User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
The cult of the suicide risk assessment
Suicide is not a trivial matter – it upends families, robs partners of a loved one, prevents children from having a parent, and can destroy a parent’s most cherished being. It is not surprising that societies have repeatedly made it a goal to study and reduce suicide within their populations.
The suicide rate in the United States is trending upward, from about 10 per 100,000 in 2000 to about 15 per 100,000 in more recent reports. The increasing suicide rates have been accompanied by increasing distress among many strata of society. From a public health level, analysts are not just witnessing increasing suicide rates, but a shocking rise in all “deaths of despair,”1 among which suicide can be considered the ultimate example.
On an individual level, many know someone who has died of suicide or suffered from a serious suicide attempt. From the public health level to the individual level, advocacy has called for various interventions in the field of psychiatry to remedy this tragic problem.
Psychiatrists have been firsthand witnesses to this increasing demand for suicide interventions. When in residency, the norm was to perform a suicide risk assessment at the time of admission to the hospital and again at the time of discharge. As the years passed, the new normal within psychiatric hospitals has shifted to asking about suicidality on a daily basis.
In what seems to us like an escalating arms race, the emerging standard of care at many facilities is now not only for daily suicide risk assessments by each psychiatrist, but also to require nurses to ask about suicidality during every 8-hour shift – in addition to documented inquiries about suicidality by other allied staff on the psychiatric unit. As a result, it is not uncommon for a patient hospitalized at an academic center to receive more than half a dozen suicide risk assessments in a day (first by the medical student, at least once – often more than once – by the resident, again by the attending psychiatrist, then the social worker and three nurses in 24 hours).
One of the concerns about such an approach is the lack of logic inherent to many risk assessment tools and symptom scales. Many of us are familiar with the Patient Health Questionnaire (PHQ-9) to assess depression.2 The PHQ-9 asks to consider “over the last 2 weeks, how often have you ...” in relation to nine symptoms associated with depression. It has always defied reason to perform a PHQ-9 every day and expect the answers to change from “nearly every day” to “not at all,” considering only 1 day has passed since the last time the patient has answered the questions. Yet daily, or near daily, PHQ-9 scores are a frequently used tool of tracking symptom improvement in response to treatments, such as electroconvulsive therapy, performed multiple times a week.
One can argue that the patient’s perspective on how symptomatic he or she has been over the past 2 weeks may change rapidly with alleviation of a depressed mood. However, the PHQ-9 is both reported to be, and often regarded as, an objective score. If one wishes to utilize it as such, the defense of its use should not be that it is a subjective report with just as much utility as “Rate your depression on a scale of 0-27.”
Similarly, many suicide scales were intended to assess thoughts of suicide in the past month3 or have been re-tooled to address this particular concern by asking “since the last contact.”4 It is baffling to see a chart with many dozens of suicide risk assessments with at times widely differing answers, yet all measuring thoughts of suicide in the past month. Is one to expect the answer to “How many times have you had these thoughts [of suicide ideation]? (1) Less than once a week (2) Once a week ...” to change between 8 a.m. and noon? Furthermore, for the purpose of assessing acute risk of suicidality in the immediate future, to only consider symptoms since the last contact – or past 2 weeks, past month, etc. – is of unclear significance.
Provider liability
Another concern is the liability placed on providers. A common problem encountered in the inpatient setting is insurance companies refusing to reimburse a hospital stay for depressed patients denying suicidality.
Any provider in the position of caring for such a patient must ask: What is the likelihood of someone providing a false negative – a false denial of suicidality? Is the likelihood of a suicidal person denying suicidality different if asked 5 or 10 or more times in a day? There are innumerable instances where a patient at a very high risk of self-harm has denied suicidality, been discharged from the hospital, and suffered terrible consequences. Ethically, the psychiatrist aware of this risk is no more at ease discharging these patients, whether it is one suicide risk scale or a dozen that suggests a patient is at low risk.
Alternatively, it may feel untenable from a medicolegal perspective for a psychiatrist to discharge a patient denying suicidality when the chart includes over a dozen previously documented elevated suicide risk assessments in the past 72 hours. By placing the job of suicide risk assessment in the hands of providers of varying levels of training and responsibility, a situation is created in which the seasoned psychiatrist who would otherwise be comfortable discharging a patient feels unable to do so because every other note-writer in the record – from the triage nurse to the medical assistant to the sitter in the emergency department – has recorded the patient as high risk for suicide. When put in such a position, the thought often occurs that systems of care, rather than individual providers, are protected most by ever escalating requirements for suicide risk documentation. To make a clinical decision contrary to the body of suicide risk documentation puts the provider at risk of being scapegoated by the system of care, which can point to its illogical and ineffective, though profusely documented, suicide prevention protocols.
Limitations of risk assessments
Considering the ongoing rise in the use of suicide risk assessments, one would expect that the evidence for their efficacy was robust and well established. Yet a thorough review of suicide risk assessments funded by the MacArthur Foundation, which examined decades of research, came to disheartening conclusions: “predictive ability has not improved over the past 50 years”; “no risk factor category or subcategory is substantially stronger than any other”; and “predicting solely according to base rates may be comparable to prediction with current risk factors.”5
Those findings were consistent with the conclusions of many other studies, which have summarized the utility of suicide risk assessments as follows: “occurrence of suicide is too low to identify those individuals who are likely to die by suicide”;6 “suicide prediction models produce accurate overall classification models, but their accuracy of predicting a future event is near zero”;7 “risk stratification is too inaccurate to be clinically useful and might even be harmful”;8 “suicide risk prediction [lacks] any items or information that to a useful degree permit the identification of persons who will complete suicide”;9 “existing suicide prediction tools have little current clinical value”;10 “our current preoccupation with risk assessment has ... created a mythology with no evidence to support it.”11 And that’s to cite just a few.
Sadly, we have known about the limitations of suicide risk assessments for many decades. In 1983 a large VA prospective study, which aimed to identify veterans who will die by suicide, examined 4,800 patients with a wide range of instruments and measures.12 This study concluded that “discriminant analysis was clearly inadequate in correctly classifying the subjects. For an event as rare as suicide, our predictive tools and guides are simply not equal to the task.” The authors described the feelings of many in stating “courts and public opinion expect physicians to be able to pick out the particular persons who will later commit suicide. Although we may reconstruct causal chains and motives, we do not possess the tools to predict suicides.”
Yet, even several decades prior, in 1954, Dr. Albert Rosen performed an elegant statistical analysis and predicted that, considering the low base rate of suicide, suicide risk assessments are “of no practical value, for it would be impossible to treat the prodigious number of false positives.”13 It seems that we continue to be unable to accept Dr. Rosen’s premonition despite decades of confirmatory evidence.
“Quantity over quality”
Regardless of those sobering reports,
One can reasonably argue that the periodic performance of a suicide risk assessment may have clinical utility in reminding us of modifiable risk factors such as intoxication, social isolation, and access to lethal means. One can also reasonably argue that these risk assessments may provide useful education to patients and their families on epidemiological risk factors such as gender, age, and marital status. But our pursuit of serial suicide risk assessments throughout the day is encouraging providers to focus on a particular risk factor that changes from moment to moment and has particularly low validity, that being self-reported suicidality.
Reported suicidality is one of the few risk factors that can change from shift to shift. But 80% of people who die by suicide had not previously expressed suicidality, and 98.3% of people who have endorsed suicidality do not die by suicide.14 While the former statistic may improve with increased assessment, the later will likely worsen.
Suicide is not a trivial matter. We admire those that study it and advocate for better interventions. We have compassion for those who have suffered the loss of a loved one to suicide. Our patients have died as a result of the human limitations surrounding suicide prevention. Recognizing the weight of suicide and making an effort to avoid minimizing its immense consequences drive our desire to be honest with ourselves, our patients and their families, and society. That includes the unfortunate truth regarding the current state of the evidence and our ability to enact change.
It is our concern that the rising fascination with repeated suicide risk assessment is misguided in its current form and serves the purpose of appeasing administrators more than reflecting a scientific understanding of the literature. More sadly, we are concerned that this “quantity-over-quality” approach is yet another barrier to practicing what may be one of the few interventions with any hope of meaningfully impacting a patient’s risk of suicide in the clinical setting – spending time connecting with our patients.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a member of the psychiatry faculty at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Badre and Dr. Compton have no conflicts of interest.
References
1. Joint Economic Committee. (2019). Long Term Trends in Deaths of Despair. SCP Report 4-19.
2. Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2013;32(9):509-15. doi: 10.3928/0048-5713-20020901-06.
3. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Lifetime/Recent.
4. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Since Last Contact.
5. Franklin JC et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017 Feb;143(2):187-232. doi: 10.1037/bul0000084.
6. Beautrais AL. Further suicidal behavior among medically serious suicide attempters. Suicide Life Threat Behav. 2004 Spring;34(1):1-11. doi: 10.1521/suli.34.1.1.27772.
7. Belsher BE. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Jun 1;76(6):642-651. doi: 10.1001/jamapsychiatry.2019.0174.
8. Carter G et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Aust N Z J Psychiatry. 2016 Oct;50(10):939-1000. doi: 10.1177/0004867416661039.
9. Fosse R et al. Predictors of suicide in the patient population admitted to a locked-door psychiatric acute ward. PLoS One. 2017 Mar 16;12(3):e0173958. doi: 10.1371/journal.pone.0173958.
10. Kessler RC et al. Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry. 2020 Jan;25(1):168-79. doi: 10.1038/s41380-019-0531-0.
11. Mulder R. Problems with suicide risk assessment. Aust N Z J Psychiatry. 2011 Aug;45(8):605-7. doi: 10.3109/00048674.2011.594786.
12. Pokorny AD. Prediction of suicide in psychiatric patients: Report of a prospective study. Arch Gen Psychiatry. 1983 Mar;40(3):249-57. doi: 10.1001/archpsyc.1983.01790030019002.
13. Rosen A. Detection of suicidal patients: An example of some limitations in the prediction of infrequent events. J Consult Psychol. 1954 Dec;18(6):397-403. doi: 10.1037/h0058579.
14. McHugh CM et al. (2019). Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open. 2019 Mar;5(2):e18. doi: 10.1192/bjo.2018.88.
Suicide is not a trivial matter – it upends families, robs partners of a loved one, prevents children from having a parent, and can destroy a parent’s most cherished being. It is not surprising that societies have repeatedly made it a goal to study and reduce suicide within their populations.
The suicide rate in the United States is trending upward, from about 10 per 100,000 in 2000 to about 15 per 100,000 in more recent reports. The increasing suicide rates have been accompanied by increasing distress among many strata of society. From a public health level, analysts are not just witnessing increasing suicide rates, but a shocking rise in all “deaths of despair,”1 among which suicide can be considered the ultimate example.
On an individual level, many know someone who has died of suicide or suffered from a serious suicide attempt. From the public health level to the individual level, advocacy has called for various interventions in the field of psychiatry to remedy this tragic problem.
Psychiatrists have been firsthand witnesses to this increasing demand for suicide interventions. When in residency, the norm was to perform a suicide risk assessment at the time of admission to the hospital and again at the time of discharge. As the years passed, the new normal within psychiatric hospitals has shifted to asking about suicidality on a daily basis.
In what seems to us like an escalating arms race, the emerging standard of care at many facilities is now not only for daily suicide risk assessments by each psychiatrist, but also to require nurses to ask about suicidality during every 8-hour shift – in addition to documented inquiries about suicidality by other allied staff on the psychiatric unit. As a result, it is not uncommon for a patient hospitalized at an academic center to receive more than half a dozen suicide risk assessments in a day (first by the medical student, at least once – often more than once – by the resident, again by the attending psychiatrist, then the social worker and three nurses in 24 hours).
One of the concerns about such an approach is the lack of logic inherent to many risk assessment tools and symptom scales. Many of us are familiar with the Patient Health Questionnaire (PHQ-9) to assess depression.2 The PHQ-9 asks to consider “over the last 2 weeks, how often have you ...” in relation to nine symptoms associated with depression. It has always defied reason to perform a PHQ-9 every day and expect the answers to change from “nearly every day” to “not at all,” considering only 1 day has passed since the last time the patient has answered the questions. Yet daily, or near daily, PHQ-9 scores are a frequently used tool of tracking symptom improvement in response to treatments, such as electroconvulsive therapy, performed multiple times a week.
One can argue that the patient’s perspective on how symptomatic he or she has been over the past 2 weeks may change rapidly with alleviation of a depressed mood. However, the PHQ-9 is both reported to be, and often regarded as, an objective score. If one wishes to utilize it as such, the defense of its use should not be that it is a subjective report with just as much utility as “Rate your depression on a scale of 0-27.”
Similarly, many suicide scales were intended to assess thoughts of suicide in the past month3 or have been re-tooled to address this particular concern by asking “since the last contact.”4 It is baffling to see a chart with many dozens of suicide risk assessments with at times widely differing answers, yet all measuring thoughts of suicide in the past month. Is one to expect the answer to “How many times have you had these thoughts [of suicide ideation]? (1) Less than once a week (2) Once a week ...” to change between 8 a.m. and noon? Furthermore, for the purpose of assessing acute risk of suicidality in the immediate future, to only consider symptoms since the last contact – or past 2 weeks, past month, etc. – is of unclear significance.
Provider liability
Another concern is the liability placed on providers. A common problem encountered in the inpatient setting is insurance companies refusing to reimburse a hospital stay for depressed patients denying suicidality.
Any provider in the position of caring for such a patient must ask: What is the likelihood of someone providing a false negative – a false denial of suicidality? Is the likelihood of a suicidal person denying suicidality different if asked 5 or 10 or more times in a day? There are innumerable instances where a patient at a very high risk of self-harm has denied suicidality, been discharged from the hospital, and suffered terrible consequences. Ethically, the psychiatrist aware of this risk is no more at ease discharging these patients, whether it is one suicide risk scale or a dozen that suggests a patient is at low risk.
Alternatively, it may feel untenable from a medicolegal perspective for a psychiatrist to discharge a patient denying suicidality when the chart includes over a dozen previously documented elevated suicide risk assessments in the past 72 hours. By placing the job of suicide risk assessment in the hands of providers of varying levels of training and responsibility, a situation is created in which the seasoned psychiatrist who would otherwise be comfortable discharging a patient feels unable to do so because every other note-writer in the record – from the triage nurse to the medical assistant to the sitter in the emergency department – has recorded the patient as high risk for suicide. When put in such a position, the thought often occurs that systems of care, rather than individual providers, are protected most by ever escalating requirements for suicide risk documentation. To make a clinical decision contrary to the body of suicide risk documentation puts the provider at risk of being scapegoated by the system of care, which can point to its illogical and ineffective, though profusely documented, suicide prevention protocols.
Limitations of risk assessments
Considering the ongoing rise in the use of suicide risk assessments, one would expect that the evidence for their efficacy was robust and well established. Yet a thorough review of suicide risk assessments funded by the MacArthur Foundation, which examined decades of research, came to disheartening conclusions: “predictive ability has not improved over the past 50 years”; “no risk factor category or subcategory is substantially stronger than any other”; and “predicting solely according to base rates may be comparable to prediction with current risk factors.”5
Those findings were consistent with the conclusions of many other studies, which have summarized the utility of suicide risk assessments as follows: “occurrence of suicide is too low to identify those individuals who are likely to die by suicide”;6 “suicide prediction models produce accurate overall classification models, but their accuracy of predicting a future event is near zero”;7 “risk stratification is too inaccurate to be clinically useful and might even be harmful”;8 “suicide risk prediction [lacks] any items or information that to a useful degree permit the identification of persons who will complete suicide”;9 “existing suicide prediction tools have little current clinical value”;10 “our current preoccupation with risk assessment has ... created a mythology with no evidence to support it.”11 And that’s to cite just a few.
Sadly, we have known about the limitations of suicide risk assessments for many decades. In 1983 a large VA prospective study, which aimed to identify veterans who will die by suicide, examined 4,800 patients with a wide range of instruments and measures.12 This study concluded that “discriminant analysis was clearly inadequate in correctly classifying the subjects. For an event as rare as suicide, our predictive tools and guides are simply not equal to the task.” The authors described the feelings of many in stating “courts and public opinion expect physicians to be able to pick out the particular persons who will later commit suicide. Although we may reconstruct causal chains and motives, we do not possess the tools to predict suicides.”
Yet, even several decades prior, in 1954, Dr. Albert Rosen performed an elegant statistical analysis and predicted that, considering the low base rate of suicide, suicide risk assessments are “of no practical value, for it would be impossible to treat the prodigious number of false positives.”13 It seems that we continue to be unable to accept Dr. Rosen’s premonition despite decades of confirmatory evidence.
“Quantity over quality”
Regardless of those sobering reports,
One can reasonably argue that the periodic performance of a suicide risk assessment may have clinical utility in reminding us of modifiable risk factors such as intoxication, social isolation, and access to lethal means. One can also reasonably argue that these risk assessments may provide useful education to patients and their families on epidemiological risk factors such as gender, age, and marital status. But our pursuit of serial suicide risk assessments throughout the day is encouraging providers to focus on a particular risk factor that changes from moment to moment and has particularly low validity, that being self-reported suicidality.
Reported suicidality is one of the few risk factors that can change from shift to shift. But 80% of people who die by suicide had not previously expressed suicidality, and 98.3% of people who have endorsed suicidality do not die by suicide.14 While the former statistic may improve with increased assessment, the later will likely worsen.
Suicide is not a trivial matter. We admire those that study it and advocate for better interventions. We have compassion for those who have suffered the loss of a loved one to suicide. Our patients have died as a result of the human limitations surrounding suicide prevention. Recognizing the weight of suicide and making an effort to avoid minimizing its immense consequences drive our desire to be honest with ourselves, our patients and their families, and society. That includes the unfortunate truth regarding the current state of the evidence and our ability to enact change.
It is our concern that the rising fascination with repeated suicide risk assessment is misguided in its current form and serves the purpose of appeasing administrators more than reflecting a scientific understanding of the literature. More sadly, we are concerned that this “quantity-over-quality” approach is yet another barrier to practicing what may be one of the few interventions with any hope of meaningfully impacting a patient’s risk of suicide in the clinical setting – spending time connecting with our patients.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a member of the psychiatry faculty at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Badre and Dr. Compton have no conflicts of interest.
References
1. Joint Economic Committee. (2019). Long Term Trends in Deaths of Despair. SCP Report 4-19.
2. Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2013;32(9):509-15. doi: 10.3928/0048-5713-20020901-06.
3. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Lifetime/Recent.
4. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Since Last Contact.
5. Franklin JC et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017 Feb;143(2):187-232. doi: 10.1037/bul0000084.
6. Beautrais AL. Further suicidal behavior among medically serious suicide attempters. Suicide Life Threat Behav. 2004 Spring;34(1):1-11. doi: 10.1521/suli.34.1.1.27772.
7. Belsher BE. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Jun 1;76(6):642-651. doi: 10.1001/jamapsychiatry.2019.0174.
8. Carter G et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Aust N Z J Psychiatry. 2016 Oct;50(10):939-1000. doi: 10.1177/0004867416661039.
9. Fosse R et al. Predictors of suicide in the patient population admitted to a locked-door psychiatric acute ward. PLoS One. 2017 Mar 16;12(3):e0173958. doi: 10.1371/journal.pone.0173958.
10. Kessler RC et al. Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry. 2020 Jan;25(1):168-79. doi: 10.1038/s41380-019-0531-0.
11. Mulder R. Problems with suicide risk assessment. Aust N Z J Psychiatry. 2011 Aug;45(8):605-7. doi: 10.3109/00048674.2011.594786.
12. Pokorny AD. Prediction of suicide in psychiatric patients: Report of a prospective study. Arch Gen Psychiatry. 1983 Mar;40(3):249-57. doi: 10.1001/archpsyc.1983.01790030019002.
13. Rosen A. Detection of suicidal patients: An example of some limitations in the prediction of infrequent events. J Consult Psychol. 1954 Dec;18(6):397-403. doi: 10.1037/h0058579.
14. McHugh CM et al. (2019). Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open. 2019 Mar;5(2):e18. doi: 10.1192/bjo.2018.88.
Suicide is not a trivial matter – it upends families, robs partners of a loved one, prevents children from having a parent, and can destroy a parent’s most cherished being. It is not surprising that societies have repeatedly made it a goal to study and reduce suicide within their populations.
The suicide rate in the United States is trending upward, from about 10 per 100,000 in 2000 to about 15 per 100,000 in more recent reports. The increasing suicide rates have been accompanied by increasing distress among many strata of society. From a public health level, analysts are not just witnessing increasing suicide rates, but a shocking rise in all “deaths of despair,”1 among which suicide can be considered the ultimate example.
On an individual level, many know someone who has died of suicide or suffered from a serious suicide attempt. From the public health level to the individual level, advocacy has called for various interventions in the field of psychiatry to remedy this tragic problem.
Psychiatrists have been firsthand witnesses to this increasing demand for suicide interventions. When in residency, the norm was to perform a suicide risk assessment at the time of admission to the hospital and again at the time of discharge. As the years passed, the new normal within psychiatric hospitals has shifted to asking about suicidality on a daily basis.
In what seems to us like an escalating arms race, the emerging standard of care at many facilities is now not only for daily suicide risk assessments by each psychiatrist, but also to require nurses to ask about suicidality during every 8-hour shift – in addition to documented inquiries about suicidality by other allied staff on the psychiatric unit. As a result, it is not uncommon for a patient hospitalized at an academic center to receive more than half a dozen suicide risk assessments in a day (first by the medical student, at least once – often more than once – by the resident, again by the attending psychiatrist, then the social worker and three nurses in 24 hours).
One of the concerns about such an approach is the lack of logic inherent to many risk assessment tools and symptom scales. Many of us are familiar with the Patient Health Questionnaire (PHQ-9) to assess depression.2 The PHQ-9 asks to consider “over the last 2 weeks, how often have you ...” in relation to nine symptoms associated with depression. It has always defied reason to perform a PHQ-9 every day and expect the answers to change from “nearly every day” to “not at all,” considering only 1 day has passed since the last time the patient has answered the questions. Yet daily, or near daily, PHQ-9 scores are a frequently used tool of tracking symptom improvement in response to treatments, such as electroconvulsive therapy, performed multiple times a week.
One can argue that the patient’s perspective on how symptomatic he or she has been over the past 2 weeks may change rapidly with alleviation of a depressed mood. However, the PHQ-9 is both reported to be, and often regarded as, an objective score. If one wishes to utilize it as such, the defense of its use should not be that it is a subjective report with just as much utility as “Rate your depression on a scale of 0-27.”
Similarly, many suicide scales were intended to assess thoughts of suicide in the past month3 or have been re-tooled to address this particular concern by asking “since the last contact.”4 It is baffling to see a chart with many dozens of suicide risk assessments with at times widely differing answers, yet all measuring thoughts of suicide in the past month. Is one to expect the answer to “How many times have you had these thoughts [of suicide ideation]? (1) Less than once a week (2) Once a week ...” to change between 8 a.m. and noon? Furthermore, for the purpose of assessing acute risk of suicidality in the immediate future, to only consider symptoms since the last contact – or past 2 weeks, past month, etc. – is of unclear significance.
Provider liability
Another concern is the liability placed on providers. A common problem encountered in the inpatient setting is insurance companies refusing to reimburse a hospital stay for depressed patients denying suicidality.
Any provider in the position of caring for such a patient must ask: What is the likelihood of someone providing a false negative – a false denial of suicidality? Is the likelihood of a suicidal person denying suicidality different if asked 5 or 10 or more times in a day? There are innumerable instances where a patient at a very high risk of self-harm has denied suicidality, been discharged from the hospital, and suffered terrible consequences. Ethically, the psychiatrist aware of this risk is no more at ease discharging these patients, whether it is one suicide risk scale or a dozen that suggests a patient is at low risk.
