User login
Virtual is the new real
Why did we fall short on maximizing telehealth’s value in the COVID-19 pandemic?
The COVID-19 pandemic catalyzed the transformation of Internet-based, remotely accessible innovative technologies. Internet-based customer service delivery technology was rapidly adopted and utilized by several services industries, but health care systems in most of the countries across the world faced unique challenges in adopting the technology for the delivery of health care services. The health care ecosystem of the United States was not immune to such challenges, and several significant barriers surfaced while the pandemic was underway.
Complexly structured, fragmented, unprepared, and overly burnt-out health systems in the United States arguably have fallen short of maximizing the value of telehealth in delivering safe, easily accessible, comprehensive, and cost-effective health care services. In this essay, we examine the reasons for such a suboptimal performance and discuss a few important strategies that may be useful in maximizing the value of telehealth value in several, appropriate health care services.
Hospitals and telehealth
Are hospitalists preparing ourselves “not to see” patients in a hospital-based health care delivery setting? If you have not yet started yet, now may be the right time! Yes, a certain percentage of doctor-patient encounters in hospital settings will remain virtual forever.
A well-established telehealth infrastructure is rarely found in most U.S. hospitals, although the COVID-19 pandemic has unexpectedly boosted the rapid growth of telehealth in the country.1 Public health emergency declarations in the United States in the face of the COVID-19 crisis have facilitated two important initiatives to restore health care delivery amidst formal and informal lockdowns that brought states to a grinding halt. These extend from expansion of virtual services, including telehealth, virtual check-ins, and e-visits, to the decision by the Department of Health & Human Services Office of Civil Rights to exercise enforcement discretion and waive penalties for the use of relatively inexpensive, non–public-facing mobile and other audiovisual technology tools.2
Hospital-based care in the United States taps nearly 33% of national health expenditure. An additional 30% of national health expenditure that is related to physicians, prescriptions, and other facilities is indirectly influenced by care delivered at health care facilities.3 Studies show that about 20% of ED visits could potentially be avoided via virtual urgent care offerings.4 A rapidly changing health care ecosystem is proving formidable for most hospital systems, and a test for their resilience and agility. Not just the implementation of telehealth is challenging, but getting it right is the key success factor.
Hospital-based telehealth
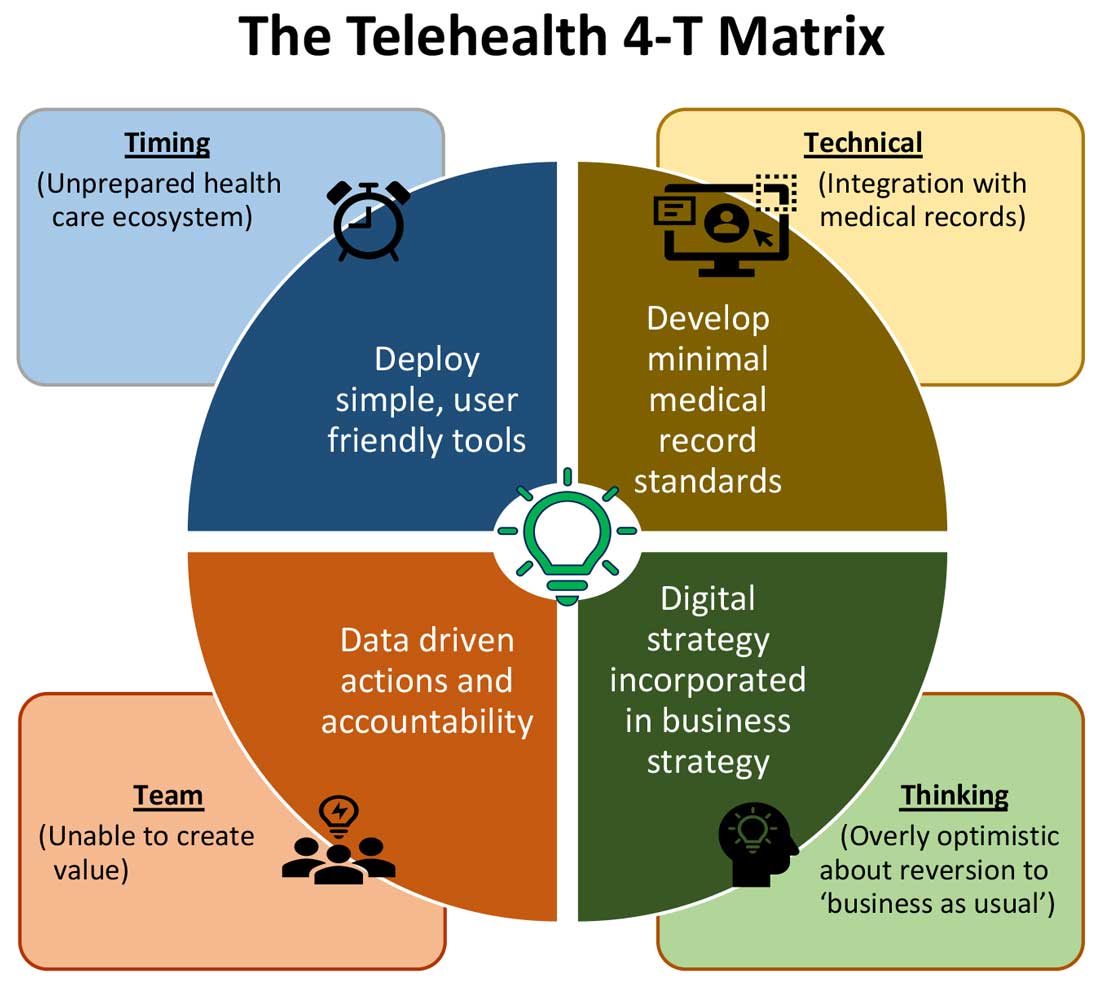
Expansion of telehealth coverage by the Centers for Medicare & Medicaid Services and most commercial payers did not quite ride the pandemic-induced momentum across the care continuum. Hospitals are lagging far behind ambulatory care in implementing telehealth. As illustrated in the “4-T Matrix” (see graphic) we would like to examine four key reasons for such a sluggish initial uptake and try to propose four important strategies that may help us to maximize the value created by telehealth technologies.
1. Timing
The health care system has always lagged far behind other service industries in terms of technology adaptation. Because of the unique nature of health care services, face-to-face interaction supersedes all other forms of communication. A rapidly evolving pandemic was not matched by simultaneous technology education for patients and providers. The enormous choice of hard-to-navigate telehealth tools; time and labor-intensive implementation; and uncertainty around payer, policy, and regulatory expectations might have precluded providers from the rapid adoption of telehealth in the hospital setting. Patients’ specific characteristics, such as the absence of technology-centered education, information, age, comorbidities, lack of technical literacy, and dependency on caregivers contributed to the suboptimal response from patients and families.
Deploying simple, ubiquitous, user-friendly, and technologically less challenging telehealth solutions may be a better approach to increase the adoption of such solutions by providers and patients. Hospitals need to develop and distribute telehealth user guides in all possible modes of communication. Provider-centric in-service sessions, workshops, and live support by “superuser teams” often work well in reducing end-user resistance.
2. Technical
Current electronic medical records vary widely in their features and offerings, and their ability to interact with third-party software and platforms. Dissatisfaction of end users with EMRs is well known, as is their likely relationship to burnout. Recent research continues to show a strong relationship between EMR usability and the odds of burnout among physicians.5 In the current climate, administrators and health informaticists have the responsibility to avoid adding increased burdens to end users.
Another issue is the limited connectivity in many remote/rural areas that would impact implementation of telehealth platforms. Studies indicate that 33% of rural Americans lack access to high-speed broadband Internet to support video visits.6 The recent successful implementation of telehealth across 530 providers in 75 ambulatory practices operated by Munson Healthcare, a rural health system in northern Michigan, sheds light on the technology’s enormous potential in providing safe access to rural populations.6,7
Privacy and safety of patient data is of paramount importance. According to a national poll on healthy aging by the University of Michigan in May 2019, targeting older adults, 47% of survey responders expressed difficulty using technology and 49% of survey responders were concerned about privacy.8 Use of certification and other tools offered by the Office of the National Coordinator for Health Information Technology would help reassure users, and the ability to capture and share images between providers would be of immense benefit in facilitating e-consults.
The need of the hour is redesigned work flow, to help providers adopt and use virtual care/telehealth efficiently. Work flow redesign must be coupled with technological advances to allow seamless integration of third-party telehealth platforms into existing EMR systems or built directly into EMRs. Use of quality metrics and analytical tools specific to telehealth would help measure the technology’s impact on patient care, outcomes, and end-user/provider experience.
3. Teams and training
Outcomes of health care interventions are often determined by the effectiveness of teams. Irrespective of how robust health care systems may have been initially, rapidly spreading infectious diseases like COVID-19 can quickly derail the system, bringing the workforce and patients to a breaking point.5 Decentralized, uncoordinated, and siloed efforts by individual teams across the care continuum were contributing factors for the partial success of telehealth care delivery pathways. The hospital systems with telehealth-ready teams at the start of the COVID-19 pandemic were so rare that the knowledge and technical training opportunities for innovators grew severalfold during the pandemic.
As per the American Medical Association, telehealth success is massively dependent on building the right team. Core, leadership, advisory, and implementation teams comprised of clinical representatives, end users, administrative personnel, executive members of the organization, technical experts, and payment/policy experts should be put together before implementing a telehealth strategy.9 Seamless integration of hospital-based care with ambulatory care via a telehealth platform is only complete when care managers are trained and deployed to fulfill the needs of a diverse group of patients. Deriving overall value from telehealth is only possible when there is a skill development, training and mentoring team put in place.
4. Thinking
In most U.S. hospitals, inpatient health care is equally distributed between nonprocedure and procedure-based services. Hospitals resorted to suspension of nonemergent procedures to mitigate the risk of spreading COVID-19. This was further compounded by many patients’ self-selection to defer care, an abrupt reduction in the influx of patients from the referral base because of suboptimally operating ambulatory care services, leading to low hospital occupancy.
Hospitals across the nation have gone through a massive short-term financial crunch and unfavorable cash-flow forecast, which prompted a paradoxical work-force reduction. While some argue that it may be akin to strategic myopia, the authors believed that such a response is strategically imperative to keep the hospital afloat. It is reasonable to attribute the paucity of innovation to constrained resources, and health systems are simply staying overly optimistic about “weathering the storm” and reverting soon to “business as usual.” The technological framework necessary for deploying a telehealth solution often comes with a price. Financially challenged hospital systems rarely exercise any capital-intensive activities. By contrast, telehealth adoption by ambulatory care can result in quicker resumption of patient care in community settings. A lack of operational and infrastructure synchrony between ambulatory and in-hospital systems has failed to capture telehealth-driven inpatient volume. For example, direct admissions from ambulatory telehealth referrals was a missed opportunity in several places. Referrals for labs, diagnostic tests, and other allied services could have helped hospitals offset their fixed costs. Similarly, work flows related to discharge and postdischarge follow up rarely embrace telehealth tools or telehealth care pathways. A brisk change in the health care ecosystem is partly responsible for this.
Digital strategy needs to be incorporated into business strategy. For the reasons already discussed, telehealth technology is not a “nice to have” anymore, but a “must have.” At present, providers are of the opinion that about 20% of their patient services can be delivered via a telehealth platform. Similar trends are observed among patients, as a new modality of access to care is increasingly beneficial to them. Telehealth must be incorporated in standardized hospital work flows. Use of telehealth for preoperative clearance will greatly minimize same-day surgery cancellations. Given the potential shortage in resources, telehealth adoption for inpatient consultations will help systems conserve personal protective equipment, minimize the risk of staff exposure to COVID-19, and improve efficiency.
Digital strategy also prompts the reengineering of care delivery.10 Excessive and unused physical capacity can be converted into digital care hubs. Health maintenance, prevention, health promotion, health education, and chronic disease management not only can serve a variety of patient groups but can also help address the “last-mile problem” in health care. A successful digital strategy usually has three important components – Commitment: Hospital leadership is committed to include digital transformation as a strategic objective; Cost: Digital strategy is added as a line item in the budget; and Control: Measurable metrics are put in place to monitor the performance, impact, and influence of the digital strategy.
Conclusion
For decades, most U.S. health systems occupied the periphery of technological transformation when compared to the rest of the service industry. While most health systems took a heroic approach to the adoption of telehealth during COVID-19, despite being unprepared, the need for a systematic telehealth deployment is far from being adequately fulfilled. The COVID-19 pandemic brought permanent changes to several business disciplines globally. Given the impact of the pandemic on the health and overall wellbeing of American society, the U.S. health care industry must leave no stone unturned in its quest for transformation.
Dr. Lingisetty is a hospitalist and physician executive at Baptist Health System, Little Rock, Ark, and is cofounder/president of SHM’s Arkansas chapter. Dr. Prasad is medical director of care management and a hospitalist at Advocate Aurora Health in Milwaukee. He is cochair of SHM’s IT Special Interest Group, sits on the HQPS committee, and is president of SHM’s Wisconsin chapter. Dr. Palabindala is the medical director, utilization management, and physician advisory services at the University of Mississippi Medical Center and an associate professor of medicine and academic hospitalist at the University of Mississippi, both in Jackson.
References
1. Finnegan M. “Telehealth booms amid COVID-19 crisis.” Computerworld. 2020 Apr 27. www.computerworld.com/article/3540315/telehealth-booms-amid-covid-19-crisis-virtual-care-is-here-to-stay.html. Accessed 2020 Sep 12.
2. Department of Health & Human Services. “OCR Announces Notification of Enforcement Discretion for Telehealth Remote Communications During the COVID-19 Nationwide Public Health Emergency.” 2020 Mar 17. www.hhs.gov/about/news/2020/03/17/ocr-announces-notification-of-enforcement-discretion-for-telehealth-remote-communications-during-the-covid-19.html. Accessed 2020 Sep 12.
3. National Center for Health Statistics. “Health Expenditures.” www.cdc.gov/nchs/fastats/health-expenditures.htm. Accessed 2020 Sep 12.
4. Bestsennyy O et al. “Telehealth: A post–COVID-19 reality?” McKinsey & Company. 2020 May 29. www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality. Accessed 2020 Sep 12.
5. Melnick ER et al. The Association Between Perceived Electronic Health Record Usability and Professional Burnout Among U.S. Physicians. Mayo Clin Proc. 2020 March;95(3):476-87.
6. Hirko KA et al. Telehealth in response to the COVID-19 pandemic: Implications for rural health disparities. J Am Med Inform Assoc. 2020 Nov;27(11):1816-8. .
7. American Academy of Family Physicians. “Study Examines Telehealth, Rural Disparities in Pandemic.” 2020 July 30. www.aafp.org/news/practice-professional-issues/20200730ruraltelehealth.html. Accessed 2020 Dec 15.
8. Kurlander J et al. “Virtual Visits: Telehealth and Older Adults.” National Poll on Healthy Aging. 2019 Oct. hdl.handle.net/2027.42/151376.
9. American Medical Association. Telehealth Implementation Playbook. 2019. www.ama-assn.org/system/files/2020-04/ama-telehealth-implementation-playbook.pdf.
10. Smith AC et al. Telehealth for global emergencies: Implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020 Jun;26(5):309-13.
Why did we fall short on maximizing telehealth’s value in the COVID-19 pandemic?
Why did we fall short on maximizing telehealth’s value in the COVID-19 pandemic?
The COVID-19 pandemic catalyzed the transformation of Internet-based, remotely accessible innovative technologies. Internet-based customer service delivery technology was rapidly adopted and utilized by several services industries, but health care systems in most of the countries across the world faced unique challenges in adopting the technology for the delivery of health care services. The health care ecosystem of the United States was not immune to such challenges, and several significant barriers surfaced while the pandemic was underway.
Complexly structured, fragmented, unprepared, and overly burnt-out health systems in the United States arguably have fallen short of maximizing the value of telehealth in delivering safe, easily accessible, comprehensive, and cost-effective health care services. In this essay, we examine the reasons for such a suboptimal performance and discuss a few important strategies that may be useful in maximizing the value of telehealth value in several, appropriate health care services.
Hospitals and telehealth
Are hospitalists preparing ourselves “not to see” patients in a hospital-based health care delivery setting? If you have not yet started yet, now may be the right time! Yes, a certain percentage of doctor-patient encounters in hospital settings will remain virtual forever.
A well-established telehealth infrastructure is rarely found in most U.S. hospitals, although the COVID-19 pandemic has unexpectedly boosted the rapid growth of telehealth in the country.1 Public health emergency declarations in the United States in the face of the COVID-19 crisis have facilitated two important initiatives to restore health care delivery amidst formal and informal lockdowns that brought states to a grinding halt. These extend from expansion of virtual services, including telehealth, virtual check-ins, and e-visits, to the decision by the Department of Health & Human Services Office of Civil Rights to exercise enforcement discretion and waive penalties for the use of relatively inexpensive, non–public-facing mobile and other audiovisual technology tools.2
Hospital-based care in the United States taps nearly 33% of national health expenditure. An additional 30% of national health expenditure that is related to physicians, prescriptions, and other facilities is indirectly influenced by care delivered at health care facilities.3 Studies show that about 20% of ED visits could potentially be avoided via virtual urgent care offerings.4 A rapidly changing health care ecosystem is proving formidable for most hospital systems, and a test for their resilience and agility. Not just the implementation of telehealth is challenging, but getting it right is the key success factor.
Hospital-based telehealth

Expansion of telehealth coverage by the Centers for Medicare & Medicaid Services and most commercial payers did not quite ride the pandemic-induced momentum across the care continuum. Hospitals are lagging far behind ambulatory care in implementing telehealth. As illustrated in the “4-T Matrix” (see graphic) we would like to examine four key reasons for such a sluggish initial uptake and try to propose four important strategies that may help us to maximize the value created by telehealth technologies.
1. Timing
The health care system has always lagged far behind other service industries in terms of technology adaptation. Because of the unique nature of health care services, face-to-face interaction supersedes all other forms of communication. A rapidly evolving pandemic was not matched by simultaneous technology education for patients and providers. The enormous choice of hard-to-navigate telehealth tools; time and labor-intensive implementation; and uncertainty around payer, policy, and regulatory expectations might have precluded providers from the rapid adoption of telehealth in the hospital setting. Patients’ specific characteristics, such as the absence of technology-centered education, information, age, comorbidities, lack of technical literacy, and dependency on caregivers contributed to the suboptimal response from patients and families.
Deploying simple, ubiquitous, user-friendly, and technologically less challenging telehealth solutions may be a better approach to increase the adoption of such solutions by providers and patients. Hospitals need to develop and distribute telehealth user guides in all possible modes of communication. Provider-centric in-service sessions, workshops, and live support by “superuser teams” often work well in reducing end-user resistance.
2. Technical
Current electronic medical records vary widely in their features and offerings, and their ability to interact with third-party software and platforms. Dissatisfaction of end users with EMRs is well known, as is their likely relationship to burnout. Recent research continues to show a strong relationship between EMR usability and the odds of burnout among physicians.5 In the current climate, administrators and health informaticists have the responsibility to avoid adding increased burdens to end users.
Another issue is the limited connectivity in many remote/rural areas that would impact implementation of telehealth platforms. Studies indicate that 33% of rural Americans lack access to high-speed broadband Internet to support video visits.6 The recent successful implementation of telehealth across 530 providers in 75 ambulatory practices operated by Munson Healthcare, a rural health system in northern Michigan, sheds light on the technology’s enormous potential in providing safe access to rural populations.6,7
Privacy and safety of patient data is of paramount importance. According to a national poll on healthy aging by the University of Michigan in May 2019, targeting older adults, 47% of survey responders expressed difficulty using technology and 49% of survey responders were concerned about privacy.8 Use of certification and other tools offered by the Office of the National Coordinator for Health Information Technology would help reassure users, and the ability to capture and share images between providers would be of immense benefit in facilitating e-consults.
The need of the hour is redesigned work flow, to help providers adopt and use virtual care/telehealth efficiently. Work flow redesign must be coupled with technological advances to allow seamless integration of third-party telehealth platforms into existing EMR systems or built directly into EMRs. Use of quality metrics and analytical tools specific to telehealth would help measure the technology’s impact on patient care, outcomes, and end-user/provider experience.
3. Teams and training
Outcomes of health care interventions are often determined by the effectiveness of teams. Irrespective of how robust health care systems may have been initially, rapidly spreading infectious diseases like COVID-19 can quickly derail the system, bringing the workforce and patients to a breaking point.5 Decentralized, uncoordinated, and siloed efforts by individual teams across the care continuum were contributing factors for the partial success of telehealth care delivery pathways. The hospital systems with telehealth-ready teams at the start of the COVID-19 pandemic were so rare that the knowledge and technical training opportunities for innovators grew severalfold during the pandemic.
As per the American Medical Association, telehealth success is massively dependent on building the right team. Core, leadership, advisory, and implementation teams comprised of clinical representatives, end users, administrative personnel, executive members of the organization, technical experts, and payment/policy experts should be put together before implementing a telehealth strategy.9 Seamless integration of hospital-based care with ambulatory care via a telehealth platform is only complete when care managers are trained and deployed to fulfill the needs of a diverse group of patients. Deriving overall value from telehealth is only possible when there is a skill development, training and mentoring team put in place.
4. Thinking
In most U.S. hospitals, inpatient health care is equally distributed between nonprocedure and procedure-based services. Hospitals resorted to suspension of nonemergent procedures to mitigate the risk of spreading COVID-19. This was further compounded by many patients’ self-selection to defer care, an abrupt reduction in the influx of patients from the referral base because of suboptimally operating ambulatory care services, leading to low hospital occupancy.
Hospitals across the nation have gone through a massive short-term financial crunch and unfavorable cash-flow forecast, which prompted a paradoxical work-force reduction. While some argue that it may be akin to strategic myopia, the authors believed that such a response is strategically imperative to keep the hospital afloat. It is reasonable to attribute the paucity of innovation to constrained resources, and health systems are simply staying overly optimistic about “weathering the storm” and reverting soon to “business as usual.” The technological framework necessary for deploying a telehealth solution often comes with a price. Financially challenged hospital systems rarely exercise any capital-intensive activities. By contrast, telehealth adoption by ambulatory care can result in quicker resumption of patient care in community settings. A lack of operational and infrastructure synchrony between ambulatory and in-hospital systems has failed to capture telehealth-driven inpatient volume. For example, direct admissions from ambulatory telehealth referrals was a missed opportunity in several places. Referrals for labs, diagnostic tests, and other allied services could have helped hospitals offset their fixed costs. Similarly, work flows related to discharge and postdischarge follow up rarely embrace telehealth tools or telehealth care pathways. A brisk change in the health care ecosystem is partly responsible for this.
Digital strategy needs to be incorporated into business strategy. For the reasons already discussed, telehealth technology is not a “nice to have” anymore, but a “must have.” At present, providers are of the opinion that about 20% of their patient services can be delivered via a telehealth platform. Similar trends are observed among patients, as a new modality of access to care is increasingly beneficial to them. Telehealth must be incorporated in standardized hospital work flows. Use of telehealth for preoperative clearance will greatly minimize same-day surgery cancellations. Given the potential shortage in resources, telehealth adoption for inpatient consultations will help systems conserve personal protective equipment, minimize the risk of staff exposure to COVID-19, and improve efficiency.
Digital strategy also prompts the reengineering of care delivery.10 Excessive and unused physical capacity can be converted into digital care hubs. Health maintenance, prevention, health promotion, health education, and chronic disease management not only can serve a variety of patient groups but can also help address the “last-mile problem” in health care. A successful digital strategy usually has three important components – Commitment: Hospital leadership is committed to include digital transformation as a strategic objective; Cost: Digital strategy is added as a line item in the budget; and Control: Measurable metrics are put in place to monitor the performance, impact, and influence of the digital strategy.
