User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Maternal perinatal mortality: A pediatric issue
Checking on the well-being of mothers is one of the important acknowledged aspects of primary pediatric care. “How are you doing?” directed to the child’s mother has long been considered an appropriate question. The AAP recommends several checks in the Bright Futures Guidelines, including conducting several formal screens for depression and asking about “getting time alone with your partner” as well as other supports.
But I have recently become aware of new data that changes my ideas about what we pediatricians need to be doing as part of our care for children and their families, especially in the first year: Considering the risks to the mother of dying.
Maternal mortality increased by 26.6% from 2000 to 2014 across the United States such that it is higher now than it was for our own mothers. The U.S. now has the highest rates of maternal mortality among high-income nations, especially for Black, American Indian, or Alaska Native women, those of lower socioeconomic status, and those under 18 or over 35 years old.
You may be thinking, well, that is an issue for ob.gyns. Indeed, the most common reasons for maternal death are cardiovascular: hemorrhage, hypertensive disorders, deep vein thrombosis, and stroke, all usually occurring at or in the first week after birth. You may have heard about sudden unexpected heart failure from postpartum cardiomyopathy, although rare (1 in 1,000-4,000), presenting from 1 month pre birth to 5 months post delivery, which is when we may be the main clinicians seeing the mother, not the ob.gyns. This can be easily missed since it presents with shortness of breath and decreased exercise tolerance, fatigue, palpitations, and/or leg swelling. Serious eclampsia may have only symptoms of headache or abdominal pain. All of these may easily be mistaken for lingering pregnancy symptoms. But in higher income countries, such as the U.S., 38% of maternal deaths occur from 8 to 42 days after birth, the period for fatal infections as well as cardiac complications. Elevated risk for all of these causes of mortality include Black race, obesity, tobacco use, congenital heart disease, and being older than 40.
As pediatric providers, we may see mothers along with their infants as newborns in the hospital, at day 2, at 2 weeks, or even at 1-2 months after birth, potentially before their one recommended postnatal obstetric visit at 3-8 weeks. Asking the mother how she is feeling at those times should not just be a social nicety but rather an additional check for serious postnatal complications.
Additional concerns
But wait, it gets worse.
Did you know that the leading cause of maternal death from pregnancy up to 1 year after a birth is homicide?
Maternal perinatal mortality figures have not usually included “perinatal-associated” deaths, a maternal death attributable to a condition that is unaffected by the pregnancy and occurring within 1 year of delivery (that I will cite as perinatal henceforth). While half of maternal deaths occur during pregnancy, another half occur in the year following. There were 3.62 homicides per 100,000 live births among females who were pregnant or within 1 year postpartum, 16% more than for similarly aged nonpregnant and nonpostpartum women (3.12 deaths/100,000 population, P < .05). Homicides made up 8.4% of reported perinatal maternal deaths from all causes, with a rate of 1.7 per 100,000 live births, twice the rate of any one of the other leading causes noted above. Black women had seven times the risk of perinatal homicide as that of White women. Females under 20, many of them our own pediatric patients, had a greater than six times higher risk and those aged 20-24 had a 65% higher risk of pregnancy-associated homicide across race and ethnic groups. Homicide is most likely before 21 weeks of pregnancy, decreases in the third trimester, but increases again after birth. Two-thirds of pregnancy-associated homicide deaths occurred in the home, with the perpetrator a current or prior partner (> 59%, with 98% being male), 45%-50% were associated with reported intimate partner violence (IPV), and the most common method was a firearm (55%). Often the same women had histories of substance abuse, serious mental illness, and/or prior IPV, all risk factors for pregnancy-associated deaths, including from homicide.
Homicide? “Not the mothers in my practice,” you may say, but, if not homicide, drug-related deaths (3.68 per 100,000 person-years) and suicide (1.42 per 100,000 person-years) together comprise 18% of all maternal deaths. Non-Hispanic White women, Medicaid-insured women, and women residing in smaller cities were especially likely to die from drugs or suicide. More than half (54.3%) of perinatal suicides involve intimate partner conflict, which increases the risk ninefold. Perinatal mood disorders, affecting up to 15% of pregnant and postpartum U.S. women, is also a risk factor in substance abuse, opioid overdose death, and suicide.
And substance use has gotten more dangerous with the increase in fentanyl lacing. Pregnancy-associated deaths (4%-10% of deaths) involving opioids more than doubled between 2007 and 2016, and, although the rates are higher for Black women, the increase has been greater for non-Hispanic White women. Two-thirds of those deaths occur between 6 and 12 months postpartum, on our watch. Although many women decrease substance use during pregnancy, they may fall back into substance use (rates increase 4 times by 7-12 months after delivery) and not continue to receive treatment. Although pharmacotherapy (e.g., methadone, buprenorphine treatment) is the current standard of care for opioid use disorder (OUD) during pregnancy, nearly half receiving treatment in publicly funded centers are not receiving these medications and others may lose insurance or access to pregnancy-related treatment programs after delivery, increasing risk of relapse. Stigma, and punitive or discriminatory approaches to pregnant women with OUD (e.g., jail, removal of children) can dissuade them from participating in treatment, increasing overdose risk.
It is important to note that in more than half of the 41 deaths from violent trauma in one study (including 22 homicides), obstetrical providers knew of or suspected IPV. Also, the vast majority (74%) of those who died by drugs or suicide had made one or more emergency department or hospital visit between their delivery and death, and 39% had made three or more visits. Without knowing if anything was done in those cases, we also know that, in addition to thorough, compassionate providers, there is sometimes segmentation of responsibility, insensitivity, discrimination, racism, stigma, inequity, lack of resources, lack of access, lack of payment mechanisms, legal issues for immigrants, time constraints, and other systemic deficits that may hinder effective care for these and subsequent women.
Awareness and action
What should we, who are primary care pediatric providers, do about these threats to the mothers and pregnant young women we care for? Clearly, their children, our main patients, would be terribly and permanently hurt by harm coming to their mothers – the extreme adverse childhood experiences and social determinants of health to which we are already committed.
I hope this article will help alert pediatric providers to what is being published, mainly as women’s health and public health issues.
First, we need awareness of the physical symptoms that may come up in our interactions with pregnant and postpartum women so that we can educate them and expedite any indicated emergency care.
Next, we need to expand our routine screening of mothers and pregnant women from just the most impactful social determinants of health (including depression, substance use, and IPV) to include anxiety, past suicide attempts and current suicidal ideation, and the presence of firearms, early and repeatedly in the first year of the child’s life. Adults and teens are more likely to disclose risk for sensitive issues through questionnaires than through interviews, perhaps even more so when the identified patient is their child rather than themselves. Any screen can have false negatives, so asking directly when risk is suspected is important. The reason for screening could be framed as caring for the caregiver who is the most important person for the child. It could be accompanied by acknowledging that pregnancy and the first year of life can be difficult for mothers and their partners and that we want to support them and connect them to resources, if needed. When substance use disorder is acknowledged, we should prescribe and teach about Narcan for overdose. When there is IPV, we should discuss firearm removal/locking as well as counseling on a personal safety plan.
Working as part of an on-site or virtual team that includes professionals who know about community resources and can coordinate care is essential, in addition to educating about 211 for services and 988 for suicide risk.
Finally, we can advocate and vote for programs, people, and laws that support and safeguard women and families, address substance use, and reduce access to firearms.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Checking on the well-being of mothers is one of the important acknowledged aspects of primary pediatric care. “How are you doing?” directed to the child’s mother has long been considered an appropriate question. The AAP recommends several checks in the Bright Futures Guidelines, including conducting several formal screens for depression and asking about “getting time alone with your partner” as well as other supports.
But I have recently become aware of new data that changes my ideas about what we pediatricians need to be doing as part of our care for children and their families, especially in the first year: Considering the risks to the mother of dying.
Maternal mortality increased by 26.6% from 2000 to 2014 across the United States such that it is higher now than it was for our own mothers. The U.S. now has the highest rates of maternal mortality among high-income nations, especially for Black, American Indian, or Alaska Native women, those of lower socioeconomic status, and those under 18 or over 35 years old.
You may be thinking, well, that is an issue for ob.gyns. Indeed, the most common reasons for maternal death are cardiovascular: hemorrhage, hypertensive disorders, deep vein thrombosis, and stroke, all usually occurring at or in the first week after birth. You may have heard about sudden unexpected heart failure from postpartum cardiomyopathy, although rare (1 in 1,000-4,000), presenting from 1 month pre birth to 5 months post delivery, which is when we may be the main clinicians seeing the mother, not the ob.gyns. This can be easily missed since it presents with shortness of breath and decreased exercise tolerance, fatigue, palpitations, and/or leg swelling. Serious eclampsia may have only symptoms of headache or abdominal pain. All of these may easily be mistaken for lingering pregnancy symptoms. But in higher income countries, such as the U.S., 38% of maternal deaths occur from 8 to 42 days after birth, the period for fatal infections as well as cardiac complications. Elevated risk for all of these causes of mortality include Black race, obesity, tobacco use, congenital heart disease, and being older than 40.
As pediatric providers, we may see mothers along with their infants as newborns in the hospital, at day 2, at 2 weeks, or even at 1-2 months after birth, potentially before their one recommended postnatal obstetric visit at 3-8 weeks. Asking the mother how she is feeling at those times should not just be a social nicety but rather an additional check for serious postnatal complications.
Additional concerns
But wait, it gets worse.
Did you know that the leading cause of maternal death from pregnancy up to 1 year after a birth is homicide?
Maternal perinatal mortality figures have not usually included “perinatal-associated” deaths, a maternal death attributable to a condition that is unaffected by the pregnancy and occurring within 1 year of delivery (that I will cite as perinatal henceforth). While half of maternal deaths occur during pregnancy, another half occur in the year following. There were 3.62 homicides per 100,000 live births among females who were pregnant or within 1 year postpartum, 16% more than for similarly aged nonpregnant and nonpostpartum women (3.12 deaths/100,000 population, P < .05). Homicides made up 8.4% of reported perinatal maternal deaths from all causes, with a rate of 1.7 per 100,000 live births, twice the rate of any one of the other leading causes noted above. Black women had seven times the risk of perinatal homicide as that of White women. Females under 20, many of them our own pediatric patients, had a greater than six times higher risk and those aged 20-24 had a 65% higher risk of pregnancy-associated homicide across race and ethnic groups. Homicide is most likely before 21 weeks of pregnancy, decreases in the third trimester, but increases again after birth. Two-thirds of pregnancy-associated homicide deaths occurred in the home, with the perpetrator a current or prior partner (> 59%, with 98% being male), 45%-50% were associated with reported intimate partner violence (IPV), and the most common method was a firearm (55%). Often the same women had histories of substance abuse, serious mental illness, and/or prior IPV, all risk factors for pregnancy-associated deaths, including from homicide.
Homicide? “Not the mothers in my practice,” you may say, but, if not homicide, drug-related deaths (3.68 per 100,000 person-years) and suicide (1.42 per 100,000 person-years) together comprise 18% of all maternal deaths. Non-Hispanic White women, Medicaid-insured women, and women residing in smaller cities were especially likely to die from drugs or suicide. More than half (54.3%) of perinatal suicides involve intimate partner conflict, which increases the risk ninefold. Perinatal mood disorders, affecting up to 15% of pregnant and postpartum U.S. women, is also a risk factor in substance abuse, opioid overdose death, and suicide.
And substance use has gotten more dangerous with the increase in fentanyl lacing. Pregnancy-associated deaths (4%-10% of deaths) involving opioids more than doubled between 2007 and 2016, and, although the rates are higher for Black women, the increase has been greater for non-Hispanic White women. Two-thirds of those deaths occur between 6 and 12 months postpartum, on our watch. Although many women decrease substance use during pregnancy, they may fall back into substance use (rates increase 4 times by 7-12 months after delivery) and not continue to receive treatment. Although pharmacotherapy (e.g., methadone, buprenorphine treatment) is the current standard of care for opioid use disorder (OUD) during pregnancy, nearly half receiving treatment in publicly funded centers are not receiving these medications and others may lose insurance or access to pregnancy-related treatment programs after delivery, increasing risk of relapse. Stigma, and punitive or discriminatory approaches to pregnant women with OUD (e.g., jail, removal of children) can dissuade them from participating in treatment, increasing overdose risk.
It is important to note that in more than half of the 41 deaths from violent trauma in one study (including 22 homicides), obstetrical providers knew of or suspected IPV. Also, the vast majority (74%) of those who died by drugs or suicide had made one or more emergency department or hospital visit between their delivery and death, and 39% had made three or more visits. Without knowing if anything was done in those cases, we also know that, in addition to thorough, compassionate providers, there is sometimes segmentation of responsibility, insensitivity, discrimination, racism, stigma, inequity, lack of resources, lack of access, lack of payment mechanisms, legal issues for immigrants, time constraints, and other systemic deficits that may hinder effective care for these and subsequent women.
Awareness and action
What should we, who are primary care pediatric providers, do about these threats to the mothers and pregnant young women we care for? Clearly, their children, our main patients, would be terribly and permanently hurt by harm coming to their mothers – the extreme adverse childhood experiences and social determinants of health to which we are already committed.
I hope this article will help alert pediatric providers to what is being published, mainly as women’s health and public health issues.
First, we need awareness of the physical symptoms that may come up in our interactions with pregnant and postpartum women so that we can educate them and expedite any indicated emergency care.
Next, we need to expand our routine screening of mothers and pregnant women from just the most impactful social determinants of health (including depression, substance use, and IPV) to include anxiety, past suicide attempts and current suicidal ideation, and the presence of firearms, early and repeatedly in the first year of the child’s life. Adults and teens are more likely to disclose risk for sensitive issues through questionnaires than through interviews, perhaps even more so when the identified patient is their child rather than themselves. Any screen can have false negatives, so asking directly when risk is suspected is important. The reason for screening could be framed as caring for the caregiver who is the most important person for the child. It could be accompanied by acknowledging that pregnancy and the first year of life can be difficult for mothers and their partners and that we want to support them and connect them to resources, if needed. When substance use disorder is acknowledged, we should prescribe and teach about Narcan for overdose. When there is IPV, we should discuss firearm removal/locking as well as counseling on a personal safety plan.
Working as part of an on-site or virtual team that includes professionals who know about community resources and can coordinate care is essential, in addition to educating about 211 for services and 988 for suicide risk.
Finally, we can advocate and vote for programs, people, and laws that support and safeguard women and families, address substance use, and reduce access to firearms.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Checking on the well-being of mothers is one of the important acknowledged aspects of primary pediatric care. “How are you doing?” directed to the child’s mother has long been considered an appropriate question. The AAP recommends several checks in the Bright Futures Guidelines, including conducting several formal screens for depression and asking about “getting time alone with your partner” as well as other supports.
But I have recently become aware of new data that changes my ideas about what we pediatricians need to be doing as part of our care for children and their families, especially in the first year: Considering the risks to the mother of dying.
Maternal mortality increased by 26.6% from 2000 to 2014 across the United States such that it is higher now than it was for our own mothers. The U.S. now has the highest rates of maternal mortality among high-income nations, especially for Black, American Indian, or Alaska Native women, those of lower socioeconomic status, and those under 18 or over 35 years old.
You may be thinking, well, that is an issue for ob.gyns. Indeed, the most common reasons for maternal death are cardiovascular: hemorrhage, hypertensive disorders, deep vein thrombosis, and stroke, all usually occurring at or in the first week after birth. You may have heard about sudden unexpected heart failure from postpartum cardiomyopathy, although rare (1 in 1,000-4,000), presenting from 1 month pre birth to 5 months post delivery, which is when we may be the main clinicians seeing the mother, not the ob.gyns. This can be easily missed since it presents with shortness of breath and decreased exercise tolerance, fatigue, palpitations, and/or leg swelling. Serious eclampsia may have only symptoms of headache or abdominal pain. All of these may easily be mistaken for lingering pregnancy symptoms. But in higher income countries, such as the U.S., 38% of maternal deaths occur from 8 to 42 days after birth, the period for fatal infections as well as cardiac complications. Elevated risk for all of these causes of mortality include Black race, obesity, tobacco use, congenital heart disease, and being older than 40.
As pediatric providers, we may see mothers along with their infants as newborns in the hospital, at day 2, at 2 weeks, or even at 1-2 months after birth, potentially before their one recommended postnatal obstetric visit at 3-8 weeks. Asking the mother how she is feeling at those times should not just be a social nicety but rather an additional check for serious postnatal complications.
Additional concerns
But wait, it gets worse.
Did you know that the leading cause of maternal death from pregnancy up to 1 year after a birth is homicide?