Alternatively, it may feel untenable from a medicolegal perspective for a psychiatrist to discharge a patient denying suicidality when the chart includes over a dozen previously documented elevated suicide risk assessments in the past 72 hours. By placing the job of suicide risk assessment in the hands of providers of varying levels of training and responsibility, a situation is created in which the seasoned psychiatrist who would otherwise be comfortable discharging a patient feels unable to do so because every other note-writer in the record – from the triage nurse to the medical assistant to the sitter in the emergency department – has recorded the patient as high risk for suicide. When put in such a position, the thought often occurs that systems of care, rather than individual providers, are protected most by ever escalating requirements for suicide risk documentation. To make a clinical decision contrary to the body of suicide risk documentation puts the provider at risk of being scapegoated by the system of care, which can point to its illogical and ineffective, though profusely documented, suicide prevention protocols.
Limitations of risk assessments
Considering the ongoing rise in the use of suicide risk assessments, one would expect that the evidence for their efficacy was robust and well established. Yet a thorough review of suicide risk assessments funded by the MacArthur Foundation, which examined decades of research, came to disheartening conclusions: “predictive ability has not improved over the past 50 years”; “no risk factor category or subcategory is substantially stronger than any other”; and “predicting solely according to base rates may be comparable to prediction with current risk factors.”5
Those findings were consistent with the conclusions of many other studies, which have summarized the utility of suicide risk assessments as follows: “occurrence of suicide is too low to identify those individuals who are likely to die by suicide”;6 “suicide prediction models produce accurate overall classification models, but their accuracy of predicting a future event is near zero”;7 “risk stratification is too inaccurate to be clinically useful and might even be harmful”;8 “suicide risk prediction [lacks] any items or information that to a useful degree permit the identification of persons who will complete suicide”;9 “existing suicide prediction tools have little current clinical value”;10 “our current preoccupation with risk assessment has ... created a mythology with no evidence to support it.”11 And that’s to cite just a few.
Sadly, we have known about the limitations of suicide risk assessments for many decades. In 1983 a large VA prospective study, which aimed to identify veterans who will die by suicide, examined 4,800 patients with a wide range of instruments and measures.12 This study concluded that “discriminant analysis was clearly inadequate in correctly classifying the subjects. For an event as rare as suicide, our predictive tools and guides are simply not equal to the task.” The authors described the feelings of many in stating “courts and public opinion expect physicians to be able to pick out the particular persons who will later commit suicide. Although we may reconstruct causal chains and motives, we do not possess the tools to predict suicides.”
Yet, even several decades prior, in 1954, Dr. Albert Rosen performed an elegant statistical analysis and predicted that, considering the low base rate of suicide, suicide risk assessments are “of no practical value, for it would be impossible to treat the prodigious number of false positives.”13 It seems that we continue to be unable to accept Dr. Rosen’s premonition despite decades of confirmatory evidence.
“Quantity over quality”
Regardless of those sobering reports,
One can reasonably argue that the periodic performance of a suicide risk assessment may have clinical utility in reminding us of modifiable risk factors such as intoxication, social isolation, and access to lethal means. One can also reasonably argue that these risk assessments may provide useful education to patients and their families on epidemiological risk factors such as gender, age, and marital status. But our pursuit of serial suicide risk assessments throughout the day is encouraging providers to focus on a particular risk factor that changes from moment to moment and has particularly low validity, that being self-reported suicidality.
Reported suicidality is one of the few risk factors that can change from shift to shift. But 80% of people who die by suicide had not previously expressed suicidality, and 98.3% of people who have endorsed suicidality do not die by suicide.14 While the former statistic may improve with increased assessment, the later will likely worsen.
Suicide is not a trivial matter. We admire those that study it and advocate for better interventions. We have compassion for those who have suffered the loss of a loved one to suicide. Our patients have died as a result of the human limitations surrounding suicide prevention. Recognizing the weight of suicide and making an effort to avoid minimizing its immense consequences drive our desire to be honest with ourselves, our patients and their families, and society. That includes the unfortunate truth regarding the current state of the evidence and our ability to enact change.
It is our concern that the rising fascination with repeated suicide risk assessment is misguided in its current form and serves the purpose of appeasing administrators more than reflecting a scientific understanding of the literature. More sadly, we are concerned that this “quantity-over-quality” approach is yet another barrier to practicing what may be one of the few interventions with any hope of meaningfully impacting a patient’s risk of suicide in the clinical setting – spending time connecting with our patients.
Dr. Badre is a clinical and forensic psychiatrist in San Diego. He holds teaching positions at the University of California, San Diego, and the University of San Diego. He teaches medical education, psychopharmacology, ethics in psychiatry, and correctional care. Dr. Badre can be reached at his website, BadreMD.com. Dr. Compton is a member of the psychiatry faculty at University of California, San Diego. His background includes medical education, mental health advocacy, work with underserved populations, and brain cancer research. Dr. Badre and Dr. Compton have no conflicts of interest.
References
1. Joint Economic Committee. (2019). Long Term Trends in Deaths of Despair. SCP Report 4-19.
2. Kroenke K and Spitzer RL. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2013;32(9):509-15. doi: 10.3928/0048-5713-20020901-06.
3. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Lifetime/Recent.
4. Columbia-Suicide Severity Rating Scale (C-SSRS) Full Since Last Contact.
5. Franklin JC et al. Risk factors for suicidal thoughts and behaviors: A meta-analysis of 50 years of research. Psychol Bull. 2017 Feb;143(2):187-232. doi: 10.1037/bul0000084.
6. Beautrais AL. Further suicidal behavior among medically serious suicide attempters. Suicide Life Threat Behav. 2004 Spring;34(1):1-11. doi: 10.1521/suli.34.1.1.27772.
7. Belsher BE. Prediction models for suicide attempts and deaths: A systematic review and simulation. JAMA Psychiatry. 2019 Jun 1;76(6):642-651. doi: 10.1001/jamapsychiatry.2019.0174.
8. Carter G et al. Royal Australian and New Zealand College of Psychiatrists clinical practice guideline for the management of deliberate self-harm. Aust N Z J Psychiatry. 2016 Oct;50(10):939-1000. doi: 10.1177/0004867416661039.
9. Fosse R et al. Predictors of suicide in the patient population admitted to a locked-door psychiatric acute ward. PLoS One. 2017 Mar 16;12(3):e0173958. doi: 10.1371/journal.pone.0173958.
10. Kessler RC et al. Suicide prediction models: A critical review of recent research with recommendations for the way forward. Mol Psychiatry. 2020 Jan;25(1):168-79. doi: 10.1038/s41380-019-0531-0.
11. Mulder R. Problems with suicide risk assessment. Aust N Z J Psychiatry. 2011 Aug;45(8):605-7. doi: 10.3109/00048674.2011.594786.
12. Pokorny AD. Prediction of suicide in psychiatric patients: Report of a prospective study. Arch Gen Psychiatry. 1983 Mar;40(3):249-57. doi: 10.1001/archpsyc.1983.01790030019002.
13. Rosen A. Detection of suicidal patients: An example of some limitations in the prediction of infrequent events. J Consult Psychol. 1954 Dec;18(6):397-403. doi: 10.1037/h0058579.
14. McHugh CM et al. (2019). Association between suicidal ideation and suicide: Meta-analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open. 2019 Mar;5(2):e18. doi: 10.1192/bjo.2018.88.
Can a decrease in dopamine lead to binge eating?
In medical school, we were repeatedly advised that there is both a science and an art to the practice of medicine. In these days of doc-in-a-box online consultations for obesity, it’s tempting to think that there’s a one-size-fits-all purely scientific approach for these new weight loss medications. Yet, for every nine patients who lose weight seemingly effortlessly on this class of medication, there is always one whose body stubbornly refuses to submit.
Adam is a 58-year-old man who came to me recently because he was having difficulty losing weight. Over the past 20 years, he’d been steadily gaining weight and now, technically has morbid obesity (a term which should arguably be obsolete). His weight gain is complicated by high blood pressure, high cholesterol, and obstructive sleep apnea. His sleep apnea has caused such profound exhaustion that he no longer has the energy to work out. He also has significant ADHD, which has been left untreated because of his ability to white-knuckle it through his many daily meetings and calls. A married father of three, he is a successful portfolio manager at a high-yield bond fund.
Adam tends to eat minimally during the day, thereby baffling his colleagues with the stark contrast between his minimal caloric intake and his large belly. However, when he returns from work late at night (kids safely tucked into bed), the floodgates open. He reports polishing off pints of ice cream, scarfing down bags of cookies, inhaling trays of brownies. No carbohydrate is off limits to him once he steps off the Metro North train and crosses the threshold from work to home.
Does Adam simply lack the desire or common-sense willpower to make the necessary changes in his lifestyle or is there something more complicated at play?
I would argue that Adam’s ADHD triggered a binge-eating disorder (BED) that festered unchecked over the past 20 years. Patients with BED typically eat massive quantities of food over short periods of time – often when they’re not even hungry. Adam admitted that he would generally continue to eat well after feeling stuffed to the brim.
The answer probably lies with dopamine, a neurotransmitter produced in the reward centers of the brain that regulates how people experience pleasure and control impulses. We believe that people with ADHD have low levels of dopamine (it’s actually a bit more complicated, but this is the general idea). These low levels of dopamine lead people to self-medicate with sugars, salt, and fats to increase dopamine levels.
Lisdexamfetamine (Vyvanse) is a Food and Drug Administration–approved treatment option for both ADHD and binge eating. It raises the levels of dopamine (as well as norepinephrine) in the brain’s reward center. Often, the strong urge to binge subsides rapidly once ADHD is properly treated.
Rather than starting Adam on a semaglutide or similar agent, I opted to start him on lisdexamfetamine. When I spoke to him 1 week later, he confided that the world suddenly shifted into focus, and he was able to plan his meals throughout the day and resist the urge to binge late at night.
I may eventually add a semaglutide-like medication if his weight loss plateaus, but for now, I will focus on raising his dopamine levels to tackle the underlying cause of his weight gain.
Dr. Messer is a clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In medical school, we were repeatedly advised that there is both a science and an art to the practice of medicine. In these days of doc-in-a-box online consultations for obesity, it’s tempting to think that there’s a one-size-fits-all purely scientific approach for these new weight loss medications. Yet, for every nine patients who lose weight seemingly effortlessly on this class of medication, there is always one whose body stubbornly refuses to submit.
Adam is a 58-year-old man who came to me recently because he was having difficulty losing weight. Over the past 20 years, he’d been steadily gaining weight and now, technically has morbid obesity (a term which should arguably be obsolete). His weight gain is complicated by high blood pressure, high cholesterol, and obstructive sleep apnea. His sleep apnea has caused such profound exhaustion that he no longer has the energy to work out. He also has significant ADHD, which has been left untreated because of his ability to white-knuckle it through his many daily meetings and calls. A married father of three, he is a successful portfolio manager at a high-yield bond fund.
Adam tends to eat minimally during the day, thereby baffling his colleagues with the stark contrast between his minimal caloric intake and his large belly. However, when he returns from work late at night (kids safely tucked into bed), the floodgates open. He reports polishing off pints of ice cream, scarfing down bags of cookies, inhaling trays of brownies. No carbohydrate is off limits to him once he steps off the Metro North train and crosses the threshold from work to home.
Does Adam simply lack the desire or common-sense willpower to make the necessary changes in his lifestyle or is there something more complicated at play?
I would argue that Adam’s ADHD triggered a binge-eating disorder (BED) that festered unchecked over the past 20 years. Patients with BED typically eat massive quantities of food over short periods of time – often when they’re not even hungry. Adam admitted that he would generally continue to eat well after feeling stuffed to the brim.
The answer probably lies with dopamine, a neurotransmitter produced in the reward centers of the brain that regulates how people experience pleasure and control impulses. We believe that people with ADHD have low levels of dopamine (it’s actually a bit more complicated, but this is the general idea). These low levels of dopamine lead people to self-medicate with sugars, salt, and fats to increase dopamine levels.
Lisdexamfetamine (Vyvanse) is a Food and Drug Administration–approved treatment option for both ADHD and binge eating. It raises the levels of dopamine (as well as norepinephrine) in the brain’s reward center. Often, the strong urge to binge subsides rapidly once ADHD is properly treated.
Rather than starting Adam on a semaglutide or similar agent, I opted to start him on lisdexamfetamine. When I spoke to him 1 week later, he confided that the world suddenly shifted into focus, and he was able to plan his meals throughout the day and resist the urge to binge late at night.
I may eventually add a semaglutide-like medication if his weight loss plateaus, but for now, I will focus on raising his dopamine levels to tackle the underlying cause of his weight gain.
Dr. Messer is a clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
In medical school, we were repeatedly advised that there is both a science and an art to the practice of medicine. In these days of doc-in-a-box online consultations for obesity, it’s tempting to think that there’s a one-size-fits-all purely scientific approach for these new weight loss medications. Yet, for every nine patients who lose weight seemingly effortlessly on this class of medication, there is always one whose body stubbornly refuses to submit.
Adam is a 58-year-old man who came to me recently because he was having difficulty losing weight. Over the past 20 years, he’d been steadily gaining weight and now, technically has morbid obesity (a term which should arguably be obsolete). His weight gain is complicated by high blood pressure, high cholesterol, and obstructive sleep apnea. His sleep apnea has caused such profound exhaustion that he no longer has the energy to work out. He also has significant ADHD, which has been left untreated because of his ability to white-knuckle it through his many daily meetings and calls. A married father of three, he is a successful portfolio manager at a high-yield bond fund.
Adam tends to eat minimally during the day, thereby baffling his colleagues with the stark contrast between his minimal caloric intake and his large belly. However, when he returns from work late at night (kids safely tucked into bed), the floodgates open. He reports polishing off pints of ice cream, scarfing down bags of cookies, inhaling trays of brownies. No carbohydrate is off limits to him once he steps off the Metro North train and crosses the threshold from work to home.
Does Adam simply lack the desire or common-sense willpower to make the necessary changes in his lifestyle or is there something more complicated at play?
I would argue that Adam’s ADHD triggered a binge-eating disorder (BED) that festered unchecked over the past 20 years. Patients with BED typically eat massive quantities of food over short periods of time – often when they’re not even hungry. Adam admitted that he would generally continue to eat well after feeling stuffed to the brim.
The answer probably lies with dopamine, a neurotransmitter produced in the reward centers of the brain that regulates how people experience pleasure and control impulses. We believe that people with ADHD have low levels of dopamine (it’s actually a bit more complicated, but this is the general idea). These low levels of dopamine lead people to self-medicate with sugars, salt, and fats to increase dopamine levels.
Lisdexamfetamine (Vyvanse) is a Food and Drug Administration–approved treatment option for both ADHD and binge eating. It raises the levels of dopamine (as well as norepinephrine) in the brain’s reward center. Often, the strong urge to binge subsides rapidly once ADHD is properly treated.
Rather than starting Adam on a semaglutide or similar agent, I opted to start him on lisdexamfetamine. When I spoke to him 1 week later, he confided that the world suddenly shifted into focus, and he was able to plan his meals throughout the day and resist the urge to binge late at night.
I may eventually add a semaglutide-like medication if his weight loss plateaus, but for now, I will focus on raising his dopamine levels to tackle the underlying cause of his weight gain.
Dr. Messer is a clinical assistant professor at the Icahn School of Medicine at Mount Sinai, New York. She disclosed no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Psilocybin reduces symptoms, disability in major depression
The randomized, phase 2 trial was conducted at 11 sites across the United States and is the latest to demonstrate the psychedelic drug’s potential as a treatment for depression.
The project was funded by Usona Institute, a nonprofit medical research organization based in Madison, Wisc. The institute issued a press statement, but researchers did not comment further on the findings.
“As the largest and most rigorous study conducted across a wide spectrum of individuals with major depressive disorder, the results show promise for all people struggling with this condition,” lead author Charles Raison, MD, director of clinical and translational research at Usona, said in the statement.
The 34 coauthors on the study are affiliated with public universities, research centers, and private companies. Eight of the investigators are identified as employees of Usona Institute.
Declining further comment, an institute spokesperson told this news organization that, “Usona has chosen the approach of no interviews, and this applies for all coauthors.”
The findings were published online in JAMA.
Largest study to date
Usona’s investigational psilocybin drug has been granted a breakthrough designation by the Food and Drug Administration, a process designed to speed drug development and review.
Previous smaller studies have suggested a rapid antidepressant response with psilocybin, but they have been small, unblinded, and have had short duration of follow-up, they write. This randomized, double-blind, phase 2 clinical trial is the largest study of psilocybin for depression to date, the researchers note.
It included 104 adults aged 21-65 years with MDD who had a current depressive episode of at least 60 days and a Montgomery-Åsberg Depression Rating Scale (MADRS) total score of 28 or more at baseline.
Participants had to be free of psychedelic drugs for at least 5 years, have had no active suicidal ideation or suicidal behavior in the prior 12 months, no personal or first-degree family history of psychosis or mania, and no history of moderate/severe alcohol or drug use disorder.
Before the study, participants had a 7- to 35-day screening period for psychiatric medication tapering, underwent baseline assessments, and received 6-8 hours of preparation with two facilitators who would be with them during dosing.
Dosing occurred within 7 days of baseline assessments. During the 6- to 8-hour session, participants received either a single 25-mg oral dose of psilocybin or 100-mg dose of niacin. One participant randomly assigned to receive psilocybin received the incorrect treatment, resulting in 50 participants receiving psilocybin and 54 receiving niacin.
Participants returned the next day, the next week, and then every 2 weeks for assessments, for a follow-up of 6 weeks.
Psychosocial support
Participants who received psilocybin reported significantly greater improvements in MDD symptoms, compared with those who received niacin. MADRS scores – a scale from 0 to 60 where higher scores indicate more severe depression – showed greater reductions with treatment vs. placebo at 8 days (mean difference, −12.0; 95% confidence interval, −16.6 to −7.4; P < .001), and at day 43 (mean difference, −12.3; 95% CI, −17.5 to −7.2; P < .001).
More participants receiving psilocybin had sustained depressive symptom response (42% vs. 11%; P = .002) and more improvement in the Sheehan Disability Scale score, which measures functional disability, 43 days after treatment (P < .001).
The effects persisted through the end of the study, although the differences between groups were no longer significant by week 6.
“This is another exciting piece of evidence that adds to the current literature regarding the potential efficacy of psilocybin for the treatment of mental health conditions, particularly depression,” said Greg Fonzo, MD, codirector of the Center for Psychedelic Research and Therapy at the University of Texas at Austin, who commented on the findings.
Significantly more people in the psilocybin group reported at least one treatment-related adverse event (AE, 82% vs. 44%), although most were mild to moderate. Headache and nausea were the most common side effects and most resolved within 1 day of dosing.
While those numbers are high, Dr. Fonzo said they’re not out of line with AEs reported in other studies.
“Particularly with the types of adverse events reported here, like headache and nausea, those are things you would typically expect to see in this treatment,” said Dr. Fonzo, who was not part of the research.
“But it is high, and it underscores that this is not a treatment without certain risks, even though it was good that they were primarily mild in severity,” he added.
A ‘stepping stone’ to FDA approval?
The use of tools to measure disability in addition to symptoms of depression severity is a strength of the study, Dr. Fonzo added. The use of an active comparator and the 6-week follow-up also offer something new over previous studies.
Despite the longer follow up, questions remain about the durability of response, something only a longer study could answer, Dr. Fonzo said. The small and homogeneous sample-size are also a concern. Nearly 90% of participants were White, and more than half had an income of $75,000 a year or higher.
“It’s another stepping stone in the process to FDA approval, but the next step in that process would be much larger phase 3 trials that would have much larger samples, a longer follow-up, and hopefully have a more inclusive swath of the population,” Dr. Fonzo said.
But perhaps one of the most significant limitations is the use of niacin as an active comparator, said Caleb Alexander, MD, codirector of the Center for Drug Safety and Effectiveness at Johns Hopkins University in Baltimore.
The use of an agent that doesn’t produce effects similar to those expected from a psychedelic introduced the potential for functional unblinding, Dr. Alexander said. Investigators did not ask participants to guess whether they received psilocybin or niacin, so the quality of the blinding was not assessed in the study.
“We’d like to see the use of [an] active comparator that might have a chance of obscuring to people as to whether they’ve been randomized to the treatment arm or control arm,” said Dr. Alexander, who wasn’t involved in the study. “Why not use a benzodiazepine or another drug that produces a transient euphoria that would better obscure whether or not people were receiving the psilocybin?”
The authors of an accompanying editorial shared these concerns, also noting that the study included “a significant number of patients who did not respond to therapy.”
“It is important to analyze and understand adverse outcomes in psychedelic trials and conduct longitudinal studies to determine how sustained the effects will be and what may initiate a recrudescence of symptoms,” write Rachel Yehuda, PhD, and Amy Lehrner, PhD, both of the Peters VA Medical Center and Icahn School of Medicine at Mount Sinai, New York.
“Future studies will help identify who is most likely to benefit from psychedelics, whether booster or repeated treatment is safe and beneficial, and what the optimal dose and therapeutic frameworks are.”
A long-term follow-up of the current trial was terminated last year because of low enrollment. The spokesperson with Usona Institute did not respond to questions about that study, and the institute’s statement only added that preparations are underway to launch another study that “will provide additional safety and efficacy data to support submission of a new drug application to the FDA.”
Usona published its manufacturing process that it used to synthesize psilocybin in an open-access journal and signed a statement on “open science and open praxis” with psilocybin and similar substances, which appears on their website. That statement was signed by 31 research and service organizations around the world and nearly 150 scientists, scholars, and practitioners.
The study was funded by Usona Institute. Dr. Raison reported receiving personal fees from Usona Institute and grants to Usona Institute from Dr. Bronner’s All-One, Fournier Family Foundation, Good Ventures, Steven and Alexandra Cohen Foundation, Tiny Blue Dot Foundation, Turnbull Family Foundation, and William A. Linton during the conduct of the study; and personal fees from Novartis, Sage/Biogen, Emory Healthcare, and Vail Health outside the submitted work. Dr. Fonzo and Dr. Alexander report no relevant financial relationships. Dr. Yehuda reports receiving nonfinancial support from the Multidisciplinary Association for Psychedelic Studies Public Benefit (MAPS PBC) and grants from COMPASS Pathways. Dr. Lehrner is an investigator on trials sponsored by MAPS PBC and COMPASS Pathways.
A version of this article first appeared on Medscape.com.
The randomized, phase 2 trial was conducted at 11 sites across the United States and is the latest to demonstrate the psychedelic drug’s potential as a treatment for depression.
The project was funded by Usona Institute, a nonprofit medical research organization based in Madison, Wisc. The institute issued a press statement, but researchers did not comment further on the findings.
“As the largest and most rigorous study conducted across a wide spectrum of individuals with major depressive disorder, the results show promise for all people struggling with this condition,” lead author Charles Raison, MD, director of clinical and translational research at Usona, said in the statement.
The 34 coauthors on the study are affiliated with public universities, research centers, and private companies. Eight of the investigators are identified as employees of Usona Institute.
Declining further comment, an institute spokesperson told this news organization that, “Usona has chosen the approach of no interviews, and this applies for all coauthors.”
The findings were published online in JAMA.
Largest study to date
Usona’s investigational psilocybin drug has been granted a breakthrough designation by the Food and Drug Administration, a process designed to speed drug development and review.
