User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Armchair epidemiology
Real epidemiologists are out knocking on doors, chasing down contacts, or hunched over their computers trying to make sense out of screens full of data and maps. A few are trying valiantly to talk some sense into our elected officials.
This leaves the rest of us with time on our hands to fabricate our own less-than-scientific explanations for the behavior of the SARS-CoV-2 virus. So I have decided to put on hold my current mental challenge of choosing which pasta shape to pair with the sauce I’ve prepared from an online recipe. Here is my educated guess based on what I can glean from media sources that may have been filtered through a variety politically biased lenses. Remember, I did go to medical school; however, when I was in college the DNA helix was still just theoretical.
From those halcyon days of mid-February when our attention was focused on the Diamond Princess quarantined in Yokohama Harbor, it didn’t take a board-certified epidemiologist to suspect that the virus was spreading through the ventilating system in the ship’s tight quarters. Subsequent outbreaks on U.S. and French military ships suggests a similar explanation.
While still not proven, it sounds like SARS-CoV-2 jumped to humans from bats. It should not surprise us that having evolved in a dense population of mammals it would thrive in other high-density populations such as New York and nursing homes. Because we have lacked a robust testing capability, it has been less obvious until recently that, while it is easily transmitted, the virus has infected many who are asymptomatic (“Antibody surveys suggesting vast undercount of coronavirus infections may be unreliable,” Gretchen Vogel, Science, April 21, 2020). Subsequent surveys seem to confirm this higher level carrier state; it suggests that the virus is far less deadly than was previously suggested. However, it seems to be a crafty little bug attacking just about any organ system it lands on.
I don’t think any of us are surprised that the elderly population with weakened immune systems, particularly those in congregate housing, has been much more vulnerable. However, many of the deaths among younger apparently healthy people have defied explanation. The anecdotal observations that physicians, particularly those who practice in-your-face medicine (e.g., ophthalmologists and otolaryngologists) may be more vulnerable raises the issue of viral load. It may be that, although it can be extremely contagious, the virus is not terribly dangerous for most people until the inoculum dose of the virus reaches a certain level. To my knowledge this dose is unknown.
A published survey of more than 300 outbreaks from 120 Chinese cities also may support my suspicion that viral load is of critical importance. The researchers found that all the “identified outbreaks of three or more cases occurred in an indoor environment, which confirms that sharing indoor space is a major SARS-CoV-2 infection risk” (Huan Qian et al. “Indoor transmission of SARS-CoV-2,” MedRxiv. 2020 Apr 7. doi: 10.1101/2020.04.04.20053058). Again, this data shouldn’t surprise us when we look back at what little we know about the outbreaks in the confined spaces on cruise ships and in nursing homes.
I’m not sure that we have any data that helps us determine whether wearing a mask in an outdoor space has any more than symbolic value when we are talking about this particular virus. We may read that the virus in a droplet can survive on the surface it lands on for 8 minutes, and we can see those slow motion videos of the impressive plume of snot spray released by a sneeze. It would seem obvious that even outside someone within 10 feet of the sneeze has a good chance of being infected. However, how much of a threat is the asymptomatic carrier who passes within three feet of you while you are out on lovely summer day stroll? This armchair epidemiologist suspects that, when we are talking about an outside space, the 6-foot guideline for small groups of a dozen or less is overly restrictive. But until we know, I’m staying put in my armchair ... outside on the porch overlooking Casco Bay.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” He has no disclosures. Email him at [email protected].
Real epidemiologists are out knocking on doors, chasing down contacts, or hunched over their computers trying to make sense out of screens full of data and maps. A few are trying valiantly to talk some sense into our elected officials.
This leaves the rest of us with time on our hands to fabricate our own less-than-scientific explanations for the behavior of the SARS-CoV-2 virus. So I have decided to put on hold my current mental challenge of choosing which pasta shape to pair with the sauce I’ve prepared from an online recipe. Here is my educated guess based on what I can glean from media sources that may have been filtered through a variety politically biased lenses. Remember, I did go to medical school; however, when I was in college the DNA helix was still just theoretical.
From those halcyon days of mid-February when our attention was focused on the Diamond Princess quarantined in Yokohama Harbor, it didn’t take a board-certified epidemiologist to suspect that the virus was spreading through the ventilating system in the ship’s tight quarters. Subsequent outbreaks on U.S. and French military ships suggests a similar explanation.
While still not proven, it sounds like SARS-CoV-2 jumped to humans from bats. It should not surprise us that having evolved in a dense population of mammals it would thrive in other high-density populations such as New York and nursing homes. Because we have lacked a robust testing capability, it has been less obvious until recently that, while it is easily transmitted, the virus has infected many who are asymptomatic (“Antibody surveys suggesting vast undercount of coronavirus infections may be unreliable,” Gretchen Vogel, Science, April 21, 2020). Subsequent surveys seem to confirm this higher level carrier state; it suggests that the virus is far less deadly than was previously suggested. However, it seems to be a crafty little bug attacking just about any organ system it lands on.
I don’t think any of us are surprised that the elderly population with weakened immune systems, particularly those in congregate housing, has been much more vulnerable. However, many of the deaths among younger apparently healthy people have defied explanation. The anecdotal observations that physicians, particularly those who practice in-your-face medicine (e.g., ophthalmologists and otolaryngologists) may be more vulnerable raises the issue of viral load. It may be that, although it can be extremely contagious, the virus is not terribly dangerous for most people until the inoculum dose of the virus reaches a certain level. To my knowledge this dose is unknown.
A published survey of more than 300 outbreaks from 120 Chinese cities also may support my suspicion that viral load is of critical importance. The researchers found that all the “identified outbreaks of three or more cases occurred in an indoor environment, which confirms that sharing indoor space is a major SARS-CoV-2 infection risk” (Huan Qian et al. “Indoor transmission of SARS-CoV-2,” MedRxiv. 2020 Apr 7. doi: 10.1101/2020.04.04.20053058). Again, this data shouldn’t surprise us when we look back at what little we know about the outbreaks in the confined spaces on cruise ships and in nursing homes.
I’m not sure that we have any data that helps us determine whether wearing a mask in an outdoor space has any more than symbolic value when we are talking about this particular virus. We may read that the virus in a droplet can survive on the surface it lands on for 8 minutes, and we can see those slow motion videos of the impressive plume of snot spray released by a sneeze. It would seem obvious that even outside someone within 10 feet of the sneeze has a good chance of being infected. However, how much of a threat is the asymptomatic carrier who passes within three feet of you while you are out on lovely summer day stroll? This armchair epidemiologist suspects that, when we are talking about an outside space, the 6-foot guideline for small groups of a dozen or less is overly restrictive. But until we know, I’m staying put in my armchair ... outside on the porch overlooking Casco Bay.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” He has no disclosures. Email him at [email protected].
Real epidemiologists are out knocking on doors, chasing down contacts, or hunched over their computers trying to make sense out of screens full of data and maps. A few are trying valiantly to talk some sense into our elected officials.
This leaves the rest of us with time on our hands to fabricate our own less-than-scientific explanations for the behavior of the SARS-CoV-2 virus. So I have decided to put on hold my current mental challenge of choosing which pasta shape to pair with the sauce I’ve prepared from an online recipe. Here is my educated guess based on what I can glean from media sources that may have been filtered through a variety politically biased lenses. Remember, I did go to medical school; however, when I was in college the DNA helix was still just theoretical.
From those halcyon days of mid-February when our attention was focused on the Diamond Princess quarantined in Yokohama Harbor, it didn’t take a board-certified epidemiologist to suspect that the virus was spreading through the ventilating system in the ship’s tight quarters. Subsequent outbreaks on U.S. and French military ships suggests a similar explanation.
While still not proven, it sounds like SARS-CoV-2 jumped to humans from bats. It should not surprise us that having evolved in a dense population of mammals it would thrive in other high-density populations such as New York and nursing homes. Because we have lacked a robust testing capability, it has been less obvious until recently that, while it is easily transmitted, the virus has infected many who are asymptomatic (“Antibody surveys suggesting vast undercount of coronavirus infections may be unreliable,” Gretchen Vogel, Science, April 21, 2020). Subsequent surveys seem to confirm this higher level carrier state; it suggests that the virus is far less deadly than was previously suggested. However, it seems to be a crafty little bug attacking just about any organ system it lands on.
I don’t think any of us are surprised that the elderly population with weakened immune systems, particularly those in congregate housing, has been much more vulnerable. However, many of the deaths among younger apparently healthy people have defied explanation. The anecdotal observations that physicians, particularly those who practice in-your-face medicine (e.g., ophthalmologists and otolaryngologists) may be more vulnerable raises the issue of viral load. It may be that, although it can be extremely contagious, the virus is not terribly dangerous for most people until the inoculum dose of the virus reaches a certain level. To my knowledge this dose is unknown.
A published survey of more than 300 outbreaks from 120 Chinese cities also may support my suspicion that viral load is of critical importance. The researchers found that all the “identified outbreaks of three or more cases occurred in an indoor environment, which confirms that sharing indoor space is a major SARS-CoV-2 infection risk” (Huan Qian et al. “Indoor transmission of SARS-CoV-2,” MedRxiv. 2020 Apr 7. doi: 10.1101/2020.04.04.20053058). Again, this data shouldn’t surprise us when we look back at what little we know about the outbreaks in the confined spaces on cruise ships and in nursing homes.
I’m not sure that we have any data that helps us determine whether wearing a mask in an outdoor space has any more than symbolic value when we are talking about this particular virus. We may read that the virus in a droplet can survive on the surface it lands on for 8 minutes, and we can see those slow motion videos of the impressive plume of snot spray released by a sneeze. It would seem obvious that even outside someone within 10 feet of the sneeze has a good chance of being infected. However, how much of a threat is the asymptomatic carrier who passes within three feet of you while you are out on lovely summer day stroll? This armchair epidemiologist suspects that, when we are talking about an outside space, the 6-foot guideline for small groups of a dozen or less is overly restrictive. But until we know, I’m staying put in my armchair ... outside on the porch overlooking Casco Bay.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” He has no disclosures. Email him at [email protected].
Time series analysis of poison control data
The US Poison Control Centers’ National Poison Data System (NPDS) publishes annual reports describing exposures to various substances among the general population.1 Table 22B of each NPDS report shows the number of outcomes from exposures to different pharmacologic treatments in the United States, including psychotropic medications.2 In this Table, the relative morbidity (RM) of a medication is calculated as the ratio of serious outcomes (SO) to single exposures (SE), where SO = moderate + major + death. In this article, I use the NPDS data to demonstrate how time series analysis of the RM ratios for hypertension and psychiatric medications can help predict SO associated with these agents, which may help guide clinicians’ prescribing decisions.2,3
Time series analysis of hypertension medications
Due to the high prevalence of hypertension, it is not surprising that more suicide deaths occur each year from calcium channel blockers (CCB) than from lithium (37 vs 2, according to 2017 NPDS data).3 I used time series analysis to compare SO during 2006-2017 for 5 classes of hypertension medications: CCB, beta blockers (BB), angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), and diuretics (Figure 1).
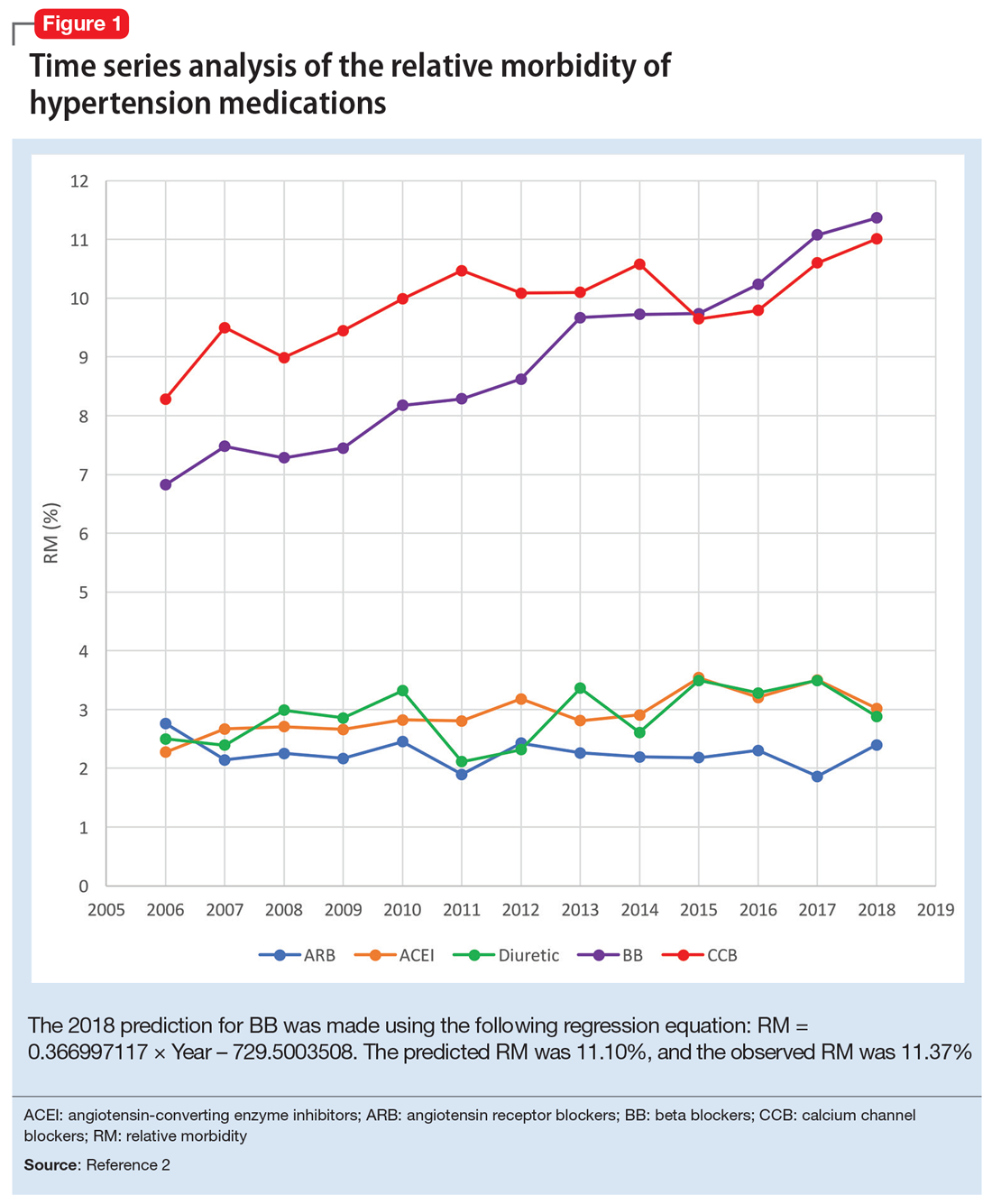
Time series analysis of 2006-2017 data predicted the following number of deaths for 2018: CCB ≥33, BB ≥17, ACEI ≤2, ARB 0, and diuretics ≤1. The observed deaths in 2018 were 41, 23, 0, 0, and 1, respectively.2 The 2018 predicted RM were CCB 10.66%, BB 11.10%, ACEI 3.51%, ARB 2.04%, and diuretics 3.38%. The 2018 observed RM for these medications were 11.01%, 11.37%, 3.02%, 2.40%, and 2.88%, respectively.2
Because the NPDS data for hypertension medications was only provided by class, in order to detect differences within each class, I used the relative lethality (RL) equation: RL = 310x / LD50, where x is the maximum daily dose of a medication prescribed for 30 days, and LD50 is the rat oral lethal dose 50. The RL equation represents the ratio of a 30-day supply of medication to the human equivalent LD50 for a 60-kg person.4 The RL equation is useful for comparing the safety of various medications, and can help clinicians avoid prescribing a lethal amount of a given medication (Figure 2). For example, the equation shows that among CCB, felodipine is 466 times safer than verapamil and 101 times safer than diltiazem. Not surprisingly, 2006-2018 data shows many deaths via intentional verapamil or diltiazem overdose vs only 1 reference to felodipine. A regression model shows significant correlation and causality between RL and SO over time.5 Integrating all 3 mathematical models suggests that the higher RM of CCB and BB may be caused by the high RL of verapamil, diltiazem, nicardipine, propranolol, and labetalol.
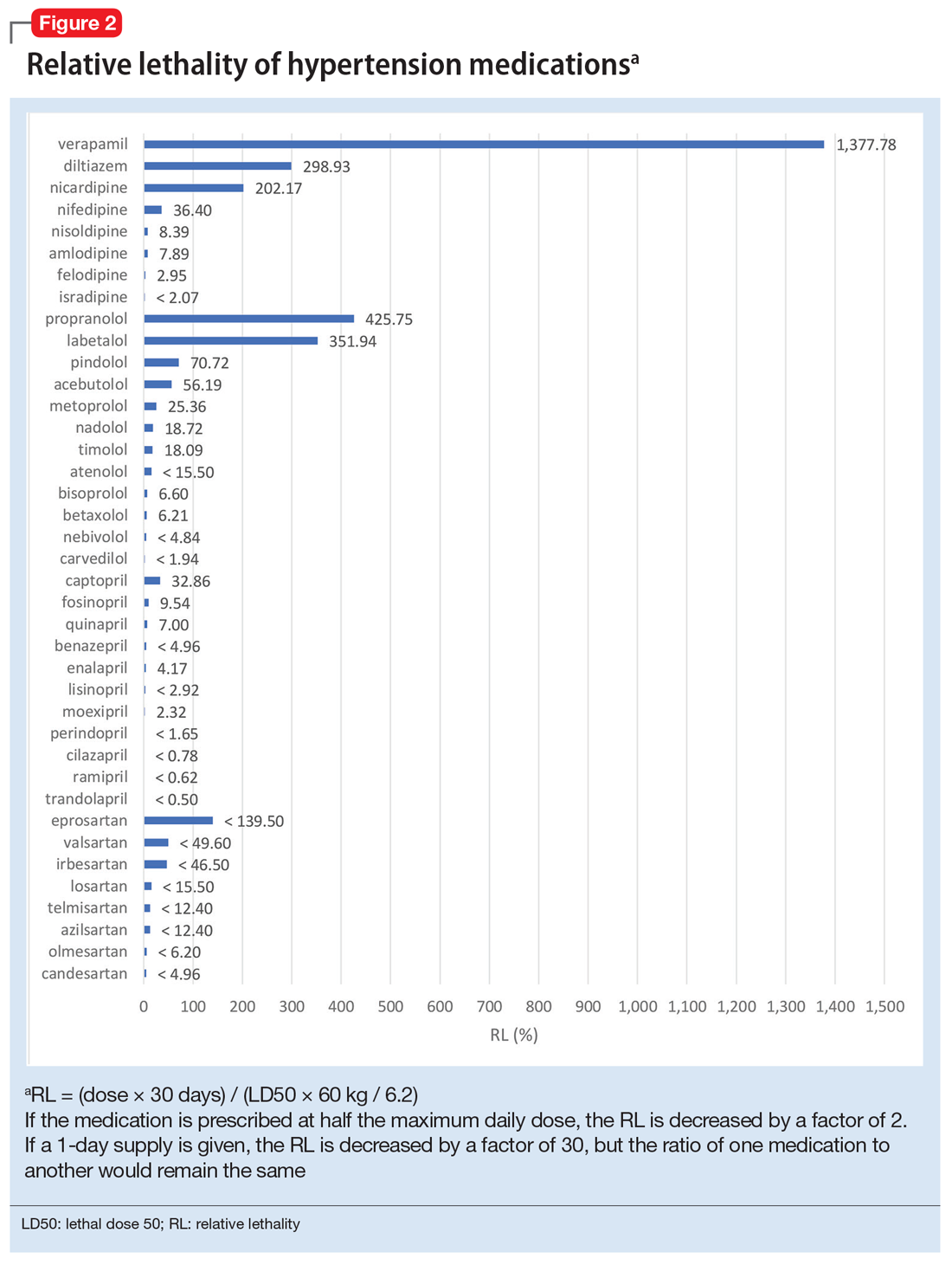
These mathematical models can help physicians consider whether to switch the patient’s current medication to another class with a lower RM. For patients who need a BB or CCB, prescribing a medication with a lower RL within the same class may be another option. The data suggest that avoiding hypertension medications with RL >100% may significantly decrease morbidity and mortality.
