User login
Hospitalists' Voices to be Heard on Capitol Hill
Every year, hundreds of thousands of people from all parts of the country travel to Washington, D.C., and visit Congress. Regardless of the organizations they represent, almost all of them have one thing in common: They go to Capitol Hill with an “ask.”
Some ask for a vote on a particular piece of legislation; some request funding for their projects. Regardless, there is almost always an ask.
But hospitalists are different, according to SHM Public Policy Committee chair Ron Greeno, MD, FCCP, MHM, and committee member Brad Flansbaum, DO, MPH, SFHM. They are featured in a new video about “Hospitalists on the Hill,” SHM’s day to meet with members of Congress.
Rather than lobbying or asking for assistance, hospitalists bring solutions to the healthcare problems currently vexing communities throughout the country. By introducing the hospitalist model and its role in quality improvement (QI) to some of the most influential government figures in public health, hospitalists who make the visit to Capitol Hill help to spread some of the best practices in hospital-based healthcare and share their personal experiences at the same time.
“Every constituency that comes in is asking them for something,” Dr. Greeno says in one of the SHM-produced videos. “We don’t ask for anything. We offer. We offer our expertise. We offer to help them make better decisions than they would make otherwise.”
That contribution matters to members of Congress and their healthcare staff. Drs. Greeno and Flansbaum are “Hill Day” veterans who have seen firsthand how legislators and their staff absorb SHM’s message and materials.
“I thought that if we are leaving materials behind, that the minute we walk out of the office, it was going in the trash,” Dr. Greeno says. “That’s not what happens. They read this stuff.”
In 2013, Hospitalists on the Hill will take place May 16, the day before the official start of HM’s annual meeting at the Gaylord National Resort & Convention Center in National Harbor, Md., just a few minutes south of Washington, D.C. The agenda is ambitious, starting with a briefing about what to expect when meeting Congress members on Capitol Hill, followed by a full day of meetings with policymakers, regulators, and their staff.
“We spend our day going back and forth, from the Senate side of the Capitol to the House side of the Capitol, in and out of the office buildings, walking the halls of Congress,” Dr. Greeno says. “It’s a blast. It’s so interesting. And we’re getting a chance to sit down and deliver our message.”
For Dr. Flansbaum, it’s an opportunity to promote action in Washington.
“It really brings government to life,” he says. “You realize that, as bottlenecked as things might be sometimes, things have to get done.”
HM13 attendees can sign-up for Hospitalists on the Hill during annual-meeing registration. For more information, visit www.hospitalmedicine2013.org/onthehill.
Brendon Shank is SHM’s associate vice president of communications.
Every year, hundreds of thousands of people from all parts of the country travel to Washington, D.C., and visit Congress. Regardless of the organizations they represent, almost all of them have one thing in common: They go to Capitol Hill with an “ask.”
Some ask for a vote on a particular piece of legislation; some request funding for their projects. Regardless, there is almost always an ask.
But hospitalists are different, according to SHM Public Policy Committee chair Ron Greeno, MD, FCCP, MHM, and committee member Brad Flansbaum, DO, MPH, SFHM. They are featured in a new video about “Hospitalists on the Hill,” SHM’s day to meet with members of Congress.
Rather than lobbying or asking for assistance, hospitalists bring solutions to the healthcare problems currently vexing communities throughout the country. By introducing the hospitalist model and its role in quality improvement (QI) to some of the most influential government figures in public health, hospitalists who make the visit to Capitol Hill help to spread some of the best practices in hospital-based healthcare and share their personal experiences at the same time.
“Every constituency that comes in is asking them for something,” Dr. Greeno says in one of the SHM-produced videos. “We don’t ask for anything. We offer. We offer our expertise. We offer to help them make better decisions than they would make otherwise.”
That contribution matters to members of Congress and their healthcare staff. Drs. Greeno and Flansbaum are “Hill Day” veterans who have seen firsthand how legislators and their staff absorb SHM’s message and materials.
“I thought that if we are leaving materials behind, that the minute we walk out of the office, it was going in the trash,” Dr. Greeno says. “That’s not what happens. They read this stuff.”
In 2013, Hospitalists on the Hill will take place May 16, the day before the official start of HM’s annual meeting at the Gaylord National Resort & Convention Center in National Harbor, Md., just a few minutes south of Washington, D.C. The agenda is ambitious, starting with a briefing about what to expect when meeting Congress members on Capitol Hill, followed by a full day of meetings with policymakers, regulators, and their staff.
“We spend our day going back and forth, from the Senate side of the Capitol to the House side of the Capitol, in and out of the office buildings, walking the halls of Congress,” Dr. Greeno says. “It’s a blast. It’s so interesting. And we’re getting a chance to sit down and deliver our message.”
For Dr. Flansbaum, it’s an opportunity to promote action in Washington.
“It really brings government to life,” he says. “You realize that, as bottlenecked as things might be sometimes, things have to get done.”
HM13 attendees can sign-up for Hospitalists on the Hill during annual-meeing registration. For more information, visit www.hospitalmedicine2013.org/onthehill.
Brendon Shank is SHM’s associate vice president of communications.
Every year, hundreds of thousands of people from all parts of the country travel to Washington, D.C., and visit Congress. Regardless of the organizations they represent, almost all of them have one thing in common: They go to Capitol Hill with an “ask.”
Some ask for a vote on a particular piece of legislation; some request funding for their projects. Regardless, there is almost always an ask.
But hospitalists are different, according to SHM Public Policy Committee chair Ron Greeno, MD, FCCP, MHM, and committee member Brad Flansbaum, DO, MPH, SFHM. They are featured in a new video about “Hospitalists on the Hill,” SHM’s day to meet with members of Congress.
Rather than lobbying or asking for assistance, hospitalists bring solutions to the healthcare problems currently vexing communities throughout the country. By introducing the hospitalist model and its role in quality improvement (QI) to some of the most influential government figures in public health, hospitalists who make the visit to Capitol Hill help to spread some of the best practices in hospital-based healthcare and share their personal experiences at the same time.
“Every constituency that comes in is asking them for something,” Dr. Greeno says in one of the SHM-produced videos. “We don’t ask for anything. We offer. We offer our expertise. We offer to help them make better decisions than they would make otherwise.”
That contribution matters to members of Congress and their healthcare staff. Drs. Greeno and Flansbaum are “Hill Day” veterans who have seen firsthand how legislators and their staff absorb SHM’s message and materials.
“I thought that if we are leaving materials behind, that the minute we walk out of the office, it was going in the trash,” Dr. Greeno says. “That’s not what happens. They read this stuff.”
In 2013, Hospitalists on the Hill will take place May 16, the day before the official start of HM’s annual meeting at the Gaylord National Resort & Convention Center in National Harbor, Md., just a few minutes south of Washington, D.C. The agenda is ambitious, starting with a briefing about what to expect when meeting Congress members on Capitol Hill, followed by a full day of meetings with policymakers, regulators, and their staff.
“We spend our day going back and forth, from the Senate side of the Capitol to the House side of the Capitol, in and out of the office buildings, walking the halls of Congress,” Dr. Greeno says. “It’s a blast. It’s so interesting. And we’re getting a chance to sit down and deliver our message.”
For Dr. Flansbaum, it’s an opportunity to promote action in Washington.
“It really brings government to life,” he says. “You realize that, as bottlenecked as things might be sometimes, things have to get done.”
HM13 attendees can sign-up for Hospitalists on the Hill during annual-meeing registration. For more information, visit www.hospitalmedicine2013.org/onthehill.
Brendon Shank is SHM’s associate vice president of communications.
We Welcome the Newest SHM Members
Enter text here
Enter text here
Enter text here
Register Now for Hospital Medicine 2013
HM12 in San Diego was the largest gathering of hospitalists in history. Approximately 2,700 hospitalists shared in the common successes and challenges facing HM and the healthcare system.
Organizers expect HM13 will have the same energy and even more opportunities for education and networking. The annual meeting returns to the nation’s capital this spring, with pre-courses beginning May 16 at the Gaylord National Resort & Convention Center in National Harbor, Md. The three-day regular meeting begins May 17.
Registration is available at www.hospitalmedicine2013.org. The meeting website is the top source for news about program updates, educational sessions, and special promotions.
Here are five tips for planning your trip to HM13:
- Request time off early and coordinate with colleagues. Now is the time to request the time off.
- Register soon. Early registration discounts only apply for the next few months. And pre-courses often fill up quickly.
- Consider your options. Come a day early for valuable pre-courses or SHM’s “Hospitalists on the Hill” visit to Washington, D.C.
- Make a vacation of it. Take advantage of all that Washington has to offer by staying through the following weekend.
- Network ahead of time. Many hospitalists use the annual meeting for valuable career networking. Get started early by booking meetings and telling colleagues via Facebook, Twitter, and LinkedIn that you’re attending.
HM12 in San Diego was the largest gathering of hospitalists in history. Approximately 2,700 hospitalists shared in the common successes and challenges facing HM and the healthcare system.
Organizers expect HM13 will have the same energy and even more opportunities for education and networking. The annual meeting returns to the nation’s capital this spring, with pre-courses beginning May 16 at the Gaylord National Resort & Convention Center in National Harbor, Md. The three-day regular meeting begins May 17.
Registration is available at www.hospitalmedicine2013.org. The meeting website is the top source for news about program updates, educational sessions, and special promotions.
Here are five tips for planning your trip to HM13:
- Request time off early and coordinate with colleagues. Now is the time to request the time off.
- Register soon. Early registration discounts only apply for the next few months. And pre-courses often fill up quickly.
- Consider your options. Come a day early for valuable pre-courses or SHM’s “Hospitalists on the Hill” visit to Washington, D.C.
- Make a vacation of it. Take advantage of all that Washington has to offer by staying through the following weekend.
- Network ahead of time. Many hospitalists use the annual meeting for valuable career networking. Get started early by booking meetings and telling colleagues via Facebook, Twitter, and LinkedIn that you’re attending.
HM12 in San Diego was the largest gathering of hospitalists in history. Approximately 2,700 hospitalists shared in the common successes and challenges facing HM and the healthcare system.
Organizers expect HM13 will have the same energy and even more opportunities for education and networking. The annual meeting returns to the nation’s capital this spring, with pre-courses beginning May 16 at the Gaylord National Resort & Convention Center in National Harbor, Md. The three-day regular meeting begins May 17.
Registration is available at www.hospitalmedicine2013.org. The meeting website is the top source for news about program updates, educational sessions, and special promotions.
Here are five tips for planning your trip to HM13:
- Request time off early and coordinate with colleagues. Now is the time to request the time off.
- Register soon. Early registration discounts only apply for the next few months. And pre-courses often fill up quickly.
- Consider your options. Come a day early for valuable pre-courses or SHM’s “Hospitalists on the Hill” visit to Washington, D.C.
- Make a vacation of it. Take advantage of all that Washington has to offer by staying through the following weekend.
- Network ahead of time. Many hospitalists use the annual meeting for valuable career networking. Get started early by booking meetings and telling colleagues via Facebook, Twitter, and LinkedIn that you’re attending.
