User login
What should be the interval between bone density screenings?
In 2010, the United States Preventive Services Task Force recommended screening for osteoporosis by measuring bone mineral density in women age 65 and older and also in younger women if their fracture risk is equal to or greater than that of a 65-year-old white woman who has no additional risk factors.
But what should be the interval between screenings? The Task Force stated that evidence on the optimum screening interval is lacking, that 2 years may be the minimum interval due to precision error, but that longer intervals may be necessary to improve fracture risk prediction.1 They also cited a study showing that repeating the test up to 8 years after an initial test did not improve the ability of screening to predict fractures.2 This was recently confirmed in a study from Canada.3
GOURLAY ET AL: TEST AGAIN IN 1 TO 15 YEARS
In response to this information void, Gourlay and colleagues4 analyzed data from the Study of Osteoporotic Fractures. Because these investigators were interested in the interval between screening measurements of bone mineral density, they included only women who did not already have osteoporosis or take medication for osteoporosis. They wanted to know how long it took for 10% of women to develop osteoporosis, and found that this interval varied from 1 to 15 years depending on the initial bone density.
I did not think these results were surprising. The durations in which osteoporosis developed were similar to what one would predict from cross-sectional reference ranges. The average woman loses a little less than 1% of bone density per year after age 65. A T score of −1.0 is 22% higher than a T score of −2.5, so on average it would take more than 20 years to go from early osteopenia to osteoporosis.
AN ONGOING DEBATE ON SCREENING
The report generated a debate about the value and timing of repeated screening.5,6
In their article “More bone density testing is needed, not less,”5 Lewiecki et al criticized the Gourlay analysis because it did not include spine measurements or screen for asymptomatic vertebral fractures, and because it did not include enough clinical risk factors.5,6 They claimed that media attention suggested that dual-energy absorptiometry (DXA) was overused and expensive, citing three news reports. One of the news reports did misinterpret the Gourlay study and suggested that fewer women should be screened.7 The others, however, accurately described the findings that many women did not need to undergo DXA every 2 years.8,9
In this issue of the Cleveland Clinic Journal of Medicine, Doshi and colleagues express their opinion that the interval between bone mineral density testings should be guided by an assessment of clinical risk factors and not just T scores.10
Doshi et al are also concerned about erroneous conclusions drawn by the media. However, when I reviewed the news reports that they cited, I thought the reports were well written and conveyed the results appropriately. One report, by Alice Park,11 cautioned: “doctors need to remain flexible in advising women about when to get tested. A patient who has a normal T score but then develops cancer and loses a lot of weight, for example, may be more vulnerable to developing osteoporosis and therefore may need to get screened before the 15-year interval.”11 The other, by Gina Kolata, also explained that those taking high doses of corticosteroids for another medical condition would lose bone rapidly, but the findings “cover most normal women.”9 Neither report discouraged patients from getting screening in the first place.
Both Lewiecki et al and Doshi et al say that clinical factors should be considered, but do not specify which factors should be included in addition to the ones already evaluated by Gourlay et al (age, body mass index, estrogen use at baseline, any fracture after 50 years of age, current smoking, current or past use of oral glucocorticoids, and self-reported rheumatoid arthritis). These did not change the estimated time to develop osteoporosis for 90% of the study participants.
Furthermore, Gourlay et al had already noted that “clinicians may choose to reevaluate patients before our estimated screening intervals if there is evidence of decreased activity or mobility, weight loss, or other risk factors not considered in our analyses.”4 Thus, patients with serious diseases should undergo DXA not for screening but for monitoring disease progression, and the Gourlay study results do not apply to them.
PATIENTS ON GLUCOCORTICOIDS: A SPECIAL SUBSET
Patients who are treated with glucocorticoids deserve further discussion. Consider the example described by Doshi et al of a woman with rheumatoid arthritis, taking prednisone, with a T score of −1.4. She would have to lose about 17% of her bone density to reach a T score at the osteoporosis level. One clinical trial in patients taking glucocorticoids, most of whom had rheumatoid arthritis, reported a loss of 2% after 2 years in the placebo group,12 so it is unlikely that this patient would have bone density in the osteoporosis range for at least several years.
However, clinicians know that these patients get fractures, especially in the spine, even with a normal bone density. Therefore, vertebral fracture assessment would be more important than bone density screening in this patient. Currently, there is uncertainty about the best time to initiate treatment in patients taking these glucocortical steroids, as well as the choice of initial medication. More research about long-term benefits of treatment are especially needed in this population.
VERTEBRAL FRACTURES: NO FIRM RECOMMENDATIONS
Doshi et al state that the Gourlay study was biased towards longer screening intervals because it included women with asymptomatic vertebral fractures. This does not make sense, because women who have untreated asymptomatic fractures would not be expected to lose bone at a slower rate. This does not mean that the asymptomatic fractures are trivial.
Instead of getting more frequent bone density measurements, I think it would be more logical to evaluate vertebral fractures using radiographs or vertebral fracture assessment, but we can’t make a firm recommendation without studies of the effectiveness of screening for vertebral fractures.
WHAT ABOUT OSTEOPENIA?
Critics of the Gourlay study point out that most fractures occur in the osteopenic population. This is true, but it does not mean that bone density should be measured more frequently. The bisphosphonates are not effective at preventing a first fracture unless the T score is lower than −2.5.13 Patients who have risk factors in addition to osteopenia may have a higher risk of fracture, but it is not clear if this can be treated with medication. For example, rodeo riders have a high fracture risk, but they would not benefit from taking alendronate. In some cases, such as people who smoke or drink alcohol to excess, treating the risk factor would be more appropriate.
As Doshi et al and others have noted, the study by Gourlay et al has limitations, and of course clinical judgment must be used in implementing the findings of any study. But doctors should not order unnecessary and expensive tests, and physicians who perform bone densitometry should not recommend frequent repeat testing that does not benefit the patient.
- US Preventive Services Task Force. Screening for osteoporosis: US preventive services task force recommendation statement. Ann Intern Med 2011; 154:356–364.
- Hillier TA, Stone KL, Bauer DC, et al. Evaluating the value of repeat bone mineral density measurement and prediction of fractures in older women: the study of osteoporotic fractures. Arch Intern Med 2007; 167:155–160.
- Leslie WD, Morin SN, Lix LM; Manitoba Bone Density Program. Rate of bone density change does not enhance fracture prediction in routine clinical practice. J Clin Endocrinol Metab 2012; 97:1211–1218.
- Gourlay ML, Fine J P, Preisser JS, et al; Study of Osteoporotic Fractures Research Group. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med 2012; 366:225–233.
- Lewiecki EM, Laster AJ, Miller PD, Bilezikian JP. More bone density testing is needed, not less. J Bone Miner Res 2012; 27:739–742.
- Yu EW, Finkelstein JS. Bone density screening intervals for osteoporosis: one size does not fit all. JAMA 2012; 307:2591–2592.
- Frier S. Women receive bone tests too often for osteoporosis, study finds. Bloomberg News; 2012. http://www.bloomberg.com/news/2012-01-18/many-women-screened-for-osteoporosis-don-t-need-it-researchers-report.html. Accessed January 3, 2013.
- Knox R. Many older women may not need frequent bone scans. National Public Radio; 2012. http://www.npr.org/blogs/health/2012/01/19/145419138/many-older-women-may-not-need-frequent-bone-scans?ps=sh_sthdl. Accessed January 3, 2013.
- Kolata G. Patients with normal bone density can delay retests, study suggests. The New York Times; 2012. http://www.nytimes.com/2012/01/19/health/bone-density-tests-for-osteoporosis-can-wait-study-says.html. Accessed January 3, 2013.
- Doshi KB, Khan LZ, Williams SE, Licata AA. Bone mineral density testing interval and transition to osteoporosis in older women: Is a T-score enough to determine a screening interval? Cleve Clin J Med 2013; 80:234–239.
- Park A. How often do women really need bone density tests? Time Healthland; 2012. http://healthland.time.com/2012/01/19/most-women-may-be-getting-too-many-bone-density-tests/. Accessed January 3, 2013.
- Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum 2001; 44:202–211.
- Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 1998; 280:2077–2082.
In 2010, the United States Preventive Services Task Force recommended screening for osteoporosis by measuring bone mineral density in women age 65 and older and also in younger women if their fracture risk is equal to or greater than that of a 65-year-old white woman who has no additional risk factors.
But what should be the interval between screenings? The Task Force stated that evidence on the optimum screening interval is lacking, that 2 years may be the minimum interval due to precision error, but that longer intervals may be necessary to improve fracture risk prediction.1 They also cited a study showing that repeating the test up to 8 years after an initial test did not improve the ability of screening to predict fractures.2 This was recently confirmed in a study from Canada.3
GOURLAY ET AL: TEST AGAIN IN 1 TO 15 YEARS
In response to this information void, Gourlay and colleagues4 analyzed data from the Study of Osteoporotic Fractures. Because these investigators were interested in the interval between screening measurements of bone mineral density, they included only women who did not already have osteoporosis or take medication for osteoporosis. They wanted to know how long it took for 10% of women to develop osteoporosis, and found that this interval varied from 1 to 15 years depending on the initial bone density.
I did not think these results were surprising. The durations in which osteoporosis developed were similar to what one would predict from cross-sectional reference ranges. The average woman loses a little less than 1% of bone density per year after age 65. A T score of −1.0 is 22% higher than a T score of −2.5, so on average it would take more than 20 years to go from early osteopenia to osteoporosis.
AN ONGOING DEBATE ON SCREENING
The report generated a debate about the value and timing of repeated screening.5,6
In their article “More bone density testing is needed, not less,”5 Lewiecki et al criticized the Gourlay analysis because it did not include spine measurements or screen for asymptomatic vertebral fractures, and because it did not include enough clinical risk factors.5,6 They claimed that media attention suggested that dual-energy absorptiometry (DXA) was overused and expensive, citing three news reports. One of the news reports did misinterpret the Gourlay study and suggested that fewer women should be screened.7 The others, however, accurately described the findings that many women did not need to undergo DXA every 2 years.8,9
In this issue of the Cleveland Clinic Journal of Medicine, Doshi and colleagues express their opinion that the interval between bone mineral density testings should be guided by an assessment of clinical risk factors and not just T scores.10
Doshi et al are also concerned about erroneous conclusions drawn by the media. However, when I reviewed the news reports that they cited, I thought the reports were well written and conveyed the results appropriately. One report, by Alice Park,11 cautioned: “doctors need to remain flexible in advising women about when to get tested. A patient who has a normal T score but then develops cancer and loses a lot of weight, for example, may be more vulnerable to developing osteoporosis and therefore may need to get screened before the 15-year interval.”11 The other, by Gina Kolata, also explained that those taking high doses of corticosteroids for another medical condition would lose bone rapidly, but the findings “cover most normal women.”9 Neither report discouraged patients from getting screening in the first place.
Both Lewiecki et al and Doshi et al say that clinical factors should be considered, but do not specify which factors should be included in addition to the ones already evaluated by Gourlay et al (age, body mass index, estrogen use at baseline, any fracture after 50 years of age, current smoking, current or past use of oral glucocorticoids, and self-reported rheumatoid arthritis). These did not change the estimated time to develop osteoporosis for 90% of the study participants.
Furthermore, Gourlay et al had already noted that “clinicians may choose to reevaluate patients before our estimated screening intervals if there is evidence of decreased activity or mobility, weight loss, or other risk factors not considered in our analyses.”4 Thus, patients with serious diseases should undergo DXA not for screening but for monitoring disease progression, and the Gourlay study results do not apply to them.
PATIENTS ON GLUCOCORTICOIDS: A SPECIAL SUBSET
Patients who are treated with glucocorticoids deserve further discussion. Consider the example described by Doshi et al of a woman with rheumatoid arthritis, taking prednisone, with a T score of −1.4. She would have to lose about 17% of her bone density to reach a T score at the osteoporosis level. One clinical trial in patients taking glucocorticoids, most of whom had rheumatoid arthritis, reported a loss of 2% after 2 years in the placebo group,12 so it is unlikely that this patient would have bone density in the osteoporosis range for at least several years.
However, clinicians know that these patients get fractures, especially in the spine, even with a normal bone density. Therefore, vertebral fracture assessment would be more important than bone density screening in this patient. Currently, there is uncertainty about the best time to initiate treatment in patients taking these glucocortical steroids, as well as the choice of initial medication. More research about long-term benefits of treatment are especially needed in this population.
VERTEBRAL FRACTURES: NO FIRM RECOMMENDATIONS
Doshi et al state that the Gourlay study was biased towards longer screening intervals because it included women with asymptomatic vertebral fractures. This does not make sense, because women who have untreated asymptomatic fractures would not be expected to lose bone at a slower rate. This does not mean that the asymptomatic fractures are trivial.
Instead of getting more frequent bone density measurements, I think it would be more logical to evaluate vertebral fractures using radiographs or vertebral fracture assessment, but we can’t make a firm recommendation without studies of the effectiveness of screening for vertebral fractures.
WHAT ABOUT OSTEOPENIA?
Critics of the Gourlay study point out that most fractures occur in the osteopenic population. This is true, but it does not mean that bone density should be measured more frequently. The bisphosphonates are not effective at preventing a first fracture unless the T score is lower than −2.5.13 Patients who have risk factors in addition to osteopenia may have a higher risk of fracture, but it is not clear if this can be treated with medication. For example, rodeo riders have a high fracture risk, but they would not benefit from taking alendronate. In some cases, such as people who smoke or drink alcohol to excess, treating the risk factor would be more appropriate.
As Doshi et al and others have noted, the study by Gourlay et al has limitations, and of course clinical judgment must be used in implementing the findings of any study. But doctors should not order unnecessary and expensive tests, and physicians who perform bone densitometry should not recommend frequent repeat testing that does not benefit the patient.
In 2010, the United States Preventive Services Task Force recommended screening for osteoporosis by measuring bone mineral density in women age 65 and older and also in younger women if their fracture risk is equal to or greater than that of a 65-year-old white woman who has no additional risk factors.
But what should be the interval between screenings? The Task Force stated that evidence on the optimum screening interval is lacking, that 2 years may be the minimum interval due to precision error, but that longer intervals may be necessary to improve fracture risk prediction.1 They also cited a study showing that repeating the test up to 8 years after an initial test did not improve the ability of screening to predict fractures.2 This was recently confirmed in a study from Canada.3
GOURLAY ET AL: TEST AGAIN IN 1 TO 15 YEARS
In response to this information void, Gourlay and colleagues4 analyzed data from the Study of Osteoporotic Fractures. Because these investigators were interested in the interval between screening measurements of bone mineral density, they included only women who did not already have osteoporosis or take medication for osteoporosis. They wanted to know how long it took for 10% of women to develop osteoporosis, and found that this interval varied from 1 to 15 years depending on the initial bone density.
I did not think these results were surprising. The durations in which osteoporosis developed were similar to what one would predict from cross-sectional reference ranges. The average woman loses a little less than 1% of bone density per year after age 65. A T score of −1.0 is 22% higher than a T score of −2.5, so on average it would take more than 20 years to go from early osteopenia to osteoporosis.
AN ONGOING DEBATE ON SCREENING
The report generated a debate about the value and timing of repeated screening.5,6
In their article “More bone density testing is needed, not less,”5 Lewiecki et al criticized the Gourlay analysis because it did not include spine measurements or screen for asymptomatic vertebral fractures, and because it did not include enough clinical risk factors.5,6 They claimed that media attention suggested that dual-energy absorptiometry (DXA) was overused and expensive, citing three news reports. One of the news reports did misinterpret the Gourlay study and suggested that fewer women should be screened.7 The others, however, accurately described the findings that many women did not need to undergo DXA every 2 years.8,9
In this issue of the Cleveland Clinic Journal of Medicine, Doshi and colleagues express their opinion that the interval between bone mineral density testings should be guided by an assessment of clinical risk factors and not just T scores.10
Doshi et al are also concerned about erroneous conclusions drawn by the media. However, when I reviewed the news reports that they cited, I thought the reports were well written and conveyed the results appropriately. One report, by Alice Park,11 cautioned: “doctors need to remain flexible in advising women about when to get tested. A patient who has a normal T score but then develops cancer and loses a lot of weight, for example, may be more vulnerable to developing osteoporosis and therefore may need to get screened before the 15-year interval.”11 The other, by Gina Kolata, also explained that those taking high doses of corticosteroids for another medical condition would lose bone rapidly, but the findings “cover most normal women.”9 Neither report discouraged patients from getting screening in the first place.
Both Lewiecki et al and Doshi et al say that clinical factors should be considered, but do not specify which factors should be included in addition to the ones already evaluated by Gourlay et al (age, body mass index, estrogen use at baseline, any fracture after 50 years of age, current smoking, current or past use of oral glucocorticoids, and self-reported rheumatoid arthritis). These did not change the estimated time to develop osteoporosis for 90% of the study participants.
Furthermore, Gourlay et al had already noted that “clinicians may choose to reevaluate patients before our estimated screening intervals if there is evidence of decreased activity or mobility, weight loss, or other risk factors not considered in our analyses.”4 Thus, patients with serious diseases should undergo DXA not for screening but for monitoring disease progression, and the Gourlay study results do not apply to them.
PATIENTS ON GLUCOCORTICOIDS: A SPECIAL SUBSET
Patients who are treated with glucocorticoids deserve further discussion. Consider the example described by Doshi et al of a woman with rheumatoid arthritis, taking prednisone, with a T score of −1.4. She would have to lose about 17% of her bone density to reach a T score at the osteoporosis level. One clinical trial in patients taking glucocorticoids, most of whom had rheumatoid arthritis, reported a loss of 2% after 2 years in the placebo group,12 so it is unlikely that this patient would have bone density in the osteoporosis range for at least several years.
However, clinicians know that these patients get fractures, especially in the spine, even with a normal bone density. Therefore, vertebral fracture assessment would be more important than bone density screening in this patient. Currently, there is uncertainty about the best time to initiate treatment in patients taking these glucocortical steroids, as well as the choice of initial medication. More research about long-term benefits of treatment are especially needed in this population.
VERTEBRAL FRACTURES: NO FIRM RECOMMENDATIONS
Doshi et al state that the Gourlay study was biased towards longer screening intervals because it included women with asymptomatic vertebral fractures. This does not make sense, because women who have untreated asymptomatic fractures would not be expected to lose bone at a slower rate. This does not mean that the asymptomatic fractures are trivial.
Instead of getting more frequent bone density measurements, I think it would be more logical to evaluate vertebral fractures using radiographs or vertebral fracture assessment, but we can’t make a firm recommendation without studies of the effectiveness of screening for vertebral fractures.
WHAT ABOUT OSTEOPENIA?
Critics of the Gourlay study point out that most fractures occur in the osteopenic population. This is true, but it does not mean that bone density should be measured more frequently. The bisphosphonates are not effective at preventing a first fracture unless the T score is lower than −2.5.13 Patients who have risk factors in addition to osteopenia may have a higher risk of fracture, but it is not clear if this can be treated with medication. For example, rodeo riders have a high fracture risk, but they would not benefit from taking alendronate. In some cases, such as people who smoke or drink alcohol to excess, treating the risk factor would be more appropriate.
As Doshi et al and others have noted, the study by Gourlay et al has limitations, and of course clinical judgment must be used in implementing the findings of any study. But doctors should not order unnecessary and expensive tests, and physicians who perform bone densitometry should not recommend frequent repeat testing that does not benefit the patient.
- US Preventive Services Task Force. Screening for osteoporosis: US preventive services task force recommendation statement. Ann Intern Med 2011; 154:356–364.
- Hillier TA, Stone KL, Bauer DC, et al. Evaluating the value of repeat bone mineral density measurement and prediction of fractures in older women: the study of osteoporotic fractures. Arch Intern Med 2007; 167:155–160.
- Leslie WD, Morin SN, Lix LM; Manitoba Bone Density Program. Rate of bone density change does not enhance fracture prediction in routine clinical practice. J Clin Endocrinol Metab 2012; 97:1211–1218.
- Gourlay ML, Fine J P, Preisser JS, et al; Study of Osteoporotic Fractures Research Group. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med 2012; 366:225–233.
- Lewiecki EM, Laster AJ, Miller PD, Bilezikian JP. More bone density testing is needed, not less. J Bone Miner Res 2012; 27:739–742.
- Yu EW, Finkelstein JS. Bone density screening intervals for osteoporosis: one size does not fit all. JAMA 2012; 307:2591–2592.
- Frier S. Women receive bone tests too often for osteoporosis, study finds. Bloomberg News; 2012. http://www.bloomberg.com/news/2012-01-18/many-women-screened-for-osteoporosis-don-t-need-it-researchers-report.html. Accessed January 3, 2013.
- Knox R. Many older women may not need frequent bone scans. National Public Radio; 2012. http://www.npr.org/blogs/health/2012/01/19/145419138/many-older-women-may-not-need-frequent-bone-scans?ps=sh_sthdl. Accessed January 3, 2013.
- Kolata G. Patients with normal bone density can delay retests, study suggests. The New York Times; 2012. http://www.nytimes.com/2012/01/19/health/bone-density-tests-for-osteoporosis-can-wait-study-says.html. Accessed January 3, 2013.
- Doshi KB, Khan LZ, Williams SE, Licata AA. Bone mineral density testing interval and transition to osteoporosis in older women: Is a T-score enough to determine a screening interval? Cleve Clin J Med 2013; 80:234–239.
- Park A. How often do women really need bone density tests? Time Healthland; 2012. http://healthland.time.com/2012/01/19/most-women-may-be-getting-too-many-bone-density-tests/. Accessed January 3, 2013.
- Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum 2001; 44:202–211.
- Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 1998; 280:2077–2082.
- US Preventive Services Task Force. Screening for osteoporosis: US preventive services task force recommendation statement. Ann Intern Med 2011; 154:356–364.
- Hillier TA, Stone KL, Bauer DC, et al. Evaluating the value of repeat bone mineral density measurement and prediction of fractures in older women: the study of osteoporotic fractures. Arch Intern Med 2007; 167:155–160.
- Leslie WD, Morin SN, Lix LM; Manitoba Bone Density Program. Rate of bone density change does not enhance fracture prediction in routine clinical practice. J Clin Endocrinol Metab 2012; 97:1211–1218.
- Gourlay ML, Fine J P, Preisser JS, et al; Study of Osteoporotic Fractures Research Group. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med 2012; 366:225–233.
- Lewiecki EM, Laster AJ, Miller PD, Bilezikian JP. More bone density testing is needed, not less. J Bone Miner Res 2012; 27:739–742.
- Yu EW, Finkelstein JS. Bone density screening intervals for osteoporosis: one size does not fit all. JAMA 2012; 307:2591–2592.
- Frier S. Women receive bone tests too often for osteoporosis, study finds. Bloomberg News; 2012. http://www.bloomberg.com/news/2012-01-18/many-women-screened-for-osteoporosis-don-t-need-it-researchers-report.html. Accessed January 3, 2013.
- Knox R. Many older women may not need frequent bone scans. National Public Radio; 2012. http://www.npr.org/blogs/health/2012/01/19/145419138/many-older-women-may-not-need-frequent-bone-scans?ps=sh_sthdl. Accessed January 3, 2013.
- Kolata G. Patients with normal bone density can delay retests, study suggests. The New York Times; 2012. http://www.nytimes.com/2012/01/19/health/bone-density-tests-for-osteoporosis-can-wait-study-says.html. Accessed January 3, 2013.
- Doshi KB, Khan LZ, Williams SE, Licata AA. Bone mineral density testing interval and transition to osteoporosis in older women: Is a T-score enough to determine a screening interval? Cleve Clin J Med 2013; 80:234–239.
- Park A. How often do women really need bone density tests? Time Healthland; 2012. http://healthland.time.com/2012/01/19/most-women-may-be-getting-too-many-bone-density-tests/. Accessed January 3, 2013.
- Adachi JD, Saag KG, Delmas PD, et al. Two-year effects of alendronate on bone mineral density and vertebral fracture in patients receiving glucocorticoids: a randomized, double-blind, placebo-controlled extension trial. Arthritis Rheum 2001; 44:202–211.
- Cummings SR, Black DM, Thompson DE, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 1998; 280:2077–2082.
A rapidly growing crusted nodule on the lip
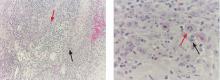
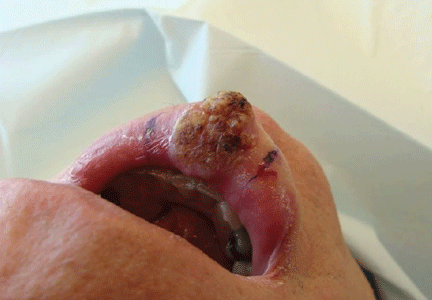
A 76-year-old man presented with a rapidly growing, indurated, crusted nodule on his lower lip (Figure 1). This combination—a rapidly growing nodule on a sun-exposed surface in an older patient—pointed to a diagnosis of squamous cell carcinoma, and biopsy study of the affected area confirmed this diagnosis (Figure 2). Clinical examination, computed tomography, and ultrasonography of the neck revealed no lymph node involvement; the tumor was staged as T2N0M0. Mohs microscopically controlled surgery was performed to remove the tumor with clear margins, and the patient has done well.
DIFFERENTIAL DIAGNOSIS
Malignant melanoma, Merkel cell carcinoma, and deep fungal infection can also cause a rapidly growing crusted nodule on the lower lip, but this typically is not an initial presentation for these conditions.
Malignant melanoma
Malignant melanoma should be considered for any rapidly growing cutaneous tumor, especially on sun-damaged skin. Most melanoma lesions have variations in pigment and may contain shades of blue, brown, red, pink, and white. Amelanotic melanomas may mimic squamous cell carcinomas, but the histologic features of atypical keratinocytes in this patient ruled out that diagnosis. Biopsy of amelanotic melanoma reveals nests of melanocytes, usually associated with an in situ component in the overlying epithelium.
Merkel cell carcinoma
Merkel cell carcinoma can mimic squamous cell carcinoma or can even arise in association with squamous cell carcinoma. Histologic examination allows for differentiation. In difficult cases, cytokeratin staining with cytokeratin 20 and CAM 5.2 can clarify the diagnosis, because Merkel cell carcinomas exhibit a characteristic paranuclear dot-like pattern not evident in squamous cell carcinoma.
Deep fungal infection
Colonization by Candida organisms was noted within the overlying crust in this patient’s lesion, but fungal organisms were not noted in the epidermis or dermis. Pseudoepitheliomatous hyperplasia can be prominent in deep fungal infection, but the marked cytologic atypia in this case excluded deep fungal infection.
Mucosal neuroma
Mucosal neuromas are typically smooth-surfaced, soft lesions on the lip. They may be associated with multiple endocrine neoplasia syndromes. Biopsy study reveals that they are composed of delicate spindle cells that lack atypia.
SQUAMOUS CELL CARCINOMA
Squamous cell carcinoma is one of the most common types of nonmelanoma skin cancer and is associated with increased sun exposure and light skin pigmentation.1 Unlike basal cell carcinoma, squamous cell carcinoma has a considerable potential to metastasize.2–4 The current cancer staging manual of the American Joint Commission on Cancer refects recent evidence-based information on staging and prognosis.5–7 Squamous cell carcinoma of the lip carries an increased risk of metastasis,8 and an increase in the number or the size of involved lymph nodes carries a worse prognosis.9 The lower lip is most commonly involved because of increased sun exposure. The most common route for the spread of squamous cell carcinoma of the head and neck is via lymphatics, so careful evaluation of the head and neck is indicated.10
STAGING OUR PATIENT’S LESION
Current staging of cutaneous squamous cell carcinoma takes into account a variety of different features. Our patient’s tumor was not associated with invasion of the maxilla, mandible, orbit, or temporal bone. This alone would have qualified the tumor as a T1 lesion, but its size of 3 cm indicated a higher risk and qualified it as a T2 lesion.
The histologic features of our patient’s lesion also indicate higher risk according to the current staging system.5 The Breslow thickness on histologic examination (measured from the granular cell layer of the epidermis to the deepest portion of the tumor) was 4 mm and the Clark’s level was IV (extension to the reticular dermis). The high-risk features and the location on the lip warrant a classification as T2 and carry a worse prognosis.
Wedge excision of the lower lip and Mohs surgery are accepted treatment options. The patient chose Mohs surgery and has done well, with excellent cosmetic and functional outcome. Radiation therapy can be useful as an adjuvant when lymph nodes are involved,11 but this was not necessary in our patient. Careful long-term follow up is warranted, as these patients are at higher risk of developing other, separate tumors.
- Schwartz RA. Squamous cell carcinoma. In:Schwartz RA, editor. Skin Cancer: Recognition and Management, 2nd ed. Malden, MA: Blackwell Publishing; 2008:47–65.
- D’Souza J, Clark J. Management of the neck in metastatic cutaneous squamous cell carcinoma of the head and neck. Curr Opin Otolaryngol Head Neck Surg 2011; 19:99–105.
- Zbar RI, Canady JW. MOC-PSSM CME article: Nonmelanoma facial skin malignancy. Plast Reconstr Surg 2008; 121(suppl 1):1–9.
- Morselli P, Masciotra L, Pinto V, Zollino I, Brunelli G, Carinci F. Clinical parameters in T1N0M0 lower lip squamous cell carcinoma. J Craniofac Surg 2007; 18:1079–1082.
- American Joint Commission on Cancer. Cancer Staging Manual. 7th ed. http://www.cancerstaging.org. Accessed February 3, 2013.
- Lardaro T, Shea SM, Sharfman W, Liégeois N, Sober AJ. Improvements in the staging of cutaneous squamous-cell carcinoma in the 7th edition of the AJCC Cancer Staging Manual. Ann Surg Oncol 2010; 17:1979–1980.
- Cutaneous squamous cell carcinoma and other cutaneous carcinomas. In:Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010:301–314.
- Frierson HF, Cooper PH. Prognostic factors in squamous cell carcinoma of the lower lip. Hum Pathol 1986; 17:346–354.
- Civantos FJ, Moffat FL, goodwin WJ. Lymphatic mapping and sentinel lymphadenectomy for 106 head and neck lesions: contrasts between oral cavity and cutaneous malignancy. Laryngoscope 2006; 112(3 Pt 2 suppl 109):1–15.
- Rowe De, Carroll RJ, Day CL. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol 1992; 26:976–990.
- Veness MJ, Palme CE, Smith M, Cakir B, Morgan GJ, Kalnins I. Cutaneous head and neck squamous cell carcinoma metastatic to cervical lymph nodes (nonparotid): a better outcome with surgery and adjuvant radiotherapy. Laryngoscope 2003; 113:1827–1833.
A 76-year-old man presented with a rapidly growing, indurated, crusted nodule on his lower lip (Figure 1). This combination—a rapidly growing nodule on a sun-exposed surface in an older patient—pointed to a diagnosis of squamous cell carcinoma, and biopsy study of the affected area confirmed this diagnosis (Figure 2). Clinical examination, computed tomography, and ultrasonography of the neck revealed no lymph node involvement; the tumor was staged as T2N0M0. Mohs microscopically controlled surgery was performed to remove the tumor with clear margins, and the patient has done well.
DIFFERENTIAL DIAGNOSIS
Malignant melanoma, Merkel cell carcinoma, and deep fungal infection can also cause a rapidly growing crusted nodule on the lower lip, but this typically is not an initial presentation for these conditions.
Malignant melanoma
Malignant melanoma should be considered for any rapidly growing cutaneous tumor, especially on sun-damaged skin. Most melanoma lesions have variations in pigment and may contain shades of blue, brown, red, pink, and white. Amelanotic melanomas may mimic squamous cell carcinomas, but the histologic features of atypical keratinocytes in this patient ruled out that diagnosis. Biopsy of amelanotic melanoma reveals nests of melanocytes, usually associated with an in situ component in the overlying epithelium.
Merkel cell carcinoma
Merkel cell carcinoma can mimic squamous cell carcinoma or can even arise in association with squamous cell carcinoma. Histologic examination allows for differentiation. In difficult cases, cytokeratin staining with cytokeratin 20 and CAM 5.2 can clarify the diagnosis, because Merkel cell carcinomas exhibit a characteristic paranuclear dot-like pattern not evident in squamous cell carcinoma.
Deep fungal infection
Colonization by Candida organisms was noted within the overlying crust in this patient’s lesion, but fungal organisms were not noted in the epidermis or dermis. Pseudoepitheliomatous hyperplasia can be prominent in deep fungal infection, but the marked cytologic atypia in this case excluded deep fungal infection.
Mucosal neuroma
Mucosal neuromas are typically smooth-surfaced, soft lesions on the lip. They may be associated with multiple endocrine neoplasia syndromes. Biopsy study reveals that they are composed of delicate spindle cells that lack atypia.
SQUAMOUS CELL CARCINOMA
Squamous cell carcinoma is one of the most common types of nonmelanoma skin cancer and is associated with increased sun exposure and light skin pigmentation.1 Unlike basal cell carcinoma, squamous cell carcinoma has a considerable potential to metastasize.2–4 The current cancer staging manual of the American Joint Commission on Cancer refects recent evidence-based information on staging and prognosis.5–7 Squamous cell carcinoma of the lip carries an increased risk of metastasis,8 and an increase in the number or the size of involved lymph nodes carries a worse prognosis.9 The lower lip is most commonly involved because of increased sun exposure. The most common route for the spread of squamous cell carcinoma of the head and neck is via lymphatics, so careful evaluation of the head and neck is indicated.10
STAGING OUR PATIENT’S LESION
Current staging of cutaneous squamous cell carcinoma takes into account a variety of different features. Our patient’s tumor was not associated with invasion of the maxilla, mandible, orbit, or temporal bone. This alone would have qualified the tumor as a T1 lesion, but its size of 3 cm indicated a higher risk and qualified it as a T2 lesion.
The histologic features of our patient’s lesion also indicate higher risk according to the current staging system.5 The Breslow thickness on histologic examination (measured from the granular cell layer of the epidermis to the deepest portion of the tumor) was 4 mm and the Clark’s level was IV (extension to the reticular dermis). The high-risk features and the location on the lip warrant a classification as T2 and carry a worse prognosis.
Wedge excision of the lower lip and Mohs surgery are accepted treatment options. The patient chose Mohs surgery and has done well, with excellent cosmetic and functional outcome. Radiation therapy can be useful as an adjuvant when lymph nodes are involved,11 but this was not necessary in our patient. Careful long-term follow up is warranted, as these patients are at higher risk of developing other, separate tumors.
A 76-year-old man presented with a rapidly growing, indurated, crusted nodule on his lower lip (Figure 1). This combination—a rapidly growing nodule on a sun-exposed surface in an older patient—pointed to a diagnosis of squamous cell carcinoma, and biopsy study of the affected area confirmed this diagnosis (Figure 2). Clinical examination, computed tomography, and ultrasonography of the neck revealed no lymph node involvement; the tumor was staged as T2N0M0. Mohs microscopically controlled surgery was performed to remove the tumor with clear margins, and the patient has done well.
DIFFERENTIAL DIAGNOSIS
Malignant melanoma, Merkel cell carcinoma, and deep fungal infection can also cause a rapidly growing crusted nodule on the lower lip, but this typically is not an initial presentation for these conditions.
Malignant melanoma
Malignant melanoma should be considered for any rapidly growing cutaneous tumor, especially on sun-damaged skin. Most melanoma lesions have variations in pigment and may contain shades of blue, brown, red, pink, and white. Amelanotic melanomas may mimic squamous cell carcinomas, but the histologic features of atypical keratinocytes in this patient ruled out that diagnosis. Biopsy of amelanotic melanoma reveals nests of melanocytes, usually associated with an in situ component in the overlying epithelium.
