User login
HN‐Associated Healthcare Burden
Hyponatremia is an electrolyte disorder most commonly defined as a serum sodium concentration <135 mEq/L.1 Its exact definition can vary across studies, but typically ranges between <130 and <138 mEq/L.2, 3 Signs and symptoms of hyponatremia can include malaise, headache, disorientation, confusion, muscle weakness, and cramps. If severe, seizures, respiratory arrest, brainstem herniation, coma, and death may result.
The incidence of hyponatremia in the general hospitalized population has been reported to range between 1% and 6% when defined as <130135 mEq/L,4, 5 and its occurrence increases with a more prolonged hospital stay to 13%.6 A recent study reported that when hyponatremia was defined with a less stringent threshold of <138 mEq/L, the incidence at admission rose to 38%.3 Hyponatremia is a comorbid condition of multiple diseases, occurring in approximately 20% of patients with heart failure,7, 8 and 40% to 57% of patients with advanced cirrhosis.9, 10 The syndrome of the inappropriate release of antidiuretic hormone (SIADH) is additionally a predominant cause of hyponatremia, with a prevalence reported as high as 35% in hospitalized patients.11
Hyponatremia is not only widespread, but also an independent predictor of mortality. In a retrospective cohort analysis, Waikar et al reported that in comparison to patients who were normonatremic, patients with serum sodium concentrations <135 mEq/L had a risk of in‐hospital mortality as high as 47%, and that this risk doubled for patients with serum sodium concentrations between 125 and 129 mEq/L.6 In the study by Wald et al, which defined hyponatremia as <138 mEq/L, the risk of in‐hospital mortality was similar.3 In both of these studies, even mild hyponatremia (130137 mEq/L) was associated with increased risk of in‐hospital mortality.3, 6
The overall cost of hyponatremia is estimated to range between $1.6 and $3.6 billion for 2011.12, 13 Hospital readmissions are a significant contributor to total healthcare costs, with some being entirely avoidable with increased standards of care. The Centers for Medicare and Medicaid Services has begun to not only publicly report hospital readmission rates, but also penalize hospitals for early readmissions.14 Strategies to reduce hospital readmissions are currently being integrated into healthcare reform policy.14 In the present study, the incremental burden of hospitalized hyponatremic (HN) versus non‐HN patients in terms of hospital resource utilization, costs, and early hospital readmission in the real‐world was evaluated.
METHODS
Study Design
This study was a retrospective analysis that examined healthcare utilization and costs among HN patients using the Premier Hospital Database. The database contains over 310 million hospital encounters from more than 700 US hospitals, or 1 out of every 4 discharges in the US. The administrative data available included patient and provider demographics, diagnoses and procedures, as well as date‐stamped billing records for all pharmacy, laboratory, imaging, procedures, and supplies.
Patient Selection and Matching
HN patients were eligible for study inclusion if they were a hospital inpatient discharged between January 1, 2007 and March 31, 2010, were >18 years of age at admission, and had either a primary or secondary diagnosis of hyponatremia or hyposmolality (defined as International Classification of Diseases, Ninth Revision (ICD‐9‐CM) code: 276.1x). Patients were excluded if they had been transferred from another acute care facility, transferred to another acute care or critical access facility, or left against medical advice. Labor and delivery patients (ICD‐9‐CM codes: 72.xx‐74.xx, V22.x, V23.x, V27.x, and V28.x), and patients classified as observational were also excluded. A second cohort of non‐hyponatremic patients was created using the same inclusion and exclusion criteria, with the exception that patients not have a primary or secondary diagnosis of hyponatremia or hyposmolality (defined as ICD‐9‐CM code: 276.1x).
The matching of hyponatremia (HN) and control (non‐HN) cohorts was accomplished using a combination of exact and propensity score matching techniques. Patients were first exact matched on age, gender, Medicare Severity‐Diagnosis Related Group (MS‐DRG) assignment, and hospital geographic region. Propensity score matching was further utilized to create the final study cohorts for outcomes comparisons. Propensity score matching is commonly used in retrospective cohort studies to correct for sample selection bias due to observable differences between groups.15, 16 The propensity score was generated using logistic regression with the dependent variable as hyponatremia (yes vs no) and the following covariates: age, race, admission source, attending physician specialty, 3M All Patient Refined‐Diagnosis Related Group (APR‐DRG) Severity of Illness and Risk of Mortality index scores, Deyo‐Charlson Comorbidity Index score, selected hyponatremia‐related comorbidity conditions, and hospital size, region, and urban/rural designation. These covariates were initially selected by an expert panel of physicians, and backward selection was utilized in the logistic regression using the most parsimonious model.17
Following generation of the propensity scores, HN patients were matched to non‐HN patients 1:1 using a nearest neighbor matching algorithm, including hospital identification and propensity score.18 Inclusion of hospital identification in the matching sequence, as well as provider characteristics, especially hospital size, attending physician specialty, and geographic region in the propensity score, was used to control for potential clustering effects at the physician and hospital level.19 During the propensity score matching process, likelihood‐ratio test, Hosmer‐Lemshow goodness of fit, and concordance c statistics were utilized to assess the fitness of the models.20 The final propensity score model produced a concordance c statistic of 0.8.
Outcome Measures and Statistical Analyses
The following outcome measures were compared between the matched HN and non‐HN patient cohorts: total and intensive care unit (ICU) hospitalization costs, total and ICU length of stay (LOS), ICU admission, and 30‐day hospital readmission. Bivariate descriptive statistics were employed to test for significant differences in demographics, patient clinical characteristics, and unadjusted costs and healthcare resource utilization and readmission rates between patient cohorts. To detect statistically significant differences in continuous and categorical variables, respectively, t tests and chi‐square tests were performed.
Multivariate analysis of outcome measures utilized generalized linear models. Due to the skewed nature of LOS and cost data, LOS was analyzed using multivariate negative binomial regression and cost was analyzed using multivariate gamma regression.21 Binary outcomes (ICU admission and 30‐day readmission) were analyzed using multivariate logistic regression. The analysis accounted for potential confounding factors by inclusion of the following covariates: age group, gender, race, admission source, and Deyo‐Charlson Comorbidity Index score. These covariates were previously identified in the Wald et al hyponatremia study,3 and were verified using likelihood‐ratio, Hosmer‐Lemshow goodness of fit, and concordance c statistics.
Subgroup Sensitivity Analysis
For the subgroup sensitivity analyses, patients were identified as having community‐acquired pneumonia (CAP), congestive heart failure (CHF), urinary tract infection (UTI), or chronic obstructive pulmonary disease (COPD) based upon principal diagnosis codes. Patients were categorized according to these subgroup definitions, and then previously matched patients with the same subgroup classification constituted the final analysis set for each subgroup. The methods that were used for the overall matched analysis were then applied to each subgroup to evaluate the incremental burden of overall cost and LOS associated with hyponatremia.
RESULTS
Patient Population
Of the 606,057 HN patients eligible for matching, a total of 558,815 HN patients were matched to 558,815 non‐HN patients, a 92% match ratio. Table 1 describes the overall characteristics of the patient populations. For both cohorts, median age was 70 years, 57% of patients were female, and approximately 67% were white. The majority of patients in either cohort had Medicare coverage (55%), and approximately 75% of patients entered the hospital via the emergency room with nearly 70% having a 3M APR‐DRG disease severity level of major or extreme. Patients of both cohorts were most often attended by an internist or a hospitalist, with a combined percentage of approximately 60%. A small, but greater proportion of HN patients had comorbidities of cancer, pulmonary disease, and SIADH. Comorbid conditions of liver cirrhosis/hepatic disease and human immunodeficiency virus (HIV) were similarly distributed among both patient cohorts.
| Hyponatremic | Non‐Hyponatremic | |
|---|---|---|
| Discharges N (%) | Discharges N (%) | |
| ||
| Sample Discharges | 558,815 (100.0%) | 558,815 (100.0%) |
| Age median (IQR) | 70.00 (57.081.0) | 70.00 (57.081.0) |
| Gender | ||
| Female | 319,069 (57.1%) | 319,069 (57.1%) |
| Male | 239,746 (42.9%) | 239,746 (42.9%) |
| Race | ||
| American Indian | 3,465 (0.6%) | 3,448 (0.6%) |
| Asian/Pacific | 10,065 (1.8%) | 9,690 (1.7%) |
| Black | 63,776 (11.4%) | 66,233 (11.9%) |
| Hispanic | 24,341 (4.4%) | 24,426 (4.4%) |
| White | 377,434 (67.5%) | 376,639 (67.4%) |
| Other/unknown | 79,734 (14.3%) | 78,379 (14.0%) |
| Primary payer | ||
| Medicaretraditional | 310,312 (55.5%) | 310,643 (55.6%) |
| Managed care | 75,476 (13.5%) | 78,184 (14.0%) |
| Medicaremanaged care | 45,439 (8.1%) | 47,947 (8.6%) |
| Non‐cap | ||
| Medicaid | 44,690 (8.0%) | 43,767 (7.8%) |
| Other | 82,898 (14.8%) | 78,274 (14.0%) |
| Admission source | ||
| Physician referral | 5,022 (0.9%) | 4,636 (0.8%) |
| Transfer from another nonacute health facility | 18,031 (3.2%) | 18,163 (3.3%) |
| Emergency room | 417,556 (74.7%) | 420,401 (75.2%) |
| Other/unknown | 118,206 (21.2%) | 115,615 (20.7%) |
| APR‐DRG severity of illness | ||
| 1Minor | 14,257 (2.6%) | 12,993 (2.3%) |
| 2Moderate | 174,859 (31.3%) | 179,356 (32.1%) |
| 3Major | 263,814 (47.2%) | 265,422 (47.5%) |
| 4Extreme | 105,885 (19.0%) | 101,044 (18.1%) |
| Attending physician specialty | ||
| Internal Medicine | 235,628 (42.1%) | 240,875 (43.1%) |
| Hospitalist | 88,250 (15.8%) | 87,720 (15.7%) |
| Family Practice (FP) | 73,346 (13.1%) | 72,828 (13.0%) |
| Orthopedic Surgery (ORS) | 23,595 (4.2%) | 22,949 (4.1%) |
| Cardiovascular Diseases (CD) | 19,521 (3.5%) | 18,057 (3.2%) |
| Comorbidities | ||
| Human immunodeficiency virus | 3,971 (0.7%) | 4,018 (0.7%) |
| Cancer/neoplasm/malignancy | 107,851 (19.3%) | 105,199 (18.8%) |
| Pulmonary disease | 77,849 (13.9%) | 77,184 (13.8%) |
| Cirrhosis/hepatic disease | 23,038 (4.1%) | 23,418 (4.2%) |
| SIADH | 1,972 (0.4%) | 1,278 (0.2%) |
| Subgroup populations | ||
| Community‐acquired pneumonia | 26,291 (4.7%) | 26,291 (4.7%) |
| Congestive heart failure | 23,020 (4.1%) | 23,020 (4.1%) |
| Urinary tract infection | 14,238 (2.6%) | 14,238 (2.6%) |
| COPD | 8,696 (1.6%) | 8,696 (1.6%) |
| CCI median (IQR) | 3.0 (1.05.0) | 3.0 (1.05.0) |
| No. of premier hospitals | 459 | |
| Provider region | ||
| East North Central | 78,332 (14.0%) | 78,332 (14.0%) |
| East South Central | 32,122 (5.8%) | 32,122 (5.8%) |
| Middle Atlantic | 73,846 (13.2%) | 73,846 (13.2%) |
| Mountain | 23,761 (4.3%) | 23,761 (4.3%) |
| New England | 9,493 (1.7%) | 9,493 (1.7%) |
| Pacific | 73,059 (13.1%) | 73,059 (13.1%) |
| South Atlantic | 175,194 (31.4%) | 175,194 (31.4%) |
| West North Central | 37,913 (6.8%) | 37,913 (6.8%) |
| West South Central | 55,095 (9.9%) | 55,095 (9.9%) |
| Population served | ||
| Rural | 69,749 (12.5%) | 68,414 (12.2%) |
| Urban | 489,066 (87.5%) | 490,401 (87.8%) |
| Teaching status | ||
| Non‐teaching | 337,620 (60.4%) | 337,513 (60.4%) |
| Teaching | 221,195 (39.6%) | 221,302 (39.6%) |
| No. of hospital beds | ||
| 699 | 22,067 (4.0%) | 21,777 (3.9%) |
| 100199 | 57,367 (10.3%) | 56,097 (10.0%) |
| 200299 | 87,563 (15.7%) | 86,639 (15.5%) |
| 300499 | 218,834 (39.2%) | 220,248 (39.4%) |
| 500+ | 172,984 (31.0%) | 174,054 (31.2%) |
Hospital Characteristics
Patient cohorts had similar distributions with respect to hospital characteristics (Table 1). Approximately 30% of patients were provided care from hospitals located in the South Atlantic region, and between 10% and 15% were serviced from hospitals in the East North Central, Middle Atlantic, Pacific, and West South Central regions. Most hospitals providing care for patient cohorts served urban populations (88%) and were large, with hospital bed numbers 300. Approximately 60% of hospitals were non‐teaching hospitals.
Healthcare Utilization, Readmission, and Cost Differences Among Patient Cohorts
The mean LOS (8.8 10.3 vs 7.7 8.5, P < 0.001), a difference of 1.1 days and mean ICU LOS (5.5 7.9 vs 4.9 7.1 days, P < 0.001), a difference of 0.6 days were significantly greater for the HN cohort in comparison to the non‐HN cohort (Table 2). The increase in healthcare resource utilization of patients with HN was reflected in their significantly higher mean total hospital costs per admission ($15,281 $24,054 vs $13,439 $22,198, P < 0.001), a difference of $1842; and mean costs incurred in the ICU ($8525 $13,342 vs $7597 $12,695, P < 0.001), a difference of $928 (Table 2). Furthermore, patients in the HN cohort were significantly more likely to be readmitted to the hospital for any cause (17.5% vs 16.4%, P < 0.001) (Table 2).
| Hyponatremic | Non‐Hyponatremic | P Value | |
|---|---|---|---|
| |||
| Total LOS (mean SD) | 8.8 10.3 | 7.7 8.5 | <0.001 |
| Total hospitalization cost (mean SD) | $15,281 $24,054 | $13,439 $22,198 | <0.001 |
| ICU admission (N, %) | 129,235 (23.1%) | 123,502 (22.1%) | <0.001 |
| ICU LOS (mean SD) | 5.5 7.9 | 4.9 7.1 | <0.001 |
| ICU cost (mean SD) | $8,525 $13,342 | $7,597 $12,695 | <0.001 |
| 30‐Day all cause readmission (N, %) | 96,063 (17.5%) | 87,058 (16.4%) | <0.001 |
Multivariate Analysis
Multivariate analysis demonstrated hyponatremia was associated with an increase in mean hospital LOS of 10.9%, [95% confidence interval: 10.4%11.5%], (P < 0.0001) and an increase in mean total hospital costs of 8.2%, [7.4%9.0%], (P < 0.0001) (Table 3). Additionally, hyponatremia was associated with an increase in ICU LOS of 10.2%, [8.7%11.8%], (P < 0.0001), and a higher ICU cost of 8.9%, [7.2%10.7%], (P < 0.0001) (Table 3). Hyponatremia was not associated with a greater likelihood of ICU admission (odds ratio = 1.0; [1.01.0], P =.5760). However, the condition was associated with a significantly greater chance of hospital readmission (odds ratio = 1.2, [1.11.2], P < 0.0001) within 30 days postdischarge (Table 3).
| Overall Cohort N = 1,117,630 | CAP N = 52,582 | CHF N = 46,040 | UTI N = 28,746 | COPD N = 17,392 | |
|---|---|---|---|---|---|
| |||||
| Total LOS difference | 10.9% [10.4%, 11.5%] P < 0.0001 | 5.4% [4.4%, 6.5%] P < 0.0001 | 20.6% [19.0%, 22.2%] P < 0.0001 | 2.7% [1.2%, 4.2%] P = 0.0003 | 6.7% [4.8%, 8.5%] P < 0.0001 |
| Total cost difference | 8.2% [7.4%, 9.0%] P < 0.0001 | 5.6% [4.0%, 7.0%] P < 0.0001 | 19.2% [17.1%, 21.4%] P < 0.0001 | 2.2% [1.4%, 4.4%] P = 0.0582 | 5.6% [3.0%, 8.2%] P < 0.0001 |
| ICU admission* odds ratio | 1.0 [1.0, 1.0] P = 0.5760 | 1.0 [1.0, 1.1] P = 0.8363 | 1.2 [1.1, 1.3] P < 0.0001 | 1.2 [1.1, 1.3] P = 0.0038 | 1.0 [0.9, 1.1] P = 0.5995 |
| ICU LOS difference | 10.2% [8.7%, 11.8%] P < 0.0001 | 8.4% [3.4%, 13.7%] P = 0.0008 | 24.8% [19.9%, 29.9%] P < 0.0001 | 4.9% [4.0%, 14.7%] P = 0.2893 | 10.1% [1.5%, 19.3%] P = 0.0204 |
| ICU cost difference | 8.9% [7.2%, 10.7%] P < 0.0001 | 8.2% [2.7%, 14.0%] P = 0.0029 | 24.1% [18.9%, 29.7%] P < 0.0001 | 2.8% [6.7%, 13.4%] P = 0.5739 | 8.4% [0.6%, 18.1%] P = 0.6680 |
| 30‐Day readmission odds ratio | 1.2 [1.1, 1.2] P < 0.0001 | 1.0 [0.9, 1.0] P = 0.5141 | 1.1 [1.1, 1.2] P < 0.0001 | 1.0 [1.0, 1.1] P = 0.5211 | 1.0 [1.0, 1.1] P = 0.6119 |
Subgroup Sensitivity Analyses
For patients with CAP (n = 52,582), CHF (n = 46,040), a UTI (n = 28,476), or COPD (n = 17,392), the percent increases in LOS and all cause hospitalization cost of the HN cohort in comparison to the non‐HN cohort were 5.4% (P < 0.0001) and 5.6% (P < 0.0001), 20.6% (P < 0.0001) and 19.2% (P < 0.0001), 2.7% (P = 0.0003) and 2.2% (P = 0.0582), and 6.7% (P < 0.0001) and 5.6% (P < 0.0001), respectively (Table 3).
DISCUSSION
This large‐scale, real‐world hospital database study provides healthcare utilization and cost data on the largest population of HN patients that has currently been studied. The results of this study are consistent with others in showing that HN patients use healthcare services more extensively, and represent a patient population which is more expensive to treat in the inpatient setting.5, 22 Additionally, this study yields new findings in that patients in the real‐world with hyponatremia resulting from various etiologies are more likely to be readmitted to the hospital than patients with similar demographics and characteristics who do not have hyponatremia. The results of the subgroup analysis were generally consistent with the results for the overall matched population, as the incremental burden estimates were directionally consistent. However, among the specific subgroups of patients, although it appears that hyponatremia is predictive of hospital readmission in patients with CHF, it did not necessarily correspond with hospital readmission rates among patients with CAP, UTI, or COPD. Therefore, hyponatremia, at least for patients with these latter conditions, may not be predictive of readmission, but remains associated with increased healthcare utilization during the initial hospitalization. Further evaluation of the incremental burden of hyponatremia among patients with specific disease conditions is needed to validate the findings.
The difference in hospital LOS at first admission between HN and non‐HN patients in this study was 1.1 days, and comparable to that reported by Shorr et al.23 In the study by Shorr et al, patients hospitalized for congestive heart failure (CHF), who were HN, had a 0.5 day increased LOS, and those who were severely HN had a 1.3 day increased LOS.23 Two other retrospective cohort analyses reported a 1.4 day5 and 2.0 day22 increased LOS for HN patients in comparison to non‐HN patients. Zilberberg et al and Callahan et al additionally reported that HN patients had a significantly greater need for ICU (4%10%).5, 22 In the present study, LOS in the ICU and associated costs were also compared among HN and non‐HN cohorts and, after adjustment for key patient characteristics, hyponatremia was associated with an incremental increase of 10.2% for ICU LOS and an 8.9% increase in ICU cost.
In this study, patients with hyponatremia of any severity were found to have a mean increase in hospital cost per admission of $1842, with multivariate analysis demonstrating an associated incremental increase of 8.2%. These results are also comparable to that reported in other retrospective cohort analyses. Shorr et al reported that in‐hospital costs attributed to hyponatremia were $509, and for severe hyponatremia were $1132.23 Callahan et al reported a $1200 increase in costs per admission for patients with mild‐to‐moderate hyponatremia, and a $3540 increase for patients with moderate‐to‐severe hyponatremia, in comparison to non‐HN patients.22
The Institute for Healthcare Improvement reports that hospitalizations account for nearly one‐third of the $882 billion spent on healthcare in the US in 2011, and that a substantial fraction of all hospitalizations are readmissions.13, 24 A recent meta‐analysis of 30‐day hospital readmission rates found 23.1% were classified as avoidable.25 Readmission rates of HN patients in comparison to non‐HN patients have been reported in 3 other studies, all of which were conducted on clinical trial patients with heart failure.7, 8, 26 Two of these studies, which evaluated only patients with acute class IV heart failure,8, 26 reported that hyponatremia was associated with a significant increase in readmission rates (up to 20% higher), and the other, which evaluated any patients hospitalized for heart failure, did not.7 The differences there may be partially attributed to the differences in heart failure severity among patient populations, as hyponatremia is an independent predictor of worsening heart failure. The only published study to date on the influence of hyponatremia on readmission rates in the real‐world was conducted by Scherz et al, who reported that the co‐occurrence of hyponatremia in patients with acute pulmonary embolism, discharged from 185 hospitals in Pennsylvania, was independently associated with an increased readmission rate of 19.3%.27 In the present study, hyponatremia was associated with an incremental increase ranging between 14% and 17% for hospital readmission for any cause. It was conducted on a patient population in which hyponatremia was resultant from many causes, and not all patients had a serious comorbid condition. Also, the HN and non‐HN cohorts were matched for comorbidity prevalence and disease severity, and in other studies they were not. Therefore, the results of this study importantly imply that hyponatremia, whether it is resultant from a serious disease or any other cause substantially increases the healthcare burden. The implementation of strategies to prevent hospital readmissions may play an important role in reducing the healthcare burden of hyponatremia, and future studies are warranted to evaluate this hypothesis alongside evaluation of the outpatient hyponatremia burden.
The limitations of this study include, firstly, that it is only representative of inpatient hospital costs, and excludes outpatient healthcare utilization and costs. Secondly, this study utilized the Premier Hospital Database for patient selection, and laboratory testing data for serum sodium level are not available in this database; therefore, the severity of hyponatremia could not be accurately established in the HN patient population. Thirdly, the occurrence of hyponatremia in patients with some diseases is a marker of disease severity, as is the case with congestive heart failure and cirrhosis.23, 28 Our study did not adjust for the specific disease (eg, CHF) severity, which may influence the results. Future research is needed to evaluate the impact of hyponatremia on underlying disease severity of other diseases, and how its co‐occurrence may influence healthcare resource utilization and cost in each case. Although the Premier Hospital Database contains information from a large number of hospitals across the US, it is possible that it may not be representative of the entire US population of HN patients. Additionally, billing and coding errors and missing data could potentially have occurred, although the large patient population size likely precludes a large impact on the results of this study. Finally, the frequency of use of fluid restriction in these hospital settings could not be chronicled, thus limiting the ability to assess therapies and treatment modalities in use.
Acknowledgements
The authors acknowledge Melissa Lingohr‐Smith from Novosys Health in the editorial support and review of this manuscript.
Disclosures: This research was supported by Otsuka America Pharmaceutical, Inc, Princeton, NJ, which manufactures tolvaptan for the treatment of hyponatremia. Drs Amin and Deitelzweig are consultants for, and have received honoraria from, Otsuka America Pharmaceutical in connection with conducting this study. Drs Christian and Friend are employees of Otsuka America Pharmaceutical. Dr Lin is an employee of Novosys Health, which has received research funds from Otsuka America Pharmaceutical in connection with conducting this study and development of this manuscript. D. Baumer and Dr. Lowe are employees of Premier Inc, which has received research funds from Otsuka America Pharmaceutical. K. Belk was previously employed with Premier Inc.
- ,,.Management of hyponatremia: providing treatment and avoiding harm.Cleve Clin J Med.2010;77(10):715–726.
- ,,.Causes and management of hyponatremia.Ann Pharmacother.2003;37(11):1694–1702.
- ,,,,.Impact of hospital‐associated hyponatremia on selected outcomes.Arch Intern Med.2010;170(3):294–302.
- ,,,.Hyponatremia: a prospective analysis of its epidemiology and the pathogenetic role of vasopressin.Ann Intern Med.1985;102(2):164–168.
- ,,, et al.Epidemiology, clinical and economic outcomes of admission hyponatremia among hospitalized patients.Curr Med Res Opin.2008;24(6):1601–1608.
- ,,.Mortality after hospitalization with mild, moderate, and severe hyponatremia.Am J Med.2009;122(9):857–865.
- ,,, et al.Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE‐HF registry.Eur Heart J.2007;28(8):980–988.
- ,,, et al.Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial.Arch Intern Med.2007;167(18):1998–2005.
- ,,,.Hyponatremia in cirrhosis: results of a patient population survey.Hepatology.2006;44(6):1535–1542.
- ,,, et al.Hyponatremia in cirrhosis: from pathogenesis to treatment.Hepatology.1998;28(3):851–864.
- ,,,,.The syndrome of inappropriate antidiuresis: pathophysiology, clinical management and new therapeutic options.Nephron Clin Pract.2011;119(1):c62–c73.
- ,,.Cost of illness of hyponatremia in the United States.Cost Eff Resour Alloc.2006;4:10.
- US Health Care Budget: US Budget Breakdown for FY12—Charts. Available at: http://www.usgovernmentspending.com/health_care_budget_2012_1.html. Accessed December 19, 2011.
- 111th Congress. Patient Protection and Affordable Care Act. Public Law 111–148.1–906.
- .An evaluation of statistical matching.JBES.1984;2(1):91–102.
- ,.Propensity score‐matching methods for nonexperimental causal studies.Rev Econ Stat.2002;84(1):151–161.
- . Applied Logistic Regression Analysis. Quantitative Applications in the Social Sciences, Vol106.2nd ed.Thousand Oaks, CA:Sage;2002.
- .Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. Paper presented at: Proceedings of the Twenty‐Sixth Annual SAS Users Group International Conference; April 22–25,2001; Long Beach, CA.
- ,,,,.The effect of clustering of outcomes on the association of procedure volume and surgical outcomes.Ann Intern Med.2003;139(8):658–665.
- .Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis.New York, NY:Springer‐Verlag,2001.
- ,,.Generalized Modeling Approaches to Risk Adjustment of Skewed Outcomes Data.Cambridge, MA:National Bureau of Economic Research, Inc;2003.
- ,,,.Economic impact of hyponatremia in hospitalized patients: a retrospective cohort study.Postgrad Med.2009;121(2):186–191.
- ,,, et al.Burden of sodium abnormalities in patients hospitalized for heart failure.Congest Heart Fail.2011;17(1):1–7.
- Institute for Healthcare Improvement. Reduce Avoidable Hospital Readmissions. Available at: http://www.ihi.org/explore/readmissions/Pages/default.aspx. Accessed December 18, 2011.
- ,,.A meta‐analysis of hospital 30‐day avoidable readmission rates.J Eval Clin Pract.2011 Nov 9. doi: 10.1111/j.1365–2753.2011.01773.x. Published online August 17, 2012.
- ,,, et al.Critical elements of clinical follow‐up after hospital discharge for heart failure: insights from the EVEREST trial.Eur J Heart Fail.2010;12(4):367–374.
- ,,, et al.Prognostic importance of hyponatremia in patients with acute pulmonary embolism.Am J Respir Crit Care Med.2010;182(9):1178–1183.
- ,,, et al.Serum sodium predicts prognosis in critically ill cirrhotic patients.J Clin Gastroenterol.2010;44(3):220–226.
Hyponatremia is an electrolyte disorder most commonly defined as a serum sodium concentration <135 mEq/L.1 Its exact definition can vary across studies, but typically ranges between <130 and <138 mEq/L.2, 3 Signs and symptoms of hyponatremia can include malaise, headache, disorientation, confusion, muscle weakness, and cramps. If severe, seizures, respiratory arrest, brainstem herniation, coma, and death may result.
The incidence of hyponatremia in the general hospitalized population has been reported to range between 1% and 6% when defined as <130135 mEq/L,4, 5 and its occurrence increases with a more prolonged hospital stay to 13%.6 A recent study reported that when hyponatremia was defined with a less stringent threshold of <138 mEq/L, the incidence at admission rose to 38%.3 Hyponatremia is a comorbid condition of multiple diseases, occurring in approximately 20% of patients with heart failure,7, 8 and 40% to 57% of patients with advanced cirrhosis.9, 10 The syndrome of the inappropriate release of antidiuretic hormone (SIADH) is additionally a predominant cause of hyponatremia, with a prevalence reported as high as 35% in hospitalized patients.11
Hyponatremia is not only widespread, but also an independent predictor of mortality. In a retrospective cohort analysis, Waikar et al reported that in comparison to patients who were normonatremic, patients with serum sodium concentrations <135 mEq/L had a risk of in‐hospital mortality as high as 47%, and that this risk doubled for patients with serum sodium concentrations between 125 and 129 mEq/L.6 In the study by Wald et al, which defined hyponatremia as <138 mEq/L, the risk of in‐hospital mortality was similar.3 In both of these studies, even mild hyponatremia (130137 mEq/L) was associated with increased risk of in‐hospital mortality.3, 6
The overall cost of hyponatremia is estimated to range between $1.6 and $3.6 billion for 2011.12, 13 Hospital readmissions are a significant contributor to total healthcare costs, with some being entirely avoidable with increased standards of care. The Centers for Medicare and Medicaid Services has begun to not only publicly report hospital readmission rates, but also penalize hospitals for early readmissions.14 Strategies to reduce hospital readmissions are currently being integrated into healthcare reform policy.14 In the present study, the incremental burden of hospitalized hyponatremic (HN) versus non‐HN patients in terms of hospital resource utilization, costs, and early hospital readmission in the real‐world was evaluated.
METHODS
Study Design
This study was a retrospective analysis that examined healthcare utilization and costs among HN patients using the Premier Hospital Database. The database contains over 310 million hospital encounters from more than 700 US hospitals, or 1 out of every 4 discharges in the US. The administrative data available included patient and provider demographics, diagnoses and procedures, as well as date‐stamped billing records for all pharmacy, laboratory, imaging, procedures, and supplies.
Patient Selection and Matching
HN patients were eligible for study inclusion if they were a hospital inpatient discharged between January 1, 2007 and March 31, 2010, were >18 years of age at admission, and had either a primary or secondary diagnosis of hyponatremia or hyposmolality (defined as International Classification of Diseases, Ninth Revision (ICD‐9‐CM) code: 276.1x). Patients were excluded if they had been transferred from another acute care facility, transferred to another acute care or critical access facility, or left against medical advice. Labor and delivery patients (ICD‐9‐CM codes: 72.xx‐74.xx, V22.x, V23.x, V27.x, and V28.x), and patients classified as observational were also excluded. A second cohort of non‐hyponatremic patients was created using the same inclusion and exclusion criteria, with the exception that patients not have a primary or secondary diagnosis of hyponatremia or hyposmolality (defined as ICD‐9‐CM code: 276.1x).
The matching of hyponatremia (HN) and control (non‐HN) cohorts was accomplished using a combination of exact and propensity score matching techniques. Patients were first exact matched on age, gender, Medicare Severity‐Diagnosis Related Group (MS‐DRG) assignment, and hospital geographic region. Propensity score matching was further utilized to create the final study cohorts for outcomes comparisons. Propensity score matching is commonly used in retrospective cohort studies to correct for sample selection bias due to observable differences between groups.15, 16 The propensity score was generated using logistic regression with the dependent variable as hyponatremia (yes vs no) and the following covariates: age, race, admission source, attending physician specialty, 3M All Patient Refined‐Diagnosis Related Group (APR‐DRG) Severity of Illness and Risk of Mortality index scores, Deyo‐Charlson Comorbidity Index score, selected hyponatremia‐related comorbidity conditions, and hospital size, region, and urban/rural designation. These covariates were initially selected by an expert panel of physicians, and backward selection was utilized in the logistic regression using the most parsimonious model.17
Following generation of the propensity scores, HN patients were matched to non‐HN patients 1:1 using a nearest neighbor matching algorithm, including hospital identification and propensity score.18 Inclusion of hospital identification in the matching sequence, as well as provider characteristics, especially hospital size, attending physician specialty, and geographic region in the propensity score, was used to control for potential clustering effects at the physician and hospital level.19 During the propensity score matching process, likelihood‐ratio test, Hosmer‐Lemshow goodness of fit, and concordance c statistics were utilized to assess the fitness of the models.20 The final propensity score model produced a concordance c statistic of 0.8.
Outcome Measures and Statistical Analyses
The following outcome measures were compared between the matched HN and non‐HN patient cohorts: total and intensive care unit (ICU) hospitalization costs, total and ICU length of stay (LOS), ICU admission, and 30‐day hospital readmission. Bivariate descriptive statistics were employed to test for significant differences in demographics, patient clinical characteristics, and unadjusted costs and healthcare resource utilization and readmission rates between patient cohorts. To detect statistically significant differences in continuous and categorical variables, respectively, t tests and chi‐square tests were performed.
Multivariate analysis of outcome measures utilized generalized linear models. Due to the skewed nature of LOS and cost data, LOS was analyzed using multivariate negative binomial regression and cost was analyzed using multivariate gamma regression.21 Binary outcomes (ICU admission and 30‐day readmission) were analyzed using multivariate logistic regression. The analysis accounted for potential confounding factors by inclusion of the following covariates: age group, gender, race, admission source, and Deyo‐Charlson Comorbidity Index score. These covariates were previously identified in the Wald et al hyponatremia study,3 and were verified using likelihood‐ratio, Hosmer‐Lemshow goodness of fit, and concordance c statistics.
