User login
Heart failure treatment: Keeping up with best practices
Heart failure (HF) affects nearly 6 million Americans and accounts for one million hospital admissions each year.1 The condition, which results from a structural or functional disorder that impairs the ventricles’ ability to fill, empty, or both,2 is a major cause of morbidity and mortality. The 5-year mortality rate ranges from 44% to 77%.3,4
Growing evidence demonstrates reduced morbidity and mortality when patients with HF with reduced ejection fraction (HFrEF) are treated with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB); a beta-blocker; and a mineralocorticoid/aldosterone receptor antagonist (MRA) in appropriate doses.5 In addition, 2 new medications representing novel drug classes have recently entered the market and are recommended in select patients who remain symptomatic despite standard treatment.
The first is sacubitril, which is available in a combination pill with the ARB valsartan, and the second is ivabradine.6 Additionally, implanted medical devices are proving useful, particularly in the management of patients with refractory symptoms.
This article will briefly review the diagnosis and initial evaluation of the patient with suspected HF and then describe how newer treatments fit within HF management priorities and strategies. But first, a word about what causes HF.
Causes are many and diverse
HF has a variety of cardiac and non-cardiac etiologies.2,7,8 Some important cardiac causes include hypertension (HTN), coronary artery disease (CAD), valvular heart disease, arrhythmias, myocarditis, Takotsubo cardiomyopathy, and postpartum cardiomyopathy. Common and important non-cardiac causes of HF include alcoholic cardiomyopathy, pulmonary embolism, pulmonary hypertension, obstructive sleep apnea, anemia, hemochromatosis, amyloidosis, sarcoidosis, thyroid dysfunction, nephrotic syndrome, and cardiac toxins (especially stimulants and certain chemotherapy drugs).2,7,8
Diagnosing an elusive culprit
HF remains a clinical diagnosis. Common symptoms include dyspnea, cough, pedal edema, and decreased exercise tolerance, but these symptoms are not at all specific. Given the varied causes and manifestations of HF, the diagnosis can be somewhat elusive. Fortunately, there are a number of objective methods to help identify patients with HF.
Framingham criteria. One commonly used tool for making the diagnosis of HF is the Framingham criteria (see https://www.mdcalc.com/framingham-heart-failure-diagnostic-criteria),9 which diagnoses HF based on historical and physical exam findings. Another well-validated decision tool is the Heart Failure Diagnostic Rule (see http://circ.ahajournals.org/content/124/25/2865.long),10 which incorporates N-terminal pro–B-type natriuretic peptide (NT-proBNP) results, as well as exam findings.
Measurement of natriuretic peptides, either B-type natriuretic peptide (BNP) or NT-proBNP, aids in the diagnosis of HF.5 Although several factors (including age, weight, and renal function) can affect BNP levels, a normal BNP value effectively rules out HF5,7 and an elevated BNP can help to make the diagnosis in the context of a patient with corresponding symptoms.
The initial evaluation: Necessary lab work and imaging studies
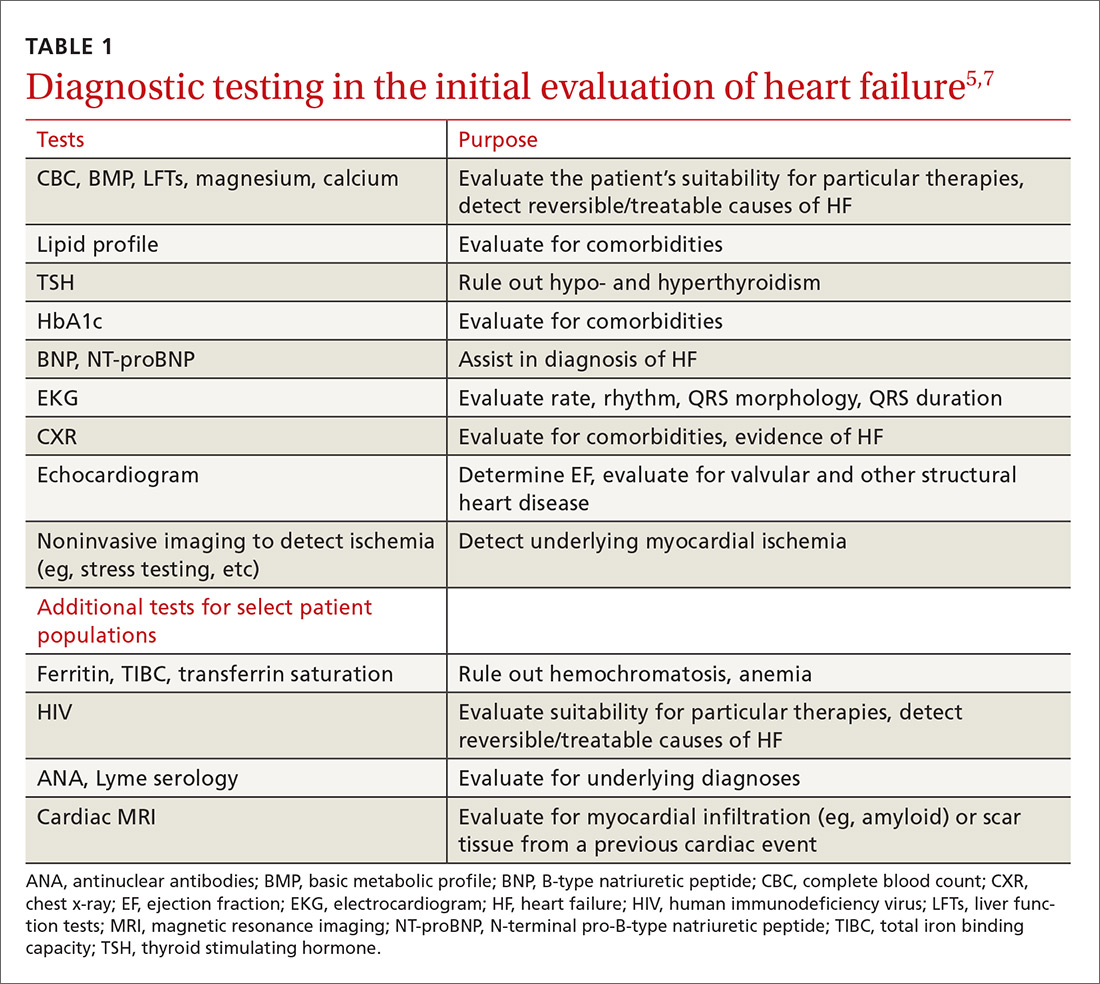
The purpose of the initial evaluation of the patient with suspected HF is to establish the diagnosis, look for underlying etiologies of HF, identify comorbidities, and establish baseline values (eg, of potassium and creatinine) for elements monitored during treatment.5,7 TABLE 15,7 lists the lab work and imaging tests that are commonly ordered in the initial evaluation of the patient with HF.
Echocardiography is useful in determining the ejection fraction (EF), which is essential in guiding treatment. Echocardiography can also identify important structural abnormalities including significant valvular disease. Refer patients with severe valvular disease for evaluation for valve repair/replacement, regardless of EF.8
Noninvasive testing (stress nuclear imaging or echocardiography) to evaluate for underlying CAD is reasonable in patients with unknown CAD status.8,11 Patients for whom there is a high suspicion of obstructive CAD should undergo coronary angiography if they are candidates for revascularization.5,7 Noninvasive testing may also be an acceptable option for assessing ischemia in patients presenting with HF who have known CAD and no angina.5
Classification of HF is determined by ejection fraction
Physicians have traditionally classified patients with HF as having either systolic or diastolic dysfunction. Patients with HF symptoms and a reduced EF were said to have systolic dysfunction; those with a normal EF were said to have diastolic dysfunction.
More recently, researchers have learned that patients with reduced EF and those with preserved EF can have both systolic and diastolic dysfunction simultaneously.8 Therefore, the current preferred terminology is HFpEF (heart failure with preserved ejection fraction) for those with an EF ≥50% and HFrEF (heart failure with reduced ejection fraction) for those with an EF ≤40%.5 Both the American Heart Association (AHA) and the European Society of Cardiology recognize a category of HF with moderately reduced ejection fraction defined as an EF between 40% and 50%.5,7 Practically speaking, this group is treated as per the guidelines for HFrEF.5
Treatment of HFrEF: The evidence is clear
The cornerstone of medical treatment for HFrEF is the combination of an ACE inhibitor or ARB with a beta-blocker.2,5,7,8 Several early trials showed clear benefits of these medications. For example, the Studies Of Left Ventricular Dysfunction trial (SOLVD), compared enalapril to placebo in patients receiving standard therapy (consisting chiefly of digitalis, diuretics, and nitrates). This study demonstrated a reduction in all-cause mortality or first hospitalization for HF (number needed to treat [NNT]=21) in the enalapril group vs the placebo group.12
Similarly, a subgroup analysis of the Valsartan Heart Failure Treatment (Val-HeFT) trial demonstrated morbidity (NNT=10) and all-cause mortality benefits (NNT=6) when valsartan (an ARB) was given to patients who were not receiving an ACE inhibitor.13
MERIT-HF (Metoprolol CR/XL Randomised Intervention Trial in congestive Heart Failure) compared the beta-blocker metoprolol succinate to placebo and found fewer deaths from HF and lower all-cause mortality (NNT=26) associated with the treatment group vs the placebo group.14
And a comparison of 2 beta-blockers—carvedilol and metoprolol tartrate—on clinical outcomes in patients with chronic HF in the Carvedilol Or Metoprolol European Trial (COMET) showed that carvedilol extended survival compared with metoprolol tartrate (NNT=19).15
Unlike ACE inhibitors and ARBs, which seem to show a class benefit, only 3 beta-blockers available in the United States have been proven to reduce mortality: sustained-release metoprolol succinate, carvedilol, and bisoprolol.2,7,8
Unless contraindicated, all patients with a reduced EF—even those without symptoms—should receive a beta-blocker and an ACE inhibitor or ARB.5,7,8
Cautionary notes
Remember the following caveats when treating patients with ACE inhibitors, ARBs, and beta-blockers:
- Use ACE inhibitors and ARBs with caution in patients with impaired renal function (serum creatinine >2.5 mg/dL) or elevated serum potassium (>5 mEq/L).16,17
- ARBs are associated with a much lower incidence of cough and angioedema than ACE inhibitors.18
- Although physicians frequently start patients on low doses of beta-blockers and ACE inhibitors or ARBs to minimize hypotension and other adverse effects, the goal of therapy is to titrate up to the therapeutic doses used in clinical trials.5-7 (For dosages of medications commonly used in the treatment of heart failure, see Table 3 in the American College of Cardiology/AHA/Heart Failure Society of America guidelines available at https://www.sciencedirect.com/science/article/pii/S0735109717370870?via%3Dihub#tbl3 and Table 7.2 in the European Society of Cardiology guidelines available at https://academic.oup.com/eurheartj/article/37/27/2129/1748921.)
- Because beta-blockers can exacerbate fluid retention, do not initiate them in patients with fluid overload unless such patients are being treated with diuretics.5,19
When more Tx is needed
For patients who remain symptomatic despite treatment with an ACE inhibitor or ARB and a beta-blocker, consider the following add-on therapies.
Diuretics are the only medications used in the treatment of HF that adequately reduce fluid overload.2,7 While thiazide diuretics confer greater blood pressure control, loop diuretics are generally preferred in the treatment of HF because they are more efficacious.5 Loop diuretics should be prescribed to all patients with fluid overload, as few patients can maintain their target (“dry”) weight without diuretic therapy.5,7 Common adverse effects include hypokalemia, dehydration, and azotemia.
Two MRAs are currently available in the United States: spironolactone and eplerenone. MRAs are used as add-on therapy for symptomatic patients with an EF ≤35% or an EF ≤40% following an acute myocardial infarction (MI).5 They significantly reduce all-cause mortality (NNT=26).20
Because hyperkalemia is a risk with MRAs, do not prescribe them for patients who are already taking both an ACE inhibitor and an ARB.5 Also, do not initiate MRA therapy in patients who have an elevated creatinine level (≥2.5 mg/dL in men; ≥2 mg/dL in women) or a potassium level ≥5 mEq/L.5,7,8 Discontinue MRA therapy if a patient’s potassium level rises to ≥5.5 mEq/L.5
Hydralazine combined with isosorbide dinitrate (H/ID) is an alternative in patients for whom ACE inhibitor/ARB therapy is contraindicated.5,8
H/ID is also an add-on option in African American patients. Trials have demonstrated that H/ID reduces both first hospitalization for HF (NNT=13) and all-cause mortality (NNT=25) when it is used as add-on therapy in African Americans already receiving standard therapy with an ACE inhibitor or ARB, a beta-blocker, and an MRA.21 Headache and dizziness are commonly reported adverse effects.
Digoxin does not reduce mortality, but it does improve both quality of life and exercise tolerance and reduces hospital admissions for patients with HF.5,7 Significant adverse effects of digoxin include anorexia, nausea, visual disturbances, and cardiac arrhythmias.22
Also, hypokalemia can intensify digoxin toxicity.23 Because of these concerns, digoxin is typically dosed at 0.125 mg/d (0.125 mg every other day in patients >70 years or patients with impaired renal function or low body weight) with a target therapeutic range of 0.5 to 0.9 ng/mL.5
New classes, new agents
Sacubitril, a neprilysin inhibitor, is the first drug from this class approved for use in the United States. Neprilysin is the enzyme responsible for the degradation of natriuretic peptides; as such it increases endogenous NPs, promoting diuresis and lowering blood pressure.24,25 Early trials with sacubitril alone showed limited clinical efficacy;25 however, when it was combined with the ARB, valsartan (the combination being called angiotensin receptor blocker + neprilysin inhibitor [ARNI] therapy), it was found to be of significant benefit.6,25
The PARADIGM-HF (Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure) trial compared outcomes in patients receiving ARNI therapy to those receiving enalapril.26 The authors stopped the trial early due to the overwhelming benefit seen in the ARNI arm.
After a median follow-up of 27 months, the researchers found a reduction in the primary outcomes of either cardiovascular death or first hospitalization for HF (26.5% in the enalapril-treated group vs 21.8% in the ARNI-treated group; NNT=21).26 There were slightly more cases of angioedema in the ARNI arm than in the enalapril arm (0.5% vs 0.2%), although there were no patients in the trial who required endotracheal intubation.26
Because of this increased risk, do not prescribe ARNI therapy for any patient with a history of angioedema.6 Hypotension was more common in the ARNI-treated group than in the enalapril group (14% vs 9.2%), but there were lower rates of hyperkalemia, elevated serum creatinine, and cough in the ARNI-treated group than in the enalapril group.26
Consider ARNI treatment for all patients with an EF ≤40% who remain symptomatic despite appropriate doses of an ACE inhibitor or ARB plus a beta-blocker. Do not administer ARNI therapy concomitantly with an ACE inhibitor or ARB. When switching, do not start ARNI therapy for at least 36 hours after the last dose of an ACE inhibitor or ARB.6
Ivabradine is a sinoatrial node modulator that provides additional heart rate reduction. It does not affect ventricular repolarization or myocardial contractility.27 Early trials with this medication have shown reduced cardiac mortality and an NNT to prevent one first HF hospitalization within one year of 27.28 Adverse effects include symptomatic and asymptomatic bradycardia and luminous phenomena.28
Recommend ivabradine as add-on therapy to all patients with an EF ≤35%, normal sinus rhythm, and resting heart rate ≥70 bpm who remain symptomatic despite taking the maximum-tolerated dose of a beta-blocker.6 The dose is adjusted to achieve a resting heart rate of 50 to 60 bpm.27
Nonpharmacologic options
Implantable cardioverter defibrillators (ICDs) are recommended as primary prevention in select HFrEF patients to reduce the risk of sudden cardiac death and all-cause mortality. The 2013 American College of Cardiology Foundation/AHA Guideline for the Management of Heart Failure recommends an ICD for primary prevention for: 1) patients with symptomatic HF and an LVEF ≤35% despite ≥3 months of optimal medical therapy, and 2) patients at least 40 days post-MI with an LVEF of ≤30%.5,29 ICDs are not recommended for patients who have a life expectancy of less than one year, and the devices are of unclear benefit for patients ≥75 years of age.5
Cardiac resynchronization therapy (CRT), although not new to the field of cardiology, is new to the treatment of heart failure. A number of patients with HFrEF have QRS prolongation and in particular, left bundle branch block (LBBB).5 CRT uses biventricular pacing to restore synchronous contraction of the left and right ventricles.30 It is strongly recommended for patients with an EF ≤35%, sinus rhythm, LBBB, QRS ≥150 ms, and a life expectancy of at least one year.5,7 It is weakly recommended for patients with an EF ≤35% and a QRS ≥150 ms but without LBBB. It’s also weakly recommended for patients with an EF ≤35% and LBBB with a QRS of 120 to 150 ms.5,31
Left ventricular assist devices (LVADs) and cardiac transplantation are considerations for patients with severe symptoms refractory to all other interventions.5 LVADs may be used either while awaiting cardiac transplantation (bridge therapy) or as definitive treatment (destination therapy). Appropriate patient selection for such therapies requires a team of experts that ideally includes HF and transplantation cardiologists, cardiothoracic surgeons, nurses, social workers, and palliative care clinicians.5
Treatment of HFpEF: Evidence is lacking
While HFpEF is common—affecting about half of all patients with HF—ideal treatment remains unclear.32 Some trials have shown promise, but to date no unequivocal evidence exists that any standard therapy reduces mortality in patients with HFpEF.33-37 Underlying mechanisms of action of HFpEF include cardiac rate and rhythm abnormalities, atrial dysfunction, and stiffening of the ventricles. In a sense, it represents an exaggerated expression of the pathophysiology seen with the normal aging of the heart and can be conceptualized as “presbycardia.”37 Indeed, HFpEF is more common in the elderly, but it is also more common in patients of African descent.38,39 Common contributing causes (which we’ll get to in a bit) include HTN, CAD, atrial fibrillation (AF), obesity, and obstructive sleep apnea (OSA).
Trials have failed to show clear benefit for ACE inhibitors, ARBs, or beta-blockers.7,33 The evidence for MRAs is somewhat unclear; however, they have recently been recommended as an option for patients who have been hospitalized in the last year to reduce the risk of subsequent hospitalizations.40 Digoxin is used primarily for rate control in the setting of AF, but otherwise is of unclear benefit.7 A low-sodium diet (ie, ≤2 g/d) may be useful in those patients who are prone to fluid overload.5,7 The cornerstone of treatment of HFpEF is the relief of volume overload with diuretics and the treatment of coexisting conditions.33
Common contributing causes of HFpEF
HTN is not only a common contributing cause, but also the most common comorbid condition affecting patients with HFpEF. As such, treatment of HTN represents the most important management goal.33,34 Based on recent data, the American College of Cardiology, the AHA, and the Heart Failure Society of America have recommended a systolic blood pressure goal <130 mm Hg for patients with HFpEF.40 Most patients with HFpEF and HTN will have some degree of fluid overload and, therefore, should receive a diuretic.
CAD. Patients with HFpEF should be evaluated for CAD and treated with medical management and coronary revascularization, as appropriate.
AF is poorly tolerated by patients with HFpEF.37 Patients with AF should receive anticoagulation and rate control medications, and those with persistent HF symptoms should be evaluated for rhythm control.33
Obesity is more prevalent in patients with HFpEF than in those with HFrEF.41 Although there is indirect evidence that weight loss improves cardiac function,34,42,43 and studies have shown bariatric surgery to improve diastolic function,44,45 there are no studies reporting clinical outcomes.
Treatment of OSA with continuous positive airway pressure appears to alleviate some symptoms of HF and to reduce all-cause mortality.46,47
Keeping HF patients out of the hospital
Many readmissions to the hospital for HF exacerbation are preventable. Patients often do not understand hospital discharge instructions or the nature of their chronic disease and its management.48-51 Routine follow-up in the office or clinic provides an opportunity to improve quality of life for patients and decrease admissions.7,52
A major role for the family physician is in the co-creation of, and adherence to, an individualized, comprehensive care plan. Make sure such a plan is easily understood not only by the patient with HF, but also by his or her care team. In addition, it should be evidence-based and reflect the patient’s culture, values, and goals of treatment.5,7
At each visit, the family physician or a member of the health care team should assess adherence to guideline-directed medical therapy, measure weight, evaluate fluid status, and provide ongoing patient education including information on the importance of activity, monitoring weight daily, and moderating fluid, salt, and alcohol intake.5,52
Research shows that cardiac rehabilitation improves functional capacity, exercise duration, quality of life, and mortality. Therefore, recommend it to all symptomatic patients with HF who are clinically stable.2
Consider collaboration with a subspecialist. Patients who remain symptomatic despite optimal medical management and patients with recurrent hospitalizations are best managed in conjunction with a subspecialist in HF treatment.2,5
CORRESPONDENCE
Darin Brink, MD, 420 Delaware St. SE, MMC 381, Minneapolis, MN 55455; [email protected].
1. Hall MJ, Levant S, DeFrances CJ. Hospitalization for congestive heart failure: United States, 2000-2010. NCHS Data Brief. 2012;(108):1-8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23102190. Accessed April 26, 2017.
2. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused Update Incorporated Into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
3. Passantino A, Guida P, Lagioia R, et al. Predictors of long-term mortality in older patients hospitalized for acutely decompensated heart failure: clinical relevance of natriuretic peptides. J Am Geriatr Soc. 2017;65:822-826.
4. Lassus JP, Siirilä-Waris K, Nieminen MS, et al. Long-term survival after hospitalization for acute heart failure—differences in prognosis of acutely decompensated chronic and new-onset acute heart failure. Int J Cardiol. 2013;168:458-462.
5. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
6. Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. J Am Coll Cardiol. 2016;68:1476-1488.
7. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129-2200.
8. Pinkerman CP, Sander JE, Breeding D, et al. Institute for Clinical Systems Improvement. Heart failure in adults. Available at: https://www.scribd.com/document/310893227/HeartFailure-pdf. Accessed December 6, 2017.
9. McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham Study. N Engl J Med. 1971;285:1441-1446.
10. Kelder JC, Cramer MJ, van Wijngaarden J, et al. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation. 2011;124:2865-2873.
11. Heart Failure Society of America, Lindenfeld J, Albert NM, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1-194.
12. Pouleur H, The SOLVD Investigators. Results of the treatment trial of the studies of left ventricular dysfunction (SOLVD). Am J Cardiol. 1992;70:135-136.
13. Maggioni AP, Anand I, Gottlieb SO, et al. Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin-converting enzyme inhibitors. J Am Coll Cardiol. 2002;40:1414-1421.
14. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001-2007.
15. Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7-13.
16. Gehr TW, Sica DA. Pharmacotherapy in congestive heart failure: Hyperkalemia in congestive heart failure. Congest Heart Fail. 2001;7:97-100.
17. National Institute for Health and Clinical Excellence (NICE). Chronic heart failure in adults: management. 2010. Available at: https://www.nice.org.uk/guidance/cg108. Accessed November 27, 2017.
18. Barreras A, Gurk-Turner C. Angiotensin II receptor blockers. Proc (Bayl Univ Med Cent). 2003;16:123-126.
19. Epstein SE, Braunwald E. The effect of beta adrenergic blockade on patterns of urinary sodium excretion: studies in normal subjects and in patients with heart disease. Ann Intern Med. 1966;65:20-27.
20. Berbenetz NM, Mrkobrada M. Mineralocorticoid receptor antagonists for heart failure: systematic review and meta-analysis. BMC Cardiovasc Disord. 2016;16:246.
21. Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049-2057.
22. Kelly RA, Smith TW. Recognition and management of digitalis toxicity. Am J Cardiol. 1992;69:108G-118G.
23. Sundar S, Burma DP, Vaish SK. Digoxin toxicity and electrolytes: a correlative study. Acta Cardiol. 1983;38:115-123.
24. McDowell G, Nicholls DP. The endopeptidase inhibitor, candoxatril, and its therapeutic potential in the treatment of chronic cardiac failure in man. Expert Opin Investig Drugs. 1999;8:79-84.
25. Prenner SB, Shah SJ, Yancy CW. Role of angiotensin receptor-neprilysin inhibition in heart failure. Curr Atheroscler Rep. 2016;18:48.
26. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004.
27. Corlanor package insert. Amgen Inc., Thousand Oaks, CA. Available at: http://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/corlanor/corlanor_pi.pdf. Accessed November 28, 2017.
28. Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875-885.
29. Kusumoto FM, Calkins H, Boehmer J, et al. HRS/ACC/AHA expert consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. Circulation. 2014;130:94-125.
30. Leyva F, Nisam S, Auricchio A. 20 years of cardiac resynchronization therapy. J Am Coll Cardiol. 2014;64:1047-1058.
31. Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS Focused Update Incorporated Into the ACCF/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013;127:e283-e352.
32. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670-679.
33. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2016;375:1868-1877.
34. Nanayakkara S, Kaye DM. Management of heart failure with preserved ejection fraction: a review. Clin Ther. 2015;37:2186-2198.
35. Cleland JG, Pellicori P, Dierckx R. Clinical trials in patients with heart failure and preserved left ventricular ejection fraction. Heart Fail Clin. 2014;10:511-523.
36. Ferrari R, Böhm M, Cleland JGF, et al. Heart failure with preserved ejection fraction: uncertainties and dilemmas. Eur J Heart Fail. 2015;17:665-671.
37. Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507-515.
38. Sharp A, Tapp R, Francis DP, et al. Ethnicity and left ventricular diastolic function in hypertension an ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J Am Coll Cardiol. 2008;52:1015-1021.
39. Zile MR. Heart failure with a preserved ejection fraction. In: Mann DL, Zipes D, Libby P BR, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 10th ed. Phila
40. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776-803.
41. Mentz RJ, Kelly JP, von Lueder TG, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281-2293.
42. de las Fuentes L, Waggoner AD, Mohammed BS, et al. Effect of moderate diet-induced weight loss and weight regain on cardiovascular structure and function. J Am Coll Cardiol. 2009;54:2376-2381.
43. Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction. JAMA. 2016;315:36-46.
44. Rider OJ, Francis JM, Ali MK, et al. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J Am Coll Cardiol. 2009;54:718-726.
45. Ristow B, Rabkin J, Haeusslein E. Improvement in dilated cardiomyopathy after bariatric surgery. J Card Fail. 2008;14:198-202.
46. Yoshihisa A, Suzuki S, Yamauchi H, et al. Beneficial effects of positive airway pressure therapy for sleep-disordered breathing in heart failure patients with preserved left ventricular ejection fraction. Clin Cardiol. 2015;38:413-421.
47. Shah RV, Abbasi SA, Heydari B, et al. Obesity and sleep apnea are independently associated with adverse left ventricular remodeling and clinical outcome in patients with atrial fibrillation and preserved ventricular function. Am Heart J. 2014;167:620-626.
48. Riegel B, Moser DK, Anker SD, et al. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120:1141-1163.
49. Moser DK, Doering LV, Chung ML. Vulnerabilities of patients recovering from an exacerbation of chronic heart failure. Am Heart J. 2005;150:984.
50. Bernheim SM, Grady JN, Lin Z, et al. National patterns of risk-standardized mortality and readmission for acute myocardial infarction and heart failure: update on publicly reported outcomes measures based on the 2010 release. Circ Cardiovasc Qual Outcomes. 2010;3:459-467.
51. Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2:407-413.
52. Cowie MR, Anker SD, Cleland JG, et al. Improving care for patients with acute heart failure: before, during and after hospitalization. Available at: http://www.oxfordhealthpolicyforum.org/files/reports/ahf-report.pdf. Accessed November 27, 2017.
Heart failure (HF) affects nearly 6 million Americans and accounts for one million hospital admissions each year.1 The condition, which results from a structural or functional disorder that impairs the ventricles’ ability to fill, empty, or both,2 is a major cause of morbidity and mortality. The 5-year mortality rate ranges from 44% to 77%.3,4
Growing evidence demonstrates reduced morbidity and mortality when patients with HF with reduced ejection fraction (HFrEF) are treated with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB); a beta-blocker; and a mineralocorticoid/aldosterone receptor antagonist (MRA) in appropriate doses.5 In addition, 2 new medications representing novel drug classes have recently entered the market and are recommended in select patients who remain symptomatic despite standard treatment.
The first is sacubitril, which is available in a combination pill with the ARB valsartan, and the second is ivabradine.6 Additionally, implanted medical devices are proving useful, particularly in the management of patients with refractory symptoms.
This article will briefly review the diagnosis and initial evaluation of the patient with suspected HF and then describe how newer treatments fit within HF management priorities and strategies. But first, a word about what causes HF.
Causes are many and diverse
HF has a variety of cardiac and non-cardiac etiologies.2,7,8 Some important cardiac causes include hypertension (HTN), coronary artery disease (CAD), valvular heart disease, arrhythmias, myocarditis, Takotsubo cardiomyopathy, and postpartum cardiomyopathy. Common and important non-cardiac causes of HF include alcoholic cardiomyopathy, pulmonary embolism, pulmonary hypertension, obstructive sleep apnea, anemia, hemochromatosis, amyloidosis, sarcoidosis, thyroid dysfunction, nephrotic syndrome, and cardiac toxins (especially stimulants and certain chemotherapy drugs).2,7,8
Diagnosing an elusive culprit
HF remains a clinical diagnosis. Common symptoms include dyspnea, cough, pedal edema, and decreased exercise tolerance, but these symptoms are not at all specific. Given the varied causes and manifestations of HF, the diagnosis can be somewhat elusive. Fortunately, there are a number of objective methods to help identify patients with HF.
Framingham criteria. One commonly used tool for making the diagnosis of HF is the Framingham criteria (see https://www.mdcalc.com/framingham-heart-failure-diagnostic-criteria),9 which diagnoses HF based on historical and physical exam findings. Another well-validated decision tool is the Heart Failure Diagnostic Rule (see http://circ.ahajournals.org/content/124/25/2865.long),10 which incorporates N-terminal pro–B-type natriuretic peptide (NT-proBNP) results, as well as exam findings.
Measurement of natriuretic peptides, either B-type natriuretic peptide (BNP) or NT-proBNP, aids in the diagnosis of HF.5 Although several factors (including age, weight, and renal function) can affect BNP levels, a normal BNP value effectively rules out HF5,7 and an elevated BNP can help to make the diagnosis in the context of a patient with corresponding symptoms.
The initial evaluation: Necessary lab work and imaging studies
The purpose of the initial evaluation of the patient with suspected HF is to establish the diagnosis, look for underlying etiologies of HF, identify comorbidities, and establish baseline values (eg, of potassium and creatinine) for elements monitored during treatment.5,7 TABLE 15,7 lists the lab work and imaging tests that are commonly ordered in the initial evaluation of the patient with HF.

Echocardiography is useful in determining the ejection fraction (EF), which is essential in guiding treatment. Echocardiography can also identify important structural abnormalities including significant valvular disease. Refer patients with severe valvular disease for evaluation for valve repair/replacement, regardless of EF.8
Noninvasive testing (stress nuclear imaging or echocardiography) to evaluate for underlying CAD is reasonable in patients with unknown CAD status.8,11 Patients for whom there is a high suspicion of obstructive CAD should undergo coronary angiography if they are candidates for revascularization.5,7 Noninvasive testing may also be an acceptable option for assessing ischemia in patients presenting with HF who have known CAD and no angina.5
Classification of HF is determined by ejection fraction
Physicians have traditionally classified patients with HF as having either systolic or diastolic dysfunction. Patients with HF symptoms and a reduced EF were said to have systolic dysfunction; those with a normal EF were said to have diastolic dysfunction.