Previous smaller studies have suggested a rapid antidepressant response with psilocybin, but they have been small, unblinded, and have had short duration of follow-up, they write. This randomized, double-blind, phase 2 clinical trial is the largest study of psilocybin for depression to date, the researchers note.
It included 104 adults aged 21-65 years with MDD who had a current depressive episode of at least 60 days and a Montgomery-Åsberg Depression Rating Scale (MADRS) total score of 28 or more at baseline.
Participants had to be free of psychedelic drugs for at least 5 years, have had no active suicidal ideation or suicidal behavior in the prior 12 months, no personal or first-degree family history of psychosis or mania, and no history of moderate/severe alcohol or drug use disorder.
Before the study, participants had a 7- to 35-day screening period for psychiatric medication tapering, underwent baseline assessments, and received 6-8 hours of preparation with two facilitators who would be with them during dosing.
Dosing occurred within 7 days of baseline assessments. During the 6- to 8-hour session, participants received either a single 25-mg oral dose of psilocybin or 100-mg dose of niacin. One participant randomly assigned to receive psilocybin received the incorrect treatment, resulting in 50 participants receiving psilocybin and 54 receiving niacin.
Participants returned the next day, the next week, and then every 2 weeks for assessments, for a follow-up of 6 weeks.
Psychosocial support
Participants who received psilocybin reported significantly greater improvements in MDD symptoms, compared with those who received niacin. MADRS scores – a scale from 0 to 60 where higher scores indicate more severe depression – showed greater reductions with treatment vs. placebo at 8 days (mean difference, −12.0; 95% confidence interval, −16.6 to −7.4; P < .001), and at day 43 (mean difference, −12.3; 95% CI, −17.5 to −7.2; P < .001).
More participants receiving psilocybin had sustained depressive symptom response (42% vs. 11%; P = .002) and more improvement in the Sheehan Disability Scale score, which measures functional disability, 43 days after treatment (P < .001).
The effects persisted through the end of the study, although the differences between groups were no longer significant by week 6.
“This is another exciting piece of evidence that adds to the current literature regarding the potential efficacy of psilocybin for the treatment of mental health conditions, particularly depression,” said Greg Fonzo, MD, codirector of the Center for Psychedelic Research and Therapy at the University of Texas at Austin, who commented on the findings.
Significantly more people in the psilocybin group reported at least one treatment-related adverse event (AE, 82% vs. 44%), although most were mild to moderate. Headache and nausea were the most common side effects and most resolved within 1 day of dosing.
While those numbers are high, Dr. Fonzo said they’re not out of line with AEs reported in other studies.
“Particularly with the types of adverse events reported here, like headache and nausea, those are things you would typically expect to see in this treatment,” said Dr. Fonzo, who was not part of the research.
“But it is high, and it underscores that this is not a treatment without certain risks, even though it was good that they were primarily mild in severity,” he added.
A ‘stepping stone’ to FDA approval?
The use of tools to measure disability in addition to symptoms of depression severity is a strength of the study, Dr. Fonzo added. The use of an active comparator and the 6-week follow-up also offer something new over previous studies.
Despite the longer follow up, questions remain about the durability of response, something only a longer study could answer, Dr. Fonzo said. The small and homogeneous sample-size are also a concern. Nearly 90% of participants were White, and more than half had an income of $75,000 a year or higher.
“It’s another stepping stone in the process to FDA approval, but the next step in that process would be much larger phase 3 trials that would have much larger samples, a longer follow-up, and hopefully have a more inclusive swath of the population,” Dr. Fonzo said.
But perhaps one of the most significant limitations is the use of niacin as an active comparator, said Caleb Alexander, MD, codirector of the Center for Drug Safety and Effectiveness at Johns Hopkins University in Baltimore.
The use of an agent that doesn’t produce effects similar to those expected from a psychedelic introduced the potential for functional unblinding, Dr. Alexander said. Investigators did not ask participants to guess whether they received psilocybin or niacin, so the quality of the blinding was not assessed in the study.
“We’d like to see the use of [an] active comparator that might have a chance of obscuring to people as to whether they’ve been randomized to the treatment arm or control arm,” said Dr. Alexander, who wasn’t involved in the study. “Why not use a benzodiazepine or another drug that produces a transient euphoria that would better obscure whether or not people were receiving the psilocybin?”
The authors of an accompanying editorial shared these concerns, also noting that the study included “a significant number of patients who did not respond to therapy.”
“It is important to analyze and understand adverse outcomes in psychedelic trials and conduct longitudinal studies to determine how sustained the effects will be and what may initiate a recrudescence of symptoms,” write Rachel Yehuda, PhD, and Amy Lehrner, PhD, both of the Peters VA Medical Center and Icahn School of Medicine at Mount Sinai, New York.
“Future studies will help identify who is most likely to benefit from psychedelics, whether booster or repeated treatment is safe and beneficial, and what the optimal dose and therapeutic frameworks are.”
A long-term follow-up of the current trial was terminated last year because of low enrollment. The spokesperson with Usona Institute did not respond to questions about that study, and the institute’s statement only added that preparations are underway to launch another study that “will provide additional safety and efficacy data to support submission of a new drug application to the FDA.”
Usona published its manufacturing process that it used to synthesize psilocybin in an open-access journal and signed a statement on “open science and open praxis” with psilocybin and similar substances, which appears on their website. That statement was signed by 31 research and service organizations around the world and nearly 150 scientists, scholars, and practitioners.
The study was funded by Usona Institute. Dr. Raison reported receiving personal fees from Usona Institute and grants to Usona Institute from Dr. Bronner’s All-One, Fournier Family Foundation, Good Ventures, Steven and Alexandra Cohen Foundation, Tiny Blue Dot Foundation, Turnbull Family Foundation, and William A. Linton during the conduct of the study; and personal fees from Novartis, Sage/Biogen, Emory Healthcare, and Vail Health outside the submitted work. Dr. Fonzo and Dr. Alexander report no relevant financial relationships. Dr. Yehuda reports receiving nonfinancial support from the Multidisciplinary Association for Psychedelic Studies Public Benefit (MAPS PBC) and grants from COMPASS Pathways. Dr. Lehrner is an investigator on trials sponsored by MAPS PBC and COMPASS Pathways.
A version of this article first appeared on Medscape.com.
The randomized, phase 2 trial was conducted at 11 sites across the United States and is the latest to demonstrate the psychedelic drug’s potential as a treatment for depression.
The project was funded by Usona Institute, a nonprofit medical research organization based in Madison, Wisc. The institute issued a press statement, but researchers did not comment further on the findings.
“As the largest and most rigorous study conducted across a wide spectrum of individuals with major depressive disorder, the results show promise for all people struggling with this condition,” lead author Charles Raison, MD, director of clinical and translational research at Usona, said in the statement.
The 34 coauthors on the study are affiliated with public universities, research centers, and private companies. Eight of the investigators are identified as employees of Usona Institute.
Declining further comment, an institute spokesperson told this news organization that, “Usona has chosen the approach of no interviews, and this applies for all coauthors.”
The findings were published online in JAMA.
Largest study to date
Usona’s investigational psilocybin drug has been granted a breakthrough designation by the Food and Drug Administration, a process designed to speed drug development and review.
Previous smaller studies have suggested a rapid antidepressant response with psilocybin, but they have been small, unblinded, and have had short duration of follow-up, they write. This randomized, double-blind, phase 2 clinical trial is the largest study of psilocybin for depression to date, the researchers note.
It included 104 adults aged 21-65 years with MDD who had a current depressive episode of at least 60 days and a Montgomery-Åsberg Depression Rating Scale (MADRS) total score of 28 or more at baseline.
Participants had to be free of psychedelic drugs for at least 5 years, have had no active suicidal ideation or suicidal behavior in the prior 12 months, no personal or first-degree family history of psychosis or mania, and no history of moderate/severe alcohol or drug use disorder.
Before the study, participants had a 7- to 35-day screening period for psychiatric medication tapering, underwent baseline assessments, and received 6-8 hours of preparation with two facilitators who would be with them during dosing.
Dosing occurred within 7 days of baseline assessments. During the 6- to 8-hour session, participants received either a single 25-mg oral dose of psilocybin or 100-mg dose of niacin. One participant randomly assigned to receive psilocybin received the incorrect treatment, resulting in 50 participants receiving psilocybin and 54 receiving niacin.
Participants returned the next day, the next week, and then every 2 weeks for assessments, for a follow-up of 6 weeks.
Psychosocial support
Participants who received psilocybin reported significantly greater improvements in MDD symptoms, compared with those who received niacin. MADRS scores – a scale from 0 to 60 where higher scores indicate more severe depression – showed greater reductions with treatment vs. placebo at 8 days (mean difference, −12.0; 95% confidence interval, −16.6 to −7.4; P < .001), and at day 43 (mean difference, −12.3; 95% CI, −17.5 to −7.2; P < .001).
More participants receiving psilocybin had sustained depressive symptom response (42% vs. 11%; P = .002) and more improvement in the Sheehan Disability Scale score, which measures functional disability, 43 days after treatment (P < .001).
The effects persisted through the end of the study, although the differences between groups were no longer significant by week 6.
“This is another exciting piece of evidence that adds to the current literature regarding the potential efficacy of psilocybin for the treatment of mental health conditions, particularly depression,” said Greg Fonzo, MD, codirector of the Center for Psychedelic Research and Therapy at the University of Texas at Austin, who commented on the findings.
Significantly more people in the psilocybin group reported at least one treatment-related adverse event (AE, 82% vs. 44%), although most were mild to moderate. Headache and nausea were the most common side effects and most resolved within 1 day of dosing.
While those numbers are high, Dr. Fonzo said they’re not out of line with AEs reported in other studies.
“Particularly with the types of adverse events reported here, like headache and nausea, those are things you would typically expect to see in this treatment,” said Dr. Fonzo, who was not part of the research.
“But it is high, and it underscores that this is not a treatment without certain risks, even though it was good that they were primarily mild in severity,” he added.
A ‘stepping stone’ to FDA approval?
The use of tools to measure disability in addition to symptoms of depression severity is a strength of the study, Dr. Fonzo added. The use of an active comparator and the 6-week follow-up also offer something new over previous studies.
Despite the longer follow up, questions remain about the durability of response, something only a longer study could answer, Dr. Fonzo said. The small and homogeneous sample-size are also a concern. Nearly 90% of participants were White, and more than half had an income of $75,000 a year or higher.
“It’s another stepping stone in the process to FDA approval, but the next step in that process would be much larger phase 3 trials that would have much larger samples, a longer follow-up, and hopefully have a more inclusive swath of the population,” Dr. Fonzo said.
But perhaps one of the most significant limitations is the use of niacin as an active comparator, said Caleb Alexander, MD, codirector of the Center for Drug Safety and Effectiveness at Johns Hopkins University in Baltimore.
The use of an agent that doesn’t produce effects similar to those expected from a psychedelic introduced the potential for functional unblinding, Dr. Alexander said. Investigators did not ask participants to guess whether they received psilocybin or niacin, so the quality of the blinding was not assessed in the study.
“We’d like to see the use of [an] active comparator that might have a chance of obscuring to people as to whether they’ve been randomized to the treatment arm or control arm,” said Dr. Alexander, who wasn’t involved in the study. “Why not use a benzodiazepine or another drug that produces a transient euphoria that would better obscure whether or not people were receiving the psilocybin?”
The authors of an accompanying editorial shared these concerns, also noting that the study included “a significant number of patients who did not respond to therapy.”
“It is important to analyze and understand adverse outcomes in psychedelic trials and conduct longitudinal studies to determine how sustained the effects will be and what may initiate a recrudescence of symptoms,” write Rachel Yehuda, PhD, and Amy Lehrner, PhD, both of the Peters VA Medical Center and Icahn School of Medicine at Mount Sinai, New York.
“Future studies will help identify who is most likely to benefit from psychedelics, whether booster or repeated treatment is safe and beneficial, and what the optimal dose and therapeutic frameworks are.”
A long-term follow-up of the current trial was terminated last year because of low enrollment. The spokesperson with Usona Institute did not respond to questions about that study, and the institute’s statement only added that preparations are underway to launch another study that “will provide additional safety and efficacy data to support submission of a new drug application to the FDA.”
Usona published its manufacturing process that it used to synthesize psilocybin in an open-access journal and signed a statement on “open science and open praxis” with psilocybin and similar substances, which appears on their website. That statement was signed by 31 research and service organizations around the world and nearly 150 scientists, scholars, and practitioners.
The study was funded by Usona Institute. Dr. Raison reported receiving personal fees from Usona Institute and grants to Usona Institute from Dr. Bronner’s All-One, Fournier Family Foundation, Good Ventures, Steven and Alexandra Cohen Foundation, Tiny Blue Dot Foundation, Turnbull Family Foundation, and William A. Linton during the conduct of the study; and personal fees from Novartis, Sage/Biogen, Emory Healthcare, and Vail Health outside the submitted work. Dr. Fonzo and Dr. Alexander report no relevant financial relationships. Dr. Yehuda reports receiving nonfinancial support from the Multidisciplinary Association for Psychedelic Studies Public Benefit (MAPS PBC) and grants from COMPASS Pathways. Dr. Lehrner is an investigator on trials sponsored by MAPS PBC and COMPASS Pathways.
A version of this article first appeared on Medscape.com.
FROM JAMA
Suicidal behavior tied to increased all-cause mortality in MDD
Investigators studied close to 143,000 patients, encompassing more than 150,000 MDD episodes. Episodes of depression with suicidal behavior (MDD-SB) were compared to MDD episodes without suicidal behavior (MDD-non-SB).
Suicidal behavior was associated with a 2.6-fold higher rate of all-cause mortality, as well as considerably higher health care resource utilization (HCRU) and work loss, compared with matched controls.
Patients with depression who had attempted suicide were younger and more commonly suffering from other psychiatric comorbidities, such as anxiety and addiction. Important risk factors for suicidal acts within a year after the onset of a depressive episode were previous suicide attempts, substance use disorder, anxiety, and sleeping disorders.
“The findings tell us that the care provided for this particular group needs to be developed,” lead author Johan Lundberg, MD, PhD, adjunct professor in psychiatry and senior physician in psychiatry, Karolinska Institute, Stockholm, told this news organization.
“The take-home message is that, when treating patients with increased risk of suicidal behavior, one should offer treatments with this in mind,” said Dr. Lundberg, also the head of the section of mood disorders, Northern Stockholm Psychiatry Clinic. “One possible option is lithium augmentation.”
The study was published online in JAMA Psychiatry.
Identifying subgroups
Depression is associated with increased all-cause mortality, the authors write. Suicidal behavior and previous suicide attempts are known to increase the risk of suicide-associated mortality, with up to 13% of patients with nonfatal suicide attempts dying of suicide at a later time.
Previous studies investigating the association between suicidal behavior and mortality have been limited by nonrandom sampling due to “nonuniversal access to health care and/or exclusion of primary care data,” they state.
For this reason, it’s not established to what extent these estimates actually represent patients with MDD as a whole, or to what extent suicidal behavior is a risk factor for all-cause mortality.
“We think there is a need to identify subgroups within the very large group of individuals with MDD in order to improve treatment outcomes,” Dr. Lundberg said.
To do so, the researchers turned to data from the Stockholm MDD Cohort (SMC), which comprises all patients diagnosed with MDD in any health care setting in the regions of Stockholm from 2010 to 2018. They identified 5 years of recorded MDD episodes (n = 158,169) in patients aged 18 years and older (n = 145,577). A single patient could contribute more than one episode.
At index, MDD-SB patients (n = 2,219; mean age, 41 years) were matched with MDD-non-SB patients (9,574; mean age, 41 years) based on age, sex, year of MDD diagnosis, and socioeconomic status. In total, 2,219 episodes (63.2% in women, 36.8% in men) were compared to 11,109 episodes (63.4% in women, 36.6% in men), respectively.
Enhanced monitoring, optimized treatment
The median time from the start of the episode until the first suicidal behavior was 165 days.
The all-cause mortality rate in the MDD-SB and MDD-non-SB groups was 2.5 per 100 person-years vs. 1 per 100 person-years, respectively (based on 466 deaths), corresponding to a hazard ratio of 2.62 (95% confidence interval, 2.15-3.20).
Patients in the MDD-SB group were younger, were more frequently diagnosed while in specialized care, and had sustained more work loss than their counterparts in the MDD-non-SB group. They also showed a gradual increase in the prevalence of comorbid conditions from about 12 months before index, with this increase being “most pronounced” for anxiety, stress, substance use, and personality disorders.
MDD-SB episodes were associated with higher HCRU and more work loss, compared with MDD-non-SB episodes.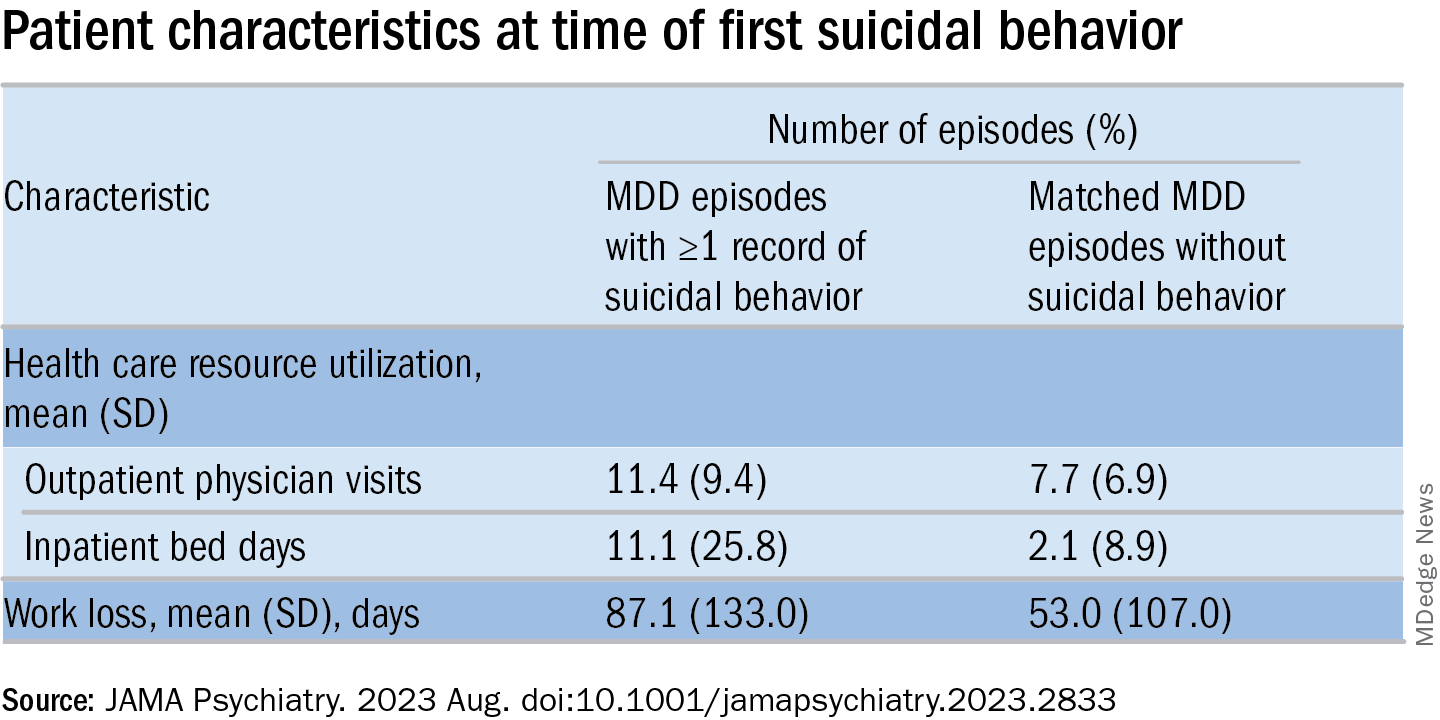
The researchers calculated a risk score for factors associated with suicidal behavior within 1 year after the start of an MDD episode (outcome). The two most important risk factors for suicidal behavior were a history of suicidal behavior together with age, which had a “U-shaped association” with the outcome, they write, with individuals younger than age 20 and older than age 70 having the highest risks.
The final risk score included additional factors that increased the risk of the outcome (in descending order): history of substance use, history of sleep disorders, health care level in which MDD was diagnosed, history of antidepressant use, and history of anxiety disorders.
These results “indicate that patients at risk for suicidal behavior can be identified at an early stage to allow for enhanced monitoring and optimized treatment with the goal of preventing suicidal behavior and reducing mortality,” the authors state.
The specific causes of death weren’t analyzed in this particular paper, Dr. Lundberg noted. A previous study conducted by the same group found the risk of death was doubled in MDD patients, compared with controls.
“We don’t speculate about which causes other than suicide might explain the difference” and account for the increased mortality risk, he said. “This should be studied in future projects.”
Complicated family of destructive behaviors
In a comment, Russell Copelan, MD, a former emergency department psychiatrist at the University of Colorado Affiliated Hospital and currently an expert consultant to the American Association of Suicidology, said a take-home message of the study is that suicide is “a complex and complicated family of destructive behaviors.”
The findings “should not suggest a wait-and-see clinical approach,” warned Dr. Copelan, who wasn’t involved with the study.
Underrecognized or misdiagnosed anxiety, agitation, and insomnia may be “barriers to remission and treatment response,” he noted.
Dr. Copelan, who is also the founder and CEO of eMed Logic, which offers assessment tools for suicide and violence, encouraged clinicians “not to minimize the proportion of patients who experience anxiety, agitation, and insomnia in response to what some may consider a personal misfortune, such as interpersonal, employment, or financial crisis.”
A version of this article first appeared on Medscape.com.
Investigators studied close to 143,000 patients, encompassing more than 150,000 MDD episodes. Episodes of depression with suicidal behavior (MDD-SB) were compared to MDD episodes without suicidal behavior (MDD-non-SB).
Suicidal behavior was associated with a 2.6-fold higher rate of all-cause mortality, as well as considerably higher health care resource utilization (HCRU) and work loss, compared with matched controls.
Patients with depression who had attempted suicide were younger and more commonly suffering from other psychiatric comorbidities, such as anxiety and addiction. Important risk factors for suicidal acts within a year after the onset of a depressive episode were previous suicide attempts, substance use disorder, anxiety, and sleeping disorders.
“The findings tell us that the care provided for this particular group needs to be developed,” lead author Johan Lundberg, MD, PhD, adjunct professor in psychiatry and senior physician in psychiatry, Karolinska Institute, Stockholm, told this news organization.
“The take-home message is that, when treating patients with increased risk of suicidal behavior, one should offer treatments with this in mind,” said Dr. Lundberg, also the head of the section of mood disorders, Northern Stockholm Psychiatry Clinic. “One possible option is lithium augmentation.”
The study was published online in JAMA Psychiatry.
Identifying subgroups
Depression is associated with increased all-cause mortality, the authors write. Suicidal behavior and previous suicide attempts are known to increase the risk of suicide-associated mortality, with up to 13% of patients with nonfatal suicide attempts dying of suicide at a later time.
Previous studies investigating the association between suicidal behavior and mortality have been limited by nonrandom sampling due to “nonuniversal access to health care and/or exclusion of primary care data,” they state.
For this reason, it’s not established to what extent these estimates actually represent patients with MDD as a whole, or to what extent suicidal behavior is a risk factor for all-cause mortality.
“We think there is a need to identify subgroups within the very large group of individuals with MDD in order to improve treatment outcomes,” Dr. Lundberg said.
