User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Psychological/neuropsychological testing: When to refer for reexamination
The evolution of illness prevention, diagnosis, and treatment has involved an increased appreciation for the clinical utility of longitudinal assessment. This has included the implementation of screening evaluations for high base rate medical conditions, such as cancer, that involve considerable morbidity and mortality.
Unfortunately, the mental health professions have been slow to embrace this approach. Baseline assessment with psychological/neuropsychological screening tests and more comprehensive test batteries to clarify diagnostic status and facilitate treatment planning is far more the exception than the rule in mental health care. This seems to be the case despite the strong evidence supporting this practice as well as multiple surveys indicating that psychiatrists and other physicians report a high level of satisfaction with the findings and recommendations of psychological/neuropsychological test reports.1-3
There is a substantial literature that reviews the relative indications and contraindications for initial psychological/neuropsychological test evaluations.4-7 However, there is a paucity of clinical and evidence-based information regarding criteria for follow-up assessment. Moreover, there are no consensus guidelines to inform decision-making regarding this issue.
In general, good clinical practice for baseline assessment and reexamination should include administration of both psychological and neuropsychological tests. Based on clinical experience, this article addresses the relative indications and contraindications for psychological/neuropsychological test reassessment of adults seen in psychiatric care. It also outlines suggested time frames for such reevaluations, based on the patient’s clinical status and circumstances.
Why are patients not referred for reassessment more often?
There are several reasons patients are not referred for follow-up testing, beginning with the failure, at times, of the psychologist to state in the recommendations section of the test report whether a reassessment is indicated, under what circumstances, and within what time frame. Empirical data is lacking, but predicated on clinical experience, even when a strong and unequivocal recommendation is made for reassessment, only a very small percentage of patients are seen for follow-up evaluation.
There are numerous reasons why this occurs. The patient and/or the psychiatrist may overlook or forget about the recommendation for reassessment, particularly if it was embedded in a lengthy list of recommendations and the suggested time frame for the reassessment was several years away. The patient and the psychiatrist may decide against going forward with a reexamination, for a variety of substantive reasons. The patient might decline, against medical advice, to be retested. The patient may fail to make or keep an appointment for the follow-up reexamination. The patient might leave treatment and become lost to follow-up. The patient might not be able to find an appropriate psychologist. The insurance company may decline to authorize follow-up testing.8
Indications for reevaluation
Follow-up testing generally is indicated in the following circumstances:
Patients who are likely to soon improve or worsen. Reassessment is indicated when, based on the initial evaluation, the patient has been identified as having a neuropsychiatric disorder that is likely to improve or worsen over the next year or 2 due to the natural trajectory of the condition and the degree to which it may respond to treatment.
Continue to: Patients who are likely...
Patients who are likely to improve include those with mental status changes referable to ≥1 medical and/or neuropsychiatric factors that are considered at least partially treatable and reversible. Patients who fall within this category include those who have mild to moderately severe head trauma or stroke, have a suspected or known medication- or substance-induced altered mental status, appear to have depression-related cognitive difficulties, or have an initial or recurrent episode of idiopathic psychosis.
Patients whose conditions can be expected to worsen over time include those with a mild neurocognitive disorder or major neurocognitive disorder of mild severity that is considered referable to a progressive neurodegenerative illness such as Alzheimer’s disease based on family and personal history, their psychometric test profile, and other factors, including findings from positron emission tomography scanning.
Older patients who were referred primarily due to a strong family history of major neurocognitive disorder but with no clear-cut concerning findings on baseline testing warrant reevaluation in the event of the emergence of significant cognitive and/or psychiatric symptoms and/or a functional decline since the baseline examination.
Patients who have been seen for initial test evaluations prior to interventions such as neurosurgery (including psychosurgery), electroconvulsive therapy (ECT), transcranial magnetic stimulation, cognitive rehabilitation, etc.
Patients undergoing a substantial transition. Reevaluation is appropriate for a broad range of patients experiencing difficulties when undergoing a significant lifestyle transition or change in level of care. This includes patients considering a return to school or work after a prolonged absence due to neuropsychiatric illness, or for whom there are questions regarding the need for a change in their level of everyday care. The latter includes patients who are returning to home care from assisted living, or transferring from home-based services to assisted living or a skilled nursing facility.
Continue to: What about patients with psychiatric disorders?
What about patients with psychiatric disorders? A “grey area” pertains to reassessment of patients with neuropsychiatric disorders such as schizophrenia and related psychotic disorders, bipolar disorder, major depressive disorder, and obsessive-compulsive disorder. Patients with these conditions often have high rates of cognitive/neuropsychological impairment on baseline testing, even when they appear to be improving from a psychiatric perspective, are reasonably stable, and may even be in remission.9-12
These deficits are frequently a mix of pre-illness, prodromal, and early-stage illness– related neurocognitive difficulties that, for the most part, remain stable over time. That said, there is emerging evidence of worsening cognitive change over time following a first episode of psychosis for some patients with schizophrenia.13
In general, reevaluation should be considered for patients with a family and/or personal history of cognitive/neuropsychological impairment, structural brain abnormalities on neuroimaging, a concerning cognitive/neuropsychological profile, or any other factors that raise the index of suspicion for a possible progressive deteriorative course of illness.13,14
Patients with personality disorders who have had a baseline psychometric evaluation do not clearly warrant reassessment unless they develop medical and/or psychosocial difficulties that are often linked to problematic personality traits/patterns and that result in significant and persistent mental status changes. For example, reassessment might be indicated for a patient with borderline personality disorder who has new-onset or worsening cognitive and/or psychiatric complaints/symptoms after sustaining a head injury while intoxicated and embroiled in a domestic conflict triggered by anger and fears related to abandonment and separation.
Reevaluation also should be considered when a patient with a personality disorder has had a baseline assessment and subsequently completes an intensive, long-term treatment program that is likely to improve their clinical status. In this context, retesting may help document these gains. Examples of such programs/services include residential psychiatric and/or substance abuse care, object relational/psychodynamically-based psychotherapy, an extended course of dialectical behavioral therapy, or a related coping skills/distress tolerance psychotherapy.
Continue to: Contraindications for reassessment
Contraindications for reassessment
Retesting generally is not indicated in the following circumstances:
Patients with advanced major neurocognitive disorder. Reassessment is not indicated for such patients when there are no new questions regarding diagnosis, prognosis, level of care, and/or related disposition issues.
Patients with transient episodes of poor functioning. For the most part, reassessment is not helpful for patients with well-established diagnoses and treatment plans who, based on their history, experience time-limited, recurrent episodes of poor functioning and then reliably return to their baseline with ongoing psychiatric care. This includes patients with borderline personality disorder and other personality difficulties with histories of transient decompensation in response to psychosocial and psychodynamic triggers.
Patients who do not improve or worsen over time. Reassessment is not indicated when there has been no clear, sustained improvement or worsening of a patient’s clinical status over an extended time, and a protracted change is not anticipated. In this situation, reassessment is unlikely to yield clinically useful information beyond what is already known or meaningfully impact case formulation and treatment planning.
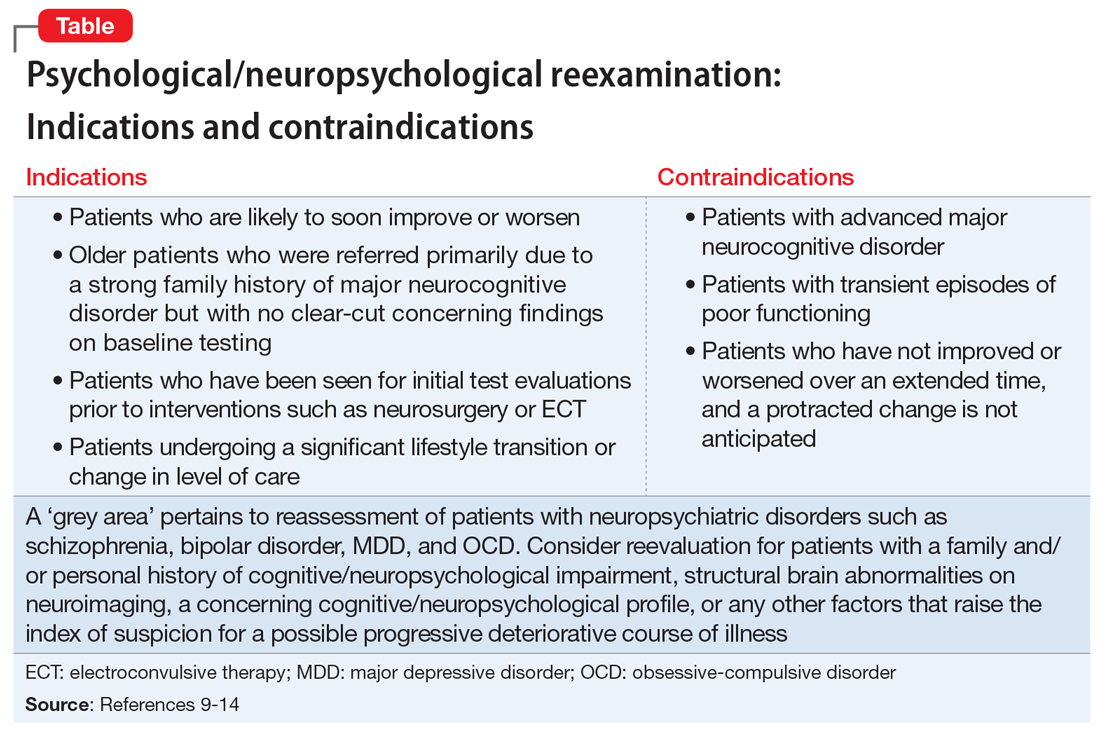
The Table9-14 summarizes the relative indications and contraindications for psychological/neuropsychological reexamination.
Continue to: Time frames for reassessment
Time frames for reassessment
Time frames for retesting vary considerably depending on factors such as diagnostic status, longitudinal course, treatment parameters, and recent/current life circumstances.
While empirical data is lacking regarding this matter, based on clinical experience, reevaluation in 18 to 24 months is generally appropriate for patients with neuropsychiatric conditions who are likely to gradually improve or slowly worsen over this time. Still, reexamination can be sooner (within 12 to 18 months) for patients who have experienced a more rapid and steep negative change in clinical status than initially anticipated.
For most patients with major mental illness, reexamination in 3 to 5 years is probably a reasonable time interval, barring a poorly understood and clinically significant negative change in functioning that warrants a shorter time frame. This suggested time frame would allow for sufficient time to better gauge improvement, stability, or deterioration in functioning and whether the reason(s) for referral have evolved. On the other hand, this time interval is somewhat arbitrary given the lack of empirical data. Therefore, on a case-by-case basis, it would be helpful for psychiatrists to consult with their patients and preferably with the psychologist who completed the baseline evaluation to determine a reasonable interval between assessments.
For patients who have undergone long-term/intensive treatment, reassessment in 3 or 6 months to as long as 1 year after the patient completes the program should be considered. Patients who undergo medical interventions such as neurosurgery or ECT—which can be associated with short-term, at least partially reversible negative effects on mental status—reassessment usually is most helpful when initiated as one or more screening level examinations for several weeks, followed by a comprehensive psychometric reassessment at the 3- to 6-month mark.
Suggestions for future research
Additional research is needed to ascertain the attitudes and opinions of psychiatrists and other physicians who use psychometric test data regarding how psychologists can most effectively communicate a recommendation for reassessment in their reports and clarify the ways psychiatrists can productively address this issue with their patients. Survey research of this kind should include questions about the frequency with which psychiatrists formally refer patients for retesting, and estimates of the rate of follow-through.
Continue to: It also would be desirable...
It also would be desirable to investigate factors that may facilitate follow-through with recommendations for reassessment, or, conversely, identify reasons that psychiatrists and their patients may decide to forgo reassessment. It would be important to try to obtain information regarding the optimum time frames for such reevaluation, depending on the patient’s circumstances and other variables. Evidence-based data pertaining to these issues would contribute to the development of consensus guidelines and a standard of care for psychological/neuropsychological test reevaluation.
Bottom Line
Only a very small percentage of patients referred for follow-up psychological/ neuropsychological test reevaluation actually undergo reexamination. Such retesting may be most helpful for certain patient populations, such as those who are likely to soon improve or worsen, were referred based on a family history of major cognitive disorder but have no concerning findings on baseline testing, or are undergoing a substantial life transition.
Related Resources
- Thom R, Farrell HM. Neuroimaging in psychiatry: potentials and pitfalls. Current Psychiatry. 2019;18(12):27-28;33-34.
- Papakostas GI. Cognitive symptoms in patients with major depressive disorder and their implications for clinical practice. J Clin Psychiatry. 2014;75(1):8-14.
- American Board of Professional Neuropsychology. https://abn-board.com
1. Schroeder RW, Martin PK, Walling A. Neuropsychological evaluations in adults. Am Fam Physician. 2019;99(2):101-198.
2. Bucher MA, Suzuki T, Samuel DB. A meta-analytic review of personality traits and their associations with mental health treatment outcomes. Clin Psychol Rev. 2019;70:51-63.
3. Pollak J. Feedback to the psychodiagnostician: a challenge for assessment psychologists in independent practice. Independent Practitioner: The community of psychologists in independent practice. 2020;40,6-9.
4. Pollak J. To test or not to test: considerations before going forward with psychometric testing. The Clinical Practitioner. 2011;6:5-10.
5. Schwarz L, Roskos PT, Grossberg GT. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):33-39.
6. Zucchella C, Federico A, Martini A, et al. Neuropsychological testing. Pract Neurol. 2018;18(3):227-237.
7. Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: a useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
8. Pollak J. Psychodiagnostic testing services: the elusive quest for clinicians. Clinical Psychiatry News. Published October 18, 2019. Accessed July 8, 2021. https://www.mdedge.com/psychiatry/article/210439/schizophrenia-other-psychotic-disorders/psychodiagnostic-testing-services
9. Mesholam-Gately RI, Giuliano AJ, Goff KP, et al. Neurocognition in first episode schizophrenia: a meta- analytic review. Neuropsychology. 2009;23(3):315-336.
10. Lam RW, Kennedy SH, McIntyre RS et al. Cognitive dysfunction in major depressive disorder: effects on psychosocial functioning and implications for treatment. Can J Psychiatry. 2014;59(12):649-654.
11. Szmulewicz AG, Samamé C, Martino DJ, et al. An updated review on the neuropsychological profile of subjects with bipolar disorder. Arch Clin Psychiatry. 2015;42(5):139-146.
12. Shin NY, Lee TY, Kim, E, et al. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol Med. 2013;44(6):1121-1130.
13. Zanelli J, Mollon J, Sandin S, et al. Cognitive change in schizophrenia and other psychoses in the decade following the first episode. Am J Psychiatry. 2019;176(10):811-819.
14. Mitleman SA, Buchsbaum MS. Very poor outcome schizophrenia: clinical and neuroimaging aspects. Int Rev Psychiatry. 2007;19(4):345-357.
The evolution of illness prevention, diagnosis, and treatment has involved an increased appreciation for the clinical utility of longitudinal assessment. This has included the implementation of screening evaluations for high base rate medical conditions, such as cancer, that involve considerable morbidity and mortality.
Unfortunately, the mental health professions have been slow to embrace this approach. Baseline assessment with psychological/neuropsychological screening tests and more comprehensive test batteries to clarify diagnostic status and facilitate treatment planning is far more the exception than the rule in mental health care. This seems to be the case despite the strong evidence supporting this practice as well as multiple surveys indicating that psychiatrists and other physicians report a high level of satisfaction with the findings and recommendations of psychological/neuropsychological test reports.1-3
There is a substantial literature that reviews the relative indications and contraindications for initial psychological/neuropsychological test evaluations.4-7 However, there is a paucity of clinical and evidence-based information regarding criteria for follow-up assessment. Moreover, there are no consensus guidelines to inform decision-making regarding this issue.
In general, good clinical practice for baseline assessment and reexamination should include administration of both psychological and neuropsychological tests. Based on clinical experience, this article addresses the relative indications and contraindications for psychological/neuropsychological test reassessment of adults seen in psychiatric care. It also outlines suggested time frames for such reevaluations, based on the patient’s clinical status and circumstances.
Why are patients not referred for reassessment more often?
There are several reasons patients are not referred for follow-up testing, beginning with the failure, at times, of the psychologist to state in the recommendations section of the test report whether a reassessment is indicated, under what circumstances, and within what time frame. Empirical data is lacking, but predicated on clinical experience, even when a strong and unequivocal recommendation is made for reassessment, only a very small percentage of patients are seen for follow-up evaluation.
There are numerous reasons why this occurs. The patient and/or the psychiatrist may overlook or forget about the recommendation for reassessment, particularly if it was embedded in a lengthy list of recommendations and the suggested time frame for the reassessment was several years away. The patient and the psychiatrist may decide against going forward with a reexamination, for a variety of substantive reasons. The patient might decline, against medical advice, to be retested. The patient may fail to make or keep an appointment for the follow-up reexamination. The patient might leave treatment and become lost to follow-up. The patient might not be able to find an appropriate psychologist. The insurance company may decline to authorize follow-up testing.8
Indications for reevaluation
Follow-up testing generally is indicated in the following circumstances:
Patients who are likely to soon improve or worsen. Reassessment is indicated when, based on the initial evaluation, the patient has been identified as having a neuropsychiatric disorder that is likely to improve or worsen over the next year or 2 due to the natural trajectory of the condition and the degree to which it may respond to treatment.
Continue to: Patients who are likely...
Patients who are likely to improve include those with mental status changes referable to ≥1 medical and/or neuropsychiatric factors that are considered at least partially treatable and reversible. Patients who fall within this category include those who have mild to moderately severe head trauma or stroke, have a suspected or known medication- or substance-induced altered mental status, appear to have depression-related cognitive difficulties, or have an initial or recurrent episode of idiopathic psychosis.
Patients whose conditions can be expected to worsen over time include those with a mild neurocognitive disorder or major neurocognitive disorder of mild severity that is considered referable to a progressive neurodegenerative illness such as Alzheimer’s disease based on family and personal history, their psychometric test profile, and other factors, including findings from positron emission tomography scanning.
Older patients who were referred primarily due to a strong family history of major neurocognitive disorder but with no clear-cut concerning findings on baseline testing warrant reevaluation in the event of the emergence of significant cognitive and/or psychiatric symptoms and/or a functional decline since the baseline examination.
Patients who have been seen for initial test evaluations prior to interventions such as neurosurgery (including psychosurgery), electroconvulsive therapy (ECT), transcranial magnetic stimulation, cognitive rehabilitation, etc.
Patients undergoing a substantial transition. Reevaluation is appropriate for a broad range of patients experiencing difficulties when undergoing a significant lifestyle transition or change in level of care. This includes patients considering a return to school or work after a prolonged absence due to neuropsychiatric illness, or for whom there are questions regarding the need for a change in their level of everyday care. The latter includes patients who are returning to home care from assisted living, or transferring from home-based services to assisted living or a skilled nursing facility.
Continue to: What about patients with psychiatric disorders?
What about patients with psychiatric disorders? A “grey area” pertains to reassessment of patients with neuropsychiatric disorders such as schizophrenia and related psychotic disorders, bipolar disorder, major depressive disorder, and obsessive-compulsive disorder. Patients with these conditions often have high rates of cognitive/neuropsychological impairment on baseline testing, even when they appear to be improving from a psychiatric perspective, are reasonably stable, and may even be in remission.9-12
These deficits are frequently a mix of pre-illness, prodromal, and early-stage illness– related neurocognitive difficulties that, for the most part, remain stable over time. That said, there is emerging evidence of worsening cognitive change over time following a first episode of psychosis for some patients with schizophrenia.13
In general, reevaluation should be considered for patients with a family and/or personal history of cognitive/neuropsychological impairment, structural brain abnormalities on neuroimaging, a concerning cognitive/neuropsychological profile, or any other factors that raise the index of suspicion for a possible progressive deteriorative course of illness.13,14
Patients with personality disorders who have had a baseline psychometric evaluation do not clearly warrant reassessment unless they develop medical and/or psychosocial difficulties that are often linked to problematic personality traits/patterns and that result in significant and persistent mental status changes. For example, reassessment might be indicated for a patient with borderline personality disorder who has new-onset or worsening cognitive and/or psychiatric complaints/symptoms after sustaining a head injury while intoxicated and embroiled in a domestic conflict triggered by anger and fears related to abandonment and separation.
Reevaluation also should be considered when a patient with a personality disorder has had a baseline assessment and subsequently completes an intensive, long-term treatment program that is likely to improve their clinical status. In this context, retesting may help document these gains. Examples of such programs/services include residential psychiatric and/or substance abuse care, object relational/psychodynamically-based psychotherapy, an extended course of dialectical behavioral therapy, or a related coping skills/distress tolerance psychotherapy.
Continue to: Contraindications for reassessment
Contraindications for reassessment
Retesting generally is not indicated in the following circumstances:
Patients with advanced major neurocognitive disorder. Reassessment is not indicated for such patients when there are no new questions regarding diagnosis, prognosis, level of care, and/or related disposition issues.
Patients with transient episodes of poor functioning. For the most part, reassessment is not helpful for patients with well-established diagnoses and treatment plans who, based on their history, experience time-limited, recurrent episodes of poor functioning and then reliably return to their baseline with ongoing psychiatric care. This includes patients with borderline personality disorder and other personality difficulties with histories of transient decompensation in response to psychosocial and psychodynamic triggers.
Patients who do not improve or worsen over time. Reassessment is not indicated when there has been no clear, sustained improvement or worsening of a patient’s clinical status over an extended time, and a protracted change is not anticipated. In this situation, reassessment is unlikely to yield clinically useful information beyond what is already known or meaningfully impact case formulation and treatment planning.
The Table9-14 summarizes the relative indications and contraindications for psychological/neuropsychological reexamination.

Continue to: Time frames for reassessment
Time frames for reassessment
Time frames for retesting vary considerably depending on factors such as diagnostic status, longitudinal course, treatment parameters, and recent/current life circumstances.
While empirical data is lacking regarding this matter, based on clinical experience, reevaluation in 18 to 24 months is generally appropriate for patients with neuropsychiatric conditions who are likely to gradually improve or slowly worsen over this time. Still, reexamination can be sooner (within 12 to 18 months) for patients who have experienced a more rapid and steep negative change in clinical status than initially anticipated.
For most patients with major mental illness, reexamination in 3 to 5 years is probably a reasonable time interval, barring a poorly understood and clinically significant negative change in functioning that warrants a shorter time frame. This suggested time frame would allow for sufficient time to better gauge improvement, stability, or deterioration in functioning and whether the reason(s) for referral have evolved. On the other hand, this time interval is somewhat arbitrary given the lack of empirical data. Therefore, on a case-by-case basis, it would be helpful for psychiatrists to consult with their patients and preferably with the psychologist who completed the baseline evaluation to determine a reasonable interval between assessments.
For patients who have undergone long-term/intensive treatment, reassessment in 3 or 6 months to as long as 1 year after the patient completes the program should be considered. Patients who undergo medical interventions such as neurosurgery or ECT—which can be associated with short-term, at least partially reversible negative effects on mental status—reassessment usually is most helpful when initiated as one or more screening level examinations for several weeks, followed by a comprehensive psychometric reassessment at the 3- to 6-month mark.
Suggestions for future research
Additional research is needed to ascertain the attitudes and opinions of psychiatrists and other physicians who use psychometric test data regarding how psychologists can most effectively communicate a recommendation for reassessment in their reports and clarify the ways psychiatrists can productively address this issue with their patients. Survey research of this kind should include questions about the frequency with which psychiatrists formally refer patients for retesting, and estimates of the rate of follow-through.
Continue to: It also would be desirable...
It also would be desirable to investigate factors that may facilitate follow-through with recommendations for reassessment, or, conversely, identify reasons that psychiatrists and their patients may decide to forgo reassessment. It would be important to try to obtain information regarding the optimum time frames for such reevaluation, depending on the patient’s circumstances and other variables. Evidence-based data pertaining to these issues would contribute to the development of consensus guidelines and a standard of care for psychological/neuropsychological test reevaluation.
Bottom Line
Only a very small percentage of patients referred for follow-up psychological/ neuropsychological test reevaluation actually undergo reexamination. Such retesting may be most helpful for certain patient populations, such as those who are likely to soon improve or worsen, were referred based on a family history of major cognitive disorder but have no concerning findings on baseline testing, or are undergoing a substantial life transition.
Related Resources
- Thom R, Farrell HM. Neuroimaging in psychiatry: potentials and pitfalls. Current Psychiatry. 2019;18(12):27-28;33-34.
- Papakostas GI. Cognitive symptoms in patients with major depressive disorder and their implications for clinical practice. J Clin Psychiatry. 2014;75(1):8-14.
- American Board of Professional Neuropsychology. https://abn-board.com
The evolution of illness prevention, diagnosis, and treatment has involved an increased appreciation for the clinical utility of longitudinal assessment. This has included the implementation of screening evaluations for high base rate medical conditions, such as cancer, that involve considerable morbidity and mortality.
Unfortunately, the mental health professions have been slow to embrace this approach. Baseline assessment with psychological/neuropsychological screening tests and more comprehensive test batteries to clarify diagnostic status and facilitate treatment planning is far more the exception than the rule in mental health care. This seems to be the case despite the strong evidence supporting this practice as well as multiple surveys indicating that psychiatrists and other physicians report a high level of satisfaction with the findings and recommendations of psychological/neuropsychological test reports.1-3
There is a substantial literature that reviews the relative indications and contraindications for initial psychological/neuropsychological test evaluations.4-7 However, there is a paucity of clinical and evidence-based information regarding criteria for follow-up assessment. Moreover, there are no consensus guidelines to inform decision-making regarding this issue.
In general, good clinical practice for baseline assessment and reexamination should include administration of both psychological and neuropsychological tests. Based on clinical experience, this article addresses the relative indications and contraindications for psychological/neuropsychological test reassessment of adults seen in psychiatric care. It also outlines suggested time frames for such reevaluations, based on the patient’s clinical status and circumstances.
Why are patients not referred for reassessment more often?
There are several reasons patients are not referred for follow-up testing, beginning with the failure, at times, of the psychologist to state in the recommendations section of the test report whether a reassessment is indicated, under what circumstances, and within what time frame. Empirical data is lacking, but predicated on clinical experience, even when a strong and unequivocal recommendation is made for reassessment, only a very small percentage of patients are seen for follow-up evaluation.
There are numerous reasons why this occurs. The patient and/or the psychiatrist may overlook or forget about the recommendation for reassessment, particularly if it was embedded in a lengthy list of recommendations and the suggested time frame for the reassessment was several years away. The patient and the psychiatrist may decide against going forward with a reexamination, for a variety of substantive reasons. The patient might decline, against medical advice, to be retested. The patient may fail to make or keep an appointment for the follow-up reexamination. The patient might leave treatment and become lost to follow-up. The patient might not be able to find an appropriate psychologist. The insurance company may decline to authorize follow-up testing.8
Indications for reevaluation
Follow-up testing generally is indicated in the following circumstances:
Patients who are likely to soon improve or worsen. Reassessment is indicated when, based on the initial evaluation, the patient has been identified as having a neuropsychiatric disorder that is likely to improve or worsen over the next year or 2 due to the natural trajectory of the condition and the degree to which it may respond to treatment.
Continue to: Patients who are likely...
Patients who are likely to improve include those with mental status changes referable to ≥1 medical and/or neuropsychiatric factors that are considered at least partially treatable and reversible. Patients who fall within this category include those who have mild to moderately severe head trauma or stroke, have a suspected or known medication- or substance-induced altered mental status, appear to have depression-related cognitive difficulties, or have an initial or recurrent episode of idiopathic psychosis.
Patients whose conditions can be expected to worsen over time include those with a mild neurocognitive disorder or major neurocognitive disorder of mild severity that is considered referable to a progressive neurodegenerative illness such as Alzheimer’s disease based on family and personal history, their psychometric test profile, and other factors, including findings from positron emission tomography scanning.
Older patients who were referred primarily due to a strong family history of major neurocognitive disorder but with no clear-cut concerning findings on baseline testing warrant reevaluation in the event of the emergence of significant cognitive and/or psychiatric symptoms and/or a functional decline since the baseline examination.
Patients who have been seen for initial test evaluations prior to interventions such as neurosurgery (including psychosurgery), electroconvulsive therapy (ECT), transcranial magnetic stimulation, cognitive rehabilitation, etc.