SHM Committee Applications due December 3
Are you looking to make a big impact on hospital medicine? Are you ready to network with innovative leaders in the specialty?
Now is the time to take the lead: Apply for a position on one of SHM’s many committees. Dec. 3 is the deadline for nominations.
The 2013-2014 term will commence May 17, 2013, and run through April 2014. Appointments will be made in February, at which time nominees will be told whether they were placed on a committee.
For more information or to apply, visit www.hospitalmedicine.org/committees.
Are you looking to make a big impact on hospital medicine? Are you ready to network with innovative leaders in the specialty?
Now is the time to take the lead: Apply for a position on one of SHM’s many committees. Dec. 3 is the deadline for nominations.
The 2013-2014 term will commence May 17, 2013, and run through April 2014. Appointments will be made in February, at which time nominees will be told whether they were placed on a committee.
For more information or to apply, visit www.hospitalmedicine.org/committees.
Are you looking to make a big impact on hospital medicine? Are you ready to network with innovative leaders in the specialty?
Now is the time to take the lead: Apply for a position on one of SHM’s many committees. Dec. 3 is the deadline for nominations.
The 2013-2014 term will commence May 17, 2013, and run through April 2014. Appointments will be made in February, at which time nominees will be told whether they were placed on a committee.
For more information or to apply, visit www.hospitalmedicine.org/committees.
Fellows Application Deadline Approaching for SHM Members
More than 1,000 SHM members have become Fellows, Senior Fellows, or Masters in Hospital Medicine. The FHM, SFHM, and MHM designations are SHM’s exclusive way for members to concretely demonstrate their experience and commitment to quality improvement (QI) and other core tenets of the specialty.
Successful candidates will be inducted as Fellows during the plenary sessions at HM13, May 16-19 in National Harbor, Md.
For the first time, hospitalists can apply online at www.hospitalmedicine.org/fellows.
More than 1,000 SHM members have become Fellows, Senior Fellows, or Masters in Hospital Medicine. The FHM, SFHM, and MHM designations are SHM’s exclusive way for members to concretely demonstrate their experience and commitment to quality improvement (QI) and other core tenets of the specialty.
Successful candidates will be inducted as Fellows during the plenary sessions at HM13, May 16-19 in National Harbor, Md.
For the first time, hospitalists can apply online at www.hospitalmedicine.org/fellows.
More than 1,000 SHM members have become Fellows, Senior Fellows, or Masters in Hospital Medicine. The FHM, SFHM, and MHM designations are SHM’s exclusive way for members to concretely demonstrate their experience and commitment to quality improvement (QI) and other core tenets of the specialty.
Successful candidates will be inducted as Fellows during the plenary sessions at HM13, May 16-19 in National Harbor, Md.
For the first time, hospitalists can apply online at www.hospitalmedicine.org/fellows.
New 'Meaningful Use' Exemption is Valuable Option for Growing Number of Hospitalists
Hospital-based eligible professionals do not qualify for the Medicare or Medicaid electronic health record (EHR) incentive program or the impending payment penalties for not being “meaningful users” of EHR technology.
A hospital-based “eligible professional” (EP) is defined by the Centers for Medicare & Medicaid Services (CMS) as an EP who furnishes 90% or more of their covered professional services in either the inpatient or emergency departments of a hospital. This exemption applies to most hospitalists and recognizes they have very little control over whether their respective institutions invest in this technology.
Although this 90% threshold should qualify most hospitalists for the exemption, it does not tell the entire story. A growing number of hospitalists are spending time rounding in skilled nursing or other post-acute facilities, and some are focusing the entirety of their practice in the post-acute setting. Under the current CMS definition, these hospitalists are not hospital-based and will, therefore, be subject to the upcoming penalties for not being meaningful users of EHR technology.
Contrary to the 90% threshold, the reality for post-acute hospitalists is that when it comes to EHRs, they are no different than their hospital-based colleagues. A hospitalist, irrespective of setting, has very little control over what kind of technology, if any, a facility invests in.
For hospitalists who are rounding or spending more of their practice time in post-acute facilities, this mechanical classification based on practice location alone is problematic. A physician-implemented EHR is not practical and does not make sense given the unique practice patterns of hospitalists. Although SHM remains strongly committed to the promise represented by health information technology (HIT), SHM consistently has noted to CMS that hospitalist practice does not always fit the confines of their rulemaking.
Hospitalists should not be penalized for failure to implement their own HIT, because they already use facility EHRs or lack control over the availability of EHR systems. It is for these reasons that SHM has been a strong advocate for an additional exemption that works for hospitalists—an exemption that recognizes lack of control of availability.
In August, CMS released the final rule for Stage 2 of Meaningful Use, and the voice of hospitalists was clearly heard. The rule includes an SHM advanced-hardship exemption acknowledging that EPs who practice in multiple locations, such as nursing homes, could face a significant hardship as they would have no way to control the use of Certified EHR Technology (CEHRT). In promulgating the exemption, CMS specifically states that a “physician merely sees patients at the center or home, and does not have any other interest in the facility; they would exert little to no influence over whether the nursing home, center, or other similar outpatient site adopts and implements CEHRT.” Hospitalists seeking this exemption would need to apply annually for up to five years.
This hardship exemption could apply to hospitalists who work in multiple facilities outside of hospitals, such as nursing homes. Although imperfect due to the time-limited nature, the exemption represents a victory in the effort to differentiate HM from traditional practice patterns. It is precisely this difference that makes HM uniquely positioned to lead changes in the healthcare system.
Josh Boswell is SHM’s interim senior manager of government relations.
Hospital-based eligible professionals do not qualify for the Medicare or Medicaid electronic health record (EHR) incentive program or the impending payment penalties for not being “meaningful users” of EHR technology.
A hospital-based “eligible professional” (EP) is defined by the Centers for Medicare & Medicaid Services (CMS) as an EP who furnishes 90% or more of their covered professional services in either the inpatient or emergency departments of a hospital. This exemption applies to most hospitalists and recognizes they have very little control over whether their respective institutions invest in this technology.
Although this 90% threshold should qualify most hospitalists for the exemption, it does not tell the entire story. A growing number of hospitalists are spending time rounding in skilled nursing or other post-acute facilities, and some are focusing the entirety of their practice in the post-acute setting. Under the current CMS definition, these hospitalists are not hospital-based and will, therefore, be subject to the upcoming penalties for not being meaningful users of EHR technology.
Contrary to the 90% threshold, the reality for post-acute hospitalists is that when it comes to EHRs, they are no different than their hospital-based colleagues. A hospitalist, irrespective of setting, has very little control over what kind of technology, if any, a facility invests in.
For hospitalists who are rounding or spending more of their practice time in post-acute facilities, this mechanical classification based on practice location alone is problematic. A physician-implemented EHR is not practical and does not make sense given the unique practice patterns of hospitalists. Although SHM remains strongly committed to the promise represented by health information technology (HIT), SHM consistently has noted to CMS that hospitalist practice does not always fit the confines of their rulemaking.
Hospitalists should not be penalized for failure to implement their own HIT, because they already use facility EHRs or lack control over the availability of EHR systems. It is for these reasons that SHM has been a strong advocate for an additional exemption that works for hospitalists—an exemption that recognizes lack of control of availability.
In August, CMS released the final rule for Stage 2 of Meaningful Use, and the voice of hospitalists was clearly heard. The rule includes an SHM advanced-hardship exemption acknowledging that EPs who practice in multiple locations, such as nursing homes, could face a significant hardship as they would have no way to control the use of Certified EHR Technology (CEHRT). In promulgating the exemption, CMS specifically states that a “physician merely sees patients at the center or home, and does not have any other interest in the facility; they would exert little to no influence over whether the nursing home, center, or other similar outpatient site adopts and implements CEHRT.” Hospitalists seeking this exemption would need to apply annually for up to five years.
This hardship exemption could apply to hospitalists who work in multiple facilities outside of hospitals, such as nursing homes. Although imperfect due to the time-limited nature, the exemption represents a victory in the effort to differentiate HM from traditional practice patterns. It is precisely this difference that makes HM uniquely positioned to lead changes in the healthcare system.
Josh Boswell is SHM’s interim senior manager of government relations.
Hospital-based eligible professionals do not qualify for the Medicare or Medicaid electronic health record (EHR) incentive program or the impending payment penalties for not being “meaningful users” of EHR technology.
A hospital-based “eligible professional” (EP) is defined by the Centers for Medicare & Medicaid Services (CMS) as an EP who furnishes 90% or more of their covered professional services in either the inpatient or emergency departments of a hospital. This exemption applies to most hospitalists and recognizes they have very little control over whether their respective institutions invest in this technology.
Although this 90% threshold should qualify most hospitalists for the exemption, it does not tell the entire story. A growing number of hospitalists are spending time rounding in skilled nursing or other post-acute facilities, and some are focusing the entirety of their practice in the post-acute setting. Under the current CMS definition, these hospitalists are not hospital-based and will, therefore, be subject to the upcoming penalties for not being meaningful users of EHR technology.
Contrary to the 90% threshold, the reality for post-acute hospitalists is that when it comes to EHRs, they are no different than their hospital-based colleagues. A hospitalist, irrespective of setting, has very little control over what kind of technology, if any, a facility invests in.
For hospitalists who are rounding or spending more of their practice time in post-acute facilities, this mechanical classification based on practice location alone is problematic. A physician-implemented EHR is not practical and does not make sense given the unique practice patterns of hospitalists. Although SHM remains strongly committed to the promise represented by health information technology (HIT), SHM consistently has noted to CMS that hospitalist practice does not always fit the confines of their rulemaking.
Hospitalists should not be penalized for failure to implement their own HIT, because they already use facility EHRs or lack control over the availability of EHR systems. It is for these reasons that SHM has been a strong advocate for an additional exemption that works for hospitalists—an exemption that recognizes lack of control of availability.
In August, CMS released the final rule for Stage 2 of Meaningful Use, and the voice of hospitalists was clearly heard. The rule includes an SHM advanced-hardship exemption acknowledging that EPs who practice in multiple locations, such as nursing homes, could face a significant hardship as they would have no way to control the use of Certified EHR Technology (CEHRT). In promulgating the exemption, CMS specifically states that a “physician merely sees patients at the center or home, and does not have any other interest in the facility; they would exert little to no influence over whether the nursing home, center, or other similar outpatient site adopts and implements CEHRT.” Hospitalists seeking this exemption would need to apply annually for up to five years.
This hardship exemption could apply to hospitalists who work in multiple facilities outside of hospitals, such as nursing homes. Although imperfect due to the time-limited nature, the exemption represents a victory in the effort to differentiate HM from traditional practice patterns. It is precisely this difference that makes HM uniquely positioned to lead changes in the healthcare system.
Josh Boswell is SHM’s interim senior manager of government relations.
Turnover for Hospitalist Groups Steady at 10%
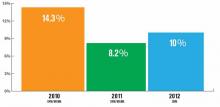
How many hospitalists left your group during the past year? If you're lucky, your group is one of the 36% of adult medicine groups that had no turnover at all. The proportion of groups with no turnover has ranged from a low of 32% (in the 2008-2009 SHM Focused Survey) to a high of 39% (2005-2006 and 2007-2008 SHM biannual surveys), so the 2012 results appear to be right in line with previous years.
