, my wife is a cancer surgeon, so she has a bit of a leg up on me here. Many of you may also know that there is actually a decent body of literature showing higher rates of various cancers among hairdressers, presumably due to the variety of chemicals they are exposed to on a continuous basis.
The No. 2 highest-risk profession on the list? Accountants, with about a twofold higher risk. That one is more of a puzzler. It could be a false positive; after all, there were multiple occupations checked and random error might give a few hits that are meaningless. But there are certainly some occupational factors unique to accountants that might bear further investigation – maybe exposure to volatile organic compounds from office printers, or just a particularly sedentary office environment.
In terms of specific exposures, there were high risks seen with mononuclear aromatic hydrocarbons, bleaches, ethanol, and fluorocarbons, among others, but we have to be a bit more careful here. These exposures were not directly measured. Rather, based on the job category a woman described, the exposures were imputed based on the job-exposure matrix. As such, the correlations between the job and the particular exposure are really quite high, making it essentially impossible to tease out whether it is, for example, being a hairdresser, or being exposed to fluorocarbons as a hairdresser, or being exposed to something else as a hairdresser, that is the problem.
This is how these types of studies work; they tend to raise more questions than they answer. But in a world where a cancer diagnosis can seem to come completely out of the blue, they provide the starting point that someday may lead to a more definitive culprit agent or group of agents. Until then, it might be wise for hairdressers to make sure their workplace is well ventilated.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
Basically, all cancers are caused by a mix of genetic and environmental factors, with some cancers driven more strongly by one or the other. When it comes to ovarian cancer, which kills more than 13,000 women per year in the United States, genetic factors like the BRCA gene mutations are well described.
Other risk factors, like early menarche and nulliparity, are difficult to modify. The only slam-dunk environmental toxin to be linked to ovarian cancer is asbestos. Still, the vast majority of women who develop ovarian cancer do not have a known high-risk gene or asbestos exposure, so other triggers may be out there. How do we find them? The answer may just be good old-fashioned epidemiology.
That’s just what researchers, led by Anita Koushik at the University of Montreal, did in a new study appearing in the journal Occupational and Environmental Medicine.
They identified 497 women in Montreal who had recently been diagnosed with ovarian cancer. They then matched those women to 897 women without ovarian cancer, based on age and address. (This approach would not work well in the United States, as diagnosis of ovarian cancer might depend on access to medical care, which is not universal here. In Canada, however, it’s safer to assume that anyone who could have gotten ovarian cancer in Montreal would have been detected.)
Cases and controls identified, the researchers took a detailed occupational history for each participant: every job they ever worked, and when, and for how long. Each occupation was mapped to a standardized set of industries and, interestingly, to a set of environmental exposures ranging from cosmetic talc to cooking fumes to cotton dust, in what is known as a job-exposure matrix. Of course, they also collected data on other ovarian cancer risk factors.
After that, it’s a simple matter of looking at the rate of ovarian cancer by occupation and occupation-associated exposures, accounting for differences in things like pregnancy rates.
A brief aside here. I was at dinner with my wife the other night and telling her about this study, and I asked, “What do you think the occupation with the highest rate of ovarian cancer is?” And without missing a beat, she said: “Hairdressers.” Which blew my mind because of how random that was, but she was also – as usual – 100% correct.
Hairdressers, at least those who had been in the industry for more than 10 years, had a threefold higher risk for ovarian cancer than matched controls who had never been hairdressers.
Of course, my wife is a cancer surgeon, so she has a bit of a leg up on me here. Many of you may also know that there is actually a decent body of literature showing higher rates of various cancers among hairdressers, presumably due to the variety of chemicals they are exposed to on a continuous basis.
The No. 2 highest-risk profession on the list? Accountants, with about a twofold higher risk. That one is more of a puzzler. It could be a false positive; after all, there were multiple occupations checked and random error might give a few hits that are meaningless. But there are certainly some occupational factors unique to accountants that might bear further investigation – maybe exposure to volatile organic compounds from office printers, or just a particularly sedentary office environment.
In terms of specific exposures, there were high risks seen with mononuclear aromatic hydrocarbons, bleaches, ethanol, and fluorocarbons, among others, but we have to be a bit more careful here. These exposures were not directly measured. Rather, based on the job category a woman described, the exposures were imputed based on the job-exposure matrix. As such, the correlations between the job and the particular exposure are really quite high, making it essentially impossible to tease out whether it is, for example, being a hairdresser, or being exposed to fluorocarbons as a hairdresser, or being exposed to something else as a hairdresser, that is the problem.
This is how these types of studies work; they tend to raise more questions than they answer. But in a world where a cancer diagnosis can seem to come completely out of the blue, they provide the starting point that someday may lead to a more definitive culprit agent or group of agents. Until then, it might be wise for hairdressers to make sure their workplace is well ventilated.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Welcome to Impact Factor, your weekly dose of commentary on a new medical study.
Basically, all cancers are caused by a mix of genetic and environmental factors, with some cancers driven more strongly by one or the other. When it comes to ovarian cancer, which kills more than 13,000 women per year in the United States, genetic factors like the BRCA gene mutations are well described.
Other risk factors, like early menarche and nulliparity, are difficult to modify. The only slam-dunk environmental toxin to be linked to ovarian cancer is asbestos. Still, the vast majority of women who develop ovarian cancer do not have a known high-risk gene or asbestos exposure, so other triggers may be out there. How do we find them? The answer may just be good old-fashioned epidemiology.
That’s just what researchers, led by Anita Koushik at the University of Montreal, did in a new study appearing in the journal Occupational and Environmental Medicine.
They identified 497 women in Montreal who had recently been diagnosed with ovarian cancer. They then matched those women to 897 women without ovarian cancer, based on age and address. (This approach would not work well in the United States, as diagnosis of ovarian cancer might depend on access to medical care, which is not universal here. In Canada, however, it’s safer to assume that anyone who could have gotten ovarian cancer in Montreal would have been detected.)
Cases and controls identified, the researchers took a detailed occupational history for each participant: every job they ever worked, and when, and for how long. Each occupation was mapped to a standardized set of industries and, interestingly, to a set of environmental exposures ranging from cosmetic talc to cooking fumes to cotton dust, in what is known as a job-exposure matrix. Of course, they also collected data on other ovarian cancer risk factors.
After that, it’s a simple matter of looking at the rate of ovarian cancer by occupation and occupation-associated exposures, accounting for differences in things like pregnancy rates.
A brief aside here. I was at dinner with my wife the other night and telling her about this study, and I asked, “What do you think the occupation with the highest rate of ovarian cancer is?” And without missing a beat, she said: “Hairdressers.” Which blew my mind because of how random that was, but she was also – as usual – 100% correct.
Hairdressers, at least those who had been in the industry for more than 10 years, had a threefold higher risk for ovarian cancer than matched controls who had never been hairdressers.
Of course, my wife is a cancer surgeon, so she has a bit of a leg up on me here. Many of you may also know that there is actually a decent body of literature showing higher rates of various cancers among hairdressers, presumably due to the variety of chemicals they are exposed to on a continuous basis.
The No. 2 highest-risk profession on the list? Accountants, with about a twofold higher risk. That one is more of a puzzler. It could be a false positive; after all, there were multiple occupations checked and random error might give a few hits that are meaningless. But there are certainly some occupational factors unique to accountants that might bear further investigation – maybe exposure to volatile organic compounds from office printers, or just a particularly sedentary office environment.
In terms of specific exposures, there were high risks seen with mononuclear aromatic hydrocarbons, bleaches, ethanol, and fluorocarbons, among others, but we have to be a bit more careful here. These exposures were not directly measured. Rather, based on the job category a woman described, the exposures were imputed based on the job-exposure matrix. As such, the correlations between the job and the particular exposure are really quite high, making it essentially impossible to tease out whether it is, for example, being a hairdresser, or being exposed to fluorocarbons as a hairdresser, or being exposed to something else as a hairdresser, that is the problem.
This is how these types of studies work; they tend to raise more questions than they answer. But in a world where a cancer diagnosis can seem to come completely out of the blue, they provide the starting point that someday may lead to a more definitive culprit agent or group of agents. Until then, it might be wise for hairdressers to make sure their workplace is well ventilated.
F. Perry Wilson, MD, MSCE, is an associate professor of medicine and director of Yale University’s Clinical and Translational Research Accelerator in New Haven, Conn. He reported no conflicts of interest.
A version of this article first appeared on Medscape.com.
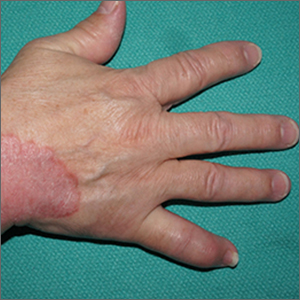
Focal plaques and finger swelling
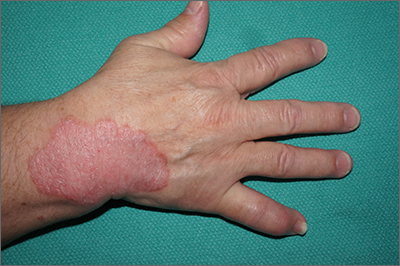
Well-demarcated symmetrical scaly plaques and dactylitis are consistent with psoriasis and psoriatic arthritis (PsA). Even in the absence of significant skin disease, a patient like this should be evaluated by Rheumatology for initiation of disease-modifying antirheumatic drugs (DMARDs).
Psoriatic arthritis manifests as a peripheral arthritis affecting the small joints of the wrists and hands, pain at the insertion of tendons and ligaments (enthesitis), or as axial arthritis. This variable presentation and the lack of specific serological marker can make diagnosis challenging. Associated symptoms beyond the musculoskeletal system include uveitis, inflammatory bowel disease, and cutaneous psoriasis.1 In contrast to osteoarthritis, PsA symptoms are often worse in the morning and improve over the course of the day. Patients with a history of psoriasis on the skin have about a 10% chance of developing PsA, with increased rates in patients who have more widespread plaques and patients with psoriasis at a young age.2 Although not pathognomonic for PsA, pitting of the fingernails may reflect episodic enthesitis in the extensor tendons of the fingers.3 Radiographs of the hands in severe cases may demonstrate narrowing of the proximal portion of the distal or proximal interphalangeal joints with a cup-like concavity of the distal half of the joint.
Conventional DMARDs (such as methotrexate and azathioprine) and biologic DMARDs (including TNF-alpha inhibitors, IL-17 inhibitors, IL-23 inhibitors) are first-line treatments and can stop or slow the progression of disease but will not reverse existing damage. For this reason, it is important to promptly start DMARD therapy after the diagnosis has been established.4
This patient was initiated on adalimumab 40 mg subcutaneously every other week. Her pain improved after 2 months of therapy and her skin plaques almost entirely resolved at 6 months.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Rida MA, Chandran V. Challenges in the clinical diagnosis of psoriatic arthritis. Clin Immunol. 2020;214:108390. doi: 10.1016/j.clim.2020.108390
2. Ogdie A, Gelfand JM. Clinical risk factors for the development of psoriatic arthritis among patients with psoriasis: a review of available evidence. Curr Rheumatol Rep. 2015;17:64. doi: 10.1007/s11926-015-0540-1
3. Elliott A, Pendleton A, Wright G, et al. The relationship between the nail and systemic enthesitis in psoriatic arthritis. Rheumatol Adv Pract. 2021;5:rkab088. doi: 10.1093/rap/rkab088
4. Coates LC, Soriano ER, Corp N, et al. GRAPPA treatment recommendations domain subcommittees. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18:465-479. doi: 10.1038/s41584-022-00798-0

Well-demarcated symmetrical scaly plaques and dactylitis are consistent with psoriasis and psoriatic arthritis (PsA). Even in the absence of significant skin disease, a patient like this should be evaluated by Rheumatology for initiation of disease-modifying antirheumatic drugs (DMARDs).
Psoriatic arthritis manifests as a peripheral arthritis affecting the small joints of the wrists and hands, pain at the insertion of tendons and ligaments (enthesitis), or as axial arthritis. This variable presentation and the lack of specific serological marker can make diagnosis challenging. Associated symptoms beyond the musculoskeletal system include uveitis, inflammatory bowel disease, and cutaneous psoriasis.1 In contrast to osteoarthritis, PsA symptoms are often worse in the morning and improve over the course of the day. Patients with a history of psoriasis on the skin have about a 10% chance of developing PsA, with increased rates in patients who have more widespread plaques and patients with psoriasis at a young age.2 Although not pathognomonic for PsA, pitting of the fingernails may reflect episodic enthesitis in the extensor tendons of the fingers.3 Radiographs of the hands in severe cases may demonstrate narrowing of the proximal portion of the distal or proximal interphalangeal joints with a cup-like concavity of the distal half of the joint.
Conventional DMARDs (such as methotrexate and azathioprine) and biologic DMARDs (including TNF-alpha inhibitors, IL-17 inhibitors, IL-23 inhibitors) are first-line treatments and can stop or slow the progression of disease but will not reverse existing damage. For this reason, it is important to promptly start DMARD therapy after the diagnosis has been established.4
This patient was initiated on adalimumab 40 mg subcutaneously every other week. Her pain improved after 2 months of therapy and her skin plaques almost entirely resolved at 6 months.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.

Well-demarcated symmetrical scaly plaques and dactylitis are consistent with psoriasis and psoriatic arthritis (PsA). Even in the absence of significant skin disease, a patient like this should be evaluated by Rheumatology for initiation of disease-modifying antirheumatic drugs (DMARDs).
Psoriatic arthritis manifests as a peripheral arthritis affecting the small joints of the wrists and hands, pain at the insertion of tendons and ligaments (enthesitis), or as axial arthritis. This variable presentation and the lack of specific serological marker can make diagnosis challenging. Associated symptoms beyond the musculoskeletal system include uveitis, inflammatory bowel disease, and cutaneous psoriasis.1 In contrast to osteoarthritis, PsA symptoms are often worse in the morning and improve over the course of the day. Patients with a history of psoriasis on the skin have about a 10% chance of developing PsA, with increased rates in patients who have more widespread plaques and patients with psoriasis at a young age.2 Although not pathognomonic for PsA, pitting of the fingernails may reflect episodic enthesitis in the extensor tendons of the fingers.3 Radiographs of the hands in severe cases may demonstrate narrowing of the proximal portion of the distal or proximal interphalangeal joints with a cup-like concavity of the distal half of the joint.
Conventional DMARDs (such as methotrexate and azathioprine) and biologic DMARDs (including TNF-alpha inhibitors, IL-17 inhibitors, IL-23 inhibitors) are first-line treatments and can stop or slow the progression of disease but will not reverse existing damage. For this reason, it is important to promptly start DMARD therapy after the diagnosis has been established.4
This patient was initiated on adalimumab 40 mg subcutaneously every other week. Her pain improved after 2 months of therapy and her skin plaques almost entirely resolved at 6 months.
Photos and text for Photo Rounds Friday courtesy of Jonathan Karnes, MD (copyright retained). Dr. Karnes is the medical director of MDFMR Dermatology Services, Augusta, ME.
1. Rida MA, Chandran V. Challenges in the clinical diagnosis of psoriatic arthritis. Clin Immunol. 2020;214:108390. doi: 10.1016/j.clim.2020.108390
2. Ogdie A, Gelfand JM. Clinical risk factors for the development of psoriatic arthritis among patients with psoriasis: a review of available evidence. Curr Rheumatol Rep. 2015;17:64. doi: 10.1007/s11926-015-0540-1
3. Elliott A, Pendleton A, Wright G, et al. The relationship between the nail and systemic enthesitis in psoriatic arthritis. Rheumatol Adv Pract. 2021;5:rkab088. doi: 10.1093/rap/rkab088
4. Coates LC, Soriano ER, Corp N, et al. GRAPPA treatment recommendations domain subcommittees. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18:465-479. doi: 10.1038/s41584-022-00798-0
1. Rida MA, Chandran V. Challenges in the clinical diagnosis of psoriatic arthritis. Clin Immunol. 2020;214:108390. doi: 10.1016/j.clim.2020.108390
2. Ogdie A, Gelfand JM. Clinical risk factors for the development of psoriatic arthritis among patients with psoriasis: a review of available evidence. Curr Rheumatol Rep. 2015;17:64. doi: 10.1007/s11926-015-0540-1
3. Elliott A, Pendleton A, Wright G, et al. The relationship between the nail and systemic enthesitis in psoriatic arthritis. Rheumatol Adv Pract. 2021;5:rkab088. doi: 10.1093/rap/rkab088
4. Coates LC, Soriano ER, Corp N, et al. GRAPPA treatment recommendations domain subcommittees. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18:465-479. doi: 10.1038/s41584-022-00798-0
Surgeon in the C-suite

“If you don’t have a seat at the table, you are probably on the menu.” I first heard this quote in 2013, and it launched my interest in health care leadership and influenced me countless times over the last 10 years.
As Chief of Staff at Cleveland Clinic, I oversee nearly 5,000 physicians and scientists across the globe. I am involved in the physician life cycle: recruiting, hiring, privileging and credentialing, talent development, promotion, professionalism, and career transitions. I also sit at the intersection of medical care and the business of medicine. This means leading 18 clinical service lines responsible for 5.6 million visits, 161,000 surgeries, and billions of dollars in operating revenue per year. How I spend most of my time is a far cry from what I spent 11 years’ training to do—gynecologic surgery. This shift in my career was not because I changed my mind about caring for patients or that I tired of being a full-time surgeon. Nothing could be further from the truth. Women’s health remains my “why,” and my leadership journey has taught me that it is critical to have a seat at the table for the sake of ObGyns and women everywhere.
Women’s health on the menu
I will start with a concrete example of when we, as women and ObGyns, were on the menu. In late 2019, the Ohio state House of Representatives introduced a bill that subjected doctors to potential murder charges if they did not try everything to save the life of a mother and fetus, “including attempting to reimplant an ectopic pregnancy into the woman’s uterus.”1 This bill was based on 2 case reports—one from 1915 and one from 1980—which were both low quality, and the latter case was deemed to be fraudulent.2 How did this happen?
An Ohio state representative developed the bill with help from a lobbyist and without input from physicians or content experts. When asked, the representative shared that “he never researched whether re-implanting an ectopic pregnancy into a woman’s uterus was a viable medical procedure before including it in the bill.”3 He added, “I heard about it over the years. I never questioned it or gave it a lot of thought.”3
This example resonates deeply with many of us; it inspires us to speak up and act. As ObGyns, we clearly understand the consequences of legal and regulatory change in women’s health and how it directly impacts our patients and each of us as physicians. Let’s shift to something that you may feel less passion about, but I believe is equally important. This is where obstetrician-gynecologists sit in the intersection of medical care and business. This is the space where I spend most of my time, and from this vantage point, I worry about our field.
