User login
Causes of Sudden Unexpected Death
The sudden unexpected death of a hospitalized patient is extremely distressing to the family and the healthcare team. It is also distressingly common. Over 200,000 treated cardiac arrests are estimated to occur each year in US hospital patients.1 Most of these patients die, and studies have shown that physicians often incorrectly diagnose the causes of death when autopsies are performed to determine the causes of death.2, 3 This study was undertaken to elucidate the causes of sudden unexpected death of adult hospital patients as determined by autopsy.
METHODS
One hundred seventy‐five consecutive cases with autopsies by the senior author (L.N.) of adult hospital patients in the University of Pittsburgh Medical Center (UPMC) Health System, who died within 1 hour after onset of symptoms and in which death was unexpected, were retrospectively analyzed. Patients under 18, or dead on arrival, or on comfort measures only, were excluded. The unexpectedness of the deaths in this series was determined by review of the medical record, usually confirmed by a pre‐autopsy discussion with a clinician (following UPMC policy), and by the fact that attempted resuscitation was carried out in all but a few cases. Patient age, sex, race, and causes of death were obtained from the autopsy report. The medical record was reviewed to determine if the patient was on cardiac monitoring at the time of death. The study was approved by the University of Pittsburgh Medical Center Committee for Oversight of Research Involving the Dead.
RESULTS
The 175 autopsies in this study included 98 male patients and 77 female patients. Their ages ranged from 19 to 95 years, with an average age of 63.8 years. Categorized by race, 139 were white, 34 black, 1 Hispanic, and 1 Filipino. The autopsies were done over a 14‐year period from 1992 to 2006, during which the autopsy rate gradually decreased from 19% to 8%. Seeking authorization for autopsy from family was the responsibility of clinicians.
The most common immediate cause of death was judged to be a cardiac arrhythmia, usually presumptive; this was the immediate cause of death in 58 (33.1%) cases, as shown in Table 1. Second most common was hemorrhage, which was the immediate cause of death in 38 (21.7%) cases, and third was pulmonary thromboembolism in 27 (15.4%) cases, as shown in Table 1. Other conditions judged to be the immediate cause of death were cardiogenic pulmonary edema/congestive heart failure in 13 cases (7.4%), sepsis in 11 cases (6.3%), pulmonary edema due to acute lung injury in 4 cases (3 with associated pneumonia), and acute respiratory failure due to pneumonia (in 3 cases), usual interstitial pneumonia (2), emphysema (1), chronic obstructive pulmonary disease (1), herpes simplex virus bronchitis (1), carbon dioxide narcosis (1), and undiagnosed massive metastatic pancreatic carcinoma infiltrating and pushing up the diaphragm (1). Miscellaneous other conditions judged to be the immediate cause of death were brain stem compression in 2 cases, aspiration in 2 cases, andin 1 case eachsubarachnoid hemorrhage, hemorrhagic cerebral infarction, fat embolism, amniotic fluid embolism, bilateral pneumothoraces, massive hemolysis, sickle cell vaso‐occlusive crisis, cardiopulmonary decompensation, cardiac tamponade (due to pericardial metastases), and shock and systemic inflammatory response syndrome (due to volvulus).
| Immediate Cause of Death | No. (n = 175) |
|---|---|
| Cardiac arrhythmia | 58 (33.1%) |
| With 75% coronary stenosis | 36 |
| With myocardial infarction | 31 |
| Remote | 23 |
| Acute or subacute | 13 |
| Hemorrhage | 38 (21.7%) |
| With endogenous coagulopathy | 13 |
| With oral or injected anticoagulation | 12 |
| With antiplatelet therapy | 6 |
| Site: Pericardial (tamponade) | 15 |
| Retroperitoneal | 6 |
| Airway | 4 |
| Gastrointestinal | 4 |
| Pleural space | 2 |
| Thoracoabdominal | 2 |
| Intra‐abdominal | 1 |
| Mediastinal | 1 |
| Nasopharyngeal | 1 |
| Retroperitoneal and pleural | 1 |
| Multi‐organ | 1 |
| Pulmonary thromboembolism | 27 (15.4%) |
The majority, 36 of the 58 patients (62%), with sudden death judged due to arrhythmias, had 75% or greater stenosis of 1 or more coronary arteries (average 2); 11 of these patients were on cardiac monitoring with their fatal arrhythmia displayed on the monitor, and 1 patient was wearing a Holter monitor at the time of her sudden death. The frequency of cardiac arrhythmia as a cause of sudden death did not change over the course of the 14 years of this study. Among the 31 of the 58 patients (53.4%) with histologically confirmed myocardial infarctions, 15 (25.9%) had a remote or subacute myocardial infarction without a history of myocardial infarction.
The most common underlying cause of death was severe coronary atherosclerosis, as shown in Table 2, but there were 14 patients whose underlying cause of death was a diverse group of other heart diseases. Five patients died postoperatively following heart surgery. One patient had a mitral valve papillary fibroelastoma, and another patient's arrhythmia was preceded by a right bundle branch block and a new first‐degree atrioventricular block attributable to mitral annular calcification. One patient with sickle cell disease had a myocardial bridge over a coronary artery. Three patients had heart transplants, 2 alcoholic cardiomyopathy, and 1 idiopathic dilated cardiomyopathy.
| Underlying Cause of Death | No. (%) n = 175 |
|---|---|
| Severe coronary atherosclerosis | 43 (24.6) |
| Neoplastic disease | 30 (17.1) |
| Various heart diseases (see text) | 14 (8.0) |
| Digestive system disorders | 14 (8.0) |
| Aortic aneurysm or dissection | 12 (6.9) |
| Chronic lung disease | 8 (4.6) |
| Infectious diseases | 7 (4.0) |
| Autoimmune diseases | 5 (2.9) |
| Diabetes mellitus | 4 (2.3) |
| Deep vein thrombosis | 3 (1.7) |
| Morbid obesity | 3 (1.7) |
| Other | 32 (18.3) |
The initial cardiac rhythm during attempted cardiopulmonary resuscitation was obtainable for 120 cases. As shown in Table 3, a higher proportion of patients judged to have died of arrhythmias had an initial rhythm of ventricular tachycardia or fibrillation than the proportion of those judged to have died of hemorrhage, pulmonary embolism, or other immediate causes of death.
| Immediate Cause of Death | Initial Cardiac Rhythm, No. (%) | ||||
|---|---|---|---|---|---|
| Ventricular Tachycardia/Fibrillation n = 28 | Bradycardia n = 33 | Pulseless Electrical Activity n = 21 | Asystole n = 31 | Total | |
| |||||
| Arrhythmia | 14 (50) | 6 (18) | 7 (33) | 16 (52) | 43 |
| Hemorrhage | 6 (21) | 10 (30) | 10 (48) | 6 (19) | 32 |
| Thromboembolism | 2 (7) | 8 (24) | 2 (10) | 2 (6) | 15* |
| Other | 6 (21) | 9 (27) | 2 (10) | 7 (23) | 30 |
DISCUSSION
The patients who had an initial cardiac rhythm of asystole during attempted resuscitation, and were judged to have died of an arrhythmia, most likely had ventricular tachycardia or some other arrhythmia before asystole. The majority of them were found in cardiac arrest at night, not on cardiac monitoring. In other studies, there has been an unexplained continuing decline in the prevalence of sudden cardiac arrest cases presenting with ventricular fibrillation and corresponding rise in the prevalence of pulseless electrical activity (PEA) arrests.4 The 7 patients who had an initial cardiac rhythm of PEA and were judged to have died of an arrhythmia were all, except 1, off cardiac monitoring. Four had myocardial infarctions, 3 heart block pre‐arrest, 1 postoperative intramyocardial hematoma, 1 myocardial metastatic melanoma, 1 acute heart transplant rejection, and 3 ventricular tachycardia or fibrillation following PEA, suggesting that PEA was a phase in the evolution of an arrhythmogenic death. The patients who had an initial cardiac rhythm of ventricular tachycardia or fibrillation, and were judged to have died of hemorrhage or pulmonary thromboembolism, generally had coronary artery disease. The hemorrhage or embolism presumably caused terminal myocardial ischemia, but the cardiac disease was judged to be a contributing cause of death, less important than the immediate cause.
The conclusion that a cardiac arrhythmia was the most common mechanism of death in this series fits with the conclusion that cardiac arrhythmias were the most common immediate cause of cardiac arrest, specifically of 49% of them, in a study of 14,720 cardiac arrests of adult inpatients.5 It is accepted by convention that the presence of 75% or greater cross‐sectional luminal narrowing of a coronary artery, even without thrombosis, can be a cause of sudden cardiac death.6 Cardiac arrhythmias commonly occur during myocardial ischemia prior to the irreversible necrosis of myocardial infarction.7 Fatal cardiac arrhythmias can also be caused by old myocardial infarctions.8 The finding that 15 of the patients in this study, 25.9% of the 58 whose sudden death was attributable to a cardiac arrhythmia, had a remote or subacute myocardial infarction without a history of myocardial infarction fits with evidence that 25%30% of myocardial infarctions are unrecognized.
Hemorrhage has been an underpublicized cause of unexpected sudden death in hospital patients. Intracranial hemorrhages, due to ruptured berry aneurysms, hypertension, tumors, or arteriovenous malformations, are well‐recognized causes of sudden death, but not specifically in hospital patients.9 In a Scottish series of 111 unexpected sudden deaths due to an acute abdomen in patients aged 70 or older, 24 died of acute gastrointestinal hemorrhage, but cases of ruptured abdominal aortic aneurysm were excluded from the study because they would have dominated the analysis.10 Retroperitoneal hemorrhage is a particularly insidious cause of sudden death. It commonly causes little or no pain, and proceeds asymptomatically until the patient reaches the limits of cardiopulmonary compensation, which can mask the hemodynamic effect of the bleeding until sudden death.
The limitations of this study include the presumptive nature of the arrhythmias in the majority of patients judged most likely to have died of arrhythmias, and a potential selection bias in the cases coming to autopsy. No data on the 81% to 92% of deaths not investigated by autopsy is available, so the possibility of some sort of selection bias in this case series cannot be excluded. The causes of death determined by autopsy also inevitably represent a judgment or opinion about causation (as opposed to mere correlation), just as the assessment of the causes of death without autopsy does, but autopsy adds the knowledge of conditions undiagnosed prior to death and the exclusion of some suspected diagnoses, substantially improving the unavoidably judgmental conclusion.
There are implications for preventing the sudden unexpected death of hospital patients from the results of this study. They suggest that more cardiac rhythm monitoring might be helpful.11 More prophylactic antiarrhythmic medication and automatic implanted cardiac defibrillators might also be helpful.12 Some UPMC intensivists believe that the sort of fatal arrhythmias seen in this study are caused by hypoxemia, which suggests that more pulse oximetry oxygen saturation monitoring might allow preventative intervention.13 More frequent and possibly automated monitoring of vital signs might provide early warning of hemorrhage or pulmonary embolization.14 Keeping a wide differential diagnosis is taught for resuscitation with an initial rhythm of PEA, but keeping a wide differential including hemorrhage in cases with other initial rhythms, especially bradycardia, for example, may be important. This study suggests the importance of keeping hemorrhage in the differential diagnosis of sudden unexpected cardiac arrest of hospital patients.
- , , , et al. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med. 2011;39:2401–2406.
- , , , . Discrepancies between clinical and autopsy diagnoses. A comparison of university, community and private autopsy practices. Am J Clin Pathol. 2008;129:102–109.
- , , , . The four horsemen: clinicopathological correlation in 407 hospital autopsies. Intern Med J. 2010;40:626–632.
- , , , et al. Factors associated with pulseless electric activity versus ventricular fibrillation: the Oregon sudden unexpected death study. Circulation. 2010;122(21):2116–2122.
- , , , et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14,720 cardiac arrests from the national registry of cardiopulmonary resuscitation. Resuscitation. 2003;58:297–308.
- , , . Coronary thrombosis: what's new? Pathol Case Rev. 2001;6:244–252.
- , , , . Myocardial ischemia and ventricular fibrillation: pathophysiology and clinical implications. Int J Cardiol. 2007;119:283–290.
- , , , . Sudden death in coronary artery disease: acute ischemia versus myocardial substrate. Circulation. 1997;96:3215–3223.
- , . Sudden unexplained death in adults. Curr Top Pathol. 2001;95:125–148.
- , , . Acute abdomen as a cause of death in sudden, unexpected deaths in the elderly. Scott Med J. 2006;52:20–23.
- . Survival after tachyarrhythmic arrest—what are we waiting for? N Engl J Med. 2008;358:77–79.
- , , , et al. Circumstances and outcomes of sudden unexpected death in patients with high‐risk myocardial infarction: implications for prevention. Circulation. 2011;123:2674–2680.
- , , , , , . Use of pulse oximetry to predict in‐hospital complications in normotensive patients with pulmonary embolism. Am J Med. 2003;115(3):203–208.
- , , , , , . Telemetry‐based vital sign monitoring for ambulatory hospital patients. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:4650–4653.
The sudden unexpected death of a hospitalized patient is extremely distressing to the family and the healthcare team. It is also distressingly common. Over 200,000 treated cardiac arrests are estimated to occur each year in US hospital patients.1 Most of these patients die, and studies have shown that physicians often incorrectly diagnose the causes of death when autopsies are performed to determine the causes of death.2, 3 This study was undertaken to elucidate the causes of sudden unexpected death of adult hospital patients as determined by autopsy.
METHODS
One hundred seventy‐five consecutive cases with autopsies by the senior author (L.N.) of adult hospital patients in the University of Pittsburgh Medical Center (UPMC) Health System, who died within 1 hour after onset of symptoms and in which death was unexpected, were retrospectively analyzed. Patients under 18, or dead on arrival, or on comfort measures only, were excluded. The unexpectedness of the deaths in this series was determined by review of the medical record, usually confirmed by a pre‐autopsy discussion with a clinician (following UPMC policy), and by the fact that attempted resuscitation was carried out in all but a few cases. Patient age, sex, race, and causes of death were obtained from the autopsy report. The medical record was reviewed to determine if the patient was on cardiac monitoring at the time of death. The study was approved by the University of Pittsburgh Medical Center Committee for Oversight of Research Involving the Dead.
RESULTS
The 175 autopsies in this study included 98 male patients and 77 female patients. Their ages ranged from 19 to 95 years, with an average age of 63.8 years. Categorized by race, 139 were white, 34 black, 1 Hispanic, and 1 Filipino. The autopsies were done over a 14‐year period from 1992 to 2006, during which the autopsy rate gradually decreased from 19% to 8%. Seeking authorization for autopsy from family was the responsibility of clinicians.
The most common immediate cause of death was judged to be a cardiac arrhythmia, usually presumptive; this was the immediate cause of death in 58 (33.1%) cases, as shown in Table 1. Second most common was hemorrhage, which was the immediate cause of death in 38 (21.7%) cases, and third was pulmonary thromboembolism in 27 (15.4%) cases, as shown in Table 1. Other conditions judged to be the immediate cause of death were cardiogenic pulmonary edema/congestive heart failure in 13 cases (7.4%), sepsis in 11 cases (6.3%), pulmonary edema due to acute lung injury in 4 cases (3 with associated pneumonia), and acute respiratory failure due to pneumonia (in 3 cases), usual interstitial pneumonia (2), emphysema (1), chronic obstructive pulmonary disease (1), herpes simplex virus bronchitis (1), carbon dioxide narcosis (1), and undiagnosed massive metastatic pancreatic carcinoma infiltrating and pushing up the diaphragm (1). Miscellaneous other conditions judged to be the immediate cause of death were brain stem compression in 2 cases, aspiration in 2 cases, andin 1 case eachsubarachnoid hemorrhage, hemorrhagic cerebral infarction, fat embolism, amniotic fluid embolism, bilateral pneumothoraces, massive hemolysis, sickle cell vaso‐occlusive crisis, cardiopulmonary decompensation, cardiac tamponade (due to pericardial metastases), and shock and systemic inflammatory response syndrome (due to volvulus).
| Immediate Cause of Death | No. (n = 175) |
|---|---|
| Cardiac arrhythmia | 58 (33.1%) |
| With 75% coronary stenosis | 36 |
| With myocardial infarction | 31 |
| Remote | 23 |
| Acute or subacute | 13 |
| Hemorrhage | 38 (21.7%) |
| With endogenous coagulopathy | 13 |
| With oral or injected anticoagulation | 12 |
| With antiplatelet therapy | 6 |
| Site: Pericardial (tamponade) | 15 |
| Retroperitoneal | 6 |
| Airway | 4 |
| Gastrointestinal | 4 |
| Pleural space | 2 |
| Thoracoabdominal | 2 |
| Intra‐abdominal | 1 |
| Mediastinal | 1 |
| Nasopharyngeal | 1 |
| Retroperitoneal and pleural | 1 |
| Multi‐organ | 1 |
| Pulmonary thromboembolism | 27 (15.4%) |
The majority, 36 of the 58 patients (62%), with sudden death judged due to arrhythmias, had 75% or greater stenosis of 1 or more coronary arteries (average 2); 11 of these patients were on cardiac monitoring with their fatal arrhythmia displayed on the monitor, and 1 patient was wearing a Holter monitor at the time of her sudden death. The frequency of cardiac arrhythmia as a cause of sudden death did not change over the course of the 14 years of this study. Among the 31 of the 58 patients (53.4%) with histologically confirmed myocardial infarctions, 15 (25.9%) had a remote or subacute myocardial infarction without a history of myocardial infarction.
The most common underlying cause of death was severe coronary atherosclerosis, as shown in Table 2, but there were 14 patients whose underlying cause of death was a diverse group of other heart diseases. Five patients died postoperatively following heart surgery. One patient had a mitral valve papillary fibroelastoma, and another patient's arrhythmia was preceded by a right bundle branch block and a new first‐degree atrioventricular block attributable to mitral annular calcification. One patient with sickle cell disease had a myocardial bridge over a coronary artery. Three patients had heart transplants, 2 alcoholic cardiomyopathy, and 1 idiopathic dilated cardiomyopathy.
| Underlying Cause of Death | No. (%) n = 175 |
|---|---|
| Severe coronary atherosclerosis | 43 (24.6) |
| Neoplastic disease | 30 (17.1) |
| Various heart diseases (see text) | 14 (8.0) |
| Digestive system disorders | 14 (8.0) |
| Aortic aneurysm or dissection | 12 (6.9) |
| Chronic lung disease | 8 (4.6) |
| Infectious diseases | 7 (4.0) |
| Autoimmune diseases | 5 (2.9) |
| Diabetes mellitus | 4 (2.3) |
| Deep vein thrombosis | 3 (1.7) |
| Morbid obesity | 3 (1.7) |
| Other | 32 (18.3) |
The initial cardiac rhythm during attempted cardiopulmonary resuscitation was obtainable for 120 cases. As shown in Table 3, a higher proportion of patients judged to have died of arrhythmias had an initial rhythm of ventricular tachycardia or fibrillation than the proportion of those judged to have died of hemorrhage, pulmonary embolism, or other immediate causes of death.
| Immediate Cause of Death | Initial Cardiac Rhythm, No. (%) | ||||
|---|---|---|---|---|---|
| Ventricular Tachycardia/Fibrillation n = 28 | Bradycardia n = 33 | Pulseless Electrical Activity n = 21 | Asystole n = 31 | Total | |
| |||||
| Arrhythmia | 14 (50) | 6 (18) | 7 (33) | 16 (52) | 43 |
| Hemorrhage | 6 (21) | 10 (30) | 10 (48) | 6 (19) | 32 |
| Thromboembolism | 2 (7) | 8 (24) | 2 (10) | 2 (6) | 15* |
| Other | 6 (21) | 9 (27) | 2 (10) | 7 (23) | 30 |
DISCUSSION
The patients who had an initial cardiac rhythm of asystole during attempted resuscitation, and were judged to have died of an arrhythmia, most likely had ventricular tachycardia or some other arrhythmia before asystole. The majority of them were found in cardiac arrest at night, not on cardiac monitoring. In other studies, there has been an unexplained continuing decline in the prevalence of sudden cardiac arrest cases presenting with ventricular fibrillation and corresponding rise in the prevalence of pulseless electrical activity (PEA) arrests.4 The 7 patients who had an initial cardiac rhythm of PEA and were judged to have died of an arrhythmia were all, except 1, off cardiac monitoring. Four had myocardial infarctions, 3 heart block pre‐arrest, 1 postoperative intramyocardial hematoma, 1 myocardial metastatic melanoma, 1 acute heart transplant rejection, and 3 ventricular tachycardia or fibrillation following PEA, suggesting that PEA was a phase in the evolution of an arrhythmogenic death. The patients who had an initial cardiac rhythm of ventricular tachycardia or fibrillation, and were judged to have died of hemorrhage or pulmonary thromboembolism, generally had coronary artery disease. The hemorrhage or embolism presumably caused terminal myocardial ischemia, but the cardiac disease was judged to be a contributing cause of death, less important than the immediate cause.
The conclusion that a cardiac arrhythmia was the most common mechanism of death in this series fits with the conclusion that cardiac arrhythmias were the most common immediate cause of cardiac arrest, specifically of 49% of them, in a study of 14,720 cardiac arrests of adult inpatients.5 It is accepted by convention that the presence of 75% or greater cross‐sectional luminal narrowing of a coronary artery, even without thrombosis, can be a cause of sudden cardiac death.6 Cardiac arrhythmias commonly occur during myocardial ischemia prior to the irreversible necrosis of myocardial infarction.7 Fatal cardiac arrhythmias can also be caused by old myocardial infarctions.8 The finding that 15 of the patients in this study, 25.9% of the 58 whose sudden death was attributable to a cardiac arrhythmia, had a remote or subacute myocardial infarction without a history of myocardial infarction fits with evidence that 25%30% of myocardial infarctions are unrecognized.
Hemorrhage has been an underpublicized cause of unexpected sudden death in hospital patients. Intracranial hemorrhages, due to ruptured berry aneurysms, hypertension, tumors, or arteriovenous malformations, are well‐recognized causes of sudden death, but not specifically in hospital patients.9 In a Scottish series of 111 unexpected sudden deaths due to an acute abdomen in patients aged 70 or older, 24 died of acute gastrointestinal hemorrhage, but cases of ruptured abdominal aortic aneurysm were excluded from the study because they would have dominated the analysis.10 Retroperitoneal hemorrhage is a particularly insidious cause of sudden death. It commonly causes little or no pain, and proceeds asymptomatically until the patient reaches the limits of cardiopulmonary compensation, which can mask the hemodynamic effect of the bleeding until sudden death.
The limitations of this study include the presumptive nature of the arrhythmias in the majority of patients judged most likely to have died of arrhythmias, and a potential selection bias in the cases coming to autopsy. No data on the 81% to 92% of deaths not investigated by autopsy is available, so the possibility of some sort of selection bias in this case series cannot be excluded. The causes of death determined by autopsy also inevitably represent a judgment or opinion about causation (as opposed to mere correlation), just as the assessment of the causes of death without autopsy does, but autopsy adds the knowledge of conditions undiagnosed prior to death and the exclusion of some suspected diagnoses, substantially improving the unavoidably judgmental conclusion.
There are implications for preventing the sudden unexpected death of hospital patients from the results of this study. They suggest that more cardiac rhythm monitoring might be helpful.11 More prophylactic antiarrhythmic medication and automatic implanted cardiac defibrillators might also be helpful.12 Some UPMC intensivists believe that the sort of fatal arrhythmias seen in this study are caused by hypoxemia, which suggests that more pulse oximetry oxygen saturation monitoring might allow preventative intervention.13 More frequent and possibly automated monitoring of vital signs might provide early warning of hemorrhage or pulmonary embolization.14 Keeping a wide differential diagnosis is taught for resuscitation with an initial rhythm of PEA, but keeping a wide differential including hemorrhage in cases with other initial rhythms, especially bradycardia, for example, may be important. This study suggests the importance of keeping hemorrhage in the differential diagnosis of sudden unexpected cardiac arrest of hospital patients.
The sudden unexpected death of a hospitalized patient is extremely distressing to the family and the healthcare team. It is also distressingly common. Over 200,000 treated cardiac arrests are estimated to occur each year in US hospital patients.1 Most of these patients die, and studies have shown that physicians often incorrectly diagnose the causes of death when autopsies are performed to determine the causes of death.2, 3 This study was undertaken to elucidate the causes of sudden unexpected death of adult hospital patients as determined by autopsy.
METHODS
One hundred seventy‐five consecutive cases with autopsies by the senior author (L.N.) of adult hospital patients in the University of Pittsburgh Medical Center (UPMC) Health System, who died within 1 hour after onset of symptoms and in which death was unexpected, were retrospectively analyzed. Patients under 18, or dead on arrival, or on comfort measures only, were excluded. The unexpectedness of the deaths in this series was determined by review of the medical record, usually confirmed by a pre‐autopsy discussion with a clinician (following UPMC policy), and by the fact that attempted resuscitation was carried out in all but a few cases. Patient age, sex, race, and causes of death were obtained from the autopsy report. The medical record was reviewed to determine if the patient was on cardiac monitoring at the time of death. The study was approved by the University of Pittsburgh Medical Center Committee for Oversight of Research Involving the Dead.
RESULTS
The 175 autopsies in this study included 98 male patients and 77 female patients. Their ages ranged from 19 to 95 years, with an average age of 63.8 years. Categorized by race, 139 were white, 34 black, 1 Hispanic, and 1 Filipino. The autopsies were done over a 14‐year period from 1992 to 2006, during which the autopsy rate gradually decreased from 19% to 8%. Seeking authorization for autopsy from family was the responsibility of clinicians.