Patients undergoing a substantial transition. Reevaluation is appropriate for a broad range of patients experiencing difficulties when undergoing a significant lifestyle transition or change in level of care. This includes patients considering a return to school or work after a prolonged absence due to neuropsychiatric illness, or for whom there are questions regarding the need for a change in their level of everyday care. The latter includes patients who are returning to home care from assisted living, or transferring from home-based services to assisted living or a skilled nursing facility.
Continue to: What about patients with psychiatric disorders?
What about patients with psychiatric disorders? A “grey area” pertains to reassessment of patients with neuropsychiatric disorders such as schizophrenia and related psychotic disorders, bipolar disorder, major depressive disorder, and obsessive-compulsive disorder. Patients with these conditions often have high rates of cognitive/neuropsychological impairment on baseline testing, even when they appear to be improving from a psychiatric perspective, are reasonably stable, and may even be in remission.9-12
These deficits are frequently a mix of pre-illness, prodromal, and early-stage illness– related neurocognitive difficulties that, for the most part, remain stable over time. That said, there is emerging evidence of worsening cognitive change over time following a first episode of psychosis for some patients with schizophrenia.13
In general, reevaluation should be considered for patients with a family and/or personal history of cognitive/neuropsychological impairment, structural brain abnormalities on neuroimaging, a concerning cognitive/neuropsychological profile, or any other factors that raise the index of suspicion for a possible progressive deteriorative course of illness.13,14
Patients with personality disorders who have had a baseline psychometric evaluation do not clearly warrant reassessment unless they develop medical and/or psychosocial difficulties that are often linked to problematic personality traits/patterns and that result in significant and persistent mental status changes. For example, reassessment might be indicated for a patient with borderline personality disorder who has new-onset or worsening cognitive and/or psychiatric complaints/symptoms after sustaining a head injury while intoxicated and embroiled in a domestic conflict triggered by anger and fears related to abandonment and separation.
Reevaluation also should be considered when a patient with a personality disorder has had a baseline assessment and subsequently completes an intensive, long-term treatment program that is likely to improve their clinical status. In this context, retesting may help document these gains. Examples of such programs/services include residential psychiatric and/or substance abuse care, object relational/psychodynamically-based psychotherapy, an extended course of dialectical behavioral therapy, or a related coping skills/distress tolerance psychotherapy.
Continue to: Contraindications for reassessment
Contraindications for reassessment
Retesting generally is not indicated in the following circumstances:
Patients with advanced major neurocognitive disorder. Reassessment is not indicated for such patients when there are no new questions regarding diagnosis, prognosis, level of care, and/or related disposition issues.
Patients with transient episodes of poor functioning. For the most part, reassessment is not helpful for patients with well-established diagnoses and treatment plans who, based on their history, experience time-limited, recurrent episodes of poor functioning and then reliably return to their baseline with ongoing psychiatric care. This includes patients with borderline personality disorder and other personality difficulties with histories of transient decompensation in response to psychosocial and psychodynamic triggers.
Patients who do not improve or worsen over time. Reassessment is not indicated when there has been no clear, sustained improvement or worsening of a patient’s clinical status over an extended time, and a protracted change is not anticipated. In this situation, reassessment is unlikely to yield clinically useful information beyond what is already known or meaningfully impact case formulation and treatment planning.
The Table9-14 summarizes the relative indications and contraindications for psychological/neuropsychological reexamination.

Continue to: Time frames for reassessment
Time frames for reassessment
Time frames for retesting vary considerably depending on factors such as diagnostic status, longitudinal course, treatment parameters, and recent/current life circumstances.
While empirical data is lacking regarding this matter, based on clinical experience, reevaluation in 18 to 24 months is generally appropriate for patients with neuropsychiatric conditions who are likely to gradually improve or slowly worsen over this time. Still, reexamination can be sooner (within 12 to 18 months) for patients who have experienced a more rapid and steep negative change in clinical status than initially anticipated.
For most patients with major mental illness, reexamination in 3 to 5 years is probably a reasonable time interval, barring a poorly understood and clinically significant negative change in functioning that warrants a shorter time frame. This suggested time frame would allow for sufficient time to better gauge improvement, stability, or deterioration in functioning and whether the reason(s) for referral have evolved. On the other hand, this time interval is somewhat arbitrary given the lack of empirical data. Therefore, on a case-by-case basis, it would be helpful for psychiatrists to consult with their patients and preferably with the psychologist who completed the baseline evaluation to determine a reasonable interval between assessments.
For patients who have undergone long-term/intensive treatment, reassessment in 3 or 6 months to as long as 1 year after the patient completes the program should be considered. Patients who undergo medical interventions such as neurosurgery or ECT—which can be associated with short-term, at least partially reversible negative effects on mental status—reassessment usually is most helpful when initiated as one or more screening level examinations for several weeks, followed by a comprehensive psychometric reassessment at the 3- to 6-month mark.
Suggestions for future research
Additional research is needed to ascertain the attitudes and opinions of psychiatrists and other physicians who use psychometric test data regarding how psychologists can most effectively communicate a recommendation for reassessment in their reports and clarify the ways psychiatrists can productively address this issue with their patients. Survey research of this kind should include questions about the frequency with which psychiatrists formally refer patients for retesting, and estimates of the rate of follow-through.
Continue to: It also would be desirable...
It also would be desirable to investigate factors that may facilitate follow-through with recommendations for reassessment, or, conversely, identify reasons that psychiatrists and their patients may decide to forgo reassessment. It would be important to try to obtain information regarding the optimum time frames for such reevaluation, depending on the patient’s circumstances and other variables. Evidence-based data pertaining to these issues would contribute to the development of consensus guidelines and a standard of care for psychological/neuropsychological test reevaluation.
Bottom Line
Only a very small percentage of patients referred for follow-up psychological/ neuropsychological test reevaluation actually undergo reexamination. Such retesting may be most helpful for certain patient populations, such as those who are likely to soon improve or worsen, were referred based on a family history of major cognitive disorder but have no concerning findings on baseline testing, or are undergoing a substantial life transition.
Related Resources
- Thom R, Farrell HM. Neuroimaging in psychiatry: potentials and pitfalls. Current Psychiatry. 2019;18(12):27-28;33-34.
- Papakostas GI. Cognitive symptoms in patients with major depressive disorder and their implications for clinical practice. J Clin Psychiatry. 2014;75(1):8-14.
- American Board of Professional Neuropsychology. https://abn-board.com
1. Schroeder RW, Martin PK, Walling A. Neuropsychological evaluations in adults. Am Fam Physician. 2019;99(2):101-198.
2. Bucher MA, Suzuki T, Samuel DB. A meta-analytic review of personality traits and their associations with mental health treatment outcomes. Clin Psychol Rev. 2019;70:51-63.
3. Pollak J. Feedback to the psychodiagnostician: a challenge for assessment psychologists in independent practice. Independent Practitioner: The community of psychologists in independent practice. 2020;40,6-9.
4. Pollak J. To test or not to test: considerations before going forward with psychometric testing. The Clinical Practitioner. 2011;6:5-10.
5. Schwarz L, Roskos PT, Grossberg GT. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):33-39.
6. Zucchella C, Federico A, Martini A, et al. Neuropsychological testing. Pract Neurol. 2018;18(3):227-237.
7. Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: a useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
8. Pollak J. Psychodiagnostic testing services: the elusive quest for clinicians. Clinical Psychiatry News. Published October 18, 2019. Accessed July 8, 2021. https://www.mdedge.com/psychiatry/article/210439/schizophrenia-other-psychotic-disorders/psychodiagnostic-testing-services
9. Mesholam-Gately RI, Giuliano AJ, Goff KP, et al. Neurocognition in first episode schizophrenia: a meta- analytic review. Neuropsychology. 2009;23(3):315-336.
10. Lam RW, Kennedy SH, McIntyre RS et al. Cognitive dysfunction in major depressive disorder: effects on psychosocial functioning and implications for treatment. Can J Psychiatry. 2014;59(12):649-654.
11. Szmulewicz AG, Samamé C, Martino DJ, et al. An updated review on the neuropsychological profile of subjects with bipolar disorder. Arch Clin Psychiatry. 2015;42(5):139-146.
12. Shin NY, Lee TY, Kim, E, et al. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol Med. 2013;44(6):1121-1130.
13. Zanelli J, Mollon J, Sandin S, et al. Cognitive change in schizophrenia and other psychoses in the decade following the first episode. Am J Psychiatry. 2019;176(10):811-819.
14. Mitleman SA, Buchsbaum MS. Very poor outcome schizophrenia: clinical and neuroimaging aspects. Int Rev Psychiatry. 2007;19(4):345-357.
1. Schroeder RW, Martin PK, Walling A. Neuropsychological evaluations in adults. Am Fam Physician. 2019;99(2):101-198.
2. Bucher MA, Suzuki T, Samuel DB. A meta-analytic review of personality traits and their associations with mental health treatment outcomes. Clin Psychol Rev. 2019;70:51-63.
3. Pollak J. Feedback to the psychodiagnostician: a challenge for assessment psychologists in independent practice. Independent Practitioner: The community of psychologists in independent practice. 2020;40,6-9.
4. Pollak J. To test or not to test: considerations before going forward with psychometric testing. The Clinical Practitioner. 2011;6:5-10.
5. Schwarz L, Roskos PT, Grossberg GT. Answers to 7 questions about using neuropsychological testing in your practice. Current Psychiatry. 2014;13(3):33-39.
6. Zucchella C, Federico A, Martini A, et al. Neuropsychological testing. Pract Neurol. 2018;18(3):227-237.
7. Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: a useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
8. Pollak J. Psychodiagnostic testing services: the elusive quest for clinicians. Clinical Psychiatry News. Published October 18, 2019. Accessed July 8, 2021. https://www.mdedge.com/psychiatry/article/210439/schizophrenia-other-psychotic-disorders/psychodiagnostic-testing-services
9. Mesholam-Gately RI, Giuliano AJ, Goff KP, et al. Neurocognition in first episode schizophrenia: a meta- analytic review. Neuropsychology. 2009;23(3):315-336.
10. Lam RW, Kennedy SH, McIntyre RS et al. Cognitive dysfunction in major depressive disorder: effects on psychosocial functioning and implications for treatment. Can J Psychiatry. 2014;59(12):649-654.
11. Szmulewicz AG, Samamé C, Martino DJ, et al. An updated review on the neuropsychological profile of subjects with bipolar disorder. Arch Clin Psychiatry. 2015;42(5):139-146.
12. Shin NY, Lee TY, Kim, E, et al. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol Med. 2013;44(6):1121-1130.
13. Zanelli J, Mollon J, Sandin S, et al. Cognitive change in schizophrenia and other psychoses in the decade following the first episode. Am J Psychiatry. 2019;176(10):811-819.
14. Mitleman SA, Buchsbaum MS. Very poor outcome schizophrenia: clinical and neuroimaging aspects. Int Rev Psychiatry. 2007;19(4):345-357.
Beyond DSM symptoms: Behavioral clues to diagnosing bipolar II disorder
The diagnosis of bipolar II disorder is one of the most common challenges in psychiatric practice. Bipolar II disorder is frequently misdiagnosed as major depressive disorder (MDD) because symptoms of transient hypomanic episodes are either insufficiently probed or are rather vague. However, there are many valuable biographical clues that can expedite the diagnosis of bipolar II disorder.
The late Hagop S. Akiskal, MD, who passed away in January 2021, was an internationally recognized expert in mood disorders, and a dear friend for decades. He was a keen observer of human behavior who delved into the “life stories” of patients seeking help for depression. By thinking “outside the DSM box,” Dr. Akiskal was the first to recognize and codify a variety of behavioral and biographical clues for the bipolar spectrum (of which he was a pioneer) in patients presenting with a chief complaint of depression. He proposed a colorful set of behavioral stigmata in most patients with bipolar II disorder by carefully canvassing the life experiences of the patients he treated in the mood disorder clinic he established in the 1970s, which is believed to have been the first mood specialty clinic in the country.
Based on a review of >1,000 patients in his clinic who presented with depressive symptoms and were ultimately diagnosed as bipolar II disorder, Dr. Akiskal highlighted what he labeled as “behavioral activation, flamboyance and extravagance” among those patients. He referred to the cluster of those behaviors as “the soft spectrum” of bipolar disorder, which manifests in a set of distinctive behaviors in addition to depressive symptoms. He found that research tools such as the DSM-based Structured Clinical Interview often fail and frequently lead to a misdiagnosis of bipolar II disorder as MDD. This often condemns the patient to multiple failed trials of antidepressant monotherapy, and a delay in improvement, thus increasing the risk of job loss, disrupted relationships, and even suicide.
Over 3 decades, Dr. Akiskal developed the Mood Clinic Data Questionnaire (MCDQ) to systematize unstructured observations of patients presenting with a chief complaint of depression. His tool expedites the diagnosis of bipolar II disorder by understanding the patient as an individual, revealing personal and behavioral features consistent with what he labeled as episodic “hyperthymia” within the context of recurrent depression. This “social and behavioral phenotype,” as Dr. Akiskal called it, is rarely observed among patients with MDD.
By examining many patients with bipolar II disorder, Dr. Akiskal identified several “triads” of behavioral traits in the patients’ biographical history and in some of their close blood relatives as well. He also noticed that temperamentally, patients with bipolar II disorder thrive on “activity” and lovingly referred to themselves as “activity junkies.” Some of them may qualify as workaholics.
Biographical features that suggest bipolar II disorder
Here is a summary of the unique biographical features of patients with bipolar II disorder that Dr. Akiskal described:
Multilingual. Speaking ≥3 languages is unusual among individuals born in the United States, but often encountered among those with bipolar II disorder.
Continue to: Eminence
Eminence. Patients with bipolar II disorder, as well as their family members, tend to have leadership roles and prominence in journalism, media, and entertainment, fields that require interpersonal charm and eloquence. Those are common features of the “hyperthymic” temperament.
Creativity. Artists, poets, painters, and musicians who experience depression are more likely to have bipolar II disorder than MDD.
Biographical instability and/or excess. This is exemplified by going to 3 colleges and not necessarily obtaining a degree, or by frequently changing one’s line of work or city of residence. A classic example is a professor of medicine who also practices law and regularly sings in the opera, or a physician who is board-certified in 3 distinct specialties.
Activity junkies. Examples include a person with boundless energy, such as a novelist who writes 3 books a year or a professional who regularly works 12 hours a day without getting exhausted but seeks treatment for depressive episodes.
Multiple substances of abuse, such as nicotine, alcohol, stimulants, and opiates.
Continue to: Multiple psychiatric comorbidities
Multiple psychiatric comorbidities, such as having 3 types of anxiety (panic attacks, social phobia, and obsessive-compulsive disorder) or bulimia, seasonal depression, and anxiety.
Multiple pleasure-seeking or “outrageous” behaviors, such as compulsive gambling, sexual addiction, car racing, or skydiving. Another example is having a history of shoplifting, paraphilia, or arrest for participating in a riot, all of which are suggestive of antisocial traits in a patient seeking help for depression.
Sexual excesses, such as dating or having sex with ≥3 individuals concurrently, sometimes on the same day, or demanding sexual intercourse from a partner several times a day. Dr. Akiskal suggested that “sexual prowess” may represent an evolutionary advantage for the perpetuation of bipolar II disorder.
Marital history, such as a history of ≥3 marriages, or maintaining ≥2 families in different cities without being married.
Flamboyance and/or ornamentation. Examples might include wearing loud, colorful clothing (especially red), wearing ≥3 rings, or having piercings in ≥3 different body parts (tongue, nipples, navel, genitalia). Having elaborate tattoos across the body is no longer unique to “hyperthymic” persons with bipolar II disorder because tattoos have become far more common in the general population than they were in the 1970s. However, some take their tattoos to extremes.
Continue to: The above behaviors...
The above behaviors are condensed in a list that Dr. Akiskal called “the rule of 3” in patients with depression (Table1). Not all patients with bipolar II disorder will meet all the criteria of the rule of 3, but the first item in the mental status exam (appearance) alone may reflect the “soft bipolar spectrum,” such as garish clothing, red sneakers, multiple rings, bizarre hair coloring, and multiple piercings. This might prompt the clinician to ask further questions about hypomanic episodes as well as other personal behaviors related to the rule of 3.
Dr. Akiskal’s contributions to psychiatry are legendary in their originality, creativity, and clinical relevance. The rule of 3 is but one of his clinical concepts that may help identify many individuals with bipolar II disorder who are misdiagnosed as having MDD and prescribed a treatment that does not help or may exacerbate their illness course and worsen their outcome.
Based on the referrals of patients who are “treatment-resistant” to our Resident Mood Clinic, there are numerous persons in the country with bipolar II disorder (possibly millions) who are mislabeled with MDD and receiving the wrong treatments, to which they failed to respond. Their lifestyles and behaviors can provide valuable clinical insights into their true psychopathology, and that will lead to developing the right treatment plan.
1. Akiskal HS. Searching for behavioral indicators of bipolar II in patients presenting with major depressive episodes: the “red sign,” the “rule of three” and other biographic signs of temperamental extravagance, activation and hypomania. J Affect Disord. 2005;84(2-3):279-290.
The diagnosis of bipolar II disorder is one of the most common challenges in psychiatric practice. Bipolar II disorder is frequently misdiagnosed as major depressive disorder (MDD) because symptoms of transient hypomanic episodes are either insufficiently probed or are rather vague. However, there are many valuable biographical clues that can expedite the diagnosis of bipolar II disorder.
The late Hagop S. Akiskal, MD, who passed away in January 2021, was an internationally recognized expert in mood disorders, and a dear friend for decades. He was a keen observer of human behavior who delved into the “life stories” of patients seeking help for depression. By thinking “outside the DSM box,” Dr. Akiskal was the first to recognize and codify a variety of behavioral and biographical clues for the bipolar spectrum (of which he was a pioneer) in patients presenting with a chief complaint of depression. He proposed a colorful set of behavioral stigmata in most patients with bipolar II disorder by carefully canvassing the life experiences of the patients he treated in the mood disorder clinic he established in the 1970s, which is believed to have been the first mood specialty clinic in the country.
Based on a review of >1,000 patients in his clinic who presented with depressive symptoms and were ultimately diagnosed as bipolar II disorder, Dr. Akiskal highlighted what he labeled as “behavioral activation, flamboyance and extravagance” among those patients. He referred to the cluster of those behaviors as “the soft spectrum” of bipolar disorder, which manifests in a set of distinctive behaviors in addition to depressive symptoms. He found that research tools such as the DSM-based Structured Clinical Interview often fail and frequently lead to a misdiagnosis of bipolar II disorder as MDD. This often condemns the patient to multiple failed trials of antidepressant monotherapy, and a delay in improvement, thus increasing the risk of job loss, disrupted relationships, and even suicide.
Over 3 decades, Dr. Akiskal developed the Mood Clinic Data Questionnaire (MCDQ) to systematize unstructured observations of patients presenting with a chief complaint of depression. His tool expedites the diagnosis of bipolar II disorder by understanding the patient as an individual, revealing personal and behavioral features consistent with what he labeled as episodic “hyperthymia” within the context of recurrent depression. This “social and behavioral phenotype,” as Dr. Akiskal called it, is rarely observed among patients with MDD.
By examining many patients with bipolar II disorder, Dr. Akiskal identified several “triads” of behavioral traits in the patients’ biographical history and in some of their close blood relatives as well. He also noticed that temperamentally, patients with bipolar II disorder thrive on “activity” and lovingly referred to themselves as “activity junkies.” Some of them may qualify as workaholics.
Biographical features that suggest bipolar II disorder
Here is a summary of the unique biographical features of patients with bipolar II disorder that Dr. Akiskal described:
Multilingual. Speaking ≥3 languages is unusual among individuals born in the United States, but often encountered among those with bipolar II disorder.
Continue to: Eminence
Eminence. Patients with bipolar II disorder, as well as their family members, tend to have leadership roles and prominence in journalism, media, and entertainment, fields that require interpersonal charm and eloquence. Those are common features of the “hyperthymic” temperament.
Creativity. Artists, poets, painters, and musicians who experience depression are more likely to have bipolar II disorder than MDD.
Biographical instability and/or excess. This is exemplified by going to 3 colleges and not necessarily obtaining a degree, or by frequently changing one’s line of work or city of residence. A classic example is a professor of medicine who also practices law and regularly sings in the opera, or a physician who is board-certified in 3 distinct specialties.
Activity junkies. Examples include a person with boundless energy, such as a novelist who writes 3 books a year or a professional who regularly works 12 hours a day without getting exhausted but seeks treatment for depressive episodes.
Multiple substances of abuse, such as nicotine, alcohol, stimulants, and opiates.
Continue to: Multiple psychiatric comorbidities
Multiple psychiatric comorbidities, such as having 3 types of anxiety (panic attacks, social phobia, and obsessive-compulsive disorder) or bulimia, seasonal depression, and anxiety.
Multiple pleasure-seeking or “outrageous” behaviors, such as compulsive gambling, sexual addiction, car racing, or skydiving. Another example is having a history of shoplifting, paraphilia, or arrest for participating in a riot, all of which are suggestive of antisocial traits in a patient seeking help for depression.
Sexual excesses, such as dating or having sex with ≥3 individuals concurrently, sometimes on the same day, or demanding sexual intercourse from a partner several times a day. Dr. Akiskal suggested that “sexual prowess” may represent an evolutionary advantage for the perpetuation of bipolar II disorder.
Marital history, such as a history of ≥3 marriages, or maintaining ≥2 families in different cities without being married.
Flamboyance and/or ornamentation. Examples might include wearing loud, colorful clothing (especially red), wearing ≥3 rings, or having piercings in ≥3 different body parts (tongue, nipples, navel, genitalia). Having elaborate tattoos across the body is no longer unique to “hyperthymic” persons with bipolar II disorder because tattoos have become far more common in the general population than they were in the 1970s. However, some take their tattoos to extremes.
Continue to: The above behaviors...
The above behaviors are condensed in a list that Dr. Akiskal called “the rule of 3” in patients with depression (Table1). Not all patients with bipolar II disorder will meet all the criteria of the rule of 3, but the first item in the mental status exam (appearance) alone may reflect the “soft bipolar spectrum,” such as garish clothing, red sneakers, multiple rings, bizarre hair coloring, and multiple piercings. This might prompt the clinician to ask further questions about hypomanic episodes as well as other personal behaviors related to the rule of 3.
Dr. Akiskal’s contributions to psychiatry are legendary in their originality, creativity, and clinical relevance. The rule of 3 is but one of his clinical concepts that may help identify many individuals with bipolar II disorder who are misdiagnosed as having MDD and prescribed a treatment that does not help or may exacerbate their illness course and worsen their outcome.
Based on the referrals of patients who are “treatment-resistant” to our Resident Mood Clinic, there are numerous persons in the country with bipolar II disorder (possibly millions) who are mislabeled with MDD and receiving the wrong treatments, to which they failed to respond. Their lifestyles and behaviors can provide valuable clinical insights into their true psychopathology, and that will lead to developing the right treatment plan.
The diagnosis of bipolar II disorder is one of the most common challenges in psychiatric practice. Bipolar II disorder is frequently misdiagnosed as major depressive disorder (MDD) because symptoms of transient hypomanic episodes are either insufficiently probed or are rather vague. However, there are many valuable biographical clues that can expedite the diagnosis of bipolar II disorder.
The late Hagop S. Akiskal, MD, who passed away in January 2021, was an internationally recognized expert in mood disorders, and a dear friend for decades. He was a keen observer of human behavior who delved into the “life stories” of patients seeking help for depression. By thinking “outside the DSM box,” Dr. Akiskal was the first to recognize and codify a variety of behavioral and biographical clues for the bipolar spectrum (of which he was a pioneer) in patients presenting with a chief complaint of depression. He proposed a colorful set of behavioral stigmata in most patients with bipolar II disorder by carefully canvassing the life experiences of the patients he treated in the mood disorder clinic he established in the 1970s, which is believed to have been the first mood specialty clinic in the country.
Based on a review of >1,000 patients in his clinic who presented with depressive symptoms and were ultimately diagnosed as bipolar II disorder, Dr. Akiskal highlighted what he labeled as “behavioral activation, flamboyance and extravagance” among those patients. He referred to the cluster of those behaviors as “the soft spectrum” of bipolar disorder, which manifests in a set of distinctive behaviors in addition to depressive symptoms. He found that research tools such as the DSM-based Structured Clinical Interview often fail and frequently lead to a misdiagnosis of bipolar II disorder as MDD. This often condemns the patient to multiple failed trials of antidepressant monotherapy, and a delay in improvement, thus increasing the risk of job loss, disrupted relationships, and even suicide.
Over 3 decades, Dr. Akiskal developed the Mood Clinic Data Questionnaire (MCDQ) to systematize unstructured observations of patients presenting with a chief complaint of depression. His tool expedites the diagnosis of bipolar II disorder by understanding the patient as an individual, revealing personal and behavioral features consistent with what he labeled as episodic “hyperthymia” within the context of recurrent depression. This “social and behavioral phenotype,” as Dr. Akiskal called it, is rarely observed among patients with MDD.
By examining many patients with bipolar II disorder, Dr. Akiskal identified several “triads” of behavioral traits in the patients’ biographical history and in some of their close blood relatives as well. He also noticed that temperamentally, patients with bipolar II disorder thrive on “activity” and lovingly referred to themselves as “activity junkies.” Some of them may qualify as workaholics.
Biographical features that suggest bipolar II disorder
Here is a summary of the unique biographical features of patients with bipolar II disorder that Dr. Akiskal described:
Multilingual. Speaking ≥3 languages is unusual among individuals born in the United States, but often encountered among those with bipolar II disorder.
Continue to: Eminence
Eminence. Patients with bipolar II disorder, as well as their family members, tend to have leadership roles and prominence in journalism, media, and entertainment, fields that require interpersonal charm and eloquence. Those are common features of the “hyperthymic” temperament.
Creativity. Artists, poets, painters, and musicians who experience depression are more likely to have bipolar II disorder than MDD.
Biographical instability and/or excess. This is exemplified by going to 3 colleges and not necessarily obtaining a degree, or by frequently changing one’s line of work or city of residence. A classic example is a professor of medicine who also practices law and regularly sings in the opera, or a physician who is board-certified in 3 distinct specialties.
Activity junkies. Examples include a person with boundless energy, such as a novelist who writes 3 books a year or a professional who regularly works 12 hours a day without getting exhausted but seeks treatment for depressive episodes.
Multiple substances of abuse, such as nicotine, alcohol, stimulants, and opiates.
Continue to: Multiple psychiatric comorbidities
Multiple psychiatric comorbidities, such as having 3 types of anxiety (panic attacks, social phobia, and obsessive-compulsive disorder) or bulimia, seasonal depression, and anxiety.
Multiple pleasure-seeking or “outrageous” behaviors, such as compulsive gambling, sexual addiction, car racing, or skydiving. Another example is having a history of shoplifting, paraphilia, or arrest for participating in a riot, all of which are suggestive of antisocial traits in a patient seeking help for depression.
Sexual excesses, such as dating or having sex with ≥3 individuals concurrently, sometimes on the same day, or demanding sexual intercourse from a partner several times a day. Dr. Akiskal suggested that “sexual prowess” may represent an evolutionary advantage for the perpetuation of bipolar II disorder.
Marital history, such as a history of ≥3 marriages, or maintaining ≥2 families in different cities without being married.
Flamboyance and/or ornamentation. Examples might include wearing loud, colorful clothing (especially red), wearing ≥3 rings, or having piercings in ≥3 different body parts (tongue, nipples, navel, genitalia). Having elaborate tattoos across the body is no longer unique to “hyperthymic” persons with bipolar II disorder because tattoos have become far more common in the general population than they were in the 1970s. However, some take their tattoos to extremes.
Continue to: The above behaviors...
The above behaviors are condensed in a list that Dr. Akiskal called “the rule of 3” in patients with depression (Table1). Not all patients with bipolar II disorder will meet all the criteria of the rule of 3, but the first item in the mental status exam (appearance) alone may reflect the “soft bipolar spectrum,” such as garish clothing, red sneakers, multiple rings, bizarre hair coloring, and multiple piercings. This might prompt the clinician to ask further questions about hypomanic episodes as well as other personal behaviors related to the rule of 3.
Dr. Akiskal’s contributions to psychiatry are legendary in their originality, creativity, and clinical relevance. The rule of 3 is but one of his clinical concepts that may help identify many individuals with bipolar II disorder who are misdiagnosed as having MDD and prescribed a treatment that does not help or may exacerbate their illness course and worsen their outcome.
