User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
A pivot in training: My path to reproductive psychiatry
In March 2020, as I was wheeling my patient into the operating room to perform a Caesarean section, covered head-to-toe in COVID personal protective equipment, my phone rang. It was Jody Schindelheim, MD, Director of the Psychiatry Residency Program at Tufts Medical Center in Boston, calling to offer me a PGY-2 spot in their program.
As COVID upended the world, I was struggling with my own major change. My path had been planned since before medical school: I would grind through a 4-year OB/GYN residency, complete a fellowship, and establish myself as a reproductive endocrinology and infertility specialist. My personal statement emphasized my dream that no woman should be made to feel useless based on infertility. OB/GYN, genetics, and ultrasound were my favorite rotations at the Albert Einstein College of Medicine in the Bronx.
However, 6 months into my OB/GYN intern year, I grew curious about the possibility of a future in reproductive psychiatry and women’s mental health. This decision was not easy. As someone who loved the adrenaline rush of delivering babies and performing surgery, I had paid little attention to psychiatry in medical school. However, my experience in gynecologic oncology in January 2020 made me realize my love of stories and trauma-informed care. I recall a woman, cachectic with only days left to live due to ovarian cancer, talking to me about her trauma and the power of her lifelong partner. Another woman, experiencing complications from chemotherapy to treat fallopian tube cancer, shared about her coping skill of chair yoga.
Fulfilling an unmet need
As I spent time with these 2 women and heard their stories, I felt compelled to help them with these psychological challenges. As a gynecologist, I addressed their physical needs, but not their personal needs. I spoke to many psychiatrists, including reproductive psychiatrists, in New York, who shared their stories and taught me about the prevalence of postpartum depression and psychosis. After caring for hundreds of pregnant and postpartum women in the Bronx, I thought about the unmet need for women’s mental health and how this career change could still fulfill my purpose of helping women feel empowered regardless of their fertility status.
In the inpatient and outpatient settings at Tufts, I have loved hearing my patients’ stories and providing continuity of care with medical management and therapy. My mentors in reproductive psychiatry inspired me to create the Reproductive Psychiatry Trainee Interest Group (https://www.repropsychtrainees.com), a national group for the burgeoning field that now has more than 650 members. With monthly lectures, journal clubs, and book clubs, I have surrounded myself with like-minded individuals who love learning about the perinatal, postpartum, and perimenopausal experiences.
As I prepare to begin a full-time faculty position in psychiatry at the University of Pennsylvania, I know I have found my joy and my calling. I once feared the life of a psychiatrist would be too sedentary for someone accustomed to the pace of OB/GYN. Now I know that my patients’ stories are all the motivation I need.
In March 2020, as I was wheeling my patient into the operating room to perform a Caesarean section, covered head-to-toe in COVID personal protective equipment, my phone rang. It was Jody Schindelheim, MD, Director of the Psychiatry Residency Program at Tufts Medical Center in Boston, calling to offer me a PGY-2 spot in their program.
As COVID upended the world, I was struggling with my own major change. My path had been planned since before medical school: I would grind through a 4-year OB/GYN residency, complete a fellowship, and establish myself as a reproductive endocrinology and infertility specialist. My personal statement emphasized my dream that no woman should be made to feel useless based on infertility. OB/GYN, genetics, and ultrasound were my favorite rotations at the Albert Einstein College of Medicine in the Bronx.
However, 6 months into my OB/GYN intern year, I grew curious about the possibility of a future in reproductive psychiatry and women’s mental health. This decision was not easy. As someone who loved the adrenaline rush of delivering babies and performing surgery, I had paid little attention to psychiatry in medical school. However, my experience in gynecologic oncology in January 2020 made me realize my love of stories and trauma-informed care. I recall a woman, cachectic with only days left to live due to ovarian cancer, talking to me about her trauma and the power of her lifelong partner. Another woman, experiencing complications from chemotherapy to treat fallopian tube cancer, shared about her coping skill of chair yoga.
Fulfilling an unmet need
As I spent time with these 2 women and heard their stories, I felt compelled to help them with these psychological challenges. As a gynecologist, I addressed their physical needs, but not their personal needs. I spoke to many psychiatrists, including reproductive psychiatrists, in New York, who shared their stories and taught me about the prevalence of postpartum depression and psychosis. After caring for hundreds of pregnant and postpartum women in the Bronx, I thought about the unmet need for women’s mental health and how this career change could still fulfill my purpose of helping women feel empowered regardless of their fertility status.
In the inpatient and outpatient settings at Tufts, I have loved hearing my patients’ stories and providing continuity of care with medical management and therapy. My mentors in reproductive psychiatry inspired me to create the Reproductive Psychiatry Trainee Interest Group (https://www.repropsychtrainees.com), a national group for the burgeoning field that now has more than 650 members. With monthly lectures, journal clubs, and book clubs, I have surrounded myself with like-minded individuals who love learning about the perinatal, postpartum, and perimenopausal experiences.
As I prepare to begin a full-time faculty position in psychiatry at the University of Pennsylvania, I know I have found my joy and my calling. I once feared the life of a psychiatrist would be too sedentary for someone accustomed to the pace of OB/GYN. Now I know that my patients’ stories are all the motivation I need.
In March 2020, as I was wheeling my patient into the operating room to perform a Caesarean section, covered head-to-toe in COVID personal protective equipment, my phone rang. It was Jody Schindelheim, MD, Director of the Psychiatry Residency Program at Tufts Medical Center in Boston, calling to offer me a PGY-2 spot in their program.
As COVID upended the world, I was struggling with my own major change. My path had been planned since before medical school: I would grind through a 4-year OB/GYN residency, complete a fellowship, and establish myself as a reproductive endocrinology and infertility specialist. My personal statement emphasized my dream that no woman should be made to feel useless based on infertility. OB/GYN, genetics, and ultrasound were my favorite rotations at the Albert Einstein College of Medicine in the Bronx.
However, 6 months into my OB/GYN intern year, I grew curious about the possibility of a future in reproductive psychiatry and women’s mental health. This decision was not easy. As someone who loved the adrenaline rush of delivering babies and performing surgery, I had paid little attention to psychiatry in medical school. However, my experience in gynecologic oncology in January 2020 made me realize my love of stories and trauma-informed care. I recall a woman, cachectic with only days left to live due to ovarian cancer, talking to me about her trauma and the power of her lifelong partner. Another woman, experiencing complications from chemotherapy to treat fallopian tube cancer, shared about her coping skill of chair yoga.
Fulfilling an unmet need
As I spent time with these 2 women and heard their stories, I felt compelled to help them with these psychological challenges. As a gynecologist, I addressed their physical needs, but not their personal needs. I spoke to many psychiatrists, including reproductive psychiatrists, in New York, who shared their stories and taught me about the prevalence of postpartum depression and psychosis. After caring for hundreds of pregnant and postpartum women in the Bronx, I thought about the unmet need for women’s mental health and how this career change could still fulfill my purpose of helping women feel empowered regardless of their fertility status.
In the inpatient and outpatient settings at Tufts, I have loved hearing my patients’ stories and providing continuity of care with medical management and therapy. My mentors in reproductive psychiatry inspired me to create the Reproductive Psychiatry Trainee Interest Group (https://www.repropsychtrainees.com), a national group for the burgeoning field that now has more than 650 members. With monthly lectures, journal clubs, and book clubs, I have surrounded myself with like-minded individuals who love learning about the perinatal, postpartum, and perimenopausal experiences.
As I prepare to begin a full-time faculty position in psychiatry at the University of Pennsylvania, I know I have found my joy and my calling. I once feared the life of a psychiatrist would be too sedentary for someone accustomed to the pace of OB/GYN. Now I know that my patients’ stories are all the motivation I need.
Using apps in clinical practice: 8 studies
COVID-19’s increased demand on the mental health care delivery system led to expanded utilization of technology-based solutions, including digital tools to deliver care.1 Technology-based solutions include both synchronous telehealth (eg, real-time interactive audio/video visits) and asynchronous tools such as smartphone applications (apps). Both real-time telehealth and apps continue to gain popularity. More than 10,000 mental health–related apps are available, and that number continues to rise.2 Numerous web- or mobile-based apps are available to aid in the treatment of various psychiatric conditions, including generalized anxiety disorder (GAD), major depressive disorder, insomnia, and posttraumatic stress disorder (PTSD).
Clinicians may find it challenging to choose the best psychiatry-related apps to recommend to patients. This dilemma calls for an approach to help clinicians select apps that are safe and effective.2 The American Psychiatric Association provides information to help mental health professionals navigate these issues and identify which aspects to consider when selecting an app for clinical use.3 The M-Health Index and Navigation Database also provides a set of objective evaluative criteria and offers guidance on choosing apps.4
In this article, we review 8 randomized controlled trials (RCTs) of mental health–related apps. We took several steps to ensure the RCTs we included were impactful and meaningful. First, we conducted a general search using mainstream search engines to assess which psychiatric apps were most popular for use in clinical practice. Using this list, we conducted a scholarly search engine query of RCTs using the name of the apps as a search parameter along with the following keywords: “mobile,” “web,” “applications,” and “psychiatry.” This search yielded approximately 50 results, which were narrowed down based on content and interest to a list of 8 articles (Table5-12). These articles were then graded using the limitations of each study as the primary substrate for evaluation.

1. Linardon J, Shatte A, Rosato J, et al. Efficacy of a transdiagnostic cognitive-behavioral intervention for eating disorder psychopathology delivered through a smartphone app: a randomized controlled trial. Psychol Med. 2022;52(9):1679-1690. doi:10.1017/S0033291720003426
Many patients with eating disorders are unable to receive effective treatment due to problems with accessing health care. Smartphone apps may help bridge the treatment gap for patients in this position. Linardon et al5 developed an app that uses the principles of cognitive-behavioral therapy (CBT) for treating eating disorders and conducted this study to evaluate its effectiveness.
Study design
- This RCT assigned individuals who reported episodes of binge eating to a group that used a mobile app (n = 197) or to a waiting list (n = 195). At baseline, 42% of participants exhibited diagnostic-level symptoms of bulimia nervosa and 31% had symptoms of binge-eating disorder.
- Assessments took place at baseline, Week 4, and Week 8.
- The primary outcome was global levels of eating disorder psychopathology.
- Secondary outcomes were other eating disorder symptoms, impairment, and distress.
Outcomes
- Compared to the control group, participants who used the mobile app reported greater reductions in global eating disorder psychopathology (d = -0.80).
- Significant effects were also observed for secondary outcomes except compensatory behavior frequency.
- Overall, participants reported they were satisfied with the app.
Continue to: Conclusions/limitations
Conclusions/limitations
- Findings show this app could potentially be a cost-effective and easily accessible option for patients who cannot receive standard treatment for eating disorders.
- Limitations: The overall posttest attrition rate was 35%.
2. Christoforou M, Sáez Fonseca JA, Tsakanikos E. Two novel cognitive behavioral therapy–based mobile apps for agoraphobia: randomized controlled trial. J Med Internet Res. 2017;19(11):e398. doi:10.2196/jmir.7747
CBT is generally the most accepted first-line treatment for agoraphobia. However, numerous barriers to obtaining CBT can prevent successful treatment. Limited research has evaluated the efficacy of apps for treating agoraphobia. Christoforou et al6 conducted an RCT to determine the effectiveness of a self-guided smartphone app for improving agoraphobic symptoms, compared to a mobile app used to treat anxiety.
Study design
- Participants (N = 170) who self-identified as having agoraphobia were randomly assigned to use a smartphone app designed to target agoraphobia (Agoraphobia Free) or a smartphone app designed to help with symptoms of anxiety (Stress Free) for 12 weeks. Both apps were based on established cognitive behavioral principles.
- Assessment occurred at baseline, midpoint, and end point.
- The primary outcome was symptom severity as measured by the Panic and Agoraphobia Scale (PAS).
Outcomes
- Both groups experienced statistically significant improvements in symptom severity over time. The differences in PAS score were -5.97 (95% CI, -8.49 to -3.44, P < .001) for Agoraphobia Free and -6.35 (95% CI, -8.82 to -3.87, P < .001) for Stress Free.
- There were no significant between-group differences in symptom severity.
Continue to: Conclusions/limitations
Conclusions/limitations
- This study is the first RCT to show that patients with agoraphobia could benefit from mobile-based interventions.
- Limitations: There was no waitlist control group. Limited information was collected about participant characteristics; there were no data on comorbid disorders, other psychological or physiological treatments, or other demographic characteristics such as ethnicity or computer literacy.
3. Everitt N, Broadbent J, Richardson B, et al. Exploring the features of an app-based just-in-time intervention for depression. J Affect Disord. 2021;291:279-287. doi:10.1016/j.jad.2021.05.021
The apps MoodTracker, ImproveYourMood, and ImproveYourMood+ deliver content “just in time” (in response to acute negative symptoms) to help patients with depression. In an RCT, Everitt et al7 evaluated delivering acute care for depressive mood states via a smartphone app. They sought to delineate whether symptom improvement was due to microintervention content, mood augmentation, or just-in-time prompts to use content.
Study design
- Participants (N = 235) from the general population who said they wanted to improve their mood were randomly assigned to a waitlist control group (n = 55) or 1 of 3 intervention groups: MoodTracker (monitoring-only; n = 58), ImproveYourMood (monitoring and content; n = 62), or ImproveYourMood+ (monitoring, content, and prompts; n = 60).
- The microintervention content provided by these apps consisted of 4 audio files of brief (2- to 3-minute) mindfulness and relaxation exercises. Participants used the assigned app for 3 weeks.
- Depressive symptoms, anxiety symptoms, and negative automatic thoughts were assessed at baseline, immediately following the intervention, and 1 month after the intervention using the 9-item Patient Health Questionnaire (PHQ-9), 7-item GAD scale (GAD-7), and 8-item Automatic Thoughts Questionnaire, respectively.
Outcomes
- Compared to the waitlist control group, participants in the ImproveYourMood group showed greater declines in depressive symptoms and anxiety symptoms (at follow-up only), and negative automatic thoughts (at both postintervention and follow-up).
- Those in the ImproveYourMood+ group only showed significantly greater improvements for automatic negative thoughts (at postintervention).
- MoodTracker participants did not differ from waitlist controls for any variables at any timepoints.
Continue to: Conclusions/limitations
Conclusions/limitations
- This study suggests that using microinterventions in acute settings can effectively reduce depressive symptoms both as they occur, and 1 to 2 months later.
- Limitations: The study featured a naturalistic design, where participants self-selected whether they wanted to use the program. Participants did not complete eligibility assessments or receive compensation, and the study had high dropout rates, ranging from 20% for the waitlist control group to 67% for the ImproveYourMood+ group.
4. McLean C, Davis CA, Miller M, et al. The effects of an exposure-based mobile app on symptoms of posttraumatic stress disorder in veterans: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2022;10(11):e38951. doi:10.2196/38951
Veterans with PTSD face barriers when receiving trauma-focused treatments such as exposure therapy or CBT. Smartphone apps may help veterans self-treat and self-manage their PTSD symptoms. McLean et al8 studied the efficacy of Renew, a smartphone app that uses exposure therapy and social support to treat PTSD.
Study design
- In this pilot RCT, 93 veterans with clinically significant PTSD symptoms were randomly assigned to use the Renew app with and without support from a research staff member (active use group) or to a waitlist (delayed use group) for 6 weeks.
- The PTSD Checklist for DSM-5 (PCL-5) was used to measure PTSD symptoms at preintervention, postintervention, and 6-week follow-up.
- Most participants (69%) were women, and the mean age was 49.
Outcomes
- Compared to the delayed use group, participants in the active use group experienced a larger decrease in PCL-5 score (-6.14 vs -1.84). However, this difference was not statistically significant (P = .29), and the effect size was small (d = -0.39).
- There was no difference in engagement with the app between participants who received support from a research staff member and those who did not receive such support.
Continue to: Conclusions/limitations
Conclusions/limitations
- Renew may show promise as a tool to reduce PTSD symptoms in veterans.
- Educating family and friends on how to best support a patient using a mobile mental health app may help improve the efficacy of Renew and increase app engagement.
- Limitations: Because the study was conducted in veterans, the results may not be generalizable to other populations. Because most data collection occurred during the first wave of the COVID-19 pandemic in the United States, COVID-19–related stress may have impacted PTSD symptoms, app engagement, or outcomes.
5. Graham AK, Greene CJ, Kwasny MJ, et al. Coached mobile app platform for the treatment of depression and anxiety among primary care patients: a randomized clinical trial. JAMA Psychiatry. 2020;77(9):906-914. doi:10.1001/jamapsychiatry.2020.1011
Many cases of depression and anxiety are initially treated in primary care settings. However, these settings may have limited resources and inadequate training, and mobile interventions might be helpful to augment patient care. Graham et al9 studied the mobile platform IntelliCare to determine its efficacy as a tool to be used in primary care settings to treat depression and anxiety.
Study design
- This RCT randomly assigned adult primary care patients (N = 146) who screened positive for depression on the PHQ-9 (score ≥10) or anxiety on the GAD-7 (score ≥8) to the coach-supported IntelliCare platform, which consisted of 5 clinically focused apps, or to a waitlist control group. Interventions were delivered over 8 weeks.
- Overall, 122 (83.6%) patients were diagnosed with depression and 131 (89.7%) were diagnosed with anxiety.
- The primary outcomes were changes in depression (as measured by change in PHQ-9 score) and anxiety (change in GAD-7 score) during the intervention period.
Outcomes
- Participants who used the IntelliCare platform had a greater reduction in depression and anxiety symptoms compared to waitlist controls, and changes were sustained over 2-month follow-up.
- The least square means (LSM) difference in depression scores at Week 4 was 2.91 (SE = 0.83; d = 0.43) and at Week 8 was 4.37 (SE = 0.83; d = 0.64). The LSM difference in anxiety scores at Week 4 was 2.51 (SE = 0.78; d = 0.41) and at Week 8 was 3.33 (SE = 0.76; d = 0.55).
- A median number of 93 and 98 sessions among participants with depression and anxiety were recorded, respectively, indicating high use of the IntelliCare platform.
Continue to: Conclusions/limitations
Conclusions/limitations
- The IntelliCare platform was shown to be effective in reducing depression and anxiety among primary care patients. Simple apps can be bundled together and used by patients in conjunction to treat their individual needs.
- Limitations: The study had a limited follow-up period and did not record participants’ use of other apps. Slightly more than one-half (56%) of participants were taking an antidepressant.
6. Wilhelm S, Weingarden H, Greenberg JL, et al. Efficacy of app-based cognitive behavioral therapy for body dysmorphic disorder with coach support: initial randomized controlled clinical trial. Psychother Psychosom. 2022;91(4):277-285. doi:10.1159/000524628
Body dysmorphic disorder (BDD) is a severe yet undertreated disorder. Apps can improve access to treatment for patients experiencing BDD. Wilhelm et al10 studied the usability and efficacy of a coach-supported app called Perspectives that was specifically designed for treating BDD. Perspectives provide CBT in 7 modules: psychoeducation, cognitive restructuring, exposure, response prevention, mindfulness, attention retraining, and relapse prevention.
Study design
- Adults (N = 80) with primary BDD were assigned to use the Perspectives app for 12 weeks or to a waitlist control group. Participants were predominately female (84%) and White (71%), with a mean age of 27.
- Coaches promoted engagement and answered questions via in-app messaging and phone calls.
- Blinded independent evaluators used the Yale-Brown Obsessive Compulsive Scale Modified for BDD (BDD-YBOCS) to measure BDD severity at baseline, midtreatment (Week 6), and end of treatment (Week 12).
- Secondary outcomes included BDD-related insight, depression, quality of life, and functioning. Various scales were used to measure these outcomes.
Outcomes
- In intent-to-treat analyses, patients who received CBT via the Perspectives app had significantly lower BDD severity at the end of treatment compared to the waitlist control group, with a mean (SD) BDD-YBOCS score of 16.8 (7.5) vs 26.7 (6.2), with P < .001 and d = 1.44.
- Slightly more than one-half (52%) of those who used Perspectives achieved full or partial remission, compared to 8% in the waitlist control group.
Continue to: Conclusions/limitations
Conclusions/limitations
- CBT delivered via the Perspectives app and a coach proved to be effective treatment for adults with BDD.
- Adoption of the application was relatively high; 86% of Perspectives users were very or mostly satisfied.
- Limitations: Because the participants in this study were predominantly female and White, the findings might not be generalizable to other populations.
7. Kuhn E, Miller KE, Puran D, et al. A pilot randomized controlled trial of the Insomnia Coach mobile app to assess its feasibility, acceptability, and potential efficacy. Behav Ther. 2022;53(3):440-457. doi:10.1016/j.beth.2021.11.003
Insomnia remains a substantial problem among military veterans. First-line treatments for the disorder are sleep hygiene modification and CBT. Access to CBT is limited, especially for veterans. Kuhn et al11 studied the effectiveness of using Insomnia Coach, a CBT for insomnia–based app, to improve insomnia symptoms.
Study design
- Fifty US veterans who were mostly male (58%) with a mean age of 44.5 and moderate insomnia symptoms were randomized to use Insomnia Coach (n = 25) or to a waitlist control group (n = 25) for 6 weeks.
- All participants completed self-report measures and sleep diaries at baseline, posttreatment, and follow-up (12 weeks). Those who used the app (n = 15) completed a qualitative interview at posttreatment.
Outcomes
- At posttreatment, 28% of participants who used Insomnia Coach achieved clinically significant improvement, vs 4% of waitlist control participants. There was also a significant treatment effect on daytime sleep-related impairment (P = .044, d = -0.6).
- Additional treatment effects emerged at follow-up for insomnia severity, sleep onset latency, global sleep quality, and depression symptoms.
- Based on self-reports and qualitative interview responses, participants’ perceptions of Insomnia Coach were favorable. Three-fourths of participants used the app through 6 weeks and engaged with active elements.
Continue to: Conclusions/limitations
Conclusions/limitations
- Insomnia Coach may provide an accessible and convenient public health intervention for patients who aren’t receiving adequate care or CBT.
- Limitations: Because this study evaluated only veterans, the findings might not be generalizable to other populations.
8. Dahne J, Lejuez CW, Diaz VA, et al. Pilot randomized trial of a self-help behavioral activation mobile app for utilization in primary care. Behav Ther. 2019;50(4):817-827. doi:10.1016/j.beth.2018.12.003
Previous mobile technologies have shown the ability to treat depression in primary care settings. Moodivate is a self-help mobile app based on the Brief Behavioral Activation Treatment for Depression, which is an evidence-based treatment. This app is designed to help the user reengage in positive, nondepressed activities by identifying, scheduling, and completing activities. Dahne et al12 investigated the feasibility and efficacy of Moodivate for depressive symptoms in primary care patients.
Study design
- Participants (N = 52) were recruited from primary care practices and randomized 2:2:1 to receive Moodivate, a CBT-based mobile app called MoodKit, or treatment as usual (no app). All participants had an initial PHQ-8 score >10.
- Participants completed assessments of depressive symptoms (PHQ-8) weekly for 8 weeks.
- App analytics data were captured to examine if the use of Moodivate was feasible. (Analytics were not available for MoodKit).
Outcomes
- Participants who used Moodivate had a mean (SD) of 46.76 (30.10) sessions throughout the trial, spent 3.50 (2.76) minutes using the app per session, and spent 120.76 (101.02) minutes using the app in total.
- Nearly 70% of Moodivate participants continued to use the app 1 month after trial enrollment and 50% at the end of the 8-week follow-up period.
- Compared to the treatment as usual group, participants who used Moodivate and those who used MoodKit experienced significant decreases in depressive symptoms over time.
Conclusions/limitations
- The results show that for primary care patients with depression, the use of Moodivate is feasible and may reduce depressive symptoms.
- Limitations: For the first 3 months of enrollment, patients who met diagnostic criteria for a current major depressive episode were excluded. This study did not assess duration of medication use (ie, whether a study participant was stabilized on medication or recently started taking a new medication) and therefore could not ascertain whether treatment gains were a result of the use of the app or of possible new medication use.
1. Torous J, Jän Myrick K, Rauseo-Ricupero N, et al. Digital mental health and COVID-19: using technology today to accelerate the curve on access and quality tomorrow. JMIR Ment Health. 2020;7(3):e18848. doi:10.2196/18848
2. Camacho E, Cohen A, Torous J. Assessment of mental health services available through smartphone apps. JAMA Netw Open. 2022;5(12):e2248784. doi:10.1001/jamanetworkopen.2022.48784
3. American Psychiatric Association. APP Advisor: An American Psychiatric Association Initiative. Accessed April 28, 2023. https://www.psychiatry.org/psychiatrists/practice/mental-health-apps
4. Lagan S, Aquino P, Emerson MR, et al. Actionable health app evaluation: translating expert frameworks into objective metrics. NPJ Digit Med. 2020;3:100. doi:10.1038/s41746-020-00312-4
5. Linardon J, Shatte A, Rosato J, et al. Efficacy of a transdiagnostic cognitive-behavioral intervention for eating disorder psychopathology delivered through a smartphone app: a randomized controlled trial. Psychol Med. 2022;52(9):1679-1690. doi:10.1017/S0033291720003426
6. Christoforou M, Sáez Fonseca JA, Tsakanikos E. Two novel cognitive behavioral therapy–based mobile apps for agoraphobia: randomized controlled trial. J Med Internet Res. 2017;19(11):e398. doi:10.2196/jmir.7747
7. Everitt N, Broadbent J, Richardson B, et al. Exploring the features of an app-based just-in-time intervention for depression. J Affect Disord. 2021;291:279-287. doi:10.1016/j.jad.2021.05.021
8. McLean C, Davis CA, Miller M, et al. The effects of an exposure-based mobile app on symptoms of posttraumatic stress disorder in veterans: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2022;10(11):e38951. doi:10.2196/38951
9. Graham AK, Greene CJ, Kwasny MJ, et al. Coached mobile app platform for the treatment of depression and anxiety among primary care patients: a randomized clinical trial. JAMA Psychiatry. 2020;77(9):906-914. doi:10.1001/jamapsychiatry.2020.1011
10. Wilhelm S, Weingarden H, Greenberg JL, et al. Efficacy of app-based cognitive behavioral therapy for body dysmorphic disorder with coach support: initial randomized controlled clinical trial. Psychother Psychosom. 2022;91(4):277-285. doi:10.1159/000524628
11. Kuhn E, Miller KE, Puran D, et al. A pilot randomized controlled trial of the Insomnia Coach mobile app to assess its feasibility, acceptability, and potential efficacy. Behav Ther. 2022;53(3):440-457. doi:10.1016/j.beth.2021.11.003
12. Dahne J, Lejuez CW, Diaz VA, et al. Pilot randomized trial of a self-help behavioral activation mobile app for utilization in primary care. Behav Ther. 2019;50(4):817-827. doi:10.1016/j.beth.2018.12.003
COVID-19’s increased demand on the mental health care delivery system led to expanded utilization of technology-based solutions, including digital tools to deliver care.1 Technology-based solutions include both synchronous telehealth (eg, real-time interactive audio/video visits) and asynchronous tools such as smartphone applications (apps). Both real-time telehealth and apps continue to gain popularity. More than 10,000 mental health–related apps are available, and that number continues to rise.2 Numerous web- or mobile-based apps are available to aid in the treatment of various psychiatric conditions, including generalized anxiety disorder (GAD), major depressive disorder, insomnia, and posttraumatic stress disorder (PTSD).
