User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Long-haul COVID-19 cases rise as stigma of chronic fatigue taunts
When Margot Gage-Witvliet began feeling run down after her family returned from a trip to the Netherlands in late February 2020, she initially chalked up her symptoms to jet lag. Three days later, however, her situation went from concerning to alarming as she struggled to breathe. “It felt like there was an elephant sitting on my chest,” she said.
Her husband and daughters also became ill with COVID-19, but Ms. Gage-Witvliet was the only one in her family who didn’t get better. After an early improvement, a rare coronavirus-induced tonic-clonic seizure in early April sent her spiraling back down. Ms. Gage-Witvliet spent the next several weeks in bed with the curtains drawn, unable to tolerate light or sound.
Today, Ms. Gage-Witvliet’s life looks nothing like it did 6 months ago when she first got sick. As one of COVID-19’s so called long-haulers, she continues to struggle with crushing fatigue, brain fog, and headaches – symptoms that worsen when she pushes herself to do more. Across the country, as many as 1 in 10 COVID-19 patients are reporting illnesses that continue for weeks and months after their initial diagnosis. Nearly all report neurologic issues like Ms. Gage-Witvliet, as well as shortness of breath and psychiatric concerns.
For Avindra Nath, MD, a neurologist at the National Institutes of Health, the experience of these long-haul COVID-19 patients feels familiar and reminds him of myalgic encephalomyelitis, also known as chronic fatigue syndrome.
Dr. Nath has long been interested in the lingering neurologic issues connected to chronic fatigue. An estimated three-quarters of all patients with chronic fatigue syndrome report that their symptoms started after a viral infection, and they suffer unrelenting exhaustion, difficulties regulating pulse and blood pressure, aches and pains, and brain fog. When Dr. Nath first read about the novel coronavirus, he began to worry that the virus would trigger symptoms in a subset of those infected. Hearing about the experiences of long-haulers like Ms. Gage-Witvliet raised his suspicions even more.
Unlike COVID-19 long-haulers, however, many patients with chronic fatigue syndrome go at least a year with these symptoms before receiving a diagnosis, according to a British survey. That means researchers have had few opportunities to study the early stages of the syndrome. “When we see patients with myalgic encephalomyelitis, whatever infection they might have had occurred in the remote past, so there’s no way for us to know how they got infected with it, what the infection was, or what the effects of it were in that early phase. We’re seeing them 2 years afterward,” Dr. Nath said.
Dr. Nath quickly realized that studying patients like Ms. Gage-Witvliet would give physicians and scientists a unique opportunity to understand not only long-term outcomes of COVID-19 infections, but also other postviral syndromes, including chronic fatigue syndrome at their earliest stages. It’s why Dr. Nath has spent the past several months scrambling to launch two NIH studies to examine the phenomenon.
Although Dr. Nath said that the parallels between COVID-19 long-haulers and those with chronic fatigue syndrome are obvious, he cautions against assuming that they are the same phenomenon. Some long-haulers might simply be taking a much slower path to recovery, or they might have a condition that looks similar on the surface but differs from chronic fatigue syndrome on a molecular level. But even if Dr. Nath fails to see links to chronic fatigue syndrome, with more than 92.5 million documented cases of COVID-19 around the world, the work will be relevant to the substantial number of infected individuals who don’t recover quickly.
“With so many people having exposure to the same virus over a similar time period, we really have the opportunity to look at these manifestations and at the very least to understand postviral syndromes,” said Mady Hornig, MD, a psychiatrist at Columbia University, New York.
The origins of chronic fatigue syndrome date back to 1985, when the Centers for Disease Control and Prevention received a request from two physicians – Paul Cheney, MD, and Daniel Peterson, MD – to investigate a mysterious disease outbreak in Nevada. In November 1984, residents in and around the idyllic vacation spot of Incline Village, a small town tucked into the north shore of Lake Tahoe, had begun reporting flu-like symptoms that persisted for weeks, even months. The doctors had searched high and low for a cause, but they couldn’t figure out what was making their patients sick.
They reported a range of symptoms – including muscle aches and pains, low-grade fevers, sore throats, and headaches – but everyone said that crippling fatigue was the most debilitating issue. This wasn’t the kind of fatigue that could be cured by a nap or even a long holiday. No matter how much their patients slept – and some were almost completely bedbound – their fatigue didn’t abate. What’s more, the fatigue got worse whenever they tried to push themselves to do more. Puzzled, the CDC sent two epidemic intelligence service (EIS) officers to try to get to the bottom of what might be happening.
Muscle aches and pains with crippling fatigue
After their visit to Incline Village, however, the CDC was just as perplexed as Dr. Cheney and Dr. Peterson. Many of the people with the condition reported flu-like symptoms right around the time they first got sick, and the physicians’ leading hypothesis was that the outbreak and its lasting symptoms were caused by chronic Epstein-Barr virus infection. But neither the CDC nor anyone else could identify the infection or any other microbial cause. The two EIS officers duly wrote up a report for the CDC’s flagship publication, Morbidity and Mortality Weekly ReportI, titled “Chronic Fatigue Possibly Related to Epstein-Barr Virus – Nevada”.
That investigators focused on the fatigue aspect made sense, says Leonard A. Jason, PhD, professor of psychology at DePaul University and director of the Center for Community Research, both in Chicago, because it was one of the few symptoms shared by all the individuals studied and it was also the most debilitating. But that focus – and the name “chronic fatigue syndrome” – led to broad public dismissal of the condition’s severity, as did an editorial note in MMWR urging physicians to look for “more definable, and possibly treatable, conditions.” Subsequent research failed to confirm a specific link to the Epstein-Barr virus, which only added to the condition’s phony reputation. Rather than being considered a potentially disabling illness, it was disregarded as a “yuppie flu” or a fancy name for malingering.
“It’s not a surprise that patients are being dismissed because there’s already this sort of grandfathered-in sense that fatigue is not real,” said Jennifer Frankovich, MD, a pediatric rheumatologist at Stanford (Calif.) University’s Lucile Packard Children’s Hospital in Palo Alto. “I’m sure that’s frustrating for them to be tired and then to have the clinician not believe them or dismiss them or think they’re making it up. It would be more helpful to the families to say: ‘You know what, we don’t know, we do not have the answer, and we believe you.’ ”
A syndrome’s shame
As time passed, patient advocacy groups began pushing back against the negative way the condition was being perceived. This criticism came as organizations like the CDC worked to develop a set of diagnostic criteria that researchers and clinicians dealing with chronic fatigue syndrome could use. With such a heterogeneous group of patients and symptoms, the task was no small challenge. The discussions, which took place over nearly 2 decades, played a key role in helping scientists home in on the single factor that was central to chronic fatigue: postexertional malaise.
“This is quite unique for chronic fatigue syndrome. With other diseases, yes, you may have fatigue as one of the components of the disease, but postexertional fatigue is very specific,” said Alain Moreau, PhD, a molecular biologist at the University of Montreal.
Of course, plenty of people have pushed themselves too hard physically and paid the price the next day. But those with chronic fatigue syndrome weren’t running marathons. To them, exertion could be anything from getting the mail to reading a book. Nor could the resulting exhaustion be resolved by an afternoon on the couch or a long vacation.
“If they do these activities, they can crash for weeks, even months,” Dr. Moreau said. It was deep, persistent, and – for 40% of those with chronic fatigue syndrome – disabling. In 2015, a study group from the Institute of Medicine proposed renaming chronic fatigue to “systemic exercise intolerance disease” because of the centrality of this symptom. Although that effort mostly stalled, their report did bring the condition out of its historic place as a scientific backwater. What resulted was an uptick in research on chronic fatigue syndrome, which helped define some of the physiological issues that either contribute to or result from the condition.
Researchers had long known about the link between infection and fatigue, said Dr. Frankovich. Work included mysterious outbreaks like the one in Lake Tahoe and well-documented issues like the wave of encephalitis lethargica (a condition that leaves patients in an almost vegetative state) that followed the 1918 H1N1 influenza pandemic.
“As a clinician, when you see someone who comes in with a chronic infection, they’re tired. I think that’s why, in the chronic-fatigue world, people are desperately looking for the infection so we can treat it, and maybe these poor suffering people will feel better,” Dr. Frankovich added. Then the pandemic struck, giving him yet another opportunity to study postviral syndromes.
Immunologic symptoms
Given the close link between a nonspecific viral illness and the onset of symptoms in chronic fatigue syndrome, scientists like Dr. Hornig opted to focus on immunologic symptoms. In a 2015 analysis published in Science, Dr. Hornig and colleagues showed that immune problems can be found in the earliest stages of chronic fatigue syndrome, and that they change as the illness progresses. Patients who had been sick for less than 3 years showed significant increases in levels of both pro- and anti-inflammatory cytokines, and the factor most strongly correlated to this inability to regulate cytokine levels was the duration of symptoms, not their severity. A series of other studies also revealed problems with regulation of the immune system, although no one could show what might have set these problems in motion.
Other researchers found signs of mitochondrial dysfunction in those with chronic fatigue syndrome. Because mitochondria make energy for cells, it wasn’t an intellectual stretch to believe that glitches in this process could contribute to fatigue. As early as 1991, scientists had discovered signs of mitochondrial degeneration in muscle biopsies from people with chronic fatigue syndrome. Subsequent studies showed that those affected by chronic fatigue were missing segments of mitochondrial DNA and had significantly reduced levels of mitochondrial activity. Although exercise normally improves mitochondrial functioning, the opposite appears to happen in chronic fatigue.
To Dr. Nath, these dual hypotheses aren’t necessarily mutually exclusive. Some studies have hinted that infection with the common human herpesvirus–6 (HHV-6) can lead to an autoimmune condition in which the body makes antibodies against the mitochondria. Mitochondria also play a key role in the ability of the innate immune system to produce interferon and other proinflammatory cytokines. It might also be that the link between immune and mitochondrial problems is more convoluted than originally thought, or that the two systems are affected independent of one another, Dr. Nath said.
Finding answers, especially those that could lead to potential treatments, wouldn’t be easy, however. In 2016, the NIH launched an in-depth study of a small number of individuals with chronic fatigue, hoping to find clues about what the condition was and how it might be treated.
For scientists like Dr. Nath, the NIH study provided a way to get at the underlying biology of chronic fatigue syndrome. Then the pandemic struck, giving him yet another opportunity to study postviral syndromes.
Chronic post-SARS syndrome
In March 2020, retired physician Harvey Moldofsky, MD, began receiving inquiries about a 2011 study he and his colleague, John Patcai, MD, had published in BMC Neurology about something they dubbed “chronic post-SARS syndrome.” The small case-control study, which involved mainly health care workers in Toronto, received little attention when it was first published, but with COVID-19, it was suddenly relevant.
Early clusters of similar cases in Miami made local physicians desperate for Dr. Moldofsky’s expertise. Luckily, he was nearby; he had fled the frigid Canadian winter for the warmth of Sarasota, Fla.
“I had people from various countries around the world writing to me and asking what they should do. And of course I don’t have any answers,” he said. But the study contained one of the world’s only references to the syndrome.
In 2003, a woman arrived in Toronto from Hong Kong. She didn’t know it at the time, but her preairport stay at the Hotel Metropole had infected her with the first SARS (severe acute respiratory syndrome) coronavirus. Her subsequent hospitalization in Toronto sparked a city-wide outbreak of SARS in which 273 people became ill and 44 died. Many of those affected were health care workers, including nurses and respiratory therapists. Although most eventually returned to work, a subset couldn’t. They complained of energy-sapping fatigue, poor sleep, brain fog, and assorted body aches and pains that persisted for more than 18 months. The aches and pains brought them to the attention of Dr. Moldofsky, then director of the Centre for the Study of Pain at the University of Toronto.
His primary interest at the time was fibromyalgia, which caused symptoms similar to those reported by the original SARS long-haulers. Intrigued, Dr. Moldofsky agreed to take a look. Their chest x-rays were clear and the nurses showed no signs of lingering viral infection. Dr. Moldofsky could see that the nurses were ill and suffering, but no lab tests or anything else could identify what was causing their symptoms.
In 2011, Dr. Moldofsky and Dr. Patcai found a strong overlap between chronic SARS, fibromyalgia, and chronic fatigue syndrome when they compared 22 patients with long-term SARS issues with 21 who had fibromyalgia. “Their problems are exactly the same. They have strange symptoms and nobody can figure out what they’re about. And these symptoms are aches and pains, and they have trouble thinking and concentrating,” Dr. Moldofsky said. Reports of COVID-19 long-haulers didn’t surprise Dr. Moldofsky, and he immediately recognized that Nath’s intention to follow these patients could provide insights into both fibromyalgia and chronic fatigue syndrome.
That’s exactly what Dr. Nath is proposing with the two NIH studies. One will focus solely on the neurologic impacts of COVID-19, including stroke, loss of taste and smell, and brain fog. The other will bring patients who have had COVID-19 symptoms for at least 6 months to the NIH Clinical Center for an inpatient stay during which they will undergo detailed physiologic tests.
Scientists around the world are launching their own post–COVID-19 studies. Dr. Moreau’s group in Montreal has laid the groundwork for such an endeavor, and the CoroNerve group in the United Kingdom is monitoring neurologic complications from the coronavirus. Many of them have the same goals as the NIH studies: Leverage the large number of COVID-19 long-haulers to better understand the earliest stages of postviral syndrome.
“At this juncture, after all the reports that we’ve seen so far, I think it’s very unlikely that there will be no relationship whatsoever between COVID-19 and chronic fatigue syndrome,” Dr. Hornig said. “I think there certainly will be some, but again, what’s the scope, what’s the size? And then, of course, even more importantly, if it is happening, what is the mechanism and how is it happening?”
For people like Ms. Gage-Witvliet, the answers can’t come soon enough. For the first time in more than a decade, the full-time professor of epidemiology didn’t prepare to teach this year because she simply can’t. It’s too taxing for her brain to deal with impromptu student questions. Ms. Gage-Witvliet hopes that, by sharing her own experiences with post COVID-19, she can help others.
“In my work, I use data to give a voice to people who don’t have a voice,” she said. “Now, I am one of those people.”
A version of this article first appeared on Medscape.com.
When Margot Gage-Witvliet began feeling run down after her family returned from a trip to the Netherlands in late February 2020, she initially chalked up her symptoms to jet lag. Three days later, however, her situation went from concerning to alarming as she struggled to breathe. “It felt like there was an elephant sitting on my chest,” she said.
Her husband and daughters also became ill with COVID-19, but Ms. Gage-Witvliet was the only one in her family who didn’t get better. After an early improvement, a rare coronavirus-induced tonic-clonic seizure in early April sent her spiraling back down. Ms. Gage-Witvliet spent the next several weeks in bed with the curtains drawn, unable to tolerate light or sound.
Today, Ms. Gage-Witvliet’s life looks nothing like it did 6 months ago when she first got sick. As one of COVID-19’s so called long-haulers, she continues to struggle with crushing fatigue, brain fog, and headaches – symptoms that worsen when she pushes herself to do more. Across the country, as many as 1 in 10 COVID-19 patients are reporting illnesses that continue for weeks and months after their initial diagnosis. Nearly all report neurologic issues like Ms. Gage-Witvliet, as well as shortness of breath and psychiatric concerns.
For Avindra Nath, MD, a neurologist at the National Institutes of Health, the experience of these long-haul COVID-19 patients feels familiar and reminds him of myalgic encephalomyelitis, also known as chronic fatigue syndrome.
Dr. Nath has long been interested in the lingering neurologic issues connected to chronic fatigue. An estimated three-quarters of all patients with chronic fatigue syndrome report that their symptoms started after a viral infection, and they suffer unrelenting exhaustion, difficulties regulating pulse and blood pressure, aches and pains, and brain fog. When Dr. Nath first read about the novel coronavirus, he began to worry that the virus would trigger symptoms in a subset of those infected. Hearing about the experiences of long-haulers like Ms. Gage-Witvliet raised his suspicions even more.
Unlike COVID-19 long-haulers, however, many patients with chronic fatigue syndrome go at least a year with these symptoms before receiving a diagnosis, according to a British survey. That means researchers have had few opportunities to study the early stages of the syndrome. “When we see patients with myalgic encephalomyelitis, whatever infection they might have had occurred in the remote past, so there’s no way for us to know how they got infected with it, what the infection was, or what the effects of it were in that early phase. We’re seeing them 2 years afterward,” Dr. Nath said.
Dr. Nath quickly realized that studying patients like Ms. Gage-Witvliet would give physicians and scientists a unique opportunity to understand not only long-term outcomes of COVID-19 infections, but also other postviral syndromes, including chronic fatigue syndrome at their earliest stages. It’s why Dr. Nath has spent the past several months scrambling to launch two NIH studies to examine the phenomenon.
Although Dr. Nath said that the parallels between COVID-19 long-haulers and those with chronic fatigue syndrome are obvious, he cautions against assuming that they are the same phenomenon. Some long-haulers might simply be taking a much slower path to recovery, or they might have a condition that looks similar on the surface but differs from chronic fatigue syndrome on a molecular level. But even if Dr. Nath fails to see links to chronic fatigue syndrome, with more than 92.5 million documented cases of COVID-19 around the world, the work will be relevant to the substantial number of infected individuals who don’t recover quickly.
“With so many people having exposure to the same virus over a similar time period, we really have the opportunity to look at these manifestations and at the very least to understand postviral syndromes,” said Mady Hornig, MD, a psychiatrist at Columbia University, New York.
The origins of chronic fatigue syndrome date back to 1985, when the Centers for Disease Control and Prevention received a request from two physicians – Paul Cheney, MD, and Daniel Peterson, MD – to investigate a mysterious disease outbreak in Nevada. In November 1984, residents in and around the idyllic vacation spot of Incline Village, a small town tucked into the north shore of Lake Tahoe, had begun reporting flu-like symptoms that persisted for weeks, even months. The doctors had searched high and low for a cause, but they couldn’t figure out what was making their patients sick.
They reported a range of symptoms – including muscle aches and pains, low-grade fevers, sore throats, and headaches – but everyone said that crippling fatigue was the most debilitating issue. This wasn’t the kind of fatigue that could be cured by a nap or even a long holiday. No matter how much their patients slept – and some were almost completely bedbound – their fatigue didn’t abate. What’s more, the fatigue got worse whenever they tried to push themselves to do more. Puzzled, the CDC sent two epidemic intelligence service (EIS) officers to try to get to the bottom of what might be happening.
Muscle aches and pains with crippling fatigue
After their visit to Incline Village, however, the CDC was just as perplexed as Dr. Cheney and Dr. Peterson. Many of the people with the condition reported flu-like symptoms right around the time they first got sick, and the physicians’ leading hypothesis was that the outbreak and its lasting symptoms were caused by chronic Epstein-Barr virus infection. But neither the CDC nor anyone else could identify the infection or any other microbial cause. The two EIS officers duly wrote up a report for the CDC’s flagship publication, Morbidity and Mortality Weekly ReportI, titled “Chronic Fatigue Possibly Related to Epstein-Barr Virus – Nevada”.
That investigators focused on the fatigue aspect made sense, says Leonard A. Jason, PhD, professor of psychology at DePaul University and director of the Center for Community Research, both in Chicago, because it was one of the few symptoms shared by all the individuals studied and it was also the most debilitating. But that focus – and the name “chronic fatigue syndrome” – led to broad public dismissal of the condition’s severity, as did an editorial note in MMWR urging physicians to look for “more definable, and possibly treatable, conditions.” Subsequent research failed to confirm a specific link to the Epstein-Barr virus, which only added to the condition’s phony reputation. Rather than being considered a potentially disabling illness, it was disregarded as a “yuppie flu” or a fancy name for malingering.
“It’s not a surprise that patients are being dismissed because there’s already this sort of grandfathered-in sense that fatigue is not real,” said Jennifer Frankovich, MD, a pediatric rheumatologist at Stanford (Calif.) University’s Lucile Packard Children’s Hospital in Palo Alto. “I’m sure that’s frustrating for them to be tired and then to have the clinician not believe them or dismiss them or think they’re making it up. It would be more helpful to the families to say: ‘You know what, we don’t know, we do not have the answer, and we believe you.’ ”
A syndrome’s shame
As time passed, patient advocacy groups began pushing back against the negative way the condition was being perceived. This criticism came as organizations like the CDC worked to develop a set of diagnostic criteria that researchers and clinicians dealing with chronic fatigue syndrome could use. With such a heterogeneous group of patients and symptoms, the task was no small challenge. The discussions, which took place over nearly 2 decades, played a key role in helping scientists home in on the single factor that was central to chronic fatigue: postexertional malaise.
“This is quite unique for chronic fatigue syndrome. With other diseases, yes, you may have fatigue as one of the components of the disease, but postexertional fatigue is very specific,” said Alain Moreau, PhD, a molecular biologist at the University of Montreal.
Of course, plenty of people have pushed themselves too hard physically and paid the price the next day. But those with chronic fatigue syndrome weren’t running marathons. To them, exertion could be anything from getting the mail to reading a book. Nor could the resulting exhaustion be resolved by an afternoon on the couch or a long vacation.
“If they do these activities, they can crash for weeks, even months,” Dr. Moreau said. It was deep, persistent, and – for 40% of those with chronic fatigue syndrome – disabling. In 2015, a study group from the Institute of Medicine proposed renaming chronic fatigue to “systemic exercise intolerance disease” because of the centrality of this symptom. Although that effort mostly stalled, their report did bring the condition out of its historic place as a scientific backwater. What resulted was an uptick in research on chronic fatigue syndrome, which helped define some of the physiological issues that either contribute to or result from the condition.
Researchers had long known about the link between infection and fatigue, said Dr. Frankovich. Work included mysterious outbreaks like the one in Lake Tahoe and well-documented issues like the wave of encephalitis lethargica (a condition that leaves patients in an almost vegetative state) that followed the 1918 H1N1 influenza pandemic.