More recently, researchers have learned that patients with reduced EF and those with preserved EF can have both systolic and diastolic dysfunction simultaneously.8 Therefore, the current preferred terminology is HFpEF (heart failure with preserved ejection fraction) for those with an EF ≥50% and HFrEF (heart failure with reduced ejection fraction) for those with an EF ≤40%.5 Both the American Heart Association (AHA) and the European Society of Cardiology recognize a category of HF with moderately reduced ejection fraction defined as an EF between 40% and 50%.5,7 Practically speaking, this group is treated as per the guidelines for HFrEF.5
Treatment of HFrEF: The evidence is clear
The cornerstone of medical treatment for HFrEF is the combination of an ACE inhibitor or ARB with a beta-blocker.2,5,7,8 Several early trials showed clear benefits of these medications. For example, the Studies Of Left Ventricular Dysfunction trial (SOLVD), compared enalapril to placebo in patients receiving standard therapy (consisting chiefly of digitalis, diuretics, and nitrates). This study demonstrated a reduction in all-cause mortality or first hospitalization for HF (number needed to treat [NNT]=21) in the enalapril group vs the placebo group.12
Similarly, a subgroup analysis of the Valsartan Heart Failure Treatment (Val-HeFT) trial demonstrated morbidity (NNT=10) and all-cause mortality benefits (NNT=6) when valsartan (an ARB) was given to patients who were not receiving an ACE inhibitor.13
MERIT-HF (Metoprolol CR/XL Randomised Intervention Trial in congestive Heart Failure) compared the beta-blocker metoprolol succinate to placebo and found fewer deaths from HF and lower all-cause mortality (NNT=26) associated with the treatment group vs the placebo group.14
And a comparison of 2 beta-blockers—carvedilol and metoprolol tartrate—on clinical outcomes in patients with chronic HF in the Carvedilol Or Metoprolol European Trial (COMET) showed that carvedilol extended survival compared with metoprolol tartrate (NNT=19).15
Unlike ACE inhibitors and ARBs, which seem to show a class benefit, only 3 beta-blockers available in the United States have been proven to reduce mortality: sustained-release metoprolol succinate, carvedilol, and bisoprolol.2,7,8
Unless contraindicated, all patients with a reduced EF—even those without symptoms—should receive a beta-blocker and an ACE inhibitor or ARB.5,7,8
Cautionary notes
Remember the following caveats when treating patients with ACE inhibitors, ARBs, and beta-blockers:
- Use ACE inhibitors and ARBs with caution in patients with impaired renal function (serum creatinine >2.5 mg/dL) or elevated serum potassium (>5 mEq/L).16,17
- ARBs are associated with a much lower incidence of cough and angioedema than ACE inhibitors.18
- Although physicians frequently start patients on low doses of beta-blockers and ACE inhibitors or ARBs to minimize hypotension and other adverse effects, the goal of therapy is to titrate up to the therapeutic doses used in clinical trials.5-7 (For dosages of medications commonly used in the treatment of heart failure, see Table 3 in the American College of Cardiology/AHA/Heart Failure Society of America guidelines available at https://www.sciencedirect.com/science/article/pii/S0735109717370870?via%3Dihub#tbl3 and Table 7.2 in the European Society of Cardiology guidelines available at https://academic.oup.com/eurheartj/article/37/27/2129/1748921.)
- Because beta-blockers can exacerbate fluid retention, do not initiate them in patients with fluid overload unless such patients are being treated with diuretics.5,19
When more Tx is needed
For patients who remain symptomatic despite treatment with an ACE inhibitor or ARB and a beta-blocker, consider the following add-on therapies.
Diuretics are the only medications used in the treatment of HF that adequately reduce fluid overload.2,7 While thiazide diuretics confer greater blood pressure control, loop diuretics are generally preferred in the treatment of HF because they are more efficacious.5 Loop diuretics should be prescribed to all patients with fluid overload, as few patients can maintain their target (“dry”) weight without diuretic therapy.5,7 Common adverse effects include hypokalemia, dehydration, and azotemia.
Two MRAs are currently available in the United States: spironolactone and eplerenone. MRAs are used as add-on therapy for symptomatic patients with an EF ≤35% or an EF ≤40% following an acute myocardial infarction (MI).5 They significantly reduce all-cause mortality (NNT=26).20
Because hyperkalemia is a risk with MRAs, do not prescribe them for patients who are already taking both an ACE inhibitor and an ARB.5 Also, do not initiate MRA therapy in patients who have an elevated creatinine level (≥2.5 mg/dL in men; ≥2 mg/dL in women) or a potassium level ≥5 mEq/L.5,7,8 Discontinue MRA therapy if a patient’s potassium level rises to ≥5.5 mEq/L.5
Hydralazine combined with isosorbide dinitrate (H/ID) is an alternative in patients for whom ACE inhibitor/ARB therapy is contraindicated.5,8
H/ID is also an add-on option in African American patients. Trials have demonstrated that H/ID reduces both first hospitalization for HF (NNT=13) and all-cause mortality (NNT=25) when it is used as add-on therapy in African Americans already receiving standard therapy with an ACE inhibitor or ARB, a beta-blocker, and an MRA.21 Headache and dizziness are commonly reported adverse effects.
Digoxin does not reduce mortality, but it does improve both quality of life and exercise tolerance and reduces hospital admissions for patients with HF.5,7 Significant adverse effects of digoxin include anorexia, nausea, visual disturbances, and cardiac arrhythmias.22
Also, hypokalemia can intensify digoxin toxicity.23 Because of these concerns, digoxin is typically dosed at 0.125 mg/d (0.125 mg every other day in patients >70 years or patients with impaired renal function or low body weight) with a target therapeutic range of 0.5 to 0.9 ng/mL.5
New classes, new agents
Sacubitril, a neprilysin inhibitor, is the first drug from this class approved for use in the United States. Neprilysin is the enzyme responsible for the degradation of natriuretic peptides; as such it increases endogenous NPs, promoting diuresis and lowering blood pressure.24,25 Early trials with sacubitril alone showed limited clinical efficacy;25 however, when it was combined with the ARB, valsartan (the combination being called angiotensin receptor blocker + neprilysin inhibitor [ARNI] therapy), it was found to be of significant benefit.6,25
The PARADIGM-HF (Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure) trial compared outcomes in patients receiving ARNI therapy to those receiving enalapril.26 The authors stopped the trial early due to the overwhelming benefit seen in the ARNI arm.
After a median follow-up of 27 months, the researchers found a reduction in the primary outcomes of either cardiovascular death or first hospitalization for HF (26.5% in the enalapril-treated group vs 21.8% in the ARNI-treated group; NNT=21).26 There were slightly more cases of angioedema in the ARNI arm than in the enalapril arm (0.5% vs 0.2%), although there were no patients in the trial who required endotracheal intubation.26
Because of this increased risk, do not prescribe ARNI therapy for any patient with a history of angioedema.6 Hypotension was more common in the ARNI-treated group than in the enalapril group (14% vs 9.2%), but there were lower rates of hyperkalemia, elevated serum creatinine, and cough in the ARNI-treated group than in the enalapril group.26
Consider ARNI treatment for all patients with an EF ≤40% who remain symptomatic despite appropriate doses of an ACE inhibitor or ARB plus a beta-blocker. Do not administer ARNI therapy concomitantly with an ACE inhibitor or ARB. When switching, do not start ARNI therapy for at least 36 hours after the last dose of an ACE inhibitor or ARB.6
Ivabradine is a sinoatrial node modulator that provides additional heart rate reduction. It does not affect ventricular repolarization or myocardial contractility.27 Early trials with this medication have shown reduced cardiac mortality and an NNT to prevent one first HF hospitalization within one year of 27.28 Adverse effects include symptomatic and asymptomatic bradycardia and luminous phenomena.28
Recommend ivabradine as add-on therapy to all patients with an EF ≤35%, normal sinus rhythm, and resting heart rate ≥70 bpm who remain symptomatic despite taking the maximum-tolerated dose of a beta-blocker.6 The dose is adjusted to achieve a resting heart rate of 50 to 60 bpm.27
Nonpharmacologic options
Implantable cardioverter defibrillators (ICDs) are recommended as primary prevention in select HFrEF patients to reduce the risk of sudden cardiac death and all-cause mortality. The 2013 American College of Cardiology Foundation/AHA Guideline for the Management of Heart Failure recommends an ICD for primary prevention for: 1) patients with symptomatic HF and an LVEF ≤35% despite ≥3 months of optimal medical therapy, and 2) patients at least 40 days post-MI with an LVEF of ≤30%.5,29 ICDs are not recommended for patients who have a life expectancy of less than one year, and the devices are of unclear benefit for patients ≥75 years of age.5
Cardiac resynchronization therapy (CRT), although not new to the field of cardiology, is new to the treatment of heart failure. A number of patients with HFrEF have QRS prolongation and in particular, left bundle branch block (LBBB).5 CRT uses biventricular pacing to restore synchronous contraction of the left and right ventricles.30 It is strongly recommended for patients with an EF ≤35%, sinus rhythm, LBBB, QRS ≥150 ms, and a life expectancy of at least one year.5,7 It is weakly recommended for patients with an EF ≤35% and a QRS ≥150 ms but without LBBB. It’s also weakly recommended for patients with an EF ≤35% and LBBB with a QRS of 120 to 150 ms.5,31
Left ventricular assist devices (LVADs) and cardiac transplantation are considerations for patients with severe symptoms refractory to all other interventions.5 LVADs may be used either while awaiting cardiac transplantation (bridge therapy) or as definitive treatment (destination therapy). Appropriate patient selection for such therapies requires a team of experts that ideally includes HF and transplantation cardiologists, cardiothoracic surgeons, nurses, social workers, and palliative care clinicians.5
Treatment of HFpEF: Evidence is lacking
While HFpEF is common—affecting about half of all patients with HF—ideal treatment remains unclear.32 Some trials have shown promise, but to date no unequivocal evidence exists that any standard therapy reduces mortality in patients with HFpEF.33-37 Underlying mechanisms of action of HFpEF include cardiac rate and rhythm abnormalities, atrial dysfunction, and stiffening of the ventricles. In a sense, it represents an exaggerated expression of the pathophysiology seen with the normal aging of the heart and can be conceptualized as “presbycardia.”37 Indeed, HFpEF is more common in the elderly, but it is also more common in patients of African descent.38,39 Common contributing causes (which we’ll get to in a bit) include HTN, CAD, atrial fibrillation (AF), obesity, and obstructive sleep apnea (OSA).
Trials have failed to show clear benefit for ACE inhibitors, ARBs, or beta-blockers.7,33 The evidence for MRAs is somewhat unclear; however, they have recently been recommended as an option for patients who have been hospitalized in the last year to reduce the risk of subsequent hospitalizations.40 Digoxin is used primarily for rate control in the setting of AF, but otherwise is of unclear benefit.7 A low-sodium diet (ie, ≤2 g/d) may be useful in those patients who are prone to fluid overload.5,7 The cornerstone of treatment of HFpEF is the relief of volume overload with diuretics and the treatment of coexisting conditions.33
Common contributing causes of HFpEF
HTN is not only a common contributing cause, but also the most common comorbid condition affecting patients with HFpEF. As such, treatment of HTN represents the most important management goal.33,34 Based on recent data, the American College of Cardiology, the AHA, and the Heart Failure Society of America have recommended a systolic blood pressure goal <130 mm Hg for patients with HFpEF.40 Most patients with HFpEF and HTN will have some degree of fluid overload and, therefore, should receive a diuretic.
CAD. Patients with HFpEF should be evaluated for CAD and treated with medical management and coronary revascularization, as appropriate.
AF is poorly tolerated by patients with HFpEF.37 Patients with AF should receive anticoagulation and rate control medications, and those with persistent HF symptoms should be evaluated for rhythm control.33
Obesity is more prevalent in patients with HFpEF than in those with HFrEF.41 Although there is indirect evidence that weight loss improves cardiac function,34,42,43 and studies have shown bariatric surgery to improve diastolic function,44,45 there are no studies reporting clinical outcomes.
Treatment of OSA with continuous positive airway pressure appears to alleviate some symptoms of HF and to reduce all-cause mortality.46,47
Keeping HF patients out of the hospital
Many readmissions to the hospital for HF exacerbation are preventable. Patients often do not understand hospital discharge instructions or the nature of their chronic disease and its management.48-51 Routine follow-up in the office or clinic provides an opportunity to improve quality of life for patients and decrease admissions.7,52
A major role for the family physician is in the co-creation of, and adherence to, an individualized, comprehensive care plan. Make sure such a plan is easily understood not only by the patient with HF, but also by his or her care team. In addition, it should be evidence-based and reflect the patient’s culture, values, and goals of treatment.5,7
At each visit, the family physician or a member of the health care team should assess adherence to guideline-directed medical therapy, measure weight, evaluate fluid status, and provide ongoing patient education including information on the importance of activity, monitoring weight daily, and moderating fluid, salt, and alcohol intake.5,52
Research shows that cardiac rehabilitation improves functional capacity, exercise duration, quality of life, and mortality. Therefore, recommend it to all symptomatic patients with HF who are clinically stable.2
Consider collaboration with a subspecialist. Patients who remain symptomatic despite optimal medical management and patients with recurrent hospitalizations are best managed in conjunction with a subspecialist in HF treatment.2,5
CORRESPONDENCE
Darin Brink, MD, 420 Delaware St. SE, MMC 381, Minneapolis, MN 55455; [email protected].
Heart failure (HF) affects nearly 6 million Americans and accounts for one million hospital admissions each year.1 The condition, which results from a structural or functional disorder that impairs the ventricles’ ability to fill, empty, or both,2 is a major cause of morbidity and mortality. The 5-year mortality rate ranges from 44% to 77%.3,4
Growing evidence demonstrates reduced morbidity and mortality when patients with HF with reduced ejection fraction (HFrEF) are treated with an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin receptor blocker (ARB); a beta-blocker; and a mineralocorticoid/aldosterone receptor antagonist (MRA) in appropriate doses.5 In addition, 2 new medications representing novel drug classes have recently entered the market and are recommended in select patients who remain symptomatic despite standard treatment.
The first is sacubitril, which is available in a combination pill with the ARB valsartan, and the second is ivabradine.6 Additionally, implanted medical devices are proving useful, particularly in the management of patients with refractory symptoms.
This article will briefly review the diagnosis and initial evaluation of the patient with suspected HF and then describe how newer treatments fit within HF management priorities and strategies. But first, a word about what causes HF.
Causes are many and diverse
HF has a variety of cardiac and non-cardiac etiologies.2,7,8 Some important cardiac causes include hypertension (HTN), coronary artery disease (CAD), valvular heart disease, arrhythmias, myocarditis, Takotsubo cardiomyopathy, and postpartum cardiomyopathy. Common and important non-cardiac causes of HF include alcoholic cardiomyopathy, pulmonary embolism, pulmonary hypertension, obstructive sleep apnea, anemia, hemochromatosis, amyloidosis, sarcoidosis, thyroid dysfunction, nephrotic syndrome, and cardiac toxins (especially stimulants and certain chemotherapy drugs).2,7,8
Diagnosing an elusive culprit
HF remains a clinical diagnosis. Common symptoms include dyspnea, cough, pedal edema, and decreased exercise tolerance, but these symptoms are not at all specific. Given the varied causes and manifestations of HF, the diagnosis can be somewhat elusive. Fortunately, there are a number of objective methods to help identify patients with HF.
Framingham criteria. One commonly used tool for making the diagnosis of HF is the Framingham criteria (see https://www.mdcalc.com/framingham-heart-failure-diagnostic-criteria),9 which diagnoses HF based on historical and physical exam findings. Another well-validated decision tool is the Heart Failure Diagnostic Rule (see http://circ.ahajournals.org/content/124/25/2865.long),10 which incorporates N-terminal pro–B-type natriuretic peptide (NT-proBNP) results, as well as exam findings.
Measurement of natriuretic peptides, either B-type natriuretic peptide (BNP) or NT-proBNP, aids in the diagnosis of HF.5 Although several factors (including age, weight, and renal function) can affect BNP levels, a normal BNP value effectively rules out HF5,7 and an elevated BNP can help to make the diagnosis in the context of a patient with corresponding symptoms.
The initial evaluation: Necessary lab work and imaging studies
The purpose of the initial evaluation of the patient with suspected HF is to establish the diagnosis, look for underlying etiologies of HF, identify comorbidities, and establish baseline values (eg, of potassium and creatinine) for elements monitored during treatment.5,7 TABLE 15,7 lists the lab work and imaging tests that are commonly ordered in the initial evaluation of the patient with HF.

Echocardiography is useful in determining the ejection fraction (EF), which is essential in guiding treatment. Echocardiography can also identify important structural abnormalities including significant valvular disease. Refer patients with severe valvular disease for evaluation for valve repair/replacement, regardless of EF.8
Noninvasive testing (stress nuclear imaging or echocardiography) to evaluate for underlying CAD is reasonable in patients with unknown CAD status.8,11 Patients for whom there is a high suspicion of obstructive CAD should undergo coronary angiography if they are candidates for revascularization.5,7 Noninvasive testing may also be an acceptable option for assessing ischemia in patients presenting with HF who have known CAD and no angina.5
Classification of HF is determined by ejection fraction
Physicians have traditionally classified patients with HF as having either systolic or diastolic dysfunction. Patients with HF symptoms and a reduced EF were said to have systolic dysfunction; those with a normal EF were said to have diastolic dysfunction.
More recently, researchers have learned that patients with reduced EF and those with preserved EF can have both systolic and diastolic dysfunction simultaneously.8 Therefore, the current preferred terminology is HFpEF (heart failure with preserved ejection fraction) for those with an EF ≥50% and HFrEF (heart failure with reduced ejection fraction) for those with an EF ≤40%.5 Both the American Heart Association (AHA) and the European Society of Cardiology recognize a category of HF with moderately reduced ejection fraction defined as an EF between 40% and 50%.5,7 Practically speaking, this group is treated as per the guidelines for HFrEF.5
Treatment of HFrEF: The evidence is clear
The cornerstone of medical treatment for HFrEF is the combination of an ACE inhibitor or ARB with a beta-blocker.2,5,7,8 Several early trials showed clear benefits of these medications. For example, the Studies Of Left Ventricular Dysfunction trial (SOLVD), compared enalapril to placebo in patients receiving standard therapy (consisting chiefly of digitalis, diuretics, and nitrates). This study demonstrated a reduction in all-cause mortality or first hospitalization for HF (number needed to treat [NNT]=21) in the enalapril group vs the placebo group.12
Similarly, a subgroup analysis of the Valsartan Heart Failure Treatment (Val-HeFT) trial demonstrated morbidity (NNT=10) and all-cause mortality benefits (NNT=6) when valsartan (an ARB) was given to patients who were not receiving an ACE inhibitor.13
MERIT-HF (Metoprolol CR/XL Randomised Intervention Trial in congestive Heart Failure) compared the beta-blocker metoprolol succinate to placebo and found fewer deaths from HF and lower all-cause mortality (NNT=26) associated with the treatment group vs the placebo group.14
And a comparison of 2 beta-blockers—carvedilol and metoprolol tartrate—on clinical outcomes in patients with chronic HF in the Carvedilol Or Metoprolol European Trial (COMET) showed that carvedilol extended survival compared with metoprolol tartrate (NNT=19).15
Unlike ACE inhibitors and ARBs, which seem to show a class benefit, only 3 beta-blockers available in the United States have been proven to reduce mortality: sustained-release metoprolol succinate, carvedilol, and bisoprolol.2,7,8
Unless contraindicated, all patients with a reduced EF—even those without symptoms—should receive a beta-blocker and an ACE inhibitor or ARB.5,7,8
Cautionary notes
Remember the following caveats when treating patients with ACE inhibitors, ARBs, and beta-blockers:
- Use ACE inhibitors and ARBs with caution in patients with impaired renal function (serum creatinine >2.5 mg/dL) or elevated serum potassium (>5 mEq/L).16,17
- ARBs are associated with a much lower incidence of cough and angioedema than ACE inhibitors.18
- Although physicians frequently start patients on low doses of beta-blockers and ACE inhibitors or ARBs to minimize hypotension and other adverse effects, the goal of therapy is to titrate up to the therapeutic doses used in clinical trials.5-7 (For dosages of medications commonly used in the treatment of heart failure, see Table 3 in the American College of Cardiology/AHA/Heart Failure Society of America guidelines available at https://www.sciencedirect.com/science/article/pii/S0735109717370870?via%3Dihub#tbl3 and Table 7.2 in the European Society of Cardiology guidelines available at https://academic.oup.com/eurheartj/article/37/27/2129/1748921.)
- Because beta-blockers can exacerbate fluid retention, do not initiate them in patients with fluid overload unless such patients are being treated with diuretics.5,19
When more Tx is needed
For patients who remain symptomatic despite treatment with an ACE inhibitor or ARB and a beta-blocker, consider the following add-on therapies.
Diuretics are the only medications used in the treatment of HF that adequately reduce fluid overload.2,7 While thiazide diuretics confer greater blood pressure control, loop diuretics are generally preferred in the treatment of HF because they are more efficacious.5 Loop diuretics should be prescribed to all patients with fluid overload, as few patients can maintain their target (“dry”) weight without diuretic therapy.5,7 Common adverse effects include hypokalemia, dehydration, and azotemia.
Two MRAs are currently available in the United States: spironolactone and eplerenone. MRAs are used as add-on therapy for symptomatic patients with an EF ≤35% or an EF ≤40% following an acute myocardial infarction (MI).5 They significantly reduce all-cause mortality (NNT=26).20
Because hyperkalemia is a risk with MRAs, do not prescribe them for patients who are already taking both an ACE inhibitor and an ARB.5 Also, do not initiate MRA therapy in patients who have an elevated creatinine level (≥2.5 mg/dL in men; ≥2 mg/dL in women) or a potassium level ≥5 mEq/L.5,7,8 Discontinue MRA therapy if a patient’s potassium level rises to ≥5.5 mEq/L.5
Hydralazine combined with isosorbide dinitrate (H/ID) is an alternative in patients for whom ACE inhibitor/ARB therapy is contraindicated.5,8
H/ID is also an add-on option in African American patients. Trials have demonstrated that H/ID reduces both first hospitalization for HF (NNT=13) and all-cause mortality (NNT=25) when it is used as add-on therapy in African Americans already receiving standard therapy with an ACE inhibitor or ARB, a beta-blocker, and an MRA.21 Headache and dizziness are commonly reported adverse effects.
Digoxin does not reduce mortality, but it does improve both quality of life and exercise tolerance and reduces hospital admissions for patients with HF.5,7 Significant adverse effects of digoxin include anorexia, nausea, visual disturbances, and cardiac arrhythmias.22
Also, hypokalemia can intensify digoxin toxicity.23 Because of these concerns, digoxin is typically dosed at 0.125 mg/d (0.125 mg every other day in patients >70 years or patients with impaired renal function or low body weight) with a target therapeutic range of 0.5 to 0.9 ng/mL.5
New classes, new agents
Sacubitril, a neprilysin inhibitor, is the first drug from this class approved for use in the United States. Neprilysin is the enzyme responsible for the degradation of natriuretic peptides; as such it increases endogenous NPs, promoting diuresis and lowering blood pressure.24,25 Early trials with sacubitril alone showed limited clinical efficacy;25 however, when it was combined with the ARB, valsartan (the combination being called angiotensin receptor blocker + neprilysin inhibitor [ARNI] therapy), it was found to be of significant benefit.6,25
The PARADIGM-HF (Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure) trial compared outcomes in patients receiving ARNI therapy to those receiving enalapril.26 The authors stopped the trial early due to the overwhelming benefit seen in the ARNI arm.
After a median follow-up of 27 months, the researchers found a reduction in the primary outcomes of either cardiovascular death or first hospitalization for HF (26.5% in the enalapril-treated group vs 21.8% in the ARNI-treated group; NNT=21).26 There were slightly more cases of angioedema in the ARNI arm than in the enalapril arm (0.5% vs 0.2%), although there were no patients in the trial who required endotracheal intubation.26
Because of this increased risk, do not prescribe ARNI therapy for any patient with a history of angioedema.6 Hypotension was more common in the ARNI-treated group than in the enalapril group (14% vs 9.2%), but there were lower rates of hyperkalemia, elevated serum creatinine, and cough in the ARNI-treated group than in the enalapril group.26
Consider ARNI treatment for all patients with an EF ≤40% who remain symptomatic despite appropriate doses of an ACE inhibitor or ARB plus a beta-blocker. Do not administer ARNI therapy concomitantly with an ACE inhibitor or ARB. When switching, do not start ARNI therapy for at least 36 hours after the last dose of an ACE inhibitor or ARB.6
Ivabradine is a sinoatrial node modulator that provides additional heart rate reduction. It does not affect ventricular repolarization or myocardial contractility.27 Early trials with this medication have shown reduced cardiac mortality and an NNT to prevent one first HF hospitalization within one year of 27.28 Adverse effects include symptomatic and asymptomatic bradycardia and luminous phenomena.28
Recommend ivabradine as add-on therapy to all patients with an EF ≤35%, normal sinus rhythm, and resting heart rate ≥70 bpm who remain symptomatic despite taking the maximum-tolerated dose of a beta-blocker.6 The dose is adjusted to achieve a resting heart rate of 50 to 60 bpm.27
Nonpharmacologic options
Implantable cardioverter defibrillators (ICDs) are recommended as primary prevention in select HFrEF patients to reduce the risk of sudden cardiac death and all-cause mortality. The 2013 American College of Cardiology Foundation/AHA Guideline for the Management of Heart Failure recommends an ICD for primary prevention for: 1) patients with symptomatic HF and an LVEF ≤35% despite ≥3 months of optimal medical therapy, and 2) patients at least 40 days post-MI with an LVEF of ≤30%.5,29 ICDs are not recommended for patients who have a life expectancy of less than one year, and the devices are of unclear benefit for patients ≥75 years of age.5
Cardiac resynchronization therapy (CRT), although not new to the field of cardiology, is new to the treatment of heart failure. A number of patients with HFrEF have QRS prolongation and in particular, left bundle branch block (LBBB).5 CRT uses biventricular pacing to restore synchronous contraction of the left and right ventricles.30 It is strongly recommended for patients with an EF ≤35%, sinus rhythm, LBBB, QRS ≥150 ms, and a life expectancy of at least one year.5,7 It is weakly recommended for patients with an EF ≤35% and a QRS ≥150 ms but without LBBB. It’s also weakly recommended for patients with an EF ≤35% and LBBB with a QRS of 120 to 150 ms.5,31
Left ventricular assist devices (LVADs) and cardiac transplantation are considerations for patients with severe symptoms refractory to all other interventions.5 LVADs may be used either while awaiting cardiac transplantation (bridge therapy) or as definitive treatment (destination therapy). Appropriate patient selection for such therapies requires a team of experts that ideally includes HF and transplantation cardiologists, cardiothoracic surgeons, nurses, social workers, and palliative care clinicians.5
Treatment of HFpEF: Evidence is lacking
While HFpEF is common—affecting about half of all patients with HF—ideal treatment remains unclear.32 Some trials have shown promise, but to date no unequivocal evidence exists that any standard therapy reduces mortality in patients with HFpEF.33-37 Underlying mechanisms of action of HFpEF include cardiac rate and rhythm abnormalities, atrial dysfunction, and stiffening of the ventricles. In a sense, it represents an exaggerated expression of the pathophysiology seen with the normal aging of the heart and can be conceptualized as “presbycardia.”37 Indeed, HFpEF is more common in the elderly, but it is also more common in patients of African descent.38,39 Common contributing causes (which we’ll get to in a bit) include HTN, CAD, atrial fibrillation (AF), obesity, and obstructive sleep apnea (OSA).
Trials have failed to show clear benefit for ACE inhibitors, ARBs, or beta-blockers.7,33 The evidence for MRAs is somewhat unclear; however, they have recently been recommended as an option for patients who have been hospitalized in the last year to reduce the risk of subsequent hospitalizations.40 Digoxin is used primarily for rate control in the setting of AF, but otherwise is of unclear benefit.7 A low-sodium diet (ie, ≤2 g/d) may be useful in those patients who are prone to fluid overload.5,7 The cornerstone of treatment of HFpEF is the relief of volume overload with diuretics and the treatment of coexisting conditions.33
Common contributing causes of HFpEF
HTN is not only a common contributing cause, but also the most common comorbid condition affecting patients with HFpEF. As such, treatment of HTN represents the most important management goal.33,34 Based on recent data, the American College of Cardiology, the AHA, and the Heart Failure Society of America have recommended a systolic blood pressure goal <130 mm Hg for patients with HFpEF.40 Most patients with HFpEF and HTN will have some degree of fluid overload and, therefore, should receive a diuretic.
CAD. Patients with HFpEF should be evaluated for CAD and treated with medical management and coronary revascularization, as appropriate.
AF is poorly tolerated by patients with HFpEF.37 Patients with AF should receive anticoagulation and rate control medications, and those with persistent HF symptoms should be evaluated for rhythm control.33
Obesity is more prevalent in patients with HFpEF than in those with HFrEF.41 Although there is indirect evidence that weight loss improves cardiac function,34,42,43 and studies have shown bariatric surgery to improve diastolic function,44,45 there are no studies reporting clinical outcomes.
Treatment of OSA with continuous positive airway pressure appears to alleviate some symptoms of HF and to reduce all-cause mortality.46,47
Keeping HF patients out of the hospital
Many readmissions to the hospital for HF exacerbation are preventable. Patients often do not understand hospital discharge instructions or the nature of their chronic disease and its management.48-51 Routine follow-up in the office or clinic provides an opportunity to improve quality of life for patients and decrease admissions.7,52
A major role for the family physician is in the co-creation of, and adherence to, an individualized, comprehensive care plan. Make sure such a plan is easily understood not only by the patient with HF, but also by his or her care team. In addition, it should be evidence-based and reflect the patient’s culture, values, and goals of treatment.5,7
At each visit, the family physician or a member of the health care team should assess adherence to guideline-directed medical therapy, measure weight, evaluate fluid status, and provide ongoing patient education including information on the importance of activity, monitoring weight daily, and moderating fluid, salt, and alcohol intake.5,52
Research shows that cardiac rehabilitation improves functional capacity, exercise duration, quality of life, and mortality. Therefore, recommend it to all symptomatic patients with HF who are clinically stable.2
Consider collaboration with a subspecialist. Patients who remain symptomatic despite optimal medical management and patients with recurrent hospitalizations are best managed in conjunction with a subspecialist in HF treatment.2,5
CORRESPONDENCE
Darin Brink, MD, 420 Delaware St. SE, MMC 381, Minneapolis, MN 55455; [email protected].
1. Hall MJ, Levant S, DeFrances CJ. Hospitalization for congestive heart failure: United States, 2000-2010. NCHS Data Brief. 2012;(108):1-8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23102190. Accessed April 26, 2017.
2. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused Update Incorporated Into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
3. Passantino A, Guida P, Lagioia R, et al. Predictors of long-term mortality in older patients hospitalized for acutely decompensated heart failure: clinical relevance of natriuretic peptides. J Am Geriatr Soc. 2017;65:822-826.
4. Lassus JP, Siirilä-Waris K, Nieminen MS, et al. Long-term survival after hospitalization for acute heart failure—differences in prognosis of acutely decompensated chronic and new-onset acute heart failure. Int J Cardiol. 2013;168:458-462.
5. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
6. Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. J Am Coll Cardiol. 2016;68:1476-1488.
7. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129-2200.
8. Pinkerman CP, Sander JE, Breeding D, et al. Institute for Clinical Systems Improvement. Heart failure in adults. Available at: https://www.scribd.com/document/310893227/HeartFailure-pdf. Accessed December 6, 2017.
9. McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham Study. N Engl J Med. 1971;285:1441-1446.
10. Kelder JC, Cramer MJ, van Wijngaarden J, et al. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation. 2011;124:2865-2873.
11. Heart Failure Society of America, Lindenfeld J, Albert NM, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1-194.
12. Pouleur H, The SOLVD Investigators. Results of the treatment trial of the studies of left ventricular dysfunction (SOLVD). Am J Cardiol. 1992;70:135-136.
13. Maggioni AP, Anand I, Gottlieb SO, et al. Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin-converting enzyme inhibitors. J Am Coll Cardiol. 2002;40:1414-1421.
14. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001-2007.
15. Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7-13.
16. Gehr TW, Sica DA. Pharmacotherapy in congestive heart failure: Hyperkalemia in congestive heart failure. Congest Heart Fail. 2001;7:97-100.
17. National Institute for Health and Clinical Excellence (NICE). Chronic heart failure in adults: management. 2010. Available at: https://www.nice.org.uk/guidance/cg108. Accessed November 27, 2017.
18. Barreras A, Gurk-Turner C. Angiotensin II receptor blockers. Proc (Bayl Univ Med Cent). 2003;16:123-126.
19. Epstein SE, Braunwald E. The effect of beta adrenergic blockade on patterns of urinary sodium excretion: studies in normal subjects and in patients with heart disease. Ann Intern Med. 1966;65:20-27.
20. Berbenetz NM, Mrkobrada M. Mineralocorticoid receptor antagonists for heart failure: systematic review and meta-analysis. BMC Cardiovasc Disord. 2016;16:246.
21. Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049-2057.
22. Kelly RA, Smith TW. Recognition and management of digitalis toxicity. Am J Cardiol. 1992;69:108G-118G.
23. Sundar S, Burma DP, Vaish SK. Digoxin toxicity and electrolytes: a correlative study. Acta Cardiol. 1983;38:115-123.
24. McDowell G, Nicholls DP. The endopeptidase inhibitor, candoxatril, and its therapeutic potential in the treatment of chronic cardiac failure in man. Expert Opin Investig Drugs. 1999;8:79-84.
25. Prenner SB, Shah SJ, Yancy CW. Role of angiotensin receptor-neprilysin inhibition in heart failure. Curr Atheroscler Rep. 2016;18:48.
26. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004.
27. Corlanor package insert. Amgen Inc., Thousand Oaks, CA. Available at: http://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/corlanor/corlanor_pi.pdf. Accessed November 28, 2017.
28. Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875-885.
29. Kusumoto FM, Calkins H, Boehmer J, et al. HRS/ACC/AHA expert consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. Circulation. 2014;130:94-125.
30. Leyva F, Nisam S, Auricchio A. 20 years of cardiac resynchronization therapy. J Am Coll Cardiol. 2014;64:1047-1058.
31. Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS Focused Update Incorporated Into the ACCF/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013;127:e283-e352.
32. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670-679.
33. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2016;375:1868-1877.
34. Nanayakkara S, Kaye DM. Management of heart failure with preserved ejection fraction: a review. Clin Ther. 2015;37:2186-2198.