Conclusion
For decades, most U.S. health systems occupied the periphery of technological transformation when compared to the rest of the service industry. While most health systems took a heroic approach to the adoption of telehealth during COVID-19, despite being unprepared, the need for a systematic telehealth deployment is far from being adequately fulfilled. The COVID-19 pandemic brought permanent changes to several business disciplines globally. Given the impact of the pandemic on the health and overall wellbeing of American society, the U.S. health care industry must leave no stone unturned in its quest for transformation.
Dr. Lingisetty is a hospitalist and physician executive at Baptist Health System, Little Rock, Ark, and is cofounder/president of SHM’s Arkansas chapter. Dr. Prasad is medical director of care management and a hospitalist at Advocate Aurora Health in Milwaukee. He is cochair of SHM’s IT Special Interest Group, sits on the HQPS committee, and is president of SHM’s Wisconsin chapter. Dr. Palabindala is the medical director, utilization management, and physician advisory services at the University of Mississippi Medical Center and an associate professor of medicine and academic hospitalist at the University of Mississippi, both in Jackson.
References
1. Finnegan M. “Telehealth booms amid COVID-19 crisis.” Computerworld. 2020 Apr 27. www.computerworld.com/article/3540315/telehealth-booms-amid-covid-19-crisis-virtual-care-is-here-to-stay.html. Accessed 2020 Sep 12.
2. Department of Health & Human Services. “OCR Announces Notification of Enforcement Discretion for Telehealth Remote Communications During the COVID-19 Nationwide Public Health Emergency.” 2020 Mar 17. www.hhs.gov/about/news/2020/03/17/ocr-announces-notification-of-enforcement-discretion-for-telehealth-remote-communications-during-the-covid-19.html. Accessed 2020 Sep 12.
3. National Center for Health Statistics. “Health Expenditures.” www.cdc.gov/nchs/fastats/health-expenditures.htm. Accessed 2020 Sep 12.
4. Bestsennyy O et al. “Telehealth: A post–COVID-19 reality?” McKinsey & Company. 2020 May 29. www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality. Accessed 2020 Sep 12.
5. Melnick ER et al. The Association Between Perceived Electronic Health Record Usability and Professional Burnout Among U.S. Physicians. Mayo Clin Proc. 2020 March;95(3):476-87.
6. Hirko KA et al. Telehealth in response to the COVID-19 pandemic: Implications for rural health disparities. J Am Med Inform Assoc. 2020 Nov;27(11):1816-8. .
7. American Academy of Family Physicians. “Study Examines Telehealth, Rural Disparities in Pandemic.” 2020 July 30. www.aafp.org/news/practice-professional-issues/20200730ruraltelehealth.html. Accessed 2020 Dec 15.
8. Kurlander J et al. “Virtual Visits: Telehealth and Older Adults.” National Poll on Healthy Aging. 2019 Oct. hdl.handle.net/2027.42/151376.
9. American Medical Association. Telehealth Implementation Playbook. 2019. www.ama-assn.org/system/files/2020-04/ama-telehealth-implementation-playbook.pdf.
10. Smith AC et al. Telehealth for global emergencies: Implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020 Jun;26(5):309-13.
The COVID-19 pandemic catalyzed the transformation of Internet-based, remotely accessible innovative technologies. Internet-based customer service delivery technology was rapidly adopted and utilized by several services industries, but health care systems in most of the countries across the world faced unique challenges in adopting the technology for the delivery of health care services. The health care ecosystem of the United States was not immune to such challenges, and several significant barriers surfaced while the pandemic was underway.
Complexly structured, fragmented, unprepared, and overly burnt-out health systems in the United States arguably have fallen short of maximizing the value of telehealth in delivering safe, easily accessible, comprehensive, and cost-effective health care services. In this essay, we examine the reasons for such a suboptimal performance and discuss a few important strategies that may be useful in maximizing the value of telehealth value in several, appropriate health care services.
Hospitals and telehealth
Are hospitalists preparing ourselves “not to see” patients in a hospital-based health care delivery setting? If you have not yet started yet, now may be the right time! Yes, a certain percentage of doctor-patient encounters in hospital settings will remain virtual forever.
A well-established telehealth infrastructure is rarely found in most U.S. hospitals, although the COVID-19 pandemic has unexpectedly boosted the rapid growth of telehealth in the country.1 Public health emergency declarations in the United States in the face of the COVID-19 crisis have facilitated two important initiatives to restore health care delivery amidst formal and informal lockdowns that brought states to a grinding halt. These extend from expansion of virtual services, including telehealth, virtual check-ins, and e-visits, to the decision by the Department of Health & Human Services Office of Civil Rights to exercise enforcement discretion and waive penalties for the use of relatively inexpensive, non–public-facing mobile and other audiovisual technology tools.2
Hospital-based care in the United States taps nearly 33% of national health expenditure. An additional 30% of national health expenditure that is related to physicians, prescriptions, and other facilities is indirectly influenced by care delivered at health care facilities.3 Studies show that about 20% of ED visits could potentially be avoided via virtual urgent care offerings.4 A rapidly changing health care ecosystem is proving formidable for most hospital systems, and a test for their resilience and agility. Not just the implementation of telehealth is challenging, but getting it right is the key success factor.
Hospital-based telehealth

Expansion of telehealth coverage by the Centers for Medicare & Medicaid Services and most commercial payers did not quite ride the pandemic-induced momentum across the care continuum. Hospitals are lagging far behind ambulatory care in implementing telehealth. As illustrated in the “4-T Matrix” (see graphic) we would like to examine four key reasons for such a sluggish initial uptake and try to propose four important strategies that may help us to maximize the value created by telehealth technologies.
1. Timing
The health care system has always lagged far behind other service industries in terms of technology adaptation. Because of the unique nature of health care services, face-to-face interaction supersedes all other forms of communication. A rapidly evolving pandemic was not matched by simultaneous technology education for patients and providers. The enormous choice of hard-to-navigate telehealth tools; time and labor-intensive implementation; and uncertainty around payer, policy, and regulatory expectations might have precluded providers from the rapid adoption of telehealth in the hospital setting. Patients’ specific characteristics, such as the absence of technology-centered education, information, age, comorbidities, lack of technical literacy, and dependency on caregivers contributed to the suboptimal response from patients and families.
Deploying simple, ubiquitous, user-friendly, and technologically less challenging telehealth solutions may be a better approach to increase the adoption of such solutions by providers and patients. Hospitals need to develop and distribute telehealth user guides in all possible modes of communication. Provider-centric in-service sessions, workshops, and live support by “superuser teams” often work well in reducing end-user resistance.
2. Technical
Current electronic medical records vary widely in their features and offerings, and their ability to interact with third-party software and platforms. Dissatisfaction of end users with EMRs is well known, as is their likely relationship to burnout. Recent research continues to show a strong relationship between EMR usability and the odds of burnout among physicians.5 In the current climate, administrators and health informaticists have the responsibility to avoid adding increased burdens to end users.
Another issue is the limited connectivity in many remote/rural areas that would impact implementation of telehealth platforms. Studies indicate that 33% of rural Americans lack access to high-speed broadband Internet to support video visits.6 The recent successful implementation of telehealth across 530 providers in 75 ambulatory practices operated by Munson Healthcare, a rural health system in northern Michigan, sheds light on the technology’s enormous potential in providing safe access to rural populations.6,7
Privacy and safety of patient data is of paramount importance. According to a national poll on healthy aging by the University of Michigan in May 2019, targeting older adults, 47% of survey responders expressed difficulty using technology and 49% of survey responders were concerned about privacy.8 Use of certification and other tools offered by the Office of the National Coordinator for Health Information Technology would help reassure users, and the ability to capture and share images between providers would be of immense benefit in facilitating e-consults.
The need of the hour is redesigned work flow, to help providers adopt and use virtual care/telehealth efficiently. Work flow redesign must be coupled with technological advances to allow seamless integration of third-party telehealth platforms into existing EMR systems or built directly into EMRs. Use of quality metrics and analytical tools specific to telehealth would help measure the technology’s impact on patient care, outcomes, and end-user/provider experience.
3. Teams and training
Outcomes of health care interventions are often determined by the effectiveness of teams. Irrespective of how robust health care systems may have been initially, rapidly spreading infectious diseases like COVID-19 can quickly derail the system, bringing the workforce and patients to a breaking point.5 Decentralized, uncoordinated, and siloed efforts by individual teams across the care continuum were contributing factors for the partial success of telehealth care delivery pathways. The hospital systems with telehealth-ready teams at the start of the COVID-19 pandemic were so rare that the knowledge and technical training opportunities for innovators grew severalfold during the pandemic.
As per the American Medical Association, telehealth success is massively dependent on building the right team. Core, leadership, advisory, and implementation teams comprised of clinical representatives, end users, administrative personnel, executive members of the organization, technical experts, and payment/policy experts should be put together before implementing a telehealth strategy.9 Seamless integration of hospital-based care with ambulatory care via a telehealth platform is only complete when care managers are trained and deployed to fulfill the needs of a diverse group of patients. Deriving overall value from telehealth is only possible when there is a skill development, training and mentoring team put in place.
4. Thinking
In most U.S. hospitals, inpatient health care is equally distributed between nonprocedure and procedure-based services. Hospitals resorted to suspension of nonemergent procedures to mitigate the risk of spreading COVID-19. This was further compounded by many patients’ self-selection to defer care, an abrupt reduction in the influx of patients from the referral base because of suboptimally operating ambulatory care services, leading to low hospital occupancy.
Hospitals across the nation have gone through a massive short-term financial crunch and unfavorable cash-flow forecast, which prompted a paradoxical work-force reduction. While some argue that it may be akin to strategic myopia, the authors believed that such a response is strategically imperative to keep the hospital afloat. It is reasonable to attribute the paucity of innovation to constrained resources, and health systems are simply staying overly optimistic about “weathering the storm” and reverting soon to “business as usual.” The technological framework necessary for deploying a telehealth solution often comes with a price. Financially challenged hospital systems rarely exercise any capital-intensive activities. By contrast, telehealth adoption by ambulatory care can result in quicker resumption of patient care in community settings. A lack of operational and infrastructure synchrony between ambulatory and in-hospital systems has failed to capture telehealth-driven inpatient volume. For example, direct admissions from ambulatory telehealth referrals was a missed opportunity in several places. Referrals for labs, diagnostic tests, and other allied services could have helped hospitals offset their fixed costs. Similarly, work flows related to discharge and postdischarge follow up rarely embrace telehealth tools or telehealth care pathways. A brisk change in the health care ecosystem is partly responsible for this.
Digital strategy needs to be incorporated into business strategy. For the reasons already discussed, telehealth technology is not a “nice to have” anymore, but a “must have.” At present, providers are of the opinion that about 20% of their patient services can be delivered via a telehealth platform. Similar trends are observed among patients, as a new modality of access to care is increasingly beneficial to them. Telehealth must be incorporated in standardized hospital work flows. Use of telehealth for preoperative clearance will greatly minimize same-day surgery cancellations. Given the potential shortage in resources, telehealth adoption for inpatient consultations will help systems conserve personal protective equipment, minimize the risk of staff exposure to COVID-19, and improve efficiency.
Digital strategy also prompts the reengineering of care delivery.10 Excessive and unused physical capacity can be converted into digital care hubs. Health maintenance, prevention, health promotion, health education, and chronic disease management not only can serve a variety of patient groups but can also help address the “last-mile problem” in health care. A successful digital strategy usually has three important components – Commitment: Hospital leadership is committed to include digital transformation as a strategic objective; Cost: Digital strategy is added as a line item in the budget; and Control: Measurable metrics are put in place to monitor the performance, impact, and influence of the digital strategy.
Conclusion
For decades, most U.S. health systems occupied the periphery of technological transformation when compared to the rest of the service industry. While most health systems took a heroic approach to the adoption of telehealth during COVID-19, despite being unprepared, the need for a systematic telehealth deployment is far from being adequately fulfilled. The COVID-19 pandemic brought permanent changes to several business disciplines globally. Given the impact of the pandemic on the health and overall wellbeing of American society, the U.S. health care industry must leave no stone unturned in its quest for transformation.
Dr. Lingisetty is a hospitalist and physician executive at Baptist Health System, Little Rock, Ark, and is cofounder/president of SHM’s Arkansas chapter. Dr. Prasad is medical director of care management and a hospitalist at Advocate Aurora Health in Milwaukee. He is cochair of SHM’s IT Special Interest Group, sits on the HQPS committee, and is president of SHM’s Wisconsin chapter. Dr. Palabindala is the medical director, utilization management, and physician advisory services at the University of Mississippi Medical Center and an associate professor of medicine and academic hospitalist at the University of Mississippi, both in Jackson.
References
1. Finnegan M. “Telehealth booms amid COVID-19 crisis.” Computerworld. 2020 Apr 27. www.computerworld.com/article/3540315/telehealth-booms-amid-covid-19-crisis-virtual-care-is-here-to-stay.html. Accessed 2020 Sep 12.
2. Department of Health & Human Services. “OCR Announces Notification of Enforcement Discretion for Telehealth Remote Communications During the COVID-19 Nationwide Public Health Emergency.” 2020 Mar 17. www.hhs.gov/about/news/2020/03/17/ocr-announces-notification-of-enforcement-discretion-for-telehealth-remote-communications-during-the-covid-19.html. Accessed 2020 Sep 12.
3. National Center for Health Statistics. “Health Expenditures.” www.cdc.gov/nchs/fastats/health-expenditures.htm. Accessed 2020 Sep 12.
4. Bestsennyy O et al. “Telehealth: A post–COVID-19 reality?” McKinsey & Company. 2020 May 29. www.mckinsey.com/industries/healthcare-systems-and-services/our-insights/telehealth-a-quarter-trillion-dollar-post-covid-19-reality. Accessed 2020 Sep 12.
5. Melnick ER et al. The Association Between Perceived Electronic Health Record Usability and Professional Burnout Among U.S. Physicians. Mayo Clin Proc. 2020 March;95(3):476-87.
6. Hirko KA et al. Telehealth in response to the COVID-19 pandemic: Implications for rural health disparities. J Am Med Inform Assoc. 2020 Nov;27(11):1816-8. .
7. American Academy of Family Physicians. “Study Examines Telehealth, Rural Disparities in Pandemic.” 2020 July 30. www.aafp.org/news/practice-professional-issues/20200730ruraltelehealth.html. Accessed 2020 Dec 15.
8. Kurlander J et al. “Virtual Visits: Telehealth and Older Adults.” National Poll on Healthy Aging. 2019 Oct. hdl.handle.net/2027.42/151376.
9. American Medical Association. Telehealth Implementation Playbook. 2019. www.ama-assn.org/system/files/2020-04/ama-telehealth-implementation-playbook.pdf.
10. Smith AC et al. Telehealth for global emergencies: Implications for coronavirus disease 2019 (COVID-19). J Telemed Telecare. 2020 Jun;26(5):309-13.
Decline in child COVID-19 cases picks up after 2-week slowdown
, according to data gathered by the American Academy of Pediatrics and the Children’s Hospital Association.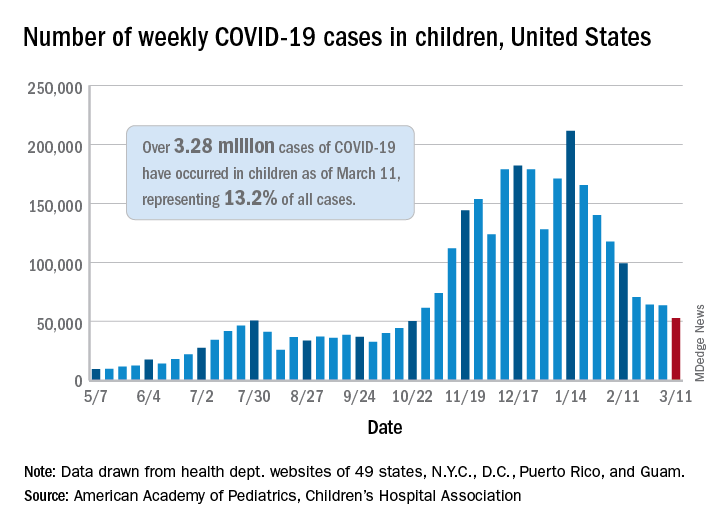
From Feb. 19 to March 4, the drop in new cases averaged just 5% each week, compared with 13.3% per week over the 5-week period from Jan. 15 to Feb. 18. For the week of March 5-11, a total of 52,695 COVID-19 cases were reported in children, down from 63,562 the previous week and the lowest number since late October, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
In those jurisdictions, 3.28 million children have been infected with SARS-CoV-2, representing 13.2% of all cases since the beginning of the pandemic. The cumulative rate of COVID-19 has now risen to 4,364 cases per 100,000 children nationally, with state rates ranging from 1,062 per 100,000 in Hawaii to 8,692 per 100,000 in North Dakota, the AAP and CHA said in their weekly COVID-19 report.
Hospitalization data are more limited – 24 states and New York City – but continue to show that serious illness is much less common in younger individuals: Children represent just 1.9% of all hospitalizations, and only 0.8% of the children who have been infected were hospitalized. Neither rate has changed since early February, the AAP and CHA said.
The number of deaths in children, however, rose from 253 to 266, the largest 1-week increase since early February in the 43 states (along with New York City, Puerto Rico, and Guam) that are tracking mortality data by age, the AAP and CHA reported.
Among those 46 jurisdictions, there are 10 (9 states and the District of Columbia) that have not yet reported a COVID-19–related child death, while Texas has almost twice as many deaths, 47, as the next state, Arizona, which has 24. Meanwhile, California’s total of 452,000 cases is almost 2½ times higher than the 183,000 recorded by Illinois, according to the report.
, according to data gathered by the American Academy of Pediatrics and the Children’s Hospital Association.
From Feb. 19 to March 4, the drop in new cases averaged just 5% each week, compared with 13.3% per week over the 5-week period from Jan. 15 to Feb. 18. For the week of March 5-11, a total of 52,695 COVID-19 cases were reported in children, down from 63,562 the previous week and the lowest number since late October, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
In those jurisdictions, 3.28 million children have been infected with SARS-CoV-2, representing 13.2% of all cases since the beginning of the pandemic. The cumulative rate of COVID-19 has now risen to 4,364 cases per 100,000 children nationally, with state rates ranging from 1,062 per 100,000 in Hawaii to 8,692 per 100,000 in North Dakota, the AAP and CHA said in their weekly COVID-19 report.
Hospitalization data are more limited – 24 states and New York City – but continue to show that serious illness is much less common in younger individuals: Children represent just 1.9% of all hospitalizations, and only 0.8% of the children who have been infected were hospitalized. Neither rate has changed since early February, the AAP and CHA said.
The number of deaths in children, however, rose from 253 to 266, the largest 1-week increase since early February in the 43 states (along with New York City, Puerto Rico, and Guam) that are tracking mortality data by age, the AAP and CHA reported.
Among those 46 jurisdictions, there are 10 (9 states and the District of Columbia) that have not yet reported a COVID-19–related child death, while Texas has almost twice as many deaths, 47, as the next state, Arizona, which has 24. Meanwhile, California’s total of 452,000 cases is almost 2½ times higher than the 183,000 recorded by Illinois, according to the report.
, according to data gathered by the American Academy of Pediatrics and the Children’s Hospital Association.
From Feb. 19 to March 4, the drop in new cases averaged just 5% each week, compared with 13.3% per week over the 5-week period from Jan. 15 to Feb. 18. For the week of March 5-11, a total of 52,695 COVID-19 cases were reported in children, down from 63,562 the previous week and the lowest number since late October, based on data from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
In those jurisdictions, 3.28 million children have been infected with SARS-CoV-2, representing 13.2% of all cases since the beginning of the pandemic. The cumulative rate of COVID-19 has now risen to 4,364 cases per 100,000 children nationally, with state rates ranging from 1,062 per 100,000 in Hawaii to 8,692 per 100,000 in North Dakota, the AAP and CHA said in their weekly COVID-19 report.
Hospitalization data are more limited – 24 states and New York City – but continue to show that serious illness is much less common in younger individuals: Children represent just 1.9% of all hospitalizations, and only 0.8% of the children who have been infected were hospitalized. Neither rate has changed since early February, the AAP and CHA said.
The number of deaths in children, however, rose from 253 to 266, the largest 1-week increase since early February in the 43 states (along with New York City, Puerto Rico, and Guam) that are tracking mortality data by age, the AAP and CHA reported.
Among those 46 jurisdictions, there are 10 (9 states and the District of Columbia) that have not yet reported a COVID-19–related child death, while Texas has almost twice as many deaths, 47, as the next state, Arizona, which has 24. Meanwhile, California’s total of 452,000 cases is almost 2½ times higher than the 183,000 recorded by Illinois, according to the report.
‘Major update’ of BP guidance for kidney disease; treat to 120 mm Hg
The new 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for blood pressure management for adults with chronic kidney disease (CKD) who are not receiving dialysis advises treating to a target systolic blood pressure of less than 120 mm Hg, provided measurements are “standardized” and that blood pressure is “measured properly.”
This blood pressure target – largely based on evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) – represents “a major update” from the 2012 KDIGO guideline, which advised clinicians to treat to a target blood pressure of less than or equal to 130/80 mm Hg for patients with albuminuria or less than or equal to 140/90 mm Hg for patients without albuminuria.
The new goal is also lower than the less than 130/80 mm Hg target in the 2017 American College of Cardiology/American Heart Association guideline.
In a study of the public health implications of the guideline, Kathryn Foti, PhD, and colleagues determined that 70% of U.S. adults with CKD would now be eligible for treatment to lower blood pressure, as opposed to 50% under the previous KDIGO guideline and 56% under the ACC/AHA guideline.
“This is a major update of an influential set of guidelines for chronic kidney disease patients” at a time when blood pressure control is worsening in the United States, Dr. Foti, a postdoctoral researcher in the department of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in a statement from her institution.
The 2021 KDIGO blood pressure guideline and executive summary and the public health implications study are published online in Kidney International.
First, ‘take blood pressure well’
The cochair of the new KDIGO guidelines, Alfred K. Cheung, MD, from the University of Utah, Salt Lake City, said in an interview that the guideline has “two important points.”
First, “take that blood pressure well,” he said. “That has a lot to do with patient preparation rather than any fancy instrument,” he emphasized.
Second, the guideline proposes a systolic blood pressure target of less than 120 mm Hg for most people with CKD not receiving dialysis, except for children and kidney transplant recipients. This target is “contingent on ‘standardized’ blood pressure measurement.”
The document provides a checklist for obtaining a standardized blood pressure measurement, adapted from the 2017 ACC/AHA blood pressure guidelines. It starts with the patient relaxed and sitting on a chair for more than 5 minutes.
In contrast to this measurement, a “routine” or “casual” office blood pressure measurement could be off by plus or minus 10 mm Hg, Dr. Cheung noted.
In a typical scenario, he continued, a patient cannot find a place to park, rushes into the clinic, and has his or her blood pressure checked right away, which would provide a “totally unreliable” reading. Adding a “fudge factor” (correction factor) would not provide an accurate reading.
Clinicians “would not settle for a potassium measurement that is 5.0 mmol/L plus or minus a few decimal points” to guide treatment, he pointed out.
Second, target 120, properly measured
“The very first chapter of the guidelines is devoted to blood pressure measurement, because we recognize if we’re going to do 120 [mm Hg] – the emphasis is on 120 measured properly – so we try to drive that point home,” Tara I. Chang, MD, guideline second author and a coauthor of the public health implications study, pointed out in an interview.
“There are a lot of other things that we base clinical decisions on where we really require some degree of precision, and blood pressure is important enough that to us it’s kind of in the same boat,” said Dr. Chang, from Stanford (Calif.) University.
