User login
MIS-C follow-up proves challenging across pediatric hospitals
The discovery of any novel disease or condition means a steep learning curve as physicians must develop protocols for diagnosis, management, and follow-up on the fly in the midst of admitting and treating patients. Medical society task forces and committees often release interim guidance during the learning process, but each institution ultimately has to determine what works for them based on their resources, clinical experience, and patient population.
But when the novel condition demands the involvement of multiple different specialties, the challenge of management grows even more complex – as does follow-up after patients are discharged. Such has been the story with multisystem inflammatory syndrome in children (MIS-C), a complication of COVID-19 that shares some features with Kawasaki disease.
The similarities to Kawasaki provided physicians a place to start in developing appropriate treatment regimens and involved a similar interdisciplinary team from, at the least, cardiology and rheumatology, plus infectious disease since MIS-C results from COVID-19.
“It literally has it in the name – multisystem essentially hints that there are multiple specialties involved, multiple hands in the pot trying to manage the kids, and so each specialty has their own kind of unique role in the patient’s care even on the outpatient side,” said Samina S. Bhumbra, MD, an infectious disease pediatrician at Riley Hospital for Children and assistant professor of clinical pediatrics at Indiana University in Indianapolis. “This isn’t a disease that falls under one specialty.”
By July, the American College of Rheumatology had issued interim clinical guidance for management that most children’s hospitals have followed or slightly adapted. But ACR guidelines could not address how each institution should handle outpatient follow-up visits, especially since those visits required, again, at least cardiology and rheumatology if not infectious disease or other specialties as well.
“When their kids are admitted to the hospital, to be told at discharge you have to be followed up by all these specialists is a lot to handle,” Dr. Bhumbra said. But just as it’s difficult for parents to deal with the need to see several different doctors after discharge, it can be difficult at some institutions for physicians to design a follow-up schedule that can accommodate families, especially families who live far from the hospital in the first place.
“Some of our follow-up is disjointed because all of our clinics had never been on the same day just because of staff availability,” Dr. Bhumbra said. “But it can be a 2- to 3-hour drive for some of our patients, depending on how far they’re coming.”
Many of them can’t make that drive more than once in the same month, much less the same week.
“If you have multiple visits, it makes it more likely that they’re not showing up,” said Ryan M. Serrano, MD, a pediatric cardiologist at Riley and assistant professor of pediatrics at Indiana University. Riley used telehealth when possible, especially if families could get labs done near home. But pediatric echocardiograms require technicians who have experience with children, so families need to come to the hospital.
Children’s hospitals have therefore had to adapt scheduling strategies or develop pediatric specialty clinics to coordinate across the multiple departments and accommodate a complex follow-up regimen that is still evolving as physicians learn more about MIS-C.
Determining a follow-up regimen
Even before determining how to coordinate appointments, hospitals had to decide what follow-up itself should be.
“How long do we follow these patients and how often do we follow them?” said Melissa S. Oliver, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University.
“We’re seeing that a lot of our patients rapidly respond when they get appropriate therapy, but we don’t know about long-term outcomes yet. We’re all still learning.”
At Children’s Hospital of Philadelphia, infectious disease follows up 4-6 weeks post discharge. The cardiology division came up with a follow-up plan that has evolved over time, said Matthew Elias, MD, an attending cardiologist at CHOP’s Cardiac Center and clinical assistant professor of pediatrics at the University of Pennsylvania, Philadelphia.
Patients get an EKG and echocardiogram at 2 weeks and, if their condition is stable, 6 weeks after discharge. After that, it depends on the patient’s clinical situation. Patients with moderately diminished left ventricular systolic function are recommended to get an MRI scan 3 months after discharge and, if old enough, exercise stress tests. Otherwise, they are seen at 6 months, but that appointment is optional for those whose prior echos have consistently been normal.
Other institutions, including Riley, are following a similar schedule of 2-week, 6-week, and 6-month postdischarge follow-ups, and most plan to do a 1-year follow-up as well, although that 1-year mark hasn’t arrived yet for most. Most do rheumatology labs at the 2-week appointment and use that to determine steroids management and whether labs are needed at the 6-week appointment. If labs have normalized, they aren’t done at 6 months. Small variations in follow-up management exist across institutions, but all are remaining open to changes. Riley, for example, is considering MRI screening for ongoing cardiac inflammation at 6 months to a year for all patients, Dr. Serrano said.
The dedicated clinic model
The two challenges Riley needed to address were the lack of a clear consensus on what MIS-C follow-up should look like and the need for continuity of care, Dr. Serrano said.
Regular discussion in departmental meetings at Riley “progressed from how do we take care of them and what treatments do we give them to how do we follow them and manage them in outpatient,” Dr. Oliver said. In the inpatient setting, they had an interdisciplinary team, but how could they maintain that for outpatients without overwhelming the families?
“I think the main challenge is for the families to identify who is leading the care for them,” said Martha M. Rodriguez, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University. That sometimes led to families picking which follow-up appointments they would attend and which they would skip if they could not make them all – and sometimes they skipped the more important ones. “They would go to the appointment with me and then miss the cardiology appointments and the echocardiogram, which was more important to follow any abnormalities in the heart,” Dr. Rodriguez said.
After trying to coordinate separate follow-up appointments for months, Riley ultimately decided to form a dedicated clinic for MIS-C follow-up – a “one-stop shop” single appointment at each follow-up, Dr. Bhumbra said, that covers labs, EKG, echocardiogram, and any other necessary tests.
“Our goal with the clinic is to make life easier for the families and to be able to coordinate the appointments,” Dr. Rodriguez said. “They will be able to see the three of us, and it would be easier for us to communicate with each other about their plan.”
The clinic began Feb. 11 and occurs twice a month. Though it’s just begun, Dr. Oliver said the first clinic went well, and it’s helping them figure out the role each specialty needs to play in follow-up care.
“For us with rheumatology, after lab values have returned to normal and they’re off steroids, sometimes we think there isn’t much more we can contribute to,” she said. And then there are the patients who didn’t see any rheumatologists while inpatients.
“That’s what we’re trying to figure out as well,” Dr. Oliver said. “Should we be seeing every single kid regardless of whether we were involved in their inpatient [stay] or only seeing the ones we’ve seen?” She expects the coming months will help them work that out.
Texas Children’s Hospital in Houston also uses a dedicated clinic, but they set it up before the first MIS-C patient came through the doors, said Sara Kristen Sexson Tejtel, MD, a pediatric cardiologist at Texas Children’s. The hospital already has other types of multidisciplinary clinics, and they anticipated the challenge of getting families to come to too many appointments in a short period of time.
“Getting someone to come back once is hard enough,” Dr. Sexson Tejtel said. “Getting them to come back twice is impossible.”
Infectious disease is less involved at Texas Children’s, so it’s primarily Dr. Sexson Tejtel and her rheumatologist colleague who see the patients. They hold the clinic once a week, twice if needed.
“It does make the appointment a little longer, but I think the patients appreciate that everything can be addressed with that one visit,” Dr. Sexson Tejtel said. “Being in the hospital as long as some of these kids are is so hard, so making any of that easy as possible is so helpful.” A single appointment also allows the doctors to work together on what labs are needed so that children don’t need multiple labs drawn.
At the appointment, she and the rheumatologist enter the patient’s room and take the patient’s history together.
“It’s nice because it makes the family not to have to repeat things and tell the same story over and over,” she said. “Sometimes I ask questions that then the rheumatologist jumps off of, and then sometimes he’ll ask questions, and I’ll think, ‘Ooh, I’ll ask more questions about that.’ ”
In fact, this team approach at all clinics has made her a more thoughtful, well-rounded physician, she said.
“I have learned so much going to all of my multidisciplinary clinics, and I think I’m able to better care for my patients because I’m not just thinking about it from a cardiac perspective,” she said. “It takes some work, but it’s not hard and I think it is beneficial both for the patient and for the physician. This team approach is definitely where we’re trying to live right now.”
Separate but coordinated appointments
A dedicated clinic isn’t the answer for all institutions, however. At Children’s Hospital of Philadelphia, the size of the networks and all its satellites made a one-stop shop impractical.
“We talked about a consolidated clinic early on, when MIS-C was first emerging and all our groups were collaborating and coming up with our inpatient and outpatient care pathways,” said Sanjeev K. Swami, MD, an infectious disease pediatrician at CHOP and associate professor of clinical pediatrics at the University of Pennsylvania. But timing varies on when each specialist wants to see the families return, and existing clinic schedules and locations varied too much.
So CHOP coordinates appointments individually for each patient, depending on where the patient lives and sometimes stacking them on the same day when possible. Sometimes infectious disease or rheumatology use telehealth, and CHOP, like the other hospitals, prioritizes cardiology, especially for the patients who had cardiac abnormalities in the hospital, Dr. Swami said.
“All three of our groups try to be as flexible as possible. We’ve had a really good collaboration between our groups,” he said, and spreading out follow-up allows specialists to ask about concerns raised at previous appointments, ensuring stronger continuity of care.
“We can make sure things are getting followed up on,” Dr. Swami said. “I think that has been beneficial to make sure things aren’t falling through the cracks.”
CHOP cardiologist Dr. Elias said that ongoing communication, among providers and with families, has been absolutely crucial.
“Everyone’s been talking so frequently about our MIS-C patients while inpatient that by the time they’re an outpatient, it seems to work smoothly, where families are hearing similar items but with a different flair, one from infectious, one from rheumatology, and one from cardiology,” he said.
Children’s Mercy in Kansas City, Mo., also has multiple satellite clinics and follows a model similar to that of CHOP. They discussed having a dedicated multidisciplinary team for each MIS-C patient, but even the logistics of that were difficult, said Emily J. Fox, MD, a rheumatologist and assistant professor of pediatrics at the University of Missouri-Kansas City.
Instead, Children’s Mercy tries to coordinate follow-up appointments to be on the same day and often use telehealth for the rheumatology appointments. Families that live closer to the hospital’s location in Joplin, Mo., go in for their cardiology appointment there, and then Dr. Fox conducts a telehealth appointment with the help of nurses in Joplin.
“We really do try hard, especially since these kids are in the hospital for a long time, to make the coordination as easy as possible,” Dr. Fox said. “This was all was very new, especially in the beginning, but I think at least our group is getting a little bit more comfortable in managing these patients.”
Looking ahead
The biggest question that still looms is what happens to these children, if anything, down the line.
“What was unique about this was this was a new disease we were all learning about together with no baseline,” Dr. Swami said. “None of us had ever seen this condition before.”
So far, the prognosis for the vast majority of children is good. “Most of these kids survive, most of them are doing well, and they almost all recover,” Dr. Serrano said. Labs tend to normalize by 6 weeks post discharge, if not much earlier, and not much cardiac involvement is showing up at later follow-ups. But not even a year has passed, so there’s plenty to learn. “We don’t know if there’s long-term risk. I would not be surprised if 20 years down the road we’re finding out things about this that we had no idea” about, Dr. Serrano said. “Everybody wants answers, and nobody has any, and the answers we have may end up being wrong. That’s how it goes when you’re dealing with something you’ve never seen.”
Research underway will ideally begin providing those answers soon. CHOP is a participating site in an NIH-NHLBI–sponsored study, called COVID MUSIC, that is tracking long-term outcomes for MIS-C at 30 centers across the United States and Canada for 5 years.
“That will really definitely be helpful in answering some of the questions about long-term outcomes,” Dr. Elias said. “We hope this is going to be a transient issue and that patients won’t have any long-term manifestations, but we don’t know that yet.”
Meanwhile, one benefit that has come out of the pandemic is strong collaboration, Dr. Bhumbra said.
“The biggest thing we’re all eagerly waiting and hoping for is standard guidelines on how best to follow-up on these kids, but I know that’s a ways away,” Dr. Bhumbra said. So for now, each institution is doing what it can to develop protocols that they feel best serve the patients’ needs, such as Riley’s new dedicated MIS-C clinic. “It takes a village to take care of these kids, and MIS-C has proven that having a clinic with all three specialties at one clinic is going to be great for the families.”
Dr. Fox serves on a committee for Pfizer unrelated to MIS-C. No other doctors interviewed for this story had relevant conflicts of interest to disclose.
The discovery of any novel disease or condition means a steep learning curve as physicians must develop protocols for diagnosis, management, and follow-up on the fly in the midst of admitting and treating patients. Medical society task forces and committees often release interim guidance during the learning process, but each institution ultimately has to determine what works for them based on their resources, clinical experience, and patient population.
But when the novel condition demands the involvement of multiple different specialties, the challenge of management grows even more complex – as does follow-up after patients are discharged. Such has been the story with multisystem inflammatory syndrome in children (MIS-C), a complication of COVID-19 that shares some features with Kawasaki disease.
The similarities to Kawasaki provided physicians a place to start in developing appropriate treatment regimens and involved a similar interdisciplinary team from, at the least, cardiology and rheumatology, plus infectious disease since MIS-C results from COVID-19.
“It literally has it in the name – multisystem essentially hints that there are multiple specialties involved, multiple hands in the pot trying to manage the kids, and so each specialty has their own kind of unique role in the patient’s care even on the outpatient side,” said Samina S. Bhumbra, MD, an infectious disease pediatrician at Riley Hospital for Children and assistant professor of clinical pediatrics at Indiana University in Indianapolis. “This isn’t a disease that falls under one specialty.”
By July, the American College of Rheumatology had issued interim clinical guidance for management that most children’s hospitals have followed or slightly adapted. But ACR guidelines could not address how each institution should handle outpatient follow-up visits, especially since those visits required, again, at least cardiology and rheumatology if not infectious disease or other specialties as well.
“When their kids are admitted to the hospital, to be told at discharge you have to be followed up by all these specialists is a lot to handle,” Dr. Bhumbra said. But just as it’s difficult for parents to deal with the need to see several different doctors after discharge, it can be difficult at some institutions for physicians to design a follow-up schedule that can accommodate families, especially families who live far from the hospital in the first place.
“Some of our follow-up is disjointed because all of our clinics had never been on the same day just because of staff availability,” Dr. Bhumbra said. “But it can be a 2- to 3-hour drive for some of our patients, depending on how far they’re coming.”
Many of them can’t make that drive more than once in the same month, much less the same week.
“If you have multiple visits, it makes it more likely that they’re not showing up,” said Ryan M. Serrano, MD, a pediatric cardiologist at Riley and assistant professor of pediatrics at Indiana University. Riley used telehealth when possible, especially if families could get labs done near home. But pediatric echocardiograms require technicians who have experience with children, so families need to come to the hospital.
Children’s hospitals have therefore had to adapt scheduling strategies or develop pediatric specialty clinics to coordinate across the multiple departments and accommodate a complex follow-up regimen that is still evolving as physicians learn more about MIS-C.
Determining a follow-up regimen
Even before determining how to coordinate appointments, hospitals had to decide what follow-up itself should be.
“How long do we follow these patients and how often do we follow them?” said Melissa S. Oliver, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University.
“We’re seeing that a lot of our patients rapidly respond when they get appropriate therapy, but we don’t know about long-term outcomes yet. We’re all still learning.”
At Children’s Hospital of Philadelphia, infectious disease follows up 4-6 weeks post discharge. The cardiology division came up with a follow-up plan that has evolved over time, said Matthew Elias, MD, an attending cardiologist at CHOP’s Cardiac Center and clinical assistant professor of pediatrics at the University of Pennsylvania, Philadelphia.
Patients get an EKG and echocardiogram at 2 weeks and, if their condition is stable, 6 weeks after discharge. After that, it depends on the patient’s clinical situation. Patients with moderately diminished left ventricular systolic function are recommended to get an MRI scan 3 months after discharge and, if old enough, exercise stress tests. Otherwise, they are seen at 6 months, but that appointment is optional for those whose prior echos have consistently been normal.
Other institutions, including Riley, are following a similar schedule of 2-week, 6-week, and 6-month postdischarge follow-ups, and most plan to do a 1-year follow-up as well, although that 1-year mark hasn’t arrived yet for most. Most do rheumatology labs at the 2-week appointment and use that to determine steroids management and whether labs are needed at the 6-week appointment. If labs have normalized, they aren’t done at 6 months. Small variations in follow-up management exist across institutions, but all are remaining open to changes. Riley, for example, is considering MRI screening for ongoing cardiac inflammation at 6 months to a year for all patients, Dr. Serrano said.
The dedicated clinic model
The two challenges Riley needed to address were the lack of a clear consensus on what MIS-C follow-up should look like and the need for continuity of care, Dr. Serrano said.
Regular discussion in departmental meetings at Riley “progressed from how do we take care of them and what treatments do we give them to how do we follow them and manage them in outpatient,” Dr. Oliver said. In the inpatient setting, they had an interdisciplinary team, but how could they maintain that for outpatients without overwhelming the families?
“I think the main challenge is for the families to identify who is leading the care for them,” said Martha M. Rodriguez, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University. That sometimes led to families picking which follow-up appointments they would attend and which they would skip if they could not make them all – and sometimes they skipped the more important ones. “They would go to the appointment with me and then miss the cardiology appointments and the echocardiogram, which was more important to follow any abnormalities in the heart,” Dr. Rodriguez said.
After trying to coordinate separate follow-up appointments for months, Riley ultimately decided to form a dedicated clinic for MIS-C follow-up – a “one-stop shop” single appointment at each follow-up, Dr. Bhumbra said, that covers labs, EKG, echocardiogram, and any other necessary tests.
“Our goal with the clinic is to make life easier for the families and to be able to coordinate the appointments,” Dr. Rodriguez said. “They will be able to see the three of us, and it would be easier for us to communicate with each other about their plan.”
The clinic began Feb. 11 and occurs twice a month. Though it’s just begun, Dr. Oliver said the first clinic went well, and it’s helping them figure out the role each specialty needs to play in follow-up care.
“For us with rheumatology, after lab values have returned to normal and they’re off steroids, sometimes we think there isn’t much more we can contribute to,” she said. And then there are the patients who didn’t see any rheumatologists while inpatients.
“That’s what we’re trying to figure out as well,” Dr. Oliver said. “Should we be seeing every single kid regardless of whether we were involved in their inpatient [stay] or only seeing the ones we’ve seen?” She expects the coming months will help them work that out.
Texas Children’s Hospital in Houston also uses a dedicated clinic, but they set it up before the first MIS-C patient came through the doors, said Sara Kristen Sexson Tejtel, MD, a pediatric cardiologist at Texas Children’s. The hospital already has other types of multidisciplinary clinics, and they anticipated the challenge of getting families to come to too many appointments in a short period of time.
“Getting someone to come back once is hard enough,” Dr. Sexson Tejtel said. “Getting them to come back twice is impossible.”
Infectious disease is less involved at Texas Children’s, so it’s primarily Dr. Sexson Tejtel and her rheumatologist colleague who see the patients. They hold the clinic once a week, twice if needed.
“It does make the appointment a little longer, but I think the patients appreciate that everything can be addressed with that one visit,” Dr. Sexson Tejtel said. “Being in the hospital as long as some of these kids are is so hard, so making any of that easy as possible is so helpful.” A single appointment also allows the doctors to work together on what labs are needed so that children don’t need multiple labs drawn.
At the appointment, she and the rheumatologist enter the patient’s room and take the patient’s history together.
“It’s nice because it makes the family not to have to repeat things and tell the same story over and over,” she said. “Sometimes I ask questions that then the rheumatologist jumps off of, and then sometimes he’ll ask questions, and I’ll think, ‘Ooh, I’ll ask more questions about that.’ ”
In fact, this team approach at all clinics has made her a more thoughtful, well-rounded physician, she said.
“I have learned so much going to all of my multidisciplinary clinics, and I think I’m able to better care for my patients because I’m not just thinking about it from a cardiac perspective,” she said. “It takes some work, but it’s not hard and I think it is beneficial both for the patient and for the physician. This team approach is definitely where we’re trying to live right now.”
Separate but coordinated appointments
A dedicated clinic isn’t the answer for all institutions, however. At Children’s Hospital of Philadelphia, the size of the networks and all its satellites made a one-stop shop impractical.
“We talked about a consolidated clinic early on, when MIS-C was first emerging and all our groups were collaborating and coming up with our inpatient and outpatient care pathways,” said Sanjeev K. Swami, MD, an infectious disease pediatrician at CHOP and associate professor of clinical pediatrics at the University of Pennsylvania. But timing varies on when each specialist wants to see the families return, and existing clinic schedules and locations varied too much.
So CHOP coordinates appointments individually for each patient, depending on where the patient lives and sometimes stacking them on the same day when possible. Sometimes infectious disease or rheumatology use telehealth, and CHOP, like the other hospitals, prioritizes cardiology, especially for the patients who had cardiac abnormalities in the hospital, Dr. Swami said.
“All three of our groups try to be as flexible as possible. We’ve had a really good collaboration between our groups,” he said, and spreading out follow-up allows specialists to ask about concerns raised at previous appointments, ensuring stronger continuity of care.
“We can make sure things are getting followed up on,” Dr. Swami said. “I think that has been beneficial to make sure things aren’t falling through the cracks.”
CHOP cardiologist Dr. Elias said that ongoing communication, among providers and with families, has been absolutely crucial.