35. Cleland JG, Pellicori P, Dierckx R. Clinical trials in patients with heart failure and preserved left ventricular ejection fraction. Heart Fail Clin. 2014;10:511-523.
36. Ferrari R, Böhm M, Cleland JGF, et al. Heart failure with preserved ejection fraction: uncertainties and dilemmas. Eur J Heart Fail. 2015;17:665-671.
37. Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507-515.
38. Sharp A, Tapp R, Francis DP, et al. Ethnicity and left ventricular diastolic function in hypertension an ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J Am Coll Cardiol. 2008;52:1015-1021.
39. Zile MR. Heart failure with a preserved ejection fraction. In: Mann DL, Zipes D, Libby P BR, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 10th ed. Phila
40. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776-803.
41. Mentz RJ, Kelly JP, von Lueder TG, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281-2293.
42. de las Fuentes L, Waggoner AD, Mohammed BS, et al. Effect of moderate diet-induced weight loss and weight regain on cardiovascular structure and function. J Am Coll Cardiol. 2009;54:2376-2381.
43. Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction. JAMA. 2016;315:36-46.
44. Rider OJ, Francis JM, Ali MK, et al. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J Am Coll Cardiol. 2009;54:718-726.
45. Ristow B, Rabkin J, Haeusslein E. Improvement in dilated cardiomyopathy after bariatric surgery. J Card Fail. 2008;14:198-202.
46. Yoshihisa A, Suzuki S, Yamauchi H, et al. Beneficial effects of positive airway pressure therapy for sleep-disordered breathing in heart failure patients with preserved left ventricular ejection fraction. Clin Cardiol. 2015;38:413-421.
47. Shah RV, Abbasi SA, Heydari B, et al. Obesity and sleep apnea are independently associated with adverse left ventricular remodeling and clinical outcome in patients with atrial fibrillation and preserved ventricular function. Am Heart J. 2014;167:620-626.
48. Riegel B, Moser DK, Anker SD, et al. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120:1141-1163.
49. Moser DK, Doering LV, Chung ML. Vulnerabilities of patients recovering from an exacerbation of chronic heart failure. Am Heart J. 2005;150:984.
50. Bernheim SM, Grady JN, Lin Z, et al. National patterns of risk-standardized mortality and readmission for acute myocardial infarction and heart failure: update on publicly reported outcomes measures based on the 2010 release. Circ Cardiovasc Qual Outcomes. 2010;3:459-467.
51. Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2:407-413.
52. Cowie MR, Anker SD, Cleland JG, et al. Improving care for patients with acute heart failure: before, during and after hospitalization. Available at: http://www.oxfordhealthpolicyforum.org/files/reports/ahf-report.pdf. Accessed November 27, 2017.
1. Hall MJ, Levant S, DeFrances CJ. Hospitalization for congestive heart failure: United States, 2000-2010. NCHS Data Brief. 2012;(108):1-8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23102190. Accessed April 26, 2017.
2. Hunt SA, Abraham WT, Chin MH, et al. 2009 Focused Update Incorporated Into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the International Society for Heart and Lung Transplantation. J Am Coll Cardiol. 2009;53:e1-e90.
3. Passantino A, Guida P, Lagioia R, et al. Predictors of long-term mortality in older patients hospitalized for acutely decompensated heart failure: clinical relevance of natriuretic peptides. J Am Geriatr Soc. 2017;65:822-826.
4. Lassus JP, Siirilä-Waris K, Nieminen MS, et al. Long-term survival after hospitalization for acute heart failure—differences in prognosis of acutely decompensated chronic and new-onset acute heart failure. Int J Cardiol. 2013;168:458-462.
5. Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240-e327.
6. Yancy CW, Jessup M, Bozkurt B, et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure. J Am Coll Cardiol. 2016;68:1476-1488.
7. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129-2200.
8. Pinkerman CP, Sander JE, Breeding D, et al. Institute for Clinical Systems Improvement. Heart failure in adults. Available at: https://www.scribd.com/document/310893227/HeartFailure-pdf. Accessed December 6, 2017.
9. McKee PA, Castelli WP, McNamara PM, et al. The natural history of congestive heart failure: the Framingham Study. N Engl J Med. 1971;285:1441-1446.
10. Kelder JC, Cramer MJ, van Wijngaarden J, et al. The diagnostic value of physical examination and additional testing in primary care patients with suspected heart failure. Circulation. 2011;124:2865-2873.
11. Heart Failure Society of America, Lindenfeld J, Albert NM, et al. HFSA 2010 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2010;16:e1-194.
12. Pouleur H, The SOLVD Investigators. Results of the treatment trial of the studies of left ventricular dysfunction (SOLVD). Am J Cardiol. 1992;70:135-136.
13. Maggioni AP, Anand I, Gottlieb SO, et al. Effects of valsartan on morbidity and mortality in patients with heart failure not receiving angiotensin-converting enzyme inhibitors. J Am Coll Cardiol. 2002;40:1414-1421.
14. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet. 1999;353:2001-2007.
15. Poole-Wilson PA, Swedberg K, Cleland JG, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7-13.
16. Gehr TW, Sica DA. Pharmacotherapy in congestive heart failure: Hyperkalemia in congestive heart failure. Congest Heart Fail. 2001;7:97-100.
17. National Institute for Health and Clinical Excellence (NICE). Chronic heart failure in adults: management. 2010. Available at: https://www.nice.org.uk/guidance/cg108. Accessed November 27, 2017.
18. Barreras A, Gurk-Turner C. Angiotensin II receptor blockers. Proc (Bayl Univ Med Cent). 2003;16:123-126.
19. Epstein SE, Braunwald E. The effect of beta adrenergic blockade on patterns of urinary sodium excretion: studies in normal subjects and in patients with heart disease. Ann Intern Med. 1966;65:20-27.
20. Berbenetz NM, Mrkobrada M. Mineralocorticoid receptor antagonists for heart failure: systematic review and meta-analysis. BMC Cardiovasc Disord. 2016;16:246.
21. Taylor AL, Ziesche S, Yancy C, et al. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med. 2004;351:2049-2057.
22. Kelly RA, Smith TW. Recognition and management of digitalis toxicity. Am J Cardiol. 1992;69:108G-118G.
23. Sundar S, Burma DP, Vaish SK. Digoxin toxicity and electrolytes: a correlative study. Acta Cardiol. 1983;38:115-123.
24. McDowell G, Nicholls DP. The endopeptidase inhibitor, candoxatril, and its therapeutic potential in the treatment of chronic cardiac failure in man. Expert Opin Investig Drugs. 1999;8:79-84.
25. Prenner SB, Shah SJ, Yancy CW. Role of angiotensin receptor-neprilysin inhibition in heart failure. Curr Atheroscler Rep. 2016;18:48.
26. McMurray JJ, Packer M, Desai AS, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004.
27. Corlanor package insert. Amgen Inc., Thousand Oaks, CA. Available at: http://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/corlanor/corlanor_pi.pdf. Accessed November 28, 2017.
28. Swedberg K, Komajda M, Böhm M, et al. Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet. 2010;376:875-885.
29. Kusumoto FM, Calkins H, Boehmer J, et al. HRS/ACC/AHA expert consensus statement on the use of implantable cardioverter-defibrillator therapy in patients who are not included or not well represented in clinical trials. Circulation. 2014;130:94-125.
30. Leyva F, Nisam S, Auricchio A. 20 years of cardiac resynchronization therapy. J Am Coll Cardiol. 2014;64:1047-1058.
31. Epstein AE, DiMarco JP, Ellenbogen KA, et al. 2012 ACCF/AHA/HRS Focused Update Incorporated Into the ACCF/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013;127:e283-e352.
32. Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32:670-679.
33. Redfield MM. Heart failure with preserved ejection fraction. N Engl J Med. 2016;375:1868-1877.
34. Nanayakkara S, Kaye DM. Management of heart failure with preserved ejection fraction: a review. Clin Ther. 2015;37:2186-2198.
35. Cleland JG, Pellicori P, Dierckx R. Clinical trials in patients with heart failure and preserved left ventricular ejection fraction. Heart Fail Clin. 2014;10:511-523.
36. Ferrari R, Böhm M, Cleland JGF, et al. Heart failure with preserved ejection fraction: uncertainties and dilemmas. Eur J Heart Fail. 2015;17:665-671.
37. Borlaug BA. The pathophysiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2014;11:507-515.
38. Sharp A, Tapp R, Francis DP, et al. Ethnicity and left ventricular diastolic function in hypertension an ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J Am Coll Cardiol. 2008;52:1015-1021.
39. Zile MR. Heart failure with a preserved ejection fraction. In: Mann DL, Zipes D, Libby P BR, eds. Braunwald’s Heart Disease: A Textbook of Cardiovascular Medicine. 10th ed. Phila
40. Yancy CW, Jessup M, Bozkurt B, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776-803.
41. Mentz RJ, Kelly JP, von Lueder TG, et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281-2293.
42. de las Fuentes L, Waggoner AD, Mohammed BS, et al. Effect of moderate diet-induced weight loss and weight regain on cardiovascular structure and function. J Am Coll Cardiol. 2009;54:2376-2381.
43. Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction. JAMA. 2016;315:36-46.
44. Rider OJ, Francis JM, Ali MK, et al. Beneficial cardiovascular effects of bariatric surgical and dietary weight loss in obesity. J Am Coll Cardiol. 2009;54:718-726.
45. Ristow B, Rabkin J, Haeusslein E. Improvement in dilated cardiomyopathy after bariatric surgery. J Card Fail. 2008;14:198-202.
46. Yoshihisa A, Suzuki S, Yamauchi H, et al. Beneficial effects of positive airway pressure therapy for sleep-disordered breathing in heart failure patients with preserved left ventricular ejection fraction. Clin Cardiol. 2015;38:413-421.
47. Shah RV, Abbasi SA, Heydari B, et al. Obesity and sleep apnea are independently associated with adverse left ventricular remodeling and clinical outcome in patients with atrial fibrillation and preserved ventricular function. Am Heart J. 2014;167:620-626.
48. Riegel B, Moser DK, Anker SD, et al. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120:1141-1163.
49. Moser DK, Doering LV, Chung ML. Vulnerabilities of patients recovering from an exacerbation of chronic heart failure. Am Heart J. 2005;150:984.
50. Bernheim SM, Grady JN, Lin Z, et al. National patterns of risk-standardized mortality and readmission for acute myocardial infarction and heart failure: update on publicly reported outcomes measures based on the 2010 release. Circ Cardiovasc Qual Outcomes. 2010;3:459-467.
51. Krumholz HM, Merrill AR, Schone EM, et al. Patterns of hospital performance in acute myocardial infarction and heart failure 30-day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2:407-413.
52. Cowie MR, Anker SD, Cleland JG, et al. Improving care for patients with acute heart failure: before, during and after hospitalization. Available at: http://www.oxfordhealthpolicyforum.org/files/reports/ahf-report.pdf. Accessed November 27, 2017.
From The Journal of Family Practice | 2018;67(1):18-26.
PRACTICE RECOMMENDATIONS
› Order a measurement of B-type natriuretic peptide or N-terminal pro-B-type natriuretic peptide in patients with dyspnea to help diagnose and manage heart failure (HF). A
› Refer patients with symptomatic HF and a left ventricular ejection fraction (LVEF) ≤35% that persists despite ≥3 months of optimal medical therapy for an implantable cardioverter defibrillator to reduce the risk of sudden death and all-cause mortality. A
› Consider cardiac resynchronization therapy for patients with an LVEF ≤35%, sinus rhythm, left bundle branch block, and a QRS duration ≥150 ms who remain symptomatic despite optimal medical therapy. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The evidence for herbal and botanical remedies, Part 1
The National Center for Complementary and Integrative Health, a division of the National Institutes of Medicine, estimates that about 38% of American adults use complementary and alternative medicine.1 That statistic includes 17.7% who say they use natural products. Despite their popularity, many physicians remain skeptical—and for good reason. Enthusiasts frequently offer dramatic anecdotes to “prove” their supplements' worth, but little scientific support is available for most herbal remedies. There are, however, exceptions. As this review of the medical literature will reveal, there is evidence to support the use of capsaicin to relieve osteoarthritis (OA) and postherpetic neuralgia (PHN) and support for green tea to serve as a lipid-lowering agent and help treat diabetes. Similarly, researchers have found that peppermint may be of value in the management of irritable bowel syndrome (IBS). (We also review the literature on butterbur for migraine headaches, but serious safety issues exist; TABLE.)

In the second part of this series, which is available here, we explore what the evidence tells us about the use of turmeric, chamomile, rosemary, coffee, and cocoa.
Worth noting as you consider this—or any—review of herbals is that while there is limited scientific evidence to establish the safety and efficacy of most herbal products, they are nonetheless freely sold without US Food & Drug Administration (FDA) approval because under current regulations, they are considered dietary supplements. That legal designation means companies can manufacture, sell, and market herbs without first demonstrating safety and efficacy, as is required for pharmaceutical drugs. Because herbal medications do not require the same testing through the large randomized controlled trials (RCTs) required for pharmaceuticals, evidence is often based on smaller RCTs and other studies of lower overall quality. Despite these limitations, we believe it’s worth keeping an open mind about the value of evidence-based herbal and botanical treatments.
Capsaicin
Overview
Capsaicin, an active compound in chili peppers, provokes a burning sensation, but also has a long history of use in pain treatment.2 Qutenza, an FDA-approved chemically synthesized 8% capsaicin patch, is identical to the naturally occurring molecule.2 Topically applied capsaicin exerts its therapeutic effect by rapidly depleting substance P, thus reducing the transmission of pain from C fibers to higher neurologic centers in the area of administration.3
Meta-analyses and systematic reviews have shown capsaicin is effective for various painful conditions, including peripheral diabetic neuropathy, OA, and PHN.
Peripheral neuropathy.
OA. Capsaicin provides mild to moderate efficacy in randomized trials for patients with hand and knee OA, when compared with placebo.5-7 A systematic review of capsaicin for all osteoarthritic conditions noted that there was consistent evidence that capsaicin gel was effective for OA.8 However, a 2013 Cochrane review of only knee OA noted that capsicum extract did not provide significant clinical improvement for pain or function in knee OA and resulted in a significant number of adverse events.9
Low back pain (LBP). Based on a 2014 Cochrane review of 3 trials (755 subjects) of moderate quality, capsicum frutescens cream or plaster appeared more efficacious than placebo in people with chronic LBP.10 Based on current (low-quality) evidence in one trial, however, it’s not clear whether topical capsicum cream is more beneficial for acute LBP than a placebo.10
PHN. Topical 8% capsaicin is an FDA-approved treatment for PHN. A review and cost-effectiveness analysis demonstrated that 8% capsaicin had significantly higher effectiveness rates than the oral agents (tricyclic antidepressants, duloxetine, gabapentin, pregabalin) used to treat PHN.11 In addition, the cost-effectiveness analysis found that the capsaicin patch was similar in cost to a topical lidocaine patch and oral products for PHN.11
A meta-analysis of 7 RCTs indicated that 8% topical capsaicin was superior to the low-dose capsaicin patch for relieving pain associated with PHN.12
Adverse effects
Very few toxic effects have been reported during a half century of capsaicin use. Those that have been reported are mainly limited to mild local reactions.2 The most common adverse effect of topical capsaicin is local irritation (burning, stinging, and erythema), which had been reported to occur in approximately 40% of patients.6 Nevertheless, more than 90% of the subjects in clinical studies were able to complete the studies, and pain rapidly resolved after patch removal.2 Washing with soap and water may help prevent the compound from spreading to other parts of the body unintentionally.
The safety of the patch has been demonstrated with repeated dosing every 3 months for up to one year. However, the long-term risks of chronic capsaicin use and its effect on epidermal innervation are uncertain.5
The bottom line
Capsaicin appears to be an effective treatment for neuropathy and chronic LBP. It is FDA approved for the treatment of PHN. It may also benefit patients with OA and acute LBP. Serious adverse effects are uncommon with topical use. Common adverse effects include burning pain and irritation in the area of application, which can be intense and cause discontinuation.2
Butterbur
Overview
Petasites hybridus, also known as butterbur, is a member of the daisy family, Asteraceae, and is a perennial plant found throughout Europe and Asia.13 It was used as a remedy for ulcers, wounds, and inflammation in ancient Greece. Its calcium channel-blocking effects may counteract vasoconstriction and play a role in preventing hyper-excitation of neurons.14 Sesquiterpenes, the pharmacologically active compounds in butterbur, have strong anti-inflammatory and vasodilatory effects through lipoxygenase and leukotriene inhibition.14
Migraine headache. Butterbur appears to be effective in migraine prophylaxis. Several studies have shown butterbur to significantly reduce the number of migraine attacks per month when compared with placebo. In a small, randomized, placebo-controlled, parallel-group study on the efficacy and tolerability of a special butterbur root extract (Petadolex) for the prevention of migraine, response rate was 45% in the butterbur group vs 15% in the placebo group. Butterbur was well tolerated.15 Similar results were found in another RCT in which Petasites (butterbur) 75 mg bid significantly reduced migraine attack frequency by 48%, compared with 26% for the placebo group.16 Petadolex was well tolerated in this study, too, and no serious adverse events occurred. Findings suggest that 75 mg bid may be a good option for migraine prevention given the agent's safety profile.
Petadolex may also be a good option in pediatric migraine. A 2005 study in children and adolescents found that 77% of patients experienced a reduction in attacks by at least 50% with butterbur. Patients were treated with 50 mg to 150 mg over 4 months.17
In their guidelines for migraine prevention, the American Academy of Neurology (AAN) and American Headache Society gave butterbur a Level A recommendation and concluded that butterbur should be offered to patients with migraine to reduce the frequency and severity of migraine attacks.18 However, the AAN has since changed its position, stating that “The 2012 AAN guideline, ‘Evidence-based guideline update: NSAIDS and other complementary treatments for episodic migraine prevention in adults’ has been retired by the AAN Board of Directors on September 16, 2015, due to serious safety concerns with a preventative treatment, butterbur, recommended by this guideline. The recommendations and conclusions in all retired guidelines are considered no longer valid and no longer supported by the AAN.”19
Allergic rhinitis. Although the data is not convincing, some studies have shown that butterbur may be beneficial for the treatment of allergic rhinitis.20,21
Adverse effects
While the butterbur plant itself contains pyrrolizidine alkaloids (PA), which are hepatotoxic and carcinogenic, extracts of butterbur root that are almost completely free from these alkaloids are available. (Patients who choose to use butterbur should be advised to use only products that are certified and labeled pyrrolizidine alkaloids free.)
Petadolex, the medication used in migraine studies, was initially approved by the German health regulatory authority, but approval was later withdrawn due to concerns about liver toxicity.22 In 2012, the United Kingdom’s Medicines and Health Care Products Regulatory Agency withdrew all butterbur products from the market due to associated cases of liver toxicity.22 Petasites (butterbur) products are still available in the US market, and the risks and benefits should be discussed with all patients considering this treatment. Liver function monitoring is recommended for all patients using butterbur.22
The herb can also cause dyspepsia, headache, itchy eyes, gastrointestinal symptoms, asthma, fatigue, and drowsiness. Additionally, people who are allergic to ragweed and daisies may have allergic reactions to butterbur. Eructation (belching) occurred in 7% of patients in a pediatric study.17
The bottom line
Butterbur appears to be efficacious for migraine prophylaxis, but long-term safety is unknown and serious concerns exist for liver toxicity.
Green tea
Overview
Most tea leaves come from the Camellia sinensis bush, but green and black tea are processed differently to produce different end products.23 It is estimated that green tea accounts for approximately a quarter of all tea consumption, and is most commonly consumed in Asian countries.23 The health-promoting effects of green tea are mainly attributed to its polyphenol content.24 While there are many types of tea due to how they are processed, green tea has the highest concentration of polyphenols, including catechins, which are powerful antioxidants.23,24 Green tea has been used in traditional Chinese and Indian medicine to control bleeding, improve digestion, and promote overall health.23
Dementia. Green tea polyphenols may enhance cognition and may protect against the development of dementia. In-vitro studies have shown that green tea reduces hydrogen peroxide and beta-amyloid peptides, which are significant in the development of Alzheimer’s disease.25 A 12-subject double-blind study found green tea increased working memory and had an impact on frontoparietal brain connections.26 Furthermore, a cohort study with 13,645 Japanese participants over a 5-year period found that frequent green tea consumption (>5 cups per day) was associated with a lower risk of dementa.27 Additional studies are needed, but green tea may be useful in the treatment or prevention of dementia in the future.
Coronary artery disease. In one study, green tea plasma and urinary concentrations were associated with plasma biomarkers of cardiovascular disease and diabetes.28 In one review, the consumption of green tea was associated with a statistically significant reduction in low-density lipoprotein cholesterol.29 Furthermore, a 2015 systematic review and meta-analysis of prospective observational studies concluded that increased tea consumption (of any type) is associated with a reduced risk of coronary heart disease, cardiac death, stroke, and total mortality.30
Cancer. Many studies have shown that green tea may reduce the risk of cancer development, although epidemiologic evidence is inconsistent. Studies have shown that cancer rates tend to be lower in those who consume higher levels of green tea.31,32 Whether this can be attributed solely to green tea remains debatable. Several other studies have shown that polyphenols in green tea can inhibit the growth of cancer cells, but the exact mechanisms by which tea interacts with cancerous cells is unknown.23
Several population-based studies have been performed, mostly in Japan, which showed green tea consumption reduced the risk of developing cancer. Fewer prostate cancer cases have been reported in men who consume green tea.33 While studies have been performed to determine whether green tea has effects on pancreatic, esophageal, ovarian, breast, bladder, and colorectal cancer, the evidence remains inadequate.32
Diabetes. Green tea has been shown in several studies to have a beneficial effect on diabetes. A retrospective Japanese cohort study showed that those who consumed green tea were one-third less likely to develop type 2 diabetes mellitus.34 A 10-year study from Taiwan found lower body fat and smaller waist circumference in those who consumed green tea regularly.35 A 2014 meta-analysis and systematic review of tea (any type) consumption and the risk of diabetes concluded that 3 cups or more of tea per day was associated with a lower risk of diabetes.36 Another meta-analysis that included 17 RCTs and that focused on green tea concluded that green tea improves glucose control and A1C values.37
Adverse effects
There have been concerns about potential hepatotoxicity induced by green tea intake.38 However, a systematic review of 34 RCTs on liver-related adverse events from green tea showed only a slight elevation in liver function tests; no serious liver-related adverse events were reported.38 This review suggested that liver-related adverse events after intake of green tea extracts are rare.38
Consuming green tea in the diet may lower the risk of adverse effects since the concentration consumed is generally much lower than that found in extracts.
Contraindications to drinking green tea are few. Individuals with caffeine sensitivities could experience insomnia, anxiety, irritability, or upset stomach. Additionally, patients who are taking anticoagulation drugs, such as warfarin, should avoid green tea due to its vitamin K content, which can counter the effects of warfarin. Pregnant or breastfeeding women, those with heart problems or high blood pressure, kidney or liver problems, stomach ulcers, or anxiety disorders should use caution with green tea consumption.
The bottom line
Green tea consumption in the diet appears to be safe and may have beneficial effects on weight, diabetes mellitus risk, cancer risk, dementia, and cardiovascular risk. Patients may want to consider drinking green tea as part of a healthy diet, in combination with exercise.
Mint/peppermint/menthol
Overview
Mentha piperita, also known as peppermint, is a hybrid between water mint and spearmint. It is found throughout Europe and North America and is commonly used in tea and toothpaste and as a flavoring for gum. It is used both orally and topically. Menthol and methyl salicylate are the main active ingredients in peppermint, and peppermint has calcium channel-blocker effects.39 Menthol has been shown to help regulate cold and pain sensation through the TRPM8 receptor.40 The peppermint herb has been studied in the treatment of multiple conditions.
IBS. It appears that peppermint inhibits spontaneous peristaltic activity, which reduces gastric emptying, decreases basal tone in the gastrointestinal tract, and slows down peristalsis in the gut.39
The American College of Gastroenterology guidelines currently note that there is moderate-quality evidence for peppermint oil in the treatment of IBS.41 A Cochrane review concluded that peppermint appears to be beneficial for IBS-related symptoms and pain.42 In a systematic review of 9 studies from 2014, peppermint oil was found to be more effective than placebo for IBS symptoms such as pain, bloating, gas, and diarrhea.43 The review also indicated that peppermint oil is safe, with heartburn being the most common complaint.43 A 2016 study also found that triple-coated microspheres containing peppermint oil reduced the frequency and intensity of IBS symptoms.44
Non-ulcer dyspepsia. In combination with caraway oil, peppermint oil can be used to reduce symptoms of non-ulcer dyspepsia.45,46 A multicenter, randomized, placebo-controlled, double-blind study found that 43.3% of subjects improved with a peppermint-caraway oil combination after 8 weeks, compared with 3.5% receiving placebo.46
Barium enema-related colonic spasm. Peppermint can relax the lower esophageal sphincter, and it has been shown to be useful as an antispasmodic agent for barium enema-related colonic spasm.47,48
Itching/skin irritation. Peppermint, when applied topically, has been used to calm pruritus and relieve irritation and inflammation. It has a soothing and cooling effect on the skin. At least one study found it to be effective in the treatment of pruritus gravidarum, although the study population consisted of only 96 subjects.49
Migraine headache. Initial small trials suggest that menthol is likely beneficial for migraine headaches. A pilot trial of 25 patients treated with topical menthol 6% gel for an acute migraine attack showed a significant improvement in headache intensity by 2 hours after gel application.50 In a randomized, triple-blind, placebo-controlled, crossover study of 35 patients, a menthol 10% solution was shown to be more efficacious as abortive treatment of migraine headaches than placebo.51
Tension headache. A randomized, placebo-controlled double-blind crossover study of topical peppermint oil showed a significant clinical reduction in tension headache pain.52 Another small randomized, double-blind trial showed that tiger balm (containing menthol as the main ingredient) also produced statistically significant improvement in tension headache discomfort compared with placebo.53
Musculoskeletal pain. A small study comparing topical menthol to ice for muscle soreness noted decreased perceived discomfort with menthol.54 Menthol has also been shown to reduce pain in patients with knee OA.55
Carpal tunnel syndrome (CTS). A triple-blind, randomized, placebo-controlled trial concluded that topical menthol acutely reduced pain intensity during the working day in slaughterhouse workers with CTS and should be considered as an effective non-systemic alternative to regular analgesics in the workplace management of chronic and neuropathic pain.56
Adverse effects
Peppermint appears to be safe for most adults when used in small doses, and serious adverse effects are rare.43,57 While peppermint tea appears to be safe in moderate to large amounts, people allergic to plants in the peppermint family (eg, mint, thyme, sage, rosemary, marjoram, basil, lavender) may experience allergic reactions with swelling, wheals, or erythema. Peppermint may also cause heartburn due to relaxation of the cardiac sphincter.
Other symptoms may include nausea, vomiting, flushing, and headache.58 The herb may also be both hepatotoxic and nephrotoxic at extremely high doses.59 Other considerations for women are that it can trigger menstruation and should be avoided during pregnancy. Due to uncertain efficacy in this population, peppermint oil should not be used on the face of infants, young children, or pregnant women.58,59
The bottom line
Peppermint appears to be safe and well-tolerated. It is useful in alleviating IBS symptoms and may be effective in the treatment of non-ulcerative dyspepsia, musculoskeletal pain, headache, and carpal tunnel syndrome.54,55
Read part 2 here.
CORRESPONDENCE
Michael Malone, MD, Family and Community Medicine, Penn State College of Medicine, 500 University Drive, Hershey, PA 17033; [email protected].
1. National Center for Complementary and Integrative Health. The Use of Complementary and Alternative Medicine in the United States. Available at: https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed November 28, 2017.
2. Wallace M, Pappagallo M. Qutenza: a capsaicin 8% patch for the management of postherpetic neuralgia. Expert Rev Neurother. 2011;11:15-27.
3. Rains C, Bryson HM. Topical capsaicin. A review of its pharmacological properties and therapeutic potential in post-herpetic neuralgia, diabetic neuropathy and osteoarthritis. Drugs Aging. 1995;7:317-328.
4. Derry S, Sven-Rice A, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013;(2):CD007393.
5. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991.
6. Deal CL, Schnitzer TJ, Lipstein E, et al. Treatment of arthritis with topical capsaicin: a double-blind trial. Clin Ther. 1991;13:383.
7. McCarthy GM, McCarty DJ. Effect of topical capsaicin in the therapy of painful osteoarthritis of the hands. J Rheumatol. 1992;19:604.
8. De Silva V, El-Metwally A, Ernst E, et al; Arthritis Research UK Working Group on Complementary and Alternative Medicines. Evidence for the efficacy of complementary and alternative medicines in the management of osteoarthritis: a systematic review. Rheumatology (Oxford). 2011;50:911-920.
9. Cameron M, Chrubasik S. Topical herbal therapies for treating osteoarthritis. Cochrane Database Syst Rev. 2013;(5):CD010538.
10. Oltean H, Robbins C, van Tulder MW, et al. Herbal medicine for low-back pain. Cochrane Database Syst Rev. 2014;(12):CD004504.
11. Armstrong EP, Malone DC, McCarberg B, et al. Cost-effectiveness analysis of a new 8% capsaicin patch compared to existing therapies for postherpetic neuralgia. Curr Med Res Opin. 2011;27:939-950.
12. Mou J, Paillard F, Turnbull B, et al. Efficacy of Qutenza (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain. 2013;154:1632-1639.
13. Sun-Edelstein C, Mauskop A. Alternative headache treatments: nutraceuticals, behavioral and physical treatments. Headache. 2011;51:469-483.
14. D’Andrea G, Cevoli S, Cologno D. Herbal therapy in migraine. Neurol Sci. 2014;35(Suppl 1):135-140.
15. Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: reanalysis of efficacy criteria. Eur Neurol. 2004;51:89-97.
16. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
17. Pothmann R, Danesch U. Migraine prevention in children and adolescents: results of an open study with a special butterbur root extract. Headache. 2005;45:196-203.
18. Holland S, Silberstein SD, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
19. American Academy of Neurology. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: [RETIRED]. Sept 16, 2015. Available at: http://n.neurology.org/content/78/17/1346. Accessed December 14, 2017.
20. Man LX. Complementary and alternative medicine for allergic rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2009;17:226-231.
21. Guo R, Pittler MH, Ernst E. Herbal medicines for the treatment of allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2007;99:483-495.
22. Daniel O, Mauskop A. Nutraceuticals in acute and prophylactic treatment of migraine. Curr Treat Options Neurol. 2016;18:14.
23. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;6:13.
24. Naghma K, Hasan M. Tea polyphenols for health promotion. Life Sciences. 2007;81:519-533.
25. Okello EJ, McDougall GJ, Kumar S, et al. In vitro protective effects of colon-available extract of Camellia sinensis (tea) against hydrogen peroxide and beta-amyloid (Aβ((1-42))) induced cytotoxicity in differentiated PC12 cells. Phytomedicine. 2011;15;18:691-696.
26. Schmidt A, Hammann F, Wölnerhanssen B, et al. Green tea extract enhances parieto-frontal connectivity during working memory processing. Psychopharmacology (Berl). 2014;231:3879-3888.
27. Tomata Y, Sugiyama K, Kaiho Y, et al. Green tea consumption and the risk of incident dementia in elderly japanese: The Ohsaki Cohort 2006 Study. Am J Geriatr Psychiatry. 2016;24:881-889.
28. Takechi R, Alfonso H, Hiramatsu N, et al. Elevated plasma and urinary concentrations of green tea catechins associated with improved plasma lipid profile in healthy Japanese women. Nutr Res. 2016;36:220-226.
29. Kim A, Chiu A, Barone MK, et al. Green tea catechins decrease total and low-density lipoprotein cholesterol: a systematic review and meta-analysis. J Am Diet Assoc. 2011;111:1720-1729.
30. Zhang C, Qin YY, Wei X, et al. Tea consumption and risk of cardiovascular outcomes and total mortality: a systematic review and meta-analysis of prospective observational studies. Eur J Epidemiol. 2015;30:103-113.
31. Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997;26:769-775.
32. Yuan JM. Cancer prevention by green tea: evidence from epidemiologic studies. Am J Clin Nutr. 2013;98(6 Suppl):1676S-1681S.
33. Kurahashi N, Sasazuki S, Iwasaki M, et al. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol. 2008;167:71-77.
34. Iso H, Date C, Wakai K, et al. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006;144:554-562.
35. Kim HM, Kim J. The effects of green tea on obesity and type 2 diabetes. Diab Metabol J. 2013;37:173-175.
36. Yang J, Mao Q, Xu H, et al. Tea consumption and risk of type 2 diabetes mellitus: a systematic review and meta-analysis update. BMJ Open. 2014;4:e005632.
37. Liu K, Zhou R, Wang B, et al. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr. 2013;98:340-348.
38. Isomura T, Suzuki S, Origasa H, et al. Liver-related safety assessment of green tea extracts in humans: a systematic review of randomized controlled trials. Eur J Clin Nutr. 2016;70:1340.