But what about the groups that did encounter turnover? SHM's 2012 State of Hospital Medicine Report, which is based on 2011 data, reported a median turnover rate of 10% for nonacademic HM groups serving adults only. That's a little higher than last year's rate but lower than the rate reported in the 2010 SHM/MGMA survey. (It's worth keeping in mind that, in 2012, SHM conducted its own survey separate from MGMA, so the results might not be directly comparable to the previous two years' SHM/MGMA surveys.)
What part of the country you practice in and who employs you matters. In 2012, groups in the Western region of the U.S. reported only about 5% turnover, compared with 10% or 11% in other parts of the country. Turnover also varied by ownership/employment model: Groups employed by management companies and university/medical schools reported higher-than-median turnover; private hospitalist groups and those employed by multispecialty groups reported lower turnover. And medium-sized groups (five to 19 hospitalist FTEs) reported higher turnover than very small or larger groups.
The report also includes turnover rates for physician assistants (PAs) and nurse practitioners (NPs). Interestingly, turnover was much lower among NPs and PAs, with around 70% of the adult medicine groups who used NP/PAs reporting no NP/PA turnover at all.
Some folks have speculated that hospitalist turnover will decline as the supply of hospitalists begins to catch up with demand, which seems reasonable. Although we can't assess turnover data at this level of granularity, I'm guessing that turnover in highly-sought-after metropolitan markets, such as Washington, New York City, and Seattle, already is trending lower than in areas that are harder to recruit to.
But as far back as its 2005-2006 survey, SHM reported median turnover of 9%—not meaningfully different from this year's survey results. I suspect hospitalist turnover will continue to hover in the 10% to 15% range for the foreseeable future. Part of that figure can be attributed to the fact that significant numbers of hospitalists working in remote locations are international medical graduates on H-1B and J-1 visas. They typically make a three-year commitment, then move on to a more desirable location. But I think it's also because of the kinds of people who are attracted to hospital medicine.
As my colleague John Nelson, MD, MHM, likes to say, "hospitalists tend to want to date their practice, rather than marry it."
HM jobs have low barriers to entry and exit, at least compared with traditional primary care, which involves building an infrastructure and a panel of patients over many years. HM likely will continue to attract clinicians who enjoy looking around and changing jobs periodically. It will be interesting to see what the next few years bring to our specialty.
Leslie Flores is a partner in Nelson Flores Hospital Medicine Consultants and SHM Practice Analysis Committee member.
How many hospitalists left your group during the past year? If you're lucky, your group is one of the 36% of adult medicine groups that had no turnover at all. The proportion of groups with no turnover has ranged from a low of 32% (in the 2008-2009 SHM Focused Survey) to a high of 39% (2005-2006 and 2007-2008 SHM biannual surveys), so the 2012 results appear to be right in line with previous years.
But what about the groups that did encounter turnover? SHM's 2012 State of Hospital Medicine Report, which is based on 2011 data, reported a median turnover rate of 10% for nonacademic HM groups serving adults only. That's a little higher than last year's rate but lower than the rate reported in the 2010 SHM/MGMA survey. (It's worth keeping in mind that, in 2012, SHM conducted its own survey separate from MGMA, so the results might not be directly comparable to the previous two years' SHM/MGMA surveys.)
What part of the country you practice in and who employs you matters. In 2012, groups in the Western region of the U.S. reported only about 5% turnover, compared with 10% or 11% in other parts of the country. Turnover also varied by ownership/employment model: Groups employed by management companies and university/medical schools reported higher-than-median turnover; private hospitalist groups and those employed by multispecialty groups reported lower turnover. And medium-sized groups (five to 19 hospitalist FTEs) reported higher turnover than very small or larger groups.
The report also includes turnover rates for physician assistants (PAs) and nurse practitioners (NPs). Interestingly, turnover was much lower among NPs and PAs, with around 70% of the adult medicine groups who used NP/PAs reporting no NP/PA turnover at all.
Some folks have speculated that hospitalist turnover will decline as the supply of hospitalists begins to catch up with demand, which seems reasonable. Although we can't assess turnover data at this level of granularity, I'm guessing that turnover in highly-sought-after metropolitan markets, such as Washington, New York City, and Seattle, already is trending lower than in areas that are harder to recruit to.
But as far back as its 2005-2006 survey, SHM reported median turnover of 9%—not meaningfully different from this year's survey results. I suspect hospitalist turnover will continue to hover in the 10% to 15% range for the foreseeable future. Part of that figure can be attributed to the fact that significant numbers of hospitalists working in remote locations are international medical graduates on H-1B and J-1 visas. They typically make a three-year commitment, then move on to a more desirable location. But I think it's also because of the kinds of people who are attracted to hospital medicine.
As my colleague John Nelson, MD, MHM, likes to say, "hospitalists tend to want to date their practice, rather than marry it."
HM jobs have low barriers to entry and exit, at least compared with traditional primary care, which involves building an infrastructure and a panel of patients over many years. HM likely will continue to attract clinicians who enjoy looking around and changing jobs periodically. It will be interesting to see what the next few years bring to our specialty.
Leslie Flores is a partner in Nelson Flores Hospital Medicine Consultants and SHM Practice Analysis Committee member.
How many hospitalists left your group during the past year? If you're lucky, your group is one of the 36% of adult medicine groups that had no turnover at all. The proportion of groups with no turnover has ranged from a low of 32% (in the 2008-2009 SHM Focused Survey) to a high of 39% (2005-2006 and 2007-2008 SHM biannual surveys), so the 2012 results appear to be right in line with previous years.
But what about the groups that did encounter turnover? SHM's 2012 State of Hospital Medicine Report, which is based on 2011 data, reported a median turnover rate of 10% for nonacademic HM groups serving adults only. That's a little higher than last year's rate but lower than the rate reported in the 2010 SHM/MGMA survey. (It's worth keeping in mind that, in 2012, SHM conducted its own survey separate from MGMA, so the results might not be directly comparable to the previous two years' SHM/MGMA surveys.)
What part of the country you practice in and who employs you matters. In 2012, groups in the Western region of the U.S. reported only about 5% turnover, compared with 10% or 11% in other parts of the country. Turnover also varied by ownership/employment model: Groups employed by management companies and university/medical schools reported higher-than-median turnover; private hospitalist groups and those employed by multispecialty groups reported lower turnover. And medium-sized groups (five to 19 hospitalist FTEs) reported higher turnover than very small or larger groups.
The report also includes turnover rates for physician assistants (PAs) and nurse practitioners (NPs). Interestingly, turnover was much lower among NPs and PAs, with around 70% of the adult medicine groups who used NP/PAs reporting no NP/PA turnover at all.
Some folks have speculated that hospitalist turnover will decline as the supply of hospitalists begins to catch up with demand, which seems reasonable. Although we can't assess turnover data at this level of granularity, I'm guessing that turnover in highly-sought-after metropolitan markets, such as Washington, New York City, and Seattle, already is trending lower than in areas that are harder to recruit to.
But as far back as its 2005-2006 survey, SHM reported median turnover of 9%—not meaningfully different from this year's survey results. I suspect hospitalist turnover will continue to hover in the 10% to 15% range for the foreseeable future. Part of that figure can be attributed to the fact that significant numbers of hospitalists working in remote locations are international medical graduates on H-1B and J-1 visas. They typically make a three-year commitment, then move on to a more desirable location. But I think it's also because of the kinds of people who are attracted to hospital medicine.
As my colleague John Nelson, MD, MHM, likes to say, "hospitalists tend to want to date their practice, rather than marry it."
HM jobs have low barriers to entry and exit, at least compared with traditional primary care, which involves building an infrastructure and a panel of patients over many years. HM likely will continue to attract clinicians who enjoy looking around and changing jobs periodically. It will be interesting to see what the next few years bring to our specialty.
Leslie Flores is a partner in Nelson Flores Hospital Medicine Consultants and SHM Practice Analysis Committee member.
Off-Label Use of Antipsychotics for Dementia Patients Discouraged
Hospitalists can play a major role in reducing deaths that come as a result of off-label prescriptions for antipsychotic drugs being given to dementia patients, according to the Centers for Medicare & Medicaid Services (CMS) and SHM.
In a letter to hospitalist leaders, SHM encouraged hospitalists to “partner with others in your clinical work environment to reduce the use of antipsychotics for treating behavioral problems in patients with dementia. We believe that hospitalists have an important role to play in this initiative; hospital-based clinicians frequently care for patients with dementia and are responsible for medications prescribed during a patient’s hospitalization and at discharge.”
The joint education effort by CMS and SHM is based on an April 2011 report from the U.S. Department of Health and Human Services’ Office of Inspector General (OIG) that found that antipsychotic medications sometimes are used to treat patients with dementia for off-label reasons (e.g. “behaviors”) or against black-box warnings despite potential dangers to patients’ health.
An earlier warning from the FDA in 2008 outlined the potential dangers as:
- Increased risk (60% to 70%) of death in older adults with dementia;
- Prolongation of the QT interval on electrocardiogram, particularly with intravenous haloperidol use;
- Increased risk of stroke and TIAs; and
- Worsening cognitive function.
The letter to hospitalists noted the necessary changes and the need for collaboration between SHM, its members, and hospital leaders. “Increased prescriber training and system practice changes will help reduce unnecessary antipsychotic drug prescribing,” the letter stated. “SHM looks forward to an ongoing collaboration with members and hospital leaders on this important patient safety concern.”
Hospitalists can play a major role in reducing deaths that come as a result of off-label prescriptions for antipsychotic drugs being given to dementia patients, according to the Centers for Medicare & Medicaid Services (CMS) and SHM.
In a letter to hospitalist leaders, SHM encouraged hospitalists to “partner with others in your clinical work environment to reduce the use of antipsychotics for treating behavioral problems in patients with dementia. We believe that hospitalists have an important role to play in this initiative; hospital-based clinicians frequently care for patients with dementia and are responsible for medications prescribed during a patient’s hospitalization and at discharge.”
The joint education effort by CMS and SHM is based on an April 2011 report from the U.S. Department of Health and Human Services’ Office of Inspector General (OIG) that found that antipsychotic medications sometimes are used to treat patients with dementia for off-label reasons (e.g. “behaviors”) or against black-box warnings despite potential dangers to patients’ health.
An earlier warning from the FDA in 2008 outlined the potential dangers as:
- Increased risk (60% to 70%) of death in older adults with dementia;
- Prolongation of the QT interval on electrocardiogram, particularly with intravenous haloperidol use;
- Increased risk of stroke and TIAs; and
- Worsening cognitive function.
The letter to hospitalists noted the necessary changes and the need for collaboration between SHM, its members, and hospital leaders. “Increased prescriber training and system practice changes will help reduce unnecessary antipsychotic drug prescribing,” the letter stated. “SHM looks forward to an ongoing collaboration with members and hospital leaders on this important patient safety concern.”
