User login
New Directions in Small-Vessel Vasculitis: ANCA, Target Organs, Treatment, and Beyond
Supplement Editor:
Carol Langford, MD, MHS
Associate Editors:
Leonard Calabrese, DO, and Gary Hoffman, MD
Contents
Diagnosis, ANCA testing, and disease activity
Clinical features and diagnosis of small-vessel vasculitis
Carol Langford, MD, MHS
Controversies in ANCA testing
Ulrich Specks, MD
Defining disease activity and damage in patients with small-vessel vasculitis
Peter A. Merkel, MD, MPH
Impact on individual organs
Upper airway manifestations of granulomatosis with polyangiitis
Daniel S. Alam, MD; Rahul Seth, MD; Raj Sindwani, MD; Erika A. Woodson, MD; and Karthik Rajasekaran, MD
Renal disease in small-vessel vasculitis
Kirsten de Groot, MD
Pulmonary disease in small-vessel vasculitis
Thomas R. Gildea, MD, MS
Ocular manifestations of small-vessel vasculitis
James A. Garrity, MD
Monitoring and safety
Monitoring patients with vasculitis
Alexandra Villa-Forte, MD, MPH
Safety issues in vasculitis: Infections and immunizations in the immunosuppressed host
Carlos M. Isada, MD, FCCP
Treatment considerations
Treating vasculitis with conventional immunosuppressive agents
David Jayne, MD, FRCP
Biologic agents in the treatment of granulomatosis with polyangiitis
Ulrich Specks, MD
Historical perspective
History of vasculitis: The life and work of Adolf Kussmaul
Eric L. Matteson, MD, MPH
Supplement Editor:
Carol Langford, MD, MHS
Associate Editors:
Leonard Calabrese, DO, and Gary Hoffman, MD
Contents
Diagnosis, ANCA testing, and disease activity
Clinical features and diagnosis of small-vessel vasculitis
Carol Langford, MD, MHS
Controversies in ANCA testing
Ulrich Specks, MD
Defining disease activity and damage in patients with small-vessel vasculitis
Peter A. Merkel, MD, MPH
Impact on individual organs
Upper airway manifestations of granulomatosis with polyangiitis
Daniel S. Alam, MD; Rahul Seth, MD; Raj Sindwani, MD; Erika A. Woodson, MD; and Karthik Rajasekaran, MD
Renal disease in small-vessel vasculitis
Kirsten de Groot, MD
Pulmonary disease in small-vessel vasculitis
Thomas R. Gildea, MD, MS
Ocular manifestations of small-vessel vasculitis
James A. Garrity, MD
Monitoring and safety
Monitoring patients with vasculitis
Alexandra Villa-Forte, MD, MPH
Safety issues in vasculitis: Infections and immunizations in the immunosuppressed host
Carlos M. Isada, MD, FCCP
Treatment considerations
Treating vasculitis with conventional immunosuppressive agents
David Jayne, MD, FRCP
Biologic agents in the treatment of granulomatosis with polyangiitis
Ulrich Specks, MD
Historical perspective
History of vasculitis: The life and work of Adolf Kussmaul
Eric L. Matteson, MD, MPH
Supplement Editor:
Carol Langford, MD, MHS
Associate Editors:
Leonard Calabrese, DO, and Gary Hoffman, MD
Contents
Diagnosis, ANCA testing, and disease activity
Clinical features and diagnosis of small-vessel vasculitis
Carol Langford, MD, MHS
Controversies in ANCA testing
Ulrich Specks, MD
Defining disease activity and damage in patients with small-vessel vasculitis
Peter A. Merkel, MD, MPH
Impact on individual organs
Upper airway manifestations of granulomatosis with polyangiitis
Daniel S. Alam, MD; Rahul Seth, MD; Raj Sindwani, MD; Erika A. Woodson, MD; and Karthik Rajasekaran, MD
Renal disease in small-vessel vasculitis
Kirsten de Groot, MD
Pulmonary disease in small-vessel vasculitis
Thomas R. Gildea, MD, MS
Ocular manifestations of small-vessel vasculitis
James A. Garrity, MD
Monitoring and safety
Monitoring patients with vasculitis
Alexandra Villa-Forte, MD, MPH
Safety issues in vasculitis: Infections and immunizations in the immunosuppressed host
Carlos M. Isada, MD, FCCP
Treatment considerations
Treating vasculitis with conventional immunosuppressive agents
David Jayne, MD, FRCP
Biologic agents in the treatment of granulomatosis with polyangiitis
Ulrich Specks, MD
Historical perspective
History of vasculitis: The life and work of Adolf Kussmaul
Eric L. Matteson, MD, MPH
Clinical features and diagnosis of small-vessel vasculitis
Vasculitis refers to inflammation of the blood vessel. This inflammation can cause vessel wall thickening that compromises or occludes the vessel lumen, ultimately resulting in organ ischemia. It also can cause vessel wall attenuation that predisposes to aneurysm formation or breach of the vessel integrity with resultant hemorrhage into the tissue.
Vasculitis can be thought of as a primary or secondary process. Primary vasculitides are unique disease entities without a currently identified underlying cause in which vasculitis forms the pathologic basis of tissue injury. Vasculitis can occur secondary to medication exposure or an underlying illness, including infections, malignancy, cryoglobulinemia, and rheumatic diseases (such as systemic lupus erythematosus, rheumatoid arthritis, Sjögren syndrome, or myositis).
Primary vasculitides may differ in epidemiology, such as the age at which they occur and the gender most likely to be affected, their clinical manifestations (including signs, symptoms, and patterns of organ involvement), the diagnostic approach (biopsy, arteriography, and laboratory investigation), treatment (supportive care, glucocorticoids alone, or in combination with other immunosuppressants), and the size of the vessels predominantly affected (large, medium, or small).
Small-vessel vasculitis affects the arteriole, capillary, and venule. An excellent example of small-vessel vasculitis and the one most commonly encountered in clinical practice is cutaneous vasculitis, in which extravasation of erythrocytes from disrupted small vessels is observed histologically, with the clinical sequelae of palpable purpura. Although categorization based on the predominant vessel size that is affected is a helpful way to view these diseases, this is not absolute and each disease has the potential to affect a diverse range of vessels.
This article explores the clinical features and diagnosis of three forms of vasculitis that predominantly affect the small vessels: granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]), microscopic polyangiitis (MPA), and eosinophilic GPA (Churg-Strauss syndrome, EGPA).
GRANULOMATOSIS WITH POLYANGIITIS
Granulomatosis with polyangiitis is characterized by granulomatous inflammation involving the respiratory tract and by vasculitis affecting small- to medium-sized vessels in which necrotizing glomerulonephritis is common.
Wide range of presentations, manifestations
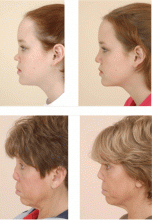
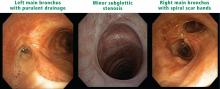
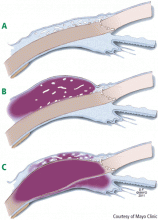
Approximately 90% of patients with GPA have upper or lower airway involvement or both.1 Upper airway or ear symptoms affect 73% of patients initially and 92% overall.1 Direct inspection of the nasal membranes shows a cobblestoned or ulcerated appearance, and computed tomography reveals mucosal thickening of the sinuses. In some instances, sinus disease can compromise blood supply to the cartilaginous portion of the nasal septum, leading to nasal septum perforations or collapse of the nasal bridge. Another manifestation of upper airway disease and GPA is subglottic stenosis, a narrowing in the subglottic region located just below the vocal cords. The narrowing typically spans about 1 cm and rarely extends or involves the remainder of the trachea.
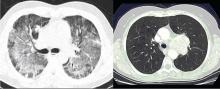
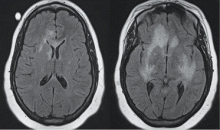
The lungs are involved in 85% of patients.1 Radiographic abnormalities can be diverse and include bilateral pulmonary nodular infiltrates, single or multiple cavities, and bilateral ground glass infiltrates as can be seen in pulmonary hemorrhage (Figure). Bronchoscopy may reveal endobronchial stenosis, and pleural disease can occur rarely.
Approximately 20% of patients with GPA may have glomerulonephritis when they first present for medical attention, but it eventually develops in nearly 80% of patients during the disease course.1 Despite its potential for rapid progression, glomerulonephritis presents a diagnostic challenge because it is asymptomatic. It is detected by evidence of proteinuria and an active urine sediment with dysmorphic red blood cells and red blood cell casts.
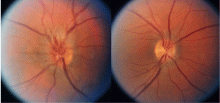
Ocular involvement occurs eventually in 52% of patients with GPA.1 Any ocular structure can be affected and ocular involvement can be visually threatening. The more prominent ocular manifestations include scleritis/episcleritis or orbital disease.
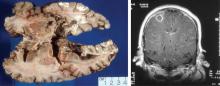
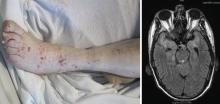
Cutaneous manifestations, observed in 46% of patients, include verrucous-appearing lesions on the elbow and infarctions in the skin and nail folds.1 Other rare manifestations can occur, such as pericarditis and cerebral vasculitis.
Although nearly all patients present with upper or lower airway symptoms, the multisystem nature of GPA explains the wide range of presentations and the varying degrees of disease severity.
Differential diagnosis
The differential diagnosis in GPA is varied. Particularly in the setting of isolated lung or sinus disease, infection is foremost in the differential diagnosis. Even in the nonimmunosuppressed host, unusual infections such as mycobacteria, histoplasmosis, and other fungal infections should be considered. Lymphadenopathy, rarely seen in GPA, should raise concern for other causes of disease. Lymphoproliferative processes and other neoplasms, other rheumatic diseases, granulomatous disease (ie, sarcoidosis), and other causes of glomerulonephritis (when present) also merit consideration. Differentiation of these entities from GPA is essential because the treatment differs in many instances.
The differential diagnosis for patients who present with midline destructive lesions must include other causes of collapse of the nasal bridge, nasal septum perforation, and possibly palate destruction. Erosions of the hard palate in particular should raise an immediate red flag for entities other than GPA, such as lymphoproliferative diseases; rare infections, particularly if the patient has studied or worked abroad; and cocaine exposure.
Diagnostic evaluation
A diagnosis of GPA is typically based on the presence of histologic features in a clinically compatible setting. Diagnostic features include necrosis, granulomatous inflammation, vasculitis, and special stains and cultures negative for microorganisms.
Biopsy sites are determined by evidence of clinical disease affecting a target organ and the likelihood of obtaining diagnostically meaningful findings from that site. One challenge is that biopsies are not always diagnostic. The changes tend to be patchy and the likelihood of a positive yield is associated with the amount of tissue that can be obtained. Tissues from the ear, nose, and throat have a yield of about 20%, depending upon the site and the biopsy size. The highest yield comes from radiographically abnormal pulmonary parenchyma. Although transbronchial biopsies are attractive because they are less invasive than open lung biopsy, they are also far less diagnostic, with fewer than 10% having a positive yield. Because cutaneous vasculitis is observed in many settings, its presence is usually insufficient evidence for diagnosis. The renal histologic appearance is a focal, segmental, crescentic, and necrotizing glomerulonephritis that has few to no immune complexes (pauciimmune glomerulonephritis).1–3
Chest imaging should be performed in any patient in whom GPA is part of the differential diagnosis, since up to one-third of patients may be asymptomatic yet have pulmonary radiographic findings.
Laboratory assessment should include serum chemistries to evaluate renal and hepatic function, complete blood count, erythrocyte sedimentation rate, measurement of C-reactive protein, and urinalysis. If the urinalysis is positive for blood, microscopy should be performed on fresh urine to look for casts. In the setting of pulmonary-renal manifestations, testing for other causes, such as antiglomerular basement antibodies and antinuclear antibodies, should be considered.
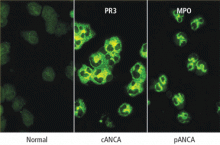
Serologic testing for antineutrophil cytoplasmic antibodies (ANCA) has provided a useful tool in suggesting the diagnosis of GPA. Two forms of ANCA have been identified in patients with vasculitis: ANCA directed against the neutrophil serine protease proteinase-3 (PR3), which results in a cytoplasmic immunofluorescence (cANCA) pattern; and ANCA directed against the neutrophil enzyme myeloperoxidase (MPO), which causes a perinuclear immunofluorescence (pANCA) pattern.4 Approximately 80% to 95% of ANCA found in patients with active severe GPA are detectable PR3-cANCA, while 5% to 20% are MPO-pANCA.5 The predictive value of ANCA for the diagnosis depends on the spectrum of clinical features. As ANCA can be seen in other settings, ANCA as the basis for diagnosis in place of tissue biopsy should be used with caution and only in selected instances where their predictive value would equal that of biopsy. The presence of ANCA is not necessary to establish the diagnosis, as up to 20% of patients with GPA may be ANCA-negative.6
MICROSCOPIC POLYANGIITIS
The history of MPA dates to 1866, with the description of periarteritis nodosa.7 The term “microscopic polyarteritis” was introduced in 1948, when glomerular disease was recognized in some patients.8 In 1994, the Chapel Hill Consensus Conference defined MPA as a necrotizing vasculitis with few or no immune deposits that affects small vessels (ie, capillaries, venules, or arterioles). Necrotizing arteritis of small- and medium-sized arteries may be present. Necrotizing glomerulonephritis and pulmonary capillaritis commonly occur.9 MPA shares many clinical features with GPA and is currently said to be distinguished by the absence of granulomatous inflammation.9
Presentations and manifestations
In one assessment of organ system involvement in 85 patients with MPA, investigators observed glomerular syndrome in 82% of patients.10 They also found a high predilection for involvement of the skin, joints, and lungs. Pulmonary hemorrhage is a particularly important manifestation of MPA because it can be immediately life-threatening.
Differential diagnosis
The differential diagnosis for MPA is similar to GPA in the inclusion of other causes of classic pulmonary-renal syndromes, such as antiglomerular basement membrane disease and systemic lupus erythematosus. Poststreptococcal glomerulonephritis should be considered when the kidney is the predominant organ involved in the absence of lung disease. In the setting of pulmonary infiltrates, infections and neoplasms remain significant in the differential diagnosis.
Diagnostic evaluation
The diagnosis of MPA is based on consistent clinical features and compatible histologic findings. The histologic renal lesion is identical to that seen in GPA. Pulmonary disease typically includes capillaritis and is notable for the absence of evidence of immune deposition, in contrast to antiglomerular basement membrane disease.
Chest imaging is indicated when MPA is part of the differential diagnosis. Computed tomography is the preferred technique, as early alveolar hemorrhage that can occur in MPA may not be visualized on a chest radiograph.
Laboratory assessment should include serum chemistries, complete blood count, erythrocyte sedimentation rate, measurement of C-reactive protein, and urinalysis. Additional testing should be pursued for other diseases as indicated by the clinical features.
Approximately 40% to 80% of patients with MPA have MPO-pANCA.5 Approximately 15% of patients are MPO-pANCA positive,6 and 0% to 20% are ANCA-negative. As with GPA, ANCA is useful to suggest—but not diagnose—disease in many instances. The absence of ANCA does not rule out MPA.
EOSINOPHILIC GPA
Eosinophilic GPA is a unique entity characterized by eosinophil-rich and granulomatous inflammation involving the respiratory tract and necrotizing vasculitis of small- to medium-size vessels. It is also associated with asthma and eosinophilia.
Different disease phases
Eosinophilic GPA is often thought of as having three phases: prodromal, eosinophilic, and vasculitic.11,12 Although helpful conceptually, these phases may not always be present and may not occur in sequence.
The prodromal phase is characterized by asthma associated with allergic rhinitis with or without polyposis. The eosinophilic phase is characterized by the presence of eosinophilia in the blood and tissue. Eosinophilia is a prominent feature, although accurate detection and assessment can be challenging in the setting of glucocorticoid use for asthma as this normalizes the eosinophil count.
The vasculitic phase distinguishes EGPA from other eosinophilic disorders. Features of vasculitis may occur in multiple organ sites, including the nerves, lungs, heart, gastrointestinal tract, and kidneys. In one series of 96 patients, nearly 100% had asthma, and peripheral nervous system involvement in the form of mononeuritis multiplex was present in 72%.12 Cardiac involvement is of particular importance as it is a prominent cause of disease-related mortality. Cardiac manifestations include myocarditis, pericarditis, endocarditis, valvulitis, and coronary vasculitis.
Differential diagnosis
The differential diagnosis of EGPA shares similarities with GPA and MPA, but also includes eosino philic disorders such as hypereosinophilic syndrome, eosinophilic leukemia, and parasitic diseases.
Diagnostic evaluation
Diagnosis is often based on the presence of asthma, a finding of peripheral eosinophilia (> 1,500 cells/mm3), and the presence of systemic vasculitis involving, ideally, two or more extrapulmonary organs. While histologic confirmation remains ideal, demonstration of characteristic findings on biopsy can be difficult. Glomerular involvement is far less common than in GPA and MPA, but, when present, the renal lesion is identical. Pulmonary histologic findings can be diverse and include the classic “allergic-granuloma” as originally described by Churg and Strauss, as well as isolated granulomatous inflammation, eosinophilic inflammation, or small-vessel vasculitis. Tissue eosinophilia is a prominent finding that typically is seen on biopsies of skin, nerve, and gastrointestinal tissues.
Chest imaging should be performed when EGPA is part of the differential diagnosis. Because of the potential for cardiac involvement, a baseline echocardiogram should be obtained. Pulmonary function tests may be useful, particularly in patients who have a strong asthmatic component.
Similar to GPA and MPA, laboratory assessment includes serum chemistries, complete blood count with differential to determine the eosinophil count, erythrocyte sedimentation rate, measurement of C-reactive protein, and urinalysis. With the allergic and asthmatic components, immunoglobulin E levels are frequently elevated. Additional testing for other eosinophilic diseases should be pursued as indicated by the clinical features.
Only about 40% of patients are ANCA-positive.13 Most of these are MPO-pANCA, with PR3-cANCA occurring less commonly. Although some reports have suggested differing clinical patterns of EGPA based on ANCA positivity, the presence or absence of ANCA is less helpful in the diagnosis.13
DIFFERENTIATION
A key histologic difference between GPA and MPA is the presence of granulomatous inflammation in GPA and its absence in MPA under the current nomenclature system.9 Granulomatous inflammation can be seen in EGPA, but it is usually accompanied by eosinophils, which are less likely to be present in GPA and MPA.
The predominance of the ANCA immunofluorescence pattern and target antigen differs between GPA and MPA, with ANCA positivity occurring in 38% of patients with EGPA.13
SUMMARY
Conceptualizing vasculitic disease based on vessel size can be useful, but it is not an absolute definition. Although GPA, MPA, and EGPA predominantly affect small- to medium-sized vessels, these disease entities are phenotypically unique, with both shared features and differences. Common to all three entities is the potential for organ- and life-threatening manifestations, particularly involving the lungs, kidneys, nerves, gastrointestinal tract, and heart. All three entities need aggressive immuno suppression for severe disease. Recognition of these entities and the distinctions among them can guide the approach to diagnosis, treatment, and future outcomes.
- Hoffman GS, Kerr GS, Leavitt RY, et al Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Travis WD, Hoffman GS, Leavitt RY, Pass HI, Fauci AS. Surgical pathology of the lung in Wegener’s granulomatosis: review of 87 open lung biopsies from 67 patients. Am J Surg Pathol 1991; 15:315–333.
- Devaney KO, Travis WD, Hoffman G, Leavitt R, Lebovics R, Fauci AS. Interpretation of head and neck biopsies in Wegener’s granulomatosis: a pathologic study of 126 biopsies in 70 patients. Am J Surg Pathol 1990; 14:555–564.
- Bosch X, Guilabert A, Font J. Antineutrophil cytoplasmic antibodies. Lancet 2006; 368:404–418.
- Hoffman GS, Specks U. Antineutrophil cytoplasmic antibodies. Arthritis Rheum 1998;1521–1537.
- Wiik A. What you should know about PR3-ANCA. An introduction. Arthritis Res 2000; 2:252–254.
- Kussmaul A, Maier R. Über eine bisher nicht beschriebene eigenthümliche Arterienerkrankung (Periarteritis nodosa), die mit Morbus Brightii und rapid fortschreitender allgemeiner Muskellähmung einhergeht. Dtsch Arch Klin Med 1866; 1:484–518.
- Davson J, Ball J, Platt R. The kidney in periarteritis nodosa. Q J Med 1948; 17:175–202.
- Jennette C, Falk RJ, Andrassy K, et al Nomenclature of systemic vasculitides: proposal of an international consensus conference. Arthritis Rheum 1994; 37:187–192.
- Guillevin L, Durand-Gasselin B, Cevallos R, et al Microscopic polyangiitis: clinical and laboratory findings in eighty-five patients. Arthritis Rheum 1999; 42:421–430.
- Keogh KA, Specks U. Churg-Strauss syndrome. Semin Respir Crit Care Med 2006; 27:148–157.
- Guillevin L, Cohen P, Gayraud M, Lhote F, Jarrousse B, Casassus P. Churg-Strauss syndrome: clinical study and long-term follow-up of 96 patients. Medicine 1999; 78:26–37.
- Sablé-Fourtassou R, Cohen P, Mahr A, et al., for the French Vasculitis Study Group. Antineutrophil cytoplasmic antibodies and the Churg-Strauss syndrome. Ann Intern Med 2005; 143:632–638.
Vasculitis refers to inflammation of the blood vessel. This inflammation can cause vessel wall thickening that compromises or occludes the vessel lumen, ultimately resulting in organ ischemia. It also can cause vessel wall attenuation that predisposes to aneurysm formation or breach of the vessel integrity with resultant hemorrhage into the tissue.
Vasculitis can be thought of as a primary or secondary process. Primary vasculitides are unique disease entities without a currently identified underlying cause in which vasculitis forms the pathologic basis of tissue injury. Vasculitis can occur secondary to medication exposure or an underlying illness, including infections, malignancy, cryoglobulinemia, and rheumatic diseases (such as systemic lupus erythematosus, rheumatoid arthritis, Sjögren syndrome, or myositis).
Primary vasculitides may differ in epidemiology, such as the age at which they occur and the gender most likely to be affected, their clinical manifestations (including signs, symptoms, and patterns of organ involvement), the diagnostic approach (biopsy, arteriography, and laboratory investigation), treatment (supportive care, glucocorticoids alone, or in combination with other immunosuppressants), and the size of the vessels predominantly affected (large, medium, or small).
Small-vessel vasculitis affects the arteriole, capillary, and venule. An excellent example of small-vessel vasculitis and the one most commonly encountered in clinical practice is cutaneous vasculitis, in which extravasation of erythrocytes from disrupted small vessels is observed histologically, with the clinical sequelae of palpable purpura. Although categorization based on the predominant vessel size that is affected is a helpful way to view these diseases, this is not absolute and each disease has the potential to affect a diverse range of vessels.
This article explores the clinical features and diagnosis of three forms of vasculitis that predominantly affect the small vessels: granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]), microscopic polyangiitis (MPA), and eosinophilic GPA (Churg-Strauss syndrome, EGPA).
GRANULOMATOSIS WITH POLYANGIITIS
Granulomatosis with polyangiitis is characterized by granulomatous inflammation involving the respiratory tract and by vasculitis affecting small- to medium-sized vessels in which necrotizing glomerulonephritis is common.
Wide range of presentations, manifestations
Approximately 90% of patients with GPA have upper or lower airway involvement or both.1 Upper airway or ear symptoms affect 73% of patients initially and 92% overall.1 Direct inspection of the nasal membranes shows a cobblestoned or ulcerated appearance, and computed tomography reveals mucosal thickening of the sinuses. In some instances, sinus disease can compromise blood supply to the cartilaginous portion of the nasal septum, leading to nasal septum perforations or collapse of the nasal bridge. Another manifestation of upper airway disease and GPA is subglottic stenosis, a narrowing in the subglottic region located just below the vocal cords. The narrowing typically spans about 1 cm and rarely extends or involves the remainder of the trachea.
The lungs are involved in 85% of patients.1 Radiographic abnormalities can be diverse and include bilateral pulmonary nodular infiltrates, single or multiple cavities, and bilateral ground glass infiltrates as can be seen in pulmonary hemorrhage (Figure). Bronchoscopy may reveal endobronchial stenosis, and pleural disease can occur rarely.
Approximately 20% of patients with GPA may have glomerulonephritis when they first present for medical attention, but it eventually develops in nearly 80% of patients during the disease course.1 Despite its potential for rapid progression, glomerulonephritis presents a diagnostic challenge because it is asymptomatic. It is detected by evidence of proteinuria and an active urine sediment with dysmorphic red blood cells and red blood cell casts.
Ocular involvement occurs eventually in 52% of patients with GPA.1 Any ocular structure can be affected and ocular involvement can be visually threatening. The more prominent ocular manifestations include scleritis/episcleritis or orbital disease.
Cutaneous manifestations, observed in 46% of patients, include verrucous-appearing lesions on the elbow and infarctions in the skin and nail folds.1 Other rare manifestations can occur, such as pericarditis and cerebral vasculitis.
Although nearly all patients present with upper or lower airway symptoms, the multisystem nature of GPA explains the wide range of presentations and the varying degrees of disease severity.
Differential diagnosis
The differential diagnosis in GPA is varied. Particularly in the setting of isolated lung or sinus disease, infection is foremost in the differential diagnosis. Even in the nonimmunosuppressed host, unusual infections such as mycobacteria, histoplasmosis, and other fungal infections should be considered. Lymphadenopathy, rarely seen in GPA, should raise concern for other causes of disease. Lymphoproliferative processes and other neoplasms, other rheumatic diseases, granulomatous disease (ie, sarcoidosis), and other causes of glomerulonephritis (when present) also merit consideration. Differentiation of these entities from GPA is essential because the treatment differs in many instances.
The differential diagnosis for patients who present with midline destructive lesions must include other causes of collapse of the nasal bridge, nasal septum perforation, and possibly palate destruction. Erosions of the hard palate in particular should raise an immediate red flag for entities other than GPA, such as lymphoproliferative diseases; rare infections, particularly if the patient has studied or worked abroad; and cocaine exposure.
Diagnostic evaluation
A diagnosis of GPA is typically based on the presence of histologic features in a clinically compatible setting. Diagnostic features include necrosis, granulomatous inflammation, vasculitis, and special stains and cultures negative for microorganisms.
Biopsy sites are determined by evidence of clinical disease affecting a target organ and the likelihood of obtaining diagnostically meaningful findings from that site. One challenge is that biopsies are not always diagnostic. The changes tend to be patchy and the likelihood of a positive yield is associated with the amount of tissue that can be obtained. Tissues from the ear, nose, and throat have a yield of about 20%, depending upon the site and the biopsy size. The highest yield comes from radiographically abnormal pulmonary parenchyma. Although transbronchial biopsies are attractive because they are less invasive than open lung biopsy, they are also far less diagnostic, with fewer than 10% having a positive yield. Because cutaneous vasculitis is observed in many settings, its presence is usually insufficient evidence for diagnosis. The renal histologic appearance is a focal, segmental, crescentic, and necrotizing glomerulonephritis that has few to no immune complexes (pauciimmune glomerulonephritis).1–3
Chest imaging should be performed in any patient in whom GPA is part of the differential diagnosis, since up to one-third of patients may be asymptomatic yet have pulmonary radiographic findings.
Laboratory assessment should include serum chemistries to evaluate renal and hepatic function, complete blood count, erythrocyte sedimentation rate, measurement of C-reactive protein, and urinalysis. If the urinalysis is positive for blood, microscopy should be performed on fresh urine to look for casts. In the setting of pulmonary-renal manifestations, testing for other causes, such as antiglomerular basement antibodies and antinuclear antibodies, should be considered.
Serologic testing for antineutrophil cytoplasmic antibodies (ANCA) has provided a useful tool in suggesting the diagnosis of GPA. Two forms of ANCA have been identified in patients with vasculitis: ANCA directed against the neutrophil serine protease proteinase-3 (PR3), which results in a cytoplasmic immunofluorescence (cANCA) pattern; and ANCA directed against the neutrophil enzyme myeloperoxidase (MPO), which causes a perinuclear immunofluorescence (pANCA) pattern.4 Approximately 80% to 95% of ANCA found in patients with active severe GPA are detectable PR3-cANCA, while 5% to 20% are MPO-pANCA.5 The predictive value of ANCA for the diagnosis depends on the spectrum of clinical features. As ANCA can be seen in other settings, ANCA as the basis for diagnosis in place of tissue biopsy should be used with caution and only in selected instances where their predictive value would equal that of biopsy. The presence of ANCA is not necessary to establish the diagnosis, as up to 20% of patients with GPA may be ANCA-negative.6
MICROSCOPIC POLYANGIITIS
The history of MPA dates to 1866, with the description of periarteritis nodosa.7 The term “microscopic polyarteritis” was introduced in 1948, when glomerular disease was recognized in some patients.8 In 1994, the Chapel Hill Consensus Conference defined MPA as a necrotizing vasculitis with few or no immune deposits that affects small vessels (ie, capillaries, venules, or arterioles). Necrotizing arteritis of small- and medium-sized arteries may be present. Necrotizing glomerulonephritis and pulmonary capillaritis commonly occur.9 MPA shares many clinical features with GPA and is currently said to be distinguished by the absence of granulomatous inflammation.9
Presentations and manifestations
In one assessment of organ system involvement in 85 patients with MPA, investigators observed glomerular syndrome in 82% of patients.10 They also found a high predilection for involvement of the skin, joints, and lungs. Pulmonary hemorrhage is a particularly important manifestation of MPA because it can be immediately life-threatening.
Differential diagnosis
The differential diagnosis for MPA is similar to GPA in the inclusion of other causes of classic pulmonary-renal syndromes, such as antiglomerular basement membrane disease and systemic lupus erythematosus. Poststreptococcal glomerulonephritis should be considered when the kidney is the predominant organ involved in the absence of lung disease. In the setting of pulmonary infiltrates, infections and neoplasms remain significant in the differential diagnosis.
Diagnostic evaluation
The diagnosis of MPA is based on consistent clinical features and compatible histologic findings. The histologic renal lesion is identical to that seen in GPA. Pulmonary disease typically includes capillaritis and is notable for the absence of evidence of immune deposition, in contrast to antiglomerular basement membrane disease.
Chest imaging is indicated when MPA is part of the differential diagnosis. Computed tomography is the preferred technique, as early alveolar hemorrhage that can occur in MPA may not be visualized on a chest radiograph.
Laboratory assessment should include serum chemistries, complete blood count, erythrocyte sedimentation rate, measurement of C-reactive protein, and urinalysis. Additional testing should be pursued for other diseases as indicated by the clinical features.
Approximately 40% to 80% of patients with MPA have MPO-pANCA.5 Approximately 15% of patients are MPO-pANCA positive,6 and 0% to 20% are ANCA-negative. As with GPA, ANCA is useful to suggest—but not diagnose—disease in many instances. The absence of ANCA does not rule out MPA.
EOSINOPHILIC GPA
Eosinophilic GPA is a unique entity characterized by eosinophil-rich and granulomatous inflammation involving the respiratory tract and necrotizing vasculitis of small- to medium-size vessels. It is also associated with asthma and eosinophilia.
Different disease phases
Eosinophilic GPA is often thought of as having three phases: prodromal, eosinophilic, and vasculitic.11,12 Although helpful conceptually, these phases may not always be present and may not occur in sequence.
The prodromal phase is characterized by asthma associated with allergic rhinitis with or without polyposis. The eosinophilic phase is characterized by the presence of eosinophilia in the blood and tissue. Eosinophilia is a prominent feature, although accurate detection and assessment can be challenging in the setting of glucocorticoid use for asthma as this normalizes the eosinophil count.
The vasculitic phase distinguishes EGPA from other eosinophilic disorders. Features of vasculitis may occur in multiple organ sites, including the nerves, lungs, heart, gastrointestinal tract, and kidneys. In one series of 96 patients, nearly 100% had asthma, and peripheral nervous system involvement in the form of mononeuritis multiplex was present in 72%.12 Cardiac involvement is of particular importance as it is a prominent cause of disease-related mortality. Cardiac manifestations include myocarditis, pericarditis, endocarditis, valvulitis, and coronary vasculitis.
Differential diagnosis
The differential diagnosis of EGPA shares similarities with GPA and MPA, but also includes eosino philic disorders such as hypereosinophilic syndrome, eosinophilic leukemia, and parasitic diseases.
Diagnostic evaluation
Diagnosis is often based on the presence of asthma, a finding of peripheral eosinophilia (> 1,500 cells/mm3), and the presence of systemic vasculitis involving, ideally, two or more extrapulmonary organs. While histologic confirmation remains ideal, demonstration of characteristic findings on biopsy can be difficult. Glomerular involvement is far less common than in GPA and MPA, but, when present, the renal lesion is identical. Pulmonary histologic findings can be diverse and include the classic “allergic-granuloma” as originally described by Churg and Strauss, as well as isolated granulomatous inflammation, eosinophilic inflammation, or small-vessel vasculitis. Tissue eosinophilia is a prominent finding that typically is seen on biopsies of skin, nerve, and gastrointestinal tissues.
Chest imaging should be performed when EGPA is part of the differential diagnosis. Because of the potential for cardiac involvement, a baseline echocardiogram should be obtained. Pulmonary function tests may be useful, particularly in patients who have a strong asthmatic component.
Similar to GPA and MPA, laboratory assessment includes serum chemistries, complete blood count with differential to determine the eosinophil count, erythrocyte sedimentation rate, measurement of C-reactive protein, and urinalysis. With the allergic and asthmatic components, immunoglobulin E levels are frequently elevated. Additional testing for other eosinophilic diseases should be pursued as indicated by the clinical features.
Only about 40% of patients are ANCA-positive.13 Most of these are MPO-pANCA, with PR3-cANCA occurring less commonly. Although some reports have suggested differing clinical patterns of EGPA based on ANCA positivity, the presence or absence of ANCA is less helpful in the diagnosis.13
DIFFERENTIATION
A key histologic difference between GPA and MPA is the presence of granulomatous inflammation in GPA and its absence in MPA under the current nomenclature system.9 Granulomatous inflammation can be seen in EGPA, but it is usually accompanied by eosinophils, which are less likely to be present in GPA and MPA.
The predominance of the ANCA immunofluorescence pattern and target antigen differs between GPA and MPA, with ANCA positivity occurring in 38% of patients with EGPA.13
SUMMARY
Conceptualizing vasculitic disease based on vessel size can be useful, but it is not an absolute definition. Although GPA, MPA, and EGPA predominantly affect small- to medium-sized vessels, these disease entities are phenotypically unique, with both shared features and differences. Common to all three entities is the potential for organ- and life-threatening manifestations, particularly involving the lungs, kidneys, nerves, gastrointestinal tract, and heart. All three entities need aggressive immuno suppression for severe disease. Recognition of these entities and the distinctions among them can guide the approach to diagnosis, treatment, and future outcomes.
Vasculitis refers to inflammation of the blood vessel. This inflammation can cause vessel wall thickening that compromises or occludes the vessel lumen, ultimately resulting in organ ischemia. It also can cause vessel wall attenuation that predisposes to aneurysm formation or breach of the vessel integrity with resultant hemorrhage into the tissue.
Vasculitis can be thought of as a primary or secondary process. Primary vasculitides are unique disease entities without a currently identified underlying cause in which vasculitis forms the pathologic basis of tissue injury. Vasculitis can occur secondary to medication exposure or an underlying illness, including infections, malignancy, cryoglobulinemia, and rheumatic diseases (such as systemic lupus erythematosus, rheumatoid arthritis, Sjögren syndrome, or myositis).
Primary vasculitides may differ in epidemiology, such as the age at which they occur and the gender most likely to be affected, their clinical manifestations (including signs, symptoms, and patterns of organ involvement), the diagnostic approach (biopsy, arteriography, and laboratory investigation), treatment (supportive care, glucocorticoids alone, or in combination with other immunosuppressants), and the size of the vessels predominantly affected (large, medium, or small).
Small-vessel vasculitis affects the arteriole, capillary, and venule. An excellent example of small-vessel vasculitis and the one most commonly encountered in clinical practice is cutaneous vasculitis, in which extravasation of erythrocytes from disrupted small vessels is observed histologically, with the clinical sequelae of palpable purpura. Although categorization based on the predominant vessel size that is affected is a helpful way to view these diseases, this is not absolute and each disease has the potential to affect a diverse range of vessels.
This article explores the clinical features and diagnosis of three forms of vasculitis that predominantly affect the small vessels: granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]), microscopic polyangiitis (MPA), and eosinophilic GPA (Churg-Strauss syndrome, EGPA).
GRANULOMATOSIS WITH POLYANGIITIS
Granulomatosis with polyangiitis is characterized by granulomatous inflammation involving the respiratory tract and by vasculitis affecting small- to medium-sized vessels in which necrotizing glomerulonephritis is common.
Wide range of presentations, manifestations
Approximately 90% of patients with GPA have upper or lower airway involvement or both.1 Upper airway or ear symptoms affect 73% of patients initially and 92% overall.1 Direct inspection of the nasal membranes shows a cobblestoned or ulcerated appearance, and computed tomography reveals mucosal thickening of the sinuses. In some instances, sinus disease can compromise blood supply to the cartilaginous portion of the nasal septum, leading to nasal septum perforations or collapse of the nasal bridge. Another manifestation of upper airway disease and GPA is subglottic stenosis, a narrowing in the subglottic region located just below the vocal cords. The narrowing typically spans about 1 cm and rarely extends or involves the remainder of the trachea.
The lungs are involved in 85% of patients.1 Radiographic abnormalities can be diverse and include bilateral pulmonary nodular infiltrates, single or multiple cavities, and bilateral ground glass infiltrates as can be seen in pulmonary hemorrhage (Figure). Bronchoscopy may reveal endobronchial stenosis, and pleural disease can occur rarely.
Approximately 20% of patients with GPA may have glomerulonephritis when they first present for medical attention, but it eventually develops in nearly 80% of patients during the disease course.1 Despite its potential for rapid progression, glomerulonephritis presents a diagnostic challenge because it is asymptomatic. It is detected by evidence of proteinuria and an active urine sediment with dysmorphic red blood cells and red blood cell casts.
Ocular involvement occurs eventually in 52% of patients with GPA.1 Any ocular structure can be affected and ocular involvement can be visually threatening. The more prominent ocular manifestations include scleritis/episcleritis or orbital disease.
Cutaneous manifestations, observed in 46% of patients, include verrucous-appearing lesions on the elbow and infarctions in the skin and nail folds.1 Other rare manifestations can occur, such as pericarditis and cerebral vasculitis.
Although nearly all patients present with upper or lower airway symptoms, the multisystem nature of GPA explains the wide range of presentations and the varying degrees of disease severity.
Differential diagnosis
The differential diagnosis in GPA is varied. Particularly in the setting of isolated lung or sinus disease, infection is foremost in the differential diagnosis. Even in the nonimmunosuppressed host, unusual infections such as mycobacteria, histoplasmosis, and other fungal infections should be considered. Lymphadenopathy, rarely seen in GPA, should raise concern for other causes of disease. Lymphoproliferative processes and other neoplasms, other rheumatic diseases, granulomatous disease (ie, sarcoidosis), and other causes of glomerulonephritis (when present) also merit consideration. Differentiation of these entities from GPA is essential because the treatment differs in many instances.
The differential diagnosis for patients who present with midline destructive lesions must include other causes of collapse of the nasal bridge, nasal septum perforation, and possibly palate destruction. Erosions of the hard palate in particular should raise an immediate red flag for entities other than GPA, such as lymphoproliferative diseases; rare infections, particularly if the patient has studied or worked abroad; and cocaine exposure.
Diagnostic evaluation
A diagnosis of GPA is typically based on the presence of histologic features in a clinically compatible setting. Diagnostic features include necrosis, granulomatous inflammation, vasculitis, and special stains and cultures negative for microorganisms.
Biopsy sites are determined by evidence of clinical disease affecting a target organ and the likelihood of obtaining diagnostically meaningful findings from that site. One challenge is that biopsies are not always diagnostic. The changes tend to be patchy and the likelihood of a positive yield is associated with the amount of tissue that can be obtained. Tissues from the ear, nose, and throat have a yield of about 20%, depending upon the site and the biopsy size. The highest yield comes from radiographically abnormal pulmonary parenchyma. Although transbronchial biopsies are attractive because they are less invasive than open lung biopsy, they are also far less diagnostic, with fewer than 10% having a positive yield. Because cutaneous vasculitis is observed in many settings, its presence is usually insufficient evidence for diagnosis. The renal histologic appearance is a focal, segmental, crescentic, and necrotizing glomerulonephritis that has few to no immune complexes (pauciimmune glomerulonephritis).1–3
Chest imaging should be performed in any patient in whom GPA is part of the differential diagnosis, since up to one-third of patients may be asymptomatic yet have pulmonary radiographic findings.
Laboratory assessment should include serum chemistries to evaluate renal and hepatic function, complete blood count, erythrocyte sedimentation rate, measurement of C-reactive protein, and urinalysis. If the urinalysis is positive for blood, microscopy should be performed on fresh urine to look for casts. In the setting of pulmonary-renal manifestations, testing for other causes, such as antiglomerular basement antibodies and antinuclear antibodies, should be considered.
Serologic testing for antineutrophil cytoplasmic antibodies (ANCA) has provided a useful tool in suggesting the diagnosis of GPA. Two forms of ANCA have been identified in patients with vasculitis: ANCA directed against the neutrophil serine protease proteinase-3 (PR3), which results in a cytoplasmic immunofluorescence (cANCA) pattern; and ANCA directed against the neutrophil enzyme myeloperoxidase (MPO), which causes a perinuclear immunofluorescence (pANCA) pattern.4 Approximately 80% to 95% of ANCA found in patients with active severe GPA are detectable PR3-cANCA, while 5% to 20% are MPO-pANCA.5 The predictive value of ANCA for the diagnosis depends on the spectrum of clinical features. As ANCA can be seen in other settings, ANCA as the basis for diagnosis in place of tissue biopsy should be used with caution and only in selected instances where their predictive value would equal that of biopsy. The presence of ANCA is not necessary to establish the diagnosis, as up to 20% of patients with GPA may be ANCA-negative.6
MICROSCOPIC POLYANGIITIS
The history of MPA dates to 1866, with the description of periarteritis nodosa.7 The term “microscopic polyarteritis” was introduced in 1948, when glomerular disease was recognized in some patients.8 In 1994, the Chapel Hill Consensus Conference defined MPA as a necrotizing vasculitis with few or no immune deposits that affects small vessels (ie, capillaries, venules, or arterioles). Necrotizing arteritis of small- and medium-sized arteries may be present. Necrotizing glomerulonephritis and pulmonary capillaritis commonly occur.9 MPA shares many clinical features with GPA and is currently said to be distinguished by the absence of granulomatous inflammation.9
Presentations and manifestations
In one assessment of organ system involvement in 85 patients with MPA, investigators observed glomerular syndrome in 82% of patients.10 They also found a high predilection for involvement of the skin, joints, and lungs. Pulmonary hemorrhage is a particularly important manifestation of MPA because it can be immediately life-threatening.
Differential diagnosis
The differential diagnosis for MPA is similar to GPA in the inclusion of other causes of classic pulmonary-renal syndromes, such as antiglomerular basement membrane disease and systemic lupus erythematosus. Poststreptococcal glomerulonephritis should be considered when the kidney is the predominant organ involved in the absence of lung disease. In the setting of pulmonary infiltrates, infections and neoplasms remain significant in the differential diagnosis.
Diagnostic evaluation
The diagnosis of MPA is based on consistent clinical features and compatible histologic findings. The histologic renal lesion is identical to that seen in GPA. Pulmonary disease typically includes capillaritis and is notable for the absence of evidence of immune deposition, in contrast to antiglomerular basement membrane disease.
Chest imaging is indicated when MPA is part of the differential diagnosis. Computed tomography is the preferred technique, as early alveolar hemorrhage that can occur in MPA may not be visualized on a chest radiograph.
Laboratory assessment should include serum chemistries, complete blood count, erythrocyte sedimentation rate, measurement of C-reactive protein, and urinalysis. Additional testing should be pursued for other diseases as indicated by the clinical features.
Approximately 40% to 80% of patients with MPA have MPO-pANCA.5 Approximately 15% of patients are MPO-pANCA positive,6 and 0% to 20% are ANCA-negative. As with GPA, ANCA is useful to suggest—but not diagnose—disease in many instances. The absence of ANCA does not rule out MPA.
EOSINOPHILIC GPA
Eosinophilic GPA is a unique entity characterized by eosinophil-rich and granulomatous inflammation involving the respiratory tract and necrotizing vasculitis of small- to medium-size vessels. It is also associated with asthma and eosinophilia.
Different disease phases
Eosinophilic GPA is often thought of as having three phases: prodromal, eosinophilic, and vasculitic.11,12 Although helpful conceptually, these phases may not always be present and may not occur in sequence.
The prodromal phase is characterized by asthma associated with allergic rhinitis with or without polyposis. The eosinophilic phase is characterized by the presence of eosinophilia in the blood and tissue. Eosinophilia is a prominent feature, although accurate detection and assessment can be challenging in the setting of glucocorticoid use for asthma as this normalizes the eosinophil count.
The vasculitic phase distinguishes EGPA from other eosinophilic disorders. Features of vasculitis may occur in multiple organ sites, including the nerves, lungs, heart, gastrointestinal tract, and kidneys. In one series of 96 patients, nearly 100% had asthma, and peripheral nervous system involvement in the form of mononeuritis multiplex was present in 72%.12 Cardiac involvement is of particular importance as it is a prominent cause of disease-related mortality. Cardiac manifestations include myocarditis, pericarditis, endocarditis, valvulitis, and coronary vasculitis.
Differential diagnosis
The differential diagnosis of EGPA shares similarities with GPA and MPA, but also includes eosino philic disorders such as hypereosinophilic syndrome, eosinophilic leukemia, and parasitic diseases.
Diagnostic evaluation
Diagnosis is often based on the presence of asthma, a finding of peripheral eosinophilia (> 1,500 cells/mm3), and the presence of systemic vasculitis involving, ideally, two or more extrapulmonary organs. While histologic confirmation remains ideal, demonstration of characteristic findings on biopsy can be difficult. Glomerular involvement is far less common than in GPA and MPA, but, when present, the renal lesion is identical. Pulmonary histologic findings can be diverse and include the classic “allergic-granuloma” as originally described by Churg and Strauss, as well as isolated granulomatous inflammation, eosinophilic inflammation, or small-vessel vasculitis. Tissue eosinophilia is a prominent finding that typically is seen on biopsies of skin, nerve, and gastrointestinal tissues.
Chest imaging should be performed when EGPA is part of the differential diagnosis. Because of the potential for cardiac involvement, a baseline echocardiogram should be obtained. Pulmonary function tests may be useful, particularly in patients who have a strong asthmatic component.
Similar to GPA and MPA, laboratory assessment includes serum chemistries, complete blood count with differential to determine the eosinophil count, erythrocyte sedimentation rate, measurement of C-reactive protein, and urinalysis. With the allergic and asthmatic components, immunoglobulin E levels are frequently elevated. Additional testing for other eosinophilic diseases should be pursued as indicated by the clinical features.
Only about 40% of patients are ANCA-positive.13 Most of these are MPO-pANCA, with PR3-cANCA occurring less commonly. Although some reports have suggested differing clinical patterns of EGPA based on ANCA positivity, the presence or absence of ANCA is less helpful in the diagnosis.13
DIFFERENTIATION
A key histologic difference between GPA and MPA is the presence of granulomatous inflammation in GPA and its absence in MPA under the current nomenclature system.9 Granulomatous inflammation can be seen in EGPA, but it is usually accompanied by eosinophils, which are less likely to be present in GPA and MPA.
The predominance of the ANCA immunofluorescence pattern and target antigen differs between GPA and MPA, with ANCA positivity occurring in 38% of patients with EGPA.13
SUMMARY
Conceptualizing vasculitic disease based on vessel size can be useful, but it is not an absolute definition. Although GPA, MPA, and EGPA predominantly affect small- to medium-sized vessels, these disease entities are phenotypically unique, with both shared features and differences. Common to all three entities is the potential for organ- and life-threatening manifestations, particularly involving the lungs, kidneys, nerves, gastrointestinal tract, and heart. All three entities need aggressive immuno suppression for severe disease. Recognition of these entities and the distinctions among them can guide the approach to diagnosis, treatment, and future outcomes.
- Hoffman GS, Kerr GS, Leavitt RY, et al Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Travis WD, Hoffman GS, Leavitt RY, Pass HI, Fauci AS. Surgical pathology of the lung in Wegener’s granulomatosis: review of 87 open lung biopsies from 67 patients. Am J Surg Pathol 1991; 15:315–333.
- Devaney KO, Travis WD, Hoffman G, Leavitt R, Lebovics R, Fauci AS. Interpretation of head and neck biopsies in Wegener’s granulomatosis: a pathologic study of 126 biopsies in 70 patients. Am J Surg Pathol 1990; 14:555–564.
- Bosch X, Guilabert A, Font J. Antineutrophil cytoplasmic antibodies. Lancet 2006; 368:404–418.
- Hoffman GS, Specks U. Antineutrophil cytoplasmic antibodies. Arthritis Rheum 1998;1521–1537.
- Wiik A. What you should know about PR3-ANCA. An introduction. Arthritis Res 2000; 2:252–254.
- Kussmaul A, Maier R. Über eine bisher nicht beschriebene eigenthümliche Arterienerkrankung (Periarteritis nodosa), die mit Morbus Brightii und rapid fortschreitender allgemeiner Muskellähmung einhergeht. Dtsch Arch Klin Med 1866; 1:484–518.
- Davson J, Ball J, Platt R. The kidney in periarteritis nodosa. Q J Med 1948; 17:175–202.
- Jennette C, Falk RJ, Andrassy K, et al Nomenclature of systemic vasculitides: proposal of an international consensus conference. Arthritis Rheum 1994; 37:187–192.
- Guillevin L, Durand-Gasselin B, Cevallos R, et al Microscopic polyangiitis: clinical and laboratory findings in eighty-five patients. Arthritis Rheum 1999; 42:421–430.
- Keogh KA, Specks U. Churg-Strauss syndrome. Semin Respir Crit Care Med 2006; 27:148–157.
- Guillevin L, Cohen P, Gayraud M, Lhote F, Jarrousse B, Casassus P. Churg-Strauss syndrome: clinical study and long-term follow-up of 96 patients. Medicine 1999; 78:26–37.
- Sablé-Fourtassou R, Cohen P, Mahr A, et al., for the French Vasculitis Study Group. Antineutrophil cytoplasmic antibodies and the Churg-Strauss syndrome. Ann Intern Med 2005; 143:632–638.
- Hoffman GS, Kerr GS, Leavitt RY, et al Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Travis WD, Hoffman GS, Leavitt RY, Pass HI, Fauci AS. Surgical pathology of the lung in Wegener’s granulomatosis: review of 87 open lung biopsies from 67 patients. Am J Surg Pathol 1991; 15:315–333.
- Devaney KO, Travis WD, Hoffman G, Leavitt R, Lebovics R, Fauci AS. Interpretation of head and neck biopsies in Wegener’s granulomatosis: a pathologic study of 126 biopsies in 70 patients. Am J Surg Pathol 1990; 14:555–564.
- Bosch X, Guilabert A, Font J. Antineutrophil cytoplasmic antibodies. Lancet 2006; 368:404–418.
- Hoffman GS, Specks U. Antineutrophil cytoplasmic antibodies. Arthritis Rheum 1998;1521–1537.
- Wiik A. What you should know about PR3-ANCA. An introduction. Arthritis Res 2000; 2:252–254.
- Kussmaul A, Maier R. Über eine bisher nicht beschriebene eigenthümliche Arterienerkrankung (Periarteritis nodosa), die mit Morbus Brightii und rapid fortschreitender allgemeiner Muskellähmung einhergeht. Dtsch Arch Klin Med 1866; 1:484–518.
- Davson J, Ball J, Platt R. The kidney in periarteritis nodosa. Q J Med 1948; 17:175–202.
- Jennette C, Falk RJ, Andrassy K, et al Nomenclature of systemic vasculitides: proposal of an international consensus conference. Arthritis Rheum 1994; 37:187–192.
- Guillevin L, Durand-Gasselin B, Cevallos R, et al Microscopic polyangiitis: clinical and laboratory findings in eighty-five patients. Arthritis Rheum 1999; 42:421–430.
- Keogh KA, Specks U. Churg-Strauss syndrome. Semin Respir Crit Care Med 2006; 27:148–157.
- Guillevin L, Cohen P, Gayraud M, Lhote F, Jarrousse B, Casassus P. Churg-Strauss syndrome: clinical study and long-term follow-up of 96 patients. Medicine 1999; 78:26–37.
- Sablé-Fourtassou R, Cohen P, Mahr A, et al., for the French Vasculitis Study Group. Antineutrophil cytoplasmic antibodies and the Churg-Strauss syndrome. Ann Intern Med 2005; 143:632–638.
Controversies in ANCA testing
Antineutrophil cytoplasmic antibody (ANCA) detection is a valuable tool for diagnosing small-vessel vasculitis,1 but measuring and interpreting ANCA levels is an inexact science. There is no single perfect ANCA test, and even a perfect test would not provide definitive clinical answers. The diagnostic utility of ANCA testing depends on the methodologic accuracy of the test and the appropriate ordering of testing in the right clinical setting. This article examines three important questions about this technology:
- What is the best ANCA test methodology?
- What is the prognostic value of serial ANCA testing?
- What is the clinical implication of ANCA type?
WHAT IS THE BEST ANCA TEST METHODOLOGY?
The diagnostic utility of ANCA testing depends on both the methodologic accuracy of the test and the appropriate ordering of tests. Methodologic accuracy comprises the analytic sensitivity and specificity of the test. Analytic sensitivity refers to the accurate identification of the presence of ANCA, and analytic specificity refers to measurement of only the entity in question (ANCA), not confounded by the presence of other entities (antibodies).
Equally as important as analytic accuracy is the appropriate ordering of the tests in the right clinical setting. Using a test that is sensitive to the presence of a specific ANCA type accurately identifies the presence of either proteinase-3 (PR3)- or myeloperoxidase (MPO)-ANCA. Once obtained, test results must be evaluated in terms of their relationship to the diagnosis being considered. If the tests are deemed diagnostically useful based on the results, the data can be used to assess the positive and negative predictive value of the tests.
Immunofluorescence or antigen-specific testing—or both?
A definitive diagnosis is more likely if an immunofluorescence staining pattern of cANCA is paired with the antigen specificity of PR3-ANCA, for example, or a perinuclear immunofluorescence pattern (pANCA) is paired with a positive MPO-ANCA. When positive test pairings have been obtained and the patient’s antigen ANCA reactivity is known, subsequent serial ANCA testing with an antigen-specific assay alone may be indicated, because the ANCA types of patients with vasculitis are unlikely to switch between PR3 and MPO during the course of their disease. If matching pairings are not obtained, the diagnostic utility of the tests remains unconfirmed.
Antigen type (PR3 or MPO) is determined through antigen-specific methods that include solid-phase assays and other methods of bringing the specific antigen in contact with the specific antibody in question. There are two categories of solid-phase assays: the enzyme-linked immunoabsorbent assay (ELISA) and the capture ELISA. In the ELISA methodology, the antigen is directly coated to a plastic plate; in the capture ELISA, an anchor, usually a monoclonal antibody or combination of antibodies, captures the target antigen on the plate. In both ELISA and capture ELISA assays, ANCA contained in the serum sample subjected to testing bind to the immobilized antigen. The amount of ANCA bound to the antigen can then be detected by a secondary antibody that is conjugated with an enzyme that can elicit a color reaction. The intensity of the color reaction is proportional to the amount of ANCA bound to the immobilized antigen.
The ELISA methodology tends to trade off analytic sensitivity for specificity, since the antigen purification process (which allows the ELISA system to increase its specificity) can cause conformational changes to the antigen being bound to the plate. This, in turn, causes a loss of some recognition of the conformationally sensitive ANCA.
In capture ELISA, a specific antibody captures the antigen; this stabilizes the conformation, boosts the analytic sensitivity, and allows a gentler purification process because it only captures the antigen in question and then binds it to the plate. This process decreases false-positive test results caused by residual contaminants in the antigen preparation. Analytic sensitivity issues may come into play if the anchoring monoclonal antibody competes for the epitope on the antigen being recognized by the serum antibody in question (ANCA), causing occasional false-negative results.
Another method now applied to commercial ANCA testing involves bead-based multiplex assays. These assays are based on principles similar to the ELISA or capture ELISA methods. In multiplex microsphere technology, the purified antigen is bound to a polystyrene microsphere instead of a plate. The microsphere is then presented to the antibody in question. The bead is then introduced to a secondary antibody labeled with a fluorescent marker (instead of an enzyme) for detection of the antibody. One advantage of this system is that various beads containing different antigens can be introduced to the same serum sample, and then different color reactions can be measured for each bead. Because only one antigen is bound to each microsphere (eg, PR3-ANCA, MPOANCA or other specific autoantibodies), only specific antibodies will react to each bead of a specific color. If there is no MPO antibody in the sample, there will be no reaction against the MPO antigen bead; however, if PR3-ANCA is present in the sample, it would react with the PR3 antigen beads. Using this methodology, a single serum sample can be tested for a multitude of autoantibodies at the same time (see “Interpreting ANCA results: Accurate tests, appropriate orders,”2–10 above).