The business of medicine
Starting at the macroeconomic level, let’s think about how we as physicians are reimbursed and who makes these decisions. Looking at the national health care expenditure data, Medicare and Medicaid spending makes up nearly 40% of the total spend, and it is growing.4 Additionally, private health insurance tends to follow Centers for Medicare and Medicaid Services (CMS) decision making, further compounding its influence.4 In simple terms, CMS decides what is covered and how much we are paid. Whether you are in a solo private practice, an employer health care organization, or an academic medical center, physician reimbursement is declining.
In fact, Congress passed its year-end omnibus legislation in the final days of 2022, including a 2% Medicare physician payment cut for 2023,5 at a time when expenses to practice medicine, including nonphysician staff and supplies, are at an all-time high and we are living in a 6% inflationary state. This translates into being asked to serve more patients and cut costs. Our day-to-day feels much tighter, and this is why: Medicare physician pay increased just 11% over the past 20 years6 (2001–2021) in comparison to the cost of running a medical practice, which increased nearly 40% during that time. In other words, adjusting for inflation in practice costs, Medicare physician payment has fallen 22% over the last 20 years.7
Depending on your employment model, you may feel insulated from these changes as increases in reimbursement have occurred in other areas, such as hospitals and ambulatory surgery centers.8 In the short term, these increases help, as organizations will see additional funds. But there are 2 main issues: First, it is not nearly enough when you consider the soaring costs of running a hospital. And second, looking at our national population, we rely tremendously on self-employed doctors to serve our patients.
More than 80% of US counties lack adequate health care infrastructure.9 More than a third of the US population has less-than-adequate access to pharmacies, primary care physicians, hospitals, trauma centers, and low-cost health centers.9 To put things into perspective, more than 20% of counties in the United States are hospital deserts, where most people must drive more than 30 minutes to reach the closest hospital.9
There is good reason for this. Operating a hospital is a challenging endeavor. Even before the COVID-19 pandemic and the most recent health care financial challenges, most health care systems and large hospitals operated with very low operating margins (2%–3%). Businesses with similar margins include grocery stores and car dealerships. These low-margin businesses, including health care, rely on high volume for sustainability. High patient volumes distribute expensive hospital costs over many encounters. If physicians cannot sustain practices across the country, it is challenging to have sufficient admission and surgical volumes to justify the cost base of hospitals.
To tie this together, we have very little influence on what we are paid for our services. Reimbursement is declining, which makes it hard to have financially sustainable practices. As hospitals struggle, there is more pressure to prioritize highly profitable service lines, like orthopedics and urology, which are associated with favorable technical revenue. As hospitals are threatened, health care deserts widen, which leaves our entire health care system in jeopardy. Not surprisingly, this most likely affects those who face additional barriers to access, such as those with lower income, limited internet access, and lack of insurance. Together, these barriers further widen disparities in health care outcomes, including outcomes for women. Additionally, this death by a thousand cuts has eroded morale and increased physician burnout.
Transforming how we practice medicine is the only viable solution. I have good news: You are the leaders you have been waiting for.
Continue to: Physicians make good managers...
Physicians make good managers
To successfully transform how we practice medicine, it is critical that those leading the transformation deeply understand how medicine is practiced. The level of understanding required can be achieved only through years of medical practice, as a doctor. We understand how medical teams interact and that different sectors of our health care system are interdependent. Also, because physicians drive patient activity and ultimately reimbursement, having a seat at the table is crucial.
Some health care systems are run by businesspeople—people with finance backgrounds—and others are led by physicians. In 2017, Becker’s Hospital Review listed the chief executive officers (CEOs) of 183 nonprofit hospital and health systems.10 Of these, only 25% were led by individuals with an MD. Looking at the 115 largest hospitals in the United States, 30% are physician led.10 Considering the top 10 hospitals ranked by U.S. News & World Report for 2022, 8 of 10 have a physician at the helm.
Beyond raters and rankers, physician-led hospitals do better. Goodall compared CEOs in the top 100 best hospitals in U.S. News & World Report in 3 key medical specialties: cancer, digestive disorders, and cardiac care.11 The study explored the question: “Are hospitals’ quality ranked more highly when they are led by a medically trained doctor or non-MD professional managers?”11 Analysis revealed that hospital quality scores are about 25% higher in physician-run hospitals than in manager-run hospitals.11 Additional research shows that good management practices correlate with hospital performance, and that “the proportion of managers with a clinical degree has the largest positive effect.”12
Several theories exist as to why doctors make good managers in the health care setting.13,14 Doctors may create a more sympathetic and productive work environment for other clinicians because they are one of them. They have peer-to-peer credibility—because they have walked the walk, they have insight and perspective into how medicine is practiced.
Physicians serve as effective change agents for their organizations in several ways:
- First, physicians take a clinical approach in their leadership roles13 and focus on patient care at the center of their decisions. We see the people behind the numbers. Simply put, we humanize the operational side of health care.
- As physicians, we understand the interconnectivity in the practice of medicine. While closing certain service lines may be financially beneficial, these services are often closely linked to profitable service lines.
- Beyond physicians taking a clinical approach to leadership, we emphasize quality.13 Because we all have experienced complications and lived through bad outcomes alongside our patients, we understand deeply how important patient safety and quality is, and we are not willing to sacrifice that for financial gain. For us, this is personal. We don’t see our solution to health care challenges as an “or” situation, instead we view it as an “and” situation.
- Physician leaders often can improve medical staff engagement.13 A 2018 national survey of physicians found that those who are satisfied with their leadership are more engaged at work, have greater job satisfaction, and are less likely to experience signs of burnout.15 Physician administrators add value here.
Continue to: Surgeons as leaders...
Surgeons as leaders
What do we know about surgeons as physician leaders? Looking at the previously mentioned lists of physician leaders, surgeons are relatively absent. In the Becker’s Hospital Review study of nonprofit hospitals, only 9% of CEOs were surgeons.10 In addition, when reviewing data that associated physician leaders and hospital performance, only 3 of the CEOs were surgeons.11 Given that surgeons make up approximately 19% of US physicians, we are underrepresented.
The omission of surgeons as leaders seems inappropriate given that most hospitals are financially reliant on revenue related to surgical care and optimizing this space is an enormous opportunity. Berger and colleagues offered 3 theories as to why there are fewer surgeon leaders16:
- The relative pay of surgeons exceeds that of most other specialties, and there may be less incentive to accept the challenges presented by leadership roles. (I will add that surgeon leadership is more costly to a system.)
- The craftsmanship nature of surgery discourages the development of other career interests beginning at the trainee level.
- Surgeons have been perceived stereotypically to exhibit arrogance, a characteristic that others may not warm to.
This last observation stings. Successful leadership takes social skill and teamwork.14 Although medical care is one of the few disciplines in which lack of teamwork might cost lives, physicians are not trained to be team players. We recognize how our training has led us to be lone wolves or gunners, situations where we as individuals had to beat others to secure our spot. We have been trained in command-and-control environments, in stepping up as a leader in highly stressful situations. This part of surgical culture may handicap surgeons in their quest to be health care leaders.
Other traits, however, make us particularly great leaders in health care. Our desire to succeed, willingness to push ourselves to extremes, ability to laser focus on a task, acceptance of delayed gratification, and aptitude for making timely decisions on limited data help us succeed in leadership roles. Seven years of surgical training helped me develop the grit I use every day in the C-suite.
We need more physician and surgeon leadership to thrive in the challenging health care landscape. Berger and colleagues proposed 3 potential solutions to increase the number of surgeons in hospital leadership positions16:
Nurture future surgical leaders through exposure to management training. Given the contribution to both expense in support services and resources and revenue related to surgical care, each organization needs a content expert to guide these decisions.
Recognize the important contributions that surgeons already make regarding quality, safety, and operational efficiency. An excellent example of this is the American College of Surgeons National Surgical Quality Improvement Program. Because surgeons are content experts in this area, we are primed to lead.
Hospitals, medical schools, and academic departments of surgery should recognize administrative efforts as an important part of the overall academic mission. As the adage states, “No margin, no mission.” We need bright minds to preserve and grow our margins so that we can further invest in our missions.
This is not easy. Given the barriers, this will not happen organically. Charan and colleagues provided an outline for a leadership pathway adapted for physicians (FIGURE).17,18 It starts with the individual practitioner who is a practicing physician and spends most of their time focused on patient care. As a physician becomes more interested in leadership, they develop new skills and take on more and more responsibility. As they increase in leadership responsibility, they tend to reduce clinical time and increase time spent on strategic and business management. This framework creates a pipeline so that physicians and surgeons can be developed strategically and given increasing responsibility as they develop their capabilities and expand their skill sets.
The leadership challenge
To thrive, we must transform health care by changing how we practice medicine. As ObGyns, we are the leaders we have been waiting for. As you ponder your future, think of your current career and the opportunities you might have. Do you have a seat at the table? What table is that? How are you using your knowledge, expertise, and privilege to advance health care and medicine? I challenge you to critically evaluate this—and lead. ●
- Law T. Ohio bill suggests doctors who perform abortions could face jail, unless they perform a non-existent treatment. December 1, 2019. Time. Accessed June 12, 2023. https://time.com/5742053 /ectopic-pregnancy-ohio-abortion-bill/
- Grossman D. Ohio abortion, ectopic pregnancy bill: ‘it’s both bad medicine and bad law-making.’ May 21, 2019. Cincinnati.com–The Enquirer. Accessed June 12, 2023. https://www .cincinnati.com/story/opinion/2019/05/21/ohio-abortion-bill -john-becker-daniel-grossman-ectopic-pregnancy-false-medicine /3753610002/
- Lobbyist had hand in bill sparking ectopic pregnancy flap. December 11, 2019. Associated Press. Accessed June 12, 2023. https://apnews .com/article/03216e44405fa184ae0ab80fa85089f8
- NHE fact sheet. CMS.gov. Updated February 17, 2023. Accessed June 12, 2023. https://www.cms.gov/research-statistics-data-and -systems/statistics-trends-and-reports/nationalhealthexpenddata /nhe-fact-sheet
- Senate passes omnibus spending bill with health provisions. December 23, 2022. American Hospital Association. Accessed June 12, 2023. https://www.aha.org/special-bulletin/2022-12-20-appropriations -committees-release-omnibus-spending-bill-health-provisions
- Medicare updates compared to inflation (2001-2021). October 2021. American Medical Association. Accessed June 12, 2023. https://www .ama-assn.org/system/files/medicare-pay-chart-2021.pdf
- Resneck Jr J. Medicare physician payment reform is long overdue. October 3, 2022. American Medical Association. Accessed June 7, 2023. https://www.ama-assn.org/about/leadership /medicare-physician-payment-reform-long-overdue
- Isenberg M. The stark reality of physician reimbursement. August 24, 2022. Zotec Partners. Accessed June 13, 2023. https://zotecpartners. com/advocacy-zpac/test-1/
- Nguyen A. Mapping healthcare deserts: 80% of the country lacks adequate access to healthcare. September 9, 2021. GoodRx Health. Accessed June 13, 2023. https://www.goodrx.com/healthcare -access/research/healthcare-deserts-80-percent-of-country-lacks -adequate-healthcare-access
- 183 nonprofit hospital and health system CEOs to know–2017. Updated June 20, 2018. Becker’s Hospital Review. Accessed June 7, 2023. https://www.beckershospitalreview.com/lists/188-nonprofit -hospital-and-health-system-ceos-to-know-2017.html
- Goodall AH. Physician-leaders and hospital performance: is there an association? Soc Sci Med. 2011;73:535-539. doi:10.1016 /j.socscimed.2011.06.025
- Bloom N, Sadun R, Van Reenen J. Does Management Matter in Healthcare? Center for Economic Performance and Harvard Business School; 2014.
- Turner J. Why healthcare C-suites should include physicians. September 3, 2019. Managed Healthcare Executive. Accessed June 13, 2023. https://www.managedhealthcareexecutive.com /view/why-healthcare-c-suites-should-include-physicians
- Stoller JK, Goodall A, Baker A. Why the best hospitals are managed by doctors. December 27, 2016. Harvard Business Review. Accessed June 13, 2023. https://hbr.org/2016/12/why-the-best-hospitals -are-managed-by-doctors
- Hayhurst C. Data confirms: leaders, physician burnout is on you. April 3, 2019. Aetnahealth. Accessed June 13, 2023. https://www .athenahealth.com/knowledge-hub/practice-management /research-confirms-leaders-burnout-you
- Berger DH, Goodall A, Tsai AY. The importance of increasing surgeon participation in hospital leadership. JAMA Surg. 2019;154:281-282. doi:10.1001/jamasurg.2018.5080
- Charan R, Drotter S, Noel J. The Leadership Pipeline: How to Build the Leadership-Powered Company. Jossey-Bass; 2001.
- Perry J, Mobley F, Brubaker M. Most doctors have little or no management training, and that’s a problem. December 15, 2017. Harvard Business Review. Accessed June 7, 2023. https://hbr.org/2017/12 /most-doctors-have-little-or-no-management-training-and-thats -a-problem

“If you don’t have a seat at the table, you are probably on the menu.” I first heard this quote in 2013, and it launched my interest in health care leadership and influenced me countless times over the last 10 years.
As Chief of Staff at Cleveland Clinic, I oversee nearly 5,000 physicians and scientists across the globe. I am involved in the physician life cycle: recruiting, hiring, privileging and credentialing, talent development, promotion, professionalism, and career transitions. I also sit at the intersection of medical care and the business of medicine. This means leading 18 clinical service lines responsible for 5.6 million visits, 161,000 surgeries, and billions of dollars in operating revenue per year. How I spend most of my time is a far cry from what I spent 11 years’ training to do—gynecologic surgery. This shift in my career was not because I changed my mind about caring for patients or that I tired of being a full-time surgeon. Nothing could be further from the truth. Women’s health remains my “why,” and my leadership journey has taught me that it is critical to have a seat at the table for the sake of ObGyns and women everywhere.
Women’s health on the menu
I will start with a concrete example of when we, as women and ObGyns, were on the menu. In late 2019, the Ohio state House of Representatives introduced a bill that subjected doctors to potential murder charges if they did not try everything to save the life of a mother and fetus, “including attempting to reimplant an ectopic pregnancy into the woman’s uterus.”1 This bill was based on 2 case reports—one from 1915 and one from 1980—which were both low quality, and the latter case was deemed to be fraudulent.2 How did this happen?
An Ohio state representative developed the bill with help from a lobbyist and without input from physicians or content experts. When asked, the representative shared that “he never researched whether re-implanting an ectopic pregnancy into a woman’s uterus was a viable medical procedure before including it in the bill.”3 He added, “I heard about it over the years. I never questioned it or gave it a lot of thought.”3
This example resonates deeply with many of us; it inspires us to speak up and act. As ObGyns, we clearly understand the consequences of legal and regulatory change in women’s health and how it directly impacts our patients and each of us as physicians. Let’s shift to something that you may feel less passion about, but I believe is equally important. This is where obstetrician-gynecologists sit in the intersection of medical care and business. This is the space where I spend most of my time, and from this vantage point, I worry about our field.
The business of medicine
Starting at the macroeconomic level, let’s think about how we as physicians are reimbursed and who makes these decisions. Looking at the national health care expenditure data, Medicare and Medicaid spending makes up nearly 40% of the total spend, and it is growing.4 Additionally, private health insurance tends to follow Centers for Medicare and Medicaid Services (CMS) decision making, further compounding its influence.4 In simple terms, CMS decides what is covered and how much we are paid. Whether you are in a solo private practice, an employer health care organization, or an academic medical center, physician reimbursement is declining.
In fact, Congress passed its year-end omnibus legislation in the final days of 2022, including a 2% Medicare physician payment cut for 2023,5 at a time when expenses to practice medicine, including nonphysician staff and supplies, are at an all-time high and we are living in a 6% inflationary state. This translates into being asked to serve more patients and cut costs. Our day-to-day feels much tighter, and this is why: Medicare physician pay increased just 11% over the past 20 years6 (2001–2021) in comparison to the cost of running a medical practice, which increased nearly 40% during that time. In other words, adjusting for inflation in practice costs, Medicare physician payment has fallen 22% over the last 20 years.7
Depending on your employment model, you may feel insulated from these changes as increases in reimbursement have occurred in other areas, such as hospitals and ambulatory surgery centers.8 In the short term, these increases help, as organizations will see additional funds. But there are 2 main issues: First, it is not nearly enough when you consider the soaring costs of running a hospital. And second, looking at our national population, we rely tremendously on self-employed doctors to serve our patients.
More than 80% of US counties lack adequate health care infrastructure.9 More than a third of the US population has less-than-adequate access to pharmacies, primary care physicians, hospitals, trauma centers, and low-cost health centers.9 To put things into perspective, more than 20% of counties in the United States are hospital deserts, where most people must drive more than 30 minutes to reach the closest hospital.9
There is good reason for this. Operating a hospital is a challenging endeavor. Even before the COVID-19 pandemic and the most recent health care financial challenges, most health care systems and large hospitals operated with very low operating margins (2%–3%). Businesses with similar margins include grocery stores and car dealerships. These low-margin businesses, including health care, rely on high volume for sustainability. High patient volumes distribute expensive hospital costs over many encounters. If physicians cannot sustain practices across the country, it is challenging to have sufficient admission and surgical volumes to justify the cost base of hospitals.
To tie this together, we have very little influence on what we are paid for our services. Reimbursement is declining, which makes it hard to have financially sustainable practices. As hospitals struggle, there is more pressure to prioritize highly profitable service lines, like orthopedics and urology, which are associated with favorable technical revenue. As hospitals are threatened, health care deserts widen, which leaves our entire health care system in jeopardy. Not surprisingly, this most likely affects those who face additional barriers to access, such as those with lower income, limited internet access, and lack of insurance. Together, these barriers further widen disparities in health care outcomes, including outcomes for women. Additionally, this death by a thousand cuts has eroded morale and increased physician burnout.
Transforming how we practice medicine is the only viable solution. I have good news: You are the leaders you have been waiting for.
Continue to: Physicians make good managers...
Physicians make good managers
To successfully transform how we practice medicine, it is critical that those leading the transformation deeply understand how medicine is practiced. The level of understanding required can be achieved only through years of medical practice, as a doctor. We understand how medical teams interact and that different sectors of our health care system are interdependent. Also, because physicians drive patient activity and ultimately reimbursement, having a seat at the table is crucial.
Some health care systems are run by businesspeople—people with finance backgrounds—and others are led by physicians. In 2017, Becker’s Hospital Review listed the chief executive officers (CEOs) of 183 nonprofit hospital and health systems.10 Of these, only 25% were led by individuals with an MD. Looking at the 115 largest hospitals in the United States, 30% are physician led.10 Considering the top 10 hospitals ranked by U.S. News & World Report for 2022, 8 of 10 have a physician at the helm.