“In SPRINT, people were randomized to less than less than 120 vs. less than 140 (they weren’t randomized to <130),” she noted.
“The recommendation should be widely adopted in clinical practice,” the guideline authors write, “since accurate measurements will ensure that proper guidance is being applied to the management of BP, as it is to the management of other risk factors.”
Still need individual treatment
Nevertheless, patients still need individualized treatment, the document stresses. “Not every patient with CKD will be appropriate to target to less than 120,” Dr. Chang said. However, “we want people to at least consider less than 120,” she added, to avoid therapeutic inertia.
“If you take the blood pressure in a standardized manner – such as in the ACCORD trial and in the SPRINT trial – even patients over 75 years old, or people over 80 years old, they have very little side effects,” Dr. Cheung noted.
“In the overall cohort,” he continued, “they do not have a significant increase in serious adverse events, do not have adverse events of postural hypotension, syncope, bradycardia, injurious falls – so people are worried about it, but it’s not borne out by the data.
“That said, I have two cautions,” Dr. Cheung noted. “One. If you drop somebody’s blood pressure rapidly over a week, you may be more likely to get in trouble. If you drop the blood pressure gradually over several weeks, several months, you’re much less likely to get into trouble.”
“Two. If the patient is old, you know the patient has carotid stenosis and already has postural dizziness, you may not want to try on that patient – but just because the patient is old is not the reason not to target 120.”
ACE inhibitors and ARBs beneficial in albuminuria, underused
“How do you get to less than 120? The short answer is, use whatever medications you need to – there is no necessarily right cocktail,” Dr. Chang said.
“We’ve known that angiotensin-converting enzyme (ACE) inhibitors and ARBs [angiotensin II receptor blockers] are beneficial in patients with CKD and in particular those with heavier albuminuria,” she continued. “We’ve known this for over 20 years.”
Yet, the study identified underutilization – “a persistent gap, just like blood pressure control and awareness,” she noted. “We’re just not making much headway.
“We are not recommending ACE inhibitors or ARBs for all the patients,” Dr. Cheung clarified. “If you are diabetic and have heavy proteinuria, that’s when the use of ACE inhibitors and ARBs are most indicated.”
Public health implications
SPRINT showed that treating to a systolic blood pressure of less than 120 mm Hg vs. less than 140 mm Hg reduced the risk for cardiovascular disease by 25% and all-cause mortality by 27% for participants with and those without CKD, Dr. Foti and colleagues stress.
They aimed to estimate how the new guideline would affect (1) the number of U.S. patients with CKD who would be eligible for blood pressure lowering treatment, and (2) the proportion of those with albuminuria who would be eligible for an ACE inhibitor or an ARB.
The researchers analyzed data from 1,699 adults with CKD (estimated glomerular filtration rate, 15-59 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio of ≥30 mg/g) who participated in the 2015-2018 National Health and Nutrition Examination Survey.
Both the 2021 and 2012 KDIGO guidelines recommend that patients with albuminuria and blood pressure higher than the target value who are not kidney transplant recipients should be treated with an ACE inhibitor or an ARB.
On the basis of the new target, 78% of patients with CKD and albuminuria were eligible for ACE inhibitor/ARB treatment by the 2021 KDIGO guideline, compared with 71% by the 2012 KDIGO guideline. However, only 39% were taking one of these drugs.
These findings show that “with the new guideline and with the lower blood pressure target, you potentially have an even larger pool of people who have blood pressure that’s not under control, and a potential larger group of people who may benefit from ACE inhibitors and ARBs,” Dr. Chang said.
“Our paper is not the only one to show that we haven’t made a whole lot of progress,” she said, “and now that the bar has been lowered, there [have] to be some renewed efforts on controlling blood pressure, because we know that blood pressure control is such an important risk factor for cardiovascular outcomes.”
Dr. Foti is supported by an NIH/National Heart, Lung, and Blood Institute grant. Dr. Cheung has received consultancy fees from Amgen, Bard, Boehringer Ingelheim, Calliditas, Tricida, and UpToDate, and grant/research support from the National Institutes of Health for SPRINT (monies paid to institution). Dr. Chang has received consultancy fees from Bayer, Gilead, Janssen Research and Development, Novo Nordisk, Tricida, and Vascular Dynamics; grant/research support from AstraZeneca and Satellite Healthcare (monies paid to institution), the NIH, and the American Heart Association; is on advisory boards for AstraZeneca and Fresenius Medical Care Renal Therapies Group; and has received workshop honoraria from Fresenius. Disclosures of relevant financial relationships of the other authors are listed in the original articles.
A version of this article first appeared on Medscape.com.
The new 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for blood pressure management for adults with chronic kidney disease (CKD) who are not receiving dialysis advises treating to a target systolic blood pressure of less than 120 mm Hg, provided measurements are “standardized” and that blood pressure is “measured properly.”
This blood pressure target – largely based on evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) – represents “a major update” from the 2012 KDIGO guideline, which advised clinicians to treat to a target blood pressure of less than or equal to 130/80 mm Hg for patients with albuminuria or less than or equal to 140/90 mm Hg for patients without albuminuria.
The new goal is also lower than the less than 130/80 mm Hg target in the 2017 American College of Cardiology/American Heart Association guideline.
In a study of the public health implications of the guideline, Kathryn Foti, PhD, and colleagues determined that 70% of U.S. adults with CKD would now be eligible for treatment to lower blood pressure, as opposed to 50% under the previous KDIGO guideline and 56% under the ACC/AHA guideline.
“This is a major update of an influential set of guidelines for chronic kidney disease patients” at a time when blood pressure control is worsening in the United States, Dr. Foti, a postdoctoral researcher in the department of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in a statement from her institution.
The 2021 KDIGO blood pressure guideline and executive summary and the public health implications study are published online in Kidney International.
First, ‘take blood pressure well’
The cochair of the new KDIGO guidelines, Alfred K. Cheung, MD, from the University of Utah, Salt Lake City, said in an interview that the guideline has “two important points.”
First, “take that blood pressure well,” he said. “That has a lot to do with patient preparation rather than any fancy instrument,” he emphasized.
Second, the guideline proposes a systolic blood pressure target of less than 120 mm Hg for most people with CKD not receiving dialysis, except for children and kidney transplant recipients. This target is “contingent on ‘standardized’ blood pressure measurement.”
The document provides a checklist for obtaining a standardized blood pressure measurement, adapted from the 2017 ACC/AHA blood pressure guidelines. It starts with the patient relaxed and sitting on a chair for more than 5 minutes.
In contrast to this measurement, a “routine” or “casual” office blood pressure measurement could be off by plus or minus 10 mm Hg, Dr. Cheung noted.
In a typical scenario, he continued, a patient cannot find a place to park, rushes into the clinic, and has his or her blood pressure checked right away, which would provide a “totally unreliable” reading. Adding a “fudge factor” (correction factor) would not provide an accurate reading.
Clinicians “would not settle for a potassium measurement that is 5.0 mmol/L plus or minus a few decimal points” to guide treatment, he pointed out.
Second, target 120, properly measured
“The very first chapter of the guidelines is devoted to blood pressure measurement, because we recognize if we’re going to do 120 [mm Hg] – the emphasis is on 120 measured properly – so we try to drive that point home,” Tara I. Chang, MD, guideline second author and a coauthor of the public health implications study, pointed out in an interview.
“There are a lot of other things that we base clinical decisions on where we really require some degree of precision, and blood pressure is important enough that to us it’s kind of in the same boat,” said Dr. Chang, from Stanford (Calif.) University.
“In SPRINT, people were randomized to less than less than 120 vs. less than 140 (they weren’t randomized to <130),” she noted.
“The recommendation should be widely adopted in clinical practice,” the guideline authors write, “since accurate measurements will ensure that proper guidance is being applied to the management of BP, as it is to the management of other risk factors.”
Still need individual treatment
Nevertheless, patients still need individualized treatment, the document stresses. “Not every patient with CKD will be appropriate to target to less than 120,” Dr. Chang said. However, “we want people to at least consider less than 120,” she added, to avoid therapeutic inertia.
“If you take the blood pressure in a standardized manner – such as in the ACCORD trial and in the SPRINT trial – even patients over 75 years old, or people over 80 years old, they have very little side effects,” Dr. Cheung noted.
“In the overall cohort,” he continued, “they do not have a significant increase in serious adverse events, do not have adverse events of postural hypotension, syncope, bradycardia, injurious falls – so people are worried about it, but it’s not borne out by the data.
“That said, I have two cautions,” Dr. Cheung noted. “One. If you drop somebody’s blood pressure rapidly over a week, you may be more likely to get in trouble. If you drop the blood pressure gradually over several weeks, several months, you’re much less likely to get into trouble.”
“Two. If the patient is old, you know the patient has carotid stenosis and already has postural dizziness, you may not want to try on that patient – but just because the patient is old is not the reason not to target 120.”
ACE inhibitors and ARBs beneficial in albuminuria, underused
“How do you get to less than 120? The short answer is, use whatever medications you need to – there is no necessarily right cocktail,” Dr. Chang said.
“We’ve known that angiotensin-converting enzyme (ACE) inhibitors and ARBs [angiotensin II receptor blockers] are beneficial in patients with CKD and in particular those with heavier albuminuria,” she continued. “We’ve known this for over 20 years.”
Yet, the study identified underutilization – “a persistent gap, just like blood pressure control and awareness,” she noted. “We’re just not making much headway.
“We are not recommending ACE inhibitors or ARBs for all the patients,” Dr. Cheung clarified. “If you are diabetic and have heavy proteinuria, that’s when the use of ACE inhibitors and ARBs are most indicated.”
Public health implications
SPRINT showed that treating to a systolic blood pressure of less than 120 mm Hg vs. less than 140 mm Hg reduced the risk for cardiovascular disease by 25% and all-cause mortality by 27% for participants with and those without CKD, Dr. Foti and colleagues stress.
They aimed to estimate how the new guideline would affect (1) the number of U.S. patients with CKD who would be eligible for blood pressure lowering treatment, and (2) the proportion of those with albuminuria who would be eligible for an ACE inhibitor or an ARB.
The researchers analyzed data from 1,699 adults with CKD (estimated glomerular filtration rate, 15-59 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio of ≥30 mg/g) who participated in the 2015-2018 National Health and Nutrition Examination Survey.
Both the 2021 and 2012 KDIGO guidelines recommend that patients with albuminuria and blood pressure higher than the target value who are not kidney transplant recipients should be treated with an ACE inhibitor or an ARB.
On the basis of the new target, 78% of patients with CKD and albuminuria were eligible for ACE inhibitor/ARB treatment by the 2021 KDIGO guideline, compared with 71% by the 2012 KDIGO guideline. However, only 39% were taking one of these drugs.
These findings show that “with the new guideline and with the lower blood pressure target, you potentially have an even larger pool of people who have blood pressure that’s not under control, and a potential larger group of people who may benefit from ACE inhibitors and ARBs,” Dr. Chang said.
“Our paper is not the only one to show that we haven’t made a whole lot of progress,” she said, “and now that the bar has been lowered, there [have] to be some renewed efforts on controlling blood pressure, because we know that blood pressure control is such an important risk factor for cardiovascular outcomes.”
Dr. Foti is supported by an NIH/National Heart, Lung, and Blood Institute grant. Dr. Cheung has received consultancy fees from Amgen, Bard, Boehringer Ingelheim, Calliditas, Tricida, and UpToDate, and grant/research support from the National Institutes of Health for SPRINT (monies paid to institution). Dr. Chang has received consultancy fees from Bayer, Gilead, Janssen Research and Development, Novo Nordisk, Tricida, and Vascular Dynamics; grant/research support from AstraZeneca and Satellite Healthcare (monies paid to institution), the NIH, and the American Heart Association; is on advisory boards for AstraZeneca and Fresenius Medical Care Renal Therapies Group; and has received workshop honoraria from Fresenius. Disclosures of relevant financial relationships of the other authors are listed in the original articles.
A version of this article first appeared on Medscape.com.
The new 2021 Kidney Disease: Improving Global Outcomes (KDIGO) clinical practice guideline for blood pressure management for adults with chronic kidney disease (CKD) who are not receiving dialysis advises treating to a target systolic blood pressure of less than 120 mm Hg, provided measurements are “standardized” and that blood pressure is “measured properly.”
This blood pressure target – largely based on evidence from the Systolic Blood Pressure Intervention Trial (SPRINT) – represents “a major update” from the 2012 KDIGO guideline, which advised clinicians to treat to a target blood pressure of less than or equal to 130/80 mm Hg for patients with albuminuria or less than or equal to 140/90 mm Hg for patients without albuminuria.
The new goal is also lower than the less than 130/80 mm Hg target in the 2017 American College of Cardiology/American Heart Association guideline.
In a study of the public health implications of the guideline, Kathryn Foti, PhD, and colleagues determined that 70% of U.S. adults with CKD would now be eligible for treatment to lower blood pressure, as opposed to 50% under the previous KDIGO guideline and 56% under the ACC/AHA guideline.
“This is a major update of an influential set of guidelines for chronic kidney disease patients” at a time when blood pressure control is worsening in the United States, Dr. Foti, a postdoctoral researcher in the department of epidemiology at Johns Hopkins Bloomberg School of Public Health, Baltimore, said in a statement from her institution.
The 2021 KDIGO blood pressure guideline and executive summary and the public health implications study are published online in Kidney International.
First, ‘take blood pressure well’
The cochair of the new KDIGO guidelines, Alfred K. Cheung, MD, from the University of Utah, Salt Lake City, said in an interview that the guideline has “two important points.”
First, “take that blood pressure well,” he said. “That has a lot to do with patient preparation rather than any fancy instrument,” he emphasized.
Second, the guideline proposes a systolic blood pressure target of less than 120 mm Hg for most people with CKD not receiving dialysis, except for children and kidney transplant recipients. This target is “contingent on ‘standardized’ blood pressure measurement.”
The document provides a checklist for obtaining a standardized blood pressure measurement, adapted from the 2017 ACC/AHA blood pressure guidelines. It starts with the patient relaxed and sitting on a chair for more than 5 minutes.
In contrast to this measurement, a “routine” or “casual” office blood pressure measurement could be off by plus or minus 10 mm Hg, Dr. Cheung noted.
In a typical scenario, he continued, a patient cannot find a place to park, rushes into the clinic, and has his or her blood pressure checked right away, which would provide a “totally unreliable” reading. Adding a “fudge factor” (correction factor) would not provide an accurate reading.
Clinicians “would not settle for a potassium measurement that is 5.0 mmol/L plus or minus a few decimal points” to guide treatment, he pointed out.
Second, target 120, properly measured
“The very first chapter of the guidelines is devoted to blood pressure measurement, because we recognize if we’re going to do 120 [mm Hg] – the emphasis is on 120 measured properly – so we try to drive that point home,” Tara I. Chang, MD, guideline second author and a coauthor of the public health implications study, pointed out in an interview.
“There are a lot of other things that we base clinical decisions on where we really require some degree of precision, and blood pressure is important enough that to us it’s kind of in the same boat,” said Dr. Chang, from Stanford (Calif.) University.
“In SPRINT, people were randomized to less than less than 120 vs. less than 140 (they weren’t randomized to <130),” she noted.
“The recommendation should be widely adopted in clinical practice,” the guideline authors write, “since accurate measurements will ensure that proper guidance is being applied to the management of BP, as it is to the management of other risk factors.”
Still need individual treatment
Nevertheless, patients still need individualized treatment, the document stresses. “Not every patient with CKD will be appropriate to target to less than 120,” Dr. Chang said. However, “we want people to at least consider less than 120,” she added, to avoid therapeutic inertia.
“If you take the blood pressure in a standardized manner – such as in the ACCORD trial and in the SPRINT trial – even patients over 75 years old, or people over 80 years old, they have very little side effects,” Dr. Cheung noted.
“In the overall cohort,” he continued, “they do not have a significant increase in serious adverse events, do not have adverse events of postural hypotension, syncope, bradycardia, injurious falls – so people are worried about it, but it’s not borne out by the data.
“That said, I have two cautions,” Dr. Cheung noted. “One. If you drop somebody’s blood pressure rapidly over a week, you may be more likely to get in trouble. If you drop the blood pressure gradually over several weeks, several months, you’re much less likely to get into trouble.”
“Two. If the patient is old, you know the patient has carotid stenosis and already has postural dizziness, you may not want to try on that patient – but just because the patient is old is not the reason not to target 120.”
ACE inhibitors and ARBs beneficial in albuminuria, underused
“How do you get to less than 120? The short answer is, use whatever medications you need to – there is no necessarily right cocktail,” Dr. Chang said.
“We’ve known that angiotensin-converting enzyme (ACE) inhibitors and ARBs [angiotensin II receptor blockers] are beneficial in patients with CKD and in particular those with heavier albuminuria,” she continued. “We’ve known this for over 20 years.”
Yet, the study identified underutilization – “a persistent gap, just like blood pressure control and awareness,” she noted. “We’re just not making much headway.
“We are not recommending ACE inhibitors or ARBs for all the patients,” Dr. Cheung clarified. “If you are diabetic and have heavy proteinuria, that’s when the use of ACE inhibitors and ARBs are most indicated.”
Public health implications
SPRINT showed that treating to a systolic blood pressure of less than 120 mm Hg vs. less than 140 mm Hg reduced the risk for cardiovascular disease by 25% and all-cause mortality by 27% for participants with and those without CKD, Dr. Foti and colleagues stress.
They aimed to estimate how the new guideline would affect (1) the number of U.S. patients with CKD who would be eligible for blood pressure lowering treatment, and (2) the proportion of those with albuminuria who would be eligible for an ACE inhibitor or an ARB.
The researchers analyzed data from 1,699 adults with CKD (estimated glomerular filtration rate, 15-59 mL/min/1.73 m2 or a urinary albumin-to-creatinine ratio of ≥30 mg/g) who participated in the 2015-2018 National Health and Nutrition Examination Survey.
Both the 2021 and 2012 KDIGO guidelines recommend that patients with albuminuria and blood pressure higher than the target value who are not kidney transplant recipients should be treated with an ACE inhibitor or an ARB.
On the basis of the new target, 78% of patients with CKD and albuminuria were eligible for ACE inhibitor/ARB treatment by the 2021 KDIGO guideline, compared with 71% by the 2012 KDIGO guideline. However, only 39% were taking one of these drugs.
These findings show that “with the new guideline and with the lower blood pressure target, you potentially have an even larger pool of people who have blood pressure that’s not under control, and a potential larger group of people who may benefit from ACE inhibitors and ARBs,” Dr. Chang said.
“Our paper is not the only one to show that we haven’t made a whole lot of progress,” she said, “and now that the bar has been lowered, there [have] to be some renewed efforts on controlling blood pressure, because we know that blood pressure control is such an important risk factor for cardiovascular outcomes.”
Dr. Foti is supported by an NIH/National Heart, Lung, and Blood Institute grant. Dr. Cheung has received consultancy fees from Amgen, Bard, Boehringer Ingelheim, Calliditas, Tricida, and UpToDate, and grant/research support from the National Institutes of Health for SPRINT (monies paid to institution). Dr. Chang has received consultancy fees from Bayer, Gilead, Janssen Research and Development, Novo Nordisk, Tricida, and Vascular Dynamics; grant/research support from AstraZeneca and Satellite Healthcare (monies paid to institution), the NIH, and the American Heart Association; is on advisory boards for AstraZeneca and Fresenius Medical Care Renal Therapies Group; and has received workshop honoraria from Fresenius. Disclosures of relevant financial relationships of the other authors are listed in the original articles.
A version of this article first appeared on Medscape.com.
Hospitalist movers and shakers – March 2021
Vivek H. Murthy, MD, was named by President Joe Biden as his selection for Surgeon General of the United States. Dr. Murthy filled the same role from 2014-17 during President Barack Obama’s administration.
Dr. Murthy was a hospitalist and an instructor at Brigham and Women’s Hospital at Harvard Medical School prior to becoming surgeon general the first time. He also is the founder of Doctors for America.
David Tupponce, MD, recently was named the new president of Allegheny Health Network’s Grove City (Pa.) Medical Center. He takes over for interim president Allan Klapper, MD, who filled the position since August 2020.
Dr. Tupponce comes to Grove City Medical Center after a successful tenure as president of Central Maine Medical Center (Lewiston, Maine), where he grew its physician group and fine-tuned the hospital quality program. Prior to that, he was chief executive officer at Tenet Healthcare’s Abrazo Scottsdale (Ariz.) Campus and CEO at Paradise Valley Hospital (Phoenix, Ariz.).
Dr. Tupponce is familiar with western Pennsylvania, having earned a master’s degree in medical management from Carnegie Mellon University in Pittsburgh. He also was chief resident at the University of Pittsburgh Medical Center.
Malcolm Mar Fan, MD, has been elevated to medical director of the Hospitalist Group at Evangelical Community Hospital (Lewisburg, Pa.). In the newly established position, Dr. Mar Fan will oversee all operations for the facility’s hospitalist program.
Dr. Mar Fan has been a hospitalist at Evangelical since 2014 after completing his internist residency at Albert Einstein Medical Center in Philadelphia. He has played a major role on Evangelical’s Peri-operative Glucose Management Committee and its Informatics Committee for Impatient and Outpatient Electronic Health Records.
Lyon County (Kansas) recently announced that Ladun Oyenuga, MD, has been appointed as public health officer for the county. She began her tenure on January 1.
Dr. Oyenuga is a hospitalist at Newman Regional Health (Emporia, Kan.). She is a native of Nigeria and did her residency at Harlem (N.Y.) Hospital Center. She has been with Newman since 2017.
Cherese Mari Laulhere BirthCare Center (Long Beach, Calif.) recently announced the addition of an OB hospitalist program at Miller Children’s & Women’s Hospital. OB hospitalists, or laborists, care for women with obstetrical issues while in the hospital.
At Cherese Mari Laulhere, OB hospitalists will be on hand 24 hours a day to assist patients’ OB/GYNs or to fill in if the personal physician cannot get to the hospital quickly.
Hospitalists at Nationwide Children’s (Columbus, Ohio) are now providing care for children who are hospitalized at Adena Regional Medical Center (Chillicothe, Ohio).
It is an expansion of an ongoing partnership between the two hospitals. Adena and Nationwide Children’s have been working together in helping to care for children in the south central and southern Ohio region since 2011. Nationwide Children’s hospitalists will round in special care and the well-baby nursery at Adena, as well as provide education programs for Adena providers and staff.
MultiCare Health System (Tacoma, Wash.) has announced that it will expand its hospitalist program partnership with Sound Physicians, also based in Tacoma, to create a region-wide, cohesive group of providers. The goal is to help ensure efficient management of inpatient populations as a region instead of at the individual hospital level, and will allow MultiCare to implement standard tools, processes and regionwide best practices.
The hospitalist programs at Tacoma General Hospital, Allenmore Hospital and Covington Medical Center will transition to Sound Physicians on April 5, 2021. Sound hospitalists are already working at three other MultiCare facilities – Tacoma General Hospital, Allenmore Hospital, and Covington Medical Center.
Vivek H. Murthy, MD, was named by President Joe Biden as his selection for Surgeon General of the United States. Dr. Murthy filled the same role from 2014-17 during President Barack Obama’s administration.
Dr. Murthy was a hospitalist and an instructor at Brigham and Women’s Hospital at Harvard Medical School prior to becoming surgeon general the first time. He also is the founder of Doctors for America.
David Tupponce, MD, recently was named the new president of Allegheny Health Network’s Grove City (Pa.) Medical Center. He takes over for interim president Allan Klapper, MD, who filled the position since August 2020.
Dr. Tupponce comes to Grove City Medical Center after a successful tenure as president of Central Maine Medical Center (Lewiston, Maine), where he grew its physician group and fine-tuned the hospital quality program. Prior to that, he was chief executive officer at Tenet Healthcare’s Abrazo Scottsdale (Ariz.) Campus and CEO at Paradise Valley Hospital (Phoenix, Ariz.).