Maternal perinatal mortality figures have not usually included “perinatal-associated” deaths, a maternal death attributable to a condition that is unaffected by the pregnancy and occurring within 1 year of delivery (that I will cite as perinatal henceforth). While half of maternal deaths occur during pregnancy, another half occur in the year following. There were 3.62 homicides per 100,000 live births among females who were pregnant or within 1 year postpartum, 16% more than for similarly aged nonpregnant and nonpostpartum women (3.12 deaths/100,000 population, P < .05). Homicides made up 8.4% of reported perinatal maternal deaths from all causes, with a rate of 1.7 per 100,000 live births, twice the rate of any one of the other leading causes noted above. Black women had seven times the risk of perinatal homicide as that of White women. Females under 20, many of them our own pediatric patients, had a greater than six times higher risk and those aged 20-24 had a 65% higher risk of pregnancy-associated homicide across race and ethnic groups. Homicide is most likely before 21 weeks of pregnancy, decreases in the third trimester, but increases again after birth. Two-thirds of pregnancy-associated homicide deaths occurred in the home, with the perpetrator a current or prior partner (> 59%, with 98% being male), 45%-50% were associated with reported intimate partner violence (IPV), and the most common method was a firearm (55%). Often the same women had histories of substance abuse, serious mental illness, and/or prior IPV, all risk factors for pregnancy-associated deaths, including from homicide.
Homicide? “Not the mothers in my practice,” you may say, but, if not homicide, drug-related deaths (3.68 per 100,000 person-years) and suicide (1.42 per 100,000 person-years) together comprise 18% of all maternal deaths. Non-Hispanic White women, Medicaid-insured women, and women residing in smaller cities were especially likely to die from drugs or suicide. More than half (54.3%) of perinatal suicides involve intimate partner conflict, which increases the risk ninefold. Perinatal mood disorders, affecting up to 15% of pregnant and postpartum U.S. women, is also a risk factor in substance abuse, opioid overdose death, and suicide.
And substance use has gotten more dangerous with the increase in fentanyl lacing. Pregnancy-associated deaths (4%-10% of deaths) involving opioids more than doubled between 2007 and 2016, and, although the rates are higher for Black women, the increase has been greater for non-Hispanic White women. Two-thirds of those deaths occur between 6 and 12 months postpartum, on our watch. Although many women decrease substance use during pregnancy, they may fall back into substance use (rates increase 4 times by 7-12 months after delivery) and not continue to receive treatment. Although pharmacotherapy (e.g., methadone, buprenorphine treatment) is the current standard of care for opioid use disorder (OUD) during pregnancy, nearly half receiving treatment in publicly funded centers are not receiving these medications and others may lose insurance or access to pregnancy-related treatment programs after delivery, increasing risk of relapse. Stigma, and punitive or discriminatory approaches to pregnant women with OUD (e.g., jail, removal of children) can dissuade them from participating in treatment, increasing overdose risk.
It is important to note that in more than half of the 41 deaths from violent trauma in one study (including 22 homicides), obstetrical providers knew of or suspected IPV. Also, the vast majority (74%) of those who died by drugs or suicide had made one or more emergency department or hospital visit between their delivery and death, and 39% had made three or more visits. Without knowing if anything was done in those cases, we also know that, in addition to thorough, compassionate providers, there is sometimes segmentation of responsibility, insensitivity, discrimination, racism, stigma, inequity, lack of resources, lack of access, lack of payment mechanisms, legal issues for immigrants, time constraints, and other systemic deficits that may hinder effective care for these and subsequent women.
Awareness and action
What should we, who are primary care pediatric providers, do about these threats to the mothers and pregnant young women we care for? Clearly, their children, our main patients, would be terribly and permanently hurt by harm coming to their mothers – the extreme adverse childhood experiences and social determinants of health to which we are already committed.
I hope this article will help alert pediatric providers to what is being published, mainly as women’s health and public health issues.
First, we need awareness of the physical symptoms that may come up in our interactions with pregnant and postpartum women so that we can educate them and expedite any indicated emergency care.
Next, we need to expand our routine screening of mothers and pregnant women from just the most impactful social determinants of health (including depression, substance use, and IPV) to include anxiety, past suicide attempts and current suicidal ideation, and the presence of firearms, early and repeatedly in the first year of the child’s life. Adults and teens are more likely to disclose risk for sensitive issues through questionnaires than through interviews, perhaps even more so when the identified patient is their child rather than themselves. Any screen can have false negatives, so asking directly when risk is suspected is important. The reason for screening could be framed as caring for the caregiver who is the most important person for the child. It could be accompanied by acknowledging that pregnancy and the first year of life can be difficult for mothers and their partners and that we want to support them and connect them to resources, if needed. When substance use disorder is acknowledged, we should prescribe and teach about Narcan for overdose. When there is IPV, we should discuss firearm removal/locking as well as counseling on a personal safety plan.
Working as part of an on-site or virtual team that includes professionals who know about community resources and can coordinate care is essential, in addition to educating about 211 for services and 988 for suicide risk.
Finally, we can advocate and vote for programs, people, and laws that support and safeguard women and families, address substance use, and reduce access to firearms.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
AI in medicine has a major Cassandra problem
This transcript has been edited for clarity.
Today I’m going to talk to you about a study at the cutting edge of modern medicine, one that uses an artificial intelligence (AI) model to guide care. But before I do, I need to take you back to the late Bronze Age, to a city located on the coast of what is now Turkey.
Troy’s towering walls made it seem unassailable, but that would not stop the Achaeans and their fleet of black ships from making landfall, and, after a siege, destroying the city. The destruction of Troy, as told in the Iliad and the Aeneid, was foretold by Cassandra, the daughter of King Priam and Priestess of Troy.
Cassandra had been given the gift of prophecy by the god Apollo in exchange for her favors. But after the gift was bestowed, she rejected the bright god and, in his rage, he added a curse to her blessing: that no one would ever believe her prophecies.
Thus it was that when her brother Paris set off to Sparta to abduct Helen, she warned him that his actions would lead to the downfall of their great city. He, of course, ignored her.
And you know the rest of the story.
Why am I telling you the story of Cassandra of Troy when we’re supposed to be talking about AI in medicine? Because AI has a major Cassandra problem.
The recent history of AI, and particularly the subset of AI known as machine learning in medicine, has been characterized by an accuracy arms race.
The electronic health record allows for the collection of volumes of data orders of magnitude greater than what we have ever been able to collect before. And all that data can be crunched by various algorithms to make predictions about, well, anything – whether a patient will be transferred to the intensive care unit, whether a GI bleed will need an intervention, whether someone will die in the next year.
Studies in this area tend to rely on retrospective datasets, and as time has gone on, better algorithms and more data have led to better and better predictions. In some simpler cases, machine-learning models have achieved near-perfect accuracy – Cassandra-level accuracy – as in the reading of chest x-rays for pneumonia, for example.
But as Cassandra teaches us, even perfect prediction is useless if no one believes you, if they don’t change their behavior. And this is the central problem of AI in medicine today. Many people are focusing on accuracy of the prediction but have forgotten that high accuracy is just table stakes for an AI model to be useful. It has to not only be accurate, but its use also has to change outcomes for patients. We need to be able to save Troy.
The best way to determine whether an AI model will help patients is to treat a model like we treat a new medication and evaluate it through a randomized trial. That’s what researchers, led by Shannon Walker of Vanderbilt University, Nashville, Tenn., did in a paper appearing in JAMA Network Open.
The model in question was one that predicted venous thromboembolism – blood clots – in hospitalized children. The model took in a variety of data points from the health record: a history of blood clot, history of cancer, presence of a central line, a variety of lab values. And the predictive model was very good – maybe not Cassandra good, but it achieved an AUC of 0.90, which means it had very high accuracy.
But again, accuracy is just table stakes.
The authors deployed the model in the live health record and recorded the results. For half of the kids, that was all that happened; no one actually saw the predictions. For those randomized to the intervention, the hematology team would be notified when the risk for clot was calculated to be greater than 2.5%. The hematology team would then contact the primary team to discuss prophylactic anticoagulation.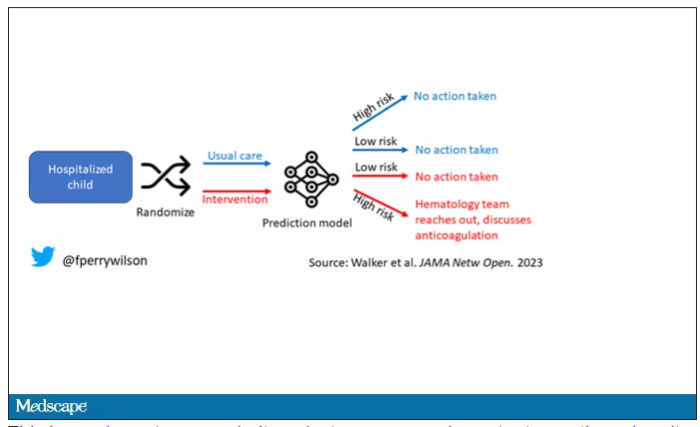
This is an elegant approach.
Let’s start with those table stakes – accuracy. The predictions were, by and large, pretty accurate in this trial. Of the 135 kids who developed blood clots, 121 had been flagged by the model in advance. That’s about 90%. The model flagged about 10% of kids who didn’t get a blood clot as well, but that’s not entirely surprising since the threshold for flagging was a 2.5% risk.
Given that the model preidentified almost every kid who would go on to develop a blood clot, it would make sense that kids randomized to the intervention would do better; after all, Cassandra was calling out her warnings.
But those kids didn’t do better. The rate of blood clot was no different between the group that used the accurate prediction model and the group that did not.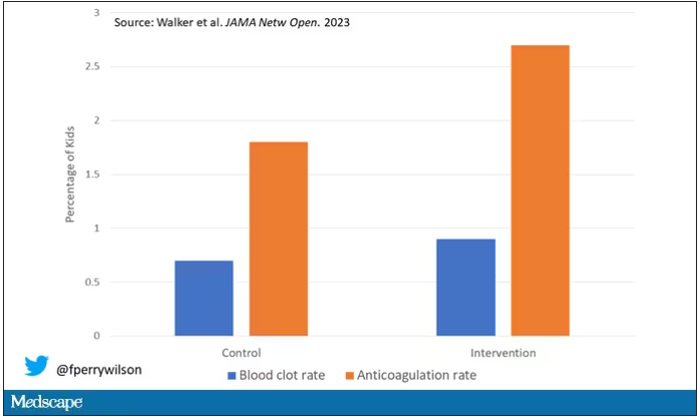
Why? Why does the use of an accurate model not necessarily improve outcomes?
First of all, a warning must lead to some change in management. Indeed, the kids in the intervention group were more likely to receive anticoagulation, but barely so. There were lots of reasons for this: physician preference, imminent discharge, active bleeding, and so on.
But let’s take a look at the 77 kids in the intervention arm who developed blood clots, because I think this is an instructive analysis.
Six of them did not meet the 2.5% threshold criteria, a case where the model missed its mark. Again, accuracy is table stakes.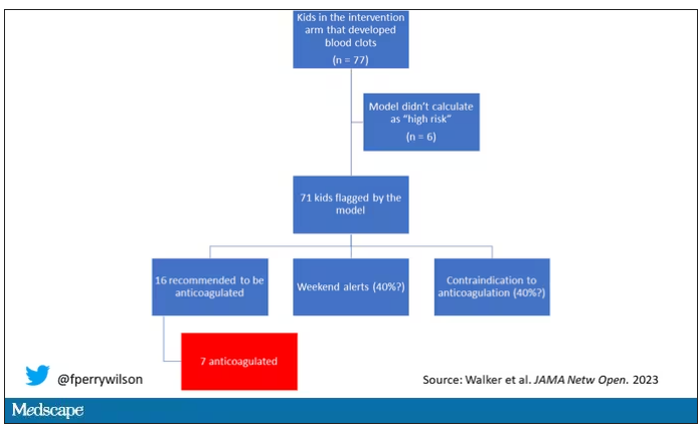
Of the remaining 71, only 16 got a recommendation from the hematologist to start anticoagulation. Why not more? Well, the model identified some of the high-risk kids on the weekend, and it seems that the study team did not contact treatment teams during that time. That may account for about 40% of these cases. The remainder had some contraindication to anticoagulation.
Most tellingly, of the 16 who did get a recommendation to start anticoagulation, the recommendation was followed in only seven patients.
This is the gap between accurate prediction and the ability to change outcomes for patients. A prediction is useless if it is wrong, for sure. But it’s also useless if you don’t tell anyone about it. It’s useless if you tell someone but they can’t do anything about it. And it’s useless if they could do something about it but choose not to.
That’s the gulf that these models need to cross at this point. So, the next time some slick company tells you how accurate their AI model is, ask them if accuracy is really the most important thing. If they say, “Well, yes, of course,” then tell them about Cassandra.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Today I’m going to talk to you about a study at the cutting edge of modern medicine, one that uses an artificial intelligence (AI) model to guide care. But before I do, I need to take you back to the late Bronze Age, to a city located on the coast of what is now Turkey.
Troy’s towering walls made it seem unassailable, but that would not stop the Achaeans and their fleet of black ships from making landfall, and, after a siege, destroying the city. The destruction of Troy, as told in the Iliad and the Aeneid, was foretold by Cassandra, the daughter of King Priam and Priestess of Troy.
Cassandra had been given the gift of prophecy by the god Apollo in exchange for her favors. But after the gift was bestowed, she rejected the bright god and, in his rage, he added a curse to her blessing: that no one would ever believe her prophecies.
Thus it was that when her brother Paris set off to Sparta to abduct Helen, she warned him that his actions would lead to the downfall of their great city. He, of course, ignored her.
And you know the rest of the story.
Why am I telling you the story of Cassandra of Troy when we’re supposed to be talking about AI in medicine? Because AI has a major Cassandra problem.
The recent history of AI, and particularly the subset of AI known as machine learning in medicine, has been characterized by an accuracy arms race.
The electronic health record allows for the collection of volumes of data orders of magnitude greater than what we have ever been able to collect before. And all that data can be crunched by various algorithms to make predictions about, well, anything – whether a patient will be transferred to the intensive care unit, whether a GI bleed will need an intervention, whether someone will die in the next year.
Studies in this area tend to rely on retrospective datasets, and as time has gone on, better algorithms and more data have led to better and better predictions. In some simpler cases, machine-learning models have achieved near-perfect accuracy – Cassandra-level accuracy – as in the reading of chest x-rays for pneumonia, for example.
But as Cassandra teaches us, even perfect prediction is useless if no one believes you, if they don’t change their behavior. And this is the central problem of AI in medicine today. Many people are focusing on accuracy of the prediction but have forgotten that high accuracy is just table stakes for an AI model to be useful. It has to not only be accurate, but its use also has to change outcomes for patients. We need to be able to save Troy.
The best way to determine whether an AI model will help patients is to treat a model like we treat a new medication and evaluate it through a randomized trial. That’s what researchers, led by Shannon Walker of Vanderbilt University, Nashville, Tenn., did in a paper appearing in JAMA Network Open.
The model in question was one that predicted venous thromboembolism – blood clots – in hospitalized children. The model took in a variety of data points from the health record: a history of blood clot, history of cancer, presence of a central line, a variety of lab values. And the predictive model was very good – maybe not Cassandra good, but it achieved an AUC of 0.90, which means it had very high accuracy.
But again, accuracy is just table stakes.
The authors deployed the model in the live health record and recorded the results. For half of the kids, that was all that happened; no one actually saw the predictions. For those randomized to the intervention, the hematology team would be notified when the risk for clot was calculated to be greater than 2.5%. The hematology team would then contact the primary team to discuss prophylactic anticoagulation.
This is an elegant approach.
Let’s start with those table stakes – accuracy. The predictions were, by and large, pretty accurate in this trial. Of the 135 kids who developed blood clots, 121 had been flagged by the model in advance. That’s about 90%. The model flagged about 10% of kids who didn’t get a blood clot as well, but that’s not entirely surprising since the threshold for flagging was a 2.5% risk.
Given that the model preidentified almost every kid who would go on to develop a blood clot, it would make sense that kids randomized to the intervention would do better; after all, Cassandra was calling out her warnings.
But those kids didn’t do better. The rate of blood clot was no different between the group that used the accurate prediction model and the group that did not.
Why? Why does the use of an accurate model not necessarily improve outcomes?
First of all, a warning must lead to some change in management. Indeed, the kids in the intervention group were more likely to receive anticoagulation, but barely so. There were lots of reasons for this: physician preference, imminent discharge, active bleeding, and so on.
But let’s take a look at the 77 kids in the intervention arm who developed blood clots, because I think this is an instructive analysis.
Six of them did not meet the 2.5% threshold criteria, a case where the model missed its mark. Again, accuracy is table stakes.
Of the remaining 71, only 16 got a recommendation from the hematologist to start anticoagulation. Why not more? Well, the model identified some of the high-risk kids on the weekend, and it seems that the study team did not contact treatment teams during that time. That may account for about 40% of these cases. The remainder had some contraindication to anticoagulation.
Most tellingly, of the 16 who did get a recommendation to start anticoagulation, the recommendation was followed in only seven patients.
This is the gap between accurate prediction and the ability to change outcomes for patients. A prediction is useless if it is wrong, for sure. But it’s also useless if you don’t tell anyone about it. It’s useless if you tell someone but they can’t do anything about it. And it’s useless if they could do something about it but choose not to.