Predicting serious outcomes of psychiatric medications
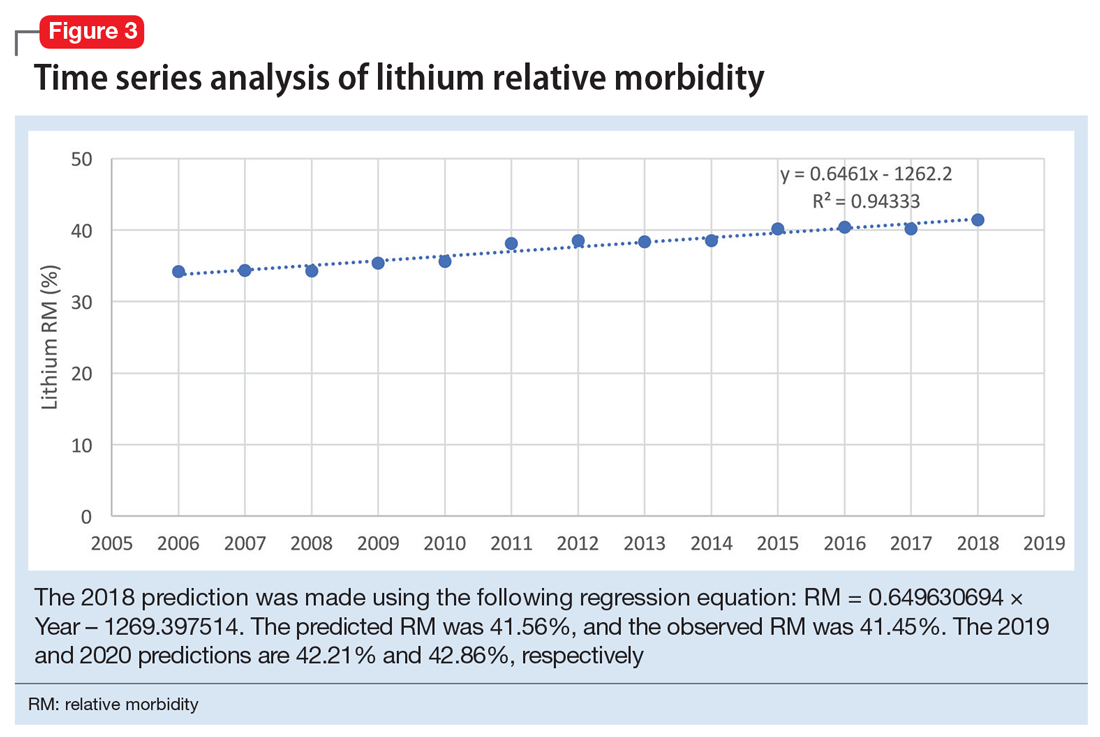
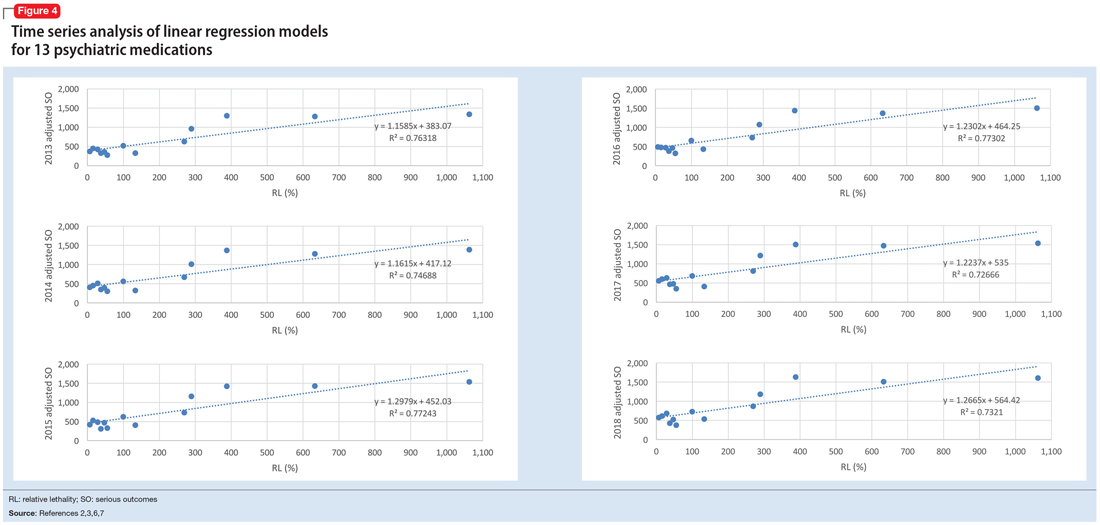
The 2018 NPDS data for psychiatric medications show similarly important results.2 For example, the lithium RM is predictable over time (Figure 3) and has been consistently the highest among psychiatric medications. Using 2006-2017 NPDS data,3 I predicted that the 2018 lithium RM would be 41.56%. The 2018 observed lithium RM was 41.45%.2 I created a linear regression model for each NPDS report from 2013 to 2018 to illustrate the correlation between RL and adjusted SO for 13 psychiatric medications.2,3,6,7 To account for different sample sizes among medications, the lithium SE for each respective year was used for all medications (adjusted SO = SE × RM). A time series analysis of these regression models shows that SO data points hover in the same y-axis region from year to year, with a corresponding RL on the x-axis: escitalopram 6.33%, citalopram 15.50%, mirtazapine 28.47%, paroxetine 37.35%, sertraline 46.72%, fluoxetine 54.87%, venlafaxine 99.64%, duloxetine 133.33%, trazodone 269.57%, bupropion 289.42%, amitriptyline 387.50%, doxepin 632.65%, and lithium 1062.86% (Figure 4). Every year, the scatter plot shape remains approximately the same, which suggests that both SO and RM can be predicted over time. Medications with RL >300% have SO ≈ 1500 (RM ≈ 40%), and those with RL <100% have SO ≈ 500 (RM ≈ 13%).
Time series analysis of NPDS data sheds light on hidden patterns. It may help clinicians discern patterns of potential SO associated with various hypertension and psychiatric medications. RL based on rat experimental data is highly correlated to RM based on human observational data, and the causality is self-evident. On a global scale, data-driven prescribing of medications with RL <100% could potentially help prevent millions of SO every year.
1. National Poison Data System Annual Reports. American Association of Poison Control Centers. https://www.aapcc.org/annual-reports. Updated November 2019. Accessed May 5, 2020.
2. Gummin DD, Mowry JB, Spyker DA, et al. 2018 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report. Clin Toxicol (Phila). 2019;57(12):1220-1413.
3. Gummin DD, Mowry JB, Spyker DA, et al. 2017 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 35th Annual Report. Clin Toxicol (Phila). 2018;56(12):1213-1415.
4. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
5. Giurca D. Data-driven prescribing. Current Psychiatry. 2018;17(10):e6-e8.
6. Mowry JB, Spyker DA, Brooks DE, et al. 2015 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd Annual Report. Clin Toxicol (Phila). 2016;54(10):924-1109.
7. Gummin DD, Mowry JB, Spyker DA, et al. 2016 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 34th Annual Report. Clin Toxicol (Phila). 2017;55(10):1072-1252.
The US Poison Control Centers’ National Poison Data System (NPDS) publishes annual reports describing exposures to various substances among the general population.1 Table 22B of each NPDS report shows the number of outcomes from exposures to different pharmacologic treatments in the United States, including psychotropic medications.2 In this Table, the relative morbidity (RM) of a medication is calculated as the ratio of serious outcomes (SO) to single exposures (SE), where SO = moderate + major + death. In this article, I use the NPDS data to demonstrate how time series analysis of the RM ratios for hypertension and psychiatric medications can help predict SO associated with these agents, which may help guide clinicians’ prescribing decisions.2,3
Time series analysis of hypertension medications
Due to the high prevalence of hypertension, it is not surprising that more suicide deaths occur each year from calcium channel blockers (CCB) than from lithium (37 vs 2, according to 2017 NPDS data).3 I used time series analysis to compare SO during 2006-2017 for 5 classes of hypertension medications: CCB, beta blockers (BB), angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), and diuretics (Figure 1).

Time series analysis of 2006-2017 data predicted the following number of deaths for 2018: CCB ≥33, BB ≥17, ACEI ≤2, ARB 0, and diuretics ≤1. The observed deaths in 2018 were 41, 23, 0, 0, and 1, respectively.2 The 2018 predicted RM were CCB 10.66%, BB 11.10%, ACEI 3.51%, ARB 2.04%, and diuretics 3.38%. The 2018 observed RM for these medications were 11.01%, 11.37%, 3.02%, 2.40%, and 2.88%, respectively.2
Because the NPDS data for hypertension medications was only provided by class, in order to detect differences within each class, I used the relative lethality (RL) equation: RL = 310x / LD50, where x is the maximum daily dose of a medication prescribed for 30 days, and LD50 is the rat oral lethal dose 50. The RL equation represents the ratio of a 30-day supply of medication to the human equivalent LD50 for a 60-kg person.4 The RL equation is useful for comparing the safety of various medications, and can help clinicians avoid prescribing a lethal amount of a given medication (Figure 2). For example, the equation shows that among CCB, felodipine is 466 times safer than verapamil and 101 times safer than diltiazem. Not surprisingly, 2006-2018 data shows many deaths via intentional verapamil or diltiazem overdose vs only 1 reference to felodipine. A regression model shows significant correlation and causality between RL and SO over time.5 Integrating all 3 mathematical models suggests that the higher RM of CCB and BB may be caused by the high RL of verapamil, diltiazem, nicardipine, propranolol, and labetalol.

These mathematical models can help physicians consider whether to switch the patient’s current medication to another class with a lower RM. For patients who need a BB or CCB, prescribing a medication with a lower RL within the same class may be another option. The data suggest that avoiding hypertension medications with RL >100% may significantly decrease morbidity and mortality.
Predicting serious outcomes of psychiatric medications
The 2018 NPDS data for psychiatric medications show similarly important results.2 For example, the lithium RM is predictable over time (Figure 3) and has been consistently the highest among psychiatric medications. Using 2006-2017 NPDS data,3 I predicted that the 2018 lithium RM would be 41.56%. The 2018 observed lithium RM was 41.45%.2 I created a linear regression model for each NPDS report from 2013 to 2018 to illustrate the correlation between RL and adjusted SO for 13 psychiatric medications.2,3,6,7 To account for different sample sizes among medications, the lithium SE for each respective year was used for all medications (adjusted SO = SE × RM). A time series analysis of these regression models shows that SO data points hover in the same y-axis region from year to year, with a corresponding RL on the x-axis: escitalopram 6.33%, citalopram 15.50%, mirtazapine 28.47%, paroxetine 37.35%, sertraline 46.72%, fluoxetine 54.87%, venlafaxine 99.64%, duloxetine 133.33%, trazodone 269.57%, bupropion 289.42%, amitriptyline 387.50%, doxepin 632.65%, and lithium 1062.86% (Figure 4). Every year, the scatter plot shape remains approximately the same, which suggests that both SO and RM can be predicted over time. Medications with RL >300% have SO ≈ 1500 (RM ≈ 40%), and those with RL <100% have SO ≈ 500 (RM ≈ 13%).

Time series analysis of NPDS data sheds light on hidden patterns. It may help clinicians discern patterns of potential SO associated with various hypertension and psychiatric medications. RL based on rat experimental data is highly correlated to RM based on human observational data, and the causality is self-evident. On a global scale, data-driven prescribing of medications with RL <100% could potentially help prevent millions of SO every year.

The US Poison Control Centers’ National Poison Data System (NPDS) publishes annual reports describing exposures to various substances among the general population.1 Table 22B of each NPDS report shows the number of outcomes from exposures to different pharmacologic treatments in the United States, including psychotropic medications.2 In this Table, the relative morbidity (RM) of a medication is calculated as the ratio of serious outcomes (SO) to single exposures (SE), where SO = moderate + major + death. In this article, I use the NPDS data to demonstrate how time series analysis of the RM ratios for hypertension and psychiatric medications can help predict SO associated with these agents, which may help guide clinicians’ prescribing decisions.2,3
Time series analysis of hypertension medications
Due to the high prevalence of hypertension, it is not surprising that more suicide deaths occur each year from calcium channel blockers (CCB) than from lithium (37 vs 2, according to 2017 NPDS data).3 I used time series analysis to compare SO during 2006-2017 for 5 classes of hypertension medications: CCB, beta blockers (BB), angiotensin-converting enzyme inhibitors (ACEI), angiotensin receptor blockers (ARB), and diuretics (Figure 1).

Time series analysis of 2006-2017 data predicted the following number of deaths for 2018: CCB ≥33, BB ≥17, ACEI ≤2, ARB 0, and diuretics ≤1. The observed deaths in 2018 were 41, 23, 0, 0, and 1, respectively.2 The 2018 predicted RM were CCB 10.66%, BB 11.10%, ACEI 3.51%, ARB 2.04%, and diuretics 3.38%. The 2018 observed RM for these medications were 11.01%, 11.37%, 3.02%, 2.40%, and 2.88%, respectively.2
Because the NPDS data for hypertension medications was only provided by class, in order to detect differences within each class, I used the relative lethality (RL) equation: RL = 310x / LD50, where x is the maximum daily dose of a medication prescribed for 30 days, and LD50 is the rat oral lethal dose 50. The RL equation represents the ratio of a 30-day supply of medication to the human equivalent LD50 for a 60-kg person.4 The RL equation is useful for comparing the safety of various medications, and can help clinicians avoid prescribing a lethal amount of a given medication (Figure 2). For example, the equation shows that among CCB, felodipine is 466 times safer than verapamil and 101 times safer than diltiazem. Not surprisingly, 2006-2018 data shows many deaths via intentional verapamil or diltiazem overdose vs only 1 reference to felodipine. A regression model shows significant correlation and causality between RL and SO over time.5 Integrating all 3 mathematical models suggests that the higher RM of CCB and BB may be caused by the high RL of verapamil, diltiazem, nicardipine, propranolol, and labetalol.

These mathematical models can help physicians consider whether to switch the patient’s current medication to another class with a lower RM. For patients who need a BB or CCB, prescribing a medication with a lower RL within the same class may be another option. The data suggest that avoiding hypertension medications with RL >100% may significantly decrease morbidity and mortality.
Predicting serious outcomes of psychiatric medications
The 2018 NPDS data for psychiatric medications show similarly important results.2 For example, the lithium RM is predictable over time (Figure 3) and has been consistently the highest among psychiatric medications. Using 2006-2017 NPDS data,3 I predicted that the 2018 lithium RM would be 41.56%. The 2018 observed lithium RM was 41.45%.2 I created a linear regression model for each NPDS report from 2013 to 2018 to illustrate the correlation between RL and adjusted SO for 13 psychiatric medications.2,3,6,7 To account for different sample sizes among medications, the lithium SE for each respective year was used for all medications (adjusted SO = SE × RM). A time series analysis of these regression models shows that SO data points hover in the same y-axis region from year to year, with a corresponding RL on the x-axis: escitalopram 6.33%, citalopram 15.50%, mirtazapine 28.47%, paroxetine 37.35%, sertraline 46.72%, fluoxetine 54.87%, venlafaxine 99.64%, duloxetine 133.33%, trazodone 269.57%, bupropion 289.42%, amitriptyline 387.50%, doxepin 632.65%, and lithium 1062.86% (Figure 4). Every year, the scatter plot shape remains approximately the same, which suggests that both SO and RM can be predicted over time. Medications with RL >300% have SO ≈ 1500 (RM ≈ 40%), and those with RL <100% have SO ≈ 500 (RM ≈ 13%).

Time series analysis of NPDS data sheds light on hidden patterns. It may help clinicians discern patterns of potential SO associated with various hypertension and psychiatric medications. RL based on rat experimental data is highly correlated to RM based on human observational data, and the causality is self-evident. On a global scale, data-driven prescribing of medications with RL <100% could potentially help prevent millions of SO every year.

1. National Poison Data System Annual Reports. American Association of Poison Control Centers. https://www.aapcc.org/annual-reports. Updated November 2019. Accessed May 5, 2020.
2. Gummin DD, Mowry JB, Spyker DA, et al. 2018 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report. Clin Toxicol (Phila). 2019;57(12):1220-1413.
3. Gummin DD, Mowry JB, Spyker DA, et al. 2017 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 35th Annual Report. Clin Toxicol (Phila). 2018;56(12):1213-1415.
4. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
5. Giurca D. Data-driven prescribing. Current Psychiatry. 2018;17(10):e6-e8.
6. Mowry JB, Spyker DA, Brooks DE, et al. 2015 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd Annual Report. Clin Toxicol (Phila). 2016;54(10):924-1109.
7. Gummin DD, Mowry JB, Spyker DA, et al. 2016 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 34th Annual Report. Clin Toxicol (Phila). 2017;55(10):1072-1252.
1. National Poison Data System Annual Reports. American Association of Poison Control Centers. https://www.aapcc.org/annual-reports. Updated November 2019. Accessed May 5, 2020.
2. Gummin DD, Mowry JB, Spyker DA, et al. 2018 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 36th Annual Report. Clin Toxicol (Phila). 2019;57(12):1220-1413.
3. Gummin DD, Mowry JB, Spyker DA, et al. 2017 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 35th Annual Report. Clin Toxicol (Phila). 2018;56(12):1213-1415.
4. Giurca D. Decreasing suicide risk with math. Current Psychiatry. 2018;17(2):57-59,A,B.
5. Giurca D. Data-driven prescribing. Current Psychiatry. 2018;17(10):e6-e8.
6. Mowry JB, Spyker DA, Brooks DE, et al. 2015 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 33rd Annual Report. Clin Toxicol (Phila). 2016;54(10):924-1109.
7. Gummin DD, Mowry JB, Spyker DA, et al. 2016 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 34th Annual Report. Clin Toxicol (Phila). 2017;55(10):1072-1252.
Telepsychiatry during COVID-19: Understanding the rules
In addition to affecting our personal lives, coronavirus disease 2019 (COVID-19) has altered the way we practice psychiatry. Telepsychiatry—the delivery of mental health services via remote communication—is being used to replace face-to-face outpatient encounters. Several rules and regulations governing the provision of care and prescribing have been temporarily modified or suspended to allow clinicians to more easily use telepsychiatry to care for their patients. Although these requirements are continually changing, here I review some of the telepsychiatry rules and regulations clinicians need to understand to minimize their risk for liability.
Changes in light of COVID-19
In March 2020, the Centers for Medicare & Medicaid Services (CMS) released guidance that allows Medicare beneficiaries to receive various services at home through telehealth without having to travel to a doctor’s office or hospital.1 Many commercial insurers also are allowing patients to receive telehealth services in their home. The US Department of Health & Human Services Office for Civil Rights, which enforces the Health Insurance Portability and Accountability Act (HIPAA), reported in March 2020 that it will not impose penalties for not complying with HIPAA requirements on clinicians who provide good-faith telepsychiatry during the COVID-19 crisis.2
Clinicians who want to use audio or video remote communication to provide any type of telehealth services (not just those related to COVID-19) should use “non-public facing” products.2 Non-public facing products (eg, Skype, WhatsApp video call, Zoom) allow only the intended parties to participate in the communication.3 Usually, these products employ end-to-end encryption, which allows only those engaging in communication to see and hear what is transmitted.3 To limit access and verify the participants, these products also support individual user accounts, login names, and passwords.3 In addition, these products usually allow participants and/or “the host” to exert some degree of control over particular features, such as choosing to record the communication, mute, or turn off the video or audio signal.3 When using these products, clinicians should enable all available encryption and privacy modes.2
“Public-facing” products (eg, Facebook Live, TikTok, Twitch) should not be used to provide telepsychiatry services because they are designed to be open to the public or allow for wide or indiscriminate access to the communication.2,3 Clinicians who desire additional privacy protections (and a more permanent solution) should choose a HIPAA-compliant telehealth vendor (eg, Doxy.me, VSee, Zoom for Healthcare) and obtain a Business Associate Agreement with the vendor to ensure data protection and security.2,4
Regardless of the product, obtain informed consent from your patients that authorizes the use of remote communication.4 Inform your patients of any potential privacy or security breaches, the need for interactions to be conducted in a location that provides privacy, and whether the specific technology used is HIPAA-compliant.4 Document that your patients understand these issues before using remote communication.4
How licensing requirements have changed
As of March 31, 2020, the CMS temporarily waived the requirement that out-of-state clinicians be licensed in the state where they are providing services to Medicare beneficiaries.5 The CMS waived this requirement for clinicians who meet the following 4 conditions5,6:
- must be enrolled in Medicare
- must possess a valid license to practice in the state that relates to his/her Medicare enrollment
- are furnishing services—whether in person or via telepsychiatry—in a state where the emergency is occurring to contribute to relief efforts in his/her professional capacity
- are not excluded from practicing in any state that is part of the nationally declared emergency area.