Clinicians may find it challenging to choose the best psychiatry-related apps to recommend to patients. This dilemma calls for an approach to help clinicians select apps that are safe and effective.2 The American Psychiatric Association provides information to help mental health professionals navigate these issues and identify which aspects to consider when selecting an app for clinical use.3 The M-Health Index and Navigation Database also provides a set of objective evaluative criteria and offers guidance on choosing apps.4
In this article, we review 8 randomized controlled trials (RCTs) of mental health–related apps. We took several steps to ensure the RCTs we included were impactful and meaningful. First, we conducted a general search using mainstream search engines to assess which psychiatric apps were most popular for use in clinical practice. Using this list, we conducted a scholarly search engine query of RCTs using the name of the apps as a search parameter along with the following keywords: “mobile,” “web,” “applications,” and “psychiatry.” This search yielded approximately 50 results, which were narrowed down based on content and interest to a list of 8 articles (Table5-12). These articles were then graded using the limitations of each study as the primary substrate for evaluation.

1. Linardon J, Shatte A, Rosato J, et al. Efficacy of a transdiagnostic cognitive-behavioral intervention for eating disorder psychopathology delivered through a smartphone app: a randomized controlled trial. Psychol Med. 2022;52(9):1679-1690. doi:10.1017/S0033291720003426
Many patients with eating disorders are unable to receive effective treatment due to problems with accessing health care. Smartphone apps may help bridge the treatment gap for patients in this position. Linardon et al5 developed an app that uses the principles of cognitive-behavioral therapy (CBT) for treating eating disorders and conducted this study to evaluate its effectiveness.
Study design
- This RCT assigned individuals who reported episodes of binge eating to a group that used a mobile app (n = 197) or to a waiting list (n = 195). At baseline, 42% of participants exhibited diagnostic-level symptoms of bulimia nervosa and 31% had symptoms of binge-eating disorder.
- Assessments took place at baseline, Week 4, and Week 8.
- The primary outcome was global levels of eating disorder psychopathology.
- Secondary outcomes were other eating disorder symptoms, impairment, and distress.
Outcomes
- Compared to the control group, participants who used the mobile app reported greater reductions in global eating disorder psychopathology (d = -0.80).
- Significant effects were also observed for secondary outcomes except compensatory behavior frequency.
- Overall, participants reported they were satisfied with the app.
Continue to: Conclusions/limitations
Conclusions/limitations
- Findings show this app could potentially be a cost-effective and easily accessible option for patients who cannot receive standard treatment for eating disorders.
- Limitations: The overall posttest attrition rate was 35%.
2. Christoforou M, Sáez Fonseca JA, Tsakanikos E. Two novel cognitive behavioral therapy–based mobile apps for agoraphobia: randomized controlled trial. J Med Internet Res. 2017;19(11):e398. doi:10.2196/jmir.7747
CBT is generally the most accepted first-line treatment for agoraphobia. However, numerous barriers to obtaining CBT can prevent successful treatment. Limited research has evaluated the efficacy of apps for treating agoraphobia. Christoforou et al6 conducted an RCT to determine the effectiveness of a self-guided smartphone app for improving agoraphobic symptoms, compared to a mobile app used to treat anxiety.
Study design
- Participants (N = 170) who self-identified as having agoraphobia were randomly assigned to use a smartphone app designed to target agoraphobia (Agoraphobia Free) or a smartphone app designed to help with symptoms of anxiety (Stress Free) for 12 weeks. Both apps were based on established cognitive behavioral principles.
- Assessment occurred at baseline, midpoint, and end point.
- The primary outcome was symptom severity as measured by the Panic and Agoraphobia Scale (PAS).
Outcomes
- Both groups experienced statistically significant improvements in symptom severity over time. The differences in PAS score were -5.97 (95% CI, -8.49 to -3.44, P < .001) for Agoraphobia Free and -6.35 (95% CI, -8.82 to -3.87, P < .001) for Stress Free.
- There were no significant between-group differences in symptom severity.
Continue to: Conclusions/limitations
Conclusions/limitations
- This study is the first RCT to show that patients with agoraphobia could benefit from mobile-based interventions.
- Limitations: There was no waitlist control group. Limited information was collected about participant characteristics; there were no data on comorbid disorders, other psychological or physiological treatments, or other demographic characteristics such as ethnicity or computer literacy.
3. Everitt N, Broadbent J, Richardson B, et al. Exploring the features of an app-based just-in-time intervention for depression. J Affect Disord. 2021;291:279-287. doi:10.1016/j.jad.2021.05.021
The apps MoodTracker, ImproveYourMood, and ImproveYourMood+ deliver content “just in time” (in response to acute negative symptoms) to help patients with depression. In an RCT, Everitt et al7 evaluated delivering acute care for depressive mood states via a smartphone app. They sought to delineate whether symptom improvement was due to microintervention content, mood augmentation, or just-in-time prompts to use content.
Study design
- Participants (N = 235) from the general population who said they wanted to improve their mood were randomly assigned to a waitlist control group (n = 55) or 1 of 3 intervention groups: MoodTracker (monitoring-only; n = 58), ImproveYourMood (monitoring and content; n = 62), or ImproveYourMood+ (monitoring, content, and prompts; n = 60).
- The microintervention content provided by these apps consisted of 4 audio files of brief (2- to 3-minute) mindfulness and relaxation exercises. Participants used the assigned app for 3 weeks.
- Depressive symptoms, anxiety symptoms, and negative automatic thoughts were assessed at baseline, immediately following the intervention, and 1 month after the intervention using the 9-item Patient Health Questionnaire (PHQ-9), 7-item GAD scale (GAD-7), and 8-item Automatic Thoughts Questionnaire, respectively.
Outcomes
- Compared to the waitlist control group, participants in the ImproveYourMood group showed greater declines in depressive symptoms and anxiety symptoms (at follow-up only), and negative automatic thoughts (at both postintervention and follow-up).
- Those in the ImproveYourMood+ group only showed significantly greater improvements for automatic negative thoughts (at postintervention).
- MoodTracker participants did not differ from waitlist controls for any variables at any timepoints.
Continue to: Conclusions/limitations
Conclusions/limitations
- This study suggests that using microinterventions in acute settings can effectively reduce depressive symptoms both as they occur, and 1 to 2 months later.
- Limitations: The study featured a naturalistic design, where participants self-selected whether they wanted to use the program. Participants did not complete eligibility assessments or receive compensation, and the study had high dropout rates, ranging from 20% for the waitlist control group to 67% for the ImproveYourMood+ group.
4. McLean C, Davis CA, Miller M, et al. The effects of an exposure-based mobile app on symptoms of posttraumatic stress disorder in veterans: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2022;10(11):e38951. doi:10.2196/38951
Veterans with PTSD face barriers when receiving trauma-focused treatments such as exposure therapy or CBT. Smartphone apps may help veterans self-treat and self-manage their PTSD symptoms. McLean et al8 studied the efficacy of Renew, a smartphone app that uses exposure therapy and social support to treat PTSD.
Study design
- In this pilot RCT, 93 veterans with clinically significant PTSD symptoms were randomly assigned to use the Renew app with and without support from a research staff member (active use group) or to a waitlist (delayed use group) for 6 weeks.
- The PTSD Checklist for DSM-5 (PCL-5) was used to measure PTSD symptoms at preintervention, postintervention, and 6-week follow-up.
- Most participants (69%) were women, and the mean age was 49.
Outcomes
- Compared to the delayed use group, participants in the active use group experienced a larger decrease in PCL-5 score (-6.14 vs -1.84). However, this difference was not statistically significant (P = .29), and the effect size was small (d = -0.39).
- There was no difference in engagement with the app between participants who received support from a research staff member and those who did not receive such support.
Continue to: Conclusions/limitations
Conclusions/limitations
- Renew may show promise as a tool to reduce PTSD symptoms in veterans.
- Educating family and friends on how to best support a patient using a mobile mental health app may help improve the efficacy of Renew and increase app engagement.
- Limitations: Because the study was conducted in veterans, the results may not be generalizable to other populations. Because most data collection occurred during the first wave of the COVID-19 pandemic in the United States, COVID-19–related stress may have impacted PTSD symptoms, app engagement, or outcomes.
5. Graham AK, Greene CJ, Kwasny MJ, et al. Coached mobile app platform for the treatment of depression and anxiety among primary care patients: a randomized clinical trial. JAMA Psychiatry. 2020;77(9):906-914. doi:10.1001/jamapsychiatry.2020.1011
Many cases of depression and anxiety are initially treated in primary care settings. However, these settings may have limited resources and inadequate training, and mobile interventions might be helpful to augment patient care. Graham et al9 studied the mobile platform IntelliCare to determine its efficacy as a tool to be used in primary care settings to treat depression and anxiety.
Study design
- This RCT randomly assigned adult primary care patients (N = 146) who screened positive for depression on the PHQ-9 (score ≥10) or anxiety on the GAD-7 (score ≥8) to the coach-supported IntelliCare platform, which consisted of 5 clinically focused apps, or to a waitlist control group. Interventions were delivered over 8 weeks.
- Overall, 122 (83.6%) patients were diagnosed with depression and 131 (89.7%) were diagnosed with anxiety.
- The primary outcomes were changes in depression (as measured by change in PHQ-9 score) and anxiety (change in GAD-7 score) during the intervention period.
Outcomes
- Participants who used the IntelliCare platform had a greater reduction in depression and anxiety symptoms compared to waitlist controls, and changes were sustained over 2-month follow-up.
- The least square means (LSM) difference in depression scores at Week 4 was 2.91 (SE = 0.83; d = 0.43) and at Week 8 was 4.37 (SE = 0.83; d = 0.64). The LSM difference in anxiety scores at Week 4 was 2.51 (SE = 0.78; d = 0.41) and at Week 8 was 3.33 (SE = 0.76; d = 0.55).
- A median number of 93 and 98 sessions among participants with depression and anxiety were recorded, respectively, indicating high use of the IntelliCare platform.
Continue to: Conclusions/limitations
Conclusions/limitations
- The IntelliCare platform was shown to be effective in reducing depression and anxiety among primary care patients. Simple apps can be bundled together and used by patients in conjunction to treat their individual needs.
- Limitations: The study had a limited follow-up period and did not record participants’ use of other apps. Slightly more than one-half (56%) of participants were taking an antidepressant.
6. Wilhelm S, Weingarden H, Greenberg JL, et al. Efficacy of app-based cognitive behavioral therapy for body dysmorphic disorder with coach support: initial randomized controlled clinical trial. Psychother Psychosom. 2022;91(4):277-285. doi:10.1159/000524628
Body dysmorphic disorder (BDD) is a severe yet undertreated disorder. Apps can improve access to treatment for patients experiencing BDD. Wilhelm et al10 studied the usability and efficacy of a coach-supported app called Perspectives that was specifically designed for treating BDD. Perspectives provide CBT in 7 modules: psychoeducation, cognitive restructuring, exposure, response prevention, mindfulness, attention retraining, and relapse prevention.
Study design
- Adults (N = 80) with primary BDD were assigned to use the Perspectives app for 12 weeks or to a waitlist control group. Participants were predominately female (84%) and White (71%), with a mean age of 27.
- Coaches promoted engagement and answered questions via in-app messaging and phone calls.
- Blinded independent evaluators used the Yale-Brown Obsessive Compulsive Scale Modified for BDD (BDD-YBOCS) to measure BDD severity at baseline, midtreatment (Week 6), and end of treatment (Week 12).
- Secondary outcomes included BDD-related insight, depression, quality of life, and functioning. Various scales were used to measure these outcomes.
Outcomes
- In intent-to-treat analyses, patients who received CBT via the Perspectives app had significantly lower BDD severity at the end of treatment compared to the waitlist control group, with a mean (SD) BDD-YBOCS score of 16.8 (7.5) vs 26.7 (6.2), with P < .001 and d = 1.44.
- Slightly more than one-half (52%) of those who used Perspectives achieved full or partial remission, compared to 8% in the waitlist control group.
Continue to: Conclusions/limitations
Conclusions/limitations
- CBT delivered via the Perspectives app and a coach proved to be effective treatment for adults with BDD.
- Adoption of the application was relatively high; 86% of Perspectives users were very or mostly satisfied.
- Limitations: Because the participants in this study were predominantly female and White, the findings might not be generalizable to other populations.
7. Kuhn E, Miller KE, Puran D, et al. A pilot randomized controlled trial of the Insomnia Coach mobile app to assess its feasibility, acceptability, and potential efficacy. Behav Ther. 2022;53(3):440-457. doi:10.1016/j.beth.2021.11.003
Insomnia remains a substantial problem among military veterans. First-line treatments for the disorder are sleep hygiene modification and CBT. Access to CBT is limited, especially for veterans. Kuhn et al11 studied the effectiveness of using Insomnia Coach, a CBT for insomnia–based app, to improve insomnia symptoms.
Study design
- Fifty US veterans who were mostly male (58%) with a mean age of 44.5 and moderate insomnia symptoms were randomized to use Insomnia Coach (n = 25) or to a waitlist control group (n = 25) for 6 weeks.
- All participants completed self-report measures and sleep diaries at baseline, posttreatment, and follow-up (12 weeks). Those who used the app (n = 15) completed a qualitative interview at posttreatment.
Outcomes
- At posttreatment, 28% of participants who used Insomnia Coach achieved clinically significant improvement, vs 4% of waitlist control participants. There was also a significant treatment effect on daytime sleep-related impairment (P = .044, d = -0.6).
- Additional treatment effects emerged at follow-up for insomnia severity, sleep onset latency, global sleep quality, and depression symptoms.
- Based on self-reports and qualitative interview responses, participants’ perceptions of Insomnia Coach were favorable. Three-fourths of participants used the app through 6 weeks and engaged with active elements.
Continue to: Conclusions/limitations
Conclusions/limitations
- Insomnia Coach may provide an accessible and convenient public health intervention for patients who aren’t receiving adequate care or CBT.
- Limitations: Because this study evaluated only veterans, the findings might not be generalizable to other populations.
8. Dahne J, Lejuez CW, Diaz VA, et al. Pilot randomized trial of a self-help behavioral activation mobile app for utilization in primary care. Behav Ther. 2019;50(4):817-827. doi:10.1016/j.beth.2018.12.003
Previous mobile technologies have shown the ability to treat depression in primary care settings. Moodivate is a self-help mobile app based on the Brief Behavioral Activation Treatment for Depression, which is an evidence-based treatment. This app is designed to help the user reengage in positive, nondepressed activities by identifying, scheduling, and completing activities. Dahne et al12 investigated the feasibility and efficacy of Moodivate for depressive symptoms in primary care patients.
Study design
- Participants (N = 52) were recruited from primary care practices and randomized 2:2:1 to receive Moodivate, a CBT-based mobile app called MoodKit, or treatment as usual (no app). All participants had an initial PHQ-8 score >10.
- Participants completed assessments of depressive symptoms (PHQ-8) weekly for 8 weeks.
- App analytics data were captured to examine if the use of Moodivate was feasible. (Analytics were not available for MoodKit).
Outcomes
- Participants who used Moodivate had a mean (SD) of 46.76 (30.10) sessions throughout the trial, spent 3.50 (2.76) minutes using the app per session, and spent 120.76 (101.02) minutes using the app in total.
- Nearly 70% of Moodivate participants continued to use the app 1 month after trial enrollment and 50% at the end of the 8-week follow-up period.
- Compared to the treatment as usual group, participants who used Moodivate and those who used MoodKit experienced significant decreases in depressive symptoms over time.
Conclusions/limitations
- The results show that for primary care patients with depression, the use of Moodivate is feasible and may reduce depressive symptoms.
- Limitations: For the first 3 months of enrollment, patients who met diagnostic criteria for a current major depressive episode were excluded. This study did not assess duration of medication use (ie, whether a study participant was stabilized on medication or recently started taking a new medication) and therefore could not ascertain whether treatment gains were a result of the use of the app or of possible new medication use.
COVID-19’s increased demand on the mental health care delivery system led to expanded utilization of technology-based solutions, including digital tools to deliver care.1 Technology-based solutions include both synchronous telehealth (eg, real-time interactive audio/video visits) and asynchronous tools such as smartphone applications (apps). Both real-time telehealth and apps continue to gain popularity. More than 10,000 mental health–related apps are available, and that number continues to rise.2 Numerous web- or mobile-based apps are available to aid in the treatment of various psychiatric conditions, including generalized anxiety disorder (GAD), major depressive disorder, insomnia, and posttraumatic stress disorder (PTSD).
Clinicians may find it challenging to choose the best psychiatry-related apps to recommend to patients. This dilemma calls for an approach to help clinicians select apps that are safe and effective.2 The American Psychiatric Association provides information to help mental health professionals navigate these issues and identify which aspects to consider when selecting an app for clinical use.3 The M-Health Index and Navigation Database also provides a set of objective evaluative criteria and offers guidance on choosing apps.4
In this article, we review 8 randomized controlled trials (RCTs) of mental health–related apps. We took several steps to ensure the RCTs we included were impactful and meaningful. First, we conducted a general search using mainstream search engines to assess which psychiatric apps were most popular for use in clinical practice. Using this list, we conducted a scholarly search engine query of RCTs using the name of the apps as a search parameter along with the following keywords: “mobile,” “web,” “applications,” and “psychiatry.” This search yielded approximately 50 results, which were narrowed down based on content and interest to a list of 8 articles (Table5-12). These articles were then graded using the limitations of each study as the primary substrate for evaluation.

1. Linardon J, Shatte A, Rosato J, et al. Efficacy of a transdiagnostic cognitive-behavioral intervention for eating disorder psychopathology delivered through a smartphone app: a randomized controlled trial. Psychol Med. 2022;52(9):1679-1690. doi:10.1017/S0033291720003426
Many patients with eating disorders are unable to receive effective treatment due to problems with accessing health care. Smartphone apps may help bridge the treatment gap for patients in this position. Linardon et al5 developed an app that uses the principles of cognitive-behavioral therapy (CBT) for treating eating disorders and conducted this study to evaluate its effectiveness.
Study design
- This RCT assigned individuals who reported episodes of binge eating to a group that used a mobile app (n = 197) or to a waiting list (n = 195). At baseline, 42% of participants exhibited diagnostic-level symptoms of bulimia nervosa and 31% had symptoms of binge-eating disorder.
- Assessments took place at baseline, Week 4, and Week 8.
- The primary outcome was global levels of eating disorder psychopathology.
- Secondary outcomes were other eating disorder symptoms, impairment, and distress.
Outcomes
- Compared to the control group, participants who used the mobile app reported greater reductions in global eating disorder psychopathology (d = -0.80).
- Significant effects were also observed for secondary outcomes except compensatory behavior frequency.
- Overall, participants reported they were satisfied with the app.
Continue to: Conclusions/limitations
Conclusions/limitations
- Findings show this app could potentially be a cost-effective and easily accessible option for patients who cannot receive standard treatment for eating disorders.
- Limitations: The overall posttest attrition rate was 35%.
2. Christoforou M, Sáez Fonseca JA, Tsakanikos E. Two novel cognitive behavioral therapy–based mobile apps for agoraphobia: randomized controlled trial. J Med Internet Res. 2017;19(11):e398. doi:10.2196/jmir.7747
CBT is generally the most accepted first-line treatment for agoraphobia. However, numerous barriers to obtaining CBT can prevent successful treatment. Limited research has evaluated the efficacy of apps for treating agoraphobia. Christoforou et al6 conducted an RCT to determine the effectiveness of a self-guided smartphone app for improving agoraphobic symptoms, compared to a mobile app used to treat anxiety.
Study design
- Participants (N = 170) who self-identified as having agoraphobia were randomly assigned to use a smartphone app designed to target agoraphobia (Agoraphobia Free) or a smartphone app designed to help with symptoms of anxiety (Stress Free) for 12 weeks. Both apps were based on established cognitive behavioral principles.
- Assessment occurred at baseline, midpoint, and end point.
- The primary outcome was symptom severity as measured by the Panic and Agoraphobia Scale (PAS).
Outcomes
- Both groups experienced statistically significant improvements in symptom severity over time. The differences in PAS score were -5.97 (95% CI, -8.49 to -3.44, P < .001) for Agoraphobia Free and -6.35 (95% CI, -8.82 to -3.87, P < .001) for Stress Free.
- There were no significant between-group differences in symptom severity.
Continue to: Conclusions/limitations
Conclusions/limitations
- This study is the first RCT to show that patients with agoraphobia could benefit from mobile-based interventions.
- Limitations: There was no waitlist control group. Limited information was collected about participant characteristics; there were no data on comorbid disorders, other psychological or physiological treatments, or other demographic characteristics such as ethnicity or computer literacy.
3. Everitt N, Broadbent J, Richardson B, et al. Exploring the features of an app-based just-in-time intervention for depression. J Affect Disord. 2021;291:279-287. doi:10.1016/j.jad.2021.05.021
The apps MoodTracker, ImproveYourMood, and ImproveYourMood+ deliver content “just in time” (in response to acute negative symptoms) to help patients with depression. In an RCT, Everitt et al7 evaluated delivering acute care for depressive mood states via a smartphone app. They sought to delineate whether symptom improvement was due to microintervention content, mood augmentation, or just-in-time prompts to use content.
Study design
- Participants (N = 235) from the general population who said they wanted to improve their mood were randomly assigned to a waitlist control group (n = 55) or 1 of 3 intervention groups: MoodTracker (monitoring-only; n = 58), ImproveYourMood (monitoring and content; n = 62), or ImproveYourMood+ (monitoring, content, and prompts; n = 60).
- The microintervention content provided by these apps consisted of 4 audio files of brief (2- to 3-minute) mindfulness and relaxation exercises. Participants used the assigned app for 3 weeks.
- Depressive symptoms, anxiety symptoms, and negative automatic thoughts were assessed at baseline, immediately following the intervention, and 1 month after the intervention using the 9-item Patient Health Questionnaire (PHQ-9), 7-item GAD scale (GAD-7), and 8-item Automatic Thoughts Questionnaire, respectively.
Outcomes
- Compared to the waitlist control group, participants in the ImproveYourMood group showed greater declines in depressive symptoms and anxiety symptoms (at follow-up only), and negative automatic thoughts (at both postintervention and follow-up).
- Those in the ImproveYourMood+ group only showed significantly greater improvements for automatic negative thoughts (at postintervention).
- MoodTracker participants did not differ from waitlist controls for any variables at any timepoints.
Continue to: Conclusions/limitations
Conclusions/limitations
- This study suggests that using microinterventions in acute settings can effectively reduce depressive symptoms both as they occur, and 1 to 2 months later.
- Limitations: The study featured a naturalistic design, where participants self-selected whether they wanted to use the program. Participants did not complete eligibility assessments or receive compensation, and the study had high dropout rates, ranging from 20% for the waitlist control group to 67% for the ImproveYourMood+ group.
4. McLean C, Davis CA, Miller M, et al. The effects of an exposure-based mobile app on symptoms of posttraumatic stress disorder in veterans: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2022;10(11):e38951. doi:10.2196/38951
Veterans with PTSD face barriers when receiving trauma-focused treatments such as exposure therapy or CBT. Smartphone apps may help veterans self-treat and self-manage their PTSD symptoms. McLean et al8 studied the efficacy of Renew, a smartphone app that uses exposure therapy and social support to treat PTSD.
Study design
- In this pilot RCT, 93 veterans with clinically significant PTSD symptoms were randomly assigned to use the Renew app with and without support from a research staff member (active use group) or to a waitlist (delayed use group) for 6 weeks.
- The PTSD Checklist for DSM-5 (PCL-5) was used to measure PTSD symptoms at preintervention, postintervention, and 6-week follow-up.
- Most participants (69%) were women, and the mean age was 49.
Outcomes
- Compared to the delayed use group, participants in the active use group experienced a larger decrease in PCL-5 score (-6.14 vs -1.84). However, this difference was not statistically significant (P = .29), and the effect size was small (d = -0.39).
- There was no difference in engagement with the app between participants who received support from a research staff member and those who did not receive such support.
Continue to: Conclusions/limitations
Conclusions/limitations
- Renew may show promise as a tool to reduce PTSD symptoms in veterans.
- Educating family and friends on how to best support a patient using a mobile mental health app may help improve the efficacy of Renew and increase app engagement.
- Limitations: Because the study was conducted in veterans, the results may not be generalizable to other populations. Because most data collection occurred during the first wave of the COVID-19 pandemic in the United States, COVID-19–related stress may have impacted PTSD symptoms, app engagement, or outcomes.
5. Graham AK, Greene CJ, Kwasny MJ, et al. Coached mobile app platform for the treatment of depression and anxiety among primary care patients: a randomized clinical trial. JAMA Psychiatry. 2020;77(9):906-914. doi:10.1001/jamapsychiatry.2020.1011
Many cases of depression and anxiety are initially treated in primary care settings. However, these settings may have limited resources and inadequate training, and mobile interventions might be helpful to augment patient care. Graham et al9 studied the mobile platform IntelliCare to determine its efficacy as a tool to be used in primary care settings to treat depression and anxiety.
Study design
- This RCT randomly assigned adult primary care patients (N = 146) who screened positive for depression on the PHQ-9 (score ≥10) or anxiety on the GAD-7 (score ≥8) to the coach-supported IntelliCare platform, which consisted of 5 clinically focused apps, or to a waitlist control group. Interventions were delivered over 8 weeks.
- Overall, 122 (83.6%) patients were diagnosed with depression and 131 (89.7%) were diagnosed with anxiety.
- The primary outcomes were changes in depression (as measured by change in PHQ-9 score) and anxiety (change in GAD-7 score) during the intervention period.
Outcomes
- Participants who used the IntelliCare platform had a greater reduction in depression and anxiety symptoms compared to waitlist controls, and changes were sustained over 2-month follow-up.
- The least square means (LSM) difference in depression scores at Week 4 was 2.91 (SE = 0.83; d = 0.43) and at Week 8 was 4.37 (SE = 0.83; d = 0.64). The LSM difference in anxiety scores at Week 4 was 2.51 (SE = 0.78; d = 0.41) and at Week 8 was 3.33 (SE = 0.76; d = 0.55).
