User login
Atorvastatin: A potential treatment in COVID-19?
For patients with COVID-19 admitted to intensive care, giving atorvastatin 20 mg/d did not result in a significant reduction in risk for venous or arterial thrombosis, for treatment with extracorporeal membrane oxygenation (ECMO), or for all-cause mortality, compared with placebo in the INSPIRATION-S study.
However, there was a suggestion of benefit in the subgroup of patients who were treated within 7 days of COVID-19 symptom onset.
The study was presented by Behnood Bikdeli, MD, Brigham and Women’s Hospital, Boston, on May 16 at the annual scientific sessions of the American College of Cardiology.
He explained that COVID-19 is characterized by an exuberant immune response and that there is a potential for thrombotic events because of enhanced endothelial activation and a prothrombotic state.
“In this context, it is interesting to think about statins as potential agents to be studied in COVID-19, because as well as having lipid-lowering actions, they are also thought to have anti-inflammatory and antithrombotic effects,” he said.
In the HARP-2 trial of simvastatin in acute respiratory distress syndrome (ARDS), published a few years ago, the main results were neutral, but in the subgroup of patients with hyperinflammatory ARDS, there was a reduction in mortality with simvastatin in comparison with placebo, Dr. Bikdeli noted.
Moreover, in a series of observational studies of patients with COVID-19, use of statins was associated with a reduction in mortality among hospitalized patients. However, there are limited high-quality data to guide clinical practice, he said.
The INSPIRATION study, conducted in 11 hospitals in Iran, had a two-by-two factorial design to investigate different anticoagulant strategies and the use of atorvastatin for COVID-19 patients in the ICU.
In the anticoagulation part of the trial, which was published in JAMA in March 2020, there was no difference in the primary endpoint of an intermediate dose and standard dose of enoxaparin.
For the statin part of the trial (INSPIRATION-S), 605 patients were randomly assigned to receive atorvastatin 20 mg daily or placebo. Patients who had been taking statins beforehand were excluded. Baseline characteristics were similar for the two groups, with around a quarter of patients taking aspirin and more than 90% taking steroids.
Results showed that atorvastatin was not associated with a significant reduction in the primary outcome – a composite of adjudicated venous or arterial thrombosis, treatment with ECMO, or mortality within 30 days – which occurred in 32.7% of the statin group versus 36.3% of the placebo group (odds ratio, 0.84; P = .35).
Atorvastatin was not associated with any significant differences in any of the individual components of the primary composite endpoint. There was also no significant difference in any of the safety endpoints, which included major bleeding and elevations in liver enzyme levels.
Subgroup analyses were mostly consistent with the main findings, with one exception.
In the subgroup of patients who presented within the first 7 days of COVID-19 symptom onset, there was a hint of a potential protective effect with atorvastatin.
In this group of 171 patients, the primary endpoint occurred in 30.9% of those taking atorvastatin versus 40.3% of those taking placebo (OR, 0.60; P = .055).
“This is an interesting observation, and it is plausible, as these patients may be in a different phase of COVID-19 disease. But we need to be cognizant of the multiplicity of comparisons, and this needs to be further investigated in subsequent studies,” Dr. Bikdeli said.
Higher dose in less sick patients a better strategy?
Discussing the study at the ACC presentation, Binita Shah, MD, said the importance of enrolling COVID-19 patients into clinical trials was paramount but that these patients in the ICU may not have been the right population in which to test a statin.
“Maybe for these very sick patients, it is just too late. Trying to rein in the inflammatory cytokine storm and the interaction with thrombosis at this point is very difficult,” Dr. Shah commented.
She suggested that it might be appropriate to try statins in an earlier phase of the disease in order to prevent the inflammatory process, rather than trying to stop it after it had already started.
Dr. Shah also questioned the use of such a low dose of atorvastatin for these patients. “In the cardiovascular literature – at least in ACS [acute coronary syndrome] – high statin doses are used to see short-term benefits. In this very inflammatory milieu, I wonder whether a high-intensity regimen would be more beneficial,” she speculated.
Dr. Bikdeli replied that a low dose of atorvastatin was chosen because early on, several antiviral agents, such as ritonavir, were being used for COVID-19 patients, and these drugs were associated with increases in liver enzyme levels.
“We didn’t want to exacerbate that with high doses of statins,” he said. “But we have now established the safety profile of atorvastatin in these patients, and in retrospect, yes, a higher dose might have been better.”
The INSPIRATION study was funded by the Rajaie Cardiovascular Medical and Research Center, Tehran, Iran. Dr. Bikdeli has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For patients with COVID-19 admitted to intensive care, giving atorvastatin 20 mg/d did not result in a significant reduction in risk for venous or arterial thrombosis, for treatment with extracorporeal membrane oxygenation (ECMO), or for all-cause mortality, compared with placebo in the INSPIRATION-S study.
However, there was a suggestion of benefit in the subgroup of patients who were treated within 7 days of COVID-19 symptom onset.
The study was presented by Behnood Bikdeli, MD, Brigham and Women’s Hospital, Boston, on May 16 at the annual scientific sessions of the American College of Cardiology.
He explained that COVID-19 is characterized by an exuberant immune response and that there is a potential for thrombotic events because of enhanced endothelial activation and a prothrombotic state.
“In this context, it is interesting to think about statins as potential agents to be studied in COVID-19, because as well as having lipid-lowering actions, they are also thought to have anti-inflammatory and antithrombotic effects,” he said.
In the HARP-2 trial of simvastatin in acute respiratory distress syndrome (ARDS), published a few years ago, the main results were neutral, but in the subgroup of patients with hyperinflammatory ARDS, there was a reduction in mortality with simvastatin in comparison with placebo, Dr. Bikdeli noted.
Moreover, in a series of observational studies of patients with COVID-19, use of statins was associated with a reduction in mortality among hospitalized patients. However, there are limited high-quality data to guide clinical practice, he said.
The INSPIRATION study, conducted in 11 hospitals in Iran, had a two-by-two factorial design to investigate different anticoagulant strategies and the use of atorvastatin for COVID-19 patients in the ICU.
In the anticoagulation part of the trial, which was published in JAMA in March 2020, there was no difference in the primary endpoint of an intermediate dose and standard dose of enoxaparin.
For the statin part of the trial (INSPIRATION-S), 605 patients were randomly assigned to receive atorvastatin 20 mg daily or placebo. Patients who had been taking statins beforehand were excluded. Baseline characteristics were similar for the two groups, with around a quarter of patients taking aspirin and more than 90% taking steroids.
Results showed that atorvastatin was not associated with a significant reduction in the primary outcome – a composite of adjudicated venous or arterial thrombosis, treatment with ECMO, or mortality within 30 days – which occurred in 32.7% of the statin group versus 36.3% of the placebo group (odds ratio, 0.84; P = .35).
Atorvastatin was not associated with any significant differences in any of the individual components of the primary composite endpoint. There was also no significant difference in any of the safety endpoints, which included major bleeding and elevations in liver enzyme levels.
Subgroup analyses were mostly consistent with the main findings, with one exception.
In the subgroup of patients who presented within the first 7 days of COVID-19 symptom onset, there was a hint of a potential protective effect with atorvastatin.
In this group of 171 patients, the primary endpoint occurred in 30.9% of those taking atorvastatin versus 40.3% of those taking placebo (OR, 0.60; P = .055).
“This is an interesting observation, and it is plausible, as these patients may be in a different phase of COVID-19 disease. But we need to be cognizant of the multiplicity of comparisons, and this needs to be further investigated in subsequent studies,” Dr. Bikdeli said.
Higher dose in less sick patients a better strategy?
Discussing the study at the ACC presentation, Binita Shah, MD, said the importance of enrolling COVID-19 patients into clinical trials was paramount but that these patients in the ICU may not have been the right population in which to test a statin.
“Maybe for these very sick patients, it is just too late. Trying to rein in the inflammatory cytokine storm and the interaction with thrombosis at this point is very difficult,” Dr. Shah commented.
She suggested that it might be appropriate to try statins in an earlier phase of the disease in order to prevent the inflammatory process, rather than trying to stop it after it had already started.
Dr. Shah also questioned the use of such a low dose of atorvastatin for these patients. “In the cardiovascular literature – at least in ACS [acute coronary syndrome] – high statin doses are used to see short-term benefits. In this very inflammatory milieu, I wonder whether a high-intensity regimen would be more beneficial,” she speculated.
Dr. Bikdeli replied that a low dose of atorvastatin was chosen because early on, several antiviral agents, such as ritonavir, were being used for COVID-19 patients, and these drugs were associated with increases in liver enzyme levels.
“We didn’t want to exacerbate that with high doses of statins,” he said. “But we have now established the safety profile of atorvastatin in these patients, and in retrospect, yes, a higher dose might have been better.”
The INSPIRATION study was funded by the Rajaie Cardiovascular Medical and Research Center, Tehran, Iran. Dr. Bikdeli has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For patients with COVID-19 admitted to intensive care, giving atorvastatin 20 mg/d did not result in a significant reduction in risk for venous or arterial thrombosis, for treatment with extracorporeal membrane oxygenation (ECMO), or for all-cause mortality, compared with placebo in the INSPIRATION-S study.
However, there was a suggestion of benefit in the subgroup of patients who were treated within 7 days of COVID-19 symptom onset.
The study was presented by Behnood Bikdeli, MD, Brigham and Women’s Hospital, Boston, on May 16 at the annual scientific sessions of the American College of Cardiology.
He explained that COVID-19 is characterized by an exuberant immune response and that there is a potential for thrombotic events because of enhanced endothelial activation and a prothrombotic state.
“In this context, it is interesting to think about statins as potential agents to be studied in COVID-19, because as well as having lipid-lowering actions, they are also thought to have anti-inflammatory and antithrombotic effects,” he said.
In the HARP-2 trial of simvastatin in acute respiratory distress syndrome (ARDS), published a few years ago, the main results were neutral, but in the subgroup of patients with hyperinflammatory ARDS, there was a reduction in mortality with simvastatin in comparison with placebo, Dr. Bikdeli noted.
Moreover, in a series of observational studies of patients with COVID-19, use of statins was associated with a reduction in mortality among hospitalized patients. However, there are limited high-quality data to guide clinical practice, he said.
The INSPIRATION study, conducted in 11 hospitals in Iran, had a two-by-two factorial design to investigate different anticoagulant strategies and the use of atorvastatin for COVID-19 patients in the ICU.
In the anticoagulation part of the trial, which was published in JAMA in March 2020, there was no difference in the primary endpoint of an intermediate dose and standard dose of enoxaparin.
For the statin part of the trial (INSPIRATION-S), 605 patients were randomly assigned to receive atorvastatin 20 mg daily or placebo. Patients who had been taking statins beforehand were excluded. Baseline characteristics were similar for the two groups, with around a quarter of patients taking aspirin and more than 90% taking steroids.
Results showed that atorvastatin was not associated with a significant reduction in the primary outcome – a composite of adjudicated venous or arterial thrombosis, treatment with ECMO, or mortality within 30 days – which occurred in 32.7% of the statin group versus 36.3% of the placebo group (odds ratio, 0.84; P = .35).
Atorvastatin was not associated with any significant differences in any of the individual components of the primary composite endpoint. There was also no significant difference in any of the safety endpoints, which included major bleeding and elevations in liver enzyme levels.
Subgroup analyses were mostly consistent with the main findings, with one exception.
In the subgroup of patients who presented within the first 7 days of COVID-19 symptom onset, there was a hint of a potential protective effect with atorvastatin.
In this group of 171 patients, the primary endpoint occurred in 30.9% of those taking atorvastatin versus 40.3% of those taking placebo (OR, 0.60; P = .055).
“This is an interesting observation, and it is plausible, as these patients may be in a different phase of COVID-19 disease. But we need to be cognizant of the multiplicity of comparisons, and this needs to be further investigated in subsequent studies,” Dr. Bikdeli said.
Higher dose in less sick patients a better strategy?
Discussing the study at the ACC presentation, Binita Shah, MD, said the importance of enrolling COVID-19 patients into clinical trials was paramount but that these patients in the ICU may not have been the right population in which to test a statin.
“Maybe for these very sick patients, it is just too late. Trying to rein in the inflammatory cytokine storm and the interaction with thrombosis at this point is very difficult,” Dr. Shah commented.
She suggested that it might be appropriate to try statins in an earlier phase of the disease in order to prevent the inflammatory process, rather than trying to stop it after it had already started.
Dr. Shah also questioned the use of such a low dose of atorvastatin for these patients. “In the cardiovascular literature – at least in ACS [acute coronary syndrome] – high statin doses are used to see short-term benefits. In this very inflammatory milieu, I wonder whether a high-intensity regimen would be more beneficial,” she speculated.
Dr. Bikdeli replied that a low dose of atorvastatin was chosen because early on, several antiviral agents, such as ritonavir, were being used for COVID-19 patients, and these drugs were associated with increases in liver enzyme levels.
“We didn’t want to exacerbate that with high doses of statins,” he said. “But we have now established the safety profile of atorvastatin in these patients, and in retrospect, yes, a higher dose might have been better.”
The INSPIRATION study was funded by the Rajaie Cardiovascular Medical and Research Center, Tehran, Iran. Dr. Bikdeli has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Procalcitonin-guided antibiotic stewardship for lower respiratory tract infection
Dynamics of the assay must be considered
Case
A 50-year-old female presents with 3 days of cough, subjective fevers, myalgias, and dyspnea. She feels she “may have caught something” while volunteering at a preschool. She has hypertension, congestive heart failure, and 20 pack-years of smoking. Chest x-ray shows bibasilar consolidation versus atelectasis. Vital signs are notable for an O2 saturation of 93%. White blood cell count and differential are normal. Procalcitonin level is 0.4 mcg/L.
Overview of the issue
Lower respiratory tract infections (LRTI) are common in the practice of hospital medicine; however, the primary symptoms of cough and dyspnea can be caused by a myriad of noninfectious conditions. Even when infection is suggested by the clinical presentation, the distinction between bacterial and viral etiologies can be challenging, complicating decisions about antibiotic use. Attention to antibiotic stewardship is a growing concern in U.S. hospitals, where the CDC estimates that as many as 50% of antibiotic orders are inappropriate or entirely unnecessary.1 Antibiotic overuse is a driver of multidrug-resistant organisms and increasing rates of Clostridium difficile infection. A diagnostic test to enhance physicians’ ability to target patients who would benefit from antibiotics could be a useful tool to combat the complications of antibiotic overuse. (See Figure 1.) 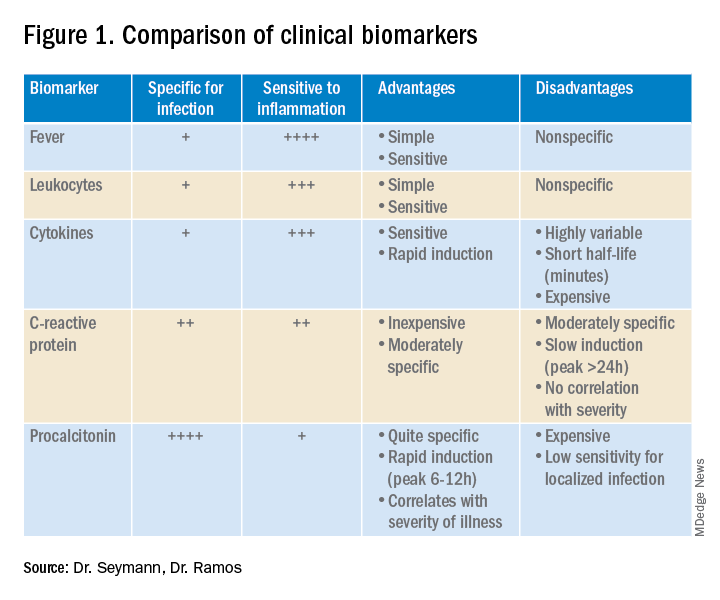
Procalcitonin is produced in the thyroidal C-cells as a prohormone which is processed intracellularly and secreted as calcitonin in response to serum calcium levels. However, intact procalcitonin protein can be secreted from many other tissues in the presence of cytokines such as interleukin 1-beta, tumor necrosis factor-alpha, and lipopolysaccharide, typically released in response to systemic bacterial infections. Conversely, cytokines present in acute viral illness (interferon-gamma) suppress procalcitonin release. This dichotomy presents an opportunity to use procalcitonin to differentiate bacterial from nonbacterial etiologies in various clinical scenarios including LRTI.
Overview of the data
Multiple studies have demonstrated that procalcitonin can be safely used to guide antibiotic prescribing in patients with LRTI. The first large multicenter randomized controlled trial to address the topic was the Swiss PROHOSP study.2 Investigators randomized 1,359 patients hospitalized with LRTI to procalcitonin (PCT) guided therapy or guideline-based therapy. After an initial PCT level was measured, antibiotic prescribing in the PCT arm of the study was directed by a prespecified protocol; specifically, clinicians were discouraged from prescribing antibiotics in patients with PCT levels less than 0.25 mcg/L. (See Figure 2.)
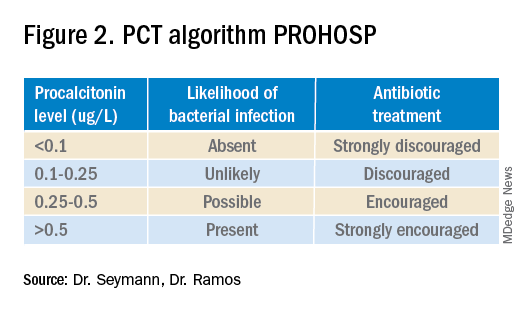
For patients who were particularly ill or unstable at admission, the protocol allowed for antibiotics despite a low PCT level, but repeat measurement within 24 hours and accompanying treatment recommendations were reinforced with the treatment team. Clinicians caring for patients in the control arm were presented with condition-specific clinical practice guidelines to reinforce antibiotic choices. In both arms, the final decision on antibiotic treatment remained with the physician.
Results from the PROHOSP study showed no difference in the combined outcome of death, intensive care unit admission, or complications in the ensuing 30 days, but antibiotic use was significantly reduced. Mean antibiotic exposure dropped from 8.7 to 5.7 days, a reduction of 35%, with the largest decrease among patients with chronic obstructive pulmonary disease (COPD) and acute bronchitis. Antibiotic-related adverse effects fell by 8.2%. Strengths of the study included a very high rate of protocol compliance (90%) by the treating clinicians.
A systematic review of all available studies of procalcitonin-guided therapy for LRTI was published in 2018 and included 26 randomized controlled trials encompassing 6,708 patients in 12 countries. Findings confirmed an overall reduction of 2.4 days in antibiotic exposure, 6% reduction in antibiotic-related adverse effects, and importantly a 17% relative risk reduction in mortality.3
Similar benefits of PCT-guided therapy have been demonstrated even among severely ill patients. A meta-analysis including 523 patients with bacteremia noted mean reduction in antibiotic exposure of 2.86 days, without excess mortality.4 A second meta-analysis of 4,482 critically ill patients admitted to the ICU with sepsis demonstrated not only a reduction in antibiotic exposure, but in mortality as well. Despite a relatively small decrease in antibiotic duration of 1.19 days, the investigators found an 11% reduction in mortality (P = .03) in the PCT-guided group.5
One notable outlier among the many positive studies on PCT-guided antibiotic therapy is the 2018 PROACT study performed in U.S. hospitals over 4 years.6 Its design was similar to the PROHOSP study, however, in contrast to the majority of other trials, the investigators were unable to demonstrate a reduction in antibiotic exposure, leading them to conclude that PCT guidance may not be a useful tool for antibiotic stewardship.
Unfortunately, significant differences in the compliance with the study protocol (90% in PROHOSP vs. 63% in PROACT), and a much healthier patient population (91% of the patients had a PCT less than 0.25, and a majority of patients had asthma which is not normally treated with antibiotics) hamper the generalizability of the PROACT findings. Rather than indicating a failure of PCT, the findings of the study underscore the fact that the utility of any lab test is limited unless it is applied in an appropriate diagnostic setting.