Subgroup Sensitivity Analysis
For the subgroup sensitivity analyses, patients were identified as having community‐acquired pneumonia (CAP), congestive heart failure (CHF), urinary tract infection (UTI), or chronic obstructive pulmonary disease (COPD) based upon principal diagnosis codes. Patients were categorized according to these subgroup definitions, and then previously matched patients with the same subgroup classification constituted the final analysis set for each subgroup. The methods that were used for the overall matched analysis were then applied to each subgroup to evaluate the incremental burden of overall cost and LOS associated with hyponatremia.
RESULTS
Patient Population
Of the 606,057 HN patients eligible for matching, a total of 558,815 HN patients were matched to 558,815 non‐HN patients, a 92% match ratio. Table 1 describes the overall characteristics of the patient populations. For both cohorts, median age was 70 years, 57% of patients were female, and approximately 67% were white. The majority of patients in either cohort had Medicare coverage (55%), and approximately 75% of patients entered the hospital via the emergency room with nearly 70% having a 3M APR‐DRG disease severity level of major or extreme. Patients of both cohorts were most often attended by an internist or a hospitalist, with a combined percentage of approximately 60%. A small, but greater proportion of HN patients had comorbidities of cancer, pulmonary disease, and SIADH. Comorbid conditions of liver cirrhosis/hepatic disease and human immunodeficiency virus (HIV) were similarly distributed among both patient cohorts.
| Hyponatremic | Non‐Hyponatremic | |
|---|---|---|
| Discharges N (%) | Discharges N (%) | |
| ||
| Sample Discharges | 558,815 (100.0%) | 558,815 (100.0%) |
| Age median (IQR) | 70.00 (57.081.0) | 70.00 (57.081.0) |
| Gender | ||
| Female | 319,069 (57.1%) | 319,069 (57.1%) |
| Male | 239,746 (42.9%) | 239,746 (42.9%) |
| Race | ||
| American Indian | 3,465 (0.6%) | 3,448 (0.6%) |
| Asian/Pacific | 10,065 (1.8%) | 9,690 (1.7%) |
| Black | 63,776 (11.4%) | 66,233 (11.9%) |
| Hispanic | 24,341 (4.4%) | 24,426 (4.4%) |
| White | 377,434 (67.5%) | 376,639 (67.4%) |
| Other/unknown | 79,734 (14.3%) | 78,379 (14.0%) |
| Primary payer | ||
| Medicaretraditional | 310,312 (55.5%) | 310,643 (55.6%) |
| Managed care | 75,476 (13.5%) | 78,184 (14.0%) |
| Medicaremanaged care | 45,439 (8.1%) | 47,947 (8.6%) |
| Non‐cap | ||
| Medicaid | 44,690 (8.0%) | 43,767 (7.8%) |
| Other | 82,898 (14.8%) | 78,274 (14.0%) |
| Admission source | ||
| Physician referral | 5,022 (0.9%) | 4,636 (0.8%) |
| Transfer from another nonacute health facility | 18,031 (3.2%) | 18,163 (3.3%) |
| Emergency room | 417,556 (74.7%) | 420,401 (75.2%) |
| Other/unknown | 118,206 (21.2%) | 115,615 (20.7%) |
| APR‐DRG severity of illness | ||
| 1Minor | 14,257 (2.6%) | 12,993 (2.3%) |
| 2Moderate | 174,859 (31.3%) | 179,356 (32.1%) |
| 3Major | 263,814 (47.2%) | 265,422 (47.5%) |
| 4Extreme | 105,885 (19.0%) | 101,044 (18.1%) |
| Attending physician specialty | ||
| Internal Medicine | 235,628 (42.1%) | 240,875 (43.1%) |
| Hospitalist | 88,250 (15.8%) | 87,720 (15.7%) |
| Family Practice (FP) | 73,346 (13.1%) | 72,828 (13.0%) |
| Orthopedic Surgery (ORS) | 23,595 (4.2%) | 22,949 (4.1%) |
| Cardiovascular Diseases (CD) | 19,521 (3.5%) | 18,057 (3.2%) |
| Comorbidities | ||
| Human immunodeficiency virus | 3,971 (0.7%) | 4,018 (0.7%) |
| Cancer/neoplasm/malignancy | 107,851 (19.3%) | 105,199 (18.8%) |
| Pulmonary disease | 77,849 (13.9%) | 77,184 (13.8%) |
| Cirrhosis/hepatic disease | 23,038 (4.1%) | 23,418 (4.2%) |
| SIADH | 1,972 (0.4%) | 1,278 (0.2%) |
| Subgroup populations | ||
| Community‐acquired pneumonia | 26,291 (4.7%) | 26,291 (4.7%) |
| Congestive heart failure | 23,020 (4.1%) | 23,020 (4.1%) |
| Urinary tract infection | 14,238 (2.6%) | 14,238 (2.6%) |
| COPD | 8,696 (1.6%) | 8,696 (1.6%) |
| CCI median (IQR) | 3.0 (1.05.0) | 3.0 (1.05.0) |
| No. of premier hospitals | 459 | |
| Provider region | ||
| East North Central | 78,332 (14.0%) | 78,332 (14.0%) |
| East South Central | 32,122 (5.8%) | 32,122 (5.8%) |
| Middle Atlantic | 73,846 (13.2%) | 73,846 (13.2%) |
| Mountain | 23,761 (4.3%) | 23,761 (4.3%) |
| New England | 9,493 (1.7%) | 9,493 (1.7%) |
| Pacific | 73,059 (13.1%) | 73,059 (13.1%) |
| South Atlantic | 175,194 (31.4%) | 175,194 (31.4%) |
| West North Central | 37,913 (6.8%) | 37,913 (6.8%) |
| West South Central | 55,095 (9.9%) | 55,095 (9.9%) |
| Population served | ||
| Rural | 69,749 (12.5%) | 68,414 (12.2%) |
| Urban | 489,066 (87.5%) | 490,401 (87.8%) |
| Teaching status | ||
| Non‐teaching | 337,620 (60.4%) | 337,513 (60.4%) |
| Teaching | 221,195 (39.6%) | 221,302 (39.6%) |
| No. of hospital beds | ||
| 699 | 22,067 (4.0%) | 21,777 (3.9%) |
| 100199 | 57,367 (10.3%) | 56,097 (10.0%) |
| 200299 | 87,563 (15.7%) | 86,639 (15.5%) |
| 300499 | 218,834 (39.2%) | 220,248 (39.4%) |
| 500+ | 172,984 (31.0%) | 174,054 (31.2%) |
Hospital Characteristics
Patient cohorts had similar distributions with respect to hospital characteristics (Table 1). Approximately 30% of patients were provided care from hospitals located in the South Atlantic region, and between 10% and 15% were serviced from hospitals in the East North Central, Middle Atlantic, Pacific, and West South Central regions. Most hospitals providing care for patient cohorts served urban populations (88%) and were large, with hospital bed numbers 300. Approximately 60% of hospitals were non‐teaching hospitals.
Healthcare Utilization, Readmission, and Cost Differences Among Patient Cohorts
The mean LOS (8.8 10.3 vs 7.7 8.5, P < 0.001), a difference of 1.1 days and mean ICU LOS (5.5 7.9 vs 4.9 7.1 days, P < 0.001), a difference of 0.6 days were significantly greater for the HN cohort in comparison to the non‐HN cohort (Table 2). The increase in healthcare resource utilization of patients with HN was reflected in their significantly higher mean total hospital costs per admission ($15,281 $24,054 vs $13,439 $22,198, P < 0.001), a difference of $1842; and mean costs incurred in the ICU ($8525 $13,342 vs $7597 $12,695, P < 0.001), a difference of $928 (Table 2). Furthermore, patients in the HN cohort were significantly more likely to be readmitted to the hospital for any cause (17.5% vs 16.4%, P < 0.001) (Table 2).
| Hyponatremic | Non‐Hyponatremic | P Value | |
|---|---|---|---|
| |||
| Total LOS (mean SD) | 8.8 10.3 | 7.7 8.5 | <0.001 |
| Total hospitalization cost (mean SD) | $15,281 $24,054 | $13,439 $22,198 | <0.001 |
| ICU admission (N, %) | 129,235 (23.1%) | 123,502 (22.1%) | <0.001 |
| ICU LOS (mean SD) | 5.5 7.9 | 4.9 7.1 | <0.001 |
| ICU cost (mean SD) | $8,525 $13,342 | $7,597 $12,695 | <0.001 |
| 30‐Day all cause readmission (N, %) | 96,063 (17.5%) | 87,058 (16.4%) | <0.001 |
Multivariate Analysis
Multivariate analysis demonstrated hyponatremia was associated with an increase in mean hospital LOS of 10.9%, [95% confidence interval: 10.4%11.5%], (P < 0.0001) and an increase in mean total hospital costs of 8.2%, [7.4%9.0%], (P < 0.0001) (Table 3). Additionally, hyponatremia was associated with an increase in ICU LOS of 10.2%, [8.7%11.8%], (P < 0.0001), and a higher ICU cost of 8.9%, [7.2%10.7%], (P < 0.0001) (Table 3). Hyponatremia was not associated with a greater likelihood of ICU admission (odds ratio = 1.0; [1.01.0], P =.5760). However, the condition was associated with a significantly greater chance of hospital readmission (odds ratio = 1.2, [1.11.2], P < 0.0001) within 30 days postdischarge (Table 3).
| Overall Cohort N = 1,117,630 | CAP N = 52,582 | CHF N = 46,040 | UTI N = 28,746 | COPD N = 17,392 | |
|---|---|---|---|---|---|
| |||||
| Total LOS difference | 10.9% [10.4%, 11.5%] P < 0.0001 | 5.4% [4.4%, 6.5%] P < 0.0001 | 20.6% [19.0%, 22.2%] P < 0.0001 | 2.7% [1.2%, 4.2%] P = 0.0003 | 6.7% [4.8%, 8.5%] P < 0.0001 |
| Total cost difference | 8.2% [7.4%, 9.0%] P < 0.0001 | 5.6% [4.0%, 7.0%] P < 0.0001 | 19.2% [17.1%, 21.4%] P < 0.0001 | 2.2% [1.4%, 4.4%] P = 0.0582 | 5.6% [3.0%, 8.2%] P < 0.0001 |
| ICU admission* odds ratio | 1.0 [1.0, 1.0] P = 0.5760 | 1.0 [1.0, 1.1] P = 0.8363 | 1.2 [1.1, 1.3] P < 0.0001 | 1.2 [1.1, 1.3] P = 0.0038 | 1.0 [0.9, 1.1] P = 0.5995 |
| ICU LOS difference | 10.2% [8.7%, 11.8%] P < 0.0001 | 8.4% [3.4%, 13.7%] P = 0.0008 | 24.8% [19.9%, 29.9%] P < 0.0001 | 4.9% [4.0%, 14.7%] P = 0.2893 | 10.1% [1.5%, 19.3%] P = 0.0204 |
| ICU cost difference | 8.9% [7.2%, 10.7%] P < 0.0001 | 8.2% [2.7%, 14.0%] P = 0.0029 | 24.1% [18.9%, 29.7%] P < 0.0001 | 2.8% [6.7%, 13.4%] P = 0.5739 | 8.4% [0.6%, 18.1%] P = 0.6680 |
| 30‐Day readmission odds ratio | 1.2 [1.1, 1.2] P < 0.0001 | 1.0 [0.9, 1.0] P = 0.5141 | 1.1 [1.1, 1.2] P < 0.0001 | 1.0 [1.0, 1.1] P = 0.5211 | 1.0 [1.0, 1.1] P = 0.6119 |
Subgroup Sensitivity Analyses
For patients with CAP (n = 52,582), CHF (n = 46,040), a UTI (n = 28,476), or COPD (n = 17,392), the percent increases in LOS and all cause hospitalization cost of the HN cohort in comparison to the non‐HN cohort were 5.4% (P < 0.0001) and 5.6% (P < 0.0001), 20.6% (P < 0.0001) and 19.2% (P < 0.0001), 2.7% (P = 0.0003) and 2.2% (P = 0.0582), and 6.7% (P < 0.0001) and 5.6% (P < 0.0001), respectively (Table 3).
DISCUSSION
This large‐scale, real‐world hospital database study provides healthcare utilization and cost data on the largest population of HN patients that has currently been studied. The results of this study are consistent with others in showing that HN patients use healthcare services more extensively, and represent a patient population which is more expensive to treat in the inpatient setting.5, 22 Additionally, this study yields new findings in that patients in the real‐world with hyponatremia resulting from various etiologies are more likely to be readmitted to the hospital than patients with similar demographics and characteristics who do not have hyponatremia. The results of the subgroup analysis were generally consistent with the results for the overall matched population, as the incremental burden estimates were directionally consistent. However, among the specific subgroups of patients, although it appears that hyponatremia is predictive of hospital readmission in patients with CHF, it did not necessarily correspond with hospital readmission rates among patients with CAP, UTI, or COPD. Therefore, hyponatremia, at least for patients with these latter conditions, may not be predictive of readmission, but remains associated with increased healthcare utilization during the initial hospitalization. Further evaluation of the incremental burden of hyponatremia among patients with specific disease conditions is needed to validate the findings.
The difference in hospital LOS at first admission between HN and non‐HN patients in this study was 1.1 days, and comparable to that reported by Shorr et al.23 In the study by Shorr et al, patients hospitalized for congestive heart failure (CHF), who were HN, had a 0.5 day increased LOS, and those who were severely HN had a 1.3 day increased LOS.23 Two other retrospective cohort analyses reported a 1.4 day5 and 2.0 day22 increased LOS for HN patients in comparison to non‐HN patients. Zilberberg et al and Callahan et al additionally reported that HN patients had a significantly greater need for ICU (4%10%).5, 22 In the present study, LOS in the ICU and associated costs were also compared among HN and non‐HN cohorts and, after adjustment for key patient characteristics, hyponatremia was associated with an incremental increase of 10.2% for ICU LOS and an 8.9% increase in ICU cost.
In this study, patients with hyponatremia of any severity were found to have a mean increase in hospital cost per admission of $1842, with multivariate analysis demonstrating an associated incremental increase of 8.2%. These results are also comparable to that reported in other retrospective cohort analyses. Shorr et al reported that in‐hospital costs attributed to hyponatremia were $509, and for severe hyponatremia were $1132.23 Callahan et al reported a $1200 increase in costs per admission for patients with mild‐to‐moderate hyponatremia, and a $3540 increase for patients with moderate‐to‐severe hyponatremia, in comparison to non‐HN patients.22
The Institute for Healthcare Improvement reports that hospitalizations account for nearly one‐third of the $882 billion spent on healthcare in the US in 2011, and that a substantial fraction of all hospitalizations are readmissions.13, 24 A recent meta‐analysis of 30‐day hospital readmission rates found 23.1% were classified as avoidable.25 Readmission rates of HN patients in comparison to non‐HN patients have been reported in 3 other studies, all of which were conducted on clinical trial patients with heart failure.7, 8, 26 Two of these studies, which evaluated only patients with acute class IV heart failure,8, 26 reported that hyponatremia was associated with a significant increase in readmission rates (up to 20% higher), and the other, which evaluated any patients hospitalized for heart failure, did not.7 The differences there may be partially attributed to the differences in heart failure severity among patient populations, as hyponatremia is an independent predictor of worsening heart failure. The only published study to date on the influence of hyponatremia on readmission rates in the real‐world was conducted by Scherz et al, who reported that the co‐occurrence of hyponatremia in patients with acute pulmonary embolism, discharged from 185 hospitals in Pennsylvania, was independently associated with an increased readmission rate of 19.3%.27 In the present study, hyponatremia was associated with an incremental increase ranging between 14% and 17% for hospital readmission for any cause. It was conducted on a patient population in which hyponatremia was resultant from many causes, and not all patients had a serious comorbid condition. Also, the HN and non‐HN cohorts were matched for comorbidity prevalence and disease severity, and in other studies they were not. Therefore, the results of this study importantly imply that hyponatremia, whether it is resultant from a serious disease or any other cause substantially increases the healthcare burden. The implementation of strategies to prevent hospital readmissions may play an important role in reducing the healthcare burden of hyponatremia, and future studies are warranted to evaluate this hypothesis alongside evaluation of the outpatient hyponatremia burden.
The limitations of this study include, firstly, that it is only representative of inpatient hospital costs, and excludes outpatient healthcare utilization and costs. Secondly, this study utilized the Premier Hospital Database for patient selection, and laboratory testing data for serum sodium level are not available in this database; therefore, the severity of hyponatremia could not be accurately established in the HN patient population. Thirdly, the occurrence of hyponatremia in patients with some diseases is a marker of disease severity, as is the case with congestive heart failure and cirrhosis.23, 28 Our study did not adjust for the specific disease (eg, CHF) severity, which may influence the results. Future research is needed to evaluate the impact of hyponatremia on underlying disease severity of other diseases, and how its co‐occurrence may influence healthcare resource utilization and cost in each case. Although the Premier Hospital Database contains information from a large number of hospitals across the US, it is possible that it may not be representative of the entire US population of HN patients. Additionally, billing and coding errors and missing data could potentially have occurred, although the large patient population size likely precludes a large impact on the results of this study. Finally, the frequency of use of fluid restriction in these hospital settings could not be chronicled, thus limiting the ability to assess therapies and treatment modalities in use.
Acknowledgements
The authors acknowledge Melissa Lingohr‐Smith from Novosys Health in the editorial support and review of this manuscript.
Disclosures: This research was supported by Otsuka America Pharmaceutical, Inc, Princeton, NJ, which manufactures tolvaptan for the treatment of hyponatremia. Drs Amin and Deitelzweig are consultants for, and have received honoraria from, Otsuka America Pharmaceutical in connection with conducting this study. Drs Christian and Friend are employees of Otsuka America Pharmaceutical. Dr Lin is an employee of Novosys Health, which has received research funds from Otsuka America Pharmaceutical in connection with conducting this study and development of this manuscript. D. Baumer and Dr. Lowe are employees of Premier Inc, which has received research funds from Otsuka America Pharmaceutical. K. Belk was previously employed with Premier Inc.
Hyponatremia is an electrolyte disorder most commonly defined as a serum sodium concentration <135 mEq/L.1 Its exact definition can vary across studies, but typically ranges between <130 and <138 mEq/L.2, 3 Signs and symptoms of hyponatremia can include malaise, headache, disorientation, confusion, muscle weakness, and cramps. If severe, seizures, respiratory arrest, brainstem herniation, coma, and death may result.
The incidence of hyponatremia in the general hospitalized population has been reported to range between 1% and 6% when defined as <130135 mEq/L,4, 5 and its occurrence increases with a more prolonged hospital stay to 13%.6 A recent study reported that when hyponatremia was defined with a less stringent threshold of <138 mEq/L, the incidence at admission rose to 38%.3 Hyponatremia is a comorbid condition of multiple diseases, occurring in approximately 20% of patients with heart failure,7, 8 and 40% to 57% of patients with advanced cirrhosis.9, 10 The syndrome of the inappropriate release of antidiuretic hormone (SIADH) is additionally a predominant cause of hyponatremia, with a prevalence reported as high as 35% in hospitalized patients.11
Hyponatremia is not only widespread, but also an independent predictor of mortality. In a retrospective cohort analysis, Waikar et al reported that in comparison to patients who were normonatremic, patients with serum sodium concentrations <135 mEq/L had a risk of in‐hospital mortality as high as 47%, and that this risk doubled for patients with serum sodium concentrations between 125 and 129 mEq/L.6 In the study by Wald et al, which defined hyponatremia as <138 mEq/L, the risk of in‐hospital mortality was similar.3 In both of these studies, even mild hyponatremia (130137 mEq/L) was associated with increased risk of in‐hospital mortality.3, 6
The overall cost of hyponatremia is estimated to range between $1.6 and $3.6 billion for 2011.12, 13 Hospital readmissions are a significant contributor to total healthcare costs, with some being entirely avoidable with increased standards of care. The Centers for Medicare and Medicaid Services has begun to not only publicly report hospital readmission rates, but also penalize hospitals for early readmissions.14 Strategies to reduce hospital readmissions are currently being integrated into healthcare reform policy.14 In the present study, the incremental burden of hospitalized hyponatremic (HN) versus non‐HN patients in terms of hospital resource utilization, costs, and early hospital readmission in the real‐world was evaluated.
METHODS
Study Design
This study was a retrospective analysis that examined healthcare utilization and costs among HN patients using the Premier Hospital Database. The database contains over 310 million hospital encounters from more than 700 US hospitals, or 1 out of every 4 discharges in the US. The administrative data available included patient and provider demographics, diagnoses and procedures, as well as date‐stamped billing records for all pharmacy, laboratory, imaging, procedures, and supplies.
Patient Selection and Matching
HN patients were eligible for study inclusion if they were a hospital inpatient discharged between January 1, 2007 and March 31, 2010, were >18 years of age at admission, and had either a primary or secondary diagnosis of hyponatremia or hyposmolality (defined as International Classification of Diseases, Ninth Revision (ICD‐9‐CM) code: 276.1x). Patients were excluded if they had been transferred from another acute care facility, transferred to another acute care or critical access facility, or left against medical advice. Labor and delivery patients (ICD‐9‐CM codes: 72.xx‐74.xx, V22.x, V23.x, V27.x, and V28.x), and patients classified as observational were also excluded. A second cohort of non‐hyponatremic patients was created using the same inclusion and exclusion criteria, with the exception that patients not have a primary or secondary diagnosis of hyponatremia or hyposmolality (defined as ICD‐9‐CM code: 276.1x).
The matching of hyponatremia (HN) and control (non‐HN) cohorts was accomplished using a combination of exact and propensity score matching techniques. Patients were first exact matched on age, gender, Medicare Severity‐Diagnosis Related Group (MS‐DRG) assignment, and hospital geographic region. Propensity score matching was further utilized to create the final study cohorts for outcomes comparisons. Propensity score matching is commonly used in retrospective cohort studies to correct for sample selection bias due to observable differences between groups.15, 16 The propensity score was generated using logistic regression with the dependent variable as hyponatremia (yes vs no) and the following covariates: age, race, admission source, attending physician specialty, 3M All Patient Refined‐Diagnosis Related Group (APR‐DRG) Severity of Illness and Risk of Mortality index scores, Deyo‐Charlson Comorbidity Index score, selected hyponatremia‐related comorbidity conditions, and hospital size, region, and urban/rural designation. These covariates were initially selected by an expert panel of physicians, and backward selection was utilized in the logistic regression using the most parsimonious model.17
Following generation of the propensity scores, HN patients were matched to non‐HN patients 1:1 using a nearest neighbor matching algorithm, including hospital identification and propensity score.18 Inclusion of hospital identification in the matching sequence, as well as provider characteristics, especially hospital size, attending physician specialty, and geographic region in the propensity score, was used to control for potential clustering effects at the physician and hospital level.19 During the propensity score matching process, likelihood‐ratio test, Hosmer‐Lemshow goodness of fit, and concordance c statistics were utilized to assess the fitness of the models.20 The final propensity score model produced a concordance c statistic of 0.8.
Outcome Measures and Statistical Analyses
The following outcome measures were compared between the matched HN and non‐HN patient cohorts: total and intensive care unit (ICU) hospitalization costs, total and ICU length of stay (LOS), ICU admission, and 30‐day hospital readmission. Bivariate descriptive statistics were employed to test for significant differences in demographics, patient clinical characteristics, and unadjusted costs and healthcare resource utilization and readmission rates between patient cohorts. To detect statistically significant differences in continuous and categorical variables, respectively, t tests and chi‐square tests were performed.
Multivariate analysis of outcome measures utilized generalized linear models. Due to the skewed nature of LOS and cost data, LOS was analyzed using multivariate negative binomial regression and cost was analyzed using multivariate gamma regression.21 Binary outcomes (ICU admission and 30‐day readmission) were analyzed using multivariate logistic regression. The analysis accounted for potential confounding factors by inclusion of the following covariates: age group, gender, race, admission source, and Deyo‐Charlson Comorbidity Index score. These covariates were previously identified in the Wald et al hyponatremia study,3 and were verified using likelihood‐ratio, Hosmer‐Lemshow goodness of fit, and concordance c statistics.
Subgroup Sensitivity Analysis
For the subgroup sensitivity analyses, patients were identified as having community‐acquired pneumonia (CAP), congestive heart failure (CHF), urinary tract infection (UTI), or chronic obstructive pulmonary disease (COPD) based upon principal diagnosis codes. Patients were categorized according to these subgroup definitions, and then previously matched patients with the same subgroup classification constituted the final analysis set for each subgroup. The methods that were used for the overall matched analysis were then applied to each subgroup to evaluate the incremental burden of overall cost and LOS associated with hyponatremia.
RESULTS
Patient Population
Of the 606,057 HN patients eligible for matching, a total of 558,815 HN patients were matched to 558,815 non‐HN patients, a 92% match ratio. Table 1 describes the overall characteristics of the patient populations. For both cohorts, median age was 70 years, 57% of patients were female, and approximately 67% were white. The majority of patients in either cohort had Medicare coverage (55%), and approximately 75% of patients entered the hospital via the emergency room with nearly 70% having a 3M APR‐DRG disease severity level of major or extreme. Patients of both cohorts were most often attended by an internist or a hospitalist, with a combined percentage of approximately 60%. A small, but greater proportion of HN patients had comorbidities of cancer, pulmonary disease, and SIADH. Comorbid conditions of liver cirrhosis/hepatic disease and human immunodeficiency virus (HIV) were similarly distributed among both patient cohorts.
| Hyponatremic | Non‐Hyponatremic | |
|---|---|---|
| Discharges N (%) | Discharges N (%) | |
| ||
| Sample Discharges | 558,815 (100.0%) | 558,815 (100.0%) |
| Age median (IQR) | 70.00 (57.081.0) | 70.00 (57.081.0) |
| Gender | ||
| Female | 319,069 (57.1%) | 319,069 (57.1%) |
| Male | 239,746 (42.9%) | 239,746 (42.9%) |
| Race | ||
| American Indian | 3,465 (0.6%) | 3,448 (0.6%) |
| Asian/Pacific | 10,065 (1.8%) | 9,690 (1.7%) |
| Black | 63,776 (11.4%) | 66,233 (11.9%) |
| Hispanic | 24,341 (4.4%) | 24,426 (4.4%) |
| White | 377,434 (67.5%) | 376,639 (67.4%) |
| Other/unknown | 79,734 (14.3%) | 78,379 (14.0%) |
| Primary payer | ||
| Medicaretraditional | 310,312 (55.5%) | 310,643 (55.6%) |
| Managed care | 75,476 (13.5%) | 78,184 (14.0%) |
| Medicaremanaged care | 45,439 (8.1%) | 47,947 (8.6%) |
| Non‐cap | ||
| Medicaid | 44,690 (8.0%) | 43,767 (7.8%) |
| Other | 82,898 (14.8%) | 78,274 (14.0%) |
| Admission source | ||
| Physician referral | 5,022 (0.9%) | 4,636 (0.8%) |
| Transfer from another nonacute health facility | 18,031 (3.2%) | 18,163 (3.3%) |
| Emergency room | 417,556 (74.7%) | 420,401 (75.2%) |
| Other/unknown | 118,206 (21.2%) | 115,615 (20.7%) |
| APR‐DRG severity of illness | ||
| 1Minor | 14,257 (2.6%) | 12,993 (2.3%) |
| 2Moderate | 174,859 (31.3%) | 179,356 (32.1%) |
| 3Major | 263,814 (47.2%) | 265,422 (47.5%) |
| 4Extreme | 105,885 (19.0%) | 101,044 (18.1%) |
| Attending physician specialty | ||
| Internal Medicine | 235,628 (42.1%) | 240,875 (43.1%) |
| Hospitalist | 88,250 (15.8%) | 87,720 (15.7%) |
| Family Practice (FP) | 73,346 (13.1%) | 72,828 (13.0%) |
| Orthopedic Surgery (ORS) | 23,595 (4.2%) | 22,949 (4.1%) |
| Cardiovascular Diseases (CD) | 19,521 (3.5%) | 18,057 (3.2%) |
| Comorbidities | ||
| Human immunodeficiency virus | 3,971 (0.7%) | 4,018 (0.7%) |
| Cancer/neoplasm/malignancy | 107,851 (19.3%) | 105,199 (18.8%) |
| Pulmonary disease | 77,849 (13.9%) | 77,184 (13.8%) |
| Cirrhosis/hepatic disease | 23,038 (4.1%) | 23,418 (4.2%) |
| SIADH | 1,972 (0.4%) | 1,278 (0.2%) |
| Subgroup populations | ||
| Community‐acquired pneumonia | 26,291 (4.7%) | 26,291 (4.7%) |
| Congestive heart failure | 23,020 (4.1%) | 23,020 (4.1%) |
| Urinary tract infection | 14,238 (2.6%) | 14,238 (2.6%) |
| COPD | 8,696 (1.6%) | 8,696 (1.6%) |
| CCI median (IQR) | 3.0 (1.05.0) | 3.0 (1.05.0) |
| No. of premier hospitals | 459 | |
| Provider region | ||
| East North Central | 78,332 (14.0%) | 78,332 (14.0%) |
| East South Central | 32,122 (5.8%) | 32,122 (5.8%) |
| Middle Atlantic | 73,846 (13.2%) | 73,846 (13.2%) |
| Mountain | 23,761 (4.3%) | 23,761 (4.3%) |
| New England | 9,493 (1.7%) | 9,493 (1.7%) |
| Pacific | 73,059 (13.1%) | 73,059 (13.1%) |
| South Atlantic | 175,194 (31.4%) | 175,194 (31.4%) |
| West North Central | 37,913 (6.8%) | 37,913 (6.8%) |
| West South Central | 55,095 (9.9%) | 55,095 (9.9%) |
| Population served | ||
| Rural | 69,749 (12.5%) | 68,414 (12.2%) |
| Urban | 489,066 (87.5%) | 490,401 (87.8%) |
| Teaching status | ||
| Non‐teaching | 337,620 (60.4%) | 337,513 (60.4%) |
| Teaching | 221,195 (39.6%) | 221,302 (39.6%) |
| No. of hospital beds | ||
| 699 | 22,067 (4.0%) | 21,777 (3.9%) |
| 100199 | 57,367 (10.3%) | 56,097 (10.0%) |
| 200299 | 87,563 (15.7%) | 86,639 (15.5%) |
| 300499 | 218,834 (39.2%) | 220,248 (39.4%) |
| 500+ | 172,984 (31.0%) | 174,054 (31.2%) |
Hospital Characteristics
Patient cohorts had similar distributions with respect to hospital characteristics (Table 1). Approximately 30% of patients were provided care from hospitals located in the South Atlantic region, and between 10% and 15% were serviced from hospitals in the East North Central, Middle Atlantic, Pacific, and West South Central regions. Most hospitals providing care for patient cohorts served urban populations (88%) and were large, with hospital bed numbers 300. Approximately 60% of hospitals were non‐teaching hospitals.
Healthcare Utilization, Readmission, and Cost Differences Among Patient Cohorts
The mean LOS (8.8 10.3 vs 7.7 8.5, P < 0.001), a difference of 1.1 days and mean ICU LOS (5.5 7.9 vs 4.9 7.1 days, P < 0.001), a difference of 0.6 days were significantly greater for the HN cohort in comparison to the non‐HN cohort (Table 2). The increase in healthcare resource utilization of patients with HN was reflected in their significantly higher mean total hospital costs per admission ($15,281 $24,054 vs $13,439 $22,198, P < 0.001), a difference of $1842; and mean costs incurred in the ICU ($8525 $13,342 vs $7597 $12,695, P < 0.001), a difference of $928 (Table 2). Furthermore, patients in the HN cohort were significantly more likely to be readmitted to the hospital for any cause (17.5% vs 16.4%, P < 0.001) (Table 2).
| Hyponatremic | Non‐Hyponatremic | P Value | |
|---|---|---|---|
| |||
| Total LOS (mean SD) | 8.8 10.3 | 7.7 8.5 | <0.001 |
| Total hospitalization cost (mean SD) | $15,281 $24,054 | $13,439 $22,198 | <0.001 |
| ICU admission (N, %) | 129,235 (23.1%) | 123,502 (22.1%) | <0.001 |
| ICU LOS (mean SD) | 5.5 7.9 | 4.9 7.1 | <0.001 |
| ICU cost (mean SD) | $8,525 $13,342 | $7,597 $12,695 | <0.001 |
| 30‐Day all cause readmission (N, %) | 96,063 (17.5%) | 87,058 (16.4%) | <0.001 |
Multivariate Analysis
Multivariate analysis demonstrated hyponatremia was associated with an increase in mean hospital LOS of 10.9%, [95% confidence interval: 10.4%11.5%], (P < 0.0001) and an increase in mean total hospital costs of 8.2%, [7.4%9.0%], (P < 0.0001) (Table 3). Additionally, hyponatremia was associated with an increase in ICU LOS of 10.2%, [8.7%11.8%], (P < 0.0001), and a higher ICU cost of 8.9%, [7.2%10.7%], (P < 0.0001) (Table 3). Hyponatremia was not associated with a greater likelihood of ICU admission (odds ratio = 1.0; [1.01.0], P =.5760). However, the condition was associated with a significantly greater chance of hospital readmission (odds ratio = 1.2, [1.11.2], P < 0.0001) within 30 days postdischarge (Table 3).
| Overall Cohort N = 1,117,630 | CAP N = 52,582 | CHF N = 46,040 | UTI N = 28,746 | COPD N = 17,392 | |
|---|---|---|---|---|---|
| |||||
| Total LOS difference | 10.9% [10.4%, 11.5%] P < 0.0001 | 5.4% [4.4%, 6.5%] P < 0.0001 | 20.6% [19.0%, 22.2%] P < 0.0001 | 2.7% [1.2%, 4.2%] P = 0.0003 | 6.7% [4.8%, 8.5%] P < 0.0001 |
| Total cost difference | 8.2% [7.4%, 9.0%] P < 0.0001 | 5.6% [4.0%, 7.0%] P < 0.0001 | 19.2% [17.1%, 21.4%] P < 0.0001 | 2.2% [1.4%, 4.4%] P = 0.0582 | 5.6% [3.0%, 8.2%] P < 0.0001 |
| ICU admission* odds ratio | 1.0 [1.0, 1.0] P = 0.5760 | 1.0 [1.0, 1.1] P = 0.8363 | 1.2 [1.1, 1.3] P < 0.0001 | 1.2 [1.1, 1.3] P = 0.0038 | 1.0 [0.9, 1.1] P = 0.5995 |
| ICU LOS difference | 10.2% [8.7%, 11.8%] P < 0.0001 | 8.4% [3.4%, 13.7%] P = 0.0008 | 24.8% [19.9%, 29.9%] P < 0.0001 | 4.9% [4.0%, 14.7%] P = 0.2893 | 10.1% [1.5%, 19.3%] P = 0.0204 |
| ICU cost difference | 8.9% [7.2%, 10.7%] P < 0.0001 | 8.2% [2.7%, 14.0%] P = 0.0029 | 24.1% [18.9%, 29.7%] P < 0.0001 | 2.8% [6.7%, 13.4%] P = 0.5739 | 8.4% [0.6%, 18.1%] P = 0.6680 |
| 30‐Day readmission odds ratio | 1.2 [1.1, 1.2] P < 0.0001 | 1.0 [0.9, 1.0] P = 0.5141 | 1.1 [1.1, 1.2] P < 0.0001 | 1.0 [1.0, 1.1] P = 0.5211 | 1.0 [1.0, 1.1] P = 0.6119 |
Subgroup Sensitivity Analyses
For patients with CAP (n = 52,582), CHF (n = 46,040), a UTI (n = 28,476), or COPD (n = 17,392), the percent increases in LOS and all cause hospitalization cost of the HN cohort in comparison to the non‐HN cohort were 5.4% (P < 0.0001) and 5.6% (P < 0.0001), 20.6% (P < 0.0001) and 19.2% (P < 0.0001), 2.7% (P = 0.0003) and 2.2% (P = 0.0582), and 6.7% (P < 0.0001) and 5.6% (P < 0.0001), respectively (Table 3).
