User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Burnout among surgeons: Lessons for psychiatrists
Burnout is an occupational phenomenon and a syndrome resulting from unsuccessfully managed chronic workplace stress. The characteristic features of burnout include feelings of exhaustion, cynicism, and reduced professional efficacy.1 A career in surgery is associated with demanding and unpredictable work hours in a high-stress environment.2-8 Research indicates that surgeons are at an elevated risk for developing burnout and mental health problems that can compromise patient care. A survey of the fellows of the American College of Surgeons found that 40% of surgeons experience burnout, 30% experience symptoms of depression, and 28% have a mental quality of life (QOL) score greater than one-half an SD below the population norm.9,10 Surgeon burnout was also found to compromise the delivery of medical care.9,10
To prevent serious harm to surgeons and patients, it is critical to understand the causative factors of burnout among surgeons and how they can be addressed. We conducted this systematic review to identify factors linked to burnout across surgical specialties and to suggest ways to mitigate these risk factors.
Methods
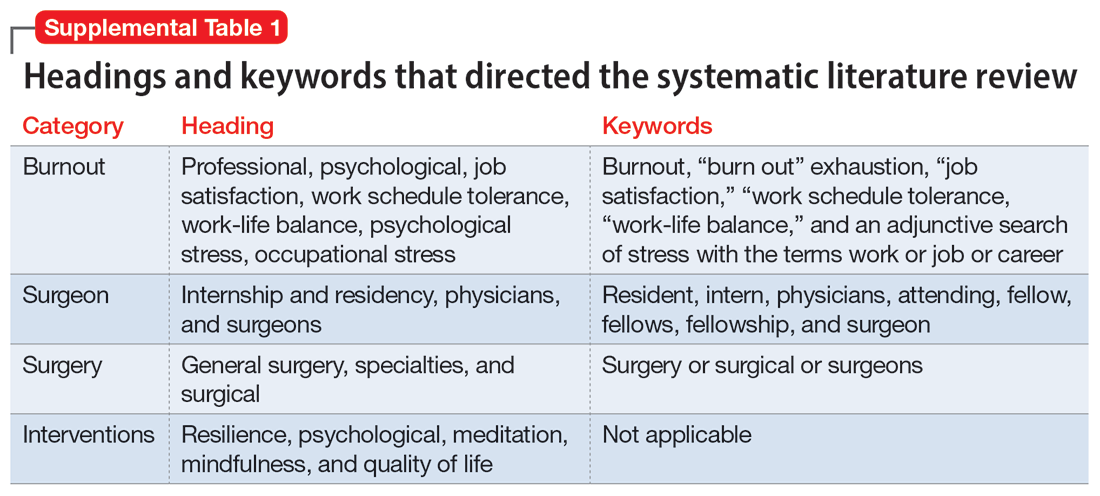
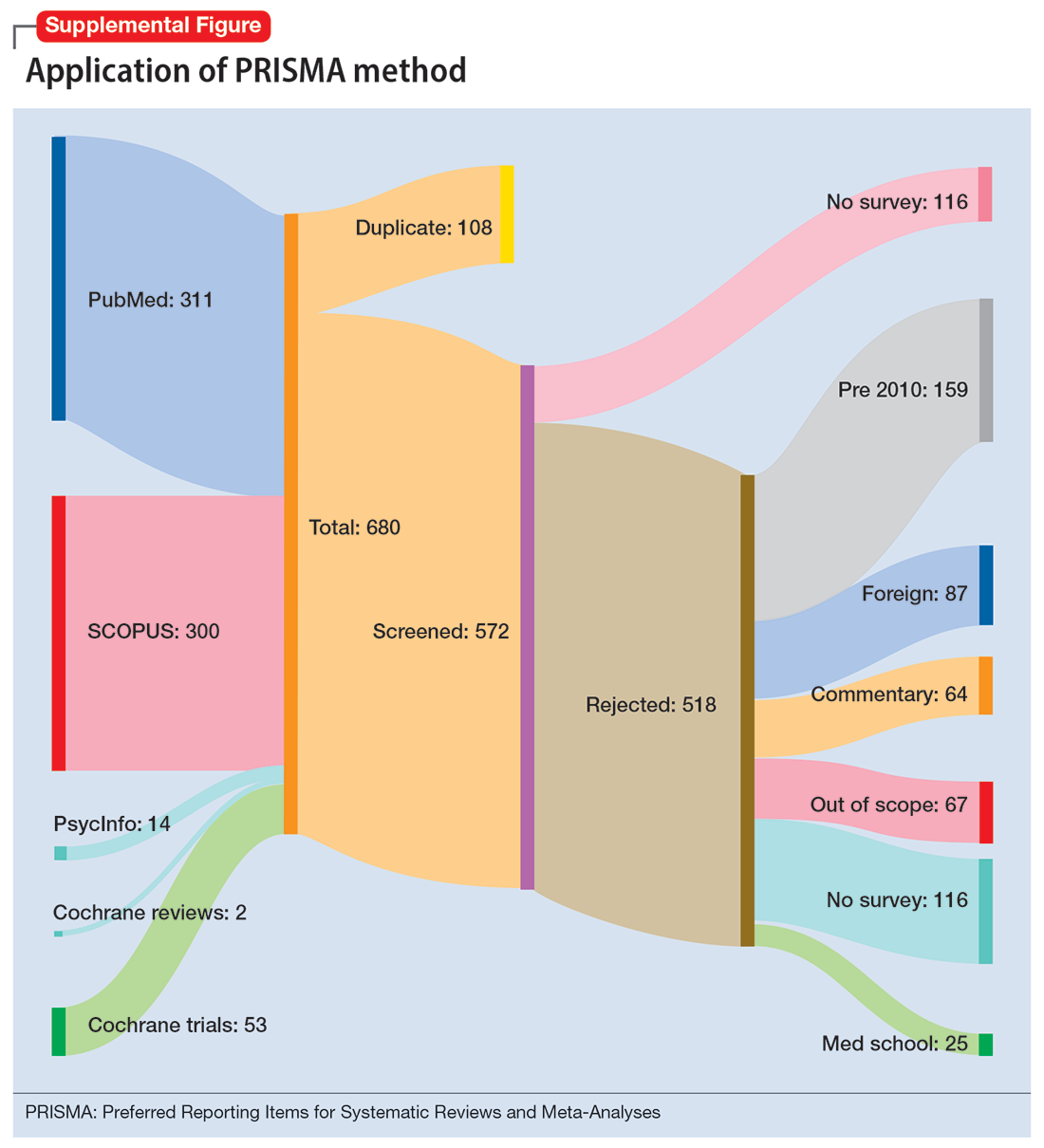
To identify studies of burnout among surgeons, we conducted an electronic search of Ovid MEDLINE, Ovid PsycInfo, SCOPUS, Cochrane Database of Systematic Reviews, and Cochrane Central Register of Controlled Trials. The headings and keywords used are listed in Supplemental Table 1. Studies met the inclusion criteria if they evaluated residents or attendings, used a tool to measure burnout, and examined any surgical specialty. Studies were excluded if they were published before 2010; were conducted outside the United States; were review articles, commentaries, or abstracts without full text articles; evaluated medical school students; were published in a language other than English; did not use a tool to measure burnout; or examined a nonsurgical specialty. Our analysis was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)11 and is outlined in the Supplemental Figure.
Results
Surgical specialties and burnout
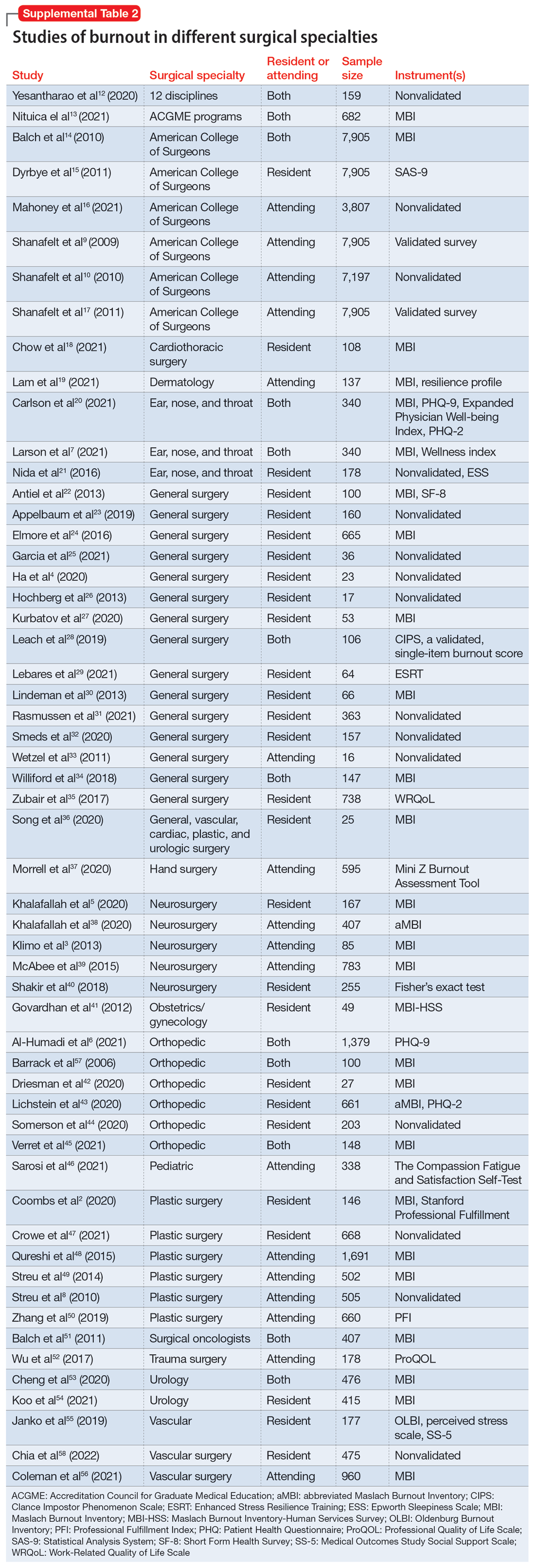
We identified 56 studies2-10,12-58 that focused on specific surgical specialties in relation to burnout. Supplemental Table 22-10,12-58 lists these studies and the surgical specialties they evaluated.
Work/life balance factors
Fifteen studies
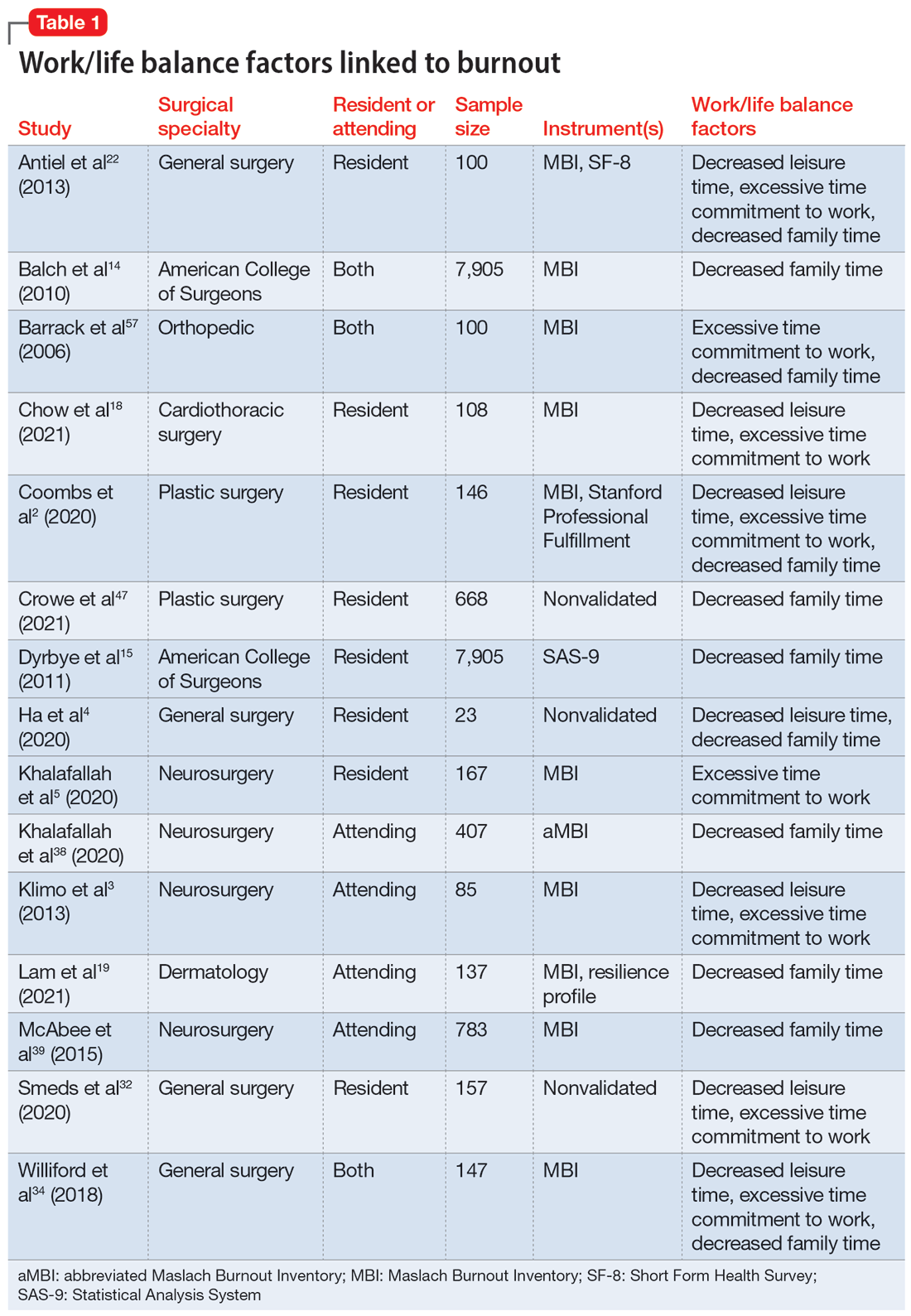
Work hours
Fifteen studies2,7,14,20,21,30,34,41,42,44-46,50,52,56 examined work hours and burnout. Of these, 142,7,14,20,21,30,34,42,44-46,50,52,56 found a correlation between increased work hours and burnout, while only 1 study41 found no correlation between these factors.
Medical errors
Six studies2,14,18,43,49,52 discussed the role of burnout in medical errors. Of these, 52,14,43,49,52 reported a correlation between burnout and medical errors, while 1 study18 found no link between burnout and medical errors. The medical errors were self-reported.14,49 They included actions that resulted in patient harm, sample collection error, and errors in medication orders and laboratory test orders.2
Continue to: Institutional and organizational factors
Institutional and organizational factors
Eighteen studies3,13,14,18,20,22,23,29,30,36-38,44,45,47,54,56,57 examined how different organizational factors play a role in burnout. Four studies3,13,20,37 discussed administrative/bureaucratic work, 420,45,54,57 mentioned electronic medical documentation, 222,30 covered duty hour regulations, 318,45,57 discussed mistreatment of physicians, and 613,18,23,44,47,56 described the importance of workplace support in addressing burnout.
Physical and mental health factors
Eighteen studies6,7,14,15,17,20,26,27,29,34,43,44,48,52,54,57-59 discussed aspects of physical and mental health linked to burnout. Among these, 334,43,59 discussed the importance of physical health and focused on how improving physical health can reduce stress and burnout. Three studies6,17,58 noted the prevalence of suicidal ideation in both residents and attendings experiencing prolonged burnout. Five studies26,29,43,44,48 described the systematic barriers that inhibit physicians from getting professional help. Two studies7,27 reported marital status as a factor for burnout; participants who were single reported higher levels of depression and suicidal ideation. Five studies6,14,15,54,57 outlined how depression is associated with burnout.
Strategies to mitigate burnout
Fifteen studies
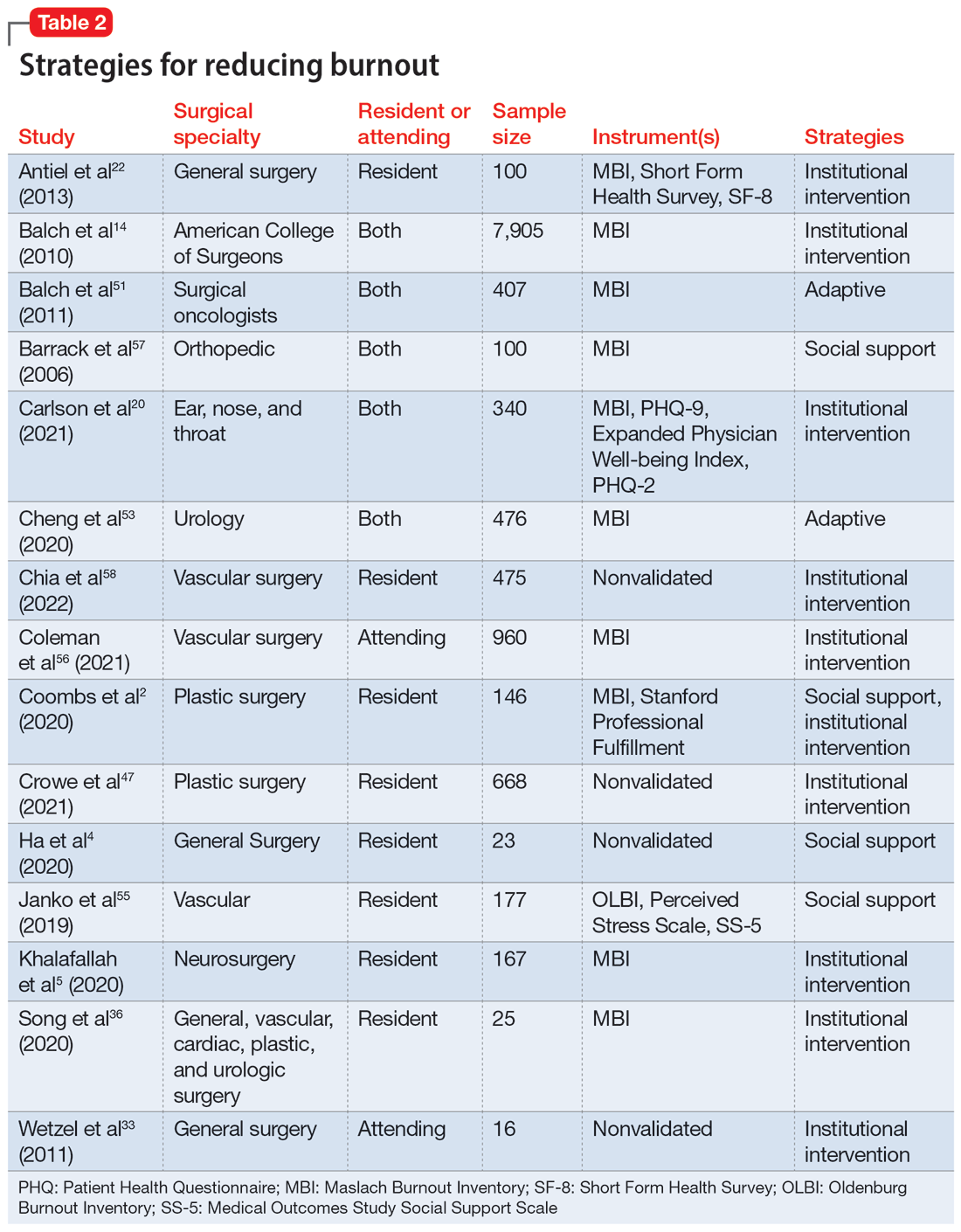
Take-home points
Research that focused on work/life balance and burnout found excessive time commitment to work is a major factor associated with poor work/life balance. Residents who worked >80 hours a week had a significantly higher burnout rate.56 One study found that 70% of residents reported not getting enough sleep, 30% reported not having enough energy for relationships, and 39% reported that they were not eating or exercising due to time constraints.4 A high correlation was found between the number of hours worked per week and rates of burnout, emotional exhaustion, and depersonalization. Emotional exhaustion and depersonalization are aspects of burnout measured by the Maslach Burnout Inventory (MBI).24 The excessive time commitment to work not only contributes to burnout but also prevents physicians from getting professional help. In 1 study, both residents (56%) and attendings (24%) reported that 1 of the biggest barriers to getting help for their burnout symptoms was the inability to take time off.34 Research indicates that the hours worked per week and work/home conflicts were independently associated with burnout and career satisfaction.15 A decrease of weekly work hours may give physicians time to meet their responsibilities at work and home, allowing for a decrease in burnout and an increase in career satisfaction.
Increased work hours have also been found to be correlated with medical errors. One study found that those who worked 60 hours per week were significantly less likely to report any major medical errors in the previous 3 months compared with those who worked 80 hours per week.9 The risk for the number of medical errors has been reported as being 2-fold if surgeons are unable to combat the burnout.49 On the other hand, a positive and supportive environment with easy access to resources to combat burnout and burnout prevention programs can reduce the frequency of medical errors, which also can reduce the risk of malpractice, thus further reducing stress and burnout.43
Continue to: In response to resident complaints...
In response to resident complaints about long duty hours, a new rule has been implemented that states residents cannot work >16 hours per shift.30 This rule has been found to increase quality of life and prevent burnout.30
The amount of time spent on electronic medical records and documentation has been a major complaint from doctors and was identified as a factor contributing to burnout.45 It can act as a time drain that impedes the physician from providing optimal patient care and cause additional stress. This suggests the need for organizations to find solutions to minimize this strain.
A concerning issue reported as an institutional factor and associated with burnout is mistreatment through discrimination, harassment, and physical or verbal abuse. A recent study found 45% of general and vascular surgeons reported being mistreated in some fashion.57 The strategies reported as helpful for institutions to combat mistreatment include resilience training, improved mentorship, and implicit bias training.57
Burnout has been positively correlated with anxiety and depression.6 A recent study reported that 13% of orthopedic surgery residents screened positive for depression.44 Higher levels of burnout and depersonalization have been found to be closely associated with increased rates of suicidal ideation.17 In a study of vascular surgeons, 8% were found to report suicidal ideation, and this increased to 15% among vascular surgeons who had higher levels of depersonalization and emotional exhaustion,58 both of which are associated with burnout. In another study, surgery residents and fellows were found to have lower levels of personal achievement and higher levels of depersonalization, depressive symptoms, alcohol abuse, and suicidal ideation compared to attending physicians and the general population.54 These findings spell out the association between burnout and depressive symptoms among surgeons and emphasize the need for institutions to create a culture that supports the mental health needs of their physicians. Without access to supportive resources, residents resort to alternative methods that may be detrimental in the long run. In a recent study, 17% of residents admitted to using alcohol, including binge drinking, to cope with their stress.4
Burnout and depression are linked to physical health risks such as cardiovascular disease, diabetes, substance abuse, and male infertility.6 Exercise has been shown to be beneficial for stress reduction, which can lead to changes in metabolism, inflammation, coagulation, and autonomic function.6 One study of surgeons found aerobic exercise and strength training were associated with lower rates of burnout and a higher quality of life.59
Continue to: The amount of burnout physicians...
The amount of burnout physicians experience can be determined by how they respond to adversities. Adaptive behaviors such as socializing, mindfulness, volunteering, and exercising have been found to be protective against burnout.6,37,54 Resilience training and maintaining low stress at work can decrease burnout.37 These findings highlight the need for physicians to be trained in the appropriate ways to combat their burnout symptoms.
Unfortunately, seeking help by health care professionals to improve mental health has been stigmatized, causing physicians to not seek help and instead resort to other ways to cope with their distress.26,34 While some of these coping methods may be positive, others—such as substance abuse or stress eating—can be maladaptive, leading to a poor quality of life, and in some cases, suicide.54 It is vital that effective mental health services become more accessible and for health care professionals to become aware of their maladaptive behaviors.34
Institutions finding ways to ease the path for their physicians to seek professional help to combat burnout may mitigate its negative impact. One strategy is to embed access to mental health services within regular wellness checks. Institutions can use wellness checks to provide resources to physicians who need it. These interventions have been found to be effective because they give physicians a safe space to seek help and become aware of any factors that could lead to burnout.18 Apart from these direct attempts to combat burnout, program-sponsored social events would also promote social connectedness with colleagues and contribute to a sense of well-being that could help decrease levels of burnout and depression.13 Mentorship has been shown to play a crucial role in decreasing burnout among residents. One study that examined the role of mentorship reported that 55% of residents felt supported, and of these, 96% felt mentorship was critical to their success.18 The role of institutions in helping to improve the well-being of surgeons is highlighted by the finding that increasing workplace support results in psychological resilience that can mitigate burnout at its roots.29
Bottom Line
Surgeons are at risk for burnout, which can impact their mental health and reduce their professional efficacy. Both institutions and surgeons themselves can take action to prevent burnout and treat burnout early when it occurs.
Related Resources
- Pahal P, Lippmann S. Building a better work/life balance. Current Psychiatry. 2021;20(8):e1-e2. doi:10.12788/cp.0158
- Gibson R, Hategan A. Physician burnout vs depression: recognize the signs. Current Psychiatry. 2019;18(10):41-42.
1. World Health Organization. International Statistical Classification of Diseases and Related Health Problems (ICD). 11th ed. World Health Organization; 2019.
2. Coombs DM, Lanni MA, Fosnot J, et al. Professional burnout in United States plastic surgery residents: is it a legitimate concern? Aesthet Surg J. 2020;40(7):802-810.
3. Klimo P Jr, DeCuypere M, Ragel BT, et al. Career satisfaction and burnout among U.S. neurosurgeons: a feasibility and pilot study. World Neurosurg. 2013;80(5):e59-e68.
4. Ha GQ, Go JT, Murayama KM, et al. Identifying sources of stress across years of general surgery residency. Hawaii J Health Soc Welf. 2020;79(3):75-81.
5. Khalafallah AM, Lam S, Gami A, et al. A national survey on the impact of the COVID-19 pandemic upon burnout and career satisfaction among neurosurgery residents. J Clin Neurosci. 2020;80:137-142.
6. Al-Humadi SM, Cáceda R, Bronson B, et al. Orthopaedic surgeon mental health during the COVID-19 pandemic. Geriatric Orthop Surg Rehabil. 2021;12:21514593211035230.
7. Larson DP, Carlson ML, Lohse CM, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part I, trainees. Otolaryngol Head Neck Surg. 2021;164(5):1019-1029.
8. Streu R, Hawley S, Gay A, et al. Satisfaction with career choice among U.S. plastic surgeons: results from a national survey. Plast Reconstr Surg. 2010;126(2):636-642.
9. Shanafelt TD, Balch CM, Bechamps GJ, et al. Burnout and career satisfaction among American surgeons. Ann Surg. 2009;250(3):463-471.
10. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251(6):995-1000.
11. Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
12. Yesantharao P, Lee E, Kraenzlin F, et al. Surgical block time satisfaction: a multi-institutional experience across twelve surgical disciplines. Perioperative Care Operating Room Manage. 2020;21:100128.
13. Nituica C, Bota OA, Blebea J. Specialty differences in resident resilience and burnout - a national survey. Am J Surg. 2021;222(2):319-328.
14. Balch CM, Shanafelt TD, Dyrbye L, et al. Surgeon distress as calibrated by hours worked and nights on call. J Am Coll Surg. 2010;211(5):609-619.
15. Dyrbye LN, Shanafelt TD, Balch CM, Satele D, Sloan J, Freischlag J. Relationship between work-home conflicts and burnout among American surgeons: a comparison by sex. Arch Surg. 2011;146(2):211-217.
16. Mahoney ST, Irish W, Strassle PD, et al. Practice characteristics and job satisfaction of private practice and academic surgeons. JAMA Surg. 2021;156(3):247-254.
17. Shanafelt TD, Balch CM, Dyrbye L, et al. Special report: suicidal ideation among American surgeons. Arch Surg. 2011;146(1):54-62.
18. Chow OS, Sudarshan M, Maxfield MW, et al. National survey of burnout and distress among cardiothoracic surgery trainees. Ann Thorac Surg. 2021;111(6):2066-2071.
19. Lam C, Kim Y, Cruz M, et al. Burnout and resiliency in Mohs surgeons: a survey study. Int J Womens Dermatol. 2021;7(3):319-322.
20. Carlson ML, Larson DP, O’Brien EK, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part II, attending physicians. Otolaryngol Head Neck Surg. 2021;164(5):1030-1039.
21. Nida AM, Googe BJ, Lewis AF, et al. Resident fatigue in otolaryngology residents: a Web based survey. Am J Otolaryngol. 2016;37(3):210-216.
22. Antiel RM, Reed DA, Van Arendonk KJ, et al. Effects of duty hour restrictions on core competencies, education, quality of life, and burnout among general surgery interns. JAMA Surg. 2013;148(5):448-455.
23. Appelbaum NP, Lee N, Amendola M, et al. Surgical resident burnout and job satisfaction: the role of workplace climate and perceived support. J Surg Res. 2019;234:20-25.
24. Elmore LC, Jeffe DB, Jin L, et al. National survey of burnout among US general surgery residents. J Am Coll Surg. 2016;223(3):440-451.
25. Garcia DI, Pannuccio A, Gallegos J, et al. Resident-driven wellness initiatives improve resident wellness and perception of work environment. J Surg Res. 2021;258:8-16.
26. Hochberg MS, Berman RS, Kalet AL, et al. The stress of residency: recognizing the signs of depression and suicide in you and your fellow residents. Am J Surg. 2013;205(2):141-146.
27. Kurbatov V, Shaughnessy M, Baratta V, et al. Application of advanced bioinformatics to understand and predict burnout among surgical trainees. J Surg Educ. 2020;77(3):499-507.
28. Leach PK, Nygaard RM, Chipman JG, et al. Impostor phenomenon and burnout in general surgeons and general surgery residents. J Surg Educ. 2019;76(1):99-106.
29. Lebares CC, Greenberg AL, Ascher NL, et al. Exploration of individual and system-level well-being initiatives at an academic surgical residency program: a mixed-methods study. JAMA Netw Open. 2021;4(1):e2032676.
30. Lindeman BM, Sacks BC, Hirose K, et al. Multifaceted longitudinal study of surgical resident education, quality of life, and patient care before and after July 2011. J Surg Educ. 2013;70(6):769-776.
31. Rasmussen JM, Najarian MM, Ties JS, et al. Career satisfaction, gender bias, and work-life balance: a contemporary assessment of general surgeons. J Surg Educ. 2021;78(1):119-125.
32. Smeds MR, Janko MR, Allen S, et al. Burnout and its relationship with perceived stress, self-efficacy, depression, social support, and programmatic factors in general surgery residents. Am J Surg. 2020;219(6):907-912.
33. Wetzel CM, George A, Hanna GB, et al. Stress management training for surgeons--a randomized, controlled, intervention study. Ann Surg. 2011;253(3):488-494.
34. Williford ML, Scarlet S, Meyers MO, et al. Multiple-institution comparison of resident and faculty perceptions of burnout and depression during surgical training. JAMA Surg. 2018;153(8):705-711.
35. Zubair MH, Hussain LR, Williams KN, et al. Work-related quality of life of US general surgery residents: is it really so bad? J Surg Educ. 2017;74(6):e138-e146.
36. Song Y, Swendiman RA, Shannon AB, et al. Can we coach resilience? An evaluation of professional resilience coaching as a well-being initiative for surgical interns. J Surg Educ. 2020;77(6):1481-1489.
37. Morrell NT, Sears ED, Desai MJ, et al. A survey of burnout among members of the American Society for Surgery of the Hand. J Hand Surg Am. 2020;45(7):573-581.e516.
38. Khalafallah AM, Lam S, Gami A, et al. Burnout and career satisfaction among attending neurosurgeons during the COVID-19 pandemic. Clin Neurol Neurosurg. 2020;198:106193.
39. McAbee JH, Ragel BT, McCartney S, et al. Factors associated with career satisfaction and burnout among US neurosurgeons: results of a nationwide survey. J Neurosurg. 2015;123(1):161-173.
40. Shakir HJ, McPheeters MJ, Shallwani H, et al. The prevalence of burnout among US neurosurgery residents. Neurosurgery. 2018;83(3):582-590.
41. Govardhan LM, Pinelli V, Schnatz PF. Burnout, depression and job satisfaction in obstetrics and gynecology residents. Conn Med. 2012;76(7):389-395.
42. Driesman AS, Strauss EJ, Konda SR, et al. Factors associated with orthopaedic resident burnout: a pilot study. J Am Acad Orthop Surg. 2020;28(21):900-906.
43. Lichstein PM, He JK, Estok D, et al. What is the prevalence of burnout, depression, and substance use among orthopaedic surgery residents and what are the risk factors? A collaborative orthopaedic educational research group survey study. Clin Orthop Relat Res. 2020;478(8):1709-1718.
44. Somerson JS, Patton A, Ahmed AA, et al. Burnout among United States orthopaedic surgery residents. J Surg Educ. 2020;77(4):961-968.
45. Verret CI, Nguyen J, Verret C, et al. How do areas of work life drive burnout in orthopaedic attending surgeons, fellows, and residents? Clin Orthop Relat Res. 2021;479(2):251-262.
46. Sarosi A, Coakley BA, Berman L, et al. A cross-sectional analysis of compassion fatigue, burnout, and compassion satisfaction in pediatric surgeons in the U.S. J Pediatr Surg. 2021;56(8):1276-1284.
47. Crowe CS, Lopez J, Morrison SD, et al. The effects of the COVID-19 pandemic on resident education and wellness: a national survey of plastic surgery residents. Plast Reconstr Surg. 2021;148(3):462e-474e.
48. Qureshi HA, Rawlani R, Mioton LM, et al. Burnout phenomenon in U.S. plastic surgeons: risk factors and impact on quality of life. Plast Reconstr Surg. 2015;135(2):619-626.
49. Streu R, Hansen J, Abrahamse P, et al. Professional burnout among US plastic surgeons: results of a national survey. Ann Plast Surg. 2014;72(3):346-350.
50. Zhang JQ, Riba L, Magrini L, ET AL. Assessing burnout and professional fulfillment in breast surgery: results from a national survey of the American Society of Breast Surgeons. Ann Surg Oncol. 2019;26(10):3089-3098.
51. Balch CM, Shanafelt TD, Sloan J, et al. Burnout and career satisfaction among surgical oncologists compared with other surgical specialties. Ann Surg Oncol. 2011;18(1):16-25.
52. Wu D, Gross B, Rittenhouse K, et al. A preliminary analysis of compassion fatigue in a surgeon population: are female surgeons at heightened risk? Am Surg. 2017;83(11):1302-1307.
53. Cheng JW, Wagner H, Hernandez BC, et al. Stressors and coping mechanisms related to burnout within urology. Urology. 2020;139:27-36.
54. Koo K, Javier-DesLoges JF, Fang R, ET AL. Professional burnout, career choice regret, and unmet needs for well-being among urology residents. Urology. 2021;157:57-63.
55. Janko MR, Smeds MR. Burnout, depression, perceived stress, and self-efficacy in vascular surgery trainees. J Vasc Surg. 2019;69(4):1233-1242.
56. Coleman DM, Money SR, Meltzer AJ, et al. Vascular surgeon wellness and burnout: a report from the Society for Vascular Surgery Wellness Task Force. J Vasc Surg. 2021;73(6):1841-1850.e3.
57. Barrack RL, Miller LS, Sotile WM, et al. Effect of duty hour standards on burnout among orthopaedic surgery residents. Clin Orthop Relat Res. 2006;449:134-137.
58. Chia MC, Hu YY, Li RD, et al. Prevalence and risk factors for burnout in U.S. vascular surgery trainees. J Vasc Surg. 2022;75(1):308-315.e4.
59. Shanafelt TD, Oreskovich MR, Dyrbye LN, et al. Avoiding burnout: the personal health habits and wellness practices of US surgeons. Ann Surg. 2012;255(4):625-633.
Burnout is an occupational phenomenon and a syndrome resulting from unsuccessfully managed chronic workplace stress. The characteristic features of burnout include feelings of exhaustion, cynicism, and reduced professional efficacy.1 A career in surgery is associated with demanding and unpredictable work hours in a high-stress environment.2-8 Research indicates that surgeons are at an elevated risk for developing burnout and mental health problems that can compromise patient care. A survey of the fellows of the American College of Surgeons found that 40% of surgeons experience burnout, 30% experience symptoms of depression, and 28% have a mental quality of life (QOL) score greater than one-half an SD below the population norm.9,10 Surgeon burnout was also found to compromise the delivery of medical care.9,10
To prevent serious harm to surgeons and patients, it is critical to understand the causative factors of burnout among surgeons and how they can be addressed. We conducted this systematic review to identify factors linked to burnout across surgical specialties and to suggest ways to mitigate these risk factors.

