User login
FDA Reports Dabigatran Bleeds Don't Exceed Warfarin Bleeds
The Food and Drug Administration is satisfied that dabigatran’s bleeding risk is no greater than that of warfarin and will not change the drug’s label.
The rates of gastrointestinal and intracranial bleeding among patients who have been prescribed the anticoagulant dabigatran "do not appear to be higher" than the rates among patients who have been prescribed warfarin, according to an analysis of insurance claims and administrative data conducted by the agency.
The results of this analysis, conducted in response to postmarketing reports of bleeding among people treated with dabigatran, are "consistent with observations" in the RE-LY trial, the study of 18,000 patients that was the basis of the approval of the anticoagulant for reducing the risk of stroke and blood clots in patients with nonvalvular atrial fibrillation (AF), the FDA said in the MedWatch safety alert, released on Nov. 2. In the RE-LY study, the rates of serious bleeding was similar among those treated with dabigatran and those with warfarin (N. Engl. J. Med. 2009;361:1139-51).
The agency is evaluating different sources of data in its review of this safety issue, which is ongoing. Dabigatran, an orally administered direct thrombin inhibitor, was approved in October 2010 for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation and is marketed as Pradaxa by Boehringer Ingelheim.
The FDA’s analysis found that the rates of bleeding were actually higher among those on warfarin, although the statement does not point this out. The FDA analyzed data from a database of nearly 100 million patients and determined that the combined incidence of intracranial and gastrointestinal hemorrhages per 100,000 days at risk was 1.8-2.6 times higher for new users of warfarin than for new users of dabigatran. When they analyzed the two events separately, they found that the incidence rate of gastrointestinal hemorrhage events per 100,000 days at risk was 1.6-2.2 times higher for new users of warfarin than for new users of dabigatran. The incidence rate of intracranial hemorrhage events per 100,000 days at risk was 2.1-3.0 times higher for new users of warfarin than for those on dabigatran.
These estimates do not account for age, medical conditions, or other differences between the patients on warfarin and dabigatran that could affect bleeding outcomes, according to the FDA. In addition, although a "large" number of reports of bleeding in treated patients were submitted to the FDA’s Adverse Events Reporting System (FAERS) after dabigatran was approved, the agency believes that a "simple comparison" between the number of postmarketing bleeding events associated with dabigatran and warfarin is "misleading" because it is likely that bleeding events associated with warfarin are under reported, since the drug has been available for so long and bleeding is a well-recognized consequence of warfarin treatment.
At this time, the FDA is not changing any recommendations on the dabigatran label and is continuing to monitor postmarketing reports of bleeding in patients on dabigatran "for evidence of inappropriate dosing, use of interacting drugs, and other clinical factors that might lead to a bleeding event," according to the statement. The recommendations in the statement include advice to clinicians that they evaluate a patient’s renal function before prescribing dabigatran, which is eliminated by the kidneys, and the dosing regimens for patients with severe renal impairment and those with a creatinine clearance above 30 mL/min.
Click here for the Medwatch safety alert. Adverse events associated with dabigatran should be reported here or to the FDA at 800-332-0178.
The Food and Drug Administration is satisfied that dabigatran’s bleeding risk is no greater than that of warfarin and will not change the drug’s label.
The rates of gastrointestinal and intracranial bleeding among patients who have been prescribed the anticoagulant dabigatran "do not appear to be higher" than the rates among patients who have been prescribed warfarin, according to an analysis of insurance claims and administrative data conducted by the agency.
The results of this analysis, conducted in response to postmarketing reports of bleeding among people treated with dabigatran, are "consistent with observations" in the RE-LY trial, the study of 18,000 patients that was the basis of the approval of the anticoagulant for reducing the risk of stroke and blood clots in patients with nonvalvular atrial fibrillation (AF), the FDA said in the MedWatch safety alert, released on Nov. 2. In the RE-LY study, the rates of serious bleeding was similar among those treated with dabigatran and those with warfarin (N. Engl. J. Med. 2009;361:1139-51).
The agency is evaluating different sources of data in its review of this safety issue, which is ongoing. Dabigatran, an orally administered direct thrombin inhibitor, was approved in October 2010 for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation and is marketed as Pradaxa by Boehringer Ingelheim.
The FDA’s analysis found that the rates of bleeding were actually higher among those on warfarin, although the statement does not point this out. The FDA analyzed data from a database of nearly 100 million patients and determined that the combined incidence of intracranial and gastrointestinal hemorrhages per 100,000 days at risk was 1.8-2.6 times higher for new users of warfarin than for new users of dabigatran. When they analyzed the two events separately, they found that the incidence rate of gastrointestinal hemorrhage events per 100,000 days at risk was 1.6-2.2 times higher for new users of warfarin than for new users of dabigatran. The incidence rate of intracranial hemorrhage events per 100,000 days at risk was 2.1-3.0 times higher for new users of warfarin than for those on dabigatran.
These estimates do not account for age, medical conditions, or other differences between the patients on warfarin and dabigatran that could affect bleeding outcomes, according to the FDA. In addition, although a "large" number of reports of bleeding in treated patients were submitted to the FDA’s Adverse Events Reporting System (FAERS) after dabigatran was approved, the agency believes that a "simple comparison" between the number of postmarketing bleeding events associated with dabigatran and warfarin is "misleading" because it is likely that bleeding events associated with warfarin are under reported, since the drug has been available for so long and bleeding is a well-recognized consequence of warfarin treatment.
At this time, the FDA is not changing any recommendations on the dabigatran label and is continuing to monitor postmarketing reports of bleeding in patients on dabigatran "for evidence of inappropriate dosing, use of interacting drugs, and other clinical factors that might lead to a bleeding event," according to the statement. The recommendations in the statement include advice to clinicians that they evaluate a patient’s renal function before prescribing dabigatran, which is eliminated by the kidneys, and the dosing regimens for patients with severe renal impairment and those with a creatinine clearance above 30 mL/min.
Click here for the Medwatch safety alert. Adverse events associated with dabigatran should be reported here or to the FDA at 800-332-0178.
The Food and Drug Administration is satisfied that dabigatran’s bleeding risk is no greater than that of warfarin and will not change the drug’s label.
The rates of gastrointestinal and intracranial bleeding among patients who have been prescribed the anticoagulant dabigatran "do not appear to be higher" than the rates among patients who have been prescribed warfarin, according to an analysis of insurance claims and administrative data conducted by the agency.
The results of this analysis, conducted in response to postmarketing reports of bleeding among people treated with dabigatran, are "consistent with observations" in the RE-LY trial, the study of 18,000 patients that was the basis of the approval of the anticoagulant for reducing the risk of stroke and blood clots in patients with nonvalvular atrial fibrillation (AF), the FDA said in the MedWatch safety alert, released on Nov. 2. In the RE-LY study, the rates of serious bleeding was similar among those treated with dabigatran and those with warfarin (N. Engl. J. Med. 2009;361:1139-51).
The agency is evaluating different sources of data in its review of this safety issue, which is ongoing. Dabigatran, an orally administered direct thrombin inhibitor, was approved in October 2010 for reducing the risk of stroke and systemic embolism in patients with nonvalvular atrial fibrillation and is marketed as Pradaxa by Boehringer Ingelheim.
The FDA’s analysis found that the rates of bleeding were actually higher among those on warfarin, although the statement does not point this out. The FDA analyzed data from a database of nearly 100 million patients and determined that the combined incidence of intracranial and gastrointestinal hemorrhages per 100,000 days at risk was 1.8-2.6 times higher for new users of warfarin than for new users of dabigatran. When they analyzed the two events separately, they found that the incidence rate of gastrointestinal hemorrhage events per 100,000 days at risk was 1.6-2.2 times higher for new users of warfarin than for new users of dabigatran. The incidence rate of intracranial hemorrhage events per 100,000 days at risk was 2.1-3.0 times higher for new users of warfarin than for those on dabigatran.
These estimates do not account for age, medical conditions, or other differences between the patients on warfarin and dabigatran that could affect bleeding outcomes, according to the FDA. In addition, although a "large" number of reports of bleeding in treated patients were submitted to the FDA’s Adverse Events Reporting System (FAERS) after dabigatran was approved, the agency believes that a "simple comparison" between the number of postmarketing bleeding events associated with dabigatran and warfarin is "misleading" because it is likely that bleeding events associated with warfarin are under reported, since the drug has been available for so long and bleeding is a well-recognized consequence of warfarin treatment.
At this time, the FDA is not changing any recommendations on the dabigatran label and is continuing to monitor postmarketing reports of bleeding in patients on dabigatran "for evidence of inappropriate dosing, use of interacting drugs, and other clinical factors that might lead to a bleeding event," according to the statement. The recommendations in the statement include advice to clinicians that they evaluate a patient’s renal function before prescribing dabigatran, which is eliminated by the kidneys, and the dosing regimens for patients with severe renal impairment and those with a creatinine clearance above 30 mL/min.
Click here for the Medwatch safety alert. Adverse events associated with dabigatran should be reported here or to the FDA at 800-332-0178.
Counsel Patients on ATV Safety
NEW ORLEANS – Adolescents, especially those in rural areas, are likely to ride all-terrain vehicles, or ATVs, and participate in risky behavior, according to researchers who advise pediatricians to provide simple safety tips to young patients and their families.
Children under 16 continue to make up as much as a quarter of ATV-related deaths and injuries, according to national data. Although there has been a slight decline in recent years, experts say that the numbers are still too high.