To do so, the researchers turned to data from the Stockholm MDD Cohort (SMC), which comprises all patients diagnosed with MDD in any health care setting in the regions of Stockholm from 2010 to 2018. They identified 5 years of recorded MDD episodes (n = 158,169) in patients aged 18 years and older (n = 145,577). A single patient could contribute more than one episode.
At index, MDD-SB patients (n = 2,219; mean age, 41 years) were matched with MDD-non-SB patients (9,574; mean age, 41 years) based on age, sex, year of MDD diagnosis, and socioeconomic status. In total, 2,219 episodes (63.2% in women, 36.8% in men) were compared to 11,109 episodes (63.4% in women, 36.6% in men), respectively.
Enhanced monitoring, optimized treatment
The median time from the start of the episode until the first suicidal behavior was 165 days.
The all-cause mortality rate in the MDD-SB and MDD-non-SB groups was 2.5 per 100 person-years vs. 1 per 100 person-years, respectively (based on 466 deaths), corresponding to a hazard ratio of 2.62 (95% confidence interval, 2.15-3.20).
Patients in the MDD-SB group were younger, were more frequently diagnosed while in specialized care, and had sustained more work loss than their counterparts in the MDD-non-SB group. They also showed a gradual increase in the prevalence of comorbid conditions from about 12 months before index, with this increase being “most pronounced” for anxiety, stress, substance use, and personality disorders.
MDD-SB episodes were associated with higher HCRU and more work loss, compared with MDD-non-SB episodes.
The researchers calculated a risk score for factors associated with suicidal behavior within 1 year after the start of an MDD episode (outcome). The two most important risk factors for suicidal behavior were a history of suicidal behavior together with age, which had a “U-shaped association” with the outcome, they write, with individuals younger than age 20 and older than age 70 having the highest risks.
The final risk score included additional factors that increased the risk of the outcome (in descending order): history of substance use, history of sleep disorders, health care level in which MDD was diagnosed, history of antidepressant use, and history of anxiety disorders.
These results “indicate that patients at risk for suicidal behavior can be identified at an early stage to allow for enhanced monitoring and optimized treatment with the goal of preventing suicidal behavior and reducing mortality,” the authors state.
The specific causes of death weren’t analyzed in this particular paper, Dr. Lundberg noted. A previous study conducted by the same group found the risk of death was doubled in MDD patients, compared with controls.
“We don’t speculate about which causes other than suicide might explain the difference” and account for the increased mortality risk, he said. “This should be studied in future projects.”
Complicated family of destructive behaviors
In a comment, Russell Copelan, MD, a former emergency department psychiatrist at the University of Colorado Affiliated Hospital and currently an expert consultant to the American Association of Suicidology, said a take-home message of the study is that suicide is “a complex and complicated family of destructive behaviors.”
The findings “should not suggest a wait-and-see clinical approach,” warned Dr. Copelan, who wasn’t involved with the study.
Underrecognized or misdiagnosed anxiety, agitation, and insomnia may be “barriers to remission and treatment response,” he noted.
Dr. Copelan, who is also the founder and CEO of eMed Logic, which offers assessment tools for suicide and violence, encouraged clinicians “not to minimize the proportion of patients who experience anxiety, agitation, and insomnia in response to what some may consider a personal misfortune, such as interpersonal, employment, or financial crisis.”
A version of this article first appeared on Medscape.com.
Investigators studied close to 143,000 patients, encompassing more than 150,000 MDD episodes. Episodes of depression with suicidal behavior (MDD-SB) were compared to MDD episodes without suicidal behavior (MDD-non-SB).
Suicidal behavior was associated with a 2.6-fold higher rate of all-cause mortality, as well as considerably higher health care resource utilization (HCRU) and work loss, compared with matched controls.
Patients with depression who had attempted suicide were younger and more commonly suffering from other psychiatric comorbidities, such as anxiety and addiction. Important risk factors for suicidal acts within a year after the onset of a depressive episode were previous suicide attempts, substance use disorder, anxiety, and sleeping disorders.
“The findings tell us that the care provided for this particular group needs to be developed,” lead author Johan Lundberg, MD, PhD, adjunct professor in psychiatry and senior physician in psychiatry, Karolinska Institute, Stockholm, told this news organization.
“The take-home message is that, when treating patients with increased risk of suicidal behavior, one should offer treatments with this in mind,” said Dr. Lundberg, also the head of the section of mood disorders, Northern Stockholm Psychiatry Clinic. “One possible option is lithium augmentation.”
The study was published online in JAMA Psychiatry.
Identifying subgroups
Depression is associated with increased all-cause mortality, the authors write. Suicidal behavior and previous suicide attempts are known to increase the risk of suicide-associated mortality, with up to 13% of patients with nonfatal suicide attempts dying of suicide at a later time.
Previous studies investigating the association between suicidal behavior and mortality have been limited by nonrandom sampling due to “nonuniversal access to health care and/or exclusion of primary care data,” they state.
For this reason, it’s not established to what extent these estimates actually represent patients with MDD as a whole, or to what extent suicidal behavior is a risk factor for all-cause mortality.
“We think there is a need to identify subgroups within the very large group of individuals with MDD in order to improve treatment outcomes,” Dr. Lundberg said.
To do so, the researchers turned to data from the Stockholm MDD Cohort (SMC), which comprises all patients diagnosed with MDD in any health care setting in the regions of Stockholm from 2010 to 2018. They identified 5 years of recorded MDD episodes (n = 158,169) in patients aged 18 years and older (n = 145,577). A single patient could contribute more than one episode.
At index, MDD-SB patients (n = 2,219; mean age, 41 years) were matched with MDD-non-SB patients (9,574; mean age, 41 years) based on age, sex, year of MDD diagnosis, and socioeconomic status. In total, 2,219 episodes (63.2% in women, 36.8% in men) were compared to 11,109 episodes (63.4% in women, 36.6% in men), respectively.
Enhanced monitoring, optimized treatment
The median time from the start of the episode until the first suicidal behavior was 165 days.
The all-cause mortality rate in the MDD-SB and MDD-non-SB groups was 2.5 per 100 person-years vs. 1 per 100 person-years, respectively (based on 466 deaths), corresponding to a hazard ratio of 2.62 (95% confidence interval, 2.15-3.20).
Patients in the MDD-SB group were younger, were more frequently diagnosed while in specialized care, and had sustained more work loss than their counterparts in the MDD-non-SB group. They also showed a gradual increase in the prevalence of comorbid conditions from about 12 months before index, with this increase being “most pronounced” for anxiety, stress, substance use, and personality disorders.
MDD-SB episodes were associated with higher HCRU and more work loss, compared with MDD-non-SB episodes.
The researchers calculated a risk score for factors associated with suicidal behavior within 1 year after the start of an MDD episode (outcome). The two most important risk factors for suicidal behavior were a history of suicidal behavior together with age, which had a “U-shaped association” with the outcome, they write, with individuals younger than age 20 and older than age 70 having the highest risks.
The final risk score included additional factors that increased the risk of the outcome (in descending order): history of substance use, history of sleep disorders, health care level in which MDD was diagnosed, history of antidepressant use, and history of anxiety disorders.
These results “indicate that patients at risk for suicidal behavior can be identified at an early stage to allow for enhanced monitoring and optimized treatment with the goal of preventing suicidal behavior and reducing mortality,” the authors state.
The specific causes of death weren’t analyzed in this particular paper, Dr. Lundberg noted. A previous study conducted by the same group found the risk of death was doubled in MDD patients, compared with controls.
“We don’t speculate about which causes other than suicide might explain the difference” and account for the increased mortality risk, he said. “This should be studied in future projects.”
Complicated family of destructive behaviors
In a comment, Russell Copelan, MD, a former emergency department psychiatrist at the University of Colorado Affiliated Hospital and currently an expert consultant to the American Association of Suicidology, said a take-home message of the study is that suicide is “a complex and complicated family of destructive behaviors.”
The findings “should not suggest a wait-and-see clinical approach,” warned Dr. Copelan, who wasn’t involved with the study.
Underrecognized or misdiagnosed anxiety, agitation, and insomnia may be “barriers to remission and treatment response,” he noted.
Dr. Copelan, who is also the founder and CEO of eMed Logic, which offers assessment tools for suicide and violence, encouraged clinicians “not to minimize the proportion of patients who experience anxiety, agitation, and insomnia in response to what some may consider a personal misfortune, such as interpersonal, employment, or financial crisis.”
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Screen bipolar patients for eating disorders
Previous research of bipolar disorder (BD) shows a high rate of comorbidities with other psychiatric disorders, including eating disorders (EDs), Valentin Flaudias, PhD, of Nantes (France) University and colleagues wrote.
“There is growing evidence that, compared with individuals with BD alone, individuals with both BD and EDs have a more severe clinical profile, including increased mood instability, alcohol use disorders, anxiety disorders, more depressive episodes, more rapid cycling, increased suicidality, and poorer response to medication,” but studies of BD type-specific ED prevalence have been inconsistent, they said.
In a study published in the Journal of Affective Disorders, the researchers reviewed data from 2,929 outpatients who underwent assessments for BD at 1 of 12 psychiatric centers in France. Of these, 1,505 met criteria for type I and 1,424 met criteria for type II. The post hoc analysis included identification of lifetime prevalence of ED. Diagnosis was based on the DSM-4-TR and the researchers considered three ED types: anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED). Subtypes of BD were type I and type II. DSM not otherwise specified diagnoses for BD and EDs were excluded. The mean age of the participants was 40.5 years, and 61% were women.
A total of 479 individuals met criteria for comorbid EDs (16.4%). ED prevalence was significantly higher in BD type II patients than in BD type I patients (20.6 % vs. 12.4 %, P < .001). The overall breakdown according to ED subtype was 30% for AN, 13% for BN, and 56% for BED. The researchers found no significant differences in patients with AN, BN, or BED according to BD subtype.
In a multivariate analysis, BD patients with ED were more likely than those without ED to be women (77% vs. 55%), especially those with AN (95% vs. 82%).
BD patients with ED also tended to be younger than those without ED (37 years vs. 41 years) and reported more frequent suicide attempts (50% vs. 35%). Younger age and more frequent suicide attempts were further significant among BD patients with AN, compared with those with BED, but BD patients with BED reported higher levels of childhood trauma.
BD patients with ED also reported higher levels of depressive symptoms than those without ED, although history of psychosis was less frequent among BD patients with AN and BED compared with BD patients without EDs.
Overall, “after controlling for other variables, the independent factors differentiating BD patients with versus without ED were primarily younger age, female gender, abnormal BMI, increased affective lability and higher comorbidity with anxiety disorders,” the researchers wrote. In addition, presence of EDs except for AN was associated with decreased current functioning.
The findings were limited by several factors including the cross-sectional design, lack of a control group of non-BD individuals, and the consideration of ED over a lifetime, and small number of BN cases, the researchers noted.
However, the results suggest a high prevalence of ED in BD patients and highlight the need to screen BD patients for ED and provide integrated care. More research is needed to explore the evolution of the two conditions as comorbidities and to examine subtypes and of both conditions and their interactions, they concluded.
The study was supported by the FondaMental Foundation, French National Institute for Health and Medical Research, Public Hospitals of Paris, and the French National Research Agency’s Investment for the Future program. The researchers had no financial conflicts to disclose.
Previous research of bipolar disorder (BD) shows a high rate of comorbidities with other psychiatric disorders, including eating disorders (EDs), Valentin Flaudias, PhD, of Nantes (France) University and colleagues wrote.
“There is growing evidence that, compared with individuals with BD alone, individuals with both BD and EDs have a more severe clinical profile, including increased mood instability, alcohol use disorders, anxiety disorders, more depressive episodes, more rapid cycling, increased suicidality, and poorer response to medication,” but studies of BD type-specific ED prevalence have been inconsistent, they said.
In a study published in the Journal of Affective Disorders, the researchers reviewed data from 2,929 outpatients who underwent assessments for BD at 1 of 12 psychiatric centers in France. Of these, 1,505 met criteria for type I and 1,424 met criteria for type II. The post hoc analysis included identification of lifetime prevalence of ED. Diagnosis was based on the DSM-4-TR and the researchers considered three ED types: anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED). Subtypes of BD were type I and type II. DSM not otherwise specified diagnoses for BD and EDs were excluded. The mean age of the participants was 40.5 years, and 61% were women.
A total of 479 individuals met criteria for comorbid EDs (16.4%). ED prevalence was significantly higher in BD type II patients than in BD type I patients (20.6 % vs. 12.4 %, P < .001). The overall breakdown according to ED subtype was 30% for AN, 13% for BN, and 56% for BED. The researchers found no significant differences in patients with AN, BN, or BED according to BD subtype.
In a multivariate analysis, BD patients with ED were more likely than those without ED to be women (77% vs. 55%), especially those with AN (95% vs. 82%).
BD patients with ED also tended to be younger than those without ED (37 years vs. 41 years) and reported more frequent suicide attempts (50% vs. 35%). Younger age and more frequent suicide attempts were further significant among BD patients with AN, compared with those with BED, but BD patients with BED reported higher levels of childhood trauma.
BD patients with ED also reported higher levels of depressive symptoms than those without ED, although history of psychosis was less frequent among BD patients with AN and BED compared with BD patients without EDs.
Overall, “after controlling for other variables, the independent factors differentiating BD patients with versus without ED were primarily younger age, female gender, abnormal BMI, increased affective lability and higher comorbidity with anxiety disorders,” the researchers wrote. In addition, presence of EDs except for AN was associated with decreased current functioning.
The findings were limited by several factors including the cross-sectional design, lack of a control group of non-BD individuals, and the consideration of ED over a lifetime, and small number of BN cases, the researchers noted.
However, the results suggest a high prevalence of ED in BD patients and highlight the need to screen BD patients for ED and provide integrated care. More research is needed to explore the evolution of the two conditions as comorbidities and to examine subtypes and of both conditions and their interactions, they concluded.
The study was supported by the FondaMental Foundation, French National Institute for Health and Medical Research, Public Hospitals of Paris, and the French National Research Agency’s Investment for the Future program. The researchers had no financial conflicts to disclose.
Previous research of bipolar disorder (BD) shows a high rate of comorbidities with other psychiatric disorders, including eating disorders (EDs), Valentin Flaudias, PhD, of Nantes (France) University and colleagues wrote.
“There is growing evidence that, compared with individuals with BD alone, individuals with both BD and EDs have a more severe clinical profile, including increased mood instability, alcohol use disorders, anxiety disorders, more depressive episodes, more rapid cycling, increased suicidality, and poorer response to medication,” but studies of BD type-specific ED prevalence have been inconsistent, they said.
In a study published in the Journal of Affective Disorders, the researchers reviewed data from 2,929 outpatients who underwent assessments for BD at 1 of 12 psychiatric centers in France. Of these, 1,505 met criteria for type I and 1,424 met criteria for type II. The post hoc analysis included identification of lifetime prevalence of ED. Diagnosis was based on the DSM-4-TR and the researchers considered three ED types: anorexia nervosa (AN), bulimia nervosa (BN), and binge-eating disorder (BED). Subtypes of BD were type I and type II. DSM not otherwise specified diagnoses for BD and EDs were excluded. The mean age of the participants was 40.5 years, and 61% were women.
A total of 479 individuals met criteria for comorbid EDs (16.4%). ED prevalence was significantly higher in BD type II patients than in BD type I patients (20.6 % vs. 12.4 %, P < .001). The overall breakdown according to ED subtype was 30% for AN, 13% for BN, and 56% for BED. The researchers found no significant differences in patients with AN, BN, or BED according to BD subtype.
In a multivariate analysis, BD patients with ED were more likely than those without ED to be women (77% vs. 55%), especially those with AN (95% vs. 82%).
BD patients with ED also tended to be younger than those without ED (37 years vs. 41 years) and reported more frequent suicide attempts (50% vs. 35%). Younger age and more frequent suicide attempts were further significant among BD patients with AN, compared with those with BED, but BD patients with BED reported higher levels of childhood trauma.
BD patients with ED also reported higher levels of depressive symptoms than those without ED, although history of psychosis was less frequent among BD patients with AN and BED compared with BD patients without EDs.
Overall, “after controlling for other variables, the independent factors differentiating BD patients with versus without ED were primarily younger age, female gender, abnormal BMI, increased affective lability and higher comorbidity with anxiety disorders,” the researchers wrote. In addition, presence of EDs except for AN was associated with decreased current functioning.
The findings were limited by several factors including the cross-sectional design, lack of a control group of non-BD individuals, and the consideration of ED over a lifetime, and small number of BN cases, the researchers noted.
However, the results suggest a high prevalence of ED in BD patients and highlight the need to screen BD patients for ED and provide integrated care. More research is needed to explore the evolution of the two conditions as comorbidities and to examine subtypes and of both conditions and their interactions, they concluded.
The study was supported by the FondaMental Foundation, French National Institute for Health and Medical Research, Public Hospitals of Paris, and the French National Research Agency’s Investment for the Future program. The researchers had no financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Your patient bequeathed money to you: Can you accept it?
Michael Victoroff, MD, described the phone call he received from an attorney asking a thorny ethics question involving a patient’s gift to another physician. Dr. Victoroff, a past member of the ethics committee of the American Academy of Family Physicians, had definite thoughts about it.
“The attorney was representing the daughters of an elderly gentleman who had moved from the East Coast to Colorado to be closer to them,” said Dr. Victoroff, who teaches bioethics in the MBA program at the University of Denver and also practices at the University of Colorado School of Medicine.
“The father visited his new primary care physician frequently because he had multiple health issues.”
The patient was happy with the doctor’s medical care and over time that they developed a friendship. Dr. Victoroff emphasized that no sexual or romantic impropriety ever took place between the patient and his physician.
“But the social relationship went beyond the ordinary doctor-patient boundaries. The patient ultimately named the doctor as his health care proxy in the event that he became unable to make decisions regarding his care. He also mentioned he was going to leave her $100,000 in his will,” says Dr. Victoroff.
The physician did accept the role of proxy, “which raises a whole host of ethical issues,” says Dr. Victoroff. As it happened, she was never called upon to exercise that decision-making authority, since the patient died suddenly and was mentally competent at the time.
for her to accept such a substantial bequest from a patient, and they hired an attorney to contest the will.
No law against it
Dennis Hursh, attorney and managing partner of Physician Agreements Health Law, a Pennsylvania-based law firm that represents physicians, noted in an interview that, “the problem isn’t legal per se. Rather, the problem is an ethical one.”
Legally speaking, there’s no prohibition against receiving a bequest or other form of gift from a patient. “People are free to dispose of their estates in whatever way they see fit, and no law technically precludes a physician from accepting a bequest,” says Dr. Victoroff. “But this presupposes there is nothing improper going on, such as extortion, deception, coercion, or exercising undue influence.”
The issue of bequeathing money to their physician gained attention in a recent case that took place in Australia. Peter Alexakis, MD, received a whopping bequest of $24 million from a patient. The elderly patient had changed his will to name Dr. Alexakis as the sole beneficiary – after Dr. Alexakis had visited him at home 92 times during the preceding months. The original heirs filed a lawsuit in Australia’s Supreme Court against Dr. Alexakis, contesting the will.
The lawsuit was unsuccessful in court, but Dr. Alexakis was found guilty of malpractice by Australia’s Health Care Complaints Commission after being reported to the HCCC by the palliative care physicians who were treating the patient. They alleged that Dr. Alexakis had interfered with their care of the patient. The more serious allegation was that the doctor had engaged in a deliberate strategy to exploit the relationship for financial gain.
Dr. Alexakis was chastised by the HCCC for engaging in “obtuse” and “suspicious” behavior and for “blurring the boundaries of the doctor-patient relationship.”
There are three domains – legal, ethical, and practical – when it comes to accepting bequests or any gifts from patients, says Dr. Victoroff.
“[In] the legal domain, for example, if you receive a bequest from anyone, patient or otherwise, you have to know your local laws about estates and taxes and so forth and obey them,” he said.
Attorney Hursh pointed out that the Australian doctor wasn’t found guilty of wrongdoing in a court of law but rather of unethical conduct by the Australian medical licensing entity.
Patients giving gifts is often a part of a physician’s life
When Ian Schorr, MD, first started out in practice, he was surprised that patients began bringing him gifts of food to express gratitude for his care.
“I thought it was unethical to accept their gifts, so I turned them down and wouldn’t accept so much as a cookie,” Dr. Schorr, a now-retired ophthalmologist, told this news organization. “But that changed because my office staff told me that some patients were feeling disappointed and insulted. I realized that some people want to express appreciation in ways that go beyond a monetary payment.”
The next time he received a gift from a patient, he “accepted it gracefully.” And he wrote a thank you note, which he continued to do any time he received a gift from a patient.
Kenneth Prager, MD, professor of clinical medicine, director of clinical ethics and chairman of the Medical Ethics Committee at Columbia University Medical Center, New York, says, “I have literally received hundreds of gifts, the vast majority being tokens of patients’ appreciation,” he said. “I’ll get boxes of chocolate or cakes, or sometimes articles of clothing.”
Occasionally, Dr. Prager receives a “somewhat larger gift” – for example, two tickets to a baseball game. “To reject these gifts would be a slap in the face to the patient,” he says, but “where it gets more ethically cloudy is when a gift is very substantial.”
Dr. Prager has never been offered a “substantial” gift or bequest personally. “But a patient whose brother I cared for has indicated that she has left instructions in her will to endow an associate chair of ethics in my honor, and I didn’t decline that,” he said.
The AMA Code of Ethics confirms that accepting gifts offered “as an expression of gratitude or a reflection of the patient’s cultural tradition” can “enhance the patient-physician relationship.” But sometimes gifts “may signal psychological needs that require the physician’s attention.” Accepting such gifts is “likely to damage the patient-physician relationship.”
Potential damage to the therapeutic relationship applies to all physicians but especially for psychiatrists and mental health professionals. “There are more stringent ethical requirements when it comes to these disciplines, where gift-giving gets into the territory of transference or may have particular psychological meaning, and accepting the gift may muddy the therapeutic waters,” Dr. Victoroff said.
Impact on the patient’s family and on other patients
The AMA statement encourages physicians to be “sensitive to the gift’s value, relative to the patient’s or physician’s means.” Physicians should decline gifts that are “disproportionately or inappropriately large, or when the physician would be uncomfortable to have colleagues know the gift had been accepted.”
They should also decline a bequest from a patient if they have reason to believe that to accept it “would present an emotional or financial hardship to the patient’s family.”
“If Bill Gates were leaving $100,000 to his doctor, I imagine Melinda would be just fine,” Mr. Hursh said. “But under ordinary circumstances, if the patient’s family might feel the impact of the bequest, it would be unethical to accept it and could be grounds for revocation of the doctor’s license.”
The AMA statement also warns physicians that by offering a gift, some patients may be seeking to “secure or influence care or to secure preferential treatment,” which can “undermine physicians’ obligation to provide services fairly to all patients.”
For this reason, bequests are “sticky,” said Laurel Lyckholm, MD, professor of hematology and oncology at West Virginia University School of Medicine. In the case of institutions where patients or community members donate money, “we know whose names are on the plaques that hang on the hospital walls, so it’s a delicate balance. What if there’s only one bed or one ventilator? Will the wife of the donor get preferential treatment?”
Follow institutional policy
A “very small gift, such as a fruitcake, is fine,” says Dr. Lyckholm, author of an essay on accepting gifts from patients. She said there’s a dollar amount ($15) that her institution mandates, above which a gift – even food – is considered too expensive to accept. “I was a nurse before I became a physician, and people always tried to give us gifts because we were so close to the minute-by-minute care of the patients,” she said. “We were not allowed to accept money or anything lavish.”