Merkel cell carcinoma
Merkel cell carcinoma can mimic squamous cell carcinoma or can even arise in association with squamous cell carcinoma. Histologic examination allows for differentiation. In difficult cases, cytokeratin staining with cytokeratin 20 and CAM 5.2 can clarify the diagnosis, because Merkel cell carcinomas exhibit a characteristic paranuclear dot-like pattern not evident in squamous cell carcinoma.
Deep fungal infection
Colonization by Candida organisms was noted within the overlying crust in this patient’s lesion, but fungal organisms were not noted in the epidermis or dermis. Pseudoepitheliomatous hyperplasia can be prominent in deep fungal infection, but the marked cytologic atypia in this case excluded deep fungal infection.
Mucosal neuroma
Mucosal neuromas are typically smooth-surfaced, soft lesions on the lip. They may be associated with multiple endocrine neoplasia syndromes. Biopsy study reveals that they are composed of delicate spindle cells that lack atypia.
SQUAMOUS CELL CARCINOMA
Squamous cell carcinoma is one of the most common types of nonmelanoma skin cancer and is associated with increased sun exposure and light skin pigmentation.1 Unlike basal cell carcinoma, squamous cell carcinoma has a considerable potential to metastasize.2–4 The current cancer staging manual of the American Joint Commission on Cancer refects recent evidence-based information on staging and prognosis.5–7 Squamous cell carcinoma of the lip carries an increased risk of metastasis,8 and an increase in the number or the size of involved lymph nodes carries a worse prognosis.9 The lower lip is most commonly involved because of increased sun exposure. The most common route for the spread of squamous cell carcinoma of the head and neck is via lymphatics, so careful evaluation of the head and neck is indicated.10
STAGING OUR PATIENT’S LESION
Current staging of cutaneous squamous cell carcinoma takes into account a variety of different features. Our patient’s tumor was not associated with invasion of the maxilla, mandible, orbit, or temporal bone. This alone would have qualified the tumor as a T1 lesion, but its size of 3 cm indicated a higher risk and qualified it as a T2 lesion.
The histologic features of our patient’s lesion also indicate higher risk according to the current staging system.5 The Breslow thickness on histologic examination (measured from the granular cell layer of the epidermis to the deepest portion of the tumor) was 4 mm and the Clark’s level was IV (extension to the reticular dermis). The high-risk features and the location on the lip warrant a classification as T2 and carry a worse prognosis.
Wedge excision of the lower lip and Mohs surgery are accepted treatment options. The patient chose Mohs surgery and has done well, with excellent cosmetic and functional outcome. Radiation therapy can be useful as an adjuvant when lymph nodes are involved,11 but this was not necessary in our patient. Careful long-term follow up is warranted, as these patients are at higher risk of developing other, separate tumors.
- Schwartz RA. Squamous cell carcinoma. In:Schwartz RA, editor. Skin Cancer: Recognition and Management, 2nd ed. Malden, MA: Blackwell Publishing; 2008:47–65.
- D’Souza J, Clark J. Management of the neck in metastatic cutaneous squamous cell carcinoma of the head and neck. Curr Opin Otolaryngol Head Neck Surg 2011; 19:99–105.
- Zbar RI, Canady JW. MOC-PSSM CME article: Nonmelanoma facial skin malignancy. Plast Reconstr Surg 2008; 121(suppl 1):1–9.
- Morselli P, Masciotra L, Pinto V, Zollino I, Brunelli G, Carinci F. Clinical parameters in T1N0M0 lower lip squamous cell carcinoma. J Craniofac Surg 2007; 18:1079–1082.
- American Joint Commission on Cancer. Cancer Staging Manual. 7th ed. http://www.cancerstaging.org. Accessed February 3, 2013.
- Lardaro T, Shea SM, Sharfman W, Liégeois N, Sober AJ. Improvements in the staging of cutaneous squamous-cell carcinoma in the 7th edition of the AJCC Cancer Staging Manual. Ann Surg Oncol 2010; 17:1979–1980.
- Cutaneous squamous cell carcinoma and other cutaneous carcinomas. In:Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010:301–314.
- Frierson HF, Cooper PH. Prognostic factors in squamous cell carcinoma of the lower lip. Hum Pathol 1986; 17:346–354.
- Civantos FJ, Moffat FL, goodwin WJ. Lymphatic mapping and sentinel lymphadenectomy for 106 head and neck lesions: contrasts between oral cavity and cutaneous malignancy. Laryngoscope 2006; 112(3 Pt 2 suppl 109):1–15.
- Rowe De, Carroll RJ, Day CL. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol 1992; 26:976–990.
- Veness MJ, Palme CE, Smith M, Cakir B, Morgan GJ, Kalnins I. Cutaneous head and neck squamous cell carcinoma metastatic to cervical lymph nodes (nonparotid): a better outcome with surgery and adjuvant radiotherapy. Laryngoscope 2003; 113:1827–1833.
- Schwartz RA. Squamous cell carcinoma. In:Schwartz RA, editor. Skin Cancer: Recognition and Management, 2nd ed. Malden, MA: Blackwell Publishing; 2008:47–65.
- D’Souza J, Clark J. Management of the neck in metastatic cutaneous squamous cell carcinoma of the head and neck. Curr Opin Otolaryngol Head Neck Surg 2011; 19:99–105.
- Zbar RI, Canady JW. MOC-PSSM CME article: Nonmelanoma facial skin malignancy. Plast Reconstr Surg 2008; 121(suppl 1):1–9.
- Morselli P, Masciotra L, Pinto V, Zollino I, Brunelli G, Carinci F. Clinical parameters in T1N0M0 lower lip squamous cell carcinoma. J Craniofac Surg 2007; 18:1079–1082.
- American Joint Commission on Cancer. Cancer Staging Manual. 7th ed. http://www.cancerstaging.org. Accessed February 3, 2013.
- Lardaro T, Shea SM, Sharfman W, Liégeois N, Sober AJ. Improvements in the staging of cutaneous squamous-cell carcinoma in the 7th edition of the AJCC Cancer Staging Manual. Ann Surg Oncol 2010; 17:1979–1980.
- Cutaneous squamous cell carcinoma and other cutaneous carcinomas. In:Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC Cancer Staging Manual. 7th ed. New York, NY: Springer; 2010:301–314.
- Frierson HF, Cooper PH. Prognostic factors in squamous cell carcinoma of the lower lip. Hum Pathol 1986; 17:346–354.
- Civantos FJ, Moffat FL, goodwin WJ. Lymphatic mapping and sentinel lymphadenectomy for 106 head and neck lesions: contrasts between oral cavity and cutaneous malignancy. Laryngoscope 2006; 112(3 Pt 2 suppl 109):1–15.
- Rowe De, Carroll RJ, Day CL. Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol 1992; 26:976–990.
- Veness MJ, Palme CE, Smith M, Cakir B, Morgan GJ, Kalnins I. Cutaneous head and neck squamous cell carcinoma metastatic to cervical lymph nodes (nonparotid): a better outcome with surgery and adjuvant radiotherapy. Laryngoscope 2003; 113:1827–1833.
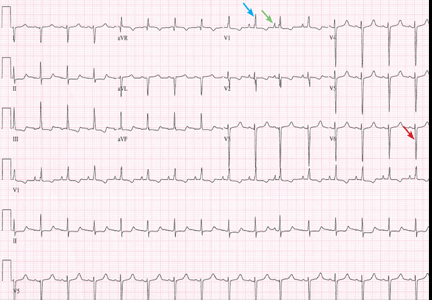
Implications of a prominent R wave in V1
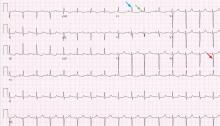
A 19-year-old woman with no significant cardiac or pulmonary history presented with exertional dyspnea, which had begun a few months earlier. Auscultation revealed a loud pulmonary component of the second heart sound and a diastolic murmur heard along the upper left sternal border. Her 12-lead electrocardiogram is shown in Figure 1.
Q: Which of the following can cause prominent R waves in lead V1?
- Normal variant in young adults
- Wolff-Parkinson-White syndrome
- Posterior wall myocardial infarction
- Right ventricular hypertrophy
- All of the above
A: The correct answer is all of the above.
The patient’s electrocardiogram shows a right atrial abnormality and right ventricular hypertrophy. Right atrial enlargement is evidenced by a prominent initial P wave in V1 with an amplitude of at least 1.5 mm (0.15 mV). A P wave taller than 2.5 mm (0.25 mV) in lead II may also suggest a right atrial abnormality.1
Multiple criteria exist for the diagnosis of right ventricular hypertrophy. Tall R waves in V1 with an R/S ratio greater than 1 (ie, the R wave amplitude is more than the S wave depth) is commonly used.2 Deep S waves with an R/S ratio less than 1 in V6 is another criterion. Tall R waves of amplitude greater than 7 mm in V1 by themselves may represent right ventricular hypertrophy. Most of the electrocardiographic criteria are specific but not sensitive for this diagnosis.3
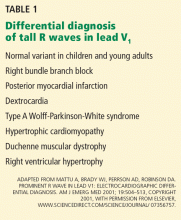
Other causes of tall R waves in V1 are given in Table 1.
Q: Which of the following diseases can present with an electrocardiographic pattern of right ventricular hypertrophy in young patients?
- Pulmonary hypertension
- Atrial septal defect
- Tetralogy of Fallot
- Pulmonary stenosis
- All of the above
A: The correct answer is all of the above.4
Our patient underwent multiple investigations. On echocardiography, her estimated right ventricular pressure was 80 mm Hg, and on cardiac catheterization her mean pulmonary arterial pressure was 55 mm Hg and her pulmonary capillary wedge pressure was 6 mm Hg. She was diagnosed with pulmonary arterial hypertension, which was the cause of her right ventricular hypertrophy. She eventually underwent bilateral lung transplantation.
- Hancock EW, Deal BJ, Mirvis DM, et al; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation 2009; 119:e251–e261.
- Milnor WR. Electrocardiogram and vectorcardiogram in right ventricular hypertrophy and right bundle-branch block. Circulation 1957; 16:348–367.
- Lehtonen J, Sutinen S, Ikäheimo M, Pääkkö P. Electrocardiographic criteria for the diagnosis of right ventricular hypertrophy verified at autopsy. Chest 1988; 93:839–842.
- Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation 2006; 114:1645–1653.
A 19-year-old woman with no significant cardiac or pulmonary history presented with exertional dyspnea, which had begun a few months earlier. Auscultation revealed a loud pulmonary component of the second heart sound and a diastolic murmur heard along the upper left sternal border. Her 12-lead electrocardiogram is shown in Figure 1.
Q: Which of the following can cause prominent R waves in lead V1?
- Normal variant in young adults
- Wolff-Parkinson-White syndrome
- Posterior wall myocardial infarction
- Right ventricular hypertrophy
- All of the above
A: The correct answer is all of the above.
The patient’s electrocardiogram shows a right atrial abnormality and right ventricular hypertrophy. Right atrial enlargement is evidenced by a prominent initial P wave in V1 with an amplitude of at least 1.5 mm (0.15 mV). A P wave taller than 2.5 mm (0.25 mV) in lead II may also suggest a right atrial abnormality.1
Multiple criteria exist for the diagnosis of right ventricular hypertrophy. Tall R waves in V1 with an R/S ratio greater than 1 (ie, the R wave amplitude is more than the S wave depth) is commonly used.2 Deep S waves with an R/S ratio less than 1 in V6 is another criterion. Tall R waves of amplitude greater than 7 mm in V1 by themselves may represent right ventricular hypertrophy. Most of the electrocardiographic criteria are specific but not sensitive for this diagnosis.3
Other causes of tall R waves in V1 are given in Table 1.
Q: Which of the following diseases can present with an electrocardiographic pattern of right ventricular hypertrophy in young patients?
- Pulmonary hypertension
- Atrial septal defect
- Tetralogy of Fallot
- Pulmonary stenosis
- All of the above
A: The correct answer is all of the above.4
Our patient underwent multiple investigations. On echocardiography, her estimated right ventricular pressure was 80 mm Hg, and on cardiac catheterization her mean pulmonary arterial pressure was 55 mm Hg and her pulmonary capillary wedge pressure was 6 mm Hg. She was diagnosed with pulmonary arterial hypertension, which was the cause of her right ventricular hypertrophy. She eventually underwent bilateral lung transplantation.
A 19-year-old woman with no significant cardiac or pulmonary history presented with exertional dyspnea, which had begun a few months earlier. Auscultation revealed a loud pulmonary component of the second heart sound and a diastolic murmur heard along the upper left sternal border. Her 12-lead electrocardiogram is shown in Figure 1.
Q: Which of the following can cause prominent R waves in lead V1?
- Normal variant in young adults
- Wolff-Parkinson-White syndrome
- Posterior wall myocardial infarction
- Right ventricular hypertrophy
- All of the above
A: The correct answer is all of the above.
The patient’s electrocardiogram shows a right atrial abnormality and right ventricular hypertrophy. Right atrial enlargement is evidenced by a prominent initial P wave in V1 with an amplitude of at least 1.5 mm (0.15 mV). A P wave taller than 2.5 mm (0.25 mV) in lead II may also suggest a right atrial abnormality.1
Multiple criteria exist for the diagnosis of right ventricular hypertrophy. Tall R waves in V1 with an R/S ratio greater than 1 (ie, the R wave amplitude is more than the S wave depth) is commonly used.2 Deep S waves with an R/S ratio less than 1 in V6 is another criterion. Tall R waves of amplitude greater than 7 mm in V1 by themselves may represent right ventricular hypertrophy. Most of the electrocardiographic criteria are specific but not sensitive for this diagnosis.3
Other causes of tall R waves in V1 are given in Table 1.
Q: Which of the following diseases can present with an electrocardiographic pattern of right ventricular hypertrophy in young patients?
- Pulmonary hypertension
- Atrial septal defect
- Tetralogy of Fallot
- Pulmonary stenosis
- All of the above
A: The correct answer is all of the above.4
Our patient underwent multiple investigations. On echocardiography, her estimated right ventricular pressure was 80 mm Hg, and on cardiac catheterization her mean pulmonary arterial pressure was 55 mm Hg and her pulmonary capillary wedge pressure was 6 mm Hg. She was diagnosed with pulmonary arterial hypertension, which was the cause of her right ventricular hypertrophy. She eventually underwent bilateral lung transplantation.
- Hancock EW, Deal BJ, Mirvis DM, et al; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation 2009; 119:e251–e261.
- Milnor WR. Electrocardiogram and vectorcardiogram in right ventricular hypertrophy and right bundle-branch block. Circulation 1957; 16:348–367.
- Lehtonen J, Sutinen S, Ikäheimo M, Pääkkö P. Electrocardiographic criteria for the diagnosis of right ventricular hypertrophy verified at autopsy. Chest 1988; 93:839–842.
- Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation 2006; 114:1645–1653.
- Hancock EW, Deal BJ, Mirvis DM, et al; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation 2009; 119:e251–e261.
- Milnor WR. Electrocardiogram and vectorcardiogram in right ventricular hypertrophy and right bundle-branch block. Circulation 1957; 16:348–367.
- Lehtonen J, Sutinen S, Ikäheimo M, Pääkkö P. Electrocardiographic criteria for the diagnosis of right ventricular hypertrophy verified at autopsy. Chest 1988; 93:839–842.
- Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation 2006; 114:1645–1653.
Resistance of man and bug
Why individual clinicians make specific decisions usually can be sorted out. But our behavior as a group is more difficult to understand and, even when there are pressing and convincing reasons to change, behavior is difficult to alter.
In this issue, Drs. Federico Perez and David Van Duin discuss the emergence of carbapenem-resistant bacteria, dubbed “superbugs” by the media. Antibiotic resistance is not new; it was reported in Staphylococcus species within several years of the introduction of penicillin.1 However, it has been increasing in prevalence and molecular complexity after years of relatively promiscuous antibiotic use. As the percentage of inpatients with immunosuppression and frailty increases in this environment of known antibiotic resistance, initial empiric antibiotic choices will by necessity include drugs likely to further promote development of resistant strains. But why do physicians still prescribe antibiotics for uncomplicated upper respiratory tract infections and asymptomatic bacteriuria, despite numerous studies and guidelines suggesting this practice has little benefit? Is it because patients expect a prescription in return for their copayment? Is it the path of least resistance? Or do physicians not accept the data showing that it is unnecessary?
Dr. Gerald Appel discusses diabetic nephropathy, an area that involves resistance of another kind, ie, the apparent resistance of physicians and patients to achieving evidence-based treatment targets. We hold controlled trials as the Holy Grail of evidence-based medicine, yet we seem to have an aversion to following guidelines based on trial-derived evidence. (I do not refer here to blind guideline adherence, ignoring individual patient characteristics.)
So how can physicians’ behavior be altered and our resistance to change be reduced? Experiments are under way, such as paying physicians based on their performance, linking patients’ insurance rates to achieving selected outcomes, and linking physician practice self-review to certification. Perhaps naively, I continue to believe that the most effective impetus to changing personal practice is the dissemination of data from high-quality trials (tempered by our accumulated experience and keeping our eyes wide open), coupled with our desire to do the best for our patients.
- Barber M. Coagulase-positive staphylococci resistant to penicillin. J Pathol Bacteriol 1947; 59:373–384.
Why individual clinicians make specific decisions usually can be sorted out. But our behavior as a group is more difficult to understand and, even when there are pressing and convincing reasons to change, behavior is difficult to alter.
In this issue, Drs. Federico Perez and David Van Duin discuss the emergence of carbapenem-resistant bacteria, dubbed “superbugs” by the media. Antibiotic resistance is not new; it was reported in Staphylococcus species within several years of the introduction of penicillin.1 However, it has been increasing in prevalence and molecular complexity after years of relatively promiscuous antibiotic use. As the percentage of inpatients with immunosuppression and frailty increases in this environment of known antibiotic resistance, initial empiric antibiotic choices will by necessity include drugs likely to further promote development of resistant strains. But why do physicians still prescribe antibiotics for uncomplicated upper respiratory tract infections and asymptomatic bacteriuria, despite numerous studies and guidelines suggesting this practice has little benefit? Is it because patients expect a prescription in return for their copayment? Is it the path of least resistance? Or do physicians not accept the data showing that it is unnecessary?
Dr. Gerald Appel discusses diabetic nephropathy, an area that involves resistance of another kind, ie, the apparent resistance of physicians and patients to achieving evidence-based treatment targets. We hold controlled trials as the Holy Grail of evidence-based medicine, yet we seem to have an aversion to following guidelines based on trial-derived evidence. (I do not refer here to blind guideline adherence, ignoring individual patient characteristics.)
So how can physicians’ behavior be altered and our resistance to change be reduced? Experiments are under way, such as paying physicians based on their performance, linking patients’ insurance rates to achieving selected outcomes, and linking physician practice self-review to certification. Perhaps naively, I continue to believe that the most effective impetus to changing personal practice is the dissemination of data from high-quality trials (tempered by our accumulated experience and keeping our eyes wide open), coupled with our desire to do the best for our patients.
Why individual clinicians make specific decisions usually can be sorted out. But our behavior as a group is more difficult to understand and, even when there are pressing and convincing reasons to change, behavior is difficult to alter.
In this issue, Drs. Federico Perez and David Van Duin discuss the emergence of carbapenem-resistant bacteria, dubbed “superbugs” by the media. Antibiotic resistance is not new; it was reported in Staphylococcus species within several years of the introduction of penicillin.1 However, it has been increasing in prevalence and molecular complexity after years of relatively promiscuous antibiotic use. As the percentage of inpatients with immunosuppression and frailty increases in this environment of known antibiotic resistance, initial empiric antibiotic choices will by necessity include drugs likely to further promote development of resistant strains. But why do physicians still prescribe antibiotics for uncomplicated upper respiratory tract infections and asymptomatic bacteriuria, despite numerous studies and guidelines suggesting this practice has little benefit? Is it because patients expect a prescription in return for their copayment? Is it the path of least resistance? Or do physicians not accept the data showing that it is unnecessary?
Dr. Gerald Appel discusses diabetic nephropathy, an area that involves resistance of another kind, ie, the apparent resistance of physicians and patients to achieving evidence-based treatment targets. We hold controlled trials as the Holy Grail of evidence-based medicine, yet we seem to have an aversion to following guidelines based on trial-derived evidence. (I do not refer here to blind guideline adherence, ignoring individual patient characteristics.)
So how can physicians’ behavior be altered and our resistance to change be reduced? Experiments are under way, such as paying physicians based on their performance, linking patients’ insurance rates to achieving selected outcomes, and linking physician practice self-review to certification. Perhaps naively, I continue to believe that the most effective impetus to changing personal practice is the dissemination of data from high-quality trials (tempered by our accumulated experience and keeping our eyes wide open), coupled with our desire to do the best for our patients.
- Barber M. Coagulase-positive staphylococci resistant to penicillin. J Pathol Bacteriol 1947; 59:373–384.
- Barber M. Coagulase-positive staphylococci resistant to penicillin. J Pathol Bacteriol 1947; 59:373–384.
Detecting and controlling diabetic nephropathy: What do we know?
Diabetes is on the rise, and so is diabetic nephropathy. In view of this epidemic, physicians should consider strategies to detect and control kidney disease in their diabetic patients.
This article will focus on kidney disease in adult-onset type 2 diabetes. Although it has different pathogenetic mechanisms than type 1 diabetes, the clinical course of the two conditions is very similar in terms of the prevalence of proteinuria after diagnosis, the progression to renal failure after the onset of proteinuria, and treatment options.1
DIABETES AND DIABETIC KIDNEY DISEASE ARE ON THE RISE
The incidence of diabetes increases with age, and with the aging of the baby boomers, its prevalence is growing dramatically. The 2005– 2008 National Health and Nutrition Examination Survey estimated the prevalence as 3.7% in adults age 20 to 44, 13.7% at age 45 to 64, and 26.9% in people age 65 and older. The obesity epidemic is also contributing to the increase in diabetes in all age groups.
Diabetic kidney disease has increased in the United States from about 4 million cases 20 years ago to about 7 million in 2005–2008.2 Diabetes is the major cause of end-stage renal disease in the developed world, accounting for 40% to 50% of cases. Other major causes are hypertension (27%) and glomerulonephritis (13%).3
Physicians in nearly every field of medicine now care for patients with diabetic nephropathy. The classic presentation—a patient who has impaired vision, fluid retention with edema, and hypertension—is commonly seen in dialysis units and ophthalmology and cardiovascular clinics.
CLINICAL PROGRESSION
Early in the course of diabetic nephropathy, blood pressure is normal and microalbuminuria is not evident, but many patients have a high glomerular filtration rate (GFR), indicating temporarily “enhanced” renal function or hyperfiltration. The next stage is characterized by microalbuminuria, correlating with glomerular mesangial expansion: the GFR falls back into the normal range and blood pressure starts to increase. Finally, macroalbuminuria occurs, accompanied by rising blood pressure and a declining GFR, correlating with the histologic appearance of glomerulosclerosis and Kimmelstiel-Wilson nodules.4
Hypertension develops in 5% of patients by 10 years after type 1 diabetes is diagnosed, 33% by 20 years, and 70% by 40 years. In contrast, 40% of patients with type 2 diabetes have high blood pressure at diagnosis.
Unfortunately, in most cases, this progression is a one-way street, so it is critical to intervene to try to slow the progression early in the course of the disease process.
SCREENING FOR DIABETIC NEPHROPATHY
Nephropathy screening guidelines for patients with diabetes are provided in Table 1.5
Blood pressure should be monitored at each office visit (Table 1). The goal for adults with diabetes should be to reduce blood pressure to 130/80 mm Hg. Reduction beyond this level may be associated with an increased mortality rate.6 Very high blood pressure (> 180 mm Hg systolic) should be lowered slowly. Lowering blood pressure delays the progression from microalbuminuria (30–299 mg/day or 20–199 μg/min) to macroalbuminuria (> 300 mg/day or > 200 μg/min) and slows the progression to renal failure.
Urinary albumin. Proteinuria takes 5 to 10 years to develop after the onset of diabetes. Because it is possible for patients with type 2 diabetes to have had the disease for some time before being diagnosed, urinary albumin screening should be performed at diagnosis and annually thereafter. Patients with type 1 are usually diagnosed with diabetes at or near onset of disease; therefore, annual screening for urinary albumin can begin 5 years after diagnosis.5
Proteinuria can be measured in different ways (Table 2). The basic screening test for clinical proteinuria is the urine dipstick, which is very sensitive to albumin and relatively insensitive to other proteins. “Trace-positive” results are common in healthy people, so proteinuria is not confirmed unless a patient has repeatedly positive results.
Microalbuminuria is important to measure, especially if it helps determine therapy. It is not detectable by the urinary dipstick, but can be measured in the following ways:
- Measurement of the albumin-creatinine ratio in a random spot collection
- 24-hour collection (creatinine should simultaneously be measured and creatinine clearance calculated)
- Timed collection (4 hours or overnight).
The first method is preferred, and any positive test result must be confirmed by repeat analyses of urinary albumin before a patient is diagnosed with microalbuminuria.
Occasionally a patient presenting with proteinuria but normal blood sugar and hemoglobin A1c will have a biopsy that reveals morphologic changes of classic diabetic nephropathy. Most such patients have a history of hyperglycemia, indicating that they actually have been diabetic.
Proteinuria—the best marker of disease progression
Proteinuria is the strongest predictor of renal outcomes. The Reduction in End Points in Noninsulin-Dependent Diabetes Mellitus With the Angiotensin II Antagonist Losartan (RENAAL) study was a randomized, placebo-controlled trial in more than 1,500 patients with type 2 diabetes to test the effects of losartan on renal outcome. Those with high albuminuria (> 3.0 g albumin/g creatinine) at baseline were five times more likely to reach a renal end point and were eight times more likely to have progression to end-stage renal disease than patients with low albuminuria (< 1.5 g/g).7 The degree of albuminuria after 6 months of treatment showed similar predictive trends, indicating that monitoring and treating proteinuria are extremely important goals.
STRATEGY 1 TO LIMIT RENAL INJURY: REDUCE BLOOD PRESSURE
Blood pressure control improves renal and cardiovascular function.
As early as 1983, Parving et al,8 in a study of only 10 insulin-dependent diabetic patients, showed strong evidence that early aggressive antihypertensive treatment improved the course of diabetic nephropathy. During the mean pretreatment period of 29 months, the GFR decreased significantly and the urinary albumin excretion rate and arterial blood pressure rose significantly. During the mean 39-month period of antihypertensive treatment with metoprolol, hydralazine, and furosemide or a thiazide, mean arterial blood pressure fell from 144/97 to 128/84 mm Hg and urinary albumin excretion from 977 to 433 μg/ min. The rate of decline in GFR slowed from 0.91 mL/min/month before treatment to 0.39 mL/min/month during treatment.
The Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial9 enrolled more than 11,000 patients internationally with type 2 diabetes at high risk for cardiovascular events. In addition to standard therapy, blood pressure was intensively controlled in one group with a combination of the angiotensin-converting enzyme (ACE) inhibitor perindopril and the diuretic indapamide. The intensive-therapy group achieved blood pressures less than 140/80 mm Hg and had a mean reduction of systolic blood pressure of 5.6 mm Hg and diastolic blood pressure of 2.2 mm Hg vs controls. Despite these apparently modest reductions, the intensively controlled group had a significant 9% reduction of the primary outcome of combined macrovascular events (cardiovascular death, myocardial infarction, and stroke) and microvascular events (new or worsening nephropathy, or retinopathy).10
A meta-analysis of studies of patients with type 2 diabetes found reduced nephropathy with systolic blood pressure control to less than 130 mm Hg.11
The United Kingdom Prospective Diabetes Study (UKPDS) is a series of studies of diabetes. The original study in 1998 enrolled 5,102 patients with newly diagnosed type 2 diabetes.12 The more than 1,000 patients with hypertension were randomized to either tight blood pressure control or regular care. The intensive treatment group had a mean blood pressure reduction of 9 mm Hg systolic and 3 mm Hg diastolic, along with major reductions in all diabetes end points, diabetes deaths, microvascular disease, and stroke over a median follow-up of 8.4 years.
Continuous blood pressure control is critical
Tight blood pressure control must be maintained to have continued benefit. During the 10 years following the UKPDS, no attempts were made to maintain the previously assigned therapies. A follow-up study13 of 884 UKPDS patients found that blood pressures were the same again between the two groups 2 years after the trial was stopped, and no beneficial legacy effect from previous blood pressure control was evident on end points.
Control below 120 mm Hg systolic not needed
Blood pressure control slows kidney disease and prevents major macrovascular disease, but there is no evidence that lowering systolic blood pressure below 120 mm Hg provides additional benefit. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,14 more than 10,000 patients with type 2 diabetes and existing cardiovascular disease or additional cardiovascular risk factors were randomized to a goal of systolic blood pressure less than 120 mm Hg or less than 140 mm Hg (actual mean systolic pressures were 119 vs 134 mm Hg, respectively). Over nearly 5 years, there was no difference in cardiovascular events or deaths between the two groups.15
Since 1997, six international organizations have revised their recommended blood pressure goals in diabetes mellitus and renal diseases. Randomized clinical trials and observational studies have demonstrated the importance of blood pressure control to the level of 125/75 to 140/80 mm Hg. The National Kidney Foundation, the American Diabetes Association, and the Canadian Hypertension Society have developed consensus guidelines for blood pressure control to less than 130/80 mm Hg.16–21 Table 3 summarizes blood pressure goals for patients with diabetes.
STRATEGY 2: CONTROL BLOOD SUGAR
Recommendations for blood sugar goals are more controversial.
The Diabetes Control and Complications Trial22 provided early evidence that tight blood sugar control slows the development of microalbuminuria and macroalbuminuria. The study randomized more than 1,400 patients with type 1 diabetes to either standard therapy (1 or 2 daily insulin injections) or intensive therapy (an external insulin pump or 3 or more insulin injections guided by frequent blood glucose monitoring) to keep blood glucose levels close to normal. About half the patients had mild retinopathy at baseline and the others had no retinopathy. After 6.5 years, intensive therapy was found to significantly delay the onset and slow the progression of diabetic retinopathy and nephropathy.
The Kumamoto Study23 randomized 110 patients with type 2 diabetes and either no retinopathy (primary prevention cohort) or simple retinopathy (secondary prevention cohort) to receive either multiple insulin injections or conventional insulin therapy over 8 years. Intensive therapy led to lower rates of retinopathy (7.7% vs 32% in primary prevention and 19% vs 44% in secondary prevention) and progressive nephropathy (7% vs 28% in primary prevention at 6 years and 11% vs 32% in secondary prevention).
In addition to studying the effects of blood pressure control, the UKPDS also studied the effects of intensive blood glucose control.24,25 Nearly 4,000 patients with newly diagnosed type 2 diabetes were randomized to intensive treatment with a sulfonylurea or insulin, or to conventional treatment with diet. Over 10 years, the mean hemoglobin A1c was reduced to 7.0% in the intensive group and 7.9% in the conventional group. The risk of any diabetes-related end point was 12% lower in the intensive group, 10% lower for diabetes-related death, and 6% lower for all-cause mortality. There was also a 25% reduction in microvascular disease (retinopathy and nephropathy). However, the intensive group had more hypoglycemic episodes than the conventional group and a tendency to some increase in macrovascular events. A legacy effect was evident: patients who had intensive treatment had less microvascular disease progression years after stopping therapy.
Tight glycemic control reduces nephropathy, but does it increase cardiovascular risk?
Earlier trials provided strong evidence that blood glucose control prevents or slows retinopathy and nephropathy. The critical question is, “At what expense?” Although diabetes is the most common cause of kidney failure in the United States, most people with diabetes do not die of kidney failure, but of cardiovascular disease. Two recent large trials had different results regarding glycemic control below hemoglobin A1c of 7.0% and macrovascular risk, creating a controversy about what recommendations are best.
The ADVANCE trial, enrolling 11,140 patients with type 2 diabetes, was largely conducted in Australia and used the sulfonylurea glipizide for glycemic control. Compared with the group that received standard therapy (n=5,569), the intensive-treatment group (n=5,571) achieved mean hemoglobin A1c levels of 6.5% compared with 7.3% in the standard group, and had less nephropathy, less microalbuminuria, less doubling of creatinine, and a lower rate of end-stage renal disease (4% vs 5% in the standard therapy group). No difference between the two groups was found in retinopathy. Rates of all-cause mortality did not differ between the groups.9
The ACCORD trial had more than 10,000 subjects with type 2 diabetes and took place mostly in the United States. Using mainly rosiglitazone for intensive therapy, the intensive group achieved hemoglobin A1c levels of 6.4% vs 7.5% in the standard-therapy group. The trial was stopped early, at 3.7 years, because of a higher risk of death and cardiovascular events in the group with intensive glycemic control. However, the intensive-therapy group did have a significant decrease in microvascular renal outcomes and a reduction in the progression of retinopathy.14,26
In summary, tighter glycemic control improves microvascular complications—both retinopathy and nephropathy—in patients with type 2 diabetes. The benefit of intensive therapy on macrovascular complications (stroke, myocardial infarction) in long-standing diabetes has not been convincingly demonstrated in randomized trials. The UKPDS suggested that maintaining a hemoglobin A1c of 7% in patients newly diagnosed with type 2 diabetes confers long-term cardiovascular benefits. The target hemoglobin A1c for type 2 diabetes should be tailored to the patient: 7% is a reasonable goal for most patients, but the goal should be higher for the elderly and frail. Reducing the risk of cardiovascular death is still best done by controlling blood pressure, reducing lipids, quitting smoking, and losing weight.
STRATEGY 3: INHIBIT THE RENIN-ANGIOTENSIN-ALDOSTERONE AXIS
Components of the renin-angiotensin-aldo-sterone system are present not only in the circulation but also in many tissues, including the heart, brain, kidney, blood vessels, and adrenal glands. The role of renin-angiotensin-aldosterone system blockers in treating and preventing diabetic nephropathy has become controversial in recent years with findings from new studies.
The renin-angiotensin-aldosterone system is important in the development or maintenance of high blood pressure and the resultant damage to the brain, heart, and kidney. Drug development has focused on inhibiting steps in the biochemical pathway. ACE inhibitors block the formation of angiotensin II—the most biologically potent angiotensin peptide—and are among the most commonly used drugs to treat hypertension and concomitant conditions, such as renal insufficiency, proteinuria, and heart failure. Angiotensin receptor blockers (ARBs) interact with the angiotensin AT1 receptor and block most of its actions. They are approved by the US Food and Drug Administration (FDA) for the treatment of hypertension, and they help prevent left ventricular hypertrophy and mesangial sclerosis. Large studies have shown that ACE inhibitors and ARBs offer similar cardiovascular benefit.
The glomerulus has the only capillary bed with a blood supply that drains into an efferent arteriole instead of a venule, providing high resistance to aid filtration. Efferent arterioles are rich in AT1 receptors. In the presence of angiotensin II they constrict, increasing pressure in the glomerulus, which can lead to proteinuria and glomerulosclerosis. ACE inhibitors and ARBs relax the efferent arteriole, allowing increased blood flow through the glomerulus. This reduction in intraglomerular pressure is associated with less proteinuria and less glomerulosclerosis.
Diabetes promotes renal disease in many ways. Glucose and advanced glycation end products can lead to increased blood flow and increased pressure in the glomerulus. Through a variety of pathways, hyperglycemia, acting on angiotensin II, leads to NF-kapa beta production, profibrotic cytokines, increased matrix, and eventual fibrosis. ACE inhibitors and ARBs counteract many of these.