Hospitalists can play a major role in reducing deaths that come as a result of off-label prescriptions for antipsychotic drugs being given to dementia patients, according to the Centers for Medicare & Medicaid Services (CMS) and SHM.
In a letter to hospitalist leaders, SHM encouraged hospitalists to “partner with others in your clinical work environment to reduce the use of antipsychotics for treating behavioral problems in patients with dementia. We believe that hospitalists have an important role to play in this initiative; hospital-based clinicians frequently care for patients with dementia and are responsible for medications prescribed during a patient’s hospitalization and at discharge.”
The joint education effort by CMS and SHM is based on an April 2011 report from the U.S. Department of Health and Human Services’ Office of Inspector General (OIG) that found that antipsychotic medications sometimes are used to treat patients with dementia for off-label reasons (e.g. “behaviors”) or against black-box warnings despite potential dangers to patients’ health.
An earlier warning from the FDA in 2008 outlined the potential dangers as:
- Increased risk (60% to 70%) of death in older adults with dementia;
- Prolongation of the QT interval on electrocardiogram, particularly with intravenous haloperidol use;
- Increased risk of stroke and TIAs; and
- Worsening cognitive function.
The letter to hospitalists noted the necessary changes and the need for collaboration between SHM, its members, and hospital leaders. “Increased prescriber training and system practice changes will help reduce unnecessary antipsychotic drug prescribing,” the letter stated. “SHM looks forward to an ongoing collaboration with members and hospital leaders on this important patient safety concern.”
Hospitalists On the Move
Hospitalist Lawrence Magras, MD, MBA, has been named medical director for the physician hospital organization at Catholic Health Services (CHS) in Rockville Centre, N.Y. Dr. Magras will serve as vice president and assistant medical director for clinical effectiveness, care coordination, and physician integration for the department of care management. Before transitioning to administration, he worked for several years as a hospitalist and held numerous positions focusing on quality improvement (QI) and efficient care management.
Randall Lorenz, MD, has been named chief hospitalist of the Sound Physicians hospitalist group at Dameron Hospital in Stockton, Calif. Dr. Lorenz completed his residency at the University of California at Irvine after receiving his medical degree from the Oregon Health and Sciences University School of Medicine in Portland. Sound Physicians has been providing hospitalist services at Dameron Hospital since April.
Bruce Mitchell, MD, is the new director of hospitalist services at Emory University Hospital Midtown in Atlanta. A Georgia native, Dr. Mitchell completed his residency at Emory’s School of Medicine. After working as a hospitalist at the Mayo Clinic in Jacksonville, Fla., for 14 years, he made his return to Emory in 2010 as assistant professor of hospital medicine.
Ryan O’Connell, MD, is now vice president of performance and risk management at Bridgeport Hospital in Bridgeport, Conn. Dr. O’Connell, who received his medical degree from New York University and completed his residency at Yale-New Haven Hospital, practiced as a hospitalist at Bridgeport Hospital before being appointed medical director for care coordination there in 2011.
Jason Krupp, MD, is the new chief medical officer at Banner Boswell Medical Center of the Banner Health System in Sun City, Ariz. Before serving as CMO at several institutions prior to Banner Boswell, Dr. Krupp worked as both a hospitalist and a privately practicing hypertension specialist.
Hospitalist Lawrence Magras, MD, MBA, has been named medical director for the physician hospital organization at Catholic Health Services (CHS) in Rockville Centre, N.Y. Dr. Magras will serve as vice president and assistant medical director for clinical effectiveness, care coordination, and physician integration for the department of care management. Before transitioning to administration, he worked for several years as a hospitalist and held numerous positions focusing on quality improvement (QI) and efficient care management.
Randall Lorenz, MD, has been named chief hospitalist of the Sound Physicians hospitalist group at Dameron Hospital in Stockton, Calif. Dr. Lorenz completed his residency at the University of California at Irvine after receiving his medical degree from the Oregon Health and Sciences University School of Medicine in Portland. Sound Physicians has been providing hospitalist services at Dameron Hospital since April.
Bruce Mitchell, MD, is the new director of hospitalist services at Emory University Hospital Midtown in Atlanta. A Georgia native, Dr. Mitchell completed his residency at Emory’s School of Medicine. After working as a hospitalist at the Mayo Clinic in Jacksonville, Fla., for 14 years, he made his return to Emory in 2010 as assistant professor of hospital medicine.
Ryan O’Connell, MD, is now vice president of performance and risk management at Bridgeport Hospital in Bridgeport, Conn. Dr. O’Connell, who received his medical degree from New York University and completed his residency at Yale-New Haven Hospital, practiced as a hospitalist at Bridgeport Hospital before being appointed medical director for care coordination there in 2011.
Jason Krupp, MD, is the new chief medical officer at Banner Boswell Medical Center of the Banner Health System in Sun City, Ariz. Before serving as CMO at several institutions prior to Banner Boswell, Dr. Krupp worked as both a hospitalist and a privately practicing hypertension specialist.
Hospitalist Lawrence Magras, MD, MBA, has been named medical director for the physician hospital organization at Catholic Health Services (CHS) in Rockville Centre, N.Y. Dr. Magras will serve as vice president and assistant medical director for clinical effectiveness, care coordination, and physician integration for the department of care management. Before transitioning to administration, he worked for several years as a hospitalist and held numerous positions focusing on quality improvement (QI) and efficient care management.
Randall Lorenz, MD, has been named chief hospitalist of the Sound Physicians hospitalist group at Dameron Hospital in Stockton, Calif. Dr. Lorenz completed his residency at the University of California at Irvine after receiving his medical degree from the Oregon Health and Sciences University School of Medicine in Portland. Sound Physicians has been providing hospitalist services at Dameron Hospital since April.
Bruce Mitchell, MD, is the new director of hospitalist services at Emory University Hospital Midtown in Atlanta. A Georgia native, Dr. Mitchell completed his residency at Emory’s School of Medicine. After working as a hospitalist at the Mayo Clinic in Jacksonville, Fla., for 14 years, he made his return to Emory in 2010 as assistant professor of hospital medicine.
Ryan O’Connell, MD, is now vice president of performance and risk management at Bridgeport Hospital in Bridgeport, Conn. Dr. O’Connell, who received his medical degree from New York University and completed his residency at Yale-New Haven Hospital, practiced as a hospitalist at Bridgeport Hospital before being appointed medical director for care coordination there in 2011.
Jason Krupp, MD, is the new chief medical officer at Banner Boswell Medical Center of the Banner Health System in Sun City, Ariz. Before serving as CMO at several institutions prior to Banner Boswell, Dr. Krupp worked as both a hospitalist and a privately practicing hypertension specialist.
ITL: Physician Reviews of HM-Relevant Research
In This Edition
Literature At A Glance
A guide to this month’s studies
- Acute myocardial infarction risk after hip/knee surgery
- Preoperative alcohol cessation minimizes complications in elective surgery
- Early laparoscopic cholecystectomy for gallstone pancreatitis
- Low-dose steroids in shock patients
- Pharmacist intervention did not reduce post-discharge errors
- Hyperbaric oxygen therapy and necrotizing soft-tissue infections
- Worse stroke outcomes on weekends
- Hospital admissions from EDs increasing
- Avoiding intensive glucose control in acute stroke
- Peri-procedural bleeding with dabigatran versus warfarin
Increased Risk of Acute Myocardial Infarction after Hip/Knee Surgery
Clinical question: How long does the risk of acute myocardial infarction (AMI) last after hip or knee replacement surgery, and what factors affect the risk?
Background: AMI is a morbid, perioperative complication of hip/knee surgery. Previous studies have demonstrated an increased risk of AMI post-surgery; however, this is the first study to assess the timing and modifiers of the risk.
Study design: Retrospective cohort study.
Setting: Data from Danish national registries from 1998 to 2007.
Synopsis: All adult patients who received total hip replacement (THR, n=66,524) or total knee replacement (TKR, n=28,703) surgery, as identified by hospital discharge records during a 10-year period, were matched with three age/sex controls without a history of THR or TKR (n=286,165). Patients with prior AMI within six weeks of the index date were excluded (n=437). The patients were followed until death, THR or TKR revision, migration, or the end of the study period.
Thromboprophylaxis was received by 99.1% of THR and TKR patients. The risk of AMI was significantly elevated for two weeks in TKR patients, and for six weeks in THR patients. Age >60 significantly increased the risk of AMI for both study populations. The risk associated with a previous AMI before THR or TKR diminished with increasing time between the two events.
The study was limited by lack of controlling for some AMI risk factors, such as smoking history or BMI.
Bottom line: AMI risk is substantially elevated for six weeks after THR and for two weeks after TKR. Preoperative risk factors to consider include age >60 and AMI within a year.
Citation: Lalmohamed A, Vestergaard P, Klop C, et al. Timing of acute myocardial infarction in patients undergoing total hip or knee replacement. Arch Intern Med. 2012;172:1229-1235.
Preoperative Alcohol Cessation Minimizes Complications of Elective Surgery in Heavy Alcohol Users
Clinical question: Does preoperative alcohol cessation minimize complication rates, mortality, or length of stay (LOS) in heavy alcohol users?
Background: Alcohol abuse is prevalent worldwide and contributes to 1.8 million deaths per year. Heavy alcohol users have an increased risk of postoperative bleeding, cardiopulmonary complications, and infections. It is unknown if preoperative cessation can reduce the risk of postoperative complications.
Study design: Systematic review.
Setting: Two Danish randomized controlled trials.
Synopsis: A Cochrane review identified 671 potential studies, of which 655 were excluded after abstract review. Of the 16 studies remaining, 14 were not randomized controlled trials with preoperative intervention for alcohol cessation. In the two studies that remained (n=69), patients who received preoperative alcohol cessation had fewer postoperative complications (odds ratio=0.22, 95% CI 0.08-0.61, P=0.004). Interventions included disulfiram and medical supervision of alcohol cessation. Investigators found no significant reductions for in-hospital or 30-day mortality.
Limitations of this review include lack of blinding to the treatments, low numbers of included women, and both studies being conducted by the same author.
Bottom line: Heavy alcohol users might benefit from a preoperative alcohol cessation program, but further studies are needed.
Citation: Oppedal K, Moller AM, Pedersen B, Tonnesen H. Preoperative alcohol cessation prior to elective surgery. Cochrane Database Syst Rev. 2012;7:CD008343.
Early Laparoscopic Cholecystectomy Is Safe and Effective for Gallstone Pancreatitis
Clinical question: Does early laparoscopic cholecystectomy (<48 hours of admission) in mild gallstone pancreatitis decrease LOS without increasing morbidity and mortality?
Background: Time to laparoscopic cholecystectomy (LC) for mild gallstone pancreatitis is greatly debated. Historically, surgeons have waited for normalization of laboratory values and decreased symptoms before performing laparoscopic or open cholecystectomy. Two previous studies demonstrated LC can be performed safely within 48 hours of admission, regardless of symptoms or laboratory values.
Study design: Retrospective cohort.
Setting: Two academic medical centers in California.