WHAT IS THE PROGNOSTIC VALUE OF SERIAL ANCA TESTING?
Persistent changes in ANCA levels in relapsing disease may have some value in predicting outcome. The issues to consider include the methodology used to determine serial ANCA levels, correlations between ANCA and disease activity, and the use of ANCA changes to guide treatment.
Does methodology matter when determining serial ANCA levels?
Methodology in serial ANCA testing is probably unimportant as long as the same method is used serially. Analysis of large groups of ANCA-positive patients show a statistically highly significant correlation among results obtained with different detection methods, including immunofluorescence, direct ELISA, or capture ELISA. However, at the individual patient level there is some variability.
Do ANCA levels correlate with disease activity?
In a prospective study, serial ANCA samples obtained during the Wegener’s Granulomatosis Etanercept Trial (WGET)11 were processed in the same manner (collected every 3 months, mean follow-up of 22 months, uniform handling of samples). All samples were analyzed by capture ELISA, and disease activity was measured by the Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG). The results indicated that an increase in PR3-ANCA levels was not a significant predictor of relapse. The frequency of a relapse within 1 year of an increase in PR3-ANCA levels was found to be approximately 50%,11 a result similar to that reported in several smaller studies of different design and methodology.
Should ANCA changes guide treatment?
The available data regarding serial ANCA testing are limited mostly to PR3-ANCA. Serial ANCA testing has limited value as a guide to treatment and, in general, changes in ANCA levels alone should not be used to guide treatment decisions. In new patients without documented serial ANCA level associations, an increase in PR3-ANCA levels has no reliable predictive value. The existing literature suggests that this lack of association is not dependent on the method used for ANCA detection. For individual patients in whom long-term serial ANCA testing has been performed and a relationship between PR3-ANCA levels and disease activity has been established, serial ANCA testing can have some predictive value and can be used to guide treatment. For example, when remission is achieved by depleting B cells in patients with chronically relapsing GPA, ANCA levels usually go down. After B-cell reconstitution, the ANCA level rises in most patients, and this rise is usually associated with a flare shortly thereafter. A flare can be preempted when this pattern is determined in a specific patient, and preemptive treatment is applied accordingly.12
WHAT IS THE IMPLICATION OF ANCA TYPE?
Available reports consistently suggest that PR3-ANCA is associated with a higher mortality than MPO-ANCA (relative risk [RR], 3.78),13 and a higher relapse rate.14,15 A more rapid loss of renal function among patients with glomerulonephritis and PR3-ANCA than those with MPO-ANCA has also been reported.16 Using remission as the starting point, the number of days from complete remission to first disease flare was plotted for patients with MPO- versus PR3-ANCA in an analysis of long-term data from the Rituximab in ANCA-Associated Vasculitis (RAVE) trial.17 The resulting curve demonstrated a divergence in the probability of remaining in remission, confirming that remission maintenance is clearly greater in patients with MPO-ANCA than in patients with PR3-ANCA.
The primary end point of the RAVE trial was remission of disease without the use of prednisone at 6 months. There was little difference in end point achieved based on comparison of diagnosis (microscopic polyangiitis or granulomatosis) or treatment arms (rituximab versus cyclophosphamide); however, an analysis of end point data separating the patients by ANCA type showed that the treatment response to rituximab was superior to that of cyclophosphamide among patients with PR3-ANCA, whereas in patients with MPO-ANCA, there was little difference in response associated with either treatment. Regarding the likelihood of attaining an ANCA-negative status after 6 months, again MPO-ANCA patients showed no difference in frequency on either treatment. Among PR3-ANCA–positive patients, 50% in the rituximab arm attained ANCA-negative status compared with only 17% in the cyclophosphamide arm.17
SUMMARY
Diagnostic utility of ANCA testing depends on the methodology and clinical setting. Only cANCA/PR3-ANCA and pANCA/MPO-ANCA pairings have positive predictive value for diagnosis of small-vessel vasculitis. Mismatches in results, findings of human neutrophil elastase–ANCA, or identification of multiple positive antigens should be considered in cases of cocaine or drug use.
The clinical utility of serial ANCA testing is unconfirmed. Good data currently exist only for PR3-ANCA, and different drugs may affect ANCA levels in different ways. ANCA type is significant in that PR3-ANCA portends a higher relapse rate and poorer patient outcomes compared with MPO-ANCA.
- Russell KA, Wiegert E, Schroeder DR, Homburger HA, Specks U. Detection of anti-neutrophil cytoplasmic antibodies under actual clinical testing conditions. Clin Immunol 2002; 103:196–203.
- Langford CA. The diagnostic utility of c-ANCA in Wegener’s granulomatosis. Cleve Clin J Med 1998; 65:135–140.
- Trimarchi M, Gregorini G, Facchetti F, et al. Cocaine-induced midline destructive lesions: clinical, radiographic, histopathologic, and serologic features and their differentiation from Wegener granulomatosis. Medicine 2001; 80:391–404.
- Wiesner O, Russell KA, Lee AS, et al. Antineutrophil cytoplasmic antibodies reacting with human neutrophil elastase as a diagnostic marker for cocaine-induced midline destructive lesions but not autoimmune vasculitis. Arthritis Rheum 2004; 50:2954–2965.
- Peikert T, Finkielman JD, Hummel AM, et al. Functional characterization of antineutrophil cytoplasmic antibodies in patients with cocaine-induced midline destructive lesions. Arthritis Rheum 2008; 58:1546–1551.
- Knowles L, Buxton JA, Skuridina N, et al. Levamisole tainted cocaine causing severe neutropenia in Alberta and British Columbia. Harm Reduct J 2009; 6 (Nov 17):30. doi: 10.1186/1477-7517-6-30.
- Zhu NY, LeGatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med 2009; 150:287–289.
- Bradford M, Rosenberg B, Moreno J, Dumyati G. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Intern Med 2010; 152:758–759.
- Waller JM, Feramisco JD, Alberta-Wszolek L, McCalmont TH, Fox LP. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit [published online ahead of print March 20, 2010]? J Am Acad Dermatol 2010; 63:530–535. doi: 10.1016/j.jaad.2010.01.055
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant [published online ahead of print July 28, 2010]. Clin Pharmacol Ther 2010; 88:408–411. doi: 10.1038/clpt.2010.156
- Finkielman JD, Merkel PA, Schroeder D, et al. Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med 2007; 147:611–619.
- Cartin-Ceba R, Golbin J, Keogh KA, et al. Rituximab for remission induction and maintenance in granulomatosis with polyangiitis (Wegener’s): a single-center ten-year experience [published online ahead of print June 21, 2012]. Arthritis Rheum. doi: 10.1002/art.34584
- Hogan SL, Nachman PH, Wilkman AS, Jennette JC, Falk RJ; the Glomerular Disease Collaborative Network. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 1996; 7:23–32.
- Booth AD, Almond MK, Burns A, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 2003; 41:776–784.
- Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349:36–44.
- Franssen CFM, Gans ROB, Arends B, et al. Differences between anti-myeloperoxidase- and anti-proteinase 3-associated renal disease. Kidney Int 1995; 47:193–199.
- Stone JH, Merkel PA, Spiera R, et al; for the RAVE–ITN Research Group. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363:221–232.
Antineutrophil cytoplasmic antibody (ANCA) detection is a valuable tool for diagnosing small-vessel vasculitis,1 but measuring and interpreting ANCA levels is an inexact science. There is no single perfect ANCA test, and even a perfect test would not provide definitive clinical answers. The diagnostic utility of ANCA testing depends on the methodologic accuracy of the test and the appropriate ordering of testing in the right clinical setting. This article examines three important questions about this technology:
- What is the best ANCA test methodology?
- What is the prognostic value of serial ANCA testing?
- What is the clinical implication of ANCA type?
WHAT IS THE BEST ANCA TEST METHODOLOGY?
The diagnostic utility of ANCA testing depends on both the methodologic accuracy of the test and the appropriate ordering of tests. Methodologic accuracy comprises the analytic sensitivity and specificity of the test. Analytic sensitivity refers to the accurate identification of the presence of ANCA, and analytic specificity refers to measurement of only the entity in question (ANCA), not confounded by the presence of other entities (antibodies).
Equally as important as analytic accuracy is the appropriate ordering of the tests in the right clinical setting. Using a test that is sensitive to the presence of a specific ANCA type accurately identifies the presence of either proteinase-3 (PR3)- or myeloperoxidase (MPO)-ANCA. Once obtained, test results must be evaluated in terms of their relationship to the diagnosis being considered. If the tests are deemed diagnostically useful based on the results, the data can be used to assess the positive and negative predictive value of the tests.
Immunofluorescence or antigen-specific testing—or both?
A definitive diagnosis is more likely if an immunofluorescence staining pattern of cANCA is paired with the antigen specificity of PR3-ANCA, for example, or a perinuclear immunofluorescence pattern (pANCA) is paired with a positive MPO-ANCA. When positive test pairings have been obtained and the patient’s antigen ANCA reactivity is known, subsequent serial ANCA testing with an antigen-specific assay alone may be indicated, because the ANCA types of patients with vasculitis are unlikely to switch between PR3 and MPO during the course of their disease. If matching pairings are not obtained, the diagnostic utility of the tests remains unconfirmed.
Antigen type (PR3 or MPO) is determined through antigen-specific methods that include solid-phase assays and other methods of bringing the specific antigen in contact with the specific antibody in question. There are two categories of solid-phase assays: the enzyme-linked immunoabsorbent assay (ELISA) and the capture ELISA. In the ELISA methodology, the antigen is directly coated to a plastic plate; in the capture ELISA, an anchor, usually a monoclonal antibody or combination of antibodies, captures the target antigen on the plate. In both ELISA and capture ELISA assays, ANCA contained in the serum sample subjected to testing bind to the immobilized antigen. The amount of ANCA bound to the antigen can then be detected by a secondary antibody that is conjugated with an enzyme that can elicit a color reaction. The intensity of the color reaction is proportional to the amount of ANCA bound to the immobilized antigen.
The ELISA methodology tends to trade off analytic sensitivity for specificity, since the antigen purification process (which allows the ELISA system to increase its specificity) can cause conformational changes to the antigen being bound to the plate. This, in turn, causes a loss of some recognition of the conformationally sensitive ANCA.
In capture ELISA, a specific antibody captures the antigen; this stabilizes the conformation, boosts the analytic sensitivity, and allows a gentler purification process because it only captures the antigen in question and then binds it to the plate. This process decreases false-positive test results caused by residual contaminants in the antigen preparation. Analytic sensitivity issues may come into play if the anchoring monoclonal antibody competes for the epitope on the antigen being recognized by the serum antibody in question (ANCA), causing occasional false-negative results.
Another method now applied to commercial ANCA testing involves bead-based multiplex assays. These assays are based on principles similar to the ELISA or capture ELISA methods. In multiplex microsphere technology, the purified antigen is bound to a polystyrene microsphere instead of a plate. The microsphere is then presented to the antibody in question. The bead is then introduced to a secondary antibody labeled with a fluorescent marker (instead of an enzyme) for detection of the antibody. One advantage of this system is that various beads containing different antigens can be introduced to the same serum sample, and then different color reactions can be measured for each bead. Because only one antigen is bound to each microsphere (eg, PR3-ANCA, MPOANCA or other specific autoantibodies), only specific antibodies will react to each bead of a specific color. If there is no MPO antibody in the sample, there will be no reaction against the MPO antigen bead; however, if PR3-ANCA is present in the sample, it would react with the PR3 antigen beads. Using this methodology, a single serum sample can be tested for a multitude of autoantibodies at the same time (see “Interpreting ANCA results: Accurate tests, appropriate orders,”2–10 above).

WHAT IS THE PROGNOSTIC VALUE OF SERIAL ANCA TESTING?
Persistent changes in ANCA levels in relapsing disease may have some value in predicting outcome. The issues to consider include the methodology used to determine serial ANCA levels, correlations between ANCA and disease activity, and the use of ANCA changes to guide treatment.
Does methodology matter when determining serial ANCA levels?
Methodology in serial ANCA testing is probably unimportant as long as the same method is used serially. Analysis of large groups of ANCA-positive patients show a statistically highly significant correlation among results obtained with different detection methods, including immunofluorescence, direct ELISA, or capture ELISA. However, at the individual patient level there is some variability.
Do ANCA levels correlate with disease activity?
In a prospective study, serial ANCA samples obtained during the Wegener’s Granulomatosis Etanercept Trial (WGET)11 were processed in the same manner (collected every 3 months, mean follow-up of 22 months, uniform handling of samples). All samples were analyzed by capture ELISA, and disease activity was measured by the Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG). The results indicated that an increase in PR3-ANCA levels was not a significant predictor of relapse. The frequency of a relapse within 1 year of an increase in PR3-ANCA levels was found to be approximately 50%,11 a result similar to that reported in several smaller studies of different design and methodology.
Should ANCA changes guide treatment?
The available data regarding serial ANCA testing are limited mostly to PR3-ANCA. Serial ANCA testing has limited value as a guide to treatment and, in general, changes in ANCA levels alone should not be used to guide treatment decisions. In new patients without documented serial ANCA level associations, an increase in PR3-ANCA levels has no reliable predictive value. The existing literature suggests that this lack of association is not dependent on the method used for ANCA detection. For individual patients in whom long-term serial ANCA testing has been performed and a relationship between PR3-ANCA levels and disease activity has been established, serial ANCA testing can have some predictive value and can be used to guide treatment. For example, when remission is achieved by depleting B cells in patients with chronically relapsing GPA, ANCA levels usually go down. After B-cell reconstitution, the ANCA level rises in most patients, and this rise is usually associated with a flare shortly thereafter. A flare can be preempted when this pattern is determined in a specific patient, and preemptive treatment is applied accordingly.12
WHAT IS THE IMPLICATION OF ANCA TYPE?
Available reports consistently suggest that PR3-ANCA is associated with a higher mortality than MPO-ANCA (relative risk [RR], 3.78),13 and a higher relapse rate.14,15 A more rapid loss of renal function among patients with glomerulonephritis and PR3-ANCA than those with MPO-ANCA has also been reported.16 Using remission as the starting point, the number of days from complete remission to first disease flare was plotted for patients with MPO- versus PR3-ANCA in an analysis of long-term data from the Rituximab in ANCA-Associated Vasculitis (RAVE) trial.17 The resulting curve demonstrated a divergence in the probability of remaining in remission, confirming that remission maintenance is clearly greater in patients with MPO-ANCA than in patients with PR3-ANCA.
The primary end point of the RAVE trial was remission of disease without the use of prednisone at 6 months. There was little difference in end point achieved based on comparison of diagnosis (microscopic polyangiitis or granulomatosis) or treatment arms (rituximab versus cyclophosphamide); however, an analysis of end point data separating the patients by ANCA type showed that the treatment response to rituximab was superior to that of cyclophosphamide among patients with PR3-ANCA, whereas in patients with MPO-ANCA, there was little difference in response associated with either treatment. Regarding the likelihood of attaining an ANCA-negative status after 6 months, again MPO-ANCA patients showed no difference in frequency on either treatment. Among PR3-ANCA–positive patients, 50% in the rituximab arm attained ANCA-negative status compared with only 17% in the cyclophosphamide arm.17
SUMMARY
Diagnostic utility of ANCA testing depends on the methodology and clinical setting. Only cANCA/PR3-ANCA and pANCA/MPO-ANCA pairings have positive predictive value for diagnosis of small-vessel vasculitis. Mismatches in results, findings of human neutrophil elastase–ANCA, or identification of multiple positive antigens should be considered in cases of cocaine or drug use.
The clinical utility of serial ANCA testing is unconfirmed. Good data currently exist only for PR3-ANCA, and different drugs may affect ANCA levels in different ways. ANCA type is significant in that PR3-ANCA portends a higher relapse rate and poorer patient outcomes compared with MPO-ANCA.
Antineutrophil cytoplasmic antibody (ANCA) detection is a valuable tool for diagnosing small-vessel vasculitis,1 but measuring and interpreting ANCA levels is an inexact science. There is no single perfect ANCA test, and even a perfect test would not provide definitive clinical answers. The diagnostic utility of ANCA testing depends on the methodologic accuracy of the test and the appropriate ordering of testing in the right clinical setting. This article examines three important questions about this technology:
- What is the best ANCA test methodology?
- What is the prognostic value of serial ANCA testing?
- What is the clinical implication of ANCA type?
WHAT IS THE BEST ANCA TEST METHODOLOGY?
The diagnostic utility of ANCA testing depends on both the methodologic accuracy of the test and the appropriate ordering of tests. Methodologic accuracy comprises the analytic sensitivity and specificity of the test. Analytic sensitivity refers to the accurate identification of the presence of ANCA, and analytic specificity refers to measurement of only the entity in question (ANCA), not confounded by the presence of other entities (antibodies).
Equally as important as analytic accuracy is the appropriate ordering of the tests in the right clinical setting. Using a test that is sensitive to the presence of a specific ANCA type accurately identifies the presence of either proteinase-3 (PR3)- or myeloperoxidase (MPO)-ANCA. Once obtained, test results must be evaluated in terms of their relationship to the diagnosis being considered. If the tests are deemed diagnostically useful based on the results, the data can be used to assess the positive and negative predictive value of the tests.
Immunofluorescence or antigen-specific testing—or both?
A definitive diagnosis is more likely if an immunofluorescence staining pattern of cANCA is paired with the antigen specificity of PR3-ANCA, for example, or a perinuclear immunofluorescence pattern (pANCA) is paired with a positive MPO-ANCA. When positive test pairings have been obtained and the patient’s antigen ANCA reactivity is known, subsequent serial ANCA testing with an antigen-specific assay alone may be indicated, because the ANCA types of patients with vasculitis are unlikely to switch between PR3 and MPO during the course of their disease. If matching pairings are not obtained, the diagnostic utility of the tests remains unconfirmed.
Antigen type (PR3 or MPO) is determined through antigen-specific methods that include solid-phase assays and other methods of bringing the specific antigen in contact with the specific antibody in question. There are two categories of solid-phase assays: the enzyme-linked immunoabsorbent assay (ELISA) and the capture ELISA. In the ELISA methodology, the antigen is directly coated to a plastic plate; in the capture ELISA, an anchor, usually a monoclonal antibody or combination of antibodies, captures the target antigen on the plate. In both ELISA and capture ELISA assays, ANCA contained in the serum sample subjected to testing bind to the immobilized antigen. The amount of ANCA bound to the antigen can then be detected by a secondary antibody that is conjugated with an enzyme that can elicit a color reaction. The intensity of the color reaction is proportional to the amount of ANCA bound to the immobilized antigen.
The ELISA methodology tends to trade off analytic sensitivity for specificity, since the antigen purification process (which allows the ELISA system to increase its specificity) can cause conformational changes to the antigen being bound to the plate. This, in turn, causes a loss of some recognition of the conformationally sensitive ANCA.
In capture ELISA, a specific antibody captures the antigen; this stabilizes the conformation, boosts the analytic sensitivity, and allows a gentler purification process because it only captures the antigen in question and then binds it to the plate. This process decreases false-positive test results caused by residual contaminants in the antigen preparation. Analytic sensitivity issues may come into play if the anchoring monoclonal antibody competes for the epitope on the antigen being recognized by the serum antibody in question (ANCA), causing occasional false-negative results.
Another method now applied to commercial ANCA testing involves bead-based multiplex assays. These assays are based on principles similar to the ELISA or capture ELISA methods. In multiplex microsphere technology, the purified antigen is bound to a polystyrene microsphere instead of a plate. The microsphere is then presented to the antibody in question. The bead is then introduced to a secondary antibody labeled with a fluorescent marker (instead of an enzyme) for detection of the antibody. One advantage of this system is that various beads containing different antigens can be introduced to the same serum sample, and then different color reactions can be measured for each bead. Because only one antigen is bound to each microsphere (eg, PR3-ANCA, MPOANCA or other specific autoantibodies), only specific antibodies will react to each bead of a specific color. If there is no MPO antibody in the sample, there will be no reaction against the MPO antigen bead; however, if PR3-ANCA is present in the sample, it would react with the PR3 antigen beads. Using this methodology, a single serum sample can be tested for a multitude of autoantibodies at the same time (see “Interpreting ANCA results: Accurate tests, appropriate orders,”2–10 above).

WHAT IS THE PROGNOSTIC VALUE OF SERIAL ANCA TESTING?
Persistent changes in ANCA levels in relapsing disease may have some value in predicting outcome. The issues to consider include the methodology used to determine serial ANCA levels, correlations between ANCA and disease activity, and the use of ANCA changes to guide treatment.
Does methodology matter when determining serial ANCA levels?
Methodology in serial ANCA testing is probably unimportant as long as the same method is used serially. Analysis of large groups of ANCA-positive patients show a statistically highly significant correlation among results obtained with different detection methods, including immunofluorescence, direct ELISA, or capture ELISA. However, at the individual patient level there is some variability.
Do ANCA levels correlate with disease activity?
In a prospective study, serial ANCA samples obtained during the Wegener’s Granulomatosis Etanercept Trial (WGET)11 were processed in the same manner (collected every 3 months, mean follow-up of 22 months, uniform handling of samples). All samples were analyzed by capture ELISA, and disease activity was measured by the Birmingham Vasculitis Activity Score for Wegener’s Granulomatosis (BVAS/WG). The results indicated that an increase in PR3-ANCA levels was not a significant predictor of relapse. The frequency of a relapse within 1 year of an increase in PR3-ANCA levels was found to be approximately 50%,11 a result similar to that reported in several smaller studies of different design and methodology.
Should ANCA changes guide treatment?
The available data regarding serial ANCA testing are limited mostly to PR3-ANCA. Serial ANCA testing has limited value as a guide to treatment and, in general, changes in ANCA levels alone should not be used to guide treatment decisions. In new patients without documented serial ANCA level associations, an increase in PR3-ANCA levels has no reliable predictive value. The existing literature suggests that this lack of association is not dependent on the method used for ANCA detection. For individual patients in whom long-term serial ANCA testing has been performed and a relationship between PR3-ANCA levels and disease activity has been established, serial ANCA testing can have some predictive value and can be used to guide treatment. For example, when remission is achieved by depleting B cells in patients with chronically relapsing GPA, ANCA levels usually go down. After B-cell reconstitution, the ANCA level rises in most patients, and this rise is usually associated with a flare shortly thereafter. A flare can be preempted when this pattern is determined in a specific patient, and preemptive treatment is applied accordingly.12
WHAT IS THE IMPLICATION OF ANCA TYPE?
Available reports consistently suggest that PR3-ANCA is associated with a higher mortality than MPO-ANCA (relative risk [RR], 3.78),13 and a higher relapse rate.14,15 A more rapid loss of renal function among patients with glomerulonephritis and PR3-ANCA than those with MPO-ANCA has also been reported.16 Using remission as the starting point, the number of days from complete remission to first disease flare was plotted for patients with MPO- versus PR3-ANCA in an analysis of long-term data from the Rituximab in ANCA-Associated Vasculitis (RAVE) trial.17 The resulting curve demonstrated a divergence in the probability of remaining in remission, confirming that remission maintenance is clearly greater in patients with MPO-ANCA than in patients with PR3-ANCA.
The primary end point of the RAVE trial was remission of disease without the use of prednisone at 6 months. There was little difference in end point achieved based on comparison of diagnosis (microscopic polyangiitis or granulomatosis) or treatment arms (rituximab versus cyclophosphamide); however, an analysis of end point data separating the patients by ANCA type showed that the treatment response to rituximab was superior to that of cyclophosphamide among patients with PR3-ANCA, whereas in patients with MPO-ANCA, there was little difference in response associated with either treatment. Regarding the likelihood of attaining an ANCA-negative status after 6 months, again MPO-ANCA patients showed no difference in frequency on either treatment. Among PR3-ANCA–positive patients, 50% in the rituximab arm attained ANCA-negative status compared with only 17% in the cyclophosphamide arm.17
SUMMARY
Diagnostic utility of ANCA testing depends on the methodology and clinical setting. Only cANCA/PR3-ANCA and pANCA/MPO-ANCA pairings have positive predictive value for diagnosis of small-vessel vasculitis. Mismatches in results, findings of human neutrophil elastase–ANCA, or identification of multiple positive antigens should be considered in cases of cocaine or drug use.
The clinical utility of serial ANCA testing is unconfirmed. Good data currently exist only for PR3-ANCA, and different drugs may affect ANCA levels in different ways. ANCA type is significant in that PR3-ANCA portends a higher relapse rate and poorer patient outcomes compared with MPO-ANCA.
- Russell KA, Wiegert E, Schroeder DR, Homburger HA, Specks U. Detection of anti-neutrophil cytoplasmic antibodies under actual clinical testing conditions. Clin Immunol 2002; 103:196–203.
- Langford CA. The diagnostic utility of c-ANCA in Wegener’s granulomatosis. Cleve Clin J Med 1998; 65:135–140.
- Trimarchi M, Gregorini G, Facchetti F, et al. Cocaine-induced midline destructive lesions: clinical, radiographic, histopathologic, and serologic features and their differentiation from Wegener granulomatosis. Medicine 2001; 80:391–404.
- Wiesner O, Russell KA, Lee AS, et al. Antineutrophil cytoplasmic antibodies reacting with human neutrophil elastase as a diagnostic marker for cocaine-induced midline destructive lesions but not autoimmune vasculitis. Arthritis Rheum 2004; 50:2954–2965.
- Peikert T, Finkielman JD, Hummel AM, et al. Functional characterization of antineutrophil cytoplasmic antibodies in patients with cocaine-induced midline destructive lesions. Arthritis Rheum 2008; 58:1546–1551.
- Knowles L, Buxton JA, Skuridina N, et al. Levamisole tainted cocaine causing severe neutropenia in Alberta and British Columbia. Harm Reduct J 2009; 6 (Nov 17):30. doi: 10.1186/1477-7517-6-30.
- Zhu NY, LeGatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med 2009; 150:287–289.
- Bradford M, Rosenberg B, Moreno J, Dumyati G. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Intern Med 2010; 152:758–759.
- Waller JM, Feramisco JD, Alberta-Wszolek L, McCalmont TH, Fox LP. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit [published online ahead of print March 20, 2010]? J Am Acad Dermatol 2010; 63:530–535. doi: 10.1016/j.jaad.2010.01.055
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant [published online ahead of print July 28, 2010]. Clin Pharmacol Ther 2010; 88:408–411. doi: 10.1038/clpt.2010.156
- Finkielman JD, Merkel PA, Schroeder D, et al. Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med 2007; 147:611–619.
- Cartin-Ceba R, Golbin J, Keogh KA, et al. Rituximab for remission induction and maintenance in granulomatosis with polyangiitis (Wegener’s): a single-center ten-year experience [published online ahead of print June 21, 2012]. Arthritis Rheum. doi: 10.1002/art.34584
- Hogan SL, Nachman PH, Wilkman AS, Jennette JC, Falk RJ; the Glomerular Disease Collaborative Network. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 1996; 7:23–32.
- Booth AD, Almond MK, Burns A, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 2003; 41:776–784.
- Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349:36–44.
- Franssen CFM, Gans ROB, Arends B, et al. Differences between anti-myeloperoxidase- and anti-proteinase 3-associated renal disease. Kidney Int 1995; 47:193–199.
- Stone JH, Merkel PA, Spiera R, et al; for the RAVE–ITN Research Group. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363:221–232.
- Russell KA, Wiegert E, Schroeder DR, Homburger HA, Specks U. Detection of anti-neutrophil cytoplasmic antibodies under actual clinical testing conditions. Clin Immunol 2002; 103:196–203.
- Langford CA. The diagnostic utility of c-ANCA in Wegener’s granulomatosis. Cleve Clin J Med 1998; 65:135–140.
- Trimarchi M, Gregorini G, Facchetti F, et al. Cocaine-induced midline destructive lesions: clinical, radiographic, histopathologic, and serologic features and their differentiation from Wegener granulomatosis. Medicine 2001; 80:391–404.
- Wiesner O, Russell KA, Lee AS, et al. Antineutrophil cytoplasmic antibodies reacting with human neutrophil elastase as a diagnostic marker for cocaine-induced midline destructive lesions but not autoimmune vasculitis. Arthritis Rheum 2004; 50:2954–2965.
- Peikert T, Finkielman JD, Hummel AM, et al. Functional characterization of antineutrophil cytoplasmic antibodies in patients with cocaine-induced midline destructive lesions. Arthritis Rheum 2008; 58:1546–1551.
- Knowles L, Buxton JA, Skuridina N, et al. Levamisole tainted cocaine causing severe neutropenia in Alberta and British Columbia. Harm Reduct J 2009; 6 (Nov 17):30. doi: 10.1186/1477-7517-6-30.
- Zhu NY, LeGatt DF, Turner AR. Agranulocytosis after consumption of cocaine adulterated with levamisole. Ann Intern Med 2009; 150:287–289.
- Bradford M, Rosenberg B, Moreno J, Dumyati G. Bilateral necrosis of earlobes and cheeks: another complication of cocaine contaminated with levamisole. Ann Intern Med 2010; 152:758–759.
- Waller JM, Feramisco JD, Alberta-Wszolek L, McCalmont TH, Fox LP. Cocaine-associated retiform purpura and neutropenia: is levamisole the culprit [published online ahead of print March 20, 2010]? J Am Acad Dermatol 2010; 63:530–535. doi: 10.1016/j.jaad.2010.01.055
- Chang A, Osterloh J, Thomas J. Levamisole: a dangerous new cocaine adulterant [published online ahead of print July 28, 2010]. Clin Pharmacol Ther 2010; 88:408–411. doi: 10.1038/clpt.2010.156
- Finkielman JD, Merkel PA, Schroeder D, et al. Antiproteinase 3 antineutrophil cytoplasmic antibodies and disease activity in Wegener granulomatosis. Ann Intern Med 2007; 147:611–619.
- Cartin-Ceba R, Golbin J, Keogh KA, et al. Rituximab for remission induction and maintenance in granulomatosis with polyangiitis (Wegener’s): a single-center ten-year experience [published online ahead of print June 21, 2012]. Arthritis Rheum. doi: 10.1002/art.34584
- Hogan SL, Nachman PH, Wilkman AS, Jennette JC, Falk RJ; the Glomerular Disease Collaborative Network. Prognostic markers in patients with antineutrophil cytoplasmic autoantibody-associated microscopic polyangiitis and glomerulonephritis. J Am Soc Nephrol 1996; 7:23–32.
- Booth AD, Almond MK, Burns A, et al. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 2003; 41:776–784.
- Jayne D, Rasmussen N, Andrassy K, et al. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med 2003; 349:36–44.
- Franssen CFM, Gans ROB, Arends B, et al. Differences between anti-myeloperoxidase- and anti-proteinase 3-associated renal disease. Kidney Int 1995; 47:193–199.
- Stone JH, Merkel PA, Spiera R, et al; for the RAVE–ITN Research Group. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med 2010; 363:221–232.
Defining disease activity and damage in patients with small-vessel vasculitis
Small-vessel vasculitides are complex diseases with highly variable clinical features and are associated with considerable morbidity and mortality. These systemic, multisystem, multiorgan diseases often threaten vital organs with manifestations that include upper airway disease, pulmonary disease, glomerulonephritis, neuropathy, arthritis/arthralgias, malaise/fatigue, eye disease, skin/mucosa irregularities, vascular disease, cardiac disease, and gastrointestinal disease.
Accurate assessment of the patient with vasculitis is a challenge for the clinician and is critical for managing therapeutic interventions throughout the course of the disease. Effective management includes repeated evaluations of the activity and severity of the disease as well as the damage it has caused. These distinct yet overlapping concepts must be measured separately but evaluated as a whole. Additional categorizations of disease course, such as whether it is active (new-onset, persistent, or flare) or in remission, further define the disease and are routinely employed in guiding treatment choices.
The importance of accurately assessing a patient’s clinical status is clear, but it is also important to standardize and quantify vasculitis assessment tools for use in clinical trials. Standardized assessments are needed to:
- Guide clinical trial enrollment criteria
- Describe and compare study populations
- Quantify and measure treatment effectiveness
- Describe long-term outcomes
- Translate standardized assessment tools into clinical practice.
Over the past few decades, improvements in clinical research have resulted in increasingly accurate data obtained from well-designed randomized controlled trials, all of which are based on better clinical assessments. Improving the quality of the assessment tools has improved both the interpretation of trial results and translation of findings into clinical practice.
DISEASE ASSESSMENT
When assessing patients with vasculitis, whether clinically or in the context of a clinical trial, it is essential to differentiate among disease activity, damage, and severity:
Disease activity, such as active bleeding or mucosal inflammation, is treatable and potentially responsive to therapy.
Disease damage is generally irreversible and not improved by treating vasculitis. Damage may be caused by the disease itself, its treatment, or a comorbid condition. In general, once damage is identified, it is considered permanent if it remains unchanged for more than 6 months. In the Wegener’s Granulomatosis Etanercept Trial,1 damage that occurred in more than 10% of the cohort included hearing loss; proteinuria (≥ 0.5 g/24 hours); nasal blockage, chronic discharge, or crusting; nasal bridge collapse or septal perforation; glomerular filtration rate at least 50% lower than premorbid baseline; subglottic stenosis; and chronic sinusitis or radiologic damage. Disease-related damage can be addressed; a saddle-nose deformity can respond to plastic surgery, for example, but treating vasculitis will have no effect on the underlying established anatomic defect.
Disease severity assesses the intensity of the disease and guides the clinician in gauging how aggressive the therapy should be.
Vasculitis has two primary disease states: remission and active disease. In remission, there is no evidence of active disease. This is often qualified by describing the remission as either complete or partial; it is further defined by introducing an element of time, such as a “sustained” remission of more than 6 months. Active disease is the presence of any ongoing expression of vasculitis that is not caused by disease damage, comorbidity, or treatment. Active disease can be graded as low, medium, or high; if active disease lasts longer than 6 months, it is described as persistent or sustained. Flare, a manifestation of active disease, describes the transition from remission to active disease and is characterized by worsening of disease activity. Flares are graded as nonsevere or severe.
These descriptions of disease status can be further broken down into whether they are occurring “on” or “off” treatment. All of these elements are important and the subtleties and differences are critical in interpreting data for use in the clinical setting or in clinical trials.
CLINICAL ASSESSMENT
Assessing the status of disease for a patient with granulomatosis with polyangiitis (GPA, Wegener’s granulomatosis [WG]) or microscopic polyangiitis (MPA) begins with a detailed medical history and physical examination every time the patient is seen. Appropriate laboratory assessments include a complete blood count, tests of renal function, acute phase reactants (possibly as disease markers, but not necessarily to guide therapy), and other laboratory tests as needed. Controversy exists regarding the role of antineutrophil cytoplasmic antibody (ANCA) testing in assessment of disease activity.
Urinalyses are key for assessing activity; if a urine dipstick result is positive, a subsequent microscopic examination should be conducted. Microscopic review may demonstrate red cell casts that a routine laboratory check may not reveal. In addition to spotting de novo hematuria, looking for a change in dipstick results may prove valuable, since hematuria may increase in patients in whom persistent hematuria has already been noted. The change may be due to renal disease from the vasculitis, cyclophosphamide-induced bladder toxicity, a kidney stone, menses, or a variety of other causes, but if the hematuria is not monitored, a key assessment will be missed.
Disease staging through diagnostic imaging of the sinuses, neck, and chest should be performed on a regular basis as appropriate, beginning at the patient’s initial visit. Restaging, in much the same way as an oncologist restages cancer, should take place regularly, because this informs whether to make a major change in therapy (eg, from cyclophosphamide, azathioprine, or rituximab). Restaging will also allow benchmarking of old, new, and changed damage so that when the disease recurs, the existing damage can be differentiated from new lesions. Once the disease has stabilized, imaging can be discontinued.
Consultations with otolaryngologists, ophthalmologists, cardiologists, and other specialists should be sought as needed. Serial audiograms, laryngoscopy, echocardiograms, and other appropriate tests should be performed as required. Biopsies are useful for assessment of patients, particularly at diagnosis, but also when it becomes necessary to reassess the progress of a patient’s disease or to identify a potential infection versus a possible malignancy. Biopsy is particularly helpful for kidney disease. If of active disease, then repeat biopsy is appropriate to determine whether the deterioration is associated with persistent active disease, the natural history of declining kidney function, or another cause. Patients with vasculitis may develop new comorbidities, particularly infections, so vigilance is always required. Importantly, documentation and awareness of disease-related damage are crucial in order to avoid overtreatment; damage should not be treated if therapy will not improve it.
ASSESSING DISEASE ACTIVITY AND DAMAGE
Birmingham Vasculitis Activity Score
Introduced in 1994, the Birmingham Vasculitis Activity Score (BVAS) is a single-page checklist that records weighted data on more than 50 items and nine organ systems; the sum of the individual items provides the final score.2 There have been two revisions of the BVAS; one focuses on GPA (BVAS/WG)3 and the other, BVAS version 3 (v.3) is more simplified.4 For all of the BVAS tools, remission is defined as a score of 0. Any score greater than 0 defines active disease. Each system is evaluated as being active or not, with items characterized as more severe being weighted more heavily. The use of the BVAS/WG is illustrated in two patients (see “Assessment with the BVAS/WG,” below).
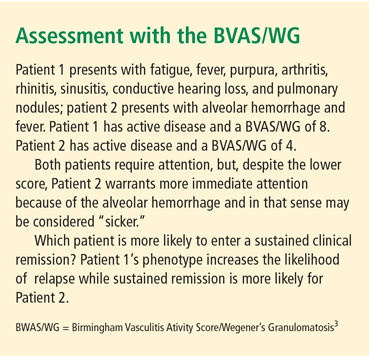
Every major randomized controlled trial in the past 15 years has used the BVAS or one of its derivatives to define outcomes, but primary outcomes were not defined strictly from the BVAS itself. There were important differences in the trials’ definitions of remission, which is the outcome of interest. For example, some trials allow for minor disease activity concurrent with partial remission, while others require full absence of disease activity to achieve “remission.”
Vasculitis Damage Index
The Vasculitis Damage Index (VDI) is a single-page catalog of damage items separated into 11 groupings. Limitations of the index include lack of attribution (to vasculitis, treatment, or comorbidities), gradation, weighting, and patient input (patient-reported outcomes).5 Revisions to the VDI have been made in the ANCA Vasculitis Index of Damage (AVID),6 which incorporates an expanded list of damage items, as well as an even more expanded version called the Combined Damage Assessment Index that combines the items from the VDI and AVID.7 While these tools provide a means to catalog damage by choosing whether an item is present or not, a more data-driven approach to damage assessment is needed that incorporates weighting into the tool.
Damage assessment may be the most important measure in evaluating the patient with vasculitis. In addition to keeping patients alive, one of the main purposes in treating active disease is to prevent damage, maintaining quality of life for the patient for the long term and improving outcomes.
PATIENT-REPORTED OUTCOMES
Patients have a different perspective on their disease than that provided by assessment tools or physicians. Because physician and patient ratings are often disparate, health-related quality of life (HRQOL) is an increasingly important outcome measure for patients as well as regulatory agencies. In a 2010 study, structured patient-reported assessments of burden of disease were obtained from 264 patients with vasculitis in the United States, Germany, and the United Kingdom. Patients ranked items in terms of most frequent burdens of disease. Across ages and countries, patients most commonly rated fatigue/energy loss, pain, musculoskeletal symptoms, and social manifestations as the most severe ramifications of their disease.11 None of the burdens of disease identified in this study are universally measured in the current assessment tools. Patients with active disease had more of the most commonly listed burden-of-disease items; however, patients still suffered from these burdens when the disease was inactive. These disease burden items are therefore mostly dynamic problems and not simply chronic issues.
Patient ratings differ considerably from physician ratings in terms of importance. For example, patients rate nasal manifestations, weight gain, and some chronic pain and fatigue items higher than renal insufficiency and stroke. There is a clear need to address not only physician-ranked issues, but also patient-ranked issues in assessing and treating vasculitis.11
When measuring HRQOL via the Medical Outcomes Study 36-item short-form health survey (SF-36) in patients with vasculitis, a correlation is noted between QOL and sustained remission. In a study by Tomasson et al, QOL was measured using the SF-36 upon treatment following a flare.12 In all patients, SF-36 increased dramatically immediately following treatment but then leveled off over time. In patients who achieved sustained remission, SF-36 scores continued to rise from baseline. In patients who did not achieve a sustained remission, the SF-36 scores did not improve. This QOL measure, therefore, captures a value that other assessments do not, further demonstrating its utility as part of the assessment process.
VALIDATED OUTCOME MEASURES
Outcome Measures in Rheumatology (OMERACT) is an international group of clinicians, trialists, epidemiologists, biostatisticians, health economists, industry executives, and FDA and European Medicines Agency officials who meet every 2 years to promote data-based validation of outcome measures for a variety of diseases. OMERACT endorses core sets of validated outcomes when data demonstrate veracity, discrimination, and feasibility.13 For each domain in the vasculitis arena, there is an associated validated instrument: for disease activity, the validated instruments are the BVAS, BVAS/WG, and BVAS v.3; for damage assessment, the instrument is the VDI; for patient-reported outcomes, the instrument is the SF-36; and finally, for mortality, the instrument is death.13 This core set of measures helps frame how future trials in vasculitis will be standardized and assists in comparing trials, which is particularly important to regulatory agencies.
The tools for disease assessment in vasculitis still need to be refined for activity and damage assessment in order to be more scalable and precise, thereby measuring smaller effects. Patient-reported outcomes and patient perspectives on disease need to be better captured, and reliable biomarkers need to be discovered or further developed. Improved outcome measures must be developed for other types of vasculitis, such as eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome), giant cell (temporal) arteritis, and Takayasu arteritis,14 in order to conduct and report trial results. These outcome measures could also translate into tools that can be used to assess patients and make treatment decisions, thereby helping the clinician at the bedside.
- Seo P, Min YI, Holbrook JT, et al. Damage caused by Wegener’s granulomatosis and its treatment: prospective data from the Wegener’s Granulomatosis Etanercept Trial (WGET). Arthritis Rheum 2005; 52:2168–2178.
- Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM 1994; 87:671–678.
- Stone JH, Hoffman GS, Merkel PA, et al. A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheum 2001; 44:912–920.
- Mukhtyar C, Lee R, Brown D, et al. Modification and valid ation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis 2009; 68:1827–1832.
- Exley AR, Bacon PA, Luqmani RA, et al. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum 1997; 40:371–380.
- Seo P, Luqmani RA, Flossmann O, et al. The future of damage assessment in vasculitis. J Rheumatol 2007; 34:1357–1371.
- Seo P, Jayne D, Luqmani R, Merkel PA. Assessment of damage in vasculitis: expert ratings of damage. Rheumatology (Oxford) 2009; 48:823–827.
- de Groot K, Gross WL, Herlyn K, Reinhold-Keller E. Development and validation of a disease extent index for Wegener’s granulomatosis. Clin Nephrol 2001; 55:31–38.
- Guillevin L, Pagnoux C, Seror R, Mahr A, Mouthon L, Le Toumelin P; French Vasculitis Study Group (FVSG). The Five-Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine (Baltimore) 2011; 90:19–27.
- Merkel PA, Cuthbertson DD, Hellmich B, et al. Comparison of disease activity measures for anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis. Ann Rheum Dis 2009; 68:103–106.
- Herlyn K, Hellmich B, Seo P, Merkel PA. Patient-reported outcome assessment in vasculitis may provide important data and a unique perspective. Arthritis Care Res (Hoboken) 2010; 62:1639–1645.
- Tomasson G, Boers M, Walsh M, et al. Assessment of health related quality of life as an outcome measure in granulomatosis with polyangiitis (Wegener’s). Arthritis Care Res (Hoboken) 2012; 64:273–279.
- Merkel PA, Aydin SZ, Boers M, et al .The OMERACT core set of outcome measures for use in clinical trials of ANCA-associated vasculitis. J Rheumatol 2011; 38:1480–1486.
- Direskeneli H, Aydin SZ, Kermani TA, et al. Development of outcome measures for large-vessel vasculitis for use in clinical trials: opportunities, challenges, and research agenda. J Rheumatol 2011; 38:1471–1479.
Small-vessel vasculitides are complex diseases with highly variable clinical features and are associated with considerable morbidity and mortality. These systemic, multisystem, multiorgan diseases often threaten vital organs with manifestations that include upper airway disease, pulmonary disease, glomerulonephritis, neuropathy, arthritis/arthralgias, malaise/fatigue, eye disease, skin/mucosa irregularities, vascular disease, cardiac disease, and gastrointestinal disease.
Accurate assessment of the patient with vasculitis is a challenge for the clinician and is critical for managing therapeutic interventions throughout the course of the disease. Effective management includes repeated evaluations of the activity and severity of the disease as well as the damage it has caused. These distinct yet overlapping concepts must be measured separately but evaluated as a whole. Additional categorizations of disease course, such as whether it is active (new-onset, persistent, or flare) or in remission, further define the disease and are routinely employed in guiding treatment choices.
The importance of accurately assessing a patient’s clinical status is clear, but it is also important to standardize and quantify vasculitis assessment tools for use in clinical trials. Standardized assessments are needed to:
- Guide clinical trial enrollment criteria
- Describe and compare study populations
- Quantify and measure treatment effectiveness
- Describe long-term outcomes
- Translate standardized assessment tools into clinical practice.
Over the past few decades, improvements in clinical research have resulted in increasingly accurate data obtained from well-designed randomized controlled trials, all of which are based on better clinical assessments. Improving the quality of the assessment tools has improved both the interpretation of trial results and translation of findings into clinical practice.
DISEASE ASSESSMENT
When assessing patients with vasculitis, whether clinically or in the context of a clinical trial, it is essential to differentiate among disease activity, damage, and severity:
Disease activity, such as active bleeding or mucosal inflammation, is treatable and potentially responsive to therapy.
Disease damage is generally irreversible and not improved by treating vasculitis. Damage may be caused by the disease itself, its treatment, or a comorbid condition. In general, once damage is identified, it is considered permanent if it remains unchanged for more than 6 months. In the Wegener’s Granulomatosis Etanercept Trial,1 damage that occurred in more than 10% of the cohort included hearing loss; proteinuria (≥ 0.5 g/24 hours); nasal blockage, chronic discharge, or crusting; nasal bridge collapse or septal perforation; glomerular filtration rate at least 50% lower than premorbid baseline; subglottic stenosis; and chronic sinusitis or radiologic damage. Disease-related damage can be addressed; a saddle-nose deformity can respond to plastic surgery, for example, but treating vasculitis will have no effect on the underlying established anatomic defect.
Disease severity assesses the intensity of the disease and guides the clinician in gauging how aggressive the therapy should be.
Vasculitis has two primary disease states: remission and active disease. In remission, there is no evidence of active disease. This is often qualified by describing the remission as either complete or partial; it is further defined by introducing an element of time, such as a “sustained” remission of more than 6 months. Active disease is the presence of any ongoing expression of vasculitis that is not caused by disease damage, comorbidity, or treatment. Active disease can be graded as low, medium, or high; if active disease lasts longer than 6 months, it is described as persistent or sustained. Flare, a manifestation of active disease, describes the transition from remission to active disease and is characterized by worsening of disease activity. Flares are graded as nonsevere or severe.
These descriptions of disease status can be further broken down into whether they are occurring “on” or “off” treatment. All of these elements are important and the subtleties and differences are critical in interpreting data for use in the clinical setting or in clinical trials.
CLINICAL ASSESSMENT
Assessing the status of disease for a patient with granulomatosis with polyangiitis (GPA, Wegener’s granulomatosis [WG]) or microscopic polyangiitis (MPA) begins with a detailed medical history and physical examination every time the patient is seen. Appropriate laboratory assessments include a complete blood count, tests of renal function, acute phase reactants (possibly as disease markers, but not necessarily to guide therapy), and other laboratory tests as needed. Controversy exists regarding the role of antineutrophil cytoplasmic antibody (ANCA) testing in assessment of disease activity.
Urinalyses are key for assessing activity; if a urine dipstick result is positive, a subsequent microscopic examination should be conducted. Microscopic review may demonstrate red cell casts that a routine laboratory check may not reveal. In addition to spotting de novo hematuria, looking for a change in dipstick results may prove valuable, since hematuria may increase in patients in whom persistent hematuria has already been noted. The change may be due to renal disease from the vasculitis, cyclophosphamide-induced bladder toxicity, a kidney stone, menses, or a variety of other causes, but if the hematuria is not monitored, a key assessment will be missed.
Disease staging through diagnostic imaging of the sinuses, neck, and chest should be performed on a regular basis as appropriate, beginning at the patient’s initial visit. Restaging, in much the same way as an oncologist restages cancer, should take place regularly, because this informs whether to make a major change in therapy (eg, from cyclophosphamide, azathioprine, or rituximab). Restaging will also allow benchmarking of old, new, and changed damage so that when the disease recurs, the existing damage can be differentiated from new lesions. Once the disease has stabilized, imaging can be discontinued.
Consultations with otolaryngologists, ophthalmologists, cardiologists, and other specialists should be sought as needed. Serial audiograms, laryngoscopy, echocardiograms, and other appropriate tests should be performed as required. Biopsies are useful for assessment of patients, particularly at diagnosis, but also when it becomes necessary to reassess the progress of a patient’s disease or to identify a potential infection versus a possible malignancy. Biopsy is particularly helpful for kidney disease. If of active disease, then repeat biopsy is appropriate to determine whether the deterioration is associated with persistent active disease, the natural history of declining kidney function, or another cause. Patients with vasculitis may develop new comorbidities, particularly infections, so vigilance is always required. Importantly, documentation and awareness of disease-related damage are crucial in order to avoid overtreatment; damage should not be treated if therapy will not improve it.
ASSESSING DISEASE ACTIVITY AND DAMAGE
Birmingham Vasculitis Activity Score
Introduced in 1994, the Birmingham Vasculitis Activity Score (BVAS) is a single-page checklist that records weighted data on more than 50 items and nine organ systems; the sum of the individual items provides the final score.2 There have been two revisions of the BVAS; one focuses on GPA (BVAS/WG)3 and the other, BVAS version 3 (v.3) is more simplified.4 For all of the BVAS tools, remission is defined as a score of 0. Any score greater than 0 defines active disease. Each system is evaluated as being active or not, with items characterized as more severe being weighted more heavily. The use of the BVAS/WG is illustrated in two patients (see “Assessment with the BVAS/WG,” below).