Methods
To identify studies of burnout among surgeons, we conducted an electronic search of Ovid MEDLINE, Ovid PsycInfo, SCOPUS, Cochrane Database of Systematic Reviews, and Cochrane Central Register of Controlled Trials. The headings and keywords used are listed in Supplemental Table 1. Studies met the inclusion criteria if they evaluated residents or attendings, used a tool to measure burnout, and examined any surgical specialty. Studies were excluded if they were published before 2010; were conducted outside the United States; were review articles, commentaries, or abstracts without full text articles; evaluated medical school students; were published in a language other than English; did not use a tool to measure burnout; or examined a nonsurgical specialty. Our analysis was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)11 and is outlined in the Supplemental Figure.

Results
Surgical specialties and burnout
We identified 56 studies2-10,12-58 that focused on specific surgical specialties in relation to burnout. Supplemental Table 22-10,12-58 lists these studies and the surgical specialties they evaluated.

Work/life balance factors
Fifteen studies

Work hours
Fifteen studies2,7,14,20,21,30,34,41,42,44-46,50,52,56 examined work hours and burnout. Of these, 142,7,14,20,21,30,34,42,44-46,50,52,56 found a correlation between increased work hours and burnout, while only 1 study41 found no correlation between these factors.
Medical errors
Six studies2,14,18,43,49,52 discussed the role of burnout in medical errors. Of these, 52,14,43,49,52 reported a correlation between burnout and medical errors, while 1 study18 found no link between burnout and medical errors. The medical errors were self-reported.14,49 They included actions that resulted in patient harm, sample collection error, and errors in medication orders and laboratory test orders.2
Continue to: Institutional and organizational factors
Institutional and organizational factors
Eighteen studies3,13,14,18,20,22,23,29,30,36-38,44,45,47,54,56,57 examined how different organizational factors play a role in burnout. Four studies3,13,20,37 discussed administrative/bureaucratic work, 420,45,54,57 mentioned electronic medical documentation, 222,30 covered duty hour regulations, 318,45,57 discussed mistreatment of physicians, and 613,18,23,44,47,56 described the importance of workplace support in addressing burnout.
Physical and mental health factors
Eighteen studies6,7,14,15,17,20,26,27,29,34,43,44,48,52,54,57-59 discussed aspects of physical and mental health linked to burnout. Among these, 334,43,59 discussed the importance of physical health and focused on how improving physical health can reduce stress and burnout. Three studies6,17,58 noted the prevalence of suicidal ideation in both residents and attendings experiencing prolonged burnout. Five studies26,29,43,44,48 described the systematic barriers that inhibit physicians from getting professional help. Two studies7,27 reported marital status as a factor for burnout; participants who were single reported higher levels of depression and suicidal ideation. Five studies6,14,15,54,57 outlined how depression is associated with burnout.
Strategies to mitigate burnout
Fifteen studies

Take-home points
Research that focused on work/life balance and burnout found excessive time commitment to work is a major factor associated with poor work/life balance. Residents who worked >80 hours a week had a significantly higher burnout rate.56 One study found that 70% of residents reported not getting enough sleep, 30% reported not having enough energy for relationships, and 39% reported that they were not eating or exercising due to time constraints.4 A high correlation was found between the number of hours worked per week and rates of burnout, emotional exhaustion, and depersonalization. Emotional exhaustion and depersonalization are aspects of burnout measured by the Maslach Burnout Inventory (MBI).24 The excessive time commitment to work not only contributes to burnout but also prevents physicians from getting professional help. In 1 study, both residents (56%) and attendings (24%) reported that 1 of the biggest barriers to getting help for their burnout symptoms was the inability to take time off.34 Research indicates that the hours worked per week and work/home conflicts were independently associated with burnout and career satisfaction.15 A decrease of weekly work hours may give physicians time to meet their responsibilities at work and home, allowing for a decrease in burnout and an increase in career satisfaction.
Increased work hours have also been found to be correlated with medical errors. One study found that those who worked 60 hours per week were significantly less likely to report any major medical errors in the previous 3 months compared with those who worked 80 hours per week.9 The risk for the number of medical errors has been reported as being 2-fold if surgeons are unable to combat the burnout.49 On the other hand, a positive and supportive environment with easy access to resources to combat burnout and burnout prevention programs can reduce the frequency of medical errors, which also can reduce the risk of malpractice, thus further reducing stress and burnout.43
Continue to: In response to resident complaints...
In response to resident complaints about long duty hours, a new rule has been implemented that states residents cannot work >16 hours per shift.30 This rule has been found to increase quality of life and prevent burnout.30
The amount of time spent on electronic medical records and documentation has been a major complaint from doctors and was identified as a factor contributing to burnout.45 It can act as a time drain that impedes the physician from providing optimal patient care and cause additional stress. This suggests the need for organizations to find solutions to minimize this strain.
A concerning issue reported as an institutional factor and associated with burnout is mistreatment through discrimination, harassment, and physical or verbal abuse. A recent study found 45% of general and vascular surgeons reported being mistreated in some fashion.57 The strategies reported as helpful for institutions to combat mistreatment include resilience training, improved mentorship, and implicit bias training.57
Burnout has been positively correlated with anxiety and depression.6 A recent study reported that 13% of orthopedic surgery residents screened positive for depression.44 Higher levels of burnout and depersonalization have been found to be closely associated with increased rates of suicidal ideation.17 In a study of vascular surgeons, 8% were found to report suicidal ideation, and this increased to 15% among vascular surgeons who had higher levels of depersonalization and emotional exhaustion,58 both of which are associated with burnout. In another study, surgery residents and fellows were found to have lower levels of personal achievement and higher levels of depersonalization, depressive symptoms, alcohol abuse, and suicidal ideation compared to attending physicians and the general population.54 These findings spell out the association between burnout and depressive symptoms among surgeons and emphasize the need for institutions to create a culture that supports the mental health needs of their physicians. Without access to supportive resources, residents resort to alternative methods that may be detrimental in the long run. In a recent study, 17% of residents admitted to using alcohol, including binge drinking, to cope with their stress.4
Burnout and depression are linked to physical health risks such as cardiovascular disease, diabetes, substance abuse, and male infertility.6 Exercise has been shown to be beneficial for stress reduction, which can lead to changes in metabolism, inflammation, coagulation, and autonomic function.6 One study of surgeons found aerobic exercise and strength training were associated with lower rates of burnout and a higher quality of life.59
Continue to: The amount of burnout physicians...
The amount of burnout physicians experience can be determined by how they respond to adversities. Adaptive behaviors such as socializing, mindfulness, volunteering, and exercising have been found to be protective against burnout.6,37,54 Resilience training and maintaining low stress at work can decrease burnout.37 These findings highlight the need for physicians to be trained in the appropriate ways to combat their burnout symptoms.
Unfortunately, seeking help by health care professionals to improve mental health has been stigmatized, causing physicians to not seek help and instead resort to other ways to cope with their distress.26,34 While some of these coping methods may be positive, others—such as substance abuse or stress eating—can be maladaptive, leading to a poor quality of life, and in some cases, suicide.54 It is vital that effective mental health services become more accessible and for health care professionals to become aware of their maladaptive behaviors.34
Institutions finding ways to ease the path for their physicians to seek professional help to combat burnout may mitigate its negative impact. One strategy is to embed access to mental health services within regular wellness checks. Institutions can use wellness checks to provide resources to physicians who need it. These interventions have been found to be effective because they give physicians a safe space to seek help and become aware of any factors that could lead to burnout.18 Apart from these direct attempts to combat burnout, program-sponsored social events would also promote social connectedness with colleagues and contribute to a sense of well-being that could help decrease levels of burnout and depression.13 Mentorship has been shown to play a crucial role in decreasing burnout among residents. One study that examined the role of mentorship reported that 55% of residents felt supported, and of these, 96% felt mentorship was critical to their success.18 The role of institutions in helping to improve the well-being of surgeons is highlighted by the finding that increasing workplace support results in psychological resilience that can mitigate burnout at its roots.29
Bottom Line
Surgeons are at risk for burnout, which can impact their mental health and reduce their professional efficacy. Both institutions and surgeons themselves can take action to prevent burnout and treat burnout early when it occurs.
Related Resources
- Pahal P, Lippmann S. Building a better work/life balance. Current Psychiatry. 2021;20(8):e1-e2. doi:10.12788/cp.0158
- Gibson R, Hategan A. Physician burnout vs depression: recognize the signs. Current Psychiatry. 2019;18(10):41-42.
Burnout is an occupational phenomenon and a syndrome resulting from unsuccessfully managed chronic workplace stress. The characteristic features of burnout include feelings of exhaustion, cynicism, and reduced professional efficacy.1 A career in surgery is associated with demanding and unpredictable work hours in a high-stress environment.2-8 Research indicates that surgeons are at an elevated risk for developing burnout and mental health problems that can compromise patient care. A survey of the fellows of the American College of Surgeons found that 40% of surgeons experience burnout, 30% experience symptoms of depression, and 28% have a mental quality of life (QOL) score greater than one-half an SD below the population norm.9,10 Surgeon burnout was also found to compromise the delivery of medical care.9,10
To prevent serious harm to surgeons and patients, it is critical to understand the causative factors of burnout among surgeons and how they can be addressed. We conducted this systematic review to identify factors linked to burnout across surgical specialties and to suggest ways to mitigate these risk factors.

Methods
To identify studies of burnout among surgeons, we conducted an electronic search of Ovid MEDLINE, Ovid PsycInfo, SCOPUS, Cochrane Database of Systematic Reviews, and Cochrane Central Register of Controlled Trials. The headings and keywords used are listed in Supplemental Table 1. Studies met the inclusion criteria if they evaluated residents or attendings, used a tool to measure burnout, and examined any surgical specialty. Studies were excluded if they were published before 2010; were conducted outside the United States; were review articles, commentaries, or abstracts without full text articles; evaluated medical school students; were published in a language other than English; did not use a tool to measure burnout; or examined a nonsurgical specialty. Our analysis was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)11 and is outlined in the Supplemental Figure.

Results
Surgical specialties and burnout
We identified 56 studies2-10,12-58 that focused on specific surgical specialties in relation to burnout. Supplemental Table 22-10,12-58 lists these studies and the surgical specialties they evaluated.

Work/life balance factors
Fifteen studies

Work hours
Fifteen studies2,7,14,20,21,30,34,41,42,44-46,50,52,56 examined work hours and burnout. Of these, 142,7,14,20,21,30,34,42,44-46,50,52,56 found a correlation between increased work hours and burnout, while only 1 study41 found no correlation between these factors.
Medical errors
Six studies2,14,18,43,49,52 discussed the role of burnout in medical errors. Of these, 52,14,43,49,52 reported a correlation between burnout and medical errors, while 1 study18 found no link between burnout and medical errors. The medical errors were self-reported.14,49 They included actions that resulted in patient harm, sample collection error, and errors in medication orders and laboratory test orders.2
Continue to: Institutional and organizational factors
Institutional and organizational factors
Eighteen studies3,13,14,18,20,22,23,29,30,36-38,44,45,47,54,56,57 examined how different organizational factors play a role in burnout. Four studies3,13,20,37 discussed administrative/bureaucratic work, 420,45,54,57 mentioned electronic medical documentation, 222,30 covered duty hour regulations, 318,45,57 discussed mistreatment of physicians, and 613,18,23,44,47,56 described the importance of workplace support in addressing burnout.
Physical and mental health factors
Eighteen studies6,7,14,15,17,20,26,27,29,34,43,44,48,52,54,57-59 discussed aspects of physical and mental health linked to burnout. Among these, 334,43,59 discussed the importance of physical health and focused on how improving physical health can reduce stress and burnout. Three studies6,17,58 noted the prevalence of suicidal ideation in both residents and attendings experiencing prolonged burnout. Five studies26,29,43,44,48 described the systematic barriers that inhibit physicians from getting professional help. Two studies7,27 reported marital status as a factor for burnout; participants who were single reported higher levels of depression and suicidal ideation. Five studies6,14,15,54,57 outlined how depression is associated with burnout.
Strategies to mitigate burnout
Fifteen studies