39. Tillisch K. Complementary and alternative medicine for gastrointestinal disorders. Clin Med (Lond). 2007;7:224-227.
40. Knowlton WM, McKemy DD. TRPM8: from cold to cancer, peppermint to pain. Curr Pharm Biotechnol. 2011;12:68-77.
41. Ford AC, Moayyedi P, Lacy BE, et al. Task Force on the Management of Functional Bowel Disorders. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(Suppl 1):S2-S26;quiz S27.
42. Ruepert L, Quartero AO, de Wit NJ, et al. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;(8):CD003460.
43. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512.
44. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Digest Dis Sci. 2016;61:560-571.
45. Holtmann G, Haag S, Adam B, et al. Effects of a fixed combination of peppermint oil and caraway oil on symptoms and quality of life in patients suffering from functional dyspepsia. Phytomedicine. 2003;10(suppl 4):56-57.
46. Madisch A, Heydenreich CJ, Wieland V, et al. Treatment of functional dyspepsia with a fixed peppermint oil and caraway oil combination preparation as compared to cisapride. A multicenter, reference-controlled double-blind equivalence study. Arzneimittelforschung. 1999;49:925-932.
47. Asao T, Kuwano H, Ide M, et al. Spasmolytic effect of peppermint oil in barium during double-contrast barium enema compared with Buscopan. Clin Radiol. 2003;58:301-305.
48. Sparks MJ, O’Sullivan P, Herrington AA, et al. Does peppermint oil relieve spasm during barium enema? Br J Radiol. 1995;68:841-843.
49. Akhavan Amjadi M, Mojab F, Kamranpour SB. The effect of peppermint oil on symptomatic treatment of pruritus in pregnant women. Iranian J Pharm Res. 2012;11:1073-1077.
50. St Cyr A, Chen A, Bradley KC, et al. Efficacy and tolerability of STOPAIN for a migraine attack. Front Neurol. 2015;6:11.
51. Borhani Haghighi A, Motazedian S, Rezaii R, et al. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: a randomised, double-blind, placebo-controlled, crossed-over study. Int J Clin Pract. 2010;64:451-456.
52. Gobel H, Fresenius J, Heinze A, et al. Effectiveness of oleum menthae piperitae and paracetamol in therapy of headache of the tension type [German]. Nervenarzt. 1996;67:672-681.
53. Schattner P, Randerson D. Tiger Balm as a treatment of tension headache. A clinical trial in general practice. Aust Fam Physician. 1996;25:216-220.
54. Johar P, Grover V, Topp R, et al. A comparison of topical menthol to ice on pain, evoked tetanic and voluntary force during delayed onset muscle soreness. Int J Sports Phys Ther. 2012;7:314-322.
55. Topp R, Brosky JA Jr, Pieschel D. The effect of either topical menthol or a placebo on functioning and knee pain among patients with knee OA. J Geriatr Phys Ther. 2013;36:92-99.
56. Sundstrup E, Jakobsen MD, Brandt M, et al. Acute effect of topical menthol on chronic pain in slaughterhouse workers with carpal tunnel syndrome: triple-blind, randomized placebo-controlled trial. Rehabil Res Pract. 2014;2014:310913.
57. Nair B. Final report on the safety assessment of mentha piperita (peppermint) oil, mentha piperita (peppermint) leaf extract, mentha piperita (peppermint) leaf, and mentha piperita (peppermint) leaf water. Int J Toxicol. 2001;20(Suppl 3):61-73.
58. Klingler B, Chadhary S. Peppermint oil. Am Fam Physician. 2007;75:1027-1030.
59. Nath SS, Pandey C, Roy D. A near fatal case of high dose peppermint oil ingestion—lessons learnt. Indian J Anaesth. 2012; 56:582-
The National Center for Complementary and Integrative Health, a division of the National Institutes of Medicine, estimates that about 38% of American adults use complementary and alternative medicine.1 That statistic includes 17.7% who say they use natural products. Despite their popularity, many physicians remain skeptical—and for good reason. Enthusiasts frequently offer dramatic anecdotes to “prove” their supplements' worth, but little scientific support is available for most herbal remedies. There are, however, exceptions. As this review of the medical literature will reveal, there is evidence to support the use of capsaicin to relieve osteoarthritis (OA) and postherpetic neuralgia (PHN) and support for green tea to serve as a lipid-lowering agent and help treat diabetes. Similarly, researchers have found that peppermint may be of value in the management of irritable bowel syndrome (IBS). (We also review the literature on butterbur for migraine headaches, but serious safety issues exist; TABLE.)

In the second part of this series, which is available here, we explore what the evidence tells us about the use of turmeric, chamomile, rosemary, coffee, and cocoa.
Worth noting as you consider this—or any—review of herbals is that while there is limited scientific evidence to establish the safety and efficacy of most herbal products, they are nonetheless freely sold without US Food & Drug Administration (FDA) approval because under current regulations, they are considered dietary supplements. That legal designation means companies can manufacture, sell, and market herbs without first demonstrating safety and efficacy, as is required for pharmaceutical drugs. Because herbal medications do not require the same testing through the large randomized controlled trials (RCTs) required for pharmaceuticals, evidence is often based on smaller RCTs and other studies of lower overall quality. Despite these limitations, we believe it’s worth keeping an open mind about the value of evidence-based herbal and botanical treatments.
Capsaicin
Overview
Capsaicin, an active compound in chili peppers, provokes a burning sensation, but also has a long history of use in pain treatment.2 Qutenza, an FDA-approved chemically synthesized 8% capsaicin patch, is identical to the naturally occurring molecule.2 Topically applied capsaicin exerts its therapeutic effect by rapidly depleting substance P, thus reducing the transmission of pain from C fibers to higher neurologic centers in the area of administration.3
Meta-analyses and systematic reviews have shown capsaicin is effective for various painful conditions, including peripheral diabetic neuropathy, OA, and PHN.
Peripheral neuropathy.
OA. Capsaicin provides mild to moderate efficacy in randomized trials for patients with hand and knee OA, when compared with placebo.5-7 A systematic review of capsaicin for all osteoarthritic conditions noted that there was consistent evidence that capsaicin gel was effective for OA.8 However, a 2013 Cochrane review of only knee OA noted that capsicum extract did not provide significant clinical improvement for pain or function in knee OA and resulted in a significant number of adverse events.9
Low back pain (LBP). Based on a 2014 Cochrane review of 3 trials (755 subjects) of moderate quality, capsicum frutescens cream or plaster appeared more efficacious than placebo in people with chronic LBP.10 Based on current (low-quality) evidence in one trial, however, it’s not clear whether topical capsicum cream is more beneficial for acute LBP than a placebo.10
PHN. Topical 8% capsaicin is an FDA-approved treatment for PHN. A review and cost-effectiveness analysis demonstrated that 8% capsaicin had significantly higher effectiveness rates than the oral agents (tricyclic antidepressants, duloxetine, gabapentin, pregabalin) used to treat PHN.11 In addition, the cost-effectiveness analysis found that the capsaicin patch was similar in cost to a topical lidocaine patch and oral products for PHN.11
A meta-analysis of 7 RCTs indicated that 8% topical capsaicin was superior to the low-dose capsaicin patch for relieving pain associated with PHN.12
Adverse effects
Very few toxic effects have been reported during a half century of capsaicin use. Those that have been reported are mainly limited to mild local reactions.2 The most common adverse effect of topical capsaicin is local irritation (burning, stinging, and erythema), which had been reported to occur in approximately 40% of patients.6 Nevertheless, more than 90% of the subjects in clinical studies were able to complete the studies, and pain rapidly resolved after patch removal.2 Washing with soap and water may help prevent the compound from spreading to other parts of the body unintentionally.
The safety of the patch has been demonstrated with repeated dosing every 3 months for up to one year. However, the long-term risks of chronic capsaicin use and its effect on epidermal innervation are uncertain.5
The bottom line
Capsaicin appears to be an effective treatment for neuropathy and chronic LBP. It is FDA approved for the treatment of PHN. It may also benefit patients with OA and acute LBP. Serious adverse effects are uncommon with topical use. Common adverse effects include burning pain and irritation in the area of application, which can be intense and cause discontinuation.2
Butterbur
Overview
Petasites hybridus, also known as butterbur, is a member of the daisy family, Asteraceae, and is a perennial plant found throughout Europe and Asia.13 It was used as a remedy for ulcers, wounds, and inflammation in ancient Greece. Its calcium channel-blocking effects may counteract vasoconstriction and play a role in preventing hyper-excitation of neurons.14 Sesquiterpenes, the pharmacologically active compounds in butterbur, have strong anti-inflammatory and vasodilatory effects through lipoxygenase and leukotriene inhibition.14
Migraine headache. Butterbur appears to be effective in migraine prophylaxis. Several studies have shown butterbur to significantly reduce the number of migraine attacks per month when compared with placebo. In a small, randomized, placebo-controlled, parallel-group study on the efficacy and tolerability of a special butterbur root extract (Petadolex) for the prevention of migraine, response rate was 45% in the butterbur group vs 15% in the placebo group. Butterbur was well tolerated.15 Similar results were found in another RCT in which Petasites (butterbur) 75 mg bid significantly reduced migraine attack frequency by 48%, compared with 26% for the placebo group.16 Petadolex was well tolerated in this study, too, and no serious adverse events occurred. Findings suggest that 75 mg bid may be a good option for migraine prevention given the agent's safety profile.
Petadolex may also be a good option in pediatric migraine. A 2005 study in children and adolescents found that 77% of patients experienced a reduction in attacks by at least 50% with butterbur. Patients were treated with 50 mg to 150 mg over 4 months.17
In their guidelines for migraine prevention, the American Academy of Neurology (AAN) and American Headache Society gave butterbur a Level A recommendation and concluded that butterbur should be offered to patients with migraine to reduce the frequency and severity of migraine attacks.18 However, the AAN has since changed its position, stating that “The 2012 AAN guideline, ‘Evidence-based guideline update: NSAIDS and other complementary treatments for episodic migraine prevention in adults’ has been retired by the AAN Board of Directors on September 16, 2015, due to serious safety concerns with a preventative treatment, butterbur, recommended by this guideline. The recommendations and conclusions in all retired guidelines are considered no longer valid and no longer supported by the AAN.”19
Allergic rhinitis. Although the data is not convincing, some studies have shown that butterbur may be beneficial for the treatment of allergic rhinitis.20,21
Adverse effects
While the butterbur plant itself contains pyrrolizidine alkaloids (PA), which are hepatotoxic and carcinogenic, extracts of butterbur root that are almost completely free from these alkaloids are available. (Patients who choose to use butterbur should be advised to use only products that are certified and labeled pyrrolizidine alkaloids free.)
Petadolex, the medication used in migraine studies, was initially approved by the German health regulatory authority, but approval was later withdrawn due to concerns about liver toxicity.22 In 2012, the United Kingdom’s Medicines and Health Care Products Regulatory Agency withdrew all butterbur products from the market due to associated cases of liver toxicity.22 Petasites (butterbur) products are still available in the US market, and the risks and benefits should be discussed with all patients considering this treatment. Liver function monitoring is recommended for all patients using butterbur.22
The herb can also cause dyspepsia, headache, itchy eyes, gastrointestinal symptoms, asthma, fatigue, and drowsiness. Additionally, people who are allergic to ragweed and daisies may have allergic reactions to butterbur. Eructation (belching) occurred in 7% of patients in a pediatric study.17
The bottom line
Butterbur appears to be efficacious for migraine prophylaxis, but long-term safety is unknown and serious concerns exist for liver toxicity.
Green tea
Overview
Most tea leaves come from the Camellia sinensis bush, but green and black tea are processed differently to produce different end products.23 It is estimated that green tea accounts for approximately a quarter of all tea consumption, and is most commonly consumed in Asian countries.23 The health-promoting effects of green tea are mainly attributed to its polyphenol content.24 While there are many types of tea due to how they are processed, green tea has the highest concentration of polyphenols, including catechins, which are powerful antioxidants.23,24 Green tea has been used in traditional Chinese and Indian medicine to control bleeding, improve digestion, and promote overall health.23
Dementia. Green tea polyphenols may enhance cognition and may protect against the development of dementia. In-vitro studies have shown that green tea reduces hydrogen peroxide and beta-amyloid peptides, which are significant in the development of Alzheimer’s disease.25 A 12-subject double-blind study found green tea increased working memory and had an impact on frontoparietal brain connections.26 Furthermore, a cohort study with 13,645 Japanese participants over a 5-year period found that frequent green tea consumption (>5 cups per day) was associated with a lower risk of dementa.27 Additional studies are needed, but green tea may be useful in the treatment or prevention of dementia in the future.
Coronary artery disease. In one study, green tea plasma and urinary concentrations were associated with plasma biomarkers of cardiovascular disease and diabetes.28 In one review, the consumption of green tea was associated with a statistically significant reduction in low-density lipoprotein cholesterol.29 Furthermore, a 2015 systematic review and meta-analysis of prospective observational studies concluded that increased tea consumption (of any type) is associated with a reduced risk of coronary heart disease, cardiac death, stroke, and total mortality.30
Cancer. Many studies have shown that green tea may reduce the risk of cancer development, although epidemiologic evidence is inconsistent. Studies have shown that cancer rates tend to be lower in those who consume higher levels of green tea.31,32 Whether this can be attributed solely to green tea remains debatable. Several other studies have shown that polyphenols in green tea can inhibit the growth of cancer cells, but the exact mechanisms by which tea interacts with cancerous cells is unknown.23
Several population-based studies have been performed, mostly in Japan, which showed green tea consumption reduced the risk of developing cancer. Fewer prostate cancer cases have been reported in men who consume green tea.33 While studies have been performed to determine whether green tea has effects on pancreatic, esophageal, ovarian, breast, bladder, and colorectal cancer, the evidence remains inadequate.32
Diabetes. Green tea has been shown in several studies to have a beneficial effect on diabetes. A retrospective Japanese cohort study showed that those who consumed green tea were one-third less likely to develop type 2 diabetes mellitus.34 A 10-year study from Taiwan found lower body fat and smaller waist circumference in those who consumed green tea regularly.35 A 2014 meta-analysis and systematic review of tea (any type) consumption and the risk of diabetes concluded that 3 cups or more of tea per day was associated with a lower risk of diabetes.36 Another meta-analysis that included 17 RCTs and that focused on green tea concluded that green tea improves glucose control and A1C values.37
Adverse effects
There have been concerns about potential hepatotoxicity induced by green tea intake.38 However, a systematic review of 34 RCTs on liver-related adverse events from green tea showed only a slight elevation in liver function tests; no serious liver-related adverse events were reported.38 This review suggested that liver-related adverse events after intake of green tea extracts are rare.38
Consuming green tea in the diet may lower the risk of adverse effects since the concentration consumed is generally much lower than that found in extracts.
Contraindications to drinking green tea are few. Individuals with caffeine sensitivities could experience insomnia, anxiety, irritability, or upset stomach. Additionally, patients who are taking anticoagulation drugs, such as warfarin, should avoid green tea due to its vitamin K content, which can counter the effects of warfarin. Pregnant or breastfeeding women, those with heart problems or high blood pressure, kidney or liver problems, stomach ulcers, or anxiety disorders should use caution with green tea consumption.
The bottom line
Green tea consumption in the diet appears to be safe and may have beneficial effects on weight, diabetes mellitus risk, cancer risk, dementia, and cardiovascular risk. Patients may want to consider drinking green tea as part of a healthy diet, in combination with exercise.
Mint/peppermint/menthol
Overview
Mentha piperita, also known as peppermint, is a hybrid between water mint and spearmint. It is found throughout Europe and North America and is commonly used in tea and toothpaste and as a flavoring for gum. It is used both orally and topically. Menthol and methyl salicylate are the main active ingredients in peppermint, and peppermint has calcium channel-blocker effects.39 Menthol has been shown to help regulate cold and pain sensation through the TRPM8 receptor.40 The peppermint herb has been studied in the treatment of multiple conditions.
IBS. It appears that peppermint inhibits spontaneous peristaltic activity, which reduces gastric emptying, decreases basal tone in the gastrointestinal tract, and slows down peristalsis in the gut.39
The American College of Gastroenterology guidelines currently note that there is moderate-quality evidence for peppermint oil in the treatment of IBS.41 A Cochrane review concluded that peppermint appears to be beneficial for IBS-related symptoms and pain.42 In a systematic review of 9 studies from 2014, peppermint oil was found to be more effective than placebo for IBS symptoms such as pain, bloating, gas, and diarrhea.43 The review also indicated that peppermint oil is safe, with heartburn being the most common complaint.43 A 2016 study also found that triple-coated microspheres containing peppermint oil reduced the frequency and intensity of IBS symptoms.44
Non-ulcer dyspepsia. In combination with caraway oil, peppermint oil can be used to reduce symptoms of non-ulcer dyspepsia.45,46 A multicenter, randomized, placebo-controlled, double-blind study found that 43.3% of subjects improved with a peppermint-caraway oil combination after 8 weeks, compared with 3.5% receiving placebo.46
Barium enema-related colonic spasm. Peppermint can relax the lower esophageal sphincter, and it has been shown to be useful as an antispasmodic agent for barium enema-related colonic spasm.47,48
Itching/skin irritation. Peppermint, when applied topically, has been used to calm pruritus and relieve irritation and inflammation. It has a soothing and cooling effect on the skin. At least one study found it to be effective in the treatment of pruritus gravidarum, although the study population consisted of only 96 subjects.49
Migraine headache. Initial small trials suggest that menthol is likely beneficial for migraine headaches. A pilot trial of 25 patients treated with topical menthol 6% gel for an acute migraine attack showed a significant improvement in headache intensity by 2 hours after gel application.50 In a randomized, triple-blind, placebo-controlled, crossover study of 35 patients, a menthol 10% solution was shown to be more efficacious as abortive treatment of migraine headaches than placebo.51
Tension headache. A randomized, placebo-controlled double-blind crossover study of topical peppermint oil showed a significant clinical reduction in tension headache pain.52 Another small randomized, double-blind trial showed that tiger balm (containing menthol as the main ingredient) also produced statistically significant improvement in tension headache discomfort compared with placebo.53
Musculoskeletal pain. A small study comparing topical menthol to ice for muscle soreness noted decreased perceived discomfort with menthol.54 Menthol has also been shown to reduce pain in patients with knee OA.55
Carpal tunnel syndrome (CTS). A triple-blind, randomized, placebo-controlled trial concluded that topical menthol acutely reduced pain intensity during the working day in slaughterhouse workers with CTS and should be considered as an effective non-systemic alternative to regular analgesics in the workplace management of chronic and neuropathic pain.56
Adverse effects
Peppermint appears to be safe for most adults when used in small doses, and serious adverse effects are rare.43,57 While peppermint tea appears to be safe in moderate to large amounts, people allergic to plants in the peppermint family (eg, mint, thyme, sage, rosemary, marjoram, basil, lavender) may experience allergic reactions with swelling, wheals, or erythema. Peppermint may also cause heartburn due to relaxation of the cardiac sphincter.
Other symptoms may include nausea, vomiting, flushing, and headache.58 The herb may also be both hepatotoxic and nephrotoxic at extremely high doses.59 Other considerations for women are that it can trigger menstruation and should be avoided during pregnancy. Due to uncertain efficacy in this population, peppermint oil should not be used on the face of infants, young children, or pregnant women.58,59
The bottom line
Peppermint appears to be safe and well-tolerated. It is useful in alleviating IBS symptoms and may be effective in the treatment of non-ulcerative dyspepsia, musculoskeletal pain, headache, and carpal tunnel syndrome.54,55
Read part 2 here.
CORRESPONDENCE
Michael Malone, MD, Family and Community Medicine, Penn State College of Medicine, 500 University Drive, Hershey, PA 17033; [email protected].
The National Center for Complementary and Integrative Health, a division of the National Institutes of Medicine, estimates that about 38% of American adults use complementary and alternative medicine.1 That statistic includes 17.7% who say they use natural products. Despite their popularity, many physicians remain skeptical—and for good reason. Enthusiasts frequently offer dramatic anecdotes to “prove” their supplements' worth, but little scientific support is available for most herbal remedies. There are, however, exceptions. As this review of the medical literature will reveal, there is evidence to support the use of capsaicin to relieve osteoarthritis (OA) and postherpetic neuralgia (PHN) and support for green tea to serve as a lipid-lowering agent and help treat diabetes. Similarly, researchers have found that peppermint may be of value in the management of irritable bowel syndrome (IBS). (We also review the literature on butterbur for migraine headaches, but serious safety issues exist; TABLE.)

In the second part of this series, which is available here, we explore what the evidence tells us about the use of turmeric, chamomile, rosemary, coffee, and cocoa.
Worth noting as you consider this—or any—review of herbals is that while there is limited scientific evidence to establish the safety and efficacy of most herbal products, they are nonetheless freely sold without US Food & Drug Administration (FDA) approval because under current regulations, they are considered dietary supplements. That legal designation means companies can manufacture, sell, and market herbs without first demonstrating safety and efficacy, as is required for pharmaceutical drugs. Because herbal medications do not require the same testing through the large randomized controlled trials (RCTs) required for pharmaceuticals, evidence is often based on smaller RCTs and other studies of lower overall quality. Despite these limitations, we believe it’s worth keeping an open mind about the value of evidence-based herbal and botanical treatments.
Capsaicin
Overview
Capsaicin, an active compound in chili peppers, provokes a burning sensation, but also has a long history of use in pain treatment.2 Qutenza, an FDA-approved chemically synthesized 8% capsaicin patch, is identical to the naturally occurring molecule.2 Topically applied capsaicin exerts its therapeutic effect by rapidly depleting substance P, thus reducing the transmission of pain from C fibers to higher neurologic centers in the area of administration.3
Meta-analyses and systematic reviews have shown capsaicin is effective for various painful conditions, including peripheral diabetic neuropathy, OA, and PHN.
Peripheral neuropathy.
OA. Capsaicin provides mild to moderate efficacy in randomized trials for patients with hand and knee OA, when compared with placebo.5-7 A systematic review of capsaicin for all osteoarthritic conditions noted that there was consistent evidence that capsaicin gel was effective for OA.8 However, a 2013 Cochrane review of only knee OA noted that capsicum extract did not provide significant clinical improvement for pain or function in knee OA and resulted in a significant number of adverse events.9
Low back pain (LBP). Based on a 2014 Cochrane review of 3 trials (755 subjects) of moderate quality, capsicum frutescens cream or plaster appeared more efficacious than placebo in people with chronic LBP.10 Based on current (low-quality) evidence in one trial, however, it’s not clear whether topical capsicum cream is more beneficial for acute LBP than a placebo.10
PHN. Topical 8% capsaicin is an FDA-approved treatment for PHN. A review and cost-effectiveness analysis demonstrated that 8% capsaicin had significantly higher effectiveness rates than the oral agents (tricyclic antidepressants, duloxetine, gabapentin, pregabalin) used to treat PHN.11 In addition, the cost-effectiveness analysis found that the capsaicin patch was similar in cost to a topical lidocaine patch and oral products for PHN.11
A meta-analysis of 7 RCTs indicated that 8% topical capsaicin was superior to the low-dose capsaicin patch for relieving pain associated with PHN.12
Adverse effects
Very few toxic effects have been reported during a half century of capsaicin use. Those that have been reported are mainly limited to mild local reactions.2 The most common adverse effect of topical capsaicin is local irritation (burning, stinging, and erythema), which had been reported to occur in approximately 40% of patients.6 Nevertheless, more than 90% of the subjects in clinical studies were able to complete the studies, and pain rapidly resolved after patch removal.2 Washing with soap and water may help prevent the compound from spreading to other parts of the body unintentionally.
The safety of the patch has been demonstrated with repeated dosing every 3 months for up to one year. However, the long-term risks of chronic capsaicin use and its effect on epidermal innervation are uncertain.5
The bottom line
Capsaicin appears to be an effective treatment for neuropathy and chronic LBP. It is FDA approved for the treatment of PHN. It may also benefit patients with OA and acute LBP. Serious adverse effects are uncommon with topical use. Common adverse effects include burning pain and irritation in the area of application, which can be intense and cause discontinuation.2
Butterbur
Overview
Petasites hybridus, also known as butterbur, is a member of the daisy family, Asteraceae, and is a perennial plant found throughout Europe and Asia.13 It was used as a remedy for ulcers, wounds, and inflammation in ancient Greece. Its calcium channel-blocking effects may counteract vasoconstriction and play a role in preventing hyper-excitation of neurons.14 Sesquiterpenes, the pharmacologically active compounds in butterbur, have strong anti-inflammatory and vasodilatory effects through lipoxygenase and leukotriene inhibition.14
Migraine headache. Butterbur appears to be effective in migraine prophylaxis. Several studies have shown butterbur to significantly reduce the number of migraine attacks per month when compared with placebo. In a small, randomized, placebo-controlled, parallel-group study on the efficacy and tolerability of a special butterbur root extract (Petadolex) for the prevention of migraine, response rate was 45% in the butterbur group vs 15% in the placebo group. Butterbur was well tolerated.15 Similar results were found in another RCT in which Petasites (butterbur) 75 mg bid significantly reduced migraine attack frequency by 48%, compared with 26% for the placebo group.16 Petadolex was well tolerated in this study, too, and no serious adverse events occurred. Findings suggest that 75 mg bid may be a good option for migraine prevention given the agent's safety profile.
Petadolex may also be a good option in pediatric migraine. A 2005 study in children and adolescents found that 77% of patients experienced a reduction in attacks by at least 50% with butterbur. Patients were treated with 50 mg to 150 mg over 4 months.17
In their guidelines for migraine prevention, the American Academy of Neurology (AAN) and American Headache Society gave butterbur a Level A recommendation and concluded that butterbur should be offered to patients with migraine to reduce the frequency and severity of migraine attacks.18 However, the AAN has since changed its position, stating that “The 2012 AAN guideline, ‘Evidence-based guideline update: NSAIDS and other complementary treatments for episodic migraine prevention in adults’ has been retired by the AAN Board of Directors on September 16, 2015, due to serious safety concerns with a preventative treatment, butterbur, recommended by this guideline. The recommendations and conclusions in all retired guidelines are considered no longer valid and no longer supported by the AAN.”19
Allergic rhinitis. Although the data is not convincing, some studies have shown that butterbur may be beneficial for the treatment of allergic rhinitis.20,21
Adverse effects
While the butterbur plant itself contains pyrrolizidine alkaloids (PA), which are hepatotoxic and carcinogenic, extracts of butterbur root that are almost completely free from these alkaloids are available. (Patients who choose to use butterbur should be advised to use only products that are certified and labeled pyrrolizidine alkaloids free.)
Petadolex, the medication used in migraine studies, was initially approved by the German health regulatory authority, but approval was later withdrawn due to concerns about liver toxicity.22 In 2012, the United Kingdom’s Medicines and Health Care Products Regulatory Agency withdrew all butterbur products from the market due to associated cases of liver toxicity.22 Petasites (butterbur) products are still available in the US market, and the risks and benefits should be discussed with all patients considering this treatment. Liver function monitoring is recommended for all patients using butterbur.22
The herb can also cause dyspepsia, headache, itchy eyes, gastrointestinal symptoms, asthma, fatigue, and drowsiness. Additionally, people who are allergic to ragweed and daisies may have allergic reactions to butterbur. Eructation (belching) occurred in 7% of patients in a pediatric study.17
The bottom line
Butterbur appears to be efficacious for migraine prophylaxis, but long-term safety is unknown and serious concerns exist for liver toxicity.
Green tea
Overview
Most tea leaves come from the Camellia sinensis bush, but green and black tea are processed differently to produce different end products.23 It is estimated that green tea accounts for approximately a quarter of all tea consumption, and is most commonly consumed in Asian countries.23 The health-promoting effects of green tea are mainly attributed to its polyphenol content.24 While there are many types of tea due to how they are processed, green tea has the highest concentration of polyphenols, including catechins, which are powerful antioxidants.23,24 Green tea has been used in traditional Chinese and Indian medicine to control bleeding, improve digestion, and promote overall health.23
Dementia. Green tea polyphenols may enhance cognition and may protect against the development of dementia. In-vitro studies have shown that green tea reduces hydrogen peroxide and beta-amyloid peptides, which are significant in the development of Alzheimer’s disease.25 A 12-subject double-blind study found green tea increased working memory and had an impact on frontoparietal brain connections.26 Furthermore, a cohort study with 13,645 Japanese participants over a 5-year period found that frequent green tea consumption (>5 cups per day) was associated with a lower risk of dementa.27 Additional studies are needed, but green tea may be useful in the treatment or prevention of dementia in the future.
Coronary artery disease. In one study, green tea plasma and urinary concentrations were associated with plasma biomarkers of cardiovascular disease and diabetes.28 In one review, the consumption of green tea was associated with a statistically significant reduction in low-density lipoprotein cholesterol.29 Furthermore, a 2015 systematic review and meta-analysis of prospective observational studies concluded that increased tea consumption (of any type) is associated with a reduced risk of coronary heart disease, cardiac death, stroke, and total mortality.30
Cancer. Many studies have shown that green tea may reduce the risk of cancer development, although epidemiologic evidence is inconsistent. Studies have shown that cancer rates tend to be lower in those who consume higher levels of green tea.31,32 Whether this can be attributed solely to green tea remains debatable. Several other studies have shown that polyphenols in green tea can inhibit the growth of cancer cells, but the exact mechanisms by which tea interacts with cancerous cells is unknown.23
Several population-based studies have been performed, mostly in Japan, which showed green tea consumption reduced the risk of developing cancer. Fewer prostate cancer cases have been reported in men who consume green tea.33 While studies have been performed to determine whether green tea has effects on pancreatic, esophageal, ovarian, breast, bladder, and colorectal cancer, the evidence remains inadequate.32
Diabetes. Green tea has been shown in several studies to have a beneficial effect on diabetes. A retrospective Japanese cohort study showed that those who consumed green tea were one-third less likely to develop type 2 diabetes mellitus.34 A 10-year study from Taiwan found lower body fat and smaller waist circumference in those who consumed green tea regularly.35 A 2014 meta-analysis and systematic review of tea (any type) consumption and the risk of diabetes concluded that 3 cups or more of tea per day was associated with a lower risk of diabetes.36 Another meta-analysis that included 17 RCTs and that focused on green tea concluded that green tea improves glucose control and A1C values.37
Adverse effects
There have been concerns about potential hepatotoxicity induced by green tea intake.38 However, a systematic review of 34 RCTs on liver-related adverse events from green tea showed only a slight elevation in liver function tests; no serious liver-related adverse events were reported.38 This review suggested that liver-related adverse events after intake of green tea extracts are rare.38
Consuming green tea in the diet may lower the risk of adverse effects since the concentration consumed is generally much lower than that found in extracts.
Contraindications to drinking green tea are few. Individuals with caffeine sensitivities could experience insomnia, anxiety, irritability, or upset stomach. Additionally, patients who are taking anticoagulation drugs, such as warfarin, should avoid green tea due to its vitamin K content, which can counter the effects of warfarin. Pregnant or breastfeeding women, those with heart problems or high blood pressure, kidney or liver problems, stomach ulcers, or anxiety disorders should use caution with green tea consumption.
The bottom line
Green tea consumption in the diet appears to be safe and may have beneficial effects on weight, diabetes mellitus risk, cancer risk, dementia, and cardiovascular risk. Patients may want to consider drinking green tea as part of a healthy diet, in combination with exercise.
Mint/peppermint/menthol
Overview
Mentha piperita, also known as peppermint, is a hybrid between water mint and spearmint. It is found throughout Europe and North America and is commonly used in tea and toothpaste and as a flavoring for gum. It is used both orally and topically. Menthol and methyl salicylate are the main active ingredients in peppermint, and peppermint has calcium channel-blocker effects.39 Menthol has been shown to help regulate cold and pain sensation through the TRPM8 receptor.40 The peppermint herb has been studied in the treatment of multiple conditions.