“As a clinician, when you see someone who comes in with a chronic infection, they’re tired. I think that’s why, in the chronic-fatigue world, people are desperately looking for the infection so we can treat it, and maybe these poor suffering people will feel better,” Dr. Frankovich added. Then the pandemic struck, giving him yet another opportunity to study postviral syndromes.
Immunologic symptoms
Given the close link between a nonspecific viral illness and the onset of symptoms in chronic fatigue syndrome, scientists like Dr. Hornig opted to focus on immunologic symptoms. In a 2015 analysis published in Science, Dr. Hornig and colleagues showed that immune problems can be found in the earliest stages of chronic fatigue syndrome, and that they change as the illness progresses. Patients who had been sick for less than 3 years showed significant increases in levels of both pro- and anti-inflammatory cytokines, and the factor most strongly correlated to this inability to regulate cytokine levels was the duration of symptoms, not their severity. A series of other studies also revealed problems with regulation of the immune system, although no one could show what might have set these problems in motion.
Other researchers found signs of mitochondrial dysfunction in those with chronic fatigue syndrome. Because mitochondria make energy for cells, it wasn’t an intellectual stretch to believe that glitches in this process could contribute to fatigue. As early as 1991, scientists had discovered signs of mitochondrial degeneration in muscle biopsies from people with chronic fatigue syndrome. Subsequent studies showed that those affected by chronic fatigue were missing segments of mitochondrial DNA and had significantly reduced levels of mitochondrial activity. Although exercise normally improves mitochondrial functioning, the opposite appears to happen in chronic fatigue.
To Dr. Nath, these dual hypotheses aren’t necessarily mutually exclusive. Some studies have hinted that infection with the common human herpesvirus–6 (HHV-6) can lead to an autoimmune condition in which the body makes antibodies against the mitochondria. Mitochondria also play a key role in the ability of the innate immune system to produce interferon and other proinflammatory cytokines. It might also be that the link between immune and mitochondrial problems is more convoluted than originally thought, or that the two systems are affected independent of one another, Dr. Nath said.
Finding answers, especially those that could lead to potential treatments, wouldn’t be easy, however. In 2016, the NIH launched an in-depth study of a small number of individuals with chronic fatigue, hoping to find clues about what the condition was and how it might be treated.
For scientists like Dr. Nath, the NIH study provided a way to get at the underlying biology of chronic fatigue syndrome. Then the pandemic struck, giving him yet another opportunity to study postviral syndromes.
Chronic post-SARS syndrome
In March 2020, retired physician Harvey Moldofsky, MD, began receiving inquiries about a 2011 study he and his colleague, John Patcai, MD, had published in BMC Neurology about something they dubbed “chronic post-SARS syndrome.” The small case-control study, which involved mainly health care workers in Toronto, received little attention when it was first published, but with COVID-19, it was suddenly relevant.
Early clusters of similar cases in Miami made local physicians desperate for Dr. Moldofsky’s expertise. Luckily, he was nearby; he had fled the frigid Canadian winter for the warmth of Sarasota, Fla.
“I had people from various countries around the world writing to me and asking what they should do. And of course I don’t have any answers,” he said. But the study contained one of the world’s only references to the syndrome.
In 2003, a woman arrived in Toronto from Hong Kong. She didn’t know it at the time, but her preairport stay at the Hotel Metropole had infected her with the first SARS (severe acute respiratory syndrome) coronavirus. Her subsequent hospitalization in Toronto sparked a city-wide outbreak of SARS in which 273 people became ill and 44 died. Many of those affected were health care workers, including nurses and respiratory therapists. Although most eventually returned to work, a subset couldn’t. They complained of energy-sapping fatigue, poor sleep, brain fog, and assorted body aches and pains that persisted for more than 18 months. The aches and pains brought them to the attention of Dr. Moldofsky, then director of the Centre for the Study of Pain at the University of Toronto.
His primary interest at the time was fibromyalgia, which caused symptoms similar to those reported by the original SARS long-haulers. Intrigued, Dr. Moldofsky agreed to take a look. Their chest x-rays were clear and the nurses showed no signs of lingering viral infection. Dr. Moldofsky could see that the nurses were ill and suffering, but no lab tests or anything else could identify what was causing their symptoms.
In 2011, Dr. Moldofsky and Dr. Patcai found a strong overlap between chronic SARS, fibromyalgia, and chronic fatigue syndrome when they compared 22 patients with long-term SARS issues with 21 who had fibromyalgia. “Their problems are exactly the same. They have strange symptoms and nobody can figure out what they’re about. And these symptoms are aches and pains, and they have trouble thinking and concentrating,” Dr. Moldofsky said. Reports of COVID-19 long-haulers didn’t surprise Dr. Moldofsky, and he immediately recognized that Nath’s intention to follow these patients could provide insights into both fibromyalgia and chronic fatigue syndrome.
That’s exactly what Dr. Nath is proposing with the two NIH studies. One will focus solely on the neurologic impacts of COVID-19, including stroke, loss of taste and smell, and brain fog. The other will bring patients who have had COVID-19 symptoms for at least 6 months to the NIH Clinical Center for an inpatient stay during which they will undergo detailed physiologic tests.
Scientists around the world are launching their own post–COVID-19 studies. Dr. Moreau’s group in Montreal has laid the groundwork for such an endeavor, and the CoroNerve group in the United Kingdom is monitoring neurologic complications from the coronavirus. Many of them have the same goals as the NIH studies: Leverage the large number of COVID-19 long-haulers to better understand the earliest stages of postviral syndrome.
“At this juncture, after all the reports that we’ve seen so far, I think it’s very unlikely that there will be no relationship whatsoever between COVID-19 and chronic fatigue syndrome,” Dr. Hornig said. “I think there certainly will be some, but again, what’s the scope, what’s the size? And then, of course, even more importantly, if it is happening, what is the mechanism and how is it happening?”
For people like Ms. Gage-Witvliet, the answers can’t come soon enough. For the first time in more than a decade, the full-time professor of epidemiology didn’t prepare to teach this year because she simply can’t. It’s too taxing for her brain to deal with impromptu student questions. Ms. Gage-Witvliet hopes that, by sharing her own experiences with post COVID-19, she can help others.
“In my work, I use data to give a voice to people who don’t have a voice,” she said. “Now, I am one of those people.”
A version of this article first appeared on Medscape.com.
When Margot Gage-Witvliet began feeling run down after her family returned from a trip to the Netherlands in late February 2020, she initially chalked up her symptoms to jet lag. Three days later, however, her situation went from concerning to alarming as she struggled to breathe. “It felt like there was an elephant sitting on my chest,” she said.
Her husband and daughters also became ill with COVID-19, but Ms. Gage-Witvliet was the only one in her family who didn’t get better. After an early improvement, a rare coronavirus-induced tonic-clonic seizure in early April sent her spiraling back down. Ms. Gage-Witvliet spent the next several weeks in bed with the curtains drawn, unable to tolerate light or sound.
Today, Ms. Gage-Witvliet’s life looks nothing like it did 6 months ago when she first got sick. As one of COVID-19’s so called long-haulers, she continues to struggle with crushing fatigue, brain fog, and headaches – symptoms that worsen when she pushes herself to do more. Across the country, as many as 1 in 10 COVID-19 patients are reporting illnesses that continue for weeks and months after their initial diagnosis. Nearly all report neurologic issues like Ms. Gage-Witvliet, as well as shortness of breath and psychiatric concerns.
For Avindra Nath, MD, a neurologist at the National Institutes of Health, the experience of these long-haul COVID-19 patients feels familiar and reminds him of myalgic encephalomyelitis, also known as chronic fatigue syndrome.
Dr. Nath has long been interested in the lingering neurologic issues connected to chronic fatigue. An estimated three-quarters of all patients with chronic fatigue syndrome report that their symptoms started after a viral infection, and they suffer unrelenting exhaustion, difficulties regulating pulse and blood pressure, aches and pains, and brain fog. When Dr. Nath first read about the novel coronavirus, he began to worry that the virus would trigger symptoms in a subset of those infected. Hearing about the experiences of long-haulers like Ms. Gage-Witvliet raised his suspicions even more.
Unlike COVID-19 long-haulers, however, many patients with chronic fatigue syndrome go at least a year with these symptoms before receiving a diagnosis, according to a British survey. That means researchers have had few opportunities to study the early stages of the syndrome. “When we see patients with myalgic encephalomyelitis, whatever infection they might have had occurred in the remote past, so there’s no way for us to know how they got infected with it, what the infection was, or what the effects of it were in that early phase. We’re seeing them 2 years afterward,” Dr. Nath said.
Dr. Nath quickly realized that studying patients like Ms. Gage-Witvliet would give physicians and scientists a unique opportunity to understand not only long-term outcomes of COVID-19 infections, but also other postviral syndromes, including chronic fatigue syndrome at their earliest stages. It’s why Dr. Nath has spent the past several months scrambling to launch two NIH studies to examine the phenomenon.
Although Dr. Nath said that the parallels between COVID-19 long-haulers and those with chronic fatigue syndrome are obvious, he cautions against assuming that they are the same phenomenon. Some long-haulers might simply be taking a much slower path to recovery, or they might have a condition that looks similar on the surface but differs from chronic fatigue syndrome on a molecular level. But even if Dr. Nath fails to see links to chronic fatigue syndrome, with more than 92.5 million documented cases of COVID-19 around the world, the work will be relevant to the substantial number of infected individuals who don’t recover quickly.
“With so many people having exposure to the same virus over a similar time period, we really have the opportunity to look at these manifestations and at the very least to understand postviral syndromes,” said Mady Hornig, MD, a psychiatrist at Columbia University, New York.
The origins of chronic fatigue syndrome date back to 1985, when the Centers for Disease Control and Prevention received a request from two physicians – Paul Cheney, MD, and Daniel Peterson, MD – to investigate a mysterious disease outbreak in Nevada. In November 1984, residents in and around the idyllic vacation spot of Incline Village, a small town tucked into the north shore of Lake Tahoe, had begun reporting flu-like symptoms that persisted for weeks, even months. The doctors had searched high and low for a cause, but they couldn’t figure out what was making their patients sick.
They reported a range of symptoms – including muscle aches and pains, low-grade fevers, sore throats, and headaches – but everyone said that crippling fatigue was the most debilitating issue. This wasn’t the kind of fatigue that could be cured by a nap or even a long holiday. No matter how much their patients slept – and some were almost completely bedbound – their fatigue didn’t abate. What’s more, the fatigue got worse whenever they tried to push themselves to do more. Puzzled, the CDC sent two epidemic intelligence service (EIS) officers to try to get to the bottom of what might be happening.
Muscle aches and pains with crippling fatigue
After their visit to Incline Village, however, the CDC was just as perplexed as Dr. Cheney and Dr. Peterson. Many of the people with the condition reported flu-like symptoms right around the time they first got sick, and the physicians’ leading hypothesis was that the outbreak and its lasting symptoms were caused by chronic Epstein-Barr virus infection. But neither the CDC nor anyone else could identify the infection or any other microbial cause. The two EIS officers duly wrote up a report for the CDC’s flagship publication, Morbidity and Mortality Weekly ReportI, titled “Chronic Fatigue Possibly Related to Epstein-Barr Virus – Nevada”.
That investigators focused on the fatigue aspect made sense, says Leonard A. Jason, PhD, professor of psychology at DePaul University and director of the Center for Community Research, both in Chicago, because it was one of the few symptoms shared by all the individuals studied and it was also the most debilitating. But that focus – and the name “chronic fatigue syndrome” – led to broad public dismissal of the condition’s severity, as did an editorial note in MMWR urging physicians to look for “more definable, and possibly treatable, conditions.” Subsequent research failed to confirm a specific link to the Epstein-Barr virus, which only added to the condition’s phony reputation. Rather than being considered a potentially disabling illness, it was disregarded as a “yuppie flu” or a fancy name for malingering.
“It’s not a surprise that patients are being dismissed because there’s already this sort of grandfathered-in sense that fatigue is not real,” said Jennifer Frankovich, MD, a pediatric rheumatologist at Stanford (Calif.) University’s Lucile Packard Children’s Hospital in Palo Alto. “I’m sure that’s frustrating for them to be tired and then to have the clinician not believe them or dismiss them or think they’re making it up. It would be more helpful to the families to say: ‘You know what, we don’t know, we do not have the answer, and we believe you.’ ”
A syndrome’s shame
As time passed, patient advocacy groups began pushing back against the negative way the condition was being perceived. This criticism came as organizations like the CDC worked to develop a set of diagnostic criteria that researchers and clinicians dealing with chronic fatigue syndrome could use. With such a heterogeneous group of patients and symptoms, the task was no small challenge. The discussions, which took place over nearly 2 decades, played a key role in helping scientists home in on the single factor that was central to chronic fatigue: postexertional malaise.
“This is quite unique for chronic fatigue syndrome. With other diseases, yes, you may have fatigue as one of the components of the disease, but postexertional fatigue is very specific,” said Alain Moreau, PhD, a molecular biologist at the University of Montreal.
Of course, plenty of people have pushed themselves too hard physically and paid the price the next day. But those with chronic fatigue syndrome weren’t running marathons. To them, exertion could be anything from getting the mail to reading a book. Nor could the resulting exhaustion be resolved by an afternoon on the couch or a long vacation.
“If they do these activities, they can crash for weeks, even months,” Dr. Moreau said. It was deep, persistent, and – for 40% of those with chronic fatigue syndrome – disabling. In 2015, a study group from the Institute of Medicine proposed renaming chronic fatigue to “systemic exercise intolerance disease” because of the centrality of this symptom. Although that effort mostly stalled, their report did bring the condition out of its historic place as a scientific backwater. What resulted was an uptick in research on chronic fatigue syndrome, which helped define some of the physiological issues that either contribute to or result from the condition.
Researchers had long known about the link between infection and fatigue, said Dr. Frankovich. Work included mysterious outbreaks like the one in Lake Tahoe and well-documented issues like the wave of encephalitis lethargica (a condition that leaves patients in an almost vegetative state) that followed the 1918 H1N1 influenza pandemic.
“As a clinician, when you see someone who comes in with a chronic infection, they’re tired. I think that’s why, in the chronic-fatigue world, people are desperately looking for the infection so we can treat it, and maybe these poor suffering people will feel better,” Dr. Frankovich added. Then the pandemic struck, giving him yet another opportunity to study postviral syndromes.
Immunologic symptoms
Given the close link between a nonspecific viral illness and the onset of symptoms in chronic fatigue syndrome, scientists like Dr. Hornig opted to focus on immunologic symptoms. In a 2015 analysis published in Science, Dr. Hornig and colleagues showed that immune problems can be found in the earliest stages of chronic fatigue syndrome, and that they change as the illness progresses. Patients who had been sick for less than 3 years showed significant increases in levels of both pro- and anti-inflammatory cytokines, and the factor most strongly correlated to this inability to regulate cytokine levels was the duration of symptoms, not their severity. A series of other studies also revealed problems with regulation of the immune system, although no one could show what might have set these problems in motion.
Other researchers found signs of mitochondrial dysfunction in those with chronic fatigue syndrome. Because mitochondria make energy for cells, it wasn’t an intellectual stretch to believe that glitches in this process could contribute to fatigue. As early as 1991, scientists had discovered signs of mitochondrial degeneration in muscle biopsies from people with chronic fatigue syndrome. Subsequent studies showed that those affected by chronic fatigue were missing segments of mitochondrial DNA and had significantly reduced levels of mitochondrial activity. Although exercise normally improves mitochondrial functioning, the opposite appears to happen in chronic fatigue.
To Dr. Nath, these dual hypotheses aren’t necessarily mutually exclusive. Some studies have hinted that infection with the common human herpesvirus–6 (HHV-6) can lead to an autoimmune condition in which the body makes antibodies against the mitochondria. Mitochondria also play a key role in the ability of the innate immune system to produce interferon and other proinflammatory cytokines. It might also be that the link between immune and mitochondrial problems is more convoluted than originally thought, or that the two systems are affected independent of one another, Dr. Nath said.
Finding answers, especially those that could lead to potential treatments, wouldn’t be easy, however. In 2016, the NIH launched an in-depth study of a small number of individuals with chronic fatigue, hoping to find clues about what the condition was and how it might be treated.
For scientists like Dr. Nath, the NIH study provided a way to get at the underlying biology of chronic fatigue syndrome. Then the pandemic struck, giving him yet another opportunity to study postviral syndromes.
Chronic post-SARS syndrome
In March 2020, retired physician Harvey Moldofsky, MD, began receiving inquiries about a 2011 study he and his colleague, John Patcai, MD, had published in BMC Neurology about something they dubbed “chronic post-SARS syndrome.” The small case-control study, which involved mainly health care workers in Toronto, received little attention when it was first published, but with COVID-19, it was suddenly relevant.
Early clusters of similar cases in Miami made local physicians desperate for Dr. Moldofsky’s expertise. Luckily, he was nearby; he had fled the frigid Canadian winter for the warmth of Sarasota, Fla.
“I had people from various countries around the world writing to me and asking what they should do. And of course I don’t have any answers,” he said. But the study contained one of the world’s only references to the syndrome.
In 2003, a woman arrived in Toronto from Hong Kong. She didn’t know it at the time, but her preairport stay at the Hotel Metropole had infected her with the first SARS (severe acute respiratory syndrome) coronavirus. Her subsequent hospitalization in Toronto sparked a city-wide outbreak of SARS in which 273 people became ill and 44 died. Many of those affected were health care workers, including nurses and respiratory therapists. Although most eventually returned to work, a subset couldn’t. They complained of energy-sapping fatigue, poor sleep, brain fog, and assorted body aches and pains that persisted for more than 18 months. The aches and pains brought them to the attention of Dr. Moldofsky, then director of the Centre for the Study of Pain at the University of Toronto.
His primary interest at the time was fibromyalgia, which caused symptoms similar to those reported by the original SARS long-haulers. Intrigued, Dr. Moldofsky agreed to take a look. Their chest x-rays were clear and the nurses showed no signs of lingering viral infection. Dr. Moldofsky could see that the nurses were ill and suffering, but no lab tests or anything else could identify what was causing their symptoms.
In 2011, Dr. Moldofsky and Dr. Patcai found a strong overlap between chronic SARS, fibromyalgia, and chronic fatigue syndrome when they compared 22 patients with long-term SARS issues with 21 who had fibromyalgia. “Their problems are exactly the same. They have strange symptoms and nobody can figure out what they’re about. And these symptoms are aches and pains, and they have trouble thinking and concentrating,” Dr. Moldofsky said. Reports of COVID-19 long-haulers didn’t surprise Dr. Moldofsky, and he immediately recognized that Nath’s intention to follow these patients could provide insights into both fibromyalgia and chronic fatigue syndrome.
That’s exactly what Dr. Nath is proposing with the two NIH studies. One will focus solely on the neurologic impacts of COVID-19, including stroke, loss of taste and smell, and brain fog. The other will bring patients who have had COVID-19 symptoms for at least 6 months to the NIH Clinical Center for an inpatient stay during which they will undergo detailed physiologic tests.
Scientists around the world are launching their own post–COVID-19 studies. Dr. Moreau’s group in Montreal has laid the groundwork for such an endeavor, and the CoroNerve group in the United Kingdom is monitoring neurologic complications from the coronavirus. Many of them have the same goals as the NIH studies: Leverage the large number of COVID-19 long-haulers to better understand the earliest stages of postviral syndrome.
“At this juncture, after all the reports that we’ve seen so far, I think it’s very unlikely that there will be no relationship whatsoever between COVID-19 and chronic fatigue syndrome,” Dr. Hornig said. “I think there certainly will be some, but again, what’s the scope, what’s the size? And then, of course, even more importantly, if it is happening, what is the mechanism and how is it happening?”
For people like Ms. Gage-Witvliet, the answers can’t come soon enough. For the first time in more than a decade, the full-time professor of epidemiology didn’t prepare to teach this year because she simply can’t. It’s too taxing for her brain to deal with impromptu student questions. Ms. Gage-Witvliet hopes that, by sharing her own experiences with post COVID-19, she can help others.
“In my work, I use data to give a voice to people who don’t have a voice,” she said. “Now, I am one of those people.”
A version of this article first appeared on Medscape.com.
The next likely COVID-19 vaccine has its advantages
Among the multiple vaccine candidates around the globe, next up in the arsenal against COVID-19 is likely the single-dose Ad26.COV2.S vaccine in development from Johnson & Johnson/Janssen, infectious disease experts predict.
And it got closer with promising interim phase 1/2a trial results, published online Jan. 13 in The New England Journal of Medicine.
A single Ad26.COV2.S dose was associated with S-binding and neutralizing antibodies in more than 90% of the participants. The finding was observed in both adults aged 18-55 years and participants 65 and older, as well as for participants given low-dose or high-dose vaccinations.
The results also suggest a durable vaccine response. “The take-home message [includes] a high neutralizing antibody responder rate to a single dose of our Ad26.COV2.S COVID-19 vaccine candidate. In addition, we see that these responses and antibody titers are stable for at least 71 days,” senior study author Hanneke Schuitemaker, PhD, global head of viral vaccine discovery and translational medicine at Johnson & Johnson in Leiden, the Netherlands, said in an interview.
If the single-dose Johnson & Johnson product gains Food and Drug Administration emergency use authorization (EUA), it could significantly boost the number of overall immunizations available. Less stringent storage requirements – only regular refrigeration vs. a need to freeze the Pfizer/BioNTech and Moderna COVID-19 vaccines – is another potential advantage. The Ad26.COV2.S vaccine can be refrigerated for up to 3 months at 36°-46 °F (2°-8 °C).
“Phase 1-2 trial data on the J&J vaccine: If it works as well as the mRNA options, it will have substantial advantages,” Jeremy Faust, MD, an emergency room physician affiliated with Brigham & Women’s Hospital and Harvard Medical School, Boston, tweeted on Jan. 13.