Dr. Tupponce is familiar with western Pennsylvania, having earned a master’s degree in medical management from Carnegie Mellon University in Pittsburgh. He also was chief resident at the University of Pittsburgh Medical Center.
Malcolm Mar Fan, MD, has been elevated to medical director of the Hospitalist Group at Evangelical Community Hospital (Lewisburg, Pa.). In the newly established position, Dr. Mar Fan will oversee all operations for the facility’s hospitalist program.
Dr. Mar Fan has been a hospitalist at Evangelical since 2014 after completing his internist residency at Albert Einstein Medical Center in Philadelphia. He has played a major role on Evangelical’s Peri-operative Glucose Management Committee and its Informatics Committee for Impatient and Outpatient Electronic Health Records.
Lyon County (Kansas) recently announced that Ladun Oyenuga, MD, has been appointed as public health officer for the county. She began her tenure on January 1.
Dr. Oyenuga is a hospitalist at Newman Regional Health (Emporia, Kan.). She is a native of Nigeria and did her residency at Harlem (N.Y.) Hospital Center. She has been with Newman since 2017.
Cherese Mari Laulhere BirthCare Center (Long Beach, Calif.) recently announced the addition of an OB hospitalist program at Miller Children’s & Women’s Hospital. OB hospitalists, or laborists, care for women with obstetrical issues while in the hospital.
At Cherese Mari Laulhere, OB hospitalists will be on hand 24 hours a day to assist patients’ OB/GYNs or to fill in if the personal physician cannot get to the hospital quickly.
Hospitalists at Nationwide Children’s (Columbus, Ohio) are now providing care for children who are hospitalized at Adena Regional Medical Center (Chillicothe, Ohio).
It is an expansion of an ongoing partnership between the two hospitals. Adena and Nationwide Children’s have been working together in helping to care for children in the south central and southern Ohio region since 2011. Nationwide Children’s hospitalists will round in special care and the well-baby nursery at Adena, as well as provide education programs for Adena providers and staff.
MultiCare Health System (Tacoma, Wash.) has announced that it will expand its hospitalist program partnership with Sound Physicians, also based in Tacoma, to create a region-wide, cohesive group of providers. The goal is to help ensure efficient management of inpatient populations as a region instead of at the individual hospital level, and will allow MultiCare to implement standard tools, processes and regionwide best practices.
The hospitalist programs at Tacoma General Hospital, Allenmore Hospital and Covington Medical Center will transition to Sound Physicians on April 5, 2021. Sound hospitalists are already working at three other MultiCare facilities – Tacoma General Hospital, Allenmore Hospital, and Covington Medical Center.
Vivek H. Murthy, MD, was named by President Joe Biden as his selection for Surgeon General of the United States. Dr. Murthy filled the same role from 2014-17 during President Barack Obama’s administration.
Dr. Murthy was a hospitalist and an instructor at Brigham and Women’s Hospital at Harvard Medical School prior to becoming surgeon general the first time. He also is the founder of Doctors for America.
David Tupponce, MD, recently was named the new president of Allegheny Health Network’s Grove City (Pa.) Medical Center. He takes over for interim president Allan Klapper, MD, who filled the position since August 2020.
Dr. Tupponce comes to Grove City Medical Center after a successful tenure as president of Central Maine Medical Center (Lewiston, Maine), where he grew its physician group and fine-tuned the hospital quality program. Prior to that, he was chief executive officer at Tenet Healthcare’s Abrazo Scottsdale (Ariz.) Campus and CEO at Paradise Valley Hospital (Phoenix, Ariz.).
Dr. Tupponce is familiar with western Pennsylvania, having earned a master’s degree in medical management from Carnegie Mellon University in Pittsburgh. He also was chief resident at the University of Pittsburgh Medical Center.
Malcolm Mar Fan, MD, has been elevated to medical director of the Hospitalist Group at Evangelical Community Hospital (Lewisburg, Pa.). In the newly established position, Dr. Mar Fan will oversee all operations for the facility’s hospitalist program.
Dr. Mar Fan has been a hospitalist at Evangelical since 2014 after completing his internist residency at Albert Einstein Medical Center in Philadelphia. He has played a major role on Evangelical’s Peri-operative Glucose Management Committee and its Informatics Committee for Impatient and Outpatient Electronic Health Records.
Lyon County (Kansas) recently announced that Ladun Oyenuga, MD, has been appointed as public health officer for the county. She began her tenure on January 1.
Dr. Oyenuga is a hospitalist at Newman Regional Health (Emporia, Kan.). She is a native of Nigeria and did her residency at Harlem (N.Y.) Hospital Center. She has been with Newman since 2017.
Cherese Mari Laulhere BirthCare Center (Long Beach, Calif.) recently announced the addition of an OB hospitalist program at Miller Children’s & Women’s Hospital. OB hospitalists, or laborists, care for women with obstetrical issues while in the hospital.
At Cherese Mari Laulhere, OB hospitalists will be on hand 24 hours a day to assist patients’ OB/GYNs or to fill in if the personal physician cannot get to the hospital quickly.
Hospitalists at Nationwide Children’s (Columbus, Ohio) are now providing care for children who are hospitalized at Adena Regional Medical Center (Chillicothe, Ohio).
It is an expansion of an ongoing partnership between the two hospitals. Adena and Nationwide Children’s have been working together in helping to care for children in the south central and southern Ohio region since 2011. Nationwide Children’s hospitalists will round in special care and the well-baby nursery at Adena, as well as provide education programs for Adena providers and staff.
MultiCare Health System (Tacoma, Wash.) has announced that it will expand its hospitalist program partnership with Sound Physicians, also based in Tacoma, to create a region-wide, cohesive group of providers. The goal is to help ensure efficient management of inpatient populations as a region instead of at the individual hospital level, and will allow MultiCare to implement standard tools, processes and regionwide best practices.
The hospitalist programs at Tacoma General Hospital, Allenmore Hospital and Covington Medical Center will transition to Sound Physicians on April 5, 2021. Sound hospitalists are already working at three other MultiCare facilities – Tacoma General Hospital, Allenmore Hospital, and Covington Medical Center.
First pill for COVID-19 could be ready by year’s end
New pills to treat patients with COVID-19 are currently in midstage clinical trials and, if successful, could be ready by the end of the year.
Only one treatment – remdesivir (Veklury) – has been fully approved by the U.S. Food and Drug Administration for patients in the hospital and it must be administered intravenously.
Hopes for a day when patients with COVID-19 can take a pill to rid their bodies of the virus got a boost when early trial results were presented at a medical conference.
Interim phase 2 results for the oral experimental COVID-19 drug molnupiravir, designed to do for patients with COVID-19 what oseltamivir (Tamiflu) can do for patients with the flu, were presented at the Conference on Retroviruses and Opportunistic Infections 2021 Annual Meeting, as reported by this news organization.
In the small study, the pill significantly reduced infectious virus in patients who were symptomatic and had tested positive for COVID-19 during the previous 4 days but were not hospitalized.
After 5 days of treatment, no participants who received molnupiravir had detectable virus, whereas 24% who received placebo did.
Two other oral agents are being developed by RedHill Biopharma: one for severe COVID-19 infection for hospitalized patients and one for patients at home with mild infection.
The first, opaganib (Yeliva), proceeded to a phase 2/3 global trial for hospitalized patients after the company announced top-line safety and efficacy data in December. In phase 2, the drug was shown to be safe in patients requiring oxygen and effectively reduced the need for oxygen by the end of the treatment period.
A key feature is that it is both an antiviral and an anti-inflammatory, Gilead Raday, RedHill’s chief operating officer, said in an interview. Data are expected midyear on its performance in 464 patients. The drug is being tested on top of remdesivir or in addition to dexamethasone.
The second, upamostat (RHB-107), is currently undergoing a phase 2/3 trial in the United States and is being investigated for use in nonhospitalized COVID-19 patients.
“I would expect data to be available in the second half of this year,” Mr. Raday said.
Upamostat is a novel serine protease inhibitor expected to be effective against emerging variants because it targets human cell factors involved in viral entry, according to the company.
Other drugs are being investigated in trials that are in earlier stages.
Urgent need for oral agents
Infectious disease specialists are watching the move toward a COVID-19 pill enthusiastically.
“We badly need an oral treatment option for COVID,” said Sarah Doernberg, MD, an infectious disease specialist from the University of California, San Francisco.
“It’s a real gap in our armamentarium for COVID in outpatient treatment, which is where most who contract COVID-19 will seek care,” she said in an interview.
Although some studies have shown the benefit of monoclonal antibodies for prevention and early treatment, there are major logistical issues because all the current options require IV administration, she explained.
“If we had a pill to treat early COVID, especially in high-risk patients, it would fill a gap,” she said, noting that a pill could help people get better faster and prevent hospital stays.
Studies of molnupiravir suggest that it decreases viral shedding in the first few days after COVID infection, Dr. Doernberg reported.
There is excitement around the drug, but it will be important to see whether the results translate into fewer people requiring hospital admission and whether people feel better faster.
“I want to see the clinical data,” Dr. Doernberg said.
She will also be watching for the upamostat and opaganib results in the coming weeks.
“If these drugs are successful, I think it’s possible we could use them – maybe under an emergency use authorization – this year,” she said.
Once antiviral pills are a viable option for COVID-19 treatment, questions will arise about their use, she said.
One question is whether patients who are getting remdesivir in the hospital and are ready to leave after 5 days should continue treatment with antiviral pills at home.
Another is whether the pills – if they are shown to be effective – will be helpful for COVID post exposure. That use would be important for people who do not have COVID-19 but who are in close contact with someone who does, such as a member of their household.
“We have that model,” Dr. Doernberg said. “We know that oseltamivir can be used for postexposure prophylaxis and can help to prevent development of clinical disease.”
But she cautioned that a challenge with COVID is that people are contagious very early. A pill would need to come with the ability to test for COVID-19 early and get patients linked to care immediately.
“Those are not small challenges,” she said.
Vaccines alone won’t end the COVID threat
Treatments are part of the “belt-and-suspenders” approach, along with vaccines to combat COVID-19, Dr. Doernberg said.
“We’re not going to eradicate COVID,” she said. “We’re still going to need treatments for people who either don’t respond to the vaccine or haven’t gotten the vaccine or developed disease despite the vaccine.”
Oral formulations are desperately needed, agreed Kenneth Johnson, PhD, professor of molecular biosciences at the University of Texas at Austin.
Right now, remdesivir treatments involve patients being hooked up to an IV for 30-120 minutes each day for 5 days. And the cost of a 5-day course of remdesivir ranges from $2340 to $3120 in the United States.
“We’re hoping we can come up with something that is a little bit easier to administer, and without as many concerns for toxic side effects,” he said.
Dr. Johnson’s team at UT-Austin recently made a key discovery about the way remdesivir stops the replication of viral RNA.
The understanding of where the virus starts to replicate in the infection chain of events and how and where it reacts with remdesivir might lead to the development of better, more concentrated pill forms of antivirals in the future, with fewer toxicities, he said.
The team used a lab dish to recreate the step-by-step process that occurs when a patient who is infected with SARS-CoV-2 receives remdesivir.
The discovery was published online in Molecular Cell in January and will be printed in the April issue of the journal.
The discovery won’t lead to an effective COVID-19 pill for our current crisis, but will be important for the next generation of drugs needed to deal with future coronaviruses, Dr. Johnson explained.
And there will be other coronaviruses, he said, noting that this one is the third in 20 years to jump from animals to humans. “It’s just a matter of time,” he said.
A version of this article first appeared on Medscape.com.
New pills to treat patients with COVID-19 are currently in midstage clinical trials and, if successful, could be ready by the end of the year.
Only one treatment – remdesivir (Veklury) – has been fully approved by the U.S. Food and Drug Administration for patients in the hospital and it must be administered intravenously.
Hopes for a day when patients with COVID-19 can take a pill to rid their bodies of the virus got a boost when early trial results were presented at a medical conference.
Interim phase 2 results for the oral experimental COVID-19 drug molnupiravir, designed to do for patients with COVID-19 what oseltamivir (Tamiflu) can do for patients with the flu, were presented at the Conference on Retroviruses and Opportunistic Infections 2021 Annual Meeting, as reported by this news organization.
In the small study, the pill significantly reduced infectious virus in patients who were symptomatic and had tested positive for COVID-19 during the previous 4 days but were not hospitalized.
After 5 days of treatment, no participants who received molnupiravir had detectable virus, whereas 24% who received placebo did.
Two other oral agents are being developed by RedHill Biopharma: one for severe COVID-19 infection for hospitalized patients and one for patients at home with mild infection.
The first, opaganib (Yeliva), proceeded to a phase 2/3 global trial for hospitalized patients after the company announced top-line safety and efficacy data in December. In phase 2, the drug was shown to be safe in patients requiring oxygen and effectively reduced the need for oxygen by the end of the treatment period.
A key feature is that it is both an antiviral and an anti-inflammatory, Gilead Raday, RedHill’s chief operating officer, said in an interview. Data are expected midyear on its performance in 464 patients. The drug is being tested on top of remdesivir or in addition to dexamethasone.
The second, upamostat (RHB-107), is currently undergoing a phase 2/3 trial in the United States and is being investigated for use in nonhospitalized COVID-19 patients.
“I would expect data to be available in the second half of this year,” Mr. Raday said.
Upamostat is a novel serine protease inhibitor expected to be effective against emerging variants because it targets human cell factors involved in viral entry, according to the company.
Other drugs are being investigated in trials that are in earlier stages.
Urgent need for oral agents
Infectious disease specialists are watching the move toward a COVID-19 pill enthusiastically.
“We badly need an oral treatment option for COVID,” said Sarah Doernberg, MD, an infectious disease specialist from the University of California, San Francisco.
“It’s a real gap in our armamentarium for COVID in outpatient treatment, which is where most who contract COVID-19 will seek care,” she said in an interview.
Although some studies have shown the benefit of monoclonal antibodies for prevention and early treatment, there are major logistical issues because all the current options require IV administration, she explained.
“If we had a pill to treat early COVID, especially in high-risk patients, it would fill a gap,” she said, noting that a pill could help people get better faster and prevent hospital stays.
Studies of molnupiravir suggest that it decreases viral shedding in the first few days after COVID infection, Dr. Doernberg reported.
There is excitement around the drug, but it will be important to see whether the results translate into fewer people requiring hospital admission and whether people feel better faster.
“I want to see the clinical data,” Dr. Doernberg said.
She will also be watching for the upamostat and opaganib results in the coming weeks.
“If these drugs are successful, I think it’s possible we could use them – maybe under an emergency use authorization – this year,” she said.
Once antiviral pills are a viable option for COVID-19 treatment, questions will arise about their use, she said.
One question is whether patients who are getting remdesivir in the hospital and are ready to leave after 5 days should continue treatment with antiviral pills at home.
Another is whether the pills – if they are shown to be effective – will be helpful for COVID post exposure. That use would be important for people who do not have COVID-19 but who are in close contact with someone who does, such as a member of their household.
“We have that model,” Dr. Doernberg said. “We know that oseltamivir can be used for postexposure prophylaxis and can help to prevent development of clinical disease.”
But she cautioned that a challenge with COVID is that people are contagious very early. A pill would need to come with the ability to test for COVID-19 early and get patients linked to care immediately.
“Those are not small challenges,” she said.
Vaccines alone won’t end the COVID threat
Treatments are part of the “belt-and-suspenders” approach, along with vaccines to combat COVID-19, Dr. Doernberg said.
“We’re not going to eradicate COVID,” she said. “We’re still going to need treatments for people who either don’t respond to the vaccine or haven’t gotten the vaccine or developed disease despite the vaccine.”
Oral formulations are desperately needed, agreed Kenneth Johnson, PhD, professor of molecular biosciences at the University of Texas at Austin.
Right now, remdesivir treatments involve patients being hooked up to an IV for 30-120 minutes each day for 5 days. And the cost of a 5-day course of remdesivir ranges from $2340 to $3120 in the United States.
“We’re hoping we can come up with something that is a little bit easier to administer, and without as many concerns for toxic side effects,” he said.
Dr. Johnson’s team at UT-Austin recently made a key discovery about the way remdesivir stops the replication of viral RNA.
The understanding of where the virus starts to replicate in the infection chain of events and how and where it reacts with remdesivir might lead to the development of better, more concentrated pill forms of antivirals in the future, with fewer toxicities, he said.
The team used a lab dish to recreate the step-by-step process that occurs when a patient who is infected with SARS-CoV-2 receives remdesivir.
The discovery was published online in Molecular Cell in January and will be printed in the April issue of the journal.
The discovery won’t lead to an effective COVID-19 pill for our current crisis, but will be important for the next generation of drugs needed to deal with future coronaviruses, Dr. Johnson explained.
And there will be other coronaviruses, he said, noting that this one is the third in 20 years to jump from animals to humans. “It’s just a matter of time,” he said.
A version of this article first appeared on Medscape.com.
New pills to treat patients with COVID-19 are currently in midstage clinical trials and, if successful, could be ready by the end of the year.
Only one treatment – remdesivir (Veklury) – has been fully approved by the U.S. Food and Drug Administration for patients in the hospital and it must be administered intravenously.
Hopes for a day when patients with COVID-19 can take a pill to rid their bodies of the virus got a boost when early trial results were presented at a medical conference.
Interim phase 2 results for the oral experimental COVID-19 drug molnupiravir, designed to do for patients with COVID-19 what oseltamivir (Tamiflu) can do for patients with the flu, were presented at the Conference on Retroviruses and Opportunistic Infections 2021 Annual Meeting, as reported by this news organization.
In the small study, the pill significantly reduced infectious virus in patients who were symptomatic and had tested positive for COVID-19 during the previous 4 days but were not hospitalized.
After 5 days of treatment, no participants who received molnupiravir had detectable virus, whereas 24% who received placebo did.
Two other oral agents are being developed by RedHill Biopharma: one for severe COVID-19 infection for hospitalized patients and one for patients at home with mild infection.
The first, opaganib (Yeliva), proceeded to a phase 2/3 global trial for hospitalized patients after the company announced top-line safety and efficacy data in December. In phase 2, the drug was shown to be safe in patients requiring oxygen and effectively reduced the need for oxygen by the end of the treatment period.
A key feature is that it is both an antiviral and an anti-inflammatory, Gilead Raday, RedHill’s chief operating officer, said in an interview. Data are expected midyear on its performance in 464 patients. The drug is being tested on top of remdesivir or in addition to dexamethasone.
The second, upamostat (RHB-107), is currently undergoing a phase 2/3 trial in the United States and is being investigated for use in nonhospitalized COVID-19 patients.
“I would expect data to be available in the second half of this year,” Mr. Raday said.
Upamostat is a novel serine protease inhibitor expected to be effective against emerging variants because it targets human cell factors involved in viral entry, according to the company.
Other drugs are being investigated in trials that are in earlier stages.
Urgent need for oral agents
Infectious disease specialists are watching the move toward a COVID-19 pill enthusiastically.
“We badly need an oral treatment option for COVID,” said Sarah Doernberg, MD, an infectious disease specialist from the University of California, San Francisco.
“It’s a real gap in our armamentarium for COVID in outpatient treatment, which is where most who contract COVID-19 will seek care,” she said in an interview.
Although some studies have shown the benefit of monoclonal antibodies for prevention and early treatment, there are major logistical issues because all the current options require IV administration, she explained.
“If we had a pill to treat early COVID, especially in high-risk patients, it would fill a gap,” she said, noting that a pill could help people get better faster and prevent hospital stays.
Studies of molnupiravir suggest that it decreases viral shedding in the first few days after COVID infection, Dr. Doernberg reported.
There is excitement around the drug, but it will be important to see whether the results translate into fewer people requiring hospital admission and whether people feel better faster.
“I want to see the clinical data,” Dr. Doernberg said.
She will also be watching for the upamostat and opaganib results in the coming weeks.
“If these drugs are successful, I think it’s possible we could use them – maybe under an emergency use authorization – this year,” she said.
Once antiviral pills are a viable option for COVID-19 treatment, questions will arise about their use, she said.
One question is whether patients who are getting remdesivir in the hospital and are ready to leave after 5 days should continue treatment with antiviral pills at home.
Another is whether the pills – if they are shown to be effective – will be helpful for COVID post exposure. That use would be important for people who do not have COVID-19 but who are in close contact with someone who does, such as a member of their household.
“We have that model,” Dr. Doernberg said. “We know that oseltamivir can be used for postexposure prophylaxis and can help to prevent development of clinical disease.”
But she cautioned that a challenge with COVID is that people are contagious very early. A pill would need to come with the ability to test for COVID-19 early and get patients linked to care immediately.
“Those are not small challenges,” she said.
Vaccines alone won’t end the COVID threat
Treatments are part of the “belt-and-suspenders” approach, along with vaccines to combat COVID-19, Dr. Doernberg said.
“We’re not going to eradicate COVID,” she said. “We’re still going to need treatments for people who either don’t respond to the vaccine or haven’t gotten the vaccine or developed disease despite the vaccine.”
Oral formulations are desperately needed, agreed Kenneth Johnson, PhD, professor of molecular biosciences at the University of Texas at Austin.
Right now, remdesivir treatments involve patients being hooked up to an IV for 30-120 minutes each day for 5 days. And the cost of a 5-day course of remdesivir ranges from $2340 to $3120 in the United States.
“We’re hoping we can come up with something that is a little bit easier to administer, and without as many concerns for toxic side effects,” he said.
Dr. Johnson’s team at UT-Austin recently made a key discovery about the way remdesivir stops the replication of viral RNA.
The understanding of where the virus starts to replicate in the infection chain of events and how and where it reacts with remdesivir might lead to the development of better, more concentrated pill forms of antivirals in the future, with fewer toxicities, he said.
The team used a lab dish to recreate the step-by-step process that occurs when a patient who is infected with SARS-CoV-2 receives remdesivir.
The discovery was published online in Molecular Cell in January and will be printed in the April issue of the journal.
The discovery won’t lead to an effective COVID-19 pill for our current crisis, but will be important for the next generation of drugs needed to deal with future coronaviruses, Dr. Johnson explained.
And there will be other coronaviruses, he said, noting that this one is the third in 20 years to jump from animals to humans. “It’s just a matter of time,” he said.
A version of this article first appeared on Medscape.com.
SHM Fellowship Class of 2021
The Society of Hospital Medicine has announced its 2021 class of Master Fellows, Senior Fellows, and Fellows in Hospital Medicine.
All Fellowship classes are listed in alphabetical order.