Note that individual state licensure requirements continue to apply unless waived by the state.6 Therefore, in order for clinicians to see Medicare patients via remote communication under the 4 conditions described above, the state also would have to waive its licensure requirements for the type of practice for which the clinicians are licensed in their own state.6 Regarding commercial payers, in general, clinicians providing telepsychiatry services need a license to practice in the state where the patient is located at the time services are provided.6 During the COVID-19 pandemic, many governors issued executive orders waiving licensure requirements, and many have accelerated granting temporary licenses to out-of-state clinicians who wish to provide telepsychiatry services to the residents of their state.4
Continue to: Prescribing via telepsychiatry
Prescribing via telepsychiatry
Effective March 31, 2020 and lasting for the duration of COVID-19 emergency declaration, the Drug Enforcement Agency (DEA) suspended the Ryan Haight Online Pharmacy Consumer Protection Act of 2008, which requires clinicians to conduct initial, in-person examinations of patients before they can prescribe controlled substances electronically.6,7 The DEA suspension allows clinicians to prescribe controlled substances after conducting an initial evaluation via remote communication. In addition, the DEA waived the requirement that a clinician needs to hold a DEA license in the state where the patient is located to be able to prescribe a controlled substance electronically.4,6 However, you still must comply with all other state laws and regulations for prescribing controlled substances.4
Staying informed
Although several telepsychiatry rules and regulations have been modified or suspended during the COVID-19 pandemic, the standard of care for services rendered via telepsychiatry remains the same as services provided via face-to-face encounters, including patient evaluation and assessment, treatment plans, medication, and documentation.4 Clinicians can keep up-to-date on how practicing telepsychiatry may evolve during these times by using the following resources from the American Psychiatric Association:
- Telepsychiatry Toolkit: www.psychiatry.org/psychiatrists/practice/telepsychiatry
- Practice Guidance for COVID-19: www.psychiatry.org/psychiatrists/covid-19-coronavirus/practice-guidance-for-covid-19.
1. Centers for Medicare and Medicaid Services. COVID-19: President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. https://www.cms.gov/outreach-and-educationoutreachffsprovpartprogprovider-partnership-email-archive/2020-03-17. Published March 17, 2020. Accessed May 6, 2020.
2. US Department of Health & Human Services. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Updated March 30, 2020. Accessed May 6, 2020.
3. US Department of Health & Human Services. What is a “non-public facing” remote communication product? https://www.hhs.gov/hipaa/for-professionals/faq/3024/what-is-a-non-public-facing-remote-communication-product/index.html. Updated April 10, 2020. Accessed May 6, 2020.
4. Huben-Kearney A. Risk management amid a global pandemic. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.5a38. Published April 28, 2020. Accessed May 6, 2020.
5. Centers for Medicare & Medicaid Services. COVID-19 emergency declaration blanket waivers for health care providers. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf. Published April 29, 2020. Accessed May 6, 2020.
6. American Psychiatric Association. Update on telehealth restrictions in response to COVID-19. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/blog/apa-resources-on-telepsychiatry-and-covid-19. Updated May 1, 2020. Accessed May 6, 2020.
7. US Drug Enforcement Agency. How to prescribe controlled substances to patients during the COVID-19 public health emergency. https://www.deadiversion.usdoj.gov/GDP/(DEA-DC-023)(DEA075)Decision_Tree_(Final)_33120_2007.pdf. Published March 31, 2020. Accessed on May 6, 2020.
In addition to affecting our personal lives, coronavirus disease 2019 (COVID-19) has altered the way we practice psychiatry. Telepsychiatry—the delivery of mental health services via remote communication—is being used to replace face-to-face outpatient encounters. Several rules and regulations governing the provision of care and prescribing have been temporarily modified or suspended to allow clinicians to more easily use telepsychiatry to care for their patients. Although these requirements are continually changing, here I review some of the telepsychiatry rules and regulations clinicians need to understand to minimize their risk for liability.
Changes in light of COVID-19
In March 2020, the Centers for Medicare & Medicaid Services (CMS) released guidance that allows Medicare beneficiaries to receive various services at home through telehealth without having to travel to a doctor’s office or hospital.1 Many commercial insurers also are allowing patients to receive telehealth services in their home. The US Department of Health & Human Services Office for Civil Rights, which enforces the Health Insurance Portability and Accountability Act (HIPAA), reported in March 2020 that it will not impose penalties for not complying with HIPAA requirements on clinicians who provide good-faith telepsychiatry during the COVID-19 crisis.2
Clinicians who want to use audio or video remote communication to provide any type of telehealth services (not just those related to COVID-19) should use “non-public facing” products.2 Non-public facing products (eg, Skype, WhatsApp video call, Zoom) allow only the intended parties to participate in the communication.3 Usually, these products employ end-to-end encryption, which allows only those engaging in communication to see and hear what is transmitted.3 To limit access and verify the participants, these products also support individual user accounts, login names, and passwords.3 In addition, these products usually allow participants and/or “the host” to exert some degree of control over particular features, such as choosing to record the communication, mute, or turn off the video or audio signal.3 When using these products, clinicians should enable all available encryption and privacy modes.2
“Public-facing” products (eg, Facebook Live, TikTok, Twitch) should not be used to provide telepsychiatry services because they are designed to be open to the public or allow for wide or indiscriminate access to the communication.2,3 Clinicians who desire additional privacy protections (and a more permanent solution) should choose a HIPAA-compliant telehealth vendor (eg, Doxy.me, VSee, Zoom for Healthcare) and obtain a Business Associate Agreement with the vendor to ensure data protection and security.2,4
Regardless of the product, obtain informed consent from your patients that authorizes the use of remote communication.4 Inform your patients of any potential privacy or security breaches, the need for interactions to be conducted in a location that provides privacy, and whether the specific technology used is HIPAA-compliant.4 Document that your patients understand these issues before using remote communication.4
How licensing requirements have changed
As of March 31, 2020, the CMS temporarily waived the requirement that out-of-state clinicians be licensed in the state where they are providing services to Medicare beneficiaries.5 The CMS waived this requirement for clinicians who meet the following 4 conditions5,6:
- must be enrolled in Medicare
- must possess a valid license to practice in the state that relates to his/her Medicare enrollment
- are furnishing services—whether in person or via telepsychiatry—in a state where the emergency is occurring to contribute to relief efforts in his/her professional capacity
- are not excluded from practicing in any state that is part of the nationally declared emergency area.
Note that individual state licensure requirements continue to apply unless waived by the state.6 Therefore, in order for clinicians to see Medicare patients via remote communication under the 4 conditions described above, the state also would have to waive its licensure requirements for the type of practice for which the clinicians are licensed in their own state.6 Regarding commercial payers, in general, clinicians providing telepsychiatry services need a license to practice in the state where the patient is located at the time services are provided.6 During the COVID-19 pandemic, many governors issued executive orders waiving licensure requirements, and many have accelerated granting temporary licenses to out-of-state clinicians who wish to provide telepsychiatry services to the residents of their state.4
Continue to: Prescribing via telepsychiatry
Prescribing via telepsychiatry
Effective March 31, 2020 and lasting for the duration of COVID-19 emergency declaration, the Drug Enforcement Agency (DEA) suspended the Ryan Haight Online Pharmacy Consumer Protection Act of 2008, which requires clinicians to conduct initial, in-person examinations of patients before they can prescribe controlled substances electronically.6,7 The DEA suspension allows clinicians to prescribe controlled substances after conducting an initial evaluation via remote communication. In addition, the DEA waived the requirement that a clinician needs to hold a DEA license in the state where the patient is located to be able to prescribe a controlled substance electronically.4,6 However, you still must comply with all other state laws and regulations for prescribing controlled substances.4
Staying informed
Although several telepsychiatry rules and regulations have been modified or suspended during the COVID-19 pandemic, the standard of care for services rendered via telepsychiatry remains the same as services provided via face-to-face encounters, including patient evaluation and assessment, treatment plans, medication, and documentation.4 Clinicians can keep up-to-date on how practicing telepsychiatry may evolve during these times by using the following resources from the American Psychiatric Association:
- Telepsychiatry Toolkit: www.psychiatry.org/psychiatrists/practice/telepsychiatry
- Practice Guidance for COVID-19: www.psychiatry.org/psychiatrists/covid-19-coronavirus/practice-guidance-for-covid-19.
In addition to affecting our personal lives, coronavirus disease 2019 (COVID-19) has altered the way we practice psychiatry. Telepsychiatry—the delivery of mental health services via remote communication—is being used to replace face-to-face outpatient encounters. Several rules and regulations governing the provision of care and prescribing have been temporarily modified or suspended to allow clinicians to more easily use telepsychiatry to care for their patients. Although these requirements are continually changing, here I review some of the telepsychiatry rules and regulations clinicians need to understand to minimize their risk for liability.
Changes in light of COVID-19
In March 2020, the Centers for Medicare & Medicaid Services (CMS) released guidance that allows Medicare beneficiaries to receive various services at home through telehealth without having to travel to a doctor’s office or hospital.1 Many commercial insurers also are allowing patients to receive telehealth services in their home. The US Department of Health & Human Services Office for Civil Rights, which enforces the Health Insurance Portability and Accountability Act (HIPAA), reported in March 2020 that it will not impose penalties for not complying with HIPAA requirements on clinicians who provide good-faith telepsychiatry during the COVID-19 crisis.2
Clinicians who want to use audio or video remote communication to provide any type of telehealth services (not just those related to COVID-19) should use “non-public facing” products.2 Non-public facing products (eg, Skype, WhatsApp video call, Zoom) allow only the intended parties to participate in the communication.3 Usually, these products employ end-to-end encryption, which allows only those engaging in communication to see and hear what is transmitted.3 To limit access and verify the participants, these products also support individual user accounts, login names, and passwords.3 In addition, these products usually allow participants and/or “the host” to exert some degree of control over particular features, such as choosing to record the communication, mute, or turn off the video or audio signal.3 When using these products, clinicians should enable all available encryption and privacy modes.2
“Public-facing” products (eg, Facebook Live, TikTok, Twitch) should not be used to provide telepsychiatry services because they are designed to be open to the public or allow for wide or indiscriminate access to the communication.2,3 Clinicians who desire additional privacy protections (and a more permanent solution) should choose a HIPAA-compliant telehealth vendor (eg, Doxy.me, VSee, Zoom for Healthcare) and obtain a Business Associate Agreement with the vendor to ensure data protection and security.2,4
Regardless of the product, obtain informed consent from your patients that authorizes the use of remote communication.4 Inform your patients of any potential privacy or security breaches, the need for interactions to be conducted in a location that provides privacy, and whether the specific technology used is HIPAA-compliant.4 Document that your patients understand these issues before using remote communication.4
How licensing requirements have changed
As of March 31, 2020, the CMS temporarily waived the requirement that out-of-state clinicians be licensed in the state where they are providing services to Medicare beneficiaries.5 The CMS waived this requirement for clinicians who meet the following 4 conditions5,6:
- must be enrolled in Medicare
- must possess a valid license to practice in the state that relates to his/her Medicare enrollment
- are furnishing services—whether in person or via telepsychiatry—in a state where the emergency is occurring to contribute to relief efforts in his/her professional capacity
- are not excluded from practicing in any state that is part of the nationally declared emergency area.
Note that individual state licensure requirements continue to apply unless waived by the state.6 Therefore, in order for clinicians to see Medicare patients via remote communication under the 4 conditions described above, the state also would have to waive its licensure requirements for the type of practice for which the clinicians are licensed in their own state.6 Regarding commercial payers, in general, clinicians providing telepsychiatry services need a license to practice in the state where the patient is located at the time services are provided.6 During the COVID-19 pandemic, many governors issued executive orders waiving licensure requirements, and many have accelerated granting temporary licenses to out-of-state clinicians who wish to provide telepsychiatry services to the residents of their state.4
Continue to: Prescribing via telepsychiatry
Prescribing via telepsychiatry
Effective March 31, 2020 and lasting for the duration of COVID-19 emergency declaration, the Drug Enforcement Agency (DEA) suspended the Ryan Haight Online Pharmacy Consumer Protection Act of 2008, which requires clinicians to conduct initial, in-person examinations of patients before they can prescribe controlled substances electronically.6,7 The DEA suspension allows clinicians to prescribe controlled substances after conducting an initial evaluation via remote communication. In addition, the DEA waived the requirement that a clinician needs to hold a DEA license in the state where the patient is located to be able to prescribe a controlled substance electronically.4,6 However, you still must comply with all other state laws and regulations for prescribing controlled substances.4
Staying informed
Although several telepsychiatry rules and regulations have been modified or suspended during the COVID-19 pandemic, the standard of care for services rendered via telepsychiatry remains the same as services provided via face-to-face encounters, including patient evaluation and assessment, treatment plans, medication, and documentation.4 Clinicians can keep up-to-date on how practicing telepsychiatry may evolve during these times by using the following resources from the American Psychiatric Association:
- Telepsychiatry Toolkit: www.psychiatry.org/psychiatrists/practice/telepsychiatry
- Practice Guidance for COVID-19: www.psychiatry.org/psychiatrists/covid-19-coronavirus/practice-guidance-for-covid-19.
1. Centers for Medicare and Medicaid Services. COVID-19: President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. https://www.cms.gov/outreach-and-educationoutreachffsprovpartprogprovider-partnership-email-archive/2020-03-17. Published March 17, 2020. Accessed May 6, 2020.
2. US Department of Health & Human Services. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Updated March 30, 2020. Accessed May 6, 2020.
3. US Department of Health & Human Services. What is a “non-public facing” remote communication product? https://www.hhs.gov/hipaa/for-professionals/faq/3024/what-is-a-non-public-facing-remote-communication-product/index.html. Updated April 10, 2020. Accessed May 6, 2020.
4. Huben-Kearney A. Risk management amid a global pandemic. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.5a38. Published April 28, 2020. Accessed May 6, 2020.
5. Centers for Medicare & Medicaid Services. COVID-19 emergency declaration blanket waivers for health care providers. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf. Published April 29, 2020. Accessed May 6, 2020.
6. American Psychiatric Association. Update on telehealth restrictions in response to COVID-19. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/blog/apa-resources-on-telepsychiatry-and-covid-19. Updated May 1, 2020. Accessed May 6, 2020.
7. US Drug Enforcement Agency. How to prescribe controlled substances to patients during the COVID-19 public health emergency. https://www.deadiversion.usdoj.gov/GDP/(DEA-DC-023)(DEA075)Decision_Tree_(Final)_33120_2007.pdf. Published March 31, 2020. Accessed on May 6, 2020.
1. Centers for Medicare and Medicaid Services. COVID-19: President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. https://www.cms.gov/outreach-and-educationoutreachffsprovpartprogprovider-partnership-email-archive/2020-03-17. Published March 17, 2020. Accessed May 6, 2020.
2. US Department of Health & Human Services. Notification of enforcement discretion for telehealth remote communications during the COVID-19 nationwide public health emergency. https://www.hhs.gov/hipaa/for-professionals/special-topics/emergency-preparedness/notification-enforcement-discretion-telehealth/index.html. Updated March 30, 2020. Accessed May 6, 2020.
3. US Department of Health & Human Services. What is a “non-public facing” remote communication product? https://www.hhs.gov/hipaa/for-professionals/faq/3024/what-is-a-non-public-facing-remote-communication-product/index.html. Updated April 10, 2020. Accessed May 6, 2020.
4. Huben-Kearney A. Risk management amid a global pandemic. Psychiatric News. https://psychnews.psychiatryonline.org/doi/10.1176/appi.pn.2020.5a38. Published April 28, 2020. Accessed May 6, 2020.
5. Centers for Medicare & Medicaid Services. COVID-19 emergency declaration blanket waivers for health care providers. https://www.cms.gov/files/document/summary-covid-19-emergency-declaration-waivers.pdf. Published April 29, 2020. Accessed May 6, 2020.
6. American Psychiatric Association. Update on telehealth restrictions in response to COVID-19. https://www.psychiatry.org/psychiatrists/practice/telepsychiatry/blog/apa-resources-on-telepsychiatry-and-covid-19. Updated May 1, 2020. Accessed May 6, 2020.
7. US Drug Enforcement Agency. How to prescribe controlled substances to patients during the COVID-19 public health emergency. https://www.deadiversion.usdoj.gov/GDP/(DEA-DC-023)(DEA075)Decision_Tree_(Final)_33120_2007.pdf. Published March 31, 2020. Accessed on May 6, 2020.
The resident’s role in combating burnout among medical students
Burnout among health care professionals has been increasingly recognized by the medical community over the past several years. The concern for burnout among medical students is equally serious. In this article, I review the prevalence of burnout among medical students, and the personal and clinical effects they experience. I also discuss how as psychiatry residents we can be more effective in preventing and identifying medical student burnout.
An underappreciated problem
Burnout has been defined as long-term unresolvable job stress that leads to exhaustion and feeling overwhelmed, cynical, and detached from work, and lacking a sense of personal accomplishment. It can lead to depression, anxiety, and suicidal ideation—one survey found that 5.8% of medical students had experienced suicidal ideation at some point in the previous 12 months.1 Burnout affects not only the individual, but also his/her team and patients. One study found that compared to medical students who didn’t report burnout, medical students who did had lower scores on measures of empathy and professionalism.2
While burnout among physicians and residents has received increasing attention, it often may go unrecognized and unreported in medical students. A literature review that included 51 studies found 28% to 45% of medical students report burnout.3 In a survey at one institution, 60% of medical students reported burnout.4 It is evident that medical schools have an important role in helping to minimize burnout rates in their students, and many schools are working toward this goal. However, what happens when students leave the classroom setting for clinical rotations?
A recent study found burnout among medical students peaks during the third year of medical school.5 This is when students are on their clinical rotations, new to the hospital environment, and without the inherent structure and support of being at school.
How residents can help
Like most medical students, while on my clinical rotations, I spent most of my day with residents, and I believe residents can help to both recognize burnout in medical students and prevent it.
The first step in addressing this problem is to understand why it occurs. A survey of medical students showed that inadequate sleep and decreased exercise play a significant role in burnout rates.6 Another study found a correlation between burnout and feeling emotionally exhausted and a decreased perceived quality of life.7 A medical student I recently worked with stated, “How can you not feel burnt out? Juggling work hours, studying, debt, health, and trying to have a life… something always gets dropped.”
So as residents, what can we do to identify and assist medical students who are experiencing burnout, or are at risk of getting there? When needed, we can utilize our psychiatry training to assess our students for depression and substance use disorders, and connect them with appropriate resources. When identifying a medical student with burnout, I believe it can become necessary to notify the attending, the site director responsible for the student, and often the school, so that the student has access to all available resources.
Continue to: It's as important to be proactive...
It’s as important to be proactive as it is to be reactive. Engaging in regular check-ins with our students about self-care and workload, as well as asking about how they are feeling, can offer them opportunities to talk about issues that they might not be getting anywhere else. One medical student I worked with told me, “It’s easy to fade into the background as the student, or to feel like I can’t complain because this is just how medical school is supposed to be.” We have the ability to change this notion with each student we work with.
It is likely that as residents we have worked with a student struggling with burnout without even realizing it. I believe we can play an important role in helping to prevent burnout by identifying at-risk students, offering assistance, and encouraging them to seek professional help. Someone’s life may depend on it.
1. Dyrbye L, Thomas M, Massie F, et al. Burnout and suicidal ideation among U.S. medical students. Ann Intern Med. 2008;149(5):334-341.
