User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'medstat-accordion-set article-series')]
Optimized Hospital Care for Gout Improves Uptake of Urate-Lowering Therapy
LIVERPOOL, ENGLAND — Optimizing how people experiencing a gout flare are managed in hospital and then followed-up afterwards can substantially increase the uptake of guideline-recommended urate-lowering therapy (ULT), researchers reported at the annual meeting of the British Society for Rheumatology (BSR).
In a prospective study, 92% of 97 people admitted to hospital for gout flares were using ULT within 6 months of discharge after a multifaceted intervention was introduced. By comparison, 49% of 94 people admitted for gout flares before the introduction of the intervention were taking ULT within the same postdischarge time frame.
Moreover, a higher proportion of individuals had urate blood tests done at least once within the 6-month postdischarge period after the intervention’s introduction (58% vs 32%) and fewer (9% vs 15%) needed repeated hospital treatment.
“Gout is the most common inflammatory arthritis affecting one in 30 adults in the United Kingdom, yet it’s one of the most poorly managed,” study investigator Mark D. Russell, MB, BChir, pointed out during a poster presentation.
“There are very effective treatments,” added Dr. Russell, a rheumatology registrar and postdoctoral research fellow at King’s College London in London, England. “Urate-lowering therapies such as allopurinol, which when taken at the correct dose, in the long term, effectively cures patients of their symptoms and prevents complications.”
Dr. Russell said in an interview that there was still work to be done as the rate of people achieving urate levels below the recommended threshold of 360 micromol/L (6 mg/dL) within 6 months was still low, at 27%, even it if was still better than the 11% seen before the intervention was introduced.
Improving the In- and Post-Hospital Pathway
“We developed and implemented an in-hospital management pathway which encouraged urate-lowering therapy initiation prior to discharge, followed by a post-discharge nurse-led review,” Dr. Russell explained.
The in-hospital pathway was based upon BSR, European Alliance of Associations for Rheumatology, and American College of Rheumatology guidelines and involved diagnosing and managing the gout flare appropriately. This may have been via early joint aspiration, medication, or both, as directed by the rheumatology team. Affected individuals also received education and were directed where to obtain further information on the use of ULT. Outpatient follow-up was considered if an individual had severe or tophaceous gout, recurrent episodes, or contraindications or intolerances to ULT. Otherwise, a rheumatology nurse telephoned the individual 2 weeks later to review symptoms and discuss next steps.
The researchers recorded improvements in in-hospital outcomes. The frequency of in-hospital serum urate level measurements rose from 66% in the 12-month preimplementation period to 93% in the 12-month period after the intervention’s introduction. Almost two thirds (62%) of patients were discharged on ULT compared with 18% preimplementation. And gout-specific recommendations were given 86% of the time compared with 59% before the intervention.
Related Work on Gout Incidence
Separately, Dr. Russell also presented data from a nationwide, population-level cohort study that used data from OpenSAFELY, the secure data analytics platform used by the National Health Service in England.
“We did an analysis previously using the CPRD [Clinical Research Practice Datalink], which is another good primary care database, showing that only a third of people with gout in the UK get urate-lowering drugs, when really it should be the vast majority,” he said in the interview.
“And then we wanted to look at, on top of that, what was the impact of the [COVID-19] pandemic,” Russell added. Specifically, the aim was to look at how the pandemic had affected the incidence, management, and prevalence of gout.
Between March 2015 and February 2023, 246,695 new cases of gout were identified among 17.9 million adults, seen in primary and secondary care.
COVID-19 Pandemic Affected Cases
“The number of new cases of gout dropped by about one third in the first year of the pandemic,” Dr. Russell said. Incidence declined from 1.78 to 1.23 per 1000 adults. “Whether that was through people not feeling comfortable going to their GP [general practitioner] or not being able to get an appointment, we don’t know.”
While there was a subsequent increase in new cases of gout since this time, the rates still haven’t reached what they were before the pandemic. This implies that there could be a substantial number of people who may be undiagnosed because of the pandemic, Dr. Russell suggested.
Moreover, he reported that in 2022-2023, the prevalence of gout was 3.21%, up slightly from the 3.07% recorded 7 years earlier in 2015-2016.
ULT Treatment Rates Low
“If you did see a GP, however, so as long as you saw someone, the treatment wasn’t any worse,” Dr. Russell said. Just under 30% of people with incident gout for whom follow-up data were available had initiated ULT within 6 months of their diagnosis. And, of these new starters, around a quarter had a serum urate level below a target of 360 micromol/L.
“This doesn’t detract from the fact that this is pretty low. Despite guidelines, we’re still not getting the majority of people on these very effective urate-lowering drugs,” Dr. Russell said.
There is perhaps too much reliance on modifying diet and lifestyle, he added, which are important for many reasons but will not do much to lower blood urate levels.
As a final word, Dr. Russell said, “It’s not just a case of preventing a bit of joint pain. People get lots of complications when they’re undertreated — erosive joint damage, work disability, impaired quality of life — and yet we’ve got very cheap, well-tolerated drugs.”
The work was independently funded. Dr. Russell acknowledged grant or research support from Eli Lilly, Janssen, Pfizer, and UCB and receipt of honoraria from AbbVie, Biogen, Eli Lilly, Galapagos, and Menarini.
A version of this article appeared on Medscape.com.
LIVERPOOL, ENGLAND — Optimizing how people experiencing a gout flare are managed in hospital and then followed-up afterwards can substantially increase the uptake of guideline-recommended urate-lowering therapy (ULT), researchers reported at the annual meeting of the British Society for Rheumatology (BSR).
In a prospective study, 92% of 97 people admitted to hospital for gout flares were using ULT within 6 months of discharge after a multifaceted intervention was introduced. By comparison, 49% of 94 people admitted for gout flares before the introduction of the intervention were taking ULT within the same postdischarge time frame.
Moreover, a higher proportion of individuals had urate blood tests done at least once within the 6-month postdischarge period after the intervention’s introduction (58% vs 32%) and fewer (9% vs 15%) needed repeated hospital treatment.
“Gout is the most common inflammatory arthritis affecting one in 30 adults in the United Kingdom, yet it’s one of the most poorly managed,” study investigator Mark D. Russell, MB, BChir, pointed out during a poster presentation.
“There are very effective treatments,” added Dr. Russell, a rheumatology registrar and postdoctoral research fellow at King’s College London in London, England. “Urate-lowering therapies such as allopurinol, which when taken at the correct dose, in the long term, effectively cures patients of their symptoms and prevents complications.”
Dr. Russell said in an interview that there was still work to be done as the rate of people achieving urate levels below the recommended threshold of 360 micromol/L (6 mg/dL) within 6 months was still low, at 27%, even it if was still better than the 11% seen before the intervention was introduced.
Improving the In- and Post-Hospital Pathway
“We developed and implemented an in-hospital management pathway which encouraged urate-lowering therapy initiation prior to discharge, followed by a post-discharge nurse-led review,” Dr. Russell explained.
The in-hospital pathway was based upon BSR, European Alliance of Associations for Rheumatology, and American College of Rheumatology guidelines and involved diagnosing and managing the gout flare appropriately. This may have been via early joint aspiration, medication, or both, as directed by the rheumatology team. Affected individuals also received education and were directed where to obtain further information on the use of ULT. Outpatient follow-up was considered if an individual had severe or tophaceous gout, recurrent episodes, or contraindications or intolerances to ULT. Otherwise, a rheumatology nurse telephoned the individual 2 weeks later to review symptoms and discuss next steps.
The researchers recorded improvements in in-hospital outcomes. The frequency of in-hospital serum urate level measurements rose from 66% in the 12-month preimplementation period to 93% in the 12-month period after the intervention’s introduction. Almost two thirds (62%) of patients were discharged on ULT compared with 18% preimplementation. And gout-specific recommendations were given 86% of the time compared with 59% before the intervention.
Related Work on Gout Incidence
Separately, Dr. Russell also presented data from a nationwide, population-level cohort study that used data from OpenSAFELY, the secure data analytics platform used by the National Health Service in England.
“We did an analysis previously using the CPRD [Clinical Research Practice Datalink], which is another good primary care database, showing that only a third of people with gout in the UK get urate-lowering drugs, when really it should be the vast majority,” he said in the interview.
“And then we wanted to look at, on top of that, what was the impact of the [COVID-19] pandemic,” Russell added. Specifically, the aim was to look at how the pandemic had affected the incidence, management, and prevalence of gout.
Between March 2015 and February 2023, 246,695 new cases of gout were identified among 17.9 million adults, seen in primary and secondary care.
COVID-19 Pandemic Affected Cases
“The number of new cases of gout dropped by about one third in the first year of the pandemic,” Dr. Russell said. Incidence declined from 1.78 to 1.23 per 1000 adults. “Whether that was through people not feeling comfortable going to their GP [general practitioner] or not being able to get an appointment, we don’t know.”
While there was a subsequent increase in new cases of gout since this time, the rates still haven’t reached what they were before the pandemic. This implies that there could be a substantial number of people who may be undiagnosed because of the pandemic, Dr. Russell suggested.
Moreover, he reported that in 2022-2023, the prevalence of gout was 3.21%, up slightly from the 3.07% recorded 7 years earlier in 2015-2016.
ULT Treatment Rates Low
“If you did see a GP, however, so as long as you saw someone, the treatment wasn’t any worse,” Dr. Russell said. Just under 30% of people with incident gout for whom follow-up data were available had initiated ULT within 6 months of their diagnosis. And, of these new starters, around a quarter had a serum urate level below a target of 360 micromol/L.
“This doesn’t detract from the fact that this is pretty low. Despite guidelines, we’re still not getting the majority of people on these very effective urate-lowering drugs,” Dr. Russell said.
There is perhaps too much reliance on modifying diet and lifestyle, he added, which are important for many reasons but will not do much to lower blood urate levels.
As a final word, Dr. Russell said, “It’s not just a case of preventing a bit of joint pain. People get lots of complications when they’re undertreated — erosive joint damage, work disability, impaired quality of life — and yet we’ve got very cheap, well-tolerated drugs.”
The work was independently funded. Dr. Russell acknowledged grant or research support from Eli Lilly, Janssen, Pfizer, and UCB and receipt of honoraria from AbbVie, Biogen, Eli Lilly, Galapagos, and Menarini.
A version of this article appeared on Medscape.com.
LIVERPOOL, ENGLAND — Optimizing how people experiencing a gout flare are managed in hospital and then followed-up afterwards can substantially increase the uptake of guideline-recommended urate-lowering therapy (ULT), researchers reported at the annual meeting of the British Society for Rheumatology (BSR).
In a prospective study, 92% of 97 people admitted to hospital for gout flares were using ULT within 6 months of discharge after a multifaceted intervention was introduced. By comparison, 49% of 94 people admitted for gout flares before the introduction of the intervention were taking ULT within the same postdischarge time frame.
Moreover, a higher proportion of individuals had urate blood tests done at least once within the 6-month postdischarge period after the intervention’s introduction (58% vs 32%) and fewer (9% vs 15%) needed repeated hospital treatment.
“Gout is the most common inflammatory arthritis affecting one in 30 adults in the United Kingdom, yet it’s one of the most poorly managed,” study investigator Mark D. Russell, MB, BChir, pointed out during a poster presentation.
“There are very effective treatments,” added Dr. Russell, a rheumatology registrar and postdoctoral research fellow at King’s College London in London, England. “Urate-lowering therapies such as allopurinol, which when taken at the correct dose, in the long term, effectively cures patients of their symptoms and prevents complications.”
Dr. Russell said in an interview that there was still work to be done as the rate of people achieving urate levels below the recommended threshold of 360 micromol/L (6 mg/dL) within 6 months was still low, at 27%, even it if was still better than the 11% seen before the intervention was introduced.
Improving the In- and Post-Hospital Pathway
“We developed and implemented an in-hospital management pathway which encouraged urate-lowering therapy initiation prior to discharge, followed by a post-discharge nurse-led review,” Dr. Russell explained.
The in-hospital pathway was based upon BSR, European Alliance of Associations for Rheumatology, and American College of Rheumatology guidelines and involved diagnosing and managing the gout flare appropriately. This may have been via early joint aspiration, medication, or both, as directed by the rheumatology team. Affected individuals also received education and were directed where to obtain further information on the use of ULT. Outpatient follow-up was considered if an individual had severe or tophaceous gout, recurrent episodes, or contraindications or intolerances to ULT. Otherwise, a rheumatology nurse telephoned the individual 2 weeks later to review symptoms and discuss next steps.
The researchers recorded improvements in in-hospital outcomes. The frequency of in-hospital serum urate level measurements rose from 66% in the 12-month preimplementation period to 93% in the 12-month period after the intervention’s introduction. Almost two thirds (62%) of patients were discharged on ULT compared with 18% preimplementation. And gout-specific recommendations were given 86% of the time compared with 59% before the intervention.
Related Work on Gout Incidence
Separately, Dr. Russell also presented data from a nationwide, population-level cohort study that used data from OpenSAFELY, the secure data analytics platform used by the National Health Service in England.
“We did an analysis previously using the CPRD [Clinical Research Practice Datalink], which is another good primary care database, showing that only a third of people with gout in the UK get urate-lowering drugs, when really it should be the vast majority,” he said in the interview.
“And then we wanted to look at, on top of that, what was the impact of the [COVID-19] pandemic,” Russell added. Specifically, the aim was to look at how the pandemic had affected the incidence, management, and prevalence of gout.
Between March 2015 and February 2023, 246,695 new cases of gout were identified among 17.9 million adults, seen in primary and secondary care.
COVID-19 Pandemic Affected Cases
“The number of new cases of gout dropped by about one third in the first year of the pandemic,” Dr. Russell said. Incidence declined from 1.78 to 1.23 per 1000 adults. “Whether that was through people not feeling comfortable going to their GP [general practitioner] or not being able to get an appointment, we don’t know.”
While there was a subsequent increase in new cases of gout since this time, the rates still haven’t reached what they were before the pandemic. This implies that there could be a substantial number of people who may be undiagnosed because of the pandemic, Dr. Russell suggested.
Moreover, he reported that in 2022-2023, the prevalence of gout was 3.21%, up slightly from the 3.07% recorded 7 years earlier in 2015-2016.
ULT Treatment Rates Low
“If you did see a GP, however, so as long as you saw someone, the treatment wasn’t any worse,” Dr. Russell said. Just under 30% of people with incident gout for whom follow-up data were available had initiated ULT within 6 months of their diagnosis. And, of these new starters, around a quarter had a serum urate level below a target of 360 micromol/L.
“This doesn’t detract from the fact that this is pretty low. Despite guidelines, we’re still not getting the majority of people on these very effective urate-lowering drugs,” Dr. Russell said.
There is perhaps too much reliance on modifying diet and lifestyle, he added, which are important for many reasons but will not do much to lower blood urate levels.
As a final word, Dr. Russell said, “It’s not just a case of preventing a bit of joint pain. People get lots of complications when they’re undertreated — erosive joint damage, work disability, impaired quality of life — and yet we’ve got very cheap, well-tolerated drugs.”
The work was independently funded. Dr. Russell acknowledged grant or research support from Eli Lilly, Janssen, Pfizer, and UCB and receipt of honoraria from AbbVie, Biogen, Eli Lilly, Galapagos, and Menarini.
A version of this article appeared on Medscape.com.
FROM BSR 2024
Blood Biomarkers Predict Knee Osteoarthritis Years in Advance
A small number of blood biomarkers can identify patients who will develop knee osteoarthritis (OA) up to 8 years before signs of the disease are detectable via X-ray, according to new research.
The study “provides more evidence for a pre-radiographic phase of disease,” wrote Virginia Byers Dr. Kraus, MD, PhD, a professor of medicine, pathology, and orthopedic surgery at Duke University School of Medicine in Durham, North Carolina, and colleagues. The results also “provide valuable information for understanding the molecular events of early disease that could inform strategies to develop disease-modifying drugs for preclinical OA,” they continued.
In the study, published in Science Advances, researchers analyzed blood samples from a population-based, longitudinal study of women in London that assessed participants annually for osteoporosis and OA. They selected individuals at low risk for radiographic knee OA, who did not have traditional risk factors for knee OA such as a history of major knee injury, knee surgery, or OA of the hand or opposite knee.
The researchers analyzed serum of 100 women who went on to develop radiographic knee OA and 100 controls who were matched by age and body mass index (BMI). Participants were, on average, aged 54 years with a BMI of 26 and all were White. They analyzed serum peptides via mass spectrometry and used machine learning to select which out of the 115 identified peptides were most predictive of OA.
Ultimately, the team zeroed in on six peptides, corresponding to six proteins, that could most accurately distinguish women who went on to develop radiographic signs of OA from controls (area under the receiver operating characteristic curve, 0.77) up to 8 years before x-rays detected these changes.
“The value of our study is a panel that, in the absence of clinical factors indicative of high-risk knee OA, has the potential to discriminate individuals at risk for incident radiographic knee OA from those not at risk,” the authors wrote.
In earlier work, a similar group of biomarkers could accurately diagnose knee OA as well as predict the progression of the disease. More than half (58%) of biomarkers that predicted incident OA also predicted OA progression.