But in the case of small gifts, “the risk-benefit analysis is that there’s much more risk not to take it and to hurt the patient’s feelings.”
Gifts above $15 are given to charity. “I explain to patients that I’m not allowed to take such a large gift, but I’d love to give it to the hospital’s Rosenbaum Family House that provides patients and their relatives with lodging, or to the homeless shelter in Morgantown.”
Dr. Lyckholm, who serves on the ethics committee at J.W. Ruby Memorial Hospital, once was offered expensive tickets and said to the patient, “This is so incredibly thoughtful and kind, but I can’t accept them. I would like to give the tickets to a charity that can auction them off.”
She advises physicians to find out their institution’s policies. Many institutions have policies about what gifts their staff – whether physicians, nurses, or other health care professionals – can accept.
Passing the ‘smell test’
Accepting a large gift from a patient could potentially make it look like you might have exercised undue influence.
“That concern brings us to the third domain, which is very practical and all about appearances and perceptions,” Dr. Victoroff said.
He noted that there is “an inherent power differential between a physician and a patient. The very nature of the relationship can create a risk of ‘undue influence’ on the doctor’s part, even if it’s not apparent to the doctor.” For this reason, it’s necessary to be utterly transparent about how the bequest came about.
He suggests that if a patient informs you that he or she would like to leave money to you, it might be wise to suggest a meeting with the patient’s family, thus establishing some transparency.
It may not be possible to meet with the patient’s family for logistical reasons or because the patient would prefer not to involve their family in their estate planning. But in any case, it’s advisable to document any conversation in the patient’s chart, Dr. Victoroff advised.
“You should make a contemporaneous note that the patient initiated the suggestion and that you counseled them about the implications, no differently than you would with an interaction of a clinical nature,” he suggests. That way, if money has been left to you and is disputed, there’s a clear record that you didn’t solicit it or use any undue influence to bring it about.
He also recommended getting advice from a trusted colleague or a member of your institution’s ethics committee. “Taking time to get a second opinion about an ethical question is a safeguard, like having a chaperone in the room during an examination.”
Ultimately, “there is no human relationship without potential conflicts of interest. Our job is to manage those as best as we can, and sunlight is the best antidote to bad appearances,” Dr. Victoroff said.
A version of this article appeared on Medscape.com.
Michael Victoroff, MD, described the phone call he received from an attorney asking a thorny ethics question involving a patient’s gift to another physician. Dr. Victoroff, a past member of the ethics committee of the American Academy of Family Physicians, had definite thoughts about it.
“The attorney was representing the daughters of an elderly gentleman who had moved from the East Coast to Colorado to be closer to them,” said Dr. Victoroff, who teaches bioethics in the MBA program at the University of Denver and also practices at the University of Colorado School of Medicine.
“The father visited his new primary care physician frequently because he had multiple health issues.”
The patient was happy with the doctor’s medical care and over time that they developed a friendship. Dr. Victoroff emphasized that no sexual or romantic impropriety ever took place between the patient and his physician.
“But the social relationship went beyond the ordinary doctor-patient boundaries. The patient ultimately named the doctor as his health care proxy in the event that he became unable to make decisions regarding his care. He also mentioned he was going to leave her $100,000 in his will,” says Dr. Victoroff.
The physician did accept the role of proxy, “which raises a whole host of ethical issues,” says Dr. Victoroff. As it happened, she was never called upon to exercise that decision-making authority, since the patient died suddenly and was mentally competent at the time.
for her to accept such a substantial bequest from a patient, and they hired an attorney to contest the will.
No law against it
Dennis Hursh, attorney and managing partner of Physician Agreements Health Law, a Pennsylvania-based law firm that represents physicians, noted in an interview that, “the problem isn’t legal per se. Rather, the problem is an ethical one.”
Legally speaking, there’s no prohibition against receiving a bequest or other form of gift from a patient. “People are free to dispose of their estates in whatever way they see fit, and no law technically precludes a physician from accepting a bequest,” says Dr. Victoroff. “But this presupposes there is nothing improper going on, such as extortion, deception, coercion, or exercising undue influence.”
The issue of bequeathing money to their physician gained attention in a recent case that took place in Australia. Peter Alexakis, MD, received a whopping bequest of $24 million from a patient. The elderly patient had changed his will to name Dr. Alexakis as the sole beneficiary – after Dr. Alexakis had visited him at home 92 times during the preceding months. The original heirs filed a lawsuit in Australia’s Supreme Court against Dr. Alexakis, contesting the will.
The lawsuit was unsuccessful in court, but Dr. Alexakis was found guilty of malpractice by Australia’s Health Care Complaints Commission after being reported to the HCCC by the palliative care physicians who were treating the patient. They alleged that Dr. Alexakis had interfered with their care of the patient. The more serious allegation was that the doctor had engaged in a deliberate strategy to exploit the relationship for financial gain.
Dr. Alexakis was chastised by the HCCC for engaging in “obtuse” and “suspicious” behavior and for “blurring the boundaries of the doctor-patient relationship.”
There are three domains – legal, ethical, and practical – when it comes to accepting bequests or any gifts from patients, says Dr. Victoroff.
“[In] the legal domain, for example, if you receive a bequest from anyone, patient or otherwise, you have to know your local laws about estates and taxes and so forth and obey them,” he said.
Attorney Hursh pointed out that the Australian doctor wasn’t found guilty of wrongdoing in a court of law but rather of unethical conduct by the Australian medical licensing entity.
Patients giving gifts is often a part of a physician’s life
When Ian Schorr, MD, first started out in practice, he was surprised that patients began bringing him gifts of food to express gratitude for his care.
“I thought it was unethical to accept their gifts, so I turned them down and wouldn’t accept so much as a cookie,” Dr. Schorr, a now-retired ophthalmologist, told this news organization. “But that changed because my office staff told me that some patients were feeling disappointed and insulted. I realized that some people want to express appreciation in ways that go beyond a monetary payment.”
The next time he received a gift from a patient, he “accepted it gracefully.” And he wrote a thank you note, which he continued to do any time he received a gift from a patient.
Kenneth Prager, MD, professor of clinical medicine, director of clinical ethics and chairman of the Medical Ethics Committee at Columbia University Medical Center, New York, says, “I have literally received hundreds of gifts, the vast majority being tokens of patients’ appreciation,” he said. “I’ll get boxes of chocolate or cakes, or sometimes articles of clothing.”
Occasionally, Dr. Prager receives a “somewhat larger gift” – for example, two tickets to a baseball game. “To reject these gifts would be a slap in the face to the patient,” he says, but “where it gets more ethically cloudy is when a gift is very substantial.”
Dr. Prager has never been offered a “substantial” gift or bequest personally. “But a patient whose brother I cared for has indicated that she has left instructions in her will to endow an associate chair of ethics in my honor, and I didn’t decline that,” he said.
The AMA Code of Ethics confirms that accepting gifts offered “as an expression of gratitude or a reflection of the patient’s cultural tradition” can “enhance the patient-physician relationship.” But sometimes gifts “may signal psychological needs that require the physician’s attention.” Accepting such gifts is “likely to damage the patient-physician relationship.”
Potential damage to the therapeutic relationship applies to all physicians but especially for psychiatrists and mental health professionals. “There are more stringent ethical requirements when it comes to these disciplines, where gift-giving gets into the territory of transference or may have particular psychological meaning, and accepting the gift may muddy the therapeutic waters,” Dr. Victoroff said.
Impact on the patient’s family and on other patients
The AMA statement encourages physicians to be “sensitive to the gift’s value, relative to the patient’s or physician’s means.” Physicians should decline gifts that are “disproportionately or inappropriately large, or when the physician would be uncomfortable to have colleagues know the gift had been accepted.”
They should also decline a bequest from a patient if they have reason to believe that to accept it “would present an emotional or financial hardship to the patient’s family.”
“If Bill Gates were leaving $100,000 to his doctor, I imagine Melinda would be just fine,” Mr. Hursh said. “But under ordinary circumstances, if the patient’s family might feel the impact of the bequest, it would be unethical to accept it and could be grounds for revocation of the doctor’s license.”
The AMA statement also warns physicians that by offering a gift, some patients may be seeking to “secure or influence care or to secure preferential treatment,” which can “undermine physicians’ obligation to provide services fairly to all patients.”
For this reason, bequests are “sticky,” said Laurel Lyckholm, MD, professor of hematology and oncology at West Virginia University School of Medicine. In the case of institutions where patients or community members donate money, “we know whose names are on the plaques that hang on the hospital walls, so it’s a delicate balance. What if there’s only one bed or one ventilator? Will the wife of the donor get preferential treatment?”
Follow institutional policy
A “very small gift, such as a fruitcake, is fine,” says Dr. Lyckholm, author of an essay on accepting gifts from patients. She said there’s a dollar amount ($15) that her institution mandates, above which a gift – even food – is considered too expensive to accept. “I was a nurse before I became a physician, and people always tried to give us gifts because we were so close to the minute-by-minute care of the patients,” she said. “We were not allowed to accept money or anything lavish.”
But in the case of small gifts, “the risk-benefit analysis is that there’s much more risk not to take it and to hurt the patient’s feelings.”
Gifts above $15 are given to charity. “I explain to patients that I’m not allowed to take such a large gift, but I’d love to give it to the hospital’s Rosenbaum Family House that provides patients and their relatives with lodging, or to the homeless shelter in Morgantown.”
Dr. Lyckholm, who serves on the ethics committee at J.W. Ruby Memorial Hospital, once was offered expensive tickets and said to the patient, “This is so incredibly thoughtful and kind, but I can’t accept them. I would like to give the tickets to a charity that can auction them off.”
She advises physicians to find out their institution’s policies. Many institutions have policies about what gifts their staff – whether physicians, nurses, or other health care professionals – can accept.
Passing the ‘smell test’
Accepting a large gift from a patient could potentially make it look like you might have exercised undue influence.
“That concern brings us to the third domain, which is very practical and all about appearances and perceptions,” Dr. Victoroff said.
He noted that there is “an inherent power differential between a physician and a patient. The very nature of the relationship can create a risk of ‘undue influence’ on the doctor’s part, even if it’s not apparent to the doctor.” For this reason, it’s necessary to be utterly transparent about how the bequest came about.
He suggests that if a patient informs you that he or she would like to leave money to you, it might be wise to suggest a meeting with the patient’s family, thus establishing some transparency.
It may not be possible to meet with the patient’s family for logistical reasons or because the patient would prefer not to involve their family in their estate planning. But in any case, it’s advisable to document any conversation in the patient’s chart, Dr. Victoroff advised.
“You should make a contemporaneous note that the patient initiated the suggestion and that you counseled them about the implications, no differently than you would with an interaction of a clinical nature,” he suggests. That way, if money has been left to you and is disputed, there’s a clear record that you didn’t solicit it or use any undue influence to bring it about.
He also recommended getting advice from a trusted colleague or a member of your institution’s ethics committee. “Taking time to get a second opinion about an ethical question is a safeguard, like having a chaperone in the room during an examination.”
Ultimately, “there is no human relationship without potential conflicts of interest. Our job is to manage those as best as we can, and sunlight is the best antidote to bad appearances,” Dr. Victoroff said.
A version of this article appeared on Medscape.com.
Michael Victoroff, MD, described the phone call he received from an attorney asking a thorny ethics question involving a patient’s gift to another physician. Dr. Victoroff, a past member of the ethics committee of the American Academy of Family Physicians, had definite thoughts about it.
“The attorney was representing the daughters of an elderly gentleman who had moved from the East Coast to Colorado to be closer to them,” said Dr. Victoroff, who teaches bioethics in the MBA program at the University of Denver and also practices at the University of Colorado School of Medicine.
“The father visited his new primary care physician frequently because he had multiple health issues.”
The patient was happy with the doctor’s medical care and over time that they developed a friendship. Dr. Victoroff emphasized that no sexual or romantic impropriety ever took place between the patient and his physician.
“But the social relationship went beyond the ordinary doctor-patient boundaries. The patient ultimately named the doctor as his health care proxy in the event that he became unable to make decisions regarding his care. He also mentioned he was going to leave her $100,000 in his will,” says Dr. Victoroff.
The physician did accept the role of proxy, “which raises a whole host of ethical issues,” says Dr. Victoroff. As it happened, she was never called upon to exercise that decision-making authority, since the patient died suddenly and was mentally competent at the time.
for her to accept such a substantial bequest from a patient, and they hired an attorney to contest the will.
No law against it
Dennis Hursh, attorney and managing partner of Physician Agreements Health Law, a Pennsylvania-based law firm that represents physicians, noted in an interview that, “the problem isn’t legal per se. Rather, the problem is an ethical one.”
Legally speaking, there’s no prohibition against receiving a bequest or other form of gift from a patient. “People are free to dispose of their estates in whatever way they see fit, and no law technically precludes a physician from accepting a bequest,” says Dr. Victoroff. “But this presupposes there is nothing improper going on, such as extortion, deception, coercion, or exercising undue influence.”
The issue of bequeathing money to their physician gained attention in a recent case that took place in Australia. Peter Alexakis, MD, received a whopping bequest of $24 million from a patient. The elderly patient had changed his will to name Dr. Alexakis as the sole beneficiary – after Dr. Alexakis had visited him at home 92 times during the preceding months. The original heirs filed a lawsuit in Australia’s Supreme Court against Dr. Alexakis, contesting the will.
The lawsuit was unsuccessful in court, but Dr. Alexakis was found guilty of malpractice by Australia’s Health Care Complaints Commission after being reported to the HCCC by the palliative care physicians who were treating the patient. They alleged that Dr. Alexakis had interfered with their care of the patient. The more serious allegation was that the doctor had engaged in a deliberate strategy to exploit the relationship for financial gain.
Dr. Alexakis was chastised by the HCCC for engaging in “obtuse” and “suspicious” behavior and for “blurring the boundaries of the doctor-patient relationship.”
There are three domains – legal, ethical, and practical – when it comes to accepting bequests or any gifts from patients, says Dr. Victoroff.
“[In] the legal domain, for example, if you receive a bequest from anyone, patient or otherwise, you have to know your local laws about estates and taxes and so forth and obey them,” he said.
Attorney Hursh pointed out that the Australian doctor wasn’t found guilty of wrongdoing in a court of law but rather of unethical conduct by the Australian medical licensing entity.
Patients giving gifts is often a part of a physician’s life
When Ian Schorr, MD, first started out in practice, he was surprised that patients began bringing him gifts of food to express gratitude for his care.
“I thought it was unethical to accept their gifts, so I turned them down and wouldn’t accept so much as a cookie,” Dr. Schorr, a now-retired ophthalmologist, told this news organization. “But that changed because my office staff told me that some patients were feeling disappointed and insulted. I realized that some people want to express appreciation in ways that go beyond a monetary payment.”
The next time he received a gift from a patient, he “accepted it gracefully.” And he wrote a thank you note, which he continued to do any time he received a gift from a patient.
Kenneth Prager, MD, professor of clinical medicine, director of clinical ethics and chairman of the Medical Ethics Committee at Columbia University Medical Center, New York, says, “I have literally received hundreds of gifts, the vast majority being tokens of patients’ appreciation,” he said. “I’ll get boxes of chocolate or cakes, or sometimes articles of clothing.”
Occasionally, Dr. Prager receives a “somewhat larger gift” – for example, two tickets to a baseball game. “To reject these gifts would be a slap in the face to the patient,” he says, but “where it gets more ethically cloudy is when a gift is very substantial.”
Dr. Prager has never been offered a “substantial” gift or bequest personally. “But a patient whose brother I cared for has indicated that she has left instructions in her will to endow an associate chair of ethics in my honor, and I didn’t decline that,” he said.
The AMA Code of Ethics confirms that accepting gifts offered “as an expression of gratitude or a reflection of the patient’s cultural tradition” can “enhance the patient-physician relationship.” But sometimes gifts “may signal psychological needs that require the physician’s attention.” Accepting such gifts is “likely to damage the patient-physician relationship.”
Potential damage to the therapeutic relationship applies to all physicians but especially for psychiatrists and mental health professionals. “There are more stringent ethical requirements when it comes to these disciplines, where gift-giving gets into the territory of transference or may have particular psychological meaning, and accepting the gift may muddy the therapeutic waters,” Dr. Victoroff said.
Impact on the patient’s family and on other patients
The AMA statement encourages physicians to be “sensitive to the gift’s value, relative to the patient’s or physician’s means.” Physicians should decline gifts that are “disproportionately or inappropriately large, or when the physician would be uncomfortable to have colleagues know the gift had been accepted.”
They should also decline a bequest from a patient if they have reason to believe that to accept it “would present an emotional or financial hardship to the patient’s family.”
“If Bill Gates were leaving $100,000 to his doctor, I imagine Melinda would be just fine,” Mr. Hursh said. “But under ordinary circumstances, if the patient’s family might feel the impact of the bequest, it would be unethical to accept it and could be grounds for revocation of the doctor’s license.”
The AMA statement also warns physicians that by offering a gift, some patients may be seeking to “secure or influence care or to secure preferential treatment,” which can “undermine physicians’ obligation to provide services fairly to all patients.”
For this reason, bequests are “sticky,” said Laurel Lyckholm, MD, professor of hematology and oncology at West Virginia University School of Medicine. In the case of institutions where patients or community members donate money, “we know whose names are on the plaques that hang on the hospital walls, so it’s a delicate balance. What if there’s only one bed or one ventilator? Will the wife of the donor get preferential treatment?”
Follow institutional policy
A “very small gift, such as a fruitcake, is fine,” says Dr. Lyckholm, author of an essay on accepting gifts from patients. She said there’s a dollar amount ($15) that her institution mandates, above which a gift – even food – is considered too expensive to accept. “I was a nurse before I became a physician, and people always tried to give us gifts because we were so close to the minute-by-minute care of the patients,” she said. “We were not allowed to accept money or anything lavish.”
But in the case of small gifts, “the risk-benefit analysis is that there’s much more risk not to take it and to hurt the patient’s feelings.”
Gifts above $15 are given to charity. “I explain to patients that I’m not allowed to take such a large gift, but I’d love to give it to the hospital’s Rosenbaum Family House that provides patients and their relatives with lodging, or to the homeless shelter in Morgantown.”
Dr. Lyckholm, who serves on the ethics committee at J.W. Ruby Memorial Hospital, once was offered expensive tickets and said to the patient, “This is so incredibly thoughtful and kind, but I can’t accept them. I would like to give the tickets to a charity that can auction them off.”
She advises physicians to find out their institution’s policies. Many institutions have policies about what gifts their staff – whether physicians, nurses, or other health care professionals – can accept.
Passing the ‘smell test’
Accepting a large gift from a patient could potentially make it look like you might have exercised undue influence.
“That concern brings us to the third domain, which is very practical and all about appearances and perceptions,” Dr. Victoroff said.
He noted that there is “an inherent power differential between a physician and a patient. The very nature of the relationship can create a risk of ‘undue influence’ on the doctor’s part, even if it’s not apparent to the doctor.” For this reason, it’s necessary to be utterly transparent about how the bequest came about.
He suggests that if a patient informs you that he or she would like to leave money to you, it might be wise to suggest a meeting with the patient’s family, thus establishing some transparency.
It may not be possible to meet with the patient’s family for logistical reasons or because the patient would prefer not to involve their family in their estate planning. But in any case, it’s advisable to document any conversation in the patient’s chart, Dr. Victoroff advised.
“You should make a contemporaneous note that the patient initiated the suggestion and that you counseled them about the implications, no differently than you would with an interaction of a clinical nature,” he suggests. That way, if money has been left to you and is disputed, there’s a clear record that you didn’t solicit it or use any undue influence to bring it about.
He also recommended getting advice from a trusted colleague or a member of your institution’s ethics committee. “Taking time to get a second opinion about an ethical question is a safeguard, like having a chaperone in the room during an examination.”
Ultimately, “there is no human relationship without potential conflicts of interest. Our job is to manage those as best as we can, and sunlight is the best antidote to bad appearances,” Dr. Victoroff said.
A version of this article appeared on Medscape.com.
Abdominal fat linked to lower brain volume in midlife
In a large study of healthy middle-aged adults, greater visceral and subcutaneous abdominal fat on abdominal MRI predicted brain atrophy on imaging, especially in women.
“The study shows that excess fat is bad for the brain and worse in women, including in Alzheimer’s disease risk regions,” lead author Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, Mo., said in an interview.
The study was published online in the journal Aging and Disease
Modifiable risk factor
Multiple studies have suggested a connection between body fat accumulation and increased dementia risk. But few have examined the relationship between types of fat (visceral and subcutaneous) and brain volume.
For the new study, 10,000 healthy adults aged 20-80 years (mean age, 52.9 years; 53% men) underwent a short whole-body MRI protocol. Regression analyses of abdominal fat types and normalized brain volumes were evaluated, controlling for age and sex.
The research team found that higher amounts of both visceral and subcutaneous abdominal fat predicted lower total gray and white matter volume, as well as lower volume in the hippocampus, frontal cortex, and temporal, parietal, and occipital lobes.
“The findings are quite dramatic,” Dr. Raji told this news organization. “Overall, we found that both subcutaneous and visceral fat has similar levels of negative relationships with brain volumes.”
Women had a higher burden of brain atrophy with increased visceral fat than men. However, it’s difficult to place the sex differences in context because of the lack of prior work specifically investigating visceral fat, brain volume loss, and sex differences, the researchers caution.
They also note that while statistically significant relationships were observed between visceral fat levels and gray matter volume changes, their effect sizes were generally small.
“Thus, the statistical significance of this work is influenced by the large sample size and less so by large effect size in any given set of regions,” the investigators write.
Other limitations include the cross-sectional nature of the study, which precludes conclusions about causality. The analysis also did not account for other lifestyle factors such as physical activity, diet, and genetic variables.
The researchers call for further investigation “to better elucidate underlying mechanisms and discover possible interventions targeting abdominal fat reduction as a strategy to maintain brain health.”
‘Helpful addition to the literature’
In a comment, Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach, noted that “previous studies have linked obesity with cognitive decline and increased risk of dementia. Rather than using BMI as a proxy for body fat, the current study examined visceral and subcutaneous fat directly using imaging techniques.”
Dr. Sexton, who was not associated with this study, said the finding that increased body fat was associated with reduced brain volumes suggests “a possible mechanism to explain the previously reported associations between obesity and cognition.”
“Though some degree of atrophy and brain shrinkage is common with old age, awareness of this association is important because reduced brain volume may be associated with problems with thinking, memory, and performing everyday tasks, and because rates of obesity continue to rise in the United States, along with obesity-related conditions including heart disease, stroke, type 2 diabetes and certain types of cancer,” she added.
“While a helpful addition to the literature, the study does have important limitations. As an observational study, it cannot establish whether higher levels of body fat directly causes reduced brain volumes,” Dr. Sexton cautioned.
In addition, the study did not take into account important related factors like physical activity and diet, which may influence any relationship between body fat and brain volumes, she noted. “Overall, it is not just one factor that is important to consider when considering risk for cognitive decline and dementia, but multiple factors.
“Obesity and the location of body fat must be considered in combination with one’s total lived experience and habits, including physical activity, education, head injury, sleep, mental health, and the health of your heart/cardiovascular system and other key bodily systems,” Dr. Sexton said.
The Alzheimer’s Association is leading a 2-year clinical trial known as U.S. POINTER to see whether combining physical activity, healthy nutrition, social and intellectual challenges, and improved self-management of medical conditions can protect cognitive function in older adults who are at increased risk for cognitive decline.
