User login
CDC: Vaccinated? You don’t need a mask indoors
the CDC announced on May 13.
“Anyone who is fully vaccinated can participate in indoor and outdoor activities, large or small, without wearing a mask or physically distancing,” CDC director Rochelle Walensky, MD, said at a press briefing. “We have all longed for this moment when we can get back to some sense of normalcy.
“This is an exciting and powerful moment,” she added, “It could only happen because of the work from so many who made sure we had the rapid administration of three safe and effective vaccines.”
Dr. Walensky cited three large studies on the effectiveness of COVID-19 vaccines against the original virus and its variants. One study from Israel found the vaccine to be 97% effective against symptomatic infection.
Those who are symptomatic should still wear masks, Dr. Walensky said, and those who are immunocompromised should talk to their doctors for further guidance. The CDC still advises travelers to wear masks while on airplanes or trains.
The COVID-19 death rates are now the lowest they have been since April 2020.
A version of this article first appeared on Medscape.com.
the CDC announced on May 13.
“Anyone who is fully vaccinated can participate in indoor and outdoor activities, large or small, without wearing a mask or physically distancing,” CDC director Rochelle Walensky, MD, said at a press briefing. “We have all longed for this moment when we can get back to some sense of normalcy.
“This is an exciting and powerful moment,” she added, “It could only happen because of the work from so many who made sure we had the rapid administration of three safe and effective vaccines.”
Dr. Walensky cited three large studies on the effectiveness of COVID-19 vaccines against the original virus and its variants. One study from Israel found the vaccine to be 97% effective against symptomatic infection.
Those who are symptomatic should still wear masks, Dr. Walensky said, and those who are immunocompromised should talk to their doctors for further guidance. The CDC still advises travelers to wear masks while on airplanes or trains.
The COVID-19 death rates are now the lowest they have been since April 2020.
A version of this article first appeared on Medscape.com.
the CDC announced on May 13.
“Anyone who is fully vaccinated can participate in indoor and outdoor activities, large or small, without wearing a mask or physically distancing,” CDC director Rochelle Walensky, MD, said at a press briefing. “We have all longed for this moment when we can get back to some sense of normalcy.
“This is an exciting and powerful moment,” she added, “It could only happen because of the work from so many who made sure we had the rapid administration of three safe and effective vaccines.”
Dr. Walensky cited three large studies on the effectiveness of COVID-19 vaccines against the original virus and its variants. One study from Israel found the vaccine to be 97% effective against symptomatic infection.
Those who are symptomatic should still wear masks, Dr. Walensky said, and those who are immunocompromised should talk to their doctors for further guidance. The CDC still advises travelers to wear masks while on airplanes or trains.
The COVID-19 death rates are now the lowest they have been since April 2020.
A version of this article first appeared on Medscape.com.
Trends in hospital medicine program operations during COVID-19
Staffing was a challenge for most groups
What a year it has been in the world of hospital medicine with all the changes, challenges, and uncertainties surrounding the COVID-19 pandemic. Some hospitalist programs were hit hard early on with an early surge, when little was known about COVID-19, and other programs have had more time to plan and adapt to later surges.
As many readers of The Hospitalist know, the Society of Hospital Medicine publishes a biennial State of Hospital Medicine (SoHM) Report – last published in September 2020 using data from 2019. The SoHM Report contains a wealth of information that many groups find useful in evaluating their programs, with topics ranging from compensation to staffing to scheduling. As some prior months’ Survey Insights columns have alluded to, with the rapid pace of change in 2020 because of the COVID-19 pandemic, the Society of Hospital Medicine made the decision to publish an addendum highlighting the myriad of adjustments and adaptations that have occurred in such a short period of time. The COVID-19 Addendum is available to all purchasers of the SoHM Report and contains data from survey responses submitted in September 2020.
Let’s take a look at what transpired in 2020, starting with staffing – no doubt a challenge for many groups. During some periods of time, patient volumes may have fallen below historical averages with stay-at-home orders, canceled procedures, and a reluctance by patients to seek medical care. In contrast, for many groups, other parts of the year were all-hands-on-deck scenarios to care for extraordinary surges in patient volume. To compound this, many hospitalist groups had physicians and staff facing quarantine or isolation requirements because of exposures or contracting COVID-19, and locums positions may have been difficult to fill because of travel restrictions and extreme demand.
What operational changes were made in response to these staffing challenges? Perhaps one notable finding from the COVID-19 Addendum was the need for contingency planning and backup systems. From the 2020 SoHM, prior to the pandemic, 47.4% of adult hospital medicine groups had backup systems in place. In our recently published addendum, we found that 61.9% of groups instituted a backup system where none previously existed. In addition, 54.2% of groups modified their existing backup system. Some 39.6% of hospital medicine groups also utilized clinicians from other service lines to help cover service needs.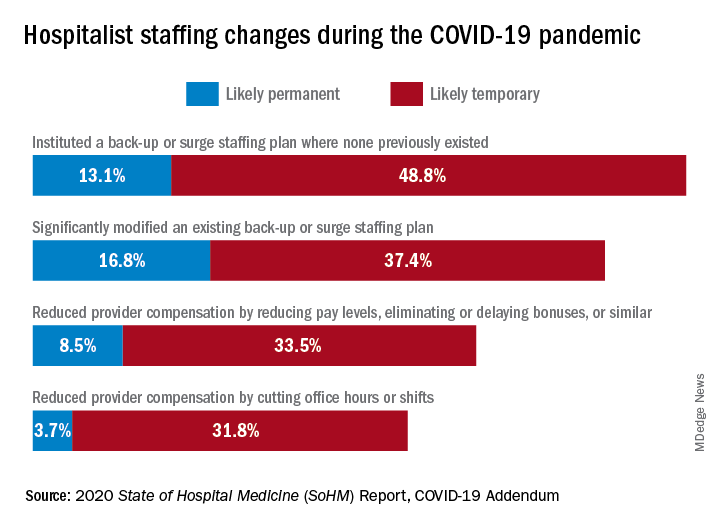
Aside from staffing, hospitals faced unprecedented financial challenges, and these effects rippled through to hospitalists. Our addendum found that 42.0% of hospitalist groups faced reductions in salary or bonuses, and 35.5% of hospital medicine groups reduced provider compensation by a reduction of work hours or shifts. I’ve personally been struck by these findings – that many hospitalists at the front-lines of COVID-19 received salary reductions, albeit temporary for many groups, during one of the most challenging years of their professional careers. Our addendum, interestingly, also found that a smaller 10.7% of groups instituted hazard pay for clinicians caring for COVID-19 patients.
So, are the changes and challenges your group faced similar to what was experienced by other hospital medicine programs? These findings and many more interesting and useful pieces of data are available in the full COVID-19 Addendum. Perhaps my biggest takeaway is that hospitalists have been perhaps the most uniquely positioned specialty to tackle the challenges of the COVID-19 pandemic. We have always been a dynamic, changing field, ready to lead and tackle change – and while change may have happened more quickly and in ways that were unforeseen just a year ago, hospitalists have undoubtedly demonstrated their strengths as leaders ready to adapt and rise to the occasion.
I am optimistic that, as we move beyond the pandemic in the coming months and years, the value that hospitalists have proven yet again will yield long-term recognition and benefits to our programs and our specialty.
Dr. Huang is a physician adviser and clinical professor of medicine in the division of hospital medicine at the University of California, San Diego. He is a member of SHM’s Practice Analysis Committee.
Staffing was a challenge for most groups
Staffing was a challenge for most groups
What a year it has been in the world of hospital medicine with all the changes, challenges, and uncertainties surrounding the COVID-19 pandemic. Some hospitalist programs were hit hard early on with an early surge, when little was known about COVID-19, and other programs have had more time to plan and adapt to later surges.
As many readers of The Hospitalist know, the Society of Hospital Medicine publishes a biennial State of Hospital Medicine (SoHM) Report – last published in September 2020 using data from 2019. The SoHM Report contains a wealth of information that many groups find useful in evaluating their programs, with topics ranging from compensation to staffing to scheduling. As some prior months’ Survey Insights columns have alluded to, with the rapid pace of change in 2020 because of the COVID-19 pandemic, the Society of Hospital Medicine made the decision to publish an addendum highlighting the myriad of adjustments and adaptations that have occurred in such a short period of time. The COVID-19 Addendum is available to all purchasers of the SoHM Report and contains data from survey responses submitted in September 2020.
Let’s take a look at what transpired in 2020, starting with staffing – no doubt a challenge for many groups. During some periods of time, patient volumes may have fallen below historical averages with stay-at-home orders, canceled procedures, and a reluctance by patients to seek medical care. In contrast, for many groups, other parts of the year were all-hands-on-deck scenarios to care for extraordinary surges in patient volume. To compound this, many hospitalist groups had physicians and staff facing quarantine or isolation requirements because of exposures or contracting COVID-19, and locums positions may have been difficult to fill because of travel restrictions and extreme demand.
What operational changes were made in response to these staffing challenges? Perhaps one notable finding from the COVID-19 Addendum was the need for contingency planning and backup systems. From the 2020 SoHM, prior to the pandemic, 47.4% of adult hospital medicine groups had backup systems in place. In our recently published addendum, we found that 61.9% of groups instituted a backup system where none previously existed. In addition, 54.2% of groups modified their existing backup system. Some 39.6% of hospital medicine groups also utilized clinicians from other service lines to help cover service needs.
Aside from staffing, hospitals faced unprecedented financial challenges, and these effects rippled through to hospitalists. Our addendum found that 42.0% of hospitalist groups faced reductions in salary or bonuses, and 35.5% of hospital medicine groups reduced provider compensation by a reduction of work hours or shifts. I’ve personally been struck by these findings – that many hospitalists at the front-lines of COVID-19 received salary reductions, albeit temporary for many groups, during one of the most challenging years of their professional careers. Our addendum, interestingly, also found that a smaller 10.7% of groups instituted hazard pay for clinicians caring for COVID-19 patients.
So, are the changes and challenges your group faced similar to what was experienced by other hospital medicine programs? These findings and many more interesting and useful pieces of data are available in the full COVID-19 Addendum. Perhaps my biggest takeaway is that hospitalists have been perhaps the most uniquely positioned specialty to tackle the challenges of the COVID-19 pandemic. We have always been a dynamic, changing field, ready to lead and tackle change – and while change may have happened more quickly and in ways that were unforeseen just a year ago, hospitalists have undoubtedly demonstrated their strengths as leaders ready to adapt and rise to the occasion.
I am optimistic that, as we move beyond the pandemic in the coming months and years, the value that hospitalists have proven yet again will yield long-term recognition and benefits to our programs and our specialty.
Dr. Huang is a physician adviser and clinical professor of medicine in the division of hospital medicine at the University of California, San Diego. He is a member of SHM’s Practice Analysis Committee.
What a year it has been in the world of hospital medicine with all the changes, challenges, and uncertainties surrounding the COVID-19 pandemic. Some hospitalist programs were hit hard early on with an early surge, when little was known about COVID-19, and other programs have had more time to plan and adapt to later surges.
As many readers of The Hospitalist know, the Society of Hospital Medicine publishes a biennial State of Hospital Medicine (SoHM) Report – last published in September 2020 using data from 2019. The SoHM Report contains a wealth of information that many groups find useful in evaluating their programs, with topics ranging from compensation to staffing to scheduling. As some prior months’ Survey Insights columns have alluded to, with the rapid pace of change in 2020 because of the COVID-19 pandemic, the Society of Hospital Medicine made the decision to publish an addendum highlighting the myriad of adjustments and adaptations that have occurred in such a short period of time. The COVID-19 Addendum is available to all purchasers of the SoHM Report and contains data from survey responses submitted in September 2020.
Let’s take a look at what transpired in 2020, starting with staffing – no doubt a challenge for many groups. During some periods of time, patient volumes may have fallen below historical averages with stay-at-home orders, canceled procedures, and a reluctance by patients to seek medical care. In contrast, for many groups, other parts of the year were all-hands-on-deck scenarios to care for extraordinary surges in patient volume. To compound this, many hospitalist groups had physicians and staff facing quarantine or isolation requirements because of exposures or contracting COVID-19, and locums positions may have been difficult to fill because of travel restrictions and extreme demand.