2. Brazeau C, Schroeder R, Rovi S. Relationships between medical student burnout, empathy, and professionalism climate. Acad Med. 2010;85(suppl 10):S33-S36. doi: 10.1097/ACM.0b013e3181ed4c47.
3. IsHak WW, Lederer S, Mandili C, et al. Burnout during residency training: a literature review. J Grad Med Educ. 2009;1(2):236-242.
4. Chang E, Eddins-Folensbee F, Coverdale J. Survey of the prevalence of burnout, stress, depression, and the use of supports by medical students at one school. Acad Psychiatry. 2012;36(3):177-182.
5. Hansell MW, Ungerleider RM, Brooks CA, et al. Temporal trends in medical student burnout. Fam Med. 2019;51(5):399-404.
6. Wolf M, Rosenstock J. Inadequate sleep and exercise associated with burnout and depression among medical students. Acad Psychiatry. 2017;41(2):174-179.
7. Colby L, Mareka M, Pillay S, et al. The association between the levels of burnout and quality of life among fourth-year medical students at the University of the Free State. S Afr J Psychiatr. 2018;24:1101.
Burnout among health care professionals has been increasingly recognized by the medical community over the past several years. The concern for burnout among medical students is equally serious. In this article, I review the prevalence of burnout among medical students, and the personal and clinical effects they experience. I also discuss how as psychiatry residents we can be more effective in preventing and identifying medical student burnout.
An underappreciated problem
Burnout has been defined as long-term unresolvable job stress that leads to exhaustion and feeling overwhelmed, cynical, and detached from work, and lacking a sense of personal accomplishment. It can lead to depression, anxiety, and suicidal ideation—one survey found that 5.8% of medical students had experienced suicidal ideation at some point in the previous 12 months.1 Burnout affects not only the individual, but also his/her team and patients. One study found that compared to medical students who didn’t report burnout, medical students who did had lower scores on measures of empathy and professionalism.2
While burnout among physicians and residents has received increasing attention, it often may go unrecognized and unreported in medical students. A literature review that included 51 studies found 28% to 45% of medical students report burnout.3 In a survey at one institution, 60% of medical students reported burnout.4 It is evident that medical schools have an important role in helping to minimize burnout rates in their students, and many schools are working toward this goal. However, what happens when students leave the classroom setting for clinical rotations?
A recent study found burnout among medical students peaks during the third year of medical school.5 This is when students are on their clinical rotations, new to the hospital environment, and without the inherent structure and support of being at school.
How residents can help
Like most medical students, while on my clinical rotations, I spent most of my day with residents, and I believe residents can help to both recognize burnout in medical students and prevent it.
The first step in addressing this problem is to understand why it occurs. A survey of medical students showed that inadequate sleep and decreased exercise play a significant role in burnout rates.6 Another study found a correlation between burnout and feeling emotionally exhausted and a decreased perceived quality of life.7 A medical student I recently worked with stated, “How can you not feel burnt out? Juggling work hours, studying, debt, health, and trying to have a life… something always gets dropped.”
So as residents, what can we do to identify and assist medical students who are experiencing burnout, or are at risk of getting there? When needed, we can utilize our psychiatry training to assess our students for depression and substance use disorders, and connect them with appropriate resources. When identifying a medical student with burnout, I believe it can become necessary to notify the attending, the site director responsible for the student, and often the school, so that the student has access to all available resources.
Continue to: It's as important to be proactive...
It’s as important to be proactive as it is to be reactive. Engaging in regular check-ins with our students about self-care and workload, as well as asking about how they are feeling, can offer them opportunities to talk about issues that they might not be getting anywhere else. One medical student I worked with told me, “It’s easy to fade into the background as the student, or to feel like I can’t complain because this is just how medical school is supposed to be.” We have the ability to change this notion with each student we work with.
It is likely that as residents we have worked with a student struggling with burnout without even realizing it. I believe we can play an important role in helping to prevent burnout by identifying at-risk students, offering assistance, and encouraging them to seek professional help. Someone’s life may depend on it.
Burnout among health care professionals has been increasingly recognized by the medical community over the past several years. The concern for burnout among medical students is equally serious. In this article, I review the prevalence of burnout among medical students, and the personal and clinical effects they experience. I also discuss how as psychiatry residents we can be more effective in preventing and identifying medical student burnout.
An underappreciated problem
Burnout has been defined as long-term unresolvable job stress that leads to exhaustion and feeling overwhelmed, cynical, and detached from work, and lacking a sense of personal accomplishment. It can lead to depression, anxiety, and suicidal ideation—one survey found that 5.8% of medical students had experienced suicidal ideation at some point in the previous 12 months.1 Burnout affects not only the individual, but also his/her team and patients. One study found that compared to medical students who didn’t report burnout, medical students who did had lower scores on measures of empathy and professionalism.2
While burnout among physicians and residents has received increasing attention, it often may go unrecognized and unreported in medical students. A literature review that included 51 studies found 28% to 45% of medical students report burnout.3 In a survey at one institution, 60% of medical students reported burnout.4 It is evident that medical schools have an important role in helping to minimize burnout rates in their students, and many schools are working toward this goal. However, what happens when students leave the classroom setting for clinical rotations?
A recent study found burnout among medical students peaks during the third year of medical school.5 This is when students are on their clinical rotations, new to the hospital environment, and without the inherent structure and support of being at school.
How residents can help
Like most medical students, while on my clinical rotations, I spent most of my day with residents, and I believe residents can help to both recognize burnout in medical students and prevent it.
The first step in addressing this problem is to understand why it occurs. A survey of medical students showed that inadequate sleep and decreased exercise play a significant role in burnout rates.6 Another study found a correlation between burnout and feeling emotionally exhausted and a decreased perceived quality of life.7 A medical student I recently worked with stated, “How can you not feel burnt out? Juggling work hours, studying, debt, health, and trying to have a life… something always gets dropped.”
So as residents, what can we do to identify and assist medical students who are experiencing burnout, or are at risk of getting there? When needed, we can utilize our psychiatry training to assess our students for depression and substance use disorders, and connect them with appropriate resources. When identifying a medical student with burnout, I believe it can become necessary to notify the attending, the site director responsible for the student, and often the school, so that the student has access to all available resources.
Continue to: It's as important to be proactive...
It’s as important to be proactive as it is to be reactive. Engaging in regular check-ins with our students about self-care and workload, as well as asking about how they are feeling, can offer them opportunities to talk about issues that they might not be getting anywhere else. One medical student I worked with told me, “It’s easy to fade into the background as the student, or to feel like I can’t complain because this is just how medical school is supposed to be.” We have the ability to change this notion with each student we work with.
It is likely that as residents we have worked with a student struggling with burnout without even realizing it. I believe we can play an important role in helping to prevent burnout by identifying at-risk students, offering assistance, and encouraging them to seek professional help. Someone’s life may depend on it.
1. Dyrbye L, Thomas M, Massie F, et al. Burnout and suicidal ideation among U.S. medical students. Ann Intern Med. 2008;149(5):334-341.
2. Brazeau C, Schroeder R, Rovi S. Relationships between medical student burnout, empathy, and professionalism climate. Acad Med. 2010;85(suppl 10):S33-S36. doi: 10.1097/ACM.0b013e3181ed4c47.
3. IsHak WW, Lederer S, Mandili C, et al. Burnout during residency training: a literature review. J Grad Med Educ. 2009;1(2):236-242.
4. Chang E, Eddins-Folensbee F, Coverdale J. Survey of the prevalence of burnout, stress, depression, and the use of supports by medical students at one school. Acad Psychiatry. 2012;36(3):177-182.
5. Hansell MW, Ungerleider RM, Brooks CA, et al. Temporal trends in medical student burnout. Fam Med. 2019;51(5):399-404.
6. Wolf M, Rosenstock J. Inadequate sleep and exercise associated with burnout and depression among medical students. Acad Psychiatry. 2017;41(2):174-179.
7. Colby L, Mareka M, Pillay S, et al. The association between the levels of burnout and quality of life among fourth-year medical students at the University of the Free State. S Afr J Psychiatr. 2018;24:1101.
1. Dyrbye L, Thomas M, Massie F, et al. Burnout and suicidal ideation among U.S. medical students. Ann Intern Med. 2008;149(5):334-341.
2. Brazeau C, Schroeder R, Rovi S. Relationships between medical student burnout, empathy, and professionalism climate. Acad Med. 2010;85(suppl 10):S33-S36. doi: 10.1097/ACM.0b013e3181ed4c47.
3. IsHak WW, Lederer S, Mandili C, et al. Burnout during residency training: a literature review. J Grad Med Educ. 2009;1(2):236-242.
4. Chang E, Eddins-Folensbee F, Coverdale J. Survey of the prevalence of burnout, stress, depression, and the use of supports by medical students at one school. Acad Psychiatry. 2012;36(3):177-182.
5. Hansell MW, Ungerleider RM, Brooks CA, et al. Temporal trends in medical student burnout. Fam Med. 2019;51(5):399-404.
6. Wolf M, Rosenstock J. Inadequate sleep and exercise associated with burnout and depression among medical students. Acad Psychiatry. 2017;41(2):174-179.
7. Colby L, Mareka M, Pillay S, et al. The association between the levels of burnout and quality of life among fourth-year medical students at the University of the Free State. S Afr J Psychiatr. 2018;24:1101.
Life during COVID-19: A pandemic of silence
Our world has radically changed during the coronavirus disease 2019 (COVID-19) crisis, and this impact has quickly transformed many lives. Whether you’re on the front lines of the COVID-19 pandemic or waiting in eager anticipation to return to practice, there is no denying that a few months ago we could never have imagined the health care and humanitarian crisis that is now before us. While we are united in our longing for a better time, we couldn’t be further apart socially and emotionally … and I’m not just talking about 6 feet.
One thing that has been truly striking to me is the silence. While experts have suggested there is a “silent pandemic” of mental illness on the horizon,1 I’ve been struck by the actual silence that exists as we walk through our stores and neighborhoods. We’re not speaking to each other anymore; it’s almost as if we’re afraid to make eye contact with one another.
Humans are social creatures, and the isolation that many people are experiencing during this pandemic could have detrimental and lasting effects if we don’t take action. While I highly encourage and support efforts to employ social distancing and mitigate the spread of this illness, I’m increasingly concerned about another kind of truly silent pandemic brewing beneath the surface of the COVID-19 crisis. Even under the best conditions, many individuals with posttraumatic stress disorder, depression, anxiety, bipolar disorder, schizophrenia, and other psychiatric disorders may lack adequate social interaction and experience feelings of isolation. These individuals need connection—not silence.
What happens to people who already felt intense isolation before COVID-19 and may have had invaluable lifelines cut off during this time of social distancing? What about individuals with alcohol or substance use disorders, or families who are sheltered in place in unsafe or violent home conditions? How can they reach out in silence? How can we help?
Fostering human connection
To address this, we must actively work to engage our patients and communities. One simple way to help is to acknowledge the people you encounter. Yes, stay 6 feet apart, and wear appropriate personal protective equipment. However, it is still OK to smile and greet someone with a nod, a smile, or a “hello.” A genuine smile can still be seen in someone’s eyes. We need these types of human connection, perhaps now more than ever before. We need each other.
Most importantly, during this time, we need to be aware of individuals who are most at risk in this silent pandemic. We can offer our patients appointments via video conferencing. We can use texting, e-mail, social media, phone calls, and video conferencing to check in with our families, friends, and neighbors. We’re at war with a terrible foe, but let’s not let the human connection become collateral damage.
1. Galea S, Merchant RM, Lurie N, et al. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention [published online April 10, 2020]. JAMA Intern Med. 2020. doi: 10.1001/jamainternmed.2020.1562.
Our world has radically changed during the coronavirus disease 2019 (COVID-19) crisis, and this impact has quickly transformed many lives. Whether you’re on the front lines of the COVID-19 pandemic or waiting in eager anticipation to return to practice, there is no denying that a few months ago we could never have imagined the health care and humanitarian crisis that is now before us. While we are united in our longing for a better time, we couldn’t be further apart socially and emotionally … and I’m not just talking about 6 feet.
One thing that has been truly striking to me is the silence. While experts have suggested there is a “silent pandemic” of mental illness on the horizon,1 I’ve been struck by the actual silence that exists as we walk through our stores and neighborhoods. We’re not speaking to each other anymore; it’s almost as if we’re afraid to make eye contact with one another.
Humans are social creatures, and the isolation that many people are experiencing during this pandemic could have detrimental and lasting effects if we don’t take action. While I highly encourage and support efforts to employ social distancing and mitigate the spread of this illness, I’m increasingly concerned about another kind of truly silent pandemic brewing beneath the surface of the COVID-19 crisis. Even under the best conditions, many individuals with posttraumatic stress disorder, depression, anxiety, bipolar disorder, schizophrenia, and other psychiatric disorders may lack adequate social interaction and experience feelings of isolation. These individuals need connection—not silence.
What happens to people who already felt intense isolation before COVID-19 and may have had invaluable lifelines cut off during this time of social distancing? What about individuals with alcohol or substance use disorders, or families who are sheltered in place in unsafe or violent home conditions? How can they reach out in silence? How can we help?
Fostering human connection
To address this, we must actively work to engage our patients and communities. One simple way to help is to acknowledge the people you encounter. Yes, stay 6 feet apart, and wear appropriate personal protective equipment. However, it is still OK to smile and greet someone with a nod, a smile, or a “hello.” A genuine smile can still be seen in someone’s eyes. We need these types of human connection, perhaps now more than ever before. We need each other.
Most importantly, during this time, we need to be aware of individuals who are most at risk in this silent pandemic. We can offer our patients appointments via video conferencing. We can use texting, e-mail, social media, phone calls, and video conferencing to check in with our families, friends, and neighbors. We’re at war with a terrible foe, but let’s not let the human connection become collateral damage.
Our world has radically changed during the coronavirus disease 2019 (COVID-19) crisis, and this impact has quickly transformed many lives. Whether you’re on the front lines of the COVID-19 pandemic or waiting in eager anticipation to return to practice, there is no denying that a few months ago we could never have imagined the health care and humanitarian crisis that is now before us. While we are united in our longing for a better time, we couldn’t be further apart socially and emotionally … and I’m not just talking about 6 feet.
One thing that has been truly striking to me is the silence. While experts have suggested there is a “silent pandemic” of mental illness on the horizon,1 I’ve been struck by the actual silence that exists as we walk through our stores and neighborhoods. We’re not speaking to each other anymore; it’s almost as if we’re afraid to make eye contact with one another.
Humans are social creatures, and the isolation that many people are experiencing during this pandemic could have detrimental and lasting effects if we don’t take action. While I highly encourage and support efforts to employ social distancing and mitigate the spread of this illness, I’m increasingly concerned about another kind of truly silent pandemic brewing beneath the surface of the COVID-19 crisis. Even under the best conditions, many individuals with posttraumatic stress disorder, depression, anxiety, bipolar disorder, schizophrenia, and other psychiatric disorders may lack adequate social interaction and experience feelings of isolation. These individuals need connection—not silence.
What happens to people who already felt intense isolation before COVID-19 and may have had invaluable lifelines cut off during this time of social distancing? What about individuals with alcohol or substance use disorders, or families who are sheltered in place in unsafe or violent home conditions? How can they reach out in silence? How can we help?
Fostering human connection
To address this, we must actively work to engage our patients and communities. One simple way to help is to acknowledge the people you encounter. Yes, stay 6 feet apart, and wear appropriate personal protective equipment. However, it is still OK to smile and greet someone with a nod, a smile, or a “hello.” A genuine smile can still be seen in someone’s eyes. We need these types of human connection, perhaps now more than ever before. We need each other.
Most importantly, during this time, we need to be aware of individuals who are most at risk in this silent pandemic. We can offer our patients appointments via video conferencing. We can use texting, e-mail, social media, phone calls, and video conferencing to check in with our families, friends, and neighbors. We’re at war with a terrible foe, but let’s not let the human connection become collateral damage.
1. Galea S, Merchant RM, Lurie N, et al. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention [published online April 10, 2020]. JAMA Intern Med. 2020. doi: 10.1001/jamainternmed.2020.1562.
1. Galea S, Merchant RM, Lurie N, et al. The mental health consequences of COVID-19 and physical distancing: the need for prevention and early intervention [published online April 10, 2020]. JAMA Intern Med. 2020. doi: 10.1001/jamainternmed.2020.1562.
Neuropsychiatric manifestations of COVID-19
On March 11, 2020, the World Health Organization declared that coronavirus disease 2019 (COVID-19) was a pandemic.1 As of mid-May 2020, the illness had claimed more than 316,000 lives worldwide.2 The main symptoms of the respiratory illness caused by COVID-19 are fever, dry cough, and shortness of breath. However, disorders of consciousness also have been reported, especially in patients who succumb to the illness.3 In fact, approximately one-third of hospitalized COVID-19 patients experience neurologic symptoms.4 Although the most common of these symptoms are dizziness, headache, and loss of smell and taste, patients with more severe cases can experience acute cerebrovascular diseases and impaired consciousness.4 As such, psychiatrists assessing confusion should include COVID-19 in their differential diagnosis as a potential cause of altered mental status.
How COVID-19 might affect the CNS
Although primarily considered a respiratory illness, COVID-19 also may have neurotropic potential. The virus that causes COVID-19, severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), is a beta-coronavirus. Two other highly pathogenic coronaviruses—SARS-CoV-1 and Middle East respiratory syndrome–related coronavirus (MERS-CoV)—are also beta-coronaviruses, and both have been reported to invade the CNS in some patients.5 These viruses are thought to invade cells via angiotensin-converting enzyme 2 (ACE2) receptors.6 These receptors are located on the epithelial cells of the respiratory and gastrointestinal (GI) tracts, but also are expressed in certain areas of the brain.7 Transmission to the brain could occur through various routes. However, the clinical symptom of loss of smell and taste hints to possible transmission of the virus from nasal cells to the olfactory bulb via trans-synaptic transmission in olfactory neurons.5,8,9
Immune injury via systemic inflammation is another proposed mechanism for nervous system damage.8,9 This has been described as “cytokine storm syndrome” and provides support to the role of immunotherapy in COVID-19 patients.10 Such inflammation has been long hypothesized as a contributor to psychiatric illnesses, especially neurocognitive disorders.11,12
Neuropsychiatric complications of COVID-19
Disorders of consciousness were identified early as a symptom of COVID-19.3 Subsequent studies and case reports have confirmed impaired consciousness as a possible symptom of COVID-19.4 The first case of encephalitis secondary to COVID-19 was reported by Chinese media on March 5, 2020 in Beijing, China.13 Subsequently, cases of encephalopathy secondary to COVID-19 have been reported in the United States. A 74-year-old man in Boca Raton, Florida who had recently returned from the Netherlands presented with altered mental status and was confirmed positive for COVID-19.14 A female airline worker in her late 50s who presented with altered mental status and tested positive for COVID-19 was found on imaging to have acute hemorrhagic necrotizing encephalopathy.15 There also have been cases of patients with confirmed COVID-19 who initially presented with complaints of seizures16 and Guillain-Barré syndrome.17 As such, neuropsychiatric complications of COVID-19 are being increasingly recognized and are important to consider during psychiatric assessments.
Consider COVID-19 when assessing altered mental status
Psychiatrists are often consulted to assess patients with impaired consciousness, mental status changes, or confusion. Acute changes to mentation raise concern for delirium. In fact, delirium should always be ruled out when assessing new psychiatric symptoms. The astute psychiatrist is aware of the myriad of medical contributors to delirium. However, because knowledge of COVID-19 is in its infancy, it can be easy to overlook this virus as a potential contributor to delirium. Even patients whose confusion seems to be more in line with a major neurocognitive disorder should be evaluated for COVID-19, because the sudden onset of cognitive impairment may be due to hypoxia, inflammatory damage, or cerebrovascular changes secondary to infection with the virus or its respiratory complications, such as acute respiratory distress syndrome (ARDS).18
The most obvious clues to the possible presence of COVID-19 in a patient who is confused would be fever, dry cough, and shortness of breath. Because ACE2 receptors are also located in the GI tract, nausea, vomiting, and diarrhea also are possible. However, patients who are confused may be poor historians, demonstrating behavioral symptoms that might make physical assessments challenging, or simply may be pre- or asymptomatic carriers of the virus. Hence, a thorough review of the patient’s history and collateral information is invaluable. A recent history of travel or contact with COVID-19–positive individuals should raise suspicion for viral infection. A patient who mentions a loss of taste or smell would also alert the psychiatrist to the possibility of COVID-19. A patient might not directly state this information, but may mention that he/she has been eating less or has not been disturbed by odors. Neuroimaging can be useful because patients with severe cases are at increased risk for acute cerebrovascular diseases.4 Also, ordering a chest CT may prove helpful because this testing is highly sensitive for COVID-19.19 If there is sufficient clinical evidence to suspect viral infection, testing for COVID-19 should be performed immediately.