Failure to use safety equipment like helmets, a lack of training, and failure to follow manufacturer safety recommendations are among the reasons for injuries in children, several studies have shown.
A survey of almost 3,000 Iowa students between ages 11 and 16 showed that nearly 85% had ridden an ATV at least a few times a year, and almost 60% of those who had been on an ATV reported having been in a crash.
"As a pediatrician, I think knowing the significant exposure children have to ATVs and their high crash incidence emphasizes the importance of our involvement in counseling families and educating them on safe practice," said Dr. Charles Jennissen, lead author of the survey and director of pediatric emergency medicine at the University of Iowa Hospitals and Clinics, Iowa City.
Growing up on a dairy farm in Central Minnesota and now working in Iowa as a pediatric emergency physician, Dr. Jennissen said he was quite familiar with ATVs and has seen his share of ATV-related injuries, not to mention losing a close family member to an ATV crash. He has published several studies on the topic.
Yet, he said he was slightly surprised by the findings from his survey.
His study showed that of those who said they had been on an ATV, more than 60% said they never or almost never wore a helmet (only 18% said they always or almost always wore a helmet), 92% said they had ridden with passengers, and 81% said they had ridden an ATV on a public road.
Dr. Jennissen said that one of his recent studies shows that 62% of all ATV-related deaths have occurred on roadways.
All-terrain vehicles were introduced in the United States about 3 decades ago for work purposes, but quickly became recreational machines for adults and children.
In 1985, the earliest year with data on atvsafety.gov, there were 250 reported deaths among all age groups and almost 106,000 injuries treated in an emergency department. In 2006, the last year for which complete data are available, those numbers rose to 833 and 147,000.
The number of reported deaths among children under 16 years old has also increased since 1990, rising from 81 to 142 in 2006. The number of emergency department visits for children rose from 22,400 to 39,300 during that period.
Meanwhile, a growing body of literature is accumulating on how dangerous these machines are, especially when children drive adult-size ATVs, said Dr. Rebeccah L. Brown, a trauma surgeon at the Cincinnati Children’s Hospital Medical Center.
A 2009 study of ATV and bicycle deaths showed that more children died annually from ATV injuries than from bicycle crashes.
Several medical associations including the American Academy of Pediatrics and the American Academy of Orthopaedic Surgeons, along with the Consumer Product Safety Commission, have called for restriction on the sale of ATVs to children (Pediatrics 2000;105:1352-4).
In a policy statement, Safe Kids USA, a global nonprofit organization focused on preventing unintentional childhood injury, said that until children "are fully licensed under state law to operate a motor vehicle, children should not operate or ride as passengers on ATVs of any size, including youth ATVs."
But the data and policies aren’t deterring adolescents from riding ATVs.
In a survey of 44 families of children who had been in an ATV crash between 2004 and 2009, Dr. Brown and her colleagues found that despite hospitalization and injuries, nearly 60% of the patients began riding again within 6 months of hospitalization.
Dr. Brown said nearly 50 families declined to participate in the survey, fearing the study would lead to legislation that would ban kids from riding ATVs.
"A lot of families see it as a family-bonding time and a fun time. They just don’t realize the danger," said Dr. Brown, who has been researching ATV injuries for more than a decade.
Her survey showed that nearly 80% of the respondents had permission to ride ATVs, and 64% were under adult supervision when they were injured.
Dr. Brown’s study also showed that none of the surveyed respondents underwent a formal ATV training course, although nearly half said they received training from a friend or relative. Only five dealers offered training.
Meanwhile, in a separate study, Dr. Jennissen and his colleagues showed that many primary care providers don’t advise their patients on ATV safety.
In an electronic survey of 218 primary care providers, 60% said they thought ATV anticipatory guidance was important for pediatric patients and their families. However, nearly 80% said they provided such counseling less than 10% of the time (J. Community Health 2012;37:968-75).
The survey also showed that families rarely ask about ATV safety; 84% of providers said they were asked about ATVs once a year or less.
"You don’t have to be an expert on ATVs, but you should be able to provide families basic safety recommendations and refer them to web sites for more detailed information," said Dr. Jennissen.
Dr. Jennissen and Dr. Brown said they had no relevant financial disclosures.
NEW ORLEANS – Adolescents, especially those in rural areas, are likely to ride all-terrain vehicles, or ATVs, and participate in risky behavior, according to researchers who advise pediatricians to provide simple safety tips to young patients and their families.
Children under 16 continue to make up as much as a quarter of ATV-related deaths and injuries, according to national data. Although there has been a slight decline in recent years, experts say that the numbers are still too high.
Failure to use safety equipment like helmets, a lack of training, and failure to follow manufacturer safety recommendations are among the reasons for injuries in children, several studies have shown.
A survey of almost 3,000 Iowa students between ages 11 and 16 showed that nearly 85% had ridden an ATV at least a few times a year, and almost 60% of those who had been on an ATV reported having been in a crash.
"As a pediatrician, I think knowing the significant exposure children have to ATVs and their high crash incidence emphasizes the importance of our involvement in counseling families and educating them on safe practice," said Dr. Charles Jennissen, lead author of the survey and director of pediatric emergency medicine at the University of Iowa Hospitals and Clinics, Iowa City.
Growing up on a dairy farm in Central Minnesota and now working in Iowa as a pediatric emergency physician, Dr. Jennissen said he was quite familiar with ATVs and has seen his share of ATV-related injuries, not to mention losing a close family member to an ATV crash. He has published several studies on the topic.
Yet, he said he was slightly surprised by the findings from his survey.
His study showed that of those who said they had been on an ATV, more than 60% said they never or almost never wore a helmet (only 18% said they always or almost always wore a helmet), 92% said they had ridden with passengers, and 81% said they had ridden an ATV on a public road.
Dr. Jennissen said that one of his recent studies shows that 62% of all ATV-related deaths have occurred on roadways.
All-terrain vehicles were introduced in the United States about 3 decades ago for work purposes, but quickly became recreational machines for adults and children.
In 1985, the earliest year with data on atvsafety.gov, there were 250 reported deaths among all age groups and almost 106,000 injuries treated in an emergency department. In 2006, the last year for which complete data are available, those numbers rose to 833 and 147,000.
The number of reported deaths among children under 16 years old has also increased since 1990, rising from 81 to 142 in 2006. The number of emergency department visits for children rose from 22,400 to 39,300 during that period.
Meanwhile, a growing body of literature is accumulating on how dangerous these machines are, especially when children drive adult-size ATVs, said Dr. Rebeccah L. Brown, a trauma surgeon at the Cincinnati Children’s Hospital Medical Center.
A 2009 study of ATV and bicycle deaths showed that more children died annually from ATV injuries than from bicycle crashes.
Several medical associations including the American Academy of Pediatrics and the American Academy of Orthopaedic Surgeons, along with the Consumer Product Safety Commission, have called for restriction on the sale of ATVs to children (Pediatrics 2000;105:1352-4).
In a policy statement, Safe Kids USA, a global nonprofit organization focused on preventing unintentional childhood injury, said that until children "are fully licensed under state law to operate a motor vehicle, children should not operate or ride as passengers on ATVs of any size, including youth ATVs."
But the data and policies aren’t deterring adolescents from riding ATVs.
In a survey of 44 families of children who had been in an ATV crash between 2004 and 2009, Dr. Brown and her colleagues found that despite hospitalization and injuries, nearly 60% of the patients began riding again within 6 months of hospitalization.
Dr. Brown said nearly 50 families declined to participate in the survey, fearing the study would lead to legislation that would ban kids from riding ATVs.
"A lot of families see it as a family-bonding time and a fun time. They just don’t realize the danger," said Dr. Brown, who has been researching ATV injuries for more than a decade.
Her survey showed that nearly 80% of the respondents had permission to ride ATVs, and 64% were under adult supervision when they were injured.
Dr. Brown’s study also showed that none of the surveyed respondents underwent a formal ATV training course, although nearly half said they received training from a friend or relative. Only five dealers offered training.
Meanwhile, in a separate study, Dr. Jennissen and his colleagues showed that many primary care providers don’t advise their patients on ATV safety.
In an electronic survey of 218 primary care providers, 60% said they thought ATV anticipatory guidance was important for pediatric patients and their families. However, nearly 80% said they provided such counseling less than 10% of the time (J. Community Health 2012;37:968-75).
The survey also showed that families rarely ask about ATV safety; 84% of providers said they were asked about ATVs once a year or less.
"You don’t have to be an expert on ATVs, but you should be able to provide families basic safety recommendations and refer them to web sites for more detailed information," said Dr. Jennissen.
Dr. Jennissen and Dr. Brown said they had no relevant financial disclosures.
NEW ORLEANS – Adolescents, especially those in rural areas, are likely to ride all-terrain vehicles, or ATVs, and participate in risky behavior, according to researchers who advise pediatricians to provide simple safety tips to young patients and their families.
Children under 16 continue to make up as much as a quarter of ATV-related deaths and injuries, according to national data. Although there has been a slight decline in recent years, experts say that the numbers are still too high.
Failure to use safety equipment like helmets, a lack of training, and failure to follow manufacturer safety recommendations are among the reasons for injuries in children, several studies have shown.
A survey of almost 3,000 Iowa students between ages 11 and 16 showed that nearly 85% had ridden an ATV at least a few times a year, and almost 60% of those who had been on an ATV reported having been in a crash.