That’s the gulf that these models need to cross at this point. So, the next time some slick company tells you how accurate their AI model is, ask them if accuracy is really the most important thing. If they say, “Well, yes, of course,” then tell them about Cassandra.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
Today I’m going to talk to you about a study at the cutting edge of modern medicine, one that uses an artificial intelligence (AI) model to guide care. But before I do, I need to take you back to the late Bronze Age, to a city located on the coast of what is now Turkey.
Troy’s towering walls made it seem unassailable, but that would not stop the Achaeans and their fleet of black ships from making landfall, and, after a siege, destroying the city. The destruction of Troy, as told in the Iliad and the Aeneid, was foretold by Cassandra, the daughter of King Priam and Priestess of Troy.
Cassandra had been given the gift of prophecy by the god Apollo in exchange for her favors. But after the gift was bestowed, she rejected the bright god and, in his rage, he added a curse to her blessing: that no one would ever believe her prophecies.
Thus it was that when her brother Paris set off to Sparta to abduct Helen, she warned him that his actions would lead to the downfall of their great city. He, of course, ignored her.
And you know the rest of the story.
Why am I telling you the story of Cassandra of Troy when we’re supposed to be talking about AI in medicine? Because AI has a major Cassandra problem.
The recent history of AI, and particularly the subset of AI known as machine learning in medicine, has been characterized by an accuracy arms race.
The electronic health record allows for the collection of volumes of data orders of magnitude greater than what we have ever been able to collect before. And all that data can be crunched by various algorithms to make predictions about, well, anything – whether a patient will be transferred to the intensive care unit, whether a GI bleed will need an intervention, whether someone will die in the next year.
Studies in this area tend to rely on retrospective datasets, and as time has gone on, better algorithms and more data have led to better and better predictions. In some simpler cases, machine-learning models have achieved near-perfect accuracy – Cassandra-level accuracy – as in the reading of chest x-rays for pneumonia, for example.
But as Cassandra teaches us, even perfect prediction is useless if no one believes you, if they don’t change their behavior. And this is the central problem of AI in medicine today. Many people are focusing on accuracy of the prediction but have forgotten that high accuracy is just table stakes for an AI model to be useful. It has to not only be accurate, but its use also has to change outcomes for patients. We need to be able to save Troy.
The best way to determine whether an AI model will help patients is to treat a model like we treat a new medication and evaluate it through a randomized trial. That’s what researchers, led by Shannon Walker of Vanderbilt University, Nashville, Tenn., did in a paper appearing in JAMA Network Open.
The model in question was one that predicted venous thromboembolism – blood clots – in hospitalized children. The model took in a variety of data points from the health record: a history of blood clot, history of cancer, presence of a central line, a variety of lab values. And the predictive model was very good – maybe not Cassandra good, but it achieved an AUC of 0.90, which means it had very high accuracy.
But again, accuracy is just table stakes.
The authors deployed the model in the live health record and recorded the results. For half of the kids, that was all that happened; no one actually saw the predictions. For those randomized to the intervention, the hematology team would be notified when the risk for clot was calculated to be greater than 2.5%. The hematology team would then contact the primary team to discuss prophylactic anticoagulation.
This is an elegant approach.
Let’s start with those table stakes – accuracy. The predictions were, by and large, pretty accurate in this trial. Of the 135 kids who developed blood clots, 121 had been flagged by the model in advance. That’s about 90%. The model flagged about 10% of kids who didn’t get a blood clot as well, but that’s not entirely surprising since the threshold for flagging was a 2.5% risk.
Given that the model preidentified almost every kid who would go on to develop a blood clot, it would make sense that kids randomized to the intervention would do better; after all, Cassandra was calling out her warnings.
But those kids didn’t do better. The rate of blood clot was no different between the group that used the accurate prediction model and the group that did not.
Why? Why does the use of an accurate model not necessarily improve outcomes?
First of all, a warning must lead to some change in management. Indeed, the kids in the intervention group were more likely to receive anticoagulation, but barely so. There were lots of reasons for this: physician preference, imminent discharge, active bleeding, and so on.
But let’s take a look at the 77 kids in the intervention arm who developed blood clots, because I think this is an instructive analysis.
Six of them did not meet the 2.5% threshold criteria, a case where the model missed its mark. Again, accuracy is table stakes.
Of the remaining 71, only 16 got a recommendation from the hematologist to start anticoagulation. Why not more? Well, the model identified some of the high-risk kids on the weekend, and it seems that the study team did not contact treatment teams during that time. That may account for about 40% of these cases. The remainder had some contraindication to anticoagulation.
Most tellingly, of the 16 who did get a recommendation to start anticoagulation, the recommendation was followed in only seven patients.
This is the gap between accurate prediction and the ability to change outcomes for patients. A prediction is useless if it is wrong, for sure. But it’s also useless if you don’t tell anyone about it. It’s useless if you tell someone but they can’t do anything about it. And it’s useless if they could do something about it but choose not to.
That’s the gulf that these models need to cross at this point. So, the next time some slick company tells you how accurate their AI model is, ask them if accuracy is really the most important thing. If they say, “Well, yes, of course,” then tell them about Cassandra.
Dr. F. Perry Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Asthma severity higher among LGBTQ+ population
HONOLULU – and asthma is especially exacerbated in SGM persons who use e-cigarettes compared with heterosexuals.
These findings come from a study of asthma severity among SGM people, with a special focus on the contribution of tobacco, reported Tugba Kaplan, MD, a resident in internal medicine at Luminis Health Anne Arundel Medical Center, Annapolis, Md.
“To the best of our knowledge, this is the first study assessing asthma severity among SGM people in a nationally representative longitudinal cohort study,” she said in an oral abstract session at the annual meeting of the American College of Chest Physicians (CHEST).
There has been only limited research on the health status and health needs of SGM people, and most of the studies conducted have focused on issues such as HIV/AIDS, sexual health, and substance use, not respiratory health, she said.
Following the PATH
Dr. Kaplan and colleagues drew on data from the Population Assessment of Tobacco and Health (PATH) Study, a nationally representative longitudinal cohort study with data on approximately 46,000 adults and adolescents in the United States.
The study uses self-reported data on tobacco use patterns; perceptions of risk and attitudes toward tobacco products; tobacco initiation, cessation, and relapse; and associated health outcomes.
The investigators combined data from three waves of the PATH Study, conducted from 2015 to 2019 on nonpregnant participants aged 18 years and older, and used mixed-effect logistic regression models to look for potential associations between sexual orientation and asthma severity.
They used standard definitions of asthma severity, based on lung function impairment measured by forced expiratory volume in 1 second and forced vital capacity, nighttime awakenings, use of a short-acting beta2-agonist for symptoms, interference with normal activity, and exacerbations requiring oral systemic corticosteroids.
The study also includes a sexual orientation question, asking participants, “do you consider yourself to be ...” with the options “straight, lesbian or gay, bisexual, something else, don’t know, or refused.”
Based on these responses, Dr. Kaplan and colleagues studied a total sample of 1,815 people who identify as SGM and 12,879 who identify as non-SGM.
Risks increased
In an analysis adjusted for age, sex, race/ethnicity, tobacco use, body mass index, physical activity, and asthma medication use, the authors found that, compared with non-SGM people, SGM respondents were significantly more likely to have had asthma attacks requiring steroid use in the past years (odds ratio, 1.47; 95% confidence interval, 1.01-2.15), asthma interfering with daily activities in the past month (OR, 1.33; CI, 1.10-1.61), and shortness of breath in any week over the 30 days (OR, 1.82; CI, 1.32-2.51). There was no significant difference between the groups in inhaler use over the past month, however.
They also found two interactions in the logistic regression models, one between urgent care visits and respondents who reported using both regular tobacco and e-cigarettes (dual users), and between exclusive e-cigarette use and waking up at night.
Among dual users, SGM respondents had a nearly fourfold greater risk for asthma attacks requiring urgent care visits, compared with non-SGM respondents (OR, 3.89; CI, 1.99-7.63). In contrast, among those who never used tobacco, there were no significant differences between the sexual orientation groups in regard to asthma attacks requiring urgent care visits.
Among those who reported using e-cigarettes exclusively, SGM respondents were nearly eight times more likely to report night awakening, compared with non-SGM users (OR, 7.81; CI, 2.93-20.8).
Among never users, in contrast, there was no significant difference in nighttime disturbances.
Possible confounders
The data suggest that “in the context of chronic illnesses like asthma, it is crucial to offer patients the knowledge and tools required to proficiently handle their conditions,” Dr. Kaplan said, adding that the differences seen between SGM and non-SGM respondents may be caused by health care disparities among SGM people that result in nonadherence to regular follow-ups.
In an interview, Jean Bourbeau, MD, MSc, who was a moderator for the session but was not involved in the study, commented that “we have to be very careful before making any conclusions, because this population could be at high risk for different reasons, and especially, do they get the same attention in terms of the care that is provided to the general population, and do they get access to the same medication?”
Nonetheless, Dr. Bourbeau continued, “I think this study is very important, because it shows us how much awareness we need to determine differences in populations, and [sexual orientation] is probably one thing that nobody had considered before, and for the first time we are now considering these potential differences in our population.”
The authors did not report a study funding source. Dr. Kaplan and Dr. Bourbeau reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
HONOLULU – and asthma is especially exacerbated in SGM persons who use e-cigarettes compared with heterosexuals.
These findings come from a study of asthma severity among SGM people, with a special focus on the contribution of tobacco, reported Tugba Kaplan, MD, a resident in internal medicine at Luminis Health Anne Arundel Medical Center, Annapolis, Md.
“To the best of our knowledge, this is the first study assessing asthma severity among SGM people in a nationally representative longitudinal cohort study,” she said in an oral abstract session at the annual meeting of the American College of Chest Physicians (CHEST).
There has been only limited research on the health status and health needs of SGM people, and most of the studies conducted have focused on issues such as HIV/AIDS, sexual health, and substance use, not respiratory health, she said.
Following the PATH
Dr. Kaplan and colleagues drew on data from the Population Assessment of Tobacco and Health (PATH) Study, a nationally representative longitudinal cohort study with data on approximately 46,000 adults and adolescents in the United States.
The study uses self-reported data on tobacco use patterns; perceptions of risk and attitudes toward tobacco products; tobacco initiation, cessation, and relapse; and associated health outcomes.
The investigators combined data from three waves of the PATH Study, conducted from 2015 to 2019 on nonpregnant participants aged 18 years and older, and used mixed-effect logistic regression models to look for potential associations between sexual orientation and asthma severity.
They used standard definitions of asthma severity, based on lung function impairment measured by forced expiratory volume in 1 second and forced vital capacity, nighttime awakenings, use of a short-acting beta2-agonist for symptoms, interference with normal activity, and exacerbations requiring oral systemic corticosteroids.
The study also includes a sexual orientation question, asking participants, “do you consider yourself to be ...” with the options “straight, lesbian or gay, bisexual, something else, don’t know, or refused.”
Based on these responses, Dr. Kaplan and colleagues studied a total sample of 1,815 people who identify as SGM and 12,879 who identify as non-SGM.
Risks increased
In an analysis adjusted for age, sex, race/ethnicity, tobacco use, body mass index, physical activity, and asthma medication use, the authors found that, compared with non-SGM people, SGM respondents were significantly more likely to have had asthma attacks requiring steroid use in the past years (odds ratio, 1.47; 95% confidence interval, 1.01-2.15), asthma interfering with daily activities in the past month (OR, 1.33; CI, 1.10-1.61), and shortness of breath in any week over the 30 days (OR, 1.82; CI, 1.32-2.51). There was no significant difference between the groups in inhaler use over the past month, however.
They also found two interactions in the logistic regression models, one between urgent care visits and respondents who reported using both regular tobacco and e-cigarettes (dual users), and between exclusive e-cigarette use and waking up at night.
Among dual users, SGM respondents had a nearly fourfold greater risk for asthma attacks requiring urgent care visits, compared with non-SGM respondents (OR, 3.89; CI, 1.99-7.63). In contrast, among those who never used tobacco, there were no significant differences between the sexual orientation groups in regard to asthma attacks requiring urgent care visits.
Among those who reported using e-cigarettes exclusively, SGM respondents were nearly eight times more likely to report night awakening, compared with non-SGM users (OR, 7.81; CI, 2.93-20.8).
Among never users, in contrast, there was no significant difference in nighttime disturbances.
Possible confounders
The data suggest that “in the context of chronic illnesses like asthma, it is crucial to offer patients the knowledge and tools required to proficiently handle their conditions,” Dr. Kaplan said, adding that the differences seen between SGM and non-SGM respondents may be caused by health care disparities among SGM people that result in nonadherence to regular follow-ups.
In an interview, Jean Bourbeau, MD, MSc, who was a moderator for the session but was not involved in the study, commented that “we have to be very careful before making any conclusions, because this population could be at high risk for different reasons, and especially, do they get the same attention in terms of the care that is provided to the general population, and do they get access to the same medication?”
Nonetheless, Dr. Bourbeau continued, “I think this study is very important, because it shows us how much awareness we need to determine differences in populations, and [sexual orientation] is probably one thing that nobody had considered before, and for the first time we are now considering these potential differences in our population.”
The authors did not report a study funding source. Dr. Kaplan and Dr. Bourbeau reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
HONOLULU – and asthma is especially exacerbated in SGM persons who use e-cigarettes compared with heterosexuals.
These findings come from a study of asthma severity among SGM people, with a special focus on the contribution of tobacco, reported Tugba Kaplan, MD, a resident in internal medicine at Luminis Health Anne Arundel Medical Center, Annapolis, Md.
“To the best of our knowledge, this is the first study assessing asthma severity among SGM people in a nationally representative longitudinal cohort study,” she said in an oral abstract session at the annual meeting of the American College of Chest Physicians (CHEST).
There has been only limited research on the health status and health needs of SGM people, and most of the studies conducted have focused on issues such as HIV/AIDS, sexual health, and substance use, not respiratory health, she said.
Following the PATH
Dr. Kaplan and colleagues drew on data from the Population Assessment of Tobacco and Health (PATH) Study, a nationally representative longitudinal cohort study with data on approximately 46,000 adults and adolescents in the United States.
The study uses self-reported data on tobacco use patterns; perceptions of risk and attitudes toward tobacco products; tobacco initiation, cessation, and relapse; and associated health outcomes.
The investigators combined data from three waves of the PATH Study, conducted from 2015 to 2019 on nonpregnant participants aged 18 years and older, and used mixed-effect logistic regression models to look for potential associations between sexual orientation and asthma severity.
They used standard definitions of asthma severity, based on lung function impairment measured by forced expiratory volume in 1 second and forced vital capacity, nighttime awakenings, use of a short-acting beta2-agonist for symptoms, interference with normal activity, and exacerbations requiring oral systemic corticosteroids.
The study also includes a sexual orientation question, asking participants, “do you consider yourself to be ...” with the options “straight, lesbian or gay, bisexual, something else, don’t know, or refused.”
Based on these responses, Dr. Kaplan and colleagues studied a total sample of 1,815 people who identify as SGM and 12,879 who identify as non-SGM.
Risks increased
In an analysis adjusted for age, sex, race/ethnicity, tobacco use, body mass index, physical activity, and asthma medication use, the authors found that, compared with non-SGM people, SGM respondents were significantly more likely to have had asthma attacks requiring steroid use in the past years (odds ratio, 1.47; 95% confidence interval, 1.01-2.15), asthma interfering with daily activities in the past month (OR, 1.33; CI, 1.10-1.61), and shortness of breath in any week over the 30 days (OR, 1.82; CI, 1.32-2.51). There was no significant difference between the groups in inhaler use over the past month, however.
They also found two interactions in the logistic regression models, one between urgent care visits and respondents who reported using both regular tobacco and e-cigarettes (dual users), and between exclusive e-cigarette use and waking up at night.
Among dual users, SGM respondents had a nearly fourfold greater risk for asthma attacks requiring urgent care visits, compared with non-SGM respondents (OR, 3.89; CI, 1.99-7.63). In contrast, among those who never used tobacco, there were no significant differences between the sexual orientation groups in regard to asthma attacks requiring urgent care visits.
Among those who reported using e-cigarettes exclusively, SGM respondents were nearly eight times more likely to report night awakening, compared with non-SGM users (OR, 7.81; CI, 2.93-20.8).
Among never users, in contrast, there was no significant difference in nighttime disturbances.
Possible confounders
The data suggest that “in the context of chronic illnesses like asthma, it is crucial to offer patients the knowledge and tools required to proficiently handle their conditions,” Dr. Kaplan said, adding that the differences seen between SGM and non-SGM respondents may be caused by health care disparities among SGM people that result in nonadherence to regular follow-ups.
In an interview, Jean Bourbeau, MD, MSc, who was a moderator for the session but was not involved in the study, commented that “we have to be very careful before making any conclusions, because this population could be at high risk for different reasons, and especially, do they get the same attention in terms of the care that is provided to the general population, and do they get access to the same medication?”
Nonetheless, Dr. Bourbeau continued, “I think this study is very important, because it shows us how much awareness we need to determine differences in populations, and [sexual orientation] is probably one thing that nobody had considered before, and for the first time we are now considering these potential differences in our population.”
The authors did not report a study funding source. Dr. Kaplan and Dr. Bourbeau reported having no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT CHEST 2023
Are migraine preventives underused in young adults?