IBS. It appears that peppermint inhibits spontaneous peristaltic activity, which reduces gastric emptying, decreases basal tone in the gastrointestinal tract, and slows down peristalsis in the gut.39
The American College of Gastroenterology guidelines currently note that there is moderate-quality evidence for peppermint oil in the treatment of IBS.41 A Cochrane review concluded that peppermint appears to be beneficial for IBS-related symptoms and pain.42 In a systematic review of 9 studies from 2014, peppermint oil was found to be more effective than placebo for IBS symptoms such as pain, bloating, gas, and diarrhea.43 The review also indicated that peppermint oil is safe, with heartburn being the most common complaint.43 A 2016 study also found that triple-coated microspheres containing peppermint oil reduced the frequency and intensity of IBS symptoms.44
Non-ulcer dyspepsia. In combination with caraway oil, peppermint oil can be used to reduce symptoms of non-ulcer dyspepsia.45,46 A multicenter, randomized, placebo-controlled, double-blind study found that 43.3% of subjects improved with a peppermint-caraway oil combination after 8 weeks, compared with 3.5% receiving placebo.46
Barium enema-related colonic spasm. Peppermint can relax the lower esophageal sphincter, and it has been shown to be useful as an antispasmodic agent for barium enema-related colonic spasm.47,48
Itching/skin irritation. Peppermint, when applied topically, has been used to calm pruritus and relieve irritation and inflammation. It has a soothing and cooling effect on the skin. At least one study found it to be effective in the treatment of pruritus gravidarum, although the study population consisted of only 96 subjects.49
Migraine headache. Initial small trials suggest that menthol is likely beneficial for migraine headaches. A pilot trial of 25 patients treated with topical menthol 6% gel for an acute migraine attack showed a significant improvement in headache intensity by 2 hours after gel application.50 In a randomized, triple-blind, placebo-controlled, crossover study of 35 patients, a menthol 10% solution was shown to be more efficacious as abortive treatment of migraine headaches than placebo.51
Tension headache. A randomized, placebo-controlled double-blind crossover study of topical peppermint oil showed a significant clinical reduction in tension headache pain.52 Another small randomized, double-blind trial showed that tiger balm (containing menthol as the main ingredient) also produced statistically significant improvement in tension headache discomfort compared with placebo.53
Musculoskeletal pain. A small study comparing topical menthol to ice for muscle soreness noted decreased perceived discomfort with menthol.54 Menthol has also been shown to reduce pain in patients with knee OA.55
Carpal tunnel syndrome (CTS). A triple-blind, randomized, placebo-controlled trial concluded that topical menthol acutely reduced pain intensity during the working day in slaughterhouse workers with CTS and should be considered as an effective non-systemic alternative to regular analgesics in the workplace management of chronic and neuropathic pain.56
Adverse effects
Peppermint appears to be safe for most adults when used in small doses, and serious adverse effects are rare.43,57 While peppermint tea appears to be safe in moderate to large amounts, people allergic to plants in the peppermint family (eg, mint, thyme, sage, rosemary, marjoram, basil, lavender) may experience allergic reactions with swelling, wheals, or erythema. Peppermint may also cause heartburn due to relaxation of the cardiac sphincter.
Other symptoms may include nausea, vomiting, flushing, and headache.58 The herb may also be both hepatotoxic and nephrotoxic at extremely high doses.59 Other considerations for women are that it can trigger menstruation and should be avoided during pregnancy. Due to uncertain efficacy in this population, peppermint oil should not be used on the face of infants, young children, or pregnant women.58,59
The bottom line
Peppermint appears to be safe and well-tolerated. It is useful in alleviating IBS symptoms and may be effective in the treatment of non-ulcerative dyspepsia, musculoskeletal pain, headache, and carpal tunnel syndrome.54,55
Read part 2 here.
CORRESPONDENCE
Michael Malone, MD, Family and Community Medicine, Penn State College of Medicine, 500 University Drive, Hershey, PA 17033; [email protected].
1. National Center for Complementary and Integrative Health. The Use of Complementary and Alternative Medicine in the United States. Available at: https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed November 28, 2017.
2. Wallace M, Pappagallo M. Qutenza: a capsaicin 8% patch for the management of postherpetic neuralgia. Expert Rev Neurother. 2011;11:15-27.
3. Rains C, Bryson HM. Topical capsaicin. A review of its pharmacological properties and therapeutic potential in post-herpetic neuralgia, diabetic neuropathy and osteoarthritis. Drugs Aging. 1995;7:317-328.
4. Derry S, Sven-Rice A, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013;(2):CD007393.
5. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991.
6. Deal CL, Schnitzer TJ, Lipstein E, et al. Treatment of arthritis with topical capsaicin: a double-blind trial. Clin Ther. 1991;13:383.
7. McCarthy GM, McCarty DJ. Effect of topical capsaicin in the therapy of painful osteoarthritis of the hands. J Rheumatol. 1992;19:604.
8. De Silva V, El-Metwally A, Ernst E, et al; Arthritis Research UK Working Group on Complementary and Alternative Medicines. Evidence for the efficacy of complementary and alternative medicines in the management of osteoarthritis: a systematic review. Rheumatology (Oxford). 2011;50:911-920.
9. Cameron M, Chrubasik S. Topical herbal therapies for treating osteoarthritis. Cochrane Database Syst Rev. 2013;(5):CD010538.
10. Oltean H, Robbins C, van Tulder MW, et al. Herbal medicine for low-back pain. Cochrane Database Syst Rev. 2014;(12):CD004504.
11. Armstrong EP, Malone DC, McCarberg B, et al. Cost-effectiveness analysis of a new 8% capsaicin patch compared to existing therapies for postherpetic neuralgia. Curr Med Res Opin. 2011;27:939-950.
12. Mou J, Paillard F, Turnbull B, et al. Efficacy of Qutenza (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain. 2013;154:1632-1639.
13. Sun-Edelstein C, Mauskop A. Alternative headache treatments: nutraceuticals, behavioral and physical treatments. Headache. 2011;51:469-483.
14. D’Andrea G, Cevoli S, Cologno D. Herbal therapy in migraine. Neurol Sci. 2014;35(Suppl 1):135-140.
15. Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: reanalysis of efficacy criteria. Eur Neurol. 2004;51:89-97.
16. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
17. Pothmann R, Danesch U. Migraine prevention in children and adolescents: results of an open study with a special butterbur root extract. Headache. 2005;45:196-203.
18. Holland S, Silberstein SD, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
19. American Academy of Neurology. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: [RETIRED]. Sept 16, 2015. Available at: http://n.neurology.org/content/78/17/1346. Accessed December 14, 2017.
20. Man LX. Complementary and alternative medicine for allergic rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2009;17:226-231.
21. Guo R, Pittler MH, Ernst E. Herbal medicines for the treatment of allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2007;99:483-495.
22. Daniel O, Mauskop A. Nutraceuticals in acute and prophylactic treatment of migraine. Curr Treat Options Neurol. 2016;18:14.
23. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;6:13.
24. Naghma K, Hasan M. Tea polyphenols for health promotion. Life Sciences. 2007;81:519-533.
25. Okello EJ, McDougall GJ, Kumar S, et al. In vitro protective effects of colon-available extract of Camellia sinensis (tea) against hydrogen peroxide and beta-amyloid (Aβ((1-42))) induced cytotoxicity in differentiated PC12 cells. Phytomedicine. 2011;15;18:691-696.
26. Schmidt A, Hammann F, Wölnerhanssen B, et al. Green tea extract enhances parieto-frontal connectivity during working memory processing. Psychopharmacology (Berl). 2014;231:3879-3888.
27. Tomata Y, Sugiyama K, Kaiho Y, et al. Green tea consumption and the risk of incident dementia in elderly japanese: The Ohsaki Cohort 2006 Study. Am J Geriatr Psychiatry. 2016;24:881-889.
28. Takechi R, Alfonso H, Hiramatsu N, et al. Elevated plasma and urinary concentrations of green tea catechins associated with improved plasma lipid profile in healthy Japanese women. Nutr Res. 2016;36:220-226.
29. Kim A, Chiu A, Barone MK, et al. Green tea catechins decrease total and low-density lipoprotein cholesterol: a systematic review and meta-analysis. J Am Diet Assoc. 2011;111:1720-1729.
30. Zhang C, Qin YY, Wei X, et al. Tea consumption and risk of cardiovascular outcomes and total mortality: a systematic review and meta-analysis of prospective observational studies. Eur J Epidemiol. 2015;30:103-113.
31. Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997;26:769-775.
32. Yuan JM. Cancer prevention by green tea: evidence from epidemiologic studies. Am J Clin Nutr. 2013;98(6 Suppl):1676S-1681S.
33. Kurahashi N, Sasazuki S, Iwasaki M, et al. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol. 2008;167:71-77.
34. Iso H, Date C, Wakai K, et al. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006;144:554-562.
35. Kim HM, Kim J. The effects of green tea on obesity and type 2 diabetes. Diab Metabol J. 2013;37:173-175.
36. Yang J, Mao Q, Xu H, et al. Tea consumption and risk of type 2 diabetes mellitus: a systematic review and meta-analysis update. BMJ Open. 2014;4:e005632.
37. Liu K, Zhou R, Wang B, et al. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr. 2013;98:340-348.
38. Isomura T, Suzuki S, Origasa H, et al. Liver-related safety assessment of green tea extracts in humans: a systematic review of randomized controlled trials. Eur J Clin Nutr. 2016;70:1340.
39. Tillisch K. Complementary and alternative medicine for gastrointestinal disorders. Clin Med (Lond). 2007;7:224-227.
40. Knowlton WM, McKemy DD. TRPM8: from cold to cancer, peppermint to pain. Curr Pharm Biotechnol. 2011;12:68-77.
41. Ford AC, Moayyedi P, Lacy BE, et al. Task Force on the Management of Functional Bowel Disorders. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(Suppl 1):S2-S26;quiz S27.
42. Ruepert L, Quartero AO, de Wit NJ, et al. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;(8):CD003460.
43. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512.
44. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Digest Dis Sci. 2016;61:560-571.
45. Holtmann G, Haag S, Adam B, et al. Effects of a fixed combination of peppermint oil and caraway oil on symptoms and quality of life in patients suffering from functional dyspepsia. Phytomedicine. 2003;10(suppl 4):56-57.
46. Madisch A, Heydenreich CJ, Wieland V, et al. Treatment of functional dyspepsia with a fixed peppermint oil and caraway oil combination preparation as compared to cisapride. A multicenter, reference-controlled double-blind equivalence study. Arzneimittelforschung. 1999;49:925-932.
47. Asao T, Kuwano H, Ide M, et al. Spasmolytic effect of peppermint oil in barium during double-contrast barium enema compared with Buscopan. Clin Radiol. 2003;58:301-305.
48. Sparks MJ, O’Sullivan P, Herrington AA, et al. Does peppermint oil relieve spasm during barium enema? Br J Radiol. 1995;68:841-843.
49. Akhavan Amjadi M, Mojab F, Kamranpour SB. The effect of peppermint oil on symptomatic treatment of pruritus in pregnant women. Iranian J Pharm Res. 2012;11:1073-1077.
50. St Cyr A, Chen A, Bradley KC, et al. Efficacy and tolerability of STOPAIN for a migraine attack. Front Neurol. 2015;6:11.
51. Borhani Haghighi A, Motazedian S, Rezaii R, et al. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: a randomised, double-blind, placebo-controlled, crossed-over study. Int J Clin Pract. 2010;64:451-456.
52. Gobel H, Fresenius J, Heinze A, et al. Effectiveness of oleum menthae piperitae and paracetamol in therapy of headache of the tension type [German]. Nervenarzt. 1996;67:672-681.
53. Schattner P, Randerson D. Tiger Balm as a treatment of tension headache. A clinical trial in general practice. Aust Fam Physician. 1996;25:216-220.
54. Johar P, Grover V, Topp R, et al. A comparison of topical menthol to ice on pain, evoked tetanic and voluntary force during delayed onset muscle soreness. Int J Sports Phys Ther. 2012;7:314-322.
55. Topp R, Brosky JA Jr, Pieschel D. The effect of either topical menthol or a placebo on functioning and knee pain among patients with knee OA. J Geriatr Phys Ther. 2013;36:92-99.
56. Sundstrup E, Jakobsen MD, Brandt M, et al. Acute effect of topical menthol on chronic pain in slaughterhouse workers with carpal tunnel syndrome: triple-blind, randomized placebo-controlled trial. Rehabil Res Pract. 2014;2014:310913.
57. Nair B. Final report on the safety assessment of mentha piperita (peppermint) oil, mentha piperita (peppermint) leaf extract, mentha piperita (peppermint) leaf, and mentha piperita (peppermint) leaf water. Int J Toxicol. 2001;20(Suppl 3):61-73.
58. Klingler B, Chadhary S. Peppermint oil. Am Fam Physician. 2007;75:1027-1030.
59. Nath SS, Pandey C, Roy D. A near fatal case of high dose peppermint oil ingestion—lessons learnt. Indian J Anaesth. 2012; 56:582-
1. National Center for Complementary and Integrative Health. The Use of Complementary and Alternative Medicine in the United States. Available at: https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed November 28, 2017.
2. Wallace M, Pappagallo M. Qutenza: a capsaicin 8% patch for the management of postherpetic neuralgia. Expert Rev Neurother. 2011;11:15-27.
3. Rains C, Bryson HM. Topical capsaicin. A review of its pharmacological properties and therapeutic potential in post-herpetic neuralgia, diabetic neuropathy and osteoarthritis. Drugs Aging. 1995;7:317-328.
4. Derry S, Sven-Rice A, Cole P, et al. Topical capsaicin (high concentration) for chronic neuropathic pain in adults. Cochrane Database Syst Rev. 2013;(2):CD007393.
5. Mason L, Moore RA, Derry S, et al. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;328:991.
6. Deal CL, Schnitzer TJ, Lipstein E, et al. Treatment of arthritis with topical capsaicin: a double-blind trial. Clin Ther. 1991;13:383.
7. McCarthy GM, McCarty DJ. Effect of topical capsaicin in the therapy of painful osteoarthritis of the hands. J Rheumatol. 1992;19:604.
8. De Silva V, El-Metwally A, Ernst E, et al; Arthritis Research UK Working Group on Complementary and Alternative Medicines. Evidence for the efficacy of complementary and alternative medicines in the management of osteoarthritis: a systematic review. Rheumatology (Oxford). 2011;50:911-920.
9. Cameron M, Chrubasik S. Topical herbal therapies for treating osteoarthritis. Cochrane Database Syst Rev. 2013;(5):CD010538.
10. Oltean H, Robbins C, van Tulder MW, et al. Herbal medicine for low-back pain. Cochrane Database Syst Rev. 2014;(12):CD004504.
11. Armstrong EP, Malone DC, McCarberg B, et al. Cost-effectiveness analysis of a new 8% capsaicin patch compared to existing therapies for postherpetic neuralgia. Curr Med Res Opin. 2011;27:939-950.
12. Mou J, Paillard F, Turnbull B, et al. Efficacy of Qutenza (capsaicin) 8% patch for neuropathic pain: a meta-analysis of the Qutenza Clinical Trials Database. Pain. 2013;154:1632-1639.
13. Sun-Edelstein C, Mauskop A. Alternative headache treatments: nutraceuticals, behavioral and physical treatments. Headache. 2011;51:469-483.
14. D’Andrea G, Cevoli S, Cologno D. Herbal therapy in migraine. Neurol Sci. 2014;35(Suppl 1):135-140.
15. Diener HC, Rahlfs VW, Danesch U. The first placebo-controlled trial of a special butterbur root extract for the prevention of migraine: reanalysis of efficacy criteria. Eur Neurol. 2004;51:89-97.
16. Lipton RB, Göbel H, Einhäupl KM, et al. Petasites hybridus root (butterbur) is an effective preventive treatment for migraine. Neurology. 2004;63:2240-2244.
17. Pothmann R, Danesch U. Migraine prevention in children and adolescents: results of an open study with a special butterbur root extract. Headache. 2005;45:196-203.
18. Holland S, Silberstein SD, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78:1346-1353.
19. American Academy of Neurology. Evidence-based guideline update: NSAIDs and other complementary treatments for episodic migraine prevention in adults: [RETIRED]. Sept 16, 2015. Available at: http://n.neurology.org/content/78/17/1346. Accessed December 14, 2017.
20. Man LX. Complementary and alternative medicine for allergic rhinitis. Curr Opin Otolaryngol Head Neck Surg. 2009;17:226-231.
21. Guo R, Pittler MH, Ernst E. Herbal medicines for the treatment of allergic rhinitis: a systematic review. Ann Allergy Asthma Immunol. 2007;99:483-495.
22. Daniel O, Mauskop A. Nutraceuticals in acute and prophylactic treatment of migraine. Curr Treat Options Neurol. 2016;18:14.
23. Chacko SM, Thambi PT, Kuttan R, et al. Beneficial effects of green tea: a literature review. Chin Med. 2010;6:13.
24. Naghma K, Hasan M. Tea polyphenols for health promotion. Life Sciences. 2007;81:519-533.
25. Okello EJ, McDougall GJ, Kumar S, et al. In vitro protective effects of colon-available extract of Camellia sinensis (tea) against hydrogen peroxide and beta-amyloid (Aβ((1-42))) induced cytotoxicity in differentiated PC12 cells. Phytomedicine. 2011;15;18:691-696.
26. Schmidt A, Hammann F, Wölnerhanssen B, et al. Green tea extract enhances parieto-frontal connectivity during working memory processing. Psychopharmacology (Berl). 2014;231:3879-3888.
27. Tomata Y, Sugiyama K, Kaiho Y, et al. Green tea consumption and the risk of incident dementia in elderly japanese: The Ohsaki Cohort 2006 Study. Am J Geriatr Psychiatry. 2016;24:881-889.
28. Takechi R, Alfonso H, Hiramatsu N, et al. Elevated plasma and urinary concentrations of green tea catechins associated with improved plasma lipid profile in healthy Japanese women. Nutr Res. 2016;36:220-226.
29. Kim A, Chiu A, Barone MK, et al. Green tea catechins decrease total and low-density lipoprotein cholesterol: a systematic review and meta-analysis. J Am Diet Assoc. 2011;111:1720-1729.
30. Zhang C, Qin YY, Wei X, et al. Tea consumption and risk of cardiovascular outcomes and total mortality: a systematic review and meta-analysis of prospective observational studies. Eur J Epidemiol. 2015;30:103-113.
31. Imai K, Suga K, Nakachi K. Cancer-preventive effects of drinking green tea among a Japanese population. Prev Med. 1997;26:769-775.
32. Yuan JM. Cancer prevention by green tea: evidence from epidemiologic studies. Am J Clin Nutr. 2013;98(6 Suppl):1676S-1681S.
33. Kurahashi N, Sasazuki S, Iwasaki M, et al. Green tea consumption and prostate cancer risk in Japanese men: a prospective study. Am J Epidemiol. 2008;167:71-77.
34. Iso H, Date C, Wakai K, et al. The relationship between green tea and total caffeine intake and risk for self-reported type 2 diabetes among Japanese adults. Ann Intern Med. 2006;144:554-562.
35. Kim HM, Kim J. The effects of green tea on obesity and type 2 diabetes. Diab Metabol J. 2013;37:173-175.
36. Yang J, Mao Q, Xu H, et al. Tea consumption and risk of type 2 diabetes mellitus: a systematic review and meta-analysis update. BMJ Open. 2014;4:e005632.
37. Liu K, Zhou R, Wang B, et al. Effect of green tea on glucose control and insulin sensitivity: a meta-analysis of 17 randomized controlled trials. Am J Clin Nutr. 2013;98:340-348.
38. Isomura T, Suzuki S, Origasa H, et al. Liver-related safety assessment of green tea extracts in humans: a systematic review of randomized controlled trials. Eur J Clin Nutr. 2016;70:1340.
39. Tillisch K. Complementary and alternative medicine for gastrointestinal disorders. Clin Med (Lond). 2007;7:224-227.
40. Knowlton WM, McKemy DD. TRPM8: from cold to cancer, peppermint to pain. Curr Pharm Biotechnol. 2011;12:68-77.
41. Ford AC, Moayyedi P, Lacy BE, et al. Task Force on the Management of Functional Bowel Disorders. American College of Gastroenterology monograph on the management of irritable bowel syndrome and chronic idiopathic constipation. Am J Gastroenterol. 2014;109(Suppl 1):S2-S26;quiz S27.
42. Ruepert L, Quartero AO, de Wit NJ, et al. Bulking agents, antispasmodics and antidepressants for the treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2011;(8):CD003460.
43. Khanna R, MacDonald JK, Levesque BG. Peppermint oil for the treatment of irritable bowel syndrome: a systematic review and meta-analysis. J Clin Gastroenterol. 2014;48:505-512.
44. Cash BD, Epstein MS, Shah SM. A novel delivery system of peppermint oil is an effective therapy for irritable bowel syndrome symptoms. Digest Dis Sci. 2016;61:560-571.
45. Holtmann G, Haag S, Adam B, et al. Effects of a fixed combination of peppermint oil and caraway oil on symptoms and quality of life in patients suffering from functional dyspepsia. Phytomedicine. 2003;10(suppl 4):56-57.
46. Madisch A, Heydenreich CJ, Wieland V, et al. Treatment of functional dyspepsia with a fixed peppermint oil and caraway oil combination preparation as compared to cisapride. A multicenter, reference-controlled double-blind equivalence study. Arzneimittelforschung. 1999;49:925-932.
47. Asao T, Kuwano H, Ide M, et al. Spasmolytic effect of peppermint oil in barium during double-contrast barium enema compared with Buscopan. Clin Radiol. 2003;58:301-305.
48. Sparks MJ, O’Sullivan P, Herrington AA, et al. Does peppermint oil relieve spasm during barium enema? Br J Radiol. 1995;68:841-843.
49. Akhavan Amjadi M, Mojab F, Kamranpour SB. The effect of peppermint oil on symptomatic treatment of pruritus in pregnant women. Iranian J Pharm Res. 2012;11:1073-1077.
50. St Cyr A, Chen A, Bradley KC, et al. Efficacy and tolerability of STOPAIN for a migraine attack. Front Neurol. 2015;6:11.
51. Borhani Haghighi A, Motazedian S, Rezaii R, et al. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: a randomised, double-blind, placebo-controlled, crossed-over study. Int J Clin Pract. 2010;64:451-456.
52. Gobel H, Fresenius J, Heinze A, et al. Effectiveness of oleum menthae piperitae and paracetamol in therapy of headache of the tension type [German]. Nervenarzt. 1996;67:672-681.
53. Schattner P, Randerson D. Tiger Balm as a treatment of tension headache. A clinical trial in general practice. Aust Fam Physician. 1996;25:216-220.
54. Johar P, Grover V, Topp R, et al. A comparison of topical menthol to ice on pain, evoked tetanic and voluntary force during delayed onset muscle soreness. Int J Sports Phys Ther. 2012;7:314-322.
55. Topp R, Brosky JA Jr, Pieschel D. The effect of either topical menthol or a placebo on functioning and knee pain among patients with knee OA. J Geriatr Phys Ther. 2013;36:92-99.
56. Sundstrup E, Jakobsen MD, Brandt M, et al. Acute effect of topical menthol on chronic pain in slaughterhouse workers with carpal tunnel syndrome: triple-blind, randomized placebo-controlled trial. Rehabil Res Pract. 2014;2014:310913.
57. Nair B. Final report on the safety assessment of mentha piperita (peppermint) oil, mentha piperita (peppermint) leaf extract, mentha piperita (peppermint) leaf, and mentha piperita (peppermint) leaf water. Int J Toxicol. 2001;20(Suppl 3):61-73.
58. Klingler B, Chadhary S. Peppermint oil. Am Fam Physician. 2007;75:1027-1030.
59. Nath SS, Pandey C, Roy D. A near fatal case of high dose peppermint oil ingestion—lessons learnt. Indian J Anaesth. 2012; 56:582-
PRACTICE RECOMMENDATIONS
› Consider capsaicin as an alternative to oral and topical nonsteroidal anti-inflammatory drugs to treat musculoskeletal pain in patients who don't respond to the latter. B
› Consider ordering liver function monitoring for patients using butterbur because of the risk of toxicity. C
› Recommend that patients consider drinking green tea as part of a healthy diet. B
› Recommend peppermint to patients with irritable bowel syndrome. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The evidence for herbal and botanical remedies, Part 2
More than a third of American adults use complementary and alternative medicine.1 Unfortunately, the public’s enthusiasm for herbal products is not always consistent with the scientific evidence supporting their use. In part one of this series, we discussed the studies that have been done on capsaicin, butterbur, green tea, and peppermint. In this installment, we outline the research on 5 additional remedies: turmeric/curcumin, which may be of benefit in ulcerative colitis; chamomile, which appears to offer relief to patients with anxiety; rosemary, which may help treat alopecia; as well as coffee and cocoa, which may have some cardiovascular benefits (TABLE).
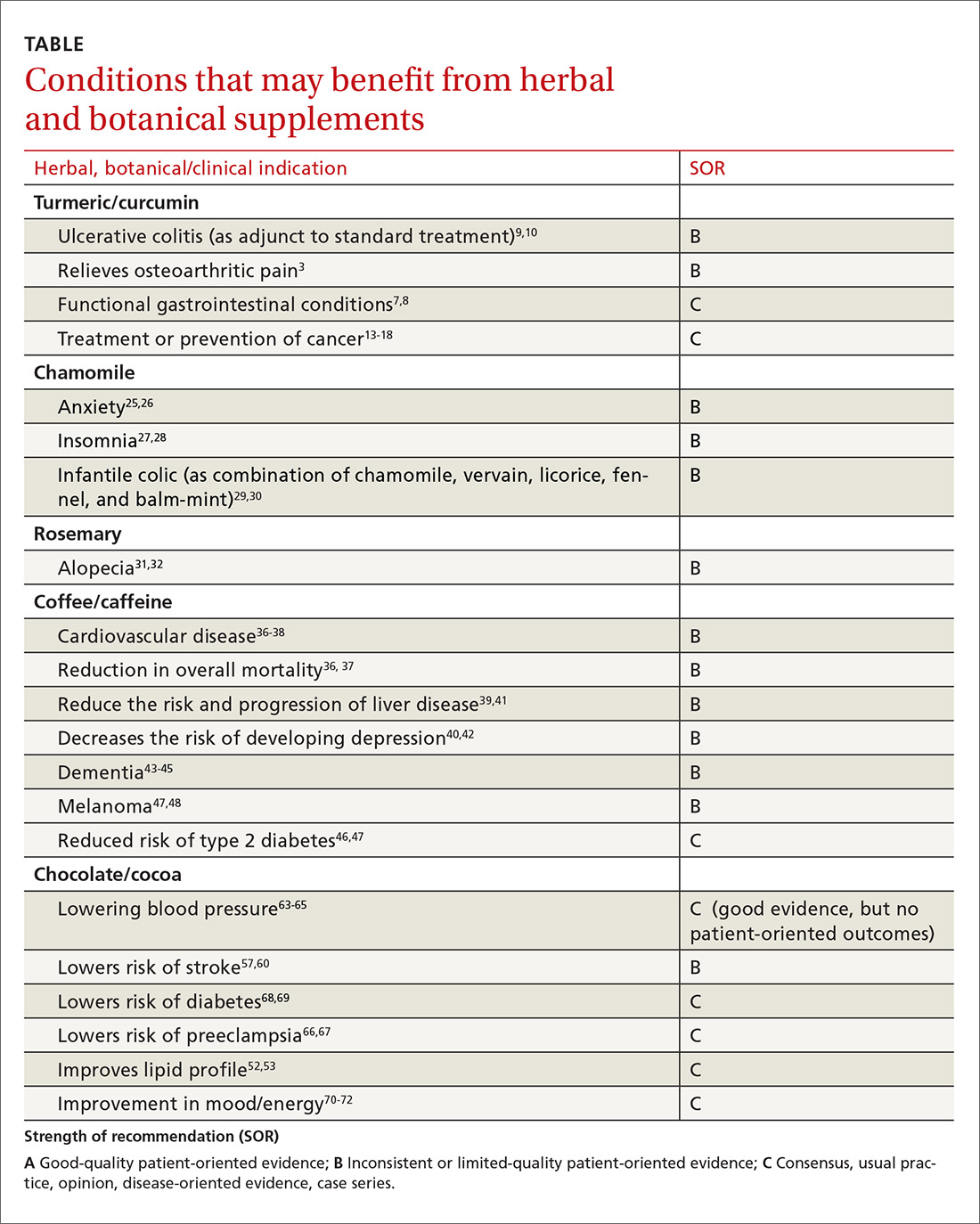
Turmeric/curcumin
Overview
Turmeric (Curcuma longa), a relative of ginger, has been used for 4000 years to treat a variety of conditions.2,3 Curcumin is the yellow pigment isolated from the rhizomes of Curcuma longa, commonly known as turmeric.3 Turmeric powder contains 5% curcumin, which is the main biologically active compound. Although it grows in many tropical locations, most turmeric is grown in India, where it is used as a main ingredient in curry. The roots and bulbs of turmeric that are used in medicine are generally boiled and dried, which results in a yellow powder.
Turmeric has been used in both Ayurvedic and Chinese medicine for its anti-inflammatory properties, in the treatment of digestive and liver problems, to fight infections, and to help heal skin diseases and wounds.3-7
Functional GI disorders. A recent review noted that curcumin has been shown in several preclinical studies and uncontrolled clinical trials to have effects on gut inflammation, gut permeability, and the brain-gut axis, especially in functional GI disorders.7 A double-blind, placebo-controlled study from 1989 found that turmeric reduced symptoms of bloating and gas in subjects suffering from undifferentiated dyspepsia.8
Ulcerative colitis (UC). A 2012 Cochrane review noted that curcumin appears to be a safe and effective therapy for maintenance of remission in quiescent UC when given as adjunctive therapy along with mesalamine or sulfasalazine.9 In a 2015 randomized controlled trial (RCT), the addition of curcumin to mesalamine therapy was superior to the combination of placebo and mesalamine in inducing clinical and endoscopic remission in patients with mild-to-moderate active UC, producing no apparent adverse effects.10
Osteoarthritis (OA). Because of turmeric’s ability to reduce inflammation, it may help relieve OA pain.3 Clinical evidence is scant for the anti-arthritic efficacy of turmeric dietary supplements, although animal studies indicate that turmeric prevents inflammation through regulation of NF-kappaB-regulated genes that regulate the immune and inflammatory response.6 Inflammatory cell influx, joint levels of prostaglandin E2, and periarticular osteoclast formation were also inhibited by turmeric extract treatment.6
A 2013 review of turmeric for OA concluded that observational studies and in vitro results are promising for the use of curcumin for OA, but well-designed clinical studies were lacking and are needed to support the efficacy of curcumin in OA patients.11 However, in a 2014 randomized trial of 367 patients, turmeric appeared to be similar in efficacy to ibuprofen for the treatment of pain and disability in adults with knee OA.12 The curcumin (turmeric) group also had fewer adverse effects.12
Cancer. There has been a great deal of research on turmeric’s anti-cancer properties, but clinical evidence is lacking. In vitro evidence, animal studies, and small clinical trials suggest that curcumin may help prevent or treat several types of cancers, but the overall evidence is poor. Nonetheless, curcumin and turmeric have been or are currently being evaluated for the treatment or prevention of prostate, liver, breast, skin, gynecologic, hematologic, pulmonary, thymic, bone, brain, and colon cancer.13-18
Oral submucous fibrosis. A small randomized trial found improvement in oral function with curcumin lozenges, when compared to placebo, indicating that turmeric may hold promise as a treatment of oral submucous fibrosis.19
Uveitis. A small pilot study of 32 patients suggested that oral curcumin may be as effective as corticosteroids for uveitis.20
Heart disease. Curcumin may have a cardiovascular protective role, as it has been shown to reduce atherosclerosis, but a reduction in myocardial infarction or stroke has not been documented.21
Alzheimer’s dementia. Animal studies have shown a reduction in amyloid plaque formation with curcumin.22
Adverse effects (and precautions)
Turmeric in food is considered safe. A variety of animal and human studies have also indicated that curcumin is safe and well tolerated, even at very high doses.13 However, taking large amounts of turmeric for long periods of time could cause stomach upset and gastric ulcers. In addition, patients with gallstones or bile obstruction should use it with caution due to increased bile production.7
Because turmeric may lower blood sugar levels, patients with diabetes should monitor for hypoglycemia when using turmeric in combination with diabetic medications. Similarly, those with bleeding disorders taking blood thinners should use turmeric and curcumin with caution, because it can inhibit platelet aggregation.23
Although it is safe to eat foods with turmeric during pregnancy, pregnant and breastfeeding women should not take turmeric supplements, as the safety of large doses in pregnancy is unknown.
The bottom line
Turmeric/curcumin has anti-inflammatory properties and may be useful as an adjunct for ulcerative colitis and to improve the symptoms of OA. It may also have anti-carcinogenic properties, although definitive data are lacking. Those with a history of gastrointestinal conditions such as gastric ulcer, patients taking blood thinners, and patients with diabetes who are prone to low blood sugar levels should use turmeric/curcumin with caution.
Chamomile
Overview
Chamomile, a member of the Asteraceae/Compositae family, is one of the oldest herbal medicines. It has been used for hay fever, inflammation, muscle spasms, menstrual disorders, insomnia, ulcers, wounds, gastrointestinal disorders, rheumatic pain, and hemorrhoids. Essential oils of chamomile are used extensively in cosmetics and aromatherapy. Many different preparations have been developed, the most popular being herbal tea.24
Individuals with a hypersensitivity to plants of the Asteraceae (Compositae) family such as ragweed (Ambrosia spp.), marigold flower (Calendula officinalis), and chrysanthemum (Chrysanthemum spp.) may show a similar reaction to chamomile.25
Anxiety. A controlled clinical trial of chamomile extract for generalized anxiety disorder (GAD) suggested that it may have modest anxiolytic activity in patients with mild to moderate GAD.26 Another randomized, double-blind, placebo-controlled trial found oral chamomile extract was efficacious and well-tolerated in patients experiencing mild to moderate GAD and may provide an alternative therapeutic anxiolytic for patients with mild GAD.25 In addition to its anxiolytic activity, chamomile may also provide clinically meaningful antidepressant activity.26
Insomnia. Chamomile may have some impact on sleep diary measures (total sleep time, sleep efficiency, sleep latency, wake after sleep onset, sleep quality, and number of awakenings) relative to placebo in adults with chronic primary insomnia, according to a small randomized, double-blind, placebo-controlled pilot trial involving 34 patients.27 However, a systematic review found no statistically significant difference between any herbal medicine (including chamomile) and placebo, for clinical efficacy in patients with insomnia. A similar, or smaller, number of adverse events per person were reported with chamomile compared with placebo, suggesting safe use.28
Infantile colic. A small prospective double-blind study on the use of chamomile-containing tea on infantile colic showed statistically significant symptom improvement in tea-treated infants. The study did note, however, that prolonged ingestion of herbal teas may lead to decreased milk intake.29,30
Adverse effects
As noted earlier, a systematic review found that the number of adverse events per person reported with chamomile was comparable to the number associated with placebo, suggesting that it is safe.28
The bottom line
Chamomile appears to be safe with minimal adverse effects and may be effective for the treatment of anxiety, insomnia, and infantile colic.