Based on the referrals of patients who are “treatment-resistant” to our Resident Mood Clinic, there are numerous persons in the country with bipolar II disorder (possibly millions) who are mislabeled with MDD and receiving the wrong treatments, to which they failed to respond. Their lifestyles and behaviors can provide valuable clinical insights into their true psychopathology, and that will lead to developing the right treatment plan.
1. Akiskal HS. Searching for behavioral indicators of bipolar II in patients presenting with major depressive episodes: the “red sign,” the “rule of three” and other biographic signs of temperamental extravagance, activation and hypomania. J Affect Disord. 2005;84(2-3):279-290.
1. Akiskal HS. Searching for behavioral indicators of bipolar II in patients presenting with major depressive episodes: the “red sign,” the “rule of three” and other biographic signs of temperamental extravagance, activation and hypomania. J Affect Disord. 2005;84(2-3):279-290.
Conspiracy theory or delusion? 3 questions to tell them apart
Many psychiatrists conceptualize mental illnesses, including psychotic disorders, across a continuum where their borders can be ambiguous.1 The same can be said of individual symptoms such as delusions, where the line separating clear-cut pathology from nonpathological or subclinical “delusion-like beliefs” is often blurred.2,3 However, the categorical distinction between mental illness and normality is fundamental to diagnostic reliability and crucial to clinical decisions about whether and how to intervene.
Conspiracy theory beliefs are delusion-like beliefs that are commonly encountered within today’s political landscape. Surveys have consistently revealed that approximately one-half of the population believes in at least 1 conspiracy theory, highlighting the normality of such beliefs despite their potential outlandishness.4 Here are 3 questions you can ask to help differentiate conspiracy theory beliefs from delusions.
1. What is the evidence for the belief?
Drawing from Karl Jaspers’ conceptualization of delusions as “impossible” and “unshareable,” the DSM-5 distinguishes delusions from culturally-sanctioned shared beliefs such as religious creeds.3 Whereas delusions often arise out of anomalous subjective experiences, individuals who come to believe in conspiracytheories have typically sought explanations and found them from secondary sources, often on the internet.5 Despite the familiar term “conspiracy theorist,” most who believe in conspiracy theories aren’t so much theorizing as they are adopting counter-narratives based on assimilated information. Unlike delusions, conspiracy theory beliefs are learned, with the “evidence” to support them easily located online.
2. Is the belief self-referential?
The stereotypical unshareability of delusions often hinges upon their self-referential content. For example, while it is easy to find others who believe in the Second Coming, it would be much harder to convince others that you are the Second Coming. Unlike delusions, conspiracy theories are beliefs about the world and explanations of real-life events; their content is rarely, if ever, directly related to the believer.
Conspiracy theory beliefs involve a negation of authoritative accounts that is rooted in “epistemic mistrust” of authoritative sources of information.5 While conspiratorial mistrust has been compared with paranoia, with paranoia found to be associated with belief in conspiracy theories,6 epistemic mistrust encompasses a range of justified cultural mistrust, unwarranted mistrust based on racial prejudice, and subclinical paranoia typical of schizotypy. The more self-referential the underlying paranoia, the more likely an associated belief is to cross the boundary from conspiracy theory to delusion.7
3. Is there overlap?
Conspiracy theory beliefs and delusions are not mutually exclusive. “Gang stalking” offers a vexing example of paranoia that is part shared conspiracy theory, part idiosyncratic delusion.8 Reliably disentangling these components requires identifying the conspiracy theory component as a widely-shared belief about government surveillance, while carefully analyzing the self-referential component to determine credibility and potential delusionality.
1. Pierre JM. The borders of mental disorder in psychiatry and the DSM: past, present, and future. J Psychiatric Practice. 2010;16(6):375-386.
2. Pierre JM. Faith or delusion? At the crossroads of religion and psychosis. J Psychiatr Practice. 2001;7(3):163-172.
3. Pierre JM. Forensic psychiatry versus the varieties of delusion-like belief. J Am Acad Psychiatry Law. 2020;48(3):327-334.
4. Oliver JE, Wood, TJ. Conspiracy theories and the paranoid style(s) of mass opinion. Am J Pol Sci. 2014;58(5);952-966.
5. Pierre JM. Mistrust and misinformation: a two-component, socio-epistemic model of belief in conspiracy theories. J Soc Polit Psychol. 2020;8(2):617-641.
6. Dagnall N, Drinkwater K, Parker A, et al. Conspiracy theory and cognitive style: a worldview. Front Psychol. 2015;6:206.
7. Imhoff R, Lamberty P. How paranoid are conspiracy believers? Toward a more fine-grained understanding of the connect and disconnect between paranoia and belief in conspiracy theories. Eur J Soc Psychol. 2018;48(7):909-926.
8. Sheridan LP, James DV. Complaints of group-stalking (‘gang-stalking’): an exploratory study of their natures and impact on complainants. J Forens Psychiatry Psychol. 2015;26(5):601-623.
Many psychiatrists conceptualize mental illnesses, including psychotic disorders, across a continuum where their borders can be ambiguous.1 The same can be said of individual symptoms such as delusions, where the line separating clear-cut pathology from nonpathological or subclinical “delusion-like beliefs” is often blurred.2,3 However, the categorical distinction between mental illness and normality is fundamental to diagnostic reliability and crucial to clinical decisions about whether and how to intervene.
Conspiracy theory beliefs are delusion-like beliefs that are commonly encountered within today’s political landscape. Surveys have consistently revealed that approximately one-half of the population believes in at least 1 conspiracy theory, highlighting the normality of such beliefs despite their potential outlandishness.4 Here are 3 questions you can ask to help differentiate conspiracy theory beliefs from delusions.
1. What is the evidence for the belief?
Drawing from Karl Jaspers’ conceptualization of delusions as “impossible” and “unshareable,” the DSM-5 distinguishes delusions from culturally-sanctioned shared beliefs such as religious creeds.3 Whereas delusions often arise out of anomalous subjective experiences, individuals who come to believe in conspiracytheories have typically sought explanations and found them from secondary sources, often on the internet.5 Despite the familiar term “conspiracy theorist,” most who believe in conspiracy theories aren’t so much theorizing as they are adopting counter-narratives based on assimilated information. Unlike delusions, conspiracy theory beliefs are learned, with the “evidence” to support them easily located online.
2. Is the belief self-referential?
The stereotypical unshareability of delusions often hinges upon their self-referential content. For example, while it is easy to find others who believe in the Second Coming, it would be much harder to convince others that you are the Second Coming. Unlike delusions, conspiracy theories are beliefs about the world and explanations of real-life events; their content is rarely, if ever, directly related to the believer.
Conspiracy theory beliefs involve a negation of authoritative accounts that is rooted in “epistemic mistrust” of authoritative sources of information.5 While conspiratorial mistrust has been compared with paranoia, with paranoia found to be associated with belief in conspiracy theories,6 epistemic mistrust encompasses a range of justified cultural mistrust, unwarranted mistrust based on racial prejudice, and subclinical paranoia typical of schizotypy. The more self-referential the underlying paranoia, the more likely an associated belief is to cross the boundary from conspiracy theory to delusion.7
3. Is there overlap?
Conspiracy theory beliefs and delusions are not mutually exclusive. “Gang stalking” offers a vexing example of paranoia that is part shared conspiracy theory, part idiosyncratic delusion.8 Reliably disentangling these components requires identifying the conspiracy theory component as a widely-shared belief about government surveillance, while carefully analyzing the self-referential component to determine credibility and potential delusionality.
Many psychiatrists conceptualize mental illnesses, including psychotic disorders, across a continuum where their borders can be ambiguous.1 The same can be said of individual symptoms such as delusions, where the line separating clear-cut pathology from nonpathological or subclinical “delusion-like beliefs” is often blurred.2,3 However, the categorical distinction between mental illness and normality is fundamental to diagnostic reliability and crucial to clinical decisions about whether and how to intervene.
Conspiracy theory beliefs are delusion-like beliefs that are commonly encountered within today’s political landscape. Surveys have consistently revealed that approximately one-half of the population believes in at least 1 conspiracy theory, highlighting the normality of such beliefs despite their potential outlandishness.4 Here are 3 questions you can ask to help differentiate conspiracy theory beliefs from delusions.
1. What is the evidence for the belief?
Drawing from Karl Jaspers’ conceptualization of delusions as “impossible” and “unshareable,” the DSM-5 distinguishes delusions from culturally-sanctioned shared beliefs such as religious creeds.3 Whereas delusions often arise out of anomalous subjective experiences, individuals who come to believe in conspiracytheories have typically sought explanations and found them from secondary sources, often on the internet.5 Despite the familiar term “conspiracy theorist,” most who believe in conspiracy theories aren’t so much theorizing as they are adopting counter-narratives based on assimilated information. Unlike delusions, conspiracy theory beliefs are learned, with the “evidence” to support them easily located online.
2. Is the belief self-referential?
The stereotypical unshareability of delusions often hinges upon their self-referential content. For example, while it is easy to find others who believe in the Second Coming, it would be much harder to convince others that you are the Second Coming. Unlike delusions, conspiracy theories are beliefs about the world and explanations of real-life events; their content is rarely, if ever, directly related to the believer.
Conspiracy theory beliefs involve a negation of authoritative accounts that is rooted in “epistemic mistrust” of authoritative sources of information.5 While conspiratorial mistrust has been compared with paranoia, with paranoia found to be associated with belief in conspiracy theories,6 epistemic mistrust encompasses a range of justified cultural mistrust, unwarranted mistrust based on racial prejudice, and subclinical paranoia typical of schizotypy. The more self-referential the underlying paranoia, the more likely an associated belief is to cross the boundary from conspiracy theory to delusion.7
3. Is there overlap?
Conspiracy theory beliefs and delusions are not mutually exclusive. “Gang stalking” offers a vexing example of paranoia that is part shared conspiracy theory, part idiosyncratic delusion.8 Reliably disentangling these components requires identifying the conspiracy theory component as a widely-shared belief about government surveillance, while carefully analyzing the self-referential component to determine credibility and potential delusionality.
1. Pierre JM. The borders of mental disorder in psychiatry and the DSM: past, present, and future. J Psychiatric Practice. 2010;16(6):375-386.
2. Pierre JM. Faith or delusion? At the crossroads of religion and psychosis. J Psychiatr Practice. 2001;7(3):163-172.
3. Pierre JM. Forensic psychiatry versus the varieties of delusion-like belief. J Am Acad Psychiatry Law. 2020;48(3):327-334.
4. Oliver JE, Wood, TJ. Conspiracy theories and the paranoid style(s) of mass opinion. Am J Pol Sci. 2014;58(5);952-966.
5. Pierre JM. Mistrust and misinformation: a two-component, socio-epistemic model of belief in conspiracy theories. J Soc Polit Psychol. 2020;8(2):617-641.
6. Dagnall N, Drinkwater K, Parker A, et al. Conspiracy theory and cognitive style: a worldview. Front Psychol. 2015;6:206.
7. Imhoff R, Lamberty P. How paranoid are conspiracy believers? Toward a more fine-grained understanding of the connect and disconnect between paranoia and belief in conspiracy theories. Eur J Soc Psychol. 2018;48(7):909-926.
8. Sheridan LP, James DV. Complaints of group-stalking (‘gang-stalking’): an exploratory study of their natures and impact on complainants. J Forens Psychiatry Psychol. 2015;26(5):601-623.
1. Pierre JM. The borders of mental disorder in psychiatry and the DSM: past, present, and future. J Psychiatric Practice. 2010;16(6):375-386.
2. Pierre JM. Faith or delusion? At the crossroads of religion and psychosis. J Psychiatr Practice. 2001;7(3):163-172.
3. Pierre JM. Forensic psychiatry versus the varieties of delusion-like belief. J Am Acad Psychiatry Law. 2020;48(3):327-334.
4. Oliver JE, Wood, TJ. Conspiracy theories and the paranoid style(s) of mass opinion. Am J Pol Sci. 2014;58(5);952-966.
5. Pierre JM. Mistrust and misinformation: a two-component, socio-epistemic model of belief in conspiracy theories. J Soc Polit Psychol. 2020;8(2):617-641.
6. Dagnall N, Drinkwater K, Parker A, et al. Conspiracy theory and cognitive style: a worldview. Front Psychol. 2015;6:206.
7. Imhoff R, Lamberty P. How paranoid are conspiracy believers? Toward a more fine-grained understanding of the connect and disconnect between paranoia and belief in conspiracy theories. Eur J Soc Psychol. 2018;48(7):909-926.
8. Sheridan LP, James DV. Complaints of group-stalking (‘gang-stalking’): an exploratory study of their natures and impact on complainants. J Forens Psychiatry Psychol. 2015;26(5):601-623.
Serotonergic antidepressants’ effects on bone health
Mrs. D, age 67, has a history of major depressive disorder. She has had adequate treatment trials with duloxetine, mirtazapine, and sertraline; each failed to produce remission. She is currently prescribed paroxetine, 40 mg/d, and aripiprazole, 10 mg/d, with good efficacy. She also has a history of hypertension and seasonal allergies, for which she receives amlodipine, 10 mg/d, and loratadine, 10 mg/d, respectively.
Mrs. D’s depressive symptoms were well controlled until 2 months ago, when she fell and fractured her hip. With encouragement from her prescriber, she enrolled in a partial hospitalization program for more intensive psychotherapy. During a medication education session, she is surprised to learn that antidepressants may affect bone health.
During a medication management meeting with her prescriber, Mrs. D asks about the risk of osteoporosis, and whether her antidepressant could have contributed to her hip fracture.
Bone is a dynamic tissue that undergoes a continuous process of remodeling. Osteoblasts are responsible for bone formation, whereas osteoclasts are responsible for bone resorption. Osteocytes—the predominant cell type in bone—along with cytokines, hormones, and growth factors help to orchestrate these actions.1 Serotonin is increasingly recognized as a factor in bone homeostasis. Bone synthesizes serotonin, expresses serotonin transporters, and contains a variety of serotonin receptors.2
Serotonin serves many physiologic functions outside of the CNS, and it appears to have opposing actions on bone metabolism (Table 11,3). Peripheral (gut-derived) serotonin inhibits bone formation through its effects on osteoblasts, whereas the actions of serotonin in the CNS promote bone growth through inhibitory effects on sympathetic output.2 Selective serotonin reuptake inhibitor (SSRI) enhancement of peripheral serotonin and its negative effect on bone may outweigh the benefits caused by SSRI enhancement of central serotonin neurotransmission.1 In vitro data suggest SSRIs inhibit osteoblast and osteoclast function, theoretically decreasing bone turnover and increasing fracture risk.4 Other data indicate SSRI treatment may decrease procollagen type 1 N-terminal propeptide, a peripheral marker of bone formation.5 Both SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs) have been associated with lower cortical bone mineral density (BMD).6Table 27,8 details the relative affinity of select antidepressants for the serotonin transporter.
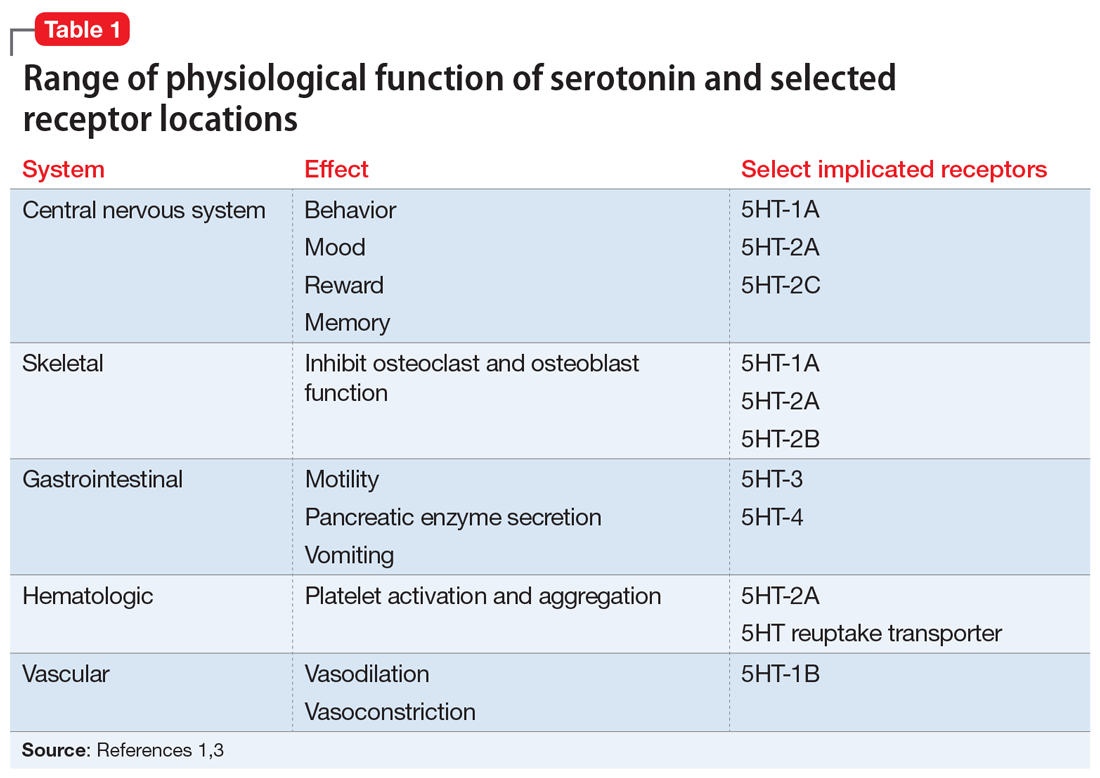
Both serotonergic antidepressants and depression have been associated with decreased BMD and increased fracture risk.1,9 Behavioral aspects of depression, such as inadequate nutrition or physical inactivity, overlap with risk factors for poor bone health. In addition, elevated levels of circulating cortisol and proinflammatory cytokines in patients with depressive symptoms may contribute to decreased bone mass.10,11 Modifiable risk factors for osteoporosis and fractures include low calcium and vitamin D intake, low body weight, and a sedentary lifestyle. Nonmodifiable risk factors include advancing age, female sex, Asian or White ethnicity, malabsorptive conditions, and chronic corticosteroid use.12
What the evidence says
Evidence for the correlation between fractures and serotonergic antidepressant use is mixed. One meta-analysis found a significant association between SSRIs and fractures, suggesting a 1.62-fold increased risk.13 Another meta-analysis investigated SSRIs and SNRIs and the risk of fracture.14 The SSRIs had a 1.67-fold increased risk; however, a lack of studies prohibited making conclusions about SNRIs. The number needed to harm was calculated at 85, 46, and 19 with 1, 2, and 5 years of SSRI exposure, respectively. A third meta-analysis found increased fracture risk related to depression and reported a hazard ratio of 1.26 after adjusting for confounders.9 This analysis suggests depression affects fracture risk and may limit the interpretation of causation from SSRI use. Studies included in these meta-analyses had significant heterogeneity.
Continue to: The effect of SSRIs...
The effect of SSRIs vs non-SSRIs on BMD also has been studied. The SSRIs were associated with significantly reduced BMD of the lumbar spine but not the total hip or femoral neck as compared to non-SSRIs; however, this BMD loss was not examined in relation to the presence of fractures. Older patients had more pronounced bone loss.15 Conversely, another meta-analysis examined BMD in women receiving SSRIs or tricyclic antidepressants.10 Neither medication class was associated with lower BMD at measured locations, including lumbar spine, femoral neck, and total hip. This analysis was limited by the lack of available trials; only 4 were included.
Other recent research has continued to explore the relationship between antidepressants and fracture in various patient populations. In a study of patients receiving maintenance dialysis treatment, short- and long-term SSRI use increased hip fracture risk. The authors speculated that short-term risk may be mediated by adverse effects that increase fall risk (eg, hyponatremia, orthostasis), whereas long-term risk may be influenced by changes in bone homeostasis.16 In two 6-month analyses of fluoxetine treatment in patients following an acute stroke, fluoxetine increased the risk of bone fractures.17,18 Finally, in women with osteoporosis receiving risedronate or teriparatide, in both groups a higher fracture risk was observed for patients who were also receiving an SSRI or SNRI.19
Monitor BMD and educate patients about bone health
Available literature has not identified any clear risk factors for fracture with SSRI use. Guidelines suggest monitoring BMD in patients with risk factors for osteoporosis, if clinically indicated, as well as monitoring BMD in those receiving long-term antidepressant treatment.20-22 Educate patients on strategies that promote optimal bone health, such as consuming a balanced diet that meets the recommended dietary allowance of calcium, vitamin D, and limits soda consumption. Teach patients to avoid tobacco and excessive alcohol use because both adversely impact BMD. Maintaining a healthy weight, physical activity, and adequate sleep also support bone health.11 Instruct patients receiving antidepressants to report unexplained bone pain, tenderness, swelling, or bruising because these symptoms may be indicative of fracture.
CASE CONTINUED
Mrs. D’s age, sex, and depression place her at higher risk of fracture. Paroxetine is the only SSRI that has bone fracture listed as a precaution in its labeling.23 In addition, it is the most anticholinergic SSRI and may have contributed to her fall. Switching to bupropion by cross titration may benefit Mrs. D because bupropion is not serotonergic. Little data exist regarding the effects of bupropion on bone. Her prescriber monitors Mrs. D’s BMD periodically, and educates her on dietary considerations. He also recommends calcium, 1,200 mg/d, and vitamin D, 800 IU/d, to help prevent fractures,24 and that she continue physical therapy exercises and increase physical activity as tolerated.
Related Resources
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2581.
- Dodd S, Mitchell PB, Bauer M, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: an international consensus statement. World J Biol Psychiatry. 2018;19(5):330-348.
- Fernandes BS, Hodge JM, Pasco JA, et al. Effects of depression and serotonergic antidepressants on bone: mechanisms and implications for the treatment of depression. Drugs Aging. 2016;33(1):21-25.
- US National Library of Medicine. DailyMed. https://dailymed.nlm.nih.gov/dailymed
Drug Brand Names
Amitriptyline • Elavil
Amlodipine • Norvasc
Aripiprazole • Abilify
Bupropion • Wellbutrin
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Doxepin • Silenor, Sinequan
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Imipramine • Tofranil
Levomilnacipran • Fetzima
Loratadine • Claritin
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Risedronate • Actonel
Sertraline • Zoloft
Teriparatide • Forteo
Trazodone • Desyrel
Venlafaxine • Effexor
Vortioxetine • Trintellix
1. Fernandes BS, Hodge JM, Pasco JA, et al. Effects of depression and serotonergic antidepressants on bone: mechanisms and implications for the treatment of depression. Drugs Aging. 2016;33(1):21-25.
2. Lavoie B, Lian JB, Mawe GM. Regulation of bone metabolism by serotonin. Adv Exp Med Biol. 2017;1033:35-46.
3. Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355-366.
4. Hodge JM, Wang Y, Berk M, et al. Selective serotonin reuptake inhibitors inhibit human osteoclast and osteoblast formation and function. Biol Psychiatry. 2013;74(1):32-39.
5. Kumar M, Jiloha RC, Kataria D, et al. Effect of selective serotonin reuptake inhibitors on markers of bone loss. Psychiatry Res. 2019;276:39-44.
6. Agarwal S, Germosen C, Kil N, et al. Current anti-depressant use is associated with cortical bone deficits and reduced physical function in elderly women. Bone. 2020;140:115552.
7. DeBattista C. Antidepressant agents. In: Katzung BG, ed. Basic and clinical pharmacology. 14th ed. McGraw-Hill; 2018.
8. Kasper S, Pail G. Milnacipran: a unique antidepressant? Neuropsychiatr Dis Treat. 2010;6(Suppl 1):23-31.
9. Wu Q, Liu B, Tonmoy S. Depression and risk of fracture and bone loss: an updated meta-analysis of prospective studies. Osteoporos Int. 2018;29(6):1303-1312.
10. Schweiger JU, Schweiger U, Hüppe M, et al. The use of antidepressant agents and bone mineral density in women: a meta-analysis. Int J Environ Res Public Health. 2018;15(7):1373.
11. Rizzoli R, Cooper C, Reginster JY, et al. Antidepressant medications and osteoporosis. Bone. 2012;51(3):606-613.
12. Rice JN, Gillett CB, Malas NM. The impact of psychotropic medications on bone health in youth. Curr Psychiatry Rep. 2018;20(11):104.
13. Kumar M, Bajpai R, Shaik AR, et al. Alliance between selective serotonin reuptake inhibitors and fracture risk: an updated systematic review and meta-analysis. Eur J Clin Pharmacol. 2020;76(10):1373-1392.
14. Khanassov V, Hu J, Reeves D, et al. Selective serotonin reuptake inhibitor and selective serotonin and norepinephrine reuptake inhibitor use and risk of fractures in adults: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2018;33(12):1688-1708.
15. Zhou C, Fang L, Chen Y, et al. Effect of selective serotonin reuptake inhibitors on bone mineral density: a systematic review and meta-analysis. Osteoporos Int. 2018;29(6):1243-1251.
16. Vangala C, Niu J, Montez-Rath ME, et al. Selective serotonin reuptake inhibitor use and hip fracture risk among patients on hemodialysis. Am J Kidney Dis. 2020;75(3):351-360.
17. Hankey GJ, Hackett ML, Almeida OP, et al. Safety and efficacy of fluoxetine on functional outcome after acute stroke (AFFINITY): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19(8):651-660.
18. Lundström E, Isaksson E, Näsman P, et al. Safety and efficacy of fluoxetine on functional recovery after acute stroke (EFFECTS): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19(8):661-669.
19. Kendler DL, Marin F, Geusens P, et al. Psychotropic medications and proton pump inhibitors and the risk of fractures in the teriparatide versus risedronate VERO clinical trial. Bone. 2020;130:115113.
20. Dodd S, Mitchell PB, Bauer M, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: an international consensus statement. World J Biol Psychiatry. 2018;19(5):330-348.
21. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. Published October 2010. Accessed February 8, 2021. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf
22. Agacayak KS, Guler R, Ilyasov B. Evaluation of the effect of long-term use of antidepressants in the SSRI group on bone density with dental volumetric tomography. Drug Des Devel Ther. 2019;13:3477-3484.
23. US National Library of Medicine. DailyMed. Accessed February 8, 2021. https://dailymed.nlm.nih.gov/dailymed
24. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2581.
Mrs. D, age 67, has a history of major depressive disorder. She has had adequate treatment trials with duloxetine, mirtazapine, and sertraline; each failed to produce remission. She is currently prescribed paroxetine, 40 mg/d, and aripiprazole, 10 mg/d, with good efficacy. She also has a history of hypertension and seasonal allergies, for which she receives amlodipine, 10 mg/d, and loratadine, 10 mg/d, respectively.
Mrs. D’s depressive symptoms were well controlled until 2 months ago, when she fell and fractured her hip. With encouragement from her prescriber, she enrolled in a partial hospitalization program for more intensive psychotherapy. During a medication education session, she is surprised to learn that antidepressants may affect bone health.
During a medication management meeting with her prescriber, Mrs. D asks about the risk of osteoporosis, and whether her antidepressant could have contributed to her hip fracture.
Bone is a dynamic tissue that undergoes a continuous process of remodeling. Osteoblasts are responsible for bone formation, whereas osteoclasts are responsible for bone resorption. Osteocytes—the predominant cell type in bone—along with cytokines, hormones, and growth factors help to orchestrate these actions.1 Serotonin is increasingly recognized as a factor in bone homeostasis. Bone synthesizes serotonin, expresses serotonin transporters, and contains a variety of serotonin receptors.2
Serotonin serves many physiologic functions outside of the CNS, and it appears to have opposing actions on bone metabolism (Table 11,3). Peripheral (gut-derived) serotonin inhibits bone formation through its effects on osteoblasts, whereas the actions of serotonin in the CNS promote bone growth through inhibitory effects on sympathetic output.2 Selective serotonin reuptake inhibitor (SSRI) enhancement of peripheral serotonin and its negative effect on bone may outweigh the benefits caused by SSRI enhancement of central serotonin neurotransmission.1 In vitro data suggest SSRIs inhibit osteoblast and osteoclast function, theoretically decreasing bone turnover and increasing fracture risk.4 Other data indicate SSRI treatment may decrease procollagen type 1 N-terminal propeptide, a peripheral marker of bone formation.5 Both SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs) have been associated with lower cortical bone mineral density (BMD).6Table 27,8 details the relative affinity of select antidepressants for the serotonin transporter.

