User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
How does lebrikizumab perform across different racial and ethnic subgroups?
.
The finding comes from an analysis of the 16-week induction periods of the phase 3 ADvocate1 and ADvocate2 trials, which Raj Chovatiya, MD, PhD, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. The efficacy of lebrikizumab monotherapy to treat moderate-to-severe AD has been established in phase 3 studies, “but disease characteristic and efficacy outcomes may vary among racial and ethnic subgroups,” said Dr. Chovatiya, assistant professor in the department of dermatology at Northwestern University, Chicago. “The goal of the current study is to report the week 16 efficacy of lebrikizumab-treated patients in racial and ethnic subgroups from ADvocate1 and ADvocate2.”
Key eligibility criteria for both trials included adults or adolescents with a diagnosis of AD as defined by the American Academy of Dermatology Consensus Criteria, for at least 1 year prior to screening. They had moderate-to-severe AD, were candidates for systemic therapy, and were dupilumab- and tralokinumab-naive. Outcomes of interest were the Investigator’s Global Assessment 0 or 1, with at least a 2-point improvement; and the proportions of patients who achieved Eczema Area and Severity Index (EASI75) and EASI90 responses, and an improvement of 4 points or more on the Pruritus Numeric Rating Scale (NRS).
For statistical analysis, the researchers pooled data from Advocate1 and Advocate2 and applied imputation methodology to the 16-week induction period. Subsequent data from patients who received topical or systemic rescue medication or discontinued treatment due to lack of efficacy were imputed as nonresponders. Subsequent data from patients who discontinued treatment for other reasons were set to missing, and the researchers handled missing data with multiple imputation. They used logistic regression to test the interaction between the treatment and subgroup and the Cochran-Mantel-Haenszel method to evaluate the treatment effect within each subgroup after adjusting for stratification factors.
Dr. Chovatiya reported findings from the 851 study participants in the combined studies. Of these, 542 were White, 192 were Asian, 84 were Black, and 33 were from other racial subgroups. By ethnic subgroup, 748 were not Hispanic or Latino, 91 were Hispanic or Latino, and ethnicity was unknown or not reported for 12 subjects. At baseline, the mean body mass index was slightly higher among Blacks (30.4 kg/m2) and Hispanics (29.4 kg/m2) compared with other racial and ethnic groups, “which reflects general epidemiologic data among these groups in the United States,” Dr. Chovatiya said. “You can also see a difference in the balance of IGA scores — they were a little bit more severe in the Black or African American and Hispanic groups as well.” The researchers also observed differences in the baseline EASI score across some of these groups, particularly in the Asian individuals, who had higher EASI scores. Prior use of systemic therapy was lower in the Black and “other” subgroups, compared with other racial subgroups.
At week 16, key efficacy endpoints were generally similar between the different racial subgroups. Specifically, 25.1% of Asians in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 4.1% of those in the placebo group (P < .001), while 33.2% of Blacks in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 13.2% of those in the placebo group (no P value was established because this subgroup represented less than 10% of the entire study population). In addition, 43.3% of Whites in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 14.1% of those in the placebo group (P < .001).
In other findings, 45.5% of Asians in the lebrikizumab treatment group achieved an EASI75, compared with 8.5% of those in the placebo group (P < .001), while 51.7% of Blacks in the lebrikizumab treatment group achieved an EASI75, compared with 18.8% of those in the placebo group. Among whites in the lebrikizumab treatment group, 59.7% of achieved an EASI75, compared with 20.4% of those in the placebo group (P < .001).
Dr. Chovatiya said that 26.5% of Asians in the lebrikizumab treatment group achieved an EASI90, compared with 4.3% of those in the placebo group (P < .001), while 26.9% of Blacks in the lebrikizumab treatment group achieved an EASI90, compared with 13.2% of those in the placebo group. In addition, 38.3% of Whites in the lebrikizumab treatment group achieved an EASI90, compared with 10.9% of those in the placebo group (P < .001).
Finally, 36.4% of Asians in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 5.7% of those in the placebo group (P <. 001), while 41.7% of Blacks in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 17.4% of those in the placebo group. In addition, 45.9% of Whites in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 14.8% of those in the placebo group (P < .001). Statistical analyses of efficacy endpoints conducted by ethnic group yielded similar results.
Dr. Chovatiya acknowledged certain limitations of the study, including the fact that differences in baseline demographics and disease characteristics limit direct comparison across racial and ethnic subgroups. “Due to the relatively small sample size of some racial and ethnic subgroups and the post hoc nature of this analysis, additional studies are needed to verify these results,” he concluded. But for now, he said, the data available indicate that “lebrikizumab is effective across racial and ethnic subgroups for the treatment of moderate-to-severe AD after 16 weeks of monotherapy treatment.”
The study was funded by Dermira, a wholly owned subsidiary of Eli Lilly and Company. Dr. Chovatiya disclosed that he is speaker for and/or a consult and advisory board member to many pharmaceutical companies, including Eli Lilly.
.
The finding comes from an analysis of the 16-week induction periods of the phase 3 ADvocate1 and ADvocate2 trials, which Raj Chovatiya, MD, PhD, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. The efficacy of lebrikizumab monotherapy to treat moderate-to-severe AD has been established in phase 3 studies, “but disease characteristic and efficacy outcomes may vary among racial and ethnic subgroups,” said Dr. Chovatiya, assistant professor in the department of dermatology at Northwestern University, Chicago. “The goal of the current study is to report the week 16 efficacy of lebrikizumab-treated patients in racial and ethnic subgroups from ADvocate1 and ADvocate2.”
Key eligibility criteria for both trials included adults or adolescents with a diagnosis of AD as defined by the American Academy of Dermatology Consensus Criteria, for at least 1 year prior to screening. They had moderate-to-severe AD, were candidates for systemic therapy, and were dupilumab- and tralokinumab-naive. Outcomes of interest were the Investigator’s Global Assessment 0 or 1, with at least a 2-point improvement; and the proportions of patients who achieved Eczema Area and Severity Index (EASI75) and EASI90 responses, and an improvement of 4 points or more on the Pruritus Numeric Rating Scale (NRS).
For statistical analysis, the researchers pooled data from Advocate1 and Advocate2 and applied imputation methodology to the 16-week induction period. Subsequent data from patients who received topical or systemic rescue medication or discontinued treatment due to lack of efficacy were imputed as nonresponders. Subsequent data from patients who discontinued treatment for other reasons were set to missing, and the researchers handled missing data with multiple imputation. They used logistic regression to test the interaction between the treatment and subgroup and the Cochran-Mantel-Haenszel method to evaluate the treatment effect within each subgroup after adjusting for stratification factors.
Dr. Chovatiya reported findings from the 851 study participants in the combined studies. Of these, 542 were White, 192 were Asian, 84 were Black, and 33 were from other racial subgroups. By ethnic subgroup, 748 were not Hispanic or Latino, 91 were Hispanic or Latino, and ethnicity was unknown or not reported for 12 subjects. At baseline, the mean body mass index was slightly higher among Blacks (30.4 kg/m2) and Hispanics (29.4 kg/m2) compared with other racial and ethnic groups, “which reflects general epidemiologic data among these groups in the United States,” Dr. Chovatiya said. “You can also see a difference in the balance of IGA scores — they were a little bit more severe in the Black or African American and Hispanic groups as well.” The researchers also observed differences in the baseline EASI score across some of these groups, particularly in the Asian individuals, who had higher EASI scores. Prior use of systemic therapy was lower in the Black and “other” subgroups, compared with other racial subgroups.
At week 16, key efficacy endpoints were generally similar between the different racial subgroups. Specifically, 25.1% of Asians in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 4.1% of those in the placebo group (P < .001), while 33.2% of Blacks in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 13.2% of those in the placebo group (no P value was established because this subgroup represented less than 10% of the entire study population). In addition, 43.3% of Whites in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 14.1% of those in the placebo group (P < .001).
In other findings, 45.5% of Asians in the lebrikizumab treatment group achieved an EASI75, compared with 8.5% of those in the placebo group (P < .001), while 51.7% of Blacks in the lebrikizumab treatment group achieved an EASI75, compared with 18.8% of those in the placebo group. Among whites in the lebrikizumab treatment group, 59.7% of achieved an EASI75, compared with 20.4% of those in the placebo group (P < .001).
Dr. Chovatiya said that 26.5% of Asians in the lebrikizumab treatment group achieved an EASI90, compared with 4.3% of those in the placebo group (P < .001), while 26.9% of Blacks in the lebrikizumab treatment group achieved an EASI90, compared with 13.2% of those in the placebo group. In addition, 38.3% of Whites in the lebrikizumab treatment group achieved an EASI90, compared with 10.9% of those in the placebo group (P < .001).
Finally, 36.4% of Asians in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 5.7% of those in the placebo group (P <. 001), while 41.7% of Blacks in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 17.4% of those in the placebo group. In addition, 45.9% of Whites in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 14.8% of those in the placebo group (P < .001). Statistical analyses of efficacy endpoints conducted by ethnic group yielded similar results.
Dr. Chovatiya acknowledged certain limitations of the study, including the fact that differences in baseline demographics and disease characteristics limit direct comparison across racial and ethnic subgroups. “Due to the relatively small sample size of some racial and ethnic subgroups and the post hoc nature of this analysis, additional studies are needed to verify these results,” he concluded. But for now, he said, the data available indicate that “lebrikizumab is effective across racial and ethnic subgroups for the treatment of moderate-to-severe AD after 16 weeks of monotherapy treatment.”
The study was funded by Dermira, a wholly owned subsidiary of Eli Lilly and Company. Dr. Chovatiya disclosed that he is speaker for and/or a consult and advisory board member to many pharmaceutical companies, including Eli Lilly.
.
The finding comes from an analysis of the 16-week induction periods of the phase 3 ADvocate1 and ADvocate2 trials, which Raj Chovatiya, MD, PhD, presented during a late-breaking abstract session at the Revolutionizing Atopic Dermatitis (RAD) Virtual Conference. The efficacy of lebrikizumab monotherapy to treat moderate-to-severe AD has been established in phase 3 studies, “but disease characteristic and efficacy outcomes may vary among racial and ethnic subgroups,” said Dr. Chovatiya, assistant professor in the department of dermatology at Northwestern University, Chicago. “The goal of the current study is to report the week 16 efficacy of lebrikizumab-treated patients in racial and ethnic subgroups from ADvocate1 and ADvocate2.”
Key eligibility criteria for both trials included adults or adolescents with a diagnosis of AD as defined by the American Academy of Dermatology Consensus Criteria, for at least 1 year prior to screening. They had moderate-to-severe AD, were candidates for systemic therapy, and were dupilumab- and tralokinumab-naive. Outcomes of interest were the Investigator’s Global Assessment 0 or 1, with at least a 2-point improvement; and the proportions of patients who achieved Eczema Area and Severity Index (EASI75) and EASI90 responses, and an improvement of 4 points or more on the Pruritus Numeric Rating Scale (NRS).
For statistical analysis, the researchers pooled data from Advocate1 and Advocate2 and applied imputation methodology to the 16-week induction period. Subsequent data from patients who received topical or systemic rescue medication or discontinued treatment due to lack of efficacy were imputed as nonresponders. Subsequent data from patients who discontinued treatment for other reasons were set to missing, and the researchers handled missing data with multiple imputation. They used logistic regression to test the interaction between the treatment and subgroup and the Cochran-Mantel-Haenszel method to evaluate the treatment effect within each subgroup after adjusting for stratification factors.
Dr. Chovatiya reported findings from the 851 study participants in the combined studies. Of these, 542 were White, 192 were Asian, 84 were Black, and 33 were from other racial subgroups. By ethnic subgroup, 748 were not Hispanic or Latino, 91 were Hispanic or Latino, and ethnicity was unknown or not reported for 12 subjects. At baseline, the mean body mass index was slightly higher among Blacks (30.4 kg/m2) and Hispanics (29.4 kg/m2) compared with other racial and ethnic groups, “which reflects general epidemiologic data among these groups in the United States,” Dr. Chovatiya said. “You can also see a difference in the balance of IGA scores — they were a little bit more severe in the Black or African American and Hispanic groups as well.” The researchers also observed differences in the baseline EASI score across some of these groups, particularly in the Asian individuals, who had higher EASI scores. Prior use of systemic therapy was lower in the Black and “other” subgroups, compared with other racial subgroups.
At week 16, key efficacy endpoints were generally similar between the different racial subgroups. Specifically, 25.1% of Asians in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 4.1% of those in the placebo group (P < .001), while 33.2% of Blacks in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 13.2% of those in the placebo group (no P value was established because this subgroup represented less than 10% of the entire study population). In addition, 43.3% of Whites in the lebrikizumab treatment group achieved an IGA of 0/1, compared with 14.1% of those in the placebo group (P < .001).
In other findings, 45.5% of Asians in the lebrikizumab treatment group achieved an EASI75, compared with 8.5% of those in the placebo group (P < .001), while 51.7% of Blacks in the lebrikizumab treatment group achieved an EASI75, compared with 18.8% of those in the placebo group. Among whites in the lebrikizumab treatment group, 59.7% of achieved an EASI75, compared with 20.4% of those in the placebo group (P < .001).