It is important to be vigilant for the possibility of COVID-19 infection in patients who present with confusion. Because the virus is highly contagious, the threshold for COVID-19 testing should be low. Viral infection in patients can manifest in ways other than classic respiratory symptoms. Psychiatrists should be aware of COVID-19’s potential to invade the CNS and cause neuropsychiatric symptoms. When assessing confusion in any setting, the clinical and historical clues for COVID-19 should be kept in mind. This will allow patients with COVID-19 to be quickly diagnosed to initiate appropriate management and minimize progression of the illness. Additionally, this will allow for efficient quarantine of the patient to prevent the spread of the virus to others. As such, psychiatrists can play an important role in containing this virus and resolving the COVID-19 pandemic.
Continue to: Bottom Line
Bottom Line
Although primarily considered a respiratory illness, coronavirus disease 2019 (COVID-19) also may have the potential to invade the CNS and cause neuropsychiatric symptoms, such as impaired consciousness, encephalitis, or a loss of taste or smell. When assessing a patient who presents with confusion, be vigilant for the possibility of COVID-19.
Related Resources
- American Psychiatry Association. APA coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-coronavirus#psych.
- Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;S0889-1591(20)30489-X. doi: 10.1016/j.bbi.2020.04.027.
1. World Health Organization. Rolling updates on coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Updated May 1, 2020. Accessed May 4, 2020.
2. John Hopkins University. Coronavirus resource center. World map. https://coronavirus.jhu.edu/map.html. Accessed May 4, 2020.
3. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091.
4. Mao L, Wang M, Chen S, et al. Neurologic manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study [published online February 25, 2020]. JAMA Neurol. 2020;e201127. doi: 10.1101/2020.02.22.20026500.
5. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients [published online February 27, 2020]. J Med Virol. 2020;92(6). doi: 10.1002/jmv.25728.
6. Baig AM, Khaleeq A, Ali E, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995-998.
7. Xia H, Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep. 2010;12(3):170-175.
8. Steardo L, Steardo L Jr, Zorec R, et al. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19 [published online March 29, 2020]. Acta Physiol (Oxf). 2020;e13473. doi: 10.1111/apha.13473.
9. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses [published online March 30, 2020]. Brain Behav Immun. 2020;S0889-1591(20)30357-3. doi: 10.1016/j.bbi.2020.03.031.
10. Mehta P, McAuley DF, Brown M, et al; HLH Across Specialty Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034.
11. McNeil JB, Hughes CG, Girard T, et al. Plasma biomarkers of inflammation, coagulation, and brain injury as predictors of delirium duration in older hospitalized patients. PLoS One. 2019;14(12):e0226412. doi: 10.1371/journal.pone.0226412.
12. Heneka MT, Carson MJ, Khoury JE, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388‐405.
13. Beijing hospital confirms nervous system infections by novel coronavirus. XINHUANET. http://www.xinhuanet.com/english/2020-03/05/c_138846529.htm. Published May 3, 2020. Accessed May 4, 2020.
14. Filatov A, Sharma P, Hindi F, et al. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3):e7352. doi: 10.7759/cureus.7352.
15. Poyiadji N, Shahin G, Noujaim D, et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features [published online March 31, 2020]. Radiology. 2020;201187. doi: 10.1148/radiol.2020201187.
16. Karimi N, Razavi AS, Rouhani N. Frequent convulsive seizures in an adult patient with COVID-19: a case report. Iran Red Crescent Med J. 2020;22(3):e102828. doi: 10.5812/ircmj.102828.
17. Zhao H, Shen D, Zhou H, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383-384.
18. Sasannejad C, Ely EW, Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit Care. 2019;23(1):352.
19. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR [published online February 19, 2020]. Radiology. 2020;200432. doi: 10.1148/radiol.2020200432.
On March 11, 2020, the World Health Organization declared that coronavirus disease 2019 (COVID-19) was a pandemic.1 As of mid-May 2020, the illness had claimed more than 316,000 lives worldwide.2 The main symptoms of the respiratory illness caused by COVID-19 are fever, dry cough, and shortness of breath. However, disorders of consciousness also have been reported, especially in patients who succumb to the illness.3 In fact, approximately one-third of hospitalized COVID-19 patients experience neurologic symptoms.4 Although the most common of these symptoms are dizziness, headache, and loss of smell and taste, patients with more severe cases can experience acute cerebrovascular diseases and impaired consciousness.4 As such, psychiatrists assessing confusion should include COVID-19 in their differential diagnosis as a potential cause of altered mental status.
How COVID-19 might affect the CNS
Although primarily considered a respiratory illness, COVID-19 also may have neurotropic potential. The virus that causes COVID-19, severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), is a beta-coronavirus. Two other highly pathogenic coronaviruses—SARS-CoV-1 and Middle East respiratory syndrome–related coronavirus (MERS-CoV)—are also beta-coronaviruses, and both have been reported to invade the CNS in some patients.5 These viruses are thought to invade cells via angiotensin-converting enzyme 2 (ACE2) receptors.6 These receptors are located on the epithelial cells of the respiratory and gastrointestinal (GI) tracts, but also are expressed in certain areas of the brain.7 Transmission to the brain could occur through various routes. However, the clinical symptom of loss of smell and taste hints to possible transmission of the virus from nasal cells to the olfactory bulb via trans-synaptic transmission in olfactory neurons.5,8,9
Immune injury via systemic inflammation is another proposed mechanism for nervous system damage.8,9 This has been described as “cytokine storm syndrome” and provides support to the role of immunotherapy in COVID-19 patients.10 Such inflammation has been long hypothesized as a contributor to psychiatric illnesses, especially neurocognitive disorders.11,12
Neuropsychiatric complications of COVID-19
Disorders of consciousness were identified early as a symptom of COVID-19.3 Subsequent studies and case reports have confirmed impaired consciousness as a possible symptom of COVID-19.4 The first case of encephalitis secondary to COVID-19 was reported by Chinese media on March 5, 2020 in Beijing, China.13 Subsequently, cases of encephalopathy secondary to COVID-19 have been reported in the United States. A 74-year-old man in Boca Raton, Florida who had recently returned from the Netherlands presented with altered mental status and was confirmed positive for COVID-19.14 A female airline worker in her late 50s who presented with altered mental status and tested positive for COVID-19 was found on imaging to have acute hemorrhagic necrotizing encephalopathy.15 There also have been cases of patients with confirmed COVID-19 who initially presented with complaints of seizures16 and Guillain-Barré syndrome.17 As such, neuropsychiatric complications of COVID-19 are being increasingly recognized and are important to consider during psychiatric assessments.
Consider COVID-19 when assessing altered mental status
Psychiatrists are often consulted to assess patients with impaired consciousness, mental status changes, or confusion. Acute changes to mentation raise concern for delirium. In fact, delirium should always be ruled out when assessing new psychiatric symptoms. The astute psychiatrist is aware of the myriad of medical contributors to delirium. However, because knowledge of COVID-19 is in its infancy, it can be easy to overlook this virus as a potential contributor to delirium. Even patients whose confusion seems to be more in line with a major neurocognitive disorder should be evaluated for COVID-19, because the sudden onset of cognitive impairment may be due to hypoxia, inflammatory damage, or cerebrovascular changes secondary to infection with the virus or its respiratory complications, such as acute respiratory distress syndrome (ARDS).18
The most obvious clues to the possible presence of COVID-19 in a patient who is confused would be fever, dry cough, and shortness of breath. Because ACE2 receptors are also located in the GI tract, nausea, vomiting, and diarrhea also are possible. However, patients who are confused may be poor historians, demonstrating behavioral symptoms that might make physical assessments challenging, or simply may be pre- or asymptomatic carriers of the virus. Hence, a thorough review of the patient’s history and collateral information is invaluable. A recent history of travel or contact with COVID-19–positive individuals should raise suspicion for viral infection. A patient who mentions a loss of taste or smell would also alert the psychiatrist to the possibility of COVID-19. A patient might not directly state this information, but may mention that he/she has been eating less or has not been disturbed by odors. Neuroimaging can be useful because patients with severe cases are at increased risk for acute cerebrovascular diseases.4 Also, ordering a chest CT may prove helpful because this testing is highly sensitive for COVID-19.19 If there is sufficient clinical evidence to suspect viral infection, testing for COVID-19 should be performed immediately.
It is important to be vigilant for the possibility of COVID-19 infection in patients who present with confusion. Because the virus is highly contagious, the threshold for COVID-19 testing should be low. Viral infection in patients can manifest in ways other than classic respiratory symptoms. Psychiatrists should be aware of COVID-19’s potential to invade the CNS and cause neuropsychiatric symptoms. When assessing confusion in any setting, the clinical and historical clues for COVID-19 should be kept in mind. This will allow patients with COVID-19 to be quickly diagnosed to initiate appropriate management and minimize progression of the illness. Additionally, this will allow for efficient quarantine of the patient to prevent the spread of the virus to others. As such, psychiatrists can play an important role in containing this virus and resolving the COVID-19 pandemic.
Continue to: Bottom Line
Bottom Line
Although primarily considered a respiratory illness, coronavirus disease 2019 (COVID-19) also may have the potential to invade the CNS and cause neuropsychiatric symptoms, such as impaired consciousness, encephalitis, or a loss of taste or smell. When assessing a patient who presents with confusion, be vigilant for the possibility of COVID-19.
Related Resources
- American Psychiatry Association. APA coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-coronavirus#psych.
- Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;S0889-1591(20)30489-X. doi: 10.1016/j.bbi.2020.04.027.
On March 11, 2020, the World Health Organization declared that coronavirus disease 2019 (COVID-19) was a pandemic.1 As of mid-May 2020, the illness had claimed more than 316,000 lives worldwide.2 The main symptoms of the respiratory illness caused by COVID-19 are fever, dry cough, and shortness of breath. However, disorders of consciousness also have been reported, especially in patients who succumb to the illness.3 In fact, approximately one-third of hospitalized COVID-19 patients experience neurologic symptoms.4 Although the most common of these symptoms are dizziness, headache, and loss of smell and taste, patients with more severe cases can experience acute cerebrovascular diseases and impaired consciousness.4 As such, psychiatrists assessing confusion should include COVID-19 in their differential diagnosis as a potential cause of altered mental status.
How COVID-19 might affect the CNS
Although primarily considered a respiratory illness, COVID-19 also may have neurotropic potential. The virus that causes COVID-19, severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2), is a beta-coronavirus. Two other highly pathogenic coronaviruses—SARS-CoV-1 and Middle East respiratory syndrome–related coronavirus (MERS-CoV)—are also beta-coronaviruses, and both have been reported to invade the CNS in some patients.5 These viruses are thought to invade cells via angiotensin-converting enzyme 2 (ACE2) receptors.6 These receptors are located on the epithelial cells of the respiratory and gastrointestinal (GI) tracts, but also are expressed in certain areas of the brain.7 Transmission to the brain could occur through various routes. However, the clinical symptom of loss of smell and taste hints to possible transmission of the virus from nasal cells to the olfactory bulb via trans-synaptic transmission in olfactory neurons.5,8,9
Immune injury via systemic inflammation is another proposed mechanism for nervous system damage.8,9 This has been described as “cytokine storm syndrome” and provides support to the role of immunotherapy in COVID-19 patients.10 Such inflammation has been long hypothesized as a contributor to psychiatric illnesses, especially neurocognitive disorders.11,12
Neuropsychiatric complications of COVID-19
Disorders of consciousness were identified early as a symptom of COVID-19.3 Subsequent studies and case reports have confirmed impaired consciousness as a possible symptom of COVID-19.4 The first case of encephalitis secondary to COVID-19 was reported by Chinese media on March 5, 2020 in Beijing, China.13 Subsequently, cases of encephalopathy secondary to COVID-19 have been reported in the United States. A 74-year-old man in Boca Raton, Florida who had recently returned from the Netherlands presented with altered mental status and was confirmed positive for COVID-19.14 A female airline worker in her late 50s who presented with altered mental status and tested positive for COVID-19 was found on imaging to have acute hemorrhagic necrotizing encephalopathy.15 There also have been cases of patients with confirmed COVID-19 who initially presented with complaints of seizures16 and Guillain-Barré syndrome.17 As such, neuropsychiatric complications of COVID-19 are being increasingly recognized and are important to consider during psychiatric assessments.
Consider COVID-19 when assessing altered mental status
Psychiatrists are often consulted to assess patients with impaired consciousness, mental status changes, or confusion. Acute changes to mentation raise concern for delirium. In fact, delirium should always be ruled out when assessing new psychiatric symptoms. The astute psychiatrist is aware of the myriad of medical contributors to delirium. However, because knowledge of COVID-19 is in its infancy, it can be easy to overlook this virus as a potential contributor to delirium. Even patients whose confusion seems to be more in line with a major neurocognitive disorder should be evaluated for COVID-19, because the sudden onset of cognitive impairment may be due to hypoxia, inflammatory damage, or cerebrovascular changes secondary to infection with the virus or its respiratory complications, such as acute respiratory distress syndrome (ARDS).18
The most obvious clues to the possible presence of COVID-19 in a patient who is confused would be fever, dry cough, and shortness of breath. Because ACE2 receptors are also located in the GI tract, nausea, vomiting, and diarrhea also are possible. However, patients who are confused may be poor historians, demonstrating behavioral symptoms that might make physical assessments challenging, or simply may be pre- or asymptomatic carriers of the virus. Hence, a thorough review of the patient’s history and collateral information is invaluable. A recent history of travel or contact with COVID-19–positive individuals should raise suspicion for viral infection. A patient who mentions a loss of taste or smell would also alert the psychiatrist to the possibility of COVID-19. A patient might not directly state this information, but may mention that he/she has been eating less or has not been disturbed by odors. Neuroimaging can be useful because patients with severe cases are at increased risk for acute cerebrovascular diseases.4 Also, ordering a chest CT may prove helpful because this testing is highly sensitive for COVID-19.19 If there is sufficient clinical evidence to suspect viral infection, testing for COVID-19 should be performed immediately.
It is important to be vigilant for the possibility of COVID-19 infection in patients who present with confusion. Because the virus is highly contagious, the threshold for COVID-19 testing should be low. Viral infection in patients can manifest in ways other than classic respiratory symptoms. Psychiatrists should be aware of COVID-19’s potential to invade the CNS and cause neuropsychiatric symptoms. When assessing confusion in any setting, the clinical and historical clues for COVID-19 should be kept in mind. This will allow patients with COVID-19 to be quickly diagnosed to initiate appropriate management and minimize progression of the illness. Additionally, this will allow for efficient quarantine of the patient to prevent the spread of the virus to others. As such, psychiatrists can play an important role in containing this virus and resolving the COVID-19 pandemic.
Continue to: Bottom Line
Bottom Line
Although primarily considered a respiratory illness, coronavirus disease 2019 (COVID-19) also may have the potential to invade the CNS and cause neuropsychiatric symptoms, such as impaired consciousness, encephalitis, or a loss of taste or smell. When assessing a patient who presents with confusion, be vigilant for the possibility of COVID-19.
Related Resources
- American Psychiatry Association. APA coronavirus resources. https://www.psychiatry.org/psychiatrists/covid-19-coronavirus#psych.
- Troyer EA, Kohn JN, Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;S0889-1591(20)30489-X. doi: 10.1016/j.bbi.2020.04.027.
1. World Health Organization. Rolling updates on coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Updated May 1, 2020. Accessed May 4, 2020.
2. John Hopkins University. Coronavirus resource center. World map. https://coronavirus.jhu.edu/map.html. Accessed May 4, 2020.
3. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091.
4. Mao L, Wang M, Chen S, et al. Neurologic manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study [published online February 25, 2020]. JAMA Neurol. 2020;e201127. doi: 10.1101/2020.02.22.20026500.
5. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients [published online February 27, 2020]. J Med Virol. 2020;92(6). doi: 10.1002/jmv.25728.
6. Baig AM, Khaleeq A, Ali E, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995-998.
7. Xia H, Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep. 2010;12(3):170-175.
8. Steardo L, Steardo L Jr, Zorec R, et al. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19 [published online March 29, 2020]. Acta Physiol (Oxf). 2020;e13473. doi: 10.1111/apha.13473.
9. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses [published online March 30, 2020]. Brain Behav Immun. 2020;S0889-1591(20)30357-3. doi: 10.1016/j.bbi.2020.03.031.
10. Mehta P, McAuley DF, Brown M, et al; HLH Across Specialty Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034.
11. McNeil JB, Hughes CG, Girard T, et al. Plasma biomarkers of inflammation, coagulation, and brain injury as predictors of delirium duration in older hospitalized patients. PLoS One. 2019;14(12):e0226412. doi: 10.1371/journal.pone.0226412.
12. Heneka MT, Carson MJ, Khoury JE, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388‐405.
13. Beijing hospital confirms nervous system infections by novel coronavirus. XINHUANET. http://www.xinhuanet.com/english/2020-03/05/c_138846529.htm. Published May 3, 2020. Accessed May 4, 2020.
14. Filatov A, Sharma P, Hindi F, et al. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3):e7352. doi: 10.7759/cureus.7352.
15. Poyiadji N, Shahin G, Noujaim D, et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features [published online March 31, 2020]. Radiology. 2020;201187. doi: 10.1148/radiol.2020201187.
16. Karimi N, Razavi AS, Rouhani N. Frequent convulsive seizures in an adult patient with COVID-19: a case report. Iran Red Crescent Med J. 2020;22(3):e102828. doi: 10.5812/ircmj.102828.
17. Zhao H, Shen D, Zhou H, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383-384.
18. Sasannejad C, Ely EW, Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit Care. 2019;23(1):352.
19. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR [published online February 19, 2020]. Radiology. 2020;200432. doi: 10.1148/radiol.2020200432.
1. World Health Organization. Rolling updates on coronavirus disease (COVID-19). https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen. Updated May 1, 2020. Accessed May 4, 2020.
2. John Hopkins University. Coronavirus resource center. World map. https://coronavirus.jhu.edu/map.html. Accessed May 4, 2020.
3. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091.
4. Mao L, Wang M, Chen S, et al. Neurologic manifestations of hospitalized patients with COVID-19 in Wuhan, China: a retrospective case series study [published online February 25, 2020]. JAMA Neurol. 2020;e201127. doi: 10.1101/2020.02.22.20026500.
5. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients [published online February 27, 2020]. J Med Virol. 2020;92(6). doi: 10.1002/jmv.25728.
6. Baig AM, Khaleeq A, Ali E, et al. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11(7):995-998.
7. Xia H, Lazartigues E. Angiotensin-converting enzyme 2: central regulator for cardiovascular function. Curr Hypertens Rep. 2010;12(3):170-175.
8. Steardo L, Steardo L Jr, Zorec R, et al. Neuroinfection may contribute to pathophysiology and clinical manifestations of COVID-19 [published online March 29, 2020]. Acta Physiol (Oxf). 2020;e13473. doi: 10.1111/apha.13473.
9. Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses [published online March 30, 2020]. Brain Behav Immun. 2020;S0889-1591(20)30357-3. doi: 10.1016/j.bbi.2020.03.031.
10. Mehta P, McAuley DF, Brown M, et al; HLH Across Specialty Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033-1034.
11. McNeil JB, Hughes CG, Girard T, et al. Plasma biomarkers of inflammation, coagulation, and brain injury as predictors of delirium duration in older hospitalized patients. PLoS One. 2019;14(12):e0226412. doi: 10.1371/journal.pone.0226412.