What operational changes were made in response to these staffing challenges? Perhaps one notable finding from the COVID-19 Addendum was the need for contingency planning and backup systems. From the 2020 SoHM, prior to the pandemic, 47.4% of adult hospital medicine groups had backup systems in place. In our recently published addendum, we found that 61.9% of groups instituted a backup system where none previously existed. In addition, 54.2% of groups modified their existing backup system. Some 39.6% of hospital medicine groups also utilized clinicians from other service lines to help cover service needs.
Aside from staffing, hospitals faced unprecedented financial challenges, and these effects rippled through to hospitalists. Our addendum found that 42.0% of hospitalist groups faced reductions in salary or bonuses, and 35.5% of hospital medicine groups reduced provider compensation by a reduction of work hours or shifts. I’ve personally been struck by these findings – that many hospitalists at the front-lines of COVID-19 received salary reductions, albeit temporary for many groups, during one of the most challenging years of their professional careers. Our addendum, interestingly, also found that a smaller 10.7% of groups instituted hazard pay for clinicians caring for COVID-19 patients.
So, are the changes and challenges your group faced similar to what was experienced by other hospital medicine programs? These findings and many more interesting and useful pieces of data are available in the full COVID-19 Addendum. Perhaps my biggest takeaway is that hospitalists have been perhaps the most uniquely positioned specialty to tackle the challenges of the COVID-19 pandemic. We have always been a dynamic, changing field, ready to lead and tackle change – and while change may have happened more quickly and in ways that were unforeseen just a year ago, hospitalists have undoubtedly demonstrated their strengths as leaders ready to adapt and rise to the occasion.
I am optimistic that, as we move beyond the pandemic in the coming months and years, the value that hospitalists have proven yet again will yield long-term recognition and benefits to our programs and our specialty.
Dr. Huang is a physician adviser and clinical professor of medicine in the division of hospital medicine at the University of California, San Diego. He is a member of SHM’s Practice Analysis Committee.
Smart prescribing strategies improve antibiotic stewardship
“Antibiotic stewardship is never easy, and sometimes it is very difficult to differentiate what is going on with a patient in the clinical setting,” said Valerie M. Vaughn, MD, of the University of Utah, Salt Lake City, at SHM Converge, the annual conference of the Society of Hospital Medicine.
“We know from studies that 20% of hospitalized patients who receive an antibiotic have an adverse drug event from that antibiotic within 30 days,” said Dr. Vaughn.
Dr. Vaughn identified several practical ways in which hospitalists can reduce antibiotic overuse, including in the management of patients hospitalized with COVID-19.
Identify asymptomatic bacteriuria
One key area in which hospitalists can improve antibiotic stewardship is in recognizing asymptomatic bacteriuria and the harms associated with treatment, Dr. Vaughn said. For example, a common scenario for hospitalists might involve and 80-year-old woman with dementia, who can provide little in the way of history, and whose chest x-ray can’t rule out an underlying infection. This patient might have a positive urine culture, but no other signs of a urinary tract infection. “We know that asymptomatic bacteriuria is very common in hospitalized patients,” especially elderly women living in nursing home settings, she noted.
In cases of asymptomatic bacteriuria, data show that antibiotic treatment does not improve outcomes, and in fact may increase the risk of subsequent UTI, said Dr. Vaughn. Elderly patients also are at increased risk for developing antibiotic-related adverse events, especially Clostridioides difficile. Asymptomatic bacteriuria is any bacteria in the urine in the absence of signs or symptoms of a UTI, even if lab tests show pyuria, nitrates, and resistant bacteria. These lab results are often associated with inappropriate antibiotic use. “The laboratory tests can’t distinguish between asymptomatic bacteriuria and a UTI, only the symptoms can,” she emphasized.
Contain treatment of community-acquired pneumonia
Another practical point for reducing antibiotics in the hospital setting is to limit treatment of community-acquired pneumonia (CAP) to 5 days when possible. Duration matters because for many diseases, shorter durations of antibiotic treatments are just as effective as longer durations based on the latest evidence. “This is a change in dogma,” from previous thinking that patients must complete a full course, and that anything less might promote antibiotic resistance, she said.
“In fact, longer antibiotic durations kill off more healthy, normal flora, select for resistant pathogens, increase the risk of C. difficile, and increase the risk of side effects,” she said.
Ultimately, the right treatment duration for pneumonia depends on several factors including patient factors, disease, clinical stability, and rate of improvement. However, a good rule of thumb is that approximately 89% of CAP patients need only 5 days of antibiotics as long as they are afebrile for 48 hours and have 1 or fewer vital sign abnormalities by day 5 of treatment. “We do need to prescribe longer durations for patients with complications,” she emphasized.
Revisit need for antibiotics at discharge
Hospitalists also can practice antibiotic stewardship by considering four points at patient discharge, said Dr. Vaughn.
First, consider whether antibiotics can be stopped. For example, antibiotics are not needed on discharge if infection is no longer the most likely diagnosis, or if the course of antibiotics has been completed, as is often the case for patients hospitalized with CAP, she noted.
Second, if the antibiotics can’t be stopped at the time of discharge, consider whether the preferred agent is being used. Third, be sure the patient is receiving the minimum duration of antibiotics, and fourth, be sure that the dose, indication, and total planned duration with start and stop dates is written in the discharge summary, said Dr. Vaughn. “This helps with communication to our outpatient providers as well as with education to the patients themselves.”
Bacterial coinfections rare in COVID-19
Dr. Vaughn concluded the session with data from a study she conducted with colleagues on the use of empiric antibacterial therapy and community-onset bacterial coinfection in hospitalized COVID-19 patients. The study included 1,667 patients at 32 hospitals in Michigan. The number of patients treated with antibiotics varied widely among hospitals, from 30% to as much as 90%, Dr. Vaughn said.
“What we found was that more than half of hospitalized patients with COVID (57%) received empiric antibiotic therapy in the first few days of hospitalization,” she said.
However, “despite all the antibiotic use, community-onset bacterial coinfections were rare,” and occurred in only 3.5% of the patients, meaning that the number needed to treat with antibiotics to prevent a single case was about 20.
Predictors of community-onset co-infections in the patients included older age, more severe disease, patients coming from nursing homes, and those with lower BMI or kidney disease, said Dr. Vaughn. She and her team also found that procalcitonin’s positive predictive value was 9.3%, but the negative predictive value was 98.3%, so these patients were extremely likely to have no coinfection.
Dr. Vaughn said that in her practice she might order procalcitonin when considering stopping antibiotics in a patient with COVID-19 and make a decision based on the negative predictive value, but she emphasized that she does not use it in the converse situation to rely on a positive value when deciding whether to start antibiotics in these patients.
Dr. Vaughn had no financial conflicts to disclose.
“Antibiotic stewardship is never easy, and sometimes it is very difficult to differentiate what is going on with a patient in the clinical setting,” said Valerie M. Vaughn, MD, of the University of Utah, Salt Lake City, at SHM Converge, the annual conference of the Society of Hospital Medicine.
“We know from studies that 20% of hospitalized patients who receive an antibiotic have an adverse drug event from that antibiotic within 30 days,” said Dr. Vaughn.
Dr. Vaughn identified several practical ways in which hospitalists can reduce antibiotic overuse, including in the management of patients hospitalized with COVID-19.
Identify asymptomatic bacteriuria
One key area in which hospitalists can improve antibiotic stewardship is in recognizing asymptomatic bacteriuria and the harms associated with treatment, Dr. Vaughn said. For example, a common scenario for hospitalists might involve and 80-year-old woman with dementia, who can provide little in the way of history, and whose chest x-ray can’t rule out an underlying infection. This patient might have a positive urine culture, but no other signs of a urinary tract infection. “We know that asymptomatic bacteriuria is very common in hospitalized patients,” especially elderly women living in nursing home settings, she noted.
In cases of asymptomatic bacteriuria, data show that antibiotic treatment does not improve outcomes, and in fact may increase the risk of subsequent UTI, said Dr. Vaughn. Elderly patients also are at increased risk for developing antibiotic-related adverse events, especially Clostridioides difficile. Asymptomatic bacteriuria is any bacteria in the urine in the absence of signs or symptoms of a UTI, even if lab tests show pyuria, nitrates, and resistant bacteria. These lab results are often associated with inappropriate antibiotic use. “The laboratory tests can’t distinguish between asymptomatic bacteriuria and a UTI, only the symptoms can,” she emphasized.
Contain treatment of community-acquired pneumonia
Another practical point for reducing antibiotics in the hospital setting is to limit treatment of community-acquired pneumonia (CAP) to 5 days when possible. Duration matters because for many diseases, shorter durations of antibiotic treatments are just as effective as longer durations based on the latest evidence. “This is a change in dogma,” from previous thinking that patients must complete a full course, and that anything less might promote antibiotic resistance, she said.
“In fact, longer antibiotic durations kill off more healthy, normal flora, select for resistant pathogens, increase the risk of C. difficile, and increase the risk of side effects,” she said.
Ultimately, the right treatment duration for pneumonia depends on several factors including patient factors, disease, clinical stability, and rate of improvement. However, a good rule of thumb is that approximately 89% of CAP patients need only 5 days of antibiotics as long as they are afebrile for 48 hours and have 1 or fewer vital sign abnormalities by day 5 of treatment. “We do need to prescribe longer durations for patients with complications,” she emphasized.
Revisit need for antibiotics at discharge
Hospitalists also can practice antibiotic stewardship by considering four points at patient discharge, said Dr. Vaughn.
First, consider whether antibiotics can be stopped. For example, antibiotics are not needed on discharge if infection is no longer the most likely diagnosis, or if the course of antibiotics has been completed, as is often the case for patients hospitalized with CAP, she noted.
Second, if the antibiotics can’t be stopped at the time of discharge, consider whether the preferred agent is being used. Third, be sure the patient is receiving the minimum duration of antibiotics, and fourth, be sure that the dose, indication, and total planned duration with start and stop dates is written in the discharge summary, said Dr. Vaughn. “This helps with communication to our outpatient providers as well as with education to the patients themselves.”
Bacterial coinfections rare in COVID-19
Dr. Vaughn concluded the session with data from a study she conducted with colleagues on the use of empiric antibacterial therapy and community-onset bacterial coinfection in hospitalized COVID-19 patients. The study included 1,667 patients at 32 hospitals in Michigan. The number of patients treated with antibiotics varied widely among hospitals, from 30% to as much as 90%, Dr. Vaughn said.
“What we found was that more than half of hospitalized patients with COVID (57%) received empiric antibiotic therapy in the first few days of hospitalization,” she said.
However, “despite all the antibiotic use, community-onset bacterial coinfections were rare,” and occurred in only 3.5% of the patients, meaning that the number needed to treat with antibiotics to prevent a single case was about 20.
Predictors of community-onset co-infections in the patients included older age, more severe disease, patients coming from nursing homes, and those with lower BMI or kidney disease, said Dr. Vaughn. She and her team also found that procalcitonin’s positive predictive value was 9.3%, but the negative predictive value was 98.3%, so these patients were extremely likely to have no coinfection.
Dr. Vaughn said that in her practice she might order procalcitonin when considering stopping antibiotics in a patient with COVID-19 and make a decision based on the negative predictive value, but she emphasized that she does not use it in the converse situation to rely on a positive value when deciding whether to start antibiotics in these patients.
Dr. Vaughn had no financial conflicts to disclose.
“Antibiotic stewardship is never easy, and sometimes it is very difficult to differentiate what is going on with a patient in the clinical setting,” said Valerie M. Vaughn, MD, of the University of Utah, Salt Lake City, at SHM Converge, the annual conference of the Society of Hospital Medicine.
“We know from studies that 20% of hospitalized patients who receive an antibiotic have an adverse drug event from that antibiotic within 30 days,” said Dr. Vaughn.
Dr. Vaughn identified several practical ways in which hospitalists can reduce antibiotic overuse, including in the management of patients hospitalized with COVID-19.
Identify asymptomatic bacteriuria
One key area in which hospitalists can improve antibiotic stewardship is in recognizing asymptomatic bacteriuria and the harms associated with treatment, Dr. Vaughn said. For example, a common scenario for hospitalists might involve and 80-year-old woman with dementia, who can provide little in the way of history, and whose chest x-ray can’t rule out an underlying infection. This patient might have a positive urine culture, but no other signs of a urinary tract infection. “We know that asymptomatic bacteriuria is very common in hospitalized patients,” especially elderly women living in nursing home settings, she noted.
