User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
News & Perspectives from Ob.Gyn. News
DRUGS, PREGNANCY, AND LACTATION
Canadian Task Force recommendation on screening for postpartum depression misses the mark
Director, Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, Boston, Massachusetts.
Postpartum/perinatal depression (PPD) remains the most common complication in modern obstetrics, with a prevalence of 10%-15% based on multiple studies over the last 2 decades. Over those same 2 decades, there has been growing interest and motivation across the country—from small community hospitals to major academic centers—to promote screening. Such screening is integrated into obstetrical practices, typically using the Edinburgh Postnatal Depression Scale (EPDS), the most widely used validated screen for PPD globally.
As mentioned in previous columns, the U.S. Preventive Services Task Force recommended screening for PPD in 2016, which includes screening women at highest risk, and both acutely treating and preventing PPD.
Since then, screening women for a common clinical problem like PPD has been widely adopted by clinicians representing a broad spectrum of interdisciplinary care. Providers who are engaged in the treatment of postpartum women—obstetricians, psychiatrists, doulas, lactation consultants, facilitators of postpartum support groups, and advocacy groups among others—are included.
An open question and one of great concern recently to our group and others has been what happens after screening. It is clear that identification of PPD per se is not necessarily a challenge, and we have multiple effective treatments from antidepressants to mindfulness-based cognitive therapy to cognitive-behavioral interventions. There is also a growing number of digital applications aimed at mitigation of depressive symptoms in women with postpartum major depressive disorder. One unanswered question is how to engage women after identification of PPD and how to facilitate access to care in a way that maximizes the likelihood that women who actually are suffering from PPD get adequate treatment.
The “perinatal treatment cascade” refers to the majority of women who, on the other side of identification of PPD, fail to receive adequate treatment and continue to have persistent depression. This is perhaps the greatest challenge to the field and to clinicians—how do we, on the other side of screening, see that these women get access to care and get well?
https://www.mdedge.com/obgyn/drugs-pregnancy-lactation
GENDER-AFFIRMING GYNECOLOGY
Caring for the aging transgender patient
Ob.Gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pennsylvania.
The elderly transgender population is rapidly expanding and remains significantly overlooked. Although emerging evidence provides some guidance for medical and surgical treatment for transgender youth, there is still a paucity of research directed at the management of gender-diverse elders.
To a large extent, the challenges that transgender elders face are no different from those experienced by the general elder population. Irrespective of gender identity, patients begin to undergo cognitive and physical changes, encounter difficulties with activities of daily living, suffer the loss of social networks and friends, and face end-of-life issues. Attributes that contribute to successful aging in the general population include good health, social engagement and support, and having a positive outlook on life. Yet, stigma surrounding gender identity and sexual orientation continues to negatively affect elder transgender people.
https://www.mdedge.com/obgyn/gender-affirming-gynecology
Continue to: LATEST NEWS...
LATEST NEWS
Study: Prenatal supplements fail to meet nutrient needs
Although drugstore shelves might suggest otherwise,affordable dietary supplements that provide critical nutrients in appropriate doses for pregnant women are virtually nonexistent, researchers have found.
In a new study published in the American Journal of Clinical Nutrition, investigators observed what many physicians have long suspected: Most prenatal vitamins and other supplements do not adequately make up the difference of what food-based intake of nutrients leave lacking. Despite patients believing they are getting everything they need with their product purchase, they fall short of guideline-recommended requirements.
“There is no magic pill,” said Katherine A. Sauder, PhD, an associate professor of pediatrics at the University of Colorado Anschutz Medical Campus, Aurora, and lead author of the study. “There is no easy answer here.”
The researchers analyzed 24-hour dietary intake data from 2,450 study participants across five states from 2007 to 2019. Dr. Sauder and colleagues focused on six of the more than 20 key nutrients recommended for pregnant people and determined the target dose for vitamin A, vitamin D, folate, calcium, iron, and omega-3 fatty acids.
The researchers tested more than 20,500 dietary supplements, of which 421 were prenatal products. Only 69 products—three prenatal—included all six nutrients. Just seven products —two prenatal—contained target doses for five nutrients. Only one product, which was not marketed as prenatal, contained target doses for all six nutrients but required seven tablets a serving and cost patients approximately $200 a month.
SARS-CoV-2 crosses placenta and infects brains of two infants: ‘This is a first’
Researchers have found for the first time that COVID infection has crossed the placenta and caused brain damage in two newborns, according to a study published online today in Pediatrics.
One of the infants died at 13 months and the other remained in hospice care at time of manuscript submission.
Lead author Merline Benny, MD,with the division of neonatology, department of pediatrics at University of Miami, and colleagues briefed reporters today ahead of the release.
“This is a first,” said senior author Shahnaz Duara, MD, medical director of the Neonatal Intensive Care Unit at Holtz Children’s Hospital, Miami, explaining it is the first study to confirm cross-placental SARS-CoV-2 transmission leading to brain injury in a newborn.
Both infants negative for the virus at birth
The two infants were admitted in the early days of the pandemic in the Delta wave to the neonatal ICU at Holtz Children’s Hospital at University of Miami/Jackson Memorial Medical Center.
Both infants tested negative for the virus at birth, but had significantly elevated SARS-CoV-2 antibodies in their blood, indicating that either antibodies crossed the placenta, or the virus crossed and the immune response was the baby’s.
Dr. Benny explained that the researchers have seen, to this point, more than 700 mother/infant pairs in whom the mother tested positive for COVID in Jackson hospital.
Most who tested positive for COVID were asymptomatic and most of the mothers and infants left the hospital without complications.
“However, (these) two babies had a very unusual clinical picture,” Dr. Benny said.
Those infants were born to mothers who became COVID positive in the second trimester and delivered a few weeks later.
Perinatal HIV nearly eradicated in U.S.
Rates of perinatal HIV have dropped so much that the disease is effectively eliminated in the United States, with less than 1 baby for every 100,000 live births having the virus, a new study released by researchers at the Centers for Disease Control and Prevention finds.
The report marks significant progress on the U.S. government’s goal to eradicate perinatal HIV, an immune-weakening and potentially deadly virus that is passed from mother to baby during pregnancy. Just 32 children in the country were diagnosed in 2019, compared with twice as many in 2010, according to the CDC.
Mothers who are HIV positive can prevent transmission of the infection by receiving antiretroviral therapy, according to Monica Gandhi, MD, MPH, a professor of medicine at University of California, San Francisco’s division of HIV, infectious disease and global medicine.
Dr. Gandhi said she could recall only one case of perinatal HIV in the San Francisco area over the last decade.
DRUGS, PREGNANCY, AND LACTATION
Canadian Task Force recommendation on screening for postpartum depression misses the mark
Director, Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, Boston, Massachusetts.
Postpartum/perinatal depression (PPD) remains the most common complication in modern obstetrics, with a prevalence of 10%-15% based on multiple studies over the last 2 decades. Over those same 2 decades, there has been growing interest and motivation across the country—from small community hospitals to major academic centers—to promote screening. Such screening is integrated into obstetrical practices, typically using the Edinburgh Postnatal Depression Scale (EPDS), the most widely used validated screen for PPD globally.
As mentioned in previous columns, the U.S. Preventive Services Task Force recommended screening for PPD in 2016, which includes screening women at highest risk, and both acutely treating and preventing PPD.
Since then, screening women for a common clinical problem like PPD has been widely adopted by clinicians representing a broad spectrum of interdisciplinary care. Providers who are engaged in the treatment of postpartum women—obstetricians, psychiatrists, doulas, lactation consultants, facilitators of postpartum support groups, and advocacy groups among others—are included.
An open question and one of great concern recently to our group and others has been what happens after screening. It is clear that identification of PPD per se is not necessarily a challenge, and we have multiple effective treatments from antidepressants to mindfulness-based cognitive therapy to cognitive-behavioral interventions. There is also a growing number of digital applications aimed at mitigation of depressive symptoms in women with postpartum major depressive disorder. One unanswered question is how to engage women after identification of PPD and how to facilitate access to care in a way that maximizes the likelihood that women who actually are suffering from PPD get adequate treatment.
The “perinatal treatment cascade” refers to the majority of women who, on the other side of identification of PPD, fail to receive adequate treatment and continue to have persistent depression. This is perhaps the greatest challenge to the field and to clinicians—how do we, on the other side of screening, see that these women get access to care and get well?
https://www.mdedge.com/obgyn/drugs-pregnancy-lactation
GENDER-AFFIRMING GYNECOLOGY
Caring for the aging transgender patient
Ob.Gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pennsylvania.
The elderly transgender population is rapidly expanding and remains significantly overlooked. Although emerging evidence provides some guidance for medical and surgical treatment for transgender youth, there is still a paucity of research directed at the management of gender-diverse elders.
To a large extent, the challenges that transgender elders face are no different from those experienced by the general elder population. Irrespective of gender identity, patients begin to undergo cognitive and physical changes, encounter difficulties with activities of daily living, suffer the loss of social networks and friends, and face end-of-life issues. Attributes that contribute to successful aging in the general population include good health, social engagement and support, and having a positive outlook on life. Yet, stigma surrounding gender identity and sexual orientation continues to negatively affect elder transgender people.
https://www.mdedge.com/obgyn/gender-affirming-gynecology
Continue to: LATEST NEWS...
LATEST NEWS
Study: Prenatal supplements fail to meet nutrient needs
Although drugstore shelves might suggest otherwise,affordable dietary supplements that provide critical nutrients in appropriate doses for pregnant women are virtually nonexistent, researchers have found.
In a new study published in the American Journal of Clinical Nutrition, investigators observed what many physicians have long suspected: Most prenatal vitamins and other supplements do not adequately make up the difference of what food-based intake of nutrients leave lacking. Despite patients believing they are getting everything they need with their product purchase, they fall short of guideline-recommended requirements.
“There is no magic pill,” said Katherine A. Sauder, PhD, an associate professor of pediatrics at the University of Colorado Anschutz Medical Campus, Aurora, and lead author of the study. “There is no easy answer here.”
The researchers analyzed 24-hour dietary intake data from 2,450 study participants across five states from 2007 to 2019. Dr. Sauder and colleagues focused on six of the more than 20 key nutrients recommended for pregnant people and determined the target dose for vitamin A, vitamin D, folate, calcium, iron, and omega-3 fatty acids.
The researchers tested more than 20,500 dietary supplements, of which 421 were prenatal products. Only 69 products—three prenatal—included all six nutrients. Just seven products —two prenatal—contained target doses for five nutrients. Only one product, which was not marketed as prenatal, contained target doses for all six nutrients but required seven tablets a serving and cost patients approximately $200 a month.
SARS-CoV-2 crosses placenta and infects brains of two infants: ‘This is a first’
Researchers have found for the first time that COVID infection has crossed the placenta and caused brain damage in two newborns, according to a study published online today in Pediatrics.
One of the infants died at 13 months and the other remained in hospice care at time of manuscript submission.
Lead author Merline Benny, MD,with the division of neonatology, department of pediatrics at University of Miami, and colleagues briefed reporters today ahead of the release.
“This is a first,” said senior author Shahnaz Duara, MD, medical director of the Neonatal Intensive Care Unit at Holtz Children’s Hospital, Miami, explaining it is the first study to confirm cross-placental SARS-CoV-2 transmission leading to brain injury in a newborn.
Both infants negative for the virus at birth
The two infants were admitted in the early days of the pandemic in the Delta wave to the neonatal ICU at Holtz Children’s Hospital at University of Miami/Jackson Memorial Medical Center.
Both infants tested negative for the virus at birth, but had significantly elevated SARS-CoV-2 antibodies in their blood, indicating that either antibodies crossed the placenta, or the virus crossed and the immune response was the baby’s.
Dr. Benny explained that the researchers have seen, to this point, more than 700 mother/infant pairs in whom the mother tested positive for COVID in Jackson hospital.
Most who tested positive for COVID were asymptomatic and most of the mothers and infants left the hospital without complications.
“However, (these) two babies had a very unusual clinical picture,” Dr. Benny said.
Those infants were born to mothers who became COVID positive in the second trimester and delivered a few weeks later.
Perinatal HIV nearly eradicated in U.S.
Rates of perinatal HIV have dropped so much that the disease is effectively eliminated in the United States, with less than 1 baby for every 100,000 live births having the virus, a new study released by researchers at the Centers for Disease Control and Prevention finds.
The report marks significant progress on the U.S. government’s goal to eradicate perinatal HIV, an immune-weakening and potentially deadly virus that is passed from mother to baby during pregnancy. Just 32 children in the country were diagnosed in 2019, compared with twice as many in 2010, according to the CDC.
Mothers who are HIV positive can prevent transmission of the infection by receiving antiretroviral therapy, according to Monica Gandhi, MD, MPH, a professor of medicine at University of California, San Francisco’s division of HIV, infectious disease and global medicine.
Dr. Gandhi said she could recall only one case of perinatal HIV in the San Francisco area over the last decade.
DRUGS, PREGNANCY, AND LACTATION
Canadian Task Force recommendation on screening for postpartum depression misses the mark
Director, Ammon-Pinizzotto Center for Women’s Mental Health at Massachusetts General Hospital, Boston, Massachusetts.
Postpartum/perinatal depression (PPD) remains the most common complication in modern obstetrics, with a prevalence of 10%-15% based on multiple studies over the last 2 decades. Over those same 2 decades, there has been growing interest and motivation across the country—from small community hospitals to major academic centers—to promote screening. Such screening is integrated into obstetrical practices, typically using the Edinburgh Postnatal Depression Scale (EPDS), the most widely used validated screen for PPD globally.
As mentioned in previous columns, the U.S. Preventive Services Task Force recommended screening for PPD in 2016, which includes screening women at highest risk, and both acutely treating and preventing PPD.
Since then, screening women for a common clinical problem like PPD has been widely adopted by clinicians representing a broad spectrum of interdisciplinary care. Providers who are engaged in the treatment of postpartum women—obstetricians, psychiatrists, doulas, lactation consultants, facilitators of postpartum support groups, and advocacy groups among others—are included.
An open question and one of great concern recently to our group and others has been what happens after screening. It is clear that identification of PPD per se is not necessarily a challenge, and we have multiple effective treatments from antidepressants to mindfulness-based cognitive therapy to cognitive-behavioral interventions. There is also a growing number of digital applications aimed at mitigation of depressive symptoms in women with postpartum major depressive disorder. One unanswered question is how to engage women after identification of PPD and how to facilitate access to care in a way that maximizes the likelihood that women who actually are suffering from PPD get adequate treatment.
The “perinatal treatment cascade” refers to the majority of women who, on the other side of identification of PPD, fail to receive adequate treatment and continue to have persistent depression. This is perhaps the greatest challenge to the field and to clinicians—how do we, on the other side of screening, see that these women get access to care and get well?
https://www.mdedge.com/obgyn/drugs-pregnancy-lactation
GENDER-AFFIRMING GYNECOLOGY
Caring for the aging transgender patient
Ob.Gyn. and fellowship-trained gender-affirming surgeon in West Reading, Pennsylvania.
The elderly transgender population is rapidly expanding and remains significantly overlooked. Although emerging evidence provides some guidance for medical and surgical treatment for transgender youth, there is still a paucity of research directed at the management of gender-diverse elders.
To a large extent, the challenges that transgender elders face are no different from those experienced by the general elder population. Irrespective of gender identity, patients begin to undergo cognitive and physical changes, encounter difficulties with activities of daily living, suffer the loss of social networks and friends, and face end-of-life issues. Attributes that contribute to successful aging in the general population include good health, social engagement and support, and having a positive outlook on life. Yet, stigma surrounding gender identity and sexual orientation continues to negatively affect elder transgender people.
https://www.mdedge.com/obgyn/gender-affirming-gynecology
Continue to: LATEST NEWS...
LATEST NEWS
Study: Prenatal supplements fail to meet nutrient needs
Although drugstore shelves might suggest otherwise,affordable dietary supplements that provide critical nutrients in appropriate doses for pregnant women are virtually nonexistent, researchers have found.
In a new study published in the American Journal of Clinical Nutrition, investigators observed what many physicians have long suspected: Most prenatal vitamins and other supplements do not adequately make up the difference of what food-based intake of nutrients leave lacking. Despite patients believing they are getting everything they need with their product purchase, they fall short of guideline-recommended requirements.
“There is no magic pill,” said Katherine A. Sauder, PhD, an associate professor of pediatrics at the University of Colorado Anschutz Medical Campus, Aurora, and lead author of the study. “There is no easy answer here.”
The researchers analyzed 24-hour dietary intake data from 2,450 study participants across five states from 2007 to 2019. Dr. Sauder and colleagues focused on six of the more than 20 key nutrients recommended for pregnant people and determined the target dose for vitamin A, vitamin D, folate, calcium, iron, and omega-3 fatty acids.
The researchers tested more than 20,500 dietary supplements, of which 421 were prenatal products. Only 69 products—three prenatal—included all six nutrients. Just seven products —two prenatal—contained target doses for five nutrients. Only one product, which was not marketed as prenatal, contained target doses for all six nutrients but required seven tablets a serving and cost patients approximately $200 a month.
SARS-CoV-2 crosses placenta and infects brains of two infants: ‘This is a first’
Researchers have found for the first time that COVID infection has crossed the placenta and caused brain damage in two newborns, according to a study published online today in Pediatrics.
One of the infants died at 13 months and the other remained in hospice care at time of manuscript submission.
Lead author Merline Benny, MD,with the division of neonatology, department of pediatrics at University of Miami, and colleagues briefed reporters today ahead of the release.
“This is a first,” said senior author Shahnaz Duara, MD, medical director of the Neonatal Intensive Care Unit at Holtz Children’s Hospital, Miami, explaining it is the first study to confirm cross-placental SARS-CoV-2 transmission leading to brain injury in a newborn.
Both infants negative for the virus at birth
The two infants were admitted in the early days of the pandemic in the Delta wave to the neonatal ICU at Holtz Children’s Hospital at University of Miami/Jackson Memorial Medical Center.
Both infants tested negative for the virus at birth, but had significantly elevated SARS-CoV-2 antibodies in their blood, indicating that either antibodies crossed the placenta, or the virus crossed and the immune response was the baby’s.
Dr. Benny explained that the researchers have seen, to this point, more than 700 mother/infant pairs in whom the mother tested positive for COVID in Jackson hospital.
Most who tested positive for COVID were asymptomatic and most of the mothers and infants left the hospital without complications.
“However, (these) two babies had a very unusual clinical picture,” Dr. Benny said.
Those infants were born to mothers who became COVID positive in the second trimester and delivered a few weeks later.
Perinatal HIV nearly eradicated in U.S.
Rates of perinatal HIV have dropped so much that the disease is effectively eliminated in the United States, with less than 1 baby for every 100,000 live births having the virus, a new study released by researchers at the Centers for Disease Control and Prevention finds.
The report marks significant progress on the U.S. government’s goal to eradicate perinatal HIV, an immune-weakening and potentially deadly virus that is passed from mother to baby during pregnancy. Just 32 children in the country were diagnosed in 2019, compared with twice as many in 2010, according to the CDC.
Mothers who are HIV positive can prevent transmission of the infection by receiving antiretroviral therapy, according to Monica Gandhi, MD, MPH, a professor of medicine at University of California, San Francisco’s division of HIV, infectious disease and global medicine.
Dr. Gandhi said she could recall only one case of perinatal HIV in the San Francisco area over the last decade.
Docs fervently hope federal ban on noncompete clauses goes through
The Federal Trade Commission’s proposed regulation that would ban noncompete agreements across the country seems like potential good news for doctors. Of course, many hospitals and employers are against it. As a result, the FTC’s sweeping proposal has tongues wagging on both sides of the issue.
Many physicians are thrilled that they may soon have more control over their career and not be stuck in jobs where they feel frustrated, underpaid, or blocked in their progress.
As of 2018, as many as 45% of primary care physicians had inked such agreements with their employers.
Typically, the agreements prevent physicians from practicing medicine with a new employer for a defined period within a specific geographic area. No matter how attractive an alternate offer of employment might be, doctors are bound by the agreements to say no if the offer exists in that defined area and time period.
The period for public comment on the proposed regulation ended on April 19, and there is currently no set date for a decision.
In a Medscape poll of 558 physicians, more than 9 out of 10 respondents said that they were either currently bound by a noncompete clause or that they had been bound by one in the past that had forced them to temporarily stop working, commute long distances, move to a different area, or switch fields.
The new proposal would make it illegal for an employer, such as a hospital or large group, to enter a noncompete with a worker; maintain a noncompete with a worker; or represent to a worker, under certain circumstances, that the worker is subject to a noncompete.
It also would not only ban future noncompete agreements but also retroactively invalidate existing ones. The FTC reasons that noncompete clauses could potentially increase worker earnings as well as lower health care costs by billions of dollars. If the ruling were to move forward, it would represent part of President Biden’s “worker-forward” priorities, focusing on how competition can be a good thing for employees. The President billed the FTC’s announcement as a “huge win for workers.”
In its statements on the proposed ban, the FTC claimed that it could lower consumer prices across the board by as much as $150 billion per year and return nearly $300 million to workers each year.
However, even if passed, the draft rule would keep in place nonsolicitation rules that many health care organizations have put into place. That means that, if a physician leaves an employer, he or she cannot reach out to former patients and colleagues to bring them along or invite them to switch to him or her in the new job.
Within that clause, however, the FTC has specified that if such nonsolicitation agreement has the “equivalent effect” of a noncompete, the agency would deem it such. That means, even if that rule stays, it could be contested and may be interpreted as violating the noncompete law. So there’s value in reading all the fine print should the ban move forward.
Could the ban bring potential downsides?
Most physicians view the potential to break free of a noncompete agreement as a victory. Peter Glennon, an employment litigation attorney with The Glennon Law Firm in Rochester, N.Y., says not so fast. “If you ask anyone if they’d prefer a noncompete agreement, of course they’re going to say no,” he said in an interview. “It sounds like a restriction, one that can hold you back.”
Mr. Glennon believes that there are actually upsides to physician noncompetes. For instance, many noncompetes come with sign-on bonuses that could potentially disappear without the agreements. There’s also the fact that when some physicians sign a noncompete agreement, they then receive pro bono training and continuing education along with marketing and promotion of their skills. Without signing a noncompete, employers may be less incentivized to provide all those benefits to their physician employers.
Those benefits – and the noncompetes – also vary by specialty, Mr. Glennon said. “In 2021, Washington, DC, banned noncompetes for doctors making less than $250,000. So, most generalists there can walk across the street and get a new job. For specialists like cardiologists or neurosurgeons, however, advanced training and marketing benefits matter, so many of them don’t want to lose noncompetes.”
Still, most physicians hope that the FTC’s ban takes hold. Manan Shah, MD, founder, and chief medical officer at Wyndly, an allergy relief startup practice, is one of them.
“Initially, it might disincentivize hospital systems from helping new physicians build up their name and practice because they might be concerned about a physician leaving and starting anew,” he said. “But in the long term, hospitals require physicians to bring their patients to them for care, so the best hospitals will always compete for the best physicians and support them as they build up their practice.”
Dr. Shah views noncompetes as overly prohibitive to physicians. “Right now, if a physician starts a job at a large hospital system and realizes they want to switch jobs, the noncompete distances are so wide they often have to move cities to continue practicing,” he said. “Picking up and starting over in a new city isn’t an option for everyone and can be especially difficult for someone with a family.”
Where Mr. Glennon argued that a physician leaving a team-based practice might harm patients, Shah takes a different perspective. “Imagine you have a doctor whom you trust and have been working with,” he said. “If something changes at their hospital and they decide to move, you literally have to find a new doctor instead of just being able to see them at another location down the street.”
Another potential burden of the noncompete agreements is that they could possibly squelch doctor’s desires to hang up their own shingle. According to Dr. Shah, the agreements make it so that if a physician wants to work independently, it’s nearly impossible to fly solo. “This is frustrating because independent practices have been shown to be more cost effective and allow patients to build better relationships with their doctors,” he claimed.
A 2016 study from Annals of Family Medicine supports that claim, at least for small general practices. Another study appearing in JAMA concurred. It does point out, however, that the cost equation is nuanced and that benefits of larger systems include more resilience to economic downturns and can provide more specialized care.
Will nonprofit hospitals be subject to this noncompete ban?
Further complicating the noncompete ban issue is how it might impact nonprofit institutions versus their for-profit peers. Most hospitals structured as nonprofits would be exempt from the rule because the FTC Act provides that it can enforce against “persons, partnerships, or corporations,” which are further defined as entities “organized to carry on business for their own profit or that of their members.”
The fallout from this, said Dr. Shah, is that it “would disproportionately affect health care providers, since many hospital systems are nonprofits. This is disconcerting because we know that many nonprofit systems make large profits anyway and can offer executive teams’ lucrative packages, while the nurses, assistants, and physicians providing the care are generally not well compensated.”
So far, about nine states plus Washington, D.C., have already put noncompete bans in place, and they may serve as a harbinger of things to come should the federal ban go into effect. Each varies in its specifics. Some, like Indiana, outright ban them, whereas others limit them based on variables like income and industry. “We’re seeing these states responding to local market conditions,” said Darryl Drevna, senior director of regulatory affairs at the American Medical Group Association. “Health care is a hyperlocal market. Depending on the situation, the bans adapt and respond specific to those states.”
Should the federal ban take hold, however, it will supersede whatever rules the individual states have in place.
Some opponents of the federal ban proposal question its authority to begin with, however, Mr. Glennon included. “Many people believe the FTC is overstepping,” he said. “Some people believe that Section 5 of the FTC Act does not give it the authority to police labor markets.”
Mr. Drevna noted that the FTC has taken an aggressive stance, one that will ultimately wind up in the courts. “How it works out is anyone’s guess,” he said. “Ideally, the FTC will consider the comments and concerns of groups like AMGA and realize that states are best suited to regulate in this area.”
In general, the ban’s supporters are employees/physicians; those who oppose it are their employers. Joining the AMGA in speaking out against the noncompete ban is the American Hospital Association, whereas the American College of Emergency Physicians has come out largely in support of the ban.
Still, doctors like Dr. Shah remain hopeful. “I am optimistic that perhaps my colleagues will not continue to be stuck in overrestrictive noncompetes, but I am also realistic,” he said. “Hospital systems are already coming out strongly against this and they have deep pockets, so I won’t be surprised if it does not come to pass.”
A version of this article first appeared on Medscape.com.