The most common immediate cause of death was judged to be a cardiac arrhythmia, usually presumptive; this was the immediate cause of death in 58 (33.1%) cases, as shown in Table 1. Second most common was hemorrhage, which was the immediate cause of death in 38 (21.7%) cases, and third was pulmonary thromboembolism in 27 (15.4%) cases, as shown in Table 1. Other conditions judged to be the immediate cause of death were cardiogenic pulmonary edema/congestive heart failure in 13 cases (7.4%), sepsis in 11 cases (6.3%), pulmonary edema due to acute lung injury in 4 cases (3 with associated pneumonia), and acute respiratory failure due to pneumonia (in 3 cases), usual interstitial pneumonia (2), emphysema (1), chronic obstructive pulmonary disease (1), herpes simplex virus bronchitis (1), carbon dioxide narcosis (1), and undiagnosed massive metastatic pancreatic carcinoma infiltrating and pushing up the diaphragm (1). Miscellaneous other conditions judged to be the immediate cause of death were brain stem compression in 2 cases, aspiration in 2 cases, andin 1 case eachsubarachnoid hemorrhage, hemorrhagic cerebral infarction, fat embolism, amniotic fluid embolism, bilateral pneumothoraces, massive hemolysis, sickle cell vaso‐occlusive crisis, cardiopulmonary decompensation, cardiac tamponade (due to pericardial metastases), and shock and systemic inflammatory response syndrome (due to volvulus).
| Immediate Cause of Death | No. (n = 175) |
|---|---|
| Cardiac arrhythmia | 58 (33.1%) |
| With 75% coronary stenosis | 36 |
| With myocardial infarction | 31 |
| Remote | 23 |
| Acute or subacute | 13 |
| Hemorrhage | 38 (21.7%) |
| With endogenous coagulopathy | 13 |
| With oral or injected anticoagulation | 12 |
| With antiplatelet therapy | 6 |
| Site: Pericardial (tamponade) | 15 |
| Retroperitoneal | 6 |
| Airway | 4 |
| Gastrointestinal | 4 |
| Pleural space | 2 |
| Thoracoabdominal | 2 |
| Intra‐abdominal | 1 |
| Mediastinal | 1 |
| Nasopharyngeal | 1 |
| Retroperitoneal and pleural | 1 |
| Multi‐organ | 1 |
| Pulmonary thromboembolism | 27 (15.4%) |
The majority, 36 of the 58 patients (62%), with sudden death judged due to arrhythmias, had 75% or greater stenosis of 1 or more coronary arteries (average 2); 11 of these patients were on cardiac monitoring with their fatal arrhythmia displayed on the monitor, and 1 patient was wearing a Holter monitor at the time of her sudden death. The frequency of cardiac arrhythmia as a cause of sudden death did not change over the course of the 14 years of this study. Among the 31 of the 58 patients (53.4%) with histologically confirmed myocardial infarctions, 15 (25.9%) had a remote or subacute myocardial infarction without a history of myocardial infarction.
The most common underlying cause of death was severe coronary atherosclerosis, as shown in Table 2, but there were 14 patients whose underlying cause of death was a diverse group of other heart diseases. Five patients died postoperatively following heart surgery. One patient had a mitral valve papillary fibroelastoma, and another patient's arrhythmia was preceded by a right bundle branch block and a new first‐degree atrioventricular block attributable to mitral annular calcification. One patient with sickle cell disease had a myocardial bridge over a coronary artery. Three patients had heart transplants, 2 alcoholic cardiomyopathy, and 1 idiopathic dilated cardiomyopathy.
| Underlying Cause of Death | No. (%) n = 175 |
|---|---|
| Severe coronary atherosclerosis | 43 (24.6) |
| Neoplastic disease | 30 (17.1) |
| Various heart diseases (see text) | 14 (8.0) |
| Digestive system disorders | 14 (8.0) |
| Aortic aneurysm or dissection | 12 (6.9) |
| Chronic lung disease | 8 (4.6) |
| Infectious diseases | 7 (4.0) |
| Autoimmune diseases | 5 (2.9) |
| Diabetes mellitus | 4 (2.3) |
| Deep vein thrombosis | 3 (1.7) |
| Morbid obesity | 3 (1.7) |
| Other | 32 (18.3) |
The initial cardiac rhythm during attempted cardiopulmonary resuscitation was obtainable for 120 cases. As shown in Table 3, a higher proportion of patients judged to have died of arrhythmias had an initial rhythm of ventricular tachycardia or fibrillation than the proportion of those judged to have died of hemorrhage, pulmonary embolism, or other immediate causes of death.
| Immediate Cause of Death | Initial Cardiac Rhythm, No. (%) | ||||
|---|---|---|---|---|---|
| Ventricular Tachycardia/Fibrillation n = 28 | Bradycardia n = 33 | Pulseless Electrical Activity n = 21 | Asystole n = 31 | Total | |
| |||||
| Arrhythmia | 14 (50) | 6 (18) | 7 (33) | 16 (52) | 43 |
| Hemorrhage | 6 (21) | 10 (30) | 10 (48) | 6 (19) | 32 |
| Thromboembolism | 2 (7) | 8 (24) | 2 (10) | 2 (6) | 15* |
| Other | 6 (21) | 9 (27) | 2 (10) | 7 (23) | 30 |
DISCUSSION
The patients who had an initial cardiac rhythm of asystole during attempted resuscitation, and were judged to have died of an arrhythmia, most likely had ventricular tachycardia or some other arrhythmia before asystole. The majority of them were found in cardiac arrest at night, not on cardiac monitoring. In other studies, there has been an unexplained continuing decline in the prevalence of sudden cardiac arrest cases presenting with ventricular fibrillation and corresponding rise in the prevalence of pulseless electrical activity (PEA) arrests.4 The 7 patients who had an initial cardiac rhythm of PEA and were judged to have died of an arrhythmia were all, except 1, off cardiac monitoring. Four had myocardial infarctions, 3 heart block pre‐arrest, 1 postoperative intramyocardial hematoma, 1 myocardial metastatic melanoma, 1 acute heart transplant rejection, and 3 ventricular tachycardia or fibrillation following PEA, suggesting that PEA was a phase in the evolution of an arrhythmogenic death. The patients who had an initial cardiac rhythm of ventricular tachycardia or fibrillation, and were judged to have died of hemorrhage or pulmonary thromboembolism, generally had coronary artery disease. The hemorrhage or embolism presumably caused terminal myocardial ischemia, but the cardiac disease was judged to be a contributing cause of death, less important than the immediate cause.
The conclusion that a cardiac arrhythmia was the most common mechanism of death in this series fits with the conclusion that cardiac arrhythmias were the most common immediate cause of cardiac arrest, specifically of 49% of them, in a study of 14,720 cardiac arrests of adult inpatients.5 It is accepted by convention that the presence of 75% or greater cross‐sectional luminal narrowing of a coronary artery, even without thrombosis, can be a cause of sudden cardiac death.6 Cardiac arrhythmias commonly occur during myocardial ischemia prior to the irreversible necrosis of myocardial infarction.7 Fatal cardiac arrhythmias can also be caused by old myocardial infarctions.8 The finding that 15 of the patients in this study, 25.9% of the 58 whose sudden death was attributable to a cardiac arrhythmia, had a remote or subacute myocardial infarction without a history of myocardial infarction fits with evidence that 25%30% of myocardial infarctions are unrecognized.
Hemorrhage has been an underpublicized cause of unexpected sudden death in hospital patients. Intracranial hemorrhages, due to ruptured berry aneurysms, hypertension, tumors, or arteriovenous malformations, are well‐recognized causes of sudden death, but not specifically in hospital patients.9 In a Scottish series of 111 unexpected sudden deaths due to an acute abdomen in patients aged 70 or older, 24 died of acute gastrointestinal hemorrhage, but cases of ruptured abdominal aortic aneurysm were excluded from the study because they would have dominated the analysis.10 Retroperitoneal hemorrhage is a particularly insidious cause of sudden death. It commonly causes little or no pain, and proceeds asymptomatically until the patient reaches the limits of cardiopulmonary compensation, which can mask the hemodynamic effect of the bleeding until sudden death.
The limitations of this study include the presumptive nature of the arrhythmias in the majority of patients judged most likely to have died of arrhythmias, and a potential selection bias in the cases coming to autopsy. No data on the 81% to 92% of deaths not investigated by autopsy is available, so the possibility of some sort of selection bias in this case series cannot be excluded. The causes of death determined by autopsy also inevitably represent a judgment or opinion about causation (as opposed to mere correlation), just as the assessment of the causes of death without autopsy does, but autopsy adds the knowledge of conditions undiagnosed prior to death and the exclusion of some suspected diagnoses, substantially improving the unavoidably judgmental conclusion.
There are implications for preventing the sudden unexpected death of hospital patients from the results of this study. They suggest that more cardiac rhythm monitoring might be helpful.11 More prophylactic antiarrhythmic medication and automatic implanted cardiac defibrillators might also be helpful.12 Some UPMC intensivists believe that the sort of fatal arrhythmias seen in this study are caused by hypoxemia, which suggests that more pulse oximetry oxygen saturation monitoring might allow preventative intervention.13 More frequent and possibly automated monitoring of vital signs might provide early warning of hemorrhage or pulmonary embolization.14 Keeping a wide differential diagnosis is taught for resuscitation with an initial rhythm of PEA, but keeping a wide differential including hemorrhage in cases with other initial rhythms, especially bradycardia, for example, may be important. This study suggests the importance of keeping hemorrhage in the differential diagnosis of sudden unexpected cardiac arrest of hospital patients.
- , , , et al. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med. 2011;39:2401–2406.
- , , , . Discrepancies between clinical and autopsy diagnoses. A comparison of university, community and private autopsy practices. Am J Clin Pathol. 2008;129:102–109.
- , , , . The four horsemen: clinicopathological correlation in 407 hospital autopsies. Intern Med J. 2010;40:626–632.
- , , , et al. Factors associated with pulseless electric activity versus ventricular fibrillation: the Oregon sudden unexpected death study. Circulation. 2010;122(21):2116–2122.
- , , , et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14,720 cardiac arrests from the national registry of cardiopulmonary resuscitation. Resuscitation. 2003;58:297–308.
- , , . Coronary thrombosis: what's new? Pathol Case Rev. 2001;6:244–252.
- , , , . Myocardial ischemia and ventricular fibrillation: pathophysiology and clinical implications. Int J Cardiol. 2007;119:283–290.
- , , , . Sudden death in coronary artery disease: acute ischemia versus myocardial substrate. Circulation. 1997;96:3215–3223.
- , . Sudden unexplained death in adults. Curr Top Pathol. 2001;95:125–148.
- , , . Acute abdomen as a cause of death in sudden, unexpected deaths in the elderly. Scott Med J. 2006;52:20–23.
- . Survival after tachyarrhythmic arrest—what are we waiting for? N Engl J Med. 2008;358:77–79.
- , , , et al. Circumstances and outcomes of sudden unexpected death in patients with high‐risk myocardial infarction: implications for prevention. Circulation. 2011;123:2674–2680.
- , , , , , . Use of pulse oximetry to predict in‐hospital complications in normotensive patients with pulmonary embolism. Am J Med. 2003;115(3):203–208.
- , , , , , . Telemetry‐based vital sign monitoring for ambulatory hospital patients. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:4650–4653.
- , , , et al. Incidence of treated cardiac arrest in hospitalized patients in the United States. Crit Care Med. 2011;39:2401–2406.
- , , , . Discrepancies between clinical and autopsy diagnoses. A comparison of university, community and private autopsy practices. Am J Clin Pathol. 2008;129:102–109.
- , , , . The four horsemen: clinicopathological correlation in 407 hospital autopsies. Intern Med J. 2010;40:626–632.
- , , , et al. Factors associated with pulseless electric activity versus ventricular fibrillation: the Oregon sudden unexpected death study. Circulation. 2010;122(21):2116–2122.
- , , , et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14,720 cardiac arrests from the national registry of cardiopulmonary resuscitation. Resuscitation. 2003;58:297–308.
- , , . Coronary thrombosis: what's new? Pathol Case Rev. 2001;6:244–252.
- , , , . Myocardial ischemia and ventricular fibrillation: pathophysiology and clinical implications. Int J Cardiol. 2007;119:283–290.
- , , , . Sudden death in coronary artery disease: acute ischemia versus myocardial substrate. Circulation. 1997;96:3215–3223.
- , . Sudden unexplained death in adults. Curr Top Pathol. 2001;95:125–148.
- , , . Acute abdomen as a cause of death in sudden, unexpected deaths in the elderly. Scott Med J. 2006;52:20–23.
- . Survival after tachyarrhythmic arrest—what are we waiting for? N Engl J Med. 2008;358:77–79.
- , , , et al. Circumstances and outcomes of sudden unexpected death in patients with high‐risk myocardial infarction: implications for prevention. Circulation. 2011;123:2674–2680.
- , , , , , . Use of pulse oximetry to predict in‐hospital complications in normotensive patients with pulmonary embolism. Am J Med. 2003;115(3):203–208.
- , , , , , . Telemetry‐based vital sign monitoring for ambulatory hospital patients. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:4650–4653.
Prediction Rule of Bacteremia
Fever is a nonspecific phenomenon that can result from many inciting causes such as infection, inflammation, malignancy, thromboembolic disease, drugs, and endocrine disease. In hospitalized patients, one of the most important clinical considerations is bacteremia. Although vital signs compose 3 of the 4 current criteria for the diagnosis of Systemic Inflammatory Response Syndrome (SIRS),1, 2 they contribute little to the diagnosis of the cause, which can be inflammation or infection. Unfortunately, the physician's clinical diagnosis of bacteremia lacks both sensitivity and specificity.35 Blood culture acquisition is a simple and basic diagnostic procedure routinely used in clinical practice that yields essential information for the evaluation of various infectious diseases.6 Positive blood cultures can demonstrate not only an infectious cause of disease but also the microbiological response to antibiotic therapy.7 However, studies have reported that 35% to 50% of positive blood cultures are falsely positive owing to contamination.711 False‐positive cultures may lead to the use of inappropriate or unnecessary antibiotics, additional testing and consultation, and prolonged hospitalizations that increase patient care costs.9, 12
Nursing staff caring for patients are generally able to assess oral intake, general clinical state, and care requirements. Moreover, the nursing staff are often able to identify problems with patients before physicians.13 In Japan, nurse‐assessed food consumption of every meal is standardized, and is frequently regarded as an indicator of the patient's clinical status, akin to a vital sign. In this context, we hypothesized that quantitative variations in food consumption could accurately distinguish those patients with or without bacteremia.
MATERIALS AND METHODS
Study Design
Between 2005 and 2009, we conducted a cross‐sectional observational study at Juntendo University Nerima Hospital in Tokyo, Japan. We evaluated 1179 consecutive Japanese patients (mean age, 67.8 16.8 years; 51% male) who underwent blood cultures. Patients with anorexia‐inducing conditions, such as gastrointestinal disease and those who were receiving chemotherapy for malignancy, were excluded. We also excluded patients who were not allowed to eat a regular diet. Patients aged <6 years old were also excluded. The indication for blood culture acquisition was at the discretion of the treating physicians. In general, when an axillary temperature >37.538C developed, blood cultures were taken. The study was approved by the ethics committee of Juntendo University Nerima Hospital, and was conducted in accordance with the Helsinki Declaration of 1971, as revised in 1983.
Definition of Bacteremia
In this study, bacteremia was defined as follows:
Identical organisms isolated from 2 sets of blood cultures (a set refers to 1 aerobic bottle and 1 anaerobic bottle).
If only 1 set of blood cultures was acquired and was positive for a pathogenic organism (such as enteric Gram‐negative bacilli or Streptococcus pneumonia) that could account for the clinical presentation, then the culture was considered positive.7, 14, 15
Definition of Contamination
We considered as contaminants organisms common to skin flora, including Bacillus species, coagulase‐negative staphylococci, Corynebacterium species, and Micrococcus species, without isolation of an identical organism with the same antibiotic susceptibilities from another potentially infected site in a patient with incompatible clinical features and no attributable risks.16 Single blood cultures positive for organisms thought unlikely to explain the patient's symptoms were also considered contaminants.
Assessment of Food Consumption and Inter‐Assessor Reliability
Nursing staff assessed the patients' food consumption by estimating the percentage intake at each meal, and we characterized the patients' oral intake based on the meal immediately prior to the blood culture. We categorized the patients into 3 groups: low food consumption group (<50% consumed), moderate food consumption group (>50% to <80% consumed), and high food consumption group (>80% food consumed). To assess the reliability of the evaluations of food consumption, 100 patients (separate from this main study) were selected randomly and evaluated independently by 2 nurses. The kappa score of agreement between the nurses was 0.79 (95% confidence interval [CI], 0.770.80) indicating a high level of concordance.
Other Predictor Variables
In addition to food consumption, we considered the following additional predictor variables: age, leukocyte count, C‐reactive protein (CRP), systolic blood pressure, heart rate, and body temperature.17 These predictor variables were obtained just prior to the blood culture acquisition. We defined systemic inflammatory response syndrome (SIRS) based on standard criteria (heart rate of 90 beats/min, temperature of 36 or 38C, and leukocyte level of 4000 or 12,000 cells/mL), and sepsis as SIRS in the context of clinical evidence or microbiological findings suggesting a primary focus of infection. Two investigators independently determined whether sepsis was present in each case, and the differences were resolved by consensus. Age subclassifications were categorized into 2 groups (<69 years and >70 years). CRP levels were dichotomized as above or below 10.0 mg/dL.
Statistical Analysis
Continuous variables were presented as medians with the associated interquartile range. Univariate analysis was performed using the Student's t test for continuous variables and the Pearson chi‐square test for categorical variables. Locally weighted regression analysis was applied for continuous variables significantly predictive of the outcome in univariate analysis, and the log odds of the outcome was performed to explore which cut‐off points were the best predictors of true‐positive blood culture results.18 Evaluation of best fit was performed using a multivariate logistic regression model with a forward stepwise procedure, with significant multivariate predictors of the outcome kept in the model and expressed as adjusted odds ratios. Calibration was evaluated using the Hosmer‐Lemeshow goodness‐of‐fit test. We calculated the sensitivity and specificity, and positive and negative predictive value for criteria to predict bacteremia. As a subgroup analysis, we repeated the above analytic approach after excluding those patients exposed to antibacterial drugs (which might independently impact food intake). All hypothesis testing was 2‐tailed, and P values of less than 0.05 were considered statistically significant. Statistical analysis was performed using the SPSS v.16.0 software package (SPSS Inc, Chicago, IL).
RESULTS
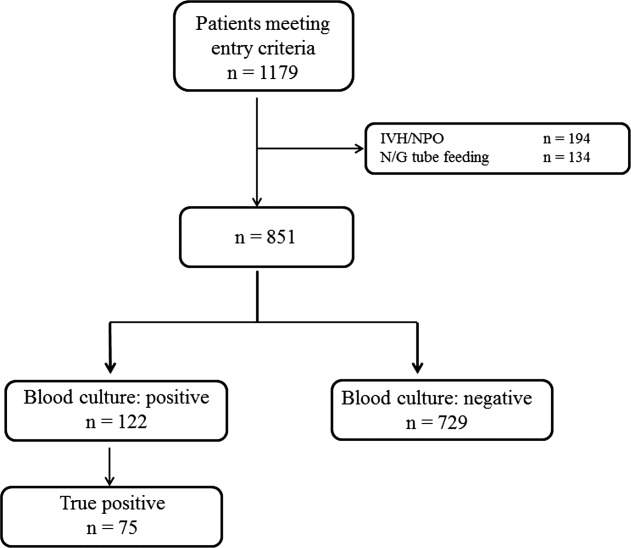
During the study period, 851 patients aged 16 to 99 years (66.8 16.6), were eligible for inclusion (Figure 1). Baseline characteristics of the subjects are given in Table 1. The mean body temperature ( standard deviation [SD]) was 38.1 1.1C, and the mean CRP level was 8.7 8.1 mg/dL. The results show that the patients had at least 2 SIRS criteria with elevations in temperature and heart rate. Of the 851 patients entered into the study, only 122 (14.3%) had positive blood cultures. Of these, 75 patients (8.8%) were considered to have true‐positive blood culture. In this study, blood cultures were taken at the time of onset of fever, whether that was a new inpatient admission, or during the course of an admission to the hospital. On average, blood cultures were drawn 12 days after admission (SD, 5.6 days). Despite the variation in onset of fever, the inverse relationship of blood culture positivity to decreased food consumption held true (data not shown). Gram‐positive and Gram‐negative organisms were obtained in near equal amounts. The main pathogens recovered from the true‐positive blood cultures were Gram‐positive cocci (26 patients [34.7% in true‐positive blood cultures]), and Gram‐negative bacilli (46 patients [61.3%]), as shown in Table 1. The underlying clinical diagnosis included 28 urinary tract infections; 9 catheter‐associated infections; 5 cases each of pneumonia and abscess; 3 cases of phlebitis; 2 cases of meningitis and osteomyelitis; 1 case each of infective endocarditis, decubitus ulcer, and pelvic infection; and 17 cases of infection with an unknown focus.
| Mean | SD | |
|---|---|---|
| ||
| Age, years | 66.8 | 16.7 |
| Male (%) | 50.6 | |
| Vital signs | ||
| Systolic blood pressure, mmHg | 122.6 | 25.9 |
| Diastolic blood pressure, mmHg | 65.3 | 14.6 |
| Heart rate, beats/min | 91.1 | 19.2 |
| Body temperature, C | 38.1 | 1.1 |
| Laboratory results | ||
| Leukocyte, 100 /L | 10.6 | 11.8 |
| C‐reactive protein, mg/L | 8.8 | 8.1 |
| Results of blood cultures | N | % |
| Blood culture positive | 122 | 14.3 |
| True positive | 75 | 8.8 |
| Gram‐positive coccus | 26 | 3.1 |
| Gram‐negative baccili | 46 | 5.4 |
| Gram‐negative coccus | 1 | 0.1 |
| Fungus | 1 | 0.1 |
| Anaerobic | 1 | 0.1 |
| Contamination | 47 | 5.6 |
| Blood culture negative | 729 | 85.7 |
| Food consumption | ||
| Low food consumption group | 344 | 4.4 |
| Moderate food consumption group | 152 | 17.9 |
| High food consumption group | 354 | 41.4 |
Low, moderate, and high food consumption groups consisted of 344 patients (40.4%), 152 patients (17.9%), and 354 patients (41.7%), respectively (Table 1). Of these, 63 patients, 6 patients, and 6 patients had bacteremia in the low, moderate, and high food consumption group, respectively. In order to distinguish those patients who had decreased food consumption compared to almost normal food consumption, low and moderate food consumption groups were combined and compared to the high food consumption group. Comparison of the combined low and moderate food consumption group versus the high food consumption group revealed a sensitivity of 92.0% and a negative predictive value of 98.3% for excluding true bacteremia. Conversely, the specificity (45.1%) and the positive predictive value (13.9%) were poor.
In the univariate analysis, the following variables were not statistically significantly associated with true bacteremia: age, heart rate, and leukocyte counts. Significant univariate predictors of bacteremia and their associated cut‐off points were body temperature of 36 or 38C (odds ratio [OR], 2.5; 95% CI, 1.54.4), CRP 10.0 mg/dL (OR, 2.0; 95% CI, 1.23.2), and food consumption (OR, 8.5; 95% CI, 3.818.6) (Table 2). There was no evidence of colinearity. In the final stepwise logistic regression (Table 3), the significant predictors of bacteremia were body temperature of 36 or 38C (OR, 2.4; 95% CI, 1.44.2; P = 0.002), C‐reactive protein of 10.0 mg/dL (OR, 1.9; 95% CI, 1.23.0; P = 0.011), and food consumption (OR, 7.5; 95% CI, 3.416.6; P < 0.001). We identified only 6 patients with bacteremia in the high food consumption group. Three of the patients had been previously treated with antibiotics for conditions including infective endocarditis, osteomyelitis, and myelodysplasic syndrome.
| Variables | Blood Culture Result | P Value | OR (95% CI) | |
|---|---|---|---|---|
| Negative (n = 729) (%) | Positive (n = 75) | |||
| ||||
| Age, years | 66.6 | 69.0 | ||
| Mean SD | 16.9 | 13.5 | ||
| 70 | 408 (56.0) | 43 (57.3) | 0.7 | |
| Heart rate, beats/min | 90.5 | 96.3 | ||
| Mean SD | 19.0 | 20.3 | ||
| 90 | 368 (50.4) | 43 (57.3) | 0.3 | |
| Temperature, C | 38.0 | 38.6 | ||
| Mean SD | 1.0 | 1.6 | ||
| 36, 38 | 444 (61.0) | 61 (81.3) | <0.001 | 2.5 (1.54.4) |
| Leukocyte count, cells/L | 10.1 | 11.2 | ||
| Mean SD, 100 | 12.1 | 7.4 | ||
| 120 103, <4 103 | 336 (46.1) | 38 (50.7) | 0.4 | |
| C‐reactive protein | ||||
| Mean SD | 7.8 | 10.0 | ||
| 10.0 | 245 (33.6) | 39 (52.0) | 0.0004 | 2.0 (1.23.2) |
| Food consumption | ||||
| Low and moderate | 426 (58.9) | 69 (92.0) | ||
| High | 350 (48.0) | 6 (8.0) | <0.001 | 8.5 (3.818.6) |
| Variables | OR (95% CI) | P Value |
|---|---|---|
| ||
| Temperature, C 36 or 38 | 2.4 (1.44.2) | 0.002 |
| C‐reactive protein, mg/dL 10.0 | 1.9 (1.23.0) | 0.011 |
| Food consumption High vs low and moderate | 7.5 (3.416.6) | <0.001 |
On further analysis, we excluded patients who had received antibiotics before blood culture acquisition. There were 661 patients in this subanalysis. Low, moderate, and high food consumption groups consisted of 282 patients (41.4%), 118 patients (17.3%), and 261 patients (38.3%), respectively. Of these, 50 patients (17.7%), 5 patients (4.2%), and 4 patients (1.5%) had bacteremia in the low, moderate, and high food consumption groups, respectively. The sensitivity and negative predictive values were 93.2% and 98.5%, respectively. In the stepwise logistic regression, significant predictors of bacteremia were body temperature of 36 or 38C (OR, 3.0; 95% CI, 1.55.6; P = 0.001), CRP 10.0 mg/dL (OR, 2.1; 95% CI, 1.23.7; P = 0.006), and food consumption (OR, 9.3; 95% CI, 3.326.1; P < 0.001).
DISCUSSION
We found that in a group of 851 Japanese patients who were suspected with bacterial infection, the estimated food consumption was negatively associated, both significantly and independently, with the subsequent isolation of microorganisms from their blood cultures. If validated in other studies, this simple rule of thumb can provide the clinician with reasonable confidence that a febrile patient has a low probability of being bacteremic, as long as the appetite remains normal. Both the sensitivity and the negative predictive value were extremely high at 92.3% and 98.3%, respectively, suggesting that adequate oral intake is a strong marker against the presence of bacteremia. In this study, it was the strongest predictor of bacteremia in multivariate analysis. After including only antibiotic‐naive patients, the sensitivity and the negative predictive values were 93.2% and 98.5%, respectively. Administration of antibiotics may lead to improved appetite in febrile patients despite bacteremia in the presence of fever, and therefore, inquiring about recent or current antimicrobial usage should be a requirement when considering oral intake as an indicator of bacteremia.