Beyond raters and rankers, physician-led hospitals do better. Goodall compared CEOs in the top 100 best hospitals in U.S. News & World Report in 3 key medical specialties: cancer, digestive disorders, and cardiac care.11 The study explored the question: “Are hospitals’ quality ranked more highly when they are led by a medically trained doctor or non-MD professional managers?”11 Analysis revealed that hospital quality scores are about 25% higher in physician-run hospitals than in manager-run hospitals.11 Additional research shows that good management practices correlate with hospital performance, and that “the proportion of managers with a clinical degree has the largest positive effect.”12
Several theories exist as to why doctors make good managers in the health care setting.13,14 Doctors may create a more sympathetic and productive work environment for other clinicians because they are one of them. They have peer-to-peer credibility—because they have walked the walk, they have insight and perspective into how medicine is practiced.
Physicians serve as effective change agents for their organizations in several ways:
- First, physicians take a clinical approach in their leadership roles13 and focus on patient care at the center of their decisions. We see the people behind the numbers. Simply put, we humanize the operational side of health care.
- As physicians, we understand the interconnectivity in the practice of medicine. While closing certain service lines may be financially beneficial, these services are often closely linked to profitable service lines.
- Beyond physicians taking a clinical approach to leadership, we emphasize quality.13 Because we all have experienced complications and lived through bad outcomes alongside our patients, we understand deeply how important patient safety and quality is, and we are not willing to sacrifice that for financial gain. For us, this is personal. We don’t see our solution to health care challenges as an “or” situation, instead we view it as an “and” situation.
- Physician leaders often can improve medical staff engagement.13 A 2018 national survey of physicians found that those who are satisfied with their leadership are more engaged at work, have greater job satisfaction, and are less likely to experience signs of burnout.15 Physician administrators add value here.
Continue to: Surgeons as leaders...
Surgeons as leaders
What do we know about surgeons as physician leaders? Looking at the previously mentioned lists of physician leaders, surgeons are relatively absent. In the Becker’s Hospital Review study of nonprofit hospitals, only 9% of CEOs were surgeons.10 In addition, when reviewing data that associated physician leaders and hospital performance, only 3 of the CEOs were surgeons.11 Given that surgeons make up approximately 19% of US physicians, we are underrepresented.
The omission of surgeons as leaders seems inappropriate given that most hospitals are financially reliant on revenue related to surgical care and optimizing this space is an enormous opportunity. Berger and colleagues offered 3 theories as to why there are fewer surgeon leaders16:
- The relative pay of surgeons exceeds that of most other specialties, and there may be less incentive to accept the challenges presented by leadership roles. (I will add that surgeon leadership is more costly to a system.)
- The craftsmanship nature of surgery discourages the development of other career interests beginning at the trainee level.
- Surgeons have been perceived stereotypically to exhibit arrogance, a characteristic that others may not warm to.
This last observation stings. Successful leadership takes social skill and teamwork.14 Although medical care is one of the few disciplines in which lack of teamwork might cost lives, physicians are not trained to be team players. We recognize how our training has led us to be lone wolves or gunners, situations where we as individuals had to beat others to secure our spot. We have been trained in command-and-control environments, in stepping up as a leader in highly stressful situations. This part of surgical culture may handicap surgeons in their quest to be health care leaders.
Other traits, however, make us particularly great leaders in health care. Our desire to succeed, willingness to push ourselves to extremes, ability to laser focus on a task, acceptance of delayed gratification, and aptitude for making timely decisions on limited data help us succeed in leadership roles. Seven years of surgical training helped me develop the grit I use every day in the C-suite.
We need more physician and surgeon leadership to thrive in the challenging health care landscape. Berger and colleagues proposed 3 potential solutions to increase the number of surgeons in hospital leadership positions16:
Nurture future surgical leaders through exposure to management training. Given the contribution to both expense in support services and resources and revenue related to surgical care, each organization needs a content expert to guide these decisions.
Recognize the important contributions that surgeons already make regarding quality, safety, and operational efficiency. An excellent example of this is the American College of Surgeons National Surgical Quality Improvement Program. Because surgeons are content experts in this area, we are primed to lead.
Hospitals, medical schools, and academic departments of surgery should recognize administrative efforts as an important part of the overall academic mission. As the adage states, “No margin, no mission.” We need bright minds to preserve and grow our margins so that we can further invest in our missions.
This is not easy. Given the barriers, this will not happen organically. Charan and colleagues provided an outline for a leadership pathway adapted for physicians (FIGURE).17,18 It starts with the individual practitioner who is a practicing physician and spends most of their time focused on patient care. As a physician becomes more interested in leadership, they develop new skills and take on more and more responsibility. As they increase in leadership responsibility, they tend to reduce clinical time and increase time spent on strategic and business management. This framework creates a pipeline so that physicians and surgeons can be developed strategically and given increasing responsibility as they develop their capabilities and expand their skill sets.

The leadership challenge
To thrive, we must transform health care by changing how we practice medicine. As ObGyns, we are the leaders we have been waiting for. As you ponder your future, think of your current career and the opportunities you might have. Do you have a seat at the table? What table is that? How are you using your knowledge, expertise, and privilege to advance health care and medicine? I challenge you to critically evaluate this—and lead. ●

“If you don’t have a seat at the table, you are probably on the menu.” I first heard this quote in 2013, and it launched my interest in health care leadership and influenced me countless times over the last 10 years.
As Chief of Staff at Cleveland Clinic, I oversee nearly 5,000 physicians and scientists across the globe. I am involved in the physician life cycle: recruiting, hiring, privileging and credentialing, talent development, promotion, professionalism, and career transitions. I also sit at the intersection of medical care and the business of medicine. This means leading 18 clinical service lines responsible for 5.6 million visits, 161,000 surgeries, and billions of dollars in operating revenue per year. How I spend most of my time is a far cry from what I spent 11 years’ training to do—gynecologic surgery. This shift in my career was not because I changed my mind about caring for patients or that I tired of being a full-time surgeon. Nothing could be further from the truth. Women’s health remains my “why,” and my leadership journey has taught me that it is critical to have a seat at the table for the sake of ObGyns and women everywhere.
Women’s health on the menu
I will start with a concrete example of when we, as women and ObGyns, were on the menu. In late 2019, the Ohio state House of Representatives introduced a bill that subjected doctors to potential murder charges if they did not try everything to save the life of a mother and fetus, “including attempting to reimplant an ectopic pregnancy into the woman’s uterus.”1 This bill was based on 2 case reports—one from 1915 and one from 1980—which were both low quality, and the latter case was deemed to be fraudulent.2 How did this happen?
An Ohio state representative developed the bill with help from a lobbyist and without input from physicians or content experts. When asked, the representative shared that “he never researched whether re-implanting an ectopic pregnancy into a woman’s uterus was a viable medical procedure before including it in the bill.”3 He added, “I heard about it over the years. I never questioned it or gave it a lot of thought.”3
This example resonates deeply with many of us; it inspires us to speak up and act. As ObGyns, we clearly understand the consequences of legal and regulatory change in women’s health and how it directly impacts our patients and each of us as physicians. Let’s shift to something that you may feel less passion about, but I believe is equally important. This is where obstetrician-gynecologists sit in the intersection of medical care and business. This is the space where I spend most of my time, and from this vantage point, I worry about our field.
The business of medicine
Starting at the macroeconomic level, let’s think about how we as physicians are reimbursed and who makes these decisions. Looking at the national health care expenditure data, Medicare and Medicaid spending makes up nearly 40% of the total spend, and it is growing.4 Additionally, private health insurance tends to follow Centers for Medicare and Medicaid Services (CMS) decision making, further compounding its influence.4 In simple terms, CMS decides what is covered and how much we are paid. Whether you are in a solo private practice, an employer health care organization, or an academic medical center, physician reimbursement is declining.
In fact, Congress passed its year-end omnibus legislation in the final days of 2022, including a 2% Medicare physician payment cut for 2023,5 at a time when expenses to practice medicine, including nonphysician staff and supplies, are at an all-time high and we are living in a 6% inflationary state. This translates into being asked to serve more patients and cut costs. Our day-to-day feels much tighter, and this is why: Medicare physician pay increased just 11% over the past 20 years6 (2001–2021) in comparison to the cost of running a medical practice, which increased nearly 40% during that time. In other words, adjusting for inflation in practice costs, Medicare physician payment has fallen 22% over the last 20 years.7
Depending on your employment model, you may feel insulated from these changes as increases in reimbursement have occurred in other areas, such as hospitals and ambulatory surgery centers.8 In the short term, these increases help, as organizations will see additional funds. But there are 2 main issues: First, it is not nearly enough when you consider the soaring costs of running a hospital. And second, looking at our national population, we rely tremendously on self-employed doctors to serve our patients.
More than 80% of US counties lack adequate health care infrastructure.9 More than a third of the US population has less-than-adequate access to pharmacies, primary care physicians, hospitals, trauma centers, and low-cost health centers.9 To put things into perspective, more than 20% of counties in the United States are hospital deserts, where most people must drive more than 30 minutes to reach the closest hospital.9
There is good reason for this. Operating a hospital is a challenging endeavor. Even before the COVID-19 pandemic and the most recent health care financial challenges, most health care systems and large hospitals operated with very low operating margins (2%–3%). Businesses with similar margins include grocery stores and car dealerships. These low-margin businesses, including health care, rely on high volume for sustainability. High patient volumes distribute expensive hospital costs over many encounters. If physicians cannot sustain practices across the country, it is challenging to have sufficient admission and surgical volumes to justify the cost base of hospitals.
To tie this together, we have very little influence on what we are paid for our services. Reimbursement is declining, which makes it hard to have financially sustainable practices. As hospitals struggle, there is more pressure to prioritize highly profitable service lines, like orthopedics and urology, which are associated with favorable technical revenue. As hospitals are threatened, health care deserts widen, which leaves our entire health care system in jeopardy. Not surprisingly, this most likely affects those who face additional barriers to access, such as those with lower income, limited internet access, and lack of insurance. Together, these barriers further widen disparities in health care outcomes, including outcomes for women. Additionally, this death by a thousand cuts has eroded morale and increased physician burnout.
Transforming how we practice medicine is the only viable solution. I have good news: You are the leaders you have been waiting for.
Continue to: Physicians make good managers...
Physicians make good managers
To successfully transform how we practice medicine, it is critical that those leading the transformation deeply understand how medicine is practiced. The level of understanding required can be achieved only through years of medical practice, as a doctor. We understand how medical teams interact and that different sectors of our health care system are interdependent. Also, because physicians drive patient activity and ultimately reimbursement, having a seat at the table is crucial.
Some health care systems are run by businesspeople—people with finance backgrounds—and others are led by physicians. In 2017, Becker’s Hospital Review listed the chief executive officers (CEOs) of 183 nonprofit hospital and health systems.10 Of these, only 25% were led by individuals with an MD. Looking at the 115 largest hospitals in the United States, 30% are physician led.10 Considering the top 10 hospitals ranked by U.S. News & World Report for 2022, 8 of 10 have a physician at the helm.
Beyond raters and rankers, physician-led hospitals do better. Goodall compared CEOs in the top 100 best hospitals in U.S. News & World Report in 3 key medical specialties: cancer, digestive disorders, and cardiac care.11 The study explored the question: “Are hospitals’ quality ranked more highly when they are led by a medically trained doctor or non-MD professional managers?”11 Analysis revealed that hospital quality scores are about 25% higher in physician-run hospitals than in manager-run hospitals.11 Additional research shows that good management practices correlate with hospital performance, and that “the proportion of managers with a clinical degree has the largest positive effect.”12
Several theories exist as to why doctors make good managers in the health care setting.13,14 Doctors may create a more sympathetic and productive work environment for other clinicians because they are one of them. They have peer-to-peer credibility—because they have walked the walk, they have insight and perspective into how medicine is practiced.
Physicians serve as effective change agents for their organizations in several ways:
- First, physicians take a clinical approach in their leadership roles13 and focus on patient care at the center of their decisions. We see the people behind the numbers. Simply put, we humanize the operational side of health care.
- As physicians, we understand the interconnectivity in the practice of medicine. While closing certain service lines may be financially beneficial, these services are often closely linked to profitable service lines.
- Beyond physicians taking a clinical approach to leadership, we emphasize quality.13 Because we all have experienced complications and lived through bad outcomes alongside our patients, we understand deeply how important patient safety and quality is, and we are not willing to sacrifice that for financial gain. For us, this is personal. We don’t see our solution to health care challenges as an “or” situation, instead we view it as an “and” situation.
- Physician leaders often can improve medical staff engagement.13 A 2018 national survey of physicians found that those who are satisfied with their leadership are more engaged at work, have greater job satisfaction, and are less likely to experience signs of burnout.15 Physician administrators add value here.
Continue to: Surgeons as leaders...
Surgeons as leaders
What do we know about surgeons as physician leaders? Looking at the previously mentioned lists of physician leaders, surgeons are relatively absent. In the Becker’s Hospital Review study of nonprofit hospitals, only 9% of CEOs were surgeons.10 In addition, when reviewing data that associated physician leaders and hospital performance, only 3 of the CEOs were surgeons.11 Given that surgeons make up approximately 19% of US physicians, we are underrepresented.
The omission of surgeons as leaders seems inappropriate given that most hospitals are financially reliant on revenue related to surgical care and optimizing this space is an enormous opportunity. Berger and colleagues offered 3 theories as to why there are fewer surgeon leaders16:
- The relative pay of surgeons exceeds that of most other specialties, and there may be less incentive to accept the challenges presented by leadership roles. (I will add that surgeon leadership is more costly to a system.)
- The craftsmanship nature of surgery discourages the development of other career interests beginning at the trainee level.
- Surgeons have been perceived stereotypically to exhibit arrogance, a characteristic that others may not warm to.
This last observation stings. Successful leadership takes social skill and teamwork.14 Although medical care is one of the few disciplines in which lack of teamwork might cost lives, physicians are not trained to be team players. We recognize how our training has led us to be lone wolves or gunners, situations where we as individuals had to beat others to secure our spot. We have been trained in command-and-control environments, in stepping up as a leader in highly stressful situations. This part of surgical culture may handicap surgeons in their quest to be health care leaders.
Other traits, however, make us particularly great leaders in health care. Our desire to succeed, willingness to push ourselves to extremes, ability to laser focus on a task, acceptance of delayed gratification, and aptitude for making timely decisions on limited data help us succeed in leadership roles. Seven years of surgical training helped me develop the grit I use every day in the C-suite.
We need more physician and surgeon leadership to thrive in the challenging health care landscape. Berger and colleagues proposed 3 potential solutions to increase the number of surgeons in hospital leadership positions16:
Nurture future surgical leaders through exposure to management training. Given the contribution to both expense in support services and resources and revenue related to surgical care, each organization needs a content expert to guide these decisions.
Recognize the important contributions that surgeons already make regarding quality, safety, and operational efficiency. An excellent example of this is the American College of Surgeons National Surgical Quality Improvement Program. Because surgeons are content experts in this area, we are primed to lead.
Hospitals, medical schools, and academic departments of surgery should recognize administrative efforts as an important part of the overall academic mission. As the adage states, “No margin, no mission.” We need bright minds to preserve and grow our margins so that we can further invest in our missions.
This is not easy. Given the barriers, this will not happen organically. Charan and colleagues provided an outline for a leadership pathway adapted for physicians (FIGURE).17,18 It starts with the individual practitioner who is a practicing physician and spends most of their time focused on patient care. As a physician becomes more interested in leadership, they develop new skills and take on more and more responsibility. As they increase in leadership responsibility, they tend to reduce clinical time and increase time spent on strategic and business management. This framework creates a pipeline so that physicians and surgeons can be developed strategically and given increasing responsibility as they develop their capabilities and expand their skill sets.

The leadership challenge
To thrive, we must transform health care by changing how we practice medicine. As ObGyns, we are the leaders we have been waiting for. As you ponder your future, think of your current career and the opportunities you might have. Do you have a seat at the table? What table is that? How are you using your knowledge, expertise, and privilege to advance health care and medicine? I challenge you to critically evaluate this—and lead. ●
- Law T. Ohio bill suggests doctors who perform abortions could face jail, unless they perform a non-existent treatment. December 1, 2019. Time. Accessed June 12, 2023. https://time.com/5742053 /ectopic-pregnancy-ohio-abortion-bill/
- Grossman D. Ohio abortion, ectopic pregnancy bill: ‘it’s both bad medicine and bad law-making.’ May 21, 2019. Cincinnati.com–The Enquirer. Accessed June 12, 2023. https://www .cincinnati.com/story/opinion/2019/05/21/ohio-abortion-bill -john-becker-daniel-grossman-ectopic-pregnancy-false-medicine /3753610002/
- Lobbyist had hand in bill sparking ectopic pregnancy flap. December 11, 2019. Associated Press. Accessed June 12, 2023. https://apnews .com/article/03216e44405fa184ae0ab80fa85089f8
- NHE fact sheet. CMS.gov. Updated February 17, 2023. Accessed June 12, 2023. https://www.cms.gov/research-statistics-data-and -systems/statistics-trends-and-reports/nationalhealthexpenddata /nhe-fact-sheet
- Senate passes omnibus spending bill with health provisions. December 23, 2022. American Hospital Association. Accessed June 12, 2023. https://www.aha.org/special-bulletin/2022-12-20-appropriations -committees-release-omnibus-spending-bill-health-provisions
- Medicare updates compared to inflation (2001-2021). October 2021. American Medical Association. Accessed June 12, 2023. https://www .ama-assn.org/system/files/medicare-pay-chart-2021.pdf
- Resneck Jr J. Medicare physician payment reform is long overdue. October 3, 2022. American Medical Association. Accessed June 7, 2023. https://www.ama-assn.org/about/leadership /medicare-physician-payment-reform-long-overdue
- Isenberg M. The stark reality of physician reimbursement. August 24, 2022. Zotec Partners. Accessed June 13, 2023. https://zotecpartners. com/advocacy-zpac/test-1/
- Nguyen A. Mapping healthcare deserts: 80% of the country lacks adequate access to healthcare. September 9, 2021. GoodRx Health. Accessed June 13, 2023. https://www.goodrx.com/healthcare -access/research/healthcare-deserts-80-percent-of-country-lacks -adequate-healthcare-access
- 183 nonprofit hospital and health system CEOs to know–2017. Updated June 20, 2018. Becker’s Hospital Review. Accessed June 7, 2023. https://www.beckershospitalreview.com/lists/188-nonprofit -hospital-and-health-system-ceos-to-know-2017.html
- Goodall AH. Physician-leaders and hospital performance: is there an association? Soc Sci Med. 2011;73:535-539. doi:10.1016 /j.socscimed.2011.06.025
- Bloom N, Sadun R, Van Reenen J. Does Management Matter in Healthcare? Center for Economic Performance and Harvard Business School; 2014.
