User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
Bipolar disorder tied to a sixfold increased risk of early death
In addition, patients with BD are three times more likely to die prematurely of all causes, compared with the general population, with alcohol-related diseases contributing to more premature deaths than cardiovascular disease (CVD), diabetes, and cancer.
The study results emphasize the need for personalized approaches to risk prediction and prevention of premature cause-specific mortality over the life-course of individuals with BD, lead investigator Tapio Paljärvi, PhD, an epidemiologist at Niuvanniemi Hospital in Kuopio, Finland, told this news organization.
The findings were published online in BMJ Mental Health.
Alcohol a major contributor to early death
A number of studies have established that those with BD have twice the risk of dying prematurely, compared with those without the disorder.
To learn more about the factors contributing to early death in this patient population, the investigators analyzed data from nationwide Finnish medical and insurance registries. They identified and tracked the health of 47,000 patients, aged 15-64 years, with BD between 2004 and 2018.
The average age at the beginning of the monitoring period was 38 years, and 57% of the cohort were women.
To determine the excess deaths directly attributable to BD, the researchers compared the ratio of deaths observed over the monitoring period in those with BD to the number expected to die in the general population, also known as the standard mortality ratio.
Of the group with BD, 3,300 died during the monitoring period. The average age at death was 50, and almost two-thirds (65%, or 2,137) of those who died were men.
Investigators grouped excess deaths in BD patients into two categories – somatic and external.
Of those with BD who died from somatic or disease-related causes, alcohol caused the highest rate of death (29%). The second-leading cause was heart disease and stroke (27%), followed by cancer (22%), respiratory diseases (4%), and diabetes (2%).
Among the 595 patients with BD who died because of alcohol consumption, liver disease was the leading cause of death (48%). The second cause was accidental alcohol poisoning (28%), followed by alcohol dependence (10%).
The leading cause of death from external causes in BD patients was suicide (58%, or 740), nearly half of which (48%) were from an overdose with prescribed psychotropic medications.
Overall, 64%, or 2,104, of the deaths in BD patients from any cause were considered excess deaths, that is, the number of deaths above those expected for those without BD of comparable age and sex.
Most of the excess deaths from somatic illness were either from alcohol-related causes (40%) – a rate three times higher than that of the general population – CVD (26%), or cancer (10%).
High suicide rate
When the team examined excess deaths from external causes, they found that 61% (651) were attributable to suicide, a rate eight times higher than that of the general population.
“In terms of absolute numbers, somatic causes of death represented the majority of all deaths in BD, as also reported in previous research,” Dr. Paljärvi said.
“However, this finding reflects the fact that in many high-income countries most of the deaths are due to somatic causes; with CVD, cancers, and diseases of the nervous system as the leading causes of death in the older age groups,” he added.
Dr. Paljärvi advised that clinicians treating patients with BD balance therapeutic response with potentially serious long-term medication side effects, to prevent premature deaths.
A stronger emphasis on identifying and treating comorbid substance abuse is also warranted, he noted.
Dr. Paljärvi noted that the underlying causes of the excess somatic mortality in people with BD are not fully understood, but may result from the “complex interaction between various established risk factors, including tobacco use, alcohol abuse, physical inactivity, unhealthy diet, obesity, hypertension, etc.”
Regarding the generalizability of the findings, he said many previous studies have been based only on inpatient data and noted that the current study included individuals from various sources including inpatient and outpatient registries as well as social insurance registries.
“While the reported excess all-cause mortality rates are strikingly similar across populations globally, there is a paucity of more detailed cause-specific analyses of excess mortality in BD,” said Dr. Paljärvi, adding that these findings should be replicated in other countries, including the United States.
Chronic inflammation
Commenting on the findings, Benjamin Goldstein, MD, PhD, professor of psychiatry and pharmacology at the University of Toronto, noted that there are clear disparities in access to, and quality of care among, patients with BD and other serious mental illnesses.
“Taking heart disease as an example, disparities exist at virtually every point of contact, ranging from the point of preventive care to the time it takes to be assessed in the ER, to the likelihood of receiving cardiac catheterization, to the quality of postdischarge care,” said Dr. Goldstein.
He also noted that CVD occurs in patients with BD, on average, 10-15 years earlier than the general population. However, he added, “there is important evidence that when people with BD receive the same standard of care as those without BD their cardiovascular outcomes are similar.”
Dr. Goldstein also noted that inflammation, which is a driver of cardiovascular risk, is elevated among patients with BD, particularly during mania and depression.
“Given that the average person with BD has some degree of mood symptoms about 40% of the time, chronically elevated inflammation likely contributes in part to the excess risk of heart disease in bipolar disorder,” he said.
Dr. Goldstein’s team’s research focuses on microvessels. “We have found that microvessel function in both the heart and the brain, determined by MRI, is reduced among teens with BD,” he said.
His team has also found that endothelial function in fingertip microvessels, an indicator of future heart disease risk, varies according to mood states.
“Collectively, these findings suggest the microvascular problems may explain, in part, the extra risk of heart disease beyond traditional risk factors in BD,” he added.
The study was funded by a Wellcome Trust Senior Clinical Research Fellowship and by the Oxford Health Biomedical Research Centre. Dr. Paljärvi and Dr. Goldstein report no relevant financial relationships.
A version of this article appeared on Medscape.com.
In addition, patients with BD are three times more likely to die prematurely of all causes, compared with the general population, with alcohol-related diseases contributing to more premature deaths than cardiovascular disease (CVD), diabetes, and cancer.
The study results emphasize the need for personalized approaches to risk prediction and prevention of premature cause-specific mortality over the life-course of individuals with BD, lead investigator Tapio Paljärvi, PhD, an epidemiologist at Niuvanniemi Hospital in Kuopio, Finland, told this news organization.
The findings were published online in BMJ Mental Health.
Alcohol a major contributor to early death
A number of studies have established that those with BD have twice the risk of dying prematurely, compared with those without the disorder.
To learn more about the factors contributing to early death in this patient population, the investigators analyzed data from nationwide Finnish medical and insurance registries. They identified and tracked the health of 47,000 patients, aged 15-64 years, with BD between 2004 and 2018.
The average age at the beginning of the monitoring period was 38 years, and 57% of the cohort were women.
To determine the excess deaths directly attributable to BD, the researchers compared the ratio of deaths observed over the monitoring period in those with BD to the number expected to die in the general population, also known as the standard mortality ratio.
Of the group with BD, 3,300 died during the monitoring period. The average age at death was 50, and almost two-thirds (65%, or 2,137) of those who died were men.
Investigators grouped excess deaths in BD patients into two categories – somatic and external.
Of those with BD who died from somatic or disease-related causes, alcohol caused the highest rate of death (29%). The second-leading cause was heart disease and stroke (27%), followed by cancer (22%), respiratory diseases (4%), and diabetes (2%).
Among the 595 patients with BD who died because of alcohol consumption, liver disease was the leading cause of death (48%). The second cause was accidental alcohol poisoning (28%), followed by alcohol dependence (10%).
The leading cause of death from external causes in BD patients was suicide (58%, or 740), nearly half of which (48%) were from an overdose with prescribed psychotropic medications.
Overall, 64%, or 2,104, of the deaths in BD patients from any cause were considered excess deaths, that is, the number of deaths above those expected for those without BD of comparable age and sex.
Most of the excess deaths from somatic illness were either from alcohol-related causes (40%) – a rate three times higher than that of the general population – CVD (26%), or cancer (10%).
High suicide rate
When the team examined excess deaths from external causes, they found that 61% (651) were attributable to suicide, a rate eight times higher than that of the general population.
“In terms of absolute numbers, somatic causes of death represented the majority of all deaths in BD, as also reported in previous research,” Dr. Paljärvi said.
“However, this finding reflects the fact that in many high-income countries most of the deaths are due to somatic causes; with CVD, cancers, and diseases of the nervous system as the leading causes of death in the older age groups,” he added.
Dr. Paljärvi advised that clinicians treating patients with BD balance therapeutic response with potentially serious long-term medication side effects, to prevent premature deaths.
A stronger emphasis on identifying and treating comorbid substance abuse is also warranted, he noted.
Dr. Paljärvi noted that the underlying causes of the excess somatic mortality in people with BD are not fully understood, but may result from the “complex interaction between various established risk factors, including tobacco use, alcohol abuse, physical inactivity, unhealthy diet, obesity, hypertension, etc.”
Regarding the generalizability of the findings, he said many previous studies have been based only on inpatient data and noted that the current study included individuals from various sources including inpatient and outpatient registries as well as social insurance registries.
“While the reported excess all-cause mortality rates are strikingly similar across populations globally, there is a paucity of more detailed cause-specific analyses of excess mortality in BD,” said Dr. Paljärvi, adding that these findings should be replicated in other countries, including the United States.
Chronic inflammation
Commenting on the findings, Benjamin Goldstein, MD, PhD, professor of psychiatry and pharmacology at the University of Toronto, noted that there are clear disparities in access to, and quality of care among, patients with BD and other serious mental illnesses.
“Taking heart disease as an example, disparities exist at virtually every point of contact, ranging from the point of preventive care to the time it takes to be assessed in the ER, to the likelihood of receiving cardiac catheterization, to the quality of postdischarge care,” said Dr. Goldstein.
He also noted that CVD occurs in patients with BD, on average, 10-15 years earlier than the general population. However, he added, “there is important evidence that when people with BD receive the same standard of care as those without BD their cardiovascular outcomes are similar.”
Dr. Goldstein also noted that inflammation, which is a driver of cardiovascular risk, is elevated among patients with BD, particularly during mania and depression.
“Given that the average person with BD has some degree of mood symptoms about 40% of the time, chronically elevated inflammation likely contributes in part to the excess risk of heart disease in bipolar disorder,” he said.
Dr. Goldstein’s team’s research focuses on microvessels. “We have found that microvessel function in both the heart and the brain, determined by MRI, is reduced among teens with BD,” he said.
His team has also found that endothelial function in fingertip microvessels, an indicator of future heart disease risk, varies according to mood states.
“Collectively, these findings suggest the microvascular problems may explain, in part, the extra risk of heart disease beyond traditional risk factors in BD,” he added.
The study was funded by a Wellcome Trust Senior Clinical Research Fellowship and by the Oxford Health Biomedical Research Centre. Dr. Paljärvi and Dr. Goldstein report no relevant financial relationships.
A version of this article appeared on Medscape.com.
In addition, patients with BD are three times more likely to die prematurely of all causes, compared with the general population, with alcohol-related diseases contributing to more premature deaths than cardiovascular disease (CVD), diabetes, and cancer.
The study results emphasize the need for personalized approaches to risk prediction and prevention of premature cause-specific mortality over the life-course of individuals with BD, lead investigator Tapio Paljärvi, PhD, an epidemiologist at Niuvanniemi Hospital in Kuopio, Finland, told this news organization.
The findings were published online in BMJ Mental Health.
Alcohol a major contributor to early death
A number of studies have established that those with BD have twice the risk of dying prematurely, compared with those without the disorder.
To learn more about the factors contributing to early death in this patient population, the investigators analyzed data from nationwide Finnish medical and insurance registries. They identified and tracked the health of 47,000 patients, aged 15-64 years, with BD between 2004 and 2018.
The average age at the beginning of the monitoring period was 38 years, and 57% of the cohort were women.
To determine the excess deaths directly attributable to BD, the researchers compared the ratio of deaths observed over the monitoring period in those with BD to the number expected to die in the general population, also known as the standard mortality ratio.
Of the group with BD, 3,300 died during the monitoring period. The average age at death was 50, and almost two-thirds (65%, or 2,137) of those who died were men.
Investigators grouped excess deaths in BD patients into two categories – somatic and external.
Of those with BD who died from somatic or disease-related causes, alcohol caused the highest rate of death (29%). The second-leading cause was heart disease and stroke (27%), followed by cancer (22%), respiratory diseases (4%), and diabetes (2%).
Among the 595 patients with BD who died because of alcohol consumption, liver disease was the leading cause of death (48%). The second cause was accidental alcohol poisoning (28%), followed by alcohol dependence (10%).
The leading cause of death from external causes in BD patients was suicide (58%, or 740), nearly half of which (48%) were from an overdose with prescribed psychotropic medications.
Overall, 64%, or 2,104, of the deaths in BD patients from any cause were considered excess deaths, that is, the number of deaths above those expected for those without BD of comparable age and sex.
Most of the excess deaths from somatic illness were either from alcohol-related causes (40%) – a rate three times higher than that of the general population – CVD (26%), or cancer (10%).
High suicide rate
When the team examined excess deaths from external causes, they found that 61% (651) were attributable to suicide, a rate eight times higher than that of the general population.
“In terms of absolute numbers, somatic causes of death represented the majority of all deaths in BD, as also reported in previous research,” Dr. Paljärvi said.
“However, this finding reflects the fact that in many high-income countries most of the deaths are due to somatic causes; with CVD, cancers, and diseases of the nervous system as the leading causes of death in the older age groups,” he added.
Dr. Paljärvi advised that clinicians treating patients with BD balance therapeutic response with potentially serious long-term medication side effects, to prevent premature deaths.
A stronger emphasis on identifying and treating comorbid substance abuse is also warranted, he noted.
Dr. Paljärvi noted that the underlying causes of the excess somatic mortality in people with BD are not fully understood, but may result from the “complex interaction between various established risk factors, including tobacco use, alcohol abuse, physical inactivity, unhealthy diet, obesity, hypertension, etc.”
Regarding the generalizability of the findings, he said many previous studies have been based only on inpatient data and noted that the current study included individuals from various sources including inpatient and outpatient registries as well as social insurance registries.
“While the reported excess all-cause mortality rates are strikingly similar across populations globally, there is a paucity of more detailed cause-specific analyses of excess mortality in BD,” said Dr. Paljärvi, adding that these findings should be replicated in other countries, including the United States.
Chronic inflammation
Commenting on the findings, Benjamin Goldstein, MD, PhD, professor of psychiatry and pharmacology at the University of Toronto, noted that there are clear disparities in access to, and quality of care among, patients with BD and other serious mental illnesses.
“Taking heart disease as an example, disparities exist at virtually every point of contact, ranging from the point of preventive care to the time it takes to be assessed in the ER, to the likelihood of receiving cardiac catheterization, to the quality of postdischarge care,” said Dr. Goldstein.
He also noted that CVD occurs in patients with BD, on average, 10-15 years earlier than the general population. However, he added, “there is important evidence that when people with BD receive the same standard of care as those without BD their cardiovascular outcomes are similar.”
Dr. Goldstein also noted that inflammation, which is a driver of cardiovascular risk, is elevated among patients with BD, particularly during mania and depression.
“Given that the average person with BD has some degree of mood symptoms about 40% of the time, chronically elevated inflammation likely contributes in part to the excess risk of heart disease in bipolar disorder,” he said.
Dr. Goldstein’s team’s research focuses on microvessels. “We have found that microvessel function in both the heart and the brain, determined by MRI, is reduced among teens with BD,” he said.
His team has also found that endothelial function in fingertip microvessels, an indicator of future heart disease risk, varies according to mood states.
“Collectively, these findings suggest the microvascular problems may explain, in part, the extra risk of heart disease beyond traditional risk factors in BD,” he added.
The study was funded by a Wellcome Trust Senior Clinical Research Fellowship and by the Oxford Health Biomedical Research Centre. Dr. Paljärvi and Dr. Goldstein report no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM BMJ MENTAL HEALTH
A new and completely different pain medicine
This transcript has been edited for clarity.
When you stub your toe or get a paper cut on your finger, you feel the pain in that part of your body. It feels like the pain is coming from that place. But, of course, that’s not really what is happening. Pain doesn’t really happen in your toe or your finger. It happens in your brain.
It’s a game of telephone, really. The afferent nerve fiber detects the noxious stimulus, passing that signal to the second-order neuron in the dorsal root ganglia of the spinal cord, which runs it up to the thalamus to be passed to the third-order neuron which brings it to the cortex for localization and conscious perception. It’s not even a very good game of telephone. It takes about 100 ms for a pain signal to get from the hand to the brain – longer from the feet, given the greater distance. You see your foot hit the corner of the coffee table and have just enough time to think: “Oh no!” before the pain hits.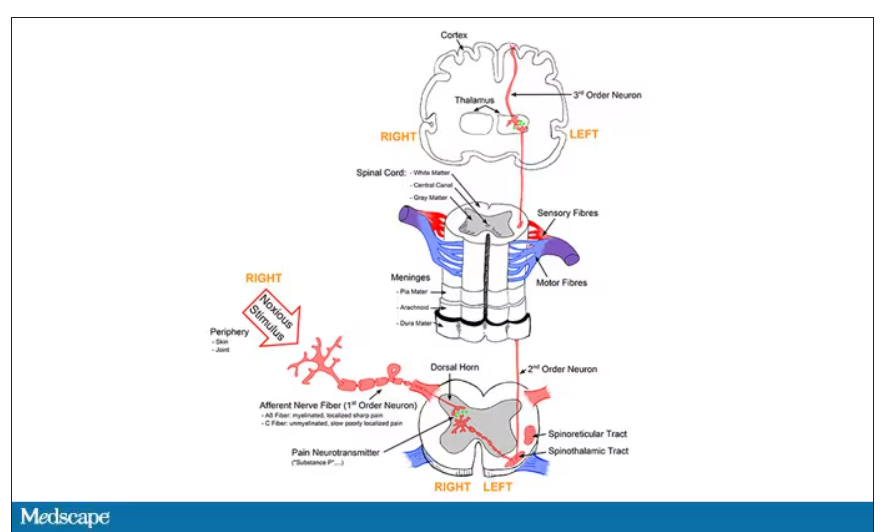
Given the Rube Goldberg nature of the process, it would seem like there are any number of places we could stop pain sensation. And sure, local anesthetics at the site of injury, or even spinal anesthetics, are powerful – if temporary and hard to administer – solutions to acute pain.
But in our everyday armamentarium, let’s be honest – we essentially have three options: opiates and opioids, which activate the mu-receptors in the brain to dull pain (and cause a host of other nasty side effects); NSAIDs, which block prostaglandin synthesis and thus limit the ability for pain-conducting neurons to get excited; and acetaminophen, which, despite being used for a century, is poorly understood.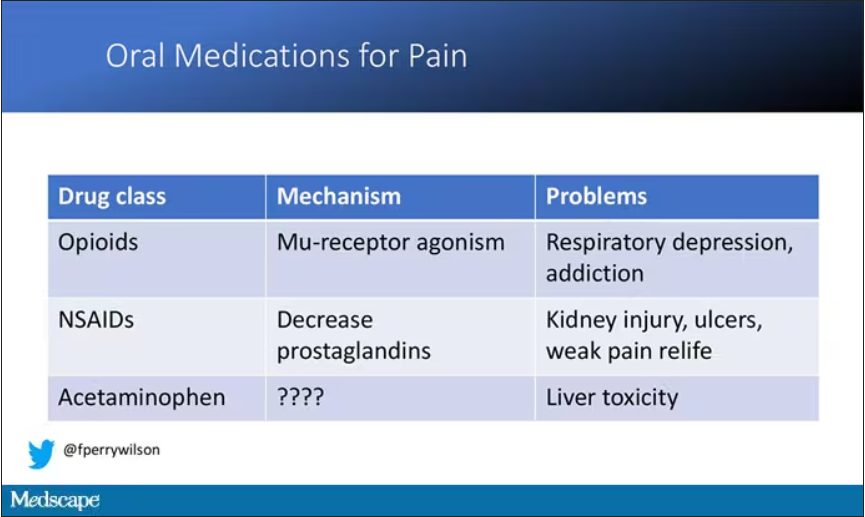
But
If you were to zoom in on the connection between that first afferent pain fiber and the secondary nerve in the spinal cord dorsal root ganglion, you would see a receptor called Nav1.8, a voltage-gated sodium channel.
This receptor is a key part of the apparatus that passes information from nerve 1 to nerve 2, but only for fibers that transmit pain signals. In fact, humans with mutations in this receptor that leave it always in the “open” state have a severe pain syndrome. Blocking the receptor, therefore, might reduce pain.
In preclinical work, researchers identified VX-548, which doesn’t have a brand name yet, as a potent blocker of that channel even in nanomolar concentrations. Importantly, the compound was highly selective for that particular channel – about 30,000 times more selective than it was for the other sodium channels in that family.
Of course, a highly selective and specific drug does not a blockbuster analgesic make. To determine how this drug would work on humans in pain, they turned to two populations: 303 individuals undergoing abdominoplasty and 274 undergoing bunionectomy, as reported in a new paper in the New England Journal of Medicine.
I know this seems a bit random, but abdominoplasty is quite painful and a good model for soft-tissue pain. Bunionectomy is also quite a painful procedure and a useful model of bone pain. After the surgeries, patients were randomized to several different doses of VX-548, hydrocodone plus acetaminophen, or placebo for 48 hours.
At 19 time points over that 48-hour period, participants were asked to rate their pain on a scale from 0 to 10. The primary outcome was the cumulative pain experienced over the 48 hours. So, higher pain would be worse here, but longer duration of pain would also be worse.
The story of the study is really told in this chart.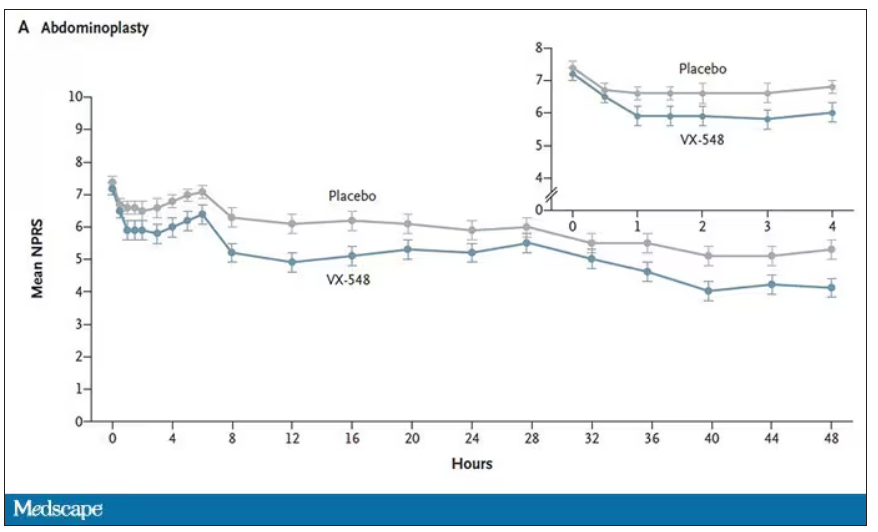
Yes, those assigned to the highest dose of VX-548 had a statistically significant lower cumulative amount of pain in the 48 hours after surgery. But the picture is really worth more than the stats here. You can see that the onset of pain relief was fairly quick, and that pain relief was sustained over time. You can also see that this is not a miracle drug. Pain scores were a bit better 48 hours out, but only by about a point and a half.
Placebo isn’t really the fair comparison here; few of us treat our postabdominoplasty patients with placebo, after all. The authors do not formally compare the effect of VX-548 with that of the opioid hydrocodone, for instance. But that doesn’t stop us.
This graph, which I put together from data in the paper, shows pain control across the four randomization categories, with higher numbers indicating more (cumulative) control. While all the active agents do a bit better than placebo, VX-548 at the higher dose appears to do the best. But I should note that 5 mg of hydrocodone may not be an adequate dose for most people.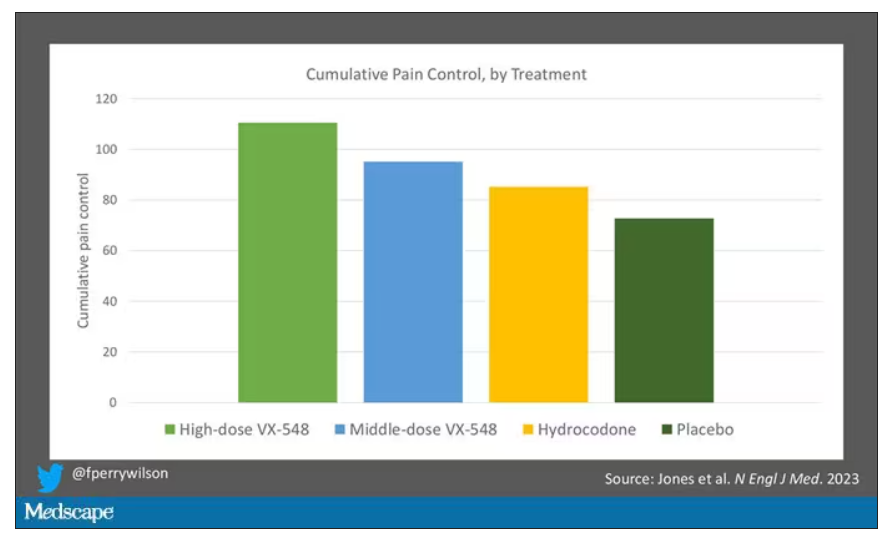
Yes, I would really have killed for an NSAID arm in this trial. Its absence, given that NSAIDs are a staple of postoperative care, is ... well, let’s just say, notable.
Although not a pain-destroying machine, VX-548 has some other things to recommend it. The receptor is really not found in the brain at all, which suggests that the drug should not carry much risk for dependency, though that has not been formally studied.
The side effects were generally mild – headache was the most common – and less prevalent than what you see even in the placebo arm.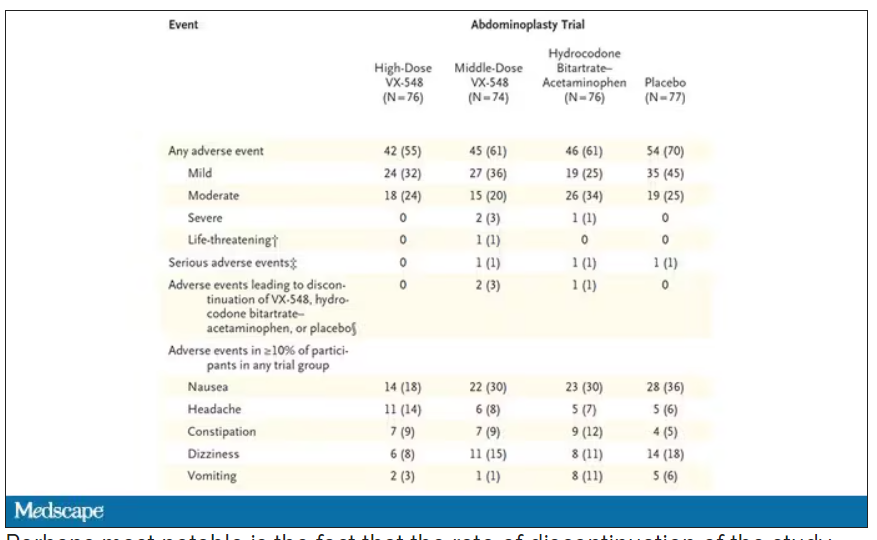
Perhaps most notable is the fact that the rate of discontinuation of the study drug was lowest in the VX-548 arm. Patients could stop taking the pill they were assigned for any reason, ranging from perceived lack of efficacy to side effects. A low discontinuation rate indicates to me a sort of “voting with your feet” that suggests this might be a well-tolerated and reasonably effective drug.
VX-548 isn’t on the market yet; phase 3 trials are ongoing. But whether it is this particular drug or another in this class, I’m happy to see researchers trying to find new ways to target that most primeval form of suffering: pain.
Dr. Wilson is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Conn. He disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
When you stub your toe or get a paper cut on your finger, you feel the pain in that part of your body. It feels like the pain is coming from that place. But, of course, that’s not really what is happening. Pain doesn’t really happen in your toe or your finger. It happens in your brain.
It’s a game of telephone, really. The afferent nerve fiber detects the noxious stimulus, passing that signal to the second-order neuron in the dorsal root ganglia of the spinal cord, which runs it up to the thalamus to be passed to the third-order neuron which brings it to the cortex for localization and conscious perception. It’s not even a very good game of telephone. It takes about 100 ms for a pain signal to get from the hand to the brain – longer from the feet, given the greater distance. You see your foot hit the corner of the coffee table and have just enough time to think: “Oh no!” before the pain hits.
Given the Rube Goldberg nature of the process, it would seem like there are any number of places we could stop pain sensation. And sure, local anesthetics at the site of injury, or even spinal anesthetics, are powerful – if temporary and hard to administer – solutions to acute pain.
But in our everyday armamentarium, let’s be honest – we essentially have three options: opiates and opioids, which activate the mu-receptors in the brain to dull pain (and cause a host of other nasty side effects); NSAIDs, which block prostaglandin synthesis and thus limit the ability for pain-conducting neurons to get excited; and acetaminophen, which, despite being used for a century, is poorly understood.