"As a pediatrician, I think knowing the significant exposure children have to ATVs and their high crash incidence emphasizes the importance of our involvement in counseling families and educating them on safe practice," said Dr. Charles Jennissen, lead author of the survey and director of pediatric emergency medicine at the University of Iowa Hospitals and Clinics, Iowa City.
Growing up on a dairy farm in Central Minnesota and now working in Iowa as a pediatric emergency physician, Dr. Jennissen said he was quite familiar with ATVs and has seen his share of ATV-related injuries, not to mention losing a close family member to an ATV crash. He has published several studies on the topic.
Yet, he said he was slightly surprised by the findings from his survey.
His study showed that of those who said they had been on an ATV, more than 60% said they never or almost never wore a helmet (only 18% said they always or almost always wore a helmet), 92% said they had ridden with passengers, and 81% said they had ridden an ATV on a public road.
Dr. Jennissen said that one of his recent studies shows that 62% of all ATV-related deaths have occurred on roadways.
All-terrain vehicles were introduced in the United States about 3 decades ago for work purposes, but quickly became recreational machines for adults and children.
In 1985, the earliest year with data on atvsafety.gov, there were 250 reported deaths among all age groups and almost 106,000 injuries treated in an emergency department. In 2006, the last year for which complete data are available, those numbers rose to 833 and 147,000.
The number of reported deaths among children under 16 years old has also increased since 1990, rising from 81 to 142 in 2006. The number of emergency department visits for children rose from 22,400 to 39,300 during that period.
Meanwhile, a growing body of literature is accumulating on how dangerous these machines are, especially when children drive adult-size ATVs, said Dr. Rebeccah L. Brown, a trauma surgeon at the Cincinnati Children’s Hospital Medical Center.
A 2009 study of ATV and bicycle deaths showed that more children died annually from ATV injuries than from bicycle crashes.
Several medical associations including the American Academy of Pediatrics and the American Academy of Orthopaedic Surgeons, along with the Consumer Product Safety Commission, have called for restriction on the sale of ATVs to children (Pediatrics 2000;105:1352-4).
In a policy statement, Safe Kids USA, a global nonprofit organization focused on preventing unintentional childhood injury, said that until children "are fully licensed under state law to operate a motor vehicle, children should not operate or ride as passengers on ATVs of any size, including youth ATVs."
But the data and policies aren’t deterring adolescents from riding ATVs.
In a survey of 44 families of children who had been in an ATV crash between 2004 and 2009, Dr. Brown and her colleagues found that despite hospitalization and injuries, nearly 60% of the patients began riding again within 6 months of hospitalization.
Dr. Brown said nearly 50 families declined to participate in the survey, fearing the study would lead to legislation that would ban kids from riding ATVs.
"A lot of families see it as a family-bonding time and a fun time. They just don’t realize the danger," said Dr. Brown, who has been researching ATV injuries for more than a decade.
Her survey showed that nearly 80% of the respondents had permission to ride ATVs, and 64% were under adult supervision when they were injured.
Dr. Brown’s study also showed that none of the surveyed respondents underwent a formal ATV training course, although nearly half said they received training from a friend or relative. Only five dealers offered training.
Meanwhile, in a separate study, Dr. Jennissen and his colleagues showed that many primary care providers don’t advise their patients on ATV safety.
In an electronic survey of 218 primary care providers, 60% said they thought ATV anticipatory guidance was important for pediatric patients and their families. However, nearly 80% said they provided such counseling less than 10% of the time (J. Community Health 2012;37:968-75).
The survey also showed that families rarely ask about ATV safety; 84% of providers said they were asked about ATVs once a year or less.
"You don’t have to be an expert on ATVs, but you should be able to provide families basic safety recommendations and refer them to web sites for more detailed information," said Dr. Jennissen.
Dr. Jennissen and Dr. Brown said they had no relevant financial disclosures.
AT THE ANNUAL MEETING OF THE AMERICAN ACADEMY OF PEDIATRICS
Major Finding: Nearly 85% of Iowa teens had ridden an ATV at least a few times a year, and almost 60% of those who had been on an ATV reported having been in a crash.
Data Source: Data are from a study of almost 3,000 Iowa students between the ages of 11 and 16 years.
Disclosures: Dr. Jennissen and Dr. Brown said they had no relevant financial disclosures.
Acetaminophen: Effect on Drinkers' ALT Levels Appears Safe
SAN DIEGO – Giving acetaminophen to patients who reported consuming ethanol did not adversely affect markers of liver damage in a meta-analysis of randomized, controlled trials.
"One of the questions we often get asked is the role of acetaminophen in patients with liver disease," according to Dr. Kennon J. Heard, who is an emergency medicine physician at the University of Colorado and director of the Medical Toxicology Fellowship at the Rocky Mountain Poison and Drug Center, Denver.
The findings of the meta-analysis suggest that "acetaminophen is safe in alcoholics," Dr. Heard said at the annual meeting of the Society of Hospital Medicine.
The meta-analysis included five trials involving 901 subjects (including patients who reported drinking ethanol) who were randomized to receive acetaminophen or placebo.
Dr. Heard and his associates looked at daily ALT measurements out to a mean of 4 days, a time period for which most of the studies had data.
They also looked for any evidence of liver injury or dysfunction, hepatotoxicity, or death.
The alanine aminotransferase (ALT) levels changed by a mean of 0.04 IU/L after starting acetaminophen or placebo, "less than a tenth of a point in ALT," reported Dr. Heard.
"Essentially, in this group of patients who consume alcohol, if you give them acetaminophen for 4 days, you don’t see any change in their ALT," according to Dr. Heard.
The study is to be published in the journal Pharmacotherapy.
When acetaminophen consumption continued beyond 4 days, ALT levels increased in most patients who consumed alcohol but also increased in 60% of nondrinkers.
"The changes in the alcoholics look exactly like the changes in the nonalcoholics," he said.
The median increase in ALT was between 10-20 IU/L.
Among patients who drink alcohol, the highest ALT level in the acetaminophen group was 312 IU/L, "which is pretty impressive until you see that in the placebo group, somebody went up 288" IU/L, he said.
The biggest increase in ALT was in a healthy nondrinking patient on acetaminophen, whose ALT increased by 638 IU/L.
Most importantly, none of the 551 people who received acetaminophen in those trials developed an increase in International Normalized Ratio, bilirubin level, or symptomatic liver injury, Dr. Heard and his associates found.
Dr. Heard said that he and his associates are now in the process of finishing a separate study that appears to confirm that these are asymptomatic, self-limiting elevations in ALT that will go away even if people stay on acetaminophen.
Such information is valuable, he said. "It is worth knowing that if you have someone who has an ALT elevation while taking acetaminophen, it may be the cause, and it is reasonable to stop the acetaminophen and see if their ALT elevations go away rather than do an extensive work-up for hepatitis," Dr. Heard said.
SAN DIEGO – Giving acetaminophen to patients who reported consuming ethanol did not adversely affect markers of liver damage in a meta-analysis of randomized, controlled trials.
"One of the questions we often get asked is the role of acetaminophen in patients with liver disease," according to Dr. Kennon J. Heard, who is an emergency medicine physician at the University of Colorado and director of the Medical Toxicology Fellowship at the Rocky Mountain Poison and Drug Center, Denver.
The findings of the meta-analysis suggest that "acetaminophen is safe in alcoholics," Dr. Heard said at the annual meeting of the Society of Hospital Medicine.
The meta-analysis included five trials involving 901 subjects (including patients who reported drinking ethanol) who were randomized to receive acetaminophen or placebo.
Dr. Heard and his associates looked at daily ALT measurements out to a mean of 4 days, a time period for which most of the studies had data.
They also looked for any evidence of liver injury or dysfunction, hepatotoxicity, or death.
The alanine aminotransferase (ALT) levels changed by a mean of 0.04 IU/L after starting acetaminophen or placebo, "less than a tenth of a point in ALT," reported Dr. Heard.
"Essentially, in this group of patients who consume alcohol, if you give them acetaminophen for 4 days, you don’t see any change in their ALT," according to Dr. Heard.
The study is to be published in the journal Pharmacotherapy.
When acetaminophen consumption continued beyond 4 days, ALT levels increased in most patients who consumed alcohol but also increased in 60% of nondrinkers.
"The changes in the alcoholics look exactly like the changes in the nonalcoholics," he said.
The median increase in ALT was between 10-20 IU/L.
Among patients who drink alcohol, the highest ALT level in the acetaminophen group was 312 IU/L, "which is pretty impressive until you see that in the placebo group, somebody went up 288" IU/L, he said.
The biggest increase in ALT was in a healthy nondrinking patient on acetaminophen, whose ALT increased by 638 IU/L.
Most importantly, none of the 551 people who received acetaminophen in those trials developed an increase in International Normalized Ratio, bilirubin level, or symptomatic liver injury, Dr. Heard and his associates found.
Dr. Heard said that he and his associates are now in the process of finishing a separate study that appears to confirm that these are asymptomatic, self-limiting elevations in ALT that will go away even if people stay on acetaminophen.
Such information is valuable, he said. "It is worth knowing that if you have someone who has an ALT elevation while taking acetaminophen, it may be the cause, and it is reasonable to stop the acetaminophen and see if their ALT elevations go away rather than do an extensive work-up for hepatitis," Dr. Heard said.
SAN DIEGO – Giving acetaminophen to patients who reported consuming ethanol did not adversely affect markers of liver damage in a meta-analysis of randomized, controlled trials.