Take-home points
Research that focused on work/life balance and burnout found excessive time commitment to work is a major factor associated with poor work/life balance. Residents who worked >80 hours a week had a significantly higher burnout rate.56 One study found that 70% of residents reported not getting enough sleep, 30% reported not having enough energy for relationships, and 39% reported that they were not eating or exercising due to time constraints.4 A high correlation was found between the number of hours worked per week and rates of burnout, emotional exhaustion, and depersonalization. Emotional exhaustion and depersonalization are aspects of burnout measured by the Maslach Burnout Inventory (MBI).24 The excessive time commitment to work not only contributes to burnout but also prevents physicians from getting professional help. In 1 study, both residents (56%) and attendings (24%) reported that 1 of the biggest barriers to getting help for their burnout symptoms was the inability to take time off.34 Research indicates that the hours worked per week and work/home conflicts were independently associated with burnout and career satisfaction.15 A decrease of weekly work hours may give physicians time to meet their responsibilities at work and home, allowing for a decrease in burnout and an increase in career satisfaction.
Increased work hours have also been found to be correlated with medical errors. One study found that those who worked 60 hours per week were significantly less likely to report any major medical errors in the previous 3 months compared with those who worked 80 hours per week.9 The risk for the number of medical errors has been reported as being 2-fold if surgeons are unable to combat the burnout.49 On the other hand, a positive and supportive environment with easy access to resources to combat burnout and burnout prevention programs can reduce the frequency of medical errors, which also can reduce the risk of malpractice, thus further reducing stress and burnout.43
Continue to: In response to resident complaints...
In response to resident complaints about long duty hours, a new rule has been implemented that states residents cannot work >16 hours per shift.30 This rule has been found to increase quality of life and prevent burnout.30
The amount of time spent on electronic medical records and documentation has been a major complaint from doctors and was identified as a factor contributing to burnout.45 It can act as a time drain that impedes the physician from providing optimal patient care and cause additional stress. This suggests the need for organizations to find solutions to minimize this strain.
A concerning issue reported as an institutional factor and associated with burnout is mistreatment through discrimination, harassment, and physical or verbal abuse. A recent study found 45% of general and vascular surgeons reported being mistreated in some fashion.57 The strategies reported as helpful for institutions to combat mistreatment include resilience training, improved mentorship, and implicit bias training.57
Burnout has been positively correlated with anxiety and depression.6 A recent study reported that 13% of orthopedic surgery residents screened positive for depression.44 Higher levels of burnout and depersonalization have been found to be closely associated with increased rates of suicidal ideation.17 In a study of vascular surgeons, 8% were found to report suicidal ideation, and this increased to 15% among vascular surgeons who had higher levels of depersonalization and emotional exhaustion,58 both of which are associated with burnout. In another study, surgery residents and fellows were found to have lower levels of personal achievement and higher levels of depersonalization, depressive symptoms, alcohol abuse, and suicidal ideation compared to attending physicians and the general population.54 These findings spell out the association between burnout and depressive symptoms among surgeons and emphasize the need for institutions to create a culture that supports the mental health needs of their physicians. Without access to supportive resources, residents resort to alternative methods that may be detrimental in the long run. In a recent study, 17% of residents admitted to using alcohol, including binge drinking, to cope with their stress.4
Burnout and depression are linked to physical health risks such as cardiovascular disease, diabetes, substance abuse, and male infertility.6 Exercise has been shown to be beneficial for stress reduction, which can lead to changes in metabolism, inflammation, coagulation, and autonomic function.6 One study of surgeons found aerobic exercise and strength training were associated with lower rates of burnout and a higher quality of life.59
Continue to: The amount of burnout physicians...
The amount of burnout physicians experience can be determined by how they respond to adversities. Adaptive behaviors such as socializing, mindfulness, volunteering, and exercising have been found to be protective against burnout.6,37,54 Resilience training and maintaining low stress at work can decrease burnout.37 These findings highlight the need for physicians to be trained in the appropriate ways to combat their burnout symptoms.
Unfortunately, seeking help by health care professionals to improve mental health has been stigmatized, causing physicians to not seek help and instead resort to other ways to cope with their distress.26,34 While some of these coping methods may be positive, others—such as substance abuse or stress eating—can be maladaptive, leading to a poor quality of life, and in some cases, suicide.54 It is vital that effective mental health services become more accessible and for health care professionals to become aware of their maladaptive behaviors.34
Institutions finding ways to ease the path for their physicians to seek professional help to combat burnout may mitigate its negative impact. One strategy is to embed access to mental health services within regular wellness checks. Institutions can use wellness checks to provide resources to physicians who need it. These interventions have been found to be effective because they give physicians a safe space to seek help and become aware of any factors that could lead to burnout.18 Apart from these direct attempts to combat burnout, program-sponsored social events would also promote social connectedness with colleagues and contribute to a sense of well-being that could help decrease levels of burnout and depression.13 Mentorship has been shown to play a crucial role in decreasing burnout among residents. One study that examined the role of mentorship reported that 55% of residents felt supported, and of these, 96% felt mentorship was critical to their success.18 The role of institutions in helping to improve the well-being of surgeons is highlighted by the finding that increasing workplace support results in psychological resilience that can mitigate burnout at its roots.29
Bottom Line
Surgeons are at risk for burnout, which can impact their mental health and reduce their professional efficacy. Both institutions and surgeons themselves can take action to prevent burnout and treat burnout early when it occurs.
Related Resources
- Pahal P, Lippmann S. Building a better work/life balance. Current Psychiatry. 2021;20(8):e1-e2. doi:10.12788/cp.0158
- Gibson R, Hategan A. Physician burnout vs depression: recognize the signs. Current Psychiatry. 2019;18(10):41-42.
1. World Health Organization. International Statistical Classification of Diseases and Related Health Problems (ICD). 11th ed. World Health Organization; 2019.
2. Coombs DM, Lanni MA, Fosnot J, et al. Professional burnout in United States plastic surgery residents: is it a legitimate concern? Aesthet Surg J. 2020;40(7):802-810.
3. Klimo P Jr, DeCuypere M, Ragel BT, et al. Career satisfaction and burnout among U.S. neurosurgeons: a feasibility and pilot study. World Neurosurg. 2013;80(5):e59-e68.
4. Ha GQ, Go JT, Murayama KM, et al. Identifying sources of stress across years of general surgery residency. Hawaii J Health Soc Welf. 2020;79(3):75-81.
5. Khalafallah AM, Lam S, Gami A, et al. A national survey on the impact of the COVID-19 pandemic upon burnout and career satisfaction among neurosurgery residents. J Clin Neurosci. 2020;80:137-142.
6. Al-Humadi SM, Cáceda R, Bronson B, et al. Orthopaedic surgeon mental health during the COVID-19 pandemic. Geriatric Orthop Surg Rehabil. 2021;12:21514593211035230.
7. Larson DP, Carlson ML, Lohse CM, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part I, trainees. Otolaryngol Head Neck Surg. 2021;164(5):1019-1029.
8. Streu R, Hawley S, Gay A, et al. Satisfaction with career choice among U.S. plastic surgeons: results from a national survey. Plast Reconstr Surg. 2010;126(2):636-642.
9. Shanafelt TD, Balch CM, Bechamps GJ, et al. Burnout and career satisfaction among American surgeons. Ann Surg. 2009;250(3):463-471.
10. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251(6):995-1000.
11. Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
12. Yesantharao P, Lee E, Kraenzlin F, et al. Surgical block time satisfaction: a multi-institutional experience across twelve surgical disciplines. Perioperative Care Operating Room Manage. 2020;21:100128.
13. Nituica C, Bota OA, Blebea J. Specialty differences in resident resilience and burnout - a national survey. Am J Surg. 2021;222(2):319-328.
14. Balch CM, Shanafelt TD, Dyrbye L, et al. Surgeon distress as calibrated by hours worked and nights on call. J Am Coll Surg. 2010;211(5):609-619.
15. Dyrbye LN, Shanafelt TD, Balch CM, Satele D, Sloan J, Freischlag J. Relationship between work-home conflicts and burnout among American surgeons: a comparison by sex. Arch Surg. 2011;146(2):211-217.
16. Mahoney ST, Irish W, Strassle PD, et al. Practice characteristics and job satisfaction of private practice and academic surgeons. JAMA Surg. 2021;156(3):247-254.
17. Shanafelt TD, Balch CM, Dyrbye L, et al. Special report: suicidal ideation among American surgeons. Arch Surg. 2011;146(1):54-62.
18. Chow OS, Sudarshan M, Maxfield MW, et al. National survey of burnout and distress among cardiothoracic surgery trainees. Ann Thorac Surg. 2021;111(6):2066-2071.
19. Lam C, Kim Y, Cruz M, et al. Burnout and resiliency in Mohs surgeons: a survey study. Int J Womens Dermatol. 2021;7(3):319-322.
20. Carlson ML, Larson DP, O’Brien EK, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part II, attending physicians. Otolaryngol Head Neck Surg. 2021;164(5):1030-1039.
21. Nida AM, Googe BJ, Lewis AF, et al. Resident fatigue in otolaryngology residents: a Web based survey. Am J Otolaryngol. 2016;37(3):210-216.
22. Antiel RM, Reed DA, Van Arendonk KJ, et al. Effects of duty hour restrictions on core competencies, education, quality of life, and burnout among general surgery interns. JAMA Surg. 2013;148(5):448-455.
23. Appelbaum NP, Lee N, Amendola M, et al. Surgical resident burnout and job satisfaction: the role of workplace climate and perceived support. J Surg Res. 2019;234:20-25.
24. Elmore LC, Jeffe DB, Jin L, et al. National survey of burnout among US general surgery residents. J Am Coll Surg. 2016;223(3):440-451.
25. Garcia DI, Pannuccio A, Gallegos J, et al. Resident-driven wellness initiatives improve resident wellness and perception of work environment. J Surg Res. 2021;258:8-16.
26. Hochberg MS, Berman RS, Kalet AL, et al. The stress of residency: recognizing the signs of depression and suicide in you and your fellow residents. Am J Surg. 2013;205(2):141-146.
27. Kurbatov V, Shaughnessy M, Baratta V, et al. Application of advanced bioinformatics to understand and predict burnout among surgical trainees. J Surg Educ. 2020;77(3):499-507.
28. Leach PK, Nygaard RM, Chipman JG, et al. Impostor phenomenon and burnout in general surgeons and general surgery residents. J Surg Educ. 2019;76(1):99-106.
29. Lebares CC, Greenberg AL, Ascher NL, et al. Exploration of individual and system-level well-being initiatives at an academic surgical residency program: a mixed-methods study. JAMA Netw Open. 2021;4(1):e2032676.
30. Lindeman BM, Sacks BC, Hirose K, et al. Multifaceted longitudinal study of surgical resident education, quality of life, and patient care before and after July 2011. J Surg Educ. 2013;70(6):769-776.
31. Rasmussen JM, Najarian MM, Ties JS, et al. Career satisfaction, gender bias, and work-life balance: a contemporary assessment of general surgeons. J Surg Educ. 2021;78(1):119-125.
32. Smeds MR, Janko MR, Allen S, et al. Burnout and its relationship with perceived stress, self-efficacy, depression, social support, and programmatic factors in general surgery residents. Am J Surg. 2020;219(6):907-912.
33. Wetzel CM, George A, Hanna GB, et al. Stress management training for surgeons--a randomized, controlled, intervention study. Ann Surg. 2011;253(3):488-494.
34. Williford ML, Scarlet S, Meyers MO, et al. Multiple-institution comparison of resident and faculty perceptions of burnout and depression during surgical training. JAMA Surg. 2018;153(8):705-711.
35. Zubair MH, Hussain LR, Williams KN, et al. Work-related quality of life of US general surgery residents: is it really so bad? J Surg Educ. 2017;74(6):e138-e146.
36. Song Y, Swendiman RA, Shannon AB, et al. Can we coach resilience? An evaluation of professional resilience coaching as a well-being initiative for surgical interns. J Surg Educ. 2020;77(6):1481-1489.
37. Morrell NT, Sears ED, Desai MJ, et al. A survey of burnout among members of the American Society for Surgery of the Hand. J Hand Surg Am. 2020;45(7):573-581.e516.
38. Khalafallah AM, Lam S, Gami A, et al. Burnout and career satisfaction among attending neurosurgeons during the COVID-19 pandemic. Clin Neurol Neurosurg. 2020;198:106193.
39. McAbee JH, Ragel BT, McCartney S, et al. Factors associated with career satisfaction and burnout among US neurosurgeons: results of a nationwide survey. J Neurosurg. 2015;123(1):161-173.
40. Shakir HJ, McPheeters MJ, Shallwani H, et al. The prevalence of burnout among US neurosurgery residents. Neurosurgery. 2018;83(3):582-590.
41. Govardhan LM, Pinelli V, Schnatz PF. Burnout, depression and job satisfaction in obstetrics and gynecology residents. Conn Med. 2012;76(7):389-395.
42. Driesman AS, Strauss EJ, Konda SR, et al. Factors associated with orthopaedic resident burnout: a pilot study. J Am Acad Orthop Surg. 2020;28(21):900-906.
43. Lichstein PM, He JK, Estok D, et al. What is the prevalence of burnout, depression, and substance use among orthopaedic surgery residents and what are the risk factors? A collaborative orthopaedic educational research group survey study. Clin Orthop Relat Res. 2020;478(8):1709-1718.
44. Somerson JS, Patton A, Ahmed AA, et al. Burnout among United States orthopaedic surgery residents. J Surg Educ. 2020;77(4):961-968.
45. Verret CI, Nguyen J, Verret C, et al. How do areas of work life drive burnout in orthopaedic attending surgeons, fellows, and residents? Clin Orthop Relat Res. 2021;479(2):251-262.
46. Sarosi A, Coakley BA, Berman L, et al. A cross-sectional analysis of compassion fatigue, burnout, and compassion satisfaction in pediatric surgeons in the U.S. J Pediatr Surg. 2021;56(8):1276-1284.
47. Crowe CS, Lopez J, Morrison SD, et al. The effects of the COVID-19 pandemic on resident education and wellness: a national survey of plastic surgery residents. Plast Reconstr Surg. 2021;148(3):462e-474e.
48. Qureshi HA, Rawlani R, Mioton LM, et al. Burnout phenomenon in U.S. plastic surgeons: risk factors and impact on quality of life. Plast Reconstr Surg. 2015;135(2):619-626.
49. Streu R, Hansen J, Abrahamse P, et al. Professional burnout among US plastic surgeons: results of a national survey. Ann Plast Surg. 2014;72(3):346-350.
50. Zhang JQ, Riba L, Magrini L, ET AL. Assessing burnout and professional fulfillment in breast surgery: results from a national survey of the American Society of Breast Surgeons. Ann Surg Oncol. 2019;26(10):3089-3098.
51. Balch CM, Shanafelt TD, Sloan J, et al. Burnout and career satisfaction among surgical oncologists compared with other surgical specialties. Ann Surg Oncol. 2011;18(1):16-25.
52. Wu D, Gross B, Rittenhouse K, et al. A preliminary analysis of compassion fatigue in a surgeon population: are female surgeons at heightened risk? Am Surg. 2017;83(11):1302-1307.
53. Cheng JW, Wagner H, Hernandez BC, et al. Stressors and coping mechanisms related to burnout within urology. Urology. 2020;139:27-36.
54. Koo K, Javier-DesLoges JF, Fang R, ET AL. Professional burnout, career choice regret, and unmet needs for well-being among urology residents. Urology. 2021;157:57-63.
55. Janko MR, Smeds MR. Burnout, depression, perceived stress, and self-efficacy in vascular surgery trainees. J Vasc Surg. 2019;69(4):1233-1242.
56. Coleman DM, Money SR, Meltzer AJ, et al. Vascular surgeon wellness and burnout: a report from the Society for Vascular Surgery Wellness Task Force. J Vasc Surg. 2021;73(6):1841-1850.e3.
57. Barrack RL, Miller LS, Sotile WM, et al. Effect of duty hour standards on burnout among orthopaedic surgery residents. Clin Orthop Relat Res. 2006;449:134-137.
58. Chia MC, Hu YY, Li RD, et al. Prevalence and risk factors for burnout in U.S. vascular surgery trainees. J Vasc Surg. 2022;75(1):308-315.e4.
59. Shanafelt TD, Oreskovich MR, Dyrbye LN, et al. Avoiding burnout: the personal health habits and wellness practices of US surgeons. Ann Surg. 2012;255(4):625-633.
1. World Health Organization. International Statistical Classification of Diseases and Related Health Problems (ICD). 11th ed. World Health Organization; 2019.
2. Coombs DM, Lanni MA, Fosnot J, et al. Professional burnout in United States plastic surgery residents: is it a legitimate concern? Aesthet Surg J. 2020;40(7):802-810.
3. Klimo P Jr, DeCuypere M, Ragel BT, et al. Career satisfaction and burnout among U.S. neurosurgeons: a feasibility and pilot study. World Neurosurg. 2013;80(5):e59-e68.
4. Ha GQ, Go JT, Murayama KM, et al. Identifying sources of stress across years of general surgery residency. Hawaii J Health Soc Welf. 2020;79(3):75-81.
5. Khalafallah AM, Lam S, Gami A, et al. A national survey on the impact of the COVID-19 pandemic upon burnout and career satisfaction among neurosurgery residents. J Clin Neurosci. 2020;80:137-142.
6. Al-Humadi SM, Cáceda R, Bronson B, et al. Orthopaedic surgeon mental health during the COVID-19 pandemic. Geriatric Orthop Surg Rehabil. 2021;12:21514593211035230.
7. Larson DP, Carlson ML, Lohse CM, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part I, trainees. Otolaryngol Head Neck Surg. 2021;164(5):1019-1029.
8. Streu R, Hawley S, Gay A, et al. Satisfaction with career choice among U.S. plastic surgeons: results from a national survey. Plast Reconstr Surg. 2010;126(2):636-642.
9. Shanafelt TD, Balch CM, Bechamps GJ, et al. Burnout and career satisfaction among American surgeons. Ann Surg. 2009;250(3):463-471.
10. Shanafelt TD, Balch CM, Bechamps G, et al. Burnout and medical errors among American surgeons. Ann Surg. 2010;251(6):995-1000.
11. Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341.
12. Yesantharao P, Lee E, Kraenzlin F, et al. Surgical block time satisfaction: a multi-institutional experience across twelve surgical disciplines. Perioperative Care Operating Room Manage. 2020;21:100128.
13. Nituica C, Bota OA, Blebea J. Specialty differences in resident resilience and burnout - a national survey. Am J Surg. 2021;222(2):319-328.
14. Balch CM, Shanafelt TD, Dyrbye L, et al. Surgeon distress as calibrated by hours worked and nights on call. J Am Coll Surg. 2010;211(5):609-619.
15. Dyrbye LN, Shanafelt TD, Balch CM, Satele D, Sloan J, Freischlag J. Relationship between work-home conflicts and burnout among American surgeons: a comparison by sex. Arch Surg. 2011;146(2):211-217.
16. Mahoney ST, Irish W, Strassle PD, et al. Practice characteristics and job satisfaction of private practice and academic surgeons. JAMA Surg. 2021;156(3):247-254.
17. Shanafelt TD, Balch CM, Dyrbye L, et al. Special report: suicidal ideation among American surgeons. Arch Surg. 2011;146(1):54-62.
18. Chow OS, Sudarshan M, Maxfield MW, et al. National survey of burnout and distress among cardiothoracic surgery trainees. Ann Thorac Surg. 2021;111(6):2066-2071.
19. Lam C, Kim Y, Cruz M, et al. Burnout and resiliency in Mohs surgeons: a survey study. Int J Womens Dermatol. 2021;7(3):319-322.
20. Carlson ML, Larson DP, O’Brien EK, et al. Prevalence of and associations with distress and professional burnout among otolaryngologists: part II, attending physicians. Otolaryngol Head Neck Surg. 2021;164(5):1030-1039.
21. Nida AM, Googe BJ, Lewis AF, et al. Resident fatigue in otolaryngology residents: a Web based survey. Am J Otolaryngol. 2016;37(3):210-216.
22. Antiel RM, Reed DA, Van Arendonk KJ, et al. Effects of duty hour restrictions on core competencies, education, quality of life, and burnout among general surgery interns. JAMA Surg. 2013;148(5):448-455.
23. Appelbaum NP, Lee N, Amendola M, et al. Surgical resident burnout and job satisfaction: the role of workplace climate and perceived support. J Surg Res. 2019;234:20-25.
24. Elmore LC, Jeffe DB, Jin L, et al. National survey of burnout among US general surgery residents. J Am Coll Surg. 2016;223(3):440-451.
25. Garcia DI, Pannuccio A, Gallegos J, et al. Resident-driven wellness initiatives improve resident wellness and perception of work environment. J Surg Res. 2021;258:8-16.
26. Hochberg MS, Berman RS, Kalet AL, et al. The stress of residency: recognizing the signs of depression and suicide in you and your fellow residents. Am J Surg. 2013;205(2):141-146.
27. Kurbatov V, Shaughnessy M, Baratta V, et al. Application of advanced bioinformatics to understand and predict burnout among surgical trainees. J Surg Educ. 2020;77(3):499-507.
28. Leach PK, Nygaard RM, Chipman JG, et al. Impostor phenomenon and burnout in general surgeons and general surgery residents. J Surg Educ. 2019;76(1):99-106.
29. Lebares CC, Greenberg AL, Ascher NL, et al. Exploration of individual and system-level well-being initiatives at an academic surgical residency program: a mixed-methods study. JAMA Netw Open. 2021;4(1):e2032676.
30. Lindeman BM, Sacks BC, Hirose K, et al. Multifaceted longitudinal study of surgical resident education, quality of life, and patient care before and after July 2011. J Surg Educ. 2013;70(6):769-776.
31. Rasmussen JM, Najarian MM, Ties JS, et al. Career satisfaction, gender bias, and work-life balance: a contemporary assessment of general surgeons. J Surg Educ. 2021;78(1):119-125.
32. Smeds MR, Janko MR, Allen S, et al. Burnout and its relationship with perceived stress, self-efficacy, depression, social support, and programmatic factors in general surgery residents. Am J Surg. 2020;219(6):907-912.
33. Wetzel CM, George A, Hanna GB, et al. Stress management training for surgeons--a randomized, controlled, intervention study. Ann Surg. 2011;253(3):488-494.
34. Williford ML, Scarlet S, Meyers MO, et al. Multiple-institution comparison of resident and faculty perceptions of burnout and depression during surgical training. JAMA Surg. 2018;153(8):705-711.
35. Zubair MH, Hussain LR, Williams KN, et al. Work-related quality of life of US general surgery residents: is it really so bad? J Surg Educ. 2017;74(6):e138-e146.
36. Song Y, Swendiman RA, Shannon AB, et al. Can we coach resilience? An evaluation of professional resilience coaching as a well-being initiative for surgical interns. J Surg Educ. 2020;77(6):1481-1489.
37. Morrell NT, Sears ED, Desai MJ, et al. A survey of burnout among members of the American Society for Surgery of the Hand. J Hand Surg Am. 2020;45(7):573-581.e516.
38. Khalafallah AM, Lam S, Gami A, et al. Burnout and career satisfaction among attending neurosurgeons during the COVID-19 pandemic. Clin Neurol Neurosurg. 2020;198:106193.
39. McAbee JH, Ragel BT, McCartney S, et al. Factors associated with career satisfaction and burnout among US neurosurgeons: results of a nationwide survey. J Neurosurg. 2015;123(1):161-173.
40. Shakir HJ, McPheeters MJ, Shallwani H, et al. The prevalence of burnout among US neurosurgery residents. Neurosurgery. 2018;83(3):582-590.
41. Govardhan LM, Pinelli V, Schnatz PF. Burnout, depression and job satisfaction in obstetrics and gynecology residents. Conn Med. 2012;76(7):389-395.
42. Driesman AS, Strauss EJ, Konda SR, et al. Factors associated with orthopaedic resident burnout: a pilot study. J Am Acad Orthop Surg. 2020;28(21):900-906.
43. Lichstein PM, He JK, Estok D, et al. What is the prevalence of burnout, depression, and substance use among orthopaedic surgery residents and what are the risk factors? A collaborative orthopaedic educational research group survey study. Clin Orthop Relat Res. 2020;478(8):1709-1718.
44. Somerson JS, Patton A, Ahmed AA, et al. Burnout among United States orthopaedic surgery residents. J Surg Educ. 2020;77(4):961-968.
45. Verret CI, Nguyen J, Verret C, et al. How do areas of work life drive burnout in orthopaedic attending surgeons, fellows, and residents? Clin Orthop Relat Res. 2021;479(2):251-262.
46. Sarosi A, Coakley BA, Berman L, et al. A cross-sectional analysis of compassion fatigue, burnout, and compassion satisfaction in pediatric surgeons in the U.S. J Pediatr Surg. 2021;56(8):1276-1284.
47. Crowe CS, Lopez J, Morrison SD, et al. The effects of the COVID-19 pandemic on resident education and wellness: a national survey of plastic surgery residents. Plast Reconstr Surg. 2021;148(3):462e-474e.
48. Qureshi HA, Rawlani R, Mioton LM, et al. Burnout phenomenon in U.S. plastic surgeons: risk factors and impact on quality of life. Plast Reconstr Surg. 2015;135(2):619-626.
49. Streu R, Hansen J, Abrahamse P, et al. Professional burnout among US plastic surgeons: results of a national survey. Ann Plast Surg. 2014;72(3):346-350.
50. Zhang JQ, Riba L, Magrini L, ET AL. Assessing burnout and professional fulfillment in breast surgery: results from a national survey of the American Society of Breast Surgeons. Ann Surg Oncol. 2019;26(10):3089-3098.
51. Balch CM, Shanafelt TD, Sloan J, et al. Burnout and career satisfaction among surgical oncologists compared with other surgical specialties. Ann Surg Oncol. 2011;18(1):16-25.
52. Wu D, Gross B, Rittenhouse K, et al. A preliminary analysis of compassion fatigue in a surgeon population: are female surgeons at heightened risk? Am Surg. 2017;83(11):1302-1307.
53. Cheng JW, Wagner H, Hernandez BC, et al. Stressors and coping mechanisms related to burnout within urology. Urology. 2020;139:27-36.
54. Koo K, Javier-DesLoges JF, Fang R, ET AL. Professional burnout, career choice regret, and unmet needs for well-being among urology residents. Urology. 2021;157:57-63.
55. Janko MR, Smeds MR. Burnout, depression, perceived stress, and self-efficacy in vascular surgery trainees. J Vasc Surg. 2019;69(4):1233-1242.
56. Coleman DM, Money SR, Meltzer AJ, et al. Vascular surgeon wellness and burnout: a report from the Society for Vascular Surgery Wellness Task Force. J Vasc Surg. 2021;73(6):1841-1850.e3.
57. Barrack RL, Miller LS, Sotile WM, et al. Effect of duty hour standards on burnout among orthopaedic surgery residents. Clin Orthop Relat Res. 2006;449:134-137.
58. Chia MC, Hu YY, Li RD, et al. Prevalence and risk factors for burnout in U.S. vascular surgery trainees. J Vasc Surg. 2022;75(1):308-315.e4.
59. Shanafelt TD, Oreskovich MR, Dyrbye LN, et al. Avoiding burnout: the personal health habits and wellness practices of US surgeons. Ann Surg. 2012;255(4):625-633.
Brain damage from recurrent relapses of bipolar mania: A call for early LAI use
Bipolar disorder (BD) is a psychotic mood disorder. Like schizophrenia, it has been shown to be associated with significant degeneration and structural brain abnormalities with multiple relapses.1,2
Just as I have always advocated preventing recurrences in schizophrenia by using long-acting injectable (LAI) antipsychotic formulations immediately after the first episode to prevent psychotic relapses and progressive brain damage,3 I strongly recommend using LAIs right after hospital discharge from the first manic episode. It is the most rational management approach for bipolar mania given the grave consequences of multiple episodes, which are so common in this psychotic mood disorder due to poor medication adherence.
In contrast to the depressive episodes of BD I, where patients have insight into their depression and seek psychiatric treatment, during a manic episode patients often have no insight (anosognosia) that they suffer from a serious brain disorder, and refuse treatment.4 In addition, young patients with BD I frequently discontinue their oral mood stabilizer or second-generation antipsychotic (which are approved for mania) because they miss the blissful euphoria and the buoyant physical and mental energy of their manic episodes. They are completely oblivious to (and uninformed about) the grave neurobiological damage of further manic episodes, which can condemn them to clinical, functional, and cognitive deterioration. These patients are also likely to become treatment-resistant, which has been labeled as “the malignant transformation of bipolar disorder.”5
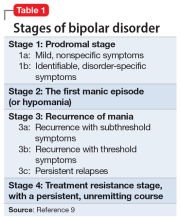
The evidence for progressive brain tissue loss, clinical deterioration, functional decline, and treatment resistance is abundant.6 I was the lead investigator of the first study to report ventricular dilatation (which is a proxy for cortical atrophy) in bipolar mania,7 a discovery that was subsequently replicated by 2 dozen researchers. This was followed by numerous neuroimaging studies reporting a loss of volume across multiple brain regions, including the frontal lobe, temporal lobe, cerebellum, thalamus, hippocampus, and basal ganglia. BD is heterogeneous8 with 4 stages (Table 19), and patients experience progressively worse brain structure and function with each stage.
Many patients with bipolar mania end up with poor clinical and functional outcomes, even when they respond well to initial treatment with lithium, anticonvulsant mood stabilizers, or second-generation antipsychotics. With their intentional nonadherence to oral medications leading to multiple recurrent relapses, these patients are at serious risk for neuroprogression and brain atrophic changes driven by multiple factors: inflammatory cytokines, increased cortical steroids, decreased neurotrophins, deceased neurogenesis, increased oxidative stress, and mitochondrial energy dysfunction. The consequences include progressive shortening of the interval between episodes with every relapse and loss of responsiveness to pharmacotherapy as the illness progresses.6,10 Predictors of a downhill progression include genetic vulnerability, perinatal complication during fetal life, childhood trauma (physical, sexual, emotional, or neglect), substance use, stress, psychiatric/medial comorbidities, and especially the number of episodes.9,11
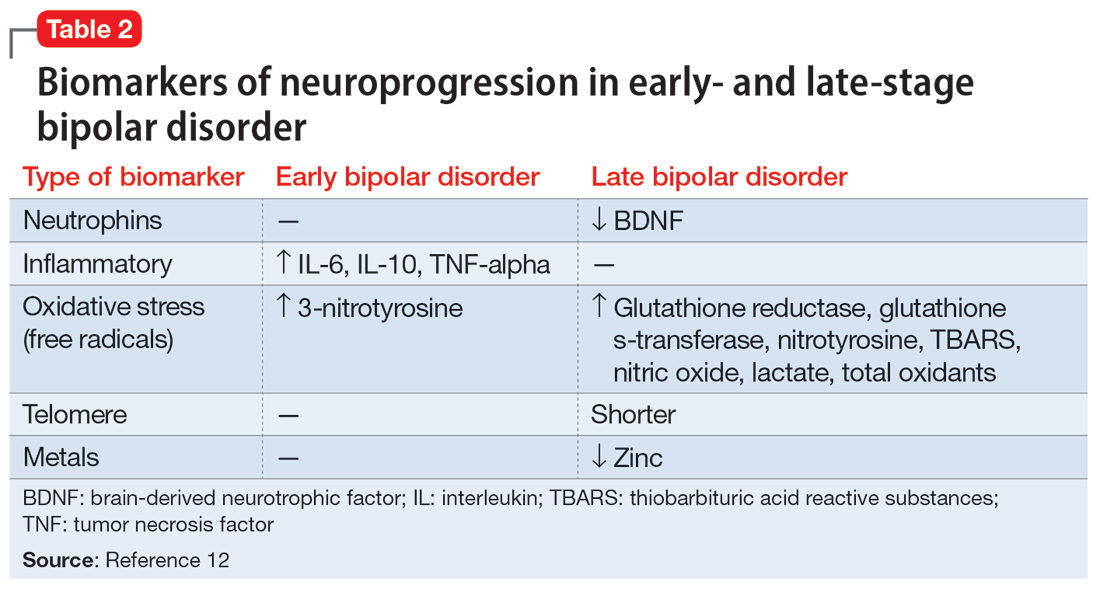
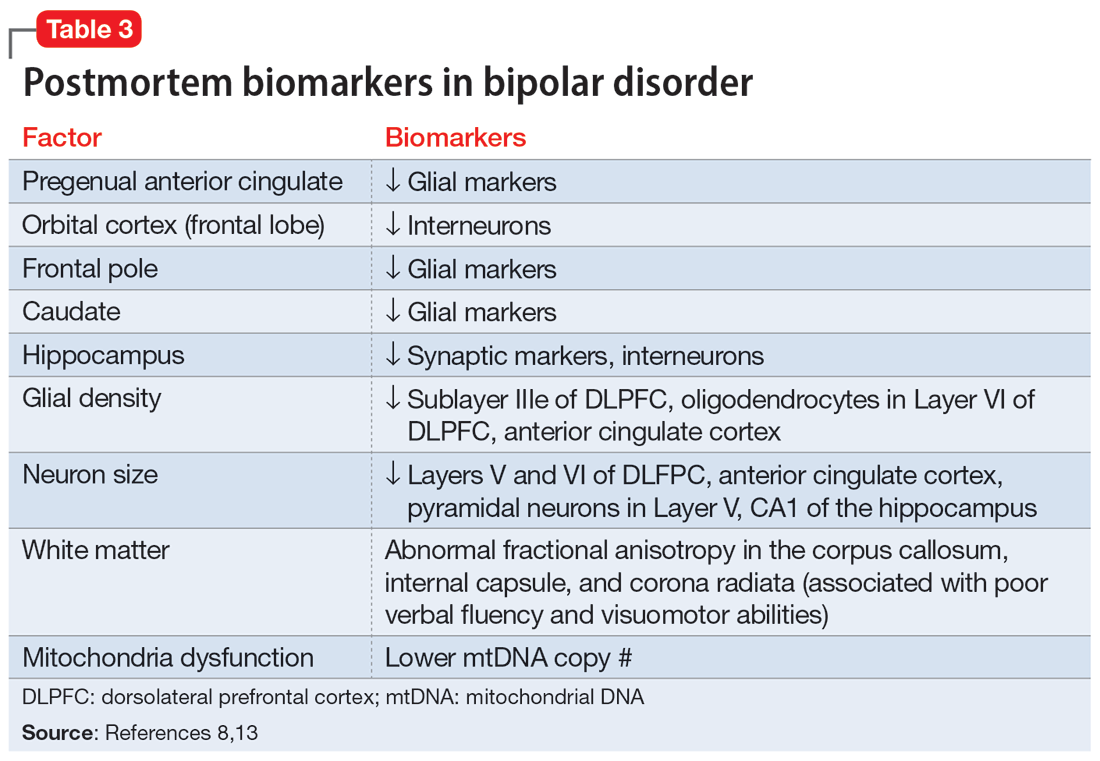
Biomarkers have been reported in both the early and late stages of BD (Table 212) as well as in postmortem studies (Table 38,13). They reflect the progressive neurodegenerative nature of recurrent BD I episodes as the disorder moves to the advanced stages. I summarize these stages in Table 19 and Table 212 for the benefit of psychiatric clinicians who do not have access to the neuroscience journals where such findings are usually published.
BD I is also believed to be associated with accelerated aging14,15 and an increased risk for dementia16 or cognitive deterioration.17 There is also an emerging hypothesis that neuroprogression and treatment resistance in BD is frequently associated with insulin resistance,18 peripheral inflammation,19 and blood-brain barrier permeability dysfunction.20
The bottom line is that like patients with schizophrenia, where relapses lead to devastating consequences,21 those with BD are at a similar high risk for neuroprogression, which includes atrophy in several brain regions, treatment resistance, and functional disability. This underscores the urgency for implementing LAI therapy early in the illness, when the first manic episode (Stage 2) emerges after the prodrome (Stage 1). This is the best strategy to preserve brain health in persons with BD22 and to allow them to remain functional with their many intellectual gifts, such as eloquence, poetry, artistic talents, humor, and social skills. It is unfortunate that the combination of patients’ and clinicians’ reluctance to use an LAI early in the illness dooms many patients with BD to a potentially avoidable malignant outcome.
1. Strakowski SM, DelBello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10(1):105-106.
2. Kapezinski NS, Mwangi B, Cassidy RM, et al. Neuroprogression and illness trajectories in bipolar disorder. Expert Rev Neurother. 2017;17(3):277-285.
3. Nasrallah HA. Errors of omission and commission in psychiatric practice. Current Psychiatry. 2017;16(11):4,6,8.
4. Nasrallah HA. Is anosognosia a delusion, a negative symptom, or a cognitive deficit? Current Psychiatry. 2022;21(1):6-8,14.
5. Post RM. Preventing the malignant transformation of bipolar disorder. JAMA. 2018;319(12):1197-1198.
6. Berk M, Kapczinski F, Andreazza AC, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35(3):804-817.
7. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cerebral ventricular enlargement in young manic males. A controlled CT study. J Affective Dis. 1982;4(1):15-19.
8. Maletic V, Raison C. Integrated neurobiology of bipolar disorder. Front Psychiatry. 2014;5:98.
9. Berk M. Neuroprogression: pathways to progressive brain changes in bipolar disorder. Int J Neuropsychopharmacol. 2009;12(4):441-445.
10. Berk M, Conus P, Kapczinski F, et al. From neuroprogression to neuroprotection: implications for clinical care. Med J Aust. 2010;193(S4):S36-S40.
11. Passos IC, Mwangi B, Vieta E, et al. Areas of controversy in neuroprogression in bipolar disorder. Acta Psychiatr Scand. 2016;134(2):91-103.
12. Fries GR, Pfaffenseller B, Stertz L, et al. Staging and neuroprogression in bipolar disorder. Curr Psychiatry Rep. 2012;14(6):667-675.
13. Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7(5):541-547.
14. Fries GR, Zamzow MJ, Andrews T, et al. Accelerated aging in bipolar disorder: a comprehensive review of molecular findings and their clinical implications. Neurosci Biobehav Rev. 2020;112:107-116.
15. Fries GR, Bauer IE, Scaini G, et al. Accelerated hippocampal biological aging in bipolar disorder. Bipolar Dis. 2020;22(5):498-507.
16. Diniz BS, Teixeira AL, Cao F, et al. History of bipolar disorder and the risk of dementia: a systematic review and meta-analysis. Am J Geriatr Psychiatry. 2017;25(4):357-362.
17. Bauer IE, Ouyang A, Mwangi B, et al. Reduced white matter integrity and verbal fluency impairment in young adults with bipolar disorder: a diffusion tensor imaging study. J Psychiatr Res. 2015;62:115-122.
18. Calkin CV. Insulin resistance takes center stage: a new paradigm in the progression of bipolar disorder. Ann Med. 2019;51(5-6):281-293.
19. Grewal S, McKinlay S, Kapczinski F, et al. Biomarkers of neuroprogression and late staging in bipolar disorder: a systematic review. Aust N Z J Psychiatry. 2023;57(3):328-343.
20. Calkin C, McClelland C, Cairns K, et al. Insulin resistance and blood-brain barrier dysfunction underlie neuroprogression in bipolar disorder. Front Psychiatry. 2021;12:636174.
21. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
22. Berk M, Hallam K, Malhi GS, et al. Evidence and implications for early intervention in bipolar disorder. J Ment Health. 2010;19(2):113-126.
Bipolar disorder (BD) is a psychotic mood disorder. Like schizophrenia, it has been shown to be associated with significant degeneration and structural brain abnormalities with multiple relapses.1,2
Just as I have always advocated preventing recurrences in schizophrenia by using long-acting injectable (LAI) antipsychotic formulations immediately after the first episode to prevent psychotic relapses and progressive brain damage,3 I strongly recommend using LAIs right after hospital discharge from the first manic episode. It is the most rational management approach for bipolar mania given the grave consequences of multiple episodes, which are so common in this psychotic mood disorder due to poor medication adherence.
In contrast to the depressive episodes of BD I, where patients have insight into their depression and seek psychiatric treatment, during a manic episode patients often have no insight (anosognosia) that they suffer from a serious brain disorder, and refuse treatment.4 In addition, young patients with BD I frequently discontinue their oral mood stabilizer or second-generation antipsychotic (which are approved for mania) because they miss the blissful euphoria and the buoyant physical and mental energy of their manic episodes. They are completely oblivious to (and uninformed about) the grave neurobiological damage of further manic episodes, which can condemn them to clinical, functional, and cognitive deterioration. These patients are also likely to become treatment-resistant, which has been labeled as “the malignant transformation of bipolar disorder.”5
The evidence for progressive brain tissue loss, clinical deterioration, functional decline, and treatment resistance is abundant.6 I was the lead investigator of the first study to report ventricular dilatation (which is a proxy for cortical atrophy) in bipolar mania,7 a discovery that was subsequently replicated by 2 dozen researchers. This was followed by numerous neuroimaging studies reporting a loss of volume across multiple brain regions, including the frontal lobe, temporal lobe, cerebellum, thalamus, hippocampus, and basal ganglia. BD is heterogeneous8 with 4 stages (Table 19), and patients experience progressively worse brain structure and function with each stage.
Many patients with bipolar mania end up with poor clinical and functional outcomes, even when they respond well to initial treatment with lithium, anticonvulsant mood stabilizers, or second-generation antipsychotics. With their intentional nonadherence to oral medications leading to multiple recurrent relapses, these patients are at serious risk for neuroprogression and brain atrophic changes driven by multiple factors: inflammatory cytokines, increased cortical steroids, decreased neurotrophins, deceased neurogenesis, increased oxidative stress, and mitochondrial energy dysfunction. The consequences include progressive shortening of the interval between episodes with every relapse and loss of responsiveness to pharmacotherapy as the illness progresses.6,10 Predictors of a downhill progression include genetic vulnerability, perinatal complication during fetal life, childhood trauma (physical, sexual, emotional, or neglect), substance use, stress, psychiatric/medial comorbidities, and especially the number of episodes.9,11