Both serotonergic antidepressants and depression have been associated with decreased BMD and increased fracture risk.1,9 Behavioral aspects of depression, such as inadequate nutrition or physical inactivity, overlap with risk factors for poor bone health. In addition, elevated levels of circulating cortisol and proinflammatory cytokines in patients with depressive symptoms may contribute to decreased bone mass.10,11 Modifiable risk factors for osteoporosis and fractures include low calcium and vitamin D intake, low body weight, and a sedentary lifestyle. Nonmodifiable risk factors include advancing age, female sex, Asian or White ethnicity, malabsorptive conditions, and chronic corticosteroid use.12

What the evidence says
Evidence for the correlation between fractures and serotonergic antidepressant use is mixed. One meta-analysis found a significant association between SSRIs and fractures, suggesting a 1.62-fold increased risk.13 Another meta-analysis investigated SSRIs and SNRIs and the risk of fracture.14 The SSRIs had a 1.67-fold increased risk; however, a lack of studies prohibited making conclusions about SNRIs. The number needed to harm was calculated at 85, 46, and 19 with 1, 2, and 5 years of SSRI exposure, respectively. A third meta-analysis found increased fracture risk related to depression and reported a hazard ratio of 1.26 after adjusting for confounders.9 This analysis suggests depression affects fracture risk and may limit the interpretation of causation from SSRI use. Studies included in these meta-analyses had significant heterogeneity.
Continue to: The effect of SSRIs...
The effect of SSRIs vs non-SSRIs on BMD also has been studied. The SSRIs were associated with significantly reduced BMD of the lumbar spine but not the total hip or femoral neck as compared to non-SSRIs; however, this BMD loss was not examined in relation to the presence of fractures. Older patients had more pronounced bone loss.15 Conversely, another meta-analysis examined BMD in women receiving SSRIs or tricyclic antidepressants.10 Neither medication class was associated with lower BMD at measured locations, including lumbar spine, femoral neck, and total hip. This analysis was limited by the lack of available trials; only 4 were included.
Other recent research has continued to explore the relationship between antidepressants and fracture in various patient populations. In a study of patients receiving maintenance dialysis treatment, short- and long-term SSRI use increased hip fracture risk. The authors speculated that short-term risk may be mediated by adverse effects that increase fall risk (eg, hyponatremia, orthostasis), whereas long-term risk may be influenced by changes in bone homeostasis.16 In two 6-month analyses of fluoxetine treatment in patients following an acute stroke, fluoxetine increased the risk of bone fractures.17,18 Finally, in women with osteoporosis receiving risedronate or teriparatide, in both groups a higher fracture risk was observed for patients who were also receiving an SSRI or SNRI.19
Monitor BMD and educate patients about bone health
Available literature has not identified any clear risk factors for fracture with SSRI use. Guidelines suggest monitoring BMD in patients with risk factors for osteoporosis, if clinically indicated, as well as monitoring BMD in those receiving long-term antidepressant treatment.20-22 Educate patients on strategies that promote optimal bone health, such as consuming a balanced diet that meets the recommended dietary allowance of calcium, vitamin D, and limits soda consumption. Teach patients to avoid tobacco and excessive alcohol use because both adversely impact BMD. Maintaining a healthy weight, physical activity, and adequate sleep also support bone health.11 Instruct patients receiving antidepressants to report unexplained bone pain, tenderness, swelling, or bruising because these symptoms may be indicative of fracture.
CASE CONTINUED
Mrs. D’s age, sex, and depression place her at higher risk of fracture. Paroxetine is the only SSRI that has bone fracture listed as a precaution in its labeling.23 In addition, it is the most anticholinergic SSRI and may have contributed to her fall. Switching to bupropion by cross titration may benefit Mrs. D because bupropion is not serotonergic. Little data exist regarding the effects of bupropion on bone. Her prescriber monitors Mrs. D’s BMD periodically, and educates her on dietary considerations. He also recommends calcium, 1,200 mg/d, and vitamin D, 800 IU/d, to help prevent fractures,24 and that she continue physical therapy exercises and increase physical activity as tolerated.
Related Resources
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2581.
- Dodd S, Mitchell PB, Bauer M, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: an international consensus statement. World J Biol Psychiatry. 2018;19(5):330-348.
- Fernandes BS, Hodge JM, Pasco JA, et al. Effects of depression and serotonergic antidepressants on bone: mechanisms and implications for the treatment of depression. Drugs Aging. 2016;33(1):21-25.
- US National Library of Medicine. DailyMed. https://dailymed.nlm.nih.gov/dailymed
Drug Brand Names
Amitriptyline • Elavil
Amlodipine • Norvasc
Aripiprazole • Abilify
Bupropion • Wellbutrin
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Doxepin • Silenor, Sinequan
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Imipramine • Tofranil
Levomilnacipran • Fetzima
Loratadine • Claritin
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Risedronate • Actonel
Sertraline • Zoloft
Teriparatide • Forteo
Trazodone • Desyrel
Venlafaxine • Effexor
Vortioxetine • Trintellix
Mrs. D, age 67, has a history of major depressive disorder. She has had adequate treatment trials with duloxetine, mirtazapine, and sertraline; each failed to produce remission. She is currently prescribed paroxetine, 40 mg/d, and aripiprazole, 10 mg/d, with good efficacy. She also has a history of hypertension and seasonal allergies, for which she receives amlodipine, 10 mg/d, and loratadine, 10 mg/d, respectively.
Mrs. D’s depressive symptoms were well controlled until 2 months ago, when she fell and fractured her hip. With encouragement from her prescriber, she enrolled in a partial hospitalization program for more intensive psychotherapy. During a medication education session, she is surprised to learn that antidepressants may affect bone health.
During a medication management meeting with her prescriber, Mrs. D asks about the risk of osteoporosis, and whether her antidepressant could have contributed to her hip fracture.
Bone is a dynamic tissue that undergoes a continuous process of remodeling. Osteoblasts are responsible for bone formation, whereas osteoclasts are responsible for bone resorption. Osteocytes—the predominant cell type in bone—along with cytokines, hormones, and growth factors help to orchestrate these actions.1 Serotonin is increasingly recognized as a factor in bone homeostasis. Bone synthesizes serotonin, expresses serotonin transporters, and contains a variety of serotonin receptors.2
Serotonin serves many physiologic functions outside of the CNS, and it appears to have opposing actions on bone metabolism (Table 11,3). Peripheral (gut-derived) serotonin inhibits bone formation through its effects on osteoblasts, whereas the actions of serotonin in the CNS promote bone growth through inhibitory effects on sympathetic output.2 Selective serotonin reuptake inhibitor (SSRI) enhancement of peripheral serotonin and its negative effect on bone may outweigh the benefits caused by SSRI enhancement of central serotonin neurotransmission.1 In vitro data suggest SSRIs inhibit osteoblast and osteoclast function, theoretically decreasing bone turnover and increasing fracture risk.4 Other data indicate SSRI treatment may decrease procollagen type 1 N-terminal propeptide, a peripheral marker of bone formation.5 Both SSRIs and serotonin-norepinephrine reuptake inhibitors (SNRIs) have been associated with lower cortical bone mineral density (BMD).6Table 27,8 details the relative affinity of select antidepressants for the serotonin transporter.

Both serotonergic antidepressants and depression have been associated with decreased BMD and increased fracture risk.1,9 Behavioral aspects of depression, such as inadequate nutrition or physical inactivity, overlap with risk factors for poor bone health. In addition, elevated levels of circulating cortisol and proinflammatory cytokines in patients with depressive symptoms may contribute to decreased bone mass.10,11 Modifiable risk factors for osteoporosis and fractures include low calcium and vitamin D intake, low body weight, and a sedentary lifestyle. Nonmodifiable risk factors include advancing age, female sex, Asian or White ethnicity, malabsorptive conditions, and chronic corticosteroid use.12

What the evidence says
Evidence for the correlation between fractures and serotonergic antidepressant use is mixed. One meta-analysis found a significant association between SSRIs and fractures, suggesting a 1.62-fold increased risk.13 Another meta-analysis investigated SSRIs and SNRIs and the risk of fracture.14 The SSRIs had a 1.67-fold increased risk; however, a lack of studies prohibited making conclusions about SNRIs. The number needed to harm was calculated at 85, 46, and 19 with 1, 2, and 5 years of SSRI exposure, respectively. A third meta-analysis found increased fracture risk related to depression and reported a hazard ratio of 1.26 after adjusting for confounders.9 This analysis suggests depression affects fracture risk and may limit the interpretation of causation from SSRI use. Studies included in these meta-analyses had significant heterogeneity.
Continue to: The effect of SSRIs...
The effect of SSRIs vs non-SSRIs on BMD also has been studied. The SSRIs were associated with significantly reduced BMD of the lumbar spine but not the total hip or femoral neck as compared to non-SSRIs; however, this BMD loss was not examined in relation to the presence of fractures. Older patients had more pronounced bone loss.15 Conversely, another meta-analysis examined BMD in women receiving SSRIs or tricyclic antidepressants.10 Neither medication class was associated with lower BMD at measured locations, including lumbar spine, femoral neck, and total hip. This analysis was limited by the lack of available trials; only 4 were included.
Other recent research has continued to explore the relationship between antidepressants and fracture in various patient populations. In a study of patients receiving maintenance dialysis treatment, short- and long-term SSRI use increased hip fracture risk. The authors speculated that short-term risk may be mediated by adverse effects that increase fall risk (eg, hyponatremia, orthostasis), whereas long-term risk may be influenced by changes in bone homeostasis.16 In two 6-month analyses of fluoxetine treatment in patients following an acute stroke, fluoxetine increased the risk of bone fractures.17,18 Finally, in women with osteoporosis receiving risedronate or teriparatide, in both groups a higher fracture risk was observed for patients who were also receiving an SSRI or SNRI.19
Monitor BMD and educate patients about bone health
Available literature has not identified any clear risk factors for fracture with SSRI use. Guidelines suggest monitoring BMD in patients with risk factors for osteoporosis, if clinically indicated, as well as monitoring BMD in those receiving long-term antidepressant treatment.20-22 Educate patients on strategies that promote optimal bone health, such as consuming a balanced diet that meets the recommended dietary allowance of calcium, vitamin D, and limits soda consumption. Teach patients to avoid tobacco and excessive alcohol use because both adversely impact BMD. Maintaining a healthy weight, physical activity, and adequate sleep also support bone health.11 Instruct patients receiving antidepressants to report unexplained bone pain, tenderness, swelling, or bruising because these symptoms may be indicative of fracture.
CASE CONTINUED
Mrs. D’s age, sex, and depression place her at higher risk of fracture. Paroxetine is the only SSRI that has bone fracture listed as a precaution in its labeling.23 In addition, it is the most anticholinergic SSRI and may have contributed to her fall. Switching to bupropion by cross titration may benefit Mrs. D because bupropion is not serotonergic. Little data exist regarding the effects of bupropion on bone. Her prescriber monitors Mrs. D’s BMD periodically, and educates her on dietary considerations. He also recommends calcium, 1,200 mg/d, and vitamin D, 800 IU/d, to help prevent fractures,24 and that she continue physical therapy exercises and increase physical activity as tolerated.
Related Resources
- Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2581.
- Dodd S, Mitchell PB, Bauer M, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: an international consensus statement. World J Biol Psychiatry. 2018;19(5):330-348.
- Fernandes BS, Hodge JM, Pasco JA, et al. Effects of depression and serotonergic antidepressants on bone: mechanisms and implications for the treatment of depression. Drugs Aging. 2016;33(1):21-25.
- US National Library of Medicine. DailyMed. https://dailymed.nlm.nih.gov/dailymed
Drug Brand Names
Amitriptyline • Elavil
Amlodipine • Norvasc
Aripiprazole • Abilify
Bupropion • Wellbutrin
Citalopram • Celexa
Clomipramine • Anafranil
Desipramine • Norpramin
Doxepin • Silenor, Sinequan
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Fluvoxamine • Luvox
Imipramine • Tofranil
Levomilnacipran • Fetzima
Loratadine • Claritin
Mirtazapine • Remeron
Nortriptyline • Pamelor
Paroxetine • Paxil
Risedronate • Actonel
Sertraline • Zoloft
Teriparatide • Forteo
Trazodone • Desyrel
Venlafaxine • Effexor
Vortioxetine • Trintellix
1. Fernandes BS, Hodge JM, Pasco JA, et al. Effects of depression and serotonergic antidepressants on bone: mechanisms and implications for the treatment of depression. Drugs Aging. 2016;33(1):21-25.
2. Lavoie B, Lian JB, Mawe GM. Regulation of bone metabolism by serotonin. Adv Exp Med Biol. 2017;1033:35-46.
3. Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355-366.
4. Hodge JM, Wang Y, Berk M, et al. Selective serotonin reuptake inhibitors inhibit human osteoclast and osteoblast formation and function. Biol Psychiatry. 2013;74(1):32-39.
5. Kumar M, Jiloha RC, Kataria D, et al. Effect of selective serotonin reuptake inhibitors on markers of bone loss. Psychiatry Res. 2019;276:39-44.
6. Agarwal S, Germosen C, Kil N, et al. Current anti-depressant use is associated with cortical bone deficits and reduced physical function in elderly women. Bone. 2020;140:115552.
7. DeBattista C. Antidepressant agents. In: Katzung BG, ed. Basic and clinical pharmacology. 14th ed. McGraw-Hill; 2018.
8. Kasper S, Pail G. Milnacipran: a unique antidepressant? Neuropsychiatr Dis Treat. 2010;6(Suppl 1):23-31.
9. Wu Q, Liu B, Tonmoy S. Depression and risk of fracture and bone loss: an updated meta-analysis of prospective studies. Osteoporos Int. 2018;29(6):1303-1312.
10. Schweiger JU, Schweiger U, Hüppe M, et al. The use of antidepressant agents and bone mineral density in women: a meta-analysis. Int J Environ Res Public Health. 2018;15(7):1373.
11. Rizzoli R, Cooper C, Reginster JY, et al. Antidepressant medications and osteoporosis. Bone. 2012;51(3):606-613.
12. Rice JN, Gillett CB, Malas NM. The impact of psychotropic medications on bone health in youth. Curr Psychiatry Rep. 2018;20(11):104.
13. Kumar M, Bajpai R, Shaik AR, et al. Alliance between selective serotonin reuptake inhibitors and fracture risk: an updated systematic review and meta-analysis. Eur J Clin Pharmacol. 2020;76(10):1373-1392.
14. Khanassov V, Hu J, Reeves D, et al. Selective serotonin reuptake inhibitor and selective serotonin and norepinephrine reuptake inhibitor use and risk of fractures in adults: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2018;33(12):1688-1708.
15. Zhou C, Fang L, Chen Y, et al. Effect of selective serotonin reuptake inhibitors on bone mineral density: a systematic review and meta-analysis. Osteoporos Int. 2018;29(6):1243-1251.
16. Vangala C, Niu J, Montez-Rath ME, et al. Selective serotonin reuptake inhibitor use and hip fracture risk among patients on hemodialysis. Am J Kidney Dis. 2020;75(3):351-360.
17. Hankey GJ, Hackett ML, Almeida OP, et al. Safety and efficacy of fluoxetine on functional outcome after acute stroke (AFFINITY): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19(8):651-660.
18. Lundström E, Isaksson E, Näsman P, et al. Safety and efficacy of fluoxetine on functional recovery after acute stroke (EFFECTS): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19(8):661-669.
19. Kendler DL, Marin F, Geusens P, et al. Psychotropic medications and proton pump inhibitors and the risk of fractures in the teriparatide versus risedronate VERO clinical trial. Bone. 2020;130:115113.
20. Dodd S, Mitchell PB, Bauer M, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: an international consensus statement. World J Biol Psychiatry. 2018;19(5):330-348.
21. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. Published October 2010. Accessed February 8, 2021. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf
22. Agacayak KS, Guler R, Ilyasov B. Evaluation of the effect of long-term use of antidepressants in the SSRI group on bone density with dental volumetric tomography. Drug Des Devel Ther. 2019;13:3477-3484.
23. US National Library of Medicine. DailyMed. Accessed February 8, 2021. https://dailymed.nlm.nih.gov/dailymed
24. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2581.
1. Fernandes BS, Hodge JM, Pasco JA, et al. Effects of depression and serotonergic antidepressants on bone: mechanisms and implications for the treatment of depression. Drugs Aging. 2016;33(1):21-25.
2. Lavoie B, Lian JB, Mawe GM. Regulation of bone metabolism by serotonin. Adv Exp Med Biol. 2017;1033:35-46.
3. Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355-366.
4. Hodge JM, Wang Y, Berk M, et al. Selective serotonin reuptake inhibitors inhibit human osteoclast and osteoblast formation and function. Biol Psychiatry. 2013;74(1):32-39.
5. Kumar M, Jiloha RC, Kataria D, et al. Effect of selective serotonin reuptake inhibitors on markers of bone loss. Psychiatry Res. 2019;276:39-44.
6. Agarwal S, Germosen C, Kil N, et al. Current anti-depressant use is associated with cortical bone deficits and reduced physical function in elderly women. Bone. 2020;140:115552.
7. DeBattista C. Antidepressant agents. In: Katzung BG, ed. Basic and clinical pharmacology. 14th ed. McGraw-Hill; 2018.
8. Kasper S, Pail G. Milnacipran: a unique antidepressant? Neuropsychiatr Dis Treat. 2010;6(Suppl 1):23-31.
9. Wu Q, Liu B, Tonmoy S. Depression and risk of fracture and bone loss: an updated meta-analysis of prospective studies. Osteoporos Int. 2018;29(6):1303-1312.
10. Schweiger JU, Schweiger U, Hüppe M, et al. The use of antidepressant agents and bone mineral density in women: a meta-analysis. Int J Environ Res Public Health. 2018;15(7):1373.
11. Rizzoli R, Cooper C, Reginster JY, et al. Antidepressant medications and osteoporosis. Bone. 2012;51(3):606-613.
12. Rice JN, Gillett CB, Malas NM. The impact of psychotropic medications on bone health in youth. Curr Psychiatry Rep. 2018;20(11):104.
13. Kumar M, Bajpai R, Shaik AR, et al. Alliance between selective serotonin reuptake inhibitors and fracture risk: an updated systematic review and meta-analysis. Eur J Clin Pharmacol. 2020;76(10):1373-1392.
14. Khanassov V, Hu J, Reeves D, et al. Selective serotonin reuptake inhibitor and selective serotonin and norepinephrine reuptake inhibitor use and risk of fractures in adults: a systematic review and meta-analysis. Int J Geriatr Psychiatry. 2018;33(12):1688-1708.
15. Zhou C, Fang L, Chen Y, et al. Effect of selective serotonin reuptake inhibitors on bone mineral density: a systematic review and meta-analysis. Osteoporos Int. 2018;29(6):1243-1251.
16. Vangala C, Niu J, Montez-Rath ME, et al. Selective serotonin reuptake inhibitor use and hip fracture risk among patients on hemodialysis. Am J Kidney Dis. 2020;75(3):351-360.
17. Hankey GJ, Hackett ML, Almeida OP, et al. Safety and efficacy of fluoxetine on functional outcome after acute stroke (AFFINITY): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19(8):651-660.
18. Lundström E, Isaksson E, Näsman P, et al. Safety and efficacy of fluoxetine on functional recovery after acute stroke (EFFECTS): a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2020;19(8):661-669.
19. Kendler DL, Marin F, Geusens P, et al. Psychotropic medications and proton pump inhibitors and the risk of fractures in the teriparatide versus risedronate VERO clinical trial. Bone. 2020;130:115113.
20. Dodd S, Mitchell PB, Bauer M, et al. Monitoring for antidepressant-associated adverse events in the treatment of patients with major depressive disorder: an international consensus statement. World J Biol Psychiatry. 2018;19(5):330-348.
21. American Psychiatric Association. Practice guideline for the treatment of patients with major depressive disorder. Published October 2010. Accessed February 8, 2021. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/mdd.pdf
22. Agacayak KS, Guler R, Ilyasov B. Evaluation of the effect of long-term use of antidepressants in the SSRI group on bone density with dental volumetric tomography. Drug Des Devel Ther. 2019;13:3477-3484.
23. US National Library of Medicine. DailyMed. Accessed February 8, 2021. https://dailymed.nlm.nih.gov/dailymed
24. Cosman F, de Beur SJ, LeBoff MS, et al. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014;25(10):2359-2581.
An unquenchable thirst
CASE Unresponsive after a presumed seizure
Mr. F, age 44, has schizophrenia. He is brought to the hospital by ambulance after he is found on the ground outside of his mother’s house following a presumed seizure and fall. On arrival to the emergency department, he is unresponsive. His laboratory values are significant for a sodium level of 110 mEq/L (reference range: 135 to 145 mEq/L), indicating hyponatremia.
HISTORY Fixated on purity
Mr. F’s mother reports that Mr. F had an unremarkable childhood. He was raised in a household with both parents and a younger sister. Mr. F did well academically and studied engineering and physics in college. There was no reported history of trauma or substance use.
During his senior year of college, Mr. F began experiencing paranoia, auditory hallucinations, and religious delusions. He required hospitalization and was diagnosed with schizophrenia. Following multiple hospitalizations over 5 years, he moved in with his mother, who was granted guardianship.
His mother said Mr. F’s religious delusions were of purity and cleansing the soul. He spent hours memorizing the Bible and would go for days without eating but would drink large amounts of water. She said she thought this was due to his desire to flush out imperfections.
In the past 3 years, Mr. F has been hospitalized several times for severe hyponatremia. At home, his mother attempted to restrict his water intake. However, Mr. F would still drink out of sinks and hoses. Mr. F’s mother reports that over the past month he had become more isolated. He would spend entire days reading the Bible, and his water intake had further increased.
Prior medication trials for Mr. F included haloperidol, up to 10 mg twice per day; aripiprazole, up to 20 mg/d; and risperidone, up to 6 mg nightly. These had been effective, but Mr. F had difficulty with adherence. He did not receive a long-acting injectable (LAI) antipsychotic initially due to lack of access at the rural clinic where he was treated, and later due to his mother’s preference for her son to receive oral medications. Prior to his current presentation, Mr. F’s medication regimen was olanzapine, 10 mg twice a day; perphenazine, 8 mg twice a day; and long-acting propranolol, 60 mg/d. Mr. F had no other chronic medical problems.
EVALUATION Hyponatremia, but why?
Mr. F is intubated and admitted to the surgical service for stabilization due to injuries from his fall. He has fractures of his right sinus and bilateral nasal bones, which are managed nonoperatively. He is delirious, with waxing and waning attention, memory disturbances, and disorientation. His psychotropic medications are held.
Continue to: Imaging of his head...
Imaging of his head does not reveal acute abnormalities suggesting a malignant or paraneoplastic process, and there are no concerns for ongoing seizures. An infection workup is negative. His urine toxicology is negative and blood alcohol level is 0. His sodium normalizes after 3 days of IV fluids and fluid restriction. Therefore, further tests to differentiate the causes of hyponatremia, such as urine electrolytes and urine osmolality, are not pursued.
[polldaddy:10910406]
The authors’ observations
The differential diagnosis for hyponatremia is broad in the setting of psychiatric illness. Low sodium levels could be due to psychotropic medications, psychiatrically-driven behaviors, or an underlying medical problem. Our differential diagnosis for Mr. F included iatrogenic syndrome of inappropriate antidiuretic hormone (SIADH), diabetes insipidus, or psychogenic polydipsia, a form of primary polydipsia. Other causes of primary polydipsia are related to substances, such as heavy beer intakeor use of 3,4-methylenedioxymethamphetamine (MDMA, also known as “ecstasy”), or brain lesions,1 but these causes were less likely given Mr. F’s negative urine toxicology and head imaging.
While psychogenic polydipsia is due to increased water consumption, both SIADH and diabetes insipidus are due to alterations in fluid homeostasis.2,3 Table 12-4 outlines distinguishing characteristics of SIADH, diabetes insipidus, and psychogenic polydipsia. Urine studies were not pursued because Mr. F’s sodium resolved and acute concerns, such as malignancy or infection, were ruled out. Mr. F’s hyponatremia was presumed to be due to psychogenic polydipsia because of his increased fluid intake and normalization of sodium with hypertonic fluids and subsequent fluid restriction. During this time, he was managed on the surgical service; the plan was to pursue urine studies and possibly a fluid challenge if his hyponatremia persisted.
EVALUATION Delirium resolves, delusions persist
While Mr. F is on the surgical service, the treatment team focuses on stabilizing his sodium level and assessing for causes of altered mental status that led to his fall. Psychiatry is consulted for management of his agitation. Following the gradual correction of his sodium level and extubation, his sensorium improves. By hospital Day 5, Mr. F’s delirium resolves.
During this time, Mr. F’s disorganization and religious delusions become apparent. He spends much of his time reading his Bible. He has poor hygiene and limited engagement in activities of daily living. Due to his psychosis and inability to care for himself, Mr. F is transferred to the psychiatric unit with consent from his mother.
Continue to: TREATMENT Olanzapine and fluid restriction
TREATMENT Olanzapine and fluid restriction
In the psychiatric unit, Mr. F is restarted on olanzapine, but not on perphenazine due to anticholinergic effects and not on propranolol due to continued orthostatic hypotension. Five days later, he is at his baseline level of functioning with residual psychosis. His fluid intake is restricted to <1.5 L per day and he is easily compliant.
Mr. F’s mother is comfortable with his discharge home on a regimen of olanzapine, 25 mg/d, and the team discusses the fluid restrictions with her. The treatment team suggests initiating an LAI before Mr. F is discharged, but this is not pursued because his mother thinks he is doing well with the oral medication. She wants to monitor him with the medication changes in the clinic before pursuing an LAI; however, she is open to it in the future.
The authors’ observations
Approximately 20% of patients with schizophrenia may experience psychogenic polydipsia.4,5 The cause of psychogenic polydipsia in patients with serious mental illness is multifactorial. It may stem from malfunction of the hypothalamic-pituitary axis, which leads to alterations in antidiuretic hormone secretion and function.4-6
Mr. F’s case highlights several challenges associated with treating psychogenic polydipsia in patients with serious mental illness. Antipsychotics with high dopamine affinity, such as risperidone and haloperidol, may increase the risk of psychogenic polydipsia, while antipsychotics with lower dopamine affinity, such as clozapine, may decrease the occurrence.5 Antipsychotics block postsynaptic dopamine receptors, which can induce supersensitivity by increasing presynaptic dopamine release in the hypothalamic areas, where thirst regulation occurs. This increase in dopamine leads to increased thirst drive and fluid intake.3
Quetiapine or clozapine may have been a better antipsychotic choice because these agents have lower D2 receptor affinity, whereas olanzapine has intermediate binding to D2 receptors.6,7 However, quetiapine and clozapine are more strongly associated with orthostasis, which was a concern during Mr. F’s hospitalization. The weekly laboratory testing required with clozapine use would have been an unfeasible burden for Mr. F because he lived in a rural environment. Perphenazine was not continued due to higher D2 affinity and anticholinergic effects, which can increase thirst.6
Continue to: In addition to switching...
In addition to switching to an antipsychotic with looser D2 binding, other medications for treating polydipsia have been studied. It is hypothesized that the alpha-2 adrenergic system may play a role in thirst regulation. For example, mianserin, an alpha-2 antagonist, may decrease water intake. However, studies have been small and inconsistent.8,9 Propranolol,10 a beta adrenergic receptor blocker; irbesartan,11 an angiotensin-II receptor blocker; demeclocycline,12 a tetracycline that inhibits antidiuretic hormone action; and naltrexone,9 a mu opioid antagonist, have been studied with inconclusive results and a variety of adverse effects5,7,13 (Table 28-13).