, according to recent research published in the journal Headache.
“Approximately two-fifths of young adults with migraine were prescribed preventive medications, and this did not differ between pediatric and adult neurologists,” Hannah F. J. Shapiro MD, of the department of neurology at the University of California, San Francisco, and the UCSF Benioff Children’s Hospitals, and colleagues wrote in their study. “This finding suggests that pediatric neurologists are providing comparable care to adult neurologists for young adults with migraine; however, this may represent the underuse of preventive medications in this patient population.”
Dr. Shapiro and colleagues conducted a retrospective study of 767 patients (mean age 20.3 years) at Mass General Brigham Hospital in Boston between 2017 and 2021 who received care from a pediatric or adult neurologist for episodic migraine. The majority of patients in the study were white (72.2%), non-Hispanic (82.1%) women (80.3%) with episodic migraine (72.8%), some of whom experienced a psychiatric comorbidity (12.7%), and had a 3.88 mean clinic visits for migraine. Researchers assessed prescription of migraine preventive medication as a primary outcome, with a secondary outcome of comparing the rate of migraine preventive prescriptions written by pediatric and adult neurologists.
Overall, 290 patients (37.8%) received care from a pediatric neurologist, and 131 of those 290 patients (45.2%) received preventive medications (95% confidence interval, 39.5%-51.0%). The remaining 477 patients received care from an adult neurologist; of these, 206 patients (43.2%) received preventive medications (95% CI, 39.0%-47.7%; P = .591). The most common preventive medication prescribed was topiramate, which was prescribed in 19.1% of cases by adult neurologists and 15.2% of cases by pediatric neurologists. Other preventive medications included tricyclic antidepressants such as amitriptyline and nortriptyline; pediatric neurologists prescribed amitriptyline more often than adult neurologists (14.5% vs. 5.5%; P < .001), and adult neurologists prescribed nortriptyline more often than pediatric neurologists (12.8% vs. 2.4%; P < .001).
Dr. Shapiro and colleagues performed a mixed effects logistic regression analysis of potential confounders, and found no significant association between clinician specialty and use of preventive medication (adjusted odds ratio, 1.20; 95% CI, 0.62-2.31), while factors such as female sex (aOR, 1.69; 95% CI, 1.07-2.66) and number of visits (aOR, 1.64; 95% CI, 1.49-1.80) carried associations with preventive medication use.
The finding that pediatric and adult neurologists use similar preventive medications is a positive one because “patients who continue care into adulthood with a pediatric neurologist should receive comparable care to the care they would receive with an adult neurologist,” Dr. Shapiro and colleagues said. “It is even more pertinent now for pediatric neurologists to have comfort prescribing preventive medication to young adults, as the newer calcitonin gene-related peptide (CGRP) pathway antagonists are currently only FDA approved for use in patients aged 18 years or older.”
Roadblocks may prevent adoption
M. Cristina Victorio, MD, a pediatric neurologist and director of the headache program at Akron (Ohio) Children’s, said in an interview that the study is well-designed, but the results cannot be generalized as the study is retrospective, was conducted at a single institution, and data about nutraceuticals and drug-free neuromodulation devices were excluded from the analysis.
Another aspect of the study to consider is that episodic migraine, defined as between 0 and 14 migraine days per month, comprised most of the diagnoses in this study, while preventive medication is usually considered in patients with migraines occurring at least 6 days per month. “[I]f migraine is only once every other month or once a month, preventive treatment may not be recommended,” she said.
There is also the element of patient preference, which is “difficult to obtain” in a retrospective study, she noted.
Citing the authors’ comments about pediatric neurologists’ comfortability prescribing preventive medications, including CGRP antagonists, Dr. Victorio said she offers CGRP antagonists to “young adult patients who have failed at least two of the guideline-recommended preventive medications.”
However, pediatric neurologists may encounter roadblocks to prescribing these medications. “A big challenge is access, as it requires prior authorization as well as writing a letter of appeal or medical necessity, which can be a nuisance for clinicians who are already inundated with clinical responsibilities,” she said.
More education is needed
“As a pediatric headache specialist and knowing the results of this study, my colleagues and I have a role in educating all clinicians as well as trainees on headache management to improve and provide optimal care for young adult patients with migraine,” Dr. Victorio said.
In her experience, more clinic visits usually mean a need for preventative medication, and psychiatric morbidities are common. “I differ in the sense that as a headache specialist I am comfortable offering various preventive treatment options when indicated, so I do not believe I am underutilizing,” she said.
Dr. Victorio said she prescribes topiramate, amitriptyline, and propranolol as migraine preventatives for adolescents and young adults, but recommends cyproheptadine for younger children “due to lesser side effects, tolerability, and convenience of formulation (both liquid and tablet forms are available), which can be challenging for younger children who are unable to swallow pills.”
“Cognizant that there are patients who are reluctant to take daily prescription medication and that consideration for preventive treatment includes patient’s preference, I include the use of nutraceuticals and drug-free neuromodulation devices when discussing preventive treatment options,” she added, noting that children and adolescents “[m]ore often than not” prefer nutraceuticals like magnesium and vitamin B2.
“I think the bottom line is that all clinicians managing young adults with migraine should know when to consider starting preventive migraine medication,” Dr. Victorio said. “Not offering preventive treatment to young adults specifically for those who have frequent migraine attacks, or those who have severe migraine despite adequate acute treatment, or those with significant adverse reactions to acute medications will only put these patients at risk to progression to chronic migraine (meaning having migraine more often than not – at least 15 days per month), and increases headache-related disability and reduces quality of life.”
The authors report no relevant financial disclosures. This study was supported by Harvard University and an award from the National Institutes of Health. Dr. Victorio reports being on the advisory board for Theranica Bio-electronics, has received honorarium serving as an author of the Merck Manual, and is involved in industry-sponsored clinical trials through Akron Children’s Hospital.
, according to recent research published in the journal Headache.
“Approximately two-fifths of young adults with migraine were prescribed preventive medications, and this did not differ between pediatric and adult neurologists,” Hannah F. J. Shapiro MD, of the department of neurology at the University of California, San Francisco, and the UCSF Benioff Children’s Hospitals, and colleagues wrote in their study. “This finding suggests that pediatric neurologists are providing comparable care to adult neurologists for young adults with migraine; however, this may represent the underuse of preventive medications in this patient population.”
Dr. Shapiro and colleagues conducted a retrospective study of 767 patients (mean age 20.3 years) at Mass General Brigham Hospital in Boston between 2017 and 2021 who received care from a pediatric or adult neurologist for episodic migraine. The majority of patients in the study were white (72.2%), non-Hispanic (82.1%) women (80.3%) with episodic migraine (72.8%), some of whom experienced a psychiatric comorbidity (12.7%), and had a 3.88 mean clinic visits for migraine. Researchers assessed prescription of migraine preventive medication as a primary outcome, with a secondary outcome of comparing the rate of migraine preventive prescriptions written by pediatric and adult neurologists.
Overall, 290 patients (37.8%) received care from a pediatric neurologist, and 131 of those 290 patients (45.2%) received preventive medications (95% confidence interval, 39.5%-51.0%). The remaining 477 patients received care from an adult neurologist; of these, 206 patients (43.2%) received preventive medications (95% CI, 39.0%-47.7%; P = .591). The most common preventive medication prescribed was topiramate, which was prescribed in 19.1% of cases by adult neurologists and 15.2% of cases by pediatric neurologists. Other preventive medications included tricyclic antidepressants such as amitriptyline and nortriptyline; pediatric neurologists prescribed amitriptyline more often than adult neurologists (14.5% vs. 5.5%; P < .001), and adult neurologists prescribed nortriptyline more often than pediatric neurologists (12.8% vs. 2.4%; P < .001).
Dr. Shapiro and colleagues performed a mixed effects logistic regression analysis of potential confounders, and found no significant association between clinician specialty and use of preventive medication (adjusted odds ratio, 1.20; 95% CI, 0.62-2.31), while factors such as female sex (aOR, 1.69; 95% CI, 1.07-2.66) and number of visits (aOR, 1.64; 95% CI, 1.49-1.80) carried associations with preventive medication use.
The finding that pediatric and adult neurologists use similar preventive medications is a positive one because “patients who continue care into adulthood with a pediatric neurologist should receive comparable care to the care they would receive with an adult neurologist,” Dr. Shapiro and colleagues said. “It is even more pertinent now for pediatric neurologists to have comfort prescribing preventive medication to young adults, as the newer calcitonin gene-related peptide (CGRP) pathway antagonists are currently only FDA approved for use in patients aged 18 years or older.”
Roadblocks may prevent adoption
M. Cristina Victorio, MD, a pediatric neurologist and director of the headache program at Akron (Ohio) Children’s, said in an interview that the study is well-designed, but the results cannot be generalized as the study is retrospective, was conducted at a single institution, and data about nutraceuticals and drug-free neuromodulation devices were excluded from the analysis.
Another aspect of the study to consider is that episodic migraine, defined as between 0 and 14 migraine days per month, comprised most of the diagnoses in this study, while preventive medication is usually considered in patients with migraines occurring at least 6 days per month. “[I]f migraine is only once every other month or once a month, preventive treatment may not be recommended,” she said.
There is also the element of patient preference, which is “difficult to obtain” in a retrospective study, she noted.
Citing the authors’ comments about pediatric neurologists’ comfortability prescribing preventive medications, including CGRP antagonists, Dr. Victorio said she offers CGRP antagonists to “young adult patients who have failed at least two of the guideline-recommended preventive medications.”
However, pediatric neurologists may encounter roadblocks to prescribing these medications. “A big challenge is access, as it requires prior authorization as well as writing a letter of appeal or medical necessity, which can be a nuisance for clinicians who are already inundated with clinical responsibilities,” she said.
More education is needed
“As a pediatric headache specialist and knowing the results of this study, my colleagues and I have a role in educating all clinicians as well as trainees on headache management to improve and provide optimal care for young adult patients with migraine,” Dr. Victorio said.
In her experience, more clinic visits usually mean a need for preventative medication, and psychiatric morbidities are common. “I differ in the sense that as a headache specialist I am comfortable offering various preventive treatment options when indicated, so I do not believe I am underutilizing,” she said.
Dr. Victorio said she prescribes topiramate, amitriptyline, and propranolol as migraine preventatives for adolescents and young adults, but recommends cyproheptadine for younger children “due to lesser side effects, tolerability, and convenience of formulation (both liquid and tablet forms are available), which can be challenging for younger children who are unable to swallow pills.”
“Cognizant that there are patients who are reluctant to take daily prescription medication and that consideration for preventive treatment includes patient’s preference, I include the use of nutraceuticals and drug-free neuromodulation devices when discussing preventive treatment options,” she added, noting that children and adolescents “[m]ore often than not” prefer nutraceuticals like magnesium and vitamin B2.
“I think the bottom line is that all clinicians managing young adults with migraine should know when to consider starting preventive migraine medication,” Dr. Victorio said. “Not offering preventive treatment to young adults specifically for those who have frequent migraine attacks, or those who have severe migraine despite adequate acute treatment, or those with significant adverse reactions to acute medications will only put these patients at risk to progression to chronic migraine (meaning having migraine more often than not – at least 15 days per month), and increases headache-related disability and reduces quality of life.”
The authors report no relevant financial disclosures. This study was supported by Harvard University and an award from the National Institutes of Health. Dr. Victorio reports being on the advisory board for Theranica Bio-electronics, has received honorarium serving as an author of the Merck Manual, and is involved in industry-sponsored clinical trials through Akron Children’s Hospital.
, according to recent research published in the journal Headache.
“Approximately two-fifths of young adults with migraine were prescribed preventive medications, and this did not differ between pediatric and adult neurologists,” Hannah F. J. Shapiro MD, of the department of neurology at the University of California, San Francisco, and the UCSF Benioff Children’s Hospitals, and colleagues wrote in their study. “This finding suggests that pediatric neurologists are providing comparable care to adult neurologists for young adults with migraine; however, this may represent the underuse of preventive medications in this patient population.”
Dr. Shapiro and colleagues conducted a retrospective study of 767 patients (mean age 20.3 years) at Mass General Brigham Hospital in Boston between 2017 and 2021 who received care from a pediatric or adult neurologist for episodic migraine. The majority of patients in the study were white (72.2%), non-Hispanic (82.1%) women (80.3%) with episodic migraine (72.8%), some of whom experienced a psychiatric comorbidity (12.7%), and had a 3.88 mean clinic visits for migraine. Researchers assessed prescription of migraine preventive medication as a primary outcome, with a secondary outcome of comparing the rate of migraine preventive prescriptions written by pediatric and adult neurologists.
Overall, 290 patients (37.8%) received care from a pediatric neurologist, and 131 of those 290 patients (45.2%) received preventive medications (95% confidence interval, 39.5%-51.0%). The remaining 477 patients received care from an adult neurologist; of these, 206 patients (43.2%) received preventive medications (95% CI, 39.0%-47.7%; P = .591). The most common preventive medication prescribed was topiramate, which was prescribed in 19.1% of cases by adult neurologists and 15.2% of cases by pediatric neurologists. Other preventive medications included tricyclic antidepressants such as amitriptyline and nortriptyline; pediatric neurologists prescribed amitriptyline more often than adult neurologists (14.5% vs. 5.5%; P < .001), and adult neurologists prescribed nortriptyline more often than pediatric neurologists (12.8% vs. 2.4%; P < .001).
Dr. Shapiro and colleagues performed a mixed effects logistic regression analysis of potential confounders, and found no significant association between clinician specialty and use of preventive medication (adjusted odds ratio, 1.20; 95% CI, 0.62-2.31), while factors such as female sex (aOR, 1.69; 95% CI, 1.07-2.66) and number of visits (aOR, 1.64; 95% CI, 1.49-1.80) carried associations with preventive medication use.
The finding that pediatric and adult neurologists use similar preventive medications is a positive one because “patients who continue care into adulthood with a pediatric neurologist should receive comparable care to the care they would receive with an adult neurologist,” Dr. Shapiro and colleagues said. “It is even more pertinent now for pediatric neurologists to have comfort prescribing preventive medication to young adults, as the newer calcitonin gene-related peptide (CGRP) pathway antagonists are currently only FDA approved for use in patients aged 18 years or older.”
Roadblocks may prevent adoption
M. Cristina Victorio, MD, a pediatric neurologist and director of the headache program at Akron (Ohio) Children’s, said in an interview that the study is well-designed, but the results cannot be generalized as the study is retrospective, was conducted at a single institution, and data about nutraceuticals and drug-free neuromodulation devices were excluded from the analysis.
Another aspect of the study to consider is that episodic migraine, defined as between 0 and 14 migraine days per month, comprised most of the diagnoses in this study, while preventive medication is usually considered in patients with migraines occurring at least 6 days per month. “[I]f migraine is only once every other month or once a month, preventive treatment may not be recommended,” she said.
There is also the element of patient preference, which is “difficult to obtain” in a retrospective study, she noted.
Citing the authors’ comments about pediatric neurologists’ comfortability prescribing preventive medications, including CGRP antagonists, Dr. Victorio said she offers CGRP antagonists to “young adult patients who have failed at least two of the guideline-recommended preventive medications.”
However, pediatric neurologists may encounter roadblocks to prescribing these medications. “A big challenge is access, as it requires prior authorization as well as writing a letter of appeal or medical necessity, which can be a nuisance for clinicians who are already inundated with clinical responsibilities,” she said.
More education is needed
“As a pediatric headache specialist and knowing the results of this study, my colleagues and I have a role in educating all clinicians as well as trainees on headache management to improve and provide optimal care for young adult patients with migraine,” Dr. Victorio said.
In her experience, more clinic visits usually mean a need for preventative medication, and psychiatric morbidities are common. “I differ in the sense that as a headache specialist I am comfortable offering various preventive treatment options when indicated, so I do not believe I am underutilizing,” she said.
Dr. Victorio said she prescribes topiramate, amitriptyline, and propranolol as migraine preventatives for adolescents and young adults, but recommends cyproheptadine for younger children “due to lesser side effects, tolerability, and convenience of formulation (both liquid and tablet forms are available), which can be challenging for younger children who are unable to swallow pills.”
“Cognizant that there are patients who are reluctant to take daily prescription medication and that consideration for preventive treatment includes patient’s preference, I include the use of nutraceuticals and drug-free neuromodulation devices when discussing preventive treatment options,” she added, noting that children and adolescents “[m]ore often than not” prefer nutraceuticals like magnesium and vitamin B2.
“I think the bottom line is that all clinicians managing young adults with migraine should know when to consider starting preventive migraine medication,” Dr. Victorio said. “Not offering preventive treatment to young adults specifically for those who have frequent migraine attacks, or those who have severe migraine despite adequate acute treatment, or those with significant adverse reactions to acute medications will only put these patients at risk to progression to chronic migraine (meaning having migraine more often than not – at least 15 days per month), and increases headache-related disability and reduces quality of life.”
The authors report no relevant financial disclosures. This study was supported by Harvard University and an award from the National Institutes of Health. Dr. Victorio reports being on the advisory board for Theranica Bio-electronics, has received honorarium serving as an author of the Merck Manual, and is involved in industry-sponsored clinical trials through Akron Children’s Hospital.