In cases of asymptomatic bacteriuria, data show that antibiotic treatment does not improve outcomes, and in fact may increase the risk of subsequent UTI, said Dr. Vaughn. Elderly patients also are at increased risk for developing antibiotic-related adverse events, especially Clostridioides difficile. Asymptomatic bacteriuria is any bacteria in the urine in the absence of signs or symptoms of a UTI, even if lab tests show pyuria, nitrates, and resistant bacteria. These lab results are often associated with inappropriate antibiotic use. “The laboratory tests can’t distinguish between asymptomatic bacteriuria and a UTI, only the symptoms can,” she emphasized.
Contain treatment of community-acquired pneumonia
Another practical point for reducing antibiotics in the hospital setting is to limit treatment of community-acquired pneumonia (CAP) to 5 days when possible. Duration matters because for many diseases, shorter durations of antibiotic treatments are just as effective as longer durations based on the latest evidence. “This is a change in dogma,” from previous thinking that patients must complete a full course, and that anything less might promote antibiotic resistance, she said.
“In fact, longer antibiotic durations kill off more healthy, normal flora, select for resistant pathogens, increase the risk of C. difficile, and increase the risk of side effects,” she said.
Ultimately, the right treatment duration for pneumonia depends on several factors including patient factors, disease, clinical stability, and rate of improvement. However, a good rule of thumb is that approximately 89% of CAP patients need only 5 days of antibiotics as long as they are afebrile for 48 hours and have 1 or fewer vital sign abnormalities by day 5 of treatment. “We do need to prescribe longer durations for patients with complications,” she emphasized.
Revisit need for antibiotics at discharge
Hospitalists also can practice antibiotic stewardship by considering four points at patient discharge, said Dr. Vaughn.
First, consider whether antibiotics can be stopped. For example, antibiotics are not needed on discharge if infection is no longer the most likely diagnosis, or if the course of antibiotics has been completed, as is often the case for patients hospitalized with CAP, she noted.
Second, if the antibiotics can’t be stopped at the time of discharge, consider whether the preferred agent is being used. Third, be sure the patient is receiving the minimum duration of antibiotics, and fourth, be sure that the dose, indication, and total planned duration with start and stop dates is written in the discharge summary, said Dr. Vaughn. “This helps with communication to our outpatient providers as well as with education to the patients themselves.”
Bacterial coinfections rare in COVID-19
Dr. Vaughn concluded the session with data from a study she conducted with colleagues on the use of empiric antibacterial therapy and community-onset bacterial coinfection in hospitalized COVID-19 patients. The study included 1,667 patients at 32 hospitals in Michigan. The number of patients treated with antibiotics varied widely among hospitals, from 30% to as much as 90%, Dr. Vaughn said.
“What we found was that more than half of hospitalized patients with COVID (57%) received empiric antibiotic therapy in the first few days of hospitalization,” she said.
However, “despite all the antibiotic use, community-onset bacterial coinfections were rare,” and occurred in only 3.5% of the patients, meaning that the number needed to treat with antibiotics to prevent a single case was about 20.
Predictors of community-onset co-infections in the patients included older age, more severe disease, patients coming from nursing homes, and those with lower BMI or kidney disease, said Dr. Vaughn. She and her team also found that procalcitonin’s positive predictive value was 9.3%, but the negative predictive value was 98.3%, so these patients were extremely likely to have no coinfection.
Dr. Vaughn said that in her practice she might order procalcitonin when considering stopping antibiotics in a patient with COVID-19 and make a decision based on the negative predictive value, but she emphasized that she does not use it in the converse situation to rely on a positive value when deciding whether to start antibiotics in these patients.
Dr. Vaughn had no financial conflicts to disclose.
FROM SHM CONVERGE 2021
Mentor-mentee relationships in hospital medicine
Your mentor has been looking for someone to help lead a new project in your division, and tells you she’s been having a hard time finding someone – but that you would be great. The project isn’t something you are very interested in doing and you’re already swamped with other projects, but the mentor seems to need the help. What do you do?
Mentor-mentee relationships can be deeply beneficial, but the dynamics – in this situation and many others – can be complex. At SHM Converge, the annual conference of the Society of Hospital Medicine, panelists offered guidance on how best to navigate this terrain.
Vineet Arora, MD, MAPP, MHM, associate chief medical officer for clinical learning environment at the University of Chicago, suggested that, in the situation involving the mentor’s request to an uncertain mentee, the mentee should not give an immediate answer, but consider the pros and cons.
“It’s tough when it’s somebody who’s directly overseeing you,” she said. “If you’re really truly the best person, they’re going to want you in the job, and maybe they’ll make it work for you.” She said it would be important to find out why the mentor is having trouble finding someone, and suggested the mentee could find someone with whom to discuss it.
Calling mentoring a “team sport,” Dr. Arora described several types: the traditional mentor who helps many aspects of a mentee’s career, a “coach” who helps on a specific project or topic, a “sponsor” that can help elevate a mentee to a bigger opportunity, and a “connector” who can help a mentee begin new career relationships.
“Don’t invest in just one person,” she said. “Try to get that personal board of directors.”
She mentioned six things all mentors should do: Choose mentees carefully, establish a mentorship team, run a tight ship, head off rifts or resolve them, prepare for transitions when they take a new position and might have a new relationship with a mentee, and don’t commit “mentorship malpractice.”
Mentoring is a two-way street, with both people benefiting and learning, but mentoring can have its troubles, either through active, dysfunctional behavior that’s easy to spot, or passive behavior, such as the “bottleneck” problem when a mentor is too preoccupied with his or her own priorities to mentor well, the “country clubber” who mentors only for popularity and social capital but doesn’t do the work required, and the “world traveler” who is sought after but has little time for day-to-day mentoring.
Valerie Vaughan, MD, MSc, assistant professor of medicine at the University of Utah, described four “golden rules” of being a mentee. First, find a CAPE mentor (for capable, availability, projects of interest, and easy to get along with). Then, be respectful of a mentor’s time, communicate effectively, and be engaged and energizing.
“Mentors typically don’t get paid to mentor and so a lot of them are doing it because they find joy for doing it,” Dr. Vaughan said. “So as much as you can as a mentee, try to be the person who brings energy to the mentor-mentee relationship. It’s up to you to drive projects forward.”
Valerie Press, MD, MPH, SFHM, associate professor of medicine at the University of Chicago, offered tips for men who are mentoring women. She said that, while cross-gender mentorship is common and important, gender-based stereotypes and “unconscious assumptions” are alive and well. Women, she noted, have less access to mentorship and sponsorship, are paid less for the same work, and have high rates of attrition.
Male mentors have to meet the challenge of thinking outside of their own lived experience, combating stereotypes, and addressing these gender-based career disparities, she said.
She suggested that male mentors, for one thing, “rewrite gender scripts,” with comments such as, “This is a difficult situation, but I have confidence in you! What do you think your next move should be?” They should also “learn from each other on how to change the power dynamic,” and start and participate in conversations involving emotions, since they can be clues to what a mentee is experiencing.
When it comes to pushing for better policies, “be an upstander, not a bystander,” Dr. Press said.
“Use your organizational power and your social capital,” she said. “Use your voice to help make more equitable policies. Don’t just leave it to the women’s committee to come up with solutions to lack of lactation rooms, or paternity and maternity leave, or better daycare. These are family issues and everybody issues.”
Maylyn S. Martinez, MD, clinical associate professor of medicine at the University of Chicago, suggested that mentors for physicians from minority groups should resist the tendency to view their interests narrowly.
“Don’t assume that their interests are going to center on their gender or minority status – invite them to be on projects that have nothing to do with that,” she said. They should also not be encouraged to do projects that won’t help with career advancement any more than others would be encouraged to take on such projects.
“Be the solution,” she said. “Not the problem.”
Your mentor has been looking for someone to help lead a new project in your division, and tells you she’s been having a hard time finding someone – but that you would be great. The project isn’t something you are very interested in doing and you’re already swamped with other projects, but the mentor seems to need the help. What do you do?
Mentor-mentee relationships can be deeply beneficial, but the dynamics – in this situation and many others – can be complex. At SHM Converge, the annual conference of the Society of Hospital Medicine, panelists offered guidance on how best to navigate this terrain.
Vineet Arora, MD, MAPP, MHM, associate chief medical officer for clinical learning environment at the University of Chicago, suggested that, in the situation involving the mentor’s request to an uncertain mentee, the mentee should not give an immediate answer, but consider the pros and cons.
“It’s tough when it’s somebody who’s directly overseeing you,” she said. “If you’re really truly the best person, they’re going to want you in the job, and maybe they’ll make it work for you.” She said it would be important to find out why the mentor is having trouble finding someone, and suggested the mentee could find someone with whom to discuss it.
Calling mentoring a “team sport,” Dr. Arora described several types: the traditional mentor who helps many aspects of a mentee’s career, a “coach” who helps on a specific project or topic, a “sponsor” that can help elevate a mentee to a bigger opportunity, and a “connector” who can help a mentee begin new career relationships.
“Don’t invest in just one person,” she said. “Try to get that personal board of directors.”
She mentioned six things all mentors should do: Choose mentees carefully, establish a mentorship team, run a tight ship, head off rifts or resolve them, prepare for transitions when they take a new position and might have a new relationship with a mentee, and don’t commit “mentorship malpractice.”
Mentoring is a two-way street, with both people benefiting and learning, but mentoring can have its troubles, either through active, dysfunctional behavior that’s easy to spot, or passive behavior, such as the “bottleneck” problem when a mentor is too preoccupied with his or her own priorities to mentor well, the “country clubber” who mentors only for popularity and social capital but doesn’t do the work required, and the “world traveler” who is sought after but has little time for day-to-day mentoring.
Valerie Vaughan, MD, MSc, assistant professor of medicine at the University of Utah, described four “golden rules” of being a mentee. First, find a CAPE mentor (for capable, availability, projects of interest, and easy to get along with). Then, be respectful of a mentor’s time, communicate effectively, and be engaged and energizing.
“Mentors typically don’t get paid to mentor and so a lot of them are doing it because they find joy for doing it,” Dr. Vaughan said. “So as much as you can as a mentee, try to be the person who brings energy to the mentor-mentee relationship. It’s up to you to drive projects forward.”
Valerie Press, MD, MPH, SFHM, associate professor of medicine at the University of Chicago, offered tips for men who are mentoring women. She said that, while cross-gender mentorship is common and important, gender-based stereotypes and “unconscious assumptions” are alive and well. Women, she noted, have less access to mentorship and sponsorship, are paid less for the same work, and have high rates of attrition.
Male mentors have to meet the challenge of thinking outside of their own lived experience, combating stereotypes, and addressing these gender-based career disparities, she said.
She suggested that male mentors, for one thing, “rewrite gender scripts,” with comments such as, “This is a difficult situation, but I have confidence in you! What do you think your next move should be?” They should also “learn from each other on how to change the power dynamic,” and start and participate in conversations involving emotions, since they can be clues to what a mentee is experiencing.
When it comes to pushing for better policies, “be an upstander, not a bystander,” Dr. Press said.
“Use your organizational power and your social capital,” she said. “Use your voice to help make more equitable policies. Don’t just leave it to the women’s committee to come up with solutions to lack of lactation rooms, or paternity and maternity leave, or better daycare. These are family issues and everybody issues.”
Maylyn S. Martinez, MD, clinical associate professor of medicine at the University of Chicago, suggested that mentors for physicians from minority groups should resist the tendency to view their interests narrowly.
“Don’t assume that their interests are going to center on their gender or minority status – invite them to be on projects that have nothing to do with that,” she said. They should also not be encouraged to do projects that won’t help with career advancement any more than others would be encouraged to take on such projects.
“Be the solution,” she said. “Not the problem.”
Your mentor has been looking for someone to help lead a new project in your division, and tells you she’s been having a hard time finding someone – but that you would be great. The project isn’t something you are very interested in doing and you’re already swamped with other projects, but the mentor seems to need the help. What do you do?
Mentor-mentee relationships can be deeply beneficial, but the dynamics – in this situation and many others – can be complex. At SHM Converge, the annual conference of the Society of Hospital Medicine, panelists offered guidance on how best to navigate this terrain.
Vineet Arora, MD, MAPP, MHM, associate chief medical officer for clinical learning environment at the University of Chicago, suggested that, in the situation involving the mentor’s request to an uncertain mentee, the mentee should not give an immediate answer, but consider the pros and cons.
“It’s tough when it’s somebody who’s directly overseeing you,” she said. “If you’re really truly the best person, they’re going to want you in the job, and maybe they’ll make it work for you.” She said it would be important to find out why the mentor is having trouble finding someone, and suggested the mentee could find someone with whom to discuss it.