Our study has limitations. Since we did not make treatment decisions based on oral intake, we cannot conclude that it is safe to withhold antibiotic treatment on the basis of food intake alone. Additionally, this study would need to be repeated across many different age groups and racial groups to ensure applicability to the general population. It is also unknown whether this rule would be applicable to patients with underlying immunosuppression. Finally, although inter‐rater reliability was high in our center, nurses in other settings may not be as diligent in their assessment of food consumption. The high inter‐assessor reliability in our setting, however, suggests that objective assessment of food intake can be performed reliably in settings in which accurate documentation of food consumption is expected.
In summary, we found that normal food intake was strongly and negatively associated with bacteremia in febrile patients. This observation, if validated in other settings, may serve as a simple aid to assist in the clinical diagnosis of bacteremia or for recruitment of patients with a high likelihood of bacteremia into clinical trials.
Acknowledgements
The authors thank Drs T. Morimoto and S. Ueda for assistance with statistical analysis, Ms M. Takigawa, and M. Kudo for collection of data, and Drs T. Oguri and Tachibana for infectious disease consultation on the pathogenicity of the microbiological organisms.
- , , , et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256.
- , , . Septic shock. Lancet. 2005;365(9453):63–78.
- , , , . A simple index to identify occult bacterial infection in adults with acute unexplained fever. Arch Intern Med. 1987;147(4):666–671.
- , , . Occult bacterial infection in adults with unexplained fever. Validation of a diagnostic index. Arch Intern Med. 1990;150(6):1270–1272.
- , , , , . Bacteremia in febrile patients. A clinical model for diagnosis. Arch Intern Med. 1991;151(9):1801–1806.
- , . Blood cultures. Ann Intern Med. 1987;106(2):246–253.
- , , , et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24(4):584–602.
- , , . Effect of iodophor vs iodine tincture skin preparation on blood culture contamination rate. JAMA. 1993;269(8):1004–1006.
- , , , et al. Predicting bacteremia in patients with sepsis syndrome. Academic Medical Center Consortium Sepsis Project Working Group. J Infect Dis. 1997;176(6):1538–1551.
- , . Clinical issues of blood cultures. Arch Intern Med. 1994;154(8):841–849.
- , , . High frequency of pseudobacteremia at a university hospital. Infect Control Hosp Epidemiol. 1997;18(3):200–202.
- , , , . Predicting bacteremia in hospitalized patients. A prospectively validated model. Ann Intern Med. 1990;113(7):495–500.
- , , , . Decisions made by critical care nurses during mechanical ventilation and weaning in an Australian intensive care unit. Am J Crit Care. 2007;16(5):434–443; quiz 444.
- , . Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788–802.
- , , , et al. Minimizing the workup of blood culture contaminants: implementation and evaluation of a laboratory‐based algorithm. J Clin Microbiol. 2002;40(7):2437–2444.
- , . Evaluation of positive blood cultures. Guidelines for early differentiation of contaminated from valid positive cultures. Arch Intern Med. 1972;130(1):84–87.
- , , , et al. Predicting bacteremia at the bedside. Clin Infect Dis. 2004;38(3):357–362.
- . Local Regression and Likelihood. New York, NY: Springer; 1999.
Fever is a nonspecific phenomenon that can result from many inciting causes such as infection, inflammation, malignancy, thromboembolic disease, drugs, and endocrine disease. In hospitalized patients, one of the most important clinical considerations is bacteremia. Although vital signs compose 3 of the 4 current criteria for the diagnosis of Systemic Inflammatory Response Syndrome (SIRS),1, 2 they contribute little to the diagnosis of the cause, which can be inflammation or infection. Unfortunately, the physician's clinical diagnosis of bacteremia lacks both sensitivity and specificity.35 Blood culture acquisition is a simple and basic diagnostic procedure routinely used in clinical practice that yields essential information for the evaluation of various infectious diseases.6 Positive blood cultures can demonstrate not only an infectious cause of disease but also the microbiological response to antibiotic therapy.7 However, studies have reported that 35% to 50% of positive blood cultures are falsely positive owing to contamination.711 False‐positive cultures may lead to the use of inappropriate or unnecessary antibiotics, additional testing and consultation, and prolonged hospitalizations that increase patient care costs.9, 12
Nursing staff caring for patients are generally able to assess oral intake, general clinical state, and care requirements. Moreover, the nursing staff are often able to identify problems with patients before physicians.13 In Japan, nurse‐assessed food consumption of every meal is standardized, and is frequently regarded as an indicator of the patient's clinical status, akin to a vital sign. In this context, we hypothesized that quantitative variations in food consumption could accurately distinguish those patients with or without bacteremia.
MATERIALS AND METHODS
Study Design
Between 2005 and 2009, we conducted a cross‐sectional observational study at Juntendo University Nerima Hospital in Tokyo, Japan. We evaluated 1179 consecutive Japanese patients (mean age, 67.8 16.8 years; 51% male) who underwent blood cultures. Patients with anorexia‐inducing conditions, such as gastrointestinal disease and those who were receiving chemotherapy for malignancy, were excluded. We also excluded patients who were not allowed to eat a regular diet. Patients aged <6 years old were also excluded. The indication for blood culture acquisition was at the discretion of the treating physicians. In general, when an axillary temperature >37.538C developed, blood cultures were taken. The study was approved by the ethics committee of Juntendo University Nerima Hospital, and was conducted in accordance with the Helsinki Declaration of 1971, as revised in 1983.
Definition of Bacteremia
In this study, bacteremia was defined as follows:
Identical organisms isolated from 2 sets of blood cultures (a set refers to 1 aerobic bottle and 1 anaerobic bottle).
If only 1 set of blood cultures was acquired and was positive for a pathogenic organism (such as enteric Gram‐negative bacilli or Streptococcus pneumonia) that could account for the clinical presentation, then the culture was considered positive.7, 14, 15
Definition of Contamination
We considered as contaminants organisms common to skin flora, including Bacillus species, coagulase‐negative staphylococci, Corynebacterium species, and Micrococcus species, without isolation of an identical organism with the same antibiotic susceptibilities from another potentially infected site in a patient with incompatible clinical features and no attributable risks.16 Single blood cultures positive for organisms thought unlikely to explain the patient's symptoms were also considered contaminants.
Assessment of Food Consumption and Inter‐Assessor Reliability
Nursing staff assessed the patients' food consumption by estimating the percentage intake at each meal, and we characterized the patients' oral intake based on the meal immediately prior to the blood culture. We categorized the patients into 3 groups: low food consumption group (<50% consumed), moderate food consumption group (>50% to <80% consumed), and high food consumption group (>80% food consumed). To assess the reliability of the evaluations of food consumption, 100 patients (separate from this main study) were selected randomly and evaluated independently by 2 nurses. The kappa score of agreement between the nurses was 0.79 (95% confidence interval [CI], 0.770.80) indicating a high level of concordance.
Other Predictor Variables
In addition to food consumption, we considered the following additional predictor variables: age, leukocyte count, C‐reactive protein (CRP), systolic blood pressure, heart rate, and body temperature.17 These predictor variables were obtained just prior to the blood culture acquisition. We defined systemic inflammatory response syndrome (SIRS) based on standard criteria (heart rate of 90 beats/min, temperature of 36 or 38C, and leukocyte level of 4000 or 12,000 cells/mL), and sepsis as SIRS in the context of clinical evidence or microbiological findings suggesting a primary focus of infection. Two investigators independently determined whether sepsis was present in each case, and the differences were resolved by consensus. Age subclassifications were categorized into 2 groups (<69 years and >70 years). CRP levels were dichotomized as above or below 10.0 mg/dL.
Statistical Analysis
Continuous variables were presented as medians with the associated interquartile range. Univariate analysis was performed using the Student's t test for continuous variables and the Pearson chi‐square test for categorical variables. Locally weighted regression analysis was applied for continuous variables significantly predictive of the outcome in univariate analysis, and the log odds of the outcome was performed to explore which cut‐off points were the best predictors of true‐positive blood culture results.18 Evaluation of best fit was performed using a multivariate logistic regression model with a forward stepwise procedure, with significant multivariate predictors of the outcome kept in the model and expressed as adjusted odds ratios. Calibration was evaluated using the Hosmer‐Lemeshow goodness‐of‐fit test. We calculated the sensitivity and specificity, and positive and negative predictive value for criteria to predict bacteremia. As a subgroup analysis, we repeated the above analytic approach after excluding those patients exposed to antibacterial drugs (which might independently impact food intake). All hypothesis testing was 2‐tailed, and P values of less than 0.05 were considered statistically significant. Statistical analysis was performed using the SPSS v.16.0 software package (SPSS Inc, Chicago, IL).
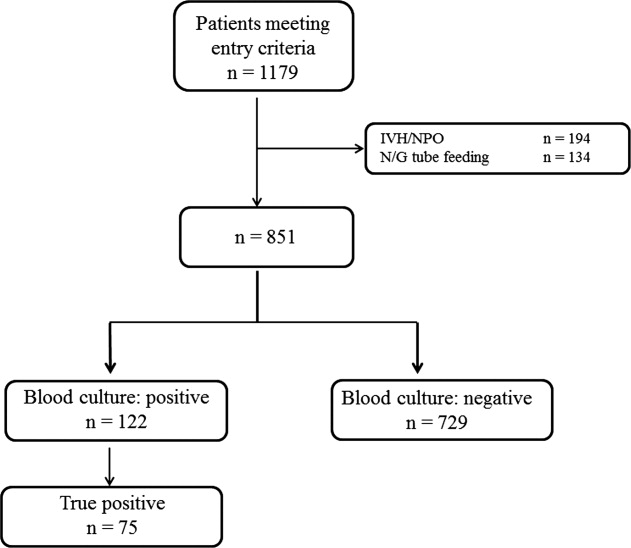
RESULTS
During the study period, 851 patients aged 16 to 99 years (66.8 16.6), were eligible for inclusion (Figure 1). Baseline characteristics of the subjects are given in Table 1. The mean body temperature ( standard deviation [SD]) was 38.1 1.1C, and the mean CRP level was 8.7 8.1 mg/dL. The results show that the patients had at least 2 SIRS criteria with elevations in temperature and heart rate. Of the 851 patients entered into the study, only 122 (14.3%) had positive blood cultures. Of these, 75 patients (8.8%) were considered to have true‐positive blood culture. In this study, blood cultures were taken at the time of onset of fever, whether that was a new inpatient admission, or during the course of an admission to the hospital. On average, blood cultures were drawn 12 days after admission (SD, 5.6 days). Despite the variation in onset of fever, the inverse relationship of blood culture positivity to decreased food consumption held true (data not shown). Gram‐positive and Gram‐negative organisms were obtained in near equal amounts. The main pathogens recovered from the true‐positive blood cultures were Gram‐positive cocci (26 patients [34.7% in true‐positive blood cultures]), and Gram‐negative bacilli (46 patients [61.3%]), as shown in Table 1. The underlying clinical diagnosis included 28 urinary tract infections; 9 catheter‐associated infections; 5 cases each of pneumonia and abscess; 3 cases of phlebitis; 2 cases of meningitis and osteomyelitis; 1 case each of infective endocarditis, decubitus ulcer, and pelvic infection; and 17 cases of infection with an unknown focus.

| Mean | SD | |
|---|---|---|
| ||
| Age, years | 66.8 | 16.7 |
| Male (%) | 50.6 | |
| Vital signs | ||
| Systolic blood pressure, mmHg | 122.6 | 25.9 |
| Diastolic blood pressure, mmHg | 65.3 | 14.6 |
| Heart rate, beats/min | 91.1 | 19.2 |
| Body temperature, C | 38.1 | 1.1 |
| Laboratory results | ||
| Leukocyte, 100 /L | 10.6 | 11.8 |
| C‐reactive protein, mg/L | 8.8 | 8.1 |
| Results of blood cultures | N | % |
| Blood culture positive | 122 | 14.3 |
| True positive | 75 | 8.8 |
| Gram‐positive coccus | 26 | 3.1 |
| Gram‐negative baccili | 46 | 5.4 |
| Gram‐negative coccus | 1 | 0.1 |
| Fungus | 1 | 0.1 |
| Anaerobic | 1 | 0.1 |
| Contamination | 47 | 5.6 |
| Blood culture negative | 729 | 85.7 |
| Food consumption | ||
| Low food consumption group | 344 | 4.4 |
| Moderate food consumption group | 152 | 17.9 |
| High food consumption group | 354 | 41.4 |
Low, moderate, and high food consumption groups consisted of 344 patients (40.4%), 152 patients (17.9%), and 354 patients (41.7%), respectively (Table 1). Of these, 63 patients, 6 patients, and 6 patients had bacteremia in the low, moderate, and high food consumption group, respectively. In order to distinguish those patients who had decreased food consumption compared to almost normal food consumption, low and moderate food consumption groups were combined and compared to the high food consumption group. Comparison of the combined low and moderate food consumption group versus the high food consumption group revealed a sensitivity of 92.0% and a negative predictive value of 98.3% for excluding true bacteremia. Conversely, the specificity (45.1%) and the positive predictive value (13.9%) were poor.
In the univariate analysis, the following variables were not statistically significantly associated with true bacteremia: age, heart rate, and leukocyte counts. Significant univariate predictors of bacteremia and their associated cut‐off points were body temperature of 36 or 38C (odds ratio [OR], 2.5; 95% CI, 1.54.4), CRP 10.0 mg/dL (OR, 2.0; 95% CI, 1.23.2), and food consumption (OR, 8.5; 95% CI, 3.818.6) (Table 2). There was no evidence of colinearity. In the final stepwise logistic regression (Table 3), the significant predictors of bacteremia were body temperature of 36 or 38C (OR, 2.4; 95% CI, 1.44.2; P = 0.002), C‐reactive protein of 10.0 mg/dL (OR, 1.9; 95% CI, 1.23.0; P = 0.011), and food consumption (OR, 7.5; 95% CI, 3.416.6; P < 0.001). We identified only 6 patients with bacteremia in the high food consumption group. Three of the patients had been previously treated with antibiotics for conditions including infective endocarditis, osteomyelitis, and myelodysplasic syndrome.
| Variables | Blood Culture Result | P Value | OR (95% CI) | |
|---|---|---|---|---|
| Negative (n = 729) (%) | Positive (n = 75) | |||
| ||||
| Age, years | 66.6 | 69.0 | ||
| Mean SD | 16.9 | 13.5 | ||
| 70 | 408 (56.0) | 43 (57.3) | 0.7 | |
| Heart rate, beats/min | 90.5 | 96.3 | ||
| Mean SD | 19.0 | 20.3 | ||
| 90 | 368 (50.4) | 43 (57.3) | 0.3 | |
| Temperature, C | 38.0 | 38.6 | ||
| Mean SD | 1.0 | 1.6 | ||
| 36, 38 | 444 (61.0) | 61 (81.3) | <0.001 | 2.5 (1.54.4) |
| Leukocyte count, cells/L | 10.1 | 11.2 | ||
| Mean SD, 100 | 12.1 | 7.4 | ||
| 120 103, <4 103 | 336 (46.1) | 38 (50.7) | 0.4 | |
| C‐reactive protein | ||||
| Mean SD | 7.8 | 10.0 | ||
| 10.0 | 245 (33.6) | 39 (52.0) | 0.0004 | 2.0 (1.23.2) |
| Food consumption | ||||
| Low and moderate | 426 (58.9) | 69 (92.0) | ||
| High | 350 (48.0) | 6 (8.0) | <0.001 | 8.5 (3.818.6) |
| Variables | OR (95% CI) | P Value |
|---|---|---|
| ||
| Temperature, C 36 or 38 | 2.4 (1.44.2) | 0.002 |
| C‐reactive protein, mg/dL 10.0 | 1.9 (1.23.0) | 0.011 |
| Food consumption High vs low and moderate | 7.5 (3.416.6) | <0.001 |
On further analysis, we excluded patients who had received antibiotics before blood culture acquisition. There were 661 patients in this subanalysis. Low, moderate, and high food consumption groups consisted of 282 patients (41.4%), 118 patients (17.3%), and 261 patients (38.3%), respectively. Of these, 50 patients (17.7%), 5 patients (4.2%), and 4 patients (1.5%) had bacteremia in the low, moderate, and high food consumption groups, respectively. The sensitivity and negative predictive values were 93.2% and 98.5%, respectively. In the stepwise logistic regression, significant predictors of bacteremia were body temperature of 36 or 38C (OR, 3.0; 95% CI, 1.55.6; P = 0.001), CRP 10.0 mg/dL (OR, 2.1; 95% CI, 1.23.7; P = 0.006), and food consumption (OR, 9.3; 95% CI, 3.326.1; P < 0.001).
DISCUSSION
We found that in a group of 851 Japanese patients who were suspected with bacterial infection, the estimated food consumption was negatively associated, both significantly and independently, with the subsequent isolation of microorganisms from their blood cultures. If validated in other studies, this simple rule of thumb can provide the clinician with reasonable confidence that a febrile patient has a low probability of being bacteremic, as long as the appetite remains normal. Both the sensitivity and the negative predictive value were extremely high at 92.3% and 98.3%, respectively, suggesting that adequate oral intake is a strong marker against the presence of bacteremia. In this study, it was the strongest predictor of bacteremia in multivariate analysis. After including only antibiotic‐naive patients, the sensitivity and the negative predictive values were 93.2% and 98.5%, respectively. Administration of antibiotics may lead to improved appetite in febrile patients despite bacteremia in the presence of fever, and therefore, inquiring about recent or current antimicrobial usage should be a requirement when considering oral intake as an indicator of bacteremia.
Our study has limitations. Since we did not make treatment decisions based on oral intake, we cannot conclude that it is safe to withhold antibiotic treatment on the basis of food intake alone. Additionally, this study would need to be repeated across many different age groups and racial groups to ensure applicability to the general population. It is also unknown whether this rule would be applicable to patients with underlying immunosuppression. Finally, although inter‐rater reliability was high in our center, nurses in other settings may not be as diligent in their assessment of food consumption. The high inter‐assessor reliability in our setting, however, suggests that objective assessment of food intake can be performed reliably in settings in which accurate documentation of food consumption is expected.
In summary, we found that normal food intake was strongly and negatively associated with bacteremia in febrile patients. This observation, if validated in other settings, may serve as a simple aid to assist in the clinical diagnosis of bacteremia or for recruitment of patients with a high likelihood of bacteremia into clinical trials.
Acknowledgements
The authors thank Drs T. Morimoto and S. Ueda for assistance with statistical analysis, Ms M. Takigawa, and M. Kudo for collection of data, and Drs T. Oguri and Tachibana for infectious disease consultation on the pathogenicity of the microbiological organisms.
Fever is a nonspecific phenomenon that can result from many inciting causes such as infection, inflammation, malignancy, thromboembolic disease, drugs, and endocrine disease. In hospitalized patients, one of the most important clinical considerations is bacteremia. Although vital signs compose 3 of the 4 current criteria for the diagnosis of Systemic Inflammatory Response Syndrome (SIRS),1, 2 they contribute little to the diagnosis of the cause, which can be inflammation or infection. Unfortunately, the physician's clinical diagnosis of bacteremia lacks both sensitivity and specificity.35 Blood culture acquisition is a simple and basic diagnostic procedure routinely used in clinical practice that yields essential information for the evaluation of various infectious diseases.6 Positive blood cultures can demonstrate not only an infectious cause of disease but also the microbiological response to antibiotic therapy.7 However, studies have reported that 35% to 50% of positive blood cultures are falsely positive owing to contamination.711 False‐positive cultures may lead to the use of inappropriate or unnecessary antibiotics, additional testing and consultation, and prolonged hospitalizations that increase patient care costs.9, 12
Nursing staff caring for patients are generally able to assess oral intake, general clinical state, and care requirements. Moreover, the nursing staff are often able to identify problems with patients before physicians.13 In Japan, nurse‐assessed food consumption of every meal is standardized, and is frequently regarded as an indicator of the patient's clinical status, akin to a vital sign. In this context, we hypothesized that quantitative variations in food consumption could accurately distinguish those patients with or without bacteremia.
MATERIALS AND METHODS
Study Design
Between 2005 and 2009, we conducted a cross‐sectional observational study at Juntendo University Nerima Hospital in Tokyo, Japan. We evaluated 1179 consecutive Japanese patients (mean age, 67.8 16.8 years; 51% male) who underwent blood cultures. Patients with anorexia‐inducing conditions, such as gastrointestinal disease and those who were receiving chemotherapy for malignancy, were excluded. We also excluded patients who were not allowed to eat a regular diet. Patients aged <6 years old were also excluded. The indication for blood culture acquisition was at the discretion of the treating physicians. In general, when an axillary temperature >37.538C developed, blood cultures were taken. The study was approved by the ethics committee of Juntendo University Nerima Hospital, and was conducted in accordance with the Helsinki Declaration of 1971, as revised in 1983.
Definition of Bacteremia
In this study, bacteremia was defined as follows:
Identical organisms isolated from 2 sets of blood cultures (a set refers to 1 aerobic bottle and 1 anaerobic bottle).
If only 1 set of blood cultures was acquired and was positive for a pathogenic organism (such as enteric Gram‐negative bacilli or Streptococcus pneumonia) that could account for the clinical presentation, then the culture was considered positive.7, 14, 15
Definition of Contamination
We considered as contaminants organisms common to skin flora, including Bacillus species, coagulase‐negative staphylococci, Corynebacterium species, and Micrococcus species, without isolation of an identical organism with the same antibiotic susceptibilities from another potentially infected site in a patient with incompatible clinical features and no attributable risks.16 Single blood cultures positive for organisms thought unlikely to explain the patient's symptoms were also considered contaminants.
Assessment of Food Consumption and Inter‐Assessor Reliability
Nursing staff assessed the patients' food consumption by estimating the percentage intake at each meal, and we characterized the patients' oral intake based on the meal immediately prior to the blood culture. We categorized the patients into 3 groups: low food consumption group (<50% consumed), moderate food consumption group (>50% to <80% consumed), and high food consumption group (>80% food consumed). To assess the reliability of the evaluations of food consumption, 100 patients (separate from this main study) were selected randomly and evaluated independently by 2 nurses. The kappa score of agreement between the nurses was 0.79 (95% confidence interval [CI], 0.770.80) indicating a high level of concordance.
Other Predictor Variables
In addition to food consumption, we considered the following additional predictor variables: age, leukocyte count, C‐reactive protein (CRP), systolic blood pressure, heart rate, and body temperature.17 These predictor variables were obtained just prior to the blood culture acquisition. We defined systemic inflammatory response syndrome (SIRS) based on standard criteria (heart rate of 90 beats/min, temperature of 36 or 38C, and leukocyte level of 4000 or 12,000 cells/mL), and sepsis as SIRS in the context of clinical evidence or microbiological findings suggesting a primary focus of infection. Two investigators independently determined whether sepsis was present in each case, and the differences were resolved by consensus. Age subclassifications were categorized into 2 groups (<69 years and >70 years). CRP levels were dichotomized as above or below 10.0 mg/dL.
Statistical Analysis
Continuous variables were presented as medians with the associated interquartile range. Univariate analysis was performed using the Student's t test for continuous variables and the Pearson chi‐square test for categorical variables. Locally weighted regression analysis was applied for continuous variables significantly predictive of the outcome in univariate analysis, and the log odds of the outcome was performed to explore which cut‐off points were the best predictors of true‐positive blood culture results.18 Evaluation of best fit was performed using a multivariate logistic regression model with a forward stepwise procedure, with significant multivariate predictors of the outcome kept in the model and expressed as adjusted odds ratios. Calibration was evaluated using the Hosmer‐Lemeshow goodness‐of‐fit test. We calculated the sensitivity and specificity, and positive and negative predictive value for criteria to predict bacteremia. As a subgroup analysis, we repeated the above analytic approach after excluding those patients exposed to antibacterial drugs (which might independently impact food intake). All hypothesis testing was 2‐tailed, and P values of less than 0.05 were considered statistically significant. Statistical analysis was performed using the SPSS v.16.0 software package (SPSS Inc, Chicago, IL).
RESULTS
During the study period, 851 patients aged 16 to 99 years (66.8 16.6), were eligible for inclusion (Figure 1). Baseline characteristics of the subjects are given in Table 1. The mean body temperature ( standard deviation [SD]) was 38.1 1.1C, and the mean CRP level was 8.7 8.1 mg/dL. The results show that the patients had at least 2 SIRS criteria with elevations in temperature and heart rate. Of the 851 patients entered into the study, only 122 (14.3%) had positive blood cultures. Of these, 75 patients (8.8%) were considered to have true‐positive blood culture. In this study, blood cultures were taken at the time of onset of fever, whether that was a new inpatient admission, or during the course of an admission to the hospital. On average, blood cultures were drawn 12 days after admission (SD, 5.6 days). Despite the variation in onset of fever, the inverse relationship of blood culture positivity to decreased food consumption held true (data not shown). Gram‐positive and Gram‐negative organisms were obtained in near equal amounts. The main pathogens recovered from the true‐positive blood cultures were Gram‐positive cocci (26 patients [34.7% in true‐positive blood cultures]), and Gram‐negative bacilli (46 patients [61.3%]), as shown in Table 1. The underlying clinical diagnosis included 28 urinary tract infections; 9 catheter‐associated infections; 5 cases each of pneumonia and abscess; 3 cases of phlebitis; 2 cases of meningitis and osteomyelitis; 1 case each of infective endocarditis, decubitus ulcer, and pelvic infection; and 17 cases of infection with an unknown focus.

| Mean | SD | |
|---|---|---|
| ||
| Age, years | 66.8 | 16.7 |
| Male (%) | 50.6 | |
| Vital signs | ||
| Systolic blood pressure, mmHg | 122.6 | 25.9 |
| Diastolic blood pressure, mmHg | 65.3 | 14.6 |
| Heart rate, beats/min | 91.1 | 19.2 |
| Body temperature, C | 38.1 | 1.1 |
| Laboratory results | ||
| Leukocyte, 100 /L | 10.6 | 11.8 |
| C‐reactive protein, mg/L | 8.8 | 8.1 |
| Results of blood cultures | N | % |
| Blood culture positive | 122 | 14.3 |
| True positive | 75 | 8.8 |
| Gram‐positive coccus | 26 | 3.1 |
| Gram‐negative baccili | 46 | 5.4 |
| Gram‐negative coccus | 1 | 0.1 |
| Fungus | 1 | 0.1 |
| Anaerobic | 1 | 0.1 |
| Contamination | 47 | 5.6 |
| Blood culture negative | 729 | 85.7 |
| Food consumption | ||
| Low food consumption group | 344 | 4.4 |
| Moderate food consumption group | 152 | 17.9 |
| High food consumption group | 354 | 41.4 |
Low, moderate, and high food consumption groups consisted of 344 patients (40.4%), 152 patients (17.9%), and 354 patients (41.7%), respectively (Table 1). Of these, 63 patients, 6 patients, and 6 patients had bacteremia in the low, moderate, and high food consumption group, respectively. In order to distinguish those patients who had decreased food consumption compared to almost normal food consumption, low and moderate food consumption groups were combined and compared to the high food consumption group. Comparison of the combined low and moderate food consumption group versus the high food consumption group revealed a sensitivity of 92.0% and a negative predictive value of 98.3% for excluding true bacteremia. Conversely, the specificity (45.1%) and the positive predictive value (13.9%) were poor.