Unlike the Pfizer/BioNTech and Moderna messenger RNA vaccines, the Johnson & Johnson product is a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector encoding a full-length and stabilized SARS-CoV-2 spike (S) protein.
Phase 3 efficacy/safety results pending
Under normal circumstances, phase 3 trial results would not be anticipated within weeks of phase 1/2a trial findings. However, the urgency of the COVID-19 pandemic accelerated the vaccine development process, so preclinical trials were conducted simultaneously and not sequentially. For this reason, phase 3 interim results for the Johnson & Johnson vaccine are expected within weeks, and a company executive told Reuters that the rollout is on track for March.
“We hope to report data from our first phase 3 study, ENSEMBLE, in which we are testing the protective efficacy of a single dose of Ad26.COV2.S, by the end of this month or early February,” Dr. Schuitemaker said.
In the meantime, the phase 1/2a ongoing, multicenter, randomized, double-blind, and placebo-controlled trial interim results have drawn positive reactions.
“Data is highly encouraging and supports the single inoculation approach that makes this vaccine unique,” Carlos del Rio, executive associate dean for Emory University at Grady in Atlanta, wrote in a tweet on Jan. 13.
“Encouraging COVID vaccine data from J&J published [Jan. 13]. Solid antibody, CD4 T cell, and CD8 T cell responses – a nice trifecta of vaccine immune responses to see! And safe!” tweeted Shane Crotty, PhD, vaccine scientist and professor at the La Jolla (Calif.) Institute for Immunology.
First results in 800+ participants
At baseline for the phase 1/2a trial, 2% of the younger group and 1% of the 65+ group were seropositive for SARS-CoV-2 S-specific antibodies.
A total of 402 people in the younger age cohort and 403 in the 65 and older group received a first dose of the Johnson & Johnson vaccine. Many participants also received a second dose 56 days later for a separate trial, ENSEMBLE2, designed to compare safety and efficacy between single- and double-dose regimens. Results of that trial are still pending.
Safety profile
A single dose was associated with a higher incidence of solicited systemic adverse events in the higher vaccine dose group. They also found that grade 3 adverse events decreased with increasing age.
Injection site pain on the day of immunization or the next day was the most common local reaction. The pain generally resolved within 24 hours. Fever was reported by 15% of the low-dose vaccine group and 39% of the high-dose cohort. Fatigue, headache, and myalgia were the most common grade 1 or 2 solicited systemic adverse events reported.
Five serious adverse events were reported, including four that investigators deemed unrelated to vaccination: hypotension, bilateral nephrolithiasis, legionella pneumonia, and one case of worsening of multiple sclerosis. The vaccine-related serious adverse event was a fever that resulted in hospitalization because of suspicion of COVID-19. The patient recovered within 12 hours.
“These data confirm our previous experience with vaccine candidates based on our Ad26 viral vector platform in the younger age group. The almost similar performance in older adults is promising,” Dr. Schuitemaker said.
A potential limitation of the phase 1/2a trial is “the lack of representation of minority groups,” the researchers noted. Johnson & Johnson is working on improving the diversity of study participants “with respect to groups that seem to be affected most by the COVID-19 pandemic.”
AstraZeneca/Oxford vaccine status
The AstraZeneca/Oxford AZD1222 vaccine in development received approval for use in the United Kingdom on Dec. 30. The approval came after Public Health England said the country was facing “unprecedented” levels of infections, the BBC reported. AstraZeneca applied for European Medical Agency approval earlier in the week of Jan. 10, which could lead to more widespread use across Europe.
The status of the vaccine remains uncertain in the United States. A phase 3 trial that started in August was paused for about 6 weeks in September and October after an adverse event in a British volunteer halted studies worldwide. On Oct. 23, the FDA permitted researchers to continue the trial with approximately 40,000 participants.
There was some suggestion in the clinical trials that a half dose of the AstraZeneca vaccine was more effective than a full dose, 90% vs. 62%, but some irregularities in the research require further investigation.
Although the AstraZeneca vaccine is delivered to cells by an adenovirus – as with the Johnson & Johnson product – it is designed to be delivered in two doses 28 days apart, like the administration schedule of the Moderna mRNA vaccine.
A need for speed, and more doses
Regardless of which vaccine product is next to gain an EUA in the United States, many experts agree the COVID-19 vaccine rollouts so far have been problematic, at a time when cases are climbing to record-breaking levels, and likely more related to logistics over administration of the vaccine than production of the doses.
“Lots of doses being manufactured. In December 20 million, January 40 million, February 80 million and J&J hopefully soon to add to the count. The shortage is the number arms not getting vaccinated. Freezers do not get COVID. They do not need all those vaccines,” Daniel Griffin, MD, PhD, an infectious disease expert in Port Washington, N.Y., tweeted on Jan. 12.
“Unfortunately, the rollout has not gone smoothly, partly due to a lack of resources for this distribution phase we’re in,” Andrew T. Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah, Salt Lake City, said during a media briefing Jan. 14 sponsored by the Infectious Diseases Society of America (IDSA).
“We’re concerned about the mismatch between the number of people who are being told they are eligible and the amount of vaccine that is being distributed,” he said.
Complicating the rollout is a directive from U.S. Health and Human Services Secretary Alex Azar that states should start vaccinating everyone 65 and older as well as those with underlying conditions.
Expanding distribution to the 15% of Americans in just this age group is a big challenge, Dr. Pavia said. “We have enough vaccine maybe to vaccinate 40 million by the end of this month. There is a huge disconnect, and that creates a lot of problems.”
“One of the biggest problems is we are trying to do this mass vaccination program in the middle of the biggest surge we’ve ever seen,” Julie Vaishampayan, MD, MPH, chair of the IDSA Public Health Committee, said during the briefing. Without sufficient time for public health officials to plan for vaccinating a larger population, “people will come and stand in extremely long lines.”
Trying to expand immunization access without a proportionate increase in available doses prompted Dr. Vaishampayan to share an analogy from a colleague: “We are trying to fill a lake with a garden hose. Rather than making the lake bigger, what we really need is more water.”
Dr. Pavia emphasized that infectious disease experts “know the measures that work.” Not using masks, physical distancing, and hand hygiene, he said, “is a bit like knowing that really good shark repellents will be available in summer, so I’m going to jump into the ocean covered in blood while the great whites are swimming around.”
An official at the World Health Organization agreed. “Vaccines are coming online and I do believe vaccines will make a huge difference. But they are not here yet in enough quantities and in enough people to make that difference,” Michael Ryan, MB, WHO executive director of health emergencies, said during an online media briefing Jan. 13, held in conjunction with Emory University.
Dr. Ryan predicted that “we’ve got weeks if not months ahead of us in which our weapon is our knowledge ... what we know about this virus, its transmission, and stopping that transmission.
“And as the vaccines roll in, we can hopefully end this horrific pandemic.”
Dr. Schuitemaker reports grants from BARDA during the conduct of the study; personal fees and other from Janssen Vaccines and Prevention, a J&J company, outside the submitted work. Johnson & Johnson and the Biomedical Advanced Research and Development Authority of the Department of Health and Human Services funded the phase 1/2a study.
A version of this article first appeared on Medscape.com.
Among the multiple vaccine candidates around the globe, next up in the arsenal against COVID-19 is likely the single-dose Ad26.COV2.S vaccine in development from Johnson & Johnson/Janssen, infectious disease experts predict.
And it got closer with promising interim phase 1/2a trial results, published online Jan. 13 in The New England Journal of Medicine.
A single Ad26.COV2.S dose was associated with S-binding and neutralizing antibodies in more than 90% of the participants. The finding was observed in both adults aged 18-55 years and participants 65 and older, as well as for participants given low-dose or high-dose vaccinations.
The results also suggest a durable vaccine response. “The take-home message [includes] a high neutralizing antibody responder rate to a single dose of our Ad26.COV2.S COVID-19 vaccine candidate. In addition, we see that these responses and antibody titers are stable for at least 71 days,” senior study author Hanneke Schuitemaker, PhD, global head of viral vaccine discovery and translational medicine at Johnson & Johnson in Leiden, the Netherlands, said in an interview.
If the single-dose Johnson & Johnson product gains Food and Drug Administration emergency use authorization (EUA), it could significantly boost the number of overall immunizations available. Less stringent storage requirements – only regular refrigeration vs. a need to freeze the Pfizer/BioNTech and Moderna COVID-19 vaccines – is another potential advantage. The Ad26.COV2.S vaccine can be refrigerated for up to 3 months at 36°-46 °F (2°-8 °C).
“Phase 1-2 trial data on the J&J vaccine: If it works as well as the mRNA options, it will have substantial advantages,” Jeremy Faust, MD, an emergency room physician affiliated with Brigham & Women’s Hospital and Harvard Medical School, Boston, tweeted on Jan. 13.
Unlike the Pfizer/BioNTech and Moderna messenger RNA vaccines, the Johnson & Johnson product is a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector encoding a full-length and stabilized SARS-CoV-2 spike (S) protein.
Phase 3 efficacy/safety results pending
Under normal circumstances, phase 3 trial results would not be anticipated within weeks of phase 1/2a trial findings. However, the urgency of the COVID-19 pandemic accelerated the vaccine development process, so preclinical trials were conducted simultaneously and not sequentially. For this reason, phase 3 interim results for the Johnson & Johnson vaccine are expected within weeks, and a company executive told Reuters that the rollout is on track for March.
“We hope to report data from our first phase 3 study, ENSEMBLE, in which we are testing the protective efficacy of a single dose of Ad26.COV2.S, by the end of this month or early February,” Dr. Schuitemaker said.
In the meantime, the phase 1/2a ongoing, multicenter, randomized, double-blind, and placebo-controlled trial interim results have drawn positive reactions.
“Data is highly encouraging and supports the single inoculation approach that makes this vaccine unique,” Carlos del Rio, executive associate dean for Emory University at Grady in Atlanta, wrote in a tweet on Jan. 13.
“Encouraging COVID vaccine data from J&J published [Jan. 13]. Solid antibody, CD4 T cell, and CD8 T cell responses – a nice trifecta of vaccine immune responses to see! And safe!” tweeted Shane Crotty, PhD, vaccine scientist and professor at the La Jolla (Calif.) Institute for Immunology.
First results in 800+ participants
At baseline for the phase 1/2a trial, 2% of the younger group and 1% of the 65+ group were seropositive for SARS-CoV-2 S-specific antibodies.
A total of 402 people in the younger age cohort and 403 in the 65 and older group received a first dose of the Johnson & Johnson vaccine. Many participants also received a second dose 56 days later for a separate trial, ENSEMBLE2, designed to compare safety and efficacy between single- and double-dose regimens. Results of that trial are still pending.
Safety profile
A single dose was associated with a higher incidence of solicited systemic adverse events in the higher vaccine dose group. They also found that grade 3 adverse events decreased with increasing age.
Injection site pain on the day of immunization or the next day was the most common local reaction. The pain generally resolved within 24 hours. Fever was reported by 15% of the low-dose vaccine group and 39% of the high-dose cohort. Fatigue, headache, and myalgia were the most common grade 1 or 2 solicited systemic adverse events reported.
Five serious adverse events were reported, including four that investigators deemed unrelated to vaccination: hypotension, bilateral nephrolithiasis, legionella pneumonia, and one case of worsening of multiple sclerosis. The vaccine-related serious adverse event was a fever that resulted in hospitalization because of suspicion of COVID-19. The patient recovered within 12 hours.
“These data confirm our previous experience with vaccine candidates based on our Ad26 viral vector platform in the younger age group. The almost similar performance in older adults is promising,” Dr. Schuitemaker said.
A potential limitation of the phase 1/2a trial is “the lack of representation of minority groups,” the researchers noted. Johnson & Johnson is working on improving the diversity of study participants “with respect to groups that seem to be affected most by the COVID-19 pandemic.”
AstraZeneca/Oxford vaccine status
The AstraZeneca/Oxford AZD1222 vaccine in development received approval for use in the United Kingdom on Dec. 30. The approval came after Public Health England said the country was facing “unprecedented” levels of infections, the BBC reported. AstraZeneca applied for European Medical Agency approval earlier in the week of Jan. 10, which could lead to more widespread use across Europe.
The status of the vaccine remains uncertain in the United States. A phase 3 trial that started in August was paused for about 6 weeks in September and October after an adverse event in a British volunteer halted studies worldwide. On Oct. 23, the FDA permitted researchers to continue the trial with approximately 40,000 participants.
There was some suggestion in the clinical trials that a half dose of the AstraZeneca vaccine was more effective than a full dose, 90% vs. 62%, but some irregularities in the research require further investigation.
Although the AstraZeneca vaccine is delivered to cells by an adenovirus – as with the Johnson & Johnson product – it is designed to be delivered in two doses 28 days apart, like the administration schedule of the Moderna mRNA vaccine.
A need for speed, and more doses
Regardless of which vaccine product is next to gain an EUA in the United States, many experts agree the COVID-19 vaccine rollouts so far have been problematic, at a time when cases are climbing to record-breaking levels, and likely more related to logistics over administration of the vaccine than production of the doses.
“Lots of doses being manufactured. In December 20 million, January 40 million, February 80 million and J&J hopefully soon to add to the count. The shortage is the number arms not getting vaccinated. Freezers do not get COVID. They do not need all those vaccines,” Daniel Griffin, MD, PhD, an infectious disease expert in Port Washington, N.Y., tweeted on Jan. 12.
“Unfortunately, the rollout has not gone smoothly, partly due to a lack of resources for this distribution phase we’re in,” Andrew T. Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah, Salt Lake City, said during a media briefing Jan. 14 sponsored by the Infectious Diseases Society of America (IDSA).
“We’re concerned about the mismatch between the number of people who are being told they are eligible and the amount of vaccine that is being distributed,” he said.
Complicating the rollout is a directive from U.S. Health and Human Services Secretary Alex Azar that states should start vaccinating everyone 65 and older as well as those with underlying conditions.
Expanding distribution to the 15% of Americans in just this age group is a big challenge, Dr. Pavia said. “We have enough vaccine maybe to vaccinate 40 million by the end of this month. There is a huge disconnect, and that creates a lot of problems.”
“One of the biggest problems is we are trying to do this mass vaccination program in the middle of the biggest surge we’ve ever seen,” Julie Vaishampayan, MD, MPH, chair of the IDSA Public Health Committee, said during the briefing. Without sufficient time for public health officials to plan for vaccinating a larger population, “people will come and stand in extremely long lines.”
Trying to expand immunization access without a proportionate increase in available doses prompted Dr. Vaishampayan to share an analogy from a colleague: “We are trying to fill a lake with a garden hose. Rather than making the lake bigger, what we really need is more water.”
Dr. Pavia emphasized that infectious disease experts “know the measures that work.” Not using masks, physical distancing, and hand hygiene, he said, “is a bit like knowing that really good shark repellents will be available in summer, so I’m going to jump into the ocean covered in blood while the great whites are swimming around.”
An official at the World Health Organization agreed. “Vaccines are coming online and I do believe vaccines will make a huge difference. But they are not here yet in enough quantities and in enough people to make that difference,” Michael Ryan, MB, WHO executive director of health emergencies, said during an online media briefing Jan. 13, held in conjunction with Emory University.
Dr. Ryan predicted that “we’ve got weeks if not months ahead of us in which our weapon is our knowledge ... what we know about this virus, its transmission, and stopping that transmission.
“And as the vaccines roll in, we can hopefully end this horrific pandemic.”
Dr. Schuitemaker reports grants from BARDA during the conduct of the study; personal fees and other from Janssen Vaccines and Prevention, a J&J company, outside the submitted work. Johnson & Johnson and the Biomedical Advanced Research and Development Authority of the Department of Health and Human Services funded the phase 1/2a study.
A version of this article first appeared on Medscape.com.
Among the multiple vaccine candidates around the globe, next up in the arsenal against COVID-19 is likely the single-dose Ad26.COV2.S vaccine in development from Johnson & Johnson/Janssen, infectious disease experts predict.
And it got closer with promising interim phase 1/2a trial results, published online Jan. 13 in The New England Journal of Medicine.
A single Ad26.COV2.S dose was associated with S-binding and neutralizing antibodies in more than 90% of the participants. The finding was observed in both adults aged 18-55 years and participants 65 and older, as well as for participants given low-dose or high-dose vaccinations.
The results also suggest a durable vaccine response. “The take-home message [includes] a high neutralizing antibody responder rate to a single dose of our Ad26.COV2.S COVID-19 vaccine candidate. In addition, we see that these responses and antibody titers are stable for at least 71 days,” senior study author Hanneke Schuitemaker, PhD, global head of viral vaccine discovery and translational medicine at Johnson & Johnson in Leiden, the Netherlands, said in an interview.
If the single-dose Johnson & Johnson product gains Food and Drug Administration emergency use authorization (EUA), it could significantly boost the number of overall immunizations available. Less stringent storage requirements – only regular refrigeration vs. a need to freeze the Pfizer/BioNTech and Moderna COVID-19 vaccines – is another potential advantage. The Ad26.COV2.S vaccine can be refrigerated for up to 3 months at 36°-46 °F (2°-8 °C).
“Phase 1-2 trial data on the J&J vaccine: If it works as well as the mRNA options, it will have substantial advantages,” Jeremy Faust, MD, an emergency room physician affiliated with Brigham & Women’s Hospital and Harvard Medical School, Boston, tweeted on Jan. 13.
Unlike the Pfizer/BioNTech and Moderna messenger RNA vaccines, the Johnson & Johnson product is a recombinant, replication-incompetent adenovirus serotype 26 (Ad26) vector encoding a full-length and stabilized SARS-CoV-2 spike (S) protein.
Phase 3 efficacy/safety results pending
Under normal circumstances, phase 3 trial results would not be anticipated within weeks of phase 1/2a trial findings. However, the urgency of the COVID-19 pandemic accelerated the vaccine development process, so preclinical trials were conducted simultaneously and not sequentially. For this reason, phase 3 interim results for the Johnson & Johnson vaccine are expected within weeks, and a company executive told Reuters that the rollout is on track for March.
“We hope to report data from our first phase 3 study, ENSEMBLE, in which we are testing the protective efficacy of a single dose of Ad26.COV2.S, by the end of this month or early February,” Dr. Schuitemaker said.
In the meantime, the phase 1/2a ongoing, multicenter, randomized, double-blind, and placebo-controlled trial interim results have drawn positive reactions.
“Data is highly encouraging and supports the single inoculation approach that makes this vaccine unique,” Carlos del Rio, executive associate dean for Emory University at Grady in Atlanta, wrote in a tweet on Jan. 13.
“Encouraging COVID vaccine data from J&J published [Jan. 13]. Solid antibody, CD4 T cell, and CD8 T cell responses – a nice trifecta of vaccine immune responses to see! And safe!” tweeted Shane Crotty, PhD, vaccine scientist and professor at the La Jolla (Calif.) Institute for Immunology.
First results in 800+ participants
At baseline for the phase 1/2a trial, 2% of the younger group and 1% of the 65+ group were seropositive for SARS-CoV-2 S-specific antibodies.
A total of 402 people in the younger age cohort and 403 in the 65 and older group received a first dose of the Johnson & Johnson vaccine. Many participants also received a second dose 56 days later for a separate trial, ENSEMBLE2, designed to compare safety and efficacy between single- and double-dose regimens. Results of that trial are still pending.
Safety profile
A single dose was associated with a higher incidence of solicited systemic adverse events in the higher vaccine dose group. They also found that grade 3 adverse events decreased with increasing age.
Injection site pain on the day of immunization or the next day was the most common local reaction. The pain generally resolved within 24 hours. Fever was reported by 15% of the low-dose vaccine group and 39% of the high-dose cohort. Fatigue, headache, and myalgia were the most common grade 1 or 2 solicited systemic adverse events reported.
Five serious adverse events were reported, including four that investigators deemed unrelated to vaccination: hypotension, bilateral nephrolithiasis, legionella pneumonia, and one case of worsening of multiple sclerosis. The vaccine-related serious adverse event was a fever that resulted in hospitalization because of suspicion of COVID-19. The patient recovered within 12 hours.
“These data confirm our previous experience with vaccine candidates based on our Ad26 viral vector platform in the younger age group. The almost similar performance in older adults is promising,” Dr. Schuitemaker said.
A potential limitation of the phase 1/2a trial is “the lack of representation of minority groups,” the researchers noted. Johnson & Johnson is working on improving the diversity of study participants “with respect to groups that seem to be affected most by the COVID-19 pandemic.”
AstraZeneca/Oxford vaccine status
The AstraZeneca/Oxford AZD1222 vaccine in development received approval for use in the United Kingdom on Dec. 30. The approval came after Public Health England said the country was facing “unprecedented” levels of infections, the BBC reported. AstraZeneca applied for European Medical Agency approval earlier in the week of Jan. 10, which could lead to more widespread use across Europe.
The status of the vaccine remains uncertain in the United States. A phase 3 trial that started in August was paused for about 6 weeks in September and October after an adverse event in a British volunteer halted studies worldwide. On Oct. 23, the FDA permitted researchers to continue the trial with approximately 40,000 participants.
There was some suggestion in the clinical trials that a half dose of the AstraZeneca vaccine was more effective than a full dose, 90% vs. 62%, but some irregularities in the research require further investigation.
Although the AstraZeneca vaccine is delivered to cells by an adenovirus – as with the Johnson & Johnson product – it is designed to be delivered in two doses 28 days apart, like the administration schedule of the Moderna mRNA vaccine.
A need for speed, and more doses
Regardless of which vaccine product is next to gain an EUA in the United States, many experts agree the COVID-19 vaccine rollouts so far have been problematic, at a time when cases are climbing to record-breaking levels, and likely more related to logistics over administration of the vaccine than production of the doses.
“Lots of doses being manufactured. In December 20 million, January 40 million, February 80 million and J&J hopefully soon to add to the count. The shortage is the number arms not getting vaccinated. Freezers do not get COVID. They do not need all those vaccines,” Daniel Griffin, MD, PhD, an infectious disease expert in Port Washington, N.Y., tweeted on Jan. 12.