Every major randomized controlled trial in the past 15 years has used the BVAS or one of its derivatives to define outcomes, but primary outcomes were not defined strictly from the BVAS itself. There were important differences in the trials’ definitions of remission, which is the outcome of interest. For example, some trials allow for minor disease activity concurrent with partial remission, while others require full absence of disease activity to achieve “remission.”
Vasculitis Damage Index
The Vasculitis Damage Index (VDI) is a single-page catalog of damage items separated into 11 groupings. Limitations of the index include lack of attribution (to vasculitis, treatment, or comorbidities), gradation, weighting, and patient input (patient-reported outcomes).5 Revisions to the VDI have been made in the ANCA Vasculitis Index of Damage (AVID),6 which incorporates an expanded list of damage items, as well as an even more expanded version called the Combined Damage Assessment Index that combines the items from the VDI and AVID.7 While these tools provide a means to catalog damage by choosing whether an item is present or not, a more data-driven approach to damage assessment is needed that incorporates weighting into the tool.
Damage assessment may be the most important measure in evaluating the patient with vasculitis. In addition to keeping patients alive, one of the main purposes in treating active disease is to prevent damage, maintaining quality of life for the patient for the long term and improving outcomes.
PATIENT-REPORTED OUTCOMES
Patients have a different perspective on their disease than that provided by assessment tools or physicians. Because physician and patient ratings are often disparate, health-related quality of life (HRQOL) is an increasingly important outcome measure for patients as well as regulatory agencies. In a 2010 study, structured patient-reported assessments of burden of disease were obtained from 264 patients with vasculitis in the United States, Germany, and the United Kingdom. Patients ranked items in terms of most frequent burdens of disease. Across ages and countries, patients most commonly rated fatigue/energy loss, pain, musculoskeletal symptoms, and social manifestations as the most severe ramifications of their disease.11 None of the burdens of disease identified in this study are universally measured in the current assessment tools. Patients with active disease had more of the most commonly listed burden-of-disease items; however, patients still suffered from these burdens when the disease was inactive. These disease burden items are therefore mostly dynamic problems and not simply chronic issues.
Patient ratings differ considerably from physician ratings in terms of importance. For example, patients rate nasal manifestations, weight gain, and some chronic pain and fatigue items higher than renal insufficiency and stroke. There is a clear need to address not only physician-ranked issues, but also patient-ranked issues in assessing and treating vasculitis.11
When measuring HRQOL via the Medical Outcomes Study 36-item short-form health survey (SF-36) in patients with vasculitis, a correlation is noted between QOL and sustained remission. In a study by Tomasson et al, QOL was measured using the SF-36 upon treatment following a flare.12 In all patients, SF-36 increased dramatically immediately following treatment but then leveled off over time. In patients who achieved sustained remission, SF-36 scores continued to rise from baseline. In patients who did not achieve a sustained remission, the SF-36 scores did not improve. This QOL measure, therefore, captures a value that other assessments do not, further demonstrating its utility as part of the assessment process.
VALIDATED OUTCOME MEASURES
Outcome Measures in Rheumatology (OMERACT) is an international group of clinicians, trialists, epidemiologists, biostatisticians, health economists, industry executives, and FDA and European Medicines Agency officials who meet every 2 years to promote data-based validation of outcome measures for a variety of diseases. OMERACT endorses core sets of validated outcomes when data demonstrate veracity, discrimination, and feasibility.13 For each domain in the vasculitis arena, there is an associated validated instrument: for disease activity, the validated instruments are the BVAS, BVAS/WG, and BVAS v.3; for damage assessment, the instrument is the VDI; for patient-reported outcomes, the instrument is the SF-36; and finally, for mortality, the instrument is death.13 This core set of measures helps frame how future trials in vasculitis will be standardized and assists in comparing trials, which is particularly important to regulatory agencies.
The tools for disease assessment in vasculitis still need to be refined for activity and damage assessment in order to be more scalable and precise, thereby measuring smaller effects. Patient-reported outcomes and patient perspectives on disease need to be better captured, and reliable biomarkers need to be discovered or further developed. Improved outcome measures must be developed for other types of vasculitis, such as eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome), giant cell (temporal) arteritis, and Takayasu arteritis,14 in order to conduct and report trial results. These outcome measures could also translate into tools that can be used to assess patients and make treatment decisions, thereby helping the clinician at the bedside.
Small-vessel vasculitides are complex diseases with highly variable clinical features and are associated with considerable morbidity and mortality. These systemic, multisystem, multiorgan diseases often threaten vital organs with manifestations that include upper airway disease, pulmonary disease, glomerulonephritis, neuropathy, arthritis/arthralgias, malaise/fatigue, eye disease, skin/mucosa irregularities, vascular disease, cardiac disease, and gastrointestinal disease.
Accurate assessment of the patient with vasculitis is a challenge for the clinician and is critical for managing therapeutic interventions throughout the course of the disease. Effective management includes repeated evaluations of the activity and severity of the disease as well as the damage it has caused. These distinct yet overlapping concepts must be measured separately but evaluated as a whole. Additional categorizations of disease course, such as whether it is active (new-onset, persistent, or flare) or in remission, further define the disease and are routinely employed in guiding treatment choices.
The importance of accurately assessing a patient’s clinical status is clear, but it is also important to standardize and quantify vasculitis assessment tools for use in clinical trials. Standardized assessments are needed to:
- Guide clinical trial enrollment criteria
- Describe and compare study populations
- Quantify and measure treatment effectiveness
- Describe long-term outcomes
- Translate standardized assessment tools into clinical practice.
Over the past few decades, improvements in clinical research have resulted in increasingly accurate data obtained from well-designed randomized controlled trials, all of which are based on better clinical assessments. Improving the quality of the assessment tools has improved both the interpretation of trial results and translation of findings into clinical practice.
DISEASE ASSESSMENT
When assessing patients with vasculitis, whether clinically or in the context of a clinical trial, it is essential to differentiate among disease activity, damage, and severity:
Disease activity, such as active bleeding or mucosal inflammation, is treatable and potentially responsive to therapy.
Disease damage is generally irreversible and not improved by treating vasculitis. Damage may be caused by the disease itself, its treatment, or a comorbid condition. In general, once damage is identified, it is considered permanent if it remains unchanged for more than 6 months. In the Wegener’s Granulomatosis Etanercept Trial,1 damage that occurred in more than 10% of the cohort included hearing loss; proteinuria (≥ 0.5 g/24 hours); nasal blockage, chronic discharge, or crusting; nasal bridge collapse or septal perforation; glomerular filtration rate at least 50% lower than premorbid baseline; subglottic stenosis; and chronic sinusitis or radiologic damage. Disease-related damage can be addressed; a saddle-nose deformity can respond to plastic surgery, for example, but treating vasculitis will have no effect on the underlying established anatomic defect.
Disease severity assesses the intensity of the disease and guides the clinician in gauging how aggressive the therapy should be.
Vasculitis has two primary disease states: remission and active disease. In remission, there is no evidence of active disease. This is often qualified by describing the remission as either complete or partial; it is further defined by introducing an element of time, such as a “sustained” remission of more than 6 months. Active disease is the presence of any ongoing expression of vasculitis that is not caused by disease damage, comorbidity, or treatment. Active disease can be graded as low, medium, or high; if active disease lasts longer than 6 months, it is described as persistent or sustained. Flare, a manifestation of active disease, describes the transition from remission to active disease and is characterized by worsening of disease activity. Flares are graded as nonsevere or severe.
These descriptions of disease status can be further broken down into whether they are occurring “on” or “off” treatment. All of these elements are important and the subtleties and differences are critical in interpreting data for use in the clinical setting or in clinical trials.
CLINICAL ASSESSMENT
Assessing the status of disease for a patient with granulomatosis with polyangiitis (GPA, Wegener’s granulomatosis [WG]) or microscopic polyangiitis (MPA) begins with a detailed medical history and physical examination every time the patient is seen. Appropriate laboratory assessments include a complete blood count, tests of renal function, acute phase reactants (possibly as disease markers, but not necessarily to guide therapy), and other laboratory tests as needed. Controversy exists regarding the role of antineutrophil cytoplasmic antibody (ANCA) testing in assessment of disease activity.
Urinalyses are key for assessing activity; if a urine dipstick result is positive, a subsequent microscopic examination should be conducted. Microscopic review may demonstrate red cell casts that a routine laboratory check may not reveal. In addition to spotting de novo hematuria, looking for a change in dipstick results may prove valuable, since hematuria may increase in patients in whom persistent hematuria has already been noted. The change may be due to renal disease from the vasculitis, cyclophosphamide-induced bladder toxicity, a kidney stone, menses, or a variety of other causes, but if the hematuria is not monitored, a key assessment will be missed.
Disease staging through diagnostic imaging of the sinuses, neck, and chest should be performed on a regular basis as appropriate, beginning at the patient’s initial visit. Restaging, in much the same way as an oncologist restages cancer, should take place regularly, because this informs whether to make a major change in therapy (eg, from cyclophosphamide, azathioprine, or rituximab). Restaging will also allow benchmarking of old, new, and changed damage so that when the disease recurs, the existing damage can be differentiated from new lesions. Once the disease has stabilized, imaging can be discontinued.
Consultations with otolaryngologists, ophthalmologists, cardiologists, and other specialists should be sought as needed. Serial audiograms, laryngoscopy, echocardiograms, and other appropriate tests should be performed as required. Biopsies are useful for assessment of patients, particularly at diagnosis, but also when it becomes necessary to reassess the progress of a patient’s disease or to identify a potential infection versus a possible malignancy. Biopsy is particularly helpful for kidney disease. If of active disease, then repeat biopsy is appropriate to determine whether the deterioration is associated with persistent active disease, the natural history of declining kidney function, or another cause. Patients with vasculitis may develop new comorbidities, particularly infections, so vigilance is always required. Importantly, documentation and awareness of disease-related damage are crucial in order to avoid overtreatment; damage should not be treated if therapy will not improve it.
ASSESSING DISEASE ACTIVITY AND DAMAGE
Birmingham Vasculitis Activity Score
Introduced in 1994, the Birmingham Vasculitis Activity Score (BVAS) is a single-page checklist that records weighted data on more than 50 items and nine organ systems; the sum of the individual items provides the final score.2 There have been two revisions of the BVAS; one focuses on GPA (BVAS/WG)3 and the other, BVAS version 3 (v.3) is more simplified.4 For all of the BVAS tools, remission is defined as a score of 0. Any score greater than 0 defines active disease. Each system is evaluated as being active or not, with items characterized as more severe being weighted more heavily. The use of the BVAS/WG is illustrated in two patients (see “Assessment with the BVAS/WG,” below).