- Turner J. Why healthcare C-suites should include physicians. September 3, 2019. Managed Healthcare Executive. Accessed June 13, 2023. https://www.managedhealthcareexecutive.com /view/why-healthcare-c-suites-should-include-physicians
- Stoller JK, Goodall A, Baker A. Why the best hospitals are managed by doctors. December 27, 2016. Harvard Business Review. Accessed June 13, 2023. https://hbr.org/2016/12/why-the-best-hospitals -are-managed-by-doctors
- Hayhurst C. Data confirms: leaders, physician burnout is on you. April 3, 2019. Aetnahealth. Accessed June 13, 2023. https://www .athenahealth.com/knowledge-hub/practice-management /research-confirms-leaders-burnout-you
- Berger DH, Goodall A, Tsai AY. The importance of increasing surgeon participation in hospital leadership. JAMA Surg. 2019;154:281-282. doi:10.1001/jamasurg.2018.5080
- Charan R, Drotter S, Noel J. The Leadership Pipeline: How to Build the Leadership-Powered Company. Jossey-Bass; 2001.
- Perry J, Mobley F, Brubaker M. Most doctors have little or no management training, and that’s a problem. December 15, 2017. Harvard Business Review. Accessed June 7, 2023. https://hbr.org/2017/12 /most-doctors-have-little-or-no-management-training-and-thats -a-problem
- Law T. Ohio bill suggests doctors who perform abortions could face jail, unless they perform a non-existent treatment. December 1, 2019. Time. Accessed June 12, 2023. https://time.com/5742053 /ectopic-pregnancy-ohio-abortion-bill/
- Grossman D. Ohio abortion, ectopic pregnancy bill: ‘it’s both bad medicine and bad law-making.’ May 21, 2019. Cincinnati.com–The Enquirer. Accessed June 12, 2023. https://www .cincinnati.com/story/opinion/2019/05/21/ohio-abortion-bill -john-becker-daniel-grossman-ectopic-pregnancy-false-medicine /3753610002/
- Lobbyist had hand in bill sparking ectopic pregnancy flap. December 11, 2019. Associated Press. Accessed June 12, 2023. https://apnews .com/article/03216e44405fa184ae0ab80fa85089f8
- NHE fact sheet. CMS.gov. Updated February 17, 2023. Accessed June 12, 2023. https://www.cms.gov/research-statistics-data-and -systems/statistics-trends-and-reports/nationalhealthexpenddata /nhe-fact-sheet
- Senate passes omnibus spending bill with health provisions. December 23, 2022. American Hospital Association. Accessed June 12, 2023. https://www.aha.org/special-bulletin/2022-12-20-appropriations -committees-release-omnibus-spending-bill-health-provisions
- Medicare updates compared to inflation (2001-2021). October 2021. American Medical Association. Accessed June 12, 2023. https://www .ama-assn.org/system/files/medicare-pay-chart-2021.pdf
- Resneck Jr J. Medicare physician payment reform is long overdue. October 3, 2022. American Medical Association. Accessed June 7, 2023. https://www.ama-assn.org/about/leadership /medicare-physician-payment-reform-long-overdue
- Isenberg M. The stark reality of physician reimbursement. August 24, 2022. Zotec Partners. Accessed June 13, 2023. https://zotecpartners. com/advocacy-zpac/test-1/
- Nguyen A. Mapping healthcare deserts: 80% of the country lacks adequate access to healthcare. September 9, 2021. GoodRx Health. Accessed June 13, 2023. https://www.goodrx.com/healthcare -access/research/healthcare-deserts-80-percent-of-country-lacks -adequate-healthcare-access
- 183 nonprofit hospital and health system CEOs to know–2017. Updated June 20, 2018. Becker’s Hospital Review. Accessed June 7, 2023. https://www.beckershospitalreview.com/lists/188-nonprofit -hospital-and-health-system-ceos-to-know-2017.html
- Goodall AH. Physician-leaders and hospital performance: is there an association? Soc Sci Med. 2011;73:535-539. doi:10.1016 /j.socscimed.2011.06.025
- Bloom N, Sadun R, Van Reenen J. Does Management Matter in Healthcare? Center for Economic Performance and Harvard Business School; 2014.
- Turner J. Why healthcare C-suites should include physicians. September 3, 2019. Managed Healthcare Executive. Accessed June 13, 2023. https://www.managedhealthcareexecutive.com /view/why-healthcare-c-suites-should-include-physicians
- Stoller JK, Goodall A, Baker A. Why the best hospitals are managed by doctors. December 27, 2016. Harvard Business Review. Accessed June 13, 2023. https://hbr.org/2016/12/why-the-best-hospitals -are-managed-by-doctors
- Hayhurst C. Data confirms: leaders, physician burnout is on you. April 3, 2019. Aetnahealth. Accessed June 13, 2023. https://www .athenahealth.com/knowledge-hub/practice-management /research-confirms-leaders-burnout-you
- Berger DH, Goodall A, Tsai AY. The importance of increasing surgeon participation in hospital leadership. JAMA Surg. 2019;154:281-282. doi:10.1001/jamasurg.2018.5080
- Charan R, Drotter S, Noel J. The Leadership Pipeline: How to Build the Leadership-Powered Company. Jossey-Bass; 2001.
- Perry J, Mobley F, Brubaker M. Most doctors have little or no management training, and that’s a problem. December 15, 2017. Harvard Business Review. Accessed June 7, 2023. https://hbr.org/2017/12 /most-doctors-have-little-or-no-management-training-and-thats -a-problem
Surgical volume and outcomes for gynecologic surgery: Is more always better?
Over the last 3 decades, abundant evidence has demonstrated the association between surgical volume and outcomes. Patients operated on by high-volume surgeons and at high-volume hospitals have superior outcomes.
Surgical volume in gynecology
The association between both hospital and surgeon volume and outcomes has been explored across a number of gynecologic procedures.3 A meta-analysis that included 741,000 patients found that low-volume surgeons had an increased rate of complications overall, a higher rate of intraoperative complications, and a higher rate of postoperative complications compared with high-volume surgeons. While there was no association between volume and mortality overall, when limited to gynecologic oncology studies, low surgeon volume was associated with increased perioperative mortality.3
While these studies demonstrated a statistically significant association between surgeon volume and perioperative outcomes, the magnitude of the effect is modest compared with other higher-risk procedures associated with greater perioperative morbidity. For example, in a large study that examined oncologic and cardiovascular surgery, perioperative mortality in patients who underwent pancreatic resection was reduced from 15% for low-volume surgeons to 5% for high-volume surgeons.1 By contrast, for gynecologic surgery, complications occurred in 97 per 1,000 patients operated on by high-volume surgeons compared with between 114 and 137 per 1,000 for low-volume surgeons. Thus, to avoid 1 in-hospital complication, 30 surgeries performed by low-volume surgeons would need to be moved to high-volume surgeons. For intraoperative complications, 38 patients would need to be moved from low- to high-volume surgeons to prevent 1 such complication.3 In addition to morbidity and mortality, higher surgeon volume is associated with greater use of minimally invasive surgery, a lower likelihood of conversion to laparotomy, and lower costs.3
Similarly, hospital volume also has been associated with outcomes for gynecologic surgery.4 In a report of patients who underwent laparoscopic hysterectomy, the authors found that the complication rate was 18% lower for patients at high- versus low-volume hospitals. In addition, cost was lower at the high-volume centers.4 Like surgeon volume, the magnitude of the differential in outcomes between high- and low-volume hospitals is often modest.4
While most studies have focused on short-term outcomes, surgical volume appears also to be associated with longer-term outcomes. For gynecologic cancer, studies have demonstrated an association between hospital volume and survival for ovarian and cervical cancer.5-7 A large report of centers across the United States found that the 5-year survival rate increased from 39% for patients treated at low-volume centers to 51% at the highest-volume hospitals.5 In urogynecology, surgeon volume has been associated with midurethral sling revision. One study noted that after an individual surgeon performed 50 procedures a year, each additional case was associated with a decline in the rate of sling revision.8 One could argue that these longer-term end points may be the measures that matter most to patients.
Although the magnitude of the association between surgical volume and outcomes in gynecology appears to be relatively modest, outcomes for very-low-volume (VLV) surgeons are substantially worse. An analysis of more than 430,000 patients who underwent hysterectomy compared outcomes between VLV surgeons (characterized as surgeons who performed only 1 hysterectomy in the prior year) and other gynecologic surgeons. The overall complication rate was 32% in VLV surgeons compared with 10% among other surgeons, while the perioperative mortality rate was 2.5% versus 0.2% in the 2 groups, respectively. Likely reflecting changing practice patterns in gynecology, a sizable number of surgeons were classified as VLV physicians.9
Continue to: Public health applications of gynecologic surgical volume...
Public health applications of gynecologic surgical volume
The large body of literature on volume and outcomes has led to a number of public health initiatives aimed at reducing perioperative morbidity and mortality. Broadly, these efforts focus on regionalization of care, targeted quality improvement, and the development of minimum volume standards. Each strategy holds promise but also the potential to lead to unwanted consequences.
Regionalization of care
Recognition of the volume-outcomes paradigm has led to efforts to regionalize care for complex procedures to high-volume surgeons and centers.10 A cohort study of surgical patterns of care for Medicare recipients who underwent cancer resections or abdominal aortic aneurysm repair from 1999 to 2008 demonstrated these shifting practice patterns. For example, in 1999–2000, pancreatectomy was performed in 1,308 hospitals, with a median case volume of 5 procedures per year. By 2007–2008, the number of hospitals in which pancreatectomy was performed declined to 978, and the median case volume rose to 16 procedures per year. Importantly, over this time period, risk-adjusted mortality for pancreatectomy declined by 19%, and increased hospital volume was responsible for more than two-thirds of the decline in mortality.10
There has similarly been a gradual concentration of some gynecologic procedures to higher-volume surgeons and centers.11,12 Among patients undergoing hysterectomy for endometrial cancer in New York State, 845 surgeons with a mean case volume of 3 procedures per year treated patients in 2000. By 2014, the number of surgeons who performed these operations declined to 317 while mean annual case volume rose to 10 procedures per year. The number of hospitals in which women with endometrial cancer were treated declined from 182 to 98 over the same time period.11 Similar trends were noted for patients undergoing ovarian cancer resection.12 While patterns of gynecologic care for some surgical procedures have clearly changed, it has been more difficult to link these changes to improvements in outcomes.11,12
Despite the intuitive appeal of regionalization of surgical care, such a strategy has a number of limitations and practical challenges. Not surprisingly, limiting the number of surgeons and hospitals that perform a given procedure necessitates that patients travel a greater distance to obtain necessary surgical care.13,14 An analysis of endometrial cancer patients in New York State stratified patients based on their area of residence into 10 hospital referral regions (HRRs), which represent health care markets for tertiary medical care. From 2000 to 2014, the distance patients traveled to receive their surgical care increased in all of the HRRs studied. This was most pronounced in 1 of the HRRs in which the median travel distance rose by 47 miles over the 15-year period (FIGURE 1; FIGURE 2).14
Whether patients are willing to travel for care remains a matter of debate and depends on the disease, the surgical procedure, and the anticipated benefit associated with a longer travel distance.15,16 In a discrete choice experiment, 100 participants were given a hypothetical scenario in which they had potentially resectable pancreatic cancer; they were queried on their willingness to travel for care based on varying differences in mortality between a local and regional hospital.15 When mortality at the local hospital was double that of the regional hospital (6% vs 3%), 45% of patients chose to remain at the local hospital. When the differential increased to a 4 times greater mortality at the local hospital (12% vs 3%), 23% of patients still chose to remain at the local hospital.15
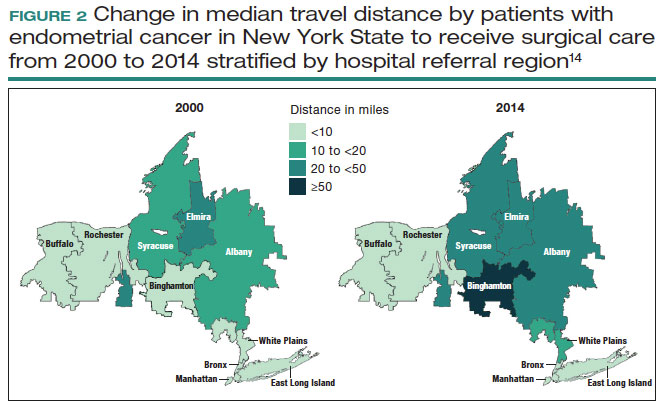
A similar study asked patients with ovarian neoplasms whether they would travel 50 miles to a regional center for surgery based on some degree of increased 5-year survival.16 Overall, 79% of patients would travel for a 4% improvement in survival while 97% would travel for a 12% improvement in survival.16
Lastly, a number of studies have shown that regionalization of surgical care disproportionately affects Black and Hispanic patients and those with low socioeconomic status.12,13,17 A simulation study on the effect of regionalizing care for pancreatectomy noted that using a hospital volume threshold of 20 procedures per year, a higher percentage of Black and Hispanic patients than White patients would be required to travel to a higher-volume center.13 Similarly, Medicaid recipients were more likely to be affected.13 Despite the inequities in who must travel for regionalized care, prior work has suggested that regionalization of cancer care to high-volume centers may reduce racial and socioeconomic disparities in survival for some cancers.18
Targeted quality improvement
Realizing the practical limitations of regionalization of care, an alternative strategy is to improve the quality of care at low-volume hospitals.5,19 Quality of care and surgical volume often are correlated, and the delivery of high-quality care can mitigate some of the influence of surgical volume on outcomes.
These principles were demonstrated in a study of more than 100,000 patients with ovarian cancer that stratified treating hospitals into volume quintiles.5 As expected, survival (both 2- and 5-year) was highest in the highest-volume quintile hospitals (FIGURE 3).5 Similarly, quality of care, measured through adherence to various process measures, was also highest in the highest-volume quintile hospitals. Interestingly, in the second-fourth volume quintile hospitals, there was substantial variation in adherence to quality metrics. Among hospitals with higher quality care, an improved survival was noted compared with lower quality care hospitals within the same volume quintile. Survival at high-quality, intermediate-volume hospitals approached that of the high-volume quintile hospitals.5
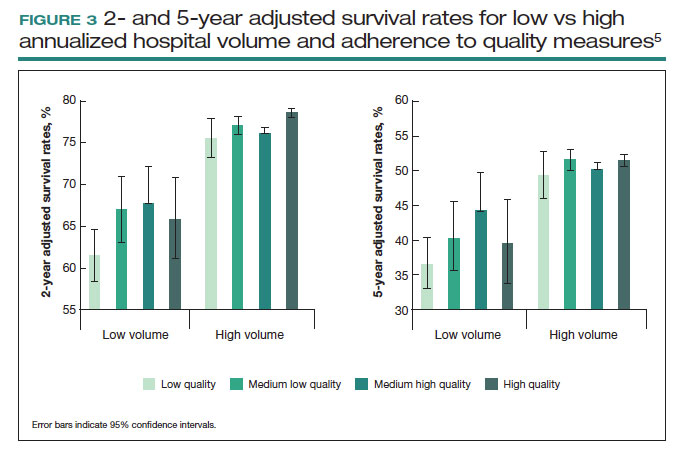
These findings highlight the importance of quality of care as well as the complex interplay of surgical volume and other factors.20 Many have argued that it may be more appropriate to measure quality of care and past performance and outcomes rather than surgical volume.21
Continue to: Minimum volume standards...
Minimum volume standards
While efforts to regionalize surgical care have gradually evolved, calls have been growing to formalize policies that limit the performance of some procedures to surgeons and centers that meet a minimum volume threshold or standard.21 One such effort, based on consensus from 3 academic hospital systems, was a campaign for hospitals to “Take the Volume Pledge.”21 The campaign’s goal is to encourage health care systems to restrict the performance of 10 procedures to surgeons and hospitals within their systems that meet a minimum volume standard for the given operations.21 In essence, procedures would be restricted for low-volume providers and centers and triaged to higher-volume surgeons and hospitals within a given health care system.21
Proponents of the Volume Pledge argue that it is a relatively straightforward way to align patients and providers to optimize outcomes. The Volume Pledge focuses on larger hospital systems and encourages referral within the given system, thus mitigating competitive and financial concerns about referring patients to outside providers. Those who have argued against the Volume Pledge point out that the volume cut points chosen are somewhat arbitrary, that these policies have the potential to negatively impact rural hospitals and those serving smaller communities, and that quality is a more appropriate metric than volume.22 The Volume Pledge does not include any gynecologic procedures, and to date it has met with only limited success.23
Perhaps more directly applicable to gynecologic surgeons are ongoing national trends to base hospital credentialing on surgical volume. In essence, individual surgeons must demonstrate that they have performed a minimum number of procedures to obtain or retain privileges.24,25 While there is strong evidence of the association between volume and outcomes for some complex surgical procedures, linking volume to credentialing has a number of potential pitfalls. Studies of surgical outcomes based on volume represent average performance, and many low-volume providers have better-than-expected outcomes. Volume measures typically represent recent performance; it is difficult to measure the overall experience of individual surgeons. Similarly, surgical outcomes depend on both the surgeon and the system in which the surgeon operates. It is difficult, if not impossible, to account for differences in the environment in which a surgeon works.25
A study of gynecologic surgeons who performed hysterectomy in New York State demonstrates many of the complexities of volume-based credentialing.26 In a cohort of more than55,000 patients who underwent abdominal hysterectomy, there was a strong association between low surgeon volume and a higher-than-expected rate of complications. If one were to consider limiting privileges to even the lowest-volume providers, there would be a significant impact on the surgical workforce. In this cohort, limiting credentialing to the lowest-volume providers, those who performed only 1 abdominal hysterectomy in the prior year would restrict the privileges of 17.5% of the surgeons in the cohort. Further, in this low-volume cohort that performed only 1 abdominal hysterectomy in the prior year, 69% of the surgeons actually had outcomes that were better than predicted.26 These data highlight not only the difficulty of applying averages to individual surgeons but also the profound impact that policy changes could have on the practice of gynecologic surgery.
Volume-outcomes paradigm discussions continue
The association between higher surgeon and hospital procedural volume for gynecologic surgeries and improved outcomes now has been convincingly demonstrated. With this knowledge, over the last decade the patterns of care for patients undergoing gynecologic surgery have clearly shifted, and these operations are now more commonly being performed by a smaller number of physicians and at fewer hospitals.
While efforts to improve quality are clearly important, many policy interventions, such as regionalization of care, have untoward consequences that must be considered. As we move forward, it will be essential to ensure that there is a robust debate among patients, providers, and policymakers on the merits of public health policies based on the volume-outcomes paradigm. ●
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117-2127.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:11281137.
- Mowat A, Maher C, Ballard E. Surgical outcomes for low-volume vs high-volume surgeons in gynecology surgery: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016;215:21-33.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Wright JD, Chen L, Hou JY, et al. Association of hospital volume and quality of care with survival for ovarian cancer. Obstet Gynecol. 2017;130:545-553.