“Unfortunately, the rollout has not gone smoothly, partly due to a lack of resources for this distribution phase we’re in,” Andrew T. Pavia, MD, chief of the division of pediatric infectious diseases at the University of Utah, Salt Lake City, said during a media briefing Jan. 14 sponsored by the Infectious Diseases Society of America (IDSA).
“We’re concerned about the mismatch between the number of people who are being told they are eligible and the amount of vaccine that is being distributed,” he said.
Complicating the rollout is a directive from U.S. Health and Human Services Secretary Alex Azar that states should start vaccinating everyone 65 and older as well as those with underlying conditions.
Expanding distribution to the 15% of Americans in just this age group is a big challenge, Dr. Pavia said. “We have enough vaccine maybe to vaccinate 40 million by the end of this month. There is a huge disconnect, and that creates a lot of problems.”
“One of the biggest problems is we are trying to do this mass vaccination program in the middle of the biggest surge we’ve ever seen,” Julie Vaishampayan, MD, MPH, chair of the IDSA Public Health Committee, said during the briefing. Without sufficient time for public health officials to plan for vaccinating a larger population, “people will come and stand in extremely long lines.”
Trying to expand immunization access without a proportionate increase in available doses prompted Dr. Vaishampayan to share an analogy from a colleague: “We are trying to fill a lake with a garden hose. Rather than making the lake bigger, what we really need is more water.”
Dr. Pavia emphasized that infectious disease experts “know the measures that work.” Not using masks, physical distancing, and hand hygiene, he said, “is a bit like knowing that really good shark repellents will be available in summer, so I’m going to jump into the ocean covered in blood while the great whites are swimming around.”
An official at the World Health Organization agreed. “Vaccines are coming online and I do believe vaccines will make a huge difference. But they are not here yet in enough quantities and in enough people to make that difference,” Michael Ryan, MB, WHO executive director of health emergencies, said during an online media briefing Jan. 13, held in conjunction with Emory University.
Dr. Ryan predicted that “we’ve got weeks if not months ahead of us in which our weapon is our knowledge ... what we know about this virus, its transmission, and stopping that transmission.
“And as the vaccines roll in, we can hopefully end this horrific pandemic.”
Dr. Schuitemaker reports grants from BARDA during the conduct of the study; personal fees and other from Janssen Vaccines and Prevention, a J&J company, outside the submitted work. Johnson & Johnson and the Biomedical Advanced Research and Development Authority of the Department of Health and Human Services funded the phase 1/2a study.
A version of this article first appeared on Medscape.com.
COVID-19 symptoms persist months after acute infection
, according to a follow-up study involving 1,733 patients.
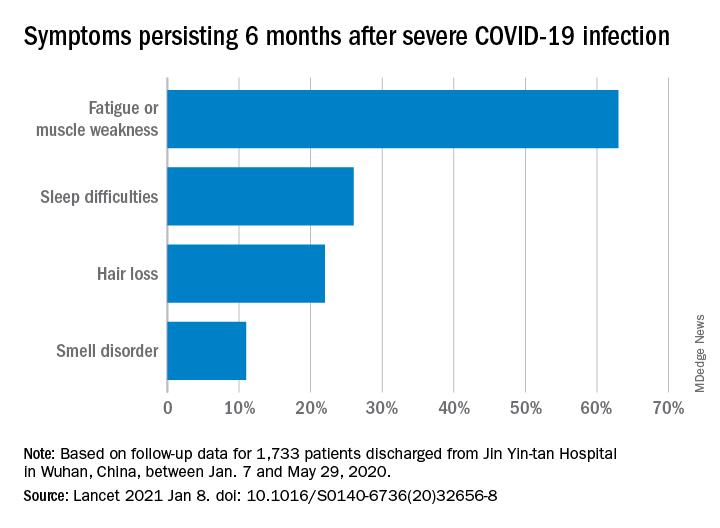
“Patients with COVID-19 had symptoms of fatigue or muscle weakness, sleep difficulties, and anxiety or depression,” and those with “more severe illness during their hospital stay had increasingly impaired pulmonary diffusion capacities and abnormal chest imaging manifestations,” Chaolin Huang, MD, of Jin Yin-tan Hospital in Wuhan, China, and associates wrote in the Lancet.
Fatigue or muscle weakness, reported by 63% of patients, was the most common symptom, followed by sleep difficulties, hair loss, and smell disorder. Altogether, 76% of those examined 6 months after discharge from Jin Yin-tan hospital – the first designated for patients with COVID-19 in Wuhan – reported at least one symptom, they said.
Symptoms were more common in women than men: 81% vs. 73% had at least one symptom, and 66% vs. 59% had fatigue or muscle weakness. Women were also more likely than men to report anxiety or depression at follow-up: 28% vs. 18% (23% overall), the investigators said.
Patients with the most severe COVID-19 were 2.4 times as likely to report any symptom later, compared with those who had the least severe levels of infection. Among the 349 participants who completed a lung function test at follow-up, lung diffusion impairment was seen in 56% of those with the most severe illness and 22% of those with the lowest level, Dr. Huang and associates reported.
In a different subset of 94 patients from whom plasma samples were collected, the “seropositivity and median titres of the neutralising antibodies were significantly lower than at the acute phase,” raising concern for reinfection, they said.
The results of the study, the investigators noted, “support that those with severe disease need post-discharge care. Longer follow-up studies in a larger population are necessary to understand the full spectrum of health consequences from COVID-19.”
, according to a follow-up study involving 1,733 patients.

“Patients with COVID-19 had symptoms of fatigue or muscle weakness, sleep difficulties, and anxiety or depression,” and those with “more severe illness during their hospital stay had increasingly impaired pulmonary diffusion capacities and abnormal chest imaging manifestations,” Chaolin Huang, MD, of Jin Yin-tan Hospital in Wuhan, China, and associates wrote in the Lancet.
Fatigue or muscle weakness, reported by 63% of patients, was the most common symptom, followed by sleep difficulties, hair loss, and smell disorder. Altogether, 76% of those examined 6 months after discharge from Jin Yin-tan hospital – the first designated for patients with COVID-19 in Wuhan – reported at least one symptom, they said.
Symptoms were more common in women than men: 81% vs. 73% had at least one symptom, and 66% vs. 59% had fatigue or muscle weakness. Women were also more likely than men to report anxiety or depression at follow-up: 28% vs. 18% (23% overall), the investigators said.
Patients with the most severe COVID-19 were 2.4 times as likely to report any symptom later, compared with those who had the least severe levels of infection. Among the 349 participants who completed a lung function test at follow-up, lung diffusion impairment was seen in 56% of those with the most severe illness and 22% of those with the lowest level, Dr. Huang and associates reported.
In a different subset of 94 patients from whom plasma samples were collected, the “seropositivity and median titres of the neutralising antibodies were significantly lower than at the acute phase,” raising concern for reinfection, they said.
The results of the study, the investigators noted, “support that those with severe disease need post-discharge care. Longer follow-up studies in a larger population are necessary to understand the full spectrum of health consequences from COVID-19.”
, according to a follow-up study involving 1,733 patients.

“Patients with COVID-19 had symptoms of fatigue or muscle weakness, sleep difficulties, and anxiety or depression,” and those with “more severe illness during their hospital stay had increasingly impaired pulmonary diffusion capacities and abnormal chest imaging manifestations,” Chaolin Huang, MD, of Jin Yin-tan Hospital in Wuhan, China, and associates wrote in the Lancet.
Fatigue or muscle weakness, reported by 63% of patients, was the most common symptom, followed by sleep difficulties, hair loss, and smell disorder. Altogether, 76% of those examined 6 months after discharge from Jin Yin-tan hospital – the first designated for patients with COVID-19 in Wuhan – reported at least one symptom, they said.
Symptoms were more common in women than men: 81% vs. 73% had at least one symptom, and 66% vs. 59% had fatigue or muscle weakness. Women were also more likely than men to report anxiety or depression at follow-up: 28% vs. 18% (23% overall), the investigators said.
Patients with the most severe COVID-19 were 2.4 times as likely to report any symptom later, compared with those who had the least severe levels of infection. Among the 349 participants who completed a lung function test at follow-up, lung diffusion impairment was seen in 56% of those with the most severe illness and 22% of those with the lowest level, Dr. Huang and associates reported.
In a different subset of 94 patients from whom plasma samples were collected, the “seropositivity and median titres of the neutralising antibodies were significantly lower than at the acute phase,” raising concern for reinfection, they said.
The results of the study, the investigators noted, “support that those with severe disease need post-discharge care. Longer follow-up studies in a larger population are necessary to understand the full spectrum of health consequences from COVID-19.”
FROM THE LANCET
CVD deaths rose, imaging declined during pandemic
While the direct toll of the COVID-19 pandemic is being tallied and shared on the nightly news, the indirect effects will undoubtedly take years to fully measure.
In two papers published online Jan. 11 in the Journal of the American College of Cardiology, researchers have started the process of quantifying the impact of the pandemic on the care of patients with cardiovascular disease (CVD).
In the first study, Rishi Wadhera, MD, MPP, MPhil, and colleagues from the Beth Israel Deaconess Medical Center and Harvard Medical School in Boston examined population-level data to determine how deaths from cardiovascular causes changed in the United States in the early months of the pandemic relative to the same periods in 2019.
In a second paper, Andrew J. Einstein, MD, PhD, from Columbia University Irving Medical Center/New York–Presbyterian Hospital and colleagues looked at the pandemic’s international impact on the diagnosis of heart disease.
Using data from the National Center for Health Statistics, Dr. Wadhera and colleagues compared death rates from cardiovascular causes in the United States from March 18, 2020, to June 2, 2020, (the first wave of the pandemic) and from Jan. 1, 2020, to March 17, 2020, (the period just before the pandemic started) and compared them to the same periods in 2019. ICD codes were used to identify underlying causes of death.
Relative to 2019, they found a significant increase in deaths from ischemic heart disease nationally (1.11; 95% confidence interval, 1.04-1.18), as well as an increase in deaths caused by hypertensive disease (1.17; 95% CI, 1.09-1.26). There was no apparent increase in deaths from heart failure, cerebrovascular disease, or other diseases of the circulatory system.
When they looked just at New York City, the area hit hardest during the early part of the pandemic, the relative increases in deaths from ischemic heart disease were more pronounced.
Deaths from ischemic heart disease or hypertensive diseases jumped 139% and 164%, respectively, between March 18, 2020, and June 2, 2020.
More modest increases in deaths were seen in the remainder of New York state, New Jersey, Michigan and Illinois, while Massachusetts and Louisiana did not see a change in cardiovascular deaths.
Several studies from different parts of the world have indicated a 40%-50% drop in hospitalization for myocardial infarction in the initial months of the pandemic, said Dr. Wadhera in an interview.
“We wanted to understand where did all the heart attacks go? And we worried that patients with urgent heart conditions were not seeking the medical care they needed. I think our data suggest that this may have been the case,” reported Dr. Wadhera.
“This very much reflects the reality of what we’re seeing on the ground,” he told this news organization. “After the initial surge ended, when hospital volumes began to return to normal, we saw patients come into the hospital who clearly had a heart attack during the surge months – and were now experiencing complications of that event – because they had initially not come into the hospital due to concerns about exposure to the virus.”
A limitation of their data, he stressed, is whether some deaths coded as CVD deaths were really deaths from undiagnosed COVID-19. “It’s possible that some portion of the increased deaths we observed really reflect the cardiovascular complications of undiagnosed COVID-19, because we know that testing was quite limited during the early first surge of cases.”
“I think that basically three factors – patients avoiding the health care system because of fear of getting COVID, health care systems being strained and overwhelmed leading to the deferral of cardiovascular care and semi-elective procedures, and the cardiovascular complications of COVID-19 itself – all probably collectively contributed to the rise in cardiovascular deaths that we observed,” said Dr. Wadhera.
In an accompanying editorial, Michael N. Young, MD, Geisel School of Medicine at Dartmouth, Lebanon, N.H., and colleagues write that these data, taken together with an earlier study showing an increase in out-of-hospital cardiac arrests at the pandemic peak in New York City, “support the notion of excess fatalities due to unattended comorbid illnesses.” That said, attribution of death in the COVID era “remains problematic.”
In the second article, Andrew Einstein, MD, PhD, and the INCAPS COVID Investigators Group took a broader approach and looked at the impact of COVID-19 on cardiac diagnostic procedures in over 100 countries.
The INCAPS (International Atomic Energy Agency Noninvasive Cardiology Protocols Study) group has for the past decade conducted numerous studies addressing the use of best practices and worldwide practice variation in CVD diagnosis.
For this effort, they sent a survey link to INCAPS participants worldwide, ultimately including 909 survey responses from 108 countries in the final analysis.
Compared with March 2019, overall procedure volume decreased 42% in March 2020 and 64% in April 2020.
The greatest decreases were seen in stress testing (78%) and transesophageal echocardiography (76%), both procedures, noted Dr. Einstein, associated with a greater risk of aerosolization.
“Whether as we reset after COVID we return to the same place in terms of the use of cardiovascular diagnostic testing remains to be seen, but it certainly poses an opportunity to improve our utilization of various modes of testing,” said Dr. Einstein.
Using regression analysis, Dr. Einstein and colleagues were able to see that sites located in low-income and lower-middle-income countries saw an additional 22% reduction in cardiac procedures and less availability of personal protective equipment (PPE) and telehealth.
Fifty-two percent of survey respondents reported significant shortages of N95 masks early in the pandemic, with fewer issues in supplies of gloves, gowns, and face shields. Lower-income countries were more likely to face significant PPE shortages and less likely to be able to implement telehealth strategies to make up for reduced in-person care. PPE shortage itself, however, was not related to lower procedural volume on multivariable regression.
“It all really begs the question of whether there is more that the world can do to help out the developing world in terms of managing the pandemic in all its facets,” said Dr. Einstein in an interview, adding he was “shocked” to learn how difficult it was for some lower-income countries to get sufficient PPE.
Did shutdowns go too far?
Calling this a “remarkable study,” an editorial written by Darryl P. Leong, MBBS, PhD, John W. Eikelboom, MBBS, and Salim Yusuf, MBBS, DPhil, all from McMaster University, Hamilton, Ont., suggests that perhaps health systems in some places went too far in closing down during the first wave of the pandemic, naming specifically Canada, Eastern Europe, and Saudi Arabia as examples.
“Although these measures were taken to prepare for the worst, overwhelming numbers of patients with COVID-19 did not materialize during the first wave of the pandemic in these countries. It is possible that delaying so-called nonessential services may have been unnecessary and potentially harmful, because it likely led to delays in providing care for the treatment of serious non–COVID-19 illnesses.”
Since then, more experience and more data have largely allowed hospital systems to “tackle the ebb and flow” of COVID-19 cases in ways that limit shutdowns of important health services, they said.
Given the more pronounced effect in low- and middle-income countries, they stressed the need to focus resources on ways to promote prevention and treatment that do not rely on diagnostic procedures.
“This calls for more emphasis on developing efficient systems of telehealth, especially in poorer countries or in remote settings in all countries,” Dr. Leong and colleagues conclude.
Dr. Wadhera has reported research support from the National Heart, Lung, and Blood Institute, along with fellow senior author Robert W. Yeh, MD, MBA, who has also received personal fees and grants from several companies not related to the submitted work. Dr. Einstein, Dr. Leong, Dr. Eikelboom, and Dr. Yusuf have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
While the direct toll of the COVID-19 pandemic is being tallied and shared on the nightly news, the indirect effects will undoubtedly take years to fully measure.
In two papers published online Jan. 11 in the Journal of the American College of Cardiology, researchers have started the process of quantifying the impact of the pandemic on the care of patients with cardiovascular disease (CVD).
In the first study, Rishi Wadhera, MD, MPP, MPhil, and colleagues from the Beth Israel Deaconess Medical Center and Harvard Medical School in Boston examined population-level data to determine how deaths from cardiovascular causes changed in the United States in the early months of the pandemic relative to the same periods in 2019.
In a second paper, Andrew J. Einstein, MD, PhD, from Columbia University Irving Medical Center/New York–Presbyterian Hospital and colleagues looked at the pandemic’s international impact on the diagnosis of heart disease.
Using data from the National Center for Health Statistics, Dr. Wadhera and colleagues compared death rates from cardiovascular causes in the United States from March 18, 2020, to June 2, 2020, (the first wave of the pandemic) and from Jan. 1, 2020, to March 17, 2020, (the period just before the pandemic started) and compared them to the same periods in 2019. ICD codes were used to identify underlying causes of death.
Relative to 2019, they found a significant increase in deaths from ischemic heart disease nationally (1.11; 95% confidence interval, 1.04-1.18), as well as an increase in deaths caused by hypertensive disease (1.17; 95% CI, 1.09-1.26). There was no apparent increase in deaths from heart failure, cerebrovascular disease, or other diseases of the circulatory system.
When they looked just at New York City, the area hit hardest during the early part of the pandemic, the relative increases in deaths from ischemic heart disease were more pronounced.
Deaths from ischemic heart disease or hypertensive diseases jumped 139% and 164%, respectively, between March 18, 2020, and June 2, 2020.
More modest increases in deaths were seen in the remainder of New York state, New Jersey, Michigan and Illinois, while Massachusetts and Louisiana did not see a change in cardiovascular deaths.
Several studies from different parts of the world have indicated a 40%-50% drop in hospitalization for myocardial infarction in the initial months of the pandemic, said Dr. Wadhera in an interview.
“We wanted to understand where did all the heart attacks go? And we worried that patients with urgent heart conditions were not seeking the medical care they needed. I think our data suggest that this may have been the case,” reported Dr. Wadhera.
“This very much reflects the reality of what we’re seeing on the ground,” he told this news organization. “After the initial surge ended, when hospital volumes began to return to normal, we saw patients come into the hospital who clearly had a heart attack during the surge months – and were now experiencing complications of that event – because they had initially not come into the hospital due to concerns about exposure to the virus.”
A limitation of their data, he stressed, is whether some deaths coded as CVD deaths were really deaths from undiagnosed COVID-19. “It’s possible that some portion of the increased deaths we observed really reflect the cardiovascular complications of undiagnosed COVID-19, because we know that testing was quite limited during the early first surge of cases.”
“I think that basically three factors – patients avoiding the health care system because of fear of getting COVID, health care systems being strained and overwhelmed leading to the deferral of cardiovascular care and semi-elective procedures, and the cardiovascular complications of COVID-19 itself – all probably collectively contributed to the rise in cardiovascular deaths that we observed,” said Dr. Wadhera.
In an accompanying editorial, Michael N. Young, MD, Geisel School of Medicine at Dartmouth, Lebanon, N.H., and colleagues write that these data, taken together with an earlier study showing an increase in out-of-hospital cardiac arrests at the pandemic peak in New York City, “support the notion of excess fatalities due to unattended comorbid illnesses.” That said, attribution of death in the COVID era “remains problematic.”
In the second article, Andrew Einstein, MD, PhD, and the INCAPS COVID Investigators Group took a broader approach and looked at the impact of COVID-19 on cardiac diagnostic procedures in over 100 countries.
The INCAPS (International Atomic Energy Agency Noninvasive Cardiology Protocols Study) group has for the past decade conducted numerous studies addressing the use of best practices and worldwide practice variation in CVD diagnosis.
For this effort, they sent a survey link to INCAPS participants worldwide, ultimately including 909 survey responses from 108 countries in the final analysis.
Compared with March 2019, overall procedure volume decreased 42% in March 2020 and 64% in April 2020.
The greatest decreases were seen in stress testing (78%) and transesophageal echocardiography (76%), both procedures, noted Dr. Einstein, associated with a greater risk of aerosolization.
“Whether as we reset after COVID we return to the same place in terms of the use of cardiovascular diagnostic testing remains to be seen, but it certainly poses an opportunity to improve our utilization of various modes of testing,” said Dr. Einstein.
Using regression analysis, Dr. Einstein and colleagues were able to see that sites located in low-income and lower-middle-income countries saw an additional 22% reduction in cardiac procedures and less availability of personal protective equipment (PPE) and telehealth.
Fifty-two percent of survey respondents reported significant shortages of N95 masks early in the pandemic, with fewer issues in supplies of gloves, gowns, and face shields. Lower-income countries were more likely to face significant PPE shortages and less likely to be able to implement telehealth strategies to make up for reduced in-person care. PPE shortage itself, however, was not related to lower procedural volume on multivariable regression.
“It all really begs the question of whether there is more that the world can do to help out the developing world in terms of managing the pandemic in all its facets,” said Dr. Einstein in an interview, adding he was “shocked” to learn how difficult it was for some lower-income countries to get sufficient PPE.
Did shutdowns go too far?
Calling this a “remarkable study,” an editorial written by Darryl P. Leong, MBBS, PhD, John W. Eikelboom, MBBS, and Salim Yusuf, MBBS, DPhil, all from McMaster University, Hamilton, Ont., suggests that perhaps health systems in some places went too far in closing down during the first wave of the pandemic, naming specifically Canada, Eastern Europe, and Saudi Arabia as examples.
“Although these measures were taken to prepare for the worst, overwhelming numbers of patients with COVID-19 did not materialize during the first wave of the pandemic in these countries. It is possible that delaying so-called nonessential services may have been unnecessary and potentially harmful, because it likely led to delays in providing care for the treatment of serious non–COVID-19 illnesses.”
Since then, more experience and more data have largely allowed hospital systems to “tackle the ebb and flow” of COVID-19 cases in ways that limit shutdowns of important health services, they said.
Given the more pronounced effect in low- and middle-income countries, they stressed the need to focus resources on ways to promote prevention and treatment that do not rely on diagnostic procedures.
“This calls for more emphasis on developing efficient systems of telehealth, especially in poorer countries or in remote settings in all countries,” Dr. Leong and colleagues conclude.