“Everyone’s been talking so frequently about our MIS-C patients while inpatient that by the time they’re an outpatient, it seems to work smoothly, where families are hearing similar items but with a different flair, one from infectious, one from rheumatology, and one from cardiology,” he said.
Children’s Mercy in Kansas City, Mo., also has multiple satellite clinics and follows a model similar to that of CHOP. They discussed having a dedicated multidisciplinary team for each MIS-C patient, but even the logistics of that were difficult, said Emily J. Fox, MD, a rheumatologist and assistant professor of pediatrics at the University of Missouri-Kansas City.
Instead, Children’s Mercy tries to coordinate follow-up appointments to be on the same day and often use telehealth for the rheumatology appointments. Families that live closer to the hospital’s location in Joplin, Mo., go in for their cardiology appointment there, and then Dr. Fox conducts a telehealth appointment with the help of nurses in Joplin.
“We really do try hard, especially since these kids are in the hospital for a long time, to make the coordination as easy as possible,” Dr. Fox said. “This was all was very new, especially in the beginning, but I think at least our group is getting a little bit more comfortable in managing these patients.”
Looking ahead
The biggest question that still looms is what happens to these children, if anything, down the line.
“What was unique about this was this was a new disease we were all learning about together with no baseline,” Dr. Swami said. “None of us had ever seen this condition before.”
So far, the prognosis for the vast majority of children is good. “Most of these kids survive, most of them are doing well, and they almost all recover,” Dr. Serrano said. Labs tend to normalize by 6 weeks post discharge, if not much earlier, and not much cardiac involvement is showing up at later follow-ups. But not even a year has passed, so there’s plenty to learn. “We don’t know if there’s long-term risk. I would not be surprised if 20 years down the road we’re finding out things about this that we had no idea” about, Dr. Serrano said. “Everybody wants answers, and nobody has any, and the answers we have may end up being wrong. That’s how it goes when you’re dealing with something you’ve never seen.”
Research underway will ideally begin providing those answers soon. CHOP is a participating site in an NIH-NHLBI–sponsored study, called COVID MUSIC, that is tracking long-term outcomes for MIS-C at 30 centers across the United States and Canada for 5 years.
“That will really definitely be helpful in answering some of the questions about long-term outcomes,” Dr. Elias said. “We hope this is going to be a transient issue and that patients won’t have any long-term manifestations, but we don’t know that yet.”
Meanwhile, one benefit that has come out of the pandemic is strong collaboration, Dr. Bhumbra said.
“The biggest thing we’re all eagerly waiting and hoping for is standard guidelines on how best to follow-up on these kids, but I know that’s a ways away,” Dr. Bhumbra said. So for now, each institution is doing what it can to develop protocols that they feel best serve the patients’ needs, such as Riley’s new dedicated MIS-C clinic. “It takes a village to take care of these kids, and MIS-C has proven that having a clinic with all three specialties at one clinic is going to be great for the families.”
Dr. Fox serves on a committee for Pfizer unrelated to MIS-C. No other doctors interviewed for this story had relevant conflicts of interest to disclose.
The discovery of any novel disease or condition means a steep learning curve as physicians must develop protocols for diagnosis, management, and follow-up on the fly in the midst of admitting and treating patients. Medical society task forces and committees often release interim guidance during the learning process, but each institution ultimately has to determine what works for them based on their resources, clinical experience, and patient population.
But when the novel condition demands the involvement of multiple different specialties, the challenge of management grows even more complex – as does follow-up after patients are discharged. Such has been the story with multisystem inflammatory syndrome in children (MIS-C), a complication of COVID-19 that shares some features with Kawasaki disease.
The similarities to Kawasaki provided physicians a place to start in developing appropriate treatment regimens and involved a similar interdisciplinary team from, at the least, cardiology and rheumatology, plus infectious disease since MIS-C results from COVID-19.
“It literally has it in the name – multisystem essentially hints that there are multiple specialties involved, multiple hands in the pot trying to manage the kids, and so each specialty has their own kind of unique role in the patient’s care even on the outpatient side,” said Samina S. Bhumbra, MD, an infectious disease pediatrician at Riley Hospital for Children and assistant professor of clinical pediatrics at Indiana University in Indianapolis. “This isn’t a disease that falls under one specialty.”
By July, the American College of Rheumatology had issued interim clinical guidance for management that most children’s hospitals have followed or slightly adapted. But ACR guidelines could not address how each institution should handle outpatient follow-up visits, especially since those visits required, again, at least cardiology and rheumatology if not infectious disease or other specialties as well.
“When their kids are admitted to the hospital, to be told at discharge you have to be followed up by all these specialists is a lot to handle,” Dr. Bhumbra said. But just as it’s difficult for parents to deal with the need to see several different doctors after discharge, it can be difficult at some institutions for physicians to design a follow-up schedule that can accommodate families, especially families who live far from the hospital in the first place.
“Some of our follow-up is disjointed because all of our clinics had never been on the same day just because of staff availability,” Dr. Bhumbra said. “But it can be a 2- to 3-hour drive for some of our patients, depending on how far they’re coming.”
Many of them can’t make that drive more than once in the same month, much less the same week.
“If you have multiple visits, it makes it more likely that they’re not showing up,” said Ryan M. Serrano, MD, a pediatric cardiologist at Riley and assistant professor of pediatrics at Indiana University. Riley used telehealth when possible, especially if families could get labs done near home. But pediatric echocardiograms require technicians who have experience with children, so families need to come to the hospital.
Children’s hospitals have therefore had to adapt scheduling strategies or develop pediatric specialty clinics to coordinate across the multiple departments and accommodate a complex follow-up regimen that is still evolving as physicians learn more about MIS-C.
Determining a follow-up regimen
Even before determining how to coordinate appointments, hospitals had to decide what follow-up itself should be.
“How long do we follow these patients and how often do we follow them?” said Melissa S. Oliver, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University.
“We’re seeing that a lot of our patients rapidly respond when they get appropriate therapy, but we don’t know about long-term outcomes yet. We’re all still learning.”
At Children’s Hospital of Philadelphia, infectious disease follows up 4-6 weeks post discharge. The cardiology division came up with a follow-up plan that has evolved over time, said Matthew Elias, MD, an attending cardiologist at CHOP’s Cardiac Center and clinical assistant professor of pediatrics at the University of Pennsylvania, Philadelphia.
Patients get an EKG and echocardiogram at 2 weeks and, if their condition is stable, 6 weeks after discharge. After that, it depends on the patient’s clinical situation. Patients with moderately diminished left ventricular systolic function are recommended to get an MRI scan 3 months after discharge and, if old enough, exercise stress tests. Otherwise, they are seen at 6 months, but that appointment is optional for those whose prior echos have consistently been normal.
Other institutions, including Riley, are following a similar schedule of 2-week, 6-week, and 6-month postdischarge follow-ups, and most plan to do a 1-year follow-up as well, although that 1-year mark hasn’t arrived yet for most. Most do rheumatology labs at the 2-week appointment and use that to determine steroids management and whether labs are needed at the 6-week appointment. If labs have normalized, they aren’t done at 6 months. Small variations in follow-up management exist across institutions, but all are remaining open to changes. Riley, for example, is considering MRI screening for ongoing cardiac inflammation at 6 months to a year for all patients, Dr. Serrano said.
The dedicated clinic model
The two challenges Riley needed to address were the lack of a clear consensus on what MIS-C follow-up should look like and the need for continuity of care, Dr. Serrano said.
Regular discussion in departmental meetings at Riley “progressed from how do we take care of them and what treatments do we give them to how do we follow them and manage them in outpatient,” Dr. Oliver said. In the inpatient setting, they had an interdisciplinary team, but how could they maintain that for outpatients without overwhelming the families?
“I think the main challenge is for the families to identify who is leading the care for them,” said Martha M. Rodriguez, MD, a rheumatologist at Riley and assistant professor of clinical pediatrics at Indiana University. That sometimes led to families picking which follow-up appointments they would attend and which they would skip if they could not make them all – and sometimes they skipped the more important ones. “They would go to the appointment with me and then miss the cardiology appointments and the echocardiogram, which was more important to follow any abnormalities in the heart,” Dr. Rodriguez said.
After trying to coordinate separate follow-up appointments for months, Riley ultimately decided to form a dedicated clinic for MIS-C follow-up – a “one-stop shop” single appointment at each follow-up, Dr. Bhumbra said, that covers labs, EKG, echocardiogram, and any other necessary tests.
“Our goal with the clinic is to make life easier for the families and to be able to coordinate the appointments,” Dr. Rodriguez said. “They will be able to see the three of us, and it would be easier for us to communicate with each other about their plan.”
The clinic began Feb. 11 and occurs twice a month. Though it’s just begun, Dr. Oliver said the first clinic went well, and it’s helping them figure out the role each specialty needs to play in follow-up care.
“For us with rheumatology, after lab values have returned to normal and they’re off steroids, sometimes we think there isn’t much more we can contribute to,” she said. And then there are the patients who didn’t see any rheumatologists while inpatients.
“That’s what we’re trying to figure out as well,” Dr. Oliver said. “Should we be seeing every single kid regardless of whether we were involved in their inpatient [stay] or only seeing the ones we’ve seen?” She expects the coming months will help them work that out.
Texas Children’s Hospital in Houston also uses a dedicated clinic, but they set it up before the first MIS-C patient came through the doors, said Sara Kristen Sexson Tejtel, MD, a pediatric cardiologist at Texas Children’s. The hospital already has other types of multidisciplinary clinics, and they anticipated the challenge of getting families to come to too many appointments in a short period of time.
“Getting someone to come back once is hard enough,” Dr. Sexson Tejtel said. “Getting them to come back twice is impossible.”
Infectious disease is less involved at Texas Children’s, so it’s primarily Dr. Sexson Tejtel and her rheumatologist colleague who see the patients. They hold the clinic once a week, twice if needed.
“It does make the appointment a little longer, but I think the patients appreciate that everything can be addressed with that one visit,” Dr. Sexson Tejtel said. “Being in the hospital as long as some of these kids are is so hard, so making any of that easy as possible is so helpful.” A single appointment also allows the doctors to work together on what labs are needed so that children don’t need multiple labs drawn.
At the appointment, she and the rheumatologist enter the patient’s room and take the patient’s history together.
“It’s nice because it makes the family not to have to repeat things and tell the same story over and over,” she said. “Sometimes I ask questions that then the rheumatologist jumps off of, and then sometimes he’ll ask questions, and I’ll think, ‘Ooh, I’ll ask more questions about that.’ ”
In fact, this team approach at all clinics has made her a more thoughtful, well-rounded physician, she said.
“I have learned so much going to all of my multidisciplinary clinics, and I think I’m able to better care for my patients because I’m not just thinking about it from a cardiac perspective,” she said. “It takes some work, but it’s not hard and I think it is beneficial both for the patient and for the physician. This team approach is definitely where we’re trying to live right now.”
Separate but coordinated appointments
A dedicated clinic isn’t the answer for all institutions, however. At Children’s Hospital of Philadelphia, the size of the networks and all its satellites made a one-stop shop impractical.
“We talked about a consolidated clinic early on, when MIS-C was first emerging and all our groups were collaborating and coming up with our inpatient and outpatient care pathways,” said Sanjeev K. Swami, MD, an infectious disease pediatrician at CHOP and associate professor of clinical pediatrics at the University of Pennsylvania. But timing varies on when each specialist wants to see the families return, and existing clinic schedules and locations varied too much.
So CHOP coordinates appointments individually for each patient, depending on where the patient lives and sometimes stacking them on the same day when possible. Sometimes infectious disease or rheumatology use telehealth, and CHOP, like the other hospitals, prioritizes cardiology, especially for the patients who had cardiac abnormalities in the hospital, Dr. Swami said.
“All three of our groups try to be as flexible as possible. We’ve had a really good collaboration between our groups,” he said, and spreading out follow-up allows specialists to ask about concerns raised at previous appointments, ensuring stronger continuity of care.
“We can make sure things are getting followed up on,” Dr. Swami said. “I think that has been beneficial to make sure things aren’t falling through the cracks.”
CHOP cardiologist Dr. Elias said that ongoing communication, among providers and with families, has been absolutely crucial.
“Everyone’s been talking so frequently about our MIS-C patients while inpatient that by the time they’re an outpatient, it seems to work smoothly, where families are hearing similar items but with a different flair, one from infectious, one from rheumatology, and one from cardiology,” he said.
Children’s Mercy in Kansas City, Mo., also has multiple satellite clinics and follows a model similar to that of CHOP. They discussed having a dedicated multidisciplinary team for each MIS-C patient, but even the logistics of that were difficult, said Emily J. Fox, MD, a rheumatologist and assistant professor of pediatrics at the University of Missouri-Kansas City.
Instead, Children’s Mercy tries to coordinate follow-up appointments to be on the same day and often use telehealth for the rheumatology appointments. Families that live closer to the hospital’s location in Joplin, Mo., go in for their cardiology appointment there, and then Dr. Fox conducts a telehealth appointment with the help of nurses in Joplin.
“We really do try hard, especially since these kids are in the hospital for a long time, to make the coordination as easy as possible,” Dr. Fox said. “This was all was very new, especially in the beginning, but I think at least our group is getting a little bit more comfortable in managing these patients.”
Looking ahead
The biggest question that still looms is what happens to these children, if anything, down the line.
“What was unique about this was this was a new disease we were all learning about together with no baseline,” Dr. Swami said. “None of us had ever seen this condition before.”
So far, the prognosis for the vast majority of children is good. “Most of these kids survive, most of them are doing well, and they almost all recover,” Dr. Serrano said. Labs tend to normalize by 6 weeks post discharge, if not much earlier, and not much cardiac involvement is showing up at later follow-ups. But not even a year has passed, so there’s plenty to learn. “We don’t know if there’s long-term risk. I would not be surprised if 20 years down the road we’re finding out things about this that we had no idea” about, Dr. Serrano said. “Everybody wants answers, and nobody has any, and the answers we have may end up being wrong. That’s how it goes when you’re dealing with something you’ve never seen.”
Research underway will ideally begin providing those answers soon. CHOP is a participating site in an NIH-NHLBI–sponsored study, called COVID MUSIC, that is tracking long-term outcomes for MIS-C at 30 centers across the United States and Canada for 5 years.
“That will really definitely be helpful in answering some of the questions about long-term outcomes,” Dr. Elias said. “We hope this is going to be a transient issue and that patients won’t have any long-term manifestations, but we don’t know that yet.”
Meanwhile, one benefit that has come out of the pandemic is strong collaboration, Dr. Bhumbra said.
“The biggest thing we’re all eagerly waiting and hoping for is standard guidelines on how best to follow-up on these kids, but I know that’s a ways away,” Dr. Bhumbra said. So for now, each institution is doing what it can to develop protocols that they feel best serve the patients’ needs, such as Riley’s new dedicated MIS-C clinic. “It takes a village to take care of these kids, and MIS-C has proven that having a clinic with all three specialties at one clinic is going to be great for the families.”
Dr. Fox serves on a committee for Pfizer unrelated to MIS-C. No other doctors interviewed for this story had relevant conflicts of interest to disclose.
Heart failure redefined with new classifications, staging
The terminology and classification scheme for heart failure (HF) is changing in ways that experts hope will directly impact patient outcomes.
In a new consensus statement, a multisociety group of experts proposed a new universal definition of heart failure and made substantial revisions to the way in which the disease is staged and classified.
The authors of the statement, led by writing committee chair and immediate past president of the Heart Failure Society of America Biykem Bozkurt, MD, PhD, hope their efforts will go far to improve standardization of terminology, but more importantly will facilitate better management of the disease in ways that keep pace with current knowledge and advances in the field.
“There is a great need for reframing and standardizing the terminology across societies and different stakeholders, and importantly for patients because a lot of the terminology we were using was understood by academicians, but were not being translated in important ways to ensure patients are being appropriately treated,” said Dr. Bozkurt, of Baylor College of Medicine, Houston.
The consensus statement was a group effort led by the HFSA, the Heart Failure Association of the European Society of Cardiology, and the Japanese Heart Failure Society, with endorsements from the Canadian Heart Failure Society, the Heart Failure Association of India, the Cardiac Society of Australia and New Zealand, and the Chinese Heart Failure Association.
The article was published March 1 in the Journal of Cardiac Failure and the European Journal of Heart Failure, authored by a writing committee of 38 individuals with domain expertise in HF, cardiomyopathy, and cardiovascular disease.
“This is a very thorough and very carefully written document that I think will be helpful for clinicians because they’ve tapped into important changes in the field that have occurred over the past 10 years and that now allow us to do more for patients than we could before,” Eugene Braunwald, MD, said in an interview.
Dr. Braunwald and Elliott M. Antman, MD, both from TIMI Study Group at Brigham and Women’s Hospital and Harvard Medical School in Boston, wrote an editorial that accompanied the European Journal of Heart Failure article.
A new universal definition
“[Heart failure] is a clinical syndrome with symptoms and or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion.”
This proposed definition, said the authors, is designed to be contemporary and simple “but conceptually comprehensive, with near universal applicability, prognostic and therapeutic viability, and acceptable sensitivity and specificity.”
Both left and right HF qualifies under this definition, said the authors, but conditions that result in marked volume overload, such as chronic kidney disease, which may present with signs and symptoms of HF, do not.
“Although some of these patients may have concomitant HF, these patients have a primary abnormality that may require a specific treatment beyond that for HF,” said the consensus statement authors.
For his part, Douglas L. Mann, MD, is happy to see what he considers a more accurate and practical definition for heart failure.
“We’ve had some wacky definitions in heart failure that haven’t made sense for 30 years, the principal of which is the definition of heart failure that says it’s the inability of the heart to meet the metabolic demands of the body,” Dr. Mann, of Washington University, St. Louis, said in an interview.
“I think this description was developed thinking about people with end-stage heart failure, but it makes no sense in clinical practice. Does it make sense to say about someone with New York Heart Association class I heart failure that their heart can’t meet the metabolic demands of the body?” said Dr. Mann, who was not involved with the writing of the consensus statement.
Proposed revised stages of the HF continuum
Overall, minimal changes have been made to the HF stages, with tweaks intended to enhance understanding and address the evolving role of biomarkers.
The authors proposed an approach to staging of HF:
- At-risk for HF (stage A), for patients at risk for HF but without current or prior symptoms or signs of HF and without structural or biomarkers evidence of heart disease.
- Pre-HF (stage B), for patients without current or prior symptoms or signs of HF, but evidence of structural heart disease or abnormal cardiac function, or elevated natriuretic peptide levels.
- HF (stage C), for patients with current or prior symptoms and/or signs of HF caused by a structural and/or functional cardiac abnormality.
- Advanced HF (stage D), for patients with severe symptoms and/or signs of HF at rest, recurrent hospitalizations despite guideline-directed management and therapy (GDMT), refractory or intolerant to GDMT, requiring advanced therapies such as consideration for transplant, mechanical circulatory support, or palliative care.
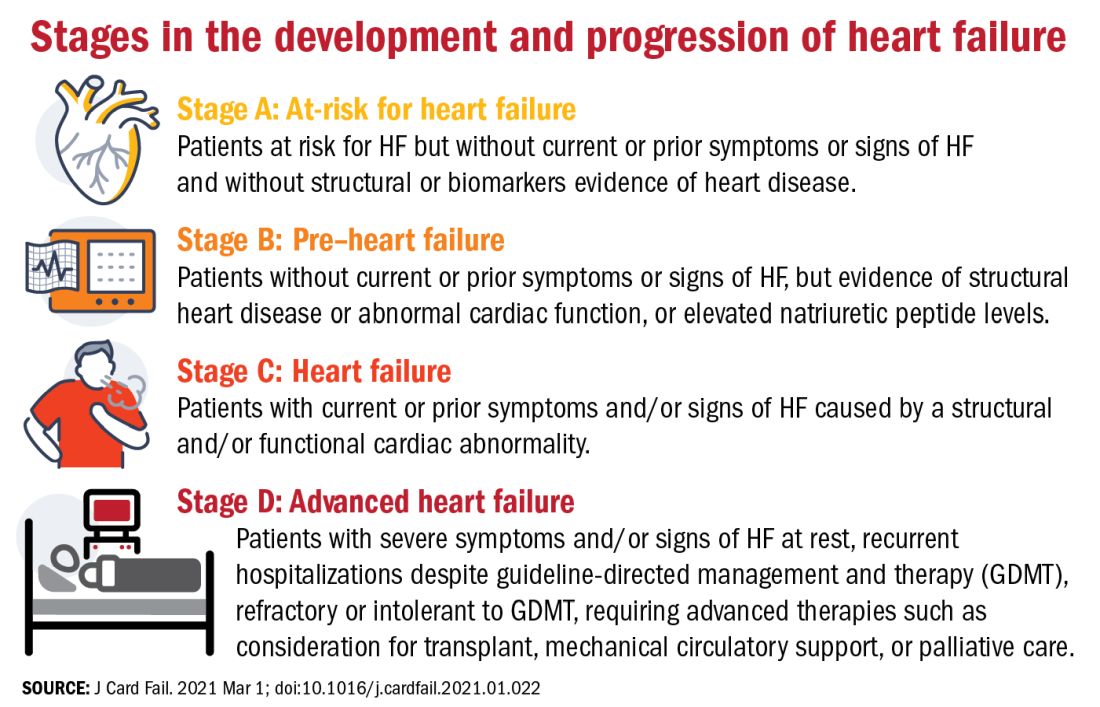
One notable change to the staging scheme is stage B, which the authors have reframed as “pre–heart failure.”
