User login
Defining and Protecting Scope of Practice Critical for Hospitalists
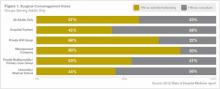
Scope creep can lead to suboptimal clinical outcomes if hospitalist practices fail to plan appropriately, Dr. Simone says. The plan must include “development of a staffing and schedule model to accommodate service expansion (when applicable), creation of policies and procedures addressing the new services, and hospitalist training (when appropriate) to ensure competently trained providers,” he adds.
Before any HM group agrees to comanagement, it should first understand the reasons for the request, Dr. Siegal says. According to a presentation he gave at HM07 on the topic, group leaders should:
- Determine if comanagement is a reasonable solution to the problem.
- Identify stakeholders and understand their goals, concerns, and expectations.
- Ask what might be jeopardized if hospitalists participate: Will it overload an already busy service, compromise care elsewhere, or set unrealistic service expectations?
- Set measurable outcomes to quantify the success (or failure) of the new arrangement.
It’s also important to define responsibilities, establish clear lines of communication, and determine how disagreements will be adjudicated. Establish your scope of practice and stick to it, Dr. Siegal says. A big red flag is when your group does things on nights, weekends, or holidays that it doesn’t do during the week.
Scope creep can lead to suboptimal clinical outcomes if hospitalist practices fail to plan appropriately, Dr. Simone says. The plan must include “development of a staffing and schedule model to accommodate service expansion (when applicable), creation of policies and procedures addressing the new services, and hospitalist training (when appropriate) to ensure competently trained providers,” he adds.
Before any HM group agrees to comanagement, it should first understand the reasons for the request, Dr. Siegal says. According to a presentation he gave at HM07 on the topic, group leaders should:
- Determine if comanagement is a reasonable solution to the problem.
- Identify stakeholders and understand their goals, concerns, and expectations.
- Ask what might be jeopardized if hospitalists participate: Will it overload an already busy service, compromise care elsewhere, or set unrealistic service expectations?
- Set measurable outcomes to quantify the success (or failure) of the new arrangement.
It’s also important to define responsibilities, establish clear lines of communication, and determine how disagreements will be adjudicated. Establish your scope of practice and stick to it, Dr. Siegal says. A big red flag is when your group does things on nights, weekends, or holidays that it doesn’t do during the week.
Scope creep can lead to suboptimal clinical outcomes if hospitalist practices fail to plan appropriately, Dr. Simone says. The plan must include “development of a staffing and schedule model to accommodate service expansion (when applicable), creation of policies and procedures addressing the new services, and hospitalist training (when appropriate) to ensure competently trained providers,” he adds.
Before any HM group agrees to comanagement, it should first understand the reasons for the request, Dr. Siegal says. According to a presentation he gave at HM07 on the topic, group leaders should:
- Determine if comanagement is a reasonable solution to the problem.
- Identify stakeholders and understand their goals, concerns, and expectations.
- Ask what might be jeopardized if hospitalists participate: Will it overload an already busy service, compromise care elsewhere, or set unrealistic service expectations?
- Set measurable outcomes to quantify the success (or failure) of the new arrangement.
It’s also important to define responsibilities, establish clear lines of communication, and determine how disagreements will be adjudicated. Establish your scope of practice and stick to it, Dr. Siegal says. A big red flag is when your group does things on nights, weekends, or holidays that it doesn’t do during the week.
Five Ways Hospitalists Can Prevent Overextending Their Services
1. Do not feel sorry for yourself; it can become a self-fulfilling prophecy.
“Most of what is happening in medicine is outside of our control,” Dr. Nelson says. “We need to realize that our role is going to change, and we should not perceive ourselves as the low person on the totem pole.”
2. Increase “face time” with your specialist colleagues.
Join them for lunch in the physician’s lounge, call your colleagues by their first names, engage in meaningful discussions about cases, and show empathy for them and their patients. Look for opportunities to do mutual education with other services.
3. Know when to draw the line.
“HM leaders should have the skills to analyze an opportunity and assess whether their program has the staffing capacity and clinical skills to successfully deliver a requested service,” Dr. Simone says. “‘No’ is an acceptable answer, if there are clear and reasonable reasons that support that decision.”
4. Make it about the patient.
Whenever your HM service is approached about comanagement, phrase your decision within the context of ensuring patient safety and delivering quality care. In that way, Dr. Siy says, you will be on solid footing.
5. Openly promote strategic “yes” answers.
Instead of digging in their heels, HM groups can periodically examine all requests, pick one or two to begin with, then promote successful outcomes to boost the group’s value.
1. Do not feel sorry for yourself; it can become a self-fulfilling prophecy.
“Most of what is happening in medicine is outside of our control,” Dr. Nelson says. “We need to realize that our role is going to change, and we should not perceive ourselves as the low person on the totem pole.”
2. Increase “face time” with your specialist colleagues.
Join them for lunch in the physician’s lounge, call your colleagues by their first names, engage in meaningful discussions about cases, and show empathy for them and their patients. Look for opportunities to do mutual education with other services.
3. Know when to draw the line.
“HM leaders should have the skills to analyze an opportunity and assess whether their program has the staffing capacity and clinical skills to successfully deliver a requested service,” Dr. Simone says. “‘No’ is an acceptable answer, if there are clear and reasonable reasons that support that decision.”
4. Make it about the patient.
Whenever your HM service is approached about comanagement, phrase your decision within the context of ensuring patient safety and delivering quality care. In that way, Dr. Siy says, you will be on solid footing.
5. Openly promote strategic “yes” answers.
Instead of digging in their heels, HM groups can periodically examine all requests, pick one or two to begin with, then promote successful outcomes to boost the group’s value.
1. Do not feel sorry for yourself; it can become a self-fulfilling prophecy.
“Most of what is happening in medicine is outside of our control,” Dr. Nelson says. “We need to realize that our role is going to change, and we should not perceive ourselves as the low person on the totem pole.”
2. Increase “face time” with your specialist colleagues.
Join them for lunch in the physician’s lounge, call your colleagues by their first names, engage in meaningful discussions about cases, and show empathy for them and their patients. Look for opportunities to do mutual education with other services.
3. Know when to draw the line.
“HM leaders should have the skills to analyze an opportunity and assess whether their program has the staffing capacity and clinical skills to successfully deliver a requested service,” Dr. Simone says. “‘No’ is an acceptable answer, if there are clear and reasonable reasons that support that decision.”
4. Make it about the patient.
Whenever your HM service is approached about comanagement, phrase your decision within the context of ensuring patient safety and delivering quality care. In that way, Dr. Siy says, you will be on solid footing.
5. Openly promote strategic “yes” answers.
Instead of digging in their heels, HM groups can periodically examine all requests, pick one or two to begin with, then promote successful outcomes to boost the group’s value.
Shaun Frost: Society of Hospital Medicine Supports the Choosing Wisely Campaign (CWC)
SHM is participating in the ABIM Foundation's Choosing Wisely Campaign (CWC).1 Launched earlier this year, the CWC aims to increase awareness about medical practices that may be of little or no benefit to patients. Presently, 26 physician organizations have teamed with the ABIM Foundation to each create a list of “five things physicians and patients should question.” In addition, Consumer Reports (the product ratings organization well known for grading the quality of such items as automobiles and vacuum cleaners) is coordinating the efforts of 11 consumer groups to advance the CWC agenda.
The CWC aims to highlight two pillars of healthcare reform that will receive enhanced attention in the near future: 1. Cost of care, and 2. Patient experience of care. Heretofore healthcare reform efforts have largely been focused on the quality and patient-safety movements. Equally important, however, to policymakers is affordability and care experience. By focusing on tests and procedures of questionable benefit, the CWC aims to directly address costly unnecessary treatment by encouraging care planning that incorporates patient preferences. This is necessary work because research suggests that physician decisions account for 80% of healthcare expenditures, while the tradition of patients entrusting their doctors with complete decision-making authority leads to care that they do not want.2
Choosing Wisely Begins with Medical Professionalism
In 2002, the ABIM Foundation collaborated with the American College of Physicians Foundation and the European Federation of Internal Medicine to jointly author “Medical Professionalism in the New Millennium: A Physician Charter.”3 The charter has since been endorsed by more than 130 organizations and triggered countless improvement initiatives to advance its fundamental principles of patient welfare, patient autonomy, and social justice.
Through project grant support, the ABIM Foundation is emphasizing two key Physician Charter commitments (see Table 1) to advance appropriate healthcare decision-making and encourage stewardship of healthcare resources. The CWC naturally augments this work by focusing on care affordability and decision-making through shared discussions between patients and providers.
SHM’s Involvement
SHM convened a workgroup of hospital medicine quality improvement experts led by John Bulger, DO, the chief quality officer at Geisinger Health System in Pennsylvania. This group solicited from SHM committee members 150 suggested tests and treatments that HM clinicians and their patients should question. After critical analysis, the list was narrowed to exclude suggestions already being advanced by the CWC while focusing on those that represent the largest opportunity for hospitalists to impact on affordability and patient experience.
The list was then submitted to SHM members for comment via survey, resulting in 11 recommended medical interventions that were subjected to comprehensive literature review. Workgroup members then rated these 11 interventions according to the following criteria: validity of supporting evidence, feasibility and degree of hospitalist impact, frequency of occurrence, and cost of occurrence.
Finally, the workgroup collaborated with the SHM board of directors to submit to the ABIM Foundation the ultimate list of “five things hospitalists and their patients should question.” Ricardo Quinonez, MD, at Baylor College of Medicine in Houston, Texas, led a similar process that generated a list of questionable practices in pediatric HM. It, too, was submitted to the ABIM Foundation.
The CWC anticipates publishing SHM’s list in February 2013. In the meantime, please consult the CWC website to find practices commonly performed by hospitalists that have been deemed to be of unclear benefit by other professional medical societies (see “2012 CWC Recommendations for Hospitalists,” left).
SHM plans to build upon this work in the future. Expect to see Choosing Wisely sessions and discussions at the HM13 SHM Annual Meeting in May (www.hospitalmedicine2013.org) focused on creating and teaching QI strategies to implement CWC recommendations. Furthermore, the Center for Hospital Innovation and Improvement will be identifying opportunities to develop mentored implementation QI programs related to Choosing Wisely and its principles.
What You Can Do
Hospitalists can make a huge impact on affordability and patient experience given that most of the country’s healthcare dollar is spent in the hospital, and patients are at their most vulnerable to receiving treatment that they may not want when they are acutely ill. Hospitalists, thus, are uniquely positioned to make a positive impact by embracing the Choosing Wisely Campaign’s principles.
Please commit to assisting SHM by visiting the CWC website and learning about other medical society’s thoughts on “things physicians and patients should question.” Pledge thereafter to engage your patients and their families in healthcare decision-making, especially in situations where the benefits of tests and therapies are unclear.
Attention to care affordability and experience are essential to reforming our broken healthcare system, so let’s lead the charge in these areas and help others who are doing the same.
Dr. Frost is president of SHM.
References
- The ABIM Foundation. Choosing Wisely: An initiative of the ABIM Foundation. Choosing Wisely website. Available at: http://www.choosingwisely.org. Accessed Sept. 25, 2012.
- The ABIM Foundation. Principles Guiding Wise Choices. ABIM Foundation website. Available at: www.abimfoundation.org/Initiatives/~/media/Files/2011-Forum/110411_ABIM%20Stewardship.ashx. Accessed Sept. 25, 2012.
- ABIM Foundation, ACP–ASIM Foundation, European Federation of Internal Medicine. Medical Professionalism in the New Millennium: A Physician Charter. Ann Intern Med. 2002;136(3):243.
SHM is participating in the ABIM Foundation's Choosing Wisely Campaign (CWC).1 Launched earlier this year, the CWC aims to increase awareness about medical practices that may be of little or no benefit to patients. Presently, 26 physician organizations have teamed with the ABIM Foundation to each create a list of “five things physicians and patients should question.” In addition, Consumer Reports (the product ratings organization well known for grading the quality of such items as automobiles and vacuum cleaners) is coordinating the efforts of 11 consumer groups to advance the CWC agenda.
The CWC aims to highlight two pillars of healthcare reform that will receive enhanced attention in the near future: 1. Cost of care, and 2. Patient experience of care. Heretofore healthcare reform efforts have largely been focused on the quality and patient-safety movements. Equally important, however, to policymakers is affordability and care experience. By focusing on tests and procedures of questionable benefit, the CWC aims to directly address costly unnecessary treatment by encouraging care planning that incorporates patient preferences. This is necessary work because research suggests that physician decisions account for 80% of healthcare expenditures, while the tradition of patients entrusting their doctors with complete decision-making authority leads to care that they do not want.2
Choosing Wisely Begins with Medical Professionalism
In 2002, the ABIM Foundation collaborated with the American College of Physicians Foundation and the European Federation of Internal Medicine to jointly author “Medical Professionalism in the New Millennium: A Physician Charter.”3 The charter has since been endorsed by more than 130 organizations and triggered countless improvement initiatives to advance its fundamental principles of patient welfare, patient autonomy, and social justice.
Through project grant support, the ABIM Foundation is emphasizing two key Physician Charter commitments (see Table 1) to advance appropriate healthcare decision-making and encourage stewardship of healthcare resources. The CWC naturally augments this work by focusing on care affordability and decision-making through shared discussions between patients and providers.
SHM’s Involvement
SHM convened a workgroup of hospital medicine quality improvement experts led by John Bulger, DO, the chief quality officer at Geisinger Health System in Pennsylvania. This group solicited from SHM committee members 150 suggested tests and treatments that HM clinicians and their patients should question. After critical analysis, the list was narrowed to exclude suggestions already being advanced by the CWC while focusing on those that represent the largest opportunity for hospitalists to impact on affordability and patient experience.
The list was then submitted to SHM members for comment via survey, resulting in 11 recommended medical interventions that were subjected to comprehensive literature review. Workgroup members then rated these 11 interventions according to the following criteria: validity of supporting evidence, feasibility and degree of hospitalist impact, frequency of occurrence, and cost of occurrence.
Finally, the workgroup collaborated with the SHM board of directors to submit to the ABIM Foundation the ultimate list of “five things hospitalists and their patients should question.” Ricardo Quinonez, MD, at Baylor College of Medicine in Houston, Texas, led a similar process that generated a list of questionable practices in pediatric HM. It, too, was submitted to the ABIM Foundation.
The CWC anticipates publishing SHM’s list in February 2013. In the meantime, please consult the CWC website to find practices commonly performed by hospitalists that have been deemed to be of unclear benefit by other professional medical societies (see “2012 CWC Recommendations for Hospitalists,” left).
SHM plans to build upon this work in the future. Expect to see Choosing Wisely sessions and discussions at the HM13 SHM Annual Meeting in May (www.hospitalmedicine2013.org) focused on creating and teaching QI strategies to implement CWC recommendations. Furthermore, the Center for Hospital Innovation and Improvement will be identifying opportunities to develop mentored implementation QI programs related to Choosing Wisely and its principles.
What You Can Do
Hospitalists can make a huge impact on affordability and patient experience given that most of the country’s healthcare dollar is spent in the hospital, and patients are at their most vulnerable to receiving treatment that they may not want when they are acutely ill. Hospitalists, thus, are uniquely positioned to make a positive impact by embracing the Choosing Wisely Campaign’s principles.
Please commit to assisting SHM by visiting the CWC website and learning about other medical society’s thoughts on “things physicians and patients should question.” Pledge thereafter to engage your patients and their families in healthcare decision-making, especially in situations where the benefits of tests and therapies are unclear.
Attention to care affordability and experience are essential to reforming our broken healthcare system, so let’s lead the charge in these areas and help others who are doing the same.
Dr. Frost is president of SHM.
References
- The ABIM Foundation. Choosing Wisely: An initiative of the ABIM Foundation. Choosing Wisely website. Available at: http://www.choosingwisely.org. Accessed Sept. 25, 2012.
- The ABIM Foundation. Principles Guiding Wise Choices. ABIM Foundation website. Available at: www.abimfoundation.org/Initiatives/~/media/Files/2011-Forum/110411_ABIM%20Stewardship.ashx. Accessed Sept. 25, 2012.
- ABIM Foundation, ACP–ASIM Foundation, European Federation of Internal Medicine. Medical Professionalism in the New Millennium: A Physician Charter. Ann Intern Med. 2002;136(3):243.
SHM is participating in the ABIM Foundation's Choosing Wisely Campaign (CWC).1 Launched earlier this year, the CWC aims to increase awareness about medical practices that may be of little or no benefit to patients. Presently, 26 physician organizations have teamed with the ABIM Foundation to each create a list of “five things physicians and patients should question.” In addition, Consumer Reports (the product ratings organization well known for grading the quality of such items as automobiles and vacuum cleaners) is coordinating the efforts of 11 consumer groups to advance the CWC agenda.
The CWC aims to highlight two pillars of healthcare reform that will receive enhanced attention in the near future: 1. Cost of care, and 2. Patient experience of care. Heretofore healthcare reform efforts have largely been focused on the quality and patient-safety movements. Equally important, however, to policymakers is affordability and care experience. By focusing on tests and procedures of questionable benefit, the CWC aims to directly address costly unnecessary treatment by encouraging care planning that incorporates patient preferences. This is necessary work because research suggests that physician decisions account for 80% of healthcare expenditures, while the tradition of patients entrusting their doctors with complete decision-making authority leads to care that they do not want.2
Choosing Wisely Begins with Medical Professionalism
In 2002, the ABIM Foundation collaborated with the American College of Physicians Foundation and the European Federation of Internal Medicine to jointly author “Medical Professionalism in the New Millennium: A Physician Charter.”3 The charter has since been endorsed by more than 130 organizations and triggered countless improvement initiatives to advance its fundamental principles of patient welfare, patient autonomy, and social justice.
Through project grant support, the ABIM Foundation is emphasizing two key Physician Charter commitments (see Table 1) to advance appropriate healthcare decision-making and encourage stewardship of healthcare resources. The CWC naturally augments this work by focusing on care affordability and decision-making through shared discussions between patients and providers.