Master Fellows Class of 2021
Nasim Afsar, MD, MBA, MHM
Shaun D. Frost, MD, MHM
Jeffrey L. Schnipper, MD, MPH, MHM
Senior Fellows Class of 2021
Akindele Adaramola, MD, MPH, SFHM
Ramesh Adhikari, MD, SFHM
Pankaj Agrawal, MD, SFHM
Robert L. Anderson, MD, SFHM
Glenda B. Atilano, MD, SFHM
Bi A. Awosika, MD, FACP, SFHM
David N. Aymond, MD, SFHM
Paula Bailey, MD, SFHM
Amit B. Bansal, MD, MBA, SFHM
Jamie K. Bartley, DO, FACP, SFHM
Stephen J. Behnke, MD, SFHM
Christina A. Beyer, MD, SFHM
Vinil K. Bhuma, MD, SFHM
John P. Biebelhausen, MD, MBA, SFHM
Matthew T. Calestino, MD, FACP, SFHM
Domingo Caparas Jr., MD, FAAFP, SFHM
Darren Caudill, DO, FACP, SFHM
Julie M. Cernanec, MD, FAAP, SFHM
Will Cushing, PA-C, SFHM
Douglas A. Dodds II, MD, FAAP, SFHM
Coley B. Duncan, MD, CPE, MMM, SFHM
Noah Finkel, MD, SFHM
Justin Glasgow, MD, PhD, SFHM
Taylor Goot, MD, SFHM
Craig G. Gunderson, MD, SFHM
Alan Hall, MD, SFHM
Vivian Hamlett, MD, SFHM
Kathrin Harrington, MD, SFHM
Hossan Hassan, MD, SFHM
Anand D. Hongalgi, MD, FACP, SFHM
Akshata Hopkins, MD, FAAP, SFHM
Neelima Kamineni, MD, SFHM
Sudheer R. Kantharajpur, MBBS, MD, MHA, SFHM
Prakash Karki, MD, SFHM
Susrutha Kotwal, MD, SFHM
Ethan Kuperman, MD, SFHM
Rumman A. Langah, MD, FACP, SFHM
Sean LaVine, MD, FACP, SFHM
Don S. Lee, MD, FACP, SFHM
Charmaine A. Lewis, MD, MPH, CLHM, SFHM
Rishi Likhi, MD, SFHM
Lenny Lopez, MD, MPH, SFHM
Anthony Macchiavelli, MD, SFHM
Brian McGillen, MD, FACP, SFHM
Parth H. Mehta, MBBS, MD, MPH, SFHM
Anuj Mehta, MBBS, MD, MBA, SFHM
Prem Nair, MD, FACP, SFHM
Don J. Neer, MD, FACP, SFHM
Shyam Odeti, MD, FAAFP, SFHM
Amy T. O’Linn, DO, SFHM
Mihir Patel, MD, FACP, MBA, MPH, SFHM
Kimberly S. Pedram, MD, FACP, SFHM
Thomas Pineo, DO, SFHM
Mauricio Pinto, MD, SFHM
Lakmali C. Ranathunga, MBBS, SFHM
Matthew Reuter, MD, SFHM
Erik P. Rufa, MD, SFHM
Dipali Ruby Sahoo, DO, MBA, SFHM
Chady Sarraf, MD, SFHM
Suchita S. Sata, MD, SFHM
Klint Schwenk, MD, FAAP, MBA, SFHM
Aaron M. Sebach, CRNP, DNP, MBA, PhD, SFHM
Kevin Sowti, MD, MBA, SFHM
Joseph G. Surber, DO, FAAFP, SFHM
Bright Thilagar, MD, SFHM
Thomas S. Trawick Jr., MD, SFHM
Rehman Usmani, MD, SFHM
Arash Velayati, MD, SFHM
Jose A. Ventura, MD, FAAFP, SFHM
Andre Wajner, MD, PhD, SFHM
Phillip D. Warr, MD, SFHM
Virginia E. Watson, MD, SFHM
Kristin R. Wise, MD, SFHM
Elham A. Yousef, MD, FACP, MBA, MSc, SFHM
Fellows Class of 2021
Elizabeth M. Aarons, MD, FHM
Suhail A. Abbasi, MD, FACP, FHM
Waqas Adeel, MD, FHM
Rajender K. Agarwal, MD, MBA, MPH, FHM
Khaalisha Ajala, MD, MBA, FHM
Faraz S. Alam, MD, FHM
Amee Amin, MD, FHM
Muhammad W. Amir, MD, FACP, FHM
Saba Asad, MD, FHM
Logan Atkins, MD, FHM
Navneet Attri, MD, FHM
Jennifer Barnett, PA, FHM
Karyn Baum, MD, FHM
Prabhjot Bedi, MD, FHM
Nicolle R. Benz, DO, FHM
Ricky Bhimani, MD, FHM
Elizabeth Blankenship, PA-C, FHM
Rahul Borsadia, MD, FHM
Kalpana Chalasani, MD, FHM
Rani Chikkanna, MD, FHM
Venu Chippa, MBBS, MD, FHM
Lisa M. Coontz, FNP, FHM
Christie Crawford, MD, FHM
Rene Daniel, MD, PhD, FHM
Elda Dede, FHM
Radha Denmark, CNP, FHM
Alvine N. Nwehla Desamours, PA-C, FHM
Satyendra Dhar, MD, FHM
Manuel Jose Diaz, MD, FHM
Tiffany Egbe, MD, FHM
Chinwe Egbo, MD, FHM
Mohammad A. Farkhondehpour, MD, FACP, FHM
Shaheen Faruque, MBBS, FHM
Chris W. Fellin, MD, FACP, FHM
Juan Carlos Fuentes-Rosales, MD, FACP, MPH, FHM
Evelyn W. Gathecha, MD, FHM
Benjamin P. Geisler, MD, FACP, MPH, FHM
Matthew George, MD, FHM
Sonia George, DO, FHM
Mirna Giordano, MD, FHM
Rebecca Gomez, MD, FHM
David Gonzales, MD, FHM
Maria A. Guevara Hernandez, MD, FACP, FHM
Shubhra Gupta, MBBS, FHM
Rohini Harvey, MD, FHM
Allison Heinen, DO, FHM
Hollie L. Hurner, PA-C, FHM
Doug Hutcheon, MD, FHM
Varalakshmi Janamanchi, MD, FHM
Brian Keegan, MD, FACP, FHM
Qasim Khalil, MD, FHM
Irfana Khan, MD, FHM
Muhammad O. Khan, MD, FAAFP, MBA, FHM
Smita Kohli, MD, FHM
Julie Kolinski, MD, FAAP, FHM
Ewa Kontny, MD, FHM
Sungmi Lian, MD, FHM
Brian Lichtenstein, MD, FHM
Fernando Madero Gorostieta, MD, FHM
Vipul Mahajan, MD, FACP, FHM
Neetu Mahendraker, MD, FHM
Victoria McCurry, MD, FHM
Bridget McGrath, PA-C, FHM
Evan Meadors, MD, FHM
Kapil Mehta, MD, FACP, MBA, FHM
Waseem Mohamed, MD, FHM
Ernest Murray, MD, FHM
Murali K. Nagubandi, MD, FHM
Jessica Nave Allen, MD, FHM
Peter Nwafor, MD, FACP, FHM
Ike Anthony Nwaobi, MBBS, MBA, FHM
Olugbenga B. Ojo, MD, FACP, MBA, FHM
Jacqueline Okere, MD, FHM
Ifedolapo S. Olanrewaju, MD, MBchB, FHM
Mobolaji Olulade, MD, FHM
Elizabeth H. Papetti, MBA, FHM
Love Patel, MBBS, FHM
Kamakshya P. Patra, MD, MMM, FHM
Charles Pizanis, MD, FHM
Rajat Prakash, MD, FHM
Chris Pribula, MD, FHM
Michael Puchaev, MD, FHM
Ryan Punsalan, MD, FHM
Bhavya Rajanna, MD, FHM
Miguel A. Ramirez, MD, FHM
Raymund Ramirez, MD, FHM
Sandeep Randhawa, MBBS, FHM
Rohit Rattan, MD, FHM
Denisha Powell Rawlings, MD, FHM
Praveen K. Reddy, MD, MPH, FHM
Michael Ree, DO, MPH, FHM
Patrick Rendon, MD, FHM
David J. Rizk, MD, FAAFP, MBA, FHM
Michael Roberts, MD, FHM
Edwin Rosas, MD, FHM
Devjit Roy, MD, FHM
Sabyasachi Roy, MD, FHM
Paul Sandroni, CMPE, MSM, FHM
Vijairam Selvaraj, MD, MPH, FHM
Megha Shah, MD, MMM, FHM
Edie Shen, MD, FHM
Gurpinder Singh, MD, FACP, FHM
Vishwas A. Singh, MD, FHM
Karen Slatkovsky, MD, FHM
Sean M. Snyder, MD, FHM
Jaclyn Spiegel, MD, FHM
Dale Stapler Jr., MD, FHM
Christina E. Stovall, MD, FHM
Daniel Suders, DO, FHM
Clayton Swalstad, CMPE, MSM, FHM
Harshil Swaminarayan, MD, FHM
Keniesha Thompson, MD, FHM
Tet Toe, MD, FACP, FHM
Christine Tsai, MD, FHM
Ajay Vaikuntam, MD, FHM
Valerie Vaughn, MD, FHM
Jane N. Wainaina, FACP, MBchB, FHM
Neshahthari Wijeyakuhan, MD, FACP, FHM
Chia-Shing Yang, MD, FHM
Jennifer Zagursky, MD, FHM
The Society of Hospital Medicine has announced its 2021 class of Master Fellows, Senior Fellows, and Fellows in Hospital Medicine.
All Fellowship classes are listed in alphabetical order.
Master Fellows Class of 2021
Nasim Afsar, MD, MBA, MHM
Shaun D. Frost, MD, MHM
Jeffrey L. Schnipper, MD, MPH, MHM
Senior Fellows Class of 2021
Akindele Adaramola, MD, MPH, SFHM
Ramesh Adhikari, MD, SFHM
Pankaj Agrawal, MD, SFHM
Robert L. Anderson, MD, SFHM
Glenda B. Atilano, MD, SFHM
Bi A. Awosika, MD, FACP, SFHM
David N. Aymond, MD, SFHM
Paula Bailey, MD, SFHM
Amit B. Bansal, MD, MBA, SFHM
Jamie K. Bartley, DO, FACP, SFHM
Stephen J. Behnke, MD, SFHM
Christina A. Beyer, MD, SFHM
Vinil K. Bhuma, MD, SFHM
John P. Biebelhausen, MD, MBA, SFHM
Matthew T. Calestino, MD, FACP, SFHM
Domingo Caparas Jr., MD, FAAFP, SFHM
Darren Caudill, DO, FACP, SFHM
Julie M. Cernanec, MD, FAAP, SFHM
Will Cushing, PA-C, SFHM
Douglas A. Dodds II, MD, FAAP, SFHM
Coley B. Duncan, MD, CPE, MMM, SFHM
Noah Finkel, MD, SFHM
Justin Glasgow, MD, PhD, SFHM
Taylor Goot, MD, SFHM
Craig G. Gunderson, MD, SFHM
Alan Hall, MD, SFHM
Vivian Hamlett, MD, SFHM
Kathrin Harrington, MD, SFHM
Hossan Hassan, MD, SFHM
Anand D. Hongalgi, MD, FACP, SFHM
Akshata Hopkins, MD, FAAP, SFHM
Neelima Kamineni, MD, SFHM
Sudheer R. Kantharajpur, MBBS, MD, MHA, SFHM
Prakash Karki, MD, SFHM
Susrutha Kotwal, MD, SFHM
Ethan Kuperman, MD, SFHM
Rumman A. Langah, MD, FACP, SFHM
Sean LaVine, MD, FACP, SFHM
Don S. Lee, MD, FACP, SFHM
Charmaine A. Lewis, MD, MPH, CLHM, SFHM
Rishi Likhi, MD, SFHM
Lenny Lopez, MD, MPH, SFHM
Anthony Macchiavelli, MD, SFHM
Brian McGillen, MD, FACP, SFHM
Parth H. Mehta, MBBS, MD, MPH, SFHM
Anuj Mehta, MBBS, MD, MBA, SFHM
Prem Nair, MD, FACP, SFHM
Don J. Neer, MD, FACP, SFHM
Shyam Odeti, MD, FAAFP, SFHM
Amy T. O’Linn, DO, SFHM
Mihir Patel, MD, FACP, MBA, MPH, SFHM
Kimberly S. Pedram, MD, FACP, SFHM
Thomas Pineo, DO, SFHM
Mauricio Pinto, MD, SFHM
Lakmali C. Ranathunga, MBBS, SFHM
Matthew Reuter, MD, SFHM
Erik P. Rufa, MD, SFHM
Dipali Ruby Sahoo, DO, MBA, SFHM
Chady Sarraf, MD, SFHM
Suchita S. Sata, MD, SFHM
Klint Schwenk, MD, FAAP, MBA, SFHM
Aaron M. Sebach, CRNP, DNP, MBA, PhD, SFHM
Kevin Sowti, MD, MBA, SFHM
Joseph G. Surber, DO, FAAFP, SFHM
Bright Thilagar, MD, SFHM
Thomas S. Trawick Jr., MD, SFHM
Rehman Usmani, MD, SFHM
Arash Velayati, MD, SFHM
Jose A. Ventura, MD, FAAFP, SFHM
Andre Wajner, MD, PhD, SFHM
Phillip D. Warr, MD, SFHM
Virginia E. Watson, MD, SFHM
Kristin R. Wise, MD, SFHM
Elham A. Yousef, MD, FACP, MBA, MSc, SFHM
Fellows Class of 2021
Elizabeth M. Aarons, MD, FHM
Suhail A. Abbasi, MD, FACP, FHM
Waqas Adeel, MD, FHM
Rajender K. Agarwal, MD, MBA, MPH, FHM
Khaalisha Ajala, MD, MBA, FHM
Faraz S. Alam, MD, FHM
Amee Amin, MD, FHM
Muhammad W. Amir, MD, FACP, FHM
Saba Asad, MD, FHM
Logan Atkins, MD, FHM
Navneet Attri, MD, FHM
Jennifer Barnett, PA, FHM
Karyn Baum, MD, FHM
Prabhjot Bedi, MD, FHM
Nicolle R. Benz, DO, FHM
Ricky Bhimani, MD, FHM
Elizabeth Blankenship, PA-C, FHM
Rahul Borsadia, MD, FHM
Kalpana Chalasani, MD, FHM
Rani Chikkanna, MD, FHM
Venu Chippa, MBBS, MD, FHM
Lisa M. Coontz, FNP, FHM
Christie Crawford, MD, FHM
Rene Daniel, MD, PhD, FHM
Elda Dede, FHM
Radha Denmark, CNP, FHM
Alvine N. Nwehla Desamours, PA-C, FHM
Satyendra Dhar, MD, FHM
Manuel Jose Diaz, MD, FHM
Tiffany Egbe, MD, FHM
Chinwe Egbo, MD, FHM
Mohammad A. Farkhondehpour, MD, FACP, FHM
Shaheen Faruque, MBBS, FHM
Chris W. Fellin, MD, FACP, FHM
Juan Carlos Fuentes-Rosales, MD, FACP, MPH, FHM
Evelyn W. Gathecha, MD, FHM
Benjamin P. Geisler, MD, FACP, MPH, FHM
Matthew George, MD, FHM
Sonia George, DO, FHM
Mirna Giordano, MD, FHM
Rebecca Gomez, MD, FHM
David Gonzales, MD, FHM
Maria A. Guevara Hernandez, MD, FACP, FHM
Shubhra Gupta, MBBS, FHM
Rohini Harvey, MD, FHM
Allison Heinen, DO, FHM
Hollie L. Hurner, PA-C, FHM
Doug Hutcheon, MD, FHM
Varalakshmi Janamanchi, MD, FHM
Brian Keegan, MD, FACP, FHM
Qasim Khalil, MD, FHM
Irfana Khan, MD, FHM
Muhammad O. Khan, MD, FAAFP, MBA, FHM
Smita Kohli, MD, FHM
Julie Kolinski, MD, FAAP, FHM
Ewa Kontny, MD, FHM
Sungmi Lian, MD, FHM
Brian Lichtenstein, MD, FHM
Fernando Madero Gorostieta, MD, FHM
Vipul Mahajan, MD, FACP, FHM
Neetu Mahendraker, MD, FHM
Victoria McCurry, MD, FHM
Bridget McGrath, PA-C, FHM
Evan Meadors, MD, FHM
Kapil Mehta, MD, FACP, MBA, FHM
Waseem Mohamed, MD, FHM
Ernest Murray, MD, FHM
Murali K. Nagubandi, MD, FHM
Jessica Nave Allen, MD, FHM
Peter Nwafor, MD, FACP, FHM
Ike Anthony Nwaobi, MBBS, MBA, FHM
Olugbenga B. Ojo, MD, FACP, MBA, FHM
Jacqueline Okere, MD, FHM
Ifedolapo S. Olanrewaju, MD, MBchB, FHM
Mobolaji Olulade, MD, FHM
Elizabeth H. Papetti, MBA, FHM
Love Patel, MBBS, FHM
Kamakshya P. Patra, MD, MMM, FHM
Charles Pizanis, MD, FHM
Rajat Prakash, MD, FHM
Chris Pribula, MD, FHM
Michael Puchaev, MD, FHM
Ryan Punsalan, MD, FHM
Bhavya Rajanna, MD, FHM
Miguel A. Ramirez, MD, FHM
Raymund Ramirez, MD, FHM
Sandeep Randhawa, MBBS, FHM
Rohit Rattan, MD, FHM
Denisha Powell Rawlings, MD, FHM
Praveen K. Reddy, MD, MPH, FHM
Michael Ree, DO, MPH, FHM
Patrick Rendon, MD, FHM
David J. Rizk, MD, FAAFP, MBA, FHM
Michael Roberts, MD, FHM
Edwin Rosas, MD, FHM
Devjit Roy, MD, FHM
Sabyasachi Roy, MD, FHM
Paul Sandroni, CMPE, MSM, FHM
Vijairam Selvaraj, MD, MPH, FHM
Megha Shah, MD, MMM, FHM
Edie Shen, MD, FHM
Gurpinder Singh, MD, FACP, FHM
Vishwas A. Singh, MD, FHM
Karen Slatkovsky, MD, FHM
Sean M. Snyder, MD, FHM
Jaclyn Spiegel, MD, FHM
Dale Stapler Jr., MD, FHM
Christina E. Stovall, MD, FHM
Daniel Suders, DO, FHM
Clayton Swalstad, CMPE, MSM, FHM
Harshil Swaminarayan, MD, FHM
Keniesha Thompson, MD, FHM
Tet Toe, MD, FACP, FHM
Christine Tsai, MD, FHM
Ajay Vaikuntam, MD, FHM
Valerie Vaughn, MD, FHM
Jane N. Wainaina, FACP, MBchB, FHM
Neshahthari Wijeyakuhan, MD, FACP, FHM
Chia-Shing Yang, MD, FHM
Jennifer Zagursky, MD, FHM
The Society of Hospital Medicine has announced its 2021 class of Master Fellows, Senior Fellows, and Fellows in Hospital Medicine.
All Fellowship classes are listed in alphabetical order.
Master Fellows Class of 2021
Nasim Afsar, MD, MBA, MHM
Shaun D. Frost, MD, MHM
Jeffrey L. Schnipper, MD, MPH, MHM
Senior Fellows Class of 2021
Akindele Adaramola, MD, MPH, SFHM
Ramesh Adhikari, MD, SFHM
Pankaj Agrawal, MD, SFHM
Robert L. Anderson, MD, SFHM
Glenda B. Atilano, MD, SFHM
Bi A. Awosika, MD, FACP, SFHM
David N. Aymond, MD, SFHM
Paula Bailey, MD, SFHM
Amit B. Bansal, MD, MBA, SFHM
Jamie K. Bartley, DO, FACP, SFHM
Stephen J. Behnke, MD, SFHM
Christina A. Beyer, MD, SFHM
Vinil K. Bhuma, MD, SFHM
John P. Biebelhausen, MD, MBA, SFHM
Matthew T. Calestino, MD, FACP, SFHM
Domingo Caparas Jr., MD, FAAFP, SFHM
Darren Caudill, DO, FACP, SFHM
Julie M. Cernanec, MD, FAAP, SFHM
Will Cushing, PA-C, SFHM
Douglas A. Dodds II, MD, FAAP, SFHM
Coley B. Duncan, MD, CPE, MMM, SFHM
Noah Finkel, MD, SFHM
Justin Glasgow, MD, PhD, SFHM
Taylor Goot, MD, SFHM
Craig G. Gunderson, MD, SFHM
Alan Hall, MD, SFHM
Vivian Hamlett, MD, SFHM
Kathrin Harrington, MD, SFHM
Hossan Hassan, MD, SFHM
Anand D. Hongalgi, MD, FACP, SFHM
Akshata Hopkins, MD, FAAP, SFHM
Neelima Kamineni, MD, SFHM
Sudheer R. Kantharajpur, MBBS, MD, MHA, SFHM
Prakash Karki, MD, SFHM
Susrutha Kotwal, MD, SFHM
Ethan Kuperman, MD, SFHM
Rumman A. Langah, MD, FACP, SFHM
Sean LaVine, MD, FACP, SFHM
Don S. Lee, MD, FACP, SFHM
Charmaine A. Lewis, MD, MPH, CLHM, SFHM
Rishi Likhi, MD, SFHM
Lenny Lopez, MD, MPH, SFHM
Anthony Macchiavelli, MD, SFHM
Brian McGillen, MD, FACP, SFHM
Parth H. Mehta, MBBS, MD, MPH, SFHM
Anuj Mehta, MBBS, MD, MBA, SFHM
Prem Nair, MD, FACP, SFHM
Don J. Neer, MD, FACP, SFHM
Shyam Odeti, MD, FAAFP, SFHM
Amy T. O’Linn, DO, SFHM
Mihir Patel, MD, FACP, MBA, MPH, SFHM
Kimberly S. Pedram, MD, FACP, SFHM
Thomas Pineo, DO, SFHM
Mauricio Pinto, MD, SFHM
Lakmali C. Ranathunga, MBBS, SFHM
Matthew Reuter, MD, SFHM
Erik P. Rufa, MD, SFHM
Dipali Ruby Sahoo, DO, MBA, SFHM
Chady Sarraf, MD, SFHM
Suchita S. Sata, MD, SFHM
Klint Schwenk, MD, FAAP, MBA, SFHM
Aaron M. Sebach, CRNP, DNP, MBA, PhD, SFHM
Kevin Sowti, MD, MBA, SFHM
Joseph G. Surber, DO, FAAFP, SFHM
Bright Thilagar, MD, SFHM
Thomas S. Trawick Jr., MD, SFHM
Rehman Usmani, MD, SFHM
Arash Velayati, MD, SFHM
Jose A. Ventura, MD, FAAFP, SFHM
Andre Wajner, MD, PhD, SFHM
Phillip D. Warr, MD, SFHM
Virginia E. Watson, MD, SFHM
Kristin R. Wise, MD, SFHM
Elham A. Yousef, MD, FACP, MBA, MSc, SFHM
Fellows Class of 2021
Elizabeth M. Aarons, MD, FHM
Suhail A. Abbasi, MD, FACP, FHM
Waqas Adeel, MD, FHM
Rajender K. Agarwal, MD, MBA, MPH, FHM
Khaalisha Ajala, MD, MBA, FHM
Faraz S. Alam, MD, FHM
Amee Amin, MD, FHM
Muhammad W. Amir, MD, FACP, FHM
Saba Asad, MD, FHM
Logan Atkins, MD, FHM
Navneet Attri, MD, FHM
Jennifer Barnett, PA, FHM
Karyn Baum, MD, FHM
Prabhjot Bedi, MD, FHM
Nicolle R. Benz, DO, FHM
Ricky Bhimani, MD, FHM
Elizabeth Blankenship, PA-C, FHM
Rahul Borsadia, MD, FHM
Kalpana Chalasani, MD, FHM
Rani Chikkanna, MD, FHM
Venu Chippa, MBBS, MD, FHM
Lisa M. Coontz, FNP, FHM
Christie Crawford, MD, FHM
Rene Daniel, MD, PhD, FHM
Elda Dede, FHM
Radha Denmark, CNP, FHM
Alvine N. Nwehla Desamours, PA-C, FHM
Satyendra Dhar, MD, FHM
Manuel Jose Diaz, MD, FHM
Tiffany Egbe, MD, FHM
Chinwe Egbo, MD, FHM
Mohammad A. Farkhondehpour, MD, FACP, FHM
Shaheen Faruque, MBBS, FHM
Chris W. Fellin, MD, FACP, FHM
Juan Carlos Fuentes-Rosales, MD, FACP, MPH, FHM
Evelyn W. Gathecha, MD, FHM
Benjamin P. Geisler, MD, FACP, MPH, FHM
Matthew George, MD, FHM
Sonia George, DO, FHM
Mirna Giordano, MD, FHM
Rebecca Gomez, MD, FHM
David Gonzales, MD, FHM
Maria A. Guevara Hernandez, MD, FACP, FHM
Shubhra Gupta, MBBS, FHM
Rohini Harvey, MD, FHM
Allison Heinen, DO, FHM
Hollie L. Hurner, PA-C, FHM
Doug Hutcheon, MD, FHM
Varalakshmi Janamanchi, MD, FHM
Brian Keegan, MD, FACP, FHM
Qasim Khalil, MD, FHM
Irfana Khan, MD, FHM
Muhammad O. Khan, MD, FAAFP, MBA, FHM
Smita Kohli, MD, FHM
Julie Kolinski, MD, FAAP, FHM
Ewa Kontny, MD, FHM
Sungmi Lian, MD, FHM
Brian Lichtenstein, MD, FHM
Fernando Madero Gorostieta, MD, FHM
Vipul Mahajan, MD, FACP, FHM
Neetu Mahendraker, MD, FHM
Victoria McCurry, MD, FHM
Bridget McGrath, PA-C, FHM
Evan Meadors, MD, FHM
Kapil Mehta, MD, FACP, MBA, FHM
Waseem Mohamed, MD, FHM
Ernest Murray, MD, FHM
Murali K. Nagubandi, MD, FHM
Jessica Nave Allen, MD, FHM
Peter Nwafor, MD, FACP, FHM
Ike Anthony Nwaobi, MBBS, MBA, FHM
Olugbenga B. Ojo, MD, FACP, MBA, FHM
Jacqueline Okere, MD, FHM
Ifedolapo S. Olanrewaju, MD, MBchB, FHM
Mobolaji Olulade, MD, FHM
Elizabeth H. Papetti, MBA, FHM
Love Patel, MBBS, FHM
Kamakshya P. Patra, MD, MMM, FHM
Charles Pizanis, MD, FHM
Rajat Prakash, MD, FHM
Chris Pribula, MD, FHM
Michael Puchaev, MD, FHM
Ryan Punsalan, MD, FHM
Bhavya Rajanna, MD, FHM
Miguel A. Ramirez, MD, FHM
Raymund Ramirez, MD, FHM
Sandeep Randhawa, MBBS, FHM
Rohit Rattan, MD, FHM
Denisha Powell Rawlings, MD, FHM
Praveen K. Reddy, MD, MPH, FHM
Michael Ree, DO, MPH, FHM
Patrick Rendon, MD, FHM
David J. Rizk, MD, FAAFP, MBA, FHM
Michael Roberts, MD, FHM
Edwin Rosas, MD, FHM
Devjit Roy, MD, FHM
Sabyasachi Roy, MD, FHM
Paul Sandroni, CMPE, MSM, FHM
Vijairam Selvaraj, MD, MPH, FHM
Megha Shah, MD, MMM, FHM
Edie Shen, MD, FHM
Gurpinder Singh, MD, FACP, FHM
Vishwas A. Singh, MD, FHM
Karen Slatkovsky, MD, FHM
Sean M. Snyder, MD, FHM
Jaclyn Spiegel, MD, FHM
Dale Stapler Jr., MD, FHM
Christina E. Stovall, MD, FHM
Daniel Suders, DO, FHM
Clayton Swalstad, CMPE, MSM, FHM
Harshil Swaminarayan, MD, FHM
Keniesha Thompson, MD, FHM
Tet Toe, MD, FACP, FHM
Christine Tsai, MD, FHM
Ajay Vaikuntam, MD, FHM
Valerie Vaughn, MD, FHM
Jane N. Wainaina, FACP, MBchB, FHM
Neshahthari Wijeyakuhan, MD, FACP, FHM
Chia-Shing Yang, MD, FHM
Jennifer Zagursky, MD, FHM
Don’t discontinue osteoporosis meds for COVID-19 vaccines, expert guidance says
COVID-19 vaccines are safe and effective for patients taking osteoporosis medications, according to joint guidance from six endocrine and osteoporosis societies and foundations.