Rosemary
Overview
Rosemary, officially known as Rosmarinus officinalis, is a medicinal evergreen plant native to the Mediterranean area that appears to increase microcapillary perfusion.31
Alopecia. A randomized double-blind controlled trial found that essential oils including rosemary oil (as well as thyme, lavender, and cedarwood) massaged into the scalp improved hair growth in almost half of patients with alopecia areata after 7 months.32 Another randomized trial comparing rosemary oil to minoxidil 2% for androgenetic alopecia showed a significant increase in hair count at the 6-month endpoint compared with the baseline, but no significant difference was found between the study groups regarding hair count either at Month 3 or Month 6 (P >.05). 31
Adverse effects
In the randomized trial described above comparing rosemary oil to minoxidil 2%, adverse effects appeared to be rare for topical rosemary oil. Scalp itching was more frequent in the minoxidil group.31
The bottom line
Topical rosemary oil may be useful in the treatment of alopecia with minimal adverse effects.
Coffee/caffeine
Overview
Coffee is one of the most widely used botanicals with approximately 3.5 billion cups of coffee consumed per day worldwide. It is a popular beverage because of its unique aromatic taste and its use as a central nervous system stimulant. The coffee tree (genus coffea) is found throughout Latin America, Africa, and eastern Asia. Two of the most common commercially grown species are Coffea arabica (Arabicas) and Coffea canephora (Robusta). Processing and roasting methods may differ and produce variations in flavor and aroma. The degree of roasting also affects the caffeine content.
Coffee consumption leads to increased alertness and can boost mental performance. Based on the literature and US Food and Drug Administration recommendations, four 8-oz cups of coffee (about 400 mg of caffeine) daily is an acceptable average amount of caffeine. More than 500 mg/d is considered excessive use of coffee.33,34
Overall mortality. A 2008 study showed that regular coffee was not associated with increased or decreased mortality in both men and women.35 However, more recent studies show an inverse relationship between mortality and coffee consumption.
Specifically, a 2014 meta-analysis found an inverse relationship between coffee and mortality.36 A large prospective cohort study from 2015 that included 79,234 women and 76,704 men found that drinking coffee was inversely associated with overall mortality.37 In this cohort study, an inverse association were observed for deaths from heart disease, respiratory disease, diabetes, and self-harm.37 While mechanisms were not analyzed, coffee may reduce mortality risk by affecting inflammation, lung function, insulin sensitivity, and depression.
Cardiovascular disease. Coffee consumption may modestly reduce the risk of stroke, according to a prospective cohort study of 83,076 women from the Nurses’ Health Study who were followed for 24 years.38 Reduced cardiovascular mortality was also found in a large prospective cohort study, as noted in the mortality discussion above.37 A 2014 meta-analysis concluded that coffee consumption is inversely associated with cardiovascular mortality. Drinking 3 or 4 cups a day appears to be the amount that may decrease one’s risk of death when compared to those who do not drink coffee at all.36
Liver disease. Friedrich et al performed a study involving 379 patients with end stage liver disease, and found that coffee consumption delayed the progression of disease in patients with both alcoholic liver disease and primary sclerosing cholangitis.39 Coffee consumption also increased long-term survival after liver transplantation.39 However, the study found that coffee did not have any effect on patients with chronic viral hepatitis.
In a 2016 meta-analysis, caffeinated coffee consumption reduced hepatic fibrosis of nonalcoholic fatty liver disease, although caffeine consumption did not reduce the prevalence of nonalcoholic fatty liver disease.40 Another meta-analysis, including 16 studies, also found caffeine reduced the risk for hepatic fibrosis and cirrhosis.41
Depression. Based on 2 different systematic reviews and meta-analyses from 2016, coffee consumption appears to have a significant protective effect, decreasing the risk of developing depression.40,42
Alzheimer’s disease/dementia. Coffee, tea, and caffeine consumption show promise in reducing the risk of cognitive decline and dementia. Individuals who consume one to 2 cups of coffee per day had a decreased incidence of mild cognitive impairment compared to non-drinkers.43 A 2015 Japanese study also found an inverse association between coffee consumption and dementia among women, nonsmokers, and those who do not drink alcohol.44 Most recently, a 2016 study, the Women’s Health Initiative Memory Study, looked at incident dementia rates in women >65 years of age with high vs low caffeine intake. Women with higher caffeine intake were less likely to develop dementia or any cognitive impairment compared with those consuming <64 mg/day.45
Type 2 diabetes. A 2009 prospective cohort study, which included 40,011 participants followed for more than 10 years, found that drinking at least 3 cups of coffee or tea was associated with a lowered risk of type 2 diabetes.46 A 2009 systematic review of 20 cohort studies showed that high intakes of coffee, decaffeinated coffee, and tea are associated with a reduced risk of diabetes.47
Melanoma.
Adverse effects
Despite the many potential benefits of coffee, caffeine is a potent drug that should be used with caution.49 People with underlying heart problems should avoid caffeine due to concern that it may cause palpitations from tachycardia. It may worsen anxiety problems or depression. Coffee may increase the production of stomach acids, which can worsen acid reflux or stomach ulcers.
Caffeine is a potent diuretic and may decrease absorption of calcium and cause OA. Caffeine may cause dependence and withdrawal symptoms. Some of the symptoms of withdrawal include drowsiness, headaches, irritability, nausea, and vomiting. It may disrupt sleeping patterns by causing jitters and sleeplessness.49 Additionally, large amounts of caffeine may cause overdose and death.
The bottom line
Regular coffee intake is associated with a lower risk of mortality, reduced cardiovascular events, and a reduction in liver disease progression. Coffee may also have some utility for improving cognitive function and reducing the risk of type 2 diabetes. Caffeinated coffee should be limited to no more than 32 oz per day, due to the risk of insomnia, palpitations, anxiety, and gastritis.
Chocolate/cocoa
Overview
Few natural products have been claimed to successfully treat as many disorders as chocolate. The modern concept of chocolate as food has overshadowed its traditional medicinal use, although recent trials have looked at evidence for some of its traditional uses. Chocolate is processed from the pod of the cacao plant. The earliest evidence for its medical use is in Mayan civilizations, and for most of its approximately 4000-year history, chocolate was consumed as a bitter drink referred to as the “drink of the Gods.” The traditional drink was mixed with water, vanilla, honey, chili peppers, and other spices. Important components in chocolate include flavonoids (antioxidants), cocoa butter, caffeine, theobromine, and phenylethylamine.
Chocolate has stimulating, anti-inflammatory, neuroprotective, and cardioprotective effects, and improves the bioavailability of nitric oxide, which can improve blood pressure and platelet function.50 Epicatechin (an antioxidant) in cocoa is primarily responsible for its favorable impact on vascular endothelium via its effect on both acute and chronic upregulation of nitric oxide production. Other cardiovascular effects are mediated by the anti-inflammatory effects of cocoa polyphenols, and modulated through the activity of NF-kappaB.51
Dark chocolate appears to have the greatest benefit, as milk binds to antioxidants in chocolate, making them unavailable. Therefore, milk chocolate is not a good antioxidant source. There is no specific amount of chocolate that is known to be ideal, but an average of one to 2 ounces per day is often used in studies.
Cardiovascular effects. Chocolate does contain saturated fat, but a comparative, double-blind study found that short-term use of cocoa powder lowered plasma low-density lipoprotein (LDL) cholesterol, oxidized LDL, and apo B concentrations, and the plasma high-density lipoprotein (HDL) cholesterol concentration increased, relative to baseline in the low-, middle-, and high-cocoa groups.52 A small randomized crossover trial without clinical outcomes indicated that chocolate may increase HDL cholesterol without increasing weight.53
A meta-analysis of short-term (2-12 weeks) treatment with dark chocolate/cocoa products showed reductions in LDL and total cholesterol, but no changes in HDL or triglycerides.54 Another meta-analysis of RCTs, however, showed no short-term effect of cocoa/chocolate on lipid concentrations.55 A randomized, placebo-controlled double-blind study of 62 patients with diabetes and hypertension showed that high polyphenol chocolate improved triglyceride levels.56
Multiple studies have shown that chocolate is associated with a reduction in cardiovascular risk.57-59 A best case scenario analysis using a Markov model to predict the long-term effectiveness and cost effectiveness of daily dark chocolate consumption in a population with metabolic syndrome at high risk of cardiovascular disease concluded that daily consumption of dark chocolate can reduce cardiovascular events by 85 per 10,000 population treated over 10 years. The study concluded that $42 could be cost effectively spent per person per year on prevention strategies using dark chocolate.59
In addition, a meta-analysis of 7 observational studies showed that high levels of chocolate consumption (any type) were associated with a 29% reduction in stroke compared with the lowest levels of chocolate intake.57 Results of a similar meta-analysis from Neurology in 2012 also suggested that moderate chocolate consumption (any type) may lower the risk of stroke.60
That said, 2 systematic reviews specifically relating to the risk of coronary heart disease and chocolate intake were inconclusive.61-62
Blood pressure (BP). An RCT published in JAMA indicates that inclusion of small amounts of polyphenol-rich dark chocolate as part of a usual diet efficiently reduced BP and improved the formation of vasodilative nitric oxide.63 A meta-analysis of 10 RCTs also showed mean BP change in the active cocoa treatment arms across all trials was -4.5 mm Hg (95% confidence interval (CI), -5.9 to -3.2; P<.001) for systolic BP and -2.5 mm Hg (95% CI, -3.9 to -1.2; P<.001) for diastolic BP.64
A Cochrane Review meta-analysis of 20 studies revealed a statistically significant BP-reducing effect of flavanol-rich cocoa products compared with control in short-term trials of 2 to 18 weeks' duration.65 Because studies have shown improvement in BP with chocolate intake, investigations into a role of chocolate in the prevention of preeclampsia have been undertaken. In some studies, chocolate intake was associated with reduced odds of preeclampsia and gestational hypertension.66,67
Diabetes. Chocolate may exert significant vascular protection because of its antioxidant properties and possible increase of nitric oxide bioavailability, which can influence glucose uptake. A small trial comparing the effects of either dark or white chocolate bars (which do not contain the polyphenols) showed improved BP and glucose and insulin responses to an oral glucose tolerance test in healthy subjects on dark chocolate, but not white chocolate.68 A comparison of chocolate consumption and risk of diabetes in the Physicians’ Health Study showed an inverse relationship between chocolate intake with incident disease, but this association appeared only to apply in younger and normal-body weight men after controlling for comprehensive lifestyles, including total energy consumption.69
Fatigue. The effect of chocolate on a person’s energy level has been noted for centuries.70 A small randomized trial showed improved energy levels in those treated with higher chocolate intakes. In a double-blind, randomized, clinical pilot crossover study, high cocoa liquor/polyphenol rich chocolate, reduced fatigue in subjects with chronic fatigue syndrome.71
Anxiety. A small randomized trial showed chocolate decreased anxiety in high-anxiety trait subjects and improved the anxiety level and the energy levels of low-anxiety trait participants.72
Eye effects. The literature presents conflicting evidence regarding the effect of flavonoids on patients with glaucoma and ocular hypertension. However, a meta-analysis showed that flavonoids have a promising role in improving visual function in patients with glaucoma and ocular hypertension, and appear to play a part in both improving and slowing the progression of visual field loss.73
Cognitive decline. Chocolate intake (any type) was associated with a lower risk of cognitive decline (RR = 0.59; 95% CI, 0.38-0.92) with the greatest benefit noted in those who averaged more than one chocolate bar or one tablespoon of cocoa powder per week. This protective effect was observed only among subjects with an average daily consumption of caffeine <75 mg (69% of the participants; RR = 0.50; 95% CI, 0.31-0.82).74
The bottom line
Chocolate with high cocoa content (dark chocolate) appears to be safe and beneficial as part of a healthy diet and lifestyle that includes exercise and stress reduction to decrease cardiovascular risk and may improve energy levels.
CORRESPONDENCE
Michael Malone, MD, Family and Community Medicine, Penn State College of Medicine, 500 University Drive, Hershey, PA 17033; [email protected].
1. National Center for Complementary and Integrative Health. The use of complementary and alternative medicine in the United States. Available at: https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed Nov 28, 2017.
2. Aggarwal BB. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
3. Henrotin Y, Clutterbuck AL, Allaway D, et al. Biological actions of curcumin on articular chondrocytes. Osteoarthritis Cartilage. 2010;18:141-149.
4. Asher GN, Spelman K. Clinical utility of curcumin extract. Altern Ther Health Med. 2013;19:20-22.
5. Phan TT, See P, Lee ST, et al. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J Trauma. 2001;51:927-931.
6. Funk JL, Frye JB, Oyarzo JN, et al. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis Rheum. 2006;54:3452-3464.
7. Patcharatrakul T, Gonlachanvit S. Chili peppers, curcumins, and prebiotics in gastrointestinal health and disease. Curr Gastroenterol Rep. 2016;18:19.
8. Thamlikitkul V, Bunyapraphatsara N, Dechatiwongse T, et al. Randomized double blind study of Curcuma domestica Val. for dyspepsia. J Med Assoc Thai. 1989;72:613-620.
9. Kumar S, Ahuja V, Sankar MJ, et al. Curcumin for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;10:CD008424.
10. Lang A, Salomon N, Wu JC, et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2015;13:1444-1449.e1.
11. Henrotin Y, Priem F, Mobasheri A. Curcumin: a new paradigm and therapeutic opportunity for the treatment of osteoarthritis: curcumin for osteoarthritis management. Springerplus. 2013;2:56.
12. Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, et al. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Interv Aging. 2014;9:451-458.
13. Shehzad A, Lee J, Lee YS. Curcumin in various cancers. Biofactors. 2013;39:56-68.
14. Sordillo LA, Sordillo PP, Helson L. Curcumin for the treatment of glioblastoma. Anticancer Res. 2015;35:6373-6378.
15. Darvesh AS, Aggarwal BB, Bishayee A. Curcumin and liver cancer: a review. Curr Pharm Biotechnol. 2012;13:218-228.
16. Nagaraju GP, Aliya S, Zafar SF, et al. The impact of curcumin on breast cancer. Integr Biol (Camb). 2012;4:996-1007.
17. Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255:170-181.
18. Dorai T, Cao YC, Dorai B, et al. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate. 2001;47:293-303.
19. Hazarey VK, Sakrikar AR, Ganvir SM. Efficacy of curcumin in the treatment for oral submucous fibrosis - a randomized clinical trial. J Oral Maxillofac Pathol. 2015;19:145-152.
20. Lal B, Kapoor AK, Asthana OP, et al. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res. 1999;13:318-322.
21. Kapakos G, Youreva V, Srivastava AK. Cardiovascular protection by curcumin: molecular aspects. Indian J Biochem Biophys. 2012;49:306-315.
22. Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892-5901.
23. Heck AM, DeWitt BA, Lukes AL. Potential interactions between alternative therapies and warfarin. Am J Health Syst Pharm. 2000;57:1221-1227.
24. Srivastava JK, Shankar E, Gupta S. Chamomile: a herbal medicine of the past with bright future. Mol Med Rep. 2010;3:895-901.
25. Ross SM. Generalized anxiety disorder (GAD): efficacy of standardized matricaria recutita (german chamomile) extract in the treatment of generalized anxiety disorder. Holistic Nursing Practice. 2013;27:366- 368.
26. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29:378-382.
27. Zick SM, Wright BD, Sen A, et al. Preliminary examination of the efficacy and safety of a standardized chamomile extract for chronic primary insomnia: a randomized placebo-controlled pilot study. BMC Complement Altern Med. 2011;11:78.
28. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12.
29. Weizman Z, Alkrinawi S, Goldfarb D, et al. Efficacy of herbal tea preparation in infantile colic. J Pediatr. 1993;122:650.
30. Crotteau CA, Wright ST, Eglash A. Clinical inquiries. What is the best treatment for infants with colic? J Fam Pract. 2006;55:634-636.
31. Panahi Y, Taghizadeh M, Marzony ET, et al. Rosemary oil vs minoxidil 2% for the treatment of androgenetic alopecia: a randomized comparative trial. Skinmed. 2015;13:15-21.
32. Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. Successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
33. Caffeine and kids: FDA takes a closer look. Available at: https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm350570.htm. Accessed: November 1, 2017.
34. Torpy JM, Livingston EH. Energy Drinks. JAMA. 2013;309:297.
35. Lopez-Garcia E, van Dam RM, Li TY, et al. The relationship of coffee consumption with mortality. Ann Intern Med. 2008;148:904-914.
36. Crippa A, Discacciati A, Larsson SC, et al. Coffee consumption and mortality from all causes, cardiovascular disease, and cancer: a dose-response meta-analysis. Am J Epidemiol. 2014;180:763-775.
37. Loftfield E, Freedman ND, Graubard BI, et al. Association of coffee consumption with overall and cause-specific mortality in a large US prospective cohort study. Am J Epidemiol. 2015;182:1010-1022.
38. Lopez-Garcia E, Rodriguez-Artalejo F, Rexrode KM, et al. Coffee consumption and risk of stroke in women. Circulation. 2009;119:1116-1123.
39. Friedrich K, Smit M, Wannhoff A, et al. Coffee consumption protects against progression in liver cirrhosis and increases long-term survival after liver transplantation. J Gastroenterol Hepatol. 2016;31:1470-1475.
40. Wang L, Shen X, Wu Y, et al. Coffee and caffeine consumption and depression: a meta-analysis of observational studies. Aust N Z J Psychiatry. 2016;50:228-242.
41. Liu F, Wang X, Wu G, et al. Coffee consumption decreases risks for hepatic fibrosis and cirrhosis: a meta-analysis. PLoS One. 2015;10:e0142457.
42. Grosso G, Micek A, Castellano S, et al. Coffee, tea, caffeine and risk of depression: a systematic review and dose-response meta-analysis of observational studies. Mol Nutr Food Res. 2016;60:223-234.
43. Solfrizzi V, Panza F, Imbimbo BP, et al. Italian longitudinal study on aging working group. Coffee consumption habits and the risk of mild cognitive impairment: The Italian Longitudinal Study on Aging. J Alzheimers Dis. 2015;47:889-899.
44. Sugiyama K, Tomata Y, Kaiho Y, et al. Association between coffee consumption and incident risk of disabling dementia in elderly japanese: The Ohsaki Cohort 2006 Study. J Alzheimers Dis. 2015;50:491-500.
45. Driscoll I, Shumaker SA, Snively BM, et al. Relationships between caffeine intake and risk for probable dementia or global cognitive impairment: The Women’s Health Initiative Memory Study. J Gerontol A Biol Sci Med Sci. 2016;71:1596-1602.
46. van Dieren S, Uiterwaal CS, van der Schouw YT, et al. Coffee and tea consumption and risk of type 2 diabetes. Diabetologia. 2009;52:2561-2569.
47. Wang J, Li X, Zhang D. Coffee consumption and the risk of cutaneous melanoma: a meta-analysis. Eur J Nutr. 2016;55:1317-1329.
48. Liu J, Shen B, Shi M, et al. Higher caffeinated coffee intake is associated with reduced malignant melanoma risk: a meta-analysis study. PLoS One. 2016;11:e0147056.
49. Wikoff D, Welsh BT, Henderson R, et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem Toxical. 2017;109(Pt 1):585-648.
50. Verna R. The history and science of chocolate. Malays J Pathol. 2013;35:111-121.
51. Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal. 2011;15:2779-2811.
52. Baba S, Natsume M, Yasuda A, et al. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. J Nutr. 2007;137:1436-1441.
53. Mellor DD, Sathyapalan T, Kilpatrick ES, et al. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in type 2 diabetes patients. Diabet Med. 2010;27:1318-1321.
54. Tokede OA, Gaziano JM, Djoussé L. Effects of cocoa products/dark chocolate on serum lipids: a meta-analysis. Eur J Clin Nutr. 2011;65:879-886.
55. Jia L, Liu X, Bai YY, et al. Short-term effect of cocoa product consumption on lipid profile: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2010;92:218-225.
56. Rostami A, Khalili M, Haghighat N, et al. High-cocoa polyphenol-rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atheroscler. 2015;11:21-29.
57. Buitrago-Lopez A, Sanderson J, Johnson L, et al. Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis. BMJ. 2011;26;343:d4488.
58. Wang X, Ouyang YY, Liu J, et al. Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr. 2014;111:1-11.
59. Zomer E, Owen A, Magliano DJ, et al. The effectiveness and cost effectiveness of dark chocolate consumption as prevention therapy in people at high risk of cardiovascular disease: best case scenario analysis using a Markov model. BMJ. 2012;344:e3657.
60. Larsson SC, Virtamo J, Wolk A. Chocolate consumption and risk of stroke: a prospective cohort of men and meta-analysis. Neurology. 2012;79:1223-1229.
61. Khawaja O, Gaziano JM, Djoussé L. Chocolate and coronary heart disease: a systematic review. Curr Atheroscler Rep. 2011;13:447-452.
62. Jacques PF, Cassidy A, Rogers G, et al. Dietary flavonoid intakes and CVD incidence in the Framingham Offspring Cohort. Br J Nutr. 2015;114:1496-1503.
63. Taubert D, Roesen R, Lehmann C, et al. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA. 2007;298:49-60.
64. Desch S, Schmidt J, Kobler D, et al. Effect of cocoa products on blood pressure: systematic review and meta-analysis. Am J Hypertens. 2010;23:97-103.
65. Ried K, Sullivan TR, Fakler P, et al. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2012;8:CD008893.
66. Saftlas AF, Triche EW, Beydoun H, et al. Does chocolate intake during pregnancy reduce the risks of preeclampsia and gestational hypertension? Ann Epidemiol. 2010;20:584-591.
67. Triche EW, Grosso LM, Belanger K, et al. Chocolate consumption in pregnancy and reduced likelihood of preeclampsia. Epidemiology. 2008;19:459-464.
68. Grassi D, Lippi C, Necozione S, et al. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. 2005;81:611-614.
69. Matsumoto C, Petrone AB, Sesso HD, et al. Chocolate consumption and risk of diabetes mellitus in the Physicians’ Health Study. Am J Clin Nutr. 2015;101:362-367.
70. Lippi D. Chocolate in history: food, medicine, medi-food. Nutrients. 2013;5:1573-1584.
71. Sathyapalan T, Beckett S, Rigby AS, et al. High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutr J. 2010;9:55.
72. Martin FP, Antille N, Rezzi S, et al. Everyday eating experiences of chocolate and non-chocolate snacks impact postprandial anxiety, energy and emotional states. Nutrients. 2012;4:554-567.
73. Patel S, Mathan JJ, Vaghefi E, et al. The effect of flavonoids on visual function in patients with glaucoma or ocular hypertension: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2015;253:1841-1850.
74. Moreira A, Diógenes MJ, de Mendonça A, et al. Chocolate consumption is associated with a lower risk of cognitive decline. J Alzheimers Dis. 2016;53:85-93.
More than a third of American adults use complementary and alternative medicine.1 Unfortunately, the public’s enthusiasm for herbal products is not always consistent with the scientific evidence supporting their use. In part one of this series, we discussed the studies that have been done on capsaicin, butterbur, green tea, and peppermint. In this installment, we outline the research on 5 additional remedies: turmeric/curcumin, which may be of benefit in ulcerative colitis; chamomile, which appears to offer relief to patients with anxiety; rosemary, which may help treat alopecia; as well as coffee and cocoa, which may have some cardiovascular benefits (TABLE).

Turmeric/curcumin
Overview
Turmeric (Curcuma longa), a relative of ginger, has been used for 4000 years to treat a variety of conditions.2,3 Curcumin is the yellow pigment isolated from the rhizomes of Curcuma longa, commonly known as turmeric.3 Turmeric powder contains 5% curcumin, which is the main biologically active compound. Although it grows in many tropical locations, most turmeric is grown in India, where it is used as a main ingredient in curry. The roots and bulbs of turmeric that are used in medicine are generally boiled and dried, which results in a yellow powder.
Turmeric has been used in both Ayurvedic and Chinese medicine for its anti-inflammatory properties, in the treatment of digestive and liver problems, to fight infections, and to help heal skin diseases and wounds.3-7
Functional GI disorders. A recent review noted that curcumin has been shown in several preclinical studies and uncontrolled clinical trials to have effects on gut inflammation, gut permeability, and the brain-gut axis, especially in functional GI disorders.7 A double-blind, placebo-controlled study from 1989 found that turmeric reduced symptoms of bloating and gas in subjects suffering from undifferentiated dyspepsia.8
Ulcerative colitis (UC). A 2012 Cochrane review noted that curcumin appears to be a safe and effective therapy for maintenance of remission in quiescent UC when given as adjunctive therapy along with mesalamine or sulfasalazine.9 In a 2015 randomized controlled trial (RCT), the addition of curcumin to mesalamine therapy was superior to the combination of placebo and mesalamine in inducing clinical and endoscopic remission in patients with mild-to-moderate active UC, producing no apparent adverse effects.10
Osteoarthritis (OA). Because of turmeric’s ability to reduce inflammation, it may help relieve OA pain.3 Clinical evidence is scant for the anti-arthritic efficacy of turmeric dietary supplements, although animal studies indicate that turmeric prevents inflammation through regulation of NF-kappaB-regulated genes that regulate the immune and inflammatory response.6 Inflammatory cell influx, joint levels of prostaglandin E2, and periarticular osteoclast formation were also inhibited by turmeric extract treatment.6
A 2013 review of turmeric for OA concluded that observational studies and in vitro results are promising for the use of curcumin for OA, but well-designed clinical studies were lacking and are needed to support the efficacy of curcumin in OA patients.11 However, in a 2014 randomized trial of 367 patients, turmeric appeared to be similar in efficacy to ibuprofen for the treatment of pain and disability in adults with knee OA.12 The curcumin (turmeric) group also had fewer adverse effects.12
Cancer. There has been a great deal of research on turmeric’s anti-cancer properties, but clinical evidence is lacking. In vitro evidence, animal studies, and small clinical trials suggest that curcumin may help prevent or treat several types of cancers, but the overall evidence is poor. Nonetheless, curcumin and turmeric have been or are currently being evaluated for the treatment or prevention of prostate, liver, breast, skin, gynecologic, hematologic, pulmonary, thymic, bone, brain, and colon cancer.13-18
Oral submucous fibrosis. A small randomized trial found improvement in oral function with curcumin lozenges, when compared to placebo, indicating that turmeric may hold promise as a treatment of oral submucous fibrosis.19
Uveitis. A small pilot study of 32 patients suggested that oral curcumin may be as effective as corticosteroids for uveitis.20
Heart disease. Curcumin may have a cardiovascular protective role, as it has been shown to reduce atherosclerosis, but a reduction in myocardial infarction or stroke has not been documented.21
Alzheimer’s dementia. Animal studies have shown a reduction in amyloid plaque formation with curcumin.22
Adverse effects (and precautions)
Turmeric in food is considered safe. A variety of animal and human studies have also indicated that curcumin is safe and well tolerated, even at very high doses.13 However, taking large amounts of turmeric for long periods of time could cause stomach upset and gastric ulcers. In addition, patients with gallstones or bile obstruction should use it with caution due to increased bile production.7
Because turmeric may lower blood sugar levels, patients with diabetes should monitor for hypoglycemia when using turmeric in combination with diabetic medications. Similarly, those with bleeding disorders taking blood thinners should use turmeric and curcumin with caution, because it can inhibit platelet aggregation.23
Although it is safe to eat foods with turmeric during pregnancy, pregnant and breastfeeding women should not take turmeric supplements, as the safety of large doses in pregnancy is unknown.
The bottom line
Turmeric/curcumin has anti-inflammatory properties and may be useful as an adjunct for ulcerative colitis and to improve the symptoms of OA. It may also have anti-carcinogenic properties, although definitive data are lacking. Those with a history of gastrointestinal conditions such as gastric ulcer, patients taking blood thinners, and patients with diabetes who are prone to low blood sugar levels should use turmeric/curcumin with caution.
Chamomile
Overview
Chamomile, a member of the Asteraceae/Compositae family, is one of the oldest herbal medicines. It has been used for hay fever, inflammation, muscle spasms, menstrual disorders, insomnia, ulcers, wounds, gastrointestinal disorders, rheumatic pain, and hemorrhoids. Essential oils of chamomile are used extensively in cosmetics and aromatherapy. Many different preparations have been developed, the most popular being herbal tea.24
Individuals with a hypersensitivity to plants of the Asteraceae (Compositae) family such as ragweed (Ambrosia spp.), marigold flower (Calendula officinalis), and chrysanthemum (Chrysanthemum spp.) may show a similar reaction to chamomile.25
Anxiety. A controlled clinical trial of chamomile extract for generalized anxiety disorder (GAD) suggested that it may have modest anxiolytic activity in patients with mild to moderate GAD.26 Another randomized, double-blind, placebo-controlled trial found oral chamomile extract was efficacious and well-tolerated in patients experiencing mild to moderate GAD and may provide an alternative therapeutic anxiolytic for patients with mild GAD.25 In addition to its anxiolytic activity, chamomile may also provide clinically meaningful antidepressant activity.26
Insomnia. Chamomile may have some impact on sleep diary measures (total sleep time, sleep efficiency, sleep latency, wake after sleep onset, sleep quality, and number of awakenings) relative to placebo in adults with chronic primary insomnia, according to a small randomized, double-blind, placebo-controlled pilot trial involving 34 patients.27 However, a systematic review found no statistically significant difference between any herbal medicine (including chamomile) and placebo, for clinical efficacy in patients with insomnia. A similar, or smaller, number of adverse events per person were reported with chamomile compared with placebo, suggesting safe use.28
Infantile colic. A small prospective double-blind study on the use of chamomile-containing tea on infantile colic showed statistically significant symptom improvement in tea-treated infants. The study did note, however, that prolonged ingestion of herbal teas may lead to decreased milk intake.29,30
Adverse effects
As noted earlier, a systematic review found that the number of adverse events per person reported with chamomile was comparable to the number associated with placebo, suggesting that it is safe.28
The bottom line
Chamomile appears to be safe with minimal adverse effects and may be effective for the treatment of anxiety, insomnia, and infantile colic.
Rosemary
Overview
Rosemary, officially known as Rosmarinus officinalis, is a medicinal evergreen plant native to the Mediterranean area that appears to increase microcapillary perfusion.31
Alopecia. A randomized double-blind controlled trial found that essential oils including rosemary oil (as well as thyme, lavender, and cedarwood) massaged into the scalp improved hair growth in almost half of patients with alopecia areata after 7 months.32 Another randomized trial comparing rosemary oil to minoxidil 2% for androgenetic alopecia showed a significant increase in hair count at the 6-month endpoint compared with the baseline, but no significant difference was found between the study groups regarding hair count either at Month 3 or Month 6 (P >.05). 31
Adverse effects
In the randomized trial described above comparing rosemary oil to minoxidil 2%, adverse effects appeared to be rare for topical rosemary oil. Scalp itching was more frequent in the minoxidil group.31
The bottom line
Topical rosemary oil may be useful in the treatment of alopecia with minimal adverse effects.
Coffee/caffeine
Overview
Coffee is one of the most widely used botanicals with approximately 3.5 billion cups of coffee consumed per day worldwide. It is a popular beverage because of its unique aromatic taste and its use as a central nervous system stimulant. The coffee tree (genus coffea) is found throughout Latin America, Africa, and eastern Asia. Two of the most common commercially grown species are Coffea arabica (Arabicas) and Coffea canephora (Robusta). Processing and roasting methods may differ and produce variations in flavor and aroma. The degree of roasting also affects the caffeine content.
Coffee consumption leads to increased alertness and can boost mental performance. Based on the literature and US Food and Drug Administration recommendations, four 8-oz cups of coffee (about 400 mg of caffeine) daily is an acceptable average amount of caffeine. More than 500 mg/d is considered excessive use of coffee.33,34
Overall mortality. A 2008 study showed that regular coffee was not associated with increased or decreased mortality in both men and women.35 However, more recent studies show an inverse relationship between mortality and coffee consumption.
Specifically, a 2014 meta-analysis found an inverse relationship between coffee and mortality.36 A large prospective cohort study from 2015 that included 79,234 women and 76,704 men found that drinking coffee was inversely associated with overall mortality.37 In this cohort study, an inverse association were observed for deaths from heart disease, respiratory disease, diabetes, and self-harm.37 While mechanisms were not analyzed, coffee may reduce mortality risk by affecting inflammation, lung function, insulin sensitivity, and depression.