The Federal Trade Commission’s proposed regulation that would ban noncompete agreements across the country seems like potential good news for doctors. Of course, many hospitals and employers are against it. As a result, the FTC’s sweeping proposal has tongues wagging on both sides of the issue.
Many physicians are thrilled that they may soon have more control over their career and not be stuck in jobs where they feel frustrated, underpaid, or blocked in their progress.
As of 2018, as many as 45% of primary care physicians had inked such agreements with their employers.
Typically, the agreements prevent physicians from practicing medicine with a new employer for a defined period within a specific geographic area. No matter how attractive an alternate offer of employment might be, doctors are bound by the agreements to say no if the offer exists in that defined area and time period.
The period for public comment on the proposed regulation ended on April 19, and there is currently no set date for a decision.
In a Medscape poll of 558 physicians, more than 9 out of 10 respondents said that they were either currently bound by a noncompete clause or that they had been bound by one in the past that had forced them to temporarily stop working, commute long distances, move to a different area, or switch fields.
The new proposal would make it illegal for an employer, such as a hospital or large group, to enter a noncompete with a worker; maintain a noncompete with a worker; or represent to a worker, under certain circumstances, that the worker is subject to a noncompete.
It also would not only ban future noncompete agreements but also retroactively invalidate existing ones. The FTC reasons that noncompete clauses could potentially increase worker earnings as well as lower health care costs by billions of dollars. If the ruling were to move forward, it would represent part of President Biden’s “worker-forward” priorities, focusing on how competition can be a good thing for employees. The President billed the FTC’s announcement as a “huge win for workers.”
In its statements on the proposed ban, the FTC claimed that it could lower consumer prices across the board by as much as $150 billion per year and return nearly $300 million to workers each year.
However, even if passed, the draft rule would keep in place nonsolicitation rules that many health care organizations have put into place. That means that, if a physician leaves an employer, he or she cannot reach out to former patients and colleagues to bring them along or invite them to switch to him or her in the new job.
Within that clause, however, the FTC has specified that if such nonsolicitation agreement has the “equivalent effect” of a noncompete, the agency would deem it such. That means, even if that rule stays, it could be contested and may be interpreted as violating the noncompete law. So there’s value in reading all the fine print should the ban move forward.
Could the ban bring potential downsides?
Most physicians view the potential to break free of a noncompete agreement as a victory. Peter Glennon, an employment litigation attorney with The Glennon Law Firm in Rochester, N.Y., says not so fast. “If you ask anyone if they’d prefer a noncompete agreement, of course they’re going to say no,” he said in an interview. “It sounds like a restriction, one that can hold you back.”
Mr. Glennon believes that there are actually upsides to physician noncompetes. For instance, many noncompetes come with sign-on bonuses that could potentially disappear without the agreements. There’s also the fact that when some physicians sign a noncompete agreement, they then receive pro bono training and continuing education along with marketing and promotion of their skills. Without signing a noncompete, employers may be less incentivized to provide all those benefits to their physician employers.
Those benefits – and the noncompetes – also vary by specialty, Mr. Glennon said. “In 2021, Washington, DC, banned noncompetes for doctors making less than $250,000. So, most generalists there can walk across the street and get a new job. For specialists like cardiologists or neurosurgeons, however, advanced training and marketing benefits matter, so many of them don’t want to lose noncompetes.”
Still, most physicians hope that the FTC’s ban takes hold. Manan Shah, MD, founder, and chief medical officer at Wyndly, an allergy relief startup practice, is one of them.
“Initially, it might disincentivize hospital systems from helping new physicians build up their name and practice because they might be concerned about a physician leaving and starting anew,” he said. “But in the long term, hospitals require physicians to bring their patients to them for care, so the best hospitals will always compete for the best physicians and support them as they build up their practice.”
Dr. Shah views noncompetes as overly prohibitive to physicians. “Right now, if a physician starts a job at a large hospital system and realizes they want to switch jobs, the noncompete distances are so wide they often have to move cities to continue practicing,” he said. “Picking up and starting over in a new city isn’t an option for everyone and can be especially difficult for someone with a family.”
Where Mr. Glennon argued that a physician leaving a team-based practice might harm patients, Shah takes a different perspective. “Imagine you have a doctor whom you trust and have been working with,” he said. “If something changes at their hospital and they decide to move, you literally have to find a new doctor instead of just being able to see them at another location down the street.”
Another potential burden of the noncompete agreements is that they could possibly squelch doctor’s desires to hang up their own shingle. According to Dr. Shah, the agreements make it so that if a physician wants to work independently, it’s nearly impossible to fly solo. “This is frustrating because independent practices have been shown to be more cost effective and allow patients to build better relationships with their doctors,” he claimed.
A 2016 study from Annals of Family Medicine supports that claim, at least for small general practices. Another study appearing in JAMA concurred. It does point out, however, that the cost equation is nuanced and that benefits of larger systems include more resilience to economic downturns and can provide more specialized care.
Will nonprofit hospitals be subject to this noncompete ban?
Further complicating the noncompete ban issue is how it might impact nonprofit institutions versus their for-profit peers. Most hospitals structured as nonprofits would be exempt from the rule because the FTC Act provides that it can enforce against “persons, partnerships, or corporations,” which are further defined as entities “organized to carry on business for their own profit or that of their members.”
The fallout from this, said Dr. Shah, is that it “would disproportionately affect health care providers, since many hospital systems are nonprofits. This is disconcerting because we know that many nonprofit systems make large profits anyway and can offer executive teams’ lucrative packages, while the nurses, assistants, and physicians providing the care are generally not well compensated.”
So far, about nine states plus Washington, D.C., have already put noncompete bans in place, and they may serve as a harbinger of things to come should the federal ban go into effect. Each varies in its specifics. Some, like Indiana, outright ban them, whereas others limit them based on variables like income and industry. “We’re seeing these states responding to local market conditions,” said Darryl Drevna, senior director of regulatory affairs at the American Medical Group Association. “Health care is a hyperlocal market. Depending on the situation, the bans adapt and respond specific to those states.”
Should the federal ban take hold, however, it will supersede whatever rules the individual states have in place.
Some opponents of the federal ban proposal question its authority to begin with, however, Mr. Glennon included. “Many people believe the FTC is overstepping,” he said. “Some people believe that Section 5 of the FTC Act does not give it the authority to police labor markets.”
Mr. Drevna noted that the FTC has taken an aggressive stance, one that will ultimately wind up in the courts. “How it works out is anyone’s guess,” he said. “Ideally, the FTC will consider the comments and concerns of groups like AMGA and realize that states are best suited to regulate in this area.”
In general, the ban’s supporters are employees/physicians; those who oppose it are their employers. Joining the AMGA in speaking out against the noncompete ban is the American Hospital Association, whereas the American College of Emergency Physicians has come out largely in support of the ban.
Still, doctors like Dr. Shah remain hopeful. “I am optimistic that perhaps my colleagues will not continue to be stuck in overrestrictive noncompetes, but I am also realistic,” he said. “Hospital systems are already coming out strongly against this and they have deep pockets, so I won’t be surprised if it does not come to pass.”
A version of this article first appeared on Medscape.com.
The Federal Trade Commission’s proposed regulation that would ban noncompete agreements across the country seems like potential good news for doctors. Of course, many hospitals and employers are against it. As a result, the FTC’s sweeping proposal has tongues wagging on both sides of the issue.
Many physicians are thrilled that they may soon have more control over their career and not be stuck in jobs where they feel frustrated, underpaid, or blocked in their progress.
As of 2018, as many as 45% of primary care physicians had inked such agreements with their employers.
Typically, the agreements prevent physicians from practicing medicine with a new employer for a defined period within a specific geographic area. No matter how attractive an alternate offer of employment might be, doctors are bound by the agreements to say no if the offer exists in that defined area and time period.
The period for public comment on the proposed regulation ended on April 19, and there is currently no set date for a decision.
In a Medscape poll of 558 physicians, more than 9 out of 10 respondents said that they were either currently bound by a noncompete clause or that they had been bound by one in the past that had forced them to temporarily stop working, commute long distances, move to a different area, or switch fields.
The new proposal would make it illegal for an employer, such as a hospital or large group, to enter a noncompete with a worker; maintain a noncompete with a worker; or represent to a worker, under certain circumstances, that the worker is subject to a noncompete.
It also would not only ban future noncompete agreements but also retroactively invalidate existing ones. The FTC reasons that noncompete clauses could potentially increase worker earnings as well as lower health care costs by billions of dollars. If the ruling were to move forward, it would represent part of President Biden’s “worker-forward” priorities, focusing on how competition can be a good thing for employees. The President billed the FTC’s announcement as a “huge win for workers.”
In its statements on the proposed ban, the FTC claimed that it could lower consumer prices across the board by as much as $150 billion per year and return nearly $300 million to workers each year.
However, even if passed, the draft rule would keep in place nonsolicitation rules that many health care organizations have put into place. That means that, if a physician leaves an employer, he or she cannot reach out to former patients and colleagues to bring them along or invite them to switch to him or her in the new job.
Within that clause, however, the FTC has specified that if such nonsolicitation agreement has the “equivalent effect” of a noncompete, the agency would deem it such. That means, even if that rule stays, it could be contested and may be interpreted as violating the noncompete law. So there’s value in reading all the fine print should the ban move forward.
Could the ban bring potential downsides?
Most physicians view the potential to break free of a noncompete agreement as a victory. Peter Glennon, an employment litigation attorney with The Glennon Law Firm in Rochester, N.Y., says not so fast. “If you ask anyone if they’d prefer a noncompete agreement, of course they’re going to say no,” he said in an interview. “It sounds like a restriction, one that can hold you back.”
Mr. Glennon believes that there are actually upsides to physician noncompetes. For instance, many noncompetes come with sign-on bonuses that could potentially disappear without the agreements. There’s also the fact that when some physicians sign a noncompete agreement, they then receive pro bono training and continuing education along with marketing and promotion of their skills. Without signing a noncompete, employers may be less incentivized to provide all those benefits to their physician employers.
Those benefits – and the noncompetes – also vary by specialty, Mr. Glennon said. “In 2021, Washington, DC, banned noncompetes for doctors making less than $250,000. So, most generalists there can walk across the street and get a new job. For specialists like cardiologists or neurosurgeons, however, advanced training and marketing benefits matter, so many of them don’t want to lose noncompetes.”
Still, most physicians hope that the FTC’s ban takes hold. Manan Shah, MD, founder, and chief medical officer at Wyndly, an allergy relief startup practice, is one of them.
“Initially, it might disincentivize hospital systems from helping new physicians build up their name and practice because they might be concerned about a physician leaving and starting anew,” he said. “But in the long term, hospitals require physicians to bring their patients to them for care, so the best hospitals will always compete for the best physicians and support them as they build up their practice.”
Dr. Shah views noncompetes as overly prohibitive to physicians. “Right now, if a physician starts a job at a large hospital system and realizes they want to switch jobs, the noncompete distances are so wide they often have to move cities to continue practicing,” he said. “Picking up and starting over in a new city isn’t an option for everyone and can be especially difficult for someone with a family.”
Where Mr. Glennon argued that a physician leaving a team-based practice might harm patients, Shah takes a different perspective. “Imagine you have a doctor whom you trust and have been working with,” he said. “If something changes at their hospital and they decide to move, you literally have to find a new doctor instead of just being able to see them at another location down the street.”
Another potential burden of the noncompete agreements is that they could possibly squelch doctor’s desires to hang up their own shingle. According to Dr. Shah, the agreements make it so that if a physician wants to work independently, it’s nearly impossible to fly solo. “This is frustrating because independent practices have been shown to be more cost effective and allow patients to build better relationships with their doctors,” he claimed.
A 2016 study from Annals of Family Medicine supports that claim, at least for small general practices. Another study appearing in JAMA concurred. It does point out, however, that the cost equation is nuanced and that benefits of larger systems include more resilience to economic downturns and can provide more specialized care.
Will nonprofit hospitals be subject to this noncompete ban?
Further complicating the noncompete ban issue is how it might impact nonprofit institutions versus their for-profit peers. Most hospitals structured as nonprofits would be exempt from the rule because the FTC Act provides that it can enforce against “persons, partnerships, or corporations,” which are further defined as entities “organized to carry on business for their own profit or that of their members.”
The fallout from this, said Dr. Shah, is that it “would disproportionately affect health care providers, since many hospital systems are nonprofits. This is disconcerting because we know that many nonprofit systems make large profits anyway and can offer executive teams’ lucrative packages, while the nurses, assistants, and physicians providing the care are generally not well compensated.”
So far, about nine states plus Washington, D.C., have already put noncompete bans in place, and they may serve as a harbinger of things to come should the federal ban go into effect. Each varies in its specifics. Some, like Indiana, outright ban them, whereas others limit them based on variables like income and industry. “We’re seeing these states responding to local market conditions,” said Darryl Drevna, senior director of regulatory affairs at the American Medical Group Association. “Health care is a hyperlocal market. Depending on the situation, the bans adapt and respond specific to those states.”
Should the federal ban take hold, however, it will supersede whatever rules the individual states have in place.
Some opponents of the federal ban proposal question its authority to begin with, however, Mr. Glennon included. “Many people believe the FTC is overstepping,” he said. “Some people believe that Section 5 of the FTC Act does not give it the authority to police labor markets.”
Mr. Drevna noted that the FTC has taken an aggressive stance, one that will ultimately wind up in the courts. “How it works out is anyone’s guess,” he said. “Ideally, the FTC will consider the comments and concerns of groups like AMGA and realize that states are best suited to regulate in this area.”
In general, the ban’s supporters are employees/physicians; those who oppose it are their employers. Joining the AMGA in speaking out against the noncompete ban is the American Hospital Association, whereas the American College of Emergency Physicians has come out largely in support of the ban.
Still, doctors like Dr. Shah remain hopeful. “I am optimistic that perhaps my colleagues will not continue to be stuck in overrestrictive noncompetes, but I am also realistic,” he said. “Hospital systems are already coming out strongly against this and they have deep pockets, so I won’t be surprised if it does not come to pass.”
A version of this article first appeared on Medscape.com.
Genomic assay changes minds on HER2+ BC treatment
The prospective pilot study is small, and the researchers didn’t report on how the patients fared, according to a poster presented at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress. Plus, the test itself hasn’t been analyzed prospectively. But the study’s lead author, Olga Martínez-Sáez, MD, PhD, said in an interview that the 56% number is significant.
“We consider this percentage to be clinically very relevant,” said Dr. Martínez-Sáez, an oncologist at Hospital Clinic of Barcelona and the University of Barcelona. “HER2DX can change practice.”
Also in an interview, Kent Hoskins, MD, associate chief of hematology/oncology at University of Illinois at Chicago, described HER2DX as a next-generation genomic test that builds on assays developed 2 decades ago to help identify patients who would benefit – or not – from adjuvant chemotherapy.
Dr. Hoskins, who isn’t connected to the new study but has studied genomic tests for breast cancer, said the HER2DX test seeks to provide guidance to oncologists about which of several treatments are most effective in treating patients with HER2+ breast cancer.
“The overall trend in the HER2+ space is escalating therapy, and the cure rates have improved quite substantially,” he said. “But do they all need that much therapy? That’s the clinical question that this assay is addressing.”
The assay examines clinical features and the expression of 4 gene signatures, Dr. Martínez-Sáez said. It provides a risk score estimating the likelihood of recurrence plus a score that estimates the likelihood of achieving pathological complete response (pCR) with trastuzumab-based neoadjuvant therapy and an ERBB2 mRNA score.
In a retrospective 2022 study published in eBioMedicine, researchers reported that the assay “predicts response following neoadjuvant letrozole in combination with dual HER2 blockade with trastuzumab and pertuzumab in early-stage HER2-positive/hormone receptor–positive breast cancer.”
In the 2022 study, researchers wrote that assay results and other scores “might help better tailor systemic therapy in this context and identify candidates for avoiding chemotherapy, a therapy associated with short- and long-term toxicities and impact in quality of life.”
For the new study, a decision-impact analysis, researchers tracked 89 patients with HER2+ breast cancer (median age = 53 years, range 30-79, and 52% postmenopausal), the poster says. Most had T1-2 tumors (87%), negative nodes (64%), grade 2 (56%) or 3 (41%) tumors, and ductal histology (87%). And most were hormone receptor positive (65%). Seventy-eight percent of patients received neoadjuvant therapy (NAT), and 22% underwent upfront surgery.
In 56% of cases, oncologists changed their treatment decisions after getting the results of the HER2DX assays. In 59% of these cases, oncologists de-escalated therapy; in 41%, they escalated therapy, opting for more intense chemotherapy 65% of the time, according to the poster.
Clinician confidence in their decisions improved in 67% of cases, the researchers reported in their poster. Among 56 patients treated with neoadjuvant therapy who could be evaluated, “HER2DX pCR score was significantly associated with pCR (81% in pCR-medium/high and 32% in pCR-low; odds ratio=9.3, P = 0.001) independently of the rest of variables.”
Dr. Hoskins said the new report suggests that the assay can change treatment decisions, although he cautioned that “this study does not in itself establish its place in standard of care.” Large, prospective, randomized research is still needed, he said.
Dr. Martínez-Sáez said, in an interview, that the HER2DX assay should cost about as much as genomic assays for other breast cancer subtypes. These kinds of tests have cost several thousand dollars each in recent years.
What’s next? The decision impact study is ongoing. As for research into the assay itself, “prospective clinical trials are planned to demonstrate its clinical utility to de-escalate and guide therapy,” Dr. Martínez-Sáez said.
No funding is reported. Reveal Genomics is the developer of the HER2DX assay. Dr. Martinez- Saez reports financial relationships with Novartis, Eisai, Roche, and Reveal Genomics. Other study authors report multiple disclosures. Dr. Hoskins discloses non-financial research support from Agendia, which makes the MammaPrint early-breast-cancer assay.
The prospective pilot study is small, and the researchers didn’t report on how the patients fared, according to a poster presented at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress. Plus, the test itself hasn’t been analyzed prospectively. But the study’s lead author, Olga Martínez-Sáez, MD, PhD, said in an interview that the 56% number is significant.
“We consider this percentage to be clinically very relevant,” said Dr. Martínez-Sáez, an oncologist at Hospital Clinic of Barcelona and the University of Barcelona. “HER2DX can change practice.”
Also in an interview, Kent Hoskins, MD, associate chief of hematology/oncology at University of Illinois at Chicago, described HER2DX as a next-generation genomic test that builds on assays developed 2 decades ago to help identify patients who would benefit – or not – from adjuvant chemotherapy.
Dr. Hoskins, who isn’t connected to the new study but has studied genomic tests for breast cancer, said the HER2DX test seeks to provide guidance to oncologists about which of several treatments are most effective in treating patients with HER2+ breast cancer.
“The overall trend in the HER2+ space is escalating therapy, and the cure rates have improved quite substantially,” he said. “But do they all need that much therapy? That’s the clinical question that this assay is addressing.”
The assay examines clinical features and the expression of 4 gene signatures, Dr. Martínez-Sáez said. It provides a risk score estimating the likelihood of recurrence plus a score that estimates the likelihood of achieving pathological complete response (pCR) with trastuzumab-based neoadjuvant therapy and an ERBB2 mRNA score.
In a retrospective 2022 study published in eBioMedicine, researchers reported that the assay “predicts response following neoadjuvant letrozole in combination with dual HER2 blockade with trastuzumab and pertuzumab in early-stage HER2-positive/hormone receptor–positive breast cancer.”
In the 2022 study, researchers wrote that assay results and other scores “might help better tailor systemic therapy in this context and identify candidates for avoiding chemotherapy, a therapy associated with short- and long-term toxicities and impact in quality of life.”
For the new study, a decision-impact analysis, researchers tracked 89 patients with HER2+ breast cancer (median age = 53 years, range 30-79, and 52% postmenopausal), the poster says. Most had T1-2 tumors (87%), negative nodes (64%), grade 2 (56%) or 3 (41%) tumors, and ductal histology (87%). And most were hormone receptor positive (65%). Seventy-eight percent of patients received neoadjuvant therapy (NAT), and 22% underwent upfront surgery.
In 56% of cases, oncologists changed their treatment decisions after getting the results of the HER2DX assays. In 59% of these cases, oncologists de-escalated therapy; in 41%, they escalated therapy, opting for more intense chemotherapy 65% of the time, according to the poster.
Clinician confidence in their decisions improved in 67% of cases, the researchers reported in their poster. Among 56 patients treated with neoadjuvant therapy who could be evaluated, “HER2DX pCR score was significantly associated with pCR (81% in pCR-medium/high and 32% in pCR-low; odds ratio=9.3, P = 0.001) independently of the rest of variables.”
Dr. Hoskins said the new report suggests that the assay can change treatment decisions, although he cautioned that “this study does not in itself establish its place in standard of care.” Large, prospective, randomized research is still needed, he said.
Dr. Martínez-Sáez said, in an interview, that the HER2DX assay should cost about as much as genomic assays for other breast cancer subtypes. These kinds of tests have cost several thousand dollars each in recent years.
What’s next? The decision impact study is ongoing. As for research into the assay itself, “prospective clinical trials are planned to demonstrate its clinical utility to de-escalate and guide therapy,” Dr. Martínez-Sáez said.
No funding is reported. Reveal Genomics is the developer of the HER2DX assay. Dr. Martinez- Saez reports financial relationships with Novartis, Eisai, Roche, and Reveal Genomics. Other study authors report multiple disclosures. Dr. Hoskins discloses non-financial research support from Agendia, which makes the MammaPrint early-breast-cancer assay.
The prospective pilot study is small, and the researchers didn’t report on how the patients fared, according to a poster presented at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress. Plus, the test itself hasn’t been analyzed prospectively. But the study’s lead author, Olga Martínez-Sáez, MD, PhD, said in an interview that the 56% number is significant.
“We consider this percentage to be clinically very relevant,” said Dr. Martínez-Sáez, an oncologist at Hospital Clinic of Barcelona and the University of Barcelona. “HER2DX can change practice.”
Also in an interview, Kent Hoskins, MD, associate chief of hematology/oncology at University of Illinois at Chicago, described HER2DX as a next-generation genomic test that builds on assays developed 2 decades ago to help identify patients who would benefit – or not – from adjuvant chemotherapy.
Dr. Hoskins, who isn’t connected to the new study but has studied genomic tests for breast cancer, said the HER2DX test seeks to provide guidance to oncologists about which of several treatments are most effective in treating patients with HER2+ breast cancer.
“The overall trend in the HER2+ space is escalating therapy, and the cure rates have improved quite substantially,” he said. “But do they all need that much therapy? That’s the clinical question that this assay is addressing.”
The assay examines clinical features and the expression of 4 gene signatures, Dr. Martínez-Sáez said. It provides a risk score estimating the likelihood of recurrence plus a score that estimates the likelihood of achieving pathological complete response (pCR) with trastuzumab-based neoadjuvant therapy and an ERBB2 mRNA score.
In a retrospective 2022 study published in eBioMedicine, researchers reported that the assay “predicts response following neoadjuvant letrozole in combination with dual HER2 blockade with trastuzumab and pertuzumab in early-stage HER2-positive/hormone receptor–positive breast cancer.”
In the 2022 study, researchers wrote that assay results and other scores “might help better tailor systemic therapy in this context and identify candidates for avoiding chemotherapy, a therapy associated with short- and long-term toxicities and impact in quality of life.”
For the new study, a decision-impact analysis, researchers tracked 89 patients with HER2+ breast cancer (median age = 53 years, range 30-79, and 52% postmenopausal), the poster says. Most had T1-2 tumors (87%), negative nodes (64%), grade 2 (56%) or 3 (41%) tumors, and ductal histology (87%). And most were hormone receptor positive (65%). Seventy-eight percent of patients received neoadjuvant therapy (NAT), and 22% underwent upfront surgery.
In 56% of cases, oncologists changed their treatment decisions after getting the results of the HER2DX assays. In 59% of these cases, oncologists de-escalated therapy; in 41%, they escalated therapy, opting for more intense chemotherapy 65% of the time, according to the poster.
Clinician confidence in their decisions improved in 67% of cases, the researchers reported in their poster. Among 56 patients treated with neoadjuvant therapy who could be evaluated, “HER2DX pCR score was significantly associated with pCR (81% in pCR-medium/high and 32% in pCR-low; odds ratio=9.3, P = 0.001) independently of the rest of variables.”
Dr. Hoskins said the new report suggests that the assay can change treatment decisions, although he cautioned that “this study does not in itself establish its place in standard of care.” Large, prospective, randomized research is still needed, he said.
Dr. Martínez-Sáez said, in an interview, that the HER2DX assay should cost about as much as genomic assays for other breast cancer subtypes. These kinds of tests have cost several thousand dollars each in recent years.
What’s next? The decision impact study is ongoing. As for research into the assay itself, “prospective clinical trials are planned to demonstrate its clinical utility to de-escalate and guide therapy,” Dr. Martínez-Sáez said.
No funding is reported. Reveal Genomics is the developer of the HER2DX assay. Dr. Martinez- Saez reports financial relationships with Novartis, Eisai, Roche, and Reveal Genomics. Other study authors report multiple disclosures. Dr. Hoskins discloses non-financial research support from Agendia, which makes the MammaPrint early-breast-cancer assay.
FROM ESMO BREAST CANCER 2023
Gestational HTN, preeclampsia worsen long-term risk for ischemic, nonischemic heart failure
, an observational study suggests.
The risks were most pronounced, jumping more than sixfold in the case of ischemic HF, during the first 6 years after the pregnancy. They then receded to plateau at a lower, still significantly elevated level of risk that persisted even years later, in the analysis of women in a Swedish medical birth registry.
The case-matching study compared women with no history of cardiovascular (CV) disease and a first successful pregnancy during which they either developed or did not experience gestational hypertension or preeclampsia.
It’s among the first studies to explore the impact of pregnancy-induced hypertensive disease on subsequent HF risk separately for both ischemic and nonischemic HF and to find that the severity of such risk differs for the two HF etiologies, according to a report published in JACC: Heart Failure.
The adjusted risk for any HF during a median of 13 years after the pregnancy rose 70% for those who had developed gestational hypertension or preeclampsia. Their risk of nonischemic HF went up 60%, and their risk of ischemic HF more than doubled.
Hypertensive disorders of pregnancy “are so much more than short-term disorders confined to the pregnancy period. They have long-term implications throughout a lifetime,” lead author Ängla Mantel, MD, PhD, said in an interview.