But
If you were to zoom in on the connection between that first afferent pain fiber and the secondary nerve in the spinal cord dorsal root ganglion, you would see a receptor called Nav1.8, a voltage-gated sodium channel.
This receptor is a key part of the apparatus that passes information from nerve 1 to nerve 2, but only for fibers that transmit pain signals. In fact, humans with mutations in this receptor that leave it always in the “open” state have a severe pain syndrome. Blocking the receptor, therefore, might reduce pain.
In preclinical work, researchers identified VX-548, which doesn’t have a brand name yet, as a potent blocker of that channel even in nanomolar concentrations. Importantly, the compound was highly selective for that particular channel – about 30,000 times more selective than it was for the other sodium channels in that family.
Of course, a highly selective and specific drug does not a blockbuster analgesic make. To determine how this drug would work on humans in pain, they turned to two populations: 303 individuals undergoing abdominoplasty and 274 undergoing bunionectomy, as reported in a new paper in the New England Journal of Medicine.
I know this seems a bit random, but abdominoplasty is quite painful and a good model for soft-tissue pain. Bunionectomy is also quite a painful procedure and a useful model of bone pain. After the surgeries, patients were randomized to several different doses of VX-548, hydrocodone plus acetaminophen, or placebo for 48 hours.
At 19 time points over that 48-hour period, participants were asked to rate their pain on a scale from 0 to 10. The primary outcome was the cumulative pain experienced over the 48 hours. So, higher pain would be worse here, but longer duration of pain would also be worse.
The story of the study is really told in this chart.
Yes, those assigned to the highest dose of VX-548 had a statistically significant lower cumulative amount of pain in the 48 hours after surgery. But the picture is really worth more than the stats here. You can see that the onset of pain relief was fairly quick, and that pain relief was sustained over time. You can also see that this is not a miracle drug. Pain scores were a bit better 48 hours out, but only by about a point and a half.
Placebo isn’t really the fair comparison here; few of us treat our postabdominoplasty patients with placebo, after all. The authors do not formally compare the effect of VX-548 with that of the opioid hydrocodone, for instance. But that doesn’t stop us.
This graph, which I put together from data in the paper, shows pain control across the four randomization categories, with higher numbers indicating more (cumulative) control. While all the active agents do a bit better than placebo, VX-548 at the higher dose appears to do the best. But I should note that 5 mg of hydrocodone may not be an adequate dose for most people.
Yes, I would really have killed for an NSAID arm in this trial. Its absence, given that NSAIDs are a staple of postoperative care, is ... well, let’s just say, notable.
Although not a pain-destroying machine, VX-548 has some other things to recommend it. The receptor is really not found in the brain at all, which suggests that the drug should not carry much risk for dependency, though that has not been formally studied.
The side effects were generally mild – headache was the most common – and less prevalent than what you see even in the placebo arm.
Perhaps most notable is the fact that the rate of discontinuation of the study drug was lowest in the VX-548 arm. Patients could stop taking the pill they were assigned for any reason, ranging from perceived lack of efficacy to side effects. A low discontinuation rate indicates to me a sort of “voting with your feet” that suggests this might be a well-tolerated and reasonably effective drug.
VX-548 isn’t on the market yet; phase 3 trials are ongoing. But whether it is this particular drug or another in this class, I’m happy to see researchers trying to find new ways to target that most primeval form of suffering: pain.
Dr. Wilson is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Conn. He disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
When you stub your toe or get a paper cut on your finger, you feel the pain in that part of your body. It feels like the pain is coming from that place. But, of course, that’s not really what is happening. Pain doesn’t really happen in your toe or your finger. It happens in your brain.
It’s a game of telephone, really. The afferent nerve fiber detects the noxious stimulus, passing that signal to the second-order neuron in the dorsal root ganglia of the spinal cord, which runs it up to the thalamus to be passed to the third-order neuron which brings it to the cortex for localization and conscious perception. It’s not even a very good game of telephone. It takes about 100 ms for a pain signal to get from the hand to the brain – longer from the feet, given the greater distance. You see your foot hit the corner of the coffee table and have just enough time to think: “Oh no!” before the pain hits.
Given the Rube Goldberg nature of the process, it would seem like there are any number of places we could stop pain sensation. And sure, local anesthetics at the site of injury, or even spinal anesthetics, are powerful – if temporary and hard to administer – solutions to acute pain.
But in our everyday armamentarium, let’s be honest – we essentially have three options: opiates and opioids, which activate the mu-receptors in the brain to dull pain (and cause a host of other nasty side effects); NSAIDs, which block prostaglandin synthesis and thus limit the ability for pain-conducting neurons to get excited; and acetaminophen, which, despite being used for a century, is poorly understood.
But
If you were to zoom in on the connection between that first afferent pain fiber and the secondary nerve in the spinal cord dorsal root ganglion, you would see a receptor called Nav1.8, a voltage-gated sodium channel.
This receptor is a key part of the apparatus that passes information from nerve 1 to nerve 2, but only for fibers that transmit pain signals. In fact, humans with mutations in this receptor that leave it always in the “open” state have a severe pain syndrome. Blocking the receptor, therefore, might reduce pain.
In preclinical work, researchers identified VX-548, which doesn’t have a brand name yet, as a potent blocker of that channel even in nanomolar concentrations. Importantly, the compound was highly selective for that particular channel – about 30,000 times more selective than it was for the other sodium channels in that family.
Of course, a highly selective and specific drug does not a blockbuster analgesic make. To determine how this drug would work on humans in pain, they turned to two populations: 303 individuals undergoing abdominoplasty and 274 undergoing bunionectomy, as reported in a new paper in the New England Journal of Medicine.
I know this seems a bit random, but abdominoplasty is quite painful and a good model for soft-tissue pain. Bunionectomy is also quite a painful procedure and a useful model of bone pain. After the surgeries, patients were randomized to several different doses of VX-548, hydrocodone plus acetaminophen, or placebo for 48 hours.
At 19 time points over that 48-hour period, participants were asked to rate their pain on a scale from 0 to 10. The primary outcome was the cumulative pain experienced over the 48 hours. So, higher pain would be worse here, but longer duration of pain would also be worse.
The story of the study is really told in this chart.
Yes, those assigned to the highest dose of VX-548 had a statistically significant lower cumulative amount of pain in the 48 hours after surgery. But the picture is really worth more than the stats here. You can see that the onset of pain relief was fairly quick, and that pain relief was sustained over time. You can also see that this is not a miracle drug. Pain scores were a bit better 48 hours out, but only by about a point and a half.
Placebo isn’t really the fair comparison here; few of us treat our postabdominoplasty patients with placebo, after all. The authors do not formally compare the effect of VX-548 with that of the opioid hydrocodone, for instance. But that doesn’t stop us.
This graph, which I put together from data in the paper, shows pain control across the four randomization categories, with higher numbers indicating more (cumulative) control. While all the active agents do a bit better than placebo, VX-548 at the higher dose appears to do the best. But I should note that 5 mg of hydrocodone may not be an adequate dose for most people.
Yes, I would really have killed for an NSAID arm in this trial. Its absence, given that NSAIDs are a staple of postoperative care, is ... well, let’s just say, notable.
Although not a pain-destroying machine, VX-548 has some other things to recommend it. The receptor is really not found in the brain at all, which suggests that the drug should not carry much risk for dependency, though that has not been formally studied.
The side effects were generally mild – headache was the most common – and less prevalent than what you see even in the placebo arm.
Perhaps most notable is the fact that the rate of discontinuation of the study drug was lowest in the VX-548 arm. Patients could stop taking the pill they were assigned for any reason, ranging from perceived lack of efficacy to side effects. A low discontinuation rate indicates to me a sort of “voting with your feet” that suggests this might be a well-tolerated and reasonably effective drug.
VX-548 isn’t on the market yet; phase 3 trials are ongoing. But whether it is this particular drug or another in this class, I’m happy to see researchers trying to find new ways to target that most primeval form of suffering: pain.
Dr. Wilson is an associate professor of medicine and public health and director of Yale’s Clinical and Translational Research Accelerator, New Haven, Conn. He disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Long COVID disability court battles just ‘tip of iceberg’
At least 30 lawsuits have been filed seeking legal resolution of disability insurance claims, according to searches of court records. In addition, the Social Security Administration said it has received about 52,000 disability claims tied to SARS-CoV-2 infections, which represents 1% of all applications.
But legal experts say those cases may not reflect the total number of cases that have gone to court. They note many claims are initially dismissed and are not appealed by claimants.
“With this system, they deny two-thirds of initial applications, then people who appeal get denied almost 90% of the time, and then they can appeal before a judge,” said Kevin LaPorte, a Social Security disability attorney at LaPorte Law Firm in Oakland, Calif. “What happens next doesn’t have a lot of precedent because long COVID is a mass disabling event, and we haven’t seen that many of these cases get all the way through the legal system yet.”
As a result, the exact number of long COVID disability claims and the number of these cases going to court isn’t clear, he said.
“It can take a year or more for cases to get to court, and even longer to reach resolution,” Mr. LaPorte added. “I suspect the few cases we’ve heard about at this point are going to be the tip of the iceberg.”
The process is convoluted and can drag on for months with multiple denials and appeals along the way. Many disabled workers find their only recourse is to take insurers to court.
Long COVID patients typically apply for disability benefits through private insurance or Social Security. But the process can drag on for months, so many find their only recourse is to take insurers to court, according to legal experts.
But even in the courts, many encounter delays and hurdles to resolution.
In one of the first federal lawsuits involving long COVID disability benefits, William Abrams, a trial and appellate attorney and active marathon runner, sued Unum Life Insurance seeking long-term disability income. Symptoms included extreme fatigue, brain fog, decreased attention and concentration, and nearly daily fevers, causing him to stop working in April 2020.
His diagnosis wasn’t definitive. Three doctors said he had long COVID, and four said he had chronic fatigue syndrome. Unum cited this inconsistency as a rationale for rejecting his claim. But the court sided with Mr. Abrams, granting him disability income. The court concluded: “Unum may be correct that [the plaintiff] has not been correctly diagnosed. But that does not mean he is not sick. If [the plaintiff’s] complaints, and [the doctor’s] assessments, are to be believed, [the plaintiff] cannot focus for more than a few minutes at a time, making it impossible for [the plaintiff] to perform the varied and complex tasks his job requires.”
Unum said in an emailed statement that the company doesn’t comment on specific claims as a matter of policy, adding that its total payouts for disability claims from March 2020 to February 2022 were 35% higher than prepandemic levels. “In general, disability and leave claims connected to COVID-19 have been primarily short-term events with the majority of claimants recovering prior to completing the normal qualification period for long-term disability insurance,” Unum said.
Mr. Abrams prevailed in part because he had detailed documentation of the numerous impairments that eventually required him to stop work, said Michelle Roberts of Roberts Disability Law in Oakland, Calif.
He submitted videos of himself taking his temperature to prove he had almost daily fevers, according to court records. He underwent neuropsychological testing, which found learning deficiencies and memory deficits.
Mr. Abrams also submitted statements from a colleague who worked with him on a complex technology patent case involving radiofrequency identification. Before he got COVID, Mr. Abrams “had the analytical ability, legal acumen, and mental energy to attack that learning curve and get up to speed very rapidly,” according to court records.
“The court focused on credulity.” Ms. Roberts said. “There was all this work to be done to show this person was high functioning and ran marathons and worked in an intense, high-pressure occupation but then couldn’t do anything after long COVID.”
Documentation was also crucial in another early federal long COVID disability lawsuit that was filed in 2022 on behalf of Wendy Haut, an educational software sales representative in California who turned to the courts seeking disability income through her company’s employee benefits plan.
Several of Ms. Haut’s doctors documented a detailed list of long COVID symptoms, including “profound fatigue and extreme cognitive difficulties,” that they said prevented her from working as a sales representative or doing any other type of job. A settlement agreement in June 2022 required Reliance Standard Life Insurance to pay Ms. Haut long-term disability benefits, including previously unpaid benefits, according to a report by the advocacy group Pandemic Patients.
Representatives of Reliance Standard didn’t respond to a request for comment.
The growing number of workers being sidelined by long COVID makes more claims and more court cases likely. Right now, an estimated 16 million working-age Americans aged 18-65 years have long COVID, and as many as 4 million of them can’t work, according to a July 2023 Census Bureau report.
Uncertainty about the volume of claims in the pipeline is part of what’s driving some insurers to fight long COVID claims, Ms. Roberts said. Another factor is the lack of clarity around how many years people with long COVID may be out of work, particularly if they’re in their 30s or 40s and might be seeking disability income until they reach retirement age.
“Doctors are not always saying that this person will be permanently disabled,” Ms. Roberts said. “If this person doesn’t get better and they’re disabled until retirement age, this could be a payout in the high six or seven figures if a person is very young and was a very high earner.”
Insurance companies routinely deny claims that can’t be backed up with objective measures, such as specific lab test results or clear findings from a physical exam. But there are steps that can increase the odds of a successful claim for long COVID disability benefits, according to New York–based law firm Hiller.
For starters, patients can document COVID test results, and if testing wasn’t conducted, patients can detail the specific symptoms that led to this diagnosis, Hiller advises. Then patients can keep a daily symptom log at home that run lists all of the specific symptoms that occur at different times during the day and night to help establish a pattern of disability. These logs should provide specific details about every job duty patients have and exactly how specific symptoms of long COVID interfere with these duties.
Even though objective testing is hard to come by for long COVID, people should undergo all the tests they can that may help document the frequency or severity of specific symptoms that make it impossible to carry on with business as usual at work, Hiller advises. This may include neuropsychological testing to document brain fog, a cardiopulmonary exercise test to demonstrate chronic fatigue and the inability to exercise, or a tilt table test to measure dizziness.
Seeking a doctor’s diagnosis can be key to collecting disability payments, in or out of court.
All of this puts a lot of pressure on doctors and patients to build strong cases, said Jonathan Whiteson, MD, codirector of the NYU Langone Health post-COVID care program in New York. “Many physicians are not familiar with the disability benefit paperwork, and so this is a challenge for the doctors to know how to complete and to build the time into their highly scheduled days to take the time needed to complete.
“It’s also challenging because most of the disability benefit forms are ‘generic’ and do not ask specific questions about COVID disability,” Dr. Whiteson added. “It can be like trying to drive a square peg into a round hole.”
Still, when it comes to long COVID, completing disability paperwork is increasingly becoming part of standard care, along with managing medication, rehabilitation therapies, and lifestyle changes to navigate daily life with this illness, Dr. Whiteson noted.
Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the Post-COVID-19 Recovery Clinic at the University of Texas Health Science Center, San Antonio, agreed with this assessment.
“I have done letter upon letter of appeal to disability insurance companies,” she said.
Some doctors, however, are reluctant to step up in such cases, in part because no standard diagnostic guidelines exist for long COVID and because it can be frustrating.
“This is the work that is not paid and causes burnout in physicians,” Dr. Verduzco-Gutierrez said. “The paperwork, the fighting with insurance companies, the resubmission of forms for disability all to get what your patient needs – and then it gets denied.
“We will keep doing this because our patients need this disability income in order to live their lives and to afford what they need for recovery,” said Dr. Verduzco-Gutierrez. “But at some point something has to change because this isn’t sustainable.”
A version of this article appeared on Medscape.com.
At least 30 lawsuits have been filed seeking legal resolution of disability insurance claims, according to searches of court records. In addition, the Social Security Administration said it has received about 52,000 disability claims tied to SARS-CoV-2 infections, which represents 1% of all applications.
But legal experts say those cases may not reflect the total number of cases that have gone to court. They note many claims are initially dismissed and are not appealed by claimants.
“With this system, they deny two-thirds of initial applications, then people who appeal get denied almost 90% of the time, and then they can appeal before a judge,” said Kevin LaPorte, a Social Security disability attorney at LaPorte Law Firm in Oakland, Calif. “What happens next doesn’t have a lot of precedent because long COVID is a mass disabling event, and we haven’t seen that many of these cases get all the way through the legal system yet.”
As a result, the exact number of long COVID disability claims and the number of these cases going to court isn’t clear, he said.
“It can take a year or more for cases to get to court, and even longer to reach resolution,” Mr. LaPorte added. “I suspect the few cases we’ve heard about at this point are going to be the tip of the iceberg.”
The process is convoluted and can drag on for months with multiple denials and appeals along the way. Many disabled workers find their only recourse is to take insurers to court.
Long COVID patients typically apply for disability benefits through private insurance or Social Security. But the process can drag on for months, so many find their only recourse is to take insurers to court, according to legal experts.
But even in the courts, many encounter delays and hurdles to resolution.
In one of the first federal lawsuits involving long COVID disability benefits, William Abrams, a trial and appellate attorney and active marathon runner, sued Unum Life Insurance seeking long-term disability income. Symptoms included extreme fatigue, brain fog, decreased attention and concentration, and nearly daily fevers, causing him to stop working in April 2020.
His diagnosis wasn’t definitive. Three doctors said he had long COVID, and four said he had chronic fatigue syndrome. Unum cited this inconsistency as a rationale for rejecting his claim. But the court sided with Mr. Abrams, granting him disability income. The court concluded: “Unum may be correct that [the plaintiff] has not been correctly diagnosed. But that does not mean he is not sick. If [the plaintiff’s] complaints, and [the doctor’s] assessments, are to be believed, [the plaintiff] cannot focus for more than a few minutes at a time, making it impossible for [the plaintiff] to perform the varied and complex tasks his job requires.”
Unum said in an emailed statement that the company doesn’t comment on specific claims as a matter of policy, adding that its total payouts for disability claims from March 2020 to February 2022 were 35% higher than prepandemic levels. “In general, disability and leave claims connected to COVID-19 have been primarily short-term events with the majority of claimants recovering prior to completing the normal qualification period for long-term disability insurance,” Unum said.
Mr. Abrams prevailed in part because he had detailed documentation of the numerous impairments that eventually required him to stop work, said Michelle Roberts of Roberts Disability Law in Oakland, Calif.
He submitted videos of himself taking his temperature to prove he had almost daily fevers, according to court records. He underwent neuropsychological testing, which found learning deficiencies and memory deficits.
Mr. Abrams also submitted statements from a colleague who worked with him on a complex technology patent case involving radiofrequency identification. Before he got COVID, Mr. Abrams “had the analytical ability, legal acumen, and mental energy to attack that learning curve and get up to speed very rapidly,” according to court records.
“The court focused on credulity.” Ms. Roberts said. “There was all this work to be done to show this person was high functioning and ran marathons and worked in an intense, high-pressure occupation but then couldn’t do anything after long COVID.”
Documentation was also crucial in another early federal long COVID disability lawsuit that was filed in 2022 on behalf of Wendy Haut, an educational software sales representative in California who turned to the courts seeking disability income through her company’s employee benefits plan.
Several of Ms. Haut’s doctors documented a detailed list of long COVID symptoms, including “profound fatigue and extreme cognitive difficulties,” that they said prevented her from working as a sales representative or doing any other type of job. A settlement agreement in June 2022 required Reliance Standard Life Insurance to pay Ms. Haut long-term disability benefits, including previously unpaid benefits, according to a report by the advocacy group Pandemic Patients.
Representatives of Reliance Standard didn’t respond to a request for comment.
The growing number of workers being sidelined by long COVID makes more claims and more court cases likely. Right now, an estimated 16 million working-age Americans aged 18-65 years have long COVID, and as many as 4 million of them can’t work, according to a July 2023 Census Bureau report.
Uncertainty about the volume of claims in the pipeline is part of what’s driving some insurers to fight long COVID claims, Ms. Roberts said. Another factor is the lack of clarity around how many years people with long COVID may be out of work, particularly if they’re in their 30s or 40s and might be seeking disability income until they reach retirement age.
“Doctors are not always saying that this person will be permanently disabled,” Ms. Roberts said. “If this person doesn’t get better and they’re disabled until retirement age, this could be a payout in the high six or seven figures if a person is very young and was a very high earner.”
Insurance companies routinely deny claims that can’t be backed up with objective measures, such as specific lab test results or clear findings from a physical exam. But there are steps that can increase the odds of a successful claim for long COVID disability benefits, according to New York–based law firm Hiller.
For starters, patients can document COVID test results, and if testing wasn’t conducted, patients can detail the specific symptoms that led to this diagnosis, Hiller advises. Then patients can keep a daily symptom log at home that run lists all of the specific symptoms that occur at different times during the day and night to help establish a pattern of disability. These logs should provide specific details about every job duty patients have and exactly how specific symptoms of long COVID interfere with these duties.
Even though objective testing is hard to come by for long COVID, people should undergo all the tests they can that may help document the frequency or severity of specific symptoms that make it impossible to carry on with business as usual at work, Hiller advises. This may include neuropsychological testing to document brain fog, a cardiopulmonary exercise test to demonstrate chronic fatigue and the inability to exercise, or a tilt table test to measure dizziness.
Seeking a doctor’s diagnosis can be key to collecting disability payments, in or out of court.
All of this puts a lot of pressure on doctors and patients to build strong cases, said Jonathan Whiteson, MD, codirector of the NYU Langone Health post-COVID care program in New York. “Many physicians are not familiar with the disability benefit paperwork, and so this is a challenge for the doctors to know how to complete and to build the time into their highly scheduled days to take the time needed to complete.
“It’s also challenging because most of the disability benefit forms are ‘generic’ and do not ask specific questions about COVID disability,” Dr. Whiteson added. “It can be like trying to drive a square peg into a round hole.”
Still, when it comes to long COVID, completing disability paperwork is increasingly becoming part of standard care, along with managing medication, rehabilitation therapies, and lifestyle changes to navigate daily life with this illness, Dr. Whiteson noted.
Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the Post-COVID-19 Recovery Clinic at the University of Texas Health Science Center, San Antonio, agreed with this assessment.
“I have done letter upon letter of appeal to disability insurance companies,” she said.
Some doctors, however, are reluctant to step up in such cases, in part because no standard diagnostic guidelines exist for long COVID and because it can be frustrating.
“This is the work that is not paid and causes burnout in physicians,” Dr. Verduzco-Gutierrez said. “The paperwork, the fighting with insurance companies, the resubmission of forms for disability all to get what your patient needs – and then it gets denied.
“We will keep doing this because our patients need this disability income in order to live their lives and to afford what they need for recovery,” said Dr. Verduzco-Gutierrez. “But at some point something has to change because this isn’t sustainable.”
A version of this article appeared on Medscape.com.
At least 30 lawsuits have been filed seeking legal resolution of disability insurance claims, according to searches of court records. In addition, the Social Security Administration said it has received about 52,000 disability claims tied to SARS-CoV-2 infections, which represents 1% of all applications.
But legal experts say those cases may not reflect the total number of cases that have gone to court. They note many claims are initially dismissed and are not appealed by claimants.
“With this system, they deny two-thirds of initial applications, then people who appeal get denied almost 90% of the time, and then they can appeal before a judge,” said Kevin LaPorte, a Social Security disability attorney at LaPorte Law Firm in Oakland, Calif. “What happens next doesn’t have a lot of precedent because long COVID is a mass disabling event, and we haven’t seen that many of these cases get all the way through the legal system yet.”
As a result, the exact number of long COVID disability claims and the number of these cases going to court isn’t clear, he said.
“It can take a year or more for cases to get to court, and even longer to reach resolution,” Mr. LaPorte added. “I suspect the few cases we’ve heard about at this point are going to be the tip of the iceberg.”
The process is convoluted and can drag on for months with multiple denials and appeals along the way. Many disabled workers find their only recourse is to take insurers to court.
Long COVID patients typically apply for disability benefits through private insurance or Social Security. But the process can drag on for months, so many find their only recourse is to take insurers to court, according to legal experts.
But even in the courts, many encounter delays and hurdles to resolution.
In one of the first federal lawsuits involving long COVID disability benefits, William Abrams, a trial and appellate attorney and active marathon runner, sued Unum Life Insurance seeking long-term disability income. Symptoms included extreme fatigue, brain fog, decreased attention and concentration, and nearly daily fevers, causing him to stop working in April 2020.
His diagnosis wasn’t definitive. Three doctors said he had long COVID, and four said he had chronic fatigue syndrome. Unum cited this inconsistency as a rationale for rejecting his claim. But the court sided with Mr. Abrams, granting him disability income. The court concluded: “Unum may be correct that [the plaintiff] has not been correctly diagnosed. But that does not mean he is not sick. If [the plaintiff’s] complaints, and [the doctor’s] assessments, are to be believed, [the plaintiff] cannot focus for more than a few minutes at a time, making it impossible for [the plaintiff] to perform the varied and complex tasks his job requires.”
Unum said in an emailed statement that the company doesn’t comment on specific claims as a matter of policy, adding that its total payouts for disability claims from March 2020 to February 2022 were 35% higher than prepandemic levels. “In general, disability and leave claims connected to COVID-19 have been primarily short-term events with the majority of claimants recovering prior to completing the normal qualification period for long-term disability insurance,” Unum said.
Mr. Abrams prevailed in part because he had detailed documentation of the numerous impairments that eventually required him to stop work, said Michelle Roberts of Roberts Disability Law in Oakland, Calif.
He submitted videos of himself taking his temperature to prove he had almost daily fevers, according to court records. He underwent neuropsychological testing, which found learning deficiencies and memory deficits.
Mr. Abrams also submitted statements from a colleague who worked with him on a complex technology patent case involving radiofrequency identification. Before he got COVID, Mr. Abrams “had the analytical ability, legal acumen, and mental energy to attack that learning curve and get up to speed very rapidly,” according to court records.
“The court focused on credulity.” Ms. Roberts said. “There was all this work to be done to show this person was high functioning and ran marathons and worked in an intense, high-pressure occupation but then couldn’t do anything after long COVID.”
Documentation was also crucial in another early federal long COVID disability lawsuit that was filed in 2022 on behalf of Wendy Haut, an educational software sales representative in California who turned to the courts seeking disability income through her company’s employee benefits plan.
Several of Ms. Haut’s doctors documented a detailed list of long COVID symptoms, including “profound fatigue and extreme cognitive difficulties,” that they said prevented her from working as a sales representative or doing any other type of job. A settlement agreement in June 2022 required Reliance Standard Life Insurance to pay Ms. Haut long-term disability benefits, including previously unpaid benefits, according to a report by the advocacy group Pandemic Patients.
Representatives of Reliance Standard didn’t respond to a request for comment.
The growing number of workers being sidelined by long COVID makes more claims and more court cases likely. Right now, an estimated 16 million working-age Americans aged 18-65 years have long COVID, and as many as 4 million of them can’t work, according to a July 2023 Census Bureau report.
Uncertainty about the volume of claims in the pipeline is part of what’s driving some insurers to fight long COVID claims, Ms. Roberts said. Another factor is the lack of clarity around how many years people with long COVID may be out of work, particularly if they’re in their 30s or 40s and might be seeking disability income until they reach retirement age.
“Doctors are not always saying that this person will be permanently disabled,” Ms. Roberts said. “If this person doesn’t get better and they’re disabled until retirement age, this could be a payout in the high six or seven figures if a person is very young and was a very high earner.”
Insurance companies routinely deny claims that can’t be backed up with objective measures, such as specific lab test results or clear findings from a physical exam. But there are steps that can increase the odds of a successful claim for long COVID disability benefits, according to New York–based law firm Hiller.
For starters, patients can document COVID test results, and if testing wasn’t conducted, patients can detail the specific symptoms that led to this diagnosis, Hiller advises. Then patients can keep a daily symptom log at home that run lists all of the specific symptoms that occur at different times during the day and night to help establish a pattern of disability. These logs should provide specific details about every job duty patients have and exactly how specific symptoms of long COVID interfere with these duties.
Even though objective testing is hard to come by for long COVID, people should undergo all the tests they can that may help document the frequency or severity of specific symptoms that make it impossible to carry on with business as usual at work, Hiller advises. This may include neuropsychological testing to document brain fog, a cardiopulmonary exercise test to demonstrate chronic fatigue and the inability to exercise, or a tilt table test to measure dizziness.
Seeking a doctor’s diagnosis can be key to collecting disability payments, in or out of court.
All of this puts a lot of pressure on doctors and patients to build strong cases, said Jonathan Whiteson, MD, codirector of the NYU Langone Health post-COVID care program in New York. “Many physicians are not familiar with the disability benefit paperwork, and so this is a challenge for the doctors to know how to complete and to build the time into their highly scheduled days to take the time needed to complete.
“It’s also challenging because most of the disability benefit forms are ‘generic’ and do not ask specific questions about COVID disability,” Dr. Whiteson added. “It can be like trying to drive a square peg into a round hole.”