For hospitalists, the most clinically useful role for PCT testing is to guide the duration of antibiotic therapy. Although the literature supports short-course antibiotic therapy in many common conditions seen by hospitalists (Table 1), data suggest overprescribing remains prevalent. Several recent studies targeting LRTI underscore this point.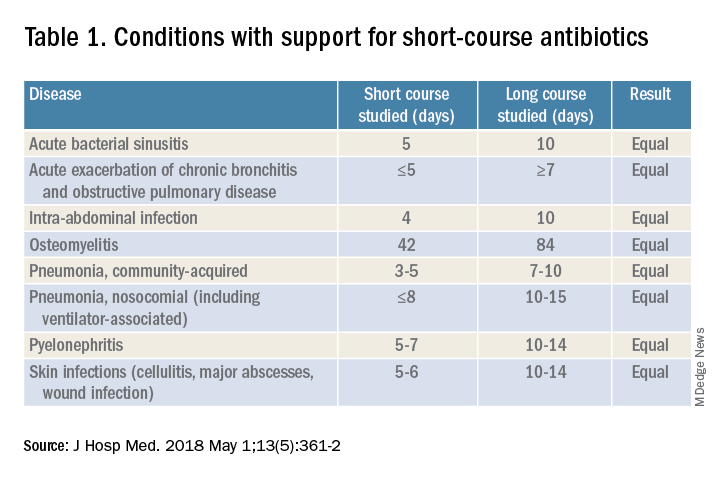
Despite guidelines advocating for treatment of uncomplicated community-acquired pneumonia (CAP) for no more than 5-7 days, two recent retrospective studies suggest most patients receive longer courses. A review of more than 150,000 patients across the United States with uncomplicated CAP documented a mean antibiotic duration of 9.5 days, with close to 70% of patients receiving more than 7 days of therapy.7 A multicenter study of CAP patients hospitalized in Michigan noted similar findings, with a mean 2-day excess duration of therapy or 2,526 excess days of treatment per 1,000 discharges.8 Though some who argue against procalcitonin’s utility cite the fact that existing guidelines already support short-course therapy, obviating the need for biomarker guidance, clinicians have not yet universally adopted this practice. Using a PCT algorithm can decrease duration of therapy and thereby reduce unnecessary antibiotic use. PCT levels less than 0.25 mcg/L support withholding or discontinuing antibiotics, or consideration of an alternative diagnosis.
The dynamics of the PCT assay must be considered in order to use it appropriately. Levels of PCT rise within 3-6 hours of infection, so patients presenting extremely early in the disease course may have falsely low levels. PCT levels correlate with severity of illness and should fall within 2-3 days of initiation of appropriate therapy. A repeat PCT in 2-3 days can be used to help time antibiotic cessation. Studies support stopping antibiotics in stable patients once the PCT level falls below 0.25 mcg/L or drops by 80% in patients with severe elevations. Lack of improvement suggests inadequate antibiotic therapy and is predictive of excess mortality.
Most drivers of false-positive PCT levels are rare and easily identifiable. (See Figure 3.) However, like troponin, patients with chronic kidney disease have delayed PCT clearance, so baseline levels may be about double the normal range. If a baseline is known, monitoring the rise and fall of PCT levels remains clinically useful in this population.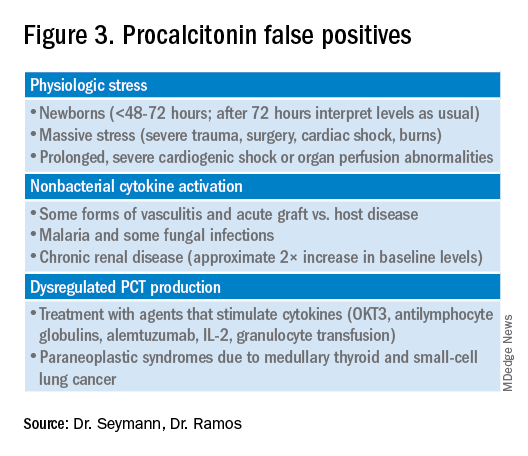
Application of data to case
In reviewing the case, the differential includes a viral upper respiratory infection, an acute exacerbation of COPD, decompensated heart failure, or bacterial pneumonia. The lab and imaging findings are nonspecific, but a PCT level less than 0.25 mcg/L raises concern for an acute bacterial pneumonia. Given that PCT levels rise in bacterial infection and are suppressed in viral infections, treating this patient with antibiotics seems prudent. In this case the relatively mild elevation suggests a less severe infection or a presentation early in the disease course. A repeat PCT in 2-3 days will guide timing for antibiotic cessation.
Bottom line
Thoughtful procalcitonin-guided antibiotic therapy for LRTI may further current antibiotic stewardship initiatives targeting reduction of inappropriate antimicrobial use, which may ultimately reduce rates of Clostridium difficile infections and the emergence of multidrug-resistant organisms.
Dr. Seymann and Dr. Ramos are clinical professors in the division of hospital medicine, department of medicine, at the University of California San Diego.
Key points
- Initial PCT level can help distinguish between viral and bacterial pneumonias.
- PCT levels rise in response to acute bacterial infections and are suppressed in viral infections.
- PCT levels below 0.25 mcg/L suggest that antibiotics can be safely withheld in otherwise stable patients.
- PCT levels correlate with severity of illness and prognosis.
- Rise of PCT is rapid (3-6 hours), and levels fall quickly with appropriate treatment (2-3 days).
- Serial PCT levels can be used to guide duration of antibiotic therapy.
References
1. CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: U.S. Department of Health & Human Services. 2014. Available at www.cdc.gov/getsmart/healthcare/ implementation/core-elements.html.
2. Schuetz P et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-66. doi: 10.1001/jama.2009.1297.
3. Schuetz P et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107. doi: 10.1016/S1473-3099(17)30592-3.
4. Meier MA et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis. 2019;69(3):388-96. doi: 10.1093/cid/ciy917.
5. Wirz Y et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7.
6. Huang DT et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018 Jul 19;379(3):236-49. doi: 10.1056/NEJMoa1802670.
7. Yi SH et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66(9):1333-41. doi: 10.1093/cid/cix986.
8. Vaughn V et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019; 171(3):153-63. doi: 10.7326/M18-3640.
Quiz
1. A 57-year-old male is hospitalized for treatment of community-acquired pneumonia with IV azithromycin and ceftriaxone. PCT level on day 1 = 0.35 mcg/L. On day 4 of antibiotics the PCT level is 0.15 mcg/L. What should be done regarding the antibiotic course?
a. Continue antibiotics for a total course of 5 days.
b. Continue antibiotics for a total course of 7 days.
c. Stop antibiotics.
d. Continue antibiotics and repeat a PCT level the next day.
Answer: The best answer is c. Evidence suggests that 5 days of therapy is adequate treatment for uncomplicated community-acquired pneumonia. Procalcitonin-guided therapy allows for further tailoring of the regimen to the individual patient. Since this patient has clinically improved, and the PCT level is less than 0.25 mcg/L, it is reasonable to discontinue treatment and avoid unnecessary antibiotic days.
2. A 42-year-old female with known CKD stage 4 is hospitalized with suspected community-acquired pneumonia. Procalcitonin level is elevated at 0.6 mcg/L. How should the patient be treated?
a. Ignore the PCT as levels are falsely elevated due to CKD.
b. Treat with antibiotics for suspected community-acquired pneumonia.
c. Repeat PCT level in the morning.
d. Check a C-reactive protein level instead.
Answer: The best answer is b. Although decreased renal function can delay clearance of PCT, levels in CKD are typically about twice normal. In this case, when pneumonia is clinically suspected, the level of 0.6 mcg/L would correspond to a level of approximately 0.3 mcg/L and support a decision to treat with antibiotics.
3. A 36-year-old male develops sudden onset of dyspnea, cough, fever, and chills and proceeds rapidly to the emergency department. He is hypoxic, febrile, and has a leukocytosis. The PCT level is checked and found to be 0.2 mcg/L. Chest imaging shows a right middle lobe consolidation. How should the patient be treated?
a. Hold antibiotics.
b. Start antibiotic therapy.
c. Hold antibiotics and repeat PCT level in the morning.
Answer: The best answer is b. The clinical scenario suggests bacterial pneumonia. Given the sudden onset and early presentation to the ED, it is likely that the PCT level has not had time to peak. PCT levels typically begin to rise in 3-6 hours from the time of infection. Withholding antibiotics until the level exceeds 0.25 mcg/L would not be recommended when clinical judgment suggests otherwise.
4. Which of the following noninfectious scenarios does NOT cause an elevated PCT level?
a. Bone marrow transplant patient with acute graft versus host disease of the skin.
b. Patient presenting with paraneoplastic syndrome from small cell lung cancer.
c. Patient with cirrhosis presenting with hepatic encephalopathy.
d. Patient presenting with severe trauma from a motor vehicle accident.
Answer: The answer is c. Cirrhosis and/or hepatic encephalopathy does not cause a falsely elevated PCT level. Acute graft versus host disease, paraneoplastic syndrome from small cell lung cancer or medullary thyroid cancer, and massive stress such as severe trauma can cause elevations in PCT.
Additional reading
Spellberg B. The maturing antibiotic mantra: Shorter is still better. J Hosp Med. 2018;13:361-2. doi: 10.12788/jhm.2904.
Soni NJ et al. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J Hosp Med. 2013;8:530-540. doi: 10.1002/jhm.2067.
Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. doi: 10.1093/ofid/ofw249.
Sager R et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15. doi: 10.1186/s12916-017-0795-7.
Dynamics of the assay must be considered
Dynamics of the assay must be considered
Case
A 50-year-old female presents with 3 days of cough, subjective fevers, myalgias, and dyspnea. She feels she “may have caught something” while volunteering at a preschool. She has hypertension, congestive heart failure, and 20 pack-years of smoking. Chest x-ray shows bibasilar consolidation versus atelectasis. Vital signs are notable for an O2 saturation of 93%. White blood cell count and differential are normal. Procalcitonin level is 0.4 mcg/L.
Overview of the issue
Lower respiratory tract infections (LRTI) are common in the practice of hospital medicine; however, the primary symptoms of cough and dyspnea can be caused by a myriad of noninfectious conditions. Even when infection is suggested by the clinical presentation, the distinction between bacterial and viral etiologies can be challenging, complicating decisions about antibiotic use. Attention to antibiotic stewardship is a growing concern in U.S. hospitals, where the CDC estimates that as many as 50% of antibiotic orders are inappropriate or entirely unnecessary.1 Antibiotic overuse is a driver of multidrug-resistant organisms and increasing rates of Clostridium difficile infection. A diagnostic test to enhance physicians’ ability to target patients who would benefit from antibiotics could be a useful tool to combat the complications of antibiotic overuse. (See Figure 1.) 
Procalcitonin is produced in the thyroidal C-cells as a prohormone which is processed intracellularly and secreted as calcitonin in response to serum calcium levels. However, intact procalcitonin protein can be secreted from many other tissues in the presence of cytokines such as interleukin 1-beta, tumor necrosis factor-alpha, and lipopolysaccharide, typically released in response to systemic bacterial infections. Conversely, cytokines present in acute viral illness (interferon-gamma) suppress procalcitonin release. This dichotomy presents an opportunity to use procalcitonin to differentiate bacterial from nonbacterial etiologies in various clinical scenarios including LRTI.
Overview of the data
Multiple studies have demonstrated that procalcitonin can be safely used to guide antibiotic prescribing in patients with LRTI. The first large multicenter randomized controlled trial to address the topic was the Swiss PROHOSP study.2 Investigators randomized 1,359 patients hospitalized with LRTI to procalcitonin (PCT) guided therapy or guideline-based therapy. After an initial PCT level was measured, antibiotic prescribing in the PCT arm of the study was directed by a prespecified protocol; specifically, clinicians were discouraged from prescribing antibiotics in patients with PCT levels less than 0.25 mcg/L. (See Figure 2.)

For patients who were particularly ill or unstable at admission, the protocol allowed for antibiotics despite a low PCT level, but repeat measurement within 24 hours and accompanying treatment recommendations were reinforced with the treatment team. Clinicians caring for patients in the control arm were presented with condition-specific clinical practice guidelines to reinforce antibiotic choices. In both arms, the final decision on antibiotic treatment remained with the physician.
Results from the PROHOSP study showed no difference in the combined outcome of death, intensive care unit admission, or complications in the ensuing 30 days, but antibiotic use was significantly reduced. Mean antibiotic exposure dropped from 8.7 to 5.7 days, a reduction of 35%, with the largest decrease among patients with chronic obstructive pulmonary disease (COPD) and acute bronchitis. Antibiotic-related adverse effects fell by 8.2%. Strengths of the study included a very high rate of protocol compliance (90%) by the treating clinicians.
A systematic review of all available studies of procalcitonin-guided therapy for LRTI was published in 2018 and included 26 randomized controlled trials encompassing 6,708 patients in 12 countries. Findings confirmed an overall reduction of 2.4 days in antibiotic exposure, 6% reduction in antibiotic-related adverse effects, and importantly a 17% relative risk reduction in mortality.3
Similar benefits of PCT-guided therapy have been demonstrated even among severely ill patients. A meta-analysis including 523 patients with bacteremia noted mean reduction in antibiotic exposure of 2.86 days, without excess mortality.4 A second meta-analysis of 4,482 critically ill patients admitted to the ICU with sepsis demonstrated not only a reduction in antibiotic exposure, but in mortality as well. Despite a relatively small decrease in antibiotic duration of 1.19 days, the investigators found an 11% reduction in mortality (P = .03) in the PCT-guided group.5
One notable outlier among the many positive studies on PCT-guided antibiotic therapy is the 2018 PROACT study performed in U.S. hospitals over 4 years.6 Its design was similar to the PROHOSP study, however, in contrast to the majority of other trials, the investigators were unable to demonstrate a reduction in antibiotic exposure, leading them to conclude that PCT guidance may not be a useful tool for antibiotic stewardship.
Unfortunately, significant differences in the compliance with the study protocol (90% in PROHOSP vs. 63% in PROACT), and a much healthier patient population (91% of the patients had a PCT less than 0.25, and a majority of patients had asthma which is not normally treated with antibiotics) hamper the generalizability of the PROACT findings. Rather than indicating a failure of PCT, the findings of the study underscore the fact that the utility of any lab test is limited unless it is applied in an appropriate diagnostic setting.
For hospitalists, the most clinically useful role for PCT testing is to guide the duration of antibiotic therapy. Although the literature supports short-course antibiotic therapy in many common conditions seen by hospitalists (Table 1), data suggest overprescribing remains prevalent. Several recent studies targeting LRTI underscore this point.
Despite guidelines advocating for treatment of uncomplicated community-acquired pneumonia (CAP) for no more than 5-7 days, two recent retrospective studies suggest most patients receive longer courses. A review of more than 150,000 patients across the United States with uncomplicated CAP documented a mean antibiotic duration of 9.5 days, with close to 70% of patients receiving more than 7 days of therapy.7 A multicenter study of CAP patients hospitalized in Michigan noted similar findings, with a mean 2-day excess duration of therapy or 2,526 excess days of treatment per 1,000 discharges.8 Though some who argue against procalcitonin’s utility cite the fact that existing guidelines already support short-course therapy, obviating the need for biomarker guidance, clinicians have not yet universally adopted this practice. Using a PCT algorithm can decrease duration of therapy and thereby reduce unnecessary antibiotic use. PCT levels less than 0.25 mcg/L support withholding or discontinuing antibiotics, or consideration of an alternative diagnosis.
The dynamics of the PCT assay must be considered in order to use it appropriately. Levels of PCT rise within 3-6 hours of infection, so patients presenting extremely early in the disease course may have falsely low levels. PCT levels correlate with severity of illness and should fall within 2-3 days of initiation of appropriate therapy. A repeat PCT in 2-3 days can be used to help time antibiotic cessation. Studies support stopping antibiotics in stable patients once the PCT level falls below 0.25 mcg/L or drops by 80% in patients with severe elevations. Lack of improvement suggests inadequate antibiotic therapy and is predictive of excess mortality.
Most drivers of false-positive PCT levels are rare and easily identifiable. (See Figure 3.) However, like troponin, patients with chronic kidney disease have delayed PCT clearance, so baseline levels may be about double the normal range. If a baseline is known, monitoring the rise and fall of PCT levels remains clinically useful in this population.
Application of data to case
In reviewing the case, the differential includes a viral upper respiratory infection, an acute exacerbation of COPD, decompensated heart failure, or bacterial pneumonia. The lab and imaging findings are nonspecific, but a PCT level less than 0.25 mcg/L raises concern for an acute bacterial pneumonia. Given that PCT levels rise in bacterial infection and are suppressed in viral infections, treating this patient with antibiotics seems prudent. In this case the relatively mild elevation suggests a less severe infection or a presentation early in the disease course. A repeat PCT in 2-3 days will guide timing for antibiotic cessation.
Bottom line
Thoughtful procalcitonin-guided antibiotic therapy for LRTI may further current antibiotic stewardship initiatives targeting reduction of inappropriate antimicrobial use, which may ultimately reduce rates of Clostridium difficile infections and the emergence of multidrug-resistant organisms.
Dr. Seymann and Dr. Ramos are clinical professors in the division of hospital medicine, department of medicine, at the University of California San Diego.
Key points
- Initial PCT level can help distinguish between viral and bacterial pneumonias.
- PCT levels rise in response to acute bacterial infections and are suppressed in viral infections.
- PCT levels below 0.25 mcg/L suggest that antibiotics can be safely withheld in otherwise stable patients.
- PCT levels correlate with severity of illness and prognosis.
- Rise of PCT is rapid (3-6 hours), and levels fall quickly with appropriate treatment (2-3 days).
- Serial PCT levels can be used to guide duration of antibiotic therapy.
References
1. CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: U.S. Department of Health & Human Services. 2014. Available at www.cdc.gov/getsmart/healthcare/ implementation/core-elements.html.
2. Schuetz P et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-66. doi: 10.1001/jama.2009.1297.
3. Schuetz P et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107. doi: 10.1016/S1473-3099(17)30592-3.
4. Meier MA et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis. 2019;69(3):388-96. doi: 10.1093/cid/ciy917.
5. Wirz Y et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7.
6. Huang DT et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018 Jul 19;379(3):236-49. doi: 10.1056/NEJMoa1802670.
7. Yi SH et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66(9):1333-41. doi: 10.1093/cid/cix986.
8. Vaughn V et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019; 171(3):153-63. doi: 10.7326/M18-3640.
Quiz
1. A 57-year-old male is hospitalized for treatment of community-acquired pneumonia with IV azithromycin and ceftriaxone. PCT level on day 1 = 0.35 mcg/L. On day 4 of antibiotics the PCT level is 0.15 mcg/L. What should be done regarding the antibiotic course?
a. Continue antibiotics for a total course of 5 days.
b. Continue antibiotics for a total course of 7 days.
c. Stop antibiotics.
d. Continue antibiotics and repeat a PCT level the next day.
Answer: The best answer is c. Evidence suggests that 5 days of therapy is adequate treatment for uncomplicated community-acquired pneumonia. Procalcitonin-guided therapy allows for further tailoring of the regimen to the individual patient. Since this patient has clinically improved, and the PCT level is less than 0.25 mcg/L, it is reasonable to discontinue treatment and avoid unnecessary antibiotic days.
2. A 42-year-old female with known CKD stage 4 is hospitalized with suspected community-acquired pneumonia. Procalcitonin level is elevated at 0.6 mcg/L. How should the patient be treated?
a. Ignore the PCT as levels are falsely elevated due to CKD.
b. Treat with antibiotics for suspected community-acquired pneumonia.
c. Repeat PCT level in the morning.
d. Check a C-reactive protein level instead.
Answer: The best answer is b. Although decreased renal function can delay clearance of PCT, levels in CKD are typically about twice normal. In this case, when pneumonia is clinically suspected, the level of 0.6 mcg/L would correspond to a level of approximately 0.3 mcg/L and support a decision to treat with antibiotics.
3. A 36-year-old male develops sudden onset of dyspnea, cough, fever, and chills and proceeds rapidly to the emergency department. He is hypoxic, febrile, and has a leukocytosis. The PCT level is checked and found to be 0.2 mcg/L. Chest imaging shows a right middle lobe consolidation. How should the patient be treated?
a. Hold antibiotics.
b. Start antibiotic therapy.
c. Hold antibiotics and repeat PCT level in the morning.
Answer: The best answer is b. The clinical scenario suggests bacterial pneumonia. Given the sudden onset and early presentation to the ED, it is likely that the PCT level has not had time to peak. PCT levels typically begin to rise in 3-6 hours from the time of infection. Withholding antibiotics until the level exceeds 0.25 mcg/L would not be recommended when clinical judgment suggests otherwise.