Synopsis: This retrospective review of 303 patients who underwent LC for mild gallstone pancreatitis from 2006 to 2011 found 117 (38.6%) underwent early LC and 186 (61.4%) underwent delayed LC (>48 hours after presentation). No significant differences in patient demographics, laboratory values, or admission Ranson scores were found between the groups. The mean LOS was significantly shorter in the early LC group (3.4 days) compared with the delayed LC group (6.3 days), and the delayed LC group had a higher rate of preoperative endoscopic retrograde cholangiopancreatography (ERCP). No differences were found with regard to mortality, complication rates, 30-day readmissions, or conversion to open cholecystectomy.
This study is limited by its retrospective design and potential for uncontrolled confounders. In addition, it did not evaluate preoperative management, postoperative recovery time, or timing of surgical consultation, which could have affected outcomes between the groups. It also is difficult to predict mild gallstone pancreatitis on admission, as some of the Ranson criteria should be calculated at 48 hours, and 5% to 10% of mild gallstone pancreatitis will progress to severe pancreatitis.
Bottom line: Early laparoscopic cholecystectomy (<48 hours of presentation) in mild gallstone pancreatitis is associated with shorter LOS and decreased ERCP use, with no difference in morbidity or mortality.
Citation: Faylor AE, de Virgilio C, Stabile BE, et al. Early laparoscopic cholecystectomy for mild gallstone pancreatitis. Time for a paradigm shift. Arch Surg. 2012;16:1-5.
Low-Dose Steroids in Shock Patients
Clinical question: Do low-dose corticosteroids improve mortality or shock reversal in adults with septic shock?
Background: Several trials have been published to evaluate the efficacy of low-dose corticosteroids in septic shock.
Study design: Systemic review and meta-analysis of randomized controlled trials.
Setting: Not identified.
Synopsis: Seven randomized controlled trials that utilized low-dose corticosteroids and reported either mortality or time-to-shock reversal in adults with septic shock were included in this review. Shock reversal was not consistently defined across trials but included time to withdrawal of vasopressors, proportion of patients on vasopressors on Day 3, or stable systolic blood pressure of >90 mm Hg for at least 24 hours without vasopressor support.
Pooled results found a significant improvement in shock reversal after low-dose corticosteroid administration (300 mg hydrocortisone in 24 hours [relative risk=1.17, 95% CI, 1.07-1.28]). Pooled results from six studies (n=965) did not find a significant difference in
mortality among those receiving low-dose corticosteroids.
Of note, two of the seven studies represented a disproportionate number of included patients, and the search only included the MEDLINE database, so some randomized controlled trials might have been missed.
Bottom line: Low-dose corticosteroids might reverse shock faster, but they do not improve mortality.
Citation: Sherwin RL, Garcia AJ, Bilkovski R. Do low-dose corticosteroids improve mortality or shock reversal in patients with septic shock? A systemic review and position statement prepared for the American Academy of Emergency Medicine. J Emerg Med. 2012;43:7-12.
Pharmacist Intervention Did Not Reduce Medication Errors after Discharge
Clinical question: Does pharmacist intervention reduce clinically important medication errors after hospital discharge for patients with acute coronary syndrome (ACS) or decompensated congestive heart failure?
Background: Research has shown adverse drug events, including discrepancies in medication regimens and nonadherence, affect 11% to 17% of discharged patients. Pharmacist interventions reduce medication errors in hospitalized patients, but their impact on recently discharged patients is unknown.
Study design: Blinded randomized controlled trial.
Setting: Two U.S. tertiary-care academic hospitals.
Synopsis: Investigators randomized 851 patients admitted with ACS or decompensated heart failure to receive pharmacist intervention or usual care. The intervention involved pharmacist medication reconciliation (“med rec”), inpatient pharmacist counseling, low-literacy adherence aids, and post-discharge follow-up phone calls.
The primary outcome of clinically important medication errors within 30 days of discharge occurred in 50.8% of all patients. The intervention and usual-care groups had similar mean number of errors, 0.87 and 0.92 events per patient, respectively. Results by intention-to-treat analysis favored the intervention but did not meet statistical significance (adjusted RR 0.92, CI 0.77-1.09).
Both hospitals had sufficient resources to support med rec and electronic health records. The study population was well-educated, with only a 10% prevalence of inadequate health literacy, compared with 26% noted in prior literature. These factors could help explain the study’s negative findings.
Study limitations include whether the results are generalizable to other settings or noncardiac populations. Further study is needed to determine characteristics of patients and settings that could benefit from pharmacist involvement to decrease medication errors.
Bottom line: A pharmacist intervention did not reduce medication errors in cardiac patients after discharge.
Citation: Kripalani S, Roumie CL, Dalal AK, et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge. Ann Int Med. 2012;157:1-10.
Hyperbaric Oxygen Therapy Saves Lives in Necrotizing Soft-Tissue Infections
Clinical question: Is the use of hyperbaric oxygen therapy in necrotizing soft-tissue infections (NSTIs) effective in improving patient outcomes?
Background: Studies evaluating the benefits of hyperbaric oxygen therapy (HBO2 therapy) in NSTIs are limited. The mainstay of management remains early surgical debridement and antibiotics. Given the significant risk of mortality and long-term disability from limb and tissue loss, alternative treatments need to be considered.
Study design: Retrospective analysis.
Setting: The Nationwide Inpatient Sample (NIS) database.
Synopsis: This study evaluated 45,913 patients in the NIS from 1988 to 2009, of which 405 patients received HBO2 therapy. Overall, those receiving HBO2 therapy had a lower mortality rate (4.5% vs. 9.4%). After adjusting for predictors and confounders, those receiving HBO2 therapy had a statistically significant lower risk of mortality, but they did have higher hospital costs and longer LOS.
The NIS database does not capture several predictors and confounders for NSTI patients. Additionally, the lack of data regarding the number of HBO2 therapy sessions received makes it impossible to estimate a dose response.
Bottom line: This retrospective analysis of NSTI patients found HBO2 therapy was associated with significantly lower mortality but higher hospitalization cost and longer LOS.
Citation: Soh CR, Pietrobon R, Freiberger JJ, et al. Hyperbaric oxygen therapy in necrotizing soft tissue infections: a study of patients in the United States Nationwide Inpatient Sample. Intensive Care Med. 2012;38:1143-1151.
Worse Stroke Outcomes on Weekends
Clinical question: Does the day of admission alter the quality and safety of care received by stroke patients?
Background: There is growing concern of disparities in care between weekday and weekend admissions in many health conditions. Recent studies have sought to determine if such a disparity exists for stroke patients.
Study design: Retrospective cohort study.
Setting: National Health Service public hospitals, England.
Synopsis: This study evaluated six quality-safety indicators among 93,621 stroke admits from April 1, 2009, to March 31, 2010. Investigators found performance on five of six indicators was significantly worse on weekends. Specifically, the percent of same-day brain scans was 43.1% on weekends, compared with 47.6% on weekdays, and seven-day in-hospital mortality for Sunday admissions was 11%, compared with an overall mean mortality of 8.9%.
It is possible that some of the observed effect could be due to unmeasured differences in patients admitted on weekends compared to weekdays.
Bottom line: This study suggests that, similar to other clinical conditions, significant disparities might exist between weekend and weekday care of stroke patients.
Citation: Palmer WL, Bottle A, Davie C, Vincent CA, Aylin P. Dying for the weekend: a retrospective cohort study on the association between day of hospital presentation and the quality and safety of stroke care. Arch Neurol. 2012;9:1-7.
Hospital Admissions from Emergency Departments Increasing
Clinical question: What proportion of current hospital admissions use the ED as an admission portal?
Background: Historically, outpatient providers’ offices admitted clinically stable patients with acute problems. From 1997 to 2007, ED visit rates increased by more than a third. Currently, a quarter of all acute-care outpatient visits occur in the ED.
Study design: Retrospective, observational cohort study.
Setting: The Nationwide Inpatient Sample (NIS) database, with approximately 8 million annual hospital stays, weighted to produce national averages.
Synopsis: NIS data from 1993 to 2006 showed the number of hospital admissions increased by 15%, while the proportion of admissions from the ED increased by 50%. The total proportion of inpatients entering from the ED increased to 43.8% from 33.5% (P<0.001). Twelve of the 13 most frequent inpatient clinical conditions also had a proportional increase in admissions from the ED (P<0.0001).
Possible explanations for the increases include difficulty arranging timely outpatient sick visits and overuse of the ED for issues that could be more appropriately addressed by primary-care offices. Furthermore, public education campaigns for heart attack or stroke emphasize early emergency care. Patients and providers expect rapid specialized care and early treatment that is readily available in ED.
More efficient use of inpatient resources is a focus of the Affordable Care Act. To inform health policy, research is needed to determine the drivers of admission trends, as well as the value and cost of emergency care.
Bottom line: The proportion of hospital admissions from the ED is increasing, but the implications for healthcare delivery are inadequately understood.
Citation: Schuur JD, Venkatesh AK. The growing role of emergency departments in hospital admissions. N Engl J Med. 2012;367(5):391-393.
Avoiding Intensive Glucose Control in Acute Stroke
Clinical question: Is intensive insulin treatment beneficial in patients with acute stroke?
Background: Although post-stroke hyperglycemia is associated with worse outcomes in acute stroke patients, it is unknown if intensive insulin treatment improves prognosis or infarct size.
Study design: Unblinded randomized controlled trial.
Setting: Single academic medical center in France.
Synopsis: Study authors randomized acute stroke patients (NIHSS from 5 to 25) to intensive insulin treatment or subcutaneous insulin therapy within 24 hours of admission. Initial median blood sugars were ~120 mg/dL in both groups. Mean blood sugars of <126 mg/dL were achieved at 24 hours in 95% of the intensive group and 67% of the subcutaneous group. The MRI infarct size at follow-up scanning was significantly larger in the intensive insulin group, but there were no differences in functional outcomes, serious adverse events, or mortality between the groups.
The study might have been underpowered to detect meaningful clinical differences between the groups.
Bottom line: Intensive blood sugar control cannot be recommended in acute stroke patients.
Citation: Rosso C, Corvol JC, Pires C, et al. Intensive versus subcutaneous insulin in patients with hyperacute stroke: results from the randomized INSULINFARCT trial. Stroke. 2012;43(9):2343-2349.
Peri-Procedural Bleeding with Dabigatran Versus Warfarin
Clinical question: Is dabigatran more likely to result in periprocedural bleeding when compared to warfarin?
Background: In a randomized clinical trial (RE-LY), dabigatran had a lower risk of major bleeding compared with warfarin. However, given that the anticoagulant effect of dabigatran is difficult to measure and there is not an effective antidote, it is unknown if dabigatran is associated with higher periprocedural rates of bleeding compared with warfarin.
Study design: Post-hoc analysis of the RE-LY trial.
Setting: Multicenter randomized controlled trial.