SHM’s Involvement
SHM convened a workgroup of hospital medicine quality improvement experts led by John Bulger, DO, the chief quality officer at Geisinger Health System in Pennsylvania. This group solicited from SHM committee members 150 suggested tests and treatments that HM clinicians and their patients should question. After critical analysis, the list was narrowed to exclude suggestions already being advanced by the CWC while focusing on those that represent the largest opportunity for hospitalists to impact on affordability and patient experience.
The list was then submitted to SHM members for comment via survey, resulting in 11 recommended medical interventions that were subjected to comprehensive literature review. Workgroup members then rated these 11 interventions according to the following criteria: validity of supporting evidence, feasibility and degree of hospitalist impact, frequency of occurrence, and cost of occurrence.
Finally, the workgroup collaborated with the SHM board of directors to submit to the ABIM Foundation the ultimate list of “five things hospitalists and their patients should question.” Ricardo Quinonez, MD, at Baylor College of Medicine in Houston, Texas, led a similar process that generated a list of questionable practices in pediatric HM. It, too, was submitted to the ABIM Foundation.
The CWC anticipates publishing SHM’s list in February 2013. In the meantime, please consult the CWC website to find practices commonly performed by hospitalists that have been deemed to be of unclear benefit by other professional medical societies (see “2012 CWC Recommendations for Hospitalists,” left).
SHM plans to build upon this work in the future. Expect to see Choosing Wisely sessions and discussions at the HM13 SHM Annual Meeting in May (www.hospitalmedicine2013.org) focused on creating and teaching QI strategies to implement CWC recommendations. Furthermore, the Center for Hospital Innovation and Improvement will be identifying opportunities to develop mentored implementation QI programs related to Choosing Wisely and its principles.
What You Can Do
Hospitalists can make a huge impact on affordability and patient experience given that most of the country’s healthcare dollar is spent in the hospital, and patients are at their most vulnerable to receiving treatment that they may not want when they are acutely ill. Hospitalists, thus, are uniquely positioned to make a positive impact by embracing the Choosing Wisely Campaign’s principles.
Please commit to assisting SHM by visiting the CWC website and learning about other medical society’s thoughts on “things physicians and patients should question.” Pledge thereafter to engage your patients and their families in healthcare decision-making, especially in situations where the benefits of tests and therapies are unclear.
Attention to care affordability and experience are essential to reforming our broken healthcare system, so let’s lead the charge in these areas and help others who are doing the same.
Dr. Frost is president of SHM.
References
- The ABIM Foundation. Choosing Wisely: An initiative of the ABIM Foundation. Choosing Wisely website. Available at: http://www.choosingwisely.org. Accessed Sept. 25, 2012.
- The ABIM Foundation. Principles Guiding Wise Choices. ABIM Foundation website. Available at: www.abimfoundation.org/Initiatives/~/media/Files/2011-Forum/110411_ABIM%20Stewardship.ashx. Accessed Sept. 25, 2012.
- ABIM Foundation, ACP–ASIM Foundation, European Federation of Internal Medicine. Medical Professionalism in the New Millennium: A Physician Charter. Ann Intern Med. 2002;136(3):243.
Why It's Hard for Healthcare Providers to Say I'm Sorry
It’s 1982, and in middle-school gyms across the country, among punch bowls and parental scrutiny, young girls and boys are slow-dancing with outstretched arms to a breathtaking song by the band Chicago. The song tells of the agonizing difficulty of apology, how despite the want and need to apologize, it is just too arduous.
Fast-forward 30 years, and it is hard to believe that cheesy No. 1 Billboard hit espoused the feelings that continue to haunt healthcare providers across the country: It’s hard for me to say I’m sorry.
Others Say It
If you look at the world around us, you see apology everywhere. Customer service representatives and customer-minded industries routinely let those words flow off their tongues with ease and grace.
While I was driving down the interstate last week, the number of traffic lanes shrunk from three to two to one. Anticipating widespread aggravation from weary travelers, the state transportation department deployed several large road signs every few miles; they read “WE APOLOGIZE FOR THE INCONVENIENCE … BEAR WITH US WHILE WE MAKE YOUR ROADS SMOOTHER AND SAFER.” Those simple messages made me feel like the congestion was not a senseless waste of time, that the state’s Department of Transportation was actually being strategic and thoughtful in their rationing of lanes during rush hour in the middle of the week.
Phone-based, customer-service departments figured out the simple apology a long time ago. While holding the line for a Lands’ End customer-service representative a few weeks ago, I heard, “We apologize for the delay. Your business is important to us. Please hold the line while we address callers ahead of you.” It validated for me that those phone representatives are not just sitting around eating lunch, completely ignoring my call, and that maybe there are others who procrastinated buying back-to-school backpacks until September—and just happened to call right before me.
I even got an apology at the dry cleaner. Amidst my last batch of clothes, my astute dry cleaner apparently found a very stubborn stain, which resisted all of their usual concoctions. It was on the back of a shirt and I probably would not have even noticed it was there. But nonetheless, they sent an apology tag, with a picture of a distraught butler who seemed to have struggled with that stain for hours.
Why Not Us?
So why is “sorry” so hard in healthcare? When things happen to patients, things that are inconvenient or downright dangerous, we have great difficulty in simply saying: “Hey, I am really sorry this happened to you,” or “I am so sorry you are still here. You must be really frustrated by our inefficiencies.”
I have the distinct pleasure of overseeing my hospital’s risk-management department for a few months. This means I get to see and hear what does and doesn’t happen to patients, which, at times, is misaligned with what should or shouldn’t happen to patients. When unanticipated events occur, the group launches into an investigation of what happened, why it happened, and the risk that it could happen again. After the initial dust settles and the facts are relayed from the care team to the risk-management team, the risk team always asks of those involved: “So what does the patient and their family know?” And we get a range of answers—some polished, some fumbled, some baffled.
The next question is: “Well, what should they know?” And that is always an easy question to answer. They should know the truth. Not just some of the truth, or half the truth, or a partial truth. Not what the care team thinks the patient “can handle.” They should just get the truth. To the best of the team’s ability, they should tell the patient:
- What (they think) happened;
- Why (they think) it happened;
- What it means for the patient; and
- What they are going to do to make it not happen again.
And then the patient (and family members) deserve an apology—sincere, compassionate, genuine. The apology should be the easy part, as most providers do not always know what happened, why it happened, or what they are going to do to prevent it from happening, but they usually truly do feel sorry that it happened at all.
“Sorry”=Positive Results
Patients are unanimous in their desire to be informed if a medical error has occurred; focus groups have found that patients believe such information would enhance their trust in their physicians and would reassure them that they were receiving complete information. And they want an apology.1
But interestingly, many physicians believe that full disclosure with apology is not warranted or appropriate, and that the apology could erode patient trust, might scare the patient, and might increase the risk of legal liability.1
There is little evidence that disclosure is harmful or detrimental, and there is some evidence that it is beneficial to the medical industry (i.e. reduces claims and litigation costs). A study published in 2010 from the University of Michigan Health System found a disclosure-with-compensation program was associated with a 36% reduction in new claims, a 65% reduction in lawsuits, and a 59% reduction in total liability cost.2
I have witnessed this phenomenon from both sides. My mother, who has Alzheimer’s and lives in an assisted-living facility, recently was given twice the dose of her medications one morning. She was “given” her night medications by being placed in her room, which she has no recollection of (the staff are supposed to watch her take her medications). The next morning, she saw the medications and took them, then took another dose when the nurse came by to give her morning medications. It was not realized until she’d already taken the medications and the staff noticed the medicine cup from the night before. My mom said she felt a little weak and dizzy for a few hours, but nothing significant, and she fully recovered. Interestingly, my mom mentioned it in passing, but no one called to let us know a medication error had occurred. Although she was not harmed, it made us, her family, lose a little trust in the facility because we found out about it indirectly, without any acknowledgement or apology.
On the other side of the equation, I have witnessed countless numbers of patient events in which providers feel worried and uncomfortable about the effects of disclosure with apology on themselves and their patients.
The bottom line is, disclosure with apology is needed and appreciated by patients, and it is absolutely the right thing to do. So download that cheesy Chicago song to your iPod and practice saying (or singing) “I’m sorry.” If the butler with chemicals can do it, so can we.
Dr. Scheurer is a hospitalist and chief quality officer at the Medical University of South Carolina in Charleston. She is physician editor of The Hospitalist. Email her at [email protected].
References
- Gallagher TH, Waterman AD, Ebers AG, Fraser VJ, Levinson W. Patients’ and physicians’ attitudes regarding the disclosure of medical errors. JAMA. 2003;289(8):1001-1007.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153(4):213-221.
It’s 1982, and in middle-school gyms across the country, among punch bowls and parental scrutiny, young girls and boys are slow-dancing with outstretched arms to a breathtaking song by the band Chicago. The song tells of the agonizing difficulty of apology, how despite the want and need to apologize, it is just too arduous.
Fast-forward 30 years, and it is hard to believe that cheesy No. 1 Billboard hit espoused the feelings that continue to haunt healthcare providers across the country: It’s hard for me to say I’m sorry.
Others Say It
If you look at the world around us, you see apology everywhere. Customer service representatives and customer-minded industries routinely let those words flow off their tongues with ease and grace.
While I was driving down the interstate last week, the number of traffic lanes shrunk from three to two to one. Anticipating widespread aggravation from weary travelers, the state transportation department deployed several large road signs every few miles; they read “WE APOLOGIZE FOR THE INCONVENIENCE … BEAR WITH US WHILE WE MAKE YOUR ROADS SMOOTHER AND SAFER.” Those simple messages made me feel like the congestion was not a senseless waste of time, that the state’s Department of Transportation was actually being strategic and thoughtful in their rationing of lanes during rush hour in the middle of the week.
Phone-based, customer-service departments figured out the simple apology a long time ago. While holding the line for a Lands’ End customer-service representative a few weeks ago, I heard, “We apologize for the delay. Your business is important to us. Please hold the line while we address callers ahead of you.” It validated for me that those phone representatives are not just sitting around eating lunch, completely ignoring my call, and that maybe there are others who procrastinated buying back-to-school backpacks until September—and just happened to call right before me.
I even got an apology at the dry cleaner. Amidst my last batch of clothes, my astute dry cleaner apparently found a very stubborn stain, which resisted all of their usual concoctions. It was on the back of a shirt and I probably would not have even noticed it was there. But nonetheless, they sent an apology tag, with a picture of a distraught butler who seemed to have struggled with that stain for hours.
Why Not Us?
So why is “sorry” so hard in healthcare? When things happen to patients, things that are inconvenient or downright dangerous, we have great difficulty in simply saying: “Hey, I am really sorry this happened to you,” or “I am so sorry you are still here. You must be really frustrated by our inefficiencies.”
I have the distinct pleasure of overseeing my hospital’s risk-management department for a few months. This means I get to see and hear what does and doesn’t happen to patients, which, at times, is misaligned with what should or shouldn’t happen to patients. When unanticipated events occur, the group launches into an investigation of what happened, why it happened, and the risk that it could happen again. After the initial dust settles and the facts are relayed from the care team to the risk-management team, the risk team always asks of those involved: “So what does the patient and their family know?” And we get a range of answers—some polished, some fumbled, some baffled.
The next question is: “Well, what should they know?” And that is always an easy question to answer. They should know the truth. Not just some of the truth, or half the truth, or a partial truth. Not what the care team thinks the patient “can handle.” They should just get the truth. To the best of the team’s ability, they should tell the patient:
- What (they think) happened;
- Why (they think) it happened;
- What it means for the patient; and
- What they are going to do to make it not happen again.
And then the patient (and family members) deserve an apology—sincere, compassionate, genuine. The apology should be the easy part, as most providers do not always know what happened, why it happened, or what they are going to do to prevent it from happening, but they usually truly do feel sorry that it happened at all.
“Sorry”=Positive Results
Patients are unanimous in their desire to be informed if a medical error has occurred; focus groups have found that patients believe such information would enhance their trust in their physicians and would reassure them that they were receiving complete information. And they want an apology.1
But interestingly, many physicians believe that full disclosure with apology is not warranted or appropriate, and that the apology could erode patient trust, might scare the patient, and might increase the risk of legal liability.1
There is little evidence that disclosure is harmful or detrimental, and there is some evidence that it is beneficial to the medical industry (i.e. reduces claims and litigation costs). A study published in 2010 from the University of Michigan Health System found a disclosure-with-compensation program was associated with a 36% reduction in new claims, a 65% reduction in lawsuits, and a 59% reduction in total liability cost.2
I have witnessed this phenomenon from both sides. My mother, who has Alzheimer’s and lives in an assisted-living facility, recently was given twice the dose of her medications one morning. She was “given” her night medications by being placed in her room, which she has no recollection of (the staff are supposed to watch her take her medications). The next morning, she saw the medications and took them, then took another dose when the nurse came by to give her morning medications. It was not realized until she’d already taken the medications and the staff noticed the medicine cup from the night before. My mom said she felt a little weak and dizzy for a few hours, but nothing significant, and she fully recovered. Interestingly, my mom mentioned it in passing, but no one called to let us know a medication error had occurred. Although she was not harmed, it made us, her family, lose a little trust in the facility because we found out about it indirectly, without any acknowledgement or apology.
On the other side of the equation, I have witnessed countless numbers of patient events in which providers feel worried and uncomfortable about the effects of disclosure with apology on themselves and their patients.
The bottom line is, disclosure with apology is needed and appreciated by patients, and it is absolutely the right thing to do. So download that cheesy Chicago song to your iPod and practice saying (or singing) “I’m sorry.” If the butler with chemicals can do it, so can we.
Dr. Scheurer is a hospitalist and chief quality officer at the Medical University of South Carolina in Charleston. She is physician editor of The Hospitalist. Email her at [email protected].
References
- Gallagher TH, Waterman AD, Ebers AG, Fraser VJ, Levinson W. Patients’ and physicians’ attitudes regarding the disclosure of medical errors. JAMA. 2003;289(8):1001-1007.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153(4):213-221.
It’s 1982, and in middle-school gyms across the country, among punch bowls and parental scrutiny, young girls and boys are slow-dancing with outstretched arms to a breathtaking song by the band Chicago. The song tells of the agonizing difficulty of apology, how despite the want and need to apologize, it is just too arduous.
Fast-forward 30 years, and it is hard to believe that cheesy No. 1 Billboard hit espoused the feelings that continue to haunt healthcare providers across the country: It’s hard for me to say I’m sorry.
Others Say It
If you look at the world around us, you see apology everywhere. Customer service representatives and customer-minded industries routinely let those words flow off their tongues with ease and grace.
While I was driving down the interstate last week, the number of traffic lanes shrunk from three to two to one. Anticipating widespread aggravation from weary travelers, the state transportation department deployed several large road signs every few miles; they read “WE APOLOGIZE FOR THE INCONVENIENCE … BEAR WITH US WHILE WE MAKE YOUR ROADS SMOOTHER AND SAFER.” Those simple messages made me feel like the congestion was not a senseless waste of time, that the state’s Department of Transportation was actually being strategic and thoughtful in their rationing of lanes during rush hour in the middle of the week.
Phone-based, customer-service departments figured out the simple apology a long time ago. While holding the line for a Lands’ End customer-service representative a few weeks ago, I heard, “We apologize for the delay. Your business is important to us. Please hold the line while we address callers ahead of you.” It validated for me that those phone representatives are not just sitting around eating lunch, completely ignoring my call, and that maybe there are others who procrastinated buying back-to-school backpacks until September—and just happened to call right before me.
I even got an apology at the dry cleaner. Amidst my last batch of clothes, my astute dry cleaner apparently found a very stubborn stain, which resisted all of their usual concoctions. It was on the back of a shirt and I probably would not have even noticed it was there. But nonetheless, they sent an apology tag, with a picture of a distraught butler who seemed to have struggled with that stain for hours.
Why Not Us?
So why is “sorry” so hard in healthcare? When things happen to patients, things that are inconvenient or downright dangerous, we have great difficulty in simply saying: “Hey, I am really sorry this happened to you,” or “I am so sorry you are still here. You must be really frustrated by our inefficiencies.”
I have the distinct pleasure of overseeing my hospital’s risk-management department for a few months. This means I get to see and hear what does and doesn’t happen to patients, which, at times, is misaligned with what should or shouldn’t happen to patients. When unanticipated events occur, the group launches into an investigation of what happened, why it happened, and the risk that it could happen again. After the initial dust settles and the facts are relayed from the care team to the risk-management team, the risk team always asks of those involved: “So what does the patient and their family know?” And we get a range of answers—some polished, some fumbled, some baffled.
The next question is: “Well, what should they know?” And that is always an easy question to answer. They should know the truth. Not just some of the truth, or half the truth, or a partial truth. Not what the care team thinks the patient “can handle.” They should just get the truth. To the best of the team’s ability, they should tell the patient:
- What (they think) happened;
- Why (they think) it happened;
- What it means for the patient; and
- What they are going to do to make it not happen again.
And then the patient (and family members) deserve an apology—sincere, compassionate, genuine. The apology should be the easy part, as most providers do not always know what happened, why it happened, or what they are going to do to prevent it from happening, but they usually truly do feel sorry that it happened at all.
“Sorry”=Positive Results
Patients are unanimous in their desire to be informed if a medical error has occurred; focus groups have found that patients believe such information would enhance their trust in their physicians and would reassure them that they were receiving complete information. And they want an apology.1
But interestingly, many physicians believe that full disclosure with apology is not warranted or appropriate, and that the apology could erode patient trust, might scare the patient, and might increase the risk of legal liability.1
There is little evidence that disclosure is harmful or detrimental, and there is some evidence that it is beneficial to the medical industry (i.e. reduces claims and litigation costs). A study published in 2010 from the University of Michigan Health System found a disclosure-with-compensation program was associated with a 36% reduction in new claims, a 65% reduction in lawsuits, and a 59% reduction in total liability cost.2
I have witnessed this phenomenon from both sides. My mother, who has Alzheimer’s and lives in an assisted-living facility, recently was given twice the dose of her medications one morning. She was “given” her night medications by being placed in her room, which she has no recollection of (the staff are supposed to watch her take her medications). The next morning, she saw the medications and took them, then took another dose when the nurse came by to give her morning medications. It was not realized until she’d already taken the medications and the staff noticed the medicine cup from the night before. My mom said she felt a little weak and dizzy for a few hours, but nothing significant, and she fully recovered. Interestingly, my mom mentioned it in passing, but no one called to let us know a medication error had occurred. Although she was not harmed, it made us, her family, lose a little trust in the facility because we found out about it indirectly, without any acknowledgement or apology.
