User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'main-prefix')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
New consensus on biomarkers for diagnosis of neurocognitive disorders
A new European consensus statement offers expert guidance on which biomarkers to use for patients presenting with cognitive complaints.
Led by Giovanni B. Frisoni, MD, laboratory of neuroimaging of aging, University of Geneva, and director of the memory clinic at Geneva University Hospital, the multidisciplinary task force set out to define a patient-centered diagnostic workflow for the rational and cost-effective use of biomarkers in memory clinics.
The new algorithm is part of a consensus statement presented at the Congress of the European Academy of Neurology 2023. An interim update was published in June in Alzheimer’s and Dementia.
Which biomarker?
Many biomarkers can aid diagnosis, said Dr. Frisoni; the challenge is choosing which biomarker to use for an individual patient.
A literature-based search, he said, yields a number of recommendations, but the vast majority of these are either disease based or biomarker based. The task force notes that “in vivo biomarkers enable early etiological diagnosis of neurocognitive disorders. While they have good analytical validity, their clinical validity and utility are uncertain.”
“When you have a patient in front of you, you don’ t know whether they have Alzheimer’s disease,” Dr. Frisoni said.
“You have a differential diagnosis to make, and you have a number of biomarkers – a number of weapons in your armamentarium – you have to choose. You can’t use all of them – we would like to, but we cannot.”
He added that trying to determine from the literature which biomarker is most appropriate given individual clinical conditions and all of the potential combinations is impossible.
“You will not find evidence of the comparative diagnostic value and the added diagnostic value” of one test vs, another, he noted.
“Is CSF [cerebrospinal fluid] better than amyloid PET in a particular clinical situation? What do I gain in terms of positive and negative predictive value in all the possible clinical conditions that I encounter in my clinical practice?”
Dr. Frisoni said the reality is that clinicians in memory clinics end up using biomarkers that are “based on clinical opportunities.”
For instance, “if you have a proficient nuclear medic, you use PET a lot.” In contrast, “if you have a proficient laboratory medic,” CSF markers will be favored – a situation that he said is “not ideal” and has resulted in large discrepancies in diagnostic approaches across Europe.
Harmonizing clinical practice
In a bid to harmonize clinical practice, 22 European experts from 11 European scientific societies and the executive director of Alzheimer Europe set out to develop a multidisciplinary consensus algorithm for the biomarker-based diagnosis of neurocognitive disorders in general, rather than specific neurocognitive disorders.
They used the Delphi method, in which a systematic literature review of the literature was followed by the drafting of a series of clinical statements by an executive board. These were then presented to the expert panel. If a majority consensus was reached on a given statement, it was considered closed. Questions for which there was no consensus were revised and presented to the panel again. The process was repeated until a consensus was reached.
A total of 56 statements underwent six rounds of discussion. A final online meeting led to the development of a diagnostic algorithm for patients who attend memory clinics for cognitive complaints.
The algorithm features three potential assessment waves. Wave 1 defines 11 clinical profiles that are based on the results of clinical and neuropsychological assessments, blood exams, brain imaging, and, in specific cases, electroencephalography. Wave 2 defines first-line biomarkers based on Wave 1 clinical profiles, and Wave 3 defines the second-line biomarker based on Wave 2 biomarker results.
When a patient’s clinical profile suggests Alzheimer’s disease and, in undefined cases, cerebrospinal fluid biomarkers are used first line. When CSF is inconclusive, 18-fluorodeoxyglucose positron emission tomography (FDG-PET) is used second line.
When the clinical profile suggests frontotemporal lobar degeneration or motor tauopathies, FDG-PET is first line and CSF biomarkers second line in atypical metabolic patter cases. When the clinical profile suggests Lewy body disease, dopamine transporter SPECT is first line and cardia I23I-metaiodobenzylguanidine scintigraphy is second line.
Dr. Frisoni noted that the panel strongly recommends performing biomarker tests for patients younger than 70. For those aged 70-85 years, biomarker testing is only recommended for patients with specific clinical features. For patients older than 85, biomarker testing is recommended only in “exceptional circumstances.”
Dr. Frisoni noted that the consensus document has a number of limitations.
“First of all, we could not capture all the theoretical possible combinations” of potential diagnosis and relevant biomarker tests. “There are so many that it’s virtually impossible.”
He also noted that the agreement among the panel for the use of some markers was “relatively low” at “barely 50%,” while for others, the agreement was approximately 70%.
The consensus document also does not explicitly address patients with “mixed pathologies,” which are common. In addition, it does not include emerging biomarkers, such as neurofilament light polypeptide levels, an indicator of axonal compromise.
“Last, but not least,” Dr. Frisoni said, the consensus document requires validation.
“This is a paper and pencil exercise. We, as self-appointed experts, can recommend ... whatever we want, but we must check whether what we write is applicable, feasible.”
In other words, it must be determined whether the “real patient journey” fits with the “ideal patient journey” set out in the consensus document.
This kind of validation, Dr. Frisoni said, is “usually not done for this type of exercise,” but “we want to do it in this case.”
Pros and cons
Bogdan Draganski, MD, consultant in neurology at the department of clinical neurosciences and director of the neuroimaging research laboratory, University Hospital of Lausanne (Switzerland), who cochaired the session, told this news organization that he was “swaying between two extremes” when considering the usefulness of the consensus document.
On one hand, the “reductionist approach” of breaking down a “complex issue into an algorithm” via the Delphi method risks introducing subjective bias.
He said machine learning and artificial intelligence could answer some of the questions posed by clinicians and, by extension, the statements included in the Delphi process by assessing the available data in a more objective manner.
On the other hand, Dr. Draganski said that reducing the options available to clinicians when making a differential diagnosis into the current algorithm is, pragmatically speaking, a “good approach.”
From this standpoint, the danger of using machine learning to answer clinical questions is that it “doesn’t take the responsibility” for the final decision, which means “we’re closing the loop of subjective decision-making for an individual doctor.”
He also applauded the idea of trying to provide more uniform patient assessment across Europe, although he believes “we have a long way to go” before it can deliver on the promise of personalized medicine.
Like Dr. Frisoni, Dr. Draganski noted the fact that patients with potential neurocognitive disorders often have multiple pathologies, which can include cardiovascular problems, depression, and cancer and that that could affect the choice of diagnostic biomarkers.
The second issue, he said, concerns implementation of the consensus document, which is a political decision that centers around “how politicians will define ‘uniformity’ and equal access to technological or nontechnological platforms.”
Achieving uniformity will require a pan-regional collaboration, he noted.
The task force was supported by unrestricted grants from F. Hoffmann-La Roche, Biogen International GmbH, Eisai Europe Limited, Life Molecular Imaging GmbH, and OM Pharma Suisse SA. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new European consensus statement offers expert guidance on which biomarkers to use for patients presenting with cognitive complaints.
Led by Giovanni B. Frisoni, MD, laboratory of neuroimaging of aging, University of Geneva, and director of the memory clinic at Geneva University Hospital, the multidisciplinary task force set out to define a patient-centered diagnostic workflow for the rational and cost-effective use of biomarkers in memory clinics.
The new algorithm is part of a consensus statement presented at the Congress of the European Academy of Neurology 2023. An interim update was published in June in Alzheimer’s and Dementia.
Which biomarker?
Many biomarkers can aid diagnosis, said Dr. Frisoni; the challenge is choosing which biomarker to use for an individual patient.
A literature-based search, he said, yields a number of recommendations, but the vast majority of these are either disease based or biomarker based. The task force notes that “in vivo biomarkers enable early etiological diagnosis of neurocognitive disorders. While they have good analytical validity, their clinical validity and utility are uncertain.”
“When you have a patient in front of you, you don’ t know whether they have Alzheimer’s disease,” Dr. Frisoni said.
“You have a differential diagnosis to make, and you have a number of biomarkers – a number of weapons in your armamentarium – you have to choose. You can’t use all of them – we would like to, but we cannot.”
He added that trying to determine from the literature which biomarker is most appropriate given individual clinical conditions and all of the potential combinations is impossible.
“You will not find evidence of the comparative diagnostic value and the added diagnostic value” of one test vs, another, he noted.
“Is CSF [cerebrospinal fluid] better than amyloid PET in a particular clinical situation? What do I gain in terms of positive and negative predictive value in all the possible clinical conditions that I encounter in my clinical practice?”
Dr. Frisoni said the reality is that clinicians in memory clinics end up using biomarkers that are “based on clinical opportunities.”
For instance, “if you have a proficient nuclear medic, you use PET a lot.” In contrast, “if you have a proficient laboratory medic,” CSF markers will be favored – a situation that he said is “not ideal” and has resulted in large discrepancies in diagnostic approaches across Europe.
Harmonizing clinical practice
In a bid to harmonize clinical practice, 22 European experts from 11 European scientific societies and the executive director of Alzheimer Europe set out to develop a multidisciplinary consensus algorithm for the biomarker-based diagnosis of neurocognitive disorders in general, rather than specific neurocognitive disorders.
They used the Delphi method, in which a systematic literature review of the literature was followed by the drafting of a series of clinical statements by an executive board. These were then presented to the expert panel. If a majority consensus was reached on a given statement, it was considered closed. Questions for which there was no consensus were revised and presented to the panel again. The process was repeated until a consensus was reached.
A total of 56 statements underwent six rounds of discussion. A final online meeting led to the development of a diagnostic algorithm for patients who attend memory clinics for cognitive complaints.
The algorithm features three potential assessment waves. Wave 1 defines 11 clinical profiles that are based on the results of clinical and neuropsychological assessments, blood exams, brain imaging, and, in specific cases, electroencephalography. Wave 2 defines first-line biomarkers based on Wave 1 clinical profiles, and Wave 3 defines the second-line biomarker based on Wave 2 biomarker results.
When a patient’s clinical profile suggests Alzheimer’s disease and, in undefined cases, cerebrospinal fluid biomarkers are used first line. When CSF is inconclusive, 18-fluorodeoxyglucose positron emission tomography (FDG-PET) is used second line.
When the clinical profile suggests frontotemporal lobar degeneration or motor tauopathies, FDG-PET is first line and CSF biomarkers second line in atypical metabolic patter cases. When the clinical profile suggests Lewy body disease, dopamine transporter SPECT is first line and cardia I23I-metaiodobenzylguanidine scintigraphy is second line.
Dr. Frisoni noted that the panel strongly recommends performing biomarker tests for patients younger than 70. For those aged 70-85 years, biomarker testing is only recommended for patients with specific clinical features. For patients older than 85, biomarker testing is recommended only in “exceptional circumstances.”
Dr. Frisoni noted that the consensus document has a number of limitations.
“First of all, we could not capture all the theoretical possible combinations” of potential diagnosis and relevant biomarker tests. “There are so many that it’s virtually impossible.”
He also noted that the agreement among the panel for the use of some markers was “relatively low” at “barely 50%,” while for others, the agreement was approximately 70%.
The consensus document also does not explicitly address patients with “mixed pathologies,” which are common. In addition, it does not include emerging biomarkers, such as neurofilament light polypeptide levels, an indicator of axonal compromise.
“Last, but not least,” Dr. Frisoni said, the consensus document requires validation.
“This is a paper and pencil exercise. We, as self-appointed experts, can recommend ... whatever we want, but we must check whether what we write is applicable, feasible.”
In other words, it must be determined whether the “real patient journey” fits with the “ideal patient journey” set out in the consensus document.
This kind of validation, Dr. Frisoni said, is “usually not done for this type of exercise,” but “we want to do it in this case.”
Pros and cons
Bogdan Draganski, MD, consultant in neurology at the department of clinical neurosciences and director of the neuroimaging research laboratory, University Hospital of Lausanne (Switzerland), who cochaired the session, told this news organization that he was “swaying between two extremes” when considering the usefulness of the consensus document.
On one hand, the “reductionist approach” of breaking down a “complex issue into an algorithm” via the Delphi method risks introducing subjective bias.
He said machine learning and artificial intelligence could answer some of the questions posed by clinicians and, by extension, the statements included in the Delphi process by assessing the available data in a more objective manner.
On the other hand, Dr. Draganski said that reducing the options available to clinicians when making a differential diagnosis into the current algorithm is, pragmatically speaking, a “good approach.”
From this standpoint, the danger of using machine learning to answer clinical questions is that it “doesn’t take the responsibility” for the final decision, which means “we’re closing the loop of subjective decision-making for an individual doctor.”
He also applauded the idea of trying to provide more uniform patient assessment across Europe, although he believes “we have a long way to go” before it can deliver on the promise of personalized medicine.
Like Dr. Frisoni, Dr. Draganski noted the fact that patients with potential neurocognitive disorders often have multiple pathologies, which can include cardiovascular problems, depression, and cancer and that that could affect the choice of diagnostic biomarkers.
The second issue, he said, concerns implementation of the consensus document, which is a political decision that centers around “how politicians will define ‘uniformity’ and equal access to technological or nontechnological platforms.”
Achieving uniformity will require a pan-regional collaboration, he noted.
The task force was supported by unrestricted grants from F. Hoffmann-La Roche, Biogen International GmbH, Eisai Europe Limited, Life Molecular Imaging GmbH, and OM Pharma Suisse SA. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new European consensus statement offers expert guidance on which biomarkers to use for patients presenting with cognitive complaints.
Led by Giovanni B. Frisoni, MD, laboratory of neuroimaging of aging, University of Geneva, and director of the memory clinic at Geneva University Hospital, the multidisciplinary task force set out to define a patient-centered diagnostic workflow for the rational and cost-effective use of biomarkers in memory clinics.
The new algorithm is part of a consensus statement presented at the Congress of the European Academy of Neurology 2023. An interim update was published in June in Alzheimer’s and Dementia.
Which biomarker?
Many biomarkers can aid diagnosis, said Dr. Frisoni; the challenge is choosing which biomarker to use for an individual patient.
A literature-based search, he said, yields a number of recommendations, but the vast majority of these are either disease based or biomarker based. The task force notes that “in vivo biomarkers enable early etiological diagnosis of neurocognitive disorders. While they have good analytical validity, their clinical validity and utility are uncertain.”
“When you have a patient in front of you, you don’ t know whether they have Alzheimer’s disease,” Dr. Frisoni said.
“You have a differential diagnosis to make, and you have a number of biomarkers – a number of weapons in your armamentarium – you have to choose. You can’t use all of them – we would like to, but we cannot.”
He added that trying to determine from the literature which biomarker is most appropriate given individual clinical conditions and all of the potential combinations is impossible.
“You will not find evidence of the comparative diagnostic value and the added diagnostic value” of one test vs, another, he noted.
“Is CSF [cerebrospinal fluid] better than amyloid PET in a particular clinical situation? What do I gain in terms of positive and negative predictive value in all the possible clinical conditions that I encounter in my clinical practice?”
Dr. Frisoni said the reality is that clinicians in memory clinics end up using biomarkers that are “based on clinical opportunities.”
For instance, “if you have a proficient nuclear medic, you use PET a lot.” In contrast, “if you have a proficient laboratory medic,” CSF markers will be favored – a situation that he said is “not ideal” and has resulted in large discrepancies in diagnostic approaches across Europe.
Harmonizing clinical practice
In a bid to harmonize clinical practice, 22 European experts from 11 European scientific societies and the executive director of Alzheimer Europe set out to develop a multidisciplinary consensus algorithm for the biomarker-based diagnosis of neurocognitive disorders in general, rather than specific neurocognitive disorders.
They used the Delphi method, in which a systematic literature review of the literature was followed by the drafting of a series of clinical statements by an executive board. These were then presented to the expert panel. If a majority consensus was reached on a given statement, it was considered closed. Questions for which there was no consensus were revised and presented to the panel again. The process was repeated until a consensus was reached.
A total of 56 statements underwent six rounds of discussion. A final online meeting led to the development of a diagnostic algorithm for patients who attend memory clinics for cognitive complaints.
The algorithm features three potential assessment waves. Wave 1 defines 11 clinical profiles that are based on the results of clinical and neuropsychological assessments, blood exams, brain imaging, and, in specific cases, electroencephalography. Wave 2 defines first-line biomarkers based on Wave 1 clinical profiles, and Wave 3 defines the second-line biomarker based on Wave 2 biomarker results.
When a patient’s clinical profile suggests Alzheimer’s disease and, in undefined cases, cerebrospinal fluid biomarkers are used first line. When CSF is inconclusive, 18-fluorodeoxyglucose positron emission tomography (FDG-PET) is used second line.
When the clinical profile suggests frontotemporal lobar degeneration or motor tauopathies, FDG-PET is first line and CSF biomarkers second line in atypical metabolic patter cases. When the clinical profile suggests Lewy body disease, dopamine transporter SPECT is first line and cardia I23I-metaiodobenzylguanidine scintigraphy is second line.
Dr. Frisoni noted that the panel strongly recommends performing biomarker tests for patients younger than 70. For those aged 70-85 years, biomarker testing is only recommended for patients with specific clinical features. For patients older than 85, biomarker testing is recommended only in “exceptional circumstances.”
Dr. Frisoni noted that the consensus document has a number of limitations.
“First of all, we could not capture all the theoretical possible combinations” of potential diagnosis and relevant biomarker tests. “There are so many that it’s virtually impossible.”
He also noted that the agreement among the panel for the use of some markers was “relatively low” at “barely 50%,” while for others, the agreement was approximately 70%.
The consensus document also does not explicitly address patients with “mixed pathologies,” which are common. In addition, it does not include emerging biomarkers, such as neurofilament light polypeptide levels, an indicator of axonal compromise.
“Last, but not least,” Dr. Frisoni said, the consensus document requires validation.
“This is a paper and pencil exercise. We, as self-appointed experts, can recommend ... whatever we want, but we must check whether what we write is applicable, feasible.”
In other words, it must be determined whether the “real patient journey” fits with the “ideal patient journey” set out in the consensus document.
This kind of validation, Dr. Frisoni said, is “usually not done for this type of exercise,” but “we want to do it in this case.”
Pros and cons
Bogdan Draganski, MD, consultant in neurology at the department of clinical neurosciences and director of the neuroimaging research laboratory, University Hospital of Lausanne (Switzerland), who cochaired the session, told this news organization that he was “swaying between two extremes” when considering the usefulness of the consensus document.
On one hand, the “reductionist approach” of breaking down a “complex issue into an algorithm” via the Delphi method risks introducing subjective bias.
He said machine learning and artificial intelligence could answer some of the questions posed by clinicians and, by extension, the statements included in the Delphi process by assessing the available data in a more objective manner.
On the other hand, Dr. Draganski said that reducing the options available to clinicians when making a differential diagnosis into the current algorithm is, pragmatically speaking, a “good approach.”
From this standpoint, the danger of using machine learning to answer clinical questions is that it “doesn’t take the responsibility” for the final decision, which means “we’re closing the loop of subjective decision-making for an individual doctor.”
He also applauded the idea of trying to provide more uniform patient assessment across Europe, although he believes “we have a long way to go” before it can deliver on the promise of personalized medicine.
Like Dr. Frisoni, Dr. Draganski noted the fact that patients with potential neurocognitive disorders often have multiple pathologies, which can include cardiovascular problems, depression, and cancer and that that could affect the choice of diagnostic biomarkers.
The second issue, he said, concerns implementation of the consensus document, which is a political decision that centers around “how politicians will define ‘uniformity’ and equal access to technological or nontechnological platforms.”
Achieving uniformity will require a pan-regional collaboration, he noted.
The task force was supported by unrestricted grants from F. Hoffmann-La Roche, Biogen International GmbH, Eisai Europe Limited, Life Molecular Imaging GmbH, and OM Pharma Suisse SA. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long COVID ‘brain fog’ confounds doctors, but new research offers hope
Kate Whitley was petrified of COVID-19 from the beginning of the pandemic because she has Hashimoto disease, an autoimmune disorder that she knew put her at high risk for complications.
She was right to be worried. Two months after contracting the infection in September 2022, the 42-year-old Nashville resident was diagnosed with long COVID. For Ms. Whitley, the resulting brain fog has been the most challenging factor. She is the owner of a successful paper goods store, and she can’t remember basic aspects of her job. She can’t tolerate loud noises and gets so distracted that she has trouble remembering what she was doing.
Ms. Whitley doesn’t like the term “brain fog” because it doesn’t begin to describe the dramatic disruption to her life over the past 7 months.
Brain fog is among the most common symptoms of long COVID, and also one of the most poorly understood. A reported 46% of those diagnosed with long COVID complain of brain fog or a loss of memory. Many clinicians agree that the term is vague and often doesn’t truly represent the condition. That, in turn, makes it harder for doctors to diagnose and treat it. There are no standard tests for it, nor are there guidelines for symptom management or treatment.
“There’s a lot of imprecision in the term because it might mean different things to different patients,” said James C. Jackson, PsyD, a neuropsychiatrist at Vanderbilt University, Nashville, Tenn., and author of a new book, “Clearing the Fog: From Surviving to Thriving With Long COVID – A Practical Guide.”
Dr. Jackson, who began treating Ms. Whitley in February 2023, said that it makes more sense to call brain fog a brain impairment or an acquired brain injury (ABI) because it doesn’t occur gradually. COVID damages the brain and causes injury. For those with long COVID who were previously in the intensive care unit and may have undergone ventilation, hypoxic brain injury may result from the lack of oxygen to the brain.
Even among those with milder cases of acute COVID, there’s some evidence that persistent neuroinflammation in the brain caused by an activated immune system may also cause damage.
In both cases, the results can be debilitating. Ms. Whitley also has dysautonomia – a disorder of the autonomic nervous system that can cause dizziness, sweating, and headaches along with fatigue and heart palpitations.
She said that she’s so forgetful that when she sees people socially, she’s nervous of what she’ll say. “I feel like I’m constantly sticking my foot in my mouth because I can’t remember details of other people’s lives,” she said.
Although brain disorders such as Alzheimer’s disease and other forms of dementia are marked by a slow decline, ABI occurs more suddenly and may include a loss of executive function and attention.
“With a brain injury, you’re doing fine, and then some event happens (in this case COVID), and immediately after that, your cognitive function is different,” said Dr. Jackson.
Additionally, ABI is an actual diagnosis, whereas brain fog is not.
“With a brain injury, there’s a treatment pathway for cognitive rehabilitation,” said Dr. Jackson.
Treatments may include speech, cognitive, and occupational therapy as well as meeting with a neuropsychiatrist for treatment of the mental and behavioral disorders that may result. Dr. Jackson said that while many patients aren’t functioning cognitively or physically at 100%, they can make enough strides that they don’t have to give up things such as driving and, in some cases, their jobs.
Other experts agree that long COVID may damage the brain. An April 2022 study published in the journal Nature found strong evidence that SARS-CoV-2 infection may cause brain-related abnormalities, for example, a reduction in gray matter in certain parts of the brain, including the prefrontal cortex, hypothalamus, and amygdala.
Additionally, white matter, which is found deeper in the brain and is responsible for the exchange of information between different parts of the brain, may also be at risk of damage as a result of the virus, according to a November 2022 study published in the journal SN Comprehensive Clinical Medicine.
Calling it a “fog” makes it easier for clinicians and the general public to dismiss its severity, said Tyler Reed Bell, PhD, a researcher who specializes in viruses that cause brain injury. He is a fellow in the department of psychiatry at the University of California, San Diego. Brain fog can make driving and returning to work especially dangerous. Because of difficulty focusing, patients are much more likely to make mistakes that cause accidents.
“The COVID virus is very invasive to the brain,” Dr. Bell said.
Others contend this may be a rush to judgment. Karla L. Thompson, PhD, lead neuropsychologist at the University of North Carolina at Chapel Hill’s COVID Recovery Clinic, agrees that in more serious cases of COVID that cause a lack of oxygen to the brain, it’s reasonable to call it a brain injury. But brain fog can also be associated with other long COVID symptoms, not just damage to the brain.
Chronic fatigue and poor sleep are both commonly reported symptoms of long COVID that negatively affect brain function, she said. Sleep disturbances, cardiac problems, dysautonomia, and emotional distress could also affect the way the brain functions post COVID. Finding the right treatment requires identifying all the factors contributing to cognitive impairment.
Part of the problem in treating long COVID brain fog is that diagnostic technology is not sensitive enough to detect inflammation that could be causing damage.
Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, said her team is working on identifying biomarkers that could detect brain inflammation in a way similar to the manner researchers have identified biomarkers to help diagnose chronic fatigue syndrome. Additionally, a new study published last month in JAMA for the first time clearly defined 12 symptoms of long COVID, and brain fog was listed among them. All of this contributes to the development of clear diagnostic criteria.
“It will make a big difference once we have some consistency among clinicians in diagnosing the condition,” said Dr. McComsey.
Ms. Whitley is thankful for the treatment that she’s received thus far. She’s seeing a cognitive rehabilitation therapist, who assesses her memory, cognition, and attention span and gives her tools to break up simple tasks, such as driving, so that they don’t feel overwhelming. She’s back behind the wheel and back to work.
But perhaps most importantly, Ms. Whitley joined a support group, led by Dr. Jackson, that includes other people experiencing the same symptoms she is. When she was at her darkest, they understood.
“Talking to other survivors has been the only solace in all this,” Ms. Whitley said. “Together, we grieve all that’s been lost.”
A version of this article first appeared on Medscape.com.
Kate Whitley was petrified of COVID-19 from the beginning of the pandemic because she has Hashimoto disease, an autoimmune disorder that she knew put her at high risk for complications.
She was right to be worried. Two months after contracting the infection in September 2022, the 42-year-old Nashville resident was diagnosed with long COVID. For Ms. Whitley, the resulting brain fog has been the most challenging factor. She is the owner of a successful paper goods store, and she can’t remember basic aspects of her job. She can’t tolerate loud noises and gets so distracted that she has trouble remembering what she was doing.
Ms. Whitley doesn’t like the term “brain fog” because it doesn’t begin to describe the dramatic disruption to her life over the past 7 months.
Brain fog is among the most common symptoms of long COVID, and also one of the most poorly understood. A reported 46% of those diagnosed with long COVID complain of brain fog or a loss of memory. Many clinicians agree that the term is vague and often doesn’t truly represent the condition. That, in turn, makes it harder for doctors to diagnose and treat it. There are no standard tests for it, nor are there guidelines for symptom management or treatment.
“There’s a lot of imprecision in the term because it might mean different things to different patients,” said James C. Jackson, PsyD, a neuropsychiatrist at Vanderbilt University, Nashville, Tenn., and author of a new book, “Clearing the Fog: From Surviving to Thriving With Long COVID – A Practical Guide.”
Dr. Jackson, who began treating Ms. Whitley in February 2023, said that it makes more sense to call brain fog a brain impairment or an acquired brain injury (ABI) because it doesn’t occur gradually. COVID damages the brain and causes injury. For those with long COVID who were previously in the intensive care unit and may have undergone ventilation, hypoxic brain injury may result from the lack of oxygen to the brain.
Even among those with milder cases of acute COVID, there’s some evidence that persistent neuroinflammation in the brain caused by an activated immune system may also cause damage.
In both cases, the results can be debilitating. Ms. Whitley also has dysautonomia – a disorder of the autonomic nervous system that can cause dizziness, sweating, and headaches along with fatigue and heart palpitations.
She said that she’s so forgetful that when she sees people socially, she’s nervous of what she’ll say. “I feel like I’m constantly sticking my foot in my mouth because I can’t remember details of other people’s lives,” she said.
Although brain disorders such as Alzheimer’s disease and other forms of dementia are marked by a slow decline, ABI occurs more suddenly and may include a loss of executive function and attention.
“With a brain injury, you’re doing fine, and then some event happens (in this case COVID), and immediately after that, your cognitive function is different,” said Dr. Jackson.
Additionally, ABI is an actual diagnosis, whereas brain fog is not.
“With a brain injury, there’s a treatment pathway for cognitive rehabilitation,” said Dr. Jackson.
Treatments may include speech, cognitive, and occupational therapy as well as meeting with a neuropsychiatrist for treatment of the mental and behavioral disorders that may result. Dr. Jackson said that while many patients aren’t functioning cognitively or physically at 100%, they can make enough strides that they don’t have to give up things such as driving and, in some cases, their jobs.
Other experts agree that long COVID may damage the brain. An April 2022 study published in the journal Nature found strong evidence that SARS-CoV-2 infection may cause brain-related abnormalities, for example, a reduction in gray matter in certain parts of the brain, including the prefrontal cortex, hypothalamus, and amygdala.
Additionally, white matter, which is found deeper in the brain and is responsible for the exchange of information between different parts of the brain, may also be at risk of damage as a result of the virus, according to a November 2022 study published in the journal SN Comprehensive Clinical Medicine.
Calling it a “fog” makes it easier for clinicians and the general public to dismiss its severity, said Tyler Reed Bell, PhD, a researcher who specializes in viruses that cause brain injury. He is a fellow in the department of psychiatry at the University of California, San Diego. Brain fog can make driving and returning to work especially dangerous. Because of difficulty focusing, patients are much more likely to make mistakes that cause accidents.
“The COVID virus is very invasive to the brain,” Dr. Bell said.
Others contend this may be a rush to judgment. Karla L. Thompson, PhD, lead neuropsychologist at the University of North Carolina at Chapel Hill’s COVID Recovery Clinic, agrees that in more serious cases of COVID that cause a lack of oxygen to the brain, it’s reasonable to call it a brain injury. But brain fog can also be associated with other long COVID symptoms, not just damage to the brain.
Chronic fatigue and poor sleep are both commonly reported symptoms of long COVID that negatively affect brain function, she said. Sleep disturbances, cardiac problems, dysautonomia, and emotional distress could also affect the way the brain functions post COVID. Finding the right treatment requires identifying all the factors contributing to cognitive impairment.
Part of the problem in treating long COVID brain fog is that diagnostic technology is not sensitive enough to detect inflammation that could be causing damage.
Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, said her team is working on identifying biomarkers that could detect brain inflammation in a way similar to the manner researchers have identified biomarkers to help diagnose chronic fatigue syndrome. Additionally, a new study published last month in JAMA for the first time clearly defined 12 symptoms of long COVID, and brain fog was listed among them. All of this contributes to the development of clear diagnostic criteria.
“It will make a big difference once we have some consistency among clinicians in diagnosing the condition,” said Dr. McComsey.
Ms. Whitley is thankful for the treatment that she’s received thus far. She’s seeing a cognitive rehabilitation therapist, who assesses her memory, cognition, and attention span and gives her tools to break up simple tasks, such as driving, so that they don’t feel overwhelming. She’s back behind the wheel and back to work.
But perhaps most importantly, Ms. Whitley joined a support group, led by Dr. Jackson, that includes other people experiencing the same symptoms she is. When she was at her darkest, they understood.
“Talking to other survivors has been the only solace in all this,” Ms. Whitley said. “Together, we grieve all that’s been lost.”
A version of this article first appeared on Medscape.com.
Kate Whitley was petrified of COVID-19 from the beginning of the pandemic because she has Hashimoto disease, an autoimmune disorder that she knew put her at high risk for complications.
She was right to be worried. Two months after contracting the infection in September 2022, the 42-year-old Nashville resident was diagnosed with long COVID. For Ms. Whitley, the resulting brain fog has been the most challenging factor. She is the owner of a successful paper goods store, and she can’t remember basic aspects of her job. She can’t tolerate loud noises and gets so distracted that she has trouble remembering what she was doing.
Ms. Whitley doesn’t like the term “brain fog” because it doesn’t begin to describe the dramatic disruption to her life over the past 7 months.
Brain fog is among the most common symptoms of long COVID, and also one of the most poorly understood. A reported 46% of those diagnosed with long COVID complain of brain fog or a loss of memory. Many clinicians agree that the term is vague and often doesn’t truly represent the condition. That, in turn, makes it harder for doctors to diagnose and treat it. There are no standard tests for it, nor are there guidelines for symptom management or treatment.
“There’s a lot of imprecision in the term because it might mean different things to different patients,” said James C. Jackson, PsyD, a neuropsychiatrist at Vanderbilt University, Nashville, Tenn., and author of a new book, “Clearing the Fog: From Surviving to Thriving With Long COVID – A Practical Guide.”
Dr. Jackson, who began treating Ms. Whitley in February 2023, said that it makes more sense to call brain fog a brain impairment or an acquired brain injury (ABI) because it doesn’t occur gradually. COVID damages the brain and causes injury. For those with long COVID who were previously in the intensive care unit and may have undergone ventilation, hypoxic brain injury may result from the lack of oxygen to the brain.
Even among those with milder cases of acute COVID, there’s some evidence that persistent neuroinflammation in the brain caused by an activated immune system may also cause damage.
In both cases, the results can be debilitating. Ms. Whitley also has dysautonomia – a disorder of the autonomic nervous system that can cause dizziness, sweating, and headaches along with fatigue and heart palpitations.
She said that she’s so forgetful that when she sees people socially, she’s nervous of what she’ll say. “I feel like I’m constantly sticking my foot in my mouth because I can’t remember details of other people’s lives,” she said.
Although brain disorders such as Alzheimer’s disease and other forms of dementia are marked by a slow decline, ABI occurs more suddenly and may include a loss of executive function and attention.
“With a brain injury, you’re doing fine, and then some event happens (in this case COVID), and immediately after that, your cognitive function is different,” said Dr. Jackson.
Additionally, ABI is an actual diagnosis, whereas brain fog is not.
“With a brain injury, there’s a treatment pathway for cognitive rehabilitation,” said Dr. Jackson.
Treatments may include speech, cognitive, and occupational therapy as well as meeting with a neuropsychiatrist for treatment of the mental and behavioral disorders that may result. Dr. Jackson said that while many patients aren’t functioning cognitively or physically at 100%, they can make enough strides that they don’t have to give up things such as driving and, in some cases, their jobs.
Other experts agree that long COVID may damage the brain. An April 2022 study published in the journal Nature found strong evidence that SARS-CoV-2 infection may cause brain-related abnormalities, for example, a reduction in gray matter in certain parts of the brain, including the prefrontal cortex, hypothalamus, and amygdala.
Additionally, white matter, which is found deeper in the brain and is responsible for the exchange of information between different parts of the brain, may also be at risk of damage as a result of the virus, according to a November 2022 study published in the journal SN Comprehensive Clinical Medicine.