"One of the questions we often get asked is the role of acetaminophen in patients with liver disease," according to Dr. Kennon J. Heard, who is an emergency medicine physician at the University of Colorado and director of the Medical Toxicology Fellowship at the Rocky Mountain Poison and Drug Center, Denver.
The findings of the meta-analysis suggest that "acetaminophen is safe in alcoholics," Dr. Heard said at the annual meeting of the Society of Hospital Medicine.
The meta-analysis included five trials involving 901 subjects (including patients who reported drinking ethanol) who were randomized to receive acetaminophen or placebo.
Dr. Heard and his associates looked at daily ALT measurements out to a mean of 4 days, a time period for which most of the studies had data.
They also looked for any evidence of liver injury or dysfunction, hepatotoxicity, or death.
The alanine aminotransferase (ALT) levels changed by a mean of 0.04 IU/L after starting acetaminophen or placebo, "less than a tenth of a point in ALT," reported Dr. Heard.
"Essentially, in this group of patients who consume alcohol, if you give them acetaminophen for 4 days, you don’t see any change in their ALT," according to Dr. Heard.
The study is to be published in the journal Pharmacotherapy.
When acetaminophen consumption continued beyond 4 days, ALT levels increased in most patients who consumed alcohol but also increased in 60% of nondrinkers.
"The changes in the alcoholics look exactly like the changes in the nonalcoholics," he said.
The median increase in ALT was between 10-20 IU/L.
Among patients who drink alcohol, the highest ALT level in the acetaminophen group was 312 IU/L, "which is pretty impressive until you see that in the placebo group, somebody went up 288" IU/L, he said.
The biggest increase in ALT was in a healthy nondrinking patient on acetaminophen, whose ALT increased by 638 IU/L.
Most importantly, none of the 551 people who received acetaminophen in those trials developed an increase in International Normalized Ratio, bilirubin level, or symptomatic liver injury, Dr. Heard and his associates found.
Dr. Heard said that he and his associates are now in the process of finishing a separate study that appears to confirm that these are asymptomatic, self-limiting elevations in ALT that will go away even if people stay on acetaminophen.
Such information is valuable, he said. "It is worth knowing that if you have someone who has an ALT elevation while taking acetaminophen, it may be the cause, and it is reasonable to stop the acetaminophen and see if their ALT elevations go away rather than do an extensive work-up for hepatitis," Dr. Heard said.
Major Finding: ALT levels changed by a median of 0.04 IU/L in alcohol drinkers and nondrinkers after taking acetaminophen for 4 days, with no significant difference between subjects on acetaminophen or placebo.
Data Source: The meta-analysis of five randomized, controlled trials included 901 subjects.
Disclosures: Dr. Heard has been a consultant or received research grants from Cadence Pharmaceuticals, McNeil Consumer Healthcare, and Cumberland Pharmaceuticals.
Pneumonia Prevalence Highest of Health Care-Associated Infections
SAN DIEGO – The overall prevalence of health care–associated infections among inpatients in the United States stands at 4%, with the most common types of infections being a combination of pneumonia and lower respiratory infections.
Those are key preliminary findings from the Centers for Disease Control and Prevention’s first large-scale health care–associated infection (HAI) prevalence survey in more than 30 years, Dr. Shelley S. Magill reported during IDWeek 2012, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The data "can help us better understand the factors that influence HAI prevalence," said Dr. Magill of the division of health care quality promotion at the Centers for Disease Control and Prevention, Atlanta. "We can also clarify the burden of different HAI types and pathogens across the hospital, which can suggest areas to target for prevention."
The phase 3 survey was conducted in 2011 in 183 hospitals in 10 states: California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New Mexico, New York, Oregon, and Tennessee. Dr. Magill reported results from 11,282 patients who were surveyed in the 183 hospitals. Of these, 452 patients had HAIs, for a prevalence of 4%.
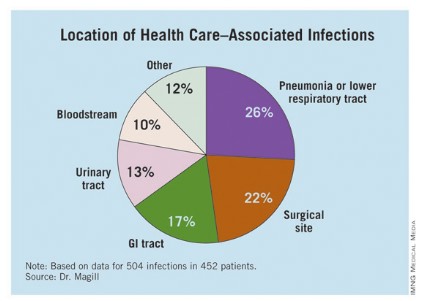
The researchers identified 504 HAIs in the 452 patients. Of these, the highest proportion (26%) were pneumonia or lower respiratory infections. "Of the pneumonia events, 39% were ventilator-associated infections," Dr. Magill said.
Surgical-site infections were the second most common infection type, representing 22% of all HAIs, followed by GI infections (17%), urinary tract infections (13%), and bloodstream infections (10%). "About two-third of UTIs were catheter associated and about 84% of the bloodstream infections were central-line associated," Dr. Magill noted.
Other infections made up the remaining 12% of HAIs.
Dr. Magill also reported that 56% of HAIs were attributed to non-ICU locations in the hospital while 53% were not directly associated with a device or with a procedure.
After multivariable regression analysis, patients with the following factors were at higher risk of having HAIs:
• Older age (risk ratio, 4.60 for patients older than age 77 compared with patients younger than 3 months old).
• Inpatient in a large hospital – defined as having 400 or more beds (RR, 1.24).
• Inpatient in a critical care unit (RR ,1.96 compared with all other units),
• Inpatient for more than 2 weeks at the time of the survey (RR, 26.09 compared with patients in the hospital for 3 days or fewer).
Pathogens were reported for 372 of the 504 HAIs. Clostridium difficile was the most common pathogen, accounting for 12% of all HAIs that were identified. Staphylococcus aureus was the second most common pathogen (11%; about half of these cases were methicillin-resistant S. aureus), followed by Klebsiella pneumoniae and Klebsiella oxytoca (10%).
Dr. Magill acknowledged certain limitations of the survey, including the fact that a small number of patients surveyed in each hospital "make results of limited use to individual facilities," she said. "We also had a relatively small number of hospitals in 10 states participate. This survey method provides a single snapshot of HAIs based on a retrospective review of medical record data, which is sometimes not complete. Finally, we were not able to collect a lot of detailed patient information, such as underlying illnesses and severity of illness."
She and her associates are currently planning a phase 4 survey, anticipated to occur in 2014.
Dr. Magill said she had no relevant financial conflicts to disclose.
SAN DIEGO – The overall prevalence of health care–associated infections among inpatients in the United States stands at 4%, with the most common types of infections being a combination of pneumonia and lower respiratory infections.
Those are key preliminary findings from the Centers for Disease Control and Prevention’s first large-scale health care–associated infection (HAI) prevalence survey in more than 30 years, Dr. Shelley S. Magill reported during IDWeek 2012, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The data "can help us better understand the factors that influence HAI prevalence," said Dr. Magill of the division of health care quality promotion at the Centers for Disease Control and Prevention, Atlanta. "We can also clarify the burden of different HAI types and pathogens across the hospital, which can suggest areas to target for prevention."
The phase 3 survey was conducted in 2011 in 183 hospitals in 10 states: California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New Mexico, New York, Oregon, and Tennessee. Dr. Magill reported results from 11,282 patients who were surveyed in the 183 hospitals. Of these, 452 patients had HAIs, for a prevalence of 4%.

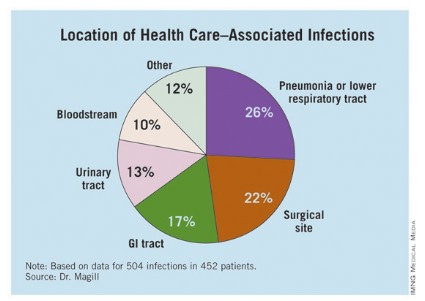
The researchers identified 504 HAIs in the 452 patients. Of these, the highest proportion (26%) were pneumonia or lower respiratory infections. "Of the pneumonia events, 39% were ventilator-associated infections," Dr. Magill said.
Surgical-site infections were the second most common infection type, representing 22% of all HAIs, followed by GI infections (17%), urinary tract infections (13%), and bloodstream infections (10%). "About two-third of UTIs were catheter associated and about 84% of the bloodstream infections were central-line associated," Dr. Magill noted.
Other infections made up the remaining 12% of HAIs.
Dr. Magill also reported that 56% of HAIs were attributed to non-ICU locations in the hospital while 53% were not directly associated with a device or with a procedure.
After multivariable regression analysis, patients with the following factors were at higher risk of having HAIs:
• Older age (risk ratio, 4.60 for patients older than age 77 compared with patients younger than 3 months old).
• Inpatient in a large hospital – defined as having 400 or more beds (RR, 1.24).
• Inpatient in a critical care unit (RR ,1.96 compared with all other units),
• Inpatient for more than 2 weeks at the time of the survey (RR, 26.09 compared with patients in the hospital for 3 days or fewer).
Pathogens were reported for 372 of the 504 HAIs. Clostridium difficile was the most common pathogen, accounting for 12% of all HAIs that were identified. Staphylococcus aureus was the second most common pathogen (11%; about half of these cases were methicillin-resistant S. aureus), followed by Klebsiella pneumoniae and Klebsiella oxytoca (10%).
Dr. Magill acknowledged certain limitations of the survey, including the fact that a small number of patients surveyed in each hospital "make results of limited use to individual facilities," she said. "We also had a relatively small number of hospitals in 10 states participate. This survey method provides a single snapshot of HAIs based on a retrospective review of medical record data, which is sometimes not complete. Finally, we were not able to collect a lot of detailed patient information, such as underlying illnesses and severity of illness."