Synopsis: Patients enrolled in the RE-LY trial all had urgent or elective-invasive procedures recorded. The protocol recommended cessation of warfarin based on local practice, and cessation of dabigatran 24 hours before the procedure; the protocol was amended in 2008 to recommend cessation of dabigatran 24 hours before low-bleeding risk procedures, but two to five hours before high-bleeding-risk procedures (depending on renal function). Periprocedural bleeding was defined as seven days prior and 30 days after the procedure. Dabigatran was interrupted a mean of 49 hours before the procedures, and warfarin for 114 hours. There was no significant difference in major bleeding rates between dabigatran (150-mg dose) and warfarin, which occurred in 5.1% versus 4.6% patients, respectively.
Bottom line: Warfarin and dabigatran have about the same rate of periprocedural bleeding rates, but interruption duration is shorter for dabigatran.
Citation: Healey JS, Eikelboom J, Douketis J, et al. Peri-procedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the randomized evaluation of long-term anticoagulation therapy (RE-LY) randomized trial. Circulation. 2012;126(3):343-348.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Acute myocardial infarction risk after hip/knee surgery
- Preoperative alcohol cessation minimizes complications in elective surgery
- Early laparoscopic cholecystectomy for gallstone pancreatitis
- Low-dose steroids in shock patients
- Pharmacist intervention did not reduce post-discharge errors
- Hyperbaric oxygen therapy and necrotizing soft-tissue infections
- Worse stroke outcomes on weekends
- Hospital admissions from EDs increasing
- Avoiding intensive glucose control in acute stroke
- Peri-procedural bleeding with dabigatran versus warfarin
Increased Risk of Acute Myocardial Infarction after Hip/Knee Surgery
Clinical question: How long does the risk of acute myocardial infarction (AMI) last after hip or knee replacement surgery, and what factors affect the risk?
Background: AMI is a morbid, perioperative complication of hip/knee surgery. Previous studies have demonstrated an increased risk of AMI post-surgery; however, this is the first study to assess the timing and modifiers of the risk.
Study design: Retrospective cohort study.
Setting: Data from Danish national registries from 1998 to 2007.
Synopsis: All adult patients who received total hip replacement (THR, n=66,524) or total knee replacement (TKR, n=28,703) surgery, as identified by hospital discharge records during a 10-year period, were matched with three age/sex controls without a history of THR or TKR (n=286,165). Patients with prior AMI within six weeks of the index date were excluded (n=437). The patients were followed until death, THR or TKR revision, migration, or the end of the study period.
Thromboprophylaxis was received by 99.1% of THR and TKR patients. The risk of AMI was significantly elevated for two weeks in TKR patients, and for six weeks in THR patients. Age >60 significantly increased the risk of AMI for both study populations. The risk associated with a previous AMI before THR or TKR diminished with increasing time between the two events.
The study was limited by lack of controlling for some AMI risk factors, such as smoking history or BMI.
Bottom line: AMI risk is substantially elevated for six weeks after THR and for two weeks after TKR. Preoperative risk factors to consider include age >60 and AMI within a year.
Citation: Lalmohamed A, Vestergaard P, Klop C, et al. Timing of acute myocardial infarction in patients undergoing total hip or knee replacement. Arch Intern Med. 2012;172:1229-1235.
Preoperative Alcohol Cessation Minimizes Complications of Elective Surgery in Heavy Alcohol Users
Clinical question: Does preoperative alcohol cessation minimize complication rates, mortality, or length of stay (LOS) in heavy alcohol users?
Background: Alcohol abuse is prevalent worldwide and contributes to 1.8 million deaths per year. Heavy alcohol users have an increased risk of postoperative bleeding, cardiopulmonary complications, and infections. It is unknown if preoperative cessation can reduce the risk of postoperative complications.
Study design: Systematic review.
Setting: Two Danish randomized controlled trials.
Synopsis: A Cochrane review identified 671 potential studies, of which 655 were excluded after abstract review. Of the 16 studies remaining, 14 were not randomized controlled trials with preoperative intervention for alcohol cessation. In the two studies that remained (n=69), patients who received preoperative alcohol cessation had fewer postoperative complications (odds ratio=0.22, 95% CI 0.08-0.61, P=0.004). Interventions included disulfiram and medical supervision of alcohol cessation. Investigators found no significant reductions for in-hospital or 30-day mortality.
Limitations of this review include lack of blinding to the treatments, low numbers of included women, and both studies being conducted by the same author.
Bottom line: Heavy alcohol users might benefit from a preoperative alcohol cessation program, but further studies are needed.
Citation: Oppedal K, Moller AM, Pedersen B, Tonnesen H. Preoperative alcohol cessation prior to elective surgery. Cochrane Database Syst Rev. 2012;7:CD008343.
Early Laparoscopic Cholecystectomy Is Safe and Effective for Gallstone Pancreatitis
Clinical question: Does early laparoscopic cholecystectomy (<48 hours of admission) in mild gallstone pancreatitis decrease LOS without increasing morbidity and mortality?
Background: Time to laparoscopic cholecystectomy (LC) for mild gallstone pancreatitis is greatly debated. Historically, surgeons have waited for normalization of laboratory values and decreased symptoms before performing laparoscopic or open cholecystectomy. Two previous studies demonstrated LC can be performed safely within 48 hours of admission, regardless of symptoms or laboratory values.
Study design: Retrospective cohort.
Setting: Two academic medical centers in California.
Synopsis: This retrospective review of 303 patients who underwent LC for mild gallstone pancreatitis from 2006 to 2011 found 117 (38.6%) underwent early LC and 186 (61.4%) underwent delayed LC (>48 hours after presentation). No significant differences in patient demographics, laboratory values, or admission Ranson scores were found between the groups. The mean LOS was significantly shorter in the early LC group (3.4 days) compared with the delayed LC group (6.3 days), and the delayed LC group had a higher rate of preoperative endoscopic retrograde cholangiopancreatography (ERCP). No differences were found with regard to mortality, complication rates, 30-day readmissions, or conversion to open cholecystectomy.
This study is limited by its retrospective design and potential for uncontrolled confounders. In addition, it did not evaluate preoperative management, postoperative recovery time, or timing of surgical consultation, which could have affected outcomes between the groups. It also is difficult to predict mild gallstone pancreatitis on admission, as some of the Ranson criteria should be calculated at 48 hours, and 5% to 10% of mild gallstone pancreatitis will progress to severe pancreatitis.
Bottom line: Early laparoscopic cholecystectomy (<48 hours of presentation) in mild gallstone pancreatitis is associated with shorter LOS and decreased ERCP use, with no difference in morbidity or mortality.
Citation: Faylor AE, de Virgilio C, Stabile BE, et al. Early laparoscopic cholecystectomy for mild gallstone pancreatitis. Time for a paradigm shift. Arch Surg. 2012;16:1-5.
Low-Dose Steroids in Shock Patients
Clinical question: Do low-dose corticosteroids improve mortality or shock reversal in adults with septic shock?
Background: Several trials have been published to evaluate the efficacy of low-dose corticosteroids in septic shock.
Study design: Systemic review and meta-analysis of randomized controlled trials.
Setting: Not identified.
Synopsis: Seven randomized controlled trials that utilized low-dose corticosteroids and reported either mortality or time-to-shock reversal in adults with septic shock were included in this review. Shock reversal was not consistently defined across trials but included time to withdrawal of vasopressors, proportion of patients on vasopressors on Day 3, or stable systolic blood pressure of >90 mm Hg for at least 24 hours without vasopressor support.
Pooled results found a significant improvement in shock reversal after low-dose corticosteroid administration (300 mg hydrocortisone in 24 hours [relative risk=1.17, 95% CI, 1.07-1.28]). Pooled results from six studies (n=965) did not find a significant difference in
mortality among those receiving low-dose corticosteroids.
Of note, two of the seven studies represented a disproportionate number of included patients, and the search only included the MEDLINE database, so some randomized controlled trials might have been missed.
Bottom line: Low-dose corticosteroids might reverse shock faster, but they do not improve mortality.
Citation: Sherwin RL, Garcia AJ, Bilkovski R. Do low-dose corticosteroids improve mortality or shock reversal in patients with septic shock? A systemic review and position statement prepared for the American Academy of Emergency Medicine. J Emerg Med. 2012;43:7-12.
Pharmacist Intervention Did Not Reduce Medication Errors after Discharge
Clinical question: Does pharmacist intervention reduce clinically important medication errors after hospital discharge for patients with acute coronary syndrome (ACS) or decompensated congestive heart failure?
Background: Research has shown adverse drug events, including discrepancies in medication regimens and nonadherence, affect 11% to 17% of discharged patients. Pharmacist interventions reduce medication errors in hospitalized patients, but their impact on recently discharged patients is unknown.
Study design: Blinded randomized controlled trial.
Setting: Two U.S. tertiary-care academic hospitals.
Synopsis: Investigators randomized 851 patients admitted with ACS or decompensated heart failure to receive pharmacist intervention or usual care. The intervention involved pharmacist medication reconciliation (“med rec”), inpatient pharmacist counseling, low-literacy adherence aids, and post-discharge follow-up phone calls.
The primary outcome of clinically important medication errors within 30 days of discharge occurred in 50.8% of all patients. The intervention and usual-care groups had similar mean number of errors, 0.87 and 0.92 events per patient, respectively. Results by intention-to-treat analysis favored the intervention but did not meet statistical significance (adjusted RR 0.92, CI 0.77-1.09).
Both hospitals had sufficient resources to support med rec and electronic health records. The study population was well-educated, with only a 10% prevalence of inadequate health literacy, compared with 26% noted in prior literature. These factors could help explain the study’s negative findings.
Study limitations include whether the results are generalizable to other settings or noncardiac populations. Further study is needed to determine characteristics of patients and settings that could benefit from pharmacist involvement to decrease medication errors.
Bottom line: A pharmacist intervention did not reduce medication errors in cardiac patients after discharge.
Citation: Kripalani S, Roumie CL, Dalal AK, et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge. Ann Int Med. 2012;157:1-10.
Hyperbaric Oxygen Therapy Saves Lives in Necrotizing Soft-Tissue Infections
Clinical question: Is the use of hyperbaric oxygen therapy in necrotizing soft-tissue infections (NSTIs) effective in improving patient outcomes?
Background: Studies evaluating the benefits of hyperbaric oxygen therapy (HBO2 therapy) in NSTIs are limited. The mainstay of management remains early surgical debridement and antibiotics. Given the significant risk of mortality and long-term disability from limb and tissue loss, alternative treatments need to be considered.
Study design: Retrospective analysis.
Setting: The Nationwide Inpatient Sample (NIS) database.
Synopsis: This study evaluated 45,913 patients in the NIS from 1988 to 2009, of which 405 patients received HBO2 therapy. Overall, those receiving HBO2 therapy had a lower mortality rate (4.5% vs. 9.4%). After adjusting for predictors and confounders, those receiving HBO2 therapy had a statistically significant lower risk of mortality, but they did have higher hospital costs and longer LOS.
The NIS database does not capture several predictors and confounders for NSTI patients. Additionally, the lack of data regarding the number of HBO2 therapy sessions received makes it impossible to estimate a dose response.
Bottom line: This retrospective analysis of NSTI patients found HBO2 therapy was associated with significantly lower mortality but higher hospitalization cost and longer LOS.