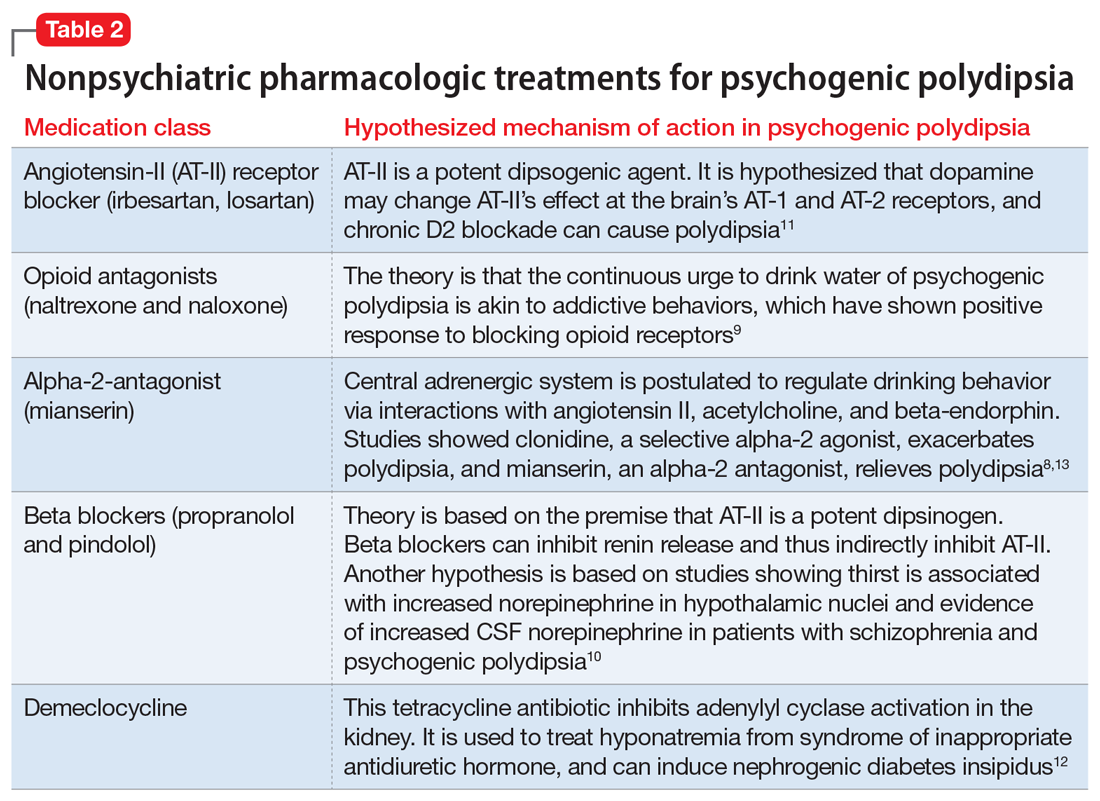
Behavioral interventions for patients with psychogenic polydipsia include fluid restriction, twice-daily weight checks, cognitive-behavioral therapy, and reinforcement schedules, which may be useful but less realistic due to need for increased supervision.11,12 Patient and family education on the signs of hyponatremia are important to prevent serious complications, such as those Mr. F experienced.
OUTCOME Repeated hospitalizations
Mr. F is discharged with follow-up in our psychiatry clinic and attends 1 appointment. At that time, his mother reports that Mr. F is compliant with his medication and has limited fluid intake. However, over the next 2 months, he is admitted to our psychiatric unit twice with similar presentations. Each time, the treatment team has extensive discussions with Mr. F’s mother about strategies to limit his water intake and the possibility of residential placement due to his need for a higher level of care. Although she acknowledges that nursing home placement may be needed in the future, she is not ready to take this step.
Three months later, Mr. F returns to our hospital with severe abdominal pain and is found to have a perforated bowel obstruction. His sodium is within normal limits on presentation, and the psychiatry team is not involved during this hospitalization. Mr. F is treated for sepsis and undergoes 3 exploratory laparotomies with continued decline in his health. He dies during this hospitalization. The cause of Mr. F’s perforated bowel obstruction is not determined, and his family does not pursue an autopsy.
The authors’ observations
At Mr. F’s final hospital presentation, his sodium was normal. It is possible Mr. F and his mother had found an acceptable fluid restriction routine, and he may have been doing better from a psychiatric perspective, but this will remain unknown.
Continue to: This case highlights...
This case highlights the clinical and ethical complexity of treating patients with psychogenic polydipsia. Because Mr. F no longer had autonomy, we had to determine if his mother was acting in his best interest as his guardian. Guardianship requirements and expectations vary by state. In our state of Missouri, a guardian is appointed by the court to act in the best interest of the ward, and may be a family member (preferred) or state-appointed. The guardian is responsible for providing the ward’s care and is in charge of financial and medical decisions. In Missouri, the guardian must assure the ward resides in the “least restrictive setting reasonably available,” which is the minimum necessary to provide the ward safe care and housing.14 Full guardianship, as in Mr. F’s case, is different from limited guardianship, which is an option in states such as Missouri. In limited guardianship, the court decides the extent of the guardian’s role in decisions for the ward.14,15
Mr. F’s mother believed she was acting in her son’s best interest by having him home with his family. She believed by living at home, he would derive more enjoyment from life than living in a nursing home. By the time Mr. F presented to our hospital, he had been living with decompensated schizophrenia for years, so some level of psychosis was likely to persist, even with treatment. Given his increasingly frequent hospitalizations for hyponatremia due to increased water intake, more intense supervision may have been needed to maintain his safety, in line with nonmaleficence. The treatment team considered Mr. F’s best interest when discussing placement and worked to understand his mother’s preferences.
His mother continued to acknowledge the need for changes and adjustments at home. She was receptive to the need for fluid restriction and increased structure at home. Therefore, we felt she continued to be acting in his best interest, and his home would be the least restrictive setting for his care. If Mr. F had continued to require repeated hospitalizations and had not passed away, we would have pursued an ethics consult to discuss the need for nursing home placement and how to best approach this with Mr. F’s mother.
Bottom Line
Patients with serious mental illness who present with hyponatremia should be evaluated for psychogenic polydipsia by assessing their dietary and fluid intakes, along with collateral from family. The use of antipsychotics with high dopamine affinity may increase the risk of psychogenic polydipsia. Behavioral interventions include fluid restriction, weight checks, cognitive-behavioral therapy, and reinforcement schedules.
Related Resources
- Sharp CS, Wilson MP. Hyponatremia. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer International Publishing; 2018:115-119. doi:10.1007/ 978-3-319-58260-3_21
- Sailer C, Winzeler B, Christ-Crain M. Primary polydipsia in the medical and psychiatric patient: characteristics, complications and therapy. Swiss Med Wkly. 2017;147:w14514. doi:10.4414/ smw.2017.14514
Drug Brand Names
Amiloride • Midamor
Aripiprazole • Abilify
Clonidine • Catapres
Clozapine • Clozaril
Demeclocycline • Declomycin
Desmopressin • DDAVP
Haloperidol • Haldol
Irbesartan • Avapro
Lithium • Eskalith, Lithobid
Losartan • Cozaar
Mianserin • Tolvon
Naloxone • Narcan
Naltrexone • Revia
Olanzapine • Zyprexa
Perphenazine • Trilafon
Propranolol • Inderal LA
Quetiapine • Seroquel
Risperidone • Risperda
1. Sharp CS, Wilson MP. Hyponatremia. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer International Publishing; 2018:115-119. doi:10.1007/978-3-319-58260-3_21
2. Gross P. Clinical management of SIADH. Ther Adv Endocrinol Metab. 2012;3(2):61-73. doi:10.1177/2042018812437561
3. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primer. 2019;5(1):54. doi:10.1038/s41572-019-0103-2
4. Ahmadi L, Goldman MB. Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101469. doi:10.1016/j.beem.2020.101469
5. Kirino S, Sakuma M, Misawa F, et al. Relationship between polydipsia and antipsychotics: a systematic review of clinical studies and case reports. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109756. doi:10.1016/j.pnpbp.2019.109756
6. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic drugs: from receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210-1223. doi:10.2174/1570159X15666170630163616
7. Seeman P, Tallerico T. Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol Psychiatry. 1998;3(2):123-134. doi:10.1038/sj.mp.4000336
8. Hayashi T, Nishikawa T, Koga I, et al. Involvement of the α 2 -adrenergic system in polydipsia in schizophrenic patients: a pilot study. Psychopharmacology (Berl). 1997;130(4):382-386. doi:10.1007/s002130050254
9. Rizvi S, Gold J, Khan AM. Role of naltrexone in improving compulsive drinking in psychogenic polydipsia. Cureus. 2019;11(8):e5320. doi:10.7759/cureus.5320
10. Kishi Y, Kurosawa H, Endo S. Is propranolol effective in primary polydipsia? Int J Psychiatry Med. 1998;28(3):315-325. doi:10.2190/QPWL-14H7-HPGG-A29D
11. Kruse D, Pantelis C, Rudd R, et al. Treatment of psychogenic polydipsia: comparison of risperidone and olanzapine, and the effects of an adjunctive angiotensin-II receptor blocking drug (irbesartan). Aust N Z J Psychiatry. 2001;35(1):65-68. doi:10.1046/j.1440-1614.2001.00847.x
12. Alexander RC, Karp BI, Thompson S, et al. A double blind, placebo-controlled trial of demeclocycline treatment of polydipsia-hyponatremia in chronically psychotic patients. Biol Psychiatry. 1991;30(4):417-420. doi:10.1016/0006-3223(91)90300-B
13. Valente S, Fisher D. Recognizing and managing psychogenic polydipsia in mental health. J Nurse Pract. 2010;6(7):546-550. doi:10.1016/j.nurpra.2010.03.004
14. Barton R, Esq SL, Lockett LL. The use of conservatorships and adult guardianships and other options in the care of the mentally ill in the United States. World Guard Congr. Published May 29, 2014. Accessed June 18, 2021. http://www.guardianship.org/IRL/Resources/Handouts/Family%20Members%20as%20Guardians_Handout.pdf
15. ABA Commission on Law & Aging. Adult Guardianship Statutory Table of Authorities. ABA. Published January 2021. Accessed June 17, 2021. https://www.americanbar.org/content/dam/aba/administrative/law_aging/2019-adult-guardianship-statutory-table-of-authorities.pdf
CASE Unresponsive after a presumed seizure
Mr. F, age 44, has schizophrenia. He is brought to the hospital by ambulance after he is found on the ground outside of his mother’s house following a presumed seizure and fall. On arrival to the emergency department, he is unresponsive. His laboratory values are significant for a sodium level of 110 mEq/L (reference range: 135 to 145 mEq/L), indicating hyponatremia.
HISTORY Fixated on purity
Mr. F’s mother reports that Mr. F had an unremarkable childhood. He was raised in a household with both parents and a younger sister. Mr. F did well academically and studied engineering and physics in college. There was no reported history of trauma or substance use.
During his senior year of college, Mr. F began experiencing paranoia, auditory hallucinations, and religious delusions. He required hospitalization and was diagnosed with schizophrenia. Following multiple hospitalizations over 5 years, he moved in with his mother, who was granted guardianship.
His mother said Mr. F’s religious delusions were of purity and cleansing the soul. He spent hours memorizing the Bible and would go for days without eating but would drink large amounts of water. She said she thought this was due to his desire to flush out imperfections.
In the past 3 years, Mr. F has been hospitalized several times for severe hyponatremia. At home, his mother attempted to restrict his water intake. However, Mr. F would still drink out of sinks and hoses. Mr. F’s mother reports that over the past month he had become more isolated. He would spend entire days reading the Bible, and his water intake had further increased.
Prior medication trials for Mr. F included haloperidol, up to 10 mg twice per day; aripiprazole, up to 20 mg/d; and risperidone, up to 6 mg nightly. These had been effective, but Mr. F had difficulty with adherence. He did not receive a long-acting injectable (LAI) antipsychotic initially due to lack of access at the rural clinic where he was treated, and later due to his mother’s preference for her son to receive oral medications. Prior to his current presentation, Mr. F’s medication regimen was olanzapine, 10 mg twice a day; perphenazine, 8 mg twice a day; and long-acting propranolol, 60 mg/d. Mr. F had no other chronic medical problems.
EVALUATION Hyponatremia, but why?
Mr. F is intubated and admitted to the surgical service for stabilization due to injuries from his fall. He has fractures of his right sinus and bilateral nasal bones, which are managed nonoperatively. He is delirious, with waxing and waning attention, memory disturbances, and disorientation. His psychotropic medications are held.
Continue to: Imaging of his head...
Imaging of his head does not reveal acute abnormalities suggesting a malignant or paraneoplastic process, and there are no concerns for ongoing seizures. An infection workup is negative. His urine toxicology is negative and blood alcohol level is 0. His sodium normalizes after 3 days of IV fluids and fluid restriction. Therefore, further tests to differentiate the causes of hyponatremia, such as urine electrolytes and urine osmolality, are not pursued.
[polldaddy:10910406]
The authors’ observations
The differential diagnosis for hyponatremia is broad in the setting of psychiatric illness. Low sodium levels could be due to psychotropic medications, psychiatrically-driven behaviors, or an underlying medical problem. Our differential diagnosis for Mr. F included iatrogenic syndrome of inappropriate antidiuretic hormone (SIADH), diabetes insipidus, or psychogenic polydipsia, a form of primary polydipsia. Other causes of primary polydipsia are related to substances, such as heavy beer intakeor use of 3,4-methylenedioxymethamphetamine (MDMA, also known as “ecstasy”), or brain lesions,1 but these causes were less likely given Mr. F’s negative urine toxicology and head imaging.
While psychogenic polydipsia is due to increased water consumption, both SIADH and diabetes insipidus are due to alterations in fluid homeostasis.2,3 Table 12-4 outlines distinguishing characteristics of SIADH, diabetes insipidus, and psychogenic polydipsia. Urine studies were not pursued because Mr. F’s sodium resolved and acute concerns, such as malignancy or infection, were ruled out. Mr. F’s hyponatremia was presumed to be due to psychogenic polydipsia because of his increased fluid intake and normalization of sodium with hypertonic fluids and subsequent fluid restriction. During this time, he was managed on the surgical service; the plan was to pursue urine studies and possibly a fluid challenge if his hyponatremia persisted.

EVALUATION Delirium resolves, delusions persist
While Mr. F is on the surgical service, the treatment team focuses on stabilizing his sodium level and assessing for causes of altered mental status that led to his fall. Psychiatry is consulted for management of his agitation. Following the gradual correction of his sodium level and extubation, his sensorium improves. By hospital Day 5, Mr. F’s delirium resolves.
During this time, Mr. F’s disorganization and religious delusions become apparent. He spends much of his time reading his Bible. He has poor hygiene and limited engagement in activities of daily living. Due to his psychosis and inability to care for himself, Mr. F is transferred to the psychiatric unit with consent from his mother.
Continue to: TREATMENT Olanzapine and fluid restriction
TREATMENT Olanzapine and fluid restriction
In the psychiatric unit, Mr. F is restarted on olanzapine, but not on perphenazine due to anticholinergic effects and not on propranolol due to continued orthostatic hypotension. Five days later, he is at his baseline level of functioning with residual psychosis. His fluid intake is restricted to <1.5 L per day and he is easily compliant.
Mr. F’s mother is comfortable with his discharge home on a regimen of olanzapine, 25 mg/d, and the team discusses the fluid restrictions with her. The treatment team suggests initiating an LAI before Mr. F is discharged, but this is not pursued because his mother thinks he is doing well with the oral medication. She wants to monitor him with the medication changes in the clinic before pursuing an LAI; however, she is open to it in the future.
The authors’ observations
Approximately 20% of patients with schizophrenia may experience psychogenic polydipsia.4,5 The cause of psychogenic polydipsia in patients with serious mental illness is multifactorial. It may stem from malfunction of the hypothalamic-pituitary axis, which leads to alterations in antidiuretic hormone secretion and function.4-6
Mr. F’s case highlights several challenges associated with treating psychogenic polydipsia in patients with serious mental illness. Antipsychotics with high dopamine affinity, such as risperidone and haloperidol, may increase the risk of psychogenic polydipsia, while antipsychotics with lower dopamine affinity, such as clozapine, may decrease the occurrence.5 Antipsychotics block postsynaptic dopamine receptors, which can induce supersensitivity by increasing presynaptic dopamine release in the hypothalamic areas, where thirst regulation occurs. This increase in dopamine leads to increased thirst drive and fluid intake.3
Quetiapine or clozapine may have been a better antipsychotic choice because these agents have lower D2 receptor affinity, whereas olanzapine has intermediate binding to D2 receptors.6,7 However, quetiapine and clozapine are more strongly associated with orthostasis, which was a concern during Mr. F’s hospitalization. The weekly laboratory testing required with clozapine use would have been an unfeasible burden for Mr. F because he lived in a rural environment. Perphenazine was not continued due to higher D2 affinity and anticholinergic effects, which can increase thirst.6
Continue to: In addition to switching...
In addition to switching to an antipsychotic with looser D2 binding, other medications for treating polydipsia have been studied. It is hypothesized that the alpha-2 adrenergic system may play a role in thirst regulation. For example, mianserin, an alpha-2 antagonist, may decrease water intake. However, studies have been small and inconsistent.8,9 Propranolol,10 a beta adrenergic receptor blocker; irbesartan,11 an angiotensin-II receptor blocker; demeclocycline,12 a tetracycline that inhibits antidiuretic hormone action; and naltrexone,9 a mu opioid antagonist, have been studied with inconclusive results and a variety of adverse effects5,7,13 (Table 28-13).

Behavioral interventions for patients with psychogenic polydipsia include fluid restriction, twice-daily weight checks, cognitive-behavioral therapy, and reinforcement schedules, which may be useful but less realistic due to need for increased supervision.11,12 Patient and family education on the signs of hyponatremia are important to prevent serious complications, such as those Mr. F experienced.
OUTCOME Repeated hospitalizations
Mr. F is discharged with follow-up in our psychiatry clinic and attends 1 appointment. At that time, his mother reports that Mr. F is compliant with his medication and has limited fluid intake. However, over the next 2 months, he is admitted to our psychiatric unit twice with similar presentations. Each time, the treatment team has extensive discussions with Mr. F’s mother about strategies to limit his water intake and the possibility of residential placement due to his need for a higher level of care. Although she acknowledges that nursing home placement may be needed in the future, she is not ready to take this step.
Three months later, Mr. F returns to our hospital with severe abdominal pain and is found to have a perforated bowel obstruction. His sodium is within normal limits on presentation, and the psychiatry team is not involved during this hospitalization. Mr. F is treated for sepsis and undergoes 3 exploratory laparotomies with continued decline in his health. He dies during this hospitalization. The cause of Mr. F’s perforated bowel obstruction is not determined, and his family does not pursue an autopsy.
The authors’ observations
At Mr. F’s final hospital presentation, his sodium was normal. It is possible Mr. F and his mother had found an acceptable fluid restriction routine, and he may have been doing better from a psychiatric perspective, but this will remain unknown.
Continue to: This case highlights...
This case highlights the clinical and ethical complexity of treating patients with psychogenic polydipsia. Because Mr. F no longer had autonomy, we had to determine if his mother was acting in his best interest as his guardian. Guardianship requirements and expectations vary by state. In our state of Missouri, a guardian is appointed by the court to act in the best interest of the ward, and may be a family member (preferred) or state-appointed. The guardian is responsible for providing the ward’s care and is in charge of financial and medical decisions. In Missouri, the guardian must assure the ward resides in the “least restrictive setting reasonably available,” which is the minimum necessary to provide the ward safe care and housing.14 Full guardianship, as in Mr. F’s case, is different from limited guardianship, which is an option in states such as Missouri. In limited guardianship, the court decides the extent of the guardian’s role in decisions for the ward.14,15
Mr. F’s mother believed she was acting in her son’s best interest by having him home with his family. She believed by living at home, he would derive more enjoyment from life than living in a nursing home. By the time Mr. F presented to our hospital, he had been living with decompensated schizophrenia for years, so some level of psychosis was likely to persist, even with treatment. Given his increasingly frequent hospitalizations for hyponatremia due to increased water intake, more intense supervision may have been needed to maintain his safety, in line with nonmaleficence. The treatment team considered Mr. F’s best interest when discussing placement and worked to understand his mother’s preferences.
His mother continued to acknowledge the need for changes and adjustments at home. She was receptive to the need for fluid restriction and increased structure at home. Therefore, we felt she continued to be acting in his best interest, and his home would be the least restrictive setting for his care. If Mr. F had continued to require repeated hospitalizations and had not passed away, we would have pursued an ethics consult to discuss the need for nursing home placement and how to best approach this with Mr. F’s mother.
Bottom Line
Patients with serious mental illness who present with hyponatremia should be evaluated for psychogenic polydipsia by assessing their dietary and fluid intakes, along with collateral from family. The use of antipsychotics with high dopamine affinity may increase the risk of psychogenic polydipsia. Behavioral interventions include fluid restriction, weight checks, cognitive-behavioral therapy, and reinforcement schedules.
Related Resources
- Sharp CS, Wilson MP. Hyponatremia. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer International Publishing; 2018:115-119. doi:10.1007/ 978-3-319-58260-3_21
- Sailer C, Winzeler B, Christ-Crain M. Primary polydipsia in the medical and psychiatric patient: characteristics, complications and therapy. Swiss Med Wkly. 2017;147:w14514. doi:10.4414/ smw.2017.14514
Drug Brand Names
Amiloride • Midamor
Aripiprazole • Abilify
Clonidine • Catapres
Clozapine • Clozaril
Demeclocycline • Declomycin
Desmopressin • DDAVP
Haloperidol • Haldol
Irbesartan • Avapro
Lithium • Eskalith, Lithobid
Losartan • Cozaar
Mianserin • Tolvon
Naloxone • Narcan
Naltrexone • Revia
Olanzapine • Zyprexa
Perphenazine • Trilafon
Propranolol • Inderal LA
Quetiapine • Seroquel
Risperidone • Risperda
CASE Unresponsive after a presumed seizure
Mr. F, age 44, has schizophrenia. He is brought to the hospital by ambulance after he is found on the ground outside of his mother’s house following a presumed seizure and fall. On arrival to the emergency department, he is unresponsive. His laboratory values are significant for a sodium level of 110 mEq/L (reference range: 135 to 145 mEq/L), indicating hyponatremia.
HISTORY Fixated on purity
Mr. F’s mother reports that Mr. F had an unremarkable childhood. He was raised in a household with both parents and a younger sister. Mr. F did well academically and studied engineering and physics in college. There was no reported history of trauma or substance use.
During his senior year of college, Mr. F began experiencing paranoia, auditory hallucinations, and religious delusions. He required hospitalization and was diagnosed with schizophrenia. Following multiple hospitalizations over 5 years, he moved in with his mother, who was granted guardianship.
His mother said Mr. F’s religious delusions were of purity and cleansing the soul. He spent hours memorizing the Bible and would go for days without eating but would drink large amounts of water. She said she thought this was due to his desire to flush out imperfections.
In the past 3 years, Mr. F has been hospitalized several times for severe hyponatremia. At home, his mother attempted to restrict his water intake. However, Mr. F would still drink out of sinks and hoses. Mr. F’s mother reports that over the past month he had become more isolated. He would spend entire days reading the Bible, and his water intake had further increased.
Prior medication trials for Mr. F included haloperidol, up to 10 mg twice per day; aripiprazole, up to 20 mg/d; and risperidone, up to 6 mg nightly. These had been effective, but Mr. F had difficulty with adherence. He did not receive a long-acting injectable (LAI) antipsychotic initially due to lack of access at the rural clinic where he was treated, and later due to his mother’s preference for her son to receive oral medications. Prior to his current presentation, Mr. F’s medication regimen was olanzapine, 10 mg twice a day; perphenazine, 8 mg twice a day; and long-acting propranolol, 60 mg/d. Mr. F had no other chronic medical problems.
EVALUATION Hyponatremia, but why?
Mr. F is intubated and admitted to the surgical service for stabilization due to injuries from his fall. He has fractures of his right sinus and bilateral nasal bones, which are managed nonoperatively. He is delirious, with waxing and waning attention, memory disturbances, and disorientation. His psychotropic medications are held.
Continue to: Imaging of his head...
Imaging of his head does not reveal acute abnormalities suggesting a malignant or paraneoplastic process, and there are no concerns for ongoing seizures. An infection workup is negative. His urine toxicology is negative and blood alcohol level is 0. His sodium normalizes after 3 days of IV fluids and fluid restriction. Therefore, further tests to differentiate the causes of hyponatremia, such as urine electrolytes and urine osmolality, are not pursued.
[polldaddy:10910406]
The authors’ observations
The differential diagnosis for hyponatremia is broad in the setting of psychiatric illness. Low sodium levels could be due to psychotropic medications, psychiatrically-driven behaviors, or an underlying medical problem. Our differential diagnosis for Mr. F included iatrogenic syndrome of inappropriate antidiuretic hormone (SIADH), diabetes insipidus, or psychogenic polydipsia, a form of primary polydipsia. Other causes of primary polydipsia are related to substances, such as heavy beer intakeor use of 3,4-methylenedioxymethamphetamine (MDMA, also known as “ecstasy”), or brain lesions,1 but these causes were less likely given Mr. F’s negative urine toxicology and head imaging.
While psychogenic polydipsia is due to increased water consumption, both SIADH and diabetes insipidus are due to alterations in fluid homeostasis.2,3 Table 12-4 outlines distinguishing characteristics of SIADH, diabetes insipidus, and psychogenic polydipsia. Urine studies were not pursued because Mr. F’s sodium resolved and acute concerns, such as malignancy or infection, were ruled out. Mr. F’s hyponatremia was presumed to be due to psychogenic polydipsia because of his increased fluid intake and normalization of sodium with hypertonic fluids and subsequent fluid restriction. During this time, he was managed on the surgical service; the plan was to pursue urine studies and possibly a fluid challenge if his hyponatremia persisted.

EVALUATION Delirium resolves, delusions persist
While Mr. F is on the surgical service, the treatment team focuses on stabilizing his sodium level and assessing for causes of altered mental status that led to his fall. Psychiatry is consulted for management of his agitation. Following the gradual correction of his sodium level and extubation, his sensorium improves. By hospital Day 5, Mr. F’s delirium resolves.
During this time, Mr. F’s disorganization and religious delusions become apparent. He spends much of his time reading his Bible. He has poor hygiene and limited engagement in activities of daily living. Due to his psychosis and inability to care for himself, Mr. F is transferred to the psychiatric unit with consent from his mother.
Continue to: TREATMENT Olanzapine and fluid restriction
TREATMENT Olanzapine and fluid restriction
In the psychiatric unit, Mr. F is restarted on olanzapine, but not on perphenazine due to anticholinergic effects and not on propranolol due to continued orthostatic hypotension. Five days later, he is at his baseline level of functioning with residual psychosis. His fluid intake is restricted to <1.5 L per day and he is easily compliant.
Mr. F’s mother is comfortable with his discharge home on a regimen of olanzapine, 25 mg/d, and the team discusses the fluid restrictions with her. The treatment team suggests initiating an LAI before Mr. F is discharged, but this is not pursued because his mother thinks he is doing well with the oral medication. She wants to monitor him with the medication changes in the clinic before pursuing an LAI; however, she is open to it in the future.
The authors’ observations
Approximately 20% of patients with schizophrenia may experience psychogenic polydipsia.4,5 The cause of psychogenic polydipsia in patients with serious mental illness is multifactorial. It may stem from malfunction of the hypothalamic-pituitary axis, which leads to alterations in antidiuretic hormone secretion and function.4-6
Mr. F’s case highlights several challenges associated with treating psychogenic polydipsia in patients with serious mental illness. Antipsychotics with high dopamine affinity, such as risperidone and haloperidol, may increase the risk of psychogenic polydipsia, while antipsychotics with lower dopamine affinity, such as clozapine, may decrease the occurrence.5 Antipsychotics block postsynaptic dopamine receptors, which can induce supersensitivity by increasing presynaptic dopamine release in the hypothalamic areas, where thirst regulation occurs. This increase in dopamine leads to increased thirst drive and fluid intake.3
Quetiapine or clozapine may have been a better antipsychotic choice because these agents have lower D2 receptor affinity, whereas olanzapine has intermediate binding to D2 receptors.6,7 However, quetiapine and clozapine are more strongly associated with orthostasis, which was a concern during Mr. F’s hospitalization. The weekly laboratory testing required with clozapine use would have been an unfeasible burden for Mr. F because he lived in a rural environment. Perphenazine was not continued due to higher D2 affinity and anticholinergic effects, which can increase thirst.6
Continue to: In addition to switching...
In addition to switching to an antipsychotic with looser D2 binding, other medications for treating polydipsia have been studied. It is hypothesized that the alpha-2 adrenergic system may play a role in thirst regulation. For example, mianserin, an alpha-2 antagonist, may decrease water intake. However, studies have been small and inconsistent.8,9 Propranolol,10 a beta adrenergic receptor blocker; irbesartan,11 an angiotensin-II receptor blocker; demeclocycline,12 a tetracycline that inhibits antidiuretic hormone action; and naltrexone,9 a mu opioid antagonist, have been studied with inconclusive results and a variety of adverse effects5,7,13 (Table 28-13).

Behavioral interventions for patients with psychogenic polydipsia include fluid restriction, twice-daily weight checks, cognitive-behavioral therapy, and reinforcement schedules, which may be useful but less realistic due to need for increased supervision.11,12 Patient and family education on the signs of hyponatremia are important to prevent serious complications, such as those Mr. F experienced.