On the other side of the equation, I have witnessed countless numbers of patient events in which providers feel worried and uncomfortable about the effects of disclosure with apology on themselves and their patients.
The bottom line is, disclosure with apology is needed and appreciated by patients, and it is absolutely the right thing to do. So download that cheesy Chicago song to your iPod and practice saying (or singing) “I’m sorry.” If the butler with chemicals can do it, so can we.
Dr. Scheurer is a hospitalist and chief quality officer at the Medical University of South Carolina in Charleston. She is physician editor of The Hospitalist. Email her at [email protected].
References
- Gallagher TH, Waterman AD, Ebers AG, Fraser VJ, Levinson W. Patients’ and physicians’ attitudes regarding the disclosure of medical errors. JAMA. 2003;289(8):1001-1007.
- Kachalia A, Kaufman SR, Boothman R, et al. Liability claims and costs before and after implementation of a medical error disclosure program. Ann Intern Med. 2010;153(4):213-221.
Why It's Hard for Healthcare Providers to Say I'm Sorry
Enter text here
Enter text here
Enter text here
John Nelson: Learning CPT Coding and Documentation Tricky for Hospitalists
There is a lot to learn when it comes to proper coding and the documentation requirements that go with it. It can even be tricky for a new residency grad to keep the difference in CPT and ICD-9 coding straight, to say nothing of the difference between documentation requirements for physician reimbursement versus hospital reimbursement. This column addresses only physician CPT coding (I’ll save documentation to support hospital billing for another column).
Although I believe that devoting the large number of brain cells required to keep this stuff straight gets in the way of maintaining necessary clinical knowledge, physicians have no real choice but to do it. (One could argue that having a professional coder read charts to determine proper CPT codes relieves a doctor of the burden of documentation and coding headaches. But this is only partially true. The doctor still needs to ensure that the documentation accurately reflects what was done for the coder to be able to select the appropriate codes, so he still needs to know a lot about this topic.)
All providers have a duty to reasonably ensure that submitted claims (bills) are true and accurate. Failing to document and code correctly risks anything from you or your employer having to return money, potentially with a penalty and interest, to being accused of criminal fraud.
Medicare and other payors generally categorize inaccurate claims as follows:
- Erroneous claims include inadvertent mistakes, innocent errors, or even negligence but still require payments associated with the error to be returned.
- Fraudulent claims are ones judged to be intentionally or recklessly false, and are subject to administrative or civil penalties, such as fines.
- Claims associated with criminal intent to defraud are subject to criminal penalties, which could include jail time.
While I haven’t heard of any hospitalists being accused of fraud, I know of several who have undergone audits and been required to return money. Whether your employer would refund the money or you would have to write a personal check to refund the money depends on your employment situation. For example, in most cases, the hospital would be liable to make the repayment for hospitalists it employs. If you’re an independent contractor, there is a good chance you could be stuck making the repayment yourself.
Trend: Increased Use of Higher-Level Codes
You might have missed it, but there was a recent study of Medicare Part B claims data from 2001 to 2010 showing that “physicians increased their billing of higher-level E/M codes in all types of E/M services.”1 For example, the report showed a steady decrease in use of the 99231 code, the lowest of the three subsequent inpatient hospital care codes, and an increase in the highest level code, 99233 (see Figure 1, below).
I can think of two reasons hospitalists might be increasing the use of higher codes. One, less-sick patients just aren’t seen in the hospital as often as they used to be, so the remaining patients require more intensive services, which could lead to the appropriate use of higher-level codes. Two, doctors have over the past 10 to 15 years invested more energy in learning appropriate documentation and coding, which might have led to correcting historical overuse of lower-level codes.
Did I tell you who conducted the study showing increased use of higher-level codes? It was the federal Office of Inspector General (OIG), which is responsible for preventing and detecting fraud and waste. Although the OIG might agree that the sicker patients and correction of historical undercoding might explain some of the trend, it’s a pretty safe bet they’re also concerned that a significant portion is inappropriate or fraudulent. Some portion of it probably is.
“CMS concurred with [OIG’s] recommendations to (1) continue to educate physicians on proper billing for E/M services and (2) encourage its contractor to review physicians’ billing for E/M services. CMS partially concurred with [OIG’s] third recommendation, to review physicians who bill higher-level E/M codes for appropriate action,” the OIG report noted.1
Plan for Education, Compliance
My sense is that most hospitalists employed by a large entity, such as a hospital or large medical group, have access to a certified coder to perform documentation and coding audits, as well as educational feedback when needed. If your practice doesn’t have access to a certified coder, you should consider photocopying some chart notes (e.g. 10 notes from each of your docs) and send them to an outside coder for an audit. Though they are very valuable, audits usually are not enough to ensure good performance.
In my March 2007 column, I described a reasonably simple chart audit allowing each doctor to compare his or her CPT coding pattern to everyone else in the group. You can compare your own coding to national coding patterns via SHM’s 2012 State of Hospital Medicine Report (www.hospitalmedicine.org/survey) or data from the CMS website, and the Medical Group Management Association (MGMA) will have data in future surveys. Such comparisons might help uncover unusual patterns that are worthy of a closer look.
Other strategies to promote proper documentation and coding include online educational programs, such as:
- SHM’s CODE-H webinars (www.hospitalmedicine.org/codeh), which are available on demand for a fee;
- American Association of Professional Coders Evaluation and Management Online Training (http://www.aapc.com/training/evaluation-management-coding-training.aspx); and
- The American Health Information Management Association’s (AHIMA) Coding Basics Program (www.ahima.org/continuinged/campus/courseinfo/cb.aspx).
If you prefer, an Internet search can turn up in-person courses to learn documentation and coding. Additionally, your in-house or external coding auditors can provide training.
To address tricky issues that come up only occasionally, several in our practice have compiled a “coding manual” by distilling guidance from our certified coders and compliance people on issues as they came up. Some issues would stump all of us, and we’d have to go to the Internet for help. All hospitalists are provided with a copy of the manual during orientation, and an electronic copy is available on the hospital’s Intranet. Topics addressed in the manual include things like how to bill the first inpatient day when a patient has changed from observation status, how to bill initial consult visits for various payors (an issue since Medicare eliminated consult codes a few years ago), how to bill when a patient is seen and discharged from the ED, etc.
Lastly, I suggest someone in your group talk with your hospital’s compliance department about its own coding and billing compliance plan. This could lead to ideas or help develop a compliance plan for your group.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is course co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
Reference
There is a lot to learn when it comes to proper coding and the documentation requirements that go with it. It can even be tricky for a new residency grad to keep the difference in CPT and ICD-9 coding straight, to say nothing of the difference between documentation requirements for physician reimbursement versus hospital reimbursement. This column addresses only physician CPT coding (I’ll save documentation to support hospital billing for another column).
Although I believe that devoting the large number of brain cells required to keep this stuff straight gets in the way of maintaining necessary clinical knowledge, physicians have no real choice but to do it. (One could argue that having a professional coder read charts to determine proper CPT codes relieves a doctor of the burden of documentation and coding headaches. But this is only partially true. The doctor still needs to ensure that the documentation accurately reflects what was done for the coder to be able to select the appropriate codes, so he still needs to know a lot about this topic.)
All providers have a duty to reasonably ensure that submitted claims (bills) are true and accurate. Failing to document and code correctly risks anything from you or your employer having to return money, potentially with a penalty and interest, to being accused of criminal fraud.
Medicare and other payors generally categorize inaccurate claims as follows:
- Erroneous claims include inadvertent mistakes, innocent errors, or even negligence but still require payments associated with the error to be returned.
- Fraudulent claims are ones judged to be intentionally or recklessly false, and are subject to administrative or civil penalties, such as fines.
- Claims associated with criminal intent to defraud are subject to criminal penalties, which could include jail time.
While I haven’t heard of any hospitalists being accused of fraud, I know of several who have undergone audits and been required to return money. Whether your employer would refund the money or you would have to write a personal check to refund the money depends on your employment situation. For example, in most cases, the hospital would be liable to make the repayment for hospitalists it employs. If you’re an independent contractor, there is a good chance you could be stuck making the repayment yourself.
Trend: Increased Use of Higher-Level Codes
You might have missed it, but there was a recent study of Medicare Part B claims data from 2001 to 2010 showing that “physicians increased their billing of higher-level E/M codes in all types of E/M services.”1 For example, the report showed a steady decrease in use of the 99231 code, the lowest of the three subsequent inpatient hospital care codes, and an increase in the highest level code, 99233 (see Figure 1, below).
I can think of two reasons hospitalists might be increasing the use of higher codes. One, less-sick patients just aren’t seen in the hospital as often as they used to be, so the remaining patients require more intensive services, which could lead to the appropriate use of higher-level codes. Two, doctors have over the past 10 to 15 years invested more energy in learning appropriate documentation and coding, which might have led to correcting historical overuse of lower-level codes.
Did I tell you who conducted the study showing increased use of higher-level codes? It was the federal Office of Inspector General (OIG), which is responsible for preventing and detecting fraud and waste. Although the OIG might agree that the sicker patients and correction of historical undercoding might explain some of the trend, it’s a pretty safe bet they’re also concerned that a significant portion is inappropriate or fraudulent. Some portion of it probably is.
“CMS concurred with [OIG’s] recommendations to (1) continue to educate physicians on proper billing for E/M services and (2) encourage its contractor to review physicians’ billing for E/M services. CMS partially concurred with [OIG’s] third recommendation, to review physicians who bill higher-level E/M codes for appropriate action,” the OIG report noted.1
Plan for Education, Compliance
My sense is that most hospitalists employed by a large entity, such as a hospital or large medical group, have access to a certified coder to perform documentation and coding audits, as well as educational feedback when needed. If your practice doesn’t have access to a certified coder, you should consider photocopying some chart notes (e.g. 10 notes from each of your docs) and send them to an outside coder for an audit. Though they are very valuable, audits usually are not enough to ensure good performance.
In my March 2007 column, I described a reasonably simple chart audit allowing each doctor to compare his or her CPT coding pattern to everyone else in the group. You can compare your own coding to national coding patterns via SHM’s 2012 State of Hospital Medicine Report (www.hospitalmedicine.org/survey) or data from the CMS website, and the Medical Group Management Association (MGMA) will have data in future surveys. Such comparisons might help uncover unusual patterns that are worthy of a closer look.
Other strategies to promote proper documentation and coding include online educational programs, such as:
- SHM’s CODE-H webinars (www.hospitalmedicine.org/codeh), which are available on demand for a fee;
- American Association of Professional Coders Evaluation and Management Online Training (http://www.aapc.com/training/evaluation-management-coding-training.aspx); and
- The American Health Information Management Association’s (AHIMA) Coding Basics Program (www.ahima.org/continuinged/campus/courseinfo/cb.aspx).
If you prefer, an Internet search can turn up in-person courses to learn documentation and coding. Additionally, your in-house or external coding auditors can provide training.
To address tricky issues that come up only occasionally, several in our practice have compiled a “coding manual” by distilling guidance from our certified coders and compliance people on issues as they came up. Some issues would stump all of us, and we’d have to go to the Internet for help. All hospitalists are provided with a copy of the manual during orientation, and an electronic copy is available on the hospital’s Intranet. Topics addressed in the manual include things like how to bill the first inpatient day when a patient has changed from observation status, how to bill initial consult visits for various payors (an issue since Medicare eliminated consult codes a few years ago), how to bill when a patient is seen and discharged from the ED, etc.
Lastly, I suggest someone in your group talk with your hospital’s compliance department about its own coding and billing compliance plan. This could lead to ideas or help develop a compliance plan for your group.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is course co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
Reference
There is a lot to learn when it comes to proper coding and the documentation requirements that go with it. It can even be tricky for a new residency grad to keep the difference in CPT and ICD-9 coding straight, to say nothing of the difference between documentation requirements for physician reimbursement versus hospital reimbursement. This column addresses only physician CPT coding (I’ll save documentation to support hospital billing for another column).
Although I believe that devoting the large number of brain cells required to keep this stuff straight gets in the way of maintaining necessary clinical knowledge, physicians have no real choice but to do it. (One could argue that having a professional coder read charts to determine proper CPT codes relieves a doctor of the burden of documentation and coding headaches. But this is only partially true. The doctor still needs to ensure that the documentation accurately reflects what was done for the coder to be able to select the appropriate codes, so he still needs to know a lot about this topic.)
All providers have a duty to reasonably ensure that submitted claims (bills) are true and accurate. Failing to document and code correctly risks anything from you or your employer having to return money, potentially with a penalty and interest, to being accused of criminal fraud.
Medicare and other payors generally categorize inaccurate claims as follows:
- Erroneous claims include inadvertent mistakes, innocent errors, or even negligence but still require payments associated with the error to be returned.
- Fraudulent claims are ones judged to be intentionally or recklessly false, and are subject to administrative or civil penalties, such as fines.
- Claims associated with criminal intent to defraud are subject to criminal penalties, which could include jail time.
While I haven’t heard of any hospitalists being accused of fraud, I know of several who have undergone audits and been required to return money. Whether your employer would refund the money or you would have to write a personal check to refund the money depends on your employment situation. For example, in most cases, the hospital would be liable to make the repayment for hospitalists it employs. If you’re an independent contractor, there is a good chance you could be stuck making the repayment yourself.
Trend: Increased Use of Higher-Level Codes
You might have missed it, but there was a recent study of Medicare Part B claims data from 2001 to 2010 showing that “physicians increased their billing of higher-level E/M codes in all types of E/M services.”1 For example, the report showed a steady decrease in use of the 99231 code, the lowest of the three subsequent inpatient hospital care codes, and an increase in the highest level code, 99233 (see Figure 1, below).
I can think of two reasons hospitalists might be increasing the use of higher codes. One, less-sick patients just aren’t seen in the hospital as often as they used to be, so the remaining patients require more intensive services, which could lead to the appropriate use of higher-level codes. Two, doctors have over the past 10 to 15 years invested more energy in learning appropriate documentation and coding, which might have led to correcting historical overuse of lower-level codes.
Did I tell you who conducted the study showing increased use of higher-level codes? It was the federal Office of Inspector General (OIG), which is responsible for preventing and detecting fraud and waste. Although the OIG might agree that the sicker patients and correction of historical undercoding might explain some of the trend, it’s a pretty safe bet they’re also concerned that a significant portion is inappropriate or fraudulent. Some portion of it probably is.
“CMS concurred with [OIG’s] recommendations to (1) continue to educate physicians on proper billing for E/M services and (2) encourage its contractor to review physicians’ billing for E/M services. CMS partially concurred with [OIG’s] third recommendation, to review physicians who bill higher-level E/M codes for appropriate action,” the OIG report noted.1
Plan for Education, Compliance
My sense is that most hospitalists employed by a large entity, such as a hospital or large medical group, have access to a certified coder to perform documentation and coding audits, as well as educational feedback when needed. If your practice doesn’t have access to a certified coder, you should consider photocopying some chart notes (e.g. 10 notes from each of your docs) and send them to an outside coder for an audit. Though they are very valuable, audits usually are not enough to ensure good performance.
In my March 2007 column, I described a reasonably simple chart audit allowing each doctor to compare his or her CPT coding pattern to everyone else in the group. You can compare your own coding to national coding patterns via SHM’s 2012 State of Hospital Medicine Report (www.hospitalmedicine.org/survey) or data from the CMS website, and the Medical Group Management Association (MGMA) will have data in future surveys. Such comparisons might help uncover unusual patterns that are worthy of a closer look.
Other strategies to promote proper documentation and coding include online educational programs, such as:
- SHM’s CODE-H webinars (www.hospitalmedicine.org/codeh), which are available on demand for a fee;
- American Association of Professional Coders Evaluation and Management Online Training (http://www.aapc.com/training/evaluation-management-coding-training.aspx); and
- The American Health Information Management Association’s (AHIMA) Coding Basics Program (www.ahima.org/continuinged/campus/courseinfo/cb.aspx).
If you prefer, an Internet search can turn up in-person courses to learn documentation and coding. Additionally, your in-house or external coding auditors can provide training.
To address tricky issues that come up only occasionally, several in our practice have compiled a “coding manual” by distilling guidance from our certified coders and compliance people on issues as they came up. Some issues would stump all of us, and we’d have to go to the Internet for help. All hospitalists are provided with a copy of the manual during orientation, and an electronic copy is available on the hospital’s Intranet. Topics addressed in the manual include things like how to bill the first inpatient day when a patient has changed from observation status, how to bill initial consult visits for various payors (an issue since Medicare eliminated consult codes a few years ago), how to bill when a patient is seen and discharged from the ED, etc.
Lastly, I suggest someone in your group talk with your hospital’s compliance department about its own coding and billing compliance plan. This could lead to ideas or help develop a compliance plan for your group.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is course co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
Reference
John Nelson: Heavy Workloads
Now that HM is moving (or has moved?) from infancy to adolescence or even maturity, you might think that we would have reached some sort of consensus on what a reasonable workload—or patient volume—for a hospitalist is. My sense is that conventional wisdom says a reasonable average daily workload for a daytime rounding/admitting hospitalist is in the range of 12 to 17 billed encounters. And to average this volume, the doctor will have a number of days with more or fewer patients.
After thinking about average workload, the next question is: What is a reasonable upper limit for patient volume on a single day? Here, opinion seems to be a little fuzzier, but I think most would say a hospitalist should be expected to see more than 20 patients in a single day only on rare occasions and on, say, no more than 10 days annually. Keep in mind that a hospitalist who has 22 patients today still has a pretty good chance they will have 20 or more tomorrow, and the day after. High volumes are not a single-day phenomenon, either, because it usually takes a number of days for those patients to reach discharge—and the doctor to realize a decline in workload.