“Pre-cancer is a term widely understood and considered actionable and we wanted to tap into this successful messaging and embrace the pre–heart failure concept as something that is treatable and preventable,” said Dr. Bozkurt.
“We want patients and clinicians to understand that there are things we can do to prevent heart failure, strategies we didn’t have before, like SGLT2 inhibitors in patients with diabetes at risk for HF,” she added.
The revision also avoids the stigma of HF before the symptoms are manifest.
“Not calling it stage A and stage B heart failure you might say is semantics, but it’s important semantics,” said Dr. Braunwald. “When you’re talking to a patient or a relative and tell them they have stage A heart failure, it’s scares them unnecessarily. They don’t hear the stage A or B part, just the heart failure part.”
New classifications according to LVEF
And finally, in what some might consider the most obviously needed modification, the document proposes a new and revised classification of HF according to left ventricular ejection fraction (LVEF). Most agree on how to classify heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF), but although the middle range has long been understood to be a clinically relevant, it has no proper name or clear delineation.
“For standardization across practice guidelines, to recognize clinical trajectories in HF, and to facilitate the recognition of different heart failure entities in a sensitive and specific manner that can guide therapy, we want to formalize the heart failure categories according to ejection fraction,” said Dr. Bozkurt.
To this end, the authors propose the following four classifications of EF:
- HF with reduced EF (HFrEF): LVEF of up to 40%.
- HF with mildly reduced EF (HFmrEF): LVEF of 41-49%.
- HF with preserved EF (HFpEF)HF with an LVEF of at least 50%.
- HF with improved EF (HFimpEF): HF with a baseline LVEF of 40% or less, an increase of at least 10 points from baseline LVEF, and a second measurement of LVEF of greater than 40%.
HFmrEF is usually a transition period, noted Dr. Bozkurt. “Patients with HF in this range may represent a population whose EF is likely to change, either increase or decrease over time and it’s important to be cognizant of that trajectory. Understanding where your patient is headed is crucial for prognosis and optimization of guideline-directed treatment,” she said.
Improved, not recovered, HF
The last classification of heart failure with improved ejection fraction (HFimpEF) represents an important change to the current classification scheme.
“We want to clarify what terms to use but also which not to use. For example, we don’t want people to use recovered heart failure or heart failure in remission, partly because we don’t want the medication to be stopped. We don’t want to give the false message that there has been full recovery,” said Dr. Bozkurt.
As seen in the TRED-HF trial, guideline-directed medical therapy should be continued in patients with HF with improved EF regardless of whether it has improved to a normal range of above 50% in subsequent measurements.
“This is a distinct group of people, and for a while the guidelines were lumping them in with HFpEF, which I think is totally wrong,” said Dr. Mann.
“I think it’s very important that we emphasize heart failure as a continuum, rather than a one-way street of [inevitable] progression. Because we do see improvements in ejection fraction and we do see that we can prevent heart failure if we do the right things, and this should be reflected in the terminology we use,” he added.
Dr. Bozkurt stressed that HFimpEF only applies if the EF improves to above 40%. A move from an EF of 10%-20% would still see the patient classified as having HFrEF, but a patient whose EF improved from, say, 30% to 45% would be classified as HFimpEF.
“The reason for this, again, is because a transition from, say an EF of 10%-20% does not change therapy, but a move upward over 40% might, especially regarding decisions for device therapies, so the trajectory as well as the absolute EF is important,” she added.
“Particularly in the early stages, people are responsive to therapy and it’s possible in some cases to reverse heart failure, so I think this change helps us understand when that’s happened,” said Dr. Braunwald.
One step toward universality
“The implementation of this terminology and nomenclature into practice will require a variety of tactics,” said Dr. Bozkurt. “For example, the current ICD 10 codes need to incorporate the at-risk and pre–heart failure categories, as well as the mid-range EF, preserved, and improved EF classifications, because the treatment differs between those three domains.”
In terms of how these proposed changes will be worked into practice guidelines, Dr. Bozkurt declined to comment on this to avoid any perception of conflict of interest as she is the cochair of the American College of Cardiology/American Heart Association HF guideline writing committee.
Dr. Braunwald and Dr. Antman suggest it may be premature to call the new terminology and classifications “universal.” In an interview, Dr. Braunwald lamented the absence of the World Heart Federation, the ACC, and the AHA as active participants in this effort and suggested this paper is only the first step of a multistep process that requires input from many stakeholders.
“It’s important that these organizations be involved, not just to bless it, but to contribute their expertise to the process,” he said.
For his part, Dr. Mann hopes these changes will gain widespread acceptance and clinical traction. “The problem sometimes with guidelines is that they’re so data driven that you just can’t come out and say the obvious, so making a position statement is a good first step. And they got good international representation on this, so I think these changes will be accepted in the next heart failure guidelines.”
To encourage further discussion and acceptance, Robert J. Mentz, MD, and Anuradha Lala, MD, editor-in-chief and deputy editor of the Journal of Cardiac Failure, respectively, announced a series of multidisciplinary perspective pieces to be published in the journal monthly, starting in May with editorials from Dr. Clyde W Yancy, MD, MSc, and Carolyn S.P. Lam, MBBS, PhD, both of whom were authors of the consensus statement.
Dr. Bozkurt reports being a consultant for Abbott, Amgen, Baxter, Bristol Myers Squibb, Liva Nova Relypsa/Vifor Pharma, Respicardia, and being on the registry steering committee for Sanofi-Aventis. Dr. Braunwald reports research grant support through Brigham and Women’s Hospital from AstraZeneca, Daiichi Sankyo, Merck, and Novartis; and consulting for Amgen, Boehringer-Ingelheim/Lilly, Cardurion, MyoKardia, Novo Nordisk, and Verve. Dr. Mann has been a consultant to Novartis, is on the steering committee for the PARADISE trial, and is on the scientific advisory board for MyoKardia/Bristol Myers Squibb.
The terminology and classification scheme for heart failure (HF) is changing in ways that experts hope will directly impact patient outcomes.
In a new consensus statement, a multisociety group of experts proposed a new universal definition of heart failure and made substantial revisions to the way in which the disease is staged and classified.
The authors of the statement, led by writing committee chair and immediate past president of the Heart Failure Society of America Biykem Bozkurt, MD, PhD, hope their efforts will go far to improve standardization of terminology, but more importantly will facilitate better management of the disease in ways that keep pace with current knowledge and advances in the field.
“There is a great need for reframing and standardizing the terminology across societies and different stakeholders, and importantly for patients because a lot of the terminology we were using was understood by academicians, but were not being translated in important ways to ensure patients are being appropriately treated,” said Dr. Bozkurt, of Baylor College of Medicine, Houston.
The consensus statement was a group effort led by the HFSA, the Heart Failure Association of the European Society of Cardiology, and the Japanese Heart Failure Society, with endorsements from the Canadian Heart Failure Society, the Heart Failure Association of India, the Cardiac Society of Australia and New Zealand, and the Chinese Heart Failure Association.
The article was published March 1 in the Journal of Cardiac Failure and the European Journal of Heart Failure, authored by a writing committee of 38 individuals with domain expertise in HF, cardiomyopathy, and cardiovascular disease.
“This is a very thorough and very carefully written document that I think will be helpful for clinicians because they’ve tapped into important changes in the field that have occurred over the past 10 years and that now allow us to do more for patients than we could before,” Eugene Braunwald, MD, said in an interview.
Dr. Braunwald and Elliott M. Antman, MD, both from TIMI Study Group at Brigham and Women’s Hospital and Harvard Medical School in Boston, wrote an editorial that accompanied the European Journal of Heart Failure article.
A new universal definition
“[Heart failure] is a clinical syndrome with symptoms and or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion.”
This proposed definition, said the authors, is designed to be contemporary and simple “but conceptually comprehensive, with near universal applicability, prognostic and therapeutic viability, and acceptable sensitivity and specificity.”
Both left and right HF qualifies under this definition, said the authors, but conditions that result in marked volume overload, such as chronic kidney disease, which may present with signs and symptoms of HF, do not.
“Although some of these patients may have concomitant HF, these patients have a primary abnormality that may require a specific treatment beyond that for HF,” said the consensus statement authors.
For his part, Douglas L. Mann, MD, is happy to see what he considers a more accurate and practical definition for heart failure.
“We’ve had some wacky definitions in heart failure that haven’t made sense for 30 years, the principal of which is the definition of heart failure that says it’s the inability of the heart to meet the metabolic demands of the body,” Dr. Mann, of Washington University, St. Louis, said in an interview.
“I think this description was developed thinking about people with end-stage heart failure, but it makes no sense in clinical practice. Does it make sense to say about someone with New York Heart Association class I heart failure that their heart can’t meet the metabolic demands of the body?” said Dr. Mann, who was not involved with the writing of the consensus statement.
Proposed revised stages of the HF continuum
Overall, minimal changes have been made to the HF stages, with tweaks intended to enhance understanding and address the evolving role of biomarkers.
The authors proposed an approach to staging of HF:
- At-risk for HF (stage A), for patients at risk for HF but without current or prior symptoms or signs of HF and without structural or biomarkers evidence of heart disease.
- Pre-HF (stage B), for patients without current or prior symptoms or signs of HF, but evidence of structural heart disease or abnormal cardiac function, or elevated natriuretic peptide levels.
- HF (stage C), for patients with current or prior symptoms and/or signs of HF caused by a structural and/or functional cardiac abnormality.
- Advanced HF (stage D), for patients with severe symptoms and/or signs of HF at rest, recurrent hospitalizations despite guideline-directed management and therapy (GDMT), refractory or intolerant to GDMT, requiring advanced therapies such as consideration for transplant, mechanical circulatory support, or palliative care.

One notable change to the staging scheme is stage B, which the authors have reframed as “pre–heart failure.”
“Pre-cancer is a term widely understood and considered actionable and we wanted to tap into this successful messaging and embrace the pre–heart failure concept as something that is treatable and preventable,” said Dr. Bozkurt.
“We want patients and clinicians to understand that there are things we can do to prevent heart failure, strategies we didn’t have before, like SGLT2 inhibitors in patients with diabetes at risk for HF,” she added.
The revision also avoids the stigma of HF before the symptoms are manifest.
“Not calling it stage A and stage B heart failure you might say is semantics, but it’s important semantics,” said Dr. Braunwald. “When you’re talking to a patient or a relative and tell them they have stage A heart failure, it’s scares them unnecessarily. They don’t hear the stage A or B part, just the heart failure part.”
New classifications according to LVEF
And finally, in what some might consider the most obviously needed modification, the document proposes a new and revised classification of HF according to left ventricular ejection fraction (LVEF). Most agree on how to classify heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF), but although the middle range has long been understood to be a clinically relevant, it has no proper name or clear delineation.
“For standardization across practice guidelines, to recognize clinical trajectories in HF, and to facilitate the recognition of different heart failure entities in a sensitive and specific manner that can guide therapy, we want to formalize the heart failure categories according to ejection fraction,” said Dr. Bozkurt.
To this end, the authors propose the following four classifications of EF:
- HF with reduced EF (HFrEF): LVEF of up to 40%.
- HF with mildly reduced EF (HFmrEF): LVEF of 41-49%.
- HF with preserved EF (HFpEF)HF with an LVEF of at least 50%.
- HF with improved EF (HFimpEF): HF with a baseline LVEF of 40% or less, an increase of at least 10 points from baseline LVEF, and a second measurement of LVEF of greater than 40%.
HFmrEF is usually a transition period, noted Dr. Bozkurt. “Patients with HF in this range may represent a population whose EF is likely to change, either increase or decrease over time and it’s important to be cognizant of that trajectory. Understanding where your patient is headed is crucial for prognosis and optimization of guideline-directed treatment,” she said.
Improved, not recovered, HF
The last classification of heart failure with improved ejection fraction (HFimpEF) represents an important change to the current classification scheme.
“We want to clarify what terms to use but also which not to use. For example, we don’t want people to use recovered heart failure or heart failure in remission, partly because we don’t want the medication to be stopped. We don’t want to give the false message that there has been full recovery,” said Dr. Bozkurt.
As seen in the TRED-HF trial, guideline-directed medical therapy should be continued in patients with HF with improved EF regardless of whether it has improved to a normal range of above 50% in subsequent measurements.
“This is a distinct group of people, and for a while the guidelines were lumping them in with HFpEF, which I think is totally wrong,” said Dr. Mann.
“I think it’s very important that we emphasize heart failure as a continuum, rather than a one-way street of [inevitable] progression. Because we do see improvements in ejection fraction and we do see that we can prevent heart failure if we do the right things, and this should be reflected in the terminology we use,” he added.
Dr. Bozkurt stressed that HFimpEF only applies if the EF improves to above 40%. A move from an EF of 10%-20% would still see the patient classified as having HFrEF, but a patient whose EF improved from, say, 30% to 45% would be classified as HFimpEF.
“The reason for this, again, is because a transition from, say an EF of 10%-20% does not change therapy, but a move upward over 40% might, especially regarding decisions for device therapies, so the trajectory as well as the absolute EF is important,” she added.
“Particularly in the early stages, people are responsive to therapy and it’s possible in some cases to reverse heart failure, so I think this change helps us understand when that’s happened,” said Dr. Braunwald.
One step toward universality
“The implementation of this terminology and nomenclature into practice will require a variety of tactics,” said Dr. Bozkurt. “For example, the current ICD 10 codes need to incorporate the at-risk and pre–heart failure categories, as well as the mid-range EF, preserved, and improved EF classifications, because the treatment differs between those three domains.”
In terms of how these proposed changes will be worked into practice guidelines, Dr. Bozkurt declined to comment on this to avoid any perception of conflict of interest as she is the cochair of the American College of Cardiology/American Heart Association HF guideline writing committee.
Dr. Braunwald and Dr. Antman suggest it may be premature to call the new terminology and classifications “universal.” In an interview, Dr. Braunwald lamented the absence of the World Heart Federation, the ACC, and the AHA as active participants in this effort and suggested this paper is only the first step of a multistep process that requires input from many stakeholders.
“It’s important that these organizations be involved, not just to bless it, but to contribute their expertise to the process,” he said.
For his part, Dr. Mann hopes these changes will gain widespread acceptance and clinical traction. “The problem sometimes with guidelines is that they’re so data driven that you just can’t come out and say the obvious, so making a position statement is a good first step. And they got good international representation on this, so I think these changes will be accepted in the next heart failure guidelines.”
To encourage further discussion and acceptance, Robert J. Mentz, MD, and Anuradha Lala, MD, editor-in-chief and deputy editor of the Journal of Cardiac Failure, respectively, announced a series of multidisciplinary perspective pieces to be published in the journal monthly, starting in May with editorials from Dr. Clyde W Yancy, MD, MSc, and Carolyn S.P. Lam, MBBS, PhD, both of whom were authors of the consensus statement.
Dr. Bozkurt reports being a consultant for Abbott, Amgen, Baxter, Bristol Myers Squibb, Liva Nova Relypsa/Vifor Pharma, Respicardia, and being on the registry steering committee for Sanofi-Aventis. Dr. Braunwald reports research grant support through Brigham and Women’s Hospital from AstraZeneca, Daiichi Sankyo, Merck, and Novartis; and consulting for Amgen, Boehringer-Ingelheim/Lilly, Cardurion, MyoKardia, Novo Nordisk, and Verve. Dr. Mann has been a consultant to Novartis, is on the steering committee for the PARADISE trial, and is on the scientific advisory board for MyoKardia/Bristol Myers Squibb.
The terminology and classification scheme for heart failure (HF) is changing in ways that experts hope will directly impact patient outcomes.
In a new consensus statement, a multisociety group of experts proposed a new universal definition of heart failure and made substantial revisions to the way in which the disease is staged and classified.
The authors of the statement, led by writing committee chair and immediate past president of the Heart Failure Society of America Biykem Bozkurt, MD, PhD, hope their efforts will go far to improve standardization of terminology, but more importantly will facilitate better management of the disease in ways that keep pace with current knowledge and advances in the field.
“There is a great need for reframing and standardizing the terminology across societies and different stakeholders, and importantly for patients because a lot of the terminology we were using was understood by academicians, but were not being translated in important ways to ensure patients are being appropriately treated,” said Dr. Bozkurt, of Baylor College of Medicine, Houston.
The consensus statement was a group effort led by the HFSA, the Heart Failure Association of the European Society of Cardiology, and the Japanese Heart Failure Society, with endorsements from the Canadian Heart Failure Society, the Heart Failure Association of India, the Cardiac Society of Australia and New Zealand, and the Chinese Heart Failure Association.
The article was published March 1 in the Journal of Cardiac Failure and the European Journal of Heart Failure, authored by a writing committee of 38 individuals with domain expertise in HF, cardiomyopathy, and cardiovascular disease.
“This is a very thorough and very carefully written document that I think will be helpful for clinicians because they’ve tapped into important changes in the field that have occurred over the past 10 years and that now allow us to do more for patients than we could before,” Eugene Braunwald, MD, said in an interview.
Dr. Braunwald and Elliott M. Antman, MD, both from TIMI Study Group at Brigham and Women’s Hospital and Harvard Medical School in Boston, wrote an editorial that accompanied the European Journal of Heart Failure article.
A new universal definition
“[Heart failure] is a clinical syndrome with symptoms and or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion.”
This proposed definition, said the authors, is designed to be contemporary and simple “but conceptually comprehensive, with near universal applicability, prognostic and therapeutic viability, and acceptable sensitivity and specificity.”
Both left and right HF qualifies under this definition, said the authors, but conditions that result in marked volume overload, such as chronic kidney disease, which may present with signs and symptoms of HF, do not.
“Although some of these patients may have concomitant HF, these patients have a primary abnormality that may require a specific treatment beyond that for HF,” said the consensus statement authors.
For his part, Douglas L. Mann, MD, is happy to see what he considers a more accurate and practical definition for heart failure.
“We’ve had some wacky definitions in heart failure that haven’t made sense for 30 years, the principal of which is the definition of heart failure that says it’s the inability of the heart to meet the metabolic demands of the body,” Dr. Mann, of Washington University, St. Louis, said in an interview.
“I think this description was developed thinking about people with end-stage heart failure, but it makes no sense in clinical practice. Does it make sense to say about someone with New York Heart Association class I heart failure that their heart can’t meet the metabolic demands of the body?” said Dr. Mann, who was not involved with the writing of the consensus statement.
Proposed revised stages of the HF continuum
Overall, minimal changes have been made to the HF stages, with tweaks intended to enhance understanding and address the evolving role of biomarkers.
The authors proposed an approach to staging of HF:
- At-risk for HF (stage A), for patients at risk for HF but without current or prior symptoms or signs of HF and without structural or biomarkers evidence of heart disease.
- Pre-HF (stage B), for patients without current or prior symptoms or signs of HF, but evidence of structural heart disease or abnormal cardiac function, or elevated natriuretic peptide levels.
- HF (stage C), for patients with current or prior symptoms and/or signs of HF caused by a structural and/or functional cardiac abnormality.
- Advanced HF (stage D), for patients with severe symptoms and/or signs of HF at rest, recurrent hospitalizations despite guideline-directed management and therapy (GDMT), refractory or intolerant to GDMT, requiring advanced therapies such as consideration for transplant, mechanical circulatory support, or palliative care.

One notable change to the staging scheme is stage B, which the authors have reframed as “pre–heart failure.”
“Pre-cancer is a term widely understood and considered actionable and we wanted to tap into this successful messaging and embrace the pre–heart failure concept as something that is treatable and preventable,” said Dr. Bozkurt.
“We want patients and clinicians to understand that there are things we can do to prevent heart failure, strategies we didn’t have before, like SGLT2 inhibitors in patients with diabetes at risk for HF,” she added.
The revision also avoids the stigma of HF before the symptoms are manifest.
“Not calling it stage A and stage B heart failure you might say is semantics, but it’s important semantics,” said Dr. Braunwald. “When you’re talking to a patient or a relative and tell them they have stage A heart failure, it’s scares them unnecessarily. They don’t hear the stage A or B part, just the heart failure part.”
New classifications according to LVEF
And finally, in what some might consider the most obviously needed modification, the document proposes a new and revised classification of HF according to left ventricular ejection fraction (LVEF). Most agree on how to classify heart failure with reduced ejection fraction (HFrEF) and heart failure with preserved ejection fraction (HFpEF), but although the middle range has long been understood to be a clinically relevant, it has no proper name or clear delineation.
“For standardization across practice guidelines, to recognize clinical trajectories in HF, and to facilitate the recognition of different heart failure entities in a sensitive and specific manner that can guide therapy, we want to formalize the heart failure categories according to ejection fraction,” said Dr. Bozkurt.
To this end, the authors propose the following four classifications of EF:
- HF with reduced EF (HFrEF): LVEF of up to 40%.
- HF with mildly reduced EF (HFmrEF): LVEF of 41-49%.
- HF with preserved EF (HFpEF)HF with an LVEF of at least 50%.
- HF with improved EF (HFimpEF): HF with a baseline LVEF of 40% or less, an increase of at least 10 points from baseline LVEF, and a second measurement of LVEF of greater than 40%.