Obstetric history doesn’t figure into any formal HF risk scoring systems, observed Dr. Mantel of Karolinska Institutet, Stockholm. Still, women who develop gestational hypertension, preeclampsia, or other pregnancy complications “should be considered a high-risk population even after the pregnancy and monitored for cardiovascular risk factors regularly throughout life.”
In many studies, she said, “knowledge of women-specific risk factors for cardiovascular disease is poor among both clinicians and patients.” The current findings should help raise awareness about such obstetric risk factors for HF, “especially” in patients with HF with preserved ejection fraction (HFpEF), which isn’t closely related to a number of traditional CV risk factors.
Even though pregnancy complications such as gestational hypertension and preeclampsia don’t feature in risk calculators, “they are actually risk enhancers per the 2019 primary prevention guidelines,” Natalie A. Bello, MD, MPH, who was not involved in the current study, said in an interview.
“We’re working to educate physicians and cardiovascular team members to take a pregnancy history” for risk stratification of women in primary prevention,” said Dr. Bello, director of hypertension research at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles.
The current study, she said, “is an important step” for its finding that hypertensive disorders of pregnancy are associated separately with both ischemic and nonischemic HF.
She pointed out, however, that because the study excluded women with peripartum cardiomyopathy, a form of nonischemic HF, it may “underestimate the impact of hypertensive disorders on the short-term risk of nonischemic heart failure.” Women who had peripartum cardiomyopathy were excluded to avoid misclassification of other HF outcomes, the authors stated.
Also, Dr. Bello said, the study’s inclusion of patients with either gestational hypertension or preeclampsia may complicate its interpretation. Compared with the former condition, she said, preeclampsia “involves more inflammation and more endothelial dysfunction. It may cause a different impact on the heart and the vasculature.”
In the analysis, about 79,000 women with gestational hypertension or preeclampsia were identified among more than 1.4 million primiparous women who entered the Swedish Medical Birth Register over a period of about 30 years. They were matched with about 396,000 women in the registry who had normotensive pregnancies.
Excluded, besides women with peripartum cardiomyopathy, were women with a prepregnancy history of HF, hypertension, ischemic heart disease, atrial fibrillation, or valvular heart disease.
Hazard ratios (HRs) for HF, ischemic HF, and nonischemic HF were significantly elevated over among the women with gestational hypertension or preeclampsia compared to those with normotensive pregnancies:
- Any HF: HR, 1.70 (95% confidence interval [CI], 1.51-1.91)
- Nonischemic HF: HR, 1.60 (95% CI, 1.40-1.83)
- Ischemic HF: HR, 2.28 (95% CI, 1.74-2.98)
The analyses were adjusted for maternal age at delivery, year of delivery, prepregnancy comorbidities, maternal education level, smoking status, and body mass index.
Sharper risk increases were seen among women with gestational hypertension or preeclampsia who delivered prior to gestational week 34:
- Any HF: HR, 2.46 (95% CI, 1.82-3.32)
- Nonischemic HF: HR, 2.33 (95% CI, 1.65-3.31)
- Ischemic HF: HR, 3.64 (95% CI, 1.97-6.74)
Risks for HF developing within 6 years of pregnancy characterized by gestational hypertension or preeclampsia were far more pronounced for ischemic HF than for nonischemic HF:
- Any HF: HR, 2.09 (95% CI, 1.52-2.89)
- Nonischemic HF: HR, 1.86 (95% CI, 1.32-2.61)
- Ischemic HF: HR, 6.52 (95% CI, 2.00-12.34).
The study couldn’t directly explore potential mechanisms for the associations between pregnancy-induced hypertensive disorders and different forms of HF, but it may have provided clues, Dr. Mantel said.
The hypertensive disorders and ischemic HF appear to share risk factors that could lead to both conditions, she noted. Also, hypertension itself is a risk factor for ischemic heart disease.
In contrast, “the risk of nonischemic heart failure might be driven by other factors, such as the inflammatory profile, endothelial dysfunction, and cardiac remodeling induced by preeclampsia or gestational hypertension.”
Those disorders, moreover, are associated with cardiac structural changes that are also seen in HFpEF, Dr. Mantel said. And both HFpEF and preeclampsia are characterized by systemic inflammation and endothelial dysfunction.
“These pathophysiological similarities,” she proposed, “might explain the link between pregnancy-induced hypertensive disorder and HFpEF.”
The authors have disclosed no relevant financial relationships. Dr. Bello has received grants from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
, an observational study suggests.
The risks were most pronounced, jumping more than sixfold in the case of ischemic HF, during the first 6 years after the pregnancy. They then receded to plateau at a lower, still significantly elevated level of risk that persisted even years later, in the analysis of women in a Swedish medical birth registry.
The case-matching study compared women with no history of cardiovascular (CV) disease and a first successful pregnancy during which they either developed or did not experience gestational hypertension or preeclampsia.
It’s among the first studies to explore the impact of pregnancy-induced hypertensive disease on subsequent HF risk separately for both ischemic and nonischemic HF and to find that the severity of such risk differs for the two HF etiologies, according to a report published in JACC: Heart Failure.
The adjusted risk for any HF during a median of 13 years after the pregnancy rose 70% for those who had developed gestational hypertension or preeclampsia. Their risk of nonischemic HF went up 60%, and their risk of ischemic HF more than doubled.
Hypertensive disorders of pregnancy “are so much more than short-term disorders confined to the pregnancy period. They have long-term implications throughout a lifetime,” lead author Ängla Mantel, MD, PhD, said in an interview.
Obstetric history doesn’t figure into any formal HF risk scoring systems, observed Dr. Mantel of Karolinska Institutet, Stockholm. Still, women who develop gestational hypertension, preeclampsia, or other pregnancy complications “should be considered a high-risk population even after the pregnancy and monitored for cardiovascular risk factors regularly throughout life.”
In many studies, she said, “knowledge of women-specific risk factors for cardiovascular disease is poor among both clinicians and patients.” The current findings should help raise awareness about such obstetric risk factors for HF, “especially” in patients with HF with preserved ejection fraction (HFpEF), which isn’t closely related to a number of traditional CV risk factors.
Even though pregnancy complications such as gestational hypertension and preeclampsia don’t feature in risk calculators, “they are actually risk enhancers per the 2019 primary prevention guidelines,” Natalie A. Bello, MD, MPH, who was not involved in the current study, said in an interview.
“We’re working to educate physicians and cardiovascular team members to take a pregnancy history” for risk stratification of women in primary prevention,” said Dr. Bello, director of hypertension research at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles.
The current study, she said, “is an important step” for its finding that hypertensive disorders of pregnancy are associated separately with both ischemic and nonischemic HF.
She pointed out, however, that because the study excluded women with peripartum cardiomyopathy, a form of nonischemic HF, it may “underestimate the impact of hypertensive disorders on the short-term risk of nonischemic heart failure.” Women who had peripartum cardiomyopathy were excluded to avoid misclassification of other HF outcomes, the authors stated.
Also, Dr. Bello said, the study’s inclusion of patients with either gestational hypertension or preeclampsia may complicate its interpretation. Compared with the former condition, she said, preeclampsia “involves more inflammation and more endothelial dysfunction. It may cause a different impact on the heart and the vasculature.”
In the analysis, about 79,000 women with gestational hypertension or preeclampsia were identified among more than 1.4 million primiparous women who entered the Swedish Medical Birth Register over a period of about 30 years. They were matched with about 396,000 women in the registry who had normotensive pregnancies.
Excluded, besides women with peripartum cardiomyopathy, were women with a prepregnancy history of HF, hypertension, ischemic heart disease, atrial fibrillation, or valvular heart disease.
Hazard ratios (HRs) for HF, ischemic HF, and nonischemic HF were significantly elevated over among the women with gestational hypertension or preeclampsia compared to those with normotensive pregnancies:
- Any HF: HR, 1.70 (95% confidence interval [CI], 1.51-1.91)
- Nonischemic HF: HR, 1.60 (95% CI, 1.40-1.83)
- Ischemic HF: HR, 2.28 (95% CI, 1.74-2.98)
The analyses were adjusted for maternal age at delivery, year of delivery, prepregnancy comorbidities, maternal education level, smoking status, and body mass index.
Sharper risk increases were seen among women with gestational hypertension or preeclampsia who delivered prior to gestational week 34:
- Any HF: HR, 2.46 (95% CI, 1.82-3.32)
- Nonischemic HF: HR, 2.33 (95% CI, 1.65-3.31)
- Ischemic HF: HR, 3.64 (95% CI, 1.97-6.74)
Risks for HF developing within 6 years of pregnancy characterized by gestational hypertension or preeclampsia were far more pronounced for ischemic HF than for nonischemic HF:
- Any HF: HR, 2.09 (95% CI, 1.52-2.89)
- Nonischemic HF: HR, 1.86 (95% CI, 1.32-2.61)
- Ischemic HF: HR, 6.52 (95% CI, 2.00-12.34).
The study couldn’t directly explore potential mechanisms for the associations between pregnancy-induced hypertensive disorders and different forms of HF, but it may have provided clues, Dr. Mantel said.
The hypertensive disorders and ischemic HF appear to share risk factors that could lead to both conditions, she noted. Also, hypertension itself is a risk factor for ischemic heart disease.
In contrast, “the risk of nonischemic heart failure might be driven by other factors, such as the inflammatory profile, endothelial dysfunction, and cardiac remodeling induced by preeclampsia or gestational hypertension.”
Those disorders, moreover, are associated with cardiac structural changes that are also seen in HFpEF, Dr. Mantel said. And both HFpEF and preeclampsia are characterized by systemic inflammation and endothelial dysfunction.
“These pathophysiological similarities,” she proposed, “might explain the link between pregnancy-induced hypertensive disorder and HFpEF.”
The authors have disclosed no relevant financial relationships. Dr. Bello has received grants from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
, an observational study suggests.
The risks were most pronounced, jumping more than sixfold in the case of ischemic HF, during the first 6 years after the pregnancy. They then receded to plateau at a lower, still significantly elevated level of risk that persisted even years later, in the analysis of women in a Swedish medical birth registry.
The case-matching study compared women with no history of cardiovascular (CV) disease and a first successful pregnancy during which they either developed or did not experience gestational hypertension or preeclampsia.
It’s among the first studies to explore the impact of pregnancy-induced hypertensive disease on subsequent HF risk separately for both ischemic and nonischemic HF and to find that the severity of such risk differs for the two HF etiologies, according to a report published in JACC: Heart Failure.
The adjusted risk for any HF during a median of 13 years after the pregnancy rose 70% for those who had developed gestational hypertension or preeclampsia. Their risk of nonischemic HF went up 60%, and their risk of ischemic HF more than doubled.
Hypertensive disorders of pregnancy “are so much more than short-term disorders confined to the pregnancy period. They have long-term implications throughout a lifetime,” lead author Ängla Mantel, MD, PhD, said in an interview.
Obstetric history doesn’t figure into any formal HF risk scoring systems, observed Dr. Mantel of Karolinska Institutet, Stockholm. Still, women who develop gestational hypertension, preeclampsia, or other pregnancy complications “should be considered a high-risk population even after the pregnancy and monitored for cardiovascular risk factors regularly throughout life.”
In many studies, she said, “knowledge of women-specific risk factors for cardiovascular disease is poor among both clinicians and patients.” The current findings should help raise awareness about such obstetric risk factors for HF, “especially” in patients with HF with preserved ejection fraction (HFpEF), which isn’t closely related to a number of traditional CV risk factors.
Even though pregnancy complications such as gestational hypertension and preeclampsia don’t feature in risk calculators, “they are actually risk enhancers per the 2019 primary prevention guidelines,” Natalie A. Bello, MD, MPH, who was not involved in the current study, said in an interview.
“We’re working to educate physicians and cardiovascular team members to take a pregnancy history” for risk stratification of women in primary prevention,” said Dr. Bello, director of hypertension research at the Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles.
The current study, she said, “is an important step” for its finding that hypertensive disorders of pregnancy are associated separately with both ischemic and nonischemic HF.
She pointed out, however, that because the study excluded women with peripartum cardiomyopathy, a form of nonischemic HF, it may “underestimate the impact of hypertensive disorders on the short-term risk of nonischemic heart failure.” Women who had peripartum cardiomyopathy were excluded to avoid misclassification of other HF outcomes, the authors stated.
Also, Dr. Bello said, the study’s inclusion of patients with either gestational hypertension or preeclampsia may complicate its interpretation. Compared with the former condition, she said, preeclampsia “involves more inflammation and more endothelial dysfunction. It may cause a different impact on the heart and the vasculature.”
In the analysis, about 79,000 women with gestational hypertension or preeclampsia were identified among more than 1.4 million primiparous women who entered the Swedish Medical Birth Register over a period of about 30 years. They were matched with about 396,000 women in the registry who had normotensive pregnancies.
Excluded, besides women with peripartum cardiomyopathy, were women with a prepregnancy history of HF, hypertension, ischemic heart disease, atrial fibrillation, or valvular heart disease.
Hazard ratios (HRs) for HF, ischemic HF, and nonischemic HF were significantly elevated over among the women with gestational hypertension or preeclampsia compared to those with normotensive pregnancies:
- Any HF: HR, 1.70 (95% confidence interval [CI], 1.51-1.91)
- Nonischemic HF: HR, 1.60 (95% CI, 1.40-1.83)
- Ischemic HF: HR, 2.28 (95% CI, 1.74-2.98)
The analyses were adjusted for maternal age at delivery, year of delivery, prepregnancy comorbidities, maternal education level, smoking status, and body mass index.
Sharper risk increases were seen among women with gestational hypertension or preeclampsia who delivered prior to gestational week 34:
- Any HF: HR, 2.46 (95% CI, 1.82-3.32)
- Nonischemic HF: HR, 2.33 (95% CI, 1.65-3.31)
- Ischemic HF: HR, 3.64 (95% CI, 1.97-6.74)
Risks for HF developing within 6 years of pregnancy characterized by gestational hypertension or preeclampsia were far more pronounced for ischemic HF than for nonischemic HF:
- Any HF: HR, 2.09 (95% CI, 1.52-2.89)
- Nonischemic HF: HR, 1.86 (95% CI, 1.32-2.61)
- Ischemic HF: HR, 6.52 (95% CI, 2.00-12.34).
The study couldn’t directly explore potential mechanisms for the associations between pregnancy-induced hypertensive disorders and different forms of HF, but it may have provided clues, Dr. Mantel said.
The hypertensive disorders and ischemic HF appear to share risk factors that could lead to both conditions, she noted. Also, hypertension itself is a risk factor for ischemic heart disease.
In contrast, “the risk of nonischemic heart failure might be driven by other factors, such as the inflammatory profile, endothelial dysfunction, and cardiac remodeling induced by preeclampsia or gestational hypertension.”
Those disorders, moreover, are associated with cardiac structural changes that are also seen in HFpEF, Dr. Mantel said. And both HFpEF and preeclampsia are characterized by systemic inflammation and endothelial dysfunction.
“These pathophysiological similarities,” she proposed, “might explain the link between pregnancy-induced hypertensive disorder and HFpEF.”
The authors have disclosed no relevant financial relationships. Dr. Bello has received grants from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM JACC: HEART FAILURE
Overcoming death anxiety: Understanding our lives and legacies
Disappointment – “I failed this exam, my life is ruined” or regret – “I am getting a divorce, I wasted so much of my life.” Patients present with a wide variety of complaints that can be understood as a form of death anxiety.
Fundamentally, patients come to see us to understand and explain their lives. One can reinterpret this as a patient asking, “If I died today, would my life have been good enough?” or “When I die, how will I look back at this moment in time and judge the choices I made?”
Other patients come to us attempting to use the same maladaptive defenses that did not serve them well in the past in the hopes of achieving a new outcome that will validate their lives. While it may be understandable that a child dissociates when facing abuse, hoping that this defense mechanism – as an adult – will work, it is unlikely to be fruitful and will certainly not validate or repair the past. This hope to repair one’s past can be interpreted as a fear of death – “I cannot die without correcting this.” This psychic conflict can intensify if one does not adopt a more adaptive understanding of his or her life.
Death anxiety is the feeling associated with the finality of life. Not only is life final, but a constant reminder of that fact is the idea that any one moment is final. Other than in science fiction, one cannot return to a prior moment and repair the past in the hope of a better future. Time goes only in one direction and death is the natural outcome of all life.
Death may have some evolutionary purpose that encourages the promotion of newer and more fitter genes, but one doesn’t have to consider its origin and reason to admit death’s constancy throughout humanity. People die and that is an anxiety-provoking fact of life. Death anxiety can feel especially tangible in our connected world. In a world of constant news, it can feel – for many people – that if your house wasn’t displaced because of global warming or that you are not a war refugee, you don’t deserve to be seen and heard.
This can be a particularly strong feeling for and among physicians, who don’t think that the mental health challenges generated by their own tough circumstances deserve to be labeled a mental disorder, so they designate themselves as having “burnout”1 – as they don’t deserve the sympathy of having the clinically significant impairments of “depression.” Our traumas don’t seem important enough to deserve notice, and thus we may feel like we could die without ever having truly mattered.
This can also be applied in the reverse fashion. Certain individuals, like celebrities, live such extravagant lives that our simpler achievements can feel futile in comparison. While the neighbor’s grass has always felt greener, we are now constantly exposed to perfectly manicured lawns on social media. When compounded, the idea that our successes and our pains are both simultaneously irrelevant can lead one to have very palpable death anxiety – my life will never matter if none of the things I do matter, or my life will never matter because I will never achieve the requisite number of “likes” or “views” on social media required to believe that one’s life was worth living.
A way of alleviating death anxiety can be through the concept of legacy, or what we leave behind. How will people remember me? Will people remember me, or will I disappear like a shadow into the distant memory of my near and dear ones? The idea of being forgotten or lost to memory is intolerable to some and can be a strong driving force to “make a name” for oneself. For those who crave fame, whether a celebrity or a generous alumnus, part of this is likely related to remaining well known after death. After all, one can argue that you are not truly dead as long as you continue to live in the memory and/or genes of others.
Legacy thus serves as a form of posthumous transitional object; a way of calming our fears about how we will be remembered. For many, reconciling their feelings towards their legacy is an avenue to tame death anxiety.
A case study
The case of Mr. B illustrates this. As a 72-year-old male with a long history of generalized anxiety, he once had a nightmare as a child, similar to the plot of Sleeping Beauty. In his dream, he walks up a spiral staircase in a castle and touches the spindle on a spinning wheel, thus ending his life. The dream was vivid and marked him.
His fear of death has subsequently reared its head throughout his life. In more recent years, he has suffered from cardiovascular disease. Although he is now quite stable on his current cardiac medications, he is constantly fearful that he will experience a cardiac event while asleep and suddenly die. He is so anxious about not waking up in the morning that falling asleep is nearly impossible.
Mr. B is single, with no close family besides a sister who lives in another state. He has a dog and few friends. He worries about what will happen to his dog if he doesn’t wake up in the morning, but perhaps most distressing to him is “there’s so much left for me to do, I have so much to write!” As an accomplished author, he continues to write, and hopes to publish many more novels in his lifetime. It is unsurprising that someone without a strong social network may fear death and feel pressured to somehow make a mark on the world before the curtain falls. It is scary to think that even without us, life goes on.
By bringing to Mr. B’s attention that his ever-present anxiety is rooted in fear of death, he was able to gain more insight into his own defensive behaviors. By confronting his death anxiety and processing his definition of a life well lived together in therapy, he’s acknowledged his lack of social connection as demoralizing, and has made significant strides to remedy this. He’s been able to focus on a more fulfilling life day to day, with less emphasis on his to-do list and aspirations. Instead, he’s connected more with his faith and members of his church. He’s gotten close to several neighbors and enjoys long dinners with them on his back patio.
At a recent meeting, he confessed that he feels “lighter” and not as fearful about sudden cardiac death, and thus has noticed that his overall anxiety has diminished greatly. He concluded that experiencing meaningful relationships in the present moment would give him greater joy than spending his remaining time engaged in preserving a future identity for himself. It seems elementary, but if we look within, we may find that we all suffer similarly: How much of our daily actions, thoughts, and fears are tied to the looming threat of death?
Conclusion
While modern psychiatry continues to advance with better understandings of our neurobiology, improved knowledge of pathophysiological processes of mental illness, and expanding discovery of novel pharmacotherapeutics, the modern psychiatrist should not forget fundamental truths of behavior and humanity that were once the staple of psychiatry.
Death anxiety is one of those truths; it is the ultimate stressor that we will all face and should be regular study and practice for psychiatrists. In this article, we explored some of those facets most meaningful to us but recommend you expand your study to the many more available.
Patients often come to physicians seeking validation of their lives or trying to use the same maladaptive defense mechanisms that did not serve them well in the past to achieve a better outcome.
In today’s world, death anxiety can feel palpable due to the constant exposure to global news and social media that can make us feel irrelevant. However, legacy, or what we leave behind, can serve as a way to alleviate death anxiety. For many, reconciling their feelings toward their legacy is an avenue to tame death anxiety. Therapy can help individuals gain insight into their defensive behaviors and process their definition of a life well lived. By focusing on a life worth living, individuals can alleviate their death anxiety and gain a sense of fulfillment.
Dr. Akkoor is a psychiatry resident at the University of California, San Diego. She is interested in immigrant mental health, ethics, consultation-liaison psychiatry, and medical education. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Badre and Dr. Akkoor have no conflicts of interest.
Reference
1. Badre N. Burnout: A concept that rebrands mental illness for professionals. Clinical Psychiatry News. 2020 Mar 5.
Disappointment – “I failed this exam, my life is ruined” or regret – “I am getting a divorce, I wasted so much of my life.” Patients present with a wide variety of complaints that can be understood as a form of death anxiety.
Fundamentally, patients come to see us to understand and explain their lives. One can reinterpret this as a patient asking, “If I died today, would my life have been good enough?” or “When I die, how will I look back at this moment in time and judge the choices I made?”
Other patients come to us attempting to use the same maladaptive defenses that did not serve them well in the past in the hopes of achieving a new outcome that will validate their lives. While it may be understandable that a child dissociates when facing abuse, hoping that this defense mechanism – as an adult – will work, it is unlikely to be fruitful and will certainly not validate or repair the past. This hope to repair one’s past can be interpreted as a fear of death – “I cannot die without correcting this.” This psychic conflict can intensify if one does not adopt a more adaptive understanding of his or her life.
Death anxiety is the feeling associated with the finality of life. Not only is life final, but a constant reminder of that fact is the idea that any one moment is final. Other than in science fiction, one cannot return to a prior moment and repair the past in the hope of a better future. Time goes only in one direction and death is the natural outcome of all life.
Death may have some evolutionary purpose that encourages the promotion of newer and more fitter genes, but one doesn’t have to consider its origin and reason to admit death’s constancy throughout humanity. People die and that is an anxiety-provoking fact of life. Death anxiety can feel especially tangible in our connected world. In a world of constant news, it can feel – for many people – that if your house wasn’t displaced because of global warming or that you are not a war refugee, you don’t deserve to be seen and heard.
This can be a particularly strong feeling for and among physicians, who don’t think that the mental health challenges generated by their own tough circumstances deserve to be labeled a mental disorder, so they designate themselves as having “burnout”1 – as they don’t deserve the sympathy of having the clinically significant impairments of “depression.” Our traumas don’t seem important enough to deserve notice, and thus we may feel like we could die without ever having truly mattered.
This can also be applied in the reverse fashion. Certain individuals, like celebrities, live such extravagant lives that our simpler achievements can feel futile in comparison. While the neighbor’s grass has always felt greener, we are now constantly exposed to perfectly manicured lawns on social media. When compounded, the idea that our successes and our pains are both simultaneously irrelevant can lead one to have very palpable death anxiety – my life will never matter if none of the things I do matter, or my life will never matter because I will never achieve the requisite number of “likes” or “views” on social media required to believe that one’s life was worth living.
A way of alleviating death anxiety can be through the concept of legacy, or what we leave behind. How will people remember me? Will people remember me, or will I disappear like a shadow into the distant memory of my near and dear ones? The idea of being forgotten or lost to memory is intolerable to some and can be a strong driving force to “make a name” for oneself. For those who crave fame, whether a celebrity or a generous alumnus, part of this is likely related to remaining well known after death. After all, one can argue that you are not truly dead as long as you continue to live in the memory and/or genes of others.
Legacy thus serves as a form of posthumous transitional object; a way of calming our fears about how we will be remembered. For many, reconciling their feelings towards their legacy is an avenue to tame death anxiety.
A case study
The case of Mr. B illustrates this. As a 72-year-old male with a long history of generalized anxiety, he once had a nightmare as a child, similar to the plot of Sleeping Beauty. In his dream, he walks up a spiral staircase in a castle and touches the spindle on a spinning wheel, thus ending his life. The dream was vivid and marked him.
His fear of death has subsequently reared its head throughout his life. In more recent years, he has suffered from cardiovascular disease. Although he is now quite stable on his current cardiac medications, he is constantly fearful that he will experience a cardiac event while asleep and suddenly die. He is so anxious about not waking up in the morning that falling asleep is nearly impossible.
Mr. B is single, with no close family besides a sister who lives in another state. He has a dog and few friends. He worries about what will happen to his dog if he doesn’t wake up in the morning, but perhaps most distressing to him is “there’s so much left for me to do, I have so much to write!” As an accomplished author, he continues to write, and hopes to publish many more novels in his lifetime. It is unsurprising that someone without a strong social network may fear death and feel pressured to somehow make a mark on the world before the curtain falls. It is scary to think that even without us, life goes on.
By bringing to Mr. B’s attention that his ever-present anxiety is rooted in fear of death, he was able to gain more insight into his own defensive behaviors. By confronting his death anxiety and processing his definition of a life well lived together in therapy, he’s acknowledged his lack of social connection as demoralizing, and has made significant strides to remedy this. He’s been able to focus on a more fulfilling life day to day, with less emphasis on his to-do list and aspirations. Instead, he’s connected more with his faith and members of his church. He’s gotten close to several neighbors and enjoys long dinners with them on his back patio.
At a recent meeting, he confessed that he feels “lighter” and not as fearful about sudden cardiac death, and thus has noticed that his overall anxiety has diminished greatly. He concluded that experiencing meaningful relationships in the present moment would give him greater joy than spending his remaining time engaged in preserving a future identity for himself. It seems elementary, but if we look within, we may find that we all suffer similarly: How much of our daily actions, thoughts, and fears are tied to the looming threat of death?