- Cliby WA, Powell MA, Al-Hammadi N, et al. Ovarian cancer in the United States: contemporary patterns of care associated with improved survival. Gynecol Oncol. 2015;136:11-17.
- Matsuo K, Shimada M, Yamaguchi S, et al. Association of radical hysterectomy surgical volume and survival for early-stage cervical cancer. Obstet Gynecol. 2019;133:1086-1098.
- Brennand EA, Quan H. Evaluation of the effect of surgeon’s operative volume and specialty on likelihood of revision after mesh midurethral sling placement. Obstet Gynecol. 2019;133:1099-1108.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:21282137.
- Wright JD, Ruiz MP, Chen L, et al. Changes in surgical volume and outcomes over time for women undergoing hysterectomy for endometrial cancer. Obstet Gynecol. 2018;132:59-69.
- Wright JD, Chen L, Buskwofie A, et al. Regionalization of care for women with ovarian cancer. Gynecol Oncol. 2019;154:394-400.
- Fong ZV, Hashimoto DA, Jin G, et al. Simulated volume-based regionalization of complex procedures: impact on spatial access to care. Ann Surg. 2021;274:312-318.
- Knisely A, Huang Y, Melamed A, et al. Effect of regionalization of endometrial cancer care on site of care and patient travel. Am J Obstet Gynecol. 2020;222:58.e1-58.e10.
- Finlayson SR, Birkmeyer JD, Tosteson AN, et al. Patient preferences for location of care: implications for regionalization. Med Care. 1999;37:204-209.
- Shalowitz DI, Nivasch E, Burger RA, et al. Are patients willing to travel for better ovarian cancer care? Gynecol Oncol. 2018;148:42-48.
- Rehmani SS, Liu B, Al-Ayoubi AM, et al. Racial disparity in utilization of high-volume hospitals for surgical treatment of esophageal cancer. Ann Thorac Surg. 2018;106:346-353.
- Nattinger AB, Rademacher N, McGinley EL, et al. Can regionalization of care reduce socioeconomic disparities in breast cancer survival? Med Care. 2021;59:77-81.
- Auerbach AD, Hilton JF, Maselli J, et al. Shop for quality or volume? Volume, quality, and outcomes of coronary artery bypass surgery. Ann Intern Med. 2009;150:696-704.
- Kurlansky PA, Argenziano M, Dunton R, et al. Quality, not volume, determines outcome of coronary artery bypass surgery in a university-based community hospital network. J Thorac Cardiovasc Surg. 2012;143:287-293.
- Urbach DR. Pledging to eliminate low-volume surgery. N Engl J Med. 2015;373:1388-1390.
- Blanco BA, Kothari AN, Blackwell RH, et al. “Take the Volume Pledge” may result in disparity in access to care. Surgery. 2017;161:837-845.
- Farjah F, Grau-Sepulveda MV, Gaissert H, et al. Volume Pledge is not associated with better short-term outcomes after lung cancer resection. J Clin Oncol. 2020;38:3518-3527.
- Tracy EE, Zephyrin LC, Rosman DA, et al. Credentialing based on surgical volume, physician workforce challenges, and patient access. Obstet Gynecol. 2013;122:947-951.
- Statement on credentialing and privileging and volume performance issues. April 1, 2018. American College of Surgeons. Accessed April 10, 2023. https://facs.org/about-acs/statements/credentialing-andprivileging-and-volume-performance-issues/
- Ruiz MP, Chen L, Hou JY, et al. Effect of minimum-volume standards on patient outcomes and surgical practice patterns for hysterectomy. Obstet Gynecol. 2018;132:1229-1237.
Over the last 3 decades, abundant evidence has demonstrated the association between surgical volume and outcomes. Patients operated on by high-volume surgeons and at high-volume hospitals have superior outcomes.
Surgical volume in gynecology
The association between both hospital and surgeon volume and outcomes has been explored across a number of gynecologic procedures.3 A meta-analysis that included 741,000 patients found that low-volume surgeons had an increased rate of complications overall, a higher rate of intraoperative complications, and a higher rate of postoperative complications compared with high-volume surgeons. While there was no association between volume and mortality overall, when limited to gynecologic oncology studies, low surgeon volume was associated with increased perioperative mortality.3
While these studies demonstrated a statistically significant association between surgeon volume and perioperative outcomes, the magnitude of the effect is modest compared with other higher-risk procedures associated with greater perioperative morbidity. For example, in a large study that examined oncologic and cardiovascular surgery, perioperative mortality in patients who underwent pancreatic resection was reduced from 15% for low-volume surgeons to 5% for high-volume surgeons.1 By contrast, for gynecologic surgery, complications occurred in 97 per 1,000 patients operated on by high-volume surgeons compared with between 114 and 137 per 1,000 for low-volume surgeons. Thus, to avoid 1 in-hospital complication, 30 surgeries performed by low-volume surgeons would need to be moved to high-volume surgeons. For intraoperative complications, 38 patients would need to be moved from low- to high-volume surgeons to prevent 1 such complication.3 In addition to morbidity and mortality, higher surgeon volume is associated with greater use of minimally invasive surgery, a lower likelihood of conversion to laparotomy, and lower costs.3

Similarly, hospital volume also has been associated with outcomes for gynecologic surgery.4 In a report of patients who underwent laparoscopic hysterectomy, the authors found that the complication rate was 18% lower for patients at high- versus low-volume hospitals. In addition, cost was lower at the high-volume centers.4 Like surgeon volume, the magnitude of the differential in outcomes between high- and low-volume hospitals is often modest.4
While most studies have focused on short-term outcomes, surgical volume appears also to be associated with longer-term outcomes. For gynecologic cancer, studies have demonstrated an association between hospital volume and survival for ovarian and cervical cancer.5-7 A large report of centers across the United States found that the 5-year survival rate increased from 39% for patients treated at low-volume centers to 51% at the highest-volume hospitals.5 In urogynecology, surgeon volume has been associated with midurethral sling revision. One study noted that after an individual surgeon performed 50 procedures a year, each additional case was associated with a decline in the rate of sling revision.8 One could argue that these longer-term end points may be the measures that matter most to patients.
Although the magnitude of the association between surgical volume and outcomes in gynecology appears to be relatively modest, outcomes for very-low-volume (VLV) surgeons are substantially worse. An analysis of more than 430,000 patients who underwent hysterectomy compared outcomes between VLV surgeons (characterized as surgeons who performed only 1 hysterectomy in the prior year) and other gynecologic surgeons. The overall complication rate was 32% in VLV surgeons compared with 10% among other surgeons, while the perioperative mortality rate was 2.5% versus 0.2% in the 2 groups, respectively. Likely reflecting changing practice patterns in gynecology, a sizable number of surgeons were classified as VLV physicians.9
Continue to: Public health applications of gynecologic surgical volume...
Public health applications of gynecologic surgical volume
The large body of literature on volume and outcomes has led to a number of public health initiatives aimed at reducing perioperative morbidity and mortality. Broadly, these efforts focus on regionalization of care, targeted quality improvement, and the development of minimum volume standards. Each strategy holds promise but also the potential to lead to unwanted consequences.
Regionalization of care
Recognition of the volume-outcomes paradigm has led to efforts to regionalize care for complex procedures to high-volume surgeons and centers.10 A cohort study of surgical patterns of care for Medicare recipients who underwent cancer resections or abdominal aortic aneurysm repair from 1999 to 2008 demonstrated these shifting practice patterns. For example, in 1999–2000, pancreatectomy was performed in 1,308 hospitals, with a median case volume of 5 procedures per year. By 2007–2008, the number of hospitals in which pancreatectomy was performed declined to 978, and the median case volume rose to 16 procedures per year. Importantly, over this time period, risk-adjusted mortality for pancreatectomy declined by 19%, and increased hospital volume was responsible for more than two-thirds of the decline in mortality.10
There has similarly been a gradual concentration of some gynecologic procedures to higher-volume surgeons and centers.11,12 Among patients undergoing hysterectomy for endometrial cancer in New York State, 845 surgeons with a mean case volume of 3 procedures per year treated patients in 2000. By 2014, the number of surgeons who performed these operations declined to 317 while mean annual case volume rose to 10 procedures per year. The number of hospitals in which women with endometrial cancer were treated declined from 182 to 98 over the same time period.11 Similar trends were noted for patients undergoing ovarian cancer resection.12 While patterns of gynecologic care for some surgical procedures have clearly changed, it has been more difficult to link these changes to improvements in outcomes.11,12
Despite the intuitive appeal of regionalization of surgical care, such a strategy has a number of limitations and practical challenges. Not surprisingly, limiting the number of surgeons and hospitals that perform a given procedure necessitates that patients travel a greater distance to obtain necessary surgical care.13,14 An analysis of endometrial cancer patients in New York State stratified patients based on their area of residence into 10 hospital referral regions (HRRs), which represent health care markets for tertiary medical care. From 2000 to 2014, the distance patients traveled to receive their surgical care increased in all of the HRRs studied. This was most pronounced in 1 of the HRRs in which the median travel distance rose by 47 miles over the 15-year period (FIGURE 1; FIGURE 2).14
Whether patients are willing to travel for care remains a matter of debate and depends on the disease, the surgical procedure, and the anticipated benefit associated with a longer travel distance.15,16 In a discrete choice experiment, 100 participants were given a hypothetical scenario in which they had potentially resectable pancreatic cancer; they were queried on their willingness to travel for care based on varying differences in mortality between a local and regional hospital.15 When mortality at the local hospital was double that of the regional hospital (6% vs 3%), 45% of patients chose to remain at the local hospital. When the differential increased to a 4 times greater mortality at the local hospital (12% vs 3%), 23% of patients still chose to remain at the local hospital.15


A similar study asked patients with ovarian neoplasms whether they would travel 50 miles to a regional center for surgery based on some degree of increased 5-year survival.16 Overall, 79% of patients would travel for a 4% improvement in survival while 97% would travel for a 12% improvement in survival.16
Lastly, a number of studies have shown that regionalization of surgical care disproportionately affects Black and Hispanic patients and those with low socioeconomic status.12,13,17 A simulation study on the effect of regionalizing care for pancreatectomy noted that using a hospital volume threshold of 20 procedures per year, a higher percentage of Black and Hispanic patients than White patients would be required to travel to a higher-volume center.13 Similarly, Medicaid recipients were more likely to be affected.13 Despite the inequities in who must travel for regionalized care, prior work has suggested that regionalization of cancer care to high-volume centers may reduce racial and socioeconomic disparities in survival for some cancers.18
Targeted quality improvement
Realizing the practical limitations of regionalization of care, an alternative strategy is to improve the quality of care at low-volume hospitals.5,19 Quality of care and surgical volume often are correlated, and the delivery of high-quality care can mitigate some of the influence of surgical volume on outcomes.
These principles were demonstrated in a study of more than 100,000 patients with ovarian cancer that stratified treating hospitals into volume quintiles.5 As expected, survival (both 2- and 5-year) was highest in the highest-volume quintile hospitals (FIGURE 3).5 Similarly, quality of care, measured through adherence to various process measures, was also highest in the highest-volume quintile hospitals. Interestingly, in the second-fourth volume quintile hospitals, there was substantial variation in adherence to quality metrics. Among hospitals with higher quality care, an improved survival was noted compared with lower quality care hospitals within the same volume quintile. Survival at high-quality, intermediate-volume hospitals approached that of the high-volume quintile hospitals.5

These findings highlight the importance of quality of care as well as the complex interplay of surgical volume and other factors.20 Many have argued that it may be more appropriate to measure quality of care and past performance and outcomes rather than surgical volume.21
Continue to: Minimum volume standards...
Minimum volume standards
While efforts to regionalize surgical care have gradually evolved, calls have been growing to formalize policies that limit the performance of some procedures to surgeons and centers that meet a minimum volume threshold or standard.21 One such effort, based on consensus from 3 academic hospital systems, was a campaign for hospitals to “Take the Volume Pledge.”21 The campaign’s goal is to encourage health care systems to restrict the performance of 10 procedures to surgeons and hospitals within their systems that meet a minimum volume standard for the given operations.21 In essence, procedures would be restricted for low-volume providers and centers and triaged to higher-volume surgeons and hospitals within a given health care system.21
Proponents of the Volume Pledge argue that it is a relatively straightforward way to align patients and providers to optimize outcomes. The Volume Pledge focuses on larger hospital systems and encourages referral within the given system, thus mitigating competitive and financial concerns about referring patients to outside providers. Those who have argued against the Volume Pledge point out that the volume cut points chosen are somewhat arbitrary, that these policies have the potential to negatively impact rural hospitals and those serving smaller communities, and that quality is a more appropriate metric than volume.22 The Volume Pledge does not include any gynecologic procedures, and to date it has met with only limited success.23
Perhaps more directly applicable to gynecologic surgeons are ongoing national trends to base hospital credentialing on surgical volume. In essence, individual surgeons must demonstrate that they have performed a minimum number of procedures to obtain or retain privileges.24,25 While there is strong evidence of the association between volume and outcomes for some complex surgical procedures, linking volume to credentialing has a number of potential pitfalls. Studies of surgical outcomes based on volume represent average performance, and many low-volume providers have better-than-expected outcomes. Volume measures typically represent recent performance; it is difficult to measure the overall experience of individual surgeons. Similarly, surgical outcomes depend on both the surgeon and the system in which the surgeon operates. It is difficult, if not impossible, to account for differences in the environment in which a surgeon works.25
A study of gynecologic surgeons who performed hysterectomy in New York State demonstrates many of the complexities of volume-based credentialing.26 In a cohort of more than55,000 patients who underwent abdominal hysterectomy, there was a strong association between low surgeon volume and a higher-than-expected rate of complications. If one were to consider limiting privileges to even the lowest-volume providers, there would be a significant impact on the surgical workforce. In this cohort, limiting credentialing to the lowest-volume providers, those who performed only 1 abdominal hysterectomy in the prior year would restrict the privileges of 17.5% of the surgeons in the cohort. Further, in this low-volume cohort that performed only 1 abdominal hysterectomy in the prior year, 69% of the surgeons actually had outcomes that were better than predicted.26 These data highlight not only the difficulty of applying averages to individual surgeons but also the profound impact that policy changes could have on the practice of gynecologic surgery.
Volume-outcomes paradigm discussions continue
The association between higher surgeon and hospital procedural volume for gynecologic surgeries and improved outcomes now has been convincingly demonstrated. With this knowledge, over the last decade the patterns of care for patients undergoing gynecologic surgery have clearly shifted, and these operations are now more commonly being performed by a smaller number of physicians and at fewer hospitals.
While efforts to improve quality are clearly important, many policy interventions, such as regionalization of care, have untoward consequences that must be considered. As we move forward, it will be essential to ensure that there is a robust debate among patients, providers, and policymakers on the merits of public health policies based on the volume-outcomes paradigm. ●
Over the last 3 decades, abundant evidence has demonstrated the association between surgical volume and outcomes. Patients operated on by high-volume surgeons and at high-volume hospitals have superior outcomes.
Surgical volume in gynecology
The association between both hospital and surgeon volume and outcomes has been explored across a number of gynecologic procedures.3 A meta-analysis that included 741,000 patients found that low-volume surgeons had an increased rate of complications overall, a higher rate of intraoperative complications, and a higher rate of postoperative complications compared with high-volume surgeons. While there was no association between volume and mortality overall, when limited to gynecologic oncology studies, low surgeon volume was associated with increased perioperative mortality.3
While these studies demonstrated a statistically significant association between surgeon volume and perioperative outcomes, the magnitude of the effect is modest compared with other higher-risk procedures associated with greater perioperative morbidity. For example, in a large study that examined oncologic and cardiovascular surgery, perioperative mortality in patients who underwent pancreatic resection was reduced from 15% for low-volume surgeons to 5% for high-volume surgeons.1 By contrast, for gynecologic surgery, complications occurred in 97 per 1,000 patients operated on by high-volume surgeons compared with between 114 and 137 per 1,000 for low-volume surgeons. Thus, to avoid 1 in-hospital complication, 30 surgeries performed by low-volume surgeons would need to be moved to high-volume surgeons. For intraoperative complications, 38 patients would need to be moved from low- to high-volume surgeons to prevent 1 such complication.3 In addition to morbidity and mortality, higher surgeon volume is associated with greater use of minimally invasive surgery, a lower likelihood of conversion to laparotomy, and lower costs.3

Similarly, hospital volume also has been associated with outcomes for gynecologic surgery.4 In a report of patients who underwent laparoscopic hysterectomy, the authors found that the complication rate was 18% lower for patients at high- versus low-volume hospitals. In addition, cost was lower at the high-volume centers.4 Like surgeon volume, the magnitude of the differential in outcomes between high- and low-volume hospitals is often modest.4
While most studies have focused on short-term outcomes, surgical volume appears also to be associated with longer-term outcomes. For gynecologic cancer, studies have demonstrated an association between hospital volume and survival for ovarian and cervical cancer.5-7 A large report of centers across the United States found that the 5-year survival rate increased from 39% for patients treated at low-volume centers to 51% at the highest-volume hospitals.5 In urogynecology, surgeon volume has been associated with midurethral sling revision. One study noted that after an individual surgeon performed 50 procedures a year, each additional case was associated with a decline in the rate of sling revision.8 One could argue that these longer-term end points may be the measures that matter most to patients.
Although the magnitude of the association between surgical volume and outcomes in gynecology appears to be relatively modest, outcomes for very-low-volume (VLV) surgeons are substantially worse. An analysis of more than 430,000 patients who underwent hysterectomy compared outcomes between VLV surgeons (characterized as surgeons who performed only 1 hysterectomy in the prior year) and other gynecologic surgeons. The overall complication rate was 32% in VLV surgeons compared with 10% among other surgeons, while the perioperative mortality rate was 2.5% versus 0.2% in the 2 groups, respectively. Likely reflecting changing practice patterns in gynecology, a sizable number of surgeons were classified as VLV physicians.9
Continue to: Public health applications of gynecologic surgical volume...
Public health applications of gynecologic surgical volume
The large body of literature on volume and outcomes has led to a number of public health initiatives aimed at reducing perioperative morbidity and mortality. Broadly, these efforts focus on regionalization of care, targeted quality improvement, and the development of minimum volume standards. Each strategy holds promise but also the potential to lead to unwanted consequences.