HFmrEF is usually a transition period, noted Dr. Bozkurt. “Patients with HF in this range may represent a population whose EF is likely to change, either increase or decrease over time and it’s important to be cognizant of that trajectory. Understanding where your patient is headed is crucial for prognosis and optimization of guideline-directed treatment,” she said.
Improved, not recovered, HF
The last classification of heart failure with improved ejection fraction (HFimpEF) represents an important change to the current classification scheme.
“We want to clarify what terms to use but also which not to use. For example, we don’t want people to use recovered heart failure or heart failure in remission, partly because we don’t want the medication to be stopped. We don’t want to give the false message that there has been full recovery,” said Dr. Bozkurt.
As seen in the TRED-HF trial, guideline-directed medical therapy should be continued in patients with HF with improved EF regardless of whether it has improved to a normal range of above 50% in subsequent measurements.
“This is a distinct group of people, and for a while the guidelines were lumping them in with HFpEF, which I think is totally wrong,” said Dr. Mann.
“I think it’s very important that we emphasize heart failure as a continuum, rather than a one-way street of [inevitable] progression. Because we do see improvements in ejection fraction and we do see that we can prevent heart failure if we do the right things, and this should be reflected in the terminology we use,” he added.
Dr. Bozkurt stressed that HFimpEF only applies if the EF improves to above 40%. A move from an EF of 10%-20% would still see the patient classified as having HFrEF, but a patient whose EF improved from, say, 30% to 45% would be classified as HFimpEF.
“The reason for this, again, is because a transition from, say an EF of 10%-20% does not change therapy, but a move upward over 40% might, especially regarding decisions for device therapies, so the trajectory as well as the absolute EF is important,” she added.
“Particularly in the early stages, people are responsive to therapy and it’s possible in some cases to reverse heart failure, so I think this change helps us understand when that’s happened,” said Dr. Braunwald.
One step toward universality
“The implementation of this terminology and nomenclature into practice will require a variety of tactics,” said Dr. Bozkurt. “For example, the current ICD 10 codes need to incorporate the at-risk and pre–heart failure categories, as well as the mid-range EF, preserved, and improved EF classifications, because the treatment differs between those three domains.”
In terms of how these proposed changes will be worked into practice guidelines, Dr. Bozkurt declined to comment on this to avoid any perception of conflict of interest as she is the cochair of the American College of Cardiology/American Heart Association HF guideline writing committee.
Dr. Braunwald and Dr. Antman suggest it may be premature to call the new terminology and classifications “universal.” In an interview, Dr. Braunwald lamented the absence of the World Heart Federation, the ACC, and the AHA as active participants in this effort and suggested this paper is only the first step of a multistep process that requires input from many stakeholders.
“It’s important that these organizations be involved, not just to bless it, but to contribute their expertise to the process,” he said.
For his part, Dr. Mann hopes these changes will gain widespread acceptance and clinical traction. “The problem sometimes with guidelines is that they’re so data driven that you just can’t come out and say the obvious, so making a position statement is a good first step. And they got good international representation on this, so I think these changes will be accepted in the next heart failure guidelines.”
To encourage further discussion and acceptance, Robert J. Mentz, MD, and Anuradha Lala, MD, editor-in-chief and deputy editor of the Journal of Cardiac Failure, respectively, announced a series of multidisciplinary perspective pieces to be published in the journal monthly, starting in May with editorials from Dr. Clyde W Yancy, MD, MSc, and Carolyn S.P. Lam, MBBS, PhD, both of whom were authors of the consensus statement.
Dr. Bozkurt reports being a consultant for Abbott, Amgen, Baxter, Bristol Myers Squibb, Liva Nova Relypsa/Vifor Pharma, Respicardia, and being on the registry steering committee for Sanofi-Aventis. Dr. Braunwald reports research grant support through Brigham and Women’s Hospital from AstraZeneca, Daiichi Sankyo, Merck, and Novartis; and consulting for Amgen, Boehringer-Ingelheim/Lilly, Cardurion, MyoKardia, Novo Nordisk, and Verve. Dr. Mann has been a consultant to Novartis, is on the steering committee for the PARADISE trial, and is on the scientific advisory board for MyoKardia/Bristol Myers Squibb.
FROM THE JOURNAL OF CARDIAC FAILURE
BMI, age, and sex affect COVID-19 vaccine antibody response
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
The capacity to mount humoral immune responses to COVID-19 vaccinations may be reduced among people who are heavier, older, and male, new findings suggest.
The data pertain specifically to the mRNA vaccine, BNT162b2, developed by BioNTech and Pfizer. The study was conducted by Italian researchers and was published Feb. 26 as a preprint.
The study involved 248 health care workers who each received two doses of the vaccine. Of the participants, 99.5% developed a humoral immune response after the second dose. Those responses varied by body mass index (BMI), age, and sex.
“The findings imply that female, lean, and young people have an increased capacity to mount humoral immune responses, compared to male, overweight, and older populations,” Raul Pellini, MD, professor at the IRCCS Regina Elena National Cancer Institute, Rome, and colleagues said.
“To our knowledge, this study is the first to analyze Covid-19 vaccine response in correlation to BMI,” they noted.
“Although further studies are needed, this data may have important implications to the development of vaccination strategies for COVID-19, particularly in obese people,” they wrote. If the data are confirmed by larger studies, “giving obese people an extra dose of the vaccine or a higher dose could be options to be evaluated in this population.”
Results contrast with Pfizer trials of vaccine
The BMI finding seemingly contrasts with final data from the phase 3 clinical trial of the vaccine, which were reported in a supplement to an article published Dec. 31, 2020, in the New England Journal of Medicine. In that study, vaccine efficacy did not differ by obesity status.
Akiko Iwasaki, PhD, professor of immunology at the Howard Hughes Medical Institute and an investigator at Yale University, New Haven, Conn., noted that, although the current Italian study showed somewhat lower levels of antibodies in people with obesity, compared with people who did not have obesity, the phase 3 trial found no difference in symptomatic infection rates.
“These results indicate that even with a slightly lower level of antibody induced in obese people, that level was sufficient to protect against symptomatic infection,” Dr. Iwasaki said in an interview.
Indeed, Dr. Pellini and colleagues pointed out that responses to vaccines against influenza, hepatitis B, and rabies are also reduced in those with obesity, compared with lean individuals.
However, they said, it was especially important to study the effectiveness of COVID-19 vaccines in people with obesity, because obesity is a major risk factor for morbidity and mortality in COVID-19.
“The constant state of low-grade inflammation, present in overweight people, can weaken some immune responses, including those launched by T cells, which can directly kill infected cells,” the authors noted.
Findings reported in British newspapers
The findings of the Italian study were widely covered in the lay press in the United Kingdom, with headlines such as “Pfizer Vaccine May Be Less Effective in People With Obesity, Says Study” and “Pfizer Vaccine: Overweight People Might Need Bigger Dose, Italian Study Says.” In tabloid newspapers, some headlines were slightly more stigmatizing.
The reports do stress that the Italian research was published as a preprint and has not been peer reviewed, or “is yet to be scrutinized by fellow scientists.”
Most make the point that there were only 26 people with obesity among the 248 persons in the study.
“We always knew that BMI was an enormous predictor of poor immune response to vaccines, so this paper is definitely interesting, although it is based on a rather small preliminary dataset,” Danny Altmann, PhD, a professor of immunology at Imperial College London, told the Guardian.
“It confirms that having a vaccinated population isn’t synonymous with having an immune population, especially in a country with high obesity, and emphasizes the vital need for long-term immune monitoring programs,” he added.
Antibody responses differ by BMI, age, and sex
In the Italian study, the participants – 158 women and 90 men – were assigned to receive a priming BNT162b2 vaccine dose with a booster at day 21. Blood and nasopharyngeal swabs were collected at baseline and 7 days after the second vaccine dose.
After the second dose, 99.5% of participants developed a humoral immune response; one person did not respond. None tested positive for SARS-CoV-2.
Titers of SARS-CoV-2–binding antibodies were greater in younger than in older participants. There were statistically significant differences between those aged 37 years and younger (453.5 AU/mL) and those aged 47-56 years (239.8 AU/mL; P = .005), those aged 37 years and younger versus those older than 56 years (453.5 vs 182.4 AU/mL; P < .0001), and those aged 37-47 years versus those older than 56 years (330.9 vs. 182.4 AU/mL; P = .01).
Antibody response was significantly greater for women than for men (338.5 vs. 212.6 AU/mL; P = .001).
Humoral responses were greater in persons of normal-weight BMI (18.5-24.9 kg/m2; 325.8 AU/mL) and those of underweight BMI (<18.5 kg/m2; 455.4 AU/mL), compared with persons with preobesity, defined as BMI of 25-29.9 (222.4 AU/mL), and those with obesity (BMI ≥30; 167.0 AU/mL; P < .0001). This association remained after adjustment for age (P = .003).
“Our data stresses the importance of close vaccination monitoring of obese people, considering the growing list of countries with obesity problems,” the researchers noted.
Hypertension was also associated with lower antibody titers (P = .006), but that lost statistical significance after matching for age (P = .22).
“We strongly believe that our results are extremely encouraging and useful for the scientific community,” Dr. Pellini and colleagues concluded.
The authors disclosed no relevant financial relationships. Dr. Iwasaki is a cofounder of RIGImmune and is a member of its scientific advisory board.
This article was updated on 3/8/21.
A version of this article first appeared on Medscape.com.
JAMA podcast on racism in medicine faces backlash
Published on Feb. 23, the episode is hosted on JAMA’s learning platform for doctors and is available for continuing medical education credits.
“No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast,” JAMA wrote in a Twitter post to promote the episode. That tweet has since been deleted.

The episode features host Ed Livingston, MD, deputy editor for clinical reviews and education at JAMA, and guest Mitchell Katz, MD, president and CEO for NYC Health + Hospitals and deputy editor for JAMA Internal Medicine. Dr. Livingston approaches the episode as “structural racism for skeptics,” and Dr. Katz tries to explain how structural racism deepens health disparities and what health systems can do about it.
“Many physicians are skeptical of structural racism, the idea that economic, educational, and other societal systems preferentially disadvantage Black Americans and other communities of color,” the episode description says.
In the podcast, Dr. Livingston and Dr. Katz speak about health care disparities and racial inequality. Dr. Livingston, who says he “didn’t understand the concept” going into the episode, suggests that racism was made illegal in the 1960s and that the discussion of “structural racism” should shift away from the term “racism” and focus on socioeconomic status instead.
“What you’re talking about isn’t so much racism ... it isn’t their race, it isn’t their color, it’s their socioeconomic status,” Dr. Livingston says. “Is that a fair statement?”
But Dr. Katz says that “acknowledging structural racism can be helpful to us. Structural racism refers to a system in which policies or practices or how we look at people perpetuates racial inequality.”
Dr. Katz points to the creation of a hospital in San Francisco in the 1880s to treat patients of Chinese ethnicity separately. Outside of health care, he talks about environmental racism between neighborhoods with inequalities in hospitals, schools, and social services.
“All of those things have an impact on that minority person,” Dr. Katz says. “The big thing we can all do is move away from trying to interrogate each other’s opinions and move to a place where we are looking at the policies of our institutions and making sure that they promote equality.”
Dr. Livingston concludes the episode by reemphasizing that “racism” should be taken out of the conversation and it should instead focus on the “structural” aspect of socioeconomics.
“Minorities ... aren’t [in those neighborhoods] because they’re not allowed to buy houses or they can’t get a job because they’re Black or Hispanic. That would be illegal,” Dr. Livingston says. “But disproportionality does exist.”
Efforts to reach Dr. Livingston were unsuccessful. Dr. Katz distanced himself from Dr. Livingston in a statement released on March 4.
“Systemic and interpersonal racism both still exist in our country — they must be rooted out. I do not share the JAMA host’s belief of doing away with the word ‘racism’ will help us be more successful in ending inequities that exists across racial and ethnic lines,” Dr. Katz said. “Further, I believe that we will only produce an equitable society when social and political structures do not continue to produce and perpetuate disparate results based on social race and ethnicity.”
Dr. Katz reiterated that both interpersonal and structural racism continue to exist in the United States, “and it is woefully naive to say that no physician is a racist just because the Civil Rights Act of 1964 forbade it.”
He also recommended JAMA use this controversy “as a learning opportunity for continued dialogue and create another podcast series as an open conversation that invites diverse experts in the field to have an open discussion about structural racism in healthcare.”
The podcast and JAMA’s tweet promoting it were widely criticized on Twitter. In interviews with WebMD, many doctors expressed disbelief that such a respected journal would lend its name to this podcast episode.
B. Bobby Chiong, MD, a radiologist in New York, said although JAMA’s effort to engage with its audience about racism is laudable, it missed the mark.
“I think the backlash comes from how they tried to make a podcast about the subject and somehow made themselves an example of unconscious bias and unfamiliarity with just how embedded in our system is structural racism,” he said.
Perhaps the podcast’s worst offense was its failure to address the painful history of racial bias in this country that still permeates the medical community, says Tamara Saint-Surin, MD, assistant professor at the University of North Carolina at Chapel Hill.
“For physicians in leadership to have the belief that structural racism does not exist in medicine, they don’t really appreciate what affects their patients and what their patients were dealing with,” Dr. Saint-Surin said in an interview. “It was a very harmful podcast and goes to show we still have so much work to do.”
Along with a flawed premise, she says, the podcast was not nearly long enough to address such a nuanced issue. And Dr. Livingston focused on interpersonal racism rather than structural racism, she said, failing to address widespread problems such as higher rates of asthma among Black populations living in areas with poor air quality.
The number of Black doctors remains low and the lack of representation adds to an environment already rife with racism, according to many medical professionals.
Shirlene Obuobi, MD, an internal medicine doctor in Chicago, said JAMA failed to live up to its own standards by publishing material that lacked research and expertise.
“I can’t submit a clinical trial to JAMA without them combing through methods with a fine-tooth comb,” Dr. Obuobi said. “They didn’t uphold the standards they normally apply to anyone else.”
Both the editor of JAMA and the head of the American Medical Association issued statements criticizing the episode and the tweet that promoted it.
JAMA Editor-in-Chief Howard Bauchner, MD, said, “The language of the tweet, and some portions of the podcast, do not reflect my commitment as editorial leader of JAMA and JAMA Network to call out and discuss the adverse effects of injustice, inequity, and racism in society and medicine as JAMA has done for many years.” He said JAMA will schedule a future podcast to address the concerns raised about the recent episode.
AMA CEO James L. Madara, MD, said, “The AMA’s House of Delegates passed policy stating that racism is structural, systemic, cultural, and interpersonal, and we are deeply disturbed – and angered – by a recent JAMA podcast that questioned the existence of structural racism and the affiliated tweet that promoted the podcast and stated ‘no physician is racist, so how can there be structural racism in health care?’ ”
He continued: “JAMA has editorial independence from AMA, but this tweet and podcast are inconsistent with the policies and views of AMA, and I’m concerned about and acknowledge the harms they have caused. Structural racism in health care and our society exists, and it is incumbent on all of us to fix it.”
This article was updated 3/5/21.
A version of this article first appeared on WebMD.com.
Published on Feb. 23, the episode is hosted on JAMA’s learning platform for doctors and is available for continuing medical education credits.
“No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast,” JAMA wrote in a Twitter post to promote the episode. That tweet has since been deleted.

The episode features host Ed Livingston, MD, deputy editor for clinical reviews and education at JAMA, and guest Mitchell Katz, MD, president and CEO for NYC Health + Hospitals and deputy editor for JAMA Internal Medicine. Dr. Livingston approaches the episode as “structural racism for skeptics,” and Dr. Katz tries to explain how structural racism deepens health disparities and what health systems can do about it.
“Many physicians are skeptical of structural racism, the idea that economic, educational, and other societal systems preferentially disadvantage Black Americans and other communities of color,” the episode description says.
In the podcast, Dr. Livingston and Dr. Katz speak about health care disparities and racial inequality. Dr. Livingston, who says he “didn’t understand the concept” going into the episode, suggests that racism was made illegal in the 1960s and that the discussion of “structural racism” should shift away from the term “racism” and focus on socioeconomic status instead.
“What you’re talking about isn’t so much racism ... it isn’t their race, it isn’t their color, it’s their socioeconomic status,” Dr. Livingston says. “Is that a fair statement?”
But Dr. Katz says that “acknowledging structural racism can be helpful to us. Structural racism refers to a system in which policies or practices or how we look at people perpetuates racial inequality.”
Dr. Katz points to the creation of a hospital in San Francisco in the 1880s to treat patients of Chinese ethnicity separately. Outside of health care, he talks about environmental racism between neighborhoods with inequalities in hospitals, schools, and social services.
“All of those things have an impact on that minority person,” Dr. Katz says. “The big thing we can all do is move away from trying to interrogate each other’s opinions and move to a place where we are looking at the policies of our institutions and making sure that they promote equality.”
Dr. Livingston concludes the episode by reemphasizing that “racism” should be taken out of the conversation and it should instead focus on the “structural” aspect of socioeconomics.
“Minorities ... aren’t [in those neighborhoods] because they’re not allowed to buy houses or they can’t get a job because they’re Black or Hispanic. That would be illegal,” Dr. Livingston says. “But disproportionality does exist.”
Efforts to reach Dr. Livingston were unsuccessful. Dr. Katz distanced himself from Dr. Livingston in a statement released on March 4.
“Systemic and interpersonal racism both still exist in our country — they must be rooted out. I do not share the JAMA host’s belief of doing away with the word ‘racism’ will help us be more successful in ending inequities that exists across racial and ethnic lines,” Dr. Katz said. “Further, I believe that we will only produce an equitable society when social and political structures do not continue to produce and perpetuate disparate results based on social race and ethnicity.”
Dr. Katz reiterated that both interpersonal and structural racism continue to exist in the United States, “and it is woefully naive to say that no physician is a racist just because the Civil Rights Act of 1964 forbade it.”
He also recommended JAMA use this controversy “as a learning opportunity for continued dialogue and create another podcast series as an open conversation that invites diverse experts in the field to have an open discussion about structural racism in healthcare.”
The podcast and JAMA’s tweet promoting it were widely criticized on Twitter. In interviews with WebMD, many doctors expressed disbelief that such a respected journal would lend its name to this podcast episode.
B. Bobby Chiong, MD, a radiologist in New York, said although JAMA’s effort to engage with its audience about racism is laudable, it missed the mark.
“I think the backlash comes from how they tried to make a podcast about the subject and somehow made themselves an example of unconscious bias and unfamiliarity with just how embedded in our system is structural racism,” he said.
Perhaps the podcast’s worst offense was its failure to address the painful history of racial bias in this country that still permeates the medical community, says Tamara Saint-Surin, MD, assistant professor at the University of North Carolina at Chapel Hill.
“For physicians in leadership to have the belief that structural racism does not exist in medicine, they don’t really appreciate what affects their patients and what their patients were dealing with,” Dr. Saint-Surin said in an interview. “It was a very harmful podcast and goes to show we still have so much work to do.”
Along with a flawed premise, she says, the podcast was not nearly long enough to address such a nuanced issue. And Dr. Livingston focused on interpersonal racism rather than structural racism, she said, failing to address widespread problems such as higher rates of asthma among Black populations living in areas with poor air quality.
The number of Black doctors remains low and the lack of representation adds to an environment already rife with racism, according to many medical professionals.
Shirlene Obuobi, MD, an internal medicine doctor in Chicago, said JAMA failed to live up to its own standards by publishing material that lacked research and expertise.
“I can’t submit a clinical trial to JAMA without them combing through methods with a fine-tooth comb,” Dr. Obuobi said. “They didn’t uphold the standards they normally apply to anyone else.”
Both the editor of JAMA and the head of the American Medical Association issued statements criticizing the episode and the tweet that promoted it.
JAMA Editor-in-Chief Howard Bauchner, MD, said, “The language of the tweet, and some portions of the podcast, do not reflect my commitment as editorial leader of JAMA and JAMA Network to call out and discuss the adverse effects of injustice, inequity, and racism in society and medicine as JAMA has done for many years.” He said JAMA will schedule a future podcast to address the concerns raised about the recent episode.
AMA CEO James L. Madara, MD, said, “The AMA’s House of Delegates passed policy stating that racism is structural, systemic, cultural, and interpersonal, and we are deeply disturbed – and angered – by a recent JAMA podcast that questioned the existence of structural racism and the affiliated tweet that promoted the podcast and stated ‘no physician is racist, so how can there be structural racism in health care?’ ”
He continued: “JAMA has editorial independence from AMA, but this tweet and podcast are inconsistent with the policies and views of AMA, and I’m concerned about and acknowledge the harms they have caused. Structural racism in health care and our society exists, and it is incumbent on all of us to fix it.”
This article was updated 3/5/21.
A version of this article first appeared on WebMD.com.
Published on Feb. 23, the episode is hosted on JAMA’s learning platform for doctors and is available for continuing medical education credits.
“No physician is racist, so how can there be structural racism in health care? An explanation of the idea by doctors for doctors in this user-friendly podcast,” JAMA wrote in a Twitter post to promote the episode. That tweet has since been deleted.