“Even for the ones that didn’t overlap with OA progression, they all pointed to the same sort of disease process, which is an unresolved acute phase response type of biological process,” Dr. Kraus told this news organization.
Commenting on the study, Andrew Grose, MD, an orthopedic trauma surgeon at the Hospital for Special Surgery in New York City, noted that the methods and conclusions seemed sound but cautioned that the study only looked for radiographic evidence of OA, and not symptomatic OA.
“Clinically relevant OA is correlated with what you see on an x-ray, but the x-ray is definitely not the whole story,” he said in an interview with this news organization.
To be clinically relevant, patients must also have symptoms, such as pain and stiffness, and interfere with daily life. But what shows up on an x-ray is not necessarily indicative of what patients are experiencing, he said. Solely focusing on radiographic findings could lead to overdiagnosis and overtreatment of OA, he said.
The study population was also small, and only included White women, he added, so further validation is necessary. Dr. Kraus and colleagues also acknowledged these limitations.
“Further validation will be needed in independent and larger cohorts, preferability prospectively collected and including male participants and the combination of incident radiographic and symptomatic OA,” they wrote. They noted that while this current study included only women, the biomarkers were not associated with sex in previous studies that used larger and mixed-sex cohorts.
“If they did more studies showing that this [test] was able to predict clinically relevant OA, then I think you could have a meaningful conversation with a patient in a primary care doctor’s office,” Dr. Grose added. “Until that time, just the fact that it predicts an x-ray finding is a little bit of a red herring.”
This work was supported by grants from the National Institutes of Health. Dr. Kraus is an inventor on a patent related to OA progression biomarkers. Dr. Grose had no relevant disclosures.
A version of this article appeared on Medscape.com.
A small number of blood biomarkers can identify patients who will develop knee osteoarthritis (OA) up to 8 years before signs of the disease are detectable via X-ray, according to new research.
The study “provides more evidence for a pre-radiographic phase of disease,” wrote Virginia Byers Dr. Kraus, MD, PhD, a professor of medicine, pathology, and orthopedic surgery at Duke University School of Medicine in Durham, North Carolina, and colleagues. The results also “provide valuable information for understanding the molecular events of early disease that could inform strategies to develop disease-modifying drugs for preclinical OA,” they continued.
In the study, published in Science Advances, researchers analyzed blood samples from a population-based, longitudinal study of women in London that assessed participants annually for osteoporosis and OA. They selected individuals at low risk for radiographic knee OA, who did not have traditional risk factors for knee OA such as a history of major knee injury, knee surgery, or OA of the hand or opposite knee.
The researchers analyzed serum of 100 women who went on to develop radiographic knee OA and 100 controls who were matched by age and body mass index (BMI). Participants were, on average, aged 54 years with a BMI of 26 and all were White. They analyzed serum peptides via mass spectrometry and used machine learning to select which out of the 115 identified peptides were most predictive of OA.
Ultimately, the team zeroed in on six peptides, corresponding to six proteins, that could most accurately distinguish women who went on to develop radiographic signs of OA from controls (area under the receiver operating characteristic curve, 0.77) up to 8 years before x-rays detected these changes.
“The value of our study is a panel that, in the absence of clinical factors indicative of high-risk knee OA, has the potential to discriminate individuals at risk for incident radiographic knee OA from those not at risk,” the authors wrote.
In earlier work, a similar group of biomarkers could accurately diagnose knee OA as well as predict the progression of the disease. More than half (58%) of biomarkers that predicted incident OA also predicted OA progression.
“Even for the ones that didn’t overlap with OA progression, they all pointed to the same sort of disease process, which is an unresolved acute phase response type of biological process,” Dr. Kraus told this news organization.
Commenting on the study, Andrew Grose, MD, an orthopedic trauma surgeon at the Hospital for Special Surgery in New York City, noted that the methods and conclusions seemed sound but cautioned that the study only looked for radiographic evidence of OA, and not symptomatic OA.
“Clinically relevant OA is correlated with what you see on an x-ray, but the x-ray is definitely not the whole story,” he said in an interview with this news organization.
To be clinically relevant, patients must also have symptoms, such as pain and stiffness, and interfere with daily life. But what shows up on an x-ray is not necessarily indicative of what patients are experiencing, he said. Solely focusing on radiographic findings could lead to overdiagnosis and overtreatment of OA, he said.
The study population was also small, and only included White women, he added, so further validation is necessary. Dr. Kraus and colleagues also acknowledged these limitations.
“Further validation will be needed in independent and larger cohorts, preferability prospectively collected and including male participants and the combination of incident radiographic and symptomatic OA,” they wrote. They noted that while this current study included only women, the biomarkers were not associated with sex in previous studies that used larger and mixed-sex cohorts.
“If they did more studies showing that this [test] was able to predict clinically relevant OA, then I think you could have a meaningful conversation with a patient in a primary care doctor’s office,” Dr. Grose added. “Until that time, just the fact that it predicts an x-ray finding is a little bit of a red herring.”
This work was supported by grants from the National Institutes of Health. Dr. Kraus is an inventor on a patent related to OA progression biomarkers. Dr. Grose had no relevant disclosures.
A version of this article appeared on Medscape.com.
A small number of blood biomarkers can identify patients who will develop knee osteoarthritis (OA) up to 8 years before signs of the disease are detectable via X-ray, according to new research.
The study “provides more evidence for a pre-radiographic phase of disease,” wrote Virginia Byers Dr. Kraus, MD, PhD, a professor of medicine, pathology, and orthopedic surgery at Duke University School of Medicine in Durham, North Carolina, and colleagues. The results also “provide valuable information for understanding the molecular events of early disease that could inform strategies to develop disease-modifying drugs for preclinical OA,” they continued.
In the study, published in Science Advances, researchers analyzed blood samples from a population-based, longitudinal study of women in London that assessed participants annually for osteoporosis and OA. They selected individuals at low risk for radiographic knee OA, who did not have traditional risk factors for knee OA such as a history of major knee injury, knee surgery, or OA of the hand or opposite knee.
The researchers analyzed serum of 100 women who went on to develop radiographic knee OA and 100 controls who were matched by age and body mass index (BMI). Participants were, on average, aged 54 years with a BMI of 26 and all were White. They analyzed serum peptides via mass spectrometry and used machine learning to select which out of the 115 identified peptides were most predictive of OA.
Ultimately, the team zeroed in on six peptides, corresponding to six proteins, that could most accurately distinguish women who went on to develop radiographic signs of OA from controls (area under the receiver operating characteristic curve, 0.77) up to 8 years before x-rays detected these changes.
“The value of our study is a panel that, in the absence of clinical factors indicative of high-risk knee OA, has the potential to discriminate individuals at risk for incident radiographic knee OA from those not at risk,” the authors wrote.
In earlier work, a similar group of biomarkers could accurately diagnose knee OA as well as predict the progression of the disease. More than half (58%) of biomarkers that predicted incident OA also predicted OA progression.
“Even for the ones that didn’t overlap with OA progression, they all pointed to the same sort of disease process, which is an unresolved acute phase response type of biological process,” Dr. Kraus told this news organization.
Commenting on the study, Andrew Grose, MD, an orthopedic trauma surgeon at the Hospital for Special Surgery in New York City, noted that the methods and conclusions seemed sound but cautioned that the study only looked for radiographic evidence of OA, and not symptomatic OA.
“Clinically relevant OA is correlated with what you see on an x-ray, but the x-ray is definitely not the whole story,” he said in an interview with this news organization.
To be clinically relevant, patients must also have symptoms, such as pain and stiffness, and interfere with daily life. But what shows up on an x-ray is not necessarily indicative of what patients are experiencing, he said. Solely focusing on radiographic findings could lead to overdiagnosis and overtreatment of OA, he said.
The study population was also small, and only included White women, he added, so further validation is necessary. Dr. Kraus and colleagues also acknowledged these limitations.
“Further validation will be needed in independent and larger cohorts, preferability prospectively collected and including male participants and the combination of incident radiographic and symptomatic OA,” they wrote. They noted that while this current study included only women, the biomarkers were not associated with sex in previous studies that used larger and mixed-sex cohorts.
“If they did more studies showing that this [test] was able to predict clinically relevant OA, then I think you could have a meaningful conversation with a patient in a primary care doctor’s office,” Dr. Grose added. “Until that time, just the fact that it predicts an x-ray finding is a little bit of a red herring.”
This work was supported by grants from the National Institutes of Health. Dr. Kraus is an inventor on a patent related to OA progression biomarkers. Dr. Grose had no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM SCIENCE ADVANCES
New British Behçet’s Disease Guidelines Emphasize Multidisciplinary Management
LIVERPOOL, ENGLAND — The British Society for Rheumatology (BSR) and the British Association of Dermatologists (BAD) have joined forces for the first time to develop the first British guidelines for the management of people living with Behçet’s disease.
The guidelines will also be the first “living guidelines” produced by either society, which means they will be regularly revised and updated when new evidence emerges that warrants inclusion.
With more than 90 recommendations being made, the new guidelines promise to be the most comprehensive and most up-to-date yet for what is regarded as a rare disease. Robert Moots, MBBS, PhD, provided a “sneak peek” of the guidelines at the annual meeting of the British Society for Rheumatology.
Dr. Moots, professor of rheumatology at the University of Liverpool and a consultant rheumatologist for Liverpool University Hospitals NHS Foundation Trust in England, noted that while the European Alliance of Associations for Rheumatology has produced a guideline for Behçet’s disease, this was last updated in 2018 and is not specific for the population for patients that is seen in the United Kingdom.
The British recommendations will cover all possible manifestations of Behçet’s disease and give practical advice on how to manage everything from the most common presentations such as skin lesions, mouth ulcers, and genital ulcers, as well as the potentially more serious eye, neurological, and vascular involvement.
Importance of Raising Awareness
“Joint and musculoskeletal problems are actually one of the least complained of symptoms in people with Behçet’s, and they often can’t understand why a rheumatologist is seeing them,” Dr. Moot said. “But of course, people do get joint problems, they can get enthesitis and arthralgia.”
Dr. Moots has been leading one of the three National Health Service (NHS) Centres of Excellence for Behçet’s Syndrome in England for more than a decade and told this news organization that diagnosing patients could be challenging. It can take up to 10 years from the first symptoms appearing to getting a diagnosis, so part of the job of the NHS Centres of Excellence is to raise awareness among both the healthcare profession and the general public.
“It’s a condition that people learn about at medical school. Most doctors will have come across it, but because it was thought to be really rare in the UK, nobody perhaps really expects to see it,” Dr. Moot said.
“But we all have these patients,” he added. “In Liverpool, we’re commissioned to be looking after an anticipated 150 people with Behçet’s — we’ve got 700. With more awareness, there’s more diagnoses being made, and people are being looked after better.”
Patient Perspective
Tony Thornburn, OBE, chair of the patient advocacy group Behçet’s UK, agreed in a separate interview that raising awareness of the syndrome was key to improving its management.
“Patients have said that it is a bit like having arthritis, lupus, MS [multiple sclerosis], and Crohn’s [disease] all at once,” Mr. Thorburn said. “So what we need is a guideline to ensure that people know what they’re looking at.”
Mr. Thorburn added, “Guidelines are important for raising awareness but also providing the detailed information that clinicians and GPs [general practitioners] need to have to treat a patient when they come in with this multifaceted condition.”
Multifaceted Means Multidisciplinary Management
Because there can be so many different aspects to managing someone with Behçet’s disease, a multispecialty team that was convened to develop the guidelines agreed that multidisciplinary management should be an overarching theme.
“The guideline development group consisted of all the specialties that you would need for a complex multisystem disease like Behçet’s,” Dr. Moot said. He highlighted that working alongside the consultants in adult and pediatric rheumatology were specialists in dermatology, gastroenterology, neurology, ophthalmology, obstetrics and gynecology, and psychology.
“We’re actually looking at psychological interactions and their impact for the first time,” Dr. Moot said, noting that clinicians needed to “take it seriously, and ask about it.”
Management of Manifestations
One of the general principles of the guidelines is to assess the involvement of each organ system and target treatment accordingly.
“One of the problems is that the evidence base to tell us what to do is pretty low,” Dr. Moots acknowledged. There have been few good quality randomized trials, so “treatment tends to be eminence-based rather than evidence-based.”
The recommendation wording bears this in mind, stating whether a treatment should or should not be offered, or just considered if there is no strong evidence to back up its use.
With regard to musculoskeletal manifestations, the recommendations say that colchicine should be offered, perhaps as a first-line option, or an intraarticular steroid injection in the case of monoarthritis. An intramuscular depot steroid may also be appropriate to offer, and there was good evidence to offer azathioprine or, as an alternative in refractory cases, a tumor necrosis factor (TNF) inhibitor. Nonsteroidal anti-inflammatory drugs, methotrexate, apremilast, secukinumab, and referral to a physiotherapist could only be considered, however, based on weaker levels of evidence for their use.
To treat mucocutaneous disease, the guidelines advise offering topical steroids in the form of ointment for genital ulcers or mouthwash or ointment for oral ulcers. For skin lesions, it is recommended to offer colchicine, azathioprine, mycophenolate mofetil, or TNF inhibitor and to consider the use of apremilast, secukinumab, or dapsone.
Future Work and Revision
“One of the key things we would like to see developing is a national registry,” Dr. Moots said. This would include biobanking samples for future research and possible genomic and phenotyping studies.
More work needs to be done in conducting clinical trials in children and young people with Behçet’s disease, studies to find prognostic factors for neurological disease, and clinical trials of potential new drug approaches such as Janus kinase inhibitors. Importantly, an auditing process needs to be set up to see what effect, if any, the guidelines will actually have onpatient management.
“It’s taken 5 years to today” to develop the guidelines, Dr. Moot said. What form the process of updating them will take still has to be decided, he said in the interview. It is likely that the necessary literature searches will be performed every 6 months or so, but it will be a compromise between the ideal situation and having the staffing time to do it.
“It’s a big ask,” Dr. Moot acknowledged, adding that even if updates were only once a year, it would still be much faster than the 5- or 6-year cycle that it traditionally takes for most guidelines to be updated.
The BSR and BAD’s processes for developing guidelines are accredited by the National Institute for Health and Care Excellence in England. Dr. Moots is the chief investigator for the Secukinumab in Behçet’s trial, which is sponsored by the Liverpool University Hospitals NHS Foundation Trust via grant funding from Novartis.
A version of this article appeared on Medscape.com.
LIVERPOOL, ENGLAND — The British Society for Rheumatology (BSR) and the British Association of Dermatologists (BAD) have joined forces for the first time to develop the first British guidelines for the management of people living with Behçet’s disease.
The guidelines will also be the first “living guidelines” produced by either society, which means they will be regularly revised and updated when new evidence emerges that warrants inclusion.
With more than 90 recommendations being made, the new guidelines promise to be the most comprehensive and most up-to-date yet for what is regarded as a rare disease. Robert Moots, MBBS, PhD, provided a “sneak peek” of the guidelines at the annual meeting of the British Society for Rheumatology.
Dr. Moots, professor of rheumatology at the University of Liverpool and a consultant rheumatologist for Liverpool University Hospitals NHS Foundation Trust in England, noted that while the European Alliance of Associations for Rheumatology has produced a guideline for Behçet’s disease, this was last updated in 2018 and is not specific for the population for patients that is seen in the United Kingdom.
The British recommendations will cover all possible manifestations of Behçet’s disease and give practical advice on how to manage everything from the most common presentations such as skin lesions, mouth ulcers, and genital ulcers, as well as the potentially more serious eye, neurological, and vascular involvement.
Importance of Raising Awareness
“Joint and musculoskeletal problems are actually one of the least complained of symptoms in people with Behçet’s, and they often can’t understand why a rheumatologist is seeing them,” Dr. Moot said. “But of course, people do get joint problems, they can get enthesitis and arthralgia.”
Dr. Moots has been leading one of the three National Health Service (NHS) Centres of Excellence for Behçet’s Syndrome in England for more than a decade and told this news organization that diagnosing patients could be challenging. It can take up to 10 years from the first symptoms appearing to getting a diagnosis, so part of the job of the NHS Centres of Excellence is to raise awareness among both the healthcare profession and the general public.
“It’s a condition that people learn about at medical school. Most doctors will have come across it, but because it was thought to be really rare in the UK, nobody perhaps really expects to see it,” Dr. Moot said.
“But we all have these patients,” he added. “In Liverpool, we’re commissioned to be looking after an anticipated 150 people with Behçet’s — we’ve got 700. With more awareness, there’s more diagnoses being made, and people are being looked after better.”
Patient Perspective
Tony Thornburn, OBE, chair of the patient advocacy group Behçet’s UK, agreed in a separate interview that raising awareness of the syndrome was key to improving its management.
“Patients have said that it is a bit like having arthritis, lupus, MS [multiple sclerosis], and Crohn’s [disease] all at once,” Mr. Thorburn said. “So what we need is a guideline to ensure that people know what they’re looking at.”