She and her associates are currently planning a phase 4 survey, anticipated to occur in 2014.
Dr. Magill said she had no relevant financial conflicts to disclose.
SAN DIEGO – The overall prevalence of health care–associated infections among inpatients in the United States stands at 4%, with the most common types of infections being a combination of pneumonia and lower respiratory infections.
Those are key preliminary findings from the Centers for Disease Control and Prevention’s first large-scale health care–associated infection (HAI) prevalence survey in more than 30 years, Dr. Shelley S. Magill reported during IDWeek 2012, the combined annual meetings of the Infectious Diseases Society of America, the Society for Healthcare Epidemiology of America, the HIV Medicine Association, and the Pediatric Infectious Diseases Society.
The data "can help us better understand the factors that influence HAI prevalence," said Dr. Magill of the division of health care quality promotion at the Centers for Disease Control and Prevention, Atlanta. "We can also clarify the burden of different HAI types and pathogens across the hospital, which can suggest areas to target for prevention."
The phase 3 survey was conducted in 2011 in 183 hospitals in 10 states: California, Colorado, Connecticut, Georgia, Maryland, Minnesota, New Mexico, New York, Oregon, and Tennessee. Dr. Magill reported results from 11,282 patients who were surveyed in the 183 hospitals. Of these, 452 patients had HAIs, for a prevalence of 4%.

The researchers identified 504 HAIs in the 452 patients. Of these, the highest proportion (26%) were pneumonia or lower respiratory infections. "Of the pneumonia events, 39% were ventilator-associated infections," Dr. Magill said.
Surgical-site infections were the second most common infection type, representing 22% of all HAIs, followed by GI infections (17%), urinary tract infections (13%), and bloodstream infections (10%). "About two-third of UTIs were catheter associated and about 84% of the bloodstream infections were central-line associated," Dr. Magill noted.
Other infections made up the remaining 12% of HAIs.
Dr. Magill also reported that 56% of HAIs were attributed to non-ICU locations in the hospital while 53% were not directly associated with a device or with a procedure.
After multivariable regression analysis, patients with the following factors were at higher risk of having HAIs:
• Older age (risk ratio, 4.60 for patients older than age 77 compared with patients younger than 3 months old).
• Inpatient in a large hospital – defined as having 400 or more beds (RR, 1.24).
• Inpatient in a critical care unit (RR ,1.96 compared with all other units),
• Inpatient for more than 2 weeks at the time of the survey (RR, 26.09 compared with patients in the hospital for 3 days or fewer).
Pathogens were reported for 372 of the 504 HAIs. Clostridium difficile was the most common pathogen, accounting for 12% of all HAIs that were identified. Staphylococcus aureus was the second most common pathogen (11%; about half of these cases were methicillin-resistant S. aureus), followed by Klebsiella pneumoniae and Klebsiella oxytoca (10%).
Dr. Magill acknowledged certain limitations of the survey, including the fact that a small number of patients surveyed in each hospital "make results of limited use to individual facilities," she said. "We also had a relatively small number of hospitals in 10 states participate. This survey method provides a single snapshot of HAIs based on a retrospective review of medical record data, which is sometimes not complete. Finally, we were not able to collect a lot of detailed patient information, such as underlying illnesses and severity of illness."
She and her associates are currently planning a phase 4 survey, anticipated to occur in 2014.
Dr. Magill said she had no relevant financial conflicts to disclose.
AT IDWEEK 2012
Major Finding: The overall prevalence of health care–associated infections among hospitalized patients nationwide was 4%.
Data Source: Preliminary results were obtained from a 2011 survey of 11,282 inpatients at 183 hospitals located in 10 states.
Disclosures: The study was conducted by the Centers for Disease Control and Prevention. Dr. Magill said she had no relevant financial conflicts to disclose.
Quality Initiative Generates Widespread Improvements for Hospitals
Aligning Forces for Quality, an 18-month virtual quality collaborative launched in 16 targeted communities, generated measurable improvements for 90% of the 100 participating hospitals. The project, sponsored by the Robert Wood Johnson Foundation (www.forces4quality.org), is focused on reducing avoidable hospital readmissions; improving ED timeliness, efficiency, and patient flow; and eliminating patient-language barriers via standardized collection of data on race, ethnicity, and language preferences.
The collaborative showed 60% of participating hospitals improved 30-day readmission rates for heart failure patients, and 75% improved adherence to heart failure care standards.
“Hospitals are willing to really take stock of what they are doing well and where they could improve,” Susan Mende, BSN, MPH, of the foundation’s senior program office noted in a prepared statement.
Hospitalist William C. Cook, DO, chief of hospital medicine for the Ohio Permanente Medical Group, served as co-chair of the steering committee for transitional care for Better Care Greater Cleveland, one of the 16 community coalitions, and led two other work groups targeting care transitions. “I also had the opportunity to meet and work with Eric Coleman, MD,” of the University of Colorado Denver and creator of the widely implemented Care Transitions Program that coaches patients discharged from the hospital.
The foundation’s program promoted a vision for addressing readmissions across Dr. Cook’s region. “From the hospitalist perspective, our role is to try to make transitions as safe and predictable as possible,” Dr. Cook says.
Aligning Forces for Quality, an 18-month virtual quality collaborative launched in 16 targeted communities, generated measurable improvements for 90% of the 100 participating hospitals. The project, sponsored by the Robert Wood Johnson Foundation (www.forces4quality.org), is focused on reducing avoidable hospital readmissions; improving ED timeliness, efficiency, and patient flow; and eliminating patient-language barriers via standardized collection of data on race, ethnicity, and language preferences.
The collaborative showed 60% of participating hospitals improved 30-day readmission rates for heart failure patients, and 75% improved adherence to heart failure care standards.
“Hospitals are willing to really take stock of what they are doing well and where they could improve,” Susan Mende, BSN, MPH, of the foundation’s senior program office noted in a prepared statement.
Hospitalist William C. Cook, DO, chief of hospital medicine for the Ohio Permanente Medical Group, served as co-chair of the steering committee for transitional care for Better Care Greater Cleveland, one of the 16 community coalitions, and led two other work groups targeting care transitions. “I also had the opportunity to meet and work with Eric Coleman, MD,” of the University of Colorado Denver and creator of the widely implemented Care Transitions Program that coaches patients discharged from the hospital.
The foundation’s program promoted a vision for addressing readmissions across Dr. Cook’s region. “From the hospitalist perspective, our role is to try to make transitions as safe and predictable as possible,” Dr. Cook says.
Aligning Forces for Quality, an 18-month virtual quality collaborative launched in 16 targeted communities, generated measurable improvements for 90% of the 100 participating hospitals. The project, sponsored by the Robert Wood Johnson Foundation (www.forces4quality.org), is focused on reducing avoidable hospital readmissions; improving ED timeliness, efficiency, and patient flow; and eliminating patient-language barriers via standardized collection of data on race, ethnicity, and language preferences.
The collaborative showed 60% of participating hospitals improved 30-day readmission rates for heart failure patients, and 75% improved adherence to heart failure care standards.
“Hospitals are willing to really take stock of what they are doing well and where they could improve,” Susan Mende, BSN, MPH, of the foundation’s senior program office noted in a prepared statement.
Hospitalist William C. Cook, DO, chief of hospital medicine for the Ohio Permanente Medical Group, served as co-chair of the steering committee for transitional care for Better Care Greater Cleveland, one of the 16 community coalitions, and led two other work groups targeting care transitions. “I also had the opportunity to meet and work with Eric Coleman, MD,” of the University of Colorado Denver and creator of the widely implemented Care Transitions Program that coaches patients discharged from the hospital.
The foundation’s program promoted a vision for addressing readmissions across Dr. Cook’s region. “From the hospitalist perspective, our role is to try to make transitions as safe and predictable as possible,” Dr. Cook says.
Palliative Care Teams in 65% of Hospitals
Portion of hospitals that had palliative-care teams in 2010, according to the latest tally from the Center to Advance Palliative Care at Mount Sinai School of Medicine in New York City (www.capc.org), an increase of 148.5% from 2000. Hospitals of 300-plus beds are more likely to have a palliative-care team than those with fewer than 300 beds (87.9% vs. 56.5%).
Portion of hospitals that had palliative-care teams in 2010, according to the latest tally from the Center to Advance Palliative Care at Mount Sinai School of Medicine in New York City (www.capc.org), an increase of 148.5% from 2000. Hospitals of 300-plus beds are more likely to have a palliative-care team than those with fewer than 300 beds (87.9% vs. 56.5%).
Portion of hospitals that had palliative-care teams in 2010, according to the latest tally from the Center to Advance Palliative Care at Mount Sinai School of Medicine in New York City (www.capc.org), an increase of 148.5% from 2000. Hospitals of 300-plus beds are more likely to have a palliative-care team than those with fewer than 300 beds (87.9% vs. 56.5%).
Professional Medical Coders Increase Hospitalist Group Reimbursement
What is the hospitalist’s optimal role in professional services billing? According to Leonard Noronha, MD, who was until recently a hospitalist practicing at the University of New Mexico (UNM), “physicians often find E/M (evaluation and management) coding rules confusing and frustrating,” leading to delinquent or tardy bills. Yet some feel apprehensive about turning billing and coding decisions over to professional coders because the physician retains legal responsibility for the accuracy of coding.