Still, when it comes to long COVID, completing disability paperwork is increasingly becoming part of standard care, along with managing medication, rehabilitation therapies, and lifestyle changes to navigate daily life with this illness, Dr. Whiteson noted.
Monica Verduzco-Gutierrez, MD, chair of rehabilitation medicine and director of the Post-COVID-19 Recovery Clinic at the University of Texas Health Science Center, San Antonio, agreed with this assessment.
“I have done letter upon letter of appeal to disability insurance companies,” she said.
Some doctors, however, are reluctant to step up in such cases, in part because no standard diagnostic guidelines exist for long COVID and because it can be frustrating.
“This is the work that is not paid and causes burnout in physicians,” Dr. Verduzco-Gutierrez said. “The paperwork, the fighting with insurance companies, the resubmission of forms for disability all to get what your patient needs – and then it gets denied.
“We will keep doing this because our patients need this disability income in order to live their lives and to afford what they need for recovery,” said Dr. Verduzco-Gutierrez. “But at some point something has to change because this isn’t sustainable.”
A version of this article appeared on Medscape.com.
Depression at any stage of life tied to increased dementia risk
Adults with depression have more than double the risk of developing dementia and the risk persists regardless of when in life depression is diagnosed, a large population-based study shows.
That the association between depression and dementia persisted even among individuals first diagnosed with depression in early or mid-life provides “strong evidence that depression is not only an early symptom of dementia, but also that depression increases dementia risk,” study investigator Holly Elser, MD, PhD, epidemiologist and resident physician, University of Pennsylvania, Philadelphia, told this news organization.
The study was published online in JAMA Neurology.
Double the risk
Several prior studies that have examined the relationship between depression and dementia over the life course have consistently shown depression later in life is associated with subsequent dementia.
“Late-life depression is generally thought to be an early symptom of dementia or a reaction to subclinical cognitive decline,” said Dr. Elser.
The investigators wanted to examine whether the association between depression and dementia persists even when depression is diagnosed earlier in life, which may suggest it increases the risk of dementia.
“To my knowledge, ours is the largest study on this topic to date, leveraging routinely and prospectively collected data from more than 1.4 million Danish citizens followed from 1977 to 2018,” Dr. Elser noted.
The cohort included 246,499 individuals diagnosed with depression and 1,190,302 individuals without depression.
In both groups, the median age was 50 years and 65% were women. Roughly two-thirds (68%) of those diagnosed with depression were diagnosed before age 60 years.
In Cox proportional hazards regression models, the overall hazard of dementia was more than doubled in those diagnosed with depression (hazard ratio [HR] 2.41). The risk of dementia with depression was more pronounced for men (HR, 2.98) than in women (HR, 2.21).
This association persisted even when the time elapsed from depression diagnosis was between 20 and 39 years (HR, 1.79) and whether depression was diagnosed in early life (18-44 years: HR, 3.08), mid-life (45-59 years: HR, 2.95), or late life (≥ 60 years: HR, 2.31).
It remains unclear whether effective treatment of depression modifies the risk of dementia, as the current study explored the role of antidepressants in a “very limited fashion,” Dr. Elser said.
Specifically, the researchers considered whether an individual was treated with an antidepressant within 6 months of the initial depression diagnosis and found no evidence of a difference in dementia risk between the treated and untreated groups.
“Research that explores implications of the timing and duration of treatment with antidepressants for dementia, treatment with cognitive behavioral therapy, and is able to evaluate the effectiveness of those treatments will be extremely important,” Dr. Elser said.
‘An assault on the brain’
Reached for comment, John Showalter, MD, chief product officer at Linus Health, said one of the most “intriguing” findings of the study is that a depression diagnosis earlier in adulthood conferred a greater risk of developing vascular dementia (HR, 3.28) than did dementia due to Alzheimer’s disease (HR, 1.73).
“The difference in risk for subtypes of dementia is a meaningful addition to our understanding of depression’s connection to dementia,” said Dr. Showalter, who was not involved in the study.
Also weighing in, Shaheen Lakhan, MD, PhD, a neurologist and researcher in Boston, said the findings from this “far-reaching investigation leave little room for doubt – depression unleashes a devastating storm within the brain, wreaking havoc on the lives of those ensnared by its grip.
“This massive, multi-decade, and high-data quality registry study adds another brick to the growing edifice of evidence attesting to the profound connection between psychiatric health and the very essence of brain health,” said Dr. Lakhan, who was not involved in the study.
“In a resounding declaration, this research underscores that psychiatric health should be perceived as an integral component of overall health – a paradigm shift that challenges long-standing misconceptions and stigmas surrounding mental disorders. Depression, once marginalized, now claims its rightful place on the pedestal of health concerns that must be addressed with unwavering resolve,” said Dr. Lakhan.
He noted that depression is “not just a mental battle, it’s a profound assault on the very fabric of the brain, leaving lives in turmoil and hearts in search of hope. No longer shrouded in silence, depression demands society’s attention.”
The study had no specific funding. Dr. Elser, Dr. Showalter, and Dr. Lakhan have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Adults with depression have more than double the risk of developing dementia and the risk persists regardless of when in life depression is diagnosed, a large population-based study shows.
That the association between depression and dementia persisted even among individuals first diagnosed with depression in early or mid-life provides “strong evidence that depression is not only an early symptom of dementia, but also that depression increases dementia risk,” study investigator Holly Elser, MD, PhD, epidemiologist and resident physician, University of Pennsylvania, Philadelphia, told this news organization.
The study was published online in JAMA Neurology.
Double the risk
Several prior studies that have examined the relationship between depression and dementia over the life course have consistently shown depression later in life is associated with subsequent dementia.
“Late-life depression is generally thought to be an early symptom of dementia or a reaction to subclinical cognitive decline,” said Dr. Elser.
The investigators wanted to examine whether the association between depression and dementia persists even when depression is diagnosed earlier in life, which may suggest it increases the risk of dementia.
“To my knowledge, ours is the largest study on this topic to date, leveraging routinely and prospectively collected data from more than 1.4 million Danish citizens followed from 1977 to 2018,” Dr. Elser noted.
The cohort included 246,499 individuals diagnosed with depression and 1,190,302 individuals without depression.
In both groups, the median age was 50 years and 65% were women. Roughly two-thirds (68%) of those diagnosed with depression were diagnosed before age 60 years.
In Cox proportional hazards regression models, the overall hazard of dementia was more than doubled in those diagnosed with depression (hazard ratio [HR] 2.41). The risk of dementia with depression was more pronounced for men (HR, 2.98) than in women (HR, 2.21).
This association persisted even when the time elapsed from depression diagnosis was between 20 and 39 years (HR, 1.79) and whether depression was diagnosed in early life (18-44 years: HR, 3.08), mid-life (45-59 years: HR, 2.95), or late life (≥ 60 years: HR, 2.31).
It remains unclear whether effective treatment of depression modifies the risk of dementia, as the current study explored the role of antidepressants in a “very limited fashion,” Dr. Elser said.
Specifically, the researchers considered whether an individual was treated with an antidepressant within 6 months of the initial depression diagnosis and found no evidence of a difference in dementia risk between the treated and untreated groups.
“Research that explores implications of the timing and duration of treatment with antidepressants for dementia, treatment with cognitive behavioral therapy, and is able to evaluate the effectiveness of those treatments will be extremely important,” Dr. Elser said.
‘An assault on the brain’
Reached for comment, John Showalter, MD, chief product officer at Linus Health, said one of the most “intriguing” findings of the study is that a depression diagnosis earlier in adulthood conferred a greater risk of developing vascular dementia (HR, 3.28) than did dementia due to Alzheimer’s disease (HR, 1.73).
“The difference in risk for subtypes of dementia is a meaningful addition to our understanding of depression’s connection to dementia,” said Dr. Showalter, who was not involved in the study.
Also weighing in, Shaheen Lakhan, MD, PhD, a neurologist and researcher in Boston, said the findings from this “far-reaching investigation leave little room for doubt – depression unleashes a devastating storm within the brain, wreaking havoc on the lives of those ensnared by its grip.
“This massive, multi-decade, and high-data quality registry study adds another brick to the growing edifice of evidence attesting to the profound connection between psychiatric health and the very essence of brain health,” said Dr. Lakhan, who was not involved in the study.
“In a resounding declaration, this research underscores that psychiatric health should be perceived as an integral component of overall health – a paradigm shift that challenges long-standing misconceptions and stigmas surrounding mental disorders. Depression, once marginalized, now claims its rightful place on the pedestal of health concerns that must be addressed with unwavering resolve,” said Dr. Lakhan.
He noted that depression is “not just a mental battle, it’s a profound assault on the very fabric of the brain, leaving lives in turmoil and hearts in search of hope. No longer shrouded in silence, depression demands society’s attention.”
The study had no specific funding. Dr. Elser, Dr. Showalter, and Dr. Lakhan have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Adults with depression have more than double the risk of developing dementia and the risk persists regardless of when in life depression is diagnosed, a large population-based study shows.
That the association between depression and dementia persisted even among individuals first diagnosed with depression in early or mid-life provides “strong evidence that depression is not only an early symptom of dementia, but also that depression increases dementia risk,” study investigator Holly Elser, MD, PhD, epidemiologist and resident physician, University of Pennsylvania, Philadelphia, told this news organization.
The study was published online in JAMA Neurology.
Double the risk
Several prior studies that have examined the relationship between depression and dementia over the life course have consistently shown depression later in life is associated with subsequent dementia.
“Late-life depression is generally thought to be an early symptom of dementia or a reaction to subclinical cognitive decline,” said Dr. Elser.
The investigators wanted to examine whether the association between depression and dementia persists even when depression is diagnosed earlier in life, which may suggest it increases the risk of dementia.
“To my knowledge, ours is the largest study on this topic to date, leveraging routinely and prospectively collected data from more than 1.4 million Danish citizens followed from 1977 to 2018,” Dr. Elser noted.
The cohort included 246,499 individuals diagnosed with depression and 1,190,302 individuals without depression.
In both groups, the median age was 50 years and 65% were women. Roughly two-thirds (68%) of those diagnosed with depression were diagnosed before age 60 years.
In Cox proportional hazards regression models, the overall hazard of dementia was more than doubled in those diagnosed with depression (hazard ratio [HR] 2.41). The risk of dementia with depression was more pronounced for men (HR, 2.98) than in women (HR, 2.21).
This association persisted even when the time elapsed from depression diagnosis was between 20 and 39 years (HR, 1.79) and whether depression was diagnosed in early life (18-44 years: HR, 3.08), mid-life (45-59 years: HR, 2.95), or late life (≥ 60 years: HR, 2.31).
It remains unclear whether effective treatment of depression modifies the risk of dementia, as the current study explored the role of antidepressants in a “very limited fashion,” Dr. Elser said.
Specifically, the researchers considered whether an individual was treated with an antidepressant within 6 months of the initial depression diagnosis and found no evidence of a difference in dementia risk between the treated and untreated groups.
“Research that explores implications of the timing and duration of treatment with antidepressants for dementia, treatment with cognitive behavioral therapy, and is able to evaluate the effectiveness of those treatments will be extremely important,” Dr. Elser said.
‘An assault on the brain’
Reached for comment, John Showalter, MD, chief product officer at Linus Health, said one of the most “intriguing” findings of the study is that a depression diagnosis earlier in adulthood conferred a greater risk of developing vascular dementia (HR, 3.28) than did dementia due to Alzheimer’s disease (HR, 1.73).
“The difference in risk for subtypes of dementia is a meaningful addition to our understanding of depression’s connection to dementia,” said Dr. Showalter, who was not involved in the study.
Also weighing in, Shaheen Lakhan, MD, PhD, a neurologist and researcher in Boston, said the findings from this “far-reaching investigation leave little room for doubt – depression unleashes a devastating storm within the brain, wreaking havoc on the lives of those ensnared by its grip.
“This massive, multi-decade, and high-data quality registry study adds another brick to the growing edifice of evidence attesting to the profound connection between psychiatric health and the very essence of brain health,” said Dr. Lakhan, who was not involved in the study.
“In a resounding declaration, this research underscores that psychiatric health should be perceived as an integral component of overall health – a paradigm shift that challenges long-standing misconceptions and stigmas surrounding mental disorders. Depression, once marginalized, now claims its rightful place on the pedestal of health concerns that must be addressed with unwavering resolve,” said Dr. Lakhan.
He noted that depression is “not just a mental battle, it’s a profound assault on the very fabric of the brain, leaving lives in turmoil and hearts in search of hope. No longer shrouded in silence, depression demands society’s attention.”
The study had no specific funding. Dr. Elser, Dr. Showalter, and Dr. Lakhan have reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Prescribing lifestyle changes: When medicine isn’t enough
In psychiatry, patients come to us with their list of symptoms, often a diagnosis they’ve made themselves, and the expectation that they will be given medication to fix their problem. Their diagnoses are often right on target – people often know if they are depressed or anxious, and Doctor Google may provide useful information.
Sometimes they want a specific medication, one they saw in a TV ad, or one that helped them in the past or has helped someone they know. As psychiatrists have focused more on their strengths as psychopharmacologists and less on psychotherapy, it gets easy for both the patient and the doctor to look to medication, cocktails, and titration as the only thing we do.
“My medicine stopped working,” is a line I commonly hear. Often the patient is on a complicated regimen that has been serving them well, and it seems unlikely that the five psychotropic medications they are taking have suddenly “stopped working.” An obvious exception is the SSRI “poop out” that can occur 6-12 months or more after beginning treatment. In addition, it’s important to make sure patients are taking their medications as prescribed, and that the generic formulations have not changed.
But as rates of mental illness increase, some of it spurred on by difficult times,
This is not to devalue our medications, but to help the patient see symptoms as having multiple factors and give them some means to intervene, in addition to medications. At the beginning of therapy, it is important to “prescribe” lifestyle changes that will facilitate the best possible outcomes.
Nonpharmaceutical prescriptions
Early in my career, people with alcohol use problems were told they needed to be substance free before they were candidates for antidepressants. While we no longer do that, it is still important to emphasize abstinence from addictive substances, and to recommend specific treatment when necessary.
Patients are often reluctant to see their use of alcohol, marijuana (it’s medical! It’s part of wellness!), or their pain medications as part of the problem, and this can be difficult. There have been times, after multiple medications have failed to help their symptoms, when I have said, “If you don’t get treatment for this problem, I am not going to be able to help you feel better” and that has been motivating for the patient.
There are other “prescriptions” to write. Regular sleep is essential for people with mood disorders, and this can be difficult for many patients, especially those who do shift work, or who have regular disruptions to their sleep from noise, pets, and children. Exercise is wonderful for the cardiovascular system, calms anxiety, and maintains strength, endurance, mobility, and quality of life as people age. But it can be a hard sell to people in a mental health crisis.
Nature is healing, and sunshine helps with maintaining circadian rhythms. For those who don’t exercise, I often “prescribe” 20 to 30 minutes a day of walking, preferably outside, during daylight hours, in a park or natural setting. For people with anxiety, it is important to check their caffeine consumption and to suggest ways to moderate it – moving to decaffeinated beverages or titrating down by mixing decaf with caffeinated.
Meditation is something that many people find helpful. For anxious people, it can be very difficult, and I will prescribe a specific instructional video course that I like on the well-being app InsightTimer – Sarah Blondin’s Learn How to Meditate in Seven Days. The sessions are approximately 10 minutes long, and that seems like the right amount of time for a beginner.
When people are very ill and don’t want to go into the hospital, I talk with them about things that happen in the hospital that are helpful, things they can try to mimic at home. In the hospital, patients don’t go to work, they don’t spend hours a day on the computer, and they are given a pass from dealing with the routine stresses of daily life.
I ask them to take time off work, to avoid as much stress as possible, to spend time with loved ones who give them comfort, and to avoid the people who leave them feeling drained or distressed. I ask them to engage in activities they find healing, to eat well, exercise, and avoid social media. In the hospital, I emphasize, they wake patients up in the morning, ask them to get out of bed and engage in therapeutic activities. They are fed and kept from intoxicants.
When it comes to nutrition, we know so little about how food affects mental health. I feel like it can’t hurt to ask people to avoid fast foods, soft drinks, and processed foods, and so I do.
And what about compliance? Of course, not everyone complies; not everyone is interested in making changes and these can be hard changes. I’ve recently started to recommend the book Atomic Habits by James Clear. Sometimes a bit of motivational interviewing can also be helpful in getting people to look at slowly moving toward making changes.
In prescribing lifestyle changes, it is important to offer most of these changes as suggestions, not as things we insist on, or that will leave the patient feeling ashamed if he doesn’t follow through. They should be discussed early in treatment so that patients don’t feel blamed for their illness or relapses. As with all the things we prescribe, some of these behavior changes help some of the people some of the time. Suggesting them, however, makes the strong statement that treating psychiatric disorders can be about more than passively swallowing a pill.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She disclosed no relevant conflicts of interest.
In psychiatry, patients come to us with their list of symptoms, often a diagnosis they’ve made themselves, and the expectation that they will be given medication to fix their problem. Their diagnoses are often right on target – people often know if they are depressed or anxious, and Doctor Google may provide useful information.
Sometimes they want a specific medication, one they saw in a TV ad, or one that helped them in the past or has helped someone they know. As psychiatrists have focused more on their strengths as psychopharmacologists and less on psychotherapy, it gets easy for both the patient and the doctor to look to medication, cocktails, and titration as the only thing we do.
“My medicine stopped working,” is a line I commonly hear. Often the patient is on a complicated regimen that has been serving them well, and it seems unlikely that the five psychotropic medications they are taking have suddenly “stopped working.” An obvious exception is the SSRI “poop out” that can occur 6-12 months or more after beginning treatment. In addition, it’s important to make sure patients are taking their medications as prescribed, and that the generic formulations have not changed.
But as rates of mental illness increase, some of it spurred on by difficult times,
This is not to devalue our medications, but to help the patient see symptoms as having multiple factors and give them some means to intervene, in addition to medications. At the beginning of therapy, it is important to “prescribe” lifestyle changes that will facilitate the best possible outcomes.
Nonpharmaceutical prescriptions
Early in my career, people with alcohol use problems were told they needed to be substance free before they were candidates for antidepressants. While we no longer do that, it is still important to emphasize abstinence from addictive substances, and to recommend specific treatment when necessary.
Patients are often reluctant to see their use of alcohol, marijuana (it’s medical! It’s part of wellness!), or their pain medications as part of the problem, and this can be difficult. There have been times, after multiple medications have failed to help their symptoms, when I have said, “If you don’t get treatment for this problem, I am not going to be able to help you feel better” and that has been motivating for the patient.
There are other “prescriptions” to write. Regular sleep is essential for people with mood disorders, and this can be difficult for many patients, especially those who do shift work, or who have regular disruptions to their sleep from noise, pets, and children. Exercise is wonderful for the cardiovascular system, calms anxiety, and maintains strength, endurance, mobility, and quality of life as people age. But it can be a hard sell to people in a mental health crisis.
Nature is healing, and sunshine helps with maintaining circadian rhythms. For those who don’t exercise, I often “prescribe” 20 to 30 minutes a day of walking, preferably outside, during daylight hours, in a park or natural setting. For people with anxiety, it is important to check their caffeine consumption and to suggest ways to moderate it – moving to decaffeinated beverages or titrating down by mixing decaf with caffeinated.
Meditation is something that many people find helpful. For anxious people, it can be very difficult, and I will prescribe a specific instructional video course that I like on the well-being app InsightTimer – Sarah Blondin’s Learn How to Meditate in Seven Days. The sessions are approximately 10 minutes long, and that seems like the right amount of time for a beginner.
When people are very ill and don’t want to go into the hospital, I talk with them about things that happen in the hospital that are helpful, things they can try to mimic at home. In the hospital, patients don’t go to work, they don’t spend hours a day on the computer, and they are given a pass from dealing with the routine stresses of daily life.
I ask them to take time off work, to avoid as much stress as possible, to spend time with loved ones who give them comfort, and to avoid the people who leave them feeling drained or distressed. I ask them to engage in activities they find healing, to eat well, exercise, and avoid social media. In the hospital, I emphasize, they wake patients up in the morning, ask them to get out of bed and engage in therapeutic activities. They are fed and kept from intoxicants.
When it comes to nutrition, we know so little about how food affects mental health. I feel like it can’t hurt to ask people to avoid fast foods, soft drinks, and processed foods, and so I do.
And what about compliance? Of course, not everyone complies; not everyone is interested in making changes and these can be hard changes. I’ve recently started to recommend the book Atomic Habits by James Clear. Sometimes a bit of motivational interviewing can also be helpful in getting people to look at slowly moving toward making changes.
In prescribing lifestyle changes, it is important to offer most of these changes as suggestions, not as things we insist on, or that will leave the patient feeling ashamed if he doesn’t follow through. They should be discussed early in treatment so that patients don’t feel blamed for their illness or relapses. As with all the things we prescribe, some of these behavior changes help some of the people some of the time. Suggesting them, however, makes the strong statement that treating psychiatric disorders can be about more than passively swallowing a pill.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She disclosed no relevant conflicts of interest.
In psychiatry, patients come to us with their list of symptoms, often a diagnosis they’ve made themselves, and the expectation that they will be given medication to fix their problem. Their diagnoses are often right on target – people often know if they are depressed or anxious, and Doctor Google may provide useful information.
Sometimes they want a specific medication, one they saw in a TV ad, or one that helped them in the past or has helped someone they know. As psychiatrists have focused more on their strengths as psychopharmacologists and less on psychotherapy, it gets easy for both the patient and the doctor to look to medication, cocktails, and titration as the only thing we do.
“My medicine stopped working,” is a line I commonly hear. Often the patient is on a complicated regimen that has been serving them well, and it seems unlikely that the five psychotropic medications they are taking have suddenly “stopped working.” An obvious exception is the SSRI “poop out” that can occur 6-12 months or more after beginning treatment. In addition, it’s important to make sure patients are taking their medications as prescribed, and that the generic formulations have not changed.
But as rates of mental illness increase, some of it spurred on by difficult times,
This is not to devalue our medications, but to help the patient see symptoms as having multiple factors and give them some means to intervene, in addition to medications. At the beginning of therapy, it is important to “prescribe” lifestyle changes that will facilitate the best possible outcomes.
Nonpharmaceutical prescriptions
Early in my career, people with alcohol use problems were told they needed to be substance free before they were candidates for antidepressants. While we no longer do that, it is still important to emphasize abstinence from addictive substances, and to recommend specific treatment when necessary.
Patients are often reluctant to see their use of alcohol, marijuana (it’s medical! It’s part of wellness!), or their pain medications as part of the problem, and this can be difficult. There have been times, after multiple medications have failed to help their symptoms, when I have said, “If you don’t get treatment for this problem, I am not going to be able to help you feel better” and that has been motivating for the patient.
There are other “prescriptions” to write. Regular sleep is essential for people with mood disorders, and this can be difficult for many patients, especially those who do shift work, or who have regular disruptions to their sleep from noise, pets, and children. Exercise is wonderful for the cardiovascular system, calms anxiety, and maintains strength, endurance, mobility, and quality of life as people age. But it can be a hard sell to people in a mental health crisis.
Nature is healing, and sunshine helps with maintaining circadian rhythms. For those who don’t exercise, I often “prescribe” 20 to 30 minutes a day of walking, preferably outside, during daylight hours, in a park or natural setting. For people with anxiety, it is important to check their caffeine consumption and to suggest ways to moderate it – moving to decaffeinated beverages or titrating down by mixing decaf with caffeinated.
Meditation is something that many people find helpful. For anxious people, it can be very difficult, and I will prescribe a specific instructional video course that I like on the well-being app InsightTimer – Sarah Blondin’s Learn How to Meditate in Seven Days. The sessions are approximately 10 minutes long, and that seems like the right amount of time for a beginner.
When people are very ill and don’t want to go into the hospital, I talk with them about things that happen in the hospital that are helpful, things they can try to mimic at home. In the hospital, patients don’t go to work, they don’t spend hours a day on the computer, and they are given a pass from dealing with the routine stresses of daily life.
I ask them to take time off work, to avoid as much stress as possible, to spend time with loved ones who give them comfort, and to avoid the people who leave them feeling drained or distressed. I ask them to engage in activities they find healing, to eat well, exercise, and avoid social media. In the hospital, I emphasize, they wake patients up in the morning, ask them to get out of bed and engage in therapeutic activities. They are fed and kept from intoxicants.
When it comes to nutrition, we know so little about how food affects mental health. I feel like it can’t hurt to ask people to avoid fast foods, soft drinks, and processed foods, and so I do.
And what about compliance? Of course, not everyone complies; not everyone is interested in making changes and these can be hard changes. I’ve recently started to recommend the book Atomic Habits by James Clear. Sometimes a bit of motivational interviewing can also be helpful in getting people to look at slowly moving toward making changes.
In prescribing lifestyle changes, it is important to offer most of these changes as suggestions, not as things we insist on, or that will leave the patient feeling ashamed if he doesn’t follow through. They should be discussed early in treatment so that patients don’t feel blamed for their illness or relapses. As with all the things we prescribe, some of these behavior changes help some of the people some of the time. Suggesting them, however, makes the strong statement that treating psychiatric disorders can be about more than passively swallowing a pill.
Dr. Miller is a coauthor of “Committed: The Battle Over Involuntary Psychiatric Care” (Baltimore: Johns Hopkins University Press, 2016). She has a private practice and is an assistant professor of psychiatry and behavioral sciences at Johns Hopkins University, Baltimore. She disclosed no relevant conflicts of interest.
Medicare to pay for at-home dementia care coordination
Under a new Medicare pilot program that will begin in 2024, the federal government will pay clinicians to coordinate at-home dementia support services, including respite care for family members.
A Department of Health & Human Services initiative, part of the aim of the Guiding an Improved Dementia Experience (GUIDE) program is to help Medicare beneficiaries with dementia stay in the community for as long as possible. It is estimated that there are 6.7 million Americans living with Alzheimer’s disease or some other form of dementia, said HHS.
The program is voluntary and will be open to Medicare-enrolled clinicians and other providers who can assemble an interdisciplinary care team and meet the program’s participation criteria.
“Our new GUIDE Model has the potential to improve the quality of life for people with dementia and alleviate the significant strain on our families,” said HHS Secretary Xavier Becerra, in a statement.
“Not only is dementia care management a proven way to improve the quality of care and quality of life for those living with Alzheimer’s and other dementia, but now we know that it would also save the federal government billions of dollars,” Robert Egge, Alzheimer’s Association chief public policy officer and Alzheimer’s Impact Movement (AIM) executive director, said in a statement.
Mr. Egge cited a recent analysis commissioned by AIM that found that dementia care management would save the federal government nearly $21 billion over 10 years.
“People living with dementia and their caregivers too often struggle to manage their health care and connect with key supports that can allow them to remain in their homes and communities,” said Centers for Medicare & Medicaid Services Administrator Chiquita Brooks-LaSure, in the HHS statement.
“Fragmented care contributes to the mental and physical health strain of caring for someone with dementia, as well as the substantial financial burden,” she said, adding that Black, Hispanic, Asian American, Native Hawaiian, and Pacific Islander populations have been especially disadvantaged.
The GUIDE Model will provide new resources and greater access to specialty care to those communities, said Ms. Brooks-LaSure.
Care teams that seek to participate in the GUIDE model must have a care navigator who has received required training in dementia, assessment, and care planning.
The teams also must have a clinician with dementia proficiency as recognized by experience caring for adults with cognitive impairment; experience caring for patients aged 65 years old or older; or specialty designation in neurology, psychiatry, geriatrics, geriatric psychiatry, behavioral neurology, or geriatric neurology.
Medicare beneficiaries will be eligible if they are not residing in a nursing home; are not enrolled in hospice; and have a confirmed dementia diagnosis.
Beneficiaries who receive care from GUIDE participants will be placed in one of five “tiers,” based on a combination of disease stage and caregiver status. Beneficiary needs, and care intensity and payment, increase by tier.
GUIDE teams will receive a monthly, per-beneficiary amount for providing care management and coordination and caregiver education and support services. They can also bill for respite services – up to an annual cap – for Medicare beneficiaries who have an unpaid caregiver.
Clinicians seeking to participate in GUIDE can apply beginning in the fall. The program will run for 8 years beginning July 1, 2024.
Alzheimer’s Association President and CEO Joanne Pike, DrPH, said in a statement that the organization had “advocated for this approach for years, believing it [to be] the key to addressing systemic challenges faced by those with dementia, their families and those who provide them with care and support.”