- A median number of 93 and 98 sessions among participants with depression and anxiety were recorded, respectively, indicating high use of the IntelliCare platform.
Continue to: Conclusions/limitations
Conclusions/limitations
- The IntelliCare platform was shown to be effective in reducing depression and anxiety among primary care patients. Simple apps can be bundled together and used by patients in conjunction to treat their individual needs.
- Limitations: The study had a limited follow-up period and did not record participants’ use of other apps. Slightly more than one-half (56%) of participants were taking an antidepressant.
6. Wilhelm S, Weingarden H, Greenberg JL, et al. Efficacy of app-based cognitive behavioral therapy for body dysmorphic disorder with coach support: initial randomized controlled clinical trial. Psychother Psychosom. 2022;91(4):277-285. doi:10.1159/000524628
Body dysmorphic disorder (BDD) is a severe yet undertreated disorder. Apps can improve access to treatment for patients experiencing BDD. Wilhelm et al10 studied the usability and efficacy of a coach-supported app called Perspectives that was specifically designed for treating BDD. Perspectives provide CBT in 7 modules: psychoeducation, cognitive restructuring, exposure, response prevention, mindfulness, attention retraining, and relapse prevention.
Study design
- Adults (N = 80) with primary BDD were assigned to use the Perspectives app for 12 weeks or to a waitlist control group. Participants were predominately female (84%) and White (71%), with a mean age of 27.
- Coaches promoted engagement and answered questions via in-app messaging and phone calls.
- Blinded independent evaluators used the Yale-Brown Obsessive Compulsive Scale Modified for BDD (BDD-YBOCS) to measure BDD severity at baseline, midtreatment (Week 6), and end of treatment (Week 12).
- Secondary outcomes included BDD-related insight, depression, quality of life, and functioning. Various scales were used to measure these outcomes.
Outcomes
- In intent-to-treat analyses, patients who received CBT via the Perspectives app had significantly lower BDD severity at the end of treatment compared to the waitlist control group, with a mean (SD) BDD-YBOCS score of 16.8 (7.5) vs 26.7 (6.2), with P < .001 and d = 1.44.
- Slightly more than one-half (52%) of those who used Perspectives achieved full or partial remission, compared to 8% in the waitlist control group.
Continue to: Conclusions/limitations
Conclusions/limitations
- CBT delivered via the Perspectives app and a coach proved to be effective treatment for adults with BDD.
- Adoption of the application was relatively high; 86% of Perspectives users were very or mostly satisfied.
- Limitations: Because the participants in this study were predominantly female and White, the findings might not be generalizable to other populations.
7. Kuhn E, Miller KE, Puran D, et al. A pilot randomized controlled trial of the Insomnia Coach mobile app to assess its feasibility, acceptability, and potential efficacy. Behav Ther. 2022;53(3):440-457. doi:10.1016/j.beth.2021.11.003
Insomnia remains a substantial problem among military veterans. First-line treatments for the disorder are sleep hygiene modification and CBT. Access to CBT is limited, especially for veterans. Kuhn et al11 studied the effectiveness of using Insomnia Coach, a CBT for insomnia–based app, to improve insomnia symptoms.
Study design
- Fifty US veterans who were mostly male (58%) with a mean age of 44.5 and moderate insomnia symptoms were randomized to use Insomnia Coach (n = 25) or to a waitlist control group (n = 25) for 6 weeks.
- All participants completed self-report measures and sleep diaries at baseline, posttreatment, and follow-up (12 weeks). Those who used the app (n = 15) completed a qualitative interview at posttreatment.
Outcomes
- At posttreatment, 28% of participants who used Insomnia Coach achieved clinically significant improvement, vs 4% of waitlist control participants. There was also a significant treatment effect on daytime sleep-related impairment (P = .044, d = -0.6).
- Additional treatment effects emerged at follow-up for insomnia severity, sleep onset latency, global sleep quality, and depression symptoms.
- Based on self-reports and qualitative interview responses, participants’ perceptions of Insomnia Coach were favorable. Three-fourths of participants used the app through 6 weeks and engaged with active elements.
Continue to: Conclusions/limitations
Conclusions/limitations
- Insomnia Coach may provide an accessible and convenient public health intervention for patients who aren’t receiving adequate care or CBT.
- Limitations: Because this study evaluated only veterans, the findings might not be generalizable to other populations.
8. Dahne J, Lejuez CW, Diaz VA, et al. Pilot randomized trial of a self-help behavioral activation mobile app for utilization in primary care. Behav Ther. 2019;50(4):817-827. doi:10.1016/j.beth.2018.12.003
Previous mobile technologies have shown the ability to treat depression in primary care settings. Moodivate is a self-help mobile app based on the Brief Behavioral Activation Treatment for Depression, which is an evidence-based treatment. This app is designed to help the user reengage in positive, nondepressed activities by identifying, scheduling, and completing activities. Dahne et al12 investigated the feasibility and efficacy of Moodivate for depressive symptoms in primary care patients.
Study design
- Participants (N = 52) were recruited from primary care practices and randomized 2:2:1 to receive Moodivate, a CBT-based mobile app called MoodKit, or treatment as usual (no app). All participants had an initial PHQ-8 score >10.
- Participants completed assessments of depressive symptoms (PHQ-8) weekly for 8 weeks.
- App analytics data were captured to examine if the use of Moodivate was feasible. (Analytics were not available for MoodKit).
Outcomes
- Participants who used Moodivate had a mean (SD) of 46.76 (30.10) sessions throughout the trial, spent 3.50 (2.76) minutes using the app per session, and spent 120.76 (101.02) minutes using the app in total.
- Nearly 70% of Moodivate participants continued to use the app 1 month after trial enrollment and 50% at the end of the 8-week follow-up period.
- Compared to the treatment as usual group, participants who used Moodivate and those who used MoodKit experienced significant decreases in depressive symptoms over time.
Conclusions/limitations
- The results show that for primary care patients with depression, the use of Moodivate is feasible and may reduce depressive symptoms.
- Limitations: For the first 3 months of enrollment, patients who met diagnostic criteria for a current major depressive episode were excluded. This study did not assess duration of medication use (ie, whether a study participant was stabilized on medication or recently started taking a new medication) and therefore could not ascertain whether treatment gains were a result of the use of the app or of possible new medication use.
1. Torous J, Jän Myrick K, Rauseo-Ricupero N, et al. Digital mental health and COVID-19: using technology today to accelerate the curve on access and quality tomorrow. JMIR Ment Health. 2020;7(3):e18848. doi:10.2196/18848
2. Camacho E, Cohen A, Torous J. Assessment of mental health services available through smartphone apps. JAMA Netw Open. 2022;5(12):e2248784. doi:10.1001/jamanetworkopen.2022.48784
3. American Psychiatric Association. APP Advisor: An American Psychiatric Association Initiative. Accessed April 28, 2023. https://www.psychiatry.org/psychiatrists/practice/mental-health-apps
4. Lagan S, Aquino P, Emerson MR, et al. Actionable health app evaluation: translating expert frameworks into objective metrics. NPJ Digit Med. 2020;3:100. doi:10.1038/s41746-020-00312-4
5. Linardon J, Shatte A, Rosato J, et al. Efficacy of a transdiagnostic cognitive-behavioral intervention for eating disorder psychopathology delivered through a smartphone app: a randomized controlled trial. Psychol Med. 2022;52(9):1679-1690. doi:10.1017/S0033291720003426
6. Christoforou M, Sáez Fonseca JA, Tsakanikos E. Two novel cognitive behavioral therapy–based mobile apps for agoraphobia: randomized controlled trial. J Med Internet Res. 2017;19(11):e398. doi:10.2196/jmir.7747
7. Everitt N, Broadbent J, Richardson B, et al. Exploring the features of an app-based just-in-time intervention for depression. J Affect Disord. 2021;291:279-287. doi:10.1016/j.jad.2021.05.021
8. McLean C, Davis CA, Miller M, et al. The effects of an exposure-based mobile app on symptoms of posttraumatic stress disorder in veterans: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2022;10(11):e38951. doi:10.2196/38951
9. Graham AK, Greene CJ, Kwasny MJ, et al. Coached mobile app platform for the treatment of depression and anxiety among primary care patients: a randomized clinical trial. JAMA Psychiatry. 2020;77(9):906-914. doi:10.1001/jamapsychiatry.2020.1011
10. Wilhelm S, Weingarden H, Greenberg JL, et al. Efficacy of app-based cognitive behavioral therapy for body dysmorphic disorder with coach support: initial randomized controlled clinical trial. Psychother Psychosom. 2022;91(4):277-285. doi:10.1159/000524628
11. Kuhn E, Miller KE, Puran D, et al. A pilot randomized controlled trial of the Insomnia Coach mobile app to assess its feasibility, acceptability, and potential efficacy. Behav Ther. 2022;53(3):440-457. doi:10.1016/j.beth.2021.11.003
12. Dahne J, Lejuez CW, Diaz VA, et al. Pilot randomized trial of a self-help behavioral activation mobile app for utilization in primary care. Behav Ther. 2019;50(4):817-827. doi:10.1016/j.beth.2018.12.003
1. Torous J, Jän Myrick K, Rauseo-Ricupero N, et al. Digital mental health and COVID-19: using technology today to accelerate the curve on access and quality tomorrow. JMIR Ment Health. 2020;7(3):e18848. doi:10.2196/18848
2. Camacho E, Cohen A, Torous J. Assessment of mental health services available through smartphone apps. JAMA Netw Open. 2022;5(12):e2248784. doi:10.1001/jamanetworkopen.2022.48784
3. American Psychiatric Association. APP Advisor: An American Psychiatric Association Initiative. Accessed April 28, 2023. https://www.psychiatry.org/psychiatrists/practice/mental-health-apps
4. Lagan S, Aquino P, Emerson MR, et al. Actionable health app evaluation: translating expert frameworks into objective metrics. NPJ Digit Med. 2020;3:100. doi:10.1038/s41746-020-00312-4
5. Linardon J, Shatte A, Rosato J, et al. Efficacy of a transdiagnostic cognitive-behavioral intervention for eating disorder psychopathology delivered through a smartphone app: a randomized controlled trial. Psychol Med. 2022;52(9):1679-1690. doi:10.1017/S0033291720003426
6. Christoforou M, Sáez Fonseca JA, Tsakanikos E. Two novel cognitive behavioral therapy–based mobile apps for agoraphobia: randomized controlled trial. J Med Internet Res. 2017;19(11):e398. doi:10.2196/jmir.7747
7. Everitt N, Broadbent J, Richardson B, et al. Exploring the features of an app-based just-in-time intervention for depression. J Affect Disord. 2021;291:279-287. doi:10.1016/j.jad.2021.05.021
8. McLean C, Davis CA, Miller M, et al. The effects of an exposure-based mobile app on symptoms of posttraumatic stress disorder in veterans: pilot randomized controlled trial. JMIR Mhealth Uhealth. 2022;10(11):e38951. doi:10.2196/38951
9. Graham AK, Greene CJ, Kwasny MJ, et al. Coached mobile app platform for the treatment of depression and anxiety among primary care patients: a randomized clinical trial. JAMA Psychiatry. 2020;77(9):906-914. doi:10.1001/jamapsychiatry.2020.1011
10. Wilhelm S, Weingarden H, Greenberg JL, et al. Efficacy of app-based cognitive behavioral therapy for body dysmorphic disorder with coach support: initial randomized controlled clinical trial. Psychother Psychosom. 2022;91(4):277-285. doi:10.1159/000524628
11. Kuhn E, Miller KE, Puran D, et al. A pilot randomized controlled trial of the Insomnia Coach mobile app to assess its feasibility, acceptability, and potential efficacy. Behav Ther. 2022;53(3):440-457. doi:10.1016/j.beth.2021.11.003
12. Dahne J, Lejuez CW, Diaz VA, et al. Pilot randomized trial of a self-help behavioral activation mobile app for utilization in primary care. Behav Ther. 2019;50(4):817-827. doi:10.1016/j.beth.2018.12.003
Lamotrigine interactions with oral contraceptives
Ms. A, age 20, presents to the clinic after experiencing difficulty sleeping, depressed mood, fatigue, and difficulty concentrating. Her psychiatric history includes bipolar II disorder (BD II), predominantly with depressive episodes. Ms. A’s current medications include a combination of lamotrigine 200 mg/d and bupropion extended-release 450 mg/d, and her symptoms were well maintained until 2 weeks ago. When her psychiatrist performs a medication reconciliation at her medication management appointment, Ms. A indicates she started taking an oral contraceptive, ethinyl estradiol and norgestimate, approximately 1 month ago for management of endometriosis symptoms. She is not currently taking any other medications or supplements.
Lamotrigine is indicated for epilepsy and as maintenance treatment for BD I. It is also used off-label to treat other mood disorders. After oral administration, lamotrigine is rapidly and fully absorbed with a high bioavailability (98%). The principal metabolic pathway is via glucuronic acid conjugation, leading to the major inactive metabolite 2-N-glucuronide. Minor metabolites include 5-N-glucuronide and a 2-N-glucuronide metabolite.1
Combined oral contraceptives contain an estrogen component, typically ethinyl estradiol, and a progestin component, which varies based on the specific formulation. The metabolism of ethinyl estradiol occurs through cytochrome P450 (CYP)3A4, CYP2C9, sulfation, and glucuronidation. For progestin—the second component of combined oral contraceptives and the lone component of progestin-only oral contraceptives—metabolism occurs via CYP3A4 and conjugation reactions.2 This article focuses on lamotrigine interactions specifically with oral contraceptives, but it is important to note that other formulations of combined hormonal contraceptives, such as the combined contraceptive patch (Ortho Evra) and vaginal ring (NuvaRing), would be expected to interact in the same way as oral formulations.3
Bidirectional interaction
While many antiseizure medications are known to interact with and potentially decrease the efficacy of oral contraceptives (Table 13-6), the interactions between lamotrigine and oral contraceptives is uniquely bidirectional. Combined oral contraceptives are thought to interact with lamotrigine primarily via the estrogen component, which causes increased metabolism of lamotrigine through induction of glucuronidation. This drug interaction decreases the plasma concentrations of lamotrigine in the body by up to 2-fold, resulting in an increased risk of seizures or inadequate mood stabilization.1 This effect on metabolism is very rapid, resulting in decreases in lamotrigine concentrations within 1 week.4,7 A recent study suggested that certain progestins may also contribute to decreased plasma levels of lamotrigine, but the mechanism for this is unknown (Table 23-7).8
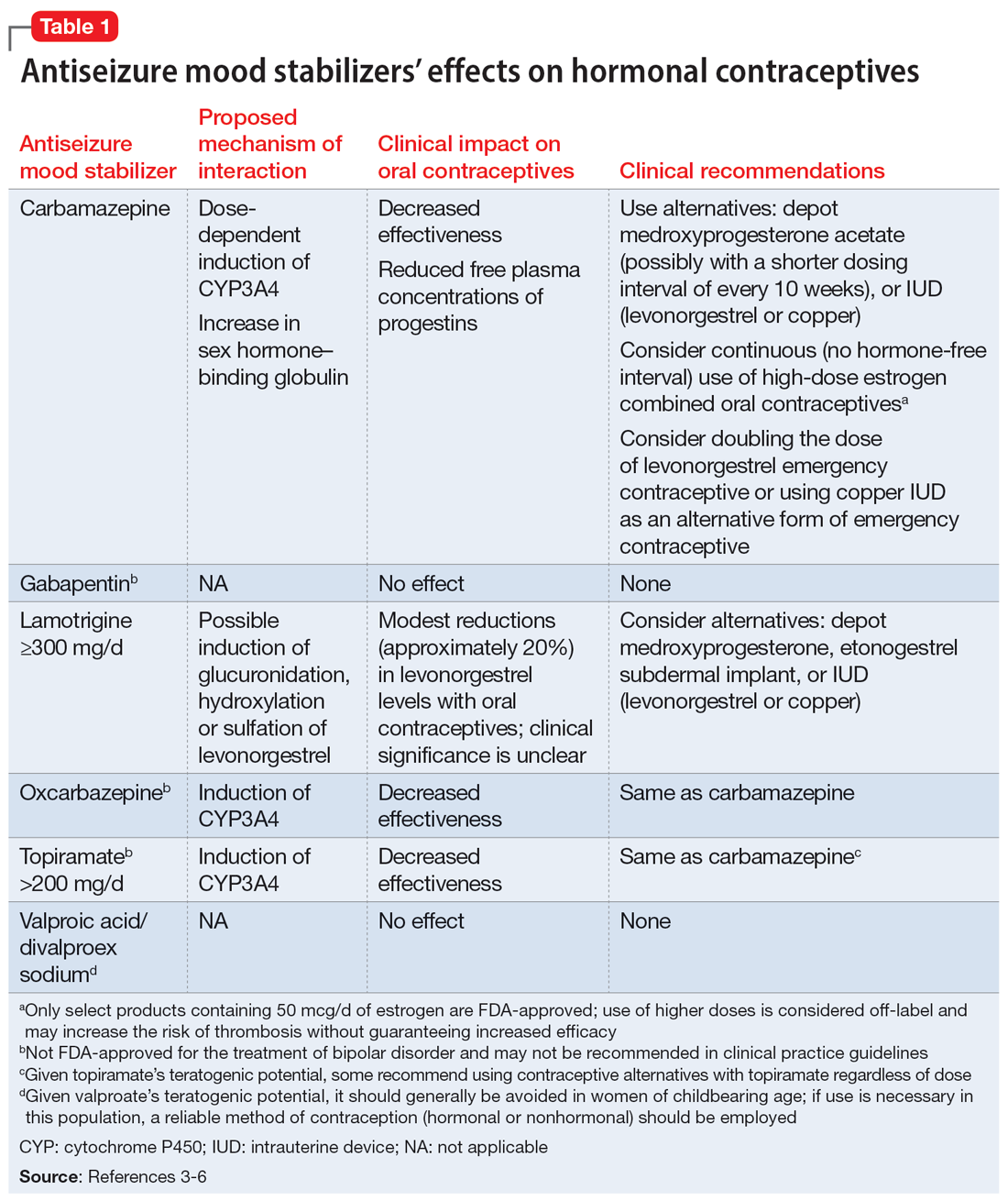
Clinicians should consider increasing the lamotrigine dose (potentially as much as 2-fold) in a patient who initiates treatment with a combined hormonal contraceptive. Dose increases should not be >50 to 100 mg/d every week.1 Collect lamotrigine blood levels before starting a hormonal contraceptive and during dose titration. While there is not a well-established therapeutic range for lamotrigine in BD, expert consensus recommends a range of 1 to 6 mcg/mL.8
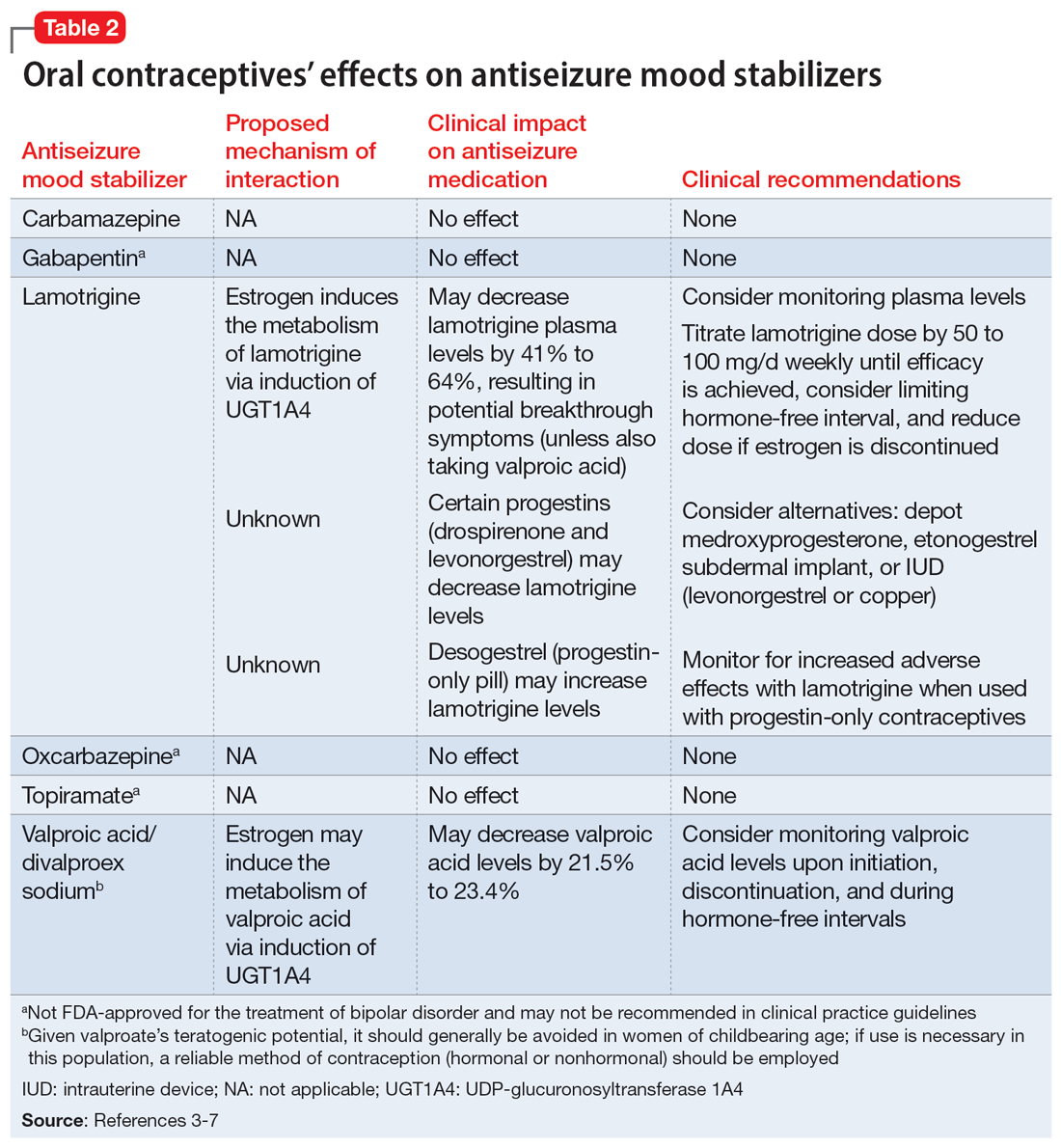
The lamotrigine dose should be decreased if combined hormonal contraceptives are discontinued. Dose decreases should not exceed 25% of the total daily dose per week.1 Desogestrel, a progestin-only medication, may increase exposure to lamotrigine, but this has not been observed in research with other progestins.5,9 When starting a progestin-only pill, monitor patients for signs of lamotrigine toxicity (ataxia, diplopia, dizziness) and consider monitoring their blood levels.
An important consideration to note with combined oral contraceptives is the hormone-free interval, also known as the pill-free week. Due to the rapid effect of estrogens, the lamotrigine concentrations have been shown to rise, even double, during this hormone-free interval, so patients should be closely monitored for adverse effects.3 Some recommend use of an extended cycle regimen (with a limited hormone-free interval), or continuous cycle regimen (with no hormone-free interval) to avoid fluctuations in lamotrigine levels.3,5 Additionally, data suggest that in patients taking lamotrigine and valproate, which inhibits glucuronidation, oral contraceptives do not cause reductions in lamotrigine concentrations.2,5 In these instances, dose increases of lamotrigine are not needed.
Continue to: The metabolism of ethinyl estradiol...
The metabolism of ethinyl estradiol and progestin are susceptible to CYP3A4 induction and increased glucuronidation. Serum concentrations may be reduced by ≥50% when used concomitantly with CYP enzyme–inducing medications, which could possibly result in subtherapeutic levels and unplanned pregnancy.3 CYP3A4 induction occurs for up to 4 weeks after discontinuation of an enzyme-inducing agent, pointing to the need for alternative or backup contraception during this time.3 Lamotrigine is not a CYP enzyme–inducing medication; it is unlikely to affect the efficacy of oral contraceptives in the same manner as other antiseizure medications. However, a study of lamotrigine and the combined hormonal contraceptive ethinyl estradiol and levonorgestrel demonstrated reduced exposure to levonorgestrel, resulting in breakthrough bleeding.5
In a study on the coadministration of lamotrigine and combined oral contraceptives, Sidhu et al4 observed a small mean reduction (20%) in progestin concentrations when lamotrigine was used at a dose of 300 mg/d. Although there is no research suggesting decreased effectiveness in preventing pregnancy when lamotrigine is used with combined oral contraceptives, progestin-only oral contraceptives, or progestin implants, additional or alternative contraceptive methods may be considered based on this pharmacokinetic data, particularly in patients who require lamotrigine doses ≥300 mg/d.5
CASE CONTINUED
Given when Ms. A started the oral contraceptive, the treatment team determines it is likely that an interaction with lamotrigine is causing her resurgence of depressive symptoms. Her care team decides to titrate the lamotrigine gradually to 300 mg/d, then 400 mg/d if needed, while carefully monitoring for signs of a serious rash. This dosage increase may help Ms. A achieve symptom remission. Monitoring plasma levels may be considered, although it is unknown what plasma level was effective for Ms. A before she started the oral contraceptive. Ms. A would need to be counseled regarding the effect of higher doses of lamotrigine on the effectiveness of the oral contraceptive.
Although it does not appear Ms. A is using the oral contraceptive specifically to prevent pregnancy, the team informs her about the possibility of unintended pregnancy with this medication combination. If Ms. A was also using the medication for this indication, alternative contraceptive options would include medroxyprogesterone acetate, levonorgestrel implants, or an intrauterine device (levonorgestrel or copper, though copper would not be effective for endometriosis symptom management). Ms. A should consult with her gynecologist regarding the most appropriate option for her endometriosis. If the decision is made to discontinue her oral contraceptive in the future, the lamotrigine dose should be decreased to her previously effective dose of 200 mg/d.
Related Resources
- Makino KK, Hatters Friedman S, Amin J. Emergency contraception for psychiatric patients. Current Psychiatry. 2022;21(11):34-39,44-45. doi:10.12788/cp.0300
- MGH Center for Women’s Mental Health. You asked: is there an interaction between lamotrigine and oral contraceptives? September 29, 2015. https://womensmentalhealth.org/posts/you-asked-is-there-an-interaction-between-lamotrigine-andoral-contraceptives/
Drug Brand Names
Bupropion extended-release • Wellbutrin XL
Carbamazepine • Equetro, Tegretol
Desogestrel • Cerazette
Divalproex sodium • Depakote
Ethinyl estradiol and etonogestrel • NuvaRing
Ethinyl estradiol and norelgestromin • Ortho Evra
Ethinyl estradiol and norgestimate • Ortho Tri-Cyclen, TriNessa, others
Etonogestrel • Implanon, Nexplanon
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levonorgestrel emergency contraceptive pill • AfterPill, Plan B
Levonorgestrel intrauterine device • Mirena, Skyla
Medroxyprogesterone acetate • Depo-Provera
Oxcarbazepine • Trileptal
Topiramate • Topamax
Valproic acid • Depakene
1. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2020.