Dr. Wadhera has reported research support from the National Heart, Lung, and Blood Institute, along with fellow senior author Robert W. Yeh, MD, MBA, who has also received personal fees and grants from several companies not related to the submitted work. Dr. Einstein, Dr. Leong, Dr. Eikelboom, and Dr. Yusuf have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
While the direct toll of the COVID-19 pandemic is being tallied and shared on the nightly news, the indirect effects will undoubtedly take years to fully measure.
In two papers published online Jan. 11 in the Journal of the American College of Cardiology, researchers have started the process of quantifying the impact of the pandemic on the care of patients with cardiovascular disease (CVD).
In the first study, Rishi Wadhera, MD, MPP, MPhil, and colleagues from the Beth Israel Deaconess Medical Center and Harvard Medical School in Boston examined population-level data to determine how deaths from cardiovascular causes changed in the United States in the early months of the pandemic relative to the same periods in 2019.
In a second paper, Andrew J. Einstein, MD, PhD, from Columbia University Irving Medical Center/New York–Presbyterian Hospital and colleagues looked at the pandemic’s international impact on the diagnosis of heart disease.
Using data from the National Center for Health Statistics, Dr. Wadhera and colleagues compared death rates from cardiovascular causes in the United States from March 18, 2020, to June 2, 2020, (the first wave of the pandemic) and from Jan. 1, 2020, to March 17, 2020, (the period just before the pandemic started) and compared them to the same periods in 2019. ICD codes were used to identify underlying causes of death.
Relative to 2019, they found a significant increase in deaths from ischemic heart disease nationally (1.11; 95% confidence interval, 1.04-1.18), as well as an increase in deaths caused by hypertensive disease (1.17; 95% CI, 1.09-1.26). There was no apparent increase in deaths from heart failure, cerebrovascular disease, or other diseases of the circulatory system.
When they looked just at New York City, the area hit hardest during the early part of the pandemic, the relative increases in deaths from ischemic heart disease were more pronounced.
Deaths from ischemic heart disease or hypertensive diseases jumped 139% and 164%, respectively, between March 18, 2020, and June 2, 2020.
More modest increases in deaths were seen in the remainder of New York state, New Jersey, Michigan and Illinois, while Massachusetts and Louisiana did not see a change in cardiovascular deaths.
Several studies from different parts of the world have indicated a 40%-50% drop in hospitalization for myocardial infarction in the initial months of the pandemic, said Dr. Wadhera in an interview.
“We wanted to understand where did all the heart attacks go? And we worried that patients with urgent heart conditions were not seeking the medical care they needed. I think our data suggest that this may have been the case,” reported Dr. Wadhera.
“This very much reflects the reality of what we’re seeing on the ground,” he told this news organization. “After the initial surge ended, when hospital volumes began to return to normal, we saw patients come into the hospital who clearly had a heart attack during the surge months – and were now experiencing complications of that event – because they had initially not come into the hospital due to concerns about exposure to the virus.”
A limitation of their data, he stressed, is whether some deaths coded as CVD deaths were really deaths from undiagnosed COVID-19. “It’s possible that some portion of the increased deaths we observed really reflect the cardiovascular complications of undiagnosed COVID-19, because we know that testing was quite limited during the early first surge of cases.”
“I think that basically three factors – patients avoiding the health care system because of fear of getting COVID, health care systems being strained and overwhelmed leading to the deferral of cardiovascular care and semi-elective procedures, and the cardiovascular complications of COVID-19 itself – all probably collectively contributed to the rise in cardiovascular deaths that we observed,” said Dr. Wadhera.
In an accompanying editorial, Michael N. Young, MD, Geisel School of Medicine at Dartmouth, Lebanon, N.H., and colleagues write that these data, taken together with an earlier study showing an increase in out-of-hospital cardiac arrests at the pandemic peak in New York City, “support the notion of excess fatalities due to unattended comorbid illnesses.” That said, attribution of death in the COVID era “remains problematic.”
In the second article, Andrew Einstein, MD, PhD, and the INCAPS COVID Investigators Group took a broader approach and looked at the impact of COVID-19 on cardiac diagnostic procedures in over 100 countries.
The INCAPS (International Atomic Energy Agency Noninvasive Cardiology Protocols Study) group has for the past decade conducted numerous studies addressing the use of best practices and worldwide practice variation in CVD diagnosis.
For this effort, they sent a survey link to INCAPS participants worldwide, ultimately including 909 survey responses from 108 countries in the final analysis.
Compared with March 2019, overall procedure volume decreased 42% in March 2020 and 64% in April 2020.
The greatest decreases were seen in stress testing (78%) and transesophageal echocardiography (76%), both procedures, noted Dr. Einstein, associated with a greater risk of aerosolization.
“Whether as we reset after COVID we return to the same place in terms of the use of cardiovascular diagnostic testing remains to be seen, but it certainly poses an opportunity to improve our utilization of various modes of testing,” said Dr. Einstein.
Using regression analysis, Dr. Einstein and colleagues were able to see that sites located in low-income and lower-middle-income countries saw an additional 22% reduction in cardiac procedures and less availability of personal protective equipment (PPE) and telehealth.
Fifty-two percent of survey respondents reported significant shortages of N95 masks early in the pandemic, with fewer issues in supplies of gloves, gowns, and face shields. Lower-income countries were more likely to face significant PPE shortages and less likely to be able to implement telehealth strategies to make up for reduced in-person care. PPE shortage itself, however, was not related to lower procedural volume on multivariable regression.
“It all really begs the question of whether there is more that the world can do to help out the developing world in terms of managing the pandemic in all its facets,” said Dr. Einstein in an interview, adding he was “shocked” to learn how difficult it was for some lower-income countries to get sufficient PPE.
Did shutdowns go too far?
Calling this a “remarkable study,” an editorial written by Darryl P. Leong, MBBS, PhD, John W. Eikelboom, MBBS, and Salim Yusuf, MBBS, DPhil, all from McMaster University, Hamilton, Ont., suggests that perhaps health systems in some places went too far in closing down during the first wave of the pandemic, naming specifically Canada, Eastern Europe, and Saudi Arabia as examples.
“Although these measures were taken to prepare for the worst, overwhelming numbers of patients with COVID-19 did not materialize during the first wave of the pandemic in these countries. It is possible that delaying so-called nonessential services may have been unnecessary and potentially harmful, because it likely led to delays in providing care for the treatment of serious non–COVID-19 illnesses.”
Since then, more experience and more data have largely allowed hospital systems to “tackle the ebb and flow” of COVID-19 cases in ways that limit shutdowns of important health services, they said.
Given the more pronounced effect in low- and middle-income countries, they stressed the need to focus resources on ways to promote prevention and treatment that do not rely on diagnostic procedures.
“This calls for more emphasis on developing efficient systems of telehealth, especially in poorer countries or in remote settings in all countries,” Dr. Leong and colleagues conclude.
Dr. Wadhera has reported research support from the National Heart, Lung, and Blood Institute, along with fellow senior author Robert W. Yeh, MD, MBA, who has also received personal fees and grants from several companies not related to the submitted work. Dr. Einstein, Dr. Leong, Dr. Eikelboom, and Dr. Yusuf have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Natural immunity from COVID-19 ‘may last months’
Infection with the SARS-CoV-2 virus may provide some immunity for at least 5 months, interim results from a study has found.
The first report from the Sarscov2 Immunity & Reinfection Evaluation (SIREN) study suggested that antibodies from people who had recovered from COVID-19 gave at least 83% protection against reinfection compared with people who had not had the disease before.
However, Public Health England (PHE) researchers said some people with antibodies may still be able to carry and transmit the SARS-CoV-2 virus.
‘Strongly encouraged’
Susan Hopkins, PhD, senior medical advisor at PHE, who is leading the study, said the overall findings were good news. She told a briefing hosted by the Science Media Centre: “I am strongly encouraged that people have immunity that is lasting much more than the few months that was speculated before the summer.”
She added: “It allows people to feel that their prior infection will protect them from future infections but at the same time it is not complete protection, and therefore they still need to be careful when they are out and about.”
PHE scientists said they would continue to assess whether protection might last longer than 5 months.
Eleanor Riley, PhD, professor of immunology and infectious disease at the University of Edinburgh, said the report suggested that “natural infection provides short-term protection against COVID-19 that is very similar to that conferred by vaccination.”
Simon Clarke, PhD, associate professor in cellular microbiology at the University of Reading, said: “The concerning finding is that some people who have COVID antibodies appear to still be able to carry the coronavirus and could spread it to others. This means that the vast majority of the population will either need to have natural immunity or have been immunised for us to fully lift restrictions on our lives.”
The analysis took place before the new variant of SARS-CoV-2 became widespread in the UK. The PHE scientists said that further work was underway to establish whether and to what extent antibodies also provide protection from the VOC202012/01 variant.
Healthcare Workers
The SIREN preprint analysed data from 20,787 health care workers from 102 NHS trusts who had undergone antibody and PCR testing from June 18 to November 9, 2020.
Of those, 6614 tested positive for COVID-19 antibodies.
Of the 44 potential reinfections identified, two were designated ‘probable’ and 42 ‘possible’, based on available evidence.
Both of the two individuals classified as probable reinfections reported having experienced COVID-19 symptoms during the first wave of the pandemic but were not tested at the time. Both reported that their symptoms were less severe the second time.
None of the 44 potential reinfection cases were PCR tested during the first wave, but all tested positive for COVID-19 antibodies at the time they were recruited to the study.
Tom Wingfield, PhD, senior clinical lecturer at the Liverpool School of Tropical Medicine, said that given the high risk of SARS-CoV-2 infection for frontline NHS staff, it was “vital that we do all that we can to understand, predict, and prevent risk of SARS-CoV-2 amongst healthcare workers”.
The study will continue to follow participants for 12 months to explore how long any immunity may last, the effectiveness of vaccines, and to what extent people with immunity are able to carry and transmit the virus.
A version of this article first appeared on Medscape.com.
Infection with the SARS-CoV-2 virus may provide some immunity for at least 5 months, interim results from a study has found.
The first report from the Sarscov2 Immunity & Reinfection Evaluation (SIREN) study suggested that antibodies from people who had recovered from COVID-19 gave at least 83% protection against reinfection compared with people who had not had the disease before.
However, Public Health England (PHE) researchers said some people with antibodies may still be able to carry and transmit the SARS-CoV-2 virus.
‘Strongly encouraged’
Susan Hopkins, PhD, senior medical advisor at PHE, who is leading the study, said the overall findings were good news. She told a briefing hosted by the Science Media Centre: “I am strongly encouraged that people have immunity that is lasting much more than the few months that was speculated before the summer.”
She added: “It allows people to feel that their prior infection will protect them from future infections but at the same time it is not complete protection, and therefore they still need to be careful when they are out and about.”
PHE scientists said they would continue to assess whether protection might last longer than 5 months.
Eleanor Riley, PhD, professor of immunology and infectious disease at the University of Edinburgh, said the report suggested that “natural infection provides short-term protection against COVID-19 that is very similar to that conferred by vaccination.”
Simon Clarke, PhD, associate professor in cellular microbiology at the University of Reading, said: “The concerning finding is that some people who have COVID antibodies appear to still be able to carry the coronavirus and could spread it to others. This means that the vast majority of the population will either need to have natural immunity or have been immunised for us to fully lift restrictions on our lives.”
The analysis took place before the new variant of SARS-CoV-2 became widespread in the UK. The PHE scientists said that further work was underway to establish whether and to what extent antibodies also provide protection from the VOC202012/01 variant.
Healthcare Workers
The SIREN preprint analysed data from 20,787 health care workers from 102 NHS trusts who had undergone antibody and PCR testing from June 18 to November 9, 2020.
Of those, 6614 tested positive for COVID-19 antibodies.
Of the 44 potential reinfections identified, two were designated ‘probable’ and 42 ‘possible’, based on available evidence.
Both of the two individuals classified as probable reinfections reported having experienced COVID-19 symptoms during the first wave of the pandemic but were not tested at the time. Both reported that their symptoms were less severe the second time.
None of the 44 potential reinfection cases were PCR tested during the first wave, but all tested positive for COVID-19 antibodies at the time they were recruited to the study.
Tom Wingfield, PhD, senior clinical lecturer at the Liverpool School of Tropical Medicine, said that given the high risk of SARS-CoV-2 infection for frontline NHS staff, it was “vital that we do all that we can to understand, predict, and prevent risk of SARS-CoV-2 amongst healthcare workers”.
The study will continue to follow participants for 12 months to explore how long any immunity may last, the effectiveness of vaccines, and to what extent people with immunity are able to carry and transmit the virus.
A version of this article first appeared on Medscape.com.
Infection with the SARS-CoV-2 virus may provide some immunity for at least 5 months, interim results from a study has found.
The first report from the Sarscov2 Immunity & Reinfection Evaluation (SIREN) study suggested that antibodies from people who had recovered from COVID-19 gave at least 83% protection against reinfection compared with people who had not had the disease before.
However, Public Health England (PHE) researchers said some people with antibodies may still be able to carry and transmit the SARS-CoV-2 virus.
‘Strongly encouraged’
Susan Hopkins, PhD, senior medical advisor at PHE, who is leading the study, said the overall findings were good news. She told a briefing hosted by the Science Media Centre: “I am strongly encouraged that people have immunity that is lasting much more than the few months that was speculated before the summer.”
She added: “It allows people to feel that their prior infection will protect them from future infections but at the same time it is not complete protection, and therefore they still need to be careful when they are out and about.”
PHE scientists said they would continue to assess whether protection might last longer than 5 months.
Eleanor Riley, PhD, professor of immunology and infectious disease at the University of Edinburgh, said the report suggested that “natural infection provides short-term protection against COVID-19 that is very similar to that conferred by vaccination.”
Simon Clarke, PhD, associate professor in cellular microbiology at the University of Reading, said: “The concerning finding is that some people who have COVID antibodies appear to still be able to carry the coronavirus and could spread it to others. This means that the vast majority of the population will either need to have natural immunity or have been immunised for us to fully lift restrictions on our lives.”
The analysis took place before the new variant of SARS-CoV-2 became widespread in the UK. The PHE scientists said that further work was underway to establish whether and to what extent antibodies also provide protection from the VOC202012/01 variant.
Healthcare Workers
The SIREN preprint analysed data from 20,787 health care workers from 102 NHS trusts who had undergone antibody and PCR testing from June 18 to November 9, 2020.
Of those, 6614 tested positive for COVID-19 antibodies.
Of the 44 potential reinfections identified, two were designated ‘probable’ and 42 ‘possible’, based on available evidence.
Both of the two individuals classified as probable reinfections reported having experienced COVID-19 symptoms during the first wave of the pandemic but were not tested at the time. Both reported that their symptoms were less severe the second time.
None of the 44 potential reinfection cases were PCR tested during the first wave, but all tested positive for COVID-19 antibodies at the time they were recruited to the study.
Tom Wingfield, PhD, senior clinical lecturer at the Liverpool School of Tropical Medicine, said that given the high risk of SARS-CoV-2 infection for frontline NHS staff, it was “vital that we do all that we can to understand, predict, and prevent risk of SARS-CoV-2 amongst healthcare workers”.
The study will continue to follow participants for 12 months to explore how long any immunity may last, the effectiveness of vaccines, and to what extent people with immunity are able to carry and transmit the virus.
A version of this article first appeared on Medscape.com.
COVID protections suppressed flu season in U.S.
Last fall, health experts said it was possible the United States could experience an easy 2020-21 flu season because health measures to fight COVID-19 would also thwart the spread of influenza.
It looks like that happened – and then some. Numbers are strikingly low for cases of the flu and other common respiratory and gastrointestinal viruses, health experts told the Washington Post.
“It’s crazy,” Lynnette Brammer, MPH, who leads the domestic influenza surveillance team at the Centers for Disease Control and Prevention, told the Washington Post. “This is my 30th flu season. I never would have expected to see flu activity this low.”
Influenza A, influenza B, parainfluenza, norovirus, respiratory syncytial virus, human metapneumovirus, and the bacteria that cause whooping cough and pneumonia are circulating at near-record-low levels.
As an example, the Washington Post said in the third week of December 2019, the CDC’s network of clinical labs reported 16.2% of almost 30,000 samples tested positive for influenza A. During the same period in 2020, only 0.3% tested positive.
But there’s a possible downside to this suppression of viruses, because flu and other viruses may rebound once the coronavirus is brought under control.
“The best analogy is to a forest fire,” Bryan Grenfell, PhD, an epidemiologist and population biologist at Princeton (N.J.) University, told the Washington Post. “For the fire to spread, it needs to have unburned wood. For epidemics to spread, they require people who haven’t previously been infected. So if people don’t get infected this year by these viruses, they likely will at some point later on.”
American health experts like Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Disease, said last fall that they noticed Australia and other nations in the southern hemisphere had easy flu seasons, apparently because of COVID protection measures. The flu season there runs March through August.
COVID-19 now has a very low presence in Australia, but in recent months the flu has been making a comeback. Flu cases among children aged 5 and younger rose sixfold by December, when such cases are usually at their lowest, the Washington Post said.
“That’s an important cautionary tale for us,” said Kevin Messacar, MD, an infectious disease doctor at Children’s Hospital Colorado, Aurora. “Just because we get through the winter and don’t see much RSV or influenza doesn’t mean we’ll be out of the woods.”
A version of this article first appeared on WebMD.com.
Last fall, health experts said it was possible the United States could experience an easy 2020-21 flu season because health measures to fight COVID-19 would also thwart the spread of influenza.
It looks like that happened – and then some. Numbers are strikingly low for cases of the flu and other common respiratory and gastrointestinal viruses, health experts told the Washington Post.
“It’s crazy,” Lynnette Brammer, MPH, who leads the domestic influenza surveillance team at the Centers for Disease Control and Prevention, told the Washington Post. “This is my 30th flu season. I never would have expected to see flu activity this low.”
Influenza A, influenza B, parainfluenza, norovirus, respiratory syncytial virus, human metapneumovirus, and the bacteria that cause whooping cough and pneumonia are circulating at near-record-low levels.
As an example, the Washington Post said in the third week of December 2019, the CDC’s network of clinical labs reported 16.2% of almost 30,000 samples tested positive for influenza A. During the same period in 2020, only 0.3% tested positive.
But there’s a possible downside to this suppression of viruses, because flu and other viruses may rebound once the coronavirus is brought under control.
“The best analogy is to a forest fire,” Bryan Grenfell, PhD, an epidemiologist and population biologist at Princeton (N.J.) University, told the Washington Post. “For the fire to spread, it needs to have unburned wood. For epidemics to spread, they require people who haven’t previously been infected. So if people don’t get infected this year by these viruses, they likely will at some point later on.”
American health experts like Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Disease, said last fall that they noticed Australia and other nations in the southern hemisphere had easy flu seasons, apparently because of COVID protection measures. The flu season there runs March through August.
COVID-19 now has a very low presence in Australia, but in recent months the flu has been making a comeback. Flu cases among children aged 5 and younger rose sixfold by December, when such cases are usually at their lowest, the Washington Post said.
“That’s an important cautionary tale for us,” said Kevin Messacar, MD, an infectious disease doctor at Children’s Hospital Colorado, Aurora. “Just because we get through the winter and don’t see much RSV or influenza doesn’t mean we’ll be out of the woods.”
A version of this article first appeared on WebMD.com.
Last fall, health experts said it was possible the United States could experience an easy 2020-21 flu season because health measures to fight COVID-19 would also thwart the spread of influenza.
It looks like that happened – and then some. Numbers are strikingly low for cases of the flu and other common respiratory and gastrointestinal viruses, health experts told the Washington Post.
“It’s crazy,” Lynnette Brammer, MPH, who leads the domestic influenza surveillance team at the Centers for Disease Control and Prevention, told the Washington Post. “This is my 30th flu season. I never would have expected to see flu activity this low.”
Influenza A, influenza B, parainfluenza, norovirus, respiratory syncytial virus, human metapneumovirus, and the bacteria that cause whooping cough and pneumonia are circulating at near-record-low levels.
As an example, the Washington Post said in the third week of December 2019, the CDC’s network of clinical labs reported 16.2% of almost 30,000 samples tested positive for influenza A. During the same period in 2020, only 0.3% tested positive.
But there’s a possible downside to this suppression of viruses, because flu and other viruses may rebound once the coronavirus is brought under control.
“The best analogy is to a forest fire,” Bryan Grenfell, PhD, an epidemiologist and population biologist at Princeton (N.J.) University, told the Washington Post. “For the fire to spread, it needs to have unburned wood. For epidemics to spread, they require people who haven’t previously been infected. So if people don’t get infected this year by these viruses, they likely will at some point later on.”
American health experts like Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Disease, said last fall that they noticed Australia and other nations in the southern hemisphere had easy flu seasons, apparently because of COVID protection measures. The flu season there runs March through August.
COVID-19 now has a very low presence in Australia, but in recent months the flu has been making a comeback. Flu cases among children aged 5 and younger rose sixfold by December, when such cases are usually at their lowest, the Washington Post said.
“That’s an important cautionary tale for us,” said Kevin Messacar, MD, an infectious disease doctor at Children’s Hospital Colorado, Aurora. “Just because we get through the winter and don’t see much RSV or influenza doesn’t mean we’ll be out of the woods.”
A version of this article first appeared on WebMD.com.
Childhood smoking and depression contribute to young adult opioid use
Depression and tobacco use in childhood significantly increased the risk for opioid use in young adults, according to data from a prospective study of approximately 1,000 individuals.
Previous research, including the annual Monitoring the Future study, documents opioid use among adolescents in the United States, but childhood risk factors for opioid use in young adults have not been well studied, wrote Lilly Shanahan, PhD, of the University of Zürich, and colleagues.
In a prospective cohort study published in JAMA Pediatrics, the researchers identified 1,252 non-Hispanic White and American Indian opioid-naive individuals aged 9-16 years in rural North Carolina. They interviewed participants and parents up to 7 times between January 1993 and December 2000, and interviewed participants only at ages 19, 21, 25, and 30 years between January 1999 and December 2015.