Calling it a “fog” makes it easier for clinicians and the general public to dismiss its severity, said Tyler Reed Bell, PhD, a researcher who specializes in viruses that cause brain injury. He is a fellow in the department of psychiatry at the University of California, San Diego. Brain fog can make driving and returning to work especially dangerous. Because of difficulty focusing, patients are much more likely to make mistakes that cause accidents.
“The COVID virus is very invasive to the brain,” Dr. Bell said.
Others contend this may be a rush to judgment. Karla L. Thompson, PhD, lead neuropsychologist at the University of North Carolina at Chapel Hill’s COVID Recovery Clinic, agrees that in more serious cases of COVID that cause a lack of oxygen to the brain, it’s reasonable to call it a brain injury. But brain fog can also be associated with other long COVID symptoms, not just damage to the brain.
Chronic fatigue and poor sleep are both commonly reported symptoms of long COVID that negatively affect brain function, she said. Sleep disturbances, cardiac problems, dysautonomia, and emotional distress could also affect the way the brain functions post COVID. Finding the right treatment requires identifying all the factors contributing to cognitive impairment.
Part of the problem in treating long COVID brain fog is that diagnostic technology is not sensitive enough to detect inflammation that could be causing damage.
Grace McComsey, MD, who leads the long COVID RECOVER study at University Hospitals Health System in Cleveland, said her team is working on identifying biomarkers that could detect brain inflammation in a way similar to the manner researchers have identified biomarkers to help diagnose chronic fatigue syndrome. Additionally, a new study published last month in JAMA for the first time clearly defined 12 symptoms of long COVID, and brain fog was listed among them. All of this contributes to the development of clear diagnostic criteria.
“It will make a big difference once we have some consistency among clinicians in diagnosing the condition,” said Dr. McComsey.
Ms. Whitley is thankful for the treatment that she’s received thus far. She’s seeing a cognitive rehabilitation therapist, who assesses her memory, cognition, and attention span and gives her tools to break up simple tasks, such as driving, so that they don’t feel overwhelming. She’s back behind the wheel and back to work.
But perhaps most importantly, Ms. Whitley joined a support group, led by Dr. Jackson, that includes other people experiencing the same symptoms she is. When she was at her darkest, they understood.
“Talking to other survivors has been the only solace in all this,” Ms. Whitley said. “Together, we grieve all that’s been lost.”
A version of this article first appeared on Medscape.com.
Medical cannabis does not reduce use of prescription meds
TOPLINE:
, according to a new study published in Annals of Internal Medicine.
METHODOLOGY:
- Cannabis advocates suggest that legal medical cannabis can be a partial solution to the opioid overdose crisis in the United States, which claimed more than 80,000 lives in 2021.
- Current research on how legalized cannabis reduces dependence on prescription pain medication is inconclusive.
- Researchers examined insurance data for the period 2010-2022 from 583,820 adults with chronic noncancer pain.
- They drew from 12 states in which medical cannabis is legal and from 17 in which it is not legal to create a hypothetical randomized trial. The control group simulated prescription rates where medical cannabis was not available.
- Authors evaluated prescription rates for opioids, nonopioid painkillers, and pain interventions, such as physical therapy.
TAKEAWAY:
In a given month during the first 3 years after legalization, for states with medical cannabis, the investigators found the following:
- There was an average decrease of 1.07 percentage points in the proportion of patients who received any opioid prescription, compared to a 1.12 percentage point decrease in the control group.
- There was an average increase of 1.14 percentage points in the proportion of patients who received any nonopioid prescription painkiller, compared to a 1.19 percentage point increase in the control group.
- There was a 0.17 percentage point decrease in the proportion of patients who received any pain procedure, compared to a 0.001 percentage point decrease in the control group.
IN PRACTICE:
“This study did not identify important effects of medical cannabis laws on receipt of opioid or nonopioid pain treatment among patients with chronic noncancer pain,” according to the researchers.
SOURCE:
The study was led by Emma E. McGinty, PhD, of Weill Cornell Medicine, New York, and was funded by the National Institute on Drug Abuse.
LIMITATIONS:
The investigators used a simulated, hypothetical control group that was based on untestable assumptions. They also drew data solely from insured individuals, so the study does not necessarily represent uninsured populations.
DISCLOSURES:
Dr. McGinty reports receiving a grant from NIDA. Her coauthors reported receiving support from NIDA and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a new study published in Annals of Internal Medicine.
METHODOLOGY:
- Cannabis advocates suggest that legal medical cannabis can be a partial solution to the opioid overdose crisis in the United States, which claimed more than 80,000 lives in 2021.
- Current research on how legalized cannabis reduces dependence on prescription pain medication is inconclusive.
- Researchers examined insurance data for the period 2010-2022 from 583,820 adults with chronic noncancer pain.
- They drew from 12 states in which medical cannabis is legal and from 17 in which it is not legal to create a hypothetical randomized trial. The control group simulated prescription rates where medical cannabis was not available.
- Authors evaluated prescription rates for opioids, nonopioid painkillers, and pain interventions, such as physical therapy.
TAKEAWAY:
In a given month during the first 3 years after legalization, for states with medical cannabis, the investigators found the following:
- There was an average decrease of 1.07 percentage points in the proportion of patients who received any opioid prescription, compared to a 1.12 percentage point decrease in the control group.
- There was an average increase of 1.14 percentage points in the proportion of patients who received any nonopioid prescription painkiller, compared to a 1.19 percentage point increase in the control group.
- There was a 0.17 percentage point decrease in the proportion of patients who received any pain procedure, compared to a 0.001 percentage point decrease in the control group.
IN PRACTICE:
“This study did not identify important effects of medical cannabis laws on receipt of opioid or nonopioid pain treatment among patients with chronic noncancer pain,” according to the researchers.
SOURCE:
The study was led by Emma E. McGinty, PhD, of Weill Cornell Medicine, New York, and was funded by the National Institute on Drug Abuse.
LIMITATIONS:
The investigators used a simulated, hypothetical control group that was based on untestable assumptions. They also drew data solely from insured individuals, so the study does not necessarily represent uninsured populations.
DISCLOSURES:
Dr. McGinty reports receiving a grant from NIDA. Her coauthors reported receiving support from NIDA and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
TOPLINE:
, according to a new study published in Annals of Internal Medicine.
METHODOLOGY:
- Cannabis advocates suggest that legal medical cannabis can be a partial solution to the opioid overdose crisis in the United States, which claimed more than 80,000 lives in 2021.
- Current research on how legalized cannabis reduces dependence on prescription pain medication is inconclusive.
- Researchers examined insurance data for the period 2010-2022 from 583,820 adults with chronic noncancer pain.
- They drew from 12 states in which medical cannabis is legal and from 17 in which it is not legal to create a hypothetical randomized trial. The control group simulated prescription rates where medical cannabis was not available.
- Authors evaluated prescription rates for opioids, nonopioid painkillers, and pain interventions, such as physical therapy.
TAKEAWAY:
In a given month during the first 3 years after legalization, for states with medical cannabis, the investigators found the following:
- There was an average decrease of 1.07 percentage points in the proportion of patients who received any opioid prescription, compared to a 1.12 percentage point decrease in the control group.
- There was an average increase of 1.14 percentage points in the proportion of patients who received any nonopioid prescription painkiller, compared to a 1.19 percentage point increase in the control group.
- There was a 0.17 percentage point decrease in the proportion of patients who received any pain procedure, compared to a 0.001 percentage point decrease in the control group.
IN PRACTICE:
“This study did not identify important effects of medical cannabis laws on receipt of opioid or nonopioid pain treatment among patients with chronic noncancer pain,” according to the researchers.
SOURCE:
The study was led by Emma E. McGinty, PhD, of Weill Cornell Medicine, New York, and was funded by the National Institute on Drug Abuse.
LIMITATIONS:
The investigators used a simulated, hypothetical control group that was based on untestable assumptions. They also drew data solely from insured individuals, so the study does not necessarily represent uninsured populations.
DISCLOSURES:
Dr. McGinty reports receiving a grant from NIDA. Her coauthors reported receiving support from NIDA and the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Lean muscle mass protective against Alzheimer’s?
Investigators analyzed data on more than 450,000 participants in the UK Biobank as well as two independent samples of more than 320,000 individuals with and without AD, and more than 260,000 individuals participating in a separate genes and intelligence study.
They estimated lean muscle and fat tissue in the arms and legs and found, in adjusted analyses, over 500 genetic variants associated with lean mass.
On average, higher genetically lean mass was associated with a “modest but statistically robust” reduction in AD risk and with superior performance on cognitive tasks.
“Using human genetic data, we found evidence for a protective effect of lean mass on risk of Alzheimer’s disease,” study investigators Iyas Daghlas, MD, a resident in the department of neurology, University of California, San Francisco, said in an interview.
Although “clinical intervention studies are needed to confirm this effect, this study supports current recommendations to maintain a healthy lifestyle to prevent dementia,” he said.
The study was published online in BMJ Medicine.
Naturally randomized research
Several measures of body composition have been investigated for their potential association with AD. Lean mass – a “proxy for muscle mass, defined as the difference between total mass and fat mass” – has been shown to be reduced in patients with AD compared with controls, the researchers noted.
“Previous research studies have tested the relationship of body mass index with Alzheimer’s disease and did not find evidence for a causal effect,” Dr. Daghlas said. “We wondered whether BMI was an insufficiently granular measure and hypothesized that disaggregating body mass into lean mass and fat mass could reveal novel associations with disease.”
Most studies have used case-control designs, which might be biased by “residual confounding or reverse causality.” Naturally randomized data “may be used as an alternative to conventional observational studies to investigate causal relations between risk factors and diseases,” the researchers wrote.
In particular, the Mendelian randomization (MR) paradigm randomly allocates germline genetic variants and uses them as proxies for a specific risk factor.
MR “is a technique that permits researchers to investigate cause-and-effect relationships using human genetic data,” Dr. Daghlas explained. “In effect, we’re studying the results of a naturally randomized experiment whereby some individuals are genetically allocated to carry more lean mass.”
The current study used MR to investigate the effect of genetically proxied lean mass on the risk of AD and the “related phenotype” of cognitive performance.
Genetic proxy
As genetic proxies for lean mass, the researchers chose single nucleotide polymorphisms (genetic variants) that were associated, in a genome-wide association study (GWAS), with appendicular lean mass.
Appendicular lean mass “more accurately reflects the effects of lean mass than whole body lean mass, which includes smooth and cardiac muscle,” the authors explained.
This GWAS used phenotypic and genetic data from 450,243 participants in the UK Biobank cohort (mean age 57 years). All participants were of European ancestry.
The researchers adjusted for age, sex, and genetic ancestry. They measured appendicular lean mass using bioimpedance – an electric current that flows at different rates through the body, depending on its composition.
In addition to the UK Biobank participants, the researchers drew on an independent sample of 21,982 people with AD; a control group of 41,944 people without AD; a replication sample of 7,329 people with and 252,879 people without AD to validate the findings; and 269,867 people taking part in a genome-wide study of cognitive performance.
The researchers identified 584 variants that met criteria for use as genetic proxies for lean mass. None were located within the APOE gene region. In the aggregate, these variants explained 10.3% of the variance in appendicular lean mass.
Each standard deviation increase in genetically proxied lean mass was associated with a 12% reduction in AD risk (odds ratio [OR], 0.88; 95% confidence interval [CI], 0.82-0.95; P < .001). This finding was replicated in the independent consortium (OR, 0.91; 95% CI, 0.83-0.99; P = .02).
The findings remained “consistent” in sensitivity analyses.
A modifiable risk factor?
Higher appendicular lean mass was associated with higher levels of cognitive performance, with each SD increase in lean mass associated with an SD increase in cognitive performance (OR, 0.09; 95% CI, 0.06-0.11; P = .001).
“Adjusting for potential mediation through performance did not reduce the association between appendicular lean mass and risk of AD,” the authors wrote.
They obtained similar results using genetically proxied trunk and whole-body lean mass, after adjusting for fat mass.
The authors noted several limitations. The bioimpedance measures “only predict, but do not directly measure, lean mass.” Moreover, the approach didn’t examine whether a “critical window of risk factor timing” exists, during which lean mass might play a role in influencing AD risk and after which “interventions would no longer be effective.” Nor could the study determine whether increasing lean mass could reverse AD pathology in patients with preclinical disease or mild cognitive impairment.
Nevertheless, the findings suggest “that lean mass might be a possible modifiable protective factor for Alzheimer’s disease,” the authors wrote. “The mechanisms underlying this finding, as well as the clinical and public health implications, warrant further investigation.”
Novel strategies
In a comment, Iva Miljkovic, MD, PhD, associate professor, department of epidemiology, University of Pittsburgh, said the investigators used “very rigorous methodology.”
The finding suggesting that lean mass is associated with better cognitive function is “important, as cognitive impairment can become stable rather than progress to a pathological state; and, in some cases, can even be reversed.”
In those cases, “identifying the underlying cause – e.g., low lean mass – can significantly improve cognitive function,” said Dr. Miljkovic, senior author of a study showing muscle fat as a risk factor for cognitive decline.
More research will enable us to “expand our understanding” of the mechanisms involved and determine whether interventions aimed at preventing muscle loss and/or increasing muscle fat may have a beneficial effect on cognitive function,” she said. “This might lead to novel strategies to prevent AD.”
Dr. Daghlas is supported by the British Heart Foundation Centre of Research Excellence at Imperial College, London, and is employed part-time by Novo Nordisk. Dr. Miljkovic reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators analyzed data on more than 450,000 participants in the UK Biobank as well as two independent samples of more than 320,000 individuals with and without AD, and more than 260,000 individuals participating in a separate genes and intelligence study.
They estimated lean muscle and fat tissue in the arms and legs and found, in adjusted analyses, over 500 genetic variants associated with lean mass.
On average, higher genetically lean mass was associated with a “modest but statistically robust” reduction in AD risk and with superior performance on cognitive tasks.
“Using human genetic data, we found evidence for a protective effect of lean mass on risk of Alzheimer’s disease,” study investigators Iyas Daghlas, MD, a resident in the department of neurology, University of California, San Francisco, said in an interview.
Although “clinical intervention studies are needed to confirm this effect, this study supports current recommendations to maintain a healthy lifestyle to prevent dementia,” he said.
The study was published online in BMJ Medicine.
Naturally randomized research
Several measures of body composition have been investigated for their potential association with AD. Lean mass – a “proxy for muscle mass, defined as the difference between total mass and fat mass” – has been shown to be reduced in patients with AD compared with controls, the researchers noted.
“Previous research studies have tested the relationship of body mass index with Alzheimer’s disease and did not find evidence for a causal effect,” Dr. Daghlas said. “We wondered whether BMI was an insufficiently granular measure and hypothesized that disaggregating body mass into lean mass and fat mass could reveal novel associations with disease.”
Most studies have used case-control designs, which might be biased by “residual confounding or reverse causality.” Naturally randomized data “may be used as an alternative to conventional observational studies to investigate causal relations between risk factors and diseases,” the researchers wrote.
In particular, the Mendelian randomization (MR) paradigm randomly allocates germline genetic variants and uses them as proxies for a specific risk factor.
MR “is a technique that permits researchers to investigate cause-and-effect relationships using human genetic data,” Dr. Daghlas explained. “In effect, we’re studying the results of a naturally randomized experiment whereby some individuals are genetically allocated to carry more lean mass.”
The current study used MR to investigate the effect of genetically proxied lean mass on the risk of AD and the “related phenotype” of cognitive performance.
Genetic proxy
As genetic proxies for lean mass, the researchers chose single nucleotide polymorphisms (genetic variants) that were associated, in a genome-wide association study (GWAS), with appendicular lean mass.
Appendicular lean mass “more accurately reflects the effects of lean mass than whole body lean mass, which includes smooth and cardiac muscle,” the authors explained.
This GWAS used phenotypic and genetic data from 450,243 participants in the UK Biobank cohort (mean age 57 years). All participants were of European ancestry.
The researchers adjusted for age, sex, and genetic ancestry. They measured appendicular lean mass using bioimpedance – an electric current that flows at different rates through the body, depending on its composition.
In addition to the UK Biobank participants, the researchers drew on an independent sample of 21,982 people with AD; a control group of 41,944 people without AD; a replication sample of 7,329 people with and 252,879 people without AD to validate the findings; and 269,867 people taking part in a genome-wide study of cognitive performance.
The researchers identified 584 variants that met criteria for use as genetic proxies for lean mass. None were located within the APOE gene region. In the aggregate, these variants explained 10.3% of the variance in appendicular lean mass.
Each standard deviation increase in genetically proxied lean mass was associated with a 12% reduction in AD risk (odds ratio [OR], 0.88; 95% confidence interval [CI], 0.82-0.95; P < .001). This finding was replicated in the independent consortium (OR, 0.91; 95% CI, 0.83-0.99; P = .02).
The findings remained “consistent” in sensitivity analyses.
A modifiable risk factor?
Higher appendicular lean mass was associated with higher levels of cognitive performance, with each SD increase in lean mass associated with an SD increase in cognitive performance (OR, 0.09; 95% CI, 0.06-0.11; P = .001).
“Adjusting for potential mediation through performance did not reduce the association between appendicular lean mass and risk of AD,” the authors wrote.
They obtained similar results using genetically proxied trunk and whole-body lean mass, after adjusting for fat mass.
The authors noted several limitations. The bioimpedance measures “only predict, but do not directly measure, lean mass.” Moreover, the approach didn’t examine whether a “critical window of risk factor timing” exists, during which lean mass might play a role in influencing AD risk and after which “interventions would no longer be effective.” Nor could the study determine whether increasing lean mass could reverse AD pathology in patients with preclinical disease or mild cognitive impairment.
Nevertheless, the findings suggest “that lean mass might be a possible modifiable protective factor for Alzheimer’s disease,” the authors wrote. “The mechanisms underlying this finding, as well as the clinical and public health implications, warrant further investigation.”
Novel strategies
In a comment, Iva Miljkovic, MD, PhD, associate professor, department of epidemiology, University of Pittsburgh, said the investigators used “very rigorous methodology.”
The finding suggesting that lean mass is associated with better cognitive function is “important, as cognitive impairment can become stable rather than progress to a pathological state; and, in some cases, can even be reversed.”
In those cases, “identifying the underlying cause – e.g., low lean mass – can significantly improve cognitive function,” said Dr. Miljkovic, senior author of a study showing muscle fat as a risk factor for cognitive decline.
More research will enable us to “expand our understanding” of the mechanisms involved and determine whether interventions aimed at preventing muscle loss and/or increasing muscle fat may have a beneficial effect on cognitive function,” she said. “This might lead to novel strategies to prevent AD.”
Dr. Daghlas is supported by the British Heart Foundation Centre of Research Excellence at Imperial College, London, and is employed part-time by Novo Nordisk. Dr. Miljkovic reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators analyzed data on more than 450,000 participants in the UK Biobank as well as two independent samples of more than 320,000 individuals with and without AD, and more than 260,000 individuals participating in a separate genes and intelligence study.
They estimated lean muscle and fat tissue in the arms and legs and found, in adjusted analyses, over 500 genetic variants associated with lean mass.
On average, higher genetically lean mass was associated with a “modest but statistically robust” reduction in AD risk and with superior performance on cognitive tasks.
“Using human genetic data, we found evidence for a protective effect of lean mass on risk of Alzheimer’s disease,” study investigators Iyas Daghlas, MD, a resident in the department of neurology, University of California, San Francisco, said in an interview.
Although “clinical intervention studies are needed to confirm this effect, this study supports current recommendations to maintain a healthy lifestyle to prevent dementia,” he said.
The study was published online in BMJ Medicine.
Naturally randomized research
Several measures of body composition have been investigated for their potential association with AD. Lean mass – a “proxy for muscle mass, defined as the difference between total mass and fat mass” – has been shown to be reduced in patients with AD compared with controls, the researchers noted.
“Previous research studies have tested the relationship of body mass index with Alzheimer’s disease and did not find evidence for a causal effect,” Dr. Daghlas said. “We wondered whether BMI was an insufficiently granular measure and hypothesized that disaggregating body mass into lean mass and fat mass could reveal novel associations with disease.”
Most studies have used case-control designs, which might be biased by “residual confounding or reverse causality.” Naturally randomized data “may be used as an alternative to conventional observational studies to investigate causal relations between risk factors and diseases,” the researchers wrote.
In particular, the Mendelian randomization (MR) paradigm randomly allocates germline genetic variants and uses them as proxies for a specific risk factor.
MR “is a technique that permits researchers to investigate cause-and-effect relationships using human genetic data,” Dr. Daghlas explained. “In effect, we’re studying the results of a naturally randomized experiment whereby some individuals are genetically allocated to carry more lean mass.”
The current study used MR to investigate the effect of genetically proxied lean mass on the risk of AD and the “related phenotype” of cognitive performance.
Genetic proxy
As genetic proxies for lean mass, the researchers chose single nucleotide polymorphisms (genetic variants) that were associated, in a genome-wide association study (GWAS), with appendicular lean mass.
Appendicular lean mass “more accurately reflects the effects of lean mass than whole body lean mass, which includes smooth and cardiac muscle,” the authors explained.
This GWAS used phenotypic and genetic data from 450,243 participants in the UK Biobank cohort (mean age 57 years). All participants were of European ancestry.
The researchers adjusted for age, sex, and genetic ancestry. They measured appendicular lean mass using bioimpedance – an electric current that flows at different rates through the body, depending on its composition.
In addition to the UK Biobank participants, the researchers drew on an independent sample of 21,982 people with AD; a control group of 41,944 people without AD; a replication sample of 7,329 people with and 252,879 people without AD to validate the findings; and 269,867 people taking part in a genome-wide study of cognitive performance.
The researchers identified 584 variants that met criteria for use as genetic proxies for lean mass. None were located within the APOE gene region. In the aggregate, these variants explained 10.3% of the variance in appendicular lean mass.
Each standard deviation increase in genetically proxied lean mass was associated with a 12% reduction in AD risk (odds ratio [OR], 0.88; 95% confidence interval [CI], 0.82-0.95; P < .001). This finding was replicated in the independent consortium (OR, 0.91; 95% CI, 0.83-0.99; P = .02).
The findings remained “consistent” in sensitivity analyses.
A modifiable risk factor?
Higher appendicular lean mass was associated with higher levels of cognitive performance, with each SD increase in lean mass associated with an SD increase in cognitive performance (OR, 0.09; 95% CI, 0.06-0.11; P = .001).
“Adjusting for potential mediation through performance did not reduce the association between appendicular lean mass and risk of AD,” the authors wrote.
They obtained similar results using genetically proxied trunk and whole-body lean mass, after adjusting for fat mass.
The authors noted several limitations. The bioimpedance measures “only predict, but do not directly measure, lean mass.” Moreover, the approach didn’t examine whether a “critical window of risk factor timing” exists, during which lean mass might play a role in influencing AD risk and after which “interventions would no longer be effective.” Nor could the study determine whether increasing lean mass could reverse AD pathology in patients with preclinical disease or mild cognitive impairment.
Nevertheless, the findings suggest “that lean mass might be a possible modifiable protective factor for Alzheimer’s disease,” the authors wrote. “The mechanisms underlying this finding, as well as the clinical and public health implications, warrant further investigation.”
Novel strategies
In a comment, Iva Miljkovic, MD, PhD, associate professor, department of epidemiology, University of Pittsburgh, said the investigators used “very rigorous methodology.”
The finding suggesting that lean mass is associated with better cognitive function is “important, as cognitive impairment can become stable rather than progress to a pathological state; and, in some cases, can even be reversed.”
In those cases, “identifying the underlying cause – e.g., low lean mass – can significantly improve cognitive function,” said Dr. Miljkovic, senior author of a study showing muscle fat as a risk factor for cognitive decline.
More research will enable us to “expand our understanding” of the mechanisms involved and determine whether interventions aimed at preventing muscle loss and/or increasing muscle fat may have a beneficial effect on cognitive function,” she said. “This might lead to novel strategies to prevent AD.”
Dr. Daghlas is supported by the British Heart Foundation Centre of Research Excellence at Imperial College, London, and is employed part-time by Novo Nordisk. Dr. Miljkovic reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM BMJ MEDICINE
Do oral contraceptives increase depression risk?
In addition, OC use in adolescence has been tied to an increased risk for depression later in life. However, some experts believe the study’s methodology may be flawed.
The investigators tracked more than 250,000 women from birth to menopause, gathering information about their use of combined contraceptive pills (progesterone and estrogen), the timing of the initial depression diagnosis, and the onset of depressive symptoms that were not formally diagnosed.
Women who began using these OCs before or at the age of 20 experienced a 130% higher incidence of depressive symptoms, whereas adult users saw a 92% increase. But the higher occurrence of depression tended to decline after the first 2 years of use, except in teenagers, who maintained an increased incidence of depression even after discontinuation.
This effect remained, even after analysis of potential familial confounding.
“Our findings suggest that the use of OCs, particularly during the first 2 years, increases the risk of depression. Additionally, OC use during adolescence might increase the risk of depression later in life,” Therese Johansson, of the department of immunology, genetics, and pathology, Science for Life Laboratory, Uppsala (Sweden) University, and colleagues wrote.
The study was published online in Epidemiology and Psychiatric Sciences.
Inconsistent findings
Previous studies suggest an association between adolescent use of hormonal contraceptives (HCs) and increased depression risk, but it’s “less clear” whether these effects are similar in adults, the authors wrote. Randomized clinical trials have “shown little or no effect” of HCs on mood. However, most of these studies didn’t consider previous use of HC.
The researchers wanted to estimate the incidence rate of depression associated with first initiation of OC use as well as the lifetime risk associated with use.
They studied 264,557 female participants in the UK Biobank (aged 37-71 years), collecting data from questionnaires, interviews, physical health measures, biological samples, imaging, and linked health records.
Most participants taking OCs had initiated use during the 1970s/early 1980s when second-generation OCs were predominantly used, consisting of levonorgestrel and ethinyl estradiol.
The researchers conducted a secondary outcome analysis on women who completed the UK Biobank Mental Health Questionnaire (MHQ) to evaluate depressive symptoms.
They estimated the associated risk for depression within 2 years after starting OCs in all women, as well as in groups stratified by age at initiation: before age 20 (adolescents) and age 20 and older (adults). In addition, the investigators estimated the lifetime risk for depression.
Time-dependent analysis compared the effect of OC use at initiation to the effect during the remaining years of use in recent and previous users.
They analyzed a subcohort of female siblings, utilizing “inference about causation from examination of familial confounding,” defined by the authors as a “regression-based approach for determining causality through the use of paired observational data collected from related individuals.”
Adolescents at highest risk
Of the participants, 80.6% had used OCs at some point.
The first 2 years of use were associated with a higher rate of depression among users, compared with never-users (hazard ration, 1.79; 95% confidence interval, 1.63-1.96). Although the risk became less pronounced after that, ever-use was still associated with increased lifetime risk for depression (HR, 1.05; 95% CI, 1.01-1.09).
Adolescents and adult OC users both experienced higher rates of depression during the first 2 years, with a more marked effect in adolescents than in adults (HR, 1.95; 95% CI, 1.64-2.32; and HR, 1.74; 95% CI, 1.54-1.95, respectively).
Previous users of OCs had a higher lifetime risk for depression, compared with never-users (HR, 1.05; 95% CI, 1.01-1.09).
Of the subcohort of women who completed the MHQ (n = 82,232), about half reported experiencing at least one of the core depressive symptoms.
OC initiation was associated with an increased risk for depressive symptoms during the first 2 years in ever- versus never-users (HR, 2.00; 95% CI, 1.91-2.10).
Those who began using OCs during adolescence had a dramatically higher rate of depressive symptoms, compared with never-users (HR, 2.30; 95% CI, 2.11-2.51), as did adult initiators (HR, 1.92; 95% CI, 2.11-2.51).
In the analysis of 7,354 first-degree sister pairs, 81% had initiated OCs. A sibling’s OC use was positively associated with a depression diagnosis, and the cosibling’s OC use was also associated with the sibling’s depression diagnosis. “These results support the hypothesis of a causal relationship between OC use and depression, such that OC use increases the risk of depression,” the authors wrote.
The main limitation is the potential for recall bias in the self-reported data, and that the UK Biobank sample consists of a healthier population than the overall U.K. population, which “hampers the generalizability” of the findings, the authors stated.
Flawed study
In a comment, Natalie Rasgon, MD, founder and director of the Stanford (Calif.) Center for Neuroscience in Women’s Health, said the study was “well researched” and “well written” but had “methodological issues.”
She questioned the sibling component, “which the researchers regard as confirming causality.” The effect may be “important but not causative.” Causality in people who are recalling retrospectively “is highly questionable by any adept researcher because it’s subject to memory. Different siblings may have different recall.”
The authors also didn’t study the indication for OC use. Several medical conditions are treated with OCs, including premenstrual dysphoric disorder, the “number one mood disorder among women of reproductive age.” Including this “could have made a huge difference in outcome data,” said Dr. Rasgon, who was not involved with the study.
Anne-Marie Amies Oelschlager, MD, professor of obstetrics and gynecology, University of Washington, Seattle, noted participants were asked to recall depressive symptoms and OC use as far back as 20-30 years ago, which lends itself to inaccurate recall.
And the researchers didn’t ascertain whether the contraceptives had been used continuously or had been started, stopped, and restarted. Nor did they look at different formulations and doses. And the observational nature of the study “limits the ability to infer causation,” continued Dr. Oelschlager, chair of the American College of Obstetrics and Gynecology Clinical Consensus Gynecology Committee. She was not involved with the study.
“This study is too flawed to use meaningfully in clinical practice,” Dr. Oelschlager concluded.
The study was primarily funded by the Swedish Research Council, the Swedish Brain Foundation, and the Uppsala University Center for Women ‘s Mental Health during the Reproductive Lifespan. The authors, Dr. Rasgon, and Dr. Oelschlager declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In addition, OC use in adolescence has been tied to an increased risk for depression later in life. However, some experts believe the study’s methodology may be flawed.
The investigators tracked more than 250,000 women from birth to menopause, gathering information about their use of combined contraceptive pills (progesterone and estrogen), the timing of the initial depression diagnosis, and the onset of depressive symptoms that were not formally diagnosed.
Women who began using these OCs before or at the age of 20 experienced a 130% higher incidence of depressive symptoms, whereas adult users saw a 92% increase. But the higher occurrence of depression tended to decline after the first 2 years of use, except in teenagers, who maintained an increased incidence of depression even after discontinuation.
This effect remained, even after analysis of potential familial confounding.
“Our findings suggest that the use of OCs, particularly during the first 2 years, increases the risk of depression. Additionally, OC use during adolescence might increase the risk of depression later in life,” Therese Johansson, of the department of immunology, genetics, and pathology, Science for Life Laboratory, Uppsala (Sweden) University, and colleagues wrote.
The study was published online in Epidemiology and Psychiatric Sciences.
Inconsistent findings
Previous studies suggest an association between adolescent use of hormonal contraceptives (HCs) and increased depression risk, but it’s “less clear” whether these effects are similar in adults, the authors wrote. Randomized clinical trials have “shown little or no effect” of HCs on mood. However, most of these studies didn’t consider previous use of HC.
The researchers wanted to estimate the incidence rate of depression associated with first initiation of OC use as well as the lifetime risk associated with use.
They studied 264,557 female participants in the UK Biobank (aged 37-71 years), collecting data from questionnaires, interviews, physical health measures, biological samples, imaging, and linked health records.
Most participants taking OCs had initiated use during the 1970s/early 1980s when second-generation OCs were predominantly used, consisting of levonorgestrel and ethinyl estradiol.
The researchers conducted a secondary outcome analysis on women who completed the UK Biobank Mental Health Questionnaire (MHQ) to evaluate depressive symptoms.
They estimated the associated risk for depression within 2 years after starting OCs in all women, as well as in groups stratified by age at initiation: before age 20 (adolescents) and age 20 and older (adults). In addition, the investigators estimated the lifetime risk for depression.
Time-dependent analysis compared the effect of OC use at initiation to the effect during the remaining years of use in recent and previous users.
They analyzed a subcohort of female siblings, utilizing “inference about causation from examination of familial confounding,” defined by the authors as a “regression-based approach for determining causality through the use of paired observational data collected from related individuals.”
Adolescents at highest risk
Of the participants, 80.6% had used OCs at some point.
The first 2 years of use were associated with a higher rate of depression among users, compared with never-users (hazard ration, 1.79; 95% confidence interval, 1.63-1.96). Although the risk became less pronounced after that, ever-use was still associated with increased lifetime risk for depression (HR, 1.05; 95% CI, 1.01-1.09).
Adolescents and adult OC users both experienced higher rates of depression during the first 2 years, with a more marked effect in adolescents than in adults (HR, 1.95; 95% CI, 1.64-2.32; and HR, 1.74; 95% CI, 1.54-1.95, respectively).
Previous users of OCs had a higher lifetime risk for depression, compared with never-users (HR, 1.05; 95% CI, 1.01-1.09).
Of the subcohort of women who completed the MHQ (n = 82,232), about half reported experiencing at least one of the core depressive symptoms.
OC initiation was associated with an increased risk for depressive symptoms during the first 2 years in ever- versus never-users (HR, 2.00; 95% CI, 1.91-2.10).
Those who began using OCs during adolescence had a dramatically higher rate of depressive symptoms, compared with never-users (HR, 2.30; 95% CI, 2.11-2.51), as did adult initiators (HR, 1.92; 95% CI, 2.11-2.51).
In the analysis of 7,354 first-degree sister pairs, 81% had initiated OCs. A sibling’s OC use was positively associated with a depression diagnosis, and the cosibling’s OC use was also associated with the sibling’s depression diagnosis. “These results support the hypothesis of a causal relationship between OC use and depression, such that OC use increases the risk of depression,” the authors wrote.
The main limitation is the potential for recall bias in the self-reported data, and that the UK Biobank sample consists of a healthier population than the overall U.K. population, which “hampers the generalizability” of the findings, the authors stated.