Biomarkers have been reported in both the early and late stages of BD (Table 212) as well as in postmortem studies (Table 38,13). They reflect the progressive neurodegenerative nature of recurrent BD I episodes as the disorder moves to the advanced stages. I summarize these stages in Table 19 and Table 212 for the benefit of psychiatric clinicians who do not have access to the neuroscience journals where such findings are usually published.

BD I is also believed to be associated with accelerated aging14,15 and an increased risk for dementia16 or cognitive deterioration.17 There is also an emerging hypothesis that neuroprogression and treatment resistance in BD is frequently associated with insulin resistance,18 peripheral inflammation,19 and blood-brain barrier permeability dysfunction.20
The bottom line is that like patients with schizophrenia, where relapses lead to devastating consequences,21 those with BD are at a similar high risk for neuroprogression, which includes atrophy in several brain regions, treatment resistance, and functional disability. This underscores the urgency for implementing LAI therapy early in the illness, when the first manic episode (Stage 2) emerges after the prodrome (Stage 1). This is the best strategy to preserve brain health in persons with BD22 and to allow them to remain functional with their many intellectual gifts, such as eloquence, poetry, artistic talents, humor, and social skills. It is unfortunate that the combination of patients’ and clinicians’ reluctance to use an LAI early in the illness dooms many patients with BD to a potentially avoidable malignant outcome.
Bipolar disorder (BD) is a psychotic mood disorder. Like schizophrenia, it has been shown to be associated with significant degeneration and structural brain abnormalities with multiple relapses.1,2
Just as I have always advocated preventing recurrences in schizophrenia by using long-acting injectable (LAI) antipsychotic formulations immediately after the first episode to prevent psychotic relapses and progressive brain damage,3 I strongly recommend using LAIs right after hospital discharge from the first manic episode. It is the most rational management approach for bipolar mania given the grave consequences of multiple episodes, which are so common in this psychotic mood disorder due to poor medication adherence.
In contrast to the depressive episodes of BD I, where patients have insight into their depression and seek psychiatric treatment, during a manic episode patients often have no insight (anosognosia) that they suffer from a serious brain disorder, and refuse treatment.4 In addition, young patients with BD I frequently discontinue their oral mood stabilizer or second-generation antipsychotic (which are approved for mania) because they miss the blissful euphoria and the buoyant physical and mental energy of their manic episodes. They are completely oblivious to (and uninformed about) the grave neurobiological damage of further manic episodes, which can condemn them to clinical, functional, and cognitive deterioration. These patients are also likely to become treatment-resistant, which has been labeled as “the malignant transformation of bipolar disorder.”5
The evidence for progressive brain tissue loss, clinical deterioration, functional decline, and treatment resistance is abundant.6 I was the lead investigator of the first study to report ventricular dilatation (which is a proxy for cortical atrophy) in bipolar mania,7 a discovery that was subsequently replicated by 2 dozen researchers. This was followed by numerous neuroimaging studies reporting a loss of volume across multiple brain regions, including the frontal lobe, temporal lobe, cerebellum, thalamus, hippocampus, and basal ganglia. BD is heterogeneous8 with 4 stages (Table 19), and patients experience progressively worse brain structure and function with each stage.
Many patients with bipolar mania end up with poor clinical and functional outcomes, even when they respond well to initial treatment with lithium, anticonvulsant mood stabilizers, or second-generation antipsychotics. With their intentional nonadherence to oral medications leading to multiple recurrent relapses, these patients are at serious risk for neuroprogression and brain atrophic changes driven by multiple factors: inflammatory cytokines, increased cortical steroids, decreased neurotrophins, deceased neurogenesis, increased oxidative stress, and mitochondrial energy dysfunction. The consequences include progressive shortening of the interval between episodes with every relapse and loss of responsiveness to pharmacotherapy as the illness progresses.6,10 Predictors of a downhill progression include genetic vulnerability, perinatal complication during fetal life, childhood trauma (physical, sexual, emotional, or neglect), substance use, stress, psychiatric/medial comorbidities, and especially the number of episodes.9,11

Biomarkers have been reported in both the early and late stages of BD (Table 212) as well as in postmortem studies (Table 38,13). They reflect the progressive neurodegenerative nature of recurrent BD I episodes as the disorder moves to the advanced stages. I summarize these stages in Table 19 and Table 212 for the benefit of psychiatric clinicians who do not have access to the neuroscience journals where such findings are usually published.