Overall, 24.2% of study participants had used a nonheroin opioid by age 30 years, and both chronic depression and dysthymia were significantly associated with this use (odds ratios 5.43 and 7.13, respectively).
In addition, 155 participants (8.8%) reported weekly use of a nonheroin opioid, and 95 (6.6%) reported weekly heroin use by age 30 years. Chronic depression and dysthymia also were strongly associated with weekly nonheroin opioid use (OR 8.89 and 11.51, respectively).
In a multivariate analysis, depression, tobacco use, and cannabis use at ages 9-16 years were strongly associated with overall opioid use at ages 19-30 years.
“One possible reason childhood chronic depression increases the risk of later opioid use is self-medication, including the use of psychoactive substances, to alleviate depression,” the researchers noted. In addition, the mood-altering properties of opioids may increase their appeal to depressed youth as a way to relieve impaired reward system function, they said.
Potential mechanisms for the association between early tobacco use and later opioid use include the alterations to neurodevelopment caused by nicotine exposure in adolescence, as well as increased risk for depression, reduced pain thresholds, and use of nicotine as a gateway to harder drugs, the researchers added.
Several childhood risk factors were not associated with young adult opioid use in multivariate analysis in this study, including alcohol use, sociodemographic status, maltreatment, family dysfunction, and anxiety, the researchers wrote. “Previous studies typically measured these risk factors retrospectively or in late adolescence and young adulthood, and most did not consider depressive disorders, which may mediate associations between select childhood risk factors and later opioid use,” they said.
The study findings were limited by several factors, including the inability to distinguish between medical and nonmedical opioid use, the incomplete list of available opioids, and the exclusion of Black participants because of low sample size, the researchers noted. However, the results were strengthened by the longitudinal, community-representative design and the inclusion of up to 11 assessments of opioid use, they said.
“Our findings suggest strong opportunities for early prevention and intervention, including in primary care settings,” using known evidence-based strategies, they concluded.
More screening is needed
“Children in the United States are at high risk of serious adult health issues as a result of childhood factors such as ACEs (adverse childhood experiences),” said Suzanne C. Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H. “This study looks prospectively at other factors in childhood over a long period of time leading to opioid usage, with its serious risks and health consequences including overdose death,” she said. “It is unclear what the effects of COVID-19 will be on the population of children growing up now and how opioid usage might change as a result,” she noted.
“Some of the links to adult usage are predictable, such as depression, tobacco use, and cannabis use in early adolescence,” said Dr. Boulter. “Surprising was the lack of correlation between anxiety, early alcohol use, child mistreatment, and sociodemographic factors with future opioid use,” she said.
The take-home message for clinicians is to screen children and adolescents for factors leading to opioid usage in young adults “with preventive strategies including avoidance of pain medication prescriptions and early referral and treatment for depression and use of cannabis and tobacco products using tools like SBIRT (Screening, Brief Intervention, and Referral to Treatment),” Dr. Boulter emphasized.
As for additional research, “It would be interesting to study e-cigarette usage and see if the correlation with future opioid usage is similar to older tobacco products,” she said. “Also helpful would be to delve deeper into connections between medical or dental diagnoses when opioids were first prescribed and later usage of those products,” Dr. Boulter noted.
The study was supported in part by the by the National Institute of Mental Health and the National Institute on Drug Abuse. The researchers had no financial conflicts to disclose. Dr. Boulter had no disclosures but serves on the Pediatric News Editorial Advisory Board.
Depression and tobacco use in childhood significantly increased the risk for opioid use in young adults, according to data from a prospective study of approximately 1,000 individuals.
Previous research, including the annual Monitoring the Future study, documents opioid use among adolescents in the United States, but childhood risk factors for opioid use in young adults have not been well studied, wrote Lilly Shanahan, PhD, of the University of Zürich, and colleagues.
In a prospective cohort study published in JAMA Pediatrics, the researchers identified 1,252 non-Hispanic White and American Indian opioid-naive individuals aged 9-16 years in rural North Carolina. They interviewed participants and parents up to 7 times between January 1993 and December 2000, and interviewed participants only at ages 19, 21, 25, and 30 years between January 1999 and December 2015.
Overall, 24.2% of study participants had used a nonheroin opioid by age 30 years, and both chronic depression and dysthymia were significantly associated with this use (odds ratios 5.43 and 7.13, respectively).
In addition, 155 participants (8.8%) reported weekly use of a nonheroin opioid, and 95 (6.6%) reported weekly heroin use by age 30 years. Chronic depression and dysthymia also were strongly associated with weekly nonheroin opioid use (OR 8.89 and 11.51, respectively).
In a multivariate analysis, depression, tobacco use, and cannabis use at ages 9-16 years were strongly associated with overall opioid use at ages 19-30 years.
“One possible reason childhood chronic depression increases the risk of later opioid use is self-medication, including the use of psychoactive substances, to alleviate depression,” the researchers noted. In addition, the mood-altering properties of opioids may increase their appeal to depressed youth as a way to relieve impaired reward system function, they said.
Potential mechanisms for the association between early tobacco use and later opioid use include the alterations to neurodevelopment caused by nicotine exposure in adolescence, as well as increased risk for depression, reduced pain thresholds, and use of nicotine as a gateway to harder drugs, the researchers added.
Several childhood risk factors were not associated with young adult opioid use in multivariate analysis in this study, including alcohol use, sociodemographic status, maltreatment, family dysfunction, and anxiety, the researchers wrote. “Previous studies typically measured these risk factors retrospectively or in late adolescence and young adulthood, and most did not consider depressive disorders, which may mediate associations between select childhood risk factors and later opioid use,” they said.
The study findings were limited by several factors, including the inability to distinguish between medical and nonmedical opioid use, the incomplete list of available opioids, and the exclusion of Black participants because of low sample size, the researchers noted. However, the results were strengthened by the longitudinal, community-representative design and the inclusion of up to 11 assessments of opioid use, they said.
“Our findings suggest strong opportunities for early prevention and intervention, including in primary care settings,” using known evidence-based strategies, they concluded.
More screening is needed
“Children in the United States are at high risk of serious adult health issues as a result of childhood factors such as ACEs (adverse childhood experiences),” said Suzanne C. Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H. “This study looks prospectively at other factors in childhood over a long period of time leading to opioid usage, with its serious risks and health consequences including overdose death,” she said. “It is unclear what the effects of COVID-19 will be on the population of children growing up now and how opioid usage might change as a result,” she noted.
“Some of the links to adult usage are predictable, such as depression, tobacco use, and cannabis use in early adolescence,” said Dr. Boulter. “Surprising was the lack of correlation between anxiety, early alcohol use, child mistreatment, and sociodemographic factors with future opioid use,” she said.
The take-home message for clinicians is to screen children and adolescents for factors leading to opioid usage in young adults “with preventive strategies including avoidance of pain medication prescriptions and early referral and treatment for depression and use of cannabis and tobacco products using tools like SBIRT (Screening, Brief Intervention, and Referral to Treatment),” Dr. Boulter emphasized.
As for additional research, “It would be interesting to study e-cigarette usage and see if the correlation with future opioid usage is similar to older tobacco products,” she said. “Also helpful would be to delve deeper into connections between medical or dental diagnoses when opioids were first prescribed and later usage of those products,” Dr. Boulter noted.
The study was supported in part by the by the National Institute of Mental Health and the National Institute on Drug Abuse. The researchers had no financial conflicts to disclose. Dr. Boulter had no disclosures but serves on the Pediatric News Editorial Advisory Board.
Depression and tobacco use in childhood significantly increased the risk for opioid use in young adults, according to data from a prospective study of approximately 1,000 individuals.
Previous research, including the annual Monitoring the Future study, documents opioid use among adolescents in the United States, but childhood risk factors for opioid use in young adults have not been well studied, wrote Lilly Shanahan, PhD, of the University of Zürich, and colleagues.
In a prospective cohort study published in JAMA Pediatrics, the researchers identified 1,252 non-Hispanic White and American Indian opioid-naive individuals aged 9-16 years in rural North Carolina. They interviewed participants and parents up to 7 times between January 1993 and December 2000, and interviewed participants only at ages 19, 21, 25, and 30 years between January 1999 and December 2015.
Overall, 24.2% of study participants had used a nonheroin opioid by age 30 years, and both chronic depression and dysthymia were significantly associated with this use (odds ratios 5.43 and 7.13, respectively).
In addition, 155 participants (8.8%) reported weekly use of a nonheroin opioid, and 95 (6.6%) reported weekly heroin use by age 30 years. Chronic depression and dysthymia also were strongly associated with weekly nonheroin opioid use (OR 8.89 and 11.51, respectively).
In a multivariate analysis, depression, tobacco use, and cannabis use at ages 9-16 years were strongly associated with overall opioid use at ages 19-30 years.
“One possible reason childhood chronic depression increases the risk of later opioid use is self-medication, including the use of psychoactive substances, to alleviate depression,” the researchers noted. In addition, the mood-altering properties of opioids may increase their appeal to depressed youth as a way to relieve impaired reward system function, they said.
Potential mechanisms for the association between early tobacco use and later opioid use include the alterations to neurodevelopment caused by nicotine exposure in adolescence, as well as increased risk for depression, reduced pain thresholds, and use of nicotine as a gateway to harder drugs, the researchers added.
Several childhood risk factors were not associated with young adult opioid use in multivariate analysis in this study, including alcohol use, sociodemographic status, maltreatment, family dysfunction, and anxiety, the researchers wrote. “Previous studies typically measured these risk factors retrospectively or in late adolescence and young adulthood, and most did not consider depressive disorders, which may mediate associations between select childhood risk factors and later opioid use,” they said.
The study findings were limited by several factors, including the inability to distinguish between medical and nonmedical opioid use, the incomplete list of available opioids, and the exclusion of Black participants because of low sample size, the researchers noted. However, the results were strengthened by the longitudinal, community-representative design and the inclusion of up to 11 assessments of opioid use, they said.
“Our findings suggest strong opportunities for early prevention and intervention, including in primary care settings,” using known evidence-based strategies, they concluded.
More screening is needed
“Children in the United States are at high risk of serious adult health issues as a result of childhood factors such as ACEs (adverse childhood experiences),” said Suzanne C. Boulter, MD, of the Geisel School of Medicine at Dartmouth, Hanover, N.H. “This study looks prospectively at other factors in childhood over a long period of time leading to opioid usage, with its serious risks and health consequences including overdose death,” she said. “It is unclear what the effects of COVID-19 will be on the population of children growing up now and how opioid usage might change as a result,” she noted.
“Some of the links to adult usage are predictable, such as depression, tobacco use, and cannabis use in early adolescence,” said Dr. Boulter. “Surprising was the lack of correlation between anxiety, early alcohol use, child mistreatment, and sociodemographic factors with future opioid use,” she said.
The take-home message for clinicians is to screen children and adolescents for factors leading to opioid usage in young adults “with preventive strategies including avoidance of pain medication prescriptions and early referral and treatment for depression and use of cannabis and tobacco products using tools like SBIRT (Screening, Brief Intervention, and Referral to Treatment),” Dr. Boulter emphasized.
As for additional research, “It would be interesting to study e-cigarette usage and see if the correlation with future opioid usage is similar to older tobacco products,” she said. “Also helpful would be to delve deeper into connections between medical or dental diagnoses when opioids were first prescribed and later usage of those products,” Dr. Boulter noted.
The study was supported in part by the by the National Institute of Mental Health and the National Institute on Drug Abuse. The researchers had no financial conflicts to disclose. Dr. Boulter had no disclosures but serves on the Pediatric News Editorial Advisory Board.
FROM JAMA PEDIATRICS
Helping interracial couples navigate racism
Joe and Esi were in the therapist’s office wanting help with their relationship. The therapist had just asked the BIG question: How does race impact your lives?”
Esi began with her story about her ethics class, a story that was at sufficient distance from her life. Depending on her husband’s response, she would move in closer. His somewhat patronizing response made her feel both angry and that he lacked any real understanding.
“Me and a mulatto girl were in ethics class,” said Esi, who grew in Kenya. We had a White professor. He seemed to think I had no education. If you are a woman of color, you are automatically thought to have no education and that you don’t know what you are talking about. He tried to shut me up. When I persisted, I know he thought from the tone of my voice that I was an angry Black woman, even although I am not Black and I am not angry! In this country, if you have any color to your skin, you are called Black and relegated to a certain place: The bottom. I was excited about what he was teaching us, but when everyone looked at me with a certain gaze, like something bad was going to happen, all those White people, just looking, I tightened up inside and sat back down.”
Esi looked down at her folded hands. Her husband, who was White, reached over and reassuringly patted her hands.
“Yes, Esi, they are wrong. They shouldn’t have treated you that way. White people can be insensitive.”
Then, she continued, “Joe, you do the same to me!”
“What do you mean, Esi?” responded Joe, with an innocent and anxious look scanning back and forth between her and the White male therapist.
“Well, Joe, do you really want to hear how I felt last week after we came back from that party at your sister’s house?”
“Yes, Esi.”
“But do you really? Are you sure you want to hear this?”
“Yes, Esi.”
“Remember when we went over, me and the kids sat with the other people of color and you sat with your sister and her side of the family?
“Yes, I remember. What is wrong with that?”
“Well, me and my colored friends got loud and excited, and you shot me a look, like ‘pipe down over there.’ THEN, after we got home, the next day, your sister called you and said that you had better control your wife; she is too loud. Do you remember all that?
“Yes, well you do get loud – especially when you are around your people.”
“So why is it I have to fit in with your relatives and not the other way round? Why do I have to conform to the whiteness in your sister’s world, not the other way round?”
“Well, we were in her house.”
“So if they came to our house and were too quiet, how would you feel if I called them up and said they needed to participate with more enthusiasm?”
“Esi, that’s not fair, and you know it isn’t.”
Esi stops and looks at the therapist.
“So I check myself. It is the same all over, White people imposing their values and beliefs on me, on us. I am not an angry Black woman. I am just frustrated. People, White people, always want an explanation for what they think is my loss of control. You can see when their demeanor changes, they pull back, sit up, back away, fidget, and won’t look you in the eye. All these little tics that show that they are trying to get out of the situation.”
Esi took a breath and saw that her husband and the therapist were listening.
“These signs are ingrained in your brain ... these signs ... I saw it when I first came here to this country. The first time I had a good dose of it ... was in that ethics class in college. You can’t use words that you are accustomed to, ’cause they mean something else here, something bad.”
“Oh, Esi, I am so sorry,” said Joe, looking concerned.
“You may be sorry but you are not willing to stand up for me against your sister and her White values. You want me to conform.”
“What do you want me to do?”
“I want you to call your sister out.”
“But she may not ever speak to me again!”
“OK, don’t then,” and Esi looked down at her hands. She was finished talking. Joe looked at the therapist, waiting for something.
The therapist resisted intervening on the issue. “Keep talking this through,” he instructed them.
Joe could see that Esi had done talking and that it was his move.
“Do you really want me to call my sister out, even if it means that she will not talk to me again?”
“Yes.”
“I don’t know if I can do that.”
The therapist now intervened: “What does that mean to you, Esi, that he doesn’t know if he can do that.”
“It means he doesn’t really love me or value me or even value our mulatto children. What do think our daughter is learning?”
“That’s not true, Esi.”
The therapist, Dr. Swarthmore, watched Esi, who has very a dark, blue-black skin tone, with a flawless complexion and a shapely body. She wears her hair cropped and she looks like that Black model, what’s her name. Joe was short, a little plump with ultra White skin and freckles on his nose. He had been brought up in the Midwest and had had little exposure to Africans before his internship abroad in Kenya. Dr. S. thought he had probably not really thought much about Esi’s dilemma.
Dr. Swarthmore encouraged Esi to talk about her immigration experience.
“Esi, can you talk more about what it is like to be an immigrant from Africa?”
“Well, I just have to check myself so that I can fit in with this White culture. If you want to see how I feel about it, you will have to see an angry Black woman and I have learned not to give you that satisfaction. You will just dismiss me. Please Dr. Swarthmore, can we move on?”
Dr. Swarthmore was caught between his desire to accept her wish to move on and his wish to have her express herself fully. He realized that it was not his desire that mattered; that the couple had to work this out between them if they were going to move forward. So he punted it back to them.
“Esi and Joe, you are both caught in an important dilemma. Esi, you want more respect from your husband and his family. Joe, you do not want to upset your family by confronting them. Is that right? You are both dammed if you do and dammed if you don’t.”
“I agree,” Joe and Esi both said, nodding.
“Do you want to work on this issue?”
They both agreed with equal enthusiasm.
“Ok, can you spend the next 10 minutes to work on this?”
They agreed.
“Ok, let’s start. What skills do you have that can help you resolve this important issue?”
Dr. Swarthmore framed the issue as one to be solved by the couple. The couple discussed that they are usually good at communication and solving problems. This problem is about whether or not Joe is more aligned with his White family than with Esi and their children.
Dr. Swarthmore encouraged them to think about this more deeply and over time; that this is such an important issue that it requires time and deep conversation.
“How do you think you can educate yourselves about the issues at hand?”
Esi’s reading list
1. “Why I’m No Longer Talking to White People About Race” by Reni Eddo-Lodge (London: Bloomsbury, 2018).
2. “Americanah” by Chimamanda Ngozi Adichie (New York: Alfred A. Knopf, 2013).
3. “How to be an Antiracist” by Ibram X. Kendi (New York: Random House, 2019).
Joe suggests that Esi think about what it might mean if his sister and their children were no longer part of their lives. She agrees to do this.
Dr. Swarthmore asks if they can each do their homework before they come back. They agree and thought they could manage that and the book for 2 weeks out.
Dr. Swarthmore decides that he will read one of the books Esi suggested, as he does not know much about racism and White privilege and he wants to learn more. Dr. Swarthmore demonstrates his desire to become more racially sensitive. The following steps can be taken by therapists who want to become more racially sensitive, according to TA Laszloffy and KV Hardy (Fam Process. 2000 Spring;39[1]:35-50):
1. Read and watch movies that address the experience of other cultural groups.
2. Go to and participate in cross-cultural events.
3. Engage in a racial self-exploration process. The following questions can begin the racial identity exploration process:
- How do I define myself racially?
- When did I first become aware of race/skin color in general, and mine in particular?
- What messages did I learn about race/skin color based on that first experience?
- What direct and indirect messages did I receive about race/skin color?
- How did the messages that I received about race/skin color affect how I thought and felt about myself racially?
- What benefits did I gain because of my race/skin color?
- What did I lose because of my race/skin?
- Have I ever dated cross-racially? Why or why not?
- How many friends of a different race do I have?
4. Internal commitment. This means committing to addressing racism in therapeutic encounters.
Lessons learned for psychiatrists
1. Therapeutic space is allocated to discuss the issue.
2. The time is strictly limited to 10 minutes, so the couple won’t feel that their emotions will overwhelm them.
3. The space is to focus on the strengths that they can bring to resolving the issue.
4. Give patients the impression that they can solve this and that it is an important issue.
5. Do not put yourself in the patients’ argument; take neither side.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest. Dr. Heru wrote the article in collaboration with Lynette Ramsingh Barros, artist and social commentator.
Joe and Esi were in the therapist’s office wanting help with their relationship. The therapist had just asked the BIG question: How does race impact your lives?”
Esi began with her story about her ethics class, a story that was at sufficient distance from her life. Depending on her husband’s response, she would move in closer. His somewhat patronizing response made her feel both angry and that he lacked any real understanding.
“Me and a mulatto girl were in ethics class,” said Esi, who grew in Kenya. We had a White professor. He seemed to think I had no education. If you are a woman of color, you are automatically thought to have no education and that you don’t know what you are talking about. He tried to shut me up. When I persisted, I know he thought from the tone of my voice that I was an angry Black woman, even although I am not Black and I am not angry! In this country, if you have any color to your skin, you are called Black and relegated to a certain place: The bottom. I was excited about what he was teaching us, but when everyone looked at me with a certain gaze, like something bad was going to happen, all those White people, just looking, I tightened up inside and sat back down.”
Esi looked down at her folded hands. Her husband, who was White, reached over and reassuringly patted her hands.
“Yes, Esi, they are wrong. They shouldn’t have treated you that way. White people can be insensitive.”
Then, she continued, “Joe, you do the same to me!”
“What do you mean, Esi?” responded Joe, with an innocent and anxious look scanning back and forth between her and the White male therapist.
“Well, Joe, do you really want to hear how I felt last week after we came back from that party at your sister’s house?”
“Yes, Esi.”
“But do you really? Are you sure you want to hear this?”
“Yes, Esi.”
“Remember when we went over, me and the kids sat with the other people of color and you sat with your sister and her side of the family?
“Yes, I remember. What is wrong with that?”
“Well, me and my colored friends got loud and excited, and you shot me a look, like ‘pipe down over there.’ THEN, after we got home, the next day, your sister called you and said that you had better control your wife; she is too loud. Do you remember all that?
“Yes, well you do get loud – especially when you are around your people.”
“So why is it I have to fit in with your relatives and not the other way round? Why do I have to conform to the whiteness in your sister’s world, not the other way round?”
“Well, we were in her house.”
“So if they came to our house and were too quiet, how would you feel if I called them up and said they needed to participate with more enthusiasm?”
“Esi, that’s not fair, and you know it isn’t.”
Esi stops and looks at the therapist.
“So I check myself. It is the same all over, White people imposing their values and beliefs on me, on us. I am not an angry Black woman. I am just frustrated. People, White people, always want an explanation for what they think is my loss of control. You can see when their demeanor changes, they pull back, sit up, back away, fidget, and won’t look you in the eye. All these little tics that show that they are trying to get out of the situation.”
Esi took a breath and saw that her husband and the therapist were listening.
“These signs are ingrained in your brain ... these signs ... I saw it when I first came here to this country. The first time I had a good dose of it ... was in that ethics class in college. You can’t use words that you are accustomed to, ’cause they mean something else here, something bad.”