FROM HEADACHE
Metformin, weight management to stop type 2 diabetes in kids
TOPLINE:
Nearly one in five adolescents are living with prediabetes, a condition where blood glucose levels are elevated, but are not high enough for a type 2 diabetes (T2D) diagnosis. According to a new study, higher levels of nonfasting glucose and hemoglobin A1c, and worsening obesity are important predictors of progression to T2D. In addition, metformin and weight stabilization may prove to be important interventions for preventing T2D in kids.
METHODOLOGY:
- Researchers did a retrospective chart review of patient data from Vanderbilt University Medical Center Pediatric Prediabetes Clinic, Nashville, Tenn., from May 2015 to August 2022.
- The study included 552 children with prediabetes, defined as abnormal blood glucose (fasting plasma glucose [FPG] ≥ 100 mg/dL, random glucose ≥ 150 mg/dL), or hemoglobin A1c equal to or greater than 5.9%.
- Based on follow-up visits, patients were classified as having progressed to T2D, or nonprogression.
- Researchers analyzed the patients’ initial visit A1c, fasting C-peptide, 2-hour glucose, fasting glucose, and body mass index (BMI), among other baseline characteristics.
TAKEAWAY:
- Thirty-six children (6.5%) progressed to T2D during the duration of the study period.
- The average time to T2D diagnosis was much longer in patients taking metformin (43 months), compared with those not taking the prescribed medication (28 months).
- Worsening obesity was strongly associated with T2D progression – patients who progressed to T2D had a higher BMI at baseline and had continued weight gain.
- A higher baseline A1c, fasting C-peptide, and 2-hour glucose were also associated with progression to T2D.
- In the multivariable analysis, both A1c and 2-hour glucose were strong independent predictors of progression.
- Fasting plasma glucose was not associated with progression to T2D.
IN PRACTICE:
“Weight stabilization and metformin therapy could be important interventions for diabetes prevention in children,” study author Ashley H. Shoemaker, MD, MSci, a pediatric endocrinologist at Vanderbilt University Medical Center in Nashville, Tenn., said in a press release.
In addition, A1c plus a nonfasting glucose may be a feasible way to identify high-risk pediatric patients in a clinical setting.
SOURCE:
This study was performed by Natasha Belsky, Jaclyn Tamaroff, and Ashley H. Shoemaker of the Vanderbilt University Medical Center and the Vanderbilt University School of Medicine in Nashville, Tenn. It was published October 12, 2023, in the Journal of the Endocrine Society
LIMITATIONS:
Additional patients who developed T2D may have been lost to follow-up, since the authors did not contact patients to confirm their disease status. The authors were also unable to establish racial differences in the progression to T2D because of missing data.
DISCLOSURES:
Funding for this study was provided by the National Center for Advancing Translational Sciences. One author has research contracts with Novo Nordisk and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
TOPLINE:
Nearly one in five adolescents are living with prediabetes, a condition where blood glucose levels are elevated, but are not high enough for a type 2 diabetes (T2D) diagnosis. According to a new study, higher levels of nonfasting glucose and hemoglobin A1c, and worsening obesity are important predictors of progression to T2D. In addition, metformin and weight stabilization may prove to be important interventions for preventing T2D in kids.
METHODOLOGY:
- Researchers did a retrospective chart review of patient data from Vanderbilt University Medical Center Pediatric Prediabetes Clinic, Nashville, Tenn., from May 2015 to August 2022.
- The study included 552 children with prediabetes, defined as abnormal blood glucose (fasting plasma glucose [FPG] ≥ 100 mg/dL, random glucose ≥ 150 mg/dL), or hemoglobin A1c equal to or greater than 5.9%.
- Based on follow-up visits, patients were classified as having progressed to T2D, or nonprogression.
- Researchers analyzed the patients’ initial visit A1c, fasting C-peptide, 2-hour glucose, fasting glucose, and body mass index (BMI), among other baseline characteristics.
TAKEAWAY:
- Thirty-six children (6.5%) progressed to T2D during the duration of the study period.
- The average time to T2D diagnosis was much longer in patients taking metformin (43 months), compared with those not taking the prescribed medication (28 months).
- Worsening obesity was strongly associated with T2D progression – patients who progressed to T2D had a higher BMI at baseline and had continued weight gain.
- A higher baseline A1c, fasting C-peptide, and 2-hour glucose were also associated with progression to T2D.
- In the multivariable analysis, both A1c and 2-hour glucose were strong independent predictors of progression.
- Fasting plasma glucose was not associated with progression to T2D.
IN PRACTICE:
“Weight stabilization and metformin therapy could be important interventions for diabetes prevention in children,” study author Ashley H. Shoemaker, MD, MSci, a pediatric endocrinologist at Vanderbilt University Medical Center in Nashville, Tenn., said in a press release.
In addition, A1c plus a nonfasting glucose may be a feasible way to identify high-risk pediatric patients in a clinical setting.
SOURCE:
This study was performed by Natasha Belsky, Jaclyn Tamaroff, and Ashley H. Shoemaker of the Vanderbilt University Medical Center and the Vanderbilt University School of Medicine in Nashville, Tenn. It was published October 12, 2023, in the Journal of the Endocrine Society
LIMITATIONS:
Additional patients who developed T2D may have been lost to follow-up, since the authors did not contact patients to confirm their disease status. The authors were also unable to establish racial differences in the progression to T2D because of missing data.
DISCLOSURES:
Funding for this study was provided by the National Center for Advancing Translational Sciences. One author has research contracts with Novo Nordisk and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
TOPLINE:
Nearly one in five adolescents are living with prediabetes, a condition where blood glucose levels are elevated, but are not high enough for a type 2 diabetes (T2D) diagnosis. According to a new study, higher levels of nonfasting glucose and hemoglobin A1c, and worsening obesity are important predictors of progression to T2D. In addition, metformin and weight stabilization may prove to be important interventions for preventing T2D in kids.
METHODOLOGY:
- Researchers did a retrospective chart review of patient data from Vanderbilt University Medical Center Pediatric Prediabetes Clinic, Nashville, Tenn., from May 2015 to August 2022.
- The study included 552 children with prediabetes, defined as abnormal blood glucose (fasting plasma glucose [FPG] ≥ 100 mg/dL, random glucose ≥ 150 mg/dL), or hemoglobin A1c equal to or greater than 5.9%.
- Based on follow-up visits, patients were classified as having progressed to T2D, or nonprogression.
- Researchers analyzed the patients’ initial visit A1c, fasting C-peptide, 2-hour glucose, fasting glucose, and body mass index (BMI), among other baseline characteristics.
TAKEAWAY:
- Thirty-six children (6.5%) progressed to T2D during the duration of the study period.
- The average time to T2D diagnosis was much longer in patients taking metformin (43 months), compared with those not taking the prescribed medication (28 months).
- Worsening obesity was strongly associated with T2D progression – patients who progressed to T2D had a higher BMI at baseline and had continued weight gain.
- A higher baseline A1c, fasting C-peptide, and 2-hour glucose were also associated with progression to T2D.
- In the multivariable analysis, both A1c and 2-hour glucose were strong independent predictors of progression.
- Fasting plasma glucose was not associated with progression to T2D.
IN PRACTICE:
“Weight stabilization and metformin therapy could be important interventions for diabetes prevention in children,” study author Ashley H. Shoemaker, MD, MSci, a pediatric endocrinologist at Vanderbilt University Medical Center in Nashville, Tenn., said in a press release.
In addition, A1c plus a nonfasting glucose may be a feasible way to identify high-risk pediatric patients in a clinical setting.
SOURCE:
This study was performed by Natasha Belsky, Jaclyn Tamaroff, and Ashley H. Shoemaker of the Vanderbilt University Medical Center and the Vanderbilt University School of Medicine in Nashville, Tenn. It was published October 12, 2023, in the Journal of the Endocrine Society
LIMITATIONS:
Additional patients who developed T2D may have been lost to follow-up, since the authors did not contact patients to confirm their disease status. The authors were also unable to establish racial differences in the progression to T2D because of missing data.
DISCLOSURES:
Funding for this study was provided by the National Center for Advancing Translational Sciences. One author has research contracts with Novo Nordisk and Boehringer Ingelheim.
A version of this article first appeared on Medscape.com.
What's the diagnosis?
The lesions on the heels are consistent with piezogenic pedal papules. They seem to be more common in women and have been described in families, though a genetic link hasn’t been elucidated. PPP manifests as small, soft, compressible papules on the lateral aspects of the skin on the heels, more noticeable when the patient is standing, and can also present on the wrists and legs. While they may not be a cause for serious concern, understanding their causes, associated conditions, and management is important.
Piezogenic pedal papules are flesh-colored or slightly reddish and can range in size from a few millimeters to a centimeter or more. They are described as benign herniations of elastic tissue and subcutaneous fat through the reticular dermis. The lesions are triggered by increased pressure and compression, such as standing or the application of pressure on the heel. The exact etiology is not known. While piezogenic pedal papules are often asymptomatic, some individuals experience discomfort, itching, or mild pain, particularly when walking or applying pressure to the affected area, especially in patients with Ehlers-Danlos syndrome (EDS).
Individuals who may be at risk of developing these lesions include obese patients, individuals with pes planus, and people who have occupations that require long periods of standing. It can also be seen in athletes who participate in long-distance running or high-impact sports. Piezogenic pedal papules have been described as one of the core skin findings in patients with hypermobile Ehlers-Danlos syndrome (hEDS), which also includes skin hyperextensibility, joint hypermobility, tissue fragility with atrophic cutaneous scars, and abnormal bruising and bleeding. Our patient presented with some of these characteristics (piezogenic papules, soft elastic skin, and some joint hypermobility) but did not fulfill all the criteria for the diagnosis of hEDS or other types of EDS.
The diagnosis of hEDS is based on the 2017 diagnostic criteria checklist. To be diagnosed with hEDS, the patient may have all three parts of the diagnostic criteria. The three domains include generalized joint hypermobility (partially met by our patient), evidence of syndromic features, musculoskeletal complications, and/or family history (she had a few of these criteria, including piezogenic papules and striae), and the exclusion of alternative diagnoses (see references for the PDF checklist). As she does have some features, we diagnosed her with hypermobility spectrum disorder. There is no genetic testing available for the hypermobile spectrum disorder or the hypermobile type of EDS. Given that these patients can present with mitral valve prolapse, she was referred to a cardiac echocardiogram, which was reported as normal.
The diagnosis of PPP is made clinically, and rarely a biopsy is required. Biopsies of the lesions show hyperkeratosis, degeneration of the thin fibrous septa between fat lobules, and subsequent coalescence of fat. If the presentation is atypical, a high-frequency ultrasound can be requested to confirm the physical exam findings.
If the lesions are fixed, firm, and solitary, a diagnosis to consider is juvenile aponeurotic fibroma, which occurs more often in children and adolescents on the wrists and is less common on the ankles. If there is suspicion for this condition, a plain radiograph will show stippled calcifications.
PPP are usually asymptomatic and need no further treatment. When they are symptomatic, conservative management should be considered first, which includes behavioral modifications, weight loss, avoidance of prolonged standing, and reduced foot trauma. If these are not successful, compression socks, heel cups, and other orthotics can be recommended. Intralesional injections of betamethasone and bupivacaine have been reported as an option in patients with symptomatic PPP and a history of EDS.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested reading
Edimo CO et al. Int J Womens Dermatol. 2021 Jan. doi: 10.1016/j.ijwd.2021.01.020. Erratum in: Int J Womens Dermatol. 2021 Jul 31;7(5Part B):869-70.
Brown F, Cook C. Piezogenic Pedal Papule. 2023 Aug 16. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing, 2023. PMID: 29489228.
Levy HP. Hypermobile Ehlers-Danlos Syndrome. 2004 Oct 22 [Updated 2018 Jun 21]. In: Adam MP, Mirzaa GM, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle: University of Washington, Seattle; 1993-2023. Available from: www.ncbi.nlm.nih.gov/books/NBK1279/.
The International Consortium on Ehlers-Danlos Syndrome & Related Disorders. Diagnostic Criteria for Hypermobile Ehlers-Danlos Syndrome (hEDS). www.ehlers-danlos.com/wp-content/uploads/2017/05/hEDS-Dx-Criteria-checklist-1.pdf.
The lesions on the heels are consistent with piezogenic pedal papules. They seem to be more common in women and have been described in families, though a genetic link hasn’t been elucidated. PPP manifests as small, soft, compressible papules on the lateral aspects of the skin on the heels, more noticeable when the patient is standing, and can also present on the wrists and legs. While they may not be a cause for serious concern, understanding their causes, associated conditions, and management is important.
Piezogenic pedal papules are flesh-colored or slightly reddish and can range in size from a few millimeters to a centimeter or more. They are described as benign herniations of elastic tissue and subcutaneous fat through the reticular dermis. The lesions are triggered by increased pressure and compression, such as standing or the application of pressure on the heel. The exact etiology is not known. While piezogenic pedal papules are often asymptomatic, some individuals experience discomfort, itching, or mild pain, particularly when walking or applying pressure to the affected area, especially in patients with Ehlers-Danlos syndrome (EDS).
Individuals who may be at risk of developing these lesions include obese patients, individuals with pes planus, and people who have occupations that require long periods of standing. It can also be seen in athletes who participate in long-distance running or high-impact sports. Piezogenic pedal papules have been described as one of the core skin findings in patients with hypermobile Ehlers-Danlos syndrome (hEDS), which also includes skin hyperextensibility, joint hypermobility, tissue fragility with atrophic cutaneous scars, and abnormal bruising and bleeding. Our patient presented with some of these characteristics (piezogenic papules, soft elastic skin, and some joint hypermobility) but did not fulfill all the criteria for the diagnosis of hEDS or other types of EDS.
The diagnosis of hEDS is based on the 2017 diagnostic criteria checklist. To be diagnosed with hEDS, the patient may have all three parts of the diagnostic criteria. The three domains include generalized joint hypermobility (partially met by our patient), evidence of syndromic features, musculoskeletal complications, and/or family history (she had a few of these criteria, including piezogenic papules and striae), and the exclusion of alternative diagnoses (see references for the PDF checklist). As she does have some features, we diagnosed her with hypermobility spectrum disorder. There is no genetic testing available for the hypermobile spectrum disorder or the hypermobile type of EDS. Given that these patients can present with mitral valve prolapse, she was referred to a cardiac echocardiogram, which was reported as normal.
The diagnosis of PPP is made clinically, and rarely a biopsy is required. Biopsies of the lesions show hyperkeratosis, degeneration of the thin fibrous septa between fat lobules, and subsequent coalescence of fat. If the presentation is atypical, a high-frequency ultrasound can be requested to confirm the physical exam findings.
If the lesions are fixed, firm, and solitary, a diagnosis to consider is juvenile aponeurotic fibroma, which occurs more often in children and adolescents on the wrists and is less common on the ankles. If there is suspicion for this condition, a plain radiograph will show stippled calcifications.
PPP are usually asymptomatic and need no further treatment. When they are symptomatic, conservative management should be considered first, which includes behavioral modifications, weight loss, avoidance of prolonged standing, and reduced foot trauma. If these are not successful, compression socks, heel cups, and other orthotics can be recommended. Intralesional injections of betamethasone and bupivacaine have been reported as an option in patients with symptomatic PPP and a history of EDS.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested reading
Edimo CO et al. Int J Womens Dermatol. 2021 Jan. doi: 10.1016/j.ijwd.2021.01.020. Erratum in: Int J Womens Dermatol. 2021 Jul 31;7(5Part B):869-70.
Brown F, Cook C. Piezogenic Pedal Papule. 2023 Aug 16. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing, 2023. PMID: 29489228.
Levy HP. Hypermobile Ehlers-Danlos Syndrome. 2004 Oct 22 [Updated 2018 Jun 21]. In: Adam MP, Mirzaa GM, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle: University of Washington, Seattle; 1993-2023. Available from: www.ncbi.nlm.nih.gov/books/NBK1279/.
The International Consortium on Ehlers-Danlos Syndrome & Related Disorders. Diagnostic Criteria for Hypermobile Ehlers-Danlos Syndrome (hEDS). www.ehlers-danlos.com/wp-content/uploads/2017/05/hEDS-Dx-Criteria-checklist-1.pdf.
The lesions on the heels are consistent with piezogenic pedal papules. They seem to be more common in women and have been described in families, though a genetic link hasn’t been elucidated. PPP manifests as small, soft, compressible papules on the lateral aspects of the skin on the heels, more noticeable when the patient is standing, and can also present on the wrists and legs. While they may not be a cause for serious concern, understanding their causes, associated conditions, and management is important.
Piezogenic pedal papules are flesh-colored or slightly reddish and can range in size from a few millimeters to a centimeter or more. They are described as benign herniations of elastic tissue and subcutaneous fat through the reticular dermis. The lesions are triggered by increased pressure and compression, such as standing or the application of pressure on the heel. The exact etiology is not known. While piezogenic pedal papules are often asymptomatic, some individuals experience discomfort, itching, or mild pain, particularly when walking or applying pressure to the affected area, especially in patients with Ehlers-Danlos syndrome (EDS).