Dr. Chovatiya said that 26.5% of Asians in the lebrikizumab treatment group achieved an EASI90, compared with 4.3% of those in the placebo group (P < .001), while 26.9% of Blacks in the lebrikizumab treatment group achieved an EASI90, compared with 13.2% of those in the placebo group. In addition, 38.3% of Whites in the lebrikizumab treatment group achieved an EASI90, compared with 10.9% of those in the placebo group (P < .001).
Finally, 36.4% of Asians in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 5.7% of those in the placebo group (P <. 001), while 41.7% of Blacks in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 17.4% of those in the placebo group. In addition, 45.9% of Whites in the lebrikizumab treatment group achieved a 4-point or greater improvement on the NRS, compared with 14.8% of those in the placebo group (P < .001). Statistical analyses of efficacy endpoints conducted by ethnic group yielded similar results.
Dr. Chovatiya acknowledged certain limitations of the study, including the fact that differences in baseline demographics and disease characteristics limit direct comparison across racial and ethnic subgroups. “Due to the relatively small sample size of some racial and ethnic subgroups and the post hoc nature of this analysis, additional studies are needed to verify these results,” he concluded. But for now, he said, the data available indicate that “lebrikizumab is effective across racial and ethnic subgroups for the treatment of moderate-to-severe AD after 16 weeks of monotherapy treatment.”
The study was funded by Dermira, a wholly owned subsidiary of Eli Lilly and Company. Dr. Chovatiya disclosed that he is speaker for and/or a consult and advisory board member to many pharmaceutical companies, including Eli Lilly.
FROM RAD 2023
10% of US physicians work for or under UnitedHealth. Is that a problem?
as more payers and private equity firms pursue medical practice acquisitions.
The company added 20,000 physicians in the last year alone, including a previously physician-owned multispecialty group practice of 400 doctors in New York. They join the growing web of doctors — about 90,000 of the 950,000 active US physicians — working for the UnitedHealth Group subsidiary, Optum Health, providing primary, specialty, urgent, and surgical care. Amar Desai, MD, chief executive officer of Optum Health, shared the updated workforce numbers during the health care conglomerate’s annual investor conference.
Health care mergers and consolidations have become more common as physician groups struggle to stay afloat amid dwindling payer reimbursements. Although private equity and health systems often acquire practices, payers like UHC are increasingly doing so as part of their model to advance value-based care.
Yashaswini Singh, PhD, health care economist and assistant professor of health services, policy, and practice at Brown University, says such moves mirror the broader trend in corporate consolidation of physician practices. She said in an interview that the integrated models could possibly enhance care coordination and improve outcomes, but the impact of payer-led consolidation has not been extensively studied.
Meanwhile, evidence considering private equity ownership is just emerging. In a 2022 study published in JAMA Health Forum, with Dr. Singh as lead author, findings showed that private equity involvement increased healthcare spending through higher prices and utilization.
Consolidation can also raise anti-trust concerns. “If payers incentivize referral patterns of their employed physicians to favor other physicians employed by the payer, it can reduce competition by restricting consumer choice,” said Dr. Singh.
A potential merger between Cigna and Humana that could happen by the end of the year will likely face intense scrutiny as it would create a company that rivals the size of UnitedHealth Group or CVS Health. If it goes through, the duo could streamline its insurance offerings and leverage each other’s care delivery platforms, clinics, and provider workforce.
The Biden Administration has sought to strengthen anti-trust statutes to prevent industry monopolies and consumer harm, and the US Department of Justice and Federal Trade Commission have proposed new merger guidelines that have yet to be finalized.
According to Dr. Singh, some of Optum’s medical practice purchases may bypass anti-trust statutes since most prospective mergers and acquisitions are reviewed only if they exceed a specific value ($101 million for 2023). Limited transparency in ownership structures further complicates matters. Plus, Dr. Singh said instances where physicians are hired instead of acquired through mergers would not be subject to current anti-trust laws.
The ‘corporatization’ of health care is not good for patients or physicians, said Robert McNamara, MD, chief medical officer of the American Academy of Emergency Medicine Physician Group and cofounder of Take Medicine Back, a physician group advocating to remove corporate interests from health care.
“If you ask a physician what causes them the most moral conflict, they’ll tell you it’s the insurance companies denying something they want to do for their patients,” he said. “To have the doctors now working for the insurance industry conflicts with a physician’s duty to put the patient first.”
Dr. McNamara, chair of emergency medicine at Temple University’s Katz School of Medicine, said in an interview that more than half the states in the United States have laws or court rulings that support protecting physician autonomy from corporate interests. Still, he hopes a federal prohibition on private equity’s involvement in healthcare can soon gain traction. In November, Take Medicine Back raised a resolution at the American Medical Association’s interim House of Delegates meeting, which he said was subsequently referred to a committee.
Emergency medicine was among the first specialties to succumb to private equity firms, but Dr. McNamara said that all types of health care providers and entities — from cardiology and urology to addiction treatment centers and nursing homes — are being swallowed up by larger organizations, including payers.
UHC was named in a class action suit recently for allegedly shirking doctors’ orders and relying on a flawed algorithm to determine the length of skilled nursing facility stays for Medicare Advantage policyholders.
At the investor meeting, Dr. Desai reiterated Optum’s desire to continue expanding care delivery options, especially in its pharmacy and behavioral health business lines, and focus on adopting value-based care. He credited the rapid growth to developing strong relationships with providers and standardizing technology and clinical systems.
A version of this article appeared on Medscape.com.
as more payers and private equity firms pursue medical practice acquisitions.
The company added 20,000 physicians in the last year alone, including a previously physician-owned multispecialty group practice of 400 doctors in New York. They join the growing web of doctors — about 90,000 of the 950,000 active US physicians — working for the UnitedHealth Group subsidiary, Optum Health, providing primary, specialty, urgent, and surgical care. Amar Desai, MD, chief executive officer of Optum Health, shared the updated workforce numbers during the health care conglomerate’s annual investor conference.
Health care mergers and consolidations have become more common as physician groups struggle to stay afloat amid dwindling payer reimbursements. Although private equity and health systems often acquire practices, payers like UHC are increasingly doing so as part of their model to advance value-based care.
Yashaswini Singh, PhD, health care economist and assistant professor of health services, policy, and practice at Brown University, says such moves mirror the broader trend in corporate consolidation of physician practices. She said in an interview that the integrated models could possibly enhance care coordination and improve outcomes, but the impact of payer-led consolidation has not been extensively studied.
Meanwhile, evidence considering private equity ownership is just emerging. In a 2022 study published in JAMA Health Forum, with Dr. Singh as lead author, findings showed that private equity involvement increased healthcare spending through higher prices and utilization.
Consolidation can also raise anti-trust concerns. “If payers incentivize referral patterns of their employed physicians to favor other physicians employed by the payer, it can reduce competition by restricting consumer choice,” said Dr. Singh.
A potential merger between Cigna and Humana that could happen by the end of the year will likely face intense scrutiny as it would create a company that rivals the size of UnitedHealth Group or CVS Health. If it goes through, the duo could streamline its insurance offerings and leverage each other’s care delivery platforms, clinics, and provider workforce.
The Biden Administration has sought to strengthen anti-trust statutes to prevent industry monopolies and consumer harm, and the US Department of Justice and Federal Trade Commission have proposed new merger guidelines that have yet to be finalized.
According to Dr. Singh, some of Optum’s medical practice purchases may bypass anti-trust statutes since most prospective mergers and acquisitions are reviewed only if they exceed a specific value ($101 million for 2023). Limited transparency in ownership structures further complicates matters. Plus, Dr. Singh said instances where physicians are hired instead of acquired through mergers would not be subject to current anti-trust laws.
The ‘corporatization’ of health care is not good for patients or physicians, said Robert McNamara, MD, chief medical officer of the American Academy of Emergency Medicine Physician Group and cofounder of Take Medicine Back, a physician group advocating to remove corporate interests from health care.
“If you ask a physician what causes them the most moral conflict, they’ll tell you it’s the insurance companies denying something they want to do for their patients,” he said. “To have the doctors now working for the insurance industry conflicts with a physician’s duty to put the patient first.”
Dr. McNamara, chair of emergency medicine at Temple University’s Katz School of Medicine, said in an interview that more than half the states in the United States have laws or court rulings that support protecting physician autonomy from corporate interests. Still, he hopes a federal prohibition on private equity’s involvement in healthcare can soon gain traction. In November, Take Medicine Back raised a resolution at the American Medical Association’s interim House of Delegates meeting, which he said was subsequently referred to a committee.
Emergency medicine was among the first specialties to succumb to private equity firms, but Dr. McNamara said that all types of health care providers and entities — from cardiology and urology to addiction treatment centers and nursing homes — are being swallowed up by larger organizations, including payers.
UHC was named in a class action suit recently for allegedly shirking doctors’ orders and relying on a flawed algorithm to determine the length of skilled nursing facility stays for Medicare Advantage policyholders.
At the investor meeting, Dr. Desai reiterated Optum’s desire to continue expanding care delivery options, especially in its pharmacy and behavioral health business lines, and focus on adopting value-based care. He credited the rapid growth to developing strong relationships with providers and standardizing technology and clinical systems.
A version of this article appeared on Medscape.com.
as more payers and private equity firms pursue medical practice acquisitions.
The company added 20,000 physicians in the last year alone, including a previously physician-owned multispecialty group practice of 400 doctors in New York. They join the growing web of doctors — about 90,000 of the 950,000 active US physicians — working for the UnitedHealth Group subsidiary, Optum Health, providing primary, specialty, urgent, and surgical care. Amar Desai, MD, chief executive officer of Optum Health, shared the updated workforce numbers during the health care conglomerate’s annual investor conference.
Health care mergers and consolidations have become more common as physician groups struggle to stay afloat amid dwindling payer reimbursements. Although private equity and health systems often acquire practices, payers like UHC are increasingly doing so as part of their model to advance value-based care.
Yashaswini Singh, PhD, health care economist and assistant professor of health services, policy, and practice at Brown University, says such moves mirror the broader trend in corporate consolidation of physician practices. She said in an interview that the integrated models could possibly enhance care coordination and improve outcomes, but the impact of payer-led consolidation has not been extensively studied.
Meanwhile, evidence considering private equity ownership is just emerging. In a 2022 study published in JAMA Health Forum, with Dr. Singh as lead author, findings showed that private equity involvement increased healthcare spending through higher prices and utilization.
Consolidation can also raise anti-trust concerns. “If payers incentivize referral patterns of their employed physicians to favor other physicians employed by the payer, it can reduce competition by restricting consumer choice,” said Dr. Singh.
A potential merger between Cigna and Humana that could happen by the end of the year will likely face intense scrutiny as it would create a company that rivals the size of UnitedHealth Group or CVS Health. If it goes through, the duo could streamline its insurance offerings and leverage each other’s care delivery platforms, clinics, and provider workforce.
The Biden Administration has sought to strengthen anti-trust statutes to prevent industry monopolies and consumer harm, and the US Department of Justice and Federal Trade Commission have proposed new merger guidelines that have yet to be finalized.
According to Dr. Singh, some of Optum’s medical practice purchases may bypass anti-trust statutes since most prospective mergers and acquisitions are reviewed only if they exceed a specific value ($101 million for 2023). Limited transparency in ownership structures further complicates matters. Plus, Dr. Singh said instances where physicians are hired instead of acquired through mergers would not be subject to current anti-trust laws.
The ‘corporatization’ of health care is not good for patients or physicians, said Robert McNamara, MD, chief medical officer of the American Academy of Emergency Medicine Physician Group and cofounder of Take Medicine Back, a physician group advocating to remove corporate interests from health care.
“If you ask a physician what causes them the most moral conflict, they’ll tell you it’s the insurance companies denying something they want to do for their patients,” he said. “To have the doctors now working for the insurance industry conflicts with a physician’s duty to put the patient first.”
Dr. McNamara, chair of emergency medicine at Temple University’s Katz School of Medicine, said in an interview that more than half the states in the United States have laws or court rulings that support protecting physician autonomy from corporate interests. Still, he hopes a federal prohibition on private equity’s involvement in healthcare can soon gain traction. In November, Take Medicine Back raised a resolution at the American Medical Association’s interim House of Delegates meeting, which he said was subsequently referred to a committee.
Emergency medicine was among the first specialties to succumb to private equity firms, but Dr. McNamara said that all types of health care providers and entities — from cardiology and urology to addiction treatment centers and nursing homes — are being swallowed up by larger organizations, including payers.
UHC was named in a class action suit recently for allegedly shirking doctors’ orders and relying on a flawed algorithm to determine the length of skilled nursing facility stays for Medicare Advantage policyholders.
At the investor meeting, Dr. Desai reiterated Optum’s desire to continue expanding care delivery options, especially in its pharmacy and behavioral health business lines, and focus on adopting value-based care. He credited the rapid growth to developing strong relationships with providers and standardizing technology and clinical systems.
A version of this article appeared on Medscape.com.
Neighborhood Disadvantage Tied to Higher Risk for ASD
TOPLINE
, a population-based prospective cohort study shows.