Calling mentoring a “team sport,” Dr. Arora described several types: the traditional mentor who helps many aspects of a mentee’s career, a “coach” who helps on a specific project or topic, a “sponsor” that can help elevate a mentee to a bigger opportunity, and a “connector” who can help a mentee begin new career relationships.
“Don’t invest in just one person,” she said. “Try to get that personal board of directors.”
She mentioned six things all mentors should do: Choose mentees carefully, establish a mentorship team, run a tight ship, head off rifts or resolve them, prepare for transitions when they take a new position and might have a new relationship with a mentee, and don’t commit “mentorship malpractice.”
Mentoring is a two-way street, with both people benefiting and learning, but mentoring can have its troubles, either through active, dysfunctional behavior that’s easy to spot, or passive behavior, such as the “bottleneck” problem when a mentor is too preoccupied with his or her own priorities to mentor well, the “country clubber” who mentors only for popularity and social capital but doesn’t do the work required, and the “world traveler” who is sought after but has little time for day-to-day mentoring.
Valerie Vaughan, MD, MSc, assistant professor of medicine at the University of Utah, described four “golden rules” of being a mentee. First, find a CAPE mentor (for capable, availability, projects of interest, and easy to get along with). Then, be respectful of a mentor’s time, communicate effectively, and be engaged and energizing.
“Mentors typically don’t get paid to mentor and so a lot of them are doing it because they find joy for doing it,” Dr. Vaughan said. “So as much as you can as a mentee, try to be the person who brings energy to the mentor-mentee relationship. It’s up to you to drive projects forward.”
Valerie Press, MD, MPH, SFHM, associate professor of medicine at the University of Chicago, offered tips for men who are mentoring women. She said that, while cross-gender mentorship is common and important, gender-based stereotypes and “unconscious assumptions” are alive and well. Women, she noted, have less access to mentorship and sponsorship, are paid less for the same work, and have high rates of attrition.
Male mentors have to meet the challenge of thinking outside of their own lived experience, combating stereotypes, and addressing these gender-based career disparities, she said.
She suggested that male mentors, for one thing, “rewrite gender scripts,” with comments such as, “This is a difficult situation, but I have confidence in you! What do you think your next move should be?” They should also “learn from each other on how to change the power dynamic,” and start and participate in conversations involving emotions, since they can be clues to what a mentee is experiencing.
When it comes to pushing for better policies, “be an upstander, not a bystander,” Dr. Press said.
“Use your organizational power and your social capital,” she said. “Use your voice to help make more equitable policies. Don’t just leave it to the women’s committee to come up with solutions to lack of lactation rooms, or paternity and maternity leave, or better daycare. These are family issues and everybody issues.”
Maylyn S. Martinez, MD, clinical associate professor of medicine at the University of Chicago, suggested that mentors for physicians from minority groups should resist the tendency to view their interests narrowly.
“Don’t assume that their interests are going to center on their gender or minority status – invite them to be on projects that have nothing to do with that,” she said. They should also not be encouraged to do projects that won’t help with career advancement any more than others would be encouraged to take on such projects.
“Be the solution,” she said. “Not the problem.”
FROM SHM CONVERGE 2021
CDC recommends use of Pfizer’s COVID vaccine in 12- to 15-year-olds
The Centers for Disease Control and Prevention’s director Rochelle Walensky, MD, signed off on an advisory panel’s recommendation May 12 endorsing the use of the Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12-15 years.
Earlier in the day the CDC’s Advisory Committee on Immunization Practices voted 14-0 in favor of the safety and effectiveness of the vaccine in younger teens.
Dr. Walensky said in an official statement.
The Food and Drug Administration on May 10 issued an emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 in individuals 12-15 years old. The FDA first cleared the Pfizer-BioNTech vaccine through an EUA in December 2020 for those ages 16 and older. Pfizer this month also initiated steps with the FDA toward a full approval of its vaccine.
Dr. Walenksy urged parents to seriously consider vaccinating their children.
“Understandably, some parents want more information before their children receive a vaccine,” she said. “I encourage parents with questions to talk to your child’s healthcare provider or your family doctor to learn more about the vaccine.”
Vaccine “safe and effective”
Separately, the American Academy of Pediatrics issued a statement May 12 in support of vaccinating all children ages 12 and older who are eligible for the federally authorized COVID-19 vaccine.
“As a pediatrician and a parent, I have looked forward to getting my own children and patients vaccinated, and I am thrilled that those ages 12 and older can now be protected,” said AAP President Lee Savio Beers, MD, in a statement. “The data continue to show that this vaccine is safe and effective. I urge all parents to call their pediatrician to learn more about how to get their children and teens vaccinated.”
The expanded clearance for the Pfizer vaccine is seen as a critical step for allowing teens to resume activities on which they missed out during the pandemic.
“We’ve seen the harm done to children’s mental and emotional health as they’ve missed out on so many experiences during the pandemic,” Dr. Beers said. “Vaccinating children will protect them and allow them to fully engage in all of the activities – school, sports, socializing with friends and family – that are so important to their health and development.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s director Rochelle Walensky, MD, signed off on an advisory panel’s recommendation May 12 endorsing the use of the Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12-15 years.
Earlier in the day the CDC’s Advisory Committee on Immunization Practices voted 14-0 in favor of the safety and effectiveness of the vaccine in younger teens.
Dr. Walensky said in an official statement.
The Food and Drug Administration on May 10 issued an emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 in individuals 12-15 years old. The FDA first cleared the Pfizer-BioNTech vaccine through an EUA in December 2020 for those ages 16 and older. Pfizer this month also initiated steps with the FDA toward a full approval of its vaccine.
Dr. Walenksy urged parents to seriously consider vaccinating their children.
“Understandably, some parents want more information before their children receive a vaccine,” she said. “I encourage parents with questions to talk to your child’s healthcare provider or your family doctor to learn more about the vaccine.”
Vaccine “safe and effective”
Separately, the American Academy of Pediatrics issued a statement May 12 in support of vaccinating all children ages 12 and older who are eligible for the federally authorized COVID-19 vaccine.
“As a pediatrician and a parent, I have looked forward to getting my own children and patients vaccinated, and I am thrilled that those ages 12 and older can now be protected,” said AAP President Lee Savio Beers, MD, in a statement. “The data continue to show that this vaccine is safe and effective. I urge all parents to call their pediatrician to learn more about how to get their children and teens vaccinated.”
The expanded clearance for the Pfizer vaccine is seen as a critical step for allowing teens to resume activities on which they missed out during the pandemic.
“We’ve seen the harm done to children’s mental and emotional health as they’ve missed out on so many experiences during the pandemic,” Dr. Beers said. “Vaccinating children will protect them and allow them to fully engage in all of the activities – school, sports, socializing with friends and family – that are so important to their health and development.”
A version of this article first appeared on Medscape.com.
The Centers for Disease Control and Prevention’s director Rochelle Walensky, MD, signed off on an advisory panel’s recommendation May 12 endorsing the use of the Pfizer-BioNTech COVID-19 vaccine in adolescents aged 12-15 years.
Earlier in the day the CDC’s Advisory Committee on Immunization Practices voted 14-0 in favor of the safety and effectiveness of the vaccine in younger teens.
Dr. Walensky said in an official statement.
The Food and Drug Administration on May 10 issued an emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine for the prevention of COVID-19 in individuals 12-15 years old. The FDA first cleared the Pfizer-BioNTech vaccine through an EUA in December 2020 for those ages 16 and older. Pfizer this month also initiated steps with the FDA toward a full approval of its vaccine.
Dr. Walenksy urged parents to seriously consider vaccinating their children.
“Understandably, some parents want more information before their children receive a vaccine,” she said. “I encourage parents with questions to talk to your child’s healthcare provider or your family doctor to learn more about the vaccine.”
Vaccine “safe and effective”
Separately, the American Academy of Pediatrics issued a statement May 12 in support of vaccinating all children ages 12 and older who are eligible for the federally authorized COVID-19 vaccine.
“As a pediatrician and a parent, I have looked forward to getting my own children and patients vaccinated, and I am thrilled that those ages 12 and older can now be protected,” said AAP President Lee Savio Beers, MD, in a statement. “The data continue to show that this vaccine is safe and effective. I urge all parents to call their pediatrician to learn more about how to get their children and teens vaccinated.”
The expanded clearance for the Pfizer vaccine is seen as a critical step for allowing teens to resume activities on which they missed out during the pandemic.
“We’ve seen the harm done to children’s mental and emotional health as they’ve missed out on so many experiences during the pandemic,” Dr. Beers said. “Vaccinating children will protect them and allow them to fully engage in all of the activities – school, sports, socializing with friends and family – that are so important to their health and development.”
A version of this article first appeared on Medscape.com.
Reassuring data on impact of mild COVID-19 on the heart
Six months after mild SARS-CoV-2 infection in a representative health care workforce, no long-term cardiovascular sequelae were detected, compared with a matched SARS-CoV-2 seronegative group.
“Mild COVID-19 left no measurable cardiovascular impact on LV structure, function, scar burden, aortic stiffness, or serum biomarkers,” the researchers reported in an article published online May 8 in JACC: Cardiovascular Imaging.
“We provide societal reassurance and support for the position that screening in asymptomatic individuals following mild disease is not indicated,” first author George Joy, MBBS, University College London, said in presenting the results at EuroCMR, the annual CMR congress of the European Association of Cardiovascular Imaging (EACVI).
Briefing comoderator Leyla Elif Sade, MD, University of Baskent, Ankara, Turkey, said, “This is the hot topic of our time because of obvious reasons and I think [this] study is quite important to avoid unnecessary further testing, surveillance testing, and to avoid a significant burden of health care costs.”
‘Alarming’ early data
Early cardiac magnetic resonance (CMR) studies in patients recovered from mild COVID-19 were “alarming,” Dr. Joy said.
As previously reported, one study showed cardiac abnormalities after mild COVID-19 in up to 78% of patients, with evidence of ongoing myocardial inflammation in 60%. The CMR findings correlated with elevations in troponin T by high-sensitivity assay (hs-TnT).
To investigate further, Dr. Joy and colleagues did a nested case-control study within the COVIDsortium, a prospective study of 731 health care workers from three London hospitals who underwent weekly symptom, polymerase chain reaction, and serology assessment over 4 months during the first wave of the pandemic.
A total of 157 (21.5%) participants seroconverted during the study period.
Six months after infection, 74 seropositive (median age, 39; 62% women) and 75 age-, sex-, and ethnicity-matched seronegative controls underwent cardiovascular phenotyping (comprehensive phantom-calibrated CMR and blood biomarkers). The analysis was blinded, using objective artificial intelligence analytics when available.
The results showed no statistically significant differences between seropositive and seronegative participants in cardiac structure (left ventricular volumes, mass, atrial area), function (ejection fraction, global longitudinal shortening, aortic distensibility), tissue characterization (T1, T2, extracellular volume fraction mapping, late gadolinium enhancement) or biomarkers (troponin, N-terminal pro–B-type natriuretic peptide).
Cardiovascular abnormalities were no more common in seropositive than seronegative otherwise healthy health care workers 6 months post mild SARS-CoV-2 infection. Measured abnormalities were “evenly distributed between both groups,” Dr. Joy said.
Therefore, it’s “important to reassure patients with mild SARS-CoV-2 infection regarding its cardiovascular effects,” Dr. Joy and colleagues concluded.
Limitations and caveats
They caution, however, that the study provides insight only into the short- to medium-term sequelae of patients aged 18-69 with mild COVID-19 who did not require hospitalization and had low numbers of comorbidities.
The study does not address the cardiovascular effects after severe COVID-19 infection requiring hospitalization or in those with multiple comorbid conditions, they noted. It also does not prove that apparently mild SARS-CoV-2 never causes chronic myocarditis.
“The study design would not distinguish between people who had sustained completely healed myocarditis and pericarditis and those in whom the heart had never been affected,” the researchers noted.
They pointed to a recent cross-sectional study of athletes 1-month post mild COVID-19 that found significant pericardial involvement (late enhancement and/or pericardial effusion), although no baseline pre-COVID-19 imaging was performed. In the current study at 6 months post infection the pericardium was normal.
The coauthors of a linked editorial say this study provides “welcome, reassuring information that in healthy individuals who experience mild infection with COVID-19, persisting evidence of cardiovascular complications is very uncommon. The results do not support cardiovascular screening in individuals with mild or asymptomatic infection with COVID-19.”