But these numbers are only conventional wisdom. There are little research data to guide our thinking about patient volumes, and thoughtful people sometimes arrive at very different conclusions. As I’ve written in this space previously, I think each individual hospitalist should have significant influence or autonomy to decide the appropriate or optimal patient volume for themselves or their group. This usually requires that doctors are connected to the economic and quality-of-care effects of their patient volume choices, something many hospitalists resist.
Divergence of Opinion
But given lots of autonomy, some hospitalists could make poor choices. I have had the experience of working with hospitalists in three practices around the country who are confident that, at least for themselves, very high patient volumes are safe and reasonable. These high-energy hospitalists see as many as 30 or 40 patients per day, day after day.
At one of these practices, I sat down with the doctors on duty that day at 1 p.m. and talked uninterrupted by pager or patient-care issues for nearly three hours. It was only at the end of the meeting that they explained each of them was seeing around 30 patients that day but had nearly finished rounds before our meeting started. I was stunned. (I probably wouldn’t stop for lunch, to say nothing of a three-hour meeting, to see just 20 patients in a day.)
So I asked just what they saw as an excessive daily patient volume. One of them seemed to deliberate carefully and said, “I probably need help when I have more than 35 patients to see in a day, but I’m OK with anything less than that.”
But the record goes to a really nice, spirited hospitalist who told me that, in addition to his usual workload, he occasionally covered weekends for an internal-medicine group. On a recent weekend, he had 88 patients to see each day, he said. Yes, you read that correctly: 88! (Fortunately, he did see that as a problem and was working to decrease the number.)
Potential Risks
I want to be clear that my own opinion is that the volumes above are unacceptable and dangerous. I think that, in most settings, routinely seeing more than 20 patients in a day probably degrades performance and increases the risk of burnout. While I think most knowledgeable people in our field share this opinion, none of us can point to compelling, generalizable research data to support our opinion.
The way I see it, excessively high workloads risk:
- Adverse patient outcomes due to increased potential for clinical errors and accompanying poor documentation;
- Failure of hospitalists to meet performance and citizenship expectations, such as length of stay (LOS), resource utilization, use of standardized order sets, attention to early discharge times, etc.;
- Lack of any excess capacity to handle transient increases in workload;
- Recruiting and/or retention challenges for hospitalists who might not want to work so hard;
- High risk of hospitalist stress and burnout, which over time could negatively impact a person’s well-being, as well as their attitudes and interactions with other members of the patient care team;
- Overdependence on a few very-hard-working doctors; if one doctor gets sick or has to stop working for a period of time, the hospital must find the equivalent of one-and-a-half doctors to replace him or her; and
- Increased malpractice risk.
Limited Data
There is some research to guide the thinking about workload. I recall one or two abstracts presented at past SHM annual meetings in which doctors in a single practice showed that LOS increased when their patient volume was high. And some sharp hospitalist researchers at Christiana Care Health System in Wilmington, Del., conducted a more robust retrospective cohort study of thousands of non-ICU adult admissions to their 1,100-bed hospital over a three-year period. Their data, which they intend to publish, showed LOS rises as hospitalist workload increases.
Others have assessed the connection between workload and well-being or burnout. Surprisingly, it has been hard to document in the peer-reviewed literature that increasing workloads are associated with increased burnout. Studies of hospitalists published in 2001 and 2011 failed to show a connection between self-reported workload and burnout.1,2 A 2009 systemic review of literature on all physician specialties concluded that “an imbalance between expected and experienced … workload is moderately associated with dissatisfaction, but there is less evidence of a significant association with objective workload.”3 (Emphasis mine.)
Rather than workload, both of the hospitalist studies found that such attributes as organizational solidarity, climate, and fairness; the feeling of being valued by the whole healthcare team; personal time; and compensation were more tightly correlated with whether hospitalists would thrive than workload.
Unfortunately, I’m not aware of any robust studies showing the relationship between hospitalist workload and quality of care (please email me if you know of any). I think the burden of proof is on those who support high workloads to show they don’t adversely affect patient incomes.
If you’d like to discuss workload further, I’ll be moderating a session titled “Who Says 15 is the Right Number?” during HM13, May 17-19, 2013, in Washington, D.C. (www.hospitalmedicine2013.org). I hope to see you there.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is course co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
References
2. Hinami K, Whelan CT, Wolosin RJ, Miller JA, Wetterneck TB. Worklife and satisfaction of hospitalists: toward flourishing careers. J Gen Intern Med. 2012;27(1):28-36.
3. Scheurer D, McKean S, Miller J, Wetterneck T. U.S. physician satisfaction: a systematic review. J Hosp Med. 2009;4(9):560-568.
Now that HM is moving (or has moved?) from infancy to adolescence or even maturity, you might think that we would have reached some sort of consensus on what a reasonable workload—or patient volume—for a hospitalist is. My sense is that conventional wisdom says a reasonable average daily workload for a daytime rounding/admitting hospitalist is in the range of 12 to 17 billed encounters. And to average this volume, the doctor will have a number of days with more or fewer patients.
After thinking about average workload, the next question is: What is a reasonable upper limit for patient volume on a single day? Here, opinion seems to be a little fuzzier, but I think most would say a hospitalist should be expected to see more than 20 patients in a single day only on rare occasions and on, say, no more than 10 days annually. Keep in mind that a hospitalist who has 22 patients today still has a pretty good chance they will have 20 or more tomorrow, and the day after. High volumes are not a single-day phenomenon, either, because it usually takes a number of days for those patients to reach discharge—and the doctor to realize a decline in workload.
But these numbers are only conventional wisdom. There are little research data to guide our thinking about patient volumes, and thoughtful people sometimes arrive at very different conclusions. As I’ve written in this space previously, I think each individual hospitalist should have significant influence or autonomy to decide the appropriate or optimal patient volume for themselves or their group. This usually requires that doctors are connected to the economic and quality-of-care effects of their patient volume choices, something many hospitalists resist.
Divergence of Opinion
But given lots of autonomy, some hospitalists could make poor choices. I have had the experience of working with hospitalists in three practices around the country who are confident that, at least for themselves, very high patient volumes are safe and reasonable. These high-energy hospitalists see as many as 30 or 40 patients per day, day after day.
At one of these practices, I sat down with the doctors on duty that day at 1 p.m. and talked uninterrupted by pager or patient-care issues for nearly three hours. It was only at the end of the meeting that they explained each of them was seeing around 30 patients that day but had nearly finished rounds before our meeting started. I was stunned. (I probably wouldn’t stop for lunch, to say nothing of a three-hour meeting, to see just 20 patients in a day.)
So I asked just what they saw as an excessive daily patient volume. One of them seemed to deliberate carefully and said, “I probably need help when I have more than 35 patients to see in a day, but I’m OK with anything less than that.”
But the record goes to a really nice, spirited hospitalist who told me that, in addition to his usual workload, he occasionally covered weekends for an internal-medicine group. On a recent weekend, he had 88 patients to see each day, he said. Yes, you read that correctly: 88! (Fortunately, he did see that as a problem and was working to decrease the number.)
Potential Risks
I want to be clear that my own opinion is that the volumes above are unacceptable and dangerous. I think that, in most settings, routinely seeing more than 20 patients in a day probably degrades performance and increases the risk of burnout. While I think most knowledgeable people in our field share this opinion, none of us can point to compelling, generalizable research data to support our opinion.
The way I see it, excessively high workloads risk:
- Adverse patient outcomes due to increased potential for clinical errors and accompanying poor documentation;
- Failure of hospitalists to meet performance and citizenship expectations, such as length of stay (LOS), resource utilization, use of standardized order sets, attention to early discharge times, etc.;
- Lack of any excess capacity to handle transient increases in workload;
- Recruiting and/or retention challenges for hospitalists who might not want to work so hard;
- High risk of hospitalist stress and burnout, which over time could negatively impact a person’s well-being, as well as their attitudes and interactions with other members of the patient care team;
- Overdependence on a few very-hard-working doctors; if one doctor gets sick or has to stop working for a period of time, the hospital must find the equivalent of one-and-a-half doctors to replace him or her; and
- Increased malpractice risk.
Limited Data
There is some research to guide the thinking about workload. I recall one or two abstracts presented at past SHM annual meetings in which doctors in a single practice showed that LOS increased when their patient volume was high. And some sharp hospitalist researchers at Christiana Care Health System in Wilmington, Del., conducted a more robust retrospective cohort study of thousands of non-ICU adult admissions to their 1,100-bed hospital over a three-year period. Their data, which they intend to publish, showed LOS rises as hospitalist workload increases.
Others have assessed the connection between workload and well-being or burnout. Surprisingly, it has been hard to document in the peer-reviewed literature that increasing workloads are associated with increased burnout. Studies of hospitalists published in 2001 and 2011 failed to show a connection between self-reported workload and burnout.1,2 A 2009 systemic review of literature on all physician specialties concluded that “an imbalance between expected and experienced … workload is moderately associated with dissatisfaction, but there is less evidence of a significant association with objective workload.”3 (Emphasis mine.)
Rather than workload, both of the hospitalist studies found that such attributes as organizational solidarity, climate, and fairness; the feeling of being valued by the whole healthcare team; personal time; and compensation were more tightly correlated with whether hospitalists would thrive than workload.
Unfortunately, I’m not aware of any robust studies showing the relationship between hospitalist workload and quality of care (please email me if you know of any). I think the burden of proof is on those who support high workloads to show they don’t adversely affect patient incomes.
If you’d like to discuss workload further, I’ll be moderating a session titled “Who Says 15 is the Right Number?” during HM13, May 17-19, 2013, in Washington, D.C. (www.hospitalmedicine2013.org). I hope to see you there.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is course co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
References
2. Hinami K, Whelan CT, Wolosin RJ, Miller JA, Wetterneck TB. Worklife and satisfaction of hospitalists: toward flourishing careers. J Gen Intern Med. 2012;27(1):28-36.
3. Scheurer D, McKean S, Miller J, Wetterneck T. U.S. physician satisfaction: a systematic review. J Hosp Med. 2009;4(9):560-568.
Now that HM is moving (or has moved?) from infancy to adolescence or even maturity, you might think that we would have reached some sort of consensus on what a reasonable workload—or patient volume—for a hospitalist is. My sense is that conventional wisdom says a reasonable average daily workload for a daytime rounding/admitting hospitalist is in the range of 12 to 17 billed encounters. And to average this volume, the doctor will have a number of days with more or fewer patients.
After thinking about average workload, the next question is: What is a reasonable upper limit for patient volume on a single day? Here, opinion seems to be a little fuzzier, but I think most would say a hospitalist should be expected to see more than 20 patients in a single day only on rare occasions and on, say, no more than 10 days annually. Keep in mind that a hospitalist who has 22 patients today still has a pretty good chance they will have 20 or more tomorrow, and the day after. High volumes are not a single-day phenomenon, either, because it usually takes a number of days for those patients to reach discharge—and the doctor to realize a decline in workload.
But these numbers are only conventional wisdom. There are little research data to guide our thinking about patient volumes, and thoughtful people sometimes arrive at very different conclusions. As I’ve written in this space previously, I think each individual hospitalist should have significant influence or autonomy to decide the appropriate or optimal patient volume for themselves or their group. This usually requires that doctors are connected to the economic and quality-of-care effects of their patient volume choices, something many hospitalists resist.
Divergence of Opinion
But given lots of autonomy, some hospitalists could make poor choices. I have had the experience of working with hospitalists in three practices around the country who are confident that, at least for themselves, very high patient volumes are safe and reasonable. These high-energy hospitalists see as many as 30 or 40 patients per day, day after day.
At one of these practices, I sat down with the doctors on duty that day at 1 p.m. and talked uninterrupted by pager or patient-care issues for nearly three hours. It was only at the end of the meeting that they explained each of them was seeing around 30 patients that day but had nearly finished rounds before our meeting started. I was stunned. (I probably wouldn’t stop for lunch, to say nothing of a three-hour meeting, to see just 20 patients in a day.)
So I asked just what they saw as an excessive daily patient volume. One of them seemed to deliberate carefully and said, “I probably need help when I have more than 35 patients to see in a day, but I’m OK with anything less than that.”
But the record goes to a really nice, spirited hospitalist who told me that, in addition to his usual workload, he occasionally covered weekends for an internal-medicine group. On a recent weekend, he had 88 patients to see each day, he said. Yes, you read that correctly: 88! (Fortunately, he did see that as a problem and was working to decrease the number.)
Potential Risks
I want to be clear that my own opinion is that the volumes above are unacceptable and dangerous. I think that, in most settings, routinely seeing more than 20 patients in a day probably degrades performance and increases the risk of burnout. While I think most knowledgeable people in our field share this opinion, none of us can point to compelling, generalizable research data to support our opinion.
The way I see it, excessively high workloads risk:
- Adverse patient outcomes due to increased potential for clinical errors and accompanying poor documentation;
- Failure of hospitalists to meet performance and citizenship expectations, such as length of stay (LOS), resource utilization, use of standardized order sets, attention to early discharge times, etc.;
- Lack of any excess capacity to handle transient increases in workload;
- Recruiting and/or retention challenges for hospitalists who might not want to work so hard;
- High risk of hospitalist stress and burnout, which over time could negatively impact a person’s well-being, as well as their attitudes and interactions with other members of the patient care team;
- Overdependence on a few very-hard-working doctors; if one doctor gets sick or has to stop working for a period of time, the hospital must find the equivalent of one-and-a-half doctors to replace him or her; and
- Increased malpractice risk.
Limited Data
There is some research to guide the thinking about workload. I recall one or two abstracts presented at past SHM annual meetings in which doctors in a single practice showed that LOS increased when their patient volume was high. And some sharp hospitalist researchers at Christiana Care Health System in Wilmington, Del., conducted a more robust retrospective cohort study of thousands of non-ICU adult admissions to their 1,100-bed hospital over a three-year period. Their data, which they intend to publish, showed LOS rises as hospitalist workload increases.
Others have assessed the connection between workload and well-being or burnout. Surprisingly, it has been hard to document in the peer-reviewed literature that increasing workloads are associated with increased burnout. Studies of hospitalists published in 2001 and 2011 failed to show a connection between self-reported workload and burnout.1,2 A 2009 systemic review of literature on all physician specialties concluded that “an imbalance between expected and experienced … workload is moderately associated with dissatisfaction, but there is less evidence of a significant association with objective workload.”3 (Emphasis mine.)
Rather than workload, both of the hospitalist studies found that such attributes as organizational solidarity, climate, and fairness; the feeling of being valued by the whole healthcare team; personal time; and compensation were more tightly correlated with whether hospitalists would thrive than workload.
Unfortunately, I’m not aware of any robust studies showing the relationship between hospitalist workload and quality of care (please email me if you know of any). I think the burden of proof is on those who support high workloads to show they don’t adversely affect patient incomes.
If you’d like to discuss workload further, I’ll be moderating a session titled “Who Says 15 is the Right Number?” during HM13, May 17-19, 2013, in Washington, D.C. (www.hospitalmedicine2013.org). I hope to see you there.
Dr. Nelson has been a practicing hospitalist since 1988. He is co-founder and past president of SHM, and principal in Nelson Flores Hospital Medicine Consultants. He is course co-director for SHM’s “Best Practices in Managing a Hospital Medicine Program” course. Write to him at [email protected].
References
2. Hinami K, Whelan CT, Wolosin RJ, Miller JA, Wetterneck TB. Worklife and satisfaction of hospitalists: toward flourishing careers. J Gen Intern Med. 2012;27(1):28-36.
3. Scheurer D, McKean S, Miller J, Wetterneck T. U.S. physician satisfaction: a systematic review. J Hosp Med. 2009;4(9):560-568.
Clarification of Consult-Code Usage
In July, I was CC’d on an email from a reader that asked for further explanation of billing for patients in the ED. I mentioned briefly in the May 2012 issue of The Hospitalist that, in the ED, a consult on a patient that ends up being sent home can be billed with CPT code 99281-99288. Another author for another hospitalist publication had previously written that hospitalists do not have consultation codes for patients evaluated in the ED, then sent home. The reader basically wanted to know how to bill for this encounter.
It should be noted that this is billing for a visit in the ED for which the patient is not admitted. It is not a consult code, as those effectively have been eliminated from the CPT manual.
Here’s some further explanation:
Effective Jan. 1, 2010, the Centers for Medicare & Medicaid Services (CMS) eliminated the use of the codes 99241-99245 (outpatient consultation) and 99215-99255 (inpatient consultation) for use with Medicare Part B beneficiaries. Those codes are now either 99201-99205 (office outpatient visit) or 99221-99223 (initial inpatient visit).
Although this might seem confusing, CMS actually made it simpler. If you or your group is seeing a patient for the first time, as either an admission or an inpatient consult, you use the 99221-99223 codes. So now there are three codes to use instead of eight. Just to note, though, this applies specifically to Medicare patients.
Similarly, if you evaluate a patient in the ED and they are not admitted to the hospital, then you use the 99281-99288 codes. Yes, these are the same E/M codes that the attending ED physician will use for their care. If you personally evaluate the patient in the ED, document as required, and if the patient is admitted, then it reverts back to the 99221-99223 codes. However, if the patient does go home from the ED (never admitted as an inpatient or under observation status), then you use the 80s codes referenced above.
Just to add one more layer of complexity, please remember that there are specific codes for patients admitted under observation status (99217-99220), as well as for patients that are admitted and discharged in the same calendar day (99234-99236). Those are distinct from what is described above.
All in all, don’t take my word for it. Here’s the link to the actual CMS bulletin:
It’s readable, too, which is nice.
In July, I was CC’d on an email from a reader that asked for further explanation of billing for patients in the ED. I mentioned briefly in the May 2012 issue of The Hospitalist that, in the ED, a consult on a patient that ends up being sent home can be billed with CPT code 99281-99288. Another author for another hospitalist publication had previously written that hospitalists do not have consultation codes for patients evaluated in the ED, then sent home. The reader basically wanted to know how to bill for this encounter.