The episode features host Ed Livingston, MD, deputy editor for clinical reviews and education at JAMA, and guest Mitchell Katz, MD, president and CEO for NYC Health + Hospitals and deputy editor for JAMA Internal Medicine. Dr. Livingston approaches the episode as “structural racism for skeptics,” and Dr. Katz tries to explain how structural racism deepens health disparities and what health systems can do about it.
“Many physicians are skeptical of structural racism, the idea that economic, educational, and other societal systems preferentially disadvantage Black Americans and other communities of color,” the episode description says.
In the podcast, Dr. Livingston and Dr. Katz speak about health care disparities and racial inequality. Dr. Livingston, who says he “didn’t understand the concept” going into the episode, suggests that racism was made illegal in the 1960s and that the discussion of “structural racism” should shift away from the term “racism” and focus on socioeconomic status instead.
“What you’re talking about isn’t so much racism ... it isn’t their race, it isn’t their color, it’s their socioeconomic status,” Dr. Livingston says. “Is that a fair statement?”
But Dr. Katz says that “acknowledging structural racism can be helpful to us. Structural racism refers to a system in which policies or practices or how we look at people perpetuates racial inequality.”
Dr. Katz points to the creation of a hospital in San Francisco in the 1880s to treat patients of Chinese ethnicity separately. Outside of health care, he talks about environmental racism between neighborhoods with inequalities in hospitals, schools, and social services.
“All of those things have an impact on that minority person,” Dr. Katz says. “The big thing we can all do is move away from trying to interrogate each other’s opinions and move to a place where we are looking at the policies of our institutions and making sure that they promote equality.”
Dr. Livingston concludes the episode by reemphasizing that “racism” should be taken out of the conversation and it should instead focus on the “structural” aspect of socioeconomics.
“Minorities ... aren’t [in those neighborhoods] because they’re not allowed to buy houses or they can’t get a job because they’re Black or Hispanic. That would be illegal,” Dr. Livingston says. “But disproportionality does exist.”
Efforts to reach Dr. Livingston were unsuccessful. Dr. Katz distanced himself from Dr. Livingston in a statement released on March 4.
“Systemic and interpersonal racism both still exist in our country — they must be rooted out. I do not share the JAMA host’s belief of doing away with the word ‘racism’ will help us be more successful in ending inequities that exists across racial and ethnic lines,” Dr. Katz said. “Further, I believe that we will only produce an equitable society when social and political structures do not continue to produce and perpetuate disparate results based on social race and ethnicity.”
Dr. Katz reiterated that both interpersonal and structural racism continue to exist in the United States, “and it is woefully naive to say that no physician is a racist just because the Civil Rights Act of 1964 forbade it.”
He also recommended JAMA use this controversy “as a learning opportunity for continued dialogue and create another podcast series as an open conversation that invites diverse experts in the field to have an open discussion about structural racism in healthcare.”
The podcast and JAMA’s tweet promoting it were widely criticized on Twitter. In interviews with WebMD, many doctors expressed disbelief that such a respected journal would lend its name to this podcast episode.
B. Bobby Chiong, MD, a radiologist in New York, said although JAMA’s effort to engage with its audience about racism is laudable, it missed the mark.
“I think the backlash comes from how they tried to make a podcast about the subject and somehow made themselves an example of unconscious bias and unfamiliarity with just how embedded in our system is structural racism,” he said.
Perhaps the podcast’s worst offense was its failure to address the painful history of racial bias in this country that still permeates the medical community, says Tamara Saint-Surin, MD, assistant professor at the University of North Carolina at Chapel Hill.
“For physicians in leadership to have the belief that structural racism does not exist in medicine, they don’t really appreciate what affects their patients and what their patients were dealing with,” Dr. Saint-Surin said in an interview. “It was a very harmful podcast and goes to show we still have so much work to do.”
Along with a flawed premise, she says, the podcast was not nearly long enough to address such a nuanced issue. And Dr. Livingston focused on interpersonal racism rather than structural racism, she said, failing to address widespread problems such as higher rates of asthma among Black populations living in areas with poor air quality.
The number of Black doctors remains low and the lack of representation adds to an environment already rife with racism, according to many medical professionals.
Shirlene Obuobi, MD, an internal medicine doctor in Chicago, said JAMA failed to live up to its own standards by publishing material that lacked research and expertise.
“I can’t submit a clinical trial to JAMA without them combing through methods with a fine-tooth comb,” Dr. Obuobi said. “They didn’t uphold the standards they normally apply to anyone else.”
Both the editor of JAMA and the head of the American Medical Association issued statements criticizing the episode and the tweet that promoted it.
JAMA Editor-in-Chief Howard Bauchner, MD, said, “The language of the tweet, and some portions of the podcast, do not reflect my commitment as editorial leader of JAMA and JAMA Network to call out and discuss the adverse effects of injustice, inequity, and racism in society and medicine as JAMA has done for many years.” He said JAMA will schedule a future podcast to address the concerns raised about the recent episode.
AMA CEO James L. Madara, MD, said, “The AMA’s House of Delegates passed policy stating that racism is structural, systemic, cultural, and interpersonal, and we are deeply disturbed – and angered – by a recent JAMA podcast that questioned the existence of structural racism and the affiliated tweet that promoted the podcast and stated ‘no physician is racist, so how can there be structural racism in health care?’ ”
He continued: “JAMA has editorial independence from AMA, but this tweet and podcast are inconsistent with the policies and views of AMA, and I’m concerned about and acknowledge the harms they have caused. Structural racism in health care and our society exists, and it is incumbent on all of us to fix it.”
This article was updated 3/5/21.
A version of this article first appeared on WebMD.com.
Happy National Hospitalist Day!
Hospitalists across the United States have been and continue to be a critical part of our nation’s response to COVID-19. On National Hospitalist Day, Thursday, March 4, 2021, the Society of Hospital Medicine invites you to celebrate the individuals and teams that make up the hospital medicine community.

On this special day, SHM encourages you to share your story, showcase your team’s efforts to improve patient care, express your pride for the specialty, or share how you are making a difference in your hospital and in the lives of patients.
Here are just a few of the ways you can celebrate:
- Register for our live roundtable, featuring Mark Shapiro, MD, hospitalist and host of the Explore the Space podcast, and four hospitalist panelists, on March 4 at 7 p.m. ET/4 p.m. PT.
- Download shareable graphics, posters, Zoom backgrounds, and coloring book pages
- Enter our social media photo contest and follow the #HowWeHospitalist hashtag across all platforms
- Read special hospitalist profiles in the Hospitalist, including: Eric E. Howell, MD, MHM; Grace Huang, MD; Bridget McGrath, PA-C, FHM; and Harry Cho, MD, SFHM
Thank you for all you do and continue to do for hospital medicine. We hope you take some time today to celebrate you and your colleagues, as well as your commendable contributions to health care and the future of the specialty.
To learn more about National Hospitalist Day, visit hospitalmedicine.org/hospitalistday.
Hospitalists across the United States have been and continue to be a critical part of our nation’s response to COVID-19. On National Hospitalist Day, Thursday, March 4, 2021, the Society of Hospital Medicine invites you to celebrate the individuals and teams that make up the hospital medicine community.

On this special day, SHM encourages you to share your story, showcase your team’s efforts to improve patient care, express your pride for the specialty, or share how you are making a difference in your hospital and in the lives of patients.
Here are just a few of the ways you can celebrate:
- Register for our live roundtable, featuring Mark Shapiro, MD, hospitalist and host of the Explore the Space podcast, and four hospitalist panelists, on March 4 at 7 p.m. ET/4 p.m. PT.
- Download shareable graphics, posters, Zoom backgrounds, and coloring book pages
- Enter our social media photo contest and follow the #HowWeHospitalist hashtag across all platforms
- Read special hospitalist profiles in the Hospitalist, including: Eric E. Howell, MD, MHM; Grace Huang, MD; Bridget McGrath, PA-C, FHM; and Harry Cho, MD, SFHM
Thank you for all you do and continue to do for hospital medicine. We hope you take some time today to celebrate you and your colleagues, as well as your commendable contributions to health care and the future of the specialty.
To learn more about National Hospitalist Day, visit hospitalmedicine.org/hospitalistday.
Hospitalists across the United States have been and continue to be a critical part of our nation’s response to COVID-19. On National Hospitalist Day, Thursday, March 4, 2021, the Society of Hospital Medicine invites you to celebrate the individuals and teams that make up the hospital medicine community.

On this special day, SHM encourages you to share your story, showcase your team’s efforts to improve patient care, express your pride for the specialty, or share how you are making a difference in your hospital and in the lives of patients.
Here are just a few of the ways you can celebrate:
- Register for our live roundtable, featuring Mark Shapiro, MD, hospitalist and host of the Explore the Space podcast, and four hospitalist panelists, on March 4 at 7 p.m. ET/4 p.m. PT.
- Download shareable graphics, posters, Zoom backgrounds, and coloring book pages
- Enter our social media photo contest and follow the #HowWeHospitalist hashtag across all platforms
- Read special hospitalist profiles in the Hospitalist, including: Eric E. Howell, MD, MHM; Grace Huang, MD; Bridget McGrath, PA-C, FHM; and Harry Cho, MD, SFHM
Thank you for all you do and continue to do for hospital medicine. We hope you take some time today to celebrate you and your colleagues, as well as your commendable contributions to health care and the future of the specialty.
To learn more about National Hospitalist Day, visit hospitalmedicine.org/hospitalistday.
Owning all aspects of patient care: Bridget McGrath, PA-C, FHM
Editor’s note: This profile is part of the Society of Hospital Medicine’s celebration of National Hospitalist Day on March 4. National Hospitalist Day occurs the first Thursday in March annually and celebrates the fastest growing specialty in modern medicine and hospitalists’ enduring contributions to the evolving health care landscape.
Bridget McGrath, PA-C, FHM, is a physician assistant and director of the nurse practitioner/physician assistant service line for the section of hospital medicine at the University of Chicago. She is a cochair of SHM’s NP/PA Special Interest Group.
Where did you receive your PA education/training? Was your intention always to be a PA?
I graduated from the PA program at Butler University, Indianapolis, in 2014. In college, whenever I shadowed a PA, I was always impressed that each one loved their job and said they would never change it. That universal passion for the PA profession really made an impression on me.
At what point in your PA education/training did you decide to practice hospital medicine? What about it appealed to you?
That occurred during my clinical rotation year at Butler. I had always thought I wanted to practice neonatology, but during my clinical rotation I really fell in love with adult medicine. I recall that during my clinical rotation, the preceptor said to me that the goal was not to have me understand every aspect of medicine, but to learn how to exist in a hospital setting. I was exposed to the breadth of hospital medicine practice and I fell in love with the complexity, the variety, and the environment itself.
I initially accepted a job as a med-peds hospitalist PA – which brought both of my passions together at that time – at Schneck Medical Center in Seymour, Ind. During that time, Schneck was a 100-bed rural community hospital which had recently been the recipient of the Malcolm Baldrige National Quality Award. It was there that I was able to practice with a phenomenal group of physicians, nurses, and social workers who really took me under their wing and taught me how to be a hospitalist PA. I practiced at Schneck for 3 years, and then moved to the University of Chicago in 2017.
I am now the director of NP/PA services for the section of hospital medicine, overseeing a group of seven on our NP/PA team, within a larger group of about 60 physicians.
What are your favorite areas of clinical practice?
Like many hospitalists, I enjoy the variety of medicine that hospitalists practice. One area that I find especially rewarding is my time in our transplant comanagement services. To be able to walk with patients on their transplant journey is very rewarding, and I am very appreciative of the mentoring I have received from some of my colleagues with a deeper understanding of transplant medicine.
In my administrative role, I have the privilege of helping to expand the professional education and training of my colleagues. I have a passion for medical education, and we have been working to develop interprofessional educational opportunities within our section. I have had time to think about the imprint of NPs and PAs in academic medicine, and how we can continue to meet the professional educational needs of our section while improving the care of our patients.
What are the most challenging aspects of practicing hospital medicine?
The volume of diagnoses that we are expected to manage on a daily basis can be challenging. This challenges you to continue learning. The complexity of discharge planning, particularly for patients in underserved communities, can also be challenging. You have to make sure your patients are ready mentally, physically and emotionally for discharge. As a hospitalist, you are continuously thinking about how to optimize patients to leave your care. For example, patients have different insurance situations, different access to care at home – you are always managing the medical needs of your patient in the context of these other issues.
How does a hospitalist PA work differently from a PA in other care settings?
We are meant to be generalists. We serve as the main provider in owning our patients’ care. A hospitalist PA serves as a cog in the wheel, with connections to specialists, consultants, nurses, social workers, pharmacists, etc., and we are tasked with synthesizing all aspects of patient care to ensure the best outcome.
What has your experience taught you about how NPs and PAs can best fit into hospital medicine groups?
Each hospital medicine group will know how to best integrate their NPs and PAs based on the skillsets of their NPs and PAs, and the needs of the section and the hospital. I personally feel that the best way to utilize NPs and PAs is to allow them to own all aspects of patient care and work at the highest scope of practice. By doing this you empower the NP or PA to continue to develop their skill set and set a precedent of collaboration and respect for interprofessional care models within your section’s culture.
Scope of practice for an NP or PA is going to be based on a conglomeration of roles and bylaws. We are certified nationally, and our scope of practice is determined at the state level and the hospital by level. For the individual NP and PA, it really depends on the hospital medicine group, and how well a practice incorporates a sense of collegiality.
What kind of resources do hospitalist PAs need to succeed, either from SHM or from their own institutions?
There are a few key things that need to happen in order for hospital medicine groups to set up their NPs and PAs for success. The first is for PAs to have exposure to inpatient rotations during clinical rotations. A hospital medicine group also should have a very intentional onboarding process for NPs and PAs. They should also establish a culture of acceptance. To do this, they should utilize resources like SHM’s NP/PA Hospital Medicine Onboarding Toolkit and the SHM/American Academy of Physician Assistants Hospitalist Bootcamp On Demand.
Mentoring is also remarkably important. I have been incredibly blessed to have mentors that helped make me into the PA that I am. I could not have done what I did in the field without people taking a chance on me, and it is important to pass that on to the next generation of PAs.
How has COVID-19 changed the practice of hospital medicine, specifically for advanced practice providers?
The pandemic has demonstrated opportunities for teamwork and utilization of NPs and PAs. The COVID pandemic forced everyone to reflect on why they originally got into medicine – to help patients. I think there will be many doors opening for NPs and PAs, and many pathways for leadership.
The hospitalist leadership at the University of Chicago truly identified that we needed to make wellness a main priority during the beginning of the pandemic. We developed a wellness work group that I have been coleading.
What’s on the horizon for NPs and PAs in hospital medicine?
We are seeing significant increases in hospitalist program utilization, so this is a time where NPs and PAs can be advocates for our profession and articulate how we can use our backgrounds and training to build better care models in order to meet the needs of our patients.
I hope we will see more NPs and PAs assuming leadership roles to ensure that our voices are heard. We should also be advocating for more collaboration and teamwork with our MD and DO colleagues.
Do you have any advice for PA students interested in hospital medicine?
I always tell my students that they should be sponges – you are not expected to know everything as a hospitalist PA, but you are expected to continue learning in order to develop into the best PA you can be. Always be open to where your career path can take you. Hospital medicine is a relatively young field within medicine, and the diversity of our field is very exciting looking forward.
Editor’s note: This profile is part of the Society of Hospital Medicine’s celebration of National Hospitalist Day on March 4. National Hospitalist Day occurs the first Thursday in March annually and celebrates the fastest growing specialty in modern medicine and hospitalists’ enduring contributions to the evolving health care landscape.
Bridget McGrath, PA-C, FHM, is a physician assistant and director of the nurse practitioner/physician assistant service line for the section of hospital medicine at the University of Chicago. She is a cochair of SHM’s NP/PA Special Interest Group.
Where did you receive your PA education/training? Was your intention always to be a PA?
I graduated from the PA program at Butler University, Indianapolis, in 2014. In college, whenever I shadowed a PA, I was always impressed that each one loved their job and said they would never change it. That universal passion for the PA profession really made an impression on me.
At what point in your PA education/training did you decide to practice hospital medicine? What about it appealed to you?
That occurred during my clinical rotation year at Butler. I had always thought I wanted to practice neonatology, but during my clinical rotation I really fell in love with adult medicine. I recall that during my clinical rotation, the preceptor said to me that the goal was not to have me understand every aspect of medicine, but to learn how to exist in a hospital setting. I was exposed to the breadth of hospital medicine practice and I fell in love with the complexity, the variety, and the environment itself.
I initially accepted a job as a med-peds hospitalist PA – which brought both of my passions together at that time – at Schneck Medical Center in Seymour, Ind. During that time, Schneck was a 100-bed rural community hospital which had recently been the recipient of the Malcolm Baldrige National Quality Award. It was there that I was able to practice with a phenomenal group of physicians, nurses, and social workers who really took me under their wing and taught me how to be a hospitalist PA. I practiced at Schneck for 3 years, and then moved to the University of Chicago in 2017.
I am now the director of NP/PA services for the section of hospital medicine, overseeing a group of seven on our NP/PA team, within a larger group of about 60 physicians.
What are your favorite areas of clinical practice?
Like many hospitalists, I enjoy the variety of medicine that hospitalists practice. One area that I find especially rewarding is my time in our transplant comanagement services. To be able to walk with patients on their transplant journey is very rewarding, and I am very appreciative of the mentoring I have received from some of my colleagues with a deeper understanding of transplant medicine.
In my administrative role, I have the privilege of helping to expand the professional education and training of my colleagues. I have a passion for medical education, and we have been working to develop interprofessional educational opportunities within our section. I have had time to think about the imprint of NPs and PAs in academic medicine, and how we can continue to meet the professional educational needs of our section while improving the care of our patients.
What are the most challenging aspects of practicing hospital medicine?
The volume of diagnoses that we are expected to manage on a daily basis can be challenging. This challenges you to continue learning. The complexity of discharge planning, particularly for patients in underserved communities, can also be challenging. You have to make sure your patients are ready mentally, physically and emotionally for discharge. As a hospitalist, you are continuously thinking about how to optimize patients to leave your care. For example, patients have different insurance situations, different access to care at home – you are always managing the medical needs of your patient in the context of these other issues.
How does a hospitalist PA work differently from a PA in other care settings?
We are meant to be generalists. We serve as the main provider in owning our patients’ care. A hospitalist PA serves as a cog in the wheel, with connections to specialists, consultants, nurses, social workers, pharmacists, etc., and we are tasked with synthesizing all aspects of patient care to ensure the best outcome.
What has your experience taught you about how NPs and PAs can best fit into hospital medicine groups?
Each hospital medicine group will know how to best integrate their NPs and PAs based on the skillsets of their NPs and PAs, and the needs of the section and the hospital. I personally feel that the best way to utilize NPs and PAs is to allow them to own all aspects of patient care and work at the highest scope of practice. By doing this you empower the NP or PA to continue to develop their skill set and set a precedent of collaboration and respect for interprofessional care models within your section’s culture.
Scope of practice for an NP or PA is going to be based on a conglomeration of roles and bylaws. We are certified nationally, and our scope of practice is determined at the state level and the hospital by level. For the individual NP and PA, it really depends on the hospital medicine group, and how well a practice incorporates a sense of collegiality.
What kind of resources do hospitalist PAs need to succeed, either from SHM or from their own institutions?
There are a few key things that need to happen in order for hospital medicine groups to set up their NPs and PAs for success. The first is for PAs to have exposure to inpatient rotations during clinical rotations. A hospital medicine group also should have a very intentional onboarding process for NPs and PAs. They should also establish a culture of acceptance. To do this, they should utilize resources like SHM’s NP/PA Hospital Medicine Onboarding Toolkit and the SHM/American Academy of Physician Assistants Hospitalist Bootcamp On Demand.
Mentoring is also remarkably important. I have been incredibly blessed to have mentors that helped make me into the PA that I am. I could not have done what I did in the field without people taking a chance on me, and it is important to pass that on to the next generation of PAs.
How has COVID-19 changed the practice of hospital medicine, specifically for advanced practice providers?
The pandemic has demonstrated opportunities for teamwork and utilization of NPs and PAs. The COVID pandemic forced everyone to reflect on why they originally got into medicine – to help patients. I think there will be many doors opening for NPs and PAs, and many pathways for leadership.
The hospitalist leadership at the University of Chicago truly identified that we needed to make wellness a main priority during the beginning of the pandemic. We developed a wellness work group that I have been coleading.
What’s on the horizon for NPs and PAs in hospital medicine?
We are seeing significant increases in hospitalist program utilization, so this is a time where NPs and PAs can be advocates for our profession and articulate how we can use our backgrounds and training to build better care models in order to meet the needs of our patients.
I hope we will see more NPs and PAs assuming leadership roles to ensure that our voices are heard. We should also be advocating for more collaboration and teamwork with our MD and DO colleagues.
Do you have any advice for PA students interested in hospital medicine?
I always tell my students that they should be sponges – you are not expected to know everything as a hospitalist PA, but you are expected to continue learning in order to develop into the best PA you can be. Always be open to where your career path can take you. Hospital medicine is a relatively young field within medicine, and the diversity of our field is very exciting looking forward.
