User login
Onychomycosis: A simpler in-office technique for sampling specimens
Background In onychomycosis, proper specimen collection is essential for an accurate diagnosis and initiation of appropriate therapy. Several techniques and locations have been suggested for specimen collection.
Objective To investigate the optimal technique of fungal sampling in onychomycosis.
Methods We reexamined 106 patients with distal and lateral subungual onychomycosis (DLSO) of the toenails. (The diagnosis had previously been confirmed by a laboratory mycological examination—both potassium hydroxide [KOH] test and fungal culture—of samples obtained by the proximal sampling approach.) We collected fungal specimens from the distal nail bed first, and later from the distal underside of the nail plate. The collected specimens underwent laboratory mycological examination.
Results KOH testing was positive in 84 (79.2%) specimens from the distal nail bed and only in 60 (56.6%) from the distal underside of the nail plate (P=.0007); cultures were positive in 93 (87.7%) and 76 (71.7%) specimens, respectively (P=.0063). Combining results from both locations showed positive KOH test results in 92 (86.8%) of the 106 patients and positive cultures in 100 (94.3%) patients.
Conclusions Based on our study, we suggest that in cases of suspected DLSO, material should be obtained by scraping nail material from the distal underside of the nail and then collecting all the material from the distal part of the nail bed.
When assessing possible onychomycosis, conventional practice is to take samples from the most proximal infected area. But this approach is usually technically difficult and may cause discomfort to patients.1-6 We therefore sought to determine the optimal location for fungal sampling from the distal part of the affected nail.
Methods
To assess the accuracy of distal sampling in diagnosing distal and lateral subungual onychomycosis (DLSO) of the toenails, we reevaluated 106 patients with DLSO previously confirmed by microscopic visualization of fungi in potassium hydroxide (KOH) solution and by fungal culture of specimens obtained using the proximal sampling approach.
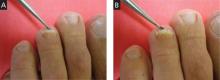
Before we obtained our samples, we cleaned the nails with alcohol and pared the most distal part of the nails in an effort to eliminate contaminant molds and bacteria. Using a 1- or 2-mm curette, we took specimens first from the distal nail bed and, second, from the distal underside of the nail plate ( FIGURE ). We separated specimens for use in either direct KOH visualization or in fungal culture using Sabouraud’s Dextrose agar (Novamed; Jerusalem, Israel), which contains chloramphenicol or streptomycin and penicillin to prevent contamination.
FIGURE
Distal sampling for distal and lateral subungual onychomycosis
Using a 2-mm curette, we collected specimens from the distal nail bed first (A), and then from the distal underside of the nail plate (B). However, our recommendation for clinical practice is to reverse this order of sampling to collect all possible material.
Statistical analyses
We recorded sociodemographic characteristics and fungal culture results in basic descriptive (prevalence) tables. In univariate analysis, we used t-tests to compare the means of continuous variables (eg, age, duration of fungal infection). To assess the distribution of categorical parameters (eg, sex) and to gauge the efficacy of the different probing techniques, we used chi-square (χ2) tests. We analyzed coded data using SPSS (Chicago, IL) for Windows software, version 12.
We conducted the study according to the rules of the local Helsinki Committee.
Results
We examined 106 patients with DLSO, of which 65 (61.3%) were male and 41 (38.7%) were female, ages 23 to 72 years (mean age, 44.6). The duration of fungal infection ranged from 3 to 30 years, with a mean of 14.9 years. In 70.8% of cases, the infection involved the first toenail. Duration of the fungal disease did not differ significantly between the sexes.
KOH test results were positive for 84 (79.2%) specimens from the distal nail bed, and for only 60 (56.6%) specimens from the distal underside of the nail plate (P=.0007); culture results were positive for 93 (87.7%) and 76 (71.7%) specimens, respectively (P=.0063). Combining results from both locations (all positive samples from the nail bed, plus positive samples from the nail underside when results from the nail bed were negative) yielded confirmation with KOH testing in 92 (86.8%) patients and with culture in 100 (94.3%) patients. There were no statistically significant differences between the combined results of both locations and the results from the distal nail bed alone (KOH, P=.143; culture, P=.149) ( TABLE ).
TABLE
Accuracy of distal sampling in 106 patients with confirmed DLSO
| Nail bed | Underside of nail plate | P value | Combined results | P value* | |
|---|---|---|---|---|---|
| Positive KOH | 84 (79.2%) | 60 (56.65%) | .0007 | 92 (86.8%) | .143 |
| Positive culture | 93 (87.7%) | 76 (71.7%) | .0063 | 100 (94.3%) | .149 |
| *Differences between results from sampling the nail bed alone and results from combined nail bed and nail plate sampling were not statistically significant. DLSO, distal and lateral subungual onychomycosis; KOH, potassium hydroxide. | |||||
Discussion
In DLSO, most dermatophyte species invade the middle and ventral layers of the nail plate adjacent to the nail bed, where the keratin is soft and close to the living cells below. In fact, the nail bed is probably the primary invasion site of dermatophytes, and it acts as a reservoir for continual reinfection of the growing nail.7 Obtaining confirmation of fungal infection before initiating antifungal treatment is the gold standard in clinical practice, given that antifungal agents have potentially serious adverse effects, that treatment is expensive, and that medicolegal issues exist.8
The standard methods used to detect a fungal nail infection are direct microscopy with KOH preparation and fungal culture. The KOH test is the simplest, least expensive method used in the detection of fungi, but it cannot identify the specific pathogen. Fungal speciation is done by culture. More accurate histopathologic evaluation is possible with periodic acid-Schiff stain, immunofluorescent microscopy with calcofluor stain, or polymerase chain reaction, but these techniques are more expensive and less feasible in outpatient clinics.9
The diagnostic accuracy of the KOH test and fungal culture ranges from 50% to 70%, depending on the experience of the laboratory technician and the methods used to collect and prepare the sample.8-10 It is better to take samples from the most proximal infected area by curettage or drilling, but this technique is usually more difficult than a distal approach, should be performed by skilled personnel, and may cause discomfort to patients.3,5,6
Our recommendation for practice. Earlier studies suggested that nail specimens should be taken from the nail bed.11-13 We sampled the nail bed first in our study because, in trying to determine an optimal location for sampling, we wanted to avoid contaminating nail-bed specimens with debris from the underside of the nail. In practice, however, we suggest that, in cases of suspected DLSO, clinicians first obtain specimens from the distal underside of the nail, and then collect all remaining material from the distal part of the nail bed. This technique is simple and can easily be performed in an office setting. If test results are negative but DLSO remains clinically likely, test a second sample after a week or 2.
CORRESPONDENCE Boaz Amichai, MD, Department of Dermatology, Sheba Medical Center, Tel-Hashomer, Israel; [email protected]
1. Lawry MA, Haneke E, Strobeck K, et al. Methods for diagnosing onychomycosis: a comparative study and review of the literature. Arch Dermatol. 2000;136:1112-1116.
2. Elewski BE. Diagnostic techniques for confirming onychomycosis. J Am Acad Dermatol. 1996;35(3 pt 2):S6-S9.
3. Heikkila H. Isolation of fungi from onychomycosis-suspected nails by two methods: clipping and drilling. Mycoses. 1996;39:479-482.
4. Mochizuki T, Kawasaki M, Tanabe H, et al. A nail drilling method suitable for the diagnosis of onychomycosis. J Dermatol. 2005;32:108-113.
5. Shemer A, Trau H, Davidovici B, et al. Nail sampling in onychomycosis: comparative study of curettage from three sites of the infected nail. J Dtsch Dermatol Ges. 2007;5:1108-1111.
6. Shemer A, Trau H, Davidovici B, et al. Collection of fungi samples from nails: comparative study of curettage and drilling techniques. J Eur Acad Dermatol Venereol. 2008;22:182-185.
7. Hay RJ, Baran R, Haneke E. Fungal (onychomycosis) and other infections involving the nail apparatus. In: Baran R, Dawber RPR, eds. Disease of the Nails and Their Management. 2nd ed. Oxford, England: Blackwell Sciences Ltd; 1994: 97–134.
8. Daniel CR, 3rd, Elewski BE. The diagnosis of nail fungus infection revisited. Arch Dermatol. 2000;136:1162-1164.
9. Weinberg JM, Koestenblatt EK, Tutrone WD, et al. Comparison of diagnostic methods in the evaluation of onychomycosis. J Am Acad Dermatol. 2003;49:193-197.
10. Arnold B, Kianifrad F, Tavakkol A. A comparison of KOH and culture results from two mycology laboratories for the diagnosis of onychomycosis during a randomized, multicenter clinical trial: a subset study. J Am Podiatr Med Assoc. 2005;95:421-423.
11. English MP. Nails and fungi. Br J Dermatol. 1976;94:697-701.
12. Elewski BE. Clinical pearl: diagnosis of onychomycosis. J Am Acad Dermatol. 1995;32:500-501.
13. Rodgers P, Bassler M. Treating onychomycosis. Am Fam Physician. 2001;63:663-672, 677–678.
Background In onychomycosis, proper specimen collection is essential for an accurate diagnosis and initiation of appropriate therapy. Several techniques and locations have been suggested for specimen collection.
Objective To investigate the optimal technique of fungal sampling in onychomycosis.
Methods We reexamined 106 patients with distal and lateral subungual onychomycosis (DLSO) of the toenails. (The diagnosis had previously been confirmed by a laboratory mycological examination—both potassium hydroxide [KOH] test and fungal culture—of samples obtained by the proximal sampling approach.) We collected fungal specimens from the distal nail bed first, and later from the distal underside of the nail plate. The collected specimens underwent laboratory mycological examination.
Results KOH testing was positive in 84 (79.2%) specimens from the distal nail bed and only in 60 (56.6%) from the distal underside of the nail plate (P=.0007); cultures were positive in 93 (87.7%) and 76 (71.7%) specimens, respectively (P=.0063). Combining results from both locations showed positive KOH test results in 92 (86.8%) of the 106 patients and positive cultures in 100 (94.3%) patients.
Conclusions Based on our study, we suggest that in cases of suspected DLSO, material should be obtained by scraping nail material from the distal underside of the nail and then collecting all the material from the distal part of the nail bed.
When assessing possible onychomycosis, conventional practice is to take samples from the most proximal infected area. But this approach is usually technically difficult and may cause discomfort to patients.1-6 We therefore sought to determine the optimal location for fungal sampling from the distal part of the affected nail.
Methods
To assess the accuracy of distal sampling in diagnosing distal and lateral subungual onychomycosis (DLSO) of the toenails, we reevaluated 106 patients with DLSO previously confirmed by microscopic visualization of fungi in potassium hydroxide (KOH) solution and by fungal culture of specimens obtained using the proximal sampling approach.
Before we obtained our samples, we cleaned the nails with alcohol and pared the most distal part of the nails in an effort to eliminate contaminant molds and bacteria. Using a 1- or 2-mm curette, we took specimens first from the distal nail bed and, second, from the distal underside of the nail plate ( FIGURE ). We separated specimens for use in either direct KOH visualization or in fungal culture using Sabouraud’s Dextrose agar (Novamed; Jerusalem, Israel), which contains chloramphenicol or streptomycin and penicillin to prevent contamination.
FIGURE
Distal sampling for distal and lateral subungual onychomycosis
Using a 2-mm curette, we collected specimens from the distal nail bed first (A), and then from the distal underside of the nail plate (B). However, our recommendation for clinical practice is to reverse this order of sampling to collect all possible material.
Statistical analyses
We recorded sociodemographic characteristics and fungal culture results in basic descriptive (prevalence) tables. In univariate analysis, we used t-tests to compare the means of continuous variables (eg, age, duration of fungal infection). To assess the distribution of categorical parameters (eg, sex) and to gauge the efficacy of the different probing techniques, we used chi-square (χ2) tests. We analyzed coded data using SPSS (Chicago, IL) for Windows software, version 12.
We conducted the study according to the rules of the local Helsinki Committee.
Results
We examined 106 patients with DLSO, of which 65 (61.3%) were male and 41 (38.7%) were female, ages 23 to 72 years (mean age, 44.6). The duration of fungal infection ranged from 3 to 30 years, with a mean of 14.9 years. In 70.8% of cases, the infection involved the first toenail. Duration of the fungal disease did not differ significantly between the sexes.
KOH test results were positive for 84 (79.2%) specimens from the distal nail bed, and for only 60 (56.6%) specimens from the distal underside of the nail plate (P=.0007); culture results were positive for 93 (87.7%) and 76 (71.7%) specimens, respectively (P=.0063). Combining results from both locations (all positive samples from the nail bed, plus positive samples from the nail underside when results from the nail bed were negative) yielded confirmation with KOH testing in 92 (86.8%) patients and with culture in 100 (94.3%) patients. There were no statistically significant differences between the combined results of both locations and the results from the distal nail bed alone (KOH, P=.143; culture, P=.149) ( TABLE ).
TABLE
Accuracy of distal sampling in 106 patients with confirmed DLSO
| Nail bed | Underside of nail plate | P value | Combined results | P value* | |
|---|---|---|---|---|---|
| Positive KOH | 84 (79.2%) | 60 (56.65%) | .0007 | 92 (86.8%) | .143 |
| Positive culture | 93 (87.7%) | 76 (71.7%) | .0063 | 100 (94.3%) | .149 |
| *Differences between results from sampling the nail bed alone and results from combined nail bed and nail plate sampling were not statistically significant. DLSO, distal and lateral subungual onychomycosis; KOH, potassium hydroxide. | |||||
Discussion
In DLSO, most dermatophyte species invade the middle and ventral layers of the nail plate adjacent to the nail bed, where the keratin is soft and close to the living cells below. In fact, the nail bed is probably the primary invasion site of dermatophytes, and it acts as a reservoir for continual reinfection of the growing nail.7 Obtaining confirmation of fungal infection before initiating antifungal treatment is the gold standard in clinical practice, given that antifungal agents have potentially serious adverse effects, that treatment is expensive, and that medicolegal issues exist.8
The standard methods used to detect a fungal nail infection are direct microscopy with KOH preparation and fungal culture. The KOH test is the simplest, least expensive method used in the detection of fungi, but it cannot identify the specific pathogen. Fungal speciation is done by culture. More accurate histopathologic evaluation is possible with periodic acid-Schiff stain, immunofluorescent microscopy with calcofluor stain, or polymerase chain reaction, but these techniques are more expensive and less feasible in outpatient clinics.9
The diagnostic accuracy of the KOH test and fungal culture ranges from 50% to 70%, depending on the experience of the laboratory technician and the methods used to collect and prepare the sample.8-10 It is better to take samples from the most proximal infected area by curettage or drilling, but this technique is usually more difficult than a distal approach, should be performed by skilled personnel, and may cause discomfort to patients.3,5,6
Our recommendation for practice. Earlier studies suggested that nail specimens should be taken from the nail bed.11-13 We sampled the nail bed first in our study because, in trying to determine an optimal location for sampling, we wanted to avoid contaminating nail-bed specimens with debris from the underside of the nail. In practice, however, we suggest that, in cases of suspected DLSO, clinicians first obtain specimens from the distal underside of the nail, and then collect all remaining material from the distal part of the nail bed. This technique is simple and can easily be performed in an office setting. If test results are negative but DLSO remains clinically likely, test a second sample after a week or 2.
CORRESPONDENCE Boaz Amichai, MD, Department of Dermatology, Sheba Medical Center, Tel-Hashomer, Israel; [email protected]
Background In onychomycosis, proper specimen collection is essential for an accurate diagnosis and initiation of appropriate therapy. Several techniques and locations have been suggested for specimen collection.
Objective To investigate the optimal technique of fungal sampling in onychomycosis.
Methods We reexamined 106 patients with distal and lateral subungual onychomycosis (DLSO) of the toenails. (The diagnosis had previously been confirmed by a laboratory mycological examination—both potassium hydroxide [KOH] test and fungal culture—of samples obtained by the proximal sampling approach.) We collected fungal specimens from the distal nail bed first, and later from the distal underside of the nail plate. The collected specimens underwent laboratory mycological examination.
Results KOH testing was positive in 84 (79.2%) specimens from the distal nail bed and only in 60 (56.6%) from the distal underside of the nail plate (P=.0007); cultures were positive in 93 (87.7%) and 76 (71.7%) specimens, respectively (P=.0063). Combining results from both locations showed positive KOH test results in 92 (86.8%) of the 106 patients and positive cultures in 100 (94.3%) patients.
Conclusions Based on our study, we suggest that in cases of suspected DLSO, material should be obtained by scraping nail material from the distal underside of the nail and then collecting all the material from the distal part of the nail bed.
When assessing possible onychomycosis, conventional practice is to take samples from the most proximal infected area. But this approach is usually technically difficult and may cause discomfort to patients.1-6 We therefore sought to determine the optimal location for fungal sampling from the distal part of the affected nail.
Methods
To assess the accuracy of distal sampling in diagnosing distal and lateral subungual onychomycosis (DLSO) of the toenails, we reevaluated 106 patients with DLSO previously confirmed by microscopic visualization of fungi in potassium hydroxide (KOH) solution and by fungal culture of specimens obtained using the proximal sampling approach.
Before we obtained our samples, we cleaned the nails with alcohol and pared the most distal part of the nails in an effort to eliminate contaminant molds and bacteria. Using a 1- or 2-mm curette, we took specimens first from the distal nail bed and, second, from the distal underside of the nail plate ( FIGURE ). We separated specimens for use in either direct KOH visualization or in fungal culture using Sabouraud’s Dextrose agar (Novamed; Jerusalem, Israel), which contains chloramphenicol or streptomycin and penicillin to prevent contamination.
FIGURE
Distal sampling for distal and lateral subungual onychomycosis
Using a 2-mm curette, we collected specimens from the distal nail bed first (A), and then from the distal underside of the nail plate (B). However, our recommendation for clinical practice is to reverse this order of sampling to collect all possible material.
Statistical analyses
We recorded sociodemographic characteristics and fungal culture results in basic descriptive (prevalence) tables. In univariate analysis, we used t-tests to compare the means of continuous variables (eg, age, duration of fungal infection). To assess the distribution of categorical parameters (eg, sex) and to gauge the efficacy of the different probing techniques, we used chi-square (χ2) tests. We analyzed coded data using SPSS (Chicago, IL) for Windows software, version 12.
We conducted the study according to the rules of the local Helsinki Committee.
Results
We examined 106 patients with DLSO, of which 65 (61.3%) were male and 41 (38.7%) were female, ages 23 to 72 years (mean age, 44.6). The duration of fungal infection ranged from 3 to 30 years, with a mean of 14.9 years. In 70.8% of cases, the infection involved the first toenail. Duration of the fungal disease did not differ significantly between the sexes.
KOH test results were positive for 84 (79.2%) specimens from the distal nail bed, and for only 60 (56.6%) specimens from the distal underside of the nail plate (P=.0007); culture results were positive for 93 (87.7%) and 76 (71.7%) specimens, respectively (P=.0063). Combining results from both locations (all positive samples from the nail bed, plus positive samples from the nail underside when results from the nail bed were negative) yielded confirmation with KOH testing in 92 (86.8%) patients and with culture in 100 (94.3%) patients. There were no statistically significant differences between the combined results of both locations and the results from the distal nail bed alone (KOH, P=.143; culture, P=.149) ( TABLE ).
TABLE
Accuracy of distal sampling in 106 patients with confirmed DLSO
| Nail bed | Underside of nail plate | P value | Combined results | P value* | |
|---|---|---|---|---|---|
| Positive KOH | 84 (79.2%) | 60 (56.65%) | .0007 | 92 (86.8%) | .143 |
| Positive culture | 93 (87.7%) | 76 (71.7%) | .0063 | 100 (94.3%) | .149 |
| *Differences between results from sampling the nail bed alone and results from combined nail bed and nail plate sampling were not statistically significant. DLSO, distal and lateral subungual onychomycosis; KOH, potassium hydroxide. | |||||
Discussion
In DLSO, most dermatophyte species invade the middle and ventral layers of the nail plate adjacent to the nail bed, where the keratin is soft and close to the living cells below. In fact, the nail bed is probably the primary invasion site of dermatophytes, and it acts as a reservoir for continual reinfection of the growing nail.7 Obtaining confirmation of fungal infection before initiating antifungal treatment is the gold standard in clinical practice, given that antifungal agents have potentially serious adverse effects, that treatment is expensive, and that medicolegal issues exist.8
The standard methods used to detect a fungal nail infection are direct microscopy with KOH preparation and fungal culture. The KOH test is the simplest, least expensive method used in the detection of fungi, but it cannot identify the specific pathogen. Fungal speciation is done by culture. More accurate histopathologic evaluation is possible with periodic acid-Schiff stain, immunofluorescent microscopy with calcofluor stain, or polymerase chain reaction, but these techniques are more expensive and less feasible in outpatient clinics.9
The diagnostic accuracy of the KOH test and fungal culture ranges from 50% to 70%, depending on the experience of the laboratory technician and the methods used to collect and prepare the sample.8-10 It is better to take samples from the most proximal infected area by curettage or drilling, but this technique is usually more difficult than a distal approach, should be performed by skilled personnel, and may cause discomfort to patients.3,5,6
Our recommendation for practice. Earlier studies suggested that nail specimens should be taken from the nail bed.11-13 We sampled the nail bed first in our study because, in trying to determine an optimal location for sampling, we wanted to avoid contaminating nail-bed specimens with debris from the underside of the nail. In practice, however, we suggest that, in cases of suspected DLSO, clinicians first obtain specimens from the distal underside of the nail, and then collect all remaining material from the distal part of the nail bed. This technique is simple and can easily be performed in an office setting. If test results are negative but DLSO remains clinically likely, test a second sample after a week or 2.
CORRESPONDENCE Boaz Amichai, MD, Department of Dermatology, Sheba Medical Center, Tel-Hashomer, Israel; [email protected]
1. Lawry MA, Haneke E, Strobeck K, et al. Methods for diagnosing onychomycosis: a comparative study and review of the literature. Arch Dermatol. 2000;136:1112-1116.
2. Elewski BE. Diagnostic techniques for confirming onychomycosis. J Am Acad Dermatol. 1996;35(3 pt 2):S6-S9.
3. Heikkila H. Isolation of fungi from onychomycosis-suspected nails by two methods: clipping and drilling. Mycoses. 1996;39:479-482.
4. Mochizuki T, Kawasaki M, Tanabe H, et al. A nail drilling method suitable for the diagnosis of onychomycosis. J Dermatol. 2005;32:108-113.