It should be noted that this is billing for a visit in the ED for which the patient is not admitted. It is not a consult code, as those effectively have been eliminated from the CPT manual.
Here’s some further explanation:
Effective Jan. 1, 2010, the Centers for Medicare & Medicaid Services (CMS) eliminated the use of the codes 99241-99245 (outpatient consultation) and 99215-99255 (inpatient consultation) for use with Medicare Part B beneficiaries. Those codes are now either 99201-99205 (office outpatient visit) or 99221-99223 (initial inpatient visit).
Although this might seem confusing, CMS actually made it simpler. If you or your group is seeing a patient for the first time, as either an admission or an inpatient consult, you use the 99221-99223 codes. So now there are three codes to use instead of eight. Just to note, though, this applies specifically to Medicare patients.
Similarly, if you evaluate a patient in the ED and they are not admitted to the hospital, then you use the 99281-99288 codes. Yes, these are the same E/M codes that the attending ED physician will use for their care. If you personally evaluate the patient in the ED, document as required, and if the patient is admitted, then it reverts back to the 99221-99223 codes. However, if the patient does go home from the ED (never admitted as an inpatient or under observation status), then you use the 80s codes referenced above.
Just to add one more layer of complexity, please remember that there are specific codes for patients admitted under observation status (99217-99220), as well as for patients that are admitted and discharged in the same calendar day (99234-99236). Those are distinct from what is described above.
All in all, don’t take my word for it. Here’s the link to the actual CMS bulletin:
It’s readable, too, which is nice.
In July, I was CC’d on an email from a reader that asked for further explanation of billing for patients in the ED. I mentioned briefly in the May 2012 issue of The Hospitalist that, in the ED, a consult on a patient that ends up being sent home can be billed with CPT code 99281-99288. Another author for another hospitalist publication had previously written that hospitalists do not have consultation codes for patients evaluated in the ED, then sent home. The reader basically wanted to know how to bill for this encounter.
It should be noted that this is billing for a visit in the ED for which the patient is not admitted. It is not a consult code, as those effectively have been eliminated from the CPT manual.
Here’s some further explanation:
Effective Jan. 1, 2010, the Centers for Medicare & Medicaid Services (CMS) eliminated the use of the codes 99241-99245 (outpatient consultation) and 99215-99255 (inpatient consultation) for use with Medicare Part B beneficiaries. Those codes are now either 99201-99205 (office outpatient visit) or 99221-99223 (initial inpatient visit).
Although this might seem confusing, CMS actually made it simpler. If you or your group is seeing a patient for the first time, as either an admission or an inpatient consult, you use the 99221-99223 codes. So now there are three codes to use instead of eight. Just to note, though, this applies specifically to Medicare patients.
Similarly, if you evaluate a patient in the ED and they are not admitted to the hospital, then you use the 99281-99288 codes. Yes, these are the same E/M codes that the attending ED physician will use for their care. If you personally evaluate the patient in the ED, document as required, and if the patient is admitted, then it reverts back to the 99221-99223 codes. However, if the patient does go home from the ED (never admitted as an inpatient or under observation status), then you use the 80s codes referenced above.
Just to add one more layer of complexity, please remember that there are specific codes for patients admitted under observation status (99217-99220), as well as for patients that are admitted and discharged in the same calendar day (99234-99236). Those are distinct from what is described above.
All in all, don’t take my word for it. Here’s the link to the actual CMS bulletin:
It’s readable, too, which is nice.
Pressure to Expand Scope of Practice Extends to Most U.S. Hospitalists
Eric M. Siegal, MD, SFHM, vividly recalls the moment when he realized “scope creep” had become a problem. A hospitalist partner who was working a night shift admitted a young man who had been in a high-speed motor vehicle accident. The hospitalist did so because the general surgeon did not want to come into the hospital.
Dr. Siegal, currently the medical director of critical-care medicine at Aurora St. Luke’s Medical Center in Milwaukee, remembers looking at his partner and asking, “What the hell are you doing admitting a trauma patient? You’re an internist!”
Dr. Siegal’s partner responded, “I’m just trying to show value.”
“That was an ‘a-ha’ moment for me,” says Dr. Siegal, a member of SHM’s board of directors. It was at that point he began to understand that the expansion strategy used by many HM services—to demonstrate value by agreeing to comanage or admit patients for their primary-care (PCP) and specialist colleagues—had produced some unintended negative consequences. “Hospitalists,” he says, “are like the spackle of the hospital. Sometimes spackle is good; it hides flaws and imperfections. But at other times, people use spackle to fix major structural problems.”
Scope creep, mission creep, scut work: There are numerous ways to describe the phenomenon. In basic terms, hospitalists have been pressured to expand their scope of practice to manage all hospitalized patients. Hospitalist leaders differ about how much of an issue this really is, as managing hospitalized patients is the definition of hospitalist work. Burke T. Kealey, MD, SFHM, medical director of hospital specialties at HealthPartners Medical Group in St. Paul, Minn., and an SHM board member, points out that “one man’s scope creep is another man’s practice-builder.” John Nelson, MD, MHM, co-founder and past president of SHM and medical director of hospitalist services at Overlake Hospital in Bellevue, Wash., says the expanding service trend is prevalent, but whether “it’s a problem depends on your point of view. The same stressful evolution occurs in every specialty. We are not unique in that regard.”
The trick, according to HM leaders, is to understand the dynamics that drive scope creep, then work proactively to address the problem.
Evolving Scope of Practice
It was not so long ago that hospitalist groups, seen by many in medicine as the new kids on the block, were perceived as a threat to their primary-care and specialist colleagues. To establish themselves, hospitalists began to demonstrate value by comanaging patients for their surgical colleagues, especially orthopedists. Some studies, notably those conducted by Mayo Clinic-based hospitalists, appeared to demonstrate that using hospitalists to help comanage orthopedic surgical patients results in improved outcomes.1,2
Dr. Siegal, however, points out that a closer parsing of those studies reveals that such outcomes as decreased time to surgery and length of stay (LOS) were better for patients with complex medical comorbidities, rather than all patients, which supports his argument that hospitalist comanagement makes most sense when applied to select groups of surgical patients.3

—Eric M. Siegal, MD, SFHM, SHM board member, medical director of critical-care medicine, Aurora St. Luke’s Medical Center, Milwaukee
As HM sprouted roots, clinicians across the country began to see an increase in requests for their services from primary-care physicians (PCPs) and subspecialists, as hospitalists freed them from rounding on patients and allowed them to concentrate on procedures for higher billings. Over the past 10 years, the expansion has been rapid, converging with multiple factors: increasing numbers of uninsured patients, an aging physician workforce, and diminishing reimbursement, to name a few.
Nailing down the extent to which comanagement has expanded HM’s scope of practice as a medical specialty is a slippery exercise. Some HM groups handle comanagement well; others do not. Dr. Kealey says that admitting and comanagement patterns are dependent on the culture of the institution. For example, in one of HealthPartners’ home hospitals, all internal-medicine subspecialties, including neurology, are admitted and managed by hospitalists with a subspecialty consult.
The 2012 State of Hospital Medicine report survey revealed that 85% of respondent hospitalist groups provide surgical comanagement services (see Figure 1, below). That figure has not changed since SHM’s 2005-2006 survey, the last time the question was asked.
Another 20% of respondent hospitalist groups reported providing medical subspecialty comanagement, according to the 2012 report. Dr. Kealey, who is board liaison to SHM’s Practice Analysis Committee, says plans are in the works to add specific questions to the survey to assess another big change in the comanagement arena: a shift from hospitalists acting as consultants with the specialist serving as attending physician to a model in which the hospitalist admits the patient and serves as attending, with the specialist/proceduralist in a consulting role.
So What’s the Problem?
Hospitalists have been both the utility player and the superstar, providing great value to their healthcare teams, says Ken Simone, DO, SFHM, a hospitalist practice-management consultant and CEO of Hospitalist and Practice Solutions in Veazie, Maine. He believes hospitalist program expansions are typically a positive thing.
“Historically, most hospital medicine programs have embraced the call for assistance from both their colleagues and the C-suite,” says Dr. Simone, a Team Hospitalist member.
Dr. Siegal, in his HM07 presentation “Managing Comanagement: How to Play in the Sandbox without Having to Eat Mud Pies” and in journal articles, has cautioned against assuming that all hospitalized patients, irrespective of diagnosis or comorbidities, should be seen by a hospitalist.3 Such a directive can produce a host of unintended negative consequences. Most notably, it can:
- Confuse patients, families, and the care team about who is ultimately responsible for oversight of the patient’s care;
- Place hospitalists in the position of assuming responsibility for patients whose conditions are outside their scope of practice;
- Delay the initiation of appropriate, specialized care;
- Overwork an already stretched hospitalist team, which can lead to burnout; and
- Increase exposure to medical liability by placing hospitalists in situations where they are in over their heads, or by creating novel opportunities for miscommunication between hospitalists and surgeons or specialists.
Pressure Points
Scope creep’s root cause has multiple layers. It can be driven by overworked physicians; by local shortages in a particular specialty; by the bottom line, when procedure-focused physicians and surgeons want to divest themselves of day-to-day management of hospitalized patients; by lifestyle preferences; or by hospitalists’ success.
Jerome C. Siy, MD, SFHM, department head of hospital medicine for HealthPartners and recipient of the 2009 SHM Award for Clinical Excellence, believes the single most important factor behind the pressure to manage more hospitalized patients is the necessity to provide more thorough care when specialists or residents cannot.
“The hours of coverage are expanding in every specialty to a 24/7 model,” he says. “Since we hospitalists were in the hospital already, it became more routine for other services to ask us to get initial orders and the history and physical started, as a bridge to a better coverage model.”
Dr. Kealey says the “bridge” is a point of concern for many HM groups, especially when the pressure comes from hospital administrators attempting to attract specialists. Hospitalists have the right in such situations, says Dr. Siy, to feel undersupported or that they lack crucial knowledge or skill sets. Still, Dr. Kealey sees requests from other physician groups as a positive thing for hospitalists.
“We’re going to be managing more in the future,” he says, noting his HM group first drew up a comanagement agreement with orthopedic surgeons 17 years ago. “We want to go there thoughtfully and carefully. We shouldn’t put our foot down and say no to new opportunity.”
Rules of Engagement
Nearly every hospitalist leader agrees that the key to protecting against scope creep resides with thoughtful, proactive planning. Make sure, they say, that your group is ready to manage the patients you’re being asked to manage (see “Define and Protect Your Scope of Practice,” p. 35).
—Michael Radzienda, MD, SFHM, regional chief medical officer, Sound Physicians, Boston
Michael Radzienda, MD, SFHM, regional chief medical officer at Sound Physicians in the greater Boston area, agrees with Dr. Kealey in that he sees opportunity where others might perceive burden. For example, he notes, the advent of value-based purchasing initiatives, linking payment to quality, will create “huge opportunities for hospitalists.” More than 50% of the quality core measures in these initiatives are related to the Surgical Care Improvement Project (SCIP).4
“Now, more than ever, hospitalists need to align with their partners in the hospital C-suite to help them be successful around those targets,” Dr. Radzienda says. However, he adds, “it behooves the HM teams to be very methodical and not rush this.”
Crafting clear rules of engagement must be handled properly and thoughtfully at the outset, Dr. Radzienda explains, and developing mutual trust and respect between the parties is the most essential step. Logistically, this can present problems.
“Getting surgeons and hospitalists together at a table is hard work,” he says. “But I can’t underscore that more: This requires a relationship. And it’s not something that is done via email exchange or memoranda through the respective practices’ business managers.”
It’s also critical to have nursing on board, says Julie Weegman, RN, MA, OCN, director of nursing and medical surgical services at HealthPartners’ Regions Hospital in St. Paul. “Communication is key in this kind of arrangement,” she says. “Nurses could potentially be put in a bad position if there are tensions between hospitalists and the specialty departments.”
That isn’t the case at Regions, though, where the comanagement agreement between orthopedics and HM has been clearly established, Weegman says. Questions about the surgical site, activity, and weight-bearing are referred to surgeons, while chronic disease management, blood pressure, glucose monitoring, etc., usually are handled by hospitalists.
Dr. Radzienda stresses that patients must remain at the center of the equation. “At three o’clock in the morning, with the post-op ortho patient who is having pain, nausea, or bleeding, it cannot be a multistep process to decide which doc is going to take that call and deliver on the patient’s needs,” he says.
Dr. Nelson, who co-founded SHM and serves as The Hospitalist’s practice-management columnist, cautions that service agreements are not a panacea. “This won’t totally solve your problems,” he says, “because every doctor is authorized to violate agreements if they see fit and if they can prove their patient is the exception to the rule.”
The bottom-line test for Dr. Siegal: Consider the patient’s best interests. Ask yourself, he advises, “if your mother came into the hospital with a head bleed, who would you want her to see first? Hospitalists are not interchangeable with neurosurgeons, and yet, unfortunately, we have started marketing ourselves as being adequate replacements for people who have spent far more time training in a specialty.
“As an intensivist, I’ve got a bit of experience with head bleeds,” he says. “But the neurosurgeon still knows more.”
Gretchen Henkel is a freelance writer in central California.
References
- Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical co-management after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1): 28-38.
- Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31.
- Siegal EM. Just because you can, doesn’t mean that you should: A call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398-402.
- The Joint Commission. Surgical Care Improvement Project. The Joint Commission website. Available at: http://www.jointcommission.org/surgical_care_improvement_project/. Accessed Sept. 30, 2012.
Eric M. Siegal, MD, SFHM, vividly recalls the moment when he realized “scope creep” had become a problem. A hospitalist partner who was working a night shift admitted a young man who had been in a high-speed motor vehicle accident. The hospitalist did so because the general surgeon did not want to come into the hospital.
Dr. Siegal, currently the medical director of critical-care medicine at Aurora St. Luke’s Medical Center in Milwaukee, remembers looking at his partner and asking, “What the hell are you doing admitting a trauma patient? You’re an internist!”
Dr. Siegal’s partner responded, “I’m just trying to show value.”
“That was an ‘a-ha’ moment for me,” says Dr. Siegal, a member of SHM’s board of directors. It was at that point he began to understand that the expansion strategy used by many HM services—to demonstrate value by agreeing to comanage or admit patients for their primary-care (PCP) and specialist colleagues—had produced some unintended negative consequences. “Hospitalists,” he says, “are like the spackle of the hospital. Sometimes spackle is good; it hides flaws and imperfections. But at other times, people use spackle to fix major structural problems.”
Scope creep, mission creep, scut work: There are numerous ways to describe the phenomenon. In basic terms, hospitalists have been pressured to expand their scope of practice to manage all hospitalized patients. Hospitalist leaders differ about how much of an issue this really is, as managing hospitalized patients is the definition of hospitalist work. Burke T. Kealey, MD, SFHM, medical director of hospital specialties at HealthPartners Medical Group in St. Paul, Minn., and an SHM board member, points out that “one man’s scope creep is another man’s practice-builder.” John Nelson, MD, MHM, co-founder and past president of SHM and medical director of hospitalist services at Overlake Hospital in Bellevue, Wash., says the expanding service trend is prevalent, but whether “it’s a problem depends on your point of view. The same stressful evolution occurs in every specialty. We are not unique in that regard.”
The trick, according to HM leaders, is to understand the dynamics that drive scope creep, then work proactively to address the problem.
Evolving Scope of Practice
It was not so long ago that hospitalist groups, seen by many in medicine as the new kids on the block, were perceived as a threat to their primary-care and specialist colleagues. To establish themselves, hospitalists began to demonstrate value by comanaging patients for their surgical colleagues, especially orthopedists. Some studies, notably those conducted by Mayo Clinic-based hospitalists, appeared to demonstrate that using hospitalists to help comanage orthopedic surgical patients results in improved outcomes.1,2
Dr. Siegal, however, points out that a closer parsing of those studies reveals that such outcomes as decreased time to surgery and length of stay (LOS) were better for patients with complex medical comorbidities, rather than all patients, which supports his argument that hospitalist comanagement makes most sense when applied to select groups of surgical patients.3

—Eric M. Siegal, MD, SFHM, SHM board member, medical director of critical-care medicine, Aurora St. Luke’s Medical Center, Milwaukee
As HM sprouted roots, clinicians across the country began to see an increase in requests for their services from primary-care physicians (PCPs) and subspecialists, as hospitalists freed them from rounding on patients and allowed them to concentrate on procedures for higher billings. Over the past 10 years, the expansion has been rapid, converging with multiple factors: increasing numbers of uninsured patients, an aging physician workforce, and diminishing reimbursement, to name a few.
Nailing down the extent to which comanagement has expanded HM’s scope of practice as a medical specialty is a slippery exercise. Some HM groups handle comanagement well; others do not. Dr. Kealey says that admitting and comanagement patterns are dependent on the culture of the institution. For example, in one of HealthPartners’ home hospitals, all internal-medicine subspecialties, including neurology, are admitted and managed by hospitalists with a subspecialty consult.
The 2012 State of Hospital Medicine report survey revealed that 85% of respondent hospitalist groups provide surgical comanagement services (see Figure 1, below). That figure has not changed since SHM’s 2005-2006 survey, the last time the question was asked.
Another 20% of respondent hospitalist groups reported providing medical subspecialty comanagement, according to the 2012 report. Dr. Kealey, who is board liaison to SHM’s Practice Analysis Committee, says plans are in the works to add specific questions to the survey to assess another big change in the comanagement arena: a shift from hospitalists acting as consultants with the specialist serving as attending physician to a model in which the hospitalist admits the patient and serves as attending, with the specialist/proceduralist in a consulting role.
So What’s the Problem?
Hospitalists have been both the utility player and the superstar, providing great value to their healthcare teams, says Ken Simone, DO, SFHM, a hospitalist practice-management consultant and CEO of Hospitalist and Practice Solutions in Veazie, Maine. He believes hospitalist program expansions are typically a positive thing.
“Historically, most hospital medicine programs have embraced the call for assistance from both their colleagues and the C-suite,” says Dr. Simone, a Team Hospitalist member.