The John A. Hartford Foundation noted that it also had long pushed for a comprehensive dementia care program. “Comprehensive dementia care supports both the medical and nonmedical needs of patients and their family caregivers,” said Foundation President Terry Fulmer, PhD, RN, FAAN, in a statement.
“Notably and necessarily, the model will help improve equity in access to care for underserved communities by addressing unpaid caregiver needs, including respite services and screening for health-related social needs,” added Dr. Fulmer.
A version of this article first appeared on Medscape.com.
Under a new Medicare pilot program that will begin in 2024, the federal government will pay clinicians to coordinate at-home dementia support services, including respite care for family members.
A Department of Health & Human Services initiative, part of the aim of the Guiding an Improved Dementia Experience (GUIDE) program is to help Medicare beneficiaries with dementia stay in the community for as long as possible. It is estimated that there are 6.7 million Americans living with Alzheimer’s disease or some other form of dementia, said HHS.
The program is voluntary and will be open to Medicare-enrolled clinicians and other providers who can assemble an interdisciplinary care team and meet the program’s participation criteria.
“Our new GUIDE Model has the potential to improve the quality of life for people with dementia and alleviate the significant strain on our families,” said HHS Secretary Xavier Becerra, in a statement.
“Not only is dementia care management a proven way to improve the quality of care and quality of life for those living with Alzheimer’s and other dementia, but now we know that it would also save the federal government billions of dollars,” Robert Egge, Alzheimer’s Association chief public policy officer and Alzheimer’s Impact Movement (AIM) executive director, said in a statement.
Mr. Egge cited a recent analysis commissioned by AIM that found that dementia care management would save the federal government nearly $21 billion over 10 years.
“People living with dementia and their caregivers too often struggle to manage their health care and connect with key supports that can allow them to remain in their homes and communities,” said Centers for Medicare & Medicaid Services Administrator Chiquita Brooks-LaSure, in the HHS statement.
“Fragmented care contributes to the mental and physical health strain of caring for someone with dementia, as well as the substantial financial burden,” she said, adding that Black, Hispanic, Asian American, Native Hawaiian, and Pacific Islander populations have been especially disadvantaged.
The GUIDE Model will provide new resources and greater access to specialty care to those communities, said Ms. Brooks-LaSure.
Care teams that seek to participate in the GUIDE model must have a care navigator who has received required training in dementia, assessment, and care planning.
The teams also must have a clinician with dementia proficiency as recognized by experience caring for adults with cognitive impairment; experience caring for patients aged 65 years old or older; or specialty designation in neurology, psychiatry, geriatrics, geriatric psychiatry, behavioral neurology, or geriatric neurology.
Medicare beneficiaries will be eligible if they are not residing in a nursing home; are not enrolled in hospice; and have a confirmed dementia diagnosis.
Beneficiaries who receive care from GUIDE participants will be placed in one of five “tiers,” based on a combination of disease stage and caregiver status. Beneficiary needs, and care intensity and payment, increase by tier.
GUIDE teams will receive a monthly, per-beneficiary amount for providing care management and coordination and caregiver education and support services. They can also bill for respite services – up to an annual cap – for Medicare beneficiaries who have an unpaid caregiver.
Clinicians seeking to participate in GUIDE can apply beginning in the fall. The program will run for 8 years beginning July 1, 2024.
Alzheimer’s Association President and CEO Joanne Pike, DrPH, said in a statement that the organization had “advocated for this approach for years, believing it [to be] the key to addressing systemic challenges faced by those with dementia, their families and those who provide them with care and support.”
The John A. Hartford Foundation noted that it also had long pushed for a comprehensive dementia care program. “Comprehensive dementia care supports both the medical and nonmedical needs of patients and their family caregivers,” said Foundation President Terry Fulmer, PhD, RN, FAAN, in a statement.
“Notably and necessarily, the model will help improve equity in access to care for underserved communities by addressing unpaid caregiver needs, including respite services and screening for health-related social needs,” added Dr. Fulmer.
A version of this article first appeared on Medscape.com.
Under a new Medicare pilot program that will begin in 2024, the federal government will pay clinicians to coordinate at-home dementia support services, including respite care for family members.
A Department of Health & Human Services initiative, part of the aim of the Guiding an Improved Dementia Experience (GUIDE) program is to help Medicare beneficiaries with dementia stay in the community for as long as possible. It is estimated that there are 6.7 million Americans living with Alzheimer’s disease or some other form of dementia, said HHS.
The program is voluntary and will be open to Medicare-enrolled clinicians and other providers who can assemble an interdisciplinary care team and meet the program’s participation criteria.
“Our new GUIDE Model has the potential to improve the quality of life for people with dementia and alleviate the significant strain on our families,” said HHS Secretary Xavier Becerra, in a statement.
“Not only is dementia care management a proven way to improve the quality of care and quality of life for those living with Alzheimer’s and other dementia, but now we know that it would also save the federal government billions of dollars,” Robert Egge, Alzheimer’s Association chief public policy officer and Alzheimer’s Impact Movement (AIM) executive director, said in a statement.
Mr. Egge cited a recent analysis commissioned by AIM that found that dementia care management would save the federal government nearly $21 billion over 10 years.
“People living with dementia and their caregivers too often struggle to manage their health care and connect with key supports that can allow them to remain in their homes and communities,” said Centers for Medicare & Medicaid Services Administrator Chiquita Brooks-LaSure, in the HHS statement.
“Fragmented care contributes to the mental and physical health strain of caring for someone with dementia, as well as the substantial financial burden,” she said, adding that Black, Hispanic, Asian American, Native Hawaiian, and Pacific Islander populations have been especially disadvantaged.
The GUIDE Model will provide new resources and greater access to specialty care to those communities, said Ms. Brooks-LaSure.
Care teams that seek to participate in the GUIDE model must have a care navigator who has received required training in dementia, assessment, and care planning.
The teams also must have a clinician with dementia proficiency as recognized by experience caring for adults with cognitive impairment; experience caring for patients aged 65 years old or older; or specialty designation in neurology, psychiatry, geriatrics, geriatric psychiatry, behavioral neurology, or geriatric neurology.
Medicare beneficiaries will be eligible if they are not residing in a nursing home; are not enrolled in hospice; and have a confirmed dementia diagnosis.
Beneficiaries who receive care from GUIDE participants will be placed in one of five “tiers,” based on a combination of disease stage and caregiver status. Beneficiary needs, and care intensity and payment, increase by tier.
GUIDE teams will receive a monthly, per-beneficiary amount for providing care management and coordination and caregiver education and support services. They can also bill for respite services – up to an annual cap – for Medicare beneficiaries who have an unpaid caregiver.
Clinicians seeking to participate in GUIDE can apply beginning in the fall. The program will run for 8 years beginning July 1, 2024.
Alzheimer’s Association President and CEO Joanne Pike, DrPH, said in a statement that the organization had “advocated for this approach for years, believing it [to be] the key to addressing systemic challenges faced by those with dementia, their families and those who provide them with care and support.”
The John A. Hartford Foundation noted that it also had long pushed for a comprehensive dementia care program. “Comprehensive dementia care supports both the medical and nonmedical needs of patients and their family caregivers,” said Foundation President Terry Fulmer, PhD, RN, FAAN, in a statement.
“Notably and necessarily, the model will help improve equity in access to care for underserved communities by addressing unpaid caregiver needs, including respite services and screening for health-related social needs,” added Dr. Fulmer.
A version of this article first appeared on Medscape.com.
Will this trial help solve chronic back pain?
Chronic pain, and back pain in particular, is among the most frequent concerns for patients in the primary care setting. Roughly 8% of adults in the United States say they suffer from chronic low back pain, and many of them say the pain is significant enough to impair their ability to move, work, and otherwise enjoy life. All this, despite decades of research and countless millions in funding to find the optimal approach to treating chronic pain.
As the United States crawls out of the opioid epidemic, a group of pain specialists is hoping to identify effective, personalized approaches to managing back pain. Daniel Clauw, MD, professor of anesthesiology, internal medicine, and psychiatry at the University of Michigan, Ann Arbor, is helping lead the BEST trial. With projected enrollment of nearly 800 patients, BEST will be the largest federally funded clinical trial of interventions to treat chronic low back pain.
In an interview, The interview has been edited for length and clarity.
What are your thoughts on the current state of primary care physicians’ understanding and management of pain?
Primary care physicians need a lot of help in demystifying the diagnosis and treatment of any kind of pain, but back pain is a really good place to start. When it comes to back pain, most primary care physicians are not any more knowledgeable than a layperson.
What has the opioid debacle-cum-tragedy taught you about pain management, particular as regards people with chronic pain?
I don’t feel opioids should ever be used to treat chronic low back pain. The few long-term studies that have been performed using opioids for longer than 3 months suggest that they often make pain worse rather than just failing to make pain better – and we know they are associated with a significantly increased all-cause mortality with increased deaths from myocardial infarction, accidents, and suicides, in addition to overdose.
Given how many patients experience back pain, how did we come to the point at which primary care physicians are so ill equipped?
We’ve had terrible pain curricula in medical schools. To give you an example: I’m one of the leading pain experts in the world and I’m not allowed to teach our medical students their pain curriculum. The students learn about neurophysiology and the anatomy of the nerves, not what’s relevant in pain.
This is notorious in medical school: Curricula are almost impossible to modify and change. So it starts with poor training in medical school. And then, regardless of what education they do or don’t get in medical school, a lot of their education about pain management is through our residencies – mainly in inpatient settings, where you’re really seeing the management of acute pain and not the management of chronic pain.
People get more accustomed to managing acute pain, where opioids are a reasonable option. It’s just that when you start managing subacute or chronic pain, opioids don’t work as well.
The other big problem is that historically, most people trained in medicine think that if you have pain in your elbow, there’s got to be something wrong in your elbow. This third mechanism of pain, central sensitization – or nociplastic pain – the kind of pain that we see in fibromyalgia, headache, and low back pain, where the pain is coming from the brain – that’s confusing to people. People can have pain without any damage or inflammation to that region of the body.
Physicians are trained that if there’s pain, there’s something wrong and we have to do surgery or there’s been some trauma. Most chronic pain is none of that. There’s a big disconnect between how people are trained, and then when they go out and are seeing a tremendous number of people with chronic pain.
What are the different types of pain, and how should they inform clinicians’ understanding about what approaches might work for managing their patients in pain?
The way the central nervous system responds to pain is analogous to the loudness of an electric guitar. You can make an electric guitar louder either by strumming the strings harder or by turning up the amplifier. For many people with fibromyalgia, low back pain, and endometriosis, for example, the problem is really more that the amplifier is turned up too high rather than its being that the guitar is strummed too strongly. That kind of pain where the pain is not due to anatomic damage or inflammation is particularly flummoxing for providers.
Can you explain the design of the new study?
It’s a 13-site study looking at four treatments: enhanced self-care, cognitive-behavioral therapy, physical therapy, and duloxetine. It’s a big precision medicine trial, trying to take everything we’ve learned and putting it all into one big study.
We’re using a SMART design, which randomizes people to two of those treatments, unless they are very much improved from the first treatment. To be eligible for the trial, you have to be able to be randomized to three of the four treatments, and people can’t choose which of the four they get.
We give them one of those treatments for 12 weeks, and at the end of 12 weeks we make the call – “Did you respond or not respond?” – and then we go back to the phenotypic data we collected at the beginning of that trial and say, “What information at baseline that we collected predicts that someone is going to respond better to duloxetine or worse to duloxetine?” And then we create the phenotype that responds best to each of those four treatments.
None of our treatments works so well that someone doesn’t end up getting randomized to a second treatment. About 85% of people so far need a second treatment because they still have enough pain that they want more relief. But the nice thing about that is we’ve already done all the functional brain imaging and all these really expensive and time-consuming things.
We’re hoping to have around 700-800 people total in this trial, which means that around 170 people will get randomized to each of the four initial treatments. No one’s ever done a study that has functional brain imaging and all these other things in it with more than 80 or 100 people. The scale of this is totally unprecedented.
Given that the individual therapies don’t appear to be all that successful on their own, what is your goal?
The primary aim is to match the phenotypic characteristics of a patient with chronic low back pain with treatment response to each of these four treatments. So at the end, we can give clinicians information on which of the patients is going to respond to physical therapy, for instance.
Right now, about one out of three people respond to most treatments for pain. We think by doing a trial like this, we can take treatments that work in one out of three people and make them work in one out of two or two out of three people just by using them in the right people.
How do you differentiate between these types of pain in your study?
We phenotype people by asking them a number of questions. We also do brain imaging, look at their back with MRI, test biomechanics, and then give them four different treatments that we know work in groups of people with low back pain.
We think one of the first parts of the phenotype is, do they have pain just in their back? Or do they have pain in their back plus a lot of other body regions? Because the more body regions that people have pain in, the more likely it is that this is an amplifier problem rather than a guitar problem.
Treatments like physical therapy, surgery, and injections are going to work better for people in whom the pain is a guitar problem rather than an amplifier problem. And drugs like duloxetine, which works in the brain, and cognitive-behavioral therapy are going to work a lot better in the people with pain in multiple sites besides the back.
To pick up on your metaphor, do any symptoms help clinicians differentiate between the guitar and the amplifier?
Sleep problems, fatigue, memory problems, and mood problems are common in patients with chronic pain and are more common with amplifier pain. Because again, those are all central nervous system problems. And so we see that the people that have anxiety, depression, and a lot of distress are more likely to have this kind of pain.
Does medical imaging help?
There’s a terrible relationship between what you see on an MRI of the back and whether someone has pain or how severe the pain is going to be. There’s always going to be individuals that have a lot of anatomic damage who don’t have any pain because they happen to be on the other end of the continuum from fibromyalgia; they’re actually pain-insensitive people.
What are your thoughts about ketamine as a possible treatment for chronic pain?
I have a mentee who’s doing a ketamine trial. We’re doing psilocybin trials in patients with fibromyalgia. Ketamine is such a dirty drug; it has so many different mechanisms of action. It does have some psychedelic effects, but it also is an NMDA blocker. It really has so many different effects.
I think it’s being thrown around like water in settings where we don’t yet know it to be efficacious. Even the data in treatment-refractory depression are pretty weak, but we’re so desperate to do something for those patients. If you’re trying to harness the psychedelic properties of ketamine, I think there’s other psychedelics that are a lot more interesting, which is why we’re using psilocybin for a subset of patients. Most of us in the pain field think that the psychedelics will work best for the people with chronic pain who have a lot of comorbid psychiatric illness, especially the ones with a lot of trauma. These drugs will allow us therapeutically to get at a lot of these patients with the side-by-side psychotherapy that’s being done as people are getting care in the medicalized setting.
Dr. Clauw reported conflicts of interest with Pfizer, Tonix, Theravance, Zynerba, Samumed, Aptinyx, Daiichi Sankyo, Intec, Regeneron, Teva, Lundbeck, Virios, and Cerephex.
A version of this article first appeared on Medscape.com.
Chronic pain, and back pain in particular, is among the most frequent concerns for patients in the primary care setting. Roughly 8% of adults in the United States say they suffer from chronic low back pain, and many of them say the pain is significant enough to impair their ability to move, work, and otherwise enjoy life. All this, despite decades of research and countless millions in funding to find the optimal approach to treating chronic pain.
As the United States crawls out of the opioid epidemic, a group of pain specialists is hoping to identify effective, personalized approaches to managing back pain. Daniel Clauw, MD, professor of anesthesiology, internal medicine, and psychiatry at the University of Michigan, Ann Arbor, is helping lead the BEST trial. With projected enrollment of nearly 800 patients, BEST will be the largest federally funded clinical trial of interventions to treat chronic low back pain.
In an interview, The interview has been edited for length and clarity.
What are your thoughts on the current state of primary care physicians’ understanding and management of pain?
Primary care physicians need a lot of help in demystifying the diagnosis and treatment of any kind of pain, but back pain is a really good place to start. When it comes to back pain, most primary care physicians are not any more knowledgeable than a layperson.
What has the opioid debacle-cum-tragedy taught you about pain management, particular as regards people with chronic pain?
I don’t feel opioids should ever be used to treat chronic low back pain. The few long-term studies that have been performed using opioids for longer than 3 months suggest that they often make pain worse rather than just failing to make pain better – and we know they are associated with a significantly increased all-cause mortality with increased deaths from myocardial infarction, accidents, and suicides, in addition to overdose.
Given how many patients experience back pain, how did we come to the point at which primary care physicians are so ill equipped?
We’ve had terrible pain curricula in medical schools. To give you an example: I’m one of the leading pain experts in the world and I’m not allowed to teach our medical students their pain curriculum. The students learn about neurophysiology and the anatomy of the nerves, not what’s relevant in pain.
This is notorious in medical school: Curricula are almost impossible to modify and change. So it starts with poor training in medical school. And then, regardless of what education they do or don’t get in medical school, a lot of their education about pain management is through our residencies – mainly in inpatient settings, where you’re really seeing the management of acute pain and not the management of chronic pain.
People get more accustomed to managing acute pain, where opioids are a reasonable option. It’s just that when you start managing subacute or chronic pain, opioids don’t work as well.
The other big problem is that historically, most people trained in medicine think that if you have pain in your elbow, there’s got to be something wrong in your elbow. This third mechanism of pain, central sensitization – or nociplastic pain – the kind of pain that we see in fibromyalgia, headache, and low back pain, where the pain is coming from the brain – that’s confusing to people. People can have pain without any damage or inflammation to that region of the body.
Physicians are trained that if there’s pain, there’s something wrong and we have to do surgery or there’s been some trauma. Most chronic pain is none of that. There’s a big disconnect between how people are trained, and then when they go out and are seeing a tremendous number of people with chronic pain.
What are the different types of pain, and how should they inform clinicians’ understanding about what approaches might work for managing their patients in pain?
The way the central nervous system responds to pain is analogous to the loudness of an electric guitar. You can make an electric guitar louder either by strumming the strings harder or by turning up the amplifier. For many people with fibromyalgia, low back pain, and endometriosis, for example, the problem is really more that the amplifier is turned up too high rather than its being that the guitar is strummed too strongly. That kind of pain where the pain is not due to anatomic damage or inflammation is particularly flummoxing for providers.
Can you explain the design of the new study?
It’s a 13-site study looking at four treatments: enhanced self-care, cognitive-behavioral therapy, physical therapy, and duloxetine. It’s a big precision medicine trial, trying to take everything we’ve learned and putting it all into one big study.
We’re using a SMART design, which randomizes people to two of those treatments, unless they are very much improved from the first treatment. To be eligible for the trial, you have to be able to be randomized to three of the four treatments, and people can’t choose which of the four they get.
We give them one of those treatments for 12 weeks, and at the end of 12 weeks we make the call – “Did you respond or not respond?” – and then we go back to the phenotypic data we collected at the beginning of that trial and say, “What information at baseline that we collected predicts that someone is going to respond better to duloxetine or worse to duloxetine?” And then we create the phenotype that responds best to each of those four treatments.
None of our treatments works so well that someone doesn’t end up getting randomized to a second treatment. About 85% of people so far need a second treatment because they still have enough pain that they want more relief. But the nice thing about that is we’ve already done all the functional brain imaging and all these really expensive and time-consuming things.
We’re hoping to have around 700-800 people total in this trial, which means that around 170 people will get randomized to each of the four initial treatments. No one’s ever done a study that has functional brain imaging and all these other things in it with more than 80 or 100 people. The scale of this is totally unprecedented.
Given that the individual therapies don’t appear to be all that successful on their own, what is your goal?
The primary aim is to match the phenotypic characteristics of a patient with chronic low back pain with treatment response to each of these four treatments. So at the end, we can give clinicians information on which of the patients is going to respond to physical therapy, for instance.
Right now, about one out of three people respond to most treatments for pain. We think by doing a trial like this, we can take treatments that work in one out of three people and make them work in one out of two or two out of three people just by using them in the right people.
How do you differentiate between these types of pain in your study?
We phenotype people by asking them a number of questions. We also do brain imaging, look at their back with MRI, test biomechanics, and then give them four different treatments that we know work in groups of people with low back pain.
We think one of the first parts of the phenotype is, do they have pain just in their back? Or do they have pain in their back plus a lot of other body regions? Because the more body regions that people have pain in, the more likely it is that this is an amplifier problem rather than a guitar problem.
Treatments like physical therapy, surgery, and injections are going to work better for people in whom the pain is a guitar problem rather than an amplifier problem. And drugs like duloxetine, which works in the brain, and cognitive-behavioral therapy are going to work a lot better in the people with pain in multiple sites besides the back.
To pick up on your metaphor, do any symptoms help clinicians differentiate between the guitar and the amplifier?
Sleep problems, fatigue, memory problems, and mood problems are common in patients with chronic pain and are more common with amplifier pain. Because again, those are all central nervous system problems. And so we see that the people that have anxiety, depression, and a lot of distress are more likely to have this kind of pain.
Does medical imaging help?
There’s a terrible relationship between what you see on an MRI of the back and whether someone has pain or how severe the pain is going to be. There’s always going to be individuals that have a lot of anatomic damage who don’t have any pain because they happen to be on the other end of the continuum from fibromyalgia; they’re actually pain-insensitive people.
What are your thoughts about ketamine as a possible treatment for chronic pain?
I have a mentee who’s doing a ketamine trial. We’re doing psilocybin trials in patients with fibromyalgia. Ketamine is such a dirty drug; it has so many different mechanisms of action. It does have some psychedelic effects, but it also is an NMDA blocker. It really has so many different effects.
I think it’s being thrown around like water in settings where we don’t yet know it to be efficacious. Even the data in treatment-refractory depression are pretty weak, but we’re so desperate to do something for those patients. If you’re trying to harness the psychedelic properties of ketamine, I think there’s other psychedelics that are a lot more interesting, which is why we’re using psilocybin for a subset of patients. Most of us in the pain field think that the psychedelics will work best for the people with chronic pain who have a lot of comorbid psychiatric illness, especially the ones with a lot of trauma. These drugs will allow us therapeutically to get at a lot of these patients with the side-by-side psychotherapy that’s being done as people are getting care in the medicalized setting.
Dr. Clauw reported conflicts of interest with Pfizer, Tonix, Theravance, Zynerba, Samumed, Aptinyx, Daiichi Sankyo, Intec, Regeneron, Teva, Lundbeck, Virios, and Cerephex.
A version of this article first appeared on Medscape.com.
Chronic pain, and back pain in particular, is among the most frequent concerns for patients in the primary care setting. Roughly 8% of adults in the United States say they suffer from chronic low back pain, and many of them say the pain is significant enough to impair their ability to move, work, and otherwise enjoy life. All this, despite decades of research and countless millions in funding to find the optimal approach to treating chronic pain.
As the United States crawls out of the opioid epidemic, a group of pain specialists is hoping to identify effective, personalized approaches to managing back pain. Daniel Clauw, MD, professor of anesthesiology, internal medicine, and psychiatry at the University of Michigan, Ann Arbor, is helping lead the BEST trial. With projected enrollment of nearly 800 patients, BEST will be the largest federally funded clinical trial of interventions to treat chronic low back pain.
In an interview, The interview has been edited for length and clarity.
What are your thoughts on the current state of primary care physicians’ understanding and management of pain?
Primary care physicians need a lot of help in demystifying the diagnosis and treatment of any kind of pain, but back pain is a really good place to start. When it comes to back pain, most primary care physicians are not any more knowledgeable than a layperson.
What has the opioid debacle-cum-tragedy taught you about pain management, particular as regards people with chronic pain?
I don’t feel opioids should ever be used to treat chronic low back pain. The few long-term studies that have been performed using opioids for longer than 3 months suggest that they often make pain worse rather than just failing to make pain better – and we know they are associated with a significantly increased all-cause mortality with increased deaths from myocardial infarction, accidents, and suicides, in addition to overdose.
Given how many patients experience back pain, how did we come to the point at which primary care physicians are so ill equipped?
We’ve had terrible pain curricula in medical schools. To give you an example: I’m one of the leading pain experts in the world and I’m not allowed to teach our medical students their pain curriculum. The students learn about neurophysiology and the anatomy of the nerves, not what’s relevant in pain.
This is notorious in medical school: Curricula are almost impossible to modify and change. So it starts with poor training in medical school. And then, regardless of what education they do or don’t get in medical school, a lot of their education about pain management is through our residencies – mainly in inpatient settings, where you’re really seeing the management of acute pain and not the management of chronic pain.
People get more accustomed to managing acute pain, where opioids are a reasonable option. It’s just that when you start managing subacute or chronic pain, opioids don’t work as well.
The other big problem is that historically, most people trained in medicine think that if you have pain in your elbow, there’s got to be something wrong in your elbow. This third mechanism of pain, central sensitization – or nociplastic pain – the kind of pain that we see in fibromyalgia, headache, and low back pain, where the pain is coming from the brain – that’s confusing to people. People can have pain without any damage or inflammation to that region of the body.
Physicians are trained that if there’s pain, there’s something wrong and we have to do surgery or there’s been some trauma. Most chronic pain is none of that. There’s a big disconnect between how people are trained, and then when they go out and are seeing a tremendous number of people with chronic pain.
What are the different types of pain, and how should they inform clinicians’ understanding about what approaches might work for managing their patients in pain?
The way the central nervous system responds to pain is analogous to the loudness of an electric guitar. You can make an electric guitar louder either by strumming the strings harder or by turning up the amplifier. For many people with fibromyalgia, low back pain, and endometriosis, for example, the problem is really more that the amplifier is turned up too high rather than its being that the guitar is strummed too strongly. That kind of pain where the pain is not due to anatomic damage or inflammation is particularly flummoxing for providers.
Can you explain the design of the new study?
It’s a 13-site study looking at four treatments: enhanced self-care, cognitive-behavioral therapy, physical therapy, and duloxetine. It’s a big precision medicine trial, trying to take everything we’ve learned and putting it all into one big study.
We’re using a SMART design, which randomizes people to two of those treatments, unless they are very much improved from the first treatment. To be eligible for the trial, you have to be able to be randomized to three of the four treatments, and people can’t choose which of the four they get.
We give them one of those treatments for 12 weeks, and at the end of 12 weeks we make the call – “Did you respond or not respond?” – and then we go back to the phenotypic data we collected at the beginning of that trial and say, “What information at baseline that we collected predicts that someone is going to respond better to duloxetine or worse to duloxetine?” And then we create the phenotype that responds best to each of those four treatments.
None of our treatments works so well that someone doesn’t end up getting randomized to a second treatment. About 85% of people so far need a second treatment because they still have enough pain that they want more relief. But the nice thing about that is we’ve already done all the functional brain imaging and all these really expensive and time-consuming things.
We’re hoping to have around 700-800 people total in this trial, which means that around 170 people will get randomized to each of the four initial treatments. No one’s ever done a study that has functional brain imaging and all these other things in it with more than 80 or 100 people. The scale of this is totally unprecedented.
Given that the individual therapies don’t appear to be all that successful on their own, what is your goal?
The primary aim is to match the phenotypic characteristics of a patient with chronic low back pain with treatment response to each of these four treatments. So at the end, we can give clinicians information on which of the patients is going to respond to physical therapy, for instance.
Right now, about one out of three people respond to most treatments for pain. We think by doing a trial like this, we can take treatments that work in one out of three people and make them work in one out of two or two out of three people just by using them in the right people.
How do you differentiate between these types of pain in your study?
We phenotype people by asking them a number of questions. We also do brain imaging, look at their back with MRI, test biomechanics, and then give them four different treatments that we know work in groups of people with low back pain.
We think one of the first parts of the phenotype is, do they have pain just in their back? Or do they have pain in their back plus a lot of other body regions? Because the more body regions that people have pain in, the more likely it is that this is an amplifier problem rather than a guitar problem.
Treatments like physical therapy, surgery, and injections are going to work better for people in whom the pain is a guitar problem rather than an amplifier problem. And drugs like duloxetine, which works in the brain, and cognitive-behavioral therapy are going to work a lot better in the people with pain in multiple sites besides the back.
To pick up on your metaphor, do any symptoms help clinicians differentiate between the guitar and the amplifier?
Sleep problems, fatigue, memory problems, and mood problems are common in patients with chronic pain and are more common with amplifier pain. Because again, those are all central nervous system problems. And so we see that the people that have anxiety, depression, and a lot of distress are more likely to have this kind of pain.
Does medical imaging help?
There’s a terrible relationship between what you see on an MRI of the back and whether someone has pain or how severe the pain is going to be. There’s always going to be individuals that have a lot of anatomic damage who don’t have any pain because they happen to be on the other end of the continuum from fibromyalgia; they’re actually pain-insensitive people.
What are your thoughts about ketamine as a possible treatment for chronic pain?