Individuals who may be at risk of developing these lesions include obese patients, individuals with pes planus, and people who have occupations that require long periods of standing. It can also be seen in athletes who participate in long-distance running or high-impact sports. Piezogenic pedal papules have been described as one of the core skin findings in patients with hypermobile Ehlers-Danlos syndrome (hEDS), which also includes skin hyperextensibility, joint hypermobility, tissue fragility with atrophic cutaneous scars, and abnormal bruising and bleeding. Our patient presented with some of these characteristics (piezogenic papules, soft elastic skin, and some joint hypermobility) but did not fulfill all the criteria for the diagnosis of hEDS or other types of EDS.
The diagnosis of hEDS is based on the 2017 diagnostic criteria checklist. To be diagnosed with hEDS, the patient may have all three parts of the diagnostic criteria. The three domains include generalized joint hypermobility (partially met by our patient), evidence of syndromic features, musculoskeletal complications, and/or family history (she had a few of these criteria, including piezogenic papules and striae), and the exclusion of alternative diagnoses (see references for the PDF checklist). As she does have some features, we diagnosed her with hypermobility spectrum disorder. There is no genetic testing available for the hypermobile spectrum disorder or the hypermobile type of EDS. Given that these patients can present with mitral valve prolapse, she was referred to a cardiac echocardiogram, which was reported as normal.
The diagnosis of PPP is made clinically, and rarely a biopsy is required. Biopsies of the lesions show hyperkeratosis, degeneration of the thin fibrous septa between fat lobules, and subsequent coalescence of fat. If the presentation is atypical, a high-frequency ultrasound can be requested to confirm the physical exam findings.
If the lesions are fixed, firm, and solitary, a diagnosis to consider is juvenile aponeurotic fibroma, which occurs more often in children and adolescents on the wrists and is less common on the ankles. If there is suspicion for this condition, a plain radiograph will show stippled calcifications.
PPP are usually asymptomatic and need no further treatment. When they are symptomatic, conservative management should be considered first, which includes behavioral modifications, weight loss, avoidance of prolonged standing, and reduced foot trauma. If these are not successful, compression socks, heel cups, and other orthotics can be recommended. Intralesional injections of betamethasone and bupivacaine have been reported as an option in patients with symptomatic PPP and a history of EDS.
Dr. Matiz is a pediatric dermatologist at Southern California Permanente Medical Group, San Diego.
Suggested reading
Edimo CO et al. Int J Womens Dermatol. 2021 Jan. doi: 10.1016/j.ijwd.2021.01.020. Erratum in: Int J Womens Dermatol. 2021 Jul 31;7(5Part B):869-70.
Brown F, Cook C. Piezogenic Pedal Papule. 2023 Aug 16. In: StatPearls [Internet]. Treasure Island, Fla.: StatPearls Publishing, 2023. PMID: 29489228.
Levy HP. Hypermobile Ehlers-Danlos Syndrome. 2004 Oct 22 [Updated 2018 Jun 21]. In: Adam MP, Mirzaa GM, Pagon RA, et al., editors. GeneReviews® [Internet]. Seattle: University of Washington, Seattle; 1993-2023. Available from: www.ncbi.nlm.nih.gov/books/NBK1279/.
The International Consortium on Ehlers-Danlos Syndrome & Related Disorders. Diagnostic Criteria for Hypermobile Ehlers-Danlos Syndrome (hEDS). www.ehlers-danlos.com/wp-content/uploads/2017/05/hEDS-Dx-Criteria-checklist-1.pdf.
On a physical exam, she has several skin-colored soft papules on the heels when she stands up (Picture 1).
She is not able to touch the floor without bending her knees, and she has normal extension of her arms and knees. She has no bruises or abnormal scars and has some striae on her legs.
Autism spectrum disorders
According to the CDC, the prevalence of autism spectrum disorders (ASD) has gone from roughly 1 in 68 children in 2010 to 1 in 36 children in 2020.1 This is nearly a 50% increase over that 10-year period. Over the last several years, there has been evidence suggesting that increasing numbers of young people with ASD or other neurodivergent conditions identify as transgender or gender diverse.2 Experts agree more careful attention must be paid to these patients.
This includes things such as difficulty with communication, possible concrete thinking, and obsessive interests. While earlier research has shown a higher incidence of ASD in those referred to specialized gender medical clinics, it is important to realize that not all of these youth are seeking medical care. They may be brought to the attention of a primary care pediatrician (PCP) if the child has discussed their gender identity at home. It is important that PCPs approach these young people with an open mind and address any coexisting mental health conditions. PCPs must be careful not to dismiss any gender identity concerns as another of the patient’s “obsessions”; rather, they should ensure the patient receives the appropriate mental health care that they need to explore these concerns. One challenge for PCPs is that there is a dearth of mental health professionals who have experience in working with young people who have both gender dysphoria and a neurodivergent condition.
For those clinicians who provide gender-affirming medical care to these young people, it is imperative that they have a thorough understanding of the patient’s gender identity and medical goals before starting any treatment. This may require extensive collaboration with the patient’s mental health provider. The clinician providing medical care may also choose to proceed slower with the introduction of hormones and their subsequent dosing to allow the young person time to continue discussing their effects with their mental health provider. To help clinicians, Dr. John Strang and a multidisciplinary group of collaborators developed a set of guidelines for co-occurring ASD and gender dysphoria in adolescents.3 More recently, Dr. Strang and other collaborators have also developed a questionnaire that can be used by clinicians in the care of these patients.4 The goal of this questionnaire is to allow the young people to “communicate their experiences and needs in a report format attuned to common autistic thinking and communication styles.”
In summary, pediatricians and those who care for children and adolescents need to be aware of the increased association between those with ASD or other neurodivergent conditions and gender dysphoria. To ensure that these young people receive optimal care, it is important to connect them to experts (if possible) in coexisting ASD and gender dysphoria. If such experts are not readily available, the National LGBTQIA+ Health Education Center has developed a resource for providing an affirmative approach to care for these young people.5 While more research is needed to better understand young people with coexisting ASD (or other neurodivergent conditions), taking an individualized approach to their care can help ensure optimal outcomes.
Dr. Cooper is assistant professor of pediatrics at University of Texas Southwestern, Dallas, and an adolescent medicine specialist at Children’s Medical Center Dallas.
References
1. Data & Statistics on Autism Spectrum Disorder. https://www.cdc.gov/ncbddd/autism/data.html.
2. Glidden D et al. Gender dysphoria and autism spectrum disorder: A systematic review of the literature. Sex Med Rev. 2016;4(1):3-14. doi:10.1016/j.sxmr.2015.10.003.
3. Strang JF et al. Initial clinical guidelines for co-occurring autism spectrum disorder and gender dysphoria or incongruence in adolescents. J Clin Child Adolesc Psychol. 2018;47(1):105-15. doi:10.1080/15374416.2016.1228462.
4. Strang JF et. al. The Gender-Diversity and Autism Questionnaire: A Community-Developed Clinical, Research, and Self-Advocacy Tool for Autistic Transgender and Gender-Diverse Young Adults. Autism Adulthood. 2023 Jun 1;5(2):175-90. doi: 10.1089/aut.2023.0002.
5. National LGBT Health Education Center. Neurodiversity & gender-diverse youth: An affirming approach to care 2020. https://www.lgbtqiahealtheducation.org/publication/neurodiversity-gender-diverse-youth-an-affirming-approach-to-care-2020/download
According to the CDC, the prevalence of autism spectrum disorders (ASD) has gone from roughly 1 in 68 children in 2010 to 1 in 36 children in 2020.1 This is nearly a 50% increase over that 10-year period. Over the last several years, there has been evidence suggesting that increasing numbers of young people with ASD or other neurodivergent conditions identify as transgender or gender diverse.2 Experts agree more careful attention must be paid to these patients.
This includes things such as difficulty with communication, possible concrete thinking, and obsessive interests. While earlier research has shown a higher incidence of ASD in those referred to specialized gender medical clinics, it is important to realize that not all of these youth are seeking medical care. They may be brought to the attention of a primary care pediatrician (PCP) if the child has discussed their gender identity at home. It is important that PCPs approach these young people with an open mind and address any coexisting mental health conditions. PCPs must be careful not to dismiss any gender identity concerns as another of the patient’s “obsessions”; rather, they should ensure the patient receives the appropriate mental health care that they need to explore these concerns. One challenge for PCPs is that there is a dearth of mental health professionals who have experience in working with young people who have both gender dysphoria and a neurodivergent condition.
For those clinicians who provide gender-affirming medical care to these young people, it is imperative that they have a thorough understanding of the patient’s gender identity and medical goals before starting any treatment. This may require extensive collaboration with the patient’s mental health provider. The clinician providing medical care may also choose to proceed slower with the introduction of hormones and their subsequent dosing to allow the young person time to continue discussing their effects with their mental health provider. To help clinicians, Dr. John Strang and a multidisciplinary group of collaborators developed a set of guidelines for co-occurring ASD and gender dysphoria in adolescents.3 More recently, Dr. Strang and other collaborators have also developed a questionnaire that can be used by clinicians in the care of these patients.4 The goal of this questionnaire is to allow the young people to “communicate their experiences and needs in a report format attuned to common autistic thinking and communication styles.”
In summary, pediatricians and those who care for children and adolescents need to be aware of the increased association between those with ASD or other neurodivergent conditions and gender dysphoria. To ensure that these young people receive optimal care, it is important to connect them to experts (if possible) in coexisting ASD and gender dysphoria. If such experts are not readily available, the National LGBTQIA+ Health Education Center has developed a resource for providing an affirmative approach to care for these young people.5 While more research is needed to better understand young people with coexisting ASD (or other neurodivergent conditions), taking an individualized approach to their care can help ensure optimal outcomes.
Dr. Cooper is assistant professor of pediatrics at University of Texas Southwestern, Dallas, and an adolescent medicine specialist at Children’s Medical Center Dallas.
References
1. Data & Statistics on Autism Spectrum Disorder. https://www.cdc.gov/ncbddd/autism/data.html.
2. Glidden D et al. Gender dysphoria and autism spectrum disorder: A systematic review of the literature. Sex Med Rev. 2016;4(1):3-14. doi:10.1016/j.sxmr.2015.10.003.
3. Strang JF et al. Initial clinical guidelines for co-occurring autism spectrum disorder and gender dysphoria or incongruence in adolescents. J Clin Child Adolesc Psychol. 2018;47(1):105-15. doi:10.1080/15374416.2016.1228462.
4. Strang JF et. al. The Gender-Diversity and Autism Questionnaire: A Community-Developed Clinical, Research, and Self-Advocacy Tool for Autistic Transgender and Gender-Diverse Young Adults. Autism Adulthood. 2023 Jun 1;5(2):175-90. doi: 10.1089/aut.2023.0002.
5. National LGBT Health Education Center. Neurodiversity & gender-diverse youth: An affirming approach to care 2020. https://www.lgbtqiahealtheducation.org/publication/neurodiversity-gender-diverse-youth-an-affirming-approach-to-care-2020/download
According to the CDC, the prevalence of autism spectrum disorders (ASD) has gone from roughly 1 in 68 children in 2010 to 1 in 36 children in 2020.1 This is nearly a 50% increase over that 10-year period. Over the last several years, there has been evidence suggesting that increasing numbers of young people with ASD or other neurodivergent conditions identify as transgender or gender diverse.2 Experts agree more careful attention must be paid to these patients.
This includes things such as difficulty with communication, possible concrete thinking, and obsessive interests. While earlier research has shown a higher incidence of ASD in those referred to specialized gender medical clinics, it is important to realize that not all of these youth are seeking medical care. They may be brought to the attention of a primary care pediatrician (PCP) if the child has discussed their gender identity at home. It is important that PCPs approach these young people with an open mind and address any coexisting mental health conditions. PCPs must be careful not to dismiss any gender identity concerns as another of the patient’s “obsessions”; rather, they should ensure the patient receives the appropriate mental health care that they need to explore these concerns. One challenge for PCPs is that there is a dearth of mental health professionals who have experience in working with young people who have both gender dysphoria and a neurodivergent condition.
For those clinicians who provide gender-affirming medical care to these young people, it is imperative that they have a thorough understanding of the patient’s gender identity and medical goals before starting any treatment. This may require extensive collaboration with the patient’s mental health provider. The clinician providing medical care may also choose to proceed slower with the introduction of hormones and their subsequent dosing to allow the young person time to continue discussing their effects with their mental health provider. To help clinicians, Dr. John Strang and a multidisciplinary group of collaborators developed a set of guidelines for co-occurring ASD and gender dysphoria in adolescents.3 More recently, Dr. Strang and other collaborators have also developed a questionnaire that can be used by clinicians in the care of these patients.4 The goal of this questionnaire is to allow the young people to “communicate their experiences and needs in a report format attuned to common autistic thinking and communication styles.”
In summary, pediatricians and those who care for children and adolescents need to be aware of the increased association between those with ASD or other neurodivergent conditions and gender dysphoria. To ensure that these young people receive optimal care, it is important to connect them to experts (if possible) in coexisting ASD and gender dysphoria. If such experts are not readily available, the National LGBTQIA+ Health Education Center has developed a resource for providing an affirmative approach to care for these young people.5 While more research is needed to better understand young people with coexisting ASD (or other neurodivergent conditions), taking an individualized approach to their care can help ensure optimal outcomes.
Dr. Cooper is assistant professor of pediatrics at University of Texas Southwestern, Dallas, and an adolescent medicine specialist at Children’s Medical Center Dallas.
References
1. Data & Statistics on Autism Spectrum Disorder. https://www.cdc.gov/ncbddd/autism/data.html.
2. Glidden D et al. Gender dysphoria and autism spectrum disorder: A systematic review of the literature. Sex Med Rev. 2016;4(1):3-14. doi:10.1016/j.sxmr.2015.10.003.
3. Strang JF et al. Initial clinical guidelines for co-occurring autism spectrum disorder and gender dysphoria or incongruence in adolescents. J Clin Child Adolesc Psychol. 2018;47(1):105-15. doi:10.1080/15374416.2016.1228462.
4. Strang JF et. al. The Gender-Diversity and Autism Questionnaire: A Community-Developed Clinical, Research, and Self-Advocacy Tool for Autistic Transgender and Gender-Diverse Young Adults. Autism Adulthood. 2023 Jun 1;5(2):175-90. doi: 10.1089/aut.2023.0002.
5. National LGBT Health Education Center. Neurodiversity & gender-diverse youth: An affirming approach to care 2020. https://www.lgbtqiahealtheducation.org/publication/neurodiversity-gender-diverse-youth-an-affirming-approach-to-care-2020/download
Novel triple-threat approach to acne beats placebo
TOPLINE:
A topical fixed-dose combination of three approved acne treatments significantly improves moderate to severe acne with a strong safety profile.
METHODOLOGY:
- The two multicenter studies included 363 individuals aged 9 years and older with moderate to severe acne from 30 centers, including 15 in North America.
- Moderate to severe acne was defined as having 30-100 inflammatory lesions (papules, pustules, or nodules), 35-150 noninflammatory lesions (open or closed comedones), and at least two nodules.
- Participants were randomly assigned to receive treatment with a combination gel containing phosphate 1.2%, 0.15%, and 3.1% (known as IDP-126) or a vehicle gel for once-daily application for 12 weeks.
- Treatment success was defined as a reduction of at least two grades from baseline on the Evaluator’s Global Severity Score (EGSS) and lesion counts of clear (0) or almost clear (1) at weeks 2, 4, 8, and 12.
TAKEAWAY:
- Treatment success occurred in 49.6% of the IDP-126 group, vs 24.9% of the vehicle group in study 1, and in 50.5% of the IDP-126 group, vs 20.5% of the vehicle group in study 2. Overall treatment compliance was 93.7% and 91.3% for studies 1 and 2, respectively (P < .01 for both).
- Patients in the IDP-126 groups for both studies 1 and 2 had significantly greater absolute mean reductions in both inflammatory and noninflammatory lesions from baseline to week 12 compared to the vehicle patients (P ≤ .001 for all).
- Significantly more patients in the IDP-126 group achieved a grade reduction of 2 or more in EGSS compared with those who received the vehicle, with treatment differences of approximately 32% in both studies. Changes in lesion reductions between the treatment and the vehicle groups were significantly greater as early as week 4.
- The most common treatment-related adverse events among patients treated with IDP-126 were erythema, application-site pain, dryness, irritation, and exfoliation. Discontinuation of the study drug as a result of adverse events occurred in 2.5% and 3.3% of these patients in studies 1 and 2, respectively.
IN PRACTICE:
“With its simple treatment regimen containing 3 recommended acne treatments (benzoyl peroxide, a topical retinoid, and a topical antibiotic), IDP-126 is a potential new treatment option for acne,” the researchers concluded.
SOURCE:
The study was led by Linda Stein Gold, MD, of Henry Ford Hospital, Detroit. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
In both studies, treatment duration was short, and the studies may not reflect patients’ real-world experiences. The results may be affected by interobserver bias or variation in assessment of acne severity.