In the univariate analysis, the following variables were not statistically significantly associated with true bacteremia: age, heart rate, and leukocyte counts. Significant univariate predictors of bacteremia and their associated cut‐off points were body temperature of 36 or 38C (odds ratio [OR], 2.5; 95% CI, 1.54.4), CRP 10.0 mg/dL (OR, 2.0; 95% CI, 1.23.2), and food consumption (OR, 8.5; 95% CI, 3.818.6) (Table 2). There was no evidence of colinearity. In the final stepwise logistic regression (Table 3), the significant predictors of bacteremia were body temperature of 36 or 38C (OR, 2.4; 95% CI, 1.44.2; P = 0.002), C‐reactive protein of 10.0 mg/dL (OR, 1.9; 95% CI, 1.23.0; P = 0.011), and food consumption (OR, 7.5; 95% CI, 3.416.6; P < 0.001). We identified only 6 patients with bacteremia in the high food consumption group. Three of the patients had been previously treated with antibiotics for conditions including infective endocarditis, osteomyelitis, and myelodysplasic syndrome.
| Variables | Blood Culture Result | P Value | OR (95% CI) | |
|---|---|---|---|---|
| Negative (n = 729) (%) | Positive (n = 75) | |||
| ||||
| Age, years | 66.6 | 69.0 | ||
| Mean SD | 16.9 | 13.5 | ||
| 70 | 408 (56.0) | 43 (57.3) | 0.7 | |
| Heart rate, beats/min | 90.5 | 96.3 | ||
| Mean SD | 19.0 | 20.3 | ||
| 90 | 368 (50.4) | 43 (57.3) | 0.3 | |
| Temperature, C | 38.0 | 38.6 | ||
| Mean SD | 1.0 | 1.6 | ||
| 36, 38 | 444 (61.0) | 61 (81.3) | <0.001 | 2.5 (1.54.4) |
| Leukocyte count, cells/L | 10.1 | 11.2 | ||
| Mean SD, 100 | 12.1 | 7.4 | ||
| 120 103, <4 103 | 336 (46.1) | 38 (50.7) | 0.4 | |
| C‐reactive protein | ||||
| Mean SD | 7.8 | 10.0 | ||
| 10.0 | 245 (33.6) | 39 (52.0) | 0.0004 | 2.0 (1.23.2) |
| Food consumption | ||||
| Low and moderate | 426 (58.9) | 69 (92.0) | ||
| High | 350 (48.0) | 6 (8.0) | <0.001 | 8.5 (3.818.6) |
| Variables | OR (95% CI) | P Value |
|---|---|---|
| ||
| Temperature, C 36 or 38 | 2.4 (1.44.2) | 0.002 |
| C‐reactive protein, mg/dL 10.0 | 1.9 (1.23.0) | 0.011 |
| Food consumption High vs low and moderate | 7.5 (3.416.6) | <0.001 |
On further analysis, we excluded patients who had received antibiotics before blood culture acquisition. There were 661 patients in this subanalysis. Low, moderate, and high food consumption groups consisted of 282 patients (41.4%), 118 patients (17.3%), and 261 patients (38.3%), respectively. Of these, 50 patients (17.7%), 5 patients (4.2%), and 4 patients (1.5%) had bacteremia in the low, moderate, and high food consumption groups, respectively. The sensitivity and negative predictive values were 93.2% and 98.5%, respectively. In the stepwise logistic regression, significant predictors of bacteremia were body temperature of 36 or 38C (OR, 3.0; 95% CI, 1.55.6; P = 0.001), CRP 10.0 mg/dL (OR, 2.1; 95% CI, 1.23.7; P = 0.006), and food consumption (OR, 9.3; 95% CI, 3.326.1; P < 0.001).
DISCUSSION
We found that in a group of 851 Japanese patients who were suspected with bacterial infection, the estimated food consumption was negatively associated, both significantly and independently, with the subsequent isolation of microorganisms from their blood cultures. If validated in other studies, this simple rule of thumb can provide the clinician with reasonable confidence that a febrile patient has a low probability of being bacteremic, as long as the appetite remains normal. Both the sensitivity and the negative predictive value were extremely high at 92.3% and 98.3%, respectively, suggesting that adequate oral intake is a strong marker against the presence of bacteremia. In this study, it was the strongest predictor of bacteremia in multivariate analysis. After including only antibiotic‐naive patients, the sensitivity and the negative predictive values were 93.2% and 98.5%, respectively. Administration of antibiotics may lead to improved appetite in febrile patients despite bacteremia in the presence of fever, and therefore, inquiring about recent or current antimicrobial usage should be a requirement when considering oral intake as an indicator of bacteremia.
Our study has limitations. Since we did not make treatment decisions based on oral intake, we cannot conclude that it is safe to withhold antibiotic treatment on the basis of food intake alone. Additionally, this study would need to be repeated across many different age groups and racial groups to ensure applicability to the general population. It is also unknown whether this rule would be applicable to patients with underlying immunosuppression. Finally, although inter‐rater reliability was high in our center, nurses in other settings may not be as diligent in their assessment of food consumption. The high inter‐assessor reliability in our setting, however, suggests that objective assessment of food intake can be performed reliably in settings in which accurate documentation of food consumption is expected.
In summary, we found that normal food intake was strongly and negatively associated with bacteremia in febrile patients. This observation, if validated in other settings, may serve as a simple aid to assist in the clinical diagnosis of bacteremia or for recruitment of patients with a high likelihood of bacteremia into clinical trials.
Acknowledgements
The authors thank Drs T. Morimoto and S. Ueda for assistance with statistical analysis, Ms M. Takigawa, and M. Kudo for collection of data, and Drs T. Oguri and Tachibana for infectious disease consultation on the pathogenicity of the microbiological organisms.
- , , , et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256.
- , , . Septic shock. Lancet. 2005;365(9453):63–78.
- , , , . A simple index to identify occult bacterial infection in adults with acute unexplained fever. Arch Intern Med. 1987;147(4):666–671.
- , , . Occult bacterial infection in adults with unexplained fever. Validation of a diagnostic index. Arch Intern Med. 1990;150(6):1270–1272.
- , , , , . Bacteremia in febrile patients. A clinical model for diagnosis. Arch Intern Med. 1991;151(9):1801–1806.
- , . Blood cultures. Ann Intern Med. 1987;106(2):246–253.
- , , , et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24(4):584–602.
- , , . Effect of iodophor vs iodine tincture skin preparation on blood culture contamination rate. JAMA. 1993;269(8):1004–1006.
- , , , et al. Predicting bacteremia in patients with sepsis syndrome. Academic Medical Center Consortium Sepsis Project Working Group. J Infect Dis. 1997;176(6):1538–1551.
- , . Clinical issues of blood cultures. Arch Intern Med. 1994;154(8):841–849.
- , , . High frequency of pseudobacteremia at a university hospital. Infect Control Hosp Epidemiol. 1997;18(3):200–202.
- , , , . Predicting bacteremia in hospitalized patients. A prospectively validated model. Ann Intern Med. 1990;113(7):495–500.
- , , , . Decisions made by critical care nurses during mechanical ventilation and weaning in an Australian intensive care unit. Am J Crit Care. 2007;16(5):434–443; quiz 444.
- , . Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788–802.
- , , , et al. Minimizing the workup of blood culture contaminants: implementation and evaluation of a laboratory‐based algorithm. J Clin Microbiol. 2002;40(7):2437–2444.
- , . Evaluation of positive blood cultures. Guidelines for early differentiation of contaminated from valid positive cultures. Arch Intern Med. 1972;130(1):84–87.
- , , , et al. Predicting bacteremia at the bedside. Clin Infect Dis. 2004;38(3):357–362.
- . Local Regression and Likelihood. New York, NY: Springer; 1999.
- , , , et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31(4):1250–1256.
- , , . Septic shock. Lancet. 2005;365(9453):63–78.
- , , , . A simple index to identify occult bacterial infection in adults with acute unexplained fever. Arch Intern Med. 1987;147(4):666–671.
- , , . Occult bacterial infection in adults with unexplained fever. Validation of a diagnostic index. Arch Intern Med. 1990;150(6):1270–1272.
- , , , , . Bacteremia in febrile patients. A clinical model for diagnosis. Arch Intern Med. 1991;151(9):1801–1806.
- , . Blood cultures. Ann Intern Med. 1987;106(2):246–253.
- , , , et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24(4):584–602.
- , , . Effect of iodophor vs iodine tincture skin preparation on blood culture contamination rate. JAMA. 1993;269(8):1004–1006.
- , , , et al. Predicting bacteremia in patients with sepsis syndrome. Academic Medical Center Consortium Sepsis Project Working Group. J Infect Dis. 1997;176(6):1538–1551.
- , . Clinical issues of blood cultures. Arch Intern Med. 1994;154(8):841–849.
- , , . High frequency of pseudobacteremia at a university hospital. Infect Control Hosp Epidemiol. 1997;18(3):200–202.
- , , , . Predicting bacteremia in hospitalized patients. A prospectively validated model. Ann Intern Med. 1990;113(7):495–500.
- , , , . Decisions made by critical care nurses during mechanical ventilation and weaning in an Australian intensive care unit. Am J Crit Care. 2007;16(5):434–443; quiz 444.
- , . Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788–802.
- , , , et al. Minimizing the workup of blood culture contaminants: implementation and evaluation of a laboratory‐based algorithm. J Clin Microbiol. 2002;40(7):2437–2444.
- , . Evaluation of positive blood cultures. Guidelines for early differentiation of contaminated from valid positive cultures. Arch Intern Med. 1972;130(1):84–87.
- , , , et al. Predicting bacteremia at the bedside. Clin Infect Dis. 2004;38(3):357–362.
- . Local Regression and Likelihood. New York, NY: Springer; 1999.
Copyright © 2012 Society of Hospital Medicine
Chance Favors the Prepared Mind
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A previously healthy 18‐year‐old woman living in the Pacific Northwest was brought in by her parents to a local hospital with a 4‐day history of acting crazy. Two weeks prior to presentation, she complained of a new‐onset severe headache, diaphoresis, and chills. Four days prior to presentation, she became progressively more impulsive, which ultimately included jumping out of a moving vehicle and running away from home. She experienced unexplained emotional outbursts and was unable to identify familiar relatives or common objects. Additionally, she began having hyperventilation spells and auditory hallucinations.
In an adolescent presenting with erratic behavior, one should consider the possibility of substance abuse or a psychiatric disease such as bipolar disorder with manic features, psychotic manifestations of severe depression, or early schizophrenia. However, it is important to first rule out non‐psychiatric disease, with a diagnostic approach dependent on her human immunodeficiency virus (HIV) status. The presence of headache, diaphoresis, and chills raises concern for an infectious or noninfectious inflammatory central nervous system process. In addition to the effects of illicit drugs such as cocaine or methamphetamine, this presentation may be consistent with a medication‐ or herbal‐induced anticholinergic syndrome, which may present with confusion, ataxia, coma, and cardiopulmonary failure. Since this case originates in the Northwest, one should be aware of the regional outbreak of Cryptococcus gattii in immunocompetent hosts, and that local hallucinogenic plants, such as jimson weed or mushrooms (Amanita muscaria) can cause anticholinergic syndromes. At this point, the differential diagnosis is broad, and evaluation should focus on potentially reversible life‐threatening conditions; in particular, herpes encephalitis. In addition to a detailed history, examination, and routine laboratory studies including HIV serology, I would obtain a drug screen, and order a computed tomography (CT) scan of the brain before performing a lumbar puncture. I would also order a magnetic resonance imaging (MRI) study to evaluate for meningeal or cerebral enhancement suggestive of encephalitis.
The patient had no past medical, psychiatric, or surgical history and took no medications. She lived with her parents who thought she neither used illicit drugs or alcohol, nor was sexually active. She had recently graduated high school and was planning to attend college. Her family history was notable for a mother with bipolar and seizure disorders, and 2 healthy younger siblings. Her family had a healthy cat and dog, and reported a large number of bats living nearby. She had never traveled outside the western United States. The patient presented in late spring, but there was no obvious history of mosquito bites. Her last menstrual period was 4 months prior to presentation. Full review of systems was otherwise negative.
The family history of mood disorder supports continued consideration of bipolar disorder with psychotic manifestations. However, infectious or inflammatory processes remain highest on the differential at this point. The duration of symptoms makes common bacterial meningitis etiologies (Streptococcus, Neisseria, Haemophilus, Listeria) less likely, but would be consistent with herpes simplex encephalitis or lupus cerebritis. Additional infectious considerations would include other viral (eg, varicella zoster virus, Epstein‐Barr virus, enteroviruses, and the arthropod‐borne encephalitides) or unusual bacterial encephalitic syndromes. Although the health status of pets is rarely helpful, dogs can carry ticks that harbor Borrelia burgdorferi (the agent of Lyme disease), which may present with central nervous system (CNS) manifestations. Other conditions associated with pets (such as leptospirosis or cat scratch disease) seem unlikely. The exposure to bats raises the possibility of rabies infection. If she is HIV‐positive, one would need to consider the possibility of opportunistic infections such as cytomegalovirus (CMV), Cryptococcus, cerebral toxoplasmosis, and progressive multifocal leukoencephalopathy (PML) caused by JC virus reaction. Finally, regardless of history, given the patient's amenorrhea, we must perform a pregnancy test.
The patient's temperature was 97.3F, heart rate 129 beats per minute, respiratory rate 19 breaths per minute, and her blood pressure 144/97 mmHg. She was an obese, well‐developed young woman, who was drowsy but arousable, with marked speech latency. Her cranium and oropharynx were normal, and her neck was supple. Aside from tachycardia, her cardiopulmonary, musculoskeletal, and skin exams were normal. Her abdomen was obese and soft, without masses or organomegaly. A pelvic examination was not performed. On neurologic exam, her strength was symmetrically diminished throughout (3+/5). Otherwise, she was oriented to person and general location, but not to day of week, month, or year. Her cranial nerves, sensation, deep tendon reflexes, and muscle tone were normal. A cerebellar examination, plantar response, and gait test were not performed. A brain MRI revealed only a small subarachnoid cyst and possible subtle enhancement of temporal lobes. Initial laboratory studies demonstrated: white blood cell count 14,000/mm3 (72% neutrophils, 17% lymphocytes, 9% monocytes, 2% eosinophils); hemoglobin 14.0 g/dL (mean corpuscular volume 87.4 fL); platelet count 417,000/mm3. Serum electrolytes, liver function tests, coagulation studies, thyroid stimulating hormone, serum ammonia, and urinalysis were normal. Her serum pregnancy test and urine toxicology screen were negative. A room air arterial blood gas revealed a pH of 7.49, PaCO2 32 mmHg, PaO2 89 mmHg; and a bicarbonate 24 mmol/L. Cerebrospinal fluid demonstrated: red cell count of 2/mm3; white cell count 17/mm3 (88% lymphocytes, 3% neutrophils, 9% monocytes); protein 19 mg/dL (normal 1555 mg/dL); and glucose of 79 mg/dL (normal 4080 mg/dL). Gram stain, fungal and bacterial cultures, and HIV serology were negative, and herpes simplex virus was not detected via polymerase chain reaction (PCR).
The tachycardia, respiratory alkalosis, and leukocytosis continue to suggest an infection or inflammatory state. Her neurological deterioration without focal findings, cerebrospinal fluid (CSF) lymphocytic pleocytosis with normal glucose and protein, and temporal lobe enhancement on MRI strongly suggest a meningoencephalitis. This would be an unusual presentation for most bacterial pathogens, but Mycobacterium, Rickettsia, Listeria, Mycoplasma, and Bartonella may rarely mimic encephalitis. Autoimmune encephalitis secondary to lupus, vasculitis, or other autoimmune disorder remains possible, but at this point an infectious encephalitis, particularly herpes encephalitis, is my highest concern. West Nile virus must be considered, but usually produces a severe illness only in immunocompromised or elderly patients. Additionally, despite the rarity of rabies, the patient's exposure to bats and the rapid clinical deterioration, suggest this possibility. In addition to routine bacterial and viral analyses (eg, enteroviral panel), samples should be sent for rabies PCR and antibody testing, West Nile virus, Lyme disease, syphilis, and mycobacterial and fungal pathogens, such as the aforementioned Cryptococcus gattii. Finally, given her presenting syndrome and MRI, immediate treatment with acyclovir and antibiotics is indicated.
The patient was treated for presumed meningoencephalitis with acyclovir and ceftriaxone, but over the following several days became unresponsive to all stimuli and developed repetitive thrusting movements of her mouth, tongue, and jaw. On hospital day 10, with concern for seizures, pentobarbital coma was induced, and the patient was intubated and transferred to our facility. On arrival, her physical examination was essentially unchanged aside from being in a medical coma. Hematology, chemistries, and thyroid‐stimulating hormone (TSH) were again unremarkable with the exception of an elevated creatine kinase (414 U/L) and a new anemia (hemoglobin 8.9 g/dL; mean corpuscular volume 87.6 fL) without evidence of iron deficiency or hemolysis. Blood and urine cultures were negative. Repeat cerebrospinal fluid analysis was essentially unchanged, revealing a red cell count of 1/mm3; white cell count 20/mm3 (86% lymphocytes, 2% neutrophils, 12% monocytes); protein 14 mg/dL; glucose 63 mg/dL, and negative Gram stain. Continuous electroencephalography revealed diffuse generalized slowing, but no seizure activity. An extensive evaluation for viral, bacterial, autoimmune, and paraneoplastic disorders was negative, including tests for anti‐acetylcholine (ACh) receptor binding antibody, anti‐striated muscle antibody, anti‐N‐type calcium channel antibody, anti‐P/Q‐type calcium channel antibodies, anto‐cancer associated retinopathy (CAR) antibody (also known as anti‐recoverin antibody), and anti‐collapsin respons mediator protein (CRMP‐5). Without confirmatory results and continued deterioration, she was empirically treated with methylprednisolone for presumed autoimmune encephalitis from hospital days 16 to 21. The patient remained unresponsive and ventilator‐dependent, despite removal of all sedation. She experienced intermittent fevers as high as 40.5C, remained tachycardic, hypertensive, and exhibited orofacial dyskinesias and jaw clenching, ultimately requiring botulinum toxin injections to prevent tongue biting. Given the lack of improvement despite attempted therapies, a working diagnosis of viral encephalitis with lasting neuropsychiatric sequelae was made. A tracheostomy and percutaneous gastrostomy tube were placed, and a long‐term ventilator care facility was identified.
I continue to wonder if this may be an autoimmune encephalitis, and am concerned about her unexplained fevers. Neuroleptic malignant syndrome secondary to misuse of her parents' medications should be considered in light of the elevated creatine kinase, although the severity and duration of the syndrome seem more profound than I would anticipate. Tetanus could present with jaw dystonia, but the rest of the case does not seem to fit. At this point, considering the patient's young age and poor prognosis without identified etiology, prior to discharge I would argue for a brain biopsy looking for evidence of rabies, or other infectious or autoimmune etiologies of the patient's progressive neurologic deterioration.
On hospital day 25, due to the persistent fevers with concern for occult abscess, an abdominopelvic CT was obtained, which identified a complex 11.8 cm 9.0 cm adnexal mass consistent with a teratoma (Figure 1).

Given the size of the mass, it is surprising that the patient did not report abdominal symptoms and that the physicians were unable to palpate it on examination. The differential diagnosis of a complex adnexal mass in an adolescent should include an ectopic pregnancy, ovarian cysts, tubo‐ovarian abscess, rarely an ovarian carcinoma or leiomyosarcoma, and a teratoma or dermoid tumor. While I mentioned the possibility of a malignancy at the outset, I did not further consider it. Common neoplasms encountered in adolescent patients include lymphoma and leukemia, germ cell tumors (including teratomas), central nervous system tumors and sarcomas, many of which have been reported to cause paraneoplastic disorders. At this point, I now think her presumed teratoma is associated with a paraneoplastic syndrome resulting in her presentation of limbic encephalitis.
A literature search was performed by the managing clinicians who rapidly identified the association between teratoma and limbic encephalitis. The patient was initially treated with intravenous immune globulin (IVIG), with transient improvement in her mental status. Serology returned positive for the anti‐N‐methyl‐D‐aspartate receptor antibody, confirming the diagnosis of anti‐N‐methyl‐D‐aspartate receptor encephalitis. On hospital day 36, her mass was resected (Figure 2). Pathology was consistent with a mature teratoma. Postoperatively, the patient improved daily, and was discharged on hospital day 43 with a near complete neurologic recovery. Four months following discharge, the patient had enrolled full time in college.
COMMENTARY
The N‐methyl‐D‐aspartate receptor (NMDAR) is an important regulator of synaptic transmission and memory within the CNS. Our patient's case illustrates the increasingly recognized syndrome of anti‐NMDAR encephalitis. NMDAR hypofunction is hypothesized to result in the cognitive and behavioral abnormalities of schizophrenia, and direct antagonism of the NMDAR by drugs such as phencyclidine (PCP) and ketamine results in symptoms such as psychosis, hallucinations, delusions, agitation, and dissociative amnesia.14 This constellation of symptoms is very similar to some of the initial neuropsychiatric symptoms observed in patients with anti‐NMDAR encephalitis.
Anti‐NMDAR encephalitis was first described in 2005 as a paraneoplastic limbic encephalitis associated with ovarian teratoma.5, 6 Characterized by the subacute onset (days to weeks) of short‐term memory loss, psychiatric symptoms, and sleep disturbances, limbic encephalitis is an inflammatory process caused by autoantibodies against intracellular or extracellar antigens in the limbic system and other brain structures. Limbic encephalitides associated with antibodies to intracellular antigens (such as Hu, Ma2, CV2/CRMP5, and Amphiphysin) are more often associated with malignancies, have worse outcomes (permanent neuropsychiatric sequelae and death), and are less responsive to immune therapy. Conversely, it appears that both the paraneoplastic and non‐paraneoplastic variants of limbic encephalitis associated with antibodies against cell membrane antigens (such as NMDAR and Voltage Gated Potassium Channels) respond more favorably to therapy.7
As with limbic encephalitis in general, anti‐NMDAR encephalitis can be non‐paraneoplastic as well as paraneoplastic in etiology. In a recently published series of 44 consecutive patients with anti‐NMDAR encephalitis, tumors were present in only 9 cases (8 teratomas).8 When associated with a teratoma, it has been postulated that anti‐NMDAR antibodies develop and cross the bloodbrain barrier to target central nervous system NMDA receptors. This process results in down‐regulation of the neuronal surface NMDAR which then causes the psychiatric and behavioral changes described.6 The mechanism by which these antibodies traverse the bloodbrain barrier is not completely understood, but likely requires some disruption of the barrier in order to trigger anti‐NMDAR encephalitis.8, 9 Non‐paraneoplastic cases evidently involve other unknown stimuli for NMDAR antibody synthesisone report has suggested that subunits of the NMDAR are expressed by normal ovarian tissue, something which may explain the female predilection even in the cohort unaffected by teratomas.10
Most patients with anti‐NMDAR encephalitis are female and young (median age 23 years), although men and children are also affected.8, 9, 11 While the exact incidence of anti‐NMDAR encephalitis is still unknown, the increasing number of case reports suggests that it may be more frequent than any other type of paraneoplastic encephalitis.12 The majority of patients with anti‐NMDAR encephalitis experience an antecedent infectious prodrome (eg, diarrheal illness or upper respiratory infection [URI]), followed 1020 days later by progressive neuropsychiatric and behavioral symptoms which include confusion, memory deficits, impaired responsiveness, seizures, central hypoventilation, and signs of autonomic instability (tachycardia, tachypnea, diaphoresis, cardiac dysrhythmia, blood pressure instability, and dysthermia). At this stage, patients may also manifest a unique constellation of choreoathetoid orofacial and limb movements such as lip licking, chewing, sustained jaw clenching, jaw opening dystonias, ocular deviation and disconjugation, grimacing, myoclonus, and bizarre arm movements. Due to cardiovascular complications and ventilator requirements, most patients require intensive care unit (ICU) level care. 8, 9, 11 As in our discussant's evaluation, other disorders to include in the differential diagnosis for this presentation includes paraneoplastic or autoimmune causes of limbic encephalitis, toxins, heavy metals, and viral causes of encephalitis; in particular, herpes simplex virus (HSV).7
The CNS imaging findings in this condition include brain MRI abnormalities in about 30%55% of patients, which can include increased signal on fluid‐attenuated inversion recovery (FLAIR) or T2 sequences of the cerebral cortex, overlying meninges, or basal ganglia. Abnormalities in the temporal lobes, corpus callosum, and brainstem have also been described. As in our patient, CSF lymphocytic pleocytosis has also been noted.6, 8, 9
Although many cases of limbic encephalitis portend a poor prognosis with permanent neuropsychiatric sequelae and death, anti‐NMDAR can be very responsive to treatment; particularly if diagnosed early. Successful treatment of anti‐NMDAR encephalitis involves immunotherapy and, preferably, early surgical resection of any tumor. 6, 8, 9 Non‐paraneoplastic cases appear to require more aggressive and prolonged immunotherapies to avoid relapse. In both groups, a trend towards improved outcome has been noted in patients treated early in disease course (40 days from symptom onset).8 There are no established guidelines for the treatment of anti‐NMDAR encephalitis, and no randomized controlled trials have evaluated anti‐NMDAR encephalitis treatment. Observational studies of immune‐modulating therapies have shown efficacy with high‐dose steroids and the addition of plasma exchange and/or intravenous immune globulin. Rituximab and cyclophosphamide can be considered if patients fail to improve on other immunotherapies.9 Data from case series seem to suggest a lower risk of relapse in patients treated with immunotherapy.13
Exploration of this patient's persistent high fevers ultimately led to the serendipitous diagnosis of the increasingly recognized syndrome of anti‐NMDAR encephalitis, although in retrospect nearly all of the features of her presentation fit well with this condition. Thus, it was only by a chance finding on her abdominal CT scan that this patient was ultimately diagnosed with a treatable, noninfectious encephalitis associated with an ovarian teratoma. This case reinforces the importance of thorough patient evaluations and being prepared to draw meaningful conclusions from unexpected findings. Given how close this patient was to being discharged to a long‐term care facility, we found this case a fascinating yet sobering reminder to guard against prematurely concluding a syndrome to be untreatable.