Conclusion
While modern psychiatry continues to advance with better understandings of our neurobiology, improved knowledge of pathophysiological processes of mental illness, and expanding discovery of novel pharmacotherapeutics, the modern psychiatrist should not forget fundamental truths of behavior and humanity that were once the staple of psychiatry.
Death anxiety is one of those truths; it is the ultimate stressor that we will all face and should be regular study and practice for psychiatrists. In this article, we explored some of those facets most meaningful to us but recommend you expand your study to the many more available.
Patients often come to physicians seeking validation of their lives or trying to use the same maladaptive defense mechanisms that did not serve them well in the past to achieve a better outcome.
In today’s world, death anxiety can feel palpable due to the constant exposure to global news and social media that can make us feel irrelevant. However, legacy, or what we leave behind, can serve as a way to alleviate death anxiety. For many, reconciling their feelings toward their legacy is an avenue to tame death anxiety. Therapy can help individuals gain insight into their defensive behaviors and process their definition of a life well lived. By focusing on a life worth living, individuals can alleviate their death anxiety and gain a sense of fulfillment.
Dr. Akkoor is a psychiatry resident at the University of California, San Diego. She is interested in immigrant mental health, ethics, consultation-liaison psychiatry, and medical education. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Badre and Dr. Akkoor have no conflicts of interest.
Reference
1. Badre N. Burnout: A concept that rebrands mental illness for professionals. Clinical Psychiatry News. 2020 Mar 5.
Disappointment – “I failed this exam, my life is ruined” or regret – “I am getting a divorce, I wasted so much of my life.” Patients present with a wide variety of complaints that can be understood as a form of death anxiety.
Fundamentally, patients come to see us to understand and explain their lives. One can reinterpret this as a patient asking, “If I died today, would my life have been good enough?” or “When I die, how will I look back at this moment in time and judge the choices I made?”
Other patients come to us attempting to use the same maladaptive defenses that did not serve them well in the past in the hopes of achieving a new outcome that will validate their lives. While it may be understandable that a child dissociates when facing abuse, hoping that this defense mechanism – as an adult – will work, it is unlikely to be fruitful and will certainly not validate or repair the past. This hope to repair one’s past can be interpreted as a fear of death – “I cannot die without correcting this.” This psychic conflict can intensify if one does not adopt a more adaptive understanding of his or her life.
Death anxiety is the feeling associated with the finality of life. Not only is life final, but a constant reminder of that fact is the idea that any one moment is final. Other than in science fiction, one cannot return to a prior moment and repair the past in the hope of a better future. Time goes only in one direction and death is the natural outcome of all life.
Death may have some evolutionary purpose that encourages the promotion of newer and more fitter genes, but one doesn’t have to consider its origin and reason to admit death’s constancy throughout humanity. People die and that is an anxiety-provoking fact of life. Death anxiety can feel especially tangible in our connected world. In a world of constant news, it can feel – for many people – that if your house wasn’t displaced because of global warming or that you are not a war refugee, you don’t deserve to be seen and heard.
This can be a particularly strong feeling for and among physicians, who don’t think that the mental health challenges generated by their own tough circumstances deserve to be labeled a mental disorder, so they designate themselves as having “burnout”1 – as they don’t deserve the sympathy of having the clinically significant impairments of “depression.” Our traumas don’t seem important enough to deserve notice, and thus we may feel like we could die without ever having truly mattered.
This can also be applied in the reverse fashion. Certain individuals, like celebrities, live such extravagant lives that our simpler achievements can feel futile in comparison. While the neighbor’s grass has always felt greener, we are now constantly exposed to perfectly manicured lawns on social media. When compounded, the idea that our successes and our pains are both simultaneously irrelevant can lead one to have very palpable death anxiety – my life will never matter if none of the things I do matter, or my life will never matter because I will never achieve the requisite number of “likes” or “views” on social media required to believe that one’s life was worth living.
A way of alleviating death anxiety can be through the concept of legacy, or what we leave behind. How will people remember me? Will people remember me, or will I disappear like a shadow into the distant memory of my near and dear ones? The idea of being forgotten or lost to memory is intolerable to some and can be a strong driving force to “make a name” for oneself. For those who crave fame, whether a celebrity or a generous alumnus, part of this is likely related to remaining well known after death. After all, one can argue that you are not truly dead as long as you continue to live in the memory and/or genes of others.
Legacy thus serves as a form of posthumous transitional object; a way of calming our fears about how we will be remembered. For many, reconciling their feelings towards their legacy is an avenue to tame death anxiety.
A case study
The case of Mr. B illustrates this. As a 72-year-old male with a long history of generalized anxiety, he once had a nightmare as a child, similar to the plot of Sleeping Beauty. In his dream, he walks up a spiral staircase in a castle and touches the spindle on a spinning wheel, thus ending his life. The dream was vivid and marked him.
His fear of death has subsequently reared its head throughout his life. In more recent years, he has suffered from cardiovascular disease. Although he is now quite stable on his current cardiac medications, he is constantly fearful that he will experience a cardiac event while asleep and suddenly die. He is so anxious about not waking up in the morning that falling asleep is nearly impossible.
Mr. B is single, with no close family besides a sister who lives in another state. He has a dog and few friends. He worries about what will happen to his dog if he doesn’t wake up in the morning, but perhaps most distressing to him is “there’s so much left for me to do, I have so much to write!” As an accomplished author, he continues to write, and hopes to publish many more novels in his lifetime. It is unsurprising that someone without a strong social network may fear death and feel pressured to somehow make a mark on the world before the curtain falls. It is scary to think that even without us, life goes on.
By bringing to Mr. B’s attention that his ever-present anxiety is rooted in fear of death, he was able to gain more insight into his own defensive behaviors. By confronting his death anxiety and processing his definition of a life well lived together in therapy, he’s acknowledged his lack of social connection as demoralizing, and has made significant strides to remedy this. He’s been able to focus on a more fulfilling life day to day, with less emphasis on his to-do list and aspirations. Instead, he’s connected more with his faith and members of his church. He’s gotten close to several neighbors and enjoys long dinners with them on his back patio.
At a recent meeting, he confessed that he feels “lighter” and not as fearful about sudden cardiac death, and thus has noticed that his overall anxiety has diminished greatly. He concluded that experiencing meaningful relationships in the present moment would give him greater joy than spending his remaining time engaged in preserving a future identity for himself. It seems elementary, but if we look within, we may find that we all suffer similarly: How much of our daily actions, thoughts, and fears are tied to the looming threat of death?
Conclusion
While modern psychiatry continues to advance with better understandings of our neurobiology, improved knowledge of pathophysiological processes of mental illness, and expanding discovery of novel pharmacotherapeutics, the modern psychiatrist should not forget fundamental truths of behavior and humanity that were once the staple of psychiatry.
Death anxiety is one of those truths; it is the ultimate stressor that we will all face and should be regular study and practice for psychiatrists. In this article, we explored some of those facets most meaningful to us but recommend you expand your study to the many more available.
Patients often come to physicians seeking validation of their lives or trying to use the same maladaptive defense mechanisms that did not serve them well in the past to achieve a better outcome.
In today’s world, death anxiety can feel palpable due to the constant exposure to global news and social media that can make us feel irrelevant. However, legacy, or what we leave behind, can serve as a way to alleviate death anxiety. For many, reconciling their feelings toward their legacy is an avenue to tame death anxiety. Therapy can help individuals gain insight into their defensive behaviors and process their definition of a life well lived. By focusing on a life worth living, individuals can alleviate their death anxiety and gain a sense of fulfillment.
Dr. Akkoor is a psychiatry resident at the University of California, San Diego. She is interested in immigrant mental health, ethics, consultation-liaison psychiatry, and medical education. Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Badre and Dr. Akkoor have no conflicts of interest.
Reference
1. Badre N. Burnout: A concept that rebrands mental illness for professionals. Clinical Psychiatry News. 2020 Mar 5.
Preventing breaks and falls in older adults
LONG BEACH, CALIF. – Ms. S had recently arrived home after a stay at a skilled nursing facility to recover from a hip fracture resulting from osteoporosis. For many patients, follow-up care would have included a DEXA scan or a prescription for a bisphosphonate from a primary care clinician not trained in geriatrics.
But the 85-year-old received care that went further and that is considered best practice for the management of geriatric fractures: A physical therapist visited her after discharge and provided education on the importance of maintaining mobility. Ms. S also underwent assessment for fall risk and gait balance, and a team of multidisciplinary clinicians managed other factors, from postural hypotension to footwear and foot problems.
Sonja Rosen, MD, professor of medicine and chief of geriatric medicine at Cedars-Sinai Medical Center, Los Angeles, talked about Ms. S as part of a panel discussion on applying the “Geriatric 5Ms” for patients with osteoporosis at the annual meeting of the American Geriatrics Society.
“You have to figure out why they are falling and help them not fall again,” Dr. Rosen said.
Approximately 10 million Americans have osteoporosis, and another 44 million have low bone density. One in two women and up to one in four men will experience a bone fracture as a result of osteoporosis, according to the Bone Health and Osteoporosis Foundation.
, which involves considering the care preferences and goals for health care outcomes of individuals.
Ms. S eventually visited a geriatrician through the Cedars-Sinai Geriatric Fracture Program, which has been shown to lower costs and shorten hospital stays. In the program, she was advised to use a walker. Initially, she saw the aid as a hindrance – she felt she should be able to walk without it, like before. But with education, she learned that it is impossible to predict falls and that the walking aid could reduce her risk of a stumble.
Dr. Rosen said clinicians should address any vision problems, prescriptions for psychotropic drugs,which can affect balance, and heart rate and rhythm abnormalities, and they should suggest modifications to the home environment, such as installing grab bars in showers and removing rugs that can easily be tripped over.
The program at Cedars-Sinai, like similar initiatives, offers a team with resources that some clinicians may not have access to, such as a care coordinator and bone-health coach. But health care providers can utilize aspects, such as making referrals to community exercise classes.
Dr. Rosen and her colleagues studied the effects of such exercise programs and found that the programs lessen loneliness and social isolation. Fear of falling decreased in 75% of participants, “which is so key to these postfracture patients in getting back out into the world and engaging in their prior level of functional status,” Dr. Rosen said.
The second ‘M’: Medication management
The second “M,” medications, can help clinicians sequence osteoporosis drugs, depending on patient characteristics and scenarios.
Cathleen Colon-Emeric, MD, MHS, chief of geriatrics at Duke University, in Durham, N.C., dived into the case history of Ms. S, who had hypertension and insomnia in addition to osteoporosis.
First-line treatment for Ms. S – and for most patients – was an oral bisphosphonate, Dr. Colon-Emeric said. Compared with placebo, the drugs decrease the risk of overall osteoporotic fractures by nearly 40% (odds ratio, 0.62). But the medications are linked to injury of the esophageal mucosa. This risk is decreased when a patient stays upright for 30 minutes after taking oral bisphosphonates. Dr. Colon-Emeric displayed a slide of a woman receiving a pedicure at a nail salon.
“The picture of the pedicure is to share the wonderful idea I got from one skilled nursing facility I was working with, who makes sure they do safe administration to prevent esophagitis in their patients by having them all go to a spa day, where they all sit up and get their nails done while they wait their 30 minutes [after taking the pill] sitting up safely,” Dr. Colon-Emeric said.
This strategy drew applause from the audience.
Dr. Colon-Emeric advised that clinicians use judgment in the interpretation of results from the Fracture Risk Assessment Tool (FRAX). Incorporating race into estimates of fracture risk has pros and cons. While there are racial and ethnic differences in average bone density, the data for race calibrations to estimate risk are dated, she said. Clinicians should compare FRAX estimates with and without race input to help patients understand a range of risks.
Some patients may be reluctant to begin taking osteoporosis drugs because of misinformation originating from inaccurate news reports or anecdotes from friends. Dr. Colon-Emeric advised clinicians to remind patients that one in five who experience a fracture will have another injury in the following 2 years.
“A major osteoporotic fracture is akin to a heart attack; it has a very similar 1-year mortality rate and a very similar rate of a subsequent secondary event,” Dr. Colon-Emeric said. “We have a class of medications that decrease both those risks by nearly a third.”
Shared decision-making can help patients understand the risks and benefits of treatment, she said.
“People are really scared about the side effects,” Michelle Keller, PhD, MPH, a research scientist at Cedars-Sinai who attended the session, said. “The idea that a “bone attack” is like a heart attack gets the message across.”
Mind and multicomplexity
Medical complexity of a patient must be considered when making decisions on treatment, according to Joshua Niznik, PharmD, PhD, assistant professor of medicine in the Center for Aging and Health at the University of North Carolina at Chapel Hill.
“Medical complexity is an acknowledgment of the entire person, the burden of their multiple chronic conditions, advanced illnesses, and also their biopsychosocial needs and how those together might augment treatment selection and decision-making,” Dr. Niznik said.
Studies by Dr. Niznik and others have shown that swallowing difficulties, severe dementia, and being older than 90 are linked with a lower likelihood of receiving treatment for osteoporosis.
But therapies for fracture prevention, especially bisphosphonates, appear to be at least as effective for adults with medical complexity as they are for people without such conditions, Dr. Niznik said. Physicians must consider the potential treatment burden and the likelihood of benefit, he said.
Dr. Niznik’s research has shown a lack of strong evidence on how clinicians can manage patients in nursing homes. In some cases, deprescribing is reasonable, such as for patients who have undergone treatment for several years and whose life expectancy is less than 2 years.
“In the absence of any of those, if they are not already treated for osteoporosis, it makes sense to initiate treatment at that time,” Dr. Niznik said.
Matters most: Patient input
Clinicians need to educate patients on how long they must undergo a treatment before they experience benefits, according to Sarah D. Berry, MD, MPH, associate professor of medicine at Harvard Medical School, in Boston.
A meta-analysis of studies that included more than 20,000 women who were randomly assigned to receive bisphosphonate or placebo found that one nonvertebral fracture was avoided during a 12-month period for every 100 persons treated. One hip fracture was avoided during a 20-month period for every 200 patients treated.
“In general, in persons with a 2-year life expectancy, time to benefit favors bisphosphonate use,” Dr. Berry said. “Anabolics may have an even quicker time to benefit.”
Dr. Berry said a shared a decision-making model can help clinicians facilitate discussions that help patients prioritize goals and compare options while considering results, benefits, and harms. And she offered a final tip: Use tools with absolute risk reduction to convey risks and benefits, as the relative risk calculations overestimate how effective treatment will be.
Dr. Rosen has disclosed no relevant financial relationships. Dr. Colon-Emeric has received grants from the National Institutes of Health and VA Health Services Research and Development Funding; has served as endpoint adjudication chair for UCB Pharma; and has received royalties from Wolters Kluwer. Dr. Niznik has received funding from the National Institute of Aging and the Centers for Disease Control and Prevention. Dr. Berry has received funding from the NIH and royalties from Wolters Kluwer.
A version of this article originally appeared on Medscape.com.
LONG BEACH, CALIF. – Ms. S had recently arrived home after a stay at a skilled nursing facility to recover from a hip fracture resulting from osteoporosis. For many patients, follow-up care would have included a DEXA scan or a prescription for a bisphosphonate from a primary care clinician not trained in geriatrics.
But the 85-year-old received care that went further and that is considered best practice for the management of geriatric fractures: A physical therapist visited her after discharge and provided education on the importance of maintaining mobility. Ms. S also underwent assessment for fall risk and gait balance, and a team of multidisciplinary clinicians managed other factors, from postural hypotension to footwear and foot problems.
Sonja Rosen, MD, professor of medicine and chief of geriatric medicine at Cedars-Sinai Medical Center, Los Angeles, talked about Ms. S as part of a panel discussion on applying the “Geriatric 5Ms” for patients with osteoporosis at the annual meeting of the American Geriatrics Society.
“You have to figure out why they are falling and help them not fall again,” Dr. Rosen said.
Approximately 10 million Americans have osteoporosis, and another 44 million have low bone density. One in two women and up to one in four men will experience a bone fracture as a result of osteoporosis, according to the Bone Health and Osteoporosis Foundation.
, which involves considering the care preferences and goals for health care outcomes of individuals.
Ms. S eventually visited a geriatrician through the Cedars-Sinai Geriatric Fracture Program, which has been shown to lower costs and shorten hospital stays. In the program, she was advised to use a walker. Initially, she saw the aid as a hindrance – she felt she should be able to walk without it, like before. But with education, she learned that it is impossible to predict falls and that the walking aid could reduce her risk of a stumble.
Dr. Rosen said clinicians should address any vision problems, prescriptions for psychotropic drugs,which can affect balance, and heart rate and rhythm abnormalities, and they should suggest modifications to the home environment, such as installing grab bars in showers and removing rugs that can easily be tripped over.
The program at Cedars-Sinai, like similar initiatives, offers a team with resources that some clinicians may not have access to, such as a care coordinator and bone-health coach. But health care providers can utilize aspects, such as making referrals to community exercise classes.
Dr. Rosen and her colleagues studied the effects of such exercise programs and found that the programs lessen loneliness and social isolation. Fear of falling decreased in 75% of participants, “which is so key to these postfracture patients in getting back out into the world and engaging in their prior level of functional status,” Dr. Rosen said.
The second ‘M’: Medication management
The second “M,” medications, can help clinicians sequence osteoporosis drugs, depending on patient characteristics and scenarios.
Cathleen Colon-Emeric, MD, MHS, chief of geriatrics at Duke University, in Durham, N.C., dived into the case history of Ms. S, who had hypertension and insomnia in addition to osteoporosis.
First-line treatment for Ms. S – and for most patients – was an oral bisphosphonate, Dr. Colon-Emeric said. Compared with placebo, the drugs decrease the risk of overall osteoporotic fractures by nearly 40% (odds ratio, 0.62). But the medications are linked to injury of the esophageal mucosa. This risk is decreased when a patient stays upright for 30 minutes after taking oral bisphosphonates. Dr. Colon-Emeric displayed a slide of a woman receiving a pedicure at a nail salon.
“The picture of the pedicure is to share the wonderful idea I got from one skilled nursing facility I was working with, who makes sure they do safe administration to prevent esophagitis in their patients by having them all go to a spa day, where they all sit up and get their nails done while they wait their 30 minutes [after taking the pill] sitting up safely,” Dr. Colon-Emeric said.
This strategy drew applause from the audience.
Dr. Colon-Emeric advised that clinicians use judgment in the interpretation of results from the Fracture Risk Assessment Tool (FRAX). Incorporating race into estimates of fracture risk has pros and cons. While there are racial and ethnic differences in average bone density, the data for race calibrations to estimate risk are dated, she said. Clinicians should compare FRAX estimates with and without race input to help patients understand a range of risks.
Some patients may be reluctant to begin taking osteoporosis drugs because of misinformation originating from inaccurate news reports or anecdotes from friends. Dr. Colon-Emeric advised clinicians to remind patients that one in five who experience a fracture will have another injury in the following 2 years.
“A major osteoporotic fracture is akin to a heart attack; it has a very similar 1-year mortality rate and a very similar rate of a subsequent secondary event,” Dr. Colon-Emeric said. “We have a class of medications that decrease both those risks by nearly a third.”
Shared decision-making can help patients understand the risks and benefits of treatment, she said.
“People are really scared about the side effects,” Michelle Keller, PhD, MPH, a research scientist at Cedars-Sinai who attended the session, said. “The idea that a “bone attack” is like a heart attack gets the message across.”
Mind and multicomplexity
Medical complexity of a patient must be considered when making decisions on treatment, according to Joshua Niznik, PharmD, PhD, assistant professor of medicine in the Center for Aging and Health at the University of North Carolina at Chapel Hill.
“Medical complexity is an acknowledgment of the entire person, the burden of their multiple chronic conditions, advanced illnesses, and also their biopsychosocial needs and how those together might augment treatment selection and decision-making,” Dr. Niznik said.
Studies by Dr. Niznik and others have shown that swallowing difficulties, severe dementia, and being older than 90 are linked with a lower likelihood of receiving treatment for osteoporosis.
But therapies for fracture prevention, especially bisphosphonates, appear to be at least as effective for adults with medical complexity as they are for people without such conditions, Dr. Niznik said. Physicians must consider the potential treatment burden and the likelihood of benefit, he said.
Dr. Niznik’s research has shown a lack of strong evidence on how clinicians can manage patients in nursing homes. In some cases, deprescribing is reasonable, such as for patients who have undergone treatment for several years and whose life expectancy is less than 2 years.
“In the absence of any of those, if they are not already treated for osteoporosis, it makes sense to initiate treatment at that time,” Dr. Niznik said.
Matters most: Patient input
Clinicians need to educate patients on how long they must undergo a treatment before they experience benefits, according to Sarah D. Berry, MD, MPH, associate professor of medicine at Harvard Medical School, in Boston.
A meta-analysis of studies that included more than 20,000 women who were randomly assigned to receive bisphosphonate or placebo found that one nonvertebral fracture was avoided during a 12-month period for every 100 persons treated. One hip fracture was avoided during a 20-month period for every 200 patients treated.
“In general, in persons with a 2-year life expectancy, time to benefit favors bisphosphonate use,” Dr. Berry said. “Anabolics may have an even quicker time to benefit.”
Dr. Berry said a shared a decision-making model can help clinicians facilitate discussions that help patients prioritize goals and compare options while considering results, benefits, and harms. And she offered a final tip: Use tools with absolute risk reduction to convey risks and benefits, as the relative risk calculations overestimate how effective treatment will be.
Dr. Rosen has disclosed no relevant financial relationships. Dr. Colon-Emeric has received grants from the National Institutes of Health and VA Health Services Research and Development Funding; has served as endpoint adjudication chair for UCB Pharma; and has received royalties from Wolters Kluwer. Dr. Niznik has received funding from the National Institute of Aging and the Centers for Disease Control and Prevention. Dr. Berry has received funding from the NIH and royalties from Wolters Kluwer.
A version of this article originally appeared on Medscape.com.
LONG BEACH, CALIF. – Ms. S had recently arrived home after a stay at a skilled nursing facility to recover from a hip fracture resulting from osteoporosis. For many patients, follow-up care would have included a DEXA scan or a prescription for a bisphosphonate from a primary care clinician not trained in geriatrics.
But the 85-year-old received care that went further and that is considered best practice for the management of geriatric fractures: A physical therapist visited her after discharge and provided education on the importance of maintaining mobility. Ms. S also underwent assessment for fall risk and gait balance, and a team of multidisciplinary clinicians managed other factors, from postural hypotension to footwear and foot problems.
Sonja Rosen, MD, professor of medicine and chief of geriatric medicine at Cedars-Sinai Medical Center, Los Angeles, talked about Ms. S as part of a panel discussion on applying the “Geriatric 5Ms” for patients with osteoporosis at the annual meeting of the American Geriatrics Society.
“You have to figure out why they are falling and help them not fall again,” Dr. Rosen said.
Approximately 10 million Americans have osteoporosis, and another 44 million have low bone density. One in two women and up to one in four men will experience a bone fracture as a result of osteoporosis, according to the Bone Health and Osteoporosis Foundation.
, which involves considering the care preferences and goals for health care outcomes of individuals.
Ms. S eventually visited a geriatrician through the Cedars-Sinai Geriatric Fracture Program, which has been shown to lower costs and shorten hospital stays. In the program, she was advised to use a walker. Initially, she saw the aid as a hindrance – she felt she should be able to walk without it, like before. But with education, she learned that it is impossible to predict falls and that the walking aid could reduce her risk of a stumble.
Dr. Rosen said clinicians should address any vision problems, prescriptions for psychotropic drugs,which can affect balance, and heart rate and rhythm abnormalities, and they should suggest modifications to the home environment, such as installing grab bars in showers and removing rugs that can easily be tripped over.
The program at Cedars-Sinai, like similar initiatives, offers a team with resources that some clinicians may not have access to, such as a care coordinator and bone-health coach. But health care providers can utilize aspects, such as making referrals to community exercise classes.
Dr. Rosen and her colleagues studied the effects of such exercise programs and found that the programs lessen loneliness and social isolation. Fear of falling decreased in 75% of participants, “which is so key to these postfracture patients in getting back out into the world and engaging in their prior level of functional status,” Dr. Rosen said.
The second ‘M’: Medication management
The second “M,” medications, can help clinicians sequence osteoporosis drugs, depending on patient characteristics and scenarios.
Cathleen Colon-Emeric, MD, MHS, chief of geriatrics at Duke University, in Durham, N.C., dived into the case history of Ms. S, who had hypertension and insomnia in addition to osteoporosis.
First-line treatment for Ms. S – and for most patients – was an oral bisphosphonate, Dr. Colon-Emeric said. Compared with placebo, the drugs decrease the risk of overall osteoporotic fractures by nearly 40% (odds ratio, 0.62). But the medications are linked to injury of the esophageal mucosa. This risk is decreased when a patient stays upright for 30 minutes after taking oral bisphosphonates. Dr. Colon-Emeric displayed a slide of a woman receiving a pedicure at a nail salon.
“The picture of the pedicure is to share the wonderful idea I got from one skilled nursing facility I was working with, who makes sure they do safe administration to prevent esophagitis in their patients by having them all go to a spa day, where they all sit up and get their nails done while they wait their 30 minutes [after taking the pill] sitting up safely,” Dr. Colon-Emeric said.
This strategy drew applause from the audience.
Dr. Colon-Emeric advised that clinicians use judgment in the interpretation of results from the Fracture Risk Assessment Tool (FRAX). Incorporating race into estimates of fracture risk has pros and cons. While there are racial and ethnic differences in average bone density, the data for race calibrations to estimate risk are dated, she said. Clinicians should compare FRAX estimates with and without race input to help patients understand a range of risks.
Some patients may be reluctant to begin taking osteoporosis drugs because of misinformation originating from inaccurate news reports or anecdotes from friends. Dr. Colon-Emeric advised clinicians to remind patients that one in five who experience a fracture will have another injury in the following 2 years.
“A major osteoporotic fracture is akin to a heart attack; it has a very similar 1-year mortality rate and a very similar rate of a subsequent secondary event,” Dr. Colon-Emeric said. “We have a class of medications that decrease both those risks by nearly a third.”
Shared decision-making can help patients understand the risks and benefits of treatment, she said.
“People are really scared about the side effects,” Michelle Keller, PhD, MPH, a research scientist at Cedars-Sinai who attended the session, said. “The idea that a “bone attack” is like a heart attack gets the message across.”
Mind and multicomplexity
Medical complexity of a patient must be considered when making decisions on treatment, according to Joshua Niznik, PharmD, PhD, assistant professor of medicine in the Center for Aging and Health at the University of North Carolina at Chapel Hill.