5. Shemer A, Trau H, Davidovici B, et al. Nail sampling in onychomycosis: comparative study of curettage from three sites of the infected nail. J Dtsch Dermatol Ges. 2007;5:1108-1111.
6. Shemer A, Trau H, Davidovici B, et al. Collection of fungi samples from nails: comparative study of curettage and drilling techniques. J Eur Acad Dermatol Venereol. 2008;22:182-185.
7. Hay RJ, Baran R, Haneke E. Fungal (onychomycosis) and other infections involving the nail apparatus. In: Baran R, Dawber RPR, eds. Disease of the Nails and Their Management. 2nd ed. Oxford, England: Blackwell Sciences Ltd; 1994: 97–134.
8. Daniel CR, 3rd, Elewski BE. The diagnosis of nail fungus infection revisited. Arch Dermatol. 2000;136:1162-1164.
9. Weinberg JM, Koestenblatt EK, Tutrone WD, et al. Comparison of diagnostic methods in the evaluation of onychomycosis. J Am Acad Dermatol. 2003;49:193-197.
10. Arnold B, Kianifrad F, Tavakkol A. A comparison of KOH and culture results from two mycology laboratories for the diagnosis of onychomycosis during a randomized, multicenter clinical trial: a subset study. J Am Podiatr Med Assoc. 2005;95:421-423.
11. English MP. Nails and fungi. Br J Dermatol. 1976;94:697-701.
12. Elewski BE. Clinical pearl: diagnosis of onychomycosis. J Am Acad Dermatol. 1995;32:500-501.
13. Rodgers P, Bassler M. Treating onychomycosis. Am Fam Physician. 2001;63:663-672, 677–678.
1. Lawry MA, Haneke E, Strobeck K, et al. Methods for diagnosing onychomycosis: a comparative study and review of the literature. Arch Dermatol. 2000;136:1112-1116.
2. Elewski BE. Diagnostic techniques for confirming onychomycosis. J Am Acad Dermatol. 1996;35(3 pt 2):S6-S9.
3. Heikkila H. Isolation of fungi from onychomycosis-suspected nails by two methods: clipping and drilling. Mycoses. 1996;39:479-482.
4. Mochizuki T, Kawasaki M, Tanabe H, et al. A nail drilling method suitable for the diagnosis of onychomycosis. J Dermatol. 2005;32:108-113.
5. Shemer A, Trau H, Davidovici B, et al. Nail sampling in onychomycosis: comparative study of curettage from three sites of the infected nail. J Dtsch Dermatol Ges. 2007;5:1108-1111.
6. Shemer A, Trau H, Davidovici B, et al. Collection of fungi samples from nails: comparative study of curettage and drilling techniques. J Eur Acad Dermatol Venereol. 2008;22:182-185.
7. Hay RJ, Baran R, Haneke E. Fungal (onychomycosis) and other infections involving the nail apparatus. In: Baran R, Dawber RPR, eds. Disease of the Nails and Their Management. 2nd ed. Oxford, England: Blackwell Sciences Ltd; 1994: 97–134.
8. Daniel CR, 3rd, Elewski BE. The diagnosis of nail fungus infection revisited. Arch Dermatol. 2000;136:1162-1164.
9. Weinberg JM, Koestenblatt EK, Tutrone WD, et al. Comparison of diagnostic methods in the evaluation of onychomycosis. J Am Acad Dermatol. 2003;49:193-197.
10. Arnold B, Kianifrad F, Tavakkol A. A comparison of KOH and culture results from two mycology laboratories for the diagnosis of onychomycosis during a randomized, multicenter clinical trial: a subset study. J Am Podiatr Med Assoc. 2005;95:421-423.
11. English MP. Nails and fungi. Br J Dermatol. 1976;94:697-701.
12. Elewski BE. Clinical pearl: diagnosis of onychomycosis. J Am Acad Dermatol. 1995;32:500-501.
13. Rodgers P, Bassler M. Treating onychomycosis. Am Fam Physician. 2001;63:663-672, 677–678.
Aspirin for primary prevention of CVD: Are the right people using it?
Purpose Aspirin is recommended for the primary prevention of cardiovascular disease (CVD) in adults at high risk, but little is known about sociodemographic disparities in prophylactic aspirin use. This study examined the association between sociodemographic characteristics and regular aspirin use among adults in Wisconsin who are free of CVD.
Methods A cross-sectional design was used, and data collected from 2008 to 2010. Regular aspirin use (aspirin therapy) was defined as taking aspirin most days of the week. We found 831 individuals for whom complete data were available for regression analyses and stratified the sample into 2 groups: those for whom aspirin therapy was indicated and those for whom it was not indicated, based on national guidelines.
Results Of the 268 patients for whom aspirin therapy was indicated, only 83 (31%) were using it regularly, and 102 (18%) of the 563 participants who did not have an aspirin indication were taking it regularly. In the group with an aspirin indication, participants who were older had higher rates of regular aspirin use than younger patients (odds ratio [OR]=1.07; P<.001), and women had significantly higher adjusted odds of regular aspirin use than men (OR=3.49; P=.021). Among those for whom aspirin therapy was not indicated, the adjusted odds of regular aspirin use were significantly higher among older participants (OR=1.07; P<.001) vs their younger counterparts, and significantly lower among Hispanic or nonwhite participants (OR=0.32; P=.063) relative to non-Hispanic whites.
Conclusions Aspirin therapy is underused by those at high risk for CVD—individuals who could gain cardioprotection from regular use—and overused by those at low risk for CVD, for whom the risk of major bleeding outweighs the potential benefit. Stronger primary care initiatives may be needed to ensure that patients undergo regular screening for aspirin use, particularly middle-aged men at high CVD risk. Patient education may be needed, as well, to better inform people (particularly older, non-Hispanic whites) about the risks of regular aspirin use that is not medically indicated.
Cardiovascular disease (CVD) is the principal cause of death in the United States.1 As the population grows older and obesity and diabetes become increasingly prevalent, the prevalence of CVD is also expected to rise.2,3 Fortunately, many CVD events can be prevented or delayed by modifying risk factors such as hyperlipidemia, hypertension, and smoking. Interventions associated with a reduction in risk have led to a reduction in CVD events4,5 and contributed to a steady decline in cardiac deaths.6
Control of platelet aggregation is a cornerstone of primary CVD prevention.7 In an outpatient setting, this usually translates into identifying patients who are at high risk for a CVD event and advising them to take low-dose aspirin daily or every other day. Although not without controversy,8,9 the US Preventive Services Task Force (USPSTF) recommends regular aspirin use for primary CVD prevention for middle-aged to older men at high risk for myocardial infarction (MI) and women at high risk for ischemic stroke.10
The efficacy of this intervention is proven: In primary prevention trials, regular aspirin use is associated with a 14% reduction in the likelihood of CVD events over 7 years.11 What’s more, aspirin therapy, as recommended by the USPSTF, is among the most cost-effective clinic-based preventive measures.12
In 2004, 41% of US adults age 40 or older reported taking aspirin regularly13 —an increase of approximately 20% since 1999.14 More recent data from a national population-based cohort study found that 41% of adults ages 45 to 90 years who did not have CVD but were at moderate to high risk for a CVD event reported taking aspirin ≥3 days per week.15 In the same study, almost one-fourth of those at low CVD risk also reported regular aspirin use.
While regular aspirin use for primary CVD prevention has been on the rise,13,14 the extent to which this intervention has penetrated various segments of the population is unclear. Several studies have found that aspirin use is consistently highest among those who are older, male, and white.15-17 Other socioeconomic variables (eg, education level, employment, marital status) have received little attention. And no previous study has used national guidelines for aspirin therapy to stratify samples.
A look at overuse and underuse. To ensure that aspirin therapy for primary CVD prevention is directed at those who are most likely to benefit from it, a better understanding of variables associated with both aspirin overuse and underuse is needed. This area of research is important, in part because direct-to-consumer aspirin marketing may be particularly influential among groups at low risk for CVD—for whom the risk of excess bleeding outweighs the potential for disease prevention.13,18
This study was undertaken to examine the association between specific sociodemographic variables and aspirin use among a representative sample of Wisconsin adults without CVD, looking both at those for whom aspirin therapy is indicated and those for whom it is not indicated based on national guidelines.
Methods
Design
We used a cross-sectional design, with data from the Survey of the Health of Wisconsin (SHOW),19 an annual survey of Wisconsin residents ages 21 to 74 years. SHOW uses a 2-stage stratified cluster sampling design to select households, with all age-eligible household members invited to participate. Recruitment for the annual survey consists of general community-wide announcements, as well as an initial letter and up to 6 visits to the randomly selected households to encourage participation.
By the end of 2010, SHOW had 1572 enrollees—about 53% of all eligible invitees. The demographic profile of SHOW enrollees was similar to US census data for all Wisconsin adults during the same time frame.19 All SHOW procedures were approved by the University of Wisconsin Institutional Review Board, and all participants provided informed consent.
Study sample
Our analyses were based on data provided by SHOW participants who were screened and enrolled between 2008 and 2010. To be included in our study, participants had to be between the ages of 35 and 74 years; not pregnant, on active military duty, or institutionalized; and have no personal history of CVD (myocardial infarction, angina, stroke, or transient ischemic attack) or CVD risk equivalent (type 1 or type 2 diabetes) at the time of recruitment. Data on key study variables had to be available, as well. (We used 35 years as the lower age limit because of the very low likelihood of CVD in younger individuals.)
We stratified the analytical sample (N=831) into 2 groups—participants for whom aspirin therapy was indicated and those for whom it was not indicated—in order to examine aspirin’s appropriate (recommended) and inappropriate use.
Measures
Outcome. The outcome variable was aspirin use. SHOW had asked participants how often they took aspirin. Similar to the methods used by Sanchez et al,15 we classified those who reported taking aspirin most (≥4) days of the week as regular aspirin users. All others were classified as nonregular aspirin users. Participants were not asked about the daily dose or weekly volume of aspirin.
Variables
Sociodemographic variables considered in our analysis were age, sex, race/ethnicity, education level, marital/partner status, employment status, health insurance, and region of residence within Wisconsin.
All participants underwent physical examinations, conducted as part of SHOW, at either a permanent or mobile exam center. Blood pressure was measured after a 5-minute rest period in a seated position, and the average of the last 2 out of 3 consecutive measurements was reported. Body mass index (BMI) was calculated, and blood samples were obtained by venipuncture, processed immediately, and sent to the Marshfield Clinic laboratory for measuring total and high-density lipoprotein (HDL) cholesterol.
Indications for aspirin therapy. We stratified the sample by those who were and those who were not candidates for aspirin therapy for primary CVD prevention based on the latest guidelines from the USPSTF ( FIGURE ).10 The Task Force recommends aspirin therapy for men ages 45 to 74 years with a moderate or greater 10-year risk of a coronary heart disease (CHD) event and women ages 55 to 74 years with a moderate or greater 10-year risk of stroke. We used the global CVD risk equation derived from the Framingham Heart Study (based on age, sex, smoking status, systolic blood pressure, and total and HDL cholesterol) to calculate each participant’s 10-year risk and, thus, determine whether aspirin therapy was or was not indicated.20 Total and HDL cholesterol values were missing for 94 participants in the analytical sample; their 10-year CVD risk was estimated using BMI, a reasonable alternative to more conventional CVD risk prediction when laboratory values are unavailable.21
FIGURE
Study (SHOW) sample, stratified based on aspirin indication10
*US Preventive Services Task Force guidelines were slightly modified for this analysis: The upper age bound was reduced from 79 to 74 years because the Survey of the Health of Wisconsin did not enroll participants >74 years.
CHD, coronary heart disease; CVD, cardiovascular disease; DM, diabetes mellitus; N/A, not applicable; SHOW, Survey of the Health of Wisconsin.
Statistical analyses
All analytical procedures were conducted using Statistical Analysis Software (SAS Version 9.2; Cary, NC). A complete-case framework was used.
We used multivariate logistic regression for survey data (PROC SURVEYLOGISTIC; SAS Institute, Cary, NC) to examine the association between aspirin use and sociodemographic variables. Two separate analyses were conducted, one of participants for whom aspirin therapy was indicated and the other for participants for whom it was not. The outcomes were modeled dichotomously, as regular vs nonregular aspirin users, and a collinearity check was done. 21
Initially, we created univariate models to gauge the crude relationship between each variable and aspirin use. Any variable with P<.20 in its univariate association with regular aspirin use was considered for inclusion in the final multivariate regression model. In the multivariate analyses, we sequentially eliminated variables with the weakest association with aspirin use until only significant (P<.10) independent predictors remained. Appropriate weighting was applied based on survey strata and cluster structure.19
Results
Of the 831 participants who met the eligibility criteria for our analysis, 268 (32%) had an aspirin indication. TABLE 1 shows the key characteristics of the analytical sample, stratified by those for whom aspirin was indicated and those for whom it was not. The sample was primarily middle-aged (mean age 52.4±0.36) and non-Hispanic white (93%). Compared with those for whom aspirin therapy was not indicated, the group with an aspirin indication was significantly older (56.9 vs 50.3) and had a significantly higher proportion of males (97% vs 19%). As expected, those for whom aspirin was indicated were also at higher risk for CHD and stroke, most notably as a result of significantly higher systolic BP (131.9 vs 121.5 mm Hg) and lower HDL cholesterol (42.5 vs 52.6 mg/dL) compared with participants without an aspirin indication.
TABLE 1
Study sample, by sociodemographic variable and aspirin indication
| Variable | Full sample (N=831) | Aspirin indicated (n=268) | Aspirin not indicated (n=563) |
|---|---|---|---|
| Mean age, y | 52.4 | 56.9 | 50.3 |
| Sex, n Male Female | 367 464 | 259 9 | 108 455 |
| Race/ethnicity, n White, non-Hispanic Nonwhite/Hispanic | 776 55 | 252 16 | 524 39 |
| Marital status, n Married/partnered Not married or partnered | 637 194 | 215 53 | 422 141 |
| Health insurance, n Uninsured Insured | 76 755 | 26 242 | 50 513 |
| Education, n ≤High school Associate’s degree ≥Bachelor’s degree | 217 312 302 | 77 107 84 | 140 205 218 |
| Employment, n Unemployed Student/retiree/home Employed | 98 147 586 | 33 52 183 | 65 95 403 |
When aspirin was indicated, use was linked to age and sex
In the group with an aspirin indication (n=268), 83 (31%) reported taking aspirin most days of the week. The initial examination of sociodemographic variables showed that age, sex, and employment status demonstrated significant univariate associations with regular aspirin use ( TABLE 2 ). In the multivariate model, however, the odds of regular aspirin use were significantly greater among participants who were older (odds ratio [OR], 1.07; P<.001) or female (OR, 3.49; P=.021) compared with participants who were younger or male, respectively.
TABLE 2
Participants who have an aspirin indication: Association between sociodemographic variables and regular aspirin use
| Variable | Regular aspirin use, OR (95% CI) | P value* |
|---|---|---|
| Age Older vs younger | 1.07 (1.04-1.11) | .001 |
| Sex Female vs male | 3.89 (1.42-10.67)† | .008 |
| Race/ethnicity Nonwhite/Hispanic vs white non-Hispanic | 0.55 (0.09-3.47) | .526 |
| Marital status Not married/partnered vs married/partner | 0.83 (0.36-1.95) | .678 |
| Health insurance Uninsured vs insured | 0.86 (0.50-1.47) | .579 |
| Education ≥Bachelor’s degree vs ≤high school Associate’s degree/some college vs ≤high school | 1.58 (0.75-3.34) 1.36 (0.74-2.49) | .234 .325 |
| Employment Student or retired vs employed Unemployed vs employed | 2.96 (1.74-5.03) 0.62 (0.25-1.56) | .001 .314 |
| *Significance was defined as P<.10. †Multivariate adjusted model: 3.49 (95% CI, 1.21-10.07; P=.021). CI, confidence interval; OR, odds ratio. | ||
When aspirin was not indicated, age and sex still affected use
Among the 563 participants for whom aspirin therapy was not indicated, 102 (18%) reported taking aspirin regularly. Age, sex, race/ethnicity, health insurance, and employment ( TABLE 3 ), as well as region of residence and study enrollment year, had significant univariate associations with regular aspirin use; these variables were tested for potential inclusion in the multivariate model. In the final multivariate regression model, the odds of regular aspirin use were significantly greater among participants who were older (OR, 1.07; P<.001) and significantly lower among participants who were Hispanic or nonwhite (OR, 0.32; P=.063).
TABLE 3
Participants who do not have an aspirin indication: Association between sociodemographic variables and regular aspirin use
| Variable | Regular aspirin use, OR (95% CI) | P value* |
|---|---|---|
| Age Older vs younger | 1.07 (1.04-1.10) | .001 |
| Sex Female vs male | 1.60 (0.84-3.04) | .152 |
| Race/ethnicity Nonwhite or Hispanic vs white non-Hispanic | 0.23 (0.07- 0.73)† | .013 |
| Marital status Not married/partnered vs married/partnered | 1.00 (0.63-1.59) | .992 |
| Health insurance Uninsured vs insured | 0.36 (0.11- 1.15) | .086 |
| Education Bachelor’s or higher vs high school or less Associate’s/some college vs high school or less | 0.74 (0.35-1.57) 0.67 (0.38-1.17) | .431 .158 |
| Employment Student/retired vs employed Unemployed vs employed | 2.35 (1.32-4.20) 0.78 (0.26- 2.34) | .004 .652 |
| *Significance was defined as P<.10. †Multivariate adjusted model: 0.32 (95% CI, 0.10-1.06; P=.063). CI, confidence interval; OR, odds ratio. | ||
Discussion
Aspirin was generally underutilized in the group with significant CVD risk (n=268) in our study, with slightly less than a third of participants for whom aspirin therapy was indicated taking it most days of the week. Despite trends of increased aspirin use among US adults in recent years,15 aspirin therapy in the 2008-2010 SHOW sample was lower than in 2005 to 2008. It was also lower than national estimates of aspirin use for primary CVD prevention15,22 —but about 20% higher than estimates of overall aspirin use in Wisconsin 20 years ago.23 Consistent with previous research, the final adjusted model and sensitivity analysis indicated that older individuals were more likely to take aspirin regularly.
Contrary to the findings in some previous studies,15-17 however, our analysis suggested that women had a higher adjusted odds of regular aspirin use compared with men. This result should be interpreted with extreme caution, however, because so few females (9 of 464 [3%]) met the current USPSTF criteria for aspirin therapy for primary CVD prevention. The previous USPSTF guidelines24,25 were less conservative, with a lower minimum age and threshold for CVD risk for women. The revision is the likely result of recent primary prevention trials10 that found regular aspirin use provided less cardioprotection for younger women.
The sample without an aspirin indication—roughly twice the size of the group with an aspirin indication (563 vs 268), which is reflective of the general population of Wisconsin—was useful in highlighting inappropriate use. There were clear indications of aspirin overuse in this group, with 18% of the sample reporting that they took aspirin regularly. The finding that inappropriate aspirin use was more likely in non-Hispanic whites vs minorities is similar to the result of an earlier study in which blacks, Hispanics, and Chinese Americans with low CVD risk were much less likely to report regular aspirin use compared with whites at low risk.15
The main concern with regular aspirin use in those for whom it is not indicated for primary CVD prevention is the risk of upper gastrointestinal bleeding and, less commonly, hemorrhagic stroke.26 To illustrate this point, consider the following: About 10% of SHOW participants ages 35 to 74 years had no history of CVD and no indication for aspirin therapy based on the latest USPSTF guidelines, but took aspirin regularly nonetheless. Extrapolating those numbers to the entire state of Wisconsin would suggest that approximately 270,000 state residents have a similar profile. Assuming an extra 1.3 major bleeding events per 1000 person-years of regular aspirin use (as a meta-analysis of studies of adverse events associated with antiplatelet therapy found),27 that would translate into an estimated 350 major bleeding events per year in Wisconsin that are attributable to aspirin overuse.
In view of the current USPSTF recommendations,10 aspirin is not optimally utilized by Wisconsin residents for the primary prevention of CVD. Aspirin therapy is not used enough by those with a high CVD risk, who could derive substantial vascular disease protection from it. Conversely, aspirin therapy is overused by those with a low CVD risk, for whom the risk of major bleeding is significantly higher than the potential for vascular disease protection. Furthermore, younger individuals at high CVD risk appear to be least likely to take aspirin regularly.
Recommendations
The strongest modifiable predictor of regular aspirin use is a recommendation from a clinician.13 Therefore, we recommend stronger primary care initiatives to ensure that patients are screened for aspirin use more frequently, particularly middle-aged men at high CVD risk. This clinic-based initiative could reach a larger proportion of the general population when combined with broader, community-oriented CVD preventive services.28
More precise marketing and education are also needed. Because aspirin is a low-cost over-the-counter product that leads the consumer market for analgesics,29 the general public (and older, non-Hispanic whites, in particular) needs to be better informed about the risks of medically inappropriate aspirin use for primary CVD prevention.
Study limitations
Selection and measurement biases were among the chief study limitations.
Study (SHOW) enrollment rate was slightly above 50%, with steady increases in enrollment each year (from 46% in 2008-2009 to 56% in 2010) due to expanded recruitment and consolidation of field operations.
Aspirin use was self-reported, and SHOW did not capture the reason for taking it (eg, CVD prevention or pain management). Some evidence of overreporting of aspirin use among older individuals exists,30 suggesting that a more objective measure of aspirin use (eg, pill bottle verification or blood platelet aggregation test) could yield different results.
Certain confounders were not measured, most notably contraindications to aspirin (eg, genetic platelet abnormalities). Such findings could explain some patterns of aspirin use in both strata, as up to 10% of any given population has a contraindication to aspirin due to allergy, intolerance, gastrointestinal ulcer, concomitant anticoagulant medication, or other high bleeding risk.18,31 Few of these variables were known about our sample.
TABLE 4W (available below) provides a breakdown of some possible aspirin contraindications, as well as possible reasons other than primary CVD prevention for regular aspirin use. Because clinical judgment is often required to assess the degree of severity of a given health condition in order to deem it an aspirin contraindication, these findings could not reliably be used to reclassify participants. We present them simply for hypothesis generation.
Some data collection predates the current USPSTF guidelines,10 which could have resulted in a misclassification of participants’ aspirin indication. However, sensitivity analyses restricted to the 2010 sample alone—the only one with data collection after the newer guidelines were released—did not reveal any meaningful differences.