ACE inhibitors and ARBs slow nephropathy progression beyond blood pressure control
Several major clinical trials27–32 examined the effects of either ACE inhibitors or ARBs in slowing the progression of diabetic nephropathy and have had consistently positive results.
The Collaborative Study Group30 was a 3-year randomized trial in 419 patients with type 1 diabetes, using the ACE inhibitor captopril vs placebo. Captopril was associated with less decline in kidney function and a 50% reduction in the risk of the combined end points of death, dialysis, and transplantation that was independent of the small difference in blood pressures between the two groups.
The Irbesartan Diabetic Nephropathy Trial (IDNT)31 studied the effect of the ARB irbesartan vs the calcium channel blocker amlodipine vs placebo over 2.6 years in 1,715 patients with type 2 diabetes. Irbesartan was found to be significantly more effective in protecting against the progression of nephropathy, independent of reduction in blood pressure.
The RENAAL trial,32 published in 2001, was a 3-year, randomized, double-blind study comparing the ARB losartan at increasing dosages with placebo (both taken in addition to conventional antihypertensive treatment) in 1,513 patients with type 2 diabetes and nephropathy. The blood pressure goal was 140/90 mm Hg in both groups, but the losartan group had a lower rate of doubling of serum creatinine, end-stage renal disease, and combined end-stage renal disease or death.
‘Aldosterone escape’ motivates the search for new therapies
An important reason for developing more ways to block the renin-angiotensin-aldosterone system is because of “aldosterone escape,” the phenomenon of angiotensin II or aldosterone returning to pretreatment levels despite continued ACE inhibition.
Biollaz et al,33 in a 1982 study of 19 patients with hypertension, showed that despite reducing blood pressure and keeping the blood level of ACE very low with twice-daily enalapril 20 mg, blood and urine levels of angiotensin II steadily rose back to baseline levels within a few months.
A growing body of evidence suggests that despite effective inhibition of angiotensin II activity, non-ACE synthetic pathways still permit angiotensin II generation via serine proteases such as chymase, cathepsin G, and tissue plasminogen activator.
Thus, efforts have been made to block the renin-angiotensin system in other places. In addition to ACE inhibitors and ARBs, two aldosterone receptor antagonists are available, spironolactone and eplerenone, both used to treat heart failure. A direct renin inhibitor, aliskiren, is also available.
Combination therapy—less proteinuria, but…
A number of studies have shown that combination treatment with agents having different targets in the renin-angiotensin-aldosterone system leads to larger reductions in albuminuria than does single-agent therapy.
Mogensen et al34 studied the effect of the ACE inhibitor lisinopril (20 mg per day) plus the ARB candesartan (16 mg per day) in subjects with microalbuminuria, hypertension, and type 2 diabetes. Combined treatment was more effective in reducing proteinuria.
Epstein et al35 studied the effects of the ACE inhibitor enalapril (20 mg/day) combined with either of two doses of the selective aldosterone receptor antagonist eplerenone (50 or 100 mg/day) or placebo. Both eplerenone dosages, when added to the enalapril treatment, significantly reduced albuminuria from baseline as early as week 4 (P < .001), but placebo treatment added to the enalapril did not result in any significant decrease in urinary albumin excretion. Systolic blood pressure decreased significantly in all treatment groups and by about the same amount.
The Aliskiren Combined With Losartan in Type 2 Diabetes and Nephropathy (AVOID) trial36 randomized more than 600 patients with type 2 diabetes and nephropathy to aliskiren (a renin inhibitor) or placebo added to the ARB losartan. Again, combination treatment was more renoprotective, independent of blood pressure lowering.
Worse outcomes with combination therapy?
More recent studies have indicated that although combination therapy reduces proteinuria to a greater extent than monotherapy, overall it worsens major renal and cardiovascular outcomes. The multicenter Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET)37 randomized more than 25,000 patients age 55 and older with established atherosclerotic vascular disease or with diabetes and end-organ damage to receive either the ARB telmisartan 80 mg daily, the ACE inhibitor ramipril 10 mg daily, or both. Mean follow-up was 56 months. The combination-treatment group had higher rates of death and renal disease than the single-therapy groups (which did not differ from one another).
Why the combination therapy had poorer outcomes is under debate. Patients may get sudden drops in blood pressure that are not detected with only periodic monitoring. Renal failure was mostly acute rather than chronic, and the estimated GFR declined more in the combined therapy group than in the single-therapy groups.
The Aliskiren Trial in Type 2 Diabetes Using Cardiovascular and Renal Disease Endpoints (ALTITUDE) was designed to test the effect of the direct renin inhibitor aliskiren or placebo, both arms combined with either an ACE inhibitor or an ARB in patients with type 2 diabetes at high risk for cardiovascular and renal events. The trial was terminated early because of more strokes and deaths in the combination therapy arms. The results led the FDA to issue black box warnings against using aliskiren with these other classes of agents, and all studies testing similar combinations have been stopped. (In one study that was stopped and has not yet been published, 100 patients with proteinuria were treated with either aliskiren, the ARB losartan, or both, to evaluate the effects of aldosterone escape. Results showed no differences: about one-third of each group had this phenomenon.)
My personal recommendation is as follows: for younger patients with proteinuria, at lower risk for cardiovascular events and with disease due not to diabetes but to immunoglobulin A nephropathy or another proteinuric kidney disease, treat with both an ACE inhibitor and ARB. But the combination should not be used for patients at high risk of cardiovascular disease, which includes almost all patients with diabetes.
If more aggressive renin-angiotensin system blockade is needed against diabetic nephropathy, adding a diuretic increases the impact of blocking the renin-angiotensin-aldosterone system on both proteinuria and progression of renal disease. The aldosterone blocker spironolactone 25 mg can be added if potassium levels are carefully monitored.
ACE inhibitor plus calcium channel blocker is safer than ACE inhibitor plus diuretic
The Avoiding Cardiovascular Events Through Combination Therapy in Patients Living With Systolic Hypertension (ACCOMPLISH) trial38 randomized more than 11,000 high-risk patients with hypertension to receive an ACE inhibitor (benazepril) plus either a calcium channel blocker (amlodipine) or thiazide diuretic (hydrochlorothiazide). Blood pressures were identical between the two groups, but the trial was terminated early, at 36 months, because of a higher risk of the combined end point of cardiovascular death, myocardial infarction, stroke, and other major cardiac events in the ACE inhibitor-thiazide group.
Although some experts believe this study is definitive and indicates that high blood pressure should never be treated with an ACE inhibitor-thiazide combination, I believe that caution is needed in interpreting these findings. This regimen should be avoided in older patients with diabetes at high risk for cardiovascular disease, but otherwise, getting blood pressure under control is critical, and this combination can be used if it works and the patient is tolerating it well.
In summary, the choice of blood pressure-lowering medications is based on reducing cardiovascular events and slowing the progression of kidney disease. Either an ACE inhibitor or an ARB is the first choice for patients with diabetes, hypertension, and any degree of proteinuria. Many experts recommend beginning one of these agents even if proteinuria is not present. However, the combination of an ACE inhibitor and ARB should not be used in diabetic patients, especially if they have cardiovascular disease, until further data clarify the results of the ONTARGET and ALTITUDE trials.
STRATEGY 4: METABOLIC MANIPULATION WITH NOVEL AGENTS
Several new agents have recently been studied for the treatment of diabetic nephropathy, including aminoguanidine, which reduces levels of advanced glycation end-products, and sulodexide, which blocks basement membrane permeability. Neither agent has been shown to be safe and effective in diabetic nephropathy. The newest agent is bardoxolone methyl. It induces the Keap1–Nrf2 pathway, which up-regulates cytoprotective factors, suppressing inflammatory and other cytokines that are major mediators of progression of chronic kidney disease.39
Pergola et al,40 in a phase 2, double-blind trial, randomized 227 adults with diabetic kidney disease and a low estimated GFR (20–45 mL/min/1.73 m2) to receive placebo or bardoxolone 25, 75, or 150 mg daily. Drug treatment was associated with improvement in the estimated GFR, a finding that persisted throughout the 52 weeks of treatment. Surprisingly, proteinuria did not decrease with drug treatment.
As of this writing, a large multicenter controlled randomized trial has been halted because of concerns by the data safety monitoring board, which found increased rates of death and fluid retention with the drug. A number of recent trials have shown a beneficial effect of sodium bicarbonate therapy in patients with late-stage chronic kidney disease. They have shown slowing of the progression of GFR decline in a number of renal diseases, including diabetes.
- Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med 1999; 341:1127–1133.
- de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011; 305:2532–2539.
- United States Renal Data System (USRDS) 2000 Annual Data Report. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases – Division of Kidney, Urologic and Hematologic Diseases. USRDS Coordinating Center operated by the Minneapolis Medical Research Foundation. www.usrds.org
- Macisaac RJ, Jerums G. Diabetic kidney disease with and without albuminuria. Curr Opin Nephrol Hypertens 2011; 20:246–257.
- Molitch ME, DeFronzo RA, Franz MJ, et al; American Diabetes Association. Nephropathy in diabetes. Diabetes Care 2004; 27(suppl 1):S79–S83.
- Vamos EP, Harris M, Millett C, et al. Association of systolic and diastolic blood pressure and all cause mortality in people with newly diagnosed type 2 diabetes: retrospective cohort study. BMJ 2012; 345:e5567.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 2004; 65:2309–2320.
- Parving HH, Andersen AR, Smidt UM, Svendsen PA. Early aggressive antihypertensive treatment reduces rate of decline in kidney function in diabetic nephropathy. Lancet 1983; 1:1175–1179.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Bangalore S, Kumar S, Lobach I, Messerli FH. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and bayesian random-effects meta-analyses of randomized trials. Circulation 2011; 123:2799–2810.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care 2012; 35(suppl 1):S11–S63. (Erratum in: Diabetes Care 2012; 35:660.)
- Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis 2000; 36:646–661.
- Ramsay L, Williams B, Johnston G, et al. Guidelines for management of hypertension: report of the third working party of the British Hypertension Society. J Hum Hypertens 1999; 13:569–592.
- Feldman RD, Campbell N, Larochelle P, et al. 1999 Canadian recommendations for the management of hypertension. Task Force for the Development of the 1999 Canadian Recommendations for the Management of Hypertension. CMAJ 1999; 161(suppl):12:S1–S17.
- Chalmers J, MacMahon S, Mancia G, et al. 1999 World Health Organization-International Society of Hypertension Guidelines for the management of hypertension. Guidelines Sub-committee of the World Health Organization. Clin Exp Hypertens 1999; 21:1009–1060.
- The seventh report of the Joint National Committee on Prevention, Detection Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993; 329:977–986.
- Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care 2000; 23(suppl 2):B21–B29.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352:837–853. Erratum in: Lancet 1999; 354:602.
- Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998; 317:703–713. Erratum in: BMJ 1999; 318:29.
- Ismail-Beigi F, Craven T, Banerji MA, et al; ACCORD trial group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010; 376:419–430. Erratum in: Lancet 2010; 376:1466.
- Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000: 355:253–259. Erratum in: Lancet2000; 356:860.
- Parving HH, Lehnert H, Bröchner-Mortensen J, et al; Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001; 345:870–878.
- Viberti G, Wheeldon NM; MicroAlbuminuria Reduction With VALsartan (MARVAL) Study Investigators. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure-independent effect. Circulation 2002; 106:672–678.
- Lewis EJ, Hunsicker LG, Bain R P, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329:1456–1462.
- Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- Biollaz J, Brunner HR, Gavras I, Waeber B, Gavras H. Antihypertensive therapy with MK 421: angiotensin II--renin relationships to evaluate efficacy of converting enzyme blockade. J Cardiovasc Pharmacol 1982; 4:966–972.
- Mogensen CE, Neldam S, Tikkanen I, et al. Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ 2000; 321:1440–1444.
- Epstein M, Williams GH, Weinberger M, et al. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol 2006; 1:940–951.
- Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK; AVOID Study Investigators. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 2008; 358:2433–2446.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Jamerson K, Weber MA, Bakris GL, et al; ACCOMPLISH Trial Investigators. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008; 359:2417–2428.
- Kim HJ, Vaziri ND. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol 2010; 298:F662–F671.
- Pergola PE, Raskin P, Toto RD, et al; BEAM Study Investigators Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med 2011; 365:327–336.
Diabetes is on the rise, and so is diabetic nephropathy. In view of this epidemic, physicians should consider strategies to detect and control kidney disease in their diabetic patients.
This article will focus on kidney disease in adult-onset type 2 diabetes. Although it has different pathogenetic mechanisms than type 1 diabetes, the clinical course of the two conditions is very similar in terms of the prevalence of proteinuria after diagnosis, the progression to renal failure after the onset of proteinuria, and treatment options.1
DIABETES AND DIABETIC KIDNEY DISEASE ARE ON THE RISE
The incidence of diabetes increases with age, and with the aging of the baby boomers, its prevalence is growing dramatically. The 2005– 2008 National Health and Nutrition Examination Survey estimated the prevalence as 3.7% in adults age 20 to 44, 13.7% at age 45 to 64, and 26.9% in people age 65 and older. The obesity epidemic is also contributing to the increase in diabetes in all age groups.
Diabetic kidney disease has increased in the United States from about 4 million cases 20 years ago to about 7 million in 2005–2008.2 Diabetes is the major cause of end-stage renal disease in the developed world, accounting for 40% to 50% of cases. Other major causes are hypertension (27%) and glomerulonephritis (13%).3
Physicians in nearly every field of medicine now care for patients with diabetic nephropathy. The classic presentation—a patient who has impaired vision, fluid retention with edema, and hypertension—is commonly seen in dialysis units and ophthalmology and cardiovascular clinics.
CLINICAL PROGRESSION
Early in the course of diabetic nephropathy, blood pressure is normal and microalbuminuria is not evident, but many patients have a high glomerular filtration rate (GFR), indicating temporarily “enhanced” renal function or hyperfiltration. The next stage is characterized by microalbuminuria, correlating with glomerular mesangial expansion: the GFR falls back into the normal range and blood pressure starts to increase. Finally, macroalbuminuria occurs, accompanied by rising blood pressure and a declining GFR, correlating with the histologic appearance of glomerulosclerosis and Kimmelstiel-Wilson nodules.4
Hypertension develops in 5% of patients by 10 years after type 1 diabetes is diagnosed, 33% by 20 years, and 70% by 40 years. In contrast, 40% of patients with type 2 diabetes have high blood pressure at diagnosis.
Unfortunately, in most cases, this progression is a one-way street, so it is critical to intervene to try to slow the progression early in the course of the disease process.
SCREENING FOR DIABETIC NEPHROPATHY
Nephropathy screening guidelines for patients with diabetes are provided in Table 1.5
Blood pressure should be monitored at each office visit (Table 1). The goal for adults with diabetes should be to reduce blood pressure to 130/80 mm Hg. Reduction beyond this level may be associated with an increased mortality rate.6 Very high blood pressure (> 180 mm Hg systolic) should be lowered slowly. Lowering blood pressure delays the progression from microalbuminuria (30–299 mg/day or 20–199 μg/min) to macroalbuminuria (> 300 mg/day or > 200 μg/min) and slows the progression to renal failure.
Urinary albumin. Proteinuria takes 5 to 10 years to develop after the onset of diabetes. Because it is possible for patients with type 2 diabetes to have had the disease for some time before being diagnosed, urinary albumin screening should be performed at diagnosis and annually thereafter. Patients with type 1 are usually diagnosed with diabetes at or near onset of disease; therefore, annual screening for urinary albumin can begin 5 years after diagnosis.5
Proteinuria can be measured in different ways (Table 2). The basic screening test for clinical proteinuria is the urine dipstick, which is very sensitive to albumin and relatively insensitive to other proteins. “Trace-positive” results are common in healthy people, so proteinuria is not confirmed unless a patient has repeatedly positive results.
Microalbuminuria is important to measure, especially if it helps determine therapy. It is not detectable by the urinary dipstick, but can be measured in the following ways:
- Measurement of the albumin-creatinine ratio in a random spot collection
- 24-hour collection (creatinine should simultaneously be measured and creatinine clearance calculated)
- Timed collection (4 hours or overnight).
The first method is preferred, and any positive test result must be confirmed by repeat analyses of urinary albumin before a patient is diagnosed with microalbuminuria.
Occasionally a patient presenting with proteinuria but normal blood sugar and hemoglobin A1c will have a biopsy that reveals morphologic changes of classic diabetic nephropathy. Most such patients have a history of hyperglycemia, indicating that they actually have been diabetic.
Proteinuria—the best marker of disease progression
Proteinuria is the strongest predictor of renal outcomes. The Reduction in End Points in Noninsulin-Dependent Diabetes Mellitus With the Angiotensin II Antagonist Losartan (RENAAL) study was a randomized, placebo-controlled trial in more than 1,500 patients with type 2 diabetes to test the effects of losartan on renal outcome. Those with high albuminuria (> 3.0 g albumin/g creatinine) at baseline were five times more likely to reach a renal end point and were eight times more likely to have progression to end-stage renal disease than patients with low albuminuria (< 1.5 g/g).7 The degree of albuminuria after 6 months of treatment showed similar predictive trends, indicating that monitoring and treating proteinuria are extremely important goals.
STRATEGY 1 TO LIMIT RENAL INJURY: REDUCE BLOOD PRESSURE
Blood pressure control improves renal and cardiovascular function.
As early as 1983, Parving et al,8 in a study of only 10 insulin-dependent diabetic patients, showed strong evidence that early aggressive antihypertensive treatment improved the course of diabetic nephropathy. During the mean pretreatment period of 29 months, the GFR decreased significantly and the urinary albumin excretion rate and arterial blood pressure rose significantly. During the mean 39-month period of antihypertensive treatment with metoprolol, hydralazine, and furosemide or a thiazide, mean arterial blood pressure fell from 144/97 to 128/84 mm Hg and urinary albumin excretion from 977 to 433 μg/ min. The rate of decline in GFR slowed from 0.91 mL/min/month before treatment to 0.39 mL/min/month during treatment.
The Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial9 enrolled more than 11,000 patients internationally with type 2 diabetes at high risk for cardiovascular events. In addition to standard therapy, blood pressure was intensively controlled in one group with a combination of the angiotensin-converting enzyme (ACE) inhibitor perindopril and the diuretic indapamide. The intensive-therapy group achieved blood pressures less than 140/80 mm Hg and had a mean reduction of systolic blood pressure of 5.6 mm Hg and diastolic blood pressure of 2.2 mm Hg vs controls. Despite these apparently modest reductions, the intensively controlled group had a significant 9% reduction of the primary outcome of combined macrovascular events (cardiovascular death, myocardial infarction, and stroke) and microvascular events (new or worsening nephropathy, or retinopathy).10
A meta-analysis of studies of patients with type 2 diabetes found reduced nephropathy with systolic blood pressure control to less than 130 mm Hg.11
The United Kingdom Prospective Diabetes Study (UKPDS) is a series of studies of diabetes. The original study in 1998 enrolled 5,102 patients with newly diagnosed type 2 diabetes.12 The more than 1,000 patients with hypertension were randomized to either tight blood pressure control or regular care. The intensive treatment group had a mean blood pressure reduction of 9 mm Hg systolic and 3 mm Hg diastolic, along with major reductions in all diabetes end points, diabetes deaths, microvascular disease, and stroke over a median follow-up of 8.4 years.
Continuous blood pressure control is critical
Tight blood pressure control must be maintained to have continued benefit. During the 10 years following the UKPDS, no attempts were made to maintain the previously assigned therapies. A follow-up study13 of 884 UKPDS patients found that blood pressures were the same again between the two groups 2 years after the trial was stopped, and no beneficial legacy effect from previous blood pressure control was evident on end points.
Control below 120 mm Hg systolic not needed
Blood pressure control slows kidney disease and prevents major macrovascular disease, but there is no evidence that lowering systolic blood pressure below 120 mm Hg provides additional benefit. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,14 more than 10,000 patients with type 2 diabetes and existing cardiovascular disease or additional cardiovascular risk factors were randomized to a goal of systolic blood pressure less than 120 mm Hg or less than 140 mm Hg (actual mean systolic pressures were 119 vs 134 mm Hg, respectively). Over nearly 5 years, there was no difference in cardiovascular events or deaths between the two groups.15
Since 1997, six international organizations have revised their recommended blood pressure goals in diabetes mellitus and renal diseases. Randomized clinical trials and observational studies have demonstrated the importance of blood pressure control to the level of 125/75 to 140/80 mm Hg. The National Kidney Foundation, the American Diabetes Association, and the Canadian Hypertension Society have developed consensus guidelines for blood pressure control to less than 130/80 mm Hg.16–21 Table 3 summarizes blood pressure goals for patients with diabetes.
STRATEGY 2: CONTROL BLOOD SUGAR
Recommendations for blood sugar goals are more controversial.
The Diabetes Control and Complications Trial22 provided early evidence that tight blood sugar control slows the development of microalbuminuria and macroalbuminuria. The study randomized more than 1,400 patients with type 1 diabetes to either standard therapy (1 or 2 daily insulin injections) or intensive therapy (an external insulin pump or 3 or more insulin injections guided by frequent blood glucose monitoring) to keep blood glucose levels close to normal. About half the patients had mild retinopathy at baseline and the others had no retinopathy. After 6.5 years, intensive therapy was found to significantly delay the onset and slow the progression of diabetic retinopathy and nephropathy.
The Kumamoto Study23 randomized 110 patients with type 2 diabetes and either no retinopathy (primary prevention cohort) or simple retinopathy (secondary prevention cohort) to receive either multiple insulin injections or conventional insulin therapy over 8 years. Intensive therapy led to lower rates of retinopathy (7.7% vs 32% in primary prevention and 19% vs 44% in secondary prevention) and progressive nephropathy (7% vs 28% in primary prevention at 6 years and 11% vs 32% in secondary prevention).
In addition to studying the effects of blood pressure control, the UKPDS also studied the effects of intensive blood glucose control.24,25 Nearly 4,000 patients with newly diagnosed type 2 diabetes were randomized to intensive treatment with a sulfonylurea or insulin, or to conventional treatment with diet. Over 10 years, the mean hemoglobin A1c was reduced to 7.0% in the intensive group and 7.9% in the conventional group. The risk of any diabetes-related end point was 12% lower in the intensive group, 10% lower for diabetes-related death, and 6% lower for all-cause mortality. There was also a 25% reduction in microvascular disease (retinopathy and nephropathy). However, the intensive group had more hypoglycemic episodes than the conventional group and a tendency to some increase in macrovascular events. A legacy effect was evident: patients who had intensive treatment had less microvascular disease progression years after stopping therapy.
Tight glycemic control reduces nephropathy, but does it increase cardiovascular risk?
Earlier trials provided strong evidence that blood glucose control prevents or slows retinopathy and nephropathy. The critical question is, “At what expense?” Although diabetes is the most common cause of kidney failure in the United States, most people with diabetes do not die of kidney failure, but of cardiovascular disease. Two recent large trials had different results regarding glycemic control below hemoglobin A1c of 7.0% and macrovascular risk, creating a controversy about what recommendations are best.
The ADVANCE trial, enrolling 11,140 patients with type 2 diabetes, was largely conducted in Australia and used the sulfonylurea glipizide for glycemic control. Compared with the group that received standard therapy (n=5,569), the intensive-treatment group (n=5,571) achieved mean hemoglobin A1c levels of 6.5% compared with 7.3% in the standard group, and had less nephropathy, less microalbuminuria, less doubling of creatinine, and a lower rate of end-stage renal disease (4% vs 5% in the standard therapy group). No difference between the two groups was found in retinopathy. Rates of all-cause mortality did not differ between the groups.9
The ACCORD trial had more than 10,000 subjects with type 2 diabetes and took place mostly in the United States. Using mainly rosiglitazone for intensive therapy, the intensive group achieved hemoglobin A1c levels of 6.4% vs 7.5% in the standard-therapy group. The trial was stopped early, at 3.7 years, because of a higher risk of death and cardiovascular events in the group with intensive glycemic control. However, the intensive-therapy group did have a significant decrease in microvascular renal outcomes and a reduction in the progression of retinopathy.14,26
In summary, tighter glycemic control improves microvascular complications—both retinopathy and nephropathy—in patients with type 2 diabetes. The benefit of intensive therapy on macrovascular complications (stroke, myocardial infarction) in long-standing diabetes has not been convincingly demonstrated in randomized trials. The UKPDS suggested that maintaining a hemoglobin A1c of 7% in patients newly diagnosed with type 2 diabetes confers long-term cardiovascular benefits. The target hemoglobin A1c for type 2 diabetes should be tailored to the patient: 7% is a reasonable goal for most patients, but the goal should be higher for the elderly and frail. Reducing the risk of cardiovascular death is still best done by controlling blood pressure, reducing lipids, quitting smoking, and losing weight.
STRATEGY 3: INHIBIT THE RENIN-ANGIOTENSIN-ALDOSTERONE AXIS
Components of the renin-angiotensin-aldo-sterone system are present not only in the circulation but also in many tissues, including the heart, brain, kidney, blood vessels, and adrenal glands. The role of renin-angiotensin-aldosterone system blockers in treating and preventing diabetic nephropathy has become controversial in recent years with findings from new studies.
The renin-angiotensin-aldosterone system is important in the development or maintenance of high blood pressure and the resultant damage to the brain, heart, and kidney. Drug development has focused on inhibiting steps in the biochemical pathway. ACE inhibitors block the formation of angiotensin II—the most biologically potent angiotensin peptide—and are among the most commonly used drugs to treat hypertension and concomitant conditions, such as renal insufficiency, proteinuria, and heart failure. Angiotensin receptor blockers (ARBs) interact with the angiotensin AT1 receptor and block most of its actions. They are approved by the US Food and Drug Administration (FDA) for the treatment of hypertension, and they help prevent left ventricular hypertrophy and mesangial sclerosis. Large studies have shown that ACE inhibitors and ARBs offer similar cardiovascular benefit.
The glomerulus has the only capillary bed with a blood supply that drains into an efferent arteriole instead of a venule, providing high resistance to aid filtration. Efferent arterioles are rich in AT1 receptors. In the presence of angiotensin II they constrict, increasing pressure in the glomerulus, which can lead to proteinuria and glomerulosclerosis. ACE inhibitors and ARBs relax the efferent arteriole, allowing increased blood flow through the glomerulus. This reduction in intraglomerular pressure is associated with less proteinuria and less glomerulosclerosis.
Diabetes promotes renal disease in many ways. Glucose and advanced glycation end products can lead to increased blood flow and increased pressure in the glomerulus. Through a variety of pathways, hyperglycemia, acting on angiotensin II, leads to NF-kapa beta production, profibrotic cytokines, increased matrix, and eventual fibrosis. ACE inhibitors and ARBs counteract many of these.
ACE inhibitors and ARBs slow nephropathy progression beyond blood pressure control
Several major clinical trials27–32 examined the effects of either ACE inhibitors or ARBs in slowing the progression of diabetic nephropathy and have had consistently positive results.
The Collaborative Study Group30 was a 3-year randomized trial in 419 patients with type 1 diabetes, using the ACE inhibitor captopril vs placebo. Captopril was associated with less decline in kidney function and a 50% reduction in the risk of the combined end points of death, dialysis, and transplantation that was independent of the small difference in blood pressures between the two groups.
The Irbesartan Diabetic Nephropathy Trial (IDNT)31 studied the effect of the ARB irbesartan vs the calcium channel blocker amlodipine vs placebo over 2.6 years in 1,715 patients with type 2 diabetes. Irbesartan was found to be significantly more effective in protecting against the progression of nephropathy, independent of reduction in blood pressure.
The RENAAL trial,32 published in 2001, was a 3-year, randomized, double-blind study comparing the ARB losartan at increasing dosages with placebo (both taken in addition to conventional antihypertensive treatment) in 1,513 patients with type 2 diabetes and nephropathy. The blood pressure goal was 140/90 mm Hg in both groups, but the losartan group had a lower rate of doubling of serum creatinine, end-stage renal disease, and combined end-stage renal disease or death.
‘Aldosterone escape’ motivates the search for new therapies
An important reason for developing more ways to block the renin-angiotensin-aldosterone system is because of “aldosterone escape,” the phenomenon of angiotensin II or aldosterone returning to pretreatment levels despite continued ACE inhibition.
Biollaz et al,33 in a 1982 study of 19 patients with hypertension, showed that despite reducing blood pressure and keeping the blood level of ACE very low with twice-daily enalapril 20 mg, blood and urine levels of angiotensin II steadily rose back to baseline levels within a few months.
A growing body of evidence suggests that despite effective inhibition of angiotensin II activity, non-ACE synthetic pathways still permit angiotensin II generation via serine proteases such as chymase, cathepsin G, and tissue plasminogen activator.
Thus, efforts have been made to block the renin-angiotensin system in other places. In addition to ACE inhibitors and ARBs, two aldosterone receptor antagonists are available, spironolactone and eplerenone, both used to treat heart failure. A direct renin inhibitor, aliskiren, is also available.
Combination therapy—less proteinuria, but…
A number of studies have shown that combination treatment with agents having different targets in the renin-angiotensin-aldosterone system leads to larger reductions in albuminuria than does single-agent therapy.
Mogensen et al34 studied the effect of the ACE inhibitor lisinopril (20 mg per day) plus the ARB candesartan (16 mg per day) in subjects with microalbuminuria, hypertension, and type 2 diabetes. Combined treatment was more effective in reducing proteinuria.
Epstein et al35 studied the effects of the ACE inhibitor enalapril (20 mg/day) combined with either of two doses of the selective aldosterone receptor antagonist eplerenone (50 or 100 mg/day) or placebo. Both eplerenone dosages, when added to the enalapril treatment, significantly reduced albuminuria from baseline as early as week 4 (P < .001), but placebo treatment added to the enalapril did not result in any significant decrease in urinary albumin excretion. Systolic blood pressure decreased significantly in all treatment groups and by about the same amount.
The Aliskiren Combined With Losartan in Type 2 Diabetes and Nephropathy (AVOID) trial36 randomized more than 600 patients with type 2 diabetes and nephropathy to aliskiren (a renin inhibitor) or placebo added to the ARB losartan. Again, combination treatment was more renoprotective, independent of blood pressure lowering.
Worse outcomes with combination therapy?
More recent studies have indicated that although combination therapy reduces proteinuria to a greater extent than monotherapy, overall it worsens major renal and cardiovascular outcomes. The multicenter Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET)37 randomized more than 25,000 patients age 55 and older with established atherosclerotic vascular disease or with diabetes and end-organ damage to receive either the ARB telmisartan 80 mg daily, the ACE inhibitor ramipril 10 mg daily, or both. Mean follow-up was 56 months. The combination-treatment group had higher rates of death and renal disease than the single-therapy groups (which did not differ from one another).
Why the combination therapy had poorer outcomes is under debate. Patients may get sudden drops in blood pressure that are not detected with only periodic monitoring. Renal failure was mostly acute rather than chronic, and the estimated GFR declined more in the combined therapy group than in the single-therapy groups.
The Aliskiren Trial in Type 2 Diabetes Using Cardiovascular and Renal Disease Endpoints (ALTITUDE) was designed to test the effect of the direct renin inhibitor aliskiren or placebo, both arms combined with either an ACE inhibitor or an ARB in patients with type 2 diabetes at high risk for cardiovascular and renal events. The trial was terminated early because of more strokes and deaths in the combination therapy arms. The results led the FDA to issue black box warnings against using aliskiren with these other classes of agents, and all studies testing similar combinations have been stopped. (In one study that was stopped and has not yet been published, 100 patients with proteinuria were treated with either aliskiren, the ARB losartan, or both, to evaluate the effects of aldosterone escape. Results showed no differences: about one-third of each group had this phenomenon.)
My personal recommendation is as follows: for younger patients with proteinuria, at lower risk for cardiovascular events and with disease due not to diabetes but to immunoglobulin A nephropathy or another proteinuric kidney disease, treat with both an ACE inhibitor and ARB. But the combination should not be used for patients at high risk of cardiovascular disease, which includes almost all patients with diabetes.
If more aggressive renin-angiotensin system blockade is needed against diabetic nephropathy, adding a diuretic increases the impact of blocking the renin-angiotensin-aldosterone system on both proteinuria and progression of renal disease. The aldosterone blocker spironolactone 25 mg can be added if potassium levels are carefully monitored.
ACE inhibitor plus calcium channel blocker is safer than ACE inhibitor plus diuretic
The Avoiding Cardiovascular Events Through Combination Therapy in Patients Living With Systolic Hypertension (ACCOMPLISH) trial38 randomized more than 11,000 high-risk patients with hypertension to receive an ACE inhibitor (benazepril) plus either a calcium channel blocker (amlodipine) or thiazide diuretic (hydrochlorothiazide). Blood pressures were identical between the two groups, but the trial was terminated early, at 36 months, because of a higher risk of the combined end point of cardiovascular death, myocardial infarction, stroke, and other major cardiac events in the ACE inhibitor-thiazide group.
Although some experts believe this study is definitive and indicates that high blood pressure should never be treated with an ACE inhibitor-thiazide combination, I believe that caution is needed in interpreting these findings. This regimen should be avoided in older patients with diabetes at high risk for cardiovascular disease, but otherwise, getting blood pressure under control is critical, and this combination can be used if it works and the patient is tolerating it well.
In summary, the choice of blood pressure-lowering medications is based on reducing cardiovascular events and slowing the progression of kidney disease. Either an ACE inhibitor or an ARB is the first choice for patients with diabetes, hypertension, and any degree of proteinuria. Many experts recommend beginning one of these agents even if proteinuria is not present. However, the combination of an ACE inhibitor and ARB should not be used in diabetic patients, especially if they have cardiovascular disease, until further data clarify the results of the ONTARGET and ALTITUDE trials.
STRATEGY 4: METABOLIC MANIPULATION WITH NOVEL AGENTS
Several new agents have recently been studied for the treatment of diabetic nephropathy, including aminoguanidine, which reduces levels of advanced glycation end-products, and sulodexide, which blocks basement membrane permeability. Neither agent has been shown to be safe and effective in diabetic nephropathy. The newest agent is bardoxolone methyl. It induces the Keap1–Nrf2 pathway, which up-regulates cytoprotective factors, suppressing inflammatory and other cytokines that are major mediators of progression of chronic kidney disease.39
Pergola et al,40 in a phase 2, double-blind trial, randomized 227 adults with diabetic kidney disease and a low estimated GFR (20–45 mL/min/1.73 m2) to receive placebo or bardoxolone 25, 75, or 150 mg daily. Drug treatment was associated with improvement in the estimated GFR, a finding that persisted throughout the 52 weeks of treatment. Surprisingly, proteinuria did not decrease with drug treatment.
As of this writing, a large multicenter controlled randomized trial has been halted because of concerns by the data safety monitoring board, which found increased rates of death and fluid retention with the drug. A number of recent trials have shown a beneficial effect of sodium bicarbonate therapy in patients with late-stage chronic kidney disease. They have shown slowing of the progression of GFR decline in a number of renal diseases, including diabetes.
Diabetes is on the rise, and so is diabetic nephropathy. In view of this epidemic, physicians should consider strategies to detect and control kidney disease in their diabetic patients.
This article will focus on kidney disease in adult-onset type 2 diabetes. Although it has different pathogenetic mechanisms than type 1 diabetes, the clinical course of the two conditions is very similar in terms of the prevalence of proteinuria after diagnosis, the progression to renal failure after the onset of proteinuria, and treatment options.1
DIABETES AND DIABETIC KIDNEY DISEASE ARE ON THE RISE
The incidence of diabetes increases with age, and with the aging of the baby boomers, its prevalence is growing dramatically. The 2005– 2008 National Health and Nutrition Examination Survey estimated the prevalence as 3.7% in adults age 20 to 44, 13.7% at age 45 to 64, and 26.9% in people age 65 and older. The obesity epidemic is also contributing to the increase in diabetes in all age groups.