Editor’s note: This profile is part of the Society of Hospital Medicine’s celebration of National Hospitalist Day on March 4. National Hospitalist Day occurs the first Thursday in March annually and celebrates the fastest growing specialty in modern medicine and hospitalists’ enduring contributions to the evolving health care landscape.
Bridget McGrath, PA-C, FHM, is a physician assistant and director of the nurse practitioner/physician assistant service line for the section of hospital medicine at the University of Chicago. She is a cochair of SHM’s NP/PA Special Interest Group.
Where did you receive your PA education/training? Was your intention always to be a PA?
I graduated from the PA program at Butler University, Indianapolis, in 2014. In college, whenever I shadowed a PA, I was always impressed that each one loved their job and said they would never change it. That universal passion for the PA profession really made an impression on me.
At what point in your PA education/training did you decide to practice hospital medicine? What about it appealed to you?
That occurred during my clinical rotation year at Butler. I had always thought I wanted to practice neonatology, but during my clinical rotation I really fell in love with adult medicine. I recall that during my clinical rotation, the preceptor said to me that the goal was not to have me understand every aspect of medicine, but to learn how to exist in a hospital setting. I was exposed to the breadth of hospital medicine practice and I fell in love with the complexity, the variety, and the environment itself.
I initially accepted a job as a med-peds hospitalist PA – which brought both of my passions together at that time – at Schneck Medical Center in Seymour, Ind. During that time, Schneck was a 100-bed rural community hospital which had recently been the recipient of the Malcolm Baldrige National Quality Award. It was there that I was able to practice with a phenomenal group of physicians, nurses, and social workers who really took me under their wing and taught me how to be a hospitalist PA. I practiced at Schneck for 3 years, and then moved to the University of Chicago in 2017.
I am now the director of NP/PA services for the section of hospital medicine, overseeing a group of seven on our NP/PA team, within a larger group of about 60 physicians.
What are your favorite areas of clinical practice?
Like many hospitalists, I enjoy the variety of medicine that hospitalists practice. One area that I find especially rewarding is my time in our transplant comanagement services. To be able to walk with patients on their transplant journey is very rewarding, and I am very appreciative of the mentoring I have received from some of my colleagues with a deeper understanding of transplant medicine.
In my administrative role, I have the privilege of helping to expand the professional education and training of my colleagues. I have a passion for medical education, and we have been working to develop interprofessional educational opportunities within our section. I have had time to think about the imprint of NPs and PAs in academic medicine, and how we can continue to meet the professional educational needs of our section while improving the care of our patients.
What are the most challenging aspects of practicing hospital medicine?
The volume of diagnoses that we are expected to manage on a daily basis can be challenging. This challenges you to continue learning. The complexity of discharge planning, particularly for patients in underserved communities, can also be challenging. You have to make sure your patients are ready mentally, physically and emotionally for discharge. As a hospitalist, you are continuously thinking about how to optimize patients to leave your care. For example, patients have different insurance situations, different access to care at home – you are always managing the medical needs of your patient in the context of these other issues.
How does a hospitalist PA work differently from a PA in other care settings?
We are meant to be generalists. We serve as the main provider in owning our patients’ care. A hospitalist PA serves as a cog in the wheel, with connections to specialists, consultants, nurses, social workers, pharmacists, etc., and we are tasked with synthesizing all aspects of patient care to ensure the best outcome.
What has your experience taught you about how NPs and PAs can best fit into hospital medicine groups?
Each hospital medicine group will know how to best integrate their NPs and PAs based on the skillsets of their NPs and PAs, and the needs of the section and the hospital. I personally feel that the best way to utilize NPs and PAs is to allow them to own all aspects of patient care and work at the highest scope of practice. By doing this you empower the NP or PA to continue to develop their skill set and set a precedent of collaboration and respect for interprofessional care models within your section’s culture.
Scope of practice for an NP or PA is going to be based on a conglomeration of roles and bylaws. We are certified nationally, and our scope of practice is determined at the state level and the hospital by level. For the individual NP and PA, it really depends on the hospital medicine group, and how well a practice incorporates a sense of collegiality.
What kind of resources do hospitalist PAs need to succeed, either from SHM or from their own institutions?
There are a few key things that need to happen in order for hospital medicine groups to set up their NPs and PAs for success. The first is for PAs to have exposure to inpatient rotations during clinical rotations. A hospital medicine group also should have a very intentional onboarding process for NPs and PAs. They should also establish a culture of acceptance. To do this, they should utilize resources like SHM’s NP/PA Hospital Medicine Onboarding Toolkit and the SHM/American Academy of Physician Assistants Hospitalist Bootcamp On Demand.
Mentoring is also remarkably important. I have been incredibly blessed to have mentors that helped make me into the PA that I am. I could not have done what I did in the field without people taking a chance on me, and it is important to pass that on to the next generation of PAs.
How has COVID-19 changed the practice of hospital medicine, specifically for advanced practice providers?
The pandemic has demonstrated opportunities for teamwork and utilization of NPs and PAs. The COVID pandemic forced everyone to reflect on why they originally got into medicine – to help patients. I think there will be many doors opening for NPs and PAs, and many pathways for leadership.
The hospitalist leadership at the University of Chicago truly identified that we needed to make wellness a main priority during the beginning of the pandemic. We developed a wellness work group that I have been coleading.
What’s on the horizon for NPs and PAs in hospital medicine?
We are seeing significant increases in hospitalist program utilization, so this is a time where NPs and PAs can be advocates for our profession and articulate how we can use our backgrounds and training to build better care models in order to meet the needs of our patients.
I hope we will see more NPs and PAs assuming leadership roles to ensure that our voices are heard. We should also be advocating for more collaboration and teamwork with our MD and DO colleagues.
Do you have any advice for PA students interested in hospital medicine?
I always tell my students that they should be sponges – you are not expected to know everything as a hospitalist PA, but you are expected to continue learning in order to develop into the best PA you can be. Always be open to where your career path can take you. Hospital medicine is a relatively young field within medicine, and the diversity of our field is very exciting looking forward.
Roundtable discussion: The Pluripotent Hospitalist
In honor of National Hospitalist Day, the Society of Hospital Medicine and the Explore the Space podcast are teaming up to bring you a roundtable discussion, featuring a diverse group of hospitalists from all stages in their careers, on Thursday, March 4, at 7 p.m. ET / 4 p.m. PT.
Registration is required. Sign up here.
Hosted by Mark Shapiro, MD, hospitalist and founder, producer, and host of Explore the Space, the roundtable will include:
- Gurpreet Dhaliwal, MD, a clinician-educator and professor of medicine at the University of California, San Francisco. He studies, writes, and speaks about how doctors think – how they make diagnoses, how they develop diagnostic expertise, and what motivates them to improve their practice and the systems in which they work.
- Anika Kumar, MD, FHM, a clinical assistant professor of pediatrics at the Cleveland Clinic Lerner College of Medicine, and a pediatric hospitalist at Cleveland Clinic Children’s. She also serves as the pediatric editor of the Hospitalist, SHM’s monthly news magazine.
- Maylyn S. Martinez, MD, a clinician-researcher and clinical associate at the University of Chicago. Her research focuses on hospital-associated disability and she recently authored a perspectives piece in the Journal of Hospital Medicine with her mentor, Vineet Arora, MD, MHM, on why the COVID-19 pandemic might exacerbate this problem.
- Ndidi Unaka, MD, MEd, an associate professor in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center. Dr. Unaka has served as the associate program director of the pediatric residency program since 2011. She is also the medical director of an inpatient unit that serves as the primary home.
For more information about SHM, please visit hospitalmedicine.org. To learn more about Explore the Space, please visit explorethespaceshow.com.
Register now.
In honor of National Hospitalist Day, the Society of Hospital Medicine and the Explore the Space podcast are teaming up to bring you a roundtable discussion, featuring a diverse group of hospitalists from all stages in their careers, on Thursday, March 4, at 7 p.m. ET / 4 p.m. PT.
Registration is required. Sign up here.
Hosted by Mark Shapiro, MD, hospitalist and founder, producer, and host of Explore the Space, the roundtable will include:
- Gurpreet Dhaliwal, MD, a clinician-educator and professor of medicine at the University of California, San Francisco. He studies, writes, and speaks about how doctors think – how they make diagnoses, how they develop diagnostic expertise, and what motivates them to improve their practice and the systems in which they work.
- Anika Kumar, MD, FHM, a clinical assistant professor of pediatrics at the Cleveland Clinic Lerner College of Medicine, and a pediatric hospitalist at Cleveland Clinic Children’s. She also serves as the pediatric editor of the Hospitalist, SHM’s monthly news magazine.
- Maylyn S. Martinez, MD, a clinician-researcher and clinical associate at the University of Chicago. Her research focuses on hospital-associated disability and she recently authored a perspectives piece in the Journal of Hospital Medicine with her mentor, Vineet Arora, MD, MHM, on why the COVID-19 pandemic might exacerbate this problem.
- Ndidi Unaka, MD, MEd, an associate professor in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center. Dr. Unaka has served as the associate program director of the pediatric residency program since 2011. She is also the medical director of an inpatient unit that serves as the primary home.
For more information about SHM, please visit hospitalmedicine.org. To learn more about Explore the Space, please visit explorethespaceshow.com.
Register now.
In honor of National Hospitalist Day, the Society of Hospital Medicine and the Explore the Space podcast are teaming up to bring you a roundtable discussion, featuring a diverse group of hospitalists from all stages in their careers, on Thursday, March 4, at 7 p.m. ET / 4 p.m. PT.
Registration is required. Sign up here.
Hosted by Mark Shapiro, MD, hospitalist and founder, producer, and host of Explore the Space, the roundtable will include:
- Gurpreet Dhaliwal, MD, a clinician-educator and professor of medicine at the University of California, San Francisco. He studies, writes, and speaks about how doctors think – how they make diagnoses, how they develop diagnostic expertise, and what motivates them to improve their practice and the systems in which they work.
- Anika Kumar, MD, FHM, a clinical assistant professor of pediatrics at the Cleveland Clinic Lerner College of Medicine, and a pediatric hospitalist at Cleveland Clinic Children’s. She also serves as the pediatric editor of the Hospitalist, SHM’s monthly news magazine.
- Maylyn S. Martinez, MD, a clinician-researcher and clinical associate at the University of Chicago. Her research focuses on hospital-associated disability and she recently authored a perspectives piece in the Journal of Hospital Medicine with her mentor, Vineet Arora, MD, MHM, on why the COVID-19 pandemic might exacerbate this problem.
- Ndidi Unaka, MD, MEd, an associate professor in the division of hospital medicine at Cincinnati Children’s Hospital Medical Center. Dr. Unaka has served as the associate program director of the pediatric residency program since 2011. She is also the medical director of an inpatient unit that serves as the primary home.
For more information about SHM, please visit hospitalmedicine.org. To learn more about Explore the Space, please visit explorethespaceshow.com.
Register now.
Inpatient telemedicine can help address hospitalist pain points
COVID-19 has increased confidence in the technology
Since the advent of COVID-19, health care has seen an unprecedented rise in virtual health. Telemedicine has come to the forefront of our conversations, and there are many speculations around its future state. One such discussion is around the sustainability and expansion of inpatient telemedicine programs post COVID, and if – and how – it is going to be helpful for health care.
Consider the following scenarios:
Scenario 1
A patient presents to an emergency department of a small community hospital. He needs to be seen by a specialist, but (s)he is not available, so patient gets transferred out to the ED of a different hospital several miles away from his hometown.
He is evaluated in the second ED by the specialist, has repeat testing done – some of those tests were already completed at the first hospital. After evaluating him, the specialist recommends that he does not need to be admitted to the hospital and can be safely followed up as an outpatient. The patient does not require any further intervention and is discharged from the ED.
Scenario 2
Dr. N is a hospitalist in a rural hospital that does not have intensivist support at night. She works 7 on/7 off and is on call 24/7 during her “on” week. Dr. N cannot be physically present in the hospital 24/7. She receives messages from the hospital around the clock and feels that this call schedule is no longer sustainable. She doesn’t feel comfortable admitting patients in the ICU who come to the hospital at night without physically seeing them and without ICU backup. Therefore, some of the patients who are sick enough to be admitted in ICU for closer monitoring but can be potentially handled in this rural hospital get transferred out to a different hospital.
Dr. N has been asking the hospital to provide her intensivist back up at night and to give her some flexibility in the call schedule. However, from hospital’s perspective, the volume isn’t high enough to hire a dedicated nocturnist, and because the hospital is in the small rural area, it is having a hard time attracting more intensivists. After multiple conversations between both parties, Dr. N finally resigns.
Scenario 3
Dr. A is a specialist who is on call covering different hospitals and seeing patients in clinic. His call is getting busier. He has received many new consults and also has to follow up on his other patients in hospital who he saw a day prior.
Dr. A started receiving many pages from the hospitals – some of his patients and their families are anxiously waiting on him so that he can let them go home once he sees them, while some are waiting to know what the next steps and plan of action are. He ends up canceling some of his clinic patients who had scheduled an appointment with him 3, 4, or even 5 months ago. It’s already afternoon.
Dr. A now drives to one hospital, sees his new consults, orders tests which may or may not get results the same day, follows up on other patients, reviews their test results, modifies treatment plans for some while clearing other patients for discharge. He then drives to the other hospital and follows the same process. Some of the patients aren’t happy because of the long wait, a few couldn’t arrange for the ride to go home and ended up staying in hospital 1 extra night, while the ER is getting backlogged waiting on discharges.
These scenarios highlight some of the important and prevalent pain points in health care as shown in Figure 1.
Scenario 1 and part of scenario 2 describe what is called potentially avoidable interfacility transfers. One study showed that around 8% of transferred patients (transferred from one ED to another) were discharged after ED evaluation in the second hospital, meaning they could have been retained locally without necessarily getting transferred if they could have been evaluated by the specialist.1
Transferring a patient from one hospital to another isn’t as simple as picking up a person from point A and dropping him off at point B. Rather it’s a very complicated, high-risk, capital-intensive, and time-consuming process that leads not only to excessive cost involved around transfer but also adds additional stress and burden on the patient and family. In these scenarios, having a specialist available via teleconsult could have eliminated much of this hassle and cost, allowing the patient to stay locally close to family and get access to necessary medical expertise from any part of the country in a timely manner.
Scenario 2 talks about the recruitment and retention challenges in low-volume, low-resourced locations because of call schedule and the lack of specialty support. It is reported in one study that 19% of common hospitalist admissions happen between 7:00 p.m. and 7:00 a.m. Eighty percent of admissions occurred prior to midnight. Nonrural facilities averaged 6.69 hospitalist admissions per night in that study, whereas rural facilities averaged 1.35 admissions.2 It’s like a double-edged sword for such facilities. While having a dedicated nocturnist is not a sustainable model for these hospitals, not having adequate support at night impacts physician wellness, which is already costing hospitals billions of dollars as well as leading to physician turnover: It could cost a hospital somewhere between $500,000 and $1 million to replace just one physician.3 Hence, the potential exists for a telehospitalist program in these settings to address this dilemma.
Scenario 3 sheds light on the operational issues resulting in reduced patient satisfaction and lost revenues, both on the outpatient and inpatient sides by cancellation of office visits and ED backlog. Telemedicine use in these situations can improve the turnaround time of physicians who can see some of those patients while staying at one location as they wait on other patients to show up in the clinic or wait on the operation room crew, or the procedure kit etcetera, hence improving the length of stay, ED throughput, patient satisfaction, and quality of care. This also can improve overall workflow and the wellness of physicians.
One common outcome in all these scenarios is emergency department overcrowding. There have been multiple studies that suggest that ED overcrowding can result in increased costs, lost revenues, and poor clinical outcomes, including delayed administration of antibiotics, delayed administration of analgesics to suffering patients, increased hospital length of stay, and even increased mortality.4-6 A crowded ED limits the ability of an institution to accept referrals and increases medicolegal risks. (See Figure 2.)
Another study showed that a 1-hour reduction in ED boarding time would result in over $9,000 of additional revenue by reducing ambulance diversion and the number of patients who left without being seen.7 Another found that using tele-emergency services can potentially result in net savings of $3,823 per avoided transfer, while accounting for the costs related to tele-emergency technology, hospital revenues, and patient-associated savings.8
There are other instances where gaps in staffing and cracks in workflow can have a negative impact on hospital operations. For example, the busier hospitals that do have a dedicated nocturnist also struggle with physician retention, since such hospitals have higher volumes and higher cross-coverage needs, and are therefore hard to manage by just one single physician at night. Since these are temporary surges, hiring another full-time nocturnist is not a viable option for the hospitals and is considered an expense in many places.
Similarly, during day shift, if a physician goes on vacation or there are surges in patient volumes, hiring a locum tenens hospitalist can be an expensive option, since the cost also includes travel and lodging. In many instances, hiring locum tenens in a given time frame is also not possible, and it leaves the physicians short staffed, fueling both physicians’ and patients’ dissatisfaction and leading to other operational and safety challenges, which I highlighted above.
Telemedicine services in these situations can provide cross-coverage while nocturnists can focus on admissions and other acute issues. Also, when physicians are on vacation or there is surge capacity (that can be forecast by using various predictive analytics models), hospitals can make plans accordingly and make use of telemedicine services. For example, Providence St. Joseph Health reported improvement in timeliness and efficiency of care after implementation of a telehospitalist program. Their 2-year study at a partner site showed a 59% improvement in patients admitted prior to midnight, about $547,000 improvement in first-day revenue capture, an increase in total revenue days and comparable patient experience scores, and a substantial increase in inpatient census and case mix index.9
Other institutions have successfully implemented some inpatient telemedicine programs – such as telepsych, telestroke, and tele-ICU – and some have also reported positive outcomes in terms of patient satisfaction, improved access, reduced length of stay in the ED, and improved quality metrics. Emory Healthcare in Atlanta reported $4.6 million savings in Medicare costs over a 15-month period from adopting a telemedicine model in the ICU, and a reduction in 60-day readmissions by 2.1%.10 Similarly, another study showed that one large health care center improved its direct contribution margins by 376% (from $7.9 million to $37.7 million) because of increased case volume, shorter lengths of stay, and higher case revenue relative to direct costs. When combined with a logistics center, they reported improved contribution margins by 665% (from $7.9 million to $60.6 million).11
There are barriers to the integration and implementation of inpatient telemedicine, including regulations, reimbursement, physician licensing, adoption of technology, and trust among staff and patients. However, I am cautiously optimistic that increased use of telehealth during the COVID-19 pandemic has allowed patients, physicians, nurses, and health care workers and leaders to gain experience with this technology, which will help them gain confidence and reduce hesitation in adapting to this new digital platform. Ultimately, the extent to which telemedicine is able to positively impact patient care will revolve around overcoming these barriers, likely through an evolution of both the technology itself and the attitudes and regulations surrounding it.
I do not suggest that telemedicine should replace the in-person encounter, but it can be implemented and used successfully in addressing the pain points in U.S. health care. (See Figure 3.)
To that end, the purpose of this article is to spark discussion around different ways of implementing telemedicine in inpatient settings to solve many of the challenges that health care faces today.
Dr. Zia is an internal medicine board-certified physician, serving as a hospitalist and physician adviser in a medically underserved area. She has also served as interim medical director of the department of hospital medicine, and medical staff president, at SIH Herrin Hospital, in Herrin, Ill., part of Southern Illinois Healthcare. She has a special interest in improving access to health care in physician shortage areas.
References
1. Kindermann DR et al. Emergency department transfers and transfer relationships in United States hospitals. Acad Emerg Med. 2015 Feb;22(2):157-65.
2. Sanders RB et al. New hospital telemedicine services: Potential market for a nighttime hospitalist service. Telemed J E Health. 2014 Oct 1;20(10):902-8.
3. Shanafelt T et al. The business case for investing in physician well-being. JAMA Intern Med. 2017;177(12):1826-32.
4. Pines JM et al. The impact of emergency department crowding measures on time to antibiotics for patients with community-acquired pneumonia. Ann Emerg Med. 2007 Nov;50(5):510-6.
5. Pines JM and Hollander JE. Emergency department crowding is associated with poor care for patients with severe pain. Ann Emerg Med. 2008 Jan;51(1):1-5.
6. Chalfin DB et al. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med. 2007 Jun;35(6):1477-83.
7. Pines JM et al. The financial consequences of lost demand and reducing boarding in hospital emergency departments. Ann Emerg Med. 2011 Oct;58(4):331-40.
8. Natafgi N et al. Using tele-emergency to avoid patient transfers in rural emergency. J Telemed Telecare. 2018 Apri;24(3):193-201.
9. Providence.org/telehealthhospitalistcasestudy.
10. Woodruff Health Sciences Center. CMS report: eICU program reduced hospital stays, saved millions, eased provider shortage. 2017 Apr 5.
11. Lilly CM et al. ICU telemedicine program financial outcomes. Chest. 2017 Feb;151(2):286-97.
COVID-19 has increased confidence in the technology
COVID-19 has increased confidence in the technology
Since the advent of COVID-19, health care has seen an unprecedented rise in virtual health. Telemedicine has come to the forefront of our conversations, and there are many speculations around its future state. One such discussion is around the sustainability and expansion of inpatient telemedicine programs post COVID, and if – and how – it is going to be helpful for health care.