This work was supported in part by Providence St. Joseph Health in Seattle; Saint John’s Health Center Foundation; Pacific Neuroscience Institute and Foundation; Will and Cary Singleton; and the McLoughlin family. Dr. Raji is a consultant for Brainreader, Apollo Health, Voxelwise, Neurevolution, Pacific Neuroscience Institute Foundation, and Icometrix. Dr. Sexton reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large study of healthy middle-aged adults, greater visceral and subcutaneous abdominal fat on abdominal MRI predicted brain atrophy on imaging, especially in women.
“The study shows that excess fat is bad for the brain and worse in women, including in Alzheimer’s disease risk regions,” lead author Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, Mo., said in an interview.
The study was published online in the journal Aging and Disease
Modifiable risk factor
Multiple studies have suggested a connection between body fat accumulation and increased dementia risk. But few have examined the relationship between types of fat (visceral and subcutaneous) and brain volume.
For the new study, 10,000 healthy adults aged 20-80 years (mean age, 52.9 years; 53% men) underwent a short whole-body MRI protocol. Regression analyses of abdominal fat types and normalized brain volumes were evaluated, controlling for age and sex.
The research team found that higher amounts of both visceral and subcutaneous abdominal fat predicted lower total gray and white matter volume, as well as lower volume in the hippocampus, frontal cortex, and temporal, parietal, and occipital lobes.
“The findings are quite dramatic,” Dr. Raji told this news organization. “Overall, we found that both subcutaneous and visceral fat has similar levels of negative relationships with brain volumes.”
Women had a higher burden of brain atrophy with increased visceral fat than men. However, it’s difficult to place the sex differences in context because of the lack of prior work specifically investigating visceral fat, brain volume loss, and sex differences, the researchers caution.
They also note that while statistically significant relationships were observed between visceral fat levels and gray matter volume changes, their effect sizes were generally small.
“Thus, the statistical significance of this work is influenced by the large sample size and less so by large effect size in any given set of regions,” the investigators write.
Other limitations include the cross-sectional nature of the study, which precludes conclusions about causality. The analysis also did not account for other lifestyle factors such as physical activity, diet, and genetic variables.
The researchers call for further investigation “to better elucidate underlying mechanisms and discover possible interventions targeting abdominal fat reduction as a strategy to maintain brain health.”
‘Helpful addition to the literature’
In a comment, Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach, noted that “previous studies have linked obesity with cognitive decline and increased risk of dementia. Rather than using BMI as a proxy for body fat, the current study examined visceral and subcutaneous fat directly using imaging techniques.”
Dr. Sexton, who was not associated with this study, said the finding that increased body fat was associated with reduced brain volumes suggests “a possible mechanism to explain the previously reported associations between obesity and cognition.”
“Though some degree of atrophy and brain shrinkage is common with old age, awareness of this association is important because reduced brain volume may be associated with problems with thinking, memory, and performing everyday tasks, and because rates of obesity continue to rise in the United States, along with obesity-related conditions including heart disease, stroke, type 2 diabetes and certain types of cancer,” she added.
“While a helpful addition to the literature, the study does have important limitations. As an observational study, it cannot establish whether higher levels of body fat directly causes reduced brain volumes,” Dr. Sexton cautioned.
In addition, the study did not take into account important related factors like physical activity and diet, which may influence any relationship between body fat and brain volumes, she noted. “Overall, it is not just one factor that is important to consider when considering risk for cognitive decline and dementia, but multiple factors.
“Obesity and the location of body fat must be considered in combination with one’s total lived experience and habits, including physical activity, education, head injury, sleep, mental health, and the health of your heart/cardiovascular system and other key bodily systems,” Dr. Sexton said.
The Alzheimer’s Association is leading a 2-year clinical trial known as U.S. POINTER to see whether combining physical activity, healthy nutrition, social and intellectual challenges, and improved self-management of medical conditions can protect cognitive function in older adults who are at increased risk for cognitive decline.
This work was supported in part by Providence St. Joseph Health in Seattle; Saint John’s Health Center Foundation; Pacific Neuroscience Institute and Foundation; Will and Cary Singleton; and the McLoughlin family. Dr. Raji is a consultant for Brainreader, Apollo Health, Voxelwise, Neurevolution, Pacific Neuroscience Institute Foundation, and Icometrix. Dr. Sexton reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a large study of healthy middle-aged adults, greater visceral and subcutaneous abdominal fat on abdominal MRI predicted brain atrophy on imaging, especially in women.
“The study shows that excess fat is bad for the brain and worse in women, including in Alzheimer’s disease risk regions,” lead author Cyrus Raji, MD, PhD, with the Mallinckrodt Institute of Radiology, Washington University, St. Louis, Mo., said in an interview.
The study was published online in the journal Aging and Disease
Modifiable risk factor
Multiple studies have suggested a connection between body fat accumulation and increased dementia risk. But few have examined the relationship between types of fat (visceral and subcutaneous) and brain volume.
For the new study, 10,000 healthy adults aged 20-80 years (mean age, 52.9 years; 53% men) underwent a short whole-body MRI protocol. Regression analyses of abdominal fat types and normalized brain volumes were evaluated, controlling for age and sex.
The research team found that higher amounts of both visceral and subcutaneous abdominal fat predicted lower total gray and white matter volume, as well as lower volume in the hippocampus, frontal cortex, and temporal, parietal, and occipital lobes.
“The findings are quite dramatic,” Dr. Raji told this news organization. “Overall, we found that both subcutaneous and visceral fat has similar levels of negative relationships with brain volumes.”
Women had a higher burden of brain atrophy with increased visceral fat than men. However, it’s difficult to place the sex differences in context because of the lack of prior work specifically investigating visceral fat, brain volume loss, and sex differences, the researchers caution.
They also note that while statistically significant relationships were observed between visceral fat levels and gray matter volume changes, their effect sizes were generally small.
“Thus, the statistical significance of this work is influenced by the large sample size and less so by large effect size in any given set of regions,” the investigators write.
Other limitations include the cross-sectional nature of the study, which precludes conclusions about causality. The analysis also did not account for other lifestyle factors such as physical activity, diet, and genetic variables.
The researchers call for further investigation “to better elucidate underlying mechanisms and discover possible interventions targeting abdominal fat reduction as a strategy to maintain brain health.”
‘Helpful addition to the literature’
In a comment, Claire Sexton, DPhil, Alzheimer’s Association senior director of scientific programs and outreach, noted that “previous studies have linked obesity with cognitive decline and increased risk of dementia. Rather than using BMI as a proxy for body fat, the current study examined visceral and subcutaneous fat directly using imaging techniques.”
Dr. Sexton, who was not associated with this study, said the finding that increased body fat was associated with reduced brain volumes suggests “a possible mechanism to explain the previously reported associations between obesity and cognition.”
“Though some degree of atrophy and brain shrinkage is common with old age, awareness of this association is important because reduced brain volume may be associated with problems with thinking, memory, and performing everyday tasks, and because rates of obesity continue to rise in the United States, along with obesity-related conditions including heart disease, stroke, type 2 diabetes and certain types of cancer,” she added.
“While a helpful addition to the literature, the study does have important limitations. As an observational study, it cannot establish whether higher levels of body fat directly causes reduced brain volumes,” Dr. Sexton cautioned.
In addition, the study did not take into account important related factors like physical activity and diet, which may influence any relationship between body fat and brain volumes, she noted. “Overall, it is not just one factor that is important to consider when considering risk for cognitive decline and dementia, but multiple factors.
“Obesity and the location of body fat must be considered in combination with one’s total lived experience and habits, including physical activity, education, head injury, sleep, mental health, and the health of your heart/cardiovascular system and other key bodily systems,” Dr. Sexton said.
The Alzheimer’s Association is leading a 2-year clinical trial known as U.S. POINTER to see whether combining physical activity, healthy nutrition, social and intellectual challenges, and improved self-management of medical conditions can protect cognitive function in older adults who are at increased risk for cognitive decline.
This work was supported in part by Providence St. Joseph Health in Seattle; Saint John’s Health Center Foundation; Pacific Neuroscience Institute and Foundation; Will and Cary Singleton; and the McLoughlin family. Dr. Raji is a consultant for Brainreader, Apollo Health, Voxelwise, Neurevolution, Pacific Neuroscience Institute Foundation, and Icometrix. Dr. Sexton reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM AGING AND DISEASES
Domestic violence in health care is real and underreported
To protect survivors’ identities, some names have been changed or shortened.
Natasha Abadilla, MD, met the man who would become her abuser while working abroad for a public health nonprofit. When he began emotionally and physically abusing her, she did everything she could to hide it.
“My coworkers knew nothing of the abuse. I became an expert in applying makeup to hide the bruises,” recalls Dr. Abadilla, now a second-year resident and pediatric neurologist at Lucile Packard Children’s Hospital at Stanford.
Dr. Abadilla says she strongly identifies as a hard worker and – to this day – hopes her work did not falter despite her partner’s constant drain on her. But the impact of the abuse continued to affect her for years. Like many survivors of domestic violence, she struggled with PTSD and depression.
Health care workers are often the first point of contact for survivors of domestic violence. Experts and advocates continue to push for more training for clinicians to identify and respond to signs among their patients. Often missing from this conversation is the reality that those tasked with screening can also be victims of intimate partner violence themselves.
What’s more: The very strengths that medical professionals often pride themselves on – perfectionism, empathy, grit – can make it harder for them to identify abuse in their own relationships and push through humiliation and shame to seek help.
Dr. Abadilla is exceptional among survivors in the medical field. Rather than keep her experience quiet, she has shared it publicly.
Awareness, she believes, can save lives.
An understudied problem in an underserved group
The majority of research on health care workers in this area has focused on workplace violence, which 62% experience worldwide. But intimate partner violence remains understudied and underdiscussed. Some medical professionals are even saddled with a “double burden,” facing trauma at work and at home, note the authors of a 2022 meta-analysis published in the journal Trauma, Violence, & Abuse.
The problem has had dire consequences. In recent years, many health care workers have been killed by their abusers:
- In 2016, Casey M. Drawert, MD, a Texas-based critical care anesthesiologist, was fatally shot by her husband in a murder-suicide.
- In 2018, Tamara O’Neal, MD, an ER physician, and Dayna Less, a first-year pharmacy resident, were killed by Dr. O’Neal’s ex-fiancé at Mercy Hospital in Chicago.
- In 2019, Sarah Hawley, MD, a first-year University of Utah resident, was fatally shot by her boyfriend in a murder-suicide.
- In 2021, Moria Kinsey, a nurse practitioner in Tahlequah, Okla., was murdered by a physician.
- In July of 2023, Gwendolyn Lavonne Riddick, DO, an ob.gyn. in North Carolina, was fatally shot by the father of her 3-year-old son.
There are others.
In the wake of these tragedies, calls for health care workers to screen each other as well as patients have grown. But for an untold number of survivors, breaking the silence is still not possible due to concerns about their reputation, professional consequences, the threat of harassment from abusers who are often in the same field, a medical culture of selfless endurance, and a lack of appropriate resources.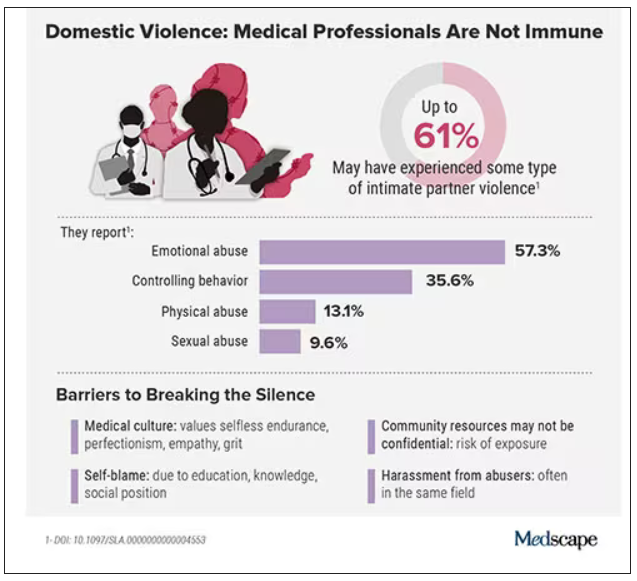
While the vast majority have stayed silent, those who have spoken out say there’s a need for targeted interventions to educate medical professionals as well as more supportive policies throughout the health care system.
Are health care workers more at risk?
Although more studies are needed, research indicates health care workers experience domestic violence at rates comparable to those of other populations, whereas some data suggest rates may be higher.
In the United States, more than one in three women and one in four men experience some form of intimate partner violence in their lifetime. Similarly, a 2020 study found that 24% of 400 physicians responding to a survey reported a history of domestic violence, with 15% reporting verbal abuse, 8% reporting physical violence, 4% reporting sexual abuse, and 4% reporting stalking.
Meanwhile, in an anonymous survey completed by 882 practicing surgeons and trainees in the United States from late 2018 to early 2019, more than 60% reported experiencing some type of intimate partner violence, most commonly emotional abuse.
Recent studies in the United Kingdom, Australia, and elsewhere show that significant numbers of medical professionals are fighting this battle. A 2019 study of more than 2,000 nurses, midwives, and health care assistants in the United Kingdom found that nurses were three times more likely to experience domestic violence than the average person.
What would help solve this problem: More study of health care worker-survivors as a unique group with unique risk factors. In general, domestic violence is most prevalent among women and people in marginalized groups. But young adults, such as medical students and trainees, can face an increased risk due to economic strain. Major life changes, such as relocating for residency, can also drive up stress and fray social connections, further isolating victims.
Why it’s so much harder for medical professionals to reveal abuse
For medical professionals accustomed to being strong and forging on, identifying as a victim of abuse can seem like a personal contradiction. It can feel easier to separate their personal and professional lives rather than face a complex reality.
In a personal essay on KevinMD.com, medical student Chloe N. L. Lee describes this emotional turmoil. “As an aspiring psychiatrist, I questioned my character judgment (how did I end up with a misogynistic abuser?) and wondered if I ought to have known better. I worried that my colleagues would deem me unfit to care for patients. And I thought that this was not supposed to happen to women like me,” Ms. Lee writes.
Kimberly, a licensed therapist, experienced a similar pattern of self-blame when her partner began exhibiting violent behavior. “For a long time, I felt guilty because I said to myself, You’re a therapist. You’re supposed to know this,” she recalls. At the same time, she felt driven to help him and sought couples therapy as his violence escalated.
Whitney, a pharmacist, recognized the “hallmarks” of abuse in her relationship, but she coped by compartmentalizing. Whitney says she was vulnerable to her abuser as a young college student who struggled financially. As he showered her with gifts, she found herself waving away red flags like aggressiveness or overprotectiveness.
After Whitney graduated, her partner’s emotional manipulation escalated into frequent physical assaults. When he gave her a black eye, she could not bring herself to go into work. She quit her job without notice. Despite a spotless record, none of her coworkers ever reached out to investigate her sudden departure.
It would take 8 years for Whitney to acknowledge the abuse and seize a moment to escape. She fled with just her purse and started over in a new city, rebuilding her life in the midst of harassment and threats from her ex. She says she’s grateful to be alive.
An imperfect system doesn’t help
Health care workers rarely ask for support or disclose abuse at work. Some have cited stigma, a lack of confidentiality (especially when the abuser is also in health care), fears about colleagues’ judgment, and a culture that doesn’t prioritize self-care.
Sometimes policies get in the way: In a 2021 qualitative study of interviews with 21 female physician-survivors in the United Kingdom, many said that despite the intense stress of abuse and recovery, they were unable to take any time off.
Of 180 UK-based midwife-survivors interviewed in a 2018 study, only 60 sought support at work and 30 received it. Many said their supervisors pressured them to report the abuse and get back to work, called social services behind their back, or reported them to their professional regulator. “I was treated like the perpetrator,” one said. Barbara Hernandez, PhD, a researcher who studies physician-survivors and director of physician vitality at Loma Linda University in southern California, says workplace violence and mistreatment from patients or colleagues – and a poor institutional response – can make those in health care feel like they have to “shut up and put up,” priming them to also tolerate abuse at home.
When survivors do reach out, there can be a disconnect between the resources they need and those they’re offered, Dr. Hernandez adds. In a recent survey of 400 physicians she conducted, respondents typically said they would advise a physician-survivor to “get to a shelter quickly.” But when roles were reversed, they admitted going to a shelter was the least feasible option. Support groups can also be problematic in smaller communities where physicians might be recognized or see their own patients.
Complicating matters further, the violence often comes from within the medical community. This can lead to particularly malicious abuse tactics like sending false accusations to a victim’s regulatory college or board; prolonged court and custody battles to drain them of all resources and their ability to hold a job; or even sabotage, harassment, or violence at work. The sheen of the abuser’s public persona, on the other hand, can guard them from any accountability.
For example, one physician-survivor said her ex-partner, a psychiatrist, coerced her into believing she was mentally ill, claimed she was “psychotic” in order to take back their children after she left, and had numerous colleagues serve as character witnesses in court for him, “saying he couldn’t have done any of these things, how great he is, and what a wonderful father he is.”
Slow progress is still progress
After Sherilyn M. Gordon-Burroughs, MD, a Texas-based transplant surgeon, mother, and educator, was killed by her husband in a murder-suicide in 2017, her friends Barbara Lee Bass, MD, president of the American College of Surgeons, and Patricia L. Turner, MD, were spurred into action. Together, they founded the ACS Intimate Partner Violence Task Force. Their mission is to educate surgeons to identify the signs of intimate partner violence (IPV) in themselves and their colleagues and connect them with resources.
“There is a concerted effort to close that gap,” says D’Andrea K. Joseph, MD, cochair of the task force and chief of trauma and acute care surgery at NYU Langone in New York. In the future, Dr. Joseph predicts, “making this a part of the curriculum, that it’s standardized for residents and trainees, that there is a safe place for victims ... and that we can band together and really recognize and assist our colleagues who are in trouble.”
Resources created by the ACS IPV task force, such as the toolkit and curriculum, provide a model for other health care leaders. But there have been few similar initiatives aimed at increasing IPV intervention within the medical system.
What you can do in your workplace
In her essay, Ms. Lee explains that a major turning point came when a physician friend explicitly asked if she was experiencing abuse. He then gently confirmed she was, and asked without judgment how he could support her, an approach that mirrors advice from the National Domestic Violence Hotline.
“Having a physician validate that this was, indeed, an abusive situation helped enormously ... I believe it may have saved my life,” she writes.
That validation can be crucial, and Dr. Abadilla urges other physicians to regularly check in with colleagues, especially those who seem particularly positive with a go-getter attitude and yet may not seem themselves. That was how she presented when she was struggling the most.
Supporting systemic changes within your organization and beyond is also important. The authors of the 2022 meta-analysis stress the need for domestic violence training, legislative changes, paid leave, and union support.
Finding strength in recovery
Over a decade after escaping her abuser, Whitney says she’s only just begun to share her experience, but what she’s learned has made her a better pharmacist. She says she’s more attuned to subtle signs something could be off with patients and coworkers. When someone makes comments about feeling anxious or that they can’t do anything right, it’s important to ask why, she says.
Recently, Kimberly has opened up to her mentor and other therapists, many of whom have shared that they’re also survivors.
“The last thing I said to [my abuser] is you think you’ve won and you’re hurting me, but what you’ve done to me – I’m going to utilize this and I’m going to help other people,” Kimberly says. “This pain that I have will go away, and I’m going to save the lives of others.”
A version of this article first appeared on Medscape.com.
To protect survivors’ identities, some names have been changed or shortened.
Natasha Abadilla, MD, met the man who would become her abuser while working abroad for a public health nonprofit. When he began emotionally and physically abusing her, she did everything she could to hide it.
“My coworkers knew nothing of the abuse. I became an expert in applying makeup to hide the bruises,” recalls Dr. Abadilla, now a second-year resident and pediatric neurologist at Lucile Packard Children’s Hospital at Stanford.
Dr. Abadilla says she strongly identifies as a hard worker and – to this day – hopes her work did not falter despite her partner’s constant drain on her. But the impact of the abuse continued to affect her for years. Like many survivors of domestic violence, she struggled with PTSD and depression.
Health care workers are often the first point of contact for survivors of domestic violence. Experts and advocates continue to push for more training for clinicians to identify and respond to signs among their patients. Often missing from this conversation is the reality that those tasked with screening can also be victims of intimate partner violence themselves.
What’s more: The very strengths that medical professionals often pride themselves on – perfectionism, empathy, grit – can make it harder for them to identify abuse in their own relationships and push through humiliation and shame to seek help.
Dr. Abadilla is exceptional among survivors in the medical field. Rather than keep her experience quiet, she has shared it publicly.
Awareness, she believes, can save lives.
An understudied problem in an underserved group
The majority of research on health care workers in this area has focused on workplace violence, which 62% experience worldwide. But intimate partner violence remains understudied and underdiscussed. Some medical professionals are even saddled with a “double burden,” facing trauma at work and at home, note the authors of a 2022 meta-analysis published in the journal Trauma, Violence, & Abuse.
The problem has had dire consequences. In recent years, many health care workers have been killed by their abusers:
- In 2016, Casey M. Drawert, MD, a Texas-based critical care anesthesiologist, was fatally shot by her husband in a murder-suicide.
- In 2018, Tamara O’Neal, MD, an ER physician, and Dayna Less, a first-year pharmacy resident, were killed by Dr. O’Neal’s ex-fiancé at Mercy Hospital in Chicago.
- In 2019, Sarah Hawley, MD, a first-year University of Utah resident, was fatally shot by her boyfriend in a murder-suicide.
- In 2021, Moria Kinsey, a nurse practitioner in Tahlequah, Okla., was murdered by a physician.
- In July of 2023, Gwendolyn Lavonne Riddick, DO, an ob.gyn. in North Carolina, was fatally shot by the father of her 3-year-old son.
There are others.
In the wake of these tragedies, calls for health care workers to screen each other as well as patients have grown. But for an untold number of survivors, breaking the silence is still not possible due to concerns about their reputation, professional consequences, the threat of harassment from abusers who are often in the same field, a medical culture of selfless endurance, and a lack of appropriate resources.
While the vast majority have stayed silent, those who have spoken out say there’s a need for targeted interventions to educate medical professionals as well as more supportive policies throughout the health care system.
Are health care workers more at risk?
Although more studies are needed, research indicates health care workers experience domestic violence at rates comparable to those of other populations, whereas some data suggest rates may be higher.
In the United States, more than one in three women and one in four men experience some form of intimate partner violence in their lifetime. Similarly, a 2020 study found that 24% of 400 physicians responding to a survey reported a history of domestic violence, with 15% reporting verbal abuse, 8% reporting physical violence, 4% reporting sexual abuse, and 4% reporting stalking.
Meanwhile, in an anonymous survey completed by 882 practicing surgeons and trainees in the United States from late 2018 to early 2019, more than 60% reported experiencing some type of intimate partner violence, most commonly emotional abuse.
Recent studies in the United Kingdom, Australia, and elsewhere show that significant numbers of medical professionals are fighting this battle. A 2019 study of more than 2,000 nurses, midwives, and health care assistants in the United Kingdom found that nurses were three times more likely to experience domestic violence than the average person.
What would help solve this problem: More study of health care worker-survivors as a unique group with unique risk factors. In general, domestic violence is most prevalent among women and people in marginalized groups. But young adults, such as medical students and trainees, can face an increased risk due to economic strain. Major life changes, such as relocating for residency, can also drive up stress and fray social connections, further isolating victims.
Why it’s so much harder for medical professionals to reveal abuse
For medical professionals accustomed to being strong and forging on, identifying as a victim of abuse can seem like a personal contradiction. It can feel easier to separate their personal and professional lives rather than face a complex reality.