Every major randomized controlled trial in the past 15 years has used the BVAS or one of its derivatives to define outcomes, but primary outcomes were not defined strictly from the BVAS itself. There were important differences in the trials’ definitions of remission, which is the outcome of interest. For example, some trials allow for minor disease activity concurrent with partial remission, while others require full absence of disease activity to achieve “remission.”
Vasculitis Damage Index
The Vasculitis Damage Index (VDI) is a single-page catalog of damage items separated into 11 groupings. Limitations of the index include lack of attribution (to vasculitis, treatment, or comorbidities), gradation, weighting, and patient input (patient-reported outcomes).5 Revisions to the VDI have been made in the ANCA Vasculitis Index of Damage (AVID),6 which incorporates an expanded list of damage items, as well as an even more expanded version called the Combined Damage Assessment Index that combines the items from the VDI and AVID.7 While these tools provide a means to catalog damage by choosing whether an item is present or not, a more data-driven approach to damage assessment is needed that incorporates weighting into the tool.
Damage assessment may be the most important measure in evaluating the patient with vasculitis. In addition to keeping patients alive, one of the main purposes in treating active disease is to prevent damage, maintaining quality of life for the patient for the long term and improving outcomes.
PATIENT-REPORTED OUTCOMES
Patients have a different perspective on their disease than that provided by assessment tools or physicians. Because physician and patient ratings are often disparate, health-related quality of life (HRQOL) is an increasingly important outcome measure for patients as well as regulatory agencies. In a 2010 study, structured patient-reported assessments of burden of disease were obtained from 264 patients with vasculitis in the United States, Germany, and the United Kingdom. Patients ranked items in terms of most frequent burdens of disease. Across ages and countries, patients most commonly rated fatigue/energy loss, pain, musculoskeletal symptoms, and social manifestations as the most severe ramifications of their disease.11 None of the burdens of disease identified in this study are universally measured in the current assessment tools. Patients with active disease had more of the most commonly listed burden-of-disease items; however, patients still suffered from these burdens when the disease was inactive. These disease burden items are therefore mostly dynamic problems and not simply chronic issues.
Patient ratings differ considerably from physician ratings in terms of importance. For example, patients rate nasal manifestations, weight gain, and some chronic pain and fatigue items higher than renal insufficiency and stroke. There is a clear need to address not only physician-ranked issues, but also patient-ranked issues in assessing and treating vasculitis.11
When measuring HRQOL via the Medical Outcomes Study 36-item short-form health survey (SF-36) in patients with vasculitis, a correlation is noted between QOL and sustained remission. In a study by Tomasson et al, QOL was measured using the SF-36 upon treatment following a flare.12 In all patients, SF-36 increased dramatically immediately following treatment but then leveled off over time. In patients who achieved sustained remission, SF-36 scores continued to rise from baseline. In patients who did not achieve a sustained remission, the SF-36 scores did not improve. This QOL measure, therefore, captures a value that other assessments do not, further demonstrating its utility as part of the assessment process.
VALIDATED OUTCOME MEASURES
Outcome Measures in Rheumatology (OMERACT) is an international group of clinicians, trialists, epidemiologists, biostatisticians, health economists, industry executives, and FDA and European Medicines Agency officials who meet every 2 years to promote data-based validation of outcome measures for a variety of diseases. OMERACT endorses core sets of validated outcomes when data demonstrate veracity, discrimination, and feasibility.13 For each domain in the vasculitis arena, there is an associated validated instrument: for disease activity, the validated instruments are the BVAS, BVAS/WG, and BVAS v.3; for damage assessment, the instrument is the VDI; for patient-reported outcomes, the instrument is the SF-36; and finally, for mortality, the instrument is death.13 This core set of measures helps frame how future trials in vasculitis will be standardized and assists in comparing trials, which is particularly important to regulatory agencies.
The tools for disease assessment in vasculitis still need to be refined for activity and damage assessment in order to be more scalable and precise, thereby measuring smaller effects. Patient-reported outcomes and patient perspectives on disease need to be better captured, and reliable biomarkers need to be discovered or further developed. Improved outcome measures must be developed for other types of vasculitis, such as eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome), giant cell (temporal) arteritis, and Takayasu arteritis,14 in order to conduct and report trial results. These outcome measures could also translate into tools that can be used to assess patients and make treatment decisions, thereby helping the clinician at the bedside.
- Seo P, Min YI, Holbrook JT, et al. Damage caused by Wegener’s granulomatosis and its treatment: prospective data from the Wegener’s Granulomatosis Etanercept Trial (WGET). Arthritis Rheum 2005; 52:2168–2178.
- Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM 1994; 87:671–678.
- Stone JH, Hoffman GS, Merkel PA, et al. A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheum 2001; 44:912–920.
- Mukhtyar C, Lee R, Brown D, et al. Modification and valid ation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis 2009; 68:1827–1832.
- Exley AR, Bacon PA, Luqmani RA, et al. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum 1997; 40:371–380.
- Seo P, Luqmani RA, Flossmann O, et al. The future of damage assessment in vasculitis. J Rheumatol 2007; 34:1357–1371.
- Seo P, Jayne D, Luqmani R, Merkel PA. Assessment of damage in vasculitis: expert ratings of damage. Rheumatology (Oxford) 2009; 48:823–827.
- de Groot K, Gross WL, Herlyn K, Reinhold-Keller E. Development and validation of a disease extent index for Wegener’s granulomatosis. Clin Nephrol 2001; 55:31–38.
- Guillevin L, Pagnoux C, Seror R, Mahr A, Mouthon L, Le Toumelin P; French Vasculitis Study Group (FVSG). The Five-Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine (Baltimore) 2011; 90:19–27.
- Merkel PA, Cuthbertson DD, Hellmich B, et al. Comparison of disease activity measures for anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis. Ann Rheum Dis 2009; 68:103–106.
- Herlyn K, Hellmich B, Seo P, Merkel PA. Patient-reported outcome assessment in vasculitis may provide important data and a unique perspective. Arthritis Care Res (Hoboken) 2010; 62:1639–1645.
- Tomasson G, Boers M, Walsh M, et al. Assessment of health related quality of life as an outcome measure in granulomatosis with polyangiitis (Wegener’s). Arthritis Care Res (Hoboken) 2012; 64:273–279.
- Merkel PA, Aydin SZ, Boers M, et al .The OMERACT core set of outcome measures for use in clinical trials of ANCA-associated vasculitis. J Rheumatol 2011; 38:1480–1486.
- Direskeneli H, Aydin SZ, Kermani TA, et al. Development of outcome measures for large-vessel vasculitis for use in clinical trials: opportunities, challenges, and research agenda. J Rheumatol 2011; 38:1471–1479.
- Seo P, Min YI, Holbrook JT, et al. Damage caused by Wegener’s granulomatosis and its treatment: prospective data from the Wegener’s Granulomatosis Etanercept Trial (WGET). Arthritis Rheum 2005; 52:2168–2178.
- Luqmani RA, Bacon PA, Moots RJ, et al. Birmingham Vasculitis Activity Score (BVAS) in systemic necrotizing vasculitis. QJM 1994; 87:671–678.
- Stone JH, Hoffman GS, Merkel PA, et al. A disease-specific activity index for Wegener’s granulomatosis: modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheum 2001; 44:912–920.
- Mukhtyar C, Lee R, Brown D, et al. Modification and valid ation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis 2009; 68:1827–1832.
- Exley AR, Bacon PA, Luqmani RA, et al. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum 1997; 40:371–380.
- Seo P, Luqmani RA, Flossmann O, et al. The future of damage assessment in vasculitis. J Rheumatol 2007; 34:1357–1371.
- Seo P, Jayne D, Luqmani R, Merkel PA. Assessment of damage in vasculitis: expert ratings of damage. Rheumatology (Oxford) 2009; 48:823–827.
- de Groot K, Gross WL, Herlyn K, Reinhold-Keller E. Development and validation of a disease extent index for Wegener’s granulomatosis. Clin Nephrol 2001; 55:31–38.
- Guillevin L, Pagnoux C, Seror R, Mahr A, Mouthon L, Le Toumelin P; French Vasculitis Study Group (FVSG). The Five-Factor Score revisited: assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine (Baltimore) 2011; 90:19–27.
- Merkel PA, Cuthbertson DD, Hellmich B, et al. Comparison of disease activity measures for anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis. Ann Rheum Dis 2009; 68:103–106.
- Herlyn K, Hellmich B, Seo P, Merkel PA. Patient-reported outcome assessment in vasculitis may provide important data and a unique perspective. Arthritis Care Res (Hoboken) 2010; 62:1639–1645.
- Tomasson G, Boers M, Walsh M, et al. Assessment of health related quality of life as an outcome measure in granulomatosis with polyangiitis (Wegener’s). Arthritis Care Res (Hoboken) 2012; 64:273–279.
- Merkel PA, Aydin SZ, Boers M, et al .The OMERACT core set of outcome measures for use in clinical trials of ANCA-associated vasculitis. J Rheumatol 2011; 38:1480–1486.
- Direskeneli H, Aydin SZ, Kermani TA, et al. Development of outcome measures for large-vessel vasculitis for use in clinical trials: opportunities, challenges, and research agenda. J Rheumatol 2011; 38:1471–1479.
Upper airway manifestations of granulomatosis with polyangiitis
The head and neck are the most common sites of involvement at initial presentation of granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]). Head and neck manifestations occur initially in 73% of patients, and eventually, up to 92% of patients with GPA are affected.1 Many of these compromise the upper airway. Although treatment is multidisciplinary, the effects on the airway make it important to understand upper airway presentations and treatments. This article examines upper airway disease presentations, their assessment, and their advocated interventions.
DISEASE COURSE
Because head and neck involvement may be associated with a less aggressive form of GPA, outcomes for patients with predominantly head and neck involvement may be better compared with those who have involvement of other systems.2
The natural course of GPA may be indolent or rapidly progressive. Regardless, left untreated, it progresses to a generalized systemic disease that often leads to significant morbidity and likely mortality. Most patients (96%) achieve remission with immunosuppressive therapy, but nearly half (49%) have at least one relapse.1 For this reason, systemic immunosuppressive medications play a dominant role in systemic and localized head and neck disease control. Patients often require maintenance medications along with additional therapies during disease exacerbation.3 Therefore, key partnerships between internists, rheumatologists, and otolaryngologists are paramount in the treatment and follow-up of these patients.
DIAGNOSIS: MAINSTAY IS SEROLOGIC EVALUATION
The differential diagnosis of GPA includes infection, lymphoproliferative disease (T-cell lymphoma), systemic lupus erythematosus, rheumatoid arthritis, sarcoidosis, and other granulomatous diseases such as eosinophilic GPA (Churg-Strauss syndrome), polyarteritis nodosa, and microscopic polyangiitis. Appropriate diagnosis is critical because treatment of these entities varies drastically.
The mainstay of GPA diagnosis is serologic evaluation for a cytoplasmic pattern of antineutrophil cytoplasmic antibodies (cANCA), which are reactive toward proteinase-3 (PR3) or myeloperoxidase (MPO). Testing for cANCA yields a pooled sensitivity of 91% and specificity of 99%. Sensitivity falls significantly (63%) when the disease is in nonacute stages, while the specificity remains high.4 These cANCA test characteristics allow a high positive predictive value for this rare disease.
Biopsy is typically reserved for cases in which serologic ANCA testing is nondiagnostic. Biopsy tissue may be readily accessible from the head and neck, but these biopsies may bear significant false-negative rates.4–6 Diagnosis requires demonstration of palisading granulomas as vascular or extravascular lesions within the upper respiratory tract tissues. The specific site biopsied from within the head and neck has been shown to influence diagnostic yield, with sinonasal biopsies producing the highest yield.
SINONASAL MANIFESTATIONS
The nose and paranasal sinuses are the most frequently affected sites in the head and neck, noted in 64% to 80% of patients. Additionally, the nose is the only site of involvement in 30% of patients.7 Given the high frequency of sinonasal manifestations, GPA should be considered as a potential diagnosis among patients with persistent sinonasal disease.
Pathophysiology and disease course
The pathophysiologic mechanisms leading to the changes in the sinonasal tract in GPA have not been established. GPA is believed to be an immunologic disease that manifests as a vasculitis of small- and medium-sized vessels. Multiple potential causative factors have been identified, including fibrinoid necrosis of small blood vessels, epithelial granulomas, chronic inflammation, and prior surgical intervention.8,9 The acute and chronic inflammation, coupled with the epithelioid granuloma formation, damages adjacent small- to medium-sized vessels. The vasculitis leads to diminished blood flow and subsequent avascular necrosis, which may promote tissue necrosis and bone destruction. This destructive process typically starts in the midseptum supplied by Kiesselbach plexus and in the turbinates. The process then eventually spreads to the paranasal sinuses.8
Patient evaluation
Examination of the nasal cavities is typically performed by rigid or flexible nasal endoscopy and often reveals nasal crusting, friable erythematous mucosa, granulation, and even signs of sinusitis. All or part of the cartilaginous septum may be involved, leading to significant septal defects. As the degree of cartilage destruction increases, nasal dorsal support decreases, leading to a visible depression of the external nose known as a “saddle-nose” deformity, which is present in 23% of patients with GPA.7,10
Imaging assessment by computed tomography (CT) is needed to establish disease extent and involvement. Atypical findings may include bony erosion and destruction of the septum and turbinates; erosion of bony partitions within the ethmoid sinuses; neo-osteogenesis of the maxillary, frontal, and sphenoid sinuses; and complete bony obliteration of the maxillary, frontal, and sphenoid sinuses.9,11
Clinical presentation
Sinonasal disease indicates the degree of disease activity.12 Clinical findings may vary, but they have a significant impact on quality of life in these patients.13 Most patients with active disease present with nasal crusting (69%), chronic rhinosinusitis (CRS) symptoms (61%), nasal obstruction (58%), and serosanguinous nasal discharge (52%).10 Patients may also complain of foul-smelling rhinorrhea, recurrent epistaxis, hyposmia, anosmia, and epiphora (from granulomatous compression or obstruction of the lacrimal system). In a series of 120 patients with GPA, Cannady et al found that four (3.3%) patients had mucoceles and three (2.5%) had orbital pseudotumor.10
Any structure in the sinonasal cavity, including mucosa, septum, turbinates, and sinuses proper, may be affected because of the vasculitic involvement of mucosal blood vessels that causes diminished blood flow and subsequent necrosis. The area of the anterior septum supplied by Kiesselbach plexus is the most common site of active nasal disease, which can eventually lead to the common presentation of an anterior nasal septal perforation.
Otologic disease secondary to sinonasal GPA
Otologic involvement is observed in 19% to 38% of patients with GPA.14,15 Most patients with GPA who exhibit otologic symptoms have middle ear or mastoid disease. It typically appears as chronic otitis media (COM) with conductive hearing loss.16 In most cases, the otologic involvement is secondary to Eustachian tube dysfunction caused by the presence of extensive disease in the nasopharynx.
Additionally, chronic mastoiditis can result from direct mastoid involvement with GPA. Facial nerve palsy secondary to infective bony destruction is a rare but repeatedly reported complication of GPA.14,15
Inner ear involvement is a relatively common otologic presentation of GPA. Patients may experience sensorineural hearing loss (SNHL) as well as vertigo, which may mimic Cogan syndrome. Importantly, patients may exhibit inner ear involvement with or without middle ear and mastoid disease. The SNHL observed in patients with GPA may be responsive to steroid or immunosuppressive therapy.
Treatment
Refractory CRS in GPA is a complex problem for which aggressive surgical intervention is often counterproductive. Unfortunately, traditional medical therapies are also often inadequate to treat progressive sinonasal symptomatology. As the nasal tissue becomes devascularized, loss of normal mucociliary function aggravates the sinus pathology, and clinical symptoms may worsen. Simple antibiotic regimens used to manage uncomplicated sinusitis are often inadequate in these patients. The subsequent progression to frank necrosis in localized regions creates an intranasal foreign body, allowing bacterial colonization, which is often refractory to antibiotics because of the inability of drug tissue penetration into these devascularized nasal structures.12,17
Medical management must be tailored to be effective in this complex intranasal milieu. Successful treatment requires a multifaceted and often prolonged treatment course. A high index of suspicion should be maintained for Staphylococcus aureus. As a rule, endoscopically obtained cultures should be used to guide antibiotic selection. Several weeks of culture-directed antibiotics followed by topical antibiotic irrigations (eg, mupirocin irrigations) can be useful to reduce the frequency of sinonasal exacerbations.
Frequent saline irrigations using high-volume, high-flow irrigation devices (as opposed to low-volume, low-flow applicators such as nasal spray bottles) can be an excellent adjunct to maintenance therapy and are effective in clearing debris and augmenting mucociliary clearance in affected nasal cavities and those with septal perforations. Occasional in-office endoscopic debridement of large crusts adherent to intranasal structures or the edges of a septal perforation can also help to improve obstructive symptoms.
Surgery for refractory cases. Surgery should be reserved for refractory cases unresponsive to maximal medical efforts or those cases with impending complications (ie, mucoceles). Overall, only 16% of patients with sinonasal GPA required surgical intervention in a large series of 120 patients at our institution. In this series, one-third of all patients had undergone previous functional nasal surgery at an outside institution without resolution of symptoms. Anecdotal evidence suggests that surgery for GPA can contribute to additional scarring and lead to protracted sinonasal symptoms.10,18
The decision to perform surgery is individualized and based on severity of the disease process, patient expectations, and surgeon expertise. In our experience at Cleveland Clinic, functional endoscopic sinus surgery in the setting of GPA is a surgical challenge, given extensive alteration of the sinonasal anatomy from previous surgery, prior and ongoing inflammation, chronic crusting, and scarring. Consequently, it has been our practice to employ conservative efforts prior to consideration of surgery. A complete surgical cure is exceedingly rare, and the patient should be counseled about the possible need for revision surgery and ongoing nonsurgical therapies. Meticulous postoperative care with weekly postoperative debridement, saline or antibiotic irrigations, and culture-directed antibiotics, is essential during the early postoperative recovery phase.
Management of epiphora. The most common ophthalmologic findings in patients with GPA include chronic epiphora and orbital pseudotumor. With the advent of advanced endoscopic techniques, the otolaryngologist plays a greater role in the surgical management of these ophthalmologic disease entities. In a series reported by Cannady et al,10 endoscopic dacrocystorhinostomy was performed successfully in seven patients, including one revision.
Nasal reconstruction for saddle-nose deformity: effective in selected patients. The progressive loss of septal support that occurs with the enlarging anterior septal perforation often results in significant collapse of the cartilaginous midvault of the nose. The tip cartilages in turn also begin to lose projection, resulting in a shortened nose with the characteristic saddle-nose deformity. The psychologic impact of this disfiguring facial abnormality is significant. The loss of midvault support also results in worsening nasal obstruction and increases the incidence of anosmia as the superior nasal vault becomes obstructed. For these reasons, patients often seek referral for potential reconstruction.
Despite the potential benefits, the general consensus in the medical community has been that surgical procedures on the nose should be avoided in GPA patients.17 Most nasal destruction in these patients is the consequence of poor tissue perfusion from the active vasculitis. Poor wound healing, reconstructive graft resorption, and worsening necrosis have been observed in patients who have undergone ill-advised surgical procedures.
These poor outcomes do not, however, preclude the potential for safe and effective surgical intervention. In three small published series, good surgical outcomes were achieved but the procedures were done in very highly selected patients and were modified to address the specific clinical issues seen in GPA patients.19–21 The critical step in achieving a good outcome is working closely with the patient’s rheumatologist to identify an appropriate clinical window during which the patient’s disease process is in a period of relative remission. The second major factor is to modify the surgical techniques to take into account the very poor vascular framework of the recipient nasal bed.
Management of COM. Because the COM in patients with GPA is frequently secondary to nasopharyngeal disease, systemic control of GPA is the first priority. Systemic control is also the first-line treatment for patients with mixed or sensorineural hearing loss, or with vertigo. For continued or symptomatic middle ear effusions that do not resolve with systemic therapy, placement of a ventilation tube may be considered. In patients with significant hearing loss, hearing amplification devices may be warranted.15,22 Cochlear implant devices in GPA patients are experimental and may pose undue risks of meningitis.
SUBGLOTTIC STENOSIS AND TRACHEAL MANIFESTATIONS
Subglottic stenosis affects 10% to 20% of patients with GPA.1,23,24 Because of its potential life-threatening airway complications, patients should be carefully assessed for this disease manifestation. It may be the only manifestation of GPA or may be part of a spectrum of other disease manifestations. Therefore, the work-up for subglottic stenosis of unknown etiology should always include an evaluation for GPA.
Pathophysiology and disease course
The etiology of subglottic stenosis in GPA is not well understood. Theories primarily involve the vulnerability of the subglottic tissues to damage, chronic inflammation, and scarring.25 The combination of vasculitis in the setting of active inflammation may synergistically produce a hyperactive reparative mechanism in GPA patients that leads to cartilaginous fibrotic scarring and stenosis. Wound healing can be divided into the phases of inflammation, proliferation, and remodeling. An imbalance or exaggerated response of any of these levels (and likely all) produces an abnormal healing response.26 Similarly, each of these phases may be targeted to improve the healing process.
Patient evaluation
The presence of subglottic stenosis must be considered in a GPA patient with respiratory symptoms. As part of the routine initial evaluation, an office-based nasopharyngeal/laryngeal endoscopy using a flexible laryngoscope should be performed to assess the presence and severity of luminal airway narrowing. Flexible laryngoscopy reveals a circumferential narrowing of the subglottis. The stenotic tissue may vary from friable with erythematous and inflamed mucosa to a rigid mature fibrotic band, depending on the inflammatory state of the stenosis.18,27
Subclinical stenosis may be identified with routine endoscopy. An appropriate baseline is needed to follow the progression of disease and to adjust the timing of any potential intervention. The ability to digitally record a patient’s examination allows further tracking of disease and is commonly used in our practices.
Although flexible fiberoptic examination is critical in diagnosis and follow-up, intraoperative direct laryngoscopy using rigid laryngoscopes and telescopes provides the optimum view of the subglottis. In particular, this view provides greater information on the length and degree of the stenosis and allows evaluation of potential stenotic segments in the inferior trachea.
Spiral CT with 3-dimensional reconstruction of the laryngotracheal lumen and virtual bronchoscopy may provide information that complements laryngoscopy. CT may permit assessment of the entire tracheobronchial pathway. Because 15% to 55% of GPA patients have additional bronchial stenotic segments, assessment of the entire airway is important.28,29
Clinical presentation
Diagnosis of GPA in patients younger than 20 years is associated with the development of subglottic stenosis.23,30 The GPA patient with subglottic stenosis may or may not have other active systemic symptoms. The efficacy of systemic therapy often does not correlate with the degree of subglottic stenosis. Importantly, when systemic disease enters remission, the subglottic stenosis may remain due to residual scarring of the subglottis.31
Patients with subglottic stenosis may present with hoarseness, cough, wheeze, stridor, or dyspnea on exertion.27,32 The stridor and wheeze may be confused with the wheeze of asthma, often leading to misdiagnosis.17
Subglottic stenosis likely begins at a small degree and increases gradually, allowing the patient to adjust his or her breathing pattern until a critical stenotic airway area is reached. Typically, and dependent on their pulmonary health, patients are asymptomatic until about 75% airway stenosis (60% in children).33,34 At this point, symptoms may become evident and correlate with the degree of stenosis, ranging from cough and mild shortness of breath to life-threatening stridor and obstruction. Importantly, as the airway caliber narrows, mucous plugging becomes a greater concern, as it can cause acute stridulous exacerbations and airway obstruction.
A significant proportion of patients with GPA who have subclinical asymptomatic stenosis may not receive laryngeal examination. Patients who have suspicious clinical histories should be referred for evaluation of subglottic stenosis prior to symptom worsening.
Patients with significant (approximately 80%) stenosis can present with respiratory symptoms that may be life-threatening. Because airway management in this setting is substantially more difficult, the goal should be to obtain a diagnosis and perform intervention before this advanced presentation develops.
Pauzner et al described a possible association between GPA tracheal stenosis and pregnancy.35 Women of childbearing age who have GPA should be counseled about this possible association and the need for close follow-up during the partum and postpartum periods.
Treatment is controversial
The treatment of subglottic stenosis of GPA requires multidisciplinary management by the rheumatologist, otolaryngologist, and pulmonologist. Systemic manifestations of disease are managed by immunosuppressive therapy, but up to 80% of patients may require surgical management of subglottic stenosis, and the remaining 20% will respond to systemic medical therapy.22,23,36,37 Overall, the treatment of this disease is controversial and varies by center. The therapeutic arsenal consists of conventional immunosuppressive therapy, endoscopic dilation, endoscopic or laser excision, and surgical resection of the stenotic segment followed by reconstruction.
Tracheotomy. Historically, tracheotomies were performed in approximately one-half of patients with airway manifestations of GPA when the patient had active disease or when airway patency could not be adequately maintained. Most of these patients were eventually decannulated.23,25 At present, tracheotomy is performed infrequently and is reserved for patients who have either a severely tenuous airway (with tracheotomy the only safe option available to obtain a secure airway) or who express a preference for tracheotomy. In a recent study by Hoffman et al,38 tracheotomy was avoided in 21 patients through the use of stenosis dilation procedures.
Dilation. Endoscopic subglottic dilation is the currently advocated method of treatment, and has shown promising results. In two studies with a total of 41 GPA patients who were able to avoid tracheotomy and open surgical procedures, 24% underwent decannulation of previously placed tracheotomies and 24% required only one procedure at an average follow-up of 3.4 and 5 years per study. In these studies, the technique of intralesional corticosteroid with mechanical dilation (ILCD) was performed.31,36,38
Preferred: Dilation plus medical therapy
Because of the inflammatory etiology of this condition, surgical intervention has the risk of potentially worsening the stenosis. However, combining dilation of the stenosis with aggressive local medical treatment to prevent scar formation and cellular proliferation has been shown to be effective and safe. This treatment modality was recently recommended as the preferred therapy based on a number of relatively small clinical trials for subglottic stenosis, without the benefit of large controlled trials.
Our patient population consists of two subsets: (1) those who respond well to ILCD and systemic medical therapy, requiring a minimal number of dilations before no longer needing procedures because of a possible “burn out” of the subglottic disease, and (2) those who continue to have recurrence of stenosis, requiring repeat ILCD. The latter group requires close long-term observation.
To counter the effects of the exaggerated healing reaction of inflammation (early) and proliferation (late) following injury, two medications are applied to the area of repaired stenosis. The stenotic lesion is first injected submucosally with a long-acting corticosteroid suspension such as methylprednisolone. The solution is injected along the submucosal-perichondrial plane. Incisions are made in a star-like fashion, employing sharp metal microlaryngeal blades or, less commonly, the carbon dioxide laser. These incisions release the constricting stenotic ring and break it up, widening the diameter of the airway and simultaneously preserving islands of intact mucous membrane between the incisions. This epithelium is intended to regenerate and resurface the expanded lumen. Progressive serial dilations are performed using semirigid, flexible, smooth dilators or high-pressure balloon dilation. The next stage involves repeated topical applications of mitomycin-C to further inhibit fibrosis and restenosis by inhibiting cellular proliferation of the vigorous injury cycles of these lesions. Application of mitomycin-C to the dilated area of a laryngotracheal stenosis has been associated with a decreased rate of stenosis relapse.39
Our group at Cleveland Clinic has never used laser surgery alone without dilation on the subglottic stenosis caused by GPA. Incidentally, patients treated with laser surgery in other institutions prior to their referral to the Cleveland Clinic have developed complicating secondary stenoses that required more extensive surgical intervention to overcome the severe secondary superimposed damage. In theory, use of the laser may create unnecessary thermal injury that likely worsens local damage. These patients required laryngotracheal reconstructive procedures or had to undergo establishment of permanent tracheotomies.
CONCLUSION
Granulomatosis with polyangiitis is a rare disease that may manifest in multiple areas of the head and neck. Careful attention to diagnosis and management is critical, as these patients tend to have progressive disease with debilitating sequelae. The rheumatologist, otolaryngologist, and internist should identify patients with any constellation of symptoms that may be typical of GPA. A collaborative effort to diagnose, treat, and follow these patients is paramount to successful disease management.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Mahr A, Girard T, Agher R, Guillevin L. Analysis of factors predictive of survival based on 49 patients with systemic Wegener’s granulomatosis and prospective follow-up. Rheumatology (Oxford) 2001; 40:492–498.
- Wung PK, Stone JH. Therapeutics of Wegener’s granulomatosis. Nat Clin Pract Rheumatol 2006; 2:192–200.
- Rao JK, Weinberger M, Oddone EZ, Allen NB, Landsman P, Feussner JR. The role of antineutrophil cytoplasmic antibody (c-ANCA) testing in the diagnosis of Wegener granulomatosis: a literature review and meta-analysis. Ann Intern Med 1995; 123:925–932.
- Devaney KO, Travis WD, Hoffman G, Leavitt R, Lebovics R, Fauci AS. Interpretation of head and neck biopsies in Wegener’s granulomatosis: a pathologic study of 126 biopsies in 70 patients. Am J Surg Pathol 1990; 14:555–564.
- Jennings CR, Jones NS, Dugar J, Powell RJ, Lowe J. Wegener’s granulomatosis—a review of diagnosis and treatment in 53 subjects. Rhinology 1998; 36:188–191.
- McDonald TJ, DeRemee RA. Wegener’s granulomatosis. Laryngoscope 1983; 93:220–231.
- Lloyd G, Lund VJ, Beale T, Howard D. Rhinologic changes in Wegener’s granulomatosis. J Laryngol Otol 2002; 116:565–569.
- Yang C, Talbot JM, Hwang PH. Bony abnormalities of the paranasal sinuses in patients with Wegener’s granulomatosis. Am J Rhinol 2001; 15:121–125.
- Cannady SB, Batra PS, Koening C, et al. Sinonasal Wegener granulomatosis: a single-institution experience with 120 cases. Laryngoscope 2009; 119:757–761.
- Grindler D, Cannady S, Batra PS. Computed tomography findings in sinonasal Wegener’s granulomatosis. Am J Rhinol Allergy 2009; 23:497–501.
- Hughes RG, Drake-Lee A. Nasal manifestations of granulomatous disease. Hosp Med 2001; 62:417–421.
- Srouji IA, Andrews P, Edwards C, Lund VJ. General and rhinosinusitis-related quality of life in patients with Wegener’s granulomatosis. Laryngoscope 2006; 116:1621–1625.
- Kornblut AD, Wolff SM, deFries HO, Fauci AS. Wegener’s granulomatosis. Laryngoscope 1980; 90:1453–1465.
- McCaffrey TV, McDonald TJ, Facer GW, DeRemee RA. Otologic manifestations of Wegener’s granulomatosis. Otolaryngol Head Neck Surg 1980; 88:586–593.
- Bradley PJ. Wegener’s granulomatosis of the ear. J Laryngol Otol 1983; 97:623–626.
- Rasmussen N. Management of the ear, nose, and throat manifestations of Wegener granulomatosis: an otorhinolaryngologist’s perspective. Curr Opin Rheumatol 2001; 13:3–11.
- Erickson VR, Hwang PH. Wegener’s granulomatosis: current trends in diagnosis and management. Curr Opin Otolaryngol Head Neck Surg 2007; 15:170–176.
- Congdon D, Sherris DA, Specks U, McDonald T. Long-term follow-up of repair of external nasal deformities in patients with Wegener’s granulomatosis. Laryngoscope 2002; 112:731–737.
- Duffy FJ, Rossi RM, Pribaz JJ. Reconstruction of Wegener’s nasal deformity using bilateral facial artery musculomucosal flaps. Plast Reconstr Surg 1998; 101:1330–1333.
- Shipchandler TZ, Chung BJ, Alam DS. Saddle nose deformity reconstruction with a split calvarial bone L-shaped strut. Arch Facial Plast Surg 2008; 10:305–311.
- Hernández-Rodríguez J, Hoffman GS, Koening CL. Surgical interventions and local therapy for Wegener’s granulomatosis. Curr Opin Rheumatol 2010; 22:29–36.
- Lebovics RS, Hoffman GS, Leavitt RY, et al. The management of subglottic stenosis in patients with Wegener’s granulomatosis. Laryngoscope 1992; 102:1341–1345.
- Waxman J, Bose WJ. Laryngeal manifestations of Wegener’s granulomatosis: case reports and review of the literature. J Rheumatol 1986; 13:408–411.
- Maronian NC, Azadeh H, Waugh P, Hillel A. Association of laryngopharyngeal reflux disease and subglottic stenosis. Ann Otol Rhinol Laryngol 2001; 110:606–612.
- Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004; 9:283–289.
- Gubbels SP, Barkhuizen A, Hwang PH. Head and neck manifestations of Wegener’s granulomatosis. Otolaryngol Clin North Am 2003; 36:685–705.
- Polychronopoulos VS, Prakash UB, Golbin JM, Edell ES, Specks U. Airway involvement in Wegener’s granulomatosis. Rheum Dis Clin North Am 2007; 33:755–775.
- Daum TE, Specks U, Colby TV, et al. Tracheobronchial involvement in Wegener’s granulomatosis. Am J Respir Crit Care Med 1995; 151:522–526.
- Rottem M, Fauci AS, Hallahan CW, et al Wegener granulomatosis in children and adolescents: clinical presentation and outcome. J Pediatr 1993; 122:26–31.
- Eliachar I, Chan J, Akst L. New approaches to the management of subglottic stenosis in Wegener’s granulomatosis. Cleve Clin J Med 2002; 69( suppl 2):SII149–SII151.
- Solans-Laqué R, Bosch-Gil J, Canela M, Lorente J, Pallisa E, Vilardell-Tarrés M. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Lupus 2008; 17:832–836.
- Sandu K, Monnier P. Cricotracheal resection. Otolaryngol Clin North Am 2008; 41:981–998.
- Brouns M, Jayaraju ST, Lacor C, De Mey J, et al. Tracheal stenosis: a flow dynamics study [published online ahead of print November 30, 2006]. J Appl Physiol 2007; 102:1178–1184. doi: 10.1152/japplphysiol.01063.2006
- Pauzner R, Mayan H, Hershko E, Alcalay M, Farfel Z. Exacerbation of Wegener’s granulomatosis during pregnancy: report of a case with tracheal stenosis and literature review. J Rheumatol 1994; 21:1153–1156.
- Langford CA, Sneller MC, Hallahan CW, et al. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Arthritis Rheum 1996; 39:1754–1760.
- Schokkenbroek AA, Franssen CF, Dikkers FG. Dilatation tracheoscopy for laryngeal and tracheal stenosis in patients with Wegener’s granulomatosis [published online ahead of print November 14, 2007]. Eur Arch Otorhinolaryngol 2008; 265:549–555. doi: 10.1007/s00405-007-0518-3
- Hoffman GS, Thomas-Golbanov CK, Chan J, Akst LM, Eliachar I. Treatment of subglottic stenosis, due to Wegener’s granulomatosis, with intralesional corticosteroids and dilation. J Rheumatol 2003; 30:1017–1021.
- Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope 2009; 119:272–283.
The head and neck are the most common sites of involvement at initial presentation of granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]). Head and neck manifestations occur initially in 73% of patients, and eventually, up to 92% of patients with GPA are affected.1 Many of these compromise the upper airway. Although treatment is multidisciplinary, the effects on the airway make it important to understand upper airway presentations and treatments. This article examines upper airway disease presentations, their assessment, and their advocated interventions.
DISEASE COURSE
Because head and neck involvement may be associated with a less aggressive form of GPA, outcomes for patients with predominantly head and neck involvement may be better compared with those who have involvement of other systems.2
The natural course of GPA may be indolent or rapidly progressive. Regardless, left untreated, it progresses to a generalized systemic disease that often leads to significant morbidity and likely mortality. Most patients (96%) achieve remission with immunosuppressive therapy, but nearly half (49%) have at least one relapse.1 For this reason, systemic immunosuppressive medications play a dominant role in systemic and localized head and neck disease control. Patients often require maintenance medications along with additional therapies during disease exacerbation.3 Therefore, key partnerships between internists, rheumatologists, and otolaryngologists are paramount in the treatment and follow-up of these patients.
DIAGNOSIS: MAINSTAY IS SEROLOGIC EVALUATION
The differential diagnosis of GPA includes infection, lymphoproliferative disease (T-cell lymphoma), systemic lupus erythematosus, rheumatoid arthritis, sarcoidosis, and other granulomatous diseases such as eosinophilic GPA (Churg-Strauss syndrome), polyarteritis nodosa, and microscopic polyangiitis. Appropriate diagnosis is critical because treatment of these entities varies drastically.
The mainstay of GPA diagnosis is serologic evaluation for a cytoplasmic pattern of antineutrophil cytoplasmic antibodies (cANCA), which are reactive toward proteinase-3 (PR3) or myeloperoxidase (MPO). Testing for cANCA yields a pooled sensitivity of 91% and specificity of 99%. Sensitivity falls significantly (63%) when the disease is in nonacute stages, while the specificity remains high.4 These cANCA test characteristics allow a high positive predictive value for this rare disease.
Biopsy is typically reserved for cases in which serologic ANCA testing is nondiagnostic. Biopsy tissue may be readily accessible from the head and neck, but these biopsies may bear significant false-negative rates.4–6 Diagnosis requires demonstration of palisading granulomas as vascular or extravascular lesions within the upper respiratory tract tissues. The specific site biopsied from within the head and neck has been shown to influence diagnostic yield, with sinonasal biopsies producing the highest yield.
SINONASAL MANIFESTATIONS
The nose and paranasal sinuses are the most frequently affected sites in the head and neck, noted in 64% to 80% of patients. Additionally, the nose is the only site of involvement in 30% of patients.7 Given the high frequency of sinonasal manifestations, GPA should be considered as a potential diagnosis among patients with persistent sinonasal disease.
Pathophysiology and disease course
The pathophysiologic mechanisms leading to the changes in the sinonasal tract in GPA have not been established. GPA is believed to be an immunologic disease that manifests as a vasculitis of small- and medium-sized vessels. Multiple potential causative factors have been identified, including fibrinoid necrosis of small blood vessels, epithelial granulomas, chronic inflammation, and prior surgical intervention.8,9 The acute and chronic inflammation, coupled with the epithelioid granuloma formation, damages adjacent small- to medium-sized vessels. The vasculitis leads to diminished blood flow and subsequent avascular necrosis, which may promote tissue necrosis and bone destruction. This destructive process typically starts in the midseptum supplied by Kiesselbach plexus and in the turbinates. The process then eventually spreads to the paranasal sinuses.8
Patient evaluation
Examination of the nasal cavities is typically performed by rigid or flexible nasal endoscopy and often reveals nasal crusting, friable erythematous mucosa, granulation, and even signs of sinusitis. All or part of the cartilaginous septum may be involved, leading to significant septal defects. As the degree of cartilage destruction increases, nasal dorsal support decreases, leading to a visible depression of the external nose known as a “saddle-nose” deformity, which is present in 23% of patients with GPA.7,10
Imaging assessment by computed tomography (CT) is needed to establish disease extent and involvement. Atypical findings may include bony erosion and destruction of the septum and turbinates; erosion of bony partitions within the ethmoid sinuses; neo-osteogenesis of the maxillary, frontal, and sphenoid sinuses; and complete bony obliteration of the maxillary, frontal, and sphenoid sinuses.9,11
Clinical presentation
Sinonasal disease indicates the degree of disease activity.12 Clinical findings may vary, but they have a significant impact on quality of life in these patients.13 Most patients with active disease present with nasal crusting (69%), chronic rhinosinusitis (CRS) symptoms (61%), nasal obstruction (58%), and serosanguinous nasal discharge (52%).10 Patients may also complain of foul-smelling rhinorrhea, recurrent epistaxis, hyposmia, anosmia, and epiphora (from granulomatous compression or obstruction of the lacrimal system). In a series of 120 patients with GPA, Cannady et al found that four (3.3%) patients had mucoceles and three (2.5%) had orbital pseudotumor.10
Any structure in the sinonasal cavity, including mucosa, septum, turbinates, and sinuses proper, may be affected because of the vasculitic involvement of mucosal blood vessels that causes diminished blood flow and subsequent necrosis. The area of the anterior septum supplied by Kiesselbach plexus is the most common site of active nasal disease, which can eventually lead to the common presentation of an anterior nasal septal perforation.
Otologic disease secondary to sinonasal GPA
Otologic involvement is observed in 19% to 38% of patients with GPA.14,15 Most patients with GPA who exhibit otologic symptoms have middle ear or mastoid disease. It typically appears as chronic otitis media (COM) with conductive hearing loss.16 In most cases, the otologic involvement is secondary to Eustachian tube dysfunction caused by the presence of extensive disease in the nasopharynx.
Additionally, chronic mastoiditis can result from direct mastoid involvement with GPA. Facial nerve palsy secondary to infective bony destruction is a rare but repeatedly reported complication of GPA.14,15
Inner ear involvement is a relatively common otologic presentation of GPA. Patients may experience sensorineural hearing loss (SNHL) as well as vertigo, which may mimic Cogan syndrome. Importantly, patients may exhibit inner ear involvement with or without middle ear and mastoid disease. The SNHL observed in patients with GPA may be responsive to steroid or immunosuppressive therapy.
Treatment
Refractory CRS in GPA is a complex problem for which aggressive surgical intervention is often counterproductive. Unfortunately, traditional medical therapies are also often inadequate to treat progressive sinonasal symptomatology. As the nasal tissue becomes devascularized, loss of normal mucociliary function aggravates the sinus pathology, and clinical symptoms may worsen. Simple antibiotic regimens used to manage uncomplicated sinusitis are often inadequate in these patients. The subsequent progression to frank necrosis in localized regions creates an intranasal foreign body, allowing bacterial colonization, which is often refractory to antibiotics because of the inability of drug tissue penetration into these devascularized nasal structures.12,17
Medical management must be tailored to be effective in this complex intranasal milieu. Successful treatment requires a multifaceted and often prolonged treatment course. A high index of suspicion should be maintained for Staphylococcus aureus. As a rule, endoscopically obtained cultures should be used to guide antibiotic selection. Several weeks of culture-directed antibiotics followed by topical antibiotic irrigations (eg, mupirocin irrigations) can be useful to reduce the frequency of sinonasal exacerbations.
Frequent saline irrigations using high-volume, high-flow irrigation devices (as opposed to low-volume, low-flow applicators such as nasal spray bottles) can be an excellent adjunct to maintenance therapy and are effective in clearing debris and augmenting mucociliary clearance in affected nasal cavities and those with septal perforations. Occasional in-office endoscopic debridement of large crusts adherent to intranasal structures or the edges of a septal perforation can also help to improve obstructive symptoms.
Surgery for refractory cases. Surgery should be reserved for refractory cases unresponsive to maximal medical efforts or those cases with impending complications (ie, mucoceles). Overall, only 16% of patients with sinonasal GPA required surgical intervention in a large series of 120 patients at our institution. In this series, one-third of all patients had undergone previous functional nasal surgery at an outside institution without resolution of symptoms. Anecdotal evidence suggests that surgery for GPA can contribute to additional scarring and lead to protracted sinonasal symptoms.10,18
The decision to perform surgery is individualized and based on severity of the disease process, patient expectations, and surgeon expertise. In our experience at Cleveland Clinic, functional endoscopic sinus surgery in the setting of GPA is a surgical challenge, given extensive alteration of the sinonasal anatomy from previous surgery, prior and ongoing inflammation, chronic crusting, and scarring. Consequently, it has been our practice to employ conservative efforts prior to consideration of surgery. A complete surgical cure is exceedingly rare, and the patient should be counseled about the possible need for revision surgery and ongoing nonsurgical therapies. Meticulous postoperative care with weekly postoperative debridement, saline or antibiotic irrigations, and culture-directed antibiotics, is essential during the early postoperative recovery phase.
Management of epiphora. The most common ophthalmologic findings in patients with GPA include chronic epiphora and orbital pseudotumor. With the advent of advanced endoscopic techniques, the otolaryngologist plays a greater role in the surgical management of these ophthalmologic disease entities. In a series reported by Cannady et al,10 endoscopic dacrocystorhinostomy was performed successfully in seven patients, including one revision.
Nasal reconstruction for saddle-nose deformity: effective in selected patients. The progressive loss of septal support that occurs with the enlarging anterior septal perforation often results in significant collapse of the cartilaginous midvault of the nose. The tip cartilages in turn also begin to lose projection, resulting in a shortened nose with the characteristic saddle-nose deformity. The psychologic impact of this disfiguring facial abnormality is significant. The loss of midvault support also results in worsening nasal obstruction and increases the incidence of anosmia as the superior nasal vault becomes obstructed. For these reasons, patients often seek referral for potential reconstruction.
Despite the potential benefits, the general consensus in the medical community has been that surgical procedures on the nose should be avoided in GPA patients.17 Most nasal destruction in these patients is the consequence of poor tissue perfusion from the active vasculitis. Poor wound healing, reconstructive graft resorption, and worsening necrosis have been observed in patients who have undergone ill-advised surgical procedures.
These poor outcomes do not, however, preclude the potential for safe and effective surgical intervention. In three small published series, good surgical outcomes were achieved but the procedures were done in very highly selected patients and were modified to address the specific clinical issues seen in GPA patients.19–21 The critical step in achieving a good outcome is working closely with the patient’s rheumatologist to identify an appropriate clinical window during which the patient’s disease process is in a period of relative remission. The second major factor is to modify the surgical techniques to take into account the very poor vascular framework of the recipient nasal bed.
Management of COM. Because the COM in patients with GPA is frequently secondary to nasopharyngeal disease, systemic control of GPA is the first priority. Systemic control is also the first-line treatment for patients with mixed or sensorineural hearing loss, or with vertigo. For continued or symptomatic middle ear effusions that do not resolve with systemic therapy, placement of a ventilation tube may be considered. In patients with significant hearing loss, hearing amplification devices may be warranted.15,22 Cochlear implant devices in GPA patients are experimental and may pose undue risks of meningitis.
SUBGLOTTIC STENOSIS AND TRACHEAL MANIFESTATIONS
Subglottic stenosis affects 10% to 20% of patients with GPA.1,23,24 Because of its potential life-threatening airway complications, patients should be carefully assessed for this disease manifestation. It may be the only manifestation of GPA or may be part of a spectrum of other disease manifestations. Therefore, the work-up for subglottic stenosis of unknown etiology should always include an evaluation for GPA.
Pathophysiology and disease course
The etiology of subglottic stenosis in GPA is not well understood. Theories primarily involve the vulnerability of the subglottic tissues to damage, chronic inflammation, and scarring.25 The combination of vasculitis in the setting of active inflammation may synergistically produce a hyperactive reparative mechanism in GPA patients that leads to cartilaginous fibrotic scarring and stenosis. Wound healing can be divided into the phases of inflammation, proliferation, and remodeling. An imbalance or exaggerated response of any of these levels (and likely all) produces an abnormal healing response.26 Similarly, each of these phases may be targeted to improve the healing process.
Patient evaluation
The presence of subglottic stenosis must be considered in a GPA patient with respiratory symptoms. As part of the routine initial evaluation, an office-based nasopharyngeal/laryngeal endoscopy using a flexible laryngoscope should be performed to assess the presence and severity of luminal airway narrowing. Flexible laryngoscopy reveals a circumferential narrowing of the subglottis. The stenotic tissue may vary from friable with erythematous and inflamed mucosa to a rigid mature fibrotic band, depending on the inflammatory state of the stenosis.18,27
Subclinical stenosis may be identified with routine endoscopy. An appropriate baseline is needed to follow the progression of disease and to adjust the timing of any potential intervention. The ability to digitally record a patient’s examination allows further tracking of disease and is commonly used in our practices.
Although flexible fiberoptic examination is critical in diagnosis and follow-up, intraoperative direct laryngoscopy using rigid laryngoscopes and telescopes provides the optimum view of the subglottis. In particular, this view provides greater information on the length and degree of the stenosis and allows evaluation of potential stenotic segments in the inferior trachea.
Spiral CT with 3-dimensional reconstruction of the laryngotracheal lumen and virtual bronchoscopy may provide information that complements laryngoscopy. CT may permit assessment of the entire tracheobronchial pathway. Because 15% to 55% of GPA patients have additional bronchial stenotic segments, assessment of the entire airway is important.28,29
Clinical presentation
Diagnosis of GPA in patients younger than 20 years is associated with the development of subglottic stenosis.23,30 The GPA patient with subglottic stenosis may or may not have other active systemic symptoms. The efficacy of systemic therapy often does not correlate with the degree of subglottic stenosis. Importantly, when systemic disease enters remission, the subglottic stenosis may remain due to residual scarring of the subglottis.31
Patients with subglottic stenosis may present with hoarseness, cough, wheeze, stridor, or dyspnea on exertion.27,32 The stridor and wheeze may be confused with the wheeze of asthma, often leading to misdiagnosis.17
Subglottic stenosis likely begins at a small degree and increases gradually, allowing the patient to adjust his or her breathing pattern until a critical stenotic airway area is reached. Typically, and dependent on their pulmonary health, patients are asymptomatic until about 75% airway stenosis (60% in children).33,34 At this point, symptoms may become evident and correlate with the degree of stenosis, ranging from cough and mild shortness of breath to life-threatening stridor and obstruction. Importantly, as the airway caliber narrows, mucous plugging becomes a greater concern, as it can cause acute stridulous exacerbations and airway obstruction.
A significant proportion of patients with GPA who have subclinical asymptomatic stenosis may not receive laryngeal examination. Patients who have suspicious clinical histories should be referred for evaluation of subglottic stenosis prior to symptom worsening.
Patients with significant (approximately 80%) stenosis can present with respiratory symptoms that may be life-threatening. Because airway management in this setting is substantially more difficult, the goal should be to obtain a diagnosis and perform intervention before this advanced presentation develops.
Pauzner et al described a possible association between GPA tracheal stenosis and pregnancy.35 Women of childbearing age who have GPA should be counseled about this possible association and the need for close follow-up during the partum and postpartum periods.
Treatment is controversial
The treatment of subglottic stenosis of GPA requires multidisciplinary management by the rheumatologist, otolaryngologist, and pulmonologist. Systemic manifestations of disease are managed by immunosuppressive therapy, but up to 80% of patients may require surgical management of subglottic stenosis, and the remaining 20% will respond to systemic medical therapy.22,23,36,37 Overall, the treatment of this disease is controversial and varies by center. The therapeutic arsenal consists of conventional immunosuppressive therapy, endoscopic dilation, endoscopic or laser excision, and surgical resection of the stenotic segment followed by reconstruction.
Tracheotomy. Historically, tracheotomies were performed in approximately one-half of patients with airway manifestations of GPA when the patient had active disease or when airway patency could not be adequately maintained. Most of these patients were eventually decannulated.23,25 At present, tracheotomy is performed infrequently and is reserved for patients who have either a severely tenuous airway (with tracheotomy the only safe option available to obtain a secure airway) or who express a preference for tracheotomy. In a recent study by Hoffman et al,38 tracheotomy was avoided in 21 patients through the use of stenosis dilation procedures.
Dilation. Endoscopic subglottic dilation is the currently advocated method of treatment, and has shown promising results. In two studies with a total of 41 GPA patients who were able to avoid tracheotomy and open surgical procedures, 24% underwent decannulation of previously placed tracheotomies and 24% required only one procedure at an average follow-up of 3.4 and 5 years per study. In these studies, the technique of intralesional corticosteroid with mechanical dilation (ILCD) was performed.31,36,38
Preferred: Dilation plus medical therapy
Because of the inflammatory etiology of this condition, surgical intervention has the risk of potentially worsening the stenosis. However, combining dilation of the stenosis with aggressive local medical treatment to prevent scar formation and cellular proliferation has been shown to be effective and safe. This treatment modality was recently recommended as the preferred therapy based on a number of relatively small clinical trials for subglottic stenosis, without the benefit of large controlled trials.
Our patient population consists of two subsets: (1) those who respond well to ILCD and systemic medical therapy, requiring a minimal number of dilations before no longer needing procedures because of a possible “burn out” of the subglottic disease, and (2) those who continue to have recurrence of stenosis, requiring repeat ILCD. The latter group requires close long-term observation.
To counter the effects of the exaggerated healing reaction of inflammation (early) and proliferation (late) following injury, two medications are applied to the area of repaired stenosis. The stenotic lesion is first injected submucosally with a long-acting corticosteroid suspension such as methylprednisolone. The solution is injected along the submucosal-perichondrial plane. Incisions are made in a star-like fashion, employing sharp metal microlaryngeal blades or, less commonly, the carbon dioxide laser. These incisions release the constricting stenotic ring and break it up, widening the diameter of the airway and simultaneously preserving islands of intact mucous membrane between the incisions. This epithelium is intended to regenerate and resurface the expanded lumen. Progressive serial dilations are performed using semirigid, flexible, smooth dilators or high-pressure balloon dilation. The next stage involves repeated topical applications of mitomycin-C to further inhibit fibrosis and restenosis by inhibiting cellular proliferation of the vigorous injury cycles of these lesions. Application of mitomycin-C to the dilated area of a laryngotracheal stenosis has been associated with a decreased rate of stenosis relapse.39
Our group at Cleveland Clinic has never used laser surgery alone without dilation on the subglottic stenosis caused by GPA. Incidentally, patients treated with laser surgery in other institutions prior to their referral to the Cleveland Clinic have developed complicating secondary stenoses that required more extensive surgical intervention to overcome the severe secondary superimposed damage. In theory, use of the laser may create unnecessary thermal injury that likely worsens local damage. These patients required laryngotracheal reconstructive procedures or had to undergo establishment of permanent tracheotomies.
CONCLUSION
Granulomatosis with polyangiitis is a rare disease that may manifest in multiple areas of the head and neck. Careful attention to diagnosis and management is critical, as these patients tend to have progressive disease with debilitating sequelae. The rheumatologist, otolaryngologist, and internist should identify patients with any constellation of symptoms that may be typical of GPA. A collaborative effort to diagnose, treat, and follow these patients is paramount to successful disease management.
The head and neck are the most common sites of involvement at initial presentation of granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]). Head and neck manifestations occur initially in 73% of patients, and eventually, up to 92% of patients with GPA are affected.1 Many of these compromise the upper airway. Although treatment is multidisciplinary, the effects on the airway make it important to understand upper airway presentations and treatments. This article examines upper airway disease presentations, their assessment, and their advocated interventions.
DISEASE COURSE
Because head and neck involvement may be associated with a less aggressive form of GPA, outcomes for patients with predominantly head and neck involvement may be better compared with those who have involvement of other systems.2
The natural course of GPA may be indolent or rapidly progressive. Regardless, left untreated, it progresses to a generalized systemic disease that often leads to significant morbidity and likely mortality. Most patients (96%) achieve remission with immunosuppressive therapy, but nearly half (49%) have at least one relapse.1 For this reason, systemic immunosuppressive medications play a dominant role in systemic and localized head and neck disease control. Patients often require maintenance medications along with additional therapies during disease exacerbation.3 Therefore, key partnerships between internists, rheumatologists, and otolaryngologists are paramount in the treatment and follow-up of these patients.
DIAGNOSIS: MAINSTAY IS SEROLOGIC EVALUATION
The differential diagnosis of GPA includes infection, lymphoproliferative disease (T-cell lymphoma), systemic lupus erythematosus, rheumatoid arthritis, sarcoidosis, and other granulomatous diseases such as eosinophilic GPA (Churg-Strauss syndrome), polyarteritis nodosa, and microscopic polyangiitis. Appropriate diagnosis is critical because treatment of these entities varies drastically.
The mainstay of GPA diagnosis is serologic evaluation for a cytoplasmic pattern of antineutrophil cytoplasmic antibodies (cANCA), which are reactive toward proteinase-3 (PR3) or myeloperoxidase (MPO). Testing for cANCA yields a pooled sensitivity of 91% and specificity of 99%. Sensitivity falls significantly (63%) when the disease is in nonacute stages, while the specificity remains high.4 These cANCA test characteristics allow a high positive predictive value for this rare disease.
Biopsy is typically reserved for cases in which serologic ANCA testing is nondiagnostic. Biopsy tissue may be readily accessible from the head and neck, but these biopsies may bear significant false-negative rates.4–6 Diagnosis requires demonstration of palisading granulomas as vascular or extravascular lesions within the upper respiratory tract tissues. The specific site biopsied from within the head and neck has been shown to influence diagnostic yield, with sinonasal biopsies producing the highest yield.
SINONASAL MANIFESTATIONS
The nose and paranasal sinuses are the most frequently affected sites in the head and neck, noted in 64% to 80% of patients. Additionally, the nose is the only site of involvement in 30% of patients.7 Given the high frequency of sinonasal manifestations, GPA should be considered as a potential diagnosis among patients with persistent sinonasal disease.
Pathophysiology and disease course
The pathophysiologic mechanisms leading to the changes in the sinonasal tract in GPA have not been established. GPA is believed to be an immunologic disease that manifests as a vasculitis of small- and medium-sized vessels. Multiple potential causative factors have been identified, including fibrinoid necrosis of small blood vessels, epithelial granulomas, chronic inflammation, and prior surgical intervention.8,9 The acute and chronic inflammation, coupled with the epithelioid granuloma formation, damages adjacent small- to medium-sized vessels. The vasculitis leads to diminished blood flow and subsequent avascular necrosis, which may promote tissue necrosis and bone destruction. This destructive process typically starts in the midseptum supplied by Kiesselbach plexus and in the turbinates. The process then eventually spreads to the paranasal sinuses.8
Patient evaluation
Examination of the nasal cavities is typically performed by rigid or flexible nasal endoscopy and often reveals nasal crusting, friable erythematous mucosa, granulation, and even signs of sinusitis. All or part of the cartilaginous septum may be involved, leading to significant septal defects. As the degree of cartilage destruction increases, nasal dorsal support decreases, leading to a visible depression of the external nose known as a “saddle-nose” deformity, which is present in 23% of patients with GPA.7,10
Imaging assessment by computed tomography (CT) is needed to establish disease extent and involvement. Atypical findings may include bony erosion and destruction of the septum and turbinates; erosion of bony partitions within the ethmoid sinuses; neo-osteogenesis of the maxillary, frontal, and sphenoid sinuses; and complete bony obliteration of the maxillary, frontal, and sphenoid sinuses.9,11
Clinical presentation
Sinonasal disease indicates the degree of disease activity.12 Clinical findings may vary, but they have a significant impact on quality of life in these patients.13 Most patients with active disease present with nasal crusting (69%), chronic rhinosinusitis (CRS) symptoms (61%), nasal obstruction (58%), and serosanguinous nasal discharge (52%).10 Patients may also complain of foul-smelling rhinorrhea, recurrent epistaxis, hyposmia, anosmia, and epiphora (from granulomatous compression or obstruction of the lacrimal system). In a series of 120 patients with GPA, Cannady et al found that four (3.3%) patients had mucoceles and three (2.5%) had orbital pseudotumor.10
Any structure in the sinonasal cavity, including mucosa, septum, turbinates, and sinuses proper, may be affected because of the vasculitic involvement of mucosal blood vessels that causes diminished blood flow and subsequent necrosis. The area of the anterior septum supplied by Kiesselbach plexus is the most common site of active nasal disease, which can eventually lead to the common presentation of an anterior nasal septal perforation.
Otologic disease secondary to sinonasal GPA
Otologic involvement is observed in 19% to 38% of patients with GPA.14,15 Most patients with GPA who exhibit otologic symptoms have middle ear or mastoid disease. It typically appears as chronic otitis media (COM) with conductive hearing loss.16 In most cases, the otologic involvement is secondary to Eustachian tube dysfunction caused by the presence of extensive disease in the nasopharynx.
Additionally, chronic mastoiditis can result from direct mastoid involvement with GPA. Facial nerve palsy secondary to infective bony destruction is a rare but repeatedly reported complication of GPA.14,15
Inner ear involvement is a relatively common otologic presentation of GPA. Patients may experience sensorineural hearing loss (SNHL) as well as vertigo, which may mimic Cogan syndrome. Importantly, patients may exhibit inner ear involvement with or without middle ear and mastoid disease. The SNHL observed in patients with GPA may be responsive to steroid or immunosuppressive therapy.
Treatment
Refractory CRS in GPA is a complex problem for which aggressive surgical intervention is often counterproductive. Unfortunately, traditional medical therapies are also often inadequate to treat progressive sinonasal symptomatology. As the nasal tissue becomes devascularized, loss of normal mucociliary function aggravates the sinus pathology, and clinical symptoms may worsen. Simple antibiotic regimens used to manage uncomplicated sinusitis are often inadequate in these patients. The subsequent progression to frank necrosis in localized regions creates an intranasal foreign body, allowing bacterial colonization, which is often refractory to antibiotics because of the inability of drug tissue penetration into these devascularized nasal structures.12,17
Medical management must be tailored to be effective in this complex intranasal milieu. Successful treatment requires a multifaceted and often prolonged treatment course. A high index of suspicion should be maintained for Staphylococcus aureus. As a rule, endoscopically obtained cultures should be used to guide antibiotic selection. Several weeks of culture-directed antibiotics followed by topical antibiotic irrigations (eg, mupirocin irrigations) can be useful to reduce the frequency of sinonasal exacerbations.
Frequent saline irrigations using high-volume, high-flow irrigation devices (as opposed to low-volume, low-flow applicators such as nasal spray bottles) can be an excellent adjunct to maintenance therapy and are effective in clearing debris and augmenting mucociliary clearance in affected nasal cavities and those with septal perforations. Occasional in-office endoscopic debridement of large crusts adherent to intranasal structures or the edges of a septal perforation can also help to improve obstructive symptoms.
Surgery for refractory cases. Surgery should be reserved for refractory cases unresponsive to maximal medical efforts or those cases with impending complications (ie, mucoceles). Overall, only 16% of patients with sinonasal GPA required surgical intervention in a large series of 120 patients at our institution. In this series, one-third of all patients had undergone previous functional nasal surgery at an outside institution without resolution of symptoms. Anecdotal evidence suggests that surgery for GPA can contribute to additional scarring and lead to protracted sinonasal symptoms.10,18
The decision to perform surgery is individualized and based on severity of the disease process, patient expectations, and surgeon expertise. In our experience at Cleveland Clinic, functional endoscopic sinus surgery in the setting of GPA is a surgical challenge, given extensive alteration of the sinonasal anatomy from previous surgery, prior and ongoing inflammation, chronic crusting, and scarring. Consequently, it has been our practice to employ conservative efforts prior to consideration of surgery. A complete surgical cure is exceedingly rare, and the patient should be counseled about the possible need for revision surgery and ongoing nonsurgical therapies. Meticulous postoperative care with weekly postoperative debridement, saline or antibiotic irrigations, and culture-directed antibiotics, is essential during the early postoperative recovery phase.
Management of epiphora. The most common ophthalmologic findings in patients with GPA include chronic epiphora and orbital pseudotumor. With the advent of advanced endoscopic techniques, the otolaryngologist plays a greater role in the surgical management of these ophthalmologic disease entities. In a series reported by Cannady et al,10 endoscopic dacrocystorhinostomy was performed successfully in seven patients, including one revision.
Nasal reconstruction for saddle-nose deformity: effective in selected patients. The progressive loss of septal support that occurs with the enlarging anterior septal perforation often results in significant collapse of the cartilaginous midvault of the nose. The tip cartilages in turn also begin to lose projection, resulting in a shortened nose with the characteristic saddle-nose deformity. The psychologic impact of this disfiguring facial abnormality is significant. The loss of midvault support also results in worsening nasal obstruction and increases the incidence of anosmia as the superior nasal vault becomes obstructed. For these reasons, patients often seek referral for potential reconstruction.
Despite the potential benefits, the general consensus in the medical community has been that surgical procedures on the nose should be avoided in GPA patients.17 Most nasal destruction in these patients is the consequence of poor tissue perfusion from the active vasculitis. Poor wound healing, reconstructive graft resorption, and worsening necrosis have been observed in patients who have undergone ill-advised surgical procedures.
These poor outcomes do not, however, preclude the potential for safe and effective surgical intervention. In three small published series, good surgical outcomes were achieved but the procedures were done in very highly selected patients and were modified to address the specific clinical issues seen in GPA patients.19–21 The critical step in achieving a good outcome is working closely with the patient’s rheumatologist to identify an appropriate clinical window during which the patient’s disease process is in a period of relative remission. The second major factor is to modify the surgical techniques to take into account the very poor vascular framework of the recipient nasal bed.
Management of COM. Because the COM in patients with GPA is frequently secondary to nasopharyngeal disease, systemic control of GPA is the first priority. Systemic control is also the first-line treatment for patients with mixed or sensorineural hearing loss, or with vertigo. For continued or symptomatic middle ear effusions that do not resolve with systemic therapy, placement of a ventilation tube may be considered. In patients with significant hearing loss, hearing amplification devices may be warranted.15,22 Cochlear implant devices in GPA patients are experimental and may pose undue risks of meningitis.
SUBGLOTTIC STENOSIS AND TRACHEAL MANIFESTATIONS
Subglottic stenosis affects 10% to 20% of patients with GPA.1,23,24 Because of its potential life-threatening airway complications, patients should be carefully assessed for this disease manifestation. It may be the only manifestation of GPA or may be part of a spectrum of other disease manifestations. Therefore, the work-up for subglottic stenosis of unknown etiology should always include an evaluation for GPA.
Pathophysiology and disease course
The etiology of subglottic stenosis in GPA is not well understood. Theories primarily involve the vulnerability of the subglottic tissues to damage, chronic inflammation, and scarring.25 The combination of vasculitis in the setting of active inflammation may synergistically produce a hyperactive reparative mechanism in GPA patients that leads to cartilaginous fibrotic scarring and stenosis. Wound healing can be divided into the phases of inflammation, proliferation, and remodeling. An imbalance or exaggerated response of any of these levels (and likely all) produces an abnormal healing response.26 Similarly, each of these phases may be targeted to improve the healing process.
Patient evaluation
The presence of subglottic stenosis must be considered in a GPA patient with respiratory symptoms. As part of the routine initial evaluation, an office-based nasopharyngeal/laryngeal endoscopy using a flexible laryngoscope should be performed to assess the presence and severity of luminal airway narrowing. Flexible laryngoscopy reveals a circumferential narrowing of the subglottis. The stenotic tissue may vary from friable with erythematous and inflamed mucosa to a rigid mature fibrotic band, depending on the inflammatory state of the stenosis.18,27
Subclinical stenosis may be identified with routine endoscopy. An appropriate baseline is needed to follow the progression of disease and to adjust the timing of any potential intervention. The ability to digitally record a patient’s examination allows further tracking of disease and is commonly used in our practices.
Although flexible fiberoptic examination is critical in diagnosis and follow-up, intraoperative direct laryngoscopy using rigid laryngoscopes and telescopes provides the optimum view of the subglottis. In particular, this view provides greater information on the length and degree of the stenosis and allows evaluation of potential stenotic segments in the inferior trachea.
Spiral CT with 3-dimensional reconstruction of the laryngotracheal lumen and virtual bronchoscopy may provide information that complements laryngoscopy. CT may permit assessment of the entire tracheobronchial pathway. Because 15% to 55% of GPA patients have additional bronchial stenotic segments, assessment of the entire airway is important.28,29
Clinical presentation
Diagnosis of GPA in patients younger than 20 years is associated with the development of subglottic stenosis.23,30 The GPA patient with subglottic stenosis may or may not have other active systemic symptoms. The efficacy of systemic therapy often does not correlate with the degree of subglottic stenosis. Importantly, when systemic disease enters remission, the subglottic stenosis may remain due to residual scarring of the subglottis.31
Patients with subglottic stenosis may present with hoarseness, cough, wheeze, stridor, or dyspnea on exertion.27,32 The stridor and wheeze may be confused with the wheeze of asthma, often leading to misdiagnosis.17
Subglottic stenosis likely begins at a small degree and increases gradually, allowing the patient to adjust his or her breathing pattern until a critical stenotic airway area is reached. Typically, and dependent on their pulmonary health, patients are asymptomatic until about 75% airway stenosis (60% in children).33,34 At this point, symptoms may become evident and correlate with the degree of stenosis, ranging from cough and mild shortness of breath to life-threatening stridor and obstruction. Importantly, as the airway caliber narrows, mucous plugging becomes a greater concern, as it can cause acute stridulous exacerbations and airway obstruction.
A significant proportion of patients with GPA who have subclinical asymptomatic stenosis may not receive laryngeal examination. Patients who have suspicious clinical histories should be referred for evaluation of subglottic stenosis prior to symptom worsening.
Patients with significant (approximately 80%) stenosis can present with respiratory symptoms that may be life-threatening. Because airway management in this setting is substantially more difficult, the goal should be to obtain a diagnosis and perform intervention before this advanced presentation develops.
Pauzner et al described a possible association between GPA tracheal stenosis and pregnancy.35 Women of childbearing age who have GPA should be counseled about this possible association and the need for close follow-up during the partum and postpartum periods.
Treatment is controversial
The treatment of subglottic stenosis of GPA requires multidisciplinary management by the rheumatologist, otolaryngologist, and pulmonologist. Systemic manifestations of disease are managed by immunosuppressive therapy, but up to 80% of patients may require surgical management of subglottic stenosis, and the remaining 20% will respond to systemic medical therapy.22,23,36,37 Overall, the treatment of this disease is controversial and varies by center. The therapeutic arsenal consists of conventional immunosuppressive therapy, endoscopic dilation, endoscopic or laser excision, and surgical resection of the stenotic segment followed by reconstruction.
Tracheotomy. Historically, tracheotomies were performed in approximately one-half of patients with airway manifestations of GPA when the patient had active disease or when airway patency could not be adequately maintained. Most of these patients were eventually decannulated.23,25 At present, tracheotomy is performed infrequently and is reserved for patients who have either a severely tenuous airway (with tracheotomy the only safe option available to obtain a secure airway) or who express a preference for tracheotomy. In a recent study by Hoffman et al,38 tracheotomy was avoided in 21 patients through the use of stenosis dilation procedures.
Dilation. Endoscopic subglottic dilation is the currently advocated method of treatment, and has shown promising results. In two studies with a total of 41 GPA patients who were able to avoid tracheotomy and open surgical procedures, 24% underwent decannulation of previously placed tracheotomies and 24% required only one procedure at an average follow-up of 3.4 and 5 years per study. In these studies, the technique of intralesional corticosteroid with mechanical dilation (ILCD) was performed.31,36,38
Preferred: Dilation plus medical therapy
Because of the inflammatory etiology of this condition, surgical intervention has the risk of potentially worsening the stenosis. However, combining dilation of the stenosis with aggressive local medical treatment to prevent scar formation and cellular proliferation has been shown to be effective and safe. This treatment modality was recently recommended as the preferred therapy based on a number of relatively small clinical trials for subglottic stenosis, without the benefit of large controlled trials.
Our patient population consists of two subsets: (1) those who respond well to ILCD and systemic medical therapy, requiring a minimal number of dilations before no longer needing procedures because of a possible “burn out” of the subglottic disease, and (2) those who continue to have recurrence of stenosis, requiring repeat ILCD. The latter group requires close long-term observation.
To counter the effects of the exaggerated healing reaction of inflammation (early) and proliferation (late) following injury, two medications are applied to the area of repaired stenosis. The stenotic lesion is first injected submucosally with a long-acting corticosteroid suspension such as methylprednisolone. The solution is injected along the submucosal-perichondrial plane. Incisions are made in a star-like fashion, employing sharp metal microlaryngeal blades or, less commonly, the carbon dioxide laser. These incisions release the constricting stenotic ring and break it up, widening the diameter of the airway and simultaneously preserving islands of intact mucous membrane between the incisions. This epithelium is intended to regenerate and resurface the expanded lumen. Progressive serial dilations are performed using semirigid, flexible, smooth dilators or high-pressure balloon dilation. The next stage involves repeated topical applications of mitomycin-C to further inhibit fibrosis and restenosis by inhibiting cellular proliferation of the vigorous injury cycles of these lesions. Application of mitomycin-C to the dilated area of a laryngotracheal stenosis has been associated with a decreased rate of stenosis relapse.39
Our group at Cleveland Clinic has never used laser surgery alone without dilation on the subglottic stenosis caused by GPA. Incidentally, patients treated with laser surgery in other institutions prior to their referral to the Cleveland Clinic have developed complicating secondary stenoses that required more extensive surgical intervention to overcome the severe secondary superimposed damage. In theory, use of the laser may create unnecessary thermal injury that likely worsens local damage. These patients required laryngotracheal reconstructive procedures or had to undergo establishment of permanent tracheotomies.
CONCLUSION
Granulomatosis with polyangiitis is a rare disease that may manifest in multiple areas of the head and neck. Careful attention to diagnosis and management is critical, as these patients tend to have progressive disease with debilitating sequelae. The rheumatologist, otolaryngologist, and internist should identify patients with any constellation of symptoms that may be typical of GPA. A collaborative effort to diagnose, treat, and follow these patients is paramount to successful disease management.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Mahr A, Girard T, Agher R, Guillevin L. Analysis of factors predictive of survival based on 49 patients with systemic Wegener’s granulomatosis and prospective follow-up. Rheumatology (Oxford) 2001; 40:492–498.
- Wung PK, Stone JH. Therapeutics of Wegener’s granulomatosis. Nat Clin Pract Rheumatol 2006; 2:192–200.
- Rao JK, Weinberger M, Oddone EZ, Allen NB, Landsman P, Feussner JR. The role of antineutrophil cytoplasmic antibody (c-ANCA) testing in the diagnosis of Wegener granulomatosis: a literature review and meta-analysis. Ann Intern Med 1995; 123:925–932.
- Devaney KO, Travis WD, Hoffman G, Leavitt R, Lebovics R, Fauci AS. Interpretation of head and neck biopsies in Wegener’s granulomatosis: a pathologic study of 126 biopsies in 70 patients. Am J Surg Pathol 1990; 14:555–564.
- Jennings CR, Jones NS, Dugar J, Powell RJ, Lowe J. Wegener’s granulomatosis—a review of diagnosis and treatment in 53 subjects. Rhinology 1998; 36:188–191.
- McDonald TJ, DeRemee RA. Wegener’s granulomatosis. Laryngoscope 1983; 93:220–231.
- Lloyd G, Lund VJ, Beale T, Howard D. Rhinologic changes in Wegener’s granulomatosis. J Laryngol Otol 2002; 116:565–569.
- Yang C, Talbot JM, Hwang PH. Bony abnormalities of the paranasal sinuses in patients with Wegener’s granulomatosis. Am J Rhinol 2001; 15:121–125.
- Cannady SB, Batra PS, Koening C, et al. Sinonasal Wegener granulomatosis: a single-institution experience with 120 cases. Laryngoscope 2009; 119:757–761.
- Grindler D, Cannady S, Batra PS. Computed tomography findings in sinonasal Wegener’s granulomatosis. Am J Rhinol Allergy 2009; 23:497–501.
- Hughes RG, Drake-Lee A. Nasal manifestations of granulomatous disease. Hosp Med 2001; 62:417–421.
- Srouji IA, Andrews P, Edwards C, Lund VJ. General and rhinosinusitis-related quality of life in patients with Wegener’s granulomatosis. Laryngoscope 2006; 116:1621–1625.
- Kornblut AD, Wolff SM, deFries HO, Fauci AS. Wegener’s granulomatosis. Laryngoscope 1980; 90:1453–1465.
- McCaffrey TV, McDonald TJ, Facer GW, DeRemee RA. Otologic manifestations of Wegener’s granulomatosis. Otolaryngol Head Neck Surg 1980; 88:586–593.
- Bradley PJ. Wegener’s granulomatosis of the ear. J Laryngol Otol 1983; 97:623–626.
- Rasmussen N. Management of the ear, nose, and throat manifestations of Wegener granulomatosis: an otorhinolaryngologist’s perspective. Curr Opin Rheumatol 2001; 13:3–11.
- Erickson VR, Hwang PH. Wegener’s granulomatosis: current trends in diagnosis and management. Curr Opin Otolaryngol Head Neck Surg 2007; 15:170–176.
- Congdon D, Sherris DA, Specks U, McDonald T. Long-term follow-up of repair of external nasal deformities in patients with Wegener’s granulomatosis. Laryngoscope 2002; 112:731–737.
- Duffy FJ, Rossi RM, Pribaz JJ. Reconstruction of Wegener’s nasal deformity using bilateral facial artery musculomucosal flaps. Plast Reconstr Surg 1998; 101:1330–1333.
- Shipchandler TZ, Chung BJ, Alam DS. Saddle nose deformity reconstruction with a split calvarial bone L-shaped strut. Arch Facial Plast Surg 2008; 10:305–311.
- Hernández-Rodríguez J, Hoffman GS, Koening CL. Surgical interventions and local therapy for Wegener’s granulomatosis. Curr Opin Rheumatol 2010; 22:29–36.
- Lebovics RS, Hoffman GS, Leavitt RY, et al. The management of subglottic stenosis in patients with Wegener’s granulomatosis. Laryngoscope 1992; 102:1341–1345.
- Waxman J, Bose WJ. Laryngeal manifestations of Wegener’s granulomatosis: case reports and review of the literature. J Rheumatol 1986; 13:408–411.
- Maronian NC, Azadeh H, Waugh P, Hillel A. Association of laryngopharyngeal reflux disease and subglottic stenosis. Ann Otol Rhinol Laryngol 2001; 110:606–612.
- Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004; 9:283–289.
- Gubbels SP, Barkhuizen A, Hwang PH. Head and neck manifestations of Wegener’s granulomatosis. Otolaryngol Clin North Am 2003; 36:685–705.
- Polychronopoulos VS, Prakash UB, Golbin JM, Edell ES, Specks U. Airway involvement in Wegener’s granulomatosis. Rheum Dis Clin North Am 2007; 33:755–775.
- Daum TE, Specks U, Colby TV, et al. Tracheobronchial involvement in Wegener’s granulomatosis. Am J Respir Crit Care Med 1995; 151:522–526.
- Rottem M, Fauci AS, Hallahan CW, et al Wegener granulomatosis in children and adolescents: clinical presentation and outcome. J Pediatr 1993; 122:26–31.
- Eliachar I, Chan J, Akst L. New approaches to the management of subglottic stenosis in Wegener’s granulomatosis. Cleve Clin J Med 2002; 69( suppl 2):SII149–SII151.
- Solans-Laqué R, Bosch-Gil J, Canela M, Lorente J, Pallisa E, Vilardell-Tarrés M. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Lupus 2008; 17:832–836.
- Sandu K, Monnier P. Cricotracheal resection. Otolaryngol Clin North Am 2008; 41:981–998.
- Brouns M, Jayaraju ST, Lacor C, De Mey J, et al. Tracheal stenosis: a flow dynamics study [published online ahead of print November 30, 2006]. J Appl Physiol 2007; 102:1178–1184. doi: 10.1152/japplphysiol.01063.2006
- Pauzner R, Mayan H, Hershko E, Alcalay M, Farfel Z. Exacerbation of Wegener’s granulomatosis during pregnancy: report of a case with tracheal stenosis and literature review. J Rheumatol 1994; 21:1153–1156.
- Langford CA, Sneller MC, Hallahan CW, et al. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Arthritis Rheum 1996; 39:1754–1760.
- Schokkenbroek AA, Franssen CF, Dikkers FG. Dilatation tracheoscopy for laryngeal and tracheal stenosis in patients with Wegener’s granulomatosis [published online ahead of print November 14, 2007]. Eur Arch Otorhinolaryngol 2008; 265:549–555. doi: 10.1007/s00405-007-0518-3
- Hoffman GS, Thomas-Golbanov CK, Chan J, Akst LM, Eliachar I. Treatment of subglottic stenosis, due to Wegener’s granulomatosis, with intralesional corticosteroids and dilation. J Rheumatol 2003; 30:1017–1021.
- Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope 2009; 119:272–283.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Mahr A, Girard T, Agher R, Guillevin L. Analysis of factors predictive of survival based on 49 patients with systemic Wegener’s granulomatosis and prospective follow-up. Rheumatology (Oxford) 2001; 40:492–498.
- Wung PK, Stone JH. Therapeutics of Wegener’s granulomatosis. Nat Clin Pract Rheumatol 2006; 2:192–200.
- Rao JK, Weinberger M, Oddone EZ, Allen NB, Landsman P, Feussner JR. The role of antineutrophil cytoplasmic antibody (c-ANCA) testing in the diagnosis of Wegener granulomatosis: a literature review and meta-analysis. Ann Intern Med 1995; 123:925–932.
- Devaney KO, Travis WD, Hoffman G, Leavitt R, Lebovics R, Fauci AS. Interpretation of head and neck biopsies in Wegener’s granulomatosis: a pathologic study of 126 biopsies in 70 patients. Am J Surg Pathol 1990; 14:555–564.
- Jennings CR, Jones NS, Dugar J, Powell RJ, Lowe J. Wegener’s granulomatosis—a review of diagnosis and treatment in 53 subjects. Rhinology 1998; 36:188–191.
- McDonald TJ, DeRemee RA. Wegener’s granulomatosis. Laryngoscope 1983; 93:220–231.
- Lloyd G, Lund VJ, Beale T, Howard D. Rhinologic changes in Wegener’s granulomatosis. J Laryngol Otol 2002; 116:565–569.
- Yang C, Talbot JM, Hwang PH. Bony abnormalities of the paranasal sinuses in patients with Wegener’s granulomatosis. Am J Rhinol 2001; 15:121–125.
- Cannady SB, Batra PS, Koening C, et al. Sinonasal Wegener granulomatosis: a single-institution experience with 120 cases. Laryngoscope 2009; 119:757–761.
- Grindler D, Cannady S, Batra PS. Computed tomography findings in sinonasal Wegener’s granulomatosis. Am J Rhinol Allergy 2009; 23:497–501.
- Hughes RG, Drake-Lee A. Nasal manifestations of granulomatous disease. Hosp Med 2001; 62:417–421.
- Srouji IA, Andrews P, Edwards C, Lund VJ. General and rhinosinusitis-related quality of life in patients with Wegener’s granulomatosis. Laryngoscope 2006; 116:1621–1625.
- Kornblut AD, Wolff SM, deFries HO, Fauci AS. Wegener’s granulomatosis. Laryngoscope 1980; 90:1453–1465.
- McCaffrey TV, McDonald TJ, Facer GW, DeRemee RA. Otologic manifestations of Wegener’s granulomatosis. Otolaryngol Head Neck Surg 1980; 88:586–593.
- Bradley PJ. Wegener’s granulomatosis of the ear. J Laryngol Otol 1983; 97:623–626.
- Rasmussen N. Management of the ear, nose, and throat manifestations of Wegener granulomatosis: an otorhinolaryngologist’s perspective. Curr Opin Rheumatol 2001; 13:3–11.
- Erickson VR, Hwang PH. Wegener’s granulomatosis: current trends in diagnosis and management. Curr Opin Otolaryngol Head Neck Surg 2007; 15:170–176.
- Congdon D, Sherris DA, Specks U, McDonald T. Long-term follow-up of repair of external nasal deformities in patients with Wegener’s granulomatosis. Laryngoscope 2002; 112:731–737.
- Duffy FJ, Rossi RM, Pribaz JJ. Reconstruction of Wegener’s nasal deformity using bilateral facial artery musculomucosal flaps. Plast Reconstr Surg 1998; 101:1330–1333.
- Shipchandler TZ, Chung BJ, Alam DS. Saddle nose deformity reconstruction with a split calvarial bone L-shaped strut. Arch Facial Plast Surg 2008; 10:305–311.
- Hernández-Rodríguez J, Hoffman GS, Koening CL. Surgical interventions and local therapy for Wegener’s granulomatosis. Curr Opin Rheumatol 2010; 22:29–36.
- Lebovics RS, Hoffman GS, Leavitt RY, et al. The management of subglottic stenosis in patients with Wegener’s granulomatosis. Laryngoscope 1992; 102:1341–1345.
- Waxman J, Bose WJ. Laryngeal manifestations of Wegener’s granulomatosis: case reports and review of the literature. J Rheumatol 1986; 13:408–411.
- Maronian NC, Azadeh H, Waugh P, Hillel A. Association of laryngopharyngeal reflux disease and subglottic stenosis. Ann Otol Rhinol Laryngol 2001; 110:606–612.
- Diegelmann RF, Evans MC. Wound healing: an overview of acute, fibrotic and delayed healing. Front Biosci 2004; 9:283–289.
- Gubbels SP, Barkhuizen A, Hwang PH. Head and neck manifestations of Wegener’s granulomatosis. Otolaryngol Clin North Am 2003; 36:685–705.
- Polychronopoulos VS, Prakash UB, Golbin JM, Edell ES, Specks U. Airway involvement in Wegener’s granulomatosis. Rheum Dis Clin North Am 2007; 33:755–775.
- Daum TE, Specks U, Colby TV, et al. Tracheobronchial involvement in Wegener’s granulomatosis. Am J Respir Crit Care Med 1995; 151:522–526.
- Rottem M, Fauci AS, Hallahan CW, et al Wegener granulomatosis in children and adolescents: clinical presentation and outcome. J Pediatr 1993; 122:26–31.
- Eliachar I, Chan J, Akst L. New approaches to the management of subglottic stenosis in Wegener’s granulomatosis. Cleve Clin J Med 2002; 69( suppl 2):SII149–SII151.
- Solans-Laqué R, Bosch-Gil J, Canela M, Lorente J, Pallisa E, Vilardell-Tarrés M. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Lupus 2008; 17:832–836.
- Sandu K, Monnier P. Cricotracheal resection. Otolaryngol Clin North Am 2008; 41:981–998.
- Brouns M, Jayaraju ST, Lacor C, De Mey J, et al. Tracheal stenosis: a flow dynamics study [published online ahead of print November 30, 2006]. J Appl Physiol 2007; 102:1178–1184. doi: 10.1152/japplphysiol.01063.2006
- Pauzner R, Mayan H, Hershko E, Alcalay M, Farfel Z. Exacerbation of Wegener’s granulomatosis during pregnancy: report of a case with tracheal stenosis and literature review. J Rheumatol 1994; 21:1153–1156.
- Langford CA, Sneller MC, Hallahan CW, et al. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Arthritis Rheum 1996; 39:1754–1760.
- Schokkenbroek AA, Franssen CF, Dikkers FG. Dilatation tracheoscopy for laryngeal and tracheal stenosis in patients with Wegener’s granulomatosis [published online ahead of print November 14, 2007]. Eur Arch Otorhinolaryngol 2008; 265:549–555. doi: 10.1007/s00405-007-0518-3
- Hoffman GS, Thomas-Golbanov CK, Chan J, Akst LM, Eliachar I. Treatment of subglottic stenosis, due to Wegener’s granulomatosis, with intralesional corticosteroids and dilation. J Rheumatol 2003; 30:1017–1021.
- Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope 2009; 119:272–283.
Renal disease in small-vessel vasculitis
Necrotizing glomerulonephritis (GN) is the classical renal manifestation of the small-vessel vasculitides, which include granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]), microscopic polyangiitis (MPA), and eosinophilic GPA (Churg-Strauss syndrome). MPA is pauci-immune (lacks antibody depositions) and causes focal segmental necrotizing GN. Other small-vessel vasculitides are Henoch-SchÖnlein purpura, which features nephritis from immunoglobulin A–dominant immune deposits, and essential cryoglobulinemic vasculitis, in which cryoglobulin immune deposits lead to membranoproliferative GN.1
Renal involvement occurs in 25% to 75% of patients with antineutrophil cytoplasmic antibody (ANCA)–associated systemic vasculitis (AASV), with the higher percentage reflecting patients who first present to a nephrologist rather than a rheumatologist. Roughly 35% are dialysis-dependent, mainly those who are elderly or proteinase-3– or myeloperoxidase-ANCA–positive. In duc tion of remission through immunosuppression allows 50% to 60% of dialysis-dependent patients to recover independent renal function.2,3
PROGNOSTIC SIGNIFICANCE OF RENAL INVOLVEMENT
Patients should be assessed at the earliest opportunity for renal involvement as it is highly predictive of survival. Diagnosis allows immunosuppressive treatment to be started early, when kidney function may still be preserved.
Reinhold-Keller et al3 examined survival by level of renal involvement at diagnosis in 155 patients with GPA who were followed for a median of 7 years. Survival in those with normal renal function, but with nephritic urinary sediment at diagnosis, declined over time compared with patients who had no renal involvement at diagnosis, with a more than twofold greater risk of death (hazard ratio [HR], 2.41; 95% confidence interval [CI] 0.53–11.06). Patients who had impaired renal function at diagnosis had a fivefold greater risk of death (HR, 5.42; 95% CI 1.76–16.68).
Maximum serum creatinine in the first month of treatment is also highly predictive of survival. An outcome analysis followed 80 patients with AASV and renal involvement for a median of 46.7 months.4 Patients were divided equally into groups by maximum serum creatinine levels after the first month of treatment: less than 299 μmol/L, 299 to 582 μmol/L, and greater than 582 μmol/L. All patients were treated for induction of remission with cyclophosphamide and oral corticosteroids. Survival was significantly worse in patients who had the highest maximum serum creatinine in the first month (P = .025).
Pooled prospective data from four European Vasculitis Study Group (EUVAS) trials of 535 patients with AASV and follow-up of 5.2 years found a mortality ratio of 2.6 (95% CI, 2.2–3.1) compared with matched subjects from the general population.5 Stage 5 chronic kidney disease (glomerular filtration rate < 15 mL/min) was a significant negative prognostic determinant of survival in these trials.
A DISEASE OF AGING
Renal vasculitis is most prevalent in people aged 50 years and older and often occurs in those aged 70 years and older.6 Because the very old may not have extrarenal symptoms, it is necessary to maintain a high index of suspicion for AASV and measure urinary sediment and renal function in this age group.
Older patients also present with more severe renal disease and have a poorer prognosis. Harper and Savage compared presentation and outcomes of patients aged 65 years and older with renal AASV with those of patients younger than 65 years.7 Older patients had more severe renal failure than did younger patients (serum creatinine 657 vs 470 μmol/L, respectively; P < .001), and this did not appear to be associated with delayed diagnosis. Survival was worse in those with serum creatinine levels greater than 400 μmol/L irrespective of age; however, when comparing younger and older groups with similar renal insufficiency, older patients were more likely to progress to end-stage renal failure (P = .039), survival was worse (P = .016), and death occurred earlier.7
HISTOPATHOLOGIC CLASSIFICATION
An international working group of renal pathologists8 developed pathologic classifications for rapidly progressive GN caused by AASV: focal (≥ 50% normal glomeruli), crescentic (≥ 50% glomeruli with cellular crescents), mixed (no predominant glomerular feature), and sclerotic (≥ 50% globally sclerotic glomeruli). These correspond to the order of severity of renal-function impairment. A study of 100 biopsies from patients with ANCA-associated GN found that the classifications at presentation closely correlated with outcomes.8
Patients with sclerotic GN had the worst renal function initially, with little improvement at 5 years; hence, immunosuppression is of little value if the kidney is more than 50% sclerotic. Patients with focal disease who had good renal function initially were found to retain good function after 5 years of treatment. Patients in the crescentic class present with rapidly progressive GN and very poor renal function. However, they were found to improve considerably after 5 years and had good recovery (P = .001). Presenting disease manifestations within the kidney are of diagnostic as well as prognostic value.
TREATMENT OF AASV
Lower dosage of cyclophosphamide
Standard immunosuppressive treatment for vasculitis is oral cyclophosphamide, 2 mg/kg per day. To reduce toxic effects and amount of the drug used, we tested whether a pulse dose could induce remission. In the EUVAS randomized trial of oral versus pulse cyclophosphamide (the CYCLOPS study),9 149 AASV patients with renal involvement received either pulse cyclophosphamide, 15 mg/kg every 2 to 3 weeks, or daily oral cyclophosphamide, 2 mg/kg per day, plus prednisone. The groups did not differ in time to remission (HR, 1.098) or in proportion of patients who had achieved remission at 9 months (88.1% vs 87.7%). The pulse-dose group needed half the amount of drug to achieve remission compared with the oral-dose group. Pulse dosing is currently the preferred method in Europe, where doses are administered in the clinic rather than at home.
In a 4.3-year follow-up, twice as many patients relapsed in the pulse-dose group compared with the oral group (HR, 0.50; P = .029), but there was no difference between groups in renal function (P = .82), end-stage renal disease (ESRD), or death.10 It should be borne in mind that there is a tendency to overtreat. An analysis of four EUVAS trials found a risk of mortality of 11% in the first year; 59% of these were due to treatment-related adverse events.11
Despite being prone to renal failure and infectious complications, elderly patients with ANCA-associated GN who do not have ESRD fare better with immunosuppressive therapy than without in terms of progression and survival. Cyclophosphamide dosage should be reduced in these patients.
Plasma exchange
In crescentic disease and rapidly progressing renal failure, plasma exchange (PE) with albumin reduces circulating antibodies by up to 60% and promotes renal recovery. The MEPEX trial compared the addition of either PE or intravenous methylprednisolone (MEP) to oral cyclophosphamide and prednisolone in 137 newly diagnosed AASV patients with severe crescentic GN (serum creatinine > 500 μmol/L).12 Two-thirds of the group were oliguric. After 3 months, 69% of PE-treated compared with 49% of MEP-treated patients were alive and had achieved independent renal function (P = .02). There was also a reduction in risk for ESRD of 24% at 12 months in the PE versus MEP group. Mortality was similarly high in both groups, however; roughly one-third had died by 12 months, reflecting the higher rates of complications in this older-aged group (median, 66 years).
For patients undergoing PE who have a sudden drop in hemoglobin, gastrointestinal bleeding is only one possible underlying condition. The patient may have bleeding into the pulmonary parenchyma, and computed tomography of the lung should be performed. This is more likely to occur when plasma is exchanged with albumin rather than fresh frozen plasma.
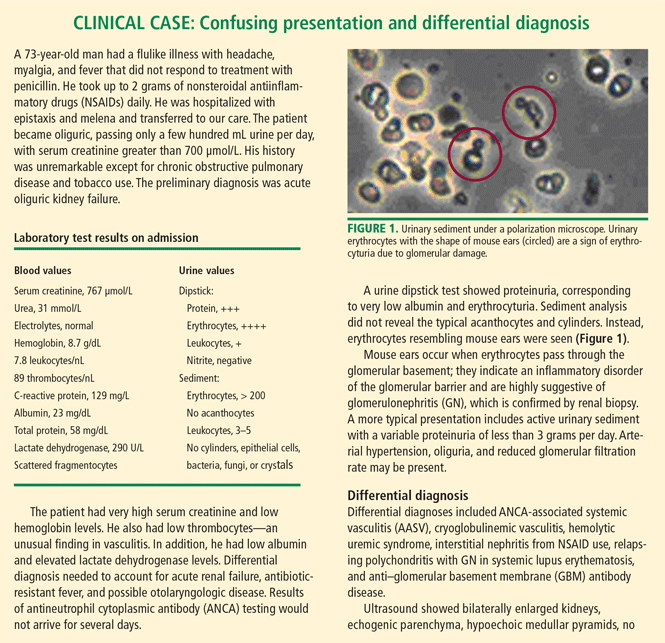
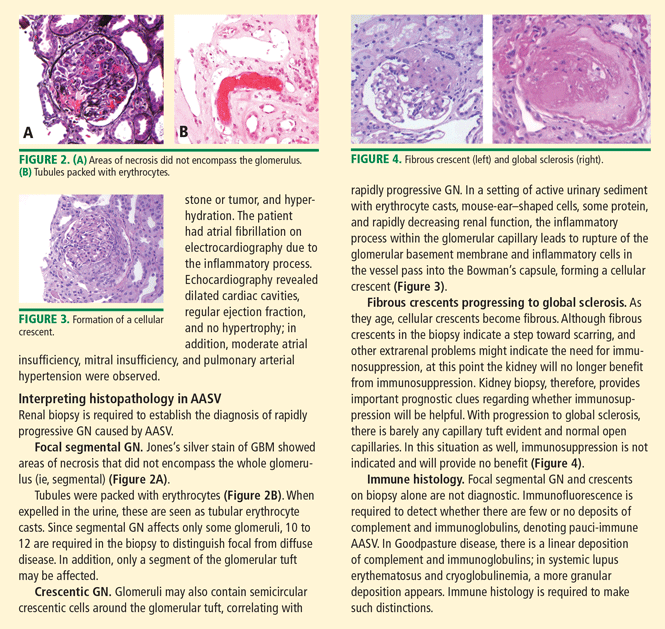
Renal replacement therapy
End-stage renal disease occurs in approximately 25% of patients 3 to 4 years after they present with AASV. Renal-limited disease occurs most often in those with MPA. When there is active rather than sclerotic disease but irreversible renal failure is suspected, immunosuppression can be tried for 3 months. If there is no response, improvement in renal function is unlikely and immunosuppressive treatment is continued only for extrarenal disease. Patients with ESRD can be treated with hemodialysis, peritoneal dialysis at home, or kidney transplant.
Lionaki et al13 described the rate of relapse in AASV patients before and after kidney dialysis compared with that in AASV patients with preserved renal function. Over a median of 40 months, 136 of 523 patients progressed to ESRD. Rate of relapse of vasculitis was significantly lower for the patients on chronic dialysis (0.08 episodes per person-year) than for the same patients before dialysis (0.2 episodes) and for the patients with preserved renal function (0.15 episodes). Infections, an important cause of death, were twice as frequent for patients on dialysis and maintenance immunosuppression.
Weidanz et al14 reported on a retrospective case series that examined whether immunosuppressive therapy with its risk of infection is beneficial for vasculitis patients on dialysis. They retrospectively examined 46 cases of AASV over 30 years and found that the patients with ESRD received less immunosuppression, but their rate of infection was twice that of pre-ESRD patients, and mortality quadrupled while on dialysis. The mode of dialysis did not affect survival, however. These results may support early discontinuation of immunosuppressive treatment in patients with ESRD and suggest that it be used only in those with active disease.
Renal transplant
At the time of transplantation, patients receive a massive immunosupressive induction regimen consisting of anti-CD25 antibody and triple conventional immunosuppressive drugs, usually a calcineurin inhibitor, antimetabolite, and prednisolone. Survival in transplant patients with vasculitis is not significantly different from that of other kidney transplant patients.15
Patients should not receive transplants until at least 12 months after induction of remission; patients who underwent transplant less than 12 months after remission had a mortality HR of 2.3 (P < .05).16 Vasculopathy occurs more frequently in ANCA-positive patients, which leads to graft loss.
Graft loss due to recurrent vasculitis is also possible. Nachman et al17 found double the rate of infection for transplant compared with nontransplant AASV patients, but the rate of relapse was lower than the rate before transplant or for patients on dialysis. There was no significant difference in rates of relapse between patients with or without circulating ANCA at the time of transplant or between those having GPA, MPA, or renal-limited disease.18
Cardiovascular risk
Renal involvement in vasculitis increases cardiovascular morbidity. Vasculitis patients with renal involvement (n =113) were matched with patients with chronic kidney disease and other contributing cardiovascular risk factors.18 After approximately 4 years of follow-up, the vasculitis patients had an HR of 2.23 (P = .017) for cardiovascular events. AASV patients with the highest excess risk had histories of cardiovascular events (HR, 4), dialysis dependency (HR, 4.3), poor renal function at admission (HR, 0.977), and history of smoking (HR, 3.9).
CONCLUSION
Level of renal function at diagnosis is an important predictor of survival. Poor renal function correlates with mortality, especially in the elderly. Pathologic classification of renal vasculitis based on histopathology obtained from the kidney biopsy correlates closely with prognosis. About 60% of initially dialysis-dependent patients with active GN can regain independent renal function. Those on dialysis have a lower rate of relapse of active vasculitis than do those with independent renal function; patients with kidney transplants have the lowest rate of relapse. There is a doubling of infection rate in patients who have ESRD and who receive any form of renal replacement therapy. Lastly, renal involvement in AASV is an independent and serious contributor to risk for cardiovascular disease.
- Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides: proposal of an International Consensus Conference. Arthritis Rheum 1994; 37:187–192.
- Booth AD, Almond MK, Burns A, et al; for the Pan-Thames Renal Research Group. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 2003; 41:776–784.
- Reinhold-Keller E, Beuge N, Latza U, et al. An interdisciplinary approach to the care of patients with Wegener’s granulomatosis: long-term outcome in 155 patients. Arthritis Rheum 2000; 43:1021–1032.
- Weidner S, Geuss S, Hafezi-Rachti S, Wonka A, Rupprecht HD. ANCA-associated vasculitis with renal involvement: an outcome analysis [published online ahead of print April 6, 2004]. Nephrol Dial Transplant 2004; 19:1403–1411. doi: 10.1093/ndt/gfh161
- Flossmann O, Berden A, de Groot K, et al; for the European Vasculitis Study Group. Long-term patient survival in ANCA-associated vasculitis [published online ahead of print November 24, 2010]. Ann Rheum Dis 2011; 70:488–494. doi: 10.1136/ard.2010.137778
- Hamour SM, Salama AD. ANCA comes of age—but with caveats. Kidney Int 2011; 79:699–701.
- Harper L, Savage CO. ANCA-associated renal vasculitis at the end of the twentieth century—a disease of older patients [published online ahead of print December 21, 2004]. Rheumatology 2005; 44:495–501. doi: 10.1093/rheumatology/keh522
- Berden AE, Ferrario F, Hagen EC, et al. Histopathologic classification of ANCA-associated glomerulonephritis [published online ahead of print July 8, 2010]. J Am Soc Nephrol 2010; 21:1628–1636. doi: 10.1681/ASN.2010050477
- de Groot K, Harper L, Jayne DRW, et al; for the EUVAS (European Vasculitis Study Group). Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody–associated vasculitis: a randomized trial. Ann Intern Med 2009; 150:670–680.
- Harper L, Morgan MD, Walsh M, et al; on behalf of the EUVAS Investigators. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: long-term follow-up [published online ahead of print November 29, 2011]. Ann Rheum Dis 2012; 71:955–960. doi: 10.1136/annrheumdis-2011-200477
- Little MA, Nightingale P, Verburgh CA, et al; for the European Vasculitis Study (EUVAS) Group. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis [published online ahead of print July 1, 2009]. Ann Rheum Dis 2010; 69:1036–1043. doi: 10.1136/ard.2009.109389
- Jayne DR, Gaskin G, Rasmussen N, et al; for the European Vasculitis Study Group. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vascultis [published online ahead of print June 20, 2007]. J Am Soc Nephrol 2007; 18:2180–2188. doi: 10.1681/ASN.2007010090
- Lionaki S, Hogan SL, Jennette CE, et al. The clinical course of ANCA small-vessel vasculitis on chronic dialysis [published online ahead of print June 17, 2009]. Kidney Int 2009; 76:644–651. doi: 10.1038/ki.2009.218
- Weidanz F, Day CJ, Hewins P, Savage CO, Harper L. Recurrences and infections during continuous immunosuppressive therapy after beginning dialysis in ANCA-associated vasculitis. Am J Kidney Dis 2007; 50:36–46.
- Schmitt WH, van der Woude FJ. Organ transplantation in the vasculitides. Curr Opin Rheumatol 2003; 15:22–28.
- Little MA, Hassan B, Jacques S, et al. Renal transplantation in systemic vasculitis: when is it safe [published online ahead of print July 13, 2009]? Nephrol Dial Transplant 2009; 24:3219–3225. doi: 10.1093/ndt/gfp347
- Nachman PH, Segelmark M, Westman K, et al. Recurrent ANCA-associated small vessel vasculitis after transplantation: a pooled analysis. Kidney Int 1999; 56:1544–1550.
- Morgan MD, Turnbull J, Selamet U, et al. Increased incidence of cardiovascular events in patients with antineutrophil cytoplasmic antibody-associated vasculitides: a matched-pair cohort study. Arthritis Rheum 2009; 60:3493–3500.
Necrotizing glomerulonephritis (GN) is the classical renal manifestation of the small-vessel vasculitides, which include granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]), microscopic polyangiitis (MPA), and eosinophilic GPA (Churg-Strauss syndrome). MPA is pauci-immune (lacks antibody depositions) and causes focal segmental necrotizing GN. Other small-vessel vasculitides are Henoch-SchÖnlein purpura, which features nephritis from immunoglobulin A–dominant immune deposits, and essential cryoglobulinemic vasculitis, in which cryoglobulin immune deposits lead to membranoproliferative GN.1
Renal involvement occurs in 25% to 75% of patients with antineutrophil cytoplasmic antibody (ANCA)–associated systemic vasculitis (AASV), with the higher percentage reflecting patients who first present to a nephrologist rather than a rheumatologist. Roughly 35% are dialysis-dependent, mainly those who are elderly or proteinase-3– or myeloperoxidase-ANCA–positive. In duc tion of remission through immunosuppression allows 50% to 60% of dialysis-dependent patients to recover independent renal function.2,3
PROGNOSTIC SIGNIFICANCE OF RENAL INVOLVEMENT
Patients should be assessed at the earliest opportunity for renal involvement as it is highly predictive of survival. Diagnosis allows immunosuppressive treatment to be started early, when kidney function may still be preserved.
Reinhold-Keller et al3 examined survival by level of renal involvement at diagnosis in 155 patients with GPA who were followed for a median of 7 years. Survival in those with normal renal function, but with nephritic urinary sediment at diagnosis, declined over time compared with patients who had no renal involvement at diagnosis, with a more than twofold greater risk of death (hazard ratio [HR], 2.41; 95% confidence interval [CI] 0.53–11.06). Patients who had impaired renal function at diagnosis had a fivefold greater risk of death (HR, 5.42; 95% CI 1.76–16.68).
Maximum serum creatinine in the first month of treatment is also highly predictive of survival. An outcome analysis followed 80 patients with AASV and renal involvement for a median of 46.7 months.4 Patients were divided equally into groups by maximum serum creatinine levels after the first month of treatment: less than 299 μmol/L, 299 to 582 μmol/L, and greater than 582 μmol/L. All patients were treated for induction of remission with cyclophosphamide and oral corticosteroids. Survival was significantly worse in patients who had the highest maximum serum creatinine in the first month (P = .025).
Pooled prospective data from four European Vasculitis Study Group (EUVAS) trials of 535 patients with AASV and follow-up of 5.2 years found a mortality ratio of 2.6 (95% CI, 2.2–3.1) compared with matched subjects from the general population.5 Stage 5 chronic kidney disease (glomerular filtration rate < 15 mL/min) was a significant negative prognostic determinant of survival in these trials.
A DISEASE OF AGING
Renal vasculitis is most prevalent in people aged 50 years and older and often occurs in those aged 70 years and older.6 Because the very old may not have extrarenal symptoms, it is necessary to maintain a high index of suspicion for AASV and measure urinary sediment and renal function in this age group.
Older patients also present with more severe renal disease and have a poorer prognosis. Harper and Savage compared presentation and outcomes of patients aged 65 years and older with renal AASV with those of patients younger than 65 years.7 Older patients had more severe renal failure than did younger patients (serum creatinine 657 vs 470 μmol/L, respectively; P < .001), and this did not appear to be associated with delayed diagnosis. Survival was worse in those with serum creatinine levels greater than 400 μmol/L irrespective of age; however, when comparing younger and older groups with similar renal insufficiency, older patients were more likely to progress to end-stage renal failure (P = .039), survival was worse (P = .016), and death occurred earlier.7
HISTOPATHOLOGIC CLASSIFICATION
An international working group of renal pathologists8 developed pathologic classifications for rapidly progressive GN caused by AASV: focal (≥ 50% normal glomeruli), crescentic (≥ 50% glomeruli with cellular crescents), mixed (no predominant glomerular feature), and sclerotic (≥ 50% globally sclerotic glomeruli). These correspond to the order of severity of renal-function impairment. A study of 100 biopsies from patients with ANCA-associated GN found that the classifications at presentation closely correlated with outcomes.8
Patients with sclerotic GN had the worst renal function initially, with little improvement at 5 years; hence, immunosuppression is of little value if the kidney is more than 50% sclerotic. Patients with focal disease who had good renal function initially were found to retain good function after 5 years of treatment. Patients in the crescentic class present with rapidly progressive GN and very poor renal function. However, they were found to improve considerably after 5 years and had good recovery (P = .001). Presenting disease manifestations within the kidney are of diagnostic as well as prognostic value.
TREATMENT OF AASV
Lower dosage of cyclophosphamide
Standard immunosuppressive treatment for vasculitis is oral cyclophosphamide, 2 mg/kg per day. To reduce toxic effects and amount of the drug used, we tested whether a pulse dose could induce remission. In the EUVAS randomized trial of oral versus pulse cyclophosphamide (the CYCLOPS study),9 149 AASV patients with renal involvement received either pulse cyclophosphamide, 15 mg/kg every 2 to 3 weeks, or daily oral cyclophosphamide, 2 mg/kg per day, plus prednisone. The groups did not differ in time to remission (HR, 1.098) or in proportion of patients who had achieved remission at 9 months (88.1% vs 87.7%). The pulse-dose group needed half the amount of drug to achieve remission compared with the oral-dose group. Pulse dosing is currently the preferred method in Europe, where doses are administered in the clinic rather than at home.
In a 4.3-year follow-up, twice as many patients relapsed in the pulse-dose group compared with the oral group (HR, 0.50; P = .029), but there was no difference between groups in renal function (P = .82), end-stage renal disease (ESRD), or death.10 It should be borne in mind that there is a tendency to overtreat. An analysis of four EUVAS trials found a risk of mortality of 11% in the first year; 59% of these were due to treatment-related adverse events.11
Despite being prone to renal failure and infectious complications, elderly patients with ANCA-associated GN who do not have ESRD fare better with immunosuppressive therapy than without in terms of progression and survival. Cyclophosphamide dosage should be reduced in these patients.
Plasma exchange
In crescentic disease and rapidly progressing renal failure, plasma exchange (PE) with albumin reduces circulating antibodies by up to 60% and promotes renal recovery. The MEPEX trial compared the addition of either PE or intravenous methylprednisolone (MEP) to oral cyclophosphamide and prednisolone in 137 newly diagnosed AASV patients with severe crescentic GN (serum creatinine > 500 μmol/L).12 Two-thirds of the group were oliguric. After 3 months, 69% of PE-treated compared with 49% of MEP-treated patients were alive and had achieved independent renal function (P = .02). There was also a reduction in risk for ESRD of 24% at 12 months in the PE versus MEP group. Mortality was similarly high in both groups, however; roughly one-third had died by 12 months, reflecting the higher rates of complications in this older-aged group (median, 66 years).
For patients undergoing PE who have a sudden drop in hemoglobin, gastrointestinal bleeding is only one possible underlying condition. The patient may have bleeding into the pulmonary parenchyma, and computed tomography of the lung should be performed. This is more likely to occur when plasma is exchanged with albumin rather than fresh frozen plasma.


