User login
DOACs best aspirin after ventricular ablation: STROKE-VT
Catheter ablation has been around a lot longer for ventricular arrhythmia than for atrial fibrillation, but far less is settled about what antithrombotic therapy should follow ventricular ablations, as there have been no big, randomized trials for guidance.
But the evidence base grew stronger this week, and it favors postprocedure treatment with a direct oral anticoagulant (DOAC) over antiplatelet therapy with aspirin for patients undergoing radiofrequency (RF) ablation to treat left ventricular (LV) arrhythmias.
The 30-day risk for ischemic stroke or transient ischemia attack (TIA) was sharply higher for patients who took daily aspirin after RF ablation for ventricular tachycardia (VT) or premature ventricular contractions (PVC) in a multicenter randomized trial.
Those of its 246 patients who received aspirin were also far more likely to show asymptomatic lesions on cerebral MRI scans performed both 24 hours and 30 days after the procedure.
The findings show the importance of DOAC therapy after ventricular ablation procedures, a setting for which there are no evidence-based guidelines, “to mitigate the risk of systemic thromboembolic events,” said Dhanunjaya Lakkireddy, MD, Kansas City Heart Rhythm Institute, Overland Park. He spoke at a media presentation on the trial, called STROKE-VT, during the Heart Rhythm Society 2021 Scientific Sessions, held virtually and on-site in Boston.
The risk for stroke and TIA went up in association with several procedural issues, including some that operators might be able to change in order to reach for better outcomes, Dr. Lakkireddy observed.
“Prolonged radiofrequency ablation times, especially in those with low left ventricle ejection fractions, are definitely higher risk,” as are procedures that involved the retrograde transaortic approach for advancing the ablation catheter, rather than a trans-septal approach.
The retrograde transaortic approach should be avoided in such procedures, “whenever it can be avoided,” said Dr. Lakkireddy, who formally presented STROKE-VT at the HRS sessions and is lead author on its report published about the same time in JACC: Clinical Electrophysiology.
The trial has limitations, but “it’s a very important study, and I think that this could become our standard of care for managing anticoagulation after VT and PVC left-sided ablations,” Mina K. Chung, MD, Cleveland Clinic, said as an invited discussant after Dr. Lakkireddy’s presentation.
How patients are treated with antithrombotics after ventricular ablations can vary widely, sometimes based on the operator’s “subjective feeling of how extensive the ablation is,” Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, not involved in the study, said during the STROKE-VT media briefing.
That’s consistent with the guidelines, which propose oral anticoagulation therapy after more extensive ventricular ablations and antiplatelets when the ablation is more limited – based more on consensus than firm evidence – as described by Jeffrey R. Winterfield, MD, Medical University of South Carolina, Charleston, and Usha Tedrow, MD, MSc, Brigham and Women’s Hospital, Boston, in an accompanying editorial.
“This is really the first randomized trial data, that I know of, that we have on this. So I do think it will be guideline-influencing,” Dr. Albert said.
“This should change practice,” agreed Jonathan P. Piccini, MD, MHS, Duke University, Durham, N.C., also not part of STROKE-VT. “A lot of evidence in the trial is consistent and provides a compelling story, not to mention that, in my opinion, the study probably underestimates the value of DOACs,” he told this news organization.
That’s because patients assigned to DOACs had far longer ablation times, “so their risk was even greater than in the aspirin arm,” Dr. Piccini said. Ablation times averaged 2,095 seconds in the DOAC group, compared with only 1,708 seconds in the aspirin group, probably because the preponderance of VT over PVC ablations for those getting a DOAC was even greater in the aspirin group.
Of the 246 patients assigned to either aspirin or a DOAC, usually a factor Xa inhibitor, 75% had undergone VT ablation and the remainder ablation for PVCs. Their mean age was 60 years and only 18% were women. None had experienced a cerebrovascular event in the previous 3 months.
The 30-day odds ratio for TIA or ischemic stroke in patients who received aspirin, compared with a DOAC, was 12.6 (95% confidence interval, 4.10-39.11; P < .001).
The corresponding OR for asymptomatic cerebral lesions by MRI at 24 hours was 2.15 (95% CI, 1.02-4.54; P = .04) and at 30 days was 3.48 (95% CI, 1.38-8.80; P = .008).
The rate of stroke or TIA was similar in patients who underwent ablation for VT and for PVCs (14% vs. 16%, respectively; P = .70). There were fewer asymptomatic cerebrovascular events by MRI at 24 hours for those undergoing VT ablations (14.7% and 25.8%, respectively; P = .046); but difference between rates attenuated by 30 days (11.4% and 14.5%, respectively; P = .52).
The OR for TIA or stroke associated with the retrograde transaortic approach, performed in about 40% of the patients, compared with the trans-septal approach in the remainder was 2.60 (95% CI, 1.06-6.37; P = .04).
“The study tells us it’s safe and indeed preferable to anticoagulate after an ablation procedure. But the more important finding, perhaps, wasn’t the one related to the core hypothesis. And that was the effect of retrograde access,” Paul A. Friedman, MD, Mayo Clinic, Rochester, Minn., said as an invited discussant after Dr. Lakkireddy’s formal presentation of the trial.
Whether a ventricular ablation is performed using the retrograde transaortic or trans-septal approach often depends on the location of the ablation targets in the left ventricle. But in some cases it’s a matter of operator preference, Dr. Piccini observed.
“There are some situations where, really, it is better to do retrograde aortic, and there are some cases that are better to do trans-septal. But now there’s going to be a higher burden of proof,” he said. Given the findings of STROKE-VT, operators may need to consider that a ventricular ablation procedure that can be done by the trans-septal route perhaps ought to be consistently done that way.
Dr. Lakkireddy discloses financial relationships with Boston Scientific, Biosense Webster, Janssen Pharmaceuticals, and more. Dr. Chung had “nothing relevant to disclose.” Dr. Piccini discloses receiving honoraria or speaking or consulting fees from Sanofi, Abbott, ARCA Biopharma, Medtronic, Philips, Biotronik, Allergan, LivaNova, and Myokardia; and research in conjunction with Bayer Healthcare, Abbott, Boston Scientific, and Philips. Dr. Friedman discloses conducting research in conjunction with Medtronic and Abbott; holding intellectual property rights with AliveCor, Inference, Medicool, Eko, and Anumana; and receiving honoraria or speaking or consulting fees from Boston Scientific. Dr. Winterfield and Dr. Tedrow had no disclosures.
A version of this article first appeared on Medscape.com.
Catheter ablation has been around a lot longer for ventricular arrhythmia than for atrial fibrillation, but far less is settled about what antithrombotic therapy should follow ventricular ablations, as there have been no big, randomized trials for guidance.
But the evidence base grew stronger this week, and it favors postprocedure treatment with a direct oral anticoagulant (DOAC) over antiplatelet therapy with aspirin for patients undergoing radiofrequency (RF) ablation to treat left ventricular (LV) arrhythmias.
The 30-day risk for ischemic stroke or transient ischemia attack (TIA) was sharply higher for patients who took daily aspirin after RF ablation for ventricular tachycardia (VT) or premature ventricular contractions (PVC) in a multicenter randomized trial.
Those of its 246 patients who received aspirin were also far more likely to show asymptomatic lesions on cerebral MRI scans performed both 24 hours and 30 days after the procedure.
The findings show the importance of DOAC therapy after ventricular ablation procedures, a setting for which there are no evidence-based guidelines, “to mitigate the risk of systemic thromboembolic events,” said Dhanunjaya Lakkireddy, MD, Kansas City Heart Rhythm Institute, Overland Park. He spoke at a media presentation on the trial, called STROKE-VT, during the Heart Rhythm Society 2021 Scientific Sessions, held virtually and on-site in Boston.
The risk for stroke and TIA went up in association with several procedural issues, including some that operators might be able to change in order to reach for better outcomes, Dr. Lakkireddy observed.
“Prolonged radiofrequency ablation times, especially in those with low left ventricle ejection fractions, are definitely higher risk,” as are procedures that involved the retrograde transaortic approach for advancing the ablation catheter, rather than a trans-septal approach.
The retrograde transaortic approach should be avoided in such procedures, “whenever it can be avoided,” said Dr. Lakkireddy, who formally presented STROKE-VT at the HRS sessions and is lead author on its report published about the same time in JACC: Clinical Electrophysiology.
The trial has limitations, but “it’s a very important study, and I think that this could become our standard of care for managing anticoagulation after VT and PVC left-sided ablations,” Mina K. Chung, MD, Cleveland Clinic, said as an invited discussant after Dr. Lakkireddy’s presentation.
How patients are treated with antithrombotics after ventricular ablations can vary widely, sometimes based on the operator’s “subjective feeling of how extensive the ablation is,” Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, not involved in the study, said during the STROKE-VT media briefing.
That’s consistent with the guidelines, which propose oral anticoagulation therapy after more extensive ventricular ablations and antiplatelets when the ablation is more limited – based more on consensus than firm evidence – as described by Jeffrey R. Winterfield, MD, Medical University of South Carolina, Charleston, and Usha Tedrow, MD, MSc, Brigham and Women’s Hospital, Boston, in an accompanying editorial.
“This is really the first randomized trial data, that I know of, that we have on this. So I do think it will be guideline-influencing,” Dr. Albert said.
“This should change practice,” agreed Jonathan P. Piccini, MD, MHS, Duke University, Durham, N.C., also not part of STROKE-VT. “A lot of evidence in the trial is consistent and provides a compelling story, not to mention that, in my opinion, the study probably underestimates the value of DOACs,” he told this news organization.
That’s because patients assigned to DOACs had far longer ablation times, “so their risk was even greater than in the aspirin arm,” Dr. Piccini said. Ablation times averaged 2,095 seconds in the DOAC group, compared with only 1,708 seconds in the aspirin group, probably because the preponderance of VT over PVC ablations for those getting a DOAC was even greater in the aspirin group.
Of the 246 patients assigned to either aspirin or a DOAC, usually a factor Xa inhibitor, 75% had undergone VT ablation and the remainder ablation for PVCs. Their mean age was 60 years and only 18% were women. None had experienced a cerebrovascular event in the previous 3 months.
The 30-day odds ratio for TIA or ischemic stroke in patients who received aspirin, compared with a DOAC, was 12.6 (95% confidence interval, 4.10-39.11; P < .001).
The corresponding OR for asymptomatic cerebral lesions by MRI at 24 hours was 2.15 (95% CI, 1.02-4.54; P = .04) and at 30 days was 3.48 (95% CI, 1.38-8.80; P = .008).
The rate of stroke or TIA was similar in patients who underwent ablation for VT and for PVCs (14% vs. 16%, respectively; P = .70). There were fewer asymptomatic cerebrovascular events by MRI at 24 hours for those undergoing VT ablations (14.7% and 25.8%, respectively; P = .046); but difference between rates attenuated by 30 days (11.4% and 14.5%, respectively; P = .52).
The OR for TIA or stroke associated with the retrograde transaortic approach, performed in about 40% of the patients, compared with the trans-septal approach in the remainder was 2.60 (95% CI, 1.06-6.37; P = .04).
“The study tells us it’s safe and indeed preferable to anticoagulate after an ablation procedure. But the more important finding, perhaps, wasn’t the one related to the core hypothesis. And that was the effect of retrograde access,” Paul A. Friedman, MD, Mayo Clinic, Rochester, Minn., said as an invited discussant after Dr. Lakkireddy’s formal presentation of the trial.
Whether a ventricular ablation is performed using the retrograde transaortic or trans-septal approach often depends on the location of the ablation targets in the left ventricle. But in some cases it’s a matter of operator preference, Dr. Piccini observed.
“There are some situations where, really, it is better to do retrograde aortic, and there are some cases that are better to do trans-septal. But now there’s going to be a higher burden of proof,” he said. Given the findings of STROKE-VT, operators may need to consider that a ventricular ablation procedure that can be done by the trans-septal route perhaps ought to be consistently done that way.
Dr. Lakkireddy discloses financial relationships with Boston Scientific, Biosense Webster, Janssen Pharmaceuticals, and more. Dr. Chung had “nothing relevant to disclose.” Dr. Piccini discloses receiving honoraria or speaking or consulting fees from Sanofi, Abbott, ARCA Biopharma, Medtronic, Philips, Biotronik, Allergan, LivaNova, and Myokardia; and research in conjunction with Bayer Healthcare, Abbott, Boston Scientific, and Philips. Dr. Friedman discloses conducting research in conjunction with Medtronic and Abbott; holding intellectual property rights with AliveCor, Inference, Medicool, Eko, and Anumana; and receiving honoraria or speaking or consulting fees from Boston Scientific. Dr. Winterfield and Dr. Tedrow had no disclosures.
A version of this article first appeared on Medscape.com.