KEY TEACHING POINTS
-
Anti‐NMDAR encephalitis is an increasingly recognized cause of autoimmune limbic encephalitis, and thus should be considered in patients with new‐onset psychiatric symptoms accompanied by seizures, autonomic instability, hypoventilation, or dyskinesias.
-
A thorough history, examination, and evaluation of data is critical to make an early diagnosis of anti‐NMDAR encephalitis, because, unlike other forms of limbic encephalitis, this condition may be very responsive to early initiation of treatment.
- , . N‐methyl‐D‐aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49.
- , . Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308.
- , , . NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533.
- , , , et al. Ketamine‐induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20:106–118.
- , , , , , . Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol. 2005;58:594–604.
- , , , et al. Paraneoplastic anti‐N‐methyl‐D‐aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36.
- , . Limbic encephalitis and variants: classification, diagnosis and treatment. Neurologist. 2007;13:261–271.
- , , , et al. N‐methyl‐D‐aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non‐paraneoplastic disorder of both sexes. Brain. 2010;133:1655–1667.
- , . NMDA receptor antibody encephalitis. Curr Neurol Neurosci Rep. 2011;11:298–304.
- , , , et al. Expression of various glutamate receptors including N‐methyl‐D‐aspartate receptor (NMDAR) in an ovarian teratoma removed from a young woman with anti‐NMDAR encephalitis. Intern Med. 2010;49:2167–2173.
- , , , et al. Anti‐NMDA‐receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1074–1075.
- , , , , . Clinical experience and laboratory investigations in patients with anti‐NMDAR encephalitis. Lancet Neurol. 2011;10:63–74.
- , , , et al. Analysis of relapses in anti‐NMDAR encephalitis. Neurology. 2011;77:996–999.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A previously healthy 18‐year‐old woman living in the Pacific Northwest was brought in by her parents to a local hospital with a 4‐day history of acting crazy. Two weeks prior to presentation, she complained of a new‐onset severe headache, diaphoresis, and chills. Four days prior to presentation, she became progressively more impulsive, which ultimately included jumping out of a moving vehicle and running away from home. She experienced unexplained emotional outbursts and was unable to identify familiar relatives or common objects. Additionally, she began having hyperventilation spells and auditory hallucinations.
In an adolescent presenting with erratic behavior, one should consider the possibility of substance abuse or a psychiatric disease such as bipolar disorder with manic features, psychotic manifestations of severe depression, or early schizophrenia. However, it is important to first rule out non‐psychiatric disease, with a diagnostic approach dependent on her human immunodeficiency virus (HIV) status. The presence of headache, diaphoresis, and chills raises concern for an infectious or noninfectious inflammatory central nervous system process. In addition to the effects of illicit drugs such as cocaine or methamphetamine, this presentation may be consistent with a medication‐ or herbal‐induced anticholinergic syndrome, which may present with confusion, ataxia, coma, and cardiopulmonary failure. Since this case originates in the Northwest, one should be aware of the regional outbreak of Cryptococcus gattii in immunocompetent hosts, and that local hallucinogenic plants, such as jimson weed or mushrooms (Amanita muscaria) can cause anticholinergic syndromes. At this point, the differential diagnosis is broad, and evaluation should focus on potentially reversible life‐threatening conditions; in particular, herpes encephalitis. In addition to a detailed history, examination, and routine laboratory studies including HIV serology, I would obtain a drug screen, and order a computed tomography (CT) scan of the brain before performing a lumbar puncture. I would also order a magnetic resonance imaging (MRI) study to evaluate for meningeal or cerebral enhancement suggestive of encephalitis.
The patient had no past medical, psychiatric, or surgical history and took no medications. She lived with her parents who thought she neither used illicit drugs or alcohol, nor was sexually active. She had recently graduated high school and was planning to attend college. Her family history was notable for a mother with bipolar and seizure disorders, and 2 healthy younger siblings. Her family had a healthy cat and dog, and reported a large number of bats living nearby. She had never traveled outside the western United States. The patient presented in late spring, but there was no obvious history of mosquito bites. Her last menstrual period was 4 months prior to presentation. Full review of systems was otherwise negative.
The family history of mood disorder supports continued consideration of bipolar disorder with psychotic manifestations. However, infectious or inflammatory processes remain highest on the differential at this point. The duration of symptoms makes common bacterial meningitis etiologies (Streptococcus, Neisseria, Haemophilus, Listeria) less likely, but would be consistent with herpes simplex encephalitis or lupus cerebritis. Additional infectious considerations would include other viral (eg, varicella zoster virus, Epstein‐Barr virus, enteroviruses, and the arthropod‐borne encephalitides) or unusual bacterial encephalitic syndromes. Although the health status of pets is rarely helpful, dogs can carry ticks that harbor Borrelia burgdorferi (the agent of Lyme disease), which may present with central nervous system (CNS) manifestations. Other conditions associated with pets (such as leptospirosis or cat scratch disease) seem unlikely. The exposure to bats raises the possibility of rabies infection. If she is HIV‐positive, one would need to consider the possibility of opportunistic infections such as cytomegalovirus (CMV), Cryptococcus, cerebral toxoplasmosis, and progressive multifocal leukoencephalopathy (PML) caused by JC virus reaction. Finally, regardless of history, given the patient's amenorrhea, we must perform a pregnancy test.
The patient's temperature was 97.3F, heart rate 129 beats per minute, respiratory rate 19 breaths per minute, and her blood pressure 144/97 mmHg. She was an obese, well‐developed young woman, who was drowsy but arousable, with marked speech latency. Her cranium and oropharynx were normal, and her neck was supple. Aside from tachycardia, her cardiopulmonary, musculoskeletal, and skin exams were normal. Her abdomen was obese and soft, without masses or organomegaly. A pelvic examination was not performed. On neurologic exam, her strength was symmetrically diminished throughout (3+/5). Otherwise, she was oriented to person and general location, but not to day of week, month, or year. Her cranial nerves, sensation, deep tendon reflexes, and muscle tone were normal. A cerebellar examination, plantar response, and gait test were not performed. A brain MRI revealed only a small subarachnoid cyst and possible subtle enhancement of temporal lobes. Initial laboratory studies demonstrated: white blood cell count 14,000/mm3 (72% neutrophils, 17% lymphocytes, 9% monocytes, 2% eosinophils); hemoglobin 14.0 g/dL (mean corpuscular volume 87.4 fL); platelet count 417,000/mm3. Serum electrolytes, liver function tests, coagulation studies, thyroid stimulating hormone, serum ammonia, and urinalysis were normal. Her serum pregnancy test and urine toxicology screen were negative. A room air arterial blood gas revealed a pH of 7.49, PaCO2 32 mmHg, PaO2 89 mmHg; and a bicarbonate 24 mmol/L. Cerebrospinal fluid demonstrated: red cell count of 2/mm3; white cell count 17/mm3 (88% lymphocytes, 3% neutrophils, 9% monocytes); protein 19 mg/dL (normal 1555 mg/dL); and glucose of 79 mg/dL (normal 4080 mg/dL). Gram stain, fungal and bacterial cultures, and HIV serology were negative, and herpes simplex virus was not detected via polymerase chain reaction (PCR).
The tachycardia, respiratory alkalosis, and leukocytosis continue to suggest an infection or inflammatory state. Her neurological deterioration without focal findings, cerebrospinal fluid (CSF) lymphocytic pleocytosis with normal glucose and protein, and temporal lobe enhancement on MRI strongly suggest a meningoencephalitis. This would be an unusual presentation for most bacterial pathogens, but Mycobacterium, Rickettsia, Listeria, Mycoplasma, and Bartonella may rarely mimic encephalitis. Autoimmune encephalitis secondary to lupus, vasculitis, or other autoimmune disorder remains possible, but at this point an infectious encephalitis, particularly herpes encephalitis, is my highest concern. West Nile virus must be considered, but usually produces a severe illness only in immunocompromised or elderly patients. Additionally, despite the rarity of rabies, the patient's exposure to bats and the rapid clinical deterioration, suggest this possibility. In addition to routine bacterial and viral analyses (eg, enteroviral panel), samples should be sent for rabies PCR and antibody testing, West Nile virus, Lyme disease, syphilis, and mycobacterial and fungal pathogens, such as the aforementioned Cryptococcus gattii. Finally, given her presenting syndrome and MRI, immediate treatment with acyclovir and antibiotics is indicated.
The patient was treated for presumed meningoencephalitis with acyclovir and ceftriaxone, but over the following several days became unresponsive to all stimuli and developed repetitive thrusting movements of her mouth, tongue, and jaw. On hospital day 10, with concern for seizures, pentobarbital coma was induced, and the patient was intubated and transferred to our facility. On arrival, her physical examination was essentially unchanged aside from being in a medical coma. Hematology, chemistries, and thyroid‐stimulating hormone (TSH) were again unremarkable with the exception of an elevated creatine kinase (414 U/L) and a new anemia (hemoglobin 8.9 g/dL; mean corpuscular volume 87.6 fL) without evidence of iron deficiency or hemolysis. Blood and urine cultures were negative. Repeat cerebrospinal fluid analysis was essentially unchanged, revealing a red cell count of 1/mm3; white cell count 20/mm3 (86% lymphocytes, 2% neutrophils, 12% monocytes); protein 14 mg/dL; glucose 63 mg/dL, and negative Gram stain. Continuous electroencephalography revealed diffuse generalized slowing, but no seizure activity. An extensive evaluation for viral, bacterial, autoimmune, and paraneoplastic disorders was negative, including tests for anti‐acetylcholine (ACh) receptor binding antibody, anti‐striated muscle antibody, anti‐N‐type calcium channel antibody, anti‐P/Q‐type calcium channel antibodies, anto‐cancer associated retinopathy (CAR) antibody (also known as anti‐recoverin antibody), and anti‐collapsin respons mediator protein (CRMP‐5). Without confirmatory results and continued deterioration, she was empirically treated with methylprednisolone for presumed autoimmune encephalitis from hospital days 16 to 21. The patient remained unresponsive and ventilator‐dependent, despite removal of all sedation. She experienced intermittent fevers as high as 40.5C, remained tachycardic, hypertensive, and exhibited orofacial dyskinesias and jaw clenching, ultimately requiring botulinum toxin injections to prevent tongue biting. Given the lack of improvement despite attempted therapies, a working diagnosis of viral encephalitis with lasting neuropsychiatric sequelae was made. A tracheostomy and percutaneous gastrostomy tube were placed, and a long‐term ventilator care facility was identified.
I continue to wonder if this may be an autoimmune encephalitis, and am concerned about her unexplained fevers. Neuroleptic malignant syndrome secondary to misuse of her parents' medications should be considered in light of the elevated creatine kinase, although the severity and duration of the syndrome seem more profound than I would anticipate. Tetanus could present with jaw dystonia, but the rest of the case does not seem to fit. At this point, considering the patient's young age and poor prognosis without identified etiology, prior to discharge I would argue for a brain biopsy looking for evidence of rabies, or other infectious or autoimmune etiologies of the patient's progressive neurologic deterioration.
On hospital day 25, due to the persistent fevers with concern for occult abscess, an abdominopelvic CT was obtained, which identified a complex 11.8 cm 9.0 cm adnexal mass consistent with a teratoma (Figure 1).

Given the size of the mass, it is surprising that the patient did not report abdominal symptoms and that the physicians were unable to palpate it on examination. The differential diagnosis of a complex adnexal mass in an adolescent should include an ectopic pregnancy, ovarian cysts, tubo‐ovarian abscess, rarely an ovarian carcinoma or leiomyosarcoma, and a teratoma or dermoid tumor. While I mentioned the possibility of a malignancy at the outset, I did not further consider it. Common neoplasms encountered in adolescent patients include lymphoma and leukemia, germ cell tumors (including teratomas), central nervous system tumors and sarcomas, many of which have been reported to cause paraneoplastic disorders. At this point, I now think her presumed teratoma is associated with a paraneoplastic syndrome resulting in her presentation of limbic encephalitis.
A literature search was performed by the managing clinicians who rapidly identified the association between teratoma and limbic encephalitis. The patient was initially treated with intravenous immune globulin (IVIG), with transient improvement in her mental status. Serology returned positive for the anti‐N‐methyl‐D‐aspartate receptor antibody, confirming the diagnosis of anti‐N‐methyl‐D‐aspartate receptor encephalitis. On hospital day 36, her mass was resected (Figure 2). Pathology was consistent with a mature teratoma. Postoperatively, the patient improved daily, and was discharged on hospital day 43 with a near complete neurologic recovery. Four months following discharge, the patient had enrolled full time in college.

COMMENTARY
The N‐methyl‐D‐aspartate receptor (NMDAR) is an important regulator of synaptic transmission and memory within the CNS. Our patient's case illustrates the increasingly recognized syndrome of anti‐NMDAR encephalitis. NMDAR hypofunction is hypothesized to result in the cognitive and behavioral abnormalities of schizophrenia, and direct antagonism of the NMDAR by drugs such as phencyclidine (PCP) and ketamine results in symptoms such as psychosis, hallucinations, delusions, agitation, and dissociative amnesia.14 This constellation of symptoms is very similar to some of the initial neuropsychiatric symptoms observed in patients with anti‐NMDAR encephalitis.
Anti‐NMDAR encephalitis was first described in 2005 as a paraneoplastic limbic encephalitis associated with ovarian teratoma.5, 6 Characterized by the subacute onset (days to weeks) of short‐term memory loss, psychiatric symptoms, and sleep disturbances, limbic encephalitis is an inflammatory process caused by autoantibodies against intracellular or extracellar antigens in the limbic system and other brain structures. Limbic encephalitides associated with antibodies to intracellular antigens (such as Hu, Ma2, CV2/CRMP5, and Amphiphysin) are more often associated with malignancies, have worse outcomes (permanent neuropsychiatric sequelae and death), and are less responsive to immune therapy. Conversely, it appears that both the paraneoplastic and non‐paraneoplastic variants of limbic encephalitis associated with antibodies against cell membrane antigens (such as NMDAR and Voltage Gated Potassium Channels) respond more favorably to therapy.7
As with limbic encephalitis in general, anti‐NMDAR encephalitis can be non‐paraneoplastic as well as paraneoplastic in etiology. In a recently published series of 44 consecutive patients with anti‐NMDAR encephalitis, tumors were present in only 9 cases (8 teratomas).8 When associated with a teratoma, it has been postulated that anti‐NMDAR antibodies develop and cross the bloodbrain barrier to target central nervous system NMDA receptors. This process results in down‐regulation of the neuronal surface NMDAR which then causes the psychiatric and behavioral changes described.6 The mechanism by which these antibodies traverse the bloodbrain barrier is not completely understood, but likely requires some disruption of the barrier in order to trigger anti‐NMDAR encephalitis.8, 9 Non‐paraneoplastic cases evidently involve other unknown stimuli for NMDAR antibody synthesisone report has suggested that subunits of the NMDAR are expressed by normal ovarian tissue, something which may explain the female predilection even in the cohort unaffected by teratomas.10
Most patients with anti‐NMDAR encephalitis are female and young (median age 23 years), although men and children are also affected.8, 9, 11 While the exact incidence of anti‐NMDAR encephalitis is still unknown, the increasing number of case reports suggests that it may be more frequent than any other type of paraneoplastic encephalitis.12 The majority of patients with anti‐NMDAR encephalitis experience an antecedent infectious prodrome (eg, diarrheal illness or upper respiratory infection [URI]), followed 1020 days later by progressive neuropsychiatric and behavioral symptoms which include confusion, memory deficits, impaired responsiveness, seizures, central hypoventilation, and signs of autonomic instability (tachycardia, tachypnea, diaphoresis, cardiac dysrhythmia, blood pressure instability, and dysthermia). At this stage, patients may also manifest a unique constellation of choreoathetoid orofacial and limb movements such as lip licking, chewing, sustained jaw clenching, jaw opening dystonias, ocular deviation and disconjugation, grimacing, myoclonus, and bizarre arm movements. Due to cardiovascular complications and ventilator requirements, most patients require intensive care unit (ICU) level care. 8, 9, 11 As in our discussant's evaluation, other disorders to include in the differential diagnosis for this presentation includes paraneoplastic or autoimmune causes of limbic encephalitis, toxins, heavy metals, and viral causes of encephalitis; in particular, herpes simplex virus (HSV).7
The CNS imaging findings in this condition include brain MRI abnormalities in about 30%55% of patients, which can include increased signal on fluid‐attenuated inversion recovery (FLAIR) or T2 sequences of the cerebral cortex, overlying meninges, or basal ganglia. Abnormalities in the temporal lobes, corpus callosum, and brainstem have also been described. As in our patient, CSF lymphocytic pleocytosis has also been noted.6, 8, 9
Although many cases of limbic encephalitis portend a poor prognosis with permanent neuropsychiatric sequelae and death, anti‐NMDAR can be very responsive to treatment; particularly if diagnosed early. Successful treatment of anti‐NMDAR encephalitis involves immunotherapy and, preferably, early surgical resection of any tumor. 6, 8, 9 Non‐paraneoplastic cases appear to require more aggressive and prolonged immunotherapies to avoid relapse. In both groups, a trend towards improved outcome has been noted in patients treated early in disease course (40 days from symptom onset).8 There are no established guidelines for the treatment of anti‐NMDAR encephalitis, and no randomized controlled trials have evaluated anti‐NMDAR encephalitis treatment. Observational studies of immune‐modulating therapies have shown efficacy with high‐dose steroids and the addition of plasma exchange and/or intravenous immune globulin. Rituximab and cyclophosphamide can be considered if patients fail to improve on other immunotherapies.9 Data from case series seem to suggest a lower risk of relapse in patients treated with immunotherapy.13
Exploration of this patient's persistent high fevers ultimately led to the serendipitous diagnosis of the increasingly recognized syndrome of anti‐NMDAR encephalitis, although in retrospect nearly all of the features of her presentation fit well with this condition. Thus, it was only by a chance finding on her abdominal CT scan that this patient was ultimately diagnosed with a treatable, noninfectious encephalitis associated with an ovarian teratoma. This case reinforces the importance of thorough patient evaluations and being prepared to draw meaningful conclusions from unexpected findings. Given how close this patient was to being discharged to a long‐term care facility, we found this case a fascinating yet sobering reminder to guard against prematurely concluding a syndrome to be untreatable.
KEY TEACHING POINTS
-
Anti‐NMDAR encephalitis is an increasingly recognized cause of autoimmune limbic encephalitis, and thus should be considered in patients with new‐onset psychiatric symptoms accompanied by seizures, autonomic instability, hypoventilation, or dyskinesias.
-
A thorough history, examination, and evaluation of data is critical to make an early diagnosis of anti‐NMDAR encephalitis, because, unlike other forms of limbic encephalitis, this condition may be very responsive to early initiation of treatment.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
A previously healthy 18‐year‐old woman living in the Pacific Northwest was brought in by her parents to a local hospital with a 4‐day history of acting crazy. Two weeks prior to presentation, she complained of a new‐onset severe headache, diaphoresis, and chills. Four days prior to presentation, she became progressively more impulsive, which ultimately included jumping out of a moving vehicle and running away from home. She experienced unexplained emotional outbursts and was unable to identify familiar relatives or common objects. Additionally, she began having hyperventilation spells and auditory hallucinations.
In an adolescent presenting with erratic behavior, one should consider the possibility of substance abuse or a psychiatric disease such as bipolar disorder with manic features, psychotic manifestations of severe depression, or early schizophrenia. However, it is important to first rule out non‐psychiatric disease, with a diagnostic approach dependent on her human immunodeficiency virus (HIV) status. The presence of headache, diaphoresis, and chills raises concern for an infectious or noninfectious inflammatory central nervous system process. In addition to the effects of illicit drugs such as cocaine or methamphetamine, this presentation may be consistent with a medication‐ or herbal‐induced anticholinergic syndrome, which may present with confusion, ataxia, coma, and cardiopulmonary failure. Since this case originates in the Northwest, one should be aware of the regional outbreak of Cryptococcus gattii in immunocompetent hosts, and that local hallucinogenic plants, such as jimson weed or mushrooms (Amanita muscaria) can cause anticholinergic syndromes. At this point, the differential diagnosis is broad, and evaluation should focus on potentially reversible life‐threatening conditions; in particular, herpes encephalitis. In addition to a detailed history, examination, and routine laboratory studies including HIV serology, I would obtain a drug screen, and order a computed tomography (CT) scan of the brain before performing a lumbar puncture. I would also order a magnetic resonance imaging (MRI) study to evaluate for meningeal or cerebral enhancement suggestive of encephalitis.
The patient had no past medical, psychiatric, or surgical history and took no medications. She lived with her parents who thought she neither used illicit drugs or alcohol, nor was sexually active. She had recently graduated high school and was planning to attend college. Her family history was notable for a mother with bipolar and seizure disorders, and 2 healthy younger siblings. Her family had a healthy cat and dog, and reported a large number of bats living nearby. She had never traveled outside the western United States. The patient presented in late spring, but there was no obvious history of mosquito bites. Her last menstrual period was 4 months prior to presentation. Full review of systems was otherwise negative.
The family history of mood disorder supports continued consideration of bipolar disorder with psychotic manifestations. However, infectious or inflammatory processes remain highest on the differential at this point. The duration of symptoms makes common bacterial meningitis etiologies (Streptococcus, Neisseria, Haemophilus, Listeria) less likely, but would be consistent with herpes simplex encephalitis or lupus cerebritis. Additional infectious considerations would include other viral (eg, varicella zoster virus, Epstein‐Barr virus, enteroviruses, and the arthropod‐borne encephalitides) or unusual bacterial encephalitic syndromes. Although the health status of pets is rarely helpful, dogs can carry ticks that harbor Borrelia burgdorferi (the agent of Lyme disease), which may present with central nervous system (CNS) manifestations. Other conditions associated with pets (such as leptospirosis or cat scratch disease) seem unlikely. The exposure to bats raises the possibility of rabies infection. If she is HIV‐positive, one would need to consider the possibility of opportunistic infections such as cytomegalovirus (CMV), Cryptococcus, cerebral toxoplasmosis, and progressive multifocal leukoencephalopathy (PML) caused by JC virus reaction. Finally, regardless of history, given the patient's amenorrhea, we must perform a pregnancy test.
The patient's temperature was 97.3F, heart rate 129 beats per minute, respiratory rate 19 breaths per minute, and her blood pressure 144/97 mmHg. She was an obese, well‐developed young woman, who was drowsy but arousable, with marked speech latency. Her cranium and oropharynx were normal, and her neck was supple. Aside from tachycardia, her cardiopulmonary, musculoskeletal, and skin exams were normal. Her abdomen was obese and soft, without masses or organomegaly. A pelvic examination was not performed. On neurologic exam, her strength was symmetrically diminished throughout (3+/5). Otherwise, she was oriented to person and general location, but not to day of week, month, or year. Her cranial nerves, sensation, deep tendon reflexes, and muscle tone were normal. A cerebellar examination, plantar response, and gait test were not performed. A brain MRI revealed only a small subarachnoid cyst and possible subtle enhancement of temporal lobes. Initial laboratory studies demonstrated: white blood cell count 14,000/mm3 (72% neutrophils, 17% lymphocytes, 9% monocytes, 2% eosinophils); hemoglobin 14.0 g/dL (mean corpuscular volume 87.4 fL); platelet count 417,000/mm3. Serum electrolytes, liver function tests, coagulation studies, thyroid stimulating hormone, serum ammonia, and urinalysis were normal. Her serum pregnancy test and urine toxicology screen were negative. A room air arterial blood gas revealed a pH of 7.49, PaCO2 32 mmHg, PaO2 89 mmHg; and a bicarbonate 24 mmol/L. Cerebrospinal fluid demonstrated: red cell count of 2/mm3; white cell count 17/mm3 (88% lymphocytes, 3% neutrophils, 9% monocytes); protein 19 mg/dL (normal 1555 mg/dL); and glucose of 79 mg/dL (normal 4080 mg/dL). Gram stain, fungal and bacterial cultures, and HIV serology were negative, and herpes simplex virus was not detected via polymerase chain reaction (PCR).
The tachycardia, respiratory alkalosis, and leukocytosis continue to suggest an infection or inflammatory state. Her neurological deterioration without focal findings, cerebrospinal fluid (CSF) lymphocytic pleocytosis with normal glucose and protein, and temporal lobe enhancement on MRI strongly suggest a meningoencephalitis. This would be an unusual presentation for most bacterial pathogens, but Mycobacterium, Rickettsia, Listeria, Mycoplasma, and Bartonella may rarely mimic encephalitis. Autoimmune encephalitis secondary to lupus, vasculitis, or other autoimmune disorder remains possible, but at this point an infectious encephalitis, particularly herpes encephalitis, is my highest concern. West Nile virus must be considered, but usually produces a severe illness only in immunocompromised or elderly patients. Additionally, despite the rarity of rabies, the patient's exposure to bats and the rapid clinical deterioration, suggest this possibility. In addition to routine bacterial and viral analyses (eg, enteroviral panel), samples should be sent for rabies PCR and antibody testing, West Nile virus, Lyme disease, syphilis, and mycobacterial and fungal pathogens, such as the aforementioned Cryptococcus gattii. Finally, given her presenting syndrome and MRI, immediate treatment with acyclovir and antibiotics is indicated.