2. Lee CR. Drug interactions and hormonal contraception. Trends in Urology Gynaecology & Sexual Health. 2009;14(3):23-26.
3. Williams D. Antiepileptic drugs and contraception. US Pharm. 2014;39(1):39-42.
4. Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61(2):191-199. doi:10.1111/j.1365-2125.2005.02539.x
5. Faculty of Sexual & Reproductive Healthcare. Clinical guidance: drug interactions with hormonal contraception. Published May 9, 2022. Accessed September 28, 2022. https://www.fsrh.org/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/
6. Johnston CA, Crawford PM. Anti-epileptic drugs and hormonal treatments. Curr Treat Options Neurol. 2014;16(5):288. doi:10.1007/s11940-014-0288-3
7. Christensen J, Petrenaite V, Atterman J, et al. Oral contraceptives induce lamotrigine metabolism: evidence from a double-blind, placebo-controlled trial. Epilepsia. 2007;48(3):484-489. doi:10.1111/j.1528-1167.2007.00997.x
8. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi:10.1055/s-0043-116492
9. Rauchenzauner M, Deichmann S, Pittschieler, et al. Bidirectional interaction between oral contraception and lamotrigine in women with epilepsy – role of progestins. Seizure. 2020;74:89-92. doi:10.1016/j.seizure.2019.11.011
Ms. A, age 20, presents to the clinic after experiencing difficulty sleeping, depressed mood, fatigue, and difficulty concentrating. Her psychiatric history includes bipolar II disorder (BD II), predominantly with depressive episodes. Ms. A’s current medications include a combination of lamotrigine 200 mg/d and bupropion extended-release 450 mg/d, and her symptoms were well maintained until 2 weeks ago. When her psychiatrist performs a medication reconciliation at her medication management appointment, Ms. A indicates she started taking an oral contraceptive, ethinyl estradiol and norgestimate, approximately 1 month ago for management of endometriosis symptoms. She is not currently taking any other medications or supplements.
Lamotrigine is indicated for epilepsy and as maintenance treatment for BD I. It is also used off-label to treat other mood disorders. After oral administration, lamotrigine is rapidly and fully absorbed with a high bioavailability (98%). The principal metabolic pathway is via glucuronic acid conjugation, leading to the major inactive metabolite 2-N-glucuronide. Minor metabolites include 5-N-glucuronide and a 2-N-glucuronide metabolite.1
Combined oral contraceptives contain an estrogen component, typically ethinyl estradiol, and a progestin component, which varies based on the specific formulation. The metabolism of ethinyl estradiol occurs through cytochrome P450 (CYP)3A4, CYP2C9, sulfation, and glucuronidation. For progestin—the second component of combined oral contraceptives and the lone component of progestin-only oral contraceptives—metabolism occurs via CYP3A4 and conjugation reactions.2 This article focuses on lamotrigine interactions specifically with oral contraceptives, but it is important to note that other formulations of combined hormonal contraceptives, such as the combined contraceptive patch (Ortho Evra) and vaginal ring (NuvaRing), would be expected to interact in the same way as oral formulations.3
Bidirectional interaction
While many antiseizure medications are known to interact with and potentially decrease the efficacy of oral contraceptives (Table 13-6), the interactions between lamotrigine and oral contraceptives is uniquely bidirectional. Combined oral contraceptives are thought to interact with lamotrigine primarily via the estrogen component, which causes increased metabolism of lamotrigine through induction of glucuronidation. This drug interaction decreases the plasma concentrations of lamotrigine in the body by up to 2-fold, resulting in an increased risk of seizures or inadequate mood stabilization.1 This effect on metabolism is very rapid, resulting in decreases in lamotrigine concentrations within 1 week.4,7 A recent study suggested that certain progestins may also contribute to decreased plasma levels of lamotrigine, but the mechanism for this is unknown (Table 23-7).8

Clinicians should consider increasing the lamotrigine dose (potentially as much as 2-fold) in a patient who initiates treatment with a combined hormonal contraceptive. Dose increases should not be >50 to 100 mg/d every week.1 Collect lamotrigine blood levels before starting a hormonal contraceptive and during dose titration. While there is not a well-established therapeutic range for lamotrigine in BD, expert consensus recommends a range of 1 to 6 mcg/mL.8

The lamotrigine dose should be decreased if combined hormonal contraceptives are discontinued. Dose decreases should not exceed 25% of the total daily dose per week.1 Desogestrel, a progestin-only medication, may increase exposure to lamotrigine, but this has not been observed in research with other progestins.5,9 When starting a progestin-only pill, monitor patients for signs of lamotrigine toxicity (ataxia, diplopia, dizziness) and consider monitoring their blood levels.
An important consideration to note with combined oral contraceptives is the hormone-free interval, also known as the pill-free week. Due to the rapid effect of estrogens, the lamotrigine concentrations have been shown to rise, even double, during this hormone-free interval, so patients should be closely monitored for adverse effects.3 Some recommend use of an extended cycle regimen (with a limited hormone-free interval), or continuous cycle regimen (with no hormone-free interval) to avoid fluctuations in lamotrigine levels.3,5 Additionally, data suggest that in patients taking lamotrigine and valproate, which inhibits glucuronidation, oral contraceptives do not cause reductions in lamotrigine concentrations.2,5 In these instances, dose increases of lamotrigine are not needed.
Continue to: The metabolism of ethinyl estradiol...
The metabolism of ethinyl estradiol and progestin are susceptible to CYP3A4 induction and increased glucuronidation. Serum concentrations may be reduced by ≥50% when used concomitantly with CYP enzyme–inducing medications, which could possibly result in subtherapeutic levels and unplanned pregnancy.3 CYP3A4 induction occurs for up to 4 weeks after discontinuation of an enzyme-inducing agent, pointing to the need for alternative or backup contraception during this time.3 Lamotrigine is not a CYP enzyme–inducing medication; it is unlikely to affect the efficacy of oral contraceptives in the same manner as other antiseizure medications. However, a study of lamotrigine and the combined hormonal contraceptive ethinyl estradiol and levonorgestrel demonstrated reduced exposure to levonorgestrel, resulting in breakthrough bleeding.5
In a study on the coadministration of lamotrigine and combined oral contraceptives, Sidhu et al4 observed a small mean reduction (20%) in progestin concentrations when lamotrigine was used at a dose of 300 mg/d. Although there is no research suggesting decreased effectiveness in preventing pregnancy when lamotrigine is used with combined oral contraceptives, progestin-only oral contraceptives, or progestin implants, additional or alternative contraceptive methods may be considered based on this pharmacokinetic data, particularly in patients who require lamotrigine doses ≥300 mg/d.5
CASE CONTINUED
Given when Ms. A started the oral contraceptive, the treatment team determines it is likely that an interaction with lamotrigine is causing her resurgence of depressive symptoms. Her care team decides to titrate the lamotrigine gradually to 300 mg/d, then 400 mg/d if needed, while carefully monitoring for signs of a serious rash. This dosage increase may help Ms. A achieve symptom remission. Monitoring plasma levels may be considered, although it is unknown what plasma level was effective for Ms. A before she started the oral contraceptive. Ms. A would need to be counseled regarding the effect of higher doses of lamotrigine on the effectiveness of the oral contraceptive.
Although it does not appear Ms. A is using the oral contraceptive specifically to prevent pregnancy, the team informs her about the possibility of unintended pregnancy with this medication combination. If Ms. A was also using the medication for this indication, alternative contraceptive options would include medroxyprogesterone acetate, levonorgestrel implants, or an intrauterine device (levonorgestrel or copper, though copper would not be effective for endometriosis symptom management). Ms. A should consult with her gynecologist regarding the most appropriate option for her endometriosis. If the decision is made to discontinue her oral contraceptive in the future, the lamotrigine dose should be decreased to her previously effective dose of 200 mg/d.
Related Resources
- Makino KK, Hatters Friedman S, Amin J. Emergency contraception for psychiatric patients. Current Psychiatry. 2022;21(11):34-39,44-45. doi:10.12788/cp.0300
- MGH Center for Women’s Mental Health. You asked: is there an interaction between lamotrigine and oral contraceptives? September 29, 2015. https://womensmentalhealth.org/posts/you-asked-is-there-an-interaction-between-lamotrigine-andoral-contraceptives/
Drug Brand Names
Bupropion extended-release • Wellbutrin XL
Carbamazepine • Equetro, Tegretol
Desogestrel • Cerazette
Divalproex sodium • Depakote
Ethinyl estradiol and etonogestrel • NuvaRing
Ethinyl estradiol and norelgestromin • Ortho Evra
Ethinyl estradiol and norgestimate • Ortho Tri-Cyclen, TriNessa, others
Etonogestrel • Implanon, Nexplanon
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levonorgestrel emergency contraceptive pill • AfterPill, Plan B
Levonorgestrel intrauterine device • Mirena, Skyla
Medroxyprogesterone acetate • Depo-Provera
Oxcarbazepine • Trileptal
Topiramate • Topamax
Valproic acid • Depakene
Ms. A, age 20, presents to the clinic after experiencing difficulty sleeping, depressed mood, fatigue, and difficulty concentrating. Her psychiatric history includes bipolar II disorder (BD II), predominantly with depressive episodes. Ms. A’s current medications include a combination of lamotrigine 200 mg/d and bupropion extended-release 450 mg/d, and her symptoms were well maintained until 2 weeks ago. When her psychiatrist performs a medication reconciliation at her medication management appointment, Ms. A indicates she started taking an oral contraceptive, ethinyl estradiol and norgestimate, approximately 1 month ago for management of endometriosis symptoms. She is not currently taking any other medications or supplements.
Lamotrigine is indicated for epilepsy and as maintenance treatment for BD I. It is also used off-label to treat other mood disorders. After oral administration, lamotrigine is rapidly and fully absorbed with a high bioavailability (98%). The principal metabolic pathway is via glucuronic acid conjugation, leading to the major inactive metabolite 2-N-glucuronide. Minor metabolites include 5-N-glucuronide and a 2-N-glucuronide metabolite.1
Combined oral contraceptives contain an estrogen component, typically ethinyl estradiol, and a progestin component, which varies based on the specific formulation. The metabolism of ethinyl estradiol occurs through cytochrome P450 (CYP)3A4, CYP2C9, sulfation, and glucuronidation. For progestin—the second component of combined oral contraceptives and the lone component of progestin-only oral contraceptives—metabolism occurs via CYP3A4 and conjugation reactions.2 This article focuses on lamotrigine interactions specifically with oral contraceptives, but it is important to note that other formulations of combined hormonal contraceptives, such as the combined contraceptive patch (Ortho Evra) and vaginal ring (NuvaRing), would be expected to interact in the same way as oral formulations.3
Bidirectional interaction
While many antiseizure medications are known to interact with and potentially decrease the efficacy of oral contraceptives (Table 13-6), the interactions between lamotrigine and oral contraceptives is uniquely bidirectional. Combined oral contraceptives are thought to interact with lamotrigine primarily via the estrogen component, which causes increased metabolism of lamotrigine through induction of glucuronidation. This drug interaction decreases the plasma concentrations of lamotrigine in the body by up to 2-fold, resulting in an increased risk of seizures or inadequate mood stabilization.1 This effect on metabolism is very rapid, resulting in decreases in lamotrigine concentrations within 1 week.4,7 A recent study suggested that certain progestins may also contribute to decreased plasma levels of lamotrigine, but the mechanism for this is unknown (Table 23-7).8

Clinicians should consider increasing the lamotrigine dose (potentially as much as 2-fold) in a patient who initiates treatment with a combined hormonal contraceptive. Dose increases should not be >50 to 100 mg/d every week.1 Collect lamotrigine blood levels before starting a hormonal contraceptive and during dose titration. While there is not a well-established therapeutic range for lamotrigine in BD, expert consensus recommends a range of 1 to 6 mcg/mL.8

The lamotrigine dose should be decreased if combined hormonal contraceptives are discontinued. Dose decreases should not exceed 25% of the total daily dose per week.1 Desogestrel, a progestin-only medication, may increase exposure to lamotrigine, but this has not been observed in research with other progestins.5,9 When starting a progestin-only pill, monitor patients for signs of lamotrigine toxicity (ataxia, diplopia, dizziness) and consider monitoring their blood levels.
An important consideration to note with combined oral contraceptives is the hormone-free interval, also known as the pill-free week. Due to the rapid effect of estrogens, the lamotrigine concentrations have been shown to rise, even double, during this hormone-free interval, so patients should be closely monitored for adverse effects.3 Some recommend use of an extended cycle regimen (with a limited hormone-free interval), or continuous cycle regimen (with no hormone-free interval) to avoid fluctuations in lamotrigine levels.3,5 Additionally, data suggest that in patients taking lamotrigine and valproate, which inhibits glucuronidation, oral contraceptives do not cause reductions in lamotrigine concentrations.2,5 In these instances, dose increases of lamotrigine are not needed.
Continue to: The metabolism of ethinyl estradiol...
The metabolism of ethinyl estradiol and progestin are susceptible to CYP3A4 induction and increased glucuronidation. Serum concentrations may be reduced by ≥50% when used concomitantly with CYP enzyme–inducing medications, which could possibly result in subtherapeutic levels and unplanned pregnancy.3 CYP3A4 induction occurs for up to 4 weeks after discontinuation of an enzyme-inducing agent, pointing to the need for alternative or backup contraception during this time.3 Lamotrigine is not a CYP enzyme–inducing medication; it is unlikely to affect the efficacy of oral contraceptives in the same manner as other antiseizure medications. However, a study of lamotrigine and the combined hormonal contraceptive ethinyl estradiol and levonorgestrel demonstrated reduced exposure to levonorgestrel, resulting in breakthrough bleeding.5
In a study on the coadministration of lamotrigine and combined oral contraceptives, Sidhu et al4 observed a small mean reduction (20%) in progestin concentrations when lamotrigine was used at a dose of 300 mg/d. Although there is no research suggesting decreased effectiveness in preventing pregnancy when lamotrigine is used with combined oral contraceptives, progestin-only oral contraceptives, or progestin implants, additional or alternative contraceptive methods may be considered based on this pharmacokinetic data, particularly in patients who require lamotrigine doses ≥300 mg/d.5
CASE CONTINUED
Given when Ms. A started the oral contraceptive, the treatment team determines it is likely that an interaction with lamotrigine is causing her resurgence of depressive symptoms. Her care team decides to titrate the lamotrigine gradually to 300 mg/d, then 400 mg/d if needed, while carefully monitoring for signs of a serious rash. This dosage increase may help Ms. A achieve symptom remission. Monitoring plasma levels may be considered, although it is unknown what plasma level was effective for Ms. A before she started the oral contraceptive. Ms. A would need to be counseled regarding the effect of higher doses of lamotrigine on the effectiveness of the oral contraceptive.
Although it does not appear Ms. A is using the oral contraceptive specifically to prevent pregnancy, the team informs her about the possibility of unintended pregnancy with this medication combination. If Ms. A was also using the medication for this indication, alternative contraceptive options would include medroxyprogesterone acetate, levonorgestrel implants, or an intrauterine device (levonorgestrel or copper, though copper would not be effective for endometriosis symptom management). Ms. A should consult with her gynecologist regarding the most appropriate option for her endometriosis. If the decision is made to discontinue her oral contraceptive in the future, the lamotrigine dose should be decreased to her previously effective dose of 200 mg/d.
Related Resources
- Makino KK, Hatters Friedman S, Amin J. Emergency contraception for psychiatric patients. Current Psychiatry. 2022;21(11):34-39,44-45. doi:10.12788/cp.0300
- MGH Center for Women’s Mental Health. You asked: is there an interaction between lamotrigine and oral contraceptives? September 29, 2015. https://womensmentalhealth.org/posts/you-asked-is-there-an-interaction-between-lamotrigine-andoral-contraceptives/
Drug Brand Names
Bupropion extended-release • Wellbutrin XL
Carbamazepine • Equetro, Tegretol
Desogestrel • Cerazette
Divalproex sodium • Depakote
Ethinyl estradiol and etonogestrel • NuvaRing
Ethinyl estradiol and norelgestromin • Ortho Evra
Ethinyl estradiol and norgestimate • Ortho Tri-Cyclen, TriNessa, others
Etonogestrel • Implanon, Nexplanon
Gabapentin • Neurontin
Lamotrigine • Lamictal
Levonorgestrel emergency contraceptive pill • AfterPill, Plan B
Levonorgestrel intrauterine device • Mirena, Skyla
Medroxyprogesterone acetate • Depo-Provera
Oxcarbazepine • Trileptal
Topiramate • Topamax
Valproic acid • Depakene
1. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2020.
2. Lee CR. Drug interactions and hormonal contraception. Trends in Urology Gynaecology & Sexual Health. 2009;14(3):23-26.
3. Williams D. Antiepileptic drugs and contraception. US Pharm. 2014;39(1):39-42.
4. Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61(2):191-199. doi:10.1111/j.1365-2125.2005.02539.x
5. Faculty of Sexual & Reproductive Healthcare. Clinical guidance: drug interactions with hormonal contraception. Published May 9, 2022. Accessed September 28, 2022. https://www.fsrh.org/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/
6. Johnston CA, Crawford PM. Anti-epileptic drugs and hormonal treatments. Curr Treat Options Neurol. 2014;16(5):288. doi:10.1007/s11940-014-0288-3
7. Christensen J, Petrenaite V, Atterman J, et al. Oral contraceptives induce lamotrigine metabolism: evidence from a double-blind, placebo-controlled trial. Epilepsia. 2007;48(3):484-489. doi:10.1111/j.1528-1167.2007.00997.x
8. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi:10.1055/s-0043-116492
9. Rauchenzauner M, Deichmann S, Pittschieler, et al. Bidirectional interaction between oral contraception and lamotrigine in women with epilepsy – role of progestins. Seizure. 2020;74:89-92. doi:10.1016/j.seizure.2019.11.011
1. Lamictal [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2020.
2. Lee CR. Drug interactions and hormonal contraception. Trends in Urology Gynaecology & Sexual Health. 2009;14(3):23-26.
3. Williams D. Antiepileptic drugs and contraception. US Pharm. 2014;39(1):39-42.
4. Sidhu J, Job S, Singh S, et al. The pharmacokinetic and pharmacodynamic consequences of the co-administration of lamotrigine and a combined oral contraceptive in healthy female subjects. Br J Clin Pharmacol. 2006;61(2):191-199. doi:10.1111/j.1365-2125.2005.02539.x
5. Faculty of Sexual & Reproductive Healthcare. Clinical guidance: drug interactions with hormonal contraception. Published May 9, 2022. Accessed September 28, 2022. https://www.fsrh.org/documents/ceu-clinical-guidance-drug-interactions-with-hormonal/
6. Johnston CA, Crawford PM. Anti-epileptic drugs and hormonal treatments. Curr Treat Options Neurol. 2014;16(5):288. doi:10.1007/s11940-014-0288-3
7. Christensen J, Petrenaite V, Atterman J, et al. Oral contraceptives induce lamotrigine metabolism: evidence from a double-blind, placebo-controlled trial. Epilepsia. 2007;48(3):484-489. doi:10.1111/j.1528-1167.2007.00997.x
8. Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9-62. doi:10.1055/s-0043-116492
9. Rauchenzauner M, Deichmann S, Pittschieler, et al. Bidirectional interaction between oral contraception and lamotrigine in women with epilepsy – role of progestins. Seizure. 2020;74:89-92. doi:10.1016/j.seizure.2019.11.011
From smiling to smizing: Assessing the affect of a patient wearing a mask
Although the guidelines for masking in hospitals and other health care settings have been revised and face masks are no longer mandatory, it is important to note that some patients and clinicians will choose to continue wearing masks for various personal or clinical reasons. While effective in reducing transmission of the coronavirus, masks have created challenges in assessing patients’ affective states, which impacts the accuracy of diagnosis and treatment. This article discusses strategies for assessing affect in patients wearing face masks.
How masks complicate assessing affect
One obvious challenge masks present is they prevent clinicians from seeing their patients’ facial expressions. Face masks cover the mouth, nose, and cheeks, all of which are involved in communicating emotions. As a result, clinicians may miss important cues that could inform their assessment of a patient’s affect. For example, when a masked patient is smiling, it is difficult to determine whether their smile is genuine or forced. A study that evaluated the interpretation of 6 emotions (angry, disgusted, fearful, happy, neutral, and sad) in masked patients found that emotion recognition was significantly reduced for all emotions except for fearful and neutral faces.1
Another challenge is the potential for misinterpretation. Health care professionals may rely more heavily on nonverbal cues, such as body language, to interpret a patient’s affect. However, these cues can be influenced by other factors, such as cultural differences and individual variations in communication style. Culture is a key component in assessing nonverbal emotion reading cues.2
Strategies to overcome these challenges
There are several strategies clinicians can use to overcome the difficulties of assessing affect while a patient is wearing a mask:
Focus on other nonverbal cues, such as a patient’s posture and hand gestures. Verbal cues—such as tone of voice, choice of words, and voice inflection—can also provide valuable insights. For example, a patient who speaks in a hesitant or monotone voice may be experiencing anxiety or depression. Clinicians can ask open-ended questions, encouraging patients to expand on their emotions and provide further information about their affect.
Maintain eye contact. Eye contact is an essential component of nonverbal communication. The eyes are “the window of the soul” and can convey various emotions including happiness, sadness, fear, anger, surprise, trust, interest, and empathy. Maintaining eye contact is crucial for building positive relationships with patients, and learning to smile with your eyes (smize) can help build rapport.
Take advantage of technology. Clinicians can leverage telemedicine to assess affect. Telemedicine platforms, which have become increasingly popular during the COVID-19 pandemic, allow clinicians to monitor patients remotely and observe nonverbal cues. Virtual reality technology can also help by documenting physiological responses such as heart rate and skin conductance.
Use standardized assessment tools, as these instruments can aid in assessing affect. For example, the Patient Health Questionnaire-9 and Generalized Anxiety Disorder 7-item scale are standardized questionnaires assessing depression and anxiety, respectively. Administering these tools to patients wearing a face mask can provide information about their affective state.
1. Carbon CC. Wearing face masks strongly confuses counterparts in reading emotions. Front Psychol. 2020;11:566886. doi:10.3389/fpsyg.2020.566886
2. Yuki M, Maddux WW, Masuda T. Are the windows to the soul the same in the East and West? Cultural differences in using the eyes and mouth as cues to recognize emotions in Japan and the United States. J Exp Soc Psychol. 2007;43(2):303-311.
Although the guidelines for masking in hospitals and other health care settings have been revised and face masks are no longer mandatory, it is important to note that some patients and clinicians will choose to continue wearing masks for various personal or clinical reasons. While effective in reducing transmission of the coronavirus, masks have created challenges in assessing patients’ affective states, which impacts the accuracy of diagnosis and treatment. This article discusses strategies for assessing affect in patients wearing face masks.
How masks complicate assessing affect
One obvious challenge masks present is they prevent clinicians from seeing their patients’ facial expressions. Face masks cover the mouth, nose, and cheeks, all of which are involved in communicating emotions. As a result, clinicians may miss important cues that could inform their assessment of a patient’s affect. For example, when a masked patient is smiling, it is difficult to determine whether their smile is genuine or forced. A study that evaluated the interpretation of 6 emotions (angry, disgusted, fearful, happy, neutral, and sad) in masked patients found that emotion recognition was significantly reduced for all emotions except for fearful and neutral faces.1
Another challenge is the potential for misinterpretation. Health care professionals may rely more heavily on nonverbal cues, such as body language, to interpret a patient’s affect. However, these cues can be influenced by other factors, such as cultural differences and individual variations in communication style. Culture is a key component in assessing nonverbal emotion reading cues.2
Strategies to overcome these challenges
There are several strategies clinicians can use to overcome the difficulties of assessing affect while a patient is wearing a mask:
Focus on other nonverbal cues, such as a patient’s posture and hand gestures. Verbal cues—such as tone of voice, choice of words, and voice inflection—can also provide valuable insights. For example, a patient who speaks in a hesitant or monotone voice may be experiencing anxiety or depression. Clinicians can ask open-ended questions, encouraging patients to expand on their emotions and provide further information about their affect.
Maintain eye contact. Eye contact is an essential component of nonverbal communication. The eyes are “the window of the soul” and can convey various emotions including happiness, sadness, fear, anger, surprise, trust, interest, and empathy. Maintaining eye contact is crucial for building positive relationships with patients, and learning to smile with your eyes (smize) can help build rapport.
Take advantage of technology. Clinicians can leverage telemedicine to assess affect. Telemedicine platforms, which have become increasingly popular during the COVID-19 pandemic, allow clinicians to monitor patients remotely and observe nonverbal cues. Virtual reality technology can also help by documenting physiological responses such as heart rate and skin conductance.
Use standardized assessment tools, as these instruments can aid in assessing affect. For example, the Patient Health Questionnaire-9 and Generalized Anxiety Disorder 7-item scale are standardized questionnaires assessing depression and anxiety, respectively. Administering these tools to patients wearing a face mask can provide information about their affective state.
Although the guidelines for masking in hospitals and other health care settings have been revised and face masks are no longer mandatory, it is important to note that some patients and clinicians will choose to continue wearing masks for various personal or clinical reasons. While effective in reducing transmission of the coronavirus, masks have created challenges in assessing patients’ affective states, which impacts the accuracy of diagnosis and treatment. This article discusses strategies for assessing affect in patients wearing face masks.
How masks complicate assessing affect
One obvious challenge masks present is they prevent clinicians from seeing their patients’ facial expressions. Face masks cover the mouth, nose, and cheeks, all of which are involved in communicating emotions. As a result, clinicians may miss important cues that could inform their assessment of a patient’s affect. For example, when a masked patient is smiling, it is difficult to determine whether their smile is genuine or forced. A study that evaluated the interpretation of 6 emotions (angry, disgusted, fearful, happy, neutral, and sad) in masked patients found that emotion recognition was significantly reduced for all emotions except for fearful and neutral faces.1
Another challenge is the potential for misinterpretation. Health care professionals may rely more heavily on nonverbal cues, such as body language, to interpret a patient’s affect. However, these cues can be influenced by other factors, such as cultural differences and individual variations in communication style. Culture is a key component in assessing nonverbal emotion reading cues.2
Strategies to overcome these challenges
There are several strategies clinicians can use to overcome the difficulties of assessing affect while a patient is wearing a mask:
Focus on other nonverbal cues, such as a patient’s posture and hand gestures. Verbal cues—such as tone of voice, choice of words, and voice inflection—can also provide valuable insights. For example, a patient who speaks in a hesitant or monotone voice may be experiencing anxiety or depression. Clinicians can ask open-ended questions, encouraging patients to expand on their emotions and provide further information about their affect.