BD I is also believed to be associated with accelerated aging14,15 and an increased risk for dementia16 or cognitive deterioration.17 There is also an emerging hypothesis that neuroprogression and treatment resistance in BD is frequently associated with insulin resistance,18 peripheral inflammation,19 and blood-brain barrier permeability dysfunction.20
The bottom line is that like patients with schizophrenia, where relapses lead to devastating consequences,21 those with BD are at a similar high risk for neuroprogression, which includes atrophy in several brain regions, treatment resistance, and functional disability. This underscores the urgency for implementing LAI therapy early in the illness, when the first manic episode (Stage 2) emerges after the prodrome (Stage 1). This is the best strategy to preserve brain health in persons with BD22 and to allow them to remain functional with their many intellectual gifts, such as eloquence, poetry, artistic talents, humor, and social skills. It is unfortunate that the combination of patients’ and clinicians’ reluctance to use an LAI early in the illness dooms many patients with BD to a potentially avoidable malignant outcome.
1. Strakowski SM, DelBello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10(1):105-106.
2. Kapezinski NS, Mwangi B, Cassidy RM, et al. Neuroprogression and illness trajectories in bipolar disorder. Expert Rev Neurother. 2017;17(3):277-285.
3. Nasrallah HA. Errors of omission and commission in psychiatric practice. Current Psychiatry. 2017;16(11):4,6,8.
4. Nasrallah HA. Is anosognosia a delusion, a negative symptom, or a cognitive deficit? Current Psychiatry. 2022;21(1):6-8,14.
5. Post RM. Preventing the malignant transformation of bipolar disorder. JAMA. 2018;319(12):1197-1198.
6. Berk M, Kapczinski F, Andreazza AC, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35(3):804-817.
7. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cerebral ventricular enlargement in young manic males. A controlled CT study. J Affective Dis. 1982;4(1):15-19.
8. Maletic V, Raison C. Integrated neurobiology of bipolar disorder. Front Psychiatry. 2014;5:98.
9. Berk M. Neuroprogression: pathways to progressive brain changes in bipolar disorder. Int J Neuropsychopharmacol. 2009;12(4):441-445.
10. Berk M, Conus P, Kapczinski F, et al. From neuroprogression to neuroprotection: implications for clinical care. Med J Aust. 2010;193(S4):S36-S40.
11. Passos IC, Mwangi B, Vieta E, et al. Areas of controversy in neuroprogression in bipolar disorder. Acta Psychiatr Scand. 2016;134(2):91-103.
12. Fries GR, Pfaffenseller B, Stertz L, et al. Staging and neuroprogression in bipolar disorder. Curr Psychiatry Rep. 2012;14(6):667-675.
13. Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7(5):541-547.
14. Fries GR, Zamzow MJ, Andrews T, et al. Accelerated aging in bipolar disorder: a comprehensive review of molecular findings and their clinical implications. Neurosci Biobehav Rev. 2020;112:107-116.
15. Fries GR, Bauer IE, Scaini G, et al. Accelerated hippocampal biological aging in bipolar disorder. Bipolar Dis. 2020;22(5):498-507.
16. Diniz BS, Teixeira AL, Cao F, et al. History of bipolar disorder and the risk of dementia: a systematic review and meta-analysis. Am J Geriatr Psychiatry. 2017;25(4):357-362.
17. Bauer IE, Ouyang A, Mwangi B, et al. Reduced white matter integrity and verbal fluency impairment in young adults with bipolar disorder: a diffusion tensor imaging study. J Psychiatr Res. 2015;62:115-122.
18. Calkin CV. Insulin resistance takes center stage: a new paradigm in the progression of bipolar disorder. Ann Med. 2019;51(5-6):281-293.
19. Grewal S, McKinlay S, Kapczinski F, et al. Biomarkers of neuroprogression and late staging in bipolar disorder: a systematic review. Aust N Z J Psychiatry. 2023;57(3):328-343.
20. Calkin C, McClelland C, Cairns K, et al. Insulin resistance and blood-brain barrier dysfunction underlie neuroprogression in bipolar disorder. Front Psychiatry. 2021;12:636174.
21. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
22. Berk M, Hallam K, Malhi GS, et al. Evidence and implications for early intervention in bipolar disorder. J Ment Health. 2010;19(2):113-126.
1. Strakowski SM, DelBello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10(1):105-106.
2. Kapezinski NS, Mwangi B, Cassidy RM, et al. Neuroprogression and illness trajectories in bipolar disorder. Expert Rev Neurother. 2017;17(3):277-285.
3. Nasrallah HA. Errors of omission and commission in psychiatric practice. Current Psychiatry. 2017;16(11):4,6,8.
4. Nasrallah HA. Is anosognosia a delusion, a negative symptom, or a cognitive deficit? Current Psychiatry. 2022;21(1):6-8,14.
5. Post RM. Preventing the malignant transformation of bipolar disorder. JAMA. 2018;319(12):1197-1198.
6. Berk M, Kapczinski F, Andreazza AC, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35(3):804-817.
7. Nasrallah HA, McCalley-Whitters M, Jacoby CG. Cerebral ventricular enlargement in young manic males. A controlled CT study. J Affective Dis. 1982;4(1):15-19.
8. Maletic V, Raison C. Integrated neurobiology of bipolar disorder. Front Psychiatry. 2014;5:98.
9. Berk M. Neuroprogression: pathways to progressive brain changes in bipolar disorder. Int J Neuropsychopharmacol. 2009;12(4):441-445.
10. Berk M, Conus P, Kapczinski F, et al. From neuroprogression to neuroprotection: implications for clinical care. Med J Aust. 2010;193(S4):S36-S40.
11. Passos IC, Mwangi B, Vieta E, et al. Areas of controversy in neuroprogression in bipolar disorder. Acta Psychiatr Scand. 2016;134(2):91-103.
12. Fries GR, Pfaffenseller B, Stertz L, et al. Staging and neuroprogression in bipolar disorder. Curr Psychiatry Rep. 2012;14(6):667-675.
13. Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7(5):541-547.
14. Fries GR, Zamzow MJ, Andrews T, et al. Accelerated aging in bipolar disorder: a comprehensive review of molecular findings and their clinical implications. Neurosci Biobehav Rev. 2020;112:107-116.
15. Fries GR, Bauer IE, Scaini G, et al. Accelerated hippocampal biological aging in bipolar disorder. Bipolar Dis. 2020;22(5):498-507.
16. Diniz BS, Teixeira AL, Cao F, et al. History of bipolar disorder and the risk of dementia: a systematic review and meta-analysis. Am J Geriatr Psychiatry. 2017;25(4):357-362.
17. Bauer IE, Ouyang A, Mwangi B, et al. Reduced white matter integrity and verbal fluency impairment in young adults with bipolar disorder: a diffusion tensor imaging study. J Psychiatr Res. 2015;62:115-122.
18. Calkin CV. Insulin resistance takes center stage: a new paradigm in the progression of bipolar disorder. Ann Med. 2019;51(5-6):281-293.
19. Grewal S, McKinlay S, Kapczinski F, et al. Biomarkers of neuroprogression and late staging in bipolar disorder: a systematic review. Aust N Z J Psychiatry. 2023;57(3):328-343.
20. Calkin C, McClelland C, Cairns K, et al. Insulin resistance and blood-brain barrier dysfunction underlie neuroprogression in bipolar disorder. Front Psychiatry. 2021;12:636174.
21. Nasrallah HA. 10 devastating consequences of psychotic relapses. Current Psychiatry. 2021;20(5):9-12.
22. Berk M, Hallam K, Malhi GS, et al. Evidence and implications for early intervention in bipolar disorder. J Ment Health. 2010;19(2):113-126.
Infested with worms, but are they really there?
CASE Detoxification and preoccupation with parasites
Mr. H, age 51, has an extensive history of alcohol and methamphetamine use. He presents to the emergency department (ED) requesting inpatient detoxification. He says he had been drinking alcohol but is unable to say how much. His blood ethanol level is 61 mg/dL (unintoxicated level: <50 mg/dL), and a urine drug screen is positive for methamphetamine; Mr. H also admits to using fentanyl. The ED team treats Mr. H’s electrolyte abnormalities, initiates thiamine supplementation, and transfers him to a unit for inpatient withdrawal management.
On the detoxification unit, Mr. H receives a total of 1,950 mg of phenobarbital for alcohol withdrawal and stabilizes on a buprenorphine/naloxone maintenance dose of 8 mg/2 mg twice daily for methamphetamine and fentanyl use. Though he was not taking any psychiatric medications prior to his arrival at the ED, Mr. H agrees to restart quetiapine
During Mr. H’s 3-day detoxification, the psychiatry team evaluates him. Mr. H says he believes he is infested with worms. He describes a prior sensation of “meth mites,” or the feeling of bugs crawling under his skin, while using methamphetamines. However, Mr. H says his current infestation feels distinctively different, and he had continued to experience these
The psychiatry team expresses concern over his preoccupation with infestations, disheveled appearance, poor hygiene, and healed scars from excoriation. Mr. H also reports poor sleep and appetite and was observed writing an incomprehensible “experiment” on a paper towel. Due to his bizarre behavior, delusional thoughts, and concerns about his inability to care for himself, the team admits Mr. H to the acute inpatient psychiatric unit on a voluntary commitment.
HISTORY Long-standing drug use and repeated hospital visits
Mr. H reports a history of drug use. His first documented ED visit was >5 years before his current admission. He has a family history of substance abuse and reports previously using methamphetamine, heroin, and alcohol. Mr. H was never diagnosed with a psychiatric illness, but when he was younger, there were suspicions of bipolar depression, with no contributing family psychiatric history. Though he took quetiapine at an unspecified younger age, Mr. H did not follow through with any outpatient mental health services or medications.
Mr. H first reported infestation
In the 6 months prior to his current admission, Mr. H came to the hospital >20 times for various reasons, including methamphetamine abuse, alcohol withdrawal, opiate overdose, cellulitis, wound checks, and 3 visits for hallucinations for which he requested physical evaluation and medical care. His substance use was the suspected cause of his tactile and visual hallucinations of infestation because formication
Continue to: The authors' observations
The authors’ observations
Delusional parasitosis (DP), also known as delusional infestation or Ekbom Syndrome, is a condition characterized by the fixed, false belief of an infestation without any objective evidence. This condition was previously defined in DSM-IV, but was removed from DSM-5-TR. In DSM-5-TR, DP is most closely associated with delusional disorder
DP is rare, affecting approximately 1.9 per 100,000 people. There has not been consistent data supporting differences in prevalence between sexes, but there is evidence for increasing incidence with age, with a mean age of diagnosis of 61.4.2,3 DP can be divided into 2 types based on the history and etiology of the symptoms: primary DP and secondary DP. Primary DP occurs when there is a failure to identify an organic cause for the occurrence of the symptoms. Therefore, primary DP requires an extensive investigation by a multidisciplinary team that commonly includes medical specialists for a nonpsychiatric workup. Secondary DP occurs when the patient has delusional symptoms associated with a primary diagnosis of schizophrenia, depression, stroke, diabetes, vitamin B12 deficiency, or substance use.4
Though Mr. H initially presented to the ED, patients with DP commonly present to a primary care physician or dermatologist with the complaint of itching or feelings of insects, worms, or unclear organisms inside them. Patients with DP may often develop poor working relationships with physicians while obtaining multiple negative results. They may seek opinions from multiple specialists; however, patients typically do not consider psychiatrists as a source of help. When patients seek psychiatric care, often after a recommendation from a primary care physician or dermatologist, mental health clinicians should listen to and evaluate the patient holistically, continuing to rule out other possible etiologies.
[polldaddy:12570072]
TREATMENT Finding the right antipsychotic
In the psychiatric unit, Mr. H says he believes worms are exiting his ears, mouth, toenail, and self-inflicted scratch wounds. He believes he has been dealing with the parasites for >1 year and they are slowly draining his energy. Mr. H insists he contracted the “infection” from his home carpet, which was wet due to a flood in his house, and after he had fallen asleep following drug use. He also believes he acquired the parasites while walking barefoot along the beach and collecting rocks, and that there are multiple species living inside him, all intelligent enough to hide, making it difficult to prove their existence. He notes they vary in size, and some have red eyes.
During admission, Mr. H voices his frustration that clinicians had not found the worms he has been seeing. He continuously requests to review imaging performed during his visit and wants a multidisciplinary team to evaluate his case. He demands to test a cup with spit-up “samples,” believing the parasites would be visible under a microscope. Throughout his admission, Mr. H continues to take buprenorphine/naloxone and does not experience withdrawal symptoms. The treatment team titrates his quetiapine to 400 mg/d. Due to the lack of improvement, the team initiates olanzapine 5 mg/d at bedtime. However, Mr. H reports significant tinnitus and requests a medication change. He is started on haloperidol 5 mg twice daily.
Continue to: Mr. H begins to see improvements...
Mr. H begins to see improvements on Day 7 of taking haloperidol. He no longer brings up infestation but still acknowledges having worms inside him when directly asked. He says the worms cause him less distress than before and he is hopeful to live without discomfort. He also demonstrates an ability to conduct activities of daily living. Because Mr. H is being monitored on an acute inpatient psychiatric basis, he is deemed appropriate for discharge even though his symptoms have not yet fully resolved. After a 19-day hospital stay, Mr. H is discharged on haloperidol 15 mg/d and quetiapine 200 mg/d.
[polldaddy:12570074]
The authors’ observations
Mr. H asked to have his sputum examined. The “specimen sign,” also called “matchbox sign” or “Ziploc bag sign,” in which patients collect what they believe to be infected tissue or organisms in a container, is a well-studied part of DP.5 Such samples should be considered during initial encounters and can be examined for formal evaluation, but cautiously. Overtesting may incur a financial burden or reinforce deleterious beliefs and behaviors.
It can be difficult to identify triggers of DP. Research shows DP may arise from nonorganic and stressful life events, home floods, or contact with people infected with parasites.6,7 Organic causes have also been found, such as patients taking multiple medications for Parkinson disease who developed delusional symptoms.8 Buscarino et al9 reported the case of a woman who started to develop symptoms of delusions and hallucinations after being on high-dose amphetamines for attention-deficit/hyperactivity disorder. Research shows that stopping the suspected medication commonly improves such symptoms.9,10 Although methamphetamine can remain detectable in urine for up to 4 days after use and potentially a few days longer for chronic users due to circulating levels,11 Mr. H’s symptoms continued for weeks after all substances of abuse should have been cleared from his system. This suggests he was experiencing a psychiatric illness and was accurate in distinguishing methamphetamine-induced from psychiatric-induced sensations. Regardless, polysubstance use has been shown to potentially increase the risk and play a role in the onset and progression of delusional illness, as seen in prior cases as well as in this case.9
It has been hypothesized that the pathophysiology of DP is associated with the deterioration of the striatal dopaminergic pathway, leading to an increase in extracellular dopamine levels. The striatum is responsible for most dopamine reuptake in the brain; therefore, certain drugs such as cocaine, methamphetamine, and methylphenidate may precipitate symptoms of DP due to their blockade of presynaptic dopamine reuptake.12 Additionally, conditions that decrease the functioning of striatal dopamine transporters, such as schizophrenia or depression, may be underlying causes of DP.13
Treatment of DP remains a topic of debate. Most current recommendations appear to be based on a small, nonrandomized placebo-controlled trial.14 The first-generation antipsychotic pimozide had been a first-line treatment for DP, but its adverse effect profile, which includes QTc prolongation and extrapyramidal symptoms, led to the exploration of second-generation antipsychotics such as olanzapine and risperidone.15,16 There is a dearth of literature about the use of haloperidol, quetiapine, or a combination of both as treatment options for DP, though the combination of these 2 medications proved effective for Mr. H. Further research is necessary to justify changes to current treatment standards, but this finding highlights a successful symptom reduction achieved with this combination.
Continue to: Patients may experience genuine symptoms...
Patients may experience genuine symptoms despite the delusional nature of DP, and it is important for clinicians to recognize the potential burden and anxiety these individuals face. Patients may present with self-inflicted bruises, cuts, and erosions to gain access to infected areas, which may be confused with skin picking disorder. Excessive cleansing or use of irritant products can also cause skin damage, leading to other dermatological conditions that reinforce the patient’s belief that something is medically wrong. During treatment, consider medications for relief of pruritus or pain. Focus on offering patients the opportunity to express their concerns, treat them with empathy, avoid stigmatizing language such as “delusions” or “psychosis,” and refrain from contradicting them until a strong rapport has been established (Table 217).
Symptoms of DP can persist for months to years. Patients who fully recovered experienced a median duration of 0.5 years until symptom resolution, compared to incompletely recovered patients, who took approximately 1 year.18 Primary DP has slower improvement rates compared to secondary DP, with the median onset of effects occurring at Week 1.5 and peak improvements occurring at Week 6.16
OUTCOME Continued ED visits
Unfortunately, Mr. H does not follow through with his outpatient psychiatry appointments. In the 7 months following discharge, he visits the ED 8 times for alcohol intoxication, alcohol withdrawal, and methamphetamine abuse, in addition to 2 admissions for inpatient detoxification, during which he was still receiving the same scheduled medications (haloperidol 15 mg/d and quetiapine 200 mg/d). At each of his ED visits, there was no documentation of DP symptoms, which suggests his symptoms may have resolved.
Bottom Line
Because delusional parasitosis symptoms feel real to patients, it is crucial to build rapport to recommend and successfully initiate treatment. After ruling out nonpsychiatric etiologies, consider traditional treatment with antipsychotics, and consider medications for relief of pruritus or pain.
Related Resources
- Sellman D, Phan SV, Inyang M. Bugs on her skin—but nobody else sees them. Current Psychiatry. 2018;17(8):48,50-53.
- Campbell EH, Elston DM, Hawthorne JD, et al. Diagnosis and management of delusional parasitosis. J Am Acad Dermatol. 2019;80(5):1428-1434. doi:10.1016/j.jaad.2018.12.012
Drug Brand Names
Buprenorphine/naloxone • Suboxone
Haloperidol • Haldol
Hydroxyzine • Vistaril
Lithium • Eskalith, Lithobid
Methylphenidate • Concerta
Olanzapine • Zyprexa
Permethrin • Elimite
Phenobarbital • Solfoton, Tedral, Luminal
Pimozide • Orap
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakote
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2013.
2. Bailey CH, Andersen LK, Lowe GC, et al. A population-based study of the incidence of delusional infestation in Olmsted County, Minnesota, 1976-2010. Br J Dermatol. 2014;170(5):1130-1135. doi:10.1111/bjd.12848
3. Kohorst JJ, Bailey CH, Andersen LK, et al. Prevalence of delusional infestation-a population-based study. JAMA Dermatol. 2018;154(5):615-617. doi:10.1001/jamadermatol.2018.0004
4. Freinhar JP. Delusions of parasitosis. Psychosomatics. 1984;25(1):47-53. doi:10.1016/S0033-3182(84)73096-9
5. Reich A, Kwiatkowska D, Pacan P. Delusions of parasitosis: an update. Dermatol Ther (Heidelb). 2019;9(4):631-638. doi:10.1007/s13555-019-00324-3
6. Berrios GE. Delusional parasitosis and physical disease. Compr Psychiatry. 1985;26(5):395-403. doi:10.1016/0010-440x(85)90077-x
7. Aizenberg D, Schwartz B, Zemishlany Z. Delusional parasitosis associated with phenelzine. Br J Psychiatry. 1991;159:716-717. doi:10.1192/bjp.159.5.716
8. Flann S, Shotbolt J, Kessel B, et al. Three cases of delusional parasitosis caused by dopamine agonists. Clin Exp Dermatol. 2010;35(7):740-742. doi:10.1111/j.1365-2230.2010.03810.x
9. Buscarino M, Saal J, Young JL. Delusional parasitosis in a female treated with mixed amphetamine salts: a case report and literature review. Case Rep Psychiatry. 2012;2012:624235. doi:10.1155/2012/624235
10. Elpern DJ. Cocaine abuse and delusions of parasitosis. Cutis. 1988;42(4):273-274.
11. Richards JR, Laurin EG. Methamphetamine toxicity. StatPearls Publishing; 2023. Updated January 8, 2023. Accessed May 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK430895/
12. Huber M, Kirchler E, Karner M, et al. Delusional parasitosis and the dopamine transporter. A new insight of etiology? Med Hypotheses. 2007;68(6):1351-1358. doi:10.1016/j.mehy.2006.07.061
13. Lipman ZM, Yosipovitch G. Substance use disorders and chronic itch. J Am Acad Dermatol. 2021;84(1):148-155. doi:10.1016/j.jaad.2020.08.117
14. Kenchaiah BK, Kumar S, Tharyan P. Atypical anti-psychotics in delusional parasitosis: a retrospective case series of 20 patients. Int J Dermatol. 2010;49(1):95-100. doi:10.1111/j.1365-4632.2009.04312.x
15. Laidler N. Delusions of parasitosis: a brief review of the literature and pathway for diagnosis and treatment. Dermatol Online J. 2018;24(1):13030/qt1fh739nx.
16. Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis: outcome and efficacy. J Clin Psychopharmacol. 2008;28(5):500-508. doi:10.1097/JCP.0b013e318185e774
17. Mumcuoglu KY, Leibovici V, Reuveni I, et al. Delusional parasitosis: diagnosis and treatment. Isr Med Assoc J. 2018;20(7):456-460.
18. Trabert W. 100 years of delusional parasitosis. Meta-analysis of 1,223 case reports. Psychopathology. 1995;28(5):238-246. doi:10.1159/000284934
CASE Detoxification and preoccupation with parasites
Mr. H, age 51, has an extensive history of alcohol and methamphetamine use. He presents to the emergency department (ED) requesting inpatient detoxification. He says he had been drinking alcohol but is unable to say how much. His blood ethanol level is 61 mg/dL (unintoxicated level: <50 mg/dL), and a urine drug screen is positive for methamphetamine; Mr. H also admits to using fentanyl. The ED team treats Mr. H’s electrolyte abnormalities, initiates thiamine supplementation, and transfers him to a unit for inpatient withdrawal management.
On the detoxification unit, Mr. H receives a total of 1,950 mg of phenobarbital for alcohol withdrawal and stabilizes on a buprenorphine/naloxone maintenance dose of 8 mg/2 mg twice daily for methamphetamine and fentanyl use. Though he was not taking any psychiatric medications prior to his arrival at the ED, Mr. H agrees to restart quetiapine
During Mr. H’s 3-day detoxification, the psychiatry team evaluates him. Mr. H says he believes he is infested with worms. He describes a prior sensation of “meth mites,” or the feeling of bugs crawling under his skin, while using methamphetamines. However, Mr. H says his current infestation feels distinctively different, and he had continued to experience these
The psychiatry team expresses concern over his preoccupation with infestations, disheveled appearance, poor hygiene, and healed scars from excoriation. Mr. H also reports poor sleep and appetite and was observed writing an incomprehensible “experiment” on a paper towel. Due to his bizarre behavior, delusional thoughts, and concerns about his inability to care for himself, the team admits Mr. H to the acute inpatient psychiatric unit on a voluntary commitment.
HISTORY Long-standing drug use and repeated hospital visits
Mr. H reports a history of drug use. His first documented ED visit was >5 years before his current admission. He has a family history of substance abuse and reports previously using methamphetamine, heroin, and alcohol. Mr. H was never diagnosed with a psychiatric illness, but when he was younger, there were suspicions of bipolar depression, with no contributing family psychiatric history. Though he took quetiapine at an unspecified younger age, Mr. H did not follow through with any outpatient mental health services or medications.
Mr. H first reported infestation
In the 6 months prior to his current admission, Mr. H came to the hospital >20 times for various reasons, including methamphetamine abuse, alcohol withdrawal, opiate overdose, cellulitis, wound checks, and 3 visits for hallucinations for which he requested physical evaluation and medical care. His substance use was the suspected cause of his tactile and visual hallucinations of infestation because formication
Continue to: The authors' observations
The authors’ observations
Delusional parasitosis (DP), also known as delusional infestation or Ekbom Syndrome, is a condition characterized by the fixed, false belief of an infestation without any objective evidence. This condition was previously defined in DSM-IV, but was removed from DSM-5-TR. In DSM-5-TR, DP is most closely associated with delusional disorder
DP is rare, affecting approximately 1.9 per 100,000 people. There has not been consistent data supporting differences in prevalence between sexes, but there is evidence for increasing incidence with age, with a mean age of diagnosis of 61.4.2,3 DP can be divided into 2 types based on the history and etiology of the symptoms: primary DP and secondary DP. Primary DP occurs when there is a failure to identify an organic cause for the occurrence of the symptoms. Therefore, primary DP requires an extensive investigation by a multidisciplinary team that commonly includes medical specialists for a nonpsychiatric workup. Secondary DP occurs when the patient has delusional symptoms associated with a primary diagnosis of schizophrenia, depression, stroke, diabetes, vitamin B12 deficiency, or substance use.4
Though Mr. H initially presented to the ED, patients with DP commonly present to a primary care physician or dermatologist with the complaint of itching or feelings of insects, worms, or unclear organisms inside them. Patients with DP may often develop poor working relationships with physicians while obtaining multiple negative results. They may seek opinions from multiple specialists; however, patients typically do not consider psychiatrists as a source of help. When patients seek psychiatric care, often after a recommendation from a primary care physician or dermatologist, mental health clinicians should listen to and evaluate the patient holistically, continuing to rule out other possible etiologies.
[polldaddy:12570072]
TREATMENT Finding the right antipsychotic
In the psychiatric unit, Mr. H says he believes worms are exiting his ears, mouth, toenail, and self-inflicted scratch wounds. He believes he has been dealing with the parasites for >1 year and they are slowly draining his energy. Mr. H insists he contracted the “infection” from his home carpet, which was wet due to a flood in his house, and after he had fallen asleep following drug use. He also believes he acquired the parasites while walking barefoot along the beach and collecting rocks, and that there are multiple species living inside him, all intelligent enough to hide, making it difficult to prove their existence. He notes they vary in size, and some have red eyes.
During admission, Mr. H voices his frustration that clinicians had not found the worms he has been seeing. He continuously requests to review imaging performed during his visit and wants a multidisciplinary team to evaluate his case. He demands to test a cup with spit-up “samples,” believing the parasites would be visible under a microscope. Throughout his admission, Mr. H continues to take buprenorphine/naloxone and does not experience withdrawal symptoms. The treatment team titrates his quetiapine to 400 mg/d. Due to the lack of improvement, the team initiates olanzapine 5 mg/d at bedtime. However, Mr. H reports significant tinnitus and requests a medication change. He is started on haloperidol 5 mg twice daily.
Continue to: Mr. H begins to see improvements...
Mr. H begins to see improvements on Day 7 of taking haloperidol. He no longer brings up infestation but still acknowledges having worms inside him when directly asked. He says the worms cause him less distress than before and he is hopeful to live without discomfort. He also demonstrates an ability to conduct activities of daily living. Because Mr. H is being monitored on an acute inpatient psychiatric basis, he is deemed appropriate for discharge even though his symptoms have not yet fully resolved. After a 19-day hospital stay, Mr. H is discharged on haloperidol 15 mg/d and quetiapine 200 mg/d.
[polldaddy:12570074]
The authors’ observations
Mr. H asked to have his sputum examined. The “specimen sign,” also called “matchbox sign” or “Ziploc bag sign,” in which patients collect what they believe to be infected tissue or organisms in a container, is a well-studied part of DP.5 Such samples should be considered during initial encounters and can be examined for formal evaluation, but cautiously. Overtesting may incur a financial burden or reinforce deleterious beliefs and behaviors.
It can be difficult to identify triggers of DP. Research shows DP may arise from nonorganic and stressful life events, home floods, or contact with people infected with parasites.6,7 Organic causes have also been found, such as patients taking multiple medications for Parkinson disease who developed delusional symptoms.8 Buscarino et al9 reported the case of a woman who started to develop symptoms of delusions and hallucinations after being on high-dose amphetamines for attention-deficit/hyperactivity disorder. Research shows that stopping the suspected medication commonly improves such symptoms.9,10 Although methamphetamine can remain detectable in urine for up to 4 days after use and potentially a few days longer for chronic users due to circulating levels,11 Mr. H’s symptoms continued for weeks after all substances of abuse should have been cleared from his system. This suggests he was experiencing a psychiatric illness and was accurate in distinguishing methamphetamine-induced from psychiatric-induced sensations. Regardless, polysubstance use has been shown to potentially increase the risk and play a role in the onset and progression of delusional illness, as seen in prior cases as well as in this case.9
It has been hypothesized that the pathophysiology of DP is associated with the deterioration of the striatal dopaminergic pathway, leading to an increase in extracellular dopamine levels. The striatum is responsible for most dopamine reuptake in the brain; therefore, certain drugs such as cocaine, methamphetamine, and methylphenidate may precipitate symptoms of DP due to their blockade of presynaptic dopamine reuptake.12 Additionally, conditions that decrease the functioning of striatal dopamine transporters, such as schizophrenia or depression, may be underlying causes of DP.13
Treatment of DP remains a topic of debate. Most current recommendations appear to be based on a small, nonrandomized placebo-controlled trial.14 The first-generation antipsychotic pimozide had been a first-line treatment for DP, but its adverse effect profile, which includes QTc prolongation and extrapyramidal symptoms, led to the exploration of second-generation antipsychotics such as olanzapine and risperidone.15,16 There is a dearth of literature about the use of haloperidol, quetiapine, or a combination of both as treatment options for DP, though the combination of these 2 medications proved effective for Mr. H. Further research is necessary to justify changes to current treatment standards, but this finding highlights a successful symptom reduction achieved with this combination.
Continue to: Patients may experience genuine symptoms...
Patients may experience genuine symptoms despite the delusional nature of DP, and it is important for clinicians to recognize the potential burden and anxiety these individuals face. Patients may present with self-inflicted bruises, cuts, and erosions to gain access to infected areas, which may be confused with skin picking disorder. Excessive cleansing or use of irritant products can also cause skin damage, leading to other dermatological conditions that reinforce the patient’s belief that something is medically wrong. During treatment, consider medications for relief of pruritus or pain. Focus on offering patients the opportunity to express their concerns, treat them with empathy, avoid stigmatizing language such as “delusions” or “psychosis,” and refrain from contradicting them until a strong rapport has been established (Table 217).
Symptoms of DP can persist for months to years. Patients who fully recovered experienced a median duration of 0.5 years until symptom resolution, compared to incompletely recovered patients, who took approximately 1 year.18 Primary DP has slower improvement rates compared to secondary DP, with the median onset of effects occurring at Week 1.5 and peak improvements occurring at Week 6.16
OUTCOME Continued ED visits
Unfortunately, Mr. H does not follow through with his outpatient psychiatry appointments. In the 7 months following discharge, he visits the ED 8 times for alcohol intoxication, alcohol withdrawal, and methamphetamine abuse, in addition to 2 admissions for inpatient detoxification, during which he was still receiving the same scheduled medications (haloperidol 15 mg/d and quetiapine 200 mg/d). At each of his ED visits, there was no documentation of DP symptoms, which suggests his symptoms may have resolved.
Bottom Line
Because delusional parasitosis symptoms feel real to patients, it is crucial to build rapport to recommend and successfully initiate treatment. After ruling out nonpsychiatric etiologies, consider traditional treatment with antipsychotics, and consider medications for relief of pruritus or pain.
Related Resources
- Sellman D, Phan SV, Inyang M. Bugs on her skin—but nobody else sees them. Current Psychiatry. 2018;17(8):48,50-53.
- Campbell EH, Elston DM, Hawthorne JD, et al. Diagnosis and management of delusional parasitosis. J Am Acad Dermatol. 2019;80(5):1428-1434. doi:10.1016/j.jaad.2018.12.012
Drug Brand Names
Buprenorphine/naloxone • Suboxone
Haloperidol • Haldol
Hydroxyzine • Vistaril
Lithium • Eskalith, Lithobid
Methylphenidate • Concerta
Olanzapine • Zyprexa
Permethrin • Elimite
Phenobarbital • Solfoton, Tedral, Luminal
Pimozide • Orap
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakote
CASE Detoxification and preoccupation with parasites
Mr. H, age 51, has an extensive history of alcohol and methamphetamine use. He presents to the emergency department (ED) requesting inpatient detoxification. He says he had been drinking alcohol but is unable to say how much. His blood ethanol level is 61 mg/dL (unintoxicated level: <50 mg/dL), and a urine drug screen is positive for methamphetamine; Mr. H also admits to using fentanyl. The ED team treats Mr. H’s electrolyte abnormalities, initiates thiamine supplementation, and transfers him to a unit for inpatient withdrawal management.
On the detoxification unit, Mr. H receives a total of 1,950 mg of phenobarbital for alcohol withdrawal and stabilizes on a buprenorphine/naloxone maintenance dose of 8 mg/2 mg twice daily for methamphetamine and fentanyl use. Though he was not taking any psychiatric medications prior to his arrival at the ED, Mr. H agrees to restart quetiapine
During Mr. H’s 3-day detoxification, the psychiatry team evaluates him. Mr. H says he believes he is infested with worms. He describes a prior sensation of “meth mites,” or the feeling of bugs crawling under his skin, while using methamphetamines. However, Mr. H says his current infestation feels distinctively different, and he had continued to experience these
The psychiatry team expresses concern over his preoccupation with infestations, disheveled appearance, poor hygiene, and healed scars from excoriation. Mr. H also reports poor sleep and appetite and was observed writing an incomprehensible “experiment” on a paper towel. Due to his bizarre behavior, delusional thoughts, and concerns about his inability to care for himself, the team admits Mr. H to the acute inpatient psychiatric unit on a voluntary commitment.
HISTORY Long-standing drug use and repeated hospital visits
Mr. H reports a history of drug use. His first documented ED visit was >5 years before his current admission. He has a family history of substance abuse and reports previously using methamphetamine, heroin, and alcohol. Mr. H was never diagnosed with a psychiatric illness, but when he was younger, there were suspicions of bipolar depression, with no contributing family psychiatric history. Though he took quetiapine at an unspecified younger age, Mr. H did not follow through with any outpatient mental health services or medications.
Mr. H first reported infestation
In the 6 months prior to his current admission, Mr. H came to the hospital >20 times for various reasons, including methamphetamine abuse, alcohol withdrawal, opiate overdose, cellulitis, wound checks, and 3 visits for hallucinations for which he requested physical evaluation and medical care. His substance use was the suspected cause of his tactile and visual hallucinations of infestation because formication
Continue to: The authors' observations
The authors’ observations
Delusional parasitosis (DP), also known as delusional infestation or Ekbom Syndrome, is a condition characterized by the fixed, false belief of an infestation without any objective evidence. This condition was previously defined in DSM-IV, but was removed from DSM-5-TR. In DSM-5-TR, DP is most closely associated with delusional disorder
DP is rare, affecting approximately 1.9 per 100,000 people. There has not been consistent data supporting differences in prevalence between sexes, but there is evidence for increasing incidence with age, with a mean age of diagnosis of 61.4.2,3 DP can be divided into 2 types based on the history and etiology of the symptoms: primary DP and secondary DP. Primary DP occurs when there is a failure to identify an organic cause for the occurrence of the symptoms. Therefore, primary DP requires an extensive investigation by a multidisciplinary team that commonly includes medical specialists for a nonpsychiatric workup. Secondary DP occurs when the patient has delusional symptoms associated with a primary diagnosis of schizophrenia, depression, stroke, diabetes, vitamin B12 deficiency, or substance use.4
Though Mr. H initially presented to the ED, patients with DP commonly present to a primary care physician or dermatologist with the complaint of itching or feelings of insects, worms, or unclear organisms inside them. Patients with DP may often develop poor working relationships with physicians while obtaining multiple negative results. They may seek opinions from multiple specialists; however, patients typically do not consider psychiatrists as a source of help. When patients seek psychiatric care, often after a recommendation from a primary care physician or dermatologist, mental health clinicians should listen to and evaluate the patient holistically, continuing to rule out other possible etiologies.
[polldaddy:12570072]
TREATMENT Finding the right antipsychotic
In the psychiatric unit, Mr. H says he believes worms are exiting his ears, mouth, toenail, and self-inflicted scratch wounds. He believes he has been dealing with the parasites for >1 year and they are slowly draining his energy. Mr. H insists he contracted the “infection” from his home carpet, which was wet due to a flood in his house, and after he had fallen asleep following drug use. He also believes he acquired the parasites while walking barefoot along the beach and collecting rocks, and that there are multiple species living inside him, all intelligent enough to hide, making it difficult to prove their existence. He notes they vary in size, and some have red eyes.
During admission, Mr. H voices his frustration that clinicians had not found the worms he has been seeing. He continuously requests to review imaging performed during his visit and wants a multidisciplinary team to evaluate his case. He demands to test a cup with spit-up “samples,” believing the parasites would be visible under a microscope. Throughout his admission, Mr. H continues to take buprenorphine/naloxone and does not experience withdrawal symptoms. The treatment team titrates his quetiapine to 400 mg/d. Due to the lack of improvement, the team initiates olanzapine 5 mg/d at bedtime. However, Mr. H reports significant tinnitus and requests a medication change. He is started on haloperidol 5 mg twice daily.
Continue to: Mr. H begins to see improvements...
Mr. H begins to see improvements on Day 7 of taking haloperidol. He no longer brings up infestation but still acknowledges having worms inside him when directly asked. He says the worms cause him less distress than before and he is hopeful to live without discomfort. He also demonstrates an ability to conduct activities of daily living. Because Mr. H is being monitored on an acute inpatient psychiatric basis, he is deemed appropriate for discharge even though his symptoms have not yet fully resolved. After a 19-day hospital stay, Mr. H is discharged on haloperidol 15 mg/d and quetiapine 200 mg/d.
[polldaddy:12570074]
The authors’ observations
Mr. H asked to have his sputum examined. The “specimen sign,” also called “matchbox sign” or “Ziploc bag sign,” in which patients collect what they believe to be infected tissue or organisms in a container, is a well-studied part of DP.5 Such samples should be considered during initial encounters and can be examined for formal evaluation, but cautiously. Overtesting may incur a financial burden or reinforce deleterious beliefs and behaviors.
It can be difficult to identify triggers of DP. Research shows DP may arise from nonorganic and stressful life events, home floods, or contact with people infected with parasites.6,7 Organic causes have also been found, such as patients taking multiple medications for Parkinson disease who developed delusional symptoms.8 Buscarino et al9 reported the case of a woman who started to develop symptoms of delusions and hallucinations after being on high-dose amphetamines for attention-deficit/hyperactivity disorder. Research shows that stopping the suspected medication commonly improves such symptoms.9,10 Although methamphetamine can remain detectable in urine for up to 4 days after use and potentially a few days longer for chronic users due to circulating levels,11 Mr. H’s symptoms continued for weeks after all substances of abuse should have been cleared from his system. This suggests he was experiencing a psychiatric illness and was accurate in distinguishing methamphetamine-induced from psychiatric-induced sensations. Regardless, polysubstance use has been shown to potentially increase the risk and play a role in the onset and progression of delusional illness, as seen in prior cases as well as in this case.9
It has been hypothesized that the pathophysiology of DP is associated with the deterioration of the striatal dopaminergic pathway, leading to an increase in extracellular dopamine levels. The striatum is responsible for most dopamine reuptake in the brain; therefore, certain drugs such as cocaine, methamphetamine, and methylphenidate may precipitate symptoms of DP due to their blockade of presynaptic dopamine reuptake.12 Additionally, conditions that decrease the functioning of striatal dopamine transporters, such as schizophrenia or depression, may be underlying causes of DP.13
Treatment of DP remains a topic of debate. Most current recommendations appear to be based on a small, nonrandomized placebo-controlled trial.14 The first-generation antipsychotic pimozide had been a first-line treatment for DP, but its adverse effect profile, which includes QTc prolongation and extrapyramidal symptoms, led to the exploration of second-generation antipsychotics such as olanzapine and risperidone.15,16 There is a dearth of literature about the use of haloperidol, quetiapine, or a combination of both as treatment options for DP, though the combination of these 2 medications proved effective for Mr. H. Further research is necessary to justify changes to current treatment standards, but this finding highlights a successful symptom reduction achieved with this combination.
Continue to: Patients may experience genuine symptoms...
Patients may experience genuine symptoms despite the delusional nature of DP, and it is important for clinicians to recognize the potential burden and anxiety these individuals face. Patients may present with self-inflicted bruises, cuts, and erosions to gain access to infected areas, which may be confused with skin picking disorder. Excessive cleansing or use of irritant products can also cause skin damage, leading to other dermatological conditions that reinforce the patient’s belief that something is medically wrong. During treatment, consider medications for relief of pruritus or pain. Focus on offering patients the opportunity to express their concerns, treat them with empathy, avoid stigmatizing language such as “delusions” or “psychosis,” and refrain from contradicting them until a strong rapport has been established (Table 217).
Symptoms of DP can persist for months to years. Patients who fully recovered experienced a median duration of 0.5 years until symptom resolution, compared to incompletely recovered patients, who took approximately 1 year.18 Primary DP has slower improvement rates compared to secondary DP, with the median onset of effects occurring at Week 1.5 and peak improvements occurring at Week 6.16
OUTCOME Continued ED visits
Unfortunately, Mr. H does not follow through with his outpatient psychiatry appointments. In the 7 months following discharge, he visits the ED 8 times for alcohol intoxication, alcohol withdrawal, and methamphetamine abuse, in addition to 2 admissions for inpatient detoxification, during which he was still receiving the same scheduled medications (haloperidol 15 mg/d and quetiapine 200 mg/d). At each of his ED visits, there was no documentation of DP symptoms, which suggests his symptoms may have resolved.
Bottom Line
Because delusional parasitosis symptoms feel real to patients, it is crucial to build rapport to recommend and successfully initiate treatment. After ruling out nonpsychiatric etiologies, consider traditional treatment with antipsychotics, and consider medications for relief of pruritus or pain.
Related Resources
- Sellman D, Phan SV, Inyang M. Bugs on her skin—but nobody else sees them. Current Psychiatry. 2018;17(8):48,50-53.
- Campbell EH, Elston DM, Hawthorne JD, et al. Diagnosis and management of delusional parasitosis. J Am Acad Dermatol. 2019;80(5):1428-1434. doi:10.1016/j.jaad.2018.12.012
Drug Brand Names
Buprenorphine/naloxone • Suboxone
Haloperidol • Haldol
Hydroxyzine • Vistaril
Lithium • Eskalith, Lithobid
Methylphenidate • Concerta
Olanzapine • Zyprexa
Permethrin • Elimite
Phenobarbital • Solfoton, Tedral, Luminal
Pimozide • Orap
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Valproic acid • Depakote
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2013.
2. Bailey CH, Andersen LK, Lowe GC, et al. A population-based study of the incidence of delusional infestation in Olmsted County, Minnesota, 1976-2010. Br J Dermatol. 2014;170(5):1130-1135. doi:10.1111/bjd.12848
3. Kohorst JJ, Bailey CH, Andersen LK, et al. Prevalence of delusional infestation-a population-based study. JAMA Dermatol. 2018;154(5):615-617. doi:10.1001/jamadermatol.2018.0004
4. Freinhar JP. Delusions of parasitosis. Psychosomatics. 1984;25(1):47-53. doi:10.1016/S0033-3182(84)73096-9
5. Reich A, Kwiatkowska D, Pacan P. Delusions of parasitosis: an update. Dermatol Ther (Heidelb). 2019;9(4):631-638. doi:10.1007/s13555-019-00324-3
6. Berrios GE. Delusional parasitosis and physical disease. Compr Psychiatry. 1985;26(5):395-403. doi:10.1016/0010-440x(85)90077-x
7. Aizenberg D, Schwartz B, Zemishlany Z. Delusional parasitosis associated with phenelzine. Br J Psychiatry. 1991;159:716-717. doi:10.1192/bjp.159.5.716
8. Flann S, Shotbolt J, Kessel B, et al. Three cases of delusional parasitosis caused by dopamine agonists. Clin Exp Dermatol. 2010;35(7):740-742. doi:10.1111/j.1365-2230.2010.03810.x
9. Buscarino M, Saal J, Young JL. Delusional parasitosis in a female treated with mixed amphetamine salts: a case report and literature review. Case Rep Psychiatry. 2012;2012:624235. doi:10.1155/2012/624235
10. Elpern DJ. Cocaine abuse and delusions of parasitosis. Cutis. 1988;42(4):273-274.
11. Richards JR, Laurin EG. Methamphetamine toxicity. StatPearls Publishing; 2023. Updated January 8, 2023. Accessed May 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK430895/
12. Huber M, Kirchler E, Karner M, et al. Delusional parasitosis and the dopamine transporter. A new insight of etiology? Med Hypotheses. 2007;68(6):1351-1358. doi:10.1016/j.mehy.2006.07.061
13. Lipman ZM, Yosipovitch G. Substance use disorders and chronic itch. J Am Acad Dermatol. 2021;84(1):148-155. doi:10.1016/j.jaad.2020.08.117
14. Kenchaiah BK, Kumar S, Tharyan P. Atypical anti-psychotics in delusional parasitosis: a retrospective case series of 20 patients. Int J Dermatol. 2010;49(1):95-100. doi:10.1111/j.1365-4632.2009.04312.x
15. Laidler N. Delusions of parasitosis: a brief review of the literature and pathway for diagnosis and treatment. Dermatol Online J. 2018;24(1):13030/qt1fh739nx.
16. Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis: outcome and efficacy. J Clin Psychopharmacol. 2008;28(5):500-508. doi:10.1097/JCP.0b013e318185e774
17. Mumcuoglu KY, Leibovici V, Reuveni I, et al. Delusional parasitosis: diagnosis and treatment. Isr Med Assoc J. 2018;20(7):456-460.
18. Trabert W. 100 years of delusional parasitosis. Meta-analysis of 1,223 case reports. Psychopathology. 1995;28(5):238-246. doi:10.1159/000284934
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2013.
2. Bailey CH, Andersen LK, Lowe GC, et al. A population-based study of the incidence of delusional infestation in Olmsted County, Minnesota, 1976-2010. Br J Dermatol. 2014;170(5):1130-1135. doi:10.1111/bjd.12848
3. Kohorst JJ, Bailey CH, Andersen LK, et al. Prevalence of delusional infestation-a population-based study. JAMA Dermatol. 2018;154(5):615-617. doi:10.1001/jamadermatol.2018.0004
4. Freinhar JP. Delusions of parasitosis. Psychosomatics. 1984;25(1):47-53. doi:10.1016/S0033-3182(84)73096-9
5. Reich A, Kwiatkowska D, Pacan P. Delusions of parasitosis: an update. Dermatol Ther (Heidelb). 2019;9(4):631-638. doi:10.1007/s13555-019-00324-3
6. Berrios GE. Delusional parasitosis and physical disease. Compr Psychiatry. 1985;26(5):395-403. doi:10.1016/0010-440x(85)90077-x
7. Aizenberg D, Schwartz B, Zemishlany Z. Delusional parasitosis associated with phenelzine. Br J Psychiatry. 1991;159:716-717. doi:10.1192/bjp.159.5.716
8. Flann S, Shotbolt J, Kessel B, et al. Three cases of delusional parasitosis caused by dopamine agonists. Clin Exp Dermatol. 2010;35(7):740-742. doi:10.1111/j.1365-2230.2010.03810.x
9. Buscarino M, Saal J, Young JL. Delusional parasitosis in a female treated with mixed amphetamine salts: a case report and literature review. Case Rep Psychiatry. 2012;2012:624235. doi:10.1155/2012/624235
10. Elpern DJ. Cocaine abuse and delusions of parasitosis. Cutis. 1988;42(4):273-274.
11. Richards JR, Laurin EG. Methamphetamine toxicity. StatPearls Publishing; 2023. Updated January 8, 2023. Accessed May 25, 2023. https://www.ncbi.nlm.nih.gov/books/NBK430895/
12. Huber M, Kirchler E, Karner M, et al. Delusional parasitosis and the dopamine transporter. A new insight of etiology? Med Hypotheses. 2007;68(6):1351-1358. doi:10.1016/j.mehy.2006.07.061
13. Lipman ZM, Yosipovitch G. Substance use disorders and chronic itch. J Am Acad Dermatol. 2021;84(1):148-155. doi:10.1016/j.jaad.2020.08.117
14. Kenchaiah BK, Kumar S, Tharyan P. Atypical anti-psychotics in delusional parasitosis: a retrospective case series of 20 patients. Int J Dermatol. 2010;49(1):95-100. doi:10.1111/j.1365-4632.2009.04312.x
15. Laidler N. Delusions of parasitosis: a brief review of the literature and pathway for diagnosis and treatment. Dermatol Online J. 2018;24(1):13030/qt1fh739nx.
16. Freudenmann RW, Lepping P. Second-generation antipsychotics in primary and secondary delusional parasitosis: outcome and efficacy. J Clin Psychopharmacol. 2008;28(5):500-508. doi:10.1097/JCP.0b013e318185e774
17. Mumcuoglu KY, Leibovici V, Reuveni I, et al. Delusional parasitosis: diagnosis and treatment. Isr Med Assoc J. 2018;20(7):456-460.
18. Trabert W. 100 years of delusional parasitosis. Meta-analysis of 1,223 case reports. Psychopathology. 1995;28(5):238-246. doi:10.1159/000284934
Perinatal psychiatry: 5 key principles
Perinatal mood and anxiety disorders are the most common complication of pregnancy and childbirth.1 Mental health concerns are a leading cause of maternal mortality in the United States, which has rising maternal mortality rates and glaring racial and socioeconomic disparities.2 Inconsistent perinatal psychiatry training likely contributes to perceived discomfort of patients who are pregnant.3 This is why it is critical for all psychiatrists to understand the principles of perinatal psychiatry. Here is a brief description of 5 key principles.
1. Discuss preconception planning
Reproductive life planning should occur with all patients who are capable of becoming pregnant. This planning should include not just a risks/benefits analysis and anticipatory planning regarding medications but also a discussion of prior perinatal symptoms, pregnancy intentions and contraception (especially in light of increasingly limited access to abortion), and the bidirectional nature of pregnancy and mental health conditions.
The acronym PATH provides a framework for these conversations:
- Pregnancy Attitudes: “Do you think you might like to have (more) children at some point?”
- Timing: “If considering future parenthood, when do you think that might be?”
- How important is prevention: “How important is it to you to prevent pregnancy (until then)?”4
2. Focus on perinatal mental health
Discussion often centers on medication risks to the fetus at the expense of considering risks of under- or nontreatment for both members of the dyad. Undertreating perinatal mental health conditions results in dual exposures (medication and illness), and untreated illness is associated with negative effects on obstetric and neonatal outcomes and the well-being of the parent and offspring.1
3. Resist experimentation
It is common for clinicians to reflexively switch patients who are pregnant from an effective medication to one viewed as the “safest” or “best” because it has more data. This exposes the fetus to 2 medications and the dyad to potential symptoms of the illness. Decisions about medication changes should instead be made on an individual basis considering the risks and benefits of all exposures as well as the patient’s current symptoms, previous treatment, and family history.
4. Collaborate and communicate
Despite effective interventions, many perinatal mental health conditions go untreated.1 Normalize perinatal mental health symptoms with patients to reduce stigma and barriers to disclosure, and respect their decisions regarding perinatal medication use. Proper communication with the obstetric team ensures appropriate perinatal mental health screening and fetal monitoring (eg, possible fetal growth ultrasounds for a patient taking prazosin, or assessing for neonatal adaptation syndrome if there is selective serotonin reuptake inhibitor exposure in utero).
5. Recognize your limitations
Our understanding of psychotropics’ teratogenicity is constantly evolving, and we must recognize when we don’t know something. In addition to medication databases such as Reprotox (https://reprotox.org/) and LactMed (https://www.ncbi.nlm.nih.gov/books/NBK501922/), several perinatal psychiatry resources are available for both patients and clinicians (Table). Additionally, Postpartum Support International maintains a National Perinatal Consult Line (1-877-499-4773) as well as a list of state perinatal psychiatry access lines (https://www.postpartum.net/professionals/state-perinatal-psychiatry-access-lines/) for clinicians. The Massachusetts General Hospital Center for Women’s Mental Health (https://womensmentalhealth.org) is also a helpful resource for clinicians.
1. Luca DL, Garlow N, Staatz C, et al. Societal costs of untreated perinatal mood and anxiety disorders in the United States. Mathematica Policy Research. April 29, 2019. Accessed July 13, 2023. https://www.mathematica.org/publications/societal-costs-of-untreated-perinatal-mood-and-anxiety-disorders-in-the-united-states
2. Singh GK. Trends and social inequalities in maternal mortality in the United States, 1969-2018. Int J MCH AIDS. 2021;10(1):29-42. doi:10.21106/ijma.444
3. Weinreb L, Byatt N, Moore Simas TA, et al. What happens to mental health treatment during pregnancy? Women’s experience with prescribing providers. Psychiatr Q. 2014;85(3):349-355. doi:10.1007/s11126-014-9293-7
4. Callegari LS, Aiken AR, Dehlendorf C, et al. Addressing potential pitfalls of reproductive life planning with patient-centered counseling. Am J Obstet Gynecol. 2017;216(2):129-134. doi:10.1016/j.ajog.2016.10.004
Perinatal mood and anxiety disorders are the most common complication of pregnancy and childbirth.1 Mental health concerns are a leading cause of maternal mortality in the United States, which has rising maternal mortality rates and glaring racial and socioeconomic disparities.2 Inconsistent perinatal psychiatry training likely contributes to perceived discomfort of patients who are pregnant.3 This is why it is critical for all psychiatrists to understand the principles of perinatal psychiatry. Here is a brief description of 5 key principles.
1. Discuss preconception planning
Reproductive life planning should occur with all patients who are capable of becoming pregnant. This planning should include not just a risks/benefits analysis and anticipatory planning regarding medications but also a discussion of prior perinatal symptoms, pregnancy intentions and contraception (especially in light of increasingly limited access to abortion), and the bidirectional nature of pregnancy and mental health conditions.
The acronym PATH provides a framework for these conversations:
- Pregnancy Attitudes: “Do you think you might like to have (more) children at some point?”
- Timing: “If considering future parenthood, when do you think that might be?”
- How important is prevention: “How important is it to you to prevent pregnancy (until then)?”4
2. Focus on perinatal mental health
Discussion often centers on medication risks to the fetus at the expense of considering risks of under- or nontreatment for both members of the dyad. Undertreating perinatal mental health conditions results in dual exposures (medication and illness), and untreated illness is associated with negative effects on obstetric and neonatal outcomes and the well-being of the parent and offspring.1
3. Resist experimentation
It is common for clinicians to reflexively switch patients who are pregnant from an effective medication to one viewed as the “safest” or “best” because it has more data. This exposes the fetus to 2 medications and the dyad to potential symptoms of the illness. Decisions about medication changes should instead be made on an individual basis considering the risks and benefits of all exposures as well as the patient’s current symptoms, previous treatment, and family history.
4. Collaborate and communicate
Despite effective interventions, many perinatal mental health conditions go untreated.1 Normalize perinatal mental health symptoms with patients to reduce stigma and barriers to disclosure, and respect their decisions regarding perinatal medication use. Proper communication with the obstetric team ensures appropriate perinatal mental health screening and fetal monitoring (eg, possible fetal growth ultrasounds for a patient taking prazosin, or assessing for neonatal adaptation syndrome if there is selective serotonin reuptake inhibitor exposure in utero).
5. Recognize your limitations
Our understanding of psychotropics’ teratogenicity is constantly evolving, and we must recognize when we don’t know something. In addition to medication databases such as Reprotox (https://reprotox.org/) and LactMed (https://www.ncbi.nlm.nih.gov/books/NBK501922/), several perinatal psychiatry resources are available for both patients and clinicians (Table). Additionally, Postpartum Support International maintains a National Perinatal Consult Line (1-877-499-4773) as well as a list of state perinatal psychiatry access lines (https://www.postpartum.net/professionals/state-perinatal-psychiatry-access-lines/) for clinicians. The Massachusetts General Hospital Center for Women’s Mental Health (https://womensmentalhealth.org) is also a helpful resource for clinicians.