Colin Berry, PhD, and Kenneth Mangion, PhD, both from University of Glasgow, cautioned that the population is restricted to health care workers; therefore, the findings may not necessarily be generalized to a community population .
“Healthcare workers do not reflect the population of individuals most clinically affected by COVID-19 illness. The severity of acute COVID-19 infection is greatest in older individuals and those with preexisting health problems. Healthcare workers are not representative of the wider, unselected, at-risk, community population,” they pointed out.
Cardiovascular risk factors and concomitant health problems (heart and respiratory disease) may be more common in the community than in health care workers, and prior studies have highlighted their potential impact for disease pathogenesis in COVID-19.
Dr. Berry and Dr. Mangion also noted that women made up nearly two-thirds of the seropositive group. This may reflect a selection bias or may naturally reflect the fact that proportionately more women are asymptomatic or have milder forms of illness, whereas severe SARS-CoV-2 infection requiring hospitalization affects men to a greater degree.
COVIDsortium funding was donated by individuals, charitable trusts, and corporations including Goldman Sachs, Citadel and Citadel Securities, The Guy Foundation, GW Pharmaceuticals, Kusuma Trust, and Jagclif Charitable Trust, and enabled by Barts Charity with support from UCLH Charity. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Six months after mild SARS-CoV-2 infection in a representative health care workforce, no long-term cardiovascular sequelae were detected, compared with a matched SARS-CoV-2 seronegative group.
“Mild COVID-19 left no measurable cardiovascular impact on LV structure, function, scar burden, aortic stiffness, or serum biomarkers,” the researchers reported in an article published online May 8 in JACC: Cardiovascular Imaging.
“We provide societal reassurance and support for the position that screening in asymptomatic individuals following mild disease is not indicated,” first author George Joy, MBBS, University College London, said in presenting the results at EuroCMR, the annual CMR congress of the European Association of Cardiovascular Imaging (EACVI).
Briefing comoderator Leyla Elif Sade, MD, University of Baskent, Ankara, Turkey, said, “This is the hot topic of our time because of obvious reasons and I think [this] study is quite important to avoid unnecessary further testing, surveillance testing, and to avoid a significant burden of health care costs.”
‘Alarming’ early data
Early cardiac magnetic resonance (CMR) studies in patients recovered from mild COVID-19 were “alarming,” Dr. Joy said.
As previously reported, one study showed cardiac abnormalities after mild COVID-19 in up to 78% of patients, with evidence of ongoing myocardial inflammation in 60%. The CMR findings correlated with elevations in troponin T by high-sensitivity assay (hs-TnT).
To investigate further, Dr. Joy and colleagues did a nested case-control study within the COVIDsortium, a prospective study of 731 health care workers from three London hospitals who underwent weekly symptom, polymerase chain reaction, and serology assessment over 4 months during the first wave of the pandemic.
A total of 157 (21.5%) participants seroconverted during the study period.
Six months after infection, 74 seropositive (median age, 39; 62% women) and 75 age-, sex-, and ethnicity-matched seronegative controls underwent cardiovascular phenotyping (comprehensive phantom-calibrated CMR and blood biomarkers). The analysis was blinded, using objective artificial intelligence analytics when available.
The results showed no statistically significant differences between seropositive and seronegative participants in cardiac structure (left ventricular volumes, mass, atrial area), function (ejection fraction, global longitudinal shortening, aortic distensibility), tissue characterization (T1, T2, extracellular volume fraction mapping, late gadolinium enhancement) or biomarkers (troponin, N-terminal pro–B-type natriuretic peptide).
Cardiovascular abnormalities were no more common in seropositive than seronegative otherwise healthy health care workers 6 months post mild SARS-CoV-2 infection. Measured abnormalities were “evenly distributed between both groups,” Dr. Joy said.
Therefore, it’s “important to reassure patients with mild SARS-CoV-2 infection regarding its cardiovascular effects,” Dr. Joy and colleagues concluded.
Limitations and caveats
They caution, however, that the study provides insight only into the short- to medium-term sequelae of patients aged 18-69 with mild COVID-19 who did not require hospitalization and had low numbers of comorbidities.
The study does not address the cardiovascular effects after severe COVID-19 infection requiring hospitalization or in those with multiple comorbid conditions, they noted. It also does not prove that apparently mild SARS-CoV-2 never causes chronic myocarditis.
“The study design would not distinguish between people who had sustained completely healed myocarditis and pericarditis and those in whom the heart had never been affected,” the researchers noted.
They pointed to a recent cross-sectional study of athletes 1-month post mild COVID-19 that found significant pericardial involvement (late enhancement and/or pericardial effusion), although no baseline pre-COVID-19 imaging was performed. In the current study at 6 months post infection the pericardium was normal.
The coauthors of a linked editorial say this study provides “welcome, reassuring information that in healthy individuals who experience mild infection with COVID-19, persisting evidence of cardiovascular complications is very uncommon. The results do not support cardiovascular screening in individuals with mild or asymptomatic infection with COVID-19.”
Colin Berry, PhD, and Kenneth Mangion, PhD, both from University of Glasgow, cautioned that the population is restricted to health care workers; therefore, the findings may not necessarily be generalized to a community population .
“Healthcare workers do not reflect the population of individuals most clinically affected by COVID-19 illness. The severity of acute COVID-19 infection is greatest in older individuals and those with preexisting health problems. Healthcare workers are not representative of the wider, unselected, at-risk, community population,” they pointed out.
Cardiovascular risk factors and concomitant health problems (heart and respiratory disease) may be more common in the community than in health care workers, and prior studies have highlighted their potential impact for disease pathogenesis in COVID-19.
Dr. Berry and Dr. Mangion also noted that women made up nearly two-thirds of the seropositive group. This may reflect a selection bias or may naturally reflect the fact that proportionately more women are asymptomatic or have milder forms of illness, whereas severe SARS-CoV-2 infection requiring hospitalization affects men to a greater degree.
COVIDsortium funding was donated by individuals, charitable trusts, and corporations including Goldman Sachs, Citadel and Citadel Securities, The Guy Foundation, GW Pharmaceuticals, Kusuma Trust, and Jagclif Charitable Trust, and enabled by Barts Charity with support from UCLH Charity. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Six months after mild SARS-CoV-2 infection in a representative health care workforce, no long-term cardiovascular sequelae were detected, compared with a matched SARS-CoV-2 seronegative group.
“Mild COVID-19 left no measurable cardiovascular impact on LV structure, function, scar burden, aortic stiffness, or serum biomarkers,” the researchers reported in an article published online May 8 in JACC: Cardiovascular Imaging.
“We provide societal reassurance and support for the position that screening in asymptomatic individuals following mild disease is not indicated,” first author George Joy, MBBS, University College London, said in presenting the results at EuroCMR, the annual CMR congress of the European Association of Cardiovascular Imaging (EACVI).
Briefing comoderator Leyla Elif Sade, MD, University of Baskent, Ankara, Turkey, said, “This is the hot topic of our time because of obvious reasons and I think [this] study is quite important to avoid unnecessary further testing, surveillance testing, and to avoid a significant burden of health care costs.”
‘Alarming’ early data
Early cardiac magnetic resonance (CMR) studies in patients recovered from mild COVID-19 were “alarming,” Dr. Joy said.
As previously reported, one study showed cardiac abnormalities after mild COVID-19 in up to 78% of patients, with evidence of ongoing myocardial inflammation in 60%. The CMR findings correlated with elevations in troponin T by high-sensitivity assay (hs-TnT).
To investigate further, Dr. Joy and colleagues did a nested case-control study within the COVIDsortium, a prospective study of 731 health care workers from three London hospitals who underwent weekly symptom, polymerase chain reaction, and serology assessment over 4 months during the first wave of the pandemic.
A total of 157 (21.5%) participants seroconverted during the study period.
Six months after infection, 74 seropositive (median age, 39; 62% women) and 75 age-, sex-, and ethnicity-matched seronegative controls underwent cardiovascular phenotyping (comprehensive phantom-calibrated CMR and blood biomarkers). The analysis was blinded, using objective artificial intelligence analytics when available.
The results showed no statistically significant differences between seropositive and seronegative participants in cardiac structure (left ventricular volumes, mass, atrial area), function (ejection fraction, global longitudinal shortening, aortic distensibility), tissue characterization (T1, T2, extracellular volume fraction mapping, late gadolinium enhancement) or biomarkers (troponin, N-terminal pro–B-type natriuretic peptide).
Cardiovascular abnormalities were no more common in seropositive than seronegative otherwise healthy health care workers 6 months post mild SARS-CoV-2 infection. Measured abnormalities were “evenly distributed between both groups,” Dr. Joy said.
Therefore, it’s “important to reassure patients with mild SARS-CoV-2 infection regarding its cardiovascular effects,” Dr. Joy and colleagues concluded.
Limitations and caveats
They caution, however, that the study provides insight only into the short- to medium-term sequelae of patients aged 18-69 with mild COVID-19 who did not require hospitalization and had low numbers of comorbidities.
The study does not address the cardiovascular effects after severe COVID-19 infection requiring hospitalization or in those with multiple comorbid conditions, they noted. It also does not prove that apparently mild SARS-CoV-2 never causes chronic myocarditis.
“The study design would not distinguish between people who had sustained completely healed myocarditis and pericarditis and those in whom the heart had never been affected,” the researchers noted.
They pointed to a recent cross-sectional study of athletes 1-month post mild COVID-19 that found significant pericardial involvement (late enhancement and/or pericardial effusion), although no baseline pre-COVID-19 imaging was performed. In the current study at 6 months post infection the pericardium was normal.
The coauthors of a linked editorial say this study provides “welcome, reassuring information that in healthy individuals who experience mild infection with COVID-19, persisting evidence of cardiovascular complications is very uncommon. The results do not support cardiovascular screening in individuals with mild or asymptomatic infection with COVID-19.”
Colin Berry, PhD, and Kenneth Mangion, PhD, both from University of Glasgow, cautioned that the population is restricted to health care workers; therefore, the findings may not necessarily be generalized to a community population .
“Healthcare workers do not reflect the population of individuals most clinically affected by COVID-19 illness. The severity of acute COVID-19 infection is greatest in older individuals and those with preexisting health problems. Healthcare workers are not representative of the wider, unselected, at-risk, community population,” they pointed out.
Cardiovascular risk factors and concomitant health problems (heart and respiratory disease) may be more common in the community than in health care workers, and prior studies have highlighted their potential impact for disease pathogenesis in COVID-19.
Dr. Berry and Dr. Mangion also noted that women made up nearly two-thirds of the seropositive group. This may reflect a selection bias or may naturally reflect the fact that proportionately more women are asymptomatic or have milder forms of illness, whereas severe SARS-CoV-2 infection requiring hospitalization affects men to a greater degree.
COVIDsortium funding was donated by individuals, charitable trusts, and corporations including Goldman Sachs, Citadel and Citadel Securities, The Guy Foundation, GW Pharmaceuticals, Kusuma Trust, and Jagclif Charitable Trust, and enabled by Barts Charity with support from UCLH Charity. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
What to know about COVID-19 vaccines and skin reactions
The good news is that these side effects tend to be minor and vanish within a few days, Esther Freeman, MD, PhD, said in a presentation at the American Academy of Dermatology Virtual Meeting Experience.
“The reality is actually very reassuring,” Dr. Freeman said, especially in light of what is currently known about when the rashes occur and how anaphylaxis is extremely uncommon. Now, she added, dermatologists can tell patients who had reactions to their initial vaccination that “we know you had this big reaction, and we know that it was upsetting and uncomfortable. But it may not happen the second time around. And if it does, [the reaction is] probably going to be smaller.”
Dr. Freeman, associate professor of dermatology at Harvard Medical School, Boston, highlighted a study published in the Journal of the American Academy of Dermatology that she coauthored with dermatologists across the United States. The researchers tracked 414 cutaneous reactions to the Moderna (83%) and Pfizer (17%) COVID-19 vaccines in a group of patients, which was 90% female, 78% White, and mostly from the United States. Their average age was 44 years. The cases were reported to the AAD–International League of Dermatological Societies registry of COVID-19 cutaneous manifestations.
While most were women, “it’s a little hard to know if this is really going to end up being a true finding,” said Dr. Freeman, the registry’s principal investigator and a member of the AAD’s COVID-19 Ad Hoc Task Force. “If you think about who got vaccinated early, it was health care providers, and the American health care workforce is over 70% female. So I think there’s a little bit of bias here. There may also be a bias because women may be slightly more likely to report or go to their health care provider for a rash.”