DISCUSSION
This large‐scale, real‐world hospital database study provides healthcare utilization and cost data on the largest population of HN patients that has currently been studied. The results of this study are consistent with others in showing that HN patients use healthcare services more extensively, and represent a patient population which is more expensive to treat in the inpatient setting.5, 22 Additionally, this study yields new findings in that patients in the real‐world with hyponatremia resulting from various etiologies are more likely to be readmitted to the hospital than patients with similar demographics and characteristics who do not have hyponatremia. The results of the subgroup analysis were generally consistent with the results for the overall matched population, as the incremental burden estimates were directionally consistent. However, among the specific subgroups of patients, although it appears that hyponatremia is predictive of hospital readmission in patients with CHF, it did not necessarily correspond with hospital readmission rates among patients with CAP, UTI, or COPD. Therefore, hyponatremia, at least for patients with these latter conditions, may not be predictive of readmission, but remains associated with increased healthcare utilization during the initial hospitalization. Further evaluation of the incremental burden of hyponatremia among patients with specific disease conditions is needed to validate the findings.
The difference in hospital LOS at first admission between HN and non‐HN patients in this study was 1.1 days, and comparable to that reported by Shorr et al.23 In the study by Shorr et al, patients hospitalized for congestive heart failure (CHF), who were HN, had a 0.5 day increased LOS, and those who were severely HN had a 1.3 day increased LOS.23 Two other retrospective cohort analyses reported a 1.4 day5 and 2.0 day22 increased LOS for HN patients in comparison to non‐HN patients. Zilberberg et al and Callahan et al additionally reported that HN patients had a significantly greater need for ICU (4%10%).5, 22 In the present study, LOS in the ICU and associated costs were also compared among HN and non‐HN cohorts and, after adjustment for key patient characteristics, hyponatremia was associated with an incremental increase of 10.2% for ICU LOS and an 8.9% increase in ICU cost.
In this study, patients with hyponatremia of any severity were found to have a mean increase in hospital cost per admission of $1842, with multivariate analysis demonstrating an associated incremental increase of 8.2%. These results are also comparable to that reported in other retrospective cohort analyses. Shorr et al reported that in‐hospital costs attributed to hyponatremia were $509, and for severe hyponatremia were $1132.23 Callahan et al reported a $1200 increase in costs per admission for patients with mild‐to‐moderate hyponatremia, and a $3540 increase for patients with moderate‐to‐severe hyponatremia, in comparison to non‐HN patients.22
The Institute for Healthcare Improvement reports that hospitalizations account for nearly one‐third of the $882 billion spent on healthcare in the US in 2011, and that a substantial fraction of all hospitalizations are readmissions.13, 24 A recent meta‐analysis of 30‐day hospital readmission rates found 23.1% were classified as avoidable.25 Readmission rates of HN patients in comparison to non‐HN patients have been reported in 3 other studies, all of which were conducted on clinical trial patients with heart failure.7, 8, 26 Two of these studies, which evaluated only patients with acute class IV heart failure,8, 26 reported that hyponatremia was associated with a significant increase in readmission rates (up to 20% higher), and the other, which evaluated any patients hospitalized for heart failure, did not.7 The differences there may be partially attributed to the differences in heart failure severity among patient populations, as hyponatremia is an independent predictor of worsening heart failure. The only published study to date on the influence of hyponatremia on readmission rates in the real‐world was conducted by Scherz et al, who reported that the co‐occurrence of hyponatremia in patients with acute pulmonary embolism, discharged from 185 hospitals in Pennsylvania, was independently associated with an increased readmission rate of 19.3%.27 In the present study, hyponatremia was associated with an incremental increase ranging between 14% and 17% for hospital readmission for any cause. It was conducted on a patient population in which hyponatremia was resultant from many causes, and not all patients had a serious comorbid condition. Also, the HN and non‐HN cohorts were matched for comorbidity prevalence and disease severity, and in other studies they were not. Therefore, the results of this study importantly imply that hyponatremia, whether it is resultant from a serious disease or any other cause substantially increases the healthcare burden. The implementation of strategies to prevent hospital readmissions may play an important role in reducing the healthcare burden of hyponatremia, and future studies are warranted to evaluate this hypothesis alongside evaluation of the outpatient hyponatremia burden.
The limitations of this study include, firstly, that it is only representative of inpatient hospital costs, and excludes outpatient healthcare utilization and costs. Secondly, this study utilized the Premier Hospital Database for patient selection, and laboratory testing data for serum sodium level are not available in this database; therefore, the severity of hyponatremia could not be accurately established in the HN patient population. Thirdly, the occurrence of hyponatremia in patients with some diseases is a marker of disease severity, as is the case with congestive heart failure and cirrhosis.23, 28 Our study did not adjust for the specific disease (eg, CHF) severity, which may influence the results. Future research is needed to evaluate the impact of hyponatremia on underlying disease severity of other diseases, and how its co‐occurrence may influence healthcare resource utilization and cost in each case. Although the Premier Hospital Database contains information from a large number of hospitals across the US, it is possible that it may not be representative of the entire US population of HN patients. Additionally, billing and coding errors and missing data could potentially have occurred, although the large patient population size likely precludes a large impact on the results of this study. Finally, the frequency of use of fluid restriction in these hospital settings could not be chronicled, thus limiting the ability to assess therapies and treatment modalities in use.
Acknowledgements
The authors acknowledge Melissa Lingohr‐Smith from Novosys Health in the editorial support and review of this manuscript.
Disclosures: This research was supported by Otsuka America Pharmaceutical, Inc, Princeton, NJ, which manufactures tolvaptan for the treatment of hyponatremia. Drs Amin and Deitelzweig are consultants for, and have received honoraria from, Otsuka America Pharmaceutical in connection with conducting this study. Drs Christian and Friend are employees of Otsuka America Pharmaceutical. Dr Lin is an employee of Novosys Health, which has received research funds from Otsuka America Pharmaceutical in connection with conducting this study and development of this manuscript. D. Baumer and Dr. Lowe are employees of Premier Inc, which has received research funds from Otsuka America Pharmaceutical. K. Belk was previously employed with Premier Inc.
- ,,.Management of hyponatremia: providing treatment and avoiding harm.Cleve Clin J Med.2010;77(10):715–726.
- ,,.Causes and management of hyponatremia.Ann Pharmacother.2003;37(11):1694–1702.
- ,,,,.Impact of hospital‐associated hyponatremia on selected outcomes.Arch Intern Med.2010;170(3):294–302.
- ,,,.Hyponatremia: a prospective analysis of its epidemiology and the pathogenetic role of vasopressin.Ann Intern Med.1985;102(2):164–168.
- ,,, et al.Epidemiology, clinical and economic outcomes of admission hyponatremia among hospitalized patients.Curr Med Res Opin.2008;24(6):1601–1608.
- ,,.Mortality after hospitalization with mild, moderate, and severe hyponatremia.Am J Med.2009;122(9):857–865.
- ,,, et al.Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE‐HF registry.Eur Heart J.2007;28(8):980–988.
- ,,, et al.Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial.Arch Intern Med.2007;167(18):1998–2005.
- ,,,.Hyponatremia in cirrhosis: results of a patient population survey.Hepatology.2006;44(6):1535–1542.
- ,,, et al.Hyponatremia in cirrhosis: from pathogenesis to treatment.Hepatology.1998;28(3):851–864.
- ,,,,.The syndrome of inappropriate antidiuresis: pathophysiology, clinical management and new therapeutic options.Nephron Clin Pract.2011;119(1):c62–c73.
- ,,.Cost of illness of hyponatremia in the United States.Cost Eff Resour Alloc.2006;4:10.
- US Health Care Budget: US Budget Breakdown for FY12—Charts. Available at: http://www.usgovernmentspending.com/health_care_budget_2012_1.html. Accessed December 19, 2011.
- 111th Congress. Patient Protection and Affordable Care Act. Public Law 111–148.1–906.
- .An evaluation of statistical matching.JBES.1984;2(1):91–102.
- ,.Propensity score‐matching methods for nonexperimental causal studies.Rev Econ Stat.2002;84(1):151–161.
- . Applied Logistic Regression Analysis. Quantitative Applications in the Social Sciences, Vol106.2nd ed.Thousand Oaks, CA:Sage;2002.
- .Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. Paper presented at: Proceedings of the Twenty‐Sixth Annual SAS Users Group International Conference; April 22–25,2001; Long Beach, CA.
- ,,,,.The effect of clustering of outcomes on the association of procedure volume and surgical outcomes.Ann Intern Med.2003;139(8):658–665.
- .Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis.New York, NY:Springer‐Verlag,2001.
- ,,.Generalized Modeling Approaches to Risk Adjustment of Skewed Outcomes Data.Cambridge, MA:National Bureau of Economic Research, Inc;2003.
- ,,,.Economic impact of hyponatremia in hospitalized patients: a retrospective cohort study.Postgrad Med.2009;121(2):186–191.
- ,,, et al.Burden of sodium abnormalities in patients hospitalized for heart failure.Congest Heart Fail.2011;17(1):1–7.
- Institute for Healthcare Improvement. Reduce Avoidable Hospital Readmissions. Available at: http://www.ihi.org/explore/readmissions/Pages/default.aspx. Accessed December 18, 2011.
- ,,.A meta‐analysis of hospital 30‐day avoidable readmission rates.J Eval Clin Pract.2011 Nov 9. doi: 10.1111/j.1365–2753.2011.01773.x. Published online August 17, 2012.
- ,,, et al.Critical elements of clinical follow‐up after hospital discharge for heart failure: insights from the EVEREST trial.Eur J Heart Fail.2010;12(4):367–374.
- ,,, et al.Prognostic importance of hyponatremia in patients with acute pulmonary embolism.Am J Respir Crit Care Med.2010;182(9):1178–1183.
- ,,, et al.Serum sodium predicts prognosis in critically ill cirrhotic patients.J Clin Gastroenterol.2010;44(3):220–226.
- ,,.Management of hyponatremia: providing treatment and avoiding harm.Cleve Clin J Med.2010;77(10):715–726.
- ,,.Causes and management of hyponatremia.Ann Pharmacother.2003;37(11):1694–1702.
- ,,,,.Impact of hospital‐associated hyponatremia on selected outcomes.Arch Intern Med.2010;170(3):294–302.
- ,,,.Hyponatremia: a prospective analysis of its epidemiology and the pathogenetic role of vasopressin.Ann Intern Med.1985;102(2):164–168.
- ,,, et al.Epidemiology, clinical and economic outcomes of admission hyponatremia among hospitalized patients.Curr Med Res Opin.2008;24(6):1601–1608.
- ,,.Mortality after hospitalization with mild, moderate, and severe hyponatremia.Am J Med.2009;122(9):857–865.
- ,,, et al.Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE‐HF registry.Eur Heart J.2007;28(8):980–988.
- ,,, et al.Characterization and prognostic value of persistent hyponatremia in patients with severe heart failure in the ESCAPE Trial.Arch Intern Med.2007;167(18):1998–2005.
- ,,,.Hyponatremia in cirrhosis: results of a patient population survey.Hepatology.2006;44(6):1535–1542.
- ,,, et al.Hyponatremia in cirrhosis: from pathogenesis to treatment.Hepatology.1998;28(3):851–864.
- ,,,,.The syndrome of inappropriate antidiuresis: pathophysiology, clinical management and new therapeutic options.Nephron Clin Pract.2011;119(1):c62–c73.
- ,,.Cost of illness of hyponatremia in the United States.Cost Eff Resour Alloc.2006;4:10.
- US Health Care Budget: US Budget Breakdown for FY12—Charts. Available at: http://www.usgovernmentspending.com/health_care_budget_2012_1.html. Accessed December 19, 2011.
- 111th Congress. Patient Protection and Affordable Care Act. Public Law 111–148.1–906.
- .An evaluation of statistical matching.JBES.1984;2(1):91–102.
- ,.Propensity score‐matching methods for nonexperimental causal studies.Rev Econ Stat.2002;84(1):151–161.
- . Applied Logistic Regression Analysis. Quantitative Applications in the Social Sciences, Vol106.2nd ed.Thousand Oaks, CA:Sage;2002.
- .Reducing bias in a propensity score matched‐pair sample using greedy matching techniques. Paper presented at: Proceedings of the Twenty‐Sixth Annual SAS Users Group International Conference; April 22–25,2001; Long Beach, CA.
- ,,,,.The effect of clustering of outcomes on the association of procedure volume and surgical outcomes.Ann Intern Med.2003;139(8):658–665.
- .Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis.New York, NY:Springer‐Verlag,2001.
- ,,.Generalized Modeling Approaches to Risk Adjustment of Skewed Outcomes Data.Cambridge, MA:National Bureau of Economic Research, Inc;2003.
- ,,,.Economic impact of hyponatremia in hospitalized patients: a retrospective cohort study.Postgrad Med.2009;121(2):186–191.
- ,,, et al.Burden of sodium abnormalities in patients hospitalized for heart failure.Congest Heart Fail.2011;17(1):1–7.
- Institute for Healthcare Improvement. Reduce Avoidable Hospital Readmissions. Available at: http://www.ihi.org/explore/readmissions/Pages/default.aspx. Accessed December 18, 2011.
- ,,.A meta‐analysis of hospital 30‐day avoidable readmission rates.J Eval Clin Pract.2011 Nov 9. doi: 10.1111/j.1365–2753.2011.01773.x. Published online August 17, 2012.
- ,,, et al.Critical elements of clinical follow‐up after hospital discharge for heart failure: insights from the EVEREST trial.Eur J Heart Fail.2010;12(4):367–374.
- ,,, et al.Prognostic importance of hyponatremia in patients with acute pulmonary embolism.Am J Respir Crit Care Med.2010;182(9):1178–1183.
- ,,, et al.Serum sodium predicts prognosis in critically ill cirrhotic patients.J Clin Gastroenterol.2010;44(3):220–226.
Copyright © 2012 Society of Hospital Medicine
Assessing Teamwork in SIDR
Teamwork is essential to delivering safe and effective hospital care,15 yet the fluidity and geographic dispersion of team members in the hospital setting presents a significant barrier to teamwork.6 Physicians, nurses, and other hospital professionals frequently lack convenient and reliable opportunities to interact, and may struggle in efforts to discuss the care of their patients in person. Research studies show that nurses and physicians on patient care units do not communicate consistently and frequently do not agree on key aspects of their patients' plans of care.7, 8
Interdisciplinary rounds (IDR), also known as multidisciplinary rounds, provide a means to assemble hospital care team members and improve collaboration.913 Prior research on the use of IDR has demonstrated improved ratings of collaboration,11, 12 but inconsistent effects on length of stay and cost.10, 12, 13 Notably, the format, frequency, and duration of IDR in prior studies has been variable and no studies, to our knowledge, have evaluated teamwork performance during IDR. Lamb and colleagues conducted observations of cancer teams during multidisciplinary meetings.14 Trained observers used a validated observation tool to rate teamwork and found significant variation in performance by subteams. However, the study focused mainly on discussion among physician team members during meetings to plan longitudinal care for oncology patients.
We recently reported on the use of structured interdisciplinary rounds (SIDR) on 2 medical units in our hospital.15, 16 SIDR combines a structured format for communication, similar to a goals‐of‐care form,17, 18 with a forum for daily interdisciplinary meetings. Though no effect was seen on length of stay or cost, SIDR was associated with significantly higher ratings of the quality of collaboration and teamwork climate, and a reduction in the rate of adverse events.19 In March 2010, we implemented SIDR across all medical units in our hospital. We subjectively noted variation in teamwork performance during SIDR after a modification of nurse manager roles. We sought to evaluate teamwork during SIDR and to determine whether variation in performance existed and, if present, to characterize it.
METHODS
Setting and Study Design
The study was conducted at Northwestern Memorial Hospital (NMH), a 920‐bed tertiary care teaching hospital in Chicago, IL, and was deemed exempt by the Institutional Review Board of Northwestern University. General medical patients were admitted to 1 of 6 units based on bed availability. Five of the medical units consisted of 30 beds, and 1 unit consisted of 23. Each unit was equipped with continuous cardiac telemetry monitoring. Three units were staffed by teaching service physician teams consisting of 1 attending, 1 resident, and 1 or 2 interns. The other 3 units were staffed by hospitalists without the assistance of resident or intern physicians. As a result of a prior intervention, physicians' patients were localized to specific units in an effort to improve communication practices among nurses and physicians.20
Beginning in March 2010, all general medical units held SIDR each weekday morning. SIDR took place in the unit conference room, was expected to last approximately 3040 minutes, and was co‐led by the unit nurse manager and a medical director. Unit nurse managers and medical directors received specific training for their roles, including 3 hours of simulation‐based exercises designed to enhance their skills in facilitating discussion during SIDR. All nurses and physicians caring for patients on the unit, as well as the pharmacist, social worker, and case manager assigned to the unit, attended SIDR. Attendees used a structured communication tool to review patients admitted in the previous 24 hours. The plan of care for other patients was also discussed in SIDR, but without the use of the structured communication tool.
Importantly, nurse management underwent restructuring in the summer of 2011. Nurse managers, who had previously been responsible for overseeing all nursing activities on a single unit, were redeployed to be responsible for specific activities across 34 units. This restructuring made it very difficult for nurse managers to colead SIDR. As a result, the unit nurse clinical coordinator assumed coleadership of SIDR with the unit medical director. Nurse clinical coordinators worked every weekday and did not have patient care responsibilities while on duty. In addition to their role in coleading SIDR, nurse clinical coordinators addressed daily staffing and scheduling challenges and other short‐term patient care needs.
Teamwork Assessment
We adapted the Observational Teamwork Assessment for Surgery (OTAS) tool, a behaviorally anchored rating scale shown to be reliable and valid in surgical settings.2123 The OTAS tool provides scores ranging from 0 to 6 (0 = problematic behavior; 3 = team function neither hindered nor enhanced by behavior; 6 = exemplary behavior) across 5 domains (communication, coordination, cooperation/backup behavior, leadership, and monitoring/situational awareness) and for prespecified subteams. We defined domains as described by the researchers who developed OTAS. Communication referred to the quality and the quantity of information exchanged by team members. Coordination referred to management and timing of activities and tasks. Cooperation and backup behavior referred to assistance provided among members of the team, supporting others and correcting errors. Leadership referred to provision of directions, assertiveness, and support among team members. Monitoring and situational awareness referred to team observation and awareness of ongoing processes. We defined subteams for each group of professionals expected to attend SIDR. Specifically, subteams included physicians, nurses, social work‐case management (SW‐CM), pharmacy, and coleaders. We combined social work and case management because these professionals have similar patient care activities. Similarly, we combined unit medical directors and nurse clinical coordinators as coleaders. By providing data on teamwork performance within specific domains and for specific subteams, the OTAS instrument helps identify factors influencing overall teamwork performance. We modified OTAS anchors to reflect behaviors during SIDR. Anchors assisted observers in their rating of teamwork behaviors during SIDR. For example, an anchor for exemplary physician communication behavior was listens actively to other team members (looks at other team members, nods, etc). An anchor for exemplary physician leadership was assigns responsibility for task completion when appropriate.
Two researchers conducted unannounced direct observations of SIDRs. One researcher (Y.N.B) was a medical librarian with previous experience conducting observational research. The other researcher (A.J.C.) had observed 170 prior SIDRs as part of a related study. Both researchers observed 10 SIDRs to practice data collection and to inform minor revisions of the anchors. We aimed to conduct 78 independent observations for each unit, and 20 joint observations to assess inter‐rater reliability. All subteams were scored for each domain. For example, all subteams received leadership domain scores because all team members exhibit leadership behaviors, depending on the situation. In addition to teamwork scores, observers recorded the number of patients on the unit, the number of patients discussed during SIDR, attendance by subteam members, and the duration of SIDR. For the SW‐CM and coleader subteams, we documented presence if one of the subteam members was present for each patients' discussion. For example, we recorded present for SW‐CM if the social worker was in attendance but the case manager was not.
Data Analysis
We calculated descriptive statistics to characterize SIDRs. We used Spearman's rank correlation coefficients to assess inter‐rater reliability for joint observations. Spearman's rank correlation is a nonparametric test of association and appropriate for assessing agreement between observers when using data that is not normally distributed. Spearman rho values range from 1 to 1, with 1 signifying perfect inverse correlation, 0 signifying no correlation, and 1 signifying perfect correlation. We used the MannWhitney U test to assess for differences in overall team scores between services (teaching vs nonteaching hospitalist service) and KruskalWallis tests to assess for differences across units, domains, and subteams. The Kruskal‐Wallis test is a nonparametric test appropriate for comparing three or more independent samples in which the outcome is not normally distributed. We used a t test to assess for difference in duration by service, and Spearman rank correlation to assess for correlation between time spent in discussion per patient and overall team score. All analyses were conducted using Stata version 11.0 (College Station, TX).
RESULTS
SIDR Characteristics
We performed 7 direct observations of SIDR for 4 units, and 8 observations for 2 units (44 total observations). Units were at 99% capacity, and SIDR attendees discussed 98% of patients on the unit. Attendance exceeded 98% for each subteam (physicians, nurses, SW‐CM, pharmacy, and coleaders). SIDR lasted a mean 41.4 11.1 minutes, with a mean 1.5 0.4 minutes spent in discussion per patient. SIDR was significantly longer in duration on teaching service units compared to the nonteaching hospitalist service units (1.7 0.3 vs 1.3 0.4 minutes per patient; P < 0.001).
Inter‐Rater Reliability
Inter‐rater reliability across unit level scores was excellent (rho = 0.75). As shown in Table 1, inter‐rater reliability across domains was good (rho = 0.530.68). Inter‐rater reliability across subteams was good to excellent (rho = 0.530.76) with the exception of the physician subteam, for which it was poor (rho = 0.35).
| Spearman's rho | P Value | |
|---|---|---|
| ||
| Domain (n = 20) | ||
| Communication | 0.62 | <0.01 |
| Coordination | 0.60 | <0.01 |
| Cooperation/backup behavior | 0.66 | <0.01 |
| Leadership | 0.68 | <0.01 |
| Monitoring/situational awareness | 0.53 | 0.02 |
| Subteam (n = 20) | ||
| Physicians | 0.35 | 0.14 |
| Nurses | 0.53 | 0.02 |
| SW‐CM | 0.60 | <0.01 |
| Pharmacy | 0.76 | <0.01 |
| Coleaders | 0.68 | <0.01 |
Assessment of Teamwork by Unit, Domain, and Subteams
Teaching and nonteaching hospitalist units had similar team scores (median [interquartile range (IQR)] = 5.2 [1.0] vs 5.2 [0.4]; P = 0.55). We found significant differences in teamwork scores across units and domains, and found differences of borderline statistical significance across subteams (see Table 2). For unit teamwork scores, the median (IQR) was 4.4 (3.94.9) for the lowest and 5.4 (5.35.5) for the highest performing unit (P = 0.008). Across domain scores, leadership received the lowest score (median [IQR] = 5.0 [4.65.3]), and cooperation/backup behavior and monitoring/situational awareness received the highest scores (median [IQR]) = 5.4 [5.05.5] and 5.4 [5.05.7]; P = 0.02). Subteam scores ranged from a median (IQR) 5.0 (4.45.8) for coleaders to 5.5 (5.05.8) for SW‐CM (P = 0.05). We found no relationship between unit teamwork score and time spent in discussion per patient (rho = 0.04; P = 0.79).
| Median (IQR) | P Value | |
|---|---|---|
| ||
| Unit (n = 44)* | ||
| A | 5.3 (5.15.4) | 0.008 |
| B | 5.4 (5.35.5) | |
| C | 5.1 (4.95.2) | |
| D | 5.4 (5.25.6) | |
| E | 4.4 (3.94.9) | |
| F | 5.3 (5.15.5) | |
| Domain (n = 44) | ||
| Communication | 5.2 (4.95.4) | 0.02 |
| Coordination | 5.2 (4.75.4) | |
| Cooperation/backup behavior | 5.4 (5.05.5) | |
| Leadership | 5.0 (4.65.3) | |
| Monitoring/situational awareness | 5.4 (5.05.7) | |
| Subteam (n = 44) | ||
| Physicians | 5.2 (4.95.4) | 0.05 |
| Nurses | 5.2 (5.05.4) | |
| SW‐CM | 5.5 (5.05.8) | |
| Pharmacy | 5.3 (4.85.8) | |
| Coleaders | 5.0 (4.45.8) | |
DISCUSSION
We found that the adapted OTAS instrument demonstrated acceptable reliability for assessing teamwork during SIDR across units, domains, and most subteams. Although teamwork scores during SIDR were generally high, we found variation in performance across units, domains, and subteams. Variation in performance is notable in light of our efforts to implement a consistent format for SIDR across units. Specifically, all units have similar timing, duration, frequency, and location of SIDR, use a structured communication tool for new patients, expect the same professions to be represented, and use coleaders to facilitate discussion. We believe teamwork within IDR likely varies across units in other hospitals, and perhaps to a larger degree, given the emphasis on purposeful design and implementation of SIDR in our hospital.
Our findings are important for several reasons. First, though commonly used in hospital settings, the effectiveness of IDR is seldom assessed. Hospitalists and other professionals may not be able to identify or characterize deficiencies in teamwork during IDR without objective assessment. The adapted OTAS instrument provides a useful tool to evaluate team performance during IDR. Second, professionals may conclude that the mere implementation of an intervention such as SIDR will improve teamwork ratings and improve patient safety. Importantly, published studies evaluating the benefits of SIDR reflected a pilot study occurring on 2 units.15, 16, 19 The current study emphasizes the need to ensure that interventions proven to be effective on a small scale are implemented consistently when put into place on a larger scale.
Despite good reliability for assessing teamwork during SIDR across units, domains, and most subteams, we found poor inter‐rater reliability for the physician subteam. The explanation for this finding is not entirely clear. We reviewed the anchors for the physician subteam behaviors and were unable to identify ambiguity in anchor definitions. An analysis of domain scores within the physician subteam did not reveal any specific pattern to explain the poor correlation.
We found that the leadership domain and coleader subteam received particularly low scores. The explanation for this finding likely relates to changes in the nurse management structure shortly before our study, which reduced attendance by nurse managers and created a need for clinical coordinators to take on a leadership role during SIDR. Although we provided simulation‐based training to unit medical directors and nurse managers prior to implementing SIDR in March 2010, clinical coordinators were not part of the initial training. Our study suggests a need to provide additional training to coleaders, including clinical coordinators, to enhance their ability to facilitate discussion in SIDR.
We found no difference in overall teamwork scores when comparing teaching service units to nonteaching hospitalist service units. Duration of SIDR was significantly longer on teaching service units, but there was no association between duration of discussion and overall team score. The difference in duration of SIDR is likely explained by less succinct discussions on the part of housestaff physicians compared to more experienced hospitalists. Importantly, the quality of input, and its impact on teamwork during SIDR, does not appear to suffer when physician discussion is less efficient.
Our study has several limitations. First, we evaluated IDR in a single, urban, academic institution, which may limit generalizability. Our version of IDR (ie, SIDR) was designed to improve teamwork and incorporate a structured communication tool with regularly held interdisciplinary meetings. Features of IDR may differ in other hospitals. Second, the high teamwork scores seen in our study may not be generalizable to hospitals which have used a less rigorous, less standardized approach to IDR. Third, SIDR did not include patients or caregivers. Research is needed to test strategies to include patients and caregivers as active team members and participants in clinical decisions during hospitalization. Finally, we used the term interdisciplinary rounds to be consistent with prior published research. The term interprofessional may be more appropriate, as it specifically describes interactions among members of different professions (eg, physicians, nurses, social workers) rather than among different disciplines within a profession (eg, cardiologists, hospitalists, surgeons).
In summary, we found that teamwork during IDR could be reliably assessed using an adapted OTAS instrument. Although scores were generally high, we found variation in performance across units and domains suggesting a need to improve consistency of teamwork performance across units, domains, and subteams. Our study fills an important gap in the literature. Although IDR is commonly used in hospitals, and research shows improvements in ratings of collaboration,11, 12 little if any research has evaluated teamwork during IDR. Beyond the mere implementation of IDR, our study suggests the need to confirm that teamwork is optimal and consistent. Furthermore, hospital leaders should consider specific training for clinicians leading discussion during IDR.
Acknowledgements
The authors express their gratitude to Nick Sevdalis, BSc, MSc, PhD for providing the OTAS instrument and detailed instructions on its use.
Disclosures: Dr O'Leary, Ms Creden, and Dr Williams received salary support from the Agency for Healthcare Research and Quality, grant R18 HS019630. All authors disclose no other relevant or financial conflicts of interest.
- Joint Commission on Accreditation of Healthcare Organizations. Sentinel Event Statistics. Available at: http://www.jointcommission.org/SentinelEvents/Statistics/. Accessed January 19,2012.
- ,,, et al.A look into the nature and causes of human errors in the intensive care unit.Crit Care Med.1995;23(2):294–300.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324(6):377–384.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- ,,,,,.The Quality in Australian Health Care Study.Med J Aust.1995;163(9):458–471.
- ,,,,,.Teamwork on inpatient medical units: assessing attitudes and barriers.Qual Saf Health Care.2010;19(2):117–121.
- ,,,,,.Can we talk? Priorities for patient care differed among health care providers. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds.Advances in Patient Safety: From Research to Implementation. Vol1: Research Findings. AHRQ Publication No. 05–0021‐1.Rockville, MD:Agency for Healthcare Research and Quality;2005.
- ,,, et al.Patterns of nurse‐physician communication and agreement on the plan of care.Qual Saf Health Care.2010;19(3):195–199.
- ,,, et al.The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs.J Nurs Adm.2006;36(2):79–85.
- ,,.A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement.Med Care.1998;36(8 suppl):AS4–AS12.
- ,,,.Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses.Am J Crit Care.2005;14(1):71–77.
- ,,,,.Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay.J Gen Intern Med.2007;22(8):1073–1079.
- ,,,.Effects of interdisciplinary rounds on length of stay in a telemetry unit.J Public Health Manag Pract.2004;10(1):63–69.
- ,,,,.Teamwork and team performance in multidisciplinary cancer teams: development and evaluation of an observational assessment tool.BMJ Qual Saf.2011 [Epub ahead of print].
- ,,,,,.Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit.J Hosp Med.2011;6(2):88–93.
- ,,,,,.Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit.J Gen Intern Med.2010;25(8):826–832.
- ,,,,.Improving nurse‐physician communication and satisfaction in the intensive care unit with a daily goals worksheet.Am J Crit Care.2006;15(2):217–222.
- ,,,,,.Improving communication in the ICU using daily goals.J Crit Care.2003;18(2):71–75.
- ,,, et al.Structured interdisciplinary rounds in a medical teaching unit: improving patient safety.Arch Intern Med.2011;171(7):678–684.
- ,,, et al.Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care.J Gen Intern Med.2009;24(11):1223–1227.
- ,,,,.Observational teamwork assessment for surgery (OTAS): refinement and application in urological surgery.World J Surg.2007;31(7):1373–1381.
- ,,,,,.Observational teamwork assessment for surgery: construct validation with expert versus novice raters.Ann Surg.2009;249(6):1047–1051.
- ,,,,.Observational teamwork assessment for surgery: content validation and tool refinement.J Am Coll Surg.2011;212(2):234–243.e1–5.