I have a mentee who’s doing a ketamine trial. We’re doing psilocybin trials in patients with fibromyalgia. Ketamine is such a dirty drug; it has so many different mechanisms of action. It does have some psychedelic effects, but it also is an NMDA blocker. It really has so many different effects.
I think it’s being thrown around like water in settings where we don’t yet know it to be efficacious. Even the data in treatment-refractory depression are pretty weak, but we’re so desperate to do something for those patients. If you’re trying to harness the psychedelic properties of ketamine, I think there’s other psychedelics that are a lot more interesting, which is why we’re using psilocybin for a subset of patients. Most of us in the pain field think that the psychedelics will work best for the people with chronic pain who have a lot of comorbid psychiatric illness, especially the ones with a lot of trauma. These drugs will allow us therapeutically to get at a lot of these patients with the side-by-side psychotherapy that’s being done as people are getting care in the medicalized setting.
Dr. Clauw reported conflicts of interest with Pfizer, Tonix, Theravance, Zynerba, Samumed, Aptinyx, Daiichi Sankyo, Intec, Regeneron, Teva, Lundbeck, Virios, and Cerephex.
A version of this article first appeared on Medscape.com.
Oxycodone tied to persistent use only after vaginal delivery
“In the last decade in Ontario, oxycodone surpassed codeine as the most commonly prescribed opioid postpartum for pain control,” Jonathan Zipursky, MD, PhD, of Sunnybrook Health Sciences Centre, ICES, Toronto, and the University of Toronto, said in an interview. “This likely had to do with concerns with codeine use during breastfeeding, many of which are unsubstantiated.
“We hypothesized that use of oxycodone would be associated with an increased risk of persistent postpartum opioid use,” he said. “However, we did not find this.”
Instead, other factors, such as the quantity of opioids initially prescribed, were probably more important risks, he said.
The team also was “a bit surprised” that oxycodone was associated with an increased risk of persistent use only among those who had a vaginal delivery, Dr. Zipursky added.
“Receipt of an opioid prescription after vaginal delivery is uncommon in Ontario. People who fill prescriptions for potent opioids, such as oxycodone, after vaginal delivery may have underlying characteristics that predispose them to chronic opioid use,” he suggested. “Some of these factors we were unable to assess using our data.”
The study was published online in the Canadian Medical Association Journal.
Oxycodone okay
The investigators analyzed data from 70,607 people (median age, 32) who filled an opioid prescription within 7 days of discharge from the hospital between 2012 and 2020. Two-thirds (69.8%) received oxycodone and one-third received (30.2%) codeine.
The median gestational age at delivery was 39 weeks, and 80% of participants had a cesarean delivery. The median opioid prescription duration was 3 days. The median opioid content per prescription was 150 morphine milligram equivalents (MMEs) among those prescribed oxycodone and 135 MMEs for codeine.
The main outcome was persistent opioid use. This was defined as one or more additional prescriptions for an opioid within 90 days of the first postpartum prescription and one or more additional prescriptions in the 91-365 days after.
Oxycodone receipt was not associated with persistent opioid use, compared with codeine (relative risk, 1.04).
However, in a secondary analysis by mode of delivery, an association was seen between a prescription for oxycodone and persistent use after vaginal (RR, 1.63), but not after cesarean (RR, 0.85), delivery.
Dr. Zipursky noted that the quantity of opioids prescribed in the initial postpartum prescription “is likely a more important modifiable risk factor for new persistent opioid use, rather than the type of opioid prescribed.”
For example, a prescription containing more than 225 MMEs (equivalent to about 30 tablets of 5 mg oxycodone and to 50 tablets of 30 mg codeine) was associated with a roughly twofold increased risk of persistent use, compared with less than 112.5 MMEs after both vaginal (odds ratio, 2.51) and cesarean (OR, 1.78) delivery.
Furthermore, a prescription duration of more than 7 days was also associated with a roughly twofold increased risk of persistent use, compared with a duration of 1-3 days after both vaginal (OR, 2.43) and cesarean (OR, 1.52) delivery.
Most risk factors for persistent opioid use – a history of mental illness, substance use disorder, and more maternal comorbidities (aggregated diagnosis groups > 10) – were consistent across modes of delivery.
“Awareness of modifiable factors associated with new, persistent opioid use may help clinicians tailor opioid prescribing while ensuring adequate analgesia after delivery,” Dr. Zipursky suggested.
Less is more
In a comment, Elaine Duryea, MD, assistant professor in the department of obstetrics and gynecology at UT Southwestern Medical Center and medical director of the Maternal-Fetal Medicine Clinic at Parkland Health and Hospital System, both in Dallas, said, “It is likely exposure to any opioid, rather than a specific opioid, that can promote continued use – that is, past the medically indicated period.”
Dr. Duryea was principal investigator of a study, published in the American Journal of Obstetrics and Gynecology, that showed a multimodal regimen that included scheduled nonsteroidal anti-inflammatory drugs and acetaminophen, with opioids used as needed, resulted in a decrease in opioid use while adequately controlling pain after cesarean delivery.
“It is important to understand how to appropriately tailor the amount of opioid given to patients at the time of hospital discharge after cesarean in order to treat pain effectively but not send patients home with more opioids than [are] really needed,” she said.
It is also important to “individualize prescribing practices and maximize the use of non-opioid medication to treat postpartum and postoperative pain. Opioids should be a last resort for breakthrough pain, not first-line therapy,” Dr. Duryea concluded.
The study was funded by a Canadian Institutes of Health Research project grant. Dr. Zipursky has received payments from private law firms for medicolegal opinions on the safety and effectiveness of analgesics, including opioids.
A version of this article first appeared on Medscape.com.
“In the last decade in Ontario, oxycodone surpassed codeine as the most commonly prescribed opioid postpartum for pain control,” Jonathan Zipursky, MD, PhD, of Sunnybrook Health Sciences Centre, ICES, Toronto, and the University of Toronto, said in an interview. “This likely had to do with concerns with codeine use during breastfeeding, many of which are unsubstantiated.
“We hypothesized that use of oxycodone would be associated with an increased risk of persistent postpartum opioid use,” he said. “However, we did not find this.”
Instead, other factors, such as the quantity of opioids initially prescribed, were probably more important risks, he said.
The team also was “a bit surprised” that oxycodone was associated with an increased risk of persistent use only among those who had a vaginal delivery, Dr. Zipursky added.
“Receipt of an opioid prescription after vaginal delivery is uncommon in Ontario. People who fill prescriptions for potent opioids, such as oxycodone, after vaginal delivery may have underlying characteristics that predispose them to chronic opioid use,” he suggested. “Some of these factors we were unable to assess using our data.”
The study was published online in the Canadian Medical Association Journal.
Oxycodone okay
The investigators analyzed data from 70,607 people (median age, 32) who filled an opioid prescription within 7 days of discharge from the hospital between 2012 and 2020. Two-thirds (69.8%) received oxycodone and one-third received (30.2%) codeine.
The median gestational age at delivery was 39 weeks, and 80% of participants had a cesarean delivery. The median opioid prescription duration was 3 days. The median opioid content per prescription was 150 morphine milligram equivalents (MMEs) among those prescribed oxycodone and 135 MMEs for codeine.
The main outcome was persistent opioid use. This was defined as one or more additional prescriptions for an opioid within 90 days of the first postpartum prescription and one or more additional prescriptions in the 91-365 days after.
Oxycodone receipt was not associated with persistent opioid use, compared with codeine (relative risk, 1.04).
However, in a secondary analysis by mode of delivery, an association was seen between a prescription for oxycodone and persistent use after vaginal (RR, 1.63), but not after cesarean (RR, 0.85), delivery.
Dr. Zipursky noted that the quantity of opioids prescribed in the initial postpartum prescription “is likely a more important modifiable risk factor for new persistent opioid use, rather than the type of opioid prescribed.”
For example, a prescription containing more than 225 MMEs (equivalent to about 30 tablets of 5 mg oxycodone and to 50 tablets of 30 mg codeine) was associated with a roughly twofold increased risk of persistent use, compared with less than 112.5 MMEs after both vaginal (odds ratio, 2.51) and cesarean (OR, 1.78) delivery.
Furthermore, a prescription duration of more than 7 days was also associated with a roughly twofold increased risk of persistent use, compared with a duration of 1-3 days after both vaginal (OR, 2.43) and cesarean (OR, 1.52) delivery.
Most risk factors for persistent opioid use – a history of mental illness, substance use disorder, and more maternal comorbidities (aggregated diagnosis groups > 10) – were consistent across modes of delivery.
“Awareness of modifiable factors associated with new, persistent opioid use may help clinicians tailor opioid prescribing while ensuring adequate analgesia after delivery,” Dr. Zipursky suggested.
Less is more
In a comment, Elaine Duryea, MD, assistant professor in the department of obstetrics and gynecology at UT Southwestern Medical Center and medical director of the Maternal-Fetal Medicine Clinic at Parkland Health and Hospital System, both in Dallas, said, “It is likely exposure to any opioid, rather than a specific opioid, that can promote continued use – that is, past the medically indicated period.”
Dr. Duryea was principal investigator of a study, published in the American Journal of Obstetrics and Gynecology, that showed a multimodal regimen that included scheduled nonsteroidal anti-inflammatory drugs and acetaminophen, with opioids used as needed, resulted in a decrease in opioid use while adequately controlling pain after cesarean delivery.
“It is important to understand how to appropriately tailor the amount of opioid given to patients at the time of hospital discharge after cesarean in order to treat pain effectively but not send patients home with more opioids than [are] really needed,” she said.
It is also important to “individualize prescribing practices and maximize the use of non-opioid medication to treat postpartum and postoperative pain. Opioids should be a last resort for breakthrough pain, not first-line therapy,” Dr. Duryea concluded.
The study was funded by a Canadian Institutes of Health Research project grant. Dr. Zipursky has received payments from private law firms for medicolegal opinions on the safety and effectiveness of analgesics, including opioids.
A version of this article first appeared on Medscape.com.
“In the last decade in Ontario, oxycodone surpassed codeine as the most commonly prescribed opioid postpartum for pain control,” Jonathan Zipursky, MD, PhD, of Sunnybrook Health Sciences Centre, ICES, Toronto, and the University of Toronto, said in an interview. “This likely had to do with concerns with codeine use during breastfeeding, many of which are unsubstantiated.
“We hypothesized that use of oxycodone would be associated with an increased risk of persistent postpartum opioid use,” he said. “However, we did not find this.”
Instead, other factors, such as the quantity of opioids initially prescribed, were probably more important risks, he said.
The team also was “a bit surprised” that oxycodone was associated with an increased risk of persistent use only among those who had a vaginal delivery, Dr. Zipursky added.
“Receipt of an opioid prescription after vaginal delivery is uncommon in Ontario. People who fill prescriptions for potent opioids, such as oxycodone, after vaginal delivery may have underlying characteristics that predispose them to chronic opioid use,” he suggested. “Some of these factors we were unable to assess using our data.”
The study was published online in the Canadian Medical Association Journal.
Oxycodone okay
The investigators analyzed data from 70,607 people (median age, 32) who filled an opioid prescription within 7 days of discharge from the hospital between 2012 and 2020. Two-thirds (69.8%) received oxycodone and one-third received (30.2%) codeine.
The median gestational age at delivery was 39 weeks, and 80% of participants had a cesarean delivery. The median opioid prescription duration was 3 days. The median opioid content per prescription was 150 morphine milligram equivalents (MMEs) among those prescribed oxycodone and 135 MMEs for codeine.
The main outcome was persistent opioid use. This was defined as one or more additional prescriptions for an opioid within 90 days of the first postpartum prescription and one or more additional prescriptions in the 91-365 days after.
Oxycodone receipt was not associated with persistent opioid use, compared with codeine (relative risk, 1.04).
However, in a secondary analysis by mode of delivery, an association was seen between a prescription for oxycodone and persistent use after vaginal (RR, 1.63), but not after cesarean (RR, 0.85), delivery.
Dr. Zipursky noted that the quantity of opioids prescribed in the initial postpartum prescription “is likely a more important modifiable risk factor for new persistent opioid use, rather than the type of opioid prescribed.”
For example, a prescription containing more than 225 MMEs (equivalent to about 30 tablets of 5 mg oxycodone and to 50 tablets of 30 mg codeine) was associated with a roughly twofold increased risk of persistent use, compared with less than 112.5 MMEs after both vaginal (odds ratio, 2.51) and cesarean (OR, 1.78) delivery.
Furthermore, a prescription duration of more than 7 days was also associated with a roughly twofold increased risk of persistent use, compared with a duration of 1-3 days after both vaginal (OR, 2.43) and cesarean (OR, 1.52) delivery.
Most risk factors for persistent opioid use – a history of mental illness, substance use disorder, and more maternal comorbidities (aggregated diagnosis groups > 10) – were consistent across modes of delivery.
“Awareness of modifiable factors associated with new, persistent opioid use may help clinicians tailor opioid prescribing while ensuring adequate analgesia after delivery,” Dr. Zipursky suggested.
Less is more
In a comment, Elaine Duryea, MD, assistant professor in the department of obstetrics and gynecology at UT Southwestern Medical Center and medical director of the Maternal-Fetal Medicine Clinic at Parkland Health and Hospital System, both in Dallas, said, “It is likely exposure to any opioid, rather than a specific opioid, that can promote continued use – that is, past the medically indicated period.”
Dr. Duryea was principal investigator of a study, published in the American Journal of Obstetrics and Gynecology, that showed a multimodal regimen that included scheduled nonsteroidal anti-inflammatory drugs and acetaminophen, with opioids used as needed, resulted in a decrease in opioid use while adequately controlling pain after cesarean delivery.
“It is important to understand how to appropriately tailor the amount of opioid given to patients at the time of hospital discharge after cesarean in order to treat pain effectively but not send patients home with more opioids than [are] really needed,” she said.
It is also important to “individualize prescribing practices and maximize the use of non-opioid medication to treat postpartum and postoperative pain. Opioids should be a last resort for breakthrough pain, not first-line therapy,” Dr. Duryea concluded.
The study was funded by a Canadian Institutes of Health Research project grant. Dr. Zipursky has received payments from private law firms for medicolegal opinions on the safety and effectiveness of analgesics, including opioids.
A version of this article first appeared on Medscape.com.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Could your practice be more profitable if you outsource?
Outsourcing certain staff functions in a practice to outside contractors working in remote locations has become commonplace in many medical practices.
Health care outsourcing services, also known as virtual assistants (VAs), were already booming in 2017, when volume grew by 36%. Then, the COVID-19 pandemic in 2020 normalized off-site work, which was a boon to outsourcing providers.
The most popular services being outsourced today by medical practices include billing, scribes, telephone calls to patients, and processing prior authorizations.
“Outsourcing is not for everyone, but I’ve seen it work for many practices,” said Lara Hochman, MD, a practice management consultant in Austin, Tex. She said that practices have used outsourcing to solve problems like high staff turnover, tight budgets, and inefficient use of staff.
When in-house staffing is insufficient or not appropriately aligned with the task, outsourcing can produce big savings, said Teri Deabler, a practice management consultant with the Texas Medical Association.
For example, she said that a client was paying an in-house accountant $80,000 a year. When the accountant retired, she was replaced with a part-time bookkeeper earning $20,000 while her accounting work was outsourced at a cost of $20,000 a year. “The practice’s costs for this service were cut in half,” Ms. Deabler said.
What functions lend themselves to outsourcing?
Clinical services are rarely outsourced by individual practices – although hospitals now outsource numerous clinical services – but virtually any kind of administrative service can be contracted out. Outsourcing used to be limited mainly to billing and off-hours phone services, but today, more services are available, such as scribing, processing prior authorizations, accounting and bookkeeping, human resources (HR) and payroll, interactions with social media, recredentialing, medical transcription, and marketing.
Meanwhile, the original outsourced services have evolved. Billing and collections may now be handled by off-shore VAs, and phone services now deal with a wider variety of tasks, such as answering patients’ questions, scheduling appointments, and making referrals.
Ron Holder, chief operating officer of Medical Group Management Association in Englewood, Colo., said that some outsourcing services can also adjust the amount of work provided based on the customer’s needs. “For instance, an IT outsourcer may allow you to scale up IT support for a new big tech project, such as installing a new electronic health record,” he said.
The outsourced service provider, who might work in another state or another country, is connected to the practice by phone and electronically, and represents the practice when dealing with patients, insurers, or other vendors.
“No one, including patients and your physicians, should know that they are dealing with an outsourced company,” said Mr. Holder. “The work, look, and feel of the outsourced functions should be seamless. Employees at the outsourcer should always identify themselves as the practice, not the outsourcing service.”
Dr. Hochman said that many outsourcing companies dedicate a particular worker to a particular practice and train them to work there. One example of this approach is Provider’s Choice Scribe Services, based in San Antonio. On its website, the company notes that each scribe is paired with a doctor and learns his or her documentation preferences, EMR use, and charting requirements.
What medical practices benefit most from outsourcing?
All kinds and sizes of practices contract with outsourcing firms, but the arrangement is particularly useful for smaller practices, Mr. Holder said. “Larger practices have the economies of scale that allow services to be in-house,” he said, “but smaller practices don’t have that opportunity.”
Dr. Hochman added that outsourcing firms can be hired part-time when the practice doesn’t have enough work for a full-time position. Alternatively, a full-time outsourcing firm can perform two or more separate tasks, such as scribing while handling prior authorizations, she said.
Outsourcing is also useful for new practices, Ms. Deabler said. “A new practice is not earning much money, so it has to have a bare-bones staff,” she said. “Billing, for example, should be contracted out, but it won’t cost that much, because the outsourcer typically charges by volume, and the volume in a new practice is low.”
Meanwhile, Mr. Holder said that the outsourcing of prior authorization work can particularly benefit specialty practices because they typically have a lot of prior authorizations to deal with.
The pros and cons of outsourcing
Experts with experience in outsourcing agree there are both pluses and minuses. “Practices with outsourced workers have less overhead, don’t have to deal with staff turnover, and costs may be lower than for in-house staff,” Ms. Deabler said. “However, you have limited control over outsourced workers and the practice may seem more anonymous to patients, so you need to consider this option very carefully.”
“With outsourcing, you lose control,” said John Machata, MD, a recently retired solo family physician in Wickford, R.I. “You’re trusting someone else to do work that you could do anyway.”
When he briefly considered outsourcing the practice’s billing many years ago, he found that billing companies wouldn’t handle bills that took a lot of work, such as getting in touch with the insurance company and explaining the patient’s situation. “They would only handle the easy bills, which the practice could do anyway,” he said.
However, he does think that answering services may be useful to outsource. “Patients are more inclined to call an anonymous entity than the doctor,” he said. When he gave patients his cell phone number, he said that some patients held off from calling because they didn’t want to bother him.
“Outsourced staff should be less expensive than in-house staff,” said Daniel Shay, an attorney at Gosfield & Associates in Philadelphia. “On the other hand, you are liable for the outsourcer’s mistakes. If your outsourced billing company is upcoding claims, your practice would be on the hook for repayment and penalties.”
Mr. Holder said: “An outsourcer ought to be more efficient at its chosen task because that is what they know how to do. This is a plus at a small practice, where the practice manager may need to do the billing, HR, IT, marketing, some legal work, and accounting,” he said. “No one person can do all of those things well.”
He added, however, “If you choose outsourcing and then decide you don’t like it, it’s difficult to unwind the arrangement. Staff that have been dismissed can’t easily be hired back, so it shouldn’t be an easy decision to make.”
Also, sometimes the staff at offshore outsourcing firms may have accents that are harder for patients to understand, and the offshore staff may not readily understand a U.S. caller. However, Dr. Hochman said that practices often have a chance to interview and select specific persons on the offshore team who best fit their needs.
Offshore outsourcing
Outsourcing firms have been moving abroad, where costs are lower. Typical venues are India and the Philippines because there are larger percentages of people who speak English. Since 2020, demand at offshore medical billing companies has been growing faster than their domestic counterparts, according to a recent analysis.
The difference in price can be substantial. In 2020, the average salary for scribes in India was $500 a month, compared with $2,500 for scribes in the United States.
However, offshore outsourcing is starting to face limitations in some places because of privacy issues, according to David J. Zetter, a practice management consultant in Mechanicsburg, Pa. He pointed to a new Florida law that limits use of offshore vendors because they deal with confidential patient information. The law, which became effective July 1, requires that any protected health information must be maintained in the United States or Canada.
“This will make it very hard for many types of offshore vendors to operate in Florida,” he said. He noted that Florida is the only state with such a restriction, but similar proposals are under consideration in a few other states, such as Texas.
How to select the right company
Mr. Zetter said that the biggest mistake practices make when choosing a company is failing to take enough time to examine their choice. “Quite often, practices don’t validate that companies know what they are doing,” he said. “They get a recommendation and go with it.”
“Choose a company with experience in your specialty,” Mr. Zetter advised. “Speak with the company’s clients, not just the ones the company gives you to speak to. You should ask for the full list of clients and speak to all of them.”
Ms. Deabler said that it’s fairly easy to find respected outsourcing companies. “Colleagues can make recommendations, state and specialty societies can provide lists of preferred vendors, and you can visit vendors’ booths at medical conferences,” she said. She added that it’s also easy to find evaluations of each company. “You can Google the company and come up with all kinds of information about it,” she said.
Mr. Shay said that practices should make sure they understand the terms of the contract with a VA. “Depending on how the contract is worded, you may be stuck with the relationship for many years,” he said. “Before you sign an outsourcing contract, you need to make sure it has a reasonable termination provision.”
Because vetting companies properly can require extensive work, Ms. Deabler said, the work can be given to an experienced practice management consultant. “The consultant can start with a cost-benefit analysis that will show you whether outsourcing would be worthwhile,” she said.
Working with outsource service providers
Mr. Holder said that doctors should keep track of what the outsourcer is doing rather than simply let them do their work. “For example, doctors should understand the billing codes they use most often, such as the five levels of evaluation and management codes, and not just blindly rely on the billing company to code and bill their work correctly,” he noted.
Ms. Deabler said that companies provide monthly reports on their work. “Doctors should be reading these reports and contacting the company if expectations aren’t met,” she said.
Even in the reports, companies can hide problems from untrained eyes, Mr. Holder said. “For example, anyone can meet a metric like days in accounts receivable simply by writing off any charge that isn’t paid after 90 days.”
“You need to be engaged with the outsourcer,” he said. “It’s also a good idea to bring in a consultant to periodically check an outsourcer’s work.”
Will outsourcing expand in the future?
Mr. Holder said that the increasing use of value-based care may require practices to rely more on outsourcing in the future. “For instance, if a practice has a value-based contract that requires providing behavioral health services to patients, it might make sense to outsource that work rather than hire psychologists in-house,” he said.
Practices rarely outsource clinical services, but Mr. Holder said that this may happen in the future: “Now that Medicare is paying less for telehealth, practices have to find a way to provide it without using expensive examining room space,” he said. “Some practices may decide to outsource telehealth instead.”
Mr. Shay said that there are many reasons why outsourcing has a strong future. “It allows you to concentrate on your clinical care, and it is a solution to problems with turnover of in-house staff,” he said. “It can also be more efficient because the service is presumably an expert in areas like billing and collections, which means it may be able to ensure more efficient and faster reimbursements. And if the work is outsourced overseas, you can save money through lower worker salaries.”
A version of this article first appeared on Medscape.com.
Outsourcing certain staff functions in a practice to outside contractors working in remote locations has become commonplace in many medical practices.
Health care outsourcing services, also known as virtual assistants (VAs), were already booming in 2017, when volume grew by 36%. Then, the COVID-19 pandemic in 2020 normalized off-site work, which was a boon to outsourcing providers.
The most popular services being outsourced today by medical practices include billing, scribes, telephone calls to patients, and processing prior authorizations.
“Outsourcing is not for everyone, but I’ve seen it work for many practices,” said Lara Hochman, MD, a practice management consultant in Austin, Tex. She said that practices have used outsourcing to solve problems like high staff turnover, tight budgets, and inefficient use of staff.
When in-house staffing is insufficient or not appropriately aligned with the task, outsourcing can produce big savings, said Teri Deabler, a practice management consultant with the Texas Medical Association.
For example, she said that a client was paying an in-house accountant $80,000 a year. When the accountant retired, she was replaced with a part-time bookkeeper earning $20,000 while her accounting work was outsourced at a cost of $20,000 a year. “The practice’s costs for this service were cut in half,” Ms. Deabler said.
What functions lend themselves to outsourcing?
Clinical services are rarely outsourced by individual practices – although hospitals now outsource numerous clinical services – but virtually any kind of administrative service can be contracted out. Outsourcing used to be limited mainly to billing and off-hours phone services, but today, more services are available, such as scribing, processing prior authorizations, accounting and bookkeeping, human resources (HR) and payroll, interactions with social media, recredentialing, medical transcription, and marketing.
Meanwhile, the original outsourced services have evolved. Billing and collections may now be handled by off-shore VAs, and phone services now deal with a wider variety of tasks, such as answering patients’ questions, scheduling appointments, and making referrals.
Ron Holder, chief operating officer of Medical Group Management Association in Englewood, Colo., said that some outsourcing services can also adjust the amount of work provided based on the customer’s needs. “For instance, an IT outsourcer may allow you to scale up IT support for a new big tech project, such as installing a new electronic health record,” he said.
The outsourced service provider, who might work in another state or another country, is connected to the practice by phone and electronically, and represents the practice when dealing with patients, insurers, or other vendors.
“No one, including patients and your physicians, should know that they are dealing with an outsourced company,” said Mr. Holder. “The work, look, and feel of the outsourced functions should be seamless. Employees at the outsourcer should always identify themselves as the practice, not the outsourcing service.”
Dr. Hochman said that many outsourcing companies dedicate a particular worker to a particular practice and train them to work there. One example of this approach is Provider’s Choice Scribe Services, based in San Antonio. On its website, the company notes that each scribe is paired with a doctor and learns his or her documentation preferences, EMR use, and charting requirements.
What medical practices benefit most from outsourcing?
All kinds and sizes of practices contract with outsourcing firms, but the arrangement is particularly useful for smaller practices, Mr. Holder said. “Larger practices have the economies of scale that allow services to be in-house,” he said, “but smaller practices don’t have that opportunity.”
Dr. Hochman added that outsourcing firms can be hired part-time when the practice doesn’t have enough work for a full-time position. Alternatively, a full-time outsourcing firm can perform two or more separate tasks, such as scribing while handling prior authorizations, she said.
Outsourcing is also useful for new practices, Ms. Deabler said. “A new practice is not earning much money, so it has to have a bare-bones staff,” she said. “Billing, for example, should be contracted out, but it won’t cost that much, because the outsourcer typically charges by volume, and the volume in a new practice is low.”
Meanwhile, Mr. Holder said that the outsourcing of prior authorization work can particularly benefit specialty practices because they typically have a lot of prior authorizations to deal with.
The pros and cons of outsourcing
Experts with experience in outsourcing agree there are both pluses and minuses. “Practices with outsourced workers have less overhead, don’t have to deal with staff turnover, and costs may be lower than for in-house staff,” Ms. Deabler said. “However, you have limited control over outsourced workers and the practice may seem more anonymous to patients, so you need to consider this option very carefully.”
“With outsourcing, you lose control,” said John Machata, MD, a recently retired solo family physician in Wickford, R.I. “You’re trusting someone else to do work that you could do anyway.”
When he briefly considered outsourcing the practice’s billing many years ago, he found that billing companies wouldn’t handle bills that took a lot of work, such as getting in touch with the insurance company and explaining the patient’s situation. “They would only handle the easy bills, which the practice could do anyway,” he said.
However, he does think that answering services may be useful to outsource. “Patients are more inclined to call an anonymous entity than the doctor,” he said. When he gave patients his cell phone number, he said that some patients held off from calling because they didn’t want to bother him.
“Outsourced staff should be less expensive than in-house staff,” said Daniel Shay, an attorney at Gosfield & Associates in Philadelphia. “On the other hand, you are liable for the outsourcer’s mistakes. If your outsourced billing company is upcoding claims, your practice would be on the hook for repayment and penalties.”
Mr. Holder said: “An outsourcer ought to be more efficient at its chosen task because that is what they know how to do. This is a plus at a small practice, where the practice manager may need to do the billing, HR, IT, marketing, some legal work, and accounting,” he said. “No one person can do all of those things well.”
He added, however, “If you choose outsourcing and then decide you don’t like it, it’s difficult to unwind the arrangement. Staff that have been dismissed can’t easily be hired back, so it shouldn’t be an easy decision to make.”