In a poster presented at HM12 in San Diego, Dr. Noronha described a 2010 decision by the academic group at UNM to have coders assign service levels to physician visits, retrieving the data from typed progress notes in the medical center’s newly implemented electronic health record (EHR). There were concerns that this new approach might lead to decreased revenue, but in practice, it led to both increased charges and collections (18%) and faster bill submissions (to 14 days from 16 days). The UNM hospitalists are incentivized to submit daily patient lists and to respond promptly to documentation completion requests.
“Working in a community hospital setting for five years and then in an academic practice for four years exposed me to a variety of approaches,” Dr. Noronha says. “My opinion is that coders have degrees and professional certifications and, thus, are capable of taking on this responsibility.”
Physicians still need to review submissions within specified time frames, and the system is yet to incorporate regular audits to ensure the quality of the coding.
What is the hospitalist’s optimal role in professional services billing? According to Leonard Noronha, MD, who was until recently a hospitalist practicing at the University of New Mexico (UNM), “physicians often find E/M (evaluation and management) coding rules confusing and frustrating,” leading to delinquent or tardy bills. Yet some feel apprehensive about turning billing and coding decisions over to professional coders because the physician retains legal responsibility for the accuracy of coding.
In a poster presented at HM12 in San Diego, Dr. Noronha described a 2010 decision by the academic group at UNM to have coders assign service levels to physician visits, retrieving the data from typed progress notes in the medical center’s newly implemented electronic health record (EHR). There were concerns that this new approach might lead to decreased revenue, but in practice, it led to both increased charges and collections (18%) and faster bill submissions (to 14 days from 16 days). The UNM hospitalists are incentivized to submit daily patient lists and to respond promptly to documentation completion requests.
“Working in a community hospital setting for five years and then in an academic practice for four years exposed me to a variety of approaches,” Dr. Noronha says. “My opinion is that coders have degrees and professional certifications and, thus, are capable of taking on this responsibility.”
Physicians still need to review submissions within specified time frames, and the system is yet to incorporate regular audits to ensure the quality of the coding.
What is the hospitalist’s optimal role in professional services billing? According to Leonard Noronha, MD, who was until recently a hospitalist practicing at the University of New Mexico (UNM), “physicians often find E/M (evaluation and management) coding rules confusing and frustrating,” leading to delinquent or tardy bills. Yet some feel apprehensive about turning billing and coding decisions over to professional coders because the physician retains legal responsibility for the accuracy of coding.
In a poster presented at HM12 in San Diego, Dr. Noronha described a 2010 decision by the academic group at UNM to have coders assign service levels to physician visits, retrieving the data from typed progress notes in the medical center’s newly implemented electronic health record (EHR). There were concerns that this new approach might lead to decreased revenue, but in practice, it led to both increased charges and collections (18%) and faster bill submissions (to 14 days from 16 days). The UNM hospitalists are incentivized to submit daily patient lists and to respond promptly to documentation completion requests.
“Working in a community hospital setting for five years and then in an academic practice for four years exposed me to a variety of approaches,” Dr. Noronha says. “My opinion is that coders have degrees and professional certifications and, thus, are capable of taking on this responsibility.”
Physicians still need to review submissions within specified time frames, and the system is yet to incorporate regular audits to ensure the quality of the coding.
U.S. Army Supports Rapid Deployment of Hospital Practice
A U.S. military combat-support hospital team based at Fort Polk near Leesville, La., works hard year-round to remain ready to erect a temporary, fully functioning tent hospital within 72 hours whenever and wherever it’s needed. That could mean an overseas war zone or closer to home for Americans hit by a tornado or hurricane.
The 115th Forward Support Battalion, led by Col. Kevin J. Stevens, has practiced assembling the temporary hospital three times this year, with another exercise planned for October. In its last run-through, a 24-hour acute-care hospital of 84 beds was erected in 66 hours. It included two operating rooms, two 24-bed ICUs with ventilators, patient wards, a six-bay ED, specialty clinics, and lab, pathology, biomedical, pharmacy, and blood services.
All of the needed equipment can be moved by truck, airplane, or boat in 32 20-foot-long vehicles, Stevens says. The staging team lays out the perimeter, perhaps in a parking lot or an existing structure, such as a school. Heating and cooling systems, water, oxygen, and power generators are brought in, and the team establishes a landing pad for helicopters.
“We bring all that wherever we go. But setting it up is the easy part,” Stevens says, adding that staffing and managing an acute-care hospital is the hard part.
When fully operational, the temporary hospital employs a professional staff of 75 to 80, including medical specialists. Some are based year-round at Fort Polk, keeping the equipment maintained. Others practice at hospitals across the country but are on the “call list” when a deployment is ordered. A new set of up-to-date, interlocking equipment for the temporary hospital was issued in March.
“Getting better at this is my mission,” says Stevens, a soldier since 1974 who has deployed with Forward Support Hospitals in both Iraq and Afghanistan. “We work to keep medical and deployment skills sharp at all times. Everything we do is meant to save soldier and civilian lives.”
Larry Beresford is a freelance writer in Oakland, Calif.
A U.S. military combat-support hospital team based at Fort Polk near Leesville, La., works hard year-round to remain ready to erect a temporary, fully functioning tent hospital within 72 hours whenever and wherever it’s needed. That could mean an overseas war zone or closer to home for Americans hit by a tornado or hurricane.
The 115th Forward Support Battalion, led by Col. Kevin J. Stevens, has practiced assembling the temporary hospital three times this year, with another exercise planned for October. In its last run-through, a 24-hour acute-care hospital of 84 beds was erected in 66 hours. It included two operating rooms, two 24-bed ICUs with ventilators, patient wards, a six-bay ED, specialty clinics, and lab, pathology, biomedical, pharmacy, and blood services.
All of the needed equipment can be moved by truck, airplane, or boat in 32 20-foot-long vehicles, Stevens says. The staging team lays out the perimeter, perhaps in a parking lot or an existing structure, such as a school. Heating and cooling systems, water, oxygen, and power generators are brought in, and the team establishes a landing pad for helicopters.
“We bring all that wherever we go. But setting it up is the easy part,” Stevens says, adding that staffing and managing an acute-care hospital is the hard part.
When fully operational, the temporary hospital employs a professional staff of 75 to 80, including medical specialists. Some are based year-round at Fort Polk, keeping the equipment maintained. Others practice at hospitals across the country but are on the “call list” when a deployment is ordered. A new set of up-to-date, interlocking equipment for the temporary hospital was issued in March.
“Getting better at this is my mission,” says Stevens, a soldier since 1974 who has deployed with Forward Support Hospitals in both Iraq and Afghanistan. “We work to keep medical and deployment skills sharp at all times. Everything we do is meant to save soldier and civilian lives.”
Larry Beresford is a freelance writer in Oakland, Calif.
A U.S. military combat-support hospital team based at Fort Polk near Leesville, La., works hard year-round to remain ready to erect a temporary, fully functioning tent hospital within 72 hours whenever and wherever it’s needed. That could mean an overseas war zone or closer to home for Americans hit by a tornado or hurricane.
The 115th Forward Support Battalion, led by Col. Kevin J. Stevens, has practiced assembling the temporary hospital three times this year, with another exercise planned for October. In its last run-through, a 24-hour acute-care hospital of 84 beds was erected in 66 hours. It included two operating rooms, two 24-bed ICUs with ventilators, patient wards, a six-bay ED, specialty clinics, and lab, pathology, biomedical, pharmacy, and blood services.
All of the needed equipment can be moved by truck, airplane, or boat in 32 20-foot-long vehicles, Stevens says. The staging team lays out the perimeter, perhaps in a parking lot or an existing structure, such as a school. Heating and cooling systems, water, oxygen, and power generators are brought in, and the team establishes a landing pad for helicopters.
“We bring all that wherever we go. But setting it up is the easy part,” Stevens says, adding that staffing and managing an acute-care hospital is the hard part.
When fully operational, the temporary hospital employs a professional staff of 75 to 80, including medical specialists. Some are based year-round at Fort Polk, keeping the equipment maintained. Others practice at hospitals across the country but are on the “call list” when a deployment is ordered. A new set of up-to-date, interlocking equipment for the temporary hospital was issued in March.
“Getting better at this is my mission,” says Stevens, a soldier since 1974 who has deployed with Forward Support Hospitals in both Iraq and Afghanistan. “We work to keep medical and deployment skills sharp at all times. Everything we do is meant to save soldier and civilian lives.”
Larry Beresford is a freelance writer in Oakland, Calif.
Win Whitcomb: Hospitalists Must Grin and Bear the Hospital-Acquired Conditions Program
The Inpatient Prospective Payment System FY2013 Final Rule charts a different future: By fiscal-year 2015 (October 2014), it will morph into a set of measures that are vetted by the National Quality Forum. Hopefully, this will be an improvement.
In recent years, hospitalists have been deluged with rules about documentation, being asked to use medical vocabulary in ways that were foreign to many of us during our training years. Much of the focus on documentation has been propelled by hospitals’ quest to optimize (“maximize” is a forbidden term) reimbursement, which is purely a function of what is written by “licensed providers” (doctors, physician assistants, and nurse practitioners) in the medical chart.
But another powerful driver of documentation practices of late is the hospital-acquired conditions (HAC) program developed by the Centers for Medicare & Medicaid Services (CMS) and enacted in 2009.
Origins of the HAC List
CMS disliked the fact that they were paying for conditions acquired in the hospital that were “reasonably preventable” if evidence-based—or at least “best”—practice was applied. After all, who likes to pay for a punctured gas tank when you brought the minivan in for an oil change? CMS worked with stakeholder groups, including SHM, to create a list of conditions known as hospital-acquired conditions (see Table 1, right).