Catheter ablation has been around a lot longer for ventricular arrhythmia than for atrial fibrillation, but far less is settled about what antithrombotic therapy should follow ventricular ablations, as there have been no big, randomized trials for guidance.
But the evidence base grew stronger this week, and it favors postprocedure treatment with a direct oral anticoagulant (DOAC) over antiplatelet therapy with aspirin for patients undergoing radiofrequency (RF) ablation to treat left ventricular (LV) arrhythmias.
The 30-day risk for ischemic stroke or transient ischemia attack (TIA) was sharply higher for patients who took daily aspirin after RF ablation for ventricular tachycardia (VT) or premature ventricular contractions (PVC) in a multicenter randomized trial.
Those of its 246 patients who received aspirin were also far more likely to show asymptomatic lesions on cerebral MRI scans performed both 24 hours and 30 days after the procedure.
The findings show the importance of DOAC therapy after ventricular ablation procedures, a setting for which there are no evidence-based guidelines, “to mitigate the risk of systemic thromboembolic events,” said Dhanunjaya Lakkireddy, MD, Kansas City Heart Rhythm Institute, Overland Park. He spoke at a media presentation on the trial, called STROKE-VT, during the Heart Rhythm Society 2021 Scientific Sessions, held virtually and on-site in Boston.
The risk for stroke and TIA went up in association with several procedural issues, including some that operators might be able to change in order to reach for better outcomes, Dr. Lakkireddy observed.
“Prolonged radiofrequency ablation times, especially in those with low left ventricle ejection fractions, are definitely higher risk,” as are procedures that involved the retrograde transaortic approach for advancing the ablation catheter, rather than a trans-septal approach.
The retrograde transaortic approach should be avoided in such procedures, “whenever it can be avoided,” said Dr. Lakkireddy, who formally presented STROKE-VT at the HRS sessions and is lead author on its report published about the same time in JACC: Clinical Electrophysiology.
The trial has limitations, but “it’s a very important study, and I think that this could become our standard of care for managing anticoagulation after VT and PVC left-sided ablations,” Mina K. Chung, MD, Cleveland Clinic, said as an invited discussant after Dr. Lakkireddy’s presentation.
How patients are treated with antithrombotics after ventricular ablations can vary widely, sometimes based on the operator’s “subjective feeling of how extensive the ablation is,” Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, not involved in the study, said during the STROKE-VT media briefing.
That’s consistent with the guidelines, which propose oral anticoagulation therapy after more extensive ventricular ablations and antiplatelets when the ablation is more limited – based more on consensus than firm evidence – as described by Jeffrey R. Winterfield, MD, Medical University of South Carolina, Charleston, and Usha Tedrow, MD, MSc, Brigham and Women’s Hospital, Boston, in an accompanying editorial.
“This is really the first randomized trial data, that I know of, that we have on this. So I do think it will be guideline-influencing,” Dr. Albert said.
“This should change practice,” agreed Jonathan P. Piccini, MD, MHS, Duke University, Durham, N.C., also not part of STROKE-VT. “A lot of evidence in the trial is consistent and provides a compelling story, not to mention that, in my opinion, the study probably underestimates the value of DOACs,” he told this news organization.
That’s because patients assigned to DOACs had far longer ablation times, “so their risk was even greater than in the aspirin arm,” Dr. Piccini said. Ablation times averaged 2,095 seconds in the DOAC group, compared with only 1,708 seconds in the aspirin group, probably because the preponderance of VT over PVC ablations for those getting a DOAC was even greater in the aspirin group.
Of the 246 patients assigned to either aspirin or a DOAC, usually a factor Xa inhibitor, 75% had undergone VT ablation and the remainder ablation for PVCs. Their mean age was 60 years and only 18% were women. None had experienced a cerebrovascular event in the previous 3 months.
The 30-day odds ratio for TIA or ischemic stroke in patients who received aspirin, compared with a DOAC, was 12.6 (95% confidence interval, 4.10-39.11; P < .001).
The corresponding OR for asymptomatic cerebral lesions by MRI at 24 hours was 2.15 (95% CI, 1.02-4.54; P = .04) and at 30 days was 3.48 (95% CI, 1.38-8.80; P = .008).
The rate of stroke or TIA was similar in patients who underwent ablation for VT and for PVCs (14% vs. 16%, respectively; P = .70). There were fewer asymptomatic cerebrovascular events by MRI at 24 hours for those undergoing VT ablations (14.7% and 25.8%, respectively; P = .046); but difference between rates attenuated by 30 days (11.4% and 14.5%, respectively; P = .52).
The OR for TIA or stroke associated with the retrograde transaortic approach, performed in about 40% of the patients, compared with the trans-septal approach in the remainder was 2.60 (95% CI, 1.06-6.37; P = .04).
“The study tells us it’s safe and indeed preferable to anticoagulate after an ablation procedure. But the more important finding, perhaps, wasn’t the one related to the core hypothesis. And that was the effect of retrograde access,” Paul A. Friedman, MD, Mayo Clinic, Rochester, Minn., said as an invited discussant after Dr. Lakkireddy’s formal presentation of the trial.
Whether a ventricular ablation is performed using the retrograde transaortic or trans-septal approach often depends on the location of the ablation targets in the left ventricle. But in some cases it’s a matter of operator preference, Dr. Piccini observed.
“There are some situations where, really, it is better to do retrograde aortic, and there are some cases that are better to do trans-septal. But now there’s going to be a higher burden of proof,” he said. Given the findings of STROKE-VT, operators may need to consider that a ventricular ablation procedure that can be done by the trans-septal route perhaps ought to be consistently done that way.
Dr. Lakkireddy discloses financial relationships with Boston Scientific, Biosense Webster, Janssen Pharmaceuticals, and more. Dr. Chung had “nothing relevant to disclose.” Dr. Piccini discloses receiving honoraria or speaking or consulting fees from Sanofi, Abbott, ARCA Biopharma, Medtronic, Philips, Biotronik, Allergan, LivaNova, and Myokardia; and research in conjunction with Bayer Healthcare, Abbott, Boston Scientific, and Philips. Dr. Friedman discloses conducting research in conjunction with Medtronic and Abbott; holding intellectual property rights with AliveCor, Inference, Medicool, Eko, and Anumana; and receiving honoraria or speaking or consulting fees from Boston Scientific. Dr. Winterfield and Dr. Tedrow had no disclosures.
A version of this article first appeared on Medscape.com.
Bronchitis the leader at putting children in the hospital
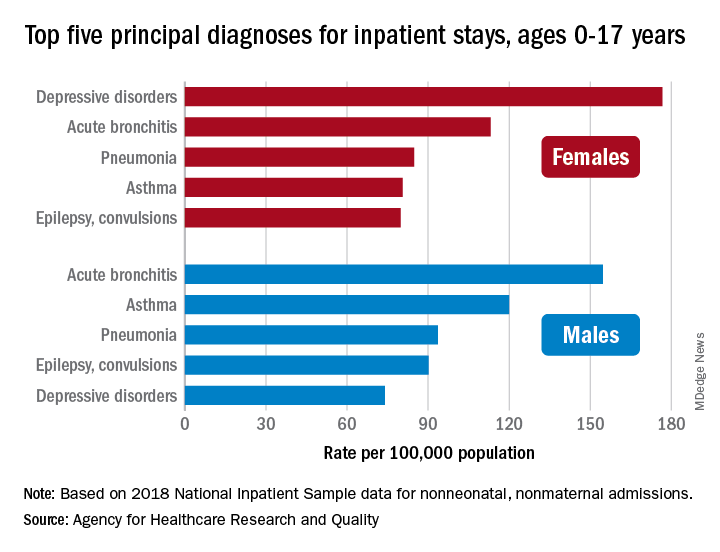
About 7% (99,000) of the 1.47 million nonmaternal, nonneonatal hospital stays in children aged 0-17 years involved a primary diagnosis of acute bronchitis in 2018, representing the leading cause of admissions in boys (154.7 stays per 100,000 population) and the second-leading diagnosis in girls (113.1 stays per 100,000), Kimberly W. McDermott, PhD, and Marc Roemer, MS, said in a statistical brief.
Depressive disorders were the most common primary diagnosis in girls, with a rate of 176.7 stays per 100,000, and the second-leading diagnosis overall, although the rate was less than half that (74.0 per 100,000) in boys. Two other respiratory conditions, asthma and pneumonia, were among the top five for both girls and boys, as was epilepsy, they reported.
The combined rate for all diagnoses was slightly higher for boys, 2,051 per 100,000, compared with 1,922 for girls, they said based on data from the National Inpatient Sample.
“Identifying the most frequent primary conditions for which patients are admitted to the hospital is important to the implementation and improvement of health care delivery, quality initiatives, and health policy,” said Dr. McDermott of IBM Watson Health and Mr. Roemer of the AHRQ.

About 7% (99,000) of the 1.47 million nonmaternal, nonneonatal hospital stays in children aged 0-17 years involved a primary diagnosis of acute bronchitis in 2018, representing the leading cause of admissions in boys (154.7 stays per 100,000 population) and the second-leading diagnosis in girls (113.1 stays per 100,000), Kimberly W. McDermott, PhD, and Marc Roemer, MS, said in a statistical brief.
Depressive disorders were the most common primary diagnosis in girls, with a rate of 176.7 stays per 100,000, and the second-leading diagnosis overall, although the rate was less than half that (74.0 per 100,000) in boys. Two other respiratory conditions, asthma and pneumonia, were among the top five for both girls and boys, as was epilepsy, they reported.
The combined rate for all diagnoses was slightly higher for boys, 2,051 per 100,000, compared with 1,922 for girls, they said based on data from the National Inpatient Sample.
“Identifying the most frequent primary conditions for which patients are admitted to the hospital is important to the implementation and improvement of health care delivery, quality initiatives, and health policy,” said Dr. McDermott of IBM Watson Health and Mr. Roemer of the AHRQ.

About 7% (99,000) of the 1.47 million nonmaternal, nonneonatal hospital stays in children aged 0-17 years involved a primary diagnosis of acute bronchitis in 2018, representing the leading cause of admissions in boys (154.7 stays per 100,000 population) and the second-leading diagnosis in girls (113.1 stays per 100,000), Kimberly W. McDermott, PhD, and Marc Roemer, MS, said in a statistical brief.
Depressive disorders were the most common primary diagnosis in girls, with a rate of 176.7 stays per 100,000, and the second-leading diagnosis overall, although the rate was less than half that (74.0 per 100,000) in boys. Two other respiratory conditions, asthma and pneumonia, were among the top five for both girls and boys, as was epilepsy, they reported.
The combined rate for all diagnoses was slightly higher for boys, 2,051 per 100,000, compared with 1,922 for girls, they said based on data from the National Inpatient Sample.
“Identifying the most frequent primary conditions for which patients are admitted to the hospital is important to the implementation and improvement of health care delivery, quality initiatives, and health policy,” said Dr. McDermott of IBM Watson Health and Mr. Roemer of the AHRQ.
Early transition to oral beta-lactams for low-risk S. aureus bacteremia may be acceptable
Background: There is consensus that LR-SAB can be safely treated with 14 days of antibiotic therapy, but the use of and/or proportion of duration of oral antibiotics is not clear. There is evidence that oral therapy has fewer treatment complications, compared with IV treatments. Objective of this study was to assess the safety of early oral switch (EOS) prior to 14 days for LR-SAB.
Study design: Retrospective cohort study.
Setting: Single institution tertiary care hospital in Wellington, New Zealand.
Synopsis: Study population included adults with health care–associated SAB deemed low risk (no positive blood cultures >72 hours after initial positive culture, no evidence of deep infection as determined by an infectious disease consultant, no nonremovable prosthetics). The primary outcome was occurrence of SAB-related complication (recurrence of SAB, deep-seated infection, readmission, attributable mortality) within 90 days.
Of the initial 469 episodes of SAB, 100 met inclusion, and 84 of those patients had EOS. Line infection was the source in a majority of patients (79% and 88% in EOS and IV, respectively). Only 5% of patients had MRSA. Overall, 86% of EOS patients were treated with an oral beta-lactam, within the EOS group, median duration of IV and oral antibiotics was 5 and 10 days, respectively. SAB recurrence within 90 days occurred in three (4%) and one (6%) patients in EOS vs. IV groups, respectively (P = .64). No deaths within 90 days were deemed attributable to SAB. Limitations include small size, single center, and observational, retrospective framework.
Bottom line: The study suggests that EOS with oral beta-lactams in selected patients with LR-SAB may be adequate; however, the study is too small to provide robust high-level evidence. Instead, the authors hope the data will lead to larger, more powerful prospective studies to examine if a simpler, cheaper, and in some ways safer treatment course is possible.