METHODOLOGY
- Investigators analyzed data from a large cohort of singleton children with insurance born in Kaiser Permanente Southern California hospitals between 2001 and 2014.
- They ascertained ASD diagnosis, maternal race and ethnicity, and maternal address at time of birth.
- Neighborhood disadvantage was determined by the percentage of families in the mother’s neighborhood considered to be living in poverty, unemployed, have female-headed households with children, using public assistance, less than a high school education, among other variables.
TAKEAWAY
- Among 318,300 mothers who delivered babies during the study period, 6350 children were diagnosed with ASD during follow-up, and median age at diagnosis was 3.5 years.
- Greater neighborhood disadvantage at birth was associated with a higher likelihood of ASD diagnosis (adjusted hazard ratio [aHR], 1.07; 95% CI, 1.02-1.11)
- ASD diagnoses were more likely among children of mothers who were Black (aHR, 1.13; 95% CI, 1.02-1.25), Asian/Pacific Islander (aHR, 1.11; 95% CI, 1.02-1.20), or Hispanic (aHR, 1.07; 95% CI, 1.00-1.15), even after the researchers controlled for neighborhood.
- While odds of an ASD diagnosis were higher among children from minority racial and ethnic groups, neighborhood disadvantage was significantly associated with ASD diagnosis only for children of White mothers (aHR, 1.17; 95% CI, 1.09-1.26).
IN PRACTICE
Investigators noted that they could only speculate about the factors driving the association between neighborhood disadvantage and a stronger risk for ASD diagnosis in children of White mothers. “They may be due to systemic racism, discrimination, and their impact on maternal health during pregnancy,” they wrote.
SOURCE
Xin Yu, MS, and Daniel Hackman, PhD, of the University of Southern California Los Angeles, led the study, which was published online November 15 in JAMA Psychiatry.
LIMITATIONS
The research was limited by a lack of information on fathers and variables such as incomes, which may have confounded the findings. The authors also acknowledged that the study should be replicated in other health service settings.
DISCLOSURES
The study was funded by the National Institutes on Environmental Health Sciences, the National Institutes of Health (NIH), and the Environmental Protection Agency. Dr. Hackman reported receiving grant funding from NIH during the conduct of the study. Other disclosures are available in the original study.
A version of this article appeared on Medscape.com.
TOPLINE
, a population-based prospective cohort study shows.
METHODOLOGY
- Investigators analyzed data from a large cohort of singleton children with insurance born in Kaiser Permanente Southern California hospitals between 2001 and 2014.
- They ascertained ASD diagnosis, maternal race and ethnicity, and maternal address at time of birth.
- Neighborhood disadvantage was determined by the percentage of families in the mother’s neighborhood considered to be living in poverty, unemployed, have female-headed households with children, using public assistance, less than a high school education, among other variables.
TAKEAWAY
- Among 318,300 mothers who delivered babies during the study period, 6350 children were diagnosed with ASD during follow-up, and median age at diagnosis was 3.5 years.
- Greater neighborhood disadvantage at birth was associated with a higher likelihood of ASD diagnosis (adjusted hazard ratio [aHR], 1.07; 95% CI, 1.02-1.11)
- ASD diagnoses were more likely among children of mothers who were Black (aHR, 1.13; 95% CI, 1.02-1.25), Asian/Pacific Islander (aHR, 1.11; 95% CI, 1.02-1.20), or Hispanic (aHR, 1.07; 95% CI, 1.00-1.15), even after the researchers controlled for neighborhood.
- While odds of an ASD diagnosis were higher among children from minority racial and ethnic groups, neighborhood disadvantage was significantly associated with ASD diagnosis only for children of White mothers (aHR, 1.17; 95% CI, 1.09-1.26).
IN PRACTICE
Investigators noted that they could only speculate about the factors driving the association between neighborhood disadvantage and a stronger risk for ASD diagnosis in children of White mothers. “They may be due to systemic racism, discrimination, and their impact on maternal health during pregnancy,” they wrote.
SOURCE
Xin Yu, MS, and Daniel Hackman, PhD, of the University of Southern California Los Angeles, led the study, which was published online November 15 in JAMA Psychiatry.
LIMITATIONS
The research was limited by a lack of information on fathers and variables such as incomes, which may have confounded the findings. The authors also acknowledged that the study should be replicated in other health service settings.
DISCLOSURES
The study was funded by the National Institutes on Environmental Health Sciences, the National Institutes of Health (NIH), and the Environmental Protection Agency. Dr. Hackman reported receiving grant funding from NIH during the conduct of the study. Other disclosures are available in the original study.
A version of this article appeared on Medscape.com.
TOPLINE
, a population-based prospective cohort study shows.
METHODOLOGY
- Investigators analyzed data from a large cohort of singleton children with insurance born in Kaiser Permanente Southern California hospitals between 2001 and 2014.
- They ascertained ASD diagnosis, maternal race and ethnicity, and maternal address at time of birth.
- Neighborhood disadvantage was determined by the percentage of families in the mother’s neighborhood considered to be living in poverty, unemployed, have female-headed households with children, using public assistance, less than a high school education, among other variables.
TAKEAWAY
- Among 318,300 mothers who delivered babies during the study period, 6350 children were diagnosed with ASD during follow-up, and median age at diagnosis was 3.5 years.
- Greater neighborhood disadvantage at birth was associated with a higher likelihood of ASD diagnosis (adjusted hazard ratio [aHR], 1.07; 95% CI, 1.02-1.11)
- ASD diagnoses were more likely among children of mothers who were Black (aHR, 1.13; 95% CI, 1.02-1.25), Asian/Pacific Islander (aHR, 1.11; 95% CI, 1.02-1.20), or Hispanic (aHR, 1.07; 95% CI, 1.00-1.15), even after the researchers controlled for neighborhood.
- While odds of an ASD diagnosis were higher among children from minority racial and ethnic groups, neighborhood disadvantage was significantly associated with ASD diagnosis only for children of White mothers (aHR, 1.17; 95% CI, 1.09-1.26).
IN PRACTICE
Investigators noted that they could only speculate about the factors driving the association between neighborhood disadvantage and a stronger risk for ASD diagnosis in children of White mothers. “They may be due to systemic racism, discrimination, and their impact on maternal health during pregnancy,” they wrote.
SOURCE
Xin Yu, MS, and Daniel Hackman, PhD, of the University of Southern California Los Angeles, led the study, which was published online November 15 in JAMA Psychiatry.
LIMITATIONS
The research was limited by a lack of information on fathers and variables such as incomes, which may have confounded the findings. The authors also acknowledged that the study should be replicated in other health service settings.
DISCLOSURES
The study was funded by the National Institutes on Environmental Health Sciences, the National Institutes of Health (NIH), and the Environmental Protection Agency. Dr. Hackman reported receiving grant funding from NIH during the conduct of the study. Other disclosures are available in the original study.
A version of this article appeared on Medscape.com.
U.S. Task Force Takes on Rising BMIs Among Children
The U.S. Preventive Services Task Force — a team of independent, volunteer experts in disease prevention who guide doctors’ decisions and influence insurance coverage — issued a draft recommendation statement outlining the interventions that should be taken when a child or teen has a high body mass index.
Nearly 20% of children between 2 and 19 years old have what are considered high BMIs, according to Centers for Disease Control and Prevention data. While adults who have a BMI of 30 or higher are considered to have obesity, childhood obesity is determined if a child is at or above the 95th percentile of others their age and gender.
Given the prevalence of the issue, the task force recommends behavioral interventions that include at least 26 hours of supervised physical activity sessions for up to a year. This differs from the task force’s previous recommendations on the topic, which emphasized the importance of screening for high BMIs rather than describing the right ways to intervene.
Some of the most effective interventions are targeted at both parents and their children, whether that be together, separately, or a combination of the two. Additionally, the task force recommends that children attend group sessions about healthy eating habits, how to read food labels, and exercise techniques. Ideally, these would be led and guided by people of various professional backgrounds like pediatricians, physical therapists, dietitians, psychologists, and social workers. Other medical organizations, namely the American Academy of Pediatrics, have recommended medication for some children with obesity; the task force, however, takes a more conservative approach. They noted that although the body of evidence shows weight loss medications and surgery are effective for many, there isn’t enough research to lean on regarding the use of these interventions in children, especially in the long term.
“There are proven ways that clinicians can help the many children and teens who have a high BMI to manage their weight and stay healthy,” said Katrina Donahue, MD, MPH, a member of the task force and professor of family medicine at the University of North Carolina at Chapel Hill. “Intensive behavioral interventions are effective in helping children achieve a healthy weight while improving quality of life.”
The guidelines are still in the draft stage and are available for public comment until Jan. 16, 2024.
A version of this article appeared on WebMD.com.
The U.S. Preventive Services Task Force — a team of independent, volunteer experts in disease prevention who guide doctors’ decisions and influence insurance coverage — issued a draft recommendation statement outlining the interventions that should be taken when a child or teen has a high body mass index.
Nearly 20% of children between 2 and 19 years old have what are considered high BMIs, according to Centers for Disease Control and Prevention data. While adults who have a BMI of 30 or higher are considered to have obesity, childhood obesity is determined if a child is at or above the 95th percentile of others their age and gender.
Given the prevalence of the issue, the task force recommends behavioral interventions that include at least 26 hours of supervised physical activity sessions for up to a year. This differs from the task force’s previous recommendations on the topic, which emphasized the importance of screening for high BMIs rather than describing the right ways to intervene.
Some of the most effective interventions are targeted at both parents and their children, whether that be together, separately, or a combination of the two. Additionally, the task force recommends that children attend group sessions about healthy eating habits, how to read food labels, and exercise techniques. Ideally, these would be led and guided by people of various professional backgrounds like pediatricians, physical therapists, dietitians, psychologists, and social workers. Other medical organizations, namely the American Academy of Pediatrics, have recommended medication for some children with obesity; the task force, however, takes a more conservative approach. They noted that although the body of evidence shows weight loss medications and surgery are effective for many, there isn’t enough research to lean on regarding the use of these interventions in children, especially in the long term.
“There are proven ways that clinicians can help the many children and teens who have a high BMI to manage their weight and stay healthy,” said Katrina Donahue, MD, MPH, a member of the task force and professor of family medicine at the University of North Carolina at Chapel Hill. “Intensive behavioral interventions are effective in helping children achieve a healthy weight while improving quality of life.”
The guidelines are still in the draft stage and are available for public comment until Jan. 16, 2024.
A version of this article appeared on WebMD.com.
The U.S. Preventive Services Task Force — a team of independent, volunteer experts in disease prevention who guide doctors’ decisions and influence insurance coverage — issued a draft recommendation statement outlining the interventions that should be taken when a child or teen has a high body mass index.
Nearly 20% of children between 2 and 19 years old have what are considered high BMIs, according to Centers for Disease Control and Prevention data. While adults who have a BMI of 30 or higher are considered to have obesity, childhood obesity is determined if a child is at or above the 95th percentile of others their age and gender.
Given the prevalence of the issue, the task force recommends behavioral interventions that include at least 26 hours of supervised physical activity sessions for up to a year. This differs from the task force’s previous recommendations on the topic, which emphasized the importance of screening for high BMIs rather than describing the right ways to intervene.
Some of the most effective interventions are targeted at both parents and their children, whether that be together, separately, or a combination of the two. Additionally, the task force recommends that children attend group sessions about healthy eating habits, how to read food labels, and exercise techniques. Ideally, these would be led and guided by people of various professional backgrounds like pediatricians, physical therapists, dietitians, psychologists, and social workers. Other medical organizations, namely the American Academy of Pediatrics, have recommended medication for some children with obesity; the task force, however, takes a more conservative approach. They noted that although the body of evidence shows weight loss medications and surgery are effective for many, there isn’t enough research to lean on regarding the use of these interventions in children, especially in the long term.
“There are proven ways that clinicians can help the many children and teens who have a high BMI to manage their weight and stay healthy,” said Katrina Donahue, MD, MPH, a member of the task force and professor of family medicine at the University of North Carolina at Chapel Hill. “Intensive behavioral interventions are effective in helping children achieve a healthy weight while improving quality of life.”
The guidelines are still in the draft stage and are available for public comment until Jan. 16, 2024.
A version of this article appeared on WebMD.com.
Fivefold Increase in Vaping During Adolescent Pregnancies
TOPLINE:
, according to research published online on December 13 in JAMA Network Open.
METHODOLOGY:
- Researchers analyzed data from the 2016-2021 Pregnancy Risk Assessment Monitoring System.
- They focused on 10,428 adolescents aged 10-19 years who had had a singleton birth and provided information about their use of e-cigarettes or cigarettes.
TAKEAWAY:
- Whereas the researchers found a roughly fivefold increase in the exclusive use of e-cigarettes, the percentage of patients using only cigarettes decreased from 9.2% in 2017 to 3.2% in 2021.
- The percentage of patients who both vaped and smoked fluctuated between 0.6% and 1.6%.
- The rate of small-for-gestational-age (SGA) births for adolescents who did not smoke or vape (12.9%) did not differ significantly from that among adolescents who exclusively used e-cigarettes (16.8%) or those who used both cigarettes and e-cigarettes (17.6%).