12. Heneka MT, Carson MJ, Khoury JE, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14(4):388‐405.
13. Beijing hospital confirms nervous system infections by novel coronavirus. XINHUANET. http://www.xinhuanet.com/english/2020-03/05/c_138846529.htm. Published May 3, 2020. Accessed May 4, 2020.
14. Filatov A, Sharma P, Hindi F, et al. Neurological complications of coronavirus disease (COVID-19): encephalopathy. Cureus. 2020;12(3):e7352. doi: 10.7759/cureus.7352.
15. Poyiadji N, Shahin G, Noujaim D, et al. COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features [published online March 31, 2020]. Radiology. 2020;201187. doi: 10.1148/radiol.2020201187.
16. Karimi N, Razavi AS, Rouhani N. Frequent convulsive seizures in an adult patient with COVID-19: a case report. Iran Red Crescent Med J. 2020;22(3):e102828. doi: 10.5812/ircmj.102828.
17. Zhao H, Shen D, Zhou H, et al. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19(5):383-384.
18. Sasannejad C, Ely EW, Lahiri S. Long-term cognitive impairment after acute respiratory distress syndrome: a review of clinical impact and pathophysiological mechanisms. Crit Care. 2019;23(1):352.
19. Fang Y, Zhang H, Xie J, et al. Sensitivity of chest CT for COVID-19: comparison to RT-PCR [published online February 19, 2020]. Radiology. 2020;200432. doi: 10.1148/radiol.2020200432.
Patient-focused precautions, testing help blunt pandemic effects on heme-onc unit
Keeping hematologic oncology patients on their treatment regimens and caring for inpatients with hematologic malignancies remained “manageable” during the first 2 months of the COVID-19 pandemic at Levine Cancer Institute in Charlotte, N.C.
That level of manageability has partly been because a surge in cases so far hasn’t arrived at Levine or in most of the surrounding North Carolina and South Carolina communities it serves. As of May 15, 2020, the total number of confirmed and reported COVID-19 cases had reached about 19,000 in North Carolina, and just under 9,000 in South Carolina, out of a total population in the two states of close to 16 million. What’s happened instead at Levine Cancer Institute (LCI) has been a steady but low drumbeat of cases that, by mid-May 2020, totaled fewer than 10 patients with hematologic malignancies diagnosed with COVID-19.
“For a large system with multiple sites throughout North and South Carolina that saw 17,200 new patients in 2019 – including solid tumor, benign hematology, and malignant hematology patients – with 198,000 total patient visits, it is safe to say that we are off to a good start. However, we remain in the early throes of the pandemic and we will need to remain vigilant going forward,” said Peter Voorhees, MD, professor of medicine and director of Medical Operations and Outreach Services in LCI’s Department of Hematologic Oncology and Blood Disorders.
The limited effects to date of COVID-19 at LCI has been thanks to a regimen of great caution for preventing infections that’s been consistently conveyed to LCI patients from before the pandemic’s onset, liberal testing that started early, a proactive plan to defer and temporarily replace infusion care when medically appropriate, a novel staffing approach designed to minimize and contain potential staff outbreaks, and an early pivot to virtual patient contact when feasible.
COVID-19 has had limited penetration into the LCI case load because patients have, in general, “been very careful,” said Dr. Voorhees.
“My impression is that the incidence has been low partly because our patients, especially those with hematologic malignancies including those on active chemotherapy, were already getting warned to be cautious even before the coronavirus using distancing, masking, and meticulous hand hygiene,” he said in an interview that reviewed the steps LCI took starting in March to confront and manage the effects of the then-nascent pandemic. “Since we started screening asymptomatic patients in the inpatient and outpatient settings we have identified only one patient with COVID-19 infection, which supports the low rate of infection in our patient population thus far.”
Another key step was the launch of “robust” testing for the COVID-19 virus starting on March 9, using an in-house assay from LCI’s parent health system, Atrium Health, that delivered results within 24 hours. Testing became available at LCI “earlier than at many other health systems.” At first, testing was limited to patients or staff presenting with symptoms, but in the following weeks, it expanded to more patients, including those without symptoms who were scheduled for treatment at the apheresis center, cell donors and cell recipients, patients arriving for inpatient chemotherapy or cellular therapy, patients arriving from a skilled nursing facility or similar environments, and more recently, outpatient chemotherapy patients. “We’re now doing a lot of screening,” Dr. Voorhees said. “In general, screening has been well received because patients recognize that it’s for their own safety.”
Another piece of COVID-19 preparedness was a move toward technology as an alternative to face-to-face encounters between patients and staff. “We adopted virtual technology early.” When medically appropriate, they provided either video consultations with more tech-savvy patients or telephone-based virtual visits for patients who preferred a more familiar interface. As LCI starts the process of reentry for patients whose face-to-face encounters were deferred, virtual visits will remain an important facet of maintaining care while limiting exposure for appropriate patients and facilitating adequate space for social distancing in the clinics and infusion centers.
Atrium Health also launched a “virtual hospital” geared to intensified remote management of COVID-19 patients who aren’t sick enough for hospitalization. “People who test positive automatically enter the virtual hospital and have regular interactions with their team of providers,” with LCI providing additional support for their patients who get infected. Patients receive an equipment kit that lets them monitor and transmit their vital signs. The virtual hospital program also helps expedite personal needs like delivery of prescriptions and food. “It helps patients manage at home, and has been incredibly useful,” said Dr. Voorhees.
Perhaps the most challenging step LCI clinicians took to preclude a potential COVID-19 case surge was to review all patients receiving infusional therapy or planned cellular therapy and triage those who could potentially tolerate a temporary change to either an oral, at-home regimen or to a brief hold on their treatment. Some patients on maintenance, outpatient infusion-therapy regimens “expressed concern about coming to the clinic. We looked at the patients scheduled to come for infusions and decided which visits were essential and which were deferrable without disrupting care by briefly using a noninfusional approach,” said Dr. Voorhees. The number of patients who had their regimens modified or held was “relatively small,” and with the recent recognition that a surge of infections has not occurred, “we’re now rolling out cautious reentry of those patients back to their originally prescribed chemotherapy.”
In addition to concerns of exposure at infusion clinics, there are concerns about the heightened susceptibility of immunosuppressed hematologic oncology patients to COVID-19 and their risk for more severe infection. “Our view is that, if patients tested positive, continuing immunosuppressive treatment would likely be detrimental,” so when possible treatment is temporarily suspended and then resumed when the infection has cleared. “When patients test positive for a prolonged period, a decision to resume treatment must be in the best interests of the patient and weigh the benefits of resuming therapy against the risks of incurring a more severe infection by restarting potentially immunosuppressive therapy,” Dr. Voorhees said.
The enhanced risk that cancer patients face if they develop COVID-19 was documented in a recent review of 218 cancer patients hospitalized for COVID-19 during parts of March and April in a large New York health system. The results showed an overall mortality rate of 28%, including a 37% rate among 54 patients with hematologic malignancies and a 25% rate among 164 patients with solid tumors. The mortality rate “may not be quite as high as they reported because that depends on how many patients you test, but there is no question that patients with more comorbidities are at higher risk. Patients with active cancer on chemotherapy are a particularly vulnerable population, and many have expressed concerns about their vulnerability,” he observed.
For the few LCI patients who developed COVID-19 infection, the medical staff has had several therapeutic options they could match to each patient’s needs, with help from the Atrium Health infectious disease team. LCI and Atrium Health are participating in several COVID-19 clinical treatment trials, including an investigational convalescent plasma protocol spearheaded by the Mayo Clinic. They have also opened a randomized, phase 2 trial evaluating the safety and efficacy of selinexor (Xpovio), an oral drug that’s Food and Drug Administration approved for patients with multiple myeloma, for treatment of moderate or severe COVID-19 infection. Additional studies evaluating blockade of granulocyte-macrophage colony-stimulating factor, as well as inhaled antiviral therapy, have recently launched, and several additional studies are poised to open in the coming weeks.
The LCI and Atrium Health team also has a supply of the antiviral agent remdesivir as part of the FDA’s expanded access protocol and emergency use authorization. They also have a supply of and experience administering the interleukin-6 receptor inhibitor tocilizumab (Actemra), which showed some suggestion of efficacy in limited experience treating patients with severe or critical COVID-19 infections. Clinicians at LCI have not used the investigational and unproven agents hydroxychloroquine, chloroquine, and azithromycin to either prevent or treat COVID-19.
LCI also instituted measures to try to minimize the risk that staff members could become infected and transmit the virus while asymptomatic. Following conversations held early on with COVID-19–experienced health authorities in China and Italy, the patient-facing LCI staff split into two teams starting on March 23 that alternated responsibility for direct patient interactions every 2 weeks. When one of these teams was off from direct patient contact they continued to care for patients remotely through virtual technologies. The concept was that, if a staffer became infected while remaining asymptomatic during their contact with patients, their status would either become diagnosable or resolve during their 2 weeks away from seeing any patients. Perhaps in part because of this approach infections among staff members “have not been a big issue. We’ve had an incredibly low infection rate among the LCI staff,” Dr. Voorhees noted.
By mid-May, with the imminent threat of a sudden CODIV-19 surge moderated, heme-onc operations at LCI began to cautiously revert to more normal operations. “We’re continuing patient screening for signs and symptoms of COVID-19 infection, testing for asymptomatic infections, and requiring masking and social distancing in the clinics and hospitals, but we’re starting to slowly restore the number of patients at our clinics [virtual and face to face[ and infusion centers,” and the staff’s division into two teams ended. “The idea was to get past a surge and make sure our system was not overwhelmed. We anticipated a local surge in late April, but then it kept getting pushed back. Current projections are for the infection rate among LCI patients to remain low provided that community spread remains stable or, ideally, decreases.” The LCI infectious disease staff is closely monitoring infection rates for early recognition of an outbreak, with plans to follow any new cases with contact tracing. So far, the COVID-19 pandemic at LCI “has been very manageable,” Dr. Voorhees concluded.
“We’re now better positioned to deal with a case surge if it were to happen. We could resume the two-team approach, hospital-wide plans are now in place for a future surge, and we are now up and running with robust testing and inpatient and outpatient virtual technology. The first time, we were all learning on the fly.”
The LCI biostatistics team has been prospectively collecting the Institutes’s COVID-19 patient data, with plans to report their findings.
Dr. Voorhees has had financial relationships with Bristol-Myers Squibb/Celgene, Janssen, Novartis, and Oncopeptides, none of which are relevant to this article.
Keeping hematologic oncology patients on their treatment regimens and caring for inpatients with hematologic malignancies remained “manageable” during the first 2 months of the COVID-19 pandemic at Levine Cancer Institute in Charlotte, N.C.
That level of manageability has partly been because a surge in cases so far hasn’t arrived at Levine or in most of the surrounding North Carolina and South Carolina communities it serves. As of May 15, 2020, the total number of confirmed and reported COVID-19 cases had reached about 19,000 in North Carolina, and just under 9,000 in South Carolina, out of a total population in the two states of close to 16 million. What’s happened instead at Levine Cancer Institute (LCI) has been a steady but low drumbeat of cases that, by mid-May 2020, totaled fewer than 10 patients with hematologic malignancies diagnosed with COVID-19.
“For a large system with multiple sites throughout North and South Carolina that saw 17,200 new patients in 2019 – including solid tumor, benign hematology, and malignant hematology patients – with 198,000 total patient visits, it is safe to say that we are off to a good start. However, we remain in the early throes of the pandemic and we will need to remain vigilant going forward,” said Peter Voorhees, MD, professor of medicine and director of Medical Operations and Outreach Services in LCI’s Department of Hematologic Oncology and Blood Disorders.
The limited effects to date of COVID-19 at LCI has been thanks to a regimen of great caution for preventing infections that’s been consistently conveyed to LCI patients from before the pandemic’s onset, liberal testing that started early, a proactive plan to defer and temporarily replace infusion care when medically appropriate, a novel staffing approach designed to minimize and contain potential staff outbreaks, and an early pivot to virtual patient contact when feasible.
COVID-19 has had limited penetration into the LCI case load because patients have, in general, “been very careful,” said Dr. Voorhees.
“My impression is that the incidence has been low partly because our patients, especially those with hematologic malignancies including those on active chemotherapy, were already getting warned to be cautious even before the coronavirus using distancing, masking, and meticulous hand hygiene,” he said in an interview that reviewed the steps LCI took starting in March to confront and manage the effects of the then-nascent pandemic. “Since we started screening asymptomatic patients in the inpatient and outpatient settings we have identified only one patient with COVID-19 infection, which supports the low rate of infection in our patient population thus far.”
Another key step was the launch of “robust” testing for the COVID-19 virus starting on March 9, using an in-house assay from LCI’s parent health system, Atrium Health, that delivered results within 24 hours. Testing became available at LCI “earlier than at many other health systems.” At first, testing was limited to patients or staff presenting with symptoms, but in the following weeks, it expanded to more patients, including those without symptoms who were scheduled for treatment at the apheresis center, cell donors and cell recipients, patients arriving for inpatient chemotherapy or cellular therapy, patients arriving from a skilled nursing facility or similar environments, and more recently, outpatient chemotherapy patients. “We’re now doing a lot of screening,” Dr. Voorhees said. “In general, screening has been well received because patients recognize that it’s for their own safety.”
Another piece of COVID-19 preparedness was a move toward technology as an alternative to face-to-face encounters between patients and staff. “We adopted virtual technology early.” When medically appropriate, they provided either video consultations with more tech-savvy patients or telephone-based virtual visits for patients who preferred a more familiar interface. As LCI starts the process of reentry for patients whose face-to-face encounters were deferred, virtual visits will remain an important facet of maintaining care while limiting exposure for appropriate patients and facilitating adequate space for social distancing in the clinics and infusion centers.
Atrium Health also launched a “virtual hospital” geared to intensified remote management of COVID-19 patients who aren’t sick enough for hospitalization. “People who test positive automatically enter the virtual hospital and have regular interactions with their team of providers,” with LCI providing additional support for their patients who get infected. Patients receive an equipment kit that lets them monitor and transmit their vital signs. The virtual hospital program also helps expedite personal needs like delivery of prescriptions and food. “It helps patients manage at home, and has been incredibly useful,” said Dr. Voorhees.
Perhaps the most challenging step LCI clinicians took to preclude a potential COVID-19 case surge was to review all patients receiving infusional therapy or planned cellular therapy and triage those who could potentially tolerate a temporary change to either an oral, at-home regimen or to a brief hold on their treatment. Some patients on maintenance, outpatient infusion-therapy regimens “expressed concern about coming to the clinic. We looked at the patients scheduled to come for infusions and decided which visits were essential and which were deferrable without disrupting care by briefly using a noninfusional approach,” said Dr. Voorhees. The number of patients who had their regimens modified or held was “relatively small,” and with the recent recognition that a surge of infections has not occurred, “we’re now rolling out cautious reentry of those patients back to their originally prescribed chemotherapy.”
In addition to concerns of exposure at infusion clinics, there are concerns about the heightened susceptibility of immunosuppressed hematologic oncology patients to COVID-19 and their risk for more severe infection. “Our view is that, if patients tested positive, continuing immunosuppressive treatment would likely be detrimental,” so when possible treatment is temporarily suspended and then resumed when the infection has cleared. “When patients test positive for a prolonged period, a decision to resume treatment must be in the best interests of the patient and weigh the benefits of resuming therapy against the risks of incurring a more severe infection by restarting potentially immunosuppressive therapy,” Dr. Voorhees said.
The enhanced risk that cancer patients face if they develop COVID-19 was documented in a recent review of 218 cancer patients hospitalized for COVID-19 during parts of March and April in a large New York health system. The results showed an overall mortality rate of 28%, including a 37% rate among 54 patients with hematologic malignancies and a 25% rate among 164 patients with solid tumors. The mortality rate “may not be quite as high as they reported because that depends on how many patients you test, but there is no question that patients with more comorbidities are at higher risk. Patients with active cancer on chemotherapy are a particularly vulnerable population, and many have expressed concerns about their vulnerability,” he observed.
For the few LCI patients who developed COVID-19 infection, the medical staff has had several therapeutic options they could match to each patient’s needs, with help from the Atrium Health infectious disease team. LCI and Atrium Health are participating in several COVID-19 clinical treatment trials, including an investigational convalescent plasma protocol spearheaded by the Mayo Clinic. They have also opened a randomized, phase 2 trial evaluating the safety and efficacy of selinexor (Xpovio), an oral drug that’s Food and Drug Administration approved for patients with multiple myeloma, for treatment of moderate or severe COVID-19 infection. Additional studies evaluating blockade of granulocyte-macrophage colony-stimulating factor, as well as inhaled antiviral therapy, have recently launched, and several additional studies are poised to open in the coming weeks.
The LCI and Atrium Health team also has a supply of the antiviral agent remdesivir as part of the FDA’s expanded access protocol and emergency use authorization. They also have a supply of and experience administering the interleukin-6 receptor inhibitor tocilizumab (Actemra), which showed some suggestion of efficacy in limited experience treating patients with severe or critical COVID-19 infections. Clinicians at LCI have not used the investigational and unproven agents hydroxychloroquine, chloroquine, and azithromycin to either prevent or treat COVID-19.
LCI also instituted measures to try to minimize the risk that staff members could become infected and transmit the virus while asymptomatic. Following conversations held early on with COVID-19–experienced health authorities in China and Italy, the patient-facing LCI staff split into two teams starting on March 23 that alternated responsibility for direct patient interactions every 2 weeks. When one of these teams was off from direct patient contact they continued to care for patients remotely through virtual technologies. The concept was that, if a staffer became infected while remaining asymptomatic during their contact with patients, their status would either become diagnosable or resolve during their 2 weeks away from seeing any patients. Perhaps in part because of this approach infections among staff members “have not been a big issue. We’ve had an incredibly low infection rate among the LCI staff,” Dr. Voorhees noted.
By mid-May, with the imminent threat of a sudden CODIV-19 surge moderated, heme-onc operations at LCI began to cautiously revert to more normal operations. “We’re continuing patient screening for signs and symptoms of COVID-19 infection, testing for asymptomatic infections, and requiring masking and social distancing in the clinics and hospitals, but we’re starting to slowly restore the number of patients at our clinics [virtual and face to face[ and infusion centers,” and the staff’s division into two teams ended. “The idea was to get past a surge and make sure our system was not overwhelmed. We anticipated a local surge in late April, but then it kept getting pushed back. Current projections are for the infection rate among LCI patients to remain low provided that community spread remains stable or, ideally, decreases.” The LCI infectious disease staff is closely monitoring infection rates for early recognition of an outbreak, with plans to follow any new cases with contact tracing. So far, the COVID-19 pandemic at LCI “has been very manageable,” Dr. Voorhees concluded.
“We’re now better positioned to deal with a case surge if it were to happen. We could resume the two-team approach, hospital-wide plans are now in place for a future surge, and we are now up and running with robust testing and inpatient and outpatient virtual technology. The first time, we were all learning on the fly.”
The LCI biostatistics team has been prospectively collecting the Institutes’s COVID-19 patient data, with plans to report their findings.
Dr. Voorhees has had financial relationships with Bristol-Myers Squibb/Celgene, Janssen, Novartis, and Oncopeptides, none of which are relevant to this article.
Keeping hematologic oncology patients on their treatment regimens and caring for inpatients with hematologic malignancies remained “manageable” during the first 2 months of the COVID-19 pandemic at Levine Cancer Institute in Charlotte, N.C.
That level of manageability has partly been because a surge in cases so far hasn’t arrived at Levine or in most of the surrounding North Carolina and South Carolina communities it serves. As of May 15, 2020, the total number of confirmed and reported COVID-19 cases had reached about 19,000 in North Carolina, and just under 9,000 in South Carolina, out of a total population in the two states of close to 16 million. What’s happened instead at Levine Cancer Institute (LCI) has been a steady but low drumbeat of cases that, by mid-May 2020, totaled fewer than 10 patients with hematologic malignancies diagnosed with COVID-19.
“For a large system with multiple sites throughout North and South Carolina that saw 17,200 new patients in 2019 – including solid tumor, benign hematology, and malignant hematology patients – with 198,000 total patient visits, it is safe to say that we are off to a good start. However, we remain in the early throes of the pandemic and we will need to remain vigilant going forward,” said Peter Voorhees, MD, professor of medicine and director of Medical Operations and Outreach Services in LCI’s Department of Hematologic Oncology and Blood Disorders.