Perinatal mood and anxiety disorders are the most common complication of pregnancy and childbirth.1 Mental health concerns are a leading cause of maternal mortality in the United States, which has rising maternal mortality rates and glaring racial and socioeconomic disparities.2 Inconsistent perinatal psychiatry training likely contributes to perceived discomfort of patients who are pregnant.3 This is why it is critical for all psychiatrists to understand the principles of perinatal psychiatry. Here is a brief description of 5 key principles.
1. Discuss preconception planning
Reproductive life planning should occur with all patients who are capable of becoming pregnant. This planning should include not just a risks/benefits analysis and anticipatory planning regarding medications but also a discussion of prior perinatal symptoms, pregnancy intentions and contraception (especially in light of increasingly limited access to abortion), and the bidirectional nature of pregnancy and mental health conditions.
The acronym PATH provides a framework for these conversations:
- Pregnancy Attitudes: “Do you think you might like to have (more) children at some point?”
- Timing: “If considering future parenthood, when do you think that might be?”
- How important is prevention: “How important is it to you to prevent pregnancy (until then)?”4
2. Focus on perinatal mental health
Discussion often centers on medication risks to the fetus at the expense of considering risks of under- or nontreatment for both members of the dyad. Undertreating perinatal mental health conditions results in dual exposures (medication and illness), and untreated illness is associated with negative effects on obstetric and neonatal outcomes and the well-being of the parent and offspring.1
3. Resist experimentation
It is common for clinicians to reflexively switch patients who are pregnant from an effective medication to one viewed as the “safest” or “best” because it has more data. This exposes the fetus to 2 medications and the dyad to potential symptoms of the illness. Decisions about medication changes should instead be made on an individual basis considering the risks and benefits of all exposures as well as the patient’s current symptoms, previous treatment, and family history.
4. Collaborate and communicate
Despite effective interventions, many perinatal mental health conditions go untreated.1 Normalize perinatal mental health symptoms with patients to reduce stigma and barriers to disclosure, and respect their decisions regarding perinatal medication use. Proper communication with the obstetric team ensures appropriate perinatal mental health screening and fetal monitoring (eg, possible fetal growth ultrasounds for a patient taking prazosin, or assessing for neonatal adaptation syndrome if there is selective serotonin reuptake inhibitor exposure in utero).
5. Recognize your limitations
Our understanding of psychotropics’ teratogenicity is constantly evolving, and we must recognize when we don’t know something. In addition to medication databases such as Reprotox (https://reprotox.org/) and LactMed (https://www.ncbi.nlm.nih.gov/books/NBK501922/), several perinatal psychiatry resources are available for both patients and clinicians (Table). Additionally, Postpartum Support International maintains a National Perinatal Consult Line (1-877-499-4773) as well as a list of state perinatal psychiatry access lines (https://www.postpartum.net/professionals/state-perinatal-psychiatry-access-lines/) for clinicians. The Massachusetts General Hospital Center for Women’s Mental Health (https://womensmentalhealth.org) is also a helpful resource for clinicians.