Diabetic kidney disease has increased in the United States from about 4 million cases 20 years ago to about 7 million in 2005–2008.2 Diabetes is the major cause of end-stage renal disease in the developed world, accounting for 40% to 50% of cases. Other major causes are hypertension (27%) and glomerulonephritis (13%).3
Physicians in nearly every field of medicine now care for patients with diabetic nephropathy. The classic presentation—a patient who has impaired vision, fluid retention with edema, and hypertension—is commonly seen in dialysis units and ophthalmology and cardiovascular clinics.
CLINICAL PROGRESSION
Early in the course of diabetic nephropathy, blood pressure is normal and microalbuminuria is not evident, but many patients have a high glomerular filtration rate (GFR), indicating temporarily “enhanced” renal function or hyperfiltration. The next stage is characterized by microalbuminuria, correlating with glomerular mesangial expansion: the GFR falls back into the normal range and blood pressure starts to increase. Finally, macroalbuminuria occurs, accompanied by rising blood pressure and a declining GFR, correlating with the histologic appearance of glomerulosclerosis and Kimmelstiel-Wilson nodules.4
Hypertension develops in 5% of patients by 10 years after type 1 diabetes is diagnosed, 33% by 20 years, and 70% by 40 years. In contrast, 40% of patients with type 2 diabetes have high blood pressure at diagnosis.
Unfortunately, in most cases, this progression is a one-way street, so it is critical to intervene to try to slow the progression early in the course of the disease process.
SCREENING FOR DIABETIC NEPHROPATHY
Nephropathy screening guidelines for patients with diabetes are provided in Table 1.5
Blood pressure should be monitored at each office visit (Table 1). The goal for adults with diabetes should be to reduce blood pressure to 130/80 mm Hg. Reduction beyond this level may be associated with an increased mortality rate.6 Very high blood pressure (> 180 mm Hg systolic) should be lowered slowly. Lowering blood pressure delays the progression from microalbuminuria (30–299 mg/day or 20–199 μg/min) to macroalbuminuria (> 300 mg/day or > 200 μg/min) and slows the progression to renal failure.
Urinary albumin. Proteinuria takes 5 to 10 years to develop after the onset of diabetes. Because it is possible for patients with type 2 diabetes to have had the disease for some time before being diagnosed, urinary albumin screening should be performed at diagnosis and annually thereafter. Patients with type 1 are usually diagnosed with diabetes at or near onset of disease; therefore, annual screening for urinary albumin can begin 5 years after diagnosis.5
Proteinuria can be measured in different ways (Table 2). The basic screening test for clinical proteinuria is the urine dipstick, which is very sensitive to albumin and relatively insensitive to other proteins. “Trace-positive” results are common in healthy people, so proteinuria is not confirmed unless a patient has repeatedly positive results.
Microalbuminuria is important to measure, especially if it helps determine therapy. It is not detectable by the urinary dipstick, but can be measured in the following ways:
- Measurement of the albumin-creatinine ratio in a random spot collection
- 24-hour collection (creatinine should simultaneously be measured and creatinine clearance calculated)
- Timed collection (4 hours or overnight).
The first method is preferred, and any positive test result must be confirmed by repeat analyses of urinary albumin before a patient is diagnosed with microalbuminuria.
Occasionally a patient presenting with proteinuria but normal blood sugar and hemoglobin A1c will have a biopsy that reveals morphologic changes of classic diabetic nephropathy. Most such patients have a history of hyperglycemia, indicating that they actually have been diabetic.
Proteinuria—the best marker of disease progression
Proteinuria is the strongest predictor of renal outcomes. The Reduction in End Points in Noninsulin-Dependent Diabetes Mellitus With the Angiotensin II Antagonist Losartan (RENAAL) study was a randomized, placebo-controlled trial in more than 1,500 patients with type 2 diabetes to test the effects of losartan on renal outcome. Those with high albuminuria (> 3.0 g albumin/g creatinine) at baseline were five times more likely to reach a renal end point and were eight times more likely to have progression to end-stage renal disease than patients with low albuminuria (< 1.5 g/g).7 The degree of albuminuria after 6 months of treatment showed similar predictive trends, indicating that monitoring and treating proteinuria are extremely important goals.
STRATEGY 1 TO LIMIT RENAL INJURY: REDUCE BLOOD PRESSURE
Blood pressure control improves renal and cardiovascular function.
As early as 1983, Parving et al,8 in a study of only 10 insulin-dependent diabetic patients, showed strong evidence that early aggressive antihypertensive treatment improved the course of diabetic nephropathy. During the mean pretreatment period of 29 months, the GFR decreased significantly and the urinary albumin excretion rate and arterial blood pressure rose significantly. During the mean 39-month period of antihypertensive treatment with metoprolol, hydralazine, and furosemide or a thiazide, mean arterial blood pressure fell from 144/97 to 128/84 mm Hg and urinary albumin excretion from 977 to 433 μg/ min. The rate of decline in GFR slowed from 0.91 mL/min/month before treatment to 0.39 mL/min/month during treatment.
The Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) trial9 enrolled more than 11,000 patients internationally with type 2 diabetes at high risk for cardiovascular events. In addition to standard therapy, blood pressure was intensively controlled in one group with a combination of the angiotensin-converting enzyme (ACE) inhibitor perindopril and the diuretic indapamide. The intensive-therapy group achieved blood pressures less than 140/80 mm Hg and had a mean reduction of systolic blood pressure of 5.6 mm Hg and diastolic blood pressure of 2.2 mm Hg vs controls. Despite these apparently modest reductions, the intensively controlled group had a significant 9% reduction of the primary outcome of combined macrovascular events (cardiovascular death, myocardial infarction, and stroke) and microvascular events (new or worsening nephropathy, or retinopathy).10
A meta-analysis of studies of patients with type 2 diabetes found reduced nephropathy with systolic blood pressure control to less than 130 mm Hg.11
The United Kingdom Prospective Diabetes Study (UKPDS) is a series of studies of diabetes. The original study in 1998 enrolled 5,102 patients with newly diagnosed type 2 diabetes.12 The more than 1,000 patients with hypertension were randomized to either tight blood pressure control or regular care. The intensive treatment group had a mean blood pressure reduction of 9 mm Hg systolic and 3 mm Hg diastolic, along with major reductions in all diabetes end points, diabetes deaths, microvascular disease, and stroke over a median follow-up of 8.4 years.
Continuous blood pressure control is critical
Tight blood pressure control must be maintained to have continued benefit. During the 10 years following the UKPDS, no attempts were made to maintain the previously assigned therapies. A follow-up study13 of 884 UKPDS patients found that blood pressures were the same again between the two groups 2 years after the trial was stopped, and no beneficial legacy effect from previous blood pressure control was evident on end points.
Control below 120 mm Hg systolic not needed
Blood pressure control slows kidney disease and prevents major macrovascular disease, but there is no evidence that lowering systolic blood pressure below 120 mm Hg provides additional benefit. In the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial,14 more than 10,000 patients with type 2 diabetes and existing cardiovascular disease or additional cardiovascular risk factors were randomized to a goal of systolic blood pressure less than 120 mm Hg or less than 140 mm Hg (actual mean systolic pressures were 119 vs 134 mm Hg, respectively). Over nearly 5 years, there was no difference in cardiovascular events or deaths between the two groups.15
Since 1997, six international organizations have revised their recommended blood pressure goals in diabetes mellitus and renal diseases. Randomized clinical trials and observational studies have demonstrated the importance of blood pressure control to the level of 125/75 to 140/80 mm Hg. The National Kidney Foundation, the American Diabetes Association, and the Canadian Hypertension Society have developed consensus guidelines for blood pressure control to less than 130/80 mm Hg.16–21 Table 3 summarizes blood pressure goals for patients with diabetes.
STRATEGY 2: CONTROL BLOOD SUGAR
Recommendations for blood sugar goals are more controversial.
The Diabetes Control and Complications Trial22 provided early evidence that tight blood sugar control slows the development of microalbuminuria and macroalbuminuria. The study randomized more than 1,400 patients with type 1 diabetes to either standard therapy (1 or 2 daily insulin injections) or intensive therapy (an external insulin pump or 3 or more insulin injections guided by frequent blood glucose monitoring) to keep blood glucose levels close to normal. About half the patients had mild retinopathy at baseline and the others had no retinopathy. After 6.5 years, intensive therapy was found to significantly delay the onset and slow the progression of diabetic retinopathy and nephropathy.
The Kumamoto Study23 randomized 110 patients with type 2 diabetes and either no retinopathy (primary prevention cohort) or simple retinopathy (secondary prevention cohort) to receive either multiple insulin injections or conventional insulin therapy over 8 years. Intensive therapy led to lower rates of retinopathy (7.7% vs 32% in primary prevention and 19% vs 44% in secondary prevention) and progressive nephropathy (7% vs 28% in primary prevention at 6 years and 11% vs 32% in secondary prevention).
In addition to studying the effects of blood pressure control, the UKPDS also studied the effects of intensive blood glucose control.24,25 Nearly 4,000 patients with newly diagnosed type 2 diabetes were randomized to intensive treatment with a sulfonylurea or insulin, or to conventional treatment with diet. Over 10 years, the mean hemoglobin A1c was reduced to 7.0% in the intensive group and 7.9% in the conventional group. The risk of any diabetes-related end point was 12% lower in the intensive group, 10% lower for diabetes-related death, and 6% lower for all-cause mortality. There was also a 25% reduction in microvascular disease (retinopathy and nephropathy). However, the intensive group had more hypoglycemic episodes than the conventional group and a tendency to some increase in macrovascular events. A legacy effect was evident: patients who had intensive treatment had less microvascular disease progression years after stopping therapy.
Tight glycemic control reduces nephropathy, but does it increase cardiovascular risk?
Earlier trials provided strong evidence that blood glucose control prevents or slows retinopathy and nephropathy. The critical question is, “At what expense?” Although diabetes is the most common cause of kidney failure in the United States, most people with diabetes do not die of kidney failure, but of cardiovascular disease. Two recent large trials had different results regarding glycemic control below hemoglobin A1c of 7.0% and macrovascular risk, creating a controversy about what recommendations are best.
The ADVANCE trial, enrolling 11,140 patients with type 2 diabetes, was largely conducted in Australia and used the sulfonylurea glipizide for glycemic control. Compared with the group that received standard therapy (n=5,569), the intensive-treatment group (n=5,571) achieved mean hemoglobin A1c levels of 6.5% compared with 7.3% in the standard group, and had less nephropathy, less microalbuminuria, less doubling of creatinine, and a lower rate of end-stage renal disease (4% vs 5% in the standard therapy group). No difference between the two groups was found in retinopathy. Rates of all-cause mortality did not differ between the groups.9
The ACCORD trial had more than 10,000 subjects with type 2 diabetes and took place mostly in the United States. Using mainly rosiglitazone for intensive therapy, the intensive group achieved hemoglobin A1c levels of 6.4% vs 7.5% in the standard-therapy group. The trial was stopped early, at 3.7 years, because of a higher risk of death and cardiovascular events in the group with intensive glycemic control. However, the intensive-therapy group did have a significant decrease in microvascular renal outcomes and a reduction in the progression of retinopathy.14,26
In summary, tighter glycemic control improves microvascular complications—both retinopathy and nephropathy—in patients with type 2 diabetes. The benefit of intensive therapy on macrovascular complications (stroke, myocardial infarction) in long-standing diabetes has not been convincingly demonstrated in randomized trials. The UKPDS suggested that maintaining a hemoglobin A1c of 7% in patients newly diagnosed with type 2 diabetes confers long-term cardiovascular benefits. The target hemoglobin A1c for type 2 diabetes should be tailored to the patient: 7% is a reasonable goal for most patients, but the goal should be higher for the elderly and frail. Reducing the risk of cardiovascular death is still best done by controlling blood pressure, reducing lipids, quitting smoking, and losing weight.
STRATEGY 3: INHIBIT THE RENIN-ANGIOTENSIN-ALDOSTERONE AXIS
Components of the renin-angiotensin-aldo-sterone system are present not only in the circulation but also in many tissues, including the heart, brain, kidney, blood vessels, and adrenal glands. The role of renin-angiotensin-aldosterone system blockers in treating and preventing diabetic nephropathy has become controversial in recent years with findings from new studies.
The renin-angiotensin-aldosterone system is important in the development or maintenance of high blood pressure and the resultant damage to the brain, heart, and kidney. Drug development has focused on inhibiting steps in the biochemical pathway. ACE inhibitors block the formation of angiotensin II—the most biologically potent angiotensin peptide—and are among the most commonly used drugs to treat hypertension and concomitant conditions, such as renal insufficiency, proteinuria, and heart failure. Angiotensin receptor blockers (ARBs) interact with the angiotensin AT1 receptor and block most of its actions. They are approved by the US Food and Drug Administration (FDA) for the treatment of hypertension, and they help prevent left ventricular hypertrophy and mesangial sclerosis. Large studies have shown that ACE inhibitors and ARBs offer similar cardiovascular benefit.
The glomerulus has the only capillary bed with a blood supply that drains into an efferent arteriole instead of a venule, providing high resistance to aid filtration. Efferent arterioles are rich in AT1 receptors. In the presence of angiotensin II they constrict, increasing pressure in the glomerulus, which can lead to proteinuria and glomerulosclerosis. ACE inhibitors and ARBs relax the efferent arteriole, allowing increased blood flow through the glomerulus. This reduction in intraglomerular pressure is associated with less proteinuria and less glomerulosclerosis.
Diabetes promotes renal disease in many ways. Glucose and advanced glycation end products can lead to increased blood flow and increased pressure in the glomerulus. Through a variety of pathways, hyperglycemia, acting on angiotensin II, leads to NF-kapa beta production, profibrotic cytokines, increased matrix, and eventual fibrosis. ACE inhibitors and ARBs counteract many of these.
ACE inhibitors and ARBs slow nephropathy progression beyond blood pressure control
Several major clinical trials27–32 examined the effects of either ACE inhibitors or ARBs in slowing the progression of diabetic nephropathy and have had consistently positive results.
The Collaborative Study Group30 was a 3-year randomized trial in 419 patients with type 1 diabetes, using the ACE inhibitor captopril vs placebo. Captopril was associated with less decline in kidney function and a 50% reduction in the risk of the combined end points of death, dialysis, and transplantation that was independent of the small difference in blood pressures between the two groups.
The Irbesartan Diabetic Nephropathy Trial (IDNT)31 studied the effect of the ARB irbesartan vs the calcium channel blocker amlodipine vs placebo over 2.6 years in 1,715 patients with type 2 diabetes. Irbesartan was found to be significantly more effective in protecting against the progression of nephropathy, independent of reduction in blood pressure.
The RENAAL trial,32 published in 2001, was a 3-year, randomized, double-blind study comparing the ARB losartan at increasing dosages with placebo (both taken in addition to conventional antihypertensive treatment) in 1,513 patients with type 2 diabetes and nephropathy. The blood pressure goal was 140/90 mm Hg in both groups, but the losartan group had a lower rate of doubling of serum creatinine, end-stage renal disease, and combined end-stage renal disease or death.
‘Aldosterone escape’ motivates the search for new therapies
An important reason for developing more ways to block the renin-angiotensin-aldosterone system is because of “aldosterone escape,” the phenomenon of angiotensin II or aldosterone returning to pretreatment levels despite continued ACE inhibition.
Biollaz et al,33 in a 1982 study of 19 patients with hypertension, showed that despite reducing blood pressure and keeping the blood level of ACE very low with twice-daily enalapril 20 mg, blood and urine levels of angiotensin II steadily rose back to baseline levels within a few months.
A growing body of evidence suggests that despite effective inhibition of angiotensin II activity, non-ACE synthetic pathways still permit angiotensin II generation via serine proteases such as chymase, cathepsin G, and tissue plasminogen activator.
Thus, efforts have been made to block the renin-angiotensin system in other places. In addition to ACE inhibitors and ARBs, two aldosterone receptor antagonists are available, spironolactone and eplerenone, both used to treat heart failure. A direct renin inhibitor, aliskiren, is also available.
Combination therapy—less proteinuria, but…
A number of studies have shown that combination treatment with agents having different targets in the renin-angiotensin-aldosterone system leads to larger reductions in albuminuria than does single-agent therapy.
Mogensen et al34 studied the effect of the ACE inhibitor lisinopril (20 mg per day) plus the ARB candesartan (16 mg per day) in subjects with microalbuminuria, hypertension, and type 2 diabetes. Combined treatment was more effective in reducing proteinuria.
Epstein et al35 studied the effects of the ACE inhibitor enalapril (20 mg/day) combined with either of two doses of the selective aldosterone receptor antagonist eplerenone (50 or 100 mg/day) or placebo. Both eplerenone dosages, when added to the enalapril treatment, significantly reduced albuminuria from baseline as early as week 4 (P < .001), but placebo treatment added to the enalapril did not result in any significant decrease in urinary albumin excretion. Systolic blood pressure decreased significantly in all treatment groups and by about the same amount.
The Aliskiren Combined With Losartan in Type 2 Diabetes and Nephropathy (AVOID) trial36 randomized more than 600 patients with type 2 diabetes and nephropathy to aliskiren (a renin inhibitor) or placebo added to the ARB losartan. Again, combination treatment was more renoprotective, independent of blood pressure lowering.
Worse outcomes with combination therapy?
More recent studies have indicated that although combination therapy reduces proteinuria to a greater extent than monotherapy, overall it worsens major renal and cardiovascular outcomes. The multicenter Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET)37 randomized more than 25,000 patients age 55 and older with established atherosclerotic vascular disease or with diabetes and end-organ damage to receive either the ARB telmisartan 80 mg daily, the ACE inhibitor ramipril 10 mg daily, or both. Mean follow-up was 56 months. The combination-treatment group had higher rates of death and renal disease than the single-therapy groups (which did not differ from one another).
Why the combination therapy had poorer outcomes is under debate. Patients may get sudden drops in blood pressure that are not detected with only periodic monitoring. Renal failure was mostly acute rather than chronic, and the estimated GFR declined more in the combined therapy group than in the single-therapy groups.
The Aliskiren Trial in Type 2 Diabetes Using Cardiovascular and Renal Disease Endpoints (ALTITUDE) was designed to test the effect of the direct renin inhibitor aliskiren or placebo, both arms combined with either an ACE inhibitor or an ARB in patients with type 2 diabetes at high risk for cardiovascular and renal events. The trial was terminated early because of more strokes and deaths in the combination therapy arms. The results led the FDA to issue black box warnings against using aliskiren with these other classes of agents, and all studies testing similar combinations have been stopped. (In one study that was stopped and has not yet been published, 100 patients with proteinuria were treated with either aliskiren, the ARB losartan, or both, to evaluate the effects of aldosterone escape. Results showed no differences: about one-third of each group had this phenomenon.)
My personal recommendation is as follows: for younger patients with proteinuria, at lower risk for cardiovascular events and with disease due not to diabetes but to immunoglobulin A nephropathy or another proteinuric kidney disease, treat with both an ACE inhibitor and ARB. But the combination should not be used for patients at high risk of cardiovascular disease, which includes almost all patients with diabetes.
If more aggressive renin-angiotensin system blockade is needed against diabetic nephropathy, adding a diuretic increases the impact of blocking the renin-angiotensin-aldosterone system on both proteinuria and progression of renal disease. The aldosterone blocker spironolactone 25 mg can be added if potassium levels are carefully monitored.
ACE inhibitor plus calcium channel blocker is safer than ACE inhibitor plus diuretic
The Avoiding Cardiovascular Events Through Combination Therapy in Patients Living With Systolic Hypertension (ACCOMPLISH) trial38 randomized more than 11,000 high-risk patients with hypertension to receive an ACE inhibitor (benazepril) plus either a calcium channel blocker (amlodipine) or thiazide diuretic (hydrochlorothiazide). Blood pressures were identical between the two groups, but the trial was terminated early, at 36 months, because of a higher risk of the combined end point of cardiovascular death, myocardial infarction, stroke, and other major cardiac events in the ACE inhibitor-thiazide group.
Although some experts believe this study is definitive and indicates that high blood pressure should never be treated with an ACE inhibitor-thiazide combination, I believe that caution is needed in interpreting these findings. This regimen should be avoided in older patients with diabetes at high risk for cardiovascular disease, but otherwise, getting blood pressure under control is critical, and this combination can be used if it works and the patient is tolerating it well.
In summary, the choice of blood pressure-lowering medications is based on reducing cardiovascular events and slowing the progression of kidney disease. Either an ACE inhibitor or an ARB is the first choice for patients with diabetes, hypertension, and any degree of proteinuria. Many experts recommend beginning one of these agents even if proteinuria is not present. However, the combination of an ACE inhibitor and ARB should not be used in diabetic patients, especially if they have cardiovascular disease, until further data clarify the results of the ONTARGET and ALTITUDE trials.
STRATEGY 4: METABOLIC MANIPULATION WITH NOVEL AGENTS
Several new agents have recently been studied for the treatment of diabetic nephropathy, including aminoguanidine, which reduces levels of advanced glycation end-products, and sulodexide, which blocks basement membrane permeability. Neither agent has been shown to be safe and effective in diabetic nephropathy. The newest agent is bardoxolone methyl. It induces the Keap1–Nrf2 pathway, which up-regulates cytoprotective factors, suppressing inflammatory and other cytokines that are major mediators of progression of chronic kidney disease.39
Pergola et al,40 in a phase 2, double-blind trial, randomized 227 adults with diabetic kidney disease and a low estimated GFR (20–45 mL/min/1.73 m2) to receive placebo or bardoxolone 25, 75, or 150 mg daily. Drug treatment was associated with improvement in the estimated GFR, a finding that persisted throughout the 52 weeks of treatment. Surprisingly, proteinuria did not decrease with drug treatment.
As of this writing, a large multicenter controlled randomized trial has been halted because of concerns by the data safety monitoring board, which found increased rates of death and fluid retention with the drug. A number of recent trials have shown a beneficial effect of sodium bicarbonate therapy in patients with late-stage chronic kidney disease. They have shown slowing of the progression of GFR decline in a number of renal diseases, including diabetes.
- Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med 1999; 341:1127–1133.
- de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011; 305:2532–2539.
- United States Renal Data System (USRDS) 2000 Annual Data Report. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases – Division of Kidney, Urologic and Hematologic Diseases. USRDS Coordinating Center operated by the Minneapolis Medical Research Foundation. www.usrds.org
- Macisaac RJ, Jerums G. Diabetic kidney disease with and without albuminuria. Curr Opin Nephrol Hypertens 2011; 20:246–257.
- Molitch ME, DeFronzo RA, Franz MJ, et al; American Diabetes Association. Nephropathy in diabetes. Diabetes Care 2004; 27(suppl 1):S79–S83.
- Vamos EP, Harris M, Millett C, et al. Association of systolic and diastolic blood pressure and all cause mortality in people with newly diagnosed type 2 diabetes: retrospective cohort study. BMJ 2012; 345:e5567.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 2004; 65:2309–2320.
- Parving HH, Andersen AR, Smidt UM, Svendsen PA. Early aggressive antihypertensive treatment reduces rate of decline in kidney function in diabetic nephropathy. Lancet 1983; 1:1175–1179.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Bangalore S, Kumar S, Lobach I, Messerli FH. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and bayesian random-effects meta-analyses of randomized trials. Circulation 2011; 123:2799–2810.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care 2012; 35(suppl 1):S11–S63. (Erratum in: Diabetes Care 2012; 35:660.)
- Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis 2000; 36:646–661.
- Ramsay L, Williams B, Johnston G, et al. Guidelines for management of hypertension: report of the third working party of the British Hypertension Society. J Hum Hypertens 1999; 13:569–592.
- Feldman RD, Campbell N, Larochelle P, et al. 1999 Canadian recommendations for the management of hypertension. Task Force for the Development of the 1999 Canadian Recommendations for the Management of Hypertension. CMAJ 1999; 161(suppl):12:S1–S17.
- Chalmers J, MacMahon S, Mancia G, et al. 1999 World Health Organization-International Society of Hypertension Guidelines for the management of hypertension. Guidelines Sub-committee of the World Health Organization. Clin Exp Hypertens 1999; 21:1009–1060.
- The seventh report of the Joint National Committee on Prevention, Detection Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993; 329:977–986.
- Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care 2000; 23(suppl 2):B21–B29.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352:837–853. Erratum in: Lancet 1999; 354:602.
- Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998; 317:703–713. Erratum in: BMJ 1999; 318:29.
- Ismail-Beigi F, Craven T, Banerji MA, et al; ACCORD trial group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010; 376:419–430. Erratum in: Lancet 2010; 376:1466.
- Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000: 355:253–259. Erratum in: Lancet2000; 356:860.
- Parving HH, Lehnert H, Bröchner-Mortensen J, et al; Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001; 345:870–878.
- Viberti G, Wheeldon NM; MicroAlbuminuria Reduction With VALsartan (MARVAL) Study Investigators. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure-independent effect. Circulation 2002; 106:672–678.
- Lewis EJ, Hunsicker LG, Bain R P, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329:1456–1462.
- Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- Biollaz J, Brunner HR, Gavras I, Waeber B, Gavras H. Antihypertensive therapy with MK 421: angiotensin II--renin relationships to evaluate efficacy of converting enzyme blockade. J Cardiovasc Pharmacol 1982; 4:966–972.
- Mogensen CE, Neldam S, Tikkanen I, et al. Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ 2000; 321:1440–1444.
- Epstein M, Williams GH, Weinberger M, et al. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol 2006; 1:940–951.
- Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK; AVOID Study Investigators. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 2008; 358:2433–2446.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Jamerson K, Weber MA, Bakris GL, et al; ACCOMPLISH Trial Investigators. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008; 359:2417–2428.
- Kim HJ, Vaziri ND. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol 2010; 298:F662–F671.
- Pergola PE, Raskin P, Toto RD, et al; BEAM Study Investigators Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med 2011; 365:327–336.
- Ritz E, Orth SR. Nephropathy in patients with type 2 diabetes mellitus. N Engl J Med 1999; 341:1127–1133.
- de Boer IH, Rue TC, Hall YN, Heagerty PJ, Weiss NS, Himmelfarb J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011; 305:2532–2539.
- United States Renal Data System (USRDS) 2000 Annual Data Report. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases – Division of Kidney, Urologic and Hematologic Diseases. USRDS Coordinating Center operated by the Minneapolis Medical Research Foundation. www.usrds.org
- Macisaac RJ, Jerums G. Diabetic kidney disease with and without albuminuria. Curr Opin Nephrol Hypertens 2011; 20:246–257.
- Molitch ME, DeFronzo RA, Franz MJ, et al; American Diabetes Association. Nephropathy in diabetes. Diabetes Care 2004; 27(suppl 1):S79–S83.
- Vamos EP, Harris M, Millett C, et al. Association of systolic and diastolic blood pressure and all cause mortality in people with newly diagnosed type 2 diabetes: retrospective cohort study. BMJ 2012; 345:e5567.
- de Zeeuw D, Remuzzi G, Parving HH, et al. Proteinuria, a target for renoprotection in patients with type 2 diabetic nephropathy: lessons from RENAAL. Kidney Int 2004; 65:2309–2320.
- Parving HH, Andersen AR, Smidt UM, Svendsen PA. Early aggressive antihypertensive treatment reduces rate of decline in kidney function in diabetic nephropathy. Lancet 1983; 1:1175–1179.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008; 358:2560–2572.
- ADVANCE Collaborative Group; Patel A, MacMahon S, Chalmers J, et al. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the ADVANCE trial): a randomised controlled trial. Lancet 2007; 370:829–840.
- Bangalore S, Kumar S, Lobach I, Messerli FH. Blood pressure targets in subjects with type 2 diabetes mellitus/impaired fasting glucose: observations from traditional and bayesian random-effects meta-analyses of randomized trials. Circulation 2011; 123:2799–2810.
- UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998; 317:703–713.
- Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 2008; 359:1577–1589.
- Action to Control Cardiovascular Risk in Diabetes Study Group; Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008; 358:2545–2559.
- ACCORD Study Group; Cushman WC, Evans GW, Byington RP, et al. Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med 2010; 362:1575–1585.
- American Diabetes Association. Standards of medical care in diabetes—2012. Diabetes Care 2012; 35(suppl 1):S11–S63. (Erratum in: Diabetes Care 2012; 35:660.)
- Bakris GL, Williams M, Dworkin L, et al. Preserving renal function in adults with hypertension and diabetes: a consensus approach. National Kidney Foundation Hypertension and Diabetes Executive Committees Working Group. Am J Kidney Dis 2000; 36:646–661.
- Ramsay L, Williams B, Johnston G, et al. Guidelines for management of hypertension: report of the third working party of the British Hypertension Society. J Hum Hypertens 1999; 13:569–592.
- Feldman RD, Campbell N, Larochelle P, et al. 1999 Canadian recommendations for the management of hypertension. Task Force for the Development of the 1999 Canadian Recommendations for the Management of Hypertension. CMAJ 1999; 161(suppl):12:S1–S17.
- Chalmers J, MacMahon S, Mancia G, et al. 1999 World Health Organization-International Society of Hypertension Guidelines for the management of hypertension. Guidelines Sub-committee of the World Health Organization. Clin Exp Hypertens 1999; 21:1009–1060.
- The seventh report of the Joint National Committee on Prevention, Detection Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 1993; 329:977–986.
- Shichiri M, Kishikawa H, Ohkubo Y, Wake N. Long-term results of the Kumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care 2000; 23(suppl 2):B21–B29.
- Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 1998; 352:837–853. Erratum in: Lancet 1999; 354:602.
- Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. UK Prospective Diabetes Study Group. BMJ 1998; 317:703–713. Erratum in: BMJ 1999; 318:29.
- Ismail-Beigi F, Craven T, Banerji MA, et al; ACCORD trial group. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010; 376:419–430. Erratum in: Lancet 2010; 376:1466.
- Effects of ramipril on cardiovascular and microvascular outcomes in people with diabetes mellitus: results of the HOPE study and MICRO-HOPE substudy. Heart Outcomes Prevention Evaluation Study Investigators. Lancet 2000: 355:253–259. Erratum in: Lancet2000; 356:860.
- Parving HH, Lehnert H, Bröchner-Mortensen J, et al; Irbesartan in Patients with Type 2 Diabetes and Microalbuminuria Study Group. The effect of irbesartan on the development of diabetic nephropathy in patients with type 2 diabetes. N Engl J Med 2001; 345:870–878.
- Viberti G, Wheeldon NM; MicroAlbuminuria Reduction With VALsartan (MARVAL) Study Investigators. Microalbuminuria reduction with valsartan in patients with type 2 diabetes mellitus: a blood pressure-independent effect. Circulation 2002; 106:672–678.
- Lewis EJ, Hunsicker LG, Bain R P, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med 1993; 329:1456–1462.
- Lewis EJ, Hunsicker LG, Clarke WR, et al; Collaborative Study Group. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001; 345:851–860.
- Brenner BM, Cooper ME, de Zeeuw D, et al; RENAAL Study Investigators. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 2001; 345:861–869.
- Biollaz J, Brunner HR, Gavras I, Waeber B, Gavras H. Antihypertensive therapy with MK 421: angiotensin II--renin relationships to evaluate efficacy of converting enzyme blockade. J Cardiovasc Pharmacol 1982; 4:966–972.
- Mogensen CE, Neldam S, Tikkanen I, et al. Randomised controlled trial of dual blockade of renin-angiotensin system in patients with hypertension, microalbuminuria, and non-insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ 2000; 321:1440–1444.
- Epstein M, Williams GH, Weinberger M, et al. Selective aldosterone blockade with eplerenone reduces albuminuria in patients with type 2 diabetes. Clin J Am Soc Nephrol 2006; 1:940–951.
- Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK; AVOID Study Investigators. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 2008; 358:2433–2446.
- Mann JF, Schmieder RE, McQueen M, et al; ONTARGET investigators. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet 2008; 372:547–553.
- Jamerson K, Weber MA, Bakris GL, et al; ACCOMPLISH Trial Investigators. Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high-risk patients. N Engl J Med 2008; 359:2417–2428.
- Kim HJ, Vaziri ND. Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure. Am J Physiol Renal Physiol 2010; 298:F662–F671.
- Pergola PE, Raskin P, Toto RD, et al; BEAM Study Investigators Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med 2011; 365:327–336.
KEY POINTS
- The progression from no proteinuria to microalbuminuria to clinical proteinuria parallels glomerular changes of thickening of the basement membrane, mesangial expansion, and the development of Kimmelstiel-Wilson nodules and sclerosis.
- Blood pressure control to 130/80 mm Hg slows microvascular and macrovascular disease, but the goal should not be lower in older patients with diabetes.
- Glycemic control slows microvascular disease: the goal for most patients for hemoglobin A1c is 7.0%. Tighter control may increase cardiovascular risk.
- Either an angiotensin-converting enzyme inhibitor or an angiotensin receptor blocker is the first-line treatment for diabetic nephropathy; combining the two is no longer recommended.
- If more aggressive treatment is needed, a diuretic or spironolactone (with potassium monitoring) can be added.
- The role of sodium bicarbonate and new agents such as blockers of transcription factors is still emerging.
Carbapenem-resistant Enterobacteriaceae: A menace to our most vulnerable patients
The past 10 years have brought a formidable challenge to the clinical arena, as carbapenems, until now the most reliable antibiotics against Klebsiella species, Escherichia coli, and other Enterobacteriaceae, are becoming increasingly ineffective.
Infections caused by carbapenem-resistant Enterobacteriaceae (CRE) pose a serious threat to hospitalized patients. Moreover, CRE often demonstrate resistance to many other classes of antibiotics, thus limiting our therapeutic options. Furthermore, few new antibiotics are in line to replace carbapenems. This public health crisis demands redefined and refocused efforts in the diagnosis, treatment, and control of infections in hospitalized patients.
Here, we present an overview of CRE and discuss avenues to escape a new era of untreatable infections.
INCREASED USE OF CARBAPENEMS AND EMERGENCE OF RESISTANCE
Developed in the 1980s, carbapenems are derivatives of thyanamycin. Imipenem and meropenem, the first members of the class, had a broad spectrum of antimicrobial activity that included coverage of Pseudomonas aeruginosa, adequately positioning them for the treatment of nosocomial infections. Back then, nearly all Enterobacteriaceae were susceptible to carbapenems.1
In the 1990s, Enterobacteriaceae started to develop resistance to cephalosporins—till then, the first-line antibiotics for these organisms—by acquiring extended-spectrum betalactamases, which inactivate those agents. Consequently, the use of cephalosporins had to be restricted, while carbapenems, which remained impervious to these enzymes, had to be used more.2 In pivotal international studies in the treatment of infections caused by strains of K pneumoniae that produced these inactivating enzymes, outcomes were better with carbapenems than with cephalosporins and fluoroquinolones.3,4
Ertapenem, a carbapenem without antipseudomonal activity and highly bound to protein, was released in 2001. Its prolonged half-life permitted once-daily dosing, which positioned it as an option for treating infections in community dwellers.5 Doripenem is the newest member of the class of carbapenems, and its spectrum of activity is similar to that of imipenem and meropenem and includes P aeruginosa.6 The use of carbapenems, measured in a representative sample of 35 university hospitals in the United States, increased by 59% between 2002 and 2006.7
In the early 2000s, carbapenem resistance in K pneumoniae and other Enterobacteriaceae was rare in North America. But then, after initial outbreaks occurred in hospitals in the Northeast (especially New York City), CRE began to spread throughout the United States. By 2009–2010, the National Healthcare Safety Network from the Centers for Disease Control and Prevention (CDC) revealed that 12.8% of K pneumoniae isolates associated with bloodstream infections were resistant to carbapenems.8
In March 2013, the CDC disclosed that 3.9% of short-stay acute-care hospitals and 17.8% of long-term acute-care hospitals reported at least one CRE health care-associated infection in 2012. CRE had extended to 42 states, and the proportion of Enterobacteriaceae that are CRE had increased fourfold over the past 10 years.9
Coinciding with the increased use of carbapenems, multiple factors and modifiers likely contributed to the dramatic increase in CRE. These include use of other antibiotics in humans and animals, their relative penetration and selective effect on the gut microbiota, case-mix and infection control practices in different health care settings, and travel patterns.