“Oh, Esi, I am so sorry,” said Joe, looking concerned.
“You may be sorry but you are not willing to stand up for me against your sister and her White values. You want me to conform.”
“What do you want me to do?”
“I want you to call your sister out.”
“But she may not ever speak to me again!”
“OK, don’t then,” and Esi looked down at her hands. She was finished talking. Joe looked at the therapist, waiting for something.
The therapist resisted intervening on the issue. “Keep talking this through,” he instructed them.
Joe could see that Esi had done talking and that it was his move.
“Do you really want me to call my sister out, even if it means that she will not talk to me again?”
“Yes.”
“I don’t know if I can do that.”
The therapist now intervened: “What does that mean to you, Esi, that he doesn’t know if he can do that.”
“It means he doesn’t really love me or value me or even value our mulatto children. What do think our daughter is learning?”
“That’s not true, Esi.”
The therapist, Dr. Swarthmore, watched Esi, who has very a dark, blue-black skin tone, with a flawless complexion and a shapely body. She wears her hair cropped and she looks like that Black model, what’s her name. Joe was short, a little plump with ultra White skin and freckles on his nose. He had been brought up in the Midwest and had had little exposure to Africans before his internship abroad in Kenya. Dr. S. thought he had probably not really thought much about Esi’s dilemma.
Dr. Swarthmore encouraged Esi to talk about her immigration experience.
“Esi, can you talk more about what it is like to be an immigrant from Africa?”
“Well, I just have to check myself so that I can fit in with this White culture. If you want to see how I feel about it, you will have to see an angry Black woman and I have learned not to give you that satisfaction. You will just dismiss me. Please Dr. Swarthmore, can we move on?”
Dr. Swarthmore was caught between his desire to accept her wish to move on and his wish to have her express herself fully. He realized that it was not his desire that mattered; that the couple had to work this out between them if they were going to move forward. So he punted it back to them.
“Esi and Joe, you are both caught in an important dilemma. Esi, you want more respect from your husband and his family. Joe, you do not want to upset your family by confronting them. Is that right? You are both dammed if you do and dammed if you don’t.”
“I agree,” Joe and Esi both said, nodding.
“Do you want to work on this issue?”
They both agreed with equal enthusiasm.
“Ok, can you spend the next 10 minutes to work on this?”
They agreed.
“Ok, let’s start. What skills do you have that can help you resolve this important issue?”
Dr. Swarthmore framed the issue as one to be solved by the couple. The couple discussed that they are usually good at communication and solving problems. This problem is about whether or not Joe is more aligned with his White family than with Esi and their children.
Dr. Swarthmore encouraged them to think about this more deeply and over time; that this is such an important issue that it requires time and deep conversation.
“How do you think you can educate yourselves about the issues at hand?”
Esi’s reading list
1. “Why I’m No Longer Talking to White People About Race” by Reni Eddo-Lodge (London: Bloomsbury, 2018).
2. “Americanah” by Chimamanda Ngozi Adichie (New York: Alfred A. Knopf, 2013).
3. “How to be an Antiracist” by Ibram X. Kendi (New York: Random House, 2019).
Joe suggests that Esi think about what it might mean if his sister and their children were no longer part of their lives. She agrees to do this.
Dr. Swarthmore asks if they can each do their homework before they come back. They agree and thought they could manage that and the book for 2 weeks out.
Dr. Swarthmore decides that he will read one of the books Esi suggested, as he does not know much about racism and White privilege and he wants to learn more. Dr. Swarthmore demonstrates his desire to become more racially sensitive. The following steps can be taken by therapists who want to become more racially sensitive, according to TA Laszloffy and KV Hardy (Fam Process. 2000 Spring;39[1]:35-50):
1. Read and watch movies that address the experience of other cultural groups.
2. Go to and participate in cross-cultural events.
3. Engage in a racial self-exploration process. The following questions can begin the racial identity exploration process:
- How do I define myself racially?
- When did I first become aware of race/skin color in general, and mine in particular?
- What messages did I learn about race/skin color based on that first experience?
- What direct and indirect messages did I receive about race/skin color?
- How did the messages that I received about race/skin color affect how I thought and felt about myself racially?
- What benefits did I gain because of my race/skin color?
- What did I lose because of my race/skin?
- Have I ever dated cross-racially? Why or why not?
- How many friends of a different race do I have?
4. Internal commitment. This means committing to addressing racism in therapeutic encounters.
Lessons learned for psychiatrists
1. Therapeutic space is allocated to discuss the issue.
2. The time is strictly limited to 10 minutes, so the couple won’t feel that their emotions will overwhelm them.
3. The space is to focus on the strengths that they can bring to resolving the issue.
4. Give patients the impression that they can solve this and that it is an important issue.
5. Do not put yourself in the patients’ argument; take neither side.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest. Dr. Heru wrote the article in collaboration with Lynette Ramsingh Barros, artist and social commentator.
Joe and Esi were in the therapist’s office wanting help with their relationship. The therapist had just asked the BIG question: How does race impact your lives?”
Esi began with her story about her ethics class, a story that was at sufficient distance from her life. Depending on her husband’s response, she would move in closer. His somewhat patronizing response made her feel both angry and that he lacked any real understanding.
“Me and a mulatto girl were in ethics class,” said Esi, who grew in Kenya. We had a White professor. He seemed to think I had no education. If you are a woman of color, you are automatically thought to have no education and that you don’t know what you are talking about. He tried to shut me up. When I persisted, I know he thought from the tone of my voice that I was an angry Black woman, even although I am not Black and I am not angry! In this country, if you have any color to your skin, you are called Black and relegated to a certain place: The bottom. I was excited about what he was teaching us, but when everyone looked at me with a certain gaze, like something bad was going to happen, all those White people, just looking, I tightened up inside and sat back down.”
Esi looked down at her folded hands. Her husband, who was White, reached over and reassuringly patted her hands.
“Yes, Esi, they are wrong. They shouldn’t have treated you that way. White people can be insensitive.”
Then, she continued, “Joe, you do the same to me!”
“What do you mean, Esi?” responded Joe, with an innocent and anxious look scanning back and forth between her and the White male therapist.
“Well, Joe, do you really want to hear how I felt last week after we came back from that party at your sister’s house?”
“Yes, Esi.”
“But do you really? Are you sure you want to hear this?”
“Yes, Esi.”
“Remember when we went over, me and the kids sat with the other people of color and you sat with your sister and her side of the family?
“Yes, I remember. What is wrong with that?”
“Well, me and my colored friends got loud and excited, and you shot me a look, like ‘pipe down over there.’ THEN, after we got home, the next day, your sister called you and said that you had better control your wife; she is too loud. Do you remember all that?
“Yes, well you do get loud – especially when you are around your people.”
“So why is it I have to fit in with your relatives and not the other way round? Why do I have to conform to the whiteness in your sister’s world, not the other way round?”
“Well, we were in her house.”
“So if they came to our house and were too quiet, how would you feel if I called them up and said they needed to participate with more enthusiasm?”
“Esi, that’s not fair, and you know it isn’t.”
Esi stops and looks at the therapist.
“So I check myself. It is the same all over, White people imposing their values and beliefs on me, on us. I am not an angry Black woman. I am just frustrated. People, White people, always want an explanation for what they think is my loss of control. You can see when their demeanor changes, they pull back, sit up, back away, fidget, and won’t look you in the eye. All these little tics that show that they are trying to get out of the situation.”
Esi took a breath and saw that her husband and the therapist were listening.
“These signs are ingrained in your brain ... these signs ... I saw it when I first came here to this country. The first time I had a good dose of it ... was in that ethics class in college. You can’t use words that you are accustomed to, ’cause they mean something else here, something bad.”
“Oh, Esi, I am so sorry,” said Joe, looking concerned.
“You may be sorry but you are not willing to stand up for me against your sister and her White values. You want me to conform.”
“What do you want me to do?”
“I want you to call your sister out.”
“But she may not ever speak to me again!”
“OK, don’t then,” and Esi looked down at her hands. She was finished talking. Joe looked at the therapist, waiting for something.
The therapist resisted intervening on the issue. “Keep talking this through,” he instructed them.
Joe could see that Esi had done talking and that it was his move.
“Do you really want me to call my sister out, even if it means that she will not talk to me again?”
“Yes.”
“I don’t know if I can do that.”
The therapist now intervened: “What does that mean to you, Esi, that he doesn’t know if he can do that.”
“It means he doesn’t really love me or value me or even value our mulatto children. What do think our daughter is learning?”
“That’s not true, Esi.”
The therapist, Dr. Swarthmore, watched Esi, who has very a dark, blue-black skin tone, with a flawless complexion and a shapely body. She wears her hair cropped and she looks like that Black model, what’s her name. Joe was short, a little plump with ultra White skin and freckles on his nose. He had been brought up in the Midwest and had had little exposure to Africans before his internship abroad in Kenya. Dr. S. thought he had probably not really thought much about Esi’s dilemma.
Dr. Swarthmore encouraged Esi to talk about her immigration experience.
“Esi, can you talk more about what it is like to be an immigrant from Africa?”
“Well, I just have to check myself so that I can fit in with this White culture. If you want to see how I feel about it, you will have to see an angry Black woman and I have learned not to give you that satisfaction. You will just dismiss me. Please Dr. Swarthmore, can we move on?”
Dr. Swarthmore was caught between his desire to accept her wish to move on and his wish to have her express herself fully. He realized that it was not his desire that mattered; that the couple had to work this out between them if they were going to move forward. So he punted it back to them.
“Esi and Joe, you are both caught in an important dilemma. Esi, you want more respect from your husband and his family. Joe, you do not want to upset your family by confronting them. Is that right? You are both dammed if you do and dammed if you don’t.”
“I agree,” Joe and Esi both said, nodding.
“Do you want to work on this issue?”
They both agreed with equal enthusiasm.
“Ok, can you spend the next 10 minutes to work on this?”
They agreed.
“Ok, let’s start. What skills do you have that can help you resolve this important issue?”
Dr. Swarthmore framed the issue as one to be solved by the couple. The couple discussed that they are usually good at communication and solving problems. This problem is about whether or not Joe is more aligned with his White family than with Esi and their children.
Dr. Swarthmore encouraged them to think about this more deeply and over time; that this is such an important issue that it requires time and deep conversation.
“How do you think you can educate yourselves about the issues at hand?”
Esi’s reading list
1. “Why I’m No Longer Talking to White People About Race” by Reni Eddo-Lodge (London: Bloomsbury, 2018).
2. “Americanah” by Chimamanda Ngozi Adichie (New York: Alfred A. Knopf, 2013).
3. “How to be an Antiracist” by Ibram X. Kendi (New York: Random House, 2019).
Joe suggests that Esi think about what it might mean if his sister and their children were no longer part of their lives. She agrees to do this.
Dr. Swarthmore asks if they can each do their homework before they come back. They agree and thought they could manage that and the book for 2 weeks out.
Dr. Swarthmore decides that he will read one of the books Esi suggested, as he does not know much about racism and White privilege and he wants to learn more. Dr. Swarthmore demonstrates his desire to become more racially sensitive. The following steps can be taken by therapists who want to become more racially sensitive, according to TA Laszloffy and KV Hardy (Fam Process. 2000 Spring;39[1]:35-50):
1. Read and watch movies that address the experience of other cultural groups.
2. Go to and participate in cross-cultural events.
3. Engage in a racial self-exploration process. The following questions can begin the racial identity exploration process:
- How do I define myself racially?
- When did I first become aware of race/skin color in general, and mine in particular?
- What messages did I learn about race/skin color based on that first experience?
- What direct and indirect messages did I receive about race/skin color?
- How did the messages that I received about race/skin color affect how I thought and felt about myself racially?
- What benefits did I gain because of my race/skin color?
- What did I lose because of my race/skin?
- Have I ever dated cross-racially? Why or why not?
- How many friends of a different race do I have?
4. Internal commitment. This means committing to addressing racism in therapeutic encounters.
Lessons learned for psychiatrists
1. Therapeutic space is allocated to discuss the issue.
2. The time is strictly limited to 10 minutes, so the couple won’t feel that their emotions will overwhelm them.
3. The space is to focus on the strengths that they can bring to resolving the issue.
4. Give patients the impression that they can solve this and that it is an important issue.
5. Do not put yourself in the patients’ argument; take neither side.
Dr. Heru is professor of psychiatry at the University of Colorado at Denver, Aurora. She is editor of “Working With Families in Medical Settings: A Multidisciplinary Guide for Psychiatrists and Other Health Professionals” (New York: Routledge, 2013). She has no conflicts of interest. Dr. Heru wrote the article in collaboration with Lynette Ramsingh Barros, artist and social commentator.
‘The Undoing’: A dramatization of ‘You Should Have Known’
Jean Hanff Korelitz’s ironic psychological thriller, “You Should Have Known,” (New York: Grand Central Publishing, 2014) was transformed into an HBO miniseries called “The Undoing,” written and produced by David E. Kelley and directed by Susanne Bier, which premiered on Oct. 25, 2020.
The television drama differed from the novel in fundamental ways, but both have themes related to the therapeutic process. In the novel, a New York City–based couples therapist, Grace Reinhart Sachs, had recently written a book called “You Should Have Known,” about women who married their spouses disregarding their gut instinct that their partner was not fundamentally right for them, or might potentially cheat on them, or whose stories contained contradictions. In the miniseries, Grace (played by Nicole Kidman), is a therapist but there is no mention of her having written a book. Grace in both the novel and the miniseries is married to a pediatric oncologist, Jonathan (his ethnicity and surname were changed in the miniseries from a Jewish New Yorker in the novel to a British Dr. Jonathan Fraser in the series, played by Hugh Grant).
[Spoiler alert]: Prepandemic New York City’s Upper East Side is scandalized when a murdered mother is found by her young son the day after a lavish fund-raising auction party for a private school. Grace and Jonathan’s son, Henry, attends this school as well, and Grace had served on the auction committee with the murdered mother. When two detectives question Grace in the course of their investigation, she assumes that they are questioning her as they would any parent in the school. However, when she tries to reach her husband about the news and the investigation, she cannot. She thought he was at a medical conference in Cleveland, but she realizes that she does not know exactly what conference and exactly where. After many failed attempts at calling and texting, she hears a familiar alert sound coming from his nightstand drawer where she retrieves the cell phone that had been deliberately placed.
In the novel, Jonathan never reappears from “Cleveland,” and although it takes Grace a while to understand that her husband is not who she thought he was, she eventually does. In the miniseries, Jonathan appears in their lake house and a trial ensues with Jonathan adamantly proclaiming his innocence despite all evidence to the contrary.
The Oxford Reference defines undoing as “an emotional conflict associated with an action is dealt with by negating the action or attempting ‘magically’ to cause it not to have occurred by substituting an approximately opposite action.” It is not that the consequences of the action are attempted to be negated (as in making amends or showing remorse), but the action itself. In this way, the miniseries is aptly named since both main characters, Grace and Jonathan, use this defense mechanism. Grace has difficulty acknowledging that her husband could be capable of any wrongdoing, even as she is faced with fact after fact that contradicts this premise – and counsels others about their relationship choices. Similarly, Jonathan’s choice of profession is likely an attempt to undo his 4-year-old sister’s death that occurred on his watch when he was 14. However, even treating children’s cancer cannot undo the many indiscretions he has apparently committed in his adult life.
In the portrayal of a doctor with narcissistic, and possibly psychopathic, traits, “The Undoing” joins multiple recent podcasts that document real-life bad doctors, including Wondery’s “The Shrink Next Door,” “Dr. Death” (seasons 1 and 2), and “Do No Harm.” While most physicians go into medicine to heal and improve peoples’ lives, others, such as the character of Dr. Jonathan Fraser, appear to become physicians for ulterior and sinister motivations. Jonathan’s difficulty with empathy was present when he was a child as a character trait – rather than being attributable to any childhood traumatic event, as Grace had let herself believe.
In a Dec. 11, 2020, New York Times op-ed, Richard A. Friedman, MD, a psychiatrist affiliated with New York Presbyterian-Cornell University, discussed three “dangerous doctors” during the pandemic who are potentially harming the nation. Scott Atlas, MD, a radiologist on leave from Stanford (Calif.) University, advised President Trump on the coronavirus despite having no training in public health or infectious disease. Before resigning, he questioned the use of face masks, contradicting scientific proof of their prevention of disease. Another doctor, a cardiologist in Washington, also publicly disputed scientific evidence of the efficacy of face masks and social distancing, and a third physician promoted hydroxychloroquine as a treatment for coronavirus despite scientific evidence that it has been ineffective and possibly even harmful to patients with the virus.
Both the novel “You Should Have Known” and the series “The Undoing” will be of interest to psychiatrists, especially therapists and forensic psychiatrists, because of the themes portrayed, such as defense mechanisms, therapeutic process, and a homicide investigation – as well as the common human experience of being an expert in something in one’s professional life, yet occasionally falling short of recognizing the same phenomena in one’s personal life.
Dr. Rosenbaum is a clinical and forensic psychiatrist in private practice in New York. She is an assistant clinical professor at New York University Langone Medical Center and is on the faculty at Weill-Cornell Medical Center. She has no conflicts of interest. Dr. Friedman serves as the Phillip Resnick Professor of Forensic Psychiatry at Case Western Reserve University, Cleveland. She is also editor of Family Murder: Pathologies of Love and Hate (Washington: American Psychiatric Publishing, 2019), which was written by the Group for the Advancement of Psychiatry’s Committee on Psychiatry & Law, and which was awarded the 2020 Manfred Gutmacher Award by the American Psychiatric Association. She has no conflicts of interest.
Jean Hanff Korelitz’s ironic psychological thriller, “You Should Have Known,” (New York: Grand Central Publishing, 2014) was transformed into an HBO miniseries called “The Undoing,” written and produced by David E. Kelley and directed by Susanne Bier, which premiered on Oct. 25, 2020.
The television drama differed from the novel in fundamental ways, but both have themes related to the therapeutic process. In the novel, a New York City–based couples therapist, Grace Reinhart Sachs, had recently written a book called “You Should Have Known,” about women who married their spouses disregarding their gut instinct that their partner was not fundamentally right for them, or might potentially cheat on them, or whose stories contained contradictions. In the miniseries, Grace (played by Nicole Kidman), is a therapist but there is no mention of her having written a book. Grace in both the novel and the miniseries is married to a pediatric oncologist, Jonathan (his ethnicity and surname were changed in the miniseries from a Jewish New Yorker in the novel to a British Dr. Jonathan Fraser in the series, played by Hugh Grant).
[Spoiler alert]: Prepandemic New York City’s Upper East Side is scandalized when a murdered mother is found by her young son the day after a lavish fund-raising auction party for a private school. Grace and Jonathan’s son, Henry, attends this school as well, and Grace had served on the auction committee with the murdered mother. When two detectives question Grace in the course of their investigation, she assumes that they are questioning her as they would any parent in the school. However, when she tries to reach her husband about the news and the investigation, she cannot. She thought he was at a medical conference in Cleveland, but she realizes that she does not know exactly what conference and exactly where. After many failed attempts at calling and texting, she hears a familiar alert sound coming from his nightstand drawer where she retrieves the cell phone that had been deliberately placed.
In the novel, Jonathan never reappears from “Cleveland,” and although it takes Grace a while to understand that her husband is not who she thought he was, she eventually does. In the miniseries, Jonathan appears in their lake house and a trial ensues with Jonathan adamantly proclaiming his innocence despite all evidence to the contrary.
The Oxford Reference defines undoing as “an emotional conflict associated with an action is dealt with by negating the action or attempting ‘magically’ to cause it not to have occurred by substituting an approximately opposite action.” It is not that the consequences of the action are attempted to be negated (as in making amends or showing remorse), but the action itself. In this way, the miniseries is aptly named since both main characters, Grace and Jonathan, use this defense mechanism. Grace has difficulty acknowledging that her husband could be capable of any wrongdoing, even as she is faced with fact after fact that contradicts this premise – and counsels others about their relationship choices. Similarly, Jonathan’s choice of profession is likely an attempt to undo his 4-year-old sister’s death that occurred on his watch when he was 14. However, even treating children’s cancer cannot undo the many indiscretions he has apparently committed in his adult life.
In the portrayal of a doctor with narcissistic, and possibly psychopathic, traits, “The Undoing” joins multiple recent podcasts that document real-life bad doctors, including Wondery’s “The Shrink Next Door,” “Dr. Death” (seasons 1 and 2), and “Do No Harm.” While most physicians go into medicine to heal and improve peoples’ lives, others, such as the character of Dr. Jonathan Fraser, appear to become physicians for ulterior and sinister motivations. Jonathan’s difficulty with empathy was present when he was a child as a character trait – rather than being attributable to any childhood traumatic event, as Grace had let herself believe.
In a Dec. 11, 2020, New York Times op-ed, Richard A. Friedman, MD, a psychiatrist affiliated with New York Presbyterian-Cornell University, discussed three “dangerous doctors” during the pandemic who are potentially harming the nation. Scott Atlas, MD, a radiologist on leave from Stanford (Calif.) University, advised President Trump on the coronavirus despite having no training in public health or infectious disease. Before resigning, he questioned the use of face masks, contradicting scientific proof of their prevention of disease. Another doctor, a cardiologist in Washington, also publicly disputed scientific evidence of the efficacy of face masks and social distancing, and a third physician promoted hydroxychloroquine as a treatment for coronavirus despite scientific evidence that it has been ineffective and possibly even harmful to patients with the virus.
Both the novel “You Should Have Known” and the series “The Undoing” will be of interest to psychiatrists, especially therapists and forensic psychiatrists, because of the themes portrayed, such as defense mechanisms, therapeutic process, and a homicide investigation – as well as the common human experience of being an expert in something in one’s professional life, yet occasionally falling short of recognizing the same phenomena in one’s personal life.