Renal replacement therapy
End-stage renal disease occurs in approximately 25% of patients 3 to 4 years after they present with AASV. Renal-limited disease occurs most often in those with MPA. When there is active rather than sclerotic disease but irreversible renal failure is suspected, immunosuppression can be tried for 3 months. If there is no response, improvement in renal function is unlikely and immunosuppressive treatment is continued only for extrarenal disease. Patients with ESRD can be treated with hemodialysis, peritoneal dialysis at home, or kidney transplant.
Lionaki et al13 described the rate of relapse in AASV patients before and after kidney dialysis compared with that in AASV patients with preserved renal function. Over a median of 40 months, 136 of 523 patients progressed to ESRD. Rate of relapse of vasculitis was significantly lower for the patients on chronic dialysis (0.08 episodes per person-year) than for the same patients before dialysis (0.2 episodes) and for the patients with preserved renal function (0.15 episodes). Infections, an important cause of death, were twice as frequent for patients on dialysis and maintenance immunosuppression.
Weidanz et al14 reported on a retrospective case series that examined whether immunosuppressive therapy with its risk of infection is beneficial for vasculitis patients on dialysis. They retrospectively examined 46 cases of AASV over 30 years and found that the patients with ESRD received less immunosuppression, but their rate of infection was twice that of pre-ESRD patients, and mortality quadrupled while on dialysis. The mode of dialysis did not affect survival, however. These results may support early discontinuation of immunosuppressive treatment in patients with ESRD and suggest that it be used only in those with active disease.
Renal transplant
At the time of transplantation, patients receive a massive immunosupressive induction regimen consisting of anti-CD25 antibody and triple conventional immunosuppressive drugs, usually a calcineurin inhibitor, antimetabolite, and prednisolone. Survival in transplant patients with vasculitis is not significantly different from that of other kidney transplant patients.15
Patients should not receive transplants until at least 12 months after induction of remission; patients who underwent transplant less than 12 months after remission had a mortality HR of 2.3 (P < .05).16 Vasculopathy occurs more frequently in ANCA-positive patients, which leads to graft loss.
Graft loss due to recurrent vasculitis is also possible. Nachman et al17 found double the rate of infection for transplant compared with nontransplant AASV patients, but the rate of relapse was lower than the rate before transplant or for patients on dialysis. There was no significant difference in rates of relapse between patients with or without circulating ANCA at the time of transplant or between those having GPA, MPA, or renal-limited disease.18
Cardiovascular risk
Renal involvement in vasculitis increases cardiovascular morbidity. Vasculitis patients with renal involvement (n =113) were matched with patients with chronic kidney disease and other contributing cardiovascular risk factors.18 After approximately 4 years of follow-up, the vasculitis patients had an HR of 2.23 (P = .017) for cardiovascular events. AASV patients with the highest excess risk had histories of cardiovascular events (HR, 4), dialysis dependency (HR, 4.3), poor renal function at admission (HR, 0.977), and history of smoking (HR, 3.9).
CONCLUSION
Level of renal function at diagnosis is an important predictor of survival. Poor renal function correlates with mortality, especially in the elderly. Pathologic classification of renal vasculitis based on histopathology obtained from the kidney biopsy correlates closely with prognosis. About 60% of initially dialysis-dependent patients with active GN can regain independent renal function. Those on dialysis have a lower rate of relapse of active vasculitis than do those with independent renal function; patients with kidney transplants have the lowest rate of relapse. There is a doubling of infection rate in patients who have ESRD and who receive any form of renal replacement therapy. Lastly, renal involvement in AASV is an independent and serious contributor to risk for cardiovascular disease.
Necrotizing glomerulonephritis (GN) is the classical renal manifestation of the small-vessel vasculitides, which include granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]), microscopic polyangiitis (MPA), and eosinophilic GPA (Churg-Strauss syndrome). MPA is pauci-immune (lacks antibody depositions) and causes focal segmental necrotizing GN. Other small-vessel vasculitides are Henoch-SchÖnlein purpura, which features nephritis from immunoglobulin A–dominant immune deposits, and essential cryoglobulinemic vasculitis, in which cryoglobulin immune deposits lead to membranoproliferative GN.1
Renal involvement occurs in 25% to 75% of patients with antineutrophil cytoplasmic antibody (ANCA)–associated systemic vasculitis (AASV), with the higher percentage reflecting patients who first present to a nephrologist rather than a rheumatologist. Roughly 35% are dialysis-dependent, mainly those who are elderly or proteinase-3– or myeloperoxidase-ANCA–positive. In duc tion of remission through immunosuppression allows 50% to 60% of dialysis-dependent patients to recover independent renal function.2,3
PROGNOSTIC SIGNIFICANCE OF RENAL INVOLVEMENT
Patients should be assessed at the earliest opportunity for renal involvement as it is highly predictive of survival. Diagnosis allows immunosuppressive treatment to be started early, when kidney function may still be preserved.
Reinhold-Keller et al3 examined survival by level of renal involvement at diagnosis in 155 patients with GPA who were followed for a median of 7 years. Survival in those with normal renal function, but with nephritic urinary sediment at diagnosis, declined over time compared with patients who had no renal involvement at diagnosis, with a more than twofold greater risk of death (hazard ratio [HR], 2.41; 95% confidence interval [CI] 0.53–11.06). Patients who had impaired renal function at diagnosis had a fivefold greater risk of death (HR, 5.42; 95% CI 1.76–16.68).
Maximum serum creatinine in the first month of treatment is also highly predictive of survival. An outcome analysis followed 80 patients with AASV and renal involvement for a median of 46.7 months.4 Patients were divided equally into groups by maximum serum creatinine levels after the first month of treatment: less than 299 μmol/L, 299 to 582 μmol/L, and greater than 582 μmol/L. All patients were treated for induction of remission with cyclophosphamide and oral corticosteroids. Survival was significantly worse in patients who had the highest maximum serum creatinine in the first month (P = .025).
Pooled prospective data from four European Vasculitis Study Group (EUVAS) trials of 535 patients with AASV and follow-up of 5.2 years found a mortality ratio of 2.6 (95% CI, 2.2–3.1) compared with matched subjects from the general population.5 Stage 5 chronic kidney disease (glomerular filtration rate < 15 mL/min) was a significant negative prognostic determinant of survival in these trials.
A DISEASE OF AGING
Renal vasculitis is most prevalent in people aged 50 years and older and often occurs in those aged 70 years and older.6 Because the very old may not have extrarenal symptoms, it is necessary to maintain a high index of suspicion for AASV and measure urinary sediment and renal function in this age group.
Older patients also present with more severe renal disease and have a poorer prognosis. Harper and Savage compared presentation and outcomes of patients aged 65 years and older with renal AASV with those of patients younger than 65 years.7 Older patients had more severe renal failure than did younger patients (serum creatinine 657 vs 470 μmol/L, respectively; P < .001), and this did not appear to be associated with delayed diagnosis. Survival was worse in those with serum creatinine levels greater than 400 μmol/L irrespective of age; however, when comparing younger and older groups with similar renal insufficiency, older patients were more likely to progress to end-stage renal failure (P = .039), survival was worse (P = .016), and death occurred earlier.7
HISTOPATHOLOGIC CLASSIFICATION
An international working group of renal pathologists8 developed pathologic classifications for rapidly progressive GN caused by AASV: focal (≥ 50% normal glomeruli), crescentic (≥ 50% glomeruli with cellular crescents), mixed (no predominant glomerular feature), and sclerotic (≥ 50% globally sclerotic glomeruli). These correspond to the order of severity of renal-function impairment. A study of 100 biopsies from patients with ANCA-associated GN found that the classifications at presentation closely correlated with outcomes.8
Patients with sclerotic GN had the worst renal function initially, with little improvement at 5 years; hence, immunosuppression is of little value if the kidney is more than 50% sclerotic. Patients with focal disease who had good renal function initially were found to retain good function after 5 years of treatment. Patients in the crescentic class present with rapidly progressive GN and very poor renal function. However, they were found to improve considerably after 5 years and had good recovery (P = .001). Presenting disease manifestations within the kidney are of diagnostic as well as prognostic value.
TREATMENT OF AASV
Lower dosage of cyclophosphamide
Standard immunosuppressive treatment for vasculitis is oral cyclophosphamide, 2 mg/kg per day. To reduce toxic effects and amount of the drug used, we tested whether a pulse dose could induce remission. In the EUVAS randomized trial of oral versus pulse cyclophosphamide (the CYCLOPS study),9 149 AASV patients with renal involvement received either pulse cyclophosphamide, 15 mg/kg every 2 to 3 weeks, or daily oral cyclophosphamide, 2 mg/kg per day, plus prednisone. The groups did not differ in time to remission (HR, 1.098) or in proportion of patients who had achieved remission at 9 months (88.1% vs 87.7%). The pulse-dose group needed half the amount of drug to achieve remission compared with the oral-dose group. Pulse dosing is currently the preferred method in Europe, where doses are administered in the clinic rather than at home.
In a 4.3-year follow-up, twice as many patients relapsed in the pulse-dose group compared with the oral group (HR, 0.50; P = .029), but there was no difference between groups in renal function (P = .82), end-stage renal disease (ESRD), or death.10 It should be borne in mind that there is a tendency to overtreat. An analysis of four EUVAS trials found a risk of mortality of 11% in the first year; 59% of these were due to treatment-related adverse events.11
Despite being prone to renal failure and infectious complications, elderly patients with ANCA-associated GN who do not have ESRD fare better with immunosuppressive therapy than without in terms of progression and survival. Cyclophosphamide dosage should be reduced in these patients.
Plasma exchange
In crescentic disease and rapidly progressing renal failure, plasma exchange (PE) with albumin reduces circulating antibodies by up to 60% and promotes renal recovery. The MEPEX trial compared the addition of either PE or intravenous methylprednisolone (MEP) to oral cyclophosphamide and prednisolone in 137 newly diagnosed AASV patients with severe crescentic GN (serum creatinine > 500 μmol/L).12 Two-thirds of the group were oliguric. After 3 months, 69% of PE-treated compared with 49% of MEP-treated patients were alive and had achieved independent renal function (P = .02). There was also a reduction in risk for ESRD of 24% at 12 months in the PE versus MEP group. Mortality was similarly high in both groups, however; roughly one-third had died by 12 months, reflecting the higher rates of complications in this older-aged group (median, 66 years).
For patients undergoing PE who have a sudden drop in hemoglobin, gastrointestinal bleeding is only one possible underlying condition. The patient may have bleeding into the pulmonary parenchyma, and computed tomography of the lung should be performed. This is more likely to occur when plasma is exchanged with albumin rather than fresh frozen plasma.