Maintain eye contact. Eye contact is an essential component of nonverbal communication. The eyes are “the window of the soul” and can convey various emotions including happiness, sadness, fear, anger, surprise, trust, interest, and empathy. Maintaining eye contact is crucial for building positive relationships with patients, and learning to smile with your eyes (smize) can help build rapport.
Take advantage of technology. Clinicians can leverage telemedicine to assess affect. Telemedicine platforms, which have become increasingly popular during the COVID-19 pandemic, allow clinicians to monitor patients remotely and observe nonverbal cues. Virtual reality technology can also help by documenting physiological responses such as heart rate and skin conductance.
Use standardized assessment tools, as these instruments can aid in assessing affect. For example, the Patient Health Questionnaire-9 and Generalized Anxiety Disorder 7-item scale are standardized questionnaires assessing depression and anxiety, respectively. Administering these tools to patients wearing a face mask can provide information about their affective state.
1. Carbon CC. Wearing face masks strongly confuses counterparts in reading emotions. Front Psychol. 2020;11:566886. doi:10.3389/fpsyg.2020.566886
2. Yuki M, Maddux WW, Masuda T. Are the windows to the soul the same in the East and West? Cultural differences in using the eyes and mouth as cues to recognize emotions in Japan and the United States. J Exp Soc Psychol. 2007;43(2):303-311.
1. Carbon CC. Wearing face masks strongly confuses counterparts in reading emotions. Front Psychol. 2020;11:566886. doi:10.3389/fpsyg.2020.566886
2. Yuki M, Maddux WW, Masuda T. Are the windows to the soul the same in the East and West? Cultural differences in using the eyes and mouth as cues to recognize emotions in Japan and the United States. J Exp Soc Psychol. 2007;43(2):303-311.
Homelessness in urban areas: The role of mental illness and need for collaboration
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
As an emergency department (ED) psychiatrist with 25 years of experience working in a large city, I am growing increasingly concerned about the escalating number of individuals experiencing homelessness in urban areas.
Homelessness remains a critical issue across the United States. The news reports from major urban areas are startling. In my own practice, I encounter approximately 10,000 patients annually, and at least one-half of them are homeless. Additionally, 75% of these patients who are homeless experience addiction, and many have lost all social support. Due to overcrowding at our area’s shelters, they resort to using the ED as a shelter because most of our shelters are overcrowded. This situation has caused an overwhelming overload in the ED and left staff disheartened and difficult to retain.
The relationship between mental illness and homelessness is complex and multifaceted. Research suggests that up to one-third of individuals who are homeless have serious mental illness.1 Mental illness can contribute to homelessness by impeding individuals’ ability to maintain employment, housing, and social relationships. Conversely, homelessness can worsen mental illness (especially in younger individuals, who are most vulnerable) by exposing individuals to traumatic experiences, substance abuse, and other stressors.2
One approach to effectively address homelessness in urban areas is provide supportive housing that incorporates access to mental health services. Research has demonstrated that offering stable housing and mental health services to individuals experiencing homelessness can significantly improve their mental and physical health and reduce their reliance on costly emergency services.3,4
Collaboration between the health care system and government is also essential. By working together, the health care system and government can develop comprehensive strategies, allocate resources, and implement interventions that address the physical and mental health needs of individuals who are homeless and provide them with the necessary support and services. This collaboration is essential to create sustainable solutions and make a meaningful impact in combating homelessness.5
Addressing homelessness in urban areas requires a comprehensive approach that recognizes the critical role of mental illness and necessity for collaborative solutions. While our ED has implemented certain measures, such as allowing patients to remain on 23-hour holds to prevent immediate re-admission, additional interventions are needed. These include expanding shelters and transitional housing programs, which are currently in short supply, and developing street medicine programs to meet individuals where they are and improve compliance with medications. By implementing these strategies, we can help minimize the impact of homelessness on individuals with mental illness and enhance the health and well-being of individuals experiencing homelessness.
1. Folsom DP, Hawthorne W, Lindamer L, et al. Prevalence and risk factors for homelessness and utilization of mental health services among 10,340 patients with serious mental illness in a large public mental health system. Am J Psychiatry. 2005;162(2):370-376. doi:10.1176/appi.ajp.162.2.370
2. Davis JP, Diguiseppi G, De Leon J, et al. Understanding pathways between PTSD, homelessness, and substance use among adolescents. Psychol Addict Behav. 2019;33(5):467-476. doi:10.1037/adb0000488
3. Larimer ME, Malone DK, Garner MD, et al. Health care and public service use and costs before and after provision of housing for chronically homeless persons with severe alcohol problems. JAMA. 2009;301(13):1349-1357. doi:10.1001/jama.2009.414
4. Wolitski RJ, Kidder DP, Pals SL, et al; Housing and Health Study Team. Randomized trial of the effects of housing assistance on the health and risk behaviors of homeless and unstably housed people living with HIV. AIDS Behav. 2010;14(3):493-503. doi:10.1007/s10461-009-9643-x
5. Sleet DA, Francescutti LH. Homelessness and public health: a focus on strategies and solutions. Int J Environ Res Public Health. 2021;18(21):11660. doi:10.3390/ijerph182111660
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
As an emergency department (ED) psychiatrist with 25 years of experience working in a large city, I am growing increasingly concerned about the escalating number of individuals experiencing homelessness in urban areas.
Homelessness remains a critical issue across the United States. The news reports from major urban areas are startling. In my own practice, I encounter approximately 10,000 patients annually, and at least one-half of them are homeless. Additionally, 75% of these patients who are homeless experience addiction, and many have lost all social support. Due to overcrowding at our area’s shelters, they resort to using the ED as a shelter because most of our shelters are overcrowded. This situation has caused an overwhelming overload in the ED and left staff disheartened and difficult to retain.
The relationship between mental illness and homelessness is complex and multifaceted. Research suggests that up to one-third of individuals who are homeless have serious mental illness.1 Mental illness can contribute to homelessness by impeding individuals’ ability to maintain employment, housing, and social relationships. Conversely, homelessness can worsen mental illness (especially in younger individuals, who are most vulnerable) by exposing individuals to traumatic experiences, substance abuse, and other stressors.2
One approach to effectively address homelessness in urban areas is provide supportive housing that incorporates access to mental health services. Research has demonstrated that offering stable housing and mental health services to individuals experiencing homelessness can significantly improve their mental and physical health and reduce their reliance on costly emergency services.3,4
Collaboration between the health care system and government is also essential. By working together, the health care system and government can develop comprehensive strategies, allocate resources, and implement interventions that address the physical and mental health needs of individuals who are homeless and provide them with the necessary support and services. This collaboration is essential to create sustainable solutions and make a meaningful impact in combating homelessness.5
Addressing homelessness in urban areas requires a comprehensive approach that recognizes the critical role of mental illness and necessity for collaborative solutions. While our ED has implemented certain measures, such as allowing patients to remain on 23-hour holds to prevent immediate re-admission, additional interventions are needed. These include expanding shelters and transitional housing programs, which are currently in short supply, and developing street medicine programs to meet individuals where they are and improve compliance with medications. By implementing these strategies, we can help minimize the impact of homelessness on individuals with mental illness and enhance the health and well-being of individuals experiencing homelessness.
Editor’s note: Readers’ Forum is a department for correspondence from readers that is not in response to articles published in
As an emergency department (ED) psychiatrist with 25 years of experience working in a large city, I am growing increasingly concerned about the escalating number of individuals experiencing homelessness in urban areas.
Homelessness remains a critical issue across the United States. The news reports from major urban areas are startling. In my own practice, I encounter approximately 10,000 patients annually, and at least one-half of them are homeless. Additionally, 75% of these patients who are homeless experience addiction, and many have lost all social support. Due to overcrowding at our area’s shelters, they resort to using the ED as a shelter because most of our shelters are overcrowded. This situation has caused an overwhelming overload in the ED and left staff disheartened and difficult to retain.
The relationship between mental illness and homelessness is complex and multifaceted. Research suggests that up to one-third of individuals who are homeless have serious mental illness.1 Mental illness can contribute to homelessness by impeding individuals’ ability to maintain employment, housing, and social relationships. Conversely, homelessness can worsen mental illness (especially in younger individuals, who are most vulnerable) by exposing individuals to traumatic experiences, substance abuse, and other stressors.2
One approach to effectively address homelessness in urban areas is provide supportive housing that incorporates access to mental health services. Research has demonstrated that offering stable housing and mental health services to individuals experiencing homelessness can significantly improve their mental and physical health and reduce their reliance on costly emergency services.3,4
Collaboration between the health care system and government is also essential. By working together, the health care system and government can develop comprehensive strategies, allocate resources, and implement interventions that address the physical and mental health needs of individuals who are homeless and provide them with the necessary support and services. This collaboration is essential to create sustainable solutions and make a meaningful impact in combating homelessness.5
Addressing homelessness in urban areas requires a comprehensive approach that recognizes the critical role of mental illness and necessity for collaborative solutions. While our ED has implemented certain measures, such as allowing patients to remain on 23-hour holds to prevent immediate re-admission, additional interventions are needed. These include expanding shelters and transitional housing programs, which are currently in short supply, and developing street medicine programs to meet individuals where they are and improve compliance with medications. By implementing these strategies, we can help minimize the impact of homelessness on individuals with mental illness and enhance the health and well-being of individuals experiencing homelessness.
1. Folsom DP, Hawthorne W, Lindamer L, et al. Prevalence and risk factors for homelessness and utilization of mental health services among 10,340 patients with serious mental illness in a large public mental health system. Am J Psychiatry. 2005;162(2):370-376. doi:10.1176/appi.ajp.162.2.370
2. Davis JP, Diguiseppi G, De Leon J, et al. Understanding pathways between PTSD, homelessness, and substance use among adolescents. Psychol Addict Behav. 2019;33(5):467-476. doi:10.1037/adb0000488
3. Larimer ME, Malone DK, Garner MD, et al. Health care and public service use and costs before and after provision of housing for chronically homeless persons with severe alcohol problems. JAMA. 2009;301(13):1349-1357. doi:10.1001/jama.2009.414
4. Wolitski RJ, Kidder DP, Pals SL, et al; Housing and Health Study Team. Randomized trial of the effects of housing assistance on the health and risk behaviors of homeless and unstably housed people living with HIV. AIDS Behav. 2010;14(3):493-503. doi:10.1007/s10461-009-9643-x
5. Sleet DA, Francescutti LH. Homelessness and public health: a focus on strategies and solutions. Int J Environ Res Public Health. 2021;18(21):11660. doi:10.3390/ijerph182111660
1. Folsom DP, Hawthorne W, Lindamer L, et al. Prevalence and risk factors for homelessness and utilization of mental health services among 10,340 patients with serious mental illness in a large public mental health system. Am J Psychiatry. 2005;162(2):370-376. doi:10.1176/appi.ajp.162.2.370
2. Davis JP, Diguiseppi G, De Leon J, et al. Understanding pathways between PTSD, homelessness, and substance use among adolescents. Psychol Addict Behav. 2019;33(5):467-476. doi:10.1037/adb0000488
3. Larimer ME, Malone DK, Garner MD, et al. Health care and public service use and costs before and after provision of housing for chronically homeless persons with severe alcohol problems. JAMA. 2009;301(13):1349-1357. doi:10.1001/jama.2009.414
4. Wolitski RJ, Kidder DP, Pals SL, et al; Housing and Health Study Team. Randomized trial of the effects of housing assistance on the health and risk behaviors of homeless and unstably housed people living with HIV. AIDS Behav. 2010;14(3):493-503. doi:10.1007/s10461-009-9643-x
5. Sleet DA, Francescutti LH. Homelessness and public health: a focus on strategies and solutions. Int J Environ Res Public Health. 2021;18(21):11660. doi:10.3390/ijerph182111660
More on an asymmetric life, transient global amnesia
More on an asymmetric life
I enjoy receiving
Too often, families bear the burden of an individual’s hyperfocused pursuits. I hope your wife has been able to pursue her occupation with the same zeal and commitment. We have all read biographies of driven individuals and, unfortunately, someone pays the price for another’s success. For every Steve Jobs, there is a Lisa Jobs.
If we were surgeons, I would applaud your essay. However, we are psychiatrists. If anything, we balance out the reductionist forces in medicine. When every other physician claims a cure with medications or procedures, we look at all aspects of the patient’s life to find the appropriate treatment. At least that’s what we should be doing.
I was part of the first class of residents to work under the 80-hours-per-week restrictions. I was grateful for the extra time to rest, exercise, and spend time with my wife. The 80-hour restrictions improved resident wellness and had no impact on patient care. There are intangible benefits of diverting the mind from a chosen pursuit (such as creativity).
There is no doubt that becoming number 1 in any field requires a tremendous amount of determination, sacrifice, and effort. But not everyone gets to be first. Our society’s single-minded focus on being the best has had a major impact on mental health, especially for children. I hope you can address that in a future editorial.
Sudhir Nagaraja, DO, MS
Fredericksburg, Virginia
Dr. Nasrallah responds
Thank you for your letter about my editorial. You obviously believe in leading a balanced life, and that’s fine if you so choose. I described why I decided at an early age to lead an intensive, “purpose-driven life,” which requires investing much more time than others do, to achieve my lofty goals and excel in my area of expertise (academic psychiatry). It is really a “calling,” and those who score an extraordinary achievement (a moonshot) in their life, including Olympic gold medalists, entrepreneurs, inventors, or Nobel laureates, must do exactly what I do. I am not urging anyone to do what I have chosen to do in my life. Everyone defines for themselves what constitutes the pursuit of happiness.
You mentioned my wife. Let me assert that she is highly successful as a mother and as a research psychologist. She is my extremely valuable life partner and very supportive of what I do. I am fortunate to have chosen well!
Continue to: More on transient global amnesia
More on transient global amnesia
Your recent article on transient global amnesia (TGA) (“Transient global amnesia: Psychiatric precipitants, features, and comorbidities,”
I witnessed TGA, experienced by my brother, while on a surf trip. After bodyboarding for about an hour in cold water, wearing a full wet suit and hood, he met me on the beach. He recognized me and knew my name but had no idea where we were, how we got there, or other events from earlier that morning. There was no stressor, just the usual surfing excitement. We went to a local emergency department, where the physical examination, usual laboratory tests, and neuroimaging were normal. After approximately 5 hours, he began to fully recall recent events. Ten years later, there has been no recurrence. The only change in his surfing habits has been to avoid using a hood with neck coverage.
In 2022, Papadis et al1 described a case of concurrent Takotsubo cardiomyopathy and TGA, noting that cardiovascular dysfunction and neurologic dysfunction may be provoked by an emotional or stressful situation. The interesting observations of capture myopathy from animal literature appear similar to human reactions to trauma.1-3
Case reports of scopolamine intoxication have been linked to TGA. Severe memory disturbances, characteristics of dry mouth, blurred vision, and tachycardia were evident. Certain South American plant extracts popularly known as “Burundanga” have anticholinergic effects. Severe anterograde amnesia and submissiveness represent the 2 most notorious clinical signs of Burundanga intoxication.4
As one reviews single and groups of case studies, several things stand out. The hallmark of TGA is the sudden inability to make new memories, which resolves in a few hours. The brief and isolated dysfunction is what distinguishes this condition from most episodic disorders, but a clinician should not prognosticate too much without screening for ischemic or metabolic disturbance. Common associated precursors include Valsalva-associated activities, emotional stress with anxiety, acute pain, cold water immersion, static neck posture, and age older than 55.5,6
Neuropsychiatric disorders involve the neuron and its connections. Major reflexes automate the processes of the “neurocardiac” axis. The vasovagal reflex (Barcroft/Edholm reflex), diving reflex, baroreceptor reflex, Cushing reflex, and others depend upon the conversion of a mechanical stimulus to neurotransmission. The reflexes have sensors, afferent paths, a central processing, and efferent paths that lead to events or experiences. CNS processing is complex but the brainstem, amygdala, prefrontal cortex, and some cortical regions are involved. Neurocardiac reactions can come from pathologic events, including ischemia, metabolic disturbance, pain signals, or emotional effects within the axis.7-11
Understanding neurocardiac reflexes may help our progress with challenging clinical conditions, such as chronic pain, trauma, and cognitive impairment. The broad use of vagus nerve stimulation is one indicator of the power of this focus.12-19 Lewis20 suggested increased susceptibility to retrograde jugular venous flow could cause regional brain ischemia, resulting in TGA. The competency of jugular venous valves during the Valsalva maneuver could be assessed with Doppler ultrasound. Abnormalities could be managed, and results assessed.20,21 Vascular shunting from memory regions in the brain to essential neurocardiac control areas should be considered.
Cholinergic processes are active in the parasympathetic nervous system, sustained attention, working memory, executive functions, and mood. Increased central cholinergic activity may lead to depression. Scopolamine, in its therapeutic range, has antidepressant effects but in toxic doses is a dissociative agent.22,23 While cholinesterase inhibitors are used in Alzheimer disease, cholinergic agonists have yet to play a large role in general psychiatry or functional neurology.
TGA continues to provide a window into memory, functional disorders, psychological defenses, and adaptive neurocardiac processes. Continued clinical care and research might include gradual adaptation to cold water immersion, caution with the Valsalva maneuver, cholinergic support, managing the trapped response, avoiding interference with normal jugular flow, and evaluation for jugular venous insufficiency.
Because a variety of medical procedures can trigger TGA, health care professionals in many fields need to understand this symptom complex.24-27 Thanks to the authors for raising the awareness of TGA for psychiatrists.
Mark Chandler, MD
Durham, North Carolina
References
1. Papadis A, Svab S, Brugger N, et al. “Broken heart” and “broken brain”: which connection? Cardiol Res. 2022;13(1):65-70. doi:10.14740/cr1336
2. Blumstein DT, Buckner J, Shah S, et al. The evolution of capture myopathy in hooved mammals: a model for human stress cardiomyopathy? Evol Med Public Health. 2015;2015(1):195-203. doi:10.1093/emph/eov015
3. Seguel M, Paredes E, Pavés H, et al. Capture-induced stress cardiomyopathy in South American fur seal pups (Arctophoca australis gracilis). Marine Mammal Science. 2014;30(3): 1149-1157. https://doi.org/10.1111/mms.12079
4. Ardila A, Moreno C. Scopolamine intoxication as a model of transient global amnesia. Brain Cogn. 1991;15(2):236-245. doi:10.1016/0278-2626(91)90028-7
5. Bartsch T, Deuschl G. Transient global amnesia: functional anatomy and clinical implications. Lancet Neurol. 2010;9(2):205-214. doi:10.1016/S1474-4422(09)70344-8
6. Spiegel DR, Smith J, Wade RR, et al. Transient global amnesia: current perspectives. Neuropsychiatr Dis Treat. 2017;13:2691-2703. doi:10.2147/NDT.S130710
7. Yartsev A. Cardiac reflexes. August 15, 2020. Updated May 19, 2023. Accessed June 12, 2023. https://derangedphysiology.com/main/cicm-primary-exam/required-reading/cardiovascular-system/Chapter%20491/cardiac-reflexes
8. Lemaitre F, Chowdhury T, Schaller B. The trigeminocardiac reflex - a comparison with the diving reflex in humans. Arch Med Sci. 2015;11(2):419-426. doi:10.5114/aoms.2015.50974
9. Lindholm P, Lundgren CE. The physiology and pathophysiology of human breath-hold diving. J Appl Physiol (1985). 2009;106(1):284-292. doi:10.1152/japplphysiol.90991.2008
10. Tansey EA, Johnson CD. Recent advances in thermoregulation. Adv Physiol Educ. 2015;39(3):139-148. doi:10.1152/advan.00126.2014
11. Alboni P, Alboni M. Vasovagal syncope as a manifestation of an evolutionary selected trait. J Atr Fibrillation. 2014;7(2):1035. doi:10.4022/jafib.1035
12. Badran BW, Austelle CW. The future is noninvasive: a brief review of the evolution and clinical utility of vagus nerve stimulation. Focus (Am Psychiatr Publ). 2022;20(1):3-7. doi:10.1176/appi.focus.20210023
13. Suarez-Roca H, Mamoun N, Sigurdson MI, et al. Baroreceptor modulation of the cardiovascular system, pain, consciousness, and cognition. Compr Physiol. 2021;11(2):1373-1423. doi:10.1002/cphy.c190038
14. Pinna T, Edwards DJ. A systematic review of associations between interoception, vagal tone, and emotional regulation: potential applications for mental health, wellbeing, psychological flexibility, and chronic conditions. Front Psychol. 2020;11:1792. doi:10.3389/fpsyg.2020.01792
15. Howland RH. Vagus nerve stimulation. Curr Behav Neurosci Rep. 2014 Jun;1(2):64-73. doi:10.1007/s40473-014-0010-5
16. Panneton WM, Gan Q. The mammalian diving response: inroads to its neural control. Front Neurosci. 2020;14:524. doi:10.3389/fnins.2020.00524
17. Khurana RK, Wu R. The cold face test: a non-baroreflex mediated test of cardiac vagal function. Clin Auton Res. 2006;16(3):202-207. doi:10.1007/s10286-006-0332-9
18. Montirosso R, Provenzi L, Tronick E, et al. Vagal tone as a biomarker of long-term memory for a stressful social event at 4 months. Dev Psychobiol. 2014;56(7):1564-1574. doi:10.1002/dev.21251
19. Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. Int J Psychophysiol. 2003;48(3):263-274. doi:10.1016/s0167-8760(03)00073-4
20. Lewis SL. Aetiology of transient global amnesia. Lancet. 1998;352(9125):397-399. doi:10.1016/S0140-6736(98)01442-1
21. Han K, Chao AC, Chang FC, et al. Obstruction of venous drainage linked to transient global amnesia. PLoS One. 2015;10(7):e0132893. doi:10.1371/journal.pone.0132893
22. Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76(1):116-129. doi:10.1016/j.neuron.2012.08.036
23. Dulawa SC, Janowsky DS. Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol Psychiatry. 2019;24(5):694-709. doi:10.1038/s41380-018-0219-x
24. Grande LA, Loeser JD, Samii A. Recurrent transient global amnesia with intrathecal baclofen. Anesth Analg. 2008;106(4):1284-1287. doi:10.1213/ane.0b013e318165e1c6
25. Carrard J, Lambert AC, Genné D. Transient global amnesia following a whole-body cryotherapy session. BMJ Case Rep. 2017;2017:bcr2017221431. doi:10.1136/bcr-2017-221431
26. Jeong M, Kim WS, Kim AR, et al. Medical procedure-related transient global amnesia. Eur Neurol. 2018;80(1-2):42-49. doi:10.1159/000493163
27. Shah B, Hussain MW. Concussion causing transient global amnesia: further insights into pathophysiology? Neurology. 2020;95(20 Suppl 1):S16. doi:10.1212/01.wnl.0000720020.86134.9d
More on an asymmetric life
I enjoy receiving
Too often, families bear the burden of an individual’s hyperfocused pursuits. I hope your wife has been able to pursue her occupation with the same zeal and commitment. We have all read biographies of driven individuals and, unfortunately, someone pays the price for another’s success. For every Steve Jobs, there is a Lisa Jobs.
If we were surgeons, I would applaud your essay. However, we are psychiatrists. If anything, we balance out the reductionist forces in medicine. When every other physician claims a cure with medications or procedures, we look at all aspects of the patient’s life to find the appropriate treatment. At least that’s what we should be doing.
I was part of the first class of residents to work under the 80-hours-per-week restrictions. I was grateful for the extra time to rest, exercise, and spend time with my wife. The 80-hour restrictions improved resident wellness and had no impact on patient care. There are intangible benefits of diverting the mind from a chosen pursuit (such as creativity).
There is no doubt that becoming number 1 in any field requires a tremendous amount of determination, sacrifice, and effort. But not everyone gets to be first. Our society’s single-minded focus on being the best has had a major impact on mental health, especially for children. I hope you can address that in a future editorial.
Sudhir Nagaraja, DO, MS
Fredericksburg, Virginia
Dr. Nasrallah responds
Thank you for your letter about my editorial. You obviously believe in leading a balanced life, and that’s fine if you so choose. I described why I decided at an early age to lead an intensive, “purpose-driven life,” which requires investing much more time than others do, to achieve my lofty goals and excel in my area of expertise (academic psychiatry). It is really a “calling,” and those who score an extraordinary achievement (a moonshot) in their life, including Olympic gold medalists, entrepreneurs, inventors, or Nobel laureates, must do exactly what I do. I am not urging anyone to do what I have chosen to do in my life. Everyone defines for themselves what constitutes the pursuit of happiness.
You mentioned my wife. Let me assert that she is highly successful as a mother and as a research psychologist. She is my extremely valuable life partner and very supportive of what I do. I am fortunate to have chosen well!
Continue to: More on transient global amnesia
More on transient global amnesia
Your recent article on transient global amnesia (TGA) (“Transient global amnesia: Psychiatric precipitants, features, and comorbidities,”
I witnessed TGA, experienced by my brother, while on a surf trip. After bodyboarding for about an hour in cold water, wearing a full wet suit and hood, he met me on the beach. He recognized me and knew my name but had no idea where we were, how we got there, or other events from earlier that morning. There was no stressor, just the usual surfing excitement. We went to a local emergency department, where the physical examination, usual laboratory tests, and neuroimaging were normal. After approximately 5 hours, he began to fully recall recent events. Ten years later, there has been no recurrence. The only change in his surfing habits has been to avoid using a hood with neck coverage.