Also, sometimes the staff at offshore outsourcing firms may have accents that are harder for patients to understand, and the offshore staff may not readily understand a U.S. caller. However, Dr. Hochman said that practices often have a chance to interview and select specific persons on the offshore team who best fit their needs.
Offshore outsourcing
Outsourcing firms have been moving abroad, where costs are lower. Typical venues are India and the Philippines because there are larger percentages of people who speak English. Since 2020, demand at offshore medical billing companies has been growing faster than their domestic counterparts, according to a recent analysis.
The difference in price can be substantial. In 2020, the average salary for scribes in India was $500 a month, compared with $2,500 for scribes in the United States.
However, offshore outsourcing is starting to face limitations in some places because of privacy issues, according to David J. Zetter, a practice management consultant in Mechanicsburg, Pa. He pointed to a new Florida law that limits use of offshore vendors because they deal with confidential patient information. The law, which became effective July 1, requires that any protected health information must be maintained in the United States or Canada.
“This will make it very hard for many types of offshore vendors to operate in Florida,” he said. He noted that Florida is the only state with such a restriction, but similar proposals are under consideration in a few other states, such as Texas.
How to select the right company
Mr. Zetter said that the biggest mistake practices make when choosing a company is failing to take enough time to examine their choice. “Quite often, practices don’t validate that companies know what they are doing,” he said. “They get a recommendation and go with it.”
“Choose a company with experience in your specialty,” Mr. Zetter advised. “Speak with the company’s clients, not just the ones the company gives you to speak to. You should ask for the full list of clients and speak to all of them.”
Ms. Deabler said that it’s fairly easy to find respected outsourcing companies. “Colleagues can make recommendations, state and specialty societies can provide lists of preferred vendors, and you can visit vendors’ booths at medical conferences,” she said. She added that it’s also easy to find evaluations of each company. “You can Google the company and come up with all kinds of information about it,” she said.
Mr. Shay said that practices should make sure they understand the terms of the contract with a VA. “Depending on how the contract is worded, you may be stuck with the relationship for many years,” he said. “Before you sign an outsourcing contract, you need to make sure it has a reasonable termination provision.”
Because vetting companies properly can require extensive work, Ms. Deabler said, the work can be given to an experienced practice management consultant. “The consultant can start with a cost-benefit analysis that will show you whether outsourcing would be worthwhile,” she said.
Working with outsource service providers
Mr. Holder said that doctors should keep track of what the outsourcer is doing rather than simply let them do their work. “For example, doctors should understand the billing codes they use most often, such as the five levels of evaluation and management codes, and not just blindly rely on the billing company to code and bill their work correctly,” he noted.
Ms. Deabler said that companies provide monthly reports on their work. “Doctors should be reading these reports and contacting the company if expectations aren’t met,” she said.
Even in the reports, companies can hide problems from untrained eyes, Mr. Holder said. “For example, anyone can meet a metric like days in accounts receivable simply by writing off any charge that isn’t paid after 90 days.”
“You need to be engaged with the outsourcer,” he said. “It’s also a good idea to bring in a consultant to periodically check an outsourcer’s work.”
Will outsourcing expand in the future?
Mr. Holder said that the increasing use of value-based care may require practices to rely more on outsourcing in the future. “For instance, if a practice has a value-based contract that requires providing behavioral health services to patients, it might make sense to outsource that work rather than hire psychologists in-house,” he said.
Practices rarely outsource clinical services, but Mr. Holder said that this may happen in the future: “Now that Medicare is paying less for telehealth, practices have to find a way to provide it without using expensive examining room space,” he said. “Some practices may decide to outsource telehealth instead.”
Mr. Shay said that there are many reasons why outsourcing has a strong future. “It allows you to concentrate on your clinical care, and it is a solution to problems with turnover of in-house staff,” he said. “It can also be more efficient because the service is presumably an expert in areas like billing and collections, which means it may be able to ensure more efficient and faster reimbursements. And if the work is outsourced overseas, you can save money through lower worker salaries.”
A version of this article first appeared on Medscape.com.
Outsourcing certain staff functions in a practice to outside contractors working in remote locations has become commonplace in many medical practices.
Health care outsourcing services, also known as virtual assistants (VAs), were already booming in 2017, when volume grew by 36%. Then, the COVID-19 pandemic in 2020 normalized off-site work, which was a boon to outsourcing providers.
The most popular services being outsourced today by medical practices include billing, scribes, telephone calls to patients, and processing prior authorizations.
“Outsourcing is not for everyone, but I’ve seen it work for many practices,” said Lara Hochman, MD, a practice management consultant in Austin, Tex. She said that practices have used outsourcing to solve problems like high staff turnover, tight budgets, and inefficient use of staff.
When in-house staffing is insufficient or not appropriately aligned with the task, outsourcing can produce big savings, said Teri Deabler, a practice management consultant with the Texas Medical Association.
For example, she said that a client was paying an in-house accountant $80,000 a year. When the accountant retired, she was replaced with a part-time bookkeeper earning $20,000 while her accounting work was outsourced at a cost of $20,000 a year. “The practice’s costs for this service were cut in half,” Ms. Deabler said.
What functions lend themselves to outsourcing?
Clinical services are rarely outsourced by individual practices – although hospitals now outsource numerous clinical services – but virtually any kind of administrative service can be contracted out. Outsourcing used to be limited mainly to billing and off-hours phone services, but today, more services are available, such as scribing, processing prior authorizations, accounting and bookkeeping, human resources (HR) and payroll, interactions with social media, recredentialing, medical transcription, and marketing.
Meanwhile, the original outsourced services have evolved. Billing and collections may now be handled by off-shore VAs, and phone services now deal with a wider variety of tasks, such as answering patients’ questions, scheduling appointments, and making referrals.
Ron Holder, chief operating officer of Medical Group Management Association in Englewood, Colo., said that some outsourcing services can also adjust the amount of work provided based on the customer’s needs. “For instance, an IT outsourcer may allow you to scale up IT support for a new big tech project, such as installing a new electronic health record,” he said.
The outsourced service provider, who might work in another state or another country, is connected to the practice by phone and electronically, and represents the practice when dealing with patients, insurers, or other vendors.
“No one, including patients and your physicians, should know that they are dealing with an outsourced company,” said Mr. Holder. “The work, look, and feel of the outsourced functions should be seamless. Employees at the outsourcer should always identify themselves as the practice, not the outsourcing service.”
Dr. Hochman said that many outsourcing companies dedicate a particular worker to a particular practice and train them to work there. One example of this approach is Provider’s Choice Scribe Services, based in San Antonio. On its website, the company notes that each scribe is paired with a doctor and learns his or her documentation preferences, EMR use, and charting requirements.
What medical practices benefit most from outsourcing?
All kinds and sizes of practices contract with outsourcing firms, but the arrangement is particularly useful for smaller practices, Mr. Holder said. “Larger practices have the economies of scale that allow services to be in-house,” he said, “but smaller practices don’t have that opportunity.”
Dr. Hochman added that outsourcing firms can be hired part-time when the practice doesn’t have enough work for a full-time position. Alternatively, a full-time outsourcing firm can perform two or more separate tasks, such as scribing while handling prior authorizations, she said.
Outsourcing is also useful for new practices, Ms. Deabler said. “A new practice is not earning much money, so it has to have a bare-bones staff,” she said. “Billing, for example, should be contracted out, but it won’t cost that much, because the outsourcer typically charges by volume, and the volume in a new practice is low.”
Meanwhile, Mr. Holder said that the outsourcing of prior authorization work can particularly benefit specialty practices because they typically have a lot of prior authorizations to deal with.
The pros and cons of outsourcing
Experts with experience in outsourcing agree there are both pluses and minuses. “Practices with outsourced workers have less overhead, don’t have to deal with staff turnover, and costs may be lower than for in-house staff,” Ms. Deabler said. “However, you have limited control over outsourced workers and the practice may seem more anonymous to patients, so you need to consider this option very carefully.”
“With outsourcing, you lose control,” said John Machata, MD, a recently retired solo family physician in Wickford, R.I. “You’re trusting someone else to do work that you could do anyway.”
When he briefly considered outsourcing the practice’s billing many years ago, he found that billing companies wouldn’t handle bills that took a lot of work, such as getting in touch with the insurance company and explaining the patient’s situation. “They would only handle the easy bills, which the practice could do anyway,” he said.
However, he does think that answering services may be useful to outsource. “Patients are more inclined to call an anonymous entity than the doctor,” he said. When he gave patients his cell phone number, he said that some patients held off from calling because they didn’t want to bother him.
“Outsourced staff should be less expensive than in-house staff,” said Daniel Shay, an attorney at Gosfield & Associates in Philadelphia. “On the other hand, you are liable for the outsourcer’s mistakes. If your outsourced billing company is upcoding claims, your practice would be on the hook for repayment and penalties.”
Mr. Holder said: “An outsourcer ought to be more efficient at its chosen task because that is what they know how to do. This is a plus at a small practice, where the practice manager may need to do the billing, HR, IT, marketing, some legal work, and accounting,” he said. “No one person can do all of those things well.”
He added, however, “If you choose outsourcing and then decide you don’t like it, it’s difficult to unwind the arrangement. Staff that have been dismissed can’t easily be hired back, so it shouldn’t be an easy decision to make.”
Also, sometimes the staff at offshore outsourcing firms may have accents that are harder for patients to understand, and the offshore staff may not readily understand a U.S. caller. However, Dr. Hochman said that practices often have a chance to interview and select specific persons on the offshore team who best fit their needs.
Offshore outsourcing
Outsourcing firms have been moving abroad, where costs are lower. Typical venues are India and the Philippines because there are larger percentages of people who speak English. Since 2020, demand at offshore medical billing companies has been growing faster than their domestic counterparts, according to a recent analysis.
The difference in price can be substantial. In 2020, the average salary for scribes in India was $500 a month, compared with $2,500 for scribes in the United States.
However, offshore outsourcing is starting to face limitations in some places because of privacy issues, according to David J. Zetter, a practice management consultant in Mechanicsburg, Pa. He pointed to a new Florida law that limits use of offshore vendors because they deal with confidential patient information. The law, which became effective July 1, requires that any protected health information must be maintained in the United States or Canada.
“This will make it very hard for many types of offshore vendors to operate in Florida,” he said. He noted that Florida is the only state with such a restriction, but similar proposals are under consideration in a few other states, such as Texas.
How to select the right company
Mr. Zetter said that the biggest mistake practices make when choosing a company is failing to take enough time to examine their choice. “Quite often, practices don’t validate that companies know what they are doing,” he said. “They get a recommendation and go with it.”
“Choose a company with experience in your specialty,” Mr. Zetter advised. “Speak with the company’s clients, not just the ones the company gives you to speak to. You should ask for the full list of clients and speak to all of them.”
Ms. Deabler said that it’s fairly easy to find respected outsourcing companies. “Colleagues can make recommendations, state and specialty societies can provide lists of preferred vendors, and you can visit vendors’ booths at medical conferences,” she said. She added that it’s also easy to find evaluations of each company. “You can Google the company and come up with all kinds of information about it,” she said.
Mr. Shay said that practices should make sure they understand the terms of the contract with a VA. “Depending on how the contract is worded, you may be stuck with the relationship for many years,” he said. “Before you sign an outsourcing contract, you need to make sure it has a reasonable termination provision.”
Because vetting companies properly can require extensive work, Ms. Deabler said, the work can be given to an experienced practice management consultant. “The consultant can start with a cost-benefit analysis that will show you whether outsourcing would be worthwhile,” she said.
Working with outsource service providers
Mr. Holder said that doctors should keep track of what the outsourcer is doing rather than simply let them do their work. “For example, doctors should understand the billing codes they use most often, such as the five levels of evaluation and management codes, and not just blindly rely on the billing company to code and bill their work correctly,” he noted.
Ms. Deabler said that companies provide monthly reports on their work. “Doctors should be reading these reports and contacting the company if expectations aren’t met,” she said.
Even in the reports, companies can hide problems from untrained eyes, Mr. Holder said. “For example, anyone can meet a metric like days in accounts receivable simply by writing off any charge that isn’t paid after 90 days.”
“You need to be engaged with the outsourcer,” he said. “It’s also a good idea to bring in a consultant to periodically check an outsourcer’s work.”
Will outsourcing expand in the future?
Mr. Holder said that the increasing use of value-based care may require practices to rely more on outsourcing in the future. “For instance, if a practice has a value-based contract that requires providing behavioral health services to patients, it might make sense to outsource that work rather than hire psychologists in-house,” he said.
Practices rarely outsource clinical services, but Mr. Holder said that this may happen in the future: “Now that Medicare is paying less for telehealth, practices have to find a way to provide it without using expensive examining room space,” he said. “Some practices may decide to outsource telehealth instead.”
Mr. Shay said that there are many reasons why outsourcing has a strong future. “It allows you to concentrate on your clinical care, and it is a solution to problems with turnover of in-house staff,” he said. “It can also be more efficient because the service is presumably an expert in areas like billing and collections, which means it may be able to ensure more efficient and faster reimbursements. And if the work is outsourced overseas, you can save money through lower worker salaries.”
A version of this article first appeared on Medscape.com.
Off-label medications for addictive disorders
Off-label prescribing (OLP) refers to the practice of using medications for indications outside of those approved by the FDA, or in dosages, dose forms, or patient populations that have not been approved by the FDA.1 OLP is common, occurring in many practice settings and nearly every medical specialty. In a 2006 review, Radley et al2 found OLP accounted for 21% of the overall use of 160 common medications. The frequency of OLP varies between medication classes. Off-label use of anticonvulsants, antidepressants, and antipsychotics tends to be higher than that of other medications.3,4 OLP is often more common
Box
Several aspects contribute to off-label prescribing (OLP). First, there is little financial incentive for pharmaceutical companies to seek new FDA indications for existing medications. In addition, there are no FDA-approved medications for many disorders included in DSM-5, and treatment of these conditions relies almost exclusively on the practice of OLP. Finally, patients enrolled in clinical trials must often meet stringent exclusion criteria, such as the lack of comorbid substance use disorders. For these reasons, using off-label medications to treat substance-related and addictive disorders is particularly necessary.
Several important medicolegal and ethical considerations surround OLP. The FDA prohibits off-label promotion, in which manufacturers advertise the use of a medication for off-label use.5 However, regulations allow physicians to use their best clinical judgment when prescribing medications for off-label use. When considering off-label use of any medication, physicians should review the most up-to-date research, including clinical trials, case reports, and reviews to safely support their decision-making. OLP should be guided by ethical principles such as autonomy, beneficence, nonmaleficence, and justice. Physicians should obtain informed consent by conducting an appropriate discussion of the risks, benefits, and alternatives of off-label medications. This conversation should be clearly documented, and physicians should provide written material regarding off-label options to patients when available. Finally, physicians should verify their patients’ understanding of this discussion, and allow patients to accept or decline off-label medications without pressure.
This article focuses on current and potential future medications available for OLP to treat patients with alcohol use disorder (AUD), gambling disorder (GD), stimulant use disorder, and cannabis use disorder.
Alcohol use disorder
CASE 1
Ms. X, age 67, has a history of severe AUD, mild renal impairment, and migraines. She presents to the outpatient clinic seeking help to drink less alcohol. Ms. X reports drinking 1 to 2 bottles of wine each day. She was previously treated for AUD but was not helped by naltrexone and did not tolerate disulfiram (abstinence was not her goal and she experienced significant adverse effects). Ms. X says she has a medical history of chronic migraines but denies other medical issues. The treatment team discusses alternative pharmacologic options, including acamprosate and topiramate. After outlining the dosing schedule and risks/benefits with Ms. X, you make the joint decision to start topiramate to reduce alcohol cravings and target her migraine symptoms.
Only 3 medications are FDA-approved for treating AUD: disulfiram, naltrexone (oral and injectable formulations), and acamprosate. Off-label options for AUD treatment include gabapentin, topiramate, and baclofen.
Gabapentin is FDA-approved for treating postherpetic neuralgia and partial seizures in patients age ≥3. The exact mechanism of action is unclear, though its effects are possibly related to its activity as a calcium channel ligand. It also carries a structural resemblance to gamma-aminobutyric acid (GABA), though it lacks activity at GABA receptors.
Several randomized controlled trials (RCTs) evaluating the efficacy of gabapentin for AUD produced promising results. In a comparison of gabapentin vs placebo for AUD, Anton et al6 found gabapentin led to significant increases in the number of participants with total alcohol abstinence and participants who reported reduced drinking. Notably, the effect was most prominent in those with heavy drinking patterns and pretreatment alcohol withdrawal symptoms. A total of 41% of participants with high alcohol withdrawal scores on pretreatment evaluation achieved total abstinence while taking gabapentin, compared to 1% in the placebo group.6 A meta-analysis of gabapentin for AUD by Kranzler et al7 included 7 RCTs and 32 effect measures. It found that although all outcome measures favored gabapentin over placebo, only the percentage of heavy drinking days was significantly different.
Gabapentin is dosed between 300 to 600 mg 3 times per day, but 1 study found that a higher dose (1,800 mg/d) was associated with better outcomes.8 Common adverse effects include sedation, dizziness, peripheral edema, and ataxia.
Continue to: Topiramate
Topiramate blocks voltage-gated sodium channels and enhances GABA-A receptor activity.9 It is indicated for the treatment of seizures, migraine prophylaxis, weight management, and weight loss. Several clinical trials, including RCTs,10-12 demonstrated that topiramate was superior to placebo in reducing the percentage of heavy drinking days and overall drinking days. Some also showed that topiramate was associated with abstinence and reduced craving levels.12,13 A meta-analysis by Blodgett et al14 found that compared to placebo, topiramate lowered the rate of heavy drinking and increased abstinence.
Topiramate is dosed from 50 to 150 mg twice daily, although some studies suggest a lower dose (≤75 mg/d) may be associated with clinical benefits.15,16 One important clinical consideration: topiramate must follow a slow titration schedule (4 to 6 weeks) to increase tolerability and avoid adverse effects. Common adverse effects include sedation, word-finding difficulty, paresthesia, increased risk for renal calculi, dizziness, anorexia, and alterations in taste.
Baclofen is a GABA-B agonist FDA-approved for the treatment of muscle spasticity related to multiple sclerosis and reversible spasticity related to spinal cord lesions and multiple sclerosis. Of note, it is approved for treatment of AUD in Europe.
In a meta-analysis of 13 RCTs, Pierce et al17 found a greater likelihood of abstinence and greater time to first lapse of drinking with baclofen compared to placebo. Interestingly, a subgroup analysis found that the positive effects were limited to trials that used 30 to 60 mg/d of baclofen, and not evident in those that used higher doses. Additionally, there was no difference between baclofen and placebo with regard to several important outcomes, including alcohol cravings, anxiety, depression, or number of total abstinent days. A review by Andrade18 proposed that individualized treatment with high-dose baclofen (30 to 300 mg/d) may be a useful second-line approach in heavy drinkers who wish to reduce their alcohol intake.
Continue to: Before starting baclofen...
Before starting baclofen, patients should be informed about its adverse effects. Common adverse effects include sedation and motor impairment. More serious but less common adverse effects include seizures, respiratory depression with sleep apnea, severe mood disorders (ie, mania, depression, or suicide risk), and mental confusion. Baclofen should be gradually discontinued, because there is some risk of clinical withdrawal symptoms (ie, agitation, confusion, seizures, or delirium).
Among the medications discussed in this section, the evidence for gabapentin and topiramate is moderate to strong, while the evidence for baclofen is overall weaker or mixed. The American Psychiatric Association’s Practice Guideline suggests offering gabapentin or topiramate to patients with moderate to severe AUD whose goal is to achieve abstinence or reduce alcohol use, or those who prefer gabapentin or topiramate or cannot tolerate or have not responded to naltrexone and acamprosate.19 Clinicians must ensure patients have no contraindications to the use of these medications. Due to the moderate quality evidence for a significant reduction in heavy drinking and increased abstinence,14,20 a practice guideline from the US Department of Veterans Affairs and US Department of Defense21 recommends topiramate as 1 of 2 first-line treatments (the other is naltrexone). This guideline suggests gabapentin as a second-line treatment for AUD.21
Gambling disorder
CASE 2
Mr. P, age 28, seeks treatment for GD and cocaine use disorder. He reports a 7-year history of sports betting that has increasingly impaired his functioning over the past year. He lost his job, savings, and familial relationships due to his impulsive and risky behavior. Mr. P also reports frequent cocaine use, about 2 to 3 days per week, mostly on the weekends. The psychiatrist tells Mr. P there is no FDA-approved pharmacologic treatment for GD or cocaine use disorder. The psychiatrist discusses the option of naltrexone as off-label treatment for GD with the goal of reducing Mr. P’s urges to gamble, and points to possible benefits for cocaine use disorder.
GD impacts approximately 0.5% of the adult US population and is often co-occurring with substance use disorders.22 It is thought to share neurobiological and clinical similarities with substance use disorders.23 There are currently no FDA-approved medications to treat the disorder. In studies of GD, treatment success with antidepressants and mood stabilizers has not been consistent,23,24 but some promising results have been published for the opioid receptor antagonist naltrexone24-29 and N-acetylcysteine (NAC).30-32
Naltrexone is thought to reduce gambling behavior and urges via downstream modulation of mesolimbic dopamine circuitry.24 It is FDA-approved for the treatment of AUD and opioid use disorder. Open-label RCTs have found a reduction in gambling urges and behavior with daily naltrexone.25-27 Dosing at 50 mg/d appears to be just as efficacious as higher doses such as 100 and 150 mg/d.27 When used as a daily as-needed medication for strong gambling urges or if an individual was planning to gamble, naltrexone 50 mg/d was not effective.28
Continue to: Naltrexone typically is started...
Naltrexone typically is started at 25 mg/d to assess tolerability and quickly titrated to 50 mg/d. When titrating, common adverse effects include nausea, vomiting, and transient elevations in transaminases. Another opioid antagonist, nalmefene, has also been studied in patients with GD. An RCT by Grant et al29 that evaluated 207 patients found that compared with placebo, nalmefene 25 mg/d for 16 weeks was associated with a significant reduction in gambling assessment scores. In Europe, nalmefene is approved for treating AUD but the oral formulation is not currently available in the US.
N-acetylcysteine is thought to potentially reverse neuronal dysfunction seen in addictive disorders by glutamatergic modulation.30 Research investigating NAC for GD is scarce. A pilot study found 16 of 27 patients with GD reduced gambling behavior with a mean dose of 1,476.9 mg/d.31 An additional study investigating the addition of NAC to behavioral therapy in nicotine-dependent individuals with pathologic gambling found a reduction in problem gambling after 18 weeks (6 weeks + 3 months follow-up).32 Common but mild adverse effects associated with NAC are nausea, vomiting, and diarrhea.
A meta-analysis by Goslar et al33 that reviewed 34 studies (1,340 participants) found pharmacologic treatments were associated with large and medium pre-post reductions in global severity, frequency, and financial loss in patients with GD. RCTs studying opioid antagonists and mood stabilizers (combined with a cognitive intervention) as well as lithium for patients with comorbid bipolar disorder and GD demonstrated promising results.33
Stimulant use disorder
There are no FDA-approved medications for stimulant use disorder. Multiple off-label options have been studied for the treatment of methamphetamine abuse and cocaine abuse.
Methamphetamine use has been expanding over the past decade with a 3.6-fold increase in positive methamphetamine screens in overdose deaths from 2011 to 2016.34 Pharmacologic options studied for OLP of methamphetamine use disorder include mirtazapine, bupropion, naltrexone, and topiramate.
Continue to: Mirtazapine
Mirtazapine is an atypical antidepressant whose mechanism of action includes modulation of the serotonin, norepinephrine, and alpha-2 adrenergic systems. It is FDA-approved for the treatment of major depressive disorder (MDD). In a randomized placebo-controlled study, mirtazapine 30 mg/d at night was found to decrease methamphetamine use for active users and led to decreased sexual risk in men who have sex with men.35 These results were supported by an additional RCT in which mirtazapine 30 mg/d significantly reduced rates of methamphetamine use vs placebo at 24 and 36 weeks despite poor medication adherence.36 Adverse effects to monitor in patients treated with mirtazapine include increased appetite, weight gain, sedation, and constipation.
Bupropion is a norepinephrine dopamine reuptake inhibitor that produces increased neurotransmission of norepinephrine and dopamine in the CNS. It is FDA-approved for the treatment of MDD and as an aid for smoking cessation. Bupropion has been studied for methamphetamine use disorder with mixed results. In a randomized placebo-controlled trial, bupropion sustained release 15
Naltrexone. Data about using oral naltrexone to treat stimulant use disorders are limited. A randomized, placebo-controlled trial by Jayaram-Lindström et al39 found naltrexone 50 mg/d significantly reduced amphetamine use compared to placebo. Additionally, naltrexone 50 and 150 mg/d have been shown to reduce cocaine use over time in combination with therapy for cocaine-dependent patients and those dependent on alcohol and cocaine.40,41
Topiramate has been studied for the treatment of cocaine use disorder. It is hypothesized that modulation of the mesocorticolimbic dopamine system may contribute to decreased cocaine cravings.42 A pilot study by Kampman et al43 found that after an 8-week titration of topiramate to 200 mg/d, individuals were more likely to achieve cocaine abstinence compared to those who receive placebo. In an RCT, Elkashef et al44 did not find topiramate assisted with increased abstinence of methamphetamine in active users at a target dose of 200 mg/d. However, it was associated with reduced relapse rates in individuals who were abstinent prior to the study.44 At a target dose of 300 mg/d, topiramate also outperformed placebo in decreasing days of cocaine use.42 Adverse effects of topiramate included paresthesia, alteration in taste, and difficulty with concentration.
Cannabis use disorder
In recent years, cannabis use in the US has greatly increased45 but no medications are FDA-approved for treating cannabis use disorder. Studies of pharmacologic options for cannabis use disorder have had mixed results.46 A meta-analysis by Bahji et al47 of 24 studies investigating pharmacotherapies for cannabis use disorder highlighted the lack of adequate evidence. In this section, we focus on a few positive trials of NAC and gabapentin.
Continue to: N-acetylcysteine
N-acetylcysteine. Studies investigating NAC 1,200 mg twice daily have been promising in adolescent and adult populations.48-50 There are some mixed results, however. A large RCT found NAC 1,200 mg twice daily was not better than placebo in helping adults achieve abstinence from cannabis.51
Gabapentin may be a viable option for treating cannabis use disorder. A pilot study by Mason et al52 found gabapentin 1,200 mg/d was more effective than placebo at reducing cannabis use among treatment-seeking adults.
When and how to consider OLP
OLP for addictive disorders is common and often necessary. This is primarily due to limitations of the FDA-approved medications and because there are no FDA-approved medications for many substance-related and addictive disorders (ie, GD, cannabis use disorder, and stimulant use disorder). When assessing pharmacotherapy options, if FDA-approved medications are available for certain diagnoses, clinicians should first consider them. The off-label medications discussed in this article are outlined in the Table.6-21,24-28,30-33,35-44,48-52
The overall level of evidence to support the use of off-label medications is lower than that of FDA-approved medications, which contributes to potential medicolegal concerns of OLP. Off-label medications should be considered when there are no FDA-approved medications available, and the decision to use off-label medications should be based on evidence from the literature and current standard of care. Additionally, OLP is necessary if a patient cannot tolerate FDA-approved medications, is not helped by FDA-approved treatments, or when there are other clinical reasons to choose a particular off-label medication. For example, if a patient has comorbid AUD and obesity (or migraines), using topiramate may be appropriate because it may target alcohol cravings and can be helpful for weight loss (and migraine prophylaxis). Similarly, for patients with AUD and neuropathic pain, using gabapentin can be considered for its dual therapeutic effects.
It is critical for clinicians to understand the landscape of off-label options for treating addictive disorders. Additional research in the form of RCTs is needed to better clarify the efficacy and adverse effects of these treatments.
Continue to: Bottom Line
Bottom Line
Off-label prescribing is prevalent in practice, including in the treatment of substance-related and addictive disorders. When considering off-label use of any medication, clinicians should review the most recent research, obtain informed consent from patients, and verify patients’ understanding of the potential risks and adverse effects associated with the particular medication.
Related Resources
- Joshi KG, Frierson RL. Off-label prescribing: how to limit your liability. Current Psychiatry. 2020;19(9):12,39. doi:10.12788/ cp.0035
- Stanciu CN, Gnanasegaram SA. Don’t balk at using medical therapy to manage alcohol use disorder. Current Psychiatry. 2017;16(2):50-52.