Mr. Thorburn added, “Guidelines are important for raising awareness but also providing the detailed information that clinicians and GPs [general practitioners] need to have to treat a patient when they come in with this multifaceted condition.”
Multifaceted Means Multidisciplinary Management
Because there can be so many different aspects to managing someone with Behçet’s disease, a multispecialty team that was convened to develop the guidelines agreed that multidisciplinary management should be an overarching theme.
“The guideline development group consisted of all the specialties that you would need for a complex multisystem disease like Behçet’s,” Dr. Moot said. He highlighted that working alongside the consultants in adult and pediatric rheumatology were specialists in dermatology, gastroenterology, neurology, ophthalmology, obstetrics and gynecology, and psychology.
“We’re actually looking at psychological interactions and their impact for the first time,” Dr. Moot said, noting that clinicians needed to “take it seriously, and ask about it.”
Management of Manifestations
One of the general principles of the guidelines is to assess the involvement of each organ system and target treatment accordingly.
“One of the problems is that the evidence base to tell us what to do is pretty low,” Dr. Moots acknowledged. There have been few good quality randomized trials, so “treatment tends to be eminence-based rather than evidence-based.”
The recommendation wording bears this in mind, stating whether a treatment should or should not be offered, or just considered if there is no strong evidence to back up its use.
With regard to musculoskeletal manifestations, the recommendations say that colchicine should be offered, perhaps as a first-line option, or an intraarticular steroid injection in the case of monoarthritis. An intramuscular depot steroid may also be appropriate to offer, and there was good evidence to offer azathioprine or, as an alternative in refractory cases, a tumor necrosis factor (TNF) inhibitor. Nonsteroidal anti-inflammatory drugs, methotrexate, apremilast, secukinumab, and referral to a physiotherapist could only be considered, however, based on weaker levels of evidence for their use.
To treat mucocutaneous disease, the guidelines advise offering topical steroids in the form of ointment for genital ulcers or mouthwash or ointment for oral ulcers. For skin lesions, it is recommended to offer colchicine, azathioprine, mycophenolate mofetil, or TNF inhibitor and to consider the use of apremilast, secukinumab, or dapsone.
Future Work and Revision
“One of the key things we would like to see developing is a national registry,” Dr. Moots said. This would include biobanking samples for future research and possible genomic and phenotyping studies.
More work needs to be done in conducting clinical trials in children and young people with Behçet’s disease, studies to find prognostic factors for neurological disease, and clinical trials of potential new drug approaches such as Janus kinase inhibitors. Importantly, an auditing process needs to be set up to see what effect, if any, the guidelines will actually have onpatient management.
“It’s taken 5 years to today” to develop the guidelines, Dr. Moot said. What form the process of updating them will take still has to be decided, he said in the interview. It is likely that the necessary literature searches will be performed every 6 months or so, but it will be a compromise between the ideal situation and having the staffing time to do it.
“It’s a big ask,” Dr. Moot acknowledged, adding that even if updates were only once a year, it would still be much faster than the 5- or 6-year cycle that it traditionally takes for most guidelines to be updated.
The BSR and BAD’s processes for developing guidelines are accredited by the National Institute for Health and Care Excellence in England. Dr. Moots is the chief investigator for the Secukinumab in Behçet’s trial, which is sponsored by the Liverpool University Hospitals NHS Foundation Trust via grant funding from Novartis.
A version of this article appeared on Medscape.com.
LIVERPOOL, ENGLAND — The British Society for Rheumatology (BSR) and the British Association of Dermatologists (BAD) have joined forces for the first time to develop the first British guidelines for the management of people living with Behçet’s disease.
The guidelines will also be the first “living guidelines” produced by either society, which means they will be regularly revised and updated when new evidence emerges that warrants inclusion.
With more than 90 recommendations being made, the new guidelines promise to be the most comprehensive and most up-to-date yet for what is regarded as a rare disease. Robert Moots, MBBS, PhD, provided a “sneak peek” of the guidelines at the annual meeting of the British Society for Rheumatology.
Dr. Moots, professor of rheumatology at the University of Liverpool and a consultant rheumatologist for Liverpool University Hospitals NHS Foundation Trust in England, noted that while the European Alliance of Associations for Rheumatology has produced a guideline for Behçet’s disease, this was last updated in 2018 and is not specific for the population for patients that is seen in the United Kingdom.
The British recommendations will cover all possible manifestations of Behçet’s disease and give practical advice on how to manage everything from the most common presentations such as skin lesions, mouth ulcers, and genital ulcers, as well as the potentially more serious eye, neurological, and vascular involvement.
Importance of Raising Awareness
“Joint and musculoskeletal problems are actually one of the least complained of symptoms in people with Behçet’s, and they often can’t understand why a rheumatologist is seeing them,” Dr. Moot said. “But of course, people do get joint problems, they can get enthesitis and arthralgia.”
Dr. Moots has been leading one of the three National Health Service (NHS) Centres of Excellence for Behçet’s Syndrome in England for more than a decade and told this news organization that diagnosing patients could be challenging. It can take up to 10 years from the first symptoms appearing to getting a diagnosis, so part of the job of the NHS Centres of Excellence is to raise awareness among both the healthcare profession and the general public.
“It’s a condition that people learn about at medical school. Most doctors will have come across it, but because it was thought to be really rare in the UK, nobody perhaps really expects to see it,” Dr. Moot said.
“But we all have these patients,” he added. “In Liverpool, we’re commissioned to be looking after an anticipated 150 people with Behçet’s — we’ve got 700. With more awareness, there’s more diagnoses being made, and people are being looked after better.”
Patient Perspective
Tony Thornburn, OBE, chair of the patient advocacy group Behçet’s UK, agreed in a separate interview that raising awareness of the syndrome was key to improving its management.
“Patients have said that it is a bit like having arthritis, lupus, MS [multiple sclerosis], and Crohn’s [disease] all at once,” Mr. Thorburn said. “So what we need is a guideline to ensure that people know what they’re looking at.”
Mr. Thorburn added, “Guidelines are important for raising awareness but also providing the detailed information that clinicians and GPs [general practitioners] need to have to treat a patient when they come in with this multifaceted condition.”
Multifaceted Means Multidisciplinary Management
Because there can be so many different aspects to managing someone with Behçet’s disease, a multispecialty team that was convened to develop the guidelines agreed that multidisciplinary management should be an overarching theme.
“The guideline development group consisted of all the specialties that you would need for a complex multisystem disease like Behçet’s,” Dr. Moot said. He highlighted that working alongside the consultants in adult and pediatric rheumatology were specialists in dermatology, gastroenterology, neurology, ophthalmology, obstetrics and gynecology, and psychology.
“We’re actually looking at psychological interactions and their impact for the first time,” Dr. Moot said, noting that clinicians needed to “take it seriously, and ask about it.”
Management of Manifestations
One of the general principles of the guidelines is to assess the involvement of each organ system and target treatment accordingly.
“One of the problems is that the evidence base to tell us what to do is pretty low,” Dr. Moots acknowledged. There have been few good quality randomized trials, so “treatment tends to be eminence-based rather than evidence-based.”
The recommendation wording bears this in mind, stating whether a treatment should or should not be offered, or just considered if there is no strong evidence to back up its use.
With regard to musculoskeletal manifestations, the recommendations say that colchicine should be offered, perhaps as a first-line option, or an intraarticular steroid injection in the case of monoarthritis. An intramuscular depot steroid may also be appropriate to offer, and there was good evidence to offer azathioprine or, as an alternative in refractory cases, a tumor necrosis factor (TNF) inhibitor. Nonsteroidal anti-inflammatory drugs, methotrexate, apremilast, secukinumab, and referral to a physiotherapist could only be considered, however, based on weaker levels of evidence for their use.
To treat mucocutaneous disease, the guidelines advise offering topical steroids in the form of ointment for genital ulcers or mouthwash or ointment for oral ulcers. For skin lesions, it is recommended to offer colchicine, azathioprine, mycophenolate mofetil, or TNF inhibitor and to consider the use of apremilast, secukinumab, or dapsone.
Future Work and Revision
“One of the key things we would like to see developing is a national registry,” Dr. Moots said. This would include biobanking samples for future research and possible genomic and phenotyping studies.
More work needs to be done in conducting clinical trials in children and young people with Behçet’s disease, studies to find prognostic factors for neurological disease, and clinical trials of potential new drug approaches such as Janus kinase inhibitors. Importantly, an auditing process needs to be set up to see what effect, if any, the guidelines will actually have onpatient management.
“It’s taken 5 years to today” to develop the guidelines, Dr. Moot said. What form the process of updating them will take still has to be decided, he said in the interview. It is likely that the necessary literature searches will be performed every 6 months or so, but it will be a compromise between the ideal situation and having the staffing time to do it.
“It’s a big ask,” Dr. Moot acknowledged, adding that even if updates were only once a year, it would still be much faster than the 5- or 6-year cycle that it traditionally takes for most guidelines to be updated.
The BSR and BAD’s processes for developing guidelines are accredited by the National Institute for Health and Care Excellence in England. Dr. Moots is the chief investigator for the Secukinumab in Behçet’s trial, which is sponsored by the Liverpool University Hospitals NHS Foundation Trust via grant funding from Novartis.
A version of this article appeared on Medscape.com.
FROM BSR 2024
Poor Use of ICD-10 Rheumatology Codes Suggests New Approach Needed for ICD-11 Adoption
Inflammatory arthritis codes increased 30-fold in the transition from the ninth to the 10th revision of the International Classification of Diseases (ICD-9 and -10), yet few were used in clinical practice, according to new research.
Most of the new codes for inflammatory arthritis in ICD-10 were rarely used, if at all, from 2015 to 2021.
“About 10-20 codes were comprising the majority of usage for inflammatory arthritis patients in ICD-10,” first author Justin Zhu, a researcher and medical student at Yale University in New Haven, Connecticut, told this news organization. “The other 380 or 400 codes just weren’t seeing a lot of use.”
The findings show the difficulties of transitioning to a new system, he added, and emphasize the need for additional training to improve adoption of ICD-11. The new coding system launched globally in January 2022, but it is not clear when it will be implemented in the United States.
ICD-10 was launched in the United States in 2015, with the goal of enabling greater specificity in identifying health conditions. For example, the new coding system allowed users to include information on laterality and anatomic location for the first time. The total number of codes increased from 14,500 with ICD-9 to 70,000 with ICD-10, with the number of inflammatory arthritis diagnosis codes growing from 14 to 425.
To see how these ICD-10 codes were utilized compared with ICD-9, Zhu and colleagues used national multi-insurance administrative claims data to find inflammatory arthritis diagnostic codes for over 5.1 million patients. About half were coded in ICD-9, while the remaining half were coded in ICD-10. Mr. Zhu and colleagues defined “higher-usage codes” as those that were used more than 1% of the time.
The findings were published in a research letter in JAMA Network Open on April 18.
For ICD-9, four of the available 14 codes (28.6%) were higher-usage codes. In contrast, only nine of the 425 ICD-10 codes (2.1%) were frequently used. Though ICD-10 allowed for increased granularity in diagnosis, data showed that nonspecific codes were most popular. Of the 20 most used ICD-10 arthritis codes, 65% contained “unspecified or other specified” in its wording.
The researchers also found that there was no significant change in these higher-usage codes throughout the study period from 2015 to 2021, suggesting there was not a detectable learning curve in ICD-10 usage among physicians and coders. They also found that clinician specialty did not change code usage patterns.
“The percentage of codes used was not better for rheumatologists (who might be expected to be more refined users of such codes) than primary care clinicians,” Mr. Zhu and colleagues wrote.
Moving to ICD-11 Brings Challenges as Well as Opportunities
Mr. Zhu noted that the study highlights the challenges of adopting new technological systems into daily practice, which can inform the eventual transition to ICD-11.
“There is this need to emphasize training as well as just invest more in improving adoption of ICD-11,” he said.
Michael Pine, MD, MBA, of MJP Healthcare Innovations, LLC in Evanston, Illinois, added that ICD-11 needs to be more user-friendly to be useful in practice. While ICD-10 allowed for greater granularity in coding, it did not result in “usable granularity, in terms of the things doctors really want to communicate,” he told this news organization.
And the transition to ICD-11 could pose greater challenges; rather than ICD-10’s taxonomy system, ICD-11 is formatted as an ontology.
“Although ICD-11 retains some precoordinated codes that convey multifaceted compound concepts, its structure and syntax also provide for post-coordination, a new feature to the ICD that supports the customized combination of concepts and modifier codes to capture previously inaccessible clinical nuance,” he wrote in a coauthored invited commentary.
This added clinical nuance, however, will potentially make coding more complex, he said. One solution is to automate coding, such that clinicians could input information in a natural clinical format that makes sense to them, which would then be translated into ICD-11 code by a program. (This would then be translated back to the user in the natural clinical format to ensure accuracy.)
This type of process would limit how much any one person would need to know about ICD-11 to code diagnoses effectively, while also taking full advantage of the increasing specificity of the new coding system, he said.
Such a program does not yet exist but could be possible with intensive investment in the transition to ICD-11.
The findings of this study serve as a cautionary tale for future transitions to new systems without considering the importance of user experience and usability, Dr. Pine noted. If the United States takes an approach for the adoption of ICD-11 that is similar to that used for ICD-10, it is likely to be “just another overhyped transition” that will make users unwilling to adopt any new system moving forward out of frustration.
But if the United States takes a different, innovative approach, the opposite could be true.
“In short, the US must decide whether it is time to invest considerable resources and effort into a 21st-century information system that could overcome such hindrances as asymmetric information for decision-making, faulty risk adjustment in performance evaluations and payment formulas, and burdens imposed by current coding and documentation practices,” the commentary reads.
“It will allow us to make the best of what computers do and the best of what clinicians do,” Dr. Pine added, “and get them to work together in ways which would not have been conceivable 50 years ago.”
No information on study funding was provided. Mr. Zhu and Dr. Pine did not disclose any competing interests.
A version of this article appeared on Medscape.com.
Inflammatory arthritis codes increased 30-fold in the transition from the ninth to the 10th revision of the International Classification of Diseases (ICD-9 and -10), yet few were used in clinical practice, according to new research.
Most of the new codes for inflammatory arthritis in ICD-10 were rarely used, if at all, from 2015 to 2021.
“About 10-20 codes were comprising the majority of usage for inflammatory arthritis patients in ICD-10,” first author Justin Zhu, a researcher and medical student at Yale University in New Haven, Connecticut, told this news organization. “The other 380 or 400 codes just weren’t seeing a lot of use.”
The findings show the difficulties of transitioning to a new system, he added, and emphasize the need for additional training to improve adoption of ICD-11. The new coding system launched globally in January 2022, but it is not clear when it will be implemented in the United States.
ICD-10 was launched in the United States in 2015, with the goal of enabling greater specificity in identifying health conditions. For example, the new coding system allowed users to include information on laterality and anatomic location for the first time. The total number of codes increased from 14,500 with ICD-9 to 70,000 with ICD-10, with the number of inflammatory arthritis diagnosis codes growing from 14 to 425.
To see how these ICD-10 codes were utilized compared with ICD-9, Zhu and colleagues used national multi-insurance administrative claims data to find inflammatory arthritis diagnostic codes for over 5.1 million patients. About half were coded in ICD-9, while the remaining half were coded in ICD-10. Mr. Zhu and colleagues defined “higher-usage codes” as those that were used more than 1% of the time.
The findings were published in a research letter in JAMA Network Open on April 18.
For ICD-9, four of the available 14 codes (28.6%) were higher-usage codes. In contrast, only nine of the 425 ICD-10 codes (2.1%) were frequently used. Though ICD-10 allowed for increased granularity in diagnosis, data showed that nonspecific codes were most popular. Of the 20 most used ICD-10 arthritis codes, 65% contained “unspecified or other specified” in its wording.
The researchers also found that there was no significant change in these higher-usage codes throughout the study period from 2015 to 2021, suggesting there was not a detectable learning curve in ICD-10 usage among physicians and coders. They also found that clinician specialty did not change code usage patterns.
“The percentage of codes used was not better for rheumatologists (who might be expected to be more refined users of such codes) than primary care clinicians,” Mr. Zhu and colleagues wrote.
Moving to ICD-11 Brings Challenges as Well as Opportunities
Mr. Zhu noted that the study highlights the challenges of adopting new technological systems into daily practice, which can inform the eventual transition to ICD-11.
“There is this need to emphasize training as well as just invest more in improving adoption of ICD-11,” he said.
Michael Pine, MD, MBA, of MJP Healthcare Innovations, LLC in Evanston, Illinois, added that ICD-11 needs to be more user-friendly to be useful in practice. While ICD-10 allowed for greater granularity in coding, it did not result in “usable granularity, in terms of the things doctors really want to communicate,” he told this news organization.
And the transition to ICD-11 could pose greater challenges; rather than ICD-10’s taxonomy system, ICD-11 is formatted as an ontology.
“Although ICD-11 retains some precoordinated codes that convey multifaceted compound concepts, its structure and syntax also provide for post-coordination, a new feature to the ICD that supports the customized combination of concepts and modifier codes to capture previously inaccessible clinical nuance,” he wrote in a coauthored invited commentary.