Flawed study
In a comment, Natalie Rasgon, MD, founder and director of the Stanford (Calif.) Center for Neuroscience in Women’s Health, said the study was “well researched” and “well written” but had “methodological issues.”
She questioned the sibling component, “which the researchers regard as confirming causality.” The effect may be “important but not causative.” Causality in people who are recalling retrospectively “is highly questionable by any adept researcher because it’s subject to memory. Different siblings may have different recall.”
The authors also didn’t study the indication for OC use. Several medical conditions are treated with OCs, including premenstrual dysphoric disorder, the “number one mood disorder among women of reproductive age.” Including this “could have made a huge difference in outcome data,” said Dr. Rasgon, who was not involved with the study.
Anne-Marie Amies Oelschlager, MD, professor of obstetrics and gynecology, University of Washington, Seattle, noted participants were asked to recall depressive symptoms and OC use as far back as 20-30 years ago, which lends itself to inaccurate recall.
And the researchers didn’t ascertain whether the contraceptives had been used continuously or had been started, stopped, and restarted. Nor did they look at different formulations and doses. And the observational nature of the study “limits the ability to infer causation,” continued Dr. Oelschlager, chair of the American College of Obstetrics and Gynecology Clinical Consensus Gynecology Committee. She was not involved with the study.
“This study is too flawed to use meaningfully in clinical practice,” Dr. Oelschlager concluded.
The study was primarily funded by the Swedish Research Council, the Swedish Brain Foundation, and the Uppsala University Center for Women ‘s Mental Health during the Reproductive Lifespan. The authors, Dr. Rasgon, and Dr. Oelschlager declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In addition, OC use in adolescence has been tied to an increased risk for depression later in life. However, some experts believe the study’s methodology may be flawed.
The investigators tracked more than 250,000 women from birth to menopause, gathering information about their use of combined contraceptive pills (progesterone and estrogen), the timing of the initial depression diagnosis, and the onset of depressive symptoms that were not formally diagnosed.
Women who began using these OCs before or at the age of 20 experienced a 130% higher incidence of depressive symptoms, whereas adult users saw a 92% increase. But the higher occurrence of depression tended to decline after the first 2 years of use, except in teenagers, who maintained an increased incidence of depression even after discontinuation.
This effect remained, even after analysis of potential familial confounding.
“Our findings suggest that the use of OCs, particularly during the first 2 years, increases the risk of depression. Additionally, OC use during adolescence might increase the risk of depression later in life,” Therese Johansson, of the department of immunology, genetics, and pathology, Science for Life Laboratory, Uppsala (Sweden) University, and colleagues wrote.
The study was published online in Epidemiology and Psychiatric Sciences.
Inconsistent findings
Previous studies suggest an association between adolescent use of hormonal contraceptives (HCs) and increased depression risk, but it’s “less clear” whether these effects are similar in adults, the authors wrote. Randomized clinical trials have “shown little or no effect” of HCs on mood. However, most of these studies didn’t consider previous use of HC.
The researchers wanted to estimate the incidence rate of depression associated with first initiation of OC use as well as the lifetime risk associated with use.
They studied 264,557 female participants in the UK Biobank (aged 37-71 years), collecting data from questionnaires, interviews, physical health measures, biological samples, imaging, and linked health records.
Most participants taking OCs had initiated use during the 1970s/early 1980s when second-generation OCs were predominantly used, consisting of levonorgestrel and ethinyl estradiol.
The researchers conducted a secondary outcome analysis on women who completed the UK Biobank Mental Health Questionnaire (MHQ) to evaluate depressive symptoms.
They estimated the associated risk for depression within 2 years after starting OCs in all women, as well as in groups stratified by age at initiation: before age 20 (adolescents) and age 20 and older (adults). In addition, the investigators estimated the lifetime risk for depression.
Time-dependent analysis compared the effect of OC use at initiation to the effect during the remaining years of use in recent and previous users.
They analyzed a subcohort of female siblings, utilizing “inference about causation from examination of familial confounding,” defined by the authors as a “regression-based approach for determining causality through the use of paired observational data collected from related individuals.”
Adolescents at highest risk
Of the participants, 80.6% had used OCs at some point.
The first 2 years of use were associated with a higher rate of depression among users, compared with never-users (hazard ration, 1.79; 95% confidence interval, 1.63-1.96). Although the risk became less pronounced after that, ever-use was still associated with increased lifetime risk for depression (HR, 1.05; 95% CI, 1.01-1.09).
Adolescents and adult OC users both experienced higher rates of depression during the first 2 years, with a more marked effect in adolescents than in adults (HR, 1.95; 95% CI, 1.64-2.32; and HR, 1.74; 95% CI, 1.54-1.95, respectively).
Previous users of OCs had a higher lifetime risk for depression, compared with never-users (HR, 1.05; 95% CI, 1.01-1.09).
Of the subcohort of women who completed the MHQ (n = 82,232), about half reported experiencing at least one of the core depressive symptoms.
OC initiation was associated with an increased risk for depressive symptoms during the first 2 years in ever- versus never-users (HR, 2.00; 95% CI, 1.91-2.10).
Those who began using OCs during adolescence had a dramatically higher rate of depressive symptoms, compared with never-users (HR, 2.30; 95% CI, 2.11-2.51), as did adult initiators (HR, 1.92; 95% CI, 2.11-2.51).
In the analysis of 7,354 first-degree sister pairs, 81% had initiated OCs. A sibling’s OC use was positively associated with a depression diagnosis, and the cosibling’s OC use was also associated with the sibling’s depression diagnosis. “These results support the hypothesis of a causal relationship between OC use and depression, such that OC use increases the risk of depression,” the authors wrote.
The main limitation is the potential for recall bias in the self-reported data, and that the UK Biobank sample consists of a healthier population than the overall U.K. population, which “hampers the generalizability” of the findings, the authors stated.
Flawed study
In a comment, Natalie Rasgon, MD, founder and director of the Stanford (Calif.) Center for Neuroscience in Women’s Health, said the study was “well researched” and “well written” but had “methodological issues.”
She questioned the sibling component, “which the researchers regard as confirming causality.” The effect may be “important but not causative.” Causality in people who are recalling retrospectively “is highly questionable by any adept researcher because it’s subject to memory. Different siblings may have different recall.”
The authors also didn’t study the indication for OC use. Several medical conditions are treated with OCs, including premenstrual dysphoric disorder, the “number one mood disorder among women of reproductive age.” Including this “could have made a huge difference in outcome data,” said Dr. Rasgon, who was not involved with the study.
Anne-Marie Amies Oelschlager, MD, professor of obstetrics and gynecology, University of Washington, Seattle, noted participants were asked to recall depressive symptoms and OC use as far back as 20-30 years ago, which lends itself to inaccurate recall.
And the researchers didn’t ascertain whether the contraceptives had been used continuously or had been started, stopped, and restarted. Nor did they look at different formulations and doses. And the observational nature of the study “limits the ability to infer causation,” continued Dr. Oelschlager, chair of the American College of Obstetrics and Gynecology Clinical Consensus Gynecology Committee. She was not involved with the study.
“This study is too flawed to use meaningfully in clinical practice,” Dr. Oelschlager concluded.
The study was primarily funded by the Swedish Research Council, the Swedish Brain Foundation, and the Uppsala University Center for Women ‘s Mental Health during the Reproductive Lifespan. The authors, Dr. Rasgon, and Dr. Oelschlager declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EPIDEMIOLOGY AND PSYCHIATRIC SCIENCES
Med students, doctor groups react to SCOTUS affirmative action ban
The U.S. Supreme Court ruled on June 29 that using race as a factor in college admissions is unconstitutional, rolling back more than 40 years of affirmative action standards and changing how medical schools evaluate applicants to attract students from diverse backgrounds.
Jesse M. Ehrenfeld, MD, MPH, president of the American Medical Association, said in a prepared statement that the Supreme Court ruling will result in a less diverse physician workforce, which is “bad for health care, bad for medicine, and undermines the health of our nation.” He cited the AMA’s recent adoption of a policy advising medical schools to increase enrollment of people from racial and ethnic groups traditionally underrepresented in medicine – even if that means considering race as a factor in admissions criteria.
“Supporting racial and ethnic diversity in the health professions – spanning classrooms, labs, and clinical settings – enriches the educational experiences of all medical and health professions students and the teaching experiences of faculty, and it is essential to improving the overall health of our nation,” the Association of American Medical Colleges (AAMC) said in a prepared statement.
The American Medical Student Association also denounced the Supreme Court decision. “As future physicians committed to justice and equality, we are profoundly outraged ... We strongly support increased representation of minority students in all levels of education, including colleges and medical schools. By fostering diversity and inclusion, institutions have the power to create more empathetic and inclusive learning environments,” the organization said in a press release.
“Diversity in the health care workforce not only benefits underserved patients but improves care for all patients” by increasing understanding and empathy for people of various cultures, Omar T. Atiq, MD, president of the American College of Physicians, said in a press release.
The Supreme Court ruling stems from a lawsuit by the Students for Fair Admissions against Harvard University and the University of North Carolina. The lawsuit alleges that considering race in the college admission process constitutes discrimination and violates the Equal Protection Clause.
Chief Justice John Roberts, who delivered the court’s decision, stated that an applicant’s personal experiences should carry the most weight in admission decisions and that historically, universities have “wrongly concluded that the touchstone of an individual’s identity is not challenges bested, skills built, or lessons learned, but the color of their skin. Our constitutional history does not tolerate that choice.”
Still, Justice Roberts said the opinion does not prohibit universities from considering how race has affected an applicant’s life, “be it through discrimination, inspiration, or otherwise.”
Diversity in medical schools increased last year, with more Black, Hispanic, and female students applying and enrolling. But continued diversity efforts were expected to prove challenging with affirmative action off the table, according to an amicus brief filed last year by the AMA, the AAMC, and dozens of other professional health care organizations.
The brief supported continued use of race in college admissions, stating that eliminating that factor could slow efforts to achieve greater health equity because fewer doctors would be training and working with colleagues from diverse backgrounds.
Several universities with medical programs, such as Yale and Johns Hopkins universities, filed a separate brief citing similar concerns. After the June 29 decision, Harvard and the University of North Carolina released statements stating they would comply with the ruling.
A version of this article first appeared on Medscape.com.
The U.S. Supreme Court ruled on June 29 that using race as a factor in college admissions is unconstitutional, rolling back more than 40 years of affirmative action standards and changing how medical schools evaluate applicants to attract students from diverse backgrounds.
Jesse M. Ehrenfeld, MD, MPH, president of the American Medical Association, said in a prepared statement that the Supreme Court ruling will result in a less diverse physician workforce, which is “bad for health care, bad for medicine, and undermines the health of our nation.” He cited the AMA’s recent adoption of a policy advising medical schools to increase enrollment of people from racial and ethnic groups traditionally underrepresented in medicine – even if that means considering race as a factor in admissions criteria.
“Supporting racial and ethnic diversity in the health professions – spanning classrooms, labs, and clinical settings – enriches the educational experiences of all medical and health professions students and the teaching experiences of faculty, and it is essential to improving the overall health of our nation,” the Association of American Medical Colleges (AAMC) said in a prepared statement.
The American Medical Student Association also denounced the Supreme Court decision. “As future physicians committed to justice and equality, we are profoundly outraged ... We strongly support increased representation of minority students in all levels of education, including colleges and medical schools. By fostering diversity and inclusion, institutions have the power to create more empathetic and inclusive learning environments,” the organization said in a press release.
“Diversity in the health care workforce not only benefits underserved patients but improves care for all patients” by increasing understanding and empathy for people of various cultures, Omar T. Atiq, MD, president of the American College of Physicians, said in a press release.
The Supreme Court ruling stems from a lawsuit by the Students for Fair Admissions against Harvard University and the University of North Carolina. The lawsuit alleges that considering race in the college admission process constitutes discrimination and violates the Equal Protection Clause.
Chief Justice John Roberts, who delivered the court’s decision, stated that an applicant’s personal experiences should carry the most weight in admission decisions and that historically, universities have “wrongly concluded that the touchstone of an individual’s identity is not challenges bested, skills built, or lessons learned, but the color of their skin. Our constitutional history does not tolerate that choice.”
Still, Justice Roberts said the opinion does not prohibit universities from considering how race has affected an applicant’s life, “be it through discrimination, inspiration, or otherwise.”
Diversity in medical schools increased last year, with more Black, Hispanic, and female students applying and enrolling. But continued diversity efforts were expected to prove challenging with affirmative action off the table, according to an amicus brief filed last year by the AMA, the AAMC, and dozens of other professional health care organizations.
The brief supported continued use of race in college admissions, stating that eliminating that factor could slow efforts to achieve greater health equity because fewer doctors would be training and working with colleagues from diverse backgrounds.
Several universities with medical programs, such as Yale and Johns Hopkins universities, filed a separate brief citing similar concerns. After the June 29 decision, Harvard and the University of North Carolina released statements stating they would comply with the ruling.
A version of this article first appeared on Medscape.com.
The U.S. Supreme Court ruled on June 29 that using race as a factor in college admissions is unconstitutional, rolling back more than 40 years of affirmative action standards and changing how medical schools evaluate applicants to attract students from diverse backgrounds.
Jesse M. Ehrenfeld, MD, MPH, president of the American Medical Association, said in a prepared statement that the Supreme Court ruling will result in a less diverse physician workforce, which is “bad for health care, bad for medicine, and undermines the health of our nation.” He cited the AMA’s recent adoption of a policy advising medical schools to increase enrollment of people from racial and ethnic groups traditionally underrepresented in medicine – even if that means considering race as a factor in admissions criteria.
“Supporting racial and ethnic diversity in the health professions – spanning classrooms, labs, and clinical settings – enriches the educational experiences of all medical and health professions students and the teaching experiences of faculty, and it is essential to improving the overall health of our nation,” the Association of American Medical Colleges (AAMC) said in a prepared statement.
The American Medical Student Association also denounced the Supreme Court decision. “As future physicians committed to justice and equality, we are profoundly outraged ... We strongly support increased representation of minority students in all levels of education, including colleges and medical schools. By fostering diversity and inclusion, institutions have the power to create more empathetic and inclusive learning environments,” the organization said in a press release.
“Diversity in the health care workforce not only benefits underserved patients but improves care for all patients” by increasing understanding and empathy for people of various cultures, Omar T. Atiq, MD, president of the American College of Physicians, said in a press release.
The Supreme Court ruling stems from a lawsuit by the Students for Fair Admissions against Harvard University and the University of North Carolina. The lawsuit alleges that considering race in the college admission process constitutes discrimination and violates the Equal Protection Clause.
Chief Justice John Roberts, who delivered the court’s decision, stated that an applicant’s personal experiences should carry the most weight in admission decisions and that historically, universities have “wrongly concluded that the touchstone of an individual’s identity is not challenges bested, skills built, or lessons learned, but the color of their skin. Our constitutional history does not tolerate that choice.”
Still, Justice Roberts said the opinion does not prohibit universities from considering how race has affected an applicant’s life, “be it through discrimination, inspiration, or otherwise.”
Diversity in medical schools increased last year, with more Black, Hispanic, and female students applying and enrolling. But continued diversity efforts were expected to prove challenging with affirmative action off the table, according to an amicus brief filed last year by the AMA, the AAMC, and dozens of other professional health care organizations.
The brief supported continued use of race in college admissions, stating that eliminating that factor could slow efforts to achieve greater health equity because fewer doctors would be training and working with colleagues from diverse backgrounds.
Several universities with medical programs, such as Yale and Johns Hopkins universities, filed a separate brief citing similar concerns. After the June 29 decision, Harvard and the University of North Carolina released statements stating they would comply with the ruling.
A version of this article first appeared on Medscape.com.
Residency match process under scrutiny again, this time by AMA
The American Medical Association is considering whether to study alternatives to the current residency matching program in an effort to improve residents’ compensation and other job-related issues. A recent call-to-action resolution by the AMA’s House of Delegates is the latest in a long string of debates about whether to change the annual process that matches future doctors with compatible residency programs.
AMA’s Resident and Fellow Section introduced the resolution in March, and the delegates approved it earlier in June at AMA’s annual meeting. The resolution states that the match process of the National Resident Matching Program (NRMP) “poses significant anticompetition concerns.” Those include preventing residents from negotiating for higher wages, better benefits, and improved working conditions, according to the approved resolution.
The full AMA board still has to consider the resolution and hasn’t set a date for that review, though it’s expected to be in the next few months, according to Jennifer Sellers, AMA’s public information officer. She said in an interview that the organization declined to comment, wanting to hold off until the board decides how to proceed.
The NRMP, which oversees the matching process, told this news organization that the AMA doesn’t play a role in the Match.
The organization doesn’t believe studying alternative placement methods benefits applicants and residents, and returning to a pre-Match environment, would harm applicants and programs, according to Donna Lamb, DHSc, MBA, BSN, president and CEO.
“The NRMP has no role in determining, publishing, or setting resident salaries nor does the NRMP have a role in the contracting or employment of residents, and it never has.”
Dr. Lamb said changing the Match would “subject applicants to undue pressure and coercion to accept an offer of training. This will exacerbate disparities in candidate selection already evident in medical education and potentially result in salary reductions in more competitive specialties and in more desirable geographic locations.”
The latest push to reform the match process dates back two decades to a 2002 class action antitrust lawsuit by residents and doctors against the NRMP and other organizations involved in the Match.
The residents argued at that time that by restraining competition among teaching hospitals, the matching system allowed hospitals to keep residents’ wages artificially low. The defendants, which included large teaching hospitals, successfully lobbied Congress for an exemption to the antitrust laws, and the case was subsequently dismissed.
The AMA was one of the defendants, so if it moves forward to review the match process, it likely would pit the organization against the NRMP.
Sherman Marek, the attorney who represented the residents, said in an interview that he was not surprised by the latest AMA resolution. “Maybe the AMA leadership has come around to the idea that it’s better for young physicians to not have the match in place,” he says. “I would applaud that sort of evolution.”
Tyler Ramsey, DO, an internal medicine resident and AMA member, said he believes the group’s current president, Jesse Ehrenfeld, MD, MPH, empathizes with doctors in training. “I think he understands [our] views and is more progressive.”
The NRMP also has considered ways to improve the match process to make it easier and more equitable for applicants. In its latest effort, the organization is studying whether programs should certify their rank order list in advance of applicants. This change would give applicants more flexibility to visit residency locations before the programs consider changing their rankings, Dr. Lamb explained. The NRMP also is mulling the possibilities of a two-phase match after deciding in 2022 not to move forward with a previous version of the proposal.
The recent House of Delegates resolution states that “residents are using other means to obtain fair wages, safe working conditions, and other benefits that are unable to be negotiated within the current system.”
Dr. Ramsey, who trains in North Carolina, said the “other means” may include negotiating through a union. “The AMA realizes that there is a problem and that people are unionizing,” he said. “Obviously, as an organization, we’re not doing something correctly, to the point where people are feeling the need to get their rights a different way.”
The Committee of Interns and Residents, which represents 30,000 members, reported a rise in medical trainee unions across the country in 2022.
Not everyone believes that ditching the Match would benefit applicants and residents. Sam Payabvash, MD, assistant professor of radiology at Yale, New Haven, Conn., School of Medicine, tweeted about the resolution as part of a larger Twitter discussion that alternatives are likely to be “more onerous and expensive for applicants.”
An advantage of the match program, Dr. Lamb argued, is that it “improves the reach of applicants into medically underserved communities through widespread program participation.”
Dr. Ramsey agreed that the match program has benefits and drawbacks, but he believes it favors programs over residents. “It comes as no surprise that numerous residents suffer from depression and our suicide rates are the highest amongst all professions due to the lack of control or negotiation of fair salary and working conditions. Overall, the way things are now, residents just do not have a lot of rights.”
A version of this article originally appeared on Medscape.com.
The American Medical Association is considering whether to study alternatives to the current residency matching program in an effort to improve residents’ compensation and other job-related issues. A recent call-to-action resolution by the AMA’s House of Delegates is the latest in a long string of debates about whether to change the annual process that matches future doctors with compatible residency programs.
AMA’s Resident and Fellow Section introduced the resolution in March, and the delegates approved it earlier in June at AMA’s annual meeting. The resolution states that the match process of the National Resident Matching Program (NRMP) “poses significant anticompetition concerns.” Those include preventing residents from negotiating for higher wages, better benefits, and improved working conditions, according to the approved resolution.
The full AMA board still has to consider the resolution and hasn’t set a date for that review, though it’s expected to be in the next few months, according to Jennifer Sellers, AMA’s public information officer. She said in an interview that the organization declined to comment, wanting to hold off until the board decides how to proceed.
The NRMP, which oversees the matching process, told this news organization that the AMA doesn’t play a role in the Match.
The organization doesn’t believe studying alternative placement methods benefits applicants and residents, and returning to a pre-Match environment, would harm applicants and programs, according to Donna Lamb, DHSc, MBA, BSN, president and CEO.
“The NRMP has no role in determining, publishing, or setting resident salaries nor does the NRMP have a role in the contracting or employment of residents, and it never has.”
Dr. Lamb said changing the Match would “subject applicants to undue pressure and coercion to accept an offer of training. This will exacerbate disparities in candidate selection already evident in medical education and potentially result in salary reductions in more competitive specialties and in more desirable geographic locations.”
The latest push to reform the match process dates back two decades to a 2002 class action antitrust lawsuit by residents and doctors against the NRMP and other organizations involved in the Match.
The residents argued at that time that by restraining competition among teaching hospitals, the matching system allowed hospitals to keep residents’ wages artificially low. The defendants, which included large teaching hospitals, successfully lobbied Congress for an exemption to the antitrust laws, and the case was subsequently dismissed.
The AMA was one of the defendants, so if it moves forward to review the match process, it likely would pit the organization against the NRMP.
Sherman Marek, the attorney who represented the residents, said in an interview that he was not surprised by the latest AMA resolution. “Maybe the AMA leadership has come around to the idea that it’s better for young physicians to not have the match in place,” he says. “I would applaud that sort of evolution.”
Tyler Ramsey, DO, an internal medicine resident and AMA member, said he believes the group’s current president, Jesse Ehrenfeld, MD, MPH, empathizes with doctors in training. “I think he understands [our] views and is more progressive.”
The NRMP also has considered ways to improve the match process to make it easier and more equitable for applicants. In its latest effort, the organization is studying whether programs should certify their rank order list in advance of applicants. This change would give applicants more flexibility to visit residency locations before the programs consider changing their rankings, Dr. Lamb explained. The NRMP also is mulling the possibilities of a two-phase match after deciding in 2022 not to move forward with a previous version of the proposal.
The recent House of Delegates resolution states that “residents are using other means to obtain fair wages, safe working conditions, and other benefits that are unable to be negotiated within the current system.”
Dr. Ramsey, who trains in North Carolina, said the “other means” may include negotiating through a union. “The AMA realizes that there is a problem and that people are unionizing,” he said. “Obviously, as an organization, we’re not doing something correctly, to the point where people are feeling the need to get their rights a different way.”
The Committee of Interns and Residents, which represents 30,000 members, reported a rise in medical trainee unions across the country in 2022.
Not everyone believes that ditching the Match would benefit applicants and residents. Sam Payabvash, MD, assistant professor of radiology at Yale, New Haven, Conn., School of Medicine, tweeted about the resolution as part of a larger Twitter discussion that alternatives are likely to be “more onerous and expensive for applicants.”
An advantage of the match program, Dr. Lamb argued, is that it “improves the reach of applicants into medically underserved communities through widespread program participation.”
Dr. Ramsey agreed that the match program has benefits and drawbacks, but he believes it favors programs over residents. “It comes as no surprise that numerous residents suffer from depression and our suicide rates are the highest amongst all professions due to the lack of control or negotiation of fair salary and working conditions. Overall, the way things are now, residents just do not have a lot of rights.”
A version of this article originally appeared on Medscape.com.
The American Medical Association is considering whether to study alternatives to the current residency matching program in an effort to improve residents’ compensation and other job-related issues. A recent call-to-action resolution by the AMA’s House of Delegates is the latest in a long string of debates about whether to change the annual process that matches future doctors with compatible residency programs.
AMA’s Resident and Fellow Section introduced the resolution in March, and the delegates approved it earlier in June at AMA’s annual meeting. The resolution states that the match process of the National Resident Matching Program (NRMP) “poses significant anticompetition concerns.” Those include preventing residents from negotiating for higher wages, better benefits, and improved working conditions, according to the approved resolution.
The full AMA board still has to consider the resolution and hasn’t set a date for that review, though it’s expected to be in the next few months, according to Jennifer Sellers, AMA’s public information officer. She said in an interview that the organization declined to comment, wanting to hold off until the board decides how to proceed.
The NRMP, which oversees the matching process, told this news organization that the AMA doesn’t play a role in the Match.
The organization doesn’t believe studying alternative placement methods benefits applicants and residents, and returning to a pre-Match environment, would harm applicants and programs, according to Donna Lamb, DHSc, MBA, BSN, president and CEO.
“The NRMP has no role in determining, publishing, or setting resident salaries nor does the NRMP have a role in the contracting or employment of residents, and it never has.”
Dr. Lamb said changing the Match would “subject applicants to undue pressure and coercion to accept an offer of training. This will exacerbate disparities in candidate selection already evident in medical education and potentially result in salary reductions in more competitive specialties and in more desirable geographic locations.”
The latest push to reform the match process dates back two decades to a 2002 class action antitrust lawsuit by residents and doctors against the NRMP and other organizations involved in the Match.
The residents argued at that time that by restraining competition among teaching hospitals, the matching system allowed hospitals to keep residents’ wages artificially low. The defendants, which included large teaching hospitals, successfully lobbied Congress for an exemption to the antitrust laws, and the case was subsequently dismissed.
The AMA was one of the defendants, so if it moves forward to review the match process, it likely would pit the organization against the NRMP.
Sherman Marek, the attorney who represented the residents, said in an interview that he was not surprised by the latest AMA resolution. “Maybe the AMA leadership has come around to the idea that it’s better for young physicians to not have the match in place,” he says. “I would applaud that sort of evolution.”
Tyler Ramsey, DO, an internal medicine resident and AMA member, said he believes the group’s current president, Jesse Ehrenfeld, MD, MPH, empathizes with doctors in training. “I think he understands [our] views and is more progressive.”
The NRMP also has considered ways to improve the match process to make it easier and more equitable for applicants. In its latest effort, the organization is studying whether programs should certify their rank order list in advance of applicants. This change would give applicants more flexibility to visit residency locations before the programs consider changing their rankings, Dr. Lamb explained. The NRMP also is mulling the possibilities of a two-phase match after deciding in 2022 not to move forward with a previous version of the proposal.
The recent House of Delegates resolution states that “residents are using other means to obtain fair wages, safe working conditions, and other benefits that are unable to be negotiated within the current system.”
Dr. Ramsey, who trains in North Carolina, said the “other means” may include negotiating through a union. “The AMA realizes that there is a problem and that people are unionizing,” he said. “Obviously, as an organization, we’re not doing something correctly, to the point where people are feeling the need to get their rights a different way.”
The Committee of Interns and Residents, which represents 30,000 members, reported a rise in medical trainee unions across the country in 2022.
Not everyone believes that ditching the Match would benefit applicants and residents. Sam Payabvash, MD, assistant professor of radiology at Yale, New Haven, Conn., School of Medicine, tweeted about the resolution as part of a larger Twitter discussion that alternatives are likely to be “more onerous and expensive for applicants.”
An advantage of the match program, Dr. Lamb argued, is that it “improves the reach of applicants into medically underserved communities through widespread program participation.”
Dr. Ramsey agreed that the match program has benefits and drawbacks, but he believes it favors programs over residents. “It comes as no surprise that numerous residents suffer from depression and our suicide rates are the highest amongst all professions due to the lack of control or negotiation of fair salary and working conditions. Overall, the way things are now, residents just do not have a lot of rights.”
A version of this article originally appeared on Medscape.com.
Risk Evaluation and Mitigation Strategy programs: How they can be improved
A Risk Evaluation and Mitigation Strategy (REMS) is a drug safety program the FDA can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks (Box1). The FDA may require medication guides, patient package inserts, communication plans for health care professionals, and/or certain packaging and safe disposal technologies for medications that pose a serious risk of abuse or overdose. The FDA may also require elements to assure safe use and/or an implementation system be included in the REMS. Pharmaceutical manufacturers then develop a proposed REMS for FDA review.2 If the FDA approves the proposed REMS, the manufacturer is responsible for implementing the REMS requirements.
Box
There are many myths and misconceptions surrounding psychiatry, the branch of medicine that deals with the diagnosis, treatment, and prevention of mental illness. Some of the most common myths include:
The FDA provides this description of a Risk Evaluation and Mitigation Strategy (REMS):
“A [REMS] is a drug safety program that the U.S. Food and Drug Administration (FDA) can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks. REMS are designed to reinforce medication use behaviors and actions that support the safe use of that medication. While all medications have labeling that informs health care stakeholders about medication risks, only a few medications require a REMS. REMS are not designed to mitigate all the adverse events of a medication, these are communicated to health care providers in the medication’s prescribing information. Rather, REMS focus on preventing, monitoring and/or managing a specific serious risk by informing, educating and/or reinforcing actions to reduce the frequency and/or severity of the event.”1
The REMS program for clozapine3 has been the subject of much discussion in the psychiatric community. The adverse impact of the 2015 update to the clozapine REMS program was emphasized at meetings of both the American Psychiatric Association and the College of Psychiatric and Neurologic Pharmacists. A white paper published by the National Association of State Mental Health Program Directors shortly after the 2015 update concluded, “clozapine is underused due to a variety of barriers related to the drug and its properties, the health care system, regulatory requirements, and reimbursement issues.”4 After an update to the clozapine REMS program in 2021, the FDA temporarily suspended enforcement of certain requirements due to concerns from health care professionals about patient access to the medication because of problems with implementing the clozapine REMS program.5,6 In November 2022, the FDA issued a second announcement of enforcement discretion related to additional requirements of the REMS program.5 The FDA had previously announced a decision to not take action regarding adherence to REMS requirements for certain laboratory tests in March 2020, during the COVID-19 pandemic.7
REMS programs for other psychiatric medications may also present challenges. The REMS programs for esketamine8 and olanzapine for extended-release (ER) injectable suspension9 include certain risks that require postadministration monitoring. Some facilities have had to dedicate additional space and clinician time to ensure REMS requirements are met.
To further understand health care professionals’ perspectives regarding the value and burden of these REMS programs, a collaborative effort of the University of Maryland (College Park and Baltimore campuses) Center of Excellence in Regulatory Science and Innovation with the FDA was undertaken. The REMS for clozapine, olanzapine for ER injectable suspension, and esketamine were examined to develop recommendations for improving patient access while ensuring safe medication use and limiting the impact on health care professionals.
Assessing the REMS programs
Focus groups were held with health care professionals nominated by professional organizations to gather their perspectives on the REMS requirements. There was 1 focus group for each of the 3 medications. A facilitator’s guide was developed that contained the details of how to conduct the focus group along with the medication-specific questions. The questions were based on the REMS requirements as of May 2021 and assessed the impact of the REMS on patient safety, patient access, and health care professional workload; effects from the COVID-19 pandemic; and suggestions to improve the REMS programs. The University of Maryland Institutional Review Board reviewed the materials and processes and made the determination of exempt.
Health care professionals were eligible to participate in a focus group if they had ≥1 year of experience working with patients who use the specific medication and ≥6 months of experience within the past year working with the REMS program for that medication. Participants were excluded if they were employed by a pharmaceutical manufacturer or the FDA. The focus groups were conducted virtually using an online conferencing service during summer 2021 and were scheduled for 90 minutes. Prior to the focus group, participants received information from the “Goals” and “Summary” tabs of the FDA REMS website10 for the specific medication along with patient/caregiver guides, which were available for clozapine and olanzapine for ER injectable suspension. For each focus group, there was a target sample size of 6 to 9 participants. However, there were only 4 participants in the olanzapine for ER injectable suspension focus group, which we believed was due to lower national utilization of this medication. Individuals were only able to participate in 1 focus group, so the unique participant count for all 3 focus groups totaled 17 (Table 1).