Other methodological limitations include the less racially diverse population of Wisconsin compared with other parts of the country and the sample size, which did not permit testing for statistical interactions and perhaps resulted in larger confidence intervals for some associations (eg, race/ethnicity) relative to the population as a whole.
TABLE 4W
Possible reasons for aspirin use—or contraindication— by aspirin indication*
| Has a doctor or other health professional ever told you that you had … | Aspirin indicated (n=268) | Aspirin not indicated (n=563) | ||
|---|---|---|---|---|
| Regular aspirin user (n=83) | Nonregular aspirin user (n=185) | Regular aspirin user (n=102) | Nonregular aspirin user (n=461) | |
| Migraine headache Yes No | 20 (24%) 63 (76%) | 28 (15%) 157 (85%) | 24 (24%) 78 (76%) | 76 (16%) 385 (84%) |
| Arthritis† Yes No | 2 (2%) 81 (98%) | 1 (1%) 184 (99%) | 12 (12%) 90 (88%) | 26 (6%) 435 (94%) |
| Stomach or intestinal ulcer Yes No | 5 (6%) 78 (94%) | 6 (3%) 179 (97%) | 7 (7%) 95 (93%) | 10 (2%) 451 (98%) |
| Reflux or GERD Yes No | 8 (10%) 75 (90%) | 14 (8%) 171 (92%) | 11 (11%) 91 (89%) | 32 (7%) 429 (93%) |
| Values presented as n (%). *Data not included in study analysis. †Osteoarthritis or rheumatoid arthritis. GERD, gastric esophageal reflux disease. | ||||
Acknowledgement
The authors thank Matt Walsh, PhD, for his assistance in creating the analytical dataset, as well as Sally Steward-Townsend, Susan Wright, Bri Deyo, Bethany Varley, and the rest of the Survey of the Health of Wisconsin staff.
CORRESPONDENCE Jeffrey J. VanWormer, PhD, Epidemiology Research Center, Marshfield Clinic Research Foundation, 1000 North Oak Avenue, Marshfield, WI 54449; [email protected]
1. Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18-e209.
2. Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933-944.
3. Sullivan PW, Ghushchyan V, Wyatt HR. The medical cost of cardiometabolic risk factor clusters in the United States. Obesity. 2007;15:3150-3158.
4. Pearson TA, Blair SN, Daniels SR, et al. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106:388-391.
5. Kriekard P, Gharacholou SM, Peterson ED. Primary and secondary prevention of cardiovascular disease in older adults: a status report. Clin Geriatr Med. 2009;25:745-755.
6. Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in US deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356:2388-2398.
7. Hennekens CH, Schneider WR. The need for wider and appropriate utilization of aspirin and statins in the treatment and prevention of cardiovascular disease. Expert Rev Cardiovasc Ther. 2008;6:95-107.
8. Barnett H, Burrill P, Iheanacho I. Don’t use aspirin for primary prevention of cardiovascular disease. BMJ. 2010;340:c1805.-
9. Sanchez-Ross M, Waller AH, Maher J, et al. Aspirin for the prevention of cardiovascular morbidity. Minerva Med. 2010;101:205-214.
10. S. Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US preventive services task force recommendation statement. Ann Intern Med. 2009;150:396-404.
11. Bartolucci AA, Tendera M, Howard G. Meta-analysis of multiple primary prevention trials of cardiovascular events using aspirin. Am J Cardiol. 2011;107:1796-1801.
12. Maciosek MV, Coffield AB, Edwards NM, et al. Priorities among effective clinical preventive services: results of a systematic review and analysis. Am J Prev Med. 2006;31:52-61.
13. Pignone M, Anderson GK, Binns K, et al. Aspirin use among adults aged 40 and older in the United States results of a national survey. Am J Prev Med. 2007;32:403-407.
14. Ajani UA, Ford ES, Greenland KJ, et al. Aspirin use among US adults: behavioral risk factor surveillance system. Am J Prev Med. 2006;30:74-77.
15. Sanchez DR, Diez Roux AV, Michos ED, et al. Comparison of the racial/ethnic prevalence of regular aspirin use for the primary prevention of coronary heart disease from the multi-ethnic study of atherosclerosis. Am J Cardiol. 2011;107:41-46.
16. Stafford RS, Monti V, Ma J. Underutilization of aspirin persists in US ambulatory care for the secondary and primary prevention of cardiovascular disease. PLoS Med. 2005;2:e353.-
17. Rodondi N, Vittinghoff E, Cornuz J, et al. Aspirin use for the primary prevention of coronary heart disease in older adults. Am J Med. 2005;118(suppl):1288e1-1288e9.
18. Rodondi N, Cornuz J, Marques-Vidal P, et al. Aspirin use for the primary prevention of coronary heart disease: a population-based study in Switzerland. Prev Med. 2008;46:137-144.
19. Nieto FJ, Peppard PE, Engelman CD, et al. The Survey of the Health of Wisconsin (SHOW), a novel infrastructure for population health research: rationale and methods. BMC Public Health. 2010;10:785.-
20. D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743-753.
21. Cody RP, Smith JK. Applied Statistics and the SAS Programming Language. New York, NY: Prentice Hall; 2005.
22. Mallonee S, Daniels CG, Mold JW, et al. Increasing aspirin use among persons at risk for cardiovascular events in Oklahoma. J Okla State Med Assoc. 2010;103:254-260.
23. Centers for Disease Control and Prevention (CDC). Prevalence of aspirin use to prevent heart disease—Wisconsin, 1991, and Michigan, 1994. MMWR Morb Mortal Wkly Rep. 1997;46:498-502.
24. US Preventive Services Task Force. Aspirin for the primary prevention of cardiovascular events: recommendation and rationale. Ann Intern Med. 2002;136:157-160.
25. Werner M, Kelsberg G, Weismantel AM. Which healthy adults should take aspirin? J Fam Pract. 2004;53:146-150.
26. Berger JS, Roncaglioni MC, Avanzini F, et al. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295:306-313.
27. McQuaid KR, Laine L. Systematic review and meta-analysis of adverse events of low-dose aspirin and clopidogrel in randomized controlled trials. Am J Med. 2006;119:624-638.
28. VanWormer JJ, Johnson PJ, Pereira RF, et al. The Heart of New Ulm project: using community-based cardiometabolic risk factor screenings in a rural population health improvement initiative. Popul Health Manag. 2012;15:135-143.
29. Jeffreys D. Aspirin: The Remarkable Story of a Wonder Drug. New York, NY: Bloomsbury Publishing; 2005.
30. Smith NL, Psaty BM, Heckbert SR, et al. The reliability of medication inventory methods compared to serum levels of cardiovascular drugs in the elderly. J Clin Epidemiol. 1999;52:143-146.
31. Hedman J, Kaprio J, Poussa T, et al. Prevalence of asthma, aspirin intolerance, nasal polyposis and chronic obstructive pulmonary disease in a population-based study. Int J Epidemiol. 1999;28:717-722.
Purpose Aspirin is recommended for the primary prevention of cardiovascular disease (CVD) in adults at high risk, but little is known about sociodemographic disparities in prophylactic aspirin use. This study examined the association between sociodemographic characteristics and regular aspirin use among adults in Wisconsin who are free of CVD.
Methods A cross-sectional design was used, and data collected from 2008 to 2010. Regular aspirin use (aspirin therapy) was defined as taking aspirin most days of the week. We found 831 individuals for whom complete data were available for regression analyses and stratified the sample into 2 groups: those for whom aspirin therapy was indicated and those for whom it was not indicated, based on national guidelines.
Results Of the 268 patients for whom aspirin therapy was indicated, only 83 (31%) were using it regularly, and 102 (18%) of the 563 participants who did not have an aspirin indication were taking it regularly. In the group with an aspirin indication, participants who were older had higher rates of regular aspirin use than younger patients (odds ratio [OR]=1.07; P<.001), and women had significantly higher adjusted odds of regular aspirin use than men (OR=3.49; P=.021). Among those for whom aspirin therapy was not indicated, the adjusted odds of regular aspirin use were significantly higher among older participants (OR=1.07; P<.001) vs their younger counterparts, and significantly lower among Hispanic or nonwhite participants (OR=0.32; P=.063) relative to non-Hispanic whites.
Conclusions Aspirin therapy is underused by those at high risk for CVD—individuals who could gain cardioprotection from regular use—and overused by those at low risk for CVD, for whom the risk of major bleeding outweighs the potential benefit. Stronger primary care initiatives may be needed to ensure that patients undergo regular screening for aspirin use, particularly middle-aged men at high CVD risk. Patient education may be needed, as well, to better inform people (particularly older, non-Hispanic whites) about the risks of regular aspirin use that is not medically indicated.
Cardiovascular disease (CVD) is the principal cause of death in the United States.1 As the population grows older and obesity and diabetes become increasingly prevalent, the prevalence of CVD is also expected to rise.2,3 Fortunately, many CVD events can be prevented or delayed by modifying risk factors such as hyperlipidemia, hypertension, and smoking. Interventions associated with a reduction in risk have led to a reduction in CVD events4,5 and contributed to a steady decline in cardiac deaths.6
Control of platelet aggregation is a cornerstone of primary CVD prevention.7 In an outpatient setting, this usually translates into identifying patients who are at high risk for a CVD event and advising them to take low-dose aspirin daily or every other day. Although not without controversy,8,9 the US Preventive Services Task Force (USPSTF) recommends regular aspirin use for primary CVD prevention for middle-aged to older men at high risk for myocardial infarction (MI) and women at high risk for ischemic stroke.10
The efficacy of this intervention is proven: In primary prevention trials, regular aspirin use is associated with a 14% reduction in the likelihood of CVD events over 7 years.11 What’s more, aspirin therapy, as recommended by the USPSTF, is among the most cost-effective clinic-based preventive measures.12
In 2004, 41% of US adults age 40 or older reported taking aspirin regularly13 —an increase of approximately 20% since 1999.14 More recent data from a national population-based cohort study found that 41% of adults ages 45 to 90 years who did not have CVD but were at moderate to high risk for a CVD event reported taking aspirin ≥3 days per week.15 In the same study, almost one-fourth of those at low CVD risk also reported regular aspirin use.
While regular aspirin use for primary CVD prevention has been on the rise,13,14 the extent to which this intervention has penetrated various segments of the population is unclear. Several studies have found that aspirin use is consistently highest among those who are older, male, and white.15-17 Other socioeconomic variables (eg, education level, employment, marital status) have received little attention. And no previous study has used national guidelines for aspirin therapy to stratify samples.
A look at overuse and underuse. To ensure that aspirin therapy for primary CVD prevention is directed at those who are most likely to benefit from it, a better understanding of variables associated with both aspirin overuse and underuse is needed. This area of research is important, in part because direct-to-consumer aspirin marketing may be particularly influential among groups at low risk for CVD—for whom the risk of excess bleeding outweighs the potential for disease prevention.13,18
This study was undertaken to examine the association between specific sociodemographic variables and aspirin use among a representative sample of Wisconsin adults without CVD, looking both at those for whom aspirin therapy is indicated and those for whom it is not indicated based on national guidelines.
Methods
Design
We used a cross-sectional design, with data from the Survey of the Health of Wisconsin (SHOW),19 an annual survey of Wisconsin residents ages 21 to 74 years. SHOW uses a 2-stage stratified cluster sampling design to select households, with all age-eligible household members invited to participate. Recruitment for the annual survey consists of general community-wide announcements, as well as an initial letter and up to 6 visits to the randomly selected households to encourage participation.
By the end of 2010, SHOW had 1572 enrollees—about 53% of all eligible invitees. The demographic profile of SHOW enrollees was similar to US census data for all Wisconsin adults during the same time frame.19 All SHOW procedures were approved by the University of Wisconsin Institutional Review Board, and all participants provided informed consent.
Study sample
Our analyses were based on data provided by SHOW participants who were screened and enrolled between 2008 and 2010. To be included in our study, participants had to be between the ages of 35 and 74 years; not pregnant, on active military duty, or institutionalized; and have no personal history of CVD (myocardial infarction, angina, stroke, or transient ischemic attack) or CVD risk equivalent (type 1 or type 2 diabetes) at the time of recruitment. Data on key study variables had to be available, as well. (We used 35 years as the lower age limit because of the very low likelihood of CVD in younger individuals.)
We stratified the analytical sample (N=831) into 2 groups—participants for whom aspirin therapy was indicated and those for whom it was not indicated—in order to examine aspirin’s appropriate (recommended) and inappropriate use.
Measures
Outcome. The outcome variable was aspirin use. SHOW had asked participants how often they took aspirin. Similar to the methods used by Sanchez et al,15 we classified those who reported taking aspirin most (≥4) days of the week as regular aspirin users. All others were classified as nonregular aspirin users. Participants were not asked about the daily dose or weekly volume of aspirin.
Variables
Sociodemographic variables considered in our analysis were age, sex, race/ethnicity, education level, marital/partner status, employment status, health insurance, and region of residence within Wisconsin.
All participants underwent physical examinations, conducted as part of SHOW, at either a permanent or mobile exam center. Blood pressure was measured after a 5-minute rest period in a seated position, and the average of the last 2 out of 3 consecutive measurements was reported. Body mass index (BMI) was calculated, and blood samples were obtained by venipuncture, processed immediately, and sent to the Marshfield Clinic laboratory for measuring total and high-density lipoprotein (HDL) cholesterol.
Indications for aspirin therapy. We stratified the sample by those who were and those who were not candidates for aspirin therapy for primary CVD prevention based on the latest guidelines from the USPSTF ( FIGURE ).10 The Task Force recommends aspirin therapy for men ages 45 to 74 years with a moderate or greater 10-year risk of a coronary heart disease (CHD) event and women ages 55 to 74 years with a moderate or greater 10-year risk of stroke. We used the global CVD risk equation derived from the Framingham Heart Study (based on age, sex, smoking status, systolic blood pressure, and total and HDL cholesterol) to calculate each participant’s 10-year risk and, thus, determine whether aspirin therapy was or was not indicated.20 Total and HDL cholesterol values were missing for 94 participants in the analytical sample; their 10-year CVD risk was estimated using BMI, a reasonable alternative to more conventional CVD risk prediction when laboratory values are unavailable.21
FIGURE
Study (SHOW) sample, stratified based on aspirin indication10
*US Preventive Services Task Force guidelines were slightly modified for this analysis: The upper age bound was reduced from 79 to 74 years because the Survey of the Health of Wisconsin did not enroll participants >74 years.
CHD, coronary heart disease; CVD, cardiovascular disease; DM, diabetes mellitus; N/A, not applicable; SHOW, Survey of the Health of Wisconsin.
Statistical analyses
All analytical procedures were conducted using Statistical Analysis Software (SAS Version 9.2; Cary, NC). A complete-case framework was used.
We used multivariate logistic regression for survey data (PROC SURVEYLOGISTIC; SAS Institute, Cary, NC) to examine the association between aspirin use and sociodemographic variables. Two separate analyses were conducted, one of participants for whom aspirin therapy was indicated and the other for participants for whom it was not. The outcomes were modeled dichotomously, as regular vs nonregular aspirin users, and a collinearity check was done. 21
Initially, we created univariate models to gauge the crude relationship between each variable and aspirin use. Any variable with P<.20 in its univariate association with regular aspirin use was considered for inclusion in the final multivariate regression model. In the multivariate analyses, we sequentially eliminated variables with the weakest association with aspirin use until only significant (P<.10) independent predictors remained. Appropriate weighting was applied based on survey strata and cluster structure.19
Results
Of the 831 participants who met the eligibility criteria for our analysis, 268 (32%) had an aspirin indication. TABLE 1 shows the key characteristics of the analytical sample, stratified by those for whom aspirin was indicated and those for whom it was not. The sample was primarily middle-aged (mean age 52.4±0.36) and non-Hispanic white (93%). Compared with those for whom aspirin therapy was not indicated, the group with an aspirin indication was significantly older (56.9 vs 50.3) and had a significantly higher proportion of males (97% vs 19%). As expected, those for whom aspirin was indicated were also at higher risk for CHD and stroke, most notably as a result of significantly higher systolic BP (131.9 vs 121.5 mm Hg) and lower HDL cholesterol (42.5 vs 52.6 mg/dL) compared with participants without an aspirin indication.
TABLE 1
Study sample, by sociodemographic variable and aspirin indication
| Variable | Full sample (N=831) | Aspirin indicated (n=268) | Aspirin not indicated (n=563) |
|---|---|---|---|
| Mean age, y | 52.4 | 56.9 | 50.3 |
| Sex, n Male Female | 367 464 | 259 9 | 108 455 |
| Race/ethnicity, n White, non-Hispanic Nonwhite/Hispanic | 776 55 | 252 16 | 524 39 |
| Marital status, n Married/partnered Not married or partnered | 637 194 | 215 53 | 422 141 |
| Health insurance, n Uninsured Insured | 76 755 | 26 242 | 50 513 |
| Education, n ≤High school Associate’s degree ≥Bachelor’s degree | 217 312 302 | 77 107 84 | 140 205 218 |
| Employment, n Unemployed Student/retiree/home Employed | 98 147 586 | 33 52 183 | 65 95 403 |
When aspirin was indicated, use was linked to age and sex
In the group with an aspirin indication (n=268), 83 (31%) reported taking aspirin most days of the week. The initial examination of sociodemographic variables showed that age, sex, and employment status demonstrated significant univariate associations with regular aspirin use ( TABLE 2 ). In the multivariate model, however, the odds of regular aspirin use were significantly greater among participants who were older (odds ratio [OR], 1.07; P<.001) or female (OR, 3.49; P=.021) compared with participants who were younger or male, respectively.
TABLE 2
Participants who have an aspirin indication: Association between sociodemographic variables and regular aspirin use
| Variable | Regular aspirin use, OR (95% CI) | P value* |
|---|---|---|
| Age Older vs younger | 1.07 (1.04-1.11) | .001 |
| Sex Female vs male | 3.89 (1.42-10.67)† | .008 |
| Race/ethnicity Nonwhite/Hispanic vs white non-Hispanic | 0.55 (0.09-3.47) | .526 |
| Marital status Not married/partnered vs married/partner | 0.83 (0.36-1.95) | .678 |
| Health insurance Uninsured vs insured | 0.86 (0.50-1.47) | .579 |
| Education ≥Bachelor’s degree vs ≤high school Associate’s degree/some college vs ≤high school | 1.58 (0.75-3.34) 1.36 (0.74-2.49) | .234 .325 |
| Employment Student or retired vs employed Unemployed vs employed | 2.96 (1.74-5.03) 0.62 (0.25-1.56) | .001 .314 |
| *Significance was defined as P<.10. †Multivariate adjusted model: 3.49 (95% CI, 1.21-10.07; P=.021). CI, confidence interval; OR, odds ratio. | ||
When aspirin was not indicated, age and sex still affected use
Among the 563 participants for whom aspirin therapy was not indicated, 102 (18%) reported taking aspirin regularly. Age, sex, race/ethnicity, health insurance, and employment ( TABLE 3 ), as well as region of residence and study enrollment year, had significant univariate associations with regular aspirin use; these variables were tested for potential inclusion in the multivariate model. In the final multivariate regression model, the odds of regular aspirin use were significantly greater among participants who were older (OR, 1.07; P<.001) and significantly lower among participants who were Hispanic or nonwhite (OR, 0.32; P=.063).
TABLE 3
Participants who do not have an aspirin indication: Association between sociodemographic variables and regular aspirin use
| Variable | Regular aspirin use, OR (95% CI) | P value* |
|---|---|---|
| Age Older vs younger | 1.07 (1.04-1.10) | .001 |
| Sex Female vs male | 1.60 (0.84-3.04) | .152 |
| Race/ethnicity Nonwhite or Hispanic vs white non-Hispanic | 0.23 (0.07- 0.73)† | .013 |
| Marital status Not married/partnered vs married/partnered | 1.00 (0.63-1.59) | .992 |
| Health insurance Uninsured vs insured | 0.36 (0.11- 1.15) | .086 |
| Education Bachelor’s or higher vs high school or less Associate’s/some college vs high school or less | 0.74 (0.35-1.57) 0.67 (0.38-1.17) | .431 .158 |
| Employment Student/retired vs employed Unemployed vs employed | 2.35 (1.32-4.20) 0.78 (0.26- 2.34) | .004 .652 |
| *Significance was defined as P<.10. †Multivariate adjusted model: 0.32 (95% CI, 0.10-1.06; P=.063). CI, confidence interval; OR, odds ratio. | ||
Discussion
Aspirin was generally underutilized in the group with significant CVD risk (n=268) in our study, with slightly less than a third of participants for whom aspirin therapy was indicated taking it most days of the week. Despite trends of increased aspirin use among US adults in recent years,15 aspirin therapy in the 2008-2010 SHOW sample was lower than in 2005 to 2008. It was also lower than national estimates of aspirin use for primary CVD prevention15,22 —but about 20% higher than estimates of overall aspirin use in Wisconsin 20 years ago.23 Consistent with previous research, the final adjusted model and sensitivity analysis indicated that older individuals were more likely to take aspirin regularly.
Contrary to the findings in some previous studies,15-17 however, our analysis suggested that women had a higher adjusted odds of regular aspirin use compared with men. This result should be interpreted with extreme caution, however, because so few females (9 of 464 [3%]) met the current USPSTF criteria for aspirin therapy for primary CVD prevention. The previous USPSTF guidelines24,25 were less conservative, with a lower minimum age and threshold for CVD risk for women. The revision is the likely result of recent primary prevention trials10 that found regular aspirin use provided less cardioprotection for younger women.
The sample without an aspirin indication—roughly twice the size of the group with an aspirin indication (563 vs 268), which is reflective of the general population of Wisconsin—was useful in highlighting inappropriate use. There were clear indications of aspirin overuse in this group, with 18% of the sample reporting that they took aspirin regularly. The finding that inappropriate aspirin use was more likely in non-Hispanic whites vs minorities is similar to the result of an earlier study in which blacks, Hispanics, and Chinese Americans with low CVD risk were much less likely to report regular aspirin use compared with whites at low risk.15
The main concern with regular aspirin use in those for whom it is not indicated for primary CVD prevention is the risk of upper gastrointestinal bleeding and, less commonly, hemorrhagic stroke.26 To illustrate this point, consider the following: About 10% of SHOW participants ages 35 to 74 years had no history of CVD and no indication for aspirin therapy based on the latest USPSTF guidelines, but took aspirin regularly nonetheless. Extrapolating those numbers to the entire state of Wisconsin would suggest that approximately 270,000 state residents have a similar profile. Assuming an extra 1.3 major bleeding events per 1000 person-years of regular aspirin use (as a meta-analysis of studies of adverse events associated with antiplatelet therapy found),27 that would translate into an estimated 350 major bleeding events per year in Wisconsin that are attributable to aspirin overuse.