Regionalization of care
Recognition of the volume-outcomes paradigm has led to efforts to regionalize care for complex procedures to high-volume surgeons and centers.10 A cohort study of surgical patterns of care for Medicare recipients who underwent cancer resections or abdominal aortic aneurysm repair from 1999 to 2008 demonstrated these shifting practice patterns. For example, in 1999–2000, pancreatectomy was performed in 1,308 hospitals, with a median case volume of 5 procedures per year. By 2007–2008, the number of hospitals in which pancreatectomy was performed declined to 978, and the median case volume rose to 16 procedures per year. Importantly, over this time period, risk-adjusted mortality for pancreatectomy declined by 19%, and increased hospital volume was responsible for more than two-thirds of the decline in mortality.10
There has similarly been a gradual concentration of some gynecologic procedures to higher-volume surgeons and centers.11,12 Among patients undergoing hysterectomy for endometrial cancer in New York State, 845 surgeons with a mean case volume of 3 procedures per year treated patients in 2000. By 2014, the number of surgeons who performed these operations declined to 317 while mean annual case volume rose to 10 procedures per year. The number of hospitals in which women with endometrial cancer were treated declined from 182 to 98 over the same time period.11 Similar trends were noted for patients undergoing ovarian cancer resection.12 While patterns of gynecologic care for some surgical procedures have clearly changed, it has been more difficult to link these changes to improvements in outcomes.11,12
Despite the intuitive appeal of regionalization of surgical care, such a strategy has a number of limitations and practical challenges. Not surprisingly, limiting the number of surgeons and hospitals that perform a given procedure necessitates that patients travel a greater distance to obtain necessary surgical care.13,14 An analysis of endometrial cancer patients in New York State stratified patients based on their area of residence into 10 hospital referral regions (HRRs), which represent health care markets for tertiary medical care. From 2000 to 2014, the distance patients traveled to receive their surgical care increased in all of the HRRs studied. This was most pronounced in 1 of the HRRs in which the median travel distance rose by 47 miles over the 15-year period (FIGURE 1; FIGURE 2).14
Whether patients are willing to travel for care remains a matter of debate and depends on the disease, the surgical procedure, and the anticipated benefit associated with a longer travel distance.15,16 In a discrete choice experiment, 100 participants were given a hypothetical scenario in which they had potentially resectable pancreatic cancer; they were queried on their willingness to travel for care based on varying differences in mortality between a local and regional hospital.15 When mortality at the local hospital was double that of the regional hospital (6% vs 3%), 45% of patients chose to remain at the local hospital. When the differential increased to a 4 times greater mortality at the local hospital (12% vs 3%), 23% of patients still chose to remain at the local hospital.15


A similar study asked patients with ovarian neoplasms whether they would travel 50 miles to a regional center for surgery based on some degree of increased 5-year survival.16 Overall, 79% of patients would travel for a 4% improvement in survival while 97% would travel for a 12% improvement in survival.16
Lastly, a number of studies have shown that regionalization of surgical care disproportionately affects Black and Hispanic patients and those with low socioeconomic status.12,13,17 A simulation study on the effect of regionalizing care for pancreatectomy noted that using a hospital volume threshold of 20 procedures per year, a higher percentage of Black and Hispanic patients than White patients would be required to travel to a higher-volume center.13 Similarly, Medicaid recipients were more likely to be affected.13 Despite the inequities in who must travel for regionalized care, prior work has suggested that regionalization of cancer care to high-volume centers may reduce racial and socioeconomic disparities in survival for some cancers.18
Targeted quality improvement
Realizing the practical limitations of regionalization of care, an alternative strategy is to improve the quality of care at low-volume hospitals.5,19 Quality of care and surgical volume often are correlated, and the delivery of high-quality care can mitigate some of the influence of surgical volume on outcomes.
These principles were demonstrated in a study of more than 100,000 patients with ovarian cancer that stratified treating hospitals into volume quintiles.5 As expected, survival (both 2- and 5-year) was highest in the highest-volume quintile hospitals (FIGURE 3).5 Similarly, quality of care, measured through adherence to various process measures, was also highest in the highest-volume quintile hospitals. Interestingly, in the second-fourth volume quintile hospitals, there was substantial variation in adherence to quality metrics. Among hospitals with higher quality care, an improved survival was noted compared with lower quality care hospitals within the same volume quintile. Survival at high-quality, intermediate-volume hospitals approached that of the high-volume quintile hospitals.5

These findings highlight the importance of quality of care as well as the complex interplay of surgical volume and other factors.20 Many have argued that it may be more appropriate to measure quality of care and past performance and outcomes rather than surgical volume.21
Continue to: Minimum volume standards...
Minimum volume standards
While efforts to regionalize surgical care have gradually evolved, calls have been growing to formalize policies that limit the performance of some procedures to surgeons and centers that meet a minimum volume threshold or standard.21 One such effort, based on consensus from 3 academic hospital systems, was a campaign for hospitals to “Take the Volume Pledge.”21 The campaign’s goal is to encourage health care systems to restrict the performance of 10 procedures to surgeons and hospitals within their systems that meet a minimum volume standard for the given operations.21 In essence, procedures would be restricted for low-volume providers and centers and triaged to higher-volume surgeons and hospitals within a given health care system.21
Proponents of the Volume Pledge argue that it is a relatively straightforward way to align patients and providers to optimize outcomes. The Volume Pledge focuses on larger hospital systems and encourages referral within the given system, thus mitigating competitive and financial concerns about referring patients to outside providers. Those who have argued against the Volume Pledge point out that the volume cut points chosen are somewhat arbitrary, that these policies have the potential to negatively impact rural hospitals and those serving smaller communities, and that quality is a more appropriate metric than volume.22 The Volume Pledge does not include any gynecologic procedures, and to date it has met with only limited success.23
Perhaps more directly applicable to gynecologic surgeons are ongoing national trends to base hospital credentialing on surgical volume. In essence, individual surgeons must demonstrate that they have performed a minimum number of procedures to obtain or retain privileges.24,25 While there is strong evidence of the association between volume and outcomes for some complex surgical procedures, linking volume to credentialing has a number of potential pitfalls. Studies of surgical outcomes based on volume represent average performance, and many low-volume providers have better-than-expected outcomes. Volume measures typically represent recent performance; it is difficult to measure the overall experience of individual surgeons. Similarly, surgical outcomes depend on both the surgeon and the system in which the surgeon operates. It is difficult, if not impossible, to account for differences in the environment in which a surgeon works.25
A study of gynecologic surgeons who performed hysterectomy in New York State demonstrates many of the complexities of volume-based credentialing.26 In a cohort of more than55,000 patients who underwent abdominal hysterectomy, there was a strong association between low surgeon volume and a higher-than-expected rate of complications. If one were to consider limiting privileges to even the lowest-volume providers, there would be a significant impact on the surgical workforce. In this cohort, limiting credentialing to the lowest-volume providers, those who performed only 1 abdominal hysterectomy in the prior year would restrict the privileges of 17.5% of the surgeons in the cohort. Further, in this low-volume cohort that performed only 1 abdominal hysterectomy in the prior year, 69% of the surgeons actually had outcomes that were better than predicted.26 These data highlight not only the difficulty of applying averages to individual surgeons but also the profound impact that policy changes could have on the practice of gynecologic surgery.
Volume-outcomes paradigm discussions continue
The association between higher surgeon and hospital procedural volume for gynecologic surgeries and improved outcomes now has been convincingly demonstrated. With this knowledge, over the last decade the patterns of care for patients undergoing gynecologic surgery have clearly shifted, and these operations are now more commonly being performed by a smaller number of physicians and at fewer hospitals.
While efforts to improve quality are clearly important, many policy interventions, such as regionalization of care, have untoward consequences that must be considered. As we move forward, it will be essential to ensure that there is a robust debate among patients, providers, and policymakers on the merits of public health policies based on the volume-outcomes paradigm. ●
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117-2127.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:11281137.
- Mowat A, Maher C, Ballard E. Surgical outcomes for low-volume vs high-volume surgeons in gynecology surgery: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016;215:21-33.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Wright JD, Chen L, Hou JY, et al. Association of hospital volume and quality of care with survival for ovarian cancer. Obstet Gynecol. 2017;130:545-553.
- Cliby WA, Powell MA, Al-Hammadi N, et al. Ovarian cancer in the United States: contemporary patterns of care associated with improved survival. Gynecol Oncol. 2015;136:11-17.
- Matsuo K, Shimada M, Yamaguchi S, et al. Association of radical hysterectomy surgical volume and survival for early-stage cervical cancer. Obstet Gynecol. 2019;133:1086-1098.
- Brennand EA, Quan H. Evaluation of the effect of surgeon’s operative volume and specialty on likelihood of revision after mesh midurethral sling placement. Obstet Gynecol. 2019;133:1099-1108.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:21282137.
- Wright JD, Ruiz MP, Chen L, et al. Changes in surgical volume and outcomes over time for women undergoing hysterectomy for endometrial cancer. Obstet Gynecol. 2018;132:59-69.
- Wright JD, Chen L, Buskwofie A, et al. Regionalization of care for women with ovarian cancer. Gynecol Oncol. 2019;154:394-400.
- Fong ZV, Hashimoto DA, Jin G, et al. Simulated volume-based regionalization of complex procedures: impact on spatial access to care. Ann Surg. 2021;274:312-318.
- Knisely A, Huang Y, Melamed A, et al. Effect of regionalization of endometrial cancer care on site of care and patient travel. Am J Obstet Gynecol. 2020;222:58.e1-58.e10.
- Finlayson SR, Birkmeyer JD, Tosteson AN, et al. Patient preferences for location of care: implications for regionalization. Med Care. 1999;37:204-209.
- Shalowitz DI, Nivasch E, Burger RA, et al. Are patients willing to travel for better ovarian cancer care? Gynecol Oncol. 2018;148:42-48.
- Rehmani SS, Liu B, Al-Ayoubi AM, et al. Racial disparity in utilization of high-volume hospitals for surgical treatment of esophageal cancer. Ann Thorac Surg. 2018;106:346-353.
- Nattinger AB, Rademacher N, McGinley EL, et al. Can regionalization of care reduce socioeconomic disparities in breast cancer survival? Med Care. 2021;59:77-81.
- Auerbach AD, Hilton JF, Maselli J, et al. Shop for quality or volume? Volume, quality, and outcomes of coronary artery bypass surgery. Ann Intern Med. 2009;150:696-704.
- Kurlansky PA, Argenziano M, Dunton R, et al. Quality, not volume, determines outcome of coronary artery bypass surgery in a university-based community hospital network. J Thorac Cardiovasc Surg. 2012;143:287-293.
- Urbach DR. Pledging to eliminate low-volume surgery. N Engl J Med. 2015;373:1388-1390.
- Blanco BA, Kothari AN, Blackwell RH, et al. “Take the Volume Pledge” may result in disparity in access to care. Surgery. 2017;161:837-845.
- Farjah F, Grau-Sepulveda MV, Gaissert H, et al. Volume Pledge is not associated with better short-term outcomes after lung cancer resection. J Clin Oncol. 2020;38:3518-3527.
- Tracy EE, Zephyrin LC, Rosman DA, et al. Credentialing based on surgical volume, physician workforce challenges, and patient access. Obstet Gynecol. 2013;122:947-951.
- Statement on credentialing and privileging and volume performance issues. April 1, 2018. American College of Surgeons. Accessed April 10, 2023. https://facs.org/about-acs/statements/credentialing-andprivileging-and-volume-performance-issues/
- Ruiz MP, Chen L, Hou JY, et al. Effect of minimum-volume standards on patient outcomes and surgical practice patterns for hysterectomy. Obstet Gynecol. 2018;132:1229-1237.
- Birkmeyer JD, Stukel TA, Siewers AE, et al. Surgeon volume and operative mortality in the United States. N Engl J Med. 2003;349:2117-2127.
- Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346:11281137.
- Mowat A, Maher C, Ballard E. Surgical outcomes for low-volume vs high-volume surgeons in gynecology surgery: a systematic review and meta-analysis. Am J Obstet Gynecol. 2016;215:21-33.
- Wallenstein MR, Ananth CV, Kim JH, et al. Effect of surgical volume on outcomes for laparoscopic hysterectomy for benign indications. Obstet Gynecol. 2012;119:709-716.
- Wright JD, Chen L, Hou JY, et al. Association of hospital volume and quality of care with survival for ovarian cancer. Obstet Gynecol. 2017;130:545-553.
- Cliby WA, Powell MA, Al-Hammadi N, et al. Ovarian cancer in the United States: contemporary patterns of care associated with improved survival. Gynecol Oncol. 2015;136:11-17.
- Matsuo K, Shimada M, Yamaguchi S, et al. Association of radical hysterectomy surgical volume and survival for early-stage cervical cancer. Obstet Gynecol. 2019;133:1086-1098.
- Brennand EA, Quan H. Evaluation of the effect of surgeon’s operative volume and specialty on likelihood of revision after mesh midurethral sling placement. Obstet Gynecol. 2019;133:1099-1108.
- Ruiz MP, Chen L, Hou JY, et al. Outcomes of hysterectomy performed by very low-volume surgeons. Obstet Gynecol. 2018;131:981-990.
- Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:21282137.
- Wright JD, Ruiz MP, Chen L, et al. Changes in surgical volume and outcomes over time for women undergoing hysterectomy for endometrial cancer. Obstet Gynecol. 2018;132:59-69.
- Wright JD, Chen L, Buskwofie A, et al. Regionalization of care for women with ovarian cancer. Gynecol Oncol. 2019;154:394-400.
- Fong ZV, Hashimoto DA, Jin G, et al. Simulated volume-based regionalization of complex procedures: impact on spatial access to care. Ann Surg. 2021;274:312-318.
- Knisely A, Huang Y, Melamed A, et al. Effect of regionalization of endometrial cancer care on site of care and patient travel. Am J Obstet Gynecol. 2020;222:58.e1-58.e10.
- Finlayson SR, Birkmeyer JD, Tosteson AN, et al. Patient preferences for location of care: implications for regionalization. Med Care. 1999;37:204-209.
- Shalowitz DI, Nivasch E, Burger RA, et al. Are patients willing to travel for better ovarian cancer care? Gynecol Oncol. 2018;148:42-48.
- Rehmani SS, Liu B, Al-Ayoubi AM, et al. Racial disparity in utilization of high-volume hospitals for surgical treatment of esophageal cancer. Ann Thorac Surg. 2018;106:346-353.
- Nattinger AB, Rademacher N, McGinley EL, et al. Can regionalization of care reduce socioeconomic disparities in breast cancer survival? Med Care. 2021;59:77-81.
- Auerbach AD, Hilton JF, Maselli J, et al. Shop for quality or volume? Volume, quality, and outcomes of coronary artery bypass surgery. Ann Intern Med. 2009;150:696-704.
- Kurlansky PA, Argenziano M, Dunton R, et al. Quality, not volume, determines outcome of coronary artery bypass surgery in a university-based community hospital network. J Thorac Cardiovasc Surg. 2012;143:287-293.
- Urbach DR. Pledging to eliminate low-volume surgery. N Engl J Med. 2015;373:1388-1390.
- Blanco BA, Kothari AN, Blackwell RH, et al. “Take the Volume Pledge” may result in disparity in access to care. Surgery. 2017;161:837-845.
- Farjah F, Grau-Sepulveda MV, Gaissert H, et al. Volume Pledge is not associated with better short-term outcomes after lung cancer resection. J Clin Oncol. 2020;38:3518-3527.
- Tracy EE, Zephyrin LC, Rosman DA, et al. Credentialing based on surgical volume, physician workforce challenges, and patient access. Obstet Gynecol. 2013;122:947-951.
- Statement on credentialing and privileging and volume performance issues. April 1, 2018. American College of Surgeons. Accessed April 10, 2023. https://facs.org/about-acs/statements/credentialing-andprivileging-and-volume-performance-issues/
- Ruiz MP, Chen L, Hou JY, et al. Effect of minimum-volume standards on patient outcomes and surgical practice patterns for hysterectomy. Obstet Gynecol. 2018;132:1229-1237.
SCD, beta-thalassemia: CRISPR-based gene therapy `transformative’
Results from the prespecified interim analyses of the phase 3 CLIMB THAL-111 and CLIMB SCD-121 studies, presented at the European Hematology Association annual congress, show that patients with beta-thalassemia who received exa-cel were able to remain transfusion-free for up to 40.7 consecutive months, while in patients with sickle cell disease, the treatment likewise provided up to 36.5 months of freedom from vaso-occlusive crises.
The findings underscore that “exa-cel can provide a one-time, functional cure to patients with beta-thalassemia and sickle cell disease,” said coauthor Franco Locatelli, MD, of Catholic University of the Sacred Heart, Bambino Gesù Children’s Hospital, Rome.
In a comment, senior investigator Haydar Frangoul, MD, noted that, “with almost 4 years of follow-up on patients with beta-thalassemia and sickle cell disease, it appears that the benefit is holding.”
“The engraftment of our edited cells appears very stable over time. There is no reason to believe it will change,” said Dr. Frangoul, who is medical director of pediatric hematology/oncology, Sarah Cannon Center for Blood Cancer at The Children’s Hospital at TriStar Centennial, Nashville, Tenn.
Burden is high; current curative options have caveats
Patients with transfusion-dependent beta-thalassemia may require blood transfusions as often as every 2-5 weeks because of genetic mutations causing the absence of functional hemoglobin and subsequent depletions in red blood cells. And with hemoglobin being an iron-rich protein, patients are also at risk of an iron accumulation in the body, adding the possible need for uncomfortable iron chelation therapy to prevent organ damage.
The measures are burdensome, but the need is dire. Life expectancy in beta-thalassemia without them is only about 5 years.
With SCD, patients can face severe pain from vaso-occlusive crises as sickled red blood cells block blood flow, potentially causing hospitalization and complications including kidney failure or stroke.
A cure does already exist for both genetic disorders in the form of allogeneic stem cell transplantation. However, that option requires a matched related stem cell donor, and fewer than 20% of patients have accessibility to such donors.
Gene therapy
Gene therapy offers a potentially ideal alternative, providing a possible “functional cure” without the need for a donor, by instead harvesting patients’ cells, fixing the mutation and transferring them back to the patient.
The Food and Drug Administration already approved a first gene therapy, betibeglogene autotemcel (beti-cel), for children and adults with transfusion dependent beta-thalassemia, in August 2022.
While beti-cel utilizes a viral vector to insert functional copies of a modified gene into patients’ extracted hematopoietic stem cells before transfusing them back, exa-cel instead uses CRISPR-CAS9 technology to edit the gene, allowing the body to produce fetal hemoglobin, in an approach believed to be more precise and efficient.
“As we explain to patients, it’s a difference between gene addition, which is what beti-cel is, or gene editing, which is what exa-cel is,” Dr. Frangoul explained.
Phase 3 trial interim results
In investigating exa-cel for beta-thalassemia, the ongoing CLIMB THAL-111 has enrolled 48 patients with a mean baseline age of 20, with 16 between the ages of 12 and 18. Of the patients, 28 (58.3%) had severe genotypes of disease.
Among 27 patients who were evaluable for the study endpoints of the current interim analysis, 24 (88.9%), achieved the primary endpoint of maintaining a weighted average hemoglobin of at least 9 g/dL without the need for a transfusion for at least 12 months (P < .0001).