The patient was treated for presumed meningoencephalitis with acyclovir and ceftriaxone, but over the following several days became unresponsive to all stimuli and developed repetitive thrusting movements of her mouth, tongue, and jaw. On hospital day 10, with concern for seizures, pentobarbital coma was induced, and the patient was intubated and transferred to our facility. On arrival, her physical examination was essentially unchanged aside from being in a medical coma. Hematology, chemistries, and thyroid‐stimulating hormone (TSH) were again unremarkable with the exception of an elevated creatine kinase (414 U/L) and a new anemia (hemoglobin 8.9 g/dL; mean corpuscular volume 87.6 fL) without evidence of iron deficiency or hemolysis. Blood and urine cultures were negative. Repeat cerebrospinal fluid analysis was essentially unchanged, revealing a red cell count of 1/mm3; white cell count 20/mm3 (86% lymphocytes, 2% neutrophils, 12% monocytes); protein 14 mg/dL; glucose 63 mg/dL, and negative Gram stain. Continuous electroencephalography revealed diffuse generalized slowing, but no seizure activity. An extensive evaluation for viral, bacterial, autoimmune, and paraneoplastic disorders was negative, including tests for anti‐acetylcholine (ACh) receptor binding antibody, anti‐striated muscle antibody, anti‐N‐type calcium channel antibody, anti‐P/Q‐type calcium channel antibodies, anto‐cancer associated retinopathy (CAR) antibody (also known as anti‐recoverin antibody), and anti‐collapsin respons mediator protein (CRMP‐5). Without confirmatory results and continued deterioration, she was empirically treated with methylprednisolone for presumed autoimmune encephalitis from hospital days 16 to 21. The patient remained unresponsive and ventilator‐dependent, despite removal of all sedation. She experienced intermittent fevers as high as 40.5C, remained tachycardic, hypertensive, and exhibited orofacial dyskinesias and jaw clenching, ultimately requiring botulinum toxin injections to prevent tongue biting. Given the lack of improvement despite attempted therapies, a working diagnosis of viral encephalitis with lasting neuropsychiatric sequelae was made. A tracheostomy and percutaneous gastrostomy tube were placed, and a long‐term ventilator care facility was identified.
I continue to wonder if this may be an autoimmune encephalitis, and am concerned about her unexplained fevers. Neuroleptic malignant syndrome secondary to misuse of her parents' medications should be considered in light of the elevated creatine kinase, although the severity and duration of the syndrome seem more profound than I would anticipate. Tetanus could present with jaw dystonia, but the rest of the case does not seem to fit. At this point, considering the patient's young age and poor prognosis without identified etiology, prior to discharge I would argue for a brain biopsy looking for evidence of rabies, or other infectious or autoimmune etiologies of the patient's progressive neurologic deterioration.
On hospital day 25, due to the persistent fevers with concern for occult abscess, an abdominopelvic CT was obtained, which identified a complex 11.8 cm 9.0 cm adnexal mass consistent with a teratoma (Figure 1).

Given the size of the mass, it is surprising that the patient did not report abdominal symptoms and that the physicians were unable to palpate it on examination. The differential diagnosis of a complex adnexal mass in an adolescent should include an ectopic pregnancy, ovarian cysts, tubo‐ovarian abscess, rarely an ovarian carcinoma or leiomyosarcoma, and a teratoma or dermoid tumor. While I mentioned the possibility of a malignancy at the outset, I did not further consider it. Common neoplasms encountered in adolescent patients include lymphoma and leukemia, germ cell tumors (including teratomas), central nervous system tumors and sarcomas, many of which have been reported to cause paraneoplastic disorders. At this point, I now think her presumed teratoma is associated with a paraneoplastic syndrome resulting in her presentation of limbic encephalitis.
A literature search was performed by the managing clinicians who rapidly identified the association between teratoma and limbic encephalitis. The patient was initially treated with intravenous immune globulin (IVIG), with transient improvement in her mental status. Serology returned positive for the anti‐N‐methyl‐D‐aspartate receptor antibody, confirming the diagnosis of anti‐N‐methyl‐D‐aspartate receptor encephalitis. On hospital day 36, her mass was resected (Figure 2). Pathology was consistent with a mature teratoma. Postoperatively, the patient improved daily, and was discharged on hospital day 43 with a near complete neurologic recovery. Four months following discharge, the patient had enrolled full time in college.

COMMENTARY
The N‐methyl‐D‐aspartate receptor (NMDAR) is an important regulator of synaptic transmission and memory within the CNS. Our patient's case illustrates the increasingly recognized syndrome of anti‐NMDAR encephalitis. NMDAR hypofunction is hypothesized to result in the cognitive and behavioral abnormalities of schizophrenia, and direct antagonism of the NMDAR by drugs such as phencyclidine (PCP) and ketamine results in symptoms such as psychosis, hallucinations, delusions, agitation, and dissociative amnesia.14 This constellation of symptoms is very similar to some of the initial neuropsychiatric symptoms observed in patients with anti‐NMDAR encephalitis.
Anti‐NMDAR encephalitis was first described in 2005 as a paraneoplastic limbic encephalitis associated with ovarian teratoma.5, 6 Characterized by the subacute onset (days to weeks) of short‐term memory loss, psychiatric symptoms, and sleep disturbances, limbic encephalitis is an inflammatory process caused by autoantibodies against intracellular or extracellar antigens in the limbic system and other brain structures. Limbic encephalitides associated with antibodies to intracellular antigens (such as Hu, Ma2, CV2/CRMP5, and Amphiphysin) are more often associated with malignancies, have worse outcomes (permanent neuropsychiatric sequelae and death), and are less responsive to immune therapy. Conversely, it appears that both the paraneoplastic and non‐paraneoplastic variants of limbic encephalitis associated with antibodies against cell membrane antigens (such as NMDAR and Voltage Gated Potassium Channels) respond more favorably to therapy.7
As with limbic encephalitis in general, anti‐NMDAR encephalitis can be non‐paraneoplastic as well as paraneoplastic in etiology. In a recently published series of 44 consecutive patients with anti‐NMDAR encephalitis, tumors were present in only 9 cases (8 teratomas).8 When associated with a teratoma, it has been postulated that anti‐NMDAR antibodies develop and cross the bloodbrain barrier to target central nervous system NMDA receptors. This process results in down‐regulation of the neuronal surface NMDAR which then causes the psychiatric and behavioral changes described.6 The mechanism by which these antibodies traverse the bloodbrain barrier is not completely understood, but likely requires some disruption of the barrier in order to trigger anti‐NMDAR encephalitis.8, 9 Non‐paraneoplastic cases evidently involve other unknown stimuli for NMDAR antibody synthesisone report has suggested that subunits of the NMDAR are expressed by normal ovarian tissue, something which may explain the female predilection even in the cohort unaffected by teratomas.10
Most patients with anti‐NMDAR encephalitis are female and young (median age 23 years), although men and children are also affected.8, 9, 11 While the exact incidence of anti‐NMDAR encephalitis is still unknown, the increasing number of case reports suggests that it may be more frequent than any other type of paraneoplastic encephalitis.12 The majority of patients with anti‐NMDAR encephalitis experience an antecedent infectious prodrome (eg, diarrheal illness or upper respiratory infection [URI]), followed 1020 days later by progressive neuropsychiatric and behavioral symptoms which include confusion, memory deficits, impaired responsiveness, seizures, central hypoventilation, and signs of autonomic instability (tachycardia, tachypnea, diaphoresis, cardiac dysrhythmia, blood pressure instability, and dysthermia). At this stage, patients may also manifest a unique constellation of choreoathetoid orofacial and limb movements such as lip licking, chewing, sustained jaw clenching, jaw opening dystonias, ocular deviation and disconjugation, grimacing, myoclonus, and bizarre arm movements. Due to cardiovascular complications and ventilator requirements, most patients require intensive care unit (ICU) level care. 8, 9, 11 As in our discussant's evaluation, other disorders to include in the differential diagnosis for this presentation includes paraneoplastic or autoimmune causes of limbic encephalitis, toxins, heavy metals, and viral causes of encephalitis; in particular, herpes simplex virus (HSV).7
The CNS imaging findings in this condition include brain MRI abnormalities in about 30%55% of patients, which can include increased signal on fluid‐attenuated inversion recovery (FLAIR) or T2 sequences of the cerebral cortex, overlying meninges, or basal ganglia. Abnormalities in the temporal lobes, corpus callosum, and brainstem have also been described. As in our patient, CSF lymphocytic pleocytosis has also been noted.6, 8, 9
Although many cases of limbic encephalitis portend a poor prognosis with permanent neuropsychiatric sequelae and death, anti‐NMDAR can be very responsive to treatment; particularly if diagnosed early. Successful treatment of anti‐NMDAR encephalitis involves immunotherapy and, preferably, early surgical resection of any tumor. 6, 8, 9 Non‐paraneoplastic cases appear to require more aggressive and prolonged immunotherapies to avoid relapse. In both groups, a trend towards improved outcome has been noted in patients treated early in disease course (40 days from symptom onset).8 There are no established guidelines for the treatment of anti‐NMDAR encephalitis, and no randomized controlled trials have evaluated anti‐NMDAR encephalitis treatment. Observational studies of immune‐modulating therapies have shown efficacy with high‐dose steroids and the addition of plasma exchange and/or intravenous immune globulin. Rituximab and cyclophosphamide can be considered if patients fail to improve on other immunotherapies.9 Data from case series seem to suggest a lower risk of relapse in patients treated with immunotherapy.13
Exploration of this patient's persistent high fevers ultimately led to the serendipitous diagnosis of the increasingly recognized syndrome of anti‐NMDAR encephalitis, although in retrospect nearly all of the features of her presentation fit well with this condition. Thus, it was only by a chance finding on her abdominal CT scan that this patient was ultimately diagnosed with a treatable, noninfectious encephalitis associated with an ovarian teratoma. This case reinforces the importance of thorough patient evaluations and being prepared to draw meaningful conclusions from unexpected findings. Given how close this patient was to being discharged to a long‐term care facility, we found this case a fascinating yet sobering reminder to guard against prematurely concluding a syndrome to be untreatable.
KEY TEACHING POINTS
-
Anti‐NMDAR encephalitis is an increasingly recognized cause of autoimmune limbic encephalitis, and thus should be considered in patients with new‐onset psychiatric symptoms accompanied by seizures, autonomic instability, hypoventilation, or dyskinesias.
-
A thorough history, examination, and evaluation of data is critical to make an early diagnosis of anti‐NMDAR encephalitis, because, unlike other forms of limbic encephalitis, this condition may be very responsive to early initiation of treatment.
- , . N‐methyl‐D‐aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49.
- , . Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308.
- , , . NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533.
- , , , et al. Ketamine‐induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20:106–118.
- , , , , , . Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol. 2005;58:594–604.
- , , , et al. Paraneoplastic anti‐N‐methyl‐D‐aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36.
- , . Limbic encephalitis and variants: classification, diagnosis and treatment. Neurologist. 2007;13:261–271.
- , , , et al. N‐methyl‐D‐aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non‐paraneoplastic disorder of both sexes. Brain. 2010;133:1655–1667.
- , . NMDA receptor antibody encephalitis. Curr Neurol Neurosci Rep. 2011;11:298–304.
- , , , et al. Expression of various glutamate receptors including N‐methyl‐D‐aspartate receptor (NMDAR) in an ovarian teratoma removed from a young woman with anti‐NMDAR encephalitis. Intern Med. 2010;49:2167–2173.
- , , , et al. Anti‐NMDA‐receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1074–1075.
- , , , , . Clinical experience and laboratory investigations in patients with anti‐NMDAR encephalitis. Lancet Neurol. 2011;10:63–74.
- , , , et al. Analysis of relapses in anti‐NMDAR encephalitis. Neurology. 2011;77:996–999.
- , . N‐methyl‐D‐aspartate receptor subtypes: multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49.
- , . Recent advances in the phencyclidine model of schizophrenia. Am J Psychiatry. 1991;148:1301–1308.
- , , . NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res. 1999;33:523–533.
- , , , et al. Ketamine‐induced NMDA receptor hypofunction as a model of memory impairment and psychosis. Neuropsychopharmacology. 1999;20:106–118.
- , , , , , . Paraneoplastic encephalitis, psychiatric symptoms, and hypoventilation in ovarian teratoma. Ann Neurol. 2005;58:594–604.
- , , , et al. Paraneoplastic anti‐N‐methyl‐D‐aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol. 2007;61:25–36.
- , . Limbic encephalitis and variants: classification, diagnosis and treatment. Neurologist. 2007;13:261–271.
- , , , et al. N‐methyl‐D‐aspartate antibody encephalitis: temporal progression of clinical and paraclinical observations in a predominantly non‐paraneoplastic disorder of both sexes. Brain. 2010;133:1655–1667.
- , . NMDA receptor antibody encephalitis. Curr Neurol Neurosci Rep. 2011;11:298–304.
- , , , et al. Expression of various glutamate receptors including N‐methyl‐D‐aspartate receptor (NMDAR) in an ovarian teratoma removed from a young woman with anti‐NMDAR encephalitis. Intern Med. 2010;49:2167–2173.
- , , , et al. Anti‐NMDA‐receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008;7:1074–1075.
- , , , , . Clinical experience and laboratory investigations in patients with anti‐NMDAR encephalitis. Lancet Neurol. 2011;10:63–74.
- , , , et al. Analysis of relapses in anti‐NMDAR encephalitis. Neurology. 2011;77:996–999.
Managing Serious Surgical Illness
Editor's Note: This article is the first in a series on surgical palliative care that will appear periodically in Surgery News. The series, which will include contributions from members of the American College of Surgeons' Surgical Palliative Care Task Force, is intended to inform readers about the variety of issues involved in managing patients with serious or terminal illnesses and the role surgeons can play in providing the best possible care for these individuals.
If I were asked what has made the biggest impact on the field of surgery during my 30-year career, I would say the transition to evidence-based practice as a benchmark of quality and the recognition of quality of life outcomes as meaningful measures of care have been equally important. These represent an evolution from the surgeon-centric practice of the past to the patient-centered practice of the present.
But I believe we are at the point of another pendulum swing, because the "patient-centeredness" concept not only has failed to prevent vast and often ineffectual health care expenditures, but might actually be contributing to them.
I recently asked a physically and emotionally exhausted family member of an "ICU to nowhere" patient why he thought patients get "stuck" in the ICU. He answered eloquently, "People just don’t think they should die."
The current conceptual framework for care of the seriously ill is unable to respond to the psychological and spiritual questions raised by this comment. Disease management alone will not break this type of gridlock, nor will it leave patients and families with a lasting sense of support.
Surgical palliative care is an evidence-based and interdisciplinary approach (consisting of surgery, nursing, social work, chaplaincy, counseling, and others) to caring for patients who are seriously or terminally ill.
Palliative care includes communication skills (disclosure of prognosis, setting goals, advance care planning), pain and non-pain symptom management, ethics and conflict resolution, and self-awareness. Palliative care emphasizes continuity of care across clinical settings and services, attention to spiritual needs, psychosocial support for patients and families, and bereavement support for families of the deceased and team members who cared for them.
Although the origins of palliative care are identifiable in the modern hospice movement, its applicability goes far beyond the hospice-appropriate population. For example, in my own in-hospital practice, only about half of my palliative care consultations are appropriate for hospice referral. Some patients I have subsequently referred for liver and kidney transplantation; numerous others have proceeded to primary surgical management of cancer; and still others have returned to work following trauma rehabilitation.
During the past 15 years, the American College of Surgeons has strongly supported the concept of palliative care through position statements, ACS Bulletin articles, and education initiatives for surgeons. The Commission on Cancer has endorsed palliative care in its Cancer Program Standards 2012 by requiring the availability of palliative care services. The ABS has joined nine other member boards of the American Board of Medical Specialties (ABMS) in offering subspecialty certification in Hospice and Palliative Medicine (HPM). Although the number of surgeons specializing in palliative medicine will be very small, the need for expertise in this area will grow as the public and practitioners recognize the rewards of an evidence-based palliative care for seriously ill patients, their families, and surgical practitioners.
Dr. Dunn, an ACS Fellow based in Erie, Pa., is chair of the ACS Surgical Palliative Care Task Force.
Editor's Note: This article is the first in a series on surgical palliative care that will appear periodically in Surgery News. The series, which will include contributions from members of the American College of Surgeons' Surgical Palliative Care Task Force, is intended to inform readers about the variety of issues involved in managing patients with serious or terminal illnesses and the role surgeons can play in providing the best possible care for these individuals.
If I were asked what has made the biggest impact on the field of surgery during my 30-year career, I would say the transition to evidence-based practice as a benchmark of quality and the recognition of quality of life outcomes as meaningful measures of care have been equally important. These represent an evolution from the surgeon-centric practice of the past to the patient-centered practice of the present.
But I believe we are at the point of another pendulum swing, because the "patient-centeredness" concept not only has failed to prevent vast and often ineffectual health care expenditures, but might actually be contributing to them.
I recently asked a physically and emotionally exhausted family member of an "ICU to nowhere" patient why he thought patients get "stuck" in the ICU. He answered eloquently, "People just don’t think they should die."
The current conceptual framework for care of the seriously ill is unable to respond to the psychological and spiritual questions raised by this comment. Disease management alone will not break this type of gridlock, nor will it leave patients and families with a lasting sense of support.
Surgical palliative care is an evidence-based and interdisciplinary approach (consisting of surgery, nursing, social work, chaplaincy, counseling, and others) to caring for patients who are seriously or terminally ill.
Palliative care includes communication skills (disclosure of prognosis, setting goals, advance care planning), pain and non-pain symptom management, ethics and conflict resolution, and self-awareness. Palliative care emphasizes continuity of care across clinical settings and services, attention to spiritual needs, psychosocial support for patients and families, and bereavement support for families of the deceased and team members who cared for them.
Although the origins of palliative care are identifiable in the modern hospice movement, its applicability goes far beyond the hospice-appropriate population. For example, in my own in-hospital practice, only about half of my palliative care consultations are appropriate for hospice referral. Some patients I have subsequently referred for liver and kidney transplantation; numerous others have proceeded to primary surgical management of cancer; and still others have returned to work following trauma rehabilitation.
During the past 15 years, the American College of Surgeons has strongly supported the concept of palliative care through position statements, ACS Bulletin articles, and education initiatives for surgeons. The Commission on Cancer has endorsed palliative care in its Cancer Program Standards 2012 by requiring the availability of palliative care services. The ABS has joined nine other member boards of the American Board of Medical Specialties (ABMS) in offering subspecialty certification in Hospice and Palliative Medicine (HPM). Although the number of surgeons specializing in palliative medicine will be very small, the need for expertise in this area will grow as the public and practitioners recognize the rewards of an evidence-based palliative care for seriously ill patients, their families, and surgical practitioners.
Dr. Dunn, an ACS Fellow based in Erie, Pa., is chair of the ACS Surgical Palliative Care Task Force.
Editor's Note: This article is the first in a series on surgical palliative care that will appear periodically in Surgery News. The series, which will include contributions from members of the American College of Surgeons' Surgical Palliative Care Task Force, is intended to inform readers about the variety of issues involved in managing patients with serious or terminal illnesses and the role surgeons can play in providing the best possible care for these individuals.
If I were asked what has made the biggest impact on the field of surgery during my 30-year career, I would say the transition to evidence-based practice as a benchmark of quality and the recognition of quality of life outcomes as meaningful measures of care have been equally important. These represent an evolution from the surgeon-centric practice of the past to the patient-centered practice of the present.
But I believe we are at the point of another pendulum swing, because the "patient-centeredness" concept not only has failed to prevent vast and often ineffectual health care expenditures, but might actually be contributing to them.
I recently asked a physically and emotionally exhausted family member of an "ICU to nowhere" patient why he thought patients get "stuck" in the ICU. He answered eloquently, "People just don’t think they should die."
The current conceptual framework for care of the seriously ill is unable to respond to the psychological and spiritual questions raised by this comment. Disease management alone will not break this type of gridlock, nor will it leave patients and families with a lasting sense of support.
Surgical palliative care is an evidence-based and interdisciplinary approach (consisting of surgery, nursing, social work, chaplaincy, counseling, and others) to caring for patients who are seriously or terminally ill.
Palliative care includes communication skills (disclosure of prognosis, setting goals, advance care planning), pain and non-pain symptom management, ethics and conflict resolution, and self-awareness. Palliative care emphasizes continuity of care across clinical settings and services, attention to spiritual needs, psychosocial support for patients and families, and bereavement support for families of the deceased and team members who cared for them.
Although the origins of palliative care are identifiable in the modern hospice movement, its applicability goes far beyond the hospice-appropriate population. For example, in my own in-hospital practice, only about half of my palliative care consultations are appropriate for hospice referral. Some patients I have subsequently referred for liver and kidney transplantation; numerous others have proceeded to primary surgical management of cancer; and still others have returned to work following trauma rehabilitation.
During the past 15 years, the American College of Surgeons has strongly supported the concept of palliative care through position statements, ACS Bulletin articles, and education initiatives for surgeons. The Commission on Cancer has endorsed palliative care in its Cancer Program Standards 2012 by requiring the availability of palliative care services. The ABS has joined nine other member boards of the American Board of Medical Specialties (ABMS) in offering subspecialty certification in Hospice and Palliative Medicine (HPM). Although the number of surgeons specializing in palliative medicine will be very small, the need for expertise in this area will grow as the public and practitioners recognize the rewards of an evidence-based palliative care for seriously ill patients, their families, and surgical practitioners.
Dr. Dunn, an ACS Fellow based in Erie, Pa., is chair of the ACS Surgical Palliative Care Task Force.
Factor appears safe, effective in hemophilia B
A novel therapy can prevent bleeding episodes in patients with hemophilia B, results of an industry-funded study suggest.
The goal of the phase 3 study, called B-LONG, was to evaluate the safety and efficacy of a long-lasting recombinant factor IX Fc fusion protein (rFIXFc, also known as eftrenonacog alfa and Alprolix).
Researchers found that a single injection of rFIXFc was sufficient to control more than 90% of bleeding episodes in males with hemophilia B.
In addition, none of the patients developed inhibitors, and the factor was generally well-tolerated.
The companies developing rFIXFc, Biogen Idec and Sobi, announced these results on September 26.
“Our companies are pioneering the application of Fc fusion technology to extend the half-life of clotting factors,” said Geoffrey McDonough, MD, Chief Executive Officer of Sobi, a biopharmaceutical company based in Stockholm, Sweden.
“Fc fusion technology utilizes a naturally occurring recycling pathway that has been successfully employed in other therapeutic areas. This approach holds promise for combining more consistent protection with fewer injections.”
Study design
To determine if rFIXFc does, in fact, provide bleeding protection with fewer injections, researchers administered the factor to 123 male patients with hemophilia B.
Patients were 12 years of age and older and had a history of at least 100 prior days during which they received 1 or more injections with any currently marketed factor IX product.
The investigators divided patients into 4 treatment arms. In Arm 1, 63 patients received weekly prophylaxis with rFIXFc. The starting dose was 50 IU/kg, and it was adjusted to maintain trough factor levels sufficient to prevent bleeding.
In Arm 2, 29 patients received individualized interval prophylaxis with rFIXFc. The factor was administered at 100 IU/kg at an initial interval of 10 days. It was subsequently individualized to maintain trough factor levels sufficient to prevent bleeding.
In Arm 3, 27 patients received episodic rFIXFc as needed to control bleeding. And in Arm 4, 12 patients who required major surgery received perioperative management with rFIXFc. Eight patients in this arm were also enrolled in other treatment arms.
Efficacy data
Overall, 93.5% of patients completed the study. Researchers assessed control of bleeding in all patients who experienced a bleeding episode while on study. In total, 90.4% of bleeding episodes were controlled by a single injection of rFIXFc.
The overall median annualized bleeding rates—including spontaneous and traumatic bleeds—were 2.95 in the weekly prophylaxis arm, 1.38 in the individualized interval prophylaxis arm, and 17.69 in the episodic treatment arm.
The perioperative management arm consisted of 12 patients undergoing 14 major surgical procedures. The treating physicians rated the hemostatic efficacy of rFIXFc as excellent or good in 100% of these surgeries.
The researchers also analyzed the pharmacokinetics of rFIXFc. The approximate terminal half-life of rFIXFc was 82 hours, compared to 34 hours for another recombinant factor IX product called BeneFIX.
This suggests that rFIXFc offers longer-lasting protection from bleeding in patients with hemophilia B, said Glenn Pierce, MD, PhD, Chief Medical Officer of Biogen Idec’s hemophilia therapeutic area.
“Currently, prophylactic treatment of hemophilia B requires intravenous injections up to 3 times a week,” he said, “which makes the prospect of a longer-lasting factor IX therapy very exciting.”
Safety data
The researchers found that rFIXFc was generally well-tolerated. None of the patients developed inhibitors, and none reported anaphylaxis.
The most common adverse events—with an incidence of 5% or greater—occurring outside of the perioperative management arm were nasopharyngitis, influenza, arthralgia, upper respiratory infection, hypertension, and headache.
One serious adverse event may have been drug-related. The patient experienced obstructive uropathy in the setting of hematuria. However, he continued to receive rFIXFc, and the event resolved with medical management.
Next steps
Based on the overall favorable outcomes of this study, Biogen Idec and Sobi are planning to submit a Biologics License Application for rFIXFc to the FDA in the first half of 2013. They also plan to submit an application to the EMA following results of a similar study in children, called Kids B-LONG.
Kids B-LONG is a phase 3, open-label study in previously treated children who are younger than 12 years of age and have been diagnosed with hemophilia B. The study is actively recruiting patients.
Researchers are also evaluating rFIXFc in the B-YOND study, which is a long-term, open-label extension study for patients who completed the B-LONG study or who will complete the Kids B-LONG study. ![]()

A novel therapy can prevent bleeding episodes in patients with hemophilia B, results of an industry-funded study suggest.
The goal of the phase 3 study, called B-LONG, was to evaluate the safety and efficacy of a long-lasting recombinant factor IX Fc fusion protein (rFIXFc, also known as eftrenonacog alfa and Alprolix).
Researchers found that a single injection of rFIXFc was sufficient to control more than 90% of bleeding episodes in males with hemophilia B.
In addition, none of the patients developed inhibitors, and the factor was generally well-tolerated.
The companies developing rFIXFc, Biogen Idec and Sobi, announced these results on September 26.
“Our companies are pioneering the application of Fc fusion technology to extend the half-life of clotting factors,” said Geoffrey McDonough, MD, Chief Executive Officer of Sobi, a biopharmaceutical company based in Stockholm, Sweden.
“Fc fusion technology utilizes a naturally occurring recycling pathway that has been successfully employed in other therapeutic areas. This approach holds promise for combining more consistent protection with fewer injections.”
Study design
To determine if rFIXFc does, in fact, provide bleeding protection with fewer injections, researchers administered the factor to 123 male patients with hemophilia B.
Patients were 12 years of age and older and had a history of at least 100 prior days during which they received 1 or more injections with any currently marketed factor IX product.
The investigators divided patients into 4 treatment arms. In Arm 1, 63 patients received weekly prophylaxis with rFIXFc. The starting dose was 50 IU/kg, and it was adjusted to maintain trough factor levels sufficient to prevent bleeding.