Dr. Rosenbaum is a clinical and forensic psychiatrist in private practice in New York. She is an assistant clinical professor at New York University Langone Medical Center and is on the faculty at Weill-Cornell Medical Center. She has no conflicts of interest. Dr. Friedman serves as the Phillip Resnick Professor of Forensic Psychiatry at Case Western Reserve University, Cleveland. She is also editor of Family Murder: Pathologies of Love and Hate (Washington: American Psychiatric Publishing, 2019), which was written by the Group for the Advancement of Psychiatry’s Committee on Psychiatry & Law, and which was awarded the 2020 Manfred Gutmacher Award by the American Psychiatric Association. She has no conflicts of interest.
Jean Hanff Korelitz’s ironic psychological thriller, “You Should Have Known,” (New York: Grand Central Publishing, 2014) was transformed into an HBO miniseries called “The Undoing,” written and produced by David E. Kelley and directed by Susanne Bier, which premiered on Oct. 25, 2020.
The television drama differed from the novel in fundamental ways, but both have themes related to the therapeutic process. In the novel, a New York City–based couples therapist, Grace Reinhart Sachs, had recently written a book called “You Should Have Known,” about women who married their spouses disregarding their gut instinct that their partner was not fundamentally right for them, or might potentially cheat on them, or whose stories contained contradictions. In the miniseries, Grace (played by Nicole Kidman), is a therapist but there is no mention of her having written a book. Grace in both the novel and the miniseries is married to a pediatric oncologist, Jonathan (his ethnicity and surname were changed in the miniseries from a Jewish New Yorker in the novel to a British Dr. Jonathan Fraser in the series, played by Hugh Grant).
[Spoiler alert]: Prepandemic New York City’s Upper East Side is scandalized when a murdered mother is found by her young son the day after a lavish fund-raising auction party for a private school. Grace and Jonathan’s son, Henry, attends this school as well, and Grace had served on the auction committee with the murdered mother. When two detectives question Grace in the course of their investigation, she assumes that they are questioning her as they would any parent in the school. However, when she tries to reach her husband about the news and the investigation, she cannot. She thought he was at a medical conference in Cleveland, but she realizes that she does not know exactly what conference and exactly where. After many failed attempts at calling and texting, she hears a familiar alert sound coming from his nightstand drawer where she retrieves the cell phone that had been deliberately placed.
In the novel, Jonathan never reappears from “Cleveland,” and although it takes Grace a while to understand that her husband is not who she thought he was, she eventually does. In the miniseries, Jonathan appears in their lake house and a trial ensues with Jonathan adamantly proclaiming his innocence despite all evidence to the contrary.
The Oxford Reference defines undoing as “an emotional conflict associated with an action is dealt with by negating the action or attempting ‘magically’ to cause it not to have occurred by substituting an approximately opposite action.” It is not that the consequences of the action are attempted to be negated (as in making amends or showing remorse), but the action itself. In this way, the miniseries is aptly named since both main characters, Grace and Jonathan, use this defense mechanism. Grace has difficulty acknowledging that her husband could be capable of any wrongdoing, even as she is faced with fact after fact that contradicts this premise – and counsels others about their relationship choices. Similarly, Jonathan’s choice of profession is likely an attempt to undo his 4-year-old sister’s death that occurred on his watch when he was 14. However, even treating children’s cancer cannot undo the many indiscretions he has apparently committed in his adult life.
In the portrayal of a doctor with narcissistic, and possibly psychopathic, traits, “The Undoing” joins multiple recent podcasts that document real-life bad doctors, including Wondery’s “The Shrink Next Door,” “Dr. Death” (seasons 1 and 2), and “Do No Harm.” While most physicians go into medicine to heal and improve peoples’ lives, others, such as the character of Dr. Jonathan Fraser, appear to become physicians for ulterior and sinister motivations. Jonathan’s difficulty with empathy was present when he was a child as a character trait – rather than being attributable to any childhood traumatic event, as Grace had let herself believe.
In a Dec. 11, 2020, New York Times op-ed, Richard A. Friedman, MD, a psychiatrist affiliated with New York Presbyterian-Cornell University, discussed three “dangerous doctors” during the pandemic who are potentially harming the nation. Scott Atlas, MD, a radiologist on leave from Stanford (Calif.) University, advised President Trump on the coronavirus despite having no training in public health or infectious disease. Before resigning, he questioned the use of face masks, contradicting scientific proof of their prevention of disease. Another doctor, a cardiologist in Washington, also publicly disputed scientific evidence of the efficacy of face masks and social distancing, and a third physician promoted hydroxychloroquine as a treatment for coronavirus despite scientific evidence that it has been ineffective and possibly even harmful to patients with the virus.
Both the novel “You Should Have Known” and the series “The Undoing” will be of interest to psychiatrists, especially therapists and forensic psychiatrists, because of the themes portrayed, such as defense mechanisms, therapeutic process, and a homicide investigation – as well as the common human experience of being an expert in something in one’s professional life, yet occasionally falling short of recognizing the same phenomena in one’s personal life.
Dr. Rosenbaum is a clinical and forensic psychiatrist in private practice in New York. She is an assistant clinical professor at New York University Langone Medical Center and is on the faculty at Weill-Cornell Medical Center. She has no conflicts of interest. Dr. Friedman serves as the Phillip Resnick Professor of Forensic Psychiatry at Case Western Reserve University, Cleveland. She is also editor of Family Murder: Pathologies of Love and Hate (Washington: American Psychiatric Publishing, 2019), which was written by the Group for the Advancement of Psychiatry’s Committee on Psychiatry & Law, and which was awarded the 2020 Manfred Gutmacher Award by the American Psychiatric Association. She has no conflicts of interest.
Machine learning flags key risk factors for suicide attempts
A history of suicidal behaviors or ideation, functional impairment related to mental health disorders, and socioeconomic disadvantage are the three most important risk factors predicting subsequent suicide attempts, new research suggests.
Investigators applied a machine-learning model to data on over 34,500 adults drawn from a large national survey database. After analyzing more than 2,500 survey questions, key areas were identified that yielded the most accurate predictions of who might be at risk for later suicide attempt.
These predictors included experiencing previous suicidal behaviors and ideation or functional impairment because of emotional problems, being at a younger age, having a lower educational achievement, and experiencing a recent financial crisis.
“Our machine learning model confirmed well-known risk factors of suicide attempt, including previous suicidal behavior and depression; and we also identified functional impairment, such as doing activities less carefully or accomplishing less because of emotional problems, as a new important risk,” lead author Angel Garcia de la Garza, PhD candidate in the department of biostatistics, Columbia University, New York, said in an interview.
“We hope our results provide a novel avenue for future suicide risk assessment,” Mr. Garcia de la Garza said.
The findings were published online Jan. 6 in JAMA Psychiatry.
‘Rich’ dataset
Previous research using machine learning approaches to study nonfatal suicide attempt prediction has focused on high-risk patients in clinical treatment. However, more than one-third of individuals making nonfatal suicide attempts do not receive mental health treatment, Mr. Garcia de la Garza noted.
To gain further insight into predictors of suicide risk in nonclinical populations, the researchers turned to the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), a longitudinal survey of noninstitutionalized U.S. adults.
“We wanted to extend our understanding of suicide attempt risk factors beyond high-risk clinical populations to the general adult population; and the richness of the NESARC dataset provides a unique opportunity to do so,” Mr. Garcia de la Garza said.
The NESARC surveys were conducted in two waves: Wave 1 (2001-2002) and wave 2 (2004-2005), in which participants self-reported nonfatal suicide attempts in the preceding 3 years since wave 1.
Assessment of wave 1 participants was based on the Alcohol Use Disorder and Associated Disabilities Interview Schedule DSM-IV.
“This survey’s extensive assessment instrument contained a detailed evaluation of substance use, psychiatric disorders, and symptoms not routinely available in electronic health records,” Mr. Garcia de la Garza noted.
The wave 1 survey contained 2,805 separate questions. From participants’ responses, the investigators derived 180 variables for three categories: past-year, prior-to-past-year, and lifetime mental disorders.
They then identified 2,978 factors associated with suicide attempts and used a statistical method called balanced random forest to classify suicide attempts at wave 2. Each variable was accorded an “importance score” using identified wave 1 features.
The outcome variable of attempted suicide at any point during the 3 years prior to the wave 2 interview was defined by combining responses to three wave 2 questions:
- In your entire life, did you ever attempt suicide?
- If yes, how old were you the first time?
- If the most recent event occurred within the last 3 years, how old were you during the most recent time?
Suicide risk severity was classified into four groups (low, medium, high, and very high) on the basis of the top-performing risk factors.
A statistical model combining survey design and nonresponse weights enabled estimates to be representative of the U.S. population, based on the 2000 census.
Out-of-fold model prediction assessed performance of the model, using area under receiver operator curve (AUC), sensitivity, and specificity.
Daily functioning
Of all participants, 70.2% (n = 34,653; almost 60% women) completed wave 2 interviews. The weighted mean ages at waves 1 and 2 were 45.1 and 48.2 years, respectively.
Of wave 2 respondents, 0.6% (n = 222) attempted suicide during the preceding 3 years.
Half of those who attempted suicide within the first year were classified as “very high risk,” while 33.2% of those who attempted suicide between the first and second year and 33.3% of those who attempted suicide between the second and third year were classified as “very high risk.”
Among participants who attempted suicide between the third year and follow-up, 16.48% were classified as “very high risk.”
The model accurately captured classification of participants, even across demographic characteristics, such as age, sex, race, and income.
Younger individuals (aged 18-36 years) were at higher risk, compared with older individuals. In addition, women were at higher risk than were men, White participants were at higher risk than were non-White participants, and individuals with lower income were at greater risk than were those with higher income.
The model found that 1.8% of the U.S. population had a 10% or greater risk of a suicide attempt.
The most important risk factors identified were the three questions about previous suicidal ideation or behavior; three items from the 12-Item Short Form Health Survey (feeling downhearted, doing activities less carefully, or accomplishing less because of emotional problems); younger age; lower educational achievement; and recent financial crisis.
“The clinical assessment of suicide risk typically focuses on acute suicidal symptoms, together with depression, anxiety, substance misuse, and recent stressful events,” coinvestigator Mark Olfson, MD, PhD, professor of epidemiology, Columbia University Irving Medical Center, New York, said in an interview.
Dr. Olfson said.
Extra vigilance
Commenting on the study in an interview, April C. Foreman, PhD, an executive board member of the American Association of Suicidology, noted that some of the findings were not surprising.
“When discharging a patient from inpatient care, or seeing them in primary care, bring up mental health concerns proactively and ask whether they have ever attempted suicide or harmed themselves – even a long time ago – just as you ask about a family history of heart disease or cancer, or other health issues,” said Dr. Foreman, chief medical officer of the Kevin and Margaret Hines Foundation.
She noted that half of people who die by suicide have a primary care visit within the preceding month.
“Primary care is a great place to get a suicide history and follow the patient with extra vigilance, just as you would with any other risk factors,” Dr. Foreman said.
The study was funded by the National Institute on Alcohol Abuse and Alcoholism and its Intramural Program. The study authors and Dr. Foreman have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A history of suicidal behaviors or ideation, functional impairment related to mental health disorders, and socioeconomic disadvantage are the three most important risk factors predicting subsequent suicide attempts, new research suggests.
Investigators applied a machine-learning model to data on over 34,500 adults drawn from a large national survey database. After analyzing more than 2,500 survey questions, key areas were identified that yielded the most accurate predictions of who might be at risk for later suicide attempt.
These predictors included experiencing previous suicidal behaviors and ideation or functional impairment because of emotional problems, being at a younger age, having a lower educational achievement, and experiencing a recent financial crisis.
“Our machine learning model confirmed well-known risk factors of suicide attempt, including previous suicidal behavior and depression; and we also identified functional impairment, such as doing activities less carefully or accomplishing less because of emotional problems, as a new important risk,” lead author Angel Garcia de la Garza, PhD candidate in the department of biostatistics, Columbia University, New York, said in an interview.
“We hope our results provide a novel avenue for future suicide risk assessment,” Mr. Garcia de la Garza said.
The findings were published online Jan. 6 in JAMA Psychiatry.
‘Rich’ dataset
Previous research using machine learning approaches to study nonfatal suicide attempt prediction has focused on high-risk patients in clinical treatment. However, more than one-third of individuals making nonfatal suicide attempts do not receive mental health treatment, Mr. Garcia de la Garza noted.
To gain further insight into predictors of suicide risk in nonclinical populations, the researchers turned to the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), a longitudinal survey of noninstitutionalized U.S. adults.
“We wanted to extend our understanding of suicide attempt risk factors beyond high-risk clinical populations to the general adult population; and the richness of the NESARC dataset provides a unique opportunity to do so,” Mr. Garcia de la Garza said.
The NESARC surveys were conducted in two waves: Wave 1 (2001-2002) and wave 2 (2004-2005), in which participants self-reported nonfatal suicide attempts in the preceding 3 years since wave 1.
Assessment of wave 1 participants was based on the Alcohol Use Disorder and Associated Disabilities Interview Schedule DSM-IV.
“This survey’s extensive assessment instrument contained a detailed evaluation of substance use, psychiatric disorders, and symptoms not routinely available in electronic health records,” Mr. Garcia de la Garza noted.
The wave 1 survey contained 2,805 separate questions. From participants’ responses, the investigators derived 180 variables for three categories: past-year, prior-to-past-year, and lifetime mental disorders.
They then identified 2,978 factors associated with suicide attempts and used a statistical method called balanced random forest to classify suicide attempts at wave 2. Each variable was accorded an “importance score” using identified wave 1 features.
The outcome variable of attempted suicide at any point during the 3 years prior to the wave 2 interview was defined by combining responses to three wave 2 questions:
- In your entire life, did you ever attempt suicide?
- If yes, how old were you the first time?
- If the most recent event occurred within the last 3 years, how old were you during the most recent time?
Suicide risk severity was classified into four groups (low, medium, high, and very high) on the basis of the top-performing risk factors.
A statistical model combining survey design and nonresponse weights enabled estimates to be representative of the U.S. population, based on the 2000 census.
Out-of-fold model prediction assessed performance of the model, using area under receiver operator curve (AUC), sensitivity, and specificity.
Daily functioning
Of all participants, 70.2% (n = 34,653; almost 60% women) completed wave 2 interviews. The weighted mean ages at waves 1 and 2 were 45.1 and 48.2 years, respectively.
Of wave 2 respondents, 0.6% (n = 222) attempted suicide during the preceding 3 years.
Half of those who attempted suicide within the first year were classified as “very high risk,” while 33.2% of those who attempted suicide between the first and second year and 33.3% of those who attempted suicide between the second and third year were classified as “very high risk.”
Among participants who attempted suicide between the third year and follow-up, 16.48% were classified as “very high risk.”
The model accurately captured classification of participants, even across demographic characteristics, such as age, sex, race, and income.
Younger individuals (aged 18-36 years) were at higher risk, compared with older individuals. In addition, women were at higher risk than were men, White participants were at higher risk than were non-White participants, and individuals with lower income were at greater risk than were those with higher income.
The model found that 1.8% of the U.S. population had a 10% or greater risk of a suicide attempt.
The most important risk factors identified were the three questions about previous suicidal ideation or behavior; three items from the 12-Item Short Form Health Survey (feeling downhearted, doing activities less carefully, or accomplishing less because of emotional problems); younger age; lower educational achievement; and recent financial crisis.
“The clinical assessment of suicide risk typically focuses on acute suicidal symptoms, together with depression, anxiety, substance misuse, and recent stressful events,” coinvestigator Mark Olfson, MD, PhD, professor of epidemiology, Columbia University Irving Medical Center, New York, said in an interview.
Dr. Olfson said.
Extra vigilance
Commenting on the study in an interview, April C. Foreman, PhD, an executive board member of the American Association of Suicidology, noted that some of the findings were not surprising.
“When discharging a patient from inpatient care, or seeing them in primary care, bring up mental health concerns proactively and ask whether they have ever attempted suicide or harmed themselves – even a long time ago – just as you ask about a family history of heart disease or cancer, or other health issues,” said Dr. Foreman, chief medical officer of the Kevin and Margaret Hines Foundation.
She noted that half of people who die by suicide have a primary care visit within the preceding month.
“Primary care is a great place to get a suicide history and follow the patient with extra vigilance, just as you would with any other risk factors,” Dr. Foreman said.
The study was funded by the National Institute on Alcohol Abuse and Alcoholism and its Intramural Program. The study authors and Dr. Foreman have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A history of suicidal behaviors or ideation, functional impairment related to mental health disorders, and socioeconomic disadvantage are the three most important risk factors predicting subsequent suicide attempts, new research suggests.
Investigators applied a machine-learning model to data on over 34,500 adults drawn from a large national survey database. After analyzing more than 2,500 survey questions, key areas were identified that yielded the most accurate predictions of who might be at risk for later suicide attempt.
These predictors included experiencing previous suicidal behaviors and ideation or functional impairment because of emotional problems, being at a younger age, having a lower educational achievement, and experiencing a recent financial crisis.
“Our machine learning model confirmed well-known risk factors of suicide attempt, including previous suicidal behavior and depression; and we also identified functional impairment, such as doing activities less carefully or accomplishing less because of emotional problems, as a new important risk,” lead author Angel Garcia de la Garza, PhD candidate in the department of biostatistics, Columbia University, New York, said in an interview.
“We hope our results provide a novel avenue for future suicide risk assessment,” Mr. Garcia de la Garza said.
The findings were published online Jan. 6 in JAMA Psychiatry.
‘Rich’ dataset
Previous research using machine learning approaches to study nonfatal suicide attempt prediction has focused on high-risk patients in clinical treatment. However, more than one-third of individuals making nonfatal suicide attempts do not receive mental health treatment, Mr. Garcia de la Garza noted.
To gain further insight into predictors of suicide risk in nonclinical populations, the researchers turned to the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC), a longitudinal survey of noninstitutionalized U.S. adults.
“We wanted to extend our understanding of suicide attempt risk factors beyond high-risk clinical populations to the general adult population; and the richness of the NESARC dataset provides a unique opportunity to do so,” Mr. Garcia de la Garza said.
The NESARC surveys were conducted in two waves: Wave 1 (2001-2002) and wave 2 (2004-2005), in which participants self-reported nonfatal suicide attempts in the preceding 3 years since wave 1.
Assessment of wave 1 participants was based on the Alcohol Use Disorder and Associated Disabilities Interview Schedule DSM-IV.
“This survey’s extensive assessment instrument contained a detailed evaluation of substance use, psychiatric disorders, and symptoms not routinely available in electronic health records,” Mr. Garcia de la Garza noted.
The wave 1 survey contained 2,805 separate questions. From participants’ responses, the investigators derived 180 variables for three categories: past-year, prior-to-past-year, and lifetime mental disorders.
They then identified 2,978 factors associated with suicide attempts and used a statistical method called balanced random forest to classify suicide attempts at wave 2. Each variable was accorded an “importance score” using identified wave 1 features.
The outcome variable of attempted suicide at any point during the 3 years prior to the wave 2 interview was defined by combining responses to three wave 2 questions:
- In your entire life, did you ever attempt suicide?
- If yes, how old were you the first time?
- If the most recent event occurred within the last 3 years, how old were you during the most recent time?
Suicide risk severity was classified into four groups (low, medium, high, and very high) on the basis of the top-performing risk factors.
A statistical model combining survey design and nonresponse weights enabled estimates to be representative of the U.S. population, based on the 2000 census.
Out-of-fold model prediction assessed performance of the model, using area under receiver operator curve (AUC), sensitivity, and specificity.
Daily functioning
Of all participants, 70.2% (n = 34,653; almost 60% women) completed wave 2 interviews. The weighted mean ages at waves 1 and 2 were 45.1 and 48.2 years, respectively.
Of wave 2 respondents, 0.6% (n = 222) attempted suicide during the preceding 3 years.
Half of those who attempted suicide within the first year were classified as “very high risk,” while 33.2% of those who attempted suicide between the first and second year and 33.3% of those who attempted suicide between the second and third year were classified as “very high risk.”
Among participants who attempted suicide between the third year and follow-up, 16.48% were classified as “very high risk.”
The model accurately captured classification of participants, even across demographic characteristics, such as age, sex, race, and income.
Younger individuals (aged 18-36 years) were at higher risk, compared with older individuals. In addition, women were at higher risk than were men, White participants were at higher risk than were non-White participants, and individuals with lower income were at greater risk than were those with higher income.
The model found that 1.8% of the U.S. population had a 10% or greater risk of a suicide attempt.
The most important risk factors identified were the three questions about previous suicidal ideation or behavior; three items from the 12-Item Short Form Health Survey (feeling downhearted, doing activities less carefully, or accomplishing less because of emotional problems); younger age; lower educational achievement; and recent financial crisis.
“The clinical assessment of suicide risk typically focuses on acute suicidal symptoms, together with depression, anxiety, substance misuse, and recent stressful events,” coinvestigator Mark Olfson, MD, PhD, professor of epidemiology, Columbia University Irving Medical Center, New York, said in an interview.
Dr. Olfson said.
Extra vigilance
Commenting on the study in an interview, April C. Foreman, PhD, an executive board member of the American Association of Suicidology, noted that some of the findings were not surprising.
“When discharging a patient from inpatient care, or seeing them in primary care, bring up mental health concerns proactively and ask whether they have ever attempted suicide or harmed themselves – even a long time ago – just as you ask about a family history of heart disease or cancer, or other health issues,” said Dr. Foreman, chief medical officer of the Kevin and Margaret Hines Foundation.
She noted that half of people who die by suicide have a primary care visit within the preceding month.
“Primary care is a great place to get a suicide history and follow the patient with extra vigilance, just as you would with any other risk factors,” Dr. Foreman said.
The study was funded by the National Institute on Alcohol Abuse and Alcoholism and its Intramural Program. The study authors and Dr. Foreman have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.