- The researchers found use of cigarettes only was associated with a significantly higher rate of SGA births: 24.6%.
IN PRACTICE:
“Exclusive e-cigarette use and dual use of cigarettes and e-cigarettes did not seem to be statistically significantly associated with SGA birth in our analysis, but this finding should be interpreted with caution given the low prevalence of use and the limited sample size,” the study authors wrote.
SOURCE:
Xiaozhong Wen, MD, PhD, with the Jacobs School of Medicine and Biomedical Sciences at the State University of New York at Buffalo, was the corresponding author of the study.
LIMITATIONS:
Participants may have underreported their use of e-cigarettes and cigarettes because of fears of social stigma. The researchers lacked information about vaping in the first and second trimesters, exposure to secondhand smoke, cannabis use, and diet.
DISCLOSURES:
The research was supported by the National Institute on Drug Abuse; the Food and Drug Administration Center for Tobacco Products; the National Heart, Lung, and Blood Institute; and the American Heart Association. A study coauthor has received grants from Pfizer and personal fees from Johnson & Johnson, the World Health Organization, and the Campaign for Tobacco-Free Kids.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to research published online on December 13 in JAMA Network Open.
METHODOLOGY:
- Researchers analyzed data from the 2016-2021 Pregnancy Risk Assessment Monitoring System.
- They focused on 10,428 adolescents aged 10-19 years who had had a singleton birth and provided information about their use of e-cigarettes or cigarettes.
TAKEAWAY:
- Whereas the researchers found a roughly fivefold increase in the exclusive use of e-cigarettes, the percentage of patients using only cigarettes decreased from 9.2% in 2017 to 3.2% in 2021.
- The percentage of patients who both vaped and smoked fluctuated between 0.6% and 1.6%.
- The rate of small-for-gestational-age (SGA) births for adolescents who did not smoke or vape (12.9%) did not differ significantly from that among adolescents who exclusively used e-cigarettes (16.8%) or those who used both cigarettes and e-cigarettes (17.6%).
- The researchers found use of cigarettes only was associated with a significantly higher rate of SGA births: 24.6%.
IN PRACTICE:
“Exclusive e-cigarette use and dual use of cigarettes and e-cigarettes did not seem to be statistically significantly associated with SGA birth in our analysis, but this finding should be interpreted with caution given the low prevalence of use and the limited sample size,” the study authors wrote.
SOURCE:
Xiaozhong Wen, MD, PhD, with the Jacobs School of Medicine and Biomedical Sciences at the State University of New York at Buffalo, was the corresponding author of the study.
LIMITATIONS:
Participants may have underreported their use of e-cigarettes and cigarettes because of fears of social stigma. The researchers lacked information about vaping in the first and second trimesters, exposure to secondhand smoke, cannabis use, and diet.
DISCLOSURES:
The research was supported by the National Institute on Drug Abuse; the Food and Drug Administration Center for Tobacco Products; the National Heart, Lung, and Blood Institute; and the American Heart Association. A study coauthor has received grants from Pfizer and personal fees from Johnson & Johnson, the World Health Organization, and the Campaign for Tobacco-Free Kids.
A version of this article appeared on Medscape.com.
TOPLINE:
, according to research published online on December 13 in JAMA Network Open.
METHODOLOGY:
- Researchers analyzed data from the 2016-2021 Pregnancy Risk Assessment Monitoring System.
- They focused on 10,428 adolescents aged 10-19 years who had had a singleton birth and provided information about their use of e-cigarettes or cigarettes.
TAKEAWAY:
- Whereas the researchers found a roughly fivefold increase in the exclusive use of e-cigarettes, the percentage of patients using only cigarettes decreased from 9.2% in 2017 to 3.2% in 2021.
- The percentage of patients who both vaped and smoked fluctuated between 0.6% and 1.6%.
- The rate of small-for-gestational-age (SGA) births for adolescents who did not smoke or vape (12.9%) did not differ significantly from that among adolescents who exclusively used e-cigarettes (16.8%) or those who used both cigarettes and e-cigarettes (17.6%).
- The researchers found use of cigarettes only was associated with a significantly higher rate of SGA births: 24.6%.
IN PRACTICE:
“Exclusive e-cigarette use and dual use of cigarettes and e-cigarettes did not seem to be statistically significantly associated with SGA birth in our analysis, but this finding should be interpreted with caution given the low prevalence of use and the limited sample size,” the study authors wrote.
SOURCE:
Xiaozhong Wen, MD, PhD, with the Jacobs School of Medicine and Biomedical Sciences at the State University of New York at Buffalo, was the corresponding author of the study.
LIMITATIONS:
Participants may have underreported their use of e-cigarettes and cigarettes because of fears of social stigma. The researchers lacked information about vaping in the first and second trimesters, exposure to secondhand smoke, cannabis use, and diet.
DISCLOSURES:
The research was supported by the National Institute on Drug Abuse; the Food and Drug Administration Center for Tobacco Products; the National Heart, Lung, and Blood Institute; and the American Heart Association. A study coauthor has received grants from Pfizer and personal fees from Johnson & Johnson, the World Health Organization, and the Campaign for Tobacco-Free Kids.
A version of this article appeared on Medscape.com.
Teen and young adult rheumatology patients report gaps in sexual health counseling
SAN DIEGO — Only half of teens and young adults on teratogenic medication report being asked about sexual activity by their rheumatologist, and 38% did not know that their medication would be harmful to a fetus, according to a new survey.
While pediatric rheumatology providers may think that health screenings and contraceptive counseling are happening elsewhere, “this study suggests that a lot of patients are being missed, including those on teratogens,” noted Brittany M. Huynh, MD, MPH, a pediatric rheumatology fellow at the Indiana University School of Medicine in Indianapolis. She led the study and presented the findings at the American College of Rheumatology annual meeting.
For the study, Dr. Huynh and colleagues recruited patients aged 14-23 years who were assigned female at birth and were followed at pediatric rheumatology clinics affiliated with Indiana University. Participants completed a one-time survey between October 2020 and July 2022 and were asked about their sexual reproductive health experience and knowledge. Notably, all but four surveys were completed prior to the US Supreme Court Dobbs decision overturning Roe v. Wade.
Of responses from 108 participants, the most common diagnoses were juvenile idiopathic arthritis (52%) and systemic lupus erythematosus (16%). About one third (36%) of patients were on teratogenic medication, with the most common being methotrexate. About three fourths (76%) were White, and the average age of respondents was 16.7.
Most participants (82%) said they had been asked about sexual activity by a health care provider, but only 38% said their pediatric rheumatologist discussed this topic with them. Of the 39 patients on teratogenic medication, 54% said they had been asked about sexual activity by their pediatric rheumatologist, and only 51% said they had received teratogenicity counseling.
A larger percentage (85%) of this group reported receiving sexual activity screenings by any provider, but there was little difference in counseling about teratogenic medication.
This suggests that this type of risk counseling “is almost exclusively done by (pediatric rheumatologists), if at all,” Dr. Huynh noted during her presentation.
In total, 56% of all patients said a provider had talked to them about how to prevent pregnancy, and 20% said they had been counseled about how to get and use emergency contraception. Only 6% of patients said their pediatric rheumatologist had discussed emergency contraception during appointments.
Although sexual activity screenings were associated with current teratogen use, pregnancy prevention counseling and emergency contraceptive counseling were not associated with teratogen use or reported sexual activity.
The survey also revealed that there were gaps in knowledge about the health effects of rheumatic medication. Of the patients on teratogens, 38% did not know that their medication could harm a fetus if they became pregnant. Only 9% of patients not on teratogens correctly answered that their medication would not harm a fetus.
Previous studies have also shown that rheumatology patients do not know that their medications can be teratogenic, noted Cuoghi Edens, MD, a rheumatologist at the University of Chicago, who sees both adult and pediatric patients. She was not involved with the study. The larger challenge is how to best educate patients, she said.
While hopefully a patient’s primary care provider is discussing these issues with them, these patients often see their rheumatologist more frequently and more consistently than other providers, Dr. Edens said.
“We are sometimes the continuity of care for the patient versus their primary care, even though it should be a group effort of trying to some of these questions,” she said.
Conducting reproductive health screenings in pediatric rheumatology clinics can be difficult though, Dr. Edens noted, not only because of time constraints but also because parents often attend appointments with their child and likely have been for years. These screenings are most accurate when done one-on-one, so pivoting and removing the parents from the room can be awkward for providers, Dr. Edens said.
She advised that starting these conversations early on can be one way to ease into talking about reproductive health. In her own practice, Dr. Huynh sets aside time during appointments to speak with adolescent patients privately.
“We always discuss teratogenic medication. I always talk to them about the fact that I’m going to be doing pregnancy testing with their other screening labs because of the risks associated,” she said. “I also specifically set time aside for patients on teratogens to talk about emergency contraception and offer a prescription, if they’re interested.”
Dr. Huynh emphasized that providing easy access to emergency contraception is key. The ACR reproductive health guidelines — although geared toward adults — recommend discussing emergency contraception with patients, and Dr. Huynh advocates writing prescriptions for interested patients.
“They can fill it and have it easily accessible, so that there are no additional barriers, particularly for people who have these higher risks,” she said.
While emergency contraceptives are also available over the counter, it can be awkward for young people to ask for them, she said, and they can be expensive if not covered under insurance. Providing a prescription is one way to avoid those issues, Dr. Huynh said.
“Certainly, you have to have some parent buy-in, because if there is going to be a script, it’s probably going to be under insurance,” she said. “But in my experience, parents are happy to have it around as long as you’re talking it through with them as well as the young person.”
Dr. Huynh and Dr. Edens had no disclosures.
A version of this article appeared on Medscape.com.
SAN DIEGO — Only half of teens and young adults on teratogenic medication report being asked about sexual activity by their rheumatologist, and 38% did not know that their medication would be harmful to a fetus, according to a new survey.
While pediatric rheumatology providers may think that health screenings and contraceptive counseling are happening elsewhere, “this study suggests that a lot of patients are being missed, including those on teratogens,” noted Brittany M. Huynh, MD, MPH, a pediatric rheumatology fellow at the Indiana University School of Medicine in Indianapolis. She led the study and presented the findings at the American College of Rheumatology annual meeting.
For the study, Dr. Huynh and colleagues recruited patients aged 14-23 years who were assigned female at birth and were followed at pediatric rheumatology clinics affiliated with Indiana University. Participants completed a one-time survey between October 2020 and July 2022 and were asked about their sexual reproductive health experience and knowledge. Notably, all but four surveys were completed prior to the US Supreme Court Dobbs decision overturning Roe v. Wade.
Of responses from 108 participants, the most common diagnoses were juvenile idiopathic arthritis (52%) and systemic lupus erythematosus (16%). About one third (36%) of patients were on teratogenic medication, with the most common being methotrexate. About three fourths (76%) were White, and the average age of respondents was 16.7.
Most participants (82%) said they had been asked about sexual activity by a health care provider, but only 38% said their pediatric rheumatologist discussed this topic with them. Of the 39 patients on teratogenic medication, 54% said they had been asked about sexual activity by their pediatric rheumatologist, and only 51% said they had received teratogenicity counseling.
A larger percentage (85%) of this group reported receiving sexual activity screenings by any provider, but there was little difference in counseling about teratogenic medication.
This suggests that this type of risk counseling “is almost exclusively done by (pediatric rheumatologists), if at all,” Dr. Huynh noted during her presentation.
In total, 56% of all patients said a provider had talked to them about how to prevent pregnancy, and 20% said they had been counseled about how to get and use emergency contraception. Only 6% of patients said their pediatric rheumatologist had discussed emergency contraception during appointments.
Although sexual activity screenings were associated with current teratogen use, pregnancy prevention counseling and emergency contraceptive counseling were not associated with teratogen use or reported sexual activity.
The survey also revealed that there were gaps in knowledge about the health effects of rheumatic medication. Of the patients on teratogens, 38% did not know that their medication could harm a fetus if they became pregnant. Only 9% of patients not on teratogens correctly answered that their medication would not harm a fetus.
Previous studies have also shown that rheumatology patients do not know that their medications can be teratogenic, noted Cuoghi Edens, MD, a rheumatologist at the University of Chicago, who sees both adult and pediatric patients. She was not involved with the study. The larger challenge is how to best educate patients, she said.
While hopefully a patient’s primary care provider is discussing these issues with them, these patients often see their rheumatologist more frequently and more consistently than other providers, Dr. Edens said.
“We are sometimes the continuity of care for the patient versus their primary care, even though it should be a group effort of trying to some of these questions,” she said.
Conducting reproductive health screenings in pediatric rheumatology clinics can be difficult though, Dr. Edens noted, not only because of time constraints but also because parents often attend appointments with their child and likely have been for years. These screenings are most accurate when done one-on-one, so pivoting and removing the parents from the room can be awkward for providers, Dr. Edens said.
She advised that starting these conversations early on can be one way to ease into talking about reproductive health. In her own practice, Dr. Huynh sets aside time during appointments to speak with adolescent patients privately.