In view of the current USPSTF recommendations,10 aspirin is not optimally utilized by Wisconsin residents for the primary prevention of CVD. Aspirin therapy is not used enough by those with a high CVD risk, who could derive substantial vascular disease protection from it. Conversely, aspirin therapy is overused by those with a low CVD risk, for whom the risk of major bleeding is significantly higher than the potential for vascular disease protection. Furthermore, younger individuals at high CVD risk appear to be least likely to take aspirin regularly.
Recommendations
The strongest modifiable predictor of regular aspirin use is a recommendation from a clinician.13 Therefore, we recommend stronger primary care initiatives to ensure that patients are screened for aspirin use more frequently, particularly middle-aged men at high CVD risk. This clinic-based initiative could reach a larger proportion of the general population when combined with broader, community-oriented CVD preventive services.28
More precise marketing and education are also needed. Because aspirin is a low-cost over-the-counter product that leads the consumer market for analgesics,29 the general public (and older, non-Hispanic whites, in particular) needs to be better informed about the risks of medically inappropriate aspirin use for primary CVD prevention.
Study limitations
Selection and measurement biases were among the chief study limitations.
Study (SHOW) enrollment rate was slightly above 50%, with steady increases in enrollment each year (from 46% in 2008-2009 to 56% in 2010) due to expanded recruitment and consolidation of field operations.
Aspirin use was self-reported, and SHOW did not capture the reason for taking it (eg, CVD prevention or pain management). Some evidence of overreporting of aspirin use among older individuals exists,30 suggesting that a more objective measure of aspirin use (eg, pill bottle verification or blood platelet aggregation test) could yield different results.
Certain confounders were not measured, most notably contraindications to aspirin (eg, genetic platelet abnormalities). Such findings could explain some patterns of aspirin use in both strata, as up to 10% of any given population has a contraindication to aspirin due to allergy, intolerance, gastrointestinal ulcer, concomitant anticoagulant medication, or other high bleeding risk.18,31 Few of these variables were known about our sample.
TABLE 4W (available below) provides a breakdown of some possible aspirin contraindications, as well as possible reasons other than primary CVD prevention for regular aspirin use. Because clinical judgment is often required to assess the degree of severity of a given health condition in order to deem it an aspirin contraindication, these findings could not reliably be used to reclassify participants. We present them simply for hypothesis generation.
Some data collection predates the current USPSTF guidelines,10 which could have resulted in a misclassification of participants’ aspirin indication. However, sensitivity analyses restricted to the 2010 sample alone—the only one with data collection after the newer guidelines were released—did not reveal any meaningful differences.
Other methodological limitations include the less racially diverse population of Wisconsin compared with other parts of the country and the sample size, which did not permit testing for statistical interactions and perhaps resulted in larger confidence intervals for some associations (eg, race/ethnicity) relative to the population as a whole.
TABLE 4W
Possible reasons for aspirin use—or contraindication— by aspirin indication*
| Has a doctor or other health professional ever told you that you had … | Aspirin indicated (n=268) | Aspirin not indicated (n=563) | ||
|---|---|---|---|---|
| Regular aspirin user (n=83) | Nonregular aspirin user (n=185) | Regular aspirin user (n=102) | Nonregular aspirin user (n=461) | |
| Migraine headache Yes No | 20 (24%) 63 (76%) | 28 (15%) 157 (85%) | 24 (24%) 78 (76%) | 76 (16%) 385 (84%) |
| Arthritis† Yes No | 2 (2%) 81 (98%) | 1 (1%) 184 (99%) | 12 (12%) 90 (88%) | 26 (6%) 435 (94%) |
| Stomach or intestinal ulcer Yes No | 5 (6%) 78 (94%) | 6 (3%) 179 (97%) | 7 (7%) 95 (93%) | 10 (2%) 451 (98%) |
| Reflux or GERD Yes No | 8 (10%) 75 (90%) | 14 (8%) 171 (92%) | 11 (11%) 91 (89%) | 32 (7%) 429 (93%) |
| Values presented as n (%). *Data not included in study analysis. †Osteoarthritis or rheumatoid arthritis. GERD, gastric esophageal reflux disease. | ||||
Acknowledgement
The authors thank Matt Walsh, PhD, for his assistance in creating the analytical dataset, as well as Sally Steward-Townsend, Susan Wright, Bri Deyo, Bethany Varley, and the rest of the Survey of the Health of Wisconsin staff.
CORRESPONDENCE Jeffrey J. VanWormer, PhD, Epidemiology Research Center, Marshfield Clinic Research Foundation, 1000 North Oak Avenue, Marshfield, WI 54449; [email protected]
Purpose Aspirin is recommended for the primary prevention of cardiovascular disease (CVD) in adults at high risk, but little is known about sociodemographic disparities in prophylactic aspirin use. This study examined the association between sociodemographic characteristics and regular aspirin use among adults in Wisconsin who are free of CVD.
Methods A cross-sectional design was used, and data collected from 2008 to 2010. Regular aspirin use (aspirin therapy) was defined as taking aspirin most days of the week. We found 831 individuals for whom complete data were available for regression analyses and stratified the sample into 2 groups: those for whom aspirin therapy was indicated and those for whom it was not indicated, based on national guidelines.
Results Of the 268 patients for whom aspirin therapy was indicated, only 83 (31%) were using it regularly, and 102 (18%) of the 563 participants who did not have an aspirin indication were taking it regularly. In the group with an aspirin indication, participants who were older had higher rates of regular aspirin use than younger patients (odds ratio [OR]=1.07; P<.001), and women had significantly higher adjusted odds of regular aspirin use than men (OR=3.49; P=.021). Among those for whom aspirin therapy was not indicated, the adjusted odds of regular aspirin use were significantly higher among older participants (OR=1.07; P<.001) vs their younger counterparts, and significantly lower among Hispanic or nonwhite participants (OR=0.32; P=.063) relative to non-Hispanic whites.
Conclusions Aspirin therapy is underused by those at high risk for CVD—individuals who could gain cardioprotection from regular use—and overused by those at low risk for CVD, for whom the risk of major bleeding outweighs the potential benefit. Stronger primary care initiatives may be needed to ensure that patients undergo regular screening for aspirin use, particularly middle-aged men at high CVD risk. Patient education may be needed, as well, to better inform people (particularly older, non-Hispanic whites) about the risks of regular aspirin use that is not medically indicated.
Cardiovascular disease (CVD) is the principal cause of death in the United States.1 As the population grows older and obesity and diabetes become increasingly prevalent, the prevalence of CVD is also expected to rise.2,3 Fortunately, many CVD events can be prevented or delayed by modifying risk factors such as hyperlipidemia, hypertension, and smoking. Interventions associated with a reduction in risk have led to a reduction in CVD events4,5 and contributed to a steady decline in cardiac deaths.6
Control of platelet aggregation is a cornerstone of primary CVD prevention.7 In an outpatient setting, this usually translates into identifying patients who are at high risk for a CVD event and advising them to take low-dose aspirin daily or every other day. Although not without controversy,8,9 the US Preventive Services Task Force (USPSTF) recommends regular aspirin use for primary CVD prevention for middle-aged to older men at high risk for myocardial infarction (MI) and women at high risk for ischemic stroke.10
The efficacy of this intervention is proven: In primary prevention trials, regular aspirin use is associated with a 14% reduction in the likelihood of CVD events over 7 years.11 What’s more, aspirin therapy, as recommended by the USPSTF, is among the most cost-effective clinic-based preventive measures.12
In 2004, 41% of US adults age 40 or older reported taking aspirin regularly13 —an increase of approximately 20% since 1999.14 More recent data from a national population-based cohort study found that 41% of adults ages 45 to 90 years who did not have CVD but were at moderate to high risk for a CVD event reported taking aspirin ≥3 days per week.15 In the same study, almost one-fourth of those at low CVD risk also reported regular aspirin use.
While regular aspirin use for primary CVD prevention has been on the rise,13,14 the extent to which this intervention has penetrated various segments of the population is unclear. Several studies have found that aspirin use is consistently highest among those who are older, male, and white.15-17 Other socioeconomic variables (eg, education level, employment, marital status) have received little attention. And no previous study has used national guidelines for aspirin therapy to stratify samples.
A look at overuse and underuse. To ensure that aspirin therapy for primary CVD prevention is directed at those who are most likely to benefit from it, a better understanding of variables associated with both aspirin overuse and underuse is needed. This area of research is important, in part because direct-to-consumer aspirin marketing may be particularly influential among groups at low risk for CVD—for whom the risk of excess bleeding outweighs the potential for disease prevention.13,18
This study was undertaken to examine the association between specific sociodemographic variables and aspirin use among a representative sample of Wisconsin adults without CVD, looking both at those for whom aspirin therapy is indicated and those for whom it is not indicated based on national guidelines.
Methods
Design
We used a cross-sectional design, with data from the Survey of the Health of Wisconsin (SHOW),19 an annual survey of Wisconsin residents ages 21 to 74 years. SHOW uses a 2-stage stratified cluster sampling design to select households, with all age-eligible household members invited to participate. Recruitment for the annual survey consists of general community-wide announcements, as well as an initial letter and up to 6 visits to the randomly selected households to encourage participation.
By the end of 2010, SHOW had 1572 enrollees—about 53% of all eligible invitees. The demographic profile of SHOW enrollees was similar to US census data for all Wisconsin adults during the same time frame.19 All SHOW procedures were approved by the University of Wisconsin Institutional Review Board, and all participants provided informed consent.
Study sample
Our analyses were based on data provided by SHOW participants who were screened and enrolled between 2008 and 2010. To be included in our study, participants had to be between the ages of 35 and 74 years; not pregnant, on active military duty, or institutionalized; and have no personal history of CVD (myocardial infarction, angina, stroke, or transient ischemic attack) or CVD risk equivalent (type 1 or type 2 diabetes) at the time of recruitment. Data on key study variables had to be available, as well. (We used 35 years as the lower age limit because of the very low likelihood of CVD in younger individuals.)
We stratified the analytical sample (N=831) into 2 groups—participants for whom aspirin therapy was indicated and those for whom it was not indicated—in order to examine aspirin’s appropriate (recommended) and inappropriate use.
Measures
Outcome. The outcome variable was aspirin use. SHOW had asked participants how often they took aspirin. Similar to the methods used by Sanchez et al,15 we classified those who reported taking aspirin most (≥4) days of the week as regular aspirin users. All others were classified as nonregular aspirin users. Participants were not asked about the daily dose or weekly volume of aspirin.
Variables
Sociodemographic variables considered in our analysis were age, sex, race/ethnicity, education level, marital/partner status, employment status, health insurance, and region of residence within Wisconsin.
All participants underwent physical examinations, conducted as part of SHOW, at either a permanent or mobile exam center. Blood pressure was measured after a 5-minute rest period in a seated position, and the average of the last 2 out of 3 consecutive measurements was reported. Body mass index (BMI) was calculated, and blood samples were obtained by venipuncture, processed immediately, and sent to the Marshfield Clinic laboratory for measuring total and high-density lipoprotein (HDL) cholesterol.
Indications for aspirin therapy. We stratified the sample by those who were and those who were not candidates for aspirin therapy for primary CVD prevention based on the latest guidelines from the USPSTF ( FIGURE ).10 The Task Force recommends aspirin therapy for men ages 45 to 74 years with a moderate or greater 10-year risk of a coronary heart disease (CHD) event and women ages 55 to 74 years with a moderate or greater 10-year risk of stroke. We used the global CVD risk equation derived from the Framingham Heart Study (based on age, sex, smoking status, systolic blood pressure, and total and HDL cholesterol) to calculate each participant’s 10-year risk and, thus, determine whether aspirin therapy was or was not indicated.20 Total and HDL cholesterol values were missing for 94 participants in the analytical sample; their 10-year CVD risk was estimated using BMI, a reasonable alternative to more conventional CVD risk prediction when laboratory values are unavailable.21
FIGURE
Study (SHOW) sample, stratified based on aspirin indication10
*US Preventive Services Task Force guidelines were slightly modified for this analysis: The upper age bound was reduced from 79 to 74 years because the Survey of the Health of Wisconsin did not enroll participants >74 years.
CHD, coronary heart disease; CVD, cardiovascular disease; DM, diabetes mellitus; N/A, not applicable; SHOW, Survey of the Health of Wisconsin.
Statistical analyses
All analytical procedures were conducted using Statistical Analysis Software (SAS Version 9.2; Cary, NC). A complete-case framework was used.
We used multivariate logistic regression for survey data (PROC SURVEYLOGISTIC; SAS Institute, Cary, NC) to examine the association between aspirin use and sociodemographic variables. Two separate analyses were conducted, one of participants for whom aspirin therapy was indicated and the other for participants for whom it was not. The outcomes were modeled dichotomously, as regular vs nonregular aspirin users, and a collinearity check was done. 21
Initially, we created univariate models to gauge the crude relationship between each variable and aspirin use. Any variable with P<.20 in its univariate association with regular aspirin use was considered for inclusion in the final multivariate regression model. In the multivariate analyses, we sequentially eliminated variables with the weakest association with aspirin use until only significant (P<.10) independent predictors remained. Appropriate weighting was applied based on survey strata and cluster structure.19
Results
Of the 831 participants who met the eligibility criteria for our analysis, 268 (32%) had an aspirin indication. TABLE 1 shows the key characteristics of the analytical sample, stratified by those for whom aspirin was indicated and those for whom it was not. The sample was primarily middle-aged (mean age 52.4±0.36) and non-Hispanic white (93%). Compared with those for whom aspirin therapy was not indicated, the group with an aspirin indication was significantly older (56.9 vs 50.3) and had a significantly higher proportion of males (97% vs 19%). As expected, those for whom aspirin was indicated were also at higher risk for CHD and stroke, most notably as a result of significantly higher systolic BP (131.9 vs 121.5 mm Hg) and lower HDL cholesterol (42.5 vs 52.6 mg/dL) compared with participants without an aspirin indication.
TABLE 1
Study sample, by sociodemographic variable and aspirin indication
| Variable | Full sample (N=831) | Aspirin indicated (n=268) | Aspirin not indicated (n=563) |
|---|---|---|---|
| Mean age, y | 52.4 | 56.9 | 50.3 |
| Sex, n Male Female | 367 464 | 259 9 | 108 455 |
| Race/ethnicity, n White, non-Hispanic Nonwhite/Hispanic | 776 55 | 252 16 | 524 39 |
| Marital status, n Married/partnered Not married or partnered | 637 194 | 215 53 | 422 141 |
| Health insurance, n Uninsured Insured | 76 755 | 26 242 | 50 513 |
| Education, n ≤High school Associate’s degree ≥Bachelor’s degree | 217 312 302 | 77 107 84 | 140 205 218 |
| Employment, n Unemployed Student/retiree/home Employed | 98 147 586 | 33 52 183 | 65 95 403 |
When aspirin was indicated, use was linked to age and sex
In the group with an aspirin indication (n=268), 83 (31%) reported taking aspirin most days of the week. The initial examination of sociodemographic variables showed that age, sex, and employment status demonstrated significant univariate associations with regular aspirin use ( TABLE 2 ). In the multivariate model, however, the odds of regular aspirin use were significantly greater among participants who were older (odds ratio [OR], 1.07; P<.001) or female (OR, 3.49; P=.021) compared with participants who were younger or male, respectively.
TABLE 2
Participants who have an aspirin indication: Association between sociodemographic variables and regular aspirin use
| Variable | Regular aspirin use, OR (95% CI) | P value* |
|---|---|---|
| Age Older vs younger | 1.07 (1.04-1.11) | .001 |
| Sex Female vs male | 3.89 (1.42-10.67)† | .008 |
| Race/ethnicity Nonwhite/Hispanic vs white non-Hispanic | 0.55 (0.09-3.47) | .526 |
| Marital status Not married/partnered vs married/partner | 0.83 (0.36-1.95) | .678 |
| Health insurance Uninsured vs insured | 0.86 (0.50-1.47) | .579 |
| Education ≥Bachelor’s degree vs ≤high school Associate’s degree/some college vs ≤high school | 1.58 (0.75-3.34) 1.36 (0.74-2.49) | .234 .325 |
| Employment Student or retired vs employed Unemployed vs employed | 2.96 (1.74-5.03) 0.62 (0.25-1.56) | .001 .314 |
| *Significance was defined as P<.10. †Multivariate adjusted model: 3.49 (95% CI, 1.21-10.07; P=.021). CI, confidence interval; OR, odds ratio. | ||
When aspirin was not indicated, age and sex still affected use
Among the 563 participants for whom aspirin therapy was not indicated, 102 (18%) reported taking aspirin regularly. Age, sex, race/ethnicity, health insurance, and employment ( TABLE 3 ), as well as region of residence and study enrollment year, had significant univariate associations with regular aspirin use; these variables were tested for potential inclusion in the multivariate model. In the final multivariate regression model, the odds of regular aspirin use were significantly greater among participants who were older (OR, 1.07; P<.001) and significantly lower among participants who were Hispanic or nonwhite (OR, 0.32; P=.063).
TABLE 3
Participants who do not have an aspirin indication: Association between sociodemographic variables and regular aspirin use
| Variable | Regular aspirin use, OR (95% CI) | P value* |
|---|---|---|
| Age Older vs younger | 1.07 (1.04-1.10) | .001 |
| Sex Female vs male | 1.60 (0.84-3.04) | .152 |
| Race/ethnicity Nonwhite or Hispanic vs white non-Hispanic | 0.23 (0.07- 0.73)† | .013 |
| Marital status Not married/partnered vs married/partnered | 1.00 (0.63-1.59) | .992 |
| Health insurance Uninsured vs insured | 0.36 (0.11- 1.15) | .086 |
| Education Bachelor’s or higher vs high school or less Associate’s/some college vs high school or less | 0.74 (0.35-1.57) 0.67 (0.38-1.17) | .431 .158 |
| Employment Student/retired vs employed Unemployed vs employed | 2.35 (1.32-4.20) 0.78 (0.26- 2.34) | .004 .652 |
| *Significance was defined as P<.10. †Multivariate adjusted model: 0.32 (95% CI, 0.10-1.06; P=.063). CI, confidence interval; OR, odds ratio. | ||
Discussion
Aspirin was generally underutilized in the group with significant CVD risk (n=268) in our study, with slightly less than a third of participants for whom aspirin therapy was indicated taking it most days of the week. Despite trends of increased aspirin use among US adults in recent years,15 aspirin therapy in the 2008-2010 SHOW sample was lower than in 2005 to 2008. It was also lower than national estimates of aspirin use for primary CVD prevention15,22 —but about 20% higher than estimates of overall aspirin use in Wisconsin 20 years ago.23 Consistent with previous research, the final adjusted model and sensitivity analysis indicated that older individuals were more likely to take aspirin regularly.
Contrary to the findings in some previous studies,15-17 however, our analysis suggested that women had a higher adjusted odds of regular aspirin use compared with men. This result should be interpreted with extreme caution, however, because so few females (9 of 464 [3%]) met the current USPSTF criteria for aspirin therapy for primary CVD prevention. The previous USPSTF guidelines24,25 were less conservative, with a lower minimum age and threshold for CVD risk for women. The revision is the likely result of recent primary prevention trials10 that found regular aspirin use provided less cardioprotection for younger women.
The sample without an aspirin indication—roughly twice the size of the group with an aspirin indication (563 vs 268), which is reflective of the general population of Wisconsin—was useful in highlighting inappropriate use. There were clear indications of aspirin overuse in this group, with 18% of the sample reporting that they took aspirin regularly. The finding that inappropriate aspirin use was more likely in non-Hispanic whites vs minorities is similar to the result of an earlier study in which blacks, Hispanics, and Chinese Americans with low CVD risk were much less likely to report regular aspirin use compared with whites at low risk.15
The main concern with regular aspirin use in those for whom it is not indicated for primary CVD prevention is the risk of upper gastrointestinal bleeding and, less commonly, hemorrhagic stroke.26 To illustrate this point, consider the following: About 10% of SHOW participants ages 35 to 74 years had no history of CVD and no indication for aspirin therapy based on the latest USPSTF guidelines, but took aspirin regularly nonetheless. Extrapolating those numbers to the entire state of Wisconsin would suggest that approximately 270,000 state residents have a similar profile. Assuming an extra 1.3 major bleeding events per 1000 person-years of regular aspirin use (as a meta-analysis of studies of adverse events associated with antiplatelet therapy found),27 that would translate into an estimated 350 major bleeding events per year in Wisconsin that are attributable to aspirin overuse.
In view of the current USPSTF recommendations,10 aspirin is not optimally utilized by Wisconsin residents for the primary prevention of CVD. Aspirin therapy is not used enough by those with a high CVD risk, who could derive substantial vascular disease protection from it. Conversely, aspirin therapy is overused by those with a low CVD risk, for whom the risk of major bleeding is significantly higher than the potential for vascular disease protection. Furthermore, younger individuals at high CVD risk appear to be least likely to take aspirin regularly.
Recommendations
The strongest modifiable predictor of regular aspirin use is a recommendation from a clinician.13 Therefore, we recommend stronger primary care initiatives to ensure that patients are screened for aspirin use more frequently, particularly middle-aged men at high CVD risk. This clinic-based initiative could reach a larger proportion of the general population when combined with broader, community-oriented CVD preventive services.28
More precise marketing and education are also needed. Because aspirin is a low-cost over-the-counter product that leads the consumer market for analgesics,29 the general public (and older, non-Hispanic whites, in particular) needs to be better informed about the risks of medically inappropriate aspirin use for primary CVD prevention.
Study limitations
Selection and measurement biases were among the chief study limitations.
Study (SHOW) enrollment rate was slightly above 50%, with steady increases in enrollment each year (from 46% in 2008-2009 to 56% in 2010) due to expanded recruitment and consolidation of field operations.
Aspirin use was self-reported, and SHOW did not capture the reason for taking it (eg, CVD prevention or pain management). Some evidence of overreporting of aspirin use among older individuals exists,30 suggesting that a more objective measure of aspirin use (eg, pill bottle verification or blood platelet aggregation test) could yield different results.
Certain confounders were not measured, most notably contraindications to aspirin (eg, genetic platelet abnormalities). Such findings could explain some patterns of aspirin use in both strata, as up to 10% of any given population has a contraindication to aspirin due to allergy, intolerance, gastrointestinal ulcer, concomitant anticoagulant medication, or other high bleeding risk.18,31 Few of these variables were known about our sample.