Dr. Siegal, in his HM07 presentation “Managing Comanagement: How to Play in the Sandbox without Having to Eat Mud Pies” and in journal articles, has cautioned against assuming that all hospitalized patients, irrespective of diagnosis or comorbidities, should be seen by a hospitalist.3 Such a directive can produce a host of unintended negative consequences. Most notably, it can:
- Confuse patients, families, and the care team about who is ultimately responsible for oversight of the patient’s care;
- Place hospitalists in the position of assuming responsibility for patients whose conditions are outside their scope of practice;
- Delay the initiation of appropriate, specialized care;
- Overwork an already stretched hospitalist team, which can lead to burnout; and
- Increase exposure to medical liability by placing hospitalists in situations where they are in over their heads, or by creating novel opportunities for miscommunication between hospitalists and surgeons or specialists.
Pressure Points
Scope creep’s root cause has multiple layers. It can be driven by overworked physicians; by local shortages in a particular specialty; by the bottom line, when procedure-focused physicians and surgeons want to divest themselves of day-to-day management of hospitalized patients; by lifestyle preferences; or by hospitalists’ success.
Jerome C. Siy, MD, SFHM, department head of hospital medicine for HealthPartners and recipient of the 2009 SHM Award for Clinical Excellence, believes the single most important factor behind the pressure to manage more hospitalized patients is the necessity to provide more thorough care when specialists or residents cannot.
“The hours of coverage are expanding in every specialty to a 24/7 model,” he says. “Since we hospitalists were in the hospital already, it became more routine for other services to ask us to get initial orders and the history and physical started, as a bridge to a better coverage model.”
Dr. Kealey says the “bridge” is a point of concern for many HM groups, especially when the pressure comes from hospital administrators attempting to attract specialists. Hospitalists have the right in such situations, says Dr. Siy, to feel undersupported or that they lack crucial knowledge or skill sets. Still, Dr. Kealey sees requests from other physician groups as a positive thing for hospitalists.
“We’re going to be managing more in the future,” he says, noting his HM group first drew up a comanagement agreement with orthopedic surgeons 17 years ago. “We want to go there thoughtfully and carefully. We shouldn’t put our foot down and say no to new opportunity.”
Rules of Engagement
Nearly every hospitalist leader agrees that the key to protecting against scope creep resides with thoughtful, proactive planning. Make sure, they say, that your group is ready to manage the patients you’re being asked to manage (see “Define and Protect Your Scope of Practice,” p. 35).
—Michael Radzienda, MD, SFHM, regional chief medical officer, Sound Physicians, Boston
Michael Radzienda, MD, SFHM, regional chief medical officer at Sound Physicians in the greater Boston area, agrees with Dr. Kealey in that he sees opportunity where others might perceive burden. For example, he notes, the advent of value-based purchasing initiatives, linking payment to quality, will create “huge opportunities for hospitalists.” More than 50% of the quality core measures in these initiatives are related to the Surgical Care Improvement Project (SCIP).4
“Now, more than ever, hospitalists need to align with their partners in the hospital C-suite to help them be successful around those targets,” Dr. Radzienda says. However, he adds, “it behooves the HM teams to be very methodical and not rush this.”
Crafting clear rules of engagement must be handled properly and thoughtfully at the outset, Dr. Radzienda explains, and developing mutual trust and respect between the parties is the most essential step. Logistically, this can present problems.
“Getting surgeons and hospitalists together at a table is hard work,” he says. “But I can’t underscore that more: This requires a relationship. And it’s not something that is done via email exchange or memoranda through the respective practices’ business managers.”
It’s also critical to have nursing on board, says Julie Weegman, RN, MA, OCN, director of nursing and medical surgical services at HealthPartners’ Regions Hospital in St. Paul. “Communication is key in this kind of arrangement,” she says. “Nurses could potentially be put in a bad position if there are tensions between hospitalists and the specialty departments.”
That isn’t the case at Regions, though, where the comanagement agreement between orthopedics and HM has been clearly established, Weegman says. Questions about the surgical site, activity, and weight-bearing are referred to surgeons, while chronic disease management, blood pressure, glucose monitoring, etc., usually are handled by hospitalists.
Dr. Radzienda stresses that patients must remain at the center of the equation. “At three o’clock in the morning, with the post-op ortho patient who is having pain, nausea, or bleeding, it cannot be a multistep process to decide which doc is going to take that call and deliver on the patient’s needs,” he says.
Dr. Nelson, who co-founded SHM and serves as The Hospitalist’s practice-management columnist, cautions that service agreements are not a panacea. “This won’t totally solve your problems,” he says, “because every doctor is authorized to violate agreements if they see fit and if they can prove their patient is the exception to the rule.”
The bottom-line test for Dr. Siegal: Consider the patient’s best interests. Ask yourself, he advises, “if your mother came into the hospital with a head bleed, who would you want her to see first? Hospitalists are not interchangeable with neurosurgeons, and yet, unfortunately, we have started marketing ourselves as being adequate replacements for people who have spent far more time training in a specialty.
“As an intensivist, I’ve got a bit of experience with head bleeds,” he says. “But the neurosurgeon still knows more.”
Gretchen Henkel is a freelance writer in central California.
References
- Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical co-management after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1): 28-38.
- Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31.
- Siegal EM. Just because you can, doesn’t mean that you should: A call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398-402.
- The Joint Commission. Surgical Care Improvement Project. The Joint Commission website. Available at: http://www.jointcommission.org/surgical_care_improvement_project/. Accessed Sept. 30, 2012.
Eric M. Siegal, MD, SFHM, vividly recalls the moment when he realized “scope creep” had become a problem. A hospitalist partner who was working a night shift admitted a young man who had been in a high-speed motor vehicle accident. The hospitalist did so because the general surgeon did not want to come into the hospital.
Dr. Siegal, currently the medical director of critical-care medicine at Aurora St. Luke’s Medical Center in Milwaukee, remembers looking at his partner and asking, “What the hell are you doing admitting a trauma patient? You’re an internist!”
Dr. Siegal’s partner responded, “I’m just trying to show value.”
“That was an ‘a-ha’ moment for me,” says Dr. Siegal, a member of SHM’s board of directors. It was at that point he began to understand that the expansion strategy used by many HM services—to demonstrate value by agreeing to comanage or admit patients for their primary-care (PCP) and specialist colleagues—had produced some unintended negative consequences. “Hospitalists,” he says, “are like the spackle of the hospital. Sometimes spackle is good; it hides flaws and imperfections. But at other times, people use spackle to fix major structural problems.”
Scope creep, mission creep, scut work: There are numerous ways to describe the phenomenon. In basic terms, hospitalists have been pressured to expand their scope of practice to manage all hospitalized patients. Hospitalist leaders differ about how much of an issue this really is, as managing hospitalized patients is the definition of hospitalist work. Burke T. Kealey, MD, SFHM, medical director of hospital specialties at HealthPartners Medical Group in St. Paul, Minn., and an SHM board member, points out that “one man’s scope creep is another man’s practice-builder.” John Nelson, MD, MHM, co-founder and past president of SHM and medical director of hospitalist services at Overlake Hospital in Bellevue, Wash., says the expanding service trend is prevalent, but whether “it’s a problem depends on your point of view. The same stressful evolution occurs in every specialty. We are not unique in that regard.”
The trick, according to HM leaders, is to understand the dynamics that drive scope creep, then work proactively to address the problem.
Evolving Scope of Practice
It was not so long ago that hospitalist groups, seen by many in medicine as the new kids on the block, were perceived as a threat to their primary-care and specialist colleagues. To establish themselves, hospitalists began to demonstrate value by comanaging patients for their surgical colleagues, especially orthopedists. Some studies, notably those conducted by Mayo Clinic-based hospitalists, appeared to demonstrate that using hospitalists to help comanage orthopedic surgical patients results in improved outcomes.1,2
Dr. Siegal, however, points out that a closer parsing of those studies reveals that such outcomes as decreased time to surgery and length of stay (LOS) were better for patients with complex medical comorbidities, rather than all patients, which supports his argument that hospitalist comanagement makes most sense when applied to select groups of surgical patients.3

—Eric M. Siegal, MD, SFHM, SHM board member, medical director of critical-care medicine, Aurora St. Luke’s Medical Center, Milwaukee
As HM sprouted roots, clinicians across the country began to see an increase in requests for their services from primary-care physicians (PCPs) and subspecialists, as hospitalists freed them from rounding on patients and allowed them to concentrate on procedures for higher billings. Over the past 10 years, the expansion has been rapid, converging with multiple factors: increasing numbers of uninsured patients, an aging physician workforce, and diminishing reimbursement, to name a few.
Nailing down the extent to which comanagement has expanded HM’s scope of practice as a medical specialty is a slippery exercise. Some HM groups handle comanagement well; others do not. Dr. Kealey says that admitting and comanagement patterns are dependent on the culture of the institution. For example, in one of HealthPartners’ home hospitals, all internal-medicine subspecialties, including neurology, are admitted and managed by hospitalists with a subspecialty consult.
The 2012 State of Hospital Medicine report survey revealed that 85% of respondent hospitalist groups provide surgical comanagement services (see Figure 1, below). That figure has not changed since SHM’s 2005-2006 survey, the last time the question was asked.
Another 20% of respondent hospitalist groups reported providing medical subspecialty comanagement, according to the 2012 report. Dr. Kealey, who is board liaison to SHM’s Practice Analysis Committee, says plans are in the works to add specific questions to the survey to assess another big change in the comanagement arena: a shift from hospitalists acting as consultants with the specialist serving as attending physician to a model in which the hospitalist admits the patient and serves as attending, with the specialist/proceduralist in a consulting role.
So What’s the Problem?
Hospitalists have been both the utility player and the superstar, providing great value to their healthcare teams, says Ken Simone, DO, SFHM, a hospitalist practice-management consultant and CEO of Hospitalist and Practice Solutions in Veazie, Maine. He believes hospitalist program expansions are typically a positive thing.
“Historically, most hospital medicine programs have embraced the call for assistance from both their colleagues and the C-suite,” says Dr. Simone, a Team Hospitalist member.
Dr. Siegal, in his HM07 presentation “Managing Comanagement: How to Play in the Sandbox without Having to Eat Mud Pies” and in journal articles, has cautioned against assuming that all hospitalized patients, irrespective of diagnosis or comorbidities, should be seen by a hospitalist.3 Such a directive can produce a host of unintended negative consequences. Most notably, it can:
- Confuse patients, families, and the care team about who is ultimately responsible for oversight of the patient’s care;
- Place hospitalists in the position of assuming responsibility for patients whose conditions are outside their scope of practice;
- Delay the initiation of appropriate, specialized care;
- Overwork an already stretched hospitalist team, which can lead to burnout; and
- Increase exposure to medical liability by placing hospitalists in situations where they are in over their heads, or by creating novel opportunities for miscommunication between hospitalists and surgeons or specialists.
Pressure Points
Scope creep’s root cause has multiple layers. It can be driven by overworked physicians; by local shortages in a particular specialty; by the bottom line, when procedure-focused physicians and surgeons want to divest themselves of day-to-day management of hospitalized patients; by lifestyle preferences; or by hospitalists’ success.
Jerome C. Siy, MD, SFHM, department head of hospital medicine for HealthPartners and recipient of the 2009 SHM Award for Clinical Excellence, believes the single most important factor behind the pressure to manage more hospitalized patients is the necessity to provide more thorough care when specialists or residents cannot.
“The hours of coverage are expanding in every specialty to a 24/7 model,” he says. “Since we hospitalists were in the hospital already, it became more routine for other services to ask us to get initial orders and the history and physical started, as a bridge to a better coverage model.”
Dr. Kealey says the “bridge” is a point of concern for many HM groups, especially when the pressure comes from hospital administrators attempting to attract specialists. Hospitalists have the right in such situations, says Dr. Siy, to feel undersupported or that they lack crucial knowledge or skill sets. Still, Dr. Kealey sees requests from other physician groups as a positive thing for hospitalists.
“We’re going to be managing more in the future,” he says, noting his HM group first drew up a comanagement agreement with orthopedic surgeons 17 years ago. “We want to go there thoughtfully and carefully. We shouldn’t put our foot down and say no to new opportunity.”
Rules of Engagement
Nearly every hospitalist leader agrees that the key to protecting against scope creep resides with thoughtful, proactive planning. Make sure, they say, that your group is ready to manage the patients you’re being asked to manage (see “Define and Protect Your Scope of Practice,” p. 35).
—Michael Radzienda, MD, SFHM, regional chief medical officer, Sound Physicians, Boston
Michael Radzienda, MD, SFHM, regional chief medical officer at Sound Physicians in the greater Boston area, agrees with Dr. Kealey in that he sees opportunity where others might perceive burden. For example, he notes, the advent of value-based purchasing initiatives, linking payment to quality, will create “huge opportunities for hospitalists.” More than 50% of the quality core measures in these initiatives are related to the Surgical Care Improvement Project (SCIP).4
“Now, more than ever, hospitalists need to align with their partners in the hospital C-suite to help them be successful around those targets,” Dr. Radzienda says. However, he adds, “it behooves the HM teams to be very methodical and not rush this.”
Crafting clear rules of engagement must be handled properly and thoughtfully at the outset, Dr. Radzienda explains, and developing mutual trust and respect between the parties is the most essential step. Logistically, this can present problems.
“Getting surgeons and hospitalists together at a table is hard work,” he says. “But I can’t underscore that more: This requires a relationship. And it’s not something that is done via email exchange or memoranda through the respective practices’ business managers.”
It’s also critical to have nursing on board, says Julie Weegman, RN, MA, OCN, director of nursing and medical surgical services at HealthPartners’ Regions Hospital in St. Paul. “Communication is key in this kind of arrangement,” she says. “Nurses could potentially be put in a bad position if there are tensions between hospitalists and the specialty departments.”
That isn’t the case at Regions, though, where the comanagement agreement between orthopedics and HM has been clearly established, Weegman says. Questions about the surgical site, activity, and weight-bearing are referred to surgeons, while chronic disease management, blood pressure, glucose monitoring, etc., usually are handled by hospitalists.
Dr. Radzienda stresses that patients must remain at the center of the equation. “At three o’clock in the morning, with the post-op ortho patient who is having pain, nausea, or bleeding, it cannot be a multistep process to decide which doc is going to take that call and deliver on the patient’s needs,” he says.
Dr. Nelson, who co-founded SHM and serves as The Hospitalist’s practice-management columnist, cautions that service agreements are not a panacea. “This won’t totally solve your problems,” he says, “because every doctor is authorized to violate agreements if they see fit and if they can prove their patient is the exception to the rule.”
The bottom-line test for Dr. Siegal: Consider the patient’s best interests. Ask yourself, he advises, “if your mother came into the hospital with a head bleed, who would you want her to see first? Hospitalists are not interchangeable with neurosurgeons, and yet, unfortunately, we have started marketing ourselves as being adequate replacements for people who have spent far more time training in a specialty.
“As an intensivist, I’ve got a bit of experience with head bleeds,” he says. “But the neurosurgeon still knows more.”
Gretchen Henkel is a freelance writer in central California.
References
- Huddleston JM, Long KH, Naessens JM, et al. Medical and surgical co-management after elective hip and knee arthroplasty: a randomized, controlled trial. Ann Intern Med. 2004;141(1): 28-38.
- Roy A, Heckman MG, Roy V. Associations between the hospitalist model of care and quality-of-care-related outcomes in patients undergoing hip fracture surgery. Mayo Clin Proc. 2006;81(1):28-31.
- Siegal EM. Just because you can, doesn’t mean that you should: A call for the rational application of hospitalist comanagement. J Hosp Med. 2008;3(5):398-402.
- The Joint Commission. Surgical Care Improvement Project. The Joint Commission website. Available at: http://www.jointcommission.org/surgical_care_improvement_project/. Accessed Sept. 30, 2012.
Five Ways to Enhance Your Hospital Medicine Group's Efficiency
Is there a role in HM for industrial engineering or industrial efficiency models? Jonathan Turner, PhD, thinks so. He is an industrial-engineer-turned-hospital-engineer whose job is to help make hospital care safer, faster, less costly, and more satisfying. He has few peers in this role, although any hospitalist group in the vicinity of a college department of engineering could seek out similar expertise there. Real-world problems make the best research projects, he says.
“I wrote my thesis on how a vascular surgery department could use computer simulation models to help balance multiple objectives in scheduling residents,” says Turner, who earned his doctorate at Northwestern University in Evanston, Ill.
His thesis examined, among other things, the need for pre-operative continuity of care and resident exposure to a variety of surgical experiences. He also found himself hanging around Northwestern’s Feinberg School of Medicine in Chicago at a time when Mark Williams, MD, MHM, chief of the division of hospital medicine, was looking for ways to build collaboration between Northwestern’s medical school, hospital, and department of engineering.
“Our objectives were the same,” says Turner, who was hired by Dr. Williams in May 2011.
Turner says many of the challenges of managing an HM group—making patient rounds better, improving length of stay (LOS) and throughput, or deciding how to incorporate technology into practice—can be scrutinized with an efficiency lens. At Northwestern, he optimized the HM group schedule and made it more appealing to the physicians. He examined the incremental costs incurred by patient handoffs and the effectiveness of consultations with medical specialists. He tackled technology, teamwork, and wait-time issues. He even helped surgeons standardize their instrument trays.
But every hospitalist group—from the three-FTE teams covering rural hospitals to high-volume groups with dozens of moving parts—has developed inefficient habits. Experts say most groups have never even thought of the problems, let alone the solutions—for example, regularly inputting data into spreadsheets that no one ever looks at.
Efficiency is an essential target for quality initiatives in the hospital, although the word means different things to different people. It typically involves trade-offs that need to be balanced if the system as a whole is to benefit. What makes an individual practice more efficient could make a group’s less so—and vice versa. What helps one department’s bottom line can harm another’s. Enhancing hospitalists’ work-life balance through schedule modification could make life harder for nurses.