Citation: Soh CR, Pietrobon R, Freiberger JJ, et al. Hyperbaric oxygen therapy in necrotizing soft tissue infections: a study of patients in the United States Nationwide Inpatient Sample. Intensive Care Med. 2012;38:1143-1151.
Worse Stroke Outcomes on Weekends
Clinical question: Does the day of admission alter the quality and safety of care received by stroke patients?
Background: There is growing concern of disparities in care between weekday and weekend admissions in many health conditions. Recent studies have sought to determine if such a disparity exists for stroke patients.
Study design: Retrospective cohort study.
Setting: National Health Service public hospitals, England.
Synopsis: This study evaluated six quality-safety indicators among 93,621 stroke admits from April 1, 2009, to March 31, 2010. Investigators found performance on five of six indicators was significantly worse on weekends. Specifically, the percent of same-day brain scans was 43.1% on weekends, compared with 47.6% on weekdays, and seven-day in-hospital mortality for Sunday admissions was 11%, compared with an overall mean mortality of 8.9%.
It is possible that some of the observed effect could be due to unmeasured differences in patients admitted on weekends compared to weekdays.
Bottom line: This study suggests that, similar to other clinical conditions, significant disparities might exist between weekend and weekday care of stroke patients.
Citation: Palmer WL, Bottle A, Davie C, Vincent CA, Aylin P. Dying for the weekend: a retrospective cohort study on the association between day of hospital presentation and the quality and safety of stroke care. Arch Neurol. 2012;9:1-7.
Hospital Admissions from Emergency Departments Increasing
Clinical question: What proportion of current hospital admissions use the ED as an admission portal?
Background: Historically, outpatient providers’ offices admitted clinically stable patients with acute problems. From 1997 to 2007, ED visit rates increased by more than a third. Currently, a quarter of all acute-care outpatient visits occur in the ED.
Study design: Retrospective, observational cohort study.
Setting: The Nationwide Inpatient Sample (NIS) database, with approximately 8 million annual hospital stays, weighted to produce national averages.
Synopsis: NIS data from 1993 to 2006 showed the number of hospital admissions increased by 15%, while the proportion of admissions from the ED increased by 50%. The total proportion of inpatients entering from the ED increased to 43.8% from 33.5% (P<0.001). Twelve of the 13 most frequent inpatient clinical conditions also had a proportional increase in admissions from the ED (P<0.0001).
Possible explanations for the increases include difficulty arranging timely outpatient sick visits and overuse of the ED for issues that could be more appropriately addressed by primary-care offices. Furthermore, public education campaigns for heart attack or stroke emphasize early emergency care. Patients and providers expect rapid specialized care and early treatment that is readily available in ED.
More efficient use of inpatient resources is a focus of the Affordable Care Act. To inform health policy, research is needed to determine the drivers of admission trends, as well as the value and cost of emergency care.
Bottom line: The proportion of hospital admissions from the ED is increasing, but the implications for healthcare delivery are inadequately understood.
Citation: Schuur JD, Venkatesh AK. The growing role of emergency departments in hospital admissions. N Engl J Med. 2012;367(5):391-393.
Avoiding Intensive Glucose Control in Acute Stroke
Clinical question: Is intensive insulin treatment beneficial in patients with acute stroke?
Background: Although post-stroke hyperglycemia is associated with worse outcomes in acute stroke patients, it is unknown if intensive insulin treatment improves prognosis or infarct size.
Study design: Unblinded randomized controlled trial.
Setting: Single academic medical center in France.
Synopsis: Study authors randomized acute stroke patients (NIHSS from 5 to 25) to intensive insulin treatment or subcutaneous insulin therapy within 24 hours of admission. Initial median blood sugars were ~120 mg/dL in both groups. Mean blood sugars of <126 mg/dL were achieved at 24 hours in 95% of the intensive group and 67% of the subcutaneous group. The MRI infarct size at follow-up scanning was significantly larger in the intensive insulin group, but there were no differences in functional outcomes, serious adverse events, or mortality between the groups.
The study might have been underpowered to detect meaningful clinical differences between the groups.
Bottom line: Intensive blood sugar control cannot be recommended in acute stroke patients.
Citation: Rosso C, Corvol JC, Pires C, et al. Intensive versus subcutaneous insulin in patients with hyperacute stroke: results from the randomized INSULINFARCT trial. Stroke. 2012;43(9):2343-2349.
Peri-Procedural Bleeding with Dabigatran Versus Warfarin
Clinical question: Is dabigatran more likely to result in periprocedural bleeding when compared to warfarin?
Background: In a randomized clinical trial (RE-LY), dabigatran had a lower risk of major bleeding compared with warfarin. However, given that the anticoagulant effect of dabigatran is difficult to measure and there is not an effective antidote, it is unknown if dabigatran is associated with higher periprocedural rates of bleeding compared with warfarin.
Study design: Post-hoc analysis of the RE-LY trial.
Setting: Multicenter randomized controlled trial.
Synopsis: Patients enrolled in the RE-LY trial all had urgent or elective-invasive procedures recorded. The protocol recommended cessation of warfarin based on local practice, and cessation of dabigatran 24 hours before the procedure; the protocol was amended in 2008 to recommend cessation of dabigatran 24 hours before low-bleeding risk procedures, but two to five hours before high-bleeding-risk procedures (depending on renal function). Periprocedural bleeding was defined as seven days prior and 30 days after the procedure. Dabigatran was interrupted a mean of 49 hours before the procedures, and warfarin for 114 hours. There was no significant difference in major bleeding rates between dabigatran (150-mg dose) and warfarin, which occurred in 5.1% versus 4.6% patients, respectively.
Bottom line: Warfarin and dabigatran have about the same rate of periprocedural bleeding rates, but interruption duration is shorter for dabigatran.
Citation: Healey JS, Eikelboom J, Douketis J, et al. Peri-procedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the randomized evaluation of long-term anticoagulation therapy (RE-LY) randomized trial. Circulation. 2012;126(3):343-348.
In This Edition
Literature At A Glance
A guide to this month’s studies
- Acute myocardial infarction risk after hip/knee surgery
- Preoperative alcohol cessation minimizes complications in elective surgery
- Early laparoscopic cholecystectomy for gallstone pancreatitis
- Low-dose steroids in shock patients
- Pharmacist intervention did not reduce post-discharge errors
- Hyperbaric oxygen therapy and necrotizing soft-tissue infections
- Worse stroke outcomes on weekends
- Hospital admissions from EDs increasing
- Avoiding intensive glucose control in acute stroke
- Peri-procedural bleeding with dabigatran versus warfarin
Increased Risk of Acute Myocardial Infarction after Hip/Knee Surgery
Clinical question: How long does the risk of acute myocardial infarction (AMI) last after hip or knee replacement surgery, and what factors affect the risk?
Background: AMI is a morbid, perioperative complication of hip/knee surgery. Previous studies have demonstrated an increased risk of AMI post-surgery; however, this is the first study to assess the timing and modifiers of the risk.
Study design: Retrospective cohort study.
Setting: Data from Danish national registries from 1998 to 2007.
Synopsis: All adult patients who received total hip replacement (THR, n=66,524) or total knee replacement (TKR, n=28,703) surgery, as identified by hospital discharge records during a 10-year period, were matched with three age/sex controls without a history of THR or TKR (n=286,165). Patients with prior AMI within six weeks of the index date were excluded (n=437). The patients were followed until death, THR or TKR revision, migration, or the end of the study period.
Thromboprophylaxis was received by 99.1% of THR and TKR patients. The risk of AMI was significantly elevated for two weeks in TKR patients, and for six weeks in THR patients. Age >60 significantly increased the risk of AMI for both study populations. The risk associated with a previous AMI before THR or TKR diminished with increasing time between the two events.
The study was limited by lack of controlling for some AMI risk factors, such as smoking history or BMI.
Bottom line: AMI risk is substantially elevated for six weeks after THR and for two weeks after TKR. Preoperative risk factors to consider include age >60 and AMI within a year.
Citation: Lalmohamed A, Vestergaard P, Klop C, et al. Timing of acute myocardial infarction in patients undergoing total hip or knee replacement. Arch Intern Med. 2012;172:1229-1235.
Preoperative Alcohol Cessation Minimizes Complications of Elective Surgery in Heavy Alcohol Users
Clinical question: Does preoperative alcohol cessation minimize complication rates, mortality, or length of stay (LOS) in heavy alcohol users?
Background: Alcohol abuse is prevalent worldwide and contributes to 1.8 million deaths per year. Heavy alcohol users have an increased risk of postoperative bleeding, cardiopulmonary complications, and infections. It is unknown if preoperative cessation can reduce the risk of postoperative complications.
Study design: Systematic review.
Setting: Two Danish randomized controlled trials.
Synopsis: A Cochrane review identified 671 potential studies, of which 655 were excluded after abstract review. Of the 16 studies remaining, 14 were not randomized controlled trials with preoperative intervention for alcohol cessation. In the two studies that remained (n=69), patients who received preoperative alcohol cessation had fewer postoperative complications (odds ratio=0.22, 95% CI 0.08-0.61, P=0.004). Interventions included disulfiram and medical supervision of alcohol cessation. Investigators found no significant reductions for in-hospital or 30-day mortality.
Limitations of this review include lack of blinding to the treatments, low numbers of included women, and both studies being conducted by the same author.
Bottom line: Heavy alcohol users might benefit from a preoperative alcohol cessation program, but further studies are needed.
Citation: Oppedal K, Moller AM, Pedersen B, Tonnesen H. Preoperative alcohol cessation prior to elective surgery. Cochrane Database Syst Rev. 2012;7:CD008343.
Early Laparoscopic Cholecystectomy Is Safe and Effective for Gallstone Pancreatitis
Clinical question: Does early laparoscopic cholecystectomy (<48 hours of admission) in mild gallstone pancreatitis decrease LOS without increasing morbidity and mortality?
Background: Time to laparoscopic cholecystectomy (LC) for mild gallstone pancreatitis is greatly debated. Historically, surgeons have waited for normalization of laboratory values and decreased symptoms before performing laparoscopic or open cholecystectomy. Two previous studies demonstrated LC can be performed safely within 48 hours of admission, regardless of symptoms or laboratory values.
Study design: Retrospective cohort.
Setting: Two academic medical centers in California.
Synopsis: This retrospective review of 303 patients who underwent LC for mild gallstone pancreatitis from 2006 to 2011 found 117 (38.6%) underwent early LC and 186 (61.4%) underwent delayed LC (>48 hours after presentation). No significant differences in patient demographics, laboratory values, or admission Ranson scores were found between the groups. The mean LOS was significantly shorter in the early LC group (3.4 days) compared with the delayed LC group (6.3 days), and the delayed LC group had a higher rate of preoperative endoscopic retrograde cholangiopancreatography (ERCP). No differences were found with regard to mortality, complication rates, 30-day readmissions, or conversion to open cholecystectomy.
This study is limited by its retrospective design and potential for uncontrolled confounders. In addition, it did not evaluate preoperative management, postoperative recovery time, or timing of surgical consultation, which could have affected outcomes between the groups. It also is difficult to predict mild gallstone pancreatitis on admission, as some of the Ranson criteria should be calculated at 48 hours, and 5% to 10% of mild gallstone pancreatitis will progress to severe pancreatitis.