Cardiovascular disease. Coffee consumption may modestly reduce the risk of stroke, according to a prospective cohort study of 83,076 women from the Nurses’ Health Study who were followed for 24 years.38 Reduced cardiovascular mortality was also found in a large prospective cohort study, as noted in the mortality discussion above.37 A 2014 meta-analysis concluded that coffee consumption is inversely associated with cardiovascular mortality. Drinking 3 or 4 cups a day appears to be the amount that may decrease one’s risk of death when compared to those who do not drink coffee at all.36
Liver disease. Friedrich et al performed a study involving 379 patients with end stage liver disease, and found that coffee consumption delayed the progression of disease in patients with both alcoholic liver disease and primary sclerosing cholangitis.39 Coffee consumption also increased long-term survival after liver transplantation.39 However, the study found that coffee did not have any effect on patients with chronic viral hepatitis.
In a 2016 meta-analysis, caffeinated coffee consumption reduced hepatic fibrosis of nonalcoholic fatty liver disease, although caffeine consumption did not reduce the prevalence of nonalcoholic fatty liver disease.40 Another meta-analysis, including 16 studies, also found caffeine reduced the risk for hepatic fibrosis and cirrhosis.41
Depression. Based on 2 different systematic reviews and meta-analyses from 2016, coffee consumption appears to have a significant protective effect, decreasing the risk of developing depression.40,42
Alzheimer’s disease/dementia. Coffee, tea, and caffeine consumption show promise in reducing the risk of cognitive decline and dementia. Individuals who consume one to 2 cups of coffee per day had a decreased incidence of mild cognitive impairment compared to non-drinkers.43 A 2015 Japanese study also found an inverse association between coffee consumption and dementia among women, nonsmokers, and those who do not drink alcohol.44 Most recently, a 2016 study, the Women’s Health Initiative Memory Study, looked at incident dementia rates in women >65 years of age with high vs low caffeine intake. Women with higher caffeine intake were less likely to develop dementia or any cognitive impairment compared with those consuming <64 mg/day.45
Type 2 diabetes. A 2009 prospective cohort study, which included 40,011 participants followed for more than 10 years, found that drinking at least 3 cups of coffee or tea was associated with a lowered risk of type 2 diabetes.46 A 2009 systematic review of 20 cohort studies showed that high intakes of coffee, decaffeinated coffee, and tea are associated with a reduced risk of diabetes.47
Melanoma.
Adverse effects
Despite the many potential benefits of coffee, caffeine is a potent drug that should be used with caution.49 People with underlying heart problems should avoid caffeine due to concern that it may cause palpitations from tachycardia. It may worsen anxiety problems or depression. Coffee may increase the production of stomach acids, which can worsen acid reflux or stomach ulcers.
Caffeine is a potent diuretic and may decrease absorption of calcium and cause OA. Caffeine may cause dependence and withdrawal symptoms. Some of the symptoms of withdrawal include drowsiness, headaches, irritability, nausea, and vomiting. It may disrupt sleeping patterns by causing jitters and sleeplessness.49 Additionally, large amounts of caffeine may cause overdose and death.
The bottom line
Regular coffee intake is associated with a lower risk of mortality, reduced cardiovascular events, and a reduction in liver disease progression. Coffee may also have some utility for improving cognitive function and reducing the risk of type 2 diabetes. Caffeinated coffee should be limited to no more than 32 oz per day, due to the risk of insomnia, palpitations, anxiety, and gastritis.
Chocolate/cocoa
Overview
Few natural products have been claimed to successfully treat as many disorders as chocolate. The modern concept of chocolate as food has overshadowed its traditional medicinal use, although recent trials have looked at evidence for some of its traditional uses. Chocolate is processed from the pod of the cacao plant. The earliest evidence for its medical use is in Mayan civilizations, and for most of its approximately 4000-year history, chocolate was consumed as a bitter drink referred to as the “drink of the Gods.” The traditional drink was mixed with water, vanilla, honey, chili peppers, and other spices. Important components in chocolate include flavonoids (antioxidants), cocoa butter, caffeine, theobromine, and phenylethylamine.
Chocolate has stimulating, anti-inflammatory, neuroprotective, and cardioprotective effects, and improves the bioavailability of nitric oxide, which can improve blood pressure and platelet function.50 Epicatechin (an antioxidant) in cocoa is primarily responsible for its favorable impact on vascular endothelium via its effect on both acute and chronic upregulation of nitric oxide production. Other cardiovascular effects are mediated by the anti-inflammatory effects of cocoa polyphenols, and modulated through the activity of NF-kappaB.51
Dark chocolate appears to have the greatest benefit, as milk binds to antioxidants in chocolate, making them unavailable. Therefore, milk chocolate is not a good antioxidant source. There is no specific amount of chocolate that is known to be ideal, but an average of one to 2 ounces per day is often used in studies.
Cardiovascular effects. Chocolate does contain saturated fat, but a comparative, double-blind study found that short-term use of cocoa powder lowered plasma low-density lipoprotein (LDL) cholesterol, oxidized LDL, and apo B concentrations, and the plasma high-density lipoprotein (HDL) cholesterol concentration increased, relative to baseline in the low-, middle-, and high-cocoa groups.52 A small randomized crossover trial without clinical outcomes indicated that chocolate may increase HDL cholesterol without increasing weight.53
A meta-analysis of short-term (2-12 weeks) treatment with dark chocolate/cocoa products showed reductions in LDL and total cholesterol, but no changes in HDL or triglycerides.54 Another meta-analysis of RCTs, however, showed no short-term effect of cocoa/chocolate on lipid concentrations.55 A randomized, placebo-controlled double-blind study of 62 patients with diabetes and hypertension showed that high polyphenol chocolate improved triglyceride levels.56
Multiple studies have shown that chocolate is associated with a reduction in cardiovascular risk.57-59 A best case scenario analysis using a Markov model to predict the long-term effectiveness and cost effectiveness of daily dark chocolate consumption in a population with metabolic syndrome at high risk of cardiovascular disease concluded that daily consumption of dark chocolate can reduce cardiovascular events by 85 per 10,000 population treated over 10 years. The study concluded that $42 could be cost effectively spent per person per year on prevention strategies using dark chocolate.59
In addition, a meta-analysis of 7 observational studies showed that high levels of chocolate consumption (any type) were associated with a 29% reduction in stroke compared with the lowest levels of chocolate intake.57 Results of a similar meta-analysis from Neurology in 2012 also suggested that moderate chocolate consumption (any type) may lower the risk of stroke.60
That said, 2 systematic reviews specifically relating to the risk of coronary heart disease and chocolate intake were inconclusive.61-62
Blood pressure (BP). An RCT published in JAMA indicates that inclusion of small amounts of polyphenol-rich dark chocolate as part of a usual diet efficiently reduced BP and improved the formation of vasodilative nitric oxide.63 A meta-analysis of 10 RCTs also showed mean BP change in the active cocoa treatment arms across all trials was -4.5 mm Hg (95% confidence interval (CI), -5.9 to -3.2; P<.001) for systolic BP and -2.5 mm Hg (95% CI, -3.9 to -1.2; P<.001) for diastolic BP.64
A Cochrane Review meta-analysis of 20 studies revealed a statistically significant BP-reducing effect of flavanol-rich cocoa products compared with control in short-term trials of 2 to 18 weeks' duration.65 Because studies have shown improvement in BP with chocolate intake, investigations into a role of chocolate in the prevention of preeclampsia have been undertaken. In some studies, chocolate intake was associated with reduced odds of preeclampsia and gestational hypertension.66,67
Diabetes. Chocolate may exert significant vascular protection because of its antioxidant properties and possible increase of nitric oxide bioavailability, which can influence glucose uptake. A small trial comparing the effects of either dark or white chocolate bars (which do not contain the polyphenols) showed improved BP and glucose and insulin responses to an oral glucose tolerance test in healthy subjects on dark chocolate, but not white chocolate.68 A comparison of chocolate consumption and risk of diabetes in the Physicians’ Health Study showed an inverse relationship between chocolate intake with incident disease, but this association appeared only to apply in younger and normal-body weight men after controlling for comprehensive lifestyles, including total energy consumption.69
Fatigue. The effect of chocolate on a person’s energy level has been noted for centuries.70 A small randomized trial showed improved energy levels in those treated with higher chocolate intakes. In a double-blind, randomized, clinical pilot crossover study, high cocoa liquor/polyphenol rich chocolate, reduced fatigue in subjects with chronic fatigue syndrome.71
Anxiety. A small randomized trial showed chocolate decreased anxiety in high-anxiety trait subjects and improved the anxiety level and the energy levels of low-anxiety trait participants.72
Eye effects. The literature presents conflicting evidence regarding the effect of flavonoids on patients with glaucoma and ocular hypertension. However, a meta-analysis showed that flavonoids have a promising role in improving visual function in patients with glaucoma and ocular hypertension, and appear to play a part in both improving and slowing the progression of visual field loss.73
Cognitive decline. Chocolate intake (any type) was associated with a lower risk of cognitive decline (RR = 0.59; 95% CI, 0.38-0.92) with the greatest benefit noted in those who averaged more than one chocolate bar or one tablespoon of cocoa powder per week. This protective effect was observed only among subjects with an average daily consumption of caffeine <75 mg (69% of the participants; RR = 0.50; 95% CI, 0.31-0.82).74
The bottom line
Chocolate with high cocoa content (dark chocolate) appears to be safe and beneficial as part of a healthy diet and lifestyle that includes exercise and stress reduction to decrease cardiovascular risk and may improve energy levels.
CORRESPONDENCE
Michael Malone, MD, Family and Community Medicine, Penn State College of Medicine, 500 University Drive, Hershey, PA 17033; [email protected].
More than a third of American adults use complementary and alternative medicine.1 Unfortunately, the public’s enthusiasm for herbal products is not always consistent with the scientific evidence supporting their use. In part one of this series, we discussed the studies that have been done on capsaicin, butterbur, green tea, and peppermint. In this installment, we outline the research on 5 additional remedies: turmeric/curcumin, which may be of benefit in ulcerative colitis; chamomile, which appears to offer relief to patients with anxiety; rosemary, which may help treat alopecia; as well as coffee and cocoa, which may have some cardiovascular benefits (TABLE).

Turmeric/curcumin
Overview
Turmeric (Curcuma longa), a relative of ginger, has been used for 4000 years to treat a variety of conditions.2,3 Curcumin is the yellow pigment isolated from the rhizomes of Curcuma longa, commonly known as turmeric.3 Turmeric powder contains 5% curcumin, which is the main biologically active compound. Although it grows in many tropical locations, most turmeric is grown in India, where it is used as a main ingredient in curry. The roots and bulbs of turmeric that are used in medicine are generally boiled and dried, which results in a yellow powder.
Turmeric has been used in both Ayurvedic and Chinese medicine for its anti-inflammatory properties, in the treatment of digestive and liver problems, to fight infections, and to help heal skin diseases and wounds.3-7
Functional GI disorders. A recent review noted that curcumin has been shown in several preclinical studies and uncontrolled clinical trials to have effects on gut inflammation, gut permeability, and the brain-gut axis, especially in functional GI disorders.7 A double-blind, placebo-controlled study from 1989 found that turmeric reduced symptoms of bloating and gas in subjects suffering from undifferentiated dyspepsia.8
Ulcerative colitis (UC). A 2012 Cochrane review noted that curcumin appears to be a safe and effective therapy for maintenance of remission in quiescent UC when given as adjunctive therapy along with mesalamine or sulfasalazine.9 In a 2015 randomized controlled trial (RCT), the addition of curcumin to mesalamine therapy was superior to the combination of placebo and mesalamine in inducing clinical and endoscopic remission in patients with mild-to-moderate active UC, producing no apparent adverse effects.10
Osteoarthritis (OA). Because of turmeric’s ability to reduce inflammation, it may help relieve OA pain.3 Clinical evidence is scant for the anti-arthritic efficacy of turmeric dietary supplements, although animal studies indicate that turmeric prevents inflammation through regulation of NF-kappaB-regulated genes that regulate the immune and inflammatory response.6 Inflammatory cell influx, joint levels of prostaglandin E2, and periarticular osteoclast formation were also inhibited by turmeric extract treatment.6
A 2013 review of turmeric for OA concluded that observational studies and in vitro results are promising for the use of curcumin for OA, but well-designed clinical studies were lacking and are needed to support the efficacy of curcumin in OA patients.11 However, in a 2014 randomized trial of 367 patients, turmeric appeared to be similar in efficacy to ibuprofen for the treatment of pain and disability in adults with knee OA.12 The curcumin (turmeric) group also had fewer adverse effects.12
Cancer. There has been a great deal of research on turmeric’s anti-cancer properties, but clinical evidence is lacking. In vitro evidence, animal studies, and small clinical trials suggest that curcumin may help prevent or treat several types of cancers, but the overall evidence is poor. Nonetheless, curcumin and turmeric have been or are currently being evaluated for the treatment or prevention of prostate, liver, breast, skin, gynecologic, hematologic, pulmonary, thymic, bone, brain, and colon cancer.13-18
Oral submucous fibrosis. A small randomized trial found improvement in oral function with curcumin lozenges, when compared to placebo, indicating that turmeric may hold promise as a treatment of oral submucous fibrosis.19
Uveitis. A small pilot study of 32 patients suggested that oral curcumin may be as effective as corticosteroids for uveitis.20
Heart disease. Curcumin may have a cardiovascular protective role, as it has been shown to reduce atherosclerosis, but a reduction in myocardial infarction or stroke has not been documented.21
Alzheimer’s dementia. Animal studies have shown a reduction in amyloid plaque formation with curcumin.22
Adverse effects (and precautions)
Turmeric in food is considered safe. A variety of animal and human studies have also indicated that curcumin is safe and well tolerated, even at very high doses.13 However, taking large amounts of turmeric for long periods of time could cause stomach upset and gastric ulcers. In addition, patients with gallstones or bile obstruction should use it with caution due to increased bile production.7
Because turmeric may lower blood sugar levels, patients with diabetes should monitor for hypoglycemia when using turmeric in combination with diabetic medications. Similarly, those with bleeding disorders taking blood thinners should use turmeric and curcumin with caution, because it can inhibit platelet aggregation.23
Although it is safe to eat foods with turmeric during pregnancy, pregnant and breastfeeding women should not take turmeric supplements, as the safety of large doses in pregnancy is unknown.
The bottom line
Turmeric/curcumin has anti-inflammatory properties and may be useful as an adjunct for ulcerative colitis and to improve the symptoms of OA. It may also have anti-carcinogenic properties, although definitive data are lacking. Those with a history of gastrointestinal conditions such as gastric ulcer, patients taking blood thinners, and patients with diabetes who are prone to low blood sugar levels should use turmeric/curcumin with caution.
Chamomile
Overview
Chamomile, a member of the Asteraceae/Compositae family, is one of the oldest herbal medicines. It has been used for hay fever, inflammation, muscle spasms, menstrual disorders, insomnia, ulcers, wounds, gastrointestinal disorders, rheumatic pain, and hemorrhoids. Essential oils of chamomile are used extensively in cosmetics and aromatherapy. Many different preparations have been developed, the most popular being herbal tea.24
Individuals with a hypersensitivity to plants of the Asteraceae (Compositae) family such as ragweed (Ambrosia spp.), marigold flower (Calendula officinalis), and chrysanthemum (Chrysanthemum spp.) may show a similar reaction to chamomile.25
Anxiety. A controlled clinical trial of chamomile extract for generalized anxiety disorder (GAD) suggested that it may have modest anxiolytic activity in patients with mild to moderate GAD.26 Another randomized, double-blind, placebo-controlled trial found oral chamomile extract was efficacious and well-tolerated in patients experiencing mild to moderate GAD and may provide an alternative therapeutic anxiolytic for patients with mild GAD.25 In addition to its anxiolytic activity, chamomile may also provide clinically meaningful antidepressant activity.26
Insomnia. Chamomile may have some impact on sleep diary measures (total sleep time, sleep efficiency, sleep latency, wake after sleep onset, sleep quality, and number of awakenings) relative to placebo in adults with chronic primary insomnia, according to a small randomized, double-blind, placebo-controlled pilot trial involving 34 patients.27 However, a systematic review found no statistically significant difference between any herbal medicine (including chamomile) and placebo, for clinical efficacy in patients with insomnia. A similar, or smaller, number of adverse events per person were reported with chamomile compared with placebo, suggesting safe use.28
Infantile colic. A small prospective double-blind study on the use of chamomile-containing tea on infantile colic showed statistically significant symptom improvement in tea-treated infants. The study did note, however, that prolonged ingestion of herbal teas may lead to decreased milk intake.29,30
Adverse effects
As noted earlier, a systematic review found that the number of adverse events per person reported with chamomile was comparable to the number associated with placebo, suggesting that it is safe.28
The bottom line
Chamomile appears to be safe with minimal adverse effects and may be effective for the treatment of anxiety, insomnia, and infantile colic.
Rosemary
Overview
Rosemary, officially known as Rosmarinus officinalis, is a medicinal evergreen plant native to the Mediterranean area that appears to increase microcapillary perfusion.31
Alopecia. A randomized double-blind controlled trial found that essential oils including rosemary oil (as well as thyme, lavender, and cedarwood) massaged into the scalp improved hair growth in almost half of patients with alopecia areata after 7 months.32 Another randomized trial comparing rosemary oil to minoxidil 2% for androgenetic alopecia showed a significant increase in hair count at the 6-month endpoint compared with the baseline, but no significant difference was found between the study groups regarding hair count either at Month 3 or Month 6 (P >.05). 31
Adverse effects
In the randomized trial described above comparing rosemary oil to minoxidil 2%, adverse effects appeared to be rare for topical rosemary oil. Scalp itching was more frequent in the minoxidil group.31
The bottom line
Topical rosemary oil may be useful in the treatment of alopecia with minimal adverse effects.
Coffee/caffeine
Overview
Coffee is one of the most widely used botanicals with approximately 3.5 billion cups of coffee consumed per day worldwide. It is a popular beverage because of its unique aromatic taste and its use as a central nervous system stimulant. The coffee tree (genus coffea) is found throughout Latin America, Africa, and eastern Asia. Two of the most common commercially grown species are Coffea arabica (Arabicas) and Coffea canephora (Robusta). Processing and roasting methods may differ and produce variations in flavor and aroma. The degree of roasting also affects the caffeine content.
Coffee consumption leads to increased alertness and can boost mental performance. Based on the literature and US Food and Drug Administration recommendations, four 8-oz cups of coffee (about 400 mg of caffeine) daily is an acceptable average amount of caffeine. More than 500 mg/d is considered excessive use of coffee.33,34
Overall mortality. A 2008 study showed that regular coffee was not associated with increased or decreased mortality in both men and women.35 However, more recent studies show an inverse relationship between mortality and coffee consumption.
Specifically, a 2014 meta-analysis found an inverse relationship between coffee and mortality.36 A large prospective cohort study from 2015 that included 79,234 women and 76,704 men found that drinking coffee was inversely associated with overall mortality.37 In this cohort study, an inverse association were observed for deaths from heart disease, respiratory disease, diabetes, and self-harm.37 While mechanisms were not analyzed, coffee may reduce mortality risk by affecting inflammation, lung function, insulin sensitivity, and depression.
Cardiovascular disease. Coffee consumption may modestly reduce the risk of stroke, according to a prospective cohort study of 83,076 women from the Nurses’ Health Study who were followed for 24 years.38 Reduced cardiovascular mortality was also found in a large prospective cohort study, as noted in the mortality discussion above.37 A 2014 meta-analysis concluded that coffee consumption is inversely associated with cardiovascular mortality. Drinking 3 or 4 cups a day appears to be the amount that may decrease one’s risk of death when compared to those who do not drink coffee at all.36
Liver disease. Friedrich et al performed a study involving 379 patients with end stage liver disease, and found that coffee consumption delayed the progression of disease in patients with both alcoholic liver disease and primary sclerosing cholangitis.39 Coffee consumption also increased long-term survival after liver transplantation.39 However, the study found that coffee did not have any effect on patients with chronic viral hepatitis.
In a 2016 meta-analysis, caffeinated coffee consumption reduced hepatic fibrosis of nonalcoholic fatty liver disease, although caffeine consumption did not reduce the prevalence of nonalcoholic fatty liver disease.40 Another meta-analysis, including 16 studies, also found caffeine reduced the risk for hepatic fibrosis and cirrhosis.41
Depression. Based on 2 different systematic reviews and meta-analyses from 2016, coffee consumption appears to have a significant protective effect, decreasing the risk of developing depression.40,42
Alzheimer’s disease/dementia. Coffee, tea, and caffeine consumption show promise in reducing the risk of cognitive decline and dementia. Individuals who consume one to 2 cups of coffee per day had a decreased incidence of mild cognitive impairment compared to non-drinkers.43 A 2015 Japanese study also found an inverse association between coffee consumption and dementia among women, nonsmokers, and those who do not drink alcohol.44 Most recently, a 2016 study, the Women’s Health Initiative Memory Study, looked at incident dementia rates in women >65 years of age with high vs low caffeine intake. Women with higher caffeine intake were less likely to develop dementia or any cognitive impairment compared with those consuming <64 mg/day.45
Type 2 diabetes. A 2009 prospective cohort study, which included 40,011 participants followed for more than 10 years, found that drinking at least 3 cups of coffee or tea was associated with a lowered risk of type 2 diabetes.46 A 2009 systematic review of 20 cohort studies showed that high intakes of coffee, decaffeinated coffee, and tea are associated with a reduced risk of diabetes.47
Melanoma.
Adverse effects
Despite the many potential benefits of coffee, caffeine is a potent drug that should be used with caution.49 People with underlying heart problems should avoid caffeine due to concern that it may cause palpitations from tachycardia. It may worsen anxiety problems or depression. Coffee may increase the production of stomach acids, which can worsen acid reflux or stomach ulcers.
Caffeine is a potent diuretic and may decrease absorption of calcium and cause OA. Caffeine may cause dependence and withdrawal symptoms. Some of the symptoms of withdrawal include drowsiness, headaches, irritability, nausea, and vomiting. It may disrupt sleeping patterns by causing jitters and sleeplessness.49 Additionally, large amounts of caffeine may cause overdose and death.
The bottom line
Regular coffee intake is associated with a lower risk of mortality, reduced cardiovascular events, and a reduction in liver disease progression. Coffee may also have some utility for improving cognitive function and reducing the risk of type 2 diabetes. Caffeinated coffee should be limited to no more than 32 oz per day, due to the risk of insomnia, palpitations, anxiety, and gastritis.
Chocolate/cocoa
Overview
Few natural products have been claimed to successfully treat as many disorders as chocolate. The modern concept of chocolate as food has overshadowed its traditional medicinal use, although recent trials have looked at evidence for some of its traditional uses. Chocolate is processed from the pod of the cacao plant. The earliest evidence for its medical use is in Mayan civilizations, and for most of its approximately 4000-year history, chocolate was consumed as a bitter drink referred to as the “drink of the Gods.” The traditional drink was mixed with water, vanilla, honey, chili peppers, and other spices. Important components in chocolate include flavonoids (antioxidants), cocoa butter, caffeine, theobromine, and phenylethylamine.
Chocolate has stimulating, anti-inflammatory, neuroprotective, and cardioprotective effects, and improves the bioavailability of nitric oxide, which can improve blood pressure and platelet function.50 Epicatechin (an antioxidant) in cocoa is primarily responsible for its favorable impact on vascular endothelium via its effect on both acute and chronic upregulation of nitric oxide production. Other cardiovascular effects are mediated by the anti-inflammatory effects of cocoa polyphenols, and modulated through the activity of NF-kappaB.51
Dark chocolate appears to have the greatest benefit, as milk binds to antioxidants in chocolate, making them unavailable. Therefore, milk chocolate is not a good antioxidant source. There is no specific amount of chocolate that is known to be ideal, but an average of one to 2 ounces per day is often used in studies.
Cardiovascular effects. Chocolate does contain saturated fat, but a comparative, double-blind study found that short-term use of cocoa powder lowered plasma low-density lipoprotein (LDL) cholesterol, oxidized LDL, and apo B concentrations, and the plasma high-density lipoprotein (HDL) cholesterol concentration increased, relative to baseline in the low-, middle-, and high-cocoa groups.52 A small randomized crossover trial without clinical outcomes indicated that chocolate may increase HDL cholesterol without increasing weight.53
A meta-analysis of short-term (2-12 weeks) treatment with dark chocolate/cocoa products showed reductions in LDL and total cholesterol, but no changes in HDL or triglycerides.54 Another meta-analysis of RCTs, however, showed no short-term effect of cocoa/chocolate on lipid concentrations.55 A randomized, placebo-controlled double-blind study of 62 patients with diabetes and hypertension showed that high polyphenol chocolate improved triglyceride levels.56
Multiple studies have shown that chocolate is associated with a reduction in cardiovascular risk.57-59 A best case scenario analysis using a Markov model to predict the long-term effectiveness and cost effectiveness of daily dark chocolate consumption in a population with metabolic syndrome at high risk of cardiovascular disease concluded that daily consumption of dark chocolate can reduce cardiovascular events by 85 per 10,000 population treated over 10 years. The study concluded that $42 could be cost effectively spent per person per year on prevention strategies using dark chocolate.59
In addition, a meta-analysis of 7 observational studies showed that high levels of chocolate consumption (any type) were associated with a 29% reduction in stroke compared with the lowest levels of chocolate intake.57 Results of a similar meta-analysis from Neurology in 2012 also suggested that moderate chocolate consumption (any type) may lower the risk of stroke.60
That said, 2 systematic reviews specifically relating to the risk of coronary heart disease and chocolate intake were inconclusive.61-62
Blood pressure (BP). An RCT published in JAMA indicates that inclusion of small amounts of polyphenol-rich dark chocolate as part of a usual diet efficiently reduced BP and improved the formation of vasodilative nitric oxide.63 A meta-analysis of 10 RCTs also showed mean BP change in the active cocoa treatment arms across all trials was -4.5 mm Hg (95% confidence interval (CI), -5.9 to -3.2; P<.001) for systolic BP and -2.5 mm Hg (95% CI, -3.9 to -1.2; P<.001) for diastolic BP.64
A Cochrane Review meta-analysis of 20 studies revealed a statistically significant BP-reducing effect of flavanol-rich cocoa products compared with control in short-term trials of 2 to 18 weeks' duration.65 Because studies have shown improvement in BP with chocolate intake, investigations into a role of chocolate in the prevention of preeclampsia have been undertaken. In some studies, chocolate intake was associated with reduced odds of preeclampsia and gestational hypertension.66,67
Diabetes. Chocolate may exert significant vascular protection because of its antioxidant properties and possible increase of nitric oxide bioavailability, which can influence glucose uptake. A small trial comparing the effects of either dark or white chocolate bars (which do not contain the polyphenols) showed improved BP and glucose and insulin responses to an oral glucose tolerance test in healthy subjects on dark chocolate, but not white chocolate.68 A comparison of chocolate consumption and risk of diabetes in the Physicians’ Health Study showed an inverse relationship between chocolate intake with incident disease, but this association appeared only to apply in younger and normal-body weight men after controlling for comprehensive lifestyles, including total energy consumption.69
Fatigue. The effect of chocolate on a person’s energy level has been noted for centuries.70 A small randomized trial showed improved energy levels in those treated with higher chocolate intakes. In a double-blind, randomized, clinical pilot crossover study, high cocoa liquor/polyphenol rich chocolate, reduced fatigue in subjects with chronic fatigue syndrome.71
Anxiety. A small randomized trial showed chocolate decreased anxiety in high-anxiety trait subjects and improved the anxiety level and the energy levels of low-anxiety trait participants.72
Eye effects. The literature presents conflicting evidence regarding the effect of flavonoids on patients with glaucoma and ocular hypertension. However, a meta-analysis showed that flavonoids have a promising role in improving visual function in patients with glaucoma and ocular hypertension, and appear to play a part in both improving and slowing the progression of visual field loss.73
Cognitive decline. Chocolate intake (any type) was associated with a lower risk of cognitive decline (RR = 0.59; 95% CI, 0.38-0.92) with the greatest benefit noted in those who averaged more than one chocolate bar or one tablespoon of cocoa powder per week. This protective effect was observed only among subjects with an average daily consumption of caffeine <75 mg (69% of the participants; RR = 0.50; 95% CI, 0.31-0.82).74
The bottom line
Chocolate with high cocoa content (dark chocolate) appears to be safe and beneficial as part of a healthy diet and lifestyle that includes exercise and stress reduction to decrease cardiovascular risk and may improve energy levels.
CORRESPONDENCE
Michael Malone, MD, Family and Community Medicine, Penn State College of Medicine, 500 University Drive, Hershey, PA 17033; [email protected].
1. National Center for Complementary and Integrative Health. The use of complementary and alternative medicine in the United States. Available at: https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed Nov 28, 2017.
2. Aggarwal BB. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
3. Henrotin Y, Clutterbuck AL, Allaway D, et al. Biological actions of curcumin on articular chondrocytes. Osteoarthritis Cartilage. 2010;18:141-149.
4. Asher GN, Spelman K. Clinical utility of curcumin extract. Altern Ther Health Med. 2013;19:20-22.
5. Phan TT, See P, Lee ST, et al. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J Trauma. 2001;51:927-931.
6. Funk JL, Frye JB, Oyarzo JN, et al. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis Rheum. 2006;54:3452-3464.
7. Patcharatrakul T, Gonlachanvit S. Chili peppers, curcumins, and prebiotics in gastrointestinal health and disease. Curr Gastroenterol Rep. 2016;18:19.
8. Thamlikitkul V, Bunyapraphatsara N, Dechatiwongse T, et al. Randomized double blind study of Curcuma domestica Val. for dyspepsia. J Med Assoc Thai. 1989;72:613-620.
9. Kumar S, Ahuja V, Sankar MJ, et al. Curcumin for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;10:CD008424.
10. Lang A, Salomon N, Wu JC, et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2015;13:1444-1449.e1.
11. Henrotin Y, Priem F, Mobasheri A. Curcumin: a new paradigm and therapeutic opportunity for the treatment of osteoarthritis: curcumin for osteoarthritis management. Springerplus. 2013;2:56.
12. Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, et al. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Interv Aging. 2014;9:451-458.
13. Shehzad A, Lee J, Lee YS. Curcumin in various cancers. Biofactors. 2013;39:56-68.
14. Sordillo LA, Sordillo PP, Helson L. Curcumin for the treatment of glioblastoma. Anticancer Res. 2015;35:6373-6378.
15. Darvesh AS, Aggarwal BB, Bishayee A. Curcumin and liver cancer: a review. Curr Pharm Biotechnol. 2012;13:218-228.
16. Nagaraju GP, Aliya S, Zafar SF, et al. The impact of curcumin on breast cancer. Integr Biol (Camb). 2012;4:996-1007.
17. Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255:170-181.
18. Dorai T, Cao YC, Dorai B, et al. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate. 2001;47:293-303.
19. Hazarey VK, Sakrikar AR, Ganvir SM. Efficacy of curcumin in the treatment for oral submucous fibrosis - a randomized clinical trial. J Oral Maxillofac Pathol. 2015;19:145-152.
20. Lal B, Kapoor AK, Asthana OP, et al. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res. 1999;13:318-322.
21. Kapakos G, Youreva V, Srivastava AK. Cardiovascular protection by curcumin: molecular aspects. Indian J Biochem Biophys. 2012;49:306-315.
22. Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892-5901.
23. Heck AM, DeWitt BA, Lukes AL. Potential interactions between alternative therapies and warfarin. Am J Health Syst Pharm. 2000;57:1221-1227.
24. Srivastava JK, Shankar E, Gupta S. Chamomile: a herbal medicine of the past with bright future. Mol Med Rep. 2010;3:895-901.
25. Ross SM. Generalized anxiety disorder (GAD): efficacy of standardized matricaria recutita (german chamomile) extract in the treatment of generalized anxiety disorder. Holistic Nursing Practice. 2013;27:366- 368.
26. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29:378-382.
27. Zick SM, Wright BD, Sen A, et al. Preliminary examination of the efficacy and safety of a standardized chamomile extract for chronic primary insomnia: a randomized placebo-controlled pilot study. BMC Complement Altern Med. 2011;11:78.
28. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12.
29. Weizman Z, Alkrinawi S, Goldfarb D, et al. Efficacy of herbal tea preparation in infantile colic. J Pediatr. 1993;122:650.
30. Crotteau CA, Wright ST, Eglash A. Clinical inquiries. What is the best treatment for infants with colic? J Fam Pract. 2006;55:634-636.
31. Panahi Y, Taghizadeh M, Marzony ET, et al. Rosemary oil vs minoxidil 2% for the treatment of androgenetic alopecia: a randomized comparative trial. Skinmed. 2015;13:15-21.
32. Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. Successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
33. Caffeine and kids: FDA takes a closer look. Available at: https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm350570.htm. Accessed: November 1, 2017.
34. Torpy JM, Livingston EH. Energy Drinks. JAMA. 2013;309:297.
35. Lopez-Garcia E, van Dam RM, Li TY, et al. The relationship of coffee consumption with mortality. Ann Intern Med. 2008;148:904-914.