Delayed large local reactions were the most common, accounting for 66% (175 cases) of the 267 skin reactions reported after the first Moderna vaccine dose and 30% (31 cases) of the 102 reactions reported after the second dose. These reactions represented 15% (5 cases) of the 34 skin reactions reported after the first Pfizer vaccine dose and 18% (7 cases) of the 40 reactions after the second dose.
There are two peaks with that first dose, Dr. Freeman said. “There’s a peak around day 2 or 3. And there’s another peak around day 7 or 8 with some of these reactions. Only 27% who had a reaction with the first dose had the same reaction with the second.” She added that these reactions “are not cellulitis and don’t require antibiotics.”
Other more common reactions included local injection-site reactions (swelling, erythema, and pain), urticaria (after 24 hours in almost all cases, occurring at a higher rate in patients who received the Pfizer vaccine), and morbilliform eruptions.
Dr. Freeman said that patients may experience redness and swelling in the hands and feet that can be “very uncomfortable.” She described one patient “who was having a hard time actually closing his fist, just because of the amount of swelling and redness in his hand. It did resolve, and it’s important to reassure your patients it will go away.”
According to this study, less common reports of other cutaneous findings with both vaccines included 9 reports of swelling at the site of cosmetic fillers, 8 reports of pernio/chilblains, 10 reports of varicella zoster, 4 reports of herpes simplex flares, 4 pityriasis rosea–like reactions, and 4 rashes in infants of vaccinated breastfeeding mothers.
The study noted that “patients responded well to topical corticosteroids, oral antihistamines, and/or pain-relieving medications. These reactions resolved after a median of 3-4 days.”
It’s important to understand that none of the patients developed anaphylaxis after the second dose even if they’d had a reaction to the first dose, Dr. Freeman said. “But I should point out that we’re talking about reactions that have started more than 4 hours after the vaccine. If a rash such as a urticaria specifically starts within 4 hours of vaccination, that’s in a different category. Those are considered more immediate allergic reactions, and those patients need to be seen by allergy before a second dose.”
Dr. Freeman added that “it’s really interesting to think about how our bodies are really reacting to the vaccine in a way that’s mimicking our body’s reactions to COVID-19.” For example, some patients who got vaccinated developed chilblains similar to the “COVID toes” described in infected patients, apparently as part of the body’s immune response to the virus. “We’ve seen this in patients who actually had COVID and had prior COVID toes and then actually got a flare with their vaccine. And then we’ve also seen it in patients who never had COVID.”
In regard to general advice for patients, she said, “I do still encourage my patients who previously had COVID to go ahead and get the vaccine even if they had a skin manifestation with COVID.”
Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, said she has have seen only a handful of cases of delayed large local reactions and local injection site reactions after COVID-19 vaccination. “I have seen a significant number of cases of acute urticaria following the first and second doses,” she said in an interview. “However, it is important to keep in mind that we cannot determine cause and effect for the cases of acute urticaria. They may or may not be vaccine related.”
Fortunately, none of the adverse effects she’s seen have been severe. “It is important that dermatologists educate the public and their patients that most people do not develop any skin reaction in response to the vaccine,” she said. In the minority who do, “reactions tend to be mild and are not life-threatening. Many of these skin reactions resolve on their own without treatment.”
She added that “patients with pernio/chilblains or herpes zoster following vaccination should be referred by a board-certified dermatologist for prompt treatment and to avoid sequelae.”
‘COVID vaccine arm’
Delayed local reactions to the Moderna vaccine were also described in a report published online on May 12, 2021, in JAMA Dermatology, after the AAD meeting, in 16 patients referred to the Yale New Haven (Conn.) Hospital Dermatology service who experienced delayed localized cutaneous hypersensitivity reactions a median of 7 days after receiving the vaccine (range, 2-12 days), from Jan. 20 to Feb. 12, 2021. No such cases were reported in Pfizer vaccine recipients.
Of the 16 patients, whose median age was 38 years and who were mostly women, 15 developed the reaction after the first dose, described as “pruritic and variably painful erythematous reactions near the injection site,” which lasted a median of 5 days (range, 1-21 days). After the second dose, 12 of the 16 patients developed injection-site reactions (including one patient who had no reaction after dose 1), a median of 2 days after the vaccine was administered (range, 0-5 days). Histologic results of a biopsy in one patient with a reaction to the second dose “ demonstrated mild predominantly perivascular and focal interstitial mixed infiltrate with lymphocytes and eosinophils consistent with a dermal hypersensitivity reaction,” wrote Alicia J. Little, MD, PhD, of the department of dermatology, Yale University, New Haven, and coauthors.
Compared with immediate hypersensitivity reactions, occurring within 4 hours of vaccination, such as anaphylaxis and urticaria, they concluded that “these delayed localized hypersensitivity reactions are not a contraindication to subsequent vaccination,” and they proposed that they be named “COVID vaccine arm.”
Dr. Freeman reported no disclosures. Dr. Lipner also had no relevant disclosures. Dr. Little reported receiving a grant from the National Center for Advancing Translational Science and a Women’s Health Career Development Award from the Dermatology Foundation while the study was conducted; another author reported equity in Johnson & Johnson in his spouse’s retirement fund outside the submitted work.
The good news is that these side effects tend to be minor and vanish within a few days, Esther Freeman, MD, PhD, said in a presentation at the American Academy of Dermatology Virtual Meeting Experience.
“The reality is actually very reassuring,” Dr. Freeman said, especially in light of what is currently known about when the rashes occur and how anaphylaxis is extremely uncommon. Now, she added, dermatologists can tell patients who had reactions to their initial vaccination that “we know you had this big reaction, and we know that it was upsetting and uncomfortable. But it may not happen the second time around. And if it does, [the reaction is] probably going to be smaller.”
Dr. Freeman, associate professor of dermatology at Harvard Medical School, Boston, highlighted a study published in the Journal of the American Academy of Dermatology that she coauthored with dermatologists across the United States. The researchers tracked 414 cutaneous reactions to the Moderna (83%) and Pfizer (17%) COVID-19 vaccines in a group of patients, which was 90% female, 78% White, and mostly from the United States. Their average age was 44 years. The cases were reported to the AAD–International League of Dermatological Societies registry of COVID-19 cutaneous manifestations.
While most were women, “it’s a little hard to know if this is really going to end up being a true finding,” said Dr. Freeman, the registry’s principal investigator and a member of the AAD’s COVID-19 Ad Hoc Task Force. “If you think about who got vaccinated early, it was health care providers, and the American health care workforce is over 70% female. So I think there’s a little bit of bias here. There may also be a bias because women may be slightly more likely to report or go to their health care provider for a rash.”
Delayed large local reactions were the most common, accounting for 66% (175 cases) of the 267 skin reactions reported after the first Moderna vaccine dose and 30% (31 cases) of the 102 reactions reported after the second dose. These reactions represented 15% (5 cases) of the 34 skin reactions reported after the first Pfizer vaccine dose and 18% (7 cases) of the 40 reactions after the second dose.
There are two peaks with that first dose, Dr. Freeman said. “There’s a peak around day 2 or 3. And there’s another peak around day 7 or 8 with some of these reactions. Only 27% who had a reaction with the first dose had the same reaction with the second.” She added that these reactions “are not cellulitis and don’t require antibiotics.”
Other more common reactions included local injection-site reactions (swelling, erythema, and pain), urticaria (after 24 hours in almost all cases, occurring at a higher rate in patients who received the Pfizer vaccine), and morbilliform eruptions.
Dr. Freeman said that patients may experience redness and swelling in the hands and feet that can be “very uncomfortable.” She described one patient “who was having a hard time actually closing his fist, just because of the amount of swelling and redness in his hand. It did resolve, and it’s important to reassure your patients it will go away.”
According to this study, less common reports of other cutaneous findings with both vaccines included 9 reports of swelling at the site of cosmetic fillers, 8 reports of pernio/chilblains, 10 reports of varicella zoster, 4 reports of herpes simplex flares, 4 pityriasis rosea–like reactions, and 4 rashes in infants of vaccinated breastfeeding mothers.
The study noted that “patients responded well to topical corticosteroids, oral antihistamines, and/or pain-relieving medications. These reactions resolved after a median of 3-4 days.”
It’s important to understand that none of the patients developed anaphylaxis after the second dose even if they’d had a reaction to the first dose, Dr. Freeman said. “But I should point out that we’re talking about reactions that have started more than 4 hours after the vaccine. If a rash such as a urticaria specifically starts within 4 hours of vaccination, that’s in a different category. Those are considered more immediate allergic reactions, and those patients need to be seen by allergy before a second dose.”
Dr. Freeman added that “it’s really interesting to think about how our bodies are really reacting to the vaccine in a way that’s mimicking our body’s reactions to COVID-19.” For example, some patients who got vaccinated developed chilblains similar to the “COVID toes” described in infected patients, apparently as part of the body’s immune response to the virus. “We’ve seen this in patients who actually had COVID and had prior COVID toes and then actually got a flare with their vaccine. And then we’ve also seen it in patients who never had COVID.”
In regard to general advice for patients, she said, “I do still encourage my patients who previously had COVID to go ahead and get the vaccine even if they had a skin manifestation with COVID.”
Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, said she has have seen only a handful of cases of delayed large local reactions and local injection site reactions after COVID-19 vaccination. “I have seen a significant number of cases of acute urticaria following the first and second doses,” she said in an interview. “However, it is important to keep in mind that we cannot determine cause and effect for the cases of acute urticaria. They may or may not be vaccine related.”
Fortunately, none of the adverse effects she’s seen have been severe. “It is important that dermatologists educate the public and their patients that most people do not develop any skin reaction in response to the vaccine,” she said. In the minority who do, “reactions tend to be mild and are not life-threatening. Many of these skin reactions resolve on their own without treatment.”
She added that “patients with pernio/chilblains or herpes zoster following vaccination should be referred by a board-certified dermatologist for prompt treatment and to avoid sequelae.”
‘COVID vaccine arm’
Delayed local reactions to the Moderna vaccine were also described in a report published online on May 12, 2021, in JAMA Dermatology, after the AAD meeting, in 16 patients referred to the Yale New Haven (Conn.) Hospital Dermatology service who experienced delayed localized cutaneous hypersensitivity reactions a median of 7 days after receiving the vaccine (range, 2-12 days), from Jan. 20 to Feb. 12, 2021. No such cases were reported in Pfizer vaccine recipients.
Of the 16 patients, whose median age was 38 years and who were mostly women, 15 developed the reaction after the first dose, described as “pruritic and variably painful erythematous reactions near the injection site,” which lasted a median of 5 days (range, 1-21 days). After the second dose, 12 of the 16 patients developed injection-site reactions (including one patient who had no reaction after dose 1), a median of 2 days after the vaccine was administered (range, 0-5 days). Histologic results of a biopsy in one patient with a reaction to the second dose “ demonstrated mild predominantly perivascular and focal interstitial mixed infiltrate with lymphocytes and eosinophils consistent with a dermal hypersensitivity reaction,” wrote Alicia J. Little, MD, PhD, of the department of dermatology, Yale University, New Haven, and coauthors.
Compared with immediate hypersensitivity reactions, occurring within 4 hours of vaccination, such as anaphylaxis and urticaria, they concluded that “these delayed localized hypersensitivity reactions are not a contraindication to subsequent vaccination,” and they proposed that they be named “COVID vaccine arm.”
Dr. Freeman reported no disclosures. Dr. Lipner also had no relevant disclosures. Dr. Little reported receiving a grant from the National Center for Advancing Translational Science and a Women’s Health Career Development Award from the Dermatology Foundation while the study was conducted; another author reported equity in Johnson & Johnson in his spouse’s retirement fund outside the submitted work.
The good news is that these side effects tend to be minor and vanish within a few days, Esther Freeman, MD, PhD, said in a presentation at the American Academy of Dermatology Virtual Meeting Experience.
“The reality is actually very reassuring,” Dr. Freeman said, especially in light of what is currently known about when the rashes occur and how anaphylaxis is extremely uncommon. Now, she added, dermatologists can tell patients who had reactions to their initial vaccination that “we know you had this big reaction, and we know that it was upsetting and uncomfortable. But it may not happen the second time around. And if it does, [the reaction is] probably going to be smaller.”