The limited effects to date of COVID-19 at LCI has been thanks to a regimen of great caution for preventing infections that’s been consistently conveyed to LCI patients from before the pandemic’s onset, liberal testing that started early, a proactive plan to defer and temporarily replace infusion care when medically appropriate, a novel staffing approach designed to minimize and contain potential staff outbreaks, and an early pivot to virtual patient contact when feasible.
COVID-19 has had limited penetration into the LCI case load because patients have, in general, “been very careful,” said Dr. Voorhees.
“My impression is that the incidence has been low partly because our patients, especially those with hematologic malignancies including those on active chemotherapy, were already getting warned to be cautious even before the coronavirus using distancing, masking, and meticulous hand hygiene,” he said in an interview that reviewed the steps LCI took starting in March to confront and manage the effects of the then-nascent pandemic. “Since we started screening asymptomatic patients in the inpatient and outpatient settings we have identified only one patient with COVID-19 infection, which supports the low rate of infection in our patient population thus far.”
Another key step was the launch of “robust” testing for the COVID-19 virus starting on March 9, using an in-house assay from LCI’s parent health system, Atrium Health, that delivered results within 24 hours. Testing became available at LCI “earlier than at many other health systems.” At first, testing was limited to patients or staff presenting with symptoms, but in the following weeks, it expanded to more patients, including those without symptoms who were scheduled for treatment at the apheresis center, cell donors and cell recipients, patients arriving for inpatient chemotherapy or cellular therapy, patients arriving from a skilled nursing facility or similar environments, and more recently, outpatient chemotherapy patients. “We’re now doing a lot of screening,” Dr. Voorhees said. “In general, screening has been well received because patients recognize that it’s for their own safety.”
Another piece of COVID-19 preparedness was a move toward technology as an alternative to face-to-face encounters between patients and staff. “We adopted virtual technology early.” When medically appropriate, they provided either video consultations with more tech-savvy patients or telephone-based virtual visits for patients who preferred a more familiar interface. As LCI starts the process of reentry for patients whose face-to-face encounters were deferred, virtual visits will remain an important facet of maintaining care while limiting exposure for appropriate patients and facilitating adequate space for social distancing in the clinics and infusion centers.
Atrium Health also launched a “virtual hospital” geared to intensified remote management of COVID-19 patients who aren’t sick enough for hospitalization. “People who test positive automatically enter the virtual hospital and have regular interactions with their team of providers,” with LCI providing additional support for their patients who get infected. Patients receive an equipment kit that lets them monitor and transmit their vital signs. The virtual hospital program also helps expedite personal needs like delivery of prescriptions and food. “It helps patients manage at home, and has been incredibly useful,” said Dr. Voorhees.
Perhaps the most challenging step LCI clinicians took to preclude a potential COVID-19 case surge was to review all patients receiving infusional therapy or planned cellular therapy and triage those who could potentially tolerate a temporary change to either an oral, at-home regimen or to a brief hold on their treatment. Some patients on maintenance, outpatient infusion-therapy regimens “expressed concern about coming to the clinic. We looked at the patients scheduled to come for infusions and decided which visits were essential and which were deferrable without disrupting care by briefly using a noninfusional approach,” said Dr. Voorhees. The number of patients who had their regimens modified or held was “relatively small,” and with the recent recognition that a surge of infections has not occurred, “we’re now rolling out cautious reentry of those patients back to their originally prescribed chemotherapy.”
In addition to concerns of exposure at infusion clinics, there are concerns about the heightened susceptibility of immunosuppressed hematologic oncology patients to COVID-19 and their risk for more severe infection. “Our view is that, if patients tested positive, continuing immunosuppressive treatment would likely be detrimental,” so when possible treatment is temporarily suspended and then resumed when the infection has cleared. “When patients test positive for a prolonged period, a decision to resume treatment must be in the best interests of the patient and weigh the benefits of resuming therapy against the risks of incurring a more severe infection by restarting potentially immunosuppressive therapy,” Dr. Voorhees said.
The enhanced risk that cancer patients face if they develop COVID-19 was documented in a recent review of 218 cancer patients hospitalized for COVID-19 during parts of March and April in a large New York health system. The results showed an overall mortality rate of 28%, including a 37% rate among 54 patients with hematologic malignancies and a 25% rate among 164 patients with solid tumors. The mortality rate “may not be quite as high as they reported because that depends on how many patients you test, but there is no question that patients with more comorbidities are at higher risk. Patients with active cancer on chemotherapy are a particularly vulnerable population, and many have expressed concerns about their vulnerability,” he observed.
For the few LCI patients who developed COVID-19 infection, the medical staff has had several therapeutic options they could match to each patient’s needs, with help from the Atrium Health infectious disease team. LCI and Atrium Health are participating in several COVID-19 clinical treatment trials, including an investigational convalescent plasma protocol spearheaded by the Mayo Clinic. They have also opened a randomized, phase 2 trial evaluating the safety and efficacy of selinexor (Xpovio), an oral drug that’s Food and Drug Administration approved for patients with multiple myeloma, for treatment of moderate or severe COVID-19 infection. Additional studies evaluating blockade of granulocyte-macrophage colony-stimulating factor, as well as inhaled antiviral therapy, have recently launched, and several additional studies are poised to open in the coming weeks.
The LCI and Atrium Health team also has a supply of the antiviral agent remdesivir as part of the FDA’s expanded access protocol and emergency use authorization. They also have a supply of and experience administering the interleukin-6 receptor inhibitor tocilizumab (Actemra), which showed some suggestion of efficacy in limited experience treating patients with severe or critical COVID-19 infections. Clinicians at LCI have not used the investigational and unproven agents hydroxychloroquine, chloroquine, and azithromycin to either prevent or treat COVID-19.
LCI also instituted measures to try to minimize the risk that staff members could become infected and transmit the virus while asymptomatic. Following conversations held early on with COVID-19–experienced health authorities in China and Italy, the patient-facing LCI staff split into two teams starting on March 23 that alternated responsibility for direct patient interactions every 2 weeks. When one of these teams was off from direct patient contact they continued to care for patients remotely through virtual technologies. The concept was that, if a staffer became infected while remaining asymptomatic during their contact with patients, their status would either become diagnosable or resolve during their 2 weeks away from seeing any patients. Perhaps in part because of this approach infections among staff members “have not been a big issue. We’ve had an incredibly low infection rate among the LCI staff,” Dr. Voorhees noted.
By mid-May, with the imminent threat of a sudden CODIV-19 surge moderated, heme-onc operations at LCI began to cautiously revert to more normal operations. “We’re continuing patient screening for signs and symptoms of COVID-19 infection, testing for asymptomatic infections, and requiring masking and social distancing in the clinics and hospitals, but we’re starting to slowly restore the number of patients at our clinics [virtual and face to face[ and infusion centers,” and the staff’s division into two teams ended. “The idea was to get past a surge and make sure our system was not overwhelmed. We anticipated a local surge in late April, but then it kept getting pushed back. Current projections are for the infection rate among LCI patients to remain low provided that community spread remains stable or, ideally, decreases.” The LCI infectious disease staff is closely monitoring infection rates for early recognition of an outbreak, with plans to follow any new cases with contact tracing. So far, the COVID-19 pandemic at LCI “has been very manageable,” Dr. Voorhees concluded.
“We’re now better positioned to deal with a case surge if it were to happen. We could resume the two-team approach, hospital-wide plans are now in place for a future surge, and we are now up and running with robust testing and inpatient and outpatient virtual technology. The first time, we were all learning on the fly.”
The LCI biostatistics team has been prospectively collecting the Institutes’s COVID-19 patient data, with plans to report their findings.
Dr. Voorhees has had financial relationships with Bristol-Myers Squibb/Celgene, Janssen, Novartis, and Oncopeptides, none of which are relevant to this article.
Psychiatrists’ pay increases, most happy with income, career
Psychiatrists continue to rank close to the bottom of the compensation ladder, but they made more this year than last year and they continue to enjoy their profession, findings from the newly released Medscape Psychiatrist Compensation Report 2020 show.
Psychiatrists’ average annual income this year rose to $268,000, up from $260,000 last year. Two-thirds of psychiatrists feel fairly compensated, similar to last year’s percentage.
Psychiatrists are below the middle earners of all physician specialties, ranking eighth from the bottom, just below neurologists ($280,000), but ahead of rheumatologists ($262,000) and internists ($251,000).
Orthopedists are the top earners ($511,000 annual pay), followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according to the overall Medscape Physician Compensation Report 2020, which covers U.S. physicians as a whole. The survey included more than 17,000 physicians in more than 30 specialties.
COVID-19 impact
An important caveat is that data for this year’s report were collected prior to Feb. 10, 2020, and therefore reflect physician salary and income prior to the COVID-19 crisis, which has had a huge impact on physicians.
For example, since the start of the crisis, data show that physician practices have seen a 55% dip in revenue and a 60% dip in patient volume on average. Hospitals and physician groups nationwide have implemented layoffs, furloughs, and pay cuts.
In March, 43,000 health care workers were laid off; 9% of independent medical practices reported that they have closed their practices, at least temporarily.
There continues to be a gender pay gap in psychiatry, with male psychiatrists earning about 21% more than their female peers ($289,000 vs. $239,000). Among all specialists, men earn 31% more than women, similar to last year’s figure of 33%. There continues to be a 25% gender pay gap among primary care physicians.
Psychiatrists report that they are eligible for $26,000 in annual incentive bonuses. Such bonuses are highest among orthopedists ($96,000) and lowest among family medicine physicians ($24,000).
Close to one-third of psychiatrists (and physicians overall) who have incentive bonuses say the prospect of the bonus has encouraged them to work longer hours.
Two thirds of psychiatrists say they receive more than three quarters of their potential annual incentive bonus. On average, psychiatrists achieve 70% of their potential bonus, similar to physicians overall (67%).
However, COVID-19 may change that. Experts recently interviewed by Medscape Medical News noted that productivity benchmarks for physicians are likely to be lowered in light of plunging patient numbers from COVID-19, and bonuses are expected to take a hit.
Happy at work
On average, male psychiatrists spend 34.5 hours per week seeing patients, somewhat higher than female psychiatrists (31.5 hours); the average for all physicians is 37.9 hours per week.
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, psychiatrists spend 15.9 hours per week on paperwork and administration, about the same as physicians overall (15.6 hours).
Intensivists top the list regarding such tasks (19.1 hours), followed by internists (18.5 hours), infectious disease physicians (18.5 hours), and psychiatrists (18.3 hours). Anesthesiologists and ophthalmologists spend the least amount of time on paperwork/administration (10.0 and 9.8 hours per week, respectively).
What is most rewarding about being a psychiatrist? Making the world a better place (helping others) tops the list (28%), followed closely by relationships with and gratitude from patients (24%), being good at what they do/finding answers, diagnoses (20%), and making good money at a job they like (15%). A few cited teaching (6%) and pride in their profession (4%).
The most challenging part of practicing psychiatry is having so many rules and regulations (29%). Other challenges include dealing with difficult patients (18%), working with EHRs (13%), having to work long hours (11%), and trouble getting fair reimbursement (10%).
Despite the challenges,
Other key findings in the latest report regarding psychiatrists include the following:
- At 16%, psychiatrists rank toward the middle of physicians, potentially losing money on denied or resubmitted claims. Plastic surgery and emergency medicine have the highest percentage of claims denied or resubmitted (28% and 22%, respectively). One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.
- Only 14% of psychiatrists say they use physician assistants to treat patients in their practices, while 46% use nurse practitioners; about half (51%) use neither for patient care. Of psychiatrists who work with physician assistants and nurse practitioners in their offices, 34% say these employees have helped boost profitability.
- 56% of psychiatrists say they will continue taking new and current Medicare/Medicaid patients; only 1% say they won’t take new Medicare patients and 22% are undecided.
- The large majority of psychiatrists rely on payers; 30% rely on fee-for-service arrangements and 14% on accountable care organizations for patient-based income.
- Only 12% of psychiatrists expect to participate in the merit-based incentive payment system and only 1% expect to participate in alternative payment models.
A version of this article originally appeared on Medscape.com.
Psychiatrists continue to rank close to the bottom of the compensation ladder, but they made more this year than last year and they continue to enjoy their profession, findings from the newly released Medscape Psychiatrist Compensation Report 2020 show.
Psychiatrists’ average annual income this year rose to $268,000, up from $260,000 last year. Two-thirds of psychiatrists feel fairly compensated, similar to last year’s percentage.
Psychiatrists are below the middle earners of all physician specialties, ranking eighth from the bottom, just below neurologists ($280,000), but ahead of rheumatologists ($262,000) and internists ($251,000).
Orthopedists are the top earners ($511,000 annual pay), followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according to the overall Medscape Physician Compensation Report 2020, which covers U.S. physicians as a whole. The survey included more than 17,000 physicians in more than 30 specialties.
COVID-19 impact
An important caveat is that data for this year’s report were collected prior to Feb. 10, 2020, and therefore reflect physician salary and income prior to the COVID-19 crisis, which has had a huge impact on physicians.
For example, since the start of the crisis, data show that physician practices have seen a 55% dip in revenue and a 60% dip in patient volume on average. Hospitals and physician groups nationwide have implemented layoffs, furloughs, and pay cuts.
In March, 43,000 health care workers were laid off; 9% of independent medical practices reported that they have closed their practices, at least temporarily.
There continues to be a gender pay gap in psychiatry, with male psychiatrists earning about 21% more than their female peers ($289,000 vs. $239,000). Among all specialists, men earn 31% more than women, similar to last year’s figure of 33%. There continues to be a 25% gender pay gap among primary care physicians.
Psychiatrists report that they are eligible for $26,000 in annual incentive bonuses. Such bonuses are highest among orthopedists ($96,000) and lowest among family medicine physicians ($24,000).
Close to one-third of psychiatrists (and physicians overall) who have incentive bonuses say the prospect of the bonus has encouraged them to work longer hours.
Two thirds of psychiatrists say they receive more than three quarters of their potential annual incentive bonus. On average, psychiatrists achieve 70% of their potential bonus, similar to physicians overall (67%).
However, COVID-19 may change that. Experts recently interviewed by Medscape Medical News noted that productivity benchmarks for physicians are likely to be lowered in light of plunging patient numbers from COVID-19, and bonuses are expected to take a hit.
Happy at work
On average, male psychiatrists spend 34.5 hours per week seeing patients, somewhat higher than female psychiatrists (31.5 hours); the average for all physicians is 37.9 hours per week.
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, psychiatrists spend 15.9 hours per week on paperwork and administration, about the same as physicians overall (15.6 hours).
Intensivists top the list regarding such tasks (19.1 hours), followed by internists (18.5 hours), infectious disease physicians (18.5 hours), and psychiatrists (18.3 hours). Anesthesiologists and ophthalmologists spend the least amount of time on paperwork/administration (10.0 and 9.8 hours per week, respectively).
What is most rewarding about being a psychiatrist? Making the world a better place (helping others) tops the list (28%), followed closely by relationships with and gratitude from patients (24%), being good at what they do/finding answers, diagnoses (20%), and making good money at a job they like (15%). A few cited teaching (6%) and pride in their profession (4%).
The most challenging part of practicing psychiatry is having so many rules and regulations (29%). Other challenges include dealing with difficult patients (18%), working with EHRs (13%), having to work long hours (11%), and trouble getting fair reimbursement (10%).
Despite the challenges,
Other key findings in the latest report regarding psychiatrists include the following:
- At 16%, psychiatrists rank toward the middle of physicians, potentially losing money on denied or resubmitted claims. Plastic surgery and emergency medicine have the highest percentage of claims denied or resubmitted (28% and 22%, respectively). One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.
- Only 14% of psychiatrists say they use physician assistants to treat patients in their practices, while 46% use nurse practitioners; about half (51%) use neither for patient care. Of psychiatrists who work with physician assistants and nurse practitioners in their offices, 34% say these employees have helped boost profitability.
- 56% of psychiatrists say they will continue taking new and current Medicare/Medicaid patients; only 1% say they won’t take new Medicare patients and 22% are undecided.
- The large majority of psychiatrists rely on payers; 30% rely on fee-for-service arrangements and 14% on accountable care organizations for patient-based income.
- Only 12% of psychiatrists expect to participate in the merit-based incentive payment system and only 1% expect to participate in alternative payment models.
A version of this article originally appeared on Medscape.com.
Psychiatrists continue to rank close to the bottom of the compensation ladder, but they made more this year than last year and they continue to enjoy their profession, findings from the newly released Medscape Psychiatrist Compensation Report 2020 show.
Psychiatrists’ average annual income this year rose to $268,000, up from $260,000 last year. Two-thirds of psychiatrists feel fairly compensated, similar to last year’s percentage.
Psychiatrists are below the middle earners of all physician specialties, ranking eighth from the bottom, just below neurologists ($280,000), but ahead of rheumatologists ($262,000) and internists ($251,000).
Orthopedists are the top earners ($511,000 annual pay), followed by plastic surgeons ($479,000), otolaryngologists ($455,000), and cardiologists ($438,000), according to the overall Medscape Physician Compensation Report 2020, which covers U.S. physicians as a whole. The survey included more than 17,000 physicians in more than 30 specialties.
COVID-19 impact
An important caveat is that data for this year’s report were collected prior to Feb. 10, 2020, and therefore reflect physician salary and income prior to the COVID-19 crisis, which has had a huge impact on physicians.
For example, since the start of the crisis, data show that physician practices have seen a 55% dip in revenue and a 60% dip in patient volume on average. Hospitals and physician groups nationwide have implemented layoffs, furloughs, and pay cuts.
In March, 43,000 health care workers were laid off; 9% of independent medical practices reported that they have closed their practices, at least temporarily.
There continues to be a gender pay gap in psychiatry, with male psychiatrists earning about 21% more than their female peers ($289,000 vs. $239,000). Among all specialists, men earn 31% more than women, similar to last year’s figure of 33%. There continues to be a 25% gender pay gap among primary care physicians.
Psychiatrists report that they are eligible for $26,000 in annual incentive bonuses. Such bonuses are highest among orthopedists ($96,000) and lowest among family medicine physicians ($24,000).
Close to one-third of psychiatrists (and physicians overall) who have incentive bonuses say the prospect of the bonus has encouraged them to work longer hours.
Two thirds of psychiatrists say they receive more than three quarters of their potential annual incentive bonus. On average, psychiatrists achieve 70% of their potential bonus, similar to physicians overall (67%).
However, COVID-19 may change that. Experts recently interviewed by Medscape Medical News noted that productivity benchmarks for physicians are likely to be lowered in light of plunging patient numbers from COVID-19, and bonuses are expected to take a hit.
Happy at work
On average, male psychiatrists spend 34.5 hours per week seeing patients, somewhat higher than female psychiatrists (31.5 hours); the average for all physicians is 37.9 hours per week.
Bureaucratic tasks continue to be a burden for physicians in all specialties. On average, psychiatrists spend 15.9 hours per week on paperwork and administration, about the same as physicians overall (15.6 hours).
Intensivists top the list regarding such tasks (19.1 hours), followed by internists (18.5 hours), infectious disease physicians (18.5 hours), and psychiatrists (18.3 hours). Anesthesiologists and ophthalmologists spend the least amount of time on paperwork/administration (10.0 and 9.8 hours per week, respectively).
What is most rewarding about being a psychiatrist? Making the world a better place (helping others) tops the list (28%), followed closely by relationships with and gratitude from patients (24%), being good at what they do/finding answers, diagnoses (20%), and making good money at a job they like (15%). A few cited teaching (6%) and pride in their profession (4%).
The most challenging part of practicing psychiatry is having so many rules and regulations (29%). Other challenges include dealing with difficult patients (18%), working with EHRs (13%), having to work long hours (11%), and trouble getting fair reimbursement (10%).
Despite the challenges,
Other key findings in the latest report regarding psychiatrists include the following:
- At 16%, psychiatrists rank toward the middle of physicians, potentially losing money on denied or resubmitted claims. Plastic surgery and emergency medicine have the highest percentage of claims denied or resubmitted (28% and 22%, respectively). One study found that, on average, 63% of denied claims are recoverable, but healthcare professionals spend about $118 per claim on appeals.