Renal replacement therapy
End-stage renal disease occurs in approximately 25% of patients 3 to 4 years after they present with AASV. Renal-limited disease occurs most often in those with MPA. When there is active rather than sclerotic disease but irreversible renal failure is suspected, immunosuppression can be tried for 3 months. If there is no response, improvement in renal function is unlikely and immunosuppressive treatment is continued only for extrarenal disease. Patients with ESRD can be treated with hemodialysis, peritoneal dialysis at home, or kidney transplant.
Lionaki et al13 described the rate of relapse in AASV patients before and after kidney dialysis compared with that in AASV patients with preserved renal function. Over a median of 40 months, 136 of 523 patients progressed to ESRD. Rate of relapse of vasculitis was significantly lower for the patients on chronic dialysis (0.08 episodes per person-year) than for the same patients before dialysis (0.2 episodes) and for the patients with preserved renal function (0.15 episodes). Infections, an important cause of death, were twice as frequent for patients on dialysis and maintenance immunosuppression.
Weidanz et al14 reported on a retrospective case series that examined whether immunosuppressive therapy with its risk of infection is beneficial for vasculitis patients on dialysis. They retrospectively examined 46 cases of AASV over 30 years and found that the patients with ESRD received less immunosuppression, but their rate of infection was twice that of pre-ESRD patients, and mortality quadrupled while on dialysis. The mode of dialysis did not affect survival, however. These results may support early discontinuation of immunosuppressive treatment in patients with ESRD and suggest that it be used only in those with active disease.
Renal transplant
At the time of transplantation, patients receive a massive immunosupressive induction regimen consisting of anti-CD25 antibody and triple conventional immunosuppressive drugs, usually a calcineurin inhibitor, antimetabolite, and prednisolone. Survival in transplant patients with vasculitis is not significantly different from that of other kidney transplant patients.15
Patients should not receive transplants until at least 12 months after induction of remission; patients who underwent transplant less than 12 months after remission had a mortality HR of 2.3 (P < .05).16 Vasculopathy occurs more frequently in ANCA-positive patients, which leads to graft loss.
Graft loss due to recurrent vasculitis is also possible. Nachman et al17 found double the rate of infection for transplant compared with nontransplant AASV patients, but the rate of relapse was lower than the rate before transplant or for patients on dialysis. There was no significant difference in rates of relapse between patients with or without circulating ANCA at the time of transplant or between those having GPA, MPA, or renal-limited disease.18
Cardiovascular risk
Renal involvement in vasculitis increases cardiovascular morbidity. Vasculitis patients with renal involvement (n =113) were matched with patients with chronic kidney disease and other contributing cardiovascular risk factors.18 After approximately 4 years of follow-up, the vasculitis patients had an HR of 2.23 (P = .017) for cardiovascular events. AASV patients with the highest excess risk had histories of cardiovascular events (HR, 4), dialysis dependency (HR, 4.3), poor renal function at admission (HR, 0.977), and history of smoking (HR, 3.9).
CONCLUSION
Level of renal function at diagnosis is an important predictor of survival. Poor renal function correlates with mortality, especially in the elderly. Pathologic classification of renal vasculitis based on histopathology obtained from the kidney biopsy correlates closely with prognosis. About 60% of initially dialysis-dependent patients with active GN can regain independent renal function. Those on dialysis have a lower rate of relapse of active vasculitis than do those with independent renal function; patients with kidney transplants have the lowest rate of relapse. There is a doubling of infection rate in patients who have ESRD and who receive any form of renal replacement therapy. Lastly, renal involvement in AASV is an independent and serious contributor to risk for cardiovascular disease.
- Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides: proposal of an International Consensus Conference. Arthritis Rheum 1994; 37:187–192.
- Booth AD, Almond MK, Burns A, et al; for the Pan-Thames Renal Research Group. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 2003; 41:776–784.
- Reinhold-Keller E, Beuge N, Latza U, et al. An interdisciplinary approach to the care of patients with Wegener’s granulomatosis: long-term outcome in 155 patients. Arthritis Rheum 2000; 43:1021–1032.
- Weidner S, Geuss S, Hafezi-Rachti S, Wonka A, Rupprecht HD. ANCA-associated vasculitis with renal involvement: an outcome analysis [published online ahead of print April 6, 2004]. Nephrol Dial Transplant 2004; 19:1403–1411. doi: 10.1093/ndt/gfh161
- Flossmann O, Berden A, de Groot K, et al; for the European Vasculitis Study Group. Long-term patient survival in ANCA-associated vasculitis [published online ahead of print November 24, 2010]. Ann Rheum Dis 2011; 70:488–494. doi: 10.1136/ard.2010.137778
- Hamour SM, Salama AD. ANCA comes of age—but with caveats. Kidney Int 2011; 79:699–701.
- Harper L, Savage CO. ANCA-associated renal vasculitis at the end of the twentieth century—a disease of older patients [published online ahead of print December 21, 2004]. Rheumatology 2005; 44:495–501. doi: 10.1093/rheumatology/keh522
- Berden AE, Ferrario F, Hagen EC, et al. Histopathologic classification of ANCA-associated glomerulonephritis [published online ahead of print July 8, 2010]. J Am Soc Nephrol 2010; 21:1628–1636. doi: 10.1681/ASN.2010050477
- de Groot K, Harper L, Jayne DRW, et al; for the EUVAS (European Vasculitis Study Group). Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody–associated vasculitis: a randomized trial. Ann Intern Med 2009; 150:670–680.
- Harper L, Morgan MD, Walsh M, et al; on behalf of the EUVAS Investigators. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: long-term follow-up [published online ahead of print November 29, 2011]. Ann Rheum Dis 2012; 71:955–960. doi: 10.1136/annrheumdis-2011-200477
- Little MA, Nightingale P, Verburgh CA, et al; for the European Vasculitis Study (EUVAS) Group. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis [published online ahead of print July 1, 2009]. Ann Rheum Dis 2010; 69:1036–1043. doi: 10.1136/ard.2009.109389
- Jayne DR, Gaskin G, Rasmussen N, et al; for the European Vasculitis Study Group. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vascultis [published online ahead of print June 20, 2007]. J Am Soc Nephrol 2007; 18:2180–2188. doi: 10.1681/ASN.2007010090
- Lionaki S, Hogan SL, Jennette CE, et al. The clinical course of ANCA small-vessel vasculitis on chronic dialysis [published online ahead of print June 17, 2009]. Kidney Int 2009; 76:644–651. doi: 10.1038/ki.2009.218
- Weidanz F, Day CJ, Hewins P, Savage CO, Harper L. Recurrences and infections during continuous immunosuppressive therapy after beginning dialysis in ANCA-associated vasculitis. Am J Kidney Dis 2007; 50:36–46.
- Schmitt WH, van der Woude FJ. Organ transplantation in the vasculitides. Curr Opin Rheumatol 2003; 15:22–28.
- Little MA, Hassan B, Jacques S, et al. Renal transplantation in systemic vasculitis: when is it safe [published online ahead of print July 13, 2009]? Nephrol Dial Transplant 2009; 24:3219–3225. doi: 10.1093/ndt/gfp347
- Nachman PH, Segelmark M, Westman K, et al. Recurrent ANCA-associated small vessel vasculitis after transplantation: a pooled analysis. Kidney Int 1999; 56:1544–1550.
- Morgan MD, Turnbull J, Selamet U, et al. Increased incidence of cardiovascular events in patients with antineutrophil cytoplasmic antibody-associated vasculitides: a matched-pair cohort study. Arthritis Rheum 2009; 60:3493–3500.
- Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides: proposal of an International Consensus Conference. Arthritis Rheum 1994; 37:187–192.
- Booth AD, Almond MK, Burns A, et al; for the Pan-Thames Renal Research Group. Outcome of ANCA-associated renal vasculitis: a 5-year retrospective study. Am J Kidney Dis 2003; 41:776–784.
- Reinhold-Keller E, Beuge N, Latza U, et al. An interdisciplinary approach to the care of patients with Wegener’s granulomatosis: long-term outcome in 155 patients. Arthritis Rheum 2000; 43:1021–1032.
- Weidner S, Geuss S, Hafezi-Rachti S, Wonka A, Rupprecht HD. ANCA-associated vasculitis with renal involvement: an outcome analysis [published online ahead of print April 6, 2004]. Nephrol Dial Transplant 2004; 19:1403–1411. doi: 10.1093/ndt/gfh161
- Flossmann O, Berden A, de Groot K, et al; for the European Vasculitis Study Group. Long-term patient survival in ANCA-associated vasculitis [published online ahead of print November 24, 2010]. Ann Rheum Dis 2011; 70:488–494. doi: 10.1136/ard.2010.137778
- Hamour SM, Salama AD. ANCA comes of age—but with caveats. Kidney Int 2011; 79:699–701.
- Harper L, Savage CO. ANCA-associated renal vasculitis at the end of the twentieth century—a disease of older patients [published online ahead of print December 21, 2004]. Rheumatology 2005; 44:495–501. doi: 10.1093/rheumatology/keh522
- Berden AE, Ferrario F, Hagen EC, et al. Histopathologic classification of ANCA-associated glomerulonephritis [published online ahead of print July 8, 2010]. J Am Soc Nephrol 2010; 21:1628–1636. doi: 10.1681/ASN.2010050477
- de Groot K, Harper L, Jayne DRW, et al; for the EUVAS (European Vasculitis Study Group). Pulse versus daily oral cyclophosphamide for induction of remission in antineutrophil cytoplasmic antibody–associated vasculitis: a randomized trial. Ann Intern Med 2009; 150:670–680.
- Harper L, Morgan MD, Walsh M, et al; on behalf of the EUVAS Investigators. Pulse versus daily oral cyclophosphamide for induction of remission in ANCA-associated vasculitis: long-term follow-up [published online ahead of print November 29, 2011]. Ann Rheum Dis 2012; 71:955–960. doi: 10.1136/annrheumdis-2011-200477
- Little MA, Nightingale P, Verburgh CA, et al; for the European Vasculitis Study (EUVAS) Group. Early mortality in systemic vasculitis: relative contribution of adverse events and active vasculitis [published online ahead of print July 1, 2009]. Ann Rheum Dis 2010; 69:1036–1043. doi: 10.1136/ard.2009.109389
- Jayne DR, Gaskin G, Rasmussen N, et al; for the European Vasculitis Study Group. Randomized trial of plasma exchange or high-dosage methylprednisolone as adjunctive therapy for severe renal vascultis [published online ahead of print June 20, 2007]. J Am Soc Nephrol 2007; 18:2180–2188. doi: 10.1681/ASN.2007010090
- Lionaki S, Hogan SL, Jennette CE, et al. The clinical course of ANCA small-vessel vasculitis on chronic dialysis [published online ahead of print June 17, 2009]. Kidney Int 2009; 76:644–651. doi: 10.1038/ki.2009.218
- Weidanz F, Day CJ, Hewins P, Savage CO, Harper L. Recurrences and infections during continuous immunosuppressive therapy after beginning dialysis in ANCA-associated vasculitis. Am J Kidney Dis 2007; 50:36–46.
- Schmitt WH, van der Woude FJ. Organ transplantation in the vasculitides. Curr Opin Rheumatol 2003; 15:22–28.
- Little MA, Hassan B, Jacques S, et al. Renal transplantation in systemic vasculitis: when is it safe [published online ahead of print July 13, 2009]? Nephrol Dial Transplant 2009; 24:3219–3225. doi: 10.1093/ndt/gfp347
- Nachman PH, Segelmark M, Westman K, et al. Recurrent ANCA-associated small vessel vasculitis after transplantation: a pooled analysis. Kidney Int 1999; 56:1544–1550.
- Morgan MD, Turnbull J, Selamet U, et al. Increased incidence of cardiovascular events in patients with antineutrophil cytoplasmic antibody-associated vasculitides: a matched-pair cohort study. Arthritis Rheum 2009; 60:3493–3500.
Pulmonary disease in small-vessel vasculitis
The pulmonary manifestations of small-vessel vasculitis are nonspecific and often overlap with other conditions. Consequently, the diagnosis and management of pulmonary vasculitis are complex and require special attention to detail. This article reviews clinical experience with vasculitis as it manifests in the pulmonary setting, with the goal of providing a sound clinical approach to diagnosis and management.
DIAGNOSTIC CONSIDERATIONS
Accurate diagnosis is enhanced with imaging technology, judicious use of bronchoscopy, and awareness of disorders that mimic or masquerade as pulmonary vasculitis. The diagnosis can be approached on the basis of pattern recognition. For example, microscopic polyangiitis (MPA) is characterized solely by alveolar hemorrhage syndrome. However, other diagnostic possibilities must be considered, such as infection, acute respiratory distress syndrome, and complications of medicines. The hallmark manifestation of granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is necrotizing granulomatous inflammations, but the pulmonary manifestations can include nodules, cavitary masses, airway stenosis, and alveolar hemorrhage. Asthma with eosinophilia is the distinguishing feature of eosinophilic GPA (Churg-Strauss syndrome, EGPA), and Goodpasture syndrome involves deposition of complement and immunoglobulins.
The use of imaging
The best imaging tool for suspected pulmonary vasculitides is high-resolution computed tomography (CT). As a general rule, CT for patients with suspected vasculitis should be ordered without contrast medium as contrast is not needed to assess the lung parenchyma. Vasculitis patients often have renal insufficiency, and contrast-free CT will help protect the kidneys. Another option, which will enhance evaluation of the distribution and location of pulmonary disease, is multiplanar reconstructions of images with virtual bronchoscopy or airway reconstruction. Certain findings on imaging will help to differentiate the vasculitides from one another as well as from mimicking diagnoses.
Eosinophilic GPA. Chest images of patients with EGPA appear as patchy, nonsegmental, often peripheral consolidations of ground-glass opacity. These tend to reside in all lobes of the lungs, close to the surface and occasionally accompanied by septal markings.
Microscopic polyangiitis. Although classically a disease of alveolar hemorrhage, MPA often does not manifest with hemoptysis. Approximately one-third of patients with MPA do not cough up blood, even after a large amount of hemorrhage directly into the parenchyma. Patients may present with nonspecific symptoms such as fatigue and shortness of breath. Chest imaging will enhance diagnostic accuracy, particularly when considered in conjunction with laboratory test results. MPA patients usually have low hematocrit levels and may actually have an increased diffusing capacity of the lung for carbon monoxide (Dlco).
Granulomatosis with polyangiitis. This form of vasculitis has characteristic nodules, cavitary lesions, and, in the worst cases, multifocal masses in the lungs. These can be identified with contrast-free CT, with examination for possible airway involvement.
Multiple lung cavity nodules and pronounced airway narrowing are significant diagnostic clues for GPA. Nodules up to 10 cm in diameter tend to be near sub-pleural and peripheral areas. Microbes and fungus may complicate the nodules’ primary presentation. While bronchoscopy may be helpful with imaging, surgical biopsy remains the gold standard to rule out infections.
The disease may be multifocal, occurring outside the lungs from the larynx to bronchi and anywhere in the lung. Subglottic stenosis caused by inflammation and scarring affects 16% of patients with GPA, but it also often develops independently of other features of GPA and may have its own course independent of systemic symptoms.1
Bronchoscopy
Bronchoscopy is a relatively low-risk way to assess airways and nodules, but it has had a limited role in the diagnosis of nonfocal interstitial lung disease and rheumatologic lung disease in general. New technologies that augment traditional bronchoscopy and enhance its utility for diagnosis for focal entities are described below.
Electromagnetic navigation bronchoscopy (ENB) uses electromagnetic technology to localize and guide a catheter through the bronchial pathways. With the help of a virtual, 3-dimensional bronchial map reconstructed from a chest CT, the clinician can navigate to a desired location within the lung for biopsy and diagnosis of pulmonary nodules. The result is a diagnostic yield per nodule of nearly 80%.2 Seijo et al showed that diagnostic yields by ENB increase with the presence of the bronchus sign, or a bronchus leading directly to a peripheral lung lesion, as viewed on CT imaging.2 If nodules are bronchocentric, or surround airways, there is greater likelihood of reaching a diagnosis without resorting to surgery.
In peripheral radial ultrasound, a catheter is threaded through another catheter sheath in order to visualize the lesion. This technology can precisely localize lung lesions and often give some clues about the final pathology.
Bronchoscopic confocal fluorescence microscopy3 is a new form of microscopy that uses a fiberoptic miniprobe instead of an objective lens. High-quality images are achieved by the use of autofluorescence. Researchers have used the technology to detect changes in the respiratory bronchioles and other structures, but a clear atlas of many disease states does not yet exist. Oddly, endobronchial GPA images have been catalogued.3
Virtual bronchoscopy is a 3-dimensional image reconstruction and display technique that converts standard CT images into multiplanar images, which can be stacked. Virtual bronchoscopy augments conventional CT because of its ability to enhance detection in the subglottic region and more accurately measure stenosis.4 The technique cannot replace traditional bronchoscopy, however, because mucus and secretions can appear as abnormalities and cause false-positive results.
Detecting mimics
Diagnoses that masquerade as EGPA include chronic eosinophilic pneumonia, bronchiolitis obliterans with organizing pneumonia, and other interstitial lung diseases. Allergic bronchopulmonary aspergillosis—an asthma syndrome sometimes associated with eosinophilia and high immunoglobulin-E levels—also mimics EGPA. This diagnostic possibility is particularly relevant if the patient is taking immunosuppressive agents or corticosteroids.
Although alveolar hemorrhage is the sole pulmonary manifestation of MPA, the diagnosis is not limited to MPA alone. Alveolar hemorrhage may have other causes, including infection or acute respiratory distress syndrome. Bronchial lavage is recommended for accurate diagnosis, with the introduction of successive volumes of saline into the lungs and examination for increasing amounts of heme in each of the aliquots of alveolar lavage fluid.
Several diagnoses can mimic GPA. Many infections, including those caused by mycobacteria and Cryptococcus, can mimic endobronchial GPA. Biopsy of all new ulcers is recommended to minimize the possibility of missing these diagnoses. Tuberculosis in its latent form can closely resemble scarred GPA. Other mimickers of cavitary lung lesions can include metastatic melanoma, metastatic renal and thyroid cancers, squamous cell carcinoma, and rheumatoid arthritis with necrobiotic nodules that open in the lungs.
TREATMENT STRATEGIES
Medications
Although many patients with GPA are surgical candidates because of dyspnea related to fixed endobronchial or endotracheal obstructions, any surgical treatment carries the risk of inciting further flares. Treatment should focus first on mitigating the systemic inflammatory disorder with pharmacologic intervention. Standard pharmacologic therapy includes corticosteroids, azathioprine, cyclophosphamide, and rituximab. Patients with subglottic stenosis are frequently unresponsive to standard immunosuppressive therapy (glucocorticoids in combination with a cytotoxic agent).1
Surgical reconstruction
When medication falls short and surgery is needed to reverse strictures, a number of tools are at our disposal. Some involve heat, such as laser, cauterization, and argon plasma coagulation. In argon plasma coagulation, a jet of ionized argon gas (plasma) is directed through a probe passed through an endoscope. Other techniques rely on cold: cryoprobes, microdebriders, and rigid scissors. In general, freeze therapies cause less scarring than heat therapy. With any surgical technique, there is risk of scars that will contract and cause structural collapse, resulting in restenosis.
Dilation
The high rate of stenosis relapse has spurred interest in alternatives to surgical treatment. One of these, dilation via endoscopy, also may mitigate the wound healing process. Other techniques for clearing the obstructed area include rigid bronchoscopy, the use of bougies (increasingly larger dilators), and balloon dilation. Balloon dilation has some advantages over the other techniques. It permits maximal radial direction and pressure, causes less damage to trachea wall mucosa, and achieves better overall results; however, the procedure usually needs to be repeated.5 It must be done quickly, and it requires flawless communication between the otolaryngologist or pulmonologist and anesthesiologist in order to stabilize the airway below the vocal cords.
Intratracheal dilation-injection therapy
Dilation can be augmented with glucocorticoid injections. In 1991, researchers at the National Institutes of Health utilized a combination dilation-injection therapy for 20 patients who had GPA and subglottic stenosis.1 Patients were first treated with mercury-filled dilators coated with 1% triamcinolone cream. Methylprednisolone acetate was then injected into the stenotic area. None of the patients treated with intratracheal dilation-injection therapy required a tracheostomy and six who already had tracheostomies were decannulated. In contrast, 56% of patients who received standard immunosuppressive therapy and no intratracheal dilation-injection therapy required tracheostomy. Intratracheal dilation-injection therapy is considered a safe and effective treatment of GPA-associated subglottic stenosis and, in the absence of major organ disease activity, could be used without systemic immunosuppressive agents.
Mitomycin-C is a controversial alternative to corticosteroids during dilation. Mitomycin-C is an alkylating agent that inhibits fibroblast proliferation and extracellular matrix protein synthesis, with the potential for reduced scarring. In a recent trial of 26 patients, two doses given 3 to 4 weeks apart reduced the rate of stenosis for 2 to 3 years compared with a single dose.6 Restenosis occurred in both groups, however, and after 5 years, the relapse rates were the same.
Nd:YAG laser photoresection versus endobronchial electrosurgery
One of the most effective therapies for treating obstructive lesions is Nd:YAG laser photoresection (LPR) in which a laser that utilizes the crystal neodymium-doped yttrium aluminum garnet (Nd:Y3Al5O12) is paired with a flexible bronchoscope. The procedure can produce favorable outcomes,7 but it has not gained favor because of perceptions that the lasers require rigid bronchoscopy, expensive equipment, and special training. There are also concerns about complications.
The lower-cost endobronchial electrosurgery (EBES) also failed to gain acceptance because of cumbersome delivery systems and complications associated with power units. Recently, engineers have spawned a new generation of electrosurgical devices, prompting renewed interest in EBES.
A recent study compared LPR and EBES in patients who represented 118 evaluations for LPR.8 Forty percent were considered amenable to EBES and so did not go on to receive the more costly LPR. Of those, 89% achieved success in alleviating the obstruction. The authors concluded that EBES can potentially eliminate the need for LPR in 36% of procedures, and that it could achieve significant savings in cost and time. We use these ablative therapies only in dire circumstances; we use non–heat-based therapies, including repeated dilation, prior to considering use of other therapies.
Cryotherapy
Cryotherapy spray was initially thought to have great therapeutic potential, but the high pressures of the spray caused complications. This modality remains under investigation, however. Some probe-based cryotherapy techniques have been effective anecdotally. These use a metal-tipped probe attached to a cryogen; the Joule-Thompson effect causes delayed tissue destruction.
Stents
A small number of case reports note patient improvement after stenting.9,10 We use stents in rare circumstances, but because complications are frequent and sometimes severe, we consider stenting a last-resort option. In 2005, the US Food and Drug Administration mandated a Black Box warning against the use of metallic stents in patients who have benign tracheal strictures.
Multimodality therapies
In general, when intervention is required to salvage airways, a combination of dilation and steroid injection with or without topical mitomycin-C is standard. We try to avoid use of thermal therapy with laser or electrocautery because of the risk of exuberant inflammation and restenosis from thermal injury. No specific standard of care exists in these cases; reliance on clinical judgment is critical because of the presentation and variety of airway lesions. Further, no large-scale randomized trials exist to guide therapy, so it is best to work with a multidisciplinary team whose members have experience in managing these complex patients.
CONCLUSION
The differential diagnosis of pulmonary manifestations of small-vessel vasculitis is complex. Several diagnoses can mimic various forms of pulmonary vasculitis, and the manifestations and symptoms often overlap with other organ systems.
Imaging is useful for analysis of common patterns of small and midsize vasculitis, although the results may be confounded by disorders that mimic pulmonary vasculitis. To enhance diagnostic accuracy, laboratory and clinical findings should be considered along with images. Ideally, treatment will be minimally destructive and mucosa-sparing. Dilation therapies can be augmented with corticosteroid injections or, possibly, mitomycin-C.
- Langford CA, Sneller MC, Hallahan CW, et al. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Arthritis Rheum 1996; 39:1754–1760.
- Seijo LM, de Torres JP, Lozano MD, et al. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a bronchus sign on CT imaging: results from a prospective study. Chest 2010; 138:1316–1321.
- Filner JJ, Bonura EJ, Lau ST, et al. Bronchoscopic fibered confocal fluorescence microscopy image characteristics and pathologic correlations. J Bronchol Intervent Pulmonol 2011; 18:23–30.
- Summers RM, Aggarwal NR, Sneller MC, et al. CT virtual bronchoscopy of the central airway in patients with Wegener’s granulomatosis. Chest 2002; 121:242–250.
- Schokkenbroek AA, Franssen CFM, Dikkers FG. Dilatation tracheoscopy for laryngeal and tracheal stenosis in patients with Wegener’s granulomatosis. Eur Arch Otorhinolaryngol 2008; 265:549–555.
- Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope 2009; 119:272–283.
- Shvero J, Shitrit D, Koren R, Shalomi D, Kramer MR. Endoscopic laser surgery for subglottic stenosis in Wegener’s granulomatosis. Yonsei Med J 2007; 48:748–753.
- Coulter TD, Mehta AC. The heat is on: impact of endobronchial electrosurgery on the need for Nd-YAG laser photoresection. Chest 2000; 118:516–521.
- Tierman J, Shah C, Elborn JS. Successful stenting in endobronchial Wegener’s granulomatosis. Ulster Med J 2006; 75:155–157.
- Daum TE, Specks U, Colby TV, et al. Tracheobronchial involvement in Wegener’s granulomatosis. Am J Respir Crit Care Med 1995; 151:522–526.
The pulmonary manifestations of small-vessel vasculitis are nonspecific and often overlap with other conditions. Consequently, the diagnosis and management of pulmonary vasculitis are complex and require special attention to detail. This article reviews clinical experience with vasculitis as it manifests in the pulmonary setting, with the goal of providing a sound clinical approach to diagnosis and management.
DIAGNOSTIC CONSIDERATIONS
Accurate diagnosis is enhanced with imaging technology, judicious use of bronchoscopy, and awareness of disorders that mimic or masquerade as pulmonary vasculitis. The diagnosis can be approached on the basis of pattern recognition. For example, microscopic polyangiitis (MPA) is characterized solely by alveolar hemorrhage syndrome. However, other diagnostic possibilities must be considered, such as infection, acute respiratory distress syndrome, and complications of medicines. The hallmark manifestation of granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is necrotizing granulomatous inflammations, but the pulmonary manifestations can include nodules, cavitary masses, airway stenosis, and alveolar hemorrhage. Asthma with eosinophilia is the distinguishing feature of eosinophilic GPA (Churg-Strauss syndrome, EGPA), and Goodpasture syndrome involves deposition of complement and immunoglobulins.
The use of imaging
The best imaging tool for suspected pulmonary vasculitides is high-resolution computed tomography (CT). As a general rule, CT for patients with suspected vasculitis should be ordered without contrast medium as contrast is not needed to assess the lung parenchyma. Vasculitis patients often have renal insufficiency, and contrast-free CT will help protect the kidneys. Another option, which will enhance evaluation of the distribution and location of pulmonary disease, is multiplanar reconstructions of images with virtual bronchoscopy or airway reconstruction. Certain findings on imaging will help to differentiate the vasculitides from one another as well as from mimicking diagnoses.
Eosinophilic GPA. Chest images of patients with EGPA appear as patchy, nonsegmental, often peripheral consolidations of ground-glass opacity. These tend to reside in all lobes of the lungs, close to the surface and occasionally accompanied by septal markings.
Microscopic polyangiitis. Although classically a disease of alveolar hemorrhage, MPA often does not manifest with hemoptysis. Approximately one-third of patients with MPA do not cough up blood, even after a large amount of hemorrhage directly into the parenchyma. Patients may present with nonspecific symptoms such as fatigue and shortness of breath. Chest imaging will enhance diagnostic accuracy, particularly when considered in conjunction with laboratory test results. MPA patients usually have low hematocrit levels and may actually have an increased diffusing capacity of the lung for carbon monoxide (Dlco).
Granulomatosis with polyangiitis. This form of vasculitis has characteristic nodules, cavitary lesions, and, in the worst cases, multifocal masses in the lungs. These can be identified with contrast-free CT, with examination for possible airway involvement.
Multiple lung cavity nodules and pronounced airway narrowing are significant diagnostic clues for GPA. Nodules up to 10 cm in diameter tend to be near sub-pleural and peripheral areas. Microbes and fungus may complicate the nodules’ primary presentation. While bronchoscopy may be helpful with imaging, surgical biopsy remains the gold standard to rule out infections.
The disease may be multifocal, occurring outside the lungs from the larynx to bronchi and anywhere in the lung. Subglottic stenosis caused by inflammation and scarring affects 16% of patients with GPA, but it also often develops independently of other features of GPA and may have its own course independent of systemic symptoms.1
Bronchoscopy
Bronchoscopy is a relatively low-risk way to assess airways and nodules, but it has had a limited role in the diagnosis of nonfocal interstitial lung disease and rheumatologic lung disease in general. New technologies that augment traditional bronchoscopy and enhance its utility for diagnosis for focal entities are described below.
Electromagnetic navigation bronchoscopy (ENB) uses electromagnetic technology to localize and guide a catheter through the bronchial pathways. With the help of a virtual, 3-dimensional bronchial map reconstructed from a chest CT, the clinician can navigate to a desired location within the lung for biopsy and diagnosis of pulmonary nodules. The result is a diagnostic yield per nodule of nearly 80%.2 Seijo et al showed that diagnostic yields by ENB increase with the presence of the bronchus sign, or a bronchus leading directly to a peripheral lung lesion, as viewed on CT imaging.2 If nodules are bronchocentric, or surround airways, there is greater likelihood of reaching a diagnosis without resorting to surgery.
In peripheral radial ultrasound, a catheter is threaded through another catheter sheath in order to visualize the lesion. This technology can precisely localize lung lesions and often give some clues about the final pathology.
Bronchoscopic confocal fluorescence microscopy3 is a new form of microscopy that uses a fiberoptic miniprobe instead of an objective lens. High-quality images are achieved by the use of autofluorescence. Researchers have used the technology to detect changes in the respiratory bronchioles and other structures, but a clear atlas of many disease states does not yet exist. Oddly, endobronchial GPA images have been catalogued.3
Virtual bronchoscopy is a 3-dimensional image reconstruction and display technique that converts standard CT images into multiplanar images, which can be stacked. Virtual bronchoscopy augments conventional CT because of its ability to enhance detection in the subglottic region and more accurately measure stenosis.4 The technique cannot replace traditional bronchoscopy, however, because mucus and secretions can appear as abnormalities and cause false-positive results.
Detecting mimics
Diagnoses that masquerade as EGPA include chronic eosinophilic pneumonia, bronchiolitis obliterans with organizing pneumonia, and other interstitial lung diseases. Allergic bronchopulmonary aspergillosis—an asthma syndrome sometimes associated with eosinophilia and high immunoglobulin-E levels—also mimics EGPA. This diagnostic possibility is particularly relevant if the patient is taking immunosuppressive agents or corticosteroids.
Although alveolar hemorrhage is the sole pulmonary manifestation of MPA, the diagnosis is not limited to MPA alone. Alveolar hemorrhage may have other causes, including infection or acute respiratory distress syndrome. Bronchial lavage is recommended for accurate diagnosis, with the introduction of successive volumes of saline into the lungs and examination for increasing amounts of heme in each of the aliquots of alveolar lavage fluid.
Several diagnoses can mimic GPA. Many infections, including those caused by mycobacteria and Cryptococcus, can mimic endobronchial GPA. Biopsy of all new ulcers is recommended to minimize the possibility of missing these diagnoses. Tuberculosis in its latent form can closely resemble scarred GPA. Other mimickers of cavitary lung lesions can include metastatic melanoma, metastatic renal and thyroid cancers, squamous cell carcinoma, and rheumatoid arthritis with necrobiotic nodules that open in the lungs.
TREATMENT STRATEGIES
Medications
Although many patients with GPA are surgical candidates because of dyspnea related to fixed endobronchial or endotracheal obstructions, any surgical treatment carries the risk of inciting further flares. Treatment should focus first on mitigating the systemic inflammatory disorder with pharmacologic intervention. Standard pharmacologic therapy includes corticosteroids, azathioprine, cyclophosphamide, and rituximab. Patients with subglottic stenosis are frequently unresponsive to standard immunosuppressive therapy (glucocorticoids in combination with a cytotoxic agent).1
Surgical reconstruction
When medication falls short and surgery is needed to reverse strictures, a number of tools are at our disposal. Some involve heat, such as laser, cauterization, and argon plasma coagulation. In argon plasma coagulation, a jet of ionized argon gas (plasma) is directed through a probe passed through an endoscope. Other techniques rely on cold: cryoprobes, microdebriders, and rigid scissors. In general, freeze therapies cause less scarring than heat therapy. With any surgical technique, there is risk of scars that will contract and cause structural collapse, resulting in restenosis.
Dilation
The high rate of stenosis relapse has spurred interest in alternatives to surgical treatment. One of these, dilation via endoscopy, also may mitigate the wound healing process. Other techniques for clearing the obstructed area include rigid bronchoscopy, the use of bougies (increasingly larger dilators), and balloon dilation. Balloon dilation has some advantages over the other techniques. It permits maximal radial direction and pressure, causes less damage to trachea wall mucosa, and achieves better overall results; however, the procedure usually needs to be repeated.5 It must be done quickly, and it requires flawless communication between the otolaryngologist or pulmonologist and anesthesiologist in order to stabilize the airway below the vocal cords.
Intratracheal dilation-injection therapy
Dilation can be augmented with glucocorticoid injections. In 1991, researchers at the National Institutes of Health utilized a combination dilation-injection therapy for 20 patients who had GPA and subglottic stenosis.1 Patients were first treated with mercury-filled dilators coated with 1% triamcinolone cream. Methylprednisolone acetate was then injected into the stenotic area. None of the patients treated with intratracheal dilation-injection therapy required a tracheostomy and six who already had tracheostomies were decannulated. In contrast, 56% of patients who received standard immunosuppressive therapy and no intratracheal dilation-injection therapy required tracheostomy. Intratracheal dilation-injection therapy is considered a safe and effective treatment of GPA-associated subglottic stenosis and, in the absence of major organ disease activity, could be used without systemic immunosuppressive agents.
Mitomycin-C is a controversial alternative to corticosteroids during dilation. Mitomycin-C is an alkylating agent that inhibits fibroblast proliferation and extracellular matrix protein synthesis, with the potential for reduced scarring. In a recent trial of 26 patients, two doses given 3 to 4 weeks apart reduced the rate of stenosis for 2 to 3 years compared with a single dose.6 Restenosis occurred in both groups, however, and after 5 years, the relapse rates were the same.
Nd:YAG laser photoresection versus endobronchial electrosurgery
One of the most effective therapies for treating obstructive lesions is Nd:YAG laser photoresection (LPR) in which a laser that utilizes the crystal neodymium-doped yttrium aluminum garnet (Nd:Y3Al5O12) is paired with a flexible bronchoscope. The procedure can produce favorable outcomes,7 but it has not gained favor because of perceptions that the lasers require rigid bronchoscopy, expensive equipment, and special training. There are also concerns about complications.
The lower-cost endobronchial electrosurgery (EBES) also failed to gain acceptance because of cumbersome delivery systems and complications associated with power units. Recently, engineers have spawned a new generation of electrosurgical devices, prompting renewed interest in EBES.
A recent study compared LPR and EBES in patients who represented 118 evaluations for LPR.8 Forty percent were considered amenable to EBES and so did not go on to receive the more costly LPR. Of those, 89% achieved success in alleviating the obstruction. The authors concluded that EBES can potentially eliminate the need for LPR in 36% of procedures, and that it could achieve significant savings in cost and time. We use these ablative therapies only in dire circumstances; we use non–heat-based therapies, including repeated dilation, prior to considering use of other therapies.
Cryotherapy
Cryotherapy spray was initially thought to have great therapeutic potential, but the high pressures of the spray caused complications. This modality remains under investigation, however. Some probe-based cryotherapy techniques have been effective anecdotally. These use a metal-tipped probe attached to a cryogen; the Joule-Thompson effect causes delayed tissue destruction.
Stents
A small number of case reports note patient improvement after stenting.9,10 We use stents in rare circumstances, but because complications are frequent and sometimes severe, we consider stenting a last-resort option. In 2005, the US Food and Drug Administration mandated a Black Box warning against the use of metallic stents in patients who have benign tracheal strictures.
Multimodality therapies
In general, when intervention is required to salvage airways, a combination of dilation and steroid injection with or without topical mitomycin-C is standard. We try to avoid use of thermal therapy with laser or electrocautery because of the risk of exuberant inflammation and restenosis from thermal injury. No specific standard of care exists in these cases; reliance on clinical judgment is critical because of the presentation and variety of airway lesions. Further, no large-scale randomized trials exist to guide therapy, so it is best to work with a multidisciplinary team whose members have experience in managing these complex patients.
CONCLUSION
The differential diagnosis of pulmonary manifestations of small-vessel vasculitis is complex. Several diagnoses can mimic various forms of pulmonary vasculitis, and the manifestations and symptoms often overlap with other organ systems.
Imaging is useful for analysis of common patterns of small and midsize vasculitis, although the results may be confounded by disorders that mimic pulmonary vasculitis. To enhance diagnostic accuracy, laboratory and clinical findings should be considered along with images. Ideally, treatment will be minimally destructive and mucosa-sparing. Dilation therapies can be augmented with corticosteroid injections or, possibly, mitomycin-C.
The pulmonary manifestations of small-vessel vasculitis are nonspecific and often overlap with other conditions. Consequently, the diagnosis and management of pulmonary vasculitis are complex and require special attention to detail. This article reviews clinical experience with vasculitis as it manifests in the pulmonary setting, with the goal of providing a sound clinical approach to diagnosis and management.
DIAGNOSTIC CONSIDERATIONS
Accurate diagnosis is enhanced with imaging technology, judicious use of bronchoscopy, and awareness of disorders that mimic or masquerade as pulmonary vasculitis. The diagnosis can be approached on the basis of pattern recognition. For example, microscopic polyangiitis (MPA) is characterized solely by alveolar hemorrhage syndrome. However, other diagnostic possibilities must be considered, such as infection, acute respiratory distress syndrome, and complications of medicines. The hallmark manifestation of granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is necrotizing granulomatous inflammations, but the pulmonary manifestations can include nodules, cavitary masses, airway stenosis, and alveolar hemorrhage. Asthma with eosinophilia is the distinguishing feature of eosinophilic GPA (Churg-Strauss syndrome, EGPA), and Goodpasture syndrome involves deposition of complement and immunoglobulins.
The use of imaging
The best imaging tool for suspected pulmonary vasculitides is high-resolution computed tomography (CT). As a general rule, CT for patients with suspected vasculitis should be ordered without contrast medium as contrast is not needed to assess the lung parenchyma. Vasculitis patients often have renal insufficiency, and contrast-free CT will help protect the kidneys. Another option, which will enhance evaluation of the distribution and location of pulmonary disease, is multiplanar reconstructions of images with virtual bronchoscopy or airway reconstruction. Certain findings on imaging will help to differentiate the vasculitides from one another as well as from mimicking diagnoses.
Eosinophilic GPA. Chest images of patients with EGPA appear as patchy, nonsegmental, often peripheral consolidations of ground-glass opacity. These tend to reside in all lobes of the lungs, close to the surface and occasionally accompanied by septal markings.
Microscopic polyangiitis. Although classically a disease of alveolar hemorrhage, MPA often does not manifest with hemoptysis. Approximately one-third of patients with MPA do not cough up blood, even after a large amount of hemorrhage directly into the parenchyma. Patients may present with nonspecific symptoms such as fatigue and shortness of breath. Chest imaging will enhance diagnostic accuracy, particularly when considered in conjunction with laboratory test results. MPA patients usually have low hematocrit levels and may actually have an increased diffusing capacity of the lung for carbon monoxide (Dlco).
Granulomatosis with polyangiitis. This form of vasculitis has characteristic nodules, cavitary lesions, and, in the worst cases, multifocal masses in the lungs. These can be identified with contrast-free CT, with examination for possible airway involvement.
Multiple lung cavity nodules and pronounced airway narrowing are significant diagnostic clues for GPA. Nodules up to 10 cm in diameter tend to be near sub-pleural and peripheral areas. Microbes and fungus may complicate the nodules’ primary presentation. While bronchoscopy may be helpful with imaging, surgical biopsy remains the gold standard to rule out infections.
The disease may be multifocal, occurring outside the lungs from the larynx to bronchi and anywhere in the lung. Subglottic stenosis caused by inflammation and scarring affects 16% of patients with GPA, but it also often develops independently of other features of GPA and may have its own course independent of systemic symptoms.1
Bronchoscopy
Bronchoscopy is a relatively low-risk way to assess airways and nodules, but it has had a limited role in the diagnosis of nonfocal interstitial lung disease and rheumatologic lung disease in general. New technologies that augment traditional bronchoscopy and enhance its utility for diagnosis for focal entities are described below.
Electromagnetic navigation bronchoscopy (ENB) uses electromagnetic technology to localize and guide a catheter through the bronchial pathways. With the help of a virtual, 3-dimensional bronchial map reconstructed from a chest CT, the clinician can navigate to a desired location within the lung for biopsy and diagnosis of pulmonary nodules. The result is a diagnostic yield per nodule of nearly 80%.2 Seijo et al showed that diagnostic yields by ENB increase with the presence of the bronchus sign, or a bronchus leading directly to a peripheral lung lesion, as viewed on CT imaging.2 If nodules are bronchocentric, or surround airways, there is greater likelihood of reaching a diagnosis without resorting to surgery.
In peripheral radial ultrasound, a catheter is threaded through another catheter sheath in order to visualize the lesion. This technology can precisely localize lung lesions and often give some clues about the final pathology.
Bronchoscopic confocal fluorescence microscopy3 is a new form of microscopy that uses a fiberoptic miniprobe instead of an objective lens. High-quality images are achieved by the use of autofluorescence. Researchers have used the technology to detect changes in the respiratory bronchioles and other structures, but a clear atlas of many disease states does not yet exist. Oddly, endobronchial GPA images have been catalogued.3
Virtual bronchoscopy is a 3-dimensional image reconstruction and display technique that converts standard CT images into multiplanar images, which can be stacked. Virtual bronchoscopy augments conventional CT because of its ability to enhance detection in the subglottic region and more accurately measure stenosis.4 The technique cannot replace traditional bronchoscopy, however, because mucus and secretions can appear as abnormalities and cause false-positive results.
Detecting mimics
Diagnoses that masquerade as EGPA include chronic eosinophilic pneumonia, bronchiolitis obliterans with organizing pneumonia, and other interstitial lung diseases. Allergic bronchopulmonary aspergillosis—an asthma syndrome sometimes associated with eosinophilia and high immunoglobulin-E levels—also mimics EGPA. This diagnostic possibility is particularly relevant if the patient is taking immunosuppressive agents or corticosteroids.
Although alveolar hemorrhage is the sole pulmonary manifestation of MPA, the diagnosis is not limited to MPA alone. Alveolar hemorrhage may have other causes, including infection or acute respiratory distress syndrome. Bronchial lavage is recommended for accurate diagnosis, with the introduction of successive volumes of saline into the lungs and examination for increasing amounts of heme in each of the aliquots of alveolar lavage fluid.
Several diagnoses can mimic GPA. Many infections, including those caused by mycobacteria and Cryptococcus, can mimic endobronchial GPA. Biopsy of all new ulcers is recommended to minimize the possibility of missing these diagnoses. Tuberculosis in its latent form can closely resemble scarred GPA. Other mimickers of cavitary lung lesions can include metastatic melanoma, metastatic renal and thyroid cancers, squamous cell carcinoma, and rheumatoid arthritis with necrobiotic nodules that open in the lungs.
TREATMENT STRATEGIES
Medications
Although many patients with GPA are surgical candidates because of dyspnea related to fixed endobronchial or endotracheal obstructions, any surgical treatment carries the risk of inciting further flares. Treatment should focus first on mitigating the systemic inflammatory disorder with pharmacologic intervention. Standard pharmacologic therapy includes corticosteroids, azathioprine, cyclophosphamide, and rituximab. Patients with subglottic stenosis are frequently unresponsive to standard immunosuppressive therapy (glucocorticoids in combination with a cytotoxic agent).1
Surgical reconstruction
When medication falls short and surgery is needed to reverse strictures, a number of tools are at our disposal. Some involve heat, such as laser, cauterization, and argon plasma coagulation. In argon plasma coagulation, a jet of ionized argon gas (plasma) is directed through a probe passed through an endoscope. Other techniques rely on cold: cryoprobes, microdebriders, and rigid scissors. In general, freeze therapies cause less scarring than heat therapy. With any surgical technique, there is risk of scars that will contract and cause structural collapse, resulting in restenosis.
Dilation
The high rate of stenosis relapse has spurred interest in alternatives to surgical treatment. One of these, dilation via endoscopy, also may mitigate the wound healing process. Other techniques for clearing the obstructed area include rigid bronchoscopy, the use of bougies (increasingly larger dilators), and balloon dilation. Balloon dilation has some advantages over the other techniques. It permits maximal radial direction and pressure, causes less damage to trachea wall mucosa, and achieves better overall results; however, the procedure usually needs to be repeated.5 It must be done quickly, and it requires flawless communication between the otolaryngologist or pulmonologist and anesthesiologist in order to stabilize the airway below the vocal cords.
Intratracheal dilation-injection therapy
Dilation can be augmented with glucocorticoid injections. In 1991, researchers at the National Institutes of Health utilized a combination dilation-injection therapy for 20 patients who had GPA and subglottic stenosis.1 Patients were first treated with mercury-filled dilators coated with 1% triamcinolone cream. Methylprednisolone acetate was then injected into the stenotic area. None of the patients treated with intratracheal dilation-injection therapy required a tracheostomy and six who already had tracheostomies were decannulated. In contrast, 56% of patients who received standard immunosuppressive therapy and no intratracheal dilation-injection therapy required tracheostomy. Intratracheal dilation-injection therapy is considered a safe and effective treatment of GPA-associated subglottic stenosis and, in the absence of major organ disease activity, could be used without systemic immunosuppressive agents.
Mitomycin-C is a controversial alternative to corticosteroids during dilation. Mitomycin-C is an alkylating agent that inhibits fibroblast proliferation and extracellular matrix protein synthesis, with the potential for reduced scarring. In a recent trial of 26 patients, two doses given 3 to 4 weeks apart reduced the rate of stenosis for 2 to 3 years compared with a single dose.6 Restenosis occurred in both groups, however, and after 5 years, the relapse rates were the same.
Nd:YAG laser photoresection versus endobronchial electrosurgery
One of the most effective therapies for treating obstructive lesions is Nd:YAG laser photoresection (LPR) in which a laser that utilizes the crystal neodymium-doped yttrium aluminum garnet (Nd:Y3Al5O12) is paired with a flexible bronchoscope. The procedure can produce favorable outcomes,7 but it has not gained favor because of perceptions that the lasers require rigid bronchoscopy, expensive equipment, and special training. There are also concerns about complications.
The lower-cost endobronchial electrosurgery (EBES) also failed to gain acceptance because of cumbersome delivery systems and complications associated with power units. Recently, engineers have spawned a new generation of electrosurgical devices, prompting renewed interest in EBES.
A recent study compared LPR and EBES in patients who represented 118 evaluations for LPR.8 Forty percent were considered amenable to EBES and so did not go on to receive the more costly LPR. Of those, 89% achieved success in alleviating the obstruction. The authors concluded that EBES can potentially eliminate the need for LPR in 36% of procedures, and that it could achieve significant savings in cost and time. We use these ablative therapies only in dire circumstances; we use non–heat-based therapies, including repeated dilation, prior to considering use of other therapies.
Cryotherapy
Cryotherapy spray was initially thought to have great therapeutic potential, but the high pressures of the spray caused complications. This modality remains under investigation, however. Some probe-based cryotherapy techniques have been effective anecdotally. These use a metal-tipped probe attached to a cryogen; the Joule-Thompson effect causes delayed tissue destruction.
Stents
A small number of case reports note patient improvement after stenting.9,10 We use stents in rare circumstances, but because complications are frequent and sometimes severe, we consider stenting a last-resort option. In 2005, the US Food and Drug Administration mandated a Black Box warning against the use of metallic stents in patients who have benign tracheal strictures.
Multimodality therapies
In general, when intervention is required to salvage airways, a combination of dilation and steroid injection with or without topical mitomycin-C is standard. We try to avoid use of thermal therapy with laser or electrocautery because of the risk of exuberant inflammation and restenosis from thermal injury. No specific standard of care exists in these cases; reliance on clinical judgment is critical because of the presentation and variety of airway lesions. Further, no large-scale randomized trials exist to guide therapy, so it is best to work with a multidisciplinary team whose members have experience in managing these complex patients.
CONCLUSION
The differential diagnosis of pulmonary manifestations of small-vessel vasculitis is complex. Several diagnoses can mimic various forms of pulmonary vasculitis, and the manifestations and symptoms often overlap with other organ systems.
Imaging is useful for analysis of common patterns of small and midsize vasculitis, although the results may be confounded by disorders that mimic pulmonary vasculitis. To enhance diagnostic accuracy, laboratory and clinical findings should be considered along with images. Ideally, treatment will be minimally destructive and mucosa-sparing. Dilation therapies can be augmented with corticosteroid injections or, possibly, mitomycin-C.
- Langford CA, Sneller MC, Hallahan CW, et al. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Arthritis Rheum 1996; 39:1754–1760.
- Seijo LM, de Torres JP, Lozano MD, et al. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a bronchus sign on CT imaging: results from a prospective study. Chest 2010; 138:1316–1321.
- Filner JJ, Bonura EJ, Lau ST, et al. Bronchoscopic fibered confocal fluorescence microscopy image characteristics and pathologic correlations. J Bronchol Intervent Pulmonol 2011; 18:23–30.
- Summers RM, Aggarwal NR, Sneller MC, et al. CT virtual bronchoscopy of the central airway in patients with Wegener’s granulomatosis. Chest 2002; 121:242–250.
- Schokkenbroek AA, Franssen CFM, Dikkers FG. Dilatation tracheoscopy for laryngeal and tracheal stenosis in patients with Wegener’s granulomatosis. Eur Arch Otorhinolaryngol 2008; 265:549–555.
- Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope 2009; 119:272–283.
- Shvero J, Shitrit D, Koren R, Shalomi D, Kramer MR. Endoscopic laser surgery for subglottic stenosis in Wegener’s granulomatosis. Yonsei Med J 2007; 48:748–753.
- Coulter TD, Mehta AC. The heat is on: impact of endobronchial electrosurgery on the need for Nd-YAG laser photoresection. Chest 2000; 118:516–521.
- Tierman J, Shah C, Elborn JS. Successful stenting in endobronchial Wegener’s granulomatosis. Ulster Med J 2006; 75:155–157.
- Daum TE, Specks U, Colby TV, et al. Tracheobronchial involvement in Wegener’s granulomatosis. Am J Respir Crit Care Med 1995; 151:522–526.
- Langford CA, Sneller MC, Hallahan CW, et al. Clinical features and therapeutic management of subglottic stenosis in patients with Wegener’s granulomatosis. Arthritis Rheum 1996; 39:1754–1760.
- Seijo LM, de Torres JP, Lozano MD, et al. Diagnostic yield of electromagnetic navigation bronchoscopy is highly dependent on the presence of a bronchus sign on CT imaging: results from a prospective study. Chest 2010; 138:1316–1321.
- Filner JJ, Bonura EJ, Lau ST, et al. Bronchoscopic fibered confocal fluorescence microscopy image characteristics and pathologic correlations. J Bronchol Intervent Pulmonol 2011; 18:23–30.
- Summers RM, Aggarwal NR, Sneller MC, et al. CT virtual bronchoscopy of the central airway in patients with Wegener’s granulomatosis. Chest 2002; 121:242–250.
- Schokkenbroek AA, Franssen CFM, Dikkers FG. Dilatation tracheoscopy for laryngeal and tracheal stenosis in patients with Wegener’s granulomatosis. Eur Arch Otorhinolaryngol 2008; 265:549–555.
- Smith ME, Elstad M. Mitomycin C and the endoscopic treatment of laryngotracheal stenosis: are two applications better than one? Laryngoscope 2009; 119:272–283.
- Shvero J, Shitrit D, Koren R, Shalomi D, Kramer MR. Endoscopic laser surgery for subglottic stenosis in Wegener’s granulomatosis. Yonsei Med J 2007; 48:748–753.
- Coulter TD, Mehta AC. The heat is on: impact of endobronchial electrosurgery on the need for Nd-YAG laser photoresection. Chest 2000; 118:516–521.
- Tierman J, Shah C, Elborn JS. Successful stenting in endobronchial Wegener’s granulomatosis. Ulster Med J 2006; 75:155–157.
- Daum TE, Specks U, Colby TV, et al. Tracheobronchial involvement in Wegener’s granulomatosis. Am J Respir Crit Care Med 1995; 151:522–526.
Ocular manifestations of small-vessel vasculitis
We have long understood that vasculitic conditions have various clinical manifestations. The Chapel Hill Consensus Conference classification of systemic vasculitis in 19941 contributed significantly to our understanding of the spectrum of vasculitides and their manifestations, enhancing our diagnostic ability and the likelihood of appropriate treatment.
The ophthalmic manifestations of vasculitis are protean and nonspecific, and should be considered in the overall context of the disease. Patients should be evaluated with the following questions in mind:
- Are the manifestations related to the vasculitis itself?
- Are the manifestations a result or complication of therapy?
- Are the manifestations signs of a completely unrelated and superimposed condition?
This article reviews the three areas of ocular inflammation related to vasculitis and comments on the role of tissue biopsy in the management of these patients.
THREE AREAS OF OCULAR INFLAMMATION
Orbital inflammation
Orbital disease can affect the lacrimal gland (inflammatory dacryoadenitis), extraocular muscles (orbital myositis), and the orbital soft tissues (inflammatory orbital pseudotumor). Orbital inflammation is characterized by relatively sudden onset (within days) of pain, erythema, and proptosis. Diplopia and visual loss from either compression or inflammation of the optic nerve or nerve sheath may be present. Depending upon the structures involved and the degree of involvement, orbital inflammation can be sight-threatening.
Either computed tomography or magnetic resonance imaging should be performed to assess orbital or extraorbital involvement. The orbital structures are particularly amenable to biopsy, which, in this author’s opinion, should be performed whenever possible. The biopsy may need to be interpreted within the context of previous or concurrent immunosuppressive therapy, which can alter the histologic picture, minimize inflammation, and make detection of vasculitis difficult. In addition to identifying inflammation, biopsy helps to identify fungal infection or lymphoma that can follow prolonged immunosuppressive therapy.
Treatment of orbital inflammation requires corticosteroid therapy or some other type of systemic immunosuppression.
Ocular, or globe, inflammation
Episcleritis: observation or topical therapy. Episcleritis usually manifests as an otherwise asymptomatic red eye with typical sector-shaped inflammation. Pain is generally not an issue, although patients often report that the eye does not feel normal. Vision is unaffected and there is no potential threat to sight.
The slit-lamp examination shows dilated vessels in the episcleral tissues that blanch after instillation of a drop of 10% phenylephrine. Simple observation may be the best management course, but topical nonsteroidal anti-inflammatory drugs (NSAIDs) or topical corticosteroids may help some patients who have discomfort. There is probably a spectrum of disease in that some patients may have either severe episcleritis or mild scleritis (Figure 1B). At times it can be difficult to differentiate between severe episcleritis and mild scleritis. Although scleritis generally requires systemic therapy, topical therapy is justified for mild scleritis. Episcleritis is associated with systemic disease in approximately 36% of patients.2–4
Scleritis: may be sight-threatening; requires systemic therapy. Scleritis characteristically presents with intense pain and a red eye.3,5–7 Patients may be sensitive to light and their vision may be compromised. Cataracts and glaucoma can complicate the course of scleritis.
With slit-lamp examination, the redness does not blanch upon instillation of topical 10% phenylephrine as it does with episcleritis. The adjacent cornea may also be affected (Figure 1C). Healed scleritis leaves an area of thinned sclera that appears as a visible blue spot, so if the patient’s history includes red eye with pain and a blue area is visible, the clinician can be confident that a prior episode of scleritis occurred.
Scleritis can be anterior or posterior, and the implications are slightly different for each type. Anterior scleritis can be subclassified as diffuse, nodular, or necrotizing. The necrotizing type can be characterized by painful inflammation or, in the case of scleromalacia perforans, no inflammation and no pain. Posterior scleritis may have minimal pain.
Akpek et al5 reported on a group of 243 patients with scleritis (average age, 52 years; range, 5 to 93 years) who were followed for an average of 1.7 years (range, 0 to 16.6 years). An associated medical condition was present in 107 (44%) patients. Rheumatologic conditions accounted for 37%, with rheumatoid arthritis being most common; infectious disease, with herpes zoster ophthalmicus being most common, accounted for 7%. Of those with an associated medical condition, 78% had been diagnosed previously; the remaining 22% were diagnosed at presentation or the condition developed during follow-up.
Treatment typically requires systemic therapy with NSAIDs, but more often oral or intravenous corticosteroids or even methotrexate, mycophenolate mofetil, cyclophosphamide, or rituximab may be required. Patients with antineutrophil cytoplasmic antibody (ANCA)–positive disease may require more intensive therapy than those with ANCA-negative disease.
Keratitis: may be sight-threatening. Patients with keratitis should be evaluated in the same spirit as patients with scleritis (Figure 1C). Although many patients may have superficial keratitis, which is often related to a dry eye and has no prognostic significance, deep or peripheral ulcerative keratitis is not only consistent with systemic vasculitis but also sight-threatening. Symptoms similar to those observed with scleritis typically include severe pain and photophobia and, as with scleritis, treatment usually involves systemic therapy.
Intraocular inflammation
There is no specific treatment for the eye other than treating the underlying condition. Vascular occlusions can sometimes give rise to neovascularization and patients should be followed for this possibility. As with a central nervous system ischemic event, recovery can be variable.
Uveitis. The term “uvea,” derived from the Greek word for grape, describes the shape of the iris, ciliary body, and choroid. Uveitis is a generic term for intraocular inflammation affecting any or all of these structures.
Iritis, or anterior uveitis, is a frequent accompaniment of keratitis or scleritis. Primarily uveitic involvement with retinal vessel vasculitis involving both arteries and veins is uncommon in general but typical of Behçet disease, especially if a hypopyon uveitis is present.
Anterior uveitis can be treated with topical corticosteroids and cycloplegic drugs, but middle and posterior uveitis almost always requires systemic therapy. Most recently, use of anti–tumor necrosis factor-α drugs has been effective in treating Behçet uveitis.8 The visual prognosis with Behçet disease remains guarded.
GRANULOMATOSIS WITH POLYANGIITIS: EYE INVOLVEMENT IS COMMON
In terms of specific small-vessel vasculitic diseases that affect the eye, granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is the quintessential condition. In data obtained from the Wegener Granulomatosis Support Group,9 eye involvement was noted at presentation in 211 of 701 patients (30%), and during the course of their disease an additional 147 patients developed eye involvement. From the time of initial presentation through the course of follow-up, 359 of the 701 patients (51%) eventually had some type of eye involvement.
In a series of patients seen at the Mayo Clinic,10 orbital inflammatory disease and scleritis were the two most frequent manifestations of eye involvement with GPA. Orbital involvement typically presents with pain, erythema, swelling, and proptosis. Varying degrees of ptosis, diplopia, or visual loss may also be present. Imaging may show an infiltrate that is usually adjacent to the maxillary or ethmoid sinus. This same process can affect the superior temporal orbital quadrant, an area apart from any sinus, and involve the lacrimal gland.
BIOPSY IS ADVISED
Biopsy, either incisional, at times to include debulking, or excisional if possible, is recommended to establish a diagnosis or aid in the selection of therapy. Orbital disease has been observed to progress in patients who are receiving maintenance therapy with methotrexate and have no evidence of systemic disease activity. Acute and chronic inflammation with evidence of active vasculitis is usually seen histologically. Personal observations suggest that intraorbital corticosteroid injection followed by rituximab has been effective therapy for this limited subset of patients. Diagnostic biopsies often must be interpreted in light of partial treatment, making histopathologic diagnosis challenging at times. Biopsy is important for exclusion of lymphoproliferative disease or fungal infection.
CONCLUSION
Underlying vasculitis might play a role in patients with nonspecific ocular presentations. It is essential that the ophthalmologist collaborate with a specialist in vasculitis (and vice versa) for evaluation and subsequent therapy, which often involves some form of immunosuppression.
- Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides: proposal of an International Consensus Conference. Arthritis Rheum 1994; 37:187–192.
- Pavesio CE, Meier FM. Systemic disorders associated with episcleritis and scleritis. Curr Opin Ophthalmol 2001; 12:471–478.
- Jabs DA, Mudun A, Dunn JP, Marsh MJ. Episcleritis and scleritis: clinical features and treatment results. Am J Ophthalmol 2000; 130:469–476.
- Akpek EK, Uy HS, Christen W, Gurdal C, Foster CS. Severity of episcleritis and systemic disease association. Ophthalmology 1999; 106:729–731.
- Akpek EK, Thorne JE, Qazi FA, Do DV, Jabs DA. Evaluation of patients with scleritis for systemic disease. Ophthalmology 2004; 111:501–506.
- McCluskey PJ, Watson PG, Lightman S, Haybittle J, Restori M, Branley M. Posterior scleritis: clinical features, systemic associations, and outcome in a large series of patients. Ophthalmology 1999; 106:2380–2386.
- Riono WP, Hidayat AA, Rao NA. Scleritis: a clinicopathologic study of 55 cases. Ophthalmology 1999; 106:1328–1333.
- Tabbara KF, Al-Hemidan AI. Infliximab effects compared to conventional therapy in the management of retinal vasculitis in Behçet disease [published online ahead of print October 17, 2008]. Am J Ophthalmol 2008; 146:845–850. doi: 10.1016/j.ajo.2008.09.010
- Abdou NI, Kullman GJ, Hoffman GS, et al. Wegener’s granulomatosis— survey of 701 patients in North America: changes in outcome in the 1990s. J Rheumatol 2002; 29:309–316.
- Bullen CL, Liesegang TJ, McDonald TJ, DeRemee RA. Ocular complications of Wegener’s granulomatosis. Ophthalmology 1983; 90:279–290.
We have long understood that vasculitic conditions have various clinical manifestations. The Chapel Hill Consensus Conference classification of systemic vasculitis in 19941 contributed significantly to our understanding of the spectrum of vasculitides and their manifestations, enhancing our diagnostic ability and the likelihood of appropriate treatment.
The ophthalmic manifestations of vasculitis are protean and nonspecific, and should be considered in the overall context of the disease. Patients should be evaluated with the following questions in mind:
- Are the manifestations related to the vasculitis itself?
- Are the manifestations a result or complication of therapy?
- Are the manifestations signs of a completely unrelated and superimposed condition?
This article reviews the three areas of ocular inflammation related to vasculitis and comments on the role of tissue biopsy in the management of these patients.
THREE AREAS OF OCULAR INFLAMMATION
Orbital inflammation
Orbital disease can affect the lacrimal gland (inflammatory dacryoadenitis), extraocular muscles (orbital myositis), and the orbital soft tissues (inflammatory orbital pseudotumor). Orbital inflammation is characterized by relatively sudden onset (within days) of pain, erythema, and proptosis. Diplopia and visual loss from either compression or inflammation of the optic nerve or nerve sheath may be present. Depending upon the structures involved and the degree of involvement, orbital inflammation can be sight-threatening.
Either computed tomography or magnetic resonance imaging should be performed to assess orbital or extraorbital involvement. The orbital structures are particularly amenable to biopsy, which, in this author’s opinion, should be performed whenever possible. The biopsy may need to be interpreted within the context of previous or concurrent immunosuppressive therapy, which can alter the histologic picture, minimize inflammation, and make detection of vasculitis difficult. In addition to identifying inflammation, biopsy helps to identify fungal infection or lymphoma that can follow prolonged immunosuppressive therapy.
Treatment of orbital inflammation requires corticosteroid therapy or some other type of systemic immunosuppression.
Ocular, or globe, inflammation
Episcleritis: observation or topical therapy. Episcleritis usually manifests as an otherwise asymptomatic red eye with typical sector-shaped inflammation. Pain is generally not an issue, although patients often report that the eye does not feel normal. Vision is unaffected and there is no potential threat to sight.
The slit-lamp examination shows dilated vessels in the episcleral tissues that blanch after instillation of a drop of 10% phenylephrine. Simple observation may be the best management course, but topical nonsteroidal anti-inflammatory drugs (NSAIDs) or topical corticosteroids may help some patients who have discomfort. There is probably a spectrum of disease in that some patients may have either severe episcleritis or mild scleritis (Figure 1B). At times it can be difficult to differentiate between severe episcleritis and mild scleritis. Although scleritis generally requires systemic therapy, topical therapy is justified for mild scleritis. Episcleritis is associated with systemic disease in approximately 36% of patients.2–4
Scleritis: may be sight-threatening; requires systemic therapy. Scleritis characteristically presents with intense pain and a red eye.3,5–7 Patients may be sensitive to light and their vision may be compromised. Cataracts and glaucoma can complicate the course of scleritis.
With slit-lamp examination, the redness does not blanch upon instillation of topical 10% phenylephrine as it does with episcleritis. The adjacent cornea may also be affected (Figure 1C). Healed scleritis leaves an area of thinned sclera that appears as a visible blue spot, so if the patient’s history includes red eye with pain and a blue area is visible, the clinician can be confident that a prior episode of scleritis occurred.
Scleritis can be anterior or posterior, and the implications are slightly different for each type. Anterior scleritis can be subclassified as diffuse, nodular, or necrotizing. The necrotizing type can be characterized by painful inflammation or, in the case of scleromalacia perforans, no inflammation and no pain. Posterior scleritis may have minimal pain.
Akpek et al5 reported on a group of 243 patients with scleritis (average age, 52 years; range, 5 to 93 years) who were followed for an average of 1.7 years (range, 0 to 16.6 years). An associated medical condition was present in 107 (44%) patients. Rheumatologic conditions accounted for 37%, with rheumatoid arthritis being most common; infectious disease, with herpes zoster ophthalmicus being most common, accounted for 7%. Of those with an associated medical condition, 78% had been diagnosed previously; the remaining 22% were diagnosed at presentation or the condition developed during follow-up.
Treatment typically requires systemic therapy with NSAIDs, but more often oral or intravenous corticosteroids or even methotrexate, mycophenolate mofetil, cyclophosphamide, or rituximab may be required. Patients with antineutrophil cytoplasmic antibody (ANCA)–positive disease may require more intensive therapy than those with ANCA-negative disease.
Keratitis: may be sight-threatening. Patients with keratitis should be evaluated in the same spirit as patients with scleritis (Figure 1C). Although many patients may have superficial keratitis, which is often related to a dry eye and has no prognostic significance, deep or peripheral ulcerative keratitis is not only consistent with systemic vasculitis but also sight-threatening. Symptoms similar to those observed with scleritis typically include severe pain and photophobia and, as with scleritis, treatment usually involves systemic therapy.
Intraocular inflammation
There is no specific treatment for the eye other than treating the underlying condition. Vascular occlusions can sometimes give rise to neovascularization and patients should be followed for this possibility. As with a central nervous system ischemic event, recovery can be variable.
Uveitis. The term “uvea,” derived from the Greek word for grape, describes the shape of the iris, ciliary body, and choroid. Uveitis is a generic term for intraocular inflammation affecting any or all of these structures.
Iritis, or anterior uveitis, is a frequent accompaniment of keratitis or scleritis. Primarily uveitic involvement with retinal vessel vasculitis involving both arteries and veins is uncommon in general but typical of Behçet disease, especially if a hypopyon uveitis is present.
Anterior uveitis can be treated with topical corticosteroids and cycloplegic drugs, but middle and posterior uveitis almost always requires systemic therapy. Most recently, use of anti–tumor necrosis factor-α drugs has been effective in treating Behçet uveitis.8 The visual prognosis with Behçet disease remains guarded.
GRANULOMATOSIS WITH POLYANGIITIS: EYE INVOLVEMENT IS COMMON
In terms of specific small-vessel vasculitic diseases that affect the eye, granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is the quintessential condition. In data obtained from the Wegener Granulomatosis Support Group,9 eye involvement was noted at presentation in 211 of 701 patients (30%), and during the course of their disease an additional 147 patients developed eye involvement. From the time of initial presentation through the course of follow-up, 359 of the 701 patients (51%) eventually had some type of eye involvement.
In a series of patients seen at the Mayo Clinic,10 orbital inflammatory disease and scleritis were the two most frequent manifestations of eye involvement with GPA. Orbital involvement typically presents with pain, erythema, swelling, and proptosis. Varying degrees of ptosis, diplopia, or visual loss may also be present. Imaging may show an infiltrate that is usually adjacent to the maxillary or ethmoid sinus. This same process can affect the superior temporal orbital quadrant, an area apart from any sinus, and involve the lacrimal gland.
BIOPSY IS ADVISED
Biopsy, either incisional, at times to include debulking, or excisional if possible, is recommended to establish a diagnosis or aid in the selection of therapy. Orbital disease has been observed to progress in patients who are receiving maintenance therapy with methotrexate and have no evidence of systemic disease activity. Acute and chronic inflammation with evidence of active vasculitis is usually seen histologically. Personal observations suggest that intraorbital corticosteroid injection followed by rituximab has been effective therapy for this limited subset of patients. Diagnostic biopsies often must be interpreted in light of partial treatment, making histopathologic diagnosis challenging at times. Biopsy is important for exclusion of lymphoproliferative disease or fungal infection.
CONCLUSION
Underlying vasculitis might play a role in patients with nonspecific ocular presentations. It is essential that the ophthalmologist collaborate with a specialist in vasculitis (and vice versa) for evaluation and subsequent therapy, which often involves some form of immunosuppression.
We have long understood that vasculitic conditions have various clinical manifestations. The Chapel Hill Consensus Conference classification of systemic vasculitis in 19941 contributed significantly to our understanding of the spectrum of vasculitides and their manifestations, enhancing our diagnostic ability and the likelihood of appropriate treatment.
The ophthalmic manifestations of vasculitis are protean and nonspecific, and should be considered in the overall context of the disease. Patients should be evaluated with the following questions in mind:
- Are the manifestations related to the vasculitis itself?
- Are the manifestations a result or complication of therapy?
- Are the manifestations signs of a completely unrelated and superimposed condition?
This article reviews the three areas of ocular inflammation related to vasculitis and comments on the role of tissue biopsy in the management of these patients.
THREE AREAS OF OCULAR INFLAMMATION
Orbital inflammation
Orbital disease can affect the lacrimal gland (inflammatory dacryoadenitis), extraocular muscles (orbital myositis), and the orbital soft tissues (inflammatory orbital pseudotumor). Orbital inflammation is characterized by relatively sudden onset (within days) of pain, erythema, and proptosis. Diplopia and visual loss from either compression or inflammation of the optic nerve or nerve sheath may be present. Depending upon the structures involved and the degree of involvement, orbital inflammation can be sight-threatening.
Either computed tomography or magnetic resonance imaging should be performed to assess orbital or extraorbital involvement. The orbital structures are particularly amenable to biopsy, which, in this author’s opinion, should be performed whenever possible. The biopsy may need to be interpreted within the context of previous or concurrent immunosuppressive therapy, which can alter the histologic picture, minimize inflammation, and make detection of vasculitis difficult. In addition to identifying inflammation, biopsy helps to identify fungal infection or lymphoma that can follow prolonged immunosuppressive therapy.
Treatment of orbital inflammation requires corticosteroid therapy or some other type of systemic immunosuppression.
Ocular, or globe, inflammation
Episcleritis: observation or topical therapy. Episcleritis usually manifests as an otherwise asymptomatic red eye with typical sector-shaped inflammation. Pain is generally not an issue, although patients often report that the eye does not feel normal. Vision is unaffected and there is no potential threat to sight.
The slit-lamp examination shows dilated vessels in the episcleral tissues that blanch after instillation of a drop of 10% phenylephrine. Simple observation may be the best management course, but topical nonsteroidal anti-inflammatory drugs (NSAIDs) or topical corticosteroids may help some patients who have discomfort. There is probably a spectrum of disease in that some patients may have either severe episcleritis or mild scleritis (Figure 1B). At times it can be difficult to differentiate between severe episcleritis and mild scleritis. Although scleritis generally requires systemic therapy, topical therapy is justified for mild scleritis. Episcleritis is associated with systemic disease in approximately 36% of patients.2–4
Scleritis: may be sight-threatening; requires systemic therapy. Scleritis characteristically presents with intense pain and a red eye.3,5–7 Patients may be sensitive to light and their vision may be compromised. Cataracts and glaucoma can complicate the course of scleritis.
With slit-lamp examination, the redness does not blanch upon instillation of topical 10% phenylephrine as it does with episcleritis. The adjacent cornea may also be affected (Figure 1C). Healed scleritis leaves an area of thinned sclera that appears as a visible blue spot, so if the patient’s history includes red eye with pain and a blue area is visible, the clinician can be confident that a prior episode of scleritis occurred.
Scleritis can be anterior or posterior, and the implications are slightly different for each type. Anterior scleritis can be subclassified as diffuse, nodular, or necrotizing. The necrotizing type can be characterized by painful inflammation or, in the case of scleromalacia perforans, no inflammation and no pain. Posterior scleritis may have minimal pain.
Akpek et al5 reported on a group of 243 patients with scleritis (average age, 52 years; range, 5 to 93 years) who were followed for an average of 1.7 years (range, 0 to 16.6 years). An associated medical condition was present in 107 (44%) patients. Rheumatologic conditions accounted for 37%, with rheumatoid arthritis being most common; infectious disease, with herpes zoster ophthalmicus being most common, accounted for 7%. Of those with an associated medical condition, 78% had been diagnosed previously; the remaining 22% were diagnosed at presentation or the condition developed during follow-up.
Treatment typically requires systemic therapy with NSAIDs, but more often oral or intravenous corticosteroids or even methotrexate, mycophenolate mofetil, cyclophosphamide, or rituximab may be required. Patients with antineutrophil cytoplasmic antibody (ANCA)–positive disease may require more intensive therapy than those with ANCA-negative disease.
Keratitis: may be sight-threatening. Patients with keratitis should be evaluated in the same spirit as patients with scleritis (Figure 1C). Although many patients may have superficial keratitis, which is often related to a dry eye and has no prognostic significance, deep or peripheral ulcerative keratitis is not only consistent with systemic vasculitis but also sight-threatening. Symptoms similar to those observed with scleritis typically include severe pain and photophobia and, as with scleritis, treatment usually involves systemic therapy.
Intraocular inflammation
There is no specific treatment for the eye other than treating the underlying condition. Vascular occlusions can sometimes give rise to neovascularization and patients should be followed for this possibility. As with a central nervous system ischemic event, recovery can be variable.
Uveitis. The term “uvea,” derived from the Greek word for grape, describes the shape of the iris, ciliary body, and choroid. Uveitis is a generic term for intraocular inflammation affecting any or all of these structures.
Iritis, or anterior uveitis, is a frequent accompaniment of keratitis or scleritis. Primarily uveitic involvement with retinal vessel vasculitis involving both arteries and veins is uncommon in general but typical of Behçet disease, especially if a hypopyon uveitis is present.
Anterior uveitis can be treated with topical corticosteroids and cycloplegic drugs, but middle and posterior uveitis almost always requires systemic therapy. Most recently, use of anti–tumor necrosis factor-α drugs has been effective in treating Behçet uveitis.8 The visual prognosis with Behçet disease remains guarded.
GRANULOMATOSIS WITH POLYANGIITIS: EYE INVOLVEMENT IS COMMON
In terms of specific small-vessel vasculitic diseases that affect the eye, granulomatosis with polyangiitis (GPA [Wegener’s granulomatosis]) is the quintessential condition. In data obtained from the Wegener Granulomatosis Support Group,9 eye involvement was noted at presentation in 211 of 701 patients (30%), and during the course of their disease an additional 147 patients developed eye involvement. From the time of initial presentation through the course of follow-up, 359 of the 701 patients (51%) eventually had some type of eye involvement.
In a series of patients seen at the Mayo Clinic,10 orbital inflammatory disease and scleritis were the two most frequent manifestations of eye involvement with GPA. Orbital involvement typically presents with pain, erythema, swelling, and proptosis. Varying degrees of ptosis, diplopia, or visual loss may also be present. Imaging may show an infiltrate that is usually adjacent to the maxillary or ethmoid sinus. This same process can affect the superior temporal orbital quadrant, an area apart from any sinus, and involve the lacrimal gland.
BIOPSY IS ADVISED
Biopsy, either incisional, at times to include debulking, or excisional if possible, is recommended to establish a diagnosis or aid in the selection of therapy. Orbital disease has been observed to progress in patients who are receiving maintenance therapy with methotrexate and have no evidence of systemic disease activity. Acute and chronic inflammation with evidence of active vasculitis is usually seen histologically. Personal observations suggest that intraorbital corticosteroid injection followed by rituximab has been effective therapy for this limited subset of patients. Diagnostic biopsies often must be interpreted in light of partial treatment, making histopathologic diagnosis challenging at times. Biopsy is important for exclusion of lymphoproliferative disease or fungal infection.
CONCLUSION
Underlying vasculitis might play a role in patients with nonspecific ocular presentations. It is essential that the ophthalmologist collaborate with a specialist in vasculitis (and vice versa) for evaluation and subsequent therapy, which often involves some form of immunosuppression.
- Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides: proposal of an International Consensus Conference. Arthritis Rheum 1994; 37:187–192.
- Pavesio CE, Meier FM. Systemic disorders associated with episcleritis and scleritis. Curr Opin Ophthalmol 2001; 12:471–478.
- Jabs DA, Mudun A, Dunn JP, Marsh MJ. Episcleritis and scleritis: clinical features and treatment results. Am J Ophthalmol 2000; 130:469–476.
- Akpek EK, Uy HS, Christen W, Gurdal C, Foster CS. Severity of episcleritis and systemic disease association. Ophthalmology 1999; 106:729–731.
- Akpek EK, Thorne JE, Qazi FA, Do DV, Jabs DA. Evaluation of patients with scleritis for systemic disease. Ophthalmology 2004; 111:501–506.
- McCluskey PJ, Watson PG, Lightman S, Haybittle J, Restori M, Branley M. Posterior scleritis: clinical features, systemic associations, and outcome in a large series of patients. Ophthalmology 1999; 106:2380–2386.
- Riono WP, Hidayat AA, Rao NA. Scleritis: a clinicopathologic study of 55 cases. Ophthalmology 1999; 106:1328–1333.
- Tabbara KF, Al-Hemidan AI. Infliximab effects compared to conventional therapy in the management of retinal vasculitis in Behçet disease [published online ahead of print October 17, 2008]. Am J Ophthalmol 2008; 146:845–850. doi: 10.1016/j.ajo.2008.09.010
- Abdou NI, Kullman GJ, Hoffman GS, et al. Wegener’s granulomatosis— survey of 701 patients in North America: changes in outcome in the 1990s. J Rheumatol 2002; 29:309–316.
- Bullen CL, Liesegang TJ, McDonald TJ, DeRemee RA. Ocular complications of Wegener’s granulomatosis. Ophthalmology 1983; 90:279–290.
- Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of systemic vasculitides: proposal of an International Consensus Conference. Arthritis Rheum 1994; 37:187–192.
- Pavesio CE, Meier FM. Systemic disorders associated with episcleritis and scleritis. Curr Opin Ophthalmol 2001; 12:471–478.
- Jabs DA, Mudun A, Dunn JP, Marsh MJ. Episcleritis and scleritis: clinical features and treatment results. Am J Ophthalmol 2000; 130:469–476.
- Akpek EK, Uy HS, Christen W, Gurdal C, Foster CS. Severity of episcleritis and systemic disease association. Ophthalmology 1999; 106:729–731.
- Akpek EK, Thorne JE, Qazi FA, Do DV, Jabs DA. Evaluation of patients with scleritis for systemic disease. Ophthalmology 2004; 111:501–506.
- McCluskey PJ, Watson PG, Lightman S, Haybittle J, Restori M, Branley M. Posterior scleritis: clinical features, systemic associations, and outcome in a large series of patients. Ophthalmology 1999; 106:2380–2386.
- Riono WP, Hidayat AA, Rao NA. Scleritis: a clinicopathologic study of 55 cases. Ophthalmology 1999; 106:1328–1333.
- Tabbara KF, Al-Hemidan AI. Infliximab effects compared to conventional therapy in the management of retinal vasculitis in Behçet disease [published online ahead of print October 17, 2008]. Am J Ophthalmol 2008; 146:845–850. doi: 10.1016/j.ajo.2008.09.010
- Abdou NI, Kullman GJ, Hoffman GS, et al. Wegener’s granulomatosis— survey of 701 patients in North America: changes in outcome in the 1990s. J Rheumatol 2002; 29:309–316.
- Bullen CL, Liesegang TJ, McDonald TJ, DeRemee RA. Ocular complications of Wegener’s granulomatosis. Ophthalmology 1983; 90:279–290.
Monitoring patients with vasculitis
Granulomatosis with polyangiitis (GPA), is one of the most common types of small-vessel vasculitis, with an estimated prevalence in the United States of 3 per 100,000 people. It is distinguished from other necrotizing vasculitides by its tendency to affect the upper and lower respiratory system and the kidneys. Despite the success of induction and maintenance treatments with cyclophosphamide (CYC), glucocorticoids, and less toxic immunosuppressive alternative therapies in improving the disease course, significant treatment-related toxicities and frequent disease relapses demand stringent patient-specific monitoring in order to provide early treatment of relapses and prevent or decrease morbidity.
SMALL-VESSEL VASCULITIS MANAGEMENT OVERVIEW
Granulomatosis with polyangiitis (formerly Wegener’s granulomatosis, or WG) is an antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis that often affects the respiratory system and kidneys across a broad spectrum of clinical presentations, from mild through life-threatening disease. Patients with severe disease present with significant multisystem manifestations, which, in addition to the respiratory system and kidneys, may involve the joints, eyes, and other organs.
Managing patients diagnosed with systemic small-vessel vasculitides such as GPA and microscopic polyangiitis (MPA) is an inexact science. The goals of treatment are to increase survival, induce and maintain remission, reduce relapses, and minimize treatment-related toxicity. Inducing and maintaining remission have become realistic goals because of the availability of medications that prolong life. On the other hand, extended periods of treatment associated with prolonged life increase the risk of treatment-related toxicity in patients who are inadequately monitored.
MONITORING CONSIDERATIONS
Achieving treatment goals requires long-term monitoring of both disease activity and treatment-related toxicities, with constant adjustments to meet the needs of the individual patient and address the often rapidly changing disease and treatment course. The monitoring protocol consists of regularly scheduled follow-up office visits, urine sediment analyses at every office visit whether or not the patient has relapse symptoms, laboratory tests at regular intervals as indicated by the patient’s medication plan and disease presentation, additional tests such as lung computed tomography (CT), and patient education regarding new symptoms and the frequency of office visits. A consistent monitoring strategy will help detect a relapse before it can produce more severe morbidity, identify treatment-related complications, and—equally important—identify the achievement of remission. An example of the consequences of inconsistent monitoring is presented in “Relapse in a nonadherent patient.”
Because there is no definitive cure for small-vessel vasculitis, relapse is always a possibility. The early diagnosis and treatment of relapse may prevent or decrease morbidity from disease, but strict monitoring is needed to identify relapse and initiate treatment before morbidity occurs (see “Relapse in a patient with new symptoms”). Repeat induction therapy following a relapse introduces risk of drug toxicity and requires careful monitoring, as does long-term maintenance therapy.
In addition to induction and maintenance therapy, several other situations, including prior therapeutic complications, serum creatinine levels, and risk of cardiovascular disease, require special monitoring attention.