Dr. Freeman, associate professor of dermatology at Harvard Medical School, Boston, highlighted a study published in the Journal of the American Academy of Dermatology that she coauthored with dermatologists across the United States. The researchers tracked 414 cutaneous reactions to the Moderna (83%) and Pfizer (17%) COVID-19 vaccines in a group of patients, which was 90% female, 78% White, and mostly from the United States. Their average age was 44 years. The cases were reported to the AAD–International League of Dermatological Societies registry of COVID-19 cutaneous manifestations.
While most were women, “it’s a little hard to know if this is really going to end up being a true finding,” said Dr. Freeman, the registry’s principal investigator and a member of the AAD’s COVID-19 Ad Hoc Task Force. “If you think about who got vaccinated early, it was health care providers, and the American health care workforce is over 70% female. So I think there’s a little bit of bias here. There may also be a bias because women may be slightly more likely to report or go to their health care provider for a rash.”
Delayed large local reactions were the most common, accounting for 66% (175 cases) of the 267 skin reactions reported after the first Moderna vaccine dose and 30% (31 cases) of the 102 reactions reported after the second dose. These reactions represented 15% (5 cases) of the 34 skin reactions reported after the first Pfizer vaccine dose and 18% (7 cases) of the 40 reactions after the second dose.
There are two peaks with that first dose, Dr. Freeman said. “There’s a peak around day 2 or 3. And there’s another peak around day 7 or 8 with some of these reactions. Only 27% who had a reaction with the first dose had the same reaction with the second.” She added that these reactions “are not cellulitis and don’t require antibiotics.”
Other more common reactions included local injection-site reactions (swelling, erythema, and pain), urticaria (after 24 hours in almost all cases, occurring at a higher rate in patients who received the Pfizer vaccine), and morbilliform eruptions.
Dr. Freeman said that patients may experience redness and swelling in the hands and feet that can be “very uncomfortable.” She described one patient “who was having a hard time actually closing his fist, just because of the amount of swelling and redness in his hand. It did resolve, and it’s important to reassure your patients it will go away.”
According to this study, less common reports of other cutaneous findings with both vaccines included 9 reports of swelling at the site of cosmetic fillers, 8 reports of pernio/chilblains, 10 reports of varicella zoster, 4 reports of herpes simplex flares, 4 pityriasis rosea–like reactions, and 4 rashes in infants of vaccinated breastfeeding mothers.
The study noted that “patients responded well to topical corticosteroids, oral antihistamines, and/or pain-relieving medications. These reactions resolved after a median of 3-4 days.”
It’s important to understand that none of the patients developed anaphylaxis after the second dose even if they’d had a reaction to the first dose, Dr. Freeman said. “But I should point out that we’re talking about reactions that have started more than 4 hours after the vaccine. If a rash such as a urticaria specifically starts within 4 hours of vaccination, that’s in a different category. Those are considered more immediate allergic reactions, and those patients need to be seen by allergy before a second dose.”
Dr. Freeman added that “it’s really interesting to think about how our bodies are really reacting to the vaccine in a way that’s mimicking our body’s reactions to COVID-19.” For example, some patients who got vaccinated developed chilblains similar to the “COVID toes” described in infected patients, apparently as part of the body’s immune response to the virus. “We’ve seen this in patients who actually had COVID and had prior COVID toes and then actually got a flare with their vaccine. And then we’ve also seen it in patients who never had COVID.”
In regard to general advice for patients, she said, “I do still encourage my patients who previously had COVID to go ahead and get the vaccine even if they had a skin manifestation with COVID.”
Shari Lipner, MD, PhD, associate professor of clinical dermatology, Weill Cornell Medicine, New York, said she has have seen only a handful of cases of delayed large local reactions and local injection site reactions after COVID-19 vaccination. “I have seen a significant number of cases of acute urticaria following the first and second doses,” she said in an interview. “However, it is important to keep in mind that we cannot determine cause and effect for the cases of acute urticaria. They may or may not be vaccine related.”
Fortunately, none of the adverse effects she’s seen have been severe. “It is important that dermatologists educate the public and their patients that most people do not develop any skin reaction in response to the vaccine,” she said. In the minority who do, “reactions tend to be mild and are not life-threatening. Many of these skin reactions resolve on their own without treatment.”
She added that “patients with pernio/chilblains or herpes zoster following vaccination should be referred by a board-certified dermatologist for prompt treatment and to avoid sequelae.”
‘COVID vaccine arm’
Delayed local reactions to the Moderna vaccine were also described in a report published online on May 12, 2021, in JAMA Dermatology, after the AAD meeting, in 16 patients referred to the Yale New Haven (Conn.) Hospital Dermatology service who experienced delayed localized cutaneous hypersensitivity reactions a median of 7 days after receiving the vaccine (range, 2-12 days), from Jan. 20 to Feb. 12, 2021. No such cases were reported in Pfizer vaccine recipients.
Of the 16 patients, whose median age was 38 years and who were mostly women, 15 developed the reaction after the first dose, described as “pruritic and variably painful erythematous reactions near the injection site,” which lasted a median of 5 days (range, 1-21 days). After the second dose, 12 of the 16 patients developed injection-site reactions (including one patient who had no reaction after dose 1), a median of 2 days after the vaccine was administered (range, 0-5 days). Histologic results of a biopsy in one patient with a reaction to the second dose “ demonstrated mild predominantly perivascular and focal interstitial mixed infiltrate with lymphocytes and eosinophils consistent with a dermal hypersensitivity reaction,” wrote Alicia J. Little, MD, PhD, of the department of dermatology, Yale University, New Haven, and coauthors.
Compared with immediate hypersensitivity reactions, occurring within 4 hours of vaccination, such as anaphylaxis and urticaria, they concluded that “these delayed localized hypersensitivity reactions are not a contraindication to subsequent vaccination,” and they proposed that they be named “COVID vaccine arm.”
Dr. Freeman reported no disclosures. Dr. Lipner also had no relevant disclosures. Dr. Little reported receiving a grant from the National Center for Advancing Translational Science and a Women’s Health Career Development Award from the Dermatology Foundation while the study was conducted; another author reported equity in Johnson & Johnson in his spouse’s retirement fund outside the submitted work.
FROM AAD VMX 2021
Small increase seen in new COVID-19 cases among children
After 2 consecutive weeks of declines, the number of new COVID-19 cases in children rose slightly, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.
higher than at any other time during the pandemic, the AAP and CHA data show.
It is worth noting, however, that Rhode Island experienced a 30% increase in the last week, adding about 4,900 cases because of data revision and a lag in reporting, the AAP and CHA said in their weekly COVID-19 report.
All the new cases bring the total national count to just over 3.54 million in children, which represents 14.0% of all cases in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. The cumulative case rate as of May 6 was 5,122 per 100,000 children, the two organizations said.
All the new cases that were added to Rhode Island’s total give it the highest cumulative rate in the country: 9,614 cases per 100,000 children. North Dakota is right behind with 9,526 per 100,000, followed by Tennessee (8,898), Connecticut (8,281), and South Carolina (8,274). Vermont has the highest proportion of cases in children at 22.4%, with Alaska next at 20.3% and South Carolina third at 18.7%, according to the AAP and CHA.
Hawaii just reported its first COVID-19–related death in a child, which drops the number of states with zero deaths in children from 10 to 9. Two other new deaths in children from April 30 to May 6 bring the total number to 306 in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting the age distribution of deaths.
In a separate statement, AAP president Lee Savio Beers acknowledged the Food and Drug Administration’s authorization of the Pfizer-BioNTech vaccine for children aged 12-15 years as “a critically important step in bringing lifesaving vaccines to children and adolescents. ... We look forward to the discussion by the Advisory Committee on Immunization Practices of the CDC, which will make recommendations about the use of this vaccine in adolescents.”
After 2 consecutive weeks of declines, the number of new COVID-19 cases in children rose slightly, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

higher than at any other time during the pandemic, the AAP and CHA data show.
It is worth noting, however, that Rhode Island experienced a 30% increase in the last week, adding about 4,900 cases because of data revision and a lag in reporting, the AAP and CHA said in their weekly COVID-19 report.
All the new cases bring the total national count to just over 3.54 million in children, which represents 14.0% of all cases in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. The cumulative case rate as of May 6 was 5,122 per 100,000 children, the two organizations said.
All the new cases that were added to Rhode Island’s total give it the highest cumulative rate in the country: 9,614 cases per 100,000 children. North Dakota is right behind with 9,526 per 100,000, followed by Tennessee (8,898), Connecticut (8,281), and South Carolina (8,274). Vermont has the highest proportion of cases in children at 22.4%, with Alaska next at 20.3% and South Carolina third at 18.7%, according to the AAP and CHA.
Hawaii just reported its first COVID-19–related death in a child, which drops the number of states with zero deaths in children from 10 to 9. Two other new deaths in children from April 30 to May 6 bring the total number to 306 in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting the age distribution of deaths.
In a separate statement, AAP president Lee Savio Beers acknowledged the Food and Drug Administration’s authorization of the Pfizer-BioNTech vaccine for children aged 12-15 years as “a critically important step in bringing lifesaving vaccines to children and adolescents. ... We look forward to the discussion by the Advisory Committee on Immunization Practices of the CDC, which will make recommendations about the use of this vaccine in adolescents.”
After 2 consecutive weeks of declines, the number of new COVID-19 cases in children rose slightly, according to a report from the American Academy of Pediatrics and the Children’s Hospital Association.

higher than at any other time during the pandemic, the AAP and CHA data show.
It is worth noting, however, that Rhode Island experienced a 30% increase in the last week, adding about 4,900 cases because of data revision and a lag in reporting, the AAP and CHA said in their weekly COVID-19 report.
All the new cases bring the total national count to just over 3.54 million in children, which represents 14.0% of all cases in 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam. The cumulative case rate as of May 6 was 5,122 per 100,000 children, the two organizations said.
All the new cases that were added to Rhode Island’s total give it the highest cumulative rate in the country: 9,614 cases per 100,000 children. North Dakota is right behind with 9,526 per 100,000, followed by Tennessee (8,898), Connecticut (8,281), and South Carolina (8,274). Vermont has the highest proportion of cases in children at 22.4%, with Alaska next at 20.3% and South Carolina third at 18.7%, according to the AAP and CHA.
Hawaii just reported its first COVID-19–related death in a child, which drops the number of states with zero deaths in children from 10 to 9. Two other new deaths in children from April 30 to May 6 bring the total number to 306 in the 43 states, along with New York City, Puerto Rico, and Guam, that are reporting the age distribution of deaths.
In a separate statement, AAP president Lee Savio Beers acknowledged the Food and Drug Administration’s authorization of the Pfizer-BioNTech vaccine for children aged 12-15 years as “a critically important step in bringing lifesaving vaccines to children and adolescents. ... We look forward to the discussion by the Advisory Committee on Immunization Practices of the CDC, which will make recommendations about the use of this vaccine in adolescents.”
FDA authorizes Pfizer COVID vaccine for teens 12-15
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration on May 10 granted emergency use authorization (EUA) for the Pfizer coronavirus vaccine to be given to children 12-15 years old.
The much-expected decision increases the likelihood that schools in the United States will fully reopen in the fall – a goal of both the Biden and Trump administrations.
Acting FDA Commissioner Janet Woodcock, MD, called the decision “a significant step” in “returning to a sense of normalcy.”
“Today’s action allows for a younger population to be protected from COVID-19, bringing us closer to returning to a sense of normalcy and to ending the pandemic,” she said in a statement. “Parents and guardians can rest assured that the agency undertook a rigorous and thorough review of all available data, as we have with all of our COVID-19 vaccine emergency use authorizations.”
The Pfizer adolescent vaccine is not yet a done deal, though.
Next, the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices will decide on May 12 whether to recommend use of the vaccine in this age group. After that, CDC Director Rochelle Walensky, MD, will decide whether to give the green light for the vaccine to be administered to that age group.
The FDA action on May 10 amends the Dec. 11, 2020, emergency use authorization that allowed the Pfizer vaccine to be given to people 16 and older. Pfizer was the first company to receive an EUA for its adult vaccine and is the first to receive authorization for its adolescent vaccine. Pfizer is conducting clinical trials on much younger children, too.
The Moderna and Johnson & Johnson vaccines are authorized for people 18 and up. Moderna also has launched clinical trials in children.
Most health experts have said the United States needs to vaccinate children before the COVID-19 pandemic can truly be brought under control. The 12- to 15-year-old group represents 17 million people, about 5% of the population. Thus far, 58% of U.S. adults have had at least one dose of a vaccine and 34.8% of all Americans are fully vaccinated.