(As an aside, SHM was supportive of CMS. In fact, we provided direct input into the final rule, recognizing some of the drawbacks of the CMS approach but understanding the larger objective of reengineering a flawed incentive system.)
The idea was that if a hospital submitted a bill to CMS that contained one of these conditions, the hospital would not be paid the amount by which that condition increased total reimbursement for that hospitalization. Note that if you’ve been told your hospital isn’t getting paid at all for patients with one of these conditions, that is not quite correct. Instead, your hospital may not get paid the added amount that is derived from having one of the diagnoses on the list submitted in your hospital’s bill to CMS for a given patient. At the end of the day, this might be a few hundred dollars each time one of these is documented—or $0, if your hospital biller can add another diagnosis in its place to capture the higher payment.
How big a hit to a hospital’s bottom line is this? Meddings and colleagues recently reported that a measly 0.003% of all hospitalizations in Michigan in 2009 saw payments lowered as a result of hospital-acquired catheter-associated UTI, one of the list’s HACs (Ann Int Med. 2012;157:305-312). When all the HACs are added together, one can extrapolate that they haven’t exactly had a big impact on hospital payments.
If the specter of nonpayment for one of these is not enough of a motivator (and it shouldn’t be, given the paltry financial stakes), the rate of HACs are now reported for all hospitals on the Hospital Compare website (www.hospitalcompare.hhs.gov). If a small poke to the pocketbook doesn’t work, maybe public humiliation will.
The Problem with HACs
Although CMS’ intent in creating the HAC program—to eliminate payment for “reasonably preventable” hospital-acquired conditions, thereby improving patient safety—was good, in practice, the program has turned out to be as much about documentation as it is about providing good care. For example, if I forget to write that a Stage III pressure ulcer was present on admission, it gets coded as hospital-acquired and my hospital gets dinged.
It’s important to note that HACs as quality measures were never endorsed by the National Quality Forum (NQF), and without such an endorsement, a quality measure suffers from Rodney Dangerfield syndrome: It don’t get no respect.
Finally, it is disquieting that Meddings et al showed that hospital-acquired catheter-associated UTI rates derived from chart documentation for HACs were but a small fraction of rates determined from rigorous epidemiologic studies, demonstrating that using claims data for determining rates for that specific HAC is flawed. We can only wonder how divergent reported vs. actual rates for the other HACs are.
The Future of the HAC Program
The Affordable Care Act specifies that the lowest-performing quartile of U.S. hospitals for HAC rates will see a 1% Medicare reimbursement reduction beginning in fiscal-year 2015. That’s right: Hospitals facing possible readmissions penalties and losses under value-based purchasing also will face a HAC penalty.
Thankfully, the recently released Inpatient Prospective Payment System FY2013 Final Rule, CMS’ annual update of how hospitals are paid, specifies that the HAC measures are to be removed from public reporting on the Hospital Compare website effective Oct. 1, 2014. They will be replaced by a new set of measures that will (hopefully) be more methodologically sound, because they will require the scrutiny required for endorsement by the NQF. Exactly how these measures will look is not certain, as the rule-making has not yet occurred.
We do know that the three infection measures—catheter-associated UTI, surgical-site infection, and vascular catheter infection—will be generated from clinical data and, therefore, more methodologically sound under the Centers for Disease Control and Prevention’s (CDC) National Healthcare Safety Network. The derivation of the other measures will have to wait until the rule is written next year.
So, until further notice, pay attention to the queries of your hospital’s documentation experts when they approach you about a potential HAC!
Dr. Whitcomb is medical director of healthcare quality at Baystate Medical Center in Springfield, Mass. He is a co-founder and past president of SHM. Email him at [email protected].
The Inpatient Prospective Payment System FY2013 Final Rule charts a different future: By fiscal-year 2015 (October 2014), it will morph into a set of measures that are vetted by the National Quality Forum. Hopefully, this will be an improvement.
In recent years, hospitalists have been deluged with rules about documentation, being asked to use medical vocabulary in ways that were foreign to many of us during our training years. Much of the focus on documentation has been propelled by hospitals’ quest to optimize (“maximize” is a forbidden term) reimbursement, which is purely a function of what is written by “licensed providers” (doctors, physician assistants, and nurse practitioners) in the medical chart.
But another powerful driver of documentation practices of late is the hospital-acquired conditions (HAC) program developed by the Centers for Medicare & Medicaid Services (CMS) and enacted in 2009.
Origins of the HAC List
CMS disliked the fact that they were paying for conditions acquired in the hospital that were “reasonably preventable” if evidence-based—or at least “best”—practice was applied. After all, who likes to pay for a punctured gas tank when you brought the minivan in for an oil change? CMS worked with stakeholder groups, including SHM, to create a list of conditions known as hospital-acquired conditions (see Table 1, right).
(As an aside, SHM was supportive of CMS. In fact, we provided direct input into the final rule, recognizing some of the drawbacks of the CMS approach but understanding the larger objective of reengineering a flawed incentive system.)
The idea was that if a hospital submitted a bill to CMS that contained one of these conditions, the hospital would not be paid the amount by which that condition increased total reimbursement for that hospitalization. Note that if you’ve been told your hospital isn’t getting paid at all for patients with one of these conditions, that is not quite correct. Instead, your hospital may not get paid the added amount that is derived from having one of the diagnoses on the list submitted in your hospital’s bill to CMS for a given patient. At the end of the day, this might be a few hundred dollars each time one of these is documented—or $0, if your hospital biller can add another diagnosis in its place to capture the higher payment.
How big a hit to a hospital’s bottom line is this? Meddings and colleagues recently reported that a measly 0.003% of all hospitalizations in Michigan in 2009 saw payments lowered as a result of hospital-acquired catheter-associated UTI, one of the list’s HACs (Ann Int Med. 2012;157:305-312). When all the HACs are added together, one can extrapolate that they haven’t exactly had a big impact on hospital payments.
If the specter of nonpayment for one of these is not enough of a motivator (and it shouldn’t be, given the paltry financial stakes), the rate of HACs are now reported for all hospitals on the Hospital Compare website (www.hospitalcompare.hhs.gov). If a small poke to the pocketbook doesn’t work, maybe public humiliation will.
The Problem with HACs
Although CMS’ intent in creating the HAC program—to eliminate payment for “reasonably preventable” hospital-acquired conditions, thereby improving patient safety—was good, in practice, the program has turned out to be as much about documentation as it is about providing good care. For example, if I forget to write that a Stage III pressure ulcer was present on admission, it gets coded as hospital-acquired and my hospital gets dinged.
It’s important to note that HACs as quality measures were never endorsed by the National Quality Forum (NQF), and without such an endorsement, a quality measure suffers from Rodney Dangerfield syndrome: It don’t get no respect.
Finally, it is disquieting that Meddings et al showed that hospital-acquired catheter-associated UTI rates derived from chart documentation for HACs were but a small fraction of rates determined from rigorous epidemiologic studies, demonstrating that using claims data for determining rates for that specific HAC is flawed. We can only wonder how divergent reported vs. actual rates for the other HACs are.
The Future of the HAC Program
The Affordable Care Act specifies that the lowest-performing quartile of U.S. hospitals for HAC rates will see a 1% Medicare reimbursement reduction beginning in fiscal-year 2015. That’s right: Hospitals facing possible readmissions penalties and losses under value-based purchasing also will face a HAC penalty.
Thankfully, the recently released Inpatient Prospective Payment System FY2013 Final Rule, CMS’ annual update of how hospitals are paid, specifies that the HAC measures are to be removed from public reporting on the Hospital Compare website effective Oct. 1, 2014. They will be replaced by a new set of measures that will (hopefully) be more methodologically sound, because they will require the scrutiny required for endorsement by the NQF. Exactly how these measures will look is not certain, as the rule-making has not yet occurred.
We do know that the three infection measures—catheter-associated UTI, surgical-site infection, and vascular catheter infection—will be generated from clinical data and, therefore, more methodologically sound under the Centers for Disease Control and Prevention’s (CDC) National Healthcare Safety Network. The derivation of the other measures will have to wait until the rule is written next year.
So, until further notice, pay attention to the queries of your hospital’s documentation experts when they approach you about a potential HAC!
Dr. Whitcomb is medical director of healthcare quality at Baystate Medical Center in Springfield, Mass. He is a co-founder and past president of SHM. Email him at [email protected].
The Inpatient Prospective Payment System FY2013 Final Rule charts a different future: By fiscal-year 2015 (October 2014), it will morph into a set of measures that are vetted by the National Quality Forum. Hopefully, this will be an improvement.
In recent years, hospitalists have been deluged with rules about documentation, being asked to use medical vocabulary in ways that were foreign to many of us during our training years. Much of the focus on documentation has been propelled by hospitals’ quest to optimize (“maximize” is a forbidden term) reimbursement, which is purely a function of what is written by “licensed providers” (doctors, physician assistants, and nurse practitioners) in the medical chart.
But another powerful driver of documentation practices of late is the hospital-acquired conditions (HAC) program developed by the Centers for Medicare & Medicaid Services (CMS) and enacted in 2009.
Origins of the HAC List
CMS disliked the fact that they were paying for conditions acquired in the hospital that were “reasonably preventable” if evidence-based—or at least “best”—practice was applied. After all, who likes to pay for a punctured gas tank when you brought the minivan in for an oil change? CMS worked with stakeholder groups, including SHM, to create a list of conditions known as hospital-acquired conditions (see Table 1, right).