Citation: Bupha-Intr O et al. Efficacy of early oral switch with beta-lactams for low-risk Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2020 Feb 3;AAC.02345-19. doi: 10.1128/AAC.02345-19.
Dr. Sneed is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: There is consensus that LR-SAB can be safely treated with 14 days of antibiotic therapy, but the use of and/or proportion of duration of oral antibiotics is not clear. There is evidence that oral therapy has fewer treatment complications, compared with IV treatments. Objective of this study was to assess the safety of early oral switch (EOS) prior to 14 days for LR-SAB.
Study design: Retrospective cohort study.
Setting: Single institution tertiary care hospital in Wellington, New Zealand.
Synopsis: Study population included adults with health care–associated SAB deemed low risk (no positive blood cultures >72 hours after initial positive culture, no evidence of deep infection as determined by an infectious disease consultant, no nonremovable prosthetics). The primary outcome was occurrence of SAB-related complication (recurrence of SAB, deep-seated infection, readmission, attributable mortality) within 90 days.
Of the initial 469 episodes of SAB, 100 met inclusion, and 84 of those patients had EOS. Line infection was the source in a majority of patients (79% and 88% in EOS and IV, respectively). Only 5% of patients had MRSA. Overall, 86% of EOS patients were treated with an oral beta-lactam, within the EOS group, median duration of IV and oral antibiotics was 5 and 10 days, respectively. SAB recurrence within 90 days occurred in three (4%) and one (6%) patients in EOS vs. IV groups, respectively (P = .64). No deaths within 90 days were deemed attributable to SAB. Limitations include small size, single center, and observational, retrospective framework.
Bottom line: The study suggests that EOS with oral beta-lactams in selected patients with LR-SAB may be adequate; however, the study is too small to provide robust high-level evidence. Instead, the authors hope the data will lead to larger, more powerful prospective studies to examine if a simpler, cheaper, and in some ways safer treatment course is possible.
Citation: Bupha-Intr O et al. Efficacy of early oral switch with beta-lactams for low-risk Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2020 Feb 3;AAC.02345-19. doi: 10.1128/AAC.02345-19.
Dr. Sneed is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: There is consensus that LR-SAB can be safely treated with 14 days of antibiotic therapy, but the use of and/or proportion of duration of oral antibiotics is not clear. There is evidence that oral therapy has fewer treatment complications, compared with IV treatments. Objective of this study was to assess the safety of early oral switch (EOS) prior to 14 days for LR-SAB.
Study design: Retrospective cohort study.
Setting: Single institution tertiary care hospital in Wellington, New Zealand.
Synopsis: Study population included adults with health care–associated SAB deemed low risk (no positive blood cultures >72 hours after initial positive culture, no evidence of deep infection as determined by an infectious disease consultant, no nonremovable prosthetics). The primary outcome was occurrence of SAB-related complication (recurrence of SAB, deep-seated infection, readmission, attributable mortality) within 90 days.
Of the initial 469 episodes of SAB, 100 met inclusion, and 84 of those patients had EOS. Line infection was the source in a majority of patients (79% and 88% in EOS and IV, respectively). Only 5% of patients had MRSA. Overall, 86% of EOS patients were treated with an oral beta-lactam, within the EOS group, median duration of IV and oral antibiotics was 5 and 10 days, respectively. SAB recurrence within 90 days occurred in three (4%) and one (6%) patients in EOS vs. IV groups, respectively (P = .64). No deaths within 90 days were deemed attributable to SAB. Limitations include small size, single center, and observational, retrospective framework.
Bottom line: The study suggests that EOS with oral beta-lactams in selected patients with LR-SAB may be adequate; however, the study is too small to provide robust high-level evidence. Instead, the authors hope the data will lead to larger, more powerful prospective studies to examine if a simpler, cheaper, and in some ways safer treatment course is possible.
Citation: Bupha-Intr O et al. Efficacy of early oral switch with beta-lactams for low-risk Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2020 Feb 3;AAC.02345-19. doi: 10.1128/AAC.02345-19.
Dr. Sneed is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
‘A few mutations away’: The threat of a vaccine-proof variant
The Centers for Disease Control and Prevention Director Rochelle Walensky, MD, MPH, made a dire prediction during a media briefing this week that, if we weren’t already living within the reality of the COVID-19 pandemic, would sound more like a pitch for a movie about a dystopian future.
“For the amount of virus circulating in this country right now largely among unvaccinated people, the largest concern that we in public health and science are worried about is that the virus … [becomes] a very transmissible virus that has the potential to evade our vaccines in terms of how it protects us from severe disease and death,” Dr. Walensky told reporters on July 27.
A new, more elusive variant could be “just a few mutations away,” she said.
“That’s a very prescient comment,” Lewis Nelson, MD, professor and clinical chair of emergency medicine and chief of the division of medical toxicology at Rutgers New Jersey Medical School in Newark, told this news organization.
“We’ve gone through a few mutations already that have been named, and each one of them gets a little more transmissible,” he said. “That’s normal, natural selection and what you would expect to happen as viruses mutate from one strain to another.”
“What we’ve mostly seen this virus do is evolve to become more infectious,” said Stuart Ray, MD, when also asked to comment. “That is the remarkable feature of Delta – that it is so infectious.”
He said that the SARS-CoV-2 has evolved largely as expected, at least so far. “The potential for this virus to mutate has been something that has been a concern from early on.”
“The viral evolution is a bit like a ticking clock. The more we allow infections to occur, the more likely changes will occur. When we have lots of people infected, we give more chances to the virus to diversify and then adapt to selective pressures,” said Dr. Ray, vice-chair of medicine for data integrity and analytics and professor in the division of infectious diseases at Johns Hopkins School of Medicine in Baltimore.
Dr. Nelson said.
If this occurs, he added, “we will have an ineffective vaccine, essentially. And we’ll be back to where we were last March with a brand-new disease.”
Technology to the rescue?
The flexibility of mRNA vaccines is one potential solution. These vaccines could be more easily and quickly adapted to respond to a new, more vaccine-elusive variant.
“That’s absolutely reassuring,” Dr. Nelson said. For example, if a mutation changes the spike protein and vaccines no longer recognize it, a manufacturer could identify the new protein and incorporate that in a new mRNA vaccine.
“The problem is that some people are not taking the current vaccine,” he added. “I’m not sure what is going to make them take the next vaccine.”
Nothing appears certain
When asked how likely a new strain of SARS-CoV-2 could emerge that gets around vaccine protection, Dr. Nelson said, “I think [what] we’ve learned so far there is no way to predict anything” about this pandemic.
“The best way to prevent the virus from mutating is to prevent hosts, people, from getting sick with it,” he said. “That’s why it’s so important people should get immunized and wear masks.”
Both Dr. Nelson and Dr. Ray pointed out that it is in the best interest of the virus to evolve to be more transmissible and spread to more people. In contrast, a virus that causes people to get so sick that they isolate or die, thus halting transmission, works against viruses surviving evolutionarily.
Some viruses also mutate to become milder over time, but that has not been the case with SARS-CoV-2, Dr. Ray said.
Mutations not the only concern
Viruses have another mechanism that produces new strains, and it works even more quickly than mutations. Recombination, as it’s known, can occur when a person is infected with two different strains of the same virus. If the two versions enter the same cell, the viruses can swap genetic material and produce a third, altogether different strain.
Recombination has already been seen with influenza strains, where H and N genetic segments are swapped to yield H1N1, H1N2, and H3N2 versions of the flu, for example.
“In the early days of SARS-CoV-2 there was so little diversity that recombination did not matter,” Dr. Ray said. However, there are now distinct lineages of the virus circulating globally. If two of these lineages swap segments “this would make a very new viral sequence in one step without having to mutate to gain those differences.”
“The more diverse the strains that are circulating, the bigger a possibility this is,” Dr. Ray said.
Protected, for now
Dr. Walensky’s sober warning came at the same time the CDC released new guidance calling for the wearing of masks indoors in schools and in any location in the country where COVID-19 cases surpass 50 people per 100,000, also known as substantial or high transmission areas.
On a positive note, Dr. Walensky said: “Right now, fortunately, we are not there. The vaccines operate really well in protecting us from severe disease and death.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention Director Rochelle Walensky, MD, MPH, made a dire prediction during a media briefing this week that, if we weren’t already living within the reality of the COVID-19 pandemic, would sound more like a pitch for a movie about a dystopian future.
“For the amount of virus circulating in this country right now largely among unvaccinated people, the largest concern that we in public health and science are worried about is that the virus … [becomes] a very transmissible virus that has the potential to evade our vaccines in terms of how it protects us from severe disease and death,” Dr. Walensky told reporters on July 27.
A new, more elusive variant could be “just a few mutations away,” she said.
“That’s a very prescient comment,” Lewis Nelson, MD, professor and clinical chair of emergency medicine and chief of the division of medical toxicology at Rutgers New Jersey Medical School in Newark, told this news organization.
“We’ve gone through a few mutations already that have been named, and each one of them gets a little more transmissible,” he said. “That’s normal, natural selection and what you would expect to happen as viruses mutate from one strain to another.”
“What we’ve mostly seen this virus do is evolve to become more infectious,” said Stuart Ray, MD, when also asked to comment. “That is the remarkable feature of Delta – that it is so infectious.”
He said that the SARS-CoV-2 has evolved largely as expected, at least so far. “The potential for this virus to mutate has been something that has been a concern from early on.”
“The viral evolution is a bit like a ticking clock. The more we allow infections to occur, the more likely changes will occur. When we have lots of people infected, we give more chances to the virus to diversify and then adapt to selective pressures,” said Dr. Ray, vice-chair of medicine for data integrity and analytics and professor in the division of infectious diseases at Johns Hopkins School of Medicine in Baltimore.
Dr. Nelson said.
If this occurs, he added, “we will have an ineffective vaccine, essentially. And we’ll be back to where we were last March with a brand-new disease.”
Technology to the rescue?
The flexibility of mRNA vaccines is one potential solution. These vaccines could be more easily and quickly adapted to respond to a new, more vaccine-elusive variant.
“That’s absolutely reassuring,” Dr. Nelson said. For example, if a mutation changes the spike protein and vaccines no longer recognize it, a manufacturer could identify the new protein and incorporate that in a new mRNA vaccine.
“The problem is that some people are not taking the current vaccine,” he added. “I’m not sure what is going to make them take the next vaccine.”
Nothing appears certain
When asked how likely a new strain of SARS-CoV-2 could emerge that gets around vaccine protection, Dr. Nelson said, “I think [what] we’ve learned so far there is no way to predict anything” about this pandemic.
“The best way to prevent the virus from mutating is to prevent hosts, people, from getting sick with it,” he said. “That’s why it’s so important people should get immunized and wear masks.”
Both Dr. Nelson and Dr. Ray pointed out that it is in the best interest of the virus to evolve to be more transmissible and spread to more people. In contrast, a virus that causes people to get so sick that they isolate or die, thus halting transmission, works against viruses surviving evolutionarily.
Some viruses also mutate to become milder over time, but that has not been the case with SARS-CoV-2, Dr. Ray said.
Mutations not the only concern
Viruses have another mechanism that produces new strains, and it works even more quickly than mutations. Recombination, as it’s known, can occur when a person is infected with two different strains of the same virus. If the two versions enter the same cell, the viruses can swap genetic material and produce a third, altogether different strain.
Recombination has already been seen with influenza strains, where H and N genetic segments are swapped to yield H1N1, H1N2, and H3N2 versions of the flu, for example.
“In the early days of SARS-CoV-2 there was so little diversity that recombination did not matter,” Dr. Ray said. However, there are now distinct lineages of the virus circulating globally. If two of these lineages swap segments “this would make a very new viral sequence in one step without having to mutate to gain those differences.”
“The more diverse the strains that are circulating, the bigger a possibility this is,” Dr. Ray said.
Protected, for now
Dr. Walensky’s sober warning came at the same time the CDC released new guidance calling for the wearing of masks indoors in schools and in any location in the country where COVID-19 cases surpass 50 people per 100,000, also known as substantial or high transmission areas.
On a positive note, Dr. Walensky said: “Right now, fortunately, we are not there. The vaccines operate really well in protecting us from severe disease and death.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention Director Rochelle Walensky, MD, MPH, made a dire prediction during a media briefing this week that, if we weren’t already living within the reality of the COVID-19 pandemic, would sound more like a pitch for a movie about a dystopian future.
“For the amount of virus circulating in this country right now largely among unvaccinated people, the largest concern that we in public health and science are worried about is that the virus … [becomes] a very transmissible virus that has the potential to evade our vaccines in terms of how it protects us from severe disease and death,” Dr. Walensky told reporters on July 27.
A new, more elusive variant could be “just a few mutations away,” she said.