“Medical complexity is an acknowledgment of the entire person, the burden of their multiple chronic conditions, advanced illnesses, and also their biopsychosocial needs and how those together might augment treatment selection and decision-making,” Dr. Niznik said.
Studies by Dr. Niznik and others have shown that swallowing difficulties, severe dementia, and being older than 90 are linked with a lower likelihood of receiving treatment for osteoporosis.
But therapies for fracture prevention, especially bisphosphonates, appear to be at least as effective for adults with medical complexity as they are for people without such conditions, Dr. Niznik said. Physicians must consider the potential treatment burden and the likelihood of benefit, he said.
Dr. Niznik’s research has shown a lack of strong evidence on how clinicians can manage patients in nursing homes. In some cases, deprescribing is reasonable, such as for patients who have undergone treatment for several years and whose life expectancy is less than 2 years.
“In the absence of any of those, if they are not already treated for osteoporosis, it makes sense to initiate treatment at that time,” Dr. Niznik said.
Matters most: Patient input
Clinicians need to educate patients on how long they must undergo a treatment before they experience benefits, according to Sarah D. Berry, MD, MPH, associate professor of medicine at Harvard Medical School, in Boston.
A meta-analysis of studies that included more than 20,000 women who were randomly assigned to receive bisphosphonate or placebo found that one nonvertebral fracture was avoided during a 12-month period for every 100 persons treated. One hip fracture was avoided during a 20-month period for every 200 patients treated.
“In general, in persons with a 2-year life expectancy, time to benefit favors bisphosphonate use,” Dr. Berry said. “Anabolics may have an even quicker time to benefit.”
Dr. Berry said a shared a decision-making model can help clinicians facilitate discussions that help patients prioritize goals and compare options while considering results, benefits, and harms. And she offered a final tip: Use tools with absolute risk reduction to convey risks and benefits, as the relative risk calculations overestimate how effective treatment will be.
Dr. Rosen has disclosed no relevant financial relationships. Dr. Colon-Emeric has received grants from the National Institutes of Health and VA Health Services Research and Development Funding; has served as endpoint adjudication chair for UCB Pharma; and has received royalties from Wolters Kluwer. Dr. Niznik has received funding from the National Institute of Aging and the Centers for Disease Control and Prevention. Dr. Berry has received funding from the NIH and royalties from Wolters Kluwer.
A version of this article originally appeared on Medscape.com.
AT AGS 2023
Atezolizumab is associated with enhanced response in triple-negative breast cancer
based on final data from a randomized trial.
The IMpassion031 trial showed significant improvement in pathological complete response (pCR) with the addition of atezolizumab to chemotherapy, as well as an acceptable safety profile, said Carlos H. Barrios, MD, of the Latin American Cooperative Oncology Group, Oncoclinicas, in Porto Allegre, Brazil, at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress. Those findings were published in the Lancet in 2020.
Dr. Barrios reported data from a final analysis of the IMpassion031 trial, with data on event-free survival (EFS), disease-free survival (DFS) and overall survival (OS) in the intent-to-treat (ITT) and PD-L1–positive populations.
In the study, patients with early triple-negative breast cancer (eTNBC) and a primary tumor greater than 2 cm were randomized to 840 mg of atezolizumab once every 2 weeks plus a neoadjuvant chemotherapy regimen of nab-paclitaxel 125 mg/m2 once weekly for 12 weeks, followed by doxorubicin 60 mg/m2 plus cyclophosphamide 600 mg/m2 once every 2 weeks for 8 weeks. A total of 333 patients were randomized (165 atezolizumab and 168 placebo). Patients were stratified by stage II versus stage III, and by status of PD-L1, a protein that can predict treatment response (PD-L1 less than 1% vs. 1% or higher).
The primary endpoints (previously reported) were pathological complete response (pCR) in the ITT and PD-L1 populations. After a median follow-up of 39 months, the pCR was 58% in patients treated with atezolizumab versus 41% in those treated with neoadjuvant chemotherapy alone (P = .0044) in the ITT population, Dr. Barrios said. The added benefit from atezolizumab occurred regardless of the status of PD-L1.
Dr. Barrios reported the secondary outcomes of EFS, DFS, and OS in the intent-to-treat and PD-L1–positive populations. “This is a descriptive analysis, with no statistical comparison,” he emphasized.
The 2-year data on EFS, DFS, and OS consistently favored atezolizumab across key clinical subgroups, Dr. Barrios said. In the ITT population, 2-year EFS, DFS, and OS was 85%, 87%, and 95%, respectively, in the atezolizumab group and 80%, 83%, and 90%, respectively, in the placebo group. The results were similar, irrespective of PD-L1 status.
In the PD-L1–positive population, 2-year EFS, DFS, and OS was 89%, 91%, and 95%, respectively, in atezolizumab patients and 80%, 87%, and 91% in placebo patients.
Among patients without pCR at the time of surgery, 14 of 70 patients (20%) in the atezolizumab group and 33 of 99 patients (33%) in the placebo group received additional adjuvant systemic therapy. The most common adjunctive therapy was capecitabine.
As for safety, no new safety signals or treatment-related deaths were observed in the study. Overall, 70% of atezolizumab patients and 62% of placebo patients experienced grade 3 or 4 adverse events (AEs); 59% and 54% of which were treatment related. A total of 1% of patients in each group experienced grade 5 AEs. A total of 25% of atezolizumab patients and 20% of placebo patients experienced AEs leading to treatment discontinuation.
In a further exploratory analysis, pCR was highly predictive of long-term outcomes. Exploratory analysis of circulating tumor DNA (ctDNA) showed clearance in 89% of atezolizumab patients and 86% of placebo patients by the time of surgery.
Looking at the relationship between ctDNA, DFS, and OS, positive ctDNA was associated with a worse prognosis following surgery. As demonstrated in previous studies, pCR patients with negative ctDNA had the best DFS and OS. “In non-pCR patients with positive ctDNA, a numerical trend suggests improved overall survival with atezolizumab,” although the caveat is the very small numbers, Dr. Barrios said.
More research is needed, but in the final analysis, the significant pCR benefit seen with the addition of atezolizumab to chemotherapy for eTNBC translated into numerically improved EFS, DFS and OS, said Dr. Barrios. Additionally, “we should further analyze ctDNA to help select patients for further therapy.”
In a question-and-answer session, Dr. Barrios was asked how the results compared with other studies.
“We should not overinterpret the results,” he said. However, “what the IMpassion031 study shows is consistency; the results are aligned with previous studies addressing the same question of introducing immunotherapy,” in the patient population. Although the numbers in the IMpassion031 study did not reach statistical significance, it is important to recognize that they reflect previous research.
“In my opinion, looking at the whole field, immunotherapy is something we need to consider as part of the treatment of these patients,” said Dr. Barrios. However, more research is needed to better identify which patients do and do not need chemotherapy.
Phase 2 data show increased response with added atezolizumab for PD-L1–negative patients
In a second study known as ABSCG-52/ATHENE, researchers evaluated neoadjuvant atezolizumab in combination with dual HER2 blockade plus epirubicin for the treatment of patients with early HER2-positive breast cancer.
For most of these patients, the current standard of care is neoadjuvant dual HER2 blockade with trastuzumab (T) and pertuzumab (P) plus poly-chemotherapy, said Gabriel Rinnerthaler, MD, of the Salzburg (Austria) Cancer Research Institute, said in his presentation at the meeting. However, de-escalation of chemotherapy has been a major focus of research in recent years, and more research is needed on a combination of anthracyclines, such as epirubicin and idarubicin, and immune-checkpoint modulators.
In the phase 2 study, the researchers randomized patients with previously untreated, histologically confirmed HER2-positive early breast cancer (defined as a clinical prognostic stage cT1c–4a-d, N0-3, M0) in a 1:1 ratio to two 3-weekly cycles of a chemotherapy-free induction phase (part 1) with TP plus 1,200 mg atezolizumab (TP-A) or TP alone.
“We hypothesized that the additive effect of immune checkpoint inhibitors plus anti-HER2 therapy and chemotherapy would not be linear,” he said.
At the end of this period, all patients underwent four cycles of TP-A in combination with epirubicin (part 2). The primary endpoint was pCR (defined as absence of invasive cancer in the breast and axillary nodes, or ypT0/Tis ypN0) in the overall study population, and a pCR of 40% was considered a positive result.
A total of 29 patients were randomized to TP-A and 29 to TP alone in nine treatment centers in Austria. The study population ranged from 33 to 82 years, with a median age of 57 years. Most patients (72.4%) had hormone receptor (HR)–positive tumors; a total of 45 patients had stage IIA cancer, and 13 had stage IIB.
The primary endpoint of pCR occurred in 35 patients overall (60.3%). In a univariate analysis, the response rates were lower in HR-positive patients, in premenopausal patients, and in histologies other than NST (invasive carcinoma of no special type), Dr. Rinnerthaler said, but none of the differences were statistically significant, likely because of the small numbers in each group.
In an exploratory analysis of the ITT population with available PD-L1 data, the pCR was 69.2% for PD-L1–negative patients and 55.2% for PD-L1–positive patients.
“We observed the highest pCR rates in PD-L1–negative patients treated in the TP-A group and the lowest in PD-L1–positive patients treated with TP alone,” Dr. Rinnerthaler said.
No new safety concerns were observed during the study, Dr. Rinnerthaler noted. AEs of grade 3 or higher occurred in 17 patients (29.3%), including 9 in the TP-A group and 8 in the TP group. The most common AEs in both groups were nausea, diarrhea, and fatigue. No AEs of special interest of grade 3 or higher (defined as immune-related AEs, cardiac disorders, or infusion-related reactions) were observed.
The study findings were limited by the small sample size, but the resulting pCR rate of 60.3% was higher than the predefined threshold of 40% and supports additional research, said Dr. Rinnerthaler.
“For HER2-positive early breast cancer, a neoadjuvant chemotherapy de-escalation immunotherapy regimen with trastuzumab, pertuzumab, atezolizumab, and epirubicin is highly effective and safe and merits further investigation,” he concluded.
During a question-and-answer session, Dr. Rinnerthaler was asked why pCR increased in PD-L1 negative patients.
Previous data have shown that PD-L1 is up-regulated in certain tumors, and may serve as a surrogate for sensitivity, he said. In previous studies the additional effect of atezolizumab was seen in a PD-L1–negative group.
Dr. Rinnerthaler said he hopes to clarify this question when his research team reviews biopsy data from baseline and after the induction phase.
Defining response is key to de-escalation
In the IMpassion031 trial, “what we saw is a tendency to better outcomes for those patients who received atezolizumab,” said Matteo Lambertini, MD, of the University of Genova (Italy), who served as discussant for the two studies. The IMpassion031 study raises the question of where we are in the use of immuno-oncology for eTNBC. The study is now one of five neoadjuvant trials in this population.
Dr. Lambertini cited the KEYNOTE-522 study, which showed significant results in EFS. However, sample sizes and statistical design were different between these studies. “I think we need large studies of data in the adjuvant and postneoadjuvant setting for patients with triple-negative breast cancer.”
Postneoadjuvant considerations from the IMpassion031 trial showed good outcomes with no additional benefit of an immune checkpoint inhibitors.
For those patients with a pCR, it is definitely time to de-escalate treatment,” he said. In patients without pCR, escalation is needed, but an improved definition of pCR is also needed.
With regard to the ATHENE study, “it may be considered a positive study because the threshold of 40% was reached,” he said. The question is what is the optimum chemotherapy backbone. There appears to be no added benefit to adding an immune checkpoint inhibitor.
There are needs for defining the role of immunotherapy in HER2-positive breast cancer and more biomarker research to inform patient selection and study design, he said.
Finally, “I am not sure that the addition of an immune checkpoint inhibitor can be considered a de-escalation,” he noted.
IMpassion031 was supported by F. Hoffmann–La Roche. Dr. Barrio disclosed financial relationships with numerous companies. ABSCG-52/ATHENE was supported by the Austrian Breast and Colorectal Cancer Study Group and Roche Austria. Dr. Rinnerthaler disclosed relationships with multiple companies including Amgen, Daiichi Sankyo, Lilly, Gilead, MSD, Novartis, Pfizer, Roche, Seagen, and Pierre Fabre. Dr. Lambertini disclosed relationships with multiple companies including Roche, Novartis, AstraZeneca, Lilly, Exact Sciences, Pfizer, MSD, Seagen, Gilead, Takeda, Sandoz, Ipsen, Libbs, Knight, and Daiichi Sankyo.
based on final data from a randomized trial.
The IMpassion031 trial showed significant improvement in pathological complete response (pCR) with the addition of atezolizumab to chemotherapy, as well as an acceptable safety profile, said Carlos H. Barrios, MD, of the Latin American Cooperative Oncology Group, Oncoclinicas, in Porto Allegre, Brazil, at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress. Those findings were published in the Lancet in 2020.
Dr. Barrios reported data from a final analysis of the IMpassion031 trial, with data on event-free survival (EFS), disease-free survival (DFS) and overall survival (OS) in the intent-to-treat (ITT) and PD-L1–positive populations.
In the study, patients with early triple-negative breast cancer (eTNBC) and a primary tumor greater than 2 cm were randomized to 840 mg of atezolizumab once every 2 weeks plus a neoadjuvant chemotherapy regimen of nab-paclitaxel 125 mg/m2 once weekly for 12 weeks, followed by doxorubicin 60 mg/m2 plus cyclophosphamide 600 mg/m2 once every 2 weeks for 8 weeks. A total of 333 patients were randomized (165 atezolizumab and 168 placebo). Patients were stratified by stage II versus stage III, and by status of PD-L1, a protein that can predict treatment response (PD-L1 less than 1% vs. 1% or higher).
The primary endpoints (previously reported) were pathological complete response (pCR) in the ITT and PD-L1 populations. After a median follow-up of 39 months, the pCR was 58% in patients treated with atezolizumab versus 41% in those treated with neoadjuvant chemotherapy alone (P = .0044) in the ITT population, Dr. Barrios said. The added benefit from atezolizumab occurred regardless of the status of PD-L1.
Dr. Barrios reported the secondary outcomes of EFS, DFS, and OS in the intent-to-treat and PD-L1–positive populations. “This is a descriptive analysis, with no statistical comparison,” he emphasized.
The 2-year data on EFS, DFS, and OS consistently favored atezolizumab across key clinical subgroups, Dr. Barrios said. In the ITT population, 2-year EFS, DFS, and OS was 85%, 87%, and 95%, respectively, in the atezolizumab group and 80%, 83%, and 90%, respectively, in the placebo group. The results were similar, irrespective of PD-L1 status.
In the PD-L1–positive population, 2-year EFS, DFS, and OS was 89%, 91%, and 95%, respectively, in atezolizumab patients and 80%, 87%, and 91% in placebo patients.
Among patients without pCR at the time of surgery, 14 of 70 patients (20%) in the atezolizumab group and 33 of 99 patients (33%) in the placebo group received additional adjuvant systemic therapy. The most common adjunctive therapy was capecitabine.
As for safety, no new safety signals or treatment-related deaths were observed in the study. Overall, 70% of atezolizumab patients and 62% of placebo patients experienced grade 3 or 4 adverse events (AEs); 59% and 54% of which were treatment related. A total of 1% of patients in each group experienced grade 5 AEs. A total of 25% of atezolizumab patients and 20% of placebo patients experienced AEs leading to treatment discontinuation.
In a further exploratory analysis, pCR was highly predictive of long-term outcomes. Exploratory analysis of circulating tumor DNA (ctDNA) showed clearance in 89% of atezolizumab patients and 86% of placebo patients by the time of surgery.
Looking at the relationship between ctDNA, DFS, and OS, positive ctDNA was associated with a worse prognosis following surgery. As demonstrated in previous studies, pCR patients with negative ctDNA had the best DFS and OS. “In non-pCR patients with positive ctDNA, a numerical trend suggests improved overall survival with atezolizumab,” although the caveat is the very small numbers, Dr. Barrios said.
More research is needed, but in the final analysis, the significant pCR benefit seen with the addition of atezolizumab to chemotherapy for eTNBC translated into numerically improved EFS, DFS and OS, said Dr. Barrios. Additionally, “we should further analyze ctDNA to help select patients for further therapy.”
In a question-and-answer session, Dr. Barrios was asked how the results compared with other studies.
“We should not overinterpret the results,” he said. However, “what the IMpassion031 study shows is consistency; the results are aligned with previous studies addressing the same question of introducing immunotherapy,” in the patient population. Although the numbers in the IMpassion031 study did not reach statistical significance, it is important to recognize that they reflect previous research.
“In my opinion, looking at the whole field, immunotherapy is something we need to consider as part of the treatment of these patients,” said Dr. Barrios. However, more research is needed to better identify which patients do and do not need chemotherapy.
Phase 2 data show increased response with added atezolizumab for PD-L1–negative patients
In a second study known as ABSCG-52/ATHENE, researchers evaluated neoadjuvant atezolizumab in combination with dual HER2 blockade plus epirubicin for the treatment of patients with early HER2-positive breast cancer.
For most of these patients, the current standard of care is neoadjuvant dual HER2 blockade with trastuzumab (T) and pertuzumab (P) plus poly-chemotherapy, said Gabriel Rinnerthaler, MD, of the Salzburg (Austria) Cancer Research Institute, said in his presentation at the meeting. However, de-escalation of chemotherapy has been a major focus of research in recent years, and more research is needed on a combination of anthracyclines, such as epirubicin and idarubicin, and immune-checkpoint modulators.
In the phase 2 study, the researchers randomized patients with previously untreated, histologically confirmed HER2-positive early breast cancer (defined as a clinical prognostic stage cT1c–4a-d, N0-3, M0) in a 1:1 ratio to two 3-weekly cycles of a chemotherapy-free induction phase (part 1) with TP plus 1,200 mg atezolizumab (TP-A) or TP alone.
“We hypothesized that the additive effect of immune checkpoint inhibitors plus anti-HER2 therapy and chemotherapy would not be linear,” he said.
At the end of this period, all patients underwent four cycles of TP-A in combination with epirubicin (part 2). The primary endpoint was pCR (defined as absence of invasive cancer in the breast and axillary nodes, or ypT0/Tis ypN0) in the overall study population, and a pCR of 40% was considered a positive result.
A total of 29 patients were randomized to TP-A and 29 to TP alone in nine treatment centers in Austria. The study population ranged from 33 to 82 years, with a median age of 57 years. Most patients (72.4%) had hormone receptor (HR)–positive tumors; a total of 45 patients had stage IIA cancer, and 13 had stage IIB.
The primary endpoint of pCR occurred in 35 patients overall (60.3%). In a univariate analysis, the response rates were lower in HR-positive patients, in premenopausal patients, and in histologies other than NST (invasive carcinoma of no special type), Dr. Rinnerthaler said, but none of the differences were statistically significant, likely because of the small numbers in each group.
In an exploratory analysis of the ITT population with available PD-L1 data, the pCR was 69.2% for PD-L1–negative patients and 55.2% for PD-L1–positive patients.
“We observed the highest pCR rates in PD-L1–negative patients treated in the TP-A group and the lowest in PD-L1–positive patients treated with TP alone,” Dr. Rinnerthaler said.
No new safety concerns were observed during the study, Dr. Rinnerthaler noted. AEs of grade 3 or higher occurred in 17 patients (29.3%), including 9 in the TP-A group and 8 in the TP group. The most common AEs in both groups were nausea, diarrhea, and fatigue. No AEs of special interest of grade 3 or higher (defined as immune-related AEs, cardiac disorders, or infusion-related reactions) were observed.
The study findings were limited by the small sample size, but the resulting pCR rate of 60.3% was higher than the predefined threshold of 40% and supports additional research, said Dr. Rinnerthaler.
“For HER2-positive early breast cancer, a neoadjuvant chemotherapy de-escalation immunotherapy regimen with trastuzumab, pertuzumab, atezolizumab, and epirubicin is highly effective and safe and merits further investigation,” he concluded.
During a question-and-answer session, Dr. Rinnerthaler was asked why pCR increased in PD-L1 negative patients.
Previous data have shown that PD-L1 is up-regulated in certain tumors, and may serve as a surrogate for sensitivity, he said. In previous studies the additional effect of atezolizumab was seen in a PD-L1–negative group.
Dr. Rinnerthaler said he hopes to clarify this question when his research team reviews biopsy data from baseline and after the induction phase.
Defining response is key to de-escalation
In the IMpassion031 trial, “what we saw is a tendency to better outcomes for those patients who received atezolizumab,” said Matteo Lambertini, MD, of the University of Genova (Italy), who served as discussant for the two studies. The IMpassion031 study raises the question of where we are in the use of immuno-oncology for eTNBC. The study is now one of five neoadjuvant trials in this population.
Dr. Lambertini cited the KEYNOTE-522 study, which showed significant results in EFS. However, sample sizes and statistical design were different between these studies. “I think we need large studies of data in the adjuvant and postneoadjuvant setting for patients with triple-negative breast cancer.”
Postneoadjuvant considerations from the IMpassion031 trial showed good outcomes with no additional benefit of an immune checkpoint inhibitors.
For those patients with a pCR, it is definitely time to de-escalate treatment,” he said. In patients without pCR, escalation is needed, but an improved definition of pCR is also needed.
With regard to the ATHENE study, “it may be considered a positive study because the threshold of 40% was reached,” he said. The question is what is the optimum chemotherapy backbone. There appears to be no added benefit to adding an immune checkpoint inhibitor.
There are needs for defining the role of immunotherapy in HER2-positive breast cancer and more biomarker research to inform patient selection and study design, he said.
Finally, “I am not sure that the addition of an immune checkpoint inhibitor can be considered a de-escalation,” he noted.
IMpassion031 was supported by F. Hoffmann–La Roche. Dr. Barrio disclosed financial relationships with numerous companies. ABSCG-52/ATHENE was supported by the Austrian Breast and Colorectal Cancer Study Group and Roche Austria. Dr. Rinnerthaler disclosed relationships with multiple companies including Amgen, Daiichi Sankyo, Lilly, Gilead, MSD, Novartis, Pfizer, Roche, Seagen, and Pierre Fabre. Dr. Lambertini disclosed relationships with multiple companies including Roche, Novartis, AstraZeneca, Lilly, Exact Sciences, Pfizer, MSD, Seagen, Gilead, Takeda, Sandoz, Ipsen, Libbs, Knight, and Daiichi Sankyo.
based on final data from a randomized trial.
The IMpassion031 trial showed significant improvement in pathological complete response (pCR) with the addition of atezolizumab to chemotherapy, as well as an acceptable safety profile, said Carlos H. Barrios, MD, of the Latin American Cooperative Oncology Group, Oncoclinicas, in Porto Allegre, Brazil, at the European Society for Medical Oncology (ESMO) Breast Cancer annual congress. Those findings were published in the Lancet in 2020.
Dr. Barrios reported data from a final analysis of the IMpassion031 trial, with data on event-free survival (EFS), disease-free survival (DFS) and overall survival (OS) in the intent-to-treat (ITT) and PD-L1–positive populations.
In the study, patients with early triple-negative breast cancer (eTNBC) and a primary tumor greater than 2 cm were randomized to 840 mg of atezolizumab once every 2 weeks plus a neoadjuvant chemotherapy regimen of nab-paclitaxel 125 mg/m2 once weekly for 12 weeks, followed by doxorubicin 60 mg/m2 plus cyclophosphamide 600 mg/m2 once every 2 weeks for 8 weeks. A total of 333 patients were randomized (165 atezolizumab and 168 placebo). Patients were stratified by stage II versus stage III, and by status of PD-L1, a protein that can predict treatment response (PD-L1 less than 1% vs. 1% or higher).
The primary endpoints (previously reported) were pathological complete response (pCR) in the ITT and PD-L1 populations. After a median follow-up of 39 months, the pCR was 58% in patients treated with atezolizumab versus 41% in those treated with neoadjuvant chemotherapy alone (P = .0044) in the ITT population, Dr. Barrios said. The added benefit from atezolizumab occurred regardless of the status of PD-L1.
Dr. Barrios reported the secondary outcomes of EFS, DFS, and OS in the intent-to-treat and PD-L1–positive populations. “This is a descriptive analysis, with no statistical comparison,” he emphasized.
The 2-year data on EFS, DFS, and OS consistently favored atezolizumab across key clinical subgroups, Dr. Barrios said. In the ITT population, 2-year EFS, DFS, and OS was 85%, 87%, and 95%, respectively, in the atezolizumab group and 80%, 83%, and 90%, respectively, in the placebo group. The results were similar, irrespective of PD-L1 status.
In the PD-L1–positive population, 2-year EFS, DFS, and OS was 89%, 91%, and 95%, respectively, in atezolizumab patients and 80%, 87%, and 91% in placebo patients.
Among patients without pCR at the time of surgery, 14 of 70 patients (20%) in the atezolizumab group and 33 of 99 patients (33%) in the placebo group received additional adjuvant systemic therapy. The most common adjunctive therapy was capecitabine.
As for safety, no new safety signals or treatment-related deaths were observed in the study. Overall, 70% of atezolizumab patients and 62% of placebo patients experienced grade 3 or 4 adverse events (AEs); 59% and 54% of which were treatment related. A total of 1% of patients in each group experienced grade 5 AEs. A total of 25% of atezolizumab patients and 20% of placebo patients experienced AEs leading to treatment discontinuation.
In a further exploratory analysis, pCR was highly predictive of long-term outcomes. Exploratory analysis of circulating tumor DNA (ctDNA) showed clearance in 89% of atezolizumab patients and 86% of placebo patients by the time of surgery.
Looking at the relationship between ctDNA, DFS, and OS, positive ctDNA was associated with a worse prognosis following surgery. As demonstrated in previous studies, pCR patients with negative ctDNA had the best DFS and OS. “In non-pCR patients with positive ctDNA, a numerical trend suggests improved overall survival with atezolizumab,” although the caveat is the very small numbers, Dr. Barrios said.
More research is needed, but in the final analysis, the significant pCR benefit seen with the addition of atezolizumab to chemotherapy for eTNBC translated into numerically improved EFS, DFS and OS, said Dr. Barrios. Additionally, “we should further analyze ctDNA to help select patients for further therapy.”
In a question-and-answer session, Dr. Barrios was asked how the results compared with other studies.
“We should not overinterpret the results,” he said. However, “what the IMpassion031 study shows is consistency; the results are aligned with previous studies addressing the same question of introducing immunotherapy,” in the patient population. Although the numbers in the IMpassion031 study did not reach statistical significance, it is important to recognize that they reflect previous research.