Consider the following scenarios:
Scenario 1
A patient presents to an emergency department of a small community hospital. He needs to be seen by a specialist, but (s)he is not available, so patient gets transferred out to the ED of a different hospital several miles away from his hometown.
He is evaluated in the second ED by the specialist, has repeat testing done – some of those tests were already completed at the first hospital. After evaluating him, the specialist recommends that he does not need to be admitted to the hospital and can be safely followed up as an outpatient. The patient does not require any further intervention and is discharged from the ED.
Scenario 2
Dr. N is a hospitalist in a rural hospital that does not have intensivist support at night. She works 7 on/7 off and is on call 24/7 during her “on” week. Dr. N cannot be physically present in the hospital 24/7. She receives messages from the hospital around the clock and feels that this call schedule is no longer sustainable. She doesn’t feel comfortable admitting patients in the ICU who come to the hospital at night without physically seeing them and without ICU backup. Therefore, some of the patients who are sick enough to be admitted in ICU for closer monitoring but can be potentially handled in this rural hospital get transferred out to a different hospital.
Dr. N has been asking the hospital to provide her intensivist back up at night and to give her some flexibility in the call schedule. However, from hospital’s perspective, the volume isn’t high enough to hire a dedicated nocturnist, and because the hospital is in the small rural area, it is having a hard time attracting more intensivists. After multiple conversations between both parties, Dr. N finally resigns.
Scenario 3
Dr. A is a specialist who is on call covering different hospitals and seeing patients in clinic. His call is getting busier. He has received many new consults and also has to follow up on his other patients in hospital who he saw a day prior.
Dr. A started receiving many pages from the hospitals – some of his patients and their families are anxiously waiting on him so that he can let them go home once he sees them, while some are waiting to know what the next steps and plan of action are. He ends up canceling some of his clinic patients who had scheduled an appointment with him 3, 4, or even 5 months ago. It’s already afternoon.
Dr. A now drives to one hospital, sees his new consults, orders tests which may or may not get results the same day, follows up on other patients, reviews their test results, modifies treatment plans for some while clearing other patients for discharge. He then drives to the other hospital and follows the same process. Some of the patients aren’t happy because of the long wait, a few couldn’t arrange for the ride to go home and ended up staying in hospital 1 extra night, while the ER is getting backlogged waiting on discharges.
These scenarios highlight some of the important and prevalent pain points in health care as shown in Figure 1.

Scenario 1 and part of scenario 2 describe what is called potentially avoidable interfacility transfers. One study showed that around 8% of transferred patients (transferred from one ED to another) were discharged after ED evaluation in the second hospital, meaning they could have been retained locally without necessarily getting transferred if they could have been evaluated by the specialist.1
Transferring a patient from one hospital to another isn’t as simple as picking up a person from point A and dropping him off at point B. Rather it’s a very complicated, high-risk, capital-intensive, and time-consuming process that leads not only to excessive cost involved around transfer but also adds additional stress and burden on the patient and family. In these scenarios, having a specialist available via teleconsult could have eliminated much of this hassle and cost, allowing the patient to stay locally close to family and get access to necessary medical expertise from any part of the country in a timely manner.
Scenario 2 talks about the recruitment and retention challenges in low-volume, low-resourced locations because of call schedule and the lack of specialty support. It is reported in one study that 19% of common hospitalist admissions happen between 7:00 p.m. and 7:00 a.m. Eighty percent of admissions occurred prior to midnight. Nonrural facilities averaged 6.69 hospitalist admissions per night in that study, whereas rural facilities averaged 1.35 admissions.2 It’s like a double-edged sword for such facilities. While having a dedicated nocturnist is not a sustainable model for these hospitals, not having adequate support at night impacts physician wellness, which is already costing hospitals billions of dollars as well as leading to physician turnover: It could cost a hospital somewhere between $500,000 and $1 million to replace just one physician.3 Hence, the potential exists for a telehospitalist program in these settings to address this dilemma.
Scenario 3 sheds light on the operational issues resulting in reduced patient satisfaction and lost revenues, both on the outpatient and inpatient sides by cancellation of office visits and ED backlog. Telemedicine use in these situations can improve the turnaround time of physicians who can see some of those patients while staying at one location as they wait on other patients to show up in the clinic or wait on the operation room crew, or the procedure kit etcetera, hence improving the length of stay, ED throughput, patient satisfaction, and quality of care. This also can improve overall workflow and the wellness of physicians.
One common outcome in all these scenarios is emergency department overcrowding. There have been multiple studies that suggest that ED overcrowding can result in increased costs, lost revenues, and poor clinical outcomes, including delayed administration of antibiotics, delayed administration of analgesics to suffering patients, increased hospital length of stay, and even increased mortality.4-6 A crowded ED limits the ability of an institution to accept referrals and increases medicolegal risks. (See Figure 2.)

Another study showed that a 1-hour reduction in ED boarding time would result in over $9,000 of additional revenue by reducing ambulance diversion and the number of patients who left without being seen.7 Another found that using tele-emergency services can potentially result in net savings of $3,823 per avoided transfer, while accounting for the costs related to tele-emergency technology, hospital revenues, and patient-associated savings.8
There are other instances where gaps in staffing and cracks in workflow can have a negative impact on hospital operations. For example, the busier hospitals that do have a dedicated nocturnist also struggle with physician retention, since such hospitals have higher volumes and higher cross-coverage needs, and are therefore hard to manage by just one single physician at night. Since these are temporary surges, hiring another full-time nocturnist is not a viable option for the hospitals and is considered an expense in many places.
Similarly, during day shift, if a physician goes on vacation or there are surges in patient volumes, hiring a locum tenens hospitalist can be an expensive option, since the cost also includes travel and lodging. In many instances, hiring locum tenens in a given time frame is also not possible, and it leaves the physicians short staffed, fueling both physicians’ and patients’ dissatisfaction and leading to other operational and safety challenges, which I highlighted above.
Telemedicine services in these situations can provide cross-coverage while nocturnists can focus on admissions and other acute issues. Also, when physicians are on vacation or there is surge capacity (that can be forecast by using various predictive analytics models), hospitals can make plans accordingly and make use of telemedicine services. For example, Providence St. Joseph Health reported improvement in timeliness and efficiency of care after implementation of a telehospitalist program. Their 2-year study at a partner site showed a 59% improvement in patients admitted prior to midnight, about $547,000 improvement in first-day revenue capture, an increase in total revenue days and comparable patient experience scores, and a substantial increase in inpatient census and case mix index.9
Other institutions have successfully implemented some inpatient telemedicine programs – such as telepsych, telestroke, and tele-ICU – and some have also reported positive outcomes in terms of patient satisfaction, improved access, reduced length of stay in the ED, and improved quality metrics. Emory Healthcare in Atlanta reported $4.6 million savings in Medicare costs over a 15-month period from adopting a telemedicine model in the ICU, and a reduction in 60-day readmissions by 2.1%.10 Similarly, another study showed that one large health care center improved its direct contribution margins by 376% (from $7.9 million to $37.7 million) because of increased case volume, shorter lengths of stay, and higher case revenue relative to direct costs. When combined with a logistics center, they reported improved contribution margins by 665% (from $7.9 million to $60.6 million).11
There are barriers to the integration and implementation of inpatient telemedicine, including regulations, reimbursement, physician licensing, adoption of technology, and trust among staff and patients. However, I am cautiously optimistic that increased use of telehealth during the COVID-19 pandemic has allowed patients, physicians, nurses, and health care workers and leaders to gain experience with this technology, which will help them gain confidence and reduce hesitation in adapting to this new digital platform. Ultimately, the extent to which telemedicine is able to positively impact patient care will revolve around overcoming these barriers, likely through an evolution of both the technology itself and the attitudes and regulations surrounding it.
I do not suggest that telemedicine should replace the in-person encounter, but it can be implemented and used successfully in addressing the pain points in U.S. health care. (See Figure 3.)

To that end, the purpose of this article is to spark discussion around different ways of implementing telemedicine in inpatient settings to solve many of the challenges that health care faces today.
Dr. Zia is an internal medicine board-certified physician, serving as a hospitalist and physician adviser in a medically underserved area. She has also served as interim medical director of the department of hospital medicine, and medical staff president, at SIH Herrin Hospital, in Herrin, Ill., part of Southern Illinois Healthcare. She has a special interest in improving access to health care in physician shortage areas.
References
1. Kindermann DR et al. Emergency department transfers and transfer relationships in United States hospitals. Acad Emerg Med. 2015 Feb;22(2):157-65.
2. Sanders RB et al. New hospital telemedicine services: Potential market for a nighttime hospitalist service. Telemed J E Health. 2014 Oct 1;20(10):902-8.
3. Shanafelt T et al. The business case for investing in physician well-being. JAMA Intern Med. 2017;177(12):1826-32.
4. Pines JM et al. The impact of emergency department crowding measures on time to antibiotics for patients with community-acquired pneumonia. Ann Emerg Med. 2007 Nov;50(5):510-6.
5. Pines JM and Hollander JE. Emergency department crowding is associated with poor care for patients with severe pain. Ann Emerg Med. 2008 Jan;51(1):1-5.
6. Chalfin DB et al. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med. 2007 Jun;35(6):1477-83.
7. Pines JM et al. The financial consequences of lost demand and reducing boarding in hospital emergency departments. Ann Emerg Med. 2011 Oct;58(4):331-40.
8. Natafgi N et al. Using tele-emergency to avoid patient transfers in rural emergency. J Telemed Telecare. 2018 Apri;24(3):193-201.
9. Providence.org/telehealthhospitalistcasestudy.
10. Woodruff Health Sciences Center. CMS report: eICU program reduced hospital stays, saved millions, eased provider shortage. 2017 Apr 5.
11. Lilly CM et al. ICU telemedicine program financial outcomes. Chest. 2017 Feb;151(2):286-97.
Since the advent of COVID-19, health care has seen an unprecedented rise in virtual health. Telemedicine has come to the forefront of our conversations, and there are many speculations around its future state. One such discussion is around the sustainability and expansion of inpatient telemedicine programs post COVID, and if – and how – it is going to be helpful for health care.
Consider the following scenarios:
Scenario 1
A patient presents to an emergency department of a small community hospital. He needs to be seen by a specialist, but (s)he is not available, so patient gets transferred out to the ED of a different hospital several miles away from his hometown.
He is evaluated in the second ED by the specialist, has repeat testing done – some of those tests were already completed at the first hospital. After evaluating him, the specialist recommends that he does not need to be admitted to the hospital and can be safely followed up as an outpatient. The patient does not require any further intervention and is discharged from the ED.
Scenario 2
Dr. N is a hospitalist in a rural hospital that does not have intensivist support at night. She works 7 on/7 off and is on call 24/7 during her “on” week. Dr. N cannot be physically present in the hospital 24/7. She receives messages from the hospital around the clock and feels that this call schedule is no longer sustainable. She doesn’t feel comfortable admitting patients in the ICU who come to the hospital at night without physically seeing them and without ICU backup. Therefore, some of the patients who are sick enough to be admitted in ICU for closer monitoring but can be potentially handled in this rural hospital get transferred out to a different hospital.
Dr. N has been asking the hospital to provide her intensivist back up at night and to give her some flexibility in the call schedule. However, from hospital’s perspective, the volume isn’t high enough to hire a dedicated nocturnist, and because the hospital is in the small rural area, it is having a hard time attracting more intensivists. After multiple conversations between both parties, Dr. N finally resigns.
Scenario 3
Dr. A is a specialist who is on call covering different hospitals and seeing patients in clinic. His call is getting busier. He has received many new consults and also has to follow up on his other patients in hospital who he saw a day prior.
Dr. A started receiving many pages from the hospitals – some of his patients and their families are anxiously waiting on him so that he can let them go home once he sees them, while some are waiting to know what the next steps and plan of action are. He ends up canceling some of his clinic patients who had scheduled an appointment with him 3, 4, or even 5 months ago. It’s already afternoon.
Dr. A now drives to one hospital, sees his new consults, orders tests which may or may not get results the same day, follows up on other patients, reviews their test results, modifies treatment plans for some while clearing other patients for discharge. He then drives to the other hospital and follows the same process. Some of the patients aren’t happy because of the long wait, a few couldn’t arrange for the ride to go home and ended up staying in hospital 1 extra night, while the ER is getting backlogged waiting on discharges.
These scenarios highlight some of the important and prevalent pain points in health care as shown in Figure 1.

Scenario 1 and part of scenario 2 describe what is called potentially avoidable interfacility transfers. One study showed that around 8% of transferred patients (transferred from one ED to another) were discharged after ED evaluation in the second hospital, meaning they could have been retained locally without necessarily getting transferred if they could have been evaluated by the specialist.1
Transferring a patient from one hospital to another isn’t as simple as picking up a person from point A and dropping him off at point B. Rather it’s a very complicated, high-risk, capital-intensive, and time-consuming process that leads not only to excessive cost involved around transfer but also adds additional stress and burden on the patient and family. In these scenarios, having a specialist available via teleconsult could have eliminated much of this hassle and cost, allowing the patient to stay locally close to family and get access to necessary medical expertise from any part of the country in a timely manner.
Scenario 2 talks about the recruitment and retention challenges in low-volume, low-resourced locations because of call schedule and the lack of specialty support. It is reported in one study that 19% of common hospitalist admissions happen between 7:00 p.m. and 7:00 a.m. Eighty percent of admissions occurred prior to midnight. Nonrural facilities averaged 6.69 hospitalist admissions per night in that study, whereas rural facilities averaged 1.35 admissions.2 It’s like a double-edged sword for such facilities. While having a dedicated nocturnist is not a sustainable model for these hospitals, not having adequate support at night impacts physician wellness, which is already costing hospitals billions of dollars as well as leading to physician turnover: It could cost a hospital somewhere between $500,000 and $1 million to replace just one physician.3 Hence, the potential exists for a telehospitalist program in these settings to address this dilemma.
Scenario 3 sheds light on the operational issues resulting in reduced patient satisfaction and lost revenues, both on the outpatient and inpatient sides by cancellation of office visits and ED backlog. Telemedicine use in these situations can improve the turnaround time of physicians who can see some of those patients while staying at one location as they wait on other patients to show up in the clinic or wait on the operation room crew, or the procedure kit etcetera, hence improving the length of stay, ED throughput, patient satisfaction, and quality of care. This also can improve overall workflow and the wellness of physicians.
One common outcome in all these scenarios is emergency department overcrowding. There have been multiple studies that suggest that ED overcrowding can result in increased costs, lost revenues, and poor clinical outcomes, including delayed administration of antibiotics, delayed administration of analgesics to suffering patients, increased hospital length of stay, and even increased mortality.4-6 A crowded ED limits the ability of an institution to accept referrals and increases medicolegal risks. (See Figure 2.)

Another study showed that a 1-hour reduction in ED boarding time would result in over $9,000 of additional revenue by reducing ambulance diversion and the number of patients who left without being seen.7 Another found that using tele-emergency services can potentially result in net savings of $3,823 per avoided transfer, while accounting for the costs related to tele-emergency technology, hospital revenues, and patient-associated savings.8
There are other instances where gaps in staffing and cracks in workflow can have a negative impact on hospital operations. For example, the busier hospitals that do have a dedicated nocturnist also struggle with physician retention, since such hospitals have higher volumes and higher cross-coverage needs, and are therefore hard to manage by just one single physician at night. Since these are temporary surges, hiring another full-time nocturnist is not a viable option for the hospitals and is considered an expense in many places.
Similarly, during day shift, if a physician goes on vacation or there are surges in patient volumes, hiring a locum tenens hospitalist can be an expensive option, since the cost also includes travel and lodging. In many instances, hiring locum tenens in a given time frame is also not possible, and it leaves the physicians short staffed, fueling both physicians’ and patients’ dissatisfaction and leading to other operational and safety challenges, which I highlighted above.
Telemedicine services in these situations can provide cross-coverage while nocturnists can focus on admissions and other acute issues. Also, when physicians are on vacation or there is surge capacity (that can be forecast by using various predictive analytics models), hospitals can make plans accordingly and make use of telemedicine services. For example, Providence St. Joseph Health reported improvement in timeliness and efficiency of care after implementation of a telehospitalist program. Their 2-year study at a partner site showed a 59% improvement in patients admitted prior to midnight, about $547,000 improvement in first-day revenue capture, an increase in total revenue days and comparable patient experience scores, and a substantial increase in inpatient census and case mix index.9
Other institutions have successfully implemented some inpatient telemedicine programs – such as telepsych, telestroke, and tele-ICU – and some have also reported positive outcomes in terms of patient satisfaction, improved access, reduced length of stay in the ED, and improved quality metrics. Emory Healthcare in Atlanta reported $4.6 million savings in Medicare costs over a 15-month period from adopting a telemedicine model in the ICU, and a reduction in 60-day readmissions by 2.1%.10 Similarly, another study showed that one large health care center improved its direct contribution margins by 376% (from $7.9 million to $37.7 million) because of increased case volume, shorter lengths of stay, and higher case revenue relative to direct costs. When combined with a logistics center, they reported improved contribution margins by 665% (from $7.9 million to $60.6 million).11
There are barriers to the integration and implementation of inpatient telemedicine, including regulations, reimbursement, physician licensing, adoption of technology, and trust among staff and patients. However, I am cautiously optimistic that increased use of telehealth during the COVID-19 pandemic has allowed patients, physicians, nurses, and health care workers and leaders to gain experience with this technology, which will help them gain confidence and reduce hesitation in adapting to this new digital platform. Ultimately, the extent to which telemedicine is able to positively impact patient care will revolve around overcoming these barriers, likely through an evolution of both the technology itself and the attitudes and regulations surrounding it.
I do not suggest that telemedicine should replace the in-person encounter, but it can be implemented and used successfully in addressing the pain points in U.S. health care. (See Figure 3.)

To that end, the purpose of this article is to spark discussion around different ways of implementing telemedicine in inpatient settings to solve many of the challenges that health care faces today.
Dr. Zia is an internal medicine board-certified physician, serving as a hospitalist and physician adviser in a medically underserved area. She has also served as interim medical director of the department of hospital medicine, and medical staff president, at SIH Herrin Hospital, in Herrin, Ill., part of Southern Illinois Healthcare. She has a special interest in improving access to health care in physician shortage areas.
References
1. Kindermann DR et al. Emergency department transfers and transfer relationships in United States hospitals. Acad Emerg Med. 2015 Feb;22(2):157-65.
2. Sanders RB et al. New hospital telemedicine services: Potential market for a nighttime hospitalist service. Telemed J E Health. 2014 Oct 1;20(10):902-8.
3. Shanafelt T et al. The business case for investing in physician well-being. JAMA Intern Med. 2017;177(12):1826-32.
4. Pines JM et al. The impact of emergency department crowding measures on time to antibiotics for patients with community-acquired pneumonia. Ann Emerg Med. 2007 Nov;50(5):510-6.
5. Pines JM and Hollander JE. Emergency department crowding is associated with poor care for patients with severe pain. Ann Emerg Med. 2008 Jan;51(1):1-5.
6. Chalfin DB et al. Impact of delayed transfer of critically ill patients from the emergency department to the intensive care unit. Crit Care Med. 2007 Jun;35(6):1477-83.
7. Pines JM et al. The financial consequences of lost demand and reducing boarding in hospital emergency departments. Ann Emerg Med. 2011 Oct;58(4):331-40.
8. Natafgi N et al. Using tele-emergency to avoid patient transfers in rural emergency. J Telemed Telecare. 2018 Apri;24(3):193-201.
9. Providence.org/telehealthhospitalistcasestudy.
10. Woodruff Health Sciences Center. CMS report: eICU program reduced hospital stays, saved millions, eased provider shortage. 2017 Apr 5.
11. Lilly CM et al. ICU telemedicine program financial outcomes. Chest. 2017 Feb;151(2):286-97.
Late-window stroke thrombolysis not linked to clot migration
In patients with acute ischemic stroke, the use of thrombolysis in the late window of 4.5-9 hours after symptom onset was not associated with an increase in clot migration that would cause reduced clot accessibility to endovascular therapy, a new analysis from the EXTEND trial shows.
“There was no significant difference in the incidence of clot migration leading to clot inaccessibility in patients who received placebo or (intravenous) thrombolysis,” the authors report.
“Our results found no convincing evidence against the use of bridging thrombolysis before endovascular therapy in patients with acute ischemic stroke who present outside the 4.5-hour window,” they conclude.
“This information is important because it provides some comfort for neurointerventionists that IV thrombolysis does not unduly increase the risk of clot migration,” senior author, Bernard Yan, DMedSci, FRACP, told this news organization.
The study was published online in Stroke on Feb. 16.
The Australian researchers explain that endovascular thrombectomy is the standard of care in patients presenting with acute ischemic stroke caused by large-vessel occlusion, and current treatment guidelines recommend bridging thrombolysis for all patients receiving thrombectomy within the 4.5-hour time window.
While thrombectomy is also recommended in selected patients up to 24 hours after onset of symptoms, it remains unclear whether thrombolysis pretreatment should be administered in this setting.
One of the issues that might affect use of thrombolysis is distal clot migration. As proximal clot location is a crucial factor determining suitability for endovascular clot retrieval, distal migration may prevent successful thrombectomy, they note.