Teamwork is essential to delivering safe and effective hospital care,15 yet the fluidity and geographic dispersion of team members in the hospital setting presents a significant barrier to teamwork.6 Physicians, nurses, and other hospital professionals frequently lack convenient and reliable opportunities to interact, and may struggle in efforts to discuss the care of their patients in person. Research studies show that nurses and physicians on patient care units do not communicate consistently and frequently do not agree on key aspects of their patients' plans of care.7, 8
Interdisciplinary rounds (IDR), also known as multidisciplinary rounds, provide a means to assemble hospital care team members and improve collaboration.913 Prior research on the use of IDR has demonstrated improved ratings of collaboration,11, 12 but inconsistent effects on length of stay and cost.10, 12, 13 Notably, the format, frequency, and duration of IDR in prior studies has been variable and no studies, to our knowledge, have evaluated teamwork performance during IDR. Lamb and colleagues conducted observations of cancer teams during multidisciplinary meetings.14 Trained observers used a validated observation tool to rate teamwork and found significant variation in performance by subteams. However, the study focused mainly on discussion among physician team members during meetings to plan longitudinal care for oncology patients.
We recently reported on the use of structured interdisciplinary rounds (SIDR) on 2 medical units in our hospital.15, 16 SIDR combines a structured format for communication, similar to a goals‐of‐care form,17, 18 with a forum for daily interdisciplinary meetings. Though no effect was seen on length of stay or cost, SIDR was associated with significantly higher ratings of the quality of collaboration and teamwork climate, and a reduction in the rate of adverse events.19 In March 2010, we implemented SIDR across all medical units in our hospital. We subjectively noted variation in teamwork performance during SIDR after a modification of nurse manager roles. We sought to evaluate teamwork during SIDR and to determine whether variation in performance existed and, if present, to characterize it.
METHODS
Setting and Study Design
The study was conducted at Northwestern Memorial Hospital (NMH), a 920‐bed tertiary care teaching hospital in Chicago, IL, and was deemed exempt by the Institutional Review Board of Northwestern University. General medical patients were admitted to 1 of 6 units based on bed availability. Five of the medical units consisted of 30 beds, and 1 unit consisted of 23. Each unit was equipped with continuous cardiac telemetry monitoring. Three units were staffed by teaching service physician teams consisting of 1 attending, 1 resident, and 1 or 2 interns. The other 3 units were staffed by hospitalists without the assistance of resident or intern physicians. As a result of a prior intervention, physicians' patients were localized to specific units in an effort to improve communication practices among nurses and physicians.20
Beginning in March 2010, all general medical units held SIDR each weekday morning. SIDR took place in the unit conference room, was expected to last approximately 3040 minutes, and was co‐led by the unit nurse manager and a medical director. Unit nurse managers and medical directors received specific training for their roles, including 3 hours of simulation‐based exercises designed to enhance their skills in facilitating discussion during SIDR. All nurses and physicians caring for patients on the unit, as well as the pharmacist, social worker, and case manager assigned to the unit, attended SIDR. Attendees used a structured communication tool to review patients admitted in the previous 24 hours. The plan of care for other patients was also discussed in SIDR, but without the use of the structured communication tool.
Importantly, nurse management underwent restructuring in the summer of 2011. Nurse managers, who had previously been responsible for overseeing all nursing activities on a single unit, were redeployed to be responsible for specific activities across 34 units. This restructuring made it very difficult for nurse managers to colead SIDR. As a result, the unit nurse clinical coordinator assumed coleadership of SIDR with the unit medical director. Nurse clinical coordinators worked every weekday and did not have patient care responsibilities while on duty. In addition to their role in coleading SIDR, nurse clinical coordinators addressed daily staffing and scheduling challenges and other short‐term patient care needs.
Teamwork Assessment
We adapted the Observational Teamwork Assessment for Surgery (OTAS) tool, a behaviorally anchored rating scale shown to be reliable and valid in surgical settings.2123 The OTAS tool provides scores ranging from 0 to 6 (0 = problematic behavior; 3 = team function neither hindered nor enhanced by behavior; 6 = exemplary behavior) across 5 domains (communication, coordination, cooperation/backup behavior, leadership, and monitoring/situational awareness) and for prespecified subteams. We defined domains as described by the researchers who developed OTAS. Communication referred to the quality and the quantity of information exchanged by team members. Coordination referred to management and timing of activities and tasks. Cooperation and backup behavior referred to assistance provided among members of the team, supporting others and correcting errors. Leadership referred to provision of directions, assertiveness, and support among team members. Monitoring and situational awareness referred to team observation and awareness of ongoing processes. We defined subteams for each group of professionals expected to attend SIDR. Specifically, subteams included physicians, nurses, social work‐case management (SW‐CM), pharmacy, and coleaders. We combined social work and case management because these professionals have similar patient care activities. Similarly, we combined unit medical directors and nurse clinical coordinators as coleaders. By providing data on teamwork performance within specific domains and for specific subteams, the OTAS instrument helps identify factors influencing overall teamwork performance. We modified OTAS anchors to reflect behaviors during SIDR. Anchors assisted observers in their rating of teamwork behaviors during SIDR. For example, an anchor for exemplary physician communication behavior was listens actively to other team members (looks at other team members, nods, etc). An anchor for exemplary physician leadership was assigns responsibility for task completion when appropriate.
Two researchers conducted unannounced direct observations of SIDRs. One researcher (Y.N.B) was a medical librarian with previous experience conducting observational research. The other researcher (A.J.C.) had observed 170 prior SIDRs as part of a related study. Both researchers observed 10 SIDRs to practice data collection and to inform minor revisions of the anchors. We aimed to conduct 78 independent observations for each unit, and 20 joint observations to assess inter‐rater reliability. All subteams were scored for each domain. For example, all subteams received leadership domain scores because all team members exhibit leadership behaviors, depending on the situation. In addition to teamwork scores, observers recorded the number of patients on the unit, the number of patients discussed during SIDR, attendance by subteam members, and the duration of SIDR. For the SW‐CM and coleader subteams, we documented presence if one of the subteam members was present for each patients' discussion. For example, we recorded present for SW‐CM if the social worker was in attendance but the case manager was not.
Data Analysis
We calculated descriptive statistics to characterize SIDRs. We used Spearman's rank correlation coefficients to assess inter‐rater reliability for joint observations. Spearman's rank correlation is a nonparametric test of association and appropriate for assessing agreement between observers when using data that is not normally distributed. Spearman rho values range from 1 to 1, with 1 signifying perfect inverse correlation, 0 signifying no correlation, and 1 signifying perfect correlation. We used the MannWhitney U test to assess for differences in overall team scores between services (teaching vs nonteaching hospitalist service) and KruskalWallis tests to assess for differences across units, domains, and subteams. The Kruskal‐Wallis test is a nonparametric test appropriate for comparing three or more independent samples in which the outcome is not normally distributed. We used a t test to assess for difference in duration by service, and Spearman rank correlation to assess for correlation between time spent in discussion per patient and overall team score. All analyses were conducted using Stata version 11.0 (College Station, TX).
RESULTS
SIDR Characteristics
We performed 7 direct observations of SIDR for 4 units, and 8 observations for 2 units (44 total observations). Units were at 99% capacity, and SIDR attendees discussed 98% of patients on the unit. Attendance exceeded 98% for each subteam (physicians, nurses, SW‐CM, pharmacy, and coleaders). SIDR lasted a mean 41.4 11.1 minutes, with a mean 1.5 0.4 minutes spent in discussion per patient. SIDR was significantly longer in duration on teaching service units compared to the nonteaching hospitalist service units (1.7 0.3 vs 1.3 0.4 minutes per patient; P < 0.001).
Inter‐Rater Reliability
Inter‐rater reliability across unit level scores was excellent (rho = 0.75). As shown in Table 1, inter‐rater reliability across domains was good (rho = 0.530.68). Inter‐rater reliability across subteams was good to excellent (rho = 0.530.76) with the exception of the physician subteam, for which it was poor (rho = 0.35).
| Spearman's rho | P Value | |
|---|---|---|
| ||
| Domain (n = 20) | ||
| Communication | 0.62 | <0.01 |
| Coordination | 0.60 | <0.01 |
| Cooperation/backup behavior | 0.66 | <0.01 |
| Leadership | 0.68 | <0.01 |
| Monitoring/situational awareness | 0.53 | 0.02 |
| Subteam (n = 20) | ||
| Physicians | 0.35 | 0.14 |
| Nurses | 0.53 | 0.02 |
| SW‐CM | 0.60 | <0.01 |
| Pharmacy | 0.76 | <0.01 |
| Coleaders | 0.68 | <0.01 |
Assessment of Teamwork by Unit, Domain, and Subteams
Teaching and nonteaching hospitalist units had similar team scores (median [interquartile range (IQR)] = 5.2 [1.0] vs 5.2 [0.4]; P = 0.55). We found significant differences in teamwork scores across units and domains, and found differences of borderline statistical significance across subteams (see Table 2). For unit teamwork scores, the median (IQR) was 4.4 (3.94.9) for the lowest and 5.4 (5.35.5) for the highest performing unit (P = 0.008). Across domain scores, leadership received the lowest score (median [IQR] = 5.0 [4.65.3]), and cooperation/backup behavior and monitoring/situational awareness received the highest scores (median [IQR]) = 5.4 [5.05.5] and 5.4 [5.05.7]; P = 0.02). Subteam scores ranged from a median (IQR) 5.0 (4.45.8) for coleaders to 5.5 (5.05.8) for SW‐CM (P = 0.05). We found no relationship between unit teamwork score and time spent in discussion per patient (rho = 0.04; P = 0.79).
| Median (IQR) | P Value | |
|---|---|---|
| ||
| Unit (n = 44)* | ||
| A | 5.3 (5.15.4) | 0.008 |
| B | 5.4 (5.35.5) | |
| C | 5.1 (4.95.2) | |
| D | 5.4 (5.25.6) | |
| E | 4.4 (3.94.9) | |
| F | 5.3 (5.15.5) | |
| Domain (n = 44) | ||
| Communication | 5.2 (4.95.4) | 0.02 |
| Coordination | 5.2 (4.75.4) | |
| Cooperation/backup behavior | 5.4 (5.05.5) | |
| Leadership | 5.0 (4.65.3) | |
| Monitoring/situational awareness | 5.4 (5.05.7) | |
| Subteam (n = 44) | ||
| Physicians | 5.2 (4.95.4) | 0.05 |
| Nurses | 5.2 (5.05.4) | |
| SW‐CM | 5.5 (5.05.8) | |
| Pharmacy | 5.3 (4.85.8) | |
| Coleaders | 5.0 (4.45.8) | |
DISCUSSION
We found that the adapted OTAS instrument demonstrated acceptable reliability for assessing teamwork during SIDR across units, domains, and most subteams. Although teamwork scores during SIDR were generally high, we found variation in performance across units, domains, and subteams. Variation in performance is notable in light of our efforts to implement a consistent format for SIDR across units. Specifically, all units have similar timing, duration, frequency, and location of SIDR, use a structured communication tool for new patients, expect the same professions to be represented, and use coleaders to facilitate discussion. We believe teamwork within IDR likely varies across units in other hospitals, and perhaps to a larger degree, given the emphasis on purposeful design and implementation of SIDR in our hospital.
Our findings are important for several reasons. First, though commonly used in hospital settings, the effectiveness of IDR is seldom assessed. Hospitalists and other professionals may not be able to identify or characterize deficiencies in teamwork during IDR without objective assessment. The adapted OTAS instrument provides a useful tool to evaluate team performance during IDR. Second, professionals may conclude that the mere implementation of an intervention such as SIDR will improve teamwork ratings and improve patient safety. Importantly, published studies evaluating the benefits of SIDR reflected a pilot study occurring on 2 units.15, 16, 19 The current study emphasizes the need to ensure that interventions proven to be effective on a small scale are implemented consistently when put into place on a larger scale.
Despite good reliability for assessing teamwork during SIDR across units, domains, and most subteams, we found poor inter‐rater reliability for the physician subteam. The explanation for this finding is not entirely clear. We reviewed the anchors for the physician subteam behaviors and were unable to identify ambiguity in anchor definitions. An analysis of domain scores within the physician subteam did not reveal any specific pattern to explain the poor correlation.
We found that the leadership domain and coleader subteam received particularly low scores. The explanation for this finding likely relates to changes in the nurse management structure shortly before our study, which reduced attendance by nurse managers and created a need for clinical coordinators to take on a leadership role during SIDR. Although we provided simulation‐based training to unit medical directors and nurse managers prior to implementing SIDR in March 2010, clinical coordinators were not part of the initial training. Our study suggests a need to provide additional training to coleaders, including clinical coordinators, to enhance their ability to facilitate discussion in SIDR.
We found no difference in overall teamwork scores when comparing teaching service units to nonteaching hospitalist service units. Duration of SIDR was significantly longer on teaching service units, but there was no association between duration of discussion and overall team score. The difference in duration of SIDR is likely explained by less succinct discussions on the part of housestaff physicians compared to more experienced hospitalists. Importantly, the quality of input, and its impact on teamwork during SIDR, does not appear to suffer when physician discussion is less efficient.
Our study has several limitations. First, we evaluated IDR in a single, urban, academic institution, which may limit generalizability. Our version of IDR (ie, SIDR) was designed to improve teamwork and incorporate a structured communication tool with regularly held interdisciplinary meetings. Features of IDR may differ in other hospitals. Second, the high teamwork scores seen in our study may not be generalizable to hospitals which have used a less rigorous, less standardized approach to IDR. Third, SIDR did not include patients or caregivers. Research is needed to test strategies to include patients and caregivers as active team members and participants in clinical decisions during hospitalization. Finally, we used the term interdisciplinary rounds to be consistent with prior published research. The term interprofessional may be more appropriate, as it specifically describes interactions among members of different professions (eg, physicians, nurses, social workers) rather than among different disciplines within a profession (eg, cardiologists, hospitalists, surgeons).
In summary, we found that teamwork during IDR could be reliably assessed using an adapted OTAS instrument. Although scores were generally high, we found variation in performance across units and domains suggesting a need to improve consistency of teamwork performance across units, domains, and subteams. Our study fills an important gap in the literature. Although IDR is commonly used in hospitals, and research shows improvements in ratings of collaboration,11, 12 little if any research has evaluated teamwork during IDR. Beyond the mere implementation of IDR, our study suggests the need to confirm that teamwork is optimal and consistent. Furthermore, hospital leaders should consider specific training for clinicians leading discussion during IDR.
Acknowledgements
The authors express their gratitude to Nick Sevdalis, BSc, MSc, PhD for providing the OTAS instrument and detailed instructions on its use.
Disclosures: Dr O'Leary, Ms Creden, and Dr Williams received salary support from the Agency for Healthcare Research and Quality, grant R18 HS019630. All authors disclose no other relevant or financial conflicts of interest.
Teamwork is essential to delivering safe and effective hospital care,15 yet the fluidity and geographic dispersion of team members in the hospital setting presents a significant barrier to teamwork.6 Physicians, nurses, and other hospital professionals frequently lack convenient and reliable opportunities to interact, and may struggle in efforts to discuss the care of their patients in person. Research studies show that nurses and physicians on patient care units do not communicate consistently and frequently do not agree on key aspects of their patients' plans of care.7, 8
Interdisciplinary rounds (IDR), also known as multidisciplinary rounds, provide a means to assemble hospital care team members and improve collaboration.913 Prior research on the use of IDR has demonstrated improved ratings of collaboration,11, 12 but inconsistent effects on length of stay and cost.10, 12, 13 Notably, the format, frequency, and duration of IDR in prior studies has been variable and no studies, to our knowledge, have evaluated teamwork performance during IDR. Lamb and colleagues conducted observations of cancer teams during multidisciplinary meetings.14 Trained observers used a validated observation tool to rate teamwork and found significant variation in performance by subteams. However, the study focused mainly on discussion among physician team members during meetings to plan longitudinal care for oncology patients.
We recently reported on the use of structured interdisciplinary rounds (SIDR) on 2 medical units in our hospital.15, 16 SIDR combines a structured format for communication, similar to a goals‐of‐care form,17, 18 with a forum for daily interdisciplinary meetings. Though no effect was seen on length of stay or cost, SIDR was associated with significantly higher ratings of the quality of collaboration and teamwork climate, and a reduction in the rate of adverse events.19 In March 2010, we implemented SIDR across all medical units in our hospital. We subjectively noted variation in teamwork performance during SIDR after a modification of nurse manager roles. We sought to evaluate teamwork during SIDR and to determine whether variation in performance existed and, if present, to characterize it.
METHODS
Setting and Study Design
The study was conducted at Northwestern Memorial Hospital (NMH), a 920‐bed tertiary care teaching hospital in Chicago, IL, and was deemed exempt by the Institutional Review Board of Northwestern University. General medical patients were admitted to 1 of 6 units based on bed availability. Five of the medical units consisted of 30 beds, and 1 unit consisted of 23. Each unit was equipped with continuous cardiac telemetry monitoring. Three units were staffed by teaching service physician teams consisting of 1 attending, 1 resident, and 1 or 2 interns. The other 3 units were staffed by hospitalists without the assistance of resident or intern physicians. As a result of a prior intervention, physicians' patients were localized to specific units in an effort to improve communication practices among nurses and physicians.20
Beginning in March 2010, all general medical units held SIDR each weekday morning. SIDR took place in the unit conference room, was expected to last approximately 3040 minutes, and was co‐led by the unit nurse manager and a medical director. Unit nurse managers and medical directors received specific training for their roles, including 3 hours of simulation‐based exercises designed to enhance their skills in facilitating discussion during SIDR. All nurses and physicians caring for patients on the unit, as well as the pharmacist, social worker, and case manager assigned to the unit, attended SIDR. Attendees used a structured communication tool to review patients admitted in the previous 24 hours. The plan of care for other patients was also discussed in SIDR, but without the use of the structured communication tool.
Importantly, nurse management underwent restructuring in the summer of 2011. Nurse managers, who had previously been responsible for overseeing all nursing activities on a single unit, were redeployed to be responsible for specific activities across 34 units. This restructuring made it very difficult for nurse managers to colead SIDR. As a result, the unit nurse clinical coordinator assumed coleadership of SIDR with the unit medical director. Nurse clinical coordinators worked every weekday and did not have patient care responsibilities while on duty. In addition to their role in coleading SIDR, nurse clinical coordinators addressed daily staffing and scheduling challenges and other short‐term patient care needs.
Teamwork Assessment
We adapted the Observational Teamwork Assessment for Surgery (OTAS) tool, a behaviorally anchored rating scale shown to be reliable and valid in surgical settings.2123 The OTAS tool provides scores ranging from 0 to 6 (0 = problematic behavior; 3 = team function neither hindered nor enhanced by behavior; 6 = exemplary behavior) across 5 domains (communication, coordination, cooperation/backup behavior, leadership, and monitoring/situational awareness) and for prespecified subteams. We defined domains as described by the researchers who developed OTAS. Communication referred to the quality and the quantity of information exchanged by team members. Coordination referred to management and timing of activities and tasks. Cooperation and backup behavior referred to assistance provided among members of the team, supporting others and correcting errors. Leadership referred to provision of directions, assertiveness, and support among team members. Monitoring and situational awareness referred to team observation and awareness of ongoing processes. We defined subteams for each group of professionals expected to attend SIDR. Specifically, subteams included physicians, nurses, social work‐case management (SW‐CM), pharmacy, and coleaders. We combined social work and case management because these professionals have similar patient care activities. Similarly, we combined unit medical directors and nurse clinical coordinators as coleaders. By providing data on teamwork performance within specific domains and for specific subteams, the OTAS instrument helps identify factors influencing overall teamwork performance. We modified OTAS anchors to reflect behaviors during SIDR. Anchors assisted observers in their rating of teamwork behaviors during SIDR. For example, an anchor for exemplary physician communication behavior was listens actively to other team members (looks at other team members, nods, etc). An anchor for exemplary physician leadership was assigns responsibility for task completion when appropriate.
Two researchers conducted unannounced direct observations of SIDRs. One researcher (Y.N.B) was a medical librarian with previous experience conducting observational research. The other researcher (A.J.C.) had observed 170 prior SIDRs as part of a related study. Both researchers observed 10 SIDRs to practice data collection and to inform minor revisions of the anchors. We aimed to conduct 78 independent observations for each unit, and 20 joint observations to assess inter‐rater reliability. All subteams were scored for each domain. For example, all subteams received leadership domain scores because all team members exhibit leadership behaviors, depending on the situation. In addition to teamwork scores, observers recorded the number of patients on the unit, the number of patients discussed during SIDR, attendance by subteam members, and the duration of SIDR. For the SW‐CM and coleader subteams, we documented presence if one of the subteam members was present for each patients' discussion. For example, we recorded present for SW‐CM if the social worker was in attendance but the case manager was not.
Data Analysis
We calculated descriptive statistics to characterize SIDRs. We used Spearman's rank correlation coefficients to assess inter‐rater reliability for joint observations. Spearman's rank correlation is a nonparametric test of association and appropriate for assessing agreement between observers when using data that is not normally distributed. Spearman rho values range from 1 to 1, with 1 signifying perfect inverse correlation, 0 signifying no correlation, and 1 signifying perfect correlation. We used the MannWhitney U test to assess for differences in overall team scores between services (teaching vs nonteaching hospitalist service) and KruskalWallis tests to assess for differences across units, domains, and subteams. The Kruskal‐Wallis test is a nonparametric test appropriate for comparing three or more independent samples in which the outcome is not normally distributed. We used a t test to assess for difference in duration by service, and Spearman rank correlation to assess for correlation between time spent in discussion per patient and overall team score. All analyses were conducted using Stata version 11.0 (College Station, TX).
RESULTS
SIDR Characteristics
We performed 7 direct observations of SIDR for 4 units, and 8 observations for 2 units (44 total observations). Units were at 99% capacity, and SIDR attendees discussed 98% of patients on the unit. Attendance exceeded 98% for each subteam (physicians, nurses, SW‐CM, pharmacy, and coleaders). SIDR lasted a mean 41.4 11.1 minutes, with a mean 1.5 0.4 minutes spent in discussion per patient. SIDR was significantly longer in duration on teaching service units compared to the nonteaching hospitalist service units (1.7 0.3 vs 1.3 0.4 minutes per patient; P < 0.001).
Inter‐Rater Reliability
Inter‐rater reliability across unit level scores was excellent (rho = 0.75). As shown in Table 1, inter‐rater reliability across domains was good (rho = 0.530.68). Inter‐rater reliability across subteams was good to excellent (rho = 0.530.76) with the exception of the physician subteam, for which it was poor (rho = 0.35).
| Spearman's rho | P Value | |
|---|---|---|
| ||
| Domain (n = 20) | ||
| Communication | 0.62 | <0.01 |
| Coordination | 0.60 | <0.01 |
| Cooperation/backup behavior | 0.66 | <0.01 |
| Leadership | 0.68 | <0.01 |
| Monitoring/situational awareness | 0.53 | 0.02 |
| Subteam (n = 20) | ||
| Physicians | 0.35 | 0.14 |
| Nurses | 0.53 | 0.02 |
| SW‐CM | 0.60 | <0.01 |
| Pharmacy | 0.76 | <0.01 |
| Coleaders | 0.68 | <0.01 |
Assessment of Teamwork by Unit, Domain, and Subteams
Teaching and nonteaching hospitalist units had similar team scores (median [interquartile range (IQR)] = 5.2 [1.0] vs 5.2 [0.4]; P = 0.55). We found significant differences in teamwork scores across units and domains, and found differences of borderline statistical significance across subteams (see Table 2). For unit teamwork scores, the median (IQR) was 4.4 (3.94.9) for the lowest and 5.4 (5.35.5) for the highest performing unit (P = 0.008). Across domain scores, leadership received the lowest score (median [IQR] = 5.0 [4.65.3]), and cooperation/backup behavior and monitoring/situational awareness received the highest scores (median [IQR]) = 5.4 [5.05.5] and 5.4 [5.05.7]; P = 0.02). Subteam scores ranged from a median (IQR) 5.0 (4.45.8) for coleaders to 5.5 (5.05.8) for SW‐CM (P = 0.05). We found no relationship between unit teamwork score and time spent in discussion per patient (rho = 0.04; P = 0.79).
| Median (IQR) | P Value | |
|---|---|---|
| ||
| Unit (n = 44)* | ||
| A | 5.3 (5.15.4) | 0.008 |
| B | 5.4 (5.35.5) | |
| C | 5.1 (4.95.2) | |
| D | 5.4 (5.25.6) | |
| E | 4.4 (3.94.9) | |
| F | 5.3 (5.15.5) | |
| Domain (n = 44) | ||
| Communication | 5.2 (4.95.4) | 0.02 |
| Coordination | 5.2 (4.75.4) | |
| Cooperation/backup behavior | 5.4 (5.05.5) | |
| Leadership | 5.0 (4.65.3) | |
| Monitoring/situational awareness | 5.4 (5.05.7) | |
| Subteam (n = 44) | ||
| Physicians | 5.2 (4.95.4) | 0.05 |
| Nurses | 5.2 (5.05.4) | |
| SW‐CM | 5.5 (5.05.8) | |
| Pharmacy | 5.3 (4.85.8) | |
| Coleaders | 5.0 (4.45.8) | |
DISCUSSION
We found that the adapted OTAS instrument demonstrated acceptable reliability for assessing teamwork during SIDR across units, domains, and most subteams. Although teamwork scores during SIDR were generally high, we found variation in performance across units, domains, and subteams. Variation in performance is notable in light of our efforts to implement a consistent format for SIDR across units. Specifically, all units have similar timing, duration, frequency, and location of SIDR, use a structured communication tool for new patients, expect the same professions to be represented, and use coleaders to facilitate discussion. We believe teamwork within IDR likely varies across units in other hospitals, and perhaps to a larger degree, given the emphasis on purposeful design and implementation of SIDR in our hospital.
Our findings are important for several reasons. First, though commonly used in hospital settings, the effectiveness of IDR is seldom assessed. Hospitalists and other professionals may not be able to identify or characterize deficiencies in teamwork during IDR without objective assessment. The adapted OTAS instrument provides a useful tool to evaluate team performance during IDR. Second, professionals may conclude that the mere implementation of an intervention such as SIDR will improve teamwork ratings and improve patient safety. Importantly, published studies evaluating the benefits of SIDR reflected a pilot study occurring on 2 units.15, 16, 19 The current study emphasizes the need to ensure that interventions proven to be effective on a small scale are implemented consistently when put into place on a larger scale.
Despite good reliability for assessing teamwork during SIDR across units, domains, and most subteams, we found poor inter‐rater reliability for the physician subteam. The explanation for this finding is not entirely clear. We reviewed the anchors for the physician subteam behaviors and were unable to identify ambiguity in anchor definitions. An analysis of domain scores within the physician subteam did not reveal any specific pattern to explain the poor correlation.
We found that the leadership domain and coleader subteam received particularly low scores. The explanation for this finding likely relates to changes in the nurse management structure shortly before our study, which reduced attendance by nurse managers and created a need for clinical coordinators to take on a leadership role during SIDR. Although we provided simulation‐based training to unit medical directors and nurse managers prior to implementing SIDR in March 2010, clinical coordinators were not part of the initial training. Our study suggests a need to provide additional training to coleaders, including clinical coordinators, to enhance their ability to facilitate discussion in SIDR.
We found no difference in overall teamwork scores when comparing teaching service units to nonteaching hospitalist service units. Duration of SIDR was significantly longer on teaching service units, but there was no association between duration of discussion and overall team score. The difference in duration of SIDR is likely explained by less succinct discussions on the part of housestaff physicians compared to more experienced hospitalists. Importantly, the quality of input, and its impact on teamwork during SIDR, does not appear to suffer when physician discussion is less efficient.
Our study has several limitations. First, we evaluated IDR in a single, urban, academic institution, which may limit generalizability. Our version of IDR (ie, SIDR) was designed to improve teamwork and incorporate a structured communication tool with regularly held interdisciplinary meetings. Features of IDR may differ in other hospitals. Second, the high teamwork scores seen in our study may not be generalizable to hospitals which have used a less rigorous, less standardized approach to IDR. Third, SIDR did not include patients or caregivers. Research is needed to test strategies to include patients and caregivers as active team members and participants in clinical decisions during hospitalization. Finally, we used the term interdisciplinary rounds to be consistent with prior published research. The term interprofessional may be more appropriate, as it specifically describes interactions among members of different professions (eg, physicians, nurses, social workers) rather than among different disciplines within a profession (eg, cardiologists, hospitalists, surgeons).
In summary, we found that teamwork during IDR could be reliably assessed using an adapted OTAS instrument. Although scores were generally high, we found variation in performance across units and domains suggesting a need to improve consistency of teamwork performance across units, domains, and subteams. Our study fills an important gap in the literature. Although IDR is commonly used in hospitals, and research shows improvements in ratings of collaboration,11, 12 little if any research has evaluated teamwork during IDR. Beyond the mere implementation of IDR, our study suggests the need to confirm that teamwork is optimal and consistent. Furthermore, hospital leaders should consider specific training for clinicians leading discussion during IDR.
Acknowledgements
The authors express their gratitude to Nick Sevdalis, BSc, MSc, PhD for providing the OTAS instrument and detailed instructions on its use.
Disclosures: Dr O'Leary, Ms Creden, and Dr Williams received salary support from the Agency for Healthcare Research and Quality, grant R18 HS019630. All authors disclose no other relevant or financial conflicts of interest.
- Joint Commission on Accreditation of Healthcare Organizations. Sentinel Event Statistics. Available at: http://www.jointcommission.org/SentinelEvents/Statistics/. Accessed January 19,2012.
- ,,, et al.A look into the nature and causes of human errors in the intensive care unit.Crit Care Med.1995;23(2):294–300.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324(6):377–384.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- ,,,,,.The Quality in Australian Health Care Study.Med J Aust.1995;163(9):458–471.
- ,,,,,.Teamwork on inpatient medical units: assessing attitudes and barriers.Qual Saf Health Care.2010;19(2):117–121.
- ,,,,,.Can we talk? Priorities for patient care differed among health care providers. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds.Advances in Patient Safety: From Research to Implementation. Vol1: Research Findings. AHRQ Publication No. 05–0021‐1.Rockville, MD:Agency for Healthcare Research and Quality;2005.
- ,,, et al.Patterns of nurse‐physician communication and agreement on the plan of care.Qual Saf Health Care.2010;19(3):195–199.
- ,,, et al.The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs.J Nurs Adm.2006;36(2):79–85.
- ,,.A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement.Med Care.1998;36(8 suppl):AS4–AS12.
- ,,,.Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses.Am J Crit Care.2005;14(1):71–77.
- ,,,,.Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay.J Gen Intern Med.2007;22(8):1073–1079.
- ,,,.Effects of interdisciplinary rounds on length of stay in a telemetry unit.J Public Health Manag Pract.2004;10(1):63–69.
- ,,,,.Teamwork and team performance in multidisciplinary cancer teams: development and evaluation of an observational assessment tool.BMJ Qual Saf.2011 [Epub ahead of print].
- ,,,,,.Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit.J Hosp Med.2011;6(2):88–93.
- ,,,,,.Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit.J Gen Intern Med.2010;25(8):826–832.
- ,,,,.Improving nurse‐physician communication and satisfaction in the intensive care unit with a daily goals worksheet.Am J Crit Care.2006;15(2):217–222.
- ,,,,,.Improving communication in the ICU using daily goals.J Crit Care.2003;18(2):71–75.
- ,,, et al.Structured interdisciplinary rounds in a medical teaching unit: improving patient safety.Arch Intern Med.2011;171(7):678–684.
- ,,, et al.Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care.J Gen Intern Med.2009;24(11):1223–1227.
- ,,,,.Observational teamwork assessment for surgery (OTAS): refinement and application in urological surgery.World J Surg.2007;31(7):1373–1381.
- ,,,,,.Observational teamwork assessment for surgery: construct validation with expert versus novice raters.Ann Surg.2009;249(6):1047–1051.
- ,,,,.Observational teamwork assessment for surgery: content validation and tool refinement.J Am Coll Surg.2011;212(2):234–243.e1–5.
- Joint Commission on Accreditation of Healthcare Organizations. Sentinel Event Statistics. Available at: http://www.jointcommission.org/SentinelEvents/Statistics/. Accessed January 19,2012.
- ,,, et al.A look into the nature and causes of human errors in the intensive care unit.Crit Care Med.1995;23(2):294–300.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324(6):377–384.
- ,,.Communication failures: an insidious contributor to medical mishaps.Acad Med.2004;79(2):186–194.
- ,,,,,.The Quality in Australian Health Care Study.Med J Aust.1995;163(9):458–471.
- ,,,,,.Teamwork on inpatient medical units: assessing attitudes and barriers.Qual Saf Health Care.2010;19(2):117–121.
- ,,,,,.Can we talk? Priorities for patient care differed among health care providers. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds.Advances in Patient Safety: From Research to Implementation. Vol1: Research Findings. AHRQ Publication No. 05–0021‐1.Rockville, MD:Agency for Healthcare Research and Quality;2005.
- ,,, et al.Patterns of nurse‐physician communication and agreement on the plan of care.Qual Saf Health Care.2010;19(3):195–199.
- ,,, et al.The effect of a multidisciplinary hospitalist/physician and advanced practice nurse collaboration on hospital costs.J Nurs Adm.2006;36(2):79–85.
- ,,.A firm trial of interdisciplinary rounds on the inpatient medical wards: an intervention designed using continuous quality improvement.Med Care.1998;36(8 suppl):AS4–AS12.
- ,,,.Effect of a multidisciplinary intervention on communication and collaboration among physicians and nurses.Am J Crit Care.2005;14(1):71–77.
- ,,,,.Use of multidisciplinary rounds to simultaneously improve quality outcomes, enhance resident education, and shorten length of stay.J Gen Intern Med.2007;22(8):1073–1079.