OUTCOME Repeated hospitalizations
Mr. F is discharged with follow-up in our psychiatry clinic and attends 1 appointment. At that time, his mother reports that Mr. F is compliant with his medication and has limited fluid intake. However, over the next 2 months, he is admitted to our psychiatric unit twice with similar presentations. Each time, the treatment team has extensive discussions with Mr. F’s mother about strategies to limit his water intake and the possibility of residential placement due to his need for a higher level of care. Although she acknowledges that nursing home placement may be needed in the future, she is not ready to take this step.
Three months later, Mr. F returns to our hospital with severe abdominal pain and is found to have a perforated bowel obstruction. His sodium is within normal limits on presentation, and the psychiatry team is not involved during this hospitalization. Mr. F is treated for sepsis and undergoes 3 exploratory laparotomies with continued decline in his health. He dies during this hospitalization. The cause of Mr. F’s perforated bowel obstruction is not determined, and his family does not pursue an autopsy.
The authors’ observations
At Mr. F’s final hospital presentation, his sodium was normal. It is possible Mr. F and his mother had found an acceptable fluid restriction routine, and he may have been doing better from a psychiatric perspective, but this will remain unknown.
Continue to: This case highlights...
This case highlights the clinical and ethical complexity of treating patients with psychogenic polydipsia. Because Mr. F no longer had autonomy, we had to determine if his mother was acting in his best interest as his guardian. Guardianship requirements and expectations vary by state. In our state of Missouri, a guardian is appointed by the court to act in the best interest of the ward, and may be a family member (preferred) or state-appointed. The guardian is responsible for providing the ward’s care and is in charge of financial and medical decisions. In Missouri, the guardian must assure the ward resides in the “least restrictive setting reasonably available,” which is the minimum necessary to provide the ward safe care and housing.14 Full guardianship, as in Mr. F’s case, is different from limited guardianship, which is an option in states such as Missouri. In limited guardianship, the court decides the extent of the guardian’s role in decisions for the ward.14,15
Mr. F’s mother believed she was acting in her son’s best interest by having him home with his family. She believed by living at home, he would derive more enjoyment from life than living in a nursing home. By the time Mr. F presented to our hospital, he had been living with decompensated schizophrenia for years, so some level of psychosis was likely to persist, even with treatment. Given his increasingly frequent hospitalizations for hyponatremia due to increased water intake, more intense supervision may have been needed to maintain his safety, in line with nonmaleficence. The treatment team considered Mr. F’s best interest when discussing placement and worked to understand his mother’s preferences.
His mother continued to acknowledge the need for changes and adjustments at home. She was receptive to the need for fluid restriction and increased structure at home. Therefore, we felt she continued to be acting in his best interest, and his home would be the least restrictive setting for his care. If Mr. F had continued to require repeated hospitalizations and had not passed away, we would have pursued an ethics consult to discuss the need for nursing home placement and how to best approach this with Mr. F’s mother.
Bottom Line
Patients with serious mental illness who present with hyponatremia should be evaluated for psychogenic polydipsia by assessing their dietary and fluid intakes, along with collateral from family. The use of antipsychotics with high dopamine affinity may increase the risk of psychogenic polydipsia. Behavioral interventions include fluid restriction, weight checks, cognitive-behavioral therapy, and reinforcement schedules.
Related Resources
- Sharp CS, Wilson MP. Hyponatremia. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer International Publishing; 2018:115-119. doi:10.1007/ 978-3-319-58260-3_21
- Sailer C, Winzeler B, Christ-Crain M. Primary polydipsia in the medical and psychiatric patient: characteristics, complications and therapy. Swiss Med Wkly. 2017;147:w14514. doi:10.4414/ smw.2017.14514
Drug Brand Names
Amiloride • Midamor
Aripiprazole • Abilify
Clonidine • Catapres
Clozapine • Clozaril
Demeclocycline • Declomycin
Desmopressin • DDAVP
Haloperidol • Haldol
Irbesartan • Avapro
Lithium • Eskalith, Lithobid
Losartan • Cozaar
Mianserin • Tolvon
Naloxone • Narcan
Naltrexone • Revia
Olanzapine • Zyprexa
Perphenazine • Trilafon
Propranolol • Inderal LA
Quetiapine • Seroquel
Risperidone • Risperda
1. Sharp CS, Wilson MP. Hyponatremia. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer International Publishing; 2018:115-119. doi:10.1007/978-3-319-58260-3_21
2. Gross P. Clinical management of SIADH. Ther Adv Endocrinol Metab. 2012;3(2):61-73. doi:10.1177/2042018812437561
3. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primer. 2019;5(1):54. doi:10.1038/s41572-019-0103-2
4. Ahmadi L, Goldman MB. Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101469. doi:10.1016/j.beem.2020.101469
5. Kirino S, Sakuma M, Misawa F, et al. Relationship between polydipsia and antipsychotics: a systematic review of clinical studies and case reports. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109756. doi:10.1016/j.pnpbp.2019.109756
6. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic drugs: from receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210-1223. doi:10.2174/1570159X15666170630163616
7. Seeman P, Tallerico T. Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol Psychiatry. 1998;3(2):123-134. doi:10.1038/sj.mp.4000336
8. Hayashi T, Nishikawa T, Koga I, et al. Involvement of the α 2 -adrenergic system in polydipsia in schizophrenic patients: a pilot study. Psychopharmacology (Berl). 1997;130(4):382-386. doi:10.1007/s002130050254
9. Rizvi S, Gold J, Khan AM. Role of naltrexone in improving compulsive drinking in psychogenic polydipsia. Cureus. 2019;11(8):e5320. doi:10.7759/cureus.5320
10. Kishi Y, Kurosawa H, Endo S. Is propranolol effective in primary polydipsia? Int J Psychiatry Med. 1998;28(3):315-325. doi:10.2190/QPWL-14H7-HPGG-A29D
11. Kruse D, Pantelis C, Rudd R, et al. Treatment of psychogenic polydipsia: comparison of risperidone and olanzapine, and the effects of an adjunctive angiotensin-II receptor blocking drug (irbesartan). Aust N Z J Psychiatry. 2001;35(1):65-68. doi:10.1046/j.1440-1614.2001.00847.x
12. Alexander RC, Karp BI, Thompson S, et al. A double blind, placebo-controlled trial of demeclocycline treatment of polydipsia-hyponatremia in chronically psychotic patients. Biol Psychiatry. 1991;30(4):417-420. doi:10.1016/0006-3223(91)90300-B
13. Valente S, Fisher D. Recognizing and managing psychogenic polydipsia in mental health. J Nurse Pract. 2010;6(7):546-550. doi:10.1016/j.nurpra.2010.03.004
14. Barton R, Esq SL, Lockett LL. The use of conservatorships and adult guardianships and other options in the care of the mentally ill in the United States. World Guard Congr. Published May 29, 2014. Accessed June 18, 2021. http://www.guardianship.org/IRL/Resources/Handouts/Family%20Members%20as%20Guardians_Handout.pdf
15. ABA Commission on Law & Aging. Adult Guardianship Statutory Table of Authorities. ABA. Published January 2021. Accessed June 17, 2021. https://www.americanbar.org/content/dam/aba/administrative/law_aging/2019-adult-guardianship-statutory-table-of-authorities.pdf
1. Sharp CS, Wilson MP. Hyponatremia. In: Nordstrom KD, Wilson MP, eds. Quick guide to psychiatric emergencies. Springer International Publishing; 2018:115-119. doi:10.1007/978-3-319-58260-3_21
2. Gross P. Clinical management of SIADH. Ther Adv Endocrinol Metab. 2012;3(2):61-73. doi:10.1177/2042018812437561
3. Christ-Crain M, Bichet DG, Fenske WK, et al. Diabetes insipidus. Nat Rev Dis Primer. 2019;5(1):54. doi:10.1038/s41572-019-0103-2
4. Ahmadi L, Goldman MB. Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab. 2020;34(5):101469. doi:10.1016/j.beem.2020.101469
5. Kirino S, Sakuma M, Misawa F, et al. Relationship between polydipsia and antipsychotics: a systematic review of clinical studies and case reports. Prog Neuropsychopharmacol Biol Psychiatry. 2020;96:109756. doi:10.1016/j.pnpbp.2019.109756
6. Siafis S, Tzachanis D, Samara M, et al. Antipsychotic drugs: from receptor-binding profiles to metabolic side effects. Curr Neuropharmacol. 2018;16(8):1210-1223. doi:10.2174/1570159X15666170630163616
7. Seeman P, Tallerico T. Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol Psychiatry. 1998;3(2):123-134. doi:10.1038/sj.mp.4000336
8. Hayashi T, Nishikawa T, Koga I, et al. Involvement of the α 2 -adrenergic system in polydipsia in schizophrenic patients: a pilot study. Psychopharmacology (Berl). 1997;130(4):382-386. doi:10.1007/s002130050254
9. Rizvi S, Gold J, Khan AM. Role of naltrexone in improving compulsive drinking in psychogenic polydipsia. Cureus. 2019;11(8):e5320. doi:10.7759/cureus.5320
10. Kishi Y, Kurosawa H, Endo S. Is propranolol effective in primary polydipsia? Int J Psychiatry Med. 1998;28(3):315-325. doi:10.2190/QPWL-14H7-HPGG-A29D
11. Kruse D, Pantelis C, Rudd R, et al. Treatment of psychogenic polydipsia: comparison of risperidone and olanzapine, and the effects of an adjunctive angiotensin-II receptor blocking drug (irbesartan). Aust N Z J Psychiatry. 2001;35(1):65-68. doi:10.1046/j.1440-1614.2001.00847.x
12. Alexander RC, Karp BI, Thompson S, et al. A double blind, placebo-controlled trial of demeclocycline treatment of polydipsia-hyponatremia in chronically psychotic patients. Biol Psychiatry. 1991;30(4):417-420. doi:10.1016/0006-3223(91)90300-B
13. Valente S, Fisher D. Recognizing and managing psychogenic polydipsia in mental health. J Nurse Pract. 2010;6(7):546-550. doi:10.1016/j.nurpra.2010.03.004
14. Barton R, Esq SL, Lockett LL. The use of conservatorships and adult guardianships and other options in the care of the mentally ill in the United States. World Guard Congr. Published May 29, 2014. Accessed June 18, 2021. http://www.guardianship.org/IRL/Resources/Handouts/Family%20Members%20as%20Guardians_Handout.pdf
15. ABA Commission on Law & Aging. Adult Guardianship Statutory Table of Authorities. ABA. Published January 2021. Accessed June 17, 2021. https://www.americanbar.org/content/dam/aba/administrative/law_aging/2019-adult-guardianship-statutory-table-of-authorities.pdf
Administration of ketamine for depression should be limited to psychiatrists
In the modern-day practice of medicine, turf wars are more common than one may realize. Presently, an ongoing battle over who should be prescribing and administering ketamine for novel treatment uses is being waged among psychiatrists, anesthesiologists, family physicians, and emergency physicians. Whoever emerges victorious will determine whether psychiatric care is administered in a safe and cost-effective manner, or if it will merely benefit the bottom line of the prescriber. In this article, we discuss how ketamine may have a role for treatment-resistant depression (TRD), and why psychiatrists are uniquely qualified to prescribe and administer this medication for this purpose.
New approaches to treatment-resistant depression
Antidepressant medications, long the mainstay of depression treatment, have been shown to be safe and relatively equally effective, with varying tolerability. However, 33% percent of patients do not achieve remission after 4 trials of antidepressant therapy.1 Most antidepressant efficacy studies report remission rates of 35% to 40%,2 which means many patients require subsequent switching and/or augmentation of their treatment.3 The STAR*D trial demonstrated that after 2 adequate antidepressant trials, the likelihood of remission diminishes.4
After a patient’s depression is found to be treatment-resistant, the onus of guiding treatment shifts away from the patient’s primary care physician to the more specialized psychiatrist. Few would question the suitability of a psychiatrist’s expertise in handling complicated and nuanced mental illness. In order to manage TRD, psychiatrists enter a terrain of emerging novel therapies with rapid onset, different mechanisms of action, and parenteral routes of administration.
One such therapy is esketamine, the S-enantiomer of ketamine. The FDA approved the intranasal (IN) formulation of esketamine in March 2019 after the medication had been designated as a breakthrough therapy for TRD in 2013 and studied in 6 Phase III clinical trials.5 The S-enantiomer of ketamine is known to bind to the N-methyl-
Ketamine may be administered intranasally, intravenously, or orally. A meta-analysis aimed at assessing differences in ketamine efficacy for depression based on route of administration have shown that both IV and IN ketamine are effective, though it is not possible to draw conclusions regarding a direct comparison based on available data.9 Despite several landmark published studies, such as those by Zarate et al,10 IV ketamine is not FDA-approved for TRD.
Continue to: Why psychiatrists?
Why psychiatrists?
Psychiatrists have been prescribing IN esketamine, which is covered by most commercial insurances and administered in certified healthcare settings under a Risk Evaluation and Mitigation Strategy program.5 However, anesthesiologists and emergency physicians have opened a crop of boutique and concierge health clinics offering various “packages” of IV ketamine infusions for a slew of mental ailments, including depression, anxiety, bipolar disorder, and posttraumatic stress disorder.11 Minimal investigation reveals that these services are being prescribed mainly by practitioners in fields other than psychiatry. Intravenous ketamine has long been used off-label as a treatment for depression not by psychiatrists but by practitioners of anesthesiology or emergency medicine. Although these clinicians are likely familiar with ketamine as an anesthetic, they have no foundation or expertise in the diagnosis and treatment of complex mood disorders. The FDA-approved indication for esketamine falls firmly in the realm of psychiatric treatment. Physicians who have not completed a psychiatry residency have neither the training nor experience necessary to determine whether a patient is a candidate for this treatment.
One potential adverse effect of ketamine is an emergence phenomenon, colloquially named a “K-hole,” that can induce symptoms of psychosis such as disturbing hallucinations. Patients who have a history of psychosis need to be carefully evaluated for appropriateness to receive this treatment.
Furthermore, ketamine treatments administered by physicians who are not psychiatrists are billed not through insurance but mostly via private pay. A patient may therefore be charged $350 to $1,000 per infusion, to be paid out of pocket.11 Tally that up over the standard 6 to 12 initial treatment infusions, followed by maintenance infusions, and these patients with profound depression are potentially building up significant debt. Does this practice align with the ethical principles of autonomy, justice, beneficence, and nonmaleficence that all physicians swore to uphold? Will psychiatrists take a stand against the financial exploitation of a vulnerable group that is desperate to find any potential relief from their depression?
1. Hillhouse TM, Porter JH. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol. 2015;23(1):1-21.
2. Fava M, Rush A, Trivedi M, et al. Background and rationale for the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. Psychiatr Clin North Am. 2003;26(2):457-494.
3. Gaynes BN, Rush AJ, Trivedi MH, et al. Primary versus specialty care outcomes for depressed outpatients managed with measurement-based care: results from STAR*D. J Gen Intern Med. 2008;23(5):551-560.
4. Gaynes BN, Warden D, Trivedi MH, et al. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60(11):1439-1445.
5. US Food and Drug Administration. Center for Drug Evaluation and Research. Esketamine clinical review. Published March 5, 2019. Accessed August 9, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211243Orig1s000MedR.pdf
6. Zanos P, Moaddel R, Morris PJ, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70(3):621-660.
7. Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4):801-811.
8. Kaur U, Pathak BK, Singh A, et al. Esketamine: a glimmer of hope in treatment-resistant depression. Eur Arch Psychiatry Clin Neurosci. 2021;271(3):417-429.
9. McIntyre RS, Carvalho IP, Lui LMW, et al. The effect of intravenous, intranasal, and oral ketamine/esketamine in mood disorders: a meta-analysis. J Affect Disord. 2020;276:576-584.
10. Zarate CA Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856-864.
11. Thielking M. Ketamine gives hope to patients with severe depression. But some clinics stray from the science and hype its benefits. STAT+. Published September 18, 2018. Accessed August 5, 2021. www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment/
In the modern-day practice of medicine, turf wars are more common than one may realize. Presently, an ongoing battle over who should be prescribing and administering ketamine for novel treatment uses is being waged among psychiatrists, anesthesiologists, family physicians, and emergency physicians. Whoever emerges victorious will determine whether psychiatric care is administered in a safe and cost-effective manner, or if it will merely benefit the bottom line of the prescriber. In this article, we discuss how ketamine may have a role for treatment-resistant depression (TRD), and why psychiatrists are uniquely qualified to prescribe and administer this medication for this purpose.
New approaches to treatment-resistant depression
Antidepressant medications, long the mainstay of depression treatment, have been shown to be safe and relatively equally effective, with varying tolerability. However, 33% percent of patients do not achieve remission after 4 trials of antidepressant therapy.1 Most antidepressant efficacy studies report remission rates of 35% to 40%,2 which means many patients require subsequent switching and/or augmentation of their treatment.3 The STAR*D trial demonstrated that after 2 adequate antidepressant trials, the likelihood of remission diminishes.4
After a patient’s depression is found to be treatment-resistant, the onus of guiding treatment shifts away from the patient’s primary care physician to the more specialized psychiatrist. Few would question the suitability of a psychiatrist’s expertise in handling complicated and nuanced mental illness. In order to manage TRD, psychiatrists enter a terrain of emerging novel therapies with rapid onset, different mechanisms of action, and parenteral routes of administration.
One such therapy is esketamine, the S-enantiomer of ketamine. The FDA approved the intranasal (IN) formulation of esketamine in March 2019 after the medication had been designated as a breakthrough therapy for TRD in 2013 and studied in 6 Phase III clinical trials.5 The S-enantiomer of ketamine is known to bind to the N-methyl-
Ketamine may be administered intranasally, intravenously, or orally. A meta-analysis aimed at assessing differences in ketamine efficacy for depression based on route of administration have shown that both IV and IN ketamine are effective, though it is not possible to draw conclusions regarding a direct comparison based on available data.9 Despite several landmark published studies, such as those by Zarate et al,10 IV ketamine is not FDA-approved for TRD.
Continue to: Why psychiatrists?
Why psychiatrists?
Psychiatrists have been prescribing IN esketamine, which is covered by most commercial insurances and administered in certified healthcare settings under a Risk Evaluation and Mitigation Strategy program.5 However, anesthesiologists and emergency physicians have opened a crop of boutique and concierge health clinics offering various “packages” of IV ketamine infusions for a slew of mental ailments, including depression, anxiety, bipolar disorder, and posttraumatic stress disorder.11 Minimal investigation reveals that these services are being prescribed mainly by practitioners in fields other than psychiatry. Intravenous ketamine has long been used off-label as a treatment for depression not by psychiatrists but by practitioners of anesthesiology or emergency medicine. Although these clinicians are likely familiar with ketamine as an anesthetic, they have no foundation or expertise in the diagnosis and treatment of complex mood disorders. The FDA-approved indication for esketamine falls firmly in the realm of psychiatric treatment. Physicians who have not completed a psychiatry residency have neither the training nor experience necessary to determine whether a patient is a candidate for this treatment.
One potential adverse effect of ketamine is an emergence phenomenon, colloquially named a “K-hole,” that can induce symptoms of psychosis such as disturbing hallucinations. Patients who have a history of psychosis need to be carefully evaluated for appropriateness to receive this treatment.
Furthermore, ketamine treatments administered by physicians who are not psychiatrists are billed not through insurance but mostly via private pay. A patient may therefore be charged $350 to $1,000 per infusion, to be paid out of pocket.11 Tally that up over the standard 6 to 12 initial treatment infusions, followed by maintenance infusions, and these patients with profound depression are potentially building up significant debt. Does this practice align with the ethical principles of autonomy, justice, beneficence, and nonmaleficence that all physicians swore to uphold? Will psychiatrists take a stand against the financial exploitation of a vulnerable group that is desperate to find any potential relief from their depression?
In the modern-day practice of medicine, turf wars are more common than one may realize. Presently, an ongoing battle over who should be prescribing and administering ketamine for novel treatment uses is being waged among psychiatrists, anesthesiologists, family physicians, and emergency physicians. Whoever emerges victorious will determine whether psychiatric care is administered in a safe and cost-effective manner, or if it will merely benefit the bottom line of the prescriber. In this article, we discuss how ketamine may have a role for treatment-resistant depression (TRD), and why psychiatrists are uniquely qualified to prescribe and administer this medication for this purpose.
New approaches to treatment-resistant depression
Antidepressant medications, long the mainstay of depression treatment, have been shown to be safe and relatively equally effective, with varying tolerability. However, 33% percent of patients do not achieve remission after 4 trials of antidepressant therapy.1 Most antidepressant efficacy studies report remission rates of 35% to 40%,2 which means many patients require subsequent switching and/or augmentation of their treatment.3 The STAR*D trial demonstrated that after 2 adequate antidepressant trials, the likelihood of remission diminishes.4
After a patient’s depression is found to be treatment-resistant, the onus of guiding treatment shifts away from the patient’s primary care physician to the more specialized psychiatrist. Few would question the suitability of a psychiatrist’s expertise in handling complicated and nuanced mental illness. In order to manage TRD, psychiatrists enter a terrain of emerging novel therapies with rapid onset, different mechanisms of action, and parenteral routes of administration.
One such therapy is esketamine, the S-enantiomer of ketamine. The FDA approved the intranasal (IN) formulation of esketamine in March 2019 after the medication had been designated as a breakthrough therapy for TRD in 2013 and studied in 6 Phase III clinical trials.5 The S-enantiomer of ketamine is known to bind to the N-methyl-
Ketamine may be administered intranasally, intravenously, or orally. A meta-analysis aimed at assessing differences in ketamine efficacy for depression based on route of administration have shown that both IV and IN ketamine are effective, though it is not possible to draw conclusions regarding a direct comparison based on available data.9 Despite several landmark published studies, such as those by Zarate et al,10 IV ketamine is not FDA-approved for TRD.
Continue to: Why psychiatrists?
Why psychiatrists?
Psychiatrists have been prescribing IN esketamine, which is covered by most commercial insurances and administered in certified healthcare settings under a Risk Evaluation and Mitigation Strategy program.5 However, anesthesiologists and emergency physicians have opened a crop of boutique and concierge health clinics offering various “packages” of IV ketamine infusions for a slew of mental ailments, including depression, anxiety, bipolar disorder, and posttraumatic stress disorder.11 Minimal investigation reveals that these services are being prescribed mainly by practitioners in fields other than psychiatry. Intravenous ketamine has long been used off-label as a treatment for depression not by psychiatrists but by practitioners of anesthesiology or emergency medicine. Although these clinicians are likely familiar with ketamine as an anesthetic, they have no foundation or expertise in the diagnosis and treatment of complex mood disorders. The FDA-approved indication for esketamine falls firmly in the realm of psychiatric treatment. Physicians who have not completed a psychiatry residency have neither the training nor experience necessary to determine whether a patient is a candidate for this treatment.
One potential adverse effect of ketamine is an emergence phenomenon, colloquially named a “K-hole,” that can induce symptoms of psychosis such as disturbing hallucinations. Patients who have a history of psychosis need to be carefully evaluated for appropriateness to receive this treatment.
Furthermore, ketamine treatments administered by physicians who are not psychiatrists are billed not through insurance but mostly via private pay. A patient may therefore be charged $350 to $1,000 per infusion, to be paid out of pocket.11 Tally that up over the standard 6 to 12 initial treatment infusions, followed by maintenance infusions, and these patients with profound depression are potentially building up significant debt. Does this practice align with the ethical principles of autonomy, justice, beneficence, and nonmaleficence that all physicians swore to uphold? Will psychiatrists take a stand against the financial exploitation of a vulnerable group that is desperate to find any potential relief from their depression?
1. Hillhouse TM, Porter JH. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol. 2015;23(1):1-21.
2. Fava M, Rush A, Trivedi M, et al. Background and rationale for the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. Psychiatr Clin North Am. 2003;26(2):457-494.
3. Gaynes BN, Rush AJ, Trivedi MH, et al. Primary versus specialty care outcomes for depressed outpatients managed with measurement-based care: results from STAR*D. J Gen Intern Med. 2008;23(5):551-560.
4. Gaynes BN, Warden D, Trivedi MH, et al. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60(11):1439-1445.
5. US Food and Drug Administration. Center for Drug Evaluation and Research. Esketamine clinical review. Published March 5, 2019. Accessed August 9, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211243Orig1s000MedR.pdf
6. Zanos P, Moaddel R, Morris PJ, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70(3):621-660.
7. Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4):801-811.
8. Kaur U, Pathak BK, Singh A, et al. Esketamine: a glimmer of hope in treatment-resistant depression. Eur Arch Psychiatry Clin Neurosci. 2021;271(3):417-429.
9. McIntyre RS, Carvalho IP, Lui LMW, et al. The effect of intravenous, intranasal, and oral ketamine/esketamine in mood disorders: a meta-analysis. J Affect Disord. 2020;276:576-584.
10. Zarate CA Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856-864.
11. Thielking M. Ketamine gives hope to patients with severe depression. But some clinics stray from the science and hype its benefits. STAT+. Published September 18, 2018. Accessed August 5, 2021. www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment/
1. Hillhouse TM, Porter JH. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol. 2015;23(1):1-21.
2. Fava M, Rush A, Trivedi M, et al. Background and rationale for the Sequenced Treatment Alternatives to Relieve Depression (STAR*D) study. Psychiatr Clin North Am. 2003;26(2):457-494.
3. Gaynes BN, Rush AJ, Trivedi MH, et al. Primary versus specialty care outcomes for depressed outpatients managed with measurement-based care: results from STAR*D. J Gen Intern Med. 2008;23(5):551-560.
4. Gaynes BN, Warden D, Trivedi MH, et al. What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60(11):1439-1445.
5. US Food and Drug Administration. Center for Drug Evaluation and Research. Esketamine clinical review. Published March 5, 2019. Accessed August 9, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/211243Orig1s000MedR.pdf
6. Zanos P, Moaddel R, Morris PJ, et al. Ketamine and ketamine metabolite pharmacology: insights into therapeutic mechanisms. Pharmacol Rev. 2018;70(3):621-660.
7. Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4):801-811.
8. Kaur U, Pathak BK, Singh A, et al. Esketamine: a glimmer of hope in treatment-resistant depression. Eur Arch Psychiatry Clin Neurosci. 2021;271(3):417-429.
9. McIntyre RS, Carvalho IP, Lui LMW, et al. The effect of intravenous, intranasal, and oral ketamine/esketamine in mood disorders: a meta-analysis. J Affect Disord. 2020;276:576-584.
10. Zarate CA Jr, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856-864.
11. Thielking M. Ketamine gives hope to patients with severe depression. But some clinics stray from the science and hype its benefits. STAT+. Published September 18, 2018. Accessed August 5, 2021. www.statnews.com/2018/09/24/ketamine-clinics-severe-depression-treatment/
APA, ABPN, and Maintenance of Certification: Stop this MOCkery
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
The Accreditation Council for Graduate Medical Education (ACGME) is entrusted with assuring that upon graduation every resident is a competent doctor, a trained professional, and prepared to practice in their own field at a level that assures patient safety and meets the standard of care. The American Board of Psychiatry and Neurology (ABPN) is a private company that sells certificates claiming to attest the capacity or competence of the doctor but does not make public the test questions or algorithms used to win its qualifications or approval. The certifying business and the newer Maintenance of Certification (MOC) process developed by ABPN have unfortunately been embraced by ACGME and many hospitals, despite the lack of any good scientific support that board certification or MOC are meaningful for quality of patient care or outcomes. By that I mean there is no evidence that the voluntary board certification process or MOC have been shown to produce better outcomes for patients, save money for the country drowning in an ocean of health care costs, or allow doctors to get paid at a higher level by insurers for the same billing codes compared with those who bill without possessing these qualifications. The only entity that “profits” from the board certification/MOC process is ABPN, a private corporation that is supposed to be a nonprofit, but was sitting on a treasury of more than $140M in assets in 2019,1 with revenues growing annually. Including the interest earned on the investment and added revenues every year, the estimated total assets of ABPN will be in the range of $150M at the end of 2021!
Collaboration between ACGME and ABPN
The collaboration between ACGME and ABPN for graduate education for designing training programs for residents and fellows, with progressively increasing competencies and their assessments to dovetail with the board examinations offered by ABPN, sounds very legitimate. This arrangement is designed to enhance the quality of training and establish a minimum level of competence in each trainee who completes the training program. However, ACGME is catering to a monopoly recognized by the US Department of Justice (DOJ) Antitrust Division.2 ACGME has not entertained other evaluators of competence to discourage competition to the monopolistic ABPN. ACGME is only involved with the accredited training programs and has no business in assessing the continued competence of graduated trainees after they leave the program, although most will voluntarily opt to become board-certified by ABPN. Maintenance of Certification definitely does not come within the purview of “graduate medical education” for ACGME to be getting drawn into this collaboration.