Induction therapy: monitor response

Response to treatment during induction must be monitored to identify whether remission is achieved. Induction monitoring requires complete assessment of organ-system involvement at every visit with tools such as the Birmingham Vasculitis Activity Score (BVAS) and, when appropriate, the BVAS/WG. If new or worsening symptoms develop during induction therapy, then the patient needs assessment for continued disease activity as well as treatment complications such as infections related to immunosuppressive therapy.
During induction therapy with daily oral CYC, monitoring should include weekly complete blood cell counts to ensure early identification of leukopenia and other cytopenias. The risk of morbidities increases with the cumulative dose, so a stable blood count for 2 months does not obviate the risk of leukopenia. If persistent hematuria is present without cellular casts, cystoscopy is indicated to look for signs of hemorrhagic cystitis. Prophylaxis against Pneumocystis jirovecii is recommended in all patients who receive immunosuppressive therapy. Finally, bone density measurements should be done at baseline.
Maintenance therapy: frequency can be extended
Monitoring during maintenance therapy is similar to induction monitoring; however, when the dosage of methotrexate or azathioprine is stabilized, the frequency of some tests can be extended to monthly rather than weekly. For example, a complete blood cell count, comprehensive metabolic panel, sedimentation rate, C-reactive protein measurement, and urinalysis should be performed monthly. Follow-up visits should include urine sediment analyses and monitoring for cardiovascular disease risk factors. Medication monitoring should include cystoscopy for persistent hematuria without cellular casts, bone density measurements, and ophthalmologic examinations as frequently as indicated for each individual’s needs. P jirovecii prophylaxis should continue as long as the patient receives immunosuppressive medication.

Therapy-related complications
Bladder complications. In a retrospective analysis of 145 patients with GPA treated with CYC and followed for 0.5 to 27 years (median 8.5 years), nonglomerular hematuria developed in 50% of the patients and bladder carcinoma in 5%.2 The cumulative CYC dose (19 to 251 g) in this group was much higher than what is currently used. Cytologic examination of the urine showed 43% sensitivity for dysplasia (specificity 100%) and 29% sensitivity for atypia (specificity 89%). In contrast, in a retrospective outcomes analysis involving newly diagnosed patients with GPA treated with CYC or methotrexate, 82 patients followed for up to 12 years had no incidents of cystitis or bladder cancer.3 Patients in this study were treated with CYC for only 3 to 6 months and therefore received a lower cumulative dose.
To prevent cystitis during treatment with CYC, the patient should be well hydrated, especially in the morning when CYC should be taken. The bladder should be emptied frequently. The addition of mesna when administering intravenous CYC decreases the risk of cystitis. Serial cystoscopy and urine cytology should be used only in patients with nonglomerular hematuria.
Infertility. Preservation of ovarian function is a concern with CYC therapy in women of childbearing age. The cumulative dose threshold for gonadal failure is unknown, because data from cancer studies4 demonstrating gonadal failure involve higher cumulative CYC doses than are typical for vasculitis treatment. It is also unknown whether duration of amenorrhea predicts the recovery of menses or fertility. The primary option for preservation of ovarian function is the use of gonadotropin-releasing hormone agonists. Oral contraceptives also may be used, but the best prevention is to avoid CYC in these patients if possible.
Osteoporosis. At glucocorticoid dosages of 5 mg/day or greater, bone mineral density begins a rapid decline within the first 3 months and peaks at 6 months.5 The American College of Rheumatology has provided recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis.5 Table 2 presents recommendations for postmenopausal women and men aged 50 years and older who will use glucocorticoids for 3 months or more.5 Recommendations are also available for premenopausal women and men younger than 50 years of age who have a history of fragility fracture.
Leukopenia. Leukopenia should be avoided during CYC treatment. The target white blood cell count should be within the normal range. During treatment with daily oral CYC, the patient should be monitored with a weekly complete blood cell count and medication should be adjusted to maintain the target white blood cell count.
Upon completion of induction therapy, after 3 to 6 months, the patient is switched to maintenance therapy with an alternative immunosuppressive agent such as azathioprine or methotrexate, depending on the serum creatinine concentration and other factors. This transition, characterized by full-dose immunosuppressive therapy when the bone marrow has been previously suppressed by CYC treatment, may induce pancytopenia. Monitoring with weekly complete blood counts for at least 4 weeks after initiating maintenance therapy can help ensure stability during the transition period.

Monitor serum creatinine and adjust dosages
The serum creatinine concentration may increase as CYC treatment progresses; in some cases, the serum creatinine concentration increases before a response to treatment is seen. The CYC dosages should be adjusted as necessary in response to serum creatinine changes. Careful monitoring of serum creatinine is necessary during methotrexate therapy, as methotrexate treatment in the setting of renal insufficiency increases the risk of bone marrow suppression.
Cardiovascular disease in GPA and MPA
Premature atherosclerosis has been well described in patients with GPA.6 Within 5 years of diagnosis of GPA or MPA, a cardiovascular event will occur in 14% of patients.7 In the absence of specific guidelines for prevention of cardiovascular disease in patients with vasculitis, it is essential to monitor patients and treat modifiable traditional risk factors aggressively, especially in younger patients. Suppiah et al found that independent determinants of cardiovascular outcome included older age, diastolic hypertension, and positive proteinase-3–ANCA status in patients without prior cardiovascular disease.7
In the Wegener’s Clinical Occurrence of Thrombosis (WeCLOT) study, Merkel et al showed an increased incidence of thrombosis in patients with active GPA8 (see “Relapse presenting as thrombosis,” left). As with cardiovascular disease, there are no specific guidelines for monitoring asymptomatic patients for thrombosis or for duration of anticoagulation in patients with GPA. It is recommended that patients be evaluated for active GPA or relapse in the setting of acute thrombosis whether or not symptoms of active GPA are present.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Talar-Williams C, Hijazi YM, Walther MM, et al. Cyclophosphamide-induced cystitis and bladder cancer in patients with Wegener granulomatosis. Ann Intern Med 1996; 124:477–484.
- Villa-Forte A, Clark TM, Gomes M, et al. Substitution of methotrexate for cyclophosphamide in Wegener granulomatosis: a 12-year single-practice experience. Medicine 2007; 86:269–277.
- Harel S, Fermé C, Poirot C. Management of fertility in patients treated for Hodgkin’s lymphoma [published online ahead of print August 9, 2011]. Haematologica 2011; 96:1692–1699. doi: 10.3324/haematol.2011.045856
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis [published online ahead of print July 26, 2010]. Arthritis Care Res (Hoboken) 2010; 62:1515–1526. doi: 10.1002/acr.20295
- Faurschou M, Mellemkjaer L, Sorensen IJ, Svalgaard Thomsen B, Dreyer L, Baslund B. Increased morbidity from ischemic heart disease in patients with Wegener’s granulomatosis. Arthritis Rheum 2009; 60:1187–1192.
- Suppiah R, Judge A, Batra R, et al. A model to predict cardiovascular events in patients with newly diagnosed Wegener’s granulomatosis and microscopic polyangiitis. Arthritis Care Res (Hoboken) 2011; 63:588–596.
- Merkel PA, Lo GH, Holbrook JT, et al; for Wegener’s Granulomatosis Etanercept Trial Research Group. Brief communication: high incidence of venous thrombotic events among patients with Wegener granulomatosis: the Wegener’s Clinical Occurrence of Thrombosis (WeCLOT) study. Ann Intern Med 2005; 142:620–626.
Granulomatosis with polyangiitis (GPA), is one of the most common types of small-vessel vasculitis, with an estimated prevalence in the United States of 3 per 100,000 people. It is distinguished from other necrotizing vasculitides by its tendency to affect the upper and lower respiratory system and the kidneys. Despite the success of induction and maintenance treatments with cyclophosphamide (CYC), glucocorticoids, and less toxic immunosuppressive alternative therapies in improving the disease course, significant treatment-related toxicities and frequent disease relapses demand stringent patient-specific monitoring in order to provide early treatment of relapses and prevent or decrease morbidity.
SMALL-VESSEL VASCULITIS MANAGEMENT OVERVIEW
Granulomatosis with polyangiitis (formerly Wegener’s granulomatosis, or WG) is an antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis that often affects the respiratory system and kidneys across a broad spectrum of clinical presentations, from mild through life-threatening disease. Patients with severe disease present with significant multisystem manifestations, which, in addition to the respiratory system and kidneys, may involve the joints, eyes, and other organs.
Managing patients diagnosed with systemic small-vessel vasculitides such as GPA and microscopic polyangiitis (MPA) is an inexact science. The goals of treatment are to increase survival, induce and maintain remission, reduce relapses, and minimize treatment-related toxicity. Inducing and maintaining remission have become realistic goals because of the availability of medications that prolong life. On the other hand, extended periods of treatment associated with prolonged life increase the risk of treatment-related toxicity in patients who are inadequately monitored.
MONITORING CONSIDERATIONS
Achieving treatment goals requires long-term monitoring of both disease activity and treatment-related toxicities, with constant adjustments to meet the needs of the individual patient and address the often rapidly changing disease and treatment course. The monitoring protocol consists of regularly scheduled follow-up office visits, urine sediment analyses at every office visit whether or not the patient has relapse symptoms, laboratory tests at regular intervals as indicated by the patient’s medication plan and disease presentation, additional tests such as lung computed tomography (CT), and patient education regarding new symptoms and the frequency of office visits. A consistent monitoring strategy will help detect a relapse before it can produce more severe morbidity, identify treatment-related complications, and—equally important—identify the achievement of remission. An example of the consequences of inconsistent monitoring is presented in “Relapse in a nonadherent patient.”
Because there is no definitive cure for small-vessel vasculitis, relapse is always a possibility. The early diagnosis and treatment of relapse may prevent or decrease morbidity from disease, but strict monitoring is needed to identify relapse and initiate treatment before morbidity occurs (see “Relapse in a patient with new symptoms”). Repeat induction therapy following a relapse introduces risk of drug toxicity and requires careful monitoring, as does long-term maintenance therapy.
In addition to induction and maintenance therapy, several other situations, including prior therapeutic complications, serum creatinine levels, and risk of cardiovascular disease, require special monitoring attention.

Induction therapy: monitor response

Response to treatment during induction must be monitored to identify whether remission is achieved. Induction monitoring requires complete assessment of organ-system involvement at every visit with tools such as the Birmingham Vasculitis Activity Score (BVAS) and, when appropriate, the BVAS/WG. If new or worsening symptoms develop during induction therapy, then the patient needs assessment for continued disease activity as well as treatment complications such as infections related to immunosuppressive therapy.
During induction therapy with daily oral CYC, monitoring should include weekly complete blood cell counts to ensure early identification of leukopenia and other cytopenias. The risk of morbidities increases with the cumulative dose, so a stable blood count for 2 months does not obviate the risk of leukopenia. If persistent hematuria is present without cellular casts, cystoscopy is indicated to look for signs of hemorrhagic cystitis. Prophylaxis against Pneumocystis jirovecii is recommended in all patients who receive immunosuppressive therapy. Finally, bone density measurements should be done at baseline.
Maintenance therapy: frequency can be extended
Monitoring during maintenance therapy is similar to induction monitoring; however, when the dosage of methotrexate or azathioprine is stabilized, the frequency of some tests can be extended to monthly rather than weekly. For example, a complete blood cell count, comprehensive metabolic panel, sedimentation rate, C-reactive protein measurement, and urinalysis should be performed monthly. Follow-up visits should include urine sediment analyses and monitoring for cardiovascular disease risk factors. Medication monitoring should include cystoscopy for persistent hematuria without cellular casts, bone density measurements, and ophthalmologic examinations as frequently as indicated for each individual’s needs. P jirovecii prophylaxis should continue as long as the patient receives immunosuppressive medication.

Therapy-related complications
Bladder complications. In a retrospective analysis of 145 patients with GPA treated with CYC and followed for 0.5 to 27 years (median 8.5 years), nonglomerular hematuria developed in 50% of the patients and bladder carcinoma in 5%.2 The cumulative CYC dose (19 to 251 g) in this group was much higher than what is currently used. Cytologic examination of the urine showed 43% sensitivity for dysplasia (specificity 100%) and 29% sensitivity for atypia (specificity 89%). In contrast, in a retrospective outcomes analysis involving newly diagnosed patients with GPA treated with CYC or methotrexate, 82 patients followed for up to 12 years had no incidents of cystitis or bladder cancer.3 Patients in this study were treated with CYC for only 3 to 6 months and therefore received a lower cumulative dose.
To prevent cystitis during treatment with CYC, the patient should be well hydrated, especially in the morning when CYC should be taken. The bladder should be emptied frequently. The addition of mesna when administering intravenous CYC decreases the risk of cystitis. Serial cystoscopy and urine cytology should be used only in patients with nonglomerular hematuria.
Infertility. Preservation of ovarian function is a concern with CYC therapy in women of childbearing age. The cumulative dose threshold for gonadal failure is unknown, because data from cancer studies4 demonstrating gonadal failure involve higher cumulative CYC doses than are typical for vasculitis treatment. It is also unknown whether duration of amenorrhea predicts the recovery of menses or fertility. The primary option for preservation of ovarian function is the use of gonadotropin-releasing hormone agonists. Oral contraceptives also may be used, but the best prevention is to avoid CYC in these patients if possible.
Osteoporosis. At glucocorticoid dosages of 5 mg/day or greater, bone mineral density begins a rapid decline within the first 3 months and peaks at 6 months.5 The American College of Rheumatology has provided recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis.5 Table 2 presents recommendations for postmenopausal women and men aged 50 years and older who will use glucocorticoids for 3 months or more.5 Recommendations are also available for premenopausal women and men younger than 50 years of age who have a history of fragility fracture.
Leukopenia. Leukopenia should be avoided during CYC treatment. The target white blood cell count should be within the normal range. During treatment with daily oral CYC, the patient should be monitored with a weekly complete blood cell count and medication should be adjusted to maintain the target white blood cell count.
Upon completion of induction therapy, after 3 to 6 months, the patient is switched to maintenance therapy with an alternative immunosuppressive agent such as azathioprine or methotrexate, depending on the serum creatinine concentration and other factors. This transition, characterized by full-dose immunosuppressive therapy when the bone marrow has been previously suppressed by CYC treatment, may induce pancytopenia. Monitoring with weekly complete blood counts for at least 4 weeks after initiating maintenance therapy can help ensure stability during the transition period.

Monitor serum creatinine and adjust dosages
The serum creatinine concentration may increase as CYC treatment progresses; in some cases, the serum creatinine concentration increases before a response to treatment is seen. The CYC dosages should be adjusted as necessary in response to serum creatinine changes. Careful monitoring of serum creatinine is necessary during methotrexate therapy, as methotrexate treatment in the setting of renal insufficiency increases the risk of bone marrow suppression.
Cardiovascular disease in GPA and MPA
Premature atherosclerosis has been well described in patients with GPA.6 Within 5 years of diagnosis of GPA or MPA, a cardiovascular event will occur in 14% of patients.7 In the absence of specific guidelines for prevention of cardiovascular disease in patients with vasculitis, it is essential to monitor patients and treat modifiable traditional risk factors aggressively, especially in younger patients. Suppiah et al found that independent determinants of cardiovascular outcome included older age, diastolic hypertension, and positive proteinase-3–ANCA status in patients without prior cardiovascular disease.7
In the Wegener’s Clinical Occurrence of Thrombosis (WeCLOT) study, Merkel et al showed an increased incidence of thrombosis in patients with active GPA8 (see “Relapse presenting as thrombosis,” left). As with cardiovascular disease, there are no specific guidelines for monitoring asymptomatic patients for thrombosis or for duration of anticoagulation in patients with GPA. It is recommended that patients be evaluated for active GPA or relapse in the setting of acute thrombosis whether or not symptoms of active GPA are present.
Granulomatosis with polyangiitis (GPA), is one of the most common types of small-vessel vasculitis, with an estimated prevalence in the United States of 3 per 100,000 people. It is distinguished from other necrotizing vasculitides by its tendency to affect the upper and lower respiratory system and the kidneys. Despite the success of induction and maintenance treatments with cyclophosphamide (CYC), glucocorticoids, and less toxic immunosuppressive alternative therapies in improving the disease course, significant treatment-related toxicities and frequent disease relapses demand stringent patient-specific monitoring in order to provide early treatment of relapses and prevent or decrease morbidity.
SMALL-VESSEL VASCULITIS MANAGEMENT OVERVIEW
Granulomatosis with polyangiitis (formerly Wegener’s granulomatosis, or WG) is an antineutrophil cytoplasmic antibody (ANCA)–associated vasculitis that often affects the respiratory system and kidneys across a broad spectrum of clinical presentations, from mild through life-threatening disease. Patients with severe disease present with significant multisystem manifestations, which, in addition to the respiratory system and kidneys, may involve the joints, eyes, and other organs.
Managing patients diagnosed with systemic small-vessel vasculitides such as GPA and microscopic polyangiitis (MPA) is an inexact science. The goals of treatment are to increase survival, induce and maintain remission, reduce relapses, and minimize treatment-related toxicity. Inducing and maintaining remission have become realistic goals because of the availability of medications that prolong life. On the other hand, extended periods of treatment associated with prolonged life increase the risk of treatment-related toxicity in patients who are inadequately monitored.
MONITORING CONSIDERATIONS
Achieving treatment goals requires long-term monitoring of both disease activity and treatment-related toxicities, with constant adjustments to meet the needs of the individual patient and address the often rapidly changing disease and treatment course. The monitoring protocol consists of regularly scheduled follow-up office visits, urine sediment analyses at every office visit whether or not the patient has relapse symptoms, laboratory tests at regular intervals as indicated by the patient’s medication plan and disease presentation, additional tests such as lung computed tomography (CT), and patient education regarding new symptoms and the frequency of office visits. A consistent monitoring strategy will help detect a relapse before it can produce more severe morbidity, identify treatment-related complications, and—equally important—identify the achievement of remission. An example of the consequences of inconsistent monitoring is presented in “Relapse in a nonadherent patient.”
Because there is no definitive cure for small-vessel vasculitis, relapse is always a possibility. The early diagnosis and treatment of relapse may prevent or decrease morbidity from disease, but strict monitoring is needed to identify relapse and initiate treatment before morbidity occurs (see “Relapse in a patient with new symptoms”). Repeat induction therapy following a relapse introduces risk of drug toxicity and requires careful monitoring, as does long-term maintenance therapy.
In addition to induction and maintenance therapy, several other situations, including prior therapeutic complications, serum creatinine levels, and risk of cardiovascular disease, require special monitoring attention.

Induction therapy: monitor response

Response to treatment during induction must be monitored to identify whether remission is achieved. Induction monitoring requires complete assessment of organ-system involvement at every visit with tools such as the Birmingham Vasculitis Activity Score (BVAS) and, when appropriate, the BVAS/WG. If new or worsening symptoms develop during induction therapy, then the patient needs assessment for continued disease activity as well as treatment complications such as infections related to immunosuppressive therapy.
During induction therapy with daily oral CYC, monitoring should include weekly complete blood cell counts to ensure early identification of leukopenia and other cytopenias. The risk of morbidities increases with the cumulative dose, so a stable blood count for 2 months does not obviate the risk of leukopenia. If persistent hematuria is present without cellular casts, cystoscopy is indicated to look for signs of hemorrhagic cystitis. Prophylaxis against Pneumocystis jirovecii is recommended in all patients who receive immunosuppressive therapy. Finally, bone density measurements should be done at baseline.
Maintenance therapy: frequency can be extended
Monitoring during maintenance therapy is similar to induction monitoring; however, when the dosage of methotrexate or azathioprine is stabilized, the frequency of some tests can be extended to monthly rather than weekly. For example, a complete blood cell count, comprehensive metabolic panel, sedimentation rate, C-reactive protein measurement, and urinalysis should be performed monthly. Follow-up visits should include urine sediment analyses and monitoring for cardiovascular disease risk factors. Medication monitoring should include cystoscopy for persistent hematuria without cellular casts, bone density measurements, and ophthalmologic examinations as frequently as indicated for each individual’s needs. P jirovecii prophylaxis should continue as long as the patient receives immunosuppressive medication.

Therapy-related complications
Bladder complications. In a retrospective analysis of 145 patients with GPA treated with CYC and followed for 0.5 to 27 years (median 8.5 years), nonglomerular hematuria developed in 50% of the patients and bladder carcinoma in 5%.2 The cumulative CYC dose (19 to 251 g) in this group was much higher than what is currently used. Cytologic examination of the urine showed 43% sensitivity for dysplasia (specificity 100%) and 29% sensitivity for atypia (specificity 89%). In contrast, in a retrospective outcomes analysis involving newly diagnosed patients with GPA treated with CYC or methotrexate, 82 patients followed for up to 12 years had no incidents of cystitis or bladder cancer.3 Patients in this study were treated with CYC for only 3 to 6 months and therefore received a lower cumulative dose.
To prevent cystitis during treatment with CYC, the patient should be well hydrated, especially in the morning when CYC should be taken. The bladder should be emptied frequently. The addition of mesna when administering intravenous CYC decreases the risk of cystitis. Serial cystoscopy and urine cytology should be used only in patients with nonglomerular hematuria.
Infertility. Preservation of ovarian function is a concern with CYC therapy in women of childbearing age. The cumulative dose threshold for gonadal failure is unknown, because data from cancer studies4 demonstrating gonadal failure involve higher cumulative CYC doses than are typical for vasculitis treatment. It is also unknown whether duration of amenorrhea predicts the recovery of menses or fertility. The primary option for preservation of ovarian function is the use of gonadotropin-releasing hormone agonists. Oral contraceptives also may be used, but the best prevention is to avoid CYC in these patients if possible.
Osteoporosis. At glucocorticoid dosages of 5 mg/day or greater, bone mineral density begins a rapid decline within the first 3 months and peaks at 6 months.5 The American College of Rheumatology has provided recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis.5 Table 2 presents recommendations for postmenopausal women and men aged 50 years and older who will use glucocorticoids for 3 months or more.5 Recommendations are also available for premenopausal women and men younger than 50 years of age who have a history of fragility fracture.
Leukopenia. Leukopenia should be avoided during CYC treatment. The target white blood cell count should be within the normal range. During treatment with daily oral CYC, the patient should be monitored with a weekly complete blood cell count and medication should be adjusted to maintain the target white blood cell count.
Upon completion of induction therapy, after 3 to 6 months, the patient is switched to maintenance therapy with an alternative immunosuppressive agent such as azathioprine or methotrexate, depending on the serum creatinine concentration and other factors. This transition, characterized by full-dose immunosuppressive therapy when the bone marrow has been previously suppressed by CYC treatment, may induce pancytopenia. Monitoring with weekly complete blood counts for at least 4 weeks after initiating maintenance therapy can help ensure stability during the transition period.