- ,,,.Effects of interdisciplinary rounds on length of stay in a telemetry unit.J Public Health Manag Pract.2004;10(1):63–69.
- ,,,,.Teamwork and team performance in multidisciplinary cancer teams: development and evaluation of an observational assessment tool.BMJ Qual Saf.2011 [Epub ahead of print].
- ,,,,,.Improving teamwork: impact of structured interdisciplinary rounds on a hospitalist unit.J Hosp Med.2011;6(2):88–93.
- ,,,,,.Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit.J Gen Intern Med.2010;25(8):826–832.
- ,,,,.Improving nurse‐physician communication and satisfaction in the intensive care unit with a daily goals worksheet.Am J Crit Care.2006;15(2):217–222.
- ,,,,,.Improving communication in the ICU using daily goals.J Crit Care.2003;18(2):71–75.
- ,,, et al.Structured interdisciplinary rounds in a medical teaching unit: improving patient safety.Arch Intern Med.2011;171(7):678–684.
- ,,, et al.Impact of localizing physicians to hospital units on nurse‐physician communication and agreement on the plan of care.J Gen Intern Med.2009;24(11):1223–1227.
- ,,,,.Observational teamwork assessment for surgery (OTAS): refinement and application in urological surgery.World J Surg.2007;31(7):1373–1381.
- ,,,,,.Observational teamwork assessment for surgery: construct validation with expert versus novice raters.Ann Surg.2009;249(6):1047–1051.
- ,,,,.Observational teamwork assessment for surgery: content validation and tool refinement.J Am Coll Surg.2011;212(2):234–243.e1–5.
Copyright © 2012 Society of Hospital Medicine
Hospitalist‐Run Preoperative Clinic
Anesthesiologists typically initiate an assessment in the immediate preoperative period, focused on management of the airway, physiologic parameters, and choice of anesthetic. Given the growing complexity of medical issues in the surgical patient, the preoperative assessment may need to be initiated weeks to months prior to surgery. Early evaluation allows time to implement required interventions, optimize medical conditions, adjust medications, and collaborate with the surgical team.
Most studies of Preoperative clinics are in the Anesthesiology literature.1 Anesthesia‐run Preoperative clinics have demonstrated a reduction in surgical cancellations and length of stay (LOS).2 Auerbach and colleagues found medical consultation to have inconsistent effects on quality of care in surgical patients, but consultations occurred, at the earliest, 1 day prior to surgery.3 A randomized trial, performed at the Pittsburgh Veterans Administration (VA) medical center using an outpatient Internal Medicine Preoperative clinic, demonstrated a shortening of preoperative LOS but no change in total LOS, and increased use of consultants. However, there were reduced numbers of unnecessary admissions, defined as patients who were discharged without having had surgery.4 An analysis of a population‐based administrative database found that voluntary preoperative consultations were associated with a significant, albeit small, increase in mortality. Although this study used a matched cohort, the unmatched cohort that underwent consultation was higher risk; also, selection bias was possible, as the reasons for initial consultation were unknown.5
Historically, the Preoperative clinic at VA Greater Los Angeles Healthcare System (VAGLAHS) was supervised by the Department of Anesthesiology. In July 2004, the Preoperative clinic was restructured with Hospitalist oversight. The Anesthesia staff continued to evaluate all surgical patients, but did so only on the day of surgery, and after the patient was deemed an acceptable risk by the Preoperative clinic.
We undertook this study to measure the institutional impact of the addition of a Hospitalist‐run Preoperative clinic to our standard practice. The VA is an ideal setting, given the closed system with reliable longitudinal data. The VA electronic medical record also allows for comprehensive calculations of clinical covariants and outcomes.
MATERIALS AND METHODS
Setting
VAGLAHS is a tertiary care, academic medical center that serves patients referred from a 110,000 square mile area of Southern California and Southern Nevada. The Preoperative clinic evaluates all outpatients scheduled for inpatient or outpatient noncardiac surgery. Evaluations are performed by mid‐level providers with physician oversight. Patients are seen within 30 days of surgery, with a goal of 2 to 3 weeks prior to the operative date. Two of the 3 mid‐level providers remained after the change in leadership; a third was hired. All were retrained to perform a detailed medical preoperative assessment. Patients awaiting cardiothoracic surgery had their evaluation performed outside the Preoperative clinic by the Cardiology or Pulmonary services during both periods.
With the change in oversight, mid‐level providers were given weekly lectures on medical disease management and preoperative assessment. A syllabus of key articles in perioperative literature was compiled. Evidence‐based protocols were developed to standardize the evaluation. Examples of guidelines include: laboratory and radiological testing guidelines,610 initiation of perioperative beta blockers,11 selection criteria for pulmonary function tests,12 protocols for bridging with low‐molecular‐weight heparin for patients on oral vitamin K antagonists,13 the cardiovascular evaluation based on American College of Cardiology/American Heart Association (ACC/AHA) guidelines,14 as well as adjustment of diabetic medications.
Prior to the change in oversight, patients who required Cardiology evaluations were referred directly to the Cardiology service generally without any prior testing. After institution of the Hospitalist‐run clinic, the mid‐level providers ordered cardiac studies after discussion with the attending to ensure necessity and compliance with ACC/AHA guidelines. Patients were referred to Cardiology only if the results required further evaluation. In addition, entry to the Preoperative clinic was denied to patients awaiting elective surgeries whose hemoglobin A1c percentage was greater than 9%; such patients were referred to their primary care provider. For patients awaiting urgent surgeries, the Preoperative clinic would expedite evaluations in order to honor the surgical date. Providers would document perioperative recommendations for patients anticipated to require an inpatient stay. Occasionally, the patient was deemed too high risk to proceed with surgery, and the case was canceled or delayed after discussion with the patient and surgical team. Once deemed a medically acceptable candidate, the patient was evaluated on the day of surgery by Anesthesia.
Methods
We extracted de‐identified data from Veterans Health Administration (VHA) national databases, and specifically from the Veterans Integrated Service Network (VISN) 22 warehouse. All patients seen in the Preoperative clinic at VAGLAHS, from July 2003 to July 2005, were included. The patients were analyzed in 2 groups: patients seen from July 2003 to June 2004, when the Anesthesia Department staff supervised the Preoperative clinic (Period A); and from July 2004 to June 2005, the first year of the new Hospitalist‐run system (Period B). We collected data on age; gender; American Society of Anesthesia (ASA) score15; perioperative beta blocker use; cardiology studies ordered; and surgical mortality defined as death within the index hospital stay. The length of stay (LOS) was calculated for patients who required an inpatient stay after surgery. As an internal control, we assessed the LOS of the cardiothoracic patients in our facility since this group of patients does not utilize the Preoperative clinic and maintained the same preoperative evaluation process during both time periods. In addition, same‐day surgical cancellations were tracked by the Anesthesia Department, which documents daily operating room utilization and determines whether a cancellation was avoidable.
Statistical Analysis
Differences in demographic, clinical, and preoperative resource utilization characteristics were compared between Periods A and B using chi‐square for categorical variables and t test (or Wilcoxon test) for continuous variables. A subgroup analysis was performed for patients who required an inpatient stay after surgery. The primary outcome was inpatient LOS and the secondary outcome was inpatient death. A mixed‐effects regression model with patient‐level random effects to account for clustering of visits by the same patient was used to assess the impact of certain patient characteristics on inpatient LOS. Covariates included age, gender, time period (A vs B), ASA classification, and perioperative period‐by‐ASA classification interaction. Comparisons of inpatient LOS between periods for different ASA classes were done through model contrasts. Chi‐square test was used to compare the inpatient mortality between periods. A subgroup analysis was performed on postoperative inpatient deaths during the study period using a logistics regression model with age, ASA, and time period. All statistical analyses were performed using SAS Version 9.2 (SAS Institute, Cary, NC).
RESULTS
Table 1 describes the demographics and clinical characteristics of the patients evaluated in the Preoperative clinic. Number of surgeries performed in Periods A and B were 3568 and 3337, respectively, with an average of 1.3 surgeries per patient for both periods. The most common surgical specialties were Ophthalmology, Orthopedics, Urology, and General Surgery. The average ages of patients in Periods A and B were 63.9 and 61.4 years, respectively (P < 0.0001). The patients were predominantly male. ASA classifications were similar in the 2 periods, with over 60% of patients having an ASA score of 3 or higher.
| Period A N (%) | Period B N (%) | P | |
|---|---|---|---|
| |||
| No. of patients | 2658 | 2565 | |
| Total no. of surgeries | 3568 | 3337 | |
| Service | 0.0746 | ||
| Ophthalmology | 756 (21.1) | 637 (19.1) | |
| Urology | 526 (14.7) | 478 (14.3) | |
| Orthopedics | 527 (14.8) | 502 (15.0) | |
| General surgery | 469 (13.1) | 495 (14.8) | |
| ENT | 363 (10.2) | 312 (9.4) | |
| Other | 927 (26.0) | 913 (27.4) | |
| Age, mean (SD) | 63.9 (13.2) | 61.4 (13.5) | <0.0001 |
| Male | 2486 (93.5) | 2335 (93.0) | 0.4100 |
| ASA classification | 0.1836 | ||
| 1. No disturbance | 59 (2.3) | 81 (3.3) | |
| 2. Mild | 896 (35.3) | 864 (35.3) | |
| 3. Severe | 1505 (59.3) | 1425 (58.1) | |
| 4. Life‐threatening or worse | 77 (3.0) | 81 (3.3) | |
| 5. Missing scores | 121 (4.6) | 114 (4.4) | |
Table 2 presents the selected preoperative resource utilization. Less than 3% of patients referred to the Preoperative clinic were referred for Cardiology consultation during both time periods. However, during Period A, some patients required multiple Cardiology referrals resulting in 85 referrals in Period A and 64 referrals in Period B. In contrast, Preoperative clinic providers ordered more cardiac studies in Period B than in Period A (P = 0.012). There was a significant increase in the number of patients on perioperative beta blockers, with 26% in Period A and 33% in Period B (P < 0.0001). Although there was no significant difference in the number of same‐day surgical cancellations between the 2 periods, there was a trend towards a reduction of cancellations for medically avoidable reasons, 34 (8.5%) and 18 (4.9%) cases during Periods A and B, respectively (P = 0.065).
| Period A N (%) | Period B N (%) | P | |
|---|---|---|---|
| |||
| No. of patients | 2658 | 2565 | |
| No. of patients that had at least 1 cardiology referral | 70 (2.6) | 62 (2.4) | 0.660 |
| No. of cardiology referrals | 85 | 64 | |
| Cardiac testing orders | 40 | 88 | 0.012 |
| Nuclear medicine | 20 (50.0) | 58 (65.9) | |
| Nuclear treadmill | 2 (5.0) | 12 (13.6) | |
| ETT | 18 (45.0) | 18 (20.5) | |
| Perioperative beta blocker | 696 (26.2) | 852 (33.2) | <0.0001 |
| Cases canceled day of surgery | |||
| Total | 400 (15.0) | 368 (14.3) | |
| Medical avoidable | 34 (8.5) | 18 (4.9) | 0.065 |
Table 3 describes the clinical characteristics, inpatient LOS, and inpatient mortality for the surgical inpatients assessed in the Preoperative clinic. There were 1101 patients with 1200 inpatient surgeries in Period A, and 1126 patients with 1245 inpatient surgeries in Period B. The mean ages were 63.3 and 61.4 years in Periods A and B, respectively (P = 0.0004). More than 90% of patients were male. Over 62% of patients had ASA scores of 3 or higher in both periods. Both mean and median LOS was reduced in Period B. Results from the mixed‐effects regression model indicated no age and gender effects. ASA classification was significantly associated with LOS (P < 0.0001). There were reductions in LOS from Period A to Period B across all ASA classifications, however, the levels of reduction were different among them (ie, significant interaction effect, P = 0.0005). Patients who were ASA 3 or higher had a significantly shorter LOS in Period B as compared to those in Period A (P < 0.0001).
| Period A | Period B | P | |
|---|---|---|---|
| |||
| No. of patients | 1101 | 1126 | |
| No. of inpatient surgeries | 1200 | 1245 | |
| Age, mean (SD)* | 63.3 (12.7) | 61.4 (12.8) | 0.0004 |
| Male (%) | 1022 (92.8) | 1024 (90.9) | 0.1039 |
| ASA classification | 0.0510 | ||
| 1. No disturbance | 15 (1.36) | 27 (2.40) | |
| 2. Mild | 324 (29.4) | 364 (32.3) | |
| 3. Severe | 710 (64.5) | 697 (61.9) | |
| 4. Life‐threatening | 52 (4.72) | 38 (3.37) | |
| Primary outcome | |||
| In‐patient LOS (days) | |||
| Mean (SD) | 9.87 (25.4) | 5.28 (9.24) | |
| Median (minmax) | 3.0 (1516) | 2.0 (1120) | |
| Mixed‐effects regression | Period AB Estimated difference (SE) | ||
| 1. No disturbance | 1.31 (5.90) | 0.8247 | |
| 2. Mild | 2.52 (1.39) | 0.0717 | |
| 3. Severe | 4.22 (0.96) | <0.0001 | |
| 4. Life‐threatening | 19.7 (3.81) | <0.0001 | |
| Secondary outcome | |||
| Mortality, N (%) | 14 (1.27) | 4 (0.36) | 0.0158 |
| ASA classification | |||
| 3. Severe | 7 (0.99) | 2 (0.29) | |
| 4. Life‐threatening | 7 (13.5) | 2 (5.26) | |
| Logistic regression | Estimated OR (95% CI) | ||
| Period (A vs B) | 3.13 (1.01, 9.73) | 0.0488 | |
| ASA classification (3 vs 4) | 0.06 (0.02, 0.16) | <0.0001 | |
The LOS on the Cardiothoracic services was also evaluated. No significant difference in LOS was observed between the 2 periods (average LOS of 18 days) after adjusting for the patients' age and ASA score.
Inpatient mortality was reduced in Period B, from 14 cases (1.27%) to 4 cases (0.36%) (P = 0.0158). No patients who were ASA 2 or less died. Deaths in each period were evenly split between ASA categories 3 and 4 (Table 3). Subgroup analysis on inpatient deaths showed no age effect, but a significant period effect (odds ratio [OR] = 3.13, 95% confidence interval [CI]: 1.019.73 for Periods A vs B; P = 0.0488) and ASA status effect (OR = 0.06, 95% CI: 0.020.16 for ASA severe vs life‐threatening; P < 0.0001).
DISCUSSION
The addition of a Hospitalist‐run, medical Preoperative clinic was associated with more perioperative beta blocker use, shortened LOS, and lower mortality rates for our veteran patients undergoing noncardiac surgery. Such LOS reduction was not apparent in our internal control of cardiothoracic surgery patients or in the VA National Surgical Quality Improvement Program (NSQIP), a national representative sample of a similar patient population. While median unadjusted LOS in the VA NSQIP did not change over the same time periods, surgical mortality rates decreased, but by a smaller magnitude (15%) than seen in our study. While mortality in our study was reduced, the absolute numbers are relatively small. However, a subgroup analysis accounting for age and ASA score demonstrated a reduction in mortality.
As multiple structure and process changes were made in the Preoperative program, it is not definitively known which specific factor or factors could have affected inpatient surgical care. The Preoperative clinic evaluation was a one‐time consult, but included recommendations for perioperative management, including medication adjustments and infrequent suggestions for perioperative consultation. The decision to follow such recommendations was voluntary on the part of the surgical team. Alternatively, preoperative optimization may have played a role. By performing a multisystem evaluation with evidence‐based protocols, we possibly identified patients that were at increased risk of perioperative harm, and were able to intervene or recommend deferral of the procedure. This could have resulted in better surgical candidate selection with fewer postoperative complications, especially among patients with significant medical comorbidities.
Better patient selection is also suggested by a trend toward fewer same‐day cancellations for medically avoidable reasons during Period B. The distinction between medical versus patient‐related causes and avoidable versus unavoidable causes may be imprecise; however, the same Anesthesia staff assigned the categories over both periods and therefore any possible inconsistencies should have averaged out.
Increased usage of perioperative beta blockers may also have contributed to reduced mortality rates. We anticipated that more patients in Period B would be placed on perioperative beta blockers, given the guidelines in place at the time. More recently, the evidence for perioperative beta blockade has been further refined,16, 17 but during study Periods A and B, it was considered best practice for wider patient populations.
Fewer repeat referrals to Cardiology clinic and more cardiac testing were ordered by the Preoperative clinic providers during Period B. Ordering cardiac studies from Preoperative clinic and referring only when guideline‐driven could have streamlined the evaluation process and prevented the need for repeat referrals. We expect the number of stress tests and Cardiology consultations to have decreased even more in recent years as the 2007 ACC/AHA guidelines further emphasize medical optimization and de‐emphasize cardiac testing and prophylactic revascularization prior to surgery.18
Our results suggest that similar healthcare systems may benefit from adding medical expertise to their preoperative clinical operations. As the LOS reduction was most noticeable in patients with higher ASA scores, the largest impact would likely be with healthcare environments with medically complex patients and variable access to primary care. The shortage of primary care physicians and the increase in chronic disease burden in the US population may cause more patients to present to a surgeon in a nonoptimized condition. Arguably, such clinics could be supervised by any discipline that is familiar with the perioperative literature, chronic disease management, and postoperative inpatient care. Other options include clinics in which Anesthesiologists jointly collaborate with Hospitalists19 or General Internists with expertise in perioperative management.
Our study has many limitations. The VA has a largely male population and an electronic medical record, and thus results are not generalizable. Patients were younger in Period B than in Period A; however, the 2‐ to 3‐year difference might not be clinically significant, and the standard deviation was wide in both groups. This study is a retrospective observational study, and thus we cannot identify the specific processes that could have lead to any associated outcomes. There was no ideal contemporaneous control group, but examination of trends in cardiothoracic surgery at our institution and the national VA database does not reveal changes of this magnitude. Unforeseen biases could have resulted in upcoding of ASA scores by the mid‐level providers. Beta blocker usage was determined by patients prescribed beta blockers perioperatively, and did not exclude those on the medication prior to presentation. However, the significant increase in usage in Period B points to an increase in prescriptions originating from the Preoperative clinic. We do not have a breakdown of postoperative days in the intensive care unit (ICU) or ward settings, or the readmission rates. Thus, a true cost‐effectiveness analysis cannot be done. However, the reduction in postoperative LOS and decline in same‐day cancellations suggests that our institution benefited to some degree. Since the mid‐level providers were present prior to the change from Anesthesia to Hospitalist leadership, the only cost of the intervention was the hiring of a Hospitalist. However, the change freed an Anesthesiologist to work in the operating room or procedure suite. We do not have precise data regarding the number of surgeries delayed or canceled by the Preoperative clinic, but surgical workload was similar between both periods. Hopefully future studies will include richer data to minimize study limitations.
CONCLUSION
The addition of a Hospitalist‐run, medical Preoperative clinic was associated with improvements in perioperative processes and outcomes. Postoperative LOS was reduced in the sickest patients, as was inpatient mortality. Perioperative beta blocker use was increased. Adding Hospitalist expertise to preoperative clinical operations may be a viable model to improve perioperative care.
Acknowledgements
The authors thank Manyee Gee for retrieving much needed data. The authors also thank our staff in the Preoperative clinic for their exceptional hard work and dedication to our veteran patients.
- ,,,,.Value of preoperative clinic visits in identifying issues with potential impact on operating room efficiency.Anesthesiology.2006;105:1254–1259.
- ,,, et al.The effect of outpatient perioperative evaluation of hospital inpatients on cancellation of surgery and length of hospital stay.Anesth Analg.2002;94(3):644–649.
- ,,,,,.Opportunity missed: medical consultation, resource use, and quality of care of patients undergoing major surgery.Arch Int Med.2007;167(21):2338–2344.
- ,.Outpatient internal medicine preoperative evaluation: a randomized clinical trial.Med Care.1994;32(5):498–507.
- ,,,,.Outcomes and processes of care related to preoperative medical consultation.Arch Intern Med.2010;170(15):1365–1374.
- .Cost‐effective preoperative evaluation and testing.Chest.1999;115(5):96S–100S.
- ,,.Optimizing postoperative outcomes with efficient preoperative assessment and management.Crit Care Med.2004;32(4):S76–S86.
- .Preoperative laboratory testing: general issues and considerations.Anesthesiol Clin North Am.2004;22(1):13–25.
- .Preoperative medical evaluation of the healthy patient. Available at: http://www.uptodate.com. Accessed July 15, 2004.
- ,.The case against routine preoperative laboratory testing.Med Clin North Am.2003;87(1):7–40.
- ,.Blockers and reduction of cardiac events in noncardiac surgery.JAMA.2002;287:1435–1444.
- .Preoperative pulmonary evaluation.N Engl J Med.1999;340(12):937–944.
- ,,,,,.The pharmacology and management of the vitamin K antagonists. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy: evidence‐based guidelines.Chest.2004;126(3 suppl):204S–233S.
- ,,, et al; for theCommittee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2002;105(10):1257–1267.
- American Society of Anesthesiology House of Delegates.New classification of physical status.Anesthesiology.1963;24:111.
- ,,, et al; for thePOISE Study Group.Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial.Lancet.2008;371(9627):1839–1847.
- ,,,,,.Perioperative beta blockers in patients having non‐cardiac surgery: a meta‐analysis.Lancet.2008;372(9654):1962–1976.
- ,,, et al; for theWriting Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2007;116(17):1971–1996.
- ,.Hospitalists and anesthesiologists as perioperative physicians: are their roles complimentary?Proc (Bayl Univ Med Cent).2007;20(2):140–142.
Anesthesiologists typically initiate an assessment in the immediate preoperative period, focused on management of the airway, physiologic parameters, and choice of anesthetic. Given the growing complexity of medical issues in the surgical patient, the preoperative assessment may need to be initiated weeks to months prior to surgery. Early evaluation allows time to implement required interventions, optimize medical conditions, adjust medications, and collaborate with the surgical team.
Most studies of Preoperative clinics are in the Anesthesiology literature.1 Anesthesia‐run Preoperative clinics have demonstrated a reduction in surgical cancellations and length of stay (LOS).2 Auerbach and colleagues found medical consultation to have inconsistent effects on quality of care in surgical patients, but consultations occurred, at the earliest, 1 day prior to surgery.3 A randomized trial, performed at the Pittsburgh Veterans Administration (VA) medical center using an outpatient Internal Medicine Preoperative clinic, demonstrated a shortening of preoperative LOS but no change in total LOS, and increased use of consultants. However, there were reduced numbers of unnecessary admissions, defined as patients who were discharged without having had surgery.4 An analysis of a population‐based administrative database found that voluntary preoperative consultations were associated with a significant, albeit small, increase in mortality. Although this study used a matched cohort, the unmatched cohort that underwent consultation was higher risk; also, selection bias was possible, as the reasons for initial consultation were unknown.5
Historically, the Preoperative clinic at VA Greater Los Angeles Healthcare System (VAGLAHS) was supervised by the Department of Anesthesiology. In July 2004, the Preoperative clinic was restructured with Hospitalist oversight. The Anesthesia staff continued to evaluate all surgical patients, but did so only on the day of surgery, and after the patient was deemed an acceptable risk by the Preoperative clinic.
We undertook this study to measure the institutional impact of the addition of a Hospitalist‐run Preoperative clinic to our standard practice. The VA is an ideal setting, given the closed system with reliable longitudinal data. The VA electronic medical record also allows for comprehensive calculations of clinical covariants and outcomes.
MATERIALS AND METHODS
Setting
VAGLAHS is a tertiary care, academic medical center that serves patients referred from a 110,000 square mile area of Southern California and Southern Nevada. The Preoperative clinic evaluates all outpatients scheduled for inpatient or outpatient noncardiac surgery. Evaluations are performed by mid‐level providers with physician oversight. Patients are seen within 30 days of surgery, with a goal of 2 to 3 weeks prior to the operative date. Two of the 3 mid‐level providers remained after the change in leadership; a third was hired. All were retrained to perform a detailed medical preoperative assessment. Patients awaiting cardiothoracic surgery had their evaluation performed outside the Preoperative clinic by the Cardiology or Pulmonary services during both periods.
With the change in oversight, mid‐level providers were given weekly lectures on medical disease management and preoperative assessment. A syllabus of key articles in perioperative literature was compiled. Evidence‐based protocols were developed to standardize the evaluation. Examples of guidelines include: laboratory and radiological testing guidelines,610 initiation of perioperative beta blockers,11 selection criteria for pulmonary function tests,12 protocols for bridging with low‐molecular‐weight heparin for patients on oral vitamin K antagonists,13 the cardiovascular evaluation based on American College of Cardiology/American Heart Association (ACC/AHA) guidelines,14 as well as adjustment of diabetic medications.
Prior to the change in oversight, patients who required Cardiology evaluations were referred directly to the Cardiology service generally without any prior testing. After institution of the Hospitalist‐run clinic, the mid‐level providers ordered cardiac studies after discussion with the attending to ensure necessity and compliance with ACC/AHA guidelines. Patients were referred to Cardiology only if the results required further evaluation. In addition, entry to the Preoperative clinic was denied to patients awaiting elective surgeries whose hemoglobin A1c percentage was greater than 9%; such patients were referred to their primary care provider. For patients awaiting urgent surgeries, the Preoperative clinic would expedite evaluations in order to honor the surgical date. Providers would document perioperative recommendations for patients anticipated to require an inpatient stay. Occasionally, the patient was deemed too high risk to proceed with surgery, and the case was canceled or delayed after discussion with the patient and surgical team. Once deemed a medically acceptable candidate, the patient was evaluated on the day of surgery by Anesthesia.
Methods
We extracted de‐identified data from Veterans Health Administration (VHA) national databases, and specifically from the Veterans Integrated Service Network (VISN) 22 warehouse. All patients seen in the Preoperative clinic at VAGLAHS, from July 2003 to July 2005, were included. The patients were analyzed in 2 groups: patients seen from July 2003 to June 2004, when the Anesthesia Department staff supervised the Preoperative clinic (Period A); and from July 2004 to June 2005, the first year of the new Hospitalist‐run system (Period B). We collected data on age; gender; American Society of Anesthesia (ASA) score15; perioperative beta blocker use; cardiology studies ordered; and surgical mortality defined as death within the index hospital stay. The length of stay (LOS) was calculated for patients who required an inpatient stay after surgery. As an internal control, we assessed the LOS of the cardiothoracic patients in our facility since this group of patients does not utilize the Preoperative clinic and maintained the same preoperative evaluation process during both time periods. In addition, same‐day surgical cancellations were tracked by the Anesthesia Department, which documents daily operating room utilization and determines whether a cancellation was avoidable.
Statistical Analysis
Differences in demographic, clinical, and preoperative resource utilization characteristics were compared between Periods A and B using chi‐square for categorical variables and t test (or Wilcoxon test) for continuous variables. A subgroup analysis was performed for patients who required an inpatient stay after surgery. The primary outcome was inpatient LOS and the secondary outcome was inpatient death. A mixed‐effects regression model with patient‐level random effects to account for clustering of visits by the same patient was used to assess the impact of certain patient characteristics on inpatient LOS. Covariates included age, gender, time period (A vs B), ASA classification, and perioperative period‐by‐ASA classification interaction. Comparisons of inpatient LOS between periods for different ASA classes were done through model contrasts. Chi‐square test was used to compare the inpatient mortality between periods. A subgroup analysis was performed on postoperative inpatient deaths during the study period using a logistics regression model with age, ASA, and time period. All statistical analyses were performed using SAS Version 9.2 (SAS Institute, Cary, NC).
RESULTS
Table 1 describes the demographics and clinical characteristics of the patients evaluated in the Preoperative clinic. Number of surgeries performed in Periods A and B were 3568 and 3337, respectively, with an average of 1.3 surgeries per patient for both periods. The most common surgical specialties were Ophthalmology, Orthopedics, Urology, and General Surgery. The average ages of patients in Periods A and B were 63.9 and 61.4 years, respectively (P < 0.0001). The patients were predominantly male. ASA classifications were similar in the 2 periods, with over 60% of patients having an ASA score of 3 or higher.
| Period A N (%) | Period B N (%) | P | |
|---|---|---|---|
| |||
| No. of patients | 2658 | 2565 | |
| Total no. of surgeries | 3568 | 3337 | |
| Service | 0.0746 | ||
| Ophthalmology | 756 (21.1) | 637 (19.1) | |
| Urology | 526 (14.7) | 478 (14.3) | |
| Orthopedics | 527 (14.8) | 502 (15.0) | |
| General surgery | 469 (13.1) | 495 (14.8) | |
| ENT | 363 (10.2) | 312 (9.4) | |
| Other | 927 (26.0) | 913 (27.4) | |
| Age, mean (SD) | 63.9 (13.2) | 61.4 (13.5) | <0.0001 |
| Male | 2486 (93.5) | 2335 (93.0) | 0.4100 |
| ASA classification | 0.1836 | ||
| 1. No disturbance | 59 (2.3) | 81 (3.3) | |
| 2. Mild | 896 (35.3) | 864 (35.3) | |
| 3. Severe | 1505 (59.3) | 1425 (58.1) | |
| 4. Life‐threatening or worse | 77 (3.0) | 81 (3.3) | |
| 5. Missing scores | 121 (4.6) | 114 (4.4) | |
Table 2 presents the selected preoperative resource utilization. Less than 3% of patients referred to the Preoperative clinic were referred for Cardiology consultation during both time periods. However, during Period A, some patients required multiple Cardiology referrals resulting in 85 referrals in Period A and 64 referrals in Period B. In contrast, Preoperative clinic providers ordered more cardiac studies in Period B than in Period A (P = 0.012). There was a significant increase in the number of patients on perioperative beta blockers, with 26% in Period A and 33% in Period B (P < 0.0001). Although there was no significant difference in the number of same‐day surgical cancellations between the 2 periods, there was a trend towards a reduction of cancellations for medically avoidable reasons, 34 (8.5%) and 18 (4.9%) cases during Periods A and B, respectively (P = 0.065).
| Period A N (%) | Period B N (%) | P | |
|---|---|---|---|
| |||
| No. of patients | 2658 | 2565 | |
| No. of patients that had at least 1 cardiology referral | 70 (2.6) | 62 (2.4) | 0.660 |
| No. of cardiology referrals | 85 | 64 | |
| Cardiac testing orders | 40 | 88 | 0.012 |
| Nuclear medicine | 20 (50.0) | 58 (65.9) | |
| Nuclear treadmill | 2 (5.0) | 12 (13.6) | |
| ETT | 18 (45.0) | 18 (20.5) | |
| Perioperative beta blocker | 696 (26.2) | 852 (33.2) | <0.0001 |
| Cases canceled day of surgery | |||
| Total | 400 (15.0) | 368 (14.3) | |
| Medical avoidable | 34 (8.5) | 18 (4.9) | 0.065 |
Table 3 describes the clinical characteristics, inpatient LOS, and inpatient mortality for the surgical inpatients assessed in the Preoperative clinic. There were 1101 patients with 1200 inpatient surgeries in Period A, and 1126 patients with 1245 inpatient surgeries in Period B. The mean ages were 63.3 and 61.4 years in Periods A and B, respectively (P = 0.0004). More than 90% of patients were male. Over 62% of patients had ASA scores of 3 or higher in both periods. Both mean and median LOS was reduced in Period B. Results from the mixed‐effects regression model indicated no age and gender effects. ASA classification was significantly associated with LOS (P < 0.0001). There were reductions in LOS from Period A to Period B across all ASA classifications, however, the levels of reduction were different among them (ie, significant interaction effect, P = 0.0005). Patients who were ASA 3 or higher had a significantly shorter LOS in Period B as compared to those in Period A (P < 0.0001).
| Period A | Period B | P | |
|---|---|---|---|
| |||
| No. of patients | 1101 | 1126 | |
| No. of inpatient surgeries | 1200 | 1245 | |
| Age, mean (SD)* | 63.3 (12.7) | 61.4 (12.8) | 0.0004 |
| Male (%) | 1022 (92.8) | 1024 (90.9) | 0.1039 |
| ASA classification | 0.0510 | ||
| 1. No disturbance | 15 (1.36) | 27 (2.40) | |
| 2. Mild | 324 (29.4) | 364 (32.3) | |
| 3. Severe | 710 (64.5) | 697 (61.9) | |
| 4. Life‐threatening | 52 (4.72) | 38 (3.37) | |
| Primary outcome | |||
| In‐patient LOS (days) | |||
| Mean (SD) | 9.87 (25.4) | 5.28 (9.24) | |
| Median (minmax) | 3.0 (1516) | 2.0 (1120) | |
| Mixed‐effects regression | Period AB Estimated difference (SE) | ||
| 1. No disturbance | 1.31 (5.90) | 0.8247 | |
| 2. Mild | 2.52 (1.39) | 0.0717 | |
| 3. Severe | 4.22 (0.96) | <0.0001 | |
| 4. Life‐threatening | 19.7 (3.81) | <0.0001 | |
| Secondary outcome | |||
| Mortality, N (%) | 14 (1.27) | 4 (0.36) | 0.0158 |
| ASA classification | |||
| 3. Severe | 7 (0.99) | 2 (0.29) | |
| 4. Life‐threatening | 7 (13.5) | 2 (5.26) | |
| Logistic regression | Estimated OR (95% CI) | ||
| Period (A vs B) | 3.13 (1.01, 9.73) | 0.0488 | |
| ASA classification (3 vs 4) | 0.06 (0.02, 0.16) | <0.0001 | |
The LOS on the Cardiothoracic services was also evaluated. No significant difference in LOS was observed between the 2 periods (average LOS of 18 days) after adjusting for the patients' age and ASA score.