Patients who achieved the transfusion independence for at least 12 months remained transfusion-free for a mean duration of 20.5 months, with a range of 12.1-40.7 months.
Of 3 patients who did not achieve the 12-month transfusion-free endpoint, substantial reductions in transfusion volume were nevertheless achieved, of 70.3%, 79.6%, and 95.5%, among the 3.
And for the CLIMB SCD-121 trial of SCD, 35 participants have been dosed with exa-cel; in the primary efficacy set of 17 patients, 16 of the 17 (94.1%) achieved the primary endpoint of having no severe vaso-occlusive crises for at least 12 months (P < .0001).
All patients, however, achieved the secondary endpoint of being free from in-patient hospitalizations for severe vaso-occlusive crises for at least 12 months (P < .0001).
Patients who achieved freedom from vaso-occlusive crises for at least 12 months remained free of the events for a mean of 18.7 months, ranging from 13.1 months to 36.5 months.
Durability, patient-reported outcomes favorable
Importantly, in both studies, hemoglobin levels, as well as levels of the edited BCL11A alleles in bone marrow CD34+ and peripheral blood nucleated cells, showed sustained stability over time, indicating durable editing of the cells, Dr. Locatelli said.
In terms of patient-reported quality-of-life, measures significantly improved during both trials at 24 months of follow-up, with significant improvements on the EuroQol visual analog scale, Functional Assessment of Cancer Therapy–General, and the Bone Marrow Transplantation Subscale.
Safety results were consistent with those observed with myeloablative busulfan-based conditioning regimen and autologous transplantation procedures, with adverse events that were manageable.
In the beta-thalassemia study, two patients experienced serious adverse events that were determined to be related to exa-cel, including one patient having symptoms in the context of hemophagocytic lymphohistiocytosis.
For the other patient, the serious adverse events consisted of delayed engraftment and thrombocytopenia, each also considered related to busulfan. None of the patients with SCD had serious adverse events related to exa-cel.
All serious adverse events were resolved, with no reports of deaths, study discontinuations, or malignancies.
Potentially first ever CRISPR-based FDA approval
While CRISPR-CAS9 gene editing is being investigated in multiple other trials in humans for various disorders, to date none have received FDA approval, which would make an approval for exa-cel a landmark development.
The therapy is currently under review, and Dr. Frangoul said the FDA has stated that a decision on the indication for SCD is expected by Dec. 8, 2023, and for beta-thalassemia, by March 2024.
Commenting on the research, Raffaella Colombatti, MD, a pediatric hematologist-oncologist and assistant professor of pediatrics at the University of Padova (Italy), underscored the need for a better curative alternative.
“Unfortunately, the other curative option, bone marrow transplant, is not available for all candidates due to the lack of suitable donors,” Dr. Colombatti said in an interview.
“And, although there are promising results from alternative donors and new conditioning regimens, a further option for selected patients with sickle cell disease and thalassemia utilizing gene therapy and gene editing is needed.”
Caveats regarding gene therapy for the two diseases that still need consideration include: “long-term safety results are still not available and eligibility criteria still needs to be explored outside clinical trials,” she said.
Furthermore, “costs and sustainability are also an issue,” Dr. Colombatti added.
The price of gene therapy is not cheap. With beti-cel priced at more than $2 million for the treatment, its manufacturer, Bluebird Bio, has reportedly already indicated that it will not pursue marketing in Europe because of unfavorable reimbursement policies, and a similar high price is anticipated for exa-cel.
Overall, however, the findings bode well for groundbreaking improvements in treatment of the two red blood cell disorders, Michael J. Eckrich, MD, MPH, medical director of pediatric stem cell transplant & cellular therapy at Atrium Health Levine Children’s Hospital Cancer and Blood Disorders in Charlotte, N.C., said in an interview.
“I do think that this is transformative therapy and will change our approach for patients with severe sickle cell disease in need of transplant,” said Dr. Eckrich, who has also been an investigator on the research of exa-cel for sickle cell disease.
“It might not be hard to imagine, that with the progress in gene therapies and gene editing, that allogeneic transplant will soon become obsolete for patients with sickle cell disease and beta-thalassemia.”
Dr. Locatelli is on the advisory board for Vertex Pharma and the speaker’s bureau for BluebirdBio. Dr. Frangoul and Dr. Colombatti are or have been consultants for Vertex Pharma.
Results from the prespecified interim analyses of the phase 3 CLIMB THAL-111 and CLIMB SCD-121 studies, presented at the European Hematology Association annual congress, show that patients with beta-thalassemia who received exa-cel were able to remain transfusion-free for up to 40.7 consecutive months, while in patients with sickle cell disease, the treatment likewise provided up to 36.5 months of freedom from vaso-occlusive crises.
The findings underscore that “exa-cel can provide a one-time, functional cure to patients with beta-thalassemia and sickle cell disease,” said coauthor Franco Locatelli, MD, of Catholic University of the Sacred Heart, Bambino Gesù Children’s Hospital, Rome.
In a comment, senior investigator Haydar Frangoul, MD, noted that, “with almost 4 years of follow-up on patients with beta-thalassemia and sickle cell disease, it appears that the benefit is holding.”
“The engraftment of our edited cells appears very stable over time. There is no reason to believe it will change,” said Dr. Frangoul, who is medical director of pediatric hematology/oncology, Sarah Cannon Center for Blood Cancer at The Children’s Hospital at TriStar Centennial, Nashville, Tenn.
Burden is high; current curative options have caveats
Patients with transfusion-dependent beta-thalassemia may require blood transfusions as often as every 2-5 weeks because of genetic mutations causing the absence of functional hemoglobin and subsequent depletions in red blood cells. And with hemoglobin being an iron-rich protein, patients are also at risk of an iron accumulation in the body, adding the possible need for uncomfortable iron chelation therapy to prevent organ damage.
The measures are burdensome, but the need is dire. Life expectancy in beta-thalassemia without them is only about 5 years.
With SCD, patients can face severe pain from vaso-occlusive crises as sickled red blood cells block blood flow, potentially causing hospitalization and complications including kidney failure or stroke.
A cure does already exist for both genetic disorders in the form of allogeneic stem cell transplantation. However, that option requires a matched related stem cell donor, and fewer than 20% of patients have accessibility to such donors.
Gene therapy
Gene therapy offers a potentially ideal alternative, providing a possible “functional cure” without the need for a donor, by instead harvesting patients’ cells, fixing the mutation and transferring them back to the patient.
The Food and Drug Administration already approved a first gene therapy, betibeglogene autotemcel (beti-cel), for children and adults with transfusion dependent beta-thalassemia, in August 2022.
While beti-cel utilizes a viral vector to insert functional copies of a modified gene into patients’ extracted hematopoietic stem cells before transfusing them back, exa-cel instead uses CRISPR-CAS9 technology to edit the gene, allowing the body to produce fetal hemoglobin, in an approach believed to be more precise and efficient.
“As we explain to patients, it’s a difference between gene addition, which is what beti-cel is, or gene editing, which is what exa-cel is,” Dr. Frangoul explained.
Phase 3 trial interim results
In investigating exa-cel for beta-thalassemia, the ongoing CLIMB THAL-111 has enrolled 48 patients with a mean baseline age of 20, with 16 between the ages of 12 and 18. Of the patients, 28 (58.3%) had severe genotypes of disease.
Among 27 patients who were evaluable for the study endpoints of the current interim analysis, 24 (88.9%), achieved the primary endpoint of maintaining a weighted average hemoglobin of at least 9 g/dL without the need for a transfusion for at least 12 months (P < .0001).
Patients who achieved the transfusion independence for at least 12 months remained transfusion-free for a mean duration of 20.5 months, with a range of 12.1-40.7 months.
Of 3 patients who did not achieve the 12-month transfusion-free endpoint, substantial reductions in transfusion volume were nevertheless achieved, of 70.3%, 79.6%, and 95.5%, among the 3.
And for the CLIMB SCD-121 trial of SCD, 35 participants have been dosed with exa-cel; in the primary efficacy set of 17 patients, 16 of the 17 (94.1%) achieved the primary endpoint of having no severe vaso-occlusive crises for at least 12 months (P < .0001).
All patients, however, achieved the secondary endpoint of being free from in-patient hospitalizations for severe vaso-occlusive crises for at least 12 months (P < .0001).
Patients who achieved freedom from vaso-occlusive crises for at least 12 months remained free of the events for a mean of 18.7 months, ranging from 13.1 months to 36.5 months.
Durability, patient-reported outcomes favorable
Importantly, in both studies, hemoglobin levels, as well as levels of the edited BCL11A alleles in bone marrow CD34+ and peripheral blood nucleated cells, showed sustained stability over time, indicating durable editing of the cells, Dr. Locatelli said.
In terms of patient-reported quality-of-life, measures significantly improved during both trials at 24 months of follow-up, with significant improvements on the EuroQol visual analog scale, Functional Assessment of Cancer Therapy–General, and the Bone Marrow Transplantation Subscale.
Safety results were consistent with those observed with myeloablative busulfan-based conditioning regimen and autologous transplantation procedures, with adverse events that were manageable.
In the beta-thalassemia study, two patients experienced serious adverse events that were determined to be related to exa-cel, including one patient having symptoms in the context of hemophagocytic lymphohistiocytosis.
For the other patient, the serious adverse events consisted of delayed engraftment and thrombocytopenia, each also considered related to busulfan. None of the patients with SCD had serious adverse events related to exa-cel.
All serious adverse events were resolved, with no reports of deaths, study discontinuations, or malignancies.
Potentially first ever CRISPR-based FDA approval
While CRISPR-CAS9 gene editing is being investigated in multiple other trials in humans for various disorders, to date none have received FDA approval, which would make an approval for exa-cel a landmark development.
The therapy is currently under review, and Dr. Frangoul said the FDA has stated that a decision on the indication for SCD is expected by Dec. 8, 2023, and for beta-thalassemia, by March 2024.
Commenting on the research, Raffaella Colombatti, MD, a pediatric hematologist-oncologist and assistant professor of pediatrics at the University of Padova (Italy), underscored the need for a better curative alternative.
“Unfortunately, the other curative option, bone marrow transplant, is not available for all candidates due to the lack of suitable donors,” Dr. Colombatti said in an interview.
“And, although there are promising results from alternative donors and new conditioning regimens, a further option for selected patients with sickle cell disease and thalassemia utilizing gene therapy and gene editing is needed.”
Caveats regarding gene therapy for the two diseases that still need consideration include: “long-term safety results are still not available and eligibility criteria still needs to be explored outside clinical trials,” she said.
Furthermore, “costs and sustainability are also an issue,” Dr. Colombatti added.
The price of gene therapy is not cheap. With beti-cel priced at more than $2 million for the treatment, its manufacturer, Bluebird Bio, has reportedly already indicated that it will not pursue marketing in Europe because of unfavorable reimbursement policies, and a similar high price is anticipated for exa-cel.
Overall, however, the findings bode well for groundbreaking improvements in treatment of the two red blood cell disorders, Michael J. Eckrich, MD, MPH, medical director of pediatric stem cell transplant & cellular therapy at Atrium Health Levine Children’s Hospital Cancer and Blood Disorders in Charlotte, N.C., said in an interview.
“I do think that this is transformative therapy and will change our approach for patients with severe sickle cell disease in need of transplant,” said Dr. Eckrich, who has also been an investigator on the research of exa-cel for sickle cell disease.
“It might not be hard to imagine, that with the progress in gene therapies and gene editing, that allogeneic transplant will soon become obsolete for patients with sickle cell disease and beta-thalassemia.”
Dr. Locatelli is on the advisory board for Vertex Pharma and the speaker’s bureau for BluebirdBio. Dr. Frangoul and Dr. Colombatti are or have been consultants for Vertex Pharma.
Results from the prespecified interim analyses of the phase 3 CLIMB THAL-111 and CLIMB SCD-121 studies, presented at the European Hematology Association annual congress, show that patients with beta-thalassemia who received exa-cel were able to remain transfusion-free for up to 40.7 consecutive months, while in patients with sickle cell disease, the treatment likewise provided up to 36.5 months of freedom from vaso-occlusive crises.
The findings underscore that “exa-cel can provide a one-time, functional cure to patients with beta-thalassemia and sickle cell disease,” said coauthor Franco Locatelli, MD, of Catholic University of the Sacred Heart, Bambino Gesù Children’s Hospital, Rome.
In a comment, senior investigator Haydar Frangoul, MD, noted that, “with almost 4 years of follow-up on patients with beta-thalassemia and sickle cell disease, it appears that the benefit is holding.”
“The engraftment of our edited cells appears very stable over time. There is no reason to believe it will change,” said Dr. Frangoul, who is medical director of pediatric hematology/oncology, Sarah Cannon Center for Blood Cancer at The Children’s Hospital at TriStar Centennial, Nashville, Tenn.
Burden is high; current curative options have caveats
Patients with transfusion-dependent beta-thalassemia may require blood transfusions as often as every 2-5 weeks because of genetic mutations causing the absence of functional hemoglobin and subsequent depletions in red blood cells. And with hemoglobin being an iron-rich protein, patients are also at risk of an iron accumulation in the body, adding the possible need for uncomfortable iron chelation therapy to prevent organ damage.
The measures are burdensome, but the need is dire. Life expectancy in beta-thalassemia without them is only about 5 years.
With SCD, patients can face severe pain from vaso-occlusive crises as sickled red blood cells block blood flow, potentially causing hospitalization and complications including kidney failure or stroke.
A cure does already exist for both genetic disorders in the form of allogeneic stem cell transplantation. However, that option requires a matched related stem cell donor, and fewer than 20% of patients have accessibility to such donors.
Gene therapy
Gene therapy offers a potentially ideal alternative, providing a possible “functional cure” without the need for a donor, by instead harvesting patients’ cells, fixing the mutation and transferring them back to the patient.
The Food and Drug Administration already approved a first gene therapy, betibeglogene autotemcel (beti-cel), for children and adults with transfusion dependent beta-thalassemia, in August 2022.
While beti-cel utilizes a viral vector to insert functional copies of a modified gene into patients’ extracted hematopoietic stem cells before transfusing them back, exa-cel instead uses CRISPR-CAS9 technology to edit the gene, allowing the body to produce fetal hemoglobin, in an approach believed to be more precise and efficient.
“As we explain to patients, it’s a difference between gene addition, which is what beti-cel is, or gene editing, which is what exa-cel is,” Dr. Frangoul explained.
Phase 3 trial interim results
In investigating exa-cel for beta-thalassemia, the ongoing CLIMB THAL-111 has enrolled 48 patients with a mean baseline age of 20, with 16 between the ages of 12 and 18. Of the patients, 28 (58.3%) had severe genotypes of disease.
Among 27 patients who were evaluable for the study endpoints of the current interim analysis, 24 (88.9%), achieved the primary endpoint of maintaining a weighted average hemoglobin of at least 9 g/dL without the need for a transfusion for at least 12 months (P < .0001).
Patients who achieved the transfusion independence for at least 12 months remained transfusion-free for a mean duration of 20.5 months, with a range of 12.1-40.7 months.
Of 3 patients who did not achieve the 12-month transfusion-free endpoint, substantial reductions in transfusion volume were nevertheless achieved, of 70.3%, 79.6%, and 95.5%, among the 3.
And for the CLIMB SCD-121 trial of SCD, 35 participants have been dosed with exa-cel; in the primary efficacy set of 17 patients, 16 of the 17 (94.1%) achieved the primary endpoint of having no severe vaso-occlusive crises for at least 12 months (P < .0001).
All patients, however, achieved the secondary endpoint of being free from in-patient hospitalizations for severe vaso-occlusive crises for at least 12 months (P < .0001).
Patients who achieved freedom from vaso-occlusive crises for at least 12 months remained free of the events for a mean of 18.7 months, ranging from 13.1 months to 36.5 months.
Durability, patient-reported outcomes favorable
Importantly, in both studies, hemoglobin levels, as well as levels of the edited BCL11A alleles in bone marrow CD34+ and peripheral blood nucleated cells, showed sustained stability over time, indicating durable editing of the cells, Dr. Locatelli said.
In terms of patient-reported quality-of-life, measures significantly improved during both trials at 24 months of follow-up, with significant improvements on the EuroQol visual analog scale, Functional Assessment of Cancer Therapy–General, and the Bone Marrow Transplantation Subscale.
Safety results were consistent with those observed with myeloablative busulfan-based conditioning regimen and autologous transplantation procedures, with adverse events that were manageable.
In the beta-thalassemia study, two patients experienced serious adverse events that were determined to be related to exa-cel, including one patient having symptoms in the context of hemophagocytic lymphohistiocytosis.
For the other patient, the serious adverse events consisted of delayed engraftment and thrombocytopenia, each also considered related to busulfan. None of the patients with SCD had serious adverse events related to exa-cel.
All serious adverse events were resolved, with no reports of deaths, study discontinuations, or malignancies.
Potentially first ever CRISPR-based FDA approval
While CRISPR-CAS9 gene editing is being investigated in multiple other trials in humans for various disorders, to date none have received FDA approval, which would make an approval for exa-cel a landmark development.
The therapy is currently under review, and Dr. Frangoul said the FDA has stated that a decision on the indication for SCD is expected by Dec. 8, 2023, and for beta-thalassemia, by March 2024.
Commenting on the research, Raffaella Colombatti, MD, a pediatric hematologist-oncologist and assistant professor of pediatrics at the University of Padova (Italy), underscored the need for a better curative alternative.
“Unfortunately, the other curative option, bone marrow transplant, is not available for all candidates due to the lack of suitable donors,” Dr. Colombatti said in an interview.
“And, although there are promising results from alternative donors and new conditioning regimens, a further option for selected patients with sickle cell disease and thalassemia utilizing gene therapy and gene editing is needed.”
Caveats regarding gene therapy for the two diseases that still need consideration include: “long-term safety results are still not available and eligibility criteria still needs to be explored outside clinical trials,” she said.
Furthermore, “costs and sustainability are also an issue,” Dr. Colombatti added.
The price of gene therapy is not cheap. With beti-cel priced at more than $2 million for the treatment, its manufacturer, Bluebird Bio, has reportedly already indicated that it will not pursue marketing in Europe because of unfavorable reimbursement policies, and a similar high price is anticipated for exa-cel.
Overall, however, the findings bode well for groundbreaking improvements in treatment of the two red blood cell disorders, Michael J. Eckrich, MD, MPH, medical director of pediatric stem cell transplant & cellular therapy at Atrium Health Levine Children’s Hospital Cancer and Blood Disorders in Charlotte, N.C., said in an interview.
“I do think that this is transformative therapy and will change our approach for patients with severe sickle cell disease in need of transplant,” said Dr. Eckrich, who has also been an investigator on the research of exa-cel for sickle cell disease.