This added clinical nuance, however, will potentially make coding more complex, he said. One solution is to automate coding, such that clinicians could input information in a natural clinical format that makes sense to them, which would then be translated into ICD-11 code by a program. (This would then be translated back to the user in the natural clinical format to ensure accuracy.)
This type of process would limit how much any one person would need to know about ICD-11 to code diagnoses effectively, while also taking full advantage of the increasing specificity of the new coding system, he said.
Such a program does not yet exist but could be possible with intensive investment in the transition to ICD-11.
The findings of this study serve as a cautionary tale for future transitions to new systems without considering the importance of user experience and usability, Dr. Pine noted. If the United States takes an approach for the adoption of ICD-11 that is similar to that used for ICD-10, it is likely to be “just another overhyped transition” that will make users unwilling to adopt any new system moving forward out of frustration.
But if the United States takes a different, innovative approach, the opposite could be true.
“In short, the US must decide whether it is time to invest considerable resources and effort into a 21st-century information system that could overcome such hindrances as asymmetric information for decision-making, faulty risk adjustment in performance evaluations and payment formulas, and burdens imposed by current coding and documentation practices,” the commentary reads.
“It will allow us to make the best of what computers do and the best of what clinicians do,” Dr. Pine added, “and get them to work together in ways which would not have been conceivable 50 years ago.”
No information on study funding was provided. Mr. Zhu and Dr. Pine did not disclose any competing interests.
A version of this article appeared on Medscape.com.
Inflammatory arthritis codes increased 30-fold in the transition from the ninth to the 10th revision of the International Classification of Diseases (ICD-9 and -10), yet few were used in clinical practice, according to new research.
Most of the new codes for inflammatory arthritis in ICD-10 were rarely used, if at all, from 2015 to 2021.
“About 10-20 codes were comprising the majority of usage for inflammatory arthritis patients in ICD-10,” first author Justin Zhu, a researcher and medical student at Yale University in New Haven, Connecticut, told this news organization. “The other 380 or 400 codes just weren’t seeing a lot of use.”
The findings show the difficulties of transitioning to a new system, he added, and emphasize the need for additional training to improve adoption of ICD-11. The new coding system launched globally in January 2022, but it is not clear when it will be implemented in the United States.
ICD-10 was launched in the United States in 2015, with the goal of enabling greater specificity in identifying health conditions. For example, the new coding system allowed users to include information on laterality and anatomic location for the first time. The total number of codes increased from 14,500 with ICD-9 to 70,000 with ICD-10, with the number of inflammatory arthritis diagnosis codes growing from 14 to 425.
To see how these ICD-10 codes were utilized compared with ICD-9, Zhu and colleagues used national multi-insurance administrative claims data to find inflammatory arthritis diagnostic codes for over 5.1 million patients. About half were coded in ICD-9, while the remaining half were coded in ICD-10. Mr. Zhu and colleagues defined “higher-usage codes” as those that were used more than 1% of the time.
The findings were published in a research letter in JAMA Network Open on April 18.
For ICD-9, four of the available 14 codes (28.6%) were higher-usage codes. In contrast, only nine of the 425 ICD-10 codes (2.1%) were frequently used. Though ICD-10 allowed for increased granularity in diagnosis, data showed that nonspecific codes were most popular. Of the 20 most used ICD-10 arthritis codes, 65% contained “unspecified or other specified” in its wording.
The researchers also found that there was no significant change in these higher-usage codes throughout the study period from 2015 to 2021, suggesting there was not a detectable learning curve in ICD-10 usage among physicians and coders. They also found that clinician specialty did not change code usage patterns.
“The percentage of codes used was not better for rheumatologists (who might be expected to be more refined users of such codes) than primary care clinicians,” Mr. Zhu and colleagues wrote.
Moving to ICD-11 Brings Challenges as Well as Opportunities
Mr. Zhu noted that the study highlights the challenges of adopting new technological systems into daily practice, which can inform the eventual transition to ICD-11.
“There is this need to emphasize training as well as just invest more in improving adoption of ICD-11,” he said.
Michael Pine, MD, MBA, of MJP Healthcare Innovations, LLC in Evanston, Illinois, added that ICD-11 needs to be more user-friendly to be useful in practice. While ICD-10 allowed for greater granularity in coding, it did not result in “usable granularity, in terms of the things doctors really want to communicate,” he told this news organization.
And the transition to ICD-11 could pose greater challenges; rather than ICD-10’s taxonomy system, ICD-11 is formatted as an ontology.
“Although ICD-11 retains some precoordinated codes that convey multifaceted compound concepts, its structure and syntax also provide for post-coordination, a new feature to the ICD that supports the customized combination of concepts and modifier codes to capture previously inaccessible clinical nuance,” he wrote in a coauthored invited commentary.
This added clinical nuance, however, will potentially make coding more complex, he said. One solution is to automate coding, such that clinicians could input information in a natural clinical format that makes sense to them, which would then be translated into ICD-11 code by a program. (This would then be translated back to the user in the natural clinical format to ensure accuracy.)
This type of process would limit how much any one person would need to know about ICD-11 to code diagnoses effectively, while also taking full advantage of the increasing specificity of the new coding system, he said.
Such a program does not yet exist but could be possible with intensive investment in the transition to ICD-11.
The findings of this study serve as a cautionary tale for future transitions to new systems without considering the importance of user experience and usability, Dr. Pine noted. If the United States takes an approach for the adoption of ICD-11 that is similar to that used for ICD-10, it is likely to be “just another overhyped transition” that will make users unwilling to adopt any new system moving forward out of frustration.
But if the United States takes a different, innovative approach, the opposite could be true.
“In short, the US must decide whether it is time to invest considerable resources and effort into a 21st-century information system that could overcome such hindrances as asymmetric information for decision-making, faulty risk adjustment in performance evaluations and payment formulas, and burdens imposed by current coding and documentation practices,” the commentary reads.
“It will allow us to make the best of what computers do and the best of what clinicians do,” Dr. Pine added, “and get them to work together in ways which would not have been conceivable 50 years ago.”
No information on study funding was provided. Mr. Zhu and Dr. Pine did not disclose any competing interests.
A version of this article appeared on Medscape.com.
FROM JAMA NETWORK OPEN
Oregon Physician Assistants Get Name Change
On April 4, Oregon’s Governor Tina Kotek signed a bill into law that officially changed the title of “physician assistants” to “physician associates” in the state.
In the Medscape Physician Assistant Career Satisfaction Report 2023, a diverse range of opinions on the title switch was reflected. Only 40% of PAs favored the name change at the time, 45% neither opposed nor favored it, and 15% opposed the name change, reflecting the complexity of the issue.
According to the AAPA, the change came about to better reflect the work PAs do in not just “assisting” physicians but in working independently with patients. Some also felt that the word “assistant” implies dependence. However, despite associate’s more accurate reflection of the job, PAs mostly remain split on whether they want the new moniker.
Many say that the name change will be confusing for the public and their patients, while others say that physician assistant was already not well understood, as patients often thought of the profession as a doctor’s helper or an assistant, like a medical assistant.
Yet many long-time PAs say that they prefer the title they’ve always had and that explaining to patients the new associate title will be equally confusing. Some mentioned patients may think they’re a business associate of the physician.
Oregon PAs won’t immediately switch to the new name. The new law takes effect on June 6, 2024. The Oregon Medical Board will establish regulations and guidance before PAs adopt the new name in their practices.
The law only changes the name of PAs in Oregon, not in other states. In fact, prematurely using the title of physician associate could subject a PA to regulatory challenges or disciplinary actions.
A version of this article appeared on Medscape.com.
On April 4, Oregon’s Governor Tina Kotek signed a bill into law that officially changed the title of “physician assistants” to “physician associates” in the state.
In the Medscape Physician Assistant Career Satisfaction Report 2023, a diverse range of opinions on the title switch was reflected. Only 40% of PAs favored the name change at the time, 45% neither opposed nor favored it, and 15% opposed the name change, reflecting the complexity of the issue.
According to the AAPA, the change came about to better reflect the work PAs do in not just “assisting” physicians but in working independently with patients. Some also felt that the word “assistant” implies dependence. However, despite associate’s more accurate reflection of the job, PAs mostly remain split on whether they want the new moniker.
Many say that the name change will be confusing for the public and their patients, while others say that physician assistant was already not well understood, as patients often thought of the profession as a doctor’s helper or an assistant, like a medical assistant.
Yet many long-time PAs say that they prefer the title they’ve always had and that explaining to patients the new associate title will be equally confusing. Some mentioned patients may think they’re a business associate of the physician.
Oregon PAs won’t immediately switch to the new name. The new law takes effect on June 6, 2024. The Oregon Medical Board will establish regulations and guidance before PAs adopt the new name in their practices.
The law only changes the name of PAs in Oregon, not in other states. In fact, prematurely using the title of physician associate could subject a PA to regulatory challenges or disciplinary actions.
A version of this article appeared on Medscape.com.
On April 4, Oregon’s Governor Tina Kotek signed a bill into law that officially changed the title of “physician assistants” to “physician associates” in the state.
In the Medscape Physician Assistant Career Satisfaction Report 2023, a diverse range of opinions on the title switch was reflected. Only 40% of PAs favored the name change at the time, 45% neither opposed nor favored it, and 15% opposed the name change, reflecting the complexity of the issue.
According to the AAPA, the change came about to better reflect the work PAs do in not just “assisting” physicians but in working independently with patients. Some also felt that the word “assistant” implies dependence. However, despite associate’s more accurate reflection of the job, PAs mostly remain split on whether they want the new moniker.
Many say that the name change will be confusing for the public and their patients, while others say that physician assistant was already not well understood, as patients often thought of the profession as a doctor’s helper or an assistant, like a medical assistant.
Yet many long-time PAs say that they prefer the title they’ve always had and that explaining to patients the new associate title will be equally confusing. Some mentioned patients may think they’re a business associate of the physician.
Oregon PAs won’t immediately switch to the new name. The new law takes effect on June 6, 2024. The Oregon Medical Board will establish regulations and guidance before PAs adopt the new name in their practices.
The law only changes the name of PAs in Oregon, not in other states. In fact, prematurely using the title of physician associate could subject a PA to regulatory challenges or disciplinary actions.
A version of this article appeared on Medscape.com.
Commentary: Comparisons Among PsA Therapies, May 2024
Bimekizumab is a novel biologic therapy that inhibits interleukin (IL)–17A and IL-17F and is efficacious in the treatment of psoriasis, PsA, and axial spondyloarthritis. In the absence of a formal head-to-head study, matching-adjusted indirect comparison is a method to evaluate comparative effectiveness. Warren and colleagues ran a study that included biological DMARD-naive patients and patients with inadequate response to tumor necrosis factor inhibitors (TNFi-IR) with PsA who received bimekizumab (160 mg every 4 weeks; 431 and 267 patients, respectively) and guselkumab (100 mg every 4 weeks or every 8 weeks; 495 and 189 patients, respectively). They demonstrate that in biological DMARD-naive patients, bimekizumab was associated with a greater likelihood of achieving ≥70% improvement in American College of Rheumatology (ACR) response and minimal disease activity outcome at week 52 compared with guselkumab. Similar outcomes were observed in the TNFi-IR subgroup. Thus, bimekizumab may be more effective than guselkumab in PsA. Formal head-to-head studies comparing bimekizumab vs guselkumab are required.
With the availability of multiple targeted therapies for PsA, choosing the most effective and safe drug for a patient is difficult, especially in the absence of many head-to-head clinical trials. To help address this problem, Lin and Ren conducted a network meta-analysis of head-to-head active comparison studies in PsA. They included 17 studies in their analysis and demonstrated that Janus kinase inhibitors had the highest probability of achieving ACR 20/50/70 response. Treatment with IL-17A inhibitors was more likely than TNFi therapy to lead to resolution of enthesitis and dactylitis and achieving combined ACR 50 and Psoriasis Area Severity Index 100 response. Patients receiving phosphodiesterase 4 inhibitors were least likely to have adverse events. They conclude that when both efficacy and safety are considered, IL-17A inhibitors may be the better agent for initial therapy for PsA. IL-17A inhibitors are indeed safe and efficacious in PsA; more direct head-to-head comparisons as well as strategy trials are required to determine choice of first and subsequent therapy in PsA.
Infections are the most important adverse effects of targeted therapies. The risk for infection in PsA in real-world settings is not well known. In a cohort study that included 12,071 patients with PsA from the French national health insurance database who were new users of targeted therapies (adalimumab, etanercept, golimumab, certolizumab pegol, infliximab, secukinumab, ixekizumab, ustekinumab, and tofacitinib), Bastard and colleagues demonstrated that the incidence of serious infections in users of targeted therapies was 17.0 per 1000 person-years. Compared with new users of adalimumab, the risk for serious infections was significantly lower in new users of etanercept (weighted hazard ratio [wHR] 0.72; 95% CI 0.53-0.97) and ustekinumab (wHR 0.57; 95% CI 0.35-0.93). Thus, the overall risk for serious infections is low, with etanercept and ustekinumab being safer treatment options than adalimumab.
Bimekizumab is a novel biologic therapy that inhibits interleukin (IL)–17A and IL-17F and is efficacious in the treatment of psoriasis, PsA, and axial spondyloarthritis. In the absence of a formal head-to-head study, matching-adjusted indirect comparison is a method to evaluate comparative effectiveness. Warren and colleagues ran a study that included biological DMARD-naive patients and patients with inadequate response to tumor necrosis factor inhibitors (TNFi-IR) with PsA who received bimekizumab (160 mg every 4 weeks; 431 and 267 patients, respectively) and guselkumab (100 mg every 4 weeks or every 8 weeks; 495 and 189 patients, respectively). They demonstrate that in biological DMARD-naive patients, bimekizumab was associated with a greater likelihood of achieving ≥70% improvement in American College of Rheumatology (ACR) response and minimal disease activity outcome at week 52 compared with guselkumab. Similar outcomes were observed in the TNFi-IR subgroup. Thus, bimekizumab may be more effective than guselkumab in PsA. Formal head-to-head studies comparing bimekizumab vs guselkumab are required.
With the availability of multiple targeted therapies for PsA, choosing the most effective and safe drug for a patient is difficult, especially in the absence of many head-to-head clinical trials. To help address this problem, Lin and Ren conducted a network meta-analysis of head-to-head active comparison studies in PsA. They included 17 studies in their analysis and demonstrated that Janus kinase inhibitors had the highest probability of achieving ACR 20/50/70 response. Treatment with IL-17A inhibitors was more likely than TNFi therapy to lead to resolution of enthesitis and dactylitis and achieving combined ACR 50 and Psoriasis Area Severity Index 100 response. Patients receiving phosphodiesterase 4 inhibitors were least likely to have adverse events. They conclude that when both efficacy and safety are considered, IL-17A inhibitors may be the better agent for initial therapy for PsA. IL-17A inhibitors are indeed safe and efficacious in PsA; more direct head-to-head comparisons as well as strategy trials are required to determine choice of first and subsequent therapy in PsA.
Infections are the most important adverse effects of targeted therapies. The risk for infection in PsA in real-world settings is not well known. In a cohort study that included 12,071 patients with PsA from the French national health insurance database who were new users of targeted therapies (adalimumab, etanercept, golimumab, certolizumab pegol, infliximab, secukinumab, ixekizumab, ustekinumab, and tofacitinib), Bastard and colleagues demonstrated that the incidence of serious infections in users of targeted therapies was 17.0 per 1000 person-years. Compared with new users of adalimumab, the risk for serious infections was significantly lower in new users of etanercept (weighted hazard ratio [wHR] 0.72; 95% CI 0.53-0.97) and ustekinumab (wHR 0.57; 95% CI 0.35-0.93). Thus, the overall risk for serious infections is low, with etanercept and ustekinumab being safer treatment options than adalimumab.
Bimekizumab is a novel biologic therapy that inhibits interleukin (IL)–17A and IL-17F and is efficacious in the treatment of psoriasis, PsA, and axial spondyloarthritis. In the absence of a formal head-to-head study, matching-adjusted indirect comparison is a method to evaluate comparative effectiveness. Warren and colleagues ran a study that included biological DMARD-naive patients and patients with inadequate response to tumor necrosis factor inhibitors (TNFi-IR) with PsA who received bimekizumab (160 mg every 4 weeks; 431 and 267 patients, respectively) and guselkumab (100 mg every 4 weeks or every 8 weeks; 495 and 189 patients, respectively). They demonstrate that in biological DMARD-naive patients, bimekizumab was associated with a greater likelihood of achieving ≥70% improvement in American College of Rheumatology (ACR) response and minimal disease activity outcome at week 52 compared with guselkumab. Similar outcomes were observed in the TNFi-IR subgroup. Thus, bimekizumab may be more effective than guselkumab in PsA. Formal head-to-head studies comparing bimekizumab vs guselkumab are required.