Inpatient mortality was reduced in Period B, from 14 cases (1.27%) to 4 cases (0.36%) (P = 0.0158). No patients who were ASA 2 or less died. Deaths in each period were evenly split between ASA categories 3 and 4 (Table 3). Subgroup analysis on inpatient deaths showed no age effect, but a significant period effect (odds ratio [OR] = 3.13, 95% confidence interval [CI]: 1.019.73 for Periods A vs B; P = 0.0488) and ASA status effect (OR = 0.06, 95% CI: 0.020.16 for ASA severe vs life‐threatening; P < 0.0001).
DISCUSSION
The addition of a Hospitalist‐run, medical Preoperative clinic was associated with more perioperative beta blocker use, shortened LOS, and lower mortality rates for our veteran patients undergoing noncardiac surgery. Such LOS reduction was not apparent in our internal control of cardiothoracic surgery patients or in the VA National Surgical Quality Improvement Program (NSQIP), a national representative sample of a similar patient population. While median unadjusted LOS in the VA NSQIP did not change over the same time periods, surgical mortality rates decreased, but by a smaller magnitude (15%) than seen in our study. While mortality in our study was reduced, the absolute numbers are relatively small. However, a subgroup analysis accounting for age and ASA score demonstrated a reduction in mortality.
As multiple structure and process changes were made in the Preoperative program, it is not definitively known which specific factor or factors could have affected inpatient surgical care. The Preoperative clinic evaluation was a one‐time consult, but included recommendations for perioperative management, including medication adjustments and infrequent suggestions for perioperative consultation. The decision to follow such recommendations was voluntary on the part of the surgical team. Alternatively, preoperative optimization may have played a role. By performing a multisystem evaluation with evidence‐based protocols, we possibly identified patients that were at increased risk of perioperative harm, and were able to intervene or recommend deferral of the procedure. This could have resulted in better surgical candidate selection with fewer postoperative complications, especially among patients with significant medical comorbidities.
Better patient selection is also suggested by a trend toward fewer same‐day cancellations for medically avoidable reasons during Period B. The distinction between medical versus patient‐related causes and avoidable versus unavoidable causes may be imprecise; however, the same Anesthesia staff assigned the categories over both periods and therefore any possible inconsistencies should have averaged out.
Increased usage of perioperative beta blockers may also have contributed to reduced mortality rates. We anticipated that more patients in Period B would be placed on perioperative beta blockers, given the guidelines in place at the time. More recently, the evidence for perioperative beta blockade has been further refined,16, 17 but during study Periods A and B, it was considered best practice for wider patient populations.
Fewer repeat referrals to Cardiology clinic and more cardiac testing were ordered by the Preoperative clinic providers during Period B. Ordering cardiac studies from Preoperative clinic and referring only when guideline‐driven could have streamlined the evaluation process and prevented the need for repeat referrals. We expect the number of stress tests and Cardiology consultations to have decreased even more in recent years as the 2007 ACC/AHA guidelines further emphasize medical optimization and de‐emphasize cardiac testing and prophylactic revascularization prior to surgery.18
Our results suggest that similar healthcare systems may benefit from adding medical expertise to their preoperative clinical operations. As the LOS reduction was most noticeable in patients with higher ASA scores, the largest impact would likely be with healthcare environments with medically complex patients and variable access to primary care. The shortage of primary care physicians and the increase in chronic disease burden in the US population may cause more patients to present to a surgeon in a nonoptimized condition. Arguably, such clinics could be supervised by any discipline that is familiar with the perioperative literature, chronic disease management, and postoperative inpatient care. Other options include clinics in which Anesthesiologists jointly collaborate with Hospitalists19 or General Internists with expertise in perioperative management.
Our study has many limitations. The VA has a largely male population and an electronic medical record, and thus results are not generalizable. Patients were younger in Period B than in Period A; however, the 2‐ to 3‐year difference might not be clinically significant, and the standard deviation was wide in both groups. This study is a retrospective observational study, and thus we cannot identify the specific processes that could have lead to any associated outcomes. There was no ideal contemporaneous control group, but examination of trends in cardiothoracic surgery at our institution and the national VA database does not reveal changes of this magnitude. Unforeseen biases could have resulted in upcoding of ASA scores by the mid‐level providers. Beta blocker usage was determined by patients prescribed beta blockers perioperatively, and did not exclude those on the medication prior to presentation. However, the significant increase in usage in Period B points to an increase in prescriptions originating from the Preoperative clinic. We do not have a breakdown of postoperative days in the intensive care unit (ICU) or ward settings, or the readmission rates. Thus, a true cost‐effectiveness analysis cannot be done. However, the reduction in postoperative LOS and decline in same‐day cancellations suggests that our institution benefited to some degree. Since the mid‐level providers were present prior to the change from Anesthesia to Hospitalist leadership, the only cost of the intervention was the hiring of a Hospitalist. However, the change freed an Anesthesiologist to work in the operating room or procedure suite. We do not have precise data regarding the number of surgeries delayed or canceled by the Preoperative clinic, but surgical workload was similar between both periods. Hopefully future studies will include richer data to minimize study limitations.
CONCLUSION
The addition of a Hospitalist‐run, medical Preoperative clinic was associated with improvements in perioperative processes and outcomes. Postoperative LOS was reduced in the sickest patients, as was inpatient mortality. Perioperative beta blocker use was increased. Adding Hospitalist expertise to preoperative clinical operations may be a viable model to improve perioperative care.
Acknowledgements
The authors thank Manyee Gee for retrieving much needed data. The authors also thank our staff in the Preoperative clinic for their exceptional hard work and dedication to our veteran patients.
Anesthesiologists typically initiate an assessment in the immediate preoperative period, focused on management of the airway, physiologic parameters, and choice of anesthetic. Given the growing complexity of medical issues in the surgical patient, the preoperative assessment may need to be initiated weeks to months prior to surgery. Early evaluation allows time to implement required interventions, optimize medical conditions, adjust medications, and collaborate with the surgical team.
Most studies of Preoperative clinics are in the Anesthesiology literature.1 Anesthesia‐run Preoperative clinics have demonstrated a reduction in surgical cancellations and length of stay (LOS).2 Auerbach and colleagues found medical consultation to have inconsistent effects on quality of care in surgical patients, but consultations occurred, at the earliest, 1 day prior to surgery.3 A randomized trial, performed at the Pittsburgh Veterans Administration (VA) medical center using an outpatient Internal Medicine Preoperative clinic, demonstrated a shortening of preoperative LOS but no change in total LOS, and increased use of consultants. However, there were reduced numbers of unnecessary admissions, defined as patients who were discharged without having had surgery.4 An analysis of a population‐based administrative database found that voluntary preoperative consultations were associated with a significant, albeit small, increase in mortality. Although this study used a matched cohort, the unmatched cohort that underwent consultation was higher risk; also, selection bias was possible, as the reasons for initial consultation were unknown.5
Historically, the Preoperative clinic at VA Greater Los Angeles Healthcare System (VAGLAHS) was supervised by the Department of Anesthesiology. In July 2004, the Preoperative clinic was restructured with Hospitalist oversight. The Anesthesia staff continued to evaluate all surgical patients, but did so only on the day of surgery, and after the patient was deemed an acceptable risk by the Preoperative clinic.
We undertook this study to measure the institutional impact of the addition of a Hospitalist‐run Preoperative clinic to our standard practice. The VA is an ideal setting, given the closed system with reliable longitudinal data. The VA electronic medical record also allows for comprehensive calculations of clinical covariants and outcomes.
MATERIALS AND METHODS
Setting
VAGLAHS is a tertiary care, academic medical center that serves patients referred from a 110,000 square mile area of Southern California and Southern Nevada. The Preoperative clinic evaluates all outpatients scheduled for inpatient or outpatient noncardiac surgery. Evaluations are performed by mid‐level providers with physician oversight. Patients are seen within 30 days of surgery, with a goal of 2 to 3 weeks prior to the operative date. Two of the 3 mid‐level providers remained after the change in leadership; a third was hired. All were retrained to perform a detailed medical preoperative assessment. Patients awaiting cardiothoracic surgery had their evaluation performed outside the Preoperative clinic by the Cardiology or Pulmonary services during both periods.
With the change in oversight, mid‐level providers were given weekly lectures on medical disease management and preoperative assessment. A syllabus of key articles in perioperative literature was compiled. Evidence‐based protocols were developed to standardize the evaluation. Examples of guidelines include: laboratory and radiological testing guidelines,610 initiation of perioperative beta blockers,11 selection criteria for pulmonary function tests,12 protocols for bridging with low‐molecular‐weight heparin for patients on oral vitamin K antagonists,13 the cardiovascular evaluation based on American College of Cardiology/American Heart Association (ACC/AHA) guidelines,14 as well as adjustment of diabetic medications.
Prior to the change in oversight, patients who required Cardiology evaluations were referred directly to the Cardiology service generally without any prior testing. After institution of the Hospitalist‐run clinic, the mid‐level providers ordered cardiac studies after discussion with the attending to ensure necessity and compliance with ACC/AHA guidelines. Patients were referred to Cardiology only if the results required further evaluation. In addition, entry to the Preoperative clinic was denied to patients awaiting elective surgeries whose hemoglobin A1c percentage was greater than 9%; such patients were referred to their primary care provider. For patients awaiting urgent surgeries, the Preoperative clinic would expedite evaluations in order to honor the surgical date. Providers would document perioperative recommendations for patients anticipated to require an inpatient stay. Occasionally, the patient was deemed too high risk to proceed with surgery, and the case was canceled or delayed after discussion with the patient and surgical team. Once deemed a medically acceptable candidate, the patient was evaluated on the day of surgery by Anesthesia.
Methods
We extracted de‐identified data from Veterans Health Administration (VHA) national databases, and specifically from the Veterans Integrated Service Network (VISN) 22 warehouse. All patients seen in the Preoperative clinic at VAGLAHS, from July 2003 to July 2005, were included. The patients were analyzed in 2 groups: patients seen from July 2003 to June 2004, when the Anesthesia Department staff supervised the Preoperative clinic (Period A); and from July 2004 to June 2005, the first year of the new Hospitalist‐run system (Period B). We collected data on age; gender; American Society of Anesthesia (ASA) score15; perioperative beta blocker use; cardiology studies ordered; and surgical mortality defined as death within the index hospital stay. The length of stay (LOS) was calculated for patients who required an inpatient stay after surgery. As an internal control, we assessed the LOS of the cardiothoracic patients in our facility since this group of patients does not utilize the Preoperative clinic and maintained the same preoperative evaluation process during both time periods. In addition, same‐day surgical cancellations were tracked by the Anesthesia Department, which documents daily operating room utilization and determines whether a cancellation was avoidable.
Statistical Analysis
Differences in demographic, clinical, and preoperative resource utilization characteristics were compared between Periods A and B using chi‐square for categorical variables and t test (or Wilcoxon test) for continuous variables. A subgroup analysis was performed for patients who required an inpatient stay after surgery. The primary outcome was inpatient LOS and the secondary outcome was inpatient death. A mixed‐effects regression model with patient‐level random effects to account for clustering of visits by the same patient was used to assess the impact of certain patient characteristics on inpatient LOS. Covariates included age, gender, time period (A vs B), ASA classification, and perioperative period‐by‐ASA classification interaction. Comparisons of inpatient LOS between periods for different ASA classes were done through model contrasts. Chi‐square test was used to compare the inpatient mortality between periods. A subgroup analysis was performed on postoperative inpatient deaths during the study period using a logistics regression model with age, ASA, and time period. All statistical analyses were performed using SAS Version 9.2 (SAS Institute, Cary, NC).
RESULTS
Table 1 describes the demographics and clinical characteristics of the patients evaluated in the Preoperative clinic. Number of surgeries performed in Periods A and B were 3568 and 3337, respectively, with an average of 1.3 surgeries per patient for both periods. The most common surgical specialties were Ophthalmology, Orthopedics, Urology, and General Surgery. The average ages of patients in Periods A and B were 63.9 and 61.4 years, respectively (P < 0.0001). The patients were predominantly male. ASA classifications were similar in the 2 periods, with over 60% of patients having an ASA score of 3 or higher.
| Period A N (%) | Period B N (%) | P | |
|---|---|---|---|
| |||
| No. of patients | 2658 | 2565 | |
| Total no. of surgeries | 3568 | 3337 | |
| Service | 0.0746 | ||
| Ophthalmology | 756 (21.1) | 637 (19.1) | |
| Urology | 526 (14.7) | 478 (14.3) | |
| Orthopedics | 527 (14.8) | 502 (15.0) | |
| General surgery | 469 (13.1) | 495 (14.8) | |
| ENT | 363 (10.2) | 312 (9.4) | |
| Other | 927 (26.0) | 913 (27.4) | |
| Age, mean (SD) | 63.9 (13.2) | 61.4 (13.5) | <0.0001 |
| Male | 2486 (93.5) | 2335 (93.0) | 0.4100 |
| ASA classification | 0.1836 | ||
| 1. No disturbance | 59 (2.3) | 81 (3.3) | |
| 2. Mild | 896 (35.3) | 864 (35.3) | |
| 3. Severe | 1505 (59.3) | 1425 (58.1) | |
| 4. Life‐threatening or worse | 77 (3.0) | 81 (3.3) | |
| 5. Missing scores | 121 (4.6) | 114 (4.4) | |
Table 2 presents the selected preoperative resource utilization. Less than 3% of patients referred to the Preoperative clinic were referred for Cardiology consultation during both time periods. However, during Period A, some patients required multiple Cardiology referrals resulting in 85 referrals in Period A and 64 referrals in Period B. In contrast, Preoperative clinic providers ordered more cardiac studies in Period B than in Period A (P = 0.012). There was a significant increase in the number of patients on perioperative beta blockers, with 26% in Period A and 33% in Period B (P < 0.0001). Although there was no significant difference in the number of same‐day surgical cancellations between the 2 periods, there was a trend towards a reduction of cancellations for medically avoidable reasons, 34 (8.5%) and 18 (4.9%) cases during Periods A and B, respectively (P = 0.065).
| Period A N (%) | Period B N (%) | P | |
|---|---|---|---|
| |||
| No. of patients | 2658 | 2565 | |
| No. of patients that had at least 1 cardiology referral | 70 (2.6) | 62 (2.4) | 0.660 |
| No. of cardiology referrals | 85 | 64 | |
| Cardiac testing orders | 40 | 88 | 0.012 |
| Nuclear medicine | 20 (50.0) | 58 (65.9) | |
| Nuclear treadmill | 2 (5.0) | 12 (13.6) | |
| ETT | 18 (45.0) | 18 (20.5) | |
| Perioperative beta blocker | 696 (26.2) | 852 (33.2) | <0.0001 |
| Cases canceled day of surgery | |||
| Total | 400 (15.0) | 368 (14.3) | |
| Medical avoidable | 34 (8.5) | 18 (4.9) | 0.065 |
Table 3 describes the clinical characteristics, inpatient LOS, and inpatient mortality for the surgical inpatients assessed in the Preoperative clinic. There were 1101 patients with 1200 inpatient surgeries in Period A, and 1126 patients with 1245 inpatient surgeries in Period B. The mean ages were 63.3 and 61.4 years in Periods A and B, respectively (P = 0.0004). More than 90% of patients were male. Over 62% of patients had ASA scores of 3 or higher in both periods. Both mean and median LOS was reduced in Period B. Results from the mixed‐effects regression model indicated no age and gender effects. ASA classification was significantly associated with LOS (P < 0.0001). There were reductions in LOS from Period A to Period B across all ASA classifications, however, the levels of reduction were different among them (ie, significant interaction effect, P = 0.0005). Patients who were ASA 3 or higher had a significantly shorter LOS in Period B as compared to those in Period A (P < 0.0001).
| Period A | Period B | P | |
|---|---|---|---|
| |||
| No. of patients | 1101 | 1126 | |
| No. of inpatient surgeries | 1200 | 1245 | |
| Age, mean (SD)* | 63.3 (12.7) | 61.4 (12.8) | 0.0004 |
| Male (%) | 1022 (92.8) | 1024 (90.9) | 0.1039 |
| ASA classification | 0.0510 | ||
| 1. No disturbance | 15 (1.36) | 27 (2.40) | |
| 2. Mild | 324 (29.4) | 364 (32.3) | |
| 3. Severe | 710 (64.5) | 697 (61.9) | |
| 4. Life‐threatening | 52 (4.72) | 38 (3.37) | |
| Primary outcome | |||
| In‐patient LOS (days) | |||
| Mean (SD) | 9.87 (25.4) | 5.28 (9.24) | |
| Median (minmax) | 3.0 (1516) | 2.0 (1120) | |
| Mixed‐effects regression | Period AB Estimated difference (SE) | ||
| 1. No disturbance | 1.31 (5.90) | 0.8247 | |
| 2. Mild | 2.52 (1.39) | 0.0717 | |
| 3. Severe | 4.22 (0.96) | <0.0001 | |
| 4. Life‐threatening | 19.7 (3.81) | <0.0001 | |
| Secondary outcome | |||
| Mortality, N (%) | 14 (1.27) | 4 (0.36) | 0.0158 |
| ASA classification | |||
| 3. Severe | 7 (0.99) | 2 (0.29) | |
| 4. Life‐threatening | 7 (13.5) | 2 (5.26) | |
| Logistic regression | Estimated OR (95% CI) | ||
| Period (A vs B) | 3.13 (1.01, 9.73) | 0.0488 | |
| ASA classification (3 vs 4) | 0.06 (0.02, 0.16) | <0.0001 | |
The LOS on the Cardiothoracic services was also evaluated. No significant difference in LOS was observed between the 2 periods (average LOS of 18 days) after adjusting for the patients' age and ASA score.
Inpatient mortality was reduced in Period B, from 14 cases (1.27%) to 4 cases (0.36%) (P = 0.0158). No patients who were ASA 2 or less died. Deaths in each period were evenly split between ASA categories 3 and 4 (Table 3). Subgroup analysis on inpatient deaths showed no age effect, but a significant period effect (odds ratio [OR] = 3.13, 95% confidence interval [CI]: 1.019.73 for Periods A vs B; P = 0.0488) and ASA status effect (OR = 0.06, 95% CI: 0.020.16 for ASA severe vs life‐threatening; P < 0.0001).
DISCUSSION
The addition of a Hospitalist‐run, medical Preoperative clinic was associated with more perioperative beta blocker use, shortened LOS, and lower mortality rates for our veteran patients undergoing noncardiac surgery. Such LOS reduction was not apparent in our internal control of cardiothoracic surgery patients or in the VA National Surgical Quality Improvement Program (NSQIP), a national representative sample of a similar patient population. While median unadjusted LOS in the VA NSQIP did not change over the same time periods, surgical mortality rates decreased, but by a smaller magnitude (15%) than seen in our study. While mortality in our study was reduced, the absolute numbers are relatively small. However, a subgroup analysis accounting for age and ASA score demonstrated a reduction in mortality.
As multiple structure and process changes were made in the Preoperative program, it is not definitively known which specific factor or factors could have affected inpatient surgical care. The Preoperative clinic evaluation was a one‐time consult, but included recommendations for perioperative management, including medication adjustments and infrequent suggestions for perioperative consultation. The decision to follow such recommendations was voluntary on the part of the surgical team. Alternatively, preoperative optimization may have played a role. By performing a multisystem evaluation with evidence‐based protocols, we possibly identified patients that were at increased risk of perioperative harm, and were able to intervene or recommend deferral of the procedure. This could have resulted in better surgical candidate selection with fewer postoperative complications, especially among patients with significant medical comorbidities.
Better patient selection is also suggested by a trend toward fewer same‐day cancellations for medically avoidable reasons during Period B. The distinction between medical versus patient‐related causes and avoidable versus unavoidable causes may be imprecise; however, the same Anesthesia staff assigned the categories over both periods and therefore any possible inconsistencies should have averaged out.
Increased usage of perioperative beta blockers may also have contributed to reduced mortality rates. We anticipated that more patients in Period B would be placed on perioperative beta blockers, given the guidelines in place at the time. More recently, the evidence for perioperative beta blockade has been further refined,16, 17 but during study Periods A and B, it was considered best practice for wider patient populations.
Fewer repeat referrals to Cardiology clinic and more cardiac testing were ordered by the Preoperative clinic providers during Period B. Ordering cardiac studies from Preoperative clinic and referring only when guideline‐driven could have streamlined the evaluation process and prevented the need for repeat referrals. We expect the number of stress tests and Cardiology consultations to have decreased even more in recent years as the 2007 ACC/AHA guidelines further emphasize medical optimization and de‐emphasize cardiac testing and prophylactic revascularization prior to surgery.18
Our results suggest that similar healthcare systems may benefit from adding medical expertise to their preoperative clinical operations. As the LOS reduction was most noticeable in patients with higher ASA scores, the largest impact would likely be with healthcare environments with medically complex patients and variable access to primary care. The shortage of primary care physicians and the increase in chronic disease burden in the US population may cause more patients to present to a surgeon in a nonoptimized condition. Arguably, such clinics could be supervised by any discipline that is familiar with the perioperative literature, chronic disease management, and postoperative inpatient care. Other options include clinics in which Anesthesiologists jointly collaborate with Hospitalists19 or General Internists with expertise in perioperative management.
Our study has many limitations. The VA has a largely male population and an electronic medical record, and thus results are not generalizable. Patients were younger in Period B than in Period A; however, the 2‐ to 3‐year difference might not be clinically significant, and the standard deviation was wide in both groups. This study is a retrospective observational study, and thus we cannot identify the specific processes that could have lead to any associated outcomes. There was no ideal contemporaneous control group, but examination of trends in cardiothoracic surgery at our institution and the national VA database does not reveal changes of this magnitude. Unforeseen biases could have resulted in upcoding of ASA scores by the mid‐level providers. Beta blocker usage was determined by patients prescribed beta blockers perioperatively, and did not exclude those on the medication prior to presentation. However, the significant increase in usage in Period B points to an increase in prescriptions originating from the Preoperative clinic. We do not have a breakdown of postoperative days in the intensive care unit (ICU) or ward settings, or the readmission rates. Thus, a true cost‐effectiveness analysis cannot be done. However, the reduction in postoperative LOS and decline in same‐day cancellations suggests that our institution benefited to some degree. Since the mid‐level providers were present prior to the change from Anesthesia to Hospitalist leadership, the only cost of the intervention was the hiring of a Hospitalist. However, the change freed an Anesthesiologist to work in the operating room or procedure suite. We do not have precise data regarding the number of surgeries delayed or canceled by the Preoperative clinic, but surgical workload was similar between both periods. Hopefully future studies will include richer data to minimize study limitations.
CONCLUSION
The addition of a Hospitalist‐run, medical Preoperative clinic was associated with improvements in perioperative processes and outcomes. Postoperative LOS was reduced in the sickest patients, as was inpatient mortality. Perioperative beta blocker use was increased. Adding Hospitalist expertise to preoperative clinical operations may be a viable model to improve perioperative care.
Acknowledgements
The authors thank Manyee Gee for retrieving much needed data. The authors also thank our staff in the Preoperative clinic for their exceptional hard work and dedication to our veteran patients.
- ,,,,.Value of preoperative clinic visits in identifying issues with potential impact on operating room efficiency.Anesthesiology.2006;105:1254–1259.
- ,,, et al.The effect of outpatient perioperative evaluation of hospital inpatients on cancellation of surgery and length of hospital stay.Anesth Analg.2002;94(3):644–649.
- ,,,,,.Opportunity missed: medical consultation, resource use, and quality of care of patients undergoing major surgery.Arch Int Med.2007;167(21):2338–2344.
- ,.Outpatient internal medicine preoperative evaluation: a randomized clinical trial.Med Care.1994;32(5):498–507.
- ,,,,.Outcomes and processes of care related to preoperative medical consultation.Arch Intern Med.2010;170(15):1365–1374.
- .Cost‐effective preoperative evaluation and testing.Chest.1999;115(5):96S–100S.
- ,,.Optimizing postoperative outcomes with efficient preoperative assessment and management.Crit Care Med.2004;32(4):S76–S86.
- .Preoperative laboratory testing: general issues and considerations.Anesthesiol Clin North Am.2004;22(1):13–25.
- .Preoperative medical evaluation of the healthy patient. Available at: http://www.uptodate.com. Accessed July 15, 2004.
- ,.The case against routine preoperative laboratory testing.Med Clin North Am.2003;87(1):7–40.
- ,.Blockers and reduction of cardiac events in noncardiac surgery.JAMA.2002;287:1435–1444.
- .Preoperative pulmonary evaluation.N Engl J Med.1999;340(12):937–944.
- ,,,,,.The pharmacology and management of the vitamin K antagonists. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy: evidence‐based guidelines.Chest.2004;126(3 suppl):204S–233S.
- ,,, et al; for theCommittee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2002;105(10):1257–1267.
- American Society of Anesthesiology House of Delegates.New classification of physical status.Anesthesiology.1963;24:111.
- ,,, et al; for thePOISE Study Group.Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial.Lancet.2008;371(9627):1839–1847.
- ,,,,,.Perioperative beta blockers in patients having non‐cardiac surgery: a meta‐analysis.Lancet.2008;372(9654):1962–1976.
- ,,, et al; for theWriting Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2007;116(17):1971–1996.
- ,.Hospitalists and anesthesiologists as perioperative physicians: are their roles complimentary?Proc (Bayl Univ Med Cent).2007;20(2):140–142.
- ,,,,.Value of preoperative clinic visits in identifying issues with potential impact on operating room efficiency.Anesthesiology.2006;105:1254–1259.
- ,,, et al.The effect of outpatient perioperative evaluation of hospital inpatients on cancellation of surgery and length of hospital stay.Anesth Analg.2002;94(3):644–649.
- ,,,,,.Opportunity missed: medical consultation, resource use, and quality of care of patients undergoing major surgery.Arch Int Med.2007;167(21):2338–2344.
- ,.Outpatient internal medicine preoperative evaluation: a randomized clinical trial.Med Care.1994;32(5):498–507.
- ,,,,.Outcomes and processes of care related to preoperative medical consultation.Arch Intern Med.2010;170(15):1365–1374.
- .Cost‐effective preoperative evaluation and testing.Chest.1999;115(5):96S–100S.
- ,,.Optimizing postoperative outcomes with efficient preoperative assessment and management.Crit Care Med.2004;32(4):S76–S86.
- .Preoperative laboratory testing: general issues and considerations.Anesthesiol Clin North Am.2004;22(1):13–25.
- .Preoperative medical evaluation of the healthy patient. Available at: http://www.uptodate.com. Accessed July 15, 2004.
- ,.The case against routine preoperative laboratory testing.Med Clin North Am.2003;87(1):7–40.
- ,.Blockers and reduction of cardiac events in noncardiac surgery.JAMA.2002;287:1435–1444.
- .Preoperative pulmonary evaluation.N Engl J Med.1999;340(12):937–944.
- ,,,,,.The pharmacology and management of the vitamin K antagonists. The Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy: evidence‐based guidelines.Chest.2004;126(3 suppl):204S–233S.
- ,,, et al; for theCommittee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery—executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2002;105(10):1257–1267.
- American Society of Anesthesiology House of Delegates.New classification of physical status.Anesthesiology.1963;24:111.
- ,,, et al; for thePOISE Study Group.Effects of extended‐release metoprolol succinate in patients undergoing non‐cardiac surgery (POISE trial): a randomised controlled trial.Lancet.2008;371(9627):1839–1847.
- ,,,,,.Perioperative beta blockers in patients having non‐cardiac surgery: a meta‐analysis.Lancet.2008;372(9654):1962–1976.
- ,,, et al; for theWriting Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines.Circulation.2007;116(17):1971–1996.
- ,.Hospitalists and anesthesiologists as perioperative physicians: are their roles complimentary?Proc (Bayl Univ Med Cent).2007;20(2):140–142.
Copyright © 2012 Society of Hospital Medicine
FDA approves 3rd-generation TKI for CML
The FDA has approved bosutinib (Bosulif), an Abl and Src kinase inhibitor, to treat patients with relapsed or refractory chronic myelogenous leukemia (CML).
Bosutinib is intended for use in patients with chronic, accelerated, or blast phase Philadelphia chromosome-positive CML who have failed therapy with first-generation and second-generation tyrosine kinase inhibitors (TKIs).
The recommended dose of bosutinib is 500 mg, taken once daily with food.
“[Bosutinib] is an important new addition to the CML treatment landscape,” said Jorge E. Cortes, MD, of MD Anderson Cancer Center in Houston.
“Despite recent advances, an unmet need remains for many CML patients who are refractory to one or more tyrosine kinase inhibitors.”
Dr Cortes was a lead investigator of the industry-sponsored study that led to bosutinib’s approval. The phase 1/2 trial included 546 adult patients who had chronic, accelerated, or blast phase CML.
Efficacy data
Patients were evaluable for efficacy if they had received at least one bosutinib dose and had a valid baseline efficacy assessment. Of the 546 patients enrolled, 503 were evaluable for efficacy.
Among the patients in chronic phase, 266 received prior treatment with imatinib only, and 108 received prior treatment with imatinib followed by dasatinib and/or nilotinib. There were 129 evaluable patients with advanced phase CML who were previously treated with at least one TKI.
The efficacy endpoints for patients with chronic phase CML were the rate of major cytogenetic response (MCyR) at week 24 and the duration of MCyR. The efficacy endpoints for patients with accelerated phase or blast phase CML were the rates of complete hematologic response (CHR) and overall hematologic response (OHR) by week 48.
In patients with chronic phase CML who received prior therapy with one TKI, 90 patients (33.8%) achieved an MCyR at week 24. Among the chronic phase CML patients who received prior therapy with more than one TKI, 29 (26.9%) achieved an MCyR by week 24.
Of the patients with chronic phase CML who had been treated with one prior TKI, 53.4% achieved an MCyR at any time during the trial. And 52.8% had a response lasting at least 18 months.
For the 32.4% of patients with chronic phase CML treated with more than one TKI who achieved an MCyR at any time, 51.4% had a response lasting at least 9 months.
In patients with accelerated phase CML who received at least one prior TKI, 21 (30.4%) achieved a CHR by week 48. And 38 patients (55.1%) achieved an OHR.
In the blast phase population, 9 patients (15%) achieved a CHR by week 48. And 17 patients (28.3%) achieved an OHR.
Safety data
The researchers evaluated bosutinib’s safety in all 546 patients. Of these patients, 287 had chronic phase CML, were previously treated with a single TKI, and had a median bosutinib treatment duration of 24 months.
There were 119 patients who had chronic phase CML, were previously treated with more than one TKI, and had a median bosutinib treatment duration of 9 months.
There were also 76 patients with accelerated phase CML and 64 patients with blast phase CML. In these patients, the median treatment duration was 10 months and 3 months, respectively.
The most common adverse events observed in more than 20% of all patients were diarrhea, nausea, thrombocytopenia, vomiting, abdominal pain, rash, anemia, pyrexia, and fatigue.
The most common grade 3 to 4 adverse events observed in more than 10% of patients were thrombocytopenia, anemia, and neutropenia. Other serious adverse events included anaphylactic shock, myelosuppression, gastrointestinal toxicity, fluid retention, hepatoxicity, and rash.
Bosutinib is marketed as Bosulif by Pfizer. For more information on bosutinib, see the package insert. ![]()
The FDA has approved bosutinib (Bosulif), an Abl and Src kinase inhibitor, to treat patients with relapsed or refractory chronic myelogenous leukemia (CML).
Bosutinib is intended for use in patients with chronic, accelerated, or blast phase Philadelphia chromosome-positive CML who have failed therapy with first-generation and second-generation tyrosine kinase inhibitors (TKIs).
The recommended dose of bosutinib is 500 mg, taken once daily with food.
“[Bosutinib] is an important new addition to the CML treatment landscape,” said Jorge E. Cortes, MD, of MD Anderson Cancer Center in Houston.
“Despite recent advances, an unmet need remains for many CML patients who are refractory to one or more tyrosine kinase inhibitors.”
Dr Cortes was a lead investigator of the industry-sponsored study that led to bosutinib’s approval. The phase 1/2 trial included 546 adult patients who had chronic, accelerated, or blast phase CML.
Efficacy data
Patients were evaluable for efficacy if they had received at least one bosutinib dose and had a valid baseline efficacy assessment. Of the 546 patients enrolled, 503 were evaluable for efficacy.
Among the patients in chronic phase, 266 received prior treatment with imatinib only, and 108 received prior treatment with imatinib followed by dasatinib and/or nilotinib. There were 129 evaluable patients with advanced phase CML who were previously treated with at least one TKI.
The efficacy endpoints for patients with chronic phase CML were the rate of major cytogenetic response (MCyR) at week 24 and the duration of MCyR. The efficacy endpoints for patients with accelerated phase or blast phase CML were the rates of complete hematologic response (CHR) and overall hematologic response (OHR) by week 48.
In patients with chronic phase CML who received prior therapy with one TKI, 90 patients (33.8%) achieved an MCyR at week 24. Among the chronic phase CML patients who received prior therapy with more than one TKI, 29 (26.9%) achieved an MCyR by week 24.
Of the patients with chronic phase CML who had been treated with one prior TKI, 53.4% achieved an MCyR at any time during the trial. And 52.8% had a response lasting at least 18 months.
For the 32.4% of patients with chronic phase CML treated with more than one TKI who achieved an MCyR at any time, 51.4% had a response lasting at least 9 months.
In patients with accelerated phase CML who received at least one prior TKI, 21 (30.4%) achieved a CHR by week 48. And 38 patients (55.1%) achieved an OHR.
In the blast phase population, 9 patients (15%) achieved a CHR by week 48. And 17 patients (28.3%) achieved an OHR.
Safety data
The researchers evaluated bosutinib’s safety in all 546 patients. Of these patients, 287 had chronic phase CML, were previously treated with a single TKI, and had a median bosutinib treatment duration of 24 months.
There were 119 patients who had chronic phase CML, were previously treated with more than one TKI, and had a median bosutinib treatment duration of 9 months.