36. Crippa A, Discacciati A, Larsson SC, et al. Coffee consumption and mortality from all causes, cardiovascular disease, and cancer: a dose-response meta-analysis. Am J Epidemiol. 2014;180:763-775.
37. Loftfield E, Freedman ND, Graubard BI, et al. Association of coffee consumption with overall and cause-specific mortality in a large US prospective cohort study. Am J Epidemiol. 2015;182:1010-1022.
38. Lopez-Garcia E, Rodriguez-Artalejo F, Rexrode KM, et al. Coffee consumption and risk of stroke in women. Circulation. 2009;119:1116-1123.
39. Friedrich K, Smit M, Wannhoff A, et al. Coffee consumption protects against progression in liver cirrhosis and increases long-term survival after liver transplantation. J Gastroenterol Hepatol. 2016;31:1470-1475.
40. Wang L, Shen X, Wu Y, et al. Coffee and caffeine consumption and depression: a meta-analysis of observational studies. Aust N Z J Psychiatry. 2016;50:228-242.
41. Liu F, Wang X, Wu G, et al. Coffee consumption decreases risks for hepatic fibrosis and cirrhosis: a meta-analysis. PLoS One. 2015;10:e0142457.
42. Grosso G, Micek A, Castellano S, et al. Coffee, tea, caffeine and risk of depression: a systematic review and dose-response meta-analysis of observational studies. Mol Nutr Food Res. 2016;60:223-234.
43. Solfrizzi V, Panza F, Imbimbo BP, et al. Italian longitudinal study on aging working group. Coffee consumption habits and the risk of mild cognitive impairment: The Italian Longitudinal Study on Aging. J Alzheimers Dis. 2015;47:889-899.
44. Sugiyama K, Tomata Y, Kaiho Y, et al. Association between coffee consumption and incident risk of disabling dementia in elderly japanese: The Ohsaki Cohort 2006 Study. J Alzheimers Dis. 2015;50:491-500.
45. Driscoll I, Shumaker SA, Snively BM, et al. Relationships between caffeine intake and risk for probable dementia or global cognitive impairment: The Women’s Health Initiative Memory Study. J Gerontol A Biol Sci Med Sci. 2016;71:1596-1602.
46. van Dieren S, Uiterwaal CS, van der Schouw YT, et al. Coffee and tea consumption and risk of type 2 diabetes. Diabetologia. 2009;52:2561-2569.
47. Wang J, Li X, Zhang D. Coffee consumption and the risk of cutaneous melanoma: a meta-analysis. Eur J Nutr. 2016;55:1317-1329.
48. Liu J, Shen B, Shi M, et al. Higher caffeinated coffee intake is associated with reduced malignant melanoma risk: a meta-analysis study. PLoS One. 2016;11:e0147056.
49. Wikoff D, Welsh BT, Henderson R, et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem Toxical. 2017;109(Pt 1):585-648.
50. Verna R. The history and science of chocolate. Malays J Pathol. 2013;35:111-121.
51. Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal. 2011;15:2779-2811.
52. Baba S, Natsume M, Yasuda A, et al. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. J Nutr. 2007;137:1436-1441.
53. Mellor DD, Sathyapalan T, Kilpatrick ES, et al. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in type 2 diabetes patients. Diabet Med. 2010;27:1318-1321.
54. Tokede OA, Gaziano JM, Djoussé L. Effects of cocoa products/dark chocolate on serum lipids: a meta-analysis. Eur J Clin Nutr. 2011;65:879-886.
55. Jia L, Liu X, Bai YY, et al. Short-term effect of cocoa product consumption on lipid profile: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2010;92:218-225.
56. Rostami A, Khalili M, Haghighat N, et al. High-cocoa polyphenol-rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atheroscler. 2015;11:21-29.
57. Buitrago-Lopez A, Sanderson J, Johnson L, et al. Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis. BMJ. 2011;26;343:d4488.
58. Wang X, Ouyang YY, Liu J, et al. Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr. 2014;111:1-11.
59. Zomer E, Owen A, Magliano DJ, et al. The effectiveness and cost effectiveness of dark chocolate consumption as prevention therapy in people at high risk of cardiovascular disease: best case scenario analysis using a Markov model. BMJ. 2012;344:e3657.
60. Larsson SC, Virtamo J, Wolk A. Chocolate consumption and risk of stroke: a prospective cohort of men and meta-analysis. Neurology. 2012;79:1223-1229.
61. Khawaja O, Gaziano JM, Djoussé L. Chocolate and coronary heart disease: a systematic review. Curr Atheroscler Rep. 2011;13:447-452.
62. Jacques PF, Cassidy A, Rogers G, et al. Dietary flavonoid intakes and CVD incidence in the Framingham Offspring Cohort. Br J Nutr. 2015;114:1496-1503.
63. Taubert D, Roesen R, Lehmann C, et al. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA. 2007;298:49-60.
64. Desch S, Schmidt J, Kobler D, et al. Effect of cocoa products on blood pressure: systematic review and meta-analysis. Am J Hypertens. 2010;23:97-103.
65. Ried K, Sullivan TR, Fakler P, et al. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2012;8:CD008893.
66. Saftlas AF, Triche EW, Beydoun H, et al. Does chocolate intake during pregnancy reduce the risks of preeclampsia and gestational hypertension? Ann Epidemiol. 2010;20:584-591.
67. Triche EW, Grosso LM, Belanger K, et al. Chocolate consumption in pregnancy and reduced likelihood of preeclampsia. Epidemiology. 2008;19:459-464.
68. Grassi D, Lippi C, Necozione S, et al. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. 2005;81:611-614.
69. Matsumoto C, Petrone AB, Sesso HD, et al. Chocolate consumption and risk of diabetes mellitus in the Physicians’ Health Study. Am J Clin Nutr. 2015;101:362-367.
70. Lippi D. Chocolate in history: food, medicine, medi-food. Nutrients. 2013;5:1573-1584.
71. Sathyapalan T, Beckett S, Rigby AS, et al. High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutr J. 2010;9:55.
72. Martin FP, Antille N, Rezzi S, et al. Everyday eating experiences of chocolate and non-chocolate snacks impact postprandial anxiety, energy and emotional states. Nutrients. 2012;4:554-567.
73. Patel S, Mathan JJ, Vaghefi E, et al. The effect of flavonoids on visual function in patients with glaucoma or ocular hypertension: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2015;253:1841-1850.
74. Moreira A, Diógenes MJ, de Mendonça A, et al. Chocolate consumption is associated with a lower risk of cognitive decline. J Alzheimers Dis. 2016;53:85-93.
1. National Center for Complementary and Integrative Health. The use of complementary and alternative medicine in the United States. Available at: https://nccih.nih.gov/research/statistics/2007/camsurvey_fs1.htm. Accessed Nov 28, 2017.
2. Aggarwal BB. Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res. 2013;57:1529-1542.
3. Henrotin Y, Clutterbuck AL, Allaway D, et al. Biological actions of curcumin on articular chondrocytes. Osteoarthritis Cartilage. 2010;18:141-149.
4. Asher GN, Spelman K. Clinical utility of curcumin extract. Altern Ther Health Med. 2013;19:20-22.
5. Phan TT, See P, Lee ST, et al. Protective effects of curcumin against oxidative damage on skin cells in vitro: its implication for wound healing. J Trauma. 2001;51:927-931.
6. Funk JL, Frye JB, Oyarzo JN, et al. Efficacy and mechanism of action of turmeric supplements in the treatment of experimental arthritis. Arthritis Rheum. 2006;54:3452-3464.
7. Patcharatrakul T, Gonlachanvit S. Chili peppers, curcumins, and prebiotics in gastrointestinal health and disease. Curr Gastroenterol Rep. 2016;18:19.
8. Thamlikitkul V, Bunyapraphatsara N, Dechatiwongse T, et al. Randomized double blind study of Curcuma domestica Val. for dyspepsia. J Med Assoc Thai. 1989;72:613-620.
9. Kumar S, Ahuja V, Sankar MJ, et al. Curcumin for maintenance of remission in ulcerative colitis. Cochrane Database Syst Rev. 2012;10:CD008424.
10. Lang A, Salomon N, Wu JC, et al. Curcumin in combination with mesalamine induces remission in patients with mild-to-moderate ulcerative colitis in a randomized controlled trial. Clin Gastroenterol Hepatol. 2015;13:1444-1449.e1.
11. Henrotin Y, Priem F, Mobasheri A. Curcumin: a new paradigm and therapeutic opportunity for the treatment of osteoarthritis: curcumin for osteoarthritis management. Springerplus. 2013;2:56.
12. Kuptniratsaikul V, Dajpratham P, Taechaarpornkul W, et al. Efficacy and safety of Curcuma domestica extracts compared with ibuprofen in patients with knee osteoarthritis: a multicenter study. Clin Interv Aging. 2014;9:451-458.
13. Shehzad A, Lee J, Lee YS. Curcumin in various cancers. Biofactors. 2013;39:56-68.
14. Sordillo LA, Sordillo PP, Helson L. Curcumin for the treatment of glioblastoma. Anticancer Res. 2015;35:6373-6378.
15. Darvesh AS, Aggarwal BB, Bishayee A. Curcumin and liver cancer: a review. Curr Pharm Biotechnol. 2012;13:218-228.
16. Nagaraju GP, Aliya S, Zafar SF, et al. The impact of curcumin on breast cancer. Integr Biol (Camb). 2012;4:996-1007.
17. Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255:170-181.
18. Dorai T, Cao YC, Dorai B, et al. Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo. Prostate. 2001;47:293-303.
19. Hazarey VK, Sakrikar AR, Ganvir SM. Efficacy of curcumin in the treatment for oral submucous fibrosis - a randomized clinical trial. J Oral Maxillofac Pathol. 2015;19:145-152.
20. Lal B, Kapoor AK, Asthana OP, et al. Efficacy of curcumin in the management of chronic anterior uveitis. Phytother Res. 1999;13:318-322.
21. Kapakos G, Youreva V, Srivastava AK. Cardiovascular protection by curcumin: molecular aspects. Indian J Biochem Biophys. 2012;49:306-315.
22. Yang F, Lim GP, Begum AN, et al. Curcumin inhibits formation of amyloid beta oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J Biol Chem. 2005;280:5892-5901.
23. Heck AM, DeWitt BA, Lukes AL. Potential interactions between alternative therapies and warfarin. Am J Health Syst Pharm. 2000;57:1221-1227.
24. Srivastava JK, Shankar E, Gupta S. Chamomile: a herbal medicine of the past with bright future. Mol Med Rep. 2010;3:895-901.
25. Ross SM. Generalized anxiety disorder (GAD): efficacy of standardized matricaria recutita (german chamomile) extract in the treatment of generalized anxiety disorder. Holistic Nursing Practice. 2013;27:366- 368.
26. Amsterdam JD, Li Y, Soeller I, et al. A randomized, double-blind, placebo-controlled trial of oral Matricaria recutita (chamomile) extract therapy for generalized anxiety disorder. J Clin Psychopharmacol. 2009;29:378-382.
27. Zick SM, Wright BD, Sen A, et al. Preliminary examination of the efficacy and safety of a standardized chamomile extract for chronic primary insomnia: a randomized placebo-controlled pilot study. BMC Complement Altern Med. 2011;11:78.
28. Leach MJ, Page AT. Herbal medicine for insomnia: a systematic review and meta-analysis. Sleep Med Rev. 2015;24:1-12.
29. Weizman Z, Alkrinawi S, Goldfarb D, et al. Efficacy of herbal tea preparation in infantile colic. J Pediatr. 1993;122:650.
30. Crotteau CA, Wright ST, Eglash A. Clinical inquiries. What is the best treatment for infants with colic? J Fam Pract. 2006;55:634-636.
31. Panahi Y, Taghizadeh M, Marzony ET, et al. Rosemary oil vs minoxidil 2% for the treatment of androgenetic alopecia: a randomized comparative trial. Skinmed. 2015;13:15-21.
32. Hay IC, Jamieson M, Ormerod AD. Randomized trial of aromatherapy. Successful treatment for alopecia areata. Arch Dermatol. 1998;134:1349-1352.
33. Caffeine and kids: FDA takes a closer look. Available at: https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm350570.htm. Accessed: November 1, 2017.
34. Torpy JM, Livingston EH. Energy Drinks. JAMA. 2013;309:297.
35. Lopez-Garcia E, van Dam RM, Li TY, et al. The relationship of coffee consumption with mortality. Ann Intern Med. 2008;148:904-914.
36. Crippa A, Discacciati A, Larsson SC, et al. Coffee consumption and mortality from all causes, cardiovascular disease, and cancer: a dose-response meta-analysis. Am J Epidemiol. 2014;180:763-775.
37. Loftfield E, Freedman ND, Graubard BI, et al. Association of coffee consumption with overall and cause-specific mortality in a large US prospective cohort study. Am J Epidemiol. 2015;182:1010-1022.
38. Lopez-Garcia E, Rodriguez-Artalejo F, Rexrode KM, et al. Coffee consumption and risk of stroke in women. Circulation. 2009;119:1116-1123.
39. Friedrich K, Smit M, Wannhoff A, et al. Coffee consumption protects against progression in liver cirrhosis and increases long-term survival after liver transplantation. J Gastroenterol Hepatol. 2016;31:1470-1475.
40. Wang L, Shen X, Wu Y, et al. Coffee and caffeine consumption and depression: a meta-analysis of observational studies. Aust N Z J Psychiatry. 2016;50:228-242.
41. Liu F, Wang X, Wu G, et al. Coffee consumption decreases risks for hepatic fibrosis and cirrhosis: a meta-analysis. PLoS One. 2015;10:e0142457.
42. Grosso G, Micek A, Castellano S, et al. Coffee, tea, caffeine and risk of depression: a systematic review and dose-response meta-analysis of observational studies. Mol Nutr Food Res. 2016;60:223-234.
43. Solfrizzi V, Panza F, Imbimbo BP, et al. Italian longitudinal study on aging working group. Coffee consumption habits and the risk of mild cognitive impairment: The Italian Longitudinal Study on Aging. J Alzheimers Dis. 2015;47:889-899.
44. Sugiyama K, Tomata Y, Kaiho Y, et al. Association between coffee consumption and incident risk of disabling dementia in elderly japanese: The Ohsaki Cohort 2006 Study. J Alzheimers Dis. 2015;50:491-500.
45. Driscoll I, Shumaker SA, Snively BM, et al. Relationships between caffeine intake and risk for probable dementia or global cognitive impairment: The Women’s Health Initiative Memory Study. J Gerontol A Biol Sci Med Sci. 2016;71:1596-1602.
46. van Dieren S, Uiterwaal CS, van der Schouw YT, et al. Coffee and tea consumption and risk of type 2 diabetes. Diabetologia. 2009;52:2561-2569.
47. Wang J, Li X, Zhang D. Coffee consumption and the risk of cutaneous melanoma: a meta-analysis. Eur J Nutr. 2016;55:1317-1329.
48. Liu J, Shen B, Shi M, et al. Higher caffeinated coffee intake is associated with reduced malignant melanoma risk: a meta-analysis study. PLoS One. 2016;11:e0147056.
49. Wikoff D, Welsh BT, Henderson R, et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem Toxical. 2017;109(Pt 1):585-648.
50. Verna R. The history and science of chocolate. Malays J Pathol. 2013;35:111-121.
51. Katz DL, Doughty K, Ali A. Cocoa and chocolate in human health and disease. Antioxid Redox Signal. 2011;15:2779-2811.
52. Baba S, Natsume M, Yasuda A, et al. Plasma LDL and HDL cholesterol and oxidized LDL concentrations are altered in normo- and hypercholesterolemic humans after intake of different levels of cocoa powder. J Nutr. 2007;137:1436-1441.
53. Mellor DD, Sathyapalan T, Kilpatrick ES, et al. High-cocoa polyphenol-rich chocolate improves HDL cholesterol in type 2 diabetes patients. Diabet Med. 2010;27:1318-1321.
54. Tokede OA, Gaziano JM, Djoussé L. Effects of cocoa products/dark chocolate on serum lipids: a meta-analysis. Eur J Clin Nutr. 2011;65:879-886.
55. Jia L, Liu X, Bai YY, et al. Short-term effect of cocoa product consumption on lipid profile: a meta-analysis of randomized controlled trials. Am J Clin Nutr. 2010;92:218-225.
56. Rostami A, Khalili M, Haghighat N, et al. High-cocoa polyphenol-rich chocolate improves blood pressure in patients with diabetes and hypertension. ARYA Atheroscler. 2015;11:21-29.
57. Buitrago-Lopez A, Sanderson J, Johnson L, et al. Chocolate consumption and cardiometabolic disorders: systematic review and meta-analysis. BMJ. 2011;26;343:d4488.
58. Wang X, Ouyang YY, Liu J, et al. Flavonoid intake and risk of CVD: a systematic review and meta-analysis of prospective cohort studies. Br J Nutr. 2014;111:1-11.
59. Zomer E, Owen A, Magliano DJ, et al. The effectiveness and cost effectiveness of dark chocolate consumption as prevention therapy in people at high risk of cardiovascular disease: best case scenario analysis using a Markov model. BMJ. 2012;344:e3657.
60. Larsson SC, Virtamo J, Wolk A. Chocolate consumption and risk of stroke: a prospective cohort of men and meta-analysis. Neurology. 2012;79:1223-1229.
61. Khawaja O, Gaziano JM, Djoussé L. Chocolate and coronary heart disease: a systematic review. Curr Atheroscler Rep. 2011;13:447-452.
62. Jacques PF, Cassidy A, Rogers G, et al. Dietary flavonoid intakes and CVD incidence in the Framingham Offspring Cohort. Br J Nutr. 2015;114:1496-1503.
63. Taubert D, Roesen R, Lehmann C, et al. Effects of low habitual cocoa intake on blood pressure and bioactive nitric oxide: a randomized controlled trial. JAMA. 2007;298:49-60.
64. Desch S, Schmidt J, Kobler D, et al. Effect of cocoa products on blood pressure: systematic review and meta-analysis. Am J Hypertens. 2010;23:97-103.
65. Ried K, Sullivan TR, Fakler P, et al. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2012;8:CD008893.
66. Saftlas AF, Triche EW, Beydoun H, et al. Does chocolate intake during pregnancy reduce the risks of preeclampsia and gestational hypertension? Ann Epidemiol. 2010;20:584-591.
67. Triche EW, Grosso LM, Belanger K, et al. Chocolate consumption in pregnancy and reduced likelihood of preeclampsia. Epidemiology. 2008;19:459-464.
68. Grassi D, Lippi C, Necozione S, et al. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am J Clin Nutr. 2005;81:611-614.
69. Matsumoto C, Petrone AB, Sesso HD, et al. Chocolate consumption and risk of diabetes mellitus in the Physicians’ Health Study. Am J Clin Nutr. 2015;101:362-367.
70. Lippi D. Chocolate in history: food, medicine, medi-food. Nutrients. 2013;5:1573-1584.
71. Sathyapalan T, Beckett S, Rigby AS, et al. High cocoa polyphenol rich chocolate may reduce the burden of the symptoms in chronic fatigue syndrome. Nutr J. 2010;9:55.
72. Martin FP, Antille N, Rezzi S, et al. Everyday eating experiences of chocolate and non-chocolate snacks impact postprandial anxiety, energy and emotional states. Nutrients. 2012;4:554-567.
73. Patel S, Mathan JJ, Vaghefi E, et al. The effect of flavonoids on visual function in patients with glaucoma or ocular hypertension: a systematic review and meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2015;253:1841-1850.
74. Moreira A, Diógenes MJ, de Mendonça A, et al. Chocolate consumption is associated with a lower risk of cognitive decline. J Alzheimers Dis. 2016;53:85-93.
PRACTICE RECOMMENDATIONS
› Inform patients that curcumin appears to be a safe and effective adjunctive therapy for ulcerative colitis when used along with mesalamine or sulfasalazine. B
› Recommend chamomile extract to patients experiencing mild to moderate generalized anxiety disorder. B
› Tell patients that coffee is associated with a lower risk of mortality, reduced cardiovascular events, and a reduction in liver disease progression (in patients with end-stage liver disease). B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Antibiotic overprescribing: Still a major concern
Despite universal agreement that antibiotic overprescribing is a problem, the practice continues to vex us. Antibiotic use—whether appropriate or not—has been linked to rising rates of antimicrobial resistance, disruption of the gut microbiome leading to Clostridium difficile infections, allergic reactions, and increased health care costs (TABLE 11-6). And yet, physicians continue to overprescribe this class of medication.
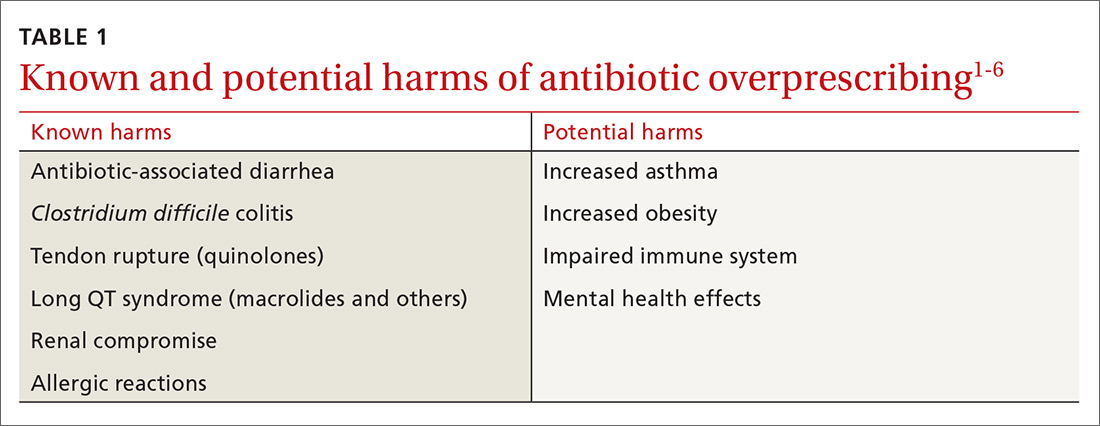
A 2016 Centers for Disease Control and Prevention (CDC) report estimates that at least 30% of antibiotics prescribed in US outpatient settings are unnecessary.7 Another report cites a slightly higher figure across a variety of health care settings.8 Pair these findings with the fact that there are currently few new drugs in development to target resistant bacteria, and you have the potential for a post-antibiotic era in which common infections could become lethal.7
In 2003, the CDC launched its “Get Smart: Know When Antibiotics Work” program, focused on decreasing inappropriate antibiotic use in the outpatient setting.9 In 2014, the White House released the National Action Plan for Combating Antibiotic-Resistant Bacteria with a goal of decreasing inappropriate outpatient antibiotic use by 50% and inappropriate inpatient use by 20% by 2020.10 And, on an international level, the World Health Organization (WHO) developed a 5-year strategic framework in 2015 for implementing its Global Action Plan on Antimicrobial Resistance.11
Family practitioners are on the front lines of this battle. Here’s what we can do now.
[polldaddy:9885811]
When and where are antibiotics most often inappropriately prescribed?
The diagnosis leading to the most frequent inappropriate prescribing of antibiotics is acute respiratory tract infection (ARTI), which includes bronchitis, otitis media, pharyngitis, sinusitis, tonsillitis, the common cold, and pneumonia. Up to 40% of antibiotic prescriptions for these conditions are unnecessary.8,12 Bronchitis is the most common ARTI diagnosis associated with inappropriate antibiotic prescriptions, while sinusitis, suppurative otitis media, and pharyngitis are the diagnoses associated with the lion’s share of all (appropriate and inappropriate) antibiotic prescriptions within the ARTI category.8,9,12,13 There are national clinical guidelines delineating when antibiotic treatment is appropriate for these conditions.14-16
With respect to setting, studies have presented conflicting results as to whether there is a difference between antibiotic prescribing in office-based vs emergency department (ED) settings. Here is a sample of some of the literature to date:
- One study found a higher rate of antibiotic prescribing during ED visits (21%) than office visits (9%), despite the fact that between 2007 and 2009, more antibiotic prescriptions were written for adults in primary care offices than in either outpatient hospital clinics or EDs.17
- A cross-sectional study focused on the frequency with which antibiotics were prescribed for uncomplicated acute rhinosinusitis. Researchers analyzed data from 2005 to 2010 National Ambulatory Medical Care Surveys (NAMCS) and National Hospital Ambulatory Medical Care Surveys (NHAMCS) and found that more than half of the patients received prescriptions for antibiotics, but that there was no overall difference in antibiotic prescriptions between primary care and ED presentation.18
- A retrospective analysis that examined antibiotic prescribing found that between 2006 and 2010, outpatient hospital practices (56%) and community-practice offices (60%) prescribed more antibiotics for ARTIs than EDs (51%).12
Stick to narrow-spectrum agents when possible
Using broad-spectrum antibiotics, such as quinolones or imipenem, first line, contributes more to the problem of antibiotic resistance than does prescribing narrow-spectrum antibiotics such as amoxicillin, cephalexin, or trimethoprim-sulfamethoxazole.7 Yet between 2007 and 2009, broad-spectrum agents were prescribed for 61% of outpatient adult visits in which patients received an antibiotic prescription.17 Quinolones (25%), macrolides (20%), and aminopenicillins (12%) were most commonly prescribed, and antibiotic prescriptions were most often written for respiratory conditions, such as bronchitis, for which we now know antibiotics are rarely indicated.17
Between 2006 and 2008, pediatric patients who received antibiotic prescriptions were given broad-spectrum agents 50% of the time, of which macrolides were the class most commonly prescribed.13
More recently, researchers examined the frequency with which physicians prescribe narrow-spectrum, first-line antibiotics for otitis media, sinusitis, and pharyngitis using 2010 to 2011 NAMCS/NHAMCS data. They found that physicians used first-line agents recommended by professional guidelines 52% of the time, although it was estimated that they would have been appropriate in 80% of cases; pediatric patients were more likely to receive appropriate first-line antibiotics than adult patients.19 Macrolides, especially azithromycin, were the most common non–first-line antibiotics prescribed.19,20 The bottom line is that when antibiotics are indicated for upper respiratory infections (otitis media, sinusitis, and pharyngitis), physicians should prescribe a narrow-spectrum antibiotic first.
Antibiotic overprescribing affects the gut and beyond
The human intestinal microbiome is composed of a diverse array of bacteria, viruses, and parasites.21 The main functions of the gut microbiome include interacting with the immune system and participating in biochemical reactions in the gut, such as absorption of fat-soluble vitamins and the production of vitamin K.
As we know, antibiotics decrease the diversity of gut bacteria, which, in turn, can cause less efficient nutrient extraction, as well as a vulnerability to enteric infections.21 It is well known, for example, that the bacterial gut microbiome can either inhibit or promote diarrheal illnesses such as those caused by C. difficile. C. difficile infection (CDI) is now the most common health care-related infection, accounting for approximately a half million health care facility infections a year.22 CDI extends hospital stays an average of almost 10 days and is estimated to cost the health care system $6.3 billion annually.23
Antibiotics can also eliminate antibiotic-susceptible organisms, allowing resistant organisms to proliferate.4 They also promote the transmission of genes for antibiotic resistance between gut bacteria.4
Beyond the gut
Less well known is that gut bacteria can promote or inhibit extraintestinal infections.
Gut bacteria and HIV. In early human immunodeficiency virus (HIV) infections, for example, gut populations of Lactobacillus and Bifidobacteria are reduced, and the gut barrier becomes compromised.24 Increasing translocation of bacterial products is associated with HIV disease progression. Preservation of Lactobacillus populations in the gut is associated with markers predictive of better HIV outcomes, including a higher CD4 count, a lower viral load, and less evidence of gut microbial translocation.24 This underscores the importance of maintaining a healthy gut flora in patients with HIV, using such steps as avoiding unnecessary antibiotics.
Gut bacteria and stress, depression. Antibiotics directly induce the expression of key genes that affect the stress response.25 While causative studies are lacking, there is a growing body of evidence suggesting that the gut microbiome is involved in 2-way communication with the brain and can affect, and be affected by, stress and depression.21,26-30 Diseases and conditions that seem to have a putative connection to a disordered microbiome (dysbiosis) include depression, anxiety, Crohn’s disease, type 2 diabetes, and obesity.
Gut bacteria and childhood obesity. Repeated use of broader-spectrum antibiotics in children <24 months of age increases the risk of developing childhood obesity.1,6 One theory for the association is that the effects of broad-spectrum antibiotics on the intestinal flora of young children may alter long-term energy homeostasis resulting in a higher risk for obesity.1
Gut bacteria and asthma. Studies demonstrate differences in the gut microbiome of asthmatic and nonasthmatic patients. These differences affect the activities of helper T-cell subsets (Th1 and Th2), which in turn affect the development of immune tolerance.31
Although additional studies are needed to confirm these findings, the evidence collected thus far should make us all pause before prescribing drugs that can alter our microbiome in complex and only partially understood ways.

What can we do right now?
The issues created by the inappropriate prescribing of antibiotics have been known for decades, and multiple attempts have been made to find solutions and implement change. Although some small successes have occurred, little overall progress has been made in reducing antibiotic prescribing in the general population. A historical review of why physicians prescribe antibiotics inappropriately and the interventions that have successfully reduced this prescribing may prove valuable as we continue to look for new, effective answers.
Why do we overprescribe antibiotics? A 2015 systematic literature review found that patient demand, pharmaceutical company marketing activities, limited up-to-date information sources, and physician fear of losing their patients are major reasons physicians cite for prescribing antibiotics.32
In a separate study that explored antibiotic prescribing habits for acute bronchitis,33 clinicians cited “patient demand” as the major reason for prescribing antibiotics. Respondents also reported that “other physicians were responsible for inappropriate antibiotic prescribing.”33
Strategies that work
Some early intervention programs directed at reducing antibiotic prescribing demonstrated success (TABLE 2).34-36 One example comes from a 1996 to 1998 study of 4 primary care practices.34 Researchers evaluated the impact of a multidimensional intervention effort targeted at clinicians and patients and aimed at lowering the use of antimicrobial agents for acute uncomplicated bronchitis in adults. It incorporated a number of elements, including office-based and household patient educational materials, and a clinician intervention involving education, practice profiling, and academic detailing. Physicians in this program reduced their rates of antibiotic prescribing for uncomplicated bronchitis from 74% to 48%.34
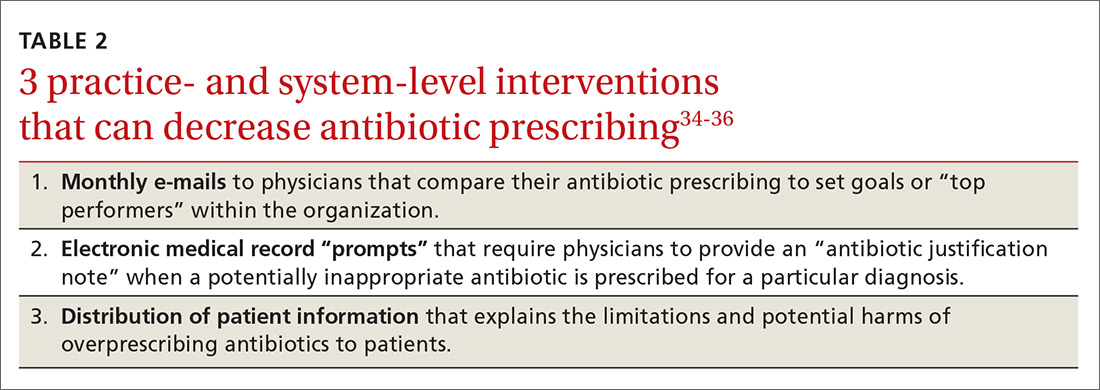
Employing EMRs. A more recent study focused on using electronic medical records (EMRs) and communications to modify physician antibiotic prescribing.35 By sending physicians monthly emails comparing their prescribing patterns to peers and “typical top performers,” inappropriate antibiotic prescriptions for ARTIs went from 19.9% to 3.7%.35
In another effort, the same researchers modified physicians’ EMRs to detect when potentially inappropriate antibiotics were prescribed. The system then prompted the physician to provide an “antibiotic justification note,” which remained visible in the patient’s chart. This approach, which encouraged physicians to follow prescribing guidelines by taking advantage of their concerns about their reputations, produced a 77% reduction in antibiotic prescribing.35
Focusing on the public. Studies have also examined the effectiveness of educating the public about when antibiotics are not likely to be helpful and of the harms of unnecessary antibiotics. Studies conducted in Tennessee and Wisconsin that combined prescriber and community education about unnecessary antibiotics for children found that the intervention reduced antibiotic prescribing in both locations by about 19% compared with about a 9% reduction in the control groups.36,37
Does prescribing antibiotics affect patient satisfaction?
The results are mixed as to whether prescribing antibiotics affects patient satisfaction. Two studies in the early 2000s found that both patients and parents reported higher satisfaction with physicians who explained why antibiotics were not indicated vs physicians who simply prescribed them, and that such explanations do not need to take a lot of time.37,38 (See TABLE 39,37,38 for patient care tips.)