ACGME and ABPN are unregulated and are not member-driven. As such, they operate outside of any real oversight. Their power derives from the status given to them by hospitals, some insurers, and many of our colleagues, who fail to see the reality that they are nothing more than diploma shops.
I am board-certified in psychiatry and child and adolescent psychiatry, and I have participated in obtaining board certification by ABPN in 3 other subspecialties (geriatric, addiction, and forensic). I decided to not participate in MOC for the latter 3 subspecialty certifications beyond 10 and 20 years for my own practical reasons. Obviously, then, I am not at all against initial certifications in any specialty, nor am I opposed to practitioners keeping up with progress in their fields and maintaining their competence. I am opposed to the continued efforts to engage professionals to pay a high price for the repeated MOC, riding on the hard work and earnings of the graduated specialists and continuously suctioning their income over their careers, with no evidence that MOC measures clinical competence or patient outcomes of their subscribers, who pay a chunk of money to the American Board of Medical Specialties (ABMS)/ABPN annually and every 10 years.
MOC and the APA
Many American Psychiatric Association (APA) members are opposed to the APA giving ABPN a piggyback ride to accomplish this profit seeking. This is becoming obvious to many APA members, who see this as a great exploitation.
Over the last 6 years, physicians have begun to question the validity of board certification and MOC by ABPN, mostly as a response to ever-increasing costs to them and ever-increasing revenues to ABPN. While APA members have long pressed the APA to push back against ABPN, the APA Board of Trustees has done the opposite by accepting yearly “unrestricted educational grants” from ABPN. In this manner, ABPN has essentially silenced the APA and has made it ineffective as our member organization in what has become a fight against ABPN’s unchecked power, influence, and intrusion. Every poll conducted by every APA District Branch or subspecialty organization has shown widespread discontent and anger at the ABPN/MOC process and APA’s deliberate inaction. Even when the APA commissioned its own member survey on the topic, wrote the questions, picked who would get the survey, decided which responses to count, and determined what statistics to apply, the results were damning. Despite its obviously transparent machinations, the APA failed to glorify the MOC process.
Continue to: The APA's membership...
The APA’s membership is declining, and the Board of Trustee’s position on MOC is partly to blame. The APA is once again not listening to its members! As a membership-driven organization, the APA must not exclusively support and promote this commercial educational product termed MOC when other, less expensive alternatives are now available. The APA can easily endorse these alternatives, in addition to offering its own less expensive products for attesting maintenance of competence. The latter effort will help eliminate the monopoly held by ABMS/ABPN in this domain and please all members as well as the DOJ.
The APA’s failure to provide less expensive alternatives or at least endorse existing ones despite repeated requests from a large number of APA members has led to frustration and a surge of strong feelings that are expressed on the APA email listservs, and especially that of the MOC caucus. These expressions are legitimate and need to be publicized to the general membership. I have collected the opinions of various loyal, long-standing APA members and put together a separate, yet-unpublished article to drive home the point that APA has resisted breaking the monopoly of ABPN, which the DOJ would encourage organizations such as the APA to do. Instead, APA is acting as an enabler to ABPN to create a multi-million dollar (and eventually a billion dollar) monopolistic industry at their members’ expense, literally endangering the careers of members if they fail to participate when employed by institutions that overvalue the MOC offered by ABPN.
I believe the recent exhibition of “collaboration” between the APA and ABPN is not similar to that between ACGME and ABPN, but is a most blatant effort on the part of the APA to help ABPN build a billion-dollar educational industry over the next 10 to 15 years. One can easily lose sight of this and get lost in the intricacies of how candidates can maintain their competency by obtaining free CME credits. The APA is distracting its members by citing this. They will continue to pay a high price for certification and recertification, with no real discount.
Most of the APA’s 38,000 members are in the dark about the above-mentioned process. They need to do their own research, especially when there are alternatives to the ABPN’s MOC program. They need to insist that the APA stop exclusively promoting ABPN products, and publicize other, much cheaper, alternatives. It will please all APA members to see the ABPN’s monopoly vanish. This is especially the case for younger psychiatrists, who average nearly $250,000 in educational loans. They need to prevent the APA/ABPN collaboration from having a far-reaching effect on their careers and finances, with potentially destructive consequences for their families, employers and—most importantly—their patients. Even some state licensing boards are being tempted to buy into the illusion.
Stop this MOCkery.
1. ProPublica. American Board of Psychiatry and Neurology. Accessed July 16, 2021. https://projects.propublica.org/nonprofits/organizations/410654864
2. US Department of Justice, Antitrust Division. Comments on Maryland House Bill 857. Published September 10, 2018. Accessed July 16, 2021. https://www.justice.gov/atr/page/file/1092791/download
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
The Accreditation Council for Graduate Medical Education (ACGME) is entrusted with assuring that upon graduation every resident is a competent doctor, a trained professional, and prepared to practice in their own field at a level that assures patient safety and meets the standard of care. The American Board of Psychiatry and Neurology (ABPN) is a private company that sells certificates claiming to attest the capacity or competence of the doctor but does not make public the test questions or algorithms used to win its qualifications or approval. The certifying business and the newer Maintenance of Certification (MOC) process developed by ABPN have unfortunately been embraced by ACGME and many hospitals, despite the lack of any good scientific support that board certification or MOC are meaningful for quality of patient care or outcomes. By that I mean there is no evidence that the voluntary board certification process or MOC have been shown to produce better outcomes for patients, save money for the country drowning in an ocean of health care costs, or allow doctors to get paid at a higher level by insurers for the same billing codes compared with those who bill without possessing these qualifications. The only entity that “profits” from the board certification/MOC process is ABPN, a private corporation that is supposed to be a nonprofit, but was sitting on a treasury of more than $140M in assets in 2019,1 with revenues growing annually. Including the interest earned on the investment and added revenues every year, the estimated total assets of ABPN will be in the range of $150M at the end of 2021!
Collaboration between ACGME and ABPN
The collaboration between ACGME and ABPN for graduate education for designing training programs for residents and fellows, with progressively increasing competencies and their assessments to dovetail with the board examinations offered by ABPN, sounds very legitimate. This arrangement is designed to enhance the quality of training and establish a minimum level of competence in each trainee who completes the training program. However, ACGME is catering to a monopoly recognized by the US Department of Justice (DOJ) Antitrust Division.2 ACGME has not entertained other evaluators of competence to discourage competition to the monopolistic ABPN. ACGME is only involved with the accredited training programs and has no business in assessing the continued competence of graduated trainees after they leave the program, although most will voluntarily opt to become board-certified by ABPN. Maintenance of Certification definitely does not come within the purview of “graduate medical education” for ACGME to be getting drawn into this collaboration.
ACGME and ABPN are unregulated and are not member-driven. As such, they operate outside of any real oversight. Their power derives from the status given to them by hospitals, some insurers, and many of our colleagues, who fail to see the reality that they are nothing more than diploma shops.
I am board-certified in psychiatry and child and adolescent psychiatry, and I have participated in obtaining board certification by ABPN in 3 other subspecialties (geriatric, addiction, and forensic). I decided to not participate in MOC for the latter 3 subspecialty certifications beyond 10 and 20 years for my own practical reasons. Obviously, then, I am not at all against initial certifications in any specialty, nor am I opposed to practitioners keeping up with progress in their fields and maintaining their competence. I am opposed to the continued efforts to engage professionals to pay a high price for the repeated MOC, riding on the hard work and earnings of the graduated specialists and continuously suctioning their income over their careers, with no evidence that MOC measures clinical competence or patient outcomes of their subscribers, who pay a chunk of money to the American Board of Medical Specialties (ABMS)/ABPN annually and every 10 years.
MOC and the APA
Many American Psychiatric Association (APA) members are opposed to the APA giving ABPN a piggyback ride to accomplish this profit seeking. This is becoming obvious to many APA members, who see this as a great exploitation.
Over the last 6 years, physicians have begun to question the validity of board certification and MOC by ABPN, mostly as a response to ever-increasing costs to them and ever-increasing revenues to ABPN. While APA members have long pressed the APA to push back against ABPN, the APA Board of Trustees has done the opposite by accepting yearly “unrestricted educational grants” from ABPN. In this manner, ABPN has essentially silenced the APA and has made it ineffective as our member organization in what has become a fight against ABPN’s unchecked power, influence, and intrusion. Every poll conducted by every APA District Branch or subspecialty organization has shown widespread discontent and anger at the ABPN/MOC process and APA’s deliberate inaction. Even when the APA commissioned its own member survey on the topic, wrote the questions, picked who would get the survey, decided which responses to count, and determined what statistics to apply, the results were damning. Despite its obviously transparent machinations, the APA failed to glorify the MOC process.
Continue to: The APA's membership...
The APA’s membership is declining, and the Board of Trustee’s position on MOC is partly to blame. The APA is once again not listening to its members! As a membership-driven organization, the APA must not exclusively support and promote this commercial educational product termed MOC when other, less expensive alternatives are now available. The APA can easily endorse these alternatives, in addition to offering its own less expensive products for attesting maintenance of competence. The latter effort will help eliminate the monopoly held by ABMS/ABPN in this domain and please all members as well as the DOJ.
The APA’s failure to provide less expensive alternatives or at least endorse existing ones despite repeated requests from a large number of APA members has led to frustration and a surge of strong feelings that are expressed on the APA email listservs, and especially that of the MOC caucus. These expressions are legitimate and need to be publicized to the general membership. I have collected the opinions of various loyal, long-standing APA members and put together a separate, yet-unpublished article to drive home the point that APA has resisted breaking the monopoly of ABPN, which the DOJ would encourage organizations such as the APA to do. Instead, APA is acting as an enabler to ABPN to create a multi-million dollar (and eventually a billion dollar) monopolistic industry at their members’ expense, literally endangering the careers of members if they fail to participate when employed by institutions that overvalue the MOC offered by ABPN.
I believe the recent exhibition of “collaboration” between the APA and ABPN is not similar to that between ACGME and ABPN, but is a most blatant effort on the part of the APA to help ABPN build a billion-dollar educational industry over the next 10 to 15 years. One can easily lose sight of this and get lost in the intricacies of how candidates can maintain their competency by obtaining free CME credits. The APA is distracting its members by citing this. They will continue to pay a high price for certification and recertification, with no real discount.
Most of the APA’s 38,000 members are in the dark about the above-mentioned process. They need to do their own research, especially when there are alternatives to the ABPN’s MOC program. They need to insist that the APA stop exclusively promoting ABPN products, and publicize other, much cheaper, alternatives. It will please all APA members to see the ABPN’s monopoly vanish. This is especially the case for younger psychiatrists, who average nearly $250,000 in educational loans. They need to prevent the APA/ABPN collaboration from having a far-reaching effect on their careers and finances, with potentially destructive consequences for their families, employers and—most importantly—their patients. Even some state licensing boards are being tempted to buy into the illusion.
Stop this MOCkery.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
The Accreditation Council for Graduate Medical Education (ACGME) is entrusted with assuring that upon graduation every resident is a competent doctor, a trained professional, and prepared to practice in their own field at a level that assures patient safety and meets the standard of care. The American Board of Psychiatry and Neurology (ABPN) is a private company that sells certificates claiming to attest the capacity or competence of the doctor but does not make public the test questions or algorithms used to win its qualifications or approval. The certifying business and the newer Maintenance of Certification (MOC) process developed by ABPN have unfortunately been embraced by ACGME and many hospitals, despite the lack of any good scientific support that board certification or MOC are meaningful for quality of patient care or outcomes. By that I mean there is no evidence that the voluntary board certification process or MOC have been shown to produce better outcomes for patients, save money for the country drowning in an ocean of health care costs, or allow doctors to get paid at a higher level by insurers for the same billing codes compared with those who bill without possessing these qualifications. The only entity that “profits” from the board certification/MOC process is ABPN, a private corporation that is supposed to be a nonprofit, but was sitting on a treasury of more than $140M in assets in 2019,1 with revenues growing annually. Including the interest earned on the investment and added revenues every year, the estimated total assets of ABPN will be in the range of $150M at the end of 2021!
Collaboration between ACGME and ABPN
The collaboration between ACGME and ABPN for graduate education for designing training programs for residents and fellows, with progressively increasing competencies and their assessments to dovetail with the board examinations offered by ABPN, sounds very legitimate. This arrangement is designed to enhance the quality of training and establish a minimum level of competence in each trainee who completes the training program. However, ACGME is catering to a monopoly recognized by the US Department of Justice (DOJ) Antitrust Division.2 ACGME has not entertained other evaluators of competence to discourage competition to the monopolistic ABPN. ACGME is only involved with the accredited training programs and has no business in assessing the continued competence of graduated trainees after they leave the program, although most will voluntarily opt to become board-certified by ABPN. Maintenance of Certification definitely does not come within the purview of “graduate medical education” for ACGME to be getting drawn into this collaboration.
ACGME and ABPN are unregulated and are not member-driven. As such, they operate outside of any real oversight. Their power derives from the status given to them by hospitals, some insurers, and many of our colleagues, who fail to see the reality that they are nothing more than diploma shops.
I am board-certified in psychiatry and child and adolescent psychiatry, and I have participated in obtaining board certification by ABPN in 3 other subspecialties (geriatric, addiction, and forensic). I decided to not participate in MOC for the latter 3 subspecialty certifications beyond 10 and 20 years for my own practical reasons. Obviously, then, I am not at all against initial certifications in any specialty, nor am I opposed to practitioners keeping up with progress in their fields and maintaining their competence. I am opposed to the continued efforts to engage professionals to pay a high price for the repeated MOC, riding on the hard work and earnings of the graduated specialists and continuously suctioning their income over their careers, with no evidence that MOC measures clinical competence or patient outcomes of their subscribers, who pay a chunk of money to the American Board of Medical Specialties (ABMS)/ABPN annually and every 10 years.
MOC and the APA
Many American Psychiatric Association (APA) members are opposed to the APA giving ABPN a piggyback ride to accomplish this profit seeking. This is becoming obvious to many APA members, who see this as a great exploitation.
Over the last 6 years, physicians have begun to question the validity of board certification and MOC by ABPN, mostly as a response to ever-increasing costs to them and ever-increasing revenues to ABPN. While APA members have long pressed the APA to push back against ABPN, the APA Board of Trustees has done the opposite by accepting yearly “unrestricted educational grants” from ABPN. In this manner, ABPN has essentially silenced the APA and has made it ineffective as our member organization in what has become a fight against ABPN’s unchecked power, influence, and intrusion. Every poll conducted by every APA District Branch or subspecialty organization has shown widespread discontent and anger at the ABPN/MOC process and APA’s deliberate inaction. Even when the APA commissioned its own member survey on the topic, wrote the questions, picked who would get the survey, decided which responses to count, and determined what statistics to apply, the results were damning. Despite its obviously transparent machinations, the APA failed to glorify the MOC process.
Continue to: The APA's membership...
The APA’s membership is declining, and the Board of Trustee’s position on MOC is partly to blame. The APA is once again not listening to its members! As a membership-driven organization, the APA must not exclusively support and promote this commercial educational product termed MOC when other, less expensive alternatives are now available. The APA can easily endorse these alternatives, in addition to offering its own less expensive products for attesting maintenance of competence. The latter effort will help eliminate the monopoly held by ABMS/ABPN in this domain and please all members as well as the DOJ.
The APA’s failure to provide less expensive alternatives or at least endorse existing ones despite repeated requests from a large number of APA members has led to frustration and a surge of strong feelings that are expressed on the APA email listservs, and especially that of the MOC caucus. These expressions are legitimate and need to be publicized to the general membership. I have collected the opinions of various loyal, long-standing APA members and put together a separate, yet-unpublished article to drive home the point that APA has resisted breaking the monopoly of ABPN, which the DOJ would encourage organizations such as the APA to do. Instead, APA is acting as an enabler to ABPN to create a multi-million dollar (and eventually a billion dollar) monopolistic industry at their members’ expense, literally endangering the careers of members if they fail to participate when employed by institutions that overvalue the MOC offered by ABPN.
I believe the recent exhibition of “collaboration” between the APA and ABPN is not similar to that between ACGME and ABPN, but is a most blatant effort on the part of the APA to help ABPN build a billion-dollar educational industry over the next 10 to 15 years. One can easily lose sight of this and get lost in the intricacies of how candidates can maintain their competency by obtaining free CME credits. The APA is distracting its members by citing this. They will continue to pay a high price for certification and recertification, with no real discount.
Most of the APA’s 38,000 members are in the dark about the above-mentioned process. They need to do their own research, especially when there are alternatives to the ABPN’s MOC program. They need to insist that the APA stop exclusively promoting ABPN products, and publicize other, much cheaper, alternatives. It will please all APA members to see the ABPN’s monopoly vanish. This is especially the case for younger psychiatrists, who average nearly $250,000 in educational loans. They need to prevent the APA/ABPN collaboration from having a far-reaching effect on their careers and finances, with potentially destructive consequences for their families, employers and—most importantly—their patients. Even some state licensing boards are being tempted to buy into the illusion.
Stop this MOCkery.
1. ProPublica. American Board of Psychiatry and Neurology. Accessed July 16, 2021. https://projects.propublica.org/nonprofits/organizations/410654864
2. US Department of Justice, Antitrust Division. Comments on Maryland House Bill 857. Published September 10, 2018. Accessed July 16, 2021. https://www.justice.gov/atr/page/file/1092791/download
1. ProPublica. American Board of Psychiatry and Neurology. Accessed July 16, 2021. https://projects.propublica.org/nonprofits/organizations/410654864
2. US Department of Justice, Antitrust Division. Comments on Maryland House Bill 857. Published September 10, 2018. Accessed July 16, 2021. https://www.justice.gov/atr/page/file/1092791/download
Neuropsychiatry affects pediatric OCD treatment
Treatment of pediatric obsessive-compulsive disorder (OCD) has evolved in recent years, with more attention given to some of the neuropsychiatric underpinnings of the condition and how they can affect treatment response.
At the Focus on Neuropsychiatry 2021 meeting, Jeffrey Strawn, MD, outlined some of the neuropsychiatry affecting disease and potential mechanisms to help control obsessions and behaviors, and how they may fit with some therapeutic regimens.
Dr. Strawn discussed the psychological construct of cognitive control, which can provide patients an “out” from the cycle of obsession/fear/worry and compulsion/avoidance. In the face of distress, compulsion and avoidance lead to relief, which reinforces the obsession/fear/worry; this in turn leads to more distress.
“We have an escape door for this circuit” in the form of cognitive control, said Dr. Strawn, who is an associate professor of pediatrics at Cincinnati Children’s Hospital Medical Center.
Cognitive control is linked to insight, which can in turn increase adaptive behaviors that help the patient resist the compulsion. Patients won’t eliminate distress, but they can be helped to make it more tolerable. Therapists can then help them move toward goal-directed thoughts and behaviors. Cognitive control is associated with several neural networks, but Dr. Strawn focused on two: the frontoparietal network, associated with top-down regulation; and the cingular-opercular network. Both of these are engaged during cognitive control processes, and play a role inhibitory control and error monitoring.
Dr. Strawn discussed a recent study that explored the neurofunctional basis of treatment. It compared the effects of a stress management therapy and cognitive-behavioral therapy (CBT) in children and adults with OCD at 6 and 12 weeks. The study found similar symptom reductions in both adults and adolescents in both intervention groups.
Before initiating treatment, the researchers conducted functional MRI scans of participants while conducting an incentive flanker task, which reveals brain activity in response to cognitive control and reward processing.
A larger therapeutic response was found in the CBT group among patients who had a larger pretreatment activation within the right temporal lobe and rostral anterior cingulate cortex during cognitive control, as well as those with more activation within the medial prefrontal, orbitofrontal, lateral prefrontal, and amygdala regions during reward processing. On the other hand, within the stress management therapy group, treatment responses were better among those who had lower pretreatment activation among overlapping regions.
“There was a difference in terms of the neurofunctional predictors of treatment response. One of the key regions is the medial prefrontal cortex as well as the rostral anterior cingulate,” said Dr. Strawn, at the meeting presented by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
On the neuropharmacology side, numerous medications have been approved for OCD. Dr. Strawn highlighted some studies to illustrate general OCD treatment concepts. That included the 2004 Pediatric OCD Treatment Study, which was one of the only trials to compare placebo with an SSRI, CBT, and the combination of SSRI and CBT. It showed the best results with combination therapy, and the difference appeared early in the treatment course.
That study had aggressive dosing, which led to some issues with sertraline tolerability. Dr. Strawn showed results of a study at his institution which showed that the drug levels of pediatric patients treated with sertraline depended on CYP2C19 metabolism, which affects overall exposure and peak dose concentration. In pediatric populations, some SSRIs clear more slowly and can have high peak concentrations. SSRIs have more side effects than serotonin and norepinephrine reuptake inhibitors in both anxiety disorders and OCD. A key difference between the two is that SSRI treatment is associated with greater frequency of activation, which is difficult to define, but includes restlessness and agitation and insomnia in the beginning stages of treatment.
SSRIs also lead to improvement early in the course of treatment, which was shown in a meta-analysis of nine trials. However, the same study showed that clomipramine is associated with a faster and greater magnitude of improvement, compared with SSRIs, even when the latter are dosed aggressively.
Clomipramine is a potent inhibitor of both serotonin and norepinephrine reuptake. It is recommended to monitor clomipramine levels in pediatric OCD patients, and Dr. Strawn suggested that monitoring should include both the parent drug and its primary metabolite, norclomipramine. At a given dose, there can be a great deal of variation in drug level. The clomipramine/norclomipramine ratio can provide information about the patient’s metabolic state, as well as drug adherence.
Dr. Strawn noted that peak levels occur around 1-3 hours after the dose, “and we really do want at least a 12-hour trough level.” EKGs should be performed at baseline and after any titration of clomipramine dose.
He also discussed pediatric OCD patients with OCD and tics. About one-third of Tourette syndrome patients experience OCD at some point. Tics often improve, whereas OCD more often persists. Tics that co-occur with OCD are associated with a lesser response to SSRI treatment, but not CBT treatment. Similarly, patients with hoarding tendencies are about one-third less likely to respond to SSRIs, CBT, or combination therapy.
Dr. Strawn discussed the concept of accommodation, in which family members cope with a patient’s behavior by altering routines to minimize distress and impairment. This may take the form of facilitating rituals, providing reassurance about a patient’s fears, acquiescing to demands, reducing the child’s day-to-day responsibilities, or helping the child complete tasks. Such actions are well intentioned, but they undermine cognitive control, negatively reinforce symptom engagement, and are associated with functional impairment. Reassurance is the most important behavior, occurring in more than half of patients, and it’s measurable. Parental involvement with rituals is also a concern. “This is associated with higher levels of child OCD severity, as well as parental psychopathology, and lower family cohesion. So
New developments in neurobiology and neuropsychology have changed the view of exposure. The old model emphasized the child’s fear rating as an index of corrective learning. The idea was that habituation would decrease anxiety and distress from future exposures. The new model revolves around inhibitory learning theory, which focuses on the variability of distress and aims to increase tolerance of distress. Another goal is to develop new, non-threat associations.
Finally, Dr. Strawn pointed out predictors of poor outcomes in pediatric OCD, including factors such as compulsion severity, oppositional behavior, frequent handwashing, functional impairment, lack of insight, externalizing symptoms, and possibly hoarding. Problematic family characteristics include higher levels of accommodation, parental anxiety, low family cohesion, and high levels of conflict. “The last three really represent a very concerning triad of family behaviors that may necessitate specific family work in order to facilitate the recovery of the pediatric patient,” Dr. Strawn said.
During the question-and-answer session after the talk, Dr. Strawn was asked whether there might be an inflammatory component to OCD, and whether pediatric autoimmune neuropsychiatric disorders associated with streptococcus (PANDAS) might be a prodromal condition. He noted that some studies have shown a relationship, but results have been mixed, with lots of heterogeneity within the studied populations. To be suspicious that a patient had OCD resulting from PANDAS would require a high threshold, including an acute onset of symptoms. “This is a situation also where I would tend to involve consultation with some other specialties, including neurology. And obviously there would be follow-up in terms of the general workup,” he said.
Dr. Strawn has received research funding from Allergan, Otsuka, and Myriad Genetics. He has consulted for Myriad Genetics, and is a speaker for CMEology and the Neuroscience Education Institute.
Treatment of pediatric obsessive-compulsive disorder (OCD) has evolved in recent years, with more attention given to some of the neuropsychiatric underpinnings of the condition and how they can affect treatment response.
At the Focus on Neuropsychiatry 2021 meeting, Jeffrey Strawn, MD, outlined some of the neuropsychiatry affecting disease and potential mechanisms to help control obsessions and behaviors, and how they may fit with some therapeutic regimens.
Dr. Strawn discussed the psychological construct of cognitive control, which can provide patients an “out” from the cycle of obsession/fear/worry and compulsion/avoidance. In the face of distress, compulsion and avoidance lead to relief, which reinforces the obsession/fear/worry; this in turn leads to more distress.
“We have an escape door for this circuit” in the form of cognitive control, said Dr. Strawn, who is an associate professor of pediatrics at Cincinnati Children’s Hospital Medical Center.
Cognitive control is linked to insight, which can in turn increase adaptive behaviors that help the patient resist the compulsion. Patients won’t eliminate distress, but they can be helped to make it more tolerable. Therapists can then help them move toward goal-directed thoughts and behaviors. Cognitive control is associated with several neural networks, but Dr. Strawn focused on two: the frontoparietal network, associated with top-down regulation; and the cingular-opercular network. Both of these are engaged during cognitive control processes, and play a role inhibitory control and error monitoring.
Dr. Strawn discussed a recent study that explored the neurofunctional basis of treatment. It compared the effects of a stress management therapy and cognitive-behavioral therapy (CBT) in children and adults with OCD at 6 and 12 weeks. The study found similar symptom reductions in both adults and adolescents in both intervention groups.
Before initiating treatment, the researchers conducted functional MRI scans of participants while conducting an incentive flanker task, which reveals brain activity in response to cognitive control and reward processing.
A larger therapeutic response was found in the CBT group among patients who had a larger pretreatment activation within the right temporal lobe and rostral anterior cingulate cortex during cognitive control, as well as those with more activation within the medial prefrontal, orbitofrontal, lateral prefrontal, and amygdala regions during reward processing. On the other hand, within the stress management therapy group, treatment responses were better among those who had lower pretreatment activation among overlapping regions.
“There was a difference in terms of the neurofunctional predictors of treatment response. One of the key regions is the medial prefrontal cortex as well as the rostral anterior cingulate,” said Dr. Strawn, at the meeting presented by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
On the neuropharmacology side, numerous medications have been approved for OCD. Dr. Strawn highlighted some studies to illustrate general OCD treatment concepts. That included the 2004 Pediatric OCD Treatment Study, which was one of the only trials to compare placebo with an SSRI, CBT, and the combination of SSRI and CBT. It showed the best results with combination therapy, and the difference appeared early in the treatment course.
That study had aggressive dosing, which led to some issues with sertraline tolerability. Dr. Strawn showed results of a study at his institution which showed that the drug levels of pediatric patients treated with sertraline depended on CYP2C19 metabolism, which affects overall exposure and peak dose concentration. In pediatric populations, some SSRIs clear more slowly and can have high peak concentrations. SSRIs have more side effects than serotonin and norepinephrine reuptake inhibitors in both anxiety disorders and OCD. A key difference between the two is that SSRI treatment is associated with greater frequency of activation, which is difficult to define, but includes restlessness and agitation and insomnia in the beginning stages of treatment.
SSRIs also lead to improvement early in the course of treatment, which was shown in a meta-analysis of nine trials. However, the same study showed that clomipramine is associated with a faster and greater magnitude of improvement, compared with SSRIs, even when the latter are dosed aggressively.
Clomipramine is a potent inhibitor of both serotonin and norepinephrine reuptake. It is recommended to monitor clomipramine levels in pediatric OCD patients, and Dr. Strawn suggested that monitoring should include both the parent drug and its primary metabolite, norclomipramine. At a given dose, there can be a great deal of variation in drug level. The clomipramine/norclomipramine ratio can provide information about the patient’s metabolic state, as well as drug adherence.
Dr. Strawn noted that peak levels occur around 1-3 hours after the dose, “and we really do want at least a 12-hour trough level.” EKGs should be performed at baseline and after any titration of clomipramine dose.