TABLE 4W (available below) provides a breakdown of some possible aspirin contraindications, as well as possible reasons other than primary CVD prevention for regular aspirin use. Because clinical judgment is often required to assess the degree of severity of a given health condition in order to deem it an aspirin contraindication, these findings could not reliably be used to reclassify participants. We present them simply for hypothesis generation.
Some data collection predates the current USPSTF guidelines,10 which could have resulted in a misclassification of participants’ aspirin indication. However, sensitivity analyses restricted to the 2010 sample alone—the only one with data collection after the newer guidelines were released—did not reveal any meaningful differences.
Other methodological limitations include the less racially diverse population of Wisconsin compared with other parts of the country and the sample size, which did not permit testing for statistical interactions and perhaps resulted in larger confidence intervals for some associations (eg, race/ethnicity) relative to the population as a whole.
TABLE 4W
Possible reasons for aspirin use—or contraindication— by aspirin indication*
| Has a doctor or other health professional ever told you that you had … | Aspirin indicated (n=268) | Aspirin not indicated (n=563) | ||
|---|---|---|---|---|
| Regular aspirin user (n=83) | Nonregular aspirin user (n=185) | Regular aspirin user (n=102) | Nonregular aspirin user (n=461) | |
| Migraine headache Yes No | 20 (24%) 63 (76%) | 28 (15%) 157 (85%) | 24 (24%) 78 (76%) | 76 (16%) 385 (84%) |
| Arthritis† Yes No | 2 (2%) 81 (98%) | 1 (1%) 184 (99%) | 12 (12%) 90 (88%) | 26 (6%) 435 (94%) |
| Stomach or intestinal ulcer Yes No | 5 (6%) 78 (94%) | 6 (3%) 179 (97%) | 7 (7%) 95 (93%) | 10 (2%) 451 (98%) |
| Reflux or GERD Yes No | 8 (10%) 75 (90%) | 14 (8%) 171 (92%) | 11 (11%) 91 (89%) | 32 (7%) 429 (93%) |
| Values presented as n (%). *Data not included in study analysis. †Osteoarthritis or rheumatoid arthritis. GERD, gastric esophageal reflux disease. | ||||
Acknowledgement
The authors thank Matt Walsh, PhD, for his assistance in creating the analytical dataset, as well as Sally Steward-Townsend, Susan Wright, Bri Deyo, Bethany Varley, and the rest of the Survey of the Health of Wisconsin staff.
CORRESPONDENCE Jeffrey J. VanWormer, PhD, Epidemiology Research Center, Marshfield Clinic Research Foundation, 1000 North Oak Avenue, Marshfield, WI 54449; [email protected]
1. Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18-e209.
2. Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933-944.
3. Sullivan PW, Ghushchyan V, Wyatt HR. The medical cost of cardiometabolic risk factor clusters in the United States. Obesity. 2007;15:3150-3158.
4. Pearson TA, Blair SN, Daniels SR, et al. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106:388-391.
5. Kriekard P, Gharacholou SM, Peterson ED. Primary and secondary prevention of cardiovascular disease in older adults: a status report. Clin Geriatr Med. 2009;25:745-755.
6. Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in US deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356:2388-2398.
7. Hennekens CH, Schneider WR. The need for wider and appropriate utilization of aspirin and statins in the treatment and prevention of cardiovascular disease. Expert Rev Cardiovasc Ther. 2008;6:95-107.
8. Barnett H, Burrill P, Iheanacho I. Don’t use aspirin for primary prevention of cardiovascular disease. BMJ. 2010;340:c1805.-
9. Sanchez-Ross M, Waller AH, Maher J, et al. Aspirin for the prevention of cardiovascular morbidity. Minerva Med. 2010;101:205-214.
10. S. Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US preventive services task force recommendation statement. Ann Intern Med. 2009;150:396-404.
11. Bartolucci AA, Tendera M, Howard G. Meta-analysis of multiple primary prevention trials of cardiovascular events using aspirin. Am J Cardiol. 2011;107:1796-1801.
12. Maciosek MV, Coffield AB, Edwards NM, et al. Priorities among effective clinical preventive services: results of a systematic review and analysis. Am J Prev Med. 2006;31:52-61.
13. Pignone M, Anderson GK, Binns K, et al. Aspirin use among adults aged 40 and older in the United States results of a national survey. Am J Prev Med. 2007;32:403-407.
14. Ajani UA, Ford ES, Greenland KJ, et al. Aspirin use among US adults: behavioral risk factor surveillance system. Am J Prev Med. 2006;30:74-77.
15. Sanchez DR, Diez Roux AV, Michos ED, et al. Comparison of the racial/ethnic prevalence of regular aspirin use for the primary prevention of coronary heart disease from the multi-ethnic study of atherosclerosis. Am J Cardiol. 2011;107:41-46.
16. Stafford RS, Monti V, Ma J. Underutilization of aspirin persists in US ambulatory care for the secondary and primary prevention of cardiovascular disease. PLoS Med. 2005;2:e353.-
17. Rodondi N, Vittinghoff E, Cornuz J, et al. Aspirin use for the primary prevention of coronary heart disease in older adults. Am J Med. 2005;118(suppl):1288e1-1288e9.
18. Rodondi N, Cornuz J, Marques-Vidal P, et al. Aspirin use for the primary prevention of coronary heart disease: a population-based study in Switzerland. Prev Med. 2008;46:137-144.
19. Nieto FJ, Peppard PE, Engelman CD, et al. The Survey of the Health of Wisconsin (SHOW), a novel infrastructure for population health research: rationale and methods. BMC Public Health. 2010;10:785.-
20. D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743-753.
21. Cody RP, Smith JK. Applied Statistics and the SAS Programming Language. New York, NY: Prentice Hall; 2005.
22. Mallonee S, Daniels CG, Mold JW, et al. Increasing aspirin use among persons at risk for cardiovascular events in Oklahoma. J Okla State Med Assoc. 2010;103:254-260.
23. Centers for Disease Control and Prevention (CDC). Prevalence of aspirin use to prevent heart disease—Wisconsin, 1991, and Michigan, 1994. MMWR Morb Mortal Wkly Rep. 1997;46:498-502.
24. US Preventive Services Task Force. Aspirin for the primary prevention of cardiovascular events: recommendation and rationale. Ann Intern Med. 2002;136:157-160.
25. Werner M, Kelsberg G, Weismantel AM. Which healthy adults should take aspirin? J Fam Pract. 2004;53:146-150.
26. Berger JS, Roncaglioni MC, Avanzini F, et al. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295:306-313.
27. McQuaid KR, Laine L. Systematic review and meta-analysis of adverse events of low-dose aspirin and clopidogrel in randomized controlled trials. Am J Med. 2006;119:624-638.
28. VanWormer JJ, Johnson PJ, Pereira RF, et al. The Heart of New Ulm project: using community-based cardiometabolic risk factor screenings in a rural population health improvement initiative. Popul Health Manag. 2012;15:135-143.
29. Jeffreys D. Aspirin: The Remarkable Story of a Wonder Drug. New York, NY: Bloomsbury Publishing; 2005.
30. Smith NL, Psaty BM, Heckbert SR, et al. The reliability of medication inventory methods compared to serum levels of cardiovascular drugs in the elderly. J Clin Epidemiol. 1999;52:143-146.
31. Hedman J, Kaprio J, Poussa T, et al. Prevalence of asthma, aspirin intolerance, nasal polyposis and chronic obstructive pulmonary disease in a population-based study. Int J Epidemiol. 1999;28:717-722.
1. Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation. 2011;123:e18-e209.
2. Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933-944.
3. Sullivan PW, Ghushchyan V, Wyatt HR. The medical cost of cardiometabolic risk factor clusters in the United States. Obesity. 2007;15:3150-3158.
4. Pearson TA, Blair SN, Daniels SR, et al. AHA guidelines for primary prevention of cardiovascular disease and stroke: 2002 update. American Heart Association Science Advisory and Coordinating Committee. Circulation. 2002;106:388-391.
5. Kriekard P, Gharacholou SM, Peterson ED. Primary and secondary prevention of cardiovascular disease in older adults: a status report. Clin Geriatr Med. 2009;25:745-755.
6. Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in US deaths from coronary disease, 1980-2000. N Engl J Med. 2007;356:2388-2398.
7. Hennekens CH, Schneider WR. The need for wider and appropriate utilization of aspirin and statins in the treatment and prevention of cardiovascular disease. Expert Rev Cardiovasc Ther. 2008;6:95-107.
8. Barnett H, Burrill P, Iheanacho I. Don’t use aspirin for primary prevention of cardiovascular disease. BMJ. 2010;340:c1805.-
9. Sanchez-Ross M, Waller AH, Maher J, et al. Aspirin for the prevention of cardiovascular morbidity. Minerva Med. 2010;101:205-214.
10. S. Preventive Services Task Force. Aspirin for the prevention of cardiovascular disease: US preventive services task force recommendation statement. Ann Intern Med. 2009;150:396-404.
11. Bartolucci AA, Tendera M, Howard G. Meta-analysis of multiple primary prevention trials of cardiovascular events using aspirin. Am J Cardiol. 2011;107:1796-1801.
12. Maciosek MV, Coffield AB, Edwards NM, et al. Priorities among effective clinical preventive services: results of a systematic review and analysis. Am J Prev Med. 2006;31:52-61.
13. Pignone M, Anderson GK, Binns K, et al. Aspirin use among adults aged 40 and older in the United States results of a national survey. Am J Prev Med. 2007;32:403-407.
14. Ajani UA, Ford ES, Greenland KJ, et al. Aspirin use among US adults: behavioral risk factor surveillance system. Am J Prev Med. 2006;30:74-77.
15. Sanchez DR, Diez Roux AV, Michos ED, et al. Comparison of the racial/ethnic prevalence of regular aspirin use for the primary prevention of coronary heart disease from the multi-ethnic study of atherosclerosis. Am J Cardiol. 2011;107:41-46.
16. Stafford RS, Monti V, Ma J. Underutilization of aspirin persists in US ambulatory care for the secondary and primary prevention of cardiovascular disease. PLoS Med. 2005;2:e353.-
17. Rodondi N, Vittinghoff E, Cornuz J, et al. Aspirin use for the primary prevention of coronary heart disease in older adults. Am J Med. 2005;118(suppl):1288e1-1288e9.
18. Rodondi N, Cornuz J, Marques-Vidal P, et al. Aspirin use for the primary prevention of coronary heart disease: a population-based study in Switzerland. Prev Med. 2008;46:137-144.
19. Nieto FJ, Peppard PE, Engelman CD, et al. The Survey of the Health of Wisconsin (SHOW), a novel infrastructure for population health research: rationale and methods. BMC Public Health. 2010;10:785.-
20. D’Agostino RB, Sr, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743-753.
21. Cody RP, Smith JK. Applied Statistics and the SAS Programming Language. New York, NY: Prentice Hall; 2005.
22. Mallonee S, Daniels CG, Mold JW, et al. Increasing aspirin use among persons at risk for cardiovascular events in Oklahoma. J Okla State Med Assoc. 2010;103:254-260.
23. Centers for Disease Control and Prevention (CDC). Prevalence of aspirin use to prevent heart disease—Wisconsin, 1991, and Michigan, 1994. MMWR Morb Mortal Wkly Rep. 1997;46:498-502.
24. US Preventive Services Task Force. Aspirin for the primary prevention of cardiovascular events: recommendation and rationale. Ann Intern Med. 2002;136:157-160.
25. Werner M, Kelsberg G, Weismantel AM. Which healthy adults should take aspirin? J Fam Pract. 2004;53:146-150.
26. Berger JS, Roncaglioni MC, Avanzini F, et al. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295:306-313.
27. McQuaid KR, Laine L. Systematic review and meta-analysis of adverse events of low-dose aspirin and clopidogrel in randomized controlled trials. Am J Med. 2006;119:624-638.
28. VanWormer JJ, Johnson PJ, Pereira RF, et al. The Heart of New Ulm project: using community-based cardiometabolic risk factor screenings in a rural population health improvement initiative. Popul Health Manag. 2012;15:135-143.
29. Jeffreys D. Aspirin: The Remarkable Story of a Wonder Drug. New York, NY: Bloomsbury Publishing; 2005.
30. Smith NL, Psaty BM, Heckbert SR, et al. The reliability of medication inventory methods compared to serum levels of cardiovascular drugs in the elderly. J Clin Epidemiol. 1999;52:143-146.
31. Hedman J, Kaprio J, Poussa T, et al. Prevalence of asthma, aspirin intolerance, nasal polyposis and chronic obstructive pulmonary disease in a population-based study. Int J Epidemiol. 1999;28:717-722.
Access to specialized treatment by adult Hispanic brain tumor patients: findings from a single-institution retrospective study
Background: The Hispanic population accounts for 15% of the population of the United States, and for as much as 75% in cities throughout California. Racial disparities that are reflected by limited access to health care and worse disease outcomes are well documented for adult Hispanic cancer patients.
Objective: To determine whether there are similar disparities—including delays in accessing surgery, radiation, and oncologic care—for adult Hispanic non English-speaking (HNES) neuro-oncology patients and white English-only–speaking (WES) patients in an academic, tertiary care center with a multidisciplinary neuro-oncology team.
Methods: This retrospective study was conducted at the Chao Family Comprehensive Cancer Center of the University of California, Irvine. All patients who were diagnosed with a primary brain tumor during January 1, 2003, to December 31, 2008, were identified and data were collected on their age, sex, ethnicity, languages spoken, diagnosis, and insurance status. The times from the date of diagnosis to the date of surgery, from the date of surgery to the date of starting radiation (if indicated), and from the date of finishing radiation to the date of starting chemotherapy (if indicated) were also recorded.
Results: Most of the HNES patients (56.4%) had state insurance for the indigent, whereas most of the WES patients (41.8%) had private insurance from a health maintenance organization. Moreover, 12.8% of HNES patients were uninsured, compared with 4.5% of WES patients. There were no significant delays in the time from diagnosis to surgery, but there was a significant delay in access to radiation treatment (P .023). There were no differences on overall survival between the 2 groups of patients.
Limitations: This is a retrospective study of a relatively small number of patients. Larger studies are needed to corroborate these findings
Conclusions: The findings demonstrate that there are disparities in insurance status and access to radiation therapy between HNES and WES neuro-oncology patients.
*To read the the full article, click on the link at the top of this introduction.
Background: The Hispanic population accounts for 15% of the population of the United States, and for as much as 75% in cities throughout California. Racial disparities that are reflected by limited access to health care and worse disease outcomes are well documented for adult Hispanic cancer patients.
Objective: To determine whether there are similar disparities—including delays in accessing surgery, radiation, and oncologic care—for adult Hispanic non English-speaking (HNES) neuro-oncology patients and white English-only–speaking (WES) patients in an academic, tertiary care center with a multidisciplinary neuro-oncology team.
Methods: This retrospective study was conducted at the Chao Family Comprehensive Cancer Center of the University of California, Irvine. All patients who were diagnosed with a primary brain tumor during January 1, 2003, to December 31, 2008, were identified and data were collected on their age, sex, ethnicity, languages spoken, diagnosis, and insurance status. The times from the date of diagnosis to the date of surgery, from the date of surgery to the date of starting radiation (if indicated), and from the date of finishing radiation to the date of starting chemotherapy (if indicated) were also recorded.
Results: Most of the HNES patients (56.4%) had state insurance for the indigent, whereas most of the WES patients (41.8%) had private insurance from a health maintenance organization. Moreover, 12.8% of HNES patients were uninsured, compared with 4.5% of WES patients. There were no significant delays in the time from diagnosis to surgery, but there was a significant delay in access to radiation treatment (P .023). There were no differences on overall survival between the 2 groups of patients.
Limitations: This is a retrospective study of a relatively small number of patients. Larger studies are needed to corroborate these findings
Conclusions: The findings demonstrate that there are disparities in insurance status and access to radiation therapy between HNES and WES neuro-oncology patients.
*To read the the full article, click on the link at the top of this introduction.
Background: The Hispanic population accounts for 15% of the population of the United States, and for as much as 75% in cities throughout California. Racial disparities that are reflected by limited access to health care and worse disease outcomes are well documented for adult Hispanic cancer patients.
Objective: To determine whether there are similar disparities—including delays in accessing surgery, radiation, and oncologic care—for adult Hispanic non English-speaking (HNES) neuro-oncology patients and white English-only–speaking (WES) patients in an academic, tertiary care center with a multidisciplinary neuro-oncology team.
Methods: This retrospective study was conducted at the Chao Family Comprehensive Cancer Center of the University of California, Irvine. All patients who were diagnosed with a primary brain tumor during January 1, 2003, to December 31, 2008, were identified and data were collected on their age, sex, ethnicity, languages spoken, diagnosis, and insurance status. The times from the date of diagnosis to the date of surgery, from the date of surgery to the date of starting radiation (if indicated), and from the date of finishing radiation to the date of starting chemotherapy (if indicated) were also recorded.
Results: Most of the HNES patients (56.4%) had state insurance for the indigent, whereas most of the WES patients (41.8%) had private insurance from a health maintenance organization. Moreover, 12.8% of HNES patients were uninsured, compared with 4.5% of WES patients. There were no significant delays in the time from diagnosis to surgery, but there was a significant delay in access to radiation treatment (P .023). There were no differences on overall survival between the 2 groups of patients.
Limitations: This is a retrospective study of a relatively small number of patients. Larger studies are needed to corroborate these findings
Conclusions: The findings demonstrate that there are disparities in insurance status and access to radiation therapy between HNES and WES neuro-oncology patients.
*To read the the full article, click on the link at the top of this introduction.
Intravenous iron in chemotherapy and cancer-related anemia
Recent guidance from the Centers for Medicare and Medicaid Services restricting erythropoiesis-stimulating agents (ESAs) in chemotherapy and cancer-related anemias has resulted in an increase in transfusions. Nine studies, without published contradictory evidence, have shown optimization of the response to ESAs by intravenous (IV) iron when the iron was added to the treatment of chemotherapy-induced anemia. The synergy observed, although greater in iron deficiency, was independent of pretreatment iron parameters. Three studies demonstrated decreased transfusions when IV iron is administered without ESAs. Discordant recommendations regarding IV iron currently exist among the American Society of Hematology/American Society of Clinical Oncology guidelines, the National Comprehensive Cancer Network, and the European Society of Medical Oncology. This discordance is at least partly the result of misconceptions about the clinical nature and incidence of adverse effects with IV iron. Other reasons for this discordance are presented in this review. Based on thousands of studied patients, we conclude that IV iron is safe and probably safer than most physicians realize. Education is needed relating to the interpretation of minor, subclinical infusion reactions that resolve without therapy. IV iron without ESAs may be an effective treatment for chemotherapy-induced anemia and warrants further study. We present evidence supporting the conclusion that baseline serum hepcidin levels may predict responses to IV iron, and we examine the published evidence supporting the conclusion that IV iron should be a standard addition to the management of chemotherapy and cancer-related anemia.
Click on the PDF icon at the top of this introduction to read the full article.
Recent guidance from the Centers for Medicare and Medicaid Services restricting erythropoiesis-stimulating agents (ESAs) in chemotherapy and cancer-related anemias has resulted in an increase in transfusions. Nine studies, without published contradictory evidence, have shown optimization of the response to ESAs by intravenous (IV) iron when the iron was added to the treatment of chemotherapy-induced anemia. The synergy observed, although greater in iron deficiency, was independent of pretreatment iron parameters. Three studies demonstrated decreased transfusions when IV iron is administered without ESAs. Discordant recommendations regarding IV iron currently exist among the American Society of Hematology/American Society of Clinical Oncology guidelines, the National Comprehensive Cancer Network, and the European Society of Medical Oncology. This discordance is at least partly the result of misconceptions about the clinical nature and incidence of adverse effects with IV iron. Other reasons for this discordance are presented in this review. Based on thousands of studied patients, we conclude that IV iron is safe and probably safer than most physicians realize. Education is needed relating to the interpretation of minor, subclinical infusion reactions that resolve without therapy. IV iron without ESAs may be an effective treatment for chemotherapy-induced anemia and warrants further study. We present evidence supporting the conclusion that baseline serum hepcidin levels may predict responses to IV iron, and we examine the published evidence supporting the conclusion that IV iron should be a standard addition to the management of chemotherapy and cancer-related anemia.
Click on the PDF icon at the top of this introduction to read the full article.
Recent guidance from the Centers for Medicare and Medicaid Services restricting erythropoiesis-stimulating agents (ESAs) in chemotherapy and cancer-related anemias has resulted in an increase in transfusions. Nine studies, without published contradictory evidence, have shown optimization of the response to ESAs by intravenous (IV) iron when the iron was added to the treatment of chemotherapy-induced anemia. The synergy observed, although greater in iron deficiency, was independent of pretreatment iron parameters. Three studies demonstrated decreased transfusions when IV iron is administered without ESAs. Discordant recommendations regarding IV iron currently exist among the American Society of Hematology/American Society of Clinical Oncology guidelines, the National Comprehensive Cancer Network, and the European Society of Medical Oncology. This discordance is at least partly the result of misconceptions about the clinical nature and incidence of adverse effects with IV iron. Other reasons for this discordance are presented in this review. Based on thousands of studied patients, we conclude that IV iron is safe and probably safer than most physicians realize. Education is needed relating to the interpretation of minor, subclinical infusion reactions that resolve without therapy. IV iron without ESAs may be an effective treatment for chemotherapy-induced anemia and warrants further study. We present evidence supporting the conclusion that baseline serum hepcidin levels may predict responses to IV iron, and we examine the published evidence supporting the conclusion that IV iron should be a standard addition to the management of chemotherapy and cancer-related anemia.
Click on the PDF icon at the top of this introduction to read the full article.
Warn Parents to Beware of Button Batteries
Counsel parents in your practice that young children can be seriously injured or die from playing with or ingesting button batteries.
Injuries from these coin-sized batteries are on the rise, with 2.5 times as many children under age 13 showing up at an emergency department in 2010 compared with 1998, according to a study in the Aug. 31 issue of Morbidity and Mortality Weekly Report (2012:61:661-6). This increase from 1,900 cases in 1998 to 4,800 in 2010 aligns with the growing popularity of these batteries in remote controls, toys, light-up jewelry, and other devices.