In 2022, Papadis et al1 described a case of concurrent Takotsubo cardiomyopathy and TGA, noting that cardiovascular dysfunction and neurologic dysfunction may be provoked by an emotional or stressful situation. The interesting observations of capture myopathy from animal literature appear similar to human reactions to trauma.1-3
Case reports of scopolamine intoxication have been linked to TGA. Severe memory disturbances, characteristics of dry mouth, blurred vision, and tachycardia were evident. Certain South American plant extracts popularly known as “Burundanga” have anticholinergic effects. Severe anterograde amnesia and submissiveness represent the 2 most notorious clinical signs of Burundanga intoxication.4
As one reviews single and groups of case studies, several things stand out. The hallmark of TGA is the sudden inability to make new memories, which resolves in a few hours. The brief and isolated dysfunction is what distinguishes this condition from most episodic disorders, but a clinician should not prognosticate too much without screening for ischemic or metabolic disturbance. Common associated precursors include Valsalva-associated activities, emotional stress with anxiety, acute pain, cold water immersion, static neck posture, and age older than 55.5,6
Neuropsychiatric disorders involve the neuron and its connections. Major reflexes automate the processes of the “neurocardiac” axis. The vasovagal reflex (Barcroft/Edholm reflex), diving reflex, baroreceptor reflex, Cushing reflex, and others depend upon the conversion of a mechanical stimulus to neurotransmission. The reflexes have sensors, afferent paths, a central processing, and efferent paths that lead to events or experiences. CNS processing is complex but the brainstem, amygdala, prefrontal cortex, and some cortical regions are involved. Neurocardiac reactions can come from pathologic events, including ischemia, metabolic disturbance, pain signals, or emotional effects within the axis.7-11
Understanding neurocardiac reflexes may help our progress with challenging clinical conditions, such as chronic pain, trauma, and cognitive impairment. The broad use of vagus nerve stimulation is one indicator of the power of this focus.12-19 Lewis20 suggested increased susceptibility to retrograde jugular venous flow could cause regional brain ischemia, resulting in TGA. The competency of jugular venous valves during the Valsalva maneuver could be assessed with Doppler ultrasound. Abnormalities could be managed, and results assessed.20,21 Vascular shunting from memory regions in the brain to essential neurocardiac control areas should be considered.
Cholinergic processes are active in the parasympathetic nervous system, sustained attention, working memory, executive functions, and mood. Increased central cholinergic activity may lead to depression. Scopolamine, in its therapeutic range, has antidepressant effects but in toxic doses is a dissociative agent.22,23 While cholinesterase inhibitors are used in Alzheimer disease, cholinergic agonists have yet to play a large role in general psychiatry or functional neurology.
TGA continues to provide a window into memory, functional disorders, psychological defenses, and adaptive neurocardiac processes. Continued clinical care and research might include gradual adaptation to cold water immersion, caution with the Valsalva maneuver, cholinergic support, managing the trapped response, avoiding interference with normal jugular flow, and evaluation for jugular venous insufficiency.
Because a variety of medical procedures can trigger TGA, health care professionals in many fields need to understand this symptom complex.24-27 Thanks to the authors for raising the awareness of TGA for psychiatrists.
Mark Chandler, MD
Durham, North Carolina
References
1. Papadis A, Svab S, Brugger N, et al. “Broken heart” and “broken brain”: which connection? Cardiol Res. 2022;13(1):65-70. doi:10.14740/cr1336
2. Blumstein DT, Buckner J, Shah S, et al. The evolution of capture myopathy in hooved mammals: a model for human stress cardiomyopathy? Evol Med Public Health. 2015;2015(1):195-203. doi:10.1093/emph/eov015
3. Seguel M, Paredes E, Pavés H, et al. Capture-induced stress cardiomyopathy in South American fur seal pups (Arctophoca australis gracilis). Marine Mammal Science. 2014;30(3): 1149-1157. https://doi.org/10.1111/mms.12079
4. Ardila A, Moreno C. Scopolamine intoxication as a model of transient global amnesia. Brain Cogn. 1991;15(2):236-245. doi:10.1016/0278-2626(91)90028-7
5. Bartsch T, Deuschl G. Transient global amnesia: functional anatomy and clinical implications. Lancet Neurol. 2010;9(2):205-214. doi:10.1016/S1474-4422(09)70344-8
6. Spiegel DR, Smith J, Wade RR, et al. Transient global amnesia: current perspectives. Neuropsychiatr Dis Treat. 2017;13:2691-2703. doi:10.2147/NDT.S130710
7. Yartsev A. Cardiac reflexes. August 15, 2020. Updated May 19, 2023. Accessed June 12, 2023. https://derangedphysiology.com/main/cicm-primary-exam/required-reading/cardiovascular-system/Chapter%20491/cardiac-reflexes
8. Lemaitre F, Chowdhury T, Schaller B. The trigeminocardiac reflex - a comparison with the diving reflex in humans. Arch Med Sci. 2015;11(2):419-426. doi:10.5114/aoms.2015.50974
9. Lindholm P, Lundgren CE. The physiology and pathophysiology of human breath-hold diving. J Appl Physiol (1985). 2009;106(1):284-292. doi:10.1152/japplphysiol.90991.2008
10. Tansey EA, Johnson CD. Recent advances in thermoregulation. Adv Physiol Educ. 2015;39(3):139-148. doi:10.1152/advan.00126.2014
11. Alboni P, Alboni M. Vasovagal syncope as a manifestation of an evolutionary selected trait. J Atr Fibrillation. 2014;7(2):1035. doi:10.4022/jafib.1035
12. Badran BW, Austelle CW. The future is noninvasive: a brief review of the evolution and clinical utility of vagus nerve stimulation. Focus (Am Psychiatr Publ). 2022;20(1):3-7. doi:10.1176/appi.focus.20210023
13. Suarez-Roca H, Mamoun N, Sigurdson MI, et al. Baroreceptor modulation of the cardiovascular system, pain, consciousness, and cognition. Compr Physiol. 2021;11(2):1373-1423. doi:10.1002/cphy.c190038
14. Pinna T, Edwards DJ. A systematic review of associations between interoception, vagal tone, and emotional regulation: potential applications for mental health, wellbeing, psychological flexibility, and chronic conditions. Front Psychol. 2020;11:1792. doi:10.3389/fpsyg.2020.01792
15. Howland RH. Vagus nerve stimulation. Curr Behav Neurosci Rep. 2014 Jun;1(2):64-73. doi:10.1007/s40473-014-0010-5
16. Panneton WM, Gan Q. The mammalian diving response: inroads to its neural control. Front Neurosci. 2020;14:524. doi:10.3389/fnins.2020.00524
17. Khurana RK, Wu R. The cold face test: a non-baroreflex mediated test of cardiac vagal function. Clin Auton Res. 2006;16(3):202-207. doi:10.1007/s10286-006-0332-9
18. Montirosso R, Provenzi L, Tronick E, et al. Vagal tone as a biomarker of long-term memory for a stressful social event at 4 months. Dev Psychobiol. 2014;56(7):1564-1574. doi:10.1002/dev.21251
19. Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. Int J Psychophysiol. 2003;48(3):263-274. doi:10.1016/s0167-8760(03)00073-4
20. Lewis SL. Aetiology of transient global amnesia. Lancet. 1998;352(9125):397-399. doi:10.1016/S0140-6736(98)01442-1
21. Han K, Chao AC, Chang FC, et al. Obstruction of venous drainage linked to transient global amnesia. PLoS One. 2015;10(7):e0132893. doi:10.1371/journal.pone.0132893
22. Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76(1):116-129. doi:10.1016/j.neuron.2012.08.036
23. Dulawa SC, Janowsky DS. Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol Psychiatry. 2019;24(5):694-709. doi:10.1038/s41380-018-0219-x
24. Grande LA, Loeser JD, Samii A. Recurrent transient global amnesia with intrathecal baclofen. Anesth Analg. 2008;106(4):1284-1287. doi:10.1213/ane.0b013e318165e1c6
25. Carrard J, Lambert AC, Genné D. Transient global amnesia following a whole-body cryotherapy session. BMJ Case Rep. 2017;2017:bcr2017221431. doi:10.1136/bcr-2017-221431
26. Jeong M, Kim WS, Kim AR, et al. Medical procedure-related transient global amnesia. Eur Neurol. 2018;80(1-2):42-49. doi:10.1159/000493163
27. Shah B, Hussain MW. Concussion causing transient global amnesia: further insights into pathophysiology? Neurology. 2020;95(20 Suppl 1):S16. doi:10.1212/01.wnl.0000720020.86134.9d
More on an asymmetric life
I enjoy receiving
Too often, families bear the burden of an individual’s hyperfocused pursuits. I hope your wife has been able to pursue her occupation with the same zeal and commitment. We have all read biographies of driven individuals and, unfortunately, someone pays the price for another’s success. For every Steve Jobs, there is a Lisa Jobs.
If we were surgeons, I would applaud your essay. However, we are psychiatrists. If anything, we balance out the reductionist forces in medicine. When every other physician claims a cure with medications or procedures, we look at all aspects of the patient’s life to find the appropriate treatment. At least that’s what we should be doing.
I was part of the first class of residents to work under the 80-hours-per-week restrictions. I was grateful for the extra time to rest, exercise, and spend time with my wife. The 80-hour restrictions improved resident wellness and had no impact on patient care. There are intangible benefits of diverting the mind from a chosen pursuit (such as creativity).
There is no doubt that becoming number 1 in any field requires a tremendous amount of determination, sacrifice, and effort. But not everyone gets to be first. Our society’s single-minded focus on being the best has had a major impact on mental health, especially for children. I hope you can address that in a future editorial.
Sudhir Nagaraja, DO, MS
Fredericksburg, Virginia
Dr. Nasrallah responds
Thank you for your letter about my editorial. You obviously believe in leading a balanced life, and that’s fine if you so choose. I described why I decided at an early age to lead an intensive, “purpose-driven life,” which requires investing much more time than others do, to achieve my lofty goals and excel in my area of expertise (academic psychiatry). It is really a “calling,” and those who score an extraordinary achievement (a moonshot) in their life, including Olympic gold medalists, entrepreneurs, inventors, or Nobel laureates, must do exactly what I do. I am not urging anyone to do what I have chosen to do in my life. Everyone defines for themselves what constitutes the pursuit of happiness.
You mentioned my wife. Let me assert that she is highly successful as a mother and as a research psychologist. She is my extremely valuable life partner and very supportive of what I do. I am fortunate to have chosen well!
Continue to: More on transient global amnesia
More on transient global amnesia
Your recent article on transient global amnesia (TGA) (“Transient global amnesia: Psychiatric precipitants, features, and comorbidities,”
I witnessed TGA, experienced by my brother, while on a surf trip. After bodyboarding for about an hour in cold water, wearing a full wet suit and hood, he met me on the beach. He recognized me and knew my name but had no idea where we were, how we got there, or other events from earlier that morning. There was no stressor, just the usual surfing excitement. We went to a local emergency department, where the physical examination, usual laboratory tests, and neuroimaging were normal. After approximately 5 hours, he began to fully recall recent events. Ten years later, there has been no recurrence. The only change in his surfing habits has been to avoid using a hood with neck coverage.
In 2022, Papadis et al1 described a case of concurrent Takotsubo cardiomyopathy and TGA, noting that cardiovascular dysfunction and neurologic dysfunction may be provoked by an emotional or stressful situation. The interesting observations of capture myopathy from animal literature appear similar to human reactions to trauma.1-3
Case reports of scopolamine intoxication have been linked to TGA. Severe memory disturbances, characteristics of dry mouth, blurred vision, and tachycardia were evident. Certain South American plant extracts popularly known as “Burundanga” have anticholinergic effects. Severe anterograde amnesia and submissiveness represent the 2 most notorious clinical signs of Burundanga intoxication.4
As one reviews single and groups of case studies, several things stand out. The hallmark of TGA is the sudden inability to make new memories, which resolves in a few hours. The brief and isolated dysfunction is what distinguishes this condition from most episodic disorders, but a clinician should not prognosticate too much without screening for ischemic or metabolic disturbance. Common associated precursors include Valsalva-associated activities, emotional stress with anxiety, acute pain, cold water immersion, static neck posture, and age older than 55.5,6
Neuropsychiatric disorders involve the neuron and its connections. Major reflexes automate the processes of the “neurocardiac” axis. The vasovagal reflex (Barcroft/Edholm reflex), diving reflex, baroreceptor reflex, Cushing reflex, and others depend upon the conversion of a mechanical stimulus to neurotransmission. The reflexes have sensors, afferent paths, a central processing, and efferent paths that lead to events or experiences. CNS processing is complex but the brainstem, amygdala, prefrontal cortex, and some cortical regions are involved. Neurocardiac reactions can come from pathologic events, including ischemia, metabolic disturbance, pain signals, or emotional effects within the axis.7-11
Understanding neurocardiac reflexes may help our progress with challenging clinical conditions, such as chronic pain, trauma, and cognitive impairment. The broad use of vagus nerve stimulation is one indicator of the power of this focus.12-19 Lewis20 suggested increased susceptibility to retrograde jugular venous flow could cause regional brain ischemia, resulting in TGA. The competency of jugular venous valves during the Valsalva maneuver could be assessed with Doppler ultrasound. Abnormalities could be managed, and results assessed.20,21 Vascular shunting from memory regions in the brain to essential neurocardiac control areas should be considered.
Cholinergic processes are active in the parasympathetic nervous system, sustained attention, working memory, executive functions, and mood. Increased central cholinergic activity may lead to depression. Scopolamine, in its therapeutic range, has antidepressant effects but in toxic doses is a dissociative agent.22,23 While cholinesterase inhibitors are used in Alzheimer disease, cholinergic agonists have yet to play a large role in general psychiatry or functional neurology.
TGA continues to provide a window into memory, functional disorders, psychological defenses, and adaptive neurocardiac processes. Continued clinical care and research might include gradual adaptation to cold water immersion, caution with the Valsalva maneuver, cholinergic support, managing the trapped response, avoiding interference with normal jugular flow, and evaluation for jugular venous insufficiency.
Because a variety of medical procedures can trigger TGA, health care professionals in many fields need to understand this symptom complex.24-27 Thanks to the authors for raising the awareness of TGA for psychiatrists.
Mark Chandler, MD
Durham, North Carolina
References
1. Papadis A, Svab S, Brugger N, et al. “Broken heart” and “broken brain”: which connection? Cardiol Res. 2022;13(1):65-70. doi:10.14740/cr1336
2. Blumstein DT, Buckner J, Shah S, et al. The evolution of capture myopathy in hooved mammals: a model for human stress cardiomyopathy? Evol Med Public Health. 2015;2015(1):195-203. doi:10.1093/emph/eov015
3. Seguel M, Paredes E, Pavés H, et al. Capture-induced stress cardiomyopathy in South American fur seal pups (Arctophoca australis gracilis). Marine Mammal Science. 2014;30(3): 1149-1157. https://doi.org/10.1111/mms.12079
4. Ardila A, Moreno C. Scopolamine intoxication as a model of transient global amnesia. Brain Cogn. 1991;15(2):236-245. doi:10.1016/0278-2626(91)90028-7
5. Bartsch T, Deuschl G. Transient global amnesia: functional anatomy and clinical implications. Lancet Neurol. 2010;9(2):205-214. doi:10.1016/S1474-4422(09)70344-8
6. Spiegel DR, Smith J, Wade RR, et al. Transient global amnesia: current perspectives. Neuropsychiatr Dis Treat. 2017;13:2691-2703. doi:10.2147/NDT.S130710
7. Yartsev A. Cardiac reflexes. August 15, 2020. Updated May 19, 2023. Accessed June 12, 2023. https://derangedphysiology.com/main/cicm-primary-exam/required-reading/cardiovascular-system/Chapter%20491/cardiac-reflexes
8. Lemaitre F, Chowdhury T, Schaller B. The trigeminocardiac reflex - a comparison with the diving reflex in humans. Arch Med Sci. 2015;11(2):419-426. doi:10.5114/aoms.2015.50974
9. Lindholm P, Lundgren CE. The physiology and pathophysiology of human breath-hold diving. J Appl Physiol (1985). 2009;106(1):284-292. doi:10.1152/japplphysiol.90991.2008
10. Tansey EA, Johnson CD. Recent advances in thermoregulation. Adv Physiol Educ. 2015;39(3):139-148. doi:10.1152/advan.00126.2014
11. Alboni P, Alboni M. Vasovagal syncope as a manifestation of an evolutionary selected trait. J Atr Fibrillation. 2014;7(2):1035. doi:10.4022/jafib.1035
12. Badran BW, Austelle CW. The future is noninvasive: a brief review of the evolution and clinical utility of vagus nerve stimulation. Focus (Am Psychiatr Publ). 2022;20(1):3-7. doi:10.1176/appi.focus.20210023
13. Suarez-Roca H, Mamoun N, Sigurdson MI, et al. Baroreceptor modulation of the cardiovascular system, pain, consciousness, and cognition. Compr Physiol. 2021;11(2):1373-1423. doi:10.1002/cphy.c190038
14. Pinna T, Edwards DJ. A systematic review of associations between interoception, vagal tone, and emotional regulation: potential applications for mental health, wellbeing, psychological flexibility, and chronic conditions. Front Psychol. 2020;11:1792. doi:10.3389/fpsyg.2020.01792
15. Howland RH. Vagus nerve stimulation. Curr Behav Neurosci Rep. 2014 Jun;1(2):64-73. doi:10.1007/s40473-014-0010-5
16. Panneton WM, Gan Q. The mammalian diving response: inroads to its neural control. Front Neurosci. 2020;14:524. doi:10.3389/fnins.2020.00524
17. Khurana RK, Wu R. The cold face test: a non-baroreflex mediated test of cardiac vagal function. Clin Auton Res. 2006;16(3):202-207. doi:10.1007/s10286-006-0332-9
18. Montirosso R, Provenzi L, Tronick E, et al. Vagal tone as a biomarker of long-term memory for a stressful social event at 4 months. Dev Psychobiol. 2014;56(7):1564-1574. doi:10.1002/dev.21251
19. Hansen AL, Johnsen BH, Thayer JF. Vagal influence on working memory and attention. Int J Psychophysiol. 2003;48(3):263-274. doi:10.1016/s0167-8760(03)00073-4
20. Lewis SL. Aetiology of transient global amnesia. Lancet. 1998;352(9125):397-399. doi:10.1016/S0140-6736(98)01442-1
21. Han K, Chao AC, Chang FC, et al. Obstruction of venous drainage linked to transient global amnesia. PLoS One. 2015;10(7):e0132893. doi:10.1371/journal.pone.0132893
22. Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76(1):116-129. doi:10.1016/j.neuron.2012.08.036
23. Dulawa SC, Janowsky DS. Cholinergic regulation of mood: from basic and clinical studies to emerging therapeutics. Mol Psychiatry. 2019;24(5):694-709. doi:10.1038/s41380-018-0219-x
24. Grande LA, Loeser JD, Samii A. Recurrent transient global amnesia with intrathecal baclofen. Anesth Analg. 2008;106(4):1284-1287. doi:10.1213/ane.0b013e318165e1c6
25. Carrard J, Lambert AC, Genné D. Transient global amnesia following a whole-body cryotherapy session. BMJ Case Rep. 2017;2017:bcr2017221431. doi:10.1136/bcr-2017-221431
26. Jeong M, Kim WS, Kim AR, et al. Medical procedure-related transient global amnesia. Eur Neurol. 2018;80(1-2):42-49. doi:10.1159/000493163
27. Shah B, Hussain MW. Concussion causing transient global amnesia: further insights into pathophysiology? Neurology. 2020;95(20 Suppl 1):S16. doi:10.1212/01.wnl.0000720020.86134.9d
AI model interprets EEGs with near-perfect accuracy
An automated artificial intelligence (AI) model trained to read electroencephalograms (EEGs) in patients with suspected epilepsy is just as accurate as trained neurologists, new data suggest.
Known as SCORE-AI, the technology distinguishes between abnormal and normal EEG recordings and classifies irregular recordings into specific categories crucial for patient decision-making.
“SCORE-AI can be used in place of experts in underprivileged areas, where expertise is missing, or to help physicians to preselect or prescore recordings in areas where the workload is high – we can all benefit from AI,” study investigator Sándor Beniczky, MD, PhD, said in a JAMA Neurology podcast.
Dr. Beniczky is professor of clinical neurophysiology at Aarhus University in Denmark.
The findings were published online in JAMA Neurology.
Gaining a foothold
Increasingly, AI is gaining a foothold in medicine by credibly addressing patient queries and aiding radiologists.
To bring AI to EEG interpretation, the researchers developed and validated an AI model that was able to assess routine, clinical EEGs in patients with suspected epilepsy.
Beyond using AI to distinguish abnormal from normal EEG recordings, the researchers wanted to train the new system to classify abnormal recordings into the major categories that are most relevant for clinical decision-making in patients who may have epilepsy. The categories included epileptiform-focal, epileptiform-generalized, nonepileptiform-focal, and nonepileptiform-diffuse abnormalities.
The researchers trained the learning model using Standardized Computer-based Organized Reporting of EEG (SCORE) software.
In the development phase, the model was trained using more than 30,490 anonymized and highly annotated EEG recordings from 14,100 men (median age, 25 years) from a single center. The recordings had an average duration of 31 minutes and were interpreted by 17 neurologists using standardized criteria. If an EEG recording was abnormal, the physicians had to specify which abnormal features were present.
SCORE-AI then performed an analysis of the recordings based on input from the experts.
To validate the findings, investigators used two independent test datasets. The first dataset consisted of 100 representative routine EEGs from 61 men (median age, 26 years), evaluated by 11 neurologists from different centers.
The consensus of these evaluations served as the reference standard. The second dataset comprised nearly 10,000 EEGs from a single center (5,170 men; median age, 35 years), independently assessed by 14 neurologists.
Near-perfect accuracy
When compared with the experts, SCORE-AI had near-perfect accuracy with an area under the receiver operating characteristic (AUROC) curve for differentiating normal from abnormal EEG recordings of 0.95.
SCORE-AI also performed well at identifying generalized epileptiform abnormalities (AUROC, 0.96), focal epileptiform abnormalities (AUROC, 0.91), focal nonepileptiform abnormalities (AUROC, 0.89), and diffuse nonepileptiform abnormalities (AUROC, 0.93).
In addition, SCORE-AI had excellent agreement with clinicians – and sometimes agreed with individual experts more than the experts agreed with one another.
When Dr. Beniczky and team tested SCORE-AI against three previously published AI models, SCORE-AI demonstrated greater specificity than those models (90% vs. 3%-63%) but was not as sensitive (86.7%) as two of the models (96.7% and 100%).
One of the study’s limitations was the fact that SCORE-AI was developed and validated on routine EEGs that excluded neonates and critically ill patients.
In the future, Dr. Beniczky said on the podcast, the team would like to train SCORE-AI to read EEGs with more granularity, and eventually use only one single channel to record EEGs. At present, SCORE-AI is being integrated with Natus Neuro, a widely used EEG equipment system, the investigators note.
In an accompanying editorial, Jonathan Kleen, MD, PhD, and Elan Guterman, MD, said, “The overall approach taken ... in developing and validating SCORE-AI sets a standard for this work going forward.”
Dr. Kleen and Dr. Guterman note that the technological gains brought about by SCORE-AI technology “could offer an exciting prospect to improve EEG availability and clinical care for the 50 million people with epilepsy worldwide.”
A version of this article originally appeared on Medscape.com.
An automated artificial intelligence (AI) model trained to read electroencephalograms (EEGs) in patients with suspected epilepsy is just as accurate as trained neurologists, new data suggest.
Known as SCORE-AI, the technology distinguishes between abnormal and normal EEG recordings and classifies irregular recordings into specific categories crucial for patient decision-making.
“SCORE-AI can be used in place of experts in underprivileged areas, where expertise is missing, or to help physicians to preselect or prescore recordings in areas where the workload is high – we can all benefit from AI,” study investigator Sándor Beniczky, MD, PhD, said in a JAMA Neurology podcast.
Dr. Beniczky is professor of clinical neurophysiology at Aarhus University in Denmark.
The findings were published online in JAMA Neurology.
Gaining a foothold
Increasingly, AI is gaining a foothold in medicine by credibly addressing patient queries and aiding radiologists.
To bring AI to EEG interpretation, the researchers developed and validated an AI model that was able to assess routine, clinical EEGs in patients with suspected epilepsy.
Beyond using AI to distinguish abnormal from normal EEG recordings, the researchers wanted to train the new system to classify abnormal recordings into the major categories that are most relevant for clinical decision-making in patients who may have epilepsy. The categories included epileptiform-focal, epileptiform-generalized, nonepileptiform-focal, and nonepileptiform-diffuse abnormalities.
The researchers trained the learning model using Standardized Computer-based Organized Reporting of EEG (SCORE) software.
In the development phase, the model was trained using more than 30,490 anonymized and highly annotated EEG recordings from 14,100 men (median age, 25 years) from a single center. The recordings had an average duration of 31 minutes and were interpreted by 17 neurologists using standardized criteria. If an EEG recording was abnormal, the physicians had to specify which abnormal features were present.
SCORE-AI then performed an analysis of the recordings based on input from the experts.
To validate the findings, investigators used two independent test datasets. The first dataset consisted of 100 representative routine EEGs from 61 men (median age, 26 years), evaluated by 11 neurologists from different centers.
The consensus of these evaluations served as the reference standard. The second dataset comprised nearly 10,000 EEGs from a single center (5,170 men; median age, 35 years), independently assessed by 14 neurologists.
Near-perfect accuracy
When compared with the experts, SCORE-AI had near-perfect accuracy with an area under the receiver operating characteristic (AUROC) curve for differentiating normal from abnormal EEG recordings of 0.95.
SCORE-AI also performed well at identifying generalized epileptiform abnormalities (AUROC, 0.96), focal epileptiform abnormalities (AUROC, 0.91), focal nonepileptiform abnormalities (AUROC, 0.89), and diffuse nonepileptiform abnormalities (AUROC, 0.93).
In addition, SCORE-AI had excellent agreement with clinicians – and sometimes agreed with individual experts more than the experts agreed with one another.
When Dr. Beniczky and team tested SCORE-AI against three previously published AI models, SCORE-AI demonstrated greater specificity than those models (90% vs. 3%-63%) but was not as sensitive (86.7%) as two of the models (96.7% and 100%).
One of the study’s limitations was the fact that SCORE-AI was developed and validated on routine EEGs that excluded neonates and critically ill patients.
In the future, Dr. Beniczky said on the podcast, the team would like to train SCORE-AI to read EEGs with more granularity, and eventually use only one single channel to record EEGs. At present, SCORE-AI is being integrated with Natus Neuro, a widely used EEG equipment system, the investigators note.
In an accompanying editorial, Jonathan Kleen, MD, PhD, and Elan Guterman, MD, said, “The overall approach taken ... in developing and validating SCORE-AI sets a standard for this work going forward.”
Dr. Kleen and Dr. Guterman note that the technological gains brought about by SCORE-AI technology “could offer an exciting prospect to improve EEG availability and clinical care for the 50 million people with epilepsy worldwide.”
A version of this article originally appeared on Medscape.com.
An automated artificial intelligence (AI) model trained to read electroencephalograms (EEGs) in patients with suspected epilepsy is just as accurate as trained neurologists, new data suggest.
Known as SCORE-AI, the technology distinguishes between abnormal and normal EEG recordings and classifies irregular recordings into specific categories crucial for patient decision-making.