With the availability of multiple targeted therapies for PsA, choosing the most effective and safe drug for a patient is difficult, especially in the absence of many head-to-head clinical trials. To help address this problem, Lin and Ren conducted a network meta-analysis of head-to-head active comparison studies in PsA. They included 17 studies in their analysis and demonstrated that Janus kinase inhibitors had the highest probability of achieving ACR 20/50/70 response. Treatment with IL-17A inhibitors was more likely than TNFi therapy to lead to resolution of enthesitis and dactylitis and achieving combined ACR 50 and Psoriasis Area Severity Index 100 response. Patients receiving phosphodiesterase 4 inhibitors were least likely to have adverse events. They conclude that when both efficacy and safety are considered, IL-17A inhibitors may be the better agent for initial therapy for PsA. IL-17A inhibitors are indeed safe and efficacious in PsA; more direct head-to-head comparisons as well as strategy trials are required to determine choice of first and subsequent therapy in PsA.
Infections are the most important adverse effects of targeted therapies. The risk for infection in PsA in real-world settings is not well known. In a cohort study that included 12,071 patients with PsA from the French national health insurance database who were new users of targeted therapies (adalimumab, etanercept, golimumab, certolizumab pegol, infliximab, secukinumab, ixekizumab, ustekinumab, and tofacitinib), Bastard and colleagues demonstrated that the incidence of serious infections in users of targeted therapies was 17.0 per 1000 person-years. Compared with new users of adalimumab, the risk for serious infections was significantly lower in new users of etanercept (weighted hazard ratio [wHR] 0.72; 95% CI 0.53-0.97) and ustekinumab (wHR 0.57; 95% CI 0.35-0.93). Thus, the overall risk for serious infections is low, with etanercept and ustekinumab being safer treatment options than adalimumab.
Are Direct-to-Consumer Microbiome Tests Clinically Useful?
Companies selling gut microbiome tests directly to consumers offer up a variety of claims to promote their products.
“We analyze the trillions of microbes in your gut microflora and craft a unique formula for your unique gut needs,” one says. “Get actionable dietary, supplement, and lifestyle recommendations from our microbiome experts based on your results, tailored to mom and baby’s biomarkers. ... Any family member like dads or siblings are welcome too,” says another.
The companies assert that they can improve gut health by offering individuals personalized treatments based on their gut microbiome test results. The trouble is, no provider, company, or technology can reliably do that yet.
Clinical Implications, Not Applications
The microbiome is the “constellation of microorganisms that call the human body home,” including many strains of bacteria, fungi, and viruses. That constellation comprises some 39 trillion cells.
Although knowledge is increasing on the oral, cutaneous, and vaginal microbiomes, the gut microbiome is arguably the most studied. However, while research is increasingly demonstrating that the gut microbiome has clinical implications, much work needs to be done before reliable applications based on that research are available.
But , Erik C. von Rosenvinge, MD, AGAF, a professor at the University of Maryland School of Medicine and chief of gastroenterology at the VA Maryland Health Care System, Baltimore, said in an interview.
“If you go to their websites, even if it’s not stated overtly, these companies at least give the impression that they’re providing actionable, useful information,” he said. “The sites recommend microbiome testing, and often supplements, probiotics, or other products that they sell. And consumers are told they need to be tested again once they start taking any of these products to see if they’re receiving any benefit.”
Dr. von Rosenvinge and colleagues authored a recent article in Science arguing that DTC microbiome tests “lack analytical and clinical validity” — and yet regulation of the industry has been “generally ignored.” They identified 31 companies globally, 17 of which are based in the United States, claiming to have products and/or services aimed at changing the intestinal microbiome.
Unreliable, Unregulated
The lack of reliability has been shown by experts who have tested the tests.
“People have taken the same stool sample, sent it to multiple companies, and gotten different results back,” Dr. von Rosenvinge said. “People also have taken a stool sample and sent it to the same company under two different names and received two different results. If the test is unreliable at its foundational level, it’s hard to use it in any clinical way.”
Test users’ methods and the companies’ procedures can affect the results, Dina Kao, MD, a professor at the University of Alberta, Edmonton, Alberta, Canada, said in an interview.
“So many biases can be introduced at every single step of the way, starting from how the stool sample was collected and how it’s preserved or not being preserved, because that can introduce a lot of noise that would change the analyses. Which primer they’re using to amplify the signals and which bioinformatic pipeline they use are also important,” said Dr. Kao, who presented at the recent Gut Microbiota for Health World Summit, organized by the American Gastroenterological Association (AGA) and the European Society of Neurogastroenterology and Motility (ESNM).
Different investigators and companies use different technologies, so it’s very difficult to compare them and to create a standard, said Mahmoud Ghannoum, PhD, a professor in the dermatology and pathology departments at Case Western Reserve University School of Medicine and director of the Center for Medical Mycology at University Hospitals in Cleveland.
The complexity of the gut microbiome makes test standardization more difficult than it is when just one organism is involved, Dr. Ghannoum, who chaired the antifungal subcommittee at the Clinical and Laboratory Standards Institute, said in an interview.
“Even though many researchers are focusing on bacteria, we also have fungi and viruses. We need standardization of methods for testing these organisms if we want to have regulations,” said Dr. Ghannoum, a cofounder of BIOHM, a microbiome company that offers nondiagnostic tests and markets a variety of probiotics, prebiotics, and immunity supplements. BIOHM is one of the 31 companies identified by Dr. von Rosenvinge and colleagues, as noted above.
Dr. Ghannoum believes that taking a systematic approach could facilitate standardization and, ultimately, regulation of the DTC microbiome testing products. He and his colleagues described such an approach by outlining the stages for designing probiotics capable of modulating the microbiome in chronic diseases, using Crohn’s disease as a model. Their strategy involved the following steps:
- Using primary microbiome data to identify, by abundance, the microorganisms underlying dysbiosis.
- Gaining insight into the interactions among the identified pathogens.
- Conducting a correlation analysis to identify potential lead probiotic strains that antagonize these pathogens and discovering metabolites that can interrupt their interactions.
- Creating a prototype formulation for testing.
- Validating the efficacy of the candidate formulation via preclinical in vitro and in vivo testing.
- Conducting clinical testing.
Dr. Ghannoum recommends that companies use a similar process “to provide evidence that what they are doing will be helpful, not only for them but also for the reputation of the whole industry.”
Potential Pitfalls
Whether test results from commercial companies are positioned as wellness aids or diagnostic tools, providing advice based on the results “is where the danger can really come in,” Dr. Kao said. “There is still so much we don’t know about which microbial signatures are associated with each condition.”
“Even when we have a solution, like the Crohn’s exclusion diet, a physician doesn’t know enough of the nuances to give advice to a patient,” she said. “That really should be done under the guidance of an expert dietitian. And if a company is selling probiotics, I personally feel that’s not ethical. I’m pretty sure there’s always going to be some kind of conflict of interest.”
Supplements and probiotics are generally safe, but negative consequences can occur, Dr. von Rosenvinge noted.
“We occasionally see people who end up with liver problems as a result of certain supplements, and rarely, probiotics have been associated with infections from those organisms, usually in those with a compromised immune system,” he said.
Other risks include people taking supplements or probiotics when they actually have a medically treatable condition or delays in diagnosis of a potentially serious underlying condition, such as colon cancer, he said. Some patients may stop taking their traditional medication in favor of taking supplements or may experience a drug-supplement interaction if they take both.
What to Tell Patients
“Doctors should be advising against this testing for their patients,” gastroenterologist Colleen R. Kelly, MD, AGAF, Brigham and Women’s Hospital, Boston, said in an interview. “I explain to patients that these tests are not validated and are clinically meaningless data and not worth the money. There is a reason they are not covered by insurance.
“Recommendations to purchase probiotics or supplements manufactured by the testing company to ‘restore a balanced or healthy microbiome’ clearly seem like a scam,” she added. “I believe some of these companies are capitalizing on patients who are desperate for answers to explain chronic symptoms, such as bloating in irritable bowel syndrome.”
Dr. von Rosenvinge said that the message to patients “is that the science isn’t there yet to support using the results of these tests in a meaningful way. We believe the microbiome is very important in health and disease, but the tests themselves in their current state are not as reliable and reproducible as we would like.”
When patients come in with test results, the first question a clinician should ask is what led them to seek out this type of information in the first place, Dr. von Rosenvinge said.
“Our patient focus groups suggested that many have not gotten clear, satisfactory answers from traditional medicine,” he said. “We don’t have a single test that says, yes, you have irritable bowel syndrome, or no, you don’t. We might suggest things that are helpful for some people and are less helpful for others.”
Dr. Kelly said she worries that “there are snake oil salesmen and cons out there who will gladly take your money. These may be smart people, capable of doing very high-level testing, and even producing very detailed and accurate results, but that doesn’t mean we know what to do with them.”
She hopes to see a microbiome-based diagnostic test in the future, particularly if the ability to therapeutically manipulate the gut microbiome in various diseases becomes a reality.
Educate Clinicians, Companies
More education is needed on the subject, so we can become “microbial clinicians,” Dr. Kao said.
“The microbiome never came up when I was going through my medical education,” she said. But we, and the next generation of physicians, “need to at least be able to understand the basics.
“Hopefully, one day, we will be in a position where we can have meaningful interpretations of the test results and make some kind of meaningful dietary interventions,” Dr. Kao added.
As for clinicians who are currently ordering these tests and products directly from the DTC companies, Dr. Kao said, “I roll my eyes.”
Dr. Ghannoum reiterated that companies offering microbiome tests and products also need to be educated and encouraged to use systematic approaches to product development and interpretation.
“Companies should be open to calls from clinicians and be ready to explain findings on a report, as well as the basis for any recommendations,” he said.
Dr. von Rosenvinge, Dr. Kao, and Dr. Kelly had no relevant conflicts of interest. Dr. Ghannoum is a cofounder of BIOHM.
A version of this article appeared on Medscape.com.
Companies selling gut microbiome tests directly to consumers offer up a variety of claims to promote their products.
“We analyze the trillions of microbes in your gut microflora and craft a unique formula for your unique gut needs,” one says. “Get actionable dietary, supplement, and lifestyle recommendations from our microbiome experts based on your results, tailored to mom and baby’s biomarkers. ... Any family member like dads or siblings are welcome too,” says another.
The companies assert that they can improve gut health by offering individuals personalized treatments based on their gut microbiome test results. The trouble is, no provider, company, or technology can reliably do that yet.
Clinical Implications, Not Applications
The microbiome is the “constellation of microorganisms that call the human body home,” including many strains of bacteria, fungi, and viruses. That constellation comprises some 39 trillion cells.
Although knowledge is increasing on the oral, cutaneous, and vaginal microbiomes, the gut microbiome is arguably the most studied. However, while research is increasingly demonstrating that the gut microbiome has clinical implications, much work needs to be done before reliable applications based on that research are available.
But , Erik C. von Rosenvinge, MD, AGAF, a professor at the University of Maryland School of Medicine and chief of gastroenterology at the VA Maryland Health Care System, Baltimore, said in an interview.
“If you go to their websites, even if it’s not stated overtly, these companies at least give the impression that they’re providing actionable, useful information,” he said. “The sites recommend microbiome testing, and often supplements, probiotics, or other products that they sell. And consumers are told they need to be tested again once they start taking any of these products to see if they’re receiving any benefit.”
Dr. von Rosenvinge and colleagues authored a recent article in Science arguing that DTC microbiome tests “lack analytical and clinical validity” — and yet regulation of the industry has been “generally ignored.” They identified 31 companies globally, 17 of which are based in the United States, claiming to have products and/or services aimed at changing the intestinal microbiome.
Unreliable, Unregulated
The lack of reliability has been shown by experts who have tested the tests.
“People have taken the same stool sample, sent it to multiple companies, and gotten different results back,” Dr. von Rosenvinge said. “People also have taken a stool sample and sent it to the same company under two different names and received two different results. If the test is unreliable at its foundational level, it’s hard to use it in any clinical way.”
Test users’ methods and the companies’ procedures can affect the results, Dina Kao, MD, a professor at the University of Alberta, Edmonton, Alberta, Canada, said in an interview.
“So many biases can be introduced at every single step of the way, starting from how the stool sample was collected and how it’s preserved or not being preserved, because that can introduce a lot of noise that would change the analyses. Which primer they’re using to amplify the signals and which bioinformatic pipeline they use are also important,” said Dr. Kao, who presented at the recent Gut Microbiota for Health World Summit, organized by the American Gastroenterological Association (AGA) and the European Society of Neurogastroenterology and Motility (ESNM).
Different investigators and companies use different technologies, so it’s very difficult to compare them and to create a standard, said Mahmoud Ghannoum, PhD, a professor in the dermatology and pathology departments at Case Western Reserve University School of Medicine and director of the Center for Medical Mycology at University Hospitals in Cleveland.
The complexity of the gut microbiome makes test standardization more difficult than it is when just one organism is involved, Dr. Ghannoum, who chaired the antifungal subcommittee at the Clinical and Laboratory Standards Institute, said in an interview.
“Even though many researchers are focusing on bacteria, we also have fungi and viruses. We need standardization of methods for testing these organisms if we want to have regulations,” said Dr. Ghannoum, a cofounder of BIOHM, a microbiome company that offers nondiagnostic tests and markets a variety of probiotics, prebiotics, and immunity supplements. BIOHM is one of the 31 companies identified by Dr. von Rosenvinge and colleagues, as noted above.
Dr. Ghannoum believes that taking a systematic approach could facilitate standardization and, ultimately, regulation of the DTC microbiome testing products. He and his colleagues described such an approach by outlining the stages for designing probiotics capable of modulating the microbiome in chronic diseases, using Crohn’s disease as a model. Their strategy involved the following steps:
- Using primary microbiome data to identify, by abundance, the microorganisms underlying dysbiosis.
- Gaining insight into the interactions among the identified pathogens.
- Conducting a correlation analysis to identify potential lead probiotic strains that antagonize these pathogens and discovering metabolites that can interrupt their interactions.
- Creating a prototype formulation for testing.
- Validating the efficacy of the candidate formulation via preclinical in vitro and in vivo testing.
- Conducting clinical testing.
Dr. Ghannoum recommends that companies use a similar process “to provide evidence that what they are doing will be helpful, not only for them but also for the reputation of the whole industry.”
Potential Pitfalls
Whether test results from commercial companies are positioned as wellness aids or diagnostic tools, providing advice based on the results “is where the danger can really come in,” Dr. Kao said. “There is still so much we don’t know about which microbial signatures are associated with each condition.”
“Even when we have a solution, like the Crohn’s exclusion diet, a physician doesn’t know enough of the nuances to give advice to a patient,” she said. “That really should be done under the guidance of an expert dietitian. And if a company is selling probiotics, I personally feel that’s not ethical. I’m pretty sure there’s always going to be some kind of conflict of interest.”
Supplements and probiotics are generally safe, but negative consequences can occur, Dr. von Rosenvinge noted.
“We occasionally see people who end up with liver problems as a result of certain supplements, and rarely, probiotics have been associated with infections from those organisms, usually in those with a compromised immune system,” he said.
Other risks include people taking supplements or probiotics when they actually have a medically treatable condition or delays in diagnosis of a potentially serious underlying condition, such as colon cancer, he said. Some patients may stop taking their traditional medication in favor of taking supplements or may experience a drug-supplement interaction if they take both.
What to Tell Patients
“Doctors should be advising against this testing for their patients,” gastroenterologist Colleen R. Kelly, MD, AGAF, Brigham and Women’s Hospital, Boston, said in an interview. “I explain to patients that these tests are not validated and are clinically meaningless data and not worth the money. There is a reason they are not covered by insurance.
“Recommendations to purchase probiotics or supplements manufactured by the testing company to ‘restore a balanced or healthy microbiome’ clearly seem like a scam,” she added. “I believe some of these companies are capitalizing on patients who are desperate for answers to explain chronic symptoms, such as bloating in irritable bowel syndrome.”
Dr. von Rosenvinge said that the message to patients “is that the science isn’t there yet to support using the results of these tests in a meaningful way. We believe the microbiome is very important in health and disease, but the tests themselves in their current state are not as reliable and reproducible as we would like.”
When patients come in with test results, the first question a clinician should ask is what led them to seek out this type of information in the first place, Dr. von Rosenvinge said.
“Our patient focus groups suggested that many have not gotten clear, satisfactory answers from traditional medicine,” he said. “We don’t have a single test that says, yes, you have irritable bowel syndrome, or no, you don’t. We might suggest things that are helpful for some people and are less helpful for others.”
Dr. Kelly said she worries that “there are snake oil salesmen and cons out there who will gladly take your money. These may be smart people, capable of doing very high-level testing, and even producing very detailed and accurate results, but that doesn’t mean we know what to do with them.”
She hopes to see a microbiome-based diagnostic test in the future, particularly if the ability to therapeutically manipulate the gut microbiome in various diseases becomes a reality.
Educate Clinicians, Companies
More education is needed on the subject, so we can become “microbial clinicians,” Dr. Kao said.
“The microbiome never came up when I was going through my medical education,” she said. But we, and the next generation of physicians, “need to at least be able to understand the basics.
“Hopefully, one day, we will be in a position where we can have meaningful interpretations of the test results and make some kind of meaningful dietary interventions,” Dr. Kao added.