"As the use of these batteries expands, so do the estimated number of ED-treated battery exposures in children, with the vast majority of these involving ingestions," Jacqueline Ferrante, Ph.D., of the U.S. Consumer Product Safety Commission (CPSC) Division of Health Sciences, and her colleagues stated in the report.
Injuries from batteries placed in a child’s nose or acid burns from ruptured batteries also are included in the report.
"Ensure that parents are aware of the problem so that batteries are kept away from young children," Dr. Ferrante said in an interview. Advise them to check remote controls and other electronic devices for easily accessible battery compartments, especially those that can be opened without a screwdriver, she advised.
An estimated 40,400 children younger than 13 years were treated at U.S. emergency departments for battery-related injuries between 1997 and 2010.
Additional CPSC databases covering 1995-2010 were searched for battery-related deaths in children under age 13. Of the 14 reported deaths, 12 children had confirmed exposure to button cell batteries. All children who died were aged 4 years and younger.
Diagnosis can be a challenge. Not all children can or are willing to report swallowing a battery or giving one to a sibling, the authors noted. In addition, the typical vomiting, abdominal pain, fever, diarrhea, respiratory distress, and dysphagia associated with battery ingestion are nonspecific symptoms (Pediatr. Emerg. Care 2008;24:313-6).
"Consider battery ingestion in the differential diagnosis of any child presenting with nonspecific GI symptoms or unexplained respiratory distress because of the serious consequences associated with a delayed or missed diagnosis," Dr. Ferrante said.
Complicating matters is the timing of injury, which can vary from 2 hours after ingestion for serious esophageal burns to more than 2 weeks for fatal hemorrhage following endoscopic removal of a button battery. The growing recognition of these injuries drove pediatric gastroenterologists at the University of Colorado Denver to develop guidelines for the management of button battery–induced hemorrhage (J. Pediatr. Gastroenterol. Nutr. 2011;52:585-9).
Nickle-sized, 3-V lithium button batteries can easily lodge in a child’s esophagus, and were most frequently associated with serious complications and death, according to the report.
Although outside the scope of the report, Dr. Ferrante also suggested pediatricians warn parents about ingestion of strong powerful magnets. This is another often-overlooked injury with similar issues and consequences, she said. "There are even cases where injury resulted from co-ingestion of a button battery and a strong magnet."
For the current study, Dr. Ferrante and her colleagues analyzed the National Electronic Injury Surveillance System (NEISS) database to quantify nonfatal, battery-related ED visits. They assessed other CPSC data to identify the battery-related deaths.
The NEISS data only comprise emergency department visits, so any child treated for battery ingestion in a doctor’s office or as an outpatient is not included in the study, a potential limitation. In addition, the number of fatal incidents involving batteries and children younger than 13 years is likely underrepresented because of the type of data collected, the authors noted.
The authors had no relevant financial disclosures. Click here for more information on battery hazards provided by the U.S. Consumer Product Safety Commission.
Counsel parents in your practice that young children can be seriously injured or die from playing with or ingesting button batteries.
Injuries from these coin-sized batteries are on the rise, with 2.5 times as many children under age 13 showing up at an emergency department in 2010 compared with 1998, according to a study in the Aug. 31 issue of Morbidity and Mortality Weekly Report (2012:61:661-6). This increase from 1,900 cases in 1998 to 4,800 in 2010 aligns with the growing popularity of these batteries in remote controls, toys, light-up jewelry, and other devices.
"As the use of these batteries expands, so do the estimated number of ED-treated battery exposures in children, with the vast majority of these involving ingestions," Jacqueline Ferrante, Ph.D., of the U.S. Consumer Product Safety Commission (CPSC) Division of Health Sciences, and her colleagues stated in the report.
Injuries from batteries placed in a child’s nose or acid burns from ruptured batteries also are included in the report.
"Ensure that parents are aware of the problem so that batteries are kept away from young children," Dr. Ferrante said in an interview. Advise them to check remote controls and other electronic devices for easily accessible battery compartments, especially those that can be opened without a screwdriver, she advised.
An estimated 40,400 children younger than 13 years were treated at U.S. emergency departments for battery-related injuries between 1997 and 2010.
Additional CPSC databases covering 1995-2010 were searched for battery-related deaths in children under age 13. Of the 14 reported deaths, 12 children had confirmed exposure to button cell batteries. All children who died were aged 4 years and younger.
Diagnosis can be a challenge. Not all children can or are willing to report swallowing a battery or giving one to a sibling, the authors noted. In addition, the typical vomiting, abdominal pain, fever, diarrhea, respiratory distress, and dysphagia associated with battery ingestion are nonspecific symptoms (Pediatr. Emerg. Care 2008;24:313-6).
"Consider battery ingestion in the differential diagnosis of any child presenting with nonspecific GI symptoms or unexplained respiratory distress because of the serious consequences associated with a delayed or missed diagnosis," Dr. Ferrante said.
Complicating matters is the timing of injury, which can vary from 2 hours after ingestion for serious esophageal burns to more than 2 weeks for fatal hemorrhage following endoscopic removal of a button battery. The growing recognition of these injuries drove pediatric gastroenterologists at the University of Colorado Denver to develop guidelines for the management of button battery–induced hemorrhage (J. Pediatr. Gastroenterol. Nutr. 2011;52:585-9).
Nickle-sized, 3-V lithium button batteries can easily lodge in a child’s esophagus, and were most frequently associated with serious complications and death, according to the report.
Although outside the scope of the report, Dr. Ferrante also suggested pediatricians warn parents about ingestion of strong powerful magnets. This is another often-overlooked injury with similar issues and consequences, she said. "There are even cases where injury resulted from co-ingestion of a button battery and a strong magnet."
For the current study, Dr. Ferrante and her colleagues analyzed the National Electronic Injury Surveillance System (NEISS) database to quantify nonfatal, battery-related ED visits. They assessed other CPSC data to identify the battery-related deaths.
The NEISS data only comprise emergency department visits, so any child treated for battery ingestion in a doctor’s office or as an outpatient is not included in the study, a potential limitation. In addition, the number of fatal incidents involving batteries and children younger than 13 years is likely underrepresented because of the type of data collected, the authors noted.
The authors had no relevant financial disclosures. Click here for more information on battery hazards provided by the U.S. Consumer Product Safety Commission.
Counsel parents in your practice that young children can be seriously injured or die from playing with or ingesting button batteries.
Injuries from these coin-sized batteries are on the rise, with 2.5 times as many children under age 13 showing up at an emergency department in 2010 compared with 1998, according to a study in the Aug. 31 issue of Morbidity and Mortality Weekly Report (2012:61:661-6). This increase from 1,900 cases in 1998 to 4,800 in 2010 aligns with the growing popularity of these batteries in remote controls, toys, light-up jewelry, and other devices.
"As the use of these batteries expands, so do the estimated number of ED-treated battery exposures in children, with the vast majority of these involving ingestions," Jacqueline Ferrante, Ph.D., of the U.S. Consumer Product Safety Commission (CPSC) Division of Health Sciences, and her colleagues stated in the report.
Injuries from batteries placed in a child’s nose or acid burns from ruptured batteries also are included in the report.
"Ensure that parents are aware of the problem so that batteries are kept away from young children," Dr. Ferrante said in an interview. Advise them to check remote controls and other electronic devices for easily accessible battery compartments, especially those that can be opened without a screwdriver, she advised.
An estimated 40,400 children younger than 13 years were treated at U.S. emergency departments for battery-related injuries between 1997 and 2010.
Additional CPSC databases covering 1995-2010 were searched for battery-related deaths in children under age 13. Of the 14 reported deaths, 12 children had confirmed exposure to button cell batteries. All children who died were aged 4 years and younger.
Diagnosis can be a challenge. Not all children can or are willing to report swallowing a battery or giving one to a sibling, the authors noted. In addition, the typical vomiting, abdominal pain, fever, diarrhea, respiratory distress, and dysphagia associated with battery ingestion are nonspecific symptoms (Pediatr. Emerg. Care 2008;24:313-6).
"Consider battery ingestion in the differential diagnosis of any child presenting with nonspecific GI symptoms or unexplained respiratory distress because of the serious consequences associated with a delayed or missed diagnosis," Dr. Ferrante said.
Complicating matters is the timing of injury, which can vary from 2 hours after ingestion for serious esophageal burns to more than 2 weeks for fatal hemorrhage following endoscopic removal of a button battery. The growing recognition of these injuries drove pediatric gastroenterologists at the University of Colorado Denver to develop guidelines for the management of button battery–induced hemorrhage (J. Pediatr. Gastroenterol. Nutr. 2011;52:585-9).
Nickle-sized, 3-V lithium button batteries can easily lodge in a child’s esophagus, and were most frequently associated with serious complications and death, according to the report.
Although outside the scope of the report, Dr. Ferrante also suggested pediatricians warn parents about ingestion of strong powerful magnets. This is another often-overlooked injury with similar issues and consequences, she said. "There are even cases where injury resulted from co-ingestion of a button battery and a strong magnet."
For the current study, Dr. Ferrante and her colleagues analyzed the National Electronic Injury Surveillance System (NEISS) database to quantify nonfatal, battery-related ED visits. They assessed other CPSC data to identify the battery-related deaths.
The NEISS data only comprise emergency department visits, so any child treated for battery ingestion in a doctor’s office or as an outpatient is not included in the study, a potential limitation. In addition, the number of fatal incidents involving batteries and children younger than 13 years is likely underrepresented because of the type of data collected, the authors noted.
The authors had no relevant financial disclosures. Click here for more information on battery hazards provided by the U.S. Consumer Product Safety Commission.
FROM MORBIDITY AND MORTALITY WEEKLY REPORT
Major Finding: An estimated 40,400 children were treated at U.S. emergency departments between 1997 and 2010 for battery-related injuries. Twelve of the 14 reported deaths involved button batteries.
Data Source: This is a study of fatal and nonfatal injuries related to battery exposure in children 13 years and younger from U.S. Consumer Product Safety Commission databases.
Disclosures: The authors had no relevant financial disclosures.
FDA Approves Linaclotide for Constipation Conditions
The Food and Drug Administration approved linaclotide on Aug. 30 to treat two conditions: chronic idiopathic constipation and irritable bowel syndrome with constipation in adults.
Linaclotide (Linzess) is administered as a capsule taken once daily on an empty stomach, at least 30 minutes before the first meal of the day. This agent helps relieve constipation by increasing the frequency of bowel movements. In irritable bowel syndrome with constipation (IBS-C), linaclotide has been shown to reduce abdominal pain, according to a statement from the FDA.
The drug is approved with a boxed warning to alert patients and health care professionals that linaclotide should not be used in patients 16 years of age and younger. The most common side effect reported during the clinical studies was diarrhea, the statement said.
According to the FDA, the safety and effectiveness of linaclotide for the management of IBS-C were established in two double-blind studies (Gastroenterology 2011;140:S138 and Gastroenterology 2011;140:S135). A total of 1,604 patients were randomly assigned to take 290 mcg of linaclotide or a placebo for at least 12 weeks. Linaclotide was more effective in reducing abdominal pain and increasing the number of complete spontaneous bowel movements, compared with placebo, in both trials.
The safety and effectiveness of linaclotide for the management of chronic idiopathic constipation also were established in two double-blind studies (N. Engl. J. Med. 2011;365:527-36). A total of 1,272 patients were randomly assigned to take 145 mcg or 290 mcg linaclotide or a placebo for 12 weeks. Patients on linaclotide had more complete spontaneous bowel movements than did those taking the placebo. The 290-mcg dose is not approved for chronic constipation because the data showed that it was no more effective than the 145-mcg dose.
Linzess is marketed by Ironwood Pharmaceuticals Inc.
Linaclotide is currently the only FDA-approved medication indicated for increasing bowel movements and decreasing abdominal pain in men and women with irritable bowel syndrome with constipation (IBS-C). It has been shown to be efficacious in relieving abdominal pain and constipation in patients with IBS-C, and constipation in those with chronic idiopathic constipation (CIC). The drug is a peripherally-acting agent that activates guanylate cyclase-C (GC-C) on intestinal epithelial cells resulting in increased intracellular and extracellular concentrations of cyclic guanosine monophosphate (cGMP).
Relief of constipation symptoms in IBS-C and CIC is believed to be due to an increase in intracellular cGMP resulting in chloride and fluid secretion through the cystic fibrosis transmembrane conductance regulator (CFTR) ion channel and acceleration of colonic transit. Linaclotide’s effect on reducing abdominal pain in IBS-C is thought to be due to increased extracellular cGMP, which has been shown to decrease firing of sensory nerves within the bowel wall in preclinical animal studies.
Patients with CIC who responded to linaclotide had at least three complete spontaneous bowel movements (CSBMs) per week and an increase in one CSBM for at least 9 out of 12 weeks. The 145 mcg and 290 mcg daily doses showed a statistically significant benefit over placebo; the FDA has approved only the lower dose for CIC. The efficacy of linaclotide was sustained throughout the 12 weeks of the trials.
The dose of 290 mcg per day was approved for the treatment of IBS-C, which is usually differentiated from CIC by the presence of predominant abdominal pain associated with constipation. The significant improvement in CSBMs occurred within the first week of treatment. The decrease in abdominal pain was more gradual and appeared to reach its maximum effect at 8 weeks. The significant effect of linaclotide on abdominal pain may be due to an additional independent effect beyond relief of constipation, but further studies are needed to better understand linaclotide’s effect on abdominal pain.
LIN CHANG, M.D., is co-director of the Oppenheimer Family Center for Neurobiology of Stress and director of the Digestive Health and Nutrition Clinic at the University of California, Los Angeles. She is a consultant for Ironwood Pharmaceuticals and Forest Laboratories and has received grant support from Ironwood Pharmaceuticals.
Linaclotide is currently the only FDA-approved medication indicated for increasing bowel movements and decreasing abdominal pain in men and women with irritable bowel syndrome with constipation (IBS-C). It has been shown to be efficacious in relieving abdominal pain and constipation in patients with IBS-C, and constipation in those with chronic idiopathic constipation (CIC). The drug is a peripherally-acting agent that activates guanylate cyclase-C (GC-C) on intestinal epithelial cells resulting in increased intracellular and extracellular concentrations of cyclic guanosine monophosphate (cGMP).
Relief of constipation symptoms in IBS-C and CIC is believed to be due to an increase in intracellular cGMP resulting in chloride and fluid secretion through the cystic fibrosis transmembrane conductance regulator (CFTR) ion channel and acceleration of colonic transit. Linaclotide’s effect on reducing abdominal pain in IBS-C is thought to be due to increased extracellular cGMP, which has been shown to decrease firing of sensory nerves within the bowel wall in preclinical animal studies.
Patients with CIC who responded to linaclotide had at least three complete spontaneous bowel movements (CSBMs) per week and an increase in one CSBM for at least 9 out of 12 weeks. The 145 mcg and 290 mcg daily doses showed a statistically significant benefit over placebo; the FDA has approved only the lower dose for CIC. The efficacy of linaclotide was sustained throughout the 12 weeks of the trials.
The dose of 290 mcg per day was approved for the treatment of IBS-C, which is usually differentiated from CIC by the presence of predominant abdominal pain associated with constipation. The significant improvement in CSBMs occurred within the first week of treatment. The decrease in abdominal pain was more gradual and appeared to reach its maximum effect at 8 weeks. The significant effect of linaclotide on abdominal pain may be due to an additional independent effect beyond relief of constipation, but further studies are needed to better understand linaclotide’s effect on abdominal pain.
LIN CHANG, M.D., is co-director of the Oppenheimer Family Center for Neurobiology of Stress and director of the Digestive Health and Nutrition Clinic at the University of California, Los Angeles. She is a consultant for Ironwood Pharmaceuticals and Forest Laboratories and has received grant support from Ironwood Pharmaceuticals.
Linaclotide is currently the only FDA-approved medication indicated for increasing bowel movements and decreasing abdominal pain in men and women with irritable bowel syndrome with constipation (IBS-C). It has been shown to be efficacious in relieving abdominal pain and constipation in patients with IBS-C, and constipation in those with chronic idiopathic constipation (CIC). The drug is a peripherally-acting agent that activates guanylate cyclase-C (GC-C) on intestinal epithelial cells resulting in increased intracellular and extracellular concentrations of cyclic guanosine monophosphate (cGMP).
Relief of constipation symptoms in IBS-C and CIC is believed to be due to an increase in intracellular cGMP resulting in chloride and fluid secretion through the cystic fibrosis transmembrane conductance regulator (CFTR) ion channel and acceleration of colonic transit. Linaclotide’s effect on reducing abdominal pain in IBS-C is thought to be due to increased extracellular cGMP, which has been shown to decrease firing of sensory nerves within the bowel wall in preclinical animal studies.
Patients with CIC who responded to linaclotide had at least three complete spontaneous bowel movements (CSBMs) per week and an increase in one CSBM for at least 9 out of 12 weeks. The 145 mcg and 290 mcg daily doses showed a statistically significant benefit over placebo; the FDA has approved only the lower dose for CIC. The efficacy of linaclotide was sustained throughout the 12 weeks of the trials.
The dose of 290 mcg per day was approved for the treatment of IBS-C, which is usually differentiated from CIC by the presence of predominant abdominal pain associated with constipation. The significant improvement in CSBMs occurred within the first week of treatment. The decrease in abdominal pain was more gradual and appeared to reach its maximum effect at 8 weeks. The significant effect of linaclotide on abdominal pain may be due to an additional independent effect beyond relief of constipation, but further studies are needed to better understand linaclotide’s effect on abdominal pain.
LIN CHANG, M.D., is co-director of the Oppenheimer Family Center for Neurobiology of Stress and director of the Digestive Health and Nutrition Clinic at the University of California, Los Angeles. She is a consultant for Ironwood Pharmaceuticals and Forest Laboratories and has received grant support from Ironwood Pharmaceuticals.
The Food and Drug Administration approved linaclotide on Aug. 30 to treat two conditions: chronic idiopathic constipation and irritable bowel syndrome with constipation in adults.
Linaclotide (Linzess) is administered as a capsule taken once daily on an empty stomach, at least 30 minutes before the first meal of the day. This agent helps relieve constipation by increasing the frequency of bowel movements. In irritable bowel syndrome with constipation (IBS-C), linaclotide has been shown to reduce abdominal pain, according to a statement from the FDA.
The drug is approved with a boxed warning to alert patients and health care professionals that linaclotide should not be used in patients 16 years of age and younger. The most common side effect reported during the clinical studies was diarrhea, the statement said.
According to the FDA, the safety and effectiveness of linaclotide for the management of IBS-C were established in two double-blind studies (Gastroenterology 2011;140:S138 and Gastroenterology 2011;140:S135). A total of 1,604 patients were randomly assigned to take 290 mcg of linaclotide or a placebo for at least 12 weeks. Linaclotide was more effective in reducing abdominal pain and increasing the number of complete spontaneous bowel movements, compared with placebo, in both trials.
The safety and effectiveness of linaclotide for the management of chronic idiopathic constipation also were established in two double-blind studies (N. Engl. J. Med. 2011;365:527-36). A total of 1,272 patients were randomly assigned to take 145 mcg or 290 mcg linaclotide or a placebo for 12 weeks. Patients on linaclotide had more complete spontaneous bowel movements than did those taking the placebo. The 290-mcg dose is not approved for chronic constipation because the data showed that it was no more effective than the 145-mcg dose.
Linzess is marketed by Ironwood Pharmaceuticals Inc.
The Food and Drug Administration approved linaclotide on Aug. 30 to treat two conditions: chronic idiopathic constipation and irritable bowel syndrome with constipation in adults.
Linaclotide (Linzess) is administered as a capsule taken once daily on an empty stomach, at least 30 minutes before the first meal of the day. This agent helps relieve constipation by increasing the frequency of bowel movements. In irritable bowel syndrome with constipation (IBS-C), linaclotide has been shown to reduce abdominal pain, according to a statement from the FDA.
The drug is approved with a boxed warning to alert patients and health care professionals that linaclotide should not be used in patients 16 years of age and younger. The most common side effect reported during the clinical studies was diarrhea, the statement said.
According to the FDA, the safety and effectiveness of linaclotide for the management of IBS-C were established in two double-blind studies (Gastroenterology 2011;140:S138 and Gastroenterology 2011;140:S135). A total of 1,604 patients were randomly assigned to take 290 mcg of linaclotide or a placebo for at least 12 weeks. Linaclotide was more effective in reducing abdominal pain and increasing the number of complete spontaneous bowel movements, compared with placebo, in both trials.
The safety and effectiveness of linaclotide for the management of chronic idiopathic constipation also were established in two double-blind studies (N. Engl. J. Med. 2011;365:527-36). A total of 1,272 patients were randomly assigned to take 145 mcg or 290 mcg linaclotide or a placebo for 12 weeks. Patients on linaclotide had more complete spontaneous bowel movements than did those taking the placebo. The 290-mcg dose is not approved for chronic constipation because the data showed that it was no more effective than the 145-mcg dose.
Linzess is marketed by Ironwood Pharmaceuticals Inc.
Study: Burnout Risk High for Hospitalists, Other “Front-Line” Physicians
An author of new research that shows physicians are more likely to be burned out by work than other professions says the findings underscore the need for hospitalists to find a balance between their professional and personal lives.
The Archives of Internal Medicine report, "Burnout and Satisfaction With Work-Life Balance Among US Physicians Relative to the General U.S. Population," found that physicians were more likely than "working U.S. adults" to exhibit at least one symptom of burnout (37.9% vs. 27.8%, P<0.01). Physicians also were more likely to be dissatisfied with their work-life balance (40.2% vs. 23.2%, P<0.01), according to the data.
"It's a balancing act," says Colin West, MD, PhD, FACP, of the Departments of Internal Medicine and Health Sciences Research at Mayo Clinic in Rochester, Minn. "Every physician is a little bit different. Every person is a little bit different. If everyone is able to exert some control … and do what's meaningful to them, that gives them the best shot to balance."
Of specific importance for hospitalists, the research found that "front-line specialties" (including internal medicine, general medicine, and emergency medicine) exhibited the highest risk factor for burnout. Dr. West says more research would be required to determine how at risk hospitalists are, but given their position in the healthcare spectrum, he suspects they are among those at highest risk. He believes that the healthcare system as a whole needs to address the burnout issue, as repercussions can include problematic alcohol use, broken relationships, and suicidal ideation.
"The best group is that which strikes a balance," Dr. West says. "It's probably because [those physicians are not] feeling like they're dropping a ball. If you pick work over home, or home over work, then, basically, one is left behind."