Monitor serum creatinine and adjust dosages
The serum creatinine concentration may increase as CYC treatment progresses; in some cases, the serum creatinine concentration increases before a response to treatment is seen. The CYC dosages should be adjusted as necessary in response to serum creatinine changes. Careful monitoring of serum creatinine is necessary during methotrexate therapy, as methotrexate treatment in the setting of renal insufficiency increases the risk of bone marrow suppression.
Cardiovascular disease in GPA and MPA
Premature atherosclerosis has been well described in patients with GPA.6 Within 5 years of diagnosis of GPA or MPA, a cardiovascular event will occur in 14% of patients.7 In the absence of specific guidelines for prevention of cardiovascular disease in patients with vasculitis, it is essential to monitor patients and treat modifiable traditional risk factors aggressively, especially in younger patients. Suppiah et al found that independent determinants of cardiovascular outcome included older age, diastolic hypertension, and positive proteinase-3–ANCA status in patients without prior cardiovascular disease.7
In the Wegener’s Clinical Occurrence of Thrombosis (WeCLOT) study, Merkel et al showed an increased incidence of thrombosis in patients with active GPA8 (see “Relapse presenting as thrombosis,” left). As with cardiovascular disease, there are no specific guidelines for monitoring asymptomatic patients for thrombosis or for duration of anticoagulation in patients with GPA. It is recommended that patients be evaluated for active GPA or relapse in the setting of acute thrombosis whether or not symptoms of active GPA are present.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Talar-Williams C, Hijazi YM, Walther MM, et al. Cyclophosphamide-induced cystitis and bladder cancer in patients with Wegener granulomatosis. Ann Intern Med 1996; 124:477–484.
- Villa-Forte A, Clark TM, Gomes M, et al. Substitution of methotrexate for cyclophosphamide in Wegener granulomatosis: a 12-year single-practice experience. Medicine 2007; 86:269–277.
- Harel S, Fermé C, Poirot C. Management of fertility in patients treated for Hodgkin’s lymphoma [published online ahead of print August 9, 2011]. Haematologica 2011; 96:1692–1699. doi: 10.3324/haematol.2011.045856
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis [published online ahead of print July 26, 2010]. Arthritis Care Res (Hoboken) 2010; 62:1515–1526. doi: 10.1002/acr.20295
- Faurschou M, Mellemkjaer L, Sorensen IJ, Svalgaard Thomsen B, Dreyer L, Baslund B. Increased morbidity from ischemic heart disease in patients with Wegener’s granulomatosis. Arthritis Rheum 2009; 60:1187–1192.
- Suppiah R, Judge A, Batra R, et al. A model to predict cardiovascular events in patients with newly diagnosed Wegener’s granulomatosis and microscopic polyangiitis. Arthritis Care Res (Hoboken) 2011; 63:588–596.
- Merkel PA, Lo GH, Holbrook JT, et al; for Wegener’s Granulomatosis Etanercept Trial Research Group. Brief communication: high incidence of venous thrombotic events among patients with Wegener granulomatosis: the Wegener’s Clinical Occurrence of Thrombosis (WeCLOT) study. Ann Intern Med 2005; 142:620–626.
- Hoffman GS, Kerr GS, Leavitt RY, et al. Wegener granulomatosis: an analysis of 158 patients. Ann Intern Med 1992; 116:488–498.
- Talar-Williams C, Hijazi YM, Walther MM, et al. Cyclophosphamide-induced cystitis and bladder cancer in patients with Wegener granulomatosis. Ann Intern Med 1996; 124:477–484.
- Villa-Forte A, Clark TM, Gomes M, et al. Substitution of methotrexate for cyclophosphamide in Wegener granulomatosis: a 12-year single-practice experience. Medicine 2007; 86:269–277.
- Harel S, Fermé C, Poirot C. Management of fertility in patients treated for Hodgkin’s lymphoma [published online ahead of print August 9, 2011]. Haematologica 2011; 96:1692–1699. doi: 10.3324/haematol.2011.045856
- Grossman JM, Gordon R, Ranganath VK, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis [published online ahead of print July 26, 2010]. Arthritis Care Res (Hoboken) 2010; 62:1515–1526. doi: 10.1002/acr.20295
- Faurschou M, Mellemkjaer L, Sorensen IJ, Svalgaard Thomsen B, Dreyer L, Baslund B. Increased morbidity from ischemic heart disease in patients with Wegener’s granulomatosis. Arthritis Rheum 2009; 60:1187–1192.
- Suppiah R, Judge A, Batra R, et al. A model to predict cardiovascular events in patients with newly diagnosed Wegener’s granulomatosis and microscopic polyangiitis. Arthritis Care Res (Hoboken) 2011; 63:588–596.
- Merkel PA, Lo GH, Holbrook JT, et al; for Wegener’s Granulomatosis Etanercept Trial Research Group. Brief communication: high incidence of venous thrombotic events among patients with Wegener granulomatosis: the Wegener’s Clinical Occurrence of Thrombosis (WeCLOT) study. Ann Intern Med 2005; 142:620–626.
Safety issues in vasculitis: Infections and immunizations in the immunosuppressed host
In 2007, Falagas et al1 provided a systematic review of studies focusing on infection-related morbidity and mortality in patients with connective tissue diseases. Many of the studies reviewed were published prior to the introduction of biologic agents for the treatment of rheumatologic disorders. In 39 studies focusing on infection incidence, patient outcomes, or both in patients with systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), polymyositis/dermatomyositis, granulomatosis with polyangiitis (GPA, [Wegener’s granulomatosis]), and systemic sclerosis, serious infection developed in 29% of patients and 24% of these died due to the infection with a median attributable mortality of 5.2%. Most of the reported infections were common bacterial syndromes such as pneumonia or bacteremia, and opportunistic fungal (Pneumocystis) infections.
Similarly, in 2006 Alarcón2 reported that 25% to 50% of patients with SLE had significant morbidity primarily from common bacterial infections, with viral, fungal, and parasitic infection less common. Staphylococcus aureus was a common cause of soft tissue infection, septic arthritis, and bacteremia. Streptococcus pneumoniae typically caused respiratory infections, although meningitis and sepsis were reported with SLE. Gram-negative bacteria such as Escherichia coli, Klebsiella species, and Pseudomonas species usually caused urinary tract infections and nosocomial pneumonia. Other bacterial infections included Nocardia species, Mycobacterium tuberculosis, and, rarely, Listeria monocytogenes. The most common viral infection was herpes zoster. Fungal infections included Pneumocystis jirovecii (formerly known as Pneumocystis carinii) and Candida species.
In scleroderma, another connective tissue disease evaluated in the literature by Alarcón,2 reports of bacterial, viral, and fungal infections are limited to case reports. In scleroderma patients, viral infections with cytomegalovirus (CMV), parvovirus B19, and P jirovecii were similar to pathogens observed with SLE.
In polymyositis/dermatomyositis, gram-positive pneumonia affected 15% to 20% of patients and S aureus occurred frequently in the juvenile form of the disease. Herpes zoster was commonly observed, but CMV was relatively rare. Other viral infections included Coxsackie virus, parvovirus B19, and hepatitis C in polymyositis/dermatomyositis. Infection with P jirovecii is frequently fatal in these patients. Other fungal infections seen in polymyositis/dermatomyositis include candidiasis and histoplasmosis.2
Since the approval of antitumor necrosis factor (anti-TNF) agents for RA in the late 1990s, as well as other more recent biologic agents, there has been heightened awareness of infectious complications in rheumatologic patients. A major concern with the anti-TNF agents is the risk of granulomatous infection, particularly mycobacterial disease and dimorphic fungal infections such as histoplasmosis and coccidioidomycosis. Formation of granulomas is the major host defense against mycobacterial infection and is mediated in large part by TNF-alpha. The precise risk of infection associated with each of the various biologic agents is still under study, and rates from randomized trials have differed from postmarketing surveillance studies. Important pathogens associated with biologic agents include Nocardia, CMV, Listeria, Aspergillus, and JC virus (JCV).3,4 Delays in the diagnosis of these infections in immunocompromised patients have led to poor outcomes.
KEY PATHOGENS IN INFECTIONS OF IMMUNOCOMPROMISED HOSTS
Pneumocystis jirovecii
For many decades, P jirovecii was classified as a protozoan but, based on gene sequencing, the organism has been reclassified as a fungus. P jirovecii is a low-virulence, unicellular organism that is the causative agent of Pneumocystis pneumonia (PCP). Epidemiologically, primary infection most likely occurs in infants and children. Colonization may be transient, entering the airways and then resolving over a period of weeks or months. Alternatively, the organism may enter a latent state similar to tuberculosis with reactivation occurring during times of intense immunosuppression. However, molecular epidemiology studies show that new cases of PCP are likely environmentally acquired through multiple exposures rather than reactivation of latent infection.5,6 Transmission is thought to be airborne from person to person. Pathogenically, the trophic form of the organism attaches to type 1 alveolar cells and remains in the extracellular compartment of the alveoli. This colonization evokes an influx of inflammatory cells (CD8 cells, neutrophils, and macrophages). However, not all colonizations result in pneumonia—even in advanced human immunodeficiency virus (HIV) infection. While there is an innate immunity through alveolar macrophages and pulmonary surfactant, alveolar macrophage response is impaired in HIV when the CD4 count is low. Cell-mediated immunity is the main defense against progression to pneumonia with assistance from costimulatory molecules (such as CD28 and CD2) as well as B cells.
Laboratory diagnosis. P jirovecii cannot be grown in culture for clinical purposes, and it is extremely difficult to culture even in the research setting. Cytologic stains such as the Wright-Giemsa and methamine silver stains are the mainstay of laboratory diagnosis. The yield for P jirovecii from routine expectorated sputum is very low and some laboratories discourage this approach. The sensitivity of nebulized sputum using hypertonic saline ranges from 50% to 90%.9
In patients with acquired immune deficiency syndrome (AIDS), bronchoscopy provides 90% to 98% sensitivity by BAL. Transbronchial biopsy may provide some additional yield over BAL in a few situations, such as patients who have been receiving partial P jirovecii prophylaxis. Immunofluorescence techniques using monoclonal antibodies to P jirovecii are commercially available and are first-line diagnostic tools in some laboratories. Recently, polymerase chain reaction (PCR) assay has been introduced into clinical practice as a reproducible test with high sensitivity.
Primary therapy. Primary therapy for PCP consists of trimethoprim-sulfamethoxazole (TMP-SMX) or pentamidine. TMP-SMX is considered the drug of choice and is usually administered intravenously for 21 days in HIV patients and 14 days for non-HIV patients. The oral form may be used in patients with less severe PCP with a functioning gastrointestinal tract. Common adverse reactions to TMP-SMX include rash, Stevens-Johnson syndrome, neutropenia, changes in pulmonary function, and nausea/vomiting/diarrhea.10 Pentamidine is as effective as TMP-SMX, but is associated with renal toxicity, hypotension, severe hypoglycemia, cardiac arrhythmias, and diabetes.11 It is generally reserved for severe cases of PCP in patients who are allergic to or otherwise intolerant of sulfa. Other treatments include atovaquone and trimethoprim-dapsone. Adjunctive corticosteroids have been shown to be beneficial in moderate to severe PCP in HIV patients to reduce the local host inflammatory response to dead or dying organisms. Recent guidelines have recommended corticosteroids for HIV patients with PCP who have an arterial oxygen pressure of 70 mm Hg or less on room air, or an alveolar-arterial (A-a) gradient of oxygen 35 mm Hg or greater.12 Little is known about the role of adjunctive corticosteroids in non-HIV patients, given a lack of clinical studies.
Prevention. Recent estimates of disease burden from a meta-analysis of 11,900 patients with connective tissue diseases found PCP in 12% of patients with GPA, in 6% of those with polydermatomyositis, in 5% of those with SLE, and in 1% of those with RA.1 Mortality due to PCP is higher in patients with rheumatic diseases, ranging from 30% in RA to 63% in GPA, than in those with HIV (10% to 20%).13 One key risk factor predisposing patients with connective tissue diseases to infection with P jirovecii is recent corticosteroid use. Among patients with connective tissue disease, more than 90% of those infected with P jirovecii have recently received steroid therapy.14 Additionally, in almost all patients with P jirovecii, lymphopenia with absolute lymphocyte counts less than 1,000/mm3 is present.15
In patients with HIV, prophylaxis is initiated at a CD4 level of 200/mm3.13 However, the cutoff is less clear for non-HIV rheumatic patients. A cutoff of less than 300 cells/mm3 has been proposed for prophylaxis of PCP. However, at that range, approximately 50% of patients with connective tissue disease would remain above the threshold.13 One possible solution is to screen by PCR and treat colonization. Other algorithms have been proposed, but there is no general consensus on treatment of non-HIV rheumatic patients.13,16 Generally, prophylaxis should be considered in patients at the highest risk for PCP. These include patients taking prednisone at doses greater than 20 mg/day for 1 month plus a cytotoxic agent, a TNF inhibitor plus glucocorticoids, and methotrexate plus glucocorticoids in GPA.13
Nocardia asteroides and Nocardia species
Classically, Nocardia infection results in abscess formation with infiltrates of polymorphonuclear cells, debris, and thin-walled abscesses. The most frequent site of primary infection is pulmonary. Characteristically, multiple pulmonary nodules or cavities are seen, and Nocardia should be considered in the differential diagnosis of an immunocompromised patient with nodular pneumonia. The nodules can also be masslike in appearance (greater than 2 cm). The presentation of new cavitary lung opacities with systemic symptoms may be mistaken for GPA.22Nocardia may disseminate to the central nervous system (CNS), skin, joints, and spine, usually causing suppurative infection at these sites. Nocardia has a very strong tropism for neural tissue. In the CNS, Nocardia can cause single or multiple brain abscesses that may be asymptomatic; patients with pulmonary nocardiosis require imaging to rule out occult CNS involvement.
Nocardia species are resistant to several antibiotics. The treatment of choice for Nocardia species is TMPSMX, but imipenem, amikacin, third-generation cephalosporins, and other options such as minocycline and linezolid may be considered depending on the species and the antimicrobial susceptibility pattern.
Histoplasma capsulatum
Histoplasma capsulatum is a dimorphic fungus that causes disease in both healthy and immunocompromised hosts. The organism differs from other pathogenic fungi in that it is an intracellular organism, mainly involving the reticuloendothelial system, and is rarely in the extracellular space. In the United States, infections are clustered endemically in areas such as the Mississippi and Ohio River Valleys, but infections are common worldwide. The fungus is found in soil, mulch, bird excrement, and bat guano. Asymptomatic or mild infections are common in healthy persons residing in endemic areas and occur on a sporadic basis. Epidemics can occur when contaminated material is aerosolized. Histoplasmosis is also an opportunistic infection in patients with impaired T-cell immunity such as persons with AIDS, organ transplant recipients, hematologic malignancies, and corticosteroid use. Clinically significant cases of histoplasmosis have been described in patients with RA while receiving methotrexate alone, corticosteroids alone, and combinations of disease-modifying agents.23 Histoplasmosis was recently identified in 240 patients in association with TNF inhibitors, translating to 17 per 100,000 patients treated with infliximab.21,24
Pathogenesis. Infection initially occurs through inhalation of contaminated material from the environment, primarily causing pulmonary infection. The organism converts from a mold form in the environment to a pathogenic yeast form in the host. Once inhaled, the mediastinal lymph nodes provide the first line of defense. Following draining of the lymph nodes, the organism enters the bloodstream in both immunocompetent and immunosuppressed patients. It is spread hematogenously into the spleen, liver, and reticulo-endothelial system, where it is eventually cleared. In immunocompetent patients, cellular immunity limits infection within 7 to 14 days and humoral immunity is not protective.25 Granuloma formation is the hallmark of host defense.
Spectrum of illness. Histoplasmosis is associated with a wide spectrum of illness, with presentation ranging from asymptomatic to mild pulmonary illness to overwhelming pneumonia. Symptomatic pulmonary histoplasmosis typically presents with fever, flulike symptoms, and cough, often with retrosternal chest pain. X-rays show patchy or nodular infiltrates, with hilar or mediastinal lymphadenopathy. In some cases the lung parenchyma is clear and the main feature is fever and bilateral hilar adenopathy. Pulmonary histoplasmosis may be difficult to distinguish from sarcoidosis and tuberculosis. Extrapulmonary disease can present as hepatitis, infective endocarditis, and chronic meningitis. In immunocompromised patients, histoplasmosis can present as a progressive disseminated disease which can be acute, subacute, or chronic. Chronic disseminated histoplasmosis is characterized by cough, persistent fever, wasting, hepatosplenomegaly, oral ulcerations, and progressive cytopenias. Acute disseminated histoplasmosis has a much more fulminant course characterized by respiratory insufficiency, hypotension, multisystem organ failure, coagulopathies, and encephalopathy. Histoplasmosis is primarily a pulmonary disease, but in disseminated disease more than 50% of patients have no pulmonary symptoms and 30% may have normal chest x-rays.26 In one series of infliximab-related cases (n = 10), all came from an endemic area 1 week to 6 months after the first dose of infliximab. Patients presented with cough, fever, and shortness of breath.27 The pathogenesis of histoplasmosis in patients receiving TNF inhibitors is not entirely clear; such patients may be suffering a new primary infection, a reinfection, or, least likely, reactivation of latent infection.
Definitive diagnosis requires culture confirmation from appropriate body fluids or identification of characteristic yeast forms from histopathologic sections of tissue biopsies. Serologic tests may also be used to confirm the diagnosis. Detection of H and M precipitins or bands by immunodiffusion is a routine test in many laboratories. M bands are present in 50% of acute cases but their presence does not distinguish acute from remote infection. H bands are present in only 10% of all acute cases, but their presence is very specific for acute histoplasmosis.28
When looking at complement fixation antibodies to yeast (HY) and mycelial (HMy) forms in pulmonary histoplasmosis, a fourfold rise in titer establishes the diagnosis retrospectively, and a single titer greater than 1:32 is strongly suggestive of active infection. However, in progressive disseminated histoplasmosis, the complement fixation antibodies are frequently negative.29 Detection of antigen in urine and serum by enzyme immunoassay has become a mainstay of diagnosis, with a sensitivity of approximately 90% in progressive disseminated disease.30 Of note, most cases of histoplasmosis associated with biologic agents have detectable urinary antigen tests.
Treatment. Acute pulmonary histoplasmosis is usually self-limited, requiring no treatment. The 2007 Infective Diseases Society of America (IDSA) guidelines recommend observation alone in most cases of mild to moderate pulmonary histoplasmosis unless symptoms persist longer than 1 month. For moderately severe or severe acute pulmonary histoplasmosis, the IDSA recommends lipid formulations of amphotericin B (3.0 to 5.0 mg/kg/day) or deosycholate amphotericin B (0.7 to 1.0 mg/kg/day) for 1 to 2 weeks followed by itraconazole 200 mg twice daily for a total of 12 weeks. Methylprednisolone at a dose of 0.5 to 1.0 mg/kg/day intravenously for 1 to 2 weeks is also recommended. For moderately severe to severe disseminated histoplasmosis, the IDSA recommends lipid formulations of amphotericin B (3.0 mg/kg/day) for 1 to 2 weeks followed by oral itraconazole 200 mg three times daily for 3 days and then 200 mg twice daily for a total of at least 12 months.31 Commonly, the immunosuppressive agent is held during treatment.
Aspergillus species
Another emerging pathogen is Aspergillus species—a ubiquitous mold spread by aerosols of spores. There are many different species of Aspergillus, but the most common human pathogens include A fumigates, A niger, and A flavus. To date, 39 cases of Aspergillus infection associated with infliximab and etanercept have been reported in the Adverse Event Reporting System, translating to 9 to 12 cases per 100,000 patients.21
Varicella zoster
JC virus
More than 80% of adults are seropositive for JCV, a DNA virus of the genus Polyomavirus that causes lytic infection of oligodendrocytes.34 In immunocompromised hosts, JCV causes progressive multifocal leukoencephalopathy (PML), a rare but devastating demyelinating disease. PML was first described in malignancy, leukemia, and various other immunocompromised states, prior to its strong association with AIDS in the 1980s. More recently, JCV has been associated with natalizumab for multiple sclerosis and Crohn disease, rituximab for oncology patients, efalizumab for psoriasis,35 and mycophenolate mofetil for transplant recipients.36
In 2006 the US Food and Drug Administration issued a safety alert regarding PML in two patients with SLE treated with rituximab and other immunosuppressives.37 In a review of PML in rheumatic disease, 36 cases were identified in patients who had not previously received a biologic agent. Most of these patients (60%) had SLE.38 Of these, many had little or no immunosuppression over the 6 months prior to the diagnosis of PML, suggesting that SLE itself may predispose to PML. Interestingly, PML is rarely associated with TNF inhibitors.
Classic presentation of PML includes motor weakness, aphasia, dysarthria, vision loss, and cognitive loss. Atypical presentation includes seizures, headaches, and brainstem involvement. PML usually spares the optic nerves, spinal cord, peripheral nerves, and muscles. In persons with underlying rheumatic diseases, PML can be difficult to distinguish from neuropsychiatric SLE or CNS vasculitis.
Treatment. In clinical trials no antiviral agent has been effective in the treatment of PML. In HIV patients who develop PML, highly active antiretroviral therapy should be initiated (if antiretroviral-naïve) or existing antiviral regimens optimized. Antiretroviral therapy in this situation may stabilize disease and possibly increase survival.42 For HIV-negative patients who develop PML, the cornerstone of management is immediate decrease or discontinuation of immunosuppression.43 Several adjunctive measures have been reported mainly in natalizumab-associated PML, including corticosteroids, mirtazapine, plasma exchange, and others.
VACCINES
Vaccination is important in the prevention of infectious disease in immunocompromised patients with connective tissue diseases. Because live vaccines are contraindicated in immunocompromised patients, inactivated or component vaccines should be used. It is recommended that patients who will start immunosuppressive therapy be vaccinated 2 to 4 weeks before beginning therapy. If this is not possible, vaccination should be administered during disease remission, 3 months after immunosuppression and 1 to 3 months after administration of high-dose corticosteroids.
- Short-term (less than 14 days)
- At a dose of less than 20 mg/day of prednisone or equivalent
- Long-term on alternate days with short-acting preparations
- At a physiologic dose of prednisone
- Topical, inhaled, intra-articular, bursal, or via tendon.44
Until definitive guidelines are developed, practitioners must evaluate and treat each patient individually to maximize the efficacy of disease treatments while preventing infection morbidity and mortality in their patients with connective tissue diseases.
- Falagas ME, Manta KG, Betsi GI, Pappas G. Infection-related morbidity and mortality in patients with connective tissue diseases: a systematic review. Clin Rheumatol 2007; 26:663–670.
- Alarcón GS. Infections in systemic connective tissue diseases: systemic lupus erythematosus, scleroderma, and polymyositis/dermatomyositis. Infect Dis Clin North Am 2006; 20:849–875.
- Crum NF, Lederman ER, Wallace MR. Infections associated with tumor necrosis factor-alpha antagonists. Medicine (Baltimore) 2005; 84:291–302.
- Rychly DJ, DiPiro JT. Infections associated with tumor necrosis factor-alpha antagonists. Pharmacotherapy 2005; 25:1181–1192.
- Wakefield AE, Lindley AR, Ambrose HE, Denis CM, Miller RF. Limited asymptomatic carriage of Pneumocystis jiroveci in human immunodeficiency virus-infected patients [published online ahead of print March 6, 2003]. J Infect Dis 2003; 187:901–908. doi: 10.1086/368165
- Beard CB, Carter JL, Keely SP, et al. Genetic variation in Pneumocystis carinii isolates from different geographic regions: implications for transmission. Emerg Infect Dis 2000; 6:265–272.
- Walzer PD, Smulian AG. Pneumocystis species. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, Pa: Elsevier Churchill Livingstone; 2009.
- Hartman TE, Primack SL, Müller NL, Staples CA. Diagnosis of thoracic complications in AIDS: accuracy of CT. Am J Roentgenol 1994; 162:547–553.
- Shelhamer JH, Gill VJ, Quinn TC, et al. The laboratory evaluation of opportunistic pulmonary infections. Ann Intern Med 1996; 124:585–599.
- Wharton JM, Coleman DL, Wofsy CB, et al. Trimethoprim-sulfamethoxazole or pentamidine for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. Ann Intern Med 1986; 105:37–44.
- Stein DS, Stevens RC. Treatment-associated toxicities: incidence and mechanisms. In:Sattler FR, Walzer PD, eds. Pneumocystis carinii. London: Bailliere Tindall; 1995:505–530.
- Consensus statement on the use of corticosteroids as adjunctive therapy for Pneumocystis pneumonia in the acquired immunodeficiency syndrome. The National Institutes of Health-University of California Expert Panel for Corticosteroids as Adjunctive Therapy for Pneumocystis Pneumonia. N Engl J Med 1990; 323:1500–1504.
- Stamp LK, Hurst M. Is there a role for consensus guidelines for P. jiroveci pneumonia prophylaxis in immunosuppressed patients with rheumatic diseases? J Rheumatol 2010; 37:686–688.
- Yale S, Limper A. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illnesses and prior corticosteroid therapy. Mayo Clin Proc 1996; 71:5–13.
- Sowden E, Carmichael A. Autoimmune inflammatory disorders, systemic corticosteroids and Pneumocystis pneumonia: a strategy for prevention [published online October 16, 2004]. BMC Infect Dis 2004; 4:42. doi: 10.1186/1471-2334-4-42
- Cettomai D, Gelber AC, Christopher-Stine L. A survey of rheumatologists’ practice for prescribing Pneumocystis prophylaxis. J Rheumatol 2010; 37:792–799.
- Keegan JM, Byrd JW. Nocardiosis associated with low dose methotrexate for rheumatoid arthritis. J Rheumatol 1988; 15:1585–1586.
- Gruberg L, Thaler M, Rozenman J, et al. Nocardia asteroides infection complicating rheumatoid arthritis. J Rheumatol 1991; 18:459–461.
- Corneliessen JJ, Bakker LJ, van der Veen MJ, et al. Nocardia asteroides pneumonia complicating low dose methotrexate treatment of refractory rheumatoid arthritis. Ann Rheum Dis 1991; 50;642–644.
- Silva C, Faccioli LH. Tumor necrosis factor and macrophage activation are important in clearance of Nocardia brasiliensis from the livers and spleens of mice. Infect Immun 1992; 60:3566–3570.
- Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis 2004; 38:1261–1265.
- Gibb W, Williams A. Nocardiosis mimicking Wegener’s granulomatosis. Scand J Infect Dis 1986; 18:583–585.
- Olson TC, Bongartz T, Crowson CS, Roberts GD, Orenstein R, Matteson EI. Histoplasmosis infection in patients with rheumatoid arthritis, 1998–2009 [published online May 23, 2011]. BMC Infectious Diseases 2011; 11:145. doi: 10.1186/1471-2334-11-145
- Information for Healthcare Professionals: Cimzia (certolizumab pegol), Enbrel etanercept), Humira (adalimumab), and Remicade (infliximab). U.S. Food and Drug Administration Web site. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124185.htm. Updated January 25, 2010. Accessed September 27, 2012.
- Paya CV, Roberts GD, Cockerill FR. Transient fungemia in acute pulmonary histoplasmosis: detection by new blood-culturing techniques. J Infect Dis 1987; 156:313–315.
- Goodwin RA, Shapiro JL, Thurman GH, Thurman SS, Des Prez RM. Disseminated histoplasmosis: clinical and pathologic correlations. Medicine (Baltimore) 1980; 59:1–33.
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum 2002; 46:2565–2570.
- Picardi JL, Kauffman CA, Schwarz J, Phair JP. Detection of precipitating antibodies to Histoplasma capsulatum by counterimmunoelectrophoresis. Am Rev Respir Dis 1976; 114:171–176.
- Deepe GS. Histoplasma capsulatum. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, Pa: Elsevier Churchill Livingstone; 2009.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America [published online ahead of print August 27, 2007]. Clin Infect Dis 2007; 45:807–825. doi: 10.1086/521259
- Johnson RW. Herpes zoster and postherpetic neuralgia. Expert Rev Vaccines 2010; 9( 3 suppl):21–26.
- Wolfe F, Michaud K, Chakravarty EF. Rates and predictors of herpes zoster in patients with rheumatoid arthritis and non-inflammatory musculoskeletal disorders. Rheumatology 2006; 45:1370–1375.
- Weber T, Trebst C, Frye S, et al. Analysis of the systemic and intrathecal humoral immune response in progressive multifocal leukoencephalopathy. J Infect Dis 1997; 176:250–254.
- Kothary N, Diak IL, Brinker A, Bezabeh S, Avigan M, Dal Pan G. Progressive multifocal leukoencephalopathy associated with efalizumab use in psoriasis patients. J Am Acad Dermatol 2011; 65:546–551.
- Neff RT, Hurst FP, Falta EM, et al. Progressive multifocal leukoencephalopathy and use of mycophenolate mofetil after kidney transplantation. Transplantation 2008; 86:1474–1478.
- Rituxan warning. FDA Consum 2007; 41:3.
- Calabrese LH, Molloy ES, Huang D, Ransohoff RM. Progressive multifocal leukoencephalopathy in rheumatic diseases. Arthritis Rheum 2007; 56:2116–2128.
- Cinque P, Scarpellini P, Vago L, Linde A, Lazzarin A. Diagnosis of central nervous system complications in HIV-infected patients: cerebrospinal fluid analysis by the polymerase chain reaction. AIDS 1997; 11:1–17.
- Ryschkewitsch C, Jensen P, Hou J, Fahle G, Fischer S, Major EO. Comparison of PCR-southern hybridization and quantitative real-time PCR for the detection of JC and BK viral nucleotide sequences in urine and cerebrospinal fluid. J Virol Methods 2004; 121:217–221.
- Major EO. History and current concepts in the pathogenesis of PML. Cleve Clin J Med 2011; 78( suppl 2):S3–S7.
- Antinori A, Ammassari A, Giancola ML, et al. Epidemiology and prognosis of AIDS-associated progressive multifocal leukoencephalopathy in the HAART era. J Neurovirol 2001; 7:323–328.
- Calabrese L. A rational approach to PML for the clinician. Cleve Clin J Med 2011; 78 (suppl 2):S38–S41.
- Kroger AT, Sumaya CV, Pickering LK, Atkinson WL. General recommendations on immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2011; 60:1–60.
In 2007, Falagas et al1 provided a systematic review of studies focusing on infection-related morbidity and mortality in patients with connective tissue diseases. Many of the studies reviewed were published prior to the introduction of biologic agents for the treatment of rheumatologic disorders. In 39 studies focusing on infection incidence, patient outcomes, or both in patients with systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), polymyositis/dermatomyositis, granulomatosis with polyangiitis (GPA, [Wegener’s granulomatosis]), and systemic sclerosis, serious infection developed in 29% of patients and 24% of these died due to the infection with a median attributable mortality of 5.2%. Most of the reported infections were common bacterial syndromes such as pneumonia or bacteremia, and opportunistic fungal (Pneumocystis) infections.
Similarly, in 2006 Alarcón2 reported that 25% to 50% of patients with SLE had significant morbidity primarily from common bacterial infections, with viral, fungal, and parasitic infection less common. Staphylococcus aureus was a common cause of soft tissue infection, septic arthritis, and bacteremia. Streptococcus pneumoniae typically caused respiratory infections, although meningitis and sepsis were reported with SLE. Gram-negative bacteria such as Escherichia coli, Klebsiella species, and Pseudomonas species usually caused urinary tract infections and nosocomial pneumonia. Other bacterial infections included Nocardia species, Mycobacterium tuberculosis, and, rarely, Listeria monocytogenes. The most common viral infection was herpes zoster. Fungal infections included Pneumocystis jirovecii (formerly known as Pneumocystis carinii) and Candida species.
In scleroderma, another connective tissue disease evaluated in the literature by Alarcón,2 reports of bacterial, viral, and fungal infections are limited to case reports. In scleroderma patients, viral infections with cytomegalovirus (CMV), parvovirus B19, and P jirovecii were similar to pathogens observed with SLE.
In polymyositis/dermatomyositis, gram-positive pneumonia affected 15% to 20% of patients and S aureus occurred frequently in the juvenile form of the disease. Herpes zoster was commonly observed, but CMV was relatively rare. Other viral infections included Coxsackie virus, parvovirus B19, and hepatitis C in polymyositis/dermatomyositis. Infection with P jirovecii is frequently fatal in these patients. Other fungal infections seen in polymyositis/dermatomyositis include candidiasis and histoplasmosis.2
Since the approval of antitumor necrosis factor (anti-TNF) agents for RA in the late 1990s, as well as other more recent biologic agents, there has been heightened awareness of infectious complications in rheumatologic patients. A major concern with the anti-TNF agents is the risk of granulomatous infection, particularly mycobacterial disease and dimorphic fungal infections such as histoplasmosis and coccidioidomycosis. Formation of granulomas is the major host defense against mycobacterial infection and is mediated in large part by TNF-alpha. The precise risk of infection associated with each of the various biologic agents is still under study, and rates from randomized trials have differed from postmarketing surveillance studies. Important pathogens associated with biologic agents include Nocardia, CMV, Listeria, Aspergillus, and JC virus (JCV).3,4 Delays in the diagnosis of these infections in immunocompromised patients have led to poor outcomes.
KEY PATHOGENS IN INFECTIONS OF IMMUNOCOMPROMISED HOSTS
Pneumocystis jirovecii
For many decades, P jirovecii was classified as a protozoan but, based on gene sequencing, the organism has been reclassified as a fungus. P jirovecii is a low-virulence, unicellular organism that is the causative agent of Pneumocystis pneumonia (PCP). Epidemiologically, primary infection most likely occurs in infants and children. Colonization may be transient, entering the airways and then resolving over a period of weeks or months. Alternatively, the organism may enter a latent state similar to tuberculosis with reactivation occurring during times of intense immunosuppression. However, molecular epidemiology studies show that new cases of PCP are likely environmentally acquired through multiple exposures rather than reactivation of latent infection.5,6 Transmission is thought to be airborne from person to person. Pathogenically, the trophic form of the organism attaches to type 1 alveolar cells and remains in the extracellular compartment of the alveoli. This colonization evokes an influx of inflammatory cells (CD8 cells, neutrophils, and macrophages). However, not all colonizations result in pneumonia—even in advanced human immunodeficiency virus (HIV) infection. While there is an innate immunity through alveolar macrophages and pulmonary surfactant, alveolar macrophage response is impaired in HIV when the CD4 count is low. Cell-mediated immunity is the main defense against progression to pneumonia with assistance from costimulatory molecules (such as CD28 and CD2) as well as B cells.
Laboratory diagnosis. P jirovecii cannot be grown in culture for clinical purposes, and it is extremely difficult to culture even in the research setting. Cytologic stains such as the Wright-Giemsa and methamine silver stains are the mainstay of laboratory diagnosis. The yield for P jirovecii from routine expectorated sputum is very low and some laboratories discourage this approach. The sensitivity of nebulized sputum using hypertonic saline ranges from 50% to 90%.9
In patients with acquired immune deficiency syndrome (AIDS), bronchoscopy provides 90% to 98% sensitivity by BAL. Transbronchial biopsy may provide some additional yield over BAL in a few situations, such as patients who have been receiving partial P jirovecii prophylaxis. Immunofluorescence techniques using monoclonal antibodies to P jirovecii are commercially available and are first-line diagnostic tools in some laboratories. Recently, polymerase chain reaction (PCR) assay has been introduced into clinical practice as a reproducible test with high sensitivity.
Primary therapy. Primary therapy for PCP consists of trimethoprim-sulfamethoxazole (TMP-SMX) or pentamidine. TMP-SMX is considered the drug of choice and is usually administered intravenously for 21 days in HIV patients and 14 days for non-HIV patients. The oral form may be used in patients with less severe PCP with a functioning gastrointestinal tract. Common adverse reactions to TMP-SMX include rash, Stevens-Johnson syndrome, neutropenia, changes in pulmonary function, and nausea/vomiting/diarrhea.10 Pentamidine is as effective as TMP-SMX, but is associated with renal toxicity, hypotension, severe hypoglycemia, cardiac arrhythmias, and diabetes.11 It is generally reserved for severe cases of PCP in patients who are allergic to or otherwise intolerant of sulfa. Other treatments include atovaquone and trimethoprim-dapsone. Adjunctive corticosteroids have been shown to be beneficial in moderate to severe PCP in HIV patients to reduce the local host inflammatory response to dead or dying organisms. Recent guidelines have recommended corticosteroids for HIV patients with PCP who have an arterial oxygen pressure of 70 mm Hg or less on room air, or an alveolar-arterial (A-a) gradient of oxygen 35 mm Hg or greater.12 Little is known about the role of adjunctive corticosteroids in non-HIV patients, given a lack of clinical studies.
Prevention. Recent estimates of disease burden from a meta-analysis of 11,900 patients with connective tissue diseases found PCP in 12% of patients with GPA, in 6% of those with polydermatomyositis, in 5% of those with SLE, and in 1% of those with RA.1 Mortality due to PCP is higher in patients with rheumatic diseases, ranging from 30% in RA to 63% in GPA, than in those with HIV (10% to 20%).13 One key risk factor predisposing patients with connective tissue diseases to infection with P jirovecii is recent corticosteroid use. Among patients with connective tissue disease, more than 90% of those infected with P jirovecii have recently received steroid therapy.14 Additionally, in almost all patients with P jirovecii, lymphopenia with absolute lymphocyte counts less than 1,000/mm3 is present.15
In patients with HIV, prophylaxis is initiated at a CD4 level of 200/mm3.13 However, the cutoff is less clear for non-HIV rheumatic patients. A cutoff of less than 300 cells/mm3 has been proposed for prophylaxis of PCP. However, at that range, approximately 50% of patients with connective tissue disease would remain above the threshold.13 One possible solution is to screen by PCR and treat colonization. Other algorithms have been proposed, but there is no general consensus on treatment of non-HIV rheumatic patients.13,16 Generally, prophylaxis should be considered in patients at the highest risk for PCP. These include patients taking prednisone at doses greater than 20 mg/day for 1 month plus a cytotoxic agent, a TNF inhibitor plus glucocorticoids, and methotrexate plus glucocorticoids in GPA.13
Nocardia asteroides and Nocardia species
Classically, Nocardia infection results in abscess formation with infiltrates of polymorphonuclear cells, debris, and thin-walled abscesses. The most frequent site of primary infection is pulmonary. Characteristically, multiple pulmonary nodules or cavities are seen, and Nocardia should be considered in the differential diagnosis of an immunocompromised patient with nodular pneumonia. The nodules can also be masslike in appearance (greater than 2 cm). The presentation of new cavitary lung opacities with systemic symptoms may be mistaken for GPA.22Nocardia may disseminate to the central nervous system (CNS), skin, joints, and spine, usually causing suppurative infection at these sites. Nocardia has a very strong tropism for neural tissue. In the CNS, Nocardia can cause single or multiple brain abscesses that may be asymptomatic; patients with pulmonary nocardiosis require imaging to rule out occult CNS involvement.
Nocardia species are resistant to several antibiotics. The treatment of choice for Nocardia species is TMPSMX, but imipenem, amikacin, third-generation cephalosporins, and other options such as minocycline and linezolid may be considered depending on the species and the antimicrobial susceptibility pattern.
Histoplasma capsulatum
Histoplasma capsulatum is a dimorphic fungus that causes disease in both healthy and immunocompromised hosts. The organism differs from other pathogenic fungi in that it is an intracellular organism, mainly involving the reticuloendothelial system, and is rarely in the extracellular space. In the United States, infections are clustered endemically in areas such as the Mississippi and Ohio River Valleys, but infections are common worldwide. The fungus is found in soil, mulch, bird excrement, and bat guano. Asymptomatic or mild infections are common in healthy persons residing in endemic areas and occur on a sporadic basis. Epidemics can occur when contaminated material is aerosolized. Histoplasmosis is also an opportunistic infection in patients with impaired T-cell immunity such as persons with AIDS, organ transplant recipients, hematologic malignancies, and corticosteroid use. Clinically significant cases of histoplasmosis have been described in patients with RA while receiving methotrexate alone, corticosteroids alone, and combinations of disease-modifying agents.23 Histoplasmosis was recently identified in 240 patients in association with TNF inhibitors, translating to 17 per 100,000 patients treated with infliximab.21,24
Pathogenesis. Infection initially occurs through inhalation of contaminated material from the environment, primarily causing pulmonary infection. The organism converts from a mold form in the environment to a pathogenic yeast form in the host. Once inhaled, the mediastinal lymph nodes provide the first line of defense. Following draining of the lymph nodes, the organism enters the bloodstream in both immunocompetent and immunosuppressed patients. It is spread hematogenously into the spleen, liver, and reticulo-endothelial system, where it is eventually cleared. In immunocompetent patients, cellular immunity limits infection within 7 to 14 days and humoral immunity is not protective.25 Granuloma formation is the hallmark of host defense.
Spectrum of illness. Histoplasmosis is associated with a wide spectrum of illness, with presentation ranging from asymptomatic to mild pulmonary illness to overwhelming pneumonia. Symptomatic pulmonary histoplasmosis typically presents with fever, flulike symptoms, and cough, often with retrosternal chest pain. X-rays show patchy or nodular infiltrates, with hilar or mediastinal lymphadenopathy. In some cases the lung parenchyma is clear and the main feature is fever and bilateral hilar adenopathy. Pulmonary histoplasmosis may be difficult to distinguish from sarcoidosis and tuberculosis. Extrapulmonary disease can present as hepatitis, infective endocarditis, and chronic meningitis. In immunocompromised patients, histoplasmosis can present as a progressive disseminated disease which can be acute, subacute, or chronic. Chronic disseminated histoplasmosis is characterized by cough, persistent fever, wasting, hepatosplenomegaly, oral ulcerations, and progressive cytopenias. Acute disseminated histoplasmosis has a much more fulminant course characterized by respiratory insufficiency, hypotension, multisystem organ failure, coagulopathies, and encephalopathy. Histoplasmosis is primarily a pulmonary disease, but in disseminated disease more than 50% of patients have no pulmonary symptoms and 30% may have normal chest x-rays.26 In one series of infliximab-related cases (n = 10), all came from an endemic area 1 week to 6 months after the first dose of infliximab. Patients presented with cough, fever, and shortness of breath.27 The pathogenesis of histoplasmosis in patients receiving TNF inhibitors is not entirely clear; such patients may be suffering a new primary infection, a reinfection, or, least likely, reactivation of latent infection.
Definitive diagnosis requires culture confirmation from appropriate body fluids or identification of characteristic yeast forms from histopathologic sections of tissue biopsies. Serologic tests may also be used to confirm the diagnosis. Detection of H and M precipitins or bands by immunodiffusion is a routine test in many laboratories. M bands are present in 50% of acute cases but their presence does not distinguish acute from remote infection. H bands are present in only 10% of all acute cases, but their presence is very specific for acute histoplasmosis.28
When looking at complement fixation antibodies to yeast (HY) and mycelial (HMy) forms in pulmonary histoplasmosis, a fourfold rise in titer establishes the diagnosis retrospectively, and a single titer greater than 1:32 is strongly suggestive of active infection. However, in progressive disseminated histoplasmosis, the complement fixation antibodies are frequently negative.29 Detection of antigen in urine and serum by enzyme immunoassay has become a mainstay of diagnosis, with a sensitivity of approximately 90% in progressive disseminated disease.30 Of note, most cases of histoplasmosis associated with biologic agents have detectable urinary antigen tests.
Treatment. Acute pulmonary histoplasmosis is usually self-limited, requiring no treatment. The 2007 Infective Diseases Society of America (IDSA) guidelines recommend observation alone in most cases of mild to moderate pulmonary histoplasmosis unless symptoms persist longer than 1 month. For moderately severe or severe acute pulmonary histoplasmosis, the IDSA recommends lipid formulations of amphotericin B (3.0 to 5.0 mg/kg/day) or deosycholate amphotericin B (0.7 to 1.0 mg/kg/day) for 1 to 2 weeks followed by itraconazole 200 mg twice daily for a total of 12 weeks. Methylprednisolone at a dose of 0.5 to 1.0 mg/kg/day intravenously for 1 to 2 weeks is also recommended. For moderately severe to severe disseminated histoplasmosis, the IDSA recommends lipid formulations of amphotericin B (3.0 mg/kg/day) for 1 to 2 weeks followed by oral itraconazole 200 mg three times daily for 3 days and then 200 mg twice daily for a total of at least 12 months.31 Commonly, the immunosuppressive agent is held during treatment.
Aspergillus species
Another emerging pathogen is Aspergillus species—a ubiquitous mold spread by aerosols of spores. There are many different species of Aspergillus, but the most common human pathogens include A fumigates, A niger, and A flavus. To date, 39 cases of Aspergillus infection associated with infliximab and etanercept have been reported in the Adverse Event Reporting System, translating to 9 to 12 cases per 100,000 patients.21
Varicella zoster
JC virus
More than 80% of adults are seropositive for JCV, a DNA virus of the genus Polyomavirus that causes lytic infection of oligodendrocytes.34 In immunocompromised hosts, JCV causes progressive multifocal leukoencephalopathy (PML), a rare but devastating demyelinating disease. PML was first described in malignancy, leukemia, and various other immunocompromised states, prior to its strong association with AIDS in the 1980s. More recently, JCV has been associated with natalizumab for multiple sclerosis and Crohn disease, rituximab for oncology patients, efalizumab for psoriasis,35 and mycophenolate mofetil for transplant recipients.36
In 2006 the US Food and Drug Administration issued a safety alert regarding PML in two patients with SLE treated with rituximab and other immunosuppressives.37 In a review of PML in rheumatic disease, 36 cases were identified in patients who had not previously received a biologic agent. Most of these patients (60%) had SLE.38 Of these, many had little or no immunosuppression over the 6 months prior to the diagnosis of PML, suggesting that SLE itself may predispose to PML. Interestingly, PML is rarely associated with TNF inhibitors.
Classic presentation of PML includes motor weakness, aphasia, dysarthria, vision loss, and cognitive loss. Atypical presentation includes seizures, headaches, and brainstem involvement. PML usually spares the optic nerves, spinal cord, peripheral nerves, and muscles. In persons with underlying rheumatic diseases, PML can be difficult to distinguish from neuropsychiatric SLE or CNS vasculitis.
Treatment. In clinical trials no antiviral agent has been effective in the treatment of PML. In HIV patients who develop PML, highly active antiretroviral therapy should be initiated (if antiretroviral-naïve) or existing antiviral regimens optimized. Antiretroviral therapy in this situation may stabilize disease and possibly increase survival.42 For HIV-negative patients who develop PML, the cornerstone of management is immediate decrease or discontinuation of immunosuppression.43 Several adjunctive measures have been reported mainly in natalizumab-associated PML, including corticosteroids, mirtazapine, plasma exchange, and others.
VACCINES
Vaccination is important in the prevention of infectious disease in immunocompromised patients with connective tissue diseases. Because live vaccines are contraindicated in immunocompromised patients, inactivated or component vaccines should be used. It is recommended that patients who will start immunosuppressive therapy be vaccinated 2 to 4 weeks before beginning therapy. If this is not possible, vaccination should be administered during disease remission, 3 months after immunosuppression and 1 to 3 months after administration of high-dose corticosteroids.
- Short-term (less than 14 days)
- At a dose of less than 20 mg/day of prednisone or equivalent
- Long-term on alternate days with short-acting preparations
- At a physiologic dose of prednisone
- Topical, inhaled, intra-articular, bursal, or via tendon.44
Until definitive guidelines are developed, practitioners must evaluate and treat each patient individually to maximize the efficacy of disease treatments while preventing infection morbidity and mortality in their patients with connective tissue diseases.
In 2007, Falagas et al1 provided a systematic review of studies focusing on infection-related morbidity and mortality in patients with connective tissue diseases. Many of the studies reviewed were published prior to the introduction of biologic agents for the treatment of rheumatologic disorders. In 39 studies focusing on infection incidence, patient outcomes, or both in patients with systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), polymyositis/dermatomyositis, granulomatosis with polyangiitis (GPA, [Wegener’s granulomatosis]), and systemic sclerosis, serious infection developed in 29% of patients and 24% of these died due to the infection with a median attributable mortality of 5.2%. Most of the reported infections were common bacterial syndromes such as pneumonia or bacteremia, and opportunistic fungal (Pneumocystis) infections.
Similarly, in 2006 Alarcón2 reported that 25% to 50% of patients with SLE had significant morbidity primarily from common bacterial infections, with viral, fungal, and parasitic infection less common. Staphylococcus aureus was a common cause of soft tissue infection, septic arthritis, and bacteremia. Streptococcus pneumoniae typically caused respiratory infections, although meningitis and sepsis were reported with SLE. Gram-negative bacteria such as Escherichia coli, Klebsiella species, and Pseudomonas species usually caused urinary tract infections and nosocomial pneumonia. Other bacterial infections included Nocardia species, Mycobacterium tuberculosis, and, rarely, Listeria monocytogenes. The most common viral infection was herpes zoster. Fungal infections included Pneumocystis jirovecii (formerly known as Pneumocystis carinii) and Candida species.
In scleroderma, another connective tissue disease evaluated in the literature by Alarcón,2 reports of bacterial, viral, and fungal infections are limited to case reports. In scleroderma patients, viral infections with cytomegalovirus (CMV), parvovirus B19, and P jirovecii were similar to pathogens observed with SLE.
In polymyositis/dermatomyositis, gram-positive pneumonia affected 15% to 20% of patients and S aureus occurred frequently in the juvenile form of the disease. Herpes zoster was commonly observed, but CMV was relatively rare. Other viral infections included Coxsackie virus, parvovirus B19, and hepatitis C in polymyositis/dermatomyositis. Infection with P jirovecii is frequently fatal in these patients. Other fungal infections seen in polymyositis/dermatomyositis include candidiasis and histoplasmosis.2
Since the approval of antitumor necrosis factor (anti-TNF) agents for RA in the late 1990s, as well as other more recent biologic agents, there has been heightened awareness of infectious complications in rheumatologic patients. A major concern with the anti-TNF agents is the risk of granulomatous infection, particularly mycobacterial disease and dimorphic fungal infections such as histoplasmosis and coccidioidomycosis. Formation of granulomas is the major host defense against mycobacterial infection and is mediated in large part by TNF-alpha. The precise risk of infection associated with each of the various biologic agents is still under study, and rates from randomized trials have differed from postmarketing surveillance studies. Important pathogens associated with biologic agents include Nocardia, CMV, Listeria, Aspergillus, and JC virus (JCV).3,4 Delays in the diagnosis of these infections in immunocompromised patients have led to poor outcomes.
KEY PATHOGENS IN INFECTIONS OF IMMUNOCOMPROMISED HOSTS
Pneumocystis jirovecii
For many decades, P jirovecii was classified as a protozoan but, based on gene sequencing, the organism has been reclassified as a fungus. P jirovecii is a low-virulence, unicellular organism that is the causative agent of Pneumocystis pneumonia (PCP). Epidemiologically, primary infection most likely occurs in infants and children. Colonization may be transient, entering the airways and then resolving over a period of weeks or months. Alternatively, the organism may enter a latent state similar to tuberculosis with reactivation occurring during times of intense immunosuppression. However, molecular epidemiology studies show that new cases of PCP are likely environmentally acquired through multiple exposures rather than reactivation of latent infection.5,6 Transmission is thought to be airborne from person to person. Pathogenically, the trophic form of the organism attaches to type 1 alveolar cells and remains in the extracellular compartment of the alveoli. This colonization evokes an influx of inflammatory cells (CD8 cells, neutrophils, and macrophages). However, not all colonizations result in pneumonia—even in advanced human immunodeficiency virus (HIV) infection. While there is an innate immunity through alveolar macrophages and pulmonary surfactant, alveolar macrophage response is impaired in HIV when the CD4 count is low. Cell-mediated immunity is the main defense against progression to pneumonia with assistance from costimulatory molecules (such as CD28 and CD2) as well as B cells.
Laboratory diagnosis. P jirovecii cannot be grown in culture for clinical purposes, and it is extremely difficult to culture even in the research setting. Cytologic stains such as the Wright-Giemsa and methamine silver stains are the mainstay of laboratory diagnosis. The yield for P jirovecii from routine expectorated sputum is very low and some laboratories discourage this approach. The sensitivity of nebulized sputum using hypertonic saline ranges from 50% to 90%.9
In patients with acquired immune deficiency syndrome (AIDS), bronchoscopy provides 90% to 98% sensitivity by BAL. Transbronchial biopsy may provide some additional yield over BAL in a few situations, such as patients who have been receiving partial P jirovecii prophylaxis. Immunofluorescence techniques using monoclonal antibodies to P jirovecii are commercially available and are first-line diagnostic tools in some laboratories. Recently, polymerase chain reaction (PCR) assay has been introduced into clinical practice as a reproducible test with high sensitivity.
Primary therapy. Primary therapy for PCP consists of trimethoprim-sulfamethoxazole (TMP-SMX) or pentamidine. TMP-SMX is considered the drug of choice and is usually administered intravenously for 21 days in HIV patients and 14 days for non-HIV patients. The oral form may be used in patients with less severe PCP with a functioning gastrointestinal tract. Common adverse reactions to TMP-SMX include rash, Stevens-Johnson syndrome, neutropenia, changes in pulmonary function, and nausea/vomiting/diarrhea.10 Pentamidine is as effective as TMP-SMX, but is associated with renal toxicity, hypotension, severe hypoglycemia, cardiac arrhythmias, and diabetes.11 It is generally reserved for severe cases of PCP in patients who are allergic to or otherwise intolerant of sulfa. Other treatments include atovaquone and trimethoprim-dapsone. Adjunctive corticosteroids have been shown to be beneficial in moderate to severe PCP in HIV patients to reduce the local host inflammatory response to dead or dying organisms. Recent guidelines have recommended corticosteroids for HIV patients with PCP who have an arterial oxygen pressure of 70 mm Hg or less on room air, or an alveolar-arterial (A-a) gradient of oxygen 35 mm Hg or greater.12 Little is known about the role of adjunctive corticosteroids in non-HIV patients, given a lack of clinical studies.
Prevention. Recent estimates of disease burden from a meta-analysis of 11,900 patients with connective tissue diseases found PCP in 12% of patients with GPA, in 6% of those with polydermatomyositis, in 5% of those with SLE, and in 1% of those with RA.1 Mortality due to PCP is higher in patients with rheumatic diseases, ranging from 30% in RA to 63% in GPA, than in those with HIV (10% to 20%).13 One key risk factor predisposing patients with connective tissue diseases to infection with P jirovecii is recent corticosteroid use. Among patients with connective tissue disease, more than 90% of those infected with P jirovecii have recently received steroid therapy.14 Additionally, in almost all patients with P jirovecii, lymphopenia with absolute lymphocyte counts less than 1,000/mm3 is present.15
In patients with HIV, prophylaxis is initiated at a CD4 level of 200/mm3.13 However, the cutoff is less clear for non-HIV rheumatic patients. A cutoff of less than 300 cells/mm3 has been proposed for prophylaxis of PCP. However, at that range, approximately 50% of patients with connective tissue disease would remain above the threshold.13 One possible solution is to screen by PCR and treat colonization. Other algorithms have been proposed, but there is no general consensus on treatment of non-HIV rheumatic patients.13,16 Generally, prophylaxis should be considered in patients at the highest risk for PCP. These include patients taking prednisone at doses greater than 20 mg/day for 1 month plus a cytotoxic agent, a TNF inhibitor plus glucocorticoids, and methotrexate plus glucocorticoids in GPA.13
Nocardia asteroides and Nocardia species
Classically, Nocardia infection results in abscess formation with infiltrates of polymorphonuclear cells, debris, and thin-walled abscesses. The most frequent site of primary infection is pulmonary. Characteristically, multiple pulmonary nodules or cavities are seen, and Nocardia should be considered in the differential diagnosis of an immunocompromised patient with nodular pneumonia. The nodules can also be masslike in appearance (greater than 2 cm). The presentation of new cavitary lung opacities with systemic symptoms may be mistaken for GPA.22Nocardia may disseminate to the central nervous system (CNS), skin, joints, and spine, usually causing suppurative infection at these sites. Nocardia has a very strong tropism for neural tissue. In the CNS, Nocardia can cause single or multiple brain abscesses that may be asymptomatic; patients with pulmonary nocardiosis require imaging to rule out occult CNS involvement.
Nocardia species are resistant to several antibiotics. The treatment of choice for Nocardia species is TMPSMX, but imipenem, amikacin, third-generation cephalosporins, and other options such as minocycline and linezolid may be considered depending on the species and the antimicrobial susceptibility pattern.
Histoplasma capsulatum
Histoplasma capsulatum is a dimorphic fungus that causes disease in both healthy and immunocompromised hosts. The organism differs from other pathogenic fungi in that it is an intracellular organism, mainly involving the reticuloendothelial system, and is rarely in the extracellular space. In the United States, infections are clustered endemically in areas such as the Mississippi and Ohio River Valleys, but infections are common worldwide. The fungus is found in soil, mulch, bird excrement, and bat guano. Asymptomatic or mild infections are common in healthy persons residing in endemic areas and occur on a sporadic basis. Epidemics can occur when contaminated material is aerosolized. Histoplasmosis is also an opportunistic infection in patients with impaired T-cell immunity such as persons with AIDS, organ transplant recipients, hematologic malignancies, and corticosteroid use. Clinically significant cases of histoplasmosis have been described in patients with RA while receiving methotrexate alone, corticosteroids alone, and combinations of disease-modifying agents.23 Histoplasmosis was recently identified in 240 patients in association with TNF inhibitors, translating to 17 per 100,000 patients treated with infliximab.21,24
Pathogenesis. Infection initially occurs through inhalation of contaminated material from the environment, primarily causing pulmonary infection. The organism converts from a mold form in the environment to a pathogenic yeast form in the host. Once inhaled, the mediastinal lymph nodes provide the first line of defense. Following draining of the lymph nodes, the organism enters the bloodstream in both immunocompetent and immunosuppressed patients. It is spread hematogenously into the spleen, liver, and reticulo-endothelial system, where it is eventually cleared. In immunocompetent patients, cellular immunity limits infection within 7 to 14 days and humoral immunity is not protective.25 Granuloma formation is the hallmark of host defense.
Spectrum of illness. Histoplasmosis is associated with a wide spectrum of illness, with presentation ranging from asymptomatic to mild pulmonary illness to overwhelming pneumonia. Symptomatic pulmonary histoplasmosis typically presents with fever, flulike symptoms, and cough, often with retrosternal chest pain. X-rays show patchy or nodular infiltrates, with hilar or mediastinal lymphadenopathy. In some cases the lung parenchyma is clear and the main feature is fever and bilateral hilar adenopathy. Pulmonary histoplasmosis may be difficult to distinguish from sarcoidosis and tuberculosis. Extrapulmonary disease can present as hepatitis, infective endocarditis, and chronic meningitis. In immunocompromised patients, histoplasmosis can present as a progressive disseminated disease which can be acute, subacute, or chronic. Chronic disseminated histoplasmosis is characterized by cough, persistent fever, wasting, hepatosplenomegaly, oral ulcerations, and progressive cytopenias. Acute disseminated histoplasmosis has a much more fulminant course characterized by respiratory insufficiency, hypotension, multisystem organ failure, coagulopathies, and encephalopathy. Histoplasmosis is primarily a pulmonary disease, but in disseminated disease more than 50% of patients have no pulmonary symptoms and 30% may have normal chest x-rays.26 In one series of infliximab-related cases (n = 10), all came from an endemic area 1 week to 6 months after the first dose of infliximab. Patients presented with cough, fever, and shortness of breath.27 The pathogenesis of histoplasmosis in patients receiving TNF inhibitors is not entirely clear; such patients may be suffering a new primary infection, a reinfection, or, least likely, reactivation of latent infection.
Definitive diagnosis requires culture confirmation from appropriate body fluids or identification of characteristic yeast forms from histopathologic sections of tissue biopsies. Serologic tests may also be used to confirm the diagnosis. Detection of H and M precipitins or bands by immunodiffusion is a routine test in many laboratories. M bands are present in 50% of acute cases but their presence does not distinguish acute from remote infection. H bands are present in only 10% of all acute cases, but their presence is very specific for acute histoplasmosis.28
When looking at complement fixation antibodies to yeast (HY) and mycelial (HMy) forms in pulmonary histoplasmosis, a fourfold rise in titer establishes the diagnosis retrospectively, and a single titer greater than 1:32 is strongly suggestive of active infection. However, in progressive disseminated histoplasmosis, the complement fixation antibodies are frequently negative.29 Detection of antigen in urine and serum by enzyme immunoassay has become a mainstay of diagnosis, with a sensitivity of approximately 90% in progressive disseminated disease.30 Of note, most cases of histoplasmosis associated with biologic agents have detectable urinary antigen tests.
Treatment. Acute pulmonary histoplasmosis is usually self-limited, requiring no treatment. The 2007 Infective Diseases Society of America (IDSA) guidelines recommend observation alone in most cases of mild to moderate pulmonary histoplasmosis unless symptoms persist longer than 1 month. For moderately severe or severe acute pulmonary histoplasmosis, the IDSA recommends lipid formulations of amphotericin B (3.0 to 5.0 mg/kg/day) or deosycholate amphotericin B (0.7 to 1.0 mg/kg/day) for 1 to 2 weeks followed by itraconazole 200 mg twice daily for a total of 12 weeks. Methylprednisolone at a dose of 0.5 to 1.0 mg/kg/day intravenously for 1 to 2 weeks is also recommended. For moderately severe to severe disseminated histoplasmosis, the IDSA recommends lipid formulations of amphotericin B (3.0 mg/kg/day) for 1 to 2 weeks followed by oral itraconazole 200 mg three times daily for 3 days and then 200 mg twice daily for a total of at least 12 months.31 Commonly, the immunosuppressive agent is held during treatment.
Aspergillus species
Another emerging pathogen is Aspergillus species—a ubiquitous mold spread by aerosols of spores. There are many different species of Aspergillus, but the most common human pathogens include A fumigates, A niger, and A flavus. To date, 39 cases of Aspergillus infection associated with infliximab and etanercept have been reported in the Adverse Event Reporting System, translating to 9 to 12 cases per 100,000 patients.21
Varicella zoster
JC virus
More than 80% of adults are seropositive for JCV, a DNA virus of the genus Polyomavirus that causes lytic infection of oligodendrocytes.34 In immunocompromised hosts, JCV causes progressive multifocal leukoencephalopathy (PML), a rare but devastating demyelinating disease. PML was first described in malignancy, leukemia, and various other immunocompromised states, prior to its strong association with AIDS in the 1980s. More recently, JCV has been associated with natalizumab for multiple sclerosis and Crohn disease, rituximab for oncology patients, efalizumab for psoriasis,35 and mycophenolate mofetil for transplant recipients.36
In 2006 the US Food and Drug Administration issued a safety alert regarding PML in two patients with SLE treated with rituximab and other immunosuppressives.37 In a review of PML in rheumatic disease, 36 cases were identified in patients who had not previously received a biologic agent. Most of these patients (60%) had SLE.38 Of these, many had little or no immunosuppression over the 6 months prior to the diagnosis of PML, suggesting that SLE itself may predispose to PML. Interestingly, PML is rarely associated with TNF inhibitors.
Classic presentation of PML includes motor weakness, aphasia, dysarthria, vision loss, and cognitive loss. Atypical presentation includes seizures, headaches, and brainstem involvement. PML usually spares the optic nerves, spinal cord, peripheral nerves, and muscles. In persons with underlying rheumatic diseases, PML can be difficult to distinguish from neuropsychiatric SLE or CNS vasculitis.
Treatment. In clinical trials no antiviral agent has been effective in the treatment of PML. In HIV patients who develop PML, highly active antiretroviral therapy should be initiated (if antiretroviral-naïve) or existing antiviral regimens optimized. Antiretroviral therapy in this situation may stabilize disease and possibly increase survival.42 For HIV-negative patients who develop PML, the cornerstone of management is immediate decrease or discontinuation of immunosuppression.43 Several adjunctive measures have been reported mainly in natalizumab-associated PML, including corticosteroids, mirtazapine, plasma exchange, and others.
VACCINES
Vaccination is important in the prevention of infectious disease in immunocompromised patients with connective tissue diseases. Because live vaccines are contraindicated in immunocompromised patients, inactivated or component vaccines should be used. It is recommended that patients who will start immunosuppressive therapy be vaccinated 2 to 4 weeks before beginning therapy. If this is not possible, vaccination should be administered during disease remission, 3 months after immunosuppression and 1 to 3 months after administration of high-dose corticosteroids.
- Short-term (less than 14 days)
- At a dose of less than 20 mg/day of prednisone or equivalent
- Long-term on alternate days with short-acting preparations
- At a physiologic dose of prednisone
- Topical, inhaled, intra-articular, bursal, or via tendon.44
Until definitive guidelines are developed, practitioners must evaluate and treat each patient individually to maximize the efficacy of disease treatments while preventing infection morbidity and mortality in their patients with connective tissue diseases.
- Falagas ME, Manta KG, Betsi GI, Pappas G. Infection-related morbidity and mortality in patients with connective tissue diseases: a systematic review. Clin Rheumatol 2007; 26:663–670.
- Alarcón GS. Infections in systemic connective tissue diseases: systemic lupus erythematosus, scleroderma, and polymyositis/dermatomyositis. Infect Dis Clin North Am 2006; 20:849–875.
- Crum NF, Lederman ER, Wallace MR. Infections associated with tumor necrosis factor-alpha antagonists. Medicine (Baltimore) 2005; 84:291–302.
- Rychly DJ, DiPiro JT. Infections associated with tumor necrosis factor-alpha antagonists. Pharmacotherapy 2005; 25:1181–1192.
- Wakefield AE, Lindley AR, Ambrose HE, Denis CM, Miller RF. Limited asymptomatic carriage of Pneumocystis jiroveci in human immunodeficiency virus-infected patients [published online ahead of print March 6, 2003]. J Infect Dis 2003; 187:901–908. doi: 10.1086/368165
- Beard CB, Carter JL, Keely SP, et al. Genetic variation in Pneumocystis carinii isolates from different geographic regions: implications for transmission. Emerg Infect Dis 2000; 6:265–272.
- Walzer PD, Smulian AG. Pneumocystis species. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, Pa: Elsevier Churchill Livingstone; 2009.
- Hartman TE, Primack SL, Müller NL, Staples CA. Diagnosis of thoracic complications in AIDS: accuracy of CT. Am J Roentgenol 1994; 162:547–553.
- Shelhamer JH, Gill VJ, Quinn TC, et al. The laboratory evaluation of opportunistic pulmonary infections. Ann Intern Med 1996; 124:585–599.
- Wharton JM, Coleman DL, Wofsy CB, et al. Trimethoprim-sulfamethoxazole or pentamidine for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. Ann Intern Med 1986; 105:37–44.
- Stein DS, Stevens RC. Treatment-associated toxicities: incidence and mechanisms. In:Sattler FR, Walzer PD, eds. Pneumocystis carinii. London: Bailliere Tindall; 1995:505–530.
- Consensus statement on the use of corticosteroids as adjunctive therapy for Pneumocystis pneumonia in the acquired immunodeficiency syndrome. The National Institutes of Health-University of California Expert Panel for Corticosteroids as Adjunctive Therapy for Pneumocystis Pneumonia. N Engl J Med 1990; 323:1500–1504.
- Stamp LK, Hurst M. Is there a role for consensus guidelines for P. jiroveci pneumonia prophylaxis in immunosuppressed patients with rheumatic diseases? J Rheumatol 2010; 37:686–688.
- Yale S, Limper A. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illnesses and prior corticosteroid therapy. Mayo Clin Proc 1996; 71:5–13.
- Sowden E, Carmichael A. Autoimmune inflammatory disorders, systemic corticosteroids and Pneumocystis pneumonia: a strategy for prevention [published online October 16, 2004]. BMC Infect Dis 2004; 4:42. doi: 10.1186/1471-2334-4-42
- Cettomai D, Gelber AC, Christopher-Stine L. A survey of rheumatologists’ practice for prescribing Pneumocystis prophylaxis. J Rheumatol 2010; 37:792–799.
- Keegan JM, Byrd JW. Nocardiosis associated with low dose methotrexate for rheumatoid arthritis. J Rheumatol 1988; 15:1585–1586.
- Gruberg L, Thaler M, Rozenman J, et al. Nocardia asteroides infection complicating rheumatoid arthritis. J Rheumatol 1991; 18:459–461.
- Corneliessen JJ, Bakker LJ, van der Veen MJ, et al. Nocardia asteroides pneumonia complicating low dose methotrexate treatment of refractory rheumatoid arthritis. Ann Rheum Dis 1991; 50;642–644.
- Silva C, Faccioli LH. Tumor necrosis factor and macrophage activation are important in clearance of Nocardia brasiliensis from the livers and spleens of mice. Infect Immun 1992; 60:3566–3570.
- Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis 2004; 38:1261–1265.
- Gibb W, Williams A. Nocardiosis mimicking Wegener’s granulomatosis. Scand J Infect Dis 1986; 18:583–585.
- Olson TC, Bongartz T, Crowson CS, Roberts GD, Orenstein R, Matteson EI. Histoplasmosis infection in patients with rheumatoid arthritis, 1998–2009 [published online May 23, 2011]. BMC Infectious Diseases 2011; 11:145. doi: 10.1186/1471-2334-11-145
- Information for Healthcare Professionals: Cimzia (certolizumab pegol), Enbrel etanercept), Humira (adalimumab), and Remicade (infliximab). U.S. Food and Drug Administration Web site. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124185.htm. Updated January 25, 2010. Accessed September 27, 2012.
- Paya CV, Roberts GD, Cockerill FR. Transient fungemia in acute pulmonary histoplasmosis: detection by new blood-culturing techniques. J Infect Dis 1987; 156:313–315.
- Goodwin RA, Shapiro JL, Thurman GH, Thurman SS, Des Prez RM. Disseminated histoplasmosis: clinical and pathologic correlations. Medicine (Baltimore) 1980; 59:1–33.
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum 2002; 46:2565–2570.
- Picardi JL, Kauffman CA, Schwarz J, Phair JP. Detection of precipitating antibodies to Histoplasma capsulatum by counterimmunoelectrophoresis. Am Rev Respir Dis 1976; 114:171–176.
- Deepe GS. Histoplasma capsulatum. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, Pa: Elsevier Churchill Livingstone; 2009.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America [published online ahead of print August 27, 2007]. Clin Infect Dis 2007; 45:807–825. doi: 10.1086/521259
- Johnson RW. Herpes zoster and postherpetic neuralgia. Expert Rev Vaccines 2010; 9( 3 suppl):21–26.
- Wolfe F, Michaud K, Chakravarty EF. Rates and predictors of herpes zoster in patients with rheumatoid arthritis and non-inflammatory musculoskeletal disorders. Rheumatology 2006; 45:1370–1375.
- Weber T, Trebst C, Frye S, et al. Analysis of the systemic and intrathecal humoral immune response in progressive multifocal leukoencephalopathy. J Infect Dis 1997; 176:250–254.
- Kothary N, Diak IL, Brinker A, Bezabeh S, Avigan M, Dal Pan G. Progressive multifocal leukoencephalopathy associated with efalizumab use in psoriasis patients. J Am Acad Dermatol 2011; 65:546–551.
- Neff RT, Hurst FP, Falta EM, et al. Progressive multifocal leukoencephalopathy and use of mycophenolate mofetil after kidney transplantation. Transplantation 2008; 86:1474–1478.
- Rituxan warning. FDA Consum 2007; 41:3.
- Calabrese LH, Molloy ES, Huang D, Ransohoff RM. Progressive multifocal leukoencephalopathy in rheumatic diseases. Arthritis Rheum 2007; 56:2116–2128.
- Cinque P, Scarpellini P, Vago L, Linde A, Lazzarin A. Diagnosis of central nervous system complications in HIV-infected patients: cerebrospinal fluid analysis by the polymerase chain reaction. AIDS 1997; 11:1–17.
- Ryschkewitsch C, Jensen P, Hou J, Fahle G, Fischer S, Major EO. Comparison of PCR-southern hybridization and quantitative real-time PCR for the detection of JC and BK viral nucleotide sequences in urine and cerebrospinal fluid. J Virol Methods 2004; 121:217–221.
- Major EO. History and current concepts in the pathogenesis of PML. Cleve Clin J Med 2011; 78( suppl 2):S3–S7.
- Antinori A, Ammassari A, Giancola ML, et al. Epidemiology and prognosis of AIDS-associated progressive multifocal leukoencephalopathy in the HAART era. J Neurovirol 2001; 7:323–328.
- Calabrese L. A rational approach to PML for the clinician. Cleve Clin J Med 2011; 78 (suppl 2):S38–S41.
- Kroger AT, Sumaya CV, Pickering LK, Atkinson WL. General recommendations on immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2011; 60:1–60.
- Falagas ME, Manta KG, Betsi GI, Pappas G. Infection-related morbidity and mortality in patients with connective tissue diseases: a systematic review. Clin Rheumatol 2007; 26:663–670.
- Alarcón GS. Infections in systemic connective tissue diseases: systemic lupus erythematosus, scleroderma, and polymyositis/dermatomyositis. Infect Dis Clin North Am 2006; 20:849–875.
- Crum NF, Lederman ER, Wallace MR. Infections associated with tumor necrosis factor-alpha antagonists. Medicine (Baltimore) 2005; 84:291–302.
- Rychly DJ, DiPiro JT. Infections associated with tumor necrosis factor-alpha antagonists. Pharmacotherapy 2005; 25:1181–1192.
- Wakefield AE, Lindley AR, Ambrose HE, Denis CM, Miller RF. Limited asymptomatic carriage of Pneumocystis jiroveci in human immunodeficiency virus-infected patients [published online ahead of print March 6, 2003]. J Infect Dis 2003; 187:901–908. doi: 10.1086/368165
- Beard CB, Carter JL, Keely SP, et al. Genetic variation in Pneumocystis carinii isolates from different geographic regions: implications for transmission. Emerg Infect Dis 2000; 6:265–272.
- Walzer PD, Smulian AG. Pneumocystis species. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, Pa: Elsevier Churchill Livingstone; 2009.
- Hartman TE, Primack SL, Müller NL, Staples CA. Diagnosis of thoracic complications in AIDS: accuracy of CT. Am J Roentgenol 1994; 162:547–553.
- Shelhamer JH, Gill VJ, Quinn TC, et al. The laboratory evaluation of opportunistic pulmonary infections. Ann Intern Med 1996; 124:585–599.
- Wharton JM, Coleman DL, Wofsy CB, et al. Trimethoprim-sulfamethoxazole or pentamidine for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome. Ann Intern Med 1986; 105:37–44.
- Stein DS, Stevens RC. Treatment-associated toxicities: incidence and mechanisms. In:Sattler FR, Walzer PD, eds. Pneumocystis carinii. London: Bailliere Tindall; 1995:505–530.
- Consensus statement on the use of corticosteroids as adjunctive therapy for Pneumocystis pneumonia in the acquired immunodeficiency syndrome. The National Institutes of Health-University of California Expert Panel for Corticosteroids as Adjunctive Therapy for Pneumocystis Pneumonia. N Engl J Med 1990; 323:1500–1504.
- Stamp LK, Hurst M. Is there a role for consensus guidelines for P. jiroveci pneumonia prophylaxis in immunosuppressed patients with rheumatic diseases? J Rheumatol 2010; 37:686–688.
- Yale S, Limper A. Pneumocystis carinii pneumonia in patients without acquired immunodeficiency syndrome: associated illnesses and prior corticosteroid therapy. Mayo Clin Proc 1996; 71:5–13.
- Sowden E, Carmichael A. Autoimmune inflammatory disorders, systemic corticosteroids and Pneumocystis pneumonia: a strategy for prevention [published online October 16, 2004]. BMC Infect Dis 2004; 4:42. doi: 10.1186/1471-2334-4-42
- Cettomai D, Gelber AC, Christopher-Stine L. A survey of rheumatologists’ practice for prescribing Pneumocystis prophylaxis. J Rheumatol 2010; 37:792–799.
- Keegan JM, Byrd JW. Nocardiosis associated with low dose methotrexate for rheumatoid arthritis. J Rheumatol 1988; 15:1585–1586.
- Gruberg L, Thaler M, Rozenman J, et al. Nocardia asteroides infection complicating rheumatoid arthritis. J Rheumatol 1991; 18:459–461.
- Corneliessen JJ, Bakker LJ, van der Veen MJ, et al. Nocardia asteroides pneumonia complicating low dose methotrexate treatment of refractory rheumatoid arthritis. Ann Rheum Dis 1991; 50;642–644.
- Silva C, Faccioli LH. Tumor necrosis factor and macrophage activation are important in clearance of Nocardia brasiliensis from the livers and spleens of mice. Infect Immun 1992; 60:3566–3570.
- Wallis RS, Broder MS, Wong JY, Hanson ME, Beenhouwer DO. Granulomatous infectious diseases associated with tumor necrosis factor antagonists. Clin Infect Dis 2004; 38:1261–1265.
- Gibb W, Williams A. Nocardiosis mimicking Wegener’s granulomatosis. Scand J Infect Dis 1986; 18:583–585.
- Olson TC, Bongartz T, Crowson CS, Roberts GD, Orenstein R, Matteson EI. Histoplasmosis infection in patients with rheumatoid arthritis, 1998–2009 [published online May 23, 2011]. BMC Infectious Diseases 2011; 11:145. doi: 10.1186/1471-2334-11-145
- Information for Healthcare Professionals: Cimzia (certolizumab pegol), Enbrel etanercept), Humira (adalimumab), and Remicade (infliximab). U.S. Food and Drug Administration Web site. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm124185.htm. Updated January 25, 2010. Accessed September 27, 2012.
- Paya CV, Roberts GD, Cockerill FR. Transient fungemia in acute pulmonary histoplasmosis: detection by new blood-culturing techniques. J Infect Dis 1987; 156:313–315.
- Goodwin RA, Shapiro JL, Thurman GH, Thurman SS, Des Prez RM. Disseminated histoplasmosis: clinical and pathologic correlations. Medicine (Baltimore) 1980; 59:1–33.
- Lee JH, Slifman NR, Gershon SK, et al. Life-threatening histoplasmosis complicating immunotherapy with tumor necrosis factor alpha antagonists infliximab and etanercept. Arthritis Rheum 2002; 46:2565–2570.
- Picardi JL, Kauffman CA, Schwarz J, Phair JP. Detection of precipitating antibodies to Histoplasma capsulatum by counterimmunoelectrophoresis. Am Rev Respir Dis 1976; 114:171–176.
- Deepe GS. Histoplasma capsulatum. In: Mandell GL, Bennett JE, Dolin R, eds. Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, Pa: Elsevier Churchill Livingstone; 2009.
- Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev 2007; 20:115–132.
- Wheat LJ, Freifeld AG, Kleiman MB, et al; Infectious Diseases Society of America. Clinical practice guidelines for the management of patients with histoplasmosis: 2007 update by the Infectious Diseases Society of America [published online ahead of print August 27, 2007]. Clin Infect Dis 2007; 45:807–825. doi: 10.1086/521259
- Johnson RW. Herpes zoster and postherpetic neuralgia. Expert Rev Vaccines 2010; 9( 3 suppl):21–26.
- Wolfe F, Michaud K, Chakravarty EF. Rates and predictors of herpes zoster in patients with rheumatoid arthritis and non-inflammatory musculoskeletal disorders. Rheumatology 2006; 45:1370–1375.
- Weber T, Trebst C, Frye S, et al. Analysis of the systemic and intrathecal humoral immune response in progressive multifocal leukoencephalopathy. J Infect Dis 1997; 176:250–254.
- Kothary N, Diak IL, Brinker A, Bezabeh S, Avigan M, Dal Pan G. Progressive multifocal leukoencephalopathy associated with efalizumab use in psoriasis patients. J Am Acad Dermatol 2011; 65:546–551.
- Neff RT, Hurst FP, Falta EM, et al. Progressive multifocal leukoencephalopathy and use of mycophenolate mofetil after kidney transplantation. Transplantation 2008; 86:1474–1478.
- Rituxan warning. FDA Consum 2007; 41:3.
- Calabrese LH, Molloy ES, Huang D, Ransohoff RM. Progressive multifocal leukoencephalopathy in rheumatic diseases. Arthritis Rheum 2007; 56:2116–2128.
- Cinque P, Scarpellini P, Vago L, Linde A, Lazzarin A. Diagnosis of central nervous system complications in HIV-infected patients: cerebrospinal fluid analysis by the polymerase chain reaction. AIDS 1997; 11:1–17.
- Ryschkewitsch C, Jensen P, Hou J, Fahle G, Fischer S, Major EO. Comparison of PCR-southern hybridization and quantitative real-time PCR for the detection of JC and BK viral nucleotide sequences in urine and cerebrospinal fluid. J Virol Methods 2004; 121:217–221.
- Major EO. History and current concepts in the pathogenesis of PML. Cleve Clin J Med 2011; 78( suppl 2):S3–S7.
- Antinori A, Ammassari A, Giancola ML, et al. Epidemiology and prognosis of AIDS-associated progressive multifocal leukoencephalopathy in the HAART era. J Neurovirol 2001; 7:323–328.
- Calabrese L. A rational approach to PML for the clinician. Cleve Clin J Med 2011; 78 (suppl 2):S38–S41.
- Kroger AT, Sumaya CV, Pickering LK, Atkinson WL. General recommendations on immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Morb Mortal Wkly Rep 2011; 60:1–60.