As for clinicians who are currently ordering these tests and products directly from the DTC companies, Dr. Kao said, “I roll my eyes.”
Dr. Ghannoum reiterated that companies offering microbiome tests and products also need to be educated and encouraged to use systematic approaches to product development and interpretation.
“Companies should be open to calls from clinicians and be ready to explain findings on a report, as well as the basis for any recommendations,” he said.
Dr. von Rosenvinge, Dr. Kao, and Dr. Kelly had no relevant conflicts of interest. Dr. Ghannoum is a cofounder of BIOHM.
A version of this article appeared on Medscape.com.
Companies selling gut microbiome tests directly to consumers offer up a variety of claims to promote their products.
“We analyze the trillions of microbes in your gut microflora and craft a unique formula for your unique gut needs,” one says. “Get actionable dietary, supplement, and lifestyle recommendations from our microbiome experts based on your results, tailored to mom and baby’s biomarkers. ... Any family member like dads or siblings are welcome too,” says another.
The companies assert that they can improve gut health by offering individuals personalized treatments based on their gut microbiome test results. The trouble is, no provider, company, or technology can reliably do that yet.
Clinical Implications, Not Applications
The microbiome is the “constellation of microorganisms that call the human body home,” including many strains of bacteria, fungi, and viruses. That constellation comprises some 39 trillion cells.
Although knowledge is increasing on the oral, cutaneous, and vaginal microbiomes, the gut microbiome is arguably the most studied. However, while research is increasingly demonstrating that the gut microbiome has clinical implications, much work needs to be done before reliable applications based on that research are available.
But , Erik C. von Rosenvinge, MD, AGAF, a professor at the University of Maryland School of Medicine and chief of gastroenterology at the VA Maryland Health Care System, Baltimore, said in an interview.
“If you go to their websites, even if it’s not stated overtly, these companies at least give the impression that they’re providing actionable, useful information,” he said. “The sites recommend microbiome testing, and often supplements, probiotics, or other products that they sell. And consumers are told they need to be tested again once they start taking any of these products to see if they’re receiving any benefit.”
Dr. von Rosenvinge and colleagues authored a recent article in Science arguing that DTC microbiome tests “lack analytical and clinical validity” — and yet regulation of the industry has been “generally ignored.” They identified 31 companies globally, 17 of which are based in the United States, claiming to have products and/or services aimed at changing the intestinal microbiome.
Unreliable, Unregulated
The lack of reliability has been shown by experts who have tested the tests.
“People have taken the same stool sample, sent it to multiple companies, and gotten different results back,” Dr. von Rosenvinge said. “People also have taken a stool sample and sent it to the same company under two different names and received two different results. If the test is unreliable at its foundational level, it’s hard to use it in any clinical way.”
Test users’ methods and the companies’ procedures can affect the results, Dina Kao, MD, a professor at the University of Alberta, Edmonton, Alberta, Canada, said in an interview.
“So many biases can be introduced at every single step of the way, starting from how the stool sample was collected and how it’s preserved or not being preserved, because that can introduce a lot of noise that would change the analyses. Which primer they’re using to amplify the signals and which bioinformatic pipeline they use are also important,” said Dr. Kao, who presented at the recent Gut Microbiota for Health World Summit, organized by the American Gastroenterological Association (AGA) and the European Society of Neurogastroenterology and Motility (ESNM).
Different investigators and companies use different technologies, so it’s very difficult to compare them and to create a standard, said Mahmoud Ghannoum, PhD, a professor in the dermatology and pathology departments at Case Western Reserve University School of Medicine and director of the Center for Medical Mycology at University Hospitals in Cleveland.
The complexity of the gut microbiome makes test standardization more difficult than it is when just one organism is involved, Dr. Ghannoum, who chaired the antifungal subcommittee at the Clinical and Laboratory Standards Institute, said in an interview.
“Even though many researchers are focusing on bacteria, we also have fungi and viruses. We need standardization of methods for testing these organisms if we want to have regulations,” said Dr. Ghannoum, a cofounder of BIOHM, a microbiome company that offers nondiagnostic tests and markets a variety of probiotics, prebiotics, and immunity supplements. BIOHM is one of the 31 companies identified by Dr. von Rosenvinge and colleagues, as noted above.
Dr. Ghannoum believes that taking a systematic approach could facilitate standardization and, ultimately, regulation of the DTC microbiome testing products. He and his colleagues described such an approach by outlining the stages for designing probiotics capable of modulating the microbiome in chronic diseases, using Crohn’s disease as a model. Their strategy involved the following steps:
- Using primary microbiome data to identify, by abundance, the microorganisms underlying dysbiosis.
- Gaining insight into the interactions among the identified pathogens.
- Conducting a correlation analysis to identify potential lead probiotic strains that antagonize these pathogens and discovering metabolites that can interrupt their interactions.
- Creating a prototype formulation for testing.
- Validating the efficacy of the candidate formulation via preclinical in vitro and in vivo testing.
- Conducting clinical testing.
Dr. Ghannoum recommends that companies use a similar process “to provide evidence that what they are doing will be helpful, not only for them but also for the reputation of the whole industry.”
Potential Pitfalls
Whether test results from commercial companies are positioned as wellness aids or diagnostic tools, providing advice based on the results “is where the danger can really come in,” Dr. Kao said. “There is still so much we don’t know about which microbial signatures are associated with each condition.”
“Even when we have a solution, like the Crohn’s exclusion diet, a physician doesn’t know enough of the nuances to give advice to a patient,” she said. “That really should be done under the guidance of an expert dietitian. And if a company is selling probiotics, I personally feel that’s not ethical. I’m pretty sure there’s always going to be some kind of conflict of interest.”
Supplements and probiotics are generally safe, but negative consequences can occur, Dr. von Rosenvinge noted.
“We occasionally see people who end up with liver problems as a result of certain supplements, and rarely, probiotics have been associated with infections from those organisms, usually in those with a compromised immune system,” he said.
Other risks include people taking supplements or probiotics when they actually have a medically treatable condition or delays in diagnosis of a potentially serious underlying condition, such as colon cancer, he said. Some patients may stop taking their traditional medication in favor of taking supplements or may experience a drug-supplement interaction if they take both.
What to Tell Patients
“Doctors should be advising against this testing for their patients,” gastroenterologist Colleen R. Kelly, MD, AGAF, Brigham and Women’s Hospital, Boston, said in an interview. “I explain to patients that these tests are not validated and are clinically meaningless data and not worth the money. There is a reason they are not covered by insurance.
“Recommendations to purchase probiotics or supplements manufactured by the testing company to ‘restore a balanced or healthy microbiome’ clearly seem like a scam,” she added. “I believe some of these companies are capitalizing on patients who are desperate for answers to explain chronic symptoms, such as bloating in irritable bowel syndrome.”
Dr. von Rosenvinge said that the message to patients “is that the science isn’t there yet to support using the results of these tests in a meaningful way. We believe the microbiome is very important in health and disease, but the tests themselves in their current state are not as reliable and reproducible as we would like.”
When patients come in with test results, the first question a clinician should ask is what led them to seek out this type of information in the first place, Dr. von Rosenvinge said.
“Our patient focus groups suggested that many have not gotten clear, satisfactory answers from traditional medicine,” he said. “We don’t have a single test that says, yes, you have irritable bowel syndrome, or no, you don’t. We might suggest things that are helpful for some people and are less helpful for others.”
Dr. Kelly said she worries that “there are snake oil salesmen and cons out there who will gladly take your money. These may be smart people, capable of doing very high-level testing, and even producing very detailed and accurate results, but that doesn’t mean we know what to do with them.”
She hopes to see a microbiome-based diagnostic test in the future, particularly if the ability to therapeutically manipulate the gut microbiome in various diseases becomes a reality.
Educate Clinicians, Companies
More education is needed on the subject, so we can become “microbial clinicians,” Dr. Kao said.
“The microbiome never came up when I was going through my medical education,” she said. But we, and the next generation of physicians, “need to at least be able to understand the basics.
“Hopefully, one day, we will be in a position where we can have meaningful interpretations of the test results and make some kind of meaningful dietary interventions,” Dr. Kao added.
As for clinicians who are currently ordering these tests and products directly from the DTC companies, Dr. Kao said, “I roll my eyes.”
Dr. Ghannoum reiterated that companies offering microbiome tests and products also need to be educated and encouraged to use systematic approaches to product development and interpretation.
“Companies should be open to calls from clinicians and be ready to explain findings on a report, as well as the basis for any recommendations,” he said.
Dr. von Rosenvinge, Dr. Kao, and Dr. Kelly had no relevant conflicts of interest. Dr. Ghannoum is a cofounder of BIOHM.
A version of this article appeared on Medscape.com.
Federal Trade Commission Bans Noncompete Agreements, Urges More Protections for Healthcare Workers
But business groups have vowed to challenge the decision in court.
The proposed final rule passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.
Tensions around noncompetes have been building for years. In 2021, President Biden issued an executive order supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency proposed ending the restrictive covenants.
While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.
US Chamber of Commerce president and CEO Suzanne P. Clark said in a statement that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.
The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.
Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or risk expensive litigation for wanting to pursue job opportunities.
For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With hospital systems merging, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to significant burnout that can shorten their [career] longevity.”
Commissioner Alvaro Bedoya said physicians have had their lives upended by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.
It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.
“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the Workforce Mobility Act of 2021 and the Freedom to Compete Act of 2023.
The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.
States, AMA Take Aim at Noncompetes
Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the Journal of the American College of Cardiology, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.
Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.
Although the American Medical Association (AMA) does not support a total ban, its House of Delegates adopted policies last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.
Challenges Await
The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a statement.
To ease the transition to the new rule, the FTC also released a model language for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.
Dr. Marcus hopes the ban improves doctors’ lives. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”
A version of this article appeared on Medscape.com.
But business groups have vowed to challenge the decision in court.
The proposed final rule passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.
Tensions around noncompetes have been building for years. In 2021, President Biden issued an executive order supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency proposed ending the restrictive covenants.
While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.
US Chamber of Commerce president and CEO Suzanne P. Clark said in a statement that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.
The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.
Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or risk expensive litigation for wanting to pursue job opportunities.
For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With hospital systems merging, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to significant burnout that can shorten their [career] longevity.”
Commissioner Alvaro Bedoya said physicians have had their lives upended by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.
It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.
“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the Workforce Mobility Act of 2021 and the Freedom to Compete Act of 2023.
The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.
States, AMA Take Aim at Noncompetes
Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the Journal of the American College of Cardiology, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.
Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.
Although the American Medical Association (AMA) does not support a total ban, its House of Delegates adopted policies last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.
Challenges Await
The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a statement.
To ease the transition to the new rule, the FTC also released a model language for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.
Dr. Marcus hopes the ban improves doctors’ lives. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”
A version of this article appeared on Medscape.com.
But business groups have vowed to challenge the decision in court.
The proposed final rule passed on a 3-2 vote, with the dissenting commissioners disputing the FTC’s authority to broadly ban noncompetes.
Tensions around noncompetes have been building for years. In 2021, President Biden issued an executive order supporting measures to improve economic competition, in which he urged the FTC to consider its rulemaking authority to address noncompete clauses that unfairly limit workers’ mobility. In January 2023, per that directive, the agency proposed ending the restrictive covenants.
While the FTC estimates that the final rule will reduce healthcare costs by up to $194 billion over the next decade and increase worker earnings by $300 million annually, the ruling faces legal hurdles.
US Chamber of Commerce president and CEO Suzanne P. Clark said in a statement that the move is a “blatant power grab” that will undermine competitive business practices, adding that the Chamber will sue to block the measure.
The FTC received more than 26,000 comments on noncompetes during the public feedback period, with about 25,000 supporting the measure, said Benjamin Cady, JD, an FTC attorney.
Mr. Cady called the feedback “compelling,” citing instances of workers who were forced to commute long distances, uproot their families, or risk expensive litigation for wanting to pursue job opportunities.
For example, a comment from a physician working in Appalachia highlights the potential real-life implications of the agreements. “With hospital systems merging, providers with aggressive noncompetes must abandon the community that they serve if they [choose] to leave their employer. Healthcare providers feel trapped in their current employment situation, leading to significant burnout that can shorten their [career] longevity.”
Commissioner Alvaro Bedoya said physicians have had their lives upended by cumbersome noncompetes, often having to move out of state to practice. “A pandemic killed a million people in this country, and there are doctors who cannot work because of a noncompete,” he said.
It’s unclear whether physicians and others who work for nonprofit healthcare groups or hospitals will be covered by the new ban. FTC Commissioner Rebecca Slaughter acknowledged that the agency’s jurisdictional limitations mean that employees of “certain nonprofit organizations” may not benefit from the rule.
“We want to be transparent about the limitation and recognize there are workers, especially healthcare workers, who are bound by anticompetitive and unfair noncompete clauses, that our rule will struggle to reach,” she said. To cover nonprofit healthcare employees, Ms. Slaughter urged Congress to pass legislation banning noncompetes, such as the Workforce Mobility Act of 2021 and the Freedom to Compete Act of 2023.
The FTC final rule will take effect 120 days after it is published in the federal register, and new noncompete agreements will be banned as of this date. However, existing contracts for senior executives will remain in effect because these individuals are less likely to experience “acute harm” due to their ability to negotiate accordingly, said Mr. Cady.
States, AMA Take Aim at Noncompetes
Before the federal ban, several states had already passed legislation limiting the reach of noncompetes. According to a recent article in the Journal of the American College of Cardiology, 12 states prohibit noncompete clauses for physicians: Alabama, California, Colorado, Delaware, Massachusetts, Montana, New Hampshire, New Mexico, North Dakota, Oklahoma, Rhode Island, and South Dakota.
The remaining states allow noncompetes in some form, often excluding them for employees earning below a certain threshold. For example, in Oregon, noncompete agreements may apply to employees earning more than $113,241. Most states have provisions to adjust the threshold annually. The District of Columbia permits 2-year noncompetes for “medical specialists” earning over $250,000 annually.
Indiana employers can no longer enter into noncompete agreements with primary care providers. Other specialties may be subject to the clauses, except when the physician terminates the contract for cause or when an employer terminates the contract without cause.
Rachel Marcus, MD, a cardiologist in Washington, DC, found out how limiting her employment contract’s noncompete clause was when she wanted to leave a former position. Due to the restrictions, she told this news organization that she couldn’t work locally for a competitor for 2 years. The closest location she could seek employment without violating the agreement was Baltimore, approximately 40 miles away.
Dr. Marcus ultimately moved to another position within the same organization because of the company’s reputation for being “aggressive” in their enforcement actions.
Although the American Medical Association (AMA) does not support a total ban, its House of Delegates adopted policies last year to support the prohibition of noncompete contracts for physicians employed by for-profit or nonprofit hospitals, hospital systems, or staffing companies.
Challenges Await
The American Hospital Association, which opposed the proposed rule, called it “bad policy.” The decision “will likely be short-lived, with courts almost certain to stop it before it can do damage to hospitals’ ability to care for their patients and communities,” the association said in a statement.
To ease the transition to the new rule, the FTC also released a model language for employers to use when discussing the changes with their employees. “All employers need to do to comply with the rule is to stop enforcing existing noncompetes with workers other than senior executives and provide notice to such workers,” he said.
Dr. Marcus hopes the ban improves doctors’ lives. “Your employer is going to have to treat you better because they know that you can easily go across town to a place that has a higher salary, and your patient can go with you.”
A version of this article appeared on Medscape.com.
Are Women Better Doctors Than Men?
This transcript has been edited for clarity.
It’s a battle of the sexes today as we dive into a paper that makes you say, “Wow, what an interesting study” and also “Boy, am I glad I didn’t do that study.” That’s because studies like this are always somewhat fraught; they say something about medicine but also something about society — and that makes this a bit precarious. But that’s never stopped us before. So, let’s go ahead and try to answer the question: Do women make better doctors than men?
On the surface, this question seems nearly impossible to answer. It’s too broad; what does it mean to be a “better” doctor? At first blush it seems that there are just too many variables to control for here: the type of doctor, the type of patient, the clinical scenario, and so on.
But this study, “Comparison of hospital mortality and readmission rates by physician and patient sex,” which appears in Annals of Internal Medicine, uses a fairly ingenious method to cut through all the bias by leveraging two simple facts: First, hospital medicine is largely conducted by hospitalists these days; second, due to the shift-based nature of hospitalist work, the hospitalist you get when you are admitted to the hospital is pretty much random.
In other words, if you are admitted to the hospital for an acute illness and get a hospitalist as your attending, you have no control over whether it is a man or a woman. Is this a randomized trial? No, but it’s not bad.
Researchers used Medicare claims data to identify adults over age 65 who had nonelective hospital admissions throughout the United States. The claims revealed the sex of the patient and the name of the attending physician. By linking to a medical provider database, they could determine the sex of the provider.
The goal was to look at outcomes across four dyads:
- Male patient – male doctor
- Male patient – female doctor
- Female patient – male doctor
- Female patient – female doctor
The primary outcome was 30-day mortality.