POWERFUL ENZYMES THAT TRAVEL FAR
Bacterial acquisition of carbapenemases, enzymes that inactivate carbapenems, is crucial to the emergence of CRE. The enzyme in the sentinel carbapenem-resistant K pneumoniae isolate found in 1996 in North Carolina was designated K pneumoniae carbapenemase (KPC-1). This mechanism also conferred resistance to all cephalosporins, aztreonam, and beta-lactamase inhibitors such as clavulanic acid and tazobactam.10
KPC-2 (later determined to be identical to KPC-1) was found in K pneumoniae from Baltimore, and KPC-3 caused an early outbreak in New York City.11,12 To date, 12 additional variants of blaKPC, the gene encoding for the KPC enzyme, have been described.13
The genes encoding carbapenemases are usually found on plasmids or other common mobile genetic elements.14 These genetic elements allow the organism to acquire genes conferring resistance to other classes of antimicrobials, such as aminoglycoside-modifying enzymes and fluoroquinolone-resistance determinants, and beta-lactamases.15,16 The result is that CRE isolates are increasingly multidrug-resistant (ie, resistant to three or more classes of antimicrobials), extensively drug-resistant (ie, resistant to all but one or two classes), or pandrug-resistant (ie, resistant to all available classes of antibiotics).17 Thus, up to 98% of KPC-producing K pneumoniae are resistant to trimethoprim-sulfamethoxazole, 90% are resistant to fluoroquinolones, and 60% are resistant to gentamicin or amikacin.15
The mobility of these genetic elements has also allowed for dispersion into diverse Enterobacteriaceae such as E coli, Klebsiella oxytoca, Enterobacter, Serratia, and Salmonella species. Furthermore, KPC has been described in non-Enterobacteriaceae such as Acinetobacter baumannii and P aeruginosa.
Extending globally, KPC is now endemic in the Mediterranean basin, including Israel, Greece, and Italy; in South America, especially Colombia, Argentina, and Brazil; and in China.18 Most interesting is the intercontinental transfer of these strains: it has been documented that the index patient with KPC-producing K pneumoniae in Medellin, Colombia, came from Israel to undergo liver transplantation.19 Likewise, KPC-producing K pneumoniae in France and Israel could be linked epidemiologically and genetically to the predominant US strain.20,21
Even more explosive has been the surge of another carbapenemase, the Ambler Class B New Delhi metallo-beta-lactamase, or NDM-1. Initially reported in a urinary isolate of K pneumoniae from a Swedish patient who had been hospitalized in New Delhi in 2008, NDM-1 was soon found throughout India, in Pakistan, and in the United Kingdom.22 Interestingly, several of the UK patients with NDM-1-harboring bacteria had received organ transplants in the Indian subcontinent. Reports from elsewhere in Europe, Australia, and Africa followed suit, usually with a connection to the Indian subcontinent epicenter. In contrast, several other cases in Europe were traced to the Balkans, where there appears to be another focus of NDM-1.23
Penetration of NDM-1 into North America has begun, with cases and outbreaks reported in several US and Canadian regions, and in a military medical facility in Afghanistan. In several of these instances, there has been a documented link with travel and hospitalizations overseas.24–27 However, no such link with travel could be established in a recent outbreak in Ontario.27
In addition, resistance to carbapenems may result from other enzymes (Table 1), or from combinations of changes in outer membrane porins and the production of extended spectrum beta-lactamases or other cephalosporinases.28
DEADLY IMPACT ON THE MOST VULNERABLE
Regardless of the resistance pattern, Enterobacteriaceae are an important cause of health care-associated infections, including urinary and bloodstream infections in patients with indwelling catheters, pneumonia (often in association with mechanical ventilation), and, less frequently, infections of skin and soft tissues and the central nervous system.29–31
Several studies have examined the clinical characteristics and outcomes of patients with CRE infections. Those typically affected are elderly and debilitated and have multiple comorbidities, including diabetes mellitus and immunosuppression. They are heavily exposed to health care with frequent antecedent hospitalizations and invasive procedures. Furthermore, they are often severely ill and require intensive care. Patients infected with carbapenem-resistant K pneumoniae, compared with those with carbapenem-susceptible strains, are more likely to have undergone organ or stem cell transplantation or mechanical ventilation, and to have had a longer hospital stay before infection.
They also experience a high mortality rate, which ranges from 30% in patients with nonbacteremic infections to 72% in series of patients with liver transplants or bloodstream infections.32–37
More recently, CRE has been reported in other vulnerable populations, such as children with critical illness or cancer and in burn patients.38–40
Elderly and critically ill patients with bacteremia originating from a high-risk source (eg, pneumonia) typically face the most adverse outcomes. With increasing drug resistance, inadequate initial antimicrobial therapy is more commonly seen and may account for some of these poor outcomes.37,41
LONG-TERM CARE FACILITIES IN THE EYE OF THE STORM
A growing body of evidence suggests that long-term care facilities play a crucial role in the spread of CRE.
In an investigation into carbapenem-resistant A baumanii and K pneumoniae in a hospital system,36 75% of patients with carbapenem-resistant K pneumoniae were admitted from long-term care facilities, and only 1 of 13 patients was discharged home.
In a series of patients with carbapenem-resistant K pneumoniae bloodstream infections, 42% survived their index hospital stay. Of these patients, only 32% were discharged home, and readmissions were very common.32
Admission from a long-term care facility or transfer from another hospital is significantly associated with carbapenem resistance in patients with Enterobacteriaceae.42 Similarly, in Israel, a large reservoir of CRE was found in postacute care facilities.43
It is clear that long-term care residents are at increased risk of colonization and infection with CRE. However, further studies are needed to evaluate whether this simply refects an overlap in risk factors, or whether significant patient-to-patient transmission occurs in these settings.
INFECTION CONTROL TAKES CENTER STAGE
It is important to note that risk factors for CRE match those of various nosocomial infections, including other resistant gram-negative bacilli, methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, Candida species, and Clostridium difficile; in fact, CRE often coexist with other multidrug-resistant organisms.44,45
Common risk factors include residence in a long-term care facility, an intensive care unit stay, use of lines and catheters, and antibiotic exposure. This commonality of risk factors implies that systematic infection-prevention measures will have an impact on the prevalence and incidence rates of multidrug-resistant organism infections across the board, CRE included. It should be emphasized that strict compliance with hand hygiene is still the foundation of any infection-prevention strategy.
Infection prevention and the control of transmission of CRE in long-term care facilities pose unique challenges. Guidelines from the Society for Healthcare Epidemiology and the Association for Professionals in Infection Control recommend the use of contact precautions for patients with multidrug-resistant organisms, including CRE, who are ill and totally dependent on health care workers for activities of daily living or whose secretions or drainage cannot be contained. These same guidelines advise against attempting to eradicate multidrug-resistant organism colonization status.46
In acute care facilities, Best Infection Control Practices from the CDC and the Healthcare Infection Control Practices Advisory Committee encourage mechanisms for the rapid recognition and reporting of CRE cases to infection prevention personnel so that contact precautions can be implemented. Furthermore, facilities without CRE cases should carry out periodic laboratory reviews to identify cases, and patients exposed to CRE cases should be screened with surveillance cultures.47
Outbreaks of CRE may require extraordinary infection control measures. An approach combining point-prevalence surveillance of colonization, detection of environmental and common-equipment contamination, with the implementation of a bundle consisting of chlorhexidine baths, cohorting of colonized patients and health care personnel, increased environmental cleaning, and staff education may be effective in controlling outbreaks of CRE.48
Nevertheless, control of CRE may prove exceptionally difficult. A recent high-profile outbreak of carbapenem-resistant K pneumoniae at the National Institutes of Health Clinical Center in Maryland caused infections in 18 patients, 11 of whom died.49 Of note, carbapenem-resistant K pneumoniae was detected in this outbreak in both respiratory equipment and sink drains. The outbreak was ultimately contained by detection through surveillance cultures and by strict cohorting of colonized patients, which minimized common medical equipment and personnel between affected patients and other patients in the hospital. Additionally, rooms were sanitized with hydrogen peroxide vapor, and sinks and drains where carbapenem-resistant K pneumoniae was detected were removed.
CHALLENGES IN THE MICROBIOLOGY LABORATORY
Adequate treatment and control of CRE infections is predicated upon their accurate and prompt diagnosis from patient samples in the clinical microbiology laboratory.50
Traditional and current culture-based methods take several days to provide that information, delaying effective antibiotic therapy and permitting the transmission of undetected CRE. Furthermore, interpretative criteria of minimal inhibitory concentrations (MICs) of carbapenems recently required readjustment, as many KPC-producing strains of K pneumoniae had MICs below the previous breakpoint of resistance. In the past, this contributed to instances of “silent” dissemination of KPC-producing K pneumoniae.51
In contrast, using the new lower breakpoints of resistance for carbapenems without using a phenotypic test such as the modified Hodge test or the carbapenem-EDTA combination tests will result in a lack of differentiation between various mechanisms of carbapenem resistance.28,52,53 This may be clinically relevant, as the clinical response to carbapenem therapy may vary depending on the mechanism of resistance.
GENERAL PRINCIPLES APPLY
In treating patients infected with CRE, clinicians need to strictly observe general principles of infectious disease management to ensure the best possible outcomes. These include:
Timely and accurate diagnosis, as discussed above.
Source control, which should include drainage of any infected collections, and removal of lines, devices, and urinary catheters.
Distinguishing between infection and colonization. CRE are often encountered as urinary isolates, and the distinction between asymptomatic bacteriuria and urinary tract infection may be extremely difficult, especially in residents of long-term care facilities with chronic indwelling catheters, who are thegroup at highest risk of CRE colonization and infection. Urinalysis may be helpful in the absence of pyuria, as this rules out an infection; however, it must be emphasized that the presence of pyuria is not a helpful feature, as pyuria is common in both asymptomatic bacteriuria and urinary tract infection.54 Symptoms should be carefully evaluated in every patient with bacteriuria, and urinary tract infection should be a diagnosis of exclusion in patients with functional symptoms such as confusion or falls.
Selection of the most appropriate antibiotic regimen. While the emphasis is often on the antibiotic regimen, the above elements should not be neglected.
A DWINDLING THERAPEUTIC ARSENAL
Clinicians treating CRE infections are left with only a few antibiotic options. These options are generally limited by a lack of clinical data on efficacy, as well as by concerns about toxicity. These “drugs of last resort” include polymyxins (such as colistin), aminoglycosides, tigecycline, and fosfomycin. The role of carbapenem therapy, potentially in combination regimens, in a high-dose prolonged infusion, or even “double carbapenem therapy” remains to be determined.37,55,56
Colistin
Colistin is one of the first-line agents for treating CRE infections. First introduced in the 1950s, its use was mostly abandoned in favor of aminoglycosides. A proportion of the data on safety and efficacy of colistin, therefore, is based on older, less rigorous studies.
Neurotoxicity and nephrotoxicity are the two main concerns with colistin, and while the incidence of these adverse events does appear to be lower with modern preparations, it is still substantial.57 Dosing issues have not been completely clarified either, especially in relation to renal clearance and in patients on renal replacement therapy.58,59 Unfortunately, there have been reports of outbreaks of CRE displaying resistance to colistin.60
Tigecycline
Tigecycline is a newer antibiotic of the glycylcycline class. Like colistin, it has no oral preparation for systemic infections.
The main side effect of tigecycline is nausea.61 Other reported issues include pancreatitis and extreme alkaline phosphatase elevations.
The efficacy of tigecycline has come into question in view of meta-analyses of clinical trials, some of which have shown higher mortality rates in patients treated with tigecycline than with comparator agents.62–65 Based on these data, the US Food and Drug Administration issued a warning in 2010 regarding the increased mortality risk. Although these meta-analyses did not include patients with CRE for whom available comparators would have been ineffective, it is an important safety signal.
The efficacy of tigecycline is further limited by increasing in vitro resistance in CRE. Serum and urinary levels of tigecycline are low, and most experts discourage the use of tigecycline as monotherapy for blood stream or urinary tract infections.
Aminoglycosides
CRE display variable in vitro susceptibility to different aminoglycosides. If the organism is susceptible, aminoglycosides may be very useful in the treatment of CRE infections, especially urinary tract infectons. In a study of carbapenem-resistant K pneumoniae urinary tract infections, patients who were treated with polymyxins or tigecycline were significantly less likely to have clearance of their urine as compared with patients treated with aminoglycosides.66
Ototoxicity and nephrotoxicity are demonstrated adverse effects of aminoglycosides. Close monitoring of serum levels, interval audiology examinations at baseline and during therapy, and the use of extended-interval dosing may help to decrease the incidence of these toxicities.
Fosfomycin
Fosfomycin is only available as an oral formulation in the United States, although intravenous administration has been used in other countries. It is exclusively used to treat urinary tract infections.
CRE often retain susceptibility to fosfomycin, and clearance of urine in cystitis may be attempted with this agent to avoid the need for intravenous treatment.29,67
Combination therapy, other topics to be explored
Recent observational reports from Greece, Italy, and the United States describe higher survival rates in patients with CRE infections treated with a combination regimen rather than monotherapy with colistin or tigecycline. This is despite reliable activity of colistin and tigecycline, and often in regimens containing carbapenems. Clinical experiments are needed to clarify the value of combination regimens that include carbapenems for the treatment of CRE infections.
Similarly, the role of carbapenems given as a high-dose prolonged infusion or as double carbapenem therapy needs to be explored further.37,55,56,68
Also to be determined is the optimal duration of treatment. To date, there is no evidence that increasing the duration of treatment beyond that recommended for infections with more susceptible bacteria results in improved outcomes. Therefore, commonly used durations include 1 week for complicated urinary tract infections, 2 weeks for bacteremia (from the first day with negative blood cultures and source control), and 8 to 14 days for pneumonia.
A SERIOUS THREAT
The emergence of CRE is a serious threat to the safety of patients in our health care system. CRE are highly successful nosocomial pathogens selected by the use of antibiotics, which burden patients debilitated by advanced age, comorbidities, and medical interventions. Infections with CRE result in poor outcomes, and available treatments of last resort such as tigecycline and colistin are of unclear efficacy and safety.
Control of CRE transmission is hindered by the transit of patients through long-term care facilities, and detection of CRE is difficult because of the myriad mechanisms involved and the imperfect methods currently available. Clinicians are concerned and frustrated, especially given the paucity of antibiotics in development to address the therapeutic dilemma posed by CRE. The challenge of CRE and other multidrug-resistant organisms requires the concerted response of professionals in various disciplines, including pharmacists, microbiologists, infection control practitioners, and infectious disease clinicians (Table 2).
Control of transmission by infection prevention strategies and by antimicrobial stewardship is going to be crucial in the years to come, not only for limiting the spread of CRE, but also for preventing the next multidrug-resistant “superbug” from emerging. However, the current reality is that health care providers will be faced with increased numbers of patients infected with CRE.
Prospective studies into transmission, molecular characteristics, and, most of all, treatment regimens are urgently needed. In addition, the development of new antimicrobials and nontraditional antimicrobial methods should have international priority.
- Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother 2011; 55:4943–4960.
- Rahal JJ, Urban C, Horn D, et al. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA 1998; 280:1233–1237.
- Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med 2004; 140:26–32.
- Endimiani A, Luzzaro F, Perilli M, et al. Bacteremia due to Klebsiella pneumoniae isolates producing the TEM-52 extended-spectrum beta-lactamase: treatment outcome of patients receiving imipenem or ciprofoxacin. Clin Infect Dis 2004; 38:243–251.
- Livermore DM, Sefton AM, Scott GM. Properties and potential of ertapenem. J Antimicrob Chemother 2003; 52:331–344.
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antibiotics: doripenem, ceftobiprole, ceftaroline, and cefepime. Infect Dis Clin North Am 2009; 23:983–996, ix.
- Pakyz AL, MacDougall C, Oinonen M, Polk RE. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med 2008; 168:2254–2260.
- Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013; 34:1–14.
- Centers for Disease Control and Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR 2013; 62:165–170.
- Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 2001; 45:1151–1161.
- Smith Moland E, Hanson ND, Herrera VL, et al. Plasmid-mediated, carbapenem-hydrolysing beta-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J Antimicrob Chemother 2003; 51:711–714.
- Woodford N, Tierno PM, Young K, et al. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York medical center. Antimicrob Agents Chemother 2004; 48:4793–4799.
- Lehey Clinic. OXA-type β-Lactamases. http://www.lahey.org/Studies/other.asp#table1. Accessed March 11, 2013.
- Mathers AJ, Cox HL, Kitchel B, et al. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. MBio 2011; 2 6:e00204–11.
- Endimiani A, Hujer AM, Perez F, et al. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J Antimicrob Chemother 2009; 63:427–437.
- Endimiani A, Carias LL, Hujer AM, et al. Presence of plasmid-mediated quinolone resistance in Klebsiella pneumoniae isolates possessing blaKPC in the United States. Antimicro Agents Chemother 2008; 52:2680–2682.
- Magiorakos A P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–281.
- Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 2012; 25:682–707.
- Lopez JA, Correa A, Navon-Venezia S, et al. Intercontinental spread from Israel to Colombia of a KPC-3-producing Klebsiella pneumoniae strain. Clin Microbiol Infect 2011; 17:52–56.
- Naas T, Nordmann P, Vedel G, Poyart C. Plasmid-mediated carbapenem-hydrolyzing beta-lactamase KPC in a Klebsiella pneumoniae isolate from France. Antimicrob Agents Chemother 2005; 49:4423–4424.
- Navon-Venezia S, Leavitt A, Schwaber MJ, et al. First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob Agents Chemother 2009; 53:818–820.
- Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 2009; 53:5046–5054.
- Livermore DM, Walsh TR, Toleman M, Woodford N. Balkan NDM-1: escape or transplant? Lancet Infect Dis 2011; 11:164.
- Centers for Disease Control and Prevention. Carbapenem-resistant enterobacteriaceae containing New Delhi metallo-beta-lactamase in two patients - Rhode Island, March 2012. MMWR Morb Mortal Wkly Rep 2012Jun 22; 61:446–448.
- Centers for Disease Control and Prevention. Detection of Enterobacteriaceae isolates carrying metallo-beta-lactamase—United States, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:750.
- McGann P, Hang J, Clifford RJ, et al. Complete sequence of a novel 178-kilobase plasmid carrying bla(NDM-1) in a Providencia stuartii strain isolated in Afghanistan. Antimicrob Agents Chemother 2012; 56:1673–1679.
- Borgia S, Lastovetska O, Richardson D, et al. Outbreak of carbapenem-resistant Enterobacteriaceae containing blaNDM-1, Ontario, Canada. Clin Infect Dis 2012; 55:e109–e117.
- Endimiani A, Perez F, Bajaksouzian S, et al. Evaluation of updated interpretative criteria for categorizing Klebsiella pneumoniae with reduced carbapenem susceptibility. J Clinic Microbiol 2010; 48:4417–4425.
- Neuner EA, Sekeres J, Hall GS, van Duin D. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother 2012; 56:5744–5748.
- Neuner EA, Yeh JY, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagnostic Microbiol Infect Dis 2011; 69:357–362.
- van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagnostic Microbiol Infect Dis 2013; 75:115–120.
- Neuner EA, Yeh J-Y, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis 2011; 69:357–362.
- Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 2008; 29:1099–1106.
- Borer A, Saidel-Odes L, Riesenberg K, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol 2009; 30:972–976.
- Marchaim D, Chopra T, Perez F, et al. Outcomes and genetic relatedness of carbapenem-resistant Enterobacteriaceae at Detroit medical center. Infect Control Hosp Epidemiol 2011; 32:861–871.
- Perez F, Endimiani A, Ray AJ, et al. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother 2010; 65:1807–1818.
- Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55:943–950.
- Little ML, Qin X, Zerr DM, Weissman SJ. Molecular diversity in mechanisms of carbapenem resistance in paediatric Enterobacteriaceae. Int J Antimicrob Agents 2012; 39:52–57.
- Logan LK. Carbapenem-resistant Enterobacteriaceae: an emerging problem in children. Clin Infect Dis 2012; 55:852–859.
- Rastegar Lari A, Azimi L, Rahbar M, Fallah F, Alaghehbandan R. Phenotypic detection of Klebsiella pneumoniae carbapenemase among burns patients: first report from Iran. Burns 2013; 39:174–176.
- Zarkotou O, Pournaras S, Tselioti P, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 2011; 17:1798–1803.
- Hyle EP, Ferraro MJ, Silver M, Lee H, Hooper DC. Ertapenem-resistant Enterobacteriaceae: risk factors for acquisition and outcomes. Infect Control Hosp Epidemiol 2010; 31:1242–1249.
- Ben-David D, Masarwa S, Navon-Venezia S, et al. Carbapenem-resistant Klebsiella pneumoniae in post-acute-care facilities in Israel. Infect Control Hosp Epidemiol 2011; 32:845–853.
- Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med 2002; 136:834–844.
- Marchaim D, Perez F, Lee J, et al. “Swimming in resistance”: co-colonization with carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii or Pseudomonas aeruginosa.” Am J Infect Control 2012; 40:830–835.
- Smith PW, Bennett G, Bradley S, et al. SHEA/APIC Guideline: Infection prevention and control in the long-term care facility. Am J Infect Control 2008; 36:504–535.
- Centers for Disease Control and Prevention. Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR 2009; 58:256–260.
- Munoz-Price LS, De La Cuesta C, Adams S, et al. Successful eradication of a monoclonal strain of Klebsiella pneumoniae during a K. pneumoniae carbapenemase-producing K. pneumoniae outbreak in a surgical intensive care unit in Miami, Florida. Infect Control Hosp Epidemiol 2010; 31:1074–1077.
- Snitkin ES, Zelazny AM, Thomas PJ, et al. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with wholegenome sequencing. Sci Transl Med 2012; 4:148ra16.
- Srinivasan A, Patel JB. Klebsiella pneumoniae carbapenemase-producing organisms: an ounce of prevention really is worth a pound of cure. Infect Control Hosp Epidemiol 2008; 29:1107–1109.
- Viau RA, Hujer AM, Marshall SH, et al. “Silent” dissemination of Klebsiella pneumoniae isolates bearing K pneumoniae carbapenemase in a long-term care facility for children and young adults in Northeast Ohio”. Clin Infect Dis 2012; 54:1314–1321.
- Galani I, Rekatsina PD, Hatzaki D, Plachouras D, Souli M, Giamarellou H. Evaluation of different laboratory tests for the detection of metallo-beta-lactamase production in Enterobacteriaceae. J Antimicrob Chemother 2008; 61:548–553.
- Anderson KF, Lonsway DR, Rasheed JK, et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol 2007; 45:2723–2725.
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40:643–654.
- Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 2011; 17:1135–1141.
- Bulik CC, Nicolau DP. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2011; 55:3002–3004.
- Pogue JM, Lee J, Marchaim D, et al. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis 2011; 53:879–884.
- Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011; 55:3284–3294.
- Dalfno L, Puntillo F, Mosca A, et al. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis 2012; 54:1720–1726.
- Marchaim D, Chopra T, Pogue JM, et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother 2011; 55:593–599.
- Bonilla MF, Avery RK, Rehm SJ, Neuner EA, Isada CM, van Duin D. Extreme alkaline phosphatase elevation associated with tigecycline. J Antimicrob Chemother 2011; 66:952–953.
- Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 2012; 54:1699–1709.
- Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis 2011; 11:834–844.
- Cai Y, Wang R, Liang B, Bai N, Liu Y. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother 2011; 55:1162–1172.
- Yahav D, Lador A, Paul M, Leibovici L. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother 2011; 66:1963–1971.
- Satlin MJ, Kubin CJ, Blumenthal JS, et al. Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother 2011; 55:5893–5899.
- Endimiani A, Patel G, Hujer KM, et al. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother 2010; 54:526–529.
- Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012; 56:2108–2113.
The past 10 years have brought a formidable challenge to the clinical arena, as carbapenems, until now the most reliable antibiotics against Klebsiella species, Escherichia coli, and other Enterobacteriaceae, are becoming increasingly ineffective.
Infections caused by carbapenem-resistant Enterobacteriaceae (CRE) pose a serious threat to hospitalized patients. Moreover, CRE often demonstrate resistance to many other classes of antibiotics, thus limiting our therapeutic options. Furthermore, few new antibiotics are in line to replace carbapenems. This public health crisis demands redefined and refocused efforts in the diagnosis, treatment, and control of infections in hospitalized patients.
Here, we present an overview of CRE and discuss avenues to escape a new era of untreatable infections.
INCREASED USE OF CARBAPENEMS AND EMERGENCE OF RESISTANCE
Developed in the 1980s, carbapenems are derivatives of thyanamycin. Imipenem and meropenem, the first members of the class, had a broad spectrum of antimicrobial activity that included coverage of Pseudomonas aeruginosa, adequately positioning them for the treatment of nosocomial infections. Back then, nearly all Enterobacteriaceae were susceptible to carbapenems.1
In the 1990s, Enterobacteriaceae started to develop resistance to cephalosporins—till then, the first-line antibiotics for these organisms—by acquiring extended-spectrum betalactamases, which inactivate those agents. Consequently, the use of cephalosporins had to be restricted, while carbapenems, which remained impervious to these enzymes, had to be used more.2 In pivotal international studies in the treatment of infections caused by strains of K pneumoniae that produced these inactivating enzymes, outcomes were better with carbapenems than with cephalosporins and fluoroquinolones.3,4
Ertapenem, a carbapenem without antipseudomonal activity and highly bound to protein, was released in 2001. Its prolonged half-life permitted once-daily dosing, which positioned it as an option for treating infections in community dwellers.5 Doripenem is the newest member of the class of carbapenems, and its spectrum of activity is similar to that of imipenem and meropenem and includes P aeruginosa.6 The use of carbapenems, measured in a representative sample of 35 university hospitals in the United States, increased by 59% between 2002 and 2006.7
In the early 2000s, carbapenem resistance in K pneumoniae and other Enterobacteriaceae was rare in North America. But then, after initial outbreaks occurred in hospitals in the Northeast (especially New York City), CRE began to spread throughout the United States. By 2009–2010, the National Healthcare Safety Network from the Centers for Disease Control and Prevention (CDC) revealed that 12.8% of K pneumoniae isolates associated with bloodstream infections were resistant to carbapenems.8
In March 2013, the CDC disclosed that 3.9% of short-stay acute-care hospitals and 17.8% of long-term acute-care hospitals reported at least one CRE health care-associated infection in 2012. CRE had extended to 42 states, and the proportion of Enterobacteriaceae that are CRE had increased fourfold over the past 10 years.9
Coinciding with the increased use of carbapenems, multiple factors and modifiers likely contributed to the dramatic increase in CRE. These include use of other antibiotics in humans and animals, their relative penetration and selective effect on the gut microbiota, case-mix and infection control practices in different health care settings, and travel patterns.
POWERFUL ENZYMES THAT TRAVEL FAR
Bacterial acquisition of carbapenemases, enzymes that inactivate carbapenems, is crucial to the emergence of CRE. The enzyme in the sentinel carbapenem-resistant K pneumoniae isolate found in 1996 in North Carolina was designated K pneumoniae carbapenemase (KPC-1). This mechanism also conferred resistance to all cephalosporins, aztreonam, and beta-lactamase inhibitors such as clavulanic acid and tazobactam.10
KPC-2 (later determined to be identical to KPC-1) was found in K pneumoniae from Baltimore, and KPC-3 caused an early outbreak in New York City.11,12 To date, 12 additional variants of blaKPC, the gene encoding for the KPC enzyme, have been described.13
The genes encoding carbapenemases are usually found on plasmids or other common mobile genetic elements.14 These genetic elements allow the organism to acquire genes conferring resistance to other classes of antimicrobials, such as aminoglycoside-modifying enzymes and fluoroquinolone-resistance determinants, and beta-lactamases.15,16 The result is that CRE isolates are increasingly multidrug-resistant (ie, resistant to three or more classes of antimicrobials), extensively drug-resistant (ie, resistant to all but one or two classes), or pandrug-resistant (ie, resistant to all available classes of antibiotics).17 Thus, up to 98% of KPC-producing K pneumoniae are resistant to trimethoprim-sulfamethoxazole, 90% are resistant to fluoroquinolones, and 60% are resistant to gentamicin or amikacin.15
The mobility of these genetic elements has also allowed for dispersion into diverse Enterobacteriaceae such as E coli, Klebsiella oxytoca, Enterobacter, Serratia, and Salmonella species. Furthermore, KPC has been described in non-Enterobacteriaceae such as Acinetobacter baumannii and P aeruginosa.
Extending globally, KPC is now endemic in the Mediterranean basin, including Israel, Greece, and Italy; in South America, especially Colombia, Argentina, and Brazil; and in China.18 Most interesting is the intercontinental transfer of these strains: it has been documented that the index patient with KPC-producing K pneumoniae in Medellin, Colombia, came from Israel to undergo liver transplantation.19 Likewise, KPC-producing K pneumoniae in France and Israel could be linked epidemiologically and genetically to the predominant US strain.20,21
Even more explosive has been the surge of another carbapenemase, the Ambler Class B New Delhi metallo-beta-lactamase, or NDM-1. Initially reported in a urinary isolate of K pneumoniae from a Swedish patient who had been hospitalized in New Delhi in 2008, NDM-1 was soon found throughout India, in Pakistan, and in the United Kingdom.22 Interestingly, several of the UK patients with NDM-1-harboring bacteria had received organ transplants in the Indian subcontinent. Reports from elsewhere in Europe, Australia, and Africa followed suit, usually with a connection to the Indian subcontinent epicenter. In contrast, several other cases in Europe were traced to the Balkans, where there appears to be another focus of NDM-1.23
Penetration of NDM-1 into North America has begun, with cases and outbreaks reported in several US and Canadian regions, and in a military medical facility in Afghanistan. In several of these instances, there has been a documented link with travel and hospitalizations overseas.24–27 However, no such link with travel could be established in a recent outbreak in Ontario.27
In addition, resistance to carbapenems may result from other enzymes (Table 1), or from combinations of changes in outer membrane porins and the production of extended spectrum beta-lactamases or other cephalosporinases.28
DEADLY IMPACT ON THE MOST VULNERABLE
Regardless of the resistance pattern, Enterobacteriaceae are an important cause of health care-associated infections, including urinary and bloodstream infections in patients with indwelling catheters, pneumonia (often in association with mechanical ventilation), and, less frequently, infections of skin and soft tissues and the central nervous system.29–31
Several studies have examined the clinical characteristics and outcomes of patients with CRE infections. Those typically affected are elderly and debilitated and have multiple comorbidities, including diabetes mellitus and immunosuppression. They are heavily exposed to health care with frequent antecedent hospitalizations and invasive procedures. Furthermore, they are often severely ill and require intensive care. Patients infected with carbapenem-resistant K pneumoniae, compared with those with carbapenem-susceptible strains, are more likely to have undergone organ or stem cell transplantation or mechanical ventilation, and to have had a longer hospital stay before infection.
They also experience a high mortality rate, which ranges from 30% in patients with nonbacteremic infections to 72% in series of patients with liver transplants or bloodstream infections.32–37
More recently, CRE has been reported in other vulnerable populations, such as children with critical illness or cancer and in burn patients.38–40
Elderly and critically ill patients with bacteremia originating from a high-risk source (eg, pneumonia) typically face the most adverse outcomes. With increasing drug resistance, inadequate initial antimicrobial therapy is more commonly seen and may account for some of these poor outcomes.37,41
LONG-TERM CARE FACILITIES IN THE EYE OF THE STORM
A growing body of evidence suggests that long-term care facilities play a crucial role in the spread of CRE.
In an investigation into carbapenem-resistant A baumanii and K pneumoniae in a hospital system,36 75% of patients with carbapenem-resistant K pneumoniae were admitted from long-term care facilities, and only 1 of 13 patients was discharged home.
In a series of patients with carbapenem-resistant K pneumoniae bloodstream infections, 42% survived their index hospital stay. Of these patients, only 32% were discharged home, and readmissions were very common.32
Admission from a long-term care facility or transfer from another hospital is significantly associated with carbapenem resistance in patients with Enterobacteriaceae.42 Similarly, in Israel, a large reservoir of CRE was found in postacute care facilities.43
It is clear that long-term care residents are at increased risk of colonization and infection with CRE. However, further studies are needed to evaluate whether this simply refects an overlap in risk factors, or whether significant patient-to-patient transmission occurs in these settings.
INFECTION CONTROL TAKES CENTER STAGE
It is important to note that risk factors for CRE match those of various nosocomial infections, including other resistant gram-negative bacilli, methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, Candida species, and Clostridium difficile; in fact, CRE often coexist with other multidrug-resistant organisms.44,45
Common risk factors include residence in a long-term care facility, an intensive care unit stay, use of lines and catheters, and antibiotic exposure. This commonality of risk factors implies that systematic infection-prevention measures will have an impact on the prevalence and incidence rates of multidrug-resistant organism infections across the board, CRE included. It should be emphasized that strict compliance with hand hygiene is still the foundation of any infection-prevention strategy.
Infection prevention and the control of transmission of CRE in long-term care facilities pose unique challenges. Guidelines from the Society for Healthcare Epidemiology and the Association for Professionals in Infection Control recommend the use of contact precautions for patients with multidrug-resistant organisms, including CRE, who are ill and totally dependent on health care workers for activities of daily living or whose secretions or drainage cannot be contained. These same guidelines advise against attempting to eradicate multidrug-resistant organism colonization status.46
In acute care facilities, Best Infection Control Practices from the CDC and the Healthcare Infection Control Practices Advisory Committee encourage mechanisms for the rapid recognition and reporting of CRE cases to infection prevention personnel so that contact precautions can be implemented. Furthermore, facilities without CRE cases should carry out periodic laboratory reviews to identify cases, and patients exposed to CRE cases should be screened with surveillance cultures.47
Outbreaks of CRE may require extraordinary infection control measures. An approach combining point-prevalence surveillance of colonization, detection of environmental and common-equipment contamination, with the implementation of a bundle consisting of chlorhexidine baths, cohorting of colonized patients and health care personnel, increased environmental cleaning, and staff education may be effective in controlling outbreaks of CRE.48
Nevertheless, control of CRE may prove exceptionally difficult. A recent high-profile outbreak of carbapenem-resistant K pneumoniae at the National Institutes of Health Clinical Center in Maryland caused infections in 18 patients, 11 of whom died.49 Of note, carbapenem-resistant K pneumoniae was detected in this outbreak in both respiratory equipment and sink drains. The outbreak was ultimately contained by detection through surveillance cultures and by strict cohorting of colonized patients, which minimized common medical equipment and personnel between affected patients and other patients in the hospital. Additionally, rooms were sanitized with hydrogen peroxide vapor, and sinks and drains where carbapenem-resistant K pneumoniae was detected were removed.