“We always discuss teratogenic medication. I always talk to them about the fact that I’m going to be doing pregnancy testing with their other screening labs because of the risks associated,” she said. “I also specifically set time aside for patients on teratogens to talk about emergency contraception and offer a prescription, if they’re interested.”
Dr. Huynh emphasized that providing easy access to emergency contraception is key. The ACR reproductive health guidelines — although geared toward adults — recommend discussing emergency contraception with patients, and Dr. Huynh advocates writing prescriptions for interested patients.
“They can fill it and have it easily accessible, so that there are no additional barriers, particularly for people who have these higher risks,” she said.
While emergency contraceptives are also available over the counter, it can be awkward for young people to ask for them, she said, and they can be expensive if not covered under insurance. Providing a prescription is one way to avoid those issues, Dr. Huynh said.
“Certainly, you have to have some parent buy-in, because if there is going to be a script, it’s probably going to be under insurance,” she said. “But in my experience, parents are happy to have it around as long as you’re talking it through with them as well as the young person.”
Dr. Huynh and Dr. Edens had no disclosures.
A version of this article appeared on Medscape.com.
SAN DIEGO — Only half of teens and young adults on teratogenic medication report being asked about sexual activity by their rheumatologist, and 38% did not know that their medication would be harmful to a fetus, according to a new survey.
While pediatric rheumatology providers may think that health screenings and contraceptive counseling are happening elsewhere, “this study suggests that a lot of patients are being missed, including those on teratogens,” noted Brittany M. Huynh, MD, MPH, a pediatric rheumatology fellow at the Indiana University School of Medicine in Indianapolis. She led the study and presented the findings at the American College of Rheumatology annual meeting.
For the study, Dr. Huynh and colleagues recruited patients aged 14-23 years who were assigned female at birth and were followed at pediatric rheumatology clinics affiliated with Indiana University. Participants completed a one-time survey between October 2020 and July 2022 and were asked about their sexual reproductive health experience and knowledge. Notably, all but four surveys were completed prior to the US Supreme Court Dobbs decision overturning Roe v. Wade.
Of responses from 108 participants, the most common diagnoses were juvenile idiopathic arthritis (52%) and systemic lupus erythematosus (16%). About one third (36%) of patients were on teratogenic medication, with the most common being methotrexate. About three fourths (76%) were White, and the average age of respondents was 16.7.
Most participants (82%) said they had been asked about sexual activity by a health care provider, but only 38% said their pediatric rheumatologist discussed this topic with them. Of the 39 patients on teratogenic medication, 54% said they had been asked about sexual activity by their pediatric rheumatologist, and only 51% said they had received teratogenicity counseling.
A larger percentage (85%) of this group reported receiving sexual activity screenings by any provider, but there was little difference in counseling about teratogenic medication.
This suggests that this type of risk counseling “is almost exclusively done by (pediatric rheumatologists), if at all,” Dr. Huynh noted during her presentation.
In total, 56% of all patients said a provider had talked to them about how to prevent pregnancy, and 20% said they had been counseled about how to get and use emergency contraception. Only 6% of patients said their pediatric rheumatologist had discussed emergency contraception during appointments.
Although sexual activity screenings were associated with current teratogen use, pregnancy prevention counseling and emergency contraceptive counseling were not associated with teratogen use or reported sexual activity.
The survey also revealed that there were gaps in knowledge about the health effects of rheumatic medication. Of the patients on teratogens, 38% did not know that their medication could harm a fetus if they became pregnant. Only 9% of patients not on teratogens correctly answered that their medication would not harm a fetus.
Previous studies have also shown that rheumatology patients do not know that their medications can be teratogenic, noted Cuoghi Edens, MD, a rheumatologist at the University of Chicago, who sees both adult and pediatric patients. She was not involved with the study. The larger challenge is how to best educate patients, she said.
While hopefully a patient’s primary care provider is discussing these issues with them, these patients often see their rheumatologist more frequently and more consistently than other providers, Dr. Edens said.
“We are sometimes the continuity of care for the patient versus their primary care, even though it should be a group effort of trying to some of these questions,” she said.
Conducting reproductive health screenings in pediatric rheumatology clinics can be difficult though, Dr. Edens noted, not only because of time constraints but also because parents often attend appointments with their child and likely have been for years. These screenings are most accurate when done one-on-one, so pivoting and removing the parents from the room can be awkward for providers, Dr. Edens said.
She advised that starting these conversations early on can be one way to ease into talking about reproductive health. In her own practice, Dr. Huynh sets aside time during appointments to speak with adolescent patients privately.
“We always discuss teratogenic medication. I always talk to them about the fact that I’m going to be doing pregnancy testing with their other screening labs because of the risks associated,” she said. “I also specifically set time aside for patients on teratogens to talk about emergency contraception and offer a prescription, if they’re interested.”
Dr. Huynh emphasized that providing easy access to emergency contraception is key. The ACR reproductive health guidelines — although geared toward adults — recommend discussing emergency contraception with patients, and Dr. Huynh advocates writing prescriptions for interested patients.
“They can fill it and have it easily accessible, so that there are no additional barriers, particularly for people who have these higher risks,” she said.
While emergency contraceptives are also available over the counter, it can be awkward for young people to ask for them, she said, and they can be expensive if not covered under insurance. Providing a prescription is one way to avoid those issues, Dr. Huynh said.
“Certainly, you have to have some parent buy-in, because if there is going to be a script, it’s probably going to be under insurance,” she said. “But in my experience, parents are happy to have it around as long as you’re talking it through with them as well as the young person.”
Dr. Huynh and Dr. Edens had no disclosures.
A version of this article appeared on Medscape.com.
FROM ACR 2023
Supercharge your medical practice with ChatGPT: Here’s why you should upgrade
Artificial intelligence (AI) has already demonstrated its potential in various areas of healthcare, from early disease detection and drug discovery to genomics and personalized care. OpenAI’s ChatGPT, a large language model, is one AI tool that has been transforming practices across the globe, including mine.
Let me walk you through it.
ChatGPT is essentially an AI-fueled assistant, capable of interpreting and generating human-like text in response to user inputs. Imagine a well-informed and competent trainee working with you, ready to tackle tasks from handling patient inquiries to summarizing intricate medical literature.
Currently, ChatGPT works on the “freemium” pricing model; there is a free version built upon GPT-3.5 as well as a subscription “ChatGPT Plus” version based on GPT-4 which offers additional features such as the use of third-party plug-ins.
Now, you may ask, “Isn’t the free version enough?” The free version is indeed impressive, but upgrading to the paid version for $20 per month unlocks the full potential of this tool, particularly if we add plug-ins.
Here are some of the best ways to incorporate ChatGPT Plus into your practice.
Time saver and efficiency multiplier. The paid version of ChatGPT is an extraordinary time-saving tool. It can help you sort through vast amounts of medical literature in a fraction of the time it would normally take. Imagine having to sift through hundreds of articles to find the latest research relevant to a patient’s case. With the paid version of ChatGPT, you can simply ask it to provide summaries of the most recent and relevant studies, all in seconds.
Did you forget about that PowerPoint you need to make but know the potential papers you would use? No problem. ChatGPT can create slides in a few minutes. It becomes your on-demand research assistant.
Of course, you need to provide the source you find most relevant to you. Using plug-ins such as ScholarAI and Link Reader are great.
Improved patient communication. Explaining complex medical terminology and procedures to patients can sometimes be a challenge. ChatGPT can generate simplified and personalized explanations for your patients, fostering their understanding and involvement in their care process.
Epic is currently collaborating with Nuance Communications, Microsoft’s speech recognition subsidiary, to use generative AI tools for medical note-taking in the electronic health record. However, you do not need to wait for it; it just takes a prompt in ChatGPT and then copying/pasting the results into the chart.
Smoother administrative management. The premium version of ChatGPT can automate administrative tasks such as creating letters of medical necessity, clearance to other physicians for services, or even communications to staff on specific topics. This frees you to focus more on your core work: providing patient care.
Precision medicine aid. ChatGPT can be a powerful ally in the field of precision medicine. Its capabilities for analyzing large datasets and unearthing valuable insights can help deliver more personalized and potentially effective treatment plans. For example, one can prompt ChatGPT to query the reported frequency of certain genomic variants and their implications; with the upgraded version and plug-ins, the results will have fewer hallucinations — inaccurate results — and key data references.
Unlimited accessibility. Uninterrupted access is a compelling reason to upgrade. While the free version may have usage limitations, the premium version provides unrestricted, round-the-clock access. Be it a late-night research quest or an early-morning patient query, your AI assistant will always be available.
Strengthened privacy and security. The premium version of ChatGPT includes heightened privacy and security measures. Just make sure to follow HIPAA and not include identifiers when making queries.
Embracing AI tools like ChatGPT in your practice can help you stay at the cutting edge of medical care, saving you time, enhancing patient communication, and supporting you in providing personalized care.
While the free version can serve as a good starting point (there are apps for both iOS and Android), upgrading to the paid version opens up a world of possibilities that can truly supercharge your practice.
I would love to hear your comments on this column or on future topics. Contact me at [email protected].
Arturo Loaiza-Bonilla, MD, MSEd, is the cofounder and chief medical officer at Massive Bio, a company connecting patients to clinical trials using artificial intelligence. His research and professional interests focus on precision medicine, clinical trial design, digital health, entrepreneurship, and patient advocacy. Dr. Loaiza-Bonilla is Assistant Professor of Medicine, Drexel University School of Medicine, Philadelphia, Pennsylvania, and serves as medical director of oncology research at Capital Health in New Jersey, where he maintains a connection to patient care by attending to patients 2 days a week. He has financial relationships with Verify, PSI CRO, Bayer, AstraZeneca, Cardinal Health, BrightInsight, The Lynx Group, Fresenius, Pfizer, Ipsen, Guardant, Amgen, Eisai, Natera, Merck, and Bristol Myers Squibb.
A version of this article appeared on Medscape.com.
Artificial intelligence (AI) has already demonstrated its potential in various areas of healthcare, from early disease detection and drug discovery to genomics and personalized care. OpenAI’s ChatGPT, a large language model, is one AI tool that has been transforming practices across the globe, including mine.
Let me walk you through it.
ChatGPT is essentially an AI-fueled assistant, capable of interpreting and generating human-like text in response to user inputs. Imagine a well-informed and competent trainee working with you, ready to tackle tasks from handling patient inquiries to summarizing intricate medical literature.
Currently, ChatGPT works on the “freemium” pricing model; there is a free version built upon GPT-3.5 as well as a subscription “ChatGPT Plus” version based on GPT-4 which offers additional features such as the use of third-party plug-ins.
Now, you may ask, “Isn’t the free version enough?” The free version is indeed impressive, but upgrading to the paid version for $20 per month unlocks the full potential of this tool, particularly if we add plug-ins.
Here are some of the best ways to incorporate ChatGPT Plus into your practice.
Time saver and efficiency multiplier. The paid version of ChatGPT is an extraordinary time-saving tool. It can help you sort through vast amounts of medical literature in a fraction of the time it would normally take. Imagine having to sift through hundreds of articles to find the latest research relevant to a patient’s case. With the paid version of ChatGPT, you can simply ask it to provide summaries of the most recent and relevant studies, all in seconds.
Did you forget about that PowerPoint you need to make but know the potential papers you would use? No problem. ChatGPT can create slides in a few minutes. It becomes your on-demand research assistant.
Of course, you need to provide the source you find most relevant to you. Using plug-ins such as ScholarAI and Link Reader are great.
Improved patient communication. Explaining complex medical terminology and procedures to patients can sometimes be a challenge. ChatGPT can generate simplified and personalized explanations for your patients, fostering their understanding and involvement in their care process.
Epic is currently collaborating with Nuance Communications, Microsoft’s speech recognition subsidiary, to use generative AI tools for medical note-taking in the electronic health record. However, you do not need to wait for it; it just takes a prompt in ChatGPT and then copying/pasting the results into the chart.
Smoother administrative management. The premium version of ChatGPT can automate administrative tasks such as creating letters of medical necessity, clearance to other physicians for services, or even communications to staff on specific topics. This frees you to focus more on your core work: providing patient care.
Precision medicine aid. ChatGPT can be a powerful ally in the field of precision medicine. Its capabilities for analyzing large datasets and unearthing valuable insights can help deliver more personalized and potentially effective treatment plans. For example, one can prompt ChatGPT to query the reported frequency of certain genomic variants and their implications; with the upgraded version and plug-ins, the results will have fewer hallucinations — inaccurate results — and key data references.
Unlimited accessibility. Uninterrupted access is a compelling reason to upgrade. While the free version may have usage limitations, the premium version provides unrestricted, round-the-clock access. Be it a late-night research quest or an early-morning patient query, your AI assistant will always be available.
Strengthened privacy and security. The premium version of ChatGPT includes heightened privacy and security measures. Just make sure to follow HIPAA and not include identifiers when making queries.
Embracing AI tools like ChatGPT in your practice can help you stay at the cutting edge of medical care, saving you time, enhancing patient communication, and supporting you in providing personalized care.
While the free version can serve as a good starting point (there are apps for both iOS and Android), upgrading to the paid version opens up a world of possibilities that can truly supercharge your practice.
I would love to hear your comments on this column or on future topics. Contact me at [email protected].