“In my opinion, looking at the whole field, immunotherapy is something we need to consider as part of the treatment of these patients,” said Dr. Barrios. However, more research is needed to better identify which patients do and do not need chemotherapy.
Phase 2 data show increased response with added atezolizumab for PD-L1–negative patients
In a second study known as ABSCG-52/ATHENE, researchers evaluated neoadjuvant atezolizumab in combination with dual HER2 blockade plus epirubicin for the treatment of patients with early HER2-positive breast cancer.
For most of these patients, the current standard of care is neoadjuvant dual HER2 blockade with trastuzumab (T) and pertuzumab (P) plus poly-chemotherapy, said Gabriel Rinnerthaler, MD, of the Salzburg (Austria) Cancer Research Institute, said in his presentation at the meeting. However, de-escalation of chemotherapy has been a major focus of research in recent years, and more research is needed on a combination of anthracyclines, such as epirubicin and idarubicin, and immune-checkpoint modulators.
In the phase 2 study, the researchers randomized patients with previously untreated, histologically confirmed HER2-positive early breast cancer (defined as a clinical prognostic stage cT1c–4a-d, N0-3, M0) in a 1:1 ratio to two 3-weekly cycles of a chemotherapy-free induction phase (part 1) with TP plus 1,200 mg atezolizumab (TP-A) or TP alone.
“We hypothesized that the additive effect of immune checkpoint inhibitors plus anti-HER2 therapy and chemotherapy would not be linear,” he said.
At the end of this period, all patients underwent four cycles of TP-A in combination with epirubicin (part 2). The primary endpoint was pCR (defined as absence of invasive cancer in the breast and axillary nodes, or ypT0/Tis ypN0) in the overall study population, and a pCR of 40% was considered a positive result.
A total of 29 patients were randomized to TP-A and 29 to TP alone in nine treatment centers in Austria. The study population ranged from 33 to 82 years, with a median age of 57 years. Most patients (72.4%) had hormone receptor (HR)–positive tumors; a total of 45 patients had stage IIA cancer, and 13 had stage IIB.
The primary endpoint of pCR occurred in 35 patients overall (60.3%). In a univariate analysis, the response rates were lower in HR-positive patients, in premenopausal patients, and in histologies other than NST (invasive carcinoma of no special type), Dr. Rinnerthaler said, but none of the differences were statistically significant, likely because of the small numbers in each group.
In an exploratory analysis of the ITT population with available PD-L1 data, the pCR was 69.2% for PD-L1–negative patients and 55.2% for PD-L1–positive patients.
“We observed the highest pCR rates in PD-L1–negative patients treated in the TP-A group and the lowest in PD-L1–positive patients treated with TP alone,” Dr. Rinnerthaler said.
No new safety concerns were observed during the study, Dr. Rinnerthaler noted. AEs of grade 3 or higher occurred in 17 patients (29.3%), including 9 in the TP-A group and 8 in the TP group. The most common AEs in both groups were nausea, diarrhea, and fatigue. No AEs of special interest of grade 3 or higher (defined as immune-related AEs, cardiac disorders, or infusion-related reactions) were observed.
The study findings were limited by the small sample size, but the resulting pCR rate of 60.3% was higher than the predefined threshold of 40% and supports additional research, said Dr. Rinnerthaler.
“For HER2-positive early breast cancer, a neoadjuvant chemotherapy de-escalation immunotherapy regimen with trastuzumab, pertuzumab, atezolizumab, and epirubicin is highly effective and safe and merits further investigation,” he concluded.
During a question-and-answer session, Dr. Rinnerthaler was asked why pCR increased in PD-L1 negative patients.
Previous data have shown that PD-L1 is up-regulated in certain tumors, and may serve as a surrogate for sensitivity, he said. In previous studies the additional effect of atezolizumab was seen in a PD-L1–negative group.
Dr. Rinnerthaler said he hopes to clarify this question when his research team reviews biopsy data from baseline and after the induction phase.
Defining response is key to de-escalation
In the IMpassion031 trial, “what we saw is a tendency to better outcomes for those patients who received atezolizumab,” said Matteo Lambertini, MD, of the University of Genova (Italy), who served as discussant for the two studies. The IMpassion031 study raises the question of where we are in the use of immuno-oncology for eTNBC. The study is now one of five neoadjuvant trials in this population.
Dr. Lambertini cited the KEYNOTE-522 study, which showed significant results in EFS. However, sample sizes and statistical design were different between these studies. “I think we need large studies of data in the adjuvant and postneoadjuvant setting for patients with triple-negative breast cancer.”
Postneoadjuvant considerations from the IMpassion031 trial showed good outcomes with no additional benefit of an immune checkpoint inhibitors.
For those patients with a pCR, it is definitely time to de-escalate treatment,” he said. In patients without pCR, escalation is needed, but an improved definition of pCR is also needed.
With regard to the ATHENE study, “it may be considered a positive study because the threshold of 40% was reached,” he said. The question is what is the optimum chemotherapy backbone. There appears to be no added benefit to adding an immune checkpoint inhibitor.
There are needs for defining the role of immunotherapy in HER2-positive breast cancer and more biomarker research to inform patient selection and study design, he said.
Finally, “I am not sure that the addition of an immune checkpoint inhibitor can be considered a de-escalation,” he noted.
IMpassion031 was supported by F. Hoffmann–La Roche. Dr. Barrio disclosed financial relationships with numerous companies. ABSCG-52/ATHENE was supported by the Austrian Breast and Colorectal Cancer Study Group and Roche Austria. Dr. Rinnerthaler disclosed relationships with multiple companies including Amgen, Daiichi Sankyo, Lilly, Gilead, MSD, Novartis, Pfizer, Roche, Seagen, and Pierre Fabre. Dr. Lambertini disclosed relationships with multiple companies including Roche, Novartis, AstraZeneca, Lilly, Exact Sciences, Pfizer, MSD, Seagen, Gilead, Takeda, Sandoz, Ipsen, Libbs, Knight, and Daiichi Sankyo.
FROM ESMO BREAST CANCER 2023
Vulvar syringoma
To the Editor:
Syringomas are common benign tumors of the eccrine sweat glands that usually manifest clinically as multiple flesh-colored papules. They are most commonly seen on the face, neck, and chest of adolescent girls. Syringomas may appear at any site of the body but are rare in the vulva. We present a case of a 51-year-old woman who was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of a tumor carrying a differential diagnosis of vulvar syringoma vs microcystic adnexal carcinoma (MAC).
A 51-year-old woman presented to dermatology (G.G.) and was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of possible vulvar syringoma vs MAC. The patient previously had been evaluated at an outside community practice due to dyspareunia, vulvar discomfort, and vulvar irregularities of 1 month’s duration. At that time, a small biopsy was performed, and the histologic differential diagnosis included syringoma vs an adnexal carcinoma. Consequently, she was referred to gynecologic oncology for further management.
Pelvic examination revealed multilobular nodular areas overlying the clitoral hood that extended down to the labia majora. The nodular processes did not involve the clitoris, labia minora, or perineum. A mobile isolated lymph node measuring 2.0×1.0 cm in the right inguinal area also was noted. The patient’s clinical history was notable for right breast carcinoma treated with a right mastectomy with axillary lymph node dissection that showed metastatic disease. She also underwent adjuvant chemotherapy with paclitaxel and doxorubicin for breast carcinoma.
After discussing the diagnostic differential and treatment options, the patient elected to undergo a bilateral partial radical vulvectomy with reconstruction and resection of the right inguinal lymph node. Gross examination of the vulvectomy specimen showed multiple flesh-colored papules (FIGURE 1). Histologic examination revealed a neoplasm with sweat gland differentiation that was broad and poorly circumscribed but confined to the dermis (FIGURES 2A and 2B). The neoplasm was composed of epithelial cells that formed ductlike structures, lined by 2 layers of cuboidal epithelium within a fibrous stroma (FIGURE 2C). A toluidine blue special stain was performed and demonstrated an increased amount of mast cells in the tissue (FIGURE 3). Immunohistochemical stains for gross cystic disease fluid protein, estrogen receptor (ER), and progesterone receptor (PR) were negative in the tumor cells. The lack of cytologic atypia, perineural invasion, and deep infiltration into the subcutis favored a syringoma. One month later, the case was presented at the Tumor Board Conference at the University of Alabama at Birmingham where a final diagnosis of vulvar syringoma was agreed upon and discussed with the patient. At that time, no recurrence was evident and follow-up was recommended.
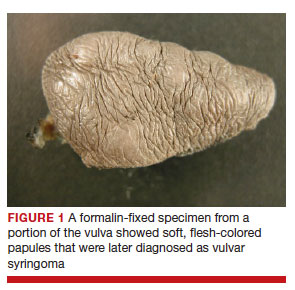
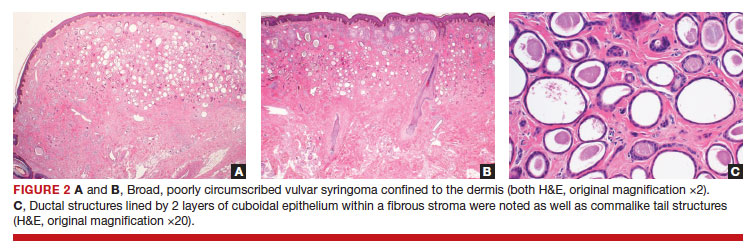
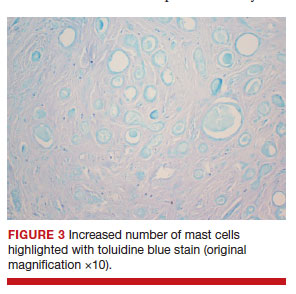
Syringomas are benign tumors of the sweat glands that are fairly common and appear to have a predilection for women. Although most of the literature classifies them as eccrine neoplasms, the term syringoma can be used to describe neoplasms of either apocrine or eccrine lineage.1 To rule out an apocrine lineage of the tumor in our patient, we performed immunohistochemistry for gross cystic disease fluid protein, a marker of apocrine differentiation. This stain highlighted normal apocrine glands that were not involved in the tumor proliferation.
Syringomas may occur at any site on the body but are prone to occur on the periorbital area, especially the eyelids.1 Some of the atypical locations for a syringoma include the anterior neck, chest, abdomen, genitals, axillae, groin, and buttocks.2 Vulvar syringomas were first reported by Carneiro3 in 1971 as usually affecting adolescent girls and middle-aged women. There have been approximately 40 reported cases affecting women aged 8 to 78 years.4,5 Vulvar syringomas classically appear as firm or soft, flesh-colored to transparent, papular lesions. The 2 other clinical variants are miliumlike, whitish, cystic papules as well as lichenoid papules.6 Pérez-Bustillo et al5 reported a case of the lichenoid papule variant on the labia majora of a 78-year-old woman who presented with intermittent vulvar pruritus of 4 years’ duration. Due to this patient’s 9-year history of urinary incontinence, the lesions had been misdiagnosed as irritant dermatitis and associated lichen simplex chronicus (LSC). This case is a reminder to consider vulvar syringoma in patients with LSC who respond poorly to oral antihistamines and topical steroids.5 Rarely, multiple clinical variants may coexist. In a case reported by Dereli et al,7 a 19-year-old woman presented with concurrent classical and miliumlike forms of vulvar syringoma.
Vulvar syringomas usually present as multiple lesions involving both sides of the labia majora; however, Blasdale and McLelland8 reported a single isolated syringoma of the vulva on the anterior right labia minora that measured 1.0×0.5 cm, leading the lesion to be described as a giant syringoma.
Vulvar syringomas usually are asymptomatic and noticed during routine gynecologic examination. Therefore, it is believed that they likely are underdiagnosed.5 When symptomatic, they commonly present with constant9 or intermittent5 pruritus, which may intensify during menstruation, pregnancy, and summertime.6,10-12 Gerdsen et al10 documented a 27-year-old woman who presented with a 2-year history of pruritic vulvar skin lesions that became exacerbated during menstruation, which raised the possibility of cyclical hormonal changes being responsible for periodic exacerbation of vulvar pruritus during menstruation. In addition, patients may experience an increase in size and number of the lesions during pregnancy. Bal et al11 reported a 24-year-old primigravida with vulvar papular lesions that intensified during pregnancy. She had experienced intermittent vulvar pruritus for 12 years but had no change in symptoms during menstruation.11 Few studies have attempted to evaluate the presence of ER and PR in the syringomas. A study of 9 nonvulvar syringomas by Wallace and Smoller13 showed ER positivity in 1 case and PR positivity in 8 cases, lending support to the hormonal theory; however, in another case series of 15 vulvar syringomas, Huang et al6 failed to show ER and PR expression by immunohistochemical staining. A case report published 3 years earlier documented the first case of PR positivity on a vulvar syringoma.14 Our patient also was negative for ER and PR, which suggested that hormonal status is important in some but not all syringomas.
Patients with vulvar syringomas also might have coexisting extragenital syringomas in the neck,4 eyelids,6,7,10 and periorbital area,6 and thorough examination of the body is essential. If an extragenital syringoma is diagnosed, a vulvar syringoma should be considered, especially when the patient presents with unexplained genital symptoms. Although no proven hereditary transmission pattern has been established, family history of syringomas has been established in several cases.15 In a case series reported by Huang et al,6 4 of 18 patients reported a family history of periorbital syringomas. In our case, the patient did not report a family history of syringomas.
The differential diagnosis of vulvar lesions with pruritus is broad and includes Fox-Fordyce disease, lichen planus, LSC, epidermal cysts, senile angiomas, dystrophic calcinosis, xanthomas, steatocytomas, soft fibromas, condyloma acuminatum, and candidiasis. Vulvar syringomas might have a nonspecific appearance, and histologic examination is essential to confirm the diagnosis and rule out any malignant process such as MAC, vulvar intraepithelial neoplasia, extramammary Paget disease, or other glandular neoplasms of the vulva.
Microcystic adnexal carcinoma was first reported in 1982 by Goldstein et al16 as a locally aggressive neoplasm that can be confused with benign adnexal neoplasms, particularly desmoplastic trichoepithelioma, trichoadenoma, and syringoma. Microcystic adnexal carcinomas present as slow-growing, flesh-colored papules that may resemble syringomas and appear in similar body sites. Histologic examination is essential to differentiate between these two entities. Syringomas are tumors confined to the dermis and are composed of multiple small ducts lined by 2 layers of cuboidal epithelium within a dense fibrous stroma. Unlike syringomas, MACs usually infiltrate diffusely into the dermis and subcutis and may extend into the underlying muscle. Although bland cytologic features predominate, perineural invasion frequently is present in MACs. A potential pitfall of misdiagnosis can be caused by a superficial biopsy that may reveal benign histologic appearance, particularly in the upper level of the tumor where it may be confused with a syringoma or a benign follicular neoplasm.17
The initial biopsy performed on our patient was possibly not deep enough to render an unequivocal diagnosis and therefore bilateral partial radical vulvectomy was considered. After surgery, histologic examination of the resection specimen revealed a poorly circumscribed tumor confined to the dermis. The tumor was broad and the lack of deep infiltration into the subcutis and perineural invasion favored a syringoma (FIGURES 2A and 2B). These findings were consistent with case reports that documented syringomas as being more wide than deep on microscopic examination, whereas the opposite pertained to MAC.18 Cases of plaque-type syringomas that initially were misdiagnosed as MACs also have been reported.19 Because misdiagnosis may affect the treatment plan and potentially result in unnecessary surgery, caution should be taken when differentiating between these two entities. When a definitive diagnosis cannot be rendered on a superficial biopsy, a recommendation should be made for a deeper biopsy sampling the subcutis.
For the majority of the patients with vulvar syringomas, treatment is seldom required due to their asymptomatic nature; however, patients who present with symptoms usually report pruritus of variable intensities and patterns. A standardized treatment does not exist for vulvar syringomas, and oral or topical treatment might be used as an initial approach. Commonly prescribed medications with variable results include topical corticosteroids, oral antihistamines, and topical retinoids. In a case reported by Iwao et al,20 vulvar syringomas were successfully treated with tranilast, which has anti-inflammatory and immunomodulatory effects. This medication could have a possible dual action—inhibiting the release of chemical mediators from the mast cells and inhibiting the release of IL-1β from the eccrine duct, which could suppress the proliferation of stromal connective tissue. Our case was stained with toluidine blue and showed an increased number of mast cells in the tissue (FIGURE 3).Patients who are unresponsive to tranilast or have extensive disease resulting in cosmetic disfigurement might benefit from more invasive treatment methods including a variety of lasers, cryotherapy, electrosurgery, and excision. Excisions should include the entire tumor to avoid recurrence. In a case reported by Garman and Metry,21 the lesions were surgically excised using small 2- to 3-mm punches; however, several weeks later the lesions recurred. Our patient presented with a 1-month evolution of dyspareunia, vulvar discomfort, and vulvar irregularities that were probably not treated with oral or topical medications before being referred for surgery.
We report a case of a vulvar syringoma that presented diagnostic challenges in the initial biopsy, which prevented the exclusion of an MAC. After partial radical vulvectomy, histologic examination was more definitive, showing lack of deep infiltration into the subcutis or perineural invasion that are commonly seen in MAC. This case is an example of a notable pitfall in the diagnosis of vulvar syringoma on a limited biopsy leading to overtreatment. Raising awareness of this entity is the only modality to prevent misdiagnosis. We encourage reporting of further cases of syringomas, particularly those with atypical locations or patterns that may cause diagnostic problems. ●
- Ensure adequate depth of biopsy to assist in the histologic diagnosis of syringoma vs microcystic adnexal carcinoma.
- Vulvar syringomas also may contribute to notable pruritus and ultimately be the underlying etiology for secondary skin changes leading to a lichen simplex chronicus–like phenotype
- Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Spain: Mosby Elsevier; 2008.
- Weedon D. Skin Pathology. 3rd ed. China: Churchill Livingstone Elsevier; 2010.
- Carneiro SJ, Gardner HL, Knox JM. Syringoma of the vulva. Arch Dermatol. 1971;103:494-496.
- Trager JD, Silvers J, Reed JA, et al. Neck and vulvar papules in an 8-year-old girl. Arch Dermatol. 1999;135:203, 206.
- Pérez-Bustillo A, Ruiz-González I, Delgado S, et al. Vulvar syringoma: a rare cause of vulvar pruritus. Actas DermoSifiliográficas. 2008; 99:580-581.
- Huang YH, Chuang YH, Kuo TT, et al. Vulvar syringoma: a clinicopathologic and immunohistologic study of 18 patients and results of treatment. J Am Acad Dermatol. 2003;48:735-739.
- Dereli T, Turk BG, Kazandi AC. Syringomas of the vulva. Int J Gynaecol Obstet. 2007;99:65-66.
- Blasdale C, McLelland J. Solitary giant vulval syringoma. Br J Dermatol. 1999;141:374-375.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Gerdsen R, Wenzel J, Uerlich M, et al. Periodic genital pruritus caused by syringoma of the vulva. Acta Obstet Gynecol Scand. 2002;81:369-370.
- Bal N, Aslan E, Kayaselcuk F, et al. Vulvar syringoma aggravated by pregnancy. Pathol Oncol Res. 2003;9:196-197.
- Turan C, Ugur M, Kutluay L, et al. Vulvar syringoma exacerbated during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:141-142.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995; 22:442-445.
- Yorganci A, Kale A, Dunder I, et al. Vulvar syringoma showing progesterone receptor positivity. BJOG. 2000;107:292-294.
- Draznin M. Hereditary syringomas: a case report. Dermatol Online J. 2004;10:19.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Hamsch C, Hartschuh W. Microcystic adnexal carcinomaaggressive infiltrative tumor often with innocent clinical appearance. J Dtsch Dermatol Ges. 2010;8:275-278.
- Henner MS, Shapiro PE, Ritter JH, et al. Solitary syringoma. report of five cases and clinicopathologic comparison with microcystic adnexal carcinoma of the skin. Am J Dermatopathol. 1995;17:465-470.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma. J Cutan Pathol. 2008;35:570-574.
- Iwao F, Onozuka T, Kawashima T. Vulval syringoma successfully treated with tranilast. Br J Dermatol. 2005;153:1228-1230.
- Garman M, Metry D. Vulvar syringomas in a 9-year-old child with review of the literature. Pediatr Dermatol. 2006;23:369372.
To the Editor:
Syringomas are common benign tumors of the eccrine sweat glands that usually manifest clinically as multiple flesh-colored papules. They are most commonly seen on the face, neck, and chest of adolescent girls. Syringomas may appear at any site of the body but are rare in the vulva. We present a case of a 51-year-old woman who was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of a tumor carrying a differential diagnosis of vulvar syringoma vs microcystic adnexal carcinoma (MAC).
A 51-year-old woman presented to dermatology (G.G.) and was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of possible vulvar syringoma vs MAC. The patient previously had been evaluated at an outside community practice due to dyspareunia, vulvar discomfort, and vulvar irregularities of 1 month’s duration. At that time, a small biopsy was performed, and the histologic differential diagnosis included syringoma vs an adnexal carcinoma. Consequently, she was referred to gynecologic oncology for further management.
Pelvic examination revealed multilobular nodular areas overlying the clitoral hood that extended down to the labia majora. The nodular processes did not involve the clitoris, labia minora, or perineum. A mobile isolated lymph node measuring 2.0×1.0 cm in the right inguinal area also was noted. The patient’s clinical history was notable for right breast carcinoma treated with a right mastectomy with axillary lymph node dissection that showed metastatic disease. She also underwent adjuvant chemotherapy with paclitaxel and doxorubicin for breast carcinoma.
After discussing the diagnostic differential and treatment options, the patient elected to undergo a bilateral partial radical vulvectomy with reconstruction and resection of the right inguinal lymph node. Gross examination of the vulvectomy specimen showed multiple flesh-colored papules (FIGURE 1). Histologic examination revealed a neoplasm with sweat gland differentiation that was broad and poorly circumscribed but confined to the dermis (FIGURES 2A and 2B). The neoplasm was composed of epithelial cells that formed ductlike structures, lined by 2 layers of cuboidal epithelium within a fibrous stroma (FIGURE 2C). A toluidine blue special stain was performed and demonstrated an increased amount of mast cells in the tissue (FIGURE 3). Immunohistochemical stains for gross cystic disease fluid protein, estrogen receptor (ER), and progesterone receptor (PR) were negative in the tumor cells. The lack of cytologic atypia, perineural invasion, and deep infiltration into the subcutis favored a syringoma. One month later, the case was presented at the Tumor Board Conference at the University of Alabama at Birmingham where a final diagnosis of vulvar syringoma was agreed upon and discussed with the patient. At that time, no recurrence was evident and follow-up was recommended.



Syringomas are benign tumors of the sweat glands that are fairly common and appear to have a predilection for women. Although most of the literature classifies them as eccrine neoplasms, the term syringoma can be used to describe neoplasms of either apocrine or eccrine lineage.1 To rule out an apocrine lineage of the tumor in our patient, we performed immunohistochemistry for gross cystic disease fluid protein, a marker of apocrine differentiation. This stain highlighted normal apocrine glands that were not involved in the tumor proliferation.
Syringomas may occur at any site on the body but are prone to occur on the periorbital area, especially the eyelids.1 Some of the atypical locations for a syringoma include the anterior neck, chest, abdomen, genitals, axillae, groin, and buttocks.2 Vulvar syringomas were first reported by Carneiro3 in 1971 as usually affecting adolescent girls and middle-aged women. There have been approximately 40 reported cases affecting women aged 8 to 78 years.4,5 Vulvar syringomas classically appear as firm or soft, flesh-colored to transparent, papular lesions. The 2 other clinical variants are miliumlike, whitish, cystic papules as well as lichenoid papules.6 Pérez-Bustillo et al5 reported a case of the lichenoid papule variant on the labia majora of a 78-year-old woman who presented with intermittent vulvar pruritus of 4 years’ duration. Due to this patient’s 9-year history of urinary incontinence, the lesions had been misdiagnosed as irritant dermatitis and associated lichen simplex chronicus (LSC). This case is a reminder to consider vulvar syringoma in patients with LSC who respond poorly to oral antihistamines and topical steroids.5 Rarely, multiple clinical variants may coexist. In a case reported by Dereli et al,7 a 19-year-old woman presented with concurrent classical and miliumlike forms of vulvar syringoma.
Vulvar syringomas usually present as multiple lesions involving both sides of the labia majora; however, Blasdale and McLelland8 reported a single isolated syringoma of the vulva on the anterior right labia minora that measured 1.0×0.5 cm, leading the lesion to be described as a giant syringoma.
Vulvar syringomas usually are asymptomatic and noticed during routine gynecologic examination. Therefore, it is believed that they likely are underdiagnosed.5 When symptomatic, they commonly present with constant9 or intermittent5 pruritus, which may intensify during menstruation, pregnancy, and summertime.6,10-12 Gerdsen et al10 documented a 27-year-old woman who presented with a 2-year history of pruritic vulvar skin lesions that became exacerbated during menstruation, which raised the possibility of cyclical hormonal changes being responsible for periodic exacerbation of vulvar pruritus during menstruation. In addition, patients may experience an increase in size and number of the lesions during pregnancy. Bal et al11 reported a 24-year-old primigravida with vulvar papular lesions that intensified during pregnancy. She had experienced intermittent vulvar pruritus for 12 years but had no change in symptoms during menstruation.11 Few studies have attempted to evaluate the presence of ER and PR in the syringomas. A study of 9 nonvulvar syringomas by Wallace and Smoller13 showed ER positivity in 1 case and PR positivity in 8 cases, lending support to the hormonal theory; however, in another case series of 15 vulvar syringomas, Huang et al6 failed to show ER and PR expression by immunohistochemical staining. A case report published 3 years earlier documented the first case of PR positivity on a vulvar syringoma.14 Our patient also was negative for ER and PR, which suggested that hormonal status is important in some but not all syringomas.
Patients with vulvar syringomas also might have coexisting extragenital syringomas in the neck,4 eyelids,6,7,10 and periorbital area,6 and thorough examination of the body is essential. If an extragenital syringoma is diagnosed, a vulvar syringoma should be considered, especially when the patient presents with unexplained genital symptoms. Although no proven hereditary transmission pattern has been established, family history of syringomas has been established in several cases.15 In a case series reported by Huang et al,6 4 of 18 patients reported a family history of periorbital syringomas. In our case, the patient did not report a family history of syringomas.