CHALLENGES IN THE MICROBIOLOGY LABORATORY
Adequate treatment and control of CRE infections is predicated upon their accurate and prompt diagnosis from patient samples in the clinical microbiology laboratory.50
Traditional and current culture-based methods take several days to provide that information, delaying effective antibiotic therapy and permitting the transmission of undetected CRE. Furthermore, interpretative criteria of minimal inhibitory concentrations (MICs) of carbapenems recently required readjustment, as many KPC-producing strains of K pneumoniae had MICs below the previous breakpoint of resistance. In the past, this contributed to instances of “silent” dissemination of KPC-producing K pneumoniae.51
In contrast, using the new lower breakpoints of resistance for carbapenems without using a phenotypic test such as the modified Hodge test or the carbapenem-EDTA combination tests will result in a lack of differentiation between various mechanisms of carbapenem resistance.28,52,53 This may be clinically relevant, as the clinical response to carbapenem therapy may vary depending on the mechanism of resistance.
GENERAL PRINCIPLES APPLY
In treating patients infected with CRE, clinicians need to strictly observe general principles of infectious disease management to ensure the best possible outcomes. These include:
Timely and accurate diagnosis, as discussed above.
Source control, which should include drainage of any infected collections, and removal of lines, devices, and urinary catheters.
Distinguishing between infection and colonization. CRE are often encountered as urinary isolates, and the distinction between asymptomatic bacteriuria and urinary tract infection may be extremely difficult, especially in residents of long-term care facilities with chronic indwelling catheters, who are thegroup at highest risk of CRE colonization and infection. Urinalysis may be helpful in the absence of pyuria, as this rules out an infection; however, it must be emphasized that the presence of pyuria is not a helpful feature, as pyuria is common in both asymptomatic bacteriuria and urinary tract infection.54 Symptoms should be carefully evaluated in every patient with bacteriuria, and urinary tract infection should be a diagnosis of exclusion in patients with functional symptoms such as confusion or falls.
Selection of the most appropriate antibiotic regimen. While the emphasis is often on the antibiotic regimen, the above elements should not be neglected.
A DWINDLING THERAPEUTIC ARSENAL
Clinicians treating CRE infections are left with only a few antibiotic options. These options are generally limited by a lack of clinical data on efficacy, as well as by concerns about toxicity. These “drugs of last resort” include polymyxins (such as colistin), aminoglycosides, tigecycline, and fosfomycin. The role of carbapenem therapy, potentially in combination regimens, in a high-dose prolonged infusion, or even “double carbapenem therapy” remains to be determined.37,55,56
Colistin
Colistin is one of the first-line agents for treating CRE infections. First introduced in the 1950s, its use was mostly abandoned in favor of aminoglycosides. A proportion of the data on safety and efficacy of colistin, therefore, is based on older, less rigorous studies.
Neurotoxicity and nephrotoxicity are the two main concerns with colistin, and while the incidence of these adverse events does appear to be lower with modern preparations, it is still substantial.57 Dosing issues have not been completely clarified either, especially in relation to renal clearance and in patients on renal replacement therapy.58,59 Unfortunately, there have been reports of outbreaks of CRE displaying resistance to colistin.60
Tigecycline
Tigecycline is a newer antibiotic of the glycylcycline class. Like colistin, it has no oral preparation for systemic infections.
The main side effect of tigecycline is nausea.61 Other reported issues include pancreatitis and extreme alkaline phosphatase elevations.
The efficacy of tigecycline has come into question in view of meta-analyses of clinical trials, some of which have shown higher mortality rates in patients treated with tigecycline than with comparator agents.62–65 Based on these data, the US Food and Drug Administration issued a warning in 2010 regarding the increased mortality risk. Although these meta-analyses did not include patients with CRE for whom available comparators would have been ineffective, it is an important safety signal.
The efficacy of tigecycline is further limited by increasing in vitro resistance in CRE. Serum and urinary levels of tigecycline are low, and most experts discourage the use of tigecycline as monotherapy for blood stream or urinary tract infections.
Aminoglycosides
CRE display variable in vitro susceptibility to different aminoglycosides. If the organism is susceptible, aminoglycosides may be very useful in the treatment of CRE infections, especially urinary tract infectons. In a study of carbapenem-resistant K pneumoniae urinary tract infections, patients who were treated with polymyxins or tigecycline were significantly less likely to have clearance of their urine as compared with patients treated with aminoglycosides.66
Ototoxicity and nephrotoxicity are demonstrated adverse effects of aminoglycosides. Close monitoring of serum levels, interval audiology examinations at baseline and during therapy, and the use of extended-interval dosing may help to decrease the incidence of these toxicities.
Fosfomycin
Fosfomycin is only available as an oral formulation in the United States, although intravenous administration has been used in other countries. It is exclusively used to treat urinary tract infections.
CRE often retain susceptibility to fosfomycin, and clearance of urine in cystitis may be attempted with this agent to avoid the need for intravenous treatment.29,67
Combination therapy, other topics to be explored
Recent observational reports from Greece, Italy, and the United States describe higher survival rates in patients with CRE infections treated with a combination regimen rather than monotherapy with colistin or tigecycline. This is despite reliable activity of colistin and tigecycline, and often in regimens containing carbapenems. Clinical experiments are needed to clarify the value of combination regimens that include carbapenems for the treatment of CRE infections.
Similarly, the role of carbapenems given as a high-dose prolonged infusion or as double carbapenem therapy needs to be explored further.37,55,56,68
Also to be determined is the optimal duration of treatment. To date, there is no evidence that increasing the duration of treatment beyond that recommended for infections with more susceptible bacteria results in improved outcomes. Therefore, commonly used durations include 1 week for complicated urinary tract infections, 2 weeks for bacteremia (from the first day with negative blood cultures and source control), and 8 to 14 days for pneumonia.
A SERIOUS THREAT
The emergence of CRE is a serious threat to the safety of patients in our health care system. CRE are highly successful nosocomial pathogens selected by the use of antibiotics, which burden patients debilitated by advanced age, comorbidities, and medical interventions. Infections with CRE result in poor outcomes, and available treatments of last resort such as tigecycline and colistin are of unclear efficacy and safety.
Control of CRE transmission is hindered by the transit of patients through long-term care facilities, and detection of CRE is difficult because of the myriad mechanisms involved and the imperfect methods currently available. Clinicians are concerned and frustrated, especially given the paucity of antibiotics in development to address the therapeutic dilemma posed by CRE. The challenge of CRE and other multidrug-resistant organisms requires the concerted response of professionals in various disciplines, including pharmacists, microbiologists, infection control practitioners, and infectious disease clinicians (Table 2).
Control of transmission by infection prevention strategies and by antimicrobial stewardship is going to be crucial in the years to come, not only for limiting the spread of CRE, but also for preventing the next multidrug-resistant “superbug” from emerging. However, the current reality is that health care providers will be faced with increased numbers of patients infected with CRE.
Prospective studies into transmission, molecular characteristics, and, most of all, treatment regimens are urgently needed. In addition, the development of new antimicrobials and nontraditional antimicrobial methods should have international priority.
The past 10 years have brought a formidable challenge to the clinical arena, as carbapenems, until now the most reliable antibiotics against Klebsiella species, Escherichia coli, and other Enterobacteriaceae, are becoming increasingly ineffective.
Infections caused by carbapenem-resistant Enterobacteriaceae (CRE) pose a serious threat to hospitalized patients. Moreover, CRE often demonstrate resistance to many other classes of antibiotics, thus limiting our therapeutic options. Furthermore, few new antibiotics are in line to replace carbapenems. This public health crisis demands redefined and refocused efforts in the diagnosis, treatment, and control of infections in hospitalized patients.
Here, we present an overview of CRE and discuss avenues to escape a new era of untreatable infections.
INCREASED USE OF CARBAPENEMS AND EMERGENCE OF RESISTANCE
Developed in the 1980s, carbapenems are derivatives of thyanamycin. Imipenem and meropenem, the first members of the class, had a broad spectrum of antimicrobial activity that included coverage of Pseudomonas aeruginosa, adequately positioning them for the treatment of nosocomial infections. Back then, nearly all Enterobacteriaceae were susceptible to carbapenems.1
In the 1990s, Enterobacteriaceae started to develop resistance to cephalosporins—till then, the first-line antibiotics for these organisms—by acquiring extended-spectrum betalactamases, which inactivate those agents. Consequently, the use of cephalosporins had to be restricted, while carbapenems, which remained impervious to these enzymes, had to be used more.2 In pivotal international studies in the treatment of infections caused by strains of K pneumoniae that produced these inactivating enzymes, outcomes were better with carbapenems than with cephalosporins and fluoroquinolones.3,4
Ertapenem, a carbapenem without antipseudomonal activity and highly bound to protein, was released in 2001. Its prolonged half-life permitted once-daily dosing, which positioned it as an option for treating infections in community dwellers.5 Doripenem is the newest member of the class of carbapenems, and its spectrum of activity is similar to that of imipenem and meropenem and includes P aeruginosa.6 The use of carbapenems, measured in a representative sample of 35 university hospitals in the United States, increased by 59% between 2002 and 2006.7
In the early 2000s, carbapenem resistance in K pneumoniae and other Enterobacteriaceae was rare in North America. But then, after initial outbreaks occurred in hospitals in the Northeast (especially New York City), CRE began to spread throughout the United States. By 2009–2010, the National Healthcare Safety Network from the Centers for Disease Control and Prevention (CDC) revealed that 12.8% of K pneumoniae isolates associated with bloodstream infections were resistant to carbapenems.8
In March 2013, the CDC disclosed that 3.9% of short-stay acute-care hospitals and 17.8% of long-term acute-care hospitals reported at least one CRE health care-associated infection in 2012. CRE had extended to 42 states, and the proportion of Enterobacteriaceae that are CRE had increased fourfold over the past 10 years.9
Coinciding with the increased use of carbapenems, multiple factors and modifiers likely contributed to the dramatic increase in CRE. These include use of other antibiotics in humans and animals, their relative penetration and selective effect on the gut microbiota, case-mix and infection control practices in different health care settings, and travel patterns.
POWERFUL ENZYMES THAT TRAVEL FAR
Bacterial acquisition of carbapenemases, enzymes that inactivate carbapenems, is crucial to the emergence of CRE. The enzyme in the sentinel carbapenem-resistant K pneumoniae isolate found in 1996 in North Carolina was designated K pneumoniae carbapenemase (KPC-1). This mechanism also conferred resistance to all cephalosporins, aztreonam, and beta-lactamase inhibitors such as clavulanic acid and tazobactam.10
KPC-2 (later determined to be identical to KPC-1) was found in K pneumoniae from Baltimore, and KPC-3 caused an early outbreak in New York City.11,12 To date, 12 additional variants of blaKPC, the gene encoding for the KPC enzyme, have been described.13
The genes encoding carbapenemases are usually found on plasmids or other common mobile genetic elements.14 These genetic elements allow the organism to acquire genes conferring resistance to other classes of antimicrobials, such as aminoglycoside-modifying enzymes and fluoroquinolone-resistance determinants, and beta-lactamases.15,16 The result is that CRE isolates are increasingly multidrug-resistant (ie, resistant to three or more classes of antimicrobials), extensively drug-resistant (ie, resistant to all but one or two classes), or pandrug-resistant (ie, resistant to all available classes of antibiotics).17 Thus, up to 98% of KPC-producing K pneumoniae are resistant to trimethoprim-sulfamethoxazole, 90% are resistant to fluoroquinolones, and 60% are resistant to gentamicin or amikacin.15
The mobility of these genetic elements has also allowed for dispersion into diverse Enterobacteriaceae such as E coli, Klebsiella oxytoca, Enterobacter, Serratia, and Salmonella species. Furthermore, KPC has been described in non-Enterobacteriaceae such as Acinetobacter baumannii and P aeruginosa.
Extending globally, KPC is now endemic in the Mediterranean basin, including Israel, Greece, and Italy; in South America, especially Colombia, Argentina, and Brazil; and in China.18 Most interesting is the intercontinental transfer of these strains: it has been documented that the index patient with KPC-producing K pneumoniae in Medellin, Colombia, came from Israel to undergo liver transplantation.19 Likewise, KPC-producing K pneumoniae in France and Israel could be linked epidemiologically and genetically to the predominant US strain.20,21
Even more explosive has been the surge of another carbapenemase, the Ambler Class B New Delhi metallo-beta-lactamase, or NDM-1. Initially reported in a urinary isolate of K pneumoniae from a Swedish patient who had been hospitalized in New Delhi in 2008, NDM-1 was soon found throughout India, in Pakistan, and in the United Kingdom.22 Interestingly, several of the UK patients with NDM-1-harboring bacteria had received organ transplants in the Indian subcontinent. Reports from elsewhere in Europe, Australia, and Africa followed suit, usually with a connection to the Indian subcontinent epicenter. In contrast, several other cases in Europe were traced to the Balkans, where there appears to be another focus of NDM-1.23
Penetration of NDM-1 into North America has begun, with cases and outbreaks reported in several US and Canadian regions, and in a military medical facility in Afghanistan. In several of these instances, there has been a documented link with travel and hospitalizations overseas.24–27 However, no such link with travel could be established in a recent outbreak in Ontario.27
In addition, resistance to carbapenems may result from other enzymes (Table 1), or from combinations of changes in outer membrane porins and the production of extended spectrum beta-lactamases or other cephalosporinases.28
DEADLY IMPACT ON THE MOST VULNERABLE
Regardless of the resistance pattern, Enterobacteriaceae are an important cause of health care-associated infections, including urinary and bloodstream infections in patients with indwelling catheters, pneumonia (often in association with mechanical ventilation), and, less frequently, infections of skin and soft tissues and the central nervous system.29–31
Several studies have examined the clinical characteristics and outcomes of patients with CRE infections. Those typically affected are elderly and debilitated and have multiple comorbidities, including diabetes mellitus and immunosuppression. They are heavily exposed to health care with frequent antecedent hospitalizations and invasive procedures. Furthermore, they are often severely ill and require intensive care. Patients infected with carbapenem-resistant K pneumoniae, compared with those with carbapenem-susceptible strains, are more likely to have undergone organ or stem cell transplantation or mechanical ventilation, and to have had a longer hospital stay before infection.
They also experience a high mortality rate, which ranges from 30% in patients with nonbacteremic infections to 72% in series of patients with liver transplants or bloodstream infections.32–37
More recently, CRE has been reported in other vulnerable populations, such as children with critical illness or cancer and in burn patients.38–40
Elderly and critically ill patients with bacteremia originating from a high-risk source (eg, pneumonia) typically face the most adverse outcomes. With increasing drug resistance, inadequate initial antimicrobial therapy is more commonly seen and may account for some of these poor outcomes.37,41
LONG-TERM CARE FACILITIES IN THE EYE OF THE STORM
A growing body of evidence suggests that long-term care facilities play a crucial role in the spread of CRE.
In an investigation into carbapenem-resistant A baumanii and K pneumoniae in a hospital system,36 75% of patients with carbapenem-resistant K pneumoniae were admitted from long-term care facilities, and only 1 of 13 patients was discharged home.
In a series of patients with carbapenem-resistant K pneumoniae bloodstream infections, 42% survived their index hospital stay. Of these patients, only 32% were discharged home, and readmissions were very common.32
Admission from a long-term care facility or transfer from another hospital is significantly associated with carbapenem resistance in patients with Enterobacteriaceae.42 Similarly, in Israel, a large reservoir of CRE was found in postacute care facilities.43
It is clear that long-term care residents are at increased risk of colonization and infection with CRE. However, further studies are needed to evaluate whether this simply refects an overlap in risk factors, or whether significant patient-to-patient transmission occurs in these settings.
INFECTION CONTROL TAKES CENTER STAGE
It is important to note that risk factors for CRE match those of various nosocomial infections, including other resistant gram-negative bacilli, methicillin-resistant Staphylococcus aureus, vancomycin-resistant enterococci, Candida species, and Clostridium difficile; in fact, CRE often coexist with other multidrug-resistant organisms.44,45
Common risk factors include residence in a long-term care facility, an intensive care unit stay, use of lines and catheters, and antibiotic exposure. This commonality of risk factors implies that systematic infection-prevention measures will have an impact on the prevalence and incidence rates of multidrug-resistant organism infections across the board, CRE included. It should be emphasized that strict compliance with hand hygiene is still the foundation of any infection-prevention strategy.
Infection prevention and the control of transmission of CRE in long-term care facilities pose unique challenges. Guidelines from the Society for Healthcare Epidemiology and the Association for Professionals in Infection Control recommend the use of contact precautions for patients with multidrug-resistant organisms, including CRE, who are ill and totally dependent on health care workers for activities of daily living or whose secretions or drainage cannot be contained. These same guidelines advise against attempting to eradicate multidrug-resistant organism colonization status.46
In acute care facilities, Best Infection Control Practices from the CDC and the Healthcare Infection Control Practices Advisory Committee encourage mechanisms for the rapid recognition and reporting of CRE cases to infection prevention personnel so that contact precautions can be implemented. Furthermore, facilities without CRE cases should carry out periodic laboratory reviews to identify cases, and patients exposed to CRE cases should be screened with surveillance cultures.47
Outbreaks of CRE may require extraordinary infection control measures. An approach combining point-prevalence surveillance of colonization, detection of environmental and common-equipment contamination, with the implementation of a bundle consisting of chlorhexidine baths, cohorting of colonized patients and health care personnel, increased environmental cleaning, and staff education may be effective in controlling outbreaks of CRE.48
Nevertheless, control of CRE may prove exceptionally difficult. A recent high-profile outbreak of carbapenem-resistant K pneumoniae at the National Institutes of Health Clinical Center in Maryland caused infections in 18 patients, 11 of whom died.49 Of note, carbapenem-resistant K pneumoniae was detected in this outbreak in both respiratory equipment and sink drains. The outbreak was ultimately contained by detection through surveillance cultures and by strict cohorting of colonized patients, which minimized common medical equipment and personnel between affected patients and other patients in the hospital. Additionally, rooms were sanitized with hydrogen peroxide vapor, and sinks and drains where carbapenem-resistant K pneumoniae was detected were removed.
CHALLENGES IN THE MICROBIOLOGY LABORATORY
Adequate treatment and control of CRE infections is predicated upon their accurate and prompt diagnosis from patient samples in the clinical microbiology laboratory.50
Traditional and current culture-based methods take several days to provide that information, delaying effective antibiotic therapy and permitting the transmission of undetected CRE. Furthermore, interpretative criteria of minimal inhibitory concentrations (MICs) of carbapenems recently required readjustment, as many KPC-producing strains of K pneumoniae had MICs below the previous breakpoint of resistance. In the past, this contributed to instances of “silent” dissemination of KPC-producing K pneumoniae.51
In contrast, using the new lower breakpoints of resistance for carbapenems without using a phenotypic test such as the modified Hodge test or the carbapenem-EDTA combination tests will result in a lack of differentiation between various mechanisms of carbapenem resistance.28,52,53 This may be clinically relevant, as the clinical response to carbapenem therapy may vary depending on the mechanism of resistance.
GENERAL PRINCIPLES APPLY
In treating patients infected with CRE, clinicians need to strictly observe general principles of infectious disease management to ensure the best possible outcomes. These include:
Timely and accurate diagnosis, as discussed above.
Source control, which should include drainage of any infected collections, and removal of lines, devices, and urinary catheters.
Distinguishing between infection and colonization. CRE are often encountered as urinary isolates, and the distinction between asymptomatic bacteriuria and urinary tract infection may be extremely difficult, especially in residents of long-term care facilities with chronic indwelling catheters, who are thegroup at highest risk of CRE colonization and infection. Urinalysis may be helpful in the absence of pyuria, as this rules out an infection; however, it must be emphasized that the presence of pyuria is not a helpful feature, as pyuria is common in both asymptomatic bacteriuria and urinary tract infection.54 Symptoms should be carefully evaluated in every patient with bacteriuria, and urinary tract infection should be a diagnosis of exclusion in patients with functional symptoms such as confusion or falls.
Selection of the most appropriate antibiotic regimen. While the emphasis is often on the antibiotic regimen, the above elements should not be neglected.
A DWINDLING THERAPEUTIC ARSENAL
Clinicians treating CRE infections are left with only a few antibiotic options. These options are generally limited by a lack of clinical data on efficacy, as well as by concerns about toxicity. These “drugs of last resort” include polymyxins (such as colistin), aminoglycosides, tigecycline, and fosfomycin. The role of carbapenem therapy, potentially in combination regimens, in a high-dose prolonged infusion, or even “double carbapenem therapy” remains to be determined.37,55,56
Colistin
Colistin is one of the first-line agents for treating CRE infections. First introduced in the 1950s, its use was mostly abandoned in favor of aminoglycosides. A proportion of the data on safety and efficacy of colistin, therefore, is based on older, less rigorous studies.
Neurotoxicity and nephrotoxicity are the two main concerns with colistin, and while the incidence of these adverse events does appear to be lower with modern preparations, it is still substantial.57 Dosing issues have not been completely clarified either, especially in relation to renal clearance and in patients on renal replacement therapy.58,59 Unfortunately, there have been reports of outbreaks of CRE displaying resistance to colistin.60
Tigecycline
Tigecycline is a newer antibiotic of the glycylcycline class. Like colistin, it has no oral preparation for systemic infections.
The main side effect of tigecycline is nausea.61 Other reported issues include pancreatitis and extreme alkaline phosphatase elevations.
The efficacy of tigecycline has come into question in view of meta-analyses of clinical trials, some of which have shown higher mortality rates in patients treated with tigecycline than with comparator agents.62–65 Based on these data, the US Food and Drug Administration issued a warning in 2010 regarding the increased mortality risk. Although these meta-analyses did not include patients with CRE for whom available comparators would have been ineffective, it is an important safety signal.
The efficacy of tigecycline is further limited by increasing in vitro resistance in CRE. Serum and urinary levels of tigecycline are low, and most experts discourage the use of tigecycline as monotherapy for blood stream or urinary tract infections.
Aminoglycosides
CRE display variable in vitro susceptibility to different aminoglycosides. If the organism is susceptible, aminoglycosides may be very useful in the treatment of CRE infections, especially urinary tract infectons. In a study of carbapenem-resistant K pneumoniae urinary tract infections, patients who were treated with polymyxins or tigecycline were significantly less likely to have clearance of their urine as compared with patients treated with aminoglycosides.66
Ototoxicity and nephrotoxicity are demonstrated adverse effects of aminoglycosides. Close monitoring of serum levels, interval audiology examinations at baseline and during therapy, and the use of extended-interval dosing may help to decrease the incidence of these toxicities.
Fosfomycin
Fosfomycin is only available as an oral formulation in the United States, although intravenous administration has been used in other countries. It is exclusively used to treat urinary tract infections.
CRE often retain susceptibility to fosfomycin, and clearance of urine in cystitis may be attempted with this agent to avoid the need for intravenous treatment.29,67
Combination therapy, other topics to be explored
Recent observational reports from Greece, Italy, and the United States describe higher survival rates in patients with CRE infections treated with a combination regimen rather than monotherapy with colistin or tigecycline. This is despite reliable activity of colistin and tigecycline, and often in regimens containing carbapenems. Clinical experiments are needed to clarify the value of combination regimens that include carbapenems for the treatment of CRE infections.
Similarly, the role of carbapenems given as a high-dose prolonged infusion or as double carbapenem therapy needs to be explored further.37,55,56,68
Also to be determined is the optimal duration of treatment. To date, there is no evidence that increasing the duration of treatment beyond that recommended for infections with more susceptible bacteria results in improved outcomes. Therefore, commonly used durations include 1 week for complicated urinary tract infections, 2 weeks for bacteremia (from the first day with negative blood cultures and source control), and 8 to 14 days for pneumonia.
A SERIOUS THREAT
The emergence of CRE is a serious threat to the safety of patients in our health care system. CRE are highly successful nosocomial pathogens selected by the use of antibiotics, which burden patients debilitated by advanced age, comorbidities, and medical interventions. Infections with CRE result in poor outcomes, and available treatments of last resort such as tigecycline and colistin are of unclear efficacy and safety.
Control of CRE transmission is hindered by the transit of patients through long-term care facilities, and detection of CRE is difficult because of the myriad mechanisms involved and the imperfect methods currently available. Clinicians are concerned and frustrated, especially given the paucity of antibiotics in development to address the therapeutic dilemma posed by CRE. The challenge of CRE and other multidrug-resistant organisms requires the concerted response of professionals in various disciplines, including pharmacists, microbiologists, infection control practitioners, and infectious disease clinicians (Table 2).
Control of transmission by infection prevention strategies and by antimicrobial stewardship is going to be crucial in the years to come, not only for limiting the spread of CRE, but also for preventing the next multidrug-resistant “superbug” from emerging. However, the current reality is that health care providers will be faced with increased numbers of patients infected with CRE.
Prospective studies into transmission, molecular characteristics, and, most of all, treatment regimens are urgently needed. In addition, the development of new antimicrobials and nontraditional antimicrobial methods should have international priority.
- Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother 2011; 55:4943–4960.
- Rahal JJ, Urban C, Horn D, et al. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA 1998; 280:1233–1237.
- Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med 2004; 140:26–32.
- Endimiani A, Luzzaro F, Perilli M, et al. Bacteremia due to Klebsiella pneumoniae isolates producing the TEM-52 extended-spectrum beta-lactamase: treatment outcome of patients receiving imipenem or ciprofoxacin. Clin Infect Dis 2004; 38:243–251.
- Livermore DM, Sefton AM, Scott GM. Properties and potential of ertapenem. J Antimicrob Chemother 2003; 52:331–344.
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antibiotics: doripenem, ceftobiprole, ceftaroline, and cefepime. Infect Dis Clin North Am 2009; 23:983–996, ix.
- Pakyz AL, MacDougall C, Oinonen M, Polk RE. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med 2008; 168:2254–2260.
- Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013; 34:1–14.
- Centers for Disease Control and Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR 2013; 62:165–170.
- Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 2001; 45:1151–1161.
- Smith Moland E, Hanson ND, Herrera VL, et al. Plasmid-mediated, carbapenem-hydrolysing beta-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J Antimicrob Chemother 2003; 51:711–714.
- Woodford N, Tierno PM, Young K, et al. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York medical center. Antimicrob Agents Chemother 2004; 48:4793–4799.
- Lehey Clinic. OXA-type β-Lactamases. http://www.lahey.org/Studies/other.asp#table1. Accessed March 11, 2013.
- Mathers AJ, Cox HL, Kitchel B, et al. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. MBio 2011; 2 6:e00204–11.
- Endimiani A, Hujer AM, Perez F, et al. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J Antimicrob Chemother 2009; 63:427–437.
- Endimiani A, Carias LL, Hujer AM, et al. Presence of plasmid-mediated quinolone resistance in Klebsiella pneumoniae isolates possessing blaKPC in the United States. Antimicro Agents Chemother 2008; 52:2680–2682.
- Magiorakos A P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–281.
- Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 2012; 25:682–707.
- Lopez JA, Correa A, Navon-Venezia S, et al. Intercontinental spread from Israel to Colombia of a KPC-3-producing Klebsiella pneumoniae strain. Clin Microbiol Infect 2011; 17:52–56.
- Naas T, Nordmann P, Vedel G, Poyart C. Plasmid-mediated carbapenem-hydrolyzing beta-lactamase KPC in a Klebsiella pneumoniae isolate from France. Antimicrob Agents Chemother 2005; 49:4423–4424.
- Navon-Venezia S, Leavitt A, Schwaber MJ, et al. First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob Agents Chemother 2009; 53:818–820.
- Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 2009; 53:5046–5054.
- Livermore DM, Walsh TR, Toleman M, Woodford N. Balkan NDM-1: escape or transplant? Lancet Infect Dis 2011; 11:164.
- Centers for Disease Control and Prevention. Carbapenem-resistant enterobacteriaceae containing New Delhi metallo-beta-lactamase in two patients - Rhode Island, March 2012. MMWR Morb Mortal Wkly Rep 2012Jun 22; 61:446–448.
- Centers for Disease Control and Prevention. Detection of Enterobacteriaceae isolates carrying metallo-beta-lactamase—United States, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:750.
- McGann P, Hang J, Clifford RJ, et al. Complete sequence of a novel 178-kilobase plasmid carrying bla(NDM-1) in a Providencia stuartii strain isolated in Afghanistan. Antimicrob Agents Chemother 2012; 56:1673–1679.
- Borgia S, Lastovetska O, Richardson D, et al. Outbreak of carbapenem-resistant Enterobacteriaceae containing blaNDM-1, Ontario, Canada. Clin Infect Dis 2012; 55:e109–e117.
- Endimiani A, Perez F, Bajaksouzian S, et al. Evaluation of updated interpretative criteria for categorizing Klebsiella pneumoniae with reduced carbapenem susceptibility. J Clinic Microbiol 2010; 48:4417–4425.
- Neuner EA, Sekeres J, Hall GS, van Duin D. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother 2012; 56:5744–5748.
- Neuner EA, Yeh JY, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagnostic Microbiol Infect Dis 2011; 69:357–362.
- van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagnostic Microbiol Infect Dis 2013; 75:115–120.
- Neuner EA, Yeh J-Y, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis 2011; 69:357–362.
- Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 2008; 29:1099–1106.
- Borer A, Saidel-Odes L, Riesenberg K, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol 2009; 30:972–976.
- Marchaim D, Chopra T, Perez F, et al. Outcomes and genetic relatedness of carbapenem-resistant Enterobacteriaceae at Detroit medical center. Infect Control Hosp Epidemiol 2011; 32:861–871.
- Perez F, Endimiani A, Ray AJ, et al. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother 2010; 65:1807–1818.
- Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55:943–950.
- Little ML, Qin X, Zerr DM, Weissman SJ. Molecular diversity in mechanisms of carbapenem resistance in paediatric Enterobacteriaceae. Int J Antimicrob Agents 2012; 39:52–57.
- Logan LK. Carbapenem-resistant Enterobacteriaceae: an emerging problem in children. Clin Infect Dis 2012; 55:852–859.
- Rastegar Lari A, Azimi L, Rahbar M, Fallah F, Alaghehbandan R. Phenotypic detection of Klebsiella pneumoniae carbapenemase among burns patients: first report from Iran. Burns 2013; 39:174–176.
- Zarkotou O, Pournaras S, Tselioti P, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 2011; 17:1798–1803.
- Hyle EP, Ferraro MJ, Silver M, Lee H, Hooper DC. Ertapenem-resistant Enterobacteriaceae: risk factors for acquisition and outcomes. Infect Control Hosp Epidemiol 2010; 31:1242–1249.
- Ben-David D, Masarwa S, Navon-Venezia S, et al. Carbapenem-resistant Klebsiella pneumoniae in post-acute-care facilities in Israel. Infect Control Hosp Epidemiol 2011; 32:845–853.
- Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med 2002; 136:834–844.
- Marchaim D, Perez F, Lee J, et al. “Swimming in resistance”: co-colonization with carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii or Pseudomonas aeruginosa.” Am J Infect Control 2012; 40:830–835.
- Smith PW, Bennett G, Bradley S, et al. SHEA/APIC Guideline: Infection prevention and control in the long-term care facility. Am J Infect Control 2008; 36:504–535.
- Centers for Disease Control and Prevention. Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR 2009; 58:256–260.
- Munoz-Price LS, De La Cuesta C, Adams S, et al. Successful eradication of a monoclonal strain of Klebsiella pneumoniae during a K. pneumoniae carbapenemase-producing K. pneumoniae outbreak in a surgical intensive care unit in Miami, Florida. Infect Control Hosp Epidemiol 2010; 31:1074–1077.
- Snitkin ES, Zelazny AM, Thomas PJ, et al. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with wholegenome sequencing. Sci Transl Med 2012; 4:148ra16.
- Srinivasan A, Patel JB. Klebsiella pneumoniae carbapenemase-producing organisms: an ounce of prevention really is worth a pound of cure. Infect Control Hosp Epidemiol 2008; 29:1107–1109.
- Viau RA, Hujer AM, Marshall SH, et al. “Silent” dissemination of Klebsiella pneumoniae isolates bearing K pneumoniae carbapenemase in a long-term care facility for children and young adults in Northeast Ohio”. Clin Infect Dis 2012; 54:1314–1321.
- Galani I, Rekatsina PD, Hatzaki D, Plachouras D, Souli M, Giamarellou H. Evaluation of different laboratory tests for the detection of metallo-beta-lactamase production in Enterobacteriaceae. J Antimicrob Chemother 2008; 61:548–553.
- Anderson KF, Lonsway DR, Rasheed JK, et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol 2007; 45:2723–2725.
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40:643–654.
- Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 2011; 17:1135–1141.
- Bulik CC, Nicolau DP. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2011; 55:3002–3004.
- Pogue JM, Lee J, Marchaim D, et al. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis 2011; 53:879–884.
- Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011; 55:3284–3294.
- Dalfno L, Puntillo F, Mosca A, et al. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis 2012; 54:1720–1726.
- Marchaim D, Chopra T, Pogue JM, et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother 2011; 55:593–599.
- Bonilla MF, Avery RK, Rehm SJ, Neuner EA, Isada CM, van Duin D. Extreme alkaline phosphatase elevation associated with tigecycline. J Antimicrob Chemother 2011; 66:952–953.
- Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 2012; 54:1699–1709.
- Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis 2011; 11:834–844.
- Cai Y, Wang R, Liang B, Bai N, Liu Y. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother 2011; 55:1162–1172.
- Yahav D, Lador A, Paul M, Leibovici L. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother 2011; 66:1963–1971.
- Satlin MJ, Kubin CJ, Blumenthal JS, et al. Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother 2011; 55:5893–5899.
- Endimiani A, Patel G, Hujer KM, et al. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother 2010; 54:526–529.
- Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012; 56:2108–2113.
- Papp-Wallace KM, Endimiani A, Taracila MA, Bonomo RA. Carbapenems: past, present, and future. Antimicrob Agents Chemother 2011; 55:4943–4960.
- Rahal JJ, Urban C, Horn D, et al. Class restriction of cephalosporin use to control total cephalosporin resistance in nosocomial Klebsiella. JAMA 1998; 280:1233–1237.
- Paterson DL, Ko WC, Von Gottberg A, et al. International prospective study of Klebsiella pneumoniae bacteremia: implications of extended-spectrum beta-lactamase production in nosocomial Infections. Ann Intern Med 2004; 140:26–32.
- Endimiani A, Luzzaro F, Perilli M, et al. Bacteremia due to Klebsiella pneumoniae isolates producing the TEM-52 extended-spectrum beta-lactamase: treatment outcome of patients receiving imipenem or ciprofoxacin. Clin Infect Dis 2004; 38:243–251.
- Livermore DM, Sefton AM, Scott GM. Properties and potential of ertapenem. J Antimicrob Chemother 2003; 52:331–344.
- Bazan JA, Martin SI, Kaye KM. Newer beta-lactam antibiotics: doripenem, ceftobiprole, ceftaroline, and cefepime. Infect Dis Clin North Am 2009; 23:983–996, ix.
- Pakyz AL, MacDougall C, Oinonen M, Polk RE. Trends in antibacterial use in US academic health centers: 2002 to 2006. Arch Intern Med 2008; 168:2254–2260.
- Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infect Control Hosp Epidemiol 2013; 34:1–14.
- Centers for Disease Control and Prevention. Vital signs: carbapenem-resistant Enterobacteriaceae. MMWR 2013; 62:165–170.
- Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumoniae. Antimicrob Agents Chemother 2001; 45:1151–1161.