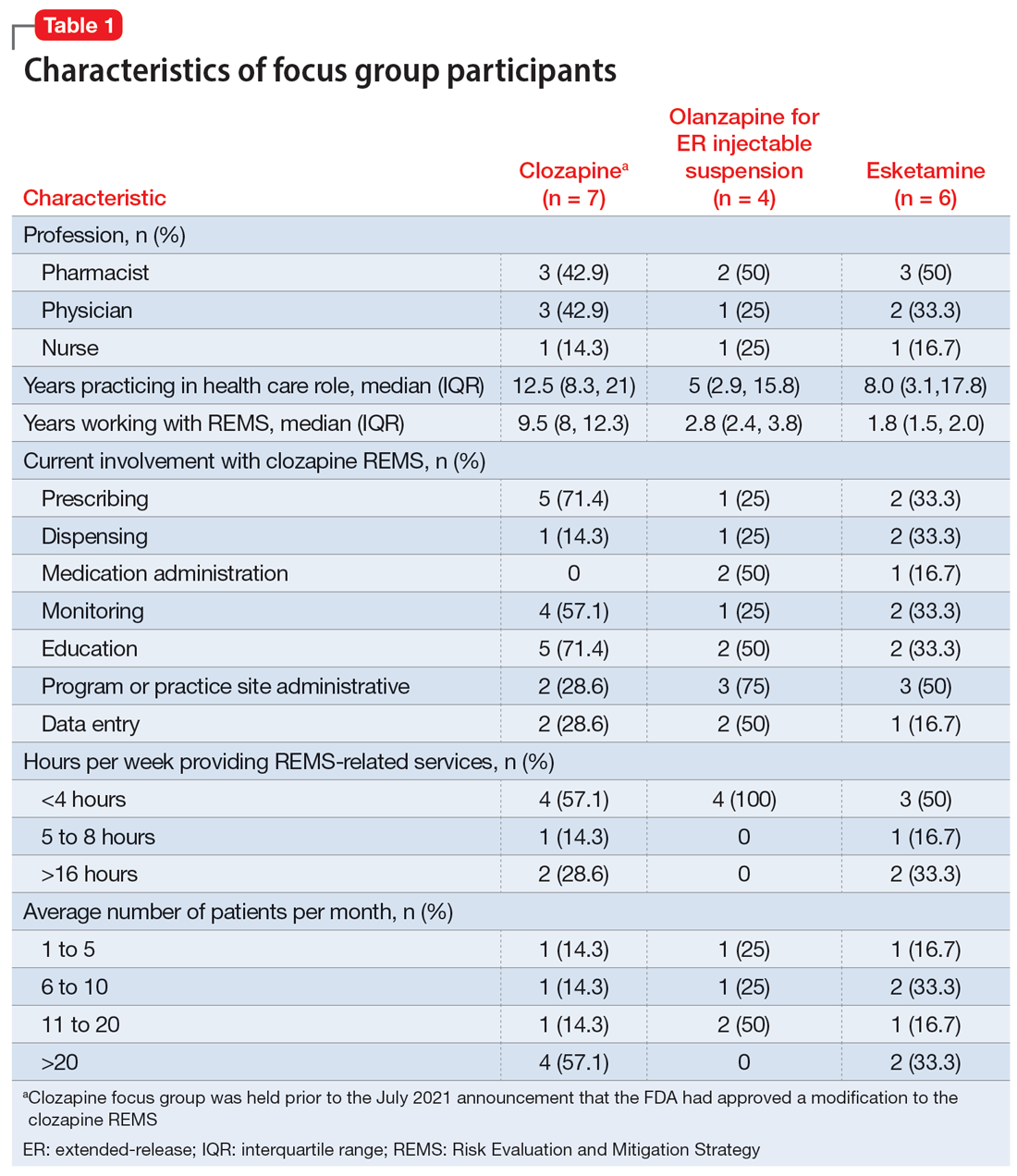
Themes extracted from qualitative analysis of the focus group responses were the value of the REMS programs; registration/enrollment processes and REMS websites; monitoring requirements; care transitions; and COVID considerations (Table 2). While the REMS programs were perceived to increase practitioner and patient awareness of potential harms, discussions centered on the relative cost-to-benefit of the required reporting and other REMS requirements. There were challenges with the registration/enrollment processes and REMS websites that also affected patient care during transitions to different health care settings or clinicians. Patient access was affected by disparities in care related to monitoring requirements and clinician availability.
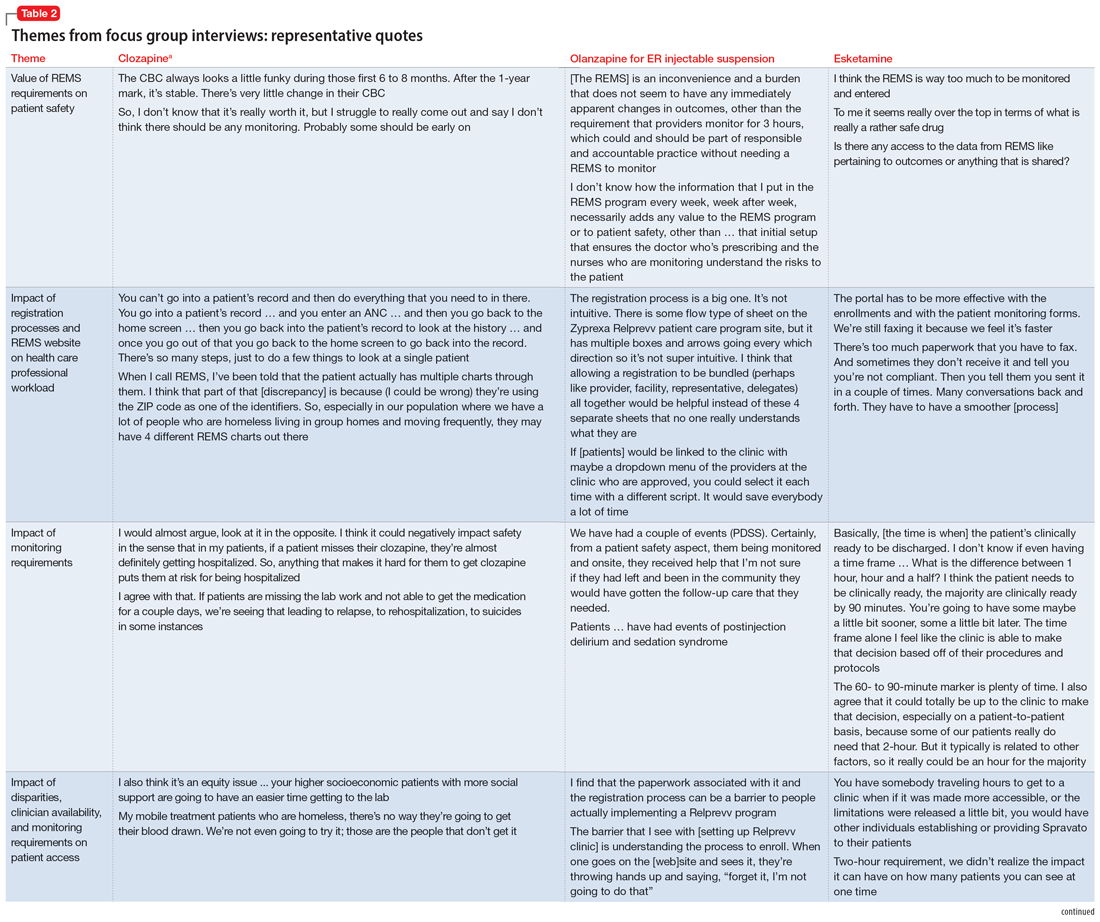

Continue to: COVID impacted all REMS...
COVID impacted all REMS programs. Physical distancing was an issue for medications that required extensive postadministration monitoring (ie, esketamine and olanzapine for ER injectable suspension). Access to laboratory services was an issue for clozapine.
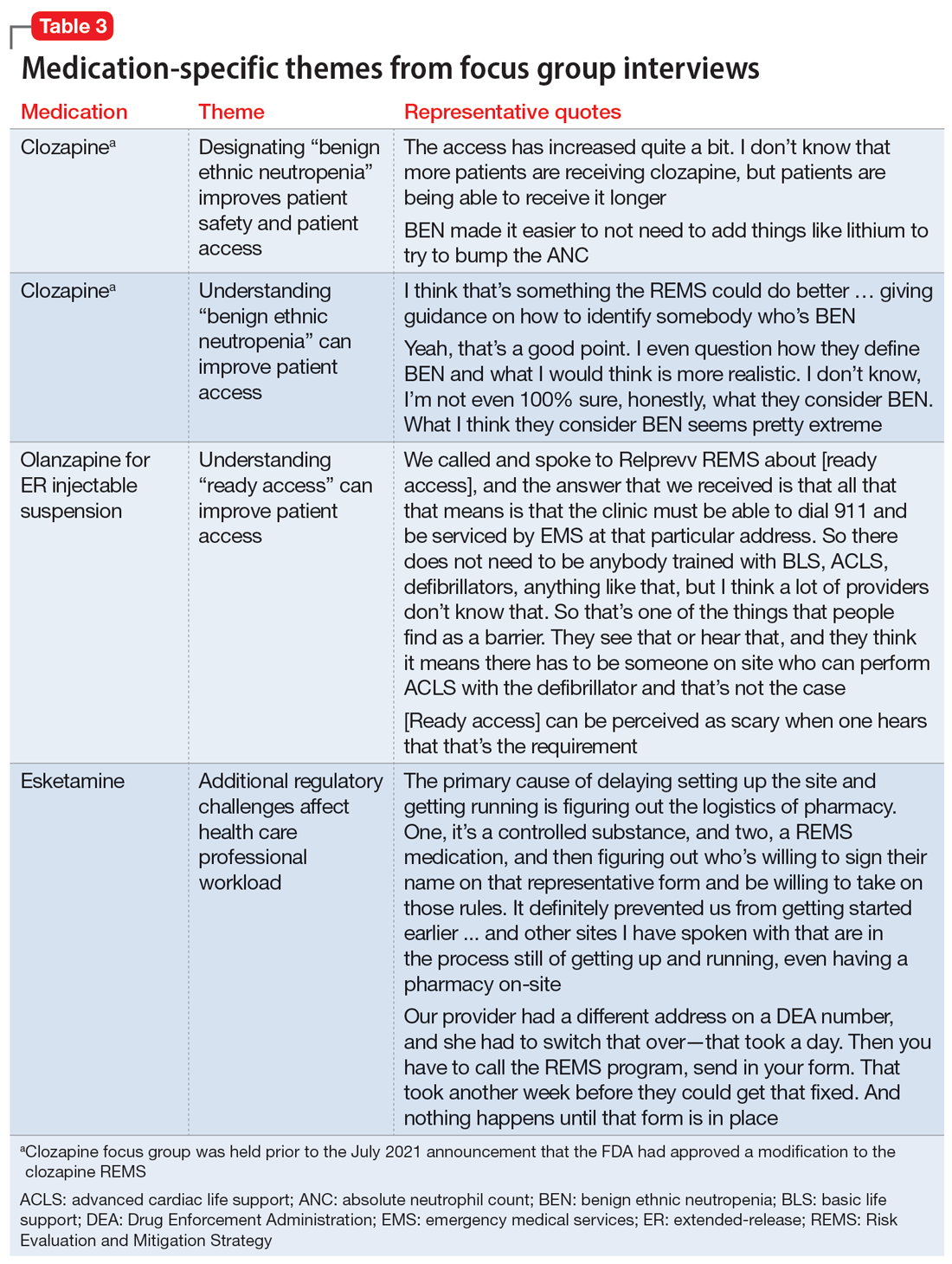
Medication-specific themes are listed in Table 3 and relate to terms and descriptions in the REMS or additional regulatory requirements from the Drug Enforcement Agency (DEA). Suggestions for improvement to the REMS are presented in Table 4.
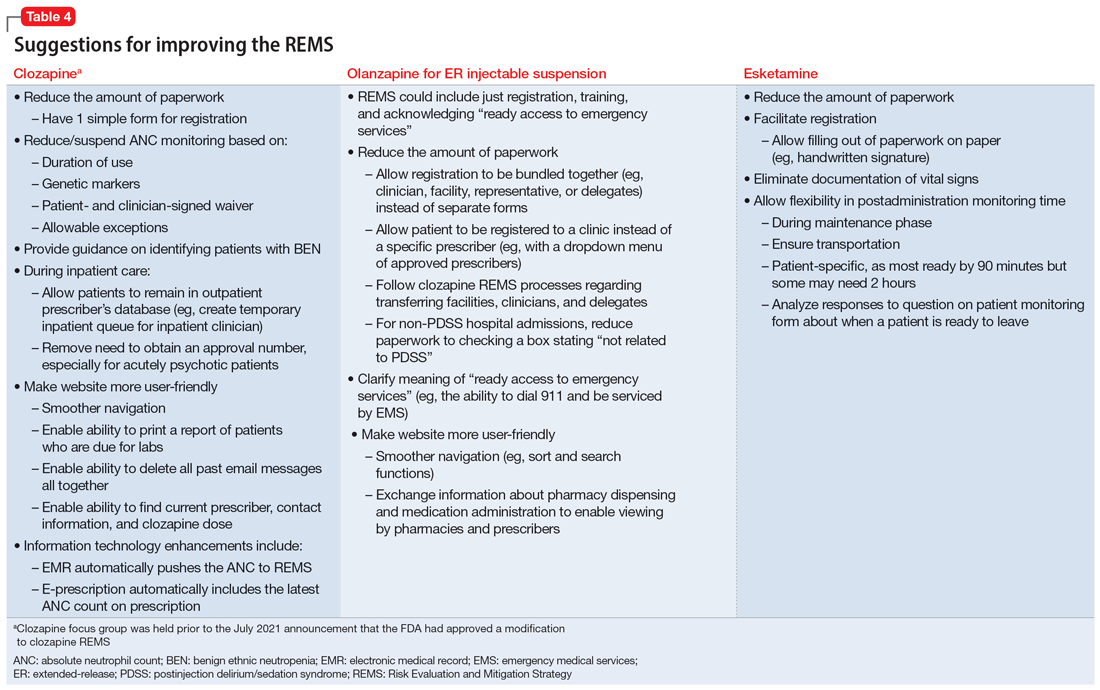
Recommendations for improving REMS
A group consisting of health care professionals, policy experts, and mental health advocates reviewed the information provided by the focus groups and developed the following recommendations.
Overarching recommendations
Each REMS should include a section providing justification for its existence, including a risk analysis of the data regarding the risk the REMS is designed to mitigate. This analysis should be repeated on a regular basis as scientific evidence regarding the risk and its epidemiology evolves. This additional section should also explain how the program requirements of the REMS as implemented (or planned) will achieve the aims of the REMS and weigh the potential benefits of the REMS requirements as implemented (or planned) by the manufacturer vs the potential risks of the REMS requirements as implemented (or planned) by the manufacturer.
Each REMS should have specific quantifiable outcomes. For example, it should specify a reduction in occurrence of the rate of the concerned risk by a specified amount.
Continue to: Ensure adequate...
Ensure adequate stakeholder input during the REMS development and real-world testing in multiple environments before implementing the REMS to identify unanticipated consequences that might impact patient access, patient safety, and health care professional burden. Implementation testing should explore issues such as purchasing and procurement, billing and reimbursement, and relevant factors such as other federal regulations or requirements (eg, the DEA or Medicare).
Ensure harmonization of the REMS forms and processes (eg, initiation and monitoring) for different medications where possible. A prescriber, pharmacist, or system should not face additional barriers to participate in a REMS based on REMS-specific intricacies (ie, prescription systems, data submission systems, or ordering systems). This streamlining will likely decrease clinical inertia to initiate care with the REMS medication, decrease health care professional burden, and improve compliance with REMS requirements.
REMS should anticipate the need for care transitions and employ provisions to ensure seamless care. Considerations should be given to transitions that occur due to:
- Different care settings (eg, inpatient, outpatient, or long-term care)
- Different geographies (eg, patient moves)
- Changes in clinicians, including leaves or absences
- Changes in facilities (eg, pharmacies).
REMS should mirror normal health care professional workflow, including how monitoring data are collected and how and with which frequency pharmacies fill prescriptions.Enhanced information technology to support REMS programs is needed. For example, REMS should be integrated with major electronic patient health record and pharmacy systems to reduce the effort required for clinicians to supply data and automate REMS processes.
For medications that are subject to other agencies and their regulations (eg, the CDC, Centers for Medicare & Medicaid Services, or the DEA), REMS should be required to meet all standards of all agencies with a single system that accommodates normal health care professional workflow.
Continue to: REMS should have a...
REMS should have a standard disclaimer that allows the health care professional to waive certain provisions of the REMS in cases when the specific provisions of the REMS pose a greater risk to the patient than the risk posed by waiving the requirement.
Assure the actions implemented by the industry to meet the requirements for each REMS program are based on peer-reviewed evidence and provide a reasonable expectation to achieve the anticipated benefit.
Ensure that manufacturers make all accumulated REMS data available in a deidentified manner for use by qualified scientific researchers. Additionally, each REMS should have a plan for data access upon initiation and termination of the REMS.
Each REMS should collect data on the performance of the centers and/or personnel who operate the REMS and submit this data for review by qualified outside reviewers. Parameters to assess could include:
- timeliness of response
- timeliness of problem resolution
- data availability and its helpfulness to patient care
- adequacy of resources.
Recommendations for clozapine REMS
These comments relate to the clozapine REMS program prior to the July 2021 announcement that FDA had approved a modification.
Provide a clear definition for “benign ethnic neutropenia.”
Ensure the REMS includes patient-specific adjustments to allow flexibility for monitoring. During COVID, the FDA allowed clinicians to “use their best medical judgment in weighing the benefits and risks of continuing treatment in the absence of laboratory testing.”7 This guidance, which allowed flexibility to absolute neutrophil count (ANC) monitoring, was perceived as positive and safe. Before the changes in the REMS requirements, patients with benign ethnic neutropenia were restricted from accessing their medication or encountered harm from additional pharmacotherapy to mitigate ANC levels.
Continue to: Recommendations for olanzapine for ER injectable suspension REMS
Recommendations for olanzapine for ER injectable suspension REMS
Provide clear explicit instructions on what is required to have “ready access to emergency services.”
Ensure the REMS include patient-specific adjustments to allow flexibility for postadministration monitoring (eg, sedation or blood pressure). Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of olanzapine for ER injectable suspension by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness are included in the REMS. How was the 3-hour cut-point determined? Has it been reevaluated?
Ensure the REMS requirements allow for seamless care during transitions, particularly when clinicians are on vacation.
Continue to: Recommendations for esketamine REMS
Recommendations for esketamine REMS
Ensure the REMS includes patient-specific adjustments to allow flexibility for postadministration monitoring. Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of esketamine by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness of requirements are included in the REMS. How was the 2-hour cut-point determined? Has it been reevaluated?
Ensure that the REMS meet all standards of the DEA, with a single system that accommodates normal health care professional workflow.
A summary of the findings
Overall, the REMS programs for these 3 medications were positively perceived for raising awareness of safe medication use for clinicians and patients. Monitoring patients for safety concerns is important and REMS requirements provide accountability.
Continue to: The use of a single shared...
The use of a single shared REMS system for documenting requirements for clozapine (compared to separate systems for each manufacturer) was a positive move forward in implementation. The focus group welcomed the increased awareness of benign ethnic neutropenia as a result of this condition being incorporated in the revised monitoring requirements of the clozapine REMS.
Focus group participants raised the issue of the real-world efficiency of the REMS programs (reduced access and increased clinician workload) vs the benefits (patient safety). They noted that excessive workload could lead to clinicians becoming unwilling to use a medication that requires a REMS. Clinician workload may be further compromised when REMS logistics disrupt the normal workflow and transitions of care between clinicians or settings. This latter aspect is of particular concern for clozapine.
The complexities of the registration and reporting system for olanzapine for ER injectable suspension and the lack of clarity about monitoring were noted to have discouraged the opening of treatment sites. This scarcity of sites may make clinicians hesitant to use this medication, and instead opt for alternative treatments in patients who may be appropriate candidates.
There has also been limited growth of esketamine treatment sites, especially in comparison to ketamine treatment sites.11-14 Esketamine is FDA-approved for treatment-resistant depression in adults and for depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior. Ketamine is not FDA-approved for treating depression but is being used off-label to treat this disorder.15 The FDA determined that ketamine does not require a REMS to ensure the benefits outweigh the risks for its approved indications as an anesthetic agent, anesthesia-inducing agent, or supplement to anesthesia. Since ketamine has no REMS requirements, there may be a lower burden for its use. Thus, clinicians are treating patients for depression with this medication without needing to comply with a REMS.16
Technology plays a role in workload burden, and integrating health care processes within current workflow systems, such as using electronic patient health records and pharmacy systems, is recommended. The FDA has been exploring technologies to facilitate the completion of REMS requirements, including mandatory education within the prescribers’ and pharmacists’ workflow.17 This is a complex task that requires multiple stakeholders with differing perspectives and incentives to align.
Continue to: The data collected for the REMS...
The data collected for the REMS program belongs to the medication’s manufacturer. Current regulations do not require manufacturers to make this data available to qualified scientific researchers. A regulatory mandate to establish data sharing methods would improve transparency and enhance efforts to better understand the outcomes of the REMS programs.
A few caveats
Both the overarching and medication-specific recommendations were based on a small number of participants’ discussions related to clozapine, olanzapine for ER injectable suspension, and esketamine. These recommendations do not include other medications with REMS that are used to treat psychiatric disorders, such as loxapine, buprenorphine ER, and buprenorphine transmucosal products. Larger-scale qualitative and quantitative research is needed to better understand health care professionals’ perspectives. Lastly, some of the recommendations outlined in this article are beyond the current purview or authority of the FDA and may require legislative or regulatory action to implement.
Bottom Line
Risk Evaluation and Mitigation Strategy (REMS) programs are designed to help reduce the occurrence and/or severity of serious risks or to inform decision-making. However, REMS requirements may adversely impact patient access to certain REMS medications and clinician burden. Health care professionals can provide informed recommendations for improving the REMS programs for clozapine, olanzapine for extended-release injectable suspension, and esketamine.
Related Resources
- FDA. Frequently asked questions (FAQs) about REMS. www.fda.gov/drugs/risk-evaluation-and-mitigation-strategies-rems/frequently-asked-questions-faqs-about-rems
Drug Brand Names
Buprenorphine extended-release • Sublocade
Buprenorphine transmucosal • Subutex, Suboxone
Clozapine • Clozaril
Esketamine • Spravato
Ketamine • Ketalar
Lithium • Eskalith, Lithobid
Loxapine • Adasuve
Olanzapine extended-release injectable suspension • Zyprexa Relprevv
1. U.S. Food and Drug Administration. Risk Evaluation and Mitigation Strategies. Accessed January 18, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems
2. U.S. Department of Health and Human Services, Food and Drug Administration. Format and Content of a REMS Document. Guidance for Industry. Accessed January 18, 2023. https://www.fda.gov/media/77846/download
3. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Clozapine. Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=RemsDetails.page&REMS=351
4. The National Association of State Mental Health Program Directors. Clozapine underutilization: addressing the barriers. Accessed September 30, 2019. https://nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
5. U.S. Food and Drug Administration. FDA is temporarily exercising enforcement discretion with respect to certain clozapine REMS program requirements to ensure continuity of care for patients taking clozapine. Updated November 22, 2022. Accessed June 1, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-temporarily-exercising-enforcement-discretion-respect-certain-clozapine-rems-program
6. Tanzi M. REMS issues affect clozapine, isotretinoin. Pharmacy Today. 2022;28(3):49.
7. U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides update on patient access to certain REMS drugs during COVID-19 public health emergency. Accessed June 1, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-update-patient-access-certain-rems-drugs-during-covid-19
8. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Spravato (esketamine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386
9. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Zyprexa Relprevv (olanzapine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74
10. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm
11. Parikh SV, Lopez D, Vande Voort JL, et al. Developing an IV ketamine clinic for treatment-resistant depression: a primer. Psychopharmacol Bull. 2021;51(3):109-124.
12. Dodge D. The ketamine cure. The New York Times. November 4, 2021. Updated November 5, 2021. Accessed June 1, 2023. https://www.nytimes.com/2021/11/04/well/ketamine-therapy-depression.html
13. Burton KW. Time for a national ketamine registry, experts say. Medscape. February 15, 2023. Accessed June 1, 2023. https://www.medscape.com/viewarticle/988310
14. Wilkinson ST, Howard DH, Busch SH. Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA. 2019;322(11):1039-1040. doi:10.1001/jama.2019.10728
15. Wilkinson ST, Toprak M, Turner MS, et al. A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am J Psychiatry. 2017;174(7):695-696. doi:10.1176/appi.ajp.2017.17020239
16. Pai SM, Gries JM; ACCP Public Policy Committee. Off-label use of ketamine: a challenging drug treatment delivery model with an inherently unfavorable risk-benefit profile. J Clin Pharmacol. 2022;62(1):10-13. doi:10.1002/jcph.1983
17. Risk Evaluation and Mitigation Strategies (REMS) Integration. Accessed June 1, 2023. https://confluence.hl7.org/display/COD/Risk+Evaluation+and+Mitigation+Strategies+%28REMS%29+Integration
A Risk Evaluation and Mitigation Strategy (REMS) is a drug safety program the FDA can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks (Box1). The FDA may require medication guides, patient package inserts, communication plans for health care professionals, and/or certain packaging and safe disposal technologies for medications that pose a serious risk of abuse or overdose. The FDA may also require elements to assure safe use and/or an implementation system be included in the REMS. Pharmaceutical manufacturers then develop a proposed REMS for FDA review.2 If the FDA approves the proposed REMS, the manufacturer is responsible for implementing the REMS requirements.
Box
There are many myths and misconceptions surrounding psychiatry, the branch of medicine that deals with the diagnosis, treatment, and prevention of mental illness. Some of the most common myths include:
The FDA provides this description of a Risk Evaluation and Mitigation Strategy (REMS):
“A [REMS] is a drug safety program that the U.S. Food and Drug Administration (FDA) can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks. REMS are designed to reinforce medication use behaviors and actions that support the safe use of that medication. While all medications have labeling that informs health care stakeholders about medication risks, only a few medications require a REMS. REMS are not designed to mitigate all the adverse events of a medication, these are communicated to health care providers in the medication’s prescribing information. Rather, REMS focus on preventing, monitoring and/or managing a specific serious risk by informing, educating and/or reinforcing actions to reduce the frequency and/or severity of the event.”1
The REMS program for clozapine3 has been the subject of much discussion in the psychiatric community. The adverse impact of the 2015 update to the clozapine REMS program was emphasized at meetings of both the American Psychiatric Association and the College of Psychiatric and Neurologic Pharmacists. A white paper published by the National Association of State Mental Health Program Directors shortly after the 2015 update concluded, “clozapine is underused due to a variety of barriers related to the drug and its properties, the health care system, regulatory requirements, and reimbursement issues.”4 After an update to the clozapine REMS program in 2021, the FDA temporarily suspended enforcement of certain requirements due to concerns from health care professionals about patient access to the medication because of problems with implementing the clozapine REMS program.5,6 In November 2022, the FDA issued a second announcement of enforcement discretion related to additional requirements of the REMS program.5 The FDA had previously announced a decision to not take action regarding adherence to REMS requirements for certain laboratory tests in March 2020, during the COVID-19 pandemic.7
REMS programs for other psychiatric medications may also present challenges. The REMS programs for esketamine8 and olanzapine for extended-release (ER) injectable suspension9 include certain risks that require postadministration monitoring. Some facilities have had to dedicate additional space and clinician time to ensure REMS requirements are met.
To further understand health care professionals’ perspectives regarding the value and burden of these REMS programs, a collaborative effort of the University of Maryland (College Park and Baltimore campuses) Center of Excellence in Regulatory Science and Innovation with the FDA was undertaken. The REMS for clozapine, olanzapine for ER injectable suspension, and esketamine were examined to develop recommendations for improving patient access while ensuring safe medication use and limiting the impact on health care professionals.
Assessing the REMS programs
Focus groups were held with health care professionals nominated by professional organizations to gather their perspectives on the REMS requirements. There was 1 focus group for each of the 3 medications. A facilitator’s guide was developed that contained the details of how to conduct the focus group along with the medication-specific questions. The questions were based on the REMS requirements as of May 2021 and assessed the impact of the REMS on patient safety, patient access, and health care professional workload; effects from the COVID-19 pandemic; and suggestions to improve the REMS programs. The University of Maryland Institutional Review Board reviewed the materials and processes and made the determination of exempt.
Health care professionals were eligible to participate in a focus group if they had ≥1 year of experience working with patients who use the specific medication and ≥6 months of experience within the past year working with the REMS program for that medication. Participants were excluded if they were employed by a pharmaceutical manufacturer or the FDA. The focus groups were conducted virtually using an online conferencing service during summer 2021 and were scheduled for 90 minutes. Prior to the focus group, participants received information from the “Goals” and “Summary” tabs of the FDA REMS website10 for the specific medication along with patient/caregiver guides, which were available for clozapine and olanzapine for ER injectable suspension. For each focus group, there was a target sample size of 6 to 9 participants. However, there were only 4 participants in the olanzapine for ER injectable suspension focus group, which we believed was due to lower national utilization of this medication. Individuals were only able to participate in 1 focus group, so the unique participant count for all 3 focus groups totaled 17 (Table 1).

Themes extracted from qualitative analysis of the focus group responses were the value of the REMS programs; registration/enrollment processes and REMS websites; monitoring requirements; care transitions; and COVID considerations (Table 2). While the REMS programs were perceived to increase practitioner and patient awareness of potential harms, discussions centered on the relative cost-to-benefit of the required reporting and other REMS requirements. There were challenges with the registration/enrollment processes and REMS websites that also affected patient care during transitions to different health care settings or clinicians. Patient access was affected by disparities in care related to monitoring requirements and clinician availability.


Continue to: COVID impacted all REMS...
COVID impacted all REMS programs. Physical distancing was an issue for medications that required extensive postadministration monitoring (ie, esketamine and olanzapine for ER injectable suspension). Access to laboratory services was an issue for clozapine.

Medication-specific themes are listed in Table 3 and relate to terms and descriptions in the REMS or additional regulatory requirements from the Drug Enforcement Agency (DEA). Suggestions for improvement to the REMS are presented in Table 4.

Recommendations for improving REMS
A group consisting of health care professionals, policy experts, and mental health advocates reviewed the information provided by the focus groups and developed the following recommendations.
Overarching recommendations
Each REMS should include a section providing justification for its existence, including a risk analysis of the data regarding the risk the REMS is designed to mitigate. This analysis should be repeated on a regular basis as scientific evidence regarding the risk and its epidemiology evolves. This additional section should also explain how the program requirements of the REMS as implemented (or planned) will achieve the aims of the REMS and weigh the potential benefits of the REMS requirements as implemented (or planned) by the manufacturer vs the potential risks of the REMS requirements as implemented (or planned) by the manufacturer.
Each REMS should have specific quantifiable outcomes. For example, it should specify a reduction in occurrence of the rate of the concerned risk by a specified amount.
Continue to: Ensure adequate...
Ensure adequate stakeholder input during the REMS development and real-world testing in multiple environments before implementing the REMS to identify unanticipated consequences that might impact patient access, patient safety, and health care professional burden. Implementation testing should explore issues such as purchasing and procurement, billing and reimbursement, and relevant factors such as other federal regulations or requirements (eg, the DEA or Medicare).
Ensure harmonization of the REMS forms and processes (eg, initiation and monitoring) for different medications where possible. A prescriber, pharmacist, or system should not face additional barriers to participate in a REMS based on REMS-specific intricacies (ie, prescription systems, data submission systems, or ordering systems). This streamlining will likely decrease clinical inertia to initiate care with the REMS medication, decrease health care professional burden, and improve compliance with REMS requirements.
REMS should anticipate the need for care transitions and employ provisions to ensure seamless care. Considerations should be given to transitions that occur due to:
- Different care settings (eg, inpatient, outpatient, or long-term care)
- Different geographies (eg, patient moves)
- Changes in clinicians, including leaves or absences
- Changes in facilities (eg, pharmacies).
REMS should mirror normal health care professional workflow, including how monitoring data are collected and how and with which frequency pharmacies fill prescriptions.Enhanced information technology to support REMS programs is needed. For example, REMS should be integrated with major electronic patient health record and pharmacy systems to reduce the effort required for clinicians to supply data and automate REMS processes.
For medications that are subject to other agencies and their regulations (eg, the CDC, Centers for Medicare & Medicaid Services, or the DEA), REMS should be required to meet all standards of all agencies with a single system that accommodates normal health care professional workflow.
Continue to: REMS should have a...
REMS should have a standard disclaimer that allows the health care professional to waive certain provisions of the REMS in cases when the specific provisions of the REMS pose a greater risk to the patient than the risk posed by waiving the requirement.
Assure the actions implemented by the industry to meet the requirements for each REMS program are based on peer-reviewed evidence and provide a reasonable expectation to achieve the anticipated benefit.
Ensure that manufacturers make all accumulated REMS data available in a deidentified manner for use by qualified scientific researchers. Additionally, each REMS should have a plan for data access upon initiation and termination of the REMS.
Each REMS should collect data on the performance of the centers and/or personnel who operate the REMS and submit this data for review by qualified outside reviewers. Parameters to assess could include:
- timeliness of response
- timeliness of problem resolution
- data availability and its helpfulness to patient care
- adequacy of resources.
Recommendations for clozapine REMS
These comments relate to the clozapine REMS program prior to the July 2021 announcement that FDA had approved a modification.
Provide a clear definition for “benign ethnic neutropenia.”
Ensure the REMS includes patient-specific adjustments to allow flexibility for monitoring. During COVID, the FDA allowed clinicians to “use their best medical judgment in weighing the benefits and risks of continuing treatment in the absence of laboratory testing.”7 This guidance, which allowed flexibility to absolute neutrophil count (ANC) monitoring, was perceived as positive and safe. Before the changes in the REMS requirements, patients with benign ethnic neutropenia were restricted from accessing their medication or encountered harm from additional pharmacotherapy to mitigate ANC levels.
Continue to: Recommendations for olanzapine for ER injectable suspension REMS
Recommendations for olanzapine for ER injectable suspension REMS
Provide clear explicit instructions on what is required to have “ready access to emergency services.”
Ensure the REMS include patient-specific adjustments to allow flexibility for postadministration monitoring (eg, sedation or blood pressure). Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of olanzapine for ER injectable suspension by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness are included in the REMS. How was the 3-hour cut-point determined? Has it been reevaluated?
Ensure the REMS requirements allow for seamless care during transitions, particularly when clinicians are on vacation.
Continue to: Recommendations for esketamine REMS
Recommendations for esketamine REMS
Ensure the REMS includes patient-specific adjustments to allow flexibility for postadministration monitoring. Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of esketamine by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness of requirements are included in the REMS. How was the 2-hour cut-point determined? Has it been reevaluated?
Ensure that the REMS meet all standards of the DEA, with a single system that accommodates normal health care professional workflow.
A summary of the findings
Overall, the REMS programs for these 3 medications were positively perceived for raising awareness of safe medication use for clinicians and patients. Monitoring patients for safety concerns is important and REMS requirements provide accountability.
Continue to: The use of a single shared...
The use of a single shared REMS system for documenting requirements for clozapine (compared to separate systems for each manufacturer) was a positive move forward in implementation. The focus group welcomed the increased awareness of benign ethnic neutropenia as a result of this condition being incorporated in the revised monitoring requirements of the clozapine REMS.
Focus group participants raised the issue of the real-world efficiency of the REMS programs (reduced access and increased clinician workload) vs the benefits (patient safety). They noted that excessive workload could lead to clinicians becoming unwilling to use a medication that requires a REMS. Clinician workload may be further compromised when REMS logistics disrupt the normal workflow and transitions of care between clinicians or settings. This latter aspect is of particular concern for clozapine.