In a personal essay on KevinMD.com, medical student Chloe N. L. Lee describes this emotional turmoil. “As an aspiring psychiatrist, I questioned my character judgment (how did I end up with a misogynistic abuser?) and wondered if I ought to have known better. I worried that my colleagues would deem me unfit to care for patients. And I thought that this was not supposed to happen to women like me,” Ms. Lee writes.
Kimberly, a licensed therapist, experienced a similar pattern of self-blame when her partner began exhibiting violent behavior. “For a long time, I felt guilty because I said to myself, You’re a therapist. You’re supposed to know this,” she recalls. At the same time, she felt driven to help him and sought couples therapy as his violence escalated.
Whitney, a pharmacist, recognized the “hallmarks” of abuse in her relationship, but she coped by compartmentalizing. Whitney says she was vulnerable to her abuser as a young college student who struggled financially. As he showered her with gifts, she found herself waving away red flags like aggressiveness or overprotectiveness.
After Whitney graduated, her partner’s emotional manipulation escalated into frequent physical assaults. When he gave her a black eye, she could not bring herself to go into work. She quit her job without notice. Despite a spotless record, none of her coworkers ever reached out to investigate her sudden departure.
It would take 8 years for Whitney to acknowledge the abuse and seize a moment to escape. She fled with just her purse and started over in a new city, rebuilding her life in the midst of harassment and threats from her ex. She says she’s grateful to be alive.
An imperfect system doesn’t help
Health care workers rarely ask for support or disclose abuse at work. Some have cited stigma, a lack of confidentiality (especially when the abuser is also in health care), fears about colleagues’ judgment, and a culture that doesn’t prioritize self-care.
Sometimes policies get in the way: In a 2021 qualitative study of interviews with 21 female physician-survivors in the United Kingdom, many said that despite the intense stress of abuse and recovery, they were unable to take any time off.
Of 180 UK-based midwife-survivors interviewed in a 2018 study, only 60 sought support at work and 30 received it. Many said their supervisors pressured them to report the abuse and get back to work, called social services behind their back, or reported them to their professional regulator. “I was treated like the perpetrator,” one said. Barbara Hernandez, PhD, a researcher who studies physician-survivors and director of physician vitality at Loma Linda University in southern California, says workplace violence and mistreatment from patients or colleagues – and a poor institutional response – can make those in health care feel like they have to “shut up and put up,” priming them to also tolerate abuse at home.
When survivors do reach out, there can be a disconnect between the resources they need and those they’re offered, Dr. Hernandez adds. In a recent survey of 400 physicians she conducted, respondents typically said they would advise a physician-survivor to “get to a shelter quickly.” But when roles were reversed, they admitted going to a shelter was the least feasible option. Support groups can also be problematic in smaller communities where physicians might be recognized or see their own patients.
Complicating matters further, the violence often comes from within the medical community. This can lead to particularly malicious abuse tactics like sending false accusations to a victim’s regulatory college or board; prolonged court and custody battles to drain them of all resources and their ability to hold a job; or even sabotage, harassment, or violence at work. The sheen of the abuser’s public persona, on the other hand, can guard them from any accountability.
For example, one physician-survivor said her ex-partner, a psychiatrist, coerced her into believing she was mentally ill, claimed she was “psychotic” in order to take back their children after she left, and had numerous colleagues serve as character witnesses in court for him, “saying he couldn’t have done any of these things, how great he is, and what a wonderful father he is.”
Slow progress is still progress
After Sherilyn M. Gordon-Burroughs, MD, a Texas-based transplant surgeon, mother, and educator, was killed by her husband in a murder-suicide in 2017, her friends Barbara Lee Bass, MD, president of the American College of Surgeons, and Patricia L. Turner, MD, were spurred into action. Together, they founded the ACS Intimate Partner Violence Task Force. Their mission is to educate surgeons to identify the signs of intimate partner violence (IPV) in themselves and their colleagues and connect them with resources.
“There is a concerted effort to close that gap,” says D’Andrea K. Joseph, MD, cochair of the task force and chief of trauma and acute care surgery at NYU Langone in New York. In the future, Dr. Joseph predicts, “making this a part of the curriculum, that it’s standardized for residents and trainees, that there is a safe place for victims ... and that we can band together and really recognize and assist our colleagues who are in trouble.”
Resources created by the ACS IPV task force, such as the toolkit and curriculum, provide a model for other health care leaders. But there have been few similar initiatives aimed at increasing IPV intervention within the medical system.
What you can do in your workplace
In her essay, Ms. Lee explains that a major turning point came when a physician friend explicitly asked if she was experiencing abuse. He then gently confirmed she was, and asked without judgment how he could support her, an approach that mirrors advice from the National Domestic Violence Hotline.
“Having a physician validate that this was, indeed, an abusive situation helped enormously ... I believe it may have saved my life,” she writes.
That validation can be crucial, and Dr. Abadilla urges other physicians to regularly check in with colleagues, especially those who seem particularly positive with a go-getter attitude and yet may not seem themselves. That was how she presented when she was struggling the most.
Supporting systemic changes within your organization and beyond is also important. The authors of the 2022 meta-analysis stress the need for domestic violence training, legislative changes, paid leave, and union support.
Finding strength in recovery
Over a decade after escaping her abuser, Whitney says she’s only just begun to share her experience, but what she’s learned has made her a better pharmacist. She says she’s more attuned to subtle signs something could be off with patients and coworkers. When someone makes comments about feeling anxious or that they can’t do anything right, it’s important to ask why, she says.
Recently, Kimberly has opened up to her mentor and other therapists, many of whom have shared that they’re also survivors.
“The last thing I said to [my abuser] is you think you’ve won and you’re hurting me, but what you’ve done to me – I’m going to utilize this and I’m going to help other people,” Kimberly says. “This pain that I have will go away, and I’m going to save the lives of others.”
A version of this article first appeared on Medscape.com.
To protect survivors’ identities, some names have been changed or shortened.
Natasha Abadilla, MD, met the man who would become her abuser while working abroad for a public health nonprofit. When he began emotionally and physically abusing her, she did everything she could to hide it.
“My coworkers knew nothing of the abuse. I became an expert in applying makeup to hide the bruises,” recalls Dr. Abadilla, now a second-year resident and pediatric neurologist at Lucile Packard Children’s Hospital at Stanford.
Dr. Abadilla says she strongly identifies as a hard worker and – to this day – hopes her work did not falter despite her partner’s constant drain on her. But the impact of the abuse continued to affect her for years. Like many survivors of domestic violence, she struggled with PTSD and depression.
Health care workers are often the first point of contact for survivors of domestic violence. Experts and advocates continue to push for more training for clinicians to identify and respond to signs among their patients. Often missing from this conversation is the reality that those tasked with screening can also be victims of intimate partner violence themselves.
What’s more: The very strengths that medical professionals often pride themselves on – perfectionism, empathy, grit – can make it harder for them to identify abuse in their own relationships and push through humiliation and shame to seek help.
Dr. Abadilla is exceptional among survivors in the medical field. Rather than keep her experience quiet, she has shared it publicly.
Awareness, she believes, can save lives.
An understudied problem in an underserved group
The majority of research on health care workers in this area has focused on workplace violence, which 62% experience worldwide. But intimate partner violence remains understudied and underdiscussed. Some medical professionals are even saddled with a “double burden,” facing trauma at work and at home, note the authors of a 2022 meta-analysis published in the journal Trauma, Violence, & Abuse.
The problem has had dire consequences. In recent years, many health care workers have been killed by their abusers:
- In 2016, Casey M. Drawert, MD, a Texas-based critical care anesthesiologist, was fatally shot by her husband in a murder-suicide.
- In 2018, Tamara O’Neal, MD, an ER physician, and Dayna Less, a first-year pharmacy resident, were killed by Dr. O’Neal’s ex-fiancé at Mercy Hospital in Chicago.
- In 2019, Sarah Hawley, MD, a first-year University of Utah resident, was fatally shot by her boyfriend in a murder-suicide.
- In 2021, Moria Kinsey, a nurse practitioner in Tahlequah, Okla., was murdered by a physician.
- In July of 2023, Gwendolyn Lavonne Riddick, DO, an ob.gyn. in North Carolina, was fatally shot by the father of her 3-year-old son.
There are others.
In the wake of these tragedies, calls for health care workers to screen each other as well as patients have grown. But for an untold number of survivors, breaking the silence is still not possible due to concerns about their reputation, professional consequences, the threat of harassment from abusers who are often in the same field, a medical culture of selfless endurance, and a lack of appropriate resources.
While the vast majority have stayed silent, those who have spoken out say there’s a need for targeted interventions to educate medical professionals as well as more supportive policies throughout the health care system.
Are health care workers more at risk?
Although more studies are needed, research indicates health care workers experience domestic violence at rates comparable to those of other populations, whereas some data suggest rates may be higher.
In the United States, more than one in three women and one in four men experience some form of intimate partner violence in their lifetime. Similarly, a 2020 study found that 24% of 400 physicians responding to a survey reported a history of domestic violence, with 15% reporting verbal abuse, 8% reporting physical violence, 4% reporting sexual abuse, and 4% reporting stalking.
Meanwhile, in an anonymous survey completed by 882 practicing surgeons and trainees in the United States from late 2018 to early 2019, more than 60% reported experiencing some type of intimate partner violence, most commonly emotional abuse.
Recent studies in the United Kingdom, Australia, and elsewhere show that significant numbers of medical professionals are fighting this battle. A 2019 study of more than 2,000 nurses, midwives, and health care assistants in the United Kingdom found that nurses were three times more likely to experience domestic violence than the average person.
What would help solve this problem: More study of health care worker-survivors as a unique group with unique risk factors. In general, domestic violence is most prevalent among women and people in marginalized groups. But young adults, such as medical students and trainees, can face an increased risk due to economic strain. Major life changes, such as relocating for residency, can also drive up stress and fray social connections, further isolating victims.
Why it’s so much harder for medical professionals to reveal abuse
For medical professionals accustomed to being strong and forging on, identifying as a victim of abuse can seem like a personal contradiction. It can feel easier to separate their personal and professional lives rather than face a complex reality.
In a personal essay on KevinMD.com, medical student Chloe N. L. Lee describes this emotional turmoil. “As an aspiring psychiatrist, I questioned my character judgment (how did I end up with a misogynistic abuser?) and wondered if I ought to have known better. I worried that my colleagues would deem me unfit to care for patients. And I thought that this was not supposed to happen to women like me,” Ms. Lee writes.
Kimberly, a licensed therapist, experienced a similar pattern of self-blame when her partner began exhibiting violent behavior. “For a long time, I felt guilty because I said to myself, You’re a therapist. You’re supposed to know this,” she recalls. At the same time, she felt driven to help him and sought couples therapy as his violence escalated.
Whitney, a pharmacist, recognized the “hallmarks” of abuse in her relationship, but she coped by compartmentalizing. Whitney says she was vulnerable to her abuser as a young college student who struggled financially. As he showered her with gifts, she found herself waving away red flags like aggressiveness or overprotectiveness.
After Whitney graduated, her partner’s emotional manipulation escalated into frequent physical assaults. When he gave her a black eye, she could not bring herself to go into work. She quit her job without notice. Despite a spotless record, none of her coworkers ever reached out to investigate her sudden departure.
It would take 8 years for Whitney to acknowledge the abuse and seize a moment to escape. She fled with just her purse and started over in a new city, rebuilding her life in the midst of harassment and threats from her ex. She says she’s grateful to be alive.
An imperfect system doesn’t help
Health care workers rarely ask for support or disclose abuse at work. Some have cited stigma, a lack of confidentiality (especially when the abuser is also in health care), fears about colleagues’ judgment, and a culture that doesn’t prioritize self-care.
Sometimes policies get in the way: In a 2021 qualitative study of interviews with 21 female physician-survivors in the United Kingdom, many said that despite the intense stress of abuse and recovery, they were unable to take any time off.
Of 180 UK-based midwife-survivors interviewed in a 2018 study, only 60 sought support at work and 30 received it. Many said their supervisors pressured them to report the abuse and get back to work, called social services behind their back, or reported them to their professional regulator. “I was treated like the perpetrator,” one said. Barbara Hernandez, PhD, a researcher who studies physician-survivors and director of physician vitality at Loma Linda University in southern California, says workplace violence and mistreatment from patients or colleagues – and a poor institutional response – can make those in health care feel like they have to “shut up and put up,” priming them to also tolerate abuse at home.
When survivors do reach out, there can be a disconnect between the resources they need and those they’re offered, Dr. Hernandez adds. In a recent survey of 400 physicians she conducted, respondents typically said they would advise a physician-survivor to “get to a shelter quickly.” But when roles were reversed, they admitted going to a shelter was the least feasible option. Support groups can also be problematic in smaller communities where physicians might be recognized or see their own patients.
Complicating matters further, the violence often comes from within the medical community. This can lead to particularly malicious abuse tactics like sending false accusations to a victim’s regulatory college or board; prolonged court and custody battles to drain them of all resources and their ability to hold a job; or even sabotage, harassment, or violence at work. The sheen of the abuser’s public persona, on the other hand, can guard them from any accountability.
For example, one physician-survivor said her ex-partner, a psychiatrist, coerced her into believing she was mentally ill, claimed she was “psychotic” in order to take back their children after she left, and had numerous colleagues serve as character witnesses in court for him, “saying he couldn’t have done any of these things, how great he is, and what a wonderful father he is.”
Slow progress is still progress
After Sherilyn M. Gordon-Burroughs, MD, a Texas-based transplant surgeon, mother, and educator, was killed by her husband in a murder-suicide in 2017, her friends Barbara Lee Bass, MD, president of the American College of Surgeons, and Patricia L. Turner, MD, were spurred into action. Together, they founded the ACS Intimate Partner Violence Task Force. Their mission is to educate surgeons to identify the signs of intimate partner violence (IPV) in themselves and their colleagues and connect them with resources.
“There is a concerted effort to close that gap,” says D’Andrea K. Joseph, MD, cochair of the task force and chief of trauma and acute care surgery at NYU Langone in New York. In the future, Dr. Joseph predicts, “making this a part of the curriculum, that it’s standardized for residents and trainees, that there is a safe place for victims ... and that we can band together and really recognize and assist our colleagues who are in trouble.”
Resources created by the ACS IPV task force, such as the toolkit and curriculum, provide a model for other health care leaders. But there have been few similar initiatives aimed at increasing IPV intervention within the medical system.
What you can do in your workplace
In her essay, Ms. Lee explains that a major turning point came when a physician friend explicitly asked if she was experiencing abuse. He then gently confirmed she was, and asked without judgment how he could support her, an approach that mirrors advice from the National Domestic Violence Hotline.
“Having a physician validate that this was, indeed, an abusive situation helped enormously ... I believe it may have saved my life,” she writes.
That validation can be crucial, and Dr. Abadilla urges other physicians to regularly check in with colleagues, especially those who seem particularly positive with a go-getter attitude and yet may not seem themselves. That was how she presented when she was struggling the most.
Supporting systemic changes within your organization and beyond is also important. The authors of the 2022 meta-analysis stress the need for domestic violence training, legislative changes, paid leave, and union support.
Finding strength in recovery
Over a decade after escaping her abuser, Whitney says she’s only just begun to share her experience, but what she’s learned has made her a better pharmacist. She says she’s more attuned to subtle signs something could be off with patients and coworkers. When someone makes comments about feeling anxious or that they can’t do anything right, it’s important to ask why, she says.
Recently, Kimberly has opened up to her mentor and other therapists, many of whom have shared that they’re also survivors.
“The last thing I said to [my abuser] is you think you’ve won and you’re hurting me, but what you’ve done to me – I’m going to utilize this and I’m going to help other people,” Kimberly says. “This pain that I have will go away, and I’m going to save the lives of others.”
A version of this article first appeared on Medscape.com.
One in five doctors with long COVID can no longer work: Survey
Crippling symptoms, lost careers, and eroded incomes: This is the harsh reality for doctors suffering with long COVID, according to the first major survey of physicians with the condition.
The survey, conducted by the British Medical Association and the Long COVID Doctors for Action support group, sheds light on the lingering effects of long COVID on more than 600 chronically ill and disabled doctors with the condition. It also spotlights what they describe as a lack of medical and financial support from their government and employers at the National Health Service.
“We feel betrayed and abandoned,” said Kelly Fearnley, MBChB, chair and cofounder of Long COVID Doctors for Action. “At a time of national crisis, when health care workers were asked to step up, we did. When the nation needed us, we stepped up. We put our lives on the line. We put our families’ lives on the line. And now that we are injured after knowingly being unprotected and deliberately and repeatedly exposed to a level 3 biohazard, we now find ourselves in this position.”
Dr. Fearnley fell ill while working in a hospital’s COVID ward in November 2020. She is one of an estimated 2 million people in the United Kingdom – including thousands of NHS employees – with long COVID. She hasn’t been able to return to work in nearly 3 years.
Long COVID affects more than 65 million people worldwide. It is estimated that 1 in 10 people infected with the virus develop long-term symptoms. In the United Kingdom, health care and social care workers are seven times more likely to have had severe COVID-19 than other types of employees.
Doctors responding to the BMA survey reported a wide range of long COVID symptoms, including fatigue, headaches, muscular pain, nerve damage, joint pain, and respiratory problems.
Among the survey’s key findings, 60% of doctors said long COVID has affected their ability to carry out day-to-day tasks on a regular basis. Almost one in five (18%) said they were no longer able to work, while fewer than one in three (31%) were working full time. This compares with more than half (57%) of respondents working full time before the onset of their COVID illness – a decline of 46%.
Nearly half (48%) of respondents said they have experienced some form of loss of earnings as a result of long COVID, and almost half of the doctors were never referred to an NHS long COVID clinic. The survey included the following first-person accounts from doctors living with the condition.
- One doctor said: “I nearly lost my life, my home, my partner and my career. I have received little support to help keep these. The impact on my mental health nearly cost [me] my life again.”
- A senior consulting physician commented: “Life is absolutely miserable. Every day is a struggle. I wake up exhausted, the insomnia and night terrors are horrendous as I live through my worst fears every night. Any activity such as eating meals, washing, etc., will mean I have to go to bed for a few hours. I am unable to look after myself or my child, exercise or maintain social relationships. I have no financial security. Long COVID has totally destroyed my life.”
- A salaried general practitioner said: “I can no longer work, finances are ruined. I didn’t have employment protection so am now unemployed and penniless.”
Calls for action from the BMA include the following:
- Financial support for doctors and health care staff with long COVID.
- The recognition of long COVID as an occupational disease among health care workers, along with a definition of the condition that covers all of the debilitating disease’s symptoms.
- Improved access to physical and mental health services to help comprehensive assessment, investigations, and treatment.
- Greater workplace protection for health care staff who risk their lives for others.
- Better support for long COVID sufferers to return to work safely if they can, including a flexible approach to the use of workplace adjustments.
“One would think, given the circumstances under which we fell ill and current workforce shortages, NHS employers would be eager to do everything to facilitate the return to work of people with long COVID,” said Dr. Fearnley. “However, NHS employers are legally required to implement only ‘reasonable adjustments,’ and so things such as extended phased return or adjustments to shift patterns are not always being facilitated. Instead, an increasing number of employers are choosing to terminate contracts.”
Raymond Agius, the BMA’s occupational medicine committee cochair, also put the blame on inadequate safety measures for doctors. Those inadequate measures persist to this day, inasmuch as U.K. hospitals have dropped masking requirements.
“During the COVID-19 pandemic, doctors were left exposed and unprotected at work,” he said in a BMA press release. “They often did not have access to the right PPE. ... Too many risk assessments of workplaces and especially of vulnerable doctors were not undertaken.”
A small minority of doctors who were surveyed said they had access to respiratory protective equipment about the time they contracted COVID-19. Only 11% had access to an FFP2 respirator (the equivalent of an N95 mask); 16% had an FFP3 respirator (the equivalent of an N99 mask).
To date, the British government hasn’t issued much of a response to the survey, saying only that it has invested more than ₤50 million to better understand long COVID.
A version of this article first appeared on Medscape.com.
Crippling symptoms, lost careers, and eroded incomes: This is the harsh reality for doctors suffering with long COVID, according to the first major survey of physicians with the condition.
The survey, conducted by the British Medical Association and the Long COVID Doctors for Action support group, sheds light on the lingering effects of long COVID on more than 600 chronically ill and disabled doctors with the condition. It also spotlights what they describe as a lack of medical and financial support from their government and employers at the National Health Service.
“We feel betrayed and abandoned,” said Kelly Fearnley, MBChB, chair and cofounder of Long COVID Doctors for Action. “At a time of national crisis, when health care workers were asked to step up, we did. When the nation needed us, we stepped up. We put our lives on the line. We put our families’ lives on the line. And now that we are injured after knowingly being unprotected and deliberately and repeatedly exposed to a level 3 biohazard, we now find ourselves in this position.”
Dr. Fearnley fell ill while working in a hospital’s COVID ward in November 2020. She is one of an estimated 2 million people in the United Kingdom – including thousands of NHS employees – with long COVID. She hasn’t been able to return to work in nearly 3 years.
Long COVID affects more than 65 million people worldwide. It is estimated that 1 in 10 people infected with the virus develop long-term symptoms. In the United Kingdom, health care and social care workers are seven times more likely to have had severe COVID-19 than other types of employees.
Doctors responding to the BMA survey reported a wide range of long COVID symptoms, including fatigue, headaches, muscular pain, nerve damage, joint pain, and respiratory problems.
Among the survey’s key findings, 60% of doctors said long COVID has affected their ability to carry out day-to-day tasks on a regular basis. Almost one in five (18%) said they were no longer able to work, while fewer than one in three (31%) were working full time. This compares with more than half (57%) of respondents working full time before the onset of their COVID illness – a decline of 46%.
Nearly half (48%) of respondents said they have experienced some form of loss of earnings as a result of long COVID, and almost half of the doctors were never referred to an NHS long COVID clinic. The survey included the following first-person accounts from doctors living with the condition.
- One doctor said: “I nearly lost my life, my home, my partner and my career. I have received little support to help keep these. The impact on my mental health nearly cost [me] my life again.”
- A senior consulting physician commented: “Life is absolutely miserable. Every day is a struggle. I wake up exhausted, the insomnia and night terrors are horrendous as I live through my worst fears every night. Any activity such as eating meals, washing, etc., will mean I have to go to bed for a few hours. I am unable to look after myself or my child, exercise or maintain social relationships. I have no financial security. Long COVID has totally destroyed my life.”
- A salaried general practitioner said: “I can no longer work, finances are ruined. I didn’t have employment protection so am now unemployed and penniless.”
Calls for action from the BMA include the following:
- Financial support for doctors and health care staff with long COVID.
- The recognition of long COVID as an occupational disease among health care workers, along with a definition of the condition that covers all of the debilitating disease’s symptoms.
- Improved access to physical and mental health services to help comprehensive assessment, investigations, and treatment.
- Greater workplace protection for health care staff who risk their lives for others.
- Better support for long COVID sufferers to return to work safely if they can, including a flexible approach to the use of workplace adjustments.
“One would think, given the circumstances under which we fell ill and current workforce shortages, NHS employers would be eager to do everything to facilitate the return to work of people with long COVID,” said Dr. Fearnley. “However, NHS employers are legally required to implement only ‘reasonable adjustments,’ and so things such as extended phased return or adjustments to shift patterns are not always being facilitated. Instead, an increasing number of employers are choosing to terminate contracts.”
Raymond Agius, the BMA’s occupational medicine committee cochair, also put the blame on inadequate safety measures for doctors. Those inadequate measures persist to this day, inasmuch as U.K. hospitals have dropped masking requirements.