An author of new research that shows physicians are more likely to be burned out by work than other professions says the findings underscore the need for hospitalists to find a balance between their professional and personal lives.
The Archives of Internal Medicine report, "Burnout and Satisfaction With Work-Life Balance Among US Physicians Relative to the General U.S. Population," found that physicians were more likely than "working U.S. adults" to exhibit at least one symptom of burnout (37.9% vs. 27.8%, P<0.01). Physicians also were more likely to be dissatisfied with their work-life balance (40.2% vs. 23.2%, P<0.01), according to the data.
"It's a balancing act," says Colin West, MD, PhD, FACP, of the Departments of Internal Medicine and Health Sciences Research at Mayo Clinic in Rochester, Minn. "Every physician is a little bit different. Every person is a little bit different. If everyone is able to exert some control … and do what's meaningful to them, that gives them the best shot to balance."
Of specific importance for hospitalists, the research found that "front-line specialties" (including internal medicine, general medicine, and emergency medicine) exhibited the highest risk factor for burnout. Dr. West says more research would be required to determine how at risk hospitalists are, but given their position in the healthcare spectrum, he suspects they are among those at highest risk. He believes that the healthcare system as a whole needs to address the burnout issue, as repercussions can include problematic alcohol use, broken relationships, and suicidal ideation.
"The best group is that which strikes a balance," Dr. West says. "It's probably because [those physicians are not] feeling like they're dropping a ball. If you pick work over home, or home over work, then, basically, one is left behind."
An author of new research that shows physicians are more likely to be burned out by work than other professions says the findings underscore the need for hospitalists to find a balance between their professional and personal lives.
The Archives of Internal Medicine report, "Burnout and Satisfaction With Work-Life Balance Among US Physicians Relative to the General U.S. Population," found that physicians were more likely than "working U.S. adults" to exhibit at least one symptom of burnout (37.9% vs. 27.8%, P<0.01). Physicians also were more likely to be dissatisfied with their work-life balance (40.2% vs. 23.2%, P<0.01), according to the data.
"It's a balancing act," says Colin West, MD, PhD, FACP, of the Departments of Internal Medicine and Health Sciences Research at Mayo Clinic in Rochester, Minn. "Every physician is a little bit different. Every person is a little bit different. If everyone is able to exert some control … and do what's meaningful to them, that gives them the best shot to balance."
Of specific importance for hospitalists, the research found that "front-line specialties" (including internal medicine, general medicine, and emergency medicine) exhibited the highest risk factor for burnout. Dr. West says more research would be required to determine how at risk hospitalists are, but given their position in the healthcare spectrum, he suspects they are among those at highest risk. He believes that the healthcare system as a whole needs to address the burnout issue, as repercussions can include problematic alcohol use, broken relationships, and suicidal ideation.
"The best group is that which strikes a balance," Dr. West says. "It's probably because [those physicians are not] feeling like they're dropping a ball. If you pick work over home, or home over work, then, basically, one is left behind."
Local Solutions Spark Readmission Reductions
Earlier this month CMS announced 17 additional awards under its Community-Based Care Transitions Program (CCTP), which now encompasses 200 acute-care hospitals and their hospitalists partnering with community agencies and coalitions to improve transitions of care in advance of the Oct. 1 start for excessive readmissions penalties. Innovative solutions to the readmissions dilemma are being tested at the local level by a variety of partnerships with hospitals and hospitalists.
For example, William C. Cook, DO, chief of hospital medicine for the Ohio Permanente Medical Group in Cleveland, is part of a community-wide quality coalition called Better Health Greater Cleveland, one of 17 such groups in the Robert Wood Johnson Foundation's Aligning Forces for Quality collaborative. The program includes 150 quality teams in 100 hospitals posting readmissions reductions and other quality metrics. Dr. Cook, who co-chairs Better Health's Steering Committee for Transitions of Care, is spearheading a transitions pilot with two local nursing homes.
"From the hospitalist perspective, our role is to make care transitions safe and predictable," Dr. Cook says. "The way I can contribute most to these transitions is by thinking ahead about what's going to happen next—and how do I prepare the patient and the next provider." One key step is taking time to complete the real-time discharge summary for each patient, he adds.
The idea, Dr. Cook explains, is to identify and communicate with collaborators across care settings so that the "coaching baton" can be passed in a manner that appears seamless to the patient.
Nearly a third of the 17 new CCTP sites participate in SHM's Project BOOST, including three hospitals in California and one each in Illinois and Pennsylvania. Project BOOST is accepting applications for its next round of sites through September.
Earlier this month CMS announced 17 additional awards under its Community-Based Care Transitions Program (CCTP), which now encompasses 200 acute-care hospitals and their hospitalists partnering with community agencies and coalitions to improve transitions of care in advance of the Oct. 1 start for excessive readmissions penalties. Innovative solutions to the readmissions dilemma are being tested at the local level by a variety of partnerships with hospitals and hospitalists.
For example, William C. Cook, DO, chief of hospital medicine for the Ohio Permanente Medical Group in Cleveland, is part of a community-wide quality coalition called Better Health Greater Cleveland, one of 17 such groups in the Robert Wood Johnson Foundation's Aligning Forces for Quality collaborative. The program includes 150 quality teams in 100 hospitals posting readmissions reductions and other quality metrics. Dr. Cook, who co-chairs Better Health's Steering Committee for Transitions of Care, is spearheading a transitions pilot with two local nursing homes.
"From the hospitalist perspective, our role is to make care transitions safe and predictable," Dr. Cook says. "The way I can contribute most to these transitions is by thinking ahead about what's going to happen next—and how do I prepare the patient and the next provider." One key step is taking time to complete the real-time discharge summary for each patient, he adds.
The idea, Dr. Cook explains, is to identify and communicate with collaborators across care settings so that the "coaching baton" can be passed in a manner that appears seamless to the patient.
Nearly a third of the 17 new CCTP sites participate in SHM's Project BOOST, including three hospitals in California and one each in Illinois and Pennsylvania. Project BOOST is accepting applications for its next round of sites through September.
Earlier this month CMS announced 17 additional awards under its Community-Based Care Transitions Program (CCTP), which now encompasses 200 acute-care hospitals and their hospitalists partnering with community agencies and coalitions to improve transitions of care in advance of the Oct. 1 start for excessive readmissions penalties. Innovative solutions to the readmissions dilemma are being tested at the local level by a variety of partnerships with hospitals and hospitalists.
For example, William C. Cook, DO, chief of hospital medicine for the Ohio Permanente Medical Group in Cleveland, is part of a community-wide quality coalition called Better Health Greater Cleveland, one of 17 such groups in the Robert Wood Johnson Foundation's Aligning Forces for Quality collaborative. The program includes 150 quality teams in 100 hospitals posting readmissions reductions and other quality metrics. Dr. Cook, who co-chairs Better Health's Steering Committee for Transitions of Care, is spearheading a transitions pilot with two local nursing homes.
"From the hospitalist perspective, our role is to make care transitions safe and predictable," Dr. Cook says. "The way I can contribute most to these transitions is by thinking ahead about what's going to happen next—and how do I prepare the patient and the next provider." One key step is taking time to complete the real-time discharge summary for each patient, he adds.
The idea, Dr. Cook explains, is to identify and communicate with collaborators across care settings so that the "coaching baton" can be passed in a manner that appears seamless to the patient.
Nearly a third of the 17 new CCTP sites participate in SHM's Project BOOST, including three hospitals in California and one each in Illinois and Pennsylvania. Project BOOST is accepting applications for its next round of sites through September.
The Paralympics and Prosthesis Pride
South African runner Oscar Pistorius fell just short of winning a medal at the London 2012 Olympics, but the double amputee did show the world that people with disabilities can achieve greatness in sports. His specially made prosthetic limbs also reflect a growing trend – highly visible in the media among returning soldiers from Afghanistan and Iraq – toward more people with amputations displaying their prostheses rather than hiding them with coverings that resemble arms and legs.
The trend will be further highlighted at the 2012 Paralympic Games, taking place in London Aug. 29 through Sept. 9, and where Mr. Pistorius will again compete.
"These are not your grandparents’ prosthetic devices that are available today. They have advanced at such a rapid pace. It’s not only exciting for users of prosthetic devices, but nonamputees are also very interested in the technology – how far it has evolved, and what prosthetic users are capable of doing, whether it be climbing Mount Everest or running in the Olympics with prosthetic limbs," according to Kevin Carroll, vice president of prosthetics at Hanger Orthopedic Group, based in Austin, Tex.*
About 4,200 athletes from 165 countries will compete in the Paralympics, across 503 medal events. Athletes with amputations will be some of the most visually distinct among the Paralympics’ 10 categories of disability: impaired muscle power, impaired passive range of motion, limb deficiency, leg length difference, short stature, hypertonia, ataxia, athetosis, vision impairment, and intellectual impairment.
"This year, we’re expecting all sorts of records at the Paralympics. There is a greater focus worldwide and countries throughout the world are seeing the importance of supporting athletes in Paralympic events ... I predict many more Pistorius-types are on their way to the Olympics," said Mr. Carroll.
In the Paralympics, athletes using specially designed prosthetic devices –that is, used specifically for the sport and not for everyday use – will be most visible in track and field events. Prosthetic devices are not used in sports such as swimming and sitting volleyball. However, athletes with amputations are using prostheses for a wide range of sports in the real world.
"We are now supplying sport-specific prostheses from early ages all the way up to adults. This includes everything from prostheses made specifically for running, cycling, swimming, mountain climbing, snowboarding, skiing, and more," Mr. Carroll said.
In fact, the emerging technology is so good that some have suggested it may soon allow disabled athletes to outdo their able-bodied counterparts.
Of course, most amputees are not elite athletes. There are approximately 1.7 million people with limb loss in the United States, with another 150,000 who undergo amputations each year. While injured soldiers tend to be the most visible amputees in the media, diabetes is by far the greatest cause of limb loss – more than 80% of all amputations are the result of diabetes. Vascular disease, trauma, and cancer are among the other top causes.
But even increasing numbers of non–athlete amputees have been showcasing their prostheses within the last 10-15 years, with comparable numbers in both the civilian and military populations. As many as 50% of amputees in the United States now choose to display their prostheses, compared with less than 10% among their European counterparts. "This number wasn’t as high in the U.S. 30 years ago. This has evolved over time," Mr. Carroll said.
A realistic-looking limb cover adds to the cost of the working part of the prosthesis itself, but he believes that’s not why we’re seeing more of the latter. "[Amputees who chose to show their prostheses] like to showcase the technology they’re walking around on. This shows they have really accepted their loss. They are not showcasing their prostheses because of the cost, they’re doing it because of pride."
His advice to physicians: "Never say never to patients. We see people who are confined to wheelchairs get up and walk every day. We’re in a whole new era of rehabilitation. We’re very excited about the future."
–Miriam E. Tucker (@MiriamETucker on Twitter)
*CORRECTION 8/30/12: The original sentence misstated the location of Hanger Orthopedic Group. The sentence should have read: "These are not your grandparents’ prosthetic devices that are available today. They have advanced at such a rapid pace. It’s not only exciting for users of prosthetic devices, but nonamputees are also very interested in the technology – how far it has evolved, and what prosthetic users are capable of doing, whether it be climbing Mount Everest or running in the Olympics with prosthetic limbs," according to Kevin Carroll, vice president of prosthetics at Hanger Orthopedic Group, based in Austin, Tex..
South African runner Oscar Pistorius fell just short of winning a medal at the London 2012 Olympics, but the double amputee did show the world that people with disabilities can achieve greatness in sports. His specially made prosthetic limbs also reflect a growing trend – highly visible in the media among returning soldiers from Afghanistan and Iraq – toward more people with amputations displaying their prostheses rather than hiding them with coverings that resemble arms and legs.
The trend will be further highlighted at the 2012 Paralympic Games, taking place in London Aug. 29 through Sept. 9, and where Mr. Pistorius will again compete.
"These are not your grandparents’ prosthetic devices that are available today. They have advanced at such a rapid pace. It’s not only exciting for users of prosthetic devices, but nonamputees are also very interested in the technology – how far it has evolved, and what prosthetic users are capable of doing, whether it be climbing Mount Everest or running in the Olympics with prosthetic limbs," according to Kevin Carroll, vice president of prosthetics at Hanger Orthopedic Group, based in Austin, Tex.*
About 4,200 athletes from 165 countries will compete in the Paralympics, across 503 medal events. Athletes with amputations will be some of the most visually distinct among the Paralympics’ 10 categories of disability: impaired muscle power, impaired passive range of motion, limb deficiency, leg length difference, short stature, hypertonia, ataxia, athetosis, vision impairment, and intellectual impairment.
"This year, we’re expecting all sorts of records at the Paralympics. There is a greater focus worldwide and countries throughout the world are seeing the importance of supporting athletes in Paralympic events ... I predict many more Pistorius-types are on their way to the Olympics," said Mr. Carroll.
In the Paralympics, athletes using specially designed prosthetic devices –that is, used specifically for the sport and not for everyday use – will be most visible in track and field events. Prosthetic devices are not used in sports such as swimming and sitting volleyball. However, athletes with amputations are using prostheses for a wide range of sports in the real world.
"We are now supplying sport-specific prostheses from early ages all the way up to adults. This includes everything from prostheses made specifically for running, cycling, swimming, mountain climbing, snowboarding, skiing, and more," Mr. Carroll said.
In fact, the emerging technology is so good that some have suggested it may soon allow disabled athletes to outdo their able-bodied counterparts.
Of course, most amputees are not elite athletes. There are approximately 1.7 million people with limb loss in the United States, with another 150,000 who undergo amputations each year. While injured soldiers tend to be the most visible amputees in the media, diabetes is by far the greatest cause of limb loss – more than 80% of all amputations are the result of diabetes. Vascular disease, trauma, and cancer are among the other top causes.
But even increasing numbers of non–athlete amputees have been showcasing their prostheses within the last 10-15 years, with comparable numbers in both the civilian and military populations. As many as 50% of amputees in the United States now choose to display their prostheses, compared with less than 10% among their European counterparts. "This number wasn’t as high in the U.S. 30 years ago. This has evolved over time," Mr. Carroll said.
A realistic-looking limb cover adds to the cost of the working part of the prosthesis itself, but he believes that’s not why we’re seeing more of the latter. "[Amputees who chose to show their prostheses] like to showcase the technology they’re walking around on. This shows they have really accepted their loss. They are not showcasing their prostheses because of the cost, they’re doing it because of pride."
His advice to physicians: "Never say never to patients. We see people who are confined to wheelchairs get up and walk every day. We’re in a whole new era of rehabilitation. We’re very excited about the future."
–Miriam E. Tucker (@MiriamETucker on Twitter)
*CORRECTION 8/30/12: The original sentence misstated the location of Hanger Orthopedic Group. The sentence should have read: "These are not your grandparents’ prosthetic devices that are available today. They have advanced at such a rapid pace. It’s not only exciting for users of prosthetic devices, but nonamputees are also very interested in the technology – how far it has evolved, and what prosthetic users are capable of doing, whether it be climbing Mount Everest or running in the Olympics with prosthetic limbs," according to Kevin Carroll, vice president of prosthetics at Hanger Orthopedic Group, based in Austin, Tex..
South African runner Oscar Pistorius fell just short of winning a medal at the London 2012 Olympics, but the double amputee did show the world that people with disabilities can achieve greatness in sports. His specially made prosthetic limbs also reflect a growing trend – highly visible in the media among returning soldiers from Afghanistan and Iraq – toward more people with amputations displaying their prostheses rather than hiding them with coverings that resemble arms and legs.
The trend will be further highlighted at the 2012 Paralympic Games, taking place in London Aug. 29 through Sept. 9, and where Mr. Pistorius will again compete.
"These are not your grandparents’ prosthetic devices that are available today. They have advanced at such a rapid pace. It’s not only exciting for users of prosthetic devices, but nonamputees are also very interested in the technology – how far it has evolved, and what prosthetic users are capable of doing, whether it be climbing Mount Everest or running in the Olympics with prosthetic limbs," according to Kevin Carroll, vice president of prosthetics at Hanger Orthopedic Group, based in Austin, Tex.*
About 4,200 athletes from 165 countries will compete in the Paralympics, across 503 medal events. Athletes with amputations will be some of the most visually distinct among the Paralympics’ 10 categories of disability: impaired muscle power, impaired passive range of motion, limb deficiency, leg length difference, short stature, hypertonia, ataxia, athetosis, vision impairment, and intellectual impairment.
"This year, we’re expecting all sorts of records at the Paralympics. There is a greater focus worldwide and countries throughout the world are seeing the importance of supporting athletes in Paralympic events ... I predict many more Pistorius-types are on their way to the Olympics," said Mr. Carroll.
In the Paralympics, athletes using specially designed prosthetic devices –that is, used specifically for the sport and not for everyday use – will be most visible in track and field events. Prosthetic devices are not used in sports such as swimming and sitting volleyball. However, athletes with amputations are using prostheses for a wide range of sports in the real world.
"We are now supplying sport-specific prostheses from early ages all the way up to adults. This includes everything from prostheses made specifically for running, cycling, swimming, mountain climbing, snowboarding, skiing, and more," Mr. Carroll said.
In fact, the emerging technology is so good that some have suggested it may soon allow disabled athletes to outdo their able-bodied counterparts.
Of course, most amputees are not elite athletes. There are approximately 1.7 million people with limb loss in the United States, with another 150,000 who undergo amputations each year. While injured soldiers tend to be the most visible amputees in the media, diabetes is by far the greatest cause of limb loss – more than 80% of all amputations are the result of diabetes. Vascular disease, trauma, and cancer are among the other top causes.
But even increasing numbers of non–athlete amputees have been showcasing their prostheses within the last 10-15 years, with comparable numbers in both the civilian and military populations. As many as 50% of amputees in the United States now choose to display their prostheses, compared with less than 10% among their European counterparts. "This number wasn’t as high in the U.S. 30 years ago. This has evolved over time," Mr. Carroll said.
A realistic-looking limb cover adds to the cost of the working part of the prosthesis itself, but he believes that’s not why we’re seeing more of the latter. "[Amputees who chose to show their prostheses] like to showcase the technology they’re walking around on. This shows they have really accepted their loss. They are not showcasing their prostheses because of the cost, they’re doing it because of pride."
His advice to physicians: "Never say never to patients. We see people who are confined to wheelchairs get up and walk every day. We’re in a whole new era of rehabilitation. We’re very excited about the future."
–Miriam E. Tucker (@MiriamETucker on Twitter)
*CORRECTION 8/30/12: The original sentence misstated the location of Hanger Orthopedic Group. The sentence should have read: "These are not your grandparents’ prosthetic devices that are available today. They have advanced at such a rapid pace. It’s not only exciting for users of prosthetic devices, but nonamputees are also very interested in the technology – how far it has evolved, and what prosthetic users are capable of doing, whether it be climbing Mount Everest or running in the Olympics with prosthetic limbs," according to Kevin Carroll, vice president of prosthetics at Hanger Orthopedic Group, based in Austin, Tex..
As Hospitalists Cement their Worth, Compensation Continues Upward Climb
Tom Smith, MD, vice president of clinical quality and hospitalist medical director at North Fulton Regional Medical Center in Alpharetta, Ga., is emotionally torn every time practice-based compensation and productivity data are published— data he will both use and defend against in negotiations with staffers and would-be staffers.
If nocturnist salaries are increasing nationally, will he have to pay his night-shift staff more?
If work relative-value units (wRVUs) are rising in his region, will he need to review how hard he is working his staff—or not hard enough?
If group leaders are receiving compensation premiums, should his top docs be paid above rank-and-file colleagues?
“There are upsides and downsides,” Dr. Smith says. “No. 1, if your compensation is good or appropriate, they sort of validate your pay and, I guess, your self-worth to some degree. But I think if you’re on the lower side, it definitely starts bringing to mind the ‘grass-is-greener scenarios.’”
For hospitalists, the grass is green in most cases. Median compensation for adult hospitalists rose 6% to $233,855 in 2011, while productivity remained nearly static, according to the Medical Group Management Association’s (MGMA) Physician Compensation and Production Survey: 2012 Report Based on 2011 Data. The report is based on data compiled from 3,402 hospitalists nationwide; slightly more than 56% of respondents work in practices owned by hospitals/integrated delivery systems (IDS); 26% work in physician-owned groups.
The data, which excludes academic hospitalists, shows hospitalist pay has jumped more than 27% since 2008, when unadjusted figures pegged median hospitalist compensation at $183,900 nationwide. The climb comes despite little movement in the number of wRVUs hospitalists are producing. In 2011, the median physician wRVU rate was 4,159 per year, a 0.17% drop from the year prior.
The MGMA survey data will be incorporated into SHM’s annual State of Hospital Medicine report (SOHM), which features information on individual physicians and HM groups. The SOHM report received submission from 396 groups that serve adults only (for more about the survey instruments, see “Apples to Apples?”). Some 40% were employed by hospitals/IDS; a third were employed by management companies; and the rest were academic or other models. The report includes group-level data valuable to hospitalist groups, including financial data (i.e. hospital support payments and CPT code distribution), and information on staffing and scheduling.
Combined, the MGMA and SHM reports show a specialty where compensation continues to be pushed by demand outstripping supply, particularly in southern states (see Table 1). More subtly, leading hospitalists say, the data shows that much of the work that physicians now do—QI initiatives, committee leadership and leading digitalization efforts—is not completely captured by the wRVU metric, long the gold standard for measuring productivity.
“I really believe this is critical, critical information for people to have,” says William “Tex” Landis, MD, FHM, chairman of SHM’s Practice Analysis Committee. “Administrators need to have information to make sure that they’re being appropriate in the compensation that they’re paying the physicians. So everybody that’s involved in hospitalist programs is interested, or should be interested in this data, because it allows them to right resource their programs.”
Dollars and Sense
First and foremost in the MGMA data is the continued trend of rising compensation. In a seemingly endless uptick, hospitalists in the South continue to earn the most (median compensation $258,793, up from $247,000 in 2011 data). Southern hospitalists, though, only saw a 4.8% increase in compensation. The largest percentage jump (7.4%) was for hospitalists in the East (median compensation $227,656, up from $212,000 the year prior). Doctors in the East typically have the lowest compensation of the country’s four geographic regions, but this year’s data showed hospitalists in the West with the lowest figure (median compensation $223,574, up from $213,405).
Dan Fuller, president of IN Compass Health of Alpharetta, Ga., and a member of SHM’s Practice Analysis Committee, says the rising compensation makes perfect sense.