4. Which of the following noninfectious scenarios does NOT cause an elevated PCT level?
a. Bone marrow transplant patient with acute graft versus host disease of the skin.
b. Patient presenting with paraneoplastic syndrome from small cell lung cancer.
c. Patient with cirrhosis presenting with hepatic encephalopathy.
d. Patient presenting with severe trauma from a motor vehicle accident.
Answer: The answer is c. Cirrhosis and/or hepatic encephalopathy does not cause a falsely elevated PCT level. Acute graft versus host disease, paraneoplastic syndrome from small cell lung cancer or medullary thyroid cancer, and massive stress such as severe trauma can cause elevations in PCT.
Additional reading
Spellberg B. The maturing antibiotic mantra: Shorter is still better. J Hosp Med. 2018;13:361-2. doi: 10.12788/jhm.2904.
Soni NJ et al. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J Hosp Med. 2013;8:530-540. doi: 10.1002/jhm.2067.
Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. doi: 10.1093/ofid/ofw249.
Sager R et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15. doi: 10.1186/s12916-017-0795-7.
Case
A 50-year-old female presents with 3 days of cough, subjective fevers, myalgias, and dyspnea. She feels she “may have caught something” while volunteering at a preschool. She has hypertension, congestive heart failure, and 20 pack-years of smoking. Chest x-ray shows bibasilar consolidation versus atelectasis. Vital signs are notable for an O2 saturation of 93%. White blood cell count and differential are normal. Procalcitonin level is 0.4 mcg/L.
Overview of the issue
Lower respiratory tract infections (LRTI) are common in the practice of hospital medicine; however, the primary symptoms of cough and dyspnea can be caused by a myriad of noninfectious conditions. Even when infection is suggested by the clinical presentation, the distinction between bacterial and viral etiologies can be challenging, complicating decisions about antibiotic use. Attention to antibiotic stewardship is a growing concern in U.S. hospitals, where the CDC estimates that as many as 50% of antibiotic orders are inappropriate or entirely unnecessary.1 Antibiotic overuse is a driver of multidrug-resistant organisms and increasing rates of Clostridium difficile infection. A diagnostic test to enhance physicians’ ability to target patients who would benefit from antibiotics could be a useful tool to combat the complications of antibiotic overuse. (See Figure 1.) 
Procalcitonin is produced in the thyroidal C-cells as a prohormone which is processed intracellularly and secreted as calcitonin in response to serum calcium levels. However, intact procalcitonin protein can be secreted from many other tissues in the presence of cytokines such as interleukin 1-beta, tumor necrosis factor-alpha, and lipopolysaccharide, typically released in response to systemic bacterial infections. Conversely, cytokines present in acute viral illness (interferon-gamma) suppress procalcitonin release. This dichotomy presents an opportunity to use procalcitonin to differentiate bacterial from nonbacterial etiologies in various clinical scenarios including LRTI.
Overview of the data
Multiple studies have demonstrated that procalcitonin can be safely used to guide antibiotic prescribing in patients with LRTI. The first large multicenter randomized controlled trial to address the topic was the Swiss PROHOSP study.2 Investigators randomized 1,359 patients hospitalized with LRTI to procalcitonin (PCT) guided therapy or guideline-based therapy. After an initial PCT level was measured, antibiotic prescribing in the PCT arm of the study was directed by a prespecified protocol; specifically, clinicians were discouraged from prescribing antibiotics in patients with PCT levels less than 0.25 mcg/L. (See Figure 2.)

For patients who were particularly ill or unstable at admission, the protocol allowed for antibiotics despite a low PCT level, but repeat measurement within 24 hours and accompanying treatment recommendations were reinforced with the treatment team. Clinicians caring for patients in the control arm were presented with condition-specific clinical practice guidelines to reinforce antibiotic choices. In both arms, the final decision on antibiotic treatment remained with the physician.
Results from the PROHOSP study showed no difference in the combined outcome of death, intensive care unit admission, or complications in the ensuing 30 days, but antibiotic use was significantly reduced. Mean antibiotic exposure dropped from 8.7 to 5.7 days, a reduction of 35%, with the largest decrease among patients with chronic obstructive pulmonary disease (COPD) and acute bronchitis. Antibiotic-related adverse effects fell by 8.2%. Strengths of the study included a very high rate of protocol compliance (90%) by the treating clinicians.
A systematic review of all available studies of procalcitonin-guided therapy for LRTI was published in 2018 and included 26 randomized controlled trials encompassing 6,708 patients in 12 countries. Findings confirmed an overall reduction of 2.4 days in antibiotic exposure, 6% reduction in antibiotic-related adverse effects, and importantly a 17% relative risk reduction in mortality.3
Similar benefits of PCT-guided therapy have been demonstrated even among severely ill patients. A meta-analysis including 523 patients with bacteremia noted mean reduction in antibiotic exposure of 2.86 days, without excess mortality.4 A second meta-analysis of 4,482 critically ill patients admitted to the ICU with sepsis demonstrated not only a reduction in antibiotic exposure, but in mortality as well. Despite a relatively small decrease in antibiotic duration of 1.19 days, the investigators found an 11% reduction in mortality (P = .03) in the PCT-guided group.5
One notable outlier among the many positive studies on PCT-guided antibiotic therapy is the 2018 PROACT study performed in U.S. hospitals over 4 years.6 Its design was similar to the PROHOSP study, however, in contrast to the majority of other trials, the investigators were unable to demonstrate a reduction in antibiotic exposure, leading them to conclude that PCT guidance may not be a useful tool for antibiotic stewardship.
Unfortunately, significant differences in the compliance with the study protocol (90% in PROHOSP vs. 63% in PROACT), and a much healthier patient population (91% of the patients had a PCT less than 0.25, and a majority of patients had asthma which is not normally treated with antibiotics) hamper the generalizability of the PROACT findings. Rather than indicating a failure of PCT, the findings of the study underscore the fact that the utility of any lab test is limited unless it is applied in an appropriate diagnostic setting.
For hospitalists, the most clinically useful role for PCT testing is to guide the duration of antibiotic therapy. Although the literature supports short-course antibiotic therapy in many common conditions seen by hospitalists (Table 1), data suggest overprescribing remains prevalent. Several recent studies targeting LRTI underscore this point.
Despite guidelines advocating for treatment of uncomplicated community-acquired pneumonia (CAP) for no more than 5-7 days, two recent retrospective studies suggest most patients receive longer courses. A review of more than 150,000 patients across the United States with uncomplicated CAP documented a mean antibiotic duration of 9.5 days, with close to 70% of patients receiving more than 7 days of therapy.7 A multicenter study of CAP patients hospitalized in Michigan noted similar findings, with a mean 2-day excess duration of therapy or 2,526 excess days of treatment per 1,000 discharges.8 Though some who argue against procalcitonin’s utility cite the fact that existing guidelines already support short-course therapy, obviating the need for biomarker guidance, clinicians have not yet universally adopted this practice. Using a PCT algorithm can decrease duration of therapy and thereby reduce unnecessary antibiotic use. PCT levels less than 0.25 mcg/L support withholding or discontinuing antibiotics, or consideration of an alternative diagnosis.
The dynamics of the PCT assay must be considered in order to use it appropriately. Levels of PCT rise within 3-6 hours of infection, so patients presenting extremely early in the disease course may have falsely low levels. PCT levels correlate with severity of illness and should fall within 2-3 days of initiation of appropriate therapy. A repeat PCT in 2-3 days can be used to help time antibiotic cessation. Studies support stopping antibiotics in stable patients once the PCT level falls below 0.25 mcg/L or drops by 80% in patients with severe elevations. Lack of improvement suggests inadequate antibiotic therapy and is predictive of excess mortality.
Most drivers of false-positive PCT levels are rare and easily identifiable. (See Figure 3.) However, like troponin, patients with chronic kidney disease have delayed PCT clearance, so baseline levels may be about double the normal range. If a baseline is known, monitoring the rise and fall of PCT levels remains clinically useful in this population.
Application of data to case
In reviewing the case, the differential includes a viral upper respiratory infection, an acute exacerbation of COPD, decompensated heart failure, or bacterial pneumonia. The lab and imaging findings are nonspecific, but a PCT level less than 0.25 mcg/L raises concern for an acute bacterial pneumonia. Given that PCT levels rise in bacterial infection and are suppressed in viral infections, treating this patient with antibiotics seems prudent. In this case the relatively mild elevation suggests a less severe infection or a presentation early in the disease course. A repeat PCT in 2-3 days will guide timing for antibiotic cessation.
Bottom line
Thoughtful procalcitonin-guided antibiotic therapy for LRTI may further current antibiotic stewardship initiatives targeting reduction of inappropriate antimicrobial use, which may ultimately reduce rates of Clostridium difficile infections and the emergence of multidrug-resistant organisms.
Dr. Seymann and Dr. Ramos are clinical professors in the division of hospital medicine, department of medicine, at the University of California San Diego.
Key points
- Initial PCT level can help distinguish between viral and bacterial pneumonias.
- PCT levels rise in response to acute bacterial infections and are suppressed in viral infections.
- PCT levels below 0.25 mcg/L suggest that antibiotics can be safely withheld in otherwise stable patients.
- PCT levels correlate with severity of illness and prognosis.
- Rise of PCT is rapid (3-6 hours), and levels fall quickly with appropriate treatment (2-3 days).
- Serial PCT levels can be used to guide duration of antibiotic therapy.
References
1. CDC. Core elements of hospital antibiotic stewardship programs. Atlanta: U.S. Department of Health & Human Services. 2014. Available at www.cdc.gov/getsmart/healthcare/ implementation/core-elements.html.
2. Schuetz P et al. Effect of procalcitonin-based guidelines vs. standard guidelines on antibiotic use in lower respiratory tract infections: The ProHOSP randomized controlled trial. JAMA. 2009;302(10):1059-66. doi: 10.1001/jama.2009.1297.
3. Schuetz P et al. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: A patient level meta-analysis. Lancet Infect Dis. 2018;18(1):95-107. doi: 10.1016/S1473-3099(17)30592-3.
4. Meier MA et al. Procalcitonin-guided antibiotic treatment in patients with positive blood cultures: A patient-level meta-analysis of randomized trials. Clin Infect Dis. 2019;69(3):388-96. doi: 10.1093/cid/ciy917.
5. Wirz Y et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: A patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7.
6. Huang DT et al. Procalcitonin-guided use of antibiotics for lower respiratory tract infection. N Engl J Med. 2018 Jul 19;379(3):236-49. doi: 10.1056/NEJMoa1802670.
7. Yi SH et al. Duration of antibiotic use among adults with uncomplicated community-acquired pneumonia requiring hospitalization in the United States. Clin Infect Dis. 2018;66(9):1333-41. doi: 10.1093/cid/cix986.
8. Vaughn V et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann Intern Med. 2019; 171(3):153-63. doi: 10.7326/M18-3640.
Quiz
1. A 57-year-old male is hospitalized for treatment of community-acquired pneumonia with IV azithromycin and ceftriaxone. PCT level on day 1 = 0.35 mcg/L. On day 4 of antibiotics the PCT level is 0.15 mcg/L. What should be done regarding the antibiotic course?
a. Continue antibiotics for a total course of 5 days.
b. Continue antibiotics for a total course of 7 days.
c. Stop antibiotics.
d. Continue antibiotics and repeat a PCT level the next day.
Answer: The best answer is c. Evidence suggests that 5 days of therapy is adequate treatment for uncomplicated community-acquired pneumonia. Procalcitonin-guided therapy allows for further tailoring of the regimen to the individual patient. Since this patient has clinically improved, and the PCT level is less than 0.25 mcg/L, it is reasonable to discontinue treatment and avoid unnecessary antibiotic days.
2. A 42-year-old female with known CKD stage 4 is hospitalized with suspected community-acquired pneumonia. Procalcitonin level is elevated at 0.6 mcg/L. How should the patient be treated?
a. Ignore the PCT as levels are falsely elevated due to CKD.
b. Treat with antibiotics for suspected community-acquired pneumonia.
c. Repeat PCT level in the morning.
d. Check a C-reactive protein level instead.
Answer: The best answer is b. Although decreased renal function can delay clearance of PCT, levels in CKD are typically about twice normal. In this case, when pneumonia is clinically suspected, the level of 0.6 mcg/L would correspond to a level of approximately 0.3 mcg/L and support a decision to treat with antibiotics.
3. A 36-year-old male develops sudden onset of dyspnea, cough, fever, and chills and proceeds rapidly to the emergency department. He is hypoxic, febrile, and has a leukocytosis. The PCT level is checked and found to be 0.2 mcg/L. Chest imaging shows a right middle lobe consolidation. How should the patient be treated?
a. Hold antibiotics.
b. Start antibiotic therapy.
c. Hold antibiotics and repeat PCT level in the morning.
Answer: The best answer is b. The clinical scenario suggests bacterial pneumonia. Given the sudden onset and early presentation to the ED, it is likely that the PCT level has not had time to peak. PCT levels typically begin to rise in 3-6 hours from the time of infection. Withholding antibiotics until the level exceeds 0.25 mcg/L would not be recommended when clinical judgment suggests otherwise.
4. Which of the following noninfectious scenarios does NOT cause an elevated PCT level?
a. Bone marrow transplant patient with acute graft versus host disease of the skin.
b. Patient presenting with paraneoplastic syndrome from small cell lung cancer.
c. Patient with cirrhosis presenting with hepatic encephalopathy.
d. Patient presenting with severe trauma from a motor vehicle accident.
Answer: The answer is c. Cirrhosis and/or hepatic encephalopathy does not cause a falsely elevated PCT level. Acute graft versus host disease, paraneoplastic syndrome from small cell lung cancer or medullary thyroid cancer, and massive stress such as severe trauma can cause elevations in PCT.
Additional reading
Spellberg B. The maturing antibiotic mantra: Shorter is still better. J Hosp Med. 2018;13:361-2. doi: 10.12788/jhm.2904.
Soni NJ et al. Procalcitonin-guided antibiotic therapy: A systematic review and meta-analysis. J Hosp Med. 2013;8:530-540. doi: 10.1002/jhm.2067.
Rhee C. Using procalcitonin to guide antibiotic therapy. Open Forum Infect Dis. 2017;4(1):ofw249. doi: 10.1093/ofid/ofw249.
Sager R et al. Procalcitonin-guided diagnosis and antibiotic stewardship revisited. BMC Med. 2017;15. doi: 10.1186/s12916-017-0795-7.
COVID-19 in children: Weekly cases drop to 6-month low
Just 1 week after it looked like the COVID-19 situation in children might be taking another turn for the worse, the number of new pediatric cases dropped to its lowest level since October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and CHA said in their weekly COVID-19 report. During the week of April 30 to May 6 – the same week Rhode Island reported a large backlog of cases and increased its total by 30% – the number of new cases went up slightly after 2 weeks of declines.
Other positive indicators come in the form of the proportion of cases occurring in children. The cumulative percentage of cases in children since the start of the pandemic remained at 14.0% for a second consecutive week, and the proportion of new cases in children held at 24.0% and did not increase for the first time in 6 weeks, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of child COVID-19 cases reported in these jurisdictions is now up to 3.9 million, for a cumulative rate of 5,187 cases per 100,000 children in the United States. Among the states, total counts range from a low of 4,070 in Hawaii to 475,619 in California. Hawaii also has the lowest rate at 1,357 per 100,000 children, while the highest, 9,778 per 100,000, can be found in Rhode Island, the AAP and CHA said.
Deaths in children continue to accumulate at a relatively slow pace, with two more added during the week of May 7-13, bringing the total to 308 for the entire pandemic in 43 states, New York City, Puerto Rico, and Guam. Children’s share of the mortality burden is currently 0.06%, a figure that has not changed since mid-December, and the death rate for children with COVID-19 is 0.01%, according to the report.
Almost two-thirds (65%) of all deaths have occurred in just nine states – Arizona (31), California (21), Colorado (13), Georgia (10), Illinois (18), Maryland (10), Pennsylvania (10), Tennessee (10), and Texas (52) – and New York City (24), while eight states have not reported any deaths yet, the two groups said.
Just 1 week after it looked like the COVID-19 situation in children might be taking another turn for the worse, the number of new pediatric cases dropped to its lowest level since October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and CHA said in their weekly COVID-19 report. During the week of April 30 to May 6 – the same week Rhode Island reported a large backlog of cases and increased its total by 30% – the number of new cases went up slightly after 2 weeks of declines.
Other positive indicators come in the form of the proportion of cases occurring in children. The cumulative percentage of cases in children since the start of the pandemic remained at 14.0% for a second consecutive week, and the proportion of new cases in children held at 24.0% and did not increase for the first time in 6 weeks, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of child COVID-19 cases reported in these jurisdictions is now up to 3.9 million, for a cumulative rate of 5,187 cases per 100,000 children in the United States. Among the states, total counts range from a low of 4,070 in Hawaii to 475,619 in California. Hawaii also has the lowest rate at 1,357 per 100,000 children, while the highest, 9,778 per 100,000, can be found in Rhode Island, the AAP and CHA said.
Deaths in children continue to accumulate at a relatively slow pace, with two more added during the week of May 7-13, bringing the total to 308 for the entire pandemic in 43 states, New York City, Puerto Rico, and Guam. Children’s share of the mortality burden is currently 0.06%, a figure that has not changed since mid-December, and the death rate for children with COVID-19 is 0.01%, according to the report.
Almost two-thirds (65%) of all deaths have occurred in just nine states – Arizona (31), California (21), Colorado (13), Georgia (10), Illinois (18), Maryland (10), Pennsylvania (10), Tennessee (10), and Texas (52) – and New York City (24), while eight states have not reported any deaths yet, the two groups said.
Just 1 week after it looked like the COVID-19 situation in children might be taking another turn for the worse, the number of new pediatric cases dropped to its lowest level since October, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

the AAP and CHA said in their weekly COVID-19 report. During the week of April 30 to May 6 – the same week Rhode Island reported a large backlog of cases and increased its total by 30% – the number of new cases went up slightly after 2 weeks of declines.
Other positive indicators come in the form of the proportion of cases occurring in children. The cumulative percentage of cases in children since the start of the pandemic remained at 14.0% for a second consecutive week, and the proportion of new cases in children held at 24.0% and did not increase for the first time in 6 weeks, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
The total number of child COVID-19 cases reported in these jurisdictions is now up to 3.9 million, for a cumulative rate of 5,187 cases per 100,000 children in the United States. Among the states, total counts range from a low of 4,070 in Hawaii to 475,619 in California. Hawaii also has the lowest rate at 1,357 per 100,000 children, while the highest, 9,778 per 100,000, can be found in Rhode Island, the AAP and CHA said.
Deaths in children continue to accumulate at a relatively slow pace, with two more added during the week of May 7-13, bringing the total to 308 for the entire pandemic in 43 states, New York City, Puerto Rico, and Guam. Children’s share of the mortality burden is currently 0.06%, a figure that has not changed since mid-December, and the death rate for children with COVID-19 is 0.01%, according to the report.
Almost two-thirds (65%) of all deaths have occurred in just nine states – Arizona (31), California (21), Colorado (13), Georgia (10), Illinois (18), Maryland (10), Pennsylvania (10), Tennessee (10), and Texas (52) – and New York City (24), while eight states have not reported any deaths yet, the two groups said.
FIDELIO-DKD: Finerenone cuts new-onset AFib in patients with type 2 diabetes and CKD
Finerenone treatment of patients with type 2 diabetes and diabetic kidney disease was linked to a significant drop in the incidence of new-onset atrial fibrillation as a prespecified, exploratory endpoint of the FIDELIO-DKD pivotal trial that randomized more than 5,700 patients.
Treatment with finerenone linked with a 29% relative reduction compared with placebo in incident cases of atrial fibrillation (AFib), Gerasimos Filippatos, MD, reported at the annual scientific sessions of the American College of Cardiology.
The absolute reduction was modest, a 1.3% reduction from the 4.5% incidence rate on placebo to a 3.2% rate on finerenone during a median 2.6 years of follow-up. Concurrently with the report, the results appeared online (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.079).
The analyses Dr. Filippatos presented also showed that whether or not patients had a history of AFib, there was no impact on either the primary benefit from finerenone treatment seen in FIDELIO-DKD, which was a significant 18% relative risk reduction compared with placebo in the combined rate of kidney failure, a 40% or greater decline from baseline in estimated glomerular filtration rate, or renal death.