The complexities of the registration and reporting system for olanzapine for ER injectable suspension and the lack of clarity about monitoring were noted to have discouraged the opening of treatment sites. This scarcity of sites may make clinicians hesitant to use this medication, and instead opt for alternative treatments in patients who may be appropriate candidates.
There has also been limited growth of esketamine treatment sites, especially in comparison to ketamine treatment sites.11-14 Esketamine is FDA-approved for treatment-resistant depression in adults and for depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior. Ketamine is not FDA-approved for treating depression but is being used off-label to treat this disorder.15 The FDA determined that ketamine does not require a REMS to ensure the benefits outweigh the risks for its approved indications as an anesthetic agent, anesthesia-inducing agent, or supplement to anesthesia. Since ketamine has no REMS requirements, there may be a lower burden for its use. Thus, clinicians are treating patients for depression with this medication without needing to comply with a REMS.16
Technology plays a role in workload burden, and integrating health care processes within current workflow systems, such as using electronic patient health records and pharmacy systems, is recommended. The FDA has been exploring technologies to facilitate the completion of REMS requirements, including mandatory education within the prescribers’ and pharmacists’ workflow.17 This is a complex task that requires multiple stakeholders with differing perspectives and incentives to align.
Continue to: The data collected for the REMS...
The data collected for the REMS program belongs to the medication’s manufacturer. Current regulations do not require manufacturers to make this data available to qualified scientific researchers. A regulatory mandate to establish data sharing methods would improve transparency and enhance efforts to better understand the outcomes of the REMS programs.
A few caveats
Both the overarching and medication-specific recommendations were based on a small number of participants’ discussions related to clozapine, olanzapine for ER injectable suspension, and esketamine. These recommendations do not include other medications with REMS that are used to treat psychiatric disorders, such as loxapine, buprenorphine ER, and buprenorphine transmucosal products. Larger-scale qualitative and quantitative research is needed to better understand health care professionals’ perspectives. Lastly, some of the recommendations outlined in this article are beyond the current purview or authority of the FDA and may require legislative or regulatory action to implement.
Bottom Line
Risk Evaluation and Mitigation Strategy (REMS) programs are designed to help reduce the occurrence and/or severity of serious risks or to inform decision-making. However, REMS requirements may adversely impact patient access to certain REMS medications and clinician burden. Health care professionals can provide informed recommendations for improving the REMS programs for clozapine, olanzapine for extended-release injectable suspension, and esketamine.
Related Resources
- FDA. Frequently asked questions (FAQs) about REMS. www.fda.gov/drugs/risk-evaluation-and-mitigation-strategies-rems/frequently-asked-questions-faqs-about-rems
Drug Brand Names
Buprenorphine extended-release • Sublocade
Buprenorphine transmucosal • Subutex, Suboxone
Clozapine • Clozaril
Esketamine • Spravato
Ketamine • Ketalar
Lithium • Eskalith, Lithobid
Loxapine • Adasuve
Olanzapine extended-release injectable suspension • Zyprexa Relprevv
A Risk Evaluation and Mitigation Strategy (REMS) is a drug safety program the FDA can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks (Box1). The FDA may require medication guides, patient package inserts, communication plans for health care professionals, and/or certain packaging and safe disposal technologies for medications that pose a serious risk of abuse or overdose. The FDA may also require elements to assure safe use and/or an implementation system be included in the REMS. Pharmaceutical manufacturers then develop a proposed REMS for FDA review.2 If the FDA approves the proposed REMS, the manufacturer is responsible for implementing the REMS requirements.
Box
There are many myths and misconceptions surrounding psychiatry, the branch of medicine that deals with the diagnosis, treatment, and prevention of mental illness. Some of the most common myths include:
The FDA provides this description of a Risk Evaluation and Mitigation Strategy (REMS):
“A [REMS] is a drug safety program that the U.S. Food and Drug Administration (FDA) can require for certain medications with serious safety concerns to help ensure the benefits of the medication outweigh its risks. REMS are designed to reinforce medication use behaviors and actions that support the safe use of that medication. While all medications have labeling that informs health care stakeholders about medication risks, only a few medications require a REMS. REMS are not designed to mitigate all the adverse events of a medication, these are communicated to health care providers in the medication’s prescribing information. Rather, REMS focus on preventing, monitoring and/or managing a specific serious risk by informing, educating and/or reinforcing actions to reduce the frequency and/or severity of the event.”1
The REMS program for clozapine3 has been the subject of much discussion in the psychiatric community. The adverse impact of the 2015 update to the clozapine REMS program was emphasized at meetings of both the American Psychiatric Association and the College of Psychiatric and Neurologic Pharmacists. A white paper published by the National Association of State Mental Health Program Directors shortly after the 2015 update concluded, “clozapine is underused due to a variety of barriers related to the drug and its properties, the health care system, regulatory requirements, and reimbursement issues.”4 After an update to the clozapine REMS program in 2021, the FDA temporarily suspended enforcement of certain requirements due to concerns from health care professionals about patient access to the medication because of problems with implementing the clozapine REMS program.5,6 In November 2022, the FDA issued a second announcement of enforcement discretion related to additional requirements of the REMS program.5 The FDA had previously announced a decision to not take action regarding adherence to REMS requirements for certain laboratory tests in March 2020, during the COVID-19 pandemic.7
REMS programs for other psychiatric medications may also present challenges. The REMS programs for esketamine8 and olanzapine for extended-release (ER) injectable suspension9 include certain risks that require postadministration monitoring. Some facilities have had to dedicate additional space and clinician time to ensure REMS requirements are met.
To further understand health care professionals’ perspectives regarding the value and burden of these REMS programs, a collaborative effort of the University of Maryland (College Park and Baltimore campuses) Center of Excellence in Regulatory Science and Innovation with the FDA was undertaken. The REMS for clozapine, olanzapine for ER injectable suspension, and esketamine were examined to develop recommendations for improving patient access while ensuring safe medication use and limiting the impact on health care professionals.
Assessing the REMS programs
Focus groups were held with health care professionals nominated by professional organizations to gather their perspectives on the REMS requirements. There was 1 focus group for each of the 3 medications. A facilitator’s guide was developed that contained the details of how to conduct the focus group along with the medication-specific questions. The questions were based on the REMS requirements as of May 2021 and assessed the impact of the REMS on patient safety, patient access, and health care professional workload; effects from the COVID-19 pandemic; and suggestions to improve the REMS programs. The University of Maryland Institutional Review Board reviewed the materials and processes and made the determination of exempt.
Health care professionals were eligible to participate in a focus group if they had ≥1 year of experience working with patients who use the specific medication and ≥6 months of experience within the past year working with the REMS program for that medication. Participants were excluded if they were employed by a pharmaceutical manufacturer or the FDA. The focus groups were conducted virtually using an online conferencing service during summer 2021 and were scheduled for 90 minutes. Prior to the focus group, participants received information from the “Goals” and “Summary” tabs of the FDA REMS website10 for the specific medication along with patient/caregiver guides, which were available for clozapine and olanzapine for ER injectable suspension. For each focus group, there was a target sample size of 6 to 9 participants. However, there were only 4 participants in the olanzapine for ER injectable suspension focus group, which we believed was due to lower national utilization of this medication. Individuals were only able to participate in 1 focus group, so the unique participant count for all 3 focus groups totaled 17 (Table 1).

Themes extracted from qualitative analysis of the focus group responses were the value of the REMS programs; registration/enrollment processes and REMS websites; monitoring requirements; care transitions; and COVID considerations (Table 2). While the REMS programs were perceived to increase practitioner and patient awareness of potential harms, discussions centered on the relative cost-to-benefit of the required reporting and other REMS requirements. There were challenges with the registration/enrollment processes and REMS websites that also affected patient care during transitions to different health care settings or clinicians. Patient access was affected by disparities in care related to monitoring requirements and clinician availability.


Continue to: COVID impacted all REMS...
COVID impacted all REMS programs. Physical distancing was an issue for medications that required extensive postadministration monitoring (ie, esketamine and olanzapine for ER injectable suspension). Access to laboratory services was an issue for clozapine.

Medication-specific themes are listed in Table 3 and relate to terms and descriptions in the REMS or additional regulatory requirements from the Drug Enforcement Agency (DEA). Suggestions for improvement to the REMS are presented in Table 4.

Recommendations for improving REMS
A group consisting of health care professionals, policy experts, and mental health advocates reviewed the information provided by the focus groups and developed the following recommendations.
Overarching recommendations
Each REMS should include a section providing justification for its existence, including a risk analysis of the data regarding the risk the REMS is designed to mitigate. This analysis should be repeated on a regular basis as scientific evidence regarding the risk and its epidemiology evolves. This additional section should also explain how the program requirements of the REMS as implemented (or planned) will achieve the aims of the REMS and weigh the potential benefits of the REMS requirements as implemented (or planned) by the manufacturer vs the potential risks of the REMS requirements as implemented (or planned) by the manufacturer.
Each REMS should have specific quantifiable outcomes. For example, it should specify a reduction in occurrence of the rate of the concerned risk by a specified amount.
Continue to: Ensure adequate...
Ensure adequate stakeholder input during the REMS development and real-world testing in multiple environments before implementing the REMS to identify unanticipated consequences that might impact patient access, patient safety, and health care professional burden. Implementation testing should explore issues such as purchasing and procurement, billing and reimbursement, and relevant factors such as other federal regulations or requirements (eg, the DEA or Medicare).
Ensure harmonization of the REMS forms and processes (eg, initiation and monitoring) for different medications where possible. A prescriber, pharmacist, or system should not face additional barriers to participate in a REMS based on REMS-specific intricacies (ie, prescription systems, data submission systems, or ordering systems). This streamlining will likely decrease clinical inertia to initiate care with the REMS medication, decrease health care professional burden, and improve compliance with REMS requirements.
REMS should anticipate the need for care transitions and employ provisions to ensure seamless care. Considerations should be given to transitions that occur due to:
- Different care settings (eg, inpatient, outpatient, or long-term care)
- Different geographies (eg, patient moves)
- Changes in clinicians, including leaves or absences
- Changes in facilities (eg, pharmacies).
REMS should mirror normal health care professional workflow, including how monitoring data are collected and how and with which frequency pharmacies fill prescriptions.Enhanced information technology to support REMS programs is needed. For example, REMS should be integrated with major electronic patient health record and pharmacy systems to reduce the effort required for clinicians to supply data and automate REMS processes.
For medications that are subject to other agencies and their regulations (eg, the CDC, Centers for Medicare & Medicaid Services, or the DEA), REMS should be required to meet all standards of all agencies with a single system that accommodates normal health care professional workflow.
Continue to: REMS should have a...
REMS should have a standard disclaimer that allows the health care professional to waive certain provisions of the REMS in cases when the specific provisions of the REMS pose a greater risk to the patient than the risk posed by waiving the requirement.
Assure the actions implemented by the industry to meet the requirements for each REMS program are based on peer-reviewed evidence and provide a reasonable expectation to achieve the anticipated benefit.
Ensure that manufacturers make all accumulated REMS data available in a deidentified manner for use by qualified scientific researchers. Additionally, each REMS should have a plan for data access upon initiation and termination of the REMS.
Each REMS should collect data on the performance of the centers and/or personnel who operate the REMS and submit this data for review by qualified outside reviewers. Parameters to assess could include:
- timeliness of response
- timeliness of problem resolution
- data availability and its helpfulness to patient care
- adequacy of resources.
Recommendations for clozapine REMS
These comments relate to the clozapine REMS program prior to the July 2021 announcement that FDA had approved a modification.
Provide a clear definition for “benign ethnic neutropenia.”
Ensure the REMS includes patient-specific adjustments to allow flexibility for monitoring. During COVID, the FDA allowed clinicians to “use their best medical judgment in weighing the benefits and risks of continuing treatment in the absence of laboratory testing.”7 This guidance, which allowed flexibility to absolute neutrophil count (ANC) monitoring, was perceived as positive and safe. Before the changes in the REMS requirements, patients with benign ethnic neutropenia were restricted from accessing their medication or encountered harm from additional pharmacotherapy to mitigate ANC levels.
Continue to: Recommendations for olanzapine for ER injectable suspension REMS
Recommendations for olanzapine for ER injectable suspension REMS
Provide clear explicit instructions on what is required to have “ready access to emergency services.”
Ensure the REMS include patient-specific adjustments to allow flexibility for postadministration monitoring (eg, sedation or blood pressure). Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of olanzapine for ER injectable suspension by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness are included in the REMS. How was the 3-hour cut-point determined? Has it been reevaluated?
Ensure the REMS requirements allow for seamless care during transitions, particularly when clinicians are on vacation.
Continue to: Recommendations for esketamine REMS
Recommendations for esketamine REMS
Ensure the REMS includes patient-specific adjustments to allow flexibility for postadministration monitoring. Specific patient groups may have differential access to certain types of facilities, transportation, or other resources. For example, consider the administration of esketamine by a mobile treatment team with an adequate protocol (eg, via videoconferencing or phone calls).
Ensure actions with peer-reviewed evidence demonstrating efficacy/effectiveness of requirements are included in the REMS. How was the 2-hour cut-point determined? Has it been reevaluated?
Ensure that the REMS meet all standards of the DEA, with a single system that accommodates normal health care professional workflow.
A summary of the findings
Overall, the REMS programs for these 3 medications were positively perceived for raising awareness of safe medication use for clinicians and patients. Monitoring patients for safety concerns is important and REMS requirements provide accountability.
Continue to: The use of a single shared...
The use of a single shared REMS system for documenting requirements for clozapine (compared to separate systems for each manufacturer) was a positive move forward in implementation. The focus group welcomed the increased awareness of benign ethnic neutropenia as a result of this condition being incorporated in the revised monitoring requirements of the clozapine REMS.
Focus group participants raised the issue of the real-world efficiency of the REMS programs (reduced access and increased clinician workload) vs the benefits (patient safety). They noted that excessive workload could lead to clinicians becoming unwilling to use a medication that requires a REMS. Clinician workload may be further compromised when REMS logistics disrupt the normal workflow and transitions of care between clinicians or settings. This latter aspect is of particular concern for clozapine.
The complexities of the registration and reporting system for olanzapine for ER injectable suspension and the lack of clarity about monitoring were noted to have discouraged the opening of treatment sites. This scarcity of sites may make clinicians hesitant to use this medication, and instead opt for alternative treatments in patients who may be appropriate candidates.
There has also been limited growth of esketamine treatment sites, especially in comparison to ketamine treatment sites.11-14 Esketamine is FDA-approved for treatment-resistant depression in adults and for depressive symptoms in adults with major depressive disorder with acute suicidal ideation or behavior. Ketamine is not FDA-approved for treating depression but is being used off-label to treat this disorder.15 The FDA determined that ketamine does not require a REMS to ensure the benefits outweigh the risks for its approved indications as an anesthetic agent, anesthesia-inducing agent, or supplement to anesthesia. Since ketamine has no REMS requirements, there may be a lower burden for its use. Thus, clinicians are treating patients for depression with this medication without needing to comply with a REMS.16
Technology plays a role in workload burden, and integrating health care processes within current workflow systems, such as using electronic patient health records and pharmacy systems, is recommended. The FDA has been exploring technologies to facilitate the completion of REMS requirements, including mandatory education within the prescribers’ and pharmacists’ workflow.17 This is a complex task that requires multiple stakeholders with differing perspectives and incentives to align.
Continue to: The data collected for the REMS...
The data collected for the REMS program belongs to the medication’s manufacturer. Current regulations do not require manufacturers to make this data available to qualified scientific researchers. A regulatory mandate to establish data sharing methods would improve transparency and enhance efforts to better understand the outcomes of the REMS programs.
A few caveats
Both the overarching and medication-specific recommendations were based on a small number of participants’ discussions related to clozapine, olanzapine for ER injectable suspension, and esketamine. These recommendations do not include other medications with REMS that are used to treat psychiatric disorders, such as loxapine, buprenorphine ER, and buprenorphine transmucosal products. Larger-scale qualitative and quantitative research is needed to better understand health care professionals’ perspectives. Lastly, some of the recommendations outlined in this article are beyond the current purview or authority of the FDA and may require legislative or regulatory action to implement.
Bottom Line
Risk Evaluation and Mitigation Strategy (REMS) programs are designed to help reduce the occurrence and/or severity of serious risks or to inform decision-making. However, REMS requirements may adversely impact patient access to certain REMS medications and clinician burden. Health care professionals can provide informed recommendations for improving the REMS programs for clozapine, olanzapine for extended-release injectable suspension, and esketamine.
Related Resources
- FDA. Frequently asked questions (FAQs) about REMS. www.fda.gov/drugs/risk-evaluation-and-mitigation-strategies-rems/frequently-asked-questions-faqs-about-rems
Drug Brand Names
Buprenorphine extended-release • Sublocade
Buprenorphine transmucosal • Subutex, Suboxone
Clozapine • Clozaril
Esketamine • Spravato
Ketamine • Ketalar
Lithium • Eskalith, Lithobid
Loxapine • Adasuve
Olanzapine extended-release injectable suspension • Zyprexa Relprevv
1. U.S. Food and Drug Administration. Risk Evaluation and Mitigation Strategies. Accessed January 18, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems
2. U.S. Department of Health and Human Services, Food and Drug Administration. Format and Content of a REMS Document. Guidance for Industry. Accessed January 18, 2023. https://www.fda.gov/media/77846/download
3. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Clozapine. Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=RemsDetails.page&REMS=351
4. The National Association of State Mental Health Program Directors. Clozapine underutilization: addressing the barriers. Accessed September 30, 2019. https://nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
5. U.S. Food and Drug Administration. FDA is temporarily exercising enforcement discretion with respect to certain clozapine REMS program requirements to ensure continuity of care for patients taking clozapine. Updated November 22, 2022. Accessed June 1, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-temporarily-exercising-enforcement-discretion-respect-certain-clozapine-rems-program
6. Tanzi M. REMS issues affect clozapine, isotretinoin. Pharmacy Today. 2022;28(3):49.
7. U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides update on patient access to certain REMS drugs during COVID-19 public health emergency. Accessed June 1, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-update-patient-access-certain-rems-drugs-during-covid-19
8. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Spravato (esketamine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386
9. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Zyprexa Relprevv (olanzapine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74
10. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm
11. Parikh SV, Lopez D, Vande Voort JL, et al. Developing an IV ketamine clinic for treatment-resistant depression: a primer. Psychopharmacol Bull. 2021;51(3):109-124.
12. Dodge D. The ketamine cure. The New York Times. November 4, 2021. Updated November 5, 2021. Accessed June 1, 2023. https://www.nytimes.com/2021/11/04/well/ketamine-therapy-depression.html
13. Burton KW. Time for a national ketamine registry, experts say. Medscape. February 15, 2023. Accessed June 1, 2023. https://www.medscape.com/viewarticle/988310
14. Wilkinson ST, Howard DH, Busch SH. Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA. 2019;322(11):1039-1040. doi:10.1001/jama.2019.10728
15. Wilkinson ST, Toprak M, Turner MS, et al. A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am J Psychiatry. 2017;174(7):695-696. doi:10.1176/appi.ajp.2017.17020239
16. Pai SM, Gries JM; ACCP Public Policy Committee. Off-label use of ketamine: a challenging drug treatment delivery model with an inherently unfavorable risk-benefit profile. J Clin Pharmacol. 2022;62(1):10-13. doi:10.1002/jcph.1983
17. Risk Evaluation and Mitigation Strategies (REMS) Integration. Accessed June 1, 2023. https://confluence.hl7.org/display/COD/Risk+Evaluation+and+Mitigation+Strategies+%28REMS%29+Integration
1. U.S. Food and Drug Administration. Risk Evaluation and Mitigation Strategies. Accessed January 18, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems
2. U.S. Department of Health and Human Services, Food and Drug Administration. Format and Content of a REMS Document. Guidance for Industry. Accessed January 18, 2023. https://www.fda.gov/media/77846/download
3. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Clozapine. Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=RemsDetails.page&REMS=351
4. The National Association of State Mental Health Program Directors. Clozapine underutilization: addressing the barriers. Accessed September 30, 2019. https://nasmhpd.org/sites/default/files/Assessment%201_Clozapine%20Underutilization.pdf
5. U.S. Food and Drug Administration. FDA is temporarily exercising enforcement discretion with respect to certain clozapine REMS program requirements to ensure continuity of care for patients taking clozapine. Updated November 22, 2022. Accessed June 1, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/fda-temporarily-exercising-enforcement-discretion-respect-certain-clozapine-rems-program
6. Tanzi M. REMS issues affect clozapine, isotretinoin. Pharmacy Today. 2022;28(3):49.
7. U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA provides update on patient access to certain REMS drugs during COVID-19 public health emergency. Accessed June 1, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-provides-update-patient-access-certain-rems-drugs-during-covid-19
8. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Spravato (esketamine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=386
9. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS), Zyprexa Relprevv (olanzapine). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm?event=IndvRemsDetails.page&REMS=74
10. U.S. Food and Drug Administration. Approved Risk Evaluation and Mitigation Strategies (REMS). Accessed January 18, 2023. https://www.accessdata.fda.gov/scripts/cder/rems/index.cfm
11. Parikh SV, Lopez D, Vande Voort JL, et al. Developing an IV ketamine clinic for treatment-resistant depression: a primer. Psychopharmacol Bull. 2021;51(3):109-124.
12. Dodge D. The ketamine cure. The New York Times. November 4, 2021. Updated November 5, 2021. Accessed June 1, 2023. https://www.nytimes.com/2021/11/04/well/ketamine-therapy-depression.html
13. Burton KW. Time for a national ketamine registry, experts say. Medscape. February 15, 2023. Accessed June 1, 2023. https://www.medscape.com/viewarticle/988310
14. Wilkinson ST, Howard DH, Busch SH. Psychiatric practice patterns and barriers to the adoption of esketamine. JAMA. 2019;322(11):1039-1040. doi:10.1001/jama.2019.10728
15. Wilkinson ST, Toprak M, Turner MS, et al. A survey of the clinical, off-label use of ketamine as a treatment for psychiatric disorders. Am J Psychiatry. 2017;174(7):695-696. doi:10.1176/appi.ajp.2017.17020239
16. Pai SM, Gries JM; ACCP Public Policy Committee. Off-label use of ketamine: a challenging drug treatment delivery model with an inherently unfavorable risk-benefit profile. J Clin Pharmacol. 2022;62(1):10-13. doi:10.1002/jcph.1983
17. Risk Evaluation and Mitigation Strategies (REMS) Integration. Accessed June 1, 2023. https://confluence.hl7.org/display/COD/Risk+Evaluation+and+Mitigation+Strategies+%28REMS%29+Integration
Interventional psychiatry (Part 2)
While most psychiatric treatments have traditionally consisted of pharmacotherapy with oral medications, a better understanding of the pathophysiology underlying many mental illnesses has led to the recent increased use of treatments that require specialized administration and the creation of a subspecialty called interventional psychiatry. In Part 1 of this 2-part article (“Interventional psychiatry [Part 1],"
Neuromodulation treatments
Neuromodulation—the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation, to specific neurologic sites—is an increasingly common approach to treating a variety of psychiatric conditions. The use of some form of neuromodulation as a medical treatment has a long history (Box1-6). Modern electric neuromodulation began in the 1930s with electroconvulsive therapy (ECT). The 1960s saw the introduction of deep brain stimulation (DBS), spinal cord stimulation, and later, vagus nerve stimulation (VNS). Target-specific noninvasive brain stimulation became possible with transcranial magnetic stimulation (TMS). These approaches are used for treating major depressive disorder (MDD), obsessive-compulsive disorder (OCD), anxiety disorders, and insomnia. Nearly all these neuromodulatory approaches require clinicians to undergo special training and patients to participate in an invasive procedure. These factors also increase cost. Nonetheless, the high rates of success of some of these approaches have led to relatively rapid and widespread acceptance.
Box
The depth and breadth of human anatomical knowledge has evolved over millennia. The time frame “thousands of years” may appear to be an overstatement, but evidence exists for successful therapeutic limb amputation as early as 31,000 years ago.1 This suggests that human knowledge of bone, muscle, and blood supply was developed much earlier than initially believed. Early Homo sapiens were altering the body—regulating or adjusting it— to serve a purpose; in this case, the purpose was survival.
In 46 AD, electrical modulation was introduced by Scribonius Largus, a physician in court of the emperor Tiberius, who used “torpedoes” (most likely electric eels) to treat headaches and pain from arthritis. Loosely, these early clinicians were modulating human function.
In the late 1800s, electrotherapeutics was a growing branch of medicine, with its own national organization—the American ElectroTherapeutic Association.2 In that era, electricity was novel, powerful, and seen as “the future.” Because such novel therapeutics were offered by both mainstream and dubious sources,3 “many of these products were marketed with the promise of curing everything from cancer to headaches.”4
Modern electric neuromodulation began in the 1930s with electroconvulsive therapy,5 followed by deep brain stimulation and spinal cord stimulation in the 1960s. Target-specific noninvasive brain stimulation became possible when Anthony Barker’s team developed the first device that permitted transcranial magnetic stimulation in 1985.6
Electroconvulsive therapy
In ECT, electric current is applied to the brain to induce a self-limiting seizure. It is the oldest and best-known interventional psychiatric treatment. ECT can also be considered one of the first treatments specifically developed to address pathophysiologic changes. In 1934, Ladislas J. Meduna, who had observed in neuropathologic studies that microglia were more numerous in patients with epilepsy compared with patients with schizophrenia, injected a patient who had been hospitalized with catatonia for 4 years with camphor, a proconvulsant.7 After 5 seizures, the patient began to recover. The therapeutic use of electricity was subsequently developed and optimized in animal models, and first used on human patients in Italy in 1939 and in the United States in 1940.8 The link between psychiatric illness and microglia, which was initially observed nearly a century ago, is making a comeback, as excessive microglial activation has been demonstrated in animal and human models of depression.9
Administering ECT requires specialized equipment, anesthesia, physician training, and nursing observation. ECT also has a negative public image.10 All of these factors conspire to reduce the availability of ECT. Despite this, approximately 100,000 patients in the United States and >1 million worldwide receive ECT each year.10 Patients generally require 6 to 12 ECT treatments11 to achieve sufficient response and may require additional maintenance treatments.12
Although ECT is used to treat psychiatric illnesses ranging from mood disorders to psychotic disorders and catatonia, it is mainly employed to treat people with severe treatment-resistant depression (TRD).13 ECT is associated with significant improvements in depressive symptoms and improvements in quality of life.14 It is superior to other treatments for TRD, such as ketamine,15 though a recent study did not show IV ketamine inferiority.16 ECT is also used to treat other neuropsychiatric disorders, such as Parkinson disease.17
Clinicians have explored alternate methods of inducing therapeutic seizures. Magnetic seizure therapy (MST) utilizes a modified magnetic stimulation device to deliver a higher energy in such a way to induce a generalized seizure under anesthesia.18 While patients receiving MST generally experience fewer adverse effects than with ECT, the procedure may be equal to19 or less effective than ECT.20
Transcranial magnetic stimulation
In neuroimaging research, certain aberrant brain circuits have been implicated in the pathogenesis of depression.21 Specifically, anatomical and functional imaging suggests connections in the prefrontal cortex are involved in the depression process. In TMS, a series of magnetic pulses are administered via the scalp to stimulate neurons in areas of the brain associated with MDD. Early case reports on using TMS to stimulate the prefrontal cortex found significant improvement of symptoms in patients with depression.22 These promising results spurred great interest in the procedure. Over time, the dose and duration of stimulation has increased, along with FDA-approved indications. TMS was first FDA-approved for TRD.23 Although the primary endpoint of the initial clinical trial did not meet criteria for FDA approval, TMS did result in improvement across multiple other measures of depression.23 After the FDA approved the first TMS device, numerous companies began to produce TMS technology. Most of these companies manufacture devices with the figure-of-eight coil, with 1 company producing the Hesed-coil helmet.24
Continue to: An unintended outcome...
An unintended outcome of the increased interest in TMS has been an increased understanding of brain regions involved in psychiatric illness. TMS was able to bring knowledge of mental health from synapses to circuits.25 Work in this area has further stratified the circuits involved in the manifestation of symptom clusters in depression.26 The exact taxonomy of these brain circuits has not been fully realized, but the default mode, salience, attention, cognitive control, and other circuits have been shown to be involved in specific symptom presentations.26,27 These circuits can be hyperactive, hypoactive, hyperconnected, or hypoconnected, with the aberrancies compared to normal controls resulting in symptoms of psychiatric illness.28
This enhanced understanding of brain function has led to further research and development of protocols and subsequent FDA approval of TMS for OCD, anxious depression, and smoking cessation.29 In addition, it has allowed for a proliferation of off-label uses for TMS, including (but not limited to) tinnitus, pain, migraines, and various substance use disorders.30 TMS treatment for these conditions involves stimulation of specific anatomical brain regions that are thought to play a role in the pathology of the target disorder. For example, subthreshold stimulation of the motor cortex has shown some utility in managing symptoms of pain disorders and movement disorders,31,32 the ventromedial prefrontal cortex has been implicated in disorders in the OCD spectrum,33 stimulation of the frontal poles may help treat substance use disorders,34 and the auditory cortex has been a target for treating tinnitus and auditory hallucinations.35
The location of stimulation for treating depression has evolved. The Talairach-Tournoux coordinate system has been used to determine the location of the dorsolateral prefrontal cortex (DLPFC) in relation to the motor cortex. This was measured to be 5 cm from the motor hotspot and subsequently became “the 5.5 cm rule,” taking skull convexity into account. The treatment paradigm for the Hesed coil also uses a measurement from the motor hotspot. Another commonly used methodology for coil placement involves using the 10 to 20 EEG coordinate system to individualize scalp landmarks. In this method, the F3 location corresponds most accurately to the DLPFC target. More recently, using fMRI-guided navigation for coil placement has been shown to lead to a significant reduction in depressive symptoms.36
For depression, the initial recommended course of treatment is 6 weeks, but most improvement is seen in the first 2 to 3 weeks.14 Therefore, many clinicians administer an initial course of 3 weeks unless the response is inadequate, in which case a 6-week course is administered. Many patients require ongoing maintenance treatment, which can be weekly or monthly based on response.37
Research to determine the optimal TMS dose for treating neuropsychiatric symptoms is ongoing. Location, intensity of stimulation, and pulse are the components of stimulation. The pulse can be subdivided into frequency, pattern (single pulse, standard, burst), train (numbers of pulse groups), interval between trains, and total number of pulses per session. The Clinical TMS Society has published TMS protocols.38 The standard intensity of stimulation is 120% of the motor threshold (MT), which is defined as the amount of stimulation over the motor cortex required to produce movement in the extensor hallucis longus. Although treatment for depression traditionally utilizes rapid TMS (3,000 pulses delivered per session at a frequency of 10 Hz in 4-second trains), in controlled studies, accelerated protocols such as intermittent theta burst stimulation (iTBS; standard stimulation parameters: triplet 50 Hz bursts at 5 Hz, with an interval of 8 seconds for 600 pulses per session) have shown noninferiority.36,39
Recent research has explored fMRI-guided iTBS in an even more accelerated format. The Stanford Neuromodulation Therapy trial involved 1,800 pulses per session for 10 sessions a day for 5 days at 90% MT.36 This treatment paradigm was shown to be more effective than standard protocols and was FDA-approved in 2022. Although this specific iTBS protocol exhibited encouraging results, the need for fMRI for adequate delivery might limit its use.
Continue to: Transcranial direct current stimulation
Transcranial direct current stimulation
Therapeutic noninvasive brain stimulation technology is plausible due to the relative lack of adverse effects and ease of administration. In transcranial direct current stimulation (tDCS), a low-intensity, constant electric current is delivered to stimulate the brain via electrodes attached to the scalp. tDCS modulates spontaneous neuronal network activity40,41 and induces polarization of resting membrane potential at the neuronal level,42 though the exact mechanism is yet to be proven. N-methyl-
tDCS has been suggested as a treatment for various psychiatric and medical conditions. However, the small sample sizes and experimental design of published studies have limited tDCS from being clinically recommended.30 No recommendation of Level A (definite efficacy) for its use was found for any indication. Level B recommendation (probable efficacy) was proposed for fibromyalgia, MDD episode without drug resistance, and addiction/craving. Level C recommendation (possible efficacy) is proposed for chronic lower limb neuropathic pain secondary to spinal cord lesion. tDCS was found to be probably ineffective as a treatment for tinnitus and drug-resistant MDD.30 Some research has suggested that tDCS targeting the DLPFC is associated with cognitive improvements in healthy individuals as well as those with schizophrenia.44 tDCS treatment remains experimental and investigational.
Deep brain stimulation
DBS is a neurosurgical procedure that uses electrical current to directly modulate specific areas of the CNS. In terms of accurate, site-specific anatomical targeting, there can be little doubt of the superiority of DBS. DBS involves the placement of leads into the brain parenchyma. Image guidance techniques are used for accurate placement. DBS is a mainstay for the symptomatic treatment of treatment-resistant movement disorders such as Parkinson disease, essential tremor, and some dystonic disorders. It also has been studied as a potential treatment for chronic pain, cluster headache, Huntington disease, and Tourette syndrome.
For treating depression, researched targets include the subgenual cingulate gyrus (SCG), ventral striatum, nucleus accumbens, inferior thalamic peduncle, medial forebrain bundle, and the red nucleus.45 In systematic reviews, improvement of depression is greatest when DBS targets the subgenual cingulate cortex and the medial forebrain bundle.46
The major limitation of DBS for treating depression is the invasive nature of the procedure. Deep TMS can achieve noninvasive stimulation of the SCG and may be associated with fewer risks, fewer adverse events, and less collateral damage. However, given the evolving concept of abnormal neurologic circuits in depression, as our understanding of circuitry in pathological psychiatric processes increases, DBS may be an attractive option for personalized targeting of symptoms in some patients.
DBS may also be beneficial for severe, treatment-resistant OCD. Electrode implantation in the region of the internal capsule/ventral striatum, including the nucleus accumbens, is used47; there is little difference in placement as a treatment for OCD vs for movement disorders.48
Continue to: A critical review of 23 trials...