Bottom line: Early laparoscopic cholecystectomy (<48 hours of presentation) in mild gallstone pancreatitis is associated with shorter LOS and decreased ERCP use, with no difference in morbidity or mortality.
Citation: Faylor AE, de Virgilio C, Stabile BE, et al. Early laparoscopic cholecystectomy for mild gallstone pancreatitis. Time for a paradigm shift. Arch Surg. 2012;16:1-5.
Low-Dose Steroids in Shock Patients
Clinical question: Do low-dose corticosteroids improve mortality or shock reversal in adults with septic shock?
Background: Several trials have been published to evaluate the efficacy of low-dose corticosteroids in septic shock.
Study design: Systemic review and meta-analysis of randomized controlled trials.
Setting: Not identified.
Synopsis: Seven randomized controlled trials that utilized low-dose corticosteroids and reported either mortality or time-to-shock reversal in adults with septic shock were included in this review. Shock reversal was not consistently defined across trials but included time to withdrawal of vasopressors, proportion of patients on vasopressors on Day 3, or stable systolic blood pressure of >90 mm Hg for at least 24 hours without vasopressor support.
Pooled results found a significant improvement in shock reversal after low-dose corticosteroid administration (300 mg hydrocortisone in 24 hours [relative risk=1.17, 95% CI, 1.07-1.28]). Pooled results from six studies (n=965) did not find a significant difference in
mortality among those receiving low-dose corticosteroids.
Of note, two of the seven studies represented a disproportionate number of included patients, and the search only included the MEDLINE database, so some randomized controlled trials might have been missed.
Bottom line: Low-dose corticosteroids might reverse shock faster, but they do not improve mortality.
Citation: Sherwin RL, Garcia AJ, Bilkovski R. Do low-dose corticosteroids improve mortality or shock reversal in patients with septic shock? A systemic review and position statement prepared for the American Academy of Emergency Medicine. J Emerg Med. 2012;43:7-12.
Pharmacist Intervention Did Not Reduce Medication Errors after Discharge
Clinical question: Does pharmacist intervention reduce clinically important medication errors after hospital discharge for patients with acute coronary syndrome (ACS) or decompensated congestive heart failure?
Background: Research has shown adverse drug events, including discrepancies in medication regimens and nonadherence, affect 11% to 17% of discharged patients. Pharmacist interventions reduce medication errors in hospitalized patients, but their impact on recently discharged patients is unknown.
Study design: Blinded randomized controlled trial.
Setting: Two U.S. tertiary-care academic hospitals.
Synopsis: Investigators randomized 851 patients admitted with ACS or decompensated heart failure to receive pharmacist intervention or usual care. The intervention involved pharmacist medication reconciliation (“med rec”), inpatient pharmacist counseling, low-literacy adherence aids, and post-discharge follow-up phone calls.
The primary outcome of clinically important medication errors within 30 days of discharge occurred in 50.8% of all patients. The intervention and usual-care groups had similar mean number of errors, 0.87 and 0.92 events per patient, respectively. Results by intention-to-treat analysis favored the intervention but did not meet statistical significance (adjusted RR 0.92, CI 0.77-1.09).
Both hospitals had sufficient resources to support med rec and electronic health records. The study population was well-educated, with only a 10% prevalence of inadequate health literacy, compared with 26% noted in prior literature. These factors could help explain the study’s negative findings.
Study limitations include whether the results are generalizable to other settings or noncardiac populations. Further study is needed to determine characteristics of patients and settings that could benefit from pharmacist involvement to decrease medication errors.
Bottom line: A pharmacist intervention did not reduce medication errors in cardiac patients after discharge.
Citation: Kripalani S, Roumie CL, Dalal AK, et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge. Ann Int Med. 2012;157:1-10.
Hyperbaric Oxygen Therapy Saves Lives in Necrotizing Soft-Tissue Infections
Clinical question: Is the use of hyperbaric oxygen therapy in necrotizing soft-tissue infections (NSTIs) effective in improving patient outcomes?
Background: Studies evaluating the benefits of hyperbaric oxygen therapy (HBO2 therapy) in NSTIs are limited. The mainstay of management remains early surgical debridement and antibiotics. Given the significant risk of mortality and long-term disability from limb and tissue loss, alternative treatments need to be considered.
Study design: Retrospective analysis.
Setting: The Nationwide Inpatient Sample (NIS) database.
Synopsis: This study evaluated 45,913 patients in the NIS from 1988 to 2009, of which 405 patients received HBO2 therapy. Overall, those receiving HBO2 therapy had a lower mortality rate (4.5% vs. 9.4%). After adjusting for predictors and confounders, those receiving HBO2 therapy had a statistically significant lower risk of mortality, but they did have higher hospital costs and longer LOS.
The NIS database does not capture several predictors and confounders for NSTI patients. Additionally, the lack of data regarding the number of HBO2 therapy sessions received makes it impossible to estimate a dose response.
Bottom line: This retrospective analysis of NSTI patients found HBO2 therapy was associated with significantly lower mortality but higher hospitalization cost and longer LOS.
Citation: Soh CR, Pietrobon R, Freiberger JJ, et al. Hyperbaric oxygen therapy in necrotizing soft tissue infections: a study of patients in the United States Nationwide Inpatient Sample. Intensive Care Med. 2012;38:1143-1151.
Worse Stroke Outcomes on Weekends
Clinical question: Does the day of admission alter the quality and safety of care received by stroke patients?
Background: There is growing concern of disparities in care between weekday and weekend admissions in many health conditions. Recent studies have sought to determine if such a disparity exists for stroke patients.
Study design: Retrospective cohort study.
Setting: National Health Service public hospitals, England.
Synopsis: This study evaluated six quality-safety indicators among 93,621 stroke admits from April 1, 2009, to March 31, 2010. Investigators found performance on five of six indicators was significantly worse on weekends. Specifically, the percent of same-day brain scans was 43.1% on weekends, compared with 47.6% on weekdays, and seven-day in-hospital mortality for Sunday admissions was 11%, compared with an overall mean mortality of 8.9%.
It is possible that some of the observed effect could be due to unmeasured differences in patients admitted on weekends compared to weekdays.
Bottom line: This study suggests that, similar to other clinical conditions, significant disparities might exist between weekend and weekday care of stroke patients.
Citation: Palmer WL, Bottle A, Davie C, Vincent CA, Aylin P. Dying for the weekend: a retrospective cohort study on the association between day of hospital presentation and the quality and safety of stroke care. Arch Neurol. 2012;9:1-7.
Hospital Admissions from Emergency Departments Increasing
Clinical question: What proportion of current hospital admissions use the ED as an admission portal?
Background: Historically, outpatient providers’ offices admitted clinically stable patients with acute problems. From 1997 to 2007, ED visit rates increased by more than a third. Currently, a quarter of all acute-care outpatient visits occur in the ED.
Study design: Retrospective, observational cohort study.
Setting: The Nationwide Inpatient Sample (NIS) database, with approximately 8 million annual hospital stays, weighted to produce national averages.
Synopsis: NIS data from 1993 to 2006 showed the number of hospital admissions increased by 15%, while the proportion of admissions from the ED increased by 50%. The total proportion of inpatients entering from the ED increased to 43.8% from 33.5% (P<0.001). Twelve of the 13 most frequent inpatient clinical conditions also had a proportional increase in admissions from the ED (P<0.0001).
Possible explanations for the increases include difficulty arranging timely outpatient sick visits and overuse of the ED for issues that could be more appropriately addressed by primary-care offices. Furthermore, public education campaigns for heart attack or stroke emphasize early emergency care. Patients and providers expect rapid specialized care and early treatment that is readily available in ED.
More efficient use of inpatient resources is a focus of the Affordable Care Act. To inform health policy, research is needed to determine the drivers of admission trends, as well as the value and cost of emergency care.
Bottom line: The proportion of hospital admissions from the ED is increasing, but the implications for healthcare delivery are inadequately understood.
Citation: Schuur JD, Venkatesh AK. The growing role of emergency departments in hospital admissions. N Engl J Med. 2012;367(5):391-393.
Avoiding Intensive Glucose Control in Acute Stroke
Clinical question: Is intensive insulin treatment beneficial in patients with acute stroke?
Background: Although post-stroke hyperglycemia is associated with worse outcomes in acute stroke patients, it is unknown if intensive insulin treatment improves prognosis or infarct size.
Study design: Unblinded randomized controlled trial.
Setting: Single academic medical center in France.
Synopsis: Study authors randomized acute stroke patients (NIHSS from 5 to 25) to intensive insulin treatment or subcutaneous insulin therapy within 24 hours of admission. Initial median blood sugars were ~120 mg/dL in both groups. Mean blood sugars of <126 mg/dL were achieved at 24 hours in 95% of the intensive group and 67% of the subcutaneous group. The MRI infarct size at follow-up scanning was significantly larger in the intensive insulin group, but there were no differences in functional outcomes, serious adverse events, or mortality between the groups.
The study might have been underpowered to detect meaningful clinical differences between the groups.
Bottom line: Intensive blood sugar control cannot be recommended in acute stroke patients.
Citation: Rosso C, Corvol JC, Pires C, et al. Intensive versus subcutaneous insulin in patients with hyperacute stroke: results from the randomized INSULINFARCT trial. Stroke. 2012;43(9):2343-2349.
Peri-Procedural Bleeding with Dabigatran Versus Warfarin
Clinical question: Is dabigatran more likely to result in periprocedural bleeding when compared to warfarin?
Background: In a randomized clinical trial (RE-LY), dabigatran had a lower risk of major bleeding compared with warfarin. However, given that the anticoagulant effect of dabigatran is difficult to measure and there is not an effective antidote, it is unknown if dabigatran is associated with higher periprocedural rates of bleeding compared with warfarin.
Study design: Post-hoc analysis of the RE-LY trial.
Setting: Multicenter randomized controlled trial.
Synopsis: Patients enrolled in the RE-LY trial all had urgent or elective-invasive procedures recorded. The protocol recommended cessation of warfarin based on local practice, and cessation of dabigatran 24 hours before the procedure; the protocol was amended in 2008 to recommend cessation of dabigatran 24 hours before low-bleeding risk procedures, but two to five hours before high-bleeding-risk procedures (depending on renal function). Periprocedural bleeding was defined as seven days prior and 30 days after the procedure. Dabigatran was interrupted a mean of 49 hours before the procedures, and warfarin for 114 hours. There was no significant difference in major bleeding rates between dabigatran (150-mg dose) and warfarin, which occurred in 5.1% versus 4.6% patients, respectively.
Bottom line: Warfarin and dabigatran have about the same rate of periprocedural bleeding rates, but interruption duration is shorter for dabigatran.
Citation: Healey JS, Eikelboom J, Douketis J, et al. Peri-procedural bleeding and thromboembolic events with dabigatran compared with warfarin: results from the randomized evaluation of long-term anticoagulation therapy (RE-LY) randomized trial. Circulation. 2012;126(3):343-348.