They noted, though, that some timing modifications with certain medications should be considered to help distinguish between adverse events from the medication versus the vaccine.
The American Society for Bone and Mineral Research “is an international organization, so we brought together our sister societies that have a vested interested in bone health. Vaccination is happening worldwide, and we wanted to present a united front and united recommendations about how to handle osteoporosis medications appropriately during vaccination,” said Suzanne Jan De Beur, MD, who is president of ASBMR and an associate professor of medicine at Johns Hopkins University, Baltimore.
There has been quite a lot of concern from the community about vaccine and medications, from both physicians and patients wondering whether treatments and vaccines should occur in a certain order, and whether there should be a time gap between the two, said Dr. Jan De Beur. “There was a dearth of information about the best practices for osteoporosis treatment management during vaccination, and we didn’t want people missing their opportunity for a vaccine, and we also didn’t want them unnecessarily delaying their osteoporosis treatment.”
There is no evidence that osteoporosis therapies affect the risk or severity of COVID-19 disease, nor do they appear to change the disease course. Osteoporosis itself does not appear associated with increased risk of infection or severe outcomes, so patients with osteoporosis do not need to be prioritized for vaccination based on that condition alone.
There is no evidence that osteoporosis therapies affect the safety or efficacy of vaccination, but given that vaccine availability is currently inconsistent, patients may need to make temporary changes to their osteoporosis regimens to ensure they can receive vaccine when it is available, such as ensuring a delay between medication and vaccination injections.
A key reason for a delay between injectable or infusion medications and a vaccine is to distinguish between adverse events that could occur, so that an adverse reaction to vaccine isn’t mistaken for an adverse reaction to a drug. Nevertheless, the real world is messy. Dr. Jan De Beur noted a recent patient who arrived at her clinic for an injectable treatment who had just received a COVID-19 vaccination that morning. “We decided to put the injection in the other arm, rather than reschedule the person and put them through the risk of coming back. We could distinguish between injection-site reactions, at least,” she said.
No changes should be made to general bone health therapies, such as calcium and vitamin D supplementation, weight-bearing exercises, and maintenance of a balanced diet.
The guidance includes some recommendations for specific osteoporosis medications.
- Oral bisphosphonates: Alendronate, risedronate, and ibandronate should be continued.
- Intravenous bisphosphonates: a 7-day interval (4-day minimum) is recommended between intravenous bisphosphonate (zoledronic acid and ibandronate) infusion and COVID-19 vaccination in order to distinguish potential autoimmune or inflammatory reactions that could be attributable to either intravenous bisphosphonate or the vaccine.
- Denosumab: There should be a 4- to 7-day delay between denosumab infusion and COVID-19 vaccination to account for injection-site reactions. Another option is to have denosumab injected into the contralateral arm or another site like the abdomen or upper thigh, if spacing the injections is not possible. In any case, denosumab injections should be performed within 7 months of the previous dose.
- Teriparatide and abaloparatide should be continued.
- Romosozumab: There should be a 4- to 7-day delay between a romosozumab injection and COVID-19 vaccine, or romosozumab can be injected in the abdomen (with the exception of a 2-inch area around the naval) or thigh if spacing is not possible.
- Raloxifene should be continued in patients receiving COVID-19 vaccination.
Guidance signatories include ASBMR, the American Association of Clinical Endocrinology, the Endocrine Society, the European Calcified Tissue Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation.
Dr. Jan De Beur has no relevant financial disclosures.
COVID-19 vaccines are safe and effective for patients taking osteoporosis medications, according to joint guidance from six endocrine and osteoporosis societies and foundations.
They noted, though, that some timing modifications with certain medications should be considered to help distinguish between adverse events from the medication versus the vaccine.
The American Society for Bone and Mineral Research “is an international organization, so we brought together our sister societies that have a vested interested in bone health. Vaccination is happening worldwide, and we wanted to present a united front and united recommendations about how to handle osteoporosis medications appropriately during vaccination,” said Suzanne Jan De Beur, MD, who is president of ASBMR and an associate professor of medicine at Johns Hopkins University, Baltimore.
There has been quite a lot of concern from the community about vaccine and medications, from both physicians and patients wondering whether treatments and vaccines should occur in a certain order, and whether there should be a time gap between the two, said Dr. Jan De Beur. “There was a dearth of information about the best practices for osteoporosis treatment management during vaccination, and we didn’t want people missing their opportunity for a vaccine, and we also didn’t want them unnecessarily delaying their osteoporosis treatment.”
There is no evidence that osteoporosis therapies affect the risk or severity of COVID-19 disease, nor do they appear to change the disease course. Osteoporosis itself does not appear associated with increased risk of infection or severe outcomes, so patients with osteoporosis do not need to be prioritized for vaccination based on that condition alone.
There is no evidence that osteoporosis therapies affect the safety or efficacy of vaccination, but given that vaccine availability is currently inconsistent, patients may need to make temporary changes to their osteoporosis regimens to ensure they can receive vaccine when it is available, such as ensuring a delay between medication and vaccination injections.
A key reason for a delay between injectable or infusion medications and a vaccine is to distinguish between adverse events that could occur, so that an adverse reaction to vaccine isn’t mistaken for an adverse reaction to a drug. Nevertheless, the real world is messy. Dr. Jan De Beur noted a recent patient who arrived at her clinic for an injectable treatment who had just received a COVID-19 vaccination that morning. “We decided to put the injection in the other arm, rather than reschedule the person and put them through the risk of coming back. We could distinguish between injection-site reactions, at least,” she said.
No changes should be made to general bone health therapies, such as calcium and vitamin D supplementation, weight-bearing exercises, and maintenance of a balanced diet.
The guidance includes some recommendations for specific osteoporosis medications.
- Oral bisphosphonates: Alendronate, risedronate, and ibandronate should be continued.
- Intravenous bisphosphonates: a 7-day interval (4-day minimum) is recommended between intravenous bisphosphonate (zoledronic acid and ibandronate) infusion and COVID-19 vaccination in order to distinguish potential autoimmune or inflammatory reactions that could be attributable to either intravenous bisphosphonate or the vaccine.
- Denosumab: There should be a 4- to 7-day delay between denosumab infusion and COVID-19 vaccination to account for injection-site reactions. Another option is to have denosumab injected into the contralateral arm or another site like the abdomen or upper thigh, if spacing the injections is not possible. In any case, denosumab injections should be performed within 7 months of the previous dose.
- Teriparatide and abaloparatide should be continued.
- Romosozumab: There should be a 4- to 7-day delay between a romosozumab injection and COVID-19 vaccine, or romosozumab can be injected in the abdomen (with the exception of a 2-inch area around the naval) or thigh if spacing is not possible.
- Raloxifene should be continued in patients receiving COVID-19 vaccination.
Guidance signatories include ASBMR, the American Association of Clinical Endocrinology, the Endocrine Society, the European Calcified Tissue Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation.
Dr. Jan De Beur has no relevant financial disclosures.
COVID-19 vaccines are safe and effective for patients taking osteoporosis medications, according to joint guidance from six endocrine and osteoporosis societies and foundations.
They noted, though, that some timing modifications with certain medications should be considered to help distinguish between adverse events from the medication versus the vaccine.
The American Society for Bone and Mineral Research “is an international organization, so we brought together our sister societies that have a vested interested in bone health. Vaccination is happening worldwide, and we wanted to present a united front and united recommendations about how to handle osteoporosis medications appropriately during vaccination,” said Suzanne Jan De Beur, MD, who is president of ASBMR and an associate professor of medicine at Johns Hopkins University, Baltimore.
There has been quite a lot of concern from the community about vaccine and medications, from both physicians and patients wondering whether treatments and vaccines should occur in a certain order, and whether there should be a time gap between the two, said Dr. Jan De Beur. “There was a dearth of information about the best practices for osteoporosis treatment management during vaccination, and we didn’t want people missing their opportunity for a vaccine, and we also didn’t want them unnecessarily delaying their osteoporosis treatment.”
There is no evidence that osteoporosis therapies affect the risk or severity of COVID-19 disease, nor do they appear to change the disease course. Osteoporosis itself does not appear associated with increased risk of infection or severe outcomes, so patients with osteoporosis do not need to be prioritized for vaccination based on that condition alone.
There is no evidence that osteoporosis therapies affect the safety or efficacy of vaccination, but given that vaccine availability is currently inconsistent, patients may need to make temporary changes to their osteoporosis regimens to ensure they can receive vaccine when it is available, such as ensuring a delay between medication and vaccination injections.
A key reason for a delay between injectable or infusion medications and a vaccine is to distinguish between adverse events that could occur, so that an adverse reaction to vaccine isn’t mistaken for an adverse reaction to a drug. Nevertheless, the real world is messy. Dr. Jan De Beur noted a recent patient who arrived at her clinic for an injectable treatment who had just received a COVID-19 vaccination that morning. “We decided to put the injection in the other arm, rather than reschedule the person and put them through the risk of coming back. We could distinguish between injection-site reactions, at least,” she said.
No changes should be made to general bone health therapies, such as calcium and vitamin D supplementation, weight-bearing exercises, and maintenance of a balanced diet.
The guidance includes some recommendations for specific osteoporosis medications.
- Oral bisphosphonates: Alendronate, risedronate, and ibandronate should be continued.
- Intravenous bisphosphonates: a 7-day interval (4-day minimum) is recommended between intravenous bisphosphonate (zoledronic acid and ibandronate) infusion and COVID-19 vaccination in order to distinguish potential autoimmune or inflammatory reactions that could be attributable to either intravenous bisphosphonate or the vaccine.
- Denosumab: There should be a 4- to 7-day delay between denosumab infusion and COVID-19 vaccination to account for injection-site reactions. Another option is to have denosumab injected into the contralateral arm or another site like the abdomen or upper thigh, if spacing the injections is not possible. In any case, denosumab injections should be performed within 7 months of the previous dose.
- Teriparatide and abaloparatide should be continued.
- Romosozumab: There should be a 4- to 7-day delay between a romosozumab injection and COVID-19 vaccine, or romosozumab can be injected in the abdomen (with the exception of a 2-inch area around the naval) or thigh if spacing is not possible.
- Raloxifene should be continued in patients receiving COVID-19 vaccination.
Guidance signatories include ASBMR, the American Association of Clinical Endocrinology, the Endocrine Society, the European Calcified Tissue Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation.
Dr. Jan De Beur has no relevant financial disclosures.
Inpatient sodium imbalances linked to adverse COVID-19 outcomes
Both high and low serum sodium levels are associated with adverse outcomes for hospitalized patients with COVID-19, new research suggests.
In the retrospective study of 488 patients hospitalized with COVID-19 at one of two London hospitals between February and May 2020, hypernatremia (defined as serum sodium level >145 mmol/L) at any time point during hospital stay was associated with a threefold increase in inpatient mortality.
Hyponatremia (serum sodium level <135 mmol/L) was associated with twice the likelihood of requiring advanced ventilatory support. In-hospital mortality was also increased among patients with hypovolemic hyponatremia.
“Serum sodium values could be used in clinical practice to identify patients with COVID-19 at high risk of poor outcomes who would benefit from more intensive monitoring and judicious rehydration,” Ploutarchos Tzoulis, MD, PhD, and colleagues wrote in their article, which was published online on Feb. 24, 2021, in the Journal of Clinical Endocrinology and Metabolism.
The findings will be presented at the upcoming news conference held by the Endocrine Society
Should sodium be included in a risk calculator for COVID-19?
Dr. Tzoulis, professor of endocrinology at the University College London Medical School, said in an interview that “sodium could be incorporated in risk calculators across other routine biomarkers, such as white cell count, lymphocytes, and CRP [C-reactive protein], in order to provide a tool for dynamic risk stratification throughout the clinical course of COVID-19 and assist clinical decision-making.”
Moreover, he said, “we should follow less conservative strategies in the rate and amount of fluid resuscitation in order to prevent hypernatremia, which is induced by negative fluid balance and can often be iatrogenic.”
Asked to comment, Steven Q. Simpson, MD, professor of medicine in the division of pulmonary, critical care, and sleep medicine at the University of Kansas, Kansas City, said that the article is missing key results that would assist in interpreting of the findings.
“Data regarding diuretic use and sparing of fluid administration are not in the paper. ... It is simply not possible to tell whether serum sodium is a ‘predictor’ ... or if it is a side effect of other issues or actions taken by physicians in patients who are progressing poorly.
“To say that sodium needs to be included in a risk calculator is to subtly suggest that there is some causal association with mortality, and that has quite clearly not been established,” stressed Dr. Simpson, who is president of the American College of Chest Physicians but was not speaking for the organization.
He added: “The data are interesting, but not actionable. It is common practice in critical care medicine to adjust water and salt intake to maintain serum sodium within the normal range, so the paper really doesn’t change any behavior.”
Dr. Tzoulis said in an interview that, despite not having electronic medical record data on diuretic use or fluid input and output, “our acute physicians and intensivists at both study sites have been adamant that they’ve not routinely used diuretics in COVID-19 patients. Diuretics have been sparingly used in our cohort, and also the frequency of pulmonary edema was reported as below 5%.”
Regarding volume of fluid intake, Dr. Tzoulis noted, “At our hospital sites, the strategy has been that of cautious fluid resuscitation. In fact, the amount of fluid given has been reported by our physicians and intensivists as ‘on purpose much more conservative than the usual one adopted in patients with community-acquired pneumonia at risk of respiratory failure.’ ”
Hyper- and hyponatremia linked to adverse COVID-19 outcomes
In the study, 5.3% of the 488 patients had hypernatremia at hospital presentation, and 24.6% had hyponatremia. Of note, only 19% of those with hyponatremia underwent laboratory workup to determine the etiology. Of those, three quarters had hypovolemic hyponatremia, determined on the basis of a urinary sodium cutoff of 30 mmol/L.
The total in-hospital mortality rate was 31.1%. There was a strong, although nonsignificant, trend toward higher mortality in association with sodium status at admission. Death rates were 28.4%, 30.8%, and 46.1% for those who were normonatremic, hyponatremic, and hypernatremic, respectively (P = .07). Baseline serum sodium levels didn’t differ between survivors (137 mmol/L) and nonsurvivors (138 mmol/L).
In multivariable analysis, the occurrence of hypernatremia at any point during the first 5 days in the hospital was among three independent risk factors for higher in-hospital mortality (adjusted hazard ratio, 2.74; P = .02). The other risk factors were older age and higher CRP level.
Overall, hyponatremia was not associated with death (P = .41).
During hospitalization, 37.9% of patients remained normonatremic; 36.9% experienced hyponatremia; 10.9% had hypernatremia; and 14.3% had both conditions at some point during their stay.
In-hospital mortality was 21% among those with normonatremia, compared with 56.6% for those with hypernatremia (odds ratio, 3.05; P = .0038) and 45.7% for those with both (OR, 2.25; P < .0001).
The 28.3% mortality rate in the overall group that experienced hyponatremia didn’t differ significantly from the 21.1% in the normonatremic group (OR, 1.34; P = .16). However, the death rate was 40.9% among the subgroup that developed hypovolemic hyponatremia, significantly higher than the normonatremic group (OR, 2.59, P = .0017).
The incidence of hyponatremia decreased from 24.6% at admission to 14.1% 5 days later, whereas the frequency of hypernatremia rose from 5.3% to 13.8%.
Key finding: Link between hospital-acquired hypernatremia and death
“The key novel finding of our study was that hospital-acquired hypernatremia, rather than hypernatremia at admission, was a predictor for in-hospital mortality, with the worst prognosis being reported in patients with the largest increase in serum sodium in the first 5 days of hospitalization,” noted Dr. Tzoulis and colleagues.
Hypernatremia was present in 29.6% of nonsurvivors, compared with 5.2% in survivors.
Among 120 patients with hyponatremia at admission, 31.7% received advanced respiratory support, compared with 17.5% and 7.7% of those with normonatremia or hypernatremia, respectively (OR, 2.18; P = .0011).
In contrast, there was no difference in the proportions needing ventilatory support between those with hypernatremia and those with normonatremia (16.7% vs. 12.4%; OR, 1.44; P = .39).
Acute kidney injury occurred in 181 patients (37.1%). It was not related to serum sodium concentration at any time point.
Dr. Tzoulis and Dr. Simpson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both high and low serum sodium levels are associated with adverse outcomes for hospitalized patients with COVID-19, new research suggests.
In the retrospective study of 488 patients hospitalized with COVID-19 at one of two London hospitals between February and May 2020, hypernatremia (defined as serum sodium level >145 mmol/L) at any time point during hospital stay was associated with a threefold increase in inpatient mortality.
Hyponatremia (serum sodium level <135 mmol/L) was associated with twice the likelihood of requiring advanced ventilatory support. In-hospital mortality was also increased among patients with hypovolemic hyponatremia.
“Serum sodium values could be used in clinical practice to identify patients with COVID-19 at high risk of poor outcomes who would benefit from more intensive monitoring and judicious rehydration,” Ploutarchos Tzoulis, MD, PhD, and colleagues wrote in their article, which was published online on Feb. 24, 2021, in the Journal of Clinical Endocrinology and Metabolism.
The findings will be presented at the upcoming news conference held by the Endocrine Society
Should sodium be included in a risk calculator for COVID-19?
Dr. Tzoulis, professor of endocrinology at the University College London Medical School, said in an interview that “sodium could be incorporated in risk calculators across other routine biomarkers, such as white cell count, lymphocytes, and CRP [C-reactive protein], in order to provide a tool for dynamic risk stratification throughout the clinical course of COVID-19 and assist clinical decision-making.”
Moreover, he said, “we should follow less conservative strategies in the rate and amount of fluid resuscitation in order to prevent hypernatremia, which is induced by negative fluid balance and can often be iatrogenic.”
Asked to comment, Steven Q. Simpson, MD, professor of medicine in the division of pulmonary, critical care, and sleep medicine at the University of Kansas, Kansas City, said that the article is missing key results that would assist in interpreting of the findings.
“Data regarding diuretic use and sparing of fluid administration are not in the paper. ... It is simply not possible to tell whether serum sodium is a ‘predictor’ ... or if it is a side effect of other issues or actions taken by physicians in patients who are progressing poorly.
“To say that sodium needs to be included in a risk calculator is to subtly suggest that there is some causal association with mortality, and that has quite clearly not been established,” stressed Dr. Simpson, who is president of the American College of Chest Physicians but was not speaking for the organization.
He added: “The data are interesting, but not actionable. It is common practice in critical care medicine to adjust water and salt intake to maintain serum sodium within the normal range, so the paper really doesn’t change any behavior.”
Dr. Tzoulis said in an interview that, despite not having electronic medical record data on diuretic use or fluid input and output, “our acute physicians and intensivists at both study sites have been adamant that they’ve not routinely used diuretics in COVID-19 patients. Diuretics have been sparingly used in our cohort, and also the frequency of pulmonary edema was reported as below 5%.”
Regarding volume of fluid intake, Dr. Tzoulis noted, “At our hospital sites, the strategy has been that of cautious fluid resuscitation. In fact, the amount of fluid given has been reported by our physicians and intensivists as ‘on purpose much more conservative than the usual one adopted in patients with community-acquired pneumonia at risk of respiratory failure.’ ”
Hyper- and hyponatremia linked to adverse COVID-19 outcomes
In the study, 5.3% of the 488 patients had hypernatremia at hospital presentation, and 24.6% had hyponatremia. Of note, only 19% of those with hyponatremia underwent laboratory workup to determine the etiology. Of those, three quarters had hypovolemic hyponatremia, determined on the basis of a urinary sodium cutoff of 30 mmol/L.
The total in-hospital mortality rate was 31.1%. There was a strong, although nonsignificant, trend toward higher mortality in association with sodium status at admission. Death rates were 28.4%, 30.8%, and 46.1% for those who were normonatremic, hyponatremic, and hypernatremic, respectively (P = .07). Baseline serum sodium levels didn’t differ between survivors (137 mmol/L) and nonsurvivors (138 mmol/L).