“That’s a very prescient comment,” Lewis Nelson, MD, professor and clinical chair of emergency medicine and chief of the division of medical toxicology at Rutgers New Jersey Medical School in Newark, told this news organization.
“We’ve gone through a few mutations already that have been named, and each one of them gets a little more transmissible,” he said. “That’s normal, natural selection and what you would expect to happen as viruses mutate from one strain to another.”
“What we’ve mostly seen this virus do is evolve to become more infectious,” said Stuart Ray, MD, when also asked to comment. “That is the remarkable feature of Delta – that it is so infectious.”
He said that the SARS-CoV-2 has evolved largely as expected, at least so far. “The potential for this virus to mutate has been something that has been a concern from early on.”
“The viral evolution is a bit like a ticking clock. The more we allow infections to occur, the more likely changes will occur. When we have lots of people infected, we give more chances to the virus to diversify and then adapt to selective pressures,” said Dr. Ray, vice-chair of medicine for data integrity and analytics and professor in the division of infectious diseases at Johns Hopkins School of Medicine in Baltimore.
Dr. Nelson said.
If this occurs, he added, “we will have an ineffective vaccine, essentially. And we’ll be back to where we were last March with a brand-new disease.”
Technology to the rescue?
The flexibility of mRNA vaccines is one potential solution. These vaccines could be more easily and quickly adapted to respond to a new, more vaccine-elusive variant.
“That’s absolutely reassuring,” Dr. Nelson said. For example, if a mutation changes the spike protein and vaccines no longer recognize it, a manufacturer could identify the new protein and incorporate that in a new mRNA vaccine.
“The problem is that some people are not taking the current vaccine,” he added. “I’m not sure what is going to make them take the next vaccine.”
Nothing appears certain
When asked how likely a new strain of SARS-CoV-2 could emerge that gets around vaccine protection, Dr. Nelson said, “I think [what] we’ve learned so far there is no way to predict anything” about this pandemic.
“The best way to prevent the virus from mutating is to prevent hosts, people, from getting sick with it,” he said. “That’s why it’s so important people should get immunized and wear masks.”
Both Dr. Nelson and Dr. Ray pointed out that it is in the best interest of the virus to evolve to be more transmissible and spread to more people. In contrast, a virus that causes people to get so sick that they isolate or die, thus halting transmission, works against viruses surviving evolutionarily.
Some viruses also mutate to become milder over time, but that has not been the case with SARS-CoV-2, Dr. Ray said.
Mutations not the only concern
Viruses have another mechanism that produces new strains, and it works even more quickly than mutations. Recombination, as it’s known, can occur when a person is infected with two different strains of the same virus. If the two versions enter the same cell, the viruses can swap genetic material and produce a third, altogether different strain.
Recombination has already been seen with influenza strains, where H and N genetic segments are swapped to yield H1N1, H1N2, and H3N2 versions of the flu, for example.
“In the early days of SARS-CoV-2 there was so little diversity that recombination did not matter,” Dr. Ray said. However, there are now distinct lineages of the virus circulating globally. If two of these lineages swap segments “this would make a very new viral sequence in one step without having to mutate to gain those differences.”
“The more diverse the strains that are circulating, the bigger a possibility this is,” Dr. Ray said.
Protected, for now
Dr. Walensky’s sober warning came at the same time the CDC released new guidance calling for the wearing of masks indoors in schools and in any location in the country where COVID-19 cases surpass 50 people per 100,000, also known as substantial or high transmission areas.
On a positive note, Dr. Walensky said: “Right now, fortunately, we are not there. The vaccines operate really well in protecting us from severe disease and death.”
A version of this article first appeared on Medscape.com.
Vaccinated people infected with Delta remain contagious
, The New York Times reported late on July 29.
The revelation is one reason the agency reversed course this week and said fully vaccinated people should go back to wearing masks in many cases.
The new findings also are a reversal from what scientists had believed to be true about other variants of the virus, the Times said. The bottom line is that the CDC data show people with so-called breakthrough cases of the Delta variant may be just as contagious as unvaccinated people, even if they do not show symptoms.
ABC News reported earlier on July 29 that the CDC’s updated mask guidance followed an outbreak in Cape Cod, where crowds gathered for the Fourth of July.
As of July 29, 882 people were tied to the outbreak centered in Provincetown, Mass. Of those who live in Massachusetts, 74% were unvaccinated. ABC said the majority were showing symptoms of COVID-19.
, The New York Times reported late on July 29.
The revelation is one reason the agency reversed course this week and said fully vaccinated people should go back to wearing masks in many cases.
The new findings also are a reversal from what scientists had believed to be true about other variants of the virus, the Times said. The bottom line is that the CDC data show people with so-called breakthrough cases of the Delta variant may be just as contagious as unvaccinated people, even if they do not show symptoms.
ABC News reported earlier on July 29 that the CDC’s updated mask guidance followed an outbreak in Cape Cod, where crowds gathered for the Fourth of July.
As of July 29, 882 people were tied to the outbreak centered in Provincetown, Mass. Of those who live in Massachusetts, 74% were unvaccinated. ABC said the majority were showing symptoms of COVID-19.
, The New York Times reported late on July 29.
The revelation is one reason the agency reversed course this week and said fully vaccinated people should go back to wearing masks in many cases.
The new findings also are a reversal from what scientists had believed to be true about other variants of the virus, the Times said. The bottom line is that the CDC data show people with so-called breakthrough cases of the Delta variant may be just as contagious as unvaccinated people, even if they do not show symptoms.
ABC News reported earlier on July 29 that the CDC’s updated mask guidance followed an outbreak in Cape Cod, where crowds gathered for the Fourth of July.
As of July 29, 882 people were tied to the outbreak centered in Provincetown, Mass. Of those who live in Massachusetts, 74% were unvaccinated. ABC said the majority were showing symptoms of COVID-19.
As common respiratory viruses resurface, children are at serious risk
Younger children may be vulnerable to the reemergence of common respiratory viruses such as influenza and respiratory syncytial virus (RSV) as COVID-19 restrictions wane, experts say. The impact could be detrimental.
The COVID-19 pandemic and the implementation of preventative measures such as social distancing, travel restrictions, mask use, and shelter in place, reduced the transmission of respiratory viruses, according to the Centers for Disease Control and Prevention. However, because older infants and toddlers have not been exposed to these bugs during the pandemic, they are vulnerable to suffering severe viral infections.
“[We’ve] been in the honeymoon for 18 months,” said Christopher J. Harrison, MD, professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. “We are going to be coming out of the honeymoon and the children who didn’t get sick are going to start packing 2 years’ worth of infections into the next 9 months so there’s going to be twice as many as would be normal.”
The CDC issued a health advisory in June for parts of the southern United States, such as Texas, the Carolinas, and Oklahoma, encouraging broader testing for RSV – a virus that usually causes mild, cold-like symptoms and is the most common cause of bronchiolitis and pneumonia in children – among those who test negative for COVID-19. Virtually all children get an RSV infection by the time they are 2 years old, according to the CDC.
In previous years, RSV usually spread during the fall and spring seasons and usually peaked late December to mid-February. However, there’s been an offseason spike in the common illness this year, with nearly 2,000 confirmed cases each week of July.
Richard J. Webby, PhD, of the infectious diseases department at St. Jude Children’s Research Hospital, Memphis, Tenn., said that although RSV transmits more easily during the winter, the virus is able to thrive during this summer because many children have limited immunity and are more vulnerable to catching the virus than before. Population immunity normally limits a virus to circulating under its most favorable conditions, which is usually the winter. However, because there are a few more “susceptible hosts,” it gives the virus the ability to spread during a time when it typically wouldn’t be able to.
“Now we have a wider range of susceptible kids because they haven’t had that exposure over the past 18 months,” said Dr. Webby, who is on the World Health Organization’s Influenza Vaccine Composition Advisory Team. “It gives the virus more chances to transmit during conditions that are less favorable.”
Dr. Harrison said that, if children continue to take preventative measures such as wearing masks and sanitizing, they can delay catching the RSV – which can be severe in infants and young children – until they’re older and symptoms won’t be as severe.
“The swelling that these viruses cause in the trachea and the bronchial tubes is much bigger in proportion to the overall size of the tubes, so it takes less swelling to clog up the trachea or bronchial tube for the 9-month-old than it does of a 9-year-old,” Dr. Harrison said. “So if a 9-year-old was to get RSV, they’re not going to have nearly the same amount symptoms as the 9-month-old.
Dr. Harrison said delaying RSV in children was never an option before because it’s a virus that’s almost impossible to avoid.
“Hopefully, the mask means that if you get exposed, instead of getting a million virus particles from your classmate or your playmate, you may only get a couple thousand,” Dr. Harrison explained. “And maybe that’s enough that you can fight it off or it may be small enough that you get a mild infection instead of a severe infection.”
A summer surge of RSV has also occurred in Australia. A study published in Clinical Infectious Diseases found that Western Australia saw a 98% reduction in RSV cases. This suggests that COVID-19 restrictions also delayed the RSV season.
Dr. Webby said the lax in penetrative measures against COVID-19 may also affect this upcoming flu season. Usually, around 10%-30% of the population gets infected with the flu each year, but that hasn’t happened the past couple of seasons, he said.
“There might be slightly less overall immunity to these viruses,” Dr. Webby said. “When these viruses do come back, there’s a little bit more room for them to take off.”
Although a severe influenza season rebound this winter is a possibility, Australia continues to experience a historically low flu season. Dr. Harrison, who said the northern hemisphere looks at what’s happening in Australia and the rest of the “southern half of the world because their influenza season is during our summer,” hopes this is an indication that the northern hemisphere will also experience a mild season.
However, there’s no indication of how this upcoming flu season will hit the United States and there isn’t any guidance on what could happen because these historically low levels of respiratory viruses have never happened before, Dr. Webby explained.
He said that, if COVID-19’s delta variant continues to circulate during the fall and winter seasons, it will keep other viruses at low levels. This is because there is rarely a peak of activity of different viruses at the same time.
“When you get infected with the virus, your body’s immune response has this nonspecific reaction that protects you from anything else for a short period of time,” Dr. Webby explained. “When you get a lot of one virus circulating, it’s really hard for these other viruses to get into that population and sort of set off an epidemic of their own.”
To prepare for an unsure influenza season, Dr. Harrison suggests making the influenza vaccine available in August as opposed to October.
Dr. Harrison and Dr. Webby reported no conflicts of interest.
Younger children may be vulnerable to the reemergence of common respiratory viruses such as influenza and respiratory syncytial virus (RSV) as COVID-19 restrictions wane, experts say. The impact could be detrimental.
The COVID-19 pandemic and the implementation of preventative measures such as social distancing, travel restrictions, mask use, and shelter in place, reduced the transmission of respiratory viruses, according to the Centers for Disease Control and Prevention. However, because older infants and toddlers have not been exposed to these bugs during the pandemic, they are vulnerable to suffering severe viral infections.
“[We’ve] been in the honeymoon for 18 months,” said Christopher J. Harrison, MD, professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. “We are going to be coming out of the honeymoon and the children who didn’t get sick are going to start packing 2 years’ worth of infections into the next 9 months so there’s going to be twice as many as would be normal.”
The CDC issued a health advisory in June for parts of the southern United States, such as Texas, the Carolinas, and Oklahoma, encouraging broader testing for RSV – a virus that usually causes mild, cold-like symptoms and is the most common cause of bronchiolitis and pneumonia in children – among those who test negative for COVID-19. Virtually all children get an RSV infection by the time they are 2 years old, according to the CDC.
In previous years, RSV usually spread during the fall and spring seasons and usually peaked late December to mid-February. However, there’s been an offseason spike in the common illness this year, with nearly 2,000 confirmed cases each week of July.
Richard J. Webby, PhD, of the infectious diseases department at St. Jude Children’s Research Hospital, Memphis, Tenn., said that although RSV transmits more easily during the winter, the virus is able to thrive during this summer because many children have limited immunity and are more vulnerable to catching the virus than before. Population immunity normally limits a virus to circulating under its most favorable conditions, which is usually the winter. However, because there are a few more “susceptible hosts,” it gives the virus the ability to spread during a time when it typically wouldn’t be able to.
“Now we have a wider range of susceptible kids because they haven’t had that exposure over the past 18 months,” said Dr. Webby, who is on the World Health Organization’s Influenza Vaccine Composition Advisory Team. “It gives the virus more chances to transmit during conditions that are less favorable.”
Dr. Harrison said that, if children continue to take preventative measures such as wearing masks and sanitizing, they can delay catching the RSV – which can be severe in infants and young children – until they’re older and symptoms won’t be as severe.
“The swelling that these viruses cause in the trachea and the bronchial tubes is much bigger in proportion to the overall size of the tubes, so it takes less swelling to clog up the trachea or bronchial tube for the 9-month-old than it does of a 9-year-old,” Dr. Harrison said. “So if a 9-year-old was to get RSV, they’re not going to have nearly the same amount symptoms as the 9-month-old.
Dr. Harrison said delaying RSV in children was never an option before because it’s a virus that’s almost impossible to avoid.
“Hopefully, the mask means that if you get exposed, instead of getting a million virus particles from your classmate or your playmate, you may only get a couple thousand,” Dr. Harrison explained. “And maybe that’s enough that you can fight it off or it may be small enough that you get a mild infection instead of a severe infection.”
A summer surge of RSV has also occurred in Australia. A study published in Clinical Infectious Diseases found that Western Australia saw a 98% reduction in RSV cases. This suggests that COVID-19 restrictions also delayed the RSV season.
Dr. Webby said the lax in penetrative measures against COVID-19 may also affect this upcoming flu season. Usually, around 10%-30% of the population gets infected with the flu each year, but that hasn’t happened the past couple of seasons, he said.
“There might be slightly less overall immunity to these viruses,” Dr. Webby said. “When these viruses do come back, there’s a little bit more room for them to take off.”
Although a severe influenza season rebound this winter is a possibility, Australia continues to experience a historically low flu season. Dr. Harrison, who said the northern hemisphere looks at what’s happening in Australia and the rest of the “southern half of the world because their influenza season is during our summer,” hopes this is an indication that the northern hemisphere will also experience a mild season.
However, there’s no indication of how this upcoming flu season will hit the United States and there isn’t any guidance on what could happen because these historically low levels of respiratory viruses have never happened before, Dr. Webby explained.
He said that, if COVID-19’s delta variant continues to circulate during the fall and winter seasons, it will keep other viruses at low levels. This is because there is rarely a peak of activity of different viruses at the same time.
“When you get infected with the virus, your body’s immune response has this nonspecific reaction that protects you from anything else for a short period of time,” Dr. Webby explained. “When you get a lot of one virus circulating, it’s really hard for these other viruses to get into that population and sort of set off an epidemic of their own.”
To prepare for an unsure influenza season, Dr. Harrison suggests making the influenza vaccine available in August as opposed to October.
Dr. Harrison and Dr. Webby reported no conflicts of interest.
Younger children may be vulnerable to the reemergence of common respiratory viruses such as influenza and respiratory syncytial virus (RSV) as COVID-19 restrictions wane, experts say. The impact could be detrimental.
The COVID-19 pandemic and the implementation of preventative measures such as social distancing, travel restrictions, mask use, and shelter in place, reduced the transmission of respiratory viruses, according to the Centers for Disease Control and Prevention. However, because older infants and toddlers have not been exposed to these bugs during the pandemic, they are vulnerable to suffering severe viral infections.
“[We’ve] been in the honeymoon for 18 months,” said Christopher J. Harrison, MD, professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics in Kansas City, Mo. “We are going to be coming out of the honeymoon and the children who didn’t get sick are going to start packing 2 years’ worth of infections into the next 9 months so there’s going to be twice as many as would be normal.”
The CDC issued a health advisory in June for parts of the southern United States, such as Texas, the Carolinas, and Oklahoma, encouraging broader testing for RSV – a virus that usually causes mild, cold-like symptoms and is the most common cause of bronchiolitis and pneumonia in children – among those who test negative for COVID-19. Virtually all children get an RSV infection by the time they are 2 years old, according to the CDC.
In previous years, RSV usually spread during the fall and spring seasons and usually peaked late December to mid-February. However, there’s been an offseason spike in the common illness this year, with nearly 2,000 confirmed cases each week of July.
Richard J. Webby, PhD, of the infectious diseases department at St. Jude Children’s Research Hospital, Memphis, Tenn., said that although RSV transmits more easily during the winter, the virus is able to thrive during this summer because many children have limited immunity and are more vulnerable to catching the virus than before. Population immunity normally limits a virus to circulating under its most favorable conditions, which is usually the winter. However, because there are a few more “susceptible hosts,” it gives the virus the ability to spread during a time when it typically wouldn’t be able to.
“Now we have a wider range of susceptible kids because they haven’t had that exposure over the past 18 months,” said Dr. Webby, who is on the World Health Organization’s Influenza Vaccine Composition Advisory Team. “It gives the virus more chances to transmit during conditions that are less favorable.”
Dr. Harrison said that, if children continue to take preventative measures such as wearing masks and sanitizing, they can delay catching the RSV – which can be severe in infants and young children – until they’re older and symptoms won’t be as severe.
“The swelling that these viruses cause in the trachea and the bronchial tubes is much bigger in proportion to the overall size of the tubes, so it takes less swelling to clog up the trachea or bronchial tube for the 9-month-old than it does of a 9-year-old,” Dr. Harrison said. “So if a 9-year-old was to get RSV, they’re not going to have nearly the same amount symptoms as the 9-month-old.
Dr. Harrison said delaying RSV in children was never an option before because it’s a virus that’s almost impossible to avoid.
“Hopefully, the mask means that if you get exposed, instead of getting a million virus particles from your classmate or your playmate, you may only get a couple thousand,” Dr. Harrison explained. “And maybe that’s enough that you can fight it off or it may be small enough that you get a mild infection instead of a severe infection.”
A summer surge of RSV has also occurred in Australia. A study published in Clinical Infectious Diseases found that Western Australia saw a 98% reduction in RSV cases. This suggests that COVID-19 restrictions also delayed the RSV season.
Dr. Webby said the lax in penetrative measures against COVID-19 may also affect this upcoming flu season. Usually, around 10%-30% of the population gets infected with the flu each year, but that hasn’t happened the past couple of seasons, he said.
“There might be slightly less overall immunity to these viruses,” Dr. Webby said. “When these viruses do come back, there’s a little bit more room for them to take off.”
Although a severe influenza season rebound this winter is a possibility, Australia continues to experience a historically low flu season. Dr. Harrison, who said the northern hemisphere looks at what’s happening in Australia and the rest of the “southern half of the world because their influenza season is during our summer,” hopes this is an indication that the northern hemisphere will also experience a mild season.
However, there’s no indication of how this upcoming flu season will hit the United States and there isn’t any guidance on what could happen because these historically low levels of respiratory viruses have never happened before, Dr. Webby explained.
He said that, if COVID-19’s delta variant continues to circulate during the fall and winter seasons, it will keep other viruses at low levels. This is because there is rarely a peak of activity of different viruses at the same time.
“When you get infected with the virus, your body’s immune response has this nonspecific reaction that protects you from anything else for a short period of time,” Dr. Webby explained. “When you get a lot of one virus circulating, it’s really hard for these other viruses to get into that population and sort of set off an epidemic of their own.”
To prepare for an unsure influenza season, Dr. Harrison suggests making the influenza vaccine available in August as opposed to October.
Dr. Harrison and Dr. Webby reported no conflicts of interest.
ESC heart failure guideline to integrate bounty of new meds
Today there are so many evidence-based drug therapies for heart failure with reduced ejection fraction (HFrEF) that physicians treating HF patients almost don’t know what to do them.
It’s an exciting new age that way, but to many vexingly unclear how best to merge the shiny new options with mainstay regimens based on time-honored renin-angiotensin system (RAS) inhibitors and beta-blockers.
To impart some clarity, the authors of a new HF guideline document recently took center stage at the Heart Failure Association of the European Society of Cardiology (ESC-HFA) annual meeting to preview their updated recommendations, with novel twists based on recent major trials, for the new age of HF pharmacotherapeutics.
The guideline committee considered the evidence base that existed “up until the end of March of this year,” Theresa A. McDonagh, MD, King’s College London, said during the presentation. The document “is now finalized, it’s with the publishers, and it will be presented in full with simultaneous publication at the ESC meeting” that starts August 27.
It describes a game plan, already followed by some clinicians in practice without official guidance, for initiating drugs from each of four classes in virtually all patients with HFrEF.
New indicated drugs, new perspective for HFrEF
Three of the drug categories are old acquaintances. Among them are the RAS inhibitors, which include angiotensin-receptor/neprilysin inhibitors, beta-blockers, and the mineralocorticoid receptor antagonists. The latter drugs are gaining new respect after having been underplayed in HF prescribing despite longstanding evidence of efficacy.
Completing the quartet of first-line HFrEF drug classes is a recent arrival to the HF arena, the sodium-glucose cotransporter 2 inhibitors.
“We now have new data and a simplified treatment algorithm for heart failure with reduced ejection fraction based on the early administration of the four major classes of drugs,” said Marco Metra, MD, University of Brescia (Italy), previewing the medical-therapy portions of the new guideline at the ESC-HFA sessions, which launched virtually and live in Florence, Italy, on July 29.
The new game plan offers a simple answer to a once-common but complex question: How and in what order are the different drug classes initiated in patients with HFrEF? In the new document, the stated goal is to get them all on board expeditiously and safely, by any means possible.
The guideline writers did not specify a sequence, preferring to leave that decision to physicians, said Dr. Metra, who stated only two guiding principles. The first is to consider the patient’s unique circumstances. The order in which the drugs are introduced might vary, depending on, for example, whether the patient has low or high blood pressure or renal dysfunction.
Second, “it is very important that we try to give all four classes of drugs to the patient in the shortest time possible, because this saves lives,” he said.
That there is no recommendation on sequencing the drugs has led some to the wrong interpretation that all should be started at once, observed coauthor Javed Butler, MD, MPH, University of Mississippi, Jackson, as a panelist during the presentation. Far from it, he said. “The doctor with the patient in front of you can make the best decision. The idea here is to get all the therapies on as soon as possible, as safely as possible.”
“The order in which they are introduced is not really important,” agreed Vijay Chopra, MD, Max Super Specialty Hospital Saket, New Delhi, another coauthor on the panel. “The important thing is that at least some dose of all the four drugs needs to be introduced in the first 4-6 weeks, and then up-titrated.”
Other medical therapy can be more tailored, Dr. Metra noted, such as loop diuretics for patients with congestion, iron for those with iron deficiency, and other drugs depending on whether there is, for example, atrial fibrillation or coronary disease.
Adoption of emerging definitions
The document adopts the emerging characterization of HFrEF by a left ventricular ejection fraction (LVEF) up to 40%.
And it will leverage an expanding evidence base for medication in a segment of patients once said to have HF with preserved ejection fraction (HFpEF), who had therefore lacked specific, guideline-directed medical therapies. Now, patients with an LVEF of 41%-49% will be said to have HF with mildly reduced ejection fraction (HFmrEF), a tweak to the recently introduced HF with “mid-range” LVEF that is designed to assert its nature as something to treat. The new document’s HFmrEF recommendations come with various class and level-of-evidence ratings.
That leaves HFpEF to be characterized by an LVEF of 50% in combination with structural or functional abnormalities associated with LV diastolic dysfunction or raised LV filling pressures, including raised natriuretic peptide levels.
The definitions are consistent with those proposed internationally by the ESC-HFA, the Heart Failure Society of America, and other groups in a statement published in March.
Expanded HFrEF med landscape
Since the 2016 ESC guideline on HF therapy, Dr. McDonagh said, “there’s been no substantial change in the evidence for many of the classical drugs that we use in heart failure. However, we had a lot of new and exciting evidence to consider,” especially in support of the SGLT2 inhibitors as one of the core medications in HFrEF.
The new data came from two controlled trials in particular. In DAPA-HF, patients with HFrEF who were initially without diabetes and who went on dapagliflozin (Farxiga, AstraZeneca) showed a 27% drop in cardiovascular (CV) death or worsening-HF events over a median of 18 months.
“That was followed up with very concordant results with empagliflozin [Jardiance, Boehringer Ingelheim/Eli Lilly] in HFrEF in the EMPEROR-Reduced trial,” Dr. McDonagh said. In that trial, comparable patients who took empagliflozin showed a 25% drop in a primary endpoint similar to that in DAPA-HF over the median 16-month follow-up.
Other HFrEF recommendations are for selected patients. They include ivabradine, already in the guidelines, for patients in sinus rhythm with an elevated resting heart rate who can’t take beta-blockers for whatever reason. But, Dr. McDonagh noted, “we had some new classes of drugs to consider as well.”
In particular, the oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo) emerged about a year ago from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization. In the trial with more than 5,000 patients, treatment with vericiguat atop standard drug and device therapy was followed by a significant 10% drop in risk for CV death or HF hospitalization.
Available now or likely to be available in the United States, the European Union, Japan, and other countries, vericiguat is recommended in the new guideline for VICTORIA-like patients who don’t adequately respond to other indicated medications.
Little for HFpEF as newly defined
“Almost nothing is new” in the guidelines for HFpEF, Dr. Metra said. The document recommends screening for and treatment of any underlying disorder and comorbidities, plus diuretics for any congestion. “That’s what we have to date.”
But that evidence base might soon change. The new HFpEF recommendations could possibly be up-staged at the ESC sessions by the August 27 scheduled presentation of EMPEROR-Preserved, a randomized test of empagliflozin in HFpEF and – it could be said – HFmrEF. The trial entered patients with chronic HF and an LVEF greater than 40%.
Eli Lilly and Boehringer Ingelheim offered the world a peek at the results, which suggest the SGLT2 inhibitor had a positive impact on the primary endpoint of CV death or HF hospitalization. They announced the cursory top-line outcomes in early July as part of its regulatory obligations, noting that the trial had “met” its primary endpoint.
But many unknowns remain, including the degree of benefit and whether it varied among subgroups, and especially whether outcomes were different for HFmrEF than for HFpEF.
Upgrades for familiar agents
Still, HFmrEF gets noteworthy attention in the document. “For the first time, we have recommendations for these patients,” Dr. Metra said. “We already knew that diuretics are indicated for the treatment of congestion. But now, ACE inhibitors, ARBs, beta-blockers, mineralocorticoid antagonists, as well as sacubitril/valsartan, may be considered to improve outcomes in these patients.” Their upgrades in the new guidelines were based on review of trials in the CHARM program and of TOPCAT and PARAGON-HF, among others, he said.

The new document also includes “treatment algorithms based on phenotypes”; that is, comorbidities and less common HF precipitants. For example, “assessment of iron status is now mandated in all patients with heart failure,” Dr. Metra said.
AFFIRM-HF is the key trial in this arena, with its more than 1,100 iron-deficient patients with LVEF less than 50% who had been recently hospitalized for HF. A year of treatment with ferric carboxymaltose (Ferinject/Injectafer, Vifor) led to a 26% drop in risk for HF hospitalization, but without affecting mortality.
For those who are iron deficient, Dr. Metra said, “ferric carboxymaltose intravenously should be considered not only in patients with low ejection fraction and outpatients, but also in patients recently hospitalized for acute heart failure.”
The SGLT2 inhibitors are recommended in HFrEF patients with type 2 diabetes. And treatment with tafamidis (Vyndaqel, Pfizer) in patients with genetic or wild-type transthyretin cardiac amyloidosis gets a class I recommendation based on survival gains seen in the ATTR-ACT trial.
Also recommended is a full CV assessment for patients with cancer who are on cardiotoxic agents or otherwise might be at risk for chemotherapy cardiotoxicity. “Beta-blockers and ACE inhibitors should be considered in those who develop left ventricular systolic dysfunction after anticancer therapy,” Dr. Metra said.
The ongoing pandemic made its mark on the document’s genesis, as it has with most everything else. “For better or worse, we were a ‘COVID guideline,’ ” Dr. McDonagh said. The writing committee consisted of “a large task force of 31 individuals, including two patients,” and there were “only two face-to-face meetings prior to the first wave of COVID hitting Europe.”
The committee voted on each of the recommendations, “and we had to have agreement of more than 75% of the task force to assign a class of recommendation or level of evidence,” she said. “I think we did the best we could in the circumstances. We had the benefit of many discussions over Zoom, and I think at the end of the day we have achieved a consensus.”
With such a large body of participants and the 75% threshold for agreement, “you end up with perhaps a conservative guideline. But that’s not a bad thing for clinical practice, for guidelines to be conservative,” Dr. McDonagh said. “They’re mainly concerned with looking at evidence and safety.”
A version of this article first appeared on Medscape.com.
Today there are so many evidence-based drug therapies for heart failure with reduced ejection fraction (HFrEF) that physicians treating HF patients almost don’t know what to do them.
It’s an exciting new age that way, but to many vexingly unclear how best to merge the shiny new options with mainstay regimens based on time-honored renin-angiotensin system (RAS) inhibitors and beta-blockers.
To impart some clarity, the authors of a new HF guideline document recently took center stage at the Heart Failure Association of the European Society of Cardiology (ESC-HFA) annual meeting to preview their updated recommendations, with novel twists based on recent major trials, for the new age of HF pharmacotherapeutics.
The guideline committee considered the evidence base that existed “up until the end of March of this year,” Theresa A. McDonagh, MD, King’s College London, said during the presentation. The document “is now finalized, it’s with the publishers, and it will be presented in full with simultaneous publication at the ESC meeting” that starts August 27.
It describes a game plan, already followed by some clinicians in practice without official guidance, for initiating drugs from each of four classes in virtually all patients with HFrEF.
New indicated drugs, new perspective for HFrEF
Three of the drug categories are old acquaintances. Among them are the RAS inhibitors, which include angiotensin-receptor/neprilysin inhibitors, beta-blockers, and the mineralocorticoid receptor antagonists. The latter drugs are gaining new respect after having been underplayed in HF prescribing despite longstanding evidence of efficacy.
Completing the quartet of first-line HFrEF drug classes is a recent arrival to the HF arena, the sodium-glucose cotransporter 2 inhibitors.
“We now have new data and a simplified treatment algorithm for heart failure with reduced ejection fraction based on the early administration of the four major classes of drugs,” said Marco Metra, MD, University of Brescia (Italy), previewing the medical-therapy portions of the new guideline at the ESC-HFA sessions, which launched virtually and live in Florence, Italy, on July 29.
The new game plan offers a simple answer to a once-common but complex question: How and in what order are the different drug classes initiated in patients with HFrEF? In the new document, the stated goal is to get them all on board expeditiously and safely, by any means possible.
The guideline writers did not specify a sequence, preferring to leave that decision to physicians, said Dr. Metra, who stated only two guiding principles. The first is to consider the patient’s unique circumstances. The order in which the drugs are introduced might vary, depending on, for example, whether the patient has low or high blood pressure or renal dysfunction.
Second, “it is very important that we try to give all four classes of drugs to the patient in the shortest time possible, because this saves lives,” he said.
That there is no recommendation on sequencing the drugs has led some to the wrong interpretation that all should be started at once, observed coauthor Javed Butler, MD, MPH, University of Mississippi, Jackson, as a panelist during the presentation. Far from it, he said. “The doctor with the patient in front of you can make the best decision. The idea here is to get all the therapies on as soon as possible, as safely as possible.”
“The order in which they are introduced is not really important,” agreed Vijay Chopra, MD, Max Super Specialty Hospital Saket, New Delhi, another coauthor on the panel. “The important thing is that at least some dose of all the four drugs needs to be introduced in the first 4-6 weeks, and then up-titrated.”
Other medical therapy can be more tailored, Dr. Metra noted, such as loop diuretics for patients with congestion, iron for those with iron deficiency, and other drugs depending on whether there is, for example, atrial fibrillation or coronary disease.
Adoption of emerging definitions
The document adopts the emerging characterization of HFrEF by a left ventricular ejection fraction (LVEF) up to 40%.
And it will leverage an expanding evidence base for medication in a segment of patients once said to have HF with preserved ejection fraction (HFpEF), who had therefore lacked specific, guideline-directed medical therapies. Now, patients with an LVEF of 41%-49% will be said to have HF with mildly reduced ejection fraction (HFmrEF), a tweak to the recently introduced HF with “mid-range” LVEF that is designed to assert its nature as something to treat. The new document’s HFmrEF recommendations come with various class and level-of-evidence ratings.
That leaves HFpEF to be characterized by an LVEF of 50% in combination with structural or functional abnormalities associated with LV diastolic dysfunction or raised LV filling pressures, including raised natriuretic peptide levels.
The definitions are consistent with those proposed internationally by the ESC-HFA, the Heart Failure Society of America, and other groups in a statement published in March.
Expanded HFrEF med landscape
Since the 2016 ESC guideline on HF therapy, Dr. McDonagh said, “there’s been no substantial change in the evidence for many of the classical drugs that we use in heart failure. However, we had a lot of new and exciting evidence to consider,” especially in support of the SGLT2 inhibitors as one of the core medications in HFrEF.
The new data came from two controlled trials in particular. In DAPA-HF, patients with HFrEF who were initially without diabetes and who went on dapagliflozin (Farxiga, AstraZeneca) showed a 27% drop in cardiovascular (CV) death or worsening-HF events over a median of 18 months.
“That was followed up with very concordant results with empagliflozin [Jardiance, Boehringer Ingelheim/Eli Lilly] in HFrEF in the EMPEROR-Reduced trial,” Dr. McDonagh said. In that trial, comparable patients who took empagliflozin showed a 25% drop in a primary endpoint similar to that in DAPA-HF over the median 16-month follow-up.
Other HFrEF recommendations are for selected patients. They include ivabradine, already in the guidelines, for patients in sinus rhythm with an elevated resting heart rate who can’t take beta-blockers for whatever reason. But, Dr. McDonagh noted, “we had some new classes of drugs to consider as well.”
In particular, the oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo) emerged about a year ago from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization. In the trial with more than 5,000 patients, treatment with vericiguat atop standard drug and device therapy was followed by a significant 10% drop in risk for CV death or HF hospitalization.
Available now or likely to be available in the United States, the European Union, Japan, and other countries, vericiguat is recommended in the new guideline for VICTORIA-like patients who don’t adequately respond to other indicated medications.
Little for HFpEF as newly defined
“Almost nothing is new” in the guidelines for HFpEF, Dr. Metra said. The document recommends screening for and treatment of any underlying disorder and comorbidities, plus diuretics for any congestion. “That’s what we have to date.”
But that evidence base might soon change. The new HFpEF recommendations could possibly be up-staged at the ESC sessions by the August 27 scheduled presentation of EMPEROR-Preserved, a randomized test of empagliflozin in HFpEF and – it could be said – HFmrEF. The trial entered patients with chronic HF and an LVEF greater than 40%.
Eli Lilly and Boehringer Ingelheim offered the world a peek at the results, which suggest the SGLT2 inhibitor had a positive impact on the primary endpoint of CV death or HF hospitalization. They announced the cursory top-line outcomes in early July as part of its regulatory obligations, noting that the trial had “met” its primary endpoint.
But many unknowns remain, including the degree of benefit and whether it varied among subgroups, and especially whether outcomes were different for HFmrEF than for HFpEF.
Upgrades for familiar agents
Still, HFmrEF gets noteworthy attention in the document. “For the first time, we have recommendations for these patients,” Dr. Metra said. “We already knew that diuretics are indicated for the treatment of congestion. But now, ACE inhibitors, ARBs, beta-blockers, mineralocorticoid antagonists, as well as sacubitril/valsartan, may be considered to improve outcomes in these patients.” Their upgrades in the new guidelines were based on review of trials in the CHARM program and of TOPCAT and PARAGON-HF, among others, he said.

The new document also includes “treatment algorithms based on phenotypes”; that is, comorbidities and less common HF precipitants. For example, “assessment of iron status is now mandated in all patients with heart failure,” Dr. Metra said.
AFFIRM-HF is the key trial in this arena, with its more than 1,100 iron-deficient patients with LVEF less than 50% who had been recently hospitalized for HF. A year of treatment with ferric carboxymaltose (Ferinject/Injectafer, Vifor) led to a 26% drop in risk for HF hospitalization, but without affecting mortality.
For those who are iron deficient, Dr. Metra said, “ferric carboxymaltose intravenously should be considered not only in patients with low ejection fraction and outpatients, but also in patients recently hospitalized for acute heart failure.”
The SGLT2 inhibitors are recommended in HFrEF patients with type 2 diabetes. And treatment with tafamidis (Vyndaqel, Pfizer) in patients with genetic or wild-type transthyretin cardiac amyloidosis gets a class I recommendation based on survival gains seen in the ATTR-ACT trial.
Also recommended is a full CV assessment for patients with cancer who are on cardiotoxic agents or otherwise might be at risk for chemotherapy cardiotoxicity. “Beta-blockers and ACE inhibitors should be considered in those who develop left ventricular systolic dysfunction after anticancer therapy,” Dr. Metra said.
The ongoing pandemic made its mark on the document’s genesis, as it has with most everything else. “For better or worse, we were a ‘COVID guideline,’ ” Dr. McDonagh said. The writing committee consisted of “a large task force of 31 individuals, including two patients,” and there were “only two face-to-face meetings prior to the first wave of COVID hitting Europe.”
The committee voted on each of the recommendations, “and we had to have agreement of more than 75% of the task force to assign a class of recommendation or level of evidence,” she said. “I think we did the best we could in the circumstances. We had the benefit of many discussions over Zoom, and I think at the end of the day we have achieved a consensus.”
With such a large body of participants and the 75% threshold for agreement, “you end up with perhaps a conservative guideline. But that’s not a bad thing for clinical practice, for guidelines to be conservative,” Dr. McDonagh said. “They’re mainly concerned with looking at evidence and safety.”
A version of this article first appeared on Medscape.com.
Today there are so many evidence-based drug therapies for heart failure with reduced ejection fraction (HFrEF) that physicians treating HF patients almost don’t know what to do them.
It’s an exciting new age that way, but to many vexingly unclear how best to merge the shiny new options with mainstay regimens based on time-honored renin-angiotensin system (RAS) inhibitors and beta-blockers.
To impart some clarity, the authors of a new HF guideline document recently took center stage at the Heart Failure Association of the European Society of Cardiology (ESC-HFA) annual meeting to preview their updated recommendations, with novel twists based on recent major trials, for the new age of HF pharmacotherapeutics.
The guideline committee considered the evidence base that existed “up until the end of March of this year,” Theresa A. McDonagh, MD, King’s College London, said during the presentation. The document “is now finalized, it’s with the publishers, and it will be presented in full with simultaneous publication at the ESC meeting” that starts August 27.
It describes a game plan, already followed by some clinicians in practice without official guidance, for initiating drugs from each of four classes in virtually all patients with HFrEF.
New indicated drugs, new perspective for HFrEF
Three of the drug categories are old acquaintances. Among them are the RAS inhibitors, which include angiotensin-receptor/neprilysin inhibitors, beta-blockers, and the mineralocorticoid receptor antagonists. The latter drugs are gaining new respect after having been underplayed in HF prescribing despite longstanding evidence of efficacy.
Completing the quartet of first-line HFrEF drug classes is a recent arrival to the HF arena, the sodium-glucose cotransporter 2 inhibitors.
“We now have new data and a simplified treatment algorithm for heart failure with reduced ejection fraction based on the early administration of the four major classes of drugs,” said Marco Metra, MD, University of Brescia (Italy), previewing the medical-therapy portions of the new guideline at the ESC-HFA sessions, which launched virtually and live in Florence, Italy, on July 29.
The new game plan offers a simple answer to a once-common but complex question: How and in what order are the different drug classes initiated in patients with HFrEF? In the new document, the stated goal is to get them all on board expeditiously and safely, by any means possible.
The guideline writers did not specify a sequence, preferring to leave that decision to physicians, said Dr. Metra, who stated only two guiding principles. The first is to consider the patient’s unique circumstances. The order in which the drugs are introduced might vary, depending on, for example, whether the patient has low or high blood pressure or renal dysfunction.
Second, “it is very important that we try to give all four classes of drugs to the patient in the shortest time possible, because this saves lives,” he said.
That there is no recommendation on sequencing the drugs has led some to the wrong interpretation that all should be started at once, observed coauthor Javed Butler, MD, MPH, University of Mississippi, Jackson, as a panelist during the presentation. Far from it, he said. “The doctor with the patient in front of you can make the best decision. The idea here is to get all the therapies on as soon as possible, as safely as possible.”
“The order in which they are introduced is not really important,” agreed Vijay Chopra, MD, Max Super Specialty Hospital Saket, New Delhi, another coauthor on the panel. “The important thing is that at least some dose of all the four drugs needs to be introduced in the first 4-6 weeks, and then up-titrated.”
Other medical therapy can be more tailored, Dr. Metra noted, such as loop diuretics for patients with congestion, iron for those with iron deficiency, and other drugs depending on whether there is, for example, atrial fibrillation or coronary disease.
Adoption of emerging definitions
The document adopts the emerging characterization of HFrEF by a left ventricular ejection fraction (LVEF) up to 40%.
And it will leverage an expanding evidence base for medication in a segment of patients once said to have HF with preserved ejection fraction (HFpEF), who had therefore lacked specific, guideline-directed medical therapies. Now, patients with an LVEF of 41%-49% will be said to have HF with mildly reduced ejection fraction (HFmrEF), a tweak to the recently introduced HF with “mid-range” LVEF that is designed to assert its nature as something to treat. The new document’s HFmrEF recommendations come with various class and level-of-evidence ratings.
That leaves HFpEF to be characterized by an LVEF of 50% in combination with structural or functional abnormalities associated with LV diastolic dysfunction or raised LV filling pressures, including raised natriuretic peptide levels.
The definitions are consistent with those proposed internationally by the ESC-HFA, the Heart Failure Society of America, and other groups in a statement published in March.
Expanded HFrEF med landscape
Since the 2016 ESC guideline on HF therapy, Dr. McDonagh said, “there’s been no substantial change in the evidence for many of the classical drugs that we use in heart failure. However, we had a lot of new and exciting evidence to consider,” especially in support of the SGLT2 inhibitors as one of the core medications in HFrEF.
The new data came from two controlled trials in particular. In DAPA-HF, patients with HFrEF who were initially without diabetes and who went on dapagliflozin (Farxiga, AstraZeneca) showed a 27% drop in cardiovascular (CV) death or worsening-HF events over a median of 18 months.
“That was followed up with very concordant results with empagliflozin [Jardiance, Boehringer Ingelheim/Eli Lilly] in HFrEF in the EMPEROR-Reduced trial,” Dr. McDonagh said. In that trial, comparable patients who took empagliflozin showed a 25% drop in a primary endpoint similar to that in DAPA-HF over the median 16-month follow-up.
Other HFrEF recommendations are for selected patients. They include ivabradine, already in the guidelines, for patients in sinus rhythm with an elevated resting heart rate who can’t take beta-blockers for whatever reason. But, Dr. McDonagh noted, “we had some new classes of drugs to consider as well.”
In particular, the oral soluble guanylate-cyclase receptor stimulator vericiguat (Verquvo) emerged about a year ago from the VICTORIA trial as a modest success for patients with HFrEF and a previous HF hospitalization. In the trial with more than 5,000 patients, treatment with vericiguat atop standard drug and device therapy was followed by a significant 10% drop in risk for CV death or HF hospitalization.
Available now or likely to be available in the United States, the European Union, Japan, and other countries, vericiguat is recommended in the new guideline for VICTORIA-like patients who don’t adequately respond to other indicated medications.
Little for HFpEF as newly defined
“Almost nothing is new” in the guidelines for HFpEF, Dr. Metra said. The document recommends screening for and treatment of any underlying disorder and comorbidities, plus diuretics for any congestion. “That’s what we have to date.”
But that evidence base might soon change. The new HFpEF recommendations could possibly be up-staged at the ESC sessions by the August 27 scheduled presentation of EMPEROR-Preserved, a randomized test of empagliflozin in HFpEF and – it could be said – HFmrEF. The trial entered patients with chronic HF and an LVEF greater than 40%.
Eli Lilly and Boehringer Ingelheim offered the world a peek at the results, which suggest the SGLT2 inhibitor had a positive impact on the primary endpoint of CV death or HF hospitalization. They announced the cursory top-line outcomes in early July as part of its regulatory obligations, noting that the trial had “met” its primary endpoint.
But many unknowns remain, including the degree of benefit and whether it varied among subgroups, and especially whether outcomes were different for HFmrEF than for HFpEF.
Upgrades for familiar agents
Still, HFmrEF gets noteworthy attention in the document. “For the first time, we have recommendations for these patients,” Dr. Metra said. “We already knew that diuretics are indicated for the treatment of congestion. But now, ACE inhibitors, ARBs, beta-blockers, mineralocorticoid antagonists, as well as sacubitril/valsartan, may be considered to improve outcomes in these patients.” Their upgrades in the new guidelines were based on review of trials in the CHARM program and of TOPCAT and PARAGON-HF, among others, he said.

The new document also includes “treatment algorithms based on phenotypes”; that is, comorbidities and less common HF precipitants. For example, “assessment of iron status is now mandated in all patients with heart failure,” Dr. Metra said.
AFFIRM-HF is the key trial in this arena, with its more than 1,100 iron-deficient patients with LVEF less than 50% who had been recently hospitalized for HF. A year of treatment with ferric carboxymaltose (Ferinject/Injectafer, Vifor) led to a 26% drop in risk for HF hospitalization, but without affecting mortality.
For those who are iron deficient, Dr. Metra said, “ferric carboxymaltose intravenously should be considered not only in patients with low ejection fraction and outpatients, but also in patients recently hospitalized for acute heart failure.”
The SGLT2 inhibitors are recommended in HFrEF patients with type 2 diabetes. And treatment with tafamidis (Vyndaqel, Pfizer) in patients with genetic or wild-type transthyretin cardiac amyloidosis gets a class I recommendation based on survival gains seen in the ATTR-ACT trial.
Also recommended is a full CV assessment for patients with cancer who are on cardiotoxic agents or otherwise might be at risk for chemotherapy cardiotoxicity. “Beta-blockers and ACE inhibitors should be considered in those who develop left ventricular systolic dysfunction after anticancer therapy,” Dr. Metra said.
The ongoing pandemic made its mark on the document’s genesis, as it has with most everything else. “For better or worse, we were a ‘COVID guideline,’ ” Dr. McDonagh said. The writing committee consisted of “a large task force of 31 individuals, including two patients,” and there were “only two face-to-face meetings prior to the first wave of COVID hitting Europe.”
The committee voted on each of the recommendations, “and we had to have agreement of more than 75% of the task force to assign a class of recommendation or level of evidence,” she said. “I think we did the best we could in the circumstances. We had the benefit of many discussions over Zoom, and I think at the end of the day we have achieved a consensus.”
With such a large body of participants and the 75% threshold for agreement, “you end up with perhaps a conservative guideline. But that’s not a bad thing for clinical practice, for guidelines to be conservative,” Dr. McDonagh said. “They’re mainly concerned with looking at evidence and safety.”
A version of this article first appeared on Medscape.com.
Unclear benefit to home NIPPV in COPD
Background: Chronic obstructive pulmonary disease (COPD) is a prevalent condition that is associated with significant mortality, morbidity, and health care utilization. Use of noninvasive positive-pressure ventilation (NIPPV) in acute hypercapnic respiratory failure caused by COPD exacerbations is well established. However, the benefits of in-home NIPPV for COPD with chronic hypercapnia is unclear.
Study design: Systematic review and meta-analysis.
Setting: Multicenter catchment of 21 randomized control trials (RCTs) and 12 observational studies involving more than 51,000 patients during 1995-2019.
Synopsis: Patients included were those with COPD and hypercapnia who used NIPPV for more than 1 month. Home bilevel positive airway pressure (BiPAP), compared to no device use was associated with lower risk of mortality, all-cause hospital admission, and intubation, but no significant difference in quality of life. Noninvasive home mechanical ventilation, compared with no device was significantly associated with lower risk of hospital admission, but not a significant difference in mortality. Of note, there was no statistically significant difference in any outcome for either BiPAP or home mechanical ventilation if evidence was limited to RCTs. Importantly, on rigorous measure, the evidence was low to moderate quality or insufficient, and some outcomes analysis was based on small numbers of studies.
Bottom line: While there is suggestion of benefit on some measures with the use of home NIPPV, the evidence is not robust enough to clearly guide use.
Citation: Wilson et al. Association of home noninvasive positive pressure ventilation with clinical outcomes in chronic obstructive pulmonary disease. JAMA. 2020 Feb 4;323(5):455-65.
Dr. Sneed is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Chronic obstructive pulmonary disease (COPD) is a prevalent condition that is associated with significant mortality, morbidity, and health care utilization. Use of noninvasive positive-pressure ventilation (NIPPV) in acute hypercapnic respiratory failure caused by COPD exacerbations is well established. However, the benefits of in-home NIPPV for COPD with chronic hypercapnia is unclear.
Study design: Systematic review and meta-analysis.
Setting: Multicenter catchment of 21 randomized control trials (RCTs) and 12 observational studies involving more than 51,000 patients during 1995-2019.
Synopsis: Patients included were those with COPD and hypercapnia who used NIPPV for more than 1 month. Home bilevel positive airway pressure (BiPAP), compared to no device use was associated with lower risk of mortality, all-cause hospital admission, and intubation, but no significant difference in quality of life. Noninvasive home mechanical ventilation, compared with no device was significantly associated with lower risk of hospital admission, but not a significant difference in mortality. Of note, there was no statistically significant difference in any outcome for either BiPAP or home mechanical ventilation if evidence was limited to RCTs. Importantly, on rigorous measure, the evidence was low to moderate quality or insufficient, and some outcomes analysis was based on small numbers of studies.
Bottom line: While there is suggestion of benefit on some measures with the use of home NIPPV, the evidence is not robust enough to clearly guide use.
Citation: Wilson et al. Association of home noninvasive positive pressure ventilation with clinical outcomes in chronic obstructive pulmonary disease. JAMA. 2020 Feb 4;323(5):455-65.
Dr. Sneed is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Chronic obstructive pulmonary disease (COPD) is a prevalent condition that is associated with significant mortality, morbidity, and health care utilization. Use of noninvasive positive-pressure ventilation (NIPPV) in acute hypercapnic respiratory failure caused by COPD exacerbations is well established. However, the benefits of in-home NIPPV for COPD with chronic hypercapnia is unclear.
Study design: Systematic review and meta-analysis.
Setting: Multicenter catchment of 21 randomized control trials (RCTs) and 12 observational studies involving more than 51,000 patients during 1995-2019.
Synopsis: Patients included were those with COPD and hypercapnia who used NIPPV for more than 1 month. Home bilevel positive airway pressure (BiPAP), compared to no device use was associated with lower risk of mortality, all-cause hospital admission, and intubation, but no significant difference in quality of life. Noninvasive home mechanical ventilation, compared with no device was significantly associated with lower risk of hospital admission, but not a significant difference in mortality. Of note, there was no statistically significant difference in any outcome for either BiPAP or home mechanical ventilation if evidence was limited to RCTs. Importantly, on rigorous measure, the evidence was low to moderate quality or insufficient, and some outcomes analysis was based on small numbers of studies.
Bottom line: While there is suggestion of benefit on some measures with the use of home NIPPV, the evidence is not robust enough to clearly guide use.
Citation: Wilson et al. Association of home noninvasive positive pressure ventilation with clinical outcomes in chronic obstructive pulmonary disease. JAMA. 2020 Feb 4;323(5):455-65.
Dr. Sneed is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Pfizer vaccine protection wanes after 6 months, study finds
, according to a new study.
The July 28 preprint report of the study, which has not been peer reviewed, suggests a gradual “declining trend in vaccine efficacy” over 6 months after two doses of the Pfizer vaccine in more than 45,000 people worldwide.
The study finds overall effectiveness falls from 96% to 84%.
At the same time, a third booster dose of the Pfizer vaccine increases neutralizing antibody levels against the Delta variant by more than five times, compared to levels after just a second dose in people aged 18-55 years, new data from Pfizer shows.
The third-dose immune response appears even more robust – more than 11 times higher than the second shot – among people aged 65-85 years.
The company noted this could mean an estimated 100-fold increase in Delta variant protection after a third dose. These new findings are outlined in a Pfizer second-quarter 2021 earnings report, which notes that the data are submitted for publication in a medical journal.
The data come from a relatively small number of people studied. There were 11 people in the 18- to 55-year-old group and 12 people in the 65- to 85-year-old group.
“These preliminary data are very encouraging as Delta continues to spread,” Mikael Dolsten, MD, chief scientific officer and president of the Worldwide Research, Development, and Medical organization at Pfizer, said during prepared remarks on a company earnings call July 28, CNN reported.
Availability of a third dose of any of the current COVID-19 vaccines would require amendment of the Food and Drug Administration’s emergency use authorization, or full FDA approval for the vaccine.
The possibility of a third dose authorization or approval has not been without controversy. For example, when Pfizer announced intentions to file for FDA authorization of a booster dose on July 8, the Centers for Disease Control and Prevention, the FDA, and the National Institutes of Health were quick to issue a joint statement saying they would decide when the timing is right for Americans to have a third immunization. The agencies stated, in part, “We are prepared for booster doses if and when the science demonstrates that they are needed.”
In addition, the World Health Organization said at a media briefing on July 12 that rich countries should prioritize sharing of COVID-19 vaccine supplies to other countries in need worldwide before allocating doses for a booster shot for its own residents.
A version of this article first appeared on WebMD.com.
, according to a new study.
The July 28 preprint report of the study, which has not been peer reviewed, suggests a gradual “declining trend in vaccine efficacy” over 6 months after two doses of the Pfizer vaccine in more than 45,000 people worldwide.
The study finds overall effectiveness falls from 96% to 84%.
At the same time, a third booster dose of the Pfizer vaccine increases neutralizing antibody levels against the Delta variant by more than five times, compared to levels after just a second dose in people aged 18-55 years, new data from Pfizer shows.
The third-dose immune response appears even more robust – more than 11 times higher than the second shot – among people aged 65-85 years.
The company noted this could mean an estimated 100-fold increase in Delta variant protection after a third dose. These new findings are outlined in a Pfizer second-quarter 2021 earnings report, which notes that the data are submitted for publication in a medical journal.
The data come from a relatively small number of people studied. There were 11 people in the 18- to 55-year-old group and 12 people in the 65- to 85-year-old group.
“These preliminary data are very encouraging as Delta continues to spread,” Mikael Dolsten, MD, chief scientific officer and president of the Worldwide Research, Development, and Medical organization at Pfizer, said during prepared remarks on a company earnings call July 28, CNN reported.
Availability of a third dose of any of the current COVID-19 vaccines would require amendment of the Food and Drug Administration’s emergency use authorization, or full FDA approval for the vaccine.
The possibility of a third dose authorization or approval has not been without controversy. For example, when Pfizer announced intentions to file for FDA authorization of a booster dose on July 8, the Centers for Disease Control and Prevention, the FDA, and the National Institutes of Health were quick to issue a joint statement saying they would decide when the timing is right for Americans to have a third immunization. The agencies stated, in part, “We are prepared for booster doses if and when the science demonstrates that they are needed.”
In addition, the World Health Organization said at a media briefing on July 12 that rich countries should prioritize sharing of COVID-19 vaccine supplies to other countries in need worldwide before allocating doses for a booster shot for its own residents.
A version of this article first appeared on WebMD.com.
, according to a new study.
The July 28 preprint report of the study, which has not been peer reviewed, suggests a gradual “declining trend in vaccine efficacy” over 6 months after two doses of the Pfizer vaccine in more than 45,000 people worldwide.
The study finds overall effectiveness falls from 96% to 84%.
At the same time, a third booster dose of the Pfizer vaccine increases neutralizing antibody levels against the Delta variant by more than five times, compared to levels after just a second dose in people aged 18-55 years, new data from Pfizer shows.
The third-dose immune response appears even more robust – more than 11 times higher than the second shot – among people aged 65-85 years.
The company noted this could mean an estimated 100-fold increase in Delta variant protection after a third dose. These new findings are outlined in a Pfizer second-quarter 2021 earnings report, which notes that the data are submitted for publication in a medical journal.
The data come from a relatively small number of people studied. There were 11 people in the 18- to 55-year-old group and 12 people in the 65- to 85-year-old group.
“These preliminary data are very encouraging as Delta continues to spread,” Mikael Dolsten, MD, chief scientific officer and president of the Worldwide Research, Development, and Medical organization at Pfizer, said during prepared remarks on a company earnings call July 28, CNN reported.
Availability of a third dose of any of the current COVID-19 vaccines would require amendment of the Food and Drug Administration’s emergency use authorization, or full FDA approval for the vaccine.
The possibility of a third dose authorization or approval has not been without controversy. For example, when Pfizer announced intentions to file for FDA authorization of a booster dose on July 8, the Centers for Disease Control and Prevention, the FDA, and the National Institutes of Health were quick to issue a joint statement saying they would decide when the timing is right for Americans to have a third immunization. The agencies stated, in part, “We are prepared for booster doses if and when the science demonstrates that they are needed.”
In addition, the World Health Organization said at a media briefing on July 12 that rich countries should prioritize sharing of COVID-19 vaccine supplies to other countries in need worldwide before allocating doses for a booster shot for its own residents.
A version of this article first appeared on WebMD.com.
No prehydration prior to contrast-enhanced CT in patients with stage 3 CKD
Background: Postcontrast acute kidney injury (PC-AKI) is known to have a mild, often self-limiting, clinical course. Despite this, preventative measures are advised by international guidelines in high-risk patients.
Study design: The Kompas trial was a multicenter, open-label, noninferiority randomized clinical trial in which 523 patients with stage 3 CKD were randomized to receive no hydration or prehydration with 250 mL of 1.4% sodium bicarbonate in a 1-hour infusion before undergoing elective contrast-enhanced CT. The primary endpoint was the mean relative increase in serum creatinine 2-5 days after contrast administration, compared with baseline.
Setting: Six hospitals in the Netherlands during April 2013–September 2016.
Synopsis: Of the 523 patients, (median age, 74 years), the mean relative increase in creatinine level 2-5 days after contrast administration compared with baseline was 3.0% in the no-prehydration group vs. 3.5% in the prehydration group. This demonstrates that withholding prehydration is noninferior to administrating prehydration. PC-AKI occurred in 7 of 262 patients in the no-prehydration group and 4 of 261 patients in the prehydration group and no patients required dialysis or developed heart failure. These results reassure us that prehydration with sodium bicarbonate can be safely omitted in patients with stage 3 CKD who undergo contrast-enhanced CT.
Bottom line: Prehydration with sodium bicarbonate is not needed to prevent additional renal injury in patients with CKD stage 3 undergoing contrast-enhanced CT imaging.
Citation: Timal RJ et al. Effect of no prehydration vs sodium bicarbonate prehydration prior to contrast-enhanced computed tomography in the prevention of postcontrast acute kidney injury in adults with chronic kidney disease: The Kompas Randomized Clinical Trial. JAMA Intern Med. 2020 Feb 17. doi: 10.1001/jamainternmed.2019.7428.
Dr. Moulder is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Postcontrast acute kidney injury (PC-AKI) is known to have a mild, often self-limiting, clinical course. Despite this, preventative measures are advised by international guidelines in high-risk patients.
Study design: The Kompas trial was a multicenter, open-label, noninferiority randomized clinical trial in which 523 patients with stage 3 CKD were randomized to receive no hydration or prehydration with 250 mL of 1.4% sodium bicarbonate in a 1-hour infusion before undergoing elective contrast-enhanced CT. The primary endpoint was the mean relative increase in serum creatinine 2-5 days after contrast administration, compared with baseline.
Setting: Six hospitals in the Netherlands during April 2013–September 2016.
Synopsis: Of the 523 patients, (median age, 74 years), the mean relative increase in creatinine level 2-5 days after contrast administration compared with baseline was 3.0% in the no-prehydration group vs. 3.5% in the prehydration group. This demonstrates that withholding prehydration is noninferior to administrating prehydration. PC-AKI occurred in 7 of 262 patients in the no-prehydration group and 4 of 261 patients in the prehydration group and no patients required dialysis or developed heart failure. These results reassure us that prehydration with sodium bicarbonate can be safely omitted in patients with stage 3 CKD who undergo contrast-enhanced CT.
Bottom line: Prehydration with sodium bicarbonate is not needed to prevent additional renal injury in patients with CKD stage 3 undergoing contrast-enhanced CT imaging.
Citation: Timal RJ et al. Effect of no prehydration vs sodium bicarbonate prehydration prior to contrast-enhanced computed tomography in the prevention of postcontrast acute kidney injury in adults with chronic kidney disease: The Kompas Randomized Clinical Trial. JAMA Intern Med. 2020 Feb 17. doi: 10.1001/jamainternmed.2019.7428.
Dr. Moulder is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.
Background: Postcontrast acute kidney injury (PC-AKI) is known to have a mild, often self-limiting, clinical course. Despite this, preventative measures are advised by international guidelines in high-risk patients.
Study design: The Kompas trial was a multicenter, open-label, noninferiority randomized clinical trial in which 523 patients with stage 3 CKD were randomized to receive no hydration or prehydration with 250 mL of 1.4% sodium bicarbonate in a 1-hour infusion before undergoing elective contrast-enhanced CT. The primary endpoint was the mean relative increase in serum creatinine 2-5 days after contrast administration, compared with baseline.
Setting: Six hospitals in the Netherlands during April 2013–September 2016.
Synopsis: Of the 523 patients, (median age, 74 years), the mean relative increase in creatinine level 2-5 days after contrast administration compared with baseline was 3.0% in the no-prehydration group vs. 3.5% in the prehydration group. This demonstrates that withholding prehydration is noninferior to administrating prehydration. PC-AKI occurred in 7 of 262 patients in the no-prehydration group and 4 of 261 patients in the prehydration group and no patients required dialysis or developed heart failure. These results reassure us that prehydration with sodium bicarbonate can be safely omitted in patients with stage 3 CKD who undergo contrast-enhanced CT.
Bottom line: Prehydration with sodium bicarbonate is not needed to prevent additional renal injury in patients with CKD stage 3 undergoing contrast-enhanced CT imaging.
Citation: Timal RJ et al. Effect of no prehydration vs sodium bicarbonate prehydration prior to contrast-enhanced computed tomography in the prevention of postcontrast acute kidney injury in adults with chronic kidney disease: The Kompas Randomized Clinical Trial. JAMA Intern Med. 2020 Feb 17. doi: 10.1001/jamainternmed.2019.7428.
Dr. Moulder is assistant professor of medicine, section of hospital medicine, at the University of Virginia School of Medicine, Charlottesville.