- Only 14% of psychiatrists say they use physician assistants to treat patients in their practices, while 46% use nurse practitioners; about half (51%) use neither for patient care. Of psychiatrists who work with physician assistants and nurse practitioners in their offices, 34% say these employees have helped boost profitability.
- 56% of psychiatrists say they will continue taking new and current Medicare/Medicaid patients; only 1% say they won’t take new Medicare patients and 22% are undecided.
- The large majority of psychiatrists rely on payers; 30% rely on fee-for-service arrangements and 14% on accountable care organizations for patient-based income.
- Only 12% of psychiatrists expect to participate in the merit-based incentive payment system and only 1% expect to participate in alternative payment models.
A version of this article originally appeared on Medscape.com.
Many clinicians still not asking about postpartum depression
Health care providers fail to ask one in five prenatal patients and one in eight postpartum patients about depression, according to the Centers for Disease Control and Prevention. Although the prevalence of screening has risen in recent years, many women could be suffering in silence.
“[U]ndetected and untreated perinatal depression can have negative health consequences for the mothers and their babies,” said coauthor Jean Y. Ko, PhD, from the division of reproductive health at the National Center for Chronic Disease Prevention and Health Promotion.
Dr. Ko and colleagues reported their findings in an article published in Morbidity and Mortality Weekly Report.
The researchers analyzed self-reported data on postpartum depressive symptoms (PDS) collected in 2018 by the Pregnancy Risk Assessment Monitoring System (PRAMS). Participants were stratified on the basis of location and maternal and infant characteristics, including age, race/ethnicity, and education level. Women who had recently given birth to one or more live infants answered questions about whether they had been screened by health care providers for depression during perinatal visits.
The prevalence of PDS among women from 31 PRAMS sites was 13.2%. States with lower prevalences included Illinois (9.7%), Massachusetts (10.3%), and Wisconsin (10.5%); states with higher prevalences included Mississippi (23.5%), West Virginia (19.4%), and Michigan (16.4%).
Some groups were at higher risk for PDS than others. The prevalence was greater than 20% among women who were aged 19 years or younger, were of American Indian or Alaska Native ethnicity, smoked during the perinatal period, experienced perinatal depression, or whose infant died after birth.
Depressive symptoms were also more common among women who received assistance from the Women, Infants, and Children program; were Medicaid beneficiaries at the time of delivery; smoked cigarettes during the last trimester of pregnancy; breastfed their infants for fewer than 8 weeks; or had experienced intimate partner violence while pregnant or before.
Small rise in screening
Overall, 79.1% of women said a health care provider had inquired about depression during the prenatal period. Prenatal screening for depression was lowest in Puerto Rico (50.7%), Mississippi (69.4%), Utah (69.5%), and Kentucky (69.5%) and was highest in Alaska (90.7%), Minnesota (90.6%), and Maine (90.5%).
Among 22 continuously reporting sites, the prevalence of prenatal depression screening rose significantly from 76.2% in 2016 to 79.3% in 2018 (P < .05) .
“It is unclear what might account for this small increase,” Dr. Ko said. “There may be additional factors, such as women may be becoming more comfortable reporting symptoms of depression. With continued awareness about the need to screen every pregnant and postpartum woman for depression, we can expect things to continue to improve.”
Overall, 90.1% of respondents reported a postpartum visit; of those, 87.4% said a health care provider had asked about depression during that visit.
Screening during the postpartum period was highest in Vermont (96.2%), Minnesota (95.9%), and Maine (95.5%) and was lowest in Puerto Rico (50.7%), New York City (73.1%), and Louisiana (75.0%).
Among the 22 sites that reported continuously, the prevalence of screening for postpartum depression rose significantly from 84.1% to 88.0% (P < .05), “with an average annual percentage point increase of 1.8%,” the authors wrote.
‘Missed opportunities’
“PRAMS responses are reported an average of 4 months postpartum, which suggests persistence of [depressive] symptoms,” the authors wrote.
Dr. Ko said that mental health conditions play a role in approximately 9% of pregnancy-related deaths and that not asking about depression represents “missed opportunities to potentially identify and treat women with depression.” The United States Preventive Services Task Force recommends screening all adults for depression, including women during pregnancy and the postpartum period, she added.
When asked what can be done to improve screening that has not already been tried, Dr. Ko said the CDC is currently evaluating a study called the Program in Support of Moms (PRISM), which “is designed to help obstetrics and gynecology practices address the significant public health issue of depression during and after pregnancy. PRISM aims to close gaps in health care delivery to ensure that women with depression during and after pregnancy receive the best treatment, which can result in improvement in their symptoms.”
Dr. Ko added that the Health Resources and Services Administration has funded seven states to begin “programs to support providers to screen, assess, refer, and treat pregnant and postpartum women for depression and other behavioral health conditions. States can use initiatives like Healthy Start, home visiting, and Title V Maternal and Child Health Services Block Grant programs as levers to improve screening and address maternal depression.
“Screening is just one part of addressing perinatal depression. Health care providers need to refer women to appropriate resources in order to get the proper diagnosis, treatment, and follow-up care for management of depression,” Dr. Ko concluded.
The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Health care providers fail to ask one in five prenatal patients and one in eight postpartum patients about depression, according to the Centers for Disease Control and Prevention. Although the prevalence of screening has risen in recent years, many women could be suffering in silence.
“[U]ndetected and untreated perinatal depression can have negative health consequences for the mothers and their babies,” said coauthor Jean Y. Ko, PhD, from the division of reproductive health at the National Center for Chronic Disease Prevention and Health Promotion.
Dr. Ko and colleagues reported their findings in an article published in Morbidity and Mortality Weekly Report.
The researchers analyzed self-reported data on postpartum depressive symptoms (PDS) collected in 2018 by the Pregnancy Risk Assessment Monitoring System (PRAMS). Participants were stratified on the basis of location and maternal and infant characteristics, including age, race/ethnicity, and education level. Women who had recently given birth to one or more live infants answered questions about whether they had been screened by health care providers for depression during perinatal visits.
The prevalence of PDS among women from 31 PRAMS sites was 13.2%. States with lower prevalences included Illinois (9.7%), Massachusetts (10.3%), and Wisconsin (10.5%); states with higher prevalences included Mississippi (23.5%), West Virginia (19.4%), and Michigan (16.4%).
Some groups were at higher risk for PDS than others. The prevalence was greater than 20% among women who were aged 19 years or younger, were of American Indian or Alaska Native ethnicity, smoked during the perinatal period, experienced perinatal depression, or whose infant died after birth.
Depressive symptoms were also more common among women who received assistance from the Women, Infants, and Children program; were Medicaid beneficiaries at the time of delivery; smoked cigarettes during the last trimester of pregnancy; breastfed their infants for fewer than 8 weeks; or had experienced intimate partner violence while pregnant or before.
Small rise in screening
Overall, 79.1% of women said a health care provider had inquired about depression during the prenatal period. Prenatal screening for depression was lowest in Puerto Rico (50.7%), Mississippi (69.4%), Utah (69.5%), and Kentucky (69.5%) and was highest in Alaska (90.7%), Minnesota (90.6%), and Maine (90.5%).
Among 22 continuously reporting sites, the prevalence of prenatal depression screening rose significantly from 76.2% in 2016 to 79.3% in 2018 (P < .05) .
“It is unclear what might account for this small increase,” Dr. Ko said. “There may be additional factors, such as women may be becoming more comfortable reporting symptoms of depression. With continued awareness about the need to screen every pregnant and postpartum woman for depression, we can expect things to continue to improve.”
Overall, 90.1% of respondents reported a postpartum visit; of those, 87.4% said a health care provider had asked about depression during that visit.
Screening during the postpartum period was highest in Vermont (96.2%), Minnesota (95.9%), and Maine (95.5%) and was lowest in Puerto Rico (50.7%), New York City (73.1%), and Louisiana (75.0%).
Among the 22 sites that reported continuously, the prevalence of screening for postpartum depression rose significantly from 84.1% to 88.0% (P < .05), “with an average annual percentage point increase of 1.8%,” the authors wrote.
‘Missed opportunities’
“PRAMS responses are reported an average of 4 months postpartum, which suggests persistence of [depressive] symptoms,” the authors wrote.
Dr. Ko said that mental health conditions play a role in approximately 9% of pregnancy-related deaths and that not asking about depression represents “missed opportunities to potentially identify and treat women with depression.” The United States Preventive Services Task Force recommends screening all adults for depression, including women during pregnancy and the postpartum period, she added.
When asked what can be done to improve screening that has not already been tried, Dr. Ko said the CDC is currently evaluating a study called the Program in Support of Moms (PRISM), which “is designed to help obstetrics and gynecology practices address the significant public health issue of depression during and after pregnancy. PRISM aims to close gaps in health care delivery to ensure that women with depression during and after pregnancy receive the best treatment, which can result in improvement in their symptoms.”
Dr. Ko added that the Health Resources and Services Administration has funded seven states to begin “programs to support providers to screen, assess, refer, and treat pregnant and postpartum women for depression and other behavioral health conditions. States can use initiatives like Healthy Start, home visiting, and Title V Maternal and Child Health Services Block Grant programs as levers to improve screening and address maternal depression.
“Screening is just one part of addressing perinatal depression. Health care providers need to refer women to appropriate resources in order to get the proper diagnosis, treatment, and follow-up care for management of depression,” Dr. Ko concluded.
The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Health care providers fail to ask one in five prenatal patients and one in eight postpartum patients about depression, according to the Centers for Disease Control and Prevention. Although the prevalence of screening has risen in recent years, many women could be suffering in silence.
“[U]ndetected and untreated perinatal depression can have negative health consequences for the mothers and their babies,” said coauthor Jean Y. Ko, PhD, from the division of reproductive health at the National Center for Chronic Disease Prevention and Health Promotion.
Dr. Ko and colleagues reported their findings in an article published in Morbidity and Mortality Weekly Report.
The researchers analyzed self-reported data on postpartum depressive symptoms (PDS) collected in 2018 by the Pregnancy Risk Assessment Monitoring System (PRAMS). Participants were stratified on the basis of location and maternal and infant characteristics, including age, race/ethnicity, and education level. Women who had recently given birth to one or more live infants answered questions about whether they had been screened by health care providers for depression during perinatal visits.
The prevalence of PDS among women from 31 PRAMS sites was 13.2%. States with lower prevalences included Illinois (9.7%), Massachusetts (10.3%), and Wisconsin (10.5%); states with higher prevalences included Mississippi (23.5%), West Virginia (19.4%), and Michigan (16.4%).
Some groups were at higher risk for PDS than others. The prevalence was greater than 20% among women who were aged 19 years or younger, were of American Indian or Alaska Native ethnicity, smoked during the perinatal period, experienced perinatal depression, or whose infant died after birth.
Depressive symptoms were also more common among women who received assistance from the Women, Infants, and Children program; were Medicaid beneficiaries at the time of delivery; smoked cigarettes during the last trimester of pregnancy; breastfed their infants for fewer than 8 weeks; or had experienced intimate partner violence while pregnant or before.
Small rise in screening
Overall, 79.1% of women said a health care provider had inquired about depression during the prenatal period. Prenatal screening for depression was lowest in Puerto Rico (50.7%), Mississippi (69.4%), Utah (69.5%), and Kentucky (69.5%) and was highest in Alaska (90.7%), Minnesota (90.6%), and Maine (90.5%).
Among 22 continuously reporting sites, the prevalence of prenatal depression screening rose significantly from 76.2% in 2016 to 79.3% in 2018 (P < .05) .
“It is unclear what might account for this small increase,” Dr. Ko said. “There may be additional factors, such as women may be becoming more comfortable reporting symptoms of depression. With continued awareness about the need to screen every pregnant and postpartum woman for depression, we can expect things to continue to improve.”
Overall, 90.1% of respondents reported a postpartum visit; of those, 87.4% said a health care provider had asked about depression during that visit.
Screening during the postpartum period was highest in Vermont (96.2%), Minnesota (95.9%), and Maine (95.5%) and was lowest in Puerto Rico (50.7%), New York City (73.1%), and Louisiana (75.0%).
Among the 22 sites that reported continuously, the prevalence of screening for postpartum depression rose significantly from 84.1% to 88.0% (P < .05), “with an average annual percentage point increase of 1.8%,” the authors wrote.
‘Missed opportunities’
“PRAMS responses are reported an average of 4 months postpartum, which suggests persistence of [depressive] symptoms,” the authors wrote.
Dr. Ko said that mental health conditions play a role in approximately 9% of pregnancy-related deaths and that not asking about depression represents “missed opportunities to potentially identify and treat women with depression.” The United States Preventive Services Task Force recommends screening all adults for depression, including women during pregnancy and the postpartum period, she added.
When asked what can be done to improve screening that has not already been tried, Dr. Ko said the CDC is currently evaluating a study called the Program in Support of Moms (PRISM), which “is designed to help obstetrics and gynecology practices address the significant public health issue of depression during and after pregnancy. PRISM aims to close gaps in health care delivery to ensure that women with depression during and after pregnancy receive the best treatment, which can result in improvement in their symptoms.”
Dr. Ko added that the Health Resources and Services Administration has funded seven states to begin “programs to support providers to screen, assess, refer, and treat pregnant and postpartum women for depression and other behavioral health conditions. States can use initiatives like Healthy Start, home visiting, and Title V Maternal and Child Health Services Block Grant programs as levers to improve screening and address maternal depression.
“Screening is just one part of addressing perinatal depression. Health care providers need to refer women to appropriate resources in order to get the proper diagnosis, treatment, and follow-up care for management of depression,” Dr. Ko concluded.
The authors disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
COVID-19 and Mental Health Awareness Month
#howareyoureally challenge seeks to increase access to care
We are months into the COVID-19 crisis, and mental health issues are proving to be rampant. In every crisis, there is opportunity, and this one is no different. The opportunity is clear. For Mental Health Awareness Month and beyond, we must convey a powerful message that mental health is key to our well-being and must be actively addressed. Because almost everyone has felt excess anxiety these last months, we have a unique chance to engage a wider audience.
To address the urgent need, the Mental Health Coalition was formed with the understanding that the mental health crisis is fueled by a pervasive and devastating stigma, preventing millions of individuals from being able to seek the critical treatment they need. Spearheaded by social activist and fashion designer, Kenneth Cole, it is a coalition of leading mental health organizations, brands, celebrities, and advocates who have joined forces to end the stigma surrounding mental health and to change the way people talk about, and care for, mental illness. The group’s mission listed on its website states: “We must increase the conversation around mental health. We must act to end silence, reduce stigma, and engage our community to inspire hope at this essential moment.”
As most of the United States has been under stay-at-home orders, our traditional relationships have been radically disrupted. New types of relationships are forming as we are relying even more on technology to connect us. Social media seems to be on the only “social” we can now safely engage in.
The coalition’s campaign, “#howareyoureally?” is harnessing the power of social media and creating a storytelling platform to allow users to more genuinely share their feelings in these unprecedented times. Celebrities include Whoopi Goldberg, Kendall Jenner, Chris Cuomo, Deepak Chopra, Kesha, and many more have already shared their stories.
“How Are You, Really?” challenges people to answer this question using social media in an open and honest fashion while still providing hope.
The second component of the initiative is to increase access to care, and they have a long list of collaborators, including leading mental health organizations such as the American Foundation for Suicide Prevention, Anxiety and Depression Association of America, Child Mind Institute, Depression and Bipolar Support Alliance, Didi Hirsch Mental Health Services, National Alliance on Mental Illness, and many more.
We have a unique opportunity this Mental Health Awareness Month, and As a community, we must be prepared to meet the escalating needs of our population.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach, Fla. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018) and is the founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world. Dr. Ritvo also is the cofounder of the Bold Beauty Project, a nonprofit group that pairs women with disabilities with photographers who create art exhibitions to raise awareness.
#howareyoureally challenge seeks to increase access to care
#howareyoureally challenge seeks to increase access to care
We are months into the COVID-19 crisis, and mental health issues are proving to be rampant. In every crisis, there is opportunity, and this one is no different. The opportunity is clear. For Mental Health Awareness Month and beyond, we must convey a powerful message that mental health is key to our well-being and must be actively addressed. Because almost everyone has felt excess anxiety these last months, we have a unique chance to engage a wider audience.
To address the urgent need, the Mental Health Coalition was formed with the understanding that the mental health crisis is fueled by a pervasive and devastating stigma, preventing millions of individuals from being able to seek the critical treatment they need. Spearheaded by social activist and fashion designer, Kenneth Cole, it is a coalition of leading mental health organizations, brands, celebrities, and advocates who have joined forces to end the stigma surrounding mental health and to change the way people talk about, and care for, mental illness. The group’s mission listed on its website states: “We must increase the conversation around mental health. We must act to end silence, reduce stigma, and engage our community to inspire hope at this essential moment.”
As most of the United States has been under stay-at-home orders, our traditional relationships have been radically disrupted. New types of relationships are forming as we are relying even more on technology to connect us. Social media seems to be on the only “social” we can now safely engage in.
The coalition’s campaign, “#howareyoureally?” is harnessing the power of social media and creating a storytelling platform to allow users to more genuinely share their feelings in these unprecedented times. Celebrities include Whoopi Goldberg, Kendall Jenner, Chris Cuomo, Deepak Chopra, Kesha, and many more have already shared their stories.
“How Are You, Really?” challenges people to answer this question using social media in an open and honest fashion while still providing hope.
The second component of the initiative is to increase access to care, and they have a long list of collaborators, including leading mental health organizations such as the American Foundation for Suicide Prevention, Anxiety and Depression Association of America, Child Mind Institute, Depression and Bipolar Support Alliance, Didi Hirsch Mental Health Services, National Alliance on Mental Illness, and many more.
We have a unique opportunity this Mental Health Awareness Month, and As a community, we must be prepared to meet the escalating needs of our population.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach, Fla. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018) and is the founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world. Dr. Ritvo also is the cofounder of the Bold Beauty Project, a nonprofit group that pairs women with disabilities with photographers who create art exhibitions to raise awareness.
We are months into the COVID-19 crisis, and mental health issues are proving to be rampant. In every crisis, there is opportunity, and this one is no different. The opportunity is clear. For Mental Health Awareness Month and beyond, we must convey a powerful message that mental health is key to our well-being and must be actively addressed. Because almost everyone has felt excess anxiety these last months, we have a unique chance to engage a wider audience.
To address the urgent need, the Mental Health Coalition was formed with the understanding that the mental health crisis is fueled by a pervasive and devastating stigma, preventing millions of individuals from being able to seek the critical treatment they need. Spearheaded by social activist and fashion designer, Kenneth Cole, it is a coalition of leading mental health organizations, brands, celebrities, and advocates who have joined forces to end the stigma surrounding mental health and to change the way people talk about, and care for, mental illness. The group’s mission listed on its website states: “We must increase the conversation around mental health. We must act to end silence, reduce stigma, and engage our community to inspire hope at this essential moment.”
As most of the United States has been under stay-at-home orders, our traditional relationships have been radically disrupted. New types of relationships are forming as we are relying even more on technology to connect us. Social media seems to be on the only “social” we can now safely engage in.
The coalition’s campaign, “#howareyoureally?” is harnessing the power of social media and creating a storytelling platform to allow users to more genuinely share their feelings in these unprecedented times. Celebrities include Whoopi Goldberg, Kendall Jenner, Chris Cuomo, Deepak Chopra, Kesha, and many more have already shared their stories.
“How Are You, Really?” challenges people to answer this question using social media in an open and honest fashion while still providing hope.
The second component of the initiative is to increase access to care, and they have a long list of collaborators, including leading mental health organizations such as the American Foundation for Suicide Prevention, Anxiety and Depression Association of America, Child Mind Institute, Depression and Bipolar Support Alliance, Didi Hirsch Mental Health Services, National Alliance on Mental Illness, and many more.
We have a unique opportunity this Mental Health Awareness Month, and As a community, we must be prepared to meet the escalating needs of our population.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach, Fla. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018) and is the founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world. Dr. Ritvo also is the cofounder of the Bold Beauty Project, a nonprofit group that pairs women with disabilities with photographers who create art exhibitions to raise awareness.