In Arm 2, 29 patients received individualized interval prophylaxis with rFIXFc. The factor was administered at 100 IU/kg at an initial interval of 10 days. It was subsequently individualized to maintain trough factor levels sufficient to prevent bleeding.
In Arm 3, 27 patients received episodic rFIXFc as needed to control bleeding. And in Arm 4, 12 patients who required major surgery received perioperative management with rFIXFc. Eight patients in this arm were also enrolled in other treatment arms.
Efficacy data
Overall, 93.5% of patients completed the study. Researchers assessed control of bleeding in all patients who experienced a bleeding episode while on study. In total, 90.4% of bleeding episodes were controlled by a single injection of rFIXFc.
The overall median annualized bleeding rates—including spontaneous and traumatic bleeds—were 2.95 in the weekly prophylaxis arm, 1.38 in the individualized interval prophylaxis arm, and 17.69 in the episodic treatment arm.
The perioperative management arm consisted of 12 patients undergoing 14 major surgical procedures. The treating physicians rated the hemostatic efficacy of rFIXFc as excellent or good in 100% of these surgeries.
The researchers also analyzed the pharmacokinetics of rFIXFc. The approximate terminal half-life of rFIXFc was 82 hours, compared to 34 hours for another recombinant factor IX product called BeneFIX.
This suggests that rFIXFc offers longer-lasting protection from bleeding in patients with hemophilia B, said Glenn Pierce, MD, PhD, Chief Medical Officer of Biogen Idec’s hemophilia therapeutic area.
“Currently, prophylactic treatment of hemophilia B requires intravenous injections up to 3 times a week,” he said, “which makes the prospect of a longer-lasting factor IX therapy very exciting.”
Safety data
The researchers found that rFIXFc was generally well-tolerated. None of the patients developed inhibitors, and none reported anaphylaxis.
The most common adverse events—with an incidence of 5% or greater—occurring outside of the perioperative management arm were nasopharyngitis, influenza, arthralgia, upper respiratory infection, hypertension, and headache.
One serious adverse event may have been drug-related. The patient experienced obstructive uropathy in the setting of hematuria. However, he continued to receive rFIXFc, and the event resolved with medical management.
Next steps
Based on the overall favorable outcomes of this study, Biogen Idec and Sobi are planning to submit a Biologics License Application for rFIXFc to the FDA in the first half of 2013. They also plan to submit an application to the EMA following results of a similar study in children, called Kids B-LONG.
Kids B-LONG is a phase 3, open-label study in previously treated children who are younger than 12 years of age and have been diagnosed with hemophilia B. The study is actively recruiting patients.
Researchers are also evaluating rFIXFc in the B-YOND study, which is a long-term, open-label extension study for patients who completed the B-LONG study or who will complete the Kids B-LONG study. ![]()

A novel therapy can prevent bleeding episodes in patients with hemophilia B, results of an industry-funded study suggest.
The goal of the phase 3 study, called B-LONG, was to evaluate the safety and efficacy of a long-lasting recombinant factor IX Fc fusion protein (rFIXFc, also known as eftrenonacog alfa and Alprolix).
Researchers found that a single injection of rFIXFc was sufficient to control more than 90% of bleeding episodes in males with hemophilia B.
In addition, none of the patients developed inhibitors, and the factor was generally well-tolerated.
The companies developing rFIXFc, Biogen Idec and Sobi, announced these results on September 26.
“Our companies are pioneering the application of Fc fusion technology to extend the half-life of clotting factors,” said Geoffrey McDonough, MD, Chief Executive Officer of Sobi, a biopharmaceutical company based in Stockholm, Sweden.
“Fc fusion technology utilizes a naturally occurring recycling pathway that has been successfully employed in other therapeutic areas. This approach holds promise for combining more consistent protection with fewer injections.”
Study design
To determine if rFIXFc does, in fact, provide bleeding protection with fewer injections, researchers administered the factor to 123 male patients with hemophilia B.
Patients were 12 years of age and older and had a history of at least 100 prior days during which they received 1 or more injections with any currently marketed factor IX product.
The investigators divided patients into 4 treatment arms. In Arm 1, 63 patients received weekly prophylaxis with rFIXFc. The starting dose was 50 IU/kg, and it was adjusted to maintain trough factor levels sufficient to prevent bleeding.
In Arm 2, 29 patients received individualized interval prophylaxis with rFIXFc. The factor was administered at 100 IU/kg at an initial interval of 10 days. It was subsequently individualized to maintain trough factor levels sufficient to prevent bleeding.
In Arm 3, 27 patients received episodic rFIXFc as needed to control bleeding. And in Arm 4, 12 patients who required major surgery received perioperative management with rFIXFc. Eight patients in this arm were also enrolled in other treatment arms.
Efficacy data
Overall, 93.5% of patients completed the study. Researchers assessed control of bleeding in all patients who experienced a bleeding episode while on study. In total, 90.4% of bleeding episodes were controlled by a single injection of rFIXFc.
The overall median annualized bleeding rates—including spontaneous and traumatic bleeds—were 2.95 in the weekly prophylaxis arm, 1.38 in the individualized interval prophylaxis arm, and 17.69 in the episodic treatment arm.
The perioperative management arm consisted of 12 patients undergoing 14 major surgical procedures. The treating physicians rated the hemostatic efficacy of rFIXFc as excellent or good in 100% of these surgeries.
The researchers also analyzed the pharmacokinetics of rFIXFc. The approximate terminal half-life of rFIXFc was 82 hours, compared to 34 hours for another recombinant factor IX product called BeneFIX.
This suggests that rFIXFc offers longer-lasting protection from bleeding in patients with hemophilia B, said Glenn Pierce, MD, PhD, Chief Medical Officer of Biogen Idec’s hemophilia therapeutic area.
“Currently, prophylactic treatment of hemophilia B requires intravenous injections up to 3 times a week,” he said, “which makes the prospect of a longer-lasting factor IX therapy very exciting.”
Safety data
The researchers found that rFIXFc was generally well-tolerated. None of the patients developed inhibitors, and none reported anaphylaxis.
The most common adverse events—with an incidence of 5% or greater—occurring outside of the perioperative management arm were nasopharyngitis, influenza, arthralgia, upper respiratory infection, hypertension, and headache.
One serious adverse event may have been drug-related. The patient experienced obstructive uropathy in the setting of hematuria. However, he continued to receive rFIXFc, and the event resolved with medical management.
Next steps
Based on the overall favorable outcomes of this study, Biogen Idec and Sobi are planning to submit a Biologics License Application for rFIXFc to the FDA in the first half of 2013. They also plan to submit an application to the EMA following results of a similar study in children, called Kids B-LONG.
Kids B-LONG is a phase 3, open-label study in previously treated children who are younger than 12 years of age and have been diagnosed with hemophilia B. The study is actively recruiting patients.
Researchers are also evaluating rFIXFc in the B-YOND study, which is a long-term, open-label extension study for patients who completed the B-LONG study or who will complete the Kids B-LONG study. ![]()
Hospitalist-Led Teams Vital to Improved ED Care
Hospitalist-led teams in the ED help reduce diversions, improve patient flow, and provide more timely care to boarded patients, according to a study in the Journal of Hospital Medicine (JHM).
The single-center study, "Hospitalist-Led Medicine Emergency Department Team: Associations with Throughput, Timeliness of Patient Care, and Satisfaction," found a reduction in diversions due to medicine bed capacity of 27% (4.5% to 3%, P=<0.01). Boarded patients were rounded a mean of 2 hours and 9 minutes earlier with hospitalist-led teams; length of stay (LOS) in the ED, LOS in the hospital, and 48-hour returns were unchanged. The study, which took place at 477-bed Denver Health Medical Center (DHMC), assigned a hospitalist and an allied health provider to the ED during dayshifts. At night, ED coverage was rolled into the existing hospitalist duties.
Lead author Smitha R. Chadaga, MD, who works in DHMC's Department of Medicine, believes the study could spur more HM groups to consider dedicating a staffer to the ED. The team in Denver was created to care for medicine patients in the ED awaiting inpatient beds, and to work with nursing supervisors to improve bed management.
"There are numerous places that hospitalists can impact hospital flow, whether it's helping with bed management, providing consultative services to the ED, or caring for boarded patients," Dr. Chadaga says. "Knowing the ins and outs of inpatient medicine really lends itself well to some areas that hospitalists might not have thought about before."
Dr. Chadaga says the research is broadly applicable because HM groups can implement its different features. For example, adding a consultative phone service can help ED physicians determine whether a patient needs to be admitted and could improve patient flow.
Hospitalist-led teams in the ED help reduce diversions, improve patient flow, and provide more timely care to boarded patients, according to a study in the Journal of Hospital Medicine (JHM).
The single-center study, "Hospitalist-Led Medicine Emergency Department Team: Associations with Throughput, Timeliness of Patient Care, and Satisfaction," found a reduction in diversions due to medicine bed capacity of 27% (4.5% to 3%, P=<0.01). Boarded patients were rounded a mean of 2 hours and 9 minutes earlier with hospitalist-led teams; length of stay (LOS) in the ED, LOS in the hospital, and 48-hour returns were unchanged. The study, which took place at 477-bed Denver Health Medical Center (DHMC), assigned a hospitalist and an allied health provider to the ED during dayshifts. At night, ED coverage was rolled into the existing hospitalist duties.
Lead author Smitha R. Chadaga, MD, who works in DHMC's Department of Medicine, believes the study could spur more HM groups to consider dedicating a staffer to the ED. The team in Denver was created to care for medicine patients in the ED awaiting inpatient beds, and to work with nursing supervisors to improve bed management.
"There are numerous places that hospitalists can impact hospital flow, whether it's helping with bed management, providing consultative services to the ED, or caring for boarded patients," Dr. Chadaga says. "Knowing the ins and outs of inpatient medicine really lends itself well to some areas that hospitalists might not have thought about before."
Dr. Chadaga says the research is broadly applicable because HM groups can implement its different features. For example, adding a consultative phone service can help ED physicians determine whether a patient needs to be admitted and could improve patient flow.
Hospitalist-led teams in the ED help reduce diversions, improve patient flow, and provide more timely care to boarded patients, according to a study in the Journal of Hospital Medicine (JHM).
The single-center study, "Hospitalist-Led Medicine Emergency Department Team: Associations with Throughput, Timeliness of Patient Care, and Satisfaction," found a reduction in diversions due to medicine bed capacity of 27% (4.5% to 3%, P=<0.01). Boarded patients were rounded a mean of 2 hours and 9 minutes earlier with hospitalist-led teams; length of stay (LOS) in the ED, LOS in the hospital, and 48-hour returns were unchanged. The study, which took place at 477-bed Denver Health Medical Center (DHMC), assigned a hospitalist and an allied health provider to the ED during dayshifts. At night, ED coverage was rolled into the existing hospitalist duties.
Lead author Smitha R. Chadaga, MD, who works in DHMC's Department of Medicine, believes the study could spur more HM groups to consider dedicating a staffer to the ED. The team in Denver was created to care for medicine patients in the ED awaiting inpatient beds, and to work with nursing supervisors to improve bed management.
"There are numerous places that hospitalists can impact hospital flow, whether it's helping with bed management, providing consultative services to the ED, or caring for boarded patients," Dr. Chadaga says. "Knowing the ins and outs of inpatient medicine really lends itself well to some areas that hospitalists might not have thought about before."
Dr. Chadaga says the research is broadly applicable because HM groups can implement its different features. For example, adding a consultative phone service can help ED physicians determine whether a patient needs to be admitted and could improve patient flow.
High-Tech Connections Give Hospitalists Broad Access to Medical Records
U.S. News and World Report recently named its 156 "most connected" U.S. hospitals, singled out for their combination of high quality and early adoption of information technology. But what does "most connected" really mean for hospitalists working on the wards?
"I've been in hospital medicine for a few years, and I can still remember the old days of going down to the medical records room and pulling a chart off the shelf," says hospitalist Kristian Feterik, MD, clinical assistant professor of medicine at the University of Pittsburgh Medical Center (UPMC) in Pittsburgh, which is on the U.S. News list. "The transition from paper to electronic medical records has just been tremendous. We're almost 100% paperless here."
Going paperless means hospitalists and other clinicians can access medical records anywhere in the hospital or at home using multiple interfaces, including their own tablet, Dr. Feterik says. "We're now piloting an application for smartphones that would notify us of pending tests. Plus, the applications support access to radiology studies, for example, without having to re-enter patient identification numbers once you're in the patient's record."
UPMC clinicians have access to a number of applications, some still in beta testing.
But technology also has its downside. "We need to be mindful about how we write our orders, because it's so easy to order things electronically," Dr. Feterik adds. "I stress to the residents: You need to take a moment and think, 'Why are you ordering this test?'"
U.S. News and World Report recently named its 156 "most connected" U.S. hospitals, singled out for their combination of high quality and early adoption of information technology. But what does "most connected" really mean for hospitalists working on the wards?
"I've been in hospital medicine for a few years, and I can still remember the old days of going down to the medical records room and pulling a chart off the shelf," says hospitalist Kristian Feterik, MD, clinical assistant professor of medicine at the University of Pittsburgh Medical Center (UPMC) in Pittsburgh, which is on the U.S. News list. "The transition from paper to electronic medical records has just been tremendous. We're almost 100% paperless here."
Going paperless means hospitalists and other clinicians can access medical records anywhere in the hospital or at home using multiple interfaces, including their own tablet, Dr. Feterik says. "We're now piloting an application for smartphones that would notify us of pending tests. Plus, the applications support access to radiology studies, for example, without having to re-enter patient identification numbers once you're in the patient's record."
UPMC clinicians have access to a number of applications, some still in beta testing.
But technology also has its downside. "We need to be mindful about how we write our orders, because it's so easy to order things electronically," Dr. Feterik adds. "I stress to the residents: You need to take a moment and think, 'Why are you ordering this test?'"
U.S. News and World Report recently named its 156 "most connected" U.S. hospitals, singled out for their combination of high quality and early adoption of information technology. But what does "most connected" really mean for hospitalists working on the wards?
"I've been in hospital medicine for a few years, and I can still remember the old days of going down to the medical records room and pulling a chart off the shelf," says hospitalist Kristian Feterik, MD, clinical assistant professor of medicine at the University of Pittsburgh Medical Center (UPMC) in Pittsburgh, which is on the U.S. News list. "The transition from paper to electronic medical records has just been tremendous. We're almost 100% paperless here."
Going paperless means hospitalists and other clinicians can access medical records anywhere in the hospital or at home using multiple interfaces, including their own tablet, Dr. Feterik says. "We're now piloting an application for smartphones that would notify us of pending tests. Plus, the applications support access to radiology studies, for example, without having to re-enter patient identification numbers once you're in the patient's record."
UPMC clinicians have access to a number of applications, some still in beta testing.
But technology also has its downside. "We need to be mindful about how we write our orders, because it's so easy to order things electronically," Dr. Feterik adds. "I stress to the residents: You need to take a moment and think, 'Why are you ordering this test?'"
Anti-Inflammatory Diet Anyone?
I’ve known Mr. G. for about a year now. When he first presented to me he’d had about 6 months of hand swelling and pain, and by the time I met him he had fusiform swelling of all his PIPs. There was no doubt that he had rheumatoid arthritis.
But we had a problem, sort of. Mr. G. had a history of drug and alcohol abuse but had turned his life around. Now that he was the model of an upright citizen, he had no interest in becoming dependent on more Western medicine.
Before he saw me, he had consulted a naturopath, who recommended that he follow an anti-inflammatory diet. After my admonitions about joint damage and internal organ involvement, I ultimately agreed to let him try the diet, but only if he agreed to regular follow-up with me.
I saw him for follow-up 6 weeks later – he had followed the diet to the letter, and, contrary to my expectations, his joints were about 50% better. In broad strokes, the anti-inflammatory diet is based on the premise that the intake of fruit and vegetables as well as whole wheat is inversely associated with the risk of inflammation, for which there is support in the literature (Int. J. Vitam. Nutr. Res. 2008;78:293-8). Some variations on the diet include using organic sources as much as possible, staying away from sugar and artificial sweeteners, and avoiding tomatoes, potatoes, and eggplant.
Now of course we as doctors are not satisfied with 50% better. Our patients want to be completely pain free, and we twist ourselves into knots trying to help them achieve that. But Mr. G. was content with the state his hands were in. He felt healthy and his discomfort was manageable. He said he could live with this state of affairs.
This got me thinking about naturopathic medicine. What is it, and how does it differ from our usual advice?
In the process of answering those questions, I got to meet with Celeste Ruland, N.D., a naturopathic doctor and personal trainer. Here’s what I learned from our conversation and my own research:
Naturopathic medicine is a 4-year graduate program. Just like students of allopathic medicine, students of naturopathic medicine study anatomy, physiology, biochemistry, microbiology, pharmacology, and other basic sciences. Students of naturopathic medicine have an organ system–based curriculum. In addition, they study Chinese medicine, herbal medicine, manipulation, and homeopathy. To practice, naturopaths have to meet licensing requirements.
The philosophy, I gathered from speaking to Dr. Ruland, is to treat illness and prevent it, using the "mind/body balance."
"Where are they out of balance, and what is the best way to balance them?" she asked. "The key is to empower the patient to stay well." While prescribing remedies such as homeopathy is sometimes part of the solution, the larger part involves altering the patient’s diet, lifestyle, and environment to restore the mind/body equilibrium.
This may sound like excessive tree-huggery, but I do think there is some value to this. After all, how often do we preach lifestyle modification? It is a very mainstream concept. Recently, Dr. Dean Ornish published a piece in the New York Times about how to eat healthily. How much evidence is there that viscosupplementation works? How much evidence is there that acupuncture works? If something does not harm the patient and could be potentially helpful, I certainly have no objections. I can’t embrace the concepts of naturopathic medicine to the exclusion of Western medicine, of course, but I see a place for it in conjunction with allopathic medicine.
In addition, I kind of like the idea that there are health care providers who don’t have the same constraints that those of us who take insurance do. These providers can spend way more time counseling patients on good sleep hygiene and avoiding highly processed foods, on exercise and stress-reduction – conversations that we would love to have with our patients but don’t have time for.
Epilogue: About 8 months after his diagnosis, Mr. G developed serositis. We managed to treat it – grudgingly on his part – with steroids, but our agreement, after all, was that when the time came he would trust me with my Western medicine. He is now on a biologic DMARD and is doing very well, much better than the initial 50% improvement that had him so satisfied.
Dr. Chan practices rheumatology in Pawtucket, R.I.
I’ve known Mr. G. for about a year now. When he first presented to me he’d had about 6 months of hand swelling and pain, and by the time I met him he had fusiform swelling of all his PIPs. There was no doubt that he had rheumatoid arthritis.
But we had a problem, sort of. Mr. G. had a history of drug and alcohol abuse but had turned his life around. Now that he was the model of an upright citizen, he had no interest in becoming dependent on more Western medicine.
Before he saw me, he had consulted a naturopath, who recommended that he follow an anti-inflammatory diet. After my admonitions about joint damage and internal organ involvement, I ultimately agreed to let him try the diet, but only if he agreed to regular follow-up with me.
I saw him for follow-up 6 weeks later – he had followed the diet to the letter, and, contrary to my expectations, his joints were about 50% better. In broad strokes, the anti-inflammatory diet is based on the premise that the intake of fruit and vegetables as well as whole wheat is inversely associated with the risk of inflammation, for which there is support in the literature (Int. J. Vitam. Nutr. Res. 2008;78:293-8). Some variations on the diet include using organic sources as much as possible, staying away from sugar and artificial sweeteners, and avoiding tomatoes, potatoes, and eggplant.
Now of course we as doctors are not satisfied with 50% better. Our patients want to be completely pain free, and we twist ourselves into knots trying to help them achieve that. But Mr. G. was content with the state his hands were in. He felt healthy and his discomfort was manageable. He said he could live with this state of affairs.
This got me thinking about naturopathic medicine. What is it, and how does it differ from our usual advice?
In the process of answering those questions, I got to meet with Celeste Ruland, N.D., a naturopathic doctor and personal trainer. Here’s what I learned from our conversation and my own research:
Naturopathic medicine is a 4-year graduate program. Just like students of allopathic medicine, students of naturopathic medicine study anatomy, physiology, biochemistry, microbiology, pharmacology, and other basic sciences. Students of naturopathic medicine have an organ system–based curriculum. In addition, they study Chinese medicine, herbal medicine, manipulation, and homeopathy. To practice, naturopaths have to meet licensing requirements.
The philosophy, I gathered from speaking to Dr. Ruland, is to treat illness and prevent it, using the "mind/body balance."
"Where are they out of balance, and what is the best way to balance them?" she asked. "The key is to empower the patient to stay well." While prescribing remedies such as homeopathy is sometimes part of the solution, the larger part involves altering the patient’s diet, lifestyle, and environment to restore the mind/body equilibrium.
This may sound like excessive tree-huggery, but I do think there is some value to this. After all, how often do we preach lifestyle modification? It is a very mainstream concept. Recently, Dr. Dean Ornish published a piece in the New York Times about how to eat healthily. How much evidence is there that viscosupplementation works? How much evidence is there that acupuncture works? If something does not harm the patient and could be potentially helpful, I certainly have no objections. I can’t embrace the concepts of naturopathic medicine to the exclusion of Western medicine, of course, but I see a place for it in conjunction with allopathic medicine.
In addition, I kind of like the idea that there are health care providers who don’t have the same constraints that those of us who take insurance do. These providers can spend way more time counseling patients on good sleep hygiene and avoiding highly processed foods, on exercise and stress-reduction – conversations that we would love to have with our patients but don’t have time for.
Epilogue: About 8 months after his diagnosis, Mr. G developed serositis. We managed to treat it – grudgingly on his part – with steroids, but our agreement, after all, was that when the time came he would trust me with my Western medicine. He is now on a biologic DMARD and is doing very well, much better than the initial 50% improvement that had him so satisfied.
Dr. Chan practices rheumatology in Pawtucket, R.I.
I’ve known Mr. G. for about a year now. When he first presented to me he’d had about 6 months of hand swelling and pain, and by the time I met him he had fusiform swelling of all his PIPs. There was no doubt that he had rheumatoid arthritis.
But we had a problem, sort of. Mr. G. had a history of drug and alcohol abuse but had turned his life around. Now that he was the model of an upright citizen, he had no interest in becoming dependent on more Western medicine.
Before he saw me, he had consulted a naturopath, who recommended that he follow an anti-inflammatory diet. After my admonitions about joint damage and internal organ involvement, I ultimately agreed to let him try the diet, but only if he agreed to regular follow-up with me.
I saw him for follow-up 6 weeks later – he had followed the diet to the letter, and, contrary to my expectations, his joints were about 50% better. In broad strokes, the anti-inflammatory diet is based on the premise that the intake of fruit and vegetables as well as whole wheat is inversely associated with the risk of inflammation, for which there is support in the literature (Int. J. Vitam. Nutr. Res. 2008;78:293-8). Some variations on the diet include using organic sources as much as possible, staying away from sugar and artificial sweeteners, and avoiding tomatoes, potatoes, and eggplant.
Now of course we as doctors are not satisfied with 50% better. Our patients want to be completely pain free, and we twist ourselves into knots trying to help them achieve that. But Mr. G. was content with the state his hands were in. He felt healthy and his discomfort was manageable. He said he could live with this state of affairs.
This got me thinking about naturopathic medicine. What is it, and how does it differ from our usual advice?
In the process of answering those questions, I got to meet with Celeste Ruland, N.D., a naturopathic doctor and personal trainer. Here’s what I learned from our conversation and my own research:
Naturopathic medicine is a 4-year graduate program. Just like students of allopathic medicine, students of naturopathic medicine study anatomy, physiology, biochemistry, microbiology, pharmacology, and other basic sciences. Students of naturopathic medicine have an organ system–based curriculum. In addition, they study Chinese medicine, herbal medicine, manipulation, and homeopathy. To practice, naturopaths have to meet licensing requirements.
The philosophy, I gathered from speaking to Dr. Ruland, is to treat illness and prevent it, using the "mind/body balance."
"Where are they out of balance, and what is the best way to balance them?" she asked. "The key is to empower the patient to stay well." While prescribing remedies such as homeopathy is sometimes part of the solution, the larger part involves altering the patient’s diet, lifestyle, and environment to restore the mind/body equilibrium.
This may sound like excessive tree-huggery, but I do think there is some value to this. After all, how often do we preach lifestyle modification? It is a very mainstream concept. Recently, Dr. Dean Ornish published a piece in the New York Times about how to eat healthily. How much evidence is there that viscosupplementation works? How much evidence is there that acupuncture works? If something does not harm the patient and could be potentially helpful, I certainly have no objections. I can’t embrace the concepts of naturopathic medicine to the exclusion of Western medicine, of course, but I see a place for it in conjunction with allopathic medicine.
In addition, I kind of like the idea that there are health care providers who don’t have the same constraints that those of us who take insurance do. These providers can spend way more time counseling patients on good sleep hygiene and avoiding highly processed foods, on exercise and stress-reduction – conversations that we would love to have with our patients but don’t have time for.
Epilogue: About 8 months after his diagnosis, Mr. G developed serositis. We managed to treat it – grudgingly on his part – with steroids, but our agreement, after all, was that when the time came he would trust me with my Western medicine. He is now on a biologic DMARD and is doing very well, much better than the initial 50% improvement that had him so satisfied.
Dr. Chan practices rheumatology in Pawtucket, R.I.
On a Scale of 1-10
When my wife was hospitalized for back surgery, I learned more about the new information revolution in health care that provides formerly unattainable precision. This investigative report includes suggestions for how we, in the outpatient world, can implement these advances.
At a 3-hour preop marathon, my wife was interviewed by several people, including two nurses. The neurosurgery nurse practitioner asked my wife how much pain she was experiencing.
"It depends on my position," my wife answered. "If I’m sitting, it’s not that painful."
"But when it does hurt?"
"Well, it isn’t that bad."
"On a scale of 1-10?"
"Four."
Later on the anesthesia nurse asked her many questions. One was, "How much pain are you in, on a scale of 1-10?"
"It depends," began my wife, a slow learner.
"On a scale of 1-10?"
"Four."
Things went smoothly after that until the final question, "Do you feel safe at home?"
I had kept my mouth shut until then, but at that point, I got clarification that she was, indeed, asking whether my wife feared being abused after discharge. Not an unfair question, though I did wonder whether someone who really wanted to know would ask the question while a potential abuser looked on.