-Jonathan Turner, PhD
One current example that cuts across HM groups of all shapes and sizes is the discharge process. Hospitalist groups speeding up discharges might lead to inadequately prepared patients leaving the hospital, which could mean post-discharge crises, which could lead to unnecessary readmissions, which certainly will mean government penalties. Pure efficiency, in terms of maximizing caseloads, also can conflict with patient safety or patient satisfaction. In many healthcare settings, approaching 100% of capacity limits the ability to respond to surges in demand, Turner says. That usually leads to backups, long waits, dissatisfaction, and even diversions from the ED, he explains.
HM groups have pursued a variety of tools and strategies to enhance efficiency. “One of the things we try to show is that you use these methods every day—but you can be more systematic in how you apply them,” Turner says. The character and personality of practicing hospitalists, who tend to be more quality-minded and focused on systems, might make them more open to becoming efficiency experts and willing to try new approaches.
Still, when engineers talk about efficiency, hospital professionals might feel that it cramps their practice style. “I need to be willing to listen to their concerns,” says Turner, who in September began a new position as director of systems engineering at University Hospital in Augusta, Ga.
“I don’t want to turn patients into widgets. I’d rather be thought of as an axe sharpener—helping people do their work more easily,” he says. “You can’t satisfy everybody, but you at least need to listen to everybody when you try to make their work lives more efficient.”
Following are some examples of how hospitalist groups have improved the efficiency of important aspects of their practice.
1. Specialized Care Plans
It is well known that some of the most challenging hospital patients consume a disproportionate share of costs and resources, says Rick Hilger, MD, SFHM, a hospitalist with HealthPartners at Regions Hospital in St. Paul, Minn. It can be controversial to suggest that these difficult patients should receive special handling, but Dr. Hilger, who presented a poster on the topic at HM12, says the current system isn’t safe for patients or sustainable in the long run.3
At Regions, about 70 high-utilizing patients have been given an ongoing, specialized care plan that is easily accessible in their electronic health record (EHR) whenever they present at a HealthPartners clinic, ED, or hospital. Patients include those with a history of drug-seeking behaviors, antisocial behavior disorders, aggression or noncompliance in the hospital, and a variety of traumatic brain injuries or memory deficits that might cause them to give a different story to every medical provider. They also include patients who simply have very complex medical conditions.
Referrals for a specialized care plan can come from any hospital staff member. A care-planning committee representing HM, case management, social work, emergency medicine, and administrative staffs meets monthly to review cases and decide if each patient would benefit from a specialized care plan. It offers quick access in the EHR to a cover page with common clinical scenarios, recent tests and procedures, and a template for optimal care that can save a lot of time and prevent duplicative or uncoordinated treatments, Dr. Hilger says. It also ensures that clinicians give a consistent message to the patient.
“Our mantra is that we want care plans that are easy to create, easy to find, easy to use, and likely to lead to better coordination of care,” he says. “We also say that if everybody has a specialized care plan, then nobody does. We want the provider—whether physician, case manager, or nurse—to walk away saying that the care plan saved them time and led to safer care.”
In its first two months, Regions saw a 68% reduction in total ED visits and hospital admissions for the 43 patients with specialized care plans.
2. Scheduling Models
A number of scheduling models are used for deploying hospitalists in larger groups, including seven on/seven off, five on/five off, weekdays versus weekends, zoned staffing, and admitters versus rounders. Research has shown an incremental cost for every handoff, and creating a work schedule that results in fewer handoffs might make patients more satisfied. But that goal needs to be balanced against provider schedules with an eye toward keeping caseloads localized in the hospital.
Shalini Chandra, MD, MS, FHM, a hospitalist at Johns Hopkins Bayview Medical Center in Baltimore, was co-presenter of a HM12 workshop on how to use performance-improvement principles to improve HM group schedules. She says the schedule needs to account for such variables as when hospitalists are assigned new patients, are required to interact with the ED, and are devoted to patient contact versus paperwork, which normally is greater at admission and discharge.
“You need to find the sweet spot between the hospital’s needs, the patient’s needs, and the doctor’s needs,” she says. “Our physicians felt they were being penalized for discharging patients in a timely manner [by receiving more new cases]. We had to go back and say, ‘OK, if somebody is doing a four-day stretch, how can we distribute patients more equitably?’”
Dr. Chandra’s quality team mapped out the entire admissions process and identified key metrics, then devised a model called CICLE (Creating Incentives and Continuity Leading to Efficiency in Hospital Medicine) for its four-day shift. The first day, which can be a long one, is front-loaded with new admissions. But on Day Two and Day Three, the hospitalist is largely protected from new admissions, thereby preserving the incentive to discharge patients when they are ready.
CICLE also results in fewer handoffs, with a third of patients seen by only one hospitalist, Dr. Chandra explains. That in turn translates into decreased LOS and cost.
The four-day schedule at Hopkins is complicated by the need to respond to other demands on the system, and Dr. Chandra says only 60 percent of the hospitalist caseload is scheduled this way.

—Rick Hilger, MD, SFHM
3. Individual Flexibility
The best schedule in the world can be turned upside down by vacations, sickness, or an open position that goes unfilled for months. Accounting for the nuances can be a full-time job.
At Northwestern, the hospitalist group is converting to scheduling software called Lightning Bolt (www.lightning-bolt.com) that provides flexibility to respond to varying needs among the 70-plus members of the group, including such needs as attending their children’s soccer games, says Charlotta Weaver, MD, assistant professor of medicine.
“An enormous amount of time, both administrative and medical, gets devoted to scheduling,” she says.
Each hospital and HM group is different, and each physician has varying desires from the schedule. “Things are constantly changing,” Dr. Weaver says. “People come and go or need to change jobs, there are changes in FTE allocations, physicians may get research grants, and there can be changes in hospital structure or service lines.”
The schedule also needs to facilitate “day trading” between members of the group, and Northwestern is experimenting with some new approaches, including pairing up two physicians on one service line and letting them work out their own schedules.
The group also needs a way to respond to admission surges beyond the capacity of scheduled physicians, which can be hard to predict, and the days when physicians call in sick.
“We have tried to develop a robust system of ‘jeopardy’ for first- and second-line backup,” Dr. Weaver says. Everyone in the hospitalist group has jeopardy one or two weeks per year, where they are in line to be called in if needed.
Franziska Jovin, MD, FHM, a hospitalist at the University of Pittsburgh Medical Center, says her group struggles with predicting patient peaks and valleys.
“One of our hospitalist teams is responsible for the transitional-care unit, and not every patient on that unit has to be seen every day,” Dr. Jovin says. “This person is already scheduled to work, but the responsibilities are not time-dependent. So we can pull in that doctor as needed to cover higher demand on the acute side.”
As most hospitalists know the winter months are busier, “and we staff accordingly,” she says, in response to higher incidence of flu, pneumonia, and the like.
4. Structured Rounds
Another challenge to scheduling is the rounding model used for daily care planning in the hospital. Various models have tried to address competing concerns of geography, schedule efficiency, and the needs of hospitalists, nurses, and other staff. At Emory Healthcare in Atlanta, an approach called Structured Interdisciplinary Bedside Rounds (SIBR) was described during an HM12 workshop and in a prize-winning poster presented by Christina Payne, MD (see “The Innovation Express,” May 2012, p. 27).
Dr. Payne described how SIBR works with two teaching hospitalist services on a 24-bed “accountable-care unit” at Emory University Hospital. Each team has a resident, three interns, a social worker, and the patient’s nurse, with the attending standing by.
“We round on each patient every day—beginning and ending on time,” 12 patients per hour, with five brisk minutes to report on each patient, she says. Rounding starts when the patient’s primary intern and nurse are both present in the patient’s room, and only ends when a plan of care for the day has been articulated—using a standardized script with safety and discharge planning checklists. The second intern enters the plan, in real time, into the EHR.
In addition to the time efficiency, this approach has posted positive outcomes, namely a 53% reduction in in-hospital mortality on the unit and an 11% reduction in LOS. With new residents and interns rotating through the unit every month, “We acknowledge to them that this will be difficult and they will be uncomfortable at first,” Dr. Payne says, “but by Week Two, we’re a well-oiled machine.”
Similar approaches have been implemented at other Emory hospitals.
5. NPP Mobilization
Many hospitalist groups have integrated nonphysician providers (NPPs, or nurse practitioners and physician assistants) into the group’s practice or are considering such a move. Tracy Cardin, ACNP-BC, a nurse practitioner in hospital practice at the University of Chicago Medical Center, says it’s important to ask why a group is considering a role for NPPs.
“Is it to promote efficiency? Is it because you can’t attract enough physicians?” she says. “Clarify your hopes for the position and how you will define success.” She also says HM group leaders need to factor in the time needed to hire, orient, and train an NPP, with mentoring that includes structured teaching and feedback.
There are a lot of models for deploying NPPs, says Cardin, a Team Hospitalist member.
“We utilize shared billing and teams of a hospitalist and nurse practitioner. This allows the physician to see a larger number of patients and brings more than one set of eyes and ears to the complex patient,” she says. “We’ve developed a process over the past six years where the hospitalist and NPP together go over the patient list every day. Both will see the patient, but the NPP commonly writes the notes and orders.”
Cardin emphasizes NPPs cost less than physicians and “can do many of the same things,” but “they are not free.” The most resourceful HM group’s use NPPs to extend the physician’s practice. “They can carry a pager and respond to small crises that come up, or see the patient on discharge day,” she says. “In other settings, the NPP does admissions, serves on quality projects, takes on a patient cohort based on diagnosis, or calls the primary-care physician at discharge.”
Efficiency can be a tough nut to crack in the hospital. Turner, the engineer, says HM groups need to “remember that the hospital is a very complex environment, with cascades of reactions and downstream effects.”
Hospitalists need the support of other professionals, and quality-improvement (QI) initiatives need sufficient time and resources to succeed.
One way to start advancing the efficiency agenda is to look for bright spots among the hospitalist group’s members. “Talk to them,” Turner says. “Find out how they do their jobs. Learn from them.”
Larry Beresford is a freelance author in Oakland, Calif.
References
- Yu D, Sanches S. Lean inpatient unit base care model [abstract]. J Hosp Med. 2012;7(Suppl 2):S107.
- Payne C, Odetoyinbo D, Castle B, et al. A dual hospital care and training model: structured interdisciplinary team rounds in an accountable care unit [abstract]. J Hosp Med. 2012;7(Suppl 2):S125.
- Hilger R, Quirk R, Dahms R. Use of restriction care plans to decrease medically unnecessary admissions and emergency department visits [abstract]. J Hosp Med. 2012;7(Suppl 2):S2.
- Premier. Year Three: QUEST Collaborative Findings. Premier website. Available at: http://www.premierinc.com/quality-safety/tools-services/quest/year3/quest-year-3-collaborative-findings.pdf. Accessed Sept. 26, 2012.
Is there a role in HM for industrial engineering or industrial efficiency models? Jonathan Turner, PhD, thinks so. He is an industrial-engineer-turned-hospital-engineer whose job is to help make hospital care safer, faster, less costly, and more satisfying. He has few peers in this role, although any hospitalist group in the vicinity of a college department of engineering could seek out similar expertise there. Real-world problems make the best research projects, he says.
“I wrote my thesis on how a vascular surgery department could use computer simulation models to help balance multiple objectives in scheduling residents,” says Turner, who earned his doctorate at Northwestern University in Evanston, Ill.
His thesis examined, among other things, the need for pre-operative continuity of care and resident exposure to a variety of surgical experiences. He also found himself hanging around Northwestern’s Feinberg School of Medicine in Chicago at a time when Mark Williams, MD, MHM, chief of the division of hospital medicine, was looking for ways to build collaboration between Northwestern’s medical school, hospital, and department of engineering.
“Our objectives were the same,” says Turner, who was hired by Dr. Williams in May 2011.
Turner says many of the challenges of managing an HM group—making patient rounds better, improving length of stay (LOS) and throughput, or deciding how to incorporate technology into practice—can be scrutinized with an efficiency lens. At Northwestern, he optimized the HM group schedule and made it more appealing to the physicians. He examined the incremental costs incurred by patient handoffs and the effectiveness of consultations with medical specialists. He tackled technology, teamwork, and wait-time issues. He even helped surgeons standardize their instrument trays.
But every hospitalist group—from the three-FTE teams covering rural hospitals to high-volume groups with dozens of moving parts—has developed inefficient habits. Experts say most groups have never even thought of the problems, let alone the solutions—for example, regularly inputting data into spreadsheets that no one ever looks at.
Efficiency is an essential target for quality initiatives in the hospital, although the word means different things to different people. It typically involves trade-offs that need to be balanced if the system as a whole is to benefit. What makes an individual practice more efficient could make a group’s less so—and vice versa. What helps one department’s bottom line can harm another’s. Enhancing hospitalists’ work-life balance through schedule modification could make life harder for nurses.
-Jonathan Turner, PhD
One current example that cuts across HM groups of all shapes and sizes is the discharge process. Hospitalist groups speeding up discharges might lead to inadequately prepared patients leaving the hospital, which could mean post-discharge crises, which could lead to unnecessary readmissions, which certainly will mean government penalties. Pure efficiency, in terms of maximizing caseloads, also can conflict with patient safety or patient satisfaction. In many healthcare settings, approaching 100% of capacity limits the ability to respond to surges in demand, Turner says. That usually leads to backups, long waits, dissatisfaction, and even diversions from the ED, he explains.
HM groups have pursued a variety of tools and strategies to enhance efficiency. “One of the things we try to show is that you use these methods every day—but you can be more systematic in how you apply them,” Turner says. The character and personality of practicing hospitalists, who tend to be more quality-minded and focused on systems, might make them more open to becoming efficiency experts and willing to try new approaches.
Still, when engineers talk about efficiency, hospital professionals might feel that it cramps their practice style. “I need to be willing to listen to their concerns,” says Turner, who in September began a new position as director of systems engineering at University Hospital in Augusta, Ga.
“I don’t want to turn patients into widgets. I’d rather be thought of as an axe sharpener—helping people do their work more easily,” he says. “You can’t satisfy everybody, but you at least need to listen to everybody when you try to make their work lives more efficient.”
Following are some examples of how hospitalist groups have improved the efficiency of important aspects of their practice.
1. Specialized Care Plans
It is well known that some of the most challenging hospital patients consume a disproportionate share of costs and resources, says Rick Hilger, MD, SFHM, a hospitalist with HealthPartners at Regions Hospital in St. Paul, Minn. It can be controversial to suggest that these difficult patients should receive special handling, but Dr. Hilger, who presented a poster on the topic at HM12, says the current system isn’t safe for patients or sustainable in the long run.3
At Regions, about 70 high-utilizing patients have been given an ongoing, specialized care plan that is easily accessible in their electronic health record (EHR) whenever they present at a HealthPartners clinic, ED, or hospital. Patients include those with a history of drug-seeking behaviors, antisocial behavior disorders, aggression or noncompliance in the hospital, and a variety of traumatic brain injuries or memory deficits that might cause them to give a different story to every medical provider. They also include patients who simply have very complex medical conditions.
Referrals for a specialized care plan can come from any hospital staff member. A care-planning committee representing HM, case management, social work, emergency medicine, and administrative staffs meets monthly to review cases and decide if each patient would benefit from a specialized care plan. It offers quick access in the EHR to a cover page with common clinical scenarios, recent tests and procedures, and a template for optimal care that can save a lot of time and prevent duplicative or uncoordinated treatments, Dr. Hilger says. It also ensures that clinicians give a consistent message to the patient.
“Our mantra is that we want care plans that are easy to create, easy to find, easy to use, and likely to lead to better coordination of care,” he says. “We also say that if everybody has a specialized care plan, then nobody does. We want the provider—whether physician, case manager, or nurse—to walk away saying that the care plan saved them time and led to safer care.”
In its first two months, Regions saw a 68% reduction in total ED visits and hospital admissions for the 43 patients with specialized care plans.
2. Scheduling Models
A number of scheduling models are used for deploying hospitalists in larger groups, including seven on/seven off, five on/five off, weekdays versus weekends, zoned staffing, and admitters versus rounders. Research has shown an incremental cost for every handoff, and creating a work schedule that results in fewer handoffs might make patients more satisfied. But that goal needs to be balanced against provider schedules with an eye toward keeping caseloads localized in the hospital.
Shalini Chandra, MD, MS, FHM, a hospitalist at Johns Hopkins Bayview Medical Center in Baltimore, was co-presenter of a HM12 workshop on how to use performance-improvement principles to improve HM group schedules. She says the schedule needs to account for such variables as when hospitalists are assigned new patients, are required to interact with the ED, and are devoted to patient contact versus paperwork, which normally is greater at admission and discharge.
“You need to find the sweet spot between the hospital’s needs, the patient’s needs, and the doctor’s needs,” she says. “Our physicians felt they were being penalized for discharging patients in a timely manner [by receiving more new cases]. We had to go back and say, ‘OK, if somebody is doing a four-day stretch, how can we distribute patients more equitably?’”
Dr. Chandra’s quality team mapped out the entire admissions process and identified key metrics, then devised a model called CICLE (Creating Incentives and Continuity Leading to Efficiency in Hospital Medicine) for its four-day shift. The first day, which can be a long one, is front-loaded with new admissions. But on Day Two and Day Three, the hospitalist is largely protected from new admissions, thereby preserving the incentive to discharge patients when they are ready.
CICLE also results in fewer handoffs, with a third of patients seen by only one hospitalist, Dr. Chandra explains. That in turn translates into decreased LOS and cost.
The four-day schedule at Hopkins is complicated by the need to respond to other demands on the system, and Dr. Chandra says only 60 percent of the hospitalist caseload is scheduled this way.

—Rick Hilger, MD, SFHM
3. Individual Flexibility
The best schedule in the world can be turned upside down by vacations, sickness, or an open position that goes unfilled for months. Accounting for the nuances can be a full-time job.
At Northwestern, the hospitalist group is converting to scheduling software called Lightning Bolt (www.lightning-bolt.com) that provides flexibility to respond to varying needs among the 70-plus members of the group, including such needs as attending their children’s soccer games, says Charlotta Weaver, MD, assistant professor of medicine.
“An enormous amount of time, both administrative and medical, gets devoted to scheduling,” she says.