“Clot migration can happen any time and makes life more difficult for the neurointerventionist who performs the endovascular clot retrieval,” added Dr. Yan, who is a neurologist and neurointerventionist at the Royal Melbourne Hospital, Australia.
In the current paper, the researchers report a retrospective analysis of data from the EXTEND trial of late thrombolysis, defined as 4.5-9 hours after symptom onset, to investigate the association between thrombolysis and clot migration leading to clot irretrievability.
The analysis included a total of 220 patients (109 patients in the placebo group and 111 in the thrombolysis group).
Results showed that retrievable clot was seen on baseline imaging in 69% of patients in the placebo group and 61% in the thrombolysis group. Clot resolution occurred in 28% of patients in the placebo group and 50% in the thrombolysis group.
No significant difference was observed in the incidence of clot migration leading to inaccessibility between groups. Clot migration from a retrievable to nonretrievable location occurred in 19% of the placebo group and 14% of the thrombolysis group, with an odds ratio for clot migration in the thrombolysis group of 0.70 (95% confidence interval, 0.35-1.44). This outcome was consistent across subgroups.
The researchers note that, to their knowledge, this is the first randomized controlled study to assess the effect of thrombolysis on clot migration and accessibility in an extended time window.
They acknowledge that a limitation of this study is that they only assessed clot migration from a retrievable to a nonretrievable location; therefore, the true frequency of any clot migration occurring was likely to be higher, and this could explain why other reports have found higher odds ratios of clot migration.
But they point out that they chose to limit their analysis in this way specifically to guide decision-making regarding bridging thrombolysis incorporating endovascular therapy in the extended time window.
“The findings of this study are highly relevant in the current clinical environment, where there are multiple ongoing trials looking at removing thrombolysis pretreatment within the 4.5-hour time window in thrombectomy patients,” the authors write.
“We have demonstrated that thrombolysis in the 4.5- to 9-hour window is not associated with reduced clot accessibility, and this information will be useful in future trial designs incorporating this extended time window,” they add.
Commenting on the study for this news organization, Michael Hill, MD, University of Calgary (Alta.), said: “Thrombus migration does happen and is likely part of the natural history of ischemic stroke, which may be influenced by therapeutics such as thrombolysis. This paper’s top-line result is that thrombus migration occurs in both treated and untreated groups – and therefore that this is really an observation of natural history.”
Dr. Hill says that, at present, patients should be treated with thrombolysis before endovascular therapy if they are eligible, and these results do not change that recommendation.
“The results of the ongoing trials comparing direct thrombectomy with thrombolysis plus thrombectomy will help to understand the potential clinical outcome relevance of this phenomenon,” he added.
The EXTEND trial was supported by grants from the Australian National Health and Medical Research Council of Australia and the Commonwealth Scientific and Industrial Research Organization Flagship Program. Dr. Yan reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with acute ischemic stroke, the use of thrombolysis in the late window of 4.5-9 hours after symptom onset was not associated with an increase in clot migration that would cause reduced clot accessibility to endovascular therapy, a new analysis from the EXTEND trial shows.
“There was no significant difference in the incidence of clot migration leading to clot inaccessibility in patients who received placebo or (intravenous) thrombolysis,” the authors report.
“Our results found no convincing evidence against the use of bridging thrombolysis before endovascular therapy in patients with acute ischemic stroke who present outside the 4.5-hour window,” they conclude.
“This information is important because it provides some comfort for neurointerventionists that IV thrombolysis does not unduly increase the risk of clot migration,” senior author, Bernard Yan, DMedSci, FRACP, told this news organization.
The study was published online in Stroke on Feb. 16.
The Australian researchers explain that endovascular thrombectomy is the standard of care in patients presenting with acute ischemic stroke caused by large-vessel occlusion, and current treatment guidelines recommend bridging thrombolysis for all patients receiving thrombectomy within the 4.5-hour time window.
While thrombectomy is also recommended in selected patients up to 24 hours after onset of symptoms, it remains unclear whether thrombolysis pretreatment should be administered in this setting.
One of the issues that might affect use of thrombolysis is distal clot migration. As proximal clot location is a crucial factor determining suitability for endovascular clot retrieval, distal migration may prevent successful thrombectomy, they note.
“Clot migration can happen any time and makes life more difficult for the neurointerventionist who performs the endovascular clot retrieval,” added Dr. Yan, who is a neurologist and neurointerventionist at the Royal Melbourne Hospital, Australia.
In the current paper, the researchers report a retrospective analysis of data from the EXTEND trial of late thrombolysis, defined as 4.5-9 hours after symptom onset, to investigate the association between thrombolysis and clot migration leading to clot irretrievability.
The analysis included a total of 220 patients (109 patients in the placebo group and 111 in the thrombolysis group).
Results showed that retrievable clot was seen on baseline imaging in 69% of patients in the placebo group and 61% in the thrombolysis group. Clot resolution occurred in 28% of patients in the placebo group and 50% in the thrombolysis group.
No significant difference was observed in the incidence of clot migration leading to inaccessibility between groups. Clot migration from a retrievable to nonretrievable location occurred in 19% of the placebo group and 14% of the thrombolysis group, with an odds ratio for clot migration in the thrombolysis group of 0.70 (95% confidence interval, 0.35-1.44). This outcome was consistent across subgroups.
The researchers note that, to their knowledge, this is the first randomized controlled study to assess the effect of thrombolysis on clot migration and accessibility in an extended time window.
They acknowledge that a limitation of this study is that they only assessed clot migration from a retrievable to a nonretrievable location; therefore, the true frequency of any clot migration occurring was likely to be higher, and this could explain why other reports have found higher odds ratios of clot migration.
But they point out that they chose to limit their analysis in this way specifically to guide decision-making regarding bridging thrombolysis incorporating endovascular therapy in the extended time window.
“The findings of this study are highly relevant in the current clinical environment, where there are multiple ongoing trials looking at removing thrombolysis pretreatment within the 4.5-hour time window in thrombectomy patients,” the authors write.
“We have demonstrated that thrombolysis in the 4.5- to 9-hour window is not associated with reduced clot accessibility, and this information will be useful in future trial designs incorporating this extended time window,” they add.
Commenting on the study for this news organization, Michael Hill, MD, University of Calgary (Alta.), said: “Thrombus migration does happen and is likely part of the natural history of ischemic stroke, which may be influenced by therapeutics such as thrombolysis. This paper’s top-line result is that thrombus migration occurs in both treated and untreated groups – and therefore that this is really an observation of natural history.”
Dr. Hill says that, at present, patients should be treated with thrombolysis before endovascular therapy if they are eligible, and these results do not change that recommendation.
“The results of the ongoing trials comparing direct thrombectomy with thrombolysis plus thrombectomy will help to understand the potential clinical outcome relevance of this phenomenon,” he added.
The EXTEND trial was supported by grants from the Australian National Health and Medical Research Council of Australia and the Commonwealth Scientific and Industrial Research Organization Flagship Program. Dr. Yan reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In patients with acute ischemic stroke, the use of thrombolysis in the late window of 4.5-9 hours after symptom onset was not associated with an increase in clot migration that would cause reduced clot accessibility to endovascular therapy, a new analysis from the EXTEND trial shows.
“There was no significant difference in the incidence of clot migration leading to clot inaccessibility in patients who received placebo or (intravenous) thrombolysis,” the authors report.
“Our results found no convincing evidence against the use of bridging thrombolysis before endovascular therapy in patients with acute ischemic stroke who present outside the 4.5-hour window,” they conclude.
“This information is important because it provides some comfort for neurointerventionists that IV thrombolysis does not unduly increase the risk of clot migration,” senior author, Bernard Yan, DMedSci, FRACP, told this news organization.
The study was published online in Stroke on Feb. 16.
The Australian researchers explain that endovascular thrombectomy is the standard of care in patients presenting with acute ischemic stroke caused by large-vessel occlusion, and current treatment guidelines recommend bridging thrombolysis for all patients receiving thrombectomy within the 4.5-hour time window.
While thrombectomy is also recommended in selected patients up to 24 hours after onset of symptoms, it remains unclear whether thrombolysis pretreatment should be administered in this setting.
One of the issues that might affect use of thrombolysis is distal clot migration. As proximal clot location is a crucial factor determining suitability for endovascular clot retrieval, distal migration may prevent successful thrombectomy, they note.
“Clot migration can happen any time and makes life more difficult for the neurointerventionist who performs the endovascular clot retrieval,” added Dr. Yan, who is a neurologist and neurointerventionist at the Royal Melbourne Hospital, Australia.
In the current paper, the researchers report a retrospective analysis of data from the EXTEND trial of late thrombolysis, defined as 4.5-9 hours after symptom onset, to investigate the association between thrombolysis and clot migration leading to clot irretrievability.
The analysis included a total of 220 patients (109 patients in the placebo group and 111 in the thrombolysis group).
Results showed that retrievable clot was seen on baseline imaging in 69% of patients in the placebo group and 61% in the thrombolysis group. Clot resolution occurred in 28% of patients in the placebo group and 50% in the thrombolysis group.
No significant difference was observed in the incidence of clot migration leading to inaccessibility between groups. Clot migration from a retrievable to nonretrievable location occurred in 19% of the placebo group and 14% of the thrombolysis group, with an odds ratio for clot migration in the thrombolysis group of 0.70 (95% confidence interval, 0.35-1.44). This outcome was consistent across subgroups.
The researchers note that, to their knowledge, this is the first randomized controlled study to assess the effect of thrombolysis on clot migration and accessibility in an extended time window.
They acknowledge that a limitation of this study is that they only assessed clot migration from a retrievable to a nonretrievable location; therefore, the true frequency of any clot migration occurring was likely to be higher, and this could explain why other reports have found higher odds ratios of clot migration.
But they point out that they chose to limit their analysis in this way specifically to guide decision-making regarding bridging thrombolysis incorporating endovascular therapy in the extended time window.
“The findings of this study are highly relevant in the current clinical environment, where there are multiple ongoing trials looking at removing thrombolysis pretreatment within the 4.5-hour time window in thrombectomy patients,” the authors write.
“We have demonstrated that thrombolysis in the 4.5- to 9-hour window is not associated with reduced clot accessibility, and this information will be useful in future trial designs incorporating this extended time window,” they add.
Commenting on the study for this news organization, Michael Hill, MD, University of Calgary (Alta.), said: “Thrombus migration does happen and is likely part of the natural history of ischemic stroke, which may be influenced by therapeutics such as thrombolysis. This paper’s top-line result is that thrombus migration occurs in both treated and untreated groups – and therefore that this is really an observation of natural history.”
Dr. Hill says that, at present, patients should be treated with thrombolysis before endovascular therapy if they are eligible, and these results do not change that recommendation.
“The results of the ongoing trials comparing direct thrombectomy with thrombolysis plus thrombectomy will help to understand the potential clinical outcome relevance of this phenomenon,” he added.
The EXTEND trial was supported by grants from the Australian National Health and Medical Research Council of Australia and the Commonwealth Scientific and Industrial Research Organization Flagship Program. Dr. Yan reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Earlier antibiotic initiation for sepsis did not lead to overuse
There has been a marked increase in the time to antibiotic administration for ICU patients with sepsis across Veterans Affairs (VA) hospitals, but there is no evidence that they are being given inappropriately, according to new findings.
Accelerating time-to-antibiotics in sepsis means that patients will be treated earlier, but it could also result in more patients receiving antibiotics, including those without infection. This in turn may contribute to antimicrobial resistance.
“The time to antibiotics for sepsis accelerated across VA hospitals, and declined from 5.8 to 4.8 hours between 2013 and 2018,” said lead study author Sarah Seelye, PhD, data scientist at the U.S. Department of Veterans Affairs, Ann Arbor, Mich. “Despite this, there was no evidence between hospital level antibiotic acceleration in sepsis and antibiotic use among all patients with potential sepsis.”
The results were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine, which was held virtually this year.
“Many hospitals have initiated programs like this to accelerate the use of antibiotics in patients with severe sepsis, but at the same time, there is growing concern that earlier antibiotic initiation may result in increased antibiotic treatment overall, including those without infection,” said Dr. Seelye. “However, to date, there is little evidence to support this claim.”
The goal of their study was to investigate whether hospital-level acceleration in antibiotic timing for sepsis was associated with increasing antibiotic use among patients hospitalized with potential infection.
They identified 1,101,239 hospitalizations for potential infection in 132 VA hospitals during the period from 2013 to 2018. Of these patients, 608,128 (55.2%) received antibiotics within 48 hours of presentation to the emergency department. A total of 117,435 (10.7%) met the criteria for sepsis.
Hospitals were classified into tertiles of antibiotic acceleration for sepsis: rapid, slow, and flat.
In the VA system, patients with severe sepsis began receiving faster antibiotic treatment in 2017, compared with earlier years. In 2017-2018 more than 20% of sepsis patients had received their first treatment within 2 hours, compared with 14% in 2013-1014.
In 2017-2018, more than 20% of sepsis patients had received their first treatment within 2 hours, compared with 14% in 2013-1014.
Hospitals categorized as rapid accelerators decreased their time to antibiotic initiation from 6.4 hours to 4.5 hours, while slow accelerators went from 5.6 to 4.6 hours from 2013 to 2018, and flat accelerators remained stable during the time period (5.3 hours down to 5.2 hours).
However, statistical analysis showed no real difference between the three groups in antibiotic prescribing.
“Despite this, there was no evidence between hospital-level antibiotic acceleration in sepsis and antibiotic use among all patients with potential sepsis,” said Dr. Seelye.
Weighing in on the study results, Craig M. Coopersmith, MD, professor of surgery at Emory University, Atlanta, noted that these results are very convincing, considering the size of the study and that it encompassed 132 different facilities.
“It’s difficult to say how generalizable these results are but they are definitely generalizable to all hospitals in the VA system,” he said. “In general, there are similarities between large health care systems, and it would be surprising if we found the opposite to be true in non-VA health systems.”
However, he emphasized that there is some possibility that the results would not be identical because different health care systems have different methods of providing care.
“This paper does show that you can get antibiotics into patients faster, which can be life saving, without inappropriately using them on everybody,” Dr. Coopersmith said.
He explained that there is more attention being paid now to antibiotic stewardship, compared with 10 or 15 years ago. “Given the choice of giving someone a single dose of antibiotics who may not need it, as opposed to withholding them from someone who is septic which is life threatening, the risk benefit ratio weighs heavily towards starting them early,” he said. “And then escalate rapidly.”
There has been a marked increase in the time to antibiotic administration for ICU patients with sepsis across Veterans Affairs (VA) hospitals, but there is no evidence that they are being given inappropriately, according to new findings.
Accelerating time-to-antibiotics in sepsis means that patients will be treated earlier, but it could also result in more patients receiving antibiotics, including those without infection. This in turn may contribute to antimicrobial resistance.
“The time to antibiotics for sepsis accelerated across VA hospitals, and declined from 5.8 to 4.8 hours between 2013 and 2018,” said lead study author Sarah Seelye, PhD, data scientist at the U.S. Department of Veterans Affairs, Ann Arbor, Mich. “Despite this, there was no evidence between hospital level antibiotic acceleration in sepsis and antibiotic use among all patients with potential sepsis.”
The results were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine, which was held virtually this year.
“Many hospitals have initiated programs like this to accelerate the use of antibiotics in patients with severe sepsis, but at the same time, there is growing concern that earlier antibiotic initiation may result in increased antibiotic treatment overall, including those without infection,” said Dr. Seelye. “However, to date, there is little evidence to support this claim.”
The goal of their study was to investigate whether hospital-level acceleration in antibiotic timing for sepsis was associated with increasing antibiotic use among patients hospitalized with potential infection.
They identified 1,101,239 hospitalizations for potential infection in 132 VA hospitals during the period from 2013 to 2018. Of these patients, 608,128 (55.2%) received antibiotics within 48 hours of presentation to the emergency department. A total of 117,435 (10.7%) met the criteria for sepsis.
Hospitals were classified into tertiles of antibiotic acceleration for sepsis: rapid, slow, and flat.
In the VA system, patients with severe sepsis began receiving faster antibiotic treatment in 2017, compared with earlier years. In 2017-2018 more than 20% of sepsis patients had received their first treatment within 2 hours, compared with 14% in 2013-1014.
In 2017-2018, more than 20% of sepsis patients had received their first treatment within 2 hours, compared with 14% in 2013-1014.
Hospitals categorized as rapid accelerators decreased their time to antibiotic initiation from 6.4 hours to 4.5 hours, while slow accelerators went from 5.6 to 4.6 hours from 2013 to 2018, and flat accelerators remained stable during the time period (5.3 hours down to 5.2 hours).
However, statistical analysis showed no real difference between the three groups in antibiotic prescribing.
“Despite this, there was no evidence between hospital-level antibiotic acceleration in sepsis and antibiotic use among all patients with potential sepsis,” said Dr. Seelye.
Weighing in on the study results, Craig M. Coopersmith, MD, professor of surgery at Emory University, Atlanta, noted that these results are very convincing, considering the size of the study and that it encompassed 132 different facilities.
“It’s difficult to say how generalizable these results are but they are definitely generalizable to all hospitals in the VA system,” he said. “In general, there are similarities between large health care systems, and it would be surprising if we found the opposite to be true in non-VA health systems.”
However, he emphasized that there is some possibility that the results would not be identical because different health care systems have different methods of providing care.
“This paper does show that you can get antibiotics into patients faster, which can be life saving, without inappropriately using them on everybody,” Dr. Coopersmith said.
He explained that there is more attention being paid now to antibiotic stewardship, compared with 10 or 15 years ago. “Given the choice of giving someone a single dose of antibiotics who may not need it, as opposed to withholding them from someone who is septic which is life threatening, the risk benefit ratio weighs heavily towards starting them early,” he said. “And then escalate rapidly.”
There has been a marked increase in the time to antibiotic administration for ICU patients with sepsis across Veterans Affairs (VA) hospitals, but there is no evidence that they are being given inappropriately, according to new findings.
Accelerating time-to-antibiotics in sepsis means that patients will be treated earlier, but it could also result in more patients receiving antibiotics, including those without infection. This in turn may contribute to antimicrobial resistance.
“The time to antibiotics for sepsis accelerated across VA hospitals, and declined from 5.8 to 4.8 hours between 2013 and 2018,” said lead study author Sarah Seelye, PhD, data scientist at the U.S. Department of Veterans Affairs, Ann Arbor, Mich. “Despite this, there was no evidence between hospital level antibiotic acceleration in sepsis and antibiotic use among all patients with potential sepsis.”
The results were presented at the Critical Care Congress sponsored by the Society of Critical Care Medicine, which was held virtually this year.
“Many hospitals have initiated programs like this to accelerate the use of antibiotics in patients with severe sepsis, but at the same time, there is growing concern that earlier antibiotic initiation may result in increased antibiotic treatment overall, including those without infection,” said Dr. Seelye. “However, to date, there is little evidence to support this claim.”
The goal of their study was to investigate whether hospital-level acceleration in antibiotic timing for sepsis was associated with increasing antibiotic use among patients hospitalized with potential infection.
They identified 1,101,239 hospitalizations for potential infection in 132 VA hospitals during the period from 2013 to 2018. Of these patients, 608,128 (55.2%) received antibiotics within 48 hours of presentation to the emergency department. A total of 117,435 (10.7%) met the criteria for sepsis.
Hospitals were classified into tertiles of antibiotic acceleration for sepsis: rapid, slow, and flat.
In the VA system, patients with severe sepsis began receiving faster antibiotic treatment in 2017, compared with earlier years. In 2017-2018 more than 20% of sepsis patients had received their first treatment within 2 hours, compared with 14% in 2013-1014.
In 2017-2018, more than 20% of sepsis patients had received their first treatment within 2 hours, compared with 14% in 2013-1014.
Hospitals categorized as rapid accelerators decreased their time to antibiotic initiation from 6.4 hours to 4.5 hours, while slow accelerators went from 5.6 to 4.6 hours from 2013 to 2018, and flat accelerators remained stable during the time period (5.3 hours down to 5.2 hours).
However, statistical analysis showed no real difference between the three groups in antibiotic prescribing.
“Despite this, there was no evidence between hospital-level antibiotic acceleration in sepsis and antibiotic use among all patients with potential sepsis,” said Dr. Seelye.
Weighing in on the study results, Craig M. Coopersmith, MD, professor of surgery at Emory University, Atlanta, noted that these results are very convincing, considering the size of the study and that it encompassed 132 different facilities.
“It’s difficult to say how generalizable these results are but they are definitely generalizable to all hospitals in the VA system,” he said. “In general, there are similarities between large health care systems, and it would be surprising if we found the opposite to be true in non-VA health systems.”
However, he emphasized that there is some possibility that the results would not be identical because different health care systems have different methods of providing care.
“This paper does show that you can get antibiotics into patients faster, which can be life saving, without inappropriately using them on everybody,” Dr. Coopersmith said.
He explained that there is more attention being paid now to antibiotic stewardship, compared with 10 or 15 years ago. “Given the choice of giving someone a single dose of antibiotics who may not need it, as opposed to withholding them from someone who is septic which is life threatening, the risk benefit ratio weighs heavily towards starting them early,” he said. “And then escalate rapidly.”
FROM CCC50






