In multivariable analysis, the occurrence of hypernatremia at any point during the first 5 days in the hospital was among three independent risk factors for higher in-hospital mortality (adjusted hazard ratio, 2.74; P = .02). The other risk factors were older age and higher CRP level.
Overall, hyponatremia was not associated with death (P = .41).
During hospitalization, 37.9% of patients remained normonatremic; 36.9% experienced hyponatremia; 10.9% had hypernatremia; and 14.3% had both conditions at some point during their stay.
In-hospital mortality was 21% among those with normonatremia, compared with 56.6% for those with hypernatremia (odds ratio, 3.05; P = .0038) and 45.7% for those with both (OR, 2.25; P < .0001).
The 28.3% mortality rate in the overall group that experienced hyponatremia didn’t differ significantly from the 21.1% in the normonatremic group (OR, 1.34; P = .16). However, the death rate was 40.9% among the subgroup that developed hypovolemic hyponatremia, significantly higher than the normonatremic group (OR, 2.59, P = .0017).
The incidence of hyponatremia decreased from 24.6% at admission to 14.1% 5 days later, whereas the frequency of hypernatremia rose from 5.3% to 13.8%.
Key finding: Link between hospital-acquired hypernatremia and death
“The key novel finding of our study was that hospital-acquired hypernatremia, rather than hypernatremia at admission, was a predictor for in-hospital mortality, with the worst prognosis being reported in patients with the largest increase in serum sodium in the first 5 days of hospitalization,” noted Dr. Tzoulis and colleagues.
Hypernatremia was present in 29.6% of nonsurvivors, compared with 5.2% in survivors.
Among 120 patients with hyponatremia at admission, 31.7% received advanced respiratory support, compared with 17.5% and 7.7% of those with normonatremia or hypernatremia, respectively (OR, 2.18; P = .0011).
In contrast, there was no difference in the proportions needing ventilatory support between those with hypernatremia and those with normonatremia (16.7% vs. 12.4%; OR, 1.44; P = .39).
Acute kidney injury occurred in 181 patients (37.1%). It was not related to serum sodium concentration at any time point.
Dr. Tzoulis and Dr. Simpson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Both high and low serum sodium levels are associated with adverse outcomes for hospitalized patients with COVID-19, new research suggests.
In the retrospective study of 488 patients hospitalized with COVID-19 at one of two London hospitals between February and May 2020, hypernatremia (defined as serum sodium level >145 mmol/L) at any time point during hospital stay was associated with a threefold increase in inpatient mortality.
Hyponatremia (serum sodium level <135 mmol/L) was associated with twice the likelihood of requiring advanced ventilatory support. In-hospital mortality was also increased among patients with hypovolemic hyponatremia.
“Serum sodium values could be used in clinical practice to identify patients with COVID-19 at high risk of poor outcomes who would benefit from more intensive monitoring and judicious rehydration,” Ploutarchos Tzoulis, MD, PhD, and colleagues wrote in their article, which was published online on Feb. 24, 2021, in the Journal of Clinical Endocrinology and Metabolism.
The findings will be presented at the upcoming news conference held by the Endocrine Society
Should sodium be included in a risk calculator for COVID-19?
Dr. Tzoulis, professor of endocrinology at the University College London Medical School, said in an interview that “sodium could be incorporated in risk calculators across other routine biomarkers, such as white cell count, lymphocytes, and CRP [C-reactive protein], in order to provide a tool for dynamic risk stratification throughout the clinical course of COVID-19 and assist clinical decision-making.”
Moreover, he said, “we should follow less conservative strategies in the rate and amount of fluid resuscitation in order to prevent hypernatremia, which is induced by negative fluid balance and can often be iatrogenic.”
Asked to comment, Steven Q. Simpson, MD, professor of medicine in the division of pulmonary, critical care, and sleep medicine at the University of Kansas, Kansas City, said that the article is missing key results that would assist in interpreting of the findings.
“Data regarding diuretic use and sparing of fluid administration are not in the paper. ... It is simply not possible to tell whether serum sodium is a ‘predictor’ ... or if it is a side effect of other issues or actions taken by physicians in patients who are progressing poorly.
“To say that sodium needs to be included in a risk calculator is to subtly suggest that there is some causal association with mortality, and that has quite clearly not been established,” stressed Dr. Simpson, who is president of the American College of Chest Physicians but was not speaking for the organization.
He added: “The data are interesting, but not actionable. It is common practice in critical care medicine to adjust water and salt intake to maintain serum sodium within the normal range, so the paper really doesn’t change any behavior.”
Dr. Tzoulis said in an interview that, despite not having electronic medical record data on diuretic use or fluid input and output, “our acute physicians and intensivists at both study sites have been adamant that they’ve not routinely used diuretics in COVID-19 patients. Diuretics have been sparingly used in our cohort, and also the frequency of pulmonary edema was reported as below 5%.”
Regarding volume of fluid intake, Dr. Tzoulis noted, “At our hospital sites, the strategy has been that of cautious fluid resuscitation. In fact, the amount of fluid given has been reported by our physicians and intensivists as ‘on purpose much more conservative than the usual one adopted in patients with community-acquired pneumonia at risk of respiratory failure.’ ”
Hyper- and hyponatremia linked to adverse COVID-19 outcomes
In the study, 5.3% of the 488 patients had hypernatremia at hospital presentation, and 24.6% had hyponatremia. Of note, only 19% of those with hyponatremia underwent laboratory workup to determine the etiology. Of those, three quarters had hypovolemic hyponatremia, determined on the basis of a urinary sodium cutoff of 30 mmol/L.
The total in-hospital mortality rate was 31.1%. There was a strong, although nonsignificant, trend toward higher mortality in association with sodium status at admission. Death rates were 28.4%, 30.8%, and 46.1% for those who were normonatremic, hyponatremic, and hypernatremic, respectively (P = .07). Baseline serum sodium levels didn’t differ between survivors (137 mmol/L) and nonsurvivors (138 mmol/L).
In multivariable analysis, the occurrence of hypernatremia at any point during the first 5 days in the hospital was among three independent risk factors for higher in-hospital mortality (adjusted hazard ratio, 2.74; P = .02). The other risk factors were older age and higher CRP level.
Overall, hyponatremia was not associated with death (P = .41).
During hospitalization, 37.9% of patients remained normonatremic; 36.9% experienced hyponatremia; 10.9% had hypernatremia; and 14.3% had both conditions at some point during their stay.
In-hospital mortality was 21% among those with normonatremia, compared with 56.6% for those with hypernatremia (odds ratio, 3.05; P = .0038) and 45.7% for those with both (OR, 2.25; P < .0001).
The 28.3% mortality rate in the overall group that experienced hyponatremia didn’t differ significantly from the 21.1% in the normonatremic group (OR, 1.34; P = .16). However, the death rate was 40.9% among the subgroup that developed hypovolemic hyponatremia, significantly higher than the normonatremic group (OR, 2.59, P = .0017).
The incidence of hyponatremia decreased from 24.6% at admission to 14.1% 5 days later, whereas the frequency of hypernatremia rose from 5.3% to 13.8%.
Key finding: Link between hospital-acquired hypernatremia and death
“The key novel finding of our study was that hospital-acquired hypernatremia, rather than hypernatremia at admission, was a predictor for in-hospital mortality, with the worst prognosis being reported in patients with the largest increase in serum sodium in the first 5 days of hospitalization,” noted Dr. Tzoulis and colleagues.
Hypernatremia was present in 29.6% of nonsurvivors, compared with 5.2% in survivors.
Among 120 patients with hyponatremia at admission, 31.7% received advanced respiratory support, compared with 17.5% and 7.7% of those with normonatremia or hypernatremia, respectively (OR, 2.18; P = .0011).
In contrast, there was no difference in the proportions needing ventilatory support between those with hypernatremia and those with normonatremia (16.7% vs. 12.4%; OR, 1.44; P = .39).
Acute kidney injury occurred in 181 patients (37.1%). It was not related to serum sodium concentration at any time point.
Dr. Tzoulis and Dr. Simpson disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Inclusivity needed in PHM fellowships
A year and a half ago, I found myself seated in a crowded hall at the national Pediatric Hospital Medicine (PHM) conference. Throughout the conference, trainees like me were warmly welcomed into small groups and lunch tables. I tried to keep my cool while PHM “celebrities” chatted with me in the elevator. Most sessions were prepared with plenty of chairs, and those that were not encouraged latecomers to grab a spot on the floor or the back wall – the more the merrier.
The intention of this “advice for applicants” meeting was to inspire and guide our next steps toward fellowship, but a discomforting reality loomed over us. It was the first year graduating pediatricians could not choose PHM board certification via the practice pathway – we needed an invitation in the form of a fellowship match.
The “hidden curriculum” was not subtle: People who scored a seat would keep their options open within the field of PHM, and those who did not had a murkier future. This message stood in stark contrast to the PHM inclusivity I had experienced all conference, and planted seeds of doubt: Was I welcome here? Did I “deserve” a seat?
I found the experience as a PHM fellowship applicant to be uncomfortable, and my all-too familiar friend “imposter syndrome” set up camp in my brain and made herself at home. I had no way of knowing how many programs to apply to, how many to interview at, or the chances of my matching at all. Once on the interview trail, I realized I was not alone in my discomfort – most applicants harbored some trepidation, and no one truly knew how the chips would fall on Match Day.
I am thrilled and relieved to have come out the other end in a great position. The team I work with and learn from is phenomenal. I am grateful that ACGME accreditation ensures structures are in place for fellows to be supported in their academic and educational efforts and have full confidence that the skills I gain in fellowship will help me contribute to progression of the field of PHM and improve my performance as a clinician-educator.
Sadly, each year PHM match day celebrations are dampened by the knowledge that a large portion of our colleagues are being left out in the cold with an “unmatched” notification in their inboxes. Approximately 200 graduating pediatricians become pediatric hospitalists each year,1 but only 68 fellowship positions were available in the United States for matriculation in 2020.2 In 2019, PHM fellowship candidates navigated the 6-month application journey with aspirations to further their training in the profession they love. Of the candidates who submitted a rank list committing to 2 or more years in PHM fellowship, 35% were denied.
Unfortunately, despite expansion of PHM fellowship programs and fifteen seats added from last year, we learned this December that there still are not enough positions to welcome qualified applicants with open arms: Thirty-three percent of candidates ranked PHM programs first in the NRMP but did not match – the highest unmatched percentage out of all pediatric subspecialties.3
The NRMP report shared a glimpse of our colleagues who received interview invitations and submitted a rank list, but this is likely an underestimation of pediatric graduates who wanted to obtain PHM board certification and wound up on a different path. Some residents anticipated the stiff competition and delayed their plans to apply for fellowship, while others matched into another subspecialty that was able to accommodate them. Many pediatric graduates joined the workforce directly as pediatric hospitalists knowing the practice pathway to certification is not available to them. Along with other physicians without board certification in PHM, they shoulder concerns of being withheld from professional advancement opportunities.
For the foreseeable future it is clear that pediatric hospitalists without board certification will be a large part of our community, and are crucial to providing high-quality care to hospitalized children nationally. In 2019, a national survey of pediatric hospital medicine groups revealed that 50% of pediatric hospitalist hires came directly out of residency, and only 8% of hires were fellowship trained.4 The same report revealed that 26% of physicians were board-certified.These percentages are likely to change over the next 5 years as the window of practice pathway certification closes and fellowship programs continue to expand. Only time will tell what the national prevalence of board-certified pediatric hospitalists settles out to be.
Historically, PHM fellowship graduates have assumed roles that include teaching and research responsibilities, and ACGME fellowship requirements have ensured that trainees graduate with skills in medical education and scholarship, and need only 4 weeks of training to be done in a community hospital.5 Pediatric hospitalists who do not pursue board certification are seeing the growing pool of PHM fellowship graduates prepared for positions in academic institutions. It is reasonable that they harbor concerns about being siloed toward primarily community hospital roles, and for community hospitalists to feel that this structure undervalues their role within the field of PHM.
At a time when inclusivity and community in medicine are receiving much-needed recognition, the current fellowship application climate has potential to create division within the PHM community. Newly graduating pediatric residents are among the populations disproportionately affected by the practice pathway cutoff. Like other subspecialties with ever-climbing steps up the “ivory tower” of academia and specialization within medicine, the inherent structure of the training pathway makes navigating it more difficult for pediatricians with professional, geographic, and economic diversity or constraints.
Med-Peds–trained colleagues have the added challenge of finding a fellowship position that is willing and able to support their concurrent internal medicine goals. International medical graduates make up about 20% of graduating residents each year, and just 11% of matched PHM fellows.3,6 Similarly, while DO medical graduates make up 20% of pediatric residents in the United States, only 10% of matched PHM fellows were DOs.3,6 New pediatricians with families or financial insecurity may be unable to invest in an expensive application process, move to a new city, and accept less than half of the average starting salary of a pediatric hospitalist for 2-3 years.7
The prevalence of implicit bias in medicine is well documented, and there is growing evidence that it negatively impacts candidate selection in medical education and contributes to minorities being underrepresented in the physician workforce.8 We must recognize the ways that adding a competitive costly hurdle may risk conflict with our mission to encourage diversity of representation within PHM leadership positions.
We have not yet successfully bridged the gap between qualified PHM fellowship candidates and available fellowship positions. I worry that this gap and the lack of transparency surrounding it is resulting in one portion of new pediatricians being welcomed by the subspecialty, and others feeling unsupported and alienated by the larger PHM community as early career physicians.
Right now, the only solution available is expansion of fellowship programs. We see progress with the new addition of fellowship positions every year, but finding funding for each position is often a lengthy endeavor, and the COVID-19 pandemic has tightened the purse strings of many children’s hospitals. It may be many years before the number of available fellowship positions more closely approximates the 200 pediatricians that become hospitalists each year.
The most equitable solution would be offering other avenues to board certification while this gap is being bridged, either by extending the practice pathway option, or making a third pathway that requires less institutional funding per fellow, but still incentivizes institutional investment in fellowship positions and resources (e.g., a pathway requiring some number of years in practice, plus 1 year in fellowship centered around a nonclinical academic curriculum).
In the absence of the solutions above, we collectively hold the responsibility of maintaining inclusivity and support of our PHM colleagues with and without board certification. One important strategy provided by Dr. Gregory Welsh9 is to incorporate community hospital medicine rotations into residency training. Sharing this side of PHM with residents may help some graduates avoid a training pathway they may not want or need. More importantly, it would raise trainee exposure and interest toward a service that is both expansive – approximately 70% of pediatric hospitalists practice in a community hospital – and crucial to children’s health nationally.
Pediatric hospitalists who are not eligible for board certification are vital and valued members of the PHM community, and as such need to maintain representation within PHM leadership. Professional development opportunities need to remain accessible outside of fellowship. The blossoming of virtual conferences and Zoom meet-ups in the face of the COVID-19 pandemic have shown us that with innovation (and a good Internet connection), networking and mentorship can be accomplished across thousands of miles.
While there’s great diversity within PHM, this subspecialty has a history of attracting pediatricians with some common core qualities: Grit, creativity, and the belief that a strong team is far greater than the sum of its parts. I have confidence that if we approach this PHM transition period with transparency about our goals and challenges, this community can emerge from it strong and united.
Dr. Ezzio is a first-year pediatric hospital medicine fellow at Helen DeVos Children’s Hospital in Grand Rapids, Mich. Her interests include medical education and advocacy. Dr. Ezzio would like to thank Dr. Jeri Kessenich and Dr. Rachel “Danielle” Fisher for their assistance in revising the article. To submit to, or for inquiries about, our PHM Fellows Column, please contact our Pediatrics Editor, Dr. Anika Kumar ([email protected]).
References
1. Leyenaar JK and Fritner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018 Mar;18(2):200-7.
2. National Resident Matching Program. Results and data: Specialties matching service 2020 appointment year. Washington, DC 2020.
3. National Resident Matching Program. Results and data: Specialties matching service 2021 appointment year. Washington, DC 2021.
4. 2020 State of Hospital Medicine report. Society of Hospital Medicine. 2020.
5. Oshimura JM et al. Current roles and perceived needs of pediatric hospital medicine fellowship graduates. Hosp Pediatr. 2016;6(10):633-7.
6. National Resident Matching Program. Results and data: 2020 main residency match. Washington, DC 2020.
7. American Academy of Pediatrics Annual Survey of graduating residents 2003-2020.
8. Quinn Capers IV. How clinicians and educators can mitigate implicit bias in patient care and candidate selection in medical education. American Thoracic Society Scholar. 2020 Jun;1(3):211-17.
9. Welsh G. The importance of community pediatric hospital medicine. The Hospitalist. 2021 Jan;25(1):27.
A year and a half ago, I found myself seated in a crowded hall at the national Pediatric Hospital Medicine (PHM) conference. Throughout the conference, trainees like me were warmly welcomed into small groups and lunch tables. I tried to keep my cool while PHM “celebrities” chatted with me in the elevator. Most sessions were prepared with plenty of chairs, and those that were not encouraged latecomers to grab a spot on the floor or the back wall – the more the merrier.
The intention of this “advice for applicants” meeting was to inspire and guide our next steps toward fellowship, but a discomforting reality loomed over us. It was the first year graduating pediatricians could not choose PHM board certification via the practice pathway – we needed an invitation in the form of a fellowship match.
The “hidden curriculum” was not subtle: People who scored a seat would keep their options open within the field of PHM, and those who did not had a murkier future. This message stood in stark contrast to the PHM inclusivity I had experienced all conference, and planted seeds of doubt: Was I welcome here? Did I “deserve” a seat?
I found the experience as a PHM fellowship applicant to be uncomfortable, and my all-too familiar friend “imposter syndrome” set up camp in my brain and made herself at home. I had no way of knowing how many programs to apply to, how many to interview at, or the chances of my matching at all. Once on the interview trail, I realized I was not alone in my discomfort – most applicants harbored some trepidation, and no one truly knew how the chips would fall on Match Day.
I am thrilled and relieved to have come out the other end in a great position. The team I work with and learn from is phenomenal. I am grateful that ACGME accreditation ensures structures are in place for fellows to be supported in their academic and educational efforts and have full confidence that the skills I gain in fellowship will help me contribute to progression of the field of PHM and improve my performance as a clinician-educator.
Sadly, each year PHM match day celebrations are dampened by the knowledge that a large portion of our colleagues are being left out in the cold with an “unmatched” notification in their inboxes. Approximately 200 graduating pediatricians become pediatric hospitalists each year,1 but only 68 fellowship positions were available in the United States for matriculation in 2020.2 In 2019, PHM fellowship candidates navigated the 6-month application journey with aspirations to further their training in the profession they love. Of the candidates who submitted a rank list committing to 2 or more years in PHM fellowship, 35% were denied.
Unfortunately, despite expansion of PHM fellowship programs and fifteen seats added from last year, we learned this December that there still are not enough positions to welcome qualified applicants with open arms: Thirty-three percent of candidates ranked PHM programs first in the NRMP but did not match – the highest unmatched percentage out of all pediatric subspecialties.3
The NRMP report shared a glimpse of our colleagues who received interview invitations and submitted a rank list, but this is likely an underestimation of pediatric graduates who wanted to obtain PHM board certification and wound up on a different path. Some residents anticipated the stiff competition and delayed their plans to apply for fellowship, while others matched into another subspecialty that was able to accommodate them. Many pediatric graduates joined the workforce directly as pediatric hospitalists knowing the practice pathway to certification is not available to them. Along with other physicians without board certification in PHM, they shoulder concerns of being withheld from professional advancement opportunities.
For the foreseeable future it is clear that pediatric hospitalists without board certification will be a large part of our community, and are crucial to providing high-quality care to hospitalized children nationally. In 2019, a national survey of pediatric hospital medicine groups revealed that 50% of pediatric hospitalist hires came directly out of residency, and only 8% of hires were fellowship trained.4 The same report revealed that 26% of physicians were board-certified.These percentages are likely to change over the next 5 years as the window of practice pathway certification closes and fellowship programs continue to expand. Only time will tell what the national prevalence of board-certified pediatric hospitalists settles out to be.
Historically, PHM fellowship graduates have assumed roles that include teaching and research responsibilities, and ACGME fellowship requirements have ensured that trainees graduate with skills in medical education and scholarship, and need only 4 weeks of training to be done in a community hospital.5 Pediatric hospitalists who do not pursue board certification are seeing the growing pool of PHM fellowship graduates prepared for positions in academic institutions. It is reasonable that they harbor concerns about being siloed toward primarily community hospital roles, and for community hospitalists to feel that this structure undervalues their role within the field of PHM.
At a time when inclusivity and community in medicine are receiving much-needed recognition, the current fellowship application climate has potential to create division within the PHM community. Newly graduating pediatric residents are among the populations disproportionately affected by the practice pathway cutoff. Like other subspecialties with ever-climbing steps up the “ivory tower” of academia and specialization within medicine, the inherent structure of the training pathway makes navigating it more difficult for pediatricians with professional, geographic, and economic diversity or constraints.
Med-Peds–trained colleagues have the added challenge of finding a fellowship position that is willing and able to support their concurrent internal medicine goals. International medical graduates make up about 20% of graduating residents each year, and just 11% of matched PHM fellows.3,6 Similarly, while DO medical graduates make up 20% of pediatric residents in the United States, only 10% of matched PHM fellows were DOs.3,6 New pediatricians with families or financial insecurity may be unable to invest in an expensive application process, move to a new city, and accept less than half of the average starting salary of a pediatric hospitalist for 2-3 years.7
The prevalence of implicit bias in medicine is well documented, and there is growing evidence that it negatively impacts candidate selection in medical education and contributes to minorities being underrepresented in the physician workforce.8 We must recognize the ways that adding a competitive costly hurdle may risk conflict with our mission to encourage diversity of representation within PHM leadership positions.
We have not yet successfully bridged the gap between qualified PHM fellowship candidates and available fellowship positions. I worry that this gap and the lack of transparency surrounding it is resulting in one portion of new pediatricians being welcomed by the subspecialty, and others feeling unsupported and alienated by the larger PHM community as early career physicians.
Right now, the only solution available is expansion of fellowship programs. We see progress with the new addition of fellowship positions every year, but finding funding for each position is often a lengthy endeavor, and the COVID-19 pandemic has tightened the purse strings of many children’s hospitals. It may be many years before the number of available fellowship positions more closely approximates the 200 pediatricians that become hospitalists each year.
The most equitable solution would be offering other avenues to board certification while this gap is being bridged, either by extending the practice pathway option, or making a third pathway that requires less institutional funding per fellow, but still incentivizes institutional investment in fellowship positions and resources (e.g., a pathway requiring some number of years in practice, plus 1 year in fellowship centered around a nonclinical academic curriculum).
In the absence of the solutions above, we collectively hold the responsibility of maintaining inclusivity and support of our PHM colleagues with and without board certification. One important strategy provided by Dr. Gregory Welsh9 is to incorporate community hospital medicine rotations into residency training. Sharing this side of PHM with residents may help some graduates avoid a training pathway they may not want or need. More importantly, it would raise trainee exposure and interest toward a service that is both expansive – approximately 70% of pediatric hospitalists practice in a community hospital – and crucial to children’s health nationally.
Pediatric hospitalists who are not eligible for board certification are vital and valued members of the PHM community, and as such need to maintain representation within PHM leadership. Professional development opportunities need to remain accessible outside of fellowship. The blossoming of virtual conferences and Zoom meet-ups in the face of the COVID-19 pandemic have shown us that with innovation (and a good Internet connection), networking and mentorship can be accomplished across thousands of miles.
While there’s great diversity within PHM, this subspecialty has a history of attracting pediatricians with some common core qualities: Grit, creativity, and the belief that a strong team is far greater than the sum of its parts. I have confidence that if we approach this PHM transition period with transparency about our goals and challenges, this community can emerge from it strong and united.