Likewise, prior AFib status had no effect on the study’s key secondary endpoint, a significant 14% relative risk reduction in the combined rate of cardiovascular death, nonfatal MI, nonfatal stroke, or hospitalization for heart failure.
The primary results from FIDELIO-DKD (Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease) appeared in a 2020 report (N Engl J Med. 2020 Dec 3;383[23];2219-29).
‘Side benefits can be very helpful’
“It’s important to know of finerenone’s benefits beyond the primary outcome of a trial because side benefits can be very helpful,” said Anne B. Curtis, MD, an electrophysiologist and professor and chair of medicine at the University of Buffalo (N.Y.) School of Medicine and Biomedical Sciences. “It’s not a huge benefit, but this could be an added benefit for selected patients,” she said during a press briefing. “Background studies had shown favorable remodeling of the heart [by finerenone] that could affect AFib.”
Possible mitigating effects by finerenone on inflammation and fibrosis might also mediate the drug’s apparent effect on AFib, said Dr. Filippatos, professor of cardiology and director of the Heart Failure and Cardio-Oncology Clinic at Attikon University Hospital and the University of Athens.
He noted that additional data addressing a possible AFib effect of finerenone will emerge soon from the FIGARO-DKD trial, which enrolled patients similar to those in FIDELIO-DKD but with more moderate stages of kidney disease, and from the FINEARTS-HF trial, which is examining the effect of finerenone in patients with heart failure with an ejection fraction of at least 40%.
“Heart failure and AFib go together tightly. It’s worth studying this specifically, so we can see whether there is an impact of finerenone on patients with heart failure who may not necessarily have kidney disease or diabetes,” Dr. Curtis said.
Hypothesis-generating findings
The new findings reported by Dr. Filippatos “should be considered hypothesis generating. Until we have more information, upstream therapies, including mineralocorticoid receptor antagonists [MRAs, the umbrella drug class that includes finerenone], should be used in appropriate patient populations based on defined benefits with the hope they will also reduce the development of AFib and atrial flutter over time,” Gerald V. Naccarelli, MD, and coauthors wrote in an editorial that accompanied the report (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.080).
The FIDELIO-DKD trial randomized 5,734 patients at 913 sites in 48 countries, including 461 patients with a history of AFib. The observed link of finerenone treatment with a reduced incidence of AFib appeared consistent regardless of patients’ age, sex, race, their kidney characteristics at baseline, baseline levels of systolic blood pressure, serum potassium, body mass index, A1c, or use of glucose-lowering medications.
Finerenone belongs to a new class of MRAs that have a nonsteroidal structure, in contrast with the MRAs spironolactone and eplerenone. This means that finerenone does not produce steroidal-associated adverse effects linked with certain other MRAs, such as gynecomastia, and may also differ in other actions.
FIDELIO-DKD was sponsored by Bayer, the company developing finerenone. Dr. Filippatos has received lecture fees from or participated in the direction of trials on behalf of Bayer, as well as for Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Curtis is an adviser to and receives honoraria from St. Jude Medical, and receives honoraria from Medtronic. Dr. Naccarelli has been a consultant to Acesion, ARCA, GlaxoSmithKline, Janssen, Milestone, Omeicos, and Sanofi. His coauthors had no disclosures.
Finerenone treatment of patients with type 2 diabetes and diabetic kidney disease was linked to a significant drop in the incidence of new-onset atrial fibrillation as a prespecified, exploratory endpoint of the FIDELIO-DKD pivotal trial that randomized more than 5,700 patients.
Treatment with finerenone linked with a 29% relative reduction compared with placebo in incident cases of atrial fibrillation (AFib), Gerasimos Filippatos, MD, reported at the annual scientific sessions of the American College of Cardiology.
The absolute reduction was modest, a 1.3% reduction from the 4.5% incidence rate on placebo to a 3.2% rate on finerenone during a median 2.6 years of follow-up. Concurrently with the report, the results appeared online (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.079).
The analyses Dr. Filippatos presented also showed that whether or not patients had a history of AFib, there was no impact on either the primary benefit from finerenone treatment seen in FIDELIO-DKD, which was a significant 18% relative risk reduction compared with placebo in the combined rate of kidney failure, a 40% or greater decline from baseline in estimated glomerular filtration rate, or renal death.
Likewise, prior AFib status had no effect on the study’s key secondary endpoint, a significant 14% relative risk reduction in the combined rate of cardiovascular death, nonfatal MI, nonfatal stroke, or hospitalization for heart failure.
The primary results from FIDELIO-DKD (Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease) appeared in a 2020 report (N Engl J Med. 2020 Dec 3;383[23];2219-29).
‘Side benefits can be very helpful’
“It’s important to know of finerenone’s benefits beyond the primary outcome of a trial because side benefits can be very helpful,” said Anne B. Curtis, MD, an electrophysiologist and professor and chair of medicine at the University of Buffalo (N.Y.) School of Medicine and Biomedical Sciences. “It’s not a huge benefit, but this could be an added benefit for selected patients,” she said during a press briefing. “Background studies had shown favorable remodeling of the heart [by finerenone] that could affect AFib.”
Possible mitigating effects by finerenone on inflammation and fibrosis might also mediate the drug’s apparent effect on AFib, said Dr. Filippatos, professor of cardiology and director of the Heart Failure and Cardio-Oncology Clinic at Attikon University Hospital and the University of Athens.
He noted that additional data addressing a possible AFib effect of finerenone will emerge soon from the FIGARO-DKD trial, which enrolled patients similar to those in FIDELIO-DKD but with more moderate stages of kidney disease, and from the FINEARTS-HF trial, which is examining the effect of finerenone in patients with heart failure with an ejection fraction of at least 40%.
“Heart failure and AFib go together tightly. It’s worth studying this specifically, so we can see whether there is an impact of finerenone on patients with heart failure who may not necessarily have kidney disease or diabetes,” Dr. Curtis said.
Hypothesis-generating findings
The new findings reported by Dr. Filippatos “should be considered hypothesis generating. Until we have more information, upstream therapies, including mineralocorticoid receptor antagonists [MRAs, the umbrella drug class that includes finerenone], should be used in appropriate patient populations based on defined benefits with the hope they will also reduce the development of AFib and atrial flutter over time,” Gerald V. Naccarelli, MD, and coauthors wrote in an editorial that accompanied the report (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.080).
The FIDELIO-DKD trial randomized 5,734 patients at 913 sites in 48 countries, including 461 patients with a history of AFib. The observed link of finerenone treatment with a reduced incidence of AFib appeared consistent regardless of patients’ age, sex, race, their kidney characteristics at baseline, baseline levels of systolic blood pressure, serum potassium, body mass index, A1c, or use of glucose-lowering medications.
Finerenone belongs to a new class of MRAs that have a nonsteroidal structure, in contrast with the MRAs spironolactone and eplerenone. This means that finerenone does not produce steroidal-associated adverse effects linked with certain other MRAs, such as gynecomastia, and may also differ in other actions.
FIDELIO-DKD was sponsored by Bayer, the company developing finerenone. Dr. Filippatos has received lecture fees from or participated in the direction of trials on behalf of Bayer, as well as for Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Curtis is an adviser to and receives honoraria from St. Jude Medical, and receives honoraria from Medtronic. Dr. Naccarelli has been a consultant to Acesion, ARCA, GlaxoSmithKline, Janssen, Milestone, Omeicos, and Sanofi. His coauthors had no disclosures.
Finerenone treatment of patients with type 2 diabetes and diabetic kidney disease was linked to a significant drop in the incidence of new-onset atrial fibrillation as a prespecified, exploratory endpoint of the FIDELIO-DKD pivotal trial that randomized more than 5,700 patients.
Treatment with finerenone linked with a 29% relative reduction compared with placebo in incident cases of atrial fibrillation (AFib), Gerasimos Filippatos, MD, reported at the annual scientific sessions of the American College of Cardiology.
The absolute reduction was modest, a 1.3% reduction from the 4.5% incidence rate on placebo to a 3.2% rate on finerenone during a median 2.6 years of follow-up. Concurrently with the report, the results appeared online (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.079).
The analyses Dr. Filippatos presented also showed that whether or not patients had a history of AFib, there was no impact on either the primary benefit from finerenone treatment seen in FIDELIO-DKD, which was a significant 18% relative risk reduction compared with placebo in the combined rate of kidney failure, a 40% or greater decline from baseline in estimated glomerular filtration rate, or renal death.
Likewise, prior AFib status had no effect on the study’s key secondary endpoint, a significant 14% relative risk reduction in the combined rate of cardiovascular death, nonfatal MI, nonfatal stroke, or hospitalization for heart failure.
The primary results from FIDELIO-DKD (Efficacy and Safety of Finerenone in Subjects With Type 2 Diabetes Mellitus and Diabetic Kidney Disease) appeared in a 2020 report (N Engl J Med. 2020 Dec 3;383[23];2219-29).
‘Side benefits can be very helpful’
“It’s important to know of finerenone’s benefits beyond the primary outcome of a trial because side benefits can be very helpful,” said Anne B. Curtis, MD, an electrophysiologist and professor and chair of medicine at the University of Buffalo (N.Y.) School of Medicine and Biomedical Sciences. “It’s not a huge benefit, but this could be an added benefit for selected patients,” she said during a press briefing. “Background studies had shown favorable remodeling of the heart [by finerenone] that could affect AFib.”
Possible mitigating effects by finerenone on inflammation and fibrosis might also mediate the drug’s apparent effect on AFib, said Dr. Filippatos, professor of cardiology and director of the Heart Failure and Cardio-Oncology Clinic at Attikon University Hospital and the University of Athens.
He noted that additional data addressing a possible AFib effect of finerenone will emerge soon from the FIGARO-DKD trial, which enrolled patients similar to those in FIDELIO-DKD but with more moderate stages of kidney disease, and from the FINEARTS-HF trial, which is examining the effect of finerenone in patients with heart failure with an ejection fraction of at least 40%.
“Heart failure and AFib go together tightly. It’s worth studying this specifically, so we can see whether there is an impact of finerenone on patients with heart failure who may not necessarily have kidney disease or diabetes,” Dr. Curtis said.
Hypothesis-generating findings
The new findings reported by Dr. Filippatos “should be considered hypothesis generating. Until we have more information, upstream therapies, including mineralocorticoid receptor antagonists [MRAs, the umbrella drug class that includes finerenone], should be used in appropriate patient populations based on defined benefits with the hope they will also reduce the development of AFib and atrial flutter over time,” Gerald V. Naccarelli, MD, and coauthors wrote in an editorial that accompanied the report (J Am Coll Cardiol. 2021 May 17. doi: 10.1016/j.jacc.2021.04.080).
The FIDELIO-DKD trial randomized 5,734 patients at 913 sites in 48 countries, including 461 patients with a history of AFib. The observed link of finerenone treatment with a reduced incidence of AFib appeared consistent regardless of patients’ age, sex, race, their kidney characteristics at baseline, baseline levels of systolic blood pressure, serum potassium, body mass index, A1c, or use of glucose-lowering medications.
Finerenone belongs to a new class of MRAs that have a nonsteroidal structure, in contrast with the MRAs spironolactone and eplerenone. This means that finerenone does not produce steroidal-associated adverse effects linked with certain other MRAs, such as gynecomastia, and may also differ in other actions.
FIDELIO-DKD was sponsored by Bayer, the company developing finerenone. Dr. Filippatos has received lecture fees from or participated in the direction of trials on behalf of Bayer, as well as for Amgen, Boehringer Ingelheim, Medtronic, Novartis, Servier, and Vifor. Dr. Curtis is an adviser to and receives honoraria from St. Jude Medical, and receives honoraria from Medtronic. Dr. Naccarelli has been a consultant to Acesion, ARCA, GlaxoSmithKline, Janssen, Milestone, Omeicos, and Sanofi. His coauthors had no disclosures.
FROM ACC 2021
Dapagliflozin misses as treatment for COVID-19 but leaves intriguing signal for benefit
In patients hospitalized with COVID-19 infection, the sodium-glucose transporter 2 inhibitor dapagliflozin showed a trend for benefit relative to placebo on multiple outcomes, including the primary outcome of time to organ failure or death, according to results from the randomized DARE-19 trial.
Because of the failure to reach statistical significance, these results have no immediate relevance, but the trends support interest in further testing SGLT2 inhibitors in acute diseases posing a high risk for organ failure, according to Mikhail Kosiborod, MD.
In a trial that did not meet its primary endpoint, Dr. Kosiborod acknowledged that positive interpretations are speculative, but he does believe that there is one immediate take-home message.
“Our results do not support discontinuation of SGLT2 inhibitors in the setting of COVID-19 as long as patients are monitored,” said Dr. Kosiborod, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo.
At many institutions, it has been common to discontinue SGLT2 inhibitors in patients admitted with COVID-19. One reason was the concern that drugs in this class could exacerbate organ damage, particularly if they were to induced ketoacidosis. However, only 2 (0.003%) of 613 patients treated with dapagliflozin developed ketoacidosis, and the signal for organ protection overall, although not significant, was consistent.
“Numerically, fewer patients treated with dapagliflozin experienced organ failure and death, and this was consistent across systems, including the kidney,” Dr. Kosiborod said in presenting the study at the annual scientific sessions of the American College of Cardiology.
Overall, the study suggests that, in the context of COVID-19, dapagliflozin did not show harm and might have potential benefit, he added.
DARE-19 was rapidly conceived, designed, and implemented during the early stages of the COVID-19 pandemic. Based on prior evidence that SGLT2 inhibitors “favorably affect a number of pathophysiologic pathways disrupted during acute illness” and that drugs in this class have provided organ protection in the context of heart failure, chronic kidney disease, and other cardiometabolic conditions, the study was designed to test the hypothesis that this mechanism might improve outcomes in patients hospitalized with COVID-19, Dr. Kosiborod said.
The entry criteria included confirmed or suspected COVID-19 with an onset of 4 days of fewer and one additional risk factor, such as atherosclerotic cardiovascular disease, hypertension, or type 2 diabetes. Patients with significant renal impairment or a history of diabetic ketoacidosis were excluded.
On top of standard treatments for COVID-19, patients were randomized to 10 mg dapagliflozin or placebo once daily. There were two primary endpoints. That of prevention was time to criteria for respiratory, cardiovascular, or renal organ failure or death. The second primary outcome, for recovery, was a hierarchical composite for four endpoints: death, organ failure, status at 30 days if hospitalized, and time to discharge if this occurred before day 30.
Of the 1,250 patients randomized at 95 sites in seven countries, 617 in the dapagliflozin group and 620 patients in the placebo group completed the study. Baseline characteristics, which included a mean of age of 62 years; types of comorbidities; and types of treatments were similar.
Results for two primary endpoints
The curves for the primary outcome of prevention had already separated by day 3 and continued to widen over the 30 days in which outcomes were compared. At the end of 30 days, 11.2% of the dapagliflozin group and 13.8% of the placebo group had an event. By hazard ratio, dapagliflozin was linked to 20% nonsignificant relative protection from events (hazard ratio, 0.80; 95% confidence interval, 0.58-1.10).
The trend (P = .168) for the primary endpoint for prevention was reflected in the individual components. For dapagliflozin related to placebo, there were generally similar or greater reductions in new or worsening organ failure (HR, 0.80), cardiac decompensation (HR, 0.81), respiratory decompensation (HR, 0.85), and kidney decompensation (HR, 0.65). None were statistically significant, but the confidence intervals were tight with the upper end never exceeding 1.20.
Moreover, the relative risk reduction for all-cause mortality moved in the same direction (HR, 0.77; 95% CI, 0.52-1.16).
In the hierarchical composite endpoint of recovery, there was no significant difference in the time to discharge, but again many recovery metrics numerically favored dapagliflozin with an overall difference producing a statistical trend (P = .14) similar to organ failure events and death.
In safety analyses, dapagliflozin consistently outperformed placebo across a broad array of safety measure, including any severe adverse event (65% vs. 82%), any adverse event with an outcome of death (32% vs. 48%), discontinuation caused by an adverse event (44% vs. 55%), and acute kidney injury (21% vs. 34%).
Data could fuel related studies
According to Ana Barac, MD, PhD, director of the cardio-oncology program in the Medstar Heart and Vascular Institute, Washington, these data are “thought provoking.” Although this was a negative trial, she said that it generates an “exciting hypothesis” about the potential of SGLT2 inhibitors to provide organ protection. She called for studies to pursue this path of research.
More immediately, Dr. Barac agreed that these data argue against stopping SGLT2 inhibitors in patients admitted to a hospital for COVID-19 infection.
“These data show that these drugs are not going to lead to harm, but they might lead to benefit,” she said.
For James Januzzi, MD, a cardiologist at Massachusetts General Hospital and professor of medicine at Harvard Medical School, both in Boston, DARE-19 was perhaps most impressive because of its rigorous design and execution in the midst of a pandemic.
Over the past year, “the medical literature was flooded with grossly underpowered, poorly designed, single-center studies” yielding results that have been hard to interpret, Dr. Januzzi said. Despite the fact that this study failed to confirm its hypothesis, he said the investigators deserve praise for the quality of the work.
Dr. Januzzi also believes the study is not without clinically relevant findings, particularly the fact that dapagliflozin was associated with a lower rate of adverse events than placebo. This, at least, provides reassurance about the safety of this drug in the setting of COVID-19 infection.
Dr. Kosiborod reported financial relationships with more than 10 pharmaceutical companies, including AstraZeneca, which provided funding for DARE-19. Dr. Barac reported financial relationships with Bristol-Myers Squibb and CTI BioPharma. Dr. Januzzi reported financial relationships with Boehringer Ingelheim, GE Healthcare, Johnson & Johnson, Merck, Novartis, Pfizer, and Roche.
In patients hospitalized with COVID-19 infection, the sodium-glucose transporter 2 inhibitor dapagliflozin showed a trend for benefit relative to placebo on multiple outcomes, including the primary outcome of time to organ failure or death, according to results from the randomized DARE-19 trial.
Because of the failure to reach statistical significance, these results have no immediate relevance, but the trends support interest in further testing SGLT2 inhibitors in acute diseases posing a high risk for organ failure, according to Mikhail Kosiborod, MD.
In a trial that did not meet its primary endpoint, Dr. Kosiborod acknowledged that positive interpretations are speculative, but he does believe that there is one immediate take-home message.
“Our results do not support discontinuation of SGLT2 inhibitors in the setting of COVID-19 as long as patients are monitored,” said Dr. Kosiborod, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo.
At many institutions, it has been common to discontinue SGLT2 inhibitors in patients admitted with COVID-19. One reason was the concern that drugs in this class could exacerbate organ damage, particularly if they were to induced ketoacidosis. However, only 2 (0.003%) of 613 patients treated with dapagliflozin developed ketoacidosis, and the signal for organ protection overall, although not significant, was consistent.
“Numerically, fewer patients treated with dapagliflozin experienced organ failure and death, and this was consistent across systems, including the kidney,” Dr. Kosiborod said in presenting the study at the annual scientific sessions of the American College of Cardiology.
Overall, the study suggests that, in the context of COVID-19, dapagliflozin did not show harm and might have potential benefit, he added.
DARE-19 was rapidly conceived, designed, and implemented during the early stages of the COVID-19 pandemic. Based on prior evidence that SGLT2 inhibitors “favorably affect a number of pathophysiologic pathways disrupted during acute illness” and that drugs in this class have provided organ protection in the context of heart failure, chronic kidney disease, and other cardiometabolic conditions, the study was designed to test the hypothesis that this mechanism might improve outcomes in patients hospitalized with COVID-19, Dr. Kosiborod said.
The entry criteria included confirmed or suspected COVID-19 with an onset of 4 days of fewer and one additional risk factor, such as atherosclerotic cardiovascular disease, hypertension, or type 2 diabetes. Patients with significant renal impairment or a history of diabetic ketoacidosis were excluded.
On top of standard treatments for COVID-19, patients were randomized to 10 mg dapagliflozin or placebo once daily. There were two primary endpoints. That of prevention was time to criteria for respiratory, cardiovascular, or renal organ failure or death. The second primary outcome, for recovery, was a hierarchical composite for four endpoints: death, organ failure, status at 30 days if hospitalized, and time to discharge if this occurred before day 30.
Of the 1,250 patients randomized at 95 sites in seven countries, 617 in the dapagliflozin group and 620 patients in the placebo group completed the study. Baseline characteristics, which included a mean of age of 62 years; types of comorbidities; and types of treatments were similar.