“SCORE-AI can be used in place of experts in underprivileged areas, where expertise is missing, or to help physicians to preselect or prescore recordings in areas where the workload is high – we can all benefit from AI,” study investigator Sándor Beniczky, MD, PhD, said in a JAMA Neurology podcast.
Dr. Beniczky is professor of clinical neurophysiology at Aarhus University in Denmark.
The findings were published online in JAMA Neurology.
Gaining a foothold
Increasingly, AI is gaining a foothold in medicine by credibly addressing patient queries and aiding radiologists.
To bring AI to EEG interpretation, the researchers developed and validated an AI model that was able to assess routine, clinical EEGs in patients with suspected epilepsy.
Beyond using AI to distinguish abnormal from normal EEG recordings, the researchers wanted to train the new system to classify abnormal recordings into the major categories that are most relevant for clinical decision-making in patients who may have epilepsy. The categories included epileptiform-focal, epileptiform-generalized, nonepileptiform-focal, and nonepileptiform-diffuse abnormalities.
The researchers trained the learning model using Standardized Computer-based Organized Reporting of EEG (SCORE) software.
In the development phase, the model was trained using more than 30,490 anonymized and highly annotated EEG recordings from 14,100 men (median age, 25 years) from a single center. The recordings had an average duration of 31 minutes and were interpreted by 17 neurologists using standardized criteria. If an EEG recording was abnormal, the physicians had to specify which abnormal features were present.
SCORE-AI then performed an analysis of the recordings based on input from the experts.
To validate the findings, investigators used two independent test datasets. The first dataset consisted of 100 representative routine EEGs from 61 men (median age, 26 years), evaluated by 11 neurologists from different centers.
The consensus of these evaluations served as the reference standard. The second dataset comprised nearly 10,000 EEGs from a single center (5,170 men; median age, 35 years), independently assessed by 14 neurologists.
Near-perfect accuracy
When compared with the experts, SCORE-AI had near-perfect accuracy with an area under the receiver operating characteristic (AUROC) curve for differentiating normal from abnormal EEG recordings of 0.95.
SCORE-AI also performed well at identifying generalized epileptiform abnormalities (AUROC, 0.96), focal epileptiform abnormalities (AUROC, 0.91), focal nonepileptiform abnormalities (AUROC, 0.89), and diffuse nonepileptiform abnormalities (AUROC, 0.93).
In addition, SCORE-AI had excellent agreement with clinicians – and sometimes agreed with individual experts more than the experts agreed with one another.
When Dr. Beniczky and team tested SCORE-AI against three previously published AI models, SCORE-AI demonstrated greater specificity than those models (90% vs. 3%-63%) but was not as sensitive (86.7%) as two of the models (96.7% and 100%).
One of the study’s limitations was the fact that SCORE-AI was developed and validated on routine EEGs that excluded neonates and critically ill patients.
In the future, Dr. Beniczky said on the podcast, the team would like to train SCORE-AI to read EEGs with more granularity, and eventually use only one single channel to record EEGs. At present, SCORE-AI is being integrated with Natus Neuro, a widely used EEG equipment system, the investigators note.
In an accompanying editorial, Jonathan Kleen, MD, PhD, and Elan Guterman, MD, said, “The overall approach taken ... in developing and validating SCORE-AI sets a standard for this work going forward.”
Dr. Kleen and Dr. Guterman note that the technological gains brought about by SCORE-AI technology “could offer an exciting prospect to improve EEG availability and clinical care for the 50 million people with epilepsy worldwide.”
A version of this article originally appeared on Medscape.com.
Placebo effect can be found in a cup of coffee
The best part of waking up is placebo in your cup
Coffee makes the world go round. It’s impossible to picture any workplace without a cast of forlorn characters huddled around the office coffee maker on a Monday morning, imbibing their beverage du jour until they’ve been lifted out of their semi-zombified stupor.
Millions upon millions of people swear by their morning coffee. And if they don’t get that sweet, sweet caffeine boost, they’ll make Garfield and the Boomtown Rats’ opinions of Mondays look tame. And it only makes sense that they’d believe that. After all, caffeine is a stimulant. It helps your brain focus and kicks it into overdrive. Of course drinking a beverage full of caffeine wakes you up. Right?
Not so fast, a group of Portuguese researchers say. That morning cup of coffee? It may actually be a placebo. Cue the dramatic sound effect.
Here’s the scoop: After recruiting a group of coffee drinkers (at least one cup a day), the researchers kept their test subjects off of coffee for at least 3 hours, then performed a brief functional MRI scan on all test subjects. Half an hour later, study participants received either a standard cup of coffee or pure caffeine. Half an hour after consuming their respective study product, the subjects underwent a second MRI.
As expected, both people who consumed coffee and those who consumed pure caffeine showed decreased connectivity in the default mode network after consumption, indicating preparation in the brain to move from resting to working on tasks. However, those who had pure caffeine did not show increased connectivity in the visual and executive control networks, while those who had coffee did. Simply put, caffeine may wake you up, but it doesn’t make you any sharper. Only coffee gets you in shape for that oh-so-important Monday meeting.
This doesn’t make a lot of sense. How can the drug part of coffee not be responsible for every effect the drink gives you? That’s where the placebo comes in, according to the scientists. It’s possible the effect they saw was caused by withdrawal – after just 3 hours? Yikes, hope not – but it’s more likely it comes down to psychology. We expect coffee to wake us up and make us ready for the day, so that’s exactly what it does. Hey, if that’s all it takes, time to convince ourselves that eating an entire pizza is actually an incredibly effective weight loss tool. Don’t let us down now, placebo effect.
Bread, milk, toilet paper, AFib diagnosis
Now consider the shopping cart. It does its job of carrying stuff around the store well enough, but can it lift you out of a semi-zombified stupor in the morning? No. Can it identify undiagnosed atrial fibrillation? Again, no.
Not so fast, say the investigators conducting the SHOPS-AF (Supermarket/Hypermarket Opportunistic Screening for Atrial Fibrillation) study. They built a better shopping cart. Except they call it a trolley, not a cart, since the study was conducted in England, where they sometimes have funny names for things.
Their improved shopping trolley – we’re just going to call it a cart from here on – has an electrocardiogram sensor embedded into the handlebar, so it can effectively detect AFib in shoppers who held it for at least 60 seconds. The sensor lights up red if it detects an irregular heartbeat and green if it does not. Let’s see a cup of coffee do that.
They put 10 of these modified carts in four supermarkets in Liverpool to see what would happen. Would shoppers be able to tell that we secretly replaced the fine coffee they usually serve with Folger’s crystals? Oops. Sorry about that. Coffee on the brain, apparently. Back to the carts.
A total of 2,155 adult shoppers used one of the carts over 2 months, and electrocardiogram data were available for 220 participants who either had a red light on the sensor and/or an irregular pulse that suggested atrial fibrillation. After further review by the SHOPS-AF cardiologist, AFib was diagnosed in 59 shoppers, of whom 39 were previously undiagnosed.
They’re already working to cut the scan time to 30 seconds for SHOPS-AF II, but we’re wondering about a possible flaw in the whole health-care-delivery-through-shopping-cart scenario. When we go to the local super/hyper/megamart, it seems like half of the people trundling up and down the aisles are store employees filling orders for customers who won’t even set foot inside. Is the shopping cart on its way out? Maybe. Who wants to tell the SHOPS-AF II team? Not us.
Put pneumonia where your mouth is
Getting dentures does not mean the end of dental care. If anything, new research reveals a huge reason for staying on top of one’s denture care: pneumonia.
It all started with swabs. Scientists in the United Kingdom took mouth, tongue, and denture specimens from frail elderly hospital patients who had pneumonia and wore dentures and from similar patients in care homes who wore dentures and did not have pneumonia. When they compared the microbial populations of the two groups, the investigators found about 20 times the number of respiratory pathogens on the dentures of those with pneumonia.
The research team suggested that dentures may play a role in causing pneumonia, but lead author Josh Twigg, BDS, PhD, also noted that “you certainly couldn’t say that people got pneumonia because they were wearing dentures. It’s just showing that there is an association there.” Improper cleaning, though, could lead to microbial colonization of the dentures, and patients could be inhaling those microbes into their lungs, thereby turning a dental issue into a respiratory issue.
More research needs to be done on the association between dentures and pneumonia, but Dr. Twigg hoped that the results of this study could be presented to the public. The message? “It is important to clean dentures thoroughly” and visit the dentist regularly, he said, but the best way to prevent denture-related infections is to avoid needing to wear dentures entirely.
The best part of waking up is placebo in your cup
Coffee makes the world go round. It’s impossible to picture any workplace without a cast of forlorn characters huddled around the office coffee maker on a Monday morning, imbibing their beverage du jour until they’ve been lifted out of their semi-zombified stupor.
Millions upon millions of people swear by their morning coffee. And if they don’t get that sweet, sweet caffeine boost, they’ll make Garfield and the Boomtown Rats’ opinions of Mondays look tame. And it only makes sense that they’d believe that. After all, caffeine is a stimulant. It helps your brain focus and kicks it into overdrive. Of course drinking a beverage full of caffeine wakes you up. Right?
Not so fast, a group of Portuguese researchers say. That morning cup of coffee? It may actually be a placebo. Cue the dramatic sound effect.
Here’s the scoop: After recruiting a group of coffee drinkers (at least one cup a day), the researchers kept their test subjects off of coffee for at least 3 hours, then performed a brief functional MRI scan on all test subjects. Half an hour later, study participants received either a standard cup of coffee or pure caffeine. Half an hour after consuming their respective study product, the subjects underwent a second MRI.
As expected, both people who consumed coffee and those who consumed pure caffeine showed decreased connectivity in the default mode network after consumption, indicating preparation in the brain to move from resting to working on tasks. However, those who had pure caffeine did not show increased connectivity in the visual and executive control networks, while those who had coffee did. Simply put, caffeine may wake you up, but it doesn’t make you any sharper. Only coffee gets you in shape for that oh-so-important Monday meeting.
This doesn’t make a lot of sense. How can the drug part of coffee not be responsible for every effect the drink gives you? That’s where the placebo comes in, according to the scientists. It’s possible the effect they saw was caused by withdrawal – after just 3 hours? Yikes, hope not – but it’s more likely it comes down to psychology. We expect coffee to wake us up and make us ready for the day, so that’s exactly what it does. Hey, if that’s all it takes, time to convince ourselves that eating an entire pizza is actually an incredibly effective weight loss tool. Don’t let us down now, placebo effect.
Bread, milk, toilet paper, AFib diagnosis
Now consider the shopping cart. It does its job of carrying stuff around the store well enough, but can it lift you out of a semi-zombified stupor in the morning? No. Can it identify undiagnosed atrial fibrillation? Again, no.
Not so fast, say the investigators conducting the SHOPS-AF (Supermarket/Hypermarket Opportunistic Screening for Atrial Fibrillation) study. They built a better shopping cart. Except they call it a trolley, not a cart, since the study was conducted in England, where they sometimes have funny names for things.
Their improved shopping trolley – we’re just going to call it a cart from here on – has an electrocardiogram sensor embedded into the handlebar, so it can effectively detect AFib in shoppers who held it for at least 60 seconds. The sensor lights up red if it detects an irregular heartbeat and green if it does not. Let’s see a cup of coffee do that.
They put 10 of these modified carts in four supermarkets in Liverpool to see what would happen. Would shoppers be able to tell that we secretly replaced the fine coffee they usually serve with Folger’s crystals? Oops. Sorry about that. Coffee on the brain, apparently. Back to the carts.
A total of 2,155 adult shoppers used one of the carts over 2 months, and electrocardiogram data were available for 220 participants who either had a red light on the sensor and/or an irregular pulse that suggested atrial fibrillation. After further review by the SHOPS-AF cardiologist, AFib was diagnosed in 59 shoppers, of whom 39 were previously undiagnosed.
They’re already working to cut the scan time to 30 seconds for SHOPS-AF II, but we’re wondering about a possible flaw in the whole health-care-delivery-through-shopping-cart scenario. When we go to the local super/hyper/megamart, it seems like half of the people trundling up and down the aisles are store employees filling orders for customers who won’t even set foot inside. Is the shopping cart on its way out? Maybe. Who wants to tell the SHOPS-AF II team? Not us.
Put pneumonia where your mouth is
Getting dentures does not mean the end of dental care. If anything, new research reveals a huge reason for staying on top of one’s denture care: pneumonia.
It all started with swabs. Scientists in the United Kingdom took mouth, tongue, and denture specimens from frail elderly hospital patients who had pneumonia and wore dentures and from similar patients in care homes who wore dentures and did not have pneumonia. When they compared the microbial populations of the two groups, the investigators found about 20 times the number of respiratory pathogens on the dentures of those with pneumonia.
The research team suggested that dentures may play a role in causing pneumonia, but lead author Josh Twigg, BDS, PhD, also noted that “you certainly couldn’t say that people got pneumonia because they were wearing dentures. It’s just showing that there is an association there.” Improper cleaning, though, could lead to microbial colonization of the dentures, and patients could be inhaling those microbes into their lungs, thereby turning a dental issue into a respiratory issue.
More research needs to be done on the association between dentures and pneumonia, but Dr. Twigg hoped that the results of this study could be presented to the public. The message? “It is important to clean dentures thoroughly” and visit the dentist regularly, he said, but the best way to prevent denture-related infections is to avoid needing to wear dentures entirely.
The best part of waking up is placebo in your cup
Coffee makes the world go round. It’s impossible to picture any workplace without a cast of forlorn characters huddled around the office coffee maker on a Monday morning, imbibing their beverage du jour until they’ve been lifted out of their semi-zombified stupor.
Millions upon millions of people swear by their morning coffee. And if they don’t get that sweet, sweet caffeine boost, they’ll make Garfield and the Boomtown Rats’ opinions of Mondays look tame. And it only makes sense that they’d believe that. After all, caffeine is a stimulant. It helps your brain focus and kicks it into overdrive. Of course drinking a beverage full of caffeine wakes you up. Right?
Not so fast, a group of Portuguese researchers say. That morning cup of coffee? It may actually be a placebo. Cue the dramatic sound effect.
Here’s the scoop: After recruiting a group of coffee drinkers (at least one cup a day), the researchers kept their test subjects off of coffee for at least 3 hours, then performed a brief functional MRI scan on all test subjects. Half an hour later, study participants received either a standard cup of coffee or pure caffeine. Half an hour after consuming their respective study product, the subjects underwent a second MRI.
As expected, both people who consumed coffee and those who consumed pure caffeine showed decreased connectivity in the default mode network after consumption, indicating preparation in the brain to move from resting to working on tasks. However, those who had pure caffeine did not show increased connectivity in the visual and executive control networks, while those who had coffee did. Simply put, caffeine may wake you up, but it doesn’t make you any sharper. Only coffee gets you in shape for that oh-so-important Monday meeting.
This doesn’t make a lot of sense. How can the drug part of coffee not be responsible for every effect the drink gives you? That’s where the placebo comes in, according to the scientists. It’s possible the effect they saw was caused by withdrawal – after just 3 hours? Yikes, hope not – but it’s more likely it comes down to psychology. We expect coffee to wake us up and make us ready for the day, so that’s exactly what it does. Hey, if that’s all it takes, time to convince ourselves that eating an entire pizza is actually an incredibly effective weight loss tool. Don’t let us down now, placebo effect.
Bread, milk, toilet paper, AFib diagnosis
Now consider the shopping cart. It does its job of carrying stuff around the store well enough, but can it lift you out of a semi-zombified stupor in the morning? No. Can it identify undiagnosed atrial fibrillation? Again, no.
Not so fast, say the investigators conducting the SHOPS-AF (Supermarket/Hypermarket Opportunistic Screening for Atrial Fibrillation) study. They built a better shopping cart. Except they call it a trolley, not a cart, since the study was conducted in England, where they sometimes have funny names for things.
Their improved shopping trolley – we’re just going to call it a cart from here on – has an electrocardiogram sensor embedded into the handlebar, so it can effectively detect AFib in shoppers who held it for at least 60 seconds. The sensor lights up red if it detects an irregular heartbeat and green if it does not. Let’s see a cup of coffee do that.
They put 10 of these modified carts in four supermarkets in Liverpool to see what would happen. Would shoppers be able to tell that we secretly replaced the fine coffee they usually serve with Folger’s crystals? Oops. Sorry about that. Coffee on the brain, apparently. Back to the carts.
A total of 2,155 adult shoppers used one of the carts over 2 months, and electrocardiogram data were available for 220 participants who either had a red light on the sensor and/or an irregular pulse that suggested atrial fibrillation. After further review by the SHOPS-AF cardiologist, AFib was diagnosed in 59 shoppers, of whom 39 were previously undiagnosed.
They’re already working to cut the scan time to 30 seconds for SHOPS-AF II, but we’re wondering about a possible flaw in the whole health-care-delivery-through-shopping-cart scenario. When we go to the local super/hyper/megamart, it seems like half of the people trundling up and down the aisles are store employees filling orders for customers who won’t even set foot inside. Is the shopping cart on its way out? Maybe. Who wants to tell the SHOPS-AF II team? Not us.
Put pneumonia where your mouth is
Getting dentures does not mean the end of dental care. If anything, new research reveals a huge reason for staying on top of one’s denture care: pneumonia.
It all started with swabs. Scientists in the United Kingdom took mouth, tongue, and denture specimens from frail elderly hospital patients who had pneumonia and wore dentures and from similar patients in care homes who wore dentures and did not have pneumonia. When they compared the microbial populations of the two groups, the investigators found about 20 times the number of respiratory pathogens on the dentures of those with pneumonia.
The research team suggested that dentures may play a role in causing pneumonia, but lead author Josh Twigg, BDS, PhD, also noted that “you certainly couldn’t say that people got pneumonia because they were wearing dentures. It’s just showing that there is an association there.” Improper cleaning, though, could lead to microbial colonization of the dentures, and patients could be inhaling those microbes into their lungs, thereby turning a dental issue into a respiratory issue.
More research needs to be done on the association between dentures and pneumonia, but Dr. Twigg hoped that the results of this study could be presented to the public. The message? “It is important to clean dentures thoroughly” and visit the dentist regularly, he said, but the best way to prevent denture-related infections is to avoid needing to wear dentures entirely.
Physician suicide roundtable: 8 important initiatives that can help
Physician suicide continues to be a challenging problem in the United States. Each year, 1 in 10 doctors think about or attempt suicide, and 400 die by suicide each year. More than half of the doctors reading this know a colleague who has attempted or died by suicide.
These are part of a public health suicide prevention strategy, the preferred method for prevention, in hospitals and institutions around the country. A public health model for preventing suicide is a multifaceted approach that includes universal education, health promotion, selective and targeted prevention, and treatment and recovery.
These physicians hope to continue creating and implementing these and other risk-reduction measures across all health care organizations.
Our physician experts for this discussion
Mary Moffit, PhD, is an associate professor in the department of psychiatry at Oregon Health & Science University, Portland. She directs the resident and faculty wellness program and is director of the OHSU peer support program. She helped design and developed a comprehensive wellness program that is now a national model for academic medical centers.
Christine Yu Moutier, MD, is the chief medical officer of the American Foundation for Suicide Prevention. She is the author of “Suicide Prevention,” a Cambridge University Press clinical handbook. She has been a practicing psychiatrist, professor of psychiatry, dean in the medical school at the University of California, San Diego, and medical director of the inpatient psychiatric unit at the VA Medical Center in La Jolla, Calif.
Michael F. Myers, MD, is a professor of clinical psychiatry in the department of psychiatry & behavioral sciences at the State University of New York, Brooklyn. He is recent past vice-chair of education and director of training in the department of psychiatry & behavioral sciences at the university. He is the author of several books, including “Why Physicians Die By Suicide,” “The Physician as Patient,” and “Touched by Suicide.”
The participants discussed these risk-reduction initiatives as having much potential for helping physicians at risk for suicide and suicidal ideations.
The importance of peer support programs
Peer support program models may differ across institutions but typically describe colleagues providing some degree of emotional first aid to peers who may be at risk.
Dr. Moffit: The Pew support program that we have in place at OHSU, similar to what’s available in many hospitals and systems nationwide, trains individual physicians across multiple specialties in a peer support model. It’s not specifically emotional first aid, although that’s integral to it. It’s also for adverse events: Having a tragic patient death, having learned that you will be named in a lawsuit, and exposure to trauma in the medical role.
Peer to peer is not where we anticipate physicians seeking someone to talk to about their marital relationship not going well. However, the peer supporter will know about resources throughout the university and the community for what is needed. We’ve got 20-30 peer supporters. We try to match them – for example, a surgeon with a surgeon, a primary care doc with a primary care doc. Physicians who use peer support aren’t tracked, and no notes are taken or documented. It takes place informally but has changed the culture and lowered a barrier. We have a waiting list of people who want to be peer supporters.
Dr. Moutier: Peer-to-peer support is usually part of a multi-pronged program and is usually not the only effort going on. Depending on how they’re set up, the goals may be slightly different for each program. Peer-to-peer can be one of the most powerful ways to augment awareness raising and education, which is almost always a basic first step.
Dr. Myers: It doesn’t feel as threatening when people start in a peer-to-peer support group. Users may have been afraid of getting a mental health diagnosis, but with peers, many of whom are often not psychiatrists, that eases distress. Peer support can break down that sense of isolation and loneliness so that someone can take the next step.
Dr. Moutier: To be connected to family, to any community resource, frankly, is a protective factor that mitigates suicide risk. So that’s the logic model from a suicide prevention standpoint. It may be the only opportunity for someone to start disclosing what they’re experiencing, receive validation and support, and not a judgmental response. It can open up the avenue toward help-seeking.
Opt-in/opt-out support for medical residents
This initiative matches residents with a counselor as part of their orientation.
Dr. Moffit: Each resident has a meet and greet with a counselor when they arrive or in their first 6 months at their university. The resident can opt out and cancel the meeting, but they’re scheduled for it as part of their “curriculum.” Institutions like Michigan, Columbia, Montefiore, Mount Sinai, and the University of California, San Diego, have this in place. It starts something like: ‘Hello. Good afternoon. How’s it going? I’m Dr. Moffitt, and here are the services available in this program.’
Dr. Myers: It’s another excellent example of normalizing the stress in the rigors of training and making it part of the wellness initiative.
Dr. Moutier: It’s just a normal part of orientation. Again, as a universal strategy, one thing that I was doing at UCSD with a particular group of medical students, who were at higher risk, was a postbaccalaureate program that found students from underrepresented, under-resourced backgrounds and brought them into this post-bacc year. I was directing it and mentoring these students.
So, I could afford a lot more intensive time and attention to them because it was a small group, but every one of them had regular meetings with me every 2 weeks. My approach was to help them uncover their unique strengths and vulnerabilities as they started this program. They all made it into med school.
It was a very intensive and more concierge-personalized approach. It’s like personalized medicine. What specific self-care, mentoring, and mental health care plan would each student codesign with me to stay on track?
And it would involve very holistic things, like if part of their vulnerability was that leaving their Chicano family was creating stress because their father had said: ‘You’re leaving our culture and our family by going into the profession of medicine,’ then we had specific plans around how to care for that aspect of their struggle. It was a much more informed, customized mentoring approach called the UCSD CAP (Conditional Acceptance Post-Baccalaureate Program). It could be a feature in a more specialized opt-in/opt-out program.
One-question survey: How full is your gas tank?
This initiative is a one-question survey emailed/texted to residents to check in on their wellness. We ask, how full is your gas tank? Select 1 to 5 (Empty to Full). If they flag low, they receive a follow-up.
Dr. Moffit: It’s certainly a metaphor that we use. It’s the idea of being depleted in combination with being extremely sleep deprived and the inability to access the usual sources of support or outlets, and how that can create a perfect storm of a level of distress that can put physicians at risk.
Dr. Moutier: It is a way to help people realize that there are things they can do proactively to keep that tank at least somewhat full enough.
Dr. Myers: Using colloquial or figurative language can get better buy-in than “Here’s a PHQ-9.” It also has a caring or intimate tone to it. Somebody could feel they’re a 1 in this rotation but a 4-5 the next. We know from a lot of the literature that when residents get a good, welcoming orientation, their satisfaction with that rotation is uniformly better than if they’re thrown to the wolves. And we know trial by fire can put trainees at risk.
A buddy to check in with
This initiative is when you’re assigned a buddy in or out of residency that you regularly check in with about how you’re doing.
Dr. Myers: Not to be cynical, but there has been some mentor/mentee research that if you’re assigned a mentor, the results are not nearly as good. And if it’s left to the individual to find a mentor, results could be marginal as well. You need a guide to say, ‘Here are some potential mentors for you, but you decide.’ We do a lot of that at (SUNY) Downstate instead of assigning a person. So, it may require some oversight. Picking a check-in buddy from a list provided rather than having one assigned may be more beneficial.
A lot of what we’re talking about are universal strategies that allow for increased interpersonal connection, which is a protective factor that normalizes help-seeking.
A platform or social media forum to share experiences
An online forum or platform where medical students, residents, and physicians can discuss mental health and suicide prevention. Physicians with personal experience could provide testimonials.
Dr. Myers: I’ve recently signed a book contract, and the working title is “Physicians With Lived Experience: How Their Stories Give Clinical Guidance.” When I talk with doctors who have published their personal stories in the New England Journal of Medicine, JAMA, or sometimes The Washington Post or The New York Times, many of them have said they had no idea at the beginning of their journey that they would do something like this: be transparent about their story. It’s a measure of their health, growth, and grace.
Dr. Moutier: The current president of the Academic Association of Surgeons, Carrie Cunningham, MD, MPH, used her platform at the annual AAS conference in 2022 to focus on suicide prevention. She told her own recent story of having gotten into recovery after having been near suicide and struggling with addiction. It was a groundbreaking moment for the field of surgery and produced a ripple effect. She risked everything to tell her story, which was highly emotional since it was still raw. It got everyone engaged, a real turning point for that field. Storytelling and a place for trainees to discuss suicide prevention, and physicians to recall their lived experiences can be highly beneficial.
Interactive Screening Program
The Interactive Screening Program (ISP) is used in higher education to allow physicians to take a safe, confidential screening test and receive a personalized response that can connect them to mental health services before a crisis emerges.
Dr. Moutier: ISP is a tool within a public health model that can afford anonymity to the user so they can safely have their needs addressed. It’s a way for high-risk individuals to sync up with treatment and support. It’s sometimes used in the universal approach because it can be offered to everyone within the health system community of physicians and staff.
It can produce a ripple effect of normalizing that we all have mental health to take care of. Its intended value is in identifying those with a higher risk for suicide, but it doesn’t stop at identifying those at risk. It helps physicians move past a stage of suffering in silence.
Our data show that 86% of a very high-risk group (currently having suicidal ideation, a recent attempt, or other high-risk factors for suicide) aren’t in any form of treatment and have not disclosed their situation to anyone. A fairly high percentage of those going through ISP request a referral to treatment. It’s a unique, very niche tool, and because users remain anonymous, that affords safety around confidentiality.