Dr. Ezzio is a first-year pediatric hospital medicine fellow at Helen DeVos Children’s Hospital in Grand Rapids, Mich. Her interests include medical education and advocacy. Dr. Ezzio would like to thank Dr. Jeri Kessenich and Dr. Rachel “Danielle” Fisher for their assistance in revising the article. To submit to, or for inquiries about, our PHM Fellows Column, please contact our Pediatrics Editor, Dr. Anika Kumar ([email protected]).
References
1. Leyenaar JK and Fritner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018 Mar;18(2):200-7.
2. National Resident Matching Program. Results and data: Specialties matching service 2020 appointment year. Washington, DC 2020.
3. National Resident Matching Program. Results and data: Specialties matching service 2021 appointment year. Washington, DC 2021.
4. 2020 State of Hospital Medicine report. Society of Hospital Medicine. 2020.
5. Oshimura JM et al. Current roles and perceived needs of pediatric hospital medicine fellowship graduates. Hosp Pediatr. 2016;6(10):633-7.
6. National Resident Matching Program. Results and data: 2020 main residency match. Washington, DC 2020.
7. American Academy of Pediatrics Annual Survey of graduating residents 2003-2020.
8. Quinn Capers IV. How clinicians and educators can mitigate implicit bias in patient care and candidate selection in medical education. American Thoracic Society Scholar. 2020 Jun;1(3):211-17.
9. Welsh G. The importance of community pediatric hospital medicine. The Hospitalist. 2021 Jan;25(1):27.
A year and a half ago, I found myself seated in a crowded hall at the national Pediatric Hospital Medicine (PHM) conference. Throughout the conference, trainees like me were warmly welcomed into small groups and lunch tables. I tried to keep my cool while PHM “celebrities” chatted with me in the elevator. Most sessions were prepared with plenty of chairs, and those that were not encouraged latecomers to grab a spot on the floor or the back wall – the more the merrier.
The intention of this “advice for applicants” meeting was to inspire and guide our next steps toward fellowship, but a discomforting reality loomed over us. It was the first year graduating pediatricians could not choose PHM board certification via the practice pathway – we needed an invitation in the form of a fellowship match.
The “hidden curriculum” was not subtle: People who scored a seat would keep their options open within the field of PHM, and those who did not had a murkier future. This message stood in stark contrast to the PHM inclusivity I had experienced all conference, and planted seeds of doubt: Was I welcome here? Did I “deserve” a seat?
I found the experience as a PHM fellowship applicant to be uncomfortable, and my all-too familiar friend “imposter syndrome” set up camp in my brain and made herself at home. I had no way of knowing how many programs to apply to, how many to interview at, or the chances of my matching at all. Once on the interview trail, I realized I was not alone in my discomfort – most applicants harbored some trepidation, and no one truly knew how the chips would fall on Match Day.
I am thrilled and relieved to have come out the other end in a great position. The team I work with and learn from is phenomenal. I am grateful that ACGME accreditation ensures structures are in place for fellows to be supported in their academic and educational efforts and have full confidence that the skills I gain in fellowship will help me contribute to progression of the field of PHM and improve my performance as a clinician-educator.
Sadly, each year PHM match day celebrations are dampened by the knowledge that a large portion of our colleagues are being left out in the cold with an “unmatched” notification in their inboxes. Approximately 200 graduating pediatricians become pediatric hospitalists each year,1 but only 68 fellowship positions were available in the United States for matriculation in 2020.2 In 2019, PHM fellowship candidates navigated the 6-month application journey with aspirations to further their training in the profession they love. Of the candidates who submitted a rank list committing to 2 or more years in PHM fellowship, 35% were denied.
Unfortunately, despite expansion of PHM fellowship programs and fifteen seats added from last year, we learned this December that there still are not enough positions to welcome qualified applicants with open arms: Thirty-three percent of candidates ranked PHM programs first in the NRMP but did not match – the highest unmatched percentage out of all pediatric subspecialties.3
The NRMP report shared a glimpse of our colleagues who received interview invitations and submitted a rank list, but this is likely an underestimation of pediatric graduates who wanted to obtain PHM board certification and wound up on a different path. Some residents anticipated the stiff competition and delayed their plans to apply for fellowship, while others matched into another subspecialty that was able to accommodate them. Many pediatric graduates joined the workforce directly as pediatric hospitalists knowing the practice pathway to certification is not available to them. Along with other physicians without board certification in PHM, they shoulder concerns of being withheld from professional advancement opportunities.
For the foreseeable future it is clear that pediatric hospitalists without board certification will be a large part of our community, and are crucial to providing high-quality care to hospitalized children nationally. In 2019, a national survey of pediatric hospital medicine groups revealed that 50% of pediatric hospitalist hires came directly out of residency, and only 8% of hires were fellowship trained.4 The same report revealed that 26% of physicians were board-certified.These percentages are likely to change over the next 5 years as the window of practice pathway certification closes and fellowship programs continue to expand. Only time will tell what the national prevalence of board-certified pediatric hospitalists settles out to be.
Historically, PHM fellowship graduates have assumed roles that include teaching and research responsibilities, and ACGME fellowship requirements have ensured that trainees graduate with skills in medical education and scholarship, and need only 4 weeks of training to be done in a community hospital.5 Pediatric hospitalists who do not pursue board certification are seeing the growing pool of PHM fellowship graduates prepared for positions in academic institutions. It is reasonable that they harbor concerns about being siloed toward primarily community hospital roles, and for community hospitalists to feel that this structure undervalues their role within the field of PHM.
At a time when inclusivity and community in medicine are receiving much-needed recognition, the current fellowship application climate has potential to create division within the PHM community. Newly graduating pediatric residents are among the populations disproportionately affected by the practice pathway cutoff. Like other subspecialties with ever-climbing steps up the “ivory tower” of academia and specialization within medicine, the inherent structure of the training pathway makes navigating it more difficult for pediatricians with professional, geographic, and economic diversity or constraints.
Med-Peds–trained colleagues have the added challenge of finding a fellowship position that is willing and able to support their concurrent internal medicine goals. International medical graduates make up about 20% of graduating residents each year, and just 11% of matched PHM fellows.3,6 Similarly, while DO medical graduates make up 20% of pediatric residents in the United States, only 10% of matched PHM fellows were DOs.3,6 New pediatricians with families or financial insecurity may be unable to invest in an expensive application process, move to a new city, and accept less than half of the average starting salary of a pediatric hospitalist for 2-3 years.7
The prevalence of implicit bias in medicine is well documented, and there is growing evidence that it negatively impacts candidate selection in medical education and contributes to minorities being underrepresented in the physician workforce.8 We must recognize the ways that adding a competitive costly hurdle may risk conflict with our mission to encourage diversity of representation within PHM leadership positions.
We have not yet successfully bridged the gap between qualified PHM fellowship candidates and available fellowship positions. I worry that this gap and the lack of transparency surrounding it is resulting in one portion of new pediatricians being welcomed by the subspecialty, and others feeling unsupported and alienated by the larger PHM community as early career physicians.
Right now, the only solution available is expansion of fellowship programs. We see progress with the new addition of fellowship positions every year, but finding funding for each position is often a lengthy endeavor, and the COVID-19 pandemic has tightened the purse strings of many children’s hospitals. It may be many years before the number of available fellowship positions more closely approximates the 200 pediatricians that become hospitalists each year.
The most equitable solution would be offering other avenues to board certification while this gap is being bridged, either by extending the practice pathway option, or making a third pathway that requires less institutional funding per fellow, but still incentivizes institutional investment in fellowship positions and resources (e.g., a pathway requiring some number of years in practice, plus 1 year in fellowship centered around a nonclinical academic curriculum).
In the absence of the solutions above, we collectively hold the responsibility of maintaining inclusivity and support of our PHM colleagues with and without board certification. One important strategy provided by Dr. Gregory Welsh9 is to incorporate community hospital medicine rotations into residency training. Sharing this side of PHM with residents may help some graduates avoid a training pathway they may not want or need. More importantly, it would raise trainee exposure and interest toward a service that is both expansive – approximately 70% of pediatric hospitalists practice in a community hospital – and crucial to children’s health nationally.
Pediatric hospitalists who are not eligible for board certification are vital and valued members of the PHM community, and as such need to maintain representation within PHM leadership. Professional development opportunities need to remain accessible outside of fellowship. The blossoming of virtual conferences and Zoom meet-ups in the face of the COVID-19 pandemic have shown us that with innovation (and a good Internet connection), networking and mentorship can be accomplished across thousands of miles.
While there’s great diversity within PHM, this subspecialty has a history of attracting pediatricians with some common core qualities: Grit, creativity, and the belief that a strong team is far greater than the sum of its parts. I have confidence that if we approach this PHM transition period with transparency about our goals and challenges, this community can emerge from it strong and united.
Dr. Ezzio is a first-year pediatric hospital medicine fellow at Helen DeVos Children’s Hospital in Grand Rapids, Mich. Her interests include medical education and advocacy. Dr. Ezzio would like to thank Dr. Jeri Kessenich and Dr. Rachel “Danielle” Fisher for their assistance in revising the article. To submit to, or for inquiries about, our PHM Fellows Column, please contact our Pediatrics Editor, Dr. Anika Kumar ([email protected]).
References
1. Leyenaar JK and Fritner MP. Graduating pediatric residents entering the hospital medicine workforce, 2006-2015. Acad Pediatr. 2018 Mar;18(2):200-7.
2. National Resident Matching Program. Results and data: Specialties matching service 2020 appointment year. Washington, DC 2020.
3. National Resident Matching Program. Results and data: Specialties matching service 2021 appointment year. Washington, DC 2021.
4. 2020 State of Hospital Medicine report. Society of Hospital Medicine. 2020.
5. Oshimura JM et al. Current roles and perceived needs of pediatric hospital medicine fellowship graduates. Hosp Pediatr. 2016;6(10):633-7.
6. National Resident Matching Program. Results and data: 2020 main residency match. Washington, DC 2020.
7. American Academy of Pediatrics Annual Survey of graduating residents 2003-2020.
8. Quinn Capers IV. How clinicians and educators can mitigate implicit bias in patient care and candidate selection in medical education. American Thoracic Society Scholar. 2020 Jun;1(3):211-17.
9. Welsh G. The importance of community pediatric hospital medicine. The Hospitalist. 2021 Jan;25(1):27.
Delay surgery by 7 weeks after COVID-19 diagnosis, study shows
Seven weeks appears to be the ideal amount of time to delay surgery, when possible, after someone tests positive for COVID-19, researchers in the United Kingdom report.
Risk for death was about 3.5 to 4 times higher in the first 6 weeks after surgery among more than 3,000 people with a preoperative COVID-19 diagnosis compared with patients without COVID-19. After 7 weeks, the 30-day mortality rate dropped to a baseline level.
The study was published online March 9 in Anaesthesia.
Surgery should be further delayed for people who remain symptomatic at 7 weeks post diagnosis, lead author Dmitri Nepogodiev, MBChB, said in an interview.
“In this group we recommend waiting until COVID-19 symptoms resolve, if possible. However, our study did not capture specific data on long COVID … so we are unable to make specific recommendations for this group,” said Dr. Nepogodiev, research fellow at the NIHR Global Health Research Unit on Global Surgery at the University of Birmingham (England).
“This should be an area for future research,” he added.
The international, multicenter, prospective cohort study is notable for its sheer size – more than 15,000 investigators reported outcomes for 140,231 surgical patients from 1,674 hospitals across 116 countries. In total, 2.2% of these patients tested positive for SARS-CoV-2 prior to surgery.
Surgery of any type performed in October 2020 was assessed. A greater proportion of patients with a preoperative COVID-19 diagnosis had emergency surgery, 44%, compared with 30% of people who never had a COVID-19 diagnosis.
Most patients were asymptomatic at the time of surgery, either because they never experienced COVID-19 symptoms or their symptoms resolved. The 30-day mortality rate was the primary outcome.
Death rates among surgical patients with preoperative COVID-19 diagnosis
Comparing the timing of surgery after COVID-19 diagnosis vs. 30-day mortality yielded the following results:
- 0 to 2 weeks – 9.1% mortality.
- 3 to 4 weeks – 6.9%.
- 5 to 6 weeks – 5.5%.
- 7 weeks or longer – 2.0%..
For comparison, the 30-day mortality rate for surgical patients without a preoperative COVID-19 diagnosis was 1.4%. A COVID-19 diagnosis more than 7 weeks before surgery did not make a significant difference on outcomes.
The ‘why’ remains unknown
The reasons for the association between a COVID-19 diagnosis and higher postoperative death rates remain unknown. However, Dr. Nepogodiev speculated that it could be related to “some degree of lung injury, even if patients are initially asymptomatic.”
Intubation and mechanical ventilation during surgery could exacerbate the existing lung injury, he said, thereby leading to more severe COVID-19.
In fact, Dr. Nepogodiev and colleagues found that postoperative pulmonary complications followed a pattern similar to the findings on death. They reported higher rates of pneumonia, acute respiratory distress syndrome, and unexpected reventilation in the first 6 weeks following a COVID-19 diagnosis. Again, at 7 weeks and beyond, the rates returned to be relatively the same as those for people who never had COVID-19.
“Waiting for 7 or more weeks may allow time for the initial COVID-19 injury to resolve,” Dr. Nepogodiev said.
‘An important study’
“This is an important study of postoperative mortality among patients recovered from COVID-19,” Adrian Diaz, MD, MPH, said in an interview when asked to comment.
The large cohort and numerous practice settings are among the strengths of the research, said Dr. Diaz, of the University of Michigan Institute for Healthcare Policy and Innovation in Ann Arbor. He was lead author of a June 2020 review article on elective surgery in the time of COVID-19, published in The American Journal of Surgery.
“As with nearly all studies of this nature, results must be interpreted on a case-by-case basis for individual patients. However, this study does add important information for patients and providers in helping them have an informed discussion on the timing of surgery,” said Dr. Diaz, a fellow in the Center for Healthcare Outcomes and Policy and a resident in general surgery at the Ohio State University, Columbus.
Dr. Nepogodiev and colleagues included both urgent and elective surgeries in the study. Dr. Diaz said this was a potential limitation because emergency operations “should never be delayed, by definition.” Lack of indications for the surgeries and information on cause of death were additional limitations.
Future research should evaluate any benefit in delaying surgery longer than 7 or more weeks, Dr. Diaz added, perhaps looking specifically at 10, 12, or 14 weeks, or considering outcomes as a continuous variable. This would help health care providers “garner more insight into risk and benefits of delaying surgery beyond 7 weeks.”
Dr. Nepogodiev and Dr. Diaz disclosed no relevant financial relationships. The study had multiple funding sources, including the National Institute for Health Research Global Health Research Unit, the Association of Upper Gastrointestinal Surgeons, the British Association of Surgical Oncology, and Medtronic.
A version of this article first appeared on Medscape.com.
Seven weeks appears to be the ideal amount of time to delay surgery, when possible, after someone tests positive for COVID-19, researchers in the United Kingdom report.
Risk for death was about 3.5 to 4 times higher in the first 6 weeks after surgery among more than 3,000 people with a preoperative COVID-19 diagnosis compared with patients without COVID-19. After 7 weeks, the 30-day mortality rate dropped to a baseline level.
The study was published online March 9 in Anaesthesia.
Surgery should be further delayed for people who remain symptomatic at 7 weeks post diagnosis, lead author Dmitri Nepogodiev, MBChB, said in an interview.
“In this group we recommend waiting until COVID-19 symptoms resolve, if possible. However, our study did not capture specific data on long COVID … so we are unable to make specific recommendations for this group,” said Dr. Nepogodiev, research fellow at the NIHR Global Health Research Unit on Global Surgery at the University of Birmingham (England).
“This should be an area for future research,” he added.
The international, multicenter, prospective cohort study is notable for its sheer size – more than 15,000 investigators reported outcomes for 140,231 surgical patients from 1,674 hospitals across 116 countries. In total, 2.2% of these patients tested positive for SARS-CoV-2 prior to surgery.
Surgery of any type performed in October 2020 was assessed. A greater proportion of patients with a preoperative COVID-19 diagnosis had emergency surgery, 44%, compared with 30% of people who never had a COVID-19 diagnosis.
Most patients were asymptomatic at the time of surgery, either because they never experienced COVID-19 symptoms or their symptoms resolved. The 30-day mortality rate was the primary outcome.
Death rates among surgical patients with preoperative COVID-19 diagnosis
Comparing the timing of surgery after COVID-19 diagnosis vs. 30-day mortality yielded the following results:
- 0 to 2 weeks – 9.1% mortality.
- 3 to 4 weeks – 6.9%.
- 5 to 6 weeks – 5.5%.
- 7 weeks or longer – 2.0%..
For comparison, the 30-day mortality rate for surgical patients without a preoperative COVID-19 diagnosis was 1.4%. A COVID-19 diagnosis more than 7 weeks before surgery did not make a significant difference on outcomes.
The ‘why’ remains unknown
The reasons for the association between a COVID-19 diagnosis and higher postoperative death rates remain unknown. However, Dr. Nepogodiev speculated that it could be related to “some degree of lung injury, even if patients are initially asymptomatic.”
Intubation and mechanical ventilation during surgery could exacerbate the existing lung injury, he said, thereby leading to more severe COVID-19.
In fact, Dr. Nepogodiev and colleagues found that postoperative pulmonary complications followed a pattern similar to the findings on death. They reported higher rates of pneumonia, acute respiratory distress syndrome, and unexpected reventilation in the first 6 weeks following a COVID-19 diagnosis. Again, at 7 weeks and beyond, the rates returned to be relatively the same as those for people who never had COVID-19.
“Waiting for 7 or more weeks may allow time for the initial COVID-19 injury to resolve,” Dr. Nepogodiev said.
‘An important study’
“This is an important study of postoperative mortality among patients recovered from COVID-19,” Adrian Diaz, MD, MPH, said in an interview when asked to comment.
The large cohort and numerous practice settings are among the strengths of the research, said Dr. Diaz, of the University of Michigan Institute for Healthcare Policy and Innovation in Ann Arbor. He was lead author of a June 2020 review article on elective surgery in the time of COVID-19, published in The American Journal of Surgery.
“As with nearly all studies of this nature, results must be interpreted on a case-by-case basis for individual patients. However, this study does add important information for patients and providers in helping them have an informed discussion on the timing of surgery,” said Dr. Diaz, a fellow in the Center for Healthcare Outcomes and Policy and a resident in general surgery at the Ohio State University, Columbus.
Dr. Nepogodiev and colleagues included both urgent and elective surgeries in the study. Dr. Diaz said this was a potential limitation because emergency operations “should never be delayed, by definition.” Lack of indications for the surgeries and information on cause of death were additional limitations.
Future research should evaluate any benefit in delaying surgery longer than 7 or more weeks, Dr. Diaz added, perhaps looking specifically at 10, 12, or 14 weeks, or considering outcomes as a continuous variable. This would help health care providers “garner more insight into risk and benefits of delaying surgery beyond 7 weeks.”
Dr. Nepogodiev and Dr. Diaz disclosed no relevant financial relationships. The study had multiple funding sources, including the National Institute for Health Research Global Health Research Unit, the Association of Upper Gastrointestinal Surgeons, the British Association of Surgical Oncology, and Medtronic.
A version of this article first appeared on Medscape.com.
Seven weeks appears to be the ideal amount of time to delay surgery, when possible, after someone tests positive for COVID-19, researchers in the United Kingdom report.
Risk for death was about 3.5 to 4 times higher in the first 6 weeks after surgery among more than 3,000 people with a preoperative COVID-19 diagnosis compared with patients without COVID-19. After 7 weeks, the 30-day mortality rate dropped to a baseline level.
The study was published online March 9 in Anaesthesia.
Surgery should be further delayed for people who remain symptomatic at 7 weeks post diagnosis, lead author Dmitri Nepogodiev, MBChB, said in an interview.
“In this group we recommend waiting until COVID-19 symptoms resolve, if possible. However, our study did not capture specific data on long COVID … so we are unable to make specific recommendations for this group,” said Dr. Nepogodiev, research fellow at the NIHR Global Health Research Unit on Global Surgery at the University of Birmingham (England).
“This should be an area for future research,” he added.
The international, multicenter, prospective cohort study is notable for its sheer size – more than 15,000 investigators reported outcomes for 140,231 surgical patients from 1,674 hospitals across 116 countries. In total, 2.2% of these patients tested positive for SARS-CoV-2 prior to surgery.
Surgery of any type performed in October 2020 was assessed. A greater proportion of patients with a preoperative COVID-19 diagnosis had emergency surgery, 44%, compared with 30% of people who never had a COVID-19 diagnosis.
Most patients were asymptomatic at the time of surgery, either because they never experienced COVID-19 symptoms or their symptoms resolved. The 30-day mortality rate was the primary outcome.
Death rates among surgical patients with preoperative COVID-19 diagnosis
Comparing the timing of surgery after COVID-19 diagnosis vs. 30-day mortality yielded the following results:
- 0 to 2 weeks – 9.1% mortality.
- 3 to 4 weeks – 6.9%.
- 5 to 6 weeks – 5.5%.
- 7 weeks or longer – 2.0%..
For comparison, the 30-day mortality rate for surgical patients without a preoperative COVID-19 diagnosis was 1.4%. A COVID-19 diagnosis more than 7 weeks before surgery did not make a significant difference on outcomes.
The ‘why’ remains unknown
The reasons for the association between a COVID-19 diagnosis and higher postoperative death rates remain unknown. However, Dr. Nepogodiev speculated that it could be related to “some degree of lung injury, even if patients are initially asymptomatic.”
Intubation and mechanical ventilation during surgery could exacerbate the existing lung injury, he said, thereby leading to more severe COVID-19.
In fact, Dr. Nepogodiev and colleagues found that postoperative pulmonary complications followed a pattern similar to the findings on death. They reported higher rates of pneumonia, acute respiratory distress syndrome, and unexpected reventilation in the first 6 weeks following a COVID-19 diagnosis. Again, at 7 weeks and beyond, the rates returned to be relatively the same as those for people who never had COVID-19.
“Waiting for 7 or more weeks may allow time for the initial COVID-19 injury to resolve,” Dr. Nepogodiev said.
‘An important study’
“This is an important study of postoperative mortality among patients recovered from COVID-19,” Adrian Diaz, MD, MPH, said in an interview when asked to comment.
The large cohort and numerous practice settings are among the strengths of the research, said Dr. Diaz, of the University of Michigan Institute for Healthcare Policy and Innovation in Ann Arbor. He was lead author of a June 2020 review article on elective surgery in the time of COVID-19, published in The American Journal of Surgery.
“As with nearly all studies of this nature, results must be interpreted on a case-by-case basis for individual patients. However, this study does add important information for patients and providers in helping them have an informed discussion on the timing of surgery,” said Dr. Diaz, a fellow in the Center for Healthcare Outcomes and Policy and a resident in general surgery at the Ohio State University, Columbus.
Dr. Nepogodiev and colleagues included both urgent and elective surgeries in the study. Dr. Diaz said this was a potential limitation because emergency operations “should never be delayed, by definition.” Lack of indications for the surgeries and information on cause of death were additional limitations.
Future research should evaluate any benefit in delaying surgery longer than 7 or more weeks, Dr. Diaz added, perhaps looking specifically at 10, 12, or 14 weeks, or considering outcomes as a continuous variable. This would help health care providers “garner more insight into risk and benefits of delaying surgery beyond 7 weeks.”
Dr. Nepogodiev and Dr. Diaz disclosed no relevant financial relationships. The study had multiple funding sources, including the National Institute for Health Research Global Health Research Unit, the Association of Upper Gastrointestinal Surgeons, the British Association of Surgical Oncology, and Medtronic.
A version of this article first appeared on Medscape.com.