A critical review of 23 trials and case reports of DBS as a treatment for OCD demonstrated a 47.7% mean reduction in score on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) and a mean response percentage (minimum 35% Y-BOCS reduction) of 58.2%.49 Most patients regained a normal quality of life after DBS.49 A more rigorous review of 15 meta-analyses of DBS found that conclusions about its efficacy or comparative effectiveness cannot be drawn.50 Because of the nature of neurosurgery, DBS has many potential complications, including cognitive changes, headache, infection, seizures, stroke, and hardware failure.
Vagus nerve stimulation
VNS, in which an implanted device stimulates the left vagus nerve with electrical impulses, was FDA-approved for treating chronic TRD in 2005.51 It had been approved for treatment-resistant epilepsy in 1997. In patients with epilepsy, VNS was shown to improve mood independent of seizure control.52 VNS requires a battery-powered pacemaker device to be implanted under the skin over the anterior chest wall, and a wire tunneled to an electrode is wrapped around the left vagus nerve in the neck.53 The pacemaker is then programmed, monitored, and reprogrammed to optimize response.
VNS is believed to stimulate deep brain nuclei that may play a role in depression.54 The onset of improvement is slow (it may take many months) but in carefully selected patients VNS can provide significant control of TRD. In addition to rare surgery-related complications such as a trauma to the vagal nerve and surrounding tissues (vocal cord paralysis, implant site infection, left facial nerve paralysis and Horner syndrome), VNS may cause hoarseness, dyspnea, and cough related to the intensity of the current output.51 Hypomania and mania were also reported; no suicidal behavior has been associated with VNS.51
Noninvasive vagus nerve stimulationIn noninvasive vagus nerve stimulation (nVNS) or transcutaneous VNS, an external handheld device is applied to the neck overlying the course of the vagus nerve to deliver a sinusoidal alternating current.55 nVNS is currently FDA-approved for treating migraine headaches.55,56 It has demonstrated actions on neurophysiology57 and inflammation in patients with MDD.58 Exploratory research has found a small beneficial effect in patients with depression.59,60 A lack of adequate reproducibility prevents this treatment from being more widely recommended, although attempts to standardize the field are evolving.61
Cranial electrical stimulation
Cranial electrical stimulation (CES) is an older form of electric stimulation developed in the 1970s. In CES, mild electrical pulses are delivered to the ear lobes bilaterally in an episodic fashion (usually 20 to 60 minutes once or twice daily). While CES can be considered a form of neuromodulation, it is not strictly interventional. Patients self-administer CES. The procedure has minimal effects on improving sleep, anxiety, and mood.62-66 Potential adverse effects include a tingling sensation in the ear lobes, lightheadedness, and fogginess. A review and meta-analysis of CES for treating addiction by Kirsch67 showed a wide range of symptoms responding positively to CES treatment, although this study was not peer-reviewed. Because of the low quality of nearly all research that evaluated CES, this form of electric stimulation cannot be viewed as an accepted treatment for any of its listed indications.
Continue to: Other neuromodulation techniques
Other
In addition to the forms of neuromodulation we have already described, there are many other techniques. Several are promising but not yet ready for clinical use. Table 1 and Table 2 summarize the neuromodulation techniques described in this article as well as several that are under development.

Acupuncture
Acupuncture is a Chinese form of medical treatment that began >3,000 years ago; there are written descriptions of it from >2,000 years ago.68 It is based on the belief that there are channels within the body through which the Qi (vital energy or life force) flow, and that inserting fine needles into these channels via the skin can rebalance Qi.68 Modern mechanistic hypotheses invoke involvement of inflammatory or pain pathways.69 Acupuncture frequently uses electric stimulation (electro-acupuncture) to increase the potency of the procedure. Alternatively, in a related procedure (acupressure), pressure can replace the needle. Accreditation in acupuncture generally requires a master’s degree in traditional Chinese medicine but does not require any specific medical training. Acupuncture training courses for physicians are widely available.
All forms of acupuncture are experimental for a wide variety of mental and medical conditions. A meta-analysis found that most research of the utility of acupuncture for depression suffered from various forms of potential bias and was considered low quality.70 Nonetheless, active acupuncture was shown to be minimally superior to placebo acupuncture.70 A meta-analysis of acupuncture for preoperative anxiety71,72 and poststroke insomnia73 reported a similar low study quality. A study of 72 patients with primary insomnia revealed that acupuncture was more effective than sham acupuncture for most sleep measures.74
Psychiatry is increasingly integrating medical tools in addition to psychological tools. Pharmacology remains a cornerstone of biological psychiatry and this will not soon change. However, nonpharmacologic psychiatric treatments such as therapeutic neuromodulation are rapidly emerging. These and novel methods of medication administration may present a challenge to psychiatrists who do not have access to medical personnel or may have forgotten general medical skills.
Our 2-part article has highlighted several interventional psychiatry tools—old and new—that may interest clinicians and benefit patients. As a rule, such treatments are reserved for the most treatment-resistant, challenging psychiatric patients, those with hard-to-treat chronic conditions, and patients who are not helped by more commonly used treatments. An additional complication is that such treatments are frequently not appropriately researched, vetted, or FDA-approved, and therefore are higher risk. Appropriate clinical judgment is always necessary, and potential benefits must be thoroughly weighed against possible adverse effects.
Bottom Line
Several forms of neuromodulation, including electroconvulsive therapy, transcranial magnetic stimulation, transcranial direct current stimulation, deep brain stimulation, and vagus nerve stimulation, may be beneficial for patients with certain treatment-resistant psychiatric disorders, including major depressive disorder and obsessive-compulsive disorder.
Related Resources
- Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
- Sharma MS, Ang-Rabanes M, Selek S, et al. Neuromodulatory options for treatment-resistant depression. Current Psychiatry. 2018;17(3):26-28,33-37.
1. Maloney TR, Dilkes-Hall IE, Vlok M, et al. Surgical amputation of a limb 31,000 years ago in Borneo. Nature. 2022;609(7927):547-551. doi:10.1038/s41586-022-05160-8
2. The American Electro-Therapeutic Association. JAMA. 1893;21(14):500. doi:10.1001/jama.1893.02420660030004
3. The American Electro-Therapeutic Association. JAMA. 1894;23(15):590-591. doi:10.1001/jama.1894.02421200024006
4. Wexler A. The medical battery in the United States (1870-1920): electrotherapy at home and in the clinic. J Hist Med Allied Sci. 2017;72(2):166-192. doi:10.1093/jhmas/jrx001
5. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
6. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106-1107. doi:10.1016/s0140-6736(85)92413-4
7. Fink M. Historical article: autobiography of L. J. Meduna. Convuls Ther. 1985;1(1):43-57.
8. Suleman R. A brief history of electroconvulsive therapy. Am J Psychiatry. 2020;16(1):6. doi:10.1176/appi.ajp-rj.2020.160103
9. Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. 2016;321:138-162. doi:10.1016/j.neuroscience.2015.05.053
10. Payne NA, Prudic J. Electroconvulsive therapy: part II: a biopsychosocial perspective. J Psychiatr Pract. 2009;15(5):369-390. doi:10.1097/01.pra.0000361278.73092.85
11. Tirmizi O, Raza A, Trevino K, et al. Electroconvulsive therapy: how modern techniques improve patient outcomes. Current Psychiatry. 2012;11(10):24-46.
12. Kolar D. Current status of electroconvulsive therapy for mood disorders: a clinical review. Evid Based Ment Health. 2017;20(1):12-14. doi:10.1136/eb-2016-102498
13. Andrade C. Active placebo, the parachute meta-analysis, the Nobel Prize, and the efficacy of electroconvulsive therapy. J Clin Psychiatry. 2021;82(2):21f13992. doi:10.4088/JCP.21f13992
14. Giacobbe P, Rakita U, Penner-Goeke K, et al. Improvements in health-related quality of life with electroconvulsive therapy: a meta-analysis. J ECT. 2018;34(2):87-94. doi:10.1097/YCT.0000000000000486
15. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79(12):1162-1172. doi:10.1001/jamapsychiatry.2022.3352
16. Anand A, Mathew SJ, Sanacora G, et al. Ketamine versus ECT for nonpsychotic treatment-resistant major depression. N Engl J Med. 2023. doi: 10.1056/NEJMoa2302399
17. Takamiya A, Seki M, Kudo S, et al. Electroconvulsive therapy for Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2021;36(1):50-58. doi:10.1002/mds.28335
18. Singh R, Sharma R, Prakash J, et al. Magnetic seizure therapy. Ind Psychiatry J. 2021;30(Suppl 1):S320-S321. doi:10.4103/0972-6748.328841
19. Chen M, Yang X, Liu C, et al. Comparative efficacy and cognitive function of magnetic seizure therapy vs. electroconvulsive therapy for major depressive disorder: a systematic review and meta-analysis. Transl Psychiatry. 2021;11(1):437. doi:10.1038/s41398-021-01560-y
20. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi:10.1155/2015/521398
21. George MS, Ketter TA, Post RM. Prefrontal cortex dysfunction in clinical depression. In: Nemeroff CB, Weiss JM, Schatzberg AF, et al, eds. Depression. 2nd ed. Wiley Online Library; 1994:59-72. https://doi.org/10.1002/depr.3050020202
22. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6(14):1853-1856.
23. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
24. Clinical TMS Society. TMS devices. Accessed January 2, 2023. https://www.clinicaltmssociety.org/devices
25. Goldstein-Piekarski AN, Ball TM, Samara Z, et al. Mapping neural circuit biotypes to symptoms and behavioral dimensions of depression and anxiety. Biol Psychiatry. 2022;91(6):561-571. doi:10.1016/j.biopsych.2021.06.024
26. Siddiqi SH, Taylor SF, Cooke D, et al. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am J Psychiatry. 2020;177(5):435-446. doi:10.1176/appi.ajp.2019.19090915
27. Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. 2017;34(1):9-24. doi:10.1002/da.22556
28. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38. doi:10.1038/nm.4246
29. Cohen SL, Bikson M, Badran BW, et al. A visual and narrative timeline of US FDA milestones for transcranial magnetic stimulation (TMS) devices. Brain Stimul. 2022;15(1):73-75. doi:10.1016/j.brs.2021.11.010
30. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56-92. doi:10.1016/j.clinph.2016.10.087
31. Li R, He Y, Qin W, et al. Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson’s disease: a meta-analysis. Neurorehabil Neural Repair. 2022;36(7):395-404. doi:10.1177/15459683221095034
32. Leung A, Shirvalkar P, Chen R, et al. Transcranial magnetic stimulation for pain, headache, and comorbid depression: INS-NANS expert consensus panel review and recommendation. Neuromodulation. 2020;23(3):267-290. doi:10.1111/ner.13094
33. Carmi L, Tendler A, Bystritsky A, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019;176(11):931-938. doi:10.1176/appi.ajp.2019.18101180
34. Harel M, Perini I, Kämpe R, et al. Repetitive transcranial magnetic stimulation in alcohol dependence: a randomized, double-blind, sham-controlled proof-of-concept trial targeting the medial prefrontal and anterior cingulate cortices. Biol Psychiatry. 2022;91(12):1061-1069. doi:10.1016/j.biopsych.2021.11.020
35. Folmer RL, Theodoroff SM, Casiana L, et al. Repetitive transcranial magnetic stimulation treatment for chronic tinnitus: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2015;141(8):716-722. doi:10.1001/jamaoto.2015.1219
36. Cole EJ, Phillips AL, Bentzley BS, et al. Stanford Neuromodulation Therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry. 2022;179(2):132-141. doi:10.1176/appi.ajp.2021.20101429
37. Wilson S, Croarkin PE, Aaronson ST, et al. Systematic review of preservation TMS that includes continuation, maintenance, relapse-prevention, and rescue TMS. J Affect Disord. 2022;296:79-88. doi:10.1016/j.jad.2021.09.040
38. Perera T, George MS, Grammer G, et al. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336-346. doi:10.1016/j.brs.2016.03.010
39. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomized non-inferiority trial. Lancet. 2018;391(10131):1683-1692. doi:10.1016/S0140-6736(18)30295-2
40. Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1(3):206-223. doi:10.1016/j.brs.2008.06.004
41. Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2009;2(4):241-245.
42. Priori A, Berardelli A, Rona S, et al. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9(10):2257-2260. doi:10.1097/00001756-199807130-00020
43. Nitsche MA, Liebetanz D, Antal A, et al. Modulation of cortical excitability by weak direct current stimulation-- technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255-276. doi:10.1016/s1567-424x(09)70230-2
44. Agarwal SM, Venkataram Shivakumar V, et al. Transcranial direct current stimulation in schizophrenia. Clin Psychopharmacol Neurosci. 2013;11(3):118-125.
45. Drobisz D, Damborská A. Deep brain stimulation targets for treating depression. Behav Brain Res. 2019;359:266-273. doi:10.1016/j.bbr.2018.11.004
46. Kisely S, Li A, Warren N, et al. A systematic review and meta-analysis of deep brain stimulation for depression. Depress Anxiety. 2018;35(5):468-480. doi:10.1002/da.22746
47. Blomstedt P, Sjöberg RL, Hansson M, et al. Deep brain stimulation in the treatment of obsessive-compulsive disorder. World Neurosurg. 2013;80(6):e245-e253. doi:10.1016/j.wneu.2012.10.006
48. Denys D, Mantione M, Figee M, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67(10):1061-1068. doi:10.1001/archgenpsychiatry.2010.122
49. van Westen M, Rietveld E, Figee M, et al. Clinical outcome and mechanisms of deep brain stimulation for obsessive-compulsive disorder. Curr Behav Neurosci Rep. 2015;2(2):41-48. doi:10.1007/s40473-015-0036-3
50. Papageorgiou PN, Deschner J, Papageorgiou SN. Effectiveness and adverse effects of deep brain stimulation: umbrella review of meta-analyses. J Neurol Surg A Cent Eur Neurosurg. 2017;78(2):180-190. doi:10.1055/s-0036-1592158
51. O’Reardon JP, Cristancho P, Peshek AD. Vagus nerve stimulation (VNS) and treatment of depression: to the brainstem and beyond. Psychiatry (Edgmont). 2006;3(5):54-63.
52. Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93-99. doi:10.1006/ebeh.2000.0046
53. Giordano F, Zicca A, Barba C, et al. Vagus nerve stimulation: surgical technique of implantation and revision and related morbidity. Epilepsia. 2017;58(S1):85-90. doi:10.1111/epi.13687
54. George MS, Nahas Z, Bohning DE, et al. Mechanisms of action of vagus nerve stimulation (VNS). Clin Neurosci Res. 2004;4(1-2):71-79.
55. Nesbitt AD, Marin JCA, Tompkins E, et al. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology. 2015;84:1249-1253. doi:10.1212/WNL.0000000000001394
56. Goadsby PJ, Grosberg BM, Mauskop A, et al. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia. 2014;34:986-993. doi:10.1177/0333102414524494
57. Fang J, Rong P, Hong Y, et al. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry. 2016;79(4):266-273. doi:10.1016/j.biopsych.2015.03.025
58. Liu CH, Yang MH, Zhang GZ, et al. Neural networks and the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation in depression. J Neuroinflammation. 2020;17(1):54. doi:10.1186/s12974-020-01732-5
59. Hein E, Nowak M, Kiess O, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm (Vienna). 2013;120(5):821-827. doi:10.1007/s00702-012-0908-6
60. Rong P, Liu J, Wang L, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J Affect Disord. 2016;195:172-179. doi:10.1016/j.jad.2016.02.031
61. Farmer AD, Strzelczyk A, Finisguerra A, et al. International consensus based review and recommendations for minimum reporting standards in research on transcutaneous vagus nerve stimulation (Version 2020). Front Hum Neurosci. 2021;14:568051. doi:10.3389/fnhum.2020.568051
62. Amr M, El-Wasify M, Elmaadawi AZ, et al. Cranial electrotherapy stimulation for the treatment of chronically symptomatic bipolar patients. J ECT. 2013;29(2):e31-e32. doi:10.1097/YCT.0b013e31828a344d
63. Kirsch DL, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013;36(1):169-176. doi:10.1016/j.psc.2013.01.006
64. Lande RG, Gragnani C. Efficacy of cranial electric stimulation for the treatment of insomnia: a randomized pilot study. Complement Ther Med. 2013;21(1):8-13. doi:10.1016/j.ctim.2012.11.007
65. Ou Y, Li, C. Sertraline combined alpha-stim clinical observations on the treatment of 30 cases of generalized anxiety disorder. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2015;24(17):73-75.
66. Price L, Briley J, Haltiwanger S, et al. A meta-analysis of cranial electrotherapy stimulation in the treatment of depression. J Psychiatr Res. 2021;135:119-134. doi:10.1016/j.jpsychires.2020.12.043
67. Kirsch D, Gilula M. CES in the treatment of addictions: a review and meta-analysis. Pract Pain Manag. 2007;7(9).
68. Hao JJ, Mittelman M. Acupuncture: past, present, and future. Glob Adv Health Med. 2014;3(4):6-8. doi:10.7453/gahmj.2014.042
69. Napadow V, Ahn A, Longhurst J, et al. The status and future of acupuncture mechanism research. J Altern Complement Med. 2008;14(7):861-869. doi:10.1089/acm.2008.SAR-3
70. Smith CA, Armour M, Lee MS, et al. Acupuncture for depression. Cochrane Database Syst Rev. 2018;3(3):CD004046. doi:10.1002/14651858.CD004046.pub4
71. Tong QY, Liu R, Zhang K, et al. Can acupuncture therapy reduce preoperative anxiety? A systematic review and meta-analysis. J Integr Med. 2021;19(1):20-28. doi:10.1016/j.joim.2020.10.007
72. Usichenko TI, Hua K, Cummings M, et al. Auricular stimulation for preoperative anxiety – a systematic review and meta-analysis of randomized controlled clinical trials. J Clin Anesth. 2022;76:110581. doi:10.1016/j.jclinane.2021.110581
73. Zhou L, Hu X, Yu Z, et al. Efficacy and safety of acupuncture in the treatment of poststroke insomnia: a systematic review and meta-analysis of twenty-six randomized controlled trials. Evid Based Complement Alternat Med. 2022;2022:5188311. doi:10.1155/2022/5188311
74. Yin X, Gou M, Xu J, et al. Efficacy and safety of acupuncture treatment on primary insomnia: a randomized controlled trial. Sleep Med. 2017;37:193-200. doi:10.1016/j.sleep.2017.02.012
While most psychiatric treatments have traditionally consisted of pharmacotherapy with oral medications, a better understanding of the pathophysiology underlying many mental illnesses has led to the recent increased use of treatments that require specialized administration and the creation of a subspecialty called interventional psychiatry. In Part 1 of this 2-part article (“Interventional psychiatry [Part 1],"
Neuromodulation treatments
Neuromodulation—the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation, to specific neurologic sites—is an increasingly common approach to treating a variety of psychiatric conditions. The use of some form of neuromodulation as a medical treatment has a long history (Box1-6). Modern electric neuromodulation began in the 1930s with electroconvulsive therapy (ECT). The 1960s saw the introduction of deep brain stimulation (DBS), spinal cord stimulation, and later, vagus nerve stimulation (VNS). Target-specific noninvasive brain stimulation became possible with transcranial magnetic stimulation (TMS). These approaches are used for treating major depressive disorder (MDD), obsessive-compulsive disorder (OCD), anxiety disorders, and insomnia. Nearly all these neuromodulatory approaches require clinicians to undergo special training and patients to participate in an invasive procedure. These factors also increase cost. Nonetheless, the high rates of success of some of these approaches have led to relatively rapid and widespread acceptance.
Box
The depth and breadth of human anatomical knowledge has evolved over millennia. The time frame “thousands of years” may appear to be an overstatement, but evidence exists for successful therapeutic limb amputation as early as 31,000 years ago.1 This suggests that human knowledge of bone, muscle, and blood supply was developed much earlier than initially believed. Early Homo sapiens were altering the body—regulating or adjusting it— to serve a purpose; in this case, the purpose was survival.
In 46 AD, electrical modulation was introduced by Scribonius Largus, a physician in court of the emperor Tiberius, who used “torpedoes” (most likely electric eels) to treat headaches and pain from arthritis. Loosely, these early clinicians were modulating human function.
In the late 1800s, electrotherapeutics was a growing branch of medicine, with its own national organization—the American ElectroTherapeutic Association.2 In that era, electricity was novel, powerful, and seen as “the future.” Because such novel therapeutics were offered by both mainstream and dubious sources,3 “many of these products were marketed with the promise of curing everything from cancer to headaches.”4
Modern electric neuromodulation began in the 1930s with electroconvulsive therapy,5 followed by deep brain stimulation and spinal cord stimulation in the 1960s. Target-specific noninvasive brain stimulation became possible when Anthony Barker’s team developed the first device that permitted transcranial magnetic stimulation in 1985.6
Electroconvulsive therapy
In ECT, electric current is applied to the brain to induce a self-limiting seizure. It is the oldest and best-known interventional psychiatric treatment. ECT can also be considered one of the first treatments specifically developed to address pathophysiologic changes. In 1934, Ladislas J. Meduna, who had observed in neuropathologic studies that microglia were more numerous in patients with epilepsy compared with patients with schizophrenia, injected a patient who had been hospitalized with catatonia for 4 years with camphor, a proconvulsant.7 After 5 seizures, the patient began to recover. The therapeutic use of electricity was subsequently developed and optimized in animal models, and first used on human patients in Italy in 1939 and in the United States in 1940.8 The link between psychiatric illness and microglia, which was initially observed nearly a century ago, is making a comeback, as excessive microglial activation has been demonstrated in animal and human models of depression.9
Administering ECT requires specialized equipment, anesthesia, physician training, and nursing observation. ECT also has a negative public image.10 All of these factors conspire to reduce the availability of ECT. Despite this, approximately 100,000 patients in the United States and >1 million worldwide receive ECT each year.10 Patients generally require 6 to 12 ECT treatments11 to achieve sufficient response and may require additional maintenance treatments.12
Although ECT is used to treat psychiatric illnesses ranging from mood disorders to psychotic disorders and catatonia, it is mainly employed to treat people with severe treatment-resistant depression (TRD).13 ECT is associated with significant improvements in depressive symptoms and improvements in quality of life.14 It is superior to other treatments for TRD, such as ketamine,15 though a recent study did not show IV ketamine inferiority.16 ECT is also used to treat other neuropsychiatric disorders, such as Parkinson disease.17
Clinicians have explored alternate methods of inducing therapeutic seizures. Magnetic seizure therapy (MST) utilizes a modified magnetic stimulation device to deliver a higher energy in such a way to induce a generalized seizure under anesthesia.18 While patients receiving MST generally experience fewer adverse effects than with ECT, the procedure may be equal to19 or less effective than ECT.20
Transcranial magnetic stimulation
In neuroimaging research, certain aberrant brain circuits have been implicated in the pathogenesis of depression.21 Specifically, anatomical and functional imaging suggests connections in the prefrontal cortex are involved in the depression process. In TMS, a series of magnetic pulses are administered via the scalp to stimulate neurons in areas of the brain associated with MDD. Early case reports on using TMS to stimulate the prefrontal cortex found significant improvement of symptoms in patients with depression.22 These promising results spurred great interest in the procedure. Over time, the dose and duration of stimulation has increased, along with FDA-approved indications. TMS was first FDA-approved for TRD.23 Although the primary endpoint of the initial clinical trial did not meet criteria for FDA approval, TMS did result in improvement across multiple other measures of depression.23 After the FDA approved the first TMS device, numerous companies began to produce TMS technology. Most of these companies manufacture devices with the figure-of-eight coil, with 1 company producing the Hesed-coil helmet.24
Continue to: An unintended outcome...
An unintended outcome of the increased interest in TMS has been an increased understanding of brain regions involved in psychiatric illness. TMS was able to bring knowledge of mental health from synapses to circuits.25 Work in this area has further stratified the circuits involved in the manifestation of symptom clusters in depression.26 The exact taxonomy of these brain circuits has not been fully realized, but the default mode, salience, attention, cognitive control, and other circuits have been shown to be involved in specific symptom presentations.26,27 These circuits can be hyperactive, hypoactive, hyperconnected, or hypoconnected, with the aberrancies compared to normal controls resulting in symptoms of psychiatric illness.28
This enhanced understanding of brain function has led to further research and development of protocols and subsequent FDA approval of TMS for OCD, anxious depression, and smoking cessation.29 In addition, it has allowed for a proliferation of off-label uses for TMS, including (but not limited to) tinnitus, pain, migraines, and various substance use disorders.30 TMS treatment for these conditions involves stimulation of specific anatomical brain regions that are thought to play a role in the pathology of the target disorder. For example, subthreshold stimulation of the motor cortex has shown some utility in managing symptoms of pain disorders and movement disorders,31,32 the ventromedial prefrontal cortex has been implicated in disorders in the OCD spectrum,33 stimulation of the frontal poles may help treat substance use disorders,34 and the auditory cortex has been a target for treating tinnitus and auditory hallucinations.35
The location of stimulation for treating depression has evolved. The Talairach-Tournoux coordinate system has been used to determine the location of the dorsolateral prefrontal cortex (DLPFC) in relation to the motor cortex. This was measured to be 5 cm from the motor hotspot and subsequently became “the 5.5 cm rule,” taking skull convexity into account. The treatment paradigm for the Hesed coil also uses a measurement from the motor hotspot. Another commonly used methodology for coil placement involves using the 10 to 20 EEG coordinate system to individualize scalp landmarks. In this method, the F3 location corresponds most accurately to the DLPFC target. More recently, using fMRI-guided navigation for coil placement has been shown to lead to a significant reduction in depressive symptoms.36
For depression, the initial recommended course of treatment is 6 weeks, but most improvement is seen in the first 2 to 3 weeks.14 Therefore, many clinicians administer an initial course of 3 weeks unless the response is inadequate, in which case a 6-week course is administered. Many patients require ongoing maintenance treatment, which can be weekly or monthly based on response.37
Research to determine the optimal TMS dose for treating neuropsychiatric symptoms is ongoing. Location, intensity of stimulation, and pulse are the components of stimulation. The pulse can be subdivided into frequency, pattern (single pulse, standard, burst), train (numbers of pulse groups), interval between trains, and total number of pulses per session. The Clinical TMS Society has published TMS protocols.38 The standard intensity of stimulation is 120% of the motor threshold (MT), which is defined as the amount of stimulation over the motor cortex required to produce movement in the extensor hallucis longus. Although treatment for depression traditionally utilizes rapid TMS (3,000 pulses delivered per session at a frequency of 10 Hz in 4-second trains), in controlled studies, accelerated protocols such as intermittent theta burst stimulation (iTBS; standard stimulation parameters: triplet 50 Hz bursts at 5 Hz, with an interval of 8 seconds for 600 pulses per session) have shown noninferiority.36,39
Recent research has explored fMRI-guided iTBS in an even more accelerated format. The Stanford Neuromodulation Therapy trial involved 1,800 pulses per session for 10 sessions a day for 5 days at 90% MT.36 This treatment paradigm was shown to be more effective than standard protocols and was FDA-approved in 2022. Although this specific iTBS protocol exhibited encouraging results, the need for fMRI for adequate delivery might limit its use.
Continue to: Transcranial direct current stimulation
Transcranial direct current stimulation
Therapeutic noninvasive brain stimulation technology is plausible due to the relative lack of adverse effects and ease of administration. In transcranial direct current stimulation (tDCS), a low-intensity, constant electric current is delivered to stimulate the brain via electrodes attached to the scalp. tDCS modulates spontaneous neuronal network activity40,41 and induces polarization of resting membrane potential at the neuronal level,42 though the exact mechanism is yet to be proven. N-methyl-
tDCS has been suggested as a treatment for various psychiatric and medical conditions. However, the small sample sizes and experimental design of published studies have limited tDCS from being clinically recommended.30 No recommendation of Level A (definite efficacy) for its use was found for any indication. Level B recommendation (probable efficacy) was proposed for fibromyalgia, MDD episode without drug resistance, and addiction/craving. Level C recommendation (possible efficacy) is proposed for chronic lower limb neuropathic pain secondary to spinal cord lesion. tDCS was found to be probably ineffective as a treatment for tinnitus and drug-resistant MDD.30 Some research has suggested that tDCS targeting the DLPFC is associated with cognitive improvements in healthy individuals as well as those with schizophrenia.44 tDCS treatment remains experimental and investigational.
Deep brain stimulation
DBS is a neurosurgical procedure that uses electrical current to directly modulate specific areas of the CNS. In terms of accurate, site-specific anatomical targeting, there can be little doubt of the superiority of DBS. DBS involves the placement of leads into the brain parenchyma. Image guidance techniques are used for accurate placement. DBS is a mainstay for the symptomatic treatment of treatment-resistant movement disorders such as Parkinson disease, essential tremor, and some dystonic disorders. It also has been studied as a potential treatment for chronic pain, cluster headache, Huntington disease, and Tourette syndrome.
For treating depression, researched targets include the subgenual cingulate gyrus (SCG), ventral striatum, nucleus accumbens, inferior thalamic peduncle, medial forebrain bundle, and the red nucleus.45 In systematic reviews, improvement of depression is greatest when DBS targets the subgenual cingulate cortex and the medial forebrain bundle.46
The major limitation of DBS for treating depression is the invasive nature of the procedure. Deep TMS can achieve noninvasive stimulation of the SCG and may be associated with fewer risks, fewer adverse events, and less collateral damage. However, given the evolving concept of abnormal neurologic circuits in depression, as our understanding of circuitry in pathological psychiatric processes increases, DBS may be an attractive option for personalized targeting of symptoms in some patients.
DBS may also be beneficial for severe, treatment-resistant OCD. Electrode implantation in the region of the internal capsule/ventral striatum, including the nucleus accumbens, is used47; there is little difference in placement as a treatment for OCD vs for movement disorders.48
Continue to: A critical review of 23 trials...
A critical review of 23 trials and case reports of DBS as a treatment for OCD demonstrated a 47.7% mean reduction in score on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) and a mean response percentage (minimum 35% Y-BOCS reduction) of 58.2%.49 Most patients regained a normal quality of life after DBS.49 A more rigorous review of 15 meta-analyses of DBS found that conclusions about its efficacy or comparative effectiveness cannot be drawn.50 Because of the nature of neurosurgery, DBS has many potential complications, including cognitive changes, headache, infection, seizures, stroke, and hardware failure.
Vagus nerve stimulation
VNS, in which an implanted device stimulates the left vagus nerve with electrical impulses, was FDA-approved for treating chronic TRD in 2005.51 It had been approved for treatment-resistant epilepsy in 1997. In patients with epilepsy, VNS was shown to improve mood independent of seizure control.52 VNS requires a battery-powered pacemaker device to be implanted under the skin over the anterior chest wall, and a wire tunneled to an electrode is wrapped around the left vagus nerve in the neck.53 The pacemaker is then programmed, monitored, and reprogrammed to optimize response.
VNS is believed to stimulate deep brain nuclei that may play a role in depression.54 The onset of improvement is slow (it may take many months) but in carefully selected patients VNS can provide significant control of TRD. In addition to rare surgery-related complications such as a trauma to the vagal nerve and surrounding tissues (vocal cord paralysis, implant site infection, left facial nerve paralysis and Horner syndrome), VNS may cause hoarseness, dyspnea, and cough related to the intensity of the current output.51 Hypomania and mania were also reported; no suicidal behavior has been associated with VNS.51
Noninvasive vagus nerve stimulationIn noninvasive vagus nerve stimulation (nVNS) or transcutaneous VNS, an external handheld device is applied to the neck overlying the course of the vagus nerve to deliver a sinusoidal alternating current.55 nVNS is currently FDA-approved for treating migraine headaches.55,56 It has demonstrated actions on neurophysiology57 and inflammation in patients with MDD.58 Exploratory research has found a small beneficial effect in patients with depression.59,60 A lack of adequate reproducibility prevents this treatment from being more widely recommended, although attempts to standardize the field are evolving.61
Cranial electrical stimulation
Cranial electrical stimulation (CES) is an older form of electric stimulation developed in the 1970s. In CES, mild electrical pulses are delivered to the ear lobes bilaterally in an episodic fashion (usually 20 to 60 minutes once or twice daily). While CES can be considered a form of neuromodulation, it is not strictly interventional. Patients self-administer CES. The procedure has minimal effects on improving sleep, anxiety, and mood.62-66 Potential adverse effects include a tingling sensation in the ear lobes, lightheadedness, and fogginess. A review and meta-analysis of CES for treating addiction by Kirsch67 showed a wide range of symptoms responding positively to CES treatment, although this study was not peer-reviewed. Because of the low quality of nearly all research that evaluated CES, this form of electric stimulation cannot be viewed as an accepted treatment for any of its listed indications.
Continue to: Other neuromodulation techniques
Other
In addition to the forms of neuromodulation we have already described, there are many other techniques. Several are promising but not yet ready for clinical use. Table 1 and Table 2 summarize the neuromodulation techniques described in this article as well as several that are under development.


Acupuncture
Acupuncture is a Chinese form of medical treatment that began >3,000 years ago; there are written descriptions of it from >2,000 years ago.68 It is based on the belief that there are channels within the body through which the Qi (vital energy or life force) flow, and that inserting fine needles into these channels via the skin can rebalance Qi.68 Modern mechanistic hypotheses invoke involvement of inflammatory or pain pathways.69 Acupuncture frequently uses electric stimulation (electro-acupuncture) to increase the potency of the procedure. Alternatively, in a related procedure (acupressure), pressure can replace the needle. Accreditation in acupuncture generally requires a master’s degree in traditional Chinese medicine but does not require any specific medical training. Acupuncture training courses for physicians are widely available.
All forms of acupuncture are experimental for a wide variety of mental and medical conditions. A meta-analysis found that most research of the utility of acupuncture for depression suffered from various forms of potential bias and was considered low quality.70 Nonetheless, active acupuncture was shown to be minimally superior to placebo acupuncture.70 A meta-analysis of acupuncture for preoperative anxiety71,72 and poststroke insomnia73 reported a similar low study quality. A study of 72 patients with primary insomnia revealed that acupuncture was more effective than sham acupuncture for most sleep measures.74
Psychiatry is increasingly integrating medical tools in addition to psychological tools. Pharmacology remains a cornerstone of biological psychiatry and this will not soon change. However, nonpharmacologic psychiatric treatments such as therapeutic neuromodulation are rapidly emerging. These and novel methods of medication administration may present a challenge to psychiatrists who do not have access to medical personnel or may have forgotten general medical skills.