Results for two primary endpoints
The curves for the primary outcome of prevention had already separated by day 3 and continued to widen over the 30 days in which outcomes were compared. At the end of 30 days, 11.2% of the dapagliflozin group and 13.8% of the placebo group had an event. By hazard ratio, dapagliflozin was linked to 20% nonsignificant relative protection from events (hazard ratio, 0.80; 95% confidence interval, 0.58-1.10).
The trend (P = .168) for the primary endpoint for prevention was reflected in the individual components. For dapagliflozin related to placebo, there were generally similar or greater reductions in new or worsening organ failure (HR, 0.80), cardiac decompensation (HR, 0.81), respiratory decompensation (HR, 0.85), and kidney decompensation (HR, 0.65). None were statistically significant, but the confidence intervals were tight with the upper end never exceeding 1.20.
Moreover, the relative risk reduction for all-cause mortality moved in the same direction (HR, 0.77; 95% CI, 0.52-1.16).
In the hierarchical composite endpoint of recovery, there was no significant difference in the time to discharge, but again many recovery metrics numerically favored dapagliflozin with an overall difference producing a statistical trend (P = .14) similar to organ failure events and death.
In safety analyses, dapagliflozin consistently outperformed placebo across a broad array of safety measure, including any severe adverse event (65% vs. 82%), any adverse event with an outcome of death (32% vs. 48%), discontinuation caused by an adverse event (44% vs. 55%), and acute kidney injury (21% vs. 34%).
Data could fuel related studies
According to Ana Barac, MD, PhD, director of the cardio-oncology program in the Medstar Heart and Vascular Institute, Washington, these data are “thought provoking.” Although this was a negative trial, she said that it generates an “exciting hypothesis” about the potential of SGLT2 inhibitors to provide organ protection. She called for studies to pursue this path of research.
More immediately, Dr. Barac agreed that these data argue against stopping SGLT2 inhibitors in patients admitted to a hospital for COVID-19 infection.
“These data show that these drugs are not going to lead to harm, but they might lead to benefit,” she said.
For James Januzzi, MD, a cardiologist at Massachusetts General Hospital and professor of medicine at Harvard Medical School, both in Boston, DARE-19 was perhaps most impressive because of its rigorous design and execution in the midst of a pandemic.
Over the past year, “the medical literature was flooded with grossly underpowered, poorly designed, single-center studies” yielding results that have been hard to interpret, Dr. Januzzi said. Despite the fact that this study failed to confirm its hypothesis, he said the investigators deserve praise for the quality of the work.
Dr. Januzzi also believes the study is not without clinically relevant findings, particularly the fact that dapagliflozin was associated with a lower rate of adverse events than placebo. This, at least, provides reassurance about the safety of this drug in the setting of COVID-19 infection.
Dr. Kosiborod reported financial relationships with more than 10 pharmaceutical companies, including AstraZeneca, which provided funding for DARE-19. Dr. Barac reported financial relationships with Bristol-Myers Squibb and CTI BioPharma. Dr. Januzzi reported financial relationships with Boehringer Ingelheim, GE Healthcare, Johnson & Johnson, Merck, Novartis, Pfizer, and Roche.
In patients hospitalized with COVID-19 infection, the sodium-glucose transporter 2 inhibitor dapagliflozin showed a trend for benefit relative to placebo on multiple outcomes, including the primary outcome of time to organ failure or death, according to results from the randomized DARE-19 trial.
Because of the failure to reach statistical significance, these results have no immediate relevance, but the trends support interest in further testing SGLT2 inhibitors in acute diseases posing a high risk for organ failure, according to Mikhail Kosiborod, MD.
In a trial that did not meet its primary endpoint, Dr. Kosiborod acknowledged that positive interpretations are speculative, but he does believe that there is one immediate take-home message.
“Our results do not support discontinuation of SGLT2 inhibitors in the setting of COVID-19 as long as patients are monitored,” said Dr. Kosiborod, director of cardiometabolic research at Saint Luke’s Mid-America Heart Institute, Kansas City, Mo.
At many institutions, it has been common to discontinue SGLT2 inhibitors in patients admitted with COVID-19. One reason was the concern that drugs in this class could exacerbate organ damage, particularly if they were to induced ketoacidosis. However, only 2 (0.003%) of 613 patients treated with dapagliflozin developed ketoacidosis, and the signal for organ protection overall, although not significant, was consistent.
“Numerically, fewer patients treated with dapagliflozin experienced organ failure and death, and this was consistent across systems, including the kidney,” Dr. Kosiborod said in presenting the study at the annual scientific sessions of the American College of Cardiology.
Overall, the study suggests that, in the context of COVID-19, dapagliflozin did not show harm and might have potential benefit, he added.
DARE-19 was rapidly conceived, designed, and implemented during the early stages of the COVID-19 pandemic. Based on prior evidence that SGLT2 inhibitors “favorably affect a number of pathophysiologic pathways disrupted during acute illness” and that drugs in this class have provided organ protection in the context of heart failure, chronic kidney disease, and other cardiometabolic conditions, the study was designed to test the hypothesis that this mechanism might improve outcomes in patients hospitalized with COVID-19, Dr. Kosiborod said.
The entry criteria included confirmed or suspected COVID-19 with an onset of 4 days of fewer and one additional risk factor, such as atherosclerotic cardiovascular disease, hypertension, or type 2 diabetes. Patients with significant renal impairment or a history of diabetic ketoacidosis were excluded.
On top of standard treatments for COVID-19, patients were randomized to 10 mg dapagliflozin or placebo once daily. There were two primary endpoints. That of prevention was time to criteria for respiratory, cardiovascular, or renal organ failure or death. The second primary outcome, for recovery, was a hierarchical composite for four endpoints: death, organ failure, status at 30 days if hospitalized, and time to discharge if this occurred before day 30.
Of the 1,250 patients randomized at 95 sites in seven countries, 617 in the dapagliflozin group and 620 patients in the placebo group completed the study. Baseline characteristics, which included a mean of age of 62 years; types of comorbidities; and types of treatments were similar.
Results for two primary endpoints
The curves for the primary outcome of prevention had already separated by day 3 and continued to widen over the 30 days in which outcomes were compared. At the end of 30 days, 11.2% of the dapagliflozin group and 13.8% of the placebo group had an event. By hazard ratio, dapagliflozin was linked to 20% nonsignificant relative protection from events (hazard ratio, 0.80; 95% confidence interval, 0.58-1.10).
The trend (P = .168) for the primary endpoint for prevention was reflected in the individual components. For dapagliflozin related to placebo, there were generally similar or greater reductions in new or worsening organ failure (HR, 0.80), cardiac decompensation (HR, 0.81), respiratory decompensation (HR, 0.85), and kidney decompensation (HR, 0.65). None were statistically significant, but the confidence intervals were tight with the upper end never exceeding 1.20.
Moreover, the relative risk reduction for all-cause mortality moved in the same direction (HR, 0.77; 95% CI, 0.52-1.16).
In the hierarchical composite endpoint of recovery, there was no significant difference in the time to discharge, but again many recovery metrics numerically favored dapagliflozin with an overall difference producing a statistical trend (P = .14) similar to organ failure events and death.
In safety analyses, dapagliflozin consistently outperformed placebo across a broad array of safety measure, including any severe adverse event (65% vs. 82%), any adverse event with an outcome of death (32% vs. 48%), discontinuation caused by an adverse event (44% vs. 55%), and acute kidney injury (21% vs. 34%).
Data could fuel related studies
According to Ana Barac, MD, PhD, director of the cardio-oncology program in the Medstar Heart and Vascular Institute, Washington, these data are “thought provoking.” Although this was a negative trial, she said that it generates an “exciting hypothesis” about the potential of SGLT2 inhibitors to provide organ protection. She called for studies to pursue this path of research.
More immediately, Dr. Barac agreed that these data argue against stopping SGLT2 inhibitors in patients admitted to a hospital for COVID-19 infection.
“These data show that these drugs are not going to lead to harm, but they might lead to benefit,” she said.
For James Januzzi, MD, a cardiologist at Massachusetts General Hospital and professor of medicine at Harvard Medical School, both in Boston, DARE-19 was perhaps most impressive because of its rigorous design and execution in the midst of a pandemic.
Over the past year, “the medical literature was flooded with grossly underpowered, poorly designed, single-center studies” yielding results that have been hard to interpret, Dr. Januzzi said. Despite the fact that this study failed to confirm its hypothesis, he said the investigators deserve praise for the quality of the work.
Dr. Januzzi also believes the study is not without clinically relevant findings, particularly the fact that dapagliflozin was associated with a lower rate of adverse events than placebo. This, at least, provides reassurance about the safety of this drug in the setting of COVID-19 infection.
Dr. Kosiborod reported financial relationships with more than 10 pharmaceutical companies, including AstraZeneca, which provided funding for DARE-19. Dr. Barac reported financial relationships with Bristol-Myers Squibb and CTI BioPharma. Dr. Januzzi reported financial relationships with Boehringer Ingelheim, GE Healthcare, Johnson & Johnson, Merck, Novartis, Pfizer, and Roche.
FROM ACC 2021
Planning for SHM Converge 2022 now underway
A hospitalist for 18 years and Annual Conference Committee (ACC) member for the last 4 years, I have always felt immense pride in this meeting. This year, we experienced constant evolution and adapted in ways unimaginable; frameshifts, detours, course corrections, wearing out words like “pivot” and “unprecedented,” whilst contending with virus lulls and surges at hospitals across the country. And SHM Converge 2021 was a landmark success despite it all.
Our SHM community successfully connected through the marvels of modern technology and enjoyed a snappy new logo and name to mark the occasion. Our unflappable course director Dan Steinberg, MD, SFHM, led an intrepid and creative team through uncertainty and produced an extraordinary educational event truly worthy of the term “unprecedented.” ACC members, talented in so many ways, each brought a unique perspective to the planning table to craft a balanced, relevant, and cutting-edge program. The only thing harder than planning a conference for thousands of hospitalists is planning TWO CONFERENCES – one in person, then one virtually.
For their facilitation of virtual adaptation of everything from clinical talks to hot dog sales, our SHM administrative staff deserve a medal. Industry sponsors likewise performed pretzel maneuvers for the virtual interface, and we thank them for their creativity and support. Freshly minted SHM CEO Eric Howell, MD, MHM, kicked off Converge by adeptly filling some very large shoes with aplomb, humor, and humility – telegraphing that our society is in good hands indeed (and that 2020 was NOT the ‘final frontier’). And, finally, each of you, in the suspended reality of a conference hall, tapped into session after session from the comfort of your hometown chairs, indefatigably learning and networking during a pandemic year.
So, beyond adaptability, what did we learn? We renewed our commitment to resilience and wellness in medicine, and reemphasized how critical diversity, equity, and inclusion are in both the workplace and in clinical practice. These topics were complemented by the usual standing-room-only clinical updates and rapid-fire sessions – where everyone could enjoy a front row seat. We talked about parenting in the pandemic, compared clinical approaches in friendly debates – for patients big and small – and deeply dived into leadership strategies for a sustainable workforce.
Here are some SHM Converge 2021 nuggets (Apologies for so few ... there were thousands!):
Plenaries
Eric Howell, MD, MHM
- Make the world a better place, be transparent and act with integrity, invest in others, do what you love.
- SHM has been leading the pack in providing e-learning options, promoting clinician self-care, and intensifying diversity, equity, and inclusion efforts before and throughout the pandemic.
- SHM has 18,000 members, 68 chapters, 26 special interest groups, 15 committees, 12 board of directors, 50 staff – growing and getting stronger every day.
- Rainbows need both rain and sunshine to form.
Gen. Mark Hertling
- Our COVID experience as hospitalists shared many features with active combat, including post-COVID combat fog.
- Use your ears, eyes, and mouth in that order: Listen more, see more, speak less.
Vineet Arora, MD, MHM
- Don’t pass up your “career gates.”
- Find “zero-gravity thinkers” – not innovation killers.
- Keep track of your state of mind using the “Bob Wachter scale.”
U.S. Surgeon Gen. Vivek Murthy, MD, and Danielle Scheurer, MD, SFHM
- Mental health and well-being of clinicians is imperative; “heal thyself” doesn’t work. Culture must support policies to truly craft a more sustaining and rewarding environment.
- We are a nation hyperfocused on episodic and salvage care (and are good at it) but must move the needle toward continuity and prevention. Sadly, nobody celebrates the heart attack that was prevented.
- What can hospitalists do about social determinants of health? Advocate for policies individually or through SHM – if you don’t know how, receive training – this is invaluable. More lobbying as a profession may yield legislation and funding aimed at such determinants and improve healthcare.
Larry Wellikson, MD, MHM
- New models hospitalists may soon inhabit: Hospital at Home, ED+, Micro-Hospitals.
- More than 50% of revenue comes from “vertical” services (outside the hospital) rather than horizontal services (in hospital) – trend to increase efforts in population health initiatives.
- Emphasis on value must go from looking at episodes of care to outcomes.
- Hospitalists Complexologists? Be relevant, add value – survive, thrive, and prosper.
Other sessions
Stroke
- Mobile stroke units are a thing!
- Neurologists are not great at predictions after stroke – but scoring tools are!
- Focus on patient-centered outcomes (100% disability free vs. able to walk vs. happy to be alive).
Drug allergies
- Penicillin allergy: 2% cross-reactivity for cephalosporins – not 10%.
Navigating work/life balance
- Have two phones for work/home – church and state – keep them separate!
Becoming an expert
- Avoid “analysis paralysis”: “Better a good decision quickly than the best decision too late” – H. Geneen
Misc. revelations
- It’s pretty cool to know the Surgeon General is a hospitalist!
- Our SHM community rocks!
- Eric Howell is an avid Star Trek and overalls enthusiast!
- It’s exceedingly difficult to become a MHM – 35 total, 3 this year.
- Danielle Scheurer is a warm and natural interviewer, sensational leader, and closet REM-rapper.
- No matter how hard I try, I’ll always be a social media Luddite: “Am I hashtagging?”
Convenience notwithstanding, this year’s conference-from-home luxury is one we hope to dispense with for SHM Converge 2022, in exchange for wandering of halls, jockeying to be closer to the front of the room, collecting freebies in exhibit halls, and seeing 50 old friends on the way to the session for which you’re already late.
Nashville, Tenn., aka Music City, will be the site of our first in-person meeting in 3 years in April 2022. I will be there with my guitar for SHM’s open mic and I hope you too bring your diverse talents from across the country to spend a week learning and energizing with us, making hospital medicine music in “Honky Tonk Hall,” “Elvis Lives Lounge,” or the “Grand Ol’ Opry-ation Suite.” The band is getting back together! Be a part of the excitement. Bring your voice, bring your talent, and let’s do Nashville in numbers!
Planning is now underway ... and we need your ideas and suggestions! Share thoughts on topics and speakers through the OPEN CALL site through June 1st ... and don’t forget to watch on-demand talks you missed from SHM Converge 2021 – a veritable treasure trove of learning.
Dr. Nye is a hospitalist and professor of medicine at the University of California, San Francisco. She is the course director of SHM Converge 2022.
A hospitalist for 18 years and Annual Conference Committee (ACC) member for the last 4 years, I have always felt immense pride in this meeting. This year, we experienced constant evolution and adapted in ways unimaginable; frameshifts, detours, course corrections, wearing out words like “pivot” and “unprecedented,” whilst contending with virus lulls and surges at hospitals across the country. And SHM Converge 2021 was a landmark success despite it all.
Our SHM community successfully connected through the marvels of modern technology and enjoyed a snappy new logo and name to mark the occasion. Our unflappable course director Dan Steinberg, MD, SFHM, led an intrepid and creative team through uncertainty and produced an extraordinary educational event truly worthy of the term “unprecedented.” ACC members, talented in so many ways, each brought a unique perspective to the planning table to craft a balanced, relevant, and cutting-edge program. The only thing harder than planning a conference for thousands of hospitalists is planning TWO CONFERENCES – one in person, then one virtually.
For their facilitation of virtual adaptation of everything from clinical talks to hot dog sales, our SHM administrative staff deserve a medal. Industry sponsors likewise performed pretzel maneuvers for the virtual interface, and we thank them for their creativity and support. Freshly minted SHM CEO Eric Howell, MD, MHM, kicked off Converge by adeptly filling some very large shoes with aplomb, humor, and humility – telegraphing that our society is in good hands indeed (and that 2020 was NOT the ‘final frontier’). And, finally, each of you, in the suspended reality of a conference hall, tapped into session after session from the comfort of your hometown chairs, indefatigably learning and networking during a pandemic year.
So, beyond adaptability, what did we learn? We renewed our commitment to resilience and wellness in medicine, and reemphasized how critical diversity, equity, and inclusion are in both the workplace and in clinical practice. These topics were complemented by the usual standing-room-only clinical updates and rapid-fire sessions – where everyone could enjoy a front row seat. We talked about parenting in the pandemic, compared clinical approaches in friendly debates – for patients big and small – and deeply dived into leadership strategies for a sustainable workforce.
Here are some SHM Converge 2021 nuggets (Apologies for so few ... there were thousands!):
Plenaries
Eric Howell, MD, MHM
- Make the world a better place, be transparent and act with integrity, invest in others, do what you love.
- SHM has been leading the pack in providing e-learning options, promoting clinician self-care, and intensifying diversity, equity, and inclusion efforts before and throughout the pandemic.
- SHM has 18,000 members, 68 chapters, 26 special interest groups, 15 committees, 12 board of directors, 50 staff – growing and getting stronger every day.
- Rainbows need both rain and sunshine to form.
Gen. Mark Hertling
- Our COVID experience as hospitalists shared many features with active combat, including post-COVID combat fog.
- Use your ears, eyes, and mouth in that order: Listen more, see more, speak less.
Vineet Arora, MD, MHM
- Don’t pass up your “career gates.”
- Find “zero-gravity thinkers” – not innovation killers.
- Keep track of your state of mind using the “Bob Wachter scale.”
U.S. Surgeon Gen. Vivek Murthy, MD, and Danielle Scheurer, MD, SFHM
- Mental health and well-being of clinicians is imperative; “heal thyself” doesn’t work. Culture must support policies to truly craft a more sustaining and rewarding environment.
- We are a nation hyperfocused on episodic and salvage care (and are good at it) but must move the needle toward continuity and prevention. Sadly, nobody celebrates the heart attack that was prevented.
- What can hospitalists do about social determinants of health? Advocate for policies individually or through SHM – if you don’t know how, receive training – this is invaluable. More lobbying as a profession may yield legislation and funding aimed at such determinants and improve healthcare.
Larry Wellikson, MD, MHM
- New models hospitalists may soon inhabit: Hospital at Home, ED+, Micro-Hospitals.
- More than 50% of revenue comes from “vertical” services (outside the hospital) rather than horizontal services (in hospital) – trend to increase efforts in population health initiatives.
- Emphasis on value must go from looking at episodes of care to outcomes.
- Hospitalists Complexologists? Be relevant, add value – survive, thrive, and prosper.
Other sessions
Stroke
- Mobile stroke units are a thing!
- Neurologists are not great at predictions after stroke – but scoring tools are!
- Focus on patient-centered outcomes (100% disability free vs. able to walk vs. happy to be alive).
Drug allergies
- Penicillin allergy: 2% cross-reactivity for cephalosporins – not 10%.
Navigating work/life balance
- Have two phones for work/home – church and state – keep them separate!
Becoming an expert
- Avoid “analysis paralysis”: “Better a good decision quickly than the best decision too late” – H. Geneen
Misc. revelations
- It’s pretty cool to know the Surgeon General is a hospitalist!
- Our SHM community rocks!
- Eric Howell is an avid Star Trek and overalls enthusiast!
- It’s exceedingly difficult to become a MHM – 35 total, 3 this year.
- Danielle Scheurer is a warm and natural interviewer, sensational leader, and closet REM-rapper.
- No matter how hard I try, I’ll always be a social media Luddite: “Am I hashtagging?”
Convenience notwithstanding, this year’s conference-from-home luxury is one we hope to dispense with for SHM Converge 2022, in exchange for wandering of halls, jockeying to be closer to the front of the room, collecting freebies in exhibit halls, and seeing 50 old friends on the way to the session for which you’re already late.