“It might not be hard to imagine, that with the progress in gene therapies and gene editing, that allogeneic transplant will soon become obsolete for patients with sickle cell disease and beta-thalassemia.”
Dr. Locatelli is on the advisory board for Vertex Pharma and the speaker’s bureau for BluebirdBio. Dr. Frangoul and Dr. Colombatti are or have been consultants for Vertex Pharma.
FROM EHA 2023
Weighing childhood obesity interventions
A teenager who weighed 300 lb and was homeschooled because he was too big to fit in a classroom chair is among the patients Manal Habib, MD, has seen in her pediatric endocrinology practice.
The boy, a social butterfly who hated isolation and blamed himself for his “poor choices,” turned out to have an MC4R mutation that interfered with proper metabolism and satiation signals.
“People often blame obese and overweight people for not having enough willpower, but it’s often a physiological problem,” said Dr. Habib, who works at the University of California, Los Angeles.
She is among the clinicians offering more aggressive forms of weight management, prescribing medications, including metformin, semaglutide, and liraglutide – often off-label – to help children and teens with obesity who do not respond to lifestyle changes.
The results of intensive interventions can be life-changing: The teen Dr. Habib treated is back at school, playing sports, and no longer needs drugs to reduce cholesterol and blood pressure. He now takes a low maintenance dose of a weight-loss medication.
But the long-term effects of these new agents on children and teens are poorly understood, and both medication and surgery are associated with significant complications. Pediatricians treating kids pre- or post-intervention should be alert to a range of physical, psychological, and behavioral risks and complications.
Keeping bones healthy
Pediatricians should be aware of the risk to bone health in patients who undergo surgery, according to Misra Madhusmita, MD, chief of pediatric endocrinology at Massachusetts General Hospital in Boston. In a recent study, Dr. Madhusmita and her colleagues found that sleeve gastrectomy reduced vertebral bone strength in adolescents and young adults.
“This is a time of life when bone mass is typically accruing rapidly,” Dr. Madhusmita told this news organization. “A deleterious effect on bone accrual at this time of life raises concerns about suboptimal acquisition of peak bone mass, which is typically attained in early adult life and is a key factor determining bone health and fracture risk in later life.”
Reduced skeletal loading and muscle mass can weaken bones, as can malabsorption of nutrients. Fat loss can trigger lower levels of bioavailable androgens and their subsequent conversion to estrogen, negatively affecting bone density. And sleeve gastrectomy in particular lowers ghrelin, another hormone influencing skeletal health.
Clinicians should advise patients who have had surgery to follow a healthy diet and consume sufficient levels of calcium and vitamin D, said Dr. Madhusmita. Weight-bearing exercises, weight training, and resistance training are also imperative to build bone mass and muscle. Any preexisting conditions or lifestyle factors that weaken the bones should be taken into consideration.
Managing expectations
The long-term effects of weight loss medications on children are less documented than with surgery, according to Caren Mangarelli, MD, a former primary care physician who is now medical director of both the adolescent bariatric program and the children’s wellness and weight management clinic at Lurie Children’s Hospital in Chicago, Ill.
But one significant known risk is the potential for rebound weight gain and the complications like high blood pressure and high blood sugar that go with it if the patient stops medication. Dr. Mangarelli said that many clinicians lack the training required to safely facilitate weight loss medications for kids.
“We have to remember that obesity is a chronic disease, especially for those with more severe forms,” she said. “They’re not likely to outgrow it. It’s not like, ‘Oh, we’ll just put a patient on medication, they’ll lose weight, and we’ll take them off of it,’ because you could create a bad cycle of losing weight, followed by metabolism slowing down, hunger cues going up, and weight going back up.”
Making the risks of stopping medication clear and supporting compliance are essential, especially when it comes to injectables like semaglutide, which can be more burdensome than taking pills, requiring weekly subcutaneous injections. Pediatricians should ensure that families understand that medication is a long-term solution, Dr. Habib said.
Many families and patients “want a quick result. They’re focused on a specific size or weight, and they want to take the medication for a short period without changing anything else,” Dr. Habib said.
But children with genetic abnormalities or severe obesity could be on medication for their entire lives. Patients who make significant healthy lifestyle changes have a greater chance of weaning off drug therapy.
But “it’s hard with children because they’re dependent on their family,” Dr. Habib said. “One of the first things that I talk about with families is that it’s very important for everyone to be involved in making healthy changes, especially the parents, because the kids are going to follow their lifestyle and choices, not necessarily what they tell them to do.”
The behavioral and mental
One of Dr. Habib’s most striking cases was a 6-year-old patient with autism spectrum disorder experiencing early-onset puberty. Her condition made it difficult for her parents to enforce behavioral and lifestyle changes, making medication the best option to normalize the young girl’s body.
“The goal in this case is not necessarily to help her lose weight, but to prevent her from having severe health risks at such a young age,” said Habib.
Though medication may be the best solution when other options have failed, the ease of using medication may mean clinicians fail to address the complex emotional and psychosocial factors that can both cause and result from obesity.
“A lot of families think that if just this one thing were better, everything else would fall into place,” Habib said. “But there often are multiple layers to treating the patient.”
According to Cambria Garrell, MD, a pediatrician at the UCLA Fit for Healthy Weight Program in Los Angeles, pediatricians should be aware of the psychosocial and mental health factors such as undiagnosed mental illness or family dysfunction.
Dr. Garrell sometimes cares for children with undiagnosed mental health disorders. Children with conditions like attention-deficit/hyperactivity disorder and autism spectrum disorders may struggle with eating because of impulse control and sensory processing issues. Family functioning, issues at school, and lack of sleep are also major contributors to obesity to screen for.
“We really like to think about the environmental and psychosocial factors contributing to obesity instead of just pathologizing the weight,” Dr. Garrell said.
Risk for alcohol abuse
Bariatric and metabolic surgeries are associated with an increased risk for alcohol use disorder (AUD). Pediatricians treating children pre- or post-op should ensure that patients receive behavioral and mental health services to minimize the risk for alcohol abuse.
The risk for AUD is likely the result of changes to the way the body metabolizes alcohol, resulting in heightened sensitivity to it, although research is not conclusive, according to Dr. Mangarelli.
The risk for AUD is likely multifactorial, Dr. Mangarelli said.
“We don’t totally understand all of it, but if you’re experiencing a high more easily, that may lead to misuse,” Dr. Mangarelli said. “It’s also important to remember that this population of patients has experienced stigma for a very long time, and they often have associated mental health and body image issues.”
“Those problems don’t disappear on their own,” she added. “You want to make sure that patients are hooked into behavioral and mental health services before surgery so that they have somebody who’s following them after surgery.”
A version of this article first appeared on Medscape.com.
A teenager who weighed 300 lb and was homeschooled because he was too big to fit in a classroom chair is among the patients Manal Habib, MD, has seen in her pediatric endocrinology practice.
The boy, a social butterfly who hated isolation and blamed himself for his “poor choices,” turned out to have an MC4R mutation that interfered with proper metabolism and satiation signals.
“People often blame obese and overweight people for not having enough willpower, but it’s often a physiological problem,” said Dr. Habib, who works at the University of California, Los Angeles.
She is among the clinicians offering more aggressive forms of weight management, prescribing medications, including metformin, semaglutide, and liraglutide – often off-label – to help children and teens with obesity who do not respond to lifestyle changes.
The results of intensive interventions can be life-changing: The teen Dr. Habib treated is back at school, playing sports, and no longer needs drugs to reduce cholesterol and blood pressure. He now takes a low maintenance dose of a weight-loss medication.
But the long-term effects of these new agents on children and teens are poorly understood, and both medication and surgery are associated with significant complications. Pediatricians treating kids pre- or post-intervention should be alert to a range of physical, psychological, and behavioral risks and complications.
Keeping bones healthy
Pediatricians should be aware of the risk to bone health in patients who undergo surgery, according to Misra Madhusmita, MD, chief of pediatric endocrinology at Massachusetts General Hospital in Boston. In a recent study, Dr. Madhusmita and her colleagues found that sleeve gastrectomy reduced vertebral bone strength in adolescents and young adults.
“This is a time of life when bone mass is typically accruing rapidly,” Dr. Madhusmita told this news organization. “A deleterious effect on bone accrual at this time of life raises concerns about suboptimal acquisition of peak bone mass, which is typically attained in early adult life and is a key factor determining bone health and fracture risk in later life.”
Reduced skeletal loading and muscle mass can weaken bones, as can malabsorption of nutrients. Fat loss can trigger lower levels of bioavailable androgens and their subsequent conversion to estrogen, negatively affecting bone density. And sleeve gastrectomy in particular lowers ghrelin, another hormone influencing skeletal health.
Clinicians should advise patients who have had surgery to follow a healthy diet and consume sufficient levels of calcium and vitamin D, said Dr. Madhusmita. Weight-bearing exercises, weight training, and resistance training are also imperative to build bone mass and muscle. Any preexisting conditions or lifestyle factors that weaken the bones should be taken into consideration.
Managing expectations
The long-term effects of weight loss medications on children are less documented than with surgery, according to Caren Mangarelli, MD, a former primary care physician who is now medical director of both the adolescent bariatric program and the children’s wellness and weight management clinic at Lurie Children’s Hospital in Chicago, Ill.
But one significant known risk is the potential for rebound weight gain and the complications like high blood pressure and high blood sugar that go with it if the patient stops medication. Dr. Mangarelli said that many clinicians lack the training required to safely facilitate weight loss medications for kids.
“We have to remember that obesity is a chronic disease, especially for those with more severe forms,” she said. “They’re not likely to outgrow it. It’s not like, ‘Oh, we’ll just put a patient on medication, they’ll lose weight, and we’ll take them off of it,’ because you could create a bad cycle of losing weight, followed by metabolism slowing down, hunger cues going up, and weight going back up.”
Making the risks of stopping medication clear and supporting compliance are essential, especially when it comes to injectables like semaglutide, which can be more burdensome than taking pills, requiring weekly subcutaneous injections. Pediatricians should ensure that families understand that medication is a long-term solution, Dr. Habib said.
Many families and patients “want a quick result. They’re focused on a specific size or weight, and they want to take the medication for a short period without changing anything else,” Dr. Habib said.
But children with genetic abnormalities or severe obesity could be on medication for their entire lives. Patients who make significant healthy lifestyle changes have a greater chance of weaning off drug therapy.
But “it’s hard with children because they’re dependent on their family,” Dr. Habib said. “One of the first things that I talk about with families is that it’s very important for everyone to be involved in making healthy changes, especially the parents, because the kids are going to follow their lifestyle and choices, not necessarily what they tell them to do.”
The behavioral and mental
One of Dr. Habib’s most striking cases was a 6-year-old patient with autism spectrum disorder experiencing early-onset puberty. Her condition made it difficult for her parents to enforce behavioral and lifestyle changes, making medication the best option to normalize the young girl’s body.
“The goal in this case is not necessarily to help her lose weight, but to prevent her from having severe health risks at such a young age,” said Habib.
Though medication may be the best solution when other options have failed, the ease of using medication may mean clinicians fail to address the complex emotional and psychosocial factors that can both cause and result from obesity.
“A lot of families think that if just this one thing were better, everything else would fall into place,” Habib said. “But there often are multiple layers to treating the patient.”
According to Cambria Garrell, MD, a pediatrician at the UCLA Fit for Healthy Weight Program in Los Angeles, pediatricians should be aware of the psychosocial and mental health factors such as undiagnosed mental illness or family dysfunction.
Dr. Garrell sometimes cares for children with undiagnosed mental health disorders. Children with conditions like attention-deficit/hyperactivity disorder and autism spectrum disorders may struggle with eating because of impulse control and sensory processing issues. Family functioning, issues at school, and lack of sleep are also major contributors to obesity to screen for.
“We really like to think about the environmental and psychosocial factors contributing to obesity instead of just pathologizing the weight,” Dr. Garrell said.
Risk for alcohol abuse
Bariatric and metabolic surgeries are associated with an increased risk for alcohol use disorder (AUD). Pediatricians treating children pre- or post-op should ensure that patients receive behavioral and mental health services to minimize the risk for alcohol abuse.
The risk for AUD is likely the result of changes to the way the body metabolizes alcohol, resulting in heightened sensitivity to it, although research is not conclusive, according to Dr. Mangarelli.
The risk for AUD is likely multifactorial, Dr. Mangarelli said.
“We don’t totally understand all of it, but if you’re experiencing a high more easily, that may lead to misuse,” Dr. Mangarelli said. “It’s also important to remember that this population of patients has experienced stigma for a very long time, and they often have associated mental health and body image issues.”
“Those problems don’t disappear on their own,” she added. “You want to make sure that patients are hooked into behavioral and mental health services before surgery so that they have somebody who’s following them after surgery.”
A version of this article first appeared on Medscape.com.
A teenager who weighed 300 lb and was homeschooled because he was too big to fit in a classroom chair is among the patients Manal Habib, MD, has seen in her pediatric endocrinology practice.
The boy, a social butterfly who hated isolation and blamed himself for his “poor choices,” turned out to have an MC4R mutation that interfered with proper metabolism and satiation signals.
“People often blame obese and overweight people for not having enough willpower, but it’s often a physiological problem,” said Dr. Habib, who works at the University of California, Los Angeles.
She is among the clinicians offering more aggressive forms of weight management, prescribing medications, including metformin, semaglutide, and liraglutide – often off-label – to help children and teens with obesity who do not respond to lifestyle changes.
The results of intensive interventions can be life-changing: The teen Dr. Habib treated is back at school, playing sports, and no longer needs drugs to reduce cholesterol and blood pressure. He now takes a low maintenance dose of a weight-loss medication.
But the long-term effects of these new agents on children and teens are poorly understood, and both medication and surgery are associated with significant complications. Pediatricians treating kids pre- or post-intervention should be alert to a range of physical, psychological, and behavioral risks and complications.
Keeping bones healthy
Pediatricians should be aware of the risk to bone health in patients who undergo surgery, according to Misra Madhusmita, MD, chief of pediatric endocrinology at Massachusetts General Hospital in Boston. In a recent study, Dr. Madhusmita and her colleagues found that sleeve gastrectomy reduced vertebral bone strength in adolescents and young adults.
“This is a time of life when bone mass is typically accruing rapidly,” Dr. Madhusmita told this news organization. “A deleterious effect on bone accrual at this time of life raises concerns about suboptimal acquisition of peak bone mass, which is typically attained in early adult life and is a key factor determining bone health and fracture risk in later life.”
Reduced skeletal loading and muscle mass can weaken bones, as can malabsorption of nutrients. Fat loss can trigger lower levels of bioavailable androgens and their subsequent conversion to estrogen, negatively affecting bone density. And sleeve gastrectomy in particular lowers ghrelin, another hormone influencing skeletal health.
Clinicians should advise patients who have had surgery to follow a healthy diet and consume sufficient levels of calcium and vitamin D, said Dr. Madhusmita. Weight-bearing exercises, weight training, and resistance training are also imperative to build bone mass and muscle. Any preexisting conditions or lifestyle factors that weaken the bones should be taken into consideration.
Managing expectations
The long-term effects of weight loss medications on children are less documented than with surgery, according to Caren Mangarelli, MD, a former primary care physician who is now medical director of both the adolescent bariatric program and the children’s wellness and weight management clinic at Lurie Children’s Hospital in Chicago, Ill.
But one significant known risk is the potential for rebound weight gain and the complications like high blood pressure and high blood sugar that go with it if the patient stops medication. Dr. Mangarelli said that many clinicians lack the training required to safely facilitate weight loss medications for kids.
“We have to remember that obesity is a chronic disease, especially for those with more severe forms,” she said. “They’re not likely to outgrow it. It’s not like, ‘Oh, we’ll just put a patient on medication, they’ll lose weight, and we’ll take them off of it,’ because you could create a bad cycle of losing weight, followed by metabolism slowing down, hunger cues going up, and weight going back up.”
Making the risks of stopping medication clear and supporting compliance are essential, especially when it comes to injectables like semaglutide, which can be more burdensome than taking pills, requiring weekly subcutaneous injections. Pediatricians should ensure that families understand that medication is a long-term solution, Dr. Habib said.
Many families and patients “want a quick result. They’re focused on a specific size or weight, and they want to take the medication for a short period without changing anything else,” Dr. Habib said.
But children with genetic abnormalities or severe obesity could be on medication for their entire lives. Patients who make significant healthy lifestyle changes have a greater chance of weaning off drug therapy.
But “it’s hard with children because they’re dependent on their family,” Dr. Habib said. “One of the first things that I talk about with families is that it’s very important for everyone to be involved in making healthy changes, especially the parents, because the kids are going to follow their lifestyle and choices, not necessarily what they tell them to do.”
The behavioral and mental
One of Dr. Habib’s most striking cases was a 6-year-old patient with autism spectrum disorder experiencing early-onset puberty. Her condition made it difficult for her parents to enforce behavioral and lifestyle changes, making medication the best option to normalize the young girl’s body.
“The goal in this case is not necessarily to help her lose weight, but to prevent her from having severe health risks at such a young age,” said Habib.
Though medication may be the best solution when other options have failed, the ease of using medication may mean clinicians fail to address the complex emotional and psychosocial factors that can both cause and result from obesity.
“A lot of families think that if just this one thing were better, everything else would fall into place,” Habib said. “But there often are multiple layers to treating the patient.”
According to Cambria Garrell, MD, a pediatrician at the UCLA Fit for Healthy Weight Program in Los Angeles, pediatricians should be aware of the psychosocial and mental health factors such as undiagnosed mental illness or family dysfunction.
Dr. Garrell sometimes cares for children with undiagnosed mental health disorders. Children with conditions like attention-deficit/hyperactivity disorder and autism spectrum disorders may struggle with eating because of impulse control and sensory processing issues. Family functioning, issues at school, and lack of sleep are also major contributors to obesity to screen for.
“We really like to think about the environmental and psychosocial factors contributing to obesity instead of just pathologizing the weight,” Dr. Garrell said.
Risk for alcohol abuse
Bariatric and metabolic surgeries are associated with an increased risk for alcohol use disorder (AUD). Pediatricians treating children pre- or post-op should ensure that patients receive behavioral and mental health services to minimize the risk for alcohol abuse.
The risk for AUD is likely the result of changes to the way the body metabolizes alcohol, resulting in heightened sensitivity to it, although research is not conclusive, according to Dr. Mangarelli.
The risk for AUD is likely multifactorial, Dr. Mangarelli said.
“We don’t totally understand all of it, but if you’re experiencing a high more easily, that may lead to misuse,” Dr. Mangarelli said. “It’s also important to remember that this population of patients has experienced stigma for a very long time, and they often have associated mental health and body image issues.”
“Those problems don’t disappear on their own,” she added. “You want to make sure that patients are hooked into behavioral and mental health services before surgery so that they have somebody who’s following them after surgery.”
A version of this article first appeared on Medscape.com.