DISCLOSURES:
Gold has served as investigator/consultant or speaker for Ortho Dermatologics, LEO Pharma, Dermavant, Incyte, Novartis, AbbVie, Pfizer, Sun Pharma, UCB, Arcutis, and Lilly. Other study coauthors have relationships with multiple companies, including Ortho Dermatologics, which provided medical writing support for the study.
A version of this article first appeared on Medscape.com.
TOPLINE:
A topical fixed-dose combination of three approved acne treatments significantly improves moderate to severe acne with a strong safety profile.
METHODOLOGY:
- The two multicenter studies included 363 individuals aged 9 years and older with moderate to severe acne from 30 centers, including 15 in North America.
- Moderate to severe acne was defined as having 30-100 inflammatory lesions (papules, pustules, or nodules), 35-150 noninflammatory lesions (open or closed comedones), and at least two nodules.
- Participants were randomly assigned to receive treatment with a combination gel containing phosphate 1.2%, 0.15%, and 3.1% (known as IDP-126) or a vehicle gel for once-daily application for 12 weeks.
- Treatment success was defined as a reduction of at least two grades from baseline on the Evaluator’s Global Severity Score (EGSS) and lesion counts of clear (0) or almost clear (1) at weeks 2, 4, 8, and 12.
TAKEAWAY:
- Treatment success occurred in 49.6% of the IDP-126 group, vs 24.9% of the vehicle group in study 1, and in 50.5% of the IDP-126 group, vs 20.5% of the vehicle group in study 2. Overall treatment compliance was 93.7% and 91.3% for studies 1 and 2, respectively (P < .01 for both).
- Patients in the IDP-126 groups for both studies 1 and 2 had significantly greater absolute mean reductions in both inflammatory and noninflammatory lesions from baseline to week 12 compared to the vehicle patients (P ≤ .001 for all).
- Significantly more patients in the IDP-126 group achieved a grade reduction of 2 or more in EGSS compared with those who received the vehicle, with treatment differences of approximately 32% in both studies. Changes in lesion reductions between the treatment and the vehicle groups were significantly greater as early as week 4.
- The most common treatment-related adverse events among patients treated with IDP-126 were erythema, application-site pain, dryness, irritation, and exfoliation. Discontinuation of the study drug as a result of adverse events occurred in 2.5% and 3.3% of these patients in studies 1 and 2, respectively.
IN PRACTICE:
“With its simple treatment regimen containing 3 recommended acne treatments (benzoyl peroxide, a topical retinoid, and a topical antibiotic), IDP-126 is a potential new treatment option for acne,” the researchers concluded.
SOURCE:
The study was led by Linda Stein Gold, MD, of Henry Ford Hospital, Detroit. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
In both studies, treatment duration was short, and the studies may not reflect patients’ real-world experiences. The results may be affected by interobserver bias or variation in assessment of acne severity.
DISCLOSURES:
Gold has served as investigator/consultant or speaker for Ortho Dermatologics, LEO Pharma, Dermavant, Incyte, Novartis, AbbVie, Pfizer, Sun Pharma, UCB, Arcutis, and Lilly. Other study coauthors have relationships with multiple companies, including Ortho Dermatologics, which provided medical writing support for the study.
A version of this article first appeared on Medscape.com.
TOPLINE:
A topical fixed-dose combination of three approved acne treatments significantly improves moderate to severe acne with a strong safety profile.
METHODOLOGY:
- The two multicenter studies included 363 individuals aged 9 years and older with moderate to severe acne from 30 centers, including 15 in North America.
- Moderate to severe acne was defined as having 30-100 inflammatory lesions (papules, pustules, or nodules), 35-150 noninflammatory lesions (open or closed comedones), and at least two nodules.
- Participants were randomly assigned to receive treatment with a combination gel containing phosphate 1.2%, 0.15%, and 3.1% (known as IDP-126) or a vehicle gel for once-daily application for 12 weeks.
- Treatment success was defined as a reduction of at least two grades from baseline on the Evaluator’s Global Severity Score (EGSS) and lesion counts of clear (0) or almost clear (1) at weeks 2, 4, 8, and 12.
TAKEAWAY:
- Treatment success occurred in 49.6% of the IDP-126 group, vs 24.9% of the vehicle group in study 1, and in 50.5% of the IDP-126 group, vs 20.5% of the vehicle group in study 2. Overall treatment compliance was 93.7% and 91.3% for studies 1 and 2, respectively (P < .01 for both).
- Patients in the IDP-126 groups for both studies 1 and 2 had significantly greater absolute mean reductions in both inflammatory and noninflammatory lesions from baseline to week 12 compared to the vehicle patients (P ≤ .001 for all).
- Significantly more patients in the IDP-126 group achieved a grade reduction of 2 or more in EGSS compared with those who received the vehicle, with treatment differences of approximately 32% in both studies. Changes in lesion reductions between the treatment and the vehicle groups were significantly greater as early as week 4.
- The most common treatment-related adverse events among patients treated with IDP-126 were erythema, application-site pain, dryness, irritation, and exfoliation. Discontinuation of the study drug as a result of adverse events occurred in 2.5% and 3.3% of these patients in studies 1 and 2, respectively.
IN PRACTICE:
“With its simple treatment regimen containing 3 recommended acne treatments (benzoyl peroxide, a topical retinoid, and a topical antibiotic), IDP-126 is a potential new treatment option for acne,” the researchers concluded.
SOURCE:
The study was led by Linda Stein Gold, MD, of Henry Ford Hospital, Detroit. The study was published online in the Journal of the American Academy of Dermatology.
LIMITATIONS:
In both studies, treatment duration was short, and the studies may not reflect patients’ real-world experiences. The results may be affected by interobserver bias or variation in assessment of acne severity.
DISCLOSURES:
Gold has served as investigator/consultant or speaker for Ortho Dermatologics, LEO Pharma, Dermavant, Incyte, Novartis, AbbVie, Pfizer, Sun Pharma, UCB, Arcutis, and Lilly. Other study coauthors have relationships with multiple companies, including Ortho Dermatologics, which provided medical writing support for the study.
A version of this article first appeared on Medscape.com.
Topical botanical drug coacillium curbs childhood alopecia
Considerable hair regrowth can be achieved in children with alopecia areata with the use of a novel plant-based drug, according to research presented during the first late-breaking news session at the annual congress of the European Academy of Dermatology and Venereology.
(–8.0%), with a significant 31% overall difference (P < .0001).
“Coacillium cutaneous solution was used for the first time for treatment of alopecia areata and also for the first time used in a pediatric population,” the presenting investigator Ulrike Blume-Peytavi, MD, said at the meeting.
“It’s well tolerated, and in fact what is interesting is, it has a durable response, even after treatment discontinuation,” added Dr. Blume-Peytavi, who is the deputy head of the department of dermatology, venereology and allergology at Charité-Universitätsmedizin Berlin.
Backing the botanical?
Paola Pasquali, MD, a dermatologist at Pius Hospital de Valls in Spain, who cochaired the session where the findings were presented, commented, “Thank you for showing that chocolate is great! I knew it. It is fantastic to see how chocolate is used.”
Dr. Pasquali was referring to the coacillium ingredient Theobroma cacao extract. The seeds of T. cacao, or the cocoa tree, are used to make various types of chocolate products. Theobroma cacao is one of four plant extracts that make up coacillium, the others being Allium cepa (onion), Citrus limon (lemon), and Paullinia cupana (guaraná, a source of caffeine).
The four plant extracts are classified as “generally regarded as safe” (GRAS), Dr. Blume-Peytavi observed, noting that the development of coacillium fell under the category of a prescription botanical drug as set out by the U.S. Food and Drug Administration or a herbal medicinal product as set out by the European Medicines Agency.
But how does it work?
The botanical’s mode of action of acting positively on hair follicle cycling and endothelial cell activation was called into question, however, by Emma Guttman-Yassky, MD, PhD, who was in the audience.
She asked, “So how do you explain that, after three large studies with topical JAK inhibitors that did not work actually in alopecia areata because it’s very hard to penetrate the scalp for a topical [drug], this one works?”
Dr. Guttman-Yassky, professor of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York, added: “Looking at the ingredients, to me, it seems that it’s more like a DPCP [diphenylcyclopropenone]-like reaction.”
DPCP, which has been used to treat alopecia, purportedly works by stimulating the immune response to target the skin surface – causing an allergic reaction – rather than the hair follicle.
It’s an interesting question as to how a molecule penetrates the hair follicle, and it depends on the size of the molecule, Dr. Blume-Peytavi responded.
“We have done a lot of studies on follicular penetration, and we are quite aware that you need a certain size of the molecule,” she said. Between 14 and 200 nanometers appears to produce “the best penetrators,” she observed.
Dr. Blume-Peytavi commented that even after topical JAK inhibitors are applied, the molecules that penetrate do not remain in the local area for very long, yet still produce an inhibitory signaling effect.
No scalp irritation was seen in the trial, which suggests that coacillium is not working in the same way as DPCP, Dr. Blume-Peytavi countered.
Evaluating efficacy and safety: The RAAINBOW study
Dr. Blume-Peytavi acknowledged that JAK inhibitors were “a tremendous advance in treating severe and very severe alopecia areata,” but because of their benefit-to-risk ratio, there was still an unmet need for new treatments, particularly in children, in whom drug safety is of critical importance.
Having a drug that could be given safely and also have an effect early on in the disease, while it is still at a mild to moderate stage, would be of considerable value, Dr. Blume-Peytavi maintained.
The RAAINBOW study was a randomized, double-blind, phase 2/3 trial conducted at 12 sites in Germany and three other countries between March 2018 and March 2022 to evaluate the efficacy and safety of coacillium in the treatment of children and adolescents with moderate to severe alopecia areata.
In all, 62 children aged 2-18 years (mean age, 11 years) participated; 42 were treated twice daily with coacillium cutaneous solution 22.5% and 20 received placebo for 24 weeks. Treatment was then stopped, and participants followed for another 24 weeks off treatment to check for disease relapse, bringing the total study duration up to 48 weeks.
Baseline characteristics were “relatively comparable for severity,” Dr. Blume-Peytavi said. Most of the children had severe alopecia areata (57% for coacillium and 65% for placebo); the remainder had moderate disease (43% vs. 35%, respectively).
The average SALT scores at the start of treatment were 56 in the coacillium group and 62 in the placebo group, and a respective 44 and 61 at the end of 24 weeks’ treatment.
Perhaps the most important results, Dr. Blume-Peytavi said, was that at 48 weeks of follow-up, which was 24 weeks after treatment had been discontinued, the mean SALT scores were 29 for coacillium and 56 for placebo (P < .0001).
“You can see the improvement in the treated group is continuing even without treatment. However, the placebo group stays relatively about the same range,” she said.
Overall, 82% of patients treated with coacillium and 37% of those who received placebo experienced hair growth after treatment had stopped, and by week 48, a respective 46.7% vs. 9.1% had a SALT score of 20 or less, and 30.0% vs. 0% had a SALT score of 10 or less.
No safety concerns were raised, with no serious treatment-related reactions, no immunosuppressant-like reactions, and no steroidlike side effects.
Beyond the RAAINBOW
Larger studies are needed, Dr. Blume-Peytavi said. According to developer Legacy Healthcare’s website, coacillium cutaneous solution is not being developed just for childhood alopecia areata. It is also under investigation as a treatment for persistent chemotherapy-induced alopecia, atopic dermatitis, and psoriasis. In addition, an oral solution is being tested for cancer-related fatigue.
The study was funded by Legacy Healthcare. Dr. Blume-Peytavi has received research funding and acts as an advisor to the company, among others; four of the study’s coauthors are employees of the company. Dr. Pasquali and Dr. Guttman-Yassky were not involved in the study and had no relevant financial ties to disclose.
A version of this article first appeared on Medscape.com.
Considerable hair regrowth can be achieved in children with alopecia areata with the use of a novel plant-based drug, according to research presented during the first late-breaking news session at the annual congress of the European Academy of Dermatology and Venereology.
(–8.0%), with a significant 31% overall difference (P < .0001).
“Coacillium cutaneous solution was used for the first time for treatment of alopecia areata and also for the first time used in a pediatric population,” the presenting investigator Ulrike Blume-Peytavi, MD, said at the meeting.
“It’s well tolerated, and in fact what is interesting is, it has a durable response, even after treatment discontinuation,” added Dr. Blume-Peytavi, who is the deputy head of the department of dermatology, venereology and allergology at Charité-Universitätsmedizin Berlin.
Backing the botanical?
Paola Pasquali, MD, a dermatologist at Pius Hospital de Valls in Spain, who cochaired the session where the findings were presented, commented, “Thank you for showing that chocolate is great! I knew it. It is fantastic to see how chocolate is used.”
Dr. Pasquali was referring to the coacillium ingredient Theobroma cacao extract. The seeds of T. cacao, or the cocoa tree, are used to make various types of chocolate products. Theobroma cacao is one of four plant extracts that make up coacillium, the others being Allium cepa (onion), Citrus limon (lemon), and Paullinia cupana (guaraná, a source of caffeine).
The four plant extracts are classified as “generally regarded as safe” (GRAS), Dr. Blume-Peytavi observed, noting that the development of coacillium fell under the category of a prescription botanical drug as set out by the U.S. Food and Drug Administration or a herbal medicinal product as set out by the European Medicines Agency.
But how does it work?
The botanical’s mode of action of acting positively on hair follicle cycling and endothelial cell activation was called into question, however, by Emma Guttman-Yassky, MD, PhD, who was in the audience.
She asked, “So how do you explain that, after three large studies with topical JAK inhibitors that did not work actually in alopecia areata because it’s very hard to penetrate the scalp for a topical [drug], this one works?”
Dr. Guttman-Yassky, professor of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York, added: “Looking at the ingredients, to me, it seems that it’s more like a DPCP [diphenylcyclopropenone]-like reaction.”
DPCP, which has been used to treat alopecia, purportedly works by stimulating the immune response to target the skin surface – causing an allergic reaction – rather than the hair follicle.
It’s an interesting question as to how a molecule penetrates the hair follicle, and it depends on the size of the molecule, Dr. Blume-Peytavi responded.
“We have done a lot of studies on follicular penetration, and we are quite aware that you need a certain size of the molecule,” she said. Between 14 and 200 nanometers appears to produce “the best penetrators,” she observed.
Dr. Blume-Peytavi commented that even after topical JAK inhibitors are applied, the molecules that penetrate do not remain in the local area for very long, yet still produce an inhibitory signaling effect.
No scalp irritation was seen in the trial, which suggests that coacillium is not working in the same way as DPCP, Dr. Blume-Peytavi countered.
Evaluating efficacy and safety: The RAAINBOW study
Dr. Blume-Peytavi acknowledged that JAK inhibitors were “a tremendous advance in treating severe and very severe alopecia areata,” but because of their benefit-to-risk ratio, there was still an unmet need for new treatments, particularly in children, in whom drug safety is of critical importance.
Having a drug that could be given safely and also have an effect early on in the disease, while it is still at a mild to moderate stage, would be of considerable value, Dr. Blume-Peytavi maintained.
The RAAINBOW study was a randomized, double-blind, phase 2/3 trial conducted at 12 sites in Germany and three other countries between March 2018 and March 2022 to evaluate the efficacy and safety of coacillium in the treatment of children and adolescents with moderate to severe alopecia areata.
In all, 62 children aged 2-18 years (mean age, 11 years) participated; 42 were treated twice daily with coacillium cutaneous solution 22.5% and 20 received placebo for 24 weeks. Treatment was then stopped, and participants followed for another 24 weeks off treatment to check for disease relapse, bringing the total study duration up to 48 weeks.
Baseline characteristics were “relatively comparable for severity,” Dr. Blume-Peytavi said. Most of the children had severe alopecia areata (57% for coacillium and 65% for placebo); the remainder had moderate disease (43% vs. 35%, respectively).
The average SALT scores at the start of treatment were 56 in the coacillium group and 62 in the placebo group, and a respective 44 and 61 at the end of 24 weeks’ treatment.
Perhaps the most important results, Dr. Blume-Peytavi said, was that at 48 weeks of follow-up, which was 24 weeks after treatment had been discontinued, the mean SALT scores were 29 for coacillium and 56 for placebo (P < .0001).
“You can see the improvement in the treated group is continuing even without treatment. However, the placebo group stays relatively about the same range,” she said.
Overall, 82% of patients treated with coacillium and 37% of those who received placebo experienced hair growth after treatment had stopped, and by week 48, a respective 46.7% vs. 9.1% had a SALT score of 20 or less, and 30.0% vs. 0% had a SALT score of 10 or less.
No safety concerns were raised, with no serious treatment-related reactions, no immunosuppressant-like reactions, and no steroidlike side effects.
Beyond the RAAINBOW
Larger studies are needed, Dr. Blume-Peytavi said. According to developer Legacy Healthcare’s website, coacillium cutaneous solution is not being developed just for childhood alopecia areata. It is also under investigation as a treatment for persistent chemotherapy-induced alopecia, atopic dermatitis, and psoriasis. In addition, an oral solution is being tested for cancer-related fatigue.