He also discussed pediatric OCD patients with OCD and tics. About one-third of Tourette syndrome patients experience OCD at some point. Tics often improve, whereas OCD more often persists. Tics that co-occur with OCD are associated with a lesser response to SSRI treatment, but not CBT treatment. Similarly, patients with hoarding tendencies are about one-third less likely to respond to SSRIs, CBT, or combination therapy.
Dr. Strawn discussed the concept of accommodation, in which family members cope with a patient’s behavior by altering routines to minimize distress and impairment. This may take the form of facilitating rituals, providing reassurance about a patient’s fears, acquiescing to demands, reducing the child’s day-to-day responsibilities, or helping the child complete tasks. Such actions are well intentioned, but they undermine cognitive control, negatively reinforce symptom engagement, and are associated with functional impairment. Reassurance is the most important behavior, occurring in more than half of patients, and it’s measurable. Parental involvement with rituals is also a concern. “This is associated with higher levels of child OCD severity, as well as parental psychopathology, and lower family cohesion. So
New developments in neurobiology and neuropsychology have changed the view of exposure. The old model emphasized the child’s fear rating as an index of corrective learning. The idea was that habituation would decrease anxiety and distress from future exposures. The new model revolves around inhibitory learning theory, which focuses on the variability of distress and aims to increase tolerance of distress. Another goal is to develop new, non-threat associations.
Finally, Dr. Strawn pointed out predictors of poor outcomes in pediatric OCD, including factors such as compulsion severity, oppositional behavior, frequent handwashing, functional impairment, lack of insight, externalizing symptoms, and possibly hoarding. Problematic family characteristics include higher levels of accommodation, parental anxiety, low family cohesion, and high levels of conflict. “The last three really represent a very concerning triad of family behaviors that may necessitate specific family work in order to facilitate the recovery of the pediatric patient,” Dr. Strawn said.
During the question-and-answer session after the talk, Dr. Strawn was asked whether there might be an inflammatory component to OCD, and whether pediatric autoimmune neuropsychiatric disorders associated with streptococcus (PANDAS) might be a prodromal condition. He noted that some studies have shown a relationship, but results have been mixed, with lots of heterogeneity within the studied populations. To be suspicious that a patient had OCD resulting from PANDAS would require a high threshold, including an acute onset of symptoms. “This is a situation also where I would tend to involve consultation with some other specialties, including neurology. And obviously there would be follow-up in terms of the general workup,” he said.
Dr. Strawn has received research funding from Allergan, Otsuka, and Myriad Genetics. He has consulted for Myriad Genetics, and is a speaker for CMEology and the Neuroscience Education Institute.
Treatment of pediatric obsessive-compulsive disorder (OCD) has evolved in recent years, with more attention given to some of the neuropsychiatric underpinnings of the condition and how they can affect treatment response.
At the Focus on Neuropsychiatry 2021 meeting, Jeffrey Strawn, MD, outlined some of the neuropsychiatry affecting disease and potential mechanisms to help control obsessions and behaviors, and how they may fit with some therapeutic regimens.
Dr. Strawn discussed the psychological construct of cognitive control, which can provide patients an “out” from the cycle of obsession/fear/worry and compulsion/avoidance. In the face of distress, compulsion and avoidance lead to relief, which reinforces the obsession/fear/worry; this in turn leads to more distress.
“We have an escape door for this circuit” in the form of cognitive control, said Dr. Strawn, who is an associate professor of pediatrics at Cincinnati Children’s Hospital Medical Center.
Cognitive control is linked to insight, which can in turn increase adaptive behaviors that help the patient resist the compulsion. Patients won’t eliminate distress, but they can be helped to make it more tolerable. Therapists can then help them move toward goal-directed thoughts and behaviors. Cognitive control is associated with several neural networks, but Dr. Strawn focused on two: the frontoparietal network, associated with top-down regulation; and the cingular-opercular network. Both of these are engaged during cognitive control processes, and play a role inhibitory control and error monitoring.
Dr. Strawn discussed a recent study that explored the neurofunctional basis of treatment. It compared the effects of a stress management therapy and cognitive-behavioral therapy (CBT) in children and adults with OCD at 6 and 12 weeks. The study found similar symptom reductions in both adults and adolescents in both intervention groups.
Before initiating treatment, the researchers conducted functional MRI scans of participants while conducting an incentive flanker task, which reveals brain activity in response to cognitive control and reward processing.
A larger therapeutic response was found in the CBT group among patients who had a larger pretreatment activation within the right temporal lobe and rostral anterior cingulate cortex during cognitive control, as well as those with more activation within the medial prefrontal, orbitofrontal, lateral prefrontal, and amygdala regions during reward processing. On the other hand, within the stress management therapy group, treatment responses were better among those who had lower pretreatment activation among overlapping regions.
“There was a difference in terms of the neurofunctional predictors of treatment response. One of the key regions is the medial prefrontal cortex as well as the rostral anterior cingulate,” said Dr. Strawn, at the meeting presented by MedscapeLive. MedscapeLive and this news organization are owned by the same parent company.
On the neuropharmacology side, numerous medications have been approved for OCD. Dr. Strawn highlighted some studies to illustrate general OCD treatment concepts. That included the 2004 Pediatric OCD Treatment Study, which was one of the only trials to compare placebo with an SSRI, CBT, and the combination of SSRI and CBT. It showed the best results with combination therapy, and the difference appeared early in the treatment course.
That study had aggressive dosing, which led to some issues with sertraline tolerability. Dr. Strawn showed results of a study at his institution which showed that the drug levels of pediatric patients treated with sertraline depended on CYP2C19 metabolism, which affects overall exposure and peak dose concentration. In pediatric populations, some SSRIs clear more slowly and can have high peak concentrations. SSRIs have more side effects than serotonin and norepinephrine reuptake inhibitors in both anxiety disorders and OCD. A key difference between the two is that SSRI treatment is associated with greater frequency of activation, which is difficult to define, but includes restlessness and agitation and insomnia in the beginning stages of treatment.
SSRIs also lead to improvement early in the course of treatment, which was shown in a meta-analysis of nine trials. However, the same study showed that clomipramine is associated with a faster and greater magnitude of improvement, compared with SSRIs, even when the latter are dosed aggressively.
Clomipramine is a potent inhibitor of both serotonin and norepinephrine reuptake. It is recommended to monitor clomipramine levels in pediatric OCD patients, and Dr. Strawn suggested that monitoring should include both the parent drug and its primary metabolite, norclomipramine. At a given dose, there can be a great deal of variation in drug level. The clomipramine/norclomipramine ratio can provide information about the patient’s metabolic state, as well as drug adherence.
Dr. Strawn noted that peak levels occur around 1-3 hours after the dose, “and we really do want at least a 12-hour trough level.” EKGs should be performed at baseline and after any titration of clomipramine dose.
He also discussed pediatric OCD patients with OCD and tics. About one-third of Tourette syndrome patients experience OCD at some point. Tics often improve, whereas OCD more often persists. Tics that co-occur with OCD are associated with a lesser response to SSRI treatment, but not CBT treatment. Similarly, patients with hoarding tendencies are about one-third less likely to respond to SSRIs, CBT, or combination therapy.
Dr. Strawn discussed the concept of accommodation, in which family members cope with a patient’s behavior by altering routines to minimize distress and impairment. This may take the form of facilitating rituals, providing reassurance about a patient’s fears, acquiescing to demands, reducing the child’s day-to-day responsibilities, or helping the child complete tasks. Such actions are well intentioned, but they undermine cognitive control, negatively reinforce symptom engagement, and are associated with functional impairment. Reassurance is the most important behavior, occurring in more than half of patients, and it’s measurable. Parental involvement with rituals is also a concern. “This is associated with higher levels of child OCD severity, as well as parental psychopathology, and lower family cohesion. So
New developments in neurobiology and neuropsychology have changed the view of exposure. The old model emphasized the child’s fear rating as an index of corrective learning. The idea was that habituation would decrease anxiety and distress from future exposures. The new model revolves around inhibitory learning theory, which focuses on the variability of distress and aims to increase tolerance of distress. Another goal is to develop new, non-threat associations.
Finally, Dr. Strawn pointed out predictors of poor outcomes in pediatric OCD, including factors such as compulsion severity, oppositional behavior, frequent handwashing, functional impairment, lack of insight, externalizing symptoms, and possibly hoarding. Problematic family characteristics include higher levels of accommodation, parental anxiety, low family cohesion, and high levels of conflict. “The last three really represent a very concerning triad of family behaviors that may necessitate specific family work in order to facilitate the recovery of the pediatric patient,” Dr. Strawn said.
During the question-and-answer session after the talk, Dr. Strawn was asked whether there might be an inflammatory component to OCD, and whether pediatric autoimmune neuropsychiatric disorders associated with streptococcus (PANDAS) might be a prodromal condition. He noted that some studies have shown a relationship, but results have been mixed, with lots of heterogeneity within the studied populations. To be suspicious that a patient had OCD resulting from PANDAS would require a high threshold, including an acute onset of symptoms. “This is a situation also where I would tend to involve consultation with some other specialties, including neurology. And obviously there would be follow-up in terms of the general workup,” he said.
Dr. Strawn has received research funding from Allergan, Otsuka, and Myriad Genetics. He has consulted for Myriad Genetics, and is a speaker for CMEology and the Neuroscience Education Institute.
FROM FOCUS ON NEUROPSYCHIATRY 2021
EDs saw more benzodiazepine overdoses, but fewer patients overall, in 2020
In a year when emergency department visits dropped by almost 18%, visits for benzodiazepine overdoses did the opposite, according to a report from the Centers for Disease Control and Prevention.

The actual increase in the number of overdose visits for benzodiazepine overdoses was quite small – from 15,547 in 2019 to 15,830 in 2020 (1.8%) – but the 11 million fewer ED visits magnified its effect, Stephen Liu, PhD, and associates said in the Morbidity and Mortality Weekly Report.
The rate of benzodiazepine overdose visits to all visits increased by 23.7% from 2019 (24.22 per 100,000 ED visits) to 2020 (29.97 per 100,000), with the larger share going to those involving opioids, which were up by 34.4%, compared with overdose visits not involving opioids (21.0%), the investigators said, based on data reported by 32 states and the District of Columbia to the CDC’s Drug Overdose Surveillance and Epidemiology system. All of the rate changes are statistically significant.
The number of overdose visits without opioid coinvolvement actually dropped, from 2019 (12,276) to 2020 (12,218), but not by enough to offset the decline in total visits, noted Dr. Liu, of the CDC’s National Center for Injury Prevention and Control and associates.
The number of deaths from benzodiazepine overdose, on the other hand, did not drop in 2020. Those data, coming from 23 states participating in the CDC’s State Unintentional Drug Overdose Reporting System, were available only for the first half of the year.
In those 6 months, The first quarter of 2020 also showed an increase, but exact numbers were not provided in the report. Overdose deaths rose by 22% for prescription forms of benzodiazepine and 520% for illicit forms in Q2 of 2020, compared with 2019, the researchers said.
Almost all of the benzodiazepine deaths (93%) in the first half of 2020 also involved opioids, mostly in the form of illicitly manufactured fentanyls (67% of all deaths). Between Q2 of 2019 and Q2 of 2020, involvement of illicit fentanyls in benzodiazepine overdose deaths increased from almost 57% to 71%, Dr. Liu and associates reported.
“Despite progress in reducing coprescribing [of opioids and benzodiazepines] before 2019, this study suggests a reversal in the decline in benzodiazepine deaths from 2017 to 2019, driven in part by increasing involvement of [illicitly manufactured fentanyls] in benzodiazepine deaths and influxes of illicit benzodiazepines,” they wrote.
In a year when emergency department visits dropped by almost 18%, visits for benzodiazepine overdoses did the opposite, according to a report from the Centers for Disease Control and Prevention.

The actual increase in the number of overdose visits for benzodiazepine overdoses was quite small – from 15,547 in 2019 to 15,830 in 2020 (1.8%) – but the 11 million fewer ED visits magnified its effect, Stephen Liu, PhD, and associates said in the Morbidity and Mortality Weekly Report.
The rate of benzodiazepine overdose visits to all visits increased by 23.7% from 2019 (24.22 per 100,000 ED visits) to 2020 (29.97 per 100,000), with the larger share going to those involving opioids, which were up by 34.4%, compared with overdose visits not involving opioids (21.0%), the investigators said, based on data reported by 32 states and the District of Columbia to the CDC’s Drug Overdose Surveillance and Epidemiology system. All of the rate changes are statistically significant.
The number of overdose visits without opioid coinvolvement actually dropped, from 2019 (12,276) to 2020 (12,218), but not by enough to offset the decline in total visits, noted Dr. Liu, of the CDC’s National Center for Injury Prevention and Control and associates.
The number of deaths from benzodiazepine overdose, on the other hand, did not drop in 2020. Those data, coming from 23 states participating in the CDC’s State Unintentional Drug Overdose Reporting System, were available only for the first half of the year.
In those 6 months, The first quarter of 2020 also showed an increase, but exact numbers were not provided in the report. Overdose deaths rose by 22% for prescription forms of benzodiazepine and 520% for illicit forms in Q2 of 2020, compared with 2019, the researchers said.
Almost all of the benzodiazepine deaths (93%) in the first half of 2020 also involved opioids, mostly in the form of illicitly manufactured fentanyls (67% of all deaths). Between Q2 of 2019 and Q2 of 2020, involvement of illicit fentanyls in benzodiazepine overdose deaths increased from almost 57% to 71%, Dr. Liu and associates reported.
“Despite progress in reducing coprescribing [of opioids and benzodiazepines] before 2019, this study suggests a reversal in the decline in benzodiazepine deaths from 2017 to 2019, driven in part by increasing involvement of [illicitly manufactured fentanyls] in benzodiazepine deaths and influxes of illicit benzodiazepines,” they wrote.
In a year when emergency department visits dropped by almost 18%, visits for benzodiazepine overdoses did the opposite, according to a report from the Centers for Disease Control and Prevention.

The actual increase in the number of overdose visits for benzodiazepine overdoses was quite small – from 15,547 in 2019 to 15,830 in 2020 (1.8%) – but the 11 million fewer ED visits magnified its effect, Stephen Liu, PhD, and associates said in the Morbidity and Mortality Weekly Report.
The rate of benzodiazepine overdose visits to all visits increased by 23.7% from 2019 (24.22 per 100,000 ED visits) to 2020 (29.97 per 100,000), with the larger share going to those involving opioids, which were up by 34.4%, compared with overdose visits not involving opioids (21.0%), the investigators said, based on data reported by 32 states and the District of Columbia to the CDC’s Drug Overdose Surveillance and Epidemiology system. All of the rate changes are statistically significant.
The number of overdose visits without opioid coinvolvement actually dropped, from 2019 (12,276) to 2020 (12,218), but not by enough to offset the decline in total visits, noted Dr. Liu, of the CDC’s National Center for Injury Prevention and Control and associates.
The number of deaths from benzodiazepine overdose, on the other hand, did not drop in 2020. Those data, coming from 23 states participating in the CDC’s State Unintentional Drug Overdose Reporting System, were available only for the first half of the year.
In those 6 months, The first quarter of 2020 also showed an increase, but exact numbers were not provided in the report. Overdose deaths rose by 22% for prescription forms of benzodiazepine and 520% for illicit forms in Q2 of 2020, compared with 2019, the researchers said.
Almost all of the benzodiazepine deaths (93%) in the first half of 2020 also involved opioids, mostly in the form of illicitly manufactured fentanyls (67% of all deaths). Between Q2 of 2019 and Q2 of 2020, involvement of illicit fentanyls in benzodiazepine overdose deaths increased from almost 57% to 71%, Dr. Liu and associates reported.
“Despite progress in reducing coprescribing [of opioids and benzodiazepines] before 2019, this study suggests a reversal in the decline in benzodiazepine deaths from 2017 to 2019, driven in part by increasing involvement of [illicitly manufactured fentanyls] in benzodiazepine deaths and influxes of illicit benzodiazepines,” they wrote.
FROM MMWR
Number of global deaths by suicide increased over 30 years
The overall global number of deaths by suicide increased by almost 20,000 during the past 30 years, new research shows.
The increase occurred despite a significant decrease in age-specific suicide rates from 1990 through 2019, according to data from the Global Burden of Disease Study 2019.
Population growth, population aging, and changes in population age structure may explain the increase in number of suicide deaths, the investigators note.
“As suicide rates are highest among the elderly (70 years or above) for both genders in almost all regions of the world, the rapidly aging population globally will pose huge challenges for the reduction in the number of suicide deaths in the future,” write the researchers, led by Paul Siu Fai Yip, PhD, of the HKJC Center for Suicide Research and Prevention, University of Hong Kong, China.
The findings were published online Aug. 16 in Injury Prevention.
Global public health concern
Around the world, approximately 800,000 individuals die by suicide each year, while many others attempt suicide. Yet suicide has not received the same level of attention as other global public health concerns, such as HIV/AIDS and cancer, the investigators write.
They examined data from the Global Burden of Disease Study 2019 to assess how demographic and epidemiologic factors contributed to the number of suicide deaths during the past 30 years.
The researchers also analyzed relationships between population growth, population age structure, income level, and gender- and age-specific suicide rates.
The Global Burden of Disease Study 2019 includes information from 204 countries about 369 diseases and injuries by age and gender. The dataset also includes population estimates for each year by location, age group, and gender.
In their analysis, the investigators looked at changes in suicide rates and the number of suicide deaths from 1990 to 2019 by gender and age group in the four income level regions defined by the World Bank. These categories include low-income, lower-middle–income, upper-middle–income, and high-income regions.
Number of deaths versus suicide rates
The number of deaths was 738,799 in 1990 and 758,696 in 2019.
The largest increase in deaths occurred in the lower-middle–income region, where the number of suicide deaths increased by 72,550 (from 232,340 to 304,890).
Population growth (300,942; 1,512.5%) was the major contributor to the overall increase in total number of suicide deaths. The second largest contributor was population age structure (189,512; 952.4%).
However, the effects of these factors were offset to a large extent by the effect of reduction in overall suicide rates (−470,556; −2,364.9%).
Interestingly, the overall suicide rate per 100,000 population decreased from 13.8 in 1990 to 9.8 in 2019.
The upper-middle–income region had the largest decline (−6.25 per 100,000), and the high-income region had the smallest decline (−1.77 per 100,000). Suicide rates also decreased in lower-middle–income (−2.51 per 100,000) and low-income regions (−1.96 per 100,000).
Reasons for the declines across all regions “have yet to be determined,” write the investigators. International efforts coordinated by the United Nations and World Health Organization likely contributed to these declines, they add.
‘Imbalance of resources’
The overall reduction in suicide rate of −4.01 per 100,000 “was mainly due” to reduction in age-specific suicide rates (−6.09; 152%), the researchers report.
This effect was partly offset, however, by the effect of the changing population age structure (2.08; −52%). In the high-income–level region, for example, the reduction in age-specific suicide rate (−3.83; 216.3%) was greater than the increase resulting from the change in population age structure (2.06; −116.3%).
“The overall contribution of population age structure mainly came from the 45-64 (565.2%) and 65+ (528.7%) age groups,” the investigators write. “This effect was observed in middle-income– as well as high-income–level regions, reflecting the global effect of population aging.”
They add that world populations will “experience pronounced and historically unprecedented aging in the coming decades” because of increasing life expectancy and declining fertility.
Men, but not women, had a notable increase in total number of suicide deaths. The significant effect of male population growth (177,128; 890.2% vs. 123,814; 622.3% for women) and male population age structure (120,186; 604.0% vs. 69,325; 348.4%) were the main factors that explained this increase, the investigators note.
However, from 1990 to 2019, the overall suicide rate per 100,000 men decreased from 16.6 to 13.5 (–3.09). The decline in overall suicide rate was even greater for women, from 11.0 to 6.1 (–4.91).
This finding was particularly notable in the upper-middle–income region (–8.12 women vs. –4.37 men per 100,000).
“This study highlighted the considerable imbalance of the resources in carrying out suicide prevention work, especially in low-income and middle-income countries,” the investigators write.
“It is time to revisit this situation to ensure that sufficient resources can be redeployed globally to meet the future challenges,” they add.
The study was funded by a Humanities and Social Sciences Prestigious Fellowship, which Dr. Yip received. He declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The overall global number of deaths by suicide increased by almost 20,000 during the past 30 years, new research shows.
The increase occurred despite a significant decrease in age-specific suicide rates from 1990 through 2019, according to data from the Global Burden of Disease Study 2019.
Population growth, population aging, and changes in population age structure may explain the increase in number of suicide deaths, the investigators note.
“As suicide rates are highest among the elderly (70 years or above) for both genders in almost all regions of the world, the rapidly aging population globally will pose huge challenges for the reduction in the number of suicide deaths in the future,” write the researchers, led by Paul Siu Fai Yip, PhD, of the HKJC Center for Suicide Research and Prevention, University of Hong Kong, China.
The findings were published online Aug. 16 in Injury Prevention.
Global public health concern
Around the world, approximately 800,000 individuals die by suicide each year, while many others attempt suicide. Yet suicide has not received the same level of attention as other global public health concerns, such as HIV/AIDS and cancer, the investigators write.
They examined data from the Global Burden of Disease Study 2019 to assess how demographic and epidemiologic factors contributed to the number of suicide deaths during the past 30 years.
The researchers also analyzed relationships between population growth, population age structure, income level, and gender- and age-specific suicide rates.
The Global Burden of Disease Study 2019 includes information from 204 countries about 369 diseases and injuries by age and gender. The dataset also includes population estimates for each year by location, age group, and gender.
In their analysis, the investigators looked at changes in suicide rates and the number of suicide deaths from 1990 to 2019 by gender and age group in the four income level regions defined by the World Bank. These categories include low-income, lower-middle–income, upper-middle–income, and high-income regions.
Number of deaths versus suicide rates
The number of deaths was 738,799 in 1990 and 758,696 in 2019.
The largest increase in deaths occurred in the lower-middle–income region, where the number of suicide deaths increased by 72,550 (from 232,340 to 304,890).
Population growth (300,942; 1,512.5%) was the major contributor to the overall increase in total number of suicide deaths. The second largest contributor was population age structure (189,512; 952.4%).
However, the effects of these factors were offset to a large extent by the effect of reduction in overall suicide rates (−470,556; −2,364.9%).
Interestingly, the overall suicide rate per 100,000 population decreased from 13.8 in 1990 to 9.8 in 2019.
The upper-middle–income region had the largest decline (−6.25 per 100,000), and the high-income region had the smallest decline (−1.77 per 100,000). Suicide rates also decreased in lower-middle–income (−2.51 per 100,000) and low-income regions (−1.96 per 100,000).
Reasons for the declines across all regions “have yet to be determined,” write the investigators. International efforts coordinated by the United Nations and World Health Organization likely contributed to these declines, they add.
‘Imbalance of resources’
The overall reduction in suicide rate of −4.01 per 100,000 “was mainly due” to reduction in age-specific suicide rates (−6.09; 152%), the researchers report.
This effect was partly offset, however, by the effect of the changing population age structure (2.08; −52%). In the high-income–level region, for example, the reduction in age-specific suicide rate (−3.83; 216.3%) was greater than the increase resulting from the change in population age structure (2.06; −116.3%).
“The overall contribution of population age structure mainly came from the 45-64 (565.2%) and 65+ (528.7%) age groups,” the investigators write. “This effect was observed in middle-income– as well as high-income–level regions, reflecting the global effect of population aging.”
They add that world populations will “experience pronounced and historically unprecedented aging in the coming decades” because of increasing life expectancy and declining fertility.
Men, but not women, had a notable increase in total number of suicide deaths. The significant effect of male population growth (177,128; 890.2% vs. 123,814; 622.3% for women) and male population age structure (120,186; 604.0% vs. 69,325; 348.4%) were the main factors that explained this increase, the investigators note.
However, from 1990 to 2019, the overall suicide rate per 100,000 men decreased from 16.6 to 13.5 (–3.09). The decline in overall suicide rate was even greater for women, from 11.0 to 6.1 (–4.91).
This finding was particularly notable in the upper-middle–income region (–8.12 women vs. –4.37 men per 100,000).
“This study highlighted the considerable imbalance of the resources in carrying out suicide prevention work, especially in low-income and middle-income countries,” the investigators write.
“It is time to revisit this situation to ensure that sufficient resources can be redeployed globally to meet the future challenges,” they add.
The study was funded by a Humanities and Social Sciences Prestigious Fellowship, which Dr. Yip received. He declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The overall global number of deaths by suicide increased by almost 20,000 during the past 30 years, new research shows.
The increase occurred despite a significant decrease in age-specific suicide rates from 1990 through 2019, according to data from the Global Burden of Disease Study 2019.
Population growth, population aging, and changes in population age structure may explain the increase in number of suicide deaths, the investigators note.
“As suicide rates are highest among the elderly (70 years or above) for both genders in almost all regions of the world, the rapidly aging population globally will pose huge challenges for the reduction in the number of suicide deaths in the future,” write the researchers, led by Paul Siu Fai Yip, PhD, of the HKJC Center for Suicide Research and Prevention, University of Hong Kong, China.
The findings were published online Aug. 16 in Injury Prevention.
Global public health concern
Around the world, approximately 800,000 individuals die by suicide each year, while many others attempt suicide. Yet suicide has not received the same level of attention as other global public health concerns, such as HIV/AIDS and cancer, the investigators write.
They examined data from the Global Burden of Disease Study 2019 to assess how demographic and epidemiologic factors contributed to the number of suicide deaths during the past 30 years.
The researchers also analyzed relationships between population growth, population age structure, income level, and gender- and age-specific suicide rates.
The Global Burden of Disease Study 2019 includes information from 204 countries about 369 diseases and injuries by age and gender. The dataset also includes population estimates for each year by location, age group, and gender.
In their analysis, the investigators looked at changes in suicide rates and the number of suicide deaths from 1990 to 2019 by gender and age group in the four income level regions defined by the World Bank. These categories include low-income, lower-middle–income, upper-middle–income, and high-income regions.
Number of deaths versus suicide rates
The number of deaths was 738,799 in 1990 and 758,696 in 2019.
The largest increase in deaths occurred in the lower-middle–income region, where the number of suicide deaths increased by 72,550 (from 232,340 to 304,890).
Population growth (300,942; 1,512.5%) was the major contributor to the overall increase in total number of suicide deaths. The second largest contributor was population age structure (189,512; 952.4%).
However, the effects of these factors were offset to a large extent by the effect of reduction in overall suicide rates (−470,556; −2,364.9%).
Interestingly, the overall suicide rate per 100,000 population decreased from 13.8 in 1990 to 9.8 in 2019.
The upper-middle–income region had the largest decline (−6.25 per 100,000), and the high-income region had the smallest decline (−1.77 per 100,000). Suicide rates also decreased in lower-middle–income (−2.51 per 100,000) and low-income regions (−1.96 per 100,000).
Reasons for the declines across all regions “have yet to be determined,” write the investigators. International efforts coordinated by the United Nations and World Health Organization likely contributed to these declines, they add.
‘Imbalance of resources’
The overall reduction in suicide rate of −4.01 per 100,000 “was mainly due” to reduction in age-specific suicide rates (−6.09; 152%), the researchers report.
This effect was partly offset, however, by the effect of the changing population age structure (2.08; −52%). In the high-income–level region, for example, the reduction in age-specific suicide rate (−3.83; 216.3%) was greater than the increase resulting from the change in population age structure (2.06; −116.3%).
“The overall contribution of population age structure mainly came from the 45-64 (565.2%) and 65+ (528.7%) age groups,” the investigators write. “This effect was observed in middle-income– as well as high-income–level regions, reflecting the global effect of population aging.”
They add that world populations will “experience pronounced and historically unprecedented aging in the coming decades” because of increasing life expectancy and declining fertility.
Men, but not women, had a notable increase in total number of suicide deaths. The significant effect of male population growth (177,128; 890.2% vs. 123,814; 622.3% for women) and male population age structure (120,186; 604.0% vs. 69,325; 348.4%) were the main factors that explained this increase, the investigators note.
However, from 1990 to 2019, the overall suicide rate per 100,000 men decreased from 16.6 to 13.5 (–3.09). The decline in overall suicide rate was even greater for women, from 11.0 to 6.1 (–4.91).
This finding was particularly notable in the upper-middle–income region (–8.12 women vs. –4.37 men per 100,000).
“This study highlighted the considerable imbalance of the resources in carrying out suicide prevention work, especially in low-income and middle-income countries,” the investigators write.
“It is time to revisit this situation to ensure that sufficient resources can be redeployed globally to meet the future challenges,” they add.
The study was funded by a Humanities and Social Sciences Prestigious Fellowship, which Dr. Yip received. He declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.