The differential diagnosis of vulvar lesions with pruritus is broad and includes Fox-Fordyce disease, lichen planus, LSC, epidermal cysts, senile angiomas, dystrophic calcinosis, xanthomas, steatocytomas, soft fibromas, condyloma acuminatum, and candidiasis. Vulvar syringomas might have a nonspecific appearance, and histologic examination is essential to confirm the diagnosis and rule out any malignant process such as MAC, vulvar intraepithelial neoplasia, extramammary Paget disease, or other glandular neoplasms of the vulva.
Microcystic adnexal carcinoma was first reported in 1982 by Goldstein et al16 as a locally aggressive neoplasm that can be confused with benign adnexal neoplasms, particularly desmoplastic trichoepithelioma, trichoadenoma, and syringoma. Microcystic adnexal carcinomas present as slow-growing, flesh-colored papules that may resemble syringomas and appear in similar body sites. Histologic examination is essential to differentiate between these two entities. Syringomas are tumors confined to the dermis and are composed of multiple small ducts lined by 2 layers of cuboidal epithelium within a dense fibrous stroma. Unlike syringomas, MACs usually infiltrate diffusely into the dermis and subcutis and may extend into the underlying muscle. Although bland cytologic features predominate, perineural invasion frequently is present in MACs. A potential pitfall of misdiagnosis can be caused by a superficial biopsy that may reveal benign histologic appearance, particularly in the upper level of the tumor where it may be confused with a syringoma or a benign follicular neoplasm.17
The initial biopsy performed on our patient was possibly not deep enough to render an unequivocal diagnosis and therefore bilateral partial radical vulvectomy was considered. After surgery, histologic examination of the resection specimen revealed a poorly circumscribed tumor confined to the dermis. The tumor was broad and the lack of deep infiltration into the subcutis and perineural invasion favored a syringoma (FIGURES 2A and 2B). These findings were consistent with case reports that documented syringomas as being more wide than deep on microscopic examination, whereas the opposite pertained to MAC.18 Cases of plaque-type syringomas that initially were misdiagnosed as MACs also have been reported.19 Because misdiagnosis may affect the treatment plan and potentially result in unnecessary surgery, caution should be taken when differentiating between these two entities. When a definitive diagnosis cannot be rendered on a superficial biopsy, a recommendation should be made for a deeper biopsy sampling the subcutis.
For the majority of the patients with vulvar syringomas, treatment is seldom required due to their asymptomatic nature; however, patients who present with symptoms usually report pruritus of variable intensities and patterns. A standardized treatment does not exist for vulvar syringomas, and oral or topical treatment might be used as an initial approach. Commonly prescribed medications with variable results include topical corticosteroids, oral antihistamines, and topical retinoids. In a case reported by Iwao et al,20 vulvar syringomas were successfully treated with tranilast, which has anti-inflammatory and immunomodulatory effects. This medication could have a possible dual action—inhibiting the release of chemical mediators from the mast cells and inhibiting the release of IL-1β from the eccrine duct, which could suppress the proliferation of stromal connective tissue. Our case was stained with toluidine blue and showed an increased number of mast cells in the tissue (FIGURE 3).Patients who are unresponsive to tranilast or have extensive disease resulting in cosmetic disfigurement might benefit from more invasive treatment methods including a variety of lasers, cryotherapy, electrosurgery, and excision. Excisions should include the entire tumor to avoid recurrence. In a case reported by Garman and Metry,21 the lesions were surgically excised using small 2- to 3-mm punches; however, several weeks later the lesions recurred. Our patient presented with a 1-month evolution of dyspareunia, vulvar discomfort, and vulvar irregularities that were probably not treated with oral or topical medications before being referred for surgery.
We report a case of a vulvar syringoma that presented diagnostic challenges in the initial biopsy, which prevented the exclusion of an MAC. After partial radical vulvectomy, histologic examination was more definitive, showing lack of deep infiltration into the subcutis or perineural invasion that are commonly seen in MAC. This case is an example of a notable pitfall in the diagnosis of vulvar syringoma on a limited biopsy leading to overtreatment. Raising awareness of this entity is the only modality to prevent misdiagnosis. We encourage reporting of further cases of syringomas, particularly those with atypical locations or patterns that may cause diagnostic problems. ●
- Ensure adequate depth of biopsy to assist in the histologic diagnosis of syringoma vs microcystic adnexal carcinoma.
- Vulvar syringomas also may contribute to notable pruritus and ultimately be the underlying etiology for secondary skin changes leading to a lichen simplex chronicus–like phenotype
To the Editor:
Syringomas are common benign tumors of the eccrine sweat glands that usually manifest clinically as multiple flesh-colored papules. They are most commonly seen on the face, neck, and chest of adolescent girls. Syringomas may appear at any site of the body but are rare in the vulva. We present a case of a 51-year-old woman who was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of a tumor carrying a differential diagnosis of vulvar syringoma vs microcystic adnexal carcinoma (MAC).
A 51-year-old woman presented to dermatology (G.G.) and was referred to the Division of Gynecologic Oncology at the University of Alabama at Birmingham for further management of possible vulvar syringoma vs MAC. The patient previously had been evaluated at an outside community practice due to dyspareunia, vulvar discomfort, and vulvar irregularities of 1 month’s duration. At that time, a small biopsy was performed, and the histologic differential diagnosis included syringoma vs an adnexal carcinoma. Consequently, she was referred to gynecologic oncology for further management.
Pelvic examination revealed multilobular nodular areas overlying the clitoral hood that extended down to the labia majora. The nodular processes did not involve the clitoris, labia minora, or perineum. A mobile isolated lymph node measuring 2.0×1.0 cm in the right inguinal area also was noted. The patient’s clinical history was notable for right breast carcinoma treated with a right mastectomy with axillary lymph node dissection that showed metastatic disease. She also underwent adjuvant chemotherapy with paclitaxel and doxorubicin for breast carcinoma.
After discussing the diagnostic differential and treatment options, the patient elected to undergo a bilateral partial radical vulvectomy with reconstruction and resection of the right inguinal lymph node. Gross examination of the vulvectomy specimen showed multiple flesh-colored papules (FIGURE 1). Histologic examination revealed a neoplasm with sweat gland differentiation that was broad and poorly circumscribed but confined to the dermis (FIGURES 2A and 2B). The neoplasm was composed of epithelial cells that formed ductlike structures, lined by 2 layers of cuboidal epithelium within a fibrous stroma (FIGURE 2C). A toluidine blue special stain was performed and demonstrated an increased amount of mast cells in the tissue (FIGURE 3). Immunohistochemical stains for gross cystic disease fluid protein, estrogen receptor (ER), and progesterone receptor (PR) were negative in the tumor cells. The lack of cytologic atypia, perineural invasion, and deep infiltration into the subcutis favored a syringoma. One month later, the case was presented at the Tumor Board Conference at the University of Alabama at Birmingham where a final diagnosis of vulvar syringoma was agreed upon and discussed with the patient. At that time, no recurrence was evident and follow-up was recommended.



Syringomas are benign tumors of the sweat glands that are fairly common and appear to have a predilection for women. Although most of the literature classifies them as eccrine neoplasms, the term syringoma can be used to describe neoplasms of either apocrine or eccrine lineage.1 To rule out an apocrine lineage of the tumor in our patient, we performed immunohistochemistry for gross cystic disease fluid protein, a marker of apocrine differentiation. This stain highlighted normal apocrine glands that were not involved in the tumor proliferation.
Syringomas may occur at any site on the body but are prone to occur on the periorbital area, especially the eyelids.1 Some of the atypical locations for a syringoma include the anterior neck, chest, abdomen, genitals, axillae, groin, and buttocks.2 Vulvar syringomas were first reported by Carneiro3 in 1971 as usually affecting adolescent girls and middle-aged women. There have been approximately 40 reported cases affecting women aged 8 to 78 years.4,5 Vulvar syringomas classically appear as firm or soft, flesh-colored to transparent, papular lesions. The 2 other clinical variants are miliumlike, whitish, cystic papules as well as lichenoid papules.6 Pérez-Bustillo et al5 reported a case of the lichenoid papule variant on the labia majora of a 78-year-old woman who presented with intermittent vulvar pruritus of 4 years’ duration. Due to this patient’s 9-year history of urinary incontinence, the lesions had been misdiagnosed as irritant dermatitis and associated lichen simplex chronicus (LSC). This case is a reminder to consider vulvar syringoma in patients with LSC who respond poorly to oral antihistamines and topical steroids.5 Rarely, multiple clinical variants may coexist. In a case reported by Dereli et al,7 a 19-year-old woman presented with concurrent classical and miliumlike forms of vulvar syringoma.
Vulvar syringomas usually present as multiple lesions involving both sides of the labia majora; however, Blasdale and McLelland8 reported a single isolated syringoma of the vulva on the anterior right labia minora that measured 1.0×0.5 cm, leading the lesion to be described as a giant syringoma.
Vulvar syringomas usually are asymptomatic and noticed during routine gynecologic examination. Therefore, it is believed that they likely are underdiagnosed.5 When symptomatic, they commonly present with constant9 or intermittent5 pruritus, which may intensify during menstruation, pregnancy, and summertime.6,10-12 Gerdsen et al10 documented a 27-year-old woman who presented with a 2-year history of pruritic vulvar skin lesions that became exacerbated during menstruation, which raised the possibility of cyclical hormonal changes being responsible for periodic exacerbation of vulvar pruritus during menstruation. In addition, patients may experience an increase in size and number of the lesions during pregnancy. Bal et al11 reported a 24-year-old primigravida with vulvar papular lesions that intensified during pregnancy. She had experienced intermittent vulvar pruritus for 12 years but had no change in symptoms during menstruation.11 Few studies have attempted to evaluate the presence of ER and PR in the syringomas. A study of 9 nonvulvar syringomas by Wallace and Smoller13 showed ER positivity in 1 case and PR positivity in 8 cases, lending support to the hormonal theory; however, in another case series of 15 vulvar syringomas, Huang et al6 failed to show ER and PR expression by immunohistochemical staining. A case report published 3 years earlier documented the first case of PR positivity on a vulvar syringoma.14 Our patient also was negative for ER and PR, which suggested that hormonal status is important in some but not all syringomas.
Patients with vulvar syringomas also might have coexisting extragenital syringomas in the neck,4 eyelids,6,7,10 and periorbital area,6 and thorough examination of the body is essential. If an extragenital syringoma is diagnosed, a vulvar syringoma should be considered, especially when the patient presents with unexplained genital symptoms. Although no proven hereditary transmission pattern has been established, family history of syringomas has been established in several cases.15 In a case series reported by Huang et al,6 4 of 18 patients reported a family history of periorbital syringomas. In our case, the patient did not report a family history of syringomas.
The differential diagnosis of vulvar lesions with pruritus is broad and includes Fox-Fordyce disease, lichen planus, LSC, epidermal cysts, senile angiomas, dystrophic calcinosis, xanthomas, steatocytomas, soft fibromas, condyloma acuminatum, and candidiasis. Vulvar syringomas might have a nonspecific appearance, and histologic examination is essential to confirm the diagnosis and rule out any malignant process such as MAC, vulvar intraepithelial neoplasia, extramammary Paget disease, or other glandular neoplasms of the vulva.
Microcystic adnexal carcinoma was first reported in 1982 by Goldstein et al16 as a locally aggressive neoplasm that can be confused with benign adnexal neoplasms, particularly desmoplastic trichoepithelioma, trichoadenoma, and syringoma. Microcystic adnexal carcinomas present as slow-growing, flesh-colored papules that may resemble syringomas and appear in similar body sites. Histologic examination is essential to differentiate between these two entities. Syringomas are tumors confined to the dermis and are composed of multiple small ducts lined by 2 layers of cuboidal epithelium within a dense fibrous stroma. Unlike syringomas, MACs usually infiltrate diffusely into the dermis and subcutis and may extend into the underlying muscle. Although bland cytologic features predominate, perineural invasion frequently is present in MACs. A potential pitfall of misdiagnosis can be caused by a superficial biopsy that may reveal benign histologic appearance, particularly in the upper level of the tumor where it may be confused with a syringoma or a benign follicular neoplasm.17
The initial biopsy performed on our patient was possibly not deep enough to render an unequivocal diagnosis and therefore bilateral partial radical vulvectomy was considered. After surgery, histologic examination of the resection specimen revealed a poorly circumscribed tumor confined to the dermis. The tumor was broad and the lack of deep infiltration into the subcutis and perineural invasion favored a syringoma (FIGURES 2A and 2B). These findings were consistent with case reports that documented syringomas as being more wide than deep on microscopic examination, whereas the opposite pertained to MAC.18 Cases of plaque-type syringomas that initially were misdiagnosed as MACs also have been reported.19 Because misdiagnosis may affect the treatment plan and potentially result in unnecessary surgery, caution should be taken when differentiating between these two entities. When a definitive diagnosis cannot be rendered on a superficial biopsy, a recommendation should be made for a deeper biopsy sampling the subcutis.
For the majority of the patients with vulvar syringomas, treatment is seldom required due to their asymptomatic nature; however, patients who present with symptoms usually report pruritus of variable intensities and patterns. A standardized treatment does not exist for vulvar syringomas, and oral or topical treatment might be used as an initial approach. Commonly prescribed medications with variable results include topical corticosteroids, oral antihistamines, and topical retinoids. In a case reported by Iwao et al,20 vulvar syringomas were successfully treated with tranilast, which has anti-inflammatory and immunomodulatory effects. This medication could have a possible dual action—inhibiting the release of chemical mediators from the mast cells and inhibiting the release of IL-1β from the eccrine duct, which could suppress the proliferation of stromal connective tissue. Our case was stained with toluidine blue and showed an increased number of mast cells in the tissue (FIGURE 3).Patients who are unresponsive to tranilast or have extensive disease resulting in cosmetic disfigurement might benefit from more invasive treatment methods including a variety of lasers, cryotherapy, electrosurgery, and excision. Excisions should include the entire tumor to avoid recurrence. In a case reported by Garman and Metry,21 the lesions were surgically excised using small 2- to 3-mm punches; however, several weeks later the lesions recurred. Our patient presented with a 1-month evolution of dyspareunia, vulvar discomfort, and vulvar irregularities that were probably not treated with oral or topical medications before being referred for surgery.
We report a case of a vulvar syringoma that presented diagnostic challenges in the initial biopsy, which prevented the exclusion of an MAC. After partial radical vulvectomy, histologic examination was more definitive, showing lack of deep infiltration into the subcutis or perineural invasion that are commonly seen in MAC. This case is an example of a notable pitfall in the diagnosis of vulvar syringoma on a limited biopsy leading to overtreatment. Raising awareness of this entity is the only modality to prevent misdiagnosis. We encourage reporting of further cases of syringomas, particularly those with atypical locations or patterns that may cause diagnostic problems. ●
- Ensure adequate depth of biopsy to assist in the histologic diagnosis of syringoma vs microcystic adnexal carcinoma.
- Vulvar syringomas also may contribute to notable pruritus and ultimately be the underlying etiology for secondary skin changes leading to a lichen simplex chronicus–like phenotype
- Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Spain: Mosby Elsevier; 2008.
- Weedon D. Skin Pathology. 3rd ed. China: Churchill Livingstone Elsevier; 2010.
- Carneiro SJ, Gardner HL, Knox JM. Syringoma of the vulva. Arch Dermatol. 1971;103:494-496.
- Trager JD, Silvers J, Reed JA, et al. Neck and vulvar papules in an 8-year-old girl. Arch Dermatol. 1999;135:203, 206.
- Pérez-Bustillo A, Ruiz-González I, Delgado S, et al. Vulvar syringoma: a rare cause of vulvar pruritus. Actas DermoSifiliográficas. 2008; 99:580-581.
- Huang YH, Chuang YH, Kuo TT, et al. Vulvar syringoma: a clinicopathologic and immunohistologic study of 18 patients and results of treatment. J Am Acad Dermatol. 2003;48:735-739.
- Dereli T, Turk BG, Kazandi AC. Syringomas of the vulva. Int J Gynaecol Obstet. 2007;99:65-66.
- Blasdale C, McLelland J. Solitary giant vulval syringoma. Br J Dermatol. 1999;141:374-375.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Gerdsen R, Wenzel J, Uerlich M, et al. Periodic genital pruritus caused by syringoma of the vulva. Acta Obstet Gynecol Scand. 2002;81:369-370.
- Bal N, Aslan E, Kayaselcuk F, et al. Vulvar syringoma aggravated by pregnancy. Pathol Oncol Res. 2003;9:196-197.
- Turan C, Ugur M, Kutluay L, et al. Vulvar syringoma exacerbated during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:141-142.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995; 22:442-445.
- Yorganci A, Kale A, Dunder I, et al. Vulvar syringoma showing progesterone receptor positivity. BJOG. 2000;107:292-294.
- Draznin M. Hereditary syringomas: a case report. Dermatol Online J. 2004;10:19.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Hamsch C, Hartschuh W. Microcystic adnexal carcinomaaggressive infiltrative tumor often with innocent clinical appearance. J Dtsch Dermatol Ges. 2010;8:275-278.
- Henner MS, Shapiro PE, Ritter JH, et al. Solitary syringoma. report of five cases and clinicopathologic comparison with microcystic adnexal carcinoma of the skin. Am J Dermatopathol. 1995;17:465-470.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma. J Cutan Pathol. 2008;35:570-574.
- Iwao F, Onozuka T, Kawashima T. Vulval syringoma successfully treated with tranilast. Br J Dermatol. 2005;153:1228-1230.
- Garman M, Metry D. Vulvar syringomas in a 9-year-old child with review of the literature. Pediatr Dermatol. 2006;23:369372.
- Bolognia JL, Jorizzo JL, Rapini RP. Dermatology. 2nd ed. Spain: Mosby Elsevier; 2008.
- Weedon D. Skin Pathology. 3rd ed. China: Churchill Livingstone Elsevier; 2010.
- Carneiro SJ, Gardner HL, Knox JM. Syringoma of the vulva. Arch Dermatol. 1971;103:494-496.
- Trager JD, Silvers J, Reed JA, et al. Neck and vulvar papules in an 8-year-old girl. Arch Dermatol. 1999;135:203, 206.
- Pérez-Bustillo A, Ruiz-González I, Delgado S, et al. Vulvar syringoma: a rare cause of vulvar pruritus. Actas DermoSifiliográficas. 2008; 99:580-581.
- Huang YH, Chuang YH, Kuo TT, et al. Vulvar syringoma: a clinicopathologic and immunohistologic study of 18 patients and results of treatment. J Am Acad Dermatol. 2003;48:735-739.
- Dereli T, Turk BG, Kazandi AC. Syringomas of the vulva. Int J Gynaecol Obstet. 2007;99:65-66.
- Blasdale C, McLelland J. Solitary giant vulval syringoma. Br J Dermatol. 1999;141:374-375.
- Kavala M, Can B, Zindanci I, et al. Vulvar pruritus caused by syringoma of the vulva. Int J Dermatol. 2008;47:831-832.
- Gerdsen R, Wenzel J, Uerlich M, et al. Periodic genital pruritus caused by syringoma of the vulva. Acta Obstet Gynecol Scand. 2002;81:369-370.
- Bal N, Aslan E, Kayaselcuk F, et al. Vulvar syringoma aggravated by pregnancy. Pathol Oncol Res. 2003;9:196-197.
- Turan C, Ugur M, Kutluay L, et al. Vulvar syringoma exacerbated during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;64:141-142.
- Wallace ML, Smoller BR. Progesterone receptor positivity supports hormonal control of syringomas. J Cutan Pathol. 1995; 22:442-445.
- Yorganci A, Kale A, Dunder I, et al. Vulvar syringoma showing progesterone receptor positivity. BJOG. 2000;107:292-294.
- Draznin M. Hereditary syringomas: a case report. Dermatol Online J. 2004;10:19.
- Goldstein DJ, Barr RJ, Santa Cruz DJ. Microcystic adnexal carcinoma: a distinct clinicopathologic entity. Cancer. 1982;50:566-572.
- Hamsch C, Hartschuh W. Microcystic adnexal carcinomaaggressive infiltrative tumor often with innocent clinical appearance. J Dtsch Dermatol Ges. 2010;8:275-278.
- Henner MS, Shapiro PE, Ritter JH, et al. Solitary syringoma. report of five cases and clinicopathologic comparison with microcystic adnexal carcinoma of the skin. Am J Dermatopathol. 1995;17:465-470.
- Suwattee P, McClelland MC, Huiras EE, et al. Plaque-type syringoma: two cases misdiagnosed as microcystic adnexal carcinoma. J Cutan Pathol. 2008;35:570-574.
- Iwao F, Onozuka T, Kawashima T. Vulval syringoma successfully treated with tranilast. Br J Dermatol. 2005;153:1228-1230.
- Garman M, Metry D. Vulvar syringomas in a 9-year-old child with review of the literature. Pediatr Dermatol. 2006;23:369372.
Risk for breast cancer reduced after bariatric surgery
In a matched cohort study of more than 69,000 Canadian women, risk for incident breast cancer at 1 year was 40% higher among women who had not undergone bariatric surgery, compared with those who had. The risk remained elevated through 5 years of follow-up.
The findings were “definitely a bit surprising,” study author Aristithes G. Doumouras, MD, MPH, assistant professor of surgery at McMaster University, Hamilton, Ont., said in an interview. “The patients that underwent bariatric surgery had better cancer outcomes than patients who weighed less than they did, so it showed that there was more at play than just weight loss. This effect was durable [and] shows how powerful the surgery is, [as well as] the fact that we haven’t even explored all of its effects.”
The study was published online in JAMA Surgery.
Protective association
To determine whether there is a residual risk for breast cancer following bariatric surgery for obesity, the investigators analyzed clinical and administrative data collected between 2010 and 2016 in Ontario. They retrospectively matched women with obesity who underwent bariatric surgery with women without a history of bariatric surgery. Participants were matched by age and breast cancer screening status. Covariates included diabetes status, neighborhood income quintile, and measures of health care use. The population included 69,260 women (mean age, 45 years).
Among participants who underwent bariatric surgery for obesity, baseline body mass index was greater than 35 for those with related comorbid conditions, and BMI was greater than 40 for those without comorbid conditions. The investigators categorized nonsurgical control patients in accordance with the following four BMI categories: less than 25, 25-29, 30-34, and greater than or equal to 35. Each control group, as well as the surgical group, included 13,852 women.
Participants in the surgical group were followed for 5 years after bariatric surgery. Those in the nonsurgical group were followed for 5 years after the index date (that is, the date of BMI measurement).
In the overall population, 659 cases of breast cancer were diagnosed in the overall population (0.95%) during the study period. This total included 103 (0.74%) cancers in the surgical cohort; 128 (0.92%) in the group with BMI less than 25; 143 (1.03%) among those with BMI 25-29; 150 (1.08%) in the group with BMI 30-34; and 135 (0.97%) among those with BMI greater than or equal to 35.
Most cancers were stage I. There were 65 cases among those with BMI less than 25; 76 for those with BMI of 25-29; 65 for BMI of 30-34; 67 for BMI greater than or equal to 35, and 60 for the surgery group.
Most tumors were of medium grade and were estrogen receptor positive, progesterone receptor positive, and ERBB2 negative. No significant differences were observed across the groups for stage, grade, or hormone status.
There was an increased hazard for incident breast cancer in the nonsurgical group, compared with the postsurgical group after washout periods of 1 year (hazard ratio, 1.40), 2 years (HR, 1.31), and 5 years (HR, 1.38).
In a comparison of the postsurgical cohort with the nonsurgical cohort with BMI less than 25, the hazard of incident breast cancer was not significantly different for any of the washout periods, but there was a reduced hazard for incident breast cancer among postsurgical patients than among nonsurgical patients in all high BMI categories (BMI ≥ 25).
“Taken together, these results demonstrate that the protective association between substantial weight loss via bariatric surgery and breast cancer risk is sustained after 5 years following surgery and that it is associated with a baseline risk similar to that of women with BMI less than 25,” the investigators write.
Nevertheless, Dr. Doumouras said “the interaction between the surgery and individuals is poorly studied, and this level of personalized medicine is simply not there yet. We are working on developing a prospective cohort that has genetic, protein, and microbiome [data] to help answer these questions.”
There are not enough women in subpopulations such as BRCA carriers to study at this point, he added. “This is where more patients and time will really help the research process.”
A universal benefit?
“Although these findings are important overall for the general population at risk for breast cancer, we raise an important caveat: The benefit of surgical weight loss may not be universal,” write Justin B. Dimick, MD, MPH, surgical innovation editor for JAMA Surgery, and Melissa L. Pilewskie, MD, both of the University of Michigan, Ann Arbor, in an accompanying commentary.
“In addition to lifestyle factors, several nonmodifiable risk factors, such as a genetic predisposition, strong family history, personal history of a high-risk breast lesion, or history of chest wall radiation, impart significant elevation in risk, and the data remain mixed on the impact of weight loss for individuals in these high-risk cohorts,” they add.
“Further study to elucidate the underlying mechanism associated with obesity, weight loss, and breast cancer risk should help guide strategies for risk reduction that are specific to unique high-risk cohorts, because modifiable risk factors may not portend the same benefit among all groups.”
Commenting on the findings, Stephen Edge, MD, breast surgeon and vice president for system quality and outcomes at Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., said, “The importance of this study is that it shows that weight loss in midlife can reduce breast cancer risk back to or even below the risk of similar people who were not obese. This has major implications for counseling women.”
The investigators did not have information on the extent of weight loss with surgery or on which participants maintained the lower weight, Dr. Edge noted; “However, overall, most people who have weight reduction surgery have major weight loss.”
At this point, he said, “we can now tell women with obesity that in addition to the many other advantages of weight loss, their risk of getting breast cancer will also be reduced.”
The study was supported by the Ontario Bariatric Registry and ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ontario Ministry of Long-Term Care. Dr. Doumouras, Dr. Dimick, Dr. Pilewskie, and Dr. Edge reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a matched cohort study of more than 69,000 Canadian women, risk for incident breast cancer at 1 year was 40% higher among women who had not undergone bariatric surgery, compared with those who had. The risk remained elevated through 5 years of follow-up.
The findings were “definitely a bit surprising,” study author Aristithes G. Doumouras, MD, MPH, assistant professor of surgery at McMaster University, Hamilton, Ont., said in an interview. “The patients that underwent bariatric surgery had better cancer outcomes than patients who weighed less than they did, so it showed that there was more at play than just weight loss. This effect was durable [and] shows how powerful the surgery is, [as well as] the fact that we haven’t even explored all of its effects.”