1. Luca DL, Garlow N, Staatz C, et al. Societal costs of untreated perinatal mood and anxiety disorders in the United States. Mathematica Policy Research. April 29, 2019. Accessed July 13, 2023. https://www.mathematica.org/publications/societal-costs-of-untreated-perinatal-mood-and-anxiety-disorders-in-the-united-states
2. Singh GK. Trends and social inequalities in maternal mortality in the United States, 1969-2018. Int J MCH AIDS. 2021;10(1):29-42. doi:10.21106/ijma.444
3. Weinreb L, Byatt N, Moore Simas TA, et al. What happens to mental health treatment during pregnancy? Women’s experience with prescribing providers. Psychiatr Q. 2014;85(3):349-355. doi:10.1007/s11126-014-9293-7
4. Callegari LS, Aiken AR, Dehlendorf C, et al. Addressing potential pitfalls of reproductive life planning with patient-centered counseling. Am J Obstet Gynecol. 2017;216(2):129-134. doi:10.1016/j.ajog.2016.10.004
1. Luca DL, Garlow N, Staatz C, et al. Societal costs of untreated perinatal mood and anxiety disorders in the United States. Mathematica Policy Research. April 29, 2019. Accessed July 13, 2023. https://www.mathematica.org/publications/societal-costs-of-untreated-perinatal-mood-and-anxiety-disorders-in-the-united-states
2. Singh GK. Trends and social inequalities in maternal mortality in the United States, 1969-2018. Int J MCH AIDS. 2021;10(1):29-42. doi:10.21106/ijma.444
3. Weinreb L, Byatt N, Moore Simas TA, et al. What happens to mental health treatment during pregnancy? Women’s experience with prescribing providers. Psychiatr Q. 2014;85(3):349-355. doi:10.1007/s11126-014-9293-7
4. Callegari LS, Aiken AR, Dehlendorf C, et al. Addressing potential pitfalls of reproductive life planning with patient-centered counseling. Am J Obstet Gynecol. 2017;216(2):129-134. doi:10.1016/j.ajog.2016.10.004
Diagnosing borderline personality disorder: Avoid these pitfalls
Borderline personality disorder (BPD) is associated with impaired psychosocial functioning, reduced quality of life, increased use of health care services, and excess mortality.1 Unfortunately, this disorder is often underrecognized and underdiagnosed, and patients with BPD may not receive an accurate diagnosis for years after first seeking treatment.1 Problems in diagnosing BPD include:
Stigma. Some patients may view the term “borderline” as stigmatizing, as if we are calling these patients borderline human beings. One of the symptoms of BPD is a “markedly and persistently unstable self-image.”2 Such patients do not need a stigmatizing label to worsen their self-image.
Terminology. The word borderline may also imply relatively mild psychiatric symptoms. However, “borderline personality disorder” does not refer to a mild personality disorder. DSM-5 describes potential BPD symptoms as “intense,” “marked,” or “severe,” and 1 of the symptoms is suicidal behavior.2
Symptoms. To meet the criteria for a BPD diagnosis, a patient must exhibit ≥5 of 9 severe symptoms2:
- frantic efforts to avoid abandonment
- unstable and intense interpersonal relationships
- unstable self-image
- impulsivity in ≥2 areas that are potentially self-damaging
- suicidal behavior
- affective instability
- chronic feelings of emptiness
- inappropriate anger
- transient paranoid ideation or dissociative symptoms.
Asking about all 9 of these criteria and their severity is not part of a routine psychiatric evaluation. A patient might not volunteer any of this information because they are concerned about potential stigma. Additionally, perhaps most of the general population has had a “BPD-like” symptom at least once during their lives. This symptom might not have been severe enough to qualify as a true BPD symptom. Clinicians might have difficulty discerning BPD-like symptoms from true BPD symptoms.
Comorbidities. Many patients with BPD also have a comorbid mood disorder or substance use disorder.1,3 Clinicians might focus on a comorbid diagnosis and not recognize BPD.
Stress. BPD symptoms may become more severe when the patient faces a stressful situation. The BPD symptoms might seem more severe than the stress would warrant.2 However, clinicians might blame the BPD symptoms solely on stress and not acknowledge the underlying BPD diagnosis.
Awareness of these factors can help clinicians keep BPD in the differential diagnosis when conducting a psychiatric evaluation, thus reducing the chances of overlooking this serious disorder.
1. Zimmerman M. Improving the recognition of borderline personality disorder. Current Psychiatry. 2017;16(10):13-19.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:663-666.
3. Grant BF, Chou SP, Goldstein RB, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008:69(4)533-545.
Borderline personality disorder (BPD) is associated with impaired psychosocial functioning, reduced quality of life, increased use of health care services, and excess mortality.1 Unfortunately, this disorder is often underrecognized and underdiagnosed, and patients with BPD may not receive an accurate diagnosis for years after first seeking treatment.1 Problems in diagnosing BPD include:
Stigma. Some patients may view the term “borderline” as stigmatizing, as if we are calling these patients borderline human beings. One of the symptoms of BPD is a “markedly and persistently unstable self-image.”2 Such patients do not need a stigmatizing label to worsen their self-image.
Terminology. The word borderline may also imply relatively mild psychiatric symptoms. However, “borderline personality disorder” does not refer to a mild personality disorder. DSM-5 describes potential BPD symptoms as “intense,” “marked,” or “severe,” and 1 of the symptoms is suicidal behavior.2
Symptoms. To meet the criteria for a BPD diagnosis, a patient must exhibit ≥5 of 9 severe symptoms2:
- frantic efforts to avoid abandonment
- unstable and intense interpersonal relationships
- unstable self-image
- impulsivity in ≥2 areas that are potentially self-damaging
- suicidal behavior
- affective instability
- chronic feelings of emptiness
- inappropriate anger
- transient paranoid ideation or dissociative symptoms.
Asking about all 9 of these criteria and their severity is not part of a routine psychiatric evaluation. A patient might not volunteer any of this information because they are concerned about potential stigma. Additionally, perhaps most of the general population has had a “BPD-like” symptom at least once during their lives. This symptom might not have been severe enough to qualify as a true BPD symptom. Clinicians might have difficulty discerning BPD-like symptoms from true BPD symptoms.
Comorbidities. Many patients with BPD also have a comorbid mood disorder or substance use disorder.1,3 Clinicians might focus on a comorbid diagnosis and not recognize BPD.
Stress. BPD symptoms may become more severe when the patient faces a stressful situation. The BPD symptoms might seem more severe than the stress would warrant.2 However, clinicians might blame the BPD symptoms solely on stress and not acknowledge the underlying BPD diagnosis.
Awareness of these factors can help clinicians keep BPD in the differential diagnosis when conducting a psychiatric evaluation, thus reducing the chances of overlooking this serious disorder.
Borderline personality disorder (BPD) is associated with impaired psychosocial functioning, reduced quality of life, increased use of health care services, and excess mortality.1 Unfortunately, this disorder is often underrecognized and underdiagnosed, and patients with BPD may not receive an accurate diagnosis for years after first seeking treatment.1 Problems in diagnosing BPD include:
Stigma. Some patients may view the term “borderline” as stigmatizing, as if we are calling these patients borderline human beings. One of the symptoms of BPD is a “markedly and persistently unstable self-image.”2 Such patients do not need a stigmatizing label to worsen their self-image.
Terminology. The word borderline may also imply relatively mild psychiatric symptoms. However, “borderline personality disorder” does not refer to a mild personality disorder. DSM-5 describes potential BPD symptoms as “intense,” “marked,” or “severe,” and 1 of the symptoms is suicidal behavior.2
Symptoms. To meet the criteria for a BPD diagnosis, a patient must exhibit ≥5 of 9 severe symptoms2:
- frantic efforts to avoid abandonment
- unstable and intense interpersonal relationships
- unstable self-image
- impulsivity in ≥2 areas that are potentially self-damaging
- suicidal behavior
- affective instability
- chronic feelings of emptiness
- inappropriate anger
- transient paranoid ideation or dissociative symptoms.
Asking about all 9 of these criteria and their severity is not part of a routine psychiatric evaluation. A patient might not volunteer any of this information because they are concerned about potential stigma. Additionally, perhaps most of the general population has had a “BPD-like” symptom at least once during their lives. This symptom might not have been severe enough to qualify as a true BPD symptom. Clinicians might have difficulty discerning BPD-like symptoms from true BPD symptoms.
Comorbidities. Many patients with BPD also have a comorbid mood disorder or substance use disorder.1,3 Clinicians might focus on a comorbid diagnosis and not recognize BPD.
Stress. BPD symptoms may become more severe when the patient faces a stressful situation. The BPD symptoms might seem more severe than the stress would warrant.2 However, clinicians might blame the BPD symptoms solely on stress and not acknowledge the underlying BPD diagnosis.
Awareness of these factors can help clinicians keep BPD in the differential diagnosis when conducting a psychiatric evaluation, thus reducing the chances of overlooking this serious disorder.
1. Zimmerman M. Improving the recognition of borderline personality disorder. Current Psychiatry. 2017;16(10):13-19.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:663-666.
3. Grant BF, Chou SP, Goldstein RB, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008:69(4)533-545.
1. Zimmerman M. Improving the recognition of borderline personality disorder. Current Psychiatry. 2017;16(10):13-19.
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; 2013:663-666.
3. Grant BF, Chou SP, Goldstein RB, et al. Prevalence, correlates, disability, and comorbidity of DSM-IV borderline personality disorder: results from the Wave 2 National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2008:69(4)533-545.
Extended-release injectable naltrexone for opioid use disorder
We appreciate the important review by Gluck et al (“Managing patients with comorbid opioid and alcohol use disorders,”
XR-NTX should be considered an equal OUD treatment alternative to buprenorphine-naloxone, especially for patients who prefer an opioid-free option.1,2 It has the added advantage of being FDA-approved for both AUD and OUD.
One obstacle to the success of XR-NTX is the induction period. The National Institute on Drug Abuse Clinical Trials Network X:BOT trial found that once the induction hurdle was surmounted, XR-NTX and buprenorphine were equally effective in a population of approximately 80% heroin users and two-thirds injection drug users.2 Patient variables that predict successful induction include young age, baseline preference for XR-NTX, fewer drug complications, and fewer family/social complications.3 If the length of the induction (usually 7 to 10 days) is a deterrent, a study supported the feasibility of a 5-day outpatient XR-NTX induction.4 Further research is needed to improve successful induction for XR-NTX.
Ashmeer Ogbuchi, MD
Karen Drexler, MD
Atlanta, Georgia
References
1. Tanum L, Solli KK, Latif Z, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence. JAMA Psychiatry. 2017;74(12):1197-1205. doi:10.1001/ jamapsychiatry.2017.3206
2. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/s0140-6736(17)32812-x
3. Murphy SM, Jeng PJ, McCollister KE, et al. Cost‐effectiveness implications of increasing the efficiency of the extended‐release naltrexone induction process for the treatment of opioid use disorder: a secondary analysis. Addiction. 2021;116(12)3444-3453. doi:10.1111/add.15531
4. Sibai M, Mishlen K, Nunes EV, et al. A week-long outpatient induction onto XR-naltrexone in patients with opioid use disorder. Am J Drug Alcohol Abuse. 2020;46(3):289-296. doi:10.1080/00952990.2019.1700265
Continue to: The authors respond
The authors respond
We appreciate Drs. Ogbuchi and Drexler for their thoughtful attention to our review. They proposed amending our original algorithm, recommending that XR-NTX be considered as another first-line option for patients with OUD. We agree with this suggestion, particularly for inpatients. However, we have some reservations about applying this suggestion to outpatient treatment. Though research evidence from Lee et al1 indicates that once initiation is completed, both medications are equally safe and effective, the initial attrition rate in the XR-NTX group was much higher (28% vs 6%, P < .0001), which suggests lower acceptability/tolerability compared with buprenorphine. Notably, the initiation of both medications in Lee et al1 was done in an inpatient setting. Moreover, although some medications are endorsed as “first-line,” the actual utilization rate is often influenced by many factors, including the ease of treatment initiation. Wakeman et al2 found the most common treatment modality received by patients with OUD was nonintensive behavioral health (59.5%), followed by inpatient withdrawal management and residential treatment (15.2%). Among all patients in the Wakeman study,2 only 12.5% received buprenorphine or methadone, and 2.4% received naltrexone.
Data from our clinic corroborate this trend. Currently, in our clinic approximately 300 patients with OUD are receiving medications, including approximately 250 on buprenorphine (including 5 to 10 on the long-acting injectable formulation), 50 on methadone, and only 1 or 2 on XR-NTX. Though this disparity may reflect bias in our clinicians’ prescribing practices, in the past few years we have had many unsuccessful attempts at initiating XR-NTX. To our disappointment, a theoretically excellent medication has not translated clinically. The recent surge in fentanyl use further complicates XR-NTX initiation for OUD, because the length of induction may be longer.
In conclusion, we agree that XR-NTX is a potential treatment option for patients with OUD, but clinicians should be cognizant of the potential barriers; inform patients of the advantages, expectations, and challenges; and respect patients’ informed decisions.
Rachel Gluck, MD
Karen Hochman, MD
Yi-lang Tang, MD, PhD
Atlanta, Georgia
References
1. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/s0140-6736(17)32812-x
2. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622. doi:10.1001/jamanetworkopen.2019.20622
We appreciate the important review by Gluck et al (“Managing patients with comorbid opioid and alcohol use disorders,”
XR-NTX should be considered an equal OUD treatment alternative to buprenorphine-naloxone, especially for patients who prefer an opioid-free option.1,2 It has the added advantage of being FDA-approved for both AUD and OUD.
One obstacle to the success of XR-NTX is the induction period. The National Institute on Drug Abuse Clinical Trials Network X:BOT trial found that once the induction hurdle was surmounted, XR-NTX and buprenorphine were equally effective in a population of approximately 80% heroin users and two-thirds injection drug users.2 Patient variables that predict successful induction include young age, baseline preference for XR-NTX, fewer drug complications, and fewer family/social complications.3 If the length of the induction (usually 7 to 10 days) is a deterrent, a study supported the feasibility of a 5-day outpatient XR-NTX induction.4 Further research is needed to improve successful induction for XR-NTX.
Ashmeer Ogbuchi, MD
Karen Drexler, MD
Atlanta, Georgia
References
1. Tanum L, Solli KK, Latif Z, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence. JAMA Psychiatry. 2017;74(12):1197-1205. doi:10.1001/ jamapsychiatry.2017.3206
2. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/s0140-6736(17)32812-x
3. Murphy SM, Jeng PJ, McCollister KE, et al. Cost‐effectiveness implications of increasing the efficiency of the extended‐release naltrexone induction process for the treatment of opioid use disorder: a secondary analysis. Addiction. 2021;116(12)3444-3453. doi:10.1111/add.15531
4. Sibai M, Mishlen K, Nunes EV, et al. A week-long outpatient induction onto XR-naltrexone in patients with opioid use disorder. Am J Drug Alcohol Abuse. 2020;46(3):289-296. doi:10.1080/00952990.2019.1700265
Continue to: The authors respond
The authors respond
We appreciate Drs. Ogbuchi and Drexler for their thoughtful attention to our review. They proposed amending our original algorithm, recommending that XR-NTX be considered as another first-line option for patients with OUD. We agree with this suggestion, particularly for inpatients. However, we have some reservations about applying this suggestion to outpatient treatment. Though research evidence from Lee et al1 indicates that once initiation is completed, both medications are equally safe and effective, the initial attrition rate in the XR-NTX group was much higher (28% vs 6%, P < .0001), which suggests lower acceptability/tolerability compared with buprenorphine. Notably, the initiation of both medications in Lee et al1 was done in an inpatient setting. Moreover, although some medications are endorsed as “first-line,” the actual utilization rate is often influenced by many factors, including the ease of treatment initiation. Wakeman et al2 found the most common treatment modality received by patients with OUD was nonintensive behavioral health (59.5%), followed by inpatient withdrawal management and residential treatment (15.2%). Among all patients in the Wakeman study,2 only 12.5% received buprenorphine or methadone, and 2.4% received naltrexone.
Data from our clinic corroborate this trend. Currently, in our clinic approximately 300 patients with OUD are receiving medications, including approximately 250 on buprenorphine (including 5 to 10 on the long-acting injectable formulation), 50 on methadone, and only 1 or 2 on XR-NTX. Though this disparity may reflect bias in our clinicians’ prescribing practices, in the past few years we have had many unsuccessful attempts at initiating XR-NTX. To our disappointment, a theoretically excellent medication has not translated clinically. The recent surge in fentanyl use further complicates XR-NTX initiation for OUD, because the length of induction may be longer.
In conclusion, we agree that XR-NTX is a potential treatment option for patients with OUD, but clinicians should be cognizant of the potential barriers; inform patients of the advantages, expectations, and challenges; and respect patients’ informed decisions.
Rachel Gluck, MD
Karen Hochman, MD
Yi-lang Tang, MD, PhD
Atlanta, Georgia
References
1. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/s0140-6736(17)32812-x
2. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622. doi:10.1001/jamanetworkopen.2019.20622
We appreciate the important review by Gluck et al (“Managing patients with comorbid opioid and alcohol use disorders,”
XR-NTX should be considered an equal OUD treatment alternative to buprenorphine-naloxone, especially for patients who prefer an opioid-free option.1,2 It has the added advantage of being FDA-approved for both AUD and OUD.
One obstacle to the success of XR-NTX is the induction period. The National Institute on Drug Abuse Clinical Trials Network X:BOT trial found that once the induction hurdle was surmounted, XR-NTX and buprenorphine were equally effective in a population of approximately 80% heroin users and two-thirds injection drug users.2 Patient variables that predict successful induction include young age, baseline preference for XR-NTX, fewer drug complications, and fewer family/social complications.3 If the length of the induction (usually 7 to 10 days) is a deterrent, a study supported the feasibility of a 5-day outpatient XR-NTX induction.4 Further research is needed to improve successful induction for XR-NTX.
Ashmeer Ogbuchi, MD
Karen Drexler, MD
Atlanta, Georgia
References
1. Tanum L, Solli KK, Latif Z, et al. Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence. JAMA Psychiatry. 2017;74(12):1197-1205. doi:10.1001/ jamapsychiatry.2017.3206
2. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/s0140-6736(17)32812-x
3. Murphy SM, Jeng PJ, McCollister KE, et al. Cost‐effectiveness implications of increasing the efficiency of the extended‐release naltrexone induction process for the treatment of opioid use disorder: a secondary analysis. Addiction. 2021;116(12)3444-3453. doi:10.1111/add.15531
4. Sibai M, Mishlen K, Nunes EV, et al. A week-long outpatient induction onto XR-naltrexone in patients with opioid use disorder. Am J Drug Alcohol Abuse. 2020;46(3):289-296. doi:10.1080/00952990.2019.1700265
Continue to: The authors respond
The authors respond
We appreciate Drs. Ogbuchi and Drexler for their thoughtful attention to our review. They proposed amending our original algorithm, recommending that XR-NTX be considered as another first-line option for patients with OUD. We agree with this suggestion, particularly for inpatients. However, we have some reservations about applying this suggestion to outpatient treatment. Though research evidence from Lee et al1 indicates that once initiation is completed, both medications are equally safe and effective, the initial attrition rate in the XR-NTX group was much higher (28% vs 6%, P < .0001), which suggests lower acceptability/tolerability compared with buprenorphine. Notably, the initiation of both medications in Lee et al1 was done in an inpatient setting. Moreover, although some medications are endorsed as “first-line,” the actual utilization rate is often influenced by many factors, including the ease of treatment initiation. Wakeman et al2 found the most common treatment modality received by patients with OUD was nonintensive behavioral health (59.5%), followed by inpatient withdrawal management and residential treatment (15.2%). Among all patients in the Wakeman study,2 only 12.5% received buprenorphine or methadone, and 2.4% received naltrexone.
Data from our clinic corroborate this trend. Currently, in our clinic approximately 300 patients with OUD are receiving medications, including approximately 250 on buprenorphine (including 5 to 10 on the long-acting injectable formulation), 50 on methadone, and only 1 or 2 on XR-NTX. Though this disparity may reflect bias in our clinicians’ prescribing practices, in the past few years we have had many unsuccessful attempts at initiating XR-NTX. To our disappointment, a theoretically excellent medication has not translated clinically. The recent surge in fentanyl use further complicates XR-NTX initiation for OUD, because the length of induction may be longer.
In conclusion, we agree that XR-NTX is a potential treatment option for patients with OUD, but clinicians should be cognizant of the potential barriers; inform patients of the advantages, expectations, and challenges; and respect patients’ informed decisions.
Rachel Gluck, MD
Karen Hochman, MD
Yi-lang Tang, MD, PhD
Atlanta, Georgia
References
1. Lee JD, Nunes EV Jr, Novo P, et al. Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): a multicentre, open-label, randomised controlled trial. Lancet. 2018;391(10118):309-318. doi:10.1016/s0140-6736(17)32812-x
2. Wakeman SE, Larochelle MR, Ameli O, et al. Comparative effectiveness of different treatment pathways for opioid use disorder. JAMA Netw Open. 2020;3(2):e1920622. doi:10.1001/jamanetworkopen.2019.20622
Reassuring data on stimulants for ADHD in kids and later substance abuse
“Throughout rigorous analyses, and after accounting for more than 70 variables in this longitudinal sample of children with ADHD taking stimulants, we did not find an association with later substance use,” lead investigator Brooke Molina, PhD, director of the youth and family research program at the University of Pittsburgh, said in an interview.
The findings were published online in JAMA Psychiatry.
Protective effect?
Owing to symptoms of impulsivity inherent to ADHD, the disorder itself carries a risk for elevated substance use, the investigators note.
They speculate that this may be why some previous research suggests prescription stimulants reduce the risk of subsequent substance use disorder. However, other studies have found no such protective link.
To shed more light on the issue, the investigators used data from the Multimodal Treatment Study of ADHD, a multicenter, 14-month randomized clinical trial of medication and behavioral therapy for children with ADHD. However, for the purposes of the present study, investigators focused only on stimulant use in children.
At the time of recruitment, the children were aged 7-9 and had been diagnosed with ADHD between 1994 and 1996.
Investigators assessed the participants prior to randomization, at months 3 and 9, and at the end of treatment. They were then followed for 16 years and were assessed at years 2, 3, 6, 8, 10, 12, 14, and 16 until a mean age of 25.
During 12-, 14-, and 16-year follow-up, participants completed a questionnaire on their use of alcohol, marijuana, cigarettes, and several illicit and prescription drugs.
Investigators collected information on participants’ stimulant treatment via the Services for Children and Adolescents Parent Interview until they reached age 18. After that, participants reported their own stimulant treatment.
A total of 579 participants were included in the analysis. Of these, 61% were White, 20% were Black, and 8% were Hispanic.
Decline in stimulant use over time
The analysis showed that stimulant use declined “precipitously” over time – from 60% at the 2- and 3-year assessments to an average of 7% during early adulthood.
The investigators also found that for some participants, substance use increased steadily through adolescence and remained stable through early adulthood. For instance, 36.5% of the adolescents in the total cohort reported smoking tobacco daily, and 29.6% reported using marijuana every week.
In addition, approximately 21% of the participants indulged in heavy drinking at least once a week, and 6% reported “other” substance use, which included sedative misuse, heroin, inhalants, hallucinogens, or other substances taken to “get high.”
After accounting for developmental trends in substance use in the sample through adolescence into early adulthood with several rigorous statistical models, the researchers found no association between current or prior stimulant treatment and cigarette, marijuana, alcohol, or other substance use, with one exception.
While cumulative stimulant treatment was associated with increased heavy drinking, the effect size of this association was small. Each additional year of cumulative stimulant use was estimated to increase participants’ likelihood of any binge drinking/drunkenness vs. none in the past year by 4% (95% confidence interval, 0.01-0.08; P =.03).
When the investigators used a causal analytic method to account for age and other time-varying characteristics, including household income, behavior problems, and parental support, there was no evidence that current (B range, –0.62-0.34) or prior stimulant treatment (B range, –0.06-0.70) or their interaction (B range, –0.49-0.86) was associated with substance use in adulthood.
Dr. Molina noted that although participants were recruited from multiple sites, the sample may not be generalizable because children and parents who present for an intensive treatment study such as this are not necessarily representative of the general ADHD population.
Reassuring findings
In a comment, Julie Schweitzer, PhD, professor of psychiatry and behavioral sciences at the University of California, Davis, said she hopes the study findings will quell the stigma surrounding stimulant use by children with ADHD.
“Parents’ fears that stimulant use will lead to a substance use disorder inhibits them from bringing their children for an ADHD evaluation, thus reducing the likelihood that they will receive timely treatment,” Dr. Schweitzer said.
“While stimulant medication is the first-line treatment most often recommended for most persons with ADHD, by not following through on evaluations, parents also miss the opportunity to learn about nonpharmacological strategies that might also be helpful to help cope with ADHD symptoms and its potential co-occurring challenges,” she added.
Dr. Schweitzer also noted that many parents hope their children will outgrow the symptoms without realizing that by not obtaining an evaluation and treatment for their child, there is an associated cost, including less than optimal academic performance, social relationships, and emotional health.
The Multimodal Treatment Study of Children with ADHD was a National Institute of Mental Health cooperative agreement randomized clinical trial, continued under an NIMH contract as a follow-up study and under a National Institute on Drug Abuse contract followed by a data analysis grant. Dr. Molina reported grants from the NIMH and the National Institute on Drug Abuse during the conduct of the study.
A version of this article first appeared on Medscape.com.
“Throughout rigorous analyses, and after accounting for more than 70 variables in this longitudinal sample of children with ADHD taking stimulants, we did not find an association with later substance use,” lead investigator Brooke Molina, PhD, director of the youth and family research program at the University of Pittsburgh, said in an interview.
The findings were published online in JAMA Psychiatry.
Protective effect?
Owing to symptoms of impulsivity inherent to ADHD, the disorder itself carries a risk for elevated substance use, the investigators note.
They speculate that this may be why some previous research suggests prescription stimulants reduce the risk of subsequent substance use disorder. However, other studies have found no such protective link.
To shed more light on the issue, the investigators used data from the Multimodal Treatment Study of ADHD, a multicenter, 14-month randomized clinical trial of medication and behavioral therapy for children with ADHD. However, for the purposes of the present study, investigators focused only on stimulant use in children.
At the time of recruitment, the children were aged 7-9 and had been diagnosed with ADHD between 1994 and 1996.
Investigators assessed the participants prior to randomization, at months 3 and 9, and at the end of treatment. They were then followed for 16 years and were assessed at years 2, 3, 6, 8, 10, 12, 14, and 16 until a mean age of 25.
During 12-, 14-, and 16-year follow-up, participants completed a questionnaire on their use of alcohol, marijuana, cigarettes, and several illicit and prescription drugs.
Investigators collected information on participants’ stimulant treatment via the Services for Children and Adolescents Parent Interview until they reached age 18. After that, participants reported their own stimulant treatment.
A total of 579 participants were included in the analysis. Of these, 61% were White, 20% were Black, and 8% were Hispanic.
Decline in stimulant use over time
The analysis showed that stimulant use declined “precipitously” over time – from 60% at the 2- and 3-year assessments to an average of 7% during early adulthood.
The investigators also found that for some participants, substance use increased steadily through adolescence and remained stable through early adulthood. For instance, 36.5% of the adolescents in the total cohort reported smoking tobacco daily, and 29.6% reported using marijuana every week.
In addition, approximately 21% of the participants indulged in heavy drinking at least once a week, and 6% reported “other” substance use, which included sedative misuse, heroin, inhalants, hallucinogens, or other substances taken to “get high.”
After accounting for developmental trends in substance use in the sample through adolescence into early adulthood with several rigorous statistical models, the researchers found no association between current or prior stimulant treatment and cigarette, marijuana, alcohol, or other substance use, with one exception.
While cumulative stimulant treatment was associated with increased heavy drinking, the effect size of this association was small. Each additional year of cumulative stimulant use was estimated to increase participants’ likelihood of any binge drinking/drunkenness vs. none in the past year by 4% (95% confidence interval, 0.01-0.08; P =.03).
When the investigators used a causal analytic method to account for age and other time-varying characteristics, including household income, behavior problems, and parental support, there was no evidence that current (B range, –0.62-0.34) or prior stimulant treatment (B range, –0.06-0.70) or their interaction (B range, –0.49-0.86) was associated with substance use in adulthood.
Dr. Molina noted that although participants were recruited from multiple sites, the sample may not be generalizable because children and parents who present for an intensive treatment study such as this are not necessarily representative of the general ADHD population.
Reassuring findings
In a comment, Julie Schweitzer, PhD, professor of psychiatry and behavioral sciences at the University of California, Davis, said she hopes the study findings will quell the stigma surrounding stimulant use by children with ADHD.
“Parents’ fears that stimulant use will lead to a substance use disorder inhibits them from bringing their children for an ADHD evaluation, thus reducing the likelihood that they will receive timely treatment,” Dr. Schweitzer said.
“While stimulant medication is the first-line treatment most often recommended for most persons with ADHD, by not following through on evaluations, parents also miss the opportunity to learn about nonpharmacological strategies that might also be helpful to help cope with ADHD symptoms and its potential co-occurring challenges,” she added.
Dr. Schweitzer also noted that many parents hope their children will outgrow the symptoms without realizing that by not obtaining an evaluation and treatment for their child, there is an associated cost, including less than optimal academic performance, social relationships, and emotional health.
The Multimodal Treatment Study of Children with ADHD was a National Institute of Mental Health cooperative agreement randomized clinical trial, continued under an NIMH contract as a follow-up study and under a National Institute on Drug Abuse contract followed by a data analysis grant. Dr. Molina reported grants from the NIMH and the National Institute on Drug Abuse during the conduct of the study.
A version of this article first appeared on Medscape.com.
“Throughout rigorous analyses, and after accounting for more than 70 variables in this longitudinal sample of children with ADHD taking stimulants, we did not find an association with later substance use,” lead investigator Brooke Molina, PhD, director of the youth and family research program at the University of Pittsburgh, said in an interview.
The findings were published online in JAMA Psychiatry.
Protective effect?
Owing to symptoms of impulsivity inherent to ADHD, the disorder itself carries a risk for elevated substance use, the investigators note.
They speculate that this may be why some previous research suggests prescription stimulants reduce the risk of subsequent substance use disorder. However, other studies have found no such protective link.
To shed more light on the issue, the investigators used data from the Multimodal Treatment Study of ADHD, a multicenter, 14-month randomized clinical trial of medication and behavioral therapy for children with ADHD. However, for the purposes of the present study, investigators focused only on stimulant use in children.
At the time of recruitment, the children were aged 7-9 and had been diagnosed with ADHD between 1994 and 1996.
Investigators assessed the participants prior to randomization, at months 3 and 9, and at the end of treatment. They were then followed for 16 years and were assessed at years 2, 3, 6, 8, 10, 12, 14, and 16 until a mean age of 25.
During 12-, 14-, and 16-year follow-up, participants completed a questionnaire on their use of alcohol, marijuana, cigarettes, and several illicit and prescription drugs.
Investigators collected information on participants’ stimulant treatment via the Services for Children and Adolescents Parent Interview until they reached age 18. After that, participants reported their own stimulant treatment.
A total of 579 participants were included in the analysis. Of these, 61% were White, 20% were Black, and 8% were Hispanic.
Decline in stimulant use over time
The analysis showed that stimulant use declined “precipitously” over time – from 60% at the 2- and 3-year assessments to an average of 7% during early adulthood.
The investigators also found that for some participants, substance use increased steadily through adolescence and remained stable through early adulthood. For instance, 36.5% of the adolescents in the total cohort reported smoking tobacco daily, and 29.6% reported using marijuana every week.
In addition, approximately 21% of the participants indulged in heavy drinking at least once a week, and 6% reported “other” substance use, which included sedative misuse, heroin, inhalants, hallucinogens, or other substances taken to “get high.”
After accounting for developmental trends in substance use in the sample through adolescence into early adulthood with several rigorous statistical models, the researchers found no association between current or prior stimulant treatment and cigarette, marijuana, alcohol, or other substance use, with one exception.
While cumulative stimulant treatment was associated with increased heavy drinking, the effect size of this association was small. Each additional year of cumulative stimulant use was estimated to increase participants’ likelihood of any binge drinking/drunkenness vs. none in the past year by 4% (95% confidence interval, 0.01-0.08; P =.03).
When the investigators used a causal analytic method to account for age and other time-varying characteristics, including household income, behavior problems, and parental support, there was no evidence that current (B range, –0.62-0.34) or prior stimulant treatment (B range, –0.06-0.70) or their interaction (B range, –0.49-0.86) was associated with substance use in adulthood.
Dr. Molina noted that although participants were recruited from multiple sites, the sample may not be generalizable because children and parents who present for an intensive treatment study such as this are not necessarily representative of the general ADHD population.
Reassuring findings
In a comment, Julie Schweitzer, PhD, professor of psychiatry and behavioral sciences at the University of California, Davis, said she hopes the study findings will quell the stigma surrounding stimulant use by children with ADHD.
“Parents’ fears that stimulant use will lead to a substance use disorder inhibits them from bringing their children for an ADHD evaluation, thus reducing the likelihood that they will receive timely treatment,” Dr. Schweitzer said.
“While stimulant medication is the first-line treatment most often recommended for most persons with ADHD, by not following through on evaluations, parents also miss the opportunity to learn about nonpharmacological strategies that might also be helpful to help cope with ADHD symptoms and its potential co-occurring challenges,” she added.
Dr. Schweitzer also noted that many parents hope their children will outgrow the symptoms without realizing that by not obtaining an evaluation and treatment for their child, there is an associated cost, including less than optimal academic performance, social relationships, and emotional health.
The Multimodal Treatment Study of Children with ADHD was a National Institute of Mental Health cooperative agreement randomized clinical trial, continued under an NIMH contract as a follow-up study and under a National Institute on Drug Abuse contract followed by a data analysis grant. Dr. Molina reported grants from the NIMH and the National Institute on Drug Abuse during the conduct of the study.
A version of this article first appeared on Medscape.com.
FROM JAMA PSYCHIATRY
Psilocybin shows early promise for anorexia nervosa
The psychedelic psilocybin may have a role in the treatment of anorexia nervosa (AN), an eating disorder that is notoriously difficult and costly to treat.
Stephanie Knatz Peck, PhD, and colleagues with the eating disorders treatment & research center, University of California San Diego, write that the “robust response” in a subset of women after a single dose of psilocybin is “notable,” given that currently available treatments for adult anorexia result in only modest improvements in symptoms and often focus on weight and nutritional rehabilitation without adequately addressing underlying psychopathology.
However, given this was a small, phase 1, open-label feasibility study, these effects are “preliminary and inconclusive,” they caution.
The study was published online in Nature Medicine.
Meaningful experience
The 10 women in the study met DSM-5 criteria for AN or partial remission of AN. They were between age 18 and 40 years with a mean body mass index (BMI) of 19.7 kg/m2.
Following the single 25-mg dose of psilocybin, no clinically significant changes were observed in ECG, vital signs, laboratory values, or suicidality.
All adverse events were mild and mirrored typical psilocybin-associated symptoms such as transient headache, nausea, and fatigue.
Psilocybin was associated with reduced levels of anxiety and preoccupations surrounding food, eating, and body shape at the 1-month follow-up.
Weight concerns decreased significantly at the 1-month (P = .036, Cohen’s d = .78) and 3-month (P = .04, d = .78) follow-up, with a medium to large effect.
Shape concerns significantly decreased at 1-month follow-up (P = .036, d = .78) but were no longer significant at 3-month follow-up (P = .081, d = .62).
Four of the 10 women (40%) had clinically significant reductions in eating disorder scores at 3 months, which qualified for remission from eating-disorder psychopathology.
However, the researchers caution that the effects on eating disorder psychopathology were “highly variable.”
On average, changes in BMI were not significant during the 3 months following psilocybin treatment. However, five women had an increase in BMI at 3 months, ranging from 0.4 to 1.2 kg/m2.
Overall, the psilocybin experience was regarded as meaningful by participants; 80% endorsed the experience as one of the top five most meaningful of life; 90% endorsed feeling more positive about life endeavors; and 70% reported experiencing a shift in personal identity and overall quality of life.
The vast majority of women (90%) felt that one dosing session was not enough.
The fact that the treatment was regarded as beneficial by most women and that there were no dropouts are “promising signs of engagement,” given that dropout rates for currently available AN treatments tend to be high, the researchers note.