Drug Brand Names
Acamprosate • Campral
Baclofen • Ozobax
Bupropion • Wellbutrin, Zyban
Disulfiram • Antabuse
Gabapentin • Neurontin
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Naltrexone • ReVia, Vivitrol
Topiramate • Topamax
1. Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc. 2012;87(10):982-990. doi:10.1016/j.mayocp.2012.04.017
2. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026. doi:10.1001/archinte.166.9.1021
3. Wang J, Jiang F, Yating Y, et al. Off-label use of antipsychotic medications in psychiatric inpatients in China: a national real-world survey. BMC Psychiatry. 2021;21(1):375. doi:10.1186/s12888-021-03374-0
4. Chen H, Reeves JH, Fincham JE, et al. Off-label use of antidepressant, anticonvulsant, and antipsychotic medications among Georgia Medicaid enrollees in 2001. J Clin Psychiatry. 2006;67(6):972-982. doi:10.4088/jcp.v67n0615
5. Ventola CL. Off-label drug information: regulation, distribution, evaluation, and related controversies. P T. 2009;34(8):428-440.
6. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736. doi:10.1001/jamainternmed.2020.0249
7. Kranzler HR, Feinn R, Morris P, et al. A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder. Addiction. 2019;114(9):1547-1555. doi:10.1111/add.14655
8. Mason BJ, Quello S, Goodell V. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
9. Fariba KA. Saadabadi A. Topiramate. StatPearls [Internet]. StatPearls Publishing LLC; 2023. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554530/
10. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685. doi:10.1016/S0140-6736(03)13370-3
11. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298(14):1641-1651. doi:10.1001/jama.298.14.1641
12. Knapp CM, Ciraulo DA, Sarid-Segal O, et al. Zonisamide, topiramate, and levetiracetam: efficacy and neuropsychological effects in alcohol use disorders. J Clin Psychopharmacol. 2015;35(1):34-42. doi:10.1097/JCP.0000000000000246
13. Kranzler HR, Covault J, Feinn R, et al. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014;171(4):445-452. doi:10.1176/appi.ajp.2013.13081014
14. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
15. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41. doi:10.1186/1471-244X-11-41
16. Tang YL, Hao W, Leggio L. Treatments for alcohol-related disorders in China: a developing story. Alcohol Alcohol. 2012;47(5):563-570. doi:10.1093/alcalc/ags066
17. Pierce M, Sutterland A, Beraha EM, et al. Efficacy, tolerability, and safety of low-dose and high-dose baclofen in the treatment of alcohol dependence: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2018;28(7):795-806. doi:10.1016/j.euroneuro.2018.03.017
18. Andrade C. Individualized, high-dose baclofen for reduction in alcohol intake in persons with high levels of consumption. J Clin Psychiatry. 2020;81(4):20f13606. doi:10.4088/JCP.20f13606
19. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
20. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
21. US Department of Veterans Affairs, US Department of Defense. Management of Substance Use Disorder (SUD) (2021). US Department of Veterans Affairs. 2021. Accessed December 24, 2022. https://www.healthquality.va.gov/guidelines/mh/sud/
22. Potenza MN, Balodis IM, Derevensky J, et al. Gambling disorder. Nat Rev Dis Primers. 2019;5(1):51. doi:10.1038/s41572-019-0099-7
23. Lupi M, Martinotti G, Acciavatti T, et al. Pharmacological treatments in gambling disorder: a qualitative review. BioMed Res Int. 2014;537306. Accessed January 18, 2023. https://www.hindawi.com/journals/bmri/2014/537306/
24. Choi SW, Shin YC, Kim DJ, et al. Treatment modalities for patients with gambling disorder. Ann Gen Psychiatry. 2017;16:23. doi:10.1186/s12991-017-0146-2
25. Kim SW, Grant JE. An open naltrexone treatment study in pathological gambling disorder. Int Clin Psychopharmacol. 2001;16(5):285-289. doi:10.1097/00004850-200109000-00006
26. Kim SW, Grant JE, Adson DE, et al. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914-921. doi:10.1016/s0006-3223(01)01079-4
27. Grant JE, Kim SW, Hartman BK. A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. J Clin Psychiatry. 2008;69(5):783-789. doi:10.4088/jcp.v69n0511
28. Kovanen L, Basnet S, Castrén S, et al. A randomised, double-blind, placebo-controlled trial of as-needed naltrexone in the treatment of pathological gambling. Eur Addict Res. 2016;22(2):70-79. doi:10.1159/000435876
29. Grant JE, Potenza MN, Hollander E, et al. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163(2):303-312. doi:10.1176/appi.ajp.163.2.303
30. Tomko RL, Jones JL, Gilmore AK, et al. N-acetylcysteine: a potential treatment for substance use disorders. Current Psychiatry. 2018;17(6):30-36,41-52,55.
31. Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62(6):652-657. doi:10.1016/j.biopsych.2006.11.021
32. G
33. Goslar M, Leibetseder M, Muench HM, et al. Pharmacological treatments for disordered gambling: a meta-analysis. J Gambling Stud. 2019;35(2):415-445. doi:10.1007/s10899-018-09815-y
34. Hedegaard H, Miniño AM, Spencer MR, et al. Drug overdose deaths in the United States, 1999-2020. Centers for Disease Control and Prevention. December 30, 2021. Accessed December 11, 2022. https://stacks.cdc.gov/view/cdc/112340
35. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175. doi:10.1001/archgenpsychiatry.2011.124
36. Coffin PO, Santos GM, Hern J, et al. Effects of mirtazapine for methamphetamine use disorder among cisgender men and transgender women who have sex with men: a placebo-controlled randomized clinical trial. JAMA Psychiatry. 2020;77(3):246-255. doi:10.1001/jamapsychiatry.2019.3655
37. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Dependence. 2008;96(3):222-232. doi:10.1016/j.drugalcdep.2008.03.010
38. Trivedi MH, Walker R, Ling W, et al. Bupropion and naltrexone in methamphetamine use disorder. N Engl J Med. 2021;384(2):140-153. doi:10.1056/NEJMoa2020214
39. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448. doi:10.1176/appi.ajp.2008.08020304
40. Schmitz JM, Stotts AL, Rhoades HM, et al. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26(2):167-180. doi:10.1016/s0306-4603(00)00098-8
41. Oslin DW, Pettinati HM, Volpicelli JR, et al. The effects of naltrexone on alcohol and cocaine use in dually addicted patients. J Subst Abuse Treat. 1999;16(2):163-167. doi:10.1016/s0740-5472(98)00039-7
42. Johnson BA, Ait-Daoud N, Wang XQ, et al. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry. 2013;70(12):1338-1346. doi:10.1001/jamapsychiatry.2013.2295
43. Kampman KM, Pettinati H, Lynch KG, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Dependence. 2004;75(3):233-240. doi:10.1016/j.drugalcdep.2004.03.008
44. Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107(7):1297-1306. doi:10.1111/j.1360-0443.2011.03771.x
45. Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology. 2018;43(1):195-212.
46. Brezing CA, Levin FR. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology. 2018;43(1):173-194. doi:10.1038/npp.2017.198
47. Bahji A, Meyyappan AC, Hawken ER, et al. Pharmacotherapies for cannabis use disorder: a systematic review and network meta-analysis. Intl J Drug Policy. 2021;97:103295. doi:10.1016/j.drugpo.2021.103295
48. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812. doi:10.1176/appi.ajp.2012.12010055
49. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791. doi:10.1016/j.addbeh.2012.11.003
50. McClure EA, Sonne SC, Winhusen T, et al. Achieving cannabis cessation—evaluating N-acetylcysteine treatment (ACCENT): design and implementation of a multi-site, randomized controlled study in the National Institute on Drug Abuse Clinical Trials Network. Contemp Clin Trials. 2014;39(2):211-223. doi:10.1016/j.cct.2014.08.011
51. Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Dependence. 2017;177:249-257. doi:10.1016/j.drugalcdep.2017.04.020
52. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689-1698. doi:10.1038/npp.2012.14
Off-label prescribing (OLP) refers to the practice of using medications for indications outside of those approved by the FDA, or in dosages, dose forms, or patient populations that have not been approved by the FDA.1 OLP is common, occurring in many practice settings and nearly every medical specialty. In a 2006 review, Radley et al2 found OLP accounted for 21% of the overall use of 160 common medications. The frequency of OLP varies between medication classes. Off-label use of anticonvulsants, antidepressants, and antipsychotics tends to be higher than that of other medications.3,4 OLP is often more common
Box
Several aspects contribute to off-label prescribing (OLP). First, there is little financial incentive for pharmaceutical companies to seek new FDA indications for existing medications. In addition, there are no FDA-approved medications for many disorders included in DSM-5, and treatment of these conditions relies almost exclusively on the practice of OLP. Finally, patients enrolled in clinical trials must often meet stringent exclusion criteria, such as the lack of comorbid substance use disorders. For these reasons, using off-label medications to treat substance-related and addictive disorders is particularly necessary.
Several important medicolegal and ethical considerations surround OLP. The FDA prohibits off-label promotion, in which manufacturers advertise the use of a medication for off-label use.5 However, regulations allow physicians to use their best clinical judgment when prescribing medications for off-label use. When considering off-label use of any medication, physicians should review the most up-to-date research, including clinical trials, case reports, and reviews to safely support their decision-making. OLP should be guided by ethical principles such as autonomy, beneficence, nonmaleficence, and justice. Physicians should obtain informed consent by conducting an appropriate discussion of the risks, benefits, and alternatives of off-label medications. This conversation should be clearly documented, and physicians should provide written material regarding off-label options to patients when available. Finally, physicians should verify their patients’ understanding of this discussion, and allow patients to accept or decline off-label medications without pressure.
This article focuses on current and potential future medications available for OLP to treat patients with alcohol use disorder (AUD), gambling disorder (GD), stimulant use disorder, and cannabis use disorder.
Alcohol use disorder
CASE 1
Ms. X, age 67, has a history of severe AUD, mild renal impairment, and migraines. She presents to the outpatient clinic seeking help to drink less alcohol. Ms. X reports drinking 1 to 2 bottles of wine each day. She was previously treated for AUD but was not helped by naltrexone and did not tolerate disulfiram (abstinence was not her goal and she experienced significant adverse effects). Ms. X says she has a medical history of chronic migraines but denies other medical issues. The treatment team discusses alternative pharmacologic options, including acamprosate and topiramate. After outlining the dosing schedule and risks/benefits with Ms. X, you make the joint decision to start topiramate to reduce alcohol cravings and target her migraine symptoms.
Only 3 medications are FDA-approved for treating AUD: disulfiram, naltrexone (oral and injectable formulations), and acamprosate. Off-label options for AUD treatment include gabapentin, topiramate, and baclofen.
Gabapentin is FDA-approved for treating postherpetic neuralgia and partial seizures in patients age ≥3. The exact mechanism of action is unclear, though its effects are possibly related to its activity as a calcium channel ligand. It also carries a structural resemblance to gamma-aminobutyric acid (GABA), though it lacks activity at GABA receptors.
Several randomized controlled trials (RCTs) evaluating the efficacy of gabapentin for AUD produced promising results. In a comparison of gabapentin vs placebo for AUD, Anton et al6 found gabapentin led to significant increases in the number of participants with total alcohol abstinence and participants who reported reduced drinking. Notably, the effect was most prominent in those with heavy drinking patterns and pretreatment alcohol withdrawal symptoms. A total of 41% of participants with high alcohol withdrawal scores on pretreatment evaluation achieved total abstinence while taking gabapentin, compared to 1% in the placebo group.6 A meta-analysis of gabapentin for AUD by Kranzler et al7 included 7 RCTs and 32 effect measures. It found that although all outcome measures favored gabapentin over placebo, only the percentage of heavy drinking days was significantly different.
Gabapentin is dosed between 300 to 600 mg 3 times per day, but 1 study found that a higher dose (1,800 mg/d) was associated with better outcomes.8 Common adverse effects include sedation, dizziness, peripheral edema, and ataxia.
Continue to: Topiramate
Topiramate blocks voltage-gated sodium channels and enhances GABA-A receptor activity.9 It is indicated for the treatment of seizures, migraine prophylaxis, weight management, and weight loss. Several clinical trials, including RCTs,10-12 demonstrated that topiramate was superior to placebo in reducing the percentage of heavy drinking days and overall drinking days. Some also showed that topiramate was associated with abstinence and reduced craving levels.12,13 A meta-analysis by Blodgett et al14 found that compared to placebo, topiramate lowered the rate of heavy drinking and increased abstinence.
Topiramate is dosed from 50 to 150 mg twice daily, although some studies suggest a lower dose (≤75 mg/d) may be associated with clinical benefits.15,16 One important clinical consideration: topiramate must follow a slow titration schedule (4 to 6 weeks) to increase tolerability and avoid adverse effects. Common adverse effects include sedation, word-finding difficulty, paresthesia, increased risk for renal calculi, dizziness, anorexia, and alterations in taste.
Baclofen is a GABA-B agonist FDA-approved for the treatment of muscle spasticity related to multiple sclerosis and reversible spasticity related to spinal cord lesions and multiple sclerosis. Of note, it is approved for treatment of AUD in Europe.
In a meta-analysis of 13 RCTs, Pierce et al17 found a greater likelihood of abstinence and greater time to first lapse of drinking with baclofen compared to placebo. Interestingly, a subgroup analysis found that the positive effects were limited to trials that used 30 to 60 mg/d of baclofen, and not evident in those that used higher doses. Additionally, there was no difference between baclofen and placebo with regard to several important outcomes, including alcohol cravings, anxiety, depression, or number of total abstinent days. A review by Andrade18 proposed that individualized treatment with high-dose baclofen (30 to 300 mg/d) may be a useful second-line approach in heavy drinkers who wish to reduce their alcohol intake.
Continue to: Before starting baclofen...
Before starting baclofen, patients should be informed about its adverse effects. Common adverse effects include sedation and motor impairment. More serious but less common adverse effects include seizures, respiratory depression with sleep apnea, severe mood disorders (ie, mania, depression, or suicide risk), and mental confusion. Baclofen should be gradually discontinued, because there is some risk of clinical withdrawal symptoms (ie, agitation, confusion, seizures, or delirium).
Among the medications discussed in this section, the evidence for gabapentin and topiramate is moderate to strong, while the evidence for baclofen is overall weaker or mixed. The American Psychiatric Association’s Practice Guideline suggests offering gabapentin or topiramate to patients with moderate to severe AUD whose goal is to achieve abstinence or reduce alcohol use, or those who prefer gabapentin or topiramate or cannot tolerate or have not responded to naltrexone and acamprosate.19 Clinicians must ensure patients have no contraindications to the use of these medications. Due to the moderate quality evidence for a significant reduction in heavy drinking and increased abstinence,14,20 a practice guideline from the US Department of Veterans Affairs and US Department of Defense21 recommends topiramate as 1 of 2 first-line treatments (the other is naltrexone). This guideline suggests gabapentin as a second-line treatment for AUD.21
Gambling disorder
CASE 2
Mr. P, age 28, seeks treatment for GD and cocaine use disorder. He reports a 7-year history of sports betting that has increasingly impaired his functioning over the past year. He lost his job, savings, and familial relationships due to his impulsive and risky behavior. Mr. P also reports frequent cocaine use, about 2 to 3 days per week, mostly on the weekends. The psychiatrist tells Mr. P there is no FDA-approved pharmacologic treatment for GD or cocaine use disorder. The psychiatrist discusses the option of naltrexone as off-label treatment for GD with the goal of reducing Mr. P’s urges to gamble, and points to possible benefits for cocaine use disorder.
GD impacts approximately 0.5% of the adult US population and is often co-occurring with substance use disorders.22 It is thought to share neurobiological and clinical similarities with substance use disorders.23 There are currently no FDA-approved medications to treat the disorder. In studies of GD, treatment success with antidepressants and mood stabilizers has not been consistent,23,24 but some promising results have been published for the opioid receptor antagonist naltrexone24-29 and N-acetylcysteine (NAC).30-32
Naltrexone is thought to reduce gambling behavior and urges via downstream modulation of mesolimbic dopamine circuitry.24 It is FDA-approved for the treatment of AUD and opioid use disorder. Open-label RCTs have found a reduction in gambling urges and behavior with daily naltrexone.25-27 Dosing at 50 mg/d appears to be just as efficacious as higher doses such as 100 and 150 mg/d.27 When used as a daily as-needed medication for strong gambling urges or if an individual was planning to gamble, naltrexone 50 mg/d was not effective.28
Continue to: Naltrexone typically is started...
Naltrexone typically is started at 25 mg/d to assess tolerability and quickly titrated to 50 mg/d. When titrating, common adverse effects include nausea, vomiting, and transient elevations in transaminases. Another opioid antagonist, nalmefene, has also been studied in patients with GD. An RCT by Grant et al29 that evaluated 207 patients found that compared with placebo, nalmefene 25 mg/d for 16 weeks was associated with a significant reduction in gambling assessment scores. In Europe, nalmefene is approved for treating AUD but the oral formulation is not currently available in the US.
N-acetylcysteine is thought to potentially reverse neuronal dysfunction seen in addictive disorders by glutamatergic modulation.30 Research investigating NAC for GD is scarce. A pilot study found 16 of 27 patients with GD reduced gambling behavior with a mean dose of 1,476.9 mg/d.31 An additional study investigating the addition of NAC to behavioral therapy in nicotine-dependent individuals with pathologic gambling found a reduction in problem gambling after 18 weeks (6 weeks + 3 months follow-up).32 Common but mild adverse effects associated with NAC are nausea, vomiting, and diarrhea.
A meta-analysis by Goslar et al33 that reviewed 34 studies (1,340 participants) found pharmacologic treatments were associated with large and medium pre-post reductions in global severity, frequency, and financial loss in patients with GD. RCTs studying opioid antagonists and mood stabilizers (combined with a cognitive intervention) as well as lithium for patients with comorbid bipolar disorder and GD demonstrated promising results.33
Stimulant use disorder
There are no FDA-approved medications for stimulant use disorder. Multiple off-label options have been studied for the treatment of methamphetamine abuse and cocaine abuse.
Methamphetamine use has been expanding over the past decade with a 3.6-fold increase in positive methamphetamine screens in overdose deaths from 2011 to 2016.34 Pharmacologic options studied for OLP of methamphetamine use disorder include mirtazapine, bupropion, naltrexone, and topiramate.
Continue to: Mirtazapine
Mirtazapine is an atypical antidepressant whose mechanism of action includes modulation of the serotonin, norepinephrine, and alpha-2 adrenergic systems. It is FDA-approved for the treatment of major depressive disorder (MDD). In a randomized placebo-controlled study, mirtazapine 30 mg/d at night was found to decrease methamphetamine use for active users and led to decreased sexual risk in men who have sex with men.35 These results were supported by an additional RCT in which mirtazapine 30 mg/d significantly reduced rates of methamphetamine use vs placebo at 24 and 36 weeks despite poor medication adherence.36 Adverse effects to monitor in patients treated with mirtazapine include increased appetite, weight gain, sedation, and constipation.
Bupropion is a norepinephrine dopamine reuptake inhibitor that produces increased neurotransmission of norepinephrine and dopamine in the CNS. It is FDA-approved for the treatment of MDD and as an aid for smoking cessation. Bupropion has been studied for methamphetamine use disorder with mixed results. In a randomized placebo-controlled trial, bupropion sustained release 15
Naltrexone. Data about using oral naltrexone to treat stimulant use disorders are limited. A randomized, placebo-controlled trial by Jayaram-Lindström et al39 found naltrexone 50 mg/d significantly reduced amphetamine use compared to placebo. Additionally, naltrexone 50 and 150 mg/d have been shown to reduce cocaine use over time in combination with therapy for cocaine-dependent patients and those dependent on alcohol and cocaine.40,41
Topiramate has been studied for the treatment of cocaine use disorder. It is hypothesized that modulation of the mesocorticolimbic dopamine system may contribute to decreased cocaine cravings.42 A pilot study by Kampman et al43 found that after an 8-week titration of topiramate to 200 mg/d, individuals were more likely to achieve cocaine abstinence compared to those who receive placebo. In an RCT, Elkashef et al44 did not find topiramate assisted with increased abstinence of methamphetamine in active users at a target dose of 200 mg/d. However, it was associated with reduced relapse rates in individuals who were abstinent prior to the study.44 At a target dose of 300 mg/d, topiramate also outperformed placebo in decreasing days of cocaine use.42 Adverse effects of topiramate included paresthesia, alteration in taste, and difficulty with concentration.
Cannabis use disorder
In recent years, cannabis use in the US has greatly increased45 but no medications are FDA-approved for treating cannabis use disorder. Studies of pharmacologic options for cannabis use disorder have had mixed results.46 A meta-analysis by Bahji et al47 of 24 studies investigating pharmacotherapies for cannabis use disorder highlighted the lack of adequate evidence. In this section, we focus on a few positive trials of NAC and gabapentin.
Continue to: N-acetylcysteine
N-acetylcysteine. Studies investigating NAC 1,200 mg twice daily have been promising in adolescent and adult populations.48-50 There are some mixed results, however. A large RCT found NAC 1,200 mg twice daily was not better than placebo in helping adults achieve abstinence from cannabis.51
Gabapentin may be a viable option for treating cannabis use disorder. A pilot study by Mason et al52 found gabapentin 1,200 mg/d was more effective than placebo at reducing cannabis use among treatment-seeking adults.
When and how to consider OLP
OLP for addictive disorders is common and often necessary. This is primarily due to limitations of the FDA-approved medications and because there are no FDA-approved medications for many substance-related and addictive disorders (ie, GD, cannabis use disorder, and stimulant use disorder). When assessing pharmacotherapy options, if FDA-approved medications are available for certain diagnoses, clinicians should first consider them. The off-label medications discussed in this article are outlined in the Table.6-21,24-28,30-33,35-44,48-52

The overall level of evidence to support the use of off-label medications is lower than that of FDA-approved medications, which contributes to potential medicolegal concerns of OLP. Off-label medications should be considered when there are no FDA-approved medications available, and the decision to use off-label medications should be based on evidence from the literature and current standard of care. Additionally, OLP is necessary if a patient cannot tolerate FDA-approved medications, is not helped by FDA-approved treatments, or when there are other clinical reasons to choose a particular off-label medication. For example, if a patient has comorbid AUD and obesity (or migraines), using topiramate may be appropriate because it may target alcohol cravings and can be helpful for weight loss (and migraine prophylaxis). Similarly, for patients with AUD and neuropathic pain, using gabapentin can be considered for its dual therapeutic effects.
It is critical for clinicians to understand the landscape of off-label options for treating addictive disorders. Additional research in the form of RCTs is needed to better clarify the efficacy and adverse effects of these treatments.
Continue to: Bottom Line
Bottom Line
Off-label prescribing is prevalent in practice, including in the treatment of substance-related and addictive disorders. When considering off-label use of any medication, clinicians should review the most recent research, obtain informed consent from patients, and verify patients’ understanding of the potential risks and adverse effects associated with the particular medication.
Related Resources
- Joshi KG, Frierson RL. Off-label prescribing: how to limit your liability. Current Psychiatry. 2020;19(9):12,39. doi:10.12788/ cp.0035
- Stanciu CN, Gnanasegaram SA. Don’t balk at using medical therapy to manage alcohol use disorder. Current Psychiatry. 2017;16(2):50-52.
Drug Brand Names
Acamprosate • Campral
Baclofen • Ozobax
Bupropion • Wellbutrin, Zyban
Disulfiram • Antabuse
Gabapentin • Neurontin
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Naltrexone • ReVia, Vivitrol
Topiramate • Topamax
Off-label prescribing (OLP) refers to the practice of using medications for indications outside of those approved by the FDA, or in dosages, dose forms, or patient populations that have not been approved by the FDA.1 OLP is common, occurring in many practice settings and nearly every medical specialty. In a 2006 review, Radley et al2 found OLP accounted for 21% of the overall use of 160 common medications. The frequency of OLP varies between medication classes. Off-label use of anticonvulsants, antidepressants, and antipsychotics tends to be higher than that of other medications.3,4 OLP is often more common
Box
Several aspects contribute to off-label prescribing (OLP). First, there is little financial incentive for pharmaceutical companies to seek new FDA indications for existing medications. In addition, there are no FDA-approved medications for many disorders included in DSM-5, and treatment of these conditions relies almost exclusively on the practice of OLP. Finally, patients enrolled in clinical trials must often meet stringent exclusion criteria, such as the lack of comorbid substance use disorders. For these reasons, using off-label medications to treat substance-related and addictive disorders is particularly necessary.
Several important medicolegal and ethical considerations surround OLP. The FDA prohibits off-label promotion, in which manufacturers advertise the use of a medication for off-label use.5 However, regulations allow physicians to use their best clinical judgment when prescribing medications for off-label use. When considering off-label use of any medication, physicians should review the most up-to-date research, including clinical trials, case reports, and reviews to safely support their decision-making. OLP should be guided by ethical principles such as autonomy, beneficence, nonmaleficence, and justice. Physicians should obtain informed consent by conducting an appropriate discussion of the risks, benefits, and alternatives of off-label medications. This conversation should be clearly documented, and physicians should provide written material regarding off-label options to patients when available. Finally, physicians should verify their patients’ understanding of this discussion, and allow patients to accept or decline off-label medications without pressure.
This article focuses on current and potential future medications available for OLP to treat patients with alcohol use disorder (AUD), gambling disorder (GD), stimulant use disorder, and cannabis use disorder.
Alcohol use disorder
CASE 1
Ms. X, age 67, has a history of severe AUD, mild renal impairment, and migraines. She presents to the outpatient clinic seeking help to drink less alcohol. Ms. X reports drinking 1 to 2 bottles of wine each day. She was previously treated for AUD but was not helped by naltrexone and did not tolerate disulfiram (abstinence was not her goal and she experienced significant adverse effects). Ms. X says she has a medical history of chronic migraines but denies other medical issues. The treatment team discusses alternative pharmacologic options, including acamprosate and topiramate. After outlining the dosing schedule and risks/benefits with Ms. X, you make the joint decision to start topiramate to reduce alcohol cravings and target her migraine symptoms.
Only 3 medications are FDA-approved for treating AUD: disulfiram, naltrexone (oral and injectable formulations), and acamprosate. Off-label options for AUD treatment include gabapentin, topiramate, and baclofen.
Gabapentin is FDA-approved for treating postherpetic neuralgia and partial seizures in patients age ≥3. The exact mechanism of action is unclear, though its effects are possibly related to its activity as a calcium channel ligand. It also carries a structural resemblance to gamma-aminobutyric acid (GABA), though it lacks activity at GABA receptors.
Several randomized controlled trials (RCTs) evaluating the efficacy of gabapentin for AUD produced promising results. In a comparison of gabapentin vs placebo for AUD, Anton et al6 found gabapentin led to significant increases in the number of participants with total alcohol abstinence and participants who reported reduced drinking. Notably, the effect was most prominent in those with heavy drinking patterns and pretreatment alcohol withdrawal symptoms. A total of 41% of participants with high alcohol withdrawal scores on pretreatment evaluation achieved total abstinence while taking gabapentin, compared to 1% in the placebo group.6 A meta-analysis of gabapentin for AUD by Kranzler et al7 included 7 RCTs and 32 effect measures. It found that although all outcome measures favored gabapentin over placebo, only the percentage of heavy drinking days was significantly different.
Gabapentin is dosed between 300 to 600 mg 3 times per day, but 1 study found that a higher dose (1,800 mg/d) was associated with better outcomes.8 Common adverse effects include sedation, dizziness, peripheral edema, and ataxia.
Continue to: Topiramate
Topiramate blocks voltage-gated sodium channels and enhances GABA-A receptor activity.9 It is indicated for the treatment of seizures, migraine prophylaxis, weight management, and weight loss. Several clinical trials, including RCTs,10-12 demonstrated that topiramate was superior to placebo in reducing the percentage of heavy drinking days and overall drinking days. Some also showed that topiramate was associated with abstinence and reduced craving levels.12,13 A meta-analysis by Blodgett et al14 found that compared to placebo, topiramate lowered the rate of heavy drinking and increased abstinence.
Topiramate is dosed from 50 to 150 mg twice daily, although some studies suggest a lower dose (≤75 mg/d) may be associated with clinical benefits.15,16 One important clinical consideration: topiramate must follow a slow titration schedule (4 to 6 weeks) to increase tolerability and avoid adverse effects. Common adverse effects include sedation, word-finding difficulty, paresthesia, increased risk for renal calculi, dizziness, anorexia, and alterations in taste.
Baclofen is a GABA-B agonist FDA-approved for the treatment of muscle spasticity related to multiple sclerosis and reversible spasticity related to spinal cord lesions and multiple sclerosis. Of note, it is approved for treatment of AUD in Europe.