- Smith Moland E, Hanson ND, Herrera VL, et al. Plasmid-mediated, carbapenem-hydrolysing beta-lactamase, KPC-2, in Klebsiella pneumoniae isolates. J Antimicrob Chemother 2003; 51:711–714.
- Woodford N, Tierno PM, Young K, et al. Outbreak of Klebsiella pneumoniae producing a new carbapenem-hydrolyzing class A beta-lactamase, KPC-3, in a New York medical center. Antimicrob Agents Chemother 2004; 48:4793–4799.
- Lehey Clinic. OXA-type β-Lactamases. http://www.lahey.org/Studies/other.asp#table1. Accessed March 11, 2013.
- Mathers AJ, Cox HL, Kitchel B, et al. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. MBio 2011; 2 6:e00204–11.
- Endimiani A, Hujer AM, Perez F, et al. Characterization of blaKPC-containing Klebsiella pneumoniae isolates detected in different institutions in the Eastern USA. J Antimicrob Chemother 2009; 63:427–437.
- Endimiani A, Carias LL, Hujer AM, et al. Presence of plasmid-mediated quinolone resistance in Klebsiella pneumoniae isolates possessing blaKPC in the United States. Antimicro Agents Chemother 2008; 52:2680–2682.
- Magiorakos A P, Srinivasan A, Carey RB, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 2012; 18:268–281.
- Tzouvelekis LS, Markogiannakis A, Psichogiou M, Tassios PT, Daikos GL. Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 2012; 25:682–707.
- Lopez JA, Correa A, Navon-Venezia S, et al. Intercontinental spread from Israel to Colombia of a KPC-3-producing Klebsiella pneumoniae strain. Clin Microbiol Infect 2011; 17:52–56.
- Naas T, Nordmann P, Vedel G, Poyart C. Plasmid-mediated carbapenem-hydrolyzing beta-lactamase KPC in a Klebsiella pneumoniae isolate from France. Antimicrob Agents Chemother 2005; 49:4423–4424.
- Navon-Venezia S, Leavitt A, Schwaber MJ, et al. First report on a hyperepidemic clone of KPC-3-producing Klebsiella pneumoniae in Israel genetically related to a strain causing outbreaks in the United States. Antimicrob Agents Chemother 2009; 53:818–820.
- Yong D, Toleman MA, Giske CG, et al. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 2009; 53:5046–5054.
- Livermore DM, Walsh TR, Toleman M, Woodford N. Balkan NDM-1: escape or transplant? Lancet Infect Dis 2011; 11:164.
- Centers for Disease Control and Prevention. Carbapenem-resistant enterobacteriaceae containing New Delhi metallo-beta-lactamase in two patients - Rhode Island, March 2012. MMWR Morb Mortal Wkly Rep 2012Jun 22; 61:446–448.
- Centers for Disease Control and Prevention. Detection of Enterobacteriaceae isolates carrying metallo-beta-lactamase—United States, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:750.
- McGann P, Hang J, Clifford RJ, et al. Complete sequence of a novel 178-kilobase plasmid carrying bla(NDM-1) in a Providencia stuartii strain isolated in Afghanistan. Antimicrob Agents Chemother 2012; 56:1673–1679.
- Borgia S, Lastovetska O, Richardson D, et al. Outbreak of carbapenem-resistant Enterobacteriaceae containing blaNDM-1, Ontario, Canada. Clin Infect Dis 2012; 55:e109–e117.
- Endimiani A, Perez F, Bajaksouzian S, et al. Evaluation of updated interpretative criteria for categorizing Klebsiella pneumoniae with reduced carbapenem susceptibility. J Clinic Microbiol 2010; 48:4417–4425.
- Neuner EA, Sekeres J, Hall GS, van Duin D. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob Agents Chemother 2012; 56:5744–5748.
- Neuner EA, Yeh JY, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagnostic Microbiol Infect Dis 2011; 69:357–362.
- van Duin D, Kaye KS, Neuner EA, Bonomo RA. Carbapenem-resistant Enterobacteriaceae: a review of treatment and outcomes. Diagnostic Microbiol Infect Dis 2013; 75:115–120.
- Neuner EA, Yeh J-Y, Hall GS, et al. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis 2011; 69:357–362.
- Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 2008; 29:1099–1106.
- Borer A, Saidel-Odes L, Riesenberg K, et al. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol 2009; 30:972–976.
- Marchaim D, Chopra T, Perez F, et al. Outcomes and genetic relatedness of carbapenem-resistant Enterobacteriaceae at Detroit medical center. Infect Control Hosp Epidemiol 2011; 32:861–871.
- Perez F, Endimiani A, Ray AJ, et al. Carbapenem-resistant Acinetobacter baumannii and Klebsiella pneumoniae across a hospital system: impact of post-acute care facilities on dissemination. J Antimicrob Chemother 2010; 65:1807–1818.
- Tumbarello M, Viale P, Viscoli C, et al. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55:943–950.
- Little ML, Qin X, Zerr DM, Weissman SJ. Molecular diversity in mechanisms of carbapenem resistance in paediatric Enterobacteriaceae. Int J Antimicrob Agents 2012; 39:52–57.
- Logan LK. Carbapenem-resistant Enterobacteriaceae: an emerging problem in children. Clin Infect Dis 2012; 55:852–859.
- Rastegar Lari A, Azimi L, Rahbar M, Fallah F, Alaghehbandan R. Phenotypic detection of Klebsiella pneumoniae carbapenemase among burns patients: first report from Iran. Burns 2013; 39:174–176.
- Zarkotou O, Pournaras S, Tselioti P, et al. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 2011; 17:1798–1803.
- Hyle EP, Ferraro MJ, Silver M, Lee H, Hooper DC. Ertapenem-resistant Enterobacteriaceae: risk factors for acquisition and outcomes. Infect Control Hosp Epidemiol 2010; 31:1242–1249.
- Ben-David D, Masarwa S, Navon-Venezia S, et al. Carbapenem-resistant Klebsiella pneumoniae in post-acute-care facilities in Israel. Infect Control Hosp Epidemiol 2011; 32:845–853.
- Safdar N, Maki DG. The commonality of risk factors for nosocomial colonization and infection with antimicrobial-resistant Staphylococcus aureus, enterococcus, gram-negative bacilli, Clostridium difficile, and Candida. Ann Intern Med 2002; 136:834–844.
- Marchaim D, Perez F, Lee J, et al. “Swimming in resistance”: co-colonization with carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii or Pseudomonas aeruginosa.” Am J Infect Control 2012; 40:830–835.
- Smith PW, Bennett G, Bradley S, et al. SHEA/APIC Guideline: Infection prevention and control in the long-term care facility. Am J Infect Control 2008; 36:504–535.
- Centers for Disease Control and Prevention. Guidance for control of infections with carbapenem-resistant or carbapenemase-producing Enterobacteriaceae in acute care facilities. MMWR 2009; 58:256–260.
- Munoz-Price LS, De La Cuesta C, Adams S, et al. Successful eradication of a monoclonal strain of Klebsiella pneumoniae during a K. pneumoniae carbapenemase-producing K. pneumoniae outbreak in a surgical intensive care unit in Miami, Florida. Infect Control Hosp Epidemiol 2010; 31:1074–1077.
- Snitkin ES, Zelazny AM, Thomas PJ, et al. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with wholegenome sequencing. Sci Transl Med 2012; 4:148ra16.
- Srinivasan A, Patel JB. Klebsiella pneumoniae carbapenemase-producing organisms: an ounce of prevention really is worth a pound of cure. Infect Control Hosp Epidemiol 2008; 29:1107–1109.
- Viau RA, Hujer AM, Marshall SH, et al. “Silent” dissemination of Klebsiella pneumoniae isolates bearing K pneumoniae carbapenemase in a long-term care facility for children and young adults in Northeast Ohio”. Clin Infect Dis 2012; 54:1314–1321.
- Galani I, Rekatsina PD, Hatzaki D, Plachouras D, Souli M, Giamarellou H. Evaluation of different laboratory tests for the detection of metallo-beta-lactamase production in Enterobacteriaceae. J Antimicrob Chemother 2008; 61:548–553.
- Anderson KF, Lonsway DR, Rasheed JK, et al. Evaluation of methods to identify the Klebsiella pneumoniae carbapenemase in Enterobacteriaceae. J Clin Microbiol 2007; 45:2723–2725.
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005; 40:643–654.
- Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 2011; 17:1135–1141.
- Bulik CC, Nicolau DP. Double-carbapenem therapy for carbapenemase-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2011; 55:3002–3004.
- Pogue JM, Lee J, Marchaim D, et al. Incidence of and risk factors for colistin-associated nephrotoxicity in a large academic health system. Clin Infect Dis 2011; 53:879–884.
- Garonzik SM, Li J, Thamlikitkul V, et al. Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011; 55:3284–3294.
- Dalfno L, Puntillo F, Mosca A, et al. High-dose, extended-interval colistin administration in critically ill patients: is this the right dosing strategy? A preliminary study. Clin Infect Dis 2012; 54:1720–1726.
- Marchaim D, Chopra T, Pogue JM, et al. Outbreak of colistin-resistant, carbapenem-resistant Klebsiella pneumoniae in metropolitan Detroit, Michigan. Antimicrob Agents Chemother 2011; 55:593–599.
- Bonilla MF, Avery RK, Rehm SJ, Neuner EA, Isada CM, van Duin D. Extreme alkaline phosphatase elevation associated with tigecycline. J Antimicrob Chemother 2011; 66:952–953.
- Prasad P, Sun J, Danner RL, Natanson C. Excess deaths associated with tigecycline after approval based on noninferiority trials. Clin Infect Dis 2012; 54:1699–1709.
- Tasina E, Haidich AB, Kokkali S, Arvanitidou M. Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect Dis 2011; 11:834–844.
- Cai Y, Wang R, Liang B, Bai N, Liu Y. Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease. Antimicrob Agents Chemother 2011; 55:1162–1172.
- Yahav D, Lador A, Paul M, Leibovici L. Efficacy and safety of tigecycline: a systematic review and meta-analysis. J Antimicrob Chemother 2011; 66:1963–1971.
- Satlin MJ, Kubin CJ, Blumenthal JS, et al. Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother 2011; 55:5893–5899.
- Endimiani A, Patel G, Hujer KM, et al. In vitro activity of fosfomycin against blaKPC-containing Klebsiella pneumoniae isolates, including those nonsusceptible to tigecycline and/or colistin. Antimicrob Agents Chemother 2010; 54:526–529.
- Qureshi ZA, Paterson DL, Potoski BA, et al. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012; 56:2108–2113.
KEY POINTS
- The utility of carbapenems is being undermined by the emergence of resistance in Enterobacteriaceae and other bacteria.
- The clinical impact of CRE falls on elderly patients exposed to these organisms in hospitals and long-term care facilities. In this vulnerable group, invasive infections with CRE exact a high death rate.
- Long-term care facilities play an important role in the transmission dynamics of CRE.
- Tigecycline and colistin are treatments of last resort against infections caused by CRE. Their use in combination with other agents, especially carbapenems, may improve outcomes and needs to be explored further.
- Early detection of CRE in the microbiology laboratory is key to guiding infection control and treatment decisions and supporting surveillance efforts.
Pros and cons of robotics in endometriosis surgery
"We need to be responsible individuals with how we shepherd in this new technology." From the Endometriosis Foundation of America's 4th Annual Medical Symposium: The American Perspective, March 11, 2013
Related article: The robot is gaining ground in gynecologic surgery. Should you be using it?
A roundtable discussion with:
Arnold P. Advincula, MD
Cheryl B. Iglesia, MD
Rosanne M. Kho, MD
Jamal Mourad, DO
Marie Fidela R. Paraiso, MD
Jason D. Wright, MD
"We need to be responsible individuals with how we shepherd in this new technology." From the Endometriosis Foundation of America's 4th Annual Medical Symposium: The American Perspective, March 11, 2013
Related article: The robot is gaining ground in gynecologic surgery. Should you be using it?
A roundtable discussion with:
Arnold P. Advincula, MD
Cheryl B. Iglesia, MD
Rosanne M. Kho, MD
Jamal Mourad, DO
Marie Fidela R. Paraiso, MD
Jason D. Wright, MD
"We need to be responsible individuals with how we shepherd in this new technology." From the Endometriosis Foundation of America's 4th Annual Medical Symposium: The American Perspective, March 11, 2013
Related article: The robot is gaining ground in gynecologic surgery. Should you be using it?
A roundtable discussion with:
Arnold P. Advincula, MD
Cheryl B. Iglesia, MD
Rosanne M. Kho, MD
Jamal Mourad, DO
Marie Fidela R. Paraiso, MD
Jason D. Wright, MD
Urology: Engineered for the New Millennium
New and Noteworthy Information—April 2013
Greater dietary fiber intake is significantly associated with a lower risk of first stroke, according to a study published online ahead of print March 28 in Stroke. Investigators searched several electronic databases for healthy participant studies published between January 1990 and May 2012 that reported fiber intake and incidence of first hemorrhagic or ischemic stroke. The group identified eight cohort studies from the United States, Europe, Australia, and Japan that met their inclusion criteria. Total dietary fiber intake was inversely associated with risk of hemorrhagic plus ischemic stroke. The researchers found evidence of heterogeneity between the studies. Soluble fiber intake of 4 g/day was not associated with stroke risk reduction, and the investigators found evidence of low heterogeneity on this point between the studies.
In women who have episodic migraine, the ratio of high molecular weight to low molecular weight ictal adiponectin (ADP) may be associated with migraine severity and predict acute treatment response, according to a study published in the March Headache. Investigators collected peripheral blood specimens from women with episodic migraine before and after acute abortive treatment with sumatriptan and naproxen sodium or placebo. In all participants, increases in the ratio of high molecular weight to low molecular weight ADP were associated with increases in pain severity. For every 0.25-μg/mL increase in low molecular weight ADP, pain severity decreased by 0.20. In treatment responders, total ADP levels were reduced at 30, 60, and 120 minutes after treatment, compared with onset.
The FDA has approved Tecfidera (dimethyl fumarate) capsules to treat adults with relapsing forms of multiple sclerosis (MS). In two clinical trials, patients with MS who took dimethyl fumarate had fewer relapses compared with people who received placebo. In one of the trials, patients who took dimethyl fumarate experienced a worsening of disability less often than patients who took a placebo. Dimethyl fumarate may decrease a person's white blood cell count, but the drug was not associated with a significant increase in infections in clinical trials. Before starting treatment, and annually thereafter, the FDA recommends that a patient's white blood cell count be assessed by a health care provider. Flushing and stomach problems were the most common adverse reactions reported. Tecfidera is manufactured by Biogen Idec (Weston, Massachusetts).
Mild cognitive impairment (MCI) at the time of Parkinson's disease diagnosis may predict a highly increased risk for early dementia, according to a study published online ahead of print March 25 in JAMA Neurology. Researchers examined data for a population-based cohort of 182 patients with incident Parkinson's disease who were monitored for three years. Significantly more patients with MCI than without MCI at baseline (27.0% versus 0.7%) progressed to dementia during follow-up. Mild cognitive impairment at the one-year visit was associated with a similar progression rate to dementia (ie, 27.8%) and reversion rate to normal cognition (ie, 19.4%). Among the 22 patients with persistent MCI at baseline and the one-year visit, 10 developed dementia and two reverted to normal cognition by the end of the study.
Higher consumption of green tea and coffee may reduce the risk of cardiovascular disease and stroke, according to a study published online ahead of print March 14 in Stroke. Investigators studied 82,369 Japanese persons between ages 45 and 74 without cardiovascular disease or cancer. Green tea and coffee consumption was assessed by a self-administered questionnaire at baseline. Compared with seldom drinking green tea, the multivariable-adjusted hazard ratios of all strokes were 0.86 and 0.80 in individuals who drank two to three and four or more cups of green tea per day, respectively. Compared with seldom drinking coffee, the multivariable-adjusted hazard ratios of all strokes were 0.89, 0.80, and 0.81 for individuals who drank coffee three to six times per week, once daily, and twice or more daily, respectively.
Updated Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injuries recommend against the use of steroids, including methylprednisolone, in acute spinal cord injury in the first 24 to 48 hours after injury. The use of steroids previously was recommended for this indication with consideration of the risk–reward profile, as evaluated by the physician. In the first new treatment guidelines in a decade, which were issued by the Joint Section on Disorders of the Spine and Peripheral Nerves of the Congress of Neurological Surgeons and the American Association of Neurological Surgeons, the standard has been revised based on the lack of medical evidence supporting the benefits of these drugs in the clinical setting. The report cites strong evidence that "high-dose steroids are associated with harmful side effects, including death."
Abnormalities in cortical surface area may indicate an individual's predisposition to developing migraine, and abnormalities in cortical thickness may result from migraine-related processes, according to research published online ahead of print March 26 in Radiology. Investigators took T2-weighted and three-dimensional T1-weighted MRIs of the brain for 63 migraineurs and 18 controls. They estimated cortical thickness and cortical surface area. Compared with control subjects, patients with migraine had reduced cortical thickness and surface area in pain-processing regions. These reductions were greater in regions involved in executive functions and visual-motion processing. Cortical thickness and cortical surface area abnormalities had minimal areas of overlap. Cortical thickness and surface area abnormalities were related to aura and white matter hyperintensities, but not to disease duration and attack frequency.
Primary stroke centers are more likely to administer t-PA than noncertified hospitals, according to research published online ahead of print March 26 in the Journal of the American Heart Association. Investigators analyzed data obtained from the Nationwide Inpatient Sample between 2004 and 2009 for patients age 18 or older with a primary diagnosis of acute ischemic stroke. IV t-PA was administered to 3.1% of patients overall. The drug was given to 2.2% of patients at noncertified hospitals and to 6.7% of patients at primary stroke centers. Between 2004 and 2009, t-PA administration increased from 1.4% to 3.3% of patients at noncertified hospitals and from 6.0% to 7.6% of patients at primary stroke centers. In a multivariable model, evaluation at a primary stroke center was significantly associated with t-PA use.
Control and prevention of risk factors such as hypertension earlier in life may limit or delay neuropathologic brain changes such as Alzheimer's disease with aging, researchers reported in a study published online ahead of print March 18 in JAMA Neurology. The investigators studied 118 cognitively normal adults ages 47 to 89. Participants were classified as having hypertension if they reported a medical diagnosis of hypertension or if blood pressure exceeded 140 mm Hg systolic/90 mm Hg diastolic on seven occasions. Participants underwent Ab PET imaging with radiotracer fluorine 18–labeled florbetapir, were genotyped for apolipoprotein E, and were classified as ε4+ or ε4−. Subjects with hypertension and at least one ε4 allele had significantly more amyloid burden than those with one or no risk factors.
Physicians can discontinue chronic antipsychotic medication for many elderly adults with Alzheimer's dementia and neuropsychiatric symptoms without causing detrimental effects on their behavior, according to a review published online March 28 in the Cochrane Database of Systematic Reviews. Investigators examined data from nine randomized controlled trials that compared antipsychotic withdrawal strategies with continuation of antipsychotics in patients with dementia. Although neurologists have concerns about the potential adverse events of antipsychotics, it is not clear whether withdrawal is beneficial for patients' cognition or psychomotor status. In two studies of patients whose agitation or psychosis had previously responded well to antipsychotic treatment, discontinuation was associated with an increased risk of relapse or shorter time to relapse. Two studies suggested that patients with severe neuropsychiatric symptoms at baseline could benefit from continuing their antipsychotic medication.
Greater exposure to pathogens associated with stroke risk and atherosclerosis may correlate with poorer cognitive performance, according to research published in the March 26 Neurology. Investigators tested for various pathogens (eg, Chlamydia pneumonia and Helicobacter pylori) in 1,625 participants in the Northern Manhattan Study. The researchers assessed patients' cognitive performance at baseline and at annual follow-up visits. Higher infectious burden index was associated with worse cognition. Each standard deviation in infectious burden correlated with a 0.77-point decline in Mini-Mental State Examination (MMSE) score. Adjustment for risk factors weakened the effect, however. Infectious burden was associated with an MMSE score of 24 or lower. Infectious burden was not associated with cognitive decline over time. Past infections may contribute to cognitive impairment, said the researchers.
Smoking cessation was associated with a decreased risk of cardiovascular disease events, and subsequent weight gain did not modify this association, researchers reported in the March 13 JAMA. Investigators analyzed data collected from 1984 through 2011 in the Framingham Offspring Study. Participants' self-reported smoking status was recorded during four-year examinations. Median four-year weight gain was 2.7 kg for recent smoking quitters without diabetes, 3.6 kg for recent quitters with diabetes, and 0.9 kg for long-term quitters. After adjustment for cardiovascular risk factors, compared with smokers, recent smoking quitters had a hazard ratio for cardiovascular disease of 0.47, and long-term quitters had a hazard ratio of 0.46. The results changed minimally after further adjustment for weight change. Similar point estimates for participants with diabetes did not reach statistical significance.
Women who enter menopause prematurely after bilateral ovariectomy may have a significantly increased risk for cognitive decline and dementia, according to a study published online ahead of print March 9 in Brain. The investigators studied rats 10 weeks after they had undergone bilateral ovariectomy and found that long-term estrogen deprivation dramatically increased the hippocampal CA3 region's sensitivity to ischemic stress, which correlated with a worse cognitive outcome. Long-term ovariectomized rats had robust hyperinduction of Alzheimer's disease-related proteins in the CA3 region. Following ischemic stress, amyloid-precursor protein processing switched from nonamyloidogenic to amyloidogenic. Replacement of 17β-estradiol at the end of the estrogen-deprivation period could not prevent CA3 hypersensitivity and amyloidogenesis, but if 17β-estradiol was initiated at ovariectomy and maintained throughout the estrogen deprivation period, it completely prevented these events.
Senior Associate Editor
Greater dietary fiber intake is significantly associated with a lower risk of first stroke, according to a study published online ahead of print March 28 in Stroke. Investigators searched several electronic databases for healthy participant studies published between January 1990 and May 2012 that reported fiber intake and incidence of first hemorrhagic or ischemic stroke. The group identified eight cohort studies from the United States, Europe, Australia, and Japan that met their inclusion criteria. Total dietary fiber intake was inversely associated with risk of hemorrhagic plus ischemic stroke. The researchers found evidence of heterogeneity between the studies. Soluble fiber intake of 4 g/day was not associated with stroke risk reduction, and the investigators found evidence of low heterogeneity on this point between the studies.
In women who have episodic migraine, the ratio of high molecular weight to low molecular weight ictal adiponectin (ADP) may be associated with migraine severity and predict acute treatment response, according to a study published in the March Headache. Investigators collected peripheral blood specimens from women with episodic migraine before and after acute abortive treatment with sumatriptan and naproxen sodium or placebo. In all participants, increases in the ratio of high molecular weight to low molecular weight ADP were associated with increases in pain severity. For every 0.25-μg/mL increase in low molecular weight ADP, pain severity decreased by 0.20. In treatment responders, total ADP levels were reduced at 30, 60, and 120 minutes after treatment, compared with onset.
The FDA has approved Tecfidera (dimethyl fumarate) capsules to treat adults with relapsing forms of multiple sclerosis (MS). In two clinical trials, patients with MS who took dimethyl fumarate had fewer relapses compared with people who received placebo. In one of the trials, patients who took dimethyl fumarate experienced a worsening of disability less often than patients who took a placebo. Dimethyl fumarate may decrease a person's white blood cell count, but the drug was not associated with a significant increase in infections in clinical trials. Before starting treatment, and annually thereafter, the FDA recommends that a patient's white blood cell count be assessed by a health care provider. Flushing and stomach problems were the most common adverse reactions reported. Tecfidera is manufactured by Biogen Idec (Weston, Massachusetts).
Mild cognitive impairment (MCI) at the time of Parkinson's disease diagnosis may predict a highly increased risk for early dementia, according to a study published online ahead of print March 25 in JAMA Neurology. Researchers examined data for a population-based cohort of 182 patients with incident Parkinson's disease who were monitored for three years. Significantly more patients with MCI than without MCI at baseline (27.0% versus 0.7%) progressed to dementia during follow-up. Mild cognitive impairment at the one-year visit was associated with a similar progression rate to dementia (ie, 27.8%) and reversion rate to normal cognition (ie, 19.4%). Among the 22 patients with persistent MCI at baseline and the one-year visit, 10 developed dementia and two reverted to normal cognition by the end of the study.
Higher consumption of green tea and coffee may reduce the risk of cardiovascular disease and stroke, according to a study published online ahead of print March 14 in Stroke. Investigators studied 82,369 Japanese persons between ages 45 and 74 without cardiovascular disease or cancer. Green tea and coffee consumption was assessed by a self-administered questionnaire at baseline. Compared with seldom drinking green tea, the multivariable-adjusted hazard ratios of all strokes were 0.86 and 0.80 in individuals who drank two to three and four or more cups of green tea per day, respectively. Compared with seldom drinking coffee, the multivariable-adjusted hazard ratios of all strokes were 0.89, 0.80, and 0.81 for individuals who drank coffee three to six times per week, once daily, and twice or more daily, respectively.
Updated Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injuries recommend against the use of steroids, including methylprednisolone, in acute spinal cord injury in the first 24 to 48 hours after injury. The use of steroids previously was recommended for this indication with consideration of the risk–reward profile, as evaluated by the physician. In the first new treatment guidelines in a decade, which were issued by the Joint Section on Disorders of the Spine and Peripheral Nerves of the Congress of Neurological Surgeons and the American Association of Neurological Surgeons, the standard has been revised based on the lack of medical evidence supporting the benefits of these drugs in the clinical setting. The report cites strong evidence that "high-dose steroids are associated with harmful side effects, including death."
Abnormalities in cortical surface area may indicate an individual's predisposition to developing migraine, and abnormalities in cortical thickness may result from migraine-related processes, according to research published online ahead of print March 26 in Radiology. Investigators took T2-weighted and three-dimensional T1-weighted MRIs of the brain for 63 migraineurs and 18 controls. They estimated cortical thickness and cortical surface area. Compared with control subjects, patients with migraine had reduced cortical thickness and surface area in pain-processing regions. These reductions were greater in regions involved in executive functions and visual-motion processing. Cortical thickness and cortical surface area abnormalities had minimal areas of overlap. Cortical thickness and surface area abnormalities were related to aura and white matter hyperintensities, but not to disease duration and attack frequency.
Primary stroke centers are more likely to administer t-PA than noncertified hospitals, according to research published online ahead of print March 26 in the Journal of the American Heart Association. Investigators analyzed data obtained from the Nationwide Inpatient Sample between 2004 and 2009 for patients age 18 or older with a primary diagnosis of acute ischemic stroke. IV t-PA was administered to 3.1% of patients overall. The drug was given to 2.2% of patients at noncertified hospitals and to 6.7% of patients at primary stroke centers. Between 2004 and 2009, t-PA administration increased from 1.4% to 3.3% of patients at noncertified hospitals and from 6.0% to 7.6% of patients at primary stroke centers. In a multivariable model, evaluation at a primary stroke center was significantly associated with t-PA use.
Control and prevention of risk factors such as hypertension earlier in life may limit or delay neuropathologic brain changes such as Alzheimer's disease with aging, researchers reported in a study published online ahead of print March 18 in JAMA Neurology. The investigators studied 118 cognitively normal adults ages 47 to 89. Participants were classified as having hypertension if they reported a medical diagnosis of hypertension or if blood pressure exceeded 140 mm Hg systolic/90 mm Hg diastolic on seven occasions. Participants underwent Ab PET imaging with radiotracer fluorine 18–labeled florbetapir, were genotyped for apolipoprotein E, and were classified as ε4+ or ε4−. Subjects with hypertension and at least one ε4 allele had significantly more amyloid burden than those with one or no risk factors.
Physicians can discontinue chronic antipsychotic medication for many elderly adults with Alzheimer's dementia and neuropsychiatric symptoms without causing detrimental effects on their behavior, according to a review published online March 28 in the Cochrane Database of Systematic Reviews. Investigators examined data from nine randomized controlled trials that compared antipsychotic withdrawal strategies with continuation of antipsychotics in patients with dementia. Although neurologists have concerns about the potential adverse events of antipsychotics, it is not clear whether withdrawal is beneficial for patients' cognition or psychomotor status. In two studies of patients whose agitation or psychosis had previously responded well to antipsychotic treatment, discontinuation was associated with an increased risk of relapse or shorter time to relapse. Two studies suggested that patients with severe neuropsychiatric symptoms at baseline could benefit from continuing their antipsychotic medication.
Greater exposure to pathogens associated with stroke risk and atherosclerosis may correlate with poorer cognitive performance, according to research published in the March 26 Neurology. Investigators tested for various pathogens (eg, Chlamydia pneumonia and Helicobacter pylori) in 1,625 participants in the Northern Manhattan Study. The researchers assessed patients' cognitive performance at baseline and at annual follow-up visits. Higher infectious burden index was associated with worse cognition. Each standard deviation in infectious burden correlated with a 0.77-point decline in Mini-Mental State Examination (MMSE) score. Adjustment for risk factors weakened the effect, however. Infectious burden was associated with an MMSE score of 24 or lower. Infectious burden was not associated with cognitive decline over time. Past infections may contribute to cognitive impairment, said the researchers.
Smoking cessation was associated with a decreased risk of cardiovascular disease events, and subsequent weight gain did not modify this association, researchers reported in the March 13 JAMA. Investigators analyzed data collected from 1984 through 2011 in the Framingham Offspring Study. Participants' self-reported smoking status was recorded during four-year examinations. Median four-year weight gain was 2.7 kg for recent smoking quitters without diabetes, 3.6 kg for recent quitters with diabetes, and 0.9 kg for long-term quitters. After adjustment for cardiovascular risk factors, compared with smokers, recent smoking quitters had a hazard ratio for cardiovascular disease of 0.47, and long-term quitters had a hazard ratio of 0.46. The results changed minimally after further adjustment for weight change. Similar point estimates for participants with diabetes did not reach statistical significance.
Women who enter menopause prematurely after bilateral ovariectomy may have a significantly increased risk for cognitive decline and dementia, according to a study published online ahead of print March 9 in Brain. The investigators studied rats 10 weeks after they had undergone bilateral ovariectomy and found that long-term estrogen deprivation dramatically increased the hippocampal CA3 region's sensitivity to ischemic stress, which correlated with a worse cognitive outcome. Long-term ovariectomized rats had robust hyperinduction of Alzheimer's disease-related proteins in the CA3 region. Following ischemic stress, amyloid-precursor protein processing switched from nonamyloidogenic to amyloidogenic. Replacement of 17β-estradiol at the end of the estrogen-deprivation period could not prevent CA3 hypersensitivity and amyloidogenesis, but if 17β-estradiol was initiated at ovariectomy and maintained throughout the estrogen deprivation period, it completely prevented these events.
Senior Associate Editor
Greater dietary fiber intake is significantly associated with a lower risk of first stroke, according to a study published online ahead of print March 28 in Stroke. Investigators searched several electronic databases for healthy participant studies published between January 1990 and May 2012 that reported fiber intake and incidence of first hemorrhagic or ischemic stroke. The group identified eight cohort studies from the United States, Europe, Australia, and Japan that met their inclusion criteria. Total dietary fiber intake was inversely associated with risk of hemorrhagic plus ischemic stroke. The researchers found evidence of heterogeneity between the studies. Soluble fiber intake of 4 g/day was not associated with stroke risk reduction, and the investigators found evidence of low heterogeneity on this point between the studies.
In women who have episodic migraine, the ratio of high molecular weight to low molecular weight ictal adiponectin (ADP) may be associated with migraine severity and predict acute treatment response, according to a study published in the March Headache. Investigators collected peripheral blood specimens from women with episodic migraine before and after acute abortive treatment with sumatriptan and naproxen sodium or placebo. In all participants, increases in the ratio of high molecular weight to low molecular weight ADP were associated with increases in pain severity. For every 0.25-μg/mL increase in low molecular weight ADP, pain severity decreased by 0.20. In treatment responders, total ADP levels were reduced at 30, 60, and 120 minutes after treatment, compared with onset.
The FDA has approved Tecfidera (dimethyl fumarate) capsules to treat adults with relapsing forms of multiple sclerosis (MS). In two clinical trials, patients with MS who took dimethyl fumarate had fewer relapses compared with people who received placebo. In one of the trials, patients who took dimethyl fumarate experienced a worsening of disability less often than patients who took a placebo. Dimethyl fumarate may decrease a person's white blood cell count, but the drug was not associated with a significant increase in infections in clinical trials. Before starting treatment, and annually thereafter, the FDA recommends that a patient's white blood cell count be assessed by a health care provider. Flushing and stomach problems were the most common adverse reactions reported. Tecfidera is manufactured by Biogen Idec (Weston, Massachusetts).
Mild cognitive impairment (MCI) at the time of Parkinson's disease diagnosis may predict a highly increased risk for early dementia, according to a study published online ahead of print March 25 in JAMA Neurology. Researchers examined data for a population-based cohort of 182 patients with incident Parkinson's disease who were monitored for three years. Significantly more patients with MCI than without MCI at baseline (27.0% versus 0.7%) progressed to dementia during follow-up. Mild cognitive impairment at the one-year visit was associated with a similar progression rate to dementia (ie, 27.8%) and reversion rate to normal cognition (ie, 19.4%). Among the 22 patients with persistent MCI at baseline and the one-year visit, 10 developed dementia and two reverted to normal cognition by the end of the study.
Higher consumption of green tea and coffee may reduce the risk of cardiovascular disease and stroke, according to a study published online ahead of print March 14 in Stroke. Investigators studied 82,369 Japanese persons between ages 45 and 74 without cardiovascular disease or cancer. Green tea and coffee consumption was assessed by a self-administered questionnaire at baseline. Compared with seldom drinking green tea, the multivariable-adjusted hazard ratios of all strokes were 0.86 and 0.80 in individuals who drank two to three and four or more cups of green tea per day, respectively. Compared with seldom drinking coffee, the multivariable-adjusted hazard ratios of all strokes were 0.89, 0.80, and 0.81 for individuals who drank coffee three to six times per week, once daily, and twice or more daily, respectively.
Updated Guidelines for the Management of Acute Cervical Spine and Spinal Cord Injuries recommend against the use of steroids, including methylprednisolone, in acute spinal cord injury in the first 24 to 48 hours after injury. The use of steroids previously was recommended for this indication with consideration of the risk–reward profile, as evaluated by the physician. In the first new treatment guidelines in a decade, which were issued by the Joint Section on Disorders of the Spine and Peripheral Nerves of the Congress of Neurological Surgeons and the American Association of Neurological Surgeons, the standard has been revised based on the lack of medical evidence supporting the benefits of these drugs in the clinical setting. The report cites strong evidence that "high-dose steroids are associated with harmful side effects, including death."
Abnormalities in cortical surface area may indicate an individual's predisposition to developing migraine, and abnormalities in cortical thickness may result from migraine-related processes, according to research published online ahead of print March 26 in Radiology. Investigators took T2-weighted and three-dimensional T1-weighted MRIs of the brain for 63 migraineurs and 18 controls. They estimated cortical thickness and cortical surface area. Compared with control subjects, patients with migraine had reduced cortical thickness and surface area in pain-processing regions. These reductions were greater in regions involved in executive functions and visual-motion processing. Cortical thickness and cortical surface area abnormalities had minimal areas of overlap. Cortical thickness and surface area abnormalities were related to aura and white matter hyperintensities, but not to disease duration and attack frequency.