They urge caution in interpreting the results considering they were based on a small sample size and did not include a placebo group. They note that larger, adequately powered, randomized controlled trials are needed to draw any conclusions about the role of psilocybin for anorexia nervosa.
Encouraging data
The coauthors of a Nature Medicine News & Views commentary say this “encouraging” phase 1 trial “underscores the necessity for more research into classic psychedelics to address the urgent need for effective treatments” for AN.
Outside experts also weighed in on the study in a statement from the U.K.-based nonprofit Science Media Centre.
Alexandra Pike, DPhil, MSc, with University of York, England, noted that this study is “a first step in showing that psilocybin may be a safe treatment for those with anorexia nervosa, but we cannot conclude from this work that it will be effective in this chronic, complex illness.”
Trevor Steward, MD, with University of Melbourne, noted that psilocybin therapy has provided “glimmers of hope in other mental health disorders, notably by providing evidence that it can improve anxiety, cognitive flexibility, and self-acceptance for some people. These are all features of anorexia nervosa and the rationale for exploring psilocybin therapy as an option in the case of anorexia is strong.”
Dr. Steward also noted that the field is only beginning to “scratch the surface in terms of understanding how psilocybin impacts the brain. Dedicated funding to exploring how it specifically acts to target anorexia nervosa symptoms is crucial to advancing this important avenue of research.
“As there are no approved medications available specifically for anorexia nervosa treatment, psilocybin therapy may prove to be a promising option, though additional research is needed to test this,” Dr. Steward said.
The study used an investigational synthetic formulation of psilocybin (COMP360 psilocybin) developed by COMPASS Pathways, which funded the study. Two coauthors have financial and scientific relationships with COMPASS Pathways. The commentary authors and Dr. Steward report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The psychedelic psilocybin may have a role in the treatment of anorexia nervosa (AN), an eating disorder that is notoriously difficult and costly to treat.
Stephanie Knatz Peck, PhD, and colleagues with the eating disorders treatment & research center, University of California San Diego, write that the “robust response” in a subset of women after a single dose of psilocybin is “notable,” given that currently available treatments for adult anorexia result in only modest improvements in symptoms and often focus on weight and nutritional rehabilitation without adequately addressing underlying psychopathology.
However, given this was a small, phase 1, open-label feasibility study, these effects are “preliminary and inconclusive,” they caution.
The study was published online in Nature Medicine.
Meaningful experience
The 10 women in the study met DSM-5 criteria for AN or partial remission of AN. They were between age 18 and 40 years with a mean body mass index (BMI) of 19.7 kg/m2.
Following the single 25-mg dose of psilocybin, no clinically significant changes were observed in ECG, vital signs, laboratory values, or suicidality.
All adverse events were mild and mirrored typical psilocybin-associated symptoms such as transient headache, nausea, and fatigue.
Psilocybin was associated with reduced levels of anxiety and preoccupations surrounding food, eating, and body shape at the 1-month follow-up.
Weight concerns decreased significantly at the 1-month (P = .036, Cohen’s d = .78) and 3-month (P = .04, d = .78) follow-up, with a medium to large effect.
Shape concerns significantly decreased at 1-month follow-up (P = .036, d = .78) but were no longer significant at 3-month follow-up (P = .081, d = .62).
Four of the 10 women (40%) had clinically significant reductions in eating disorder scores at 3 months, which qualified for remission from eating-disorder psychopathology.
However, the researchers caution that the effects on eating disorder psychopathology were “highly variable.”
On average, changes in BMI were not significant during the 3 months following psilocybin treatment. However, five women had an increase in BMI at 3 months, ranging from 0.4 to 1.2 kg/m2.
Overall, the psilocybin experience was regarded as meaningful by participants; 80% endorsed the experience as one of the top five most meaningful of life; 90% endorsed feeling more positive about life endeavors; and 70% reported experiencing a shift in personal identity and overall quality of life.
The vast majority of women (90%) felt that one dosing session was not enough.
The fact that the treatment was regarded as beneficial by most women and that there were no dropouts are “promising signs of engagement,” given that dropout rates for currently available AN treatments tend to be high, the researchers note.
They urge caution in interpreting the results considering they were based on a small sample size and did not include a placebo group. They note that larger, adequately powered, randomized controlled trials are needed to draw any conclusions about the role of psilocybin for anorexia nervosa.
Encouraging data
The coauthors of a Nature Medicine News & Views commentary say this “encouraging” phase 1 trial “underscores the necessity for more research into classic psychedelics to address the urgent need for effective treatments” for AN.
Outside experts also weighed in on the study in a statement from the U.K.-based nonprofit Science Media Centre.
Alexandra Pike, DPhil, MSc, with University of York, England, noted that this study is “a first step in showing that psilocybin may be a safe treatment for those with anorexia nervosa, but we cannot conclude from this work that it will be effective in this chronic, complex illness.”
Trevor Steward, MD, with University of Melbourne, noted that psilocybin therapy has provided “glimmers of hope in other mental health disorders, notably by providing evidence that it can improve anxiety, cognitive flexibility, and self-acceptance for some people. These are all features of anorexia nervosa and the rationale for exploring psilocybin therapy as an option in the case of anorexia is strong.”
Dr. Steward also noted that the field is only beginning to “scratch the surface in terms of understanding how psilocybin impacts the brain. Dedicated funding to exploring how it specifically acts to target anorexia nervosa symptoms is crucial to advancing this important avenue of research.
“As there are no approved medications available specifically for anorexia nervosa treatment, psilocybin therapy may prove to be a promising option, though additional research is needed to test this,” Dr. Steward said.
The study used an investigational synthetic formulation of psilocybin (COMP360 psilocybin) developed by COMPASS Pathways, which funded the study. Two coauthors have financial and scientific relationships with COMPASS Pathways. The commentary authors and Dr. Steward report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The psychedelic psilocybin may have a role in the treatment of anorexia nervosa (AN), an eating disorder that is notoriously difficult and costly to treat.
Stephanie Knatz Peck, PhD, and colleagues with the eating disorders treatment & research center, University of California San Diego, write that the “robust response” in a subset of women after a single dose of psilocybin is “notable,” given that currently available treatments for adult anorexia result in only modest improvements in symptoms and often focus on weight and nutritional rehabilitation without adequately addressing underlying psychopathology.
However, given this was a small, phase 1, open-label feasibility study, these effects are “preliminary and inconclusive,” they caution.
The study was published online in Nature Medicine.
Meaningful experience
The 10 women in the study met DSM-5 criteria for AN or partial remission of AN. They were between age 18 and 40 years with a mean body mass index (BMI) of 19.7 kg/m2.
Following the single 25-mg dose of psilocybin, no clinically significant changes were observed in ECG, vital signs, laboratory values, or suicidality.
All adverse events were mild and mirrored typical psilocybin-associated symptoms such as transient headache, nausea, and fatigue.
Psilocybin was associated with reduced levels of anxiety and preoccupations surrounding food, eating, and body shape at the 1-month follow-up.
Weight concerns decreased significantly at the 1-month (P = .036, Cohen’s d = .78) and 3-month (P = .04, d = .78) follow-up, with a medium to large effect.
Shape concerns significantly decreased at 1-month follow-up (P = .036, d = .78) but were no longer significant at 3-month follow-up (P = .081, d = .62).
Four of the 10 women (40%) had clinically significant reductions in eating disorder scores at 3 months, which qualified for remission from eating-disorder psychopathology.
However, the researchers caution that the effects on eating disorder psychopathology were “highly variable.”
On average, changes in BMI were not significant during the 3 months following psilocybin treatment. However, five women had an increase in BMI at 3 months, ranging from 0.4 to 1.2 kg/m2.
Overall, the psilocybin experience was regarded as meaningful by participants; 80% endorsed the experience as one of the top five most meaningful of life; 90% endorsed feeling more positive about life endeavors; and 70% reported experiencing a shift in personal identity and overall quality of life.
The vast majority of women (90%) felt that one dosing session was not enough.
The fact that the treatment was regarded as beneficial by most women and that there were no dropouts are “promising signs of engagement,” given that dropout rates for currently available AN treatments tend to be high, the researchers note.
They urge caution in interpreting the results considering they were based on a small sample size and did not include a placebo group. They note that larger, adequately powered, randomized controlled trials are needed to draw any conclusions about the role of psilocybin for anorexia nervosa.
Encouraging data
The coauthors of a Nature Medicine News & Views commentary say this “encouraging” phase 1 trial “underscores the necessity for more research into classic psychedelics to address the urgent need for effective treatments” for AN.
Outside experts also weighed in on the study in a statement from the U.K.-based nonprofit Science Media Centre.
Alexandra Pike, DPhil, MSc, with University of York, England, noted that this study is “a first step in showing that psilocybin may be a safe treatment for those with anorexia nervosa, but we cannot conclude from this work that it will be effective in this chronic, complex illness.”
Trevor Steward, MD, with University of Melbourne, noted that psilocybin therapy has provided “glimmers of hope in other mental health disorders, notably by providing evidence that it can improve anxiety, cognitive flexibility, and self-acceptance for some people. These are all features of anorexia nervosa and the rationale for exploring psilocybin therapy as an option in the case of anorexia is strong.”
Dr. Steward also noted that the field is only beginning to “scratch the surface in terms of understanding how psilocybin impacts the brain. Dedicated funding to exploring how it specifically acts to target anorexia nervosa symptoms is crucial to advancing this important avenue of research.
“As there are no approved medications available specifically for anorexia nervosa treatment, psilocybin therapy may prove to be a promising option, though additional research is needed to test this,” Dr. Steward said.
The study used an investigational synthetic formulation of psilocybin (COMP360 psilocybin) developed by COMPASS Pathways, which funded the study. Two coauthors have financial and scientific relationships with COMPASS Pathways. The commentary authors and Dr. Steward report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
Fast-acting postpartum depression drug is effective
The Food and Drug Administration is considering approving a postpartum depression medication that can start working rapidly – in as little as 3 days. Promising results for the drug, zuranolone, were published recently in The American Journal of Psychiatry.
Approximately 17% of women are affected by postpartum depression (PPD) during pregnancy or after birth, study authors noted. The condition often results in reduced breastfeeding, poor maternal-infant bonding, and hindering behavioral, emotional and brain development of the baby. Severe PPD can lead to suicide of the mother, which accounts for 20% of all postpartum deaths, they wrote.
The study included 196 people who had given birth in the past year, and were between the ages of 18 and 45 years old. Participants had major depression that began in the 3rd trimester of pregnancy or during the first 4 weeks of the postpartum period. Among participants, 22% were Black and 38% were Hispanic.
The average time it took for symptoms to significantly decline was 9 days. Most people who saw improvements had them continue for the entire 45-day follow-up period. The most common side effects were drowsiness, dizziness, and sleepiness.
Currently, PPD treatment includes taking antidepressants, which can take up to 12 weeks to work.
Researchers noted that the limitations of the study were that it only included women with severe PPD, and that women with a history of bipolar or psychotic disorders were excluded. Women in the study were not allowed to breastfeed, so the effect of zuranolone on lactation is unknown, they wrote.
A February news release from drugmaker Biogen indicated the FDA may decide whether to approve the medicine by Aug. 5.
A version of this article first appeared on WebMD.com.
The Food and Drug Administration is considering approving a postpartum depression medication that can start working rapidly – in as little as 3 days. Promising results for the drug, zuranolone, were published recently in The American Journal of Psychiatry.
Approximately 17% of women are affected by postpartum depression (PPD) during pregnancy or after birth, study authors noted. The condition often results in reduced breastfeeding, poor maternal-infant bonding, and hindering behavioral, emotional and brain development of the baby. Severe PPD can lead to suicide of the mother, which accounts for 20% of all postpartum deaths, they wrote.
The study included 196 people who had given birth in the past year, and were between the ages of 18 and 45 years old. Participants had major depression that began in the 3rd trimester of pregnancy or during the first 4 weeks of the postpartum period. Among participants, 22% were Black and 38% were Hispanic.
The average time it took for symptoms to significantly decline was 9 days. Most people who saw improvements had them continue for the entire 45-day follow-up period. The most common side effects were drowsiness, dizziness, and sleepiness.
Currently, PPD treatment includes taking antidepressants, which can take up to 12 weeks to work.
Researchers noted that the limitations of the study were that it only included women with severe PPD, and that women with a history of bipolar or psychotic disorders were excluded. Women in the study were not allowed to breastfeed, so the effect of zuranolone on lactation is unknown, they wrote.
A February news release from drugmaker Biogen indicated the FDA may decide whether to approve the medicine by Aug. 5.
A version of this article first appeared on WebMD.com.
The Food and Drug Administration is considering approving a postpartum depression medication that can start working rapidly – in as little as 3 days. Promising results for the drug, zuranolone, were published recently in The American Journal of Psychiatry.
Approximately 17% of women are affected by postpartum depression (PPD) during pregnancy or after birth, study authors noted. The condition often results in reduced breastfeeding, poor maternal-infant bonding, and hindering behavioral, emotional and brain development of the baby. Severe PPD can lead to suicide of the mother, which accounts for 20% of all postpartum deaths, they wrote.
The study included 196 people who had given birth in the past year, and were between the ages of 18 and 45 years old. Participants had major depression that began in the 3rd trimester of pregnancy or during the first 4 weeks of the postpartum period. Among participants, 22% were Black and 38% were Hispanic.
The average time it took for symptoms to significantly decline was 9 days. Most people who saw improvements had them continue for the entire 45-day follow-up period. The most common side effects were drowsiness, dizziness, and sleepiness.
Currently, PPD treatment includes taking antidepressants, which can take up to 12 weeks to work.
Researchers noted that the limitations of the study were that it only included women with severe PPD, and that women with a history of bipolar or psychotic disorders were excluded. Women in the study were not allowed to breastfeed, so the effect of zuranolone on lactation is unknown, they wrote.
A February news release from drugmaker Biogen indicated the FDA may decide whether to approve the medicine by Aug. 5.
A version of this article first appeared on WebMD.com.
FROM THE AMERICAN JOURNAL OF PSYCHIATRY
Hospital guards snoop through patient records, cost hospital $240K
Yakima Valley Memorial Hospital agreed to the voluntary settlement after an investigation into the actions of 23 emergency department security guards who allegedly used their login credentials to access the patient medical records of 419 patients.
The information accessed included names, dates of birth, medical record numbers, addresses, certain notes related to treatment, and insurance information, according to a release by the U.S .Department of Health & Human Services’ Office for Civil Rights (OCR). A breach notification report alerted OCR to the snooping.
As part of the agreement, OCR will monitor Yakima Valley Memorial Hospital for 2 years and the hospital must conduct a thorough risk analysis as well as develop a risk management plan to address and mitigate identified security risks and vulnerabilities. The settlement is not considered an admission of guilt by the hospital.
Is such snooping common?
The incident highlights the frequent practice of employees snooping through medical records and the steep consequences that can result for providers, said Paul Redding, vice president of partner engagement and cybersecurity at Compliancy Group, a company that offers guided HIPAA compliance software for healthcare providers and vendors.
“I think the problem is absolutely growing,” he said. “What’s crazy about this case is it’s actually a really small HIPAA violation. Less than 500 people were affected, and the hospital still must pay a quarter-of-a-million-dollar settlement. If you take the average HIPAA violation, which is in the thousands and thousands of [patients], this amount would be magnified many times over.”
In general, employees snoop through records out of curiosity or to find out information about people they know – or want to learn about, said J. David Sims, a cybersecurity expert and CEO of Security First IT, a company that provides cybersecurity solutions and IT support to health care businesses.
Mr. Sims says he has heard of cases where health professionals snooped through records to find information about the new love interests of ex-partners or to learn about people on dating websites whom they’re interested in dating.
“Most of the time, it’s people being nosy,” he said. “In a lot of cases, it’s curiosity about famous people. You see it a lot in areas where you have football players who come in with injuries or you have an actor or actress who come in for something.”
“Data breaches caused by current and former workforce members impermissibly accessing patient records are a recurring issue across the health care industry. Health care organizations must ensure that workforce members can only access the patient information needed to do their jobs,” OCR director Melanie Fontes Rainer said in a June statement. “HIPAA-covered entities must have robust policies and procedures in place to ensure patient health information is protected from identify theft and fraud.”
Yakima Valley Memorial Hospital did not return a message seeking comment.
According to OCR’s latest report to Congress, complaints about HIPAA violations increased by 39% between 2017 and 2021. Breaches affecting fewer than 500 individuals rose by 5% during the same time period, and breaches impacting 500 or more individuals increased by 58%.
Common reasons employees snoop
The OCR announcement does not specify why the 23 security guards were accessing the medical records, but the incident raises questions about why the security guards had access to protected health information (PHI) in the first place, Mr. Redding said.
“I have yet to have anyone explain to me why the security guards would have access to PHI at all, at any level,” he said. “Was it by design or was it by error?”
In 2019 for instance, dozens of employees at Northwestern Memorial Hospital in Chicago were fired for accessing the health records of former Empire actor Jussie Smollett. In another high-profile case, nearly a dozen emergency medical service employees were caught snooping through 911 records connected to the treatment and, later, death of Joan Rivers.
“Sadly, there is a lack of education around what compliance really means inside the medical industry as a whole,” Mr. Redding said. “There is a lack of employee training and a lack of emphasis on accountability for employees.”
Privacy breaches fuel lawsuits
Health professionals caught snooping through records are frequently terminated and employers can face a range of ramifications, including civil and criminal penalties.
A growing trend is class action lawsuits associated with privacy violations, Mr. Redding adds.
Because patients are unable to sue in civil court for HIPAA breaches, they frequently sue for “breach of an implied contract,” he explained. In such cases, patients allege that the privacy documents they signed with health care providers established an implied contract, and their records being exposed constituted a contract breach.
“Class action lawsuits are starting to become extremely common,” Mr. Redding said. “It’s happening in many cases, even sometimes before Health & Human Services issue a fine, that [providers] are being wrapped into a class action lawsuit.”
Mayo Clinic, for example, was recently slapped with a class action suit after a former employee inappropriately accessed the records of 1,600 patients. Mayo settled the suit in January 2023, the terms of which were not publicly disclosed.
Multiple patients also filed a class action suit against San Diego–based Scripps Health after its data were hit with a cyberattack and subsequent breach that impacted close to 2 million people. Scripps reached a $3.5 million settlement with the plaintiffs in 2023.
Some practices and employers may also face state penalties for data privacy breaches, depending on their jurisdiction. In July, Connecticut became the fifth state to enact a comprehensive data privacy law. The measure, which creates a robust framework for protecting health-related records and other data, includes civil penalties of up to $5,000 for violations. Other states, including California, Virginia, Utah, and Colorado, also have state data privacy laws on the books.
How can practices stop snooping?
A first step to preventing snooping is conducting a thorough risk assessment, said David Harlow, a health care attorney and chief compliance and privacy officer for Insulet Corporation, a medical device company. The analysis should address who has access to what data and whether they really need such access, he said.
“Then it’s putting in place the proper controls to ensure access is limited and use is limited to the appropriate individuals and circumstances,” Mr. Harlow said.
Regulators don’t expect a giant academic medical center and a small private physician practice to take an identical HIPAA compliance approach, he stressed. The ideal approach will vary by entity. Providers just need to address the standards in a way that makes sense for their operation, he said.
Training is also a critical component, adds Mr. Sims.
“Having training is key,” he said. “Oftentimes, an employee might think, ‘Well, if I can click on this data and it comes up, obviously, I can look at it.’ They need to understand what information they are and are not allowed to access.”
Keep in mind that settings or controls might change when larger transitions take place, such as moving to a new electronic health record system, Mr. Sims said. It’s essential to reevaluate controls when changes in the practice take place to ensure that everything is functioning correctly.
Mr. Sims also suggests that practices create a type of “If you see something, say something,” policy that encourages fellow physicians and employees to report anything that looks suspicious within electronic logs. If an employee, for instance, is suddenly looking at many more records than usual or at odd times of the day or night, this should raise red flags.
“It’s great to stop it early so that it doesn’t become a bigger issue for the practice to deal with, but also, from a legal standpoint, you want to have a defensible argument that you were doing all you could to stop this as quickly as possible,” he said. “It puts you in a better position to defend yourself.”
The snooping security guards case holds an important lesson for all health providers, Mr. Harlow said.
“This is a message to all of us, that you need to have done the assessment up front,” he said. You need to have the right controls in place up front. This is not a situation where somebody managed to hack into a system for some devious means. This is someone who was given keys. Why were they given the keys?”
A version of this article first appeared on Medscape.com.
Yakima Valley Memorial Hospital agreed to the voluntary settlement after an investigation into the actions of 23 emergency department security guards who allegedly used their login credentials to access the patient medical records of 419 patients.
The information accessed included names, dates of birth, medical record numbers, addresses, certain notes related to treatment, and insurance information, according to a release by the U.S .Department of Health & Human Services’ Office for Civil Rights (OCR). A breach notification report alerted OCR to the snooping.
As part of the agreement, OCR will monitor Yakima Valley Memorial Hospital for 2 years and the hospital must conduct a thorough risk analysis as well as develop a risk management plan to address and mitigate identified security risks and vulnerabilities. The settlement is not considered an admission of guilt by the hospital.
Is such snooping common?
The incident highlights the frequent practice of employees snooping through medical records and the steep consequences that can result for providers, said Paul Redding, vice president of partner engagement and cybersecurity at Compliancy Group, a company that offers guided HIPAA compliance software for healthcare providers and vendors.
“I think the problem is absolutely growing,” he said. “What’s crazy about this case is it’s actually a really small HIPAA violation. Less than 500 people were affected, and the hospital still must pay a quarter-of-a-million-dollar settlement. If you take the average HIPAA violation, which is in the thousands and thousands of [patients], this amount would be magnified many times over.”
In general, employees snoop through records out of curiosity or to find out information about people they know – or want to learn about, said J. David Sims, a cybersecurity expert and CEO of Security First IT, a company that provides cybersecurity solutions and IT support to health care businesses.
Mr. Sims says he has heard of cases where health professionals snooped through records to find information about the new love interests of ex-partners or to learn about people on dating websites whom they’re interested in dating.
“Most of the time, it’s people being nosy,” he said. “In a lot of cases, it’s curiosity about famous people. You see it a lot in areas where you have football players who come in with injuries or you have an actor or actress who come in for something.”
“Data breaches caused by current and former workforce members impermissibly accessing patient records are a recurring issue across the health care industry. Health care organizations must ensure that workforce members can only access the patient information needed to do their jobs,” OCR director Melanie Fontes Rainer said in a June statement. “HIPAA-covered entities must have robust policies and procedures in place to ensure patient health information is protected from identify theft and fraud.”
Yakima Valley Memorial Hospital did not return a message seeking comment.
According to OCR’s latest report to Congress, complaints about HIPAA violations increased by 39% between 2017 and 2021. Breaches affecting fewer than 500 individuals rose by 5% during the same time period, and breaches impacting 500 or more individuals increased by 58%.
Common reasons employees snoop
The OCR announcement does not specify why the 23 security guards were accessing the medical records, but the incident raises questions about why the security guards had access to protected health information (PHI) in the first place, Mr. Redding said.
“I have yet to have anyone explain to me why the security guards would have access to PHI at all, at any level,” he said. “Was it by design or was it by error?”
In 2019 for instance, dozens of employees at Northwestern Memorial Hospital in Chicago were fired for accessing the health records of former Empire actor Jussie Smollett. In another high-profile case, nearly a dozen emergency medical service employees were caught snooping through 911 records connected to the treatment and, later, death of Joan Rivers.
“Sadly, there is a lack of education around what compliance really means inside the medical industry as a whole,” Mr. Redding said. “There is a lack of employee training and a lack of emphasis on accountability for employees.”
Privacy breaches fuel lawsuits
Health professionals caught snooping through records are frequently terminated and employers can face a range of ramifications, including civil and criminal penalties.
A growing trend is class action lawsuits associated with privacy violations, Mr. Redding adds.
Because patients are unable to sue in civil court for HIPAA breaches, they frequently sue for “breach of an implied contract,” he explained. In such cases, patients allege that the privacy documents they signed with health care providers established an implied contract, and their records being exposed constituted a contract breach.
“Class action lawsuits are starting to become extremely common,” Mr. Redding said. “It’s happening in many cases, even sometimes before Health & Human Services issue a fine, that [providers] are being wrapped into a class action lawsuit.”
Mayo Clinic, for example, was recently slapped with a class action suit after a former employee inappropriately accessed the records of 1,600 patients. Mayo settled the suit in January 2023, the terms of which were not publicly disclosed.
Multiple patients also filed a class action suit against San Diego–based Scripps Health after its data were hit with a cyberattack and subsequent breach that impacted close to 2 million people. Scripps reached a $3.5 million settlement with the plaintiffs in 2023.
Some practices and employers may also face state penalties for data privacy breaches, depending on their jurisdiction. In July, Connecticut became the fifth state to enact a comprehensive data privacy law. The measure, which creates a robust framework for protecting health-related records and other data, includes civil penalties of up to $5,000 for violations. Other states, including California, Virginia, Utah, and Colorado, also have state data privacy laws on the books.
How can practices stop snooping?
A first step to preventing snooping is conducting a thorough risk assessment, said David Harlow, a health care attorney and chief compliance and privacy officer for Insulet Corporation, a medical device company. The analysis should address who has access to what data and whether they really need such access, he said.
“Then it’s putting in place the proper controls to ensure access is limited and use is limited to the appropriate individuals and circumstances,” Mr. Harlow said.
Regulators don’t expect a giant academic medical center and a small private physician practice to take an identical HIPAA compliance approach, he stressed. The ideal approach will vary by entity. Providers just need to address the standards in a way that makes sense for their operation, he said.
Training is also a critical component, adds Mr. Sims.
“Having training is key,” he said. “Oftentimes, an employee might think, ‘Well, if I can click on this data and it comes up, obviously, I can look at it.’ They need to understand what information they are and are not allowed to access.”
Keep in mind that settings or controls might change when larger transitions take place, such as moving to a new electronic health record system, Mr. Sims said. It’s essential to reevaluate controls when changes in the practice take place to ensure that everything is functioning correctly.
Mr. Sims also suggests that practices create a type of “If you see something, say something,” policy that encourages fellow physicians and employees to report anything that looks suspicious within electronic logs. If an employee, for instance, is suddenly looking at many more records than usual or at odd times of the day or night, this should raise red flags.
“It’s great to stop it early so that it doesn’t become a bigger issue for the practice to deal with, but also, from a legal standpoint, you want to have a defensible argument that you were doing all you could to stop this as quickly as possible,” he said. “It puts you in a better position to defend yourself.”
The snooping security guards case holds an important lesson for all health providers, Mr. Harlow said.
“This is a message to all of us, that you need to have done the assessment up front,” he said. You need to have the right controls in place up front. This is not a situation where somebody managed to hack into a system for some devious means. This is someone who was given keys. Why were they given the keys?”
A version of this article first appeared on Medscape.com.
Yakima Valley Memorial Hospital agreed to the voluntary settlement after an investigation into the actions of 23 emergency department security guards who allegedly used their login credentials to access the patient medical records of 419 patients.
The information accessed included names, dates of birth, medical record numbers, addresses, certain notes related to treatment, and insurance information, according to a release by the U.S .Department of Health & Human Services’ Office for Civil Rights (OCR). A breach notification report alerted OCR to the snooping.
As part of the agreement, OCR will monitor Yakima Valley Memorial Hospital for 2 years and the hospital must conduct a thorough risk analysis as well as develop a risk management plan to address and mitigate identified security risks and vulnerabilities. The settlement is not considered an admission of guilt by the hospital.
Is such snooping common?
The incident highlights the frequent practice of employees snooping through medical records and the steep consequences that can result for providers, said Paul Redding, vice president of partner engagement and cybersecurity at Compliancy Group, a company that offers guided HIPAA compliance software for healthcare providers and vendors.
“I think the problem is absolutely growing,” he said. “What’s crazy about this case is it’s actually a really small HIPAA violation. Less than 500 people were affected, and the hospital still must pay a quarter-of-a-million-dollar settlement. If you take the average HIPAA violation, which is in the thousands and thousands of [patients], this amount would be magnified many times over.”
In general, employees snoop through records out of curiosity or to find out information about people they know – or want to learn about, said J. David Sims, a cybersecurity expert and CEO of Security First IT, a company that provides cybersecurity solutions and IT support to health care businesses.
Mr. Sims says he has heard of cases where health professionals snooped through records to find information about the new love interests of ex-partners or to learn about people on dating websites whom they’re interested in dating.
“Most of the time, it’s people being nosy,” he said. “In a lot of cases, it’s curiosity about famous people. You see it a lot in areas where you have football players who come in with injuries or you have an actor or actress who come in for something.”
“Data breaches caused by current and former workforce members impermissibly accessing patient records are a recurring issue across the health care industry. Health care organizations must ensure that workforce members can only access the patient information needed to do their jobs,” OCR director Melanie Fontes Rainer said in a June statement. “HIPAA-covered entities must have robust policies and procedures in place to ensure patient health information is protected from identify theft and fraud.”
Yakima Valley Memorial Hospital did not return a message seeking comment.
According to OCR’s latest report to Congress, complaints about HIPAA violations increased by 39% between 2017 and 2021. Breaches affecting fewer than 500 individuals rose by 5% during the same time period, and breaches impacting 500 or more individuals increased by 58%.
Common reasons employees snoop
The OCR announcement does not specify why the 23 security guards were accessing the medical records, but the incident raises questions about why the security guards had access to protected health information (PHI) in the first place, Mr. Redding said.
“I have yet to have anyone explain to me why the security guards would have access to PHI at all, at any level,” he said. “Was it by design or was it by error?”
In 2019 for instance, dozens of employees at Northwestern Memorial Hospital in Chicago were fired for accessing the health records of former Empire actor Jussie Smollett. In another high-profile case, nearly a dozen emergency medical service employees were caught snooping through 911 records connected to the treatment and, later, death of Joan Rivers.
“Sadly, there is a lack of education around what compliance really means inside the medical industry as a whole,” Mr. Redding said. “There is a lack of employee training and a lack of emphasis on accountability for employees.”
Privacy breaches fuel lawsuits
Health professionals caught snooping through records are frequently terminated and employers can face a range of ramifications, including civil and criminal penalties.
A growing trend is class action lawsuits associated with privacy violations, Mr. Redding adds.
Because patients are unable to sue in civil court for HIPAA breaches, they frequently sue for “breach of an implied contract,” he explained. In such cases, patients allege that the privacy documents they signed with health care providers established an implied contract, and their records being exposed constituted a contract breach.
“Class action lawsuits are starting to become extremely common,” Mr. Redding said. “It’s happening in many cases, even sometimes before Health & Human Services issue a fine, that [providers] are being wrapped into a class action lawsuit.”
Mayo Clinic, for example, was recently slapped with a class action suit after a former employee inappropriately accessed the records of 1,600 patients. Mayo settled the suit in January 2023, the terms of which were not publicly disclosed.
Multiple patients also filed a class action suit against San Diego–based Scripps Health after its data were hit with a cyberattack and subsequent breach that impacted close to 2 million people. Scripps reached a $3.5 million settlement with the plaintiffs in 2023.
Some practices and employers may also face state penalties for data privacy breaches, depending on their jurisdiction. In July, Connecticut became the fifth state to enact a comprehensive data privacy law. The measure, which creates a robust framework for protecting health-related records and other data, includes civil penalties of up to $5,000 for violations. Other states, including California, Virginia, Utah, and Colorado, also have state data privacy laws on the books.
How can practices stop snooping?
A first step to preventing snooping is conducting a thorough risk assessment, said David Harlow, a health care attorney and chief compliance and privacy officer for Insulet Corporation, a medical device company. The analysis should address who has access to what data and whether they really need such access, he said.
“Then it’s putting in place the proper controls to ensure access is limited and use is limited to the appropriate individuals and circumstances,” Mr. Harlow said.
Regulators don’t expect a giant academic medical center and a small private physician practice to take an identical HIPAA compliance approach, he stressed. The ideal approach will vary by entity. Providers just need to address the standards in a way that makes sense for their operation, he said.
Training is also a critical component, adds Mr. Sims.
“Having training is key,” he said. “Oftentimes, an employee might think, ‘Well, if I can click on this data and it comes up, obviously, I can look at it.’ They need to understand what information they are and are not allowed to access.”
Keep in mind that settings or controls might change when larger transitions take place, such as moving to a new electronic health record system, Mr. Sims said. It’s essential to reevaluate controls when changes in the practice take place to ensure that everything is functioning correctly.
Mr. Sims also suggests that practices create a type of “If you see something, say something,” policy that encourages fellow physicians and employees to report anything that looks suspicious within electronic logs. If an employee, for instance, is suddenly looking at many more records than usual or at odd times of the day or night, this should raise red flags.
“It’s great to stop it early so that it doesn’t become a bigger issue for the practice to deal with, but also, from a legal standpoint, you want to have a defensible argument that you were doing all you could to stop this as quickly as possible,” he said. “It puts you in a better position to defend yourself.”
The snooping security guards case holds an important lesson for all health providers, Mr. Harlow said.
“This is a message to all of us, that you need to have done the assessment up front,” he said. You need to have the right controls in place up front. This is not a situation where somebody managed to hack into a system for some devious means. This is someone who was given keys. Why were they given the keys?”
A version of this article first appeared on Medscape.com.