The study was funded by Legacy Healthcare. Dr. Blume-Peytavi has received research funding and acts as an advisor to the company, among others; four of the study’s coauthors are employees of the company. Dr. Pasquali and Dr. Guttman-Yassky were not involved in the study and had no relevant financial ties to disclose.
A version of this article first appeared on Medscape.com.
Considerable hair regrowth can be achieved in children with alopecia areata with the use of a novel plant-based drug, according to research presented during the first late-breaking news session at the annual congress of the European Academy of Dermatology and Venereology.
(–8.0%), with a significant 31% overall difference (P < .0001).
“Coacillium cutaneous solution was used for the first time for treatment of alopecia areata and also for the first time used in a pediatric population,” the presenting investigator Ulrike Blume-Peytavi, MD, said at the meeting.
“It’s well tolerated, and in fact what is interesting is, it has a durable response, even after treatment discontinuation,” added Dr. Blume-Peytavi, who is the deputy head of the department of dermatology, venereology and allergology at Charité-Universitätsmedizin Berlin.
Backing the botanical?
Paola Pasquali, MD, a dermatologist at Pius Hospital de Valls in Spain, who cochaired the session where the findings were presented, commented, “Thank you for showing that chocolate is great! I knew it. It is fantastic to see how chocolate is used.”
Dr. Pasquali was referring to the coacillium ingredient Theobroma cacao extract. The seeds of T. cacao, or the cocoa tree, are used to make various types of chocolate products. Theobroma cacao is one of four plant extracts that make up coacillium, the others being Allium cepa (onion), Citrus limon (lemon), and Paullinia cupana (guaraná, a source of caffeine).
The four plant extracts are classified as “generally regarded as safe” (GRAS), Dr. Blume-Peytavi observed, noting that the development of coacillium fell under the category of a prescription botanical drug as set out by the U.S. Food and Drug Administration or a herbal medicinal product as set out by the European Medicines Agency.
But how does it work?
The botanical’s mode of action of acting positively on hair follicle cycling and endothelial cell activation was called into question, however, by Emma Guttman-Yassky, MD, PhD, who was in the audience.
She asked, “So how do you explain that, after three large studies with topical JAK inhibitors that did not work actually in alopecia areata because it’s very hard to penetrate the scalp for a topical [drug], this one works?”
Dr. Guttman-Yassky, professor of dermatology and immunology at the Icahn School of Medicine at Mount Sinai, New York, added: “Looking at the ingredients, to me, it seems that it’s more like a DPCP [diphenylcyclopropenone]-like reaction.”
DPCP, which has been used to treat alopecia, purportedly works by stimulating the immune response to target the skin surface – causing an allergic reaction – rather than the hair follicle.
It’s an interesting question as to how a molecule penetrates the hair follicle, and it depends on the size of the molecule, Dr. Blume-Peytavi responded.
“We have done a lot of studies on follicular penetration, and we are quite aware that you need a certain size of the molecule,” she said. Between 14 and 200 nanometers appears to produce “the best penetrators,” she observed.
Dr. Blume-Peytavi commented that even after topical JAK inhibitors are applied, the molecules that penetrate do not remain in the local area for very long, yet still produce an inhibitory signaling effect.
No scalp irritation was seen in the trial, which suggests that coacillium is not working in the same way as DPCP, Dr. Blume-Peytavi countered.
Evaluating efficacy and safety: The RAAINBOW study
Dr. Blume-Peytavi acknowledged that JAK inhibitors were “a tremendous advance in treating severe and very severe alopecia areata,” but because of their benefit-to-risk ratio, there was still an unmet need for new treatments, particularly in children, in whom drug safety is of critical importance.
Having a drug that could be given safely and also have an effect early on in the disease, while it is still at a mild to moderate stage, would be of considerable value, Dr. Blume-Peytavi maintained.
The RAAINBOW study was a randomized, double-blind, phase 2/3 trial conducted at 12 sites in Germany and three other countries between March 2018 and March 2022 to evaluate the efficacy and safety of coacillium in the treatment of children and adolescents with moderate to severe alopecia areata.
In all, 62 children aged 2-18 years (mean age, 11 years) participated; 42 were treated twice daily with coacillium cutaneous solution 22.5% and 20 received placebo for 24 weeks. Treatment was then stopped, and participants followed for another 24 weeks off treatment to check for disease relapse, bringing the total study duration up to 48 weeks.
Baseline characteristics were “relatively comparable for severity,” Dr. Blume-Peytavi said. Most of the children had severe alopecia areata (57% for coacillium and 65% for placebo); the remainder had moderate disease (43% vs. 35%, respectively).
The average SALT scores at the start of treatment were 56 in the coacillium group and 62 in the placebo group, and a respective 44 and 61 at the end of 24 weeks’ treatment.
Perhaps the most important results, Dr. Blume-Peytavi said, was that at 48 weeks of follow-up, which was 24 weeks after treatment had been discontinued, the mean SALT scores were 29 for coacillium and 56 for placebo (P < .0001).
“You can see the improvement in the treated group is continuing even without treatment. However, the placebo group stays relatively about the same range,” she said.
Overall, 82% of patients treated with coacillium and 37% of those who received placebo experienced hair growth after treatment had stopped, and by week 48, a respective 46.7% vs. 9.1% had a SALT score of 20 or less, and 30.0% vs. 0% had a SALT score of 10 or less.
No safety concerns were raised, with no serious treatment-related reactions, no immunosuppressant-like reactions, and no steroidlike side effects.
Beyond the RAAINBOW
Larger studies are needed, Dr. Blume-Peytavi said. According to developer Legacy Healthcare’s website, coacillium cutaneous solution is not being developed just for childhood alopecia areata. It is also under investigation as a treatment for persistent chemotherapy-induced alopecia, atopic dermatitis, and psoriasis. In addition, an oral solution is being tested for cancer-related fatigue.
The study was funded by Legacy Healthcare. Dr. Blume-Peytavi has received research funding and acts as an advisor to the company, among others; four of the study’s coauthors are employees of the company. Dr. Pasquali and Dr. Guttman-Yassky were not involved in the study and had no relevant financial ties to disclose.
A version of this article first appeared on Medscape.com.
AT THE EADV CONGRESS
New RSV vaccine will cut hospitalizations, study shows
, according to research presented at an annual scientific meeting on infectious diseases.
“With RSV maternal vaccination that is associated with clinical efficacy of 69% against severe RSV disease at 6 months, we estimated that up to 200,000 cases can be averted, and that is associated with almost $800 million in total,” presenting author Amy W. Law, PharmD, director of global value and evidence at Pfizer, pointed out during a news briefing.
“RSV is associated with a significant burden in the U.S. and this newly approved and recommended maternal RSV vaccine can have substantial impact in easing some of that burden,” Dr. Law explained.
This study is “particularly timely as we head into RSV peak season,” said briefing moderator Natasha Halasa, MD, MPH, professor of pediatrics, division of pediatric infectious diseases at Vanderbilt University, Nashville, Tenn.
The challenge, said Dr. Halasa, is that uptake of maternal vaccines and vaccines in general is “not optimal,” making increased awareness of this new maternal RSV vaccine important.
Strong efficacy data
Most children are infected with RSV at least once by the time they reach age 2 years. Very young children are at particular risk of severe complications, such as pneumonia or bronchitis.
As reported previously by this news organization, in the randomized, double-blind, placebo-controlled phase 3 study, Pfizer’s maternal RSV vaccine had an almost 82% efficacy against severe RSV infection in infants from birth through the first 90 days of life.
The vaccine also had a 69% efficacy against severe disease through the first 6 months of life. As part of the trial, a total of 7,400 women received a single dose of the vaccine in the late second or third trimester of their pregnancy. There were no signs of safety issues for the mothers or infants.
Based on the results, the U.S. Food and Drug Administration approved the vaccine, known as Abrysvo, in August, to be given between weeks 32 and 36 of pregnancy.
New modeling study
Dr. Law and colleagues modeled the potential public health impact – both clinical and economic – of the maternal RSV vaccine among the population of all pregnant women and their infants born during a 12-month period in the United States. The model focused on severe RSV disease in babies that required medical attention.
According to their model, without widespread use of the maternal RSV vaccine, 48,246 hospitalizations, 144,495 emergency department encounters, and 399,313 outpatient clinic visits related to RSV are projected to occur annually among the U.S. birth cohort of 3.7 million infants younger than 12 months.
With widespread use of the vaccine, annual hospitalizations resulting from infant RSV would fall by 51%, emergency department encounters would decline by 32%, and outpatient clinic visits by 32% – corresponding to a decrease in direct medical costs of about $692 million and indirect nonmedical costs of roughly $110 million.
Dr. Law highlighted two important caveats to the data. “The protections are based on the year-round administration of the vaccine to pregnant women at 32 to 36 weeks’ gestational age, and this is also assuming 100% uptake. Of course, in reality, that most likely is not the case,” she told the briefing.
Dr. Halasa noted that the peak age for severe RSV illness is 3 months and it’s tough to identify infants at highest risk for severe RSV.
Nearly 80% of infants with RSV who are hospitalized do not have an underlying medical condition, “so we don’t even know who those high-risk infants are. That’s why having this vaccine is so exciting,” she told the briefing.
Dr. Halasa said it’s also important to note that infants with severe RSV typically make not just one but multiple visits to the clinic or emergency department, leading to missed days of work for the parent, not to mention the “emotional burden of having your otherwise healthy newborn or young infant in the hospital.”
In addition to Pfizer’s maternal RSV vaccine, the FDA in July approved AstraZeneca’s monoclonal antibody nirsevimab (Beyfortus) for the prevention of RSV in neonates and infants entering their first RSV season, and in children up to 24 months who remain vulnerable to severe RSV disease through their second RSV season.
The study was funded by Pfizer. Dr. Law is employed by Pfizer. Dr. Halasa has received grant and research support from Merck.
A version of this article first appeared on Medscape.com.
, according to research presented at an annual scientific meeting on infectious diseases.
“With RSV maternal vaccination that is associated with clinical efficacy of 69% against severe RSV disease at 6 months, we estimated that up to 200,000 cases can be averted, and that is associated with almost $800 million in total,” presenting author Amy W. Law, PharmD, director of global value and evidence at Pfizer, pointed out during a news briefing.
“RSV is associated with a significant burden in the U.S. and this newly approved and recommended maternal RSV vaccine can have substantial impact in easing some of that burden,” Dr. Law explained.
This study is “particularly timely as we head into RSV peak season,” said briefing moderator Natasha Halasa, MD, MPH, professor of pediatrics, division of pediatric infectious diseases at Vanderbilt University, Nashville, Tenn.
The challenge, said Dr. Halasa, is that uptake of maternal vaccines and vaccines in general is “not optimal,” making increased awareness of this new maternal RSV vaccine important.
Strong efficacy data
Most children are infected with RSV at least once by the time they reach age 2 years. Very young children are at particular risk of severe complications, such as pneumonia or bronchitis.
As reported previously by this news organization, in the randomized, double-blind, placebo-controlled phase 3 study, Pfizer’s maternal RSV vaccine had an almost 82% efficacy against severe RSV infection in infants from birth through the first 90 days of life.
The vaccine also had a 69% efficacy against severe disease through the first 6 months of life. As part of the trial, a total of 7,400 women received a single dose of the vaccine in the late second or third trimester of their pregnancy. There were no signs of safety issues for the mothers or infants.
Based on the results, the U.S. Food and Drug Administration approved the vaccine, known as Abrysvo, in August, to be given between weeks 32 and 36 of pregnancy.
New modeling study
Dr. Law and colleagues modeled the potential public health impact – both clinical and economic – of the maternal RSV vaccine among the population of all pregnant women and their infants born during a 12-month period in the United States. The model focused on severe RSV disease in babies that required medical attention.
According to their model, without widespread use of the maternal RSV vaccine, 48,246 hospitalizations, 144,495 emergency department encounters, and 399,313 outpatient clinic visits related to RSV are projected to occur annually among the U.S. birth cohort of 3.7 million infants younger than 12 months.
With widespread use of the vaccine, annual hospitalizations resulting from infant RSV would fall by 51%, emergency department encounters would decline by 32%, and outpatient clinic visits by 32% – corresponding to a decrease in direct medical costs of about $692 million and indirect nonmedical costs of roughly $110 million.
Dr. Law highlighted two important caveats to the data. “The protections are based on the year-round administration of the vaccine to pregnant women at 32 to 36 weeks’ gestational age, and this is also assuming 100% uptake. Of course, in reality, that most likely is not the case,” she told the briefing.
Dr. Halasa noted that the peak age for severe RSV illness is 3 months and it’s tough to identify infants at highest risk for severe RSV.
Nearly 80% of infants with RSV who are hospitalized do not have an underlying medical condition, “so we don’t even know who those high-risk infants are. That’s why having this vaccine is so exciting,” she told the briefing.
Dr. Halasa said it’s also important to note that infants with severe RSV typically make not just one but multiple visits to the clinic or emergency department, leading to missed days of work for the parent, not to mention the “emotional burden of having your otherwise healthy newborn or young infant in the hospital.”
In addition to Pfizer’s maternal RSV vaccine, the FDA in July approved AstraZeneca’s monoclonal antibody nirsevimab (Beyfortus) for the prevention of RSV in neonates and infants entering their first RSV season, and in children up to 24 months who remain vulnerable to severe RSV disease through their second RSV season.
The study was funded by Pfizer. Dr. Law is employed by Pfizer. Dr. Halasa has received grant and research support from Merck.
A version of this article first appeared on Medscape.com.
, according to research presented at an annual scientific meeting on infectious diseases.
“With RSV maternal vaccination that is associated with clinical efficacy of 69% against severe RSV disease at 6 months, we estimated that up to 200,000 cases can be averted, and that is associated with almost $800 million in total,” presenting author Amy W. Law, PharmD, director of global value and evidence at Pfizer, pointed out during a news briefing.
“RSV is associated with a significant burden in the U.S. and this newly approved and recommended maternal RSV vaccine can have substantial impact in easing some of that burden,” Dr. Law explained.
This study is “particularly timely as we head into RSV peak season,” said briefing moderator Natasha Halasa, MD, MPH, professor of pediatrics, division of pediatric infectious diseases at Vanderbilt University, Nashville, Tenn.
The challenge, said Dr. Halasa, is that uptake of maternal vaccines and vaccines in general is “not optimal,” making increased awareness of this new maternal RSV vaccine important.
Strong efficacy data
Most children are infected with RSV at least once by the time they reach age 2 years. Very young children are at particular risk of severe complications, such as pneumonia or bronchitis.
As reported previously by this news organization, in the randomized, double-blind, placebo-controlled phase 3 study, Pfizer’s maternal RSV vaccine had an almost 82% efficacy against severe RSV infection in infants from birth through the first 90 days of life.
The vaccine also had a 69% efficacy against severe disease through the first 6 months of life. As part of the trial, a total of 7,400 women received a single dose of the vaccine in the late second or third trimester of their pregnancy. There were no signs of safety issues for the mothers or infants.
Based on the results, the U.S. Food and Drug Administration approved the vaccine, known as Abrysvo, in August, to be given between weeks 32 and 36 of pregnancy.
New modeling study
Dr. Law and colleagues modeled the potential public health impact – both clinical and economic – of the maternal RSV vaccine among the population of all pregnant women and their infants born during a 12-month period in the United States. The model focused on severe RSV disease in babies that required medical attention.
According to their model, without widespread use of the maternal RSV vaccine, 48,246 hospitalizations, 144,495 emergency department encounters, and 399,313 outpatient clinic visits related to RSV are projected to occur annually among the U.S. birth cohort of 3.7 million infants younger than 12 months.
With widespread use of the vaccine, annual hospitalizations resulting from infant RSV would fall by 51%, emergency department encounters would decline by 32%, and outpatient clinic visits by 32% – corresponding to a decrease in direct medical costs of about $692 million and indirect nonmedical costs of roughly $110 million.
Dr. Law highlighted two important caveats to the data. “The protections are based on the year-round administration of the vaccine to pregnant women at 32 to 36 weeks’ gestational age, and this is also assuming 100% uptake. Of course, in reality, that most likely is not the case,” she told the briefing.
Dr. Halasa noted that the peak age for severe RSV illness is 3 months and it’s tough to identify infants at highest risk for severe RSV.
Nearly 80% of infants with RSV who are hospitalized do not have an underlying medical condition, “so we don’t even know who those high-risk infants are. That’s why having this vaccine is so exciting,” she told the briefing.
Dr. Halasa said it’s also important to note that infants with severe RSV typically make not just one but multiple visits to the clinic or emergency department, leading to missed days of work for the parent, not to mention the “emotional burden of having your otherwise healthy newborn or young infant in the hospital.”
In addition to Pfizer’s maternal RSV vaccine, the FDA in July approved AstraZeneca’s monoclonal antibody nirsevimab (Beyfortus) for the prevention of RSV in neonates and infants entering their first RSV season, and in children up to 24 months who remain vulnerable to severe RSV disease through their second RSV season.
The study was funded by Pfizer. Dr. Law is employed by Pfizer. Dr. Halasa has received grant and research support from Merck.
A version of this article first appeared on Medscape.com.
FROM IDWEEK 2023