Nashville, Tenn., aka Music City, will be the site of our first in-person meeting in 3 years in April 2022. I will be there with my guitar for SHM’s open mic and I hope you too bring your diverse talents from across the country to spend a week learning and energizing with us, making hospital medicine music in “Honky Tonk Hall,” “Elvis Lives Lounge,” or the “Grand Ol’ Opry-ation Suite.” The band is getting back together! Be a part of the excitement. Bring your voice, bring your talent, and let’s do Nashville in numbers!
Planning is now underway ... and we need your ideas and suggestions! Share thoughts on topics and speakers through the OPEN CALL site through June 1st ... and don’t forget to watch on-demand talks you missed from SHM Converge 2021 – a veritable treasure trove of learning.
Dr. Nye is a hospitalist and professor of medicine at the University of California, San Francisco. She is the course director of SHM Converge 2022.
A hospitalist for 18 years and Annual Conference Committee (ACC) member for the last 4 years, I have always felt immense pride in this meeting. This year, we experienced constant evolution and adapted in ways unimaginable; frameshifts, detours, course corrections, wearing out words like “pivot” and “unprecedented,” whilst contending with virus lulls and surges at hospitals across the country. And SHM Converge 2021 was a landmark success despite it all.
Our SHM community successfully connected through the marvels of modern technology and enjoyed a snappy new logo and name to mark the occasion. Our unflappable course director Dan Steinberg, MD, SFHM, led an intrepid and creative team through uncertainty and produced an extraordinary educational event truly worthy of the term “unprecedented.” ACC members, talented in so many ways, each brought a unique perspective to the planning table to craft a balanced, relevant, and cutting-edge program. The only thing harder than planning a conference for thousands of hospitalists is planning TWO CONFERENCES – one in person, then one virtually.
For their facilitation of virtual adaptation of everything from clinical talks to hot dog sales, our SHM administrative staff deserve a medal. Industry sponsors likewise performed pretzel maneuvers for the virtual interface, and we thank them for their creativity and support. Freshly minted SHM CEO Eric Howell, MD, MHM, kicked off Converge by adeptly filling some very large shoes with aplomb, humor, and humility – telegraphing that our society is in good hands indeed (and that 2020 was NOT the ‘final frontier’). And, finally, each of you, in the suspended reality of a conference hall, tapped into session after session from the comfort of your hometown chairs, indefatigably learning and networking during a pandemic year.
So, beyond adaptability, what did we learn? We renewed our commitment to resilience and wellness in medicine, and reemphasized how critical diversity, equity, and inclusion are in both the workplace and in clinical practice. These topics were complemented by the usual standing-room-only clinical updates and rapid-fire sessions – where everyone could enjoy a front row seat. We talked about parenting in the pandemic, compared clinical approaches in friendly debates – for patients big and small – and deeply dived into leadership strategies for a sustainable workforce.
Here are some SHM Converge 2021 nuggets (Apologies for so few ... there were thousands!):
Plenaries
Eric Howell, MD, MHM
- Make the world a better place, be transparent and act with integrity, invest in others, do what you love.
- SHM has been leading the pack in providing e-learning options, promoting clinician self-care, and intensifying diversity, equity, and inclusion efforts before and throughout the pandemic.
- SHM has 18,000 members, 68 chapters, 26 special interest groups, 15 committees, 12 board of directors, 50 staff – growing and getting stronger every day.
- Rainbows need both rain and sunshine to form.
Gen. Mark Hertling
- Our COVID experience as hospitalists shared many features with active combat, including post-COVID combat fog.
- Use your ears, eyes, and mouth in that order: Listen more, see more, speak less.
Vineet Arora, MD, MHM
- Don’t pass up your “career gates.”
- Find “zero-gravity thinkers” – not innovation killers.
- Keep track of your state of mind using the “Bob Wachter scale.”
U.S. Surgeon Gen. Vivek Murthy, MD, and Danielle Scheurer, MD, SFHM
- Mental health and well-being of clinicians is imperative; “heal thyself” doesn’t work. Culture must support policies to truly craft a more sustaining and rewarding environment.
- We are a nation hyperfocused on episodic and salvage care (and are good at it) but must move the needle toward continuity and prevention. Sadly, nobody celebrates the heart attack that was prevented.
- What can hospitalists do about social determinants of health? Advocate for policies individually or through SHM – if you don’t know how, receive training – this is invaluable. More lobbying as a profession may yield legislation and funding aimed at such determinants and improve healthcare.
Larry Wellikson, MD, MHM
- New models hospitalists may soon inhabit: Hospital at Home, ED+, Micro-Hospitals.
- More than 50% of revenue comes from “vertical” services (outside the hospital) rather than horizontal services (in hospital) – trend to increase efforts in population health initiatives.
- Emphasis on value must go from looking at episodes of care to outcomes.
- Hospitalists Complexologists? Be relevant, add value – survive, thrive, and prosper.
Other sessions
Stroke
- Mobile stroke units are a thing!
- Neurologists are not great at predictions after stroke – but scoring tools are!
- Focus on patient-centered outcomes (100% disability free vs. able to walk vs. happy to be alive).
Drug allergies
- Penicillin allergy: 2% cross-reactivity for cephalosporins – not 10%.
Navigating work/life balance
- Have two phones for work/home – church and state – keep them separate!
Becoming an expert
- Avoid “analysis paralysis”: “Better a good decision quickly than the best decision too late” – H. Geneen
Misc. revelations
- It’s pretty cool to know the Surgeon General is a hospitalist!
- Our SHM community rocks!
- Eric Howell is an avid Star Trek and overalls enthusiast!
- It’s exceedingly difficult to become a MHM – 35 total, 3 this year.
- Danielle Scheurer is a warm and natural interviewer, sensational leader, and closet REM-rapper.
- No matter how hard I try, I’ll always be a social media Luddite: “Am I hashtagging?”
Convenience notwithstanding, this year’s conference-from-home luxury is one we hope to dispense with for SHM Converge 2022, in exchange for wandering of halls, jockeying to be closer to the front of the room, collecting freebies in exhibit halls, and seeing 50 old friends on the way to the session for which you’re already late.
Nashville, Tenn., aka Music City, will be the site of our first in-person meeting in 3 years in April 2022. I will be there with my guitar for SHM’s open mic and I hope you too bring your diverse talents from across the country to spend a week learning and energizing with us, making hospital medicine music in “Honky Tonk Hall,” “Elvis Lives Lounge,” or the “Grand Ol’ Opry-ation Suite.” The band is getting back together! Be a part of the excitement. Bring your voice, bring your talent, and let’s do Nashville in numbers!
Planning is now underway ... and we need your ideas and suggestions! Share thoughts on topics and speakers through the OPEN CALL site through June 1st ... and don’t forget to watch on-demand talks you missed from SHM Converge 2021 – a veritable treasure trove of learning.
Dr. Nye is a hospitalist and professor of medicine at the University of California, San Francisco. She is the course director of SHM Converge 2022.
Omics analysis links blood type to COVID-19
A new analysis of gene expression and protein content in lung and blood tissue suggests that certain variants of the ABO gene, which plays a central role in determining blood type, may also influence susceptibility to COVID-19. Researchers at the University of British Columbia, Vancouver, analyzed data from three studies to link gene and protein expression in lungs and blood with genetic regions associated with COVID-19 susceptibility.
“These genes may also prove to be good markers for disease as well as potential drug targets,” said lead author Ana Hernandez Cordero, PhD, postdoctoral fellow with the Center for Heart Lung Innovation, University of British Columbia, in a statement. Dr. Cordero presented the study at the American Thoracic Society’s virtual international conference.
Dr. Cordero noted that genomewide association studies have been used to identify genetic regions associated with COVID-19 susceptibility, but they cannot be used to identify specific genes. To pinpoint genes, the researchers employed integrated genomics, which combines Bayesian colocalization summary-based Mendelian randomization and Mendelian randomization.
Searching for candidate genes
The researchers combined genetic data and transcriptomics data, which are a measurement of the messenger RNA produced in a cell. Messenger RNA is used as a blueprint for protein production. The genetics data came from the COVID-19 Host Genetics Initiative genomewide association meta-analysis version 4 (patients with COVID-19 vs. patients without COVID-19). Blood transcriptomics data came from the INTERVAL study (n = 3301), and lung transcriptomics data came from the Lung eQTL study (n = 1038). “From the integration of these three datasets we identified the candidate genes that are most likely to influence COVID-19 through gene expression. We further investigated the most consistent candidate genes and tested the causal association between their plasma protein levels and COVID-19 susceptibility using Bayesian colocalization and Mendelian randomization,” said Dr. Cordero during her talk.
Susceptibility drivers
The researchers identified six genes expressed in the lung and five expressed in blood that colocalized with COVID-19 susceptibility loci. They found that an increase in plasma levels of ABO was associated with greater risk for COVID-19 (Mendelian randomization, P = .000025) and that expression of the SLC6A20 gene in the lung was also associated with higher COVID-19 risk. They also found novel associations at genes associated with respiratory diseases, such as asthma, as well as genes associated with the host immune responses, such as neutrophil and eosinophil counts.
Possibly protective?
Within the ABO gene, the research also turned up evidence that blood type O may be protective against COVID-19. “The most significant variant used for the Mendelian randomization test was in complete linkage disagreement with the variant responsible for the blood type O genotype, conferring reduced risk,” said Dr. Cordero.
The study’s method is a powerful technique, said Jeremy Alexander Hirota, PhD, who was asked to comment. “The present study uses integrative omics to determine COVID-19 susceptibility factors which would have been challenging to identify with a single technology,” said Dr. Hirota, who is an assistant professor of medicine at McMaster University, Hamilton, Ont.; an adjunct professor of biology at the University of Waterloo (Ont.); and an affiliate professor of medicine at the University of British Columbia. He trained with the senior author of the study but was not directly involved in the research.
The host response is widely believed to be most responsible for the symptoms of COVID-19, so it isn’t surprising that host genes can be identified, according to Dr. Hirota. The identification of variants in the ABO protein is interesting, though. It suggests ‘that systemic effects beyond respiratory mucosal immunity are a driver for susceptibility.’ To my understanding, ABO protein is not expressed in the respiratory mucosa, which is a common site of first contact for SARS-CoV-2. The links between blood ABO levels and initial infection of the respiratory mucosa by SARS-CoV-2 are unclear,” he said.
Severity link needed
Dr. Hirota also said that although the study points toward associations with susceptibility to COVID-19, it isn’t clear from the available data whether such associations are related to severity of disease. “If the [patients with gene variants] are more susceptible but [the disease is] less severe, then the results need to be interpreted accordingly. If the susceptibility is increased and the severity is also increased, maybe measured by increased risk for ICU admission, ventilator use, or mortality, then the work carries a much more important message. Future studies extending this work and integrating measures of severity are warranted to better understand the clinical utility of these findings for managing COVID-19 patients optimally,” said Dr. Hirota.
It’s also unclear whether the study populations are reflective of the populations that are currently at highest risk for COVID-19, such as residents of India, where the burden of disease is currently severe.
Dr. Cordero and Dr. Hirota disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new analysis of gene expression and protein content in lung and blood tissue suggests that certain variants of the ABO gene, which plays a central role in determining blood type, may also influence susceptibility to COVID-19. Researchers at the University of British Columbia, Vancouver, analyzed data from three studies to link gene and protein expression in lungs and blood with genetic regions associated with COVID-19 susceptibility.
“These genes may also prove to be good markers for disease as well as potential drug targets,” said lead author Ana Hernandez Cordero, PhD, postdoctoral fellow with the Center for Heart Lung Innovation, University of British Columbia, in a statement. Dr. Cordero presented the study at the American Thoracic Society’s virtual international conference.
Dr. Cordero noted that genomewide association studies have been used to identify genetic regions associated with COVID-19 susceptibility, but they cannot be used to identify specific genes. To pinpoint genes, the researchers employed integrated genomics, which combines Bayesian colocalization summary-based Mendelian randomization and Mendelian randomization.
Searching for candidate genes
The researchers combined genetic data and transcriptomics data, which are a measurement of the messenger RNA produced in a cell. Messenger RNA is used as a blueprint for protein production. The genetics data came from the COVID-19 Host Genetics Initiative genomewide association meta-analysis version 4 (patients with COVID-19 vs. patients without COVID-19). Blood transcriptomics data came from the INTERVAL study (n = 3301), and lung transcriptomics data came from the Lung eQTL study (n = 1038). “From the integration of these three datasets we identified the candidate genes that are most likely to influence COVID-19 through gene expression. We further investigated the most consistent candidate genes and tested the causal association between their plasma protein levels and COVID-19 susceptibility using Bayesian colocalization and Mendelian randomization,” said Dr. Cordero during her talk.
Susceptibility drivers
The researchers identified six genes expressed in the lung and five expressed in blood that colocalized with COVID-19 susceptibility loci. They found that an increase in plasma levels of ABO was associated with greater risk for COVID-19 (Mendelian randomization, P = .000025) and that expression of the SLC6A20 gene in the lung was also associated with higher COVID-19 risk. They also found novel associations at genes associated with respiratory diseases, such as asthma, as well as genes associated with the host immune responses, such as neutrophil and eosinophil counts.
Possibly protective?
Within the ABO gene, the research also turned up evidence that blood type O may be protective against COVID-19. “The most significant variant used for the Mendelian randomization test was in complete linkage disagreement with the variant responsible for the blood type O genotype, conferring reduced risk,” said Dr. Cordero.
The study’s method is a powerful technique, said Jeremy Alexander Hirota, PhD, who was asked to comment. “The present study uses integrative omics to determine COVID-19 susceptibility factors which would have been challenging to identify with a single technology,” said Dr. Hirota, who is an assistant professor of medicine at McMaster University, Hamilton, Ont.; an adjunct professor of biology at the University of Waterloo (Ont.); and an affiliate professor of medicine at the University of British Columbia. He trained with the senior author of the study but was not directly involved in the research.
The host response is widely believed to be most responsible for the symptoms of COVID-19, so it isn’t surprising that host genes can be identified, according to Dr. Hirota. The identification of variants in the ABO protein is interesting, though. It suggests ‘that systemic effects beyond respiratory mucosal immunity are a driver for susceptibility.’ To my understanding, ABO protein is not expressed in the respiratory mucosa, which is a common site of first contact for SARS-CoV-2. The links between blood ABO levels and initial infection of the respiratory mucosa by SARS-CoV-2 are unclear,” he said.
Severity link needed
Dr. Hirota also said that although the study points toward associations with susceptibility to COVID-19, it isn’t clear from the available data whether such associations are related to severity of disease. “If the [patients with gene variants] are more susceptible but [the disease is] less severe, then the results need to be interpreted accordingly. If the susceptibility is increased and the severity is also increased, maybe measured by increased risk for ICU admission, ventilator use, or mortality, then the work carries a much more important message. Future studies extending this work and integrating measures of severity are warranted to better understand the clinical utility of these findings for managing COVID-19 patients optimally,” said Dr. Hirota.
It’s also unclear whether the study populations are reflective of the populations that are currently at highest risk for COVID-19, such as residents of India, where the burden of disease is currently severe.
Dr. Cordero and Dr. Hirota disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new analysis of gene expression and protein content in lung and blood tissue suggests that certain variants of the ABO gene, which plays a central role in determining blood type, may also influence susceptibility to COVID-19. Researchers at the University of British Columbia, Vancouver, analyzed data from three studies to link gene and protein expression in lungs and blood with genetic regions associated with COVID-19 susceptibility.
“These genes may also prove to be good markers for disease as well as potential drug targets,” said lead author Ana Hernandez Cordero, PhD, postdoctoral fellow with the Center for Heart Lung Innovation, University of British Columbia, in a statement. Dr. Cordero presented the study at the American Thoracic Society’s virtual international conference.
Dr. Cordero noted that genomewide association studies have been used to identify genetic regions associated with COVID-19 susceptibility, but they cannot be used to identify specific genes. To pinpoint genes, the researchers employed integrated genomics, which combines Bayesian colocalization summary-based Mendelian randomization and Mendelian randomization.
Searching for candidate genes
The researchers combined genetic data and transcriptomics data, which are a measurement of the messenger RNA produced in a cell. Messenger RNA is used as a blueprint for protein production. The genetics data came from the COVID-19 Host Genetics Initiative genomewide association meta-analysis version 4 (patients with COVID-19 vs. patients without COVID-19). Blood transcriptomics data came from the INTERVAL study (n = 3301), and lung transcriptomics data came from the Lung eQTL study (n = 1038). “From the integration of these three datasets we identified the candidate genes that are most likely to influence COVID-19 through gene expression. We further investigated the most consistent candidate genes and tested the causal association between their plasma protein levels and COVID-19 susceptibility using Bayesian colocalization and Mendelian randomization,” said Dr. Cordero during her talk.
Susceptibility drivers
The researchers identified six genes expressed in the lung and five expressed in blood that colocalized with COVID-19 susceptibility loci. They found that an increase in plasma levels of ABO was associated with greater risk for COVID-19 (Mendelian randomization, P = .000025) and that expression of the SLC6A20 gene in the lung was also associated with higher COVID-19 risk. They also found novel associations at genes associated with respiratory diseases, such as asthma, as well as genes associated with the host immune responses, such as neutrophil and eosinophil counts.
Possibly protective?
Within the ABO gene, the research also turned up evidence that blood type O may be protective against COVID-19. “The most significant variant used for the Mendelian randomization test was in complete linkage disagreement with the variant responsible for the blood type O genotype, conferring reduced risk,” said Dr. Cordero.
The study’s method is a powerful technique, said Jeremy Alexander Hirota, PhD, who was asked to comment. “The present study uses integrative omics to determine COVID-19 susceptibility factors which would have been challenging to identify with a single technology,” said Dr. Hirota, who is an assistant professor of medicine at McMaster University, Hamilton, Ont.; an adjunct professor of biology at the University of Waterloo (Ont.); and an affiliate professor of medicine at the University of British Columbia. He trained with the senior author of the study but was not directly involved in the research.
The host response is widely believed to be most responsible for the symptoms of COVID-19, so it isn’t surprising that host genes can be identified, according to Dr. Hirota. The identification of variants in the ABO protein is interesting, though. It suggests ‘that systemic effects beyond respiratory mucosal immunity are a driver for susceptibility.’ To my understanding, ABO protein is not expressed in the respiratory mucosa, which is a common site of first contact for SARS-CoV-2. The links between blood ABO levels and initial infection of the respiratory mucosa by SARS-CoV-2 are unclear,” he said.
Severity link needed
Dr. Hirota also said that although the study points toward associations with susceptibility to COVID-19, it isn’t clear from the available data whether such associations are related to severity of disease. “If the [patients with gene variants] are more susceptible but [the disease is] less severe, then the results need to be interpreted accordingly. If the susceptibility is increased and the severity is also increased, maybe measured by increased risk for ICU admission, ventilator use, or mortality, then the work carries a much more important message. Future studies extending this work and integrating measures of severity are warranted to better understand the clinical utility of these findings for managing COVID-19 patients optimally,” said Dr. Hirota.
It’s also unclear whether the study populations are reflective of the populations that are currently at highest risk for COVID-19, such as residents of India, where the burden of disease is currently severe.
Dr. Cordero and Dr. Hirota disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
PARADISE-MI: Sacubitril/valsartan can’t beat ramipril in patients with acute MI
Treatment with sacubitril/valsartan, a pillar of therapy for patients with chronic heart failure with below-normal ejection fraction, came suggestively close to showing efficacy for preventing cardiovascular death or heart failure events in patients who have just had an MI but have no history of heart failure in a controlled trial with more than 5,600 patients.
Although sacubitril/valsartan (Entresto) fell short of producing a significant benefit, it did show good safety that was similar to the study’s comparator treatment, ramipril, an agent from the angiotensin-converting enzyme inhibitor class that is a mainstay of treatment in these patients.
“To say that, with no run-in, sacubitril/valsartan is as well tolerated and as safe as one of the best-studied ACE inhibitors – ramipril – in acutely ill MI patients, is a big statement,” said Marc A. Pfeffer, MD, at the annual scientific sessions of the American College of Cardiology. This high level of safety without gradual uptitration of sacubitril/valsartan (Entresto) “should lower barriers” to broader use of the dual-drug formulation for its approved indication in patients with chronic heart failure, especially patients with a left ventricular ejection fraction that is below normal. In addition, results from the PARADISE-MI trial suggested that “patients seemed to benefit before they develop heart failure. We couldn’t prove that, but we should build on this, and make it easier for patients to use this treatment,” Dr. Pfeffer said during a press briefing following his talk at the sessions.