(As an aside, SHM was supportive of CMS. In fact, we provided direct input into the final rule, recognizing some of the drawbacks of the CMS approach but understanding the larger objective of reengineering a flawed incentive system.)
The idea was that if a hospital submitted a bill to CMS that contained one of these conditions, the hospital would not be paid the amount by which that condition increased total reimbursement for that hospitalization. Note that if you’ve been told your hospital isn’t getting paid at all for patients with one of these conditions, that is not quite correct. Instead, your hospital may not get paid the added amount that is derived from having one of the diagnoses on the list submitted in your hospital’s bill to CMS for a given patient. At the end of the day, this might be a few hundred dollars each time one of these is documented—or $0, if your hospital biller can add another diagnosis in its place to capture the higher payment.
How big a hit to a hospital’s bottom line is this? Meddings and colleagues recently reported that a measly 0.003% of all hospitalizations in Michigan in 2009 saw payments lowered as a result of hospital-acquired catheter-associated UTI, one of the list’s HACs (Ann Int Med. 2012;157:305-312). When all the HACs are added together, one can extrapolate that they haven’t exactly had a big impact on hospital payments.
If the specter of nonpayment for one of these is not enough of a motivator (and it shouldn’t be, given the paltry financial stakes), the rate of HACs are now reported for all hospitals on the Hospital Compare website (www.hospitalcompare.hhs.gov). If a small poke to the pocketbook doesn’t work, maybe public humiliation will.
The Problem with HACs
Although CMS’ intent in creating the HAC program—to eliminate payment for “reasonably preventable” hospital-acquired conditions, thereby improving patient safety—was good, in practice, the program has turned out to be as much about documentation as it is about providing good care. For example, if I forget to write that a Stage III pressure ulcer was present on admission, it gets coded as hospital-acquired and my hospital gets dinged.
It’s important to note that HACs as quality measures were never endorsed by the National Quality Forum (NQF), and without such an endorsement, a quality measure suffers from Rodney Dangerfield syndrome: It don’t get no respect.
Finally, it is disquieting that Meddings et al showed that hospital-acquired catheter-associated UTI rates derived from chart documentation for HACs were but a small fraction of rates determined from rigorous epidemiologic studies, demonstrating that using claims data for determining rates for that specific HAC is flawed. We can only wonder how divergent reported vs. actual rates for the other HACs are.
The Future of the HAC Program
The Affordable Care Act specifies that the lowest-performing quartile of U.S. hospitals for HAC rates will see a 1% Medicare reimbursement reduction beginning in fiscal-year 2015. That’s right: Hospitals facing possible readmissions penalties and losses under value-based purchasing also will face a HAC penalty.
Thankfully, the recently released Inpatient Prospective Payment System FY2013 Final Rule, CMS’ annual update of how hospitals are paid, specifies that the HAC measures are to be removed from public reporting on the Hospital Compare website effective Oct. 1, 2014. They will be replaced by a new set of measures that will (hopefully) be more methodologically sound, because they will require the scrutiny required for endorsement by the NQF. Exactly how these measures will look is not certain, as the rule-making has not yet occurred.
We do know that the three infection measures—catheter-associated UTI, surgical-site infection, and vascular catheter infection—will be generated from clinical data and, therefore, more methodologically sound under the Centers for Disease Control and Prevention’s (CDC) National Healthcare Safety Network. The derivation of the other measures will have to wait until the rule is written next year.
So, until further notice, pay attention to the queries of your hospital’s documentation experts when they approach you about a potential HAC!
Dr. Whitcomb is medical director of healthcare quality at Baystate Medical Center in Springfield, Mass. He is a co-founder and past president of SHM. Email him at [email protected].
The Quality Journey of Hospitalist David J. Yu
Hospitalist David J. Yu, MD, FACP, MBA, SFHM, medical director of the adult inpatient service at Presbyterian Healthcare Services in Albuquerque, N.M., began his quality journey by earning an MBA, then spending a week at University of Toyota in Gardena, Calif., to learn its Lean process-management techniques. He presented a Research, Innovations, and Clinical Vignettes poster at HM12 that outlined the multidisciplinary quality initiative at 453-bed Presbyterian Hospital in Albuquerque.
The project identified problems of throughput, ED diversion, disjointed team rounding, inadequate communication, multiple patient handoffs, low staff morale, and greater-than-expected length of stay (LOS).
“We really dug into the issues on a granular level,” with the participation of finance, nursing, care coordinators, physical therapy, and other staffers alongside the hospitalists, Dr. Yu says. The project ended up changing the practice from a model in which 11 hospitalist teams and five admitting hospitalists cared for patients throughout the hospital’s various floors while carrying caseloads too high to manage optimally. They replaced it with a unit-based rounding model, with hospitalists and care coordinators geographically segregated on units and multidisciplinary rounds to improve the efficiency of team communication (see “A Holy Grail,” July 2012, p. 30).
The group also used data to persuade the hospital’s administration to add hospitalist FTEs. As a result, LOS on two pilot units decreased by nearly half a day, with increased inpatient volume, higher patient satisfaction scores on Press-Ganey surveys, and an estimated net financial benefit of nearly $3.5 million between April 2010 and December 2011—even counting the additional hospitalist FTEs. This model has since spread to all medical units in the hospital.
“We need to be in the business of producing ‘defect-free’ discharges,” Dr. Yu quips. “Every group needs a local solution. But the mantra for this work is standardization....That’s where the leadership of the hospitalist comes in. It’s not, ‘Follow me,’ but ‘Hey, join us in collaborating together to come up with a solution.’
“It has been a two-year journey, and we’re still learning.”
Hospitalist David J. Yu, MD, FACP, MBA, SFHM, medical director of the adult inpatient service at Presbyterian Healthcare Services in Albuquerque, N.M., began his quality journey by earning an MBA, then spending a week at University of Toyota in Gardena, Calif., to learn its Lean process-management techniques. He presented a Research, Innovations, and Clinical Vignettes poster at HM12 that outlined the multidisciplinary quality initiative at 453-bed Presbyterian Hospital in Albuquerque.
The project identified problems of throughput, ED diversion, disjointed team rounding, inadequate communication, multiple patient handoffs, low staff morale, and greater-than-expected length of stay (LOS).
“We really dug into the issues on a granular level,” with the participation of finance, nursing, care coordinators, physical therapy, and other staffers alongside the hospitalists, Dr. Yu says. The project ended up changing the practice from a model in which 11 hospitalist teams and five admitting hospitalists cared for patients throughout the hospital’s various floors while carrying caseloads too high to manage optimally. They replaced it with a unit-based rounding model, with hospitalists and care coordinators geographically segregated on units and multidisciplinary rounds to improve the efficiency of team communication (see “A Holy Grail,” July 2012, p. 30).
The group also used data to persuade the hospital’s administration to add hospitalist FTEs. As a result, LOS on two pilot units decreased by nearly half a day, with increased inpatient volume, higher patient satisfaction scores on Press-Ganey surveys, and an estimated net financial benefit of nearly $3.5 million between April 2010 and December 2011—even counting the additional hospitalist FTEs. This model has since spread to all medical units in the hospital.
“We need to be in the business of producing ‘defect-free’ discharges,” Dr. Yu quips. “Every group needs a local solution. But the mantra for this work is standardization....That’s where the leadership of the hospitalist comes in. It’s not, ‘Follow me,’ but ‘Hey, join us in collaborating together to come up with a solution.’
“It has been a two-year journey, and we’re still learning.”
Hospitalist David J. Yu, MD, FACP, MBA, SFHM, medical director of the adult inpatient service at Presbyterian Healthcare Services in Albuquerque, N.M., began his quality journey by earning an MBA, then spending a week at University of Toyota in Gardena, Calif., to learn its Lean process-management techniques. He presented a Research, Innovations, and Clinical Vignettes poster at HM12 that outlined the multidisciplinary quality initiative at 453-bed Presbyterian Hospital in Albuquerque.
The project identified problems of throughput, ED diversion, disjointed team rounding, inadequate communication, multiple patient handoffs, low staff morale, and greater-than-expected length of stay (LOS).
“We really dug into the issues on a granular level,” with the participation of finance, nursing, care coordinators, physical therapy, and other staffers alongside the hospitalists, Dr. Yu says. The project ended up changing the practice from a model in which 11 hospitalist teams and five admitting hospitalists cared for patients throughout the hospital’s various floors while carrying caseloads too high to manage optimally. They replaced it with a unit-based rounding model, with hospitalists and care coordinators geographically segregated on units and multidisciplinary rounds to improve the efficiency of team communication (see “A Holy Grail,” July 2012, p. 30).
The group also used data to persuade the hospital’s administration to add hospitalist FTEs. As a result, LOS on two pilot units decreased by nearly half a day, with increased inpatient volume, higher patient satisfaction scores on Press-Ganey surveys, and an estimated net financial benefit of nearly $3.5 million between April 2010 and December 2011—even counting the additional hospitalist FTEs. This model has since spread to all medical units in the hospital.
“We need to be in the business of producing ‘defect-free’ discharges,” Dr. Yu quips. “Every group needs a local solution. But the mantra for this work is standardization....That’s where the leadership of the hospitalist comes in. It’s not, ‘Follow me,’ but ‘Hey, join us in collaborating together to come up with a solution.’
“It has been a two-year journey, and we’re still learning.”