Each hospital and HM group is different, and each physician has varying desires from the schedule. “Things are constantly changing,” Dr. Weaver says. “People come and go or need to change jobs, there are changes in FTE allocations, physicians may get research grants, and there can be changes in hospital structure or service lines.”
The schedule also needs to facilitate “day trading” between members of the group, and Northwestern is experimenting with some new approaches, including pairing up two physicians on one service line and letting them work out their own schedules.
The group also needs a way to respond to admission surges beyond the capacity of scheduled physicians, which can be hard to predict, and the days when physicians call in sick.
“We have tried to develop a robust system of ‘jeopardy’ for first- and second-line backup,” Dr. Weaver says. Everyone in the hospitalist group has jeopardy one or two weeks per year, where they are in line to be called in if needed.
Franziska Jovin, MD, FHM, a hospitalist at the University of Pittsburgh Medical Center, says her group struggles with predicting patient peaks and valleys.
“One of our hospitalist teams is responsible for the transitional-care unit, and not every patient on that unit has to be seen every day,” Dr. Jovin says. “This person is already scheduled to work, but the responsibilities are not time-dependent. So we can pull in that doctor as needed to cover higher demand on the acute side.”
As most hospitalists know the winter months are busier, “and we staff accordingly,” she says, in response to higher incidence of flu, pneumonia, and the like.
4. Structured Rounds
Another challenge to scheduling is the rounding model used for daily care planning in the hospital. Various models have tried to address competing concerns of geography, schedule efficiency, and the needs of hospitalists, nurses, and other staff. At Emory Healthcare in Atlanta, an approach called Structured Interdisciplinary Bedside Rounds (SIBR) was described during an HM12 workshop and in a prize-winning poster presented by Christina Payne, MD (see “The Innovation Express,” May 2012, p. 27).
Dr. Payne described how SIBR works with two teaching hospitalist services on a 24-bed “accountable-care unit” at Emory University Hospital. Each team has a resident, three interns, a social worker, and the patient’s nurse, with the attending standing by.
“We round on each patient every day—beginning and ending on time,” 12 patients per hour, with five brisk minutes to report on each patient, she says. Rounding starts when the patient’s primary intern and nurse are both present in the patient’s room, and only ends when a plan of care for the day has been articulated—using a standardized script with safety and discharge planning checklists. The second intern enters the plan, in real time, into the EHR.
In addition to the time efficiency, this approach has posted positive outcomes, namely a 53% reduction in in-hospital mortality on the unit and an 11% reduction in LOS. With new residents and interns rotating through the unit every month, “We acknowledge to them that this will be difficult and they will be uncomfortable at first,” Dr. Payne says, “but by Week Two, we’re a well-oiled machine.”
Similar approaches have been implemented at other Emory hospitals.
5. NPP Mobilization
Many hospitalist groups have integrated nonphysician providers (NPPs, or nurse practitioners and physician assistants) into the group’s practice or are considering such a move. Tracy Cardin, ACNP-BC, a nurse practitioner in hospital practice at the University of Chicago Medical Center, says it’s important to ask why a group is considering a role for NPPs.
“Is it to promote efficiency? Is it because you can’t attract enough physicians?” she says. “Clarify your hopes for the position and how you will define success.” She also says HM group leaders need to factor in the time needed to hire, orient, and train an NPP, with mentoring that includes structured teaching and feedback.
There are a lot of models for deploying NPPs, says Cardin, a Team Hospitalist member.
“We utilize shared billing and teams of a hospitalist and nurse practitioner. This allows the physician to see a larger number of patients and brings more than one set of eyes and ears to the complex patient,” she says. “We’ve developed a process over the past six years where the hospitalist and NPP together go over the patient list every day. Both will see the patient, but the NPP commonly writes the notes and orders.”
Cardin emphasizes NPPs cost less than physicians and “can do many of the same things,” but “they are not free.” The most resourceful HM group’s use NPPs to extend the physician’s practice. “They can carry a pager and respond to small crises that come up, or see the patient on discharge day,” she says. “In other settings, the NPP does admissions, serves on quality projects, takes on a patient cohort based on diagnosis, or calls the primary-care physician at discharge.”
Efficiency can be a tough nut to crack in the hospital. Turner, the engineer, says HM groups need to “remember that the hospital is a very complex environment, with cascades of reactions and downstream effects.”
Hospitalists need the support of other professionals, and quality-improvement (QI) initiatives need sufficient time and resources to succeed.
One way to start advancing the efficiency agenda is to look for bright spots among the hospitalist group’s members. “Talk to them,” Turner says. “Find out how they do their jobs. Learn from them.”
Larry Beresford is a freelance author in Oakland, Calif.
References
- Yu D, Sanches S. Lean inpatient unit base care model [abstract]. J Hosp Med. 2012;7(Suppl 2):S107.
- Payne C, Odetoyinbo D, Castle B, et al. A dual hospital care and training model: structured interdisciplinary team rounds in an accountable care unit [abstract]. J Hosp Med. 2012;7(Suppl 2):S125.
- Hilger R, Quirk R, Dahms R. Use of restriction care plans to decrease medically unnecessary admissions and emergency department visits [abstract]. J Hosp Med. 2012;7(Suppl 2):S2.
- Premier. Year Three: QUEST Collaborative Findings. Premier website. Available at: http://www.premierinc.com/quality-safety/tools-services/quest/year3/quest-year-3-collaborative-findings.pdf. Accessed Sept. 26, 2012.
Is there a role in HM for industrial engineering or industrial efficiency models? Jonathan Turner, PhD, thinks so. He is an industrial-engineer-turned-hospital-engineer whose job is to help make hospital care safer, faster, less costly, and more satisfying. He has few peers in this role, although any hospitalist group in the vicinity of a college department of engineering could seek out similar expertise there. Real-world problems make the best research projects, he says.
“I wrote my thesis on how a vascular surgery department could use computer simulation models to help balance multiple objectives in scheduling residents,” says Turner, who earned his doctorate at Northwestern University in Evanston, Ill.
His thesis examined, among other things, the need for pre-operative continuity of care and resident exposure to a variety of surgical experiences. He also found himself hanging around Northwestern’s Feinberg School of Medicine in Chicago at a time when Mark Williams, MD, MHM, chief of the division of hospital medicine, was looking for ways to build collaboration between Northwestern’s medical school, hospital, and department of engineering.
“Our objectives were the same,” says Turner, who was hired by Dr. Williams in May 2011.
Turner says many of the challenges of managing an HM group—making patient rounds better, improving length of stay (LOS) and throughput, or deciding how to incorporate technology into practice—can be scrutinized with an efficiency lens. At Northwestern, he optimized the HM group schedule and made it more appealing to the physicians. He examined the incremental costs incurred by patient handoffs and the effectiveness of consultations with medical specialists. He tackled technology, teamwork, and wait-time issues. He even helped surgeons standardize their instrument trays.
But every hospitalist group—from the three-FTE teams covering rural hospitals to high-volume groups with dozens of moving parts—has developed inefficient habits. Experts say most groups have never even thought of the problems, let alone the solutions—for example, regularly inputting data into spreadsheets that no one ever looks at.
Efficiency is an essential target for quality initiatives in the hospital, although the word means different things to different people. It typically involves trade-offs that need to be balanced if the system as a whole is to benefit. What makes an individual practice more efficient could make a group’s less so—and vice versa. What helps one department’s bottom line can harm another’s. Enhancing hospitalists’ work-life balance through schedule modification could make life harder for nurses.
-Jonathan Turner, PhD
One current example that cuts across HM groups of all shapes and sizes is the discharge process. Hospitalist groups speeding up discharges might lead to inadequately prepared patients leaving the hospital, which could mean post-discharge crises, which could lead to unnecessary readmissions, which certainly will mean government penalties. Pure efficiency, in terms of maximizing caseloads, also can conflict with patient safety or patient satisfaction. In many healthcare settings, approaching 100% of capacity limits the ability to respond to surges in demand, Turner says. That usually leads to backups, long waits, dissatisfaction, and even diversions from the ED, he explains.
HM groups have pursued a variety of tools and strategies to enhance efficiency. “One of the things we try to show is that you use these methods every day—but you can be more systematic in how you apply them,” Turner says. The character and personality of practicing hospitalists, who tend to be more quality-minded and focused on systems, might make them more open to becoming efficiency experts and willing to try new approaches.
Still, when engineers talk about efficiency, hospital professionals might feel that it cramps their practice style. “I need to be willing to listen to their concerns,” says Turner, who in September began a new position as director of systems engineering at University Hospital in Augusta, Ga.
“I don’t want to turn patients into widgets. I’d rather be thought of as an axe sharpener—helping people do their work more easily,” he says. “You can’t satisfy everybody, but you at least need to listen to everybody when you try to make their work lives more efficient.”
Following are some examples of how hospitalist groups have improved the efficiency of important aspects of their practice.
1. Specialized Care Plans
It is well known that some of the most challenging hospital patients consume a disproportionate share of costs and resources, says Rick Hilger, MD, SFHM, a hospitalist with HealthPartners at Regions Hospital in St. Paul, Minn. It can be controversial to suggest that these difficult patients should receive special handling, but Dr. Hilger, who presented a poster on the topic at HM12, says the current system isn’t safe for patients or sustainable in the long run.3
At Regions, about 70 high-utilizing patients have been given an ongoing, specialized care plan that is easily accessible in their electronic health record (EHR) whenever they present at a HealthPartners clinic, ED, or hospital. Patients include those with a history of drug-seeking behaviors, antisocial behavior disorders, aggression or noncompliance in the hospital, and a variety of traumatic brain injuries or memory deficits that might cause them to give a different story to every medical provider. They also include patients who simply have very complex medical conditions.
Referrals for a specialized care plan can come from any hospital staff member. A care-planning committee representing HM, case management, social work, emergency medicine, and administrative staffs meets monthly to review cases and decide if each patient would benefit from a specialized care plan. It offers quick access in the EHR to a cover page with common clinical scenarios, recent tests and procedures, and a template for optimal care that can save a lot of time and prevent duplicative or uncoordinated treatments, Dr. Hilger says. It also ensures that clinicians give a consistent message to the patient.
“Our mantra is that we want care plans that are easy to create, easy to find, easy to use, and likely to lead to better coordination of care,” he says. “We also say that if everybody has a specialized care plan, then nobody does. We want the provider—whether physician, case manager, or nurse—to walk away saying that the care plan saved them time and led to safer care.”
In its first two months, Regions saw a 68% reduction in total ED visits and hospital admissions for the 43 patients with specialized care plans.
2. Scheduling Models
A number of scheduling models are used for deploying hospitalists in larger groups, including seven on/seven off, five on/five off, weekdays versus weekends, zoned staffing, and admitters versus rounders. Research has shown an incremental cost for every handoff, and creating a work schedule that results in fewer handoffs might make patients more satisfied. But that goal needs to be balanced against provider schedules with an eye toward keeping caseloads localized in the hospital.
Shalini Chandra, MD, MS, FHM, a hospitalist at Johns Hopkins Bayview Medical Center in Baltimore, was co-presenter of a HM12 workshop on how to use performance-improvement principles to improve HM group schedules. She says the schedule needs to account for such variables as when hospitalists are assigned new patients, are required to interact with the ED, and are devoted to patient contact versus paperwork, which normally is greater at admission and discharge.
“You need to find the sweet spot between the hospital’s needs, the patient’s needs, and the doctor’s needs,” she says. “Our physicians felt they were being penalized for discharging patients in a timely manner [by receiving more new cases]. We had to go back and say, ‘OK, if somebody is doing a four-day stretch, how can we distribute patients more equitably?’”
Dr. Chandra’s quality team mapped out the entire admissions process and identified key metrics, then devised a model called CICLE (Creating Incentives and Continuity Leading to Efficiency in Hospital Medicine) for its four-day shift. The first day, which can be a long one, is front-loaded with new admissions. But on Day Two and Day Three, the hospitalist is largely protected from new admissions, thereby preserving the incentive to discharge patients when they are ready.
CICLE also results in fewer handoffs, with a third of patients seen by only one hospitalist, Dr. Chandra explains. That in turn translates into decreased LOS and cost.
The four-day schedule at Hopkins is complicated by the need to respond to other demands on the system, and Dr. Chandra says only 60 percent of the hospitalist caseload is scheduled this way.

—Rick Hilger, MD, SFHM
3. Individual Flexibility
The best schedule in the world can be turned upside down by vacations, sickness, or an open position that goes unfilled for months. Accounting for the nuances can be a full-time job.
At Northwestern, the hospitalist group is converting to scheduling software called Lightning Bolt (www.lightning-bolt.com) that provides flexibility to respond to varying needs among the 70-plus members of the group, including such needs as attending their children’s soccer games, says Charlotta Weaver, MD, assistant professor of medicine.
“An enormous amount of time, both administrative and medical, gets devoted to scheduling,” she says.
Each hospital and HM group is different, and each physician has varying desires from the schedule. “Things are constantly changing,” Dr. Weaver says. “People come and go or need to change jobs, there are changes in FTE allocations, physicians may get research grants, and there can be changes in hospital structure or service lines.”
The schedule also needs to facilitate “day trading” between members of the group, and Northwestern is experimenting with some new approaches, including pairing up two physicians on one service line and letting them work out their own schedules.
The group also needs a way to respond to admission surges beyond the capacity of scheduled physicians, which can be hard to predict, and the days when physicians call in sick.
“We have tried to develop a robust system of ‘jeopardy’ for first- and second-line backup,” Dr. Weaver says. Everyone in the hospitalist group has jeopardy one or two weeks per year, where they are in line to be called in if needed.
Franziska Jovin, MD, FHM, a hospitalist at the University of Pittsburgh Medical Center, says her group struggles with predicting patient peaks and valleys.
“One of our hospitalist teams is responsible for the transitional-care unit, and not every patient on that unit has to be seen every day,” Dr. Jovin says. “This person is already scheduled to work, but the responsibilities are not time-dependent. So we can pull in that doctor as needed to cover higher demand on the acute side.”
As most hospitalists know the winter months are busier, “and we staff accordingly,” she says, in response to higher incidence of flu, pneumonia, and the like.
4. Structured Rounds
Another challenge to scheduling is the rounding model used for daily care planning in the hospital. Various models have tried to address competing concerns of geography, schedule efficiency, and the needs of hospitalists, nurses, and other staff. At Emory Healthcare in Atlanta, an approach called Structured Interdisciplinary Bedside Rounds (SIBR) was described during an HM12 workshop and in a prize-winning poster presented by Christina Payne, MD (see “The Innovation Express,” May 2012, p. 27).
Dr. Payne described how SIBR works with two teaching hospitalist services on a 24-bed “accountable-care unit” at Emory University Hospital. Each team has a resident, three interns, a social worker, and the patient’s nurse, with the attending standing by.
“We round on each patient every day—beginning and ending on time,” 12 patients per hour, with five brisk minutes to report on each patient, she says. Rounding starts when the patient’s primary intern and nurse are both present in the patient’s room, and only ends when a plan of care for the day has been articulated—using a standardized script with safety and discharge planning checklists. The second intern enters the plan, in real time, into the EHR.
In addition to the time efficiency, this approach has posted positive outcomes, namely a 53% reduction in in-hospital mortality on the unit and an 11% reduction in LOS. With new residents and interns rotating through the unit every month, “We acknowledge to them that this will be difficult and they will be uncomfortable at first,” Dr. Payne says, “but by Week Two, we’re a well-oiled machine.”
Similar approaches have been implemented at other Emory hospitals.
5. NPP Mobilization
Many hospitalist groups have integrated nonphysician providers (NPPs, or nurse practitioners and physician assistants) into the group’s practice or are considering such a move. Tracy Cardin, ACNP-BC, a nurse practitioner in hospital practice at the University of Chicago Medical Center, says it’s important to ask why a group is considering a role for NPPs.
“Is it to promote efficiency? Is it because you can’t attract enough physicians?” she says. “Clarify your hopes for the position and how you will define success.” She also says HM group leaders need to factor in the time needed to hire, orient, and train an NPP, with mentoring that includes structured teaching and feedback.
There are a lot of models for deploying NPPs, says Cardin, a Team Hospitalist member.
“We utilize shared billing and teams of a hospitalist and nurse practitioner. This allows the physician to see a larger number of patients and brings more than one set of eyes and ears to the complex patient,” she says. “We’ve developed a process over the past six years where the hospitalist and NPP together go over the patient list every day. Both will see the patient, but the NPP commonly writes the notes and orders.”
Cardin emphasizes NPPs cost less than physicians and “can do many of the same things,” but “they are not free.” The most resourceful HM group’s use NPPs to extend the physician’s practice. “They can carry a pager and respond to small crises that come up, or see the patient on discharge day,” she says. “In other settings, the NPP does admissions, serves on quality projects, takes on a patient cohort based on diagnosis, or calls the primary-care physician at discharge.”
Efficiency can be a tough nut to crack in the hospital. Turner, the engineer, says HM groups need to “remember that the hospital is a very complex environment, with cascades of reactions and downstream effects.”
Hospitalists need the support of other professionals, and quality-improvement (QI) initiatives need sufficient time and resources to succeed.
One way to start advancing the efficiency agenda is to look for bright spots among the hospitalist group’s members. “Talk to them,” Turner says. “Find out how they do their jobs. Learn from them.”
Larry Beresford is a freelance author in Oakland, Calif.
References
- Yu D, Sanches S. Lean inpatient unit base care model [abstract]. J Hosp Med. 2012;7(Suppl 2):S107.
- Payne C, Odetoyinbo D, Castle B, et al. A dual hospital care and training model: structured interdisciplinary team rounds in an accountable care unit [abstract]. J Hosp Med. 2012;7(Suppl 2):S125.
- Hilger R, Quirk R, Dahms R. Use of restriction care plans to decrease medically unnecessary admissions and emergency department visits [abstract]. J Hosp Med. 2012;7(Suppl 2):S2.
- Premier. Year Three: QUEST Collaborative Findings. Premier website. Available at: http://www.premierinc.com/quality-safety/tools-services/quest/year3/quest-year-3-collaborative-findings.pdf. Accessed Sept. 26, 2012.