There were also 76 patients with accelerated phase CML and 64 patients with blast phase CML. In these patients, the median treatment duration was 10 months and 3 months, respectively.
The most common adverse events observed in more than 20% of all patients were diarrhea, nausea, thrombocytopenia, vomiting, abdominal pain, rash, anemia, pyrexia, and fatigue.
The most common grade 3 to 4 adverse events observed in more than 10% of patients were thrombocytopenia, anemia, and neutropenia. Other serious adverse events included anaphylactic shock, myelosuppression, gastrointestinal toxicity, fluid retention, hepatoxicity, and rash.
Bosutinib is marketed as Bosulif by Pfizer. For more information on bosutinib, see the package insert. ![]()
The FDA has approved bosutinib (Bosulif), an Abl and Src kinase inhibitor, to treat patients with relapsed or refractory chronic myelogenous leukemia (CML).
Bosutinib is intended for use in patients with chronic, accelerated, or blast phase Philadelphia chromosome-positive CML who have failed therapy with first-generation and second-generation tyrosine kinase inhibitors (TKIs).
The recommended dose of bosutinib is 500 mg, taken once daily with food.
“[Bosutinib] is an important new addition to the CML treatment landscape,” said Jorge E. Cortes, MD, of MD Anderson Cancer Center in Houston.
“Despite recent advances, an unmet need remains for many CML patients who are refractory to one or more tyrosine kinase inhibitors.”
Dr Cortes was a lead investigator of the industry-sponsored study that led to bosutinib’s approval. The phase 1/2 trial included 546 adult patients who had chronic, accelerated, or blast phase CML.
Efficacy data
Patients were evaluable for efficacy if they had received at least one bosutinib dose and had a valid baseline efficacy assessment. Of the 546 patients enrolled, 503 were evaluable for efficacy.
Among the patients in chronic phase, 266 received prior treatment with imatinib only, and 108 received prior treatment with imatinib followed by dasatinib and/or nilotinib. There were 129 evaluable patients with advanced phase CML who were previously treated with at least one TKI.
The efficacy endpoints for patients with chronic phase CML were the rate of major cytogenetic response (MCyR) at week 24 and the duration of MCyR. The efficacy endpoints for patients with accelerated phase or blast phase CML were the rates of complete hematologic response (CHR) and overall hematologic response (OHR) by week 48.
In patients with chronic phase CML who received prior therapy with one TKI, 90 patients (33.8%) achieved an MCyR at week 24. Among the chronic phase CML patients who received prior therapy with more than one TKI, 29 (26.9%) achieved an MCyR by week 24.
Of the patients with chronic phase CML who had been treated with one prior TKI, 53.4% achieved an MCyR at any time during the trial. And 52.8% had a response lasting at least 18 months.
For the 32.4% of patients with chronic phase CML treated with more than one TKI who achieved an MCyR at any time, 51.4% had a response lasting at least 9 months.
In patients with accelerated phase CML who received at least one prior TKI, 21 (30.4%) achieved a CHR by week 48. And 38 patients (55.1%) achieved an OHR.
In the blast phase population, 9 patients (15%) achieved a CHR by week 48. And 17 patients (28.3%) achieved an OHR.
Safety data
The researchers evaluated bosutinib’s safety in all 546 patients. Of these patients, 287 had chronic phase CML, were previously treated with a single TKI, and had a median bosutinib treatment duration of 24 months.
There were 119 patients who had chronic phase CML, were previously treated with more than one TKI, and had a median bosutinib treatment duration of 9 months.
There were also 76 patients with accelerated phase CML and 64 patients with blast phase CML. In these patients, the median treatment duration was 10 months and 3 months, respectively.
The most common adverse events observed in more than 20% of all patients were diarrhea, nausea, thrombocytopenia, vomiting, abdominal pain, rash, anemia, pyrexia, and fatigue.
The most common grade 3 to 4 adverse events observed in more than 10% of patients were thrombocytopenia, anemia, and neutropenia. Other serious adverse events included anaphylactic shock, myelosuppression, gastrointestinal toxicity, fluid retention, hepatoxicity, and rash.
Bosutinib is marketed as Bosulif by Pfizer. For more information on bosutinib, see the package insert. ![]()
Attention: New Resident Medical Editors Wanted! (copy 1)
Thoracic Surgery News is seeking 2 new resident associate medical editors for a 1-year appointment for our publication. To apply, you should be a resident in a field of thoracic surgery and willing to review and potentially comment upon articles for our monthly Residents’ Corner section.
In addition, resident medical editors are expected to work with the other editors to contribute 4 to 6 short articles throughout the appointment year, whether it is case studies by themselves or solicited from other thoracic surgeons, news or opinion pieces on resident issues, or summaries of resident-oriented sessions at meetings they attend.
Please send a CV and cover letter indicating your interest to [email protected] Deadline: October 15, 2012
Thoracic Surgery News is seeking 2 new resident associate medical editors for a 1-year appointment for our publication. To apply, you should be a resident in a field of thoracic surgery and willing to review and potentially comment upon articles for our monthly Residents’ Corner section.
In addition, resident medical editors are expected to work with the other editors to contribute 4 to 6 short articles throughout the appointment year, whether it is case studies by themselves or solicited from other thoracic surgeons, news or opinion pieces on resident issues, or summaries of resident-oriented sessions at meetings they attend.
Please send a CV and cover letter indicating your interest to [email protected] Deadline: October 15, 2012
Thoracic Surgery News is seeking 2 new resident associate medical editors for a 1-year appointment for our publication. To apply, you should be a resident in a field of thoracic surgery and willing to review and potentially comment upon articles for our monthly Residents’ Corner section.
In addition, resident medical editors are expected to work with the other editors to contribute 4 to 6 short articles throughout the appointment year, whether it is case studies by themselves or solicited from other thoracic surgeons, news or opinion pieces on resident issues, or summaries of resident-oriented sessions at meetings they attend.
Please send a CV and cover letter indicating your interest to [email protected] Deadline: October 15, 2012
Attention: New Resident Medical Editors Wanted! (copy 1)
Thoracic Surgery News is seeking 2 new resident associate medical editors for a 1-year appointment for our publication. To apply, you should be a resident in a field of thoracic surgery and willing to review and potentially comment upon articles for our monthly Residents’ Corner section.
In addition, resident medical editors are expected to work with the other editors to contribute 4 to 6 short articles throughout the appointment year, whether it is case studies by themselves or solicited from other thoracic surgeons, news or opinion pieces on resident issues, or summaries of resident-oriented sessions at meetings they attend.
Please send a CV and cover letter indicating your interest to [email protected] Deadline: October 15, 2012
Thoracic Surgery News is seeking 2 new resident associate medical editors for a 1-year appointment for our publication. To apply, you should be a resident in a field of thoracic surgery and willing to review and potentially comment upon articles for our monthly Residents’ Corner section.
In addition, resident medical editors are expected to work with the other editors to contribute 4 to 6 short articles throughout the appointment year, whether it is case studies by themselves or solicited from other thoracic surgeons, news or opinion pieces on resident issues, or summaries of resident-oriented sessions at meetings they attend.
Please send a CV and cover letter indicating your interest to [email protected] Deadline: October 15, 2012
Thoracic Surgery News is seeking 2 new resident associate medical editors for a 1-year appointment for our publication. To apply, you should be a resident in a field of thoracic surgery and willing to review and potentially comment upon articles for our monthly Residents’ Corner section.
In addition, resident medical editors are expected to work with the other editors to contribute 4 to 6 short articles throughout the appointment year, whether it is case studies by themselves or solicited from other thoracic surgeons, news or opinion pieces on resident issues, or summaries of resident-oriented sessions at meetings they attend.
Please send a CV and cover letter indicating your interest to [email protected] Deadline: October 15, 2012
Attention: New Resident Medical Editors Wanted!
Thoracic Surgery News is seeking 2 new resident associate medical editors for a 1-year appointment for our publication. To apply, you should be a resident in a field of thoracic surgery and willing to review and potentially comment upon articles for our monthly Residents’ Corner section.
In addition, resident medical editors are expected to work with the other editors to contribute 4 to 6 short articles throughout the appointment year, whether it is case studies by themselves or solicited from other thoracic surgeons, news or opinion pieces on resident issues, or summaries of resident-oriented sessions at meetings they attend.
Please send a CV and cover letter indicating your interest to [email protected] Deadline: November 15, 2012
Thoracic Surgery News is seeking 2 new resident associate medical editors for a 1-year appointment for our publication. To apply, you should be a resident in a field of thoracic surgery and willing to review and potentially comment upon articles for our monthly Residents’ Corner section.
In addition, resident medical editors are expected to work with the other editors to contribute 4 to 6 short articles throughout the appointment year, whether it is case studies by themselves or solicited from other thoracic surgeons, news or opinion pieces on resident issues, or summaries of resident-oriented sessions at meetings they attend.
Please send a CV and cover letter indicating your interest to [email protected] Deadline: November 15, 2012
Thoracic Surgery News is seeking 2 new resident associate medical editors for a 1-year appointment for our publication. To apply, you should be a resident in a field of thoracic surgery and willing to review and potentially comment upon articles for our monthly Residents’ Corner section.
In addition, resident medical editors are expected to work with the other editors to contribute 4 to 6 short articles throughout the appointment year, whether it is case studies by themselves or solicited from other thoracic surgeons, news or opinion pieces on resident issues, or summaries of resident-oriented sessions at meetings they attend.
Please send a CV and cover letter indicating your interest to [email protected] Deadline: November 15, 2012
PMI After Hip Fracture Surgery
Perioperative myocardial infarction (PMI) often remains unrecognized with higher mortality in the aged.13 Perioperative ischemic symptoms are often masked by analgesia, sedation, and transient and subtle electrocardiographic (ECG) changes. Postoperative troponin measurement is not routinely done for PMI diagnosis. Hip fracture surgery is the most common non‐cardiac surgical procedure in the elderly, with limited data on clinical presentation of PMI.46 Moreover, the elderly are significantly underrepresented in clinical studies.7 We therefore examined the clinical presentation of PMI and its outcomes among elderly patients admitted for hip fracture repair.
METHODS
Study Population
A population‐based, retrospective, case‐control study was conducted of all residents in Olmsted County, Minnesota undergoing surgery for hip fracture repair from January 1, 1988 through December 31, 2002. Primary indication for the surgery was proximal femur (femoral neck or subtrochanteric) fracture. Patients who were 65 years old, had a pathological hip fracture, multiple injuries or fractures, surgery >72 hours after injury (due to higher mortality with delayed surgery),8 nonsurgical management of hip fracture repair, or incomplete data were excluded. All patients provided prior authorization to use their medical records for research, per institutional protocols.9
Criteria for Perioperative Myocardial Infarction and Death
We utilized the universal definition of acute myocardial infarction10 to define PMI within the first 7 days following hip fracture surgery. We included creatine kinase‐MB fraction (CK‐MB) as the biomarker for 1988July 2000, and troponin as the biomarker for August 20002002. Mortality was defined as death from any cause within the first year following hip fracture repair. Deaths were identified through the National Death Index.
Statistical Analysis
For each case of PMI, we identified 2 control patients who were selected at random from the non‐PMI patient population. These controls were matched to cases based on age at the time of surgery (5 years) and gender in 1:2 ratios. Baseline characteristics across PMI and non‐PMI groups were compared using the Kruskal‐Wallis test (for continuous data) and the chi‐square or Fisher's exact tests (for categorical data). Mean values were utilized in place of the missing values for the following variables: preoperative troponin (missing values 88 [17.5%]), CK‐MB (8 [1.6%]), troponin (21 [5.4%]), and postoperative hemoglobin (17 [3.4%]). Univariate predictors of PMI with P 0.2 baseline characteristics were entered into a multivariate, conditional, logistic regression analysis. Rates of outcomes were calculated using the Kaplan‐Meier method, and by a landmark survival curve for those with and without PMI. Cox proportional hazards analysis was utilized for survival analysis at 30 days and 1 year. All statistical tests were 2‐sided, and P values 0.05 were considered significant. All analyses were performed using SAS for UNIX (version 9.1.3; SAS Institute, Inc, Cary, NC).
RESULTS
In the cohort of 1212 with hip fracture surgeries, 167 (13.8%) cases of PMI occurred in the first 7 days, of which 153 (92%) occurred within the first 48 hours. A total of 334 controls were matched with 167 cases of PMI. Table 1 summarizes the demographic characteristics of the study participants. Of the patients with PMI, 25.2% experienced symptoms of ischemia; 7% reported chest pain, and 12% reported dyspnea. Only 22.8% of patients with PMI had ECG changes consistent with ischemia. ST elevation MI was present in 7.2% patients. PMI patients had a lower mean hemoglobin compared to the patients without PMI (8.9 mg/dL vs 9.4 mg/dL, P 0.001). Median length of stay (LOS) in the hospital was higher among patients who experienced PMI (11.6 vs 7.4 days, P 0.001). Overall in‐hospital mortality was 5.6%. There were 24 deaths (14.4%) in the PMI group compared to 4 (1.2%) in‐hospital deaths in patients without PMI (P 0.001). A total of 473 (94%) patients survived to discharge. At 30‐day follow‐up, there were 29 (17.4%) deaths in the PMI group and 14 (4.2%) deaths in non‐PMI group. During the follow‐up for 1 year, there were 143 (29%) deaths: PMI 66 (39.5%) and 77 (23%) non‐PMI group (P 0.01).
| Characteristics, n (%) | Patients With PMI | Patients Without PMI | P Value* |
|---|---|---|---|
| (N = 167) | (N = 334) | ||
| |||
| Age mean SD | 85.3 7.4 | 85.2 7.1 | 0.5 |
| Weight (kg) mean SD | 59.98 16.7 | 59.80 13.9 | 0.5 |
| Women | 127 (76.4) | 254 (76) | 0.5 |
| Any symptom of ischemia, n (%) | |||
| Chest/arm pain | 11 (7) | 4 (1) | 0.002 |
| Dyspnea | 20 (12) | 14 (4) | 0.001 |
| Nausea/vomiting | 8 (5) | 6 (2) | 0.08 |
| Diaphoresis | 1 (1) | 1 (0.3) | 1.0 |
| PND | 3 (2) | 1 (0.3) | 0.3 |
| ECG changes, n (%) | |||
| ST‐segment elevation MI | 12 (7.2) | 0 | 0.01 |
| New ECG changes consistent with ischemia | 38 (22.8) | 1(0.3) | 0.01 |
| Biochemical evidence of ischemia, n (%) | |||
| CK‐MB | 147 (88) | 20 (6) | 0.01 |
| Troponin | 52 (33) | 9 (3) | 0.001 |
| Laboratory markers | |||
| Hemoglobin gm/dL mean (SD) | 8.9 1.0 | 9.4 1.2 | 0.001 |
| Postoperative anemia (8.0 gm/dL), n (%) | 22 (13.2) | 37 (11.1) | 0.5 |
| Length of stay (days), mean SD | 11.6 7.7 | 7.4 6.4 | 0.001 |
| In‐hospital outcome | 0.001 | ||
| Dead | 24 (14.4) | 4 (1.2) | |
| Alive | 143 (85.6) | 330 (98.8) | |
| 30‐Day outcome | 0.001 | ||
| Dead | 29 (17.4) | 14 (4.2) | |
| Alive | 138 (82.6) | 320 (95.8) | |
| 1‐Year outcome | 0.001 | ||
| Dead | 66 (39.5) | 77 (23) | |
| Alive | 101 (60.4) | 257 (77) | |
Table 2 describes the risk factors associated with PMI in‐hospital, 30‐day, and 1‐year mortality. Risk factors for PMI were coronary artery disease (CAD) (odds ratio [OR], 3.5; confidence interval [CI], 2.25.6), and serum creatinine >2 mg/dL (OR, 2.4; CI, 1.34.4). Risk factors for in‐hospital mortality were age 8589 (OR, 5.3; CI, 1.617.7), age 90 (OR, 8.9; CI, 2.630.8), PMI (OR 15.1; CI, 4.648.8), male gender (OR 5.8; CI, 2.215.2), dyspnea (OR 5.4; CI, 1.816.9), and hemoglobin 8.0 gm/dL (OR, 3.5; CI, 1.29.9). PMI was a strong predictor for 30‐day mortality (hazard ratio [HR], 4.3; CI, 2.18.9). Risk factors for 1‐year mortality were: age 90 (HR, 2.0; CI, 1.43.1), male gender (HR, 2.1; CI, 1.53.0), and PMI (HR, 1.9; CI, 1.42.7). Figures 1 and 2 describe the Kaplan‐Meier survival curves for patients with and without PMI.
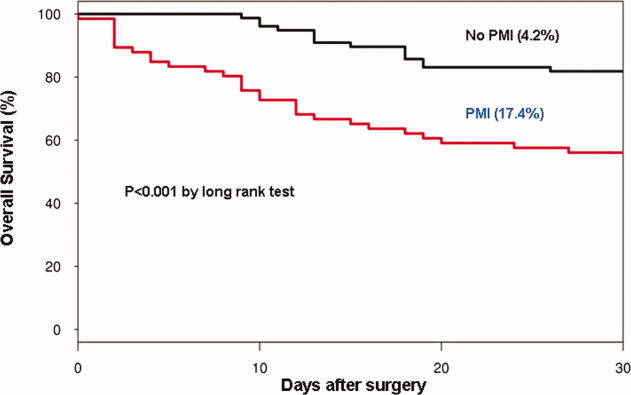
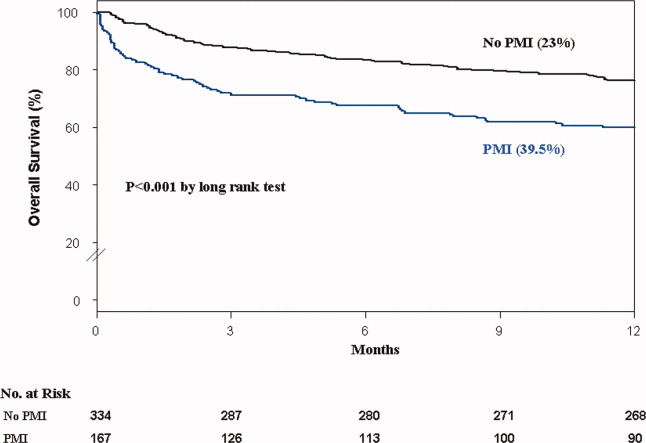
| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | P Value | |
|---|---|---|---|
| |||
| Perioperative myocardial infarction | |||
| Coronary artery disease | 3.0 (2.14.5) | 3.5 (2.25.6) | 0.001 |
| Serum creatinine >2.0 mg/dL | 2.7 (1.64.8) | 2.4 (1.34.4) | 0.003 |
| In‐hospital mortality | |||
| Age 8589 | 1.7 (0.83.7) | 5.3 (1.617.7) | 0.01 |
| Age 90 | 2.2 (1.04.8) | 8.9 (2.630.8) | 0.001 |
| Male gender | 3.0 (1.46.4) | 5.8 (2.215.2) | 0.001 |
| Postoperative anemia (8.0 gm/dL) | 4.2 (1.710.0) | 3.5 (1.29.9) | 0.02 |
| Perioperative myocardial infarction | 14.0 (5.248.0) | 15.1 (4.649.0) | 0.001 |
| 30‐Day mortality | |||
| Perioperative myocardial infarction | 4.1 (2.27.8) | 4.3 (2.18.9) | 0.001 |
| 1‐Year mortality | |||
| Age 8589 | 1.3 (0.81.9) | 1.6 (1.02.4) | 0.03 |
| Age 90 | 1.9 (1.32.9) | 2.0 (1.43.1) | 0.001 |
| Male gender | 1.9 (1.32.6) | 2.1 (1.53.0) | 0.001 |
| Dementia | 2.5 (1.83.6) | 2.7 (1.93.8) | 0.001 |
| Perioperative myocardial infarction | 2.0 (1.52.8) | 1.9 (1.42.7) | 0.001 |
DISCUSSION
We report the high incidence of PMI (13.8%) in the cohort of 1212 elderly patients (mean age 85 years) undergoing hip fracture surgery. Most PMI events (92%) occurred within the first 48 hours of surgery. Most of the events (75%) were asymptomatic. Elderly patients with PMI had an increased hospital LOS by 4.2 days, with high in‐hospital mortality (13.8%), 30‐day mortality (17.4%), and 1‐year mortality (39.5%).
Most of the PMI patients were identified with cardiac biomarkers on the basis of universal definition of MI within the first 48 hours. Although universal definition of MI does not define PMI as a separate type, PMI shares common pathophysiological pathways of Type 1 MI (primary coronary event) and Type 2 MI (myocardial oxygen supplydemand imbalance). Postoperative tachycardia, hemodynamic instability, anemia, and hypoxemia may initiate pathways causing more Type 2 MI. Our study highlights the continued need for active surveillance of clinical symptoms, postoperative ECG monitoring for STT changes, and utilizing cardiac troponin in older postoperative patients to improve diagnostic accuracy of PMI.
The current study has higher asymptomatic PMI events when compared to a study of Devereaux et al.11 The current study had an older population undergoing urgent hip fracture surgery, with a higher burden of CAD (60%) and renal failure (20%) with serum creatinine >2 gm/dL (see Supporting Information, Appendix 1, in the online version of this article). Older age and a higher burden of these risk factors may explain the higher incidence of PMI in the current study. Perioperative liberal use of analgesics in hip fracture surgery may explain more asymptomatic patients.
In light of the recently published FOCUS12 trial, an important finding from our study is that postoperative anemia among elderly (8.0 gm/dL) is associated with a 3.5‐fold increased in‐hospital mortality. It is critical to maintain perioperative hemoglobin above 8.0 gm/dL in very elderly patients, due to asymptomatic presentation of PMI.
In the current study, PMI is associated with a 15‐fold increased risk of in‐hospital death and a 4.3‐fold increased risk of 30‐day mortality in the elderly. Advanced age (85 years) is a well known strong predictor of initial hospital admission and death in elderly patients after outpatient surgery.13 Furthermore, the odds for an in‐hospital death increase by 70% for each 10‐year increase in age.14 Therefore, early detection of silent PMI among at‐risk elderly patients by cardiac biomarkers may help in optimization of cardiac pharmacotherapy known to decrease short‐ and long‐term mortality.
There are limitations inherent to the retrospective design and methodology. Data collection was done through the year 2002. CK was used for the period that spans from 1988 to mid‐2000. Troponin was used from 2000 to 2002. Statin use was not analyzed for lack of significant data. Limited use of beta‐blockers (15%) and angiotensin‐converting‐enzyme (ACE) inhibitors (25%) may also contribute to higher events (see Supporting Information, Appendix 1, in the online version of this article).
CONCLUSIONS
Elderly patients have a higher incidence of PMI and mortality after hip fracture surgery than what guidelines indicate. The majority of the elderly patients with PMI did not experience ischemic symptoms and required cardiac biomarkers for diagnosis. The results of our study support the measurement of troponin in postoperative elderly patients for the diagnosis of PMI to implement in‐hospital preventive strategies to reduce PMI‐associated mortality.
Acknowledgements
The authors gratefully acknowledge the assistance of Ms Dawn Bergen in drafting and editing the manuscript.
Disclosures: This research was supported by funding from AHA grant 03‐30103N‐04, Rochester Epidemiology Project (grant RO1‐AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases). The project was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1 RR024150. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
- , , , et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med. 2001;134(8):637–643.
- , , , et al. Meta‐analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380–390.
- , , , et al. Body mass index (BMI) and risk of noncardiac postoperative medical complications in elderly hip fracture patients: a population‐based study. J Hosp Med. 2009;4(8):E1–E9.
- . History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274.
- , , , . Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573–1579.
- , , , et al. Body mass index and risk of adverse cardiac events in elderly patients with hip fracture: a population‐based study. J Am Geriatr Soc. 2009;57(3):419–426.
- , , , et al. Acute coronary care in the elderly, part I. Non‐ST‐segment‐elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115(19):2549–2569.
- , . Hip fracture mortality. A prospective, multifactorial study to predict and minimize death risk. Clin Orthop Relat Res. 1992;280:214–222.
- , , , et al. ACC/AHA/ACP‐ASIM guidelines for the management of patients with chronic stable angina. J Am Coll Cardiol. 1999;33(7):2092–2190.
- , , ; for the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50(22):2173–2195.
- , , , et al. Characteristics and short‐term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery. Ann Intern Med. 2011;154(8):523–528.
- , , , et al. Liberal or restrictive transfusion in high‐risk patients after hip surgery. N Engl J Med. 2011;365(26):2453–2462.
- , , , . Inpatient hospital admission and death after outpatient surgery in elderly patients: importance of patient and system characteristics and location of care. Arch Surg. 2004;139(1):67–72.
- , , , et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345–2353.
Perioperative myocardial infarction (PMI) often remains unrecognized with higher mortality in the aged.13 Perioperative ischemic symptoms are often masked by analgesia, sedation, and transient and subtle electrocardiographic (ECG) changes. Postoperative troponin measurement is not routinely done for PMI diagnosis. Hip fracture surgery is the most common non‐cardiac surgical procedure in the elderly, with limited data on clinical presentation of PMI.46 Moreover, the elderly are significantly underrepresented in clinical studies.7 We therefore examined the clinical presentation of PMI and its outcomes among elderly patients admitted for hip fracture repair.
METHODS
Study Population
A population‐based, retrospective, case‐control study was conducted of all residents in Olmsted County, Minnesota undergoing surgery for hip fracture repair from January 1, 1988 through December 31, 2002. Primary indication for the surgery was proximal femur (femoral neck or subtrochanteric) fracture. Patients who were 65 years old, had a pathological hip fracture, multiple injuries or fractures, surgery >72 hours after injury (due to higher mortality with delayed surgery),8 nonsurgical management of hip fracture repair, or incomplete data were excluded. All patients provided prior authorization to use their medical records for research, per institutional protocols.9
Criteria for Perioperative Myocardial Infarction and Death
We utilized the universal definition of acute myocardial infarction10 to define PMI within the first 7 days following hip fracture surgery. We included creatine kinase‐MB fraction (CK‐MB) as the biomarker for 1988July 2000, and troponin as the biomarker for August 20002002. Mortality was defined as death from any cause within the first year following hip fracture repair. Deaths were identified through the National Death Index.
Statistical Analysis
For each case of PMI, we identified 2 control patients who were selected at random from the non‐PMI patient population. These controls were matched to cases based on age at the time of surgery (5 years) and gender in 1:2 ratios. Baseline characteristics across PMI and non‐PMI groups were compared using the Kruskal‐Wallis test (for continuous data) and the chi‐square or Fisher's exact tests (for categorical data). Mean values were utilized in place of the missing values for the following variables: preoperative troponin (missing values 88 [17.5%]), CK‐MB (8 [1.6%]), troponin (21 [5.4%]), and postoperative hemoglobin (17 [3.4%]). Univariate predictors of PMI with P 0.2 baseline characteristics were entered into a multivariate, conditional, logistic regression analysis. Rates of outcomes were calculated using the Kaplan‐Meier method, and by a landmark survival curve for those with and without PMI. Cox proportional hazards analysis was utilized for survival analysis at 30 days and 1 year. All statistical tests were 2‐sided, and P values 0.05 were considered significant. All analyses were performed using SAS for UNIX (version 9.1.3; SAS Institute, Inc, Cary, NC).
RESULTS
In the cohort of 1212 with hip fracture surgeries, 167 (13.8%) cases of PMI occurred in the first 7 days, of which 153 (92%) occurred within the first 48 hours. A total of 334 controls were matched with 167 cases of PMI. Table 1 summarizes the demographic characteristics of the study participants. Of the patients with PMI, 25.2% experienced symptoms of ischemia; 7% reported chest pain, and 12% reported dyspnea. Only 22.8% of patients with PMI had ECG changes consistent with ischemia. ST elevation MI was present in 7.2% patients. PMI patients had a lower mean hemoglobin compared to the patients without PMI (8.9 mg/dL vs 9.4 mg/dL, P 0.001). Median length of stay (LOS) in the hospital was higher among patients who experienced PMI (11.6 vs 7.4 days, P 0.001). Overall in‐hospital mortality was 5.6%. There were 24 deaths (14.4%) in the PMI group compared to 4 (1.2%) in‐hospital deaths in patients without PMI (P 0.001). A total of 473 (94%) patients survived to discharge. At 30‐day follow‐up, there were 29 (17.4%) deaths in the PMI group and 14 (4.2%) deaths in non‐PMI group. During the follow‐up for 1 year, there were 143 (29%) deaths: PMI 66 (39.5%) and 77 (23%) non‐PMI group (P 0.01).
| Characteristics, n (%) | Patients With PMI | Patients Without PMI | P Value* |
|---|---|---|---|
| (N = 167) | (N = 334) | ||
| |||
| Age mean SD | 85.3 7.4 | 85.2 7.1 | 0.5 |
| Weight (kg) mean SD | 59.98 16.7 | 59.80 13.9 | 0.5 |
| Women | 127 (76.4) | 254 (76) | 0.5 |
| Any symptom of ischemia, n (%) | |||
| Chest/arm pain | 11 (7) | 4 (1) | 0.002 |
| Dyspnea | 20 (12) | 14 (4) | 0.001 |
| Nausea/vomiting | 8 (5) | 6 (2) | 0.08 |
| Diaphoresis | 1 (1) | 1 (0.3) | 1.0 |
| PND | 3 (2) | 1 (0.3) | 0.3 |
| ECG changes, n (%) | |||
| ST‐segment elevation MI | 12 (7.2) | 0 | 0.01 |
| New ECG changes consistent with ischemia | 38 (22.8) | 1(0.3) | 0.01 |
| Biochemical evidence of ischemia, n (%) | |||
| CK‐MB | 147 (88) | 20 (6) | 0.01 |
| Troponin | 52 (33) | 9 (3) | 0.001 |
| Laboratory markers | |||
| Hemoglobin gm/dL mean (SD) | 8.9 1.0 | 9.4 1.2 | 0.001 |
| Postoperative anemia (8.0 gm/dL), n (%) | 22 (13.2) | 37 (11.1) | 0.5 |
| Length of stay (days), mean SD | 11.6 7.7 | 7.4 6.4 | 0.001 |
| In‐hospital outcome | 0.001 | ||
| Dead | 24 (14.4) | 4 (1.2) | |
| Alive | 143 (85.6) | 330 (98.8) | |
| 30‐Day outcome | 0.001 | ||
| Dead | 29 (17.4) | 14 (4.2) | |
| Alive | 138 (82.6) | 320 (95.8) | |
| 1‐Year outcome | 0.001 | ||
| Dead | 66 (39.5) | 77 (23) | |
| Alive | 101 (60.4) | 257 (77) | |
Table 2 describes the risk factors associated with PMI in‐hospital, 30‐day, and 1‐year mortality. Risk factors for PMI were coronary artery disease (CAD) (odds ratio [OR], 3.5; confidence interval [CI], 2.25.6), and serum creatinine >2 mg/dL (OR, 2.4; CI, 1.34.4). Risk factors for in‐hospital mortality were age 8589 (OR, 5.3; CI, 1.617.7), age 90 (OR, 8.9; CI, 2.630.8), PMI (OR 15.1; CI, 4.648.8), male gender (OR 5.8; CI, 2.215.2), dyspnea (OR 5.4; CI, 1.816.9), and hemoglobin 8.0 gm/dL (OR, 3.5; CI, 1.29.9). PMI was a strong predictor for 30‐day mortality (hazard ratio [HR], 4.3; CI, 2.18.9). Risk factors for 1‐year mortality were: age 90 (HR, 2.0; CI, 1.43.1), male gender (HR, 2.1; CI, 1.53.0), and PMI (HR, 1.9; CI, 1.42.7). Figures 1 and 2 describe the Kaplan‐Meier survival curves for patients with and without PMI.


| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | P Value | |
|---|---|---|---|
| |||
| Perioperative myocardial infarction | |||
| Coronary artery disease | 3.0 (2.14.5) | 3.5 (2.25.6) | 0.001 |
| Serum creatinine >2.0 mg/dL | 2.7 (1.64.8) | 2.4 (1.34.4) | 0.003 |
| In‐hospital mortality | |||
| Age 8589 | 1.7 (0.83.7) | 5.3 (1.617.7) | 0.01 |
| Age 90 | 2.2 (1.04.8) | 8.9 (2.630.8) | 0.001 |
| Male gender | 3.0 (1.46.4) | 5.8 (2.215.2) | 0.001 |
| Postoperative anemia (8.0 gm/dL) | 4.2 (1.710.0) | 3.5 (1.29.9) | 0.02 |
| Perioperative myocardial infarction | 14.0 (5.248.0) | 15.1 (4.649.0) | 0.001 |
| 30‐Day mortality | |||
| Perioperative myocardial infarction | 4.1 (2.27.8) | 4.3 (2.18.9) | 0.001 |
| 1‐Year mortality | |||
| Age 8589 | 1.3 (0.81.9) | 1.6 (1.02.4) | 0.03 |
| Age 90 | 1.9 (1.32.9) | 2.0 (1.43.1) | 0.001 |
| Male gender | 1.9 (1.32.6) | 2.1 (1.53.0) | 0.001 |
| Dementia | 2.5 (1.83.6) | 2.7 (1.93.8) | 0.001 |
| Perioperative myocardial infarction | 2.0 (1.52.8) | 1.9 (1.42.7) | 0.001 |
DISCUSSION
We report the high incidence of PMI (13.8%) in the cohort of 1212 elderly patients (mean age 85 years) undergoing hip fracture surgery. Most PMI events (92%) occurred within the first 48 hours of surgery. Most of the events (75%) were asymptomatic. Elderly patients with PMI had an increased hospital LOS by 4.2 days, with high in‐hospital mortality (13.8%), 30‐day mortality (17.4%), and 1‐year mortality (39.5%).