“During the COVID-19 pandemic, doctors were left exposed and unprotected at work,” he said in a BMA press release. “They often did not have access to the right PPE. ... Too many risk assessments of workplaces and especially of vulnerable doctors were not undertaken.”
A small minority of doctors who were surveyed said they had access to respiratory protective equipment about the time they contracted COVID-19. Only 11% had access to an FFP2 respirator (the equivalent of an N95 mask); 16% had an FFP3 respirator (the equivalent of an N99 mask).
To date, the British government hasn’t issued much of a response to the survey, saying only that it has invested more than ₤50 million to better understand long COVID.
A version of this article first appeared on Medscape.com.
Crippling symptoms, lost careers, and eroded incomes: This is the harsh reality for doctors suffering with long COVID, according to the first major survey of physicians with the condition.
The survey, conducted by the British Medical Association and the Long COVID Doctors for Action support group, sheds light on the lingering effects of long COVID on more than 600 chronically ill and disabled doctors with the condition. It also spotlights what they describe as a lack of medical and financial support from their government and employers at the National Health Service.
“We feel betrayed and abandoned,” said Kelly Fearnley, MBChB, chair and cofounder of Long COVID Doctors for Action. “At a time of national crisis, when health care workers were asked to step up, we did. When the nation needed us, we stepped up. We put our lives on the line. We put our families’ lives on the line. And now that we are injured after knowingly being unprotected and deliberately and repeatedly exposed to a level 3 biohazard, we now find ourselves in this position.”
Dr. Fearnley fell ill while working in a hospital’s COVID ward in November 2020. She is one of an estimated 2 million people in the United Kingdom – including thousands of NHS employees – with long COVID. She hasn’t been able to return to work in nearly 3 years.
Long COVID affects more than 65 million people worldwide. It is estimated that 1 in 10 people infected with the virus develop long-term symptoms. In the United Kingdom, health care and social care workers are seven times more likely to have had severe COVID-19 than other types of employees.
Doctors responding to the BMA survey reported a wide range of long COVID symptoms, including fatigue, headaches, muscular pain, nerve damage, joint pain, and respiratory problems.
Among the survey’s key findings, 60% of doctors said long COVID has affected their ability to carry out day-to-day tasks on a regular basis. Almost one in five (18%) said they were no longer able to work, while fewer than one in three (31%) were working full time. This compares with more than half (57%) of respondents working full time before the onset of their COVID illness – a decline of 46%.
Nearly half (48%) of respondents said they have experienced some form of loss of earnings as a result of long COVID, and almost half of the doctors were never referred to an NHS long COVID clinic. The survey included the following first-person accounts from doctors living with the condition.
- One doctor said: “I nearly lost my life, my home, my partner and my career. I have received little support to help keep these. The impact on my mental health nearly cost [me] my life again.”
- A senior consulting physician commented: “Life is absolutely miserable. Every day is a struggle. I wake up exhausted, the insomnia and night terrors are horrendous as I live through my worst fears every night. Any activity such as eating meals, washing, etc., will mean I have to go to bed for a few hours. I am unable to look after myself or my child, exercise or maintain social relationships. I have no financial security. Long COVID has totally destroyed my life.”
- A salaried general practitioner said: “I can no longer work, finances are ruined. I didn’t have employment protection so am now unemployed and penniless.”
Calls for action from the BMA include the following:
- Financial support for doctors and health care staff with long COVID.
- The recognition of long COVID as an occupational disease among health care workers, along with a definition of the condition that covers all of the debilitating disease’s symptoms.
- Improved access to physical and mental health services to help comprehensive assessment, investigations, and treatment.
- Greater workplace protection for health care staff who risk their lives for others.
- Better support for long COVID sufferers to return to work safely if they can, including a flexible approach to the use of workplace adjustments.
“One would think, given the circumstances under which we fell ill and current workforce shortages, NHS employers would be eager to do everything to facilitate the return to work of people with long COVID,” said Dr. Fearnley. “However, NHS employers are legally required to implement only ‘reasonable adjustments,’ and so things such as extended phased return or adjustments to shift patterns are not always being facilitated. Instead, an increasing number of employers are choosing to terminate contracts.”
Raymond Agius, the BMA’s occupational medicine committee cochair, also put the blame on inadequate safety measures for doctors. Those inadequate measures persist to this day, inasmuch as U.K. hospitals have dropped masking requirements.
“During the COVID-19 pandemic, doctors were left exposed and unprotected at work,” he said in a BMA press release. “They often did not have access to the right PPE. ... Too many risk assessments of workplaces and especially of vulnerable doctors were not undertaken.”
A small minority of doctors who were surveyed said they had access to respiratory protective equipment about the time they contracted COVID-19. Only 11% had access to an FFP2 respirator (the equivalent of an N95 mask); 16% had an FFP3 respirator (the equivalent of an N99 mask).
To date, the British government hasn’t issued much of a response to the survey, saying only that it has invested more than ₤50 million to better understand long COVID.
A version of this article first appeared on Medscape.com.
Resident creates AI alternative to U.S. News med school ranking
For decades, pre-med students depended on the annual medical school rankings by U.S. News and World Report to decide where to apply for physician education. But after several prominent med schools pulled out of the rankings, one resident began experimenting with artificial intelligence (AI) to create an alternative.
Brandon Turner MD, MSc, a radiation oncology resident at Massachusetts General Hospital in Boston, developed a free do-it-yourself tool using AI that allows prospective students to rank medical schools based on considerations that are most important to them. His research was published online in JAMA Network Open.
“One of the flaws with conventional ranking systems is that the metrics used in these tools are weighted based on the preferences and views of the people who developed these rankings, but those may not work for everyone,” Dr. Turner told this news organization.
He explained that there are different types of metrics used in the U.S. News ranking: one for research and the other for primary care. “The research rankings carry the most prestige and are the ones that most people know about,” he explained. These metrics take into account factors such as how many grant dollars the medical school receives and the average size of those grants per faculty member, Dr. Turner said.
Admission metrics are also included – for example, the median grade point average or MCAT scores of students who have been accepted. “These don’t tell you anything about the research output of the school, only about how selective the school is,” he said.
Primary care metrics might focus on how many graduates of a given school go into primary care, or how other schools rate the quality of primary care training at a given school – a process called peer assessment, Dr. Turner said.
But even though these might be helpful, students may be more interested in the cost of attendance, average debt, representation of minorities, and how many graduates pass their boards, he said. “U.S. News metrics don’t capture these things, but I included them in my algorithm.”
A U.S. News spokesperson said that the publication continues to help students and their families make decisions about their future education. The spokesperson cited U.S. News’ explanation of how it calculates its rankings. “A school’s overall Best Medical Schools rank should be one consideration and not the lone determinant in where a student applies and accepts,” the article states.
Dr. Turner agreed ranking systems are a good starting point when researching med schools, “but the values reflected in the ranking may not reflect an individual’s goals.”
Tyra-Lee Brett, a premed student at the University of South Florida, Tampa, believes an additional tool for students to evaluate medical schools is needed – and she could potentially see herself using Dr. Turner’s creation.
Still, Ms. Brett, a premed trustee of the American Medical Student Association, doesn’t regard any ranking tool as the “be all and end all.” Rather, she feels that the most effective tool would be based on students’ lived experiences. The AMSA is developing a scorecard in which students grade schools based on their opinions about such issues as housing, family planning, and environmental health, she said.
No prior judgments
To develop his algorithm, Dr. Turner used a branch of AI called “unsupervised learning.” It doesn’t make a prior judgment about what the data should look like, Dr. Turner explained.
“You’re just analyzing natural trends within the data.”
The algorithm tries to find and discover clusters or patterns within the data. “It’s like saying to the algorithm: ‘I want you to tell me what schools you think should be grouped together based on the data I feed you,’ which is the data that the user selects based on his or her personal preferences.”
U.S. News has been transparent about the metrics it uses, Dr. Turner notes. “When I started looking into how rankings are developed, I saw that there was transparency, and the reasoning for choosing the metrics used to develop the ranking was pretty sound,” he said.
“But I didn’t see any justification as to why they chose the particular metrics and weighted them in the way that they did.”
Dr. Turner extracted data from the 2023 U.S. News report, which ranked 109 allopathic medical schools, and applied several scenarios to the results to create his alternative ranking system.
In one scenario, he used the same research metrics used by U.S. News, such as a peer research assessment, median federal research activity per full-time faculty member, median GPA, median MCAT, acceptance rate, and faculty-student ratio.
In another scenario, he included four additional metrics: debt, in-state cost of attendance, USMLE Step 1 passing rate, and percentage of underrepresented students with minority race or ethnicity at the school.
For example, a user can rank the importance of the diversity of the class, amount of debt students expect to incur, and amount of research funding the medical school receives. After selecting those factors, the tool generates tiered results displayed in a circle, a shape chosen to avoid the appearance of the hierarchy associated with traditional rankings, Dr. Turner said.
“A prospective student might not care about acceptance rates and MCAT scores, and instead cares about diversity and debt,” Dr. Turner said. He looks forward to extending this approach to the ranking of colleges as well.
‘Imperfect measures’
“The model and interesting online tool that Dr. Turner created allows a premed [student] to generate custom rankings that are in line with their own priorities,” said Christopher Worsham, MD, MPH, a critical care physician in Mass General’s division of pulmonary and critical care medicine.
But Dr. Worsham, also a teaching associate at Harvard Medical School’s department of health care policy, expressed concern that factors figuring into the rankings by U.S. News and Dr. Turner’s alternative “are imperfect measures of medical school quality.”
For example, a student interested in research might favor federal research funding in their customized rankings with Dr. Turner’s model. “But higher research funding doesn’t necessarily translate into a better education for students, particularly when differentiating between two major research systems,” Dr. Worsham noted.
Dr. Worsham added that neither ranking system accurately predicts the quality of doctors graduating from the schools. Instead, he’d like to see ranking systems based on which schools’ graduates deliver the best patient outcomes, whether that’s through direct patient care, impactful research, or leadership within the health care system.
Michael Sauder, PhD, professor of sociology at the University of Iowa, Iowa City, said the model could offer a valuable alternative to the U.S. News ranking system. It might help users develop their own criteria for determining the ranking of medical schools, which is a big improvement over a “one-size-fits-all” approach, Dr. Sauder said.
And Hanna Stotland, an admission consultant based in Chicago, noted that most students rely on rankings because they “don’t have the luxury of advisers who know the ins and outs of different medical schools.” Given the role that rankings play, Ms. Stotland expects that every new ranking tool will have some influence on students.
This tool in particular “has the potential to be useful for students who have identified values they want their medical school to share.” For example, students who care about racial diversity “could use it to easily identify schools that are successful on that metric,” Ms. Stotland said.
Sujay Ratna, a 2nd-year med student at Icahn School of Medicine at Mount Sinai in New York, said he considered the U.S. News ranking his “go-to tool” when he was applying to med school.
But after reading Dr. Turner’s article, the AMSA membership vice president tried the algorithm. “I definitely would have used it had it existed when I was thinking of what schools to apply to and what [schools] to attend.”
The study had no specific funding. Dr. Turner, Dr. Worsham, Dr. Sauder, Ms. Stotland, Ms. Brett, and Mr. Ratna report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For decades, pre-med students depended on the annual medical school rankings by U.S. News and World Report to decide where to apply for physician education. But after several prominent med schools pulled out of the rankings, one resident began experimenting with artificial intelligence (AI) to create an alternative.
Brandon Turner MD, MSc, a radiation oncology resident at Massachusetts General Hospital in Boston, developed a free do-it-yourself tool using AI that allows prospective students to rank medical schools based on considerations that are most important to them. His research was published online in JAMA Network Open.
“One of the flaws with conventional ranking systems is that the metrics used in these tools are weighted based on the preferences and views of the people who developed these rankings, but those may not work for everyone,” Dr. Turner told this news organization.
He explained that there are different types of metrics used in the U.S. News ranking: one for research and the other for primary care. “The research rankings carry the most prestige and are the ones that most people know about,” he explained. These metrics take into account factors such as how many grant dollars the medical school receives and the average size of those grants per faculty member, Dr. Turner said.
Admission metrics are also included – for example, the median grade point average or MCAT scores of students who have been accepted. “These don’t tell you anything about the research output of the school, only about how selective the school is,” he said.
Primary care metrics might focus on how many graduates of a given school go into primary care, or how other schools rate the quality of primary care training at a given school – a process called peer assessment, Dr. Turner said.
But even though these might be helpful, students may be more interested in the cost of attendance, average debt, representation of minorities, and how many graduates pass their boards, he said. “U.S. News metrics don’t capture these things, but I included them in my algorithm.”
A U.S. News spokesperson said that the publication continues to help students and their families make decisions about their future education. The spokesperson cited U.S. News’ explanation of how it calculates its rankings. “A school’s overall Best Medical Schools rank should be one consideration and not the lone determinant in where a student applies and accepts,” the article states.
Dr. Turner agreed ranking systems are a good starting point when researching med schools, “but the values reflected in the ranking may not reflect an individual’s goals.”
Tyra-Lee Brett, a premed student at the University of South Florida, Tampa, believes an additional tool for students to evaluate medical schools is needed – and she could potentially see herself using Dr. Turner’s creation.
Still, Ms. Brett, a premed trustee of the American Medical Student Association, doesn’t regard any ranking tool as the “be all and end all.” Rather, she feels that the most effective tool would be based on students’ lived experiences. The AMSA is developing a scorecard in which students grade schools based on their opinions about such issues as housing, family planning, and environmental health, she said.
No prior judgments
To develop his algorithm, Dr. Turner used a branch of AI called “unsupervised learning.” It doesn’t make a prior judgment about what the data should look like, Dr. Turner explained.
“You’re just analyzing natural trends within the data.”
The algorithm tries to find and discover clusters or patterns within the data. “It’s like saying to the algorithm: ‘I want you to tell me what schools you think should be grouped together based on the data I feed you,’ which is the data that the user selects based on his or her personal preferences.”
U.S. News has been transparent about the metrics it uses, Dr. Turner notes. “When I started looking into how rankings are developed, I saw that there was transparency, and the reasoning for choosing the metrics used to develop the ranking was pretty sound,” he said.
“But I didn’t see any justification as to why they chose the particular metrics and weighted them in the way that they did.”
Dr. Turner extracted data from the 2023 U.S. News report, which ranked 109 allopathic medical schools, and applied several scenarios to the results to create his alternative ranking system.
In one scenario, he used the same research metrics used by U.S. News, such as a peer research assessment, median federal research activity per full-time faculty member, median GPA, median MCAT, acceptance rate, and faculty-student ratio.
In another scenario, he included four additional metrics: debt, in-state cost of attendance, USMLE Step 1 passing rate, and percentage of underrepresented students with minority race or ethnicity at the school.
For example, a user can rank the importance of the diversity of the class, amount of debt students expect to incur, and amount of research funding the medical school receives. After selecting those factors, the tool generates tiered results displayed in a circle, a shape chosen to avoid the appearance of the hierarchy associated with traditional rankings, Dr. Turner said.
“A prospective student might not care about acceptance rates and MCAT scores, and instead cares about diversity and debt,” Dr. Turner said. He looks forward to extending this approach to the ranking of colleges as well.
‘Imperfect measures’
“The model and interesting online tool that Dr. Turner created allows a premed [student] to generate custom rankings that are in line with their own priorities,” said Christopher Worsham, MD, MPH, a critical care physician in Mass General’s division of pulmonary and critical care medicine.
But Dr. Worsham, also a teaching associate at Harvard Medical School’s department of health care policy, expressed concern that factors figuring into the rankings by U.S. News and Dr. Turner’s alternative “are imperfect measures of medical school quality.”
For example, a student interested in research might favor federal research funding in their customized rankings with Dr. Turner’s model. “But higher research funding doesn’t necessarily translate into a better education for students, particularly when differentiating between two major research systems,” Dr. Worsham noted.
Dr. Worsham added that neither ranking system accurately predicts the quality of doctors graduating from the schools. Instead, he’d like to see ranking systems based on which schools’ graduates deliver the best patient outcomes, whether that’s through direct patient care, impactful research, or leadership within the health care system.
Michael Sauder, PhD, professor of sociology at the University of Iowa, Iowa City, said the model could offer a valuable alternative to the U.S. News ranking system. It might help users develop their own criteria for determining the ranking of medical schools, which is a big improvement over a “one-size-fits-all” approach, Dr. Sauder said.
And Hanna Stotland, an admission consultant based in Chicago, noted that most students rely on rankings because they “don’t have the luxury of advisers who know the ins and outs of different medical schools.” Given the role that rankings play, Ms. Stotland expects that every new ranking tool will have some influence on students.
This tool in particular “has the potential to be useful for students who have identified values they want their medical school to share.” For example, students who care about racial diversity “could use it to easily identify schools that are successful on that metric,” Ms. Stotland said.
Sujay Ratna, a 2nd-year med student at Icahn School of Medicine at Mount Sinai in New York, said he considered the U.S. News ranking his “go-to tool” when he was applying to med school.
But after reading Dr. Turner’s article, the AMSA membership vice president tried the algorithm. “I definitely would have used it had it existed when I was thinking of what schools to apply to and what [schools] to attend.”
The study had no specific funding. Dr. Turner, Dr. Worsham, Dr. Sauder, Ms. Stotland, Ms. Brett, and Mr. Ratna report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For decades, pre-med students depended on the annual medical school rankings by U.S. News and World Report to decide where to apply for physician education. But after several prominent med schools pulled out of the rankings, one resident began experimenting with artificial intelligence (AI) to create an alternative.
Brandon Turner MD, MSc, a radiation oncology resident at Massachusetts General Hospital in Boston, developed a free do-it-yourself tool using AI that allows prospective students to rank medical schools based on considerations that are most important to them. His research was published online in JAMA Network Open.
“One of the flaws with conventional ranking systems is that the metrics used in these tools are weighted based on the preferences and views of the people who developed these rankings, but those may not work for everyone,” Dr. Turner told this news organization.
He explained that there are different types of metrics used in the U.S. News ranking: one for research and the other for primary care. “The research rankings carry the most prestige and are the ones that most people know about,” he explained. These metrics take into account factors such as how many grant dollars the medical school receives and the average size of those grants per faculty member, Dr. Turner said.
Admission metrics are also included – for example, the median grade point average or MCAT scores of students who have been accepted. “These don’t tell you anything about the research output of the school, only about how selective the school is,” he said.
Primary care metrics might focus on how many graduates of a given school go into primary care, or how other schools rate the quality of primary care training at a given school – a process called peer assessment, Dr. Turner said.
But even though these might be helpful, students may be more interested in the cost of attendance, average debt, representation of minorities, and how many graduates pass their boards, he said. “U.S. News metrics don’t capture these things, but I included them in my algorithm.”
A U.S. News spokesperson said that the publication continues to help students and their families make decisions about their future education. The spokesperson cited U.S. News’ explanation of how it calculates its rankings. “A school’s overall Best Medical Schools rank should be one consideration and not the lone determinant in where a student applies and accepts,” the article states.
Dr. Turner agreed ranking systems are a good starting point when researching med schools, “but the values reflected in the ranking may not reflect an individual’s goals.”
Tyra-Lee Brett, a premed student at the University of South Florida, Tampa, believes an additional tool for students to evaluate medical schools is needed – and she could potentially see herself using Dr. Turner’s creation.
Still, Ms. Brett, a premed trustee of the American Medical Student Association, doesn’t regard any ranking tool as the “be all and end all.” Rather, she feels that the most effective tool would be based on students’ lived experiences. The AMSA is developing a scorecard in which students grade schools based on their opinions about such issues as housing, family planning, and environmental health, she said.
No prior judgments
To develop his algorithm, Dr. Turner used a branch of AI called “unsupervised learning.” It doesn’t make a prior judgment about what the data should look like, Dr. Turner explained.
“You’re just analyzing natural trends within the data.”
The algorithm tries to find and discover clusters or patterns within the data. “It’s like saying to the algorithm: ‘I want you to tell me what schools you think should be grouped together based on the data I feed you,’ which is the data that the user selects based on his or her personal preferences.”
U.S. News has been transparent about the metrics it uses, Dr. Turner notes. “When I started looking into how rankings are developed, I saw that there was transparency, and the reasoning for choosing the metrics used to develop the ranking was pretty sound,” he said.
“But I didn’t see any justification as to why they chose the particular metrics and weighted them in the way that they did.”
Dr. Turner extracted data from the 2023 U.S. News report, which ranked 109 allopathic medical schools, and applied several scenarios to the results to create his alternative ranking system.
In one scenario, he used the same research metrics used by U.S. News, such as a peer research assessment, median federal research activity per full-time faculty member, median GPA, median MCAT, acceptance rate, and faculty-student ratio.
In another scenario, he included four additional metrics: debt, in-state cost of attendance, USMLE Step 1 passing rate, and percentage of underrepresented students with minority race or ethnicity at the school.
For example, a user can rank the importance of the diversity of the class, amount of debt students expect to incur, and amount of research funding the medical school receives. After selecting those factors, the tool generates tiered results displayed in a circle, a shape chosen to avoid the appearance of the hierarchy associated with traditional rankings, Dr. Turner said.
“A prospective student might not care about acceptance rates and MCAT scores, and instead cares about diversity and debt,” Dr. Turner said. He looks forward to extending this approach to the ranking of colleges as well.
‘Imperfect measures’
“The model and interesting online tool that Dr. Turner created allows a premed [student] to generate custom rankings that are in line with their own priorities,” said Christopher Worsham, MD, MPH, a critical care physician in Mass General’s division of pulmonary and critical care medicine.
But Dr. Worsham, also a teaching associate at Harvard Medical School’s department of health care policy, expressed concern that factors figuring into the rankings by U.S. News and Dr. Turner’s alternative “are imperfect measures of medical school quality.”
For example, a student interested in research might favor federal research funding in their customized rankings with Dr. Turner’s model. “But higher research funding doesn’t necessarily translate into a better education for students, particularly when differentiating between two major research systems,” Dr. Worsham noted.
Dr. Worsham added that neither ranking system accurately predicts the quality of doctors graduating from the schools. Instead, he’d like to see ranking systems based on which schools’ graduates deliver the best patient outcomes, whether that’s through direct patient care, impactful research, or leadership within the health care system.
Michael Sauder, PhD, professor of sociology at the University of Iowa, Iowa City, said the model could offer a valuable alternative to the U.S. News ranking system. It might help users develop their own criteria for determining the ranking of medical schools, which is a big improvement over a “one-size-fits-all” approach, Dr. Sauder said.
And Hanna Stotland, an admission consultant based in Chicago, noted that most students rely on rankings because they “don’t have the luxury of advisers who know the ins and outs of different medical schools.” Given the role that rankings play, Ms. Stotland expects that every new ranking tool will have some influence on students.
This tool in particular “has the potential to be useful for students who have identified values they want their medical school to share.” For example, students who care about racial diversity “could use it to easily identify schools that are successful on that metric,” Ms. Stotland said.
Sujay Ratna, a 2nd-year med student at Icahn School of Medicine at Mount Sinai in New York, said he considered the U.S. News ranking his “go-to tool” when he was applying to med school.
But after reading Dr. Turner’s article, the AMSA membership vice president tried the algorithm. “I definitely would have used it had it existed when I was thinking of what schools to apply to and what [schools] to attend.”
The study had no specific funding. Dr. Turner, Dr. Worsham, Dr. Sauder, Ms. Stotland, Ms. Brett, and Mr. Ratna report no relevant financial relationships.
A version of this article first appeared on Medscape.com.