The study was published online in JAMA Surgery.
Protective association
To determine whether there is a residual risk for breast cancer following bariatric surgery for obesity, the investigators analyzed clinical and administrative data collected between 2010 and 2016 in Ontario. They retrospectively matched women with obesity who underwent bariatric surgery with women without a history of bariatric surgery. Participants were matched by age and breast cancer screening status. Covariates included diabetes status, neighborhood income quintile, and measures of health care use. The population included 69,260 women (mean age, 45 years).
Among participants who underwent bariatric surgery for obesity, baseline body mass index was greater than 35 for those with related comorbid conditions, and BMI was greater than 40 for those without comorbid conditions. The investigators categorized nonsurgical control patients in accordance with the following four BMI categories: less than 25, 25-29, 30-34, and greater than or equal to 35. Each control group, as well as the surgical group, included 13,852 women.
Participants in the surgical group were followed for 5 years after bariatric surgery. Those in the nonsurgical group were followed for 5 years after the index date (that is, the date of BMI measurement).
In the overall population, 659 cases of breast cancer were diagnosed in the overall population (0.95%) during the study period. This total included 103 (0.74%) cancers in the surgical cohort; 128 (0.92%) in the group with BMI less than 25; 143 (1.03%) among those with BMI 25-29; 150 (1.08%) in the group with BMI 30-34; and 135 (0.97%) among those with BMI greater than or equal to 35.
Most cancers were stage I. There were 65 cases among those with BMI less than 25; 76 for those with BMI of 25-29; 65 for BMI of 30-34; 67 for BMI greater than or equal to 35, and 60 for the surgery group.
Most tumors were of medium grade and were estrogen receptor positive, progesterone receptor positive, and ERBB2 negative. No significant differences were observed across the groups for stage, grade, or hormone status.
There was an increased hazard for incident breast cancer in the nonsurgical group, compared with the postsurgical group after washout periods of 1 year (hazard ratio, 1.40), 2 years (HR, 1.31), and 5 years (HR, 1.38).
In a comparison of the postsurgical cohort with the nonsurgical cohort with BMI less than 25, the hazard of incident breast cancer was not significantly different for any of the washout periods, but there was a reduced hazard for incident breast cancer among postsurgical patients than among nonsurgical patients in all high BMI categories (BMI ≥ 25).
“Taken together, these results demonstrate that the protective association between substantial weight loss via bariatric surgery and breast cancer risk is sustained after 5 years following surgery and that it is associated with a baseline risk similar to that of women with BMI less than 25,” the investigators write.
Nevertheless, Dr. Doumouras said “the interaction between the surgery and individuals is poorly studied, and this level of personalized medicine is simply not there yet. We are working on developing a prospective cohort that has genetic, protein, and microbiome [data] to help answer these questions.”
There are not enough women in subpopulations such as BRCA carriers to study at this point, he added. “This is where more patients and time will really help the research process.”
A universal benefit?
“Although these findings are important overall for the general population at risk for breast cancer, we raise an important caveat: The benefit of surgical weight loss may not be universal,” write Justin B. Dimick, MD, MPH, surgical innovation editor for JAMA Surgery, and Melissa L. Pilewskie, MD, both of the University of Michigan, Ann Arbor, in an accompanying commentary.
“In addition to lifestyle factors, several nonmodifiable risk factors, such as a genetic predisposition, strong family history, personal history of a high-risk breast lesion, or history of chest wall radiation, impart significant elevation in risk, and the data remain mixed on the impact of weight loss for individuals in these high-risk cohorts,” they add.
“Further study to elucidate the underlying mechanism associated with obesity, weight loss, and breast cancer risk should help guide strategies for risk reduction that are specific to unique high-risk cohorts, because modifiable risk factors may not portend the same benefit among all groups.”
Commenting on the findings, Stephen Edge, MD, breast surgeon and vice president for system quality and outcomes at Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., said, “The importance of this study is that it shows that weight loss in midlife can reduce breast cancer risk back to or even below the risk of similar people who were not obese. This has major implications for counseling women.”
The investigators did not have information on the extent of weight loss with surgery or on which participants maintained the lower weight, Dr. Edge noted; “However, overall, most people who have weight reduction surgery have major weight loss.”
At this point, he said, “we can now tell women with obesity that in addition to the many other advantages of weight loss, their risk of getting breast cancer will also be reduced.”
The study was supported by the Ontario Bariatric Registry and ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ontario Ministry of Long-Term Care. Dr. Doumouras, Dr. Dimick, Dr. Pilewskie, and Dr. Edge reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a matched cohort study of more than 69,000 Canadian women, risk for incident breast cancer at 1 year was 40% higher among women who had not undergone bariatric surgery, compared with those who had. The risk remained elevated through 5 years of follow-up.
The findings were “definitely a bit surprising,” study author Aristithes G. Doumouras, MD, MPH, assistant professor of surgery at McMaster University, Hamilton, Ont., said in an interview. “The patients that underwent bariatric surgery had better cancer outcomes than patients who weighed less than they did, so it showed that there was more at play than just weight loss. This effect was durable [and] shows how powerful the surgery is, [as well as] the fact that we haven’t even explored all of its effects.”
The study was published online in JAMA Surgery.
Protective association
To determine whether there is a residual risk for breast cancer following bariatric surgery for obesity, the investigators analyzed clinical and administrative data collected between 2010 and 2016 in Ontario. They retrospectively matched women with obesity who underwent bariatric surgery with women without a history of bariatric surgery. Participants were matched by age and breast cancer screening status. Covariates included diabetes status, neighborhood income quintile, and measures of health care use. The population included 69,260 women (mean age, 45 years).
Among participants who underwent bariatric surgery for obesity, baseline body mass index was greater than 35 for those with related comorbid conditions, and BMI was greater than 40 for those without comorbid conditions. The investigators categorized nonsurgical control patients in accordance with the following four BMI categories: less than 25, 25-29, 30-34, and greater than or equal to 35. Each control group, as well as the surgical group, included 13,852 women.
Participants in the surgical group were followed for 5 years after bariatric surgery. Those in the nonsurgical group were followed for 5 years after the index date (that is, the date of BMI measurement).
In the overall population, 659 cases of breast cancer were diagnosed in the overall population (0.95%) during the study period. This total included 103 (0.74%) cancers in the surgical cohort; 128 (0.92%) in the group with BMI less than 25; 143 (1.03%) among those with BMI 25-29; 150 (1.08%) in the group with BMI 30-34; and 135 (0.97%) among those with BMI greater than or equal to 35.
Most cancers were stage I. There were 65 cases among those with BMI less than 25; 76 for those with BMI of 25-29; 65 for BMI of 30-34; 67 for BMI greater than or equal to 35, and 60 for the surgery group.
Most tumors were of medium grade and were estrogen receptor positive, progesterone receptor positive, and ERBB2 negative. No significant differences were observed across the groups for stage, grade, or hormone status.
There was an increased hazard for incident breast cancer in the nonsurgical group, compared with the postsurgical group after washout periods of 1 year (hazard ratio, 1.40), 2 years (HR, 1.31), and 5 years (HR, 1.38).
In a comparison of the postsurgical cohort with the nonsurgical cohort with BMI less than 25, the hazard of incident breast cancer was not significantly different for any of the washout periods, but there was a reduced hazard for incident breast cancer among postsurgical patients than among nonsurgical patients in all high BMI categories (BMI ≥ 25).
“Taken together, these results demonstrate that the protective association between substantial weight loss via bariatric surgery and breast cancer risk is sustained after 5 years following surgery and that it is associated with a baseline risk similar to that of women with BMI less than 25,” the investigators write.
Nevertheless, Dr. Doumouras said “the interaction between the surgery and individuals is poorly studied, and this level of personalized medicine is simply not there yet. We are working on developing a prospective cohort that has genetic, protein, and microbiome [data] to help answer these questions.”
There are not enough women in subpopulations such as BRCA carriers to study at this point, he added. “This is where more patients and time will really help the research process.”
A universal benefit?
“Although these findings are important overall for the general population at risk for breast cancer, we raise an important caveat: The benefit of surgical weight loss may not be universal,” write Justin B. Dimick, MD, MPH, surgical innovation editor for JAMA Surgery, and Melissa L. Pilewskie, MD, both of the University of Michigan, Ann Arbor, in an accompanying commentary.
“In addition to lifestyle factors, several nonmodifiable risk factors, such as a genetic predisposition, strong family history, personal history of a high-risk breast lesion, or history of chest wall radiation, impart significant elevation in risk, and the data remain mixed on the impact of weight loss for individuals in these high-risk cohorts,” they add.
“Further study to elucidate the underlying mechanism associated with obesity, weight loss, and breast cancer risk should help guide strategies for risk reduction that are specific to unique high-risk cohorts, because modifiable risk factors may not portend the same benefit among all groups.”
Commenting on the findings, Stephen Edge, MD, breast surgeon and vice president for system quality and outcomes at Roswell Park Comprehensive Cancer Center, Buffalo, N.Y., said, “The importance of this study is that it shows that weight loss in midlife can reduce breast cancer risk back to or even below the risk of similar people who were not obese. This has major implications for counseling women.”
The investigators did not have information on the extent of weight loss with surgery or on which participants maintained the lower weight, Dr. Edge noted; “However, overall, most people who have weight reduction surgery have major weight loss.”
At this point, he said, “we can now tell women with obesity that in addition to the many other advantages of weight loss, their risk of getting breast cancer will also be reduced.”
The study was supported by the Ontario Bariatric Registry and ICES, which is funded by an annual grant from the Ontario Ministry of Health and the Ontario Ministry of Long-Term Care. Dr. Doumouras, Dr. Dimick, Dr. Pilewskie, and Dr. Edge reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA SURGERY
sFlt-1:PlGF ratio normal at 24 to 28 weeks: Discontinue aspirin for preterm preeclampsia prevention?

Mendoza M, Bonacina E, Garcia-Manau P, et al. Aspirin discontinuation at 24 to 28 weeks’ gestation in pregnancies at high risk of preterm preeclampsia: a randomized clinical trial. JAMA. 2023;329:542-550. doi:10.1001/jama.2023.0691.
EXPERT COMMENTARY
Aspirin is, to date, the only proven preventative treatment to reduce the risk of preeclampsia in pregnancy. While aspirin initiation, optimally prior to 16 weeks, generally is accepted, the best timing for discontinuation remains uncertain due to conflicting data on risk of bleeding and different doses used. The American College of Obstetricians and Gynecologists recommends a broad range of patients eligible for low-dose aspirin with continuation through delivery, citing data that support no increase in either maternal or fetal/neonatal complications, including bleeding complications.1 Other guidelines recommend reduction in pregnancy exposure to aspirin with strict guidelines for which patients are considered “high risk” as well as discontinuation at 36 weeks prior to labor onset to reduce the risk of potential bleeding complications.
Recently, Mendoza and colleagues tested the hypothesis that, in patients at high risk for preterm preeclampsia (based on high-risk first-trimester screening followed by a low risk of preeclampsia at 24 to 28 weeks based on a normal sFlt-1:PlGF [soluble fms-like tyrosine kinase-1 to placental growth factor] ratio), discontinuing aspirin is noninferior in preventing preterm preeclampsia compared with continuing aspirin until 36 weeks.2
Details of the study
Mendoza and colleagues conducted a multicenter, open label, randomized, phase 3, noninferiority trial that randomly assigned 968 participants prior to stopping recruitment based on the findings from a planned interim analysis.2
The patient population included women with singleton pregnancies between 24 and 28 weeks who had initiated aspirin 150 mg daily by 16 6/7 weeks due to high-risk first- trimester screening for preterm preeclampsia. Additionally, these patients also had an sFlt-1:PlGR ratio of 38 or less between 24 and 28 weeks’ gestation, which prior studies have demonstrated to exclude the diagnosis of preeclampsia.
Patients were randomly assigned to either discontinue aspirin at 24 to 28 weeks’ gestation (intervention group) or continue aspirin until 36 weeks’ gestation (control group). The primary outcome was delivery due to preeclampsia at less than 37 weeks, with secondary outcomes of preeclampsia at less than 34 weeks, preeclampsia at 37 or more weeks, or other adverse pregnancy outcomes.
Results. For the primary outcome (936 participants’ data analyzed), the incidence of preeclampsia at less than 37 weeks was 1.48% in the intervention group and 1.73% in the control group (absolute difference, -0.25%, which meets study criteria for noninferiority).
No difference occurred in the secondary outcomes of adverse outcomes at less than 34 weeks or at less than 37 weeks. While there was no difference in the incidence of the individual adverse outcomes at 37 or more weeks, the intervention group had a decrease in the incidence of having “any” adverse outcome (-5.04%) as well as a decrease in minor antepartum hemorrhage (nose and/or gum bleeding) (-4.7%).
The authors therefore concluded that aspirin discontinuation at 24 to 28 weeks’ gestation in pregnant patients at high risk for preterm preeclampsia and a normal sFlt-1:PlGF ratio is noninferior to aspirin continuation for prevention of preterm preeclampsia. They also suggested that this discontinuation may reduce the risk of adverse pregnancy outcomes at 37 or more weeks as well as minor bleeding complications.
Study strengths and limitations
The authors cited the novelty of this study at considering using aspirin for the prevention of preterm preeclampsia in a specific patient group for the shortest amount of time needed to achieve this goal. Potential benefits could be decreased bleeding complications, cost, anxiety, and visits.
They also noted the following study limitations: open-label design, a predominantly White patient population, early termination due to the interim analysis, inadequate power for more rare complications, and a query as to the appropriate choice for the threshold for noninferiority. Noninferiority trials have inherent weaknesses as a group that should be considered before major practice changes occur as a result of their findings.
Several other factors in the study limit the generalizability of the authors’ recommendations, especially to patient populations in the United States. For example, the study used an aspirin dose of 150 mg daily, which is almost double the dose recommended in the United States (81 mg). The reasoning for this was that doses higher than 100 mg have been shown to be the most effective for preeclampsia prevention but also may have higher rates of bleeding complications, including placental abruption. The demonstrated increase in complications may not hold at a lower dose.
Additionally, patients in this study were selected for aspirin by a first-trimester algorithm that may not be in general use everywhere (and differs from the US Preventive Services Task Force recommendations for low-dose aspirin use in pregnancy). Finally, although extremely interesting, the use of the sFlt-1:PlFG ratio at 24 to 28 weeks is not in widespread use in the United States and may incur an additional cost not equivalent to the low cost of a daily aspirin.
Essentially, this is an extremely limited study for a very specific population. Before globally discontinuing low-dose aspirin in high-risk patients, the different doses and eligibility criteria should be studied for effect of early discontinuation. ●
Low-dose aspirin should continue to be used for prevention of preeclampsia in high-risk pregnant patients, optimally starting at 12 to 16 weeks’ gestation and continuing either through 36 weeks or delivery. Further study is needed to determine the optimal timing for earlier discontinuation of aspirin based on dose, risk factors, and other measures of preeclampsia risk as the pregnancy progresses.
JAIMEY M. PAULI, MD
- ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52. doi:10.1097/AOG.0000000000002708.
- Mendoza M, Bonacina E, Garcia-Manau P, et al. Aspirin discontinuation at 24 to 28 weeks’ gestation in pregnancies at high risk of preterm preeclampsia: a randomized clinical trial. JAMA. 2023;329:542-550. doi:10.1001/jama.2023.0691.

Mendoza M, Bonacina E, Garcia-Manau P, et al. Aspirin discontinuation at 24 to 28 weeks’ gestation in pregnancies at high risk of preterm preeclampsia: a randomized clinical trial. JAMA. 2023;329:542-550. doi:10.1001/jama.2023.0691.
EXPERT COMMENTARY
Aspirin is, to date, the only proven preventative treatment to reduce the risk of preeclampsia in pregnancy. While aspirin initiation, optimally prior to 16 weeks, generally is accepted, the best timing for discontinuation remains uncertain due to conflicting data on risk of bleeding and different doses used. The American College of Obstetricians and Gynecologists recommends a broad range of patients eligible for low-dose aspirin with continuation through delivery, citing data that support no increase in either maternal or fetal/neonatal complications, including bleeding complications.1 Other guidelines recommend reduction in pregnancy exposure to aspirin with strict guidelines for which patients are considered “high risk” as well as discontinuation at 36 weeks prior to labor onset to reduce the risk of potential bleeding complications.
Recently, Mendoza and colleagues tested the hypothesis that, in patients at high risk for preterm preeclampsia (based on high-risk first-trimester screening followed by a low risk of preeclampsia at 24 to 28 weeks based on a normal sFlt-1:PlGF [soluble fms-like tyrosine kinase-1 to placental growth factor] ratio), discontinuing aspirin is noninferior in preventing preterm preeclampsia compared with continuing aspirin until 36 weeks.2
Details of the study
Mendoza and colleagues conducted a multicenter, open label, randomized, phase 3, noninferiority trial that randomly assigned 968 participants prior to stopping recruitment based on the findings from a planned interim analysis.2
The patient population included women with singleton pregnancies between 24 and 28 weeks who had initiated aspirin 150 mg daily by 16 6/7 weeks due to high-risk first- trimester screening for preterm preeclampsia. Additionally, these patients also had an sFlt-1:PlGR ratio of 38 or less between 24 and 28 weeks’ gestation, which prior studies have demonstrated to exclude the diagnosis of preeclampsia.
Patients were randomly assigned to either discontinue aspirin at 24 to 28 weeks’ gestation (intervention group) or continue aspirin until 36 weeks’ gestation (control group). The primary outcome was delivery due to preeclampsia at less than 37 weeks, with secondary outcomes of preeclampsia at less than 34 weeks, preeclampsia at 37 or more weeks, or other adverse pregnancy outcomes.
Results. For the primary outcome (936 participants’ data analyzed), the incidence of preeclampsia at less than 37 weeks was 1.48% in the intervention group and 1.73% in the control group (absolute difference, -0.25%, which meets study criteria for noninferiority).
No difference occurred in the secondary outcomes of adverse outcomes at less than 34 weeks or at less than 37 weeks. While there was no difference in the incidence of the individual adverse outcomes at 37 or more weeks, the intervention group had a decrease in the incidence of having “any” adverse outcome (-5.04%) as well as a decrease in minor antepartum hemorrhage (nose and/or gum bleeding) (-4.7%).
The authors therefore concluded that aspirin discontinuation at 24 to 28 weeks’ gestation in pregnant patients at high risk for preterm preeclampsia and a normal sFlt-1:PlGF ratio is noninferior to aspirin continuation for prevention of preterm preeclampsia. They also suggested that this discontinuation may reduce the risk of adverse pregnancy outcomes at 37 or more weeks as well as minor bleeding complications.
Study strengths and limitations
The authors cited the novelty of this study at considering using aspirin for the prevention of preterm preeclampsia in a specific patient group for the shortest amount of time needed to achieve this goal. Potential benefits could be decreased bleeding complications, cost, anxiety, and visits.
They also noted the following study limitations: open-label design, a predominantly White patient population, early termination due to the interim analysis, inadequate power for more rare complications, and a query as to the appropriate choice for the threshold for noninferiority. Noninferiority trials have inherent weaknesses as a group that should be considered before major practice changes occur as a result of their findings.
Several other factors in the study limit the generalizability of the authors’ recommendations, especially to patient populations in the United States. For example, the study used an aspirin dose of 150 mg daily, which is almost double the dose recommended in the United States (81 mg). The reasoning for this was that doses higher than 100 mg have been shown to be the most effective for preeclampsia prevention but also may have higher rates of bleeding complications, including placental abruption. The demonstrated increase in complications may not hold at a lower dose.
Additionally, patients in this study were selected for aspirin by a first-trimester algorithm that may not be in general use everywhere (and differs from the US Preventive Services Task Force recommendations for low-dose aspirin use in pregnancy). Finally, although extremely interesting, the use of the sFlt-1:PlFG ratio at 24 to 28 weeks is not in widespread use in the United States and may incur an additional cost not equivalent to the low cost of a daily aspirin.
Essentially, this is an extremely limited study for a very specific population. Before globally discontinuing low-dose aspirin in high-risk patients, the different doses and eligibility criteria should be studied for effect of early discontinuation. ●
Low-dose aspirin should continue to be used for prevention of preeclampsia in high-risk pregnant patients, optimally starting at 12 to 16 weeks’ gestation and continuing either through 36 weeks or delivery. Further study is needed to determine the optimal timing for earlier discontinuation of aspirin based on dose, risk factors, and other measures of preeclampsia risk as the pregnancy progresses.
JAIMEY M. PAULI, MD

Mendoza M, Bonacina E, Garcia-Manau P, et al. Aspirin discontinuation at 24 to 28 weeks’ gestation in pregnancies at high risk of preterm preeclampsia: a randomized clinical trial. JAMA. 2023;329:542-550. doi:10.1001/jama.2023.0691.
EXPERT COMMENTARY
Aspirin is, to date, the only proven preventative treatment to reduce the risk of preeclampsia in pregnancy. While aspirin initiation, optimally prior to 16 weeks, generally is accepted, the best timing for discontinuation remains uncertain due to conflicting data on risk of bleeding and different doses used. The American College of Obstetricians and Gynecologists recommends a broad range of patients eligible for low-dose aspirin with continuation through delivery, citing data that support no increase in either maternal or fetal/neonatal complications, including bleeding complications.1 Other guidelines recommend reduction in pregnancy exposure to aspirin with strict guidelines for which patients are considered “high risk” as well as discontinuation at 36 weeks prior to labor onset to reduce the risk of potential bleeding complications.
Recently, Mendoza and colleagues tested the hypothesis that, in patients at high risk for preterm preeclampsia (based on high-risk first-trimester screening followed by a low risk of preeclampsia at 24 to 28 weeks based on a normal sFlt-1:PlGF [soluble fms-like tyrosine kinase-1 to placental growth factor] ratio), discontinuing aspirin is noninferior in preventing preterm preeclampsia compared with continuing aspirin until 36 weeks.2
Details of the study
Mendoza and colleagues conducted a multicenter, open label, randomized, phase 3, noninferiority trial that randomly assigned 968 participants prior to stopping recruitment based on the findings from a planned interim analysis.2
The patient population included women with singleton pregnancies between 24 and 28 weeks who had initiated aspirin 150 mg daily by 16 6/7 weeks due to high-risk first- trimester screening for preterm preeclampsia. Additionally, these patients also had an sFlt-1:PlGR ratio of 38 or less between 24 and 28 weeks’ gestation, which prior studies have demonstrated to exclude the diagnosis of preeclampsia.
Patients were randomly assigned to either discontinue aspirin at 24 to 28 weeks’ gestation (intervention group) or continue aspirin until 36 weeks’ gestation (control group). The primary outcome was delivery due to preeclampsia at less than 37 weeks, with secondary outcomes of preeclampsia at less than 34 weeks, preeclampsia at 37 or more weeks, or other adverse pregnancy outcomes.
Results. For the primary outcome (936 participants’ data analyzed), the incidence of preeclampsia at less than 37 weeks was 1.48% in the intervention group and 1.73% in the control group (absolute difference, -0.25%, which meets study criteria for noninferiority).
No difference occurred in the secondary outcomes of adverse outcomes at less than 34 weeks or at less than 37 weeks. While there was no difference in the incidence of the individual adverse outcomes at 37 or more weeks, the intervention group had a decrease in the incidence of having “any” adverse outcome (-5.04%) as well as a decrease in minor antepartum hemorrhage (nose and/or gum bleeding) (-4.7%).
The authors therefore concluded that aspirin discontinuation at 24 to 28 weeks’ gestation in pregnant patients at high risk for preterm preeclampsia and a normal sFlt-1:PlGF ratio is noninferior to aspirin continuation for prevention of preterm preeclampsia. They also suggested that this discontinuation may reduce the risk of adverse pregnancy outcomes at 37 or more weeks as well as minor bleeding complications.
Study strengths and limitations
The authors cited the novelty of this study at considering using aspirin for the prevention of preterm preeclampsia in a specific patient group for the shortest amount of time needed to achieve this goal. Potential benefits could be decreased bleeding complications, cost, anxiety, and visits.
They also noted the following study limitations: open-label design, a predominantly White patient population, early termination due to the interim analysis, inadequate power for more rare complications, and a query as to the appropriate choice for the threshold for noninferiority. Noninferiority trials have inherent weaknesses as a group that should be considered before major practice changes occur as a result of their findings.
Several other factors in the study limit the generalizability of the authors’ recommendations, especially to patient populations in the United States. For example, the study used an aspirin dose of 150 mg daily, which is almost double the dose recommended in the United States (81 mg). The reasoning for this was that doses higher than 100 mg have been shown to be the most effective for preeclampsia prevention but also may have higher rates of bleeding complications, including placental abruption. The demonstrated increase in complications may not hold at a lower dose.
Additionally, patients in this study were selected for aspirin by a first-trimester algorithm that may not be in general use everywhere (and differs from the US Preventive Services Task Force recommendations for low-dose aspirin use in pregnancy). Finally, although extremely interesting, the use of the sFlt-1:PlFG ratio at 24 to 28 weeks is not in widespread use in the United States and may incur an additional cost not equivalent to the low cost of a daily aspirin.
Essentially, this is an extremely limited study for a very specific population. Before globally discontinuing low-dose aspirin in high-risk patients, the different doses and eligibility criteria should be studied for effect of early discontinuation. ●
Low-dose aspirin should continue to be used for prevention of preeclampsia in high-risk pregnant patients, optimally starting at 12 to 16 weeks’ gestation and continuing either through 36 weeks or delivery. Further study is needed to determine the optimal timing for earlier discontinuation of aspirin based on dose, risk factors, and other measures of preeclampsia risk as the pregnancy progresses.
JAIMEY M. PAULI, MD
- ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52. doi:10.1097/AOG.0000000000002708.
- Mendoza M, Bonacina E, Garcia-Manau P, et al. Aspirin discontinuation at 24 to 28 weeks’ gestation in pregnancies at high risk of preterm preeclampsia: a randomized clinical trial. JAMA. 2023;329:542-550. doi:10.1001/jama.2023.0691.
- ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol. 2018;132:e44-e52. doi:10.1097/AOG.0000000000002708.
- Mendoza M, Bonacina E, Garcia-Manau P, et al. Aspirin discontinuation at 24 to 28 weeks’ gestation in pregnancies at high risk of preterm preeclampsia: a randomized clinical trial. JAMA. 2023;329:542-550. doi:10.1001/jama.2023.0691.