Most of the PMI patients were identified with cardiac biomarkers on the basis of universal definition of MI within the first 48 hours. Although universal definition of MI does not define PMI as a separate type, PMI shares common pathophysiological pathways of Type 1 MI (primary coronary event) and Type 2 MI (myocardial oxygen supplydemand imbalance). Postoperative tachycardia, hemodynamic instability, anemia, and hypoxemia may initiate pathways causing more Type 2 MI. Our study highlights the continued need for active surveillance of clinical symptoms, postoperative ECG monitoring for STT changes, and utilizing cardiac troponin in older postoperative patients to improve diagnostic accuracy of PMI.
The current study has higher asymptomatic PMI events when compared to a study of Devereaux et al.11 The current study had an older population undergoing urgent hip fracture surgery, with a higher burden of CAD (60%) and renal failure (20%) with serum creatinine >2 gm/dL (see Supporting Information, Appendix 1, in the online version of this article). Older age and a higher burden of these risk factors may explain the higher incidence of PMI in the current study. Perioperative liberal use of analgesics in hip fracture surgery may explain more asymptomatic patients.
In light of the recently published FOCUS12 trial, an important finding from our study is that postoperative anemia among elderly (8.0 gm/dL) is associated with a 3.5‐fold increased in‐hospital mortality. It is critical to maintain perioperative hemoglobin above 8.0 gm/dL in very elderly patients, due to asymptomatic presentation of PMI.
In the current study, PMI is associated with a 15‐fold increased risk of in‐hospital death and a 4.3‐fold increased risk of 30‐day mortality in the elderly. Advanced age (85 years) is a well known strong predictor of initial hospital admission and death in elderly patients after outpatient surgery.13 Furthermore, the odds for an in‐hospital death increase by 70% for each 10‐year increase in age.14 Therefore, early detection of silent PMI among at‐risk elderly patients by cardiac biomarkers may help in optimization of cardiac pharmacotherapy known to decrease short‐ and long‐term mortality.
There are limitations inherent to the retrospective design and methodology. Data collection was done through the year 2002. CK was used for the period that spans from 1988 to mid‐2000. Troponin was used from 2000 to 2002. Statin use was not analyzed for lack of significant data. Limited use of beta‐blockers (15%) and angiotensin‐converting‐enzyme (ACE) inhibitors (25%) may also contribute to higher events (see Supporting Information, Appendix 1, in the online version of this article).
CONCLUSIONS
Elderly patients have a higher incidence of PMI and mortality after hip fracture surgery than what guidelines indicate. The majority of the elderly patients with PMI did not experience ischemic symptoms and required cardiac biomarkers for diagnosis. The results of our study support the measurement of troponin in postoperative elderly patients for the diagnosis of PMI to implement in‐hospital preventive strategies to reduce PMI‐associated mortality.
Acknowledgements
The authors gratefully acknowledge the assistance of Ms Dawn Bergen in drafting and editing the manuscript.
Disclosures: This research was supported by funding from AHA grant 03‐30103N‐04, Rochester Epidemiology Project (grant RO1‐AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases). The project was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1 RR024150. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Perioperative myocardial infarction (PMI) often remains unrecognized with higher mortality in the aged.13 Perioperative ischemic symptoms are often masked by analgesia, sedation, and transient and subtle electrocardiographic (ECG) changes. Postoperative troponin measurement is not routinely done for PMI diagnosis. Hip fracture surgery is the most common non‐cardiac surgical procedure in the elderly, with limited data on clinical presentation of PMI.46 Moreover, the elderly are significantly underrepresented in clinical studies.7 We therefore examined the clinical presentation of PMI and its outcomes among elderly patients admitted for hip fracture repair.
METHODS
Study Population
A population‐based, retrospective, case‐control study was conducted of all residents in Olmsted County, Minnesota undergoing surgery for hip fracture repair from January 1, 1988 through December 31, 2002. Primary indication for the surgery was proximal femur (femoral neck or subtrochanteric) fracture. Patients who were 65 years old, had a pathological hip fracture, multiple injuries or fractures, surgery >72 hours after injury (due to higher mortality with delayed surgery),8 nonsurgical management of hip fracture repair, or incomplete data were excluded. All patients provided prior authorization to use their medical records for research, per institutional protocols.9
Criteria for Perioperative Myocardial Infarction and Death
We utilized the universal definition of acute myocardial infarction10 to define PMI within the first 7 days following hip fracture surgery. We included creatine kinase‐MB fraction (CK‐MB) as the biomarker for 1988July 2000, and troponin as the biomarker for August 20002002. Mortality was defined as death from any cause within the first year following hip fracture repair. Deaths were identified through the National Death Index.
Statistical Analysis
For each case of PMI, we identified 2 control patients who were selected at random from the non‐PMI patient population. These controls were matched to cases based on age at the time of surgery (5 years) and gender in 1:2 ratios. Baseline characteristics across PMI and non‐PMI groups were compared using the Kruskal‐Wallis test (for continuous data) and the chi‐square or Fisher's exact tests (for categorical data). Mean values were utilized in place of the missing values for the following variables: preoperative troponin (missing values 88 [17.5%]), CK‐MB (8 [1.6%]), troponin (21 [5.4%]), and postoperative hemoglobin (17 [3.4%]). Univariate predictors of PMI with P 0.2 baseline characteristics were entered into a multivariate, conditional, logistic regression analysis. Rates of outcomes were calculated using the Kaplan‐Meier method, and by a landmark survival curve for those with and without PMI. Cox proportional hazards analysis was utilized for survival analysis at 30 days and 1 year. All statistical tests were 2‐sided, and P values 0.05 were considered significant. All analyses were performed using SAS for UNIX (version 9.1.3; SAS Institute, Inc, Cary, NC).
RESULTS
In the cohort of 1212 with hip fracture surgeries, 167 (13.8%) cases of PMI occurred in the first 7 days, of which 153 (92%) occurred within the first 48 hours. A total of 334 controls were matched with 167 cases of PMI. Table 1 summarizes the demographic characteristics of the study participants. Of the patients with PMI, 25.2% experienced symptoms of ischemia; 7% reported chest pain, and 12% reported dyspnea. Only 22.8% of patients with PMI had ECG changes consistent with ischemia. ST elevation MI was present in 7.2% patients. PMI patients had a lower mean hemoglobin compared to the patients without PMI (8.9 mg/dL vs 9.4 mg/dL, P 0.001). Median length of stay (LOS) in the hospital was higher among patients who experienced PMI (11.6 vs 7.4 days, P 0.001). Overall in‐hospital mortality was 5.6%. There were 24 deaths (14.4%) in the PMI group compared to 4 (1.2%) in‐hospital deaths in patients without PMI (P 0.001). A total of 473 (94%) patients survived to discharge. At 30‐day follow‐up, there were 29 (17.4%) deaths in the PMI group and 14 (4.2%) deaths in non‐PMI group. During the follow‐up for 1 year, there were 143 (29%) deaths: PMI 66 (39.5%) and 77 (23%) non‐PMI group (P 0.01).
| Characteristics, n (%) | Patients With PMI | Patients Without PMI | P Value* |
|---|---|---|---|
| (N = 167) | (N = 334) | ||
| |||
| Age mean SD | 85.3 7.4 | 85.2 7.1 | 0.5 |
| Weight (kg) mean SD | 59.98 16.7 | 59.80 13.9 | 0.5 |
| Women | 127 (76.4) | 254 (76) | 0.5 |
| Any symptom of ischemia, n (%) | |||
| Chest/arm pain | 11 (7) | 4 (1) | 0.002 |
| Dyspnea | 20 (12) | 14 (4) | 0.001 |
| Nausea/vomiting | 8 (5) | 6 (2) | 0.08 |
| Diaphoresis | 1 (1) | 1 (0.3) | 1.0 |
| PND | 3 (2) | 1 (0.3) | 0.3 |
| ECG changes, n (%) | |||
| ST‐segment elevation MI | 12 (7.2) | 0 | 0.01 |
| New ECG changes consistent with ischemia | 38 (22.8) | 1(0.3) | 0.01 |
| Biochemical evidence of ischemia, n (%) | |||
| CK‐MB | 147 (88) | 20 (6) | 0.01 |
| Troponin | 52 (33) | 9 (3) | 0.001 |
| Laboratory markers | |||
| Hemoglobin gm/dL mean (SD) | 8.9 1.0 | 9.4 1.2 | 0.001 |
| Postoperative anemia (8.0 gm/dL), n (%) | 22 (13.2) | 37 (11.1) | 0.5 |
| Length of stay (days), mean SD | 11.6 7.7 | 7.4 6.4 | 0.001 |
| In‐hospital outcome | 0.001 | ||
| Dead | 24 (14.4) | 4 (1.2) | |
| Alive | 143 (85.6) | 330 (98.8) | |
| 30‐Day outcome | 0.001 | ||
| Dead | 29 (17.4) | 14 (4.2) | |
| Alive | 138 (82.6) | 320 (95.8) | |
| 1‐Year outcome | 0.001 | ||
| Dead | 66 (39.5) | 77 (23) | |
| Alive | 101 (60.4) | 257 (77) | |
Table 2 describes the risk factors associated with PMI in‐hospital, 30‐day, and 1‐year mortality. Risk factors for PMI were coronary artery disease (CAD) (odds ratio [OR], 3.5; confidence interval [CI], 2.25.6), and serum creatinine >2 mg/dL (OR, 2.4; CI, 1.34.4). Risk factors for in‐hospital mortality were age 8589 (OR, 5.3; CI, 1.617.7), age 90 (OR, 8.9; CI, 2.630.8), PMI (OR 15.1; CI, 4.648.8), male gender (OR 5.8; CI, 2.215.2), dyspnea (OR 5.4; CI, 1.816.9), and hemoglobin 8.0 gm/dL (OR, 3.5; CI, 1.29.9). PMI was a strong predictor for 30‐day mortality (hazard ratio [HR], 4.3; CI, 2.18.9). Risk factors for 1‐year mortality were: age 90 (HR, 2.0; CI, 1.43.1), male gender (HR, 2.1; CI, 1.53.0), and PMI (HR, 1.9; CI, 1.42.7). Figures 1 and 2 describe the Kaplan‐Meier survival curves for patients with and without PMI.


| Unadjusted OR (95% CI) | Adjusted OR (95% CI) | P Value | |
|---|---|---|---|
| |||
| Perioperative myocardial infarction | |||
| Coronary artery disease | 3.0 (2.14.5) | 3.5 (2.25.6) | 0.001 |
| Serum creatinine >2.0 mg/dL | 2.7 (1.64.8) | 2.4 (1.34.4) | 0.003 |
| In‐hospital mortality | |||
| Age 8589 | 1.7 (0.83.7) | 5.3 (1.617.7) | 0.01 |
| Age 90 | 2.2 (1.04.8) | 8.9 (2.630.8) | 0.001 |
| Male gender | 3.0 (1.46.4) | 5.8 (2.215.2) | 0.001 |
| Postoperative anemia (8.0 gm/dL) | 4.2 (1.710.0) | 3.5 (1.29.9) | 0.02 |
| Perioperative myocardial infarction | 14.0 (5.248.0) | 15.1 (4.649.0) | 0.001 |
| 30‐Day mortality | |||
| Perioperative myocardial infarction | 4.1 (2.27.8) | 4.3 (2.18.9) | 0.001 |
| 1‐Year mortality | |||
| Age 8589 | 1.3 (0.81.9) | 1.6 (1.02.4) | 0.03 |
| Age 90 | 1.9 (1.32.9) | 2.0 (1.43.1) | 0.001 |
| Male gender | 1.9 (1.32.6) | 2.1 (1.53.0) | 0.001 |
| Dementia | 2.5 (1.83.6) | 2.7 (1.93.8) | 0.001 |
| Perioperative myocardial infarction | 2.0 (1.52.8) | 1.9 (1.42.7) | 0.001 |
DISCUSSION
We report the high incidence of PMI (13.8%) in the cohort of 1212 elderly patients (mean age 85 years) undergoing hip fracture surgery. Most PMI events (92%) occurred within the first 48 hours of surgery. Most of the events (75%) were asymptomatic. Elderly patients with PMI had an increased hospital LOS by 4.2 days, with high in‐hospital mortality (13.8%), 30‐day mortality (17.4%), and 1‐year mortality (39.5%).
Most of the PMI patients were identified with cardiac biomarkers on the basis of universal definition of MI within the first 48 hours. Although universal definition of MI does not define PMI as a separate type, PMI shares common pathophysiological pathways of Type 1 MI (primary coronary event) and Type 2 MI (myocardial oxygen supplydemand imbalance). Postoperative tachycardia, hemodynamic instability, anemia, and hypoxemia may initiate pathways causing more Type 2 MI. Our study highlights the continued need for active surveillance of clinical symptoms, postoperative ECG monitoring for STT changes, and utilizing cardiac troponin in older postoperative patients to improve diagnostic accuracy of PMI.
The current study has higher asymptomatic PMI events when compared to a study of Devereaux et al.11 The current study had an older population undergoing urgent hip fracture surgery, with a higher burden of CAD (60%) and renal failure (20%) with serum creatinine >2 gm/dL (see Supporting Information, Appendix 1, in the online version of this article). Older age and a higher burden of these risk factors may explain the higher incidence of PMI in the current study. Perioperative liberal use of analgesics in hip fracture surgery may explain more asymptomatic patients.
In light of the recently published FOCUS12 trial, an important finding from our study is that postoperative anemia among elderly (8.0 gm/dL) is associated with a 3.5‐fold increased in‐hospital mortality. It is critical to maintain perioperative hemoglobin above 8.0 gm/dL in very elderly patients, due to asymptomatic presentation of PMI.
In the current study, PMI is associated with a 15‐fold increased risk of in‐hospital death and a 4.3‐fold increased risk of 30‐day mortality in the elderly. Advanced age (85 years) is a well known strong predictor of initial hospital admission and death in elderly patients after outpatient surgery.13 Furthermore, the odds for an in‐hospital death increase by 70% for each 10‐year increase in age.14 Therefore, early detection of silent PMI among at‐risk elderly patients by cardiac biomarkers may help in optimization of cardiac pharmacotherapy known to decrease short‐ and long‐term mortality.
There are limitations inherent to the retrospective design and methodology. Data collection was done through the year 2002. CK was used for the period that spans from 1988 to mid‐2000. Troponin was used from 2000 to 2002. Statin use was not analyzed for lack of significant data. Limited use of beta‐blockers (15%) and angiotensin‐converting‐enzyme (ACE) inhibitors (25%) may also contribute to higher events (see Supporting Information, Appendix 1, in the online version of this article).
CONCLUSIONS
Elderly patients have a higher incidence of PMI and mortality after hip fracture surgery than what guidelines indicate. The majority of the elderly patients with PMI did not experience ischemic symptoms and required cardiac biomarkers for diagnosis. The results of our study support the measurement of troponin in postoperative elderly patients for the diagnosis of PMI to implement in‐hospital preventive strategies to reduce PMI‐associated mortality.
Acknowledgements
The authors gratefully acknowledge the assistance of Ms Dawn Bergen in drafting and editing the manuscript.
Disclosures: This research was supported by funding from AHA grant 03‐30103N‐04, Rochester Epidemiology Project (grant RO1‐AR30582 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases). The project was also supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1 RR024150. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
- , , , et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med. 2001;134(8):637–643.
- , , , et al. Meta‐analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380–390.
- , , , et al. Body mass index (BMI) and risk of noncardiac postoperative medical complications in elderly hip fracture patients: a population‐based study. J Hosp Med. 2009;4(8):E1–E9.
- . History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274.
- , , , . Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573–1579.
- , , , et al. Body mass index and risk of adverse cardiac events in elderly patients with hip fracture: a population‐based study. J Am Geriatr Soc. 2009;57(3):419–426.
- , , , et al. Acute coronary care in the elderly, part I. Non‐ST‐segment‐elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115(19):2549–2569.
- , . Hip fracture mortality. A prospective, multifactorial study to predict and minimize death risk. Clin Orthop Relat Res. 1992;280:214–222.
- , , , et al. ACC/AHA/ACP‐ASIM guidelines for the management of patients with chronic stable angina. J Am Coll Cardiol. 1999;33(7):2092–2190.
- , , ; for the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50(22):2173–2195.
- , , , et al. Characteristics and short‐term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery. Ann Intern Med. 2011;154(8):523–528.
- , , , et al. Liberal or restrictive transfusion in high‐risk patients after hip surgery. N Engl J Med. 2011;365(26):2453–2462.
- , , , . Inpatient hospital admission and death after outpatient surgery in elderly patients: importance of patient and system characteristics and location of care. Arch Surg. 2004;139(1):67–72.
- , , , et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345–2353.
- , , , et al. Impact of age on perioperative complications and length of stay in patients undergoing noncardiac surgery. Ann Intern Med. 2001;134(8):637–643.
- , , , et al. Meta‐analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380–390.
- , , , et al. Body mass index (BMI) and risk of noncardiac postoperative medical complications in elderly hip fracture patients: a population‐based study. J Hosp Med. 2009;4(8):E1–E9.
- . History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274.
- , , , . Incidence and mortality of hip fractures in the United States. JAMA. 2009;302(14):1573–1579.
- , , , et al. Body mass index and risk of adverse cardiac events in elderly patients with hip fracture: a population‐based study. J Am Geriatr Soc. 2009;57(3):419–426.
- , , , et al. Acute coronary care in the elderly, part I. Non‐ST‐segment‐elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115(19):2549–2569.
- , . Hip fracture mortality. A prospective, multifactorial study to predict and minimize death risk. Clin Orthop Relat Res. 1992;280:214–222.
- , , , et al. ACC/AHA/ACP‐ASIM guidelines for the management of patients with chronic stable angina. J Am Coll Cardiol. 1999;33(7):2092–2190.
- , , ; for the Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007;50(22):2173–2195.
- , , , et al. Characteristics and short‐term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery. Ann Intern Med. 2011;154(8):523–528.
- , , , et al. Liberal or restrictive transfusion in high‐risk patients after hip surgery. N Engl J Med. 2011;365(26):2453–2462.
- , , , . Inpatient hospital admission and death after outpatient surgery in elderly patients: importance of patient and system characteristics and location of care. Arch Surg. 2004;139(1):67–72.
- , , , et al. Predictors of hospital mortality in the global registry of acute coronary events. Arch Intern Med. 2003;163(19):2345–2353.
Ask-Tell-Ask: Simple Technique Can Help Hospitalists Communicate Difficult Messages
Sometimes a hospitalist is put in the difficult position of communicating information that involves bad news—for instance, a poor prognosis to a patient or clarifying treatment options and goals for care to a family member of a patient with an advanced illness. A workshop at HM12 offered a technique that hospitalists can use to convey such difficult messages.
“Ask-Tell-Ask” is a back-and-forth cycle between the patient and health professional that addresses four essential components: the patient’s perspective, information that needs to be delivered, response to the patient’s emotions, and recommendations by the professional.
—Kristen Schaefer, MD, palliative-care physician, Brigham and Women’s Hospital, Boston
“In the setting of an advanced illness, the patient’s perspective needs to be more fully explored so that we can figure out what information they need and want,” says Kristen Schaefer, MD, a palliative-care physician and director of residency education at Brigham and Women’s Hospital in Boston who spoke at an HM12 workshop. “That communication needs to be multidirectional to promote shared decision-making. All of these communication techniques are based on a better understanding of the patient’s perspective, but with Ask-Tell-Ask, you are clarifying their emotional response to illness, their values and personal goals in life, and how they cope with setbacks.”
Physicians should always start in an open-ended way, asking questions and listening to the response, Dr. Schaefer explains. “Then you can tailor the information you provide to what they have told you. There’s always emotional content around these issues, and you need to clarify that emotion,” she says. “If there is a big emotion in the room, and it hasn’t been addressed, it doesn’t matter what you teach the patient. You’ll never get to the underlying problems.”
Another effective technique, Dr. Schaefer says, is the judicious use of silence. She says healthcare providers can learn to listen more, talk less, and always start with the patient’s perspective as the basis for communication.
“It makes for more satisfying work—and it’s also more effective,” she says.
Larry Beresford is a freelance writer in Oakland, Calif.
Sometimes a hospitalist is put in the difficult position of communicating information that involves bad news—for instance, a poor prognosis to a patient or clarifying treatment options and goals for care to a family member of a patient with an advanced illness. A workshop at HM12 offered a technique that hospitalists can use to convey such difficult messages.
“Ask-Tell-Ask” is a back-and-forth cycle between the patient and health professional that addresses four essential components: the patient’s perspective, information that needs to be delivered, response to the patient’s emotions, and recommendations by the professional.
—Kristen Schaefer, MD, palliative-care physician, Brigham and Women’s Hospital, Boston
“In the setting of an advanced illness, the patient’s perspective needs to be more fully explored so that we can figure out what information they need and want,” says Kristen Schaefer, MD, a palliative-care physician and director of residency education at Brigham and Women’s Hospital in Boston who spoke at an HM12 workshop. “That communication needs to be multidirectional to promote shared decision-making. All of these communication techniques are based on a better understanding of the patient’s perspective, but with Ask-Tell-Ask, you are clarifying their emotional response to illness, their values and personal goals in life, and how they cope with setbacks.”
Physicians should always start in an open-ended way, asking questions and listening to the response, Dr. Schaefer explains. “Then you can tailor the information you provide to what they have told you. There’s always emotional content around these issues, and you need to clarify that emotion,” she says. “If there is a big emotion in the room, and it hasn’t been addressed, it doesn’t matter what you teach the patient. You’ll never get to the underlying problems.”
Another effective technique, Dr. Schaefer says, is the judicious use of silence. She says healthcare providers can learn to listen more, talk less, and always start with the patient’s perspective as the basis for communication.
“It makes for more satisfying work—and it’s also more effective,” she says.
Larry Beresford is a freelance writer in Oakland, Calif.
Sometimes a hospitalist is put in the difficult position of communicating information that involves bad news—for instance, a poor prognosis to a patient or clarifying treatment options and goals for care to a family member of a patient with an advanced illness. A workshop at HM12 offered a technique that hospitalists can use to convey such difficult messages.
“Ask-Tell-Ask” is a back-and-forth cycle between the patient and health professional that addresses four essential components: the patient’s perspective, information that needs to be delivered, response to the patient’s emotions, and recommendations by the professional.
—Kristen Schaefer, MD, palliative-care physician, Brigham and Women’s Hospital, Boston
“In the setting of an advanced illness, the patient’s perspective needs to be more fully explored so that we can figure out what information they need and want,” says Kristen Schaefer, MD, a palliative-care physician and director of residency education at Brigham and Women’s Hospital in Boston who spoke at an HM12 workshop. “That communication needs to be multidirectional to promote shared decision-making. All of these communication techniques are based on a better understanding of the patient’s perspective, but with Ask-Tell-Ask, you are clarifying their emotional response to illness, their values and personal goals in life, and how they cope with setbacks.”
Physicians should always start in an open-ended way, asking questions and listening to the response, Dr. Schaefer explains. “Then you can tailor the information you provide to what they have told you. There’s always emotional content around these issues, and you need to clarify that emotion,” she says. “If there is a big emotion in the room, and it hasn’t been addressed, it doesn’t matter what you teach the patient. You’ll never get to the underlying problems.”
Another effective technique, Dr. Schaefer says, is the judicious use of silence. She says healthcare providers can learn to listen more, talk less, and always start with the patient’s perspective as the basis for communication.
“It makes for more satisfying work—and it’s also more effective,” she says.
Larry Beresford is a freelance writer in Oakland, Calif.
Huber the Tuber
Recently, I had an office visit from a lovely 80-year-old woman, born and raised in Providence, R.I., whose past medical history included pulmonary tuberculosis for which she was sent to a sanatorium – 50 years ago.
Her TB had nothing to do with why she had come to see me. By and large, this is a really healthy patient whose only complaint was a 2-month history of right shoulder pain that turned out to be caused by rotator cuff tendonitis. But I lingered with her, and we chatted for awhile. She used to work at Veterans Affairs, processing claims and grievances so she was familiar with medical terminology and was in general a joy to talk with. And I was captivated by the progress in medicine that she represented.
Now, by the time I went to medical school, we were no longer sending patients to sanatoria. The word was as abstract a concept to me as, say, injecting intramuscular gold to treat rheumatic diseases. By the time I was in training, everyone in the developing world got a BCG vaccine, which prevents severe complications from TB but does not prevent infections. As long as I have been a physician, we have understood transmission well, have known about four-drug regimens, and were aware of drug-resistant TB (I am still floored when I read about XDR-TB, with the X being short for "extensively.")
Needless to say I was fascinated by her story.
When she was originally diagnosed more than half a century ago, this woman did not have the usual symptoms that we associate with active pulmonary tuberculosis. She had not had a cough and certainly did not have "wasting." She simply tripped one day and in doing so coughed up some blood. She was found to have disease in both apices and, subsequently, she spent 14 months in a local sanatorium. She remembers being treated with "PAS and streptomycin" (PAS being p-aminosalicylic acid), and "lots of fresh air."
Although tuberculosis is rare in the USA today, it was "so rampant that cautionary visual messages appeared in myriad public places, from offices to restrooms," according to the National Library of Medicine. "Huber the Tuber" was a mascot developed by TB patient and physician Harry Wilmer (1917-2005). In the educational pamphlet, Huber rides respiratory droplets along with his cohort "Nasty von Sputum, Rusty the Bloodyvitch, and Huey the Long Tuber." That final appellation is supposedly a reference to Sen. Huey Long, according to the NLM. (Can we still anthropomorphize bacteria into corrupt government officials?)
The discovery of Mycobacterium tuberculosis by German bacteriologist Dr. Robert Koch in 1882 led to a revolution of isolating patients, which in turn led to a decrease in transmission. In 1905, the American Sanatorium Association was formed – it still exists today as the American Thoracic Society! When the association started, there were 106 sanatoria in the United States, which provided 9,107 beds for patients. At its peak in 1954, there were 108,457 beds worsening (Am. J. Respir. Crit. Care Med. 2004:169;118-6). From a patient’s journal during time spent in a sanatorium in 1944, we know that there were only two rules for sanatorium residents:
1. Absolute and utter rest of mind and body – no bath, no movement except to toilet once a day, no sitting up except propped by pillows and semireclining, no deep breath. Lead the life of a log, in fact. Don’t try, therefore, to sew, knit, or write, except as occasional relief from reading and sleeping.
2. Eat nourishing food and have plenty of fresh air.
Not everyone got antibiotic treatment, unless their chest x-rays showed worsening. Some patients were treated with an induced pneumothorax, according to the women’s journal. Why this would be is not clear to me.
Then, in 1952, isoniazid was developed, and that was the start of the end of the sanatorium.
In our daily lives, we focus on individual patients, but history informs the current practice of medicine. How wonderful that we can now treat many illnesses that were once considered uniformly fatal. How fortunate are we to call this our profession, one that provides an unambiguous good.
Dr. Chan practices rheumatology in Pawtucket, R.I.
Recently, I had an office visit from a lovely 80-year-old woman, born and raised in Providence, R.I., whose past medical history included pulmonary tuberculosis for which she was sent to a sanatorium – 50 years ago.
Her TB had nothing to do with why she had come to see me. By and large, this is a really healthy patient whose only complaint was a 2-month history of right shoulder pain that turned out to be caused by rotator cuff tendonitis. But I lingered with her, and we chatted for awhile. She used to work at Veterans Affairs, processing claims and grievances so she was familiar with medical terminology and was in general a joy to talk with. And I was captivated by the progress in medicine that she represented.
Now, by the time I went to medical school, we were no longer sending patients to sanatoria. The word was as abstract a concept to me as, say, injecting intramuscular gold to treat rheumatic diseases. By the time I was in training, everyone in the developing world got a BCG vaccine, which prevents severe complications from TB but does not prevent infections. As long as I have been a physician, we have understood transmission well, have known about four-drug regimens, and were aware of drug-resistant TB (I am still floored when I read about XDR-TB, with the X being short for "extensively.")
Needless to say I was fascinated by her story.
When she was originally diagnosed more than half a century ago, this woman did not have the usual symptoms that we associate with active pulmonary tuberculosis. She had not had a cough and certainly did not have "wasting." She simply tripped one day and in doing so coughed up some blood. She was found to have disease in both apices and, subsequently, she spent 14 months in a local sanatorium. She remembers being treated with "PAS and streptomycin" (PAS being p-aminosalicylic acid), and "lots of fresh air."
Although tuberculosis is rare in the USA today, it was "so rampant that cautionary visual messages appeared in myriad public places, from offices to restrooms," according to the National Library of Medicine. "Huber the Tuber" was a mascot developed by TB patient and physician Harry Wilmer (1917-2005). In the educational pamphlet, Huber rides respiratory droplets along with his cohort "Nasty von Sputum, Rusty the Bloodyvitch, and Huey the Long Tuber." That final appellation is supposedly a reference to Sen. Huey Long, according to the NLM. (Can we still anthropomorphize bacteria into corrupt government officials?)
The discovery of Mycobacterium tuberculosis by German bacteriologist Dr. Robert Koch in 1882 led to a revolution of isolating patients, which in turn led to a decrease in transmission. In 1905, the American Sanatorium Association was formed – it still exists today as the American Thoracic Society! When the association started, there were 106 sanatoria in the United States, which provided 9,107 beds for patients. At its peak in 1954, there were 108,457 beds worsening (Am. J. Respir. Crit. Care Med. 2004:169;118-6). From a patient’s journal during time spent in a sanatorium in 1944, we know that there were only two rules for sanatorium residents:
1. Absolute and utter rest of mind and body – no bath, no movement except to toilet once a day, no sitting up except propped by pillows and semireclining, no deep breath. Lead the life of a log, in fact. Don’t try, therefore, to sew, knit, or write, except as occasional relief from reading and sleeping.
2. Eat nourishing food and have plenty of fresh air.
Not everyone got antibiotic treatment, unless their chest x-rays showed worsening. Some patients were treated with an induced pneumothorax, according to the women’s journal. Why this would be is not clear to me.
Then, in 1952, isoniazid was developed, and that was the start of the end of the sanatorium.
In our daily lives, we focus on individual patients, but history informs the current practice of medicine. How wonderful that we can now treat many illnesses that were once considered uniformly fatal. How fortunate are we to call this our profession, one that provides an unambiguous good.
Dr. Chan practices rheumatology in Pawtucket, R.I.
Recently, I had an office visit from a lovely 80-year-old woman, born and raised in Providence, R.I., whose past medical history included pulmonary tuberculosis for which she was sent to a sanatorium – 50 years ago.
Her TB had nothing to do with why she had come to see me. By and large, this is a really healthy patient whose only complaint was a 2-month history of right shoulder pain that turned out to be caused by rotator cuff tendonitis. But I lingered with her, and we chatted for awhile. She used to work at Veterans Affairs, processing claims and grievances so she was familiar with medical terminology and was in general a joy to talk with. And I was captivated by the progress in medicine that she represented.
Now, by the time I went to medical school, we were no longer sending patients to sanatoria. The word was as abstract a concept to me as, say, injecting intramuscular gold to treat rheumatic diseases. By the time I was in training, everyone in the developing world got a BCG vaccine, which prevents severe complications from TB but does not prevent infections. As long as I have been a physician, we have understood transmission well, have known about four-drug regimens, and were aware of drug-resistant TB (I am still floored when I read about XDR-TB, with the X being short for "extensively.")
Needless to say I was fascinated by her story.
When she was originally diagnosed more than half a century ago, this woman did not have the usual symptoms that we associate with active pulmonary tuberculosis. She had not had a cough and certainly did not have "wasting." She simply tripped one day and in doing so coughed up some blood. She was found to have disease in both apices and, subsequently, she spent 14 months in a local sanatorium. She remembers being treated with "PAS and streptomycin" (PAS being p-aminosalicylic acid), and "lots of fresh air."
Although tuberculosis is rare in the USA today, it was "so rampant that cautionary visual messages appeared in myriad public places, from offices to restrooms," according to the National Library of Medicine. "Huber the Tuber" was a mascot developed by TB patient and physician Harry Wilmer (1917-2005). In the educational pamphlet, Huber rides respiratory droplets along with his cohort "Nasty von Sputum, Rusty the Bloodyvitch, and Huey the Long Tuber." That final appellation is supposedly a reference to Sen. Huey Long, according to the NLM. (Can we still anthropomorphize bacteria into corrupt government officials?)
The discovery of Mycobacterium tuberculosis by German bacteriologist Dr. Robert Koch in 1882 led to a revolution of isolating patients, which in turn led to a decrease in transmission. In 1905, the American Sanatorium Association was formed – it still exists today as the American Thoracic Society! When the association started, there were 106 sanatoria in the United States, which provided 9,107 beds for patients. At its peak in 1954, there were 108,457 beds worsening (Am. J. Respir. Crit. Care Med. 2004:169;118-6). From a patient’s journal during time spent in a sanatorium in 1944, we know that there were only two rules for sanatorium residents:
1. Absolute and utter rest of mind and body – no bath, no movement except to toilet once a day, no sitting up except propped by pillows and semireclining, no deep breath. Lead the life of a log, in fact. Don’t try, therefore, to sew, knit, or write, except as occasional relief from reading and sleeping.
2. Eat nourishing food and have plenty of fresh air.
Not everyone got antibiotic treatment, unless their chest x-rays showed worsening. Some patients were treated with an induced pneumothorax, according to the women’s journal. Why this would be is not clear to me.
Then, in 1952, isoniazid was developed, and that was the start of the end of the sanatorium.
In our daily lives, we focus on individual patients, but history informs the current practice of medicine. How wonderful that we can now treat many illnesses that were once considered uniformly fatal. How fortunate are we to call this our profession, one that provides an unambiguous good.
Dr. Chan practices rheumatology in Pawtucket, R.I.