I told you that focusing on hospitalists produces some pseudorandomization, but let’s look at the data to be sure. Just under a million patients were treated by approximately 50,000 physicians, 30% of whom were female. And, though female patients and male patients differed, they did not differ with respect to the sex of their hospitalist. So, by physician sex, patients were similar in mean age, race, ethnicity, household income, eligibility for Medicaid, and comorbid conditions. The authors even created a “predicted mortality” score which was similar across the groups as well.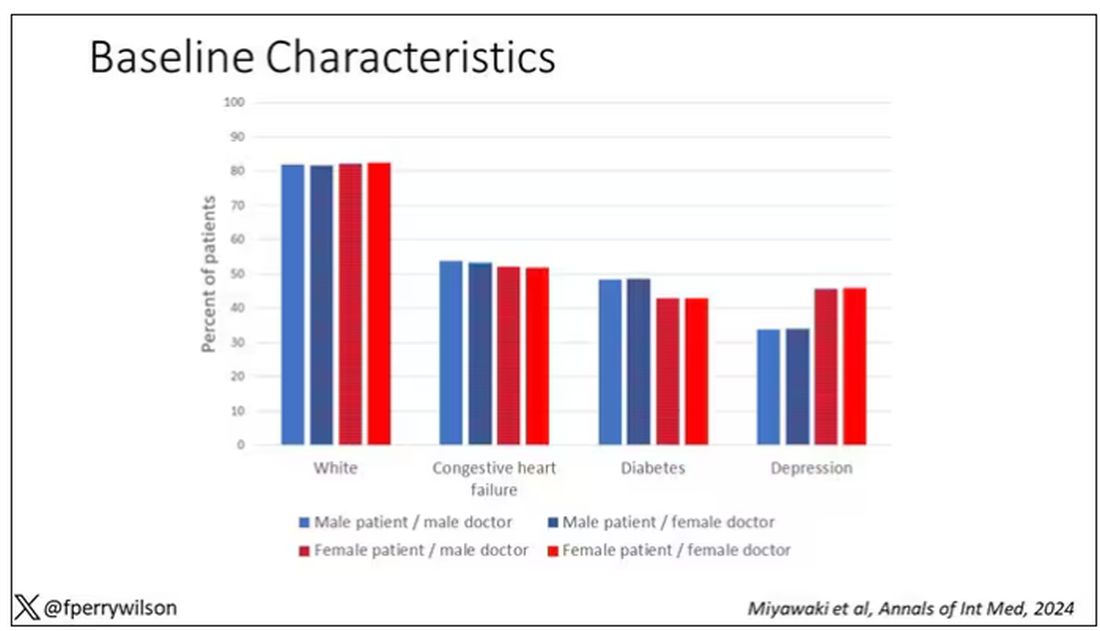
Now, the female physicians were a bit different from the male physicians. The female hospitalists were slightly more likely to have an osteopathic degree, had slightly fewer admissions per year, and were a bit younger.
So, we have broadly similar patients regardless of who their hospitalist was, but hospitalists differ by factors other than their sex. Fine.
I’ve graphed the results here. 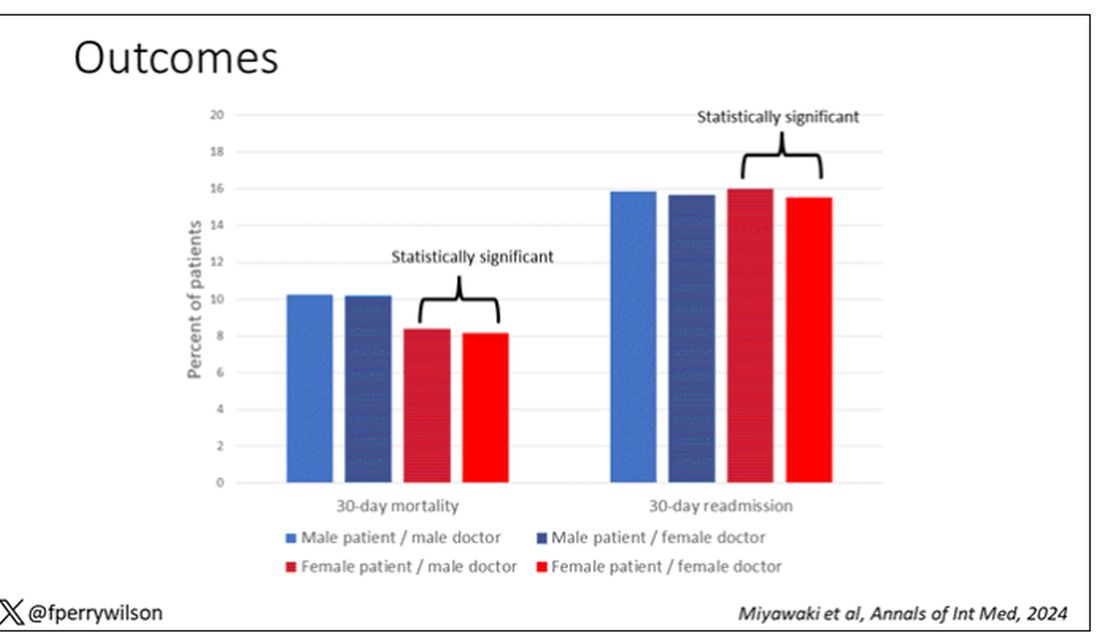
This is a relatively small effect, to be sure, but if you multiply it across the millions of hospitalist admissions per year, you can start to put up some real numbers.
So, what is going on here? I see four broad buckets of possibilities.
Let’s start with the obvious explanation: Women, on average, are better doctors than men. I am married to a woman doctor, and based on my personal experience, this explanation is undoubtedly true. But why would that be?
The authors cite data that suggest that female physicians are less likely than male physicians to dismiss patient concerns — and in particular, the concerns of female patients — perhaps leading to fewer missed diagnoses. But this is impossible to measure with administrative data, so this study can no more tell us whether these female hospitalists are more attentive than their male counterparts than it can suggest that the benefit is mediated by the shorter average height of female physicians. Perhaps the key is being closer to the patient?
The second possibility here is that this has nothing to do with the sex of the physician at all; it has to do with those other things that associate with the sex of the physician. We know, for example, that the female physicians saw fewer patients per year than the male physicians, but the study authors adjusted for this in the statistical models. Still, other unmeasured factors (confounders) could be present. By the way, confounders wouldn’t necessarily change the primary finding — you are better off being cared for by female physicians. It’s just not because they are female; it’s a convenient marker for some other quality, such as age.
The third possibility is that the study represents a phenomenon called collider bias. The idea here is that physicians only get into the study if they are hospitalists, and the quality of physicians who choose to become a hospitalist may differ by sex. When deciding on a specialty, a talented resident considering certain lifestyle issues may find hospital medicine particularly attractive — and that draw toward a more lifestyle-friendly specialty may differ by sex, as some prior studies have shown. If true, the pool of women hospitalists may be better than their male counterparts because male physicians of that caliber don’t become hospitalists.
Okay, don’t write in. I’m just trying to cite examples of how to think about collider bias. I can’t prove that this is the case, and in fact the authors do a sensitivity analysis of all physicians, not just hospitalists, and show the same thing. So this is probably not true, but epidemiology is fun, right?
And the fourth possibility: This is nothing but statistical noise. The effect size is incredibly small and just on the border of statistical significance. Especially when you’re working with very large datasets like this, you’ve got to be really careful about overinterpreting statistically significant findings that are nevertheless of small magnitude.
Regardless, it’s an interesting study, one that made me think and, of course, worry a bit about how I would present it. Forgive me if I’ve been indelicate in handling the complex issues of sex, gender, and society here. But I’m not sure what you expect; after all, I’m only a male doctor.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s a battle of the sexes today as we dive into a paper that makes you say, “Wow, what an interesting study” and also “Boy, am I glad I didn’t do that study.” That’s because studies like this are always somewhat fraught; they say something about medicine but also something about society — and that makes this a bit precarious. But that’s never stopped us before. So, let’s go ahead and try to answer the question: Do women make better doctors than men?
On the surface, this question seems nearly impossible to answer. It’s too broad; what does it mean to be a “better” doctor? At first blush it seems that there are just too many variables to control for here: the type of doctor, the type of patient, the clinical scenario, and so on.
But this study, “Comparison of hospital mortality and readmission rates by physician and patient sex,” which appears in Annals of Internal Medicine, uses a fairly ingenious method to cut through all the bias by leveraging two simple facts: First, hospital medicine is largely conducted by hospitalists these days; second, due to the shift-based nature of hospitalist work, the hospitalist you get when you are admitted to the hospital is pretty much random.
In other words, if you are admitted to the hospital for an acute illness and get a hospitalist as your attending, you have no control over whether it is a man or a woman. Is this a randomized trial? No, but it’s not bad.
Researchers used Medicare claims data to identify adults over age 65 who had nonelective hospital admissions throughout the United States. The claims revealed the sex of the patient and the name of the attending physician. By linking to a medical provider database, they could determine the sex of the provider.
The goal was to look at outcomes across four dyads:
- Male patient – male doctor
- Male patient – female doctor
- Female patient – male doctor
- Female patient – female doctor
The primary outcome was 30-day mortality.
I told you that focusing on hospitalists produces some pseudorandomization, but let’s look at the data to be sure. Just under a million patients were treated by approximately 50,000 physicians, 30% of whom were female. And, though female patients and male patients differed, they did not differ with respect to the sex of their hospitalist. So, by physician sex, patients were similar in mean age, race, ethnicity, household income, eligibility for Medicaid, and comorbid conditions. The authors even created a “predicted mortality” score which was similar across the groups as well.
Now, the female physicians were a bit different from the male physicians. The female hospitalists were slightly more likely to have an osteopathic degree, had slightly fewer admissions per year, and were a bit younger.
So, we have broadly similar patients regardless of who their hospitalist was, but hospitalists differ by factors other than their sex. Fine.
I’ve graphed the results here. 
This is a relatively small effect, to be sure, but if you multiply it across the millions of hospitalist admissions per year, you can start to put up some real numbers.
So, what is going on here? I see four broad buckets of possibilities.
Let’s start with the obvious explanation: Women, on average, are better doctors than men. I am married to a woman doctor, and based on my personal experience, this explanation is undoubtedly true. But why would that be?
The authors cite data that suggest that female physicians are less likely than male physicians to dismiss patient concerns — and in particular, the concerns of female patients — perhaps leading to fewer missed diagnoses. But this is impossible to measure with administrative data, so this study can no more tell us whether these female hospitalists are more attentive than their male counterparts than it can suggest that the benefit is mediated by the shorter average height of female physicians. Perhaps the key is being closer to the patient?
The second possibility here is that this has nothing to do with the sex of the physician at all; it has to do with those other things that associate with the sex of the physician. We know, for example, that the female physicians saw fewer patients per year than the male physicians, but the study authors adjusted for this in the statistical models. Still, other unmeasured factors (confounders) could be present. By the way, confounders wouldn’t necessarily change the primary finding — you are better off being cared for by female physicians. It’s just not because they are female; it’s a convenient marker for some other quality, such as age.
The third possibility is that the study represents a phenomenon called collider bias. The idea here is that physicians only get into the study if they are hospitalists, and the quality of physicians who choose to become a hospitalist may differ by sex. When deciding on a specialty, a talented resident considering certain lifestyle issues may find hospital medicine particularly attractive — and that draw toward a more lifestyle-friendly specialty may differ by sex, as some prior studies have shown. If true, the pool of women hospitalists may be better than their male counterparts because male physicians of that caliber don’t become hospitalists.
Okay, don’t write in. I’m just trying to cite examples of how to think about collider bias. I can’t prove that this is the case, and in fact the authors do a sensitivity analysis of all physicians, not just hospitalists, and show the same thing. So this is probably not true, but epidemiology is fun, right?
And the fourth possibility: This is nothing but statistical noise. The effect size is incredibly small and just on the border of statistical significance. Especially when you’re working with very large datasets like this, you’ve got to be really careful about overinterpreting statistically significant findings that are nevertheless of small magnitude.
Regardless, it’s an interesting study, one that made me think and, of course, worry a bit about how I would present it. Forgive me if I’ve been indelicate in handling the complex issues of sex, gender, and society here. But I’m not sure what you expect; after all, I’m only a male doctor.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
This transcript has been edited for clarity.
It’s a battle of the sexes today as we dive into a paper that makes you say, “Wow, what an interesting study” and also “Boy, am I glad I didn’t do that study.” That’s because studies like this are always somewhat fraught; they say something about medicine but also something about society — and that makes this a bit precarious. But that’s never stopped us before. So, let’s go ahead and try to answer the question: Do women make better doctors than men?
On the surface, this question seems nearly impossible to answer. It’s too broad; what does it mean to be a “better” doctor? At first blush it seems that there are just too many variables to control for here: the type of doctor, the type of patient, the clinical scenario, and so on.
But this study, “Comparison of hospital mortality and readmission rates by physician and patient sex,” which appears in Annals of Internal Medicine, uses a fairly ingenious method to cut through all the bias by leveraging two simple facts: First, hospital medicine is largely conducted by hospitalists these days; second, due to the shift-based nature of hospitalist work, the hospitalist you get when you are admitted to the hospital is pretty much random.
In other words, if you are admitted to the hospital for an acute illness and get a hospitalist as your attending, you have no control over whether it is a man or a woman. Is this a randomized trial? No, but it’s not bad.
Researchers used Medicare claims data to identify adults over age 65 who had nonelective hospital admissions throughout the United States. The claims revealed the sex of the patient and the name of the attending physician. By linking to a medical provider database, they could determine the sex of the provider.
The goal was to look at outcomes across four dyads:
- Male patient – male doctor
- Male patient – female doctor
- Female patient – male doctor
- Female patient – female doctor
The primary outcome was 30-day mortality.
I told you that focusing on hospitalists produces some pseudorandomization, but let’s look at the data to be sure. Just under a million patients were treated by approximately 50,000 physicians, 30% of whom were female. And, though female patients and male patients differed, they did not differ with respect to the sex of their hospitalist. So, by physician sex, patients were similar in mean age, race, ethnicity, household income, eligibility for Medicaid, and comorbid conditions. The authors even created a “predicted mortality” score which was similar across the groups as well.
Now, the female physicians were a bit different from the male physicians. The female hospitalists were slightly more likely to have an osteopathic degree, had slightly fewer admissions per year, and were a bit younger.
So, we have broadly similar patients regardless of who their hospitalist was, but hospitalists differ by factors other than their sex. Fine.
I’ve graphed the results here. 
This is a relatively small effect, to be sure, but if you multiply it across the millions of hospitalist admissions per year, you can start to put up some real numbers.
So, what is going on here? I see four broad buckets of possibilities.
Let’s start with the obvious explanation: Women, on average, are better doctors than men. I am married to a woman doctor, and based on my personal experience, this explanation is undoubtedly true. But why would that be?
The authors cite data that suggest that female physicians are less likely than male physicians to dismiss patient concerns — and in particular, the concerns of female patients — perhaps leading to fewer missed diagnoses. But this is impossible to measure with administrative data, so this study can no more tell us whether these female hospitalists are more attentive than their male counterparts than it can suggest that the benefit is mediated by the shorter average height of female physicians. Perhaps the key is being closer to the patient?
The second possibility here is that this has nothing to do with the sex of the physician at all; it has to do with those other things that associate with the sex of the physician. We know, for example, that the female physicians saw fewer patients per year than the male physicians, but the study authors adjusted for this in the statistical models. Still, other unmeasured factors (confounders) could be present. By the way, confounders wouldn’t necessarily change the primary finding — you are better off being cared for by female physicians. It’s just not because they are female; it’s a convenient marker for some other quality, such as age.
The third possibility is that the study represents a phenomenon called collider bias. The idea here is that physicians only get into the study if they are hospitalists, and the quality of physicians who choose to become a hospitalist may differ by sex. When deciding on a specialty, a talented resident considering certain lifestyle issues may find hospital medicine particularly attractive — and that draw toward a more lifestyle-friendly specialty may differ by sex, as some prior studies have shown. If true, the pool of women hospitalists may be better than their male counterparts because male physicians of that caliber don’t become hospitalists.
Okay, don’t write in. I’m just trying to cite examples of how to think about collider bias. I can’t prove that this is the case, and in fact the authors do a sensitivity analysis of all physicians, not just hospitalists, and show the same thing. So this is probably not true, but epidemiology is fun, right?
And the fourth possibility: This is nothing but statistical noise. The effect size is incredibly small and just on the border of statistical significance. Especially when you’re working with very large datasets like this, you’ve got to be really careful about overinterpreting statistically significant findings that are nevertheless of small magnitude.
Regardless, it’s an interesting study, one that made me think and, of course, worry a bit about how I would present it. Forgive me if I’ve been indelicate in handling the complex issues of sex, gender, and society here. But I’m not sure what you expect; after all, I’m only a male doctor.
Dr. Wilson is associate professor of medicine and public health and director of the Clinical and Translational Research Accelerator at Yale University, New Haven, Conn. He has disclosed no relevant financial relationships.
A version of this article appeared on Medscape.com.