Arturo Loaiza-Bonilla, MD, MSEd, is the cofounder and chief medical officer at Massive Bio, a company connecting patients to clinical trials using artificial intelligence. His research and professional interests focus on precision medicine, clinical trial design, digital health, entrepreneurship, and patient advocacy. Dr. Loaiza-Bonilla is Assistant Professor of Medicine, Drexel University School of Medicine, Philadelphia, Pennsylvania, and serves as medical director of oncology research at Capital Health in New Jersey, where he maintains a connection to patient care by attending to patients 2 days a week. He has financial relationships with Verify, PSI CRO, Bayer, AstraZeneca, Cardinal Health, BrightInsight, The Lynx Group, Fresenius, Pfizer, Ipsen, Guardant, Amgen, Eisai, Natera, Merck, and Bristol Myers Squibb.
A version of this article appeared on Medscape.com.
Artificial intelligence (AI) has already demonstrated its potential in various areas of healthcare, from early disease detection and drug discovery to genomics and personalized care. OpenAI’s ChatGPT, a large language model, is one AI tool that has been transforming practices across the globe, including mine.
Let me walk you through it.
ChatGPT is essentially an AI-fueled assistant, capable of interpreting and generating human-like text in response to user inputs. Imagine a well-informed and competent trainee working with you, ready to tackle tasks from handling patient inquiries to summarizing intricate medical literature.
Currently, ChatGPT works on the “freemium” pricing model; there is a free version built upon GPT-3.5 as well as a subscription “ChatGPT Plus” version based on GPT-4 which offers additional features such as the use of third-party plug-ins.
Now, you may ask, “Isn’t the free version enough?” The free version is indeed impressive, but upgrading to the paid version for $20 per month unlocks the full potential of this tool, particularly if we add plug-ins.
Here are some of the best ways to incorporate ChatGPT Plus into your practice.
Time saver and efficiency multiplier. The paid version of ChatGPT is an extraordinary time-saving tool. It can help you sort through vast amounts of medical literature in a fraction of the time it would normally take. Imagine having to sift through hundreds of articles to find the latest research relevant to a patient’s case. With the paid version of ChatGPT, you can simply ask it to provide summaries of the most recent and relevant studies, all in seconds.
Did you forget about that PowerPoint you need to make but know the potential papers you would use? No problem. ChatGPT can create slides in a few minutes. It becomes your on-demand research assistant.
Of course, you need to provide the source you find most relevant to you. Using plug-ins such as ScholarAI and Link Reader are great.
Improved patient communication. Explaining complex medical terminology and procedures to patients can sometimes be a challenge. ChatGPT can generate simplified and personalized explanations for your patients, fostering their understanding and involvement in their care process.
Epic is currently collaborating with Nuance Communications, Microsoft’s speech recognition subsidiary, to use generative AI tools for medical note-taking in the electronic health record. However, you do not need to wait for it; it just takes a prompt in ChatGPT and then copying/pasting the results into the chart.
Smoother administrative management. The premium version of ChatGPT can automate administrative tasks such as creating letters of medical necessity, clearance to other physicians for services, or even communications to staff on specific topics. This frees you to focus more on your core work: providing patient care.
Precision medicine aid. ChatGPT can be a powerful ally in the field of precision medicine. Its capabilities for analyzing large datasets and unearthing valuable insights can help deliver more personalized and potentially effective treatment plans. For example, one can prompt ChatGPT to query the reported frequency of certain genomic variants and their implications; with the upgraded version and plug-ins, the results will have fewer hallucinations — inaccurate results — and key data references.
Unlimited accessibility. Uninterrupted access is a compelling reason to upgrade. While the free version may have usage limitations, the premium version provides unrestricted, round-the-clock access. Be it a late-night research quest or an early-morning patient query, your AI assistant will always be available.
Strengthened privacy and security. The premium version of ChatGPT includes heightened privacy and security measures. Just make sure to follow HIPAA and not include identifiers when making queries.
Embracing AI tools like ChatGPT in your practice can help you stay at the cutting edge of medical care, saving you time, enhancing patient communication, and supporting you in providing personalized care.
While the free version can serve as a good starting point (there are apps for both iOS and Android), upgrading to the paid version opens up a world of possibilities that can truly supercharge your practice.
I would love to hear your comments on this column or on future topics. Contact me at [email protected].
Arturo Loaiza-Bonilla, MD, MSEd, is the cofounder and chief medical officer at Massive Bio, a company connecting patients to clinical trials using artificial intelligence. His research and professional interests focus on precision medicine, clinical trial design, digital health, entrepreneurship, and patient advocacy. Dr. Loaiza-Bonilla is Assistant Professor of Medicine, Drexel University School of Medicine, Philadelphia, Pennsylvania, and serves as medical director of oncology research at Capital Health in New Jersey, where he maintains a connection to patient care by attending to patients 2 days a week. He has financial relationships with Verify, PSI CRO, Bayer, AstraZeneca, Cardinal Health, BrightInsight, The Lynx Group, Fresenius, Pfizer, Ipsen, Guardant, Amgen, Eisai, Natera, Merck, and Bristol Myers Squibb.
A version of this article appeared on Medscape.com.
What if a single GLP-1 shot could last for months?
As revolutionary as glucagon-like peptide 1 (GLP-1) drugs are, they still last for only so long in the body. Patients with diabetes typically must be injected once or twice a day (liraglutide) or once a week (semaglutide). This could hinder proper diabetes management, as adherence tends to go down the more frequent the dose.
But what if a single GLP-1 injection could last for 4 months?
“melts away like a sugar cube dissolving in water, molecule by molecule,” said Eric Appel, PhD, the project’s principal investigator and an associate professor of materials science and engineering at Stanford (Calif.) University.
So far, the team has tested the new drug delivery system in rats, and they say human clinical trials could start within 2 years.
Mathematical modeling indicated that one shot of liraglutide could maintain exposure in humans for 120 days, or about 4 months, according to their study in Cell Reports Medicine.
“Patient adherence is of critical importance to diabetes care,” said Alex Abramson, PhD, assistant professor in the chemical and biomolecular engineering department at Georgia Tech, who was not involved in the study. “It’s very exciting to have a potential new system that can last 4 months on a single injection.”
Long-Acting Injectables Have Come a Long Way
The first long-acting injectable — Lupron Depot, a monthly treatment for advanced prostate cancer — was approved in 1989. Since then, long-acting injectable depots have revolutionized the treatment and management of conditions ranging from osteoarthritis knee pain to schizophrenia to opioid use disorder. In 2021, the US Food and Drug Administration approved Apretude — an injectable treatment for HIV pre-exposure prevention that needs to be given every 2 months, compared with daily for the pill equivalent. Other new and innovative developments are underway: Researchers at the University of Connecticut are working on a transdermal microneedle patch — with many tiny vaccine-loaded needles — that could provide multiple doses of a vaccine over time, no boosters needed.
At Stanford, Appel’s lab has spent years developing gels for drug delivery. His team uses a class of hydrogel called polymer-nanoparticle (PNP), which features weakly bound polymers and nanoparticles that can dissipate slowly over time.
The goal is to address a longstanding challenge with long-acting formulations: Achieving steady release. Because the hydrogel is “self-healing” — able to repair damages and restore its shape — it’s less likely to burst and release its drug cargo too early.
“Our PNP hydrogels possess a number of really unique characteristics,” Dr. Appel said. They have “excellent” biocompatibility, based on animal studies, and could work with a wide range of drugs. In proof-of-concept mouse studies, Dr. Appel and his team have shown that these hydrogels could also be used to make vaccines last longer, ferry cancer immunotherapies directly to tumors, and deliver antibodies for the prevention of infectious diseases like SARS-CoV-2.
Though the recent study on GLP-1s focused on treating type 2 diabetes, the same formulation could also be used to treat obesity, said Dr. Appel.
The researchers tested the tech using two GLP-1 receptor agonists — semaglutide and liraglutide. In rats, one shot maintained therapeutic serum concentrations of semaglutide or liraglutide over 42 days. With semaglutide, a significant portion was released quickly, followed by controlled release. Liraglutide, on the other hand, was released gradually as the hydrogel dissolved. This suggests the liraglutide hydrogel may be better tolerated, as a sudden peak in drug serum concentration is associated with adverse effects.
The researchers used pharmacokinetic modeling to predict how liraglutide would behave in humans with a larger injection volume, finding that a single dose could maintain therapeutic levels for about 4 months.
“Moving forward, it will be important to determine whether a burst release from the formulation causes any side effects,” Dr. Abramson noted. “Furthermore, it will be important to minimize the injection volumes in humans.”
But first, more studies in larger animals are needed. Next, Dr. Appel and his team plan to test the technology in pigs, whose skin and endocrine systems are most like humans’. If those trials go well, Dr. Appel said, human clinical trials could start within 2 years.
A version of this article appeared on Medscape.com.
As revolutionary as glucagon-like peptide 1 (GLP-1) drugs are, they still last for only so long in the body. Patients with diabetes typically must be injected once or twice a day (liraglutide) or once a week (semaglutide). This could hinder proper diabetes management, as adherence tends to go down the more frequent the dose.
But what if a single GLP-1 injection could last for 4 months?
“melts away like a sugar cube dissolving in water, molecule by molecule,” said Eric Appel, PhD, the project’s principal investigator and an associate professor of materials science and engineering at Stanford (Calif.) University.
So far, the team has tested the new drug delivery system in rats, and they say human clinical trials could start within 2 years.
Mathematical modeling indicated that one shot of liraglutide could maintain exposure in humans for 120 days, or about 4 months, according to their study in Cell Reports Medicine.
“Patient adherence is of critical importance to diabetes care,” said Alex Abramson, PhD, assistant professor in the chemical and biomolecular engineering department at Georgia Tech, who was not involved in the study. “It’s very exciting to have a potential new system that can last 4 months on a single injection.”
Long-Acting Injectables Have Come a Long Way
The first long-acting injectable — Lupron Depot, a monthly treatment for advanced prostate cancer — was approved in 1989. Since then, long-acting injectable depots have revolutionized the treatment and management of conditions ranging from osteoarthritis knee pain to schizophrenia to opioid use disorder. In 2021, the US Food and Drug Administration approved Apretude — an injectable treatment for HIV pre-exposure prevention that needs to be given every 2 months, compared with daily for the pill equivalent. Other new and innovative developments are underway: Researchers at the University of Connecticut are working on a transdermal microneedle patch — with many tiny vaccine-loaded needles — that could provide multiple doses of a vaccine over time, no boosters needed.
At Stanford, Appel’s lab has spent years developing gels for drug delivery. His team uses a class of hydrogel called polymer-nanoparticle (PNP), which features weakly bound polymers and nanoparticles that can dissipate slowly over time.
The goal is to address a longstanding challenge with long-acting formulations: Achieving steady release. Because the hydrogel is “self-healing” — able to repair damages and restore its shape — it’s less likely to burst and release its drug cargo too early.
“Our PNP hydrogels possess a number of really unique characteristics,” Dr. Appel said. They have “excellent” biocompatibility, based on animal studies, and could work with a wide range of drugs. In proof-of-concept mouse studies, Dr. Appel and his team have shown that these hydrogels could also be used to make vaccines last longer, ferry cancer immunotherapies directly to tumors, and deliver antibodies for the prevention of infectious diseases like SARS-CoV-2.
Though the recent study on GLP-1s focused on treating type 2 diabetes, the same formulation could also be used to treat obesity, said Dr. Appel.
The researchers tested the tech using two GLP-1 receptor agonists — semaglutide and liraglutide. In rats, one shot maintained therapeutic serum concentrations of semaglutide or liraglutide over 42 days. With semaglutide, a significant portion was released quickly, followed by controlled release. Liraglutide, on the other hand, was released gradually as the hydrogel dissolved. This suggests the liraglutide hydrogel may be better tolerated, as a sudden peak in drug serum concentration is associated with adverse effects.
The researchers used pharmacokinetic modeling to predict how liraglutide would behave in humans with a larger injection volume, finding that a single dose could maintain therapeutic levels for about 4 months.
“Moving forward, it will be important to determine whether a burst release from the formulation causes any side effects,” Dr. Abramson noted. “Furthermore, it will be important to minimize the injection volumes in humans.”
But first, more studies in larger animals are needed. Next, Dr. Appel and his team plan to test the technology in pigs, whose skin and endocrine systems are most like humans’. If those trials go well, Dr. Appel said, human clinical trials could start within 2 years.
A version of this article appeared on Medscape.com.
As revolutionary as glucagon-like peptide 1 (GLP-1) drugs are, they still last for only so long in the body. Patients with diabetes typically must be injected once or twice a day (liraglutide) or once a week (semaglutide). This could hinder proper diabetes management, as adherence tends to go down the more frequent the dose.
But what if a single GLP-1 injection could last for 4 months?
“melts away like a sugar cube dissolving in water, molecule by molecule,” said Eric Appel, PhD, the project’s principal investigator and an associate professor of materials science and engineering at Stanford (Calif.) University.
So far, the team has tested the new drug delivery system in rats, and they say human clinical trials could start within 2 years.