It’s usually part of a multipronged approach with education, stigma reduction, storytelling, peer support, and other modalities. In my experience with the UCSD HEAR (Healer Assessment Education and Recovery) program, which is still going strong in about its 15th year, the program went from seeing 13 physicians die by suicide in the years leading up to its launch and in the 15 years since it’s been going, one suicide. We all believe that the ISP is the heart of prevention.
Even though all of the universal strategies are important, they probably wouldn’t be sufficient by themselves because the risk [for suicide] is dynamic, and you have to catch people when they are suffering and ready to seek treatment. Suicide prevention is challenging and must be strategic, multifaceted, and sustained over time.
The importance of confidentiality for physicians
In the past, physicians may have been hesitant to seek treatment when struggling with mental health, substance use disorder and suicidal ideations because they heard stories from doctors who said they had to disclose mental health treatment to medical and state licensing boards.
Dr. Myers: There is so much dated stuff out there, and it gets propagated by people who have had a bad experience. I’m not challenging the authenticity of that, but I feel like those are in the minority. The vast majority of people are seeking help. The Federation of State Physician Health Programs is working with state boards to update and get rid of antiquated questions, and they’re working with credentialing groups.
When I was in practice and my patient was petrified of having to come into the hospital [because of confidentiality] I would just be their physician and say: “Look, I know that this is a worry for you [licensing and credentialing issues] but trust me, I’m going to help you get well; that’s my job. And I’m going to help you sort all that out afterward.” It was part of my work as their physician that if they were going to have to jump through hurdles to get their license reinstated, etc., I could help.
The Dr. Lorna Breen Heroes’ Foundation is also doing so much good work in this area, especially with their toolkits to audit, change, remove, and communicate the changes about intrusive language in licensing applications and credentialing. (Dr. Breen was a New York City ED physician who died by suicide in April 2020 during the early days and height of the COVID-19 pandemic. Her father was quoted as saying: “She was in the trenches. She was a hero.”)
Dr. Moutier: We’re seeing hundreds of physicians get therapy and psychiatric treatment annually. And the advocacy effort is incredibly important, and I think we are witnessing a swifter pace to eliminate those inappropriate and illegal questions about mental health and mental health treatment for physicians and nurses.
Dr. Moffit: We have lowered barriers, not only in individual institutions but also with programming. We have also worked with the Federation of State Medical Boards and The Lorna Breen Foundation to change the legislation. The Foundation has audited and changed 20 state medical boards to remove intrusive language from licensing applications.
Support for colleagues working to help each other
Dr. Myers: One final note for those physicians who need to take time out for medical leave: In my clinical experience, I find that they felt lonely as they were getting well. I can’t tell you how much it made a difference for those who received a phone call, a card, or an email from their colleagues at work. It doesn’t take long for a vibrant, active physician to feel out of the loop when ill.
We know from suicide literature that when somebody’s discharged from the hospital or the emergency department, caring communications, brief expressions of care and concern by email, letter, card, text message, etc., can make all the difference to their recovery. Reaching out to those struggling and those in recovery can help your fellow physician.
A version of this article originally appeared on Medscape.com.
Physician suicide continues to be a challenging problem in the United States. Each year, 1 in 10 doctors think about or attempt suicide, and 400 die by suicide each year. More than half of the doctors reading this know a colleague who has attempted or died by suicide.
These are part of a public health suicide prevention strategy, the preferred method for prevention, in hospitals and institutions around the country. A public health model for preventing suicide is a multifaceted approach that includes universal education, health promotion, selective and targeted prevention, and treatment and recovery.
These physicians hope to continue creating and implementing these and other risk-reduction measures across all health care organizations.
Our physician experts for this discussion
Mary Moffit, PhD, is an associate professor in the department of psychiatry at Oregon Health & Science University, Portland. She directs the resident and faculty wellness program and is director of the OHSU peer support program. She helped design and developed a comprehensive wellness program that is now a national model for academic medical centers.
Christine Yu Moutier, MD, is the chief medical officer of the American Foundation for Suicide Prevention. She is the author of “Suicide Prevention,” a Cambridge University Press clinical handbook. She has been a practicing psychiatrist, professor of psychiatry, dean in the medical school at the University of California, San Diego, and medical director of the inpatient psychiatric unit at the VA Medical Center in La Jolla, Calif.
Michael F. Myers, MD, is a professor of clinical psychiatry in the department of psychiatry & behavioral sciences at the State University of New York, Brooklyn. He is recent past vice-chair of education and director of training in the department of psychiatry & behavioral sciences at the university. He is the author of several books, including “Why Physicians Die By Suicide,” “The Physician as Patient,” and “Touched by Suicide.”
The participants discussed these risk-reduction initiatives as having much potential for helping physicians at risk for suicide and suicidal ideations.
The importance of peer support programs
Peer support program models may differ across institutions but typically describe colleagues providing some degree of emotional first aid to peers who may be at risk.
Dr. Moffit: The Pew support program that we have in place at OHSU, similar to what’s available in many hospitals and systems nationwide, trains individual physicians across multiple specialties in a peer support model. It’s not specifically emotional first aid, although that’s integral to it. It’s also for adverse events: Having a tragic patient death, having learned that you will be named in a lawsuit, and exposure to trauma in the medical role.
Peer to peer is not where we anticipate physicians seeking someone to talk to about their marital relationship not going well. However, the peer supporter will know about resources throughout the university and the community for what is needed. We’ve got 20-30 peer supporters. We try to match them – for example, a surgeon with a surgeon, a primary care doc with a primary care doc. Physicians who use peer support aren’t tracked, and no notes are taken or documented. It takes place informally but has changed the culture and lowered a barrier. We have a waiting list of people who want to be peer supporters.
Dr. Moutier: Peer-to-peer support is usually part of a multi-pronged program and is usually not the only effort going on. Depending on how they’re set up, the goals may be slightly different for each program. Peer-to-peer can be one of the most powerful ways to augment awareness raising and education, which is almost always a basic first step.
Dr. Myers: It doesn’t feel as threatening when people start in a peer-to-peer support group. Users may have been afraid of getting a mental health diagnosis, but with peers, many of whom are often not psychiatrists, that eases distress. Peer support can break down that sense of isolation and loneliness so that someone can take the next step.
Dr. Moutier: To be connected to family, to any community resource, frankly, is a protective factor that mitigates suicide risk. So that’s the logic model from a suicide prevention standpoint. It may be the only opportunity for someone to start disclosing what they’re experiencing, receive validation and support, and not a judgmental response. It can open up the avenue toward help-seeking.
Opt-in/opt-out support for medical residents
This initiative matches residents with a counselor as part of their orientation.
Dr. Moffit: Each resident has a meet and greet with a counselor when they arrive or in their first 6 months at their university. The resident can opt out and cancel the meeting, but they’re scheduled for it as part of their “curriculum.” Institutions like Michigan, Columbia, Montefiore, Mount Sinai, and the University of California, San Diego, have this in place. It starts something like: ‘Hello. Good afternoon. How’s it going? I’m Dr. Moffitt, and here are the services available in this program.’
Dr. Myers: It’s another excellent example of normalizing the stress in the rigors of training and making it part of the wellness initiative.
Dr. Moutier: It’s just a normal part of orientation. Again, as a universal strategy, one thing that I was doing at UCSD with a particular group of medical students, who were at higher risk, was a postbaccalaureate program that found students from underrepresented, under-resourced backgrounds and brought them into this post-bacc year. I was directing it and mentoring these students.
So, I could afford a lot more intensive time and attention to them because it was a small group, but every one of them had regular meetings with me every 2 weeks. My approach was to help them uncover their unique strengths and vulnerabilities as they started this program. They all made it into med school.
It was a very intensive and more concierge-personalized approach. It’s like personalized medicine. What specific self-care, mentoring, and mental health care plan would each student codesign with me to stay on track?
And it would involve very holistic things, like if part of their vulnerability was that leaving their Chicano family was creating stress because their father had said: ‘You’re leaving our culture and our family by going into the profession of medicine,’ then we had specific plans around how to care for that aspect of their struggle. It was a much more informed, customized mentoring approach called the UCSD CAP (Conditional Acceptance Post-Baccalaureate Program). It could be a feature in a more specialized opt-in/opt-out program.
One-question survey: How full is your gas tank?
This initiative is a one-question survey emailed/texted to residents to check in on their wellness. We ask, how full is your gas tank? Select 1 to 5 (Empty to Full). If they flag low, they receive a follow-up.
Dr. Moffit: It’s certainly a metaphor that we use. It’s the idea of being depleted in combination with being extremely sleep deprived and the inability to access the usual sources of support or outlets, and how that can create a perfect storm of a level of distress that can put physicians at risk.
Dr. Moutier: It is a way to help people realize that there are things they can do proactively to keep that tank at least somewhat full enough.
Dr. Myers: Using colloquial or figurative language can get better buy-in than “Here’s a PHQ-9.” It also has a caring or intimate tone to it. Somebody could feel they’re a 1 in this rotation but a 4-5 the next. We know from a lot of the literature that when residents get a good, welcoming orientation, their satisfaction with that rotation is uniformly better than if they’re thrown to the wolves. And we know trial by fire can put trainees at risk.
A buddy to check in with
This initiative is when you’re assigned a buddy in or out of residency that you regularly check in with about how you’re doing.
Dr. Myers: Not to be cynical, but there has been some mentor/mentee research that if you’re assigned a mentor, the results are not nearly as good. And if it’s left to the individual to find a mentor, results could be marginal as well. You need a guide to say, ‘Here are some potential mentors for you, but you decide.’ We do a lot of that at (SUNY) Downstate instead of assigning a person. So, it may require some oversight. Picking a check-in buddy from a list provided rather than having one assigned may be more beneficial.
A lot of what we’re talking about are universal strategies that allow for increased interpersonal connection, which is a protective factor that normalizes help-seeking.
A platform or social media forum to share experiences
An online forum or platform where medical students, residents, and physicians can discuss mental health and suicide prevention. Physicians with personal experience could provide testimonials.
Dr. Myers: I’ve recently signed a book contract, and the working title is “Physicians With Lived Experience: How Their Stories Give Clinical Guidance.” When I talk with doctors who have published their personal stories in the New England Journal of Medicine, JAMA, or sometimes The Washington Post or The New York Times, many of them have said they had no idea at the beginning of their journey that they would do something like this: be transparent about their story. It’s a measure of their health, growth, and grace.
Dr. Moutier: The current president of the Academic Association of Surgeons, Carrie Cunningham, MD, MPH, used her platform at the annual AAS conference in 2022 to focus on suicide prevention. She told her own recent story of having gotten into recovery after having been near suicide and struggling with addiction. It was a groundbreaking moment for the field of surgery and produced a ripple effect. She risked everything to tell her story, which was highly emotional since it was still raw. It got everyone engaged, a real turning point for that field. Storytelling and a place for trainees to discuss suicide prevention, and physicians to recall their lived experiences can be highly beneficial.
Interactive Screening Program
The Interactive Screening Program (ISP) is used in higher education to allow physicians to take a safe, confidential screening test and receive a personalized response that can connect them to mental health services before a crisis emerges.
Dr. Moutier: ISP is a tool within a public health model that can afford anonymity to the user so they can safely have their needs addressed. It’s a way for high-risk individuals to sync up with treatment and support. It’s sometimes used in the universal approach because it can be offered to everyone within the health system community of physicians and staff.
It can produce a ripple effect of normalizing that we all have mental health to take care of. Its intended value is in identifying those with a higher risk for suicide, but it doesn’t stop at identifying those at risk. It helps physicians move past a stage of suffering in silence.
Our data show that 86% of a very high-risk group (currently having suicidal ideation, a recent attempt, or other high-risk factors for suicide) aren’t in any form of treatment and have not disclosed their situation to anyone. A fairly high percentage of those going through ISP request a referral to treatment. It’s a unique, very niche tool, and because users remain anonymous, that affords safety around confidentiality.
It’s usually part of a multipronged approach with education, stigma reduction, storytelling, peer support, and other modalities. In my experience with the UCSD HEAR (Healer Assessment Education and Recovery) program, which is still going strong in about its 15th year, the program went from seeing 13 physicians die by suicide in the years leading up to its launch and in the 15 years since it’s been going, one suicide. We all believe that the ISP is the heart of prevention.
Even though all of the universal strategies are important, they probably wouldn’t be sufficient by themselves because the risk [for suicide] is dynamic, and you have to catch people when they are suffering and ready to seek treatment. Suicide prevention is challenging and must be strategic, multifaceted, and sustained over time.
The importance of confidentiality for physicians
In the past, physicians may have been hesitant to seek treatment when struggling with mental health, substance use disorder and suicidal ideations because they heard stories from doctors who said they had to disclose mental health treatment to medical and state licensing boards.
Dr. Myers: There is so much dated stuff out there, and it gets propagated by people who have had a bad experience. I’m not challenging the authenticity of that, but I feel like those are in the minority. The vast majority of people are seeking help. The Federation of State Physician Health Programs is working with state boards to update and get rid of antiquated questions, and they’re working with credentialing groups.
When I was in practice and my patient was petrified of having to come into the hospital [because of confidentiality] I would just be their physician and say: “Look, I know that this is a worry for you [licensing and credentialing issues] but trust me, I’m going to help you get well; that’s my job. And I’m going to help you sort all that out afterward.” It was part of my work as their physician that if they were going to have to jump through hurdles to get their license reinstated, etc., I could help.
The Dr. Lorna Breen Heroes’ Foundation is also doing so much good work in this area, especially with their toolkits to audit, change, remove, and communicate the changes about intrusive language in licensing applications and credentialing. (Dr. Breen was a New York City ED physician who died by suicide in April 2020 during the early days and height of the COVID-19 pandemic. Her father was quoted as saying: “She was in the trenches. She was a hero.”)
Dr. Moutier: We’re seeing hundreds of physicians get therapy and psychiatric treatment annually. And the advocacy effort is incredibly important, and I think we are witnessing a swifter pace to eliminate those inappropriate and illegal questions about mental health and mental health treatment for physicians and nurses.
Dr. Moffit: We have lowered barriers, not only in individual institutions but also with programming. We have also worked with the Federation of State Medical Boards and The Lorna Breen Foundation to change the legislation. The Foundation has audited and changed 20 state medical boards to remove intrusive language from licensing applications.
Support for colleagues working to help each other
Dr. Myers: One final note for those physicians who need to take time out for medical leave: In my clinical experience, I find that they felt lonely as they were getting well. I can’t tell you how much it made a difference for those who received a phone call, a card, or an email from their colleagues at work. It doesn’t take long for a vibrant, active physician to feel out of the loop when ill.
We know from suicide literature that when somebody’s discharged from the hospital or the emergency department, caring communications, brief expressions of care and concern by email, letter, card, text message, etc., can make all the difference to their recovery. Reaching out to those struggling and those in recovery can help your fellow physician.
A version of this article originally appeared on Medscape.com.
Physician suicide continues to be a challenging problem in the United States. Each year, 1 in 10 doctors think about or attempt suicide, and 400 die by suicide each year. More than half of the doctors reading this know a colleague who has attempted or died by suicide.
These are part of a public health suicide prevention strategy, the preferred method for prevention, in hospitals and institutions around the country. A public health model for preventing suicide is a multifaceted approach that includes universal education, health promotion, selective and targeted prevention, and treatment and recovery.
These physicians hope to continue creating and implementing these and other risk-reduction measures across all health care organizations.
Our physician experts for this discussion
Mary Moffit, PhD, is an associate professor in the department of psychiatry at Oregon Health & Science University, Portland. She directs the resident and faculty wellness program and is director of the OHSU peer support program. She helped design and developed a comprehensive wellness program that is now a national model for academic medical centers.
Christine Yu Moutier, MD, is the chief medical officer of the American Foundation for Suicide Prevention. She is the author of “Suicide Prevention,” a Cambridge University Press clinical handbook. She has been a practicing psychiatrist, professor of psychiatry, dean in the medical school at the University of California, San Diego, and medical director of the inpatient psychiatric unit at the VA Medical Center in La Jolla, Calif.
Michael F. Myers, MD, is a professor of clinical psychiatry in the department of psychiatry & behavioral sciences at the State University of New York, Brooklyn. He is recent past vice-chair of education and director of training in the department of psychiatry & behavioral sciences at the university. He is the author of several books, including “Why Physicians Die By Suicide,” “The Physician as Patient,” and “Touched by Suicide.”
The participants discussed these risk-reduction initiatives as having much potential for helping physicians at risk for suicide and suicidal ideations.
The importance of peer support programs
Peer support program models may differ across institutions but typically describe colleagues providing some degree of emotional first aid to peers who may be at risk.
Dr. Moffit: The Pew support program that we have in place at OHSU, similar to what’s available in many hospitals and systems nationwide, trains individual physicians across multiple specialties in a peer support model. It’s not specifically emotional first aid, although that’s integral to it. It’s also for adverse events: Having a tragic patient death, having learned that you will be named in a lawsuit, and exposure to trauma in the medical role.
Peer to peer is not where we anticipate physicians seeking someone to talk to about their marital relationship not going well. However, the peer supporter will know about resources throughout the university and the community for what is needed. We’ve got 20-30 peer supporters. We try to match them – for example, a surgeon with a surgeon, a primary care doc with a primary care doc. Physicians who use peer support aren’t tracked, and no notes are taken or documented. It takes place informally but has changed the culture and lowered a barrier. We have a waiting list of people who want to be peer supporters.
Dr. Moutier: Peer-to-peer support is usually part of a multi-pronged program and is usually not the only effort going on. Depending on how they’re set up, the goals may be slightly different for each program. Peer-to-peer can be one of the most powerful ways to augment awareness raising and education, which is almost always a basic first step.
Dr. Myers: It doesn’t feel as threatening when people start in a peer-to-peer support group. Users may have been afraid of getting a mental health diagnosis, but with peers, many of whom are often not psychiatrists, that eases distress. Peer support can break down that sense of isolation and loneliness so that someone can take the next step.
Dr. Moutier: To be connected to family, to any community resource, frankly, is a protective factor that mitigates suicide risk. So that’s the logic model from a suicide prevention standpoint. It may be the only opportunity for someone to start disclosing what they’re experiencing, receive validation and support, and not a judgmental response. It can open up the avenue toward help-seeking.
Opt-in/opt-out support for medical residents
This initiative matches residents with a counselor as part of their orientation.
Dr. Moffit: Each resident has a meet and greet with a counselor when they arrive or in their first 6 months at their university. The resident can opt out and cancel the meeting, but they’re scheduled for it as part of their “curriculum.” Institutions like Michigan, Columbia, Montefiore, Mount Sinai, and the University of California, San Diego, have this in place. It starts something like: ‘Hello. Good afternoon. How’s it going? I’m Dr. Moffitt, and here are the services available in this program.’
Dr. Myers: It’s another excellent example of normalizing the stress in the rigors of training and making it part of the wellness initiative.
Dr. Moutier: It’s just a normal part of orientation. Again, as a universal strategy, one thing that I was doing at UCSD with a particular group of medical students, who were at higher risk, was a postbaccalaureate program that found students from underrepresented, under-resourced backgrounds and brought them into this post-bacc year. I was directing it and mentoring these students.
So, I could afford a lot more intensive time and attention to them because it was a small group, but every one of them had regular meetings with me every 2 weeks. My approach was to help them uncover their unique strengths and vulnerabilities as they started this program. They all made it into med school.
It was a very intensive and more concierge-personalized approach. It’s like personalized medicine. What specific self-care, mentoring, and mental health care plan would each student codesign with me to stay on track?
And it would involve very holistic things, like if part of their vulnerability was that leaving their Chicano family was creating stress because their father had said: ‘You’re leaving our culture and our family by going into the profession of medicine,’ then we had specific plans around how to care for that aspect of their struggle. It was a much more informed, customized mentoring approach called the UCSD CAP (Conditional Acceptance Post-Baccalaureate Program). It could be a feature in a more specialized opt-in/opt-out program.
One-question survey: How full is your gas tank?
This initiative is a one-question survey emailed/texted to residents to check in on their wellness. We ask, how full is your gas tank? Select 1 to 5 (Empty to Full). If they flag low, they receive a follow-up.
Dr. Moffit: It’s certainly a metaphor that we use. It’s the idea of being depleted in combination with being extremely sleep deprived and the inability to access the usual sources of support or outlets, and how that can create a perfect storm of a level of distress that can put physicians at risk.
Dr. Moutier: It is a way to help people realize that there are things they can do proactively to keep that tank at least somewhat full enough.
Dr. Myers: Using colloquial or figurative language can get better buy-in than “Here’s a PHQ-9.” It also has a caring or intimate tone to it. Somebody could feel they’re a 1 in this rotation but a 4-5 the next. We know from a lot of the literature that when residents get a good, welcoming orientation, their satisfaction with that rotation is uniformly better than if they’re thrown to the wolves. And we know trial by fire can put trainees at risk.
A buddy to check in with
This initiative is when you’re assigned a buddy in or out of residency that you regularly check in with about how you’re doing.
Dr. Myers: Not to be cynical, but there has been some mentor/mentee research that if you’re assigned a mentor, the results are not nearly as good. And if it’s left to the individual to find a mentor, results could be marginal as well. You need a guide to say, ‘Here are some potential mentors for you, but you decide.’ We do a lot of that at (SUNY) Downstate instead of assigning a person. So, it may require some oversight. Picking a check-in buddy from a list provided rather than having one assigned may be more beneficial.
A lot of what we’re talking about are universal strategies that allow for increased interpersonal connection, which is a protective factor that normalizes help-seeking.
A platform or social media forum to share experiences
An online forum or platform where medical students, residents, and physicians can discuss mental health and suicide prevention. Physicians with personal experience could provide testimonials.
Dr. Myers: I’ve recently signed a book contract, and the working title is “Physicians With Lived Experience: How Their Stories Give Clinical Guidance.” When I talk with doctors who have published their personal stories in the New England Journal of Medicine, JAMA, or sometimes The Washington Post or The New York Times, many of them have said they had no idea at the beginning of their journey that they would do something like this: be transparent about their story. It’s a measure of their health, growth, and grace.
Dr. Moutier: The current president of the Academic Association of Surgeons, Carrie Cunningham, MD, MPH, used her platform at the annual AAS conference in 2022 to focus on suicide prevention. She told her own recent story of having gotten into recovery after having been near suicide and struggling with addiction. It was a groundbreaking moment for the field of surgery and produced a ripple effect. She risked everything to tell her story, which was highly emotional since it was still raw. It got everyone engaged, a real turning point for that field. Storytelling and a place for trainees to discuss suicide prevention, and physicians to recall their lived experiences can be highly beneficial.
Interactive Screening Program
The Interactive Screening Program (ISP) is used in higher education to allow physicians to take a safe, confidential screening test and receive a personalized response that can connect them to mental health services before a crisis emerges.
Dr. Moutier: ISP is a tool within a public health model that can afford anonymity to the user so they can safely have their needs addressed. It’s a way for high-risk individuals to sync up with treatment and support. It’s sometimes used in the universal approach because it can be offered to everyone within the health system community of physicians and staff.
It can produce a ripple effect of normalizing that we all have mental health to take care of. Its intended value is in identifying those with a higher risk for suicide, but it doesn’t stop at identifying those at risk. It helps physicians move past a stage of suffering in silence.
Our data show that 86% of a very high-risk group (currently having suicidal ideation, a recent attempt, or other high-risk factors for suicide) aren’t in any form of treatment and have not disclosed their situation to anyone. A fairly high percentage of those going through ISP request a referral to treatment. It’s a unique, very niche tool, and because users remain anonymous, that affords safety around confidentiality.
It’s usually part of a multipronged approach with education, stigma reduction, storytelling, peer support, and other modalities. In my experience with the UCSD HEAR (Healer Assessment Education and Recovery) program, which is still going strong in about its 15th year, the program went from seeing 13 physicians die by suicide in the years leading up to its launch and in the 15 years since it’s been going, one suicide. We all believe that the ISP is the heart of prevention.
Even though all of the universal strategies are important, they probably wouldn’t be sufficient by themselves because the risk [for suicide] is dynamic, and you have to catch people when they are suffering and ready to seek treatment. Suicide prevention is challenging and must be strategic, multifaceted, and sustained over time.
The importance of confidentiality for physicians
In the past, physicians may have been hesitant to seek treatment when struggling with mental health, substance use disorder and suicidal ideations because they heard stories from doctors who said they had to disclose mental health treatment to medical and state licensing boards.
Dr. Myers: There is so much dated stuff out there, and it gets propagated by people who have had a bad experience. I’m not challenging the authenticity of that, but I feel like those are in the minority. The vast majority of people are seeking help. The Federation of State Physician Health Programs is working with state boards to update and get rid of antiquated questions, and they’re working with credentialing groups.
When I was in practice and my patient was petrified of having to come into the hospital [because of confidentiality] I would just be their physician and say: “Look, I know that this is a worry for you [licensing and credentialing issues] but trust me, I’m going to help you get well; that’s my job. And I’m going to help you sort all that out afterward.” It was part of my work as their physician that if they were going to have to jump through hurdles to get their license reinstated, etc., I could help.
The Dr. Lorna Breen Heroes’ Foundation is also doing so much good work in this area, especially with their toolkits to audit, change, remove, and communicate the changes about intrusive language in licensing applications and credentialing. (Dr. Breen was a New York City ED physician who died by suicide in April 2020 during the early days and height of the COVID-19 pandemic. Her father was quoted as saying: “She was in the trenches. She was a hero.”)
Dr. Moutier: We’re seeing hundreds of physicians get therapy and psychiatric treatment annually. And the advocacy effort is incredibly important, and I think we are witnessing a swifter pace to eliminate those inappropriate and illegal questions about mental health and mental health treatment for physicians and nurses.
Dr. Moffit: We have lowered barriers, not only in individual institutions but also with programming. We have also worked with the Federation of State Medical Boards and The Lorna Breen Foundation to change the legislation. The Foundation has audited and changed 20 state medical boards to remove intrusive language from licensing applications.
Support for colleagues working to help each other
Dr. Myers: One final note for those physicians who need to take time out for medical leave: In my clinical experience, I find that they felt lonely as they were getting well. I can’t tell you how much it made a difference for those who received a phone call, a card, or an email from their colleagues at work. It doesn’t take long for a vibrant, active physician to feel out of the loop when ill.
We know from suicide literature that when somebody’s discharged from the hospital or the emergency department, caring communications, brief expressions of care and concern by email, letter, card, text message, etc., can make all the difference to their recovery. Reaching out to those struggling and those in recovery can help your fellow physician.
A version of this article originally appeared on Medscape.com.