Preventing heart failures to come
Treatment with sacubitril/valsartan in acute MI patients within a few days of their event “is perhaps addressing prevention of the heart failure that’s to come,” commented Lynne W. Stevenson, MD, designated discussant for the report and professor of medicine at Vanderbilt University Medical Center in Nashville. “Patients who are destined to develop heart failure are beginning their treatment early. The subgroup analyses suggest that it’s the sicker patients who benefited the most,” she said.
But Dr. Pfeffer stressed that “I don’t think this is a subgroup discussion. I would like to pursue this, but that’s up to the sponsor,” Novartis, the company that markets sacubitril/valsartan.
‘Exceedingly reassuring’ safety
The safety data that Dr. Pfeffer reported “are exceedingly reassuring. We didn’t see a signal of harm, and in some of the exploratory endpoints there was some evidence of benefit, so we need to encourage you to continue,” commented Mary N. Walsh, MD, medical director of the heart failure and cardiac transplantation program at Ascension St. Vincent Heart Center of Indiana in Indianapolis.
The PARADISE-MI (Prospective ARNI vs. ACE Inhibitor Trial to Determine Superiority in Reducing Heart Failure Events After MI) trial enrolled 5,669 patients with no history of heart failure within an average of 4 days following an acute MI at 495 sites in 41 countries during 2016-2020, with 8% of enrolled patients from the United States. Patients averaged 64 years of age, about three-quarters were men, about 43% had a history of diabetes, and only 1% were Black; Dr. Pfeffer noted that this is because most patients came from countries with low Black populations. The enrollment criteria required a left ventricular ejection fraction no greater than 40%, and among the enrolled patients this averaged about 37%.
A 10% nonsignificant relative risk reduction for the primary endpoint
The study’s primary endpoint was the combined first-event rate of cardiovascular death, hospitalization for heart failure, or an outpatient visit for heart failure. During a median follow-up of 23 months, this occurred at a rate of 7.4/100 patient years in the ramipril arm and 6.7/100 patient years in the sacubitril/valsartan arm, a 10% relative risk reduction with sacubitril/valsartan that was not significant, which meant all other efficacy analyses were exploratory, Dr. Pfeffer stressed.
Several secondary efficacy analyses showed significant benefits from sacubitril/valsartan, compared with ramipril, including the total number of events that comprised the primary endpoint, with a 21% relative risk reduction associated with sacubitril/valsartan, as well as investigator-reported events. The primary-endpoint benefit from sacubitril/valsartan was also significant in two subgroup analyses: patients aged 65 years or older (roughly half the study cohort), who had a 24% relative risk reduction on sacubitril/valsartan, compared with ramipril, and the 88% of patients who received treatment with percutaneous coronary intervention for their acute MI, who had a 19% relative risk reduction on sacubitril/valsartan, compared with patients who received ramipril.
The study’s safety data showed nearly identical rates in the two treatment arms for total adverse events, serious adverse events, adverse events that led to stopping the study drug, as well as in laboratory measures. The biggest between-treatment differences were a modest excess of hypotension on sacubitril valsartan, 28%, compared with 22% on ramipril, and a modest excess rate of cough on ramipril, 13%, compared with 9% on sacubitril/valsartan.
The added insight the results provide about sacubitril/valsartan comes at a time when U.S. patients continue to struggle to get health insurance coverage for an agent that has been approved for U.S. use in treating heart failure since 2015.
“Our patients do not have access to this important treatment,” declared Dr. Walsh during the press briefing. “The prior authorization process is unbelievable, and some patients have no access unless they pay the full cost on their own. This is an important, real-world problem that we face with this drug.”
PARADISE-MI was sponsored by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. Pfeffer has received research funding from and is a consultant to Novartis. He is also a consultant to AstraZeneca, Boehringer Ingelheim, Corvidia, DalCor, Eli Lilly, GlaxoSmithKline, Novo Nordisk, Peerbridge, and Sanofi, and he holds equity in DalCor and Peerbridge. Dr. Stevenson has received honoraria from LivaNova and has received research support from Abbott. Dr. Walsh had no disclosures.
Treatment with sacubitril/valsartan, a pillar of therapy for patients with chronic heart failure with below-normal ejection fraction, came suggestively close to showing efficacy for preventing cardiovascular death or heart failure events in patients who have just had an MI but have no history of heart failure in a controlled trial with more than 5,600 patients.
Although sacubitril/valsartan (Entresto) fell short of producing a significant benefit, it did show good safety that was similar to the study’s comparator treatment, ramipril, an agent from the angiotensin-converting enzyme inhibitor class that is a mainstay of treatment in these patients.
“To say that, with no run-in, sacubitril/valsartan is as well tolerated and as safe as one of the best-studied ACE inhibitors – ramipril – in acutely ill MI patients, is a big statement,” said Marc A. Pfeffer, MD, at the annual scientific sessions of the American College of Cardiology. This high level of safety without gradual uptitration of sacubitril/valsartan (Entresto) “should lower barriers” to broader use of the dual-drug formulation for its approved indication in patients with chronic heart failure, especially patients with a left ventricular ejection fraction that is below normal. In addition, results from the PARADISE-MI trial suggested that “patients seemed to benefit before they develop heart failure. We couldn’t prove that, but we should build on this, and make it easier for patients to use this treatment,” Dr. Pfeffer said during a press briefing following his talk at the sessions.
Preventing heart failures to come
Treatment with sacubitril/valsartan in acute MI patients within a few days of their event “is perhaps addressing prevention of the heart failure that’s to come,” commented Lynne W. Stevenson, MD, designated discussant for the report and professor of medicine at Vanderbilt University Medical Center in Nashville. “Patients who are destined to develop heart failure are beginning their treatment early. The subgroup analyses suggest that it’s the sicker patients who benefited the most,” she said.
But Dr. Pfeffer stressed that “I don’t think this is a subgroup discussion. I would like to pursue this, but that’s up to the sponsor,” Novartis, the company that markets sacubitril/valsartan.
‘Exceedingly reassuring’ safety
The safety data that Dr. Pfeffer reported “are exceedingly reassuring. We didn’t see a signal of harm, and in some of the exploratory endpoints there was some evidence of benefit, so we need to encourage you to continue,” commented Mary N. Walsh, MD, medical director of the heart failure and cardiac transplantation program at Ascension St. Vincent Heart Center of Indiana in Indianapolis.
The PARADISE-MI (Prospective ARNI vs. ACE Inhibitor Trial to Determine Superiority in Reducing Heart Failure Events After MI) trial enrolled 5,669 patients with no history of heart failure within an average of 4 days following an acute MI at 495 sites in 41 countries during 2016-2020, with 8% of enrolled patients from the United States. Patients averaged 64 years of age, about three-quarters were men, about 43% had a history of diabetes, and only 1% were Black; Dr. Pfeffer noted that this is because most patients came from countries with low Black populations. The enrollment criteria required a left ventricular ejection fraction no greater than 40%, and among the enrolled patients this averaged about 37%.
A 10% nonsignificant relative risk reduction for the primary endpoint
The study’s primary endpoint was the combined first-event rate of cardiovascular death, hospitalization for heart failure, or an outpatient visit for heart failure. During a median follow-up of 23 months, this occurred at a rate of 7.4/100 patient years in the ramipril arm and 6.7/100 patient years in the sacubitril/valsartan arm, a 10% relative risk reduction with sacubitril/valsartan that was not significant, which meant all other efficacy analyses were exploratory, Dr. Pfeffer stressed.
Several secondary efficacy analyses showed significant benefits from sacubitril/valsartan, compared with ramipril, including the total number of events that comprised the primary endpoint, with a 21% relative risk reduction associated with sacubitril/valsartan, as well as investigator-reported events. The primary-endpoint benefit from sacubitril/valsartan was also significant in two subgroup analyses: patients aged 65 years or older (roughly half the study cohort), who had a 24% relative risk reduction on sacubitril/valsartan, compared with ramipril, and the 88% of patients who received treatment with percutaneous coronary intervention for their acute MI, who had a 19% relative risk reduction on sacubitril/valsartan, compared with patients who received ramipril.
The study’s safety data showed nearly identical rates in the two treatment arms for total adverse events, serious adverse events, adverse events that led to stopping the study drug, as well as in laboratory measures. The biggest between-treatment differences were a modest excess of hypotension on sacubitril valsartan, 28%, compared with 22% on ramipril, and a modest excess rate of cough on ramipril, 13%, compared with 9% on sacubitril/valsartan.
The added insight the results provide about sacubitril/valsartan comes at a time when U.S. patients continue to struggle to get health insurance coverage for an agent that has been approved for U.S. use in treating heart failure since 2015.
“Our patients do not have access to this important treatment,” declared Dr. Walsh during the press briefing. “The prior authorization process is unbelievable, and some patients have no access unless they pay the full cost on their own. This is an important, real-world problem that we face with this drug.”
PARADISE-MI was sponsored by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. Pfeffer has received research funding from and is a consultant to Novartis. He is also a consultant to AstraZeneca, Boehringer Ingelheim, Corvidia, DalCor, Eli Lilly, GlaxoSmithKline, Novo Nordisk, Peerbridge, and Sanofi, and he holds equity in DalCor and Peerbridge. Dr. Stevenson has received honoraria from LivaNova and has received research support from Abbott. Dr. Walsh had no disclosures.
Treatment with sacubitril/valsartan, a pillar of therapy for patients with chronic heart failure with below-normal ejection fraction, came suggestively close to showing efficacy for preventing cardiovascular death or heart failure events in patients who have just had an MI but have no history of heart failure in a controlled trial with more than 5,600 patients.
Although sacubitril/valsartan (Entresto) fell short of producing a significant benefit, it did show good safety that was similar to the study’s comparator treatment, ramipril, an agent from the angiotensin-converting enzyme inhibitor class that is a mainstay of treatment in these patients.
“To say that, with no run-in, sacubitril/valsartan is as well tolerated and as safe as one of the best-studied ACE inhibitors – ramipril – in acutely ill MI patients, is a big statement,” said Marc A. Pfeffer, MD, at the annual scientific sessions of the American College of Cardiology. This high level of safety without gradual uptitration of sacubitril/valsartan (Entresto) “should lower barriers” to broader use of the dual-drug formulation for its approved indication in patients with chronic heart failure, especially patients with a left ventricular ejection fraction that is below normal. In addition, results from the PARADISE-MI trial suggested that “patients seemed to benefit before they develop heart failure. We couldn’t prove that, but we should build on this, and make it easier for patients to use this treatment,” Dr. Pfeffer said during a press briefing following his talk at the sessions.
Preventing heart failures to come
Treatment with sacubitril/valsartan in acute MI patients within a few days of their event “is perhaps addressing prevention of the heart failure that’s to come,” commented Lynne W. Stevenson, MD, designated discussant for the report and professor of medicine at Vanderbilt University Medical Center in Nashville. “Patients who are destined to develop heart failure are beginning their treatment early. The subgroup analyses suggest that it’s the sicker patients who benefited the most,” she said.
But Dr. Pfeffer stressed that “I don’t think this is a subgroup discussion. I would like to pursue this, but that’s up to the sponsor,” Novartis, the company that markets sacubitril/valsartan.
‘Exceedingly reassuring’ safety
The safety data that Dr. Pfeffer reported “are exceedingly reassuring. We didn’t see a signal of harm, and in some of the exploratory endpoints there was some evidence of benefit, so we need to encourage you to continue,” commented Mary N. Walsh, MD, medical director of the heart failure and cardiac transplantation program at Ascension St. Vincent Heart Center of Indiana in Indianapolis.
The PARADISE-MI (Prospective ARNI vs. ACE Inhibitor Trial to Determine Superiority in Reducing Heart Failure Events After MI) trial enrolled 5,669 patients with no history of heart failure within an average of 4 days following an acute MI at 495 sites in 41 countries during 2016-2020, with 8% of enrolled patients from the United States. Patients averaged 64 years of age, about three-quarters were men, about 43% had a history of diabetes, and only 1% were Black; Dr. Pfeffer noted that this is because most patients came from countries with low Black populations. The enrollment criteria required a left ventricular ejection fraction no greater than 40%, and among the enrolled patients this averaged about 37%.
A 10% nonsignificant relative risk reduction for the primary endpoint
The study’s primary endpoint was the combined first-event rate of cardiovascular death, hospitalization for heart failure, or an outpatient visit for heart failure. During a median follow-up of 23 months, this occurred at a rate of 7.4/100 patient years in the ramipril arm and 6.7/100 patient years in the sacubitril/valsartan arm, a 10% relative risk reduction with sacubitril/valsartan that was not significant, which meant all other efficacy analyses were exploratory, Dr. Pfeffer stressed.
Several secondary efficacy analyses showed significant benefits from sacubitril/valsartan, compared with ramipril, including the total number of events that comprised the primary endpoint, with a 21% relative risk reduction associated with sacubitril/valsartan, as well as investigator-reported events. The primary-endpoint benefit from sacubitril/valsartan was also significant in two subgroup analyses: patients aged 65 years or older (roughly half the study cohort), who had a 24% relative risk reduction on sacubitril/valsartan, compared with ramipril, and the 88% of patients who received treatment with percutaneous coronary intervention for their acute MI, who had a 19% relative risk reduction on sacubitril/valsartan, compared with patients who received ramipril.
The study’s safety data showed nearly identical rates in the two treatment arms for total adverse events, serious adverse events, adverse events that led to stopping the study drug, as well as in laboratory measures. The biggest between-treatment differences were a modest excess of hypotension on sacubitril valsartan, 28%, compared with 22% on ramipril, and a modest excess rate of cough on ramipril, 13%, compared with 9% on sacubitril/valsartan.
The added insight the results provide about sacubitril/valsartan comes at a time when U.S. patients continue to struggle to get health insurance coverage for an agent that has been approved for U.S. use in treating heart failure since 2015.
“Our patients do not have access to this important treatment,” declared Dr. Walsh during the press briefing. “The prior authorization process is unbelievable, and some patients have no access unless they pay the full cost on their own. This is an important, real-world problem that we face with this drug.”
PARADISE-MI was sponsored by Novartis, the company that markets sacubitril/valsartan (Entresto). Dr. Pfeffer has received research funding from and is a consultant to Novartis. He is also a consultant to AstraZeneca, Boehringer Ingelheim, Corvidia, DalCor, Eli Lilly, GlaxoSmithKline, Novo Nordisk, Peerbridge, and Sanofi, and he holds equity in DalCor and Peerbridge. Dr. Stevenson has received honoraria from LivaNova and has received research support from Abbott. Dr. Walsh had no disclosures.
FROM ACC 2021
PHM groups issue Choosing Wisely® recommendations
SHM members involved from the start
The Choosing Wisely® Pediatric Hospital Medicine (PHM) recommendations were published in January 2021. The initial Choosing Wisely® PHM recommendations were released in 2012 and the 2021 recommendations were the result of an extensive and years-long process. The Choosing Wisely® campaign, an initiative led by the American Board of Internal Medicine, was developed to enhance clinician-patient conversations, promoting care that is evidenced based, free from harm, and truly necessary.
The campaign has been embraced by the entire medical community, with more than 70 professional medical societies releasing recommendations. With its emphasis on high value care and eliminating medical waste, it is no surprise that the Choosing Wisely® campaign has found a home in a pediatric hospital medicine community that prides itself on those very traits. This article sheds light on the recommendation development process and identifies challenges and opportunities for implementation across the country.
The Choosing Wisely® process started with the selection of a committee. This group comprised nine members, with equal representation from all three societies affiliated with PHM: the Society of Hospital Medicine (SHM), the American Academy of Pediatrics’ Section on Hospital Medicine (AAP SOHM), and the Academic Pediatric Association (APA). Members of the committee intentionally represented a wide spectrum of practice variability, geography, and clinical experience.
The SHM members of the group were: James O’Callaghan, MD, FAAP, SFHM, pediatric hospitalist at Seattle Children’s Hospital and clinical professor of pediatrics at the University of Washington School of Medicine; Vivian Lee, MD, clinical pediatric hospitalist at Children’s Hospital of Los Angeles and associate professor of pediatrics at USC Keck School of Medicine; and Francisco Alvarez, MD, pediatric hospitalist at Lucile Packard Children’s Hospital, Palo Alto, Calif., and clinical associate professor of pediatrics at Stanford (Calif.) University.
According to Dr. O’Callaghan, it was important that the Choosing Wisely® recommendations come from the broader PHM community, reflecting the community’s priorities.
The committee started the process by asking the broader PHM community to submit ideas for consideration, via SHM’s HMX and the AAP SOHM listserv. The PHM community responded with more than 400 submissions.
Dr. Alvarez said the committee organized and trimmed the initial submissions, removing redundancy, into approximately 200 distinct recommendations. After initial literature review, the committee focused on approximately 70 recommendations. At that point, each member undertook an extensive literature review of the topics.
Once every potential recommendation had received a thorough review, Dr. Lee said, the committee underwent a modified Delphi process to evaluate the list. In this process, each member ranked the recommendations on validity – a measure of the quality of evidence supporting a topic – and feasibility – a measure of the PHM community’s ability to influence compliance.
At the end of this objective process, Dr. O’Callaghan said, the committee chose the five recommendations that received the highest total scores. While there were spirited discussions regarding the data available for each recommendation, all three SHM members of the committee agreed that the objective process played itself out.
Now that the Choosing Wisely® recommendations have been published, the PHM community is challenged to implement these recommendations to spur change for the care of hospitalized children throughout the country. Given the variety that exists in PHM, specifically in practice settings, it may be a daunting task. Dr. O’Callaghan said that differing opinions among physicians in a group may be a challenge to implementing change. “These recommendations allow for those conversations” to take place, he said. Dr. Lee said she hopes these recommendations provide a national panel opinion of the evidence to help support hospitalists in management discussions with others in a hospital – such as subspecialists or emergency department physicians – to increase high value care.
Since the nature of hospital medicine is one of collaboration, these recommendations will allow pediatric hospitalists to lead change throughout their hospitals and health care systems. However, it may not be a quick task. Dr. Alvarez estimates it may take 10-15 years until these recommendations are fully implemented throughout the country. However, there is reason to be optimistic, as the initial PHM Choosing Wisely® recommendations from 2012 have been broadly accepted and now represent national standards of care.
While the road ahead may be long and filled with challenges, the path forward has been clearly delineated, and the PHM community is grateful for the work done by members of the Choosing Wisely® Pediatric Hospital Medicine committee.
Dr. Casey is a pediatric hospitalist at Joe DiMaggio Children’s Hospital in Hollywood, Fla., and a member of the Society of Hospital Medicine’s Pediatric Special Interest Group’s Executive Council.
SHM members involved from the start
SHM members involved from the start
The Choosing Wisely® Pediatric Hospital Medicine (PHM) recommendations were published in January 2021. The initial Choosing Wisely® PHM recommendations were released in 2012 and the 2021 recommendations were the result of an extensive and years-long process. The Choosing Wisely® campaign, an initiative led by the American Board of Internal Medicine, was developed to enhance clinician-patient conversations, promoting care that is evidenced based, free from harm, and truly necessary.
The campaign has been embraced by the entire medical community, with more than 70 professional medical societies releasing recommendations. With its emphasis on high value care and eliminating medical waste, it is no surprise that the Choosing Wisely® campaign has found a home in a pediatric hospital medicine community that prides itself on those very traits. This article sheds light on the recommendation development process and identifies challenges and opportunities for implementation across the country.
The Choosing Wisely® process started with the selection of a committee. This group comprised nine members, with equal representation from all three societies affiliated with PHM: the Society of Hospital Medicine (SHM), the American Academy of Pediatrics’ Section on Hospital Medicine (AAP SOHM), and the Academic Pediatric Association (APA). Members of the committee intentionally represented a wide spectrum of practice variability, geography, and clinical experience.
The SHM members of the group were: James O’Callaghan, MD, FAAP, SFHM, pediatric hospitalist at Seattle Children’s Hospital and clinical professor of pediatrics at the University of Washington School of Medicine; Vivian Lee, MD, clinical pediatric hospitalist at Children’s Hospital of Los Angeles and associate professor of pediatrics at USC Keck School of Medicine; and Francisco Alvarez, MD, pediatric hospitalist at Lucile Packard Children’s Hospital, Palo Alto, Calif., and clinical associate professor of pediatrics at Stanford (Calif.) University.