Mathematical modeling indicated that one shot of liraglutide could maintain exposure in humans for 120 days, or about 4 months, according to their study in Cell Reports Medicine.
“Patient adherence is of critical importance to diabetes care,” said Alex Abramson, PhD, assistant professor in the chemical and biomolecular engineering department at Georgia Tech, who was not involved in the study. “It’s very exciting to have a potential new system that can last 4 months on a single injection.”
Long-Acting Injectables Have Come a Long Way
The first long-acting injectable — Lupron Depot, a monthly treatment for advanced prostate cancer — was approved in 1989. Since then, long-acting injectable depots have revolutionized the treatment and management of conditions ranging from osteoarthritis knee pain to schizophrenia to opioid use disorder. In 2021, the US Food and Drug Administration approved Apretude — an injectable treatment for HIV pre-exposure prevention that needs to be given every 2 months, compared with daily for the pill equivalent. Other new and innovative developments are underway: Researchers at the University of Connecticut are working on a transdermal microneedle patch — with many tiny vaccine-loaded needles — that could provide multiple doses of a vaccine over time, no boosters needed.
At Stanford, Appel’s lab has spent years developing gels for drug delivery. His team uses a class of hydrogel called polymer-nanoparticle (PNP), which features weakly bound polymers and nanoparticles that can dissipate slowly over time.
The goal is to address a longstanding challenge with long-acting formulations: Achieving steady release. Because the hydrogel is “self-healing” — able to repair damages and restore its shape — it’s less likely to burst and release its drug cargo too early.
“Our PNP hydrogels possess a number of really unique characteristics,” Dr. Appel said. They have “excellent” biocompatibility, based on animal studies, and could work with a wide range of drugs. In proof-of-concept mouse studies, Dr. Appel and his team have shown that these hydrogels could also be used to make vaccines last longer, ferry cancer immunotherapies directly to tumors, and deliver antibodies for the prevention of infectious diseases like SARS-CoV-2.
Though the recent study on GLP-1s focused on treating type 2 diabetes, the same formulation could also be used to treat obesity, said Dr. Appel.
The researchers tested the tech using two GLP-1 receptor agonists — semaglutide and liraglutide. In rats, one shot maintained therapeutic serum concentrations of semaglutide or liraglutide over 42 days. With semaglutide, a significant portion was released quickly, followed by controlled release. Liraglutide, on the other hand, was released gradually as the hydrogel dissolved. This suggests the liraglutide hydrogel may be better tolerated, as a sudden peak in drug serum concentration is associated with adverse effects.
The researchers used pharmacokinetic modeling to predict how liraglutide would behave in humans with a larger injection volume, finding that a single dose could maintain therapeutic levels for about 4 months.
“Moving forward, it will be important to determine whether a burst release from the formulation causes any side effects,” Dr. Abramson noted. “Furthermore, it will be important to minimize the injection volumes in humans.”
But first, more studies in larger animals are needed. Next, Dr. Appel and his team plan to test the technology in pigs, whose skin and endocrine systems are most like humans’. If those trials go well, Dr. Appel said, human clinical trials could start within 2 years.
A version of this article appeared on Medscape.com.
FROM CELL REPORTS MEDICINE
How to prescribe Zepbound
December marks the advent of the approval of tirzepatide (Zepbound) for on-label treatment of obesity. In November 2023, the US Food and Drug Administration (FDA) approved it for the treatment of obesity in adults.
In May 2022, the FDA approved Mounjaro, which is tirzepatide, for type 2 diabetes. Since then, many physicians, including myself, have prescribed it off-label for obesity. As an endocrinologist treating both obesity and diabetes, 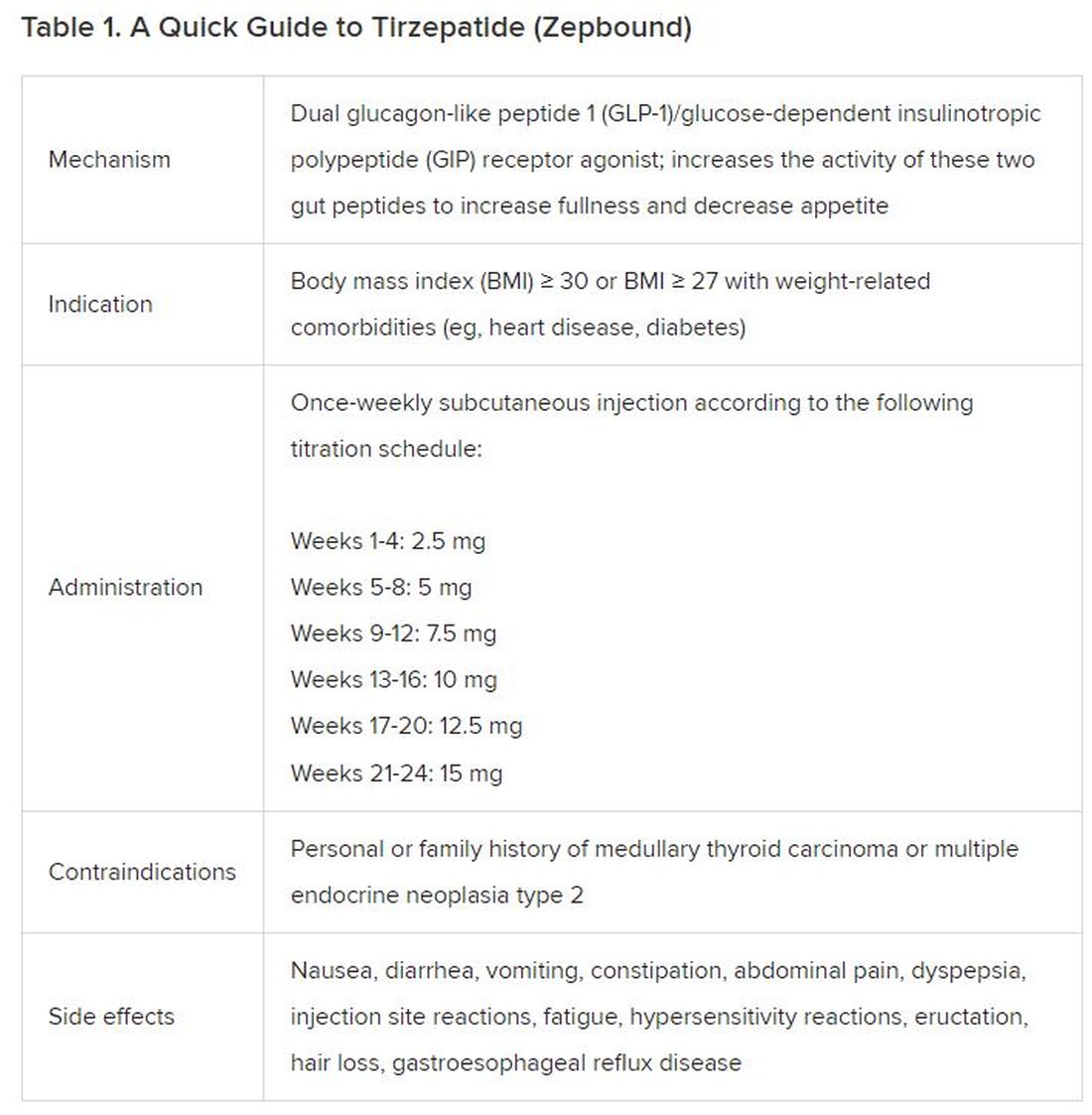
The Expertise
Because GLP-1 receptor agonists have been around since 2005, we’ve had over a decade of clinical experience with these medications. Table 2 provides more nuanced information on tirzepatide (as Zepbound, for obesity) based on our experiences with dulaglutide, liraglutide, semaglutide, and tirzepatide (as Mounjaro).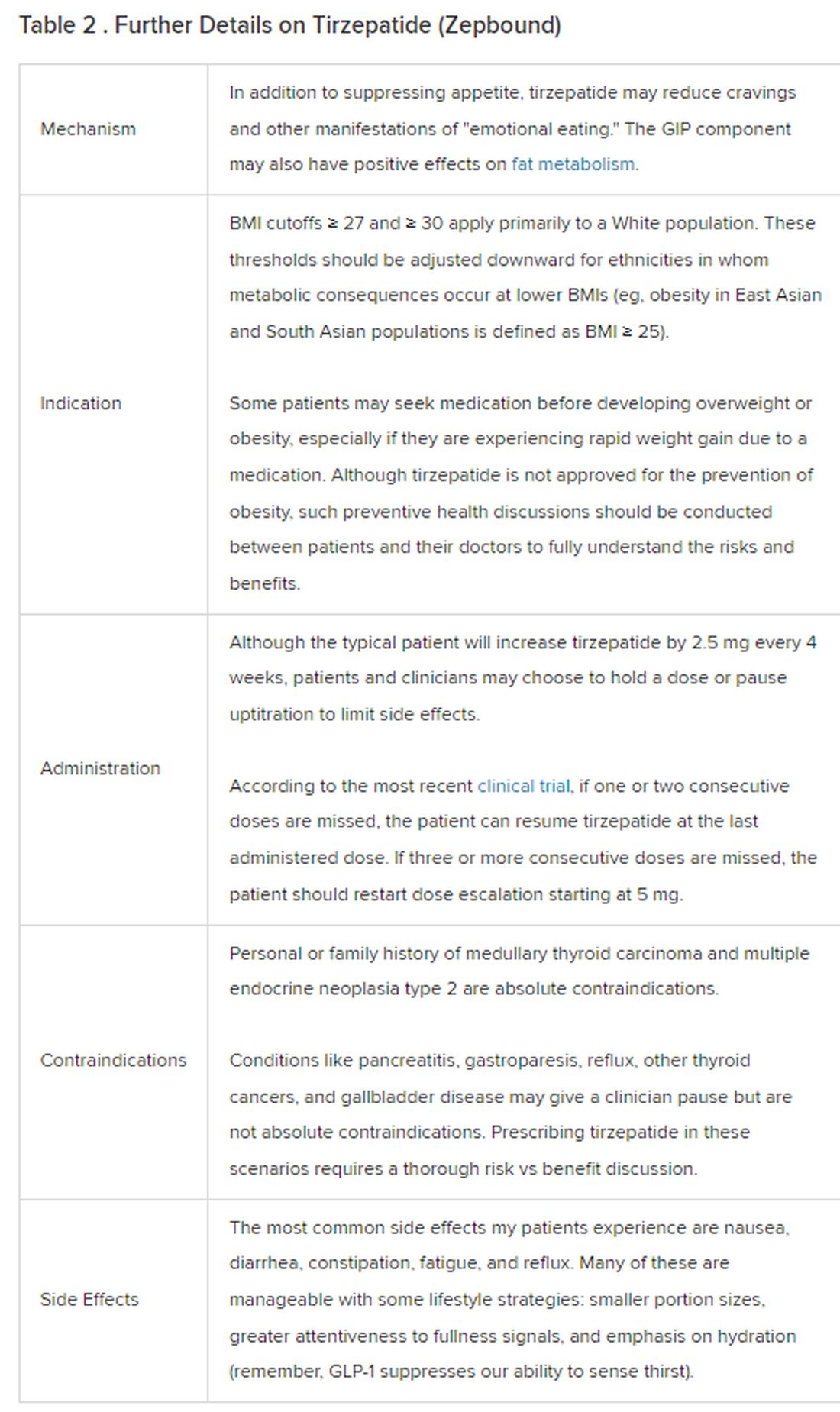
The Reality
In today’s increasingly complex healthcare system, the reality of providing high-quality obesity care is challenging. When discussing tirzepatide with patients, I use a 4 Cs schematic — comorbidities, cautions, costs, choices — to cover the most frequently asked questions.
Comorbidities
In trials, tirzepatide reduced A1c by about 2%. In one diabetes trial, tirzepatide reduced liver fat content significantly more than the comparator (insulin), and trials of tirzepatide in nonalcoholic steatohepatitis are ongoing. A prespecified meta-analysis of tirzepatide and cardiovascular disease estimated a 20% reduction in the risk for cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina. Tirzepatide as well as other GLP-1 agonists may be beneficial in alcohol use disorder. Prescribing tirzepatide to patients who have or are at risk of developing such comorbidities is an ideal way to target multiple metabolic diseases with one agent.
Cautions
The first principle of medicine is “do no harm.” Tirzepatide may be a poor option for individuals with a history of pancreatitis, gastroparesis, or severe gastroesophageal reflux disease. Because tirzepatide may interfere with the efficacy of estrogen-containing contraceptives during its uptitration phase, women should speak with their doctors about appropriate birth control options (eg, progestin-only, barrier methods). In clinical trials of tirzepatide, male participants were also advised to use reliable contraception. If patients are family-planning, tirzepatide should be discontinued 2 months (for women) and 4 months (for men) before conception, because its effects on fertility or pregnancy are currently unknown.
Costs
At a retail price of $1279 per month, Zepbound is only slightly more affordable than its main competitor, Wegovy (semaglutide 2.4 mg). Complex pharmacy negotiations may reduce this cost, but even with rebates, coupons, and commercial insurance, these costs still place tirzepatide out of reach for many patients. For patients who cannot access tirzepatide, clinicians should discuss more cost-feasible, evidence-based alternatives: for example, phentermine, phentermine-topiramate, naltrexone-bupropion, metformin, bupropion, or topiramate.