Our 2-part article has highlighted several interventional psychiatry tools—old and new—that may interest clinicians and benefit patients. As a rule, such treatments are reserved for the most treatment-resistant, challenging psychiatric patients, those with hard-to-treat chronic conditions, and patients who are not helped by more commonly used treatments. An additional complication is that such treatments are frequently not appropriately researched, vetted, or FDA-approved, and therefore are higher risk. Appropriate clinical judgment is always necessary, and potential benefits must be thoroughly weighed against possible adverse effects.
Bottom Line
Several forms of neuromodulation, including electroconvulsive therapy, transcranial magnetic stimulation, transcranial direct current stimulation, deep brain stimulation, and vagus nerve stimulation, may be beneficial for patients with certain treatment-resistant psychiatric disorders, including major depressive disorder and obsessive-compulsive disorder.
Related Resources
- Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
- Sharma MS, Ang-Rabanes M, Selek S, et al. Neuromodulatory options for treatment-resistant depression. Current Psychiatry. 2018;17(3):26-28,33-37.
While most psychiatric treatments have traditionally consisted of pharmacotherapy with oral medications, a better understanding of the pathophysiology underlying many mental illnesses has led to the recent increased use of treatments that require specialized administration and the creation of a subspecialty called interventional psychiatry. In Part 1 of this 2-part article (“Interventional psychiatry [Part 1],"
Neuromodulation treatments
Neuromodulation—the alteration of nerve activity through targeted delivery of a stimulus, such as electrical stimulation, to specific neurologic sites—is an increasingly common approach to treating a variety of psychiatric conditions. The use of some form of neuromodulation as a medical treatment has a long history (Box1-6). Modern electric neuromodulation began in the 1930s with electroconvulsive therapy (ECT). The 1960s saw the introduction of deep brain stimulation (DBS), spinal cord stimulation, and later, vagus nerve stimulation (VNS). Target-specific noninvasive brain stimulation became possible with transcranial magnetic stimulation (TMS). These approaches are used for treating major depressive disorder (MDD), obsessive-compulsive disorder (OCD), anxiety disorders, and insomnia. Nearly all these neuromodulatory approaches require clinicians to undergo special training and patients to participate in an invasive procedure. These factors also increase cost. Nonetheless, the high rates of success of some of these approaches have led to relatively rapid and widespread acceptance.
Box
The depth and breadth of human anatomical knowledge has evolved over millennia. The time frame “thousands of years” may appear to be an overstatement, but evidence exists for successful therapeutic limb amputation as early as 31,000 years ago.1 This suggests that human knowledge of bone, muscle, and blood supply was developed much earlier than initially believed. Early Homo sapiens were altering the body—regulating or adjusting it— to serve a purpose; in this case, the purpose was survival.
In 46 AD, electrical modulation was introduced by Scribonius Largus, a physician in court of the emperor Tiberius, who used “torpedoes” (most likely electric eels) to treat headaches and pain from arthritis. Loosely, these early clinicians were modulating human function.
In the late 1800s, electrotherapeutics was a growing branch of medicine, with its own national organization—the American ElectroTherapeutic Association.2 In that era, electricity was novel, powerful, and seen as “the future.” Because such novel therapeutics were offered by both mainstream and dubious sources,3 “many of these products were marketed with the promise of curing everything from cancer to headaches.”4
Modern electric neuromodulation began in the 1930s with electroconvulsive therapy,5 followed by deep brain stimulation and spinal cord stimulation in the 1960s. Target-specific noninvasive brain stimulation became possible when Anthony Barker’s team developed the first device that permitted transcranial magnetic stimulation in 1985.6
Electroconvulsive therapy
In ECT, electric current is applied to the brain to induce a self-limiting seizure. It is the oldest and best-known interventional psychiatric treatment. ECT can also be considered one of the first treatments specifically developed to address pathophysiologic changes. In 1934, Ladislas J. Meduna, who had observed in neuropathologic studies that microglia were more numerous in patients with epilepsy compared with patients with schizophrenia, injected a patient who had been hospitalized with catatonia for 4 years with camphor, a proconvulsant.7 After 5 seizures, the patient began to recover. The therapeutic use of electricity was subsequently developed and optimized in animal models, and first used on human patients in Italy in 1939 and in the United States in 1940.8 The link between psychiatric illness and microglia, which was initially observed nearly a century ago, is making a comeback, as excessive microglial activation has been demonstrated in animal and human models of depression.9
Administering ECT requires specialized equipment, anesthesia, physician training, and nursing observation. ECT also has a negative public image.10 All of these factors conspire to reduce the availability of ECT. Despite this, approximately 100,000 patients in the United States and >1 million worldwide receive ECT each year.10 Patients generally require 6 to 12 ECT treatments11 to achieve sufficient response and may require additional maintenance treatments.12
Although ECT is used to treat psychiatric illnesses ranging from mood disorders to psychotic disorders and catatonia, it is mainly employed to treat people with severe treatment-resistant depression (TRD).13 ECT is associated with significant improvements in depressive symptoms and improvements in quality of life.14 It is superior to other treatments for TRD, such as ketamine,15 though a recent study did not show IV ketamine inferiority.16 ECT is also used to treat other neuropsychiatric disorders, such as Parkinson disease.17
Clinicians have explored alternate methods of inducing therapeutic seizures. Magnetic seizure therapy (MST) utilizes a modified magnetic stimulation device to deliver a higher energy in such a way to induce a generalized seizure under anesthesia.18 While patients receiving MST generally experience fewer adverse effects than with ECT, the procedure may be equal to19 or less effective than ECT.20
Transcranial magnetic stimulation
In neuroimaging research, certain aberrant brain circuits have been implicated in the pathogenesis of depression.21 Specifically, anatomical and functional imaging suggests connections in the prefrontal cortex are involved in the depression process. In TMS, a series of magnetic pulses are administered via the scalp to stimulate neurons in areas of the brain associated with MDD. Early case reports on using TMS to stimulate the prefrontal cortex found significant improvement of symptoms in patients with depression.22 These promising results spurred great interest in the procedure. Over time, the dose and duration of stimulation has increased, along with FDA-approved indications. TMS was first FDA-approved for TRD.23 Although the primary endpoint of the initial clinical trial did not meet criteria for FDA approval, TMS did result in improvement across multiple other measures of depression.23 After the FDA approved the first TMS device, numerous companies began to produce TMS technology. Most of these companies manufacture devices with the figure-of-eight coil, with 1 company producing the Hesed-coil helmet.24
Continue to: An unintended outcome...
An unintended outcome of the increased interest in TMS has been an increased understanding of brain regions involved in psychiatric illness. TMS was able to bring knowledge of mental health from synapses to circuits.25 Work in this area has further stratified the circuits involved in the manifestation of symptom clusters in depression.26 The exact taxonomy of these brain circuits has not been fully realized, but the default mode, salience, attention, cognitive control, and other circuits have been shown to be involved in specific symptom presentations.26,27 These circuits can be hyperactive, hypoactive, hyperconnected, or hypoconnected, with the aberrancies compared to normal controls resulting in symptoms of psychiatric illness.28
This enhanced understanding of brain function has led to further research and development of protocols and subsequent FDA approval of TMS for OCD, anxious depression, and smoking cessation.29 In addition, it has allowed for a proliferation of off-label uses for TMS, including (but not limited to) tinnitus, pain, migraines, and various substance use disorders.30 TMS treatment for these conditions involves stimulation of specific anatomical brain regions that are thought to play a role in the pathology of the target disorder. For example, subthreshold stimulation of the motor cortex has shown some utility in managing symptoms of pain disorders and movement disorders,31,32 the ventromedial prefrontal cortex has been implicated in disorders in the OCD spectrum,33 stimulation of the frontal poles may help treat substance use disorders,34 and the auditory cortex has been a target for treating tinnitus and auditory hallucinations.35
The location of stimulation for treating depression has evolved. The Talairach-Tournoux coordinate system has been used to determine the location of the dorsolateral prefrontal cortex (DLPFC) in relation to the motor cortex. This was measured to be 5 cm from the motor hotspot and subsequently became “the 5.5 cm rule,” taking skull convexity into account. The treatment paradigm for the Hesed coil also uses a measurement from the motor hotspot. Another commonly used methodology for coil placement involves using the 10 to 20 EEG coordinate system to individualize scalp landmarks. In this method, the F3 location corresponds most accurately to the DLPFC target. More recently, using fMRI-guided navigation for coil placement has been shown to lead to a significant reduction in depressive symptoms.36
For depression, the initial recommended course of treatment is 6 weeks, but most improvement is seen in the first 2 to 3 weeks.14 Therefore, many clinicians administer an initial course of 3 weeks unless the response is inadequate, in which case a 6-week course is administered. Many patients require ongoing maintenance treatment, which can be weekly or monthly based on response.37
Research to determine the optimal TMS dose for treating neuropsychiatric symptoms is ongoing. Location, intensity of stimulation, and pulse are the components of stimulation. The pulse can be subdivided into frequency, pattern (single pulse, standard, burst), train (numbers of pulse groups), interval between trains, and total number of pulses per session. The Clinical TMS Society has published TMS protocols.38 The standard intensity of stimulation is 120% of the motor threshold (MT), which is defined as the amount of stimulation over the motor cortex required to produce movement in the extensor hallucis longus. Although treatment for depression traditionally utilizes rapid TMS (3,000 pulses delivered per session at a frequency of 10 Hz in 4-second trains), in controlled studies, accelerated protocols such as intermittent theta burst stimulation (iTBS; standard stimulation parameters: triplet 50 Hz bursts at 5 Hz, with an interval of 8 seconds for 600 pulses per session) have shown noninferiority.36,39
Recent research has explored fMRI-guided iTBS in an even more accelerated format. The Stanford Neuromodulation Therapy trial involved 1,800 pulses per session for 10 sessions a day for 5 days at 90% MT.36 This treatment paradigm was shown to be more effective than standard protocols and was FDA-approved in 2022. Although this specific iTBS protocol exhibited encouraging results, the need for fMRI for adequate delivery might limit its use.
Continue to: Transcranial direct current stimulation
Transcranial direct current stimulation
Therapeutic noninvasive brain stimulation technology is plausible due to the relative lack of adverse effects and ease of administration. In transcranial direct current stimulation (tDCS), a low-intensity, constant electric current is delivered to stimulate the brain via electrodes attached to the scalp. tDCS modulates spontaneous neuronal network activity40,41 and induces polarization of resting membrane potential at the neuronal level,42 though the exact mechanism is yet to be proven. N-methyl-
tDCS has been suggested as a treatment for various psychiatric and medical conditions. However, the small sample sizes and experimental design of published studies have limited tDCS from being clinically recommended.30 No recommendation of Level A (definite efficacy) for its use was found for any indication. Level B recommendation (probable efficacy) was proposed for fibromyalgia, MDD episode without drug resistance, and addiction/craving. Level C recommendation (possible efficacy) is proposed for chronic lower limb neuropathic pain secondary to spinal cord lesion. tDCS was found to be probably ineffective as a treatment for tinnitus and drug-resistant MDD.30 Some research has suggested that tDCS targeting the DLPFC is associated with cognitive improvements in healthy individuals as well as those with schizophrenia.44 tDCS treatment remains experimental and investigational.
Deep brain stimulation
DBS is a neurosurgical procedure that uses electrical current to directly modulate specific areas of the CNS. In terms of accurate, site-specific anatomical targeting, there can be little doubt of the superiority of DBS. DBS involves the placement of leads into the brain parenchyma. Image guidance techniques are used for accurate placement. DBS is a mainstay for the symptomatic treatment of treatment-resistant movement disorders such as Parkinson disease, essential tremor, and some dystonic disorders. It also has been studied as a potential treatment for chronic pain, cluster headache, Huntington disease, and Tourette syndrome.
For treating depression, researched targets include the subgenual cingulate gyrus (SCG), ventral striatum, nucleus accumbens, inferior thalamic peduncle, medial forebrain bundle, and the red nucleus.45 In systematic reviews, improvement of depression is greatest when DBS targets the subgenual cingulate cortex and the medial forebrain bundle.46
The major limitation of DBS for treating depression is the invasive nature of the procedure. Deep TMS can achieve noninvasive stimulation of the SCG and may be associated with fewer risks, fewer adverse events, and less collateral damage. However, given the evolving concept of abnormal neurologic circuits in depression, as our understanding of circuitry in pathological psychiatric processes increases, DBS may be an attractive option for personalized targeting of symptoms in some patients.
DBS may also be beneficial for severe, treatment-resistant OCD. Electrode implantation in the region of the internal capsule/ventral striatum, including the nucleus accumbens, is used47; there is little difference in placement as a treatment for OCD vs for movement disorders.48
Continue to: A critical review of 23 trials...
A critical review of 23 trials and case reports of DBS as a treatment for OCD demonstrated a 47.7% mean reduction in score on the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) and a mean response percentage (minimum 35% Y-BOCS reduction) of 58.2%.49 Most patients regained a normal quality of life after DBS.49 A more rigorous review of 15 meta-analyses of DBS found that conclusions about its efficacy or comparative effectiveness cannot be drawn.50 Because of the nature of neurosurgery, DBS has many potential complications, including cognitive changes, headache, infection, seizures, stroke, and hardware failure.
Vagus nerve stimulation
VNS, in which an implanted device stimulates the left vagus nerve with electrical impulses, was FDA-approved for treating chronic TRD in 2005.51 It had been approved for treatment-resistant epilepsy in 1997. In patients with epilepsy, VNS was shown to improve mood independent of seizure control.52 VNS requires a battery-powered pacemaker device to be implanted under the skin over the anterior chest wall, and a wire tunneled to an electrode is wrapped around the left vagus nerve in the neck.53 The pacemaker is then programmed, monitored, and reprogrammed to optimize response.
VNS is believed to stimulate deep brain nuclei that may play a role in depression.54 The onset of improvement is slow (it may take many months) but in carefully selected patients VNS can provide significant control of TRD. In addition to rare surgery-related complications such as a trauma to the vagal nerve and surrounding tissues (vocal cord paralysis, implant site infection, left facial nerve paralysis and Horner syndrome), VNS may cause hoarseness, dyspnea, and cough related to the intensity of the current output.51 Hypomania and mania were also reported; no suicidal behavior has been associated with VNS.51
Noninvasive vagus nerve stimulationIn noninvasive vagus nerve stimulation (nVNS) or transcutaneous VNS, an external handheld device is applied to the neck overlying the course of the vagus nerve to deliver a sinusoidal alternating current.55 nVNS is currently FDA-approved for treating migraine headaches.55,56 It has demonstrated actions on neurophysiology57 and inflammation in patients with MDD.58 Exploratory research has found a small beneficial effect in patients with depression.59,60 A lack of adequate reproducibility prevents this treatment from being more widely recommended, although attempts to standardize the field are evolving.61
Cranial electrical stimulation
Cranial electrical stimulation (CES) is an older form of electric stimulation developed in the 1970s. In CES, mild electrical pulses are delivered to the ear lobes bilaterally in an episodic fashion (usually 20 to 60 minutes once or twice daily). While CES can be considered a form of neuromodulation, it is not strictly interventional. Patients self-administer CES. The procedure has minimal effects on improving sleep, anxiety, and mood.62-66 Potential adverse effects include a tingling sensation in the ear lobes, lightheadedness, and fogginess. A review and meta-analysis of CES for treating addiction by Kirsch67 showed a wide range of symptoms responding positively to CES treatment, although this study was not peer-reviewed. Because of the low quality of nearly all research that evaluated CES, this form of electric stimulation cannot be viewed as an accepted treatment for any of its listed indications.
Continue to: Other neuromodulation techniques
Other
In addition to the forms of neuromodulation we have already described, there are many other techniques. Several are promising but not yet ready for clinical use. Table 1 and Table 2 summarize the neuromodulation techniques described in this article as well as several that are under development.


Acupuncture
Acupuncture is a Chinese form of medical treatment that began >3,000 years ago; there are written descriptions of it from >2,000 years ago.68 It is based on the belief that there are channels within the body through which the Qi (vital energy or life force) flow, and that inserting fine needles into these channels via the skin can rebalance Qi.68 Modern mechanistic hypotheses invoke involvement of inflammatory or pain pathways.69 Acupuncture frequently uses electric stimulation (electro-acupuncture) to increase the potency of the procedure. Alternatively, in a related procedure (acupressure), pressure can replace the needle. Accreditation in acupuncture generally requires a master’s degree in traditional Chinese medicine but does not require any specific medical training. Acupuncture training courses for physicians are widely available.
All forms of acupuncture are experimental for a wide variety of mental and medical conditions. A meta-analysis found that most research of the utility of acupuncture for depression suffered from various forms of potential bias and was considered low quality.70 Nonetheless, active acupuncture was shown to be minimally superior to placebo acupuncture.70 A meta-analysis of acupuncture for preoperative anxiety71,72 and poststroke insomnia73 reported a similar low study quality. A study of 72 patients with primary insomnia revealed that acupuncture was more effective than sham acupuncture for most sleep measures.74
Psychiatry is increasingly integrating medical tools in addition to psychological tools. Pharmacology remains a cornerstone of biological psychiatry and this will not soon change. However, nonpharmacologic psychiatric treatments such as therapeutic neuromodulation are rapidly emerging. These and novel methods of medication administration may present a challenge to psychiatrists who do not have access to medical personnel or may have forgotten general medical skills.
Our 2-part article has highlighted several interventional psychiatry tools—old and new—that may interest clinicians and benefit patients. As a rule, such treatments are reserved for the most treatment-resistant, challenging psychiatric patients, those with hard-to-treat chronic conditions, and patients who are not helped by more commonly used treatments. An additional complication is that such treatments are frequently not appropriately researched, vetted, or FDA-approved, and therefore are higher risk. Appropriate clinical judgment is always necessary, and potential benefits must be thoroughly weighed against possible adverse effects.
Bottom Line
Several forms of neuromodulation, including electroconvulsive therapy, transcranial magnetic stimulation, transcranial direct current stimulation, deep brain stimulation, and vagus nerve stimulation, may be beneficial for patients with certain treatment-resistant psychiatric disorders, including major depressive disorder and obsessive-compulsive disorder.
Related Resources
- Janicak PG. What’s new in transcranial magnetic stimulation. Current Psychiatry. 2019;18(3):10-16.
- Sharma MS, Ang-Rabanes M, Selek S, et al. Neuromodulatory options for treatment-resistant depression. Current Psychiatry. 2018;17(3):26-28,33-37.
1. Maloney TR, Dilkes-Hall IE, Vlok M, et al. Surgical amputation of a limb 31,000 years ago in Borneo. Nature. 2022;609(7927):547-551. doi:10.1038/s41586-022-05160-8
2. The American Electro-Therapeutic Association. JAMA. 1893;21(14):500. doi:10.1001/jama.1893.02420660030004
3. The American Electro-Therapeutic Association. JAMA. 1894;23(15):590-591. doi:10.1001/jama.1894.02421200024006
4. Wexler A. The medical battery in the United States (1870-1920): electrotherapy at home and in the clinic. J Hist Med Allied Sci. 2017;72(2):166-192. doi:10.1093/jhmas/jrx001
5. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
6. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106-1107. doi:10.1016/s0140-6736(85)92413-4
7. Fink M. Historical article: autobiography of L. J. Meduna. Convuls Ther. 1985;1(1):43-57.
8. Suleman R. A brief history of electroconvulsive therapy. Am J Psychiatry. 2020;16(1):6. doi:10.1176/appi.ajp-rj.2020.160103
9. Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. 2016;321:138-162. doi:10.1016/j.neuroscience.2015.05.053
10. Payne NA, Prudic J. Electroconvulsive therapy: part II: a biopsychosocial perspective. J Psychiatr Pract. 2009;15(5):369-390. doi:10.1097/01.pra.0000361278.73092.85
11. Tirmizi O, Raza A, Trevino K, et al. Electroconvulsive therapy: how modern techniques improve patient outcomes. Current Psychiatry. 2012;11(10):24-46.
12. Kolar D. Current status of electroconvulsive therapy for mood disorders: a clinical review. Evid Based Ment Health. 2017;20(1):12-14. doi:10.1136/eb-2016-102498
13. Andrade C. Active placebo, the parachute meta-analysis, the Nobel Prize, and the efficacy of electroconvulsive therapy. J Clin Psychiatry. 2021;82(2):21f13992. doi:10.4088/JCP.21f13992
14. Giacobbe P, Rakita U, Penner-Goeke K, et al. Improvements in health-related quality of life with electroconvulsive therapy: a meta-analysis. J ECT. 2018;34(2):87-94. doi:10.1097/YCT.0000000000000486
15. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79(12):1162-1172. doi:10.1001/jamapsychiatry.2022.3352
16. Anand A, Mathew SJ, Sanacora G, et al. Ketamine versus ECT for nonpsychotic treatment-resistant major depression. N Engl J Med. 2023. doi: 10.1056/NEJMoa2302399
17. Takamiya A, Seki M, Kudo S, et al. Electroconvulsive therapy for Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2021;36(1):50-58. doi:10.1002/mds.28335
18. Singh R, Sharma R, Prakash J, et al. Magnetic seizure therapy. Ind Psychiatry J. 2021;30(Suppl 1):S320-S321. doi:10.4103/0972-6748.328841
19. Chen M, Yang X, Liu C, et al. Comparative efficacy and cognitive function of magnetic seizure therapy vs. electroconvulsive therapy for major depressive disorder: a systematic review and meta-analysis. Transl Psychiatry. 2021;11(1):437. doi:10.1038/s41398-021-01560-y
20. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi:10.1155/2015/521398
21. George MS, Ketter TA, Post RM. Prefrontal cortex dysfunction in clinical depression. In: Nemeroff CB, Weiss JM, Schatzberg AF, et al, eds. Depression. 2nd ed. Wiley Online Library; 1994:59-72. https://doi.org/10.1002/depr.3050020202
22. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6(14):1853-1856.
23. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
24. Clinical TMS Society. TMS devices. Accessed January 2, 2023. https://www.clinicaltmssociety.org/devices
25. Goldstein-Piekarski AN, Ball TM, Samara Z, et al. Mapping neural circuit biotypes to symptoms and behavioral dimensions of depression and anxiety. Biol Psychiatry. 2022;91(6):561-571. doi:10.1016/j.biopsych.2021.06.024
26. Siddiqi SH, Taylor SF, Cooke D, et al. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am J Psychiatry. 2020;177(5):435-446. doi:10.1176/appi.ajp.2019.19090915
27. Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. 2017;34(1):9-24. doi:10.1002/da.22556
28. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38. doi:10.1038/nm.4246
29. Cohen SL, Bikson M, Badran BW, et al. A visual and narrative timeline of US FDA milestones for transcranial magnetic stimulation (TMS) devices. Brain Stimul. 2022;15(1):73-75. doi:10.1016/j.brs.2021.11.010
30. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56-92. doi:10.1016/j.clinph.2016.10.087
31. Li R, He Y, Qin W, et al. Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson’s disease: a meta-analysis. Neurorehabil Neural Repair. 2022;36(7):395-404. doi:10.1177/15459683221095034
32. Leung A, Shirvalkar P, Chen R, et al. Transcranial magnetic stimulation for pain, headache, and comorbid depression: INS-NANS expert consensus panel review and recommendation. Neuromodulation. 2020;23(3):267-290. doi:10.1111/ner.13094
33. Carmi L, Tendler A, Bystritsky A, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019;176(11):931-938. doi:10.1176/appi.ajp.2019.18101180
34. Harel M, Perini I, Kämpe R, et al. Repetitive transcranial magnetic stimulation in alcohol dependence: a randomized, double-blind, sham-controlled proof-of-concept trial targeting the medial prefrontal and anterior cingulate cortices. Biol Psychiatry. 2022;91(12):1061-1069. doi:10.1016/j.biopsych.2021.11.020
35. Folmer RL, Theodoroff SM, Casiana L, et al. Repetitive transcranial magnetic stimulation treatment for chronic tinnitus: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2015;141(8):716-722. doi:10.1001/jamaoto.2015.1219
36. Cole EJ, Phillips AL, Bentzley BS, et al. Stanford Neuromodulation Therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry. 2022;179(2):132-141. doi:10.1176/appi.ajp.2021.20101429
37. Wilson S, Croarkin PE, Aaronson ST, et al. Systematic review of preservation TMS that includes continuation, maintenance, relapse-prevention, and rescue TMS. J Affect Disord. 2022;296:79-88. doi:10.1016/j.jad.2021.09.040
38. Perera T, George MS, Grammer G, et al. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336-346. doi:10.1016/j.brs.2016.03.010
39. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomized non-inferiority trial. Lancet. 2018;391(10131):1683-1692. doi:10.1016/S0140-6736(18)30295-2
40. Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1(3):206-223. doi:10.1016/j.brs.2008.06.004
41. Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2009;2(4):241-245.
42. Priori A, Berardelli A, Rona S, et al. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9(10):2257-2260. doi:10.1097/00001756-199807130-00020
43. Nitsche MA, Liebetanz D, Antal A, et al. Modulation of cortical excitability by weak direct current stimulation-- technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255-276. doi:10.1016/s1567-424x(09)70230-2
44. Agarwal SM, Venkataram Shivakumar V, et al. Transcranial direct current stimulation in schizophrenia. Clin Psychopharmacol Neurosci. 2013;11(3):118-125.
45. Drobisz D, Damborská A. Deep brain stimulation targets for treating depression. Behav Brain Res. 2019;359:266-273. doi:10.1016/j.bbr.2018.11.004
46. Kisely S, Li A, Warren N, et al. A systematic review and meta-analysis of deep brain stimulation for depression. Depress Anxiety. 2018;35(5):468-480. doi:10.1002/da.22746
47. Blomstedt P, Sjöberg RL, Hansson M, et al. Deep brain stimulation in the treatment of obsessive-compulsive disorder. World Neurosurg. 2013;80(6):e245-e253. doi:10.1016/j.wneu.2012.10.006
48. Denys D, Mantione M, Figee M, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67(10):1061-1068. doi:10.1001/archgenpsychiatry.2010.122
49. van Westen M, Rietveld E, Figee M, et al. Clinical outcome and mechanisms of deep brain stimulation for obsessive-compulsive disorder. Curr Behav Neurosci Rep. 2015;2(2):41-48. doi:10.1007/s40473-015-0036-3
50. Papageorgiou PN, Deschner J, Papageorgiou SN. Effectiveness and adverse effects of deep brain stimulation: umbrella review of meta-analyses. J Neurol Surg A Cent Eur Neurosurg. 2017;78(2):180-190. doi:10.1055/s-0036-1592158
51. O’Reardon JP, Cristancho P, Peshek AD. Vagus nerve stimulation (VNS) and treatment of depression: to the brainstem and beyond. Psychiatry (Edgmont). 2006;3(5):54-63.
52. Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93-99. doi:10.1006/ebeh.2000.0046
53. Giordano F, Zicca A, Barba C, et al. Vagus nerve stimulation: surgical technique of implantation and revision and related morbidity. Epilepsia. 2017;58(S1):85-90. doi:10.1111/epi.13687
54. George MS, Nahas Z, Bohning DE, et al. Mechanisms of action of vagus nerve stimulation (VNS). Clin Neurosci Res. 2004;4(1-2):71-79.
55. Nesbitt AD, Marin JCA, Tompkins E, et al. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology. 2015;84:1249-1253. doi:10.1212/WNL.0000000000001394
56. Goadsby PJ, Grosberg BM, Mauskop A, et al. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia. 2014;34:986-993. doi:10.1177/0333102414524494
57. Fang J, Rong P, Hong Y, et al. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry. 2016;79(4):266-273. doi:10.1016/j.biopsych.2015.03.025
58. Liu CH, Yang MH, Zhang GZ, et al. Neural networks and the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation in depression. J Neuroinflammation. 2020;17(1):54. doi:10.1186/s12974-020-01732-5
59. Hein E, Nowak M, Kiess O, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm (Vienna). 2013;120(5):821-827. doi:10.1007/s00702-012-0908-6
60. Rong P, Liu J, Wang L, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J Affect Disord. 2016;195:172-179. doi:10.1016/j.jad.2016.02.031
61. Farmer AD, Strzelczyk A, Finisguerra A, et al. International consensus based review and recommendations for minimum reporting standards in research on transcutaneous vagus nerve stimulation (Version 2020). Front Hum Neurosci. 2021;14:568051. doi:10.3389/fnhum.2020.568051
62. Amr M, El-Wasify M, Elmaadawi AZ, et al. Cranial electrotherapy stimulation for the treatment of chronically symptomatic bipolar patients. J ECT. 2013;29(2):e31-e32. doi:10.1097/YCT.0b013e31828a344d
63. Kirsch DL, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013;36(1):169-176. doi:10.1016/j.psc.2013.01.006
64. Lande RG, Gragnani C. Efficacy of cranial electric stimulation for the treatment of insomnia: a randomized pilot study. Complement Ther Med. 2013;21(1):8-13. doi:10.1016/j.ctim.2012.11.007
65. Ou Y, Li, C. Sertraline combined alpha-stim clinical observations on the treatment of 30 cases of generalized anxiety disorder. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2015;24(17):73-75.
66. Price L, Briley J, Haltiwanger S, et al. A meta-analysis of cranial electrotherapy stimulation in the treatment of depression. J Psychiatr Res. 2021;135:119-134. doi:10.1016/j.jpsychires.2020.12.043
67. Kirsch D, Gilula M. CES in the treatment of addictions: a review and meta-analysis. Pract Pain Manag. 2007;7(9).
68. Hao JJ, Mittelman M. Acupuncture: past, present, and future. Glob Adv Health Med. 2014;3(4):6-8. doi:10.7453/gahmj.2014.042
69. Napadow V, Ahn A, Longhurst J, et al. The status and future of acupuncture mechanism research. J Altern Complement Med. 2008;14(7):861-869. doi:10.1089/acm.2008.SAR-3
70. Smith CA, Armour M, Lee MS, et al. Acupuncture for depression. Cochrane Database Syst Rev. 2018;3(3):CD004046. doi:10.1002/14651858.CD004046.pub4
71. Tong QY, Liu R, Zhang K, et al. Can acupuncture therapy reduce preoperative anxiety? A systematic review and meta-analysis. J Integr Med. 2021;19(1):20-28. doi:10.1016/j.joim.2020.10.007
72. Usichenko TI, Hua K, Cummings M, et al. Auricular stimulation for preoperative anxiety – a systematic review and meta-analysis of randomized controlled clinical trials. J Clin Anesth. 2022;76:110581. doi:10.1016/j.jclinane.2021.110581
73. Zhou L, Hu X, Yu Z, et al. Efficacy and safety of acupuncture in the treatment of poststroke insomnia: a systematic review and meta-analysis of twenty-six randomized controlled trials. Evid Based Complement Alternat Med. 2022;2022:5188311. doi:10.1155/2022/5188311
74. Yin X, Gou M, Xu J, et al. Efficacy and safety of acupuncture treatment on primary insomnia: a randomized controlled trial. Sleep Med. 2017;37:193-200. doi:10.1016/j.sleep.2017.02.012
1. Maloney TR, Dilkes-Hall IE, Vlok M, et al. Surgical amputation of a limb 31,000 years ago in Borneo. Nature. 2022;609(7927):547-551. doi:10.1038/s41586-022-05160-8
2. The American Electro-Therapeutic Association. JAMA. 1893;21(14):500. doi:10.1001/jama.1893.02420660030004
3. The American Electro-Therapeutic Association. JAMA. 1894;23(15):590-591. doi:10.1001/jama.1894.02421200024006
4. Wexler A. The medical battery in the United States (1870-1920): electrotherapy at home and in the clinic. J Hist Med Allied Sci. 2017;72(2):166-192. doi:10.1093/jhmas/jrx001
5. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
6. Barker AT, Jalinous R, Freeston IL. Non-invasive magnetic stimulation of human motor cortex. Lancet. 1985;1(8437):1106-1107. doi:10.1016/s0140-6736(85)92413-4
7. Fink M. Historical article: autobiography of L. J. Meduna. Convuls Ther. 1985;1(1):43-57.
8. Suleman R. A brief history of electroconvulsive therapy. Am J Psychiatry. 2020;16(1):6. doi:10.1176/appi.ajp-rj.2020.160103
9. Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. 2016;321:138-162. doi:10.1016/j.neuroscience.2015.05.053
10. Payne NA, Prudic J. Electroconvulsive therapy: part II: a biopsychosocial perspective. J Psychiatr Pract. 2009;15(5):369-390. doi:10.1097/01.pra.0000361278.73092.85
11. Tirmizi O, Raza A, Trevino K, et al. Electroconvulsive therapy: how modern techniques improve patient outcomes. Current Psychiatry. 2012;11(10):24-46.