American Academy of Pediatrics President Lee Savio Beers, MD, praised the agency’s decision, calling it a “critically important step in bringing life-saving vaccines to children and adolescents. Our youngest generations have shouldered heavy burdens over the past year, and the vaccine is a hopeful sign that they will be able to begin to experience all the activities that are so important for their health and development.”
President Joe Biden recently announced a new strategy for expanding vaccinations in which vaccinating 12- to 15-year-olds was a key component. He said the administration was ready to ship the adolescent vaccine directly to pharmacies and pediatricians to speed up the vaccination rate.
In March, Anthony S. Fauci, MD, told a Senate committee, “We don’t really know what that magical point of herd immunity is, but we do know that if we get the overwhelming population vaccinated, we’re going to be in good shape. … We ultimately would like to get and have to get children into that mix.”
Pfizer submitted data to the FDA in late March showing its mRNA vaccine was 100% effective at preventing COVID-19 infection in children ages 12-15 in clinical trials.
Though most children have milder symptoms when infected with the coronavirus, about 1.5 million cases in children aged 11-17 were reported to the CDC between March 1, 2020, and April 30 of this year, the FDA news release said.
Albert Bourla, CEO of Pfizer, tweeted that “today brings very encouraging news for families and adolescents across the United States.
“While this is a meaningful step forward, we are still in a critical period of combating #COVID19 around the world. In the coming weeks, we hope to continue to receive authorizations from global regulators to support worldwide vaccination efforts,” he said.
“It’s essential for children to be vaccinated against COVID-19. According to data compiled by the AAP and Children’s Hospital Association, more than 3.8 million children have tested positive for COVID-19 in the United States since the start of the pandemic,” said Dr. Savio Beers. “While fewer children than adults have suffered the most severe disease, this is not a benign disease in children. Thousands of children have been hospitalized, and hundreds have died. We will soon have a very safe, highly effective vaccine that can prevent so much suffering. I encourage parents to talk with their pediatricians about how to get the vaccine for their adolescents as soon as they are eligible.”
A version of this article first appeared on Medscape.com.
NSAIDs don’t make COVID-19 worse in hospitalized patients
NSAIDs don’t boost the risk of more severe disease or death in hospitalized patients with COVID-19, a new study finds.
“To our knowledge, our prospective study includes the largest number of patients admitted to hospital with COVID-19 to date, and adds to the literature on the safety of NSAIDs and in-hospital outcomes. NSAIDs do not appear to increase the risk of worse in-hospital outcomes ...” the study authors wrote. “NSAIDs are an important analgesic modality and have a vital opioid-sparing role in pain management. Patients and clinicians should be reassured by these findings that NSAIDs are safe in the context of the pandemic.”
The report was published online May 7 in The Lancet Rheumatology and led by clinical research fellow Thomas M. Drake, MBChB, of the University of Edinburgh’s Usher Institute.
For more than a year, researchers worldwide have debated about whether NSAIDs spell trouble for people at risk of COVID-19. In March 2020, French health officials announced that use of the painkillers such as NSAIDs may increase the severity of the disease, and they recommended that patients take acetaminophen instead. The National Health Service in the United Kingdom made a similar recommendation. But other agencies didn’t believe there was enough evidence to support ditching NSAIDs, and recent research studies published in Annals of the Rheumatic Diseases and PLoS Medicine suggested they may be right.
For the new study, researchers identified 72,179 patients who were treated for COVID-19 in British hospitals during January-August 2020. About 56% were men, 74% were White, and 6% took NSAIDs on a regular basis before they entered the hospital. The average age was 70.
The researchers examined whether the patients in either group were more or less likely to die in the hospital, be admitted into a critical care unit, need oxygen treatment, need a ventilator, or suffer kidney injury.
In terms of outcomes, there weren’t any major gaps between the groups overall. The differences in most comparisons were statistically insignificant. For example, 31% of those who didn’t take NSAIDs died vs. 30% of those who did (P = .227). In both groups, 14% required critical care admission (P = .476).
The researchers then focused on two matched groups of 4,205 patients: One group used NSAIDs regularly, and the other group didn’t. The difference in risk of death in those who took NSAIDs vs. those who didn’t was statistically insignificant (odds ratio, 0.95; 95% confidence interval, 0.84-1.07; P = .35). Other comparisons were also statistically insignificant.
The findings offer insight into whether the use of NSAIDs might actually be helpful for patients who develop COVID-19. Scientists believe that COVID-19 is linked to inflammation in the body, and NSAIDs, of course, reduce inflammation. But the researchers didn’t turn up any sign of a benefit.
The new study has some weaknesses: It doesn’t say anything about whether NSAIDs have an impact on whether people get COVID-19 in the first place. Researchers don’t know if high use of NSAIDs may affect the severity of the disease. And it doesn’t examine the potential effect of acetaminophen, although other research suggests the drug also may not cause harm in patients with COVID-19.
Still, the researchers say the study is the largest of its kind to look at the use of NSAIDs by patients who are admitted to the hospital with COVID-19. “Considering all the evidence, if there was an extreme effect of NSAIDs on COVID-19 outcomes or severity, this would have been observed in one or more of the studies that have been done, including the present study,” they wrote.
In a commentary that accompanied the study, three physicians from hospitals in Denmark, led by Kristian Kragholm, MD, of Aalborg University Hospital, praised the research and wrote that it adds to “a growing body of evidence” that NSAIDs don’t make things worse for patients with COVID-19.
The study was funded by the U.K. National Institute for Health Research and the U.K. Medical Research Council. The study and commentary authors reported no relevant disclosures.
NSAIDs don’t boost the risk of more severe disease or death in hospitalized patients with COVID-19, a new study finds.
“To our knowledge, our prospective study includes the largest number of patients admitted to hospital with COVID-19 to date, and adds to the literature on the safety of NSAIDs and in-hospital outcomes. NSAIDs do not appear to increase the risk of worse in-hospital outcomes ...” the study authors wrote. “NSAIDs are an important analgesic modality and have a vital opioid-sparing role in pain management. Patients and clinicians should be reassured by these findings that NSAIDs are safe in the context of the pandemic.”
The report was published online May 7 in The Lancet Rheumatology and led by clinical research fellow Thomas M. Drake, MBChB, of the University of Edinburgh’s Usher Institute.
For more than a year, researchers worldwide have debated about whether NSAIDs spell trouble for people at risk of COVID-19. In March 2020, French health officials announced that use of the painkillers such as NSAIDs may increase the severity of the disease, and they recommended that patients take acetaminophen instead. The National Health Service in the United Kingdom made a similar recommendation. But other agencies didn’t believe there was enough evidence to support ditching NSAIDs, and recent research studies published in Annals of the Rheumatic Diseases and PLoS Medicine suggested they may be right.
For the new study, researchers identified 72,179 patients who were treated for COVID-19 in British hospitals during January-August 2020. About 56% were men, 74% were White, and 6% took NSAIDs on a regular basis before they entered the hospital. The average age was 70.
The researchers examined whether the patients in either group were more or less likely to die in the hospital, be admitted into a critical care unit, need oxygen treatment, need a ventilator, or suffer kidney injury.
In terms of outcomes, there weren’t any major gaps between the groups overall. The differences in most comparisons were statistically insignificant. For example, 31% of those who didn’t take NSAIDs died vs. 30% of those who did (P = .227). In both groups, 14% required critical care admission (P = .476).
The researchers then focused on two matched groups of 4,205 patients: One group used NSAIDs regularly, and the other group didn’t. The difference in risk of death in those who took NSAIDs vs. those who didn’t was statistically insignificant (odds ratio, 0.95; 95% confidence interval, 0.84-1.07; P = .35). Other comparisons were also statistically insignificant.
The findings offer insight into whether the use of NSAIDs might actually be helpful for patients who develop COVID-19. Scientists believe that COVID-19 is linked to inflammation in the body, and NSAIDs, of course, reduce inflammation. But the researchers didn’t turn up any sign of a benefit.
The new study has some weaknesses: It doesn’t say anything about whether NSAIDs have an impact on whether people get COVID-19 in the first place. Researchers don’t know if high use of NSAIDs may affect the severity of the disease. And it doesn’t examine the potential effect of acetaminophen, although other research suggests the drug also may not cause harm in patients with COVID-19.
Still, the researchers say the study is the largest of its kind to look at the use of NSAIDs by patients who are admitted to the hospital with COVID-19. “Considering all the evidence, if there was an extreme effect of NSAIDs on COVID-19 outcomes or severity, this would have been observed in one or more of the studies that have been done, including the present study,” they wrote.
In a commentary that accompanied the study, three physicians from hospitals in Denmark, led by Kristian Kragholm, MD, of Aalborg University Hospital, praised the research and wrote that it adds to “a growing body of evidence” that NSAIDs don’t make things worse for patients with COVID-19.
The study was funded by the U.K. National Institute for Health Research and the U.K. Medical Research Council. The study and commentary authors reported no relevant disclosures.
NSAIDs don’t boost the risk of more severe disease or death in hospitalized patients with COVID-19, a new study finds.
“To our knowledge, our prospective study includes the largest number of patients admitted to hospital with COVID-19 to date, and adds to the literature on the safety of NSAIDs and in-hospital outcomes. NSAIDs do not appear to increase the risk of worse in-hospital outcomes ...” the study authors wrote. “NSAIDs are an important analgesic modality and have a vital opioid-sparing role in pain management. Patients and clinicians should be reassured by these findings that NSAIDs are safe in the context of the pandemic.”
The report was published online May 7 in The Lancet Rheumatology and led by clinical research fellow Thomas M. Drake, MBChB, of the University of Edinburgh’s Usher Institute.
For more than a year, researchers worldwide have debated about whether NSAIDs spell trouble for people at risk of COVID-19. In March 2020, French health officials announced that use of the painkillers such as NSAIDs may increase the severity of the disease, and they recommended that patients take acetaminophen instead. The National Health Service in the United Kingdom made a similar recommendation. But other agencies didn’t believe there was enough evidence to support ditching NSAIDs, and recent research studies published in Annals of the Rheumatic Diseases and PLoS Medicine suggested they may be right.
For the new study, researchers identified 72,179 patients who were treated for COVID-19 in British hospitals during January-August 2020. About 56% were men, 74% were White, and 6% took NSAIDs on a regular basis before they entered the hospital. The average age was 70.
The researchers examined whether the patients in either group were more or less likely to die in the hospital, be admitted into a critical care unit, need oxygen treatment, need a ventilator, or suffer kidney injury.
In terms of outcomes, there weren’t any major gaps between the groups overall. The differences in most comparisons were statistically insignificant. For example, 31% of those who didn’t take NSAIDs died vs. 30% of those who did (P = .227). In both groups, 14% required critical care admission (P = .476).
The researchers then focused on two matched groups of 4,205 patients: One group used NSAIDs regularly, and the other group didn’t. The difference in risk of death in those who took NSAIDs vs. those who didn’t was statistically insignificant (odds ratio, 0.95; 95% confidence interval, 0.84-1.07; P = .35). Other comparisons were also statistically insignificant.
The findings offer insight into whether the use of NSAIDs might actually be helpful for patients who develop COVID-19. Scientists believe that COVID-19 is linked to inflammation in the body, and NSAIDs, of course, reduce inflammation. But the researchers didn’t turn up any sign of a benefit.
The new study has some weaknesses: It doesn’t say anything about whether NSAIDs have an impact on whether people get COVID-19 in the first place. Researchers don’t know if high use of NSAIDs may affect the severity of the disease. And it doesn’t examine the potential effect of acetaminophen, although other research suggests the drug also may not cause harm in patients with COVID-19.
Still, the researchers say the study is the largest of its kind to look at the use of NSAIDs by patients who are admitted to the hospital with COVID-19. “Considering all the evidence, if there was an extreme effect of NSAIDs on COVID-19 outcomes or severity, this would have been observed in one or more of the studies that have been done, including the present study,” they wrote.
In a commentary that accompanied the study, three physicians from hospitals in Denmark, led by Kristian Kragholm, MD, of Aalborg University Hospital, praised the research and wrote that it adds to “a growing body of evidence” that NSAIDs don’t make things worse for patients with COVID-19.
The study was funded by the U.K. National Institute for Health Research and the U.K. Medical Research Council. The study and commentary authors reported no relevant disclosures.
FROM THE LANCET RHEUMATOLOGY