Primary stroke centers are more likely to administer t-PA than noncertified hospitals, according to research published online ahead of print March 26 in the Journal of the American Heart Association. Investigators analyzed data obtained from the Nationwide Inpatient Sample between 2004 and 2009 for patients age 18 or older with a primary diagnosis of acute ischemic stroke. IV t-PA was administered to 3.1% of patients overall. The drug was given to 2.2% of patients at noncertified hospitals and to 6.7% of patients at primary stroke centers. Between 2004 and 2009, t-PA administration increased from 1.4% to 3.3% of patients at noncertified hospitals and from 6.0% to 7.6% of patients at primary stroke centers. In a multivariable model, evaluation at a primary stroke center was significantly associated with t-PA use.
Control and prevention of risk factors such as hypertension earlier in life may limit or delay neuropathologic brain changes such as Alzheimer's disease with aging, researchers reported in a study published online ahead of print March 18 in JAMA Neurology. The investigators studied 118 cognitively normal adults ages 47 to 89. Participants were classified as having hypertension if they reported a medical diagnosis of hypertension or if blood pressure exceeded 140 mm Hg systolic/90 mm Hg diastolic on seven occasions. Participants underwent Ab PET imaging with radiotracer fluorine 18–labeled florbetapir, were genotyped for apolipoprotein E, and were classified as ε4+ or ε4−. Subjects with hypertension and at least one ε4 allele had significantly more amyloid burden than those with one or no risk factors.
Physicians can discontinue chronic antipsychotic medication for many elderly adults with Alzheimer's dementia and neuropsychiatric symptoms without causing detrimental effects on their behavior, according to a review published online March 28 in the Cochrane Database of Systematic Reviews. Investigators examined data from nine randomized controlled trials that compared antipsychotic withdrawal strategies with continuation of antipsychotics in patients with dementia. Although neurologists have concerns about the potential adverse events of antipsychotics, it is not clear whether withdrawal is beneficial for patients' cognition or psychomotor status. In two studies of patients whose agitation or psychosis had previously responded well to antipsychotic treatment, discontinuation was associated with an increased risk of relapse or shorter time to relapse. Two studies suggested that patients with severe neuropsychiatric symptoms at baseline could benefit from continuing their antipsychotic medication.
Greater exposure to pathogens associated with stroke risk and atherosclerosis may correlate with poorer cognitive performance, according to research published in the March 26 Neurology. Investigators tested for various pathogens (eg, Chlamydia pneumonia and Helicobacter pylori) in 1,625 participants in the Northern Manhattan Study. The researchers assessed patients' cognitive performance at baseline and at annual follow-up visits. Higher infectious burden index was associated with worse cognition. Each standard deviation in infectious burden correlated with a 0.77-point decline in Mini-Mental State Examination (MMSE) score. Adjustment for risk factors weakened the effect, however. Infectious burden was associated with an MMSE score of 24 or lower. Infectious burden was not associated with cognitive decline over time. Past infections may contribute to cognitive impairment, said the researchers.
Smoking cessation was associated with a decreased risk of cardiovascular disease events, and subsequent weight gain did not modify this association, researchers reported in the March 13 JAMA. Investigators analyzed data collected from 1984 through 2011 in the Framingham Offspring Study. Participants' self-reported smoking status was recorded during four-year examinations. Median four-year weight gain was 2.7 kg for recent smoking quitters without diabetes, 3.6 kg for recent quitters with diabetes, and 0.9 kg for long-term quitters. After adjustment for cardiovascular risk factors, compared with smokers, recent smoking quitters had a hazard ratio for cardiovascular disease of 0.47, and long-term quitters had a hazard ratio of 0.46. The results changed minimally after further adjustment for weight change. Similar point estimates for participants with diabetes did not reach statistical significance.
Women who enter menopause prematurely after bilateral ovariectomy may have a significantly increased risk for cognitive decline and dementia, according to a study published online ahead of print March 9 in Brain. The investigators studied rats 10 weeks after they had undergone bilateral ovariectomy and found that long-term estrogen deprivation dramatically increased the hippocampal CA3 region's sensitivity to ischemic stress, which correlated with a worse cognitive outcome. Long-term ovariectomized rats had robust hyperinduction of Alzheimer's disease-related proteins in the CA3 region. Following ischemic stress, amyloid-precursor protein processing switched from nonamyloidogenic to amyloidogenic. Replacement of 17β-estradiol at the end of the estrogen-deprivation period could not prevent CA3 hypersensitivity and amyloidogenesis, but if 17β-estradiol was initiated at ovariectomy and maintained throughout the estrogen deprivation period, it completely prevented these events.
Senior Associate Editor
Man, 57, With Dyspnea After Chiropractic Manipulation
A 57-year-old man presented to the emergency department (ED) with a two-day history of worsening shortness of breath, light-headedness, and back pain. The patient, who had a history of ankylosing spondylitis, had been receiving weekly therapy from a chiropractor for about 10 years. One week before presenting to the ED, he had begun to undergo daily manipulations under anesthesia (MUA)—an aggressive chiropractic procedure that is administered while the patient is under monitored, procedural sedation. After the second day of treatment, the patient began to experience worsening back pain and progressive light-headedness and shortness of breath.
At a follow-up visit with his chiropractor, he was found to have decreased O2 saturation and was directed to go to the hospital for evaluation. On arrival at the ED, the patient was awake and alert. He had intact motor strength in all extremities, no sensory abnormalities, intact symmetric reflexes, and no bladder or bowel dysfunction, with a negative Babinski sign. His O2 saturation was 92% on 5 L of oxygen. An absence of breath sounds was noted on the left side.
Chest x-ray (see Figure 1) was performed, which demonstrated complete opacification of the left hemithorax, consistent with a large pleural effusion or hemothorax. CT scan of the thoracic spine showed diffuse ankylosis. A complex oblique coronal and transversely oriented fracture with 7 mm of displacement was identified, beginning at the right anterior inferior lateral margin of the T8 vertebral body and extending centrally and inferiorly to the left and right into the T9 vertebral body. The fracture continued through the right T9-10 neural foramen and what was probably the right fused T9-10 facet joint. The fracture exited through the left superior and lateral margin of the T10 vertebral body and the left T10-11 neural foramen (see Figures 2, 3, and 4).
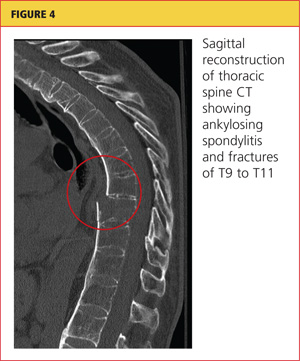
A chest tube was inserted in the ED, and 1,600 mL of old blood was immediately drained. The patient was admitted to the ICU on the trauma service. He was taken to surgery for open reduction and internal fixation of his unstable thoracic spine fracture on day 3 of hospitalization, after his pulmonary condition stabilized. Pedicle screws were placed from T7 through T12 during the spinal fusion. Good reduction of the fracture was observed following the spine surgery (see Figures 5 and 6). At the conclusion of surgery, an epidural catheter was placed in the thoracic spine to administer pain control.
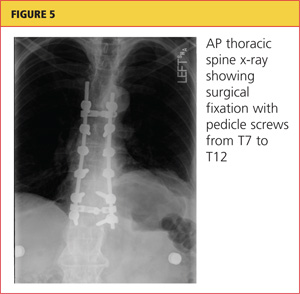
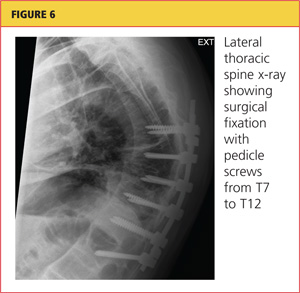
After the spine portion of the procedure, the patient was repositioned and underwent video-assisted thoracoscopic surgery of the left hemithorax for evacuation of retained hemothorax. The patient tolerated the procedure well and was taken to the ICU for recovery.
On postoperative day 2, the patient complained of chest pain and experienced hypoxemia with activity. CT angiography of the chest demonstrated bilateral segmental and subsegmental pulmonary emboli. The epidural catheter was discontinued. Six hours later, a heparin drip was started, and the patient was transitioned to therapeutic enoxaparin and warfarin. When methicillin-sensitive Staphylococcus aureus (MSSA) was detected in his hemothorax fluid, he was treated with a course of nafcillin.
The patient was discharged to home on postoperative day 12. He has remained neurologically intact and has returned to his former work activities. He is not taking narcotic pain medications.
Discussion
Chiropractic care is a popular alternative health care modality in the United States. Researchers for the 2007 National Health Interview Study1 reported an annual use of chiropractic manipulation of 8.6%, while the Medical Expenditure Panel Survey2 data yielded an estimate of 12.6 million adults using chiropractic manipulation in 2006—translating to a prevalence of 5.6%. Despite the popularity of chiropractic medicine, few well-designed studies have been conducted to support its use.3,4 Because of its designation as an alternative therapy, however, chiropractic manipulation has not been subjected to rigorous efficacy and safety evaluations.5
Given the inconsistency of the evidence to support chiropractic manipulation, the practice's safety profile is a concern. The risks associated with spinal manipulation are generally described in case reports and small series. Most serious adverse events described in the literature are cerebrovascular in nature and tend to occur after cervical manipulation.6,7 Fractures after spine manipulation are exceedingly rare, and published literature on this topic consists of a few isolated case reports, with all fractures occurring in the cervical spine in patients with an underlying pathologic condition.8-10
In 2009, Gouveia et al5 reviewed the published literature regarding all adverse events resulting from chiropractic manipulation. The authors found one randomized controlled trial, two case-control studies, six prospective studies, 12 surveys, three retrospective studies, and 100 case reports. The spectrum of complications identified ranged from benign and transient, such as local discomfort, to far more serious: stroke, myelopathy, radiculopathy, subdural hematoma, spinal fluid leakage, cauda equina syndrome, herniated disc, diaphragmatic palsy, and vertebral fractures. The authors were unable to perform a true meta-analysis because of the heterogeneity of the data, but they concluded that complications associated with chiropractic procedures are "frequent."5
Manipulations Under Anesthesia
MUA is a procedure that combines chiropractic adjustments and manipulations with general anesthesia or procedural sedation.11 The theory behind this strategy is that the anesthesia or sedation reduces pain and muscle spasm that may hinder the manipulation, allowing the practitioner to more effectively break up joint adhesions and reduce segmental dysfunction than if the patient had not undergone anesthesia.11
MUA is generally indicated in patients who have not responded to a 4- to 8-week trial of traditional manipulation therapy.12 It is also considered in patients who have "painful and restricting muscular guarding [that] interferes with the performance of spinal adjustments, mobilizations, and soft tissue release techniques."13
In the chiropractic literature, between 3% and 10% of patients are estimated to be candidates for MUA.12,14 It is not completely clear, however, what diagnoses are most likely to be treated successfully with this technique. Contraindications to MUA are generally the same as those for manipulation in conscious patients. A published list of contraindications from the Committee for Manipulation under Anesthesia (2003)15 included malignancy with bony metastasis, tuberculosis of the bone, recent fracture, acute arthritis, acute gout, diabetic neuropathy, syphilitic articular lesions, excessive spinal osteoporosis, disk fragmentation, direct nerve root impingement, and evidence of cord or caudal compression by tumor, ankylosis, or other space-occupying lesions.
MUA generally begins with deep procedural sedation, managed by an anesthesiologist. Once an adequate level of sedation is achieved, the manipulations are performed. Both high- and low-velocity thrusts are used, but it is recommended that the force exerted should be much less, and the manipulations performed with more caution, than in patients who are not anesthetized.12
For the thoracic spine, the patient is manipulated in the supine position with the arms crossed over the chest. The practitioner places one hand in a fist under the spine with the other hand on the patient's crossed arms, then delivers an anterior-to-posterior thrust. This is repeated until all affected segments have been treated.11,12
Literature to support the use of MUA for various indications is largely anecdotal. The largest published series13 is of 177 patients with chronic spinal pain who each underwent three MUA sessions followed by four to six weeks of traditional manipulations. The authors found that pain, as measured by visual analog scale, was reduced by 62% in patients with cervical spine pain, and by 60% in patients with lumbar pain. No adverse events were reported in the study.
Kohlbeck and Haldeman12 reviewed the reported complications of MUA across all published literature. They found that in 17 published papers, the overall complication rate was 0.7%, mainly represented by transitory increased pain. No spinal fractures were reported.
This case demonstrates a rare but serious complication of chiropractic MUA. It is unclear exactly what mechanism of injury led to an unstable thoracic spine fracture with massive hemothorax, and the precise cause will probably never be known. The clinicians who treated the case patient find it curious that the reported rate of adverse events following this procedure is so low, but they suspect an element of reporting bias in the chiropractic literature.
Conclusion
Iatrogenic injury after chiropractic manipulation is uncommon, but it can be devastating. Few serious complications of chiropractic MUA have been reported, but the literature is lacking in well-designed research studies. Despite the dearth of clinical trials to support its safety and efficacy, use of MUA has continued in the chiropractic community. This case demonstrates that serious adverse outcomes can occur, and more rigorous studies are needed to delineate the true benefits and risks of this set of chiropractic procedures.
References
1. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1-9.
2. Davis MA, Sirovich BE, Weeks WB. Utilization and expenditures on chiropractic care in the United States from 1997 to 2006. Health Serv Res. 2009;45:748-761.
3. Canadian Chiropractic Association; Canadian Federation of Chiropractic Regulatory Boards; Clinical Practice Guidelines Development Initiative; Guidelines Development Committee. Chiropractic clinical practice guideline: evidence-based treatment of adult neck pain not due to whiplash. J Can Chiropr Assoc. 2005;49:417-421.
4.
Hurwitz EL, Aker PD, Adams AH, et al. Manipulation and mobilization of the cervical spine: a systematic review of the literature. Spine (Phila Pa 1976). 1996;21:1746-1760.
5.Gouveia LO, Castanho P, Ferreira JJ. Safety of chiropractic interventions: a systematic review. Spine (Phila Pa 1976). 2009;34:E405-E413.
6. Di Fabio RP. Manipulation of the cervical spine: risks and benefits. Phys Ther. 1999;79:50-65.
7. Nadareishvili Z, Norris JW. Stroke from traumatic arterial dissection. Lancet. 1999;354:159-160.
8. Austin RT. Pathological vertebral fractures after spinal manipulation. Br Med J (Clin Res Ed). 1985;291:1114-1115.
9. Ea HK, Weber AJ, Yon F, Lioté F. Osteoporotic fracture of the dens revealed by cervical manipulation. Joint Bone Spine. 2004;71:246-250.
10. Schmitz A, Lutterbey G, von Engelhardt L, et al. Pathological cervical fracture after spinal manipulation in a pregnant patient. J Manipulative Physiol Ther. 2005;28:633-636.
11. Cremata E, Collins S, Clauson W, et al. Manipulation under anesthesia: a report of four cases. J Manipulative Physiol Ther. 2005;28:526-533.
12. Kohlbeck FJ, Haldeman S. Medication-assisted spinal manipulation. Spine J. 2002;2:288-302.
13. West DT, Mathews RS, Miller MR, Kent GM. Effective management of spinal pain in one hundred seventy-seven patients evaluated for manipulation under anesthesia. J Manipulative Physiol Ther. 1999;22:299-308.
14. Morey LW Jr. Osteopathic manipulation under general anesthesia. J Am Osteopath Assoc. 1973;73:116-127.
15. Tain L, Gunderson C, Cremata E, et al; Committee for Manipulation Under Anesthesia. Recommendations to the Industrial Medical Council Work Group of California for manipulation under anesthesia use for injured workers. Sacramento, CA: Industrial Medical Council; 2003.
A 57-year-old man presented to the emergency department (ED) with a two-day history of worsening shortness of breath, light-headedness, and back pain. The patient, who had a history of ankylosing spondylitis, had been receiving weekly therapy from a chiropractor for about 10 years. One week before presenting to the ED, he had begun to undergo daily manipulations under anesthesia (MUA)—an aggressive chiropractic procedure that is administered while the patient is under monitored, procedural sedation. After the second day of treatment, the patient began to experience worsening back pain and progressive light-headedness and shortness of breath.
At a follow-up visit with his chiropractor, he was found to have decreased O2 saturation and was directed to go to the hospital for evaluation. On arrival at the ED, the patient was awake and alert. He had intact motor strength in all extremities, no sensory abnormalities, intact symmetric reflexes, and no bladder or bowel dysfunction, with a negative Babinski sign. His O2 saturation was 92% on 5 L of oxygen. An absence of breath sounds was noted on the left side.
Chest x-ray (see Figure 1) was performed, which demonstrated complete opacification of the left hemithorax, consistent with a large pleural effusion or hemothorax. CT scan of the thoracic spine showed diffuse ankylosis. A complex oblique coronal and transversely oriented fracture with 7 mm of displacement was identified, beginning at the right anterior inferior lateral margin of the T8 vertebral body and extending centrally and inferiorly to the left and right into the T9 vertebral body. The fracture continued through the right T9-10 neural foramen and what was probably the right fused T9-10 facet joint. The fracture exited through the left superior and lateral margin of the T10 vertebral body and the left T10-11 neural foramen (see Figures 2, 3, and 4).




A chest tube was inserted in the ED, and 1,600 mL of old blood was immediately drained. The patient was admitted to the ICU on the trauma service. He was taken to surgery for open reduction and internal fixation of his unstable thoracic spine fracture on day 3 of hospitalization, after his pulmonary condition stabilized. Pedicle screws were placed from T7 through T12 during the spinal fusion. Good reduction of the fracture was observed following the spine surgery (see Figures 5 and 6). At the conclusion of surgery, an epidural catheter was placed in the thoracic spine to administer pain control.


After the spine portion of the procedure, the patient was repositioned and underwent video-assisted thoracoscopic surgery of the left hemithorax for evacuation of retained hemothorax. The patient tolerated the procedure well and was taken to the ICU for recovery.
On postoperative day 2, the patient complained of chest pain and experienced hypoxemia with activity. CT angiography of the chest demonstrated bilateral segmental and subsegmental pulmonary emboli. The epidural catheter was discontinued. Six hours later, a heparin drip was started, and the patient was transitioned to therapeutic enoxaparin and warfarin. When methicillin-sensitive Staphylococcus aureus (MSSA) was detected in his hemothorax fluid, he was treated with a course of nafcillin.
The patient was discharged to home on postoperative day 12. He has remained neurologically intact and has returned to his former work activities. He is not taking narcotic pain medications.
Discussion
Chiropractic care is a popular alternative health care modality in the United States. Researchers for the 2007 National Health Interview Study1 reported an annual use of chiropractic manipulation of 8.6%, while the Medical Expenditure Panel Survey2 data yielded an estimate of 12.6 million adults using chiropractic manipulation in 2006—translating to a prevalence of 5.6%. Despite the popularity of chiropractic medicine, few well-designed studies have been conducted to support its use.3,4 Because of its designation as an alternative therapy, however, chiropractic manipulation has not been subjected to rigorous efficacy and safety evaluations.5
Given the inconsistency of the evidence to support chiropractic manipulation, the practice's safety profile is a concern. The risks associated with spinal manipulation are generally described in case reports and small series. Most serious adverse events described in the literature are cerebrovascular in nature and tend to occur after cervical manipulation.6,7 Fractures after spine manipulation are exceedingly rare, and published literature on this topic consists of a few isolated case reports, with all fractures occurring in the cervical spine in patients with an underlying pathologic condition.8-10
In 2009, Gouveia et al5 reviewed the published literature regarding all adverse events resulting from chiropractic manipulation. The authors found one randomized controlled trial, two case-control studies, six prospective studies, 12 surveys, three retrospective studies, and 100 case reports. The spectrum of complications identified ranged from benign and transient, such as local discomfort, to far more serious: stroke, myelopathy, radiculopathy, subdural hematoma, spinal fluid leakage, cauda equina syndrome, herniated disc, diaphragmatic palsy, and vertebral fractures. The authors were unable to perform a true meta-analysis because of the heterogeneity of the data, but they concluded that complications associated with chiropractic procedures are "frequent."5
Manipulations Under Anesthesia
MUA is a procedure that combines chiropractic adjustments and manipulations with general anesthesia or procedural sedation.11 The theory behind this strategy is that the anesthesia or sedation reduces pain and muscle spasm that may hinder the manipulation, allowing the practitioner to more effectively break up joint adhesions and reduce segmental dysfunction than if the patient had not undergone anesthesia.11
MUA is generally indicated in patients who have not responded to a 4- to 8-week trial of traditional manipulation therapy.12 It is also considered in patients who have "painful and restricting muscular guarding [that] interferes with the performance of spinal adjustments, mobilizations, and soft tissue release techniques."13
In the chiropractic literature, between 3% and 10% of patients are estimated to be candidates for MUA.12,14 It is not completely clear, however, what diagnoses are most likely to be treated successfully with this technique. Contraindications to MUA are generally the same as those for manipulation in conscious patients. A published list of contraindications from the Committee for Manipulation under Anesthesia (2003)15 included malignancy with bony metastasis, tuberculosis of the bone, recent fracture, acute arthritis, acute gout, diabetic neuropathy, syphilitic articular lesions, excessive spinal osteoporosis, disk fragmentation, direct nerve root impingement, and evidence of cord or caudal compression by tumor, ankylosis, or other space-occupying lesions.
MUA generally begins with deep procedural sedation, managed by an anesthesiologist. Once an adequate level of sedation is achieved, the manipulations are performed. Both high- and low-velocity thrusts are used, but it is recommended that the force exerted should be much less, and the manipulations performed with more caution, than in patients who are not anesthetized.12
For the thoracic spine, the patient is manipulated in the supine position with the arms crossed over the chest. The practitioner places one hand in a fist under the spine with the other hand on the patient's crossed arms, then delivers an anterior-to-posterior thrust. This is repeated until all affected segments have been treated.11,12
Literature to support the use of MUA for various indications is largely anecdotal. The largest published series13 is of 177 patients with chronic spinal pain who each underwent three MUA sessions followed by four to six weeks of traditional manipulations. The authors found that pain, as measured by visual analog scale, was reduced by 62% in patients with cervical spine pain, and by 60% in patients with lumbar pain. No adverse events were reported in the study.
Kohlbeck and Haldeman12 reviewed the reported complications of MUA across all published literature. They found that in 17 published papers, the overall complication rate was 0.7%, mainly represented by transitory increased pain. No spinal fractures were reported.
This case demonstrates a rare but serious complication of chiropractic MUA. It is unclear exactly what mechanism of injury led to an unstable thoracic spine fracture with massive hemothorax, and the precise cause will probably never be known. The clinicians who treated the case patient find it curious that the reported rate of adverse events following this procedure is so low, but they suspect an element of reporting bias in the chiropractic literature.
Conclusion
Iatrogenic injury after chiropractic manipulation is uncommon, but it can be devastating. Few serious complications of chiropractic MUA have been reported, but the literature is lacking in well-designed research studies. Despite the dearth of clinical trials to support its safety and efficacy, use of MUA has continued in the chiropractic community. This case demonstrates that serious adverse outcomes can occur, and more rigorous studies are needed to delineate the true benefits and risks of this set of chiropractic procedures.
References
1. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1-9.
2. Davis MA, Sirovich BE, Weeks WB. Utilization and expenditures on chiropractic care in the United States from 1997 to 2006. Health Serv Res. 2009;45:748-761.
3. Canadian Chiropractic Association; Canadian Federation of Chiropractic Regulatory Boards; Clinical Practice Guidelines Development Initiative; Guidelines Development Committee. Chiropractic clinical practice guideline: evidence-based treatment of adult neck pain not due to whiplash. J Can Chiropr Assoc. 2005;49:417-421.
4.
Hurwitz EL, Aker PD, Adams AH, et al. Manipulation and mobilization of the cervical spine: a systematic review of the literature. Spine (Phila Pa 1976). 1996;21:1746-1760.
5.Gouveia LO, Castanho P, Ferreira JJ. Safety of chiropractic interventions: a systematic review. Spine (Phila Pa 1976). 2009;34:E405-E413.
6. Di Fabio RP. Manipulation of the cervical spine: risks and benefits. Phys Ther. 1999;79:50-65.
7. Nadareishvili Z, Norris JW. Stroke from traumatic arterial dissection. Lancet. 1999;354:159-160.
8. Austin RT. Pathological vertebral fractures after spinal manipulation. Br Med J (Clin Res Ed). 1985;291:1114-1115.
9. Ea HK, Weber AJ, Yon F, Lioté F. Osteoporotic fracture of the dens revealed by cervical manipulation. Joint Bone Spine. 2004;71:246-250.
10. Schmitz A, Lutterbey G, von Engelhardt L, et al. Pathological cervical fracture after spinal manipulation in a pregnant patient. J Manipulative Physiol Ther. 2005;28:633-636.
11. Cremata E, Collins S, Clauson W, et al. Manipulation under anesthesia: a report of four cases. J Manipulative Physiol Ther. 2005;28:526-533.
12. Kohlbeck FJ, Haldeman S. Medication-assisted spinal manipulation. Spine J. 2002;2:288-302.
13. West DT, Mathews RS, Miller MR, Kent GM. Effective management of spinal pain in one hundred seventy-seven patients evaluated for manipulation under anesthesia. J Manipulative Physiol Ther. 1999;22:299-308.
14. Morey LW Jr. Osteopathic manipulation under general anesthesia. J Am Osteopath Assoc. 1973;73:116-127.
15. Tain L, Gunderson C, Cremata E, et al; Committee for Manipulation Under Anesthesia. Recommendations to the Industrial Medical Council Work Group of California for manipulation under anesthesia use for injured workers. Sacramento, CA: Industrial Medical Council; 2003.
A 57-year-old man presented to the emergency department (ED) with a two-day history of worsening shortness of breath, light-headedness, and back pain. The patient, who had a history of ankylosing spondylitis, had been receiving weekly therapy from a chiropractor for about 10 years. One week before presenting to the ED, he had begun to undergo daily manipulations under anesthesia (MUA)—an aggressive chiropractic procedure that is administered while the patient is under monitored, procedural sedation. After the second day of treatment, the patient began to experience worsening back pain and progressive light-headedness and shortness of breath.
At a follow-up visit with his chiropractor, he was found to have decreased O2 saturation and was directed to go to the hospital for evaluation. On arrival at the ED, the patient was awake and alert. He had intact motor strength in all extremities, no sensory abnormalities, intact symmetric reflexes, and no bladder or bowel dysfunction, with a negative Babinski sign. His O2 saturation was 92% on 5 L of oxygen. An absence of breath sounds was noted on the left side.
Chest x-ray (see Figure 1) was performed, which demonstrated complete opacification of the left hemithorax, consistent with a large pleural effusion or hemothorax. CT scan of the thoracic spine showed diffuse ankylosis. A complex oblique coronal and transversely oriented fracture with 7 mm of displacement was identified, beginning at the right anterior inferior lateral margin of the T8 vertebral body and extending centrally and inferiorly to the left and right into the T9 vertebral body. The fracture continued through the right T9-10 neural foramen and what was probably the right fused T9-10 facet joint. The fracture exited through the left superior and lateral margin of the T10 vertebral body and the left T10-11 neural foramen (see Figures 2, 3, and 4).




A chest tube was inserted in the ED, and 1,600 mL of old blood was immediately drained. The patient was admitted to the ICU on the trauma service. He was taken to surgery for open reduction and internal fixation of his unstable thoracic spine fracture on day 3 of hospitalization, after his pulmonary condition stabilized. Pedicle screws were placed from T7 through T12 during the spinal fusion. Good reduction of the fracture was observed following the spine surgery (see Figures 5 and 6). At the conclusion of surgery, an epidural catheter was placed in the thoracic spine to administer pain control.


After the spine portion of the procedure, the patient was repositioned and underwent video-assisted thoracoscopic surgery of the left hemithorax for evacuation of retained hemothorax. The patient tolerated the procedure well and was taken to the ICU for recovery.
On postoperative day 2, the patient complained of chest pain and experienced hypoxemia with activity. CT angiography of the chest demonstrated bilateral segmental and subsegmental pulmonary emboli. The epidural catheter was discontinued. Six hours later, a heparin drip was started, and the patient was transitioned to therapeutic enoxaparin and warfarin. When methicillin-sensitive Staphylococcus aureus (MSSA) was detected in his hemothorax fluid, he was treated with a course of nafcillin.
The patient was discharged to home on postoperative day 12. He has remained neurologically intact and has returned to his former work activities. He is not taking narcotic pain medications.
Discussion
Chiropractic care is a popular alternative health care modality in the United States. Researchers for the 2007 National Health Interview Study1 reported an annual use of chiropractic manipulation of 8.6%, while the Medical Expenditure Panel Survey2 data yielded an estimate of 12.6 million adults using chiropractic manipulation in 2006—translating to a prevalence of 5.6%. Despite the popularity of chiropractic medicine, few well-designed studies have been conducted to support its use.3,4 Because of its designation as an alternative therapy, however, chiropractic manipulation has not been subjected to rigorous efficacy and safety evaluations.5
Given the inconsistency of the evidence to support chiropractic manipulation, the practice's safety profile is a concern. The risks associated with spinal manipulation are generally described in case reports and small series. Most serious adverse events described in the literature are cerebrovascular in nature and tend to occur after cervical manipulation.6,7 Fractures after spine manipulation are exceedingly rare, and published literature on this topic consists of a few isolated case reports, with all fractures occurring in the cervical spine in patients with an underlying pathologic condition.8-10
In 2009, Gouveia et al5 reviewed the published literature regarding all adverse events resulting from chiropractic manipulation. The authors found one randomized controlled trial, two case-control studies, six prospective studies, 12 surveys, three retrospective studies, and 100 case reports. The spectrum of complications identified ranged from benign and transient, such as local discomfort, to far more serious: stroke, myelopathy, radiculopathy, subdural hematoma, spinal fluid leakage, cauda equina syndrome, herniated disc, diaphragmatic palsy, and vertebral fractures. The authors were unable to perform a true meta-analysis because of the heterogeneity of the data, but they concluded that complications associated with chiropractic procedures are "frequent."5
Manipulations Under Anesthesia
MUA is a procedure that combines chiropractic adjustments and manipulations with general anesthesia or procedural sedation.11 The theory behind this strategy is that the anesthesia or sedation reduces pain and muscle spasm that may hinder the manipulation, allowing the practitioner to more effectively break up joint adhesions and reduce segmental dysfunction than if the patient had not undergone anesthesia.11
MUA is generally indicated in patients who have not responded to a 4- to 8-week trial of traditional manipulation therapy.12 It is also considered in patients who have "painful and restricting muscular guarding [that] interferes with the performance of spinal adjustments, mobilizations, and soft tissue release techniques."13
In the chiropractic literature, between 3% and 10% of patients are estimated to be candidates for MUA.12,14 It is not completely clear, however, what diagnoses are most likely to be treated successfully with this technique. Contraindications to MUA are generally the same as those for manipulation in conscious patients. A published list of contraindications from the Committee for Manipulation under Anesthesia (2003)15 included malignancy with bony metastasis, tuberculosis of the bone, recent fracture, acute arthritis, acute gout, diabetic neuropathy, syphilitic articular lesions, excessive spinal osteoporosis, disk fragmentation, direct nerve root impingement, and evidence of cord or caudal compression by tumor, ankylosis, or other space-occupying lesions.
MUA generally begins with deep procedural sedation, managed by an anesthesiologist. Once an adequate level of sedation is achieved, the manipulations are performed. Both high- and low-velocity thrusts are used, but it is recommended that the force exerted should be much less, and the manipulations performed with more caution, than in patients who are not anesthetized.12
For the thoracic spine, the patient is manipulated in the supine position with the arms crossed over the chest. The practitioner places one hand in a fist under the spine with the other hand on the patient's crossed arms, then delivers an anterior-to-posterior thrust. This is repeated until all affected segments have been treated.11,12
Literature to support the use of MUA for various indications is largely anecdotal. The largest published series13 is of 177 patients with chronic spinal pain who each underwent three MUA sessions followed by four to six weeks of traditional manipulations. The authors found that pain, as measured by visual analog scale, was reduced by 62% in patients with cervical spine pain, and by 60% in patients with lumbar pain. No adverse events were reported in the study.
Kohlbeck and Haldeman12 reviewed the reported complications of MUA across all published literature. They found that in 17 published papers, the overall complication rate was 0.7%, mainly represented by transitory increased pain. No spinal fractures were reported.
This case demonstrates a rare but serious complication of chiropractic MUA. It is unclear exactly what mechanism of injury led to an unstable thoracic spine fracture with massive hemothorax, and the precise cause will probably never be known. The clinicians who treated the case patient find it curious that the reported rate of adverse events following this procedure is so low, but they suspect an element of reporting bias in the chiropractic literature.
Conclusion
Iatrogenic injury after chiropractic manipulation is uncommon, but it can be devastating. Few serious complications of chiropractic MUA have been reported, but the literature is lacking in well-designed research studies. Despite the dearth of clinical trials to support its safety and efficacy, use of MUA has continued in the chiropractic community. This case demonstrates that serious adverse outcomes can occur, and more rigorous studies are needed to delineate the true benefits and risks of this set of chiropractic procedures.
References
1. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1-9.
2. Davis MA, Sirovich BE, Weeks WB. Utilization and expenditures on chiropractic care in the United States from 1997 to 2006. Health Serv Res. 2009;45:748-761.
3. Canadian Chiropractic Association; Canadian Federation of Chiropractic Regulatory Boards; Clinical Practice Guidelines Development Initiative; Guidelines Development Committee. Chiropractic clinical practice guideline: evidence-based treatment of adult neck pain not due to whiplash. J Can Chiropr Assoc. 2005;49:417-421.
4.
Hurwitz EL, Aker PD, Adams AH, et al. Manipulation and mobilization of the cervical spine: a systematic review of the literature. Spine (Phila Pa 1976). 1996;21:1746-1760.
5.Gouveia LO, Castanho P, Ferreira JJ. Safety of chiropractic interventions: a systematic review. Spine (Phila Pa 1976). 2009;34:E405-E413.
6. Di Fabio RP. Manipulation of the cervical spine: risks and benefits. Phys Ther. 1999;79:50-65.
7. Nadareishvili Z, Norris JW. Stroke from traumatic arterial dissection. Lancet. 1999;354:159-160.
8. Austin RT. Pathological vertebral fractures after spinal manipulation. Br Med J (Clin Res Ed). 1985;291:1114-1115.
9. Ea HK, Weber AJ, Yon F, Lioté F. Osteoporotic fracture of the dens revealed by cervical manipulation. Joint Bone Spine. 2004;71:246-250.
10. Schmitz A, Lutterbey G, von Engelhardt L, et al. Pathological cervical fracture after spinal manipulation in a pregnant patient. J Manipulative Physiol Ther. 2005;28:633-636.
11. Cremata E, Collins S, Clauson W, et al. Manipulation under anesthesia: a report of four cases. J Manipulative Physiol Ther. 2005;28:526-533.
12. Kohlbeck FJ, Haldeman S. Medication-assisted spinal manipulation. Spine J. 2002;2:288-302.
13. West DT, Mathews RS, Miller MR, Kent GM. Effective management of spinal pain in one hundred seventy-seven patients evaluated for manipulation under anesthesia. J Manipulative Physiol Ther. 1999;22:299-308.
14. Morey LW Jr. Osteopathic manipulation under general anesthesia. J Am Osteopath Assoc. 1973;73:116-127.
15. Tain L, Gunderson C, Cremata E, et al; Committee for Manipulation Under Anesthesia. Recommendations to the Industrial Medical Council Work Group of California for manipulation under anesthesia use for injured workers. Sacramento, CA: Industrial Medical Council; 2003.