Choices
Patient preference drives much of today’s clinical decision-making. Some patients may be switching from semaglutide to tirzepatide, whether by choice or on the basis of physician recommendation. Although no head-to-head obesity trial exists, data from SURPASS-2 and SUSTAIN-FORTE can inform therapeutic equivalence:
- Semaglutide 1.0 mg to tirzepatide 2.5 mg will be a step-down; 5 mg will be a step-up
- Semaglutide 2.0 or 2.4 mg to tirzepatide 5 mg is probably equivalent
The decision to switch therapeutics may depend on weight loss goals, side effect tolerability, or insurance coverage. As with all medications, the use of tirzepatide should progress with shared decision-making, thorough discussions of risks vs benefits, and individualized regimens tailored to each patient’s needs.
The newly approved Zepbound is a valuable addition to our toolbox of obesity treatments. Patients and providers alike are excited for its potential as a highly effective antiobesity medication that can cause a degree of weight loss necessary to reverse comorbidities. The medical management of obesity with agents like tirzepatide holds great promise in addressing today’s obesity epidemic.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties to Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
December marks the advent of the approval of tirzepatide (Zepbound) for on-label treatment of obesity. In November 2023, the US Food and Drug Administration (FDA) approved it for the treatment of obesity in adults.
In May 2022, the FDA approved Mounjaro, which is tirzepatide, for type 2 diabetes. Since then, many physicians, including myself, have prescribed it off-label for obesity. As an endocrinologist treating both obesity and diabetes, 
The Expertise
Because GLP-1 receptor agonists have been around since 2005, we’ve had over a decade of clinical experience with these medications. Table 2 provides more nuanced information on tirzepatide (as Zepbound, for obesity) based on our experiences with dulaglutide, liraglutide, semaglutide, and tirzepatide (as Mounjaro).
The Reality
In today’s increasingly complex healthcare system, the reality of providing high-quality obesity care is challenging. When discussing tirzepatide with patients, I use a 4 Cs schematic — comorbidities, cautions, costs, choices — to cover the most frequently asked questions.
Comorbidities
In trials, tirzepatide reduced A1c by about 2%. In one diabetes trial, tirzepatide reduced liver fat content significantly more than the comparator (insulin), and trials of tirzepatide in nonalcoholic steatohepatitis are ongoing. A prespecified meta-analysis of tirzepatide and cardiovascular disease estimated a 20% reduction in the risk for cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina. Tirzepatide as well as other GLP-1 agonists may be beneficial in alcohol use disorder. Prescribing tirzepatide to patients who have or are at risk of developing such comorbidities is an ideal way to target multiple metabolic diseases with one agent.
Cautions
The first principle of medicine is “do no harm.” Tirzepatide may be a poor option for individuals with a history of pancreatitis, gastroparesis, or severe gastroesophageal reflux disease. Because tirzepatide may interfere with the efficacy of estrogen-containing contraceptives during its uptitration phase, women should speak with their doctors about appropriate birth control options (eg, progestin-only, barrier methods). In clinical trials of tirzepatide, male participants were also advised to use reliable contraception. If patients are family-planning, tirzepatide should be discontinued 2 months (for women) and 4 months (for men) before conception, because its effects on fertility or pregnancy are currently unknown.
Costs
At a retail price of $1279 per month, Zepbound is only slightly more affordable than its main competitor, Wegovy (semaglutide 2.4 mg). Complex pharmacy negotiations may reduce this cost, but even with rebates, coupons, and commercial insurance, these costs still place tirzepatide out of reach for many patients. For patients who cannot access tirzepatide, clinicians should discuss more cost-feasible, evidence-based alternatives: for example, phentermine, phentermine-topiramate, naltrexone-bupropion, metformin, bupropion, or topiramate.
Choices
Patient preference drives much of today’s clinical decision-making. Some patients may be switching from semaglutide to tirzepatide, whether by choice or on the basis of physician recommendation. Although no head-to-head obesity trial exists, data from SURPASS-2 and SUSTAIN-FORTE can inform therapeutic equivalence:
- Semaglutide 1.0 mg to tirzepatide 2.5 mg will be a step-down; 5 mg will be a step-up
- Semaglutide 2.0 or 2.4 mg to tirzepatide 5 mg is probably equivalent
The decision to switch therapeutics may depend on weight loss goals, side effect tolerability, or insurance coverage. As with all medications, the use of tirzepatide should progress with shared decision-making, thorough discussions of risks vs benefits, and individualized regimens tailored to each patient’s needs.
The newly approved Zepbound is a valuable addition to our toolbox of obesity treatments. Patients and providers alike are excited for its potential as a highly effective antiobesity medication that can cause a degree of weight loss necessary to reverse comorbidities. The medical management of obesity with agents like tirzepatide holds great promise in addressing today’s obesity epidemic.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties to Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
December marks the advent of the approval of tirzepatide (Zepbound) for on-label treatment of obesity. In November 2023, the US Food and Drug Administration (FDA) approved it for the treatment of obesity in adults.
In May 2022, the FDA approved Mounjaro, which is tirzepatide, for type 2 diabetes. Since then, many physicians, including myself, have prescribed it off-label for obesity. As an endocrinologist treating both obesity and diabetes, 
The Expertise
Because GLP-1 receptor agonists have been around since 2005, we’ve had over a decade of clinical experience with these medications. Table 2 provides more nuanced information on tirzepatide (as Zepbound, for obesity) based on our experiences with dulaglutide, liraglutide, semaglutide, and tirzepatide (as Mounjaro).
The Reality
In today’s increasingly complex healthcare system, the reality of providing high-quality obesity care is challenging. When discussing tirzepatide with patients, I use a 4 Cs schematic — comorbidities, cautions, costs, choices — to cover the most frequently asked questions.
Comorbidities
In trials, tirzepatide reduced A1c by about 2%. In one diabetes trial, tirzepatide reduced liver fat content significantly more than the comparator (insulin), and trials of tirzepatide in nonalcoholic steatohepatitis are ongoing. A prespecified meta-analysis of tirzepatide and cardiovascular disease estimated a 20% reduction in the risk for cardiovascular death, myocardial infarction, stroke, and hospitalized unstable angina. Tirzepatide as well as other GLP-1 agonists may be beneficial in alcohol use disorder. Prescribing tirzepatide to patients who have or are at risk of developing such comorbidities is an ideal way to target multiple metabolic diseases with one agent.
Cautions
The first principle of medicine is “do no harm.” Tirzepatide may be a poor option for individuals with a history of pancreatitis, gastroparesis, or severe gastroesophageal reflux disease. Because tirzepatide may interfere with the efficacy of estrogen-containing contraceptives during its uptitration phase, women should speak with their doctors about appropriate birth control options (eg, progestin-only, barrier methods). In clinical trials of tirzepatide, male participants were also advised to use reliable contraception. If patients are family-planning, tirzepatide should be discontinued 2 months (for women) and 4 months (for men) before conception, because its effects on fertility or pregnancy are currently unknown.
Costs
At a retail price of $1279 per month, Zepbound is only slightly more affordable than its main competitor, Wegovy (semaglutide 2.4 mg). Complex pharmacy negotiations may reduce this cost, but even with rebates, coupons, and commercial insurance, these costs still place tirzepatide out of reach for many patients. For patients who cannot access tirzepatide, clinicians should discuss more cost-feasible, evidence-based alternatives: for example, phentermine, phentermine-topiramate, naltrexone-bupropion, metformin, bupropion, or topiramate.
Choices
Patient preference drives much of today’s clinical decision-making. Some patients may be switching from semaglutide to tirzepatide, whether by choice or on the basis of physician recommendation. Although no head-to-head obesity trial exists, data from SURPASS-2 and SUSTAIN-FORTE can inform therapeutic equivalence:
- Semaglutide 1.0 mg to tirzepatide 2.5 mg will be a step-down; 5 mg will be a step-up
- Semaglutide 2.0 or 2.4 mg to tirzepatide 5 mg is probably equivalent
The decision to switch therapeutics may depend on weight loss goals, side effect tolerability, or insurance coverage. As with all medications, the use of tirzepatide should progress with shared decision-making, thorough discussions of risks vs benefits, and individualized regimens tailored to each patient’s needs.
The newly approved Zepbound is a valuable addition to our toolbox of obesity treatments. Patients and providers alike are excited for its potential as a highly effective antiobesity medication that can cause a degree of weight loss necessary to reverse comorbidities. The medical management of obesity with agents like tirzepatide holds great promise in addressing today’s obesity epidemic.
Dr. Tchang is Assistant Professor, Clinical Medicine, Division of Endocrinology, Diabetes, and Metabolism, Weill Cornell Medicine; Physician, Department of Medicine, Iris Cantor Women’s Health Center, Comprehensive Weight Control Center, New York, NY. She disclosed ties to Gelesis and Novo Nordisk.
A version of this article appeared on Medscape.com.
Federal program offers free COVID, flu at-home tests, treatments
The U.S. government has expanded a program offering free COVID-19 and flu tests and treatment.
The Home Test to Treat program is virtual and offers at-home rapid tests, telehealth sessions, and at-home treatments to people nationwide. The program is a collaboration among the National Institutes of Health, the Administration for Strategic Preparedness and Response, and the CDC. It began as a pilot program in some locations this year.
“With its expansion, the Home Test to Treat program will now offer free testing, telehealth and treatment for both COVID-19 and for influenza (flu) A and B,” the NIH said in a press release. “It is the first public health program that includes home testing technology at such a scale for both COVID-19 and flu.”
The news release says that anyone 18 or over with a current positive test for COVID-19 or flu can get free telehealth care and medicine delivered to their home.
Adults who don’t have COVID-19 or the flu can get free tests if they are uninsured or are enrolled in Medicare, Medicaid, the Veterans Affairs health care system, or Indian Health Services. If they test positive later, they can get free telehealth care and, if prescribed, treatment.
“I think that these [telehealth] delivery mechanisms are going to be absolutely crucial to unburden the in-person offices and the lines that we have and wait times,” said Michael Mina, MD, chief science officer at eMed, the company that helped implement the new Home Test to Treat program, to ABC News.
ABC notes that COVID tests can also be ordered at covidtests.gov – four tests per household or eight for those who have yet to order any this fall.
A version of this article appeared on WebMD.com .
The U.S. government has expanded a program offering free COVID-19 and flu tests and treatment.
The Home Test to Treat program is virtual and offers at-home rapid tests, telehealth sessions, and at-home treatments to people nationwide. The program is a collaboration among the National Institutes of Health, the Administration for Strategic Preparedness and Response, and the CDC. It began as a pilot program in some locations this year.
“With its expansion, the Home Test to Treat program will now offer free testing, telehealth and treatment for both COVID-19 and for influenza (flu) A and B,” the NIH said in a press release. “It is the first public health program that includes home testing technology at such a scale for both COVID-19 and flu.”
The news release says that anyone 18 or over with a current positive test for COVID-19 or flu can get free telehealth care and medicine delivered to their home.
Adults who don’t have COVID-19 or the flu can get free tests if they are uninsured or are enrolled in Medicare, Medicaid, the Veterans Affairs health care system, or Indian Health Services. If they test positive later, they can get free telehealth care and, if prescribed, treatment.
“I think that these [telehealth] delivery mechanisms are going to be absolutely crucial to unburden the in-person offices and the lines that we have and wait times,” said Michael Mina, MD, chief science officer at eMed, the company that helped implement the new Home Test to Treat program, to ABC News.
ABC notes that COVID tests can also be ordered at covidtests.gov – four tests per household or eight for those who have yet to order any this fall.
A version of this article appeared on WebMD.com .
The U.S. government has expanded a program offering free COVID-19 and flu tests and treatment.
The Home Test to Treat program is virtual and offers at-home rapid tests, telehealth sessions, and at-home treatments to people nationwide. The program is a collaboration among the National Institutes of Health, the Administration for Strategic Preparedness and Response, and the CDC. It began as a pilot program in some locations this year.
“With its expansion, the Home Test to Treat program will now offer free testing, telehealth and treatment for both COVID-19 and for influenza (flu) A and B,” the NIH said in a press release. “It is the first public health program that includes home testing technology at such a scale for both COVID-19 and flu.”
The news release says that anyone 18 or over with a current positive test for COVID-19 or flu can get free telehealth care and medicine delivered to their home.
Adults who don’t have COVID-19 or the flu can get free tests if they are uninsured or are enrolled in Medicare, Medicaid, the Veterans Affairs health care system, or Indian Health Services. If they test positive later, they can get free telehealth care and, if prescribed, treatment.
“I think that these [telehealth] delivery mechanisms are going to be absolutely crucial to unburden the in-person offices and the lines that we have and wait times,” said Michael Mina, MD, chief science officer at eMed, the company that helped implement the new Home Test to Treat program, to ABC News.
ABC notes that COVID tests can also be ordered at covidtests.gov – four tests per household or eight for those who have yet to order any this fall.
A version of this article appeared on WebMD.com .