In a meta-analysis of 13 RCTs, Pierce et al17 found a greater likelihood of abstinence and greater time to first lapse of drinking with baclofen compared to placebo. Interestingly, a subgroup analysis found that the positive effects were limited to trials that used 30 to 60 mg/d of baclofen, and not evident in those that used higher doses. Additionally, there was no difference between baclofen and placebo with regard to several important outcomes, including alcohol cravings, anxiety, depression, or number of total abstinent days. A review by Andrade18 proposed that individualized treatment with high-dose baclofen (30 to 300 mg/d) may be a useful second-line approach in heavy drinkers who wish to reduce their alcohol intake.
Continue to: Before starting baclofen...
Before starting baclofen, patients should be informed about its adverse effects. Common adverse effects include sedation and motor impairment. More serious but less common adverse effects include seizures, respiratory depression with sleep apnea, severe mood disorders (ie, mania, depression, or suicide risk), and mental confusion. Baclofen should be gradually discontinued, because there is some risk of clinical withdrawal symptoms (ie, agitation, confusion, seizures, or delirium).
Among the medications discussed in this section, the evidence for gabapentin and topiramate is moderate to strong, while the evidence for baclofen is overall weaker or mixed. The American Psychiatric Association’s Practice Guideline suggests offering gabapentin or topiramate to patients with moderate to severe AUD whose goal is to achieve abstinence or reduce alcohol use, or those who prefer gabapentin or topiramate or cannot tolerate or have not responded to naltrexone and acamprosate.19 Clinicians must ensure patients have no contraindications to the use of these medications. Due to the moderate quality evidence for a significant reduction in heavy drinking and increased abstinence,14,20 a practice guideline from the US Department of Veterans Affairs and US Department of Defense21 recommends topiramate as 1 of 2 first-line treatments (the other is naltrexone). This guideline suggests gabapentin as a second-line treatment for AUD.21
Gambling disorder
CASE 2
Mr. P, age 28, seeks treatment for GD and cocaine use disorder. He reports a 7-year history of sports betting that has increasingly impaired his functioning over the past year. He lost his job, savings, and familial relationships due to his impulsive and risky behavior. Mr. P also reports frequent cocaine use, about 2 to 3 days per week, mostly on the weekends. The psychiatrist tells Mr. P there is no FDA-approved pharmacologic treatment for GD or cocaine use disorder. The psychiatrist discusses the option of naltrexone as off-label treatment for GD with the goal of reducing Mr. P’s urges to gamble, and points to possible benefits for cocaine use disorder.
GD impacts approximately 0.5% of the adult US population and is often co-occurring with substance use disorders.22 It is thought to share neurobiological and clinical similarities with substance use disorders.23 There are currently no FDA-approved medications to treat the disorder. In studies of GD, treatment success with antidepressants and mood stabilizers has not been consistent,23,24 but some promising results have been published for the opioid receptor antagonist naltrexone24-29 and N-acetylcysteine (NAC).30-32
Naltrexone is thought to reduce gambling behavior and urges via downstream modulation of mesolimbic dopamine circuitry.24 It is FDA-approved for the treatment of AUD and opioid use disorder. Open-label RCTs have found a reduction in gambling urges and behavior with daily naltrexone.25-27 Dosing at 50 mg/d appears to be just as efficacious as higher doses such as 100 and 150 mg/d.27 When used as a daily as-needed medication for strong gambling urges or if an individual was planning to gamble, naltrexone 50 mg/d was not effective.28
Continue to: Naltrexone typically is started...
Naltrexone typically is started at 25 mg/d to assess tolerability and quickly titrated to 50 mg/d. When titrating, common adverse effects include nausea, vomiting, and transient elevations in transaminases. Another opioid antagonist, nalmefene, has also been studied in patients with GD. An RCT by Grant et al29 that evaluated 207 patients found that compared with placebo, nalmefene 25 mg/d for 16 weeks was associated with a significant reduction in gambling assessment scores. In Europe, nalmefene is approved for treating AUD but the oral formulation is not currently available in the US.
N-acetylcysteine is thought to potentially reverse neuronal dysfunction seen in addictive disorders by glutamatergic modulation.30 Research investigating NAC for GD is scarce. A pilot study found 16 of 27 patients with GD reduced gambling behavior with a mean dose of 1,476.9 mg/d.31 An additional study investigating the addition of NAC to behavioral therapy in nicotine-dependent individuals with pathologic gambling found a reduction in problem gambling after 18 weeks (6 weeks + 3 months follow-up).32 Common but mild adverse effects associated with NAC are nausea, vomiting, and diarrhea.
A meta-analysis by Goslar et al33 that reviewed 34 studies (1,340 participants) found pharmacologic treatments were associated with large and medium pre-post reductions in global severity, frequency, and financial loss in patients with GD. RCTs studying opioid antagonists and mood stabilizers (combined with a cognitive intervention) as well as lithium for patients with comorbid bipolar disorder and GD demonstrated promising results.33
Stimulant use disorder
There are no FDA-approved medications for stimulant use disorder. Multiple off-label options have been studied for the treatment of methamphetamine abuse and cocaine abuse.
Methamphetamine use has been expanding over the past decade with a 3.6-fold increase in positive methamphetamine screens in overdose deaths from 2011 to 2016.34 Pharmacologic options studied for OLP of methamphetamine use disorder include mirtazapine, bupropion, naltrexone, and topiramate.
Continue to: Mirtazapine
Mirtazapine is an atypical antidepressant whose mechanism of action includes modulation of the serotonin, norepinephrine, and alpha-2 adrenergic systems. It is FDA-approved for the treatment of major depressive disorder (MDD). In a randomized placebo-controlled study, mirtazapine 30 mg/d at night was found to decrease methamphetamine use for active users and led to decreased sexual risk in men who have sex with men.35 These results were supported by an additional RCT in which mirtazapine 30 mg/d significantly reduced rates of methamphetamine use vs placebo at 24 and 36 weeks despite poor medication adherence.36 Adverse effects to monitor in patients treated with mirtazapine include increased appetite, weight gain, sedation, and constipation.
Bupropion is a norepinephrine dopamine reuptake inhibitor that produces increased neurotransmission of norepinephrine and dopamine in the CNS. It is FDA-approved for the treatment of MDD and as an aid for smoking cessation. Bupropion has been studied for methamphetamine use disorder with mixed results. In a randomized placebo-controlled trial, bupropion sustained release 15
Naltrexone. Data about using oral naltrexone to treat stimulant use disorders are limited. A randomized, placebo-controlled trial by Jayaram-Lindström et al39 found naltrexone 50 mg/d significantly reduced amphetamine use compared to placebo. Additionally, naltrexone 50 and 150 mg/d have been shown to reduce cocaine use over time in combination with therapy for cocaine-dependent patients and those dependent on alcohol and cocaine.40,41
Topiramate has been studied for the treatment of cocaine use disorder. It is hypothesized that modulation of the mesocorticolimbic dopamine system may contribute to decreased cocaine cravings.42 A pilot study by Kampman et al43 found that after an 8-week titration of topiramate to 200 mg/d, individuals were more likely to achieve cocaine abstinence compared to those who receive placebo. In an RCT, Elkashef et al44 did not find topiramate assisted with increased abstinence of methamphetamine in active users at a target dose of 200 mg/d. However, it was associated with reduced relapse rates in individuals who were abstinent prior to the study.44 At a target dose of 300 mg/d, topiramate also outperformed placebo in decreasing days of cocaine use.42 Adverse effects of topiramate included paresthesia, alteration in taste, and difficulty with concentration.
Cannabis use disorder
In recent years, cannabis use in the US has greatly increased45 but no medications are FDA-approved for treating cannabis use disorder. Studies of pharmacologic options for cannabis use disorder have had mixed results.46 A meta-analysis by Bahji et al47 of 24 studies investigating pharmacotherapies for cannabis use disorder highlighted the lack of adequate evidence. In this section, we focus on a few positive trials of NAC and gabapentin.
Continue to: N-acetylcysteine
N-acetylcysteine. Studies investigating NAC 1,200 mg twice daily have been promising in adolescent and adult populations.48-50 There are some mixed results, however. A large RCT found NAC 1,200 mg twice daily was not better than placebo in helping adults achieve abstinence from cannabis.51
Gabapentin may be a viable option for treating cannabis use disorder. A pilot study by Mason et al52 found gabapentin 1,200 mg/d was more effective than placebo at reducing cannabis use among treatment-seeking adults.
When and how to consider OLP
OLP for addictive disorders is common and often necessary. This is primarily due to limitations of the FDA-approved medications and because there are no FDA-approved medications for many substance-related and addictive disorders (ie, GD, cannabis use disorder, and stimulant use disorder). When assessing pharmacotherapy options, if FDA-approved medications are available for certain diagnoses, clinicians should first consider them. The off-label medications discussed in this article are outlined in the Table.6-21,24-28,30-33,35-44,48-52

The overall level of evidence to support the use of off-label medications is lower than that of FDA-approved medications, which contributes to potential medicolegal concerns of OLP. Off-label medications should be considered when there are no FDA-approved medications available, and the decision to use off-label medications should be based on evidence from the literature and current standard of care. Additionally, OLP is necessary if a patient cannot tolerate FDA-approved medications, is not helped by FDA-approved treatments, or when there are other clinical reasons to choose a particular off-label medication. For example, if a patient has comorbid AUD and obesity (or migraines), using topiramate may be appropriate because it may target alcohol cravings and can be helpful for weight loss (and migraine prophylaxis). Similarly, for patients with AUD and neuropathic pain, using gabapentin can be considered for its dual therapeutic effects.
It is critical for clinicians to understand the landscape of off-label options for treating addictive disorders. Additional research in the form of RCTs is needed to better clarify the efficacy and adverse effects of these treatments.
Continue to: Bottom Line
Bottom Line
Off-label prescribing is prevalent in practice, including in the treatment of substance-related and addictive disorders. When considering off-label use of any medication, clinicians should review the most recent research, obtain informed consent from patients, and verify patients’ understanding of the potential risks and adverse effects associated with the particular medication.
Related Resources
- Joshi KG, Frierson RL. Off-label prescribing: how to limit your liability. Current Psychiatry. 2020;19(9):12,39. doi:10.12788/ cp.0035
- Stanciu CN, Gnanasegaram SA. Don’t balk at using medical therapy to manage alcohol use disorder. Current Psychiatry. 2017;16(2):50-52.
Drug Brand Names
Acamprosate • Campral
Baclofen • Ozobax
Bupropion • Wellbutrin, Zyban
Disulfiram • Antabuse
Gabapentin • Neurontin
Lithium • Eskalith, Lithobid
Mirtazapine • Remeron
Naltrexone • ReVia, Vivitrol
Topiramate • Topamax
1. Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc. 2012;87(10):982-990. doi:10.1016/j.mayocp.2012.04.017
2. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026. doi:10.1001/archinte.166.9.1021
3. Wang J, Jiang F, Yating Y, et al. Off-label use of antipsychotic medications in psychiatric inpatients in China: a national real-world survey. BMC Psychiatry. 2021;21(1):375. doi:10.1186/s12888-021-03374-0
4. Chen H, Reeves JH, Fincham JE, et al. Off-label use of antidepressant, anticonvulsant, and antipsychotic medications among Georgia Medicaid enrollees in 2001. J Clin Psychiatry. 2006;67(6):972-982. doi:10.4088/jcp.v67n0615
5. Ventola CL. Off-label drug information: regulation, distribution, evaluation, and related controversies. P T. 2009;34(8):428-440.
6. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736. doi:10.1001/jamainternmed.2020.0249
7. Kranzler HR, Feinn R, Morris P, et al. A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder. Addiction. 2019;114(9):1547-1555. doi:10.1111/add.14655
8. Mason BJ, Quello S, Goodell V. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
9. Fariba KA. Saadabadi A. Topiramate. StatPearls [Internet]. StatPearls Publishing LLC; 2023. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554530/
10. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685. doi:10.1016/S0140-6736(03)13370-3
11. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298(14):1641-1651. doi:10.1001/jama.298.14.1641
12. Knapp CM, Ciraulo DA, Sarid-Segal O, et al. Zonisamide, topiramate, and levetiracetam: efficacy and neuropsychological effects in alcohol use disorders. J Clin Psychopharmacol. 2015;35(1):34-42. doi:10.1097/JCP.0000000000000246
13. Kranzler HR, Covault J, Feinn R, et al. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014;171(4):445-452. doi:10.1176/appi.ajp.2013.13081014
14. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
15. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41. doi:10.1186/1471-244X-11-41
16. Tang YL, Hao W, Leggio L. Treatments for alcohol-related disorders in China: a developing story. Alcohol Alcohol. 2012;47(5):563-570. doi:10.1093/alcalc/ags066
17. Pierce M, Sutterland A, Beraha EM, et al. Efficacy, tolerability, and safety of low-dose and high-dose baclofen in the treatment of alcohol dependence: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2018;28(7):795-806. doi:10.1016/j.euroneuro.2018.03.017
18. Andrade C. Individualized, high-dose baclofen for reduction in alcohol intake in persons with high levels of consumption. J Clin Psychiatry. 2020;81(4):20f13606. doi:10.4088/JCP.20f13606
19. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
20. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
21. US Department of Veterans Affairs, US Department of Defense. Management of Substance Use Disorder (SUD) (2021). US Department of Veterans Affairs. 2021. Accessed December 24, 2022. https://www.healthquality.va.gov/guidelines/mh/sud/
22. Potenza MN, Balodis IM, Derevensky J, et al. Gambling disorder. Nat Rev Dis Primers. 2019;5(1):51. doi:10.1038/s41572-019-0099-7
23. Lupi M, Martinotti G, Acciavatti T, et al. Pharmacological treatments in gambling disorder: a qualitative review. BioMed Res Int. 2014;537306. Accessed January 18, 2023. https://www.hindawi.com/journals/bmri/2014/537306/
24. Choi SW, Shin YC, Kim DJ, et al. Treatment modalities for patients with gambling disorder. Ann Gen Psychiatry. 2017;16:23. doi:10.1186/s12991-017-0146-2
25. Kim SW, Grant JE. An open naltrexone treatment study in pathological gambling disorder. Int Clin Psychopharmacol. 2001;16(5):285-289. doi:10.1097/00004850-200109000-00006
26. Kim SW, Grant JE, Adson DE, et al. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914-921. doi:10.1016/s0006-3223(01)01079-4
27. Grant JE, Kim SW, Hartman BK. A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. J Clin Psychiatry. 2008;69(5):783-789. doi:10.4088/jcp.v69n0511
28. Kovanen L, Basnet S, Castrén S, et al. A randomised, double-blind, placebo-controlled trial of as-needed naltrexone in the treatment of pathological gambling. Eur Addict Res. 2016;22(2):70-79. doi:10.1159/000435876
29. Grant JE, Potenza MN, Hollander E, et al. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163(2):303-312. doi:10.1176/appi.ajp.163.2.303
30. Tomko RL, Jones JL, Gilmore AK, et al. N-acetylcysteine: a potential treatment for substance use disorders. Current Psychiatry. 2018;17(6):30-36,41-52,55.
31. Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62(6):652-657. doi:10.1016/j.biopsych.2006.11.021
32. G
33. Goslar M, Leibetseder M, Muench HM, et al. Pharmacological treatments for disordered gambling: a meta-analysis. J Gambling Stud. 2019;35(2):415-445. doi:10.1007/s10899-018-09815-y
34. Hedegaard H, Miniño AM, Spencer MR, et al. Drug overdose deaths in the United States, 1999-2020. Centers for Disease Control and Prevention. December 30, 2021. Accessed December 11, 2022. https://stacks.cdc.gov/view/cdc/112340
35. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175. doi:10.1001/archgenpsychiatry.2011.124
36. Coffin PO, Santos GM, Hern J, et al. Effects of mirtazapine for methamphetamine use disorder among cisgender men and transgender women who have sex with men: a placebo-controlled randomized clinical trial. JAMA Psychiatry. 2020;77(3):246-255. doi:10.1001/jamapsychiatry.2019.3655
37. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Dependence. 2008;96(3):222-232. doi:10.1016/j.drugalcdep.2008.03.010
38. Trivedi MH, Walker R, Ling W, et al. Bupropion and naltrexone in methamphetamine use disorder. N Engl J Med. 2021;384(2):140-153. doi:10.1056/NEJMoa2020214
39. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448. doi:10.1176/appi.ajp.2008.08020304
40. Schmitz JM, Stotts AL, Rhoades HM, et al. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26(2):167-180. doi:10.1016/s0306-4603(00)00098-8
41. Oslin DW, Pettinati HM, Volpicelli JR, et al. The effects of naltrexone on alcohol and cocaine use in dually addicted patients. J Subst Abuse Treat. 1999;16(2):163-167. doi:10.1016/s0740-5472(98)00039-7
42. Johnson BA, Ait-Daoud N, Wang XQ, et al. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry. 2013;70(12):1338-1346. doi:10.1001/jamapsychiatry.2013.2295
43. Kampman KM, Pettinati H, Lynch KG, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Dependence. 2004;75(3):233-240. doi:10.1016/j.drugalcdep.2004.03.008
44. Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107(7):1297-1306. doi:10.1111/j.1360-0443.2011.03771.x
45. Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology. 2018;43(1):195-212.
46. Brezing CA, Levin FR. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology. 2018;43(1):173-194. doi:10.1038/npp.2017.198
47. Bahji A, Meyyappan AC, Hawken ER, et al. Pharmacotherapies for cannabis use disorder: a systematic review and network meta-analysis. Intl J Drug Policy. 2021;97:103295. doi:10.1016/j.drugpo.2021.103295
48. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812. doi:10.1176/appi.ajp.2012.12010055
49. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791. doi:10.1016/j.addbeh.2012.11.003
50. McClure EA, Sonne SC, Winhusen T, et al. Achieving cannabis cessation—evaluating N-acetylcysteine treatment (ACCENT): design and implementation of a multi-site, randomized controlled study in the National Institute on Drug Abuse Clinical Trials Network. Contemp Clin Trials. 2014;39(2):211-223. doi:10.1016/j.cct.2014.08.011
51. Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Dependence. 2017;177:249-257. doi:10.1016/j.drugalcdep.2017.04.020
52. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689-1698. doi:10.1038/npp.2012.14
1. Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc. 2012;87(10):982-990. doi:10.1016/j.mayocp.2012.04.017
2. Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166(9):1021-1026. doi:10.1001/archinte.166.9.1021
3. Wang J, Jiang F, Yating Y, et al. Off-label use of antipsychotic medications in psychiatric inpatients in China: a national real-world survey. BMC Psychiatry. 2021;21(1):375. doi:10.1186/s12888-021-03374-0
4. Chen H, Reeves JH, Fincham JE, et al. Off-label use of antidepressant, anticonvulsant, and antipsychotic medications among Georgia Medicaid enrollees in 2001. J Clin Psychiatry. 2006;67(6):972-982. doi:10.4088/jcp.v67n0615
5. Ventola CL. Off-label drug information: regulation, distribution, evaluation, and related controversies. P T. 2009;34(8):428-440.
6. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736. doi:10.1001/jamainternmed.2020.0249
7. Kranzler HR, Feinn R, Morris P, et al. A meta-analysis of the efficacy of gabapentin for treating alcohol use disorder. Addiction. 2019;114(9):1547-1555. doi:10.1111/add.14655
8. Mason BJ, Quello S, Goodell V. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. doi:10.1001/jamainternmed.2013.11950
9. Fariba KA. Saadabadi A. Topiramate. StatPearls [Internet]. StatPearls Publishing LLC; 2023. Accessed December 22, 2022. https://www.ncbi.nlm.nih.gov/books/NBK554530/
10. Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677-1685. doi:10.1016/S0140-6736(03)13370-3
11. Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298(14):1641-1651. doi:10.1001/jama.298.14.1641
12. Knapp CM, Ciraulo DA, Sarid-Segal O, et al. Zonisamide, topiramate, and levetiracetam: efficacy and neuropsychological effects in alcohol use disorders. J Clin Psychopharmacol. 2015;35(1):34-42. doi:10.1097/JCP.0000000000000246
13. Kranzler HR, Covault J, Feinn R, et al. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014;171(4):445-452. doi:10.1176/appi.ajp.2013.13081014
14. Blodgett JC, Del Re AC, Maisel NC, et al. A meta-analysis of topiramate’s effects for individuals with alcohol use disorders. Alcohol Clin Exp Res. 2014;38(6):1481-1488. doi:10.1111/acer.12411
15. Paparrigopoulos T, Tzavellas E, Karaiskos D, et al. Treatment of alcohol dependence with low-dose topiramate: an open-label controlled study. BMC Psychiatry. 2011;11:41. doi:10.1186/1471-244X-11-41
16. Tang YL, Hao W, Leggio L. Treatments for alcohol-related disorders in China: a developing story. Alcohol Alcohol. 2012;47(5):563-570. doi:10.1093/alcalc/ags066
17. Pierce M, Sutterland A, Beraha EM, et al. Efficacy, tolerability, and safety of low-dose and high-dose baclofen in the treatment of alcohol dependence: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2018;28(7):795-806. doi:10.1016/j.euroneuro.2018.03.017
18. Andrade C. Individualized, high-dose baclofen for reduction in alcohol intake in persons with high levels of consumption. J Clin Psychiatry. 2020;81(4):20f13606. doi:10.4088/JCP.20f13606
19. Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association Practice Guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86-90. doi:10.1176/appi.ajp.2017.1750101
20. Jonas DE, Amick HR, Feltner C, et al. Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA. 2014;311(18):1889-1900. doi:10.1001/jama.2014.3628
21. US Department of Veterans Affairs, US Department of Defense. Management of Substance Use Disorder (SUD) (2021). US Department of Veterans Affairs. 2021. Accessed December 24, 2022. https://www.healthquality.va.gov/guidelines/mh/sud/
22. Potenza MN, Balodis IM, Derevensky J, et al. Gambling disorder. Nat Rev Dis Primers. 2019;5(1):51. doi:10.1038/s41572-019-0099-7
23. Lupi M, Martinotti G, Acciavatti T, et al. Pharmacological treatments in gambling disorder: a qualitative review. BioMed Res Int. 2014;537306. Accessed January 18, 2023. https://www.hindawi.com/journals/bmri/2014/537306/
24. Choi SW, Shin YC, Kim DJ, et al. Treatment modalities for patients with gambling disorder. Ann Gen Psychiatry. 2017;16:23. doi:10.1186/s12991-017-0146-2
25. Kim SW, Grant JE. An open naltrexone treatment study in pathological gambling disorder. Int Clin Psychopharmacol. 2001;16(5):285-289. doi:10.1097/00004850-200109000-00006
26. Kim SW, Grant JE, Adson DE, et al. Double-blind naltrexone and placebo comparison study in the treatment of pathological gambling. Biol Psychiatry. 2001;49(11):914-921. doi:10.1016/s0006-3223(01)01079-4
27. Grant JE, Kim SW, Hartman BK. A double-blind, placebo-controlled study of the opiate antagonist naltrexone in the treatment of pathological gambling urges. J Clin Psychiatry. 2008;69(5):783-789. doi:10.4088/jcp.v69n0511
28. Kovanen L, Basnet S, Castrén S, et al. A randomised, double-blind, placebo-controlled trial of as-needed naltrexone in the treatment of pathological gambling. Eur Addict Res. 2016;22(2):70-79. doi:10.1159/000435876
29. Grant JE, Potenza MN, Hollander E, et al. Multicenter investigation of the opioid antagonist nalmefene in the treatment of pathological gambling. Am J Psychiatry. 2006;163(2):303-312. doi:10.1176/appi.ajp.163.2.303
30. Tomko RL, Jones JL, Gilmore AK, et al. N-acetylcysteine: a potential treatment for substance use disorders. Current Psychiatry. 2018;17(6):30-36,41-52,55.
31. Grant JE, Kim SW, Odlaug BL. N-acetyl cysteine, a glutamate-modulating agent, in the treatment of pathological gambling: a pilot study. Biol Psychiatry. 2007;62(6):652-657. doi:10.1016/j.biopsych.2006.11.021
32. G
33. Goslar M, Leibetseder M, Muench HM, et al. Pharmacological treatments for disordered gambling: a meta-analysis. J Gambling Stud. 2019;35(2):415-445. doi:10.1007/s10899-018-09815-y
34. Hedegaard H, Miniño AM, Spencer MR, et al. Drug overdose deaths in the United States, 1999-2020. Centers for Disease Control and Prevention. December 30, 2021. Accessed December 11, 2022. https://stacks.cdc.gov/view/cdc/112340
35. Colfax GN, Santos GM, Das M, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiatry. 2011;68(11):1168-1175. doi:10.1001/archgenpsychiatry.2011.124
36. Coffin PO, Santos GM, Hern J, et al. Effects of mirtazapine for methamphetamine use disorder among cisgender men and transgender women who have sex with men: a placebo-controlled randomized clinical trial. JAMA Psychiatry. 2020;77(3):246-255. doi:10.1001/jamapsychiatry.2019.3655
37. Shoptaw S, Heinzerling KG, Rotheram-Fuller E, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Dependence. 2008;96(3):222-232. doi:10.1016/j.drugalcdep.2008.03.010
38. Trivedi MH, Walker R, Ling W, et al. Bupropion and naltrexone in methamphetamine use disorder. N Engl J Med. 2021;384(2):140-153. doi:10.1056/NEJMoa2020214
39. Jayaram-Lindström N, Hammarberg A, Beck O, et al. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165(11):1442-1448. doi:10.1176/appi.ajp.2008.08020304
40. Schmitz JM, Stotts AL, Rhoades HM, et al. Naltrexone and relapse prevention treatment for cocaine-dependent patients. Addict Behav. 2001;26(2):167-180. doi:10.1016/s0306-4603(00)00098-8
41. Oslin DW, Pettinati HM, Volpicelli JR, et al. The effects of naltrexone on alcohol and cocaine use in dually addicted patients. J Subst Abuse Treat. 1999;16(2):163-167. doi:10.1016/s0740-5472(98)00039-7
42. Johnson BA, Ait-Daoud N, Wang XQ, et al. Topiramate for the treatment of cocaine addiction: a randomized clinical trial. JAMA Psychiatry. 2013;70(12):1338-1346. doi:10.1001/jamapsychiatry.2013.2295
43. Kampman KM, Pettinati H, Lynch KG, et al. A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Dependence. 2004;75(3):233-240. doi:10.1016/j.drugalcdep.2004.03.008
44. Elkashef A, Kahn R, Yu E, et al. Topiramate for the treatment of methamphetamine addiction: a multi-center placebo-controlled trial. Addiction. 2012;107(7):1297-1306. doi:10.1111/j.1360-0443.2011.03771.x
45. Hasin DS. US epidemiology of cannabis use and associated problems. Neuropsychopharmacology. 2018;43(1):195-212.
46. Brezing CA, Levin FR. The current state of pharmacological treatments for cannabis use disorder and withdrawal. Neuropsychopharmacology. 2018;43(1):173-194. doi:10.1038/npp.2017.198
47. Bahji A, Meyyappan AC, Hawken ER, et al. Pharmacotherapies for cannabis use disorder: a systematic review and network meta-analysis. Intl J Drug Policy. 2021;97:103295. doi:10.1016/j.drugpo.2021.103295
48. Gray KM, Carpenter MJ, Baker NL, et al. A double-blind randomized controlled trial of N-acetylcysteine in cannabis-dependent adolescents. Am J Psychiatry. 2012;169(8):805-812. doi:10.1176/appi.ajp.2012.12010055
49. Roten AT, Baker NL, Gray KM. Marijuana craving trajectories in an adolescent marijuana cessation pharmacotherapy trial. Addict Behav. 2013;38(3):1788-1791. doi:10.1016/j.addbeh.2012.11.003
50. McClure EA, Sonne SC, Winhusen T, et al. Achieving cannabis cessation—evaluating N-acetylcysteine treatment (ACCENT): design and implementation of a multi-site, randomized controlled study in the National Institute on Drug Abuse Clinical Trials Network. Contemp Clin Trials. 2014;39(2):211-223. doi:10.1016/j.cct.2014.08.011
51. Gray KM, Sonne SC, McClure EA, et al. A randomized placebo-controlled trial of N-acetylcysteine for cannabis use disorder in adults. Drug Alcohol Dependence. 2017;177:249-257. doi:10.1016/j.drugalcdep.2017.04.020
52. Mason BJ, Crean R, Goodell V, et al. A proof-of-concept randomized controlled study of gabapentin: effects on cannabis use, withdrawal and executive function deficits in cannabis-dependent adults. Neuropsychopharmacology. 2012;37(7):1689-1698. doi:10.1038/npp.2012.14