Semaglutide Trial for Knee Osteoarthritis Shows Improvements in Pain, Physical Function
VIENNA — The glucagon-like peptide 1 (GLP-1) receptor agonist semaglutide (Wegovy) not only induced weight loss but also improved knee pain in people with knee osteoarthritis (OA) and obesity, according to results from the STEP 9 study reported at the Osteoarthritis Research Society International (OARSI) 2024 World Congress.
From baseline to week 68, the mean change in knee pain assessed using the Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain score was a reduction of 41.7 points for semaglutide and a decrease of 27.5 points for a matching placebo. The estimated treatment difference of 14.1 points between the groups was statistically significant (P < .001).
As for weight loss, this also fell by a significantly greater amount in the people treated with semaglutide vs those given placebo, with respective reductions of 13.7% and 3.2% from baseline, with an estimated 10.5% greater weight loss with semaglutide.
“The interesting thing is whether there’s a specific action of GLP-1 receptor agonists on the joint, not through the weight loss but by itself,” principal study investigator Henning Bliddal, MD, DMSc, told this news organization ahead of reporting the results at OARSI 2024.
Weight loss is “obviously good” because “the knees suffer from the weight. But whether it’s good for the knee or just for the health or the well-being of the person is another matter,” said Dr. Bliddal, who is director of the Parker Institute at Bispebjerg Frederiksberg Hospital in Copenhagen, Denmark.
Not Approved in OA
Semaglutide and other potentially weight loss-inducing drugs are not currently indicated for use specifically in OA, Tonia Vincent, MBBS, PhD, told this news organization, and so “I think we have to be very cautious,” she said.
“Weight loss is one of the few things that has been shown to be successful in clinical trials,” said Dr. Vincent, who is a professor of musculoskeletal biology and an honorary rheumatologist at the Kennedy Institute of Rheumatology at Oxford University in Oxford, England.
“People always feel better too when they lose weight, so that helps manage pain. So, I’d be very surprised if there isn’t a benefit,” she added.
“I just think we need to know more about the long-term use of these drugs, whether the healthcare system can afford them, and how we would ration them.”
Previous Work
The STEP 9 study is not the first time that Dr. Bliddal has investigated the effects of a GLP-1 receptor agonist in people with knee OA, but it is the first to have shown a significant effect on knee pain.
Previously, results from the LOSEIT trial with liraglutide demonstrated that, after an 8-week dietary intervention run-in phase, people who were treated with the GLP-1 receptor agonist lost an average of 2.8 kg in body weight over a period of 1 year, vs a 1.2 kg gain in the placebo group. Knee injury and Osteoarthritis Outcome Scores, however, were largely unaffected.
“The study was more or less negative for knee pain because at that time we had to pretreat patients with some kind of weight loss before they were allowed to have the liraglutide,” Dr. Bliddal said.
“There’s so many different considerations with diets and the different ways that [dietary modification] is performed, that could be part of the explanation why some people didn’t find the pain relief,” Dr. Bliddal suggested.
STEP 9 Study Design
No pre-study dietary intervention was required in the STEP 9 trial, although a reduced-calorie diet and increased physical exercise were used alongside both semaglutide and placebo treatment.
STEP 9 was a multicenter, multinational phase 3 clinical trial that enrolled people if they had a body mass index (BMI) of > 30, had a clinical diagnosis of knee OA with moderate radiographic changes (Kellgren-Lawrence grade of 2-3), and were experiencing knee pain.
In addition to a baseline WOMAC pain score of at least 40 points (where 0 represents no and 100 the worst pain), the participants had to have a WOMAC numerical rating scale (NRS) score of ≥ 3.1.
A total of 407 participants were recruited and randomly allocated, 2:1, to receive once-weekly subcutaneous injections of either semaglutide 2.4 mg or placebo for a total of 68 weeks.
Dr. Bliddal presented demographic information only for the study population as a whole, showing that the mean was 56 years, 81.6% were women, 60.9% were White, 11.8% Native American, 7.6% Black, and 19.7% of other ethnic origin.
Moreover, the mean bodyweight at baseline was 108.6 kg, and the mean baseline BMI was 40.3, with 75% of participants having a BMI ≥ 35. The mean waist circumference was 118.7 cm. The mean baseline WOMAC pain score was 70.9.
Other Findings
In addition to the reductions seen in the coprimary endpoints of weight loss and knee pain, the WOMAC physical function score was also reduced from baseline to week 68 to a greater degree in the semaglutide than placebo arm, by a respective 41.5 vs 26.7 points, with a significant estimated treatment difference of -14.9 points.
“The use of pain medication went down as well; you can see the drop was faster in the semaglutide group than the placebo group, and it was maintained throughout the study,” Dr. Bliddal said during his presentation. He noted that patients had to temporarily stop taking pain relievers such as acetaminophen 3 days before their pain was assessed.
Additional findings reported in the abstract, but not presented at the meeting, were a significant estimated treatment difference of -1.0 in NRS pain intensity, more people treated with semaglutide than placebo achieving ≥ 5% (87.0% vs 29.2%) or ≥ 10% (70.4% vs 9.2%) weight loss.
“Safety and tolerability with semaglutide were consistent with the global STEP program and the GLP-1 receptor agonist class in general,” Dr. Bliddal reported.
Serious adverse events occurred in a respective 10.0% and 8.1% of participants, and adverse events leading to discontinuation were recorded in 6.7% and 3%. Around one third (2.2%) of those leading to discontinuation in the semaglutide arm were gastrointestinal adverse events.
The STEP 9 study was funded by Novo Nordisk. Henning is a principal investigator for the trial and acknowledged that research grants were received from Novo Nordisk to his institution, as well as consulting fees and honoraria. He has also received congress and travel support from Contura. Dr. Vincent was not involved in the study and had no relevant conflicts of interest to disclose.
A version of this article appeared on Medscape.com.
VIENNA — The glucagon-like peptide 1 (GLP-1) receptor agonist semaglutide (Wegovy) not only induced weight loss but also improved knee pain in people with knee osteoarthritis (OA) and obesity, according to results from the STEP 9 study reported at the Osteoarthritis Research Society International (OARSI) 2024 World Congress.
From baseline to week 68, the mean change in knee pain assessed using the Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain score was a reduction of 41.7 points for semaglutide and a decrease of 27.5 points for a matching placebo. The estimated treatment difference of 14.1 points between the groups was statistically significant (P < .001).
As for weight loss, this also fell by a significantly greater amount in the people treated with semaglutide vs those given placebo, with respective reductions of 13.7% and 3.2% from baseline, with an estimated 10.5% greater weight loss with semaglutide.
“The interesting thing is whether there’s a specific action of GLP-1 receptor agonists on the joint, not through the weight loss but by itself,” principal study investigator Henning Bliddal, MD, DMSc, told this news organization ahead of reporting the results at OARSI 2024.
Weight loss is “obviously good” because “the knees suffer from the weight. But whether it’s good for the knee or just for the health or the well-being of the person is another matter,” said Dr. Bliddal, who is director of the Parker Institute at Bispebjerg Frederiksberg Hospital in Copenhagen, Denmark.
Not Approved in OA
Semaglutide and other potentially weight loss-inducing drugs are not currently indicated for use specifically in OA, Tonia Vincent, MBBS, PhD, told this news organization, and so “I think we have to be very cautious,” she said.
“Weight loss is one of the few things that has been shown to be successful in clinical trials,” said Dr. Vincent, who is a professor of musculoskeletal biology and an honorary rheumatologist at the Kennedy Institute of Rheumatology at Oxford University in Oxford, England.
“People always feel better too when they lose weight, so that helps manage pain. So, I’d be very surprised if there isn’t a benefit,” she added.
“I just think we need to know more about the long-term use of these drugs, whether the healthcare system can afford them, and how we would ration them.”
Previous Work
The STEP 9 study is not the first time that Dr. Bliddal has investigated the effects of a GLP-1 receptor agonist in people with knee OA, but it is the first to have shown a significant effect on knee pain.
Previously, results from the LOSEIT trial with liraglutide demonstrated that, after an 8-week dietary intervention run-in phase, people who were treated with the GLP-1 receptor agonist lost an average of 2.8 kg in body weight over a period of 1 year, vs a 1.2 kg gain in the placebo group. Knee injury and Osteoarthritis Outcome Scores, however, were largely unaffected.
“The study was more or less negative for knee pain because at that time we had to pretreat patients with some kind of weight loss before they were allowed to have the liraglutide,” Dr. Bliddal said.
“There’s so many different considerations with diets and the different ways that [dietary modification] is performed, that could be part of the explanation why some people didn’t find the pain relief,” Dr. Bliddal suggested.
STEP 9 Study Design
No pre-study dietary intervention was required in the STEP 9 trial, although a reduced-calorie diet and increased physical exercise were used alongside both semaglutide and placebo treatment.
STEP 9 was a multicenter, multinational phase 3 clinical trial that enrolled people if they had a body mass index (BMI) of > 30, had a clinical diagnosis of knee OA with moderate radiographic changes (Kellgren-Lawrence grade of 2-3), and were experiencing knee pain.
In addition to a baseline WOMAC pain score of at least 40 points (where 0 represents no and 100 the worst pain), the participants had to have a WOMAC numerical rating scale (NRS) score of ≥ 3.1.
A total of 407 participants were recruited and randomly allocated, 2:1, to receive once-weekly subcutaneous injections of either semaglutide 2.4 mg or placebo for a total of 68 weeks.
Dr. Bliddal presented demographic information only for the study population as a whole, showing that the mean was 56 years, 81.6% were women, 60.9% were White, 11.8% Native American, 7.6% Black, and 19.7% of other ethnic origin.
Moreover, the mean bodyweight at baseline was 108.6 kg, and the mean baseline BMI was 40.3, with 75% of participants having a BMI ≥ 35. The mean waist circumference was 118.7 cm. The mean baseline WOMAC pain score was 70.9.
Other Findings
In addition to the reductions seen in the coprimary endpoints of weight loss and knee pain, the WOMAC physical function score was also reduced from baseline to week 68 to a greater degree in the semaglutide than placebo arm, by a respective 41.5 vs 26.7 points, with a significant estimated treatment difference of -14.9 points.
“The use of pain medication went down as well; you can see the drop was faster in the semaglutide group than the placebo group, and it was maintained throughout the study,” Dr. Bliddal said during his presentation. He noted that patients had to temporarily stop taking pain relievers such as acetaminophen 3 days before their pain was assessed.
Additional findings reported in the abstract, but not presented at the meeting, were a significant estimated treatment difference of -1.0 in NRS pain intensity, more people treated with semaglutide than placebo achieving ≥ 5% (87.0% vs 29.2%) or ≥ 10% (70.4% vs 9.2%) weight loss.
“Safety and tolerability with semaglutide were consistent with the global STEP program and the GLP-1 receptor agonist class in general,” Dr. Bliddal reported.
Serious adverse events occurred in a respective 10.0% and 8.1% of participants, and adverse events leading to discontinuation were recorded in 6.7% and 3%. Around one third (2.2%) of those leading to discontinuation in the semaglutide arm were gastrointestinal adverse events.
The STEP 9 study was funded by Novo Nordisk. Henning is a principal investigator for the trial and acknowledged that research grants were received from Novo Nordisk to his institution, as well as consulting fees and honoraria. He has also received congress and travel support from Contura. Dr. Vincent was not involved in the study and had no relevant conflicts of interest to disclose.
A version of this article appeared on Medscape.com.
VIENNA — The glucagon-like peptide 1 (GLP-1) receptor agonist semaglutide (Wegovy) not only induced weight loss but also improved knee pain in people with knee osteoarthritis (OA) and obesity, according to results from the STEP 9 study reported at the Osteoarthritis Research Society International (OARSI) 2024 World Congress.
From baseline to week 68, the mean change in knee pain assessed using the Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain score was a reduction of 41.7 points for semaglutide and a decrease of 27.5 points for a matching placebo. The estimated treatment difference of 14.1 points between the groups was statistically significant (P < .001).
As for weight loss, this also fell by a significantly greater amount in the people treated with semaglutide vs those given placebo, with respective reductions of 13.7% and 3.2% from baseline, with an estimated 10.5% greater weight loss with semaglutide.
“The interesting thing is whether there’s a specific action of GLP-1 receptor agonists on the joint, not through the weight loss but by itself,” principal study investigator Henning Bliddal, MD, DMSc, told this news organization ahead of reporting the results at OARSI 2024.
Weight loss is “obviously good” because “the knees suffer from the weight. But whether it’s good for the knee or just for the health or the well-being of the person is another matter,” said Dr. Bliddal, who is director of the Parker Institute at Bispebjerg Frederiksberg Hospital in Copenhagen, Denmark.
Not Approved in OA
Semaglutide and other potentially weight loss-inducing drugs are not currently indicated for use specifically in OA, Tonia Vincent, MBBS, PhD, told this news organization, and so “I think we have to be very cautious,” she said.
“Weight loss is one of the few things that has been shown to be successful in clinical trials,” said Dr. Vincent, who is a professor of musculoskeletal biology and an honorary rheumatologist at the Kennedy Institute of Rheumatology at Oxford University in Oxford, England.
“People always feel better too when they lose weight, so that helps manage pain. So, I’d be very surprised if there isn’t a benefit,” she added.
“I just think we need to know more about the long-term use of these drugs, whether the healthcare system can afford them, and how we would ration them.”
Previous Work
The STEP 9 study is not the first time that Dr. Bliddal has investigated the effects of a GLP-1 receptor agonist in people with knee OA, but it is the first to have shown a significant effect on knee pain.
Previously, results from the LOSEIT trial with liraglutide demonstrated that, after an 8-week dietary intervention run-in phase, people who were treated with the GLP-1 receptor agonist lost an average of 2.8 kg in body weight over a period of 1 year, vs a 1.2 kg gain in the placebo group. Knee injury and Osteoarthritis Outcome Scores, however, were largely unaffected.
“The study was more or less negative for knee pain because at that time we had to pretreat patients with some kind of weight loss before they were allowed to have the liraglutide,” Dr. Bliddal said.
“There’s so many different considerations with diets and the different ways that [dietary modification] is performed, that could be part of the explanation why some people didn’t find the pain relief,” Dr. Bliddal suggested.
STEP 9 Study Design
No pre-study dietary intervention was required in the STEP 9 trial, although a reduced-calorie diet and increased physical exercise were used alongside both semaglutide and placebo treatment.
STEP 9 was a multicenter, multinational phase 3 clinical trial that enrolled people if they had a body mass index (BMI) of > 30, had a clinical diagnosis of knee OA with moderate radiographic changes (Kellgren-Lawrence grade of 2-3), and were experiencing knee pain.
In addition to a baseline WOMAC pain score of at least 40 points (where 0 represents no and 100 the worst pain), the participants had to have a WOMAC numerical rating scale (NRS) score of ≥ 3.1.
A total of 407 participants were recruited and randomly allocated, 2:1, to receive once-weekly subcutaneous injections of either semaglutide 2.4 mg or placebo for a total of 68 weeks.
Dr. Bliddal presented demographic information only for the study population as a whole, showing that the mean was 56 years, 81.6% were women, 60.9% were White, 11.8% Native American, 7.6% Black, and 19.7% of other ethnic origin.
Moreover, the mean bodyweight at baseline was 108.6 kg, and the mean baseline BMI was 40.3, with 75% of participants having a BMI ≥ 35. The mean waist circumference was 118.7 cm. The mean baseline WOMAC pain score was 70.9.
Other Findings
In addition to the reductions seen in the coprimary endpoints of weight loss and knee pain, the WOMAC physical function score was also reduced from baseline to week 68 to a greater degree in the semaglutide than placebo arm, by a respective 41.5 vs 26.7 points, with a significant estimated treatment difference of -14.9 points.
“The use of pain medication went down as well; you can see the drop was faster in the semaglutide group than the placebo group, and it was maintained throughout the study,” Dr. Bliddal said during his presentation. He noted that patients had to temporarily stop taking pain relievers such as acetaminophen 3 days before their pain was assessed.
Additional findings reported in the abstract, but not presented at the meeting, were a significant estimated treatment difference of -1.0 in NRS pain intensity, more people treated with semaglutide than placebo achieving ≥ 5% (87.0% vs 29.2%) or ≥ 10% (70.4% vs 9.2%) weight loss.
“Safety and tolerability with semaglutide were consistent with the global STEP program and the GLP-1 receptor agonist class in general,” Dr. Bliddal reported.
Serious adverse events occurred in a respective 10.0% and 8.1% of participants, and adverse events leading to discontinuation were recorded in 6.7% and 3%. Around one third (2.2%) of those leading to discontinuation in the semaglutide arm were gastrointestinal adverse events.
The STEP 9 study was funded by Novo Nordisk. Henning is a principal investigator for the trial and acknowledged that research grants were received from Novo Nordisk to his institution, as well as consulting fees and honoraria. He has also received congress and travel support from Contura. Dr. Vincent was not involved in the study and had no relevant conflicts of interest to disclose.
A version of this article appeared on Medscape.com.
FROM OARSI 2024