12. Kolar D. Current status of electroconvulsive therapy for mood disorders: a clinical review. Evid Based Ment Health. 2017;20(1):12-14. doi:10.1136/eb-2016-102498
13. Andrade C. Active placebo, the parachute meta-analysis, the Nobel Prize, and the efficacy of electroconvulsive therapy. J Clin Psychiatry. 2021;82(2):21f13992. doi:10.4088/JCP.21f13992
14. Giacobbe P, Rakita U, Penner-Goeke K, et al. Improvements in health-related quality of life with electroconvulsive therapy: a meta-analysis. J ECT. 2018;34(2):87-94. doi:10.1097/YCT.0000000000000486
15. Rhee TG, Shim SR, Forester BP, et al. Efficacy and safety of ketamine vs electroconvulsive therapy among patients with major depressive episode: a systematic review and meta-analysis. JAMA Psychiatry. 2022;79(12):1162-1172. doi:10.1001/jamapsychiatry.2022.3352
16. Anand A, Mathew SJ, Sanacora G, et al. Ketamine versus ECT for nonpsychotic treatment-resistant major depression. N Engl J Med. 2023. doi: 10.1056/NEJMoa2302399
17. Takamiya A, Seki M, Kudo S, et al. Electroconvulsive therapy for Parkinson’s disease: a systematic review and meta-analysis. Mov Disord. 2021;36(1):50-58. doi:10.1002/mds.28335
18. Singh R, Sharma R, Prakash J, et al. Magnetic seizure therapy. Ind Psychiatry J. 2021;30(Suppl 1):S320-S321. doi:10.4103/0972-6748.328841
19. Chen M, Yang X, Liu C, et al. Comparative efficacy and cognitive function of magnetic seizure therapy vs. electroconvulsive therapy for major depressive disorder: a systematic review and meta-analysis. Transl Psychiatry. 2021;11(1):437. doi:10.1038/s41398-021-01560-y
20. Cretaz E, Brunoni AR, Lafer B. Magnetic seizure therapy for unipolar and bipolar depression: a systematic review. Neural Plast. 2015;2015:521398. doi:10.1155/2015/521398
21. George MS, Ketter TA, Post RM. Prefrontal cortex dysfunction in clinical depression. In: Nemeroff CB, Weiss JM, Schatzberg AF, et al, eds. Depression. 2nd ed. Wiley Online Library; 1994:59-72. https://doi.org/10.1002/depr.3050020202
22. George MS, Wassermann EM, Williams WA, et al. Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport. 1995;6(14):1853-1856.
23. O’Reardon JP, Solvason HB, Janicak PG, et al. Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry. 2007;62(11):1208-1216.
24. Clinical TMS Society. TMS devices. Accessed January 2, 2023. https://www.clinicaltmssociety.org/devices
25. Goldstein-Piekarski AN, Ball TM, Samara Z, et al. Mapping neural circuit biotypes to symptoms and behavioral dimensions of depression and anxiety. Biol Psychiatry. 2022;91(6):561-571. doi:10.1016/j.biopsych.2021.06.024
26. Siddiqi SH, Taylor SF, Cooke D, et al. Distinct symptom-specific treatment targets for circuit-based neuromodulation. Am J Psychiatry. 2020;177(5):435-446. doi:10.1176/appi.ajp.2019.19090915
27. Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. 2017;34(1):9-24. doi:10.1002/da.22556
28. Drysdale AT, Grosenick L, Downar J, et al. Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nat Med. 2017;23(1):28-38. doi:10.1038/nm.4246
29. Cohen SL, Bikson M, Badran BW, et al. A visual and narrative timeline of US FDA milestones for transcranial magnetic stimulation (TMS) devices. Brain Stimul. 2022;15(1):73-75. doi:10.1016/j.brs.2021.11.010
30. Lefaucheur JP, Antal A, Ayache SS, et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol. 2017;128(1):56-92. doi:10.1016/j.clinph.2016.10.087
31. Li R, He Y, Qin W, et al. Effects of repetitive transcranial magnetic stimulation on motor symptoms in Parkinson’s disease: a meta-analysis. Neurorehabil Neural Repair. 2022;36(7):395-404. doi:10.1177/15459683221095034
32. Leung A, Shirvalkar P, Chen R, et al. Transcranial magnetic stimulation for pain, headache, and comorbid depression: INS-NANS expert consensus panel review and recommendation. Neuromodulation. 2020;23(3):267-290. doi:10.1111/ner.13094
33. Carmi L, Tendler A, Bystritsky A, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019;176(11):931-938. doi:10.1176/appi.ajp.2019.18101180
34. Harel M, Perini I, Kämpe R, et al. Repetitive transcranial magnetic stimulation in alcohol dependence: a randomized, double-blind, sham-controlled proof-of-concept trial targeting the medial prefrontal and anterior cingulate cortices. Biol Psychiatry. 2022;91(12):1061-1069. doi:10.1016/j.biopsych.2021.11.020
35. Folmer RL, Theodoroff SM, Casiana L, et al. Repetitive transcranial magnetic stimulation treatment for chronic tinnitus: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg. 2015;141(8):716-722. doi:10.1001/jamaoto.2015.1219
36. Cole EJ, Phillips AL, Bentzley BS, et al. Stanford Neuromodulation Therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry. 2022;179(2):132-141. doi:10.1176/appi.ajp.2021.20101429
37. Wilson S, Croarkin PE, Aaronson ST, et al. Systematic review of preservation TMS that includes continuation, maintenance, relapse-prevention, and rescue TMS. J Affect Disord. 2022;296:79-88. doi:10.1016/j.jad.2021.09.040
38. Perera T, George MS, Grammer G, et al. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9(3):336-346. doi:10.1016/j.brs.2016.03.010
39. Blumberger DM, Vila-Rodriguez F, Thorpe KE, et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomized non-inferiority trial. Lancet. 2018;391(10131):1683-1692. doi:10.1016/S0140-6736(18)30295-2
40. Nitsche MA, Cohen LG, Wassermann EM, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimul. 2008;1(3):206-223. doi:10.1016/j.brs.2008.06.004
41. Priori A, Hallett M, Rothwell JC. Repetitive transcranial magnetic stimulation or transcranial direct current stimulation? Brain Stimul. 2009;2(4):241-245.
42. Priori A, Berardelli A, Rona S, et al. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9(10):2257-2260. doi:10.1097/00001756-199807130-00020
43. Nitsche MA, Liebetanz D, Antal A, et al. Modulation of cortical excitability by weak direct current stimulation-- technical, safety and functional aspects. Suppl Clin Neurophysiol. 2003;56:255-276. doi:10.1016/s1567-424x(09)70230-2
44. Agarwal SM, Venkataram Shivakumar V, et al. Transcranial direct current stimulation in schizophrenia. Clin Psychopharmacol Neurosci. 2013;11(3):118-125.
45. Drobisz D, Damborská A. Deep brain stimulation targets for treating depression. Behav Brain Res. 2019;359:266-273. doi:10.1016/j.bbr.2018.11.004
46. Kisely S, Li A, Warren N, et al. A systematic review and meta-analysis of deep brain stimulation for depression. Depress Anxiety. 2018;35(5):468-480. doi:10.1002/da.22746
47. Blomstedt P, Sjöberg RL, Hansson M, et al. Deep brain stimulation in the treatment of obsessive-compulsive disorder. World Neurosurg. 2013;80(6):e245-e253. doi:10.1016/j.wneu.2012.10.006
48. Denys D, Mantione M, Figee M, et al. Deep brain stimulation of the nucleus accumbens for treatment-refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 2010;67(10):1061-1068. doi:10.1001/archgenpsychiatry.2010.122
49. van Westen M, Rietveld E, Figee M, et al. Clinical outcome and mechanisms of deep brain stimulation for obsessive-compulsive disorder. Curr Behav Neurosci Rep. 2015;2(2):41-48. doi:10.1007/s40473-015-0036-3
50. Papageorgiou PN, Deschner J, Papageorgiou SN. Effectiveness and adverse effects of deep brain stimulation: umbrella review of meta-analyses. J Neurol Surg A Cent Eur Neurosurg. 2017;78(2):180-190. doi:10.1055/s-0036-1592158
51. O’Reardon JP, Cristancho P, Peshek AD. Vagus nerve stimulation (VNS) and treatment of depression: to the brainstem and beyond. Psychiatry (Edgmont). 2006;3(5):54-63.
52. Harden CL, Pulver MC, Ravdin LD, et al. A pilot study of mood in epilepsy patients treated with vagus nerve stimulation. Epilepsy Behav. 2000;1(2):93-99. doi:10.1006/ebeh.2000.0046
53. Giordano F, Zicca A, Barba C, et al. Vagus nerve stimulation: surgical technique of implantation and revision and related morbidity. Epilepsia. 2017;58(S1):85-90. doi:10.1111/epi.13687
54. George MS, Nahas Z, Bohning DE, et al. Mechanisms of action of vagus nerve stimulation (VNS). Clin Neurosci Res. 2004;4(1-2):71-79.
55. Nesbitt AD, Marin JCA, Tompkins E, et al. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology. 2015;84:1249-1253. doi:10.1212/WNL.0000000000001394
56. Goadsby PJ, Grosberg BM, Mauskop A, et al. Effect of noninvasive vagus nerve stimulation on acute migraine: an open-label pilot study. Cephalalgia. 2014;34:986-993. doi:10.1177/0333102414524494
57. Fang J, Rong P, Hong Y, et al. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry. 2016;79(4):266-273. doi:10.1016/j.biopsych.2015.03.025
58. Liu CH, Yang MH, Zhang GZ, et al. Neural networks and the anti-inflammatory effect of transcutaneous auricular vagus nerve stimulation in depression. J Neuroinflammation. 2020;17(1):54. doi:10.1186/s12974-020-01732-5
59. Hein E, Nowak M, Kiess O, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm (Vienna). 2013;120(5):821-827. doi:10.1007/s00702-012-0908-6
60. Rong P, Liu J, Wang L, et al. Effect of transcutaneous auricular vagus nerve stimulation on major depressive disorder: a nonrandomized controlled pilot study. J Affect Disord. 2016;195:172-179. doi:10.1016/j.jad.2016.02.031
61. Farmer AD, Strzelczyk A, Finisguerra A, et al. International consensus based review and recommendations for minimum reporting standards in research on transcutaneous vagus nerve stimulation (Version 2020). Front Hum Neurosci. 2021;14:568051. doi:10.3389/fnhum.2020.568051
62. Amr M, El-Wasify M, Elmaadawi AZ, et al. Cranial electrotherapy stimulation for the treatment of chronically symptomatic bipolar patients. J ECT. 2013;29(2):e31-e32. doi:10.1097/YCT.0b013e31828a344d
63. Kirsch DL, Nichols F. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013;36(1):169-176. doi:10.1016/j.psc.2013.01.006
64. Lande RG, Gragnani C. Efficacy of cranial electric stimulation for the treatment of insomnia: a randomized pilot study. Complement Ther Med. 2013;21(1):8-13. doi:10.1016/j.ctim.2012.11.007
65. Ou Y, Li, C. Sertraline combined alpha-stim clinical observations on the treatment of 30 cases of generalized anxiety disorder. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2015;24(17):73-75.
66. Price L, Briley J, Haltiwanger S, et al. A meta-analysis of cranial electrotherapy stimulation in the treatment of depression. J Psychiatr Res. 2021;135:119-134. doi:10.1016/j.jpsychires.2020.12.043
67. Kirsch D, Gilula M. CES in the treatment of addictions: a review and meta-analysis. Pract Pain Manag. 2007;7(9).
68. Hao JJ, Mittelman M. Acupuncture: past, present, and future. Glob Adv Health Med. 2014;3(4):6-8. doi:10.7453/gahmj.2014.042
69. Napadow V, Ahn A, Longhurst J, et al. The status and future of acupuncture mechanism research. J Altern Complement Med. 2008;14(7):861-869. doi:10.1089/acm.2008.SAR-3
70. Smith CA, Armour M, Lee MS, et al. Acupuncture for depression. Cochrane Database Syst Rev. 2018;3(3):CD004046. doi:10.1002/14651858.CD004046.pub4
71. Tong QY, Liu R, Zhang K, et al. Can acupuncture therapy reduce preoperative anxiety? A systematic review and meta-analysis. J Integr Med. 2021;19(1):20-28. doi:10.1016/j.joim.2020.10.007
72. Usichenko TI, Hua K, Cummings M, et al. Auricular stimulation for preoperative anxiety – a systematic review and meta-analysis of randomized controlled clinical trials. J Clin Anesth. 2022;76:110581. doi:10.1016/j.jclinane.2021.110581
73. Zhou L, Hu X, Yu Z, et al. Efficacy and safety of acupuncture in the treatment of poststroke insomnia: a systematic review and meta-analysis of twenty-six randomized controlled trials. Evid Based Complement Alternat Med. 2022;2022:5188311. doi:10.1155/2022/5188311
74. Yin X, Gou M, Xu J, et al. Efficacy and safety of acupuncture treatment on primary insomnia: a randomized controlled trial. Sleep Med. 2017;37:193-200. doi:10.1016/j.sleep.2017.02.012
Interventional psychiatry: What are the next steps?
The explosion of interest in interventional psychiatry is highlighted by 2 recent reviews published in
Psychiatry’s failure to address these changes would be a dire error, as psychiatrists could lose control of our field’s advances and growth. But this creates an even larger question: what are the next steps we need to take? We believe interventional psychiatry must be recognized as its own psychiatric subspeciality, receive greater emphasis in psychiatry residency training, and be subject to standardization by professional organizations.
Psychiatry has incorporated procedures into patient care for almost 100 years, starting with electroconvulsive therapy (ECT) and insulin shock therapy in the 1930s.3,4 However, in the last 10 years, the rapid expansion of FDA approvals of neuromodulation procedures to treat psychiatric conditions (including vagus nerve stimulation in 2005, transcranial magnetic stimulation [TMS] in 2008, and the device exception granted for the use of deep brain stimulation in 2009) has produced the moniker “interventional psychiatry” for this unofficial psychiatric subspeciality.5,6
If we are to establish interventional psychiatry as a recognized subspeciality, it is important to create a universally accepted definition. We propose the term refer to therapeutic techniques or processes that may or may not be invasive but require special training to perform. Additionally, interventional psychiatry should include even minimally invasive procedures, such as ketamine infusions, medication implants, long-acting injectable (LAI) medications, and processes that require a Risk Evaluation and Mitigation Strategy (REMS), such as those utilized with clozapine, esketamine, or olanzapine for extended-release injectable suspension7 (see “Risk Evaluation and Mitigation Strategy programs: How they can be improved”). The proportions of clinicians who prescribe clozapine (7%)8 or LAIs (32.1% to 77.7%, depending on the patient population being treated)9,10 is evidence that the interventional nature of these treatments creates obstacles to their use.
This vacuum of adequate training among psychiatrists has caused interventional psychiatry to grow beyond the confines of the psychiatric field. In most metropolitan areas of the United States, there are clinicians who focus on a specific interventional treatment, such as ketamine infusions or TMS administration. The creation of these specialized clinics has frequently been pioneered by nonpsychiatrists, such as anesthesiologists. This may be attributed to these clinicians’ level of comfort with procedures, or because they possess an infrastructure within their practice that facilitates delivery of the services. In certain states with independent-practice laws, midlevel clinicians are granted permission to open these clinics. However, having nonpsychiatrists provide these treatments to patients with complex psychiatric disorders without psychiatrist involvement makes it less likely that the appropriateness of treatment will be determined, or that the treatment will be incorporated into the patient’s overall biopsychosocial treatment plan.
A gap in training
There is evidence the growth of interventional psychiatry has exceeded the capacity of the current training infrastructure to provide trainees with adequate exposure to these procedures. The Accreditation Council for Graduate Medical Education requires that psychiatry residents be trained in the indications for and use of ECT and neuromodulation therapies but does not provide any specifics about how this training should occur,11 and the Psychiatry Milestones do not indicate how competency in these therapies can be achieved.12 Most trainees have exposure to some interventional treatments, such as ECT or clozapine administration, during residency. However, in 1 survey, only 63% of residents had prescribed clozapine, and 83% indicated they wanted additional experience.13 In a survey of 91 training programs, 75% stated that ECT was required of residents, but 37% estimated that a typical resident would participate in <10 treatments.14 Even more surprising, 27% estimated that the typical resident would care for <5 patients receiving ECT.14
Addressing the changing role of interventional practices in our field must occur on multiple levels, starting with a core curriculum during residency training, expanded learning opportunities for residents with a specific interest in interventional psychiatry, and, most important, a formal interventional psychiatry fellowship leading to certification from the American Board of Medical Specialties.5,6 There are growing numbers of 1-year fellowship programs that offer extensive experiences in neuromodulation and novel pharmacologic treatment and may produce the next generation of leaders in this field. However, training in interventional psychiatry techniques for practicing psychiatrists wishing to expand their treatment offerings is generally quite limited.
Oversight of interventional psychiatry training should be performed by peers. Therefore, creation of an interventional psychiatry society, or a work group within a larger organization, is necessary. While much of this already exists, it is fragmented into associations focused on unique aspects of interventional psychiatry, such as just ECT (eg, International Society for ECT and Neurostimulation), just TMS (eg, Clinical TMS Society), or just ketamine (eg, the American Society of Ketamine Physicians). Despite disparate foci, the goal would be for all to unite into a parent interventional organization that can face these challenges. These organizations have already united a core of individual interventional psychiatrists who can lead psychiatry into the future. They can provide input into guidelines, minimal standards, procedures, protocols, and outcome measures. They also can address any ethical issues that may arise with the use of more invasive treatments.
Change, especially the monumental changes in practice that accompany interventional psychiatry, is both exciting and intimidating. However, certain “growing pains” along the way require urgent consideration. Ultimately, as a field, we either adapt to change or get left behind.
1. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 1). Current Psychiatry. 2023;22(5):25-35. doi:10.12788/cp.0356
2. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 2). Current Psychiatry. 2023;22(7):27-35. doi:10.12788/cp.0364
3. Jones K. Insulin coma therapy in schizophrenia. J R Soc Med. 2000;93(3):147-149. doi:10.1177/014107680009300313
4. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
5. Williams NR, Taylor JJ, Snipes JM, et al. Interventional psychiatry: how should psychiatric educators incorporate neuromodulation into training? Acad Psychiatry. 2014;38(2):168-176. doi:10.1007/s40596-014-0050-x
6. Trapp NT, Williams NR. The future of training and practice in neuromodulation: an interventional psychiatry perspective. Front Psychiatry. 2021;12:734487. doi:10.3389/fpsyt.2021.734487
7. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574. doi:10.1080/00325481.2020.1727671
8. Tang Y, Horvitz-Lennon M, Gellad WF, et al. Prescribing of clozapine and antipsychotic polypharmacy for schizophrenia in a large Medicaid program. Psychiatr Serv. 2017;68(6):579-586. doi:10.1176/appi.ps.201600041
9. Zhdanava M, Starr HL, Lefebvre P, et al. Understanding the health system conditions affecting the use of long-acting injectable antipsychotics in the treatment of schizophrenia in clinical practice: a US healthcare provider survey. Neuropsychiatr Dis Treat. 2022;18:1479-1493. doi:10.2147/NDT.S369494
10. Bunting SR, Chalmers K, Yohanna D, et al. Prescription of long-acting injectable antipsychotic medications among outpatient mental health care service providers. Psychiatr Serv. 2023:appips20220586. doi:10.1176/appi.ps.20220586
11. Accreditation Council for Graduate Medical Education. Common program requirements. July 2022. Accessed June 6, 2023. https://www.acgme.org/programs-and-institutions/programs/common-program-requirements
12. Kinzie JM, DeJong SM, Edgar L, et al. Psychiatry Milestones 2.0: using the supplemental guide to create a shared model of the development of professional identity and expertise. Acad Psychiatry. 2021;45(4):500-505. doi:10.1007/s40596-021-01455-6
13. Singh B, Hughes AJ, Roerig JL. Comfort level and barriers to the appropriate use of clozapine: a preliminary survey of US psychiatric residents. Acad Psychiatry. 2020;44(1):53-58 doi:10.1007/s40596-019-01134-7
14. Dinwiddie SH, Spitz D. Resident education in electroconvulsive therapy. J ECT. 2010;26(4):310-316. doi:10.1097/YCT.0b013e3181cb5f78
The explosion of interest in interventional psychiatry is highlighted by 2 recent reviews published in
Psychiatry’s failure to address these changes would be a dire error, as psychiatrists could lose control of our field’s advances and growth. But this creates an even larger question: what are the next steps we need to take? We believe interventional psychiatry must be recognized as its own psychiatric subspeciality, receive greater emphasis in psychiatry residency training, and be subject to standardization by professional organizations.
Psychiatry has incorporated procedures into patient care for almost 100 years, starting with electroconvulsive therapy (ECT) and insulin shock therapy in the 1930s.3,4 However, in the last 10 years, the rapid expansion of FDA approvals of neuromodulation procedures to treat psychiatric conditions (including vagus nerve stimulation in 2005, transcranial magnetic stimulation [TMS] in 2008, and the device exception granted for the use of deep brain stimulation in 2009) has produced the moniker “interventional psychiatry” for this unofficial psychiatric subspeciality.5,6
If we are to establish interventional psychiatry as a recognized subspeciality, it is important to create a universally accepted definition. We propose the term refer to therapeutic techniques or processes that may or may not be invasive but require special training to perform. Additionally, interventional psychiatry should include even minimally invasive procedures, such as ketamine infusions, medication implants, long-acting injectable (LAI) medications, and processes that require a Risk Evaluation and Mitigation Strategy (REMS), such as those utilized with clozapine, esketamine, or olanzapine for extended-release injectable suspension7 (see “Risk Evaluation and Mitigation Strategy programs: How they can be improved”). The proportions of clinicians who prescribe clozapine (7%)8 or LAIs (32.1% to 77.7%, depending on the patient population being treated)9,10 is evidence that the interventional nature of these treatments creates obstacles to their use.
This vacuum of adequate training among psychiatrists has caused interventional psychiatry to grow beyond the confines of the psychiatric field. In most metropolitan areas of the United States, there are clinicians who focus on a specific interventional treatment, such as ketamine infusions or TMS administration. The creation of these specialized clinics has frequently been pioneered by nonpsychiatrists, such as anesthesiologists. This may be attributed to these clinicians’ level of comfort with procedures, or because they possess an infrastructure within their practice that facilitates delivery of the services. In certain states with independent-practice laws, midlevel clinicians are granted permission to open these clinics. However, having nonpsychiatrists provide these treatments to patients with complex psychiatric disorders without psychiatrist involvement makes it less likely that the appropriateness of treatment will be determined, or that the treatment will be incorporated into the patient’s overall biopsychosocial treatment plan.
A gap in training
There is evidence the growth of interventional psychiatry has exceeded the capacity of the current training infrastructure to provide trainees with adequate exposure to these procedures. The Accreditation Council for Graduate Medical Education requires that psychiatry residents be trained in the indications for and use of ECT and neuromodulation therapies but does not provide any specifics about how this training should occur,11 and the Psychiatry Milestones do not indicate how competency in these therapies can be achieved.12 Most trainees have exposure to some interventional treatments, such as ECT or clozapine administration, during residency. However, in 1 survey, only 63% of residents had prescribed clozapine, and 83% indicated they wanted additional experience.13 In a survey of 91 training programs, 75% stated that ECT was required of residents, but 37% estimated that a typical resident would participate in <10 treatments.14 Even more surprising, 27% estimated that the typical resident would care for <5 patients receiving ECT.14
Addressing the changing role of interventional practices in our field must occur on multiple levels, starting with a core curriculum during residency training, expanded learning opportunities for residents with a specific interest in interventional psychiatry, and, most important, a formal interventional psychiatry fellowship leading to certification from the American Board of Medical Specialties.5,6 There are growing numbers of 1-year fellowship programs that offer extensive experiences in neuromodulation and novel pharmacologic treatment and may produce the next generation of leaders in this field. However, training in interventional psychiatry techniques for practicing psychiatrists wishing to expand their treatment offerings is generally quite limited.
Oversight of interventional psychiatry training should be performed by peers. Therefore, creation of an interventional psychiatry society, or a work group within a larger organization, is necessary. While much of this already exists, it is fragmented into associations focused on unique aspects of interventional psychiatry, such as just ECT (eg, International Society for ECT and Neurostimulation), just TMS (eg, Clinical TMS Society), or just ketamine (eg, the American Society of Ketamine Physicians). Despite disparate foci, the goal would be for all to unite into a parent interventional organization that can face these challenges. These organizations have already united a core of individual interventional psychiatrists who can lead psychiatry into the future. They can provide input into guidelines, minimal standards, procedures, protocols, and outcome measures. They also can address any ethical issues that may arise with the use of more invasive treatments.
Change, especially the monumental changes in practice that accompany interventional psychiatry, is both exciting and intimidating. However, certain “growing pains” along the way require urgent consideration. Ultimately, as a field, we either adapt to change or get left behind.
The explosion of interest in interventional psychiatry is highlighted by 2 recent reviews published in
Psychiatry’s failure to address these changes would be a dire error, as psychiatrists could lose control of our field’s advances and growth. But this creates an even larger question: what are the next steps we need to take? We believe interventional psychiatry must be recognized as its own psychiatric subspeciality, receive greater emphasis in psychiatry residency training, and be subject to standardization by professional organizations.
Psychiatry has incorporated procedures into patient care for almost 100 years, starting with electroconvulsive therapy (ECT) and insulin shock therapy in the 1930s.3,4 However, in the last 10 years, the rapid expansion of FDA approvals of neuromodulation procedures to treat psychiatric conditions (including vagus nerve stimulation in 2005, transcranial magnetic stimulation [TMS] in 2008, and the device exception granted for the use of deep brain stimulation in 2009) has produced the moniker “interventional psychiatry” for this unofficial psychiatric subspeciality.5,6
If we are to establish interventional psychiatry as a recognized subspeciality, it is important to create a universally accepted definition. We propose the term refer to therapeutic techniques or processes that may or may not be invasive but require special training to perform. Additionally, interventional psychiatry should include even minimally invasive procedures, such as ketamine infusions, medication implants, long-acting injectable (LAI) medications, and processes that require a Risk Evaluation and Mitigation Strategy (REMS), such as those utilized with clozapine, esketamine, or olanzapine for extended-release injectable suspension7 (see “Risk Evaluation and Mitigation Strategy programs: How they can be improved”). The proportions of clinicians who prescribe clozapine (7%)8 or LAIs (32.1% to 77.7%, depending on the patient population being treated)9,10 is evidence that the interventional nature of these treatments creates obstacles to their use.
This vacuum of adequate training among psychiatrists has caused interventional psychiatry to grow beyond the confines of the psychiatric field. In most metropolitan areas of the United States, there are clinicians who focus on a specific interventional treatment, such as ketamine infusions or TMS administration. The creation of these specialized clinics has frequently been pioneered by nonpsychiatrists, such as anesthesiologists. This may be attributed to these clinicians’ level of comfort with procedures, or because they possess an infrastructure within their practice that facilitates delivery of the services. In certain states with independent-practice laws, midlevel clinicians are granted permission to open these clinics. However, having nonpsychiatrists provide these treatments to patients with complex psychiatric disorders without psychiatrist involvement makes it less likely that the appropriateness of treatment will be determined, or that the treatment will be incorporated into the patient’s overall biopsychosocial treatment plan.
A gap in training
There is evidence the growth of interventional psychiatry has exceeded the capacity of the current training infrastructure to provide trainees with adequate exposure to these procedures. The Accreditation Council for Graduate Medical Education requires that psychiatry residents be trained in the indications for and use of ECT and neuromodulation therapies but does not provide any specifics about how this training should occur,11 and the Psychiatry Milestones do not indicate how competency in these therapies can be achieved.12 Most trainees have exposure to some interventional treatments, such as ECT or clozapine administration, during residency. However, in 1 survey, only 63% of residents had prescribed clozapine, and 83% indicated they wanted additional experience.13 In a survey of 91 training programs, 75% stated that ECT was required of residents, but 37% estimated that a typical resident would participate in <10 treatments.14 Even more surprising, 27% estimated that the typical resident would care for <5 patients receiving ECT.14
Addressing the changing role of interventional practices in our field must occur on multiple levels, starting with a core curriculum during residency training, expanded learning opportunities for residents with a specific interest in interventional psychiatry, and, most important, a formal interventional psychiatry fellowship leading to certification from the American Board of Medical Specialties.5,6 There are growing numbers of 1-year fellowship programs that offer extensive experiences in neuromodulation and novel pharmacologic treatment and may produce the next generation of leaders in this field. However, training in interventional psychiatry techniques for practicing psychiatrists wishing to expand their treatment offerings is generally quite limited.
Oversight of interventional psychiatry training should be performed by peers. Therefore, creation of an interventional psychiatry society, or a work group within a larger organization, is necessary. While much of this already exists, it is fragmented into associations focused on unique aspects of interventional psychiatry, such as just ECT (eg, International Society for ECT and Neurostimulation), just TMS (eg, Clinical TMS Society), or just ketamine (eg, the American Society of Ketamine Physicians). Despite disparate foci, the goal would be for all to unite into a parent interventional organization that can face these challenges. These organizations have already united a core of individual interventional psychiatrists who can lead psychiatry into the future. They can provide input into guidelines, minimal standards, procedures, protocols, and outcome measures. They also can address any ethical issues that may arise with the use of more invasive treatments.
Change, especially the monumental changes in practice that accompany interventional psychiatry, is both exciting and intimidating. However, certain “growing pains” along the way require urgent consideration. Ultimately, as a field, we either adapt to change or get left behind.
1. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 1). Current Psychiatry. 2023;22(5):25-35. doi:10.12788/cp.0356
2. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 2). Current Psychiatry. 2023;22(7):27-35. doi:10.12788/cp.0364
3. Jones K. Insulin coma therapy in schizophrenia. J R Soc Med. 2000;93(3):147-149. doi:10.1177/014107680009300313
4. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
5. Williams NR, Taylor JJ, Snipes JM, et al. Interventional psychiatry: how should psychiatric educators incorporate neuromodulation into training? Acad Psychiatry. 2014;38(2):168-176. doi:10.1007/s40596-014-0050-x
6. Trapp NT, Williams NR. The future of training and practice in neuromodulation: an interventional psychiatry perspective. Front Psychiatry. 2021;12:734487. doi:10.3389/fpsyt.2021.734487
7. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574. doi:10.1080/00325481.2020.1727671
8. Tang Y, Horvitz-Lennon M, Gellad WF, et al. Prescribing of clozapine and antipsychotic polypharmacy for schizophrenia in a large Medicaid program. Psychiatr Serv. 2017;68(6):579-586. doi:10.1176/appi.ps.201600041
9. Zhdanava M, Starr HL, Lefebvre P, et al. Understanding the health system conditions affecting the use of long-acting injectable antipsychotics in the treatment of schizophrenia in clinical practice: a US healthcare provider survey. Neuropsychiatr Dis Treat. 2022;18:1479-1493. doi:10.2147/NDT.S369494
10. Bunting SR, Chalmers K, Yohanna D, et al. Prescription of long-acting injectable antipsychotic medications among outpatient mental health care service providers. Psychiatr Serv. 2023:appips20220586. doi:10.1176/appi.ps.20220586
11. Accreditation Council for Graduate Medical Education. Common program requirements. July 2022. Accessed June 6, 2023. https://www.acgme.org/programs-and-institutions/programs/common-program-requirements
12. Kinzie JM, DeJong SM, Edgar L, et al. Psychiatry Milestones 2.0: using the supplemental guide to create a shared model of the development of professional identity and expertise. Acad Psychiatry. 2021;45(4):500-505. doi:10.1007/s40596-021-01455-6
13. Singh B, Hughes AJ, Roerig JL. Comfort level and barriers to the appropriate use of clozapine: a preliminary survey of US psychiatric residents. Acad Psychiatry. 2020;44(1):53-58 doi:10.1007/s40596-019-01134-7
14. Dinwiddie SH, Spitz D. Resident education in electroconvulsive therapy. J ECT. 2010;26(4):310-316. doi:10.1097/YCT.0b013e3181cb5f78
1. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 1). Current Psychiatry. 2023;22(5):25-35. doi:10.12788/cp.0356
2. Arbuck D, Farooqui A, El-Mallakh RS. Interventional psychiatry (Part 2). Current Psychiatry. 2023;22(7):27-35. doi:10.12788/cp.0364
3. Jones K. Insulin coma therapy in schizophrenia. J R Soc Med. 2000;93(3):147-149. doi:10.1177/014107680009300313
4. Gazdag G, Ungvari GS. Electroconvulsive therapy: 80 years old and still going strong. World J Psychiatry. 2019;9(1):1-6. doi:10.5498/wjp.v9.i1.1
5. Williams NR, Taylor JJ, Snipes JM, et al. Interventional psychiatry: how should psychiatric educators incorporate neuromodulation into training? Acad Psychiatry. 2014;38(2):168-176. doi:10.1007/s40596-014-0050-x
6. Trapp NT, Williams NR. The future of training and practice in neuromodulation: an interventional psychiatry perspective. Front Psychiatry. 2021;12:734487. doi:10.3389/fpsyt.2021.734487
7. Vincent KM, Ryan M, Palmer E, et al. Interventional psychiatry. Postgrad Med. 2020;132(7):573-574. doi:10.1080/00325481.2020.1727671
8. Tang Y, Horvitz-Lennon M, Gellad WF, et al. Prescribing of clozapine and antipsychotic polypharmacy for schizophrenia in a large Medicaid program. Psychiatr Serv. 2017;68(6):579-586. doi:10.1176/appi.ps.201600041
9. Zhdanava M, Starr HL, Lefebvre P, et al. Understanding the health system conditions affecting the use of long-acting injectable antipsychotics in the treatment of schizophrenia in clinical practice: a US healthcare provider survey. Neuropsychiatr Dis Treat. 2022;18:1479-1493. doi:10.2147/NDT.S369494
10. Bunting SR, Chalmers K, Yohanna D, et al. Prescription of long-acting injectable antipsychotic medications among outpatient mental health care service providers. Psychiatr Serv. 2023:appips20220586. doi:10.1176/appi.ps.20220586
11. Accreditation Council for Graduate Medical Education. Common program requirements. July 2022. Accessed June 6, 2023. https://www.acgme.org/programs-and-institutions/programs/common-program-requirements
12. Kinzie JM, DeJong SM, Edgar L, et al. Psychiatry Milestones 2.0: using the supplemental guide to create a shared model of the development of professional identity and expertise. Acad Psychiatry. 2021;45(4):500-505. doi:10.1007/s40596-021-01455-6
13. Singh B, Hughes AJ, Roerig JL. Comfort level and barriers to the appropriate use of clozapine: a preliminary survey of US psychiatric residents. Acad Psychiatry. 2020;44(1):53-58 doi:10.1007/s40596-019-01134-7
14. Dinwiddie SH, Spitz D. Resident education in electroconvulsive therapy. J ECT. 2010;26(4):310-316. doi:10.1097/YCT.0b013e3181cb5f78