On the morning of surgery a clerk asked my wife again how much pain she was in, on a scale of one 1-10. In the recovery room they asked her again, several times.
Once my wife reached the ward, each nurse asked, once per shift, how much pain she had, on a scale of 1-10. At first, she tried to explain through her opiate stupor, what she was feeling.
"On a scale of 1-10," came the polite but insistent request. I doubt whether my wife remembered 5 minutes later what number she had given, which had in any case been duly noted and entered into the computer.
Ditto the aides. Ditto the physical therapist. Ditto the occupational therapist. At each visit.
Back home the visiting nurse’s aid called to visit and set up services. Before the nurse came, I said to my wife, "Let’s practice."
"Practice what?"
"Saying ‘four’ "
"Why four?"
"Because she’s going to ask you how much pain you’re having, on a scale of 1-10."
"But it isn’t four."
"Okay, say three."
The nurse came. Her first question was, "How much pain are you having, on a scale of 1-10?"
My wife tried again to give a nuanced answer. But eventually she did say, "Four."
The nurse asked my wife whether she was depressed. "Starting last month, they’re making us ask that."
My wife laughed. "I feel wonderful!" she said. "I don’t look depressed, do I?"
"I need a ‘yes’ or ‘no,’ " said the nurse.
"No," said my wife.
"Thank you," said the nurse, wearily. "I have a 30-page form to fill out for every case."
All of the "yes" and "no" replies, and all the numbers from 1-10, will be recorded and filed in the great information repository in the sky.
We can easily apply this method to our own practices.
Consider:
"Mr. Smith, how is your eczema?"
"Doing better, Doc, thanks."
"Great. How much does it itch, on a scale of 1-10?"
"Well, it’s worse at night."
"On a scale of 1-10, please."
"At night, or when I’m working?"
"On a scale of 1-10."
"Two."
"Excellent. How regular have you been with the application?"
"Pretty regular."
"On a scale of 1-10."
"What?"
"How happy are you with the service you received in this office?"
"Well, pretty happy, I guess."
"On a scale of 1-5, with five being ‘Very happy.’ "
"I suppose three."
"How likely are you to use our services again, or to refer a friend, on a scale of 1-6, with six being, ‘You bet!’ and one being, ‘No way, Jose!’?"
"Look, I think that’s enough."
"Just a few more questions, Mr. Smith. Mr. Smith, why are you staring at me like that? Mr. Smith, please get your hands off my neck. What? How much ... do I want ... you to stop ... throttling me? A lot! What? On a scale of 1-10? 10! 10!"
Dr. Rockoff practices dermatology in Brookline, Mass. To respond to this column, e-mail him at our editorial offices at [email protected].
When my wife was hospitalized for back surgery, I learned more about the new information revolution in health care that provides formerly unattainable precision. This investigative report includes suggestions for how we, in the outpatient world, can implement these advances.
At a 3-hour preop marathon, my wife was interviewed by several people, including two nurses. The neurosurgery nurse practitioner asked my wife how much pain she was experiencing.
"It depends on my position," my wife answered. "If I’m sitting, it’s not that painful."
"But when it does hurt?"
"Well, it isn’t that bad."
"On a scale of 1-10?"
"Four."
Later on the anesthesia nurse asked her many questions. One was, "How much pain are you in, on a scale of 1-10?"
"It depends," began my wife, a slow learner.
"On a scale of 1-10?"
"Four."
Things went smoothly after that until the final question, "Do you feel safe at home?"
I had kept my mouth shut until then, but at that point, I got clarification that she was, indeed, asking whether my wife feared being abused after discharge. Not an unfair question, though I did wonder whether someone who really wanted to know would ask the question while a potential abuser looked on.
On the morning of surgery a clerk asked my wife again how much pain she was in, on a scale of one 1-10. In the recovery room they asked her again, several times.
Once my wife reached the ward, each nurse asked, once per shift, how much pain she had, on a scale of 1-10. At first, she tried to explain through her opiate stupor, what she was feeling.
"On a scale of 1-10," came the polite but insistent request. I doubt whether my wife remembered 5 minutes later what number she had given, which had in any case been duly noted and entered into the computer.
Ditto the aides. Ditto the physical therapist. Ditto the occupational therapist. At each visit.
Back home the visiting nurse’s aid called to visit and set up services. Before the nurse came, I said to my wife, "Let’s practice."
"Practice what?"
"Saying ‘four’ "
"Why four?"
"Because she’s going to ask you how much pain you’re having, on a scale of 1-10."
"But it isn’t four."
"Okay, say three."
The nurse came. Her first question was, "How much pain are you having, on a scale of 1-10?"
My wife tried again to give a nuanced answer. But eventually she did say, "Four."
The nurse asked my wife whether she was depressed. "Starting last month, they’re making us ask that."
My wife laughed. "I feel wonderful!" she said. "I don’t look depressed, do I?"
"I need a ‘yes’ or ‘no,’ " said the nurse.
"No," said my wife.
"Thank you," said the nurse, wearily. "I have a 30-page form to fill out for every case."
All of the "yes" and "no" replies, and all the numbers from 1-10, will be recorded and filed in the great information repository in the sky.
We can easily apply this method to our own practices.
Consider:
"Mr. Smith, how is your eczema?"
"Doing better, Doc, thanks."
"Great. How much does it itch, on a scale of 1-10?"
"Well, it’s worse at night."
"On a scale of 1-10, please."
"At night, or when I’m working?"
"On a scale of 1-10."
"Two."
"Excellent. How regular have you been with the application?"
"Pretty regular."
"On a scale of 1-10."
"What?"
"How happy are you with the service you received in this office?"
"Well, pretty happy, I guess."
"On a scale of 1-5, with five being ‘Very happy.’ "
"I suppose three."
"How likely are you to use our services again, or to refer a friend, on a scale of 1-6, with six being, ‘You bet!’ and one being, ‘No way, Jose!’?"
"Look, I think that’s enough."
"Just a few more questions, Mr. Smith. Mr. Smith, why are you staring at me like that? Mr. Smith, please get your hands off my neck. What? How much ... do I want ... you to stop ... throttling me? A lot! What? On a scale of 1-10? 10! 10!"
Dr. Rockoff practices dermatology in Brookline, Mass. To respond to this column, e-mail him at our editorial offices at [email protected].
When my wife was hospitalized for back surgery, I learned more about the new information revolution in health care that provides formerly unattainable precision. This investigative report includes suggestions for how we, in the outpatient world, can implement these advances.
At a 3-hour preop marathon, my wife was interviewed by several people, including two nurses. The neurosurgery nurse practitioner asked my wife how much pain she was experiencing.
"It depends on my position," my wife answered. "If I’m sitting, it’s not that painful."
"But when it does hurt?"
"Well, it isn’t that bad."
"On a scale of 1-10?"
"Four."
Later on the anesthesia nurse asked her many questions. One was, "How much pain are you in, on a scale of 1-10?"
"It depends," began my wife, a slow learner.
"On a scale of 1-10?"
"Four."
Things went smoothly after that until the final question, "Do you feel safe at home?"
I had kept my mouth shut until then, but at that point, I got clarification that she was, indeed, asking whether my wife feared being abused after discharge. Not an unfair question, though I did wonder whether someone who really wanted to know would ask the question while a potential abuser looked on.
On the morning of surgery a clerk asked my wife again how much pain she was in, on a scale of one 1-10. In the recovery room they asked her again, several times.
Once my wife reached the ward, each nurse asked, once per shift, how much pain she had, on a scale of 1-10. At first, she tried to explain through her opiate stupor, what she was feeling.
"On a scale of 1-10," came the polite but insistent request. I doubt whether my wife remembered 5 minutes later what number she had given, which had in any case been duly noted and entered into the computer.
Ditto the aides. Ditto the physical therapist. Ditto the occupational therapist. At each visit.
Back home the visiting nurse’s aid called to visit and set up services. Before the nurse came, I said to my wife, "Let’s practice."
"Practice what?"
"Saying ‘four’ "
"Why four?"
"Because she’s going to ask you how much pain you’re having, on a scale of 1-10."
"But it isn’t four."
"Okay, say three."
The nurse came. Her first question was, "How much pain are you having, on a scale of 1-10?"
My wife tried again to give a nuanced answer. But eventually she did say, "Four."
The nurse asked my wife whether she was depressed. "Starting last month, they’re making us ask that."
My wife laughed. "I feel wonderful!" she said. "I don’t look depressed, do I?"
"I need a ‘yes’ or ‘no,’ " said the nurse.
"No," said my wife.
"Thank you," said the nurse, wearily. "I have a 30-page form to fill out for every case."
All of the "yes" and "no" replies, and all the numbers from 1-10, will be recorded and filed in the great information repository in the sky.
We can easily apply this method to our own practices.
Consider:
"Mr. Smith, how is your eczema?"
"Doing better, Doc, thanks."
"Great. How much does it itch, on a scale of 1-10?"
"Well, it’s worse at night."
"On a scale of 1-10, please."
"At night, or when I’m working?"
"On a scale of 1-10."
"Two."
"Excellent. How regular have you been with the application?"
"Pretty regular."
"On a scale of 1-10."
"What?"
"How happy are you with the service you received in this office?"
"Well, pretty happy, I guess."
"On a scale of 1-5, with five being ‘Very happy.’ "
"I suppose three."
"How likely are you to use our services again, or to refer a friend, on a scale of 1-6, with six being, ‘You bet!’ and one being, ‘No way, Jose!’?"
"Look, I think that’s enough."
"Just a few more questions, Mr. Smith. Mr. Smith, why are you staring at me like that? Mr. Smith, please get your hands off my neck. What? How much ... do I want ... you to stop ... throttling me? A lot! What? On a scale of 1-10? 10! 10!"
Dr. Rockoff practices dermatology in Brookline, Mass. To respond to this column, e-mail him at our editorial offices at [email protected].
Dr. Minuchin and the Ashtray: A History Lesson
A pod of family psychiatrists is sitting around and chatting about the state of family psychiatry. They are preparing for a plenary at the Group for the Advancement of Psychiatry with the goal of showing how far family psychiatry has come since the first psychiatrists embraced the paradigm of systemic thinking. They also debate why family psychiatry is ignored in current practice, especially since the evidence shows that family treatment dramatically improves recovery rates for many illnesses.
When family therapy had its first wave of popularity, the charismatic leaders were out front wowing the crowds. Dr. Sal Minuchin’s sessions were heavily focused on structure and boundary making, and involved much chair rearranging and pulling family members, especially children, out from between the couple dyad and into their own space and chairs in the room. One of his most famous tapes involved putting an ashtray between the chairs of two family members to literally increase the distance between them!
Jay Haley, Ph.D., delivered strategic barbed arrows that pierced the hearts of the family members. Virginia Satir demonstrated the theater of families, sculpting organic shapes that pulsed with the gestalt of the family. There was much smoking of cigarettes during the sessions, by both the family psychiatrists and the family members. Psychiatry was exciting. The possibilities for change were endless. It was the 1960s.
Unfortunately, in those early days, family therapy was oversold as the sole treatment for schizophrenia and other mental illnesses. As a result, families have felt blamed by the negative attention and are still hesitant to engage in traditional family therapy. Nevertheless, quiet pioneers, like Carol M. Anderson, Ph.D., continue to research and practice a measured educational and collaborative approach aimed at involving families in mental health treatment. Indeed, current American Psychiatric Association guidelines for many psychiatric illnesses recommend that families be brought into the treatment process.
Family research has become much more sophisticated, with Dr. Minuchin’s early research on asthma and "psychosomatic families" being refined by teams led by Betsy Wood in New York, and Dr. Fred Wamboldt and Dr. Marianne Z. Wamboldt in Denver. Family research covers a broad territory, from studies on the impact of care giving on the caregiver’s immune function, to the role of expressed emotion in the outcome of illnesses – medical and psychiatric – to the efficacy of family treatments.
However, the Big Question still remains: Which model is the best? Structural? Strategic? Experiential?
While the arguments among devotees continue, studious researchers are quietly extracting the common factors found in the original family therapy models. These common factors are defined as the variables associated with positive clinical outcomes and are shared by several or all approaches. Andrew Christensen, Ph.D., suggests five principles that evidence-based couple interventions share: a systemic rather than an individual orientation of problems; modification of emotion-driven dysfunctional behaviors by teaching partners constructive ways to deal with differences, problems, and emotions; making both partners aware of avoided, emotion-based, private behaviors of each other, and making these internal experiences accessible to each other; enhancement of constructive communication in speaking and listening; and emphasis on strengths and positive behaviors (Enhancing Couples, Cambridge, Mass.: Hogrefe Publishing, 2009).
For couples and family therapies, common factors are conceptualizing the problems in relational terms, disrupting relational patterns, expanding treatment to include family members of the identified patient and an expanded therapeutic alliance (Common Factors in Couple and Family Therapy: The Overlooked Foundation for Effective Practice, New York: Guilford Press, 2009). Relational patterns have cognitive, behavioral, and affective domains, all of which can be targets of intervention. The therapeutic alliance is with the relationship and the family, rather than with the individual family members.
Patients, families, and psychiatrists all demand treatments that have been shown to work well. Family psychiatry has moved from theatrical showmanship to evidence-based treatments. Within a broad range of family interventions are different levels of family involvement. Family inclusion is the easiest intervention – simply involving the family members as historians, supporters, and allies in treatment.
Second, family psychoeducation has amassed a substantial evidence base showing its efficacy in the treatment of schizophrenia, bipolar disorder, and many medical illnesses, such as diabetes.
Last, but certainly not least, are the family systemic therapies, which in a meta-analysis of family systems therapies, were defined as "any couple, family, group, multifamily group, or individual focused therapeutic intervention that refers to either one of the following systems-oriented authors (Tom Andersen, Dr. Ivan Böszörményi-Nagy, Steve de Shazer, Jay Haley, Ph.D., Dr. Minuchin, Ms. Satir, Dr. Mara Selvini Palazzoli, Dr. Helm Stierlin, Paul Watzlawick, Ph.D., Michael White, Gerald H. Zuk, Ph.D.) or specified the intervention by use of at least one of the following terms: systemic, structural, strategic, triadic, Milan, functional, solution focused, narrative, resource/strength oriented, McMaster model" (Fam. Process 2010;49:457-85).
Family systems therapy has come a long way from the early days. We are very clear that for serious mental illness, family therapy alone is not enough, but neither are medications. Combination treatment produces symptom reduction AND good quality of life.
However, most psychotherapies – of the individual and family variety – are delivered by non-psychiatrists. Psychiatry is in danger of losing itself, as primary care physicians prescribe medications and refer patients to psychotherapists who are often co-located in their offices. Psychiatrists, however, are still the only professionals who have the potential to see the whole person and oversee the entire treatment: medications, individual, and family interventions.
It is to our advantage to be knowledgeable about all psychotherapeutic interventions AND to use them. We must make family therapy more visible and easier to teach in residencies. Psychiatrists have been reluctant to identify themselves as family psychiatrists because our enthusiastic charismatic leaders took the promise of family therapy too far. We hope that the solid family research now available will encourage all psychiatrists to learn and implement family interventions.
Dr. Minuchin and the ashtray, however, remain potent symbols of how creativity and genius created a new paradigm in psychiatry.
Dr. Heru is with the department of psychiatry at the University of Colorado, Denver. She has been a member of the Association of Family Psychiatrists since 2002 and currently serves as the organization’s treasurer. In addition, she is the coauthor of two books on working with families and is the author of numerous articles on this topic.
A pod of family psychiatrists is sitting around and chatting about the state of family psychiatry. They are preparing for a plenary at the Group for the Advancement of Psychiatry with the goal of showing how far family psychiatry has come since the first psychiatrists embraced the paradigm of systemic thinking. They also debate why family psychiatry is ignored in current practice, especially since the evidence shows that family treatment dramatically improves recovery rates for many illnesses.
When family therapy had its first wave of popularity, the charismatic leaders were out front wowing the crowds. Dr. Sal Minuchin’s sessions were heavily focused on structure and boundary making, and involved much chair rearranging and pulling family members, especially children, out from between the couple dyad and into their own space and chairs in the room. One of his most famous tapes involved putting an ashtray between the chairs of two family members to literally increase the distance between them!
Jay Haley, Ph.D., delivered strategic barbed arrows that pierced the hearts of the family members. Virginia Satir demonstrated the theater of families, sculpting organic shapes that pulsed with the gestalt of the family. There was much smoking of cigarettes during the sessions, by both the family psychiatrists and the family members. Psychiatry was exciting. The possibilities for change were endless. It was the 1960s.
Unfortunately, in those early days, family therapy was oversold as the sole treatment for schizophrenia and other mental illnesses. As a result, families have felt blamed by the negative attention and are still hesitant to engage in traditional family therapy. Nevertheless, quiet pioneers, like Carol M. Anderson, Ph.D., continue to research and practice a measured educational and collaborative approach aimed at involving families in mental health treatment. Indeed, current American Psychiatric Association guidelines for many psychiatric illnesses recommend that families be brought into the treatment process.
Family research has become much more sophisticated, with Dr. Minuchin’s early research on asthma and "psychosomatic families" being refined by teams led by Betsy Wood in New York, and Dr. Fred Wamboldt and Dr. Marianne Z. Wamboldt in Denver. Family research covers a broad territory, from studies on the impact of care giving on the caregiver’s immune function, to the role of expressed emotion in the outcome of illnesses – medical and psychiatric – to the efficacy of family treatments.
However, the Big Question still remains: Which model is the best? Structural? Strategic? Experiential?
While the arguments among devotees continue, studious researchers are quietly extracting the common factors found in the original family therapy models. These common factors are defined as the variables associated with positive clinical outcomes and are shared by several or all approaches. Andrew Christensen, Ph.D., suggests five principles that evidence-based couple interventions share: a systemic rather than an individual orientation of problems; modification of emotion-driven dysfunctional behaviors by teaching partners constructive ways to deal with differences, problems, and emotions; making both partners aware of avoided, emotion-based, private behaviors of each other, and making these internal experiences accessible to each other; enhancement of constructive communication in speaking and listening; and emphasis on strengths and positive behaviors (Enhancing Couples, Cambridge, Mass.: Hogrefe Publishing, 2009).
For couples and family therapies, common factors are conceptualizing the problems in relational terms, disrupting relational patterns, expanding treatment to include family members of the identified patient and an expanded therapeutic alliance (Common Factors in Couple and Family Therapy: The Overlooked Foundation for Effective Practice, New York: Guilford Press, 2009). Relational patterns have cognitive, behavioral, and affective domains, all of which can be targets of intervention. The therapeutic alliance is with the relationship and the family, rather than with the individual family members.
Patients, families, and psychiatrists all demand treatments that have been shown to work well. Family psychiatry has moved from theatrical showmanship to evidence-based treatments. Within a broad range of family interventions are different levels of family involvement. Family inclusion is the easiest intervention – simply involving the family members as historians, supporters, and allies in treatment.
Second, family psychoeducation has amassed a substantial evidence base showing its efficacy in the treatment of schizophrenia, bipolar disorder, and many medical illnesses, such as diabetes.
Last, but certainly not least, are the family systemic therapies, which in a meta-analysis of family systems therapies, were defined as "any couple, family, group, multifamily group, or individual focused therapeutic intervention that refers to either one of the following systems-oriented authors (Tom Andersen, Dr. Ivan Böszörményi-Nagy, Steve de Shazer, Jay Haley, Ph.D., Dr. Minuchin, Ms. Satir, Dr. Mara Selvini Palazzoli, Dr. Helm Stierlin, Paul Watzlawick, Ph.D., Michael White, Gerald H. Zuk, Ph.D.) or specified the intervention by use of at least one of the following terms: systemic, structural, strategic, triadic, Milan, functional, solution focused, narrative, resource/strength oriented, McMaster model" (Fam. Process 2010;49:457-85).
Family systems therapy has come a long way from the early days. We are very clear that for serious mental illness, family therapy alone is not enough, but neither are medications. Combination treatment produces symptom reduction AND good quality of life.
However, most psychotherapies – of the individual and family variety – are delivered by non-psychiatrists. Psychiatry is in danger of losing itself, as primary care physicians prescribe medications and refer patients to psychotherapists who are often co-located in their offices. Psychiatrists, however, are still the only professionals who have the potential to see the whole person and oversee the entire treatment: medications, individual, and family interventions.
It is to our advantage to be knowledgeable about all psychotherapeutic interventions AND to use them. We must make family therapy more visible and easier to teach in residencies. Psychiatrists have been reluctant to identify themselves as family psychiatrists because our enthusiastic charismatic leaders took the promise of family therapy too far. We hope that the solid family research now available will encourage all psychiatrists to learn and implement family interventions.
Dr. Minuchin and the ashtray, however, remain potent symbols of how creativity and genius created a new paradigm in psychiatry.
Dr. Heru is with the department of psychiatry at the University of Colorado, Denver. She has been a member of the Association of Family Psychiatrists since 2002 and currently serves as the organization’s treasurer. In addition, she is the coauthor of two books on working with families and is the author of numerous articles on this topic.
A pod of family psychiatrists is sitting around and chatting about the state of family psychiatry. They are preparing for a plenary at the Group for the Advancement of Psychiatry with the goal of showing how far family psychiatry has come since the first psychiatrists embraced the paradigm of systemic thinking. They also debate why family psychiatry is ignored in current practice, especially since the evidence shows that family treatment dramatically improves recovery rates for many illnesses.
When family therapy had its first wave of popularity, the charismatic leaders were out front wowing the crowds. Dr. Sal Minuchin’s sessions were heavily focused on structure and boundary making, and involved much chair rearranging and pulling family members, especially children, out from between the couple dyad and into their own space and chairs in the room. One of his most famous tapes involved putting an ashtray between the chairs of two family members to literally increase the distance between them!
Jay Haley, Ph.D., delivered strategic barbed arrows that pierced the hearts of the family members. Virginia Satir demonstrated the theater of families, sculpting organic shapes that pulsed with the gestalt of the family. There was much smoking of cigarettes during the sessions, by both the family psychiatrists and the family members. Psychiatry was exciting. The possibilities for change were endless. It was the 1960s.
Unfortunately, in those early days, family therapy was oversold as the sole treatment for schizophrenia and other mental illnesses. As a result, families have felt blamed by the negative attention and are still hesitant to engage in traditional family therapy. Nevertheless, quiet pioneers, like Carol M. Anderson, Ph.D., continue to research and practice a measured educational and collaborative approach aimed at involving families in mental health treatment. Indeed, current American Psychiatric Association guidelines for many psychiatric illnesses recommend that families be brought into the treatment process.
Family research has become much more sophisticated, with Dr. Minuchin’s early research on asthma and "psychosomatic families" being refined by teams led by Betsy Wood in New York, and Dr. Fred Wamboldt and Dr. Marianne Z. Wamboldt in Denver. Family research covers a broad territory, from studies on the impact of care giving on the caregiver’s immune function, to the role of expressed emotion in the outcome of illnesses – medical and psychiatric – to the efficacy of family treatments.
However, the Big Question still remains: Which model is the best? Structural? Strategic? Experiential?
While the arguments among devotees continue, studious researchers are quietly extracting the common factors found in the original family therapy models. These common factors are defined as the variables associated with positive clinical outcomes and are shared by several or all approaches. Andrew Christensen, Ph.D., suggests five principles that evidence-based couple interventions share: a systemic rather than an individual orientation of problems; modification of emotion-driven dysfunctional behaviors by teaching partners constructive ways to deal with differences, problems, and emotions; making both partners aware of avoided, emotion-based, private behaviors of each other, and making these internal experiences accessible to each other; enhancement of constructive communication in speaking and listening; and emphasis on strengths and positive behaviors (Enhancing Couples, Cambridge, Mass.: Hogrefe Publishing, 2009).
For couples and family therapies, common factors are conceptualizing the problems in relational terms, disrupting relational patterns, expanding treatment to include family members of the identified patient and an expanded therapeutic alliance (Common Factors in Couple and Family Therapy: The Overlooked Foundation for Effective Practice, New York: Guilford Press, 2009). Relational patterns have cognitive, behavioral, and affective domains, all of which can be targets of intervention. The therapeutic alliance is with the relationship and the family, rather than with the individual family members.
Patients, families, and psychiatrists all demand treatments that have been shown to work well. Family psychiatry has moved from theatrical showmanship to evidence-based treatments. Within a broad range of family interventions are different levels of family involvement. Family inclusion is the easiest intervention – simply involving the family members as historians, supporters, and allies in treatment.
Second, family psychoeducation has amassed a substantial evidence base showing its efficacy in the treatment of schizophrenia, bipolar disorder, and many medical illnesses, such as diabetes.
Last, but certainly not least, are the family systemic therapies, which in a meta-analysis of family systems therapies, were defined as "any couple, family, group, multifamily group, or individual focused therapeutic intervention that refers to either one of the following systems-oriented authors (Tom Andersen, Dr. Ivan Böszörményi-Nagy, Steve de Shazer, Jay Haley, Ph.D., Dr. Minuchin, Ms. Satir, Dr. Mara Selvini Palazzoli, Dr. Helm Stierlin, Paul Watzlawick, Ph.D., Michael White, Gerald H. Zuk, Ph.D.) or specified the intervention by use of at least one of the following terms: systemic, structural, strategic, triadic, Milan, functional, solution focused, narrative, resource/strength oriented, McMaster model" (Fam. Process 2010;49:457-85).
Family systems therapy has come a long way from the early days. We are very clear that for serious mental illness, family therapy alone is not enough, but neither are medications. Combination treatment produces symptom reduction AND good quality of life.
However, most psychotherapies – of the individual and family variety – are delivered by non-psychiatrists. Psychiatry is in danger of losing itself, as primary care physicians prescribe medications and refer patients to psychotherapists who are often co-located in their offices. Psychiatrists, however, are still the only professionals who have the potential to see the whole person and oversee the entire treatment: medications, individual, and family interventions.
It is to our advantage to be knowledgeable about all psychotherapeutic interventions AND to use them. We must make family therapy more visible and easier to teach in residencies. Psychiatrists have been reluctant to identify themselves as family psychiatrists because our enthusiastic charismatic leaders took the promise of family therapy too far. We hope that the solid family research now available will encourage all psychiatrists to learn and implement family interventions.
Dr. Minuchin and the ashtray, however, remain potent symbols of how creativity and genius created a new paradigm in psychiatry.
Dr. Heru is with the department of psychiatry at the University of Colorado, Denver. She has been a member of the Association of Family Psychiatrists since 2002 and currently serves as the organization’s treasurer. In addition, she is the coauthor of two books on working with families and is the author of numerous articles on this topic.