“In fact, I think it’s something we’re probably going to have to deal with for the near future,” he says, noting some in the specialty believed median compensation was ready to plateau. “And, certainly, as hospitalists are asked to do more and more, and they a play a bigger and bigger role in the facility, there should be a higher expectation that they continue to make more and more, and make a bigger impact.”
The question group leaders are asking now: how high can the compensation figures climb? Todd Evenson, MGMA director of data solutions, says there is no answer, yet. Evenson says he sees no immediate obstacles to the continued growth, as hospitalists have established themselves as major players in most hospitals.
What could determine the upper limit is “the payment mechanisms that we start to see fall out of the legislation that occurs,” he says. “As that evolves, I can’t say I know the ceiling.”
A year or two ago, Dr. Landis would have told anyone who asked that the compensation limit was in sight. Now, he believes that as long as competition makes recruitment and retention difficult, it’s hard to predict an end to the compensation growth.
“I don’t think there is a hospitalist group, whether it’s hospitalist-owned, national company, or a private group part of a large, multi-specialty clinic, there’s not a group in the country that’s not struggling with recruitment and retention,” Dr. Fuller adds.
The Value of wRVUs
One data point that has stymied the expectations of some hospitalists is the relative stability of wRVUs. The national figure has ticked up 1.26% since 2010 to 4,159 per year. But the stability is geographically deceiving. In the East and Midwest regions, hospitalist wRVUs jumped 9.8% and 9.7%, respectively. In the South and West, wRVUs fell 1.8% and 2.7%, respectively.
In whole numbers, the South continues to show the highest productivity per physician. Hospitalists in the south produced 671 more wRVUs than the next-highest regional cohort, (5,192 South vs. 4,521 East). Hospitalists in the South region also produced nearly 35% more wRVUs than their Western counterparts (5,192 vs. 3,383).
Survey experts have no explanation for why regional productivity varies so much. Regardless, the trouble with wRVUs is that they are intended to serve as a proxy of billable productivity, hospitalists say.
As HM groups and physicians become more engaged in moving from a fee-for-service payment model to one that rewards quality and value, the metric becomes less precise, says Ken Simone, DO, SFHM, founder and president of Hospitalist and Practice Solutions in Veazie, Maine, an HM consulting firm.
“Productivity in some ways is difficult to measure with hospitalists because they are also providing services [while they are] working on committees, doing IT work, or doing some research,” says Dr. Simone, a member of Team Hospitalist. “I’m very careful in how I look at a hospitalist’s productivity.”
Although some are hesitant to suggest that wRVUs have leveled, Dr. Smith, who oversees a six-hospitalist group at his 208-bed institution about 25 miles north of Atlanta, believes hospitalists “are about at capacity.”
“Particularly considering the new pressures that are on patient satisfaction, time to go in the ER, length of stay, discharge, reducing readmissions,” he says. “I think it’s going to be hard to push that number up.”
Dr. Landis, medical director of Wellspan Hospitalists in York, Pa., believes it is counter-productive to push wRVUs too high. He believes a hospitalist’s role is to provide patient care, lead process improvement, and coordinate multi-disciplinary teams. Too much of a focus on any one role takes away from physician efficacy.
“The value of a hospitalist goes well beyond the wRVU number,” he explains. “That being said, we are still in the business of seeing patients. I don’t think having a hospitalist that’s generating 1,500 RVUs and paying them at the 75th percentile is going to be very effective. You’re going to need to balance those out.”
continued below...
How Much Turnover Is Too Much Turnover?
Some HM leaders were pleased last year when hospitalist turnover dropped to 8% from a 14% turnover rate the year prior. This year’s State of Hospital Medicine report pegs the turnover figure at 10%. Although just a slight increase this year, Fuller views the uptick in turnover as a burgeoning cycle. While the supply-demand curve continues to push compensation up, increased turnover will continue to impact both sides of the equation.
“I’m sure there are many fully staffed programs, but they’re dealing with turnover,” he says. “They’re dealing with attrition, physicians leaving to go to fellowships, physicians relocating...physicians wanting to retire. I think it’s a crisis, a tremendous crisis that we need to be prepared to deal with for the near future.”
Aside from turnover data, the SOHM report this year looked to break new ground by trying out new questions. The report for the first time surveyed how hospitalists perform comanagement duties. In surgical comanagement cases, the hospitalist served as the admitting or attending physician 57% of the time. The rest of the time they served as a consultant. In medical comanagement, hospitalists were the admitting/attending physician 85% of the time (see “Comanagement Roles,”).
As hospitalists find specialties even within the field, the report also looked to put data to the cohort of nocturnists. Roughly half of those covering night shifts work fewer shifts than their daytime colleagues. Moreover, 63% of nocturnists earned a differential for that work (see Figure 4).
The value of data points on emerging and existing trends is that it gives HM groups and group administrators thresholds to benchmark themselves against, Dr. Simone says.
“It also allows the HM leader to compare within a practice,” he adds. “If hospital medicine groups are performing at or above median, or are highly functional groups, it gives great feedback that they’re doing things correctly. But it also gives the leader an opportunity to make a sound business plan when he’s going to talk to the hospital [administration] for subsidy, or when he’s going to negotiate compensation for his providers for the next year. I think that’s a powerful tool.”
Interactive regional survey breakdowns
Richard Quinn is a freelance writer based in New Jersey.
Tom Smith, MD, vice president of clinical quality and hospitalist medical director at North Fulton Regional Medical Center in Alpharetta, Ga., is emotionally torn every time practice-based compensation and productivity data are published— data he will both use and defend against in negotiations with staffers and would-be staffers.
If nocturnist salaries are increasing nationally, will he have to pay his night-shift staff more?
If work relative-value units (wRVUs) are rising in his region, will he need to review how hard he is working his staff—or not hard enough?
If group leaders are receiving compensation premiums, should his top docs be paid above rank-and-file colleagues?
“There are upsides and downsides,” Dr. Smith says. “No. 1, if your compensation is good or appropriate, they sort of validate your pay and, I guess, your self-worth to some degree. But I think if you’re on the lower side, it definitely starts bringing to mind the ‘grass-is-greener scenarios.’”
For hospitalists, the grass is green in most cases. Median compensation for adult hospitalists rose 6% to $233,855 in 2011, while productivity remained nearly static, according to the Medical Group Management Association’s (MGMA) Physician Compensation and Production Survey: 2012 Report Based on 2011 Data. The report is based on data compiled from 3,402 hospitalists nationwide; slightly more than 56% of respondents work in practices owned by hospitals/integrated delivery systems (IDS); 26% work in physician-owned groups.
The data, which excludes academic hospitalists, shows hospitalist pay has jumped more than 27% since 2008, when unadjusted figures pegged median hospitalist compensation at $183,900 nationwide. The climb comes despite little movement in the number of wRVUs hospitalists are producing. In 2011, the median physician wRVU rate was 4,159 per year, a 0.17% drop from the year prior.
The MGMA survey data will be incorporated into SHM’s annual State of Hospital Medicine report (SOHM), which features information on individual physicians and HM groups. The SOHM report received submission from 396 groups that serve adults only (for more about the survey instruments, see “Apples to Apples?”). Some 40% were employed by hospitals/IDS; a third were employed by management companies; and the rest were academic or other models. The report includes group-level data valuable to hospitalist groups, including financial data (i.e. hospital support payments and CPT code distribution), and information on staffing and scheduling.
Combined, the MGMA and SHM reports show a specialty where compensation continues to be pushed by demand outstripping supply, particularly in southern states (see Table 1). More subtly, leading hospitalists say, the data shows that much of the work that physicians now do—QI initiatives, committee leadership and leading digitalization efforts—is not completely captured by the wRVU metric, long the gold standard for measuring productivity.
“I really believe this is critical, critical information for people to have,” says William “Tex” Landis, MD, FHM, chairman of SHM’s Practice Analysis Committee. “Administrators need to have information to make sure that they’re being appropriate in the compensation that they’re paying the physicians. So everybody that’s involved in hospitalist programs is interested, or should be interested in this data, because it allows them to right resource their programs.”
Dollars and Sense
First and foremost in the MGMA data is the continued trend of rising compensation. In a seemingly endless uptick, hospitalists in the South continue to earn the most (median compensation $258,793, up from $247,000 in 2011 data). Southern hospitalists, though, only saw a 4.8% increase in compensation. The largest percentage jump (7.4%) was for hospitalists in the East (median compensation $227,656, up from $212,000 the year prior). Doctors in the East typically have the lowest compensation of the country’s four geographic regions, but this year’s data showed hospitalists in the West with the lowest figure (median compensation $223,574, up from $213,405).
Dan Fuller, president of IN Compass Health of Alpharetta, Ga., and a member of SHM’s Practice Analysis Committee, says the rising compensation makes perfect sense.
“In fact, I think it’s something we’re probably going to have to deal with for the near future,” he says, noting some in the specialty believed median compensation was ready to plateau. “And, certainly, as hospitalists are asked to do more and more, and they a play a bigger and bigger role in the facility, there should be a higher expectation that they continue to make more and more, and make a bigger impact.”
The question group leaders are asking now: how high can the compensation figures climb? Todd Evenson, MGMA director of data solutions, says there is no answer, yet. Evenson says he sees no immediate obstacles to the continued growth, as hospitalists have established themselves as major players in most hospitals.
What could determine the upper limit is “the payment mechanisms that we start to see fall out of the legislation that occurs,” he says. “As that evolves, I can’t say I know the ceiling.”
A year or two ago, Dr. Landis would have told anyone who asked that the compensation limit was in sight. Now, he believes that as long as competition makes recruitment and retention difficult, it’s hard to predict an end to the compensation growth.
“I don’t think there is a hospitalist group, whether it’s hospitalist-owned, national company, or a private group part of a large, multi-specialty clinic, there’s not a group in the country that’s not struggling with recruitment and retention,” Dr. Fuller adds.
The Value of wRVUs
One data point that has stymied the expectations of some hospitalists is the relative stability of wRVUs. The national figure has ticked up 1.26% since 2010 to 4,159 per year. But the stability is geographically deceiving. In the East and Midwest regions, hospitalist wRVUs jumped 9.8% and 9.7%, respectively. In the South and West, wRVUs fell 1.8% and 2.7%, respectively.
In whole numbers, the South continues to show the highest productivity per physician. Hospitalists in the south produced 671 more wRVUs than the next-highest regional cohort, (5,192 South vs. 4,521 East). Hospitalists in the South region also produced nearly 35% more wRVUs than their Western counterparts (5,192 vs. 3,383).
Survey experts have no explanation for why regional productivity varies so much. Regardless, the trouble with wRVUs is that they are intended to serve as a proxy of billable productivity, hospitalists say.
As HM groups and physicians become more engaged in moving from a fee-for-service payment model to one that rewards quality and value, the metric becomes less precise, says Ken Simone, DO, SFHM, founder and president of Hospitalist and Practice Solutions in Veazie, Maine, an HM consulting firm.
“Productivity in some ways is difficult to measure with hospitalists because they are also providing services [while they are] working on committees, doing IT work, or doing some research,” says Dr. Simone, a member of Team Hospitalist. “I’m very careful in how I look at a hospitalist’s productivity.”
Although some are hesitant to suggest that wRVUs have leveled, Dr. Smith, who oversees a six-hospitalist group at his 208-bed institution about 25 miles north of Atlanta, believes hospitalists “are about at capacity.”
“Particularly considering the new pressures that are on patient satisfaction, time to go in the ER, length of stay, discharge, reducing readmissions,” he says. “I think it’s going to be hard to push that number up.”
Dr. Landis, medical director of Wellspan Hospitalists in York, Pa., believes it is counter-productive to push wRVUs too high. He believes a hospitalist’s role is to provide patient care, lead process improvement, and coordinate multi-disciplinary teams. Too much of a focus on any one role takes away from physician efficacy.
“The value of a hospitalist goes well beyond the wRVU number,” he explains. “That being said, we are still in the business of seeing patients. I don’t think having a hospitalist that’s generating 1,500 RVUs and paying them at the 75th percentile is going to be very effective. You’re going to need to balance those out.”
continued below...
How Much Turnover Is Too Much Turnover?
Some HM leaders were pleased last year when hospitalist turnover dropped to 8% from a 14% turnover rate the year prior. This year’s State of Hospital Medicine report pegs the turnover figure at 10%. Although just a slight increase this year, Fuller views the uptick in turnover as a burgeoning cycle. While the supply-demand curve continues to push compensation up, increased turnover will continue to impact both sides of the equation.
“I’m sure there are many fully staffed programs, but they’re dealing with turnover,” he says. “They’re dealing with attrition, physicians leaving to go to fellowships, physicians relocating...physicians wanting to retire. I think it’s a crisis, a tremendous crisis that we need to be prepared to deal with for the near future.”
Aside from turnover data, the SOHM report this year looked to break new ground by trying out new questions. The report for the first time surveyed how hospitalists perform comanagement duties. In surgical comanagement cases, the hospitalist served as the admitting or attending physician 57% of the time. The rest of the time they served as a consultant. In medical comanagement, hospitalists were the admitting/attending physician 85% of the time (see “Comanagement Roles,”).
As hospitalists find specialties even within the field, the report also looked to put data to the cohort of nocturnists. Roughly half of those covering night shifts work fewer shifts than their daytime colleagues. Moreover, 63% of nocturnists earned a differential for that work (see Figure 4).
The value of data points on emerging and existing trends is that it gives HM groups and group administrators thresholds to benchmark themselves against, Dr. Simone says.
“It also allows the HM leader to compare within a practice,” he adds. “If hospital medicine groups are performing at or above median, or are highly functional groups, it gives great feedback that they’re doing things correctly. But it also gives the leader an opportunity to make a sound business plan when he’s going to talk to the hospital [administration] for subsidy, or when he’s going to negotiate compensation for his providers for the next year. I think that’s a powerful tool.”
Interactive regional survey breakdowns
Richard Quinn is a freelance writer based in New Jersey.
Tom Smith, MD, vice president of clinical quality and hospitalist medical director at North Fulton Regional Medical Center in Alpharetta, Ga., is emotionally torn every time practice-based compensation and productivity data are published— data he will both use and defend against in negotiations with staffers and would-be staffers.
If nocturnist salaries are increasing nationally, will he have to pay his night-shift staff more?
If work relative-value units (wRVUs) are rising in his region, will he need to review how hard he is working his staff—or not hard enough?
If group leaders are receiving compensation premiums, should his top docs be paid above rank-and-file colleagues?
“There are upsides and downsides,” Dr. Smith says. “No. 1, if your compensation is good or appropriate, they sort of validate your pay and, I guess, your self-worth to some degree. But I think if you’re on the lower side, it definitely starts bringing to mind the ‘grass-is-greener scenarios.’”
For hospitalists, the grass is green in most cases. Median compensation for adult hospitalists rose 6% to $233,855 in 2011, while productivity remained nearly static, according to the Medical Group Management Association’s (MGMA) Physician Compensation and Production Survey: 2012 Report Based on 2011 Data. The report is based on data compiled from 3,402 hospitalists nationwide; slightly more than 56% of respondents work in practices owned by hospitals/integrated delivery systems (IDS); 26% work in physician-owned groups.
The data, which excludes academic hospitalists, shows hospitalist pay has jumped more than 27% since 2008, when unadjusted figures pegged median hospitalist compensation at $183,900 nationwide. The climb comes despite little movement in the number of wRVUs hospitalists are producing. In 2011, the median physician wRVU rate was 4,159 per year, a 0.17% drop from the year prior.
The MGMA survey data will be incorporated into SHM’s annual State of Hospital Medicine report (SOHM), which features information on individual physicians and HM groups. The SOHM report received submission from 396 groups that serve adults only (for more about the survey instruments, see “Apples to Apples?”). Some 40% were employed by hospitals/IDS; a third were employed by management companies; and the rest were academic or other models. The report includes group-level data valuable to hospitalist groups, including financial data (i.e. hospital support payments and CPT code distribution), and information on staffing and scheduling.
Combined, the MGMA and SHM reports show a specialty where compensation continues to be pushed by demand outstripping supply, particularly in southern states (see Table 1). More subtly, leading hospitalists say, the data shows that much of the work that physicians now do—QI initiatives, committee leadership and leading digitalization efforts—is not completely captured by the wRVU metric, long the gold standard for measuring productivity.
“I really believe this is critical, critical information for people to have,” says William “Tex” Landis, MD, FHM, chairman of SHM’s Practice Analysis Committee. “Administrators need to have information to make sure that they’re being appropriate in the compensation that they’re paying the physicians. So everybody that’s involved in hospitalist programs is interested, or should be interested in this data, because it allows them to right resource their programs.”
Dollars and Sense
First and foremost in the MGMA data is the continued trend of rising compensation. In a seemingly endless uptick, hospitalists in the South continue to earn the most (median compensation $258,793, up from $247,000 in 2011 data). Southern hospitalists, though, only saw a 4.8% increase in compensation. The largest percentage jump (7.4%) was for hospitalists in the East (median compensation $227,656, up from $212,000 the year prior). Doctors in the East typically have the lowest compensation of the country’s four geographic regions, but this year’s data showed hospitalists in the West with the lowest figure (median compensation $223,574, up from $213,405).
Dan Fuller, president of IN Compass Health of Alpharetta, Ga., and a member of SHM’s Practice Analysis Committee, says the rising compensation makes perfect sense.
“In fact, I think it’s something we’re probably going to have to deal with for the near future,” he says, noting some in the specialty believed median compensation was ready to plateau. “And, certainly, as hospitalists are asked to do more and more, and they a play a bigger and bigger role in the facility, there should be a higher expectation that they continue to make more and more, and make a bigger impact.”
The question group leaders are asking now: how high can the compensation figures climb? Todd Evenson, MGMA director of data solutions, says there is no answer, yet. Evenson says he sees no immediate obstacles to the continued growth, as hospitalists have established themselves as major players in most hospitals.
What could determine the upper limit is “the payment mechanisms that we start to see fall out of the legislation that occurs,” he says. “As that evolves, I can’t say I know the ceiling.”
A year or two ago, Dr. Landis would have told anyone who asked that the compensation limit was in sight. Now, he believes that as long as competition makes recruitment and retention difficult, it’s hard to predict an end to the compensation growth.
“I don’t think there is a hospitalist group, whether it’s hospitalist-owned, national company, or a private group part of a large, multi-specialty clinic, there’s not a group in the country that’s not struggling with recruitment and retention,” Dr. Fuller adds.
The Value of wRVUs
One data point that has stymied the expectations of some hospitalists is the relative stability of wRVUs. The national figure has ticked up 1.26% since 2010 to 4,159 per year. But the stability is geographically deceiving. In the East and Midwest regions, hospitalist wRVUs jumped 9.8% and 9.7%, respectively. In the South and West, wRVUs fell 1.8% and 2.7%, respectively.
In whole numbers, the South continues to show the highest productivity per physician. Hospitalists in the south produced 671 more wRVUs than the next-highest regional cohort, (5,192 South vs. 4,521 East). Hospitalists in the South region also produced nearly 35% more wRVUs than their Western counterparts (5,192 vs. 3,383).
Survey experts have no explanation for why regional productivity varies so much. Regardless, the trouble with wRVUs is that they are intended to serve as a proxy of billable productivity, hospitalists say.
As HM groups and physicians become more engaged in moving from a fee-for-service payment model to one that rewards quality and value, the metric becomes less precise, says Ken Simone, DO, SFHM, founder and president of Hospitalist and Practice Solutions in Veazie, Maine, an HM consulting firm.
“Productivity in some ways is difficult to measure with hospitalists because they are also providing services [while they are] working on committees, doing IT work, or doing some research,” says Dr. Simone, a member of Team Hospitalist. “I’m very careful in how I look at a hospitalist’s productivity.”
Although some are hesitant to suggest that wRVUs have leveled, Dr. Smith, who oversees a six-hospitalist group at his 208-bed institution about 25 miles north of Atlanta, believes hospitalists “are about at capacity.”
“Particularly considering the new pressures that are on patient satisfaction, time to go in the ER, length of stay, discharge, reducing readmissions,” he says. “I think it’s going to be hard to push that number up.”
Dr. Landis, medical director of Wellspan Hospitalists in York, Pa., believes it is counter-productive to push wRVUs too high. He believes a hospitalist’s role is to provide patient care, lead process improvement, and coordinate multi-disciplinary teams. Too much of a focus on any one role takes away from physician efficacy.
“The value of a hospitalist goes well beyond the wRVU number,” he explains. “That being said, we are still in the business of seeing patients. I don’t think having a hospitalist that’s generating 1,500 RVUs and paying them at the 75th percentile is going to be very effective. You’re going to need to balance those out.”
continued below...
How Much Turnover Is Too Much Turnover?
Some HM leaders were pleased last year when hospitalist turnover dropped to 8% from a 14% turnover rate the year prior. This year’s State of Hospital Medicine report pegs the turnover figure at 10%. Although just a slight increase this year, Fuller views the uptick in turnover as a burgeoning cycle. While the supply-demand curve continues to push compensation up, increased turnover will continue to impact both sides of the equation.
“I’m sure there are many fully staffed programs, but they’re dealing with turnover,” he says. “They’re dealing with attrition, physicians leaving to go to fellowships, physicians relocating...physicians wanting to retire. I think it’s a crisis, a tremendous crisis that we need to be prepared to deal with for the near future.”
Aside from turnover data, the SOHM report this year looked to break new ground by trying out new questions. The report for the first time surveyed how hospitalists perform comanagement duties. In surgical comanagement cases, the hospitalist served as the admitting or attending physician 57% of the time. The rest of the time they served as a consultant. In medical comanagement, hospitalists were the admitting/attending physician 85% of the time (see “Comanagement Roles,”).
As hospitalists find specialties even within the field, the report also looked to put data to the cohort of nocturnists. Roughly half of those covering night shifts work fewer shifts than their daytime colleagues. Moreover, 63% of nocturnists earned a differential for that work (see Figure 4).
The value of data points on emerging and existing trends is that it gives HM groups and group administrators thresholds to benchmark themselves against, Dr. Simone says.
“It also allows the HM leader to compare within a practice,” he adds. “If hospital medicine groups are performing at or above median, or are highly functional groups, it gives great feedback that they’re doing things correctly. But it also gives the leader an opportunity to make a sound business plan when he’s going to talk to the hospital [administration] for subsidy, or when he’s going to negotiate compensation for his providers for the next year. I think that’s a powerful tool.”
Interactive regional survey breakdowns
Richard Quinn is a freelance writer based in New Jersey.