According to Dr. O’Callaghan, it was important that the Choosing Wisely® recommendations come from the broader PHM community, reflecting the community’s priorities.
The committee started the process by asking the broader PHM community to submit ideas for consideration, via SHM’s HMX and the AAP SOHM listserv. The PHM community responded with more than 400 submissions.
Dr. Alvarez said the committee organized and trimmed the initial submissions, removing redundancy, into approximately 200 distinct recommendations. After initial literature review, the committee focused on approximately 70 recommendations. At that point, each member undertook an extensive literature review of the topics.
Once every potential recommendation had received a thorough review, Dr. Lee said, the committee underwent a modified Delphi process to evaluate the list. In this process, each member ranked the recommendations on validity – a measure of the quality of evidence supporting a topic – and feasibility – a measure of the PHM community’s ability to influence compliance.
At the end of this objective process, Dr. O’Callaghan said, the committee chose the five recommendations that received the highest total scores. While there were spirited discussions regarding the data available for each recommendation, all three SHM members of the committee agreed that the objective process played itself out.
Now that the Choosing Wisely® recommendations have been published, the PHM community is challenged to implement these recommendations to spur change for the care of hospitalized children throughout the country. Given the variety that exists in PHM, specifically in practice settings, it may be a daunting task. Dr. O’Callaghan said that differing opinions among physicians in a group may be a challenge to implementing change. “These recommendations allow for those conversations” to take place, he said. Dr. Lee said she hopes these recommendations provide a national panel opinion of the evidence to help support hospitalists in management discussions with others in a hospital – such as subspecialists or emergency department physicians – to increase high value care.
Since the nature of hospital medicine is one of collaboration, these recommendations will allow pediatric hospitalists to lead change throughout their hospitals and health care systems. However, it may not be a quick task. Dr. Alvarez estimates it may take 10-15 years until these recommendations are fully implemented throughout the country. However, there is reason to be optimistic, as the initial PHM Choosing Wisely® recommendations from 2012 have been broadly accepted and now represent national standards of care.
While the road ahead may be long and filled with challenges, the path forward has been clearly delineated, and the PHM community is grateful for the work done by members of the Choosing Wisely® Pediatric Hospital Medicine committee.
Dr. Casey is a pediatric hospitalist at Joe DiMaggio Children’s Hospital in Hollywood, Fla., and a member of the Society of Hospital Medicine’s Pediatric Special Interest Group’s Executive Council.
The Choosing Wisely® Pediatric Hospital Medicine (PHM) recommendations were published in January 2021. The initial Choosing Wisely® PHM recommendations were released in 2012 and the 2021 recommendations were the result of an extensive and years-long process. The Choosing Wisely® campaign, an initiative led by the American Board of Internal Medicine, was developed to enhance clinician-patient conversations, promoting care that is evidenced based, free from harm, and truly necessary.
The campaign has been embraced by the entire medical community, with more than 70 professional medical societies releasing recommendations. With its emphasis on high value care and eliminating medical waste, it is no surprise that the Choosing Wisely® campaign has found a home in a pediatric hospital medicine community that prides itself on those very traits. This article sheds light on the recommendation development process and identifies challenges and opportunities for implementation across the country.
The Choosing Wisely® process started with the selection of a committee. This group comprised nine members, with equal representation from all three societies affiliated with PHM: the Society of Hospital Medicine (SHM), the American Academy of Pediatrics’ Section on Hospital Medicine (AAP SOHM), and the Academic Pediatric Association (APA). Members of the committee intentionally represented a wide spectrum of practice variability, geography, and clinical experience.
The SHM members of the group were: James O’Callaghan, MD, FAAP, SFHM, pediatric hospitalist at Seattle Children’s Hospital and clinical professor of pediatrics at the University of Washington School of Medicine; Vivian Lee, MD, clinical pediatric hospitalist at Children’s Hospital of Los Angeles and associate professor of pediatrics at USC Keck School of Medicine; and Francisco Alvarez, MD, pediatric hospitalist at Lucile Packard Children’s Hospital, Palo Alto, Calif., and clinical associate professor of pediatrics at Stanford (Calif.) University.
According to Dr. O’Callaghan, it was important that the Choosing Wisely® recommendations come from the broader PHM community, reflecting the community’s priorities.
The committee started the process by asking the broader PHM community to submit ideas for consideration, via SHM’s HMX and the AAP SOHM listserv. The PHM community responded with more than 400 submissions.
Dr. Alvarez said the committee organized and trimmed the initial submissions, removing redundancy, into approximately 200 distinct recommendations. After initial literature review, the committee focused on approximately 70 recommendations. At that point, each member undertook an extensive literature review of the topics.
Once every potential recommendation had received a thorough review, Dr. Lee said, the committee underwent a modified Delphi process to evaluate the list. In this process, each member ranked the recommendations on validity – a measure of the quality of evidence supporting a topic – and feasibility – a measure of the PHM community’s ability to influence compliance.
At the end of this objective process, Dr. O’Callaghan said, the committee chose the five recommendations that received the highest total scores. While there were spirited discussions regarding the data available for each recommendation, all three SHM members of the committee agreed that the objective process played itself out.
Now that the Choosing Wisely® recommendations have been published, the PHM community is challenged to implement these recommendations to spur change for the care of hospitalized children throughout the country. Given the variety that exists in PHM, specifically in practice settings, it may be a daunting task. Dr. O’Callaghan said that differing opinions among physicians in a group may be a challenge to implementing change. “These recommendations allow for those conversations” to take place, he said. Dr. Lee said she hopes these recommendations provide a national panel opinion of the evidence to help support hospitalists in management discussions with others in a hospital – such as subspecialists or emergency department physicians – to increase high value care.
Since the nature of hospital medicine is one of collaboration, these recommendations will allow pediatric hospitalists to lead change throughout their hospitals and health care systems. However, it may not be a quick task. Dr. Alvarez estimates it may take 10-15 years until these recommendations are fully implemented throughout the country. However, there is reason to be optimistic, as the initial PHM Choosing Wisely® recommendations from 2012 have been broadly accepted and now represent national standards of care.
While the road ahead may be long and filled with challenges, the path forward has been clearly delineated, and the PHM community is grateful for the work done by members of the Choosing Wisely® Pediatric Hospital Medicine committee.
Dr. Casey is a pediatric hospitalist at Joe DiMaggio Children’s Hospital in Hollywood, Fla., and a member of the Society of Hospital Medicine’s Pediatric Special Interest Group’s Executive Council.
Update in Hospital Medicine relays important findings
Two experts scoured the medical journals for the practice-changing research most relevant to hospital medicine in 2020 at a recent session at SHM Converge, the annual conference of the Society of Hospital Medicine.
The presenters chose findings they considered either practice changing or practice confirming, and in areas over which hospitalists have at least some control. Here is what they highlighted:
IV iron administration before hospital discharge
In a randomized double-blind, placebo-controlled trial across 121 centers in Europe, South America, and Singapore, 1,108 patients hospitalized with acute heart failure and iron deficiency were randomized to receive intravenous ferric carboxymaltose or placebo, with a first dose before discharge and a second at 6 weeks.
Those in the intravenous iron group had a significant reduction in hospitalizations for heart failure up to 52 weeks after randomization, but there was no significant reduction in deaths because of heart failure. There was no difference in serious adverse events.
Anthony Breu, MD, assistant professor of medicine at Harvard Medical School, Boston, said the findings should alter hospitalist practice.
“In patients hospitalized with acute heart failure and left ventricular ejection fraction of less than 50%, check iron studies and start IV iron prior to discharge if they have iron deficiency, with or without anemia,” he said.
Apixaban versus dalteparin for venous thromboembolism in cancer
This noninferiority trial involved 1,155 adults with cancer who had symptomatic or incidental acute proximal deep vein thrombosis or pulmonary embolism. The patients were randomized to receive oral apixaban or subcutaneous dalteparin for 6 months.
Patients in the apixaban group had a significantly lower rate of recurrent venous thromboembolism (P = .09), with no increase in major bleeds, Dr. Breu said. He noted that those with brain cancer and leukemia were excluded.
“In patients with cancer and acute venous thromboembolism, consider apixaban as your first-line treatment, with some caveats,” he said.
Clinical decision rule for penicillin allergy
With fewer than 10% of patients who report a penicillin allergy actually testing positive on a standard allergy test, a simpler way to predict an allergy would help clinicians, said Shoshana Herzig, MD, MPH, associate professor of medicine at Harvard Medical School.
A 622-patient cohort that had undergone penicillin allergy testing was used to identify factors that could help predict an allergy. A scoring system called PEN-FAST was developed based on five factors – a penicillin allergy reported by the patient, 5 years or less since the last reaction (2 points); anaphylaxis or angioedema, or severe cutaneous adverse reaction (2 points); and treatment being required for the reaction (1 point).
Researchers, after validation at three sites, found that a score below a threshold identified a group that had a 96% negative predictive value for penicillin allergy skin testing.
“A PEN-FAST score of less than 3 can be used to identify patients with reported penicillin allergy who can likely proceed safely to oral challenge,” Dr. Herzig said. She said the findings would benefit from validation in an inpatient setting.
Prehydration before contrast-enhanced computed tomography in CKD
Previous studies have found that omitting prehydration was noninferior to volume expansion with isotonic saline, and this trial looked at omission versus sodium bicarbonate hydration.
Participants were 523 adults with stage 3 chronic kidney disease who were getting elective outpatient CT with contrast. They were randomized to either no prehydration or prehydration with 250 mL of 1.4% sodium bicarbonate an hour before CT.
Researchers found that postcontrast acute kidney injury was rare even in this high-risk patient population overall, and that withholding prehydration was noninferior to prehydration with sodium bicarbonate, Dr. Herzig said.
Gabapentin for alcohol use disorder in those with alcohol withdrawal symptoms
Dr. Breu noted that only about one in five patients with alcohol use disorder receive medications to help preserve abstinence or to reduce drinking, and many medications target cravings but not symptoms of withdrawal.
In a double-blind, randomized, placebo-controlled trial at a single academic outpatient medical center in South Carolina, 90 patients were randomized to receive titrated gabapentin or placebo for 16 weeks.
Researchers found that, among those with abstinence of at least 2 days, gabapentin reduced the number of days of heavy drinking and the days of any drinking, especially in those with high symptoms of withdrawal.
“In patients with alcohol use disorder and high alcohol withdrawal symptoms, consider gabapentin to help reduce heavy drinking or maintain abstinence,” Dr. Breu said.
Hospitalist continuity of care and patient outcomes
In a retrospective study examining all medical admissions of Medicare patients with a 3- to 6-day length of stay, and in which all general medical care was provided by hospitalists, researchers examined the effects of continuity of care. Nearly 115,000 patient stays were included in the study, which covered 229 Texas hospitals.
The stays were grouped into quartiles of continuity of care, based on the number of hospitalists involved in a patient’s stay. Greater continuity was associated with lower 30-day mortality, with a linear relationship between the two. Researchers also found costs to be lower as continuity increased.
“Efforts by hospitals and hospitalist groups to promote working schedules with more continuity,” Dr. Herzig said, “could lead to improved postdischarge outcomes.”
Two experts scoured the medical journals for the practice-changing research most relevant to hospital medicine in 2020 at a recent session at SHM Converge, the annual conference of the Society of Hospital Medicine.
The presenters chose findings they considered either practice changing or practice confirming, and in areas over which hospitalists have at least some control. Here is what they highlighted:
IV iron administration before hospital discharge
In a randomized double-blind, placebo-controlled trial across 121 centers in Europe, South America, and Singapore, 1,108 patients hospitalized with acute heart failure and iron deficiency were randomized to receive intravenous ferric carboxymaltose or placebo, with a first dose before discharge and a second at 6 weeks.
Those in the intravenous iron group had a significant reduction in hospitalizations for heart failure up to 52 weeks after randomization, but there was no significant reduction in deaths because of heart failure. There was no difference in serious adverse events.
Anthony Breu, MD, assistant professor of medicine at Harvard Medical School, Boston, said the findings should alter hospitalist practice.
“In patients hospitalized with acute heart failure and left ventricular ejection fraction of less than 50%, check iron studies and start IV iron prior to discharge if they have iron deficiency, with or without anemia,” he said.
Apixaban versus dalteparin for venous thromboembolism in cancer
This noninferiority trial involved 1,155 adults with cancer who had symptomatic or incidental acute proximal deep vein thrombosis or pulmonary embolism. The patients were randomized to receive oral apixaban or subcutaneous dalteparin for 6 months.
Patients in the apixaban group had a significantly lower rate of recurrent venous thromboembolism (P = .09), with no increase in major bleeds, Dr. Breu said. He noted that those with brain cancer and leukemia were excluded.
“In patients with cancer and acute venous thromboembolism, consider apixaban as your first-line treatment, with some caveats,” he said.
Clinical decision rule for penicillin allergy
With fewer than 10% of patients who report a penicillin allergy actually testing positive on a standard allergy test, a simpler way to predict an allergy would help clinicians, said Shoshana Herzig, MD, MPH, associate professor of medicine at Harvard Medical School.
A 622-patient cohort that had undergone penicillin allergy testing was used to identify factors that could help predict an allergy. A scoring system called PEN-FAST was developed based on five factors – a penicillin allergy reported by the patient, 5 years or less since the last reaction (2 points); anaphylaxis or angioedema, or severe cutaneous adverse reaction (2 points); and treatment being required for the reaction (1 point).
Researchers, after validation at three sites, found that a score below a threshold identified a group that had a 96% negative predictive value for penicillin allergy skin testing.
“A PEN-FAST score of less than 3 can be used to identify patients with reported penicillin allergy who can likely proceed safely to oral challenge,” Dr. Herzig said. She said the findings would benefit from validation in an inpatient setting.
Prehydration before contrast-enhanced computed tomography in CKD
Previous studies have found that omitting prehydration was noninferior to volume expansion with isotonic saline, and this trial looked at omission versus sodium bicarbonate hydration.
Participants were 523 adults with stage 3 chronic kidney disease who were getting elective outpatient CT with contrast. They were randomized to either no prehydration or prehydration with 250 mL of 1.4% sodium bicarbonate an hour before CT.
Researchers found that postcontrast acute kidney injury was rare even in this high-risk patient population overall, and that withholding prehydration was noninferior to prehydration with sodium bicarbonate, Dr. Herzig said.
Gabapentin for alcohol use disorder in those with alcohol withdrawal symptoms
Dr. Breu noted that only about one in five patients with alcohol use disorder receive medications to help preserve abstinence or to reduce drinking, and many medications target cravings but not symptoms of withdrawal.
In a double-blind, randomized, placebo-controlled trial at a single academic outpatient medical center in South Carolina, 90 patients were randomized to receive titrated gabapentin or placebo for 16 weeks.
Researchers found that, among those with abstinence of at least 2 days, gabapentin reduced the number of days of heavy drinking and the days of any drinking, especially in those with high symptoms of withdrawal.
“In patients with alcohol use disorder and high alcohol withdrawal symptoms, consider gabapentin to help reduce heavy drinking or maintain abstinence,” Dr. Breu said.
Hospitalist continuity of care and patient outcomes
In a retrospective study examining all medical admissions of Medicare patients with a 3- to 6-day length of stay, and in which all general medical care was provided by hospitalists, researchers examined the effects of continuity of care. Nearly 115,000 patient stays were included in the study, which covered 229 Texas hospitals.
The stays were grouped into quartiles of continuity of care, based on the number of hospitalists involved in a patient’s stay. Greater continuity was associated with lower 30-day mortality, with a linear relationship between the two. Researchers also found costs to be lower as continuity increased.
“Efforts by hospitals and hospitalist groups to promote working schedules with more continuity,” Dr. Herzig said, “could lead to improved postdischarge outcomes.”
Two experts scoured the medical journals for the practice-changing research most relevant to hospital medicine in 2020 at a recent session at SHM Converge, the annual conference of the Society of Hospital Medicine.
The presenters chose findings they considered either practice changing or practice confirming, and in areas over which hospitalists have at least some control. Here is what they highlighted:
IV iron administration before hospital discharge
In a randomized double-blind, placebo-controlled trial across 121 centers in Europe, South America, and Singapore, 1,108 patients hospitalized with acute heart failure and iron deficiency were randomized to receive intravenous ferric carboxymaltose or placebo, with a first dose before discharge and a second at 6 weeks.
Those in the intravenous iron group had a significant reduction in hospitalizations for heart failure up to 52 weeks after randomization, but there was no significant reduction in deaths because of heart failure. There was no difference in serious adverse events.
Anthony Breu, MD, assistant professor of medicine at Harvard Medical School, Boston, said the findings should alter hospitalist practice.
“In patients hospitalized with acute heart failure and left ventricular ejection fraction of less than 50%, check iron studies and start IV iron prior to discharge if they have iron deficiency, with or without anemia,” he said.
Apixaban versus dalteparin for venous thromboembolism in cancer
This noninferiority trial involved 1,155 adults with cancer who had symptomatic or incidental acute proximal deep vein thrombosis or pulmonary embolism. The patients were randomized to receive oral apixaban or subcutaneous dalteparin for 6 months.
Patients in the apixaban group had a significantly lower rate of recurrent venous thromboembolism (P = .09), with no increase in major bleeds, Dr. Breu said. He noted that those with brain cancer and leukemia were excluded.
“In patients with cancer and acute venous thromboembolism, consider apixaban as your first-line treatment, with some caveats,” he said.
Clinical decision rule for penicillin allergy
With fewer than 10% of patients who report a penicillin allergy actually testing positive on a standard allergy test, a simpler way to predict an allergy would help clinicians, said Shoshana Herzig, MD, MPH, associate professor of medicine at Harvard Medical School.
A 622-patient cohort that had undergone penicillin allergy testing was used to identify factors that could help predict an allergy. A scoring system called PEN-FAST was developed based on five factors – a penicillin allergy reported by the patient, 5 years or less since the last reaction (2 points); anaphylaxis or angioedema, or severe cutaneous adverse reaction (2 points); and treatment being required for the reaction (1 point).
Researchers, after validation at three sites, found that a score below a threshold identified a group that had a 96% negative predictive value for penicillin allergy skin testing.
“A PEN-FAST score of less than 3 can be used to identify patients with reported penicillin allergy who can likely proceed safely to oral challenge,” Dr. Herzig said. She said the findings would benefit from validation in an inpatient setting.
Prehydration before contrast-enhanced computed tomography in CKD
Previous studies have found that omitting prehydration was noninferior to volume expansion with isotonic saline, and this trial looked at omission versus sodium bicarbonate hydration.
Participants were 523 adults with stage 3 chronic kidney disease who were getting elective outpatient CT with contrast. They were randomized to either no prehydration or prehydration with 250 mL of 1.4% sodium bicarbonate an hour before CT.
Researchers found that postcontrast acute kidney injury was rare even in this high-risk patient population overall, and that withholding prehydration was noninferior to prehydration with sodium bicarbonate, Dr. Herzig said.
Gabapentin for alcohol use disorder in those with alcohol withdrawal symptoms
Dr. Breu noted that only about one in five patients with alcohol use disorder receive medications to help preserve abstinence or to reduce drinking, and many medications target cravings but not symptoms of withdrawal.
In a double-blind, randomized, placebo-controlled trial at a single academic outpatient medical center in South Carolina, 90 patients were randomized to receive titrated gabapentin or placebo for 16 weeks.
Researchers found that, among those with abstinence of at least 2 days, gabapentin reduced the number of days of heavy drinking and the days of any drinking, especially in those with high symptoms of withdrawal.
“In patients with alcohol use disorder and high alcohol withdrawal symptoms, consider gabapentin to help reduce heavy drinking or maintain abstinence,” Dr. Breu said.
Hospitalist continuity of care and patient outcomes
In a retrospective study examining all medical admissions of Medicare patients with a 3- to 6-day length of stay, and in which all general medical care was provided by hospitalists, researchers examined the effects of continuity of care. Nearly 115,000 patient stays were included in the study, which covered 229 Texas hospitals.
The stays were grouped into quartiles of continuity of care, based on the number of hospitalists involved in a patient’s stay. Greater continuity was associated with lower 30-day mortality, with a linear relationship between the two. Researchers also found costs to be lower as continuity increased.
“Efforts by hospitals and hospitalist groups to promote working schedules with more continuity,” Dr. Herzig said, “could lead to improved postdischarge outcomes.”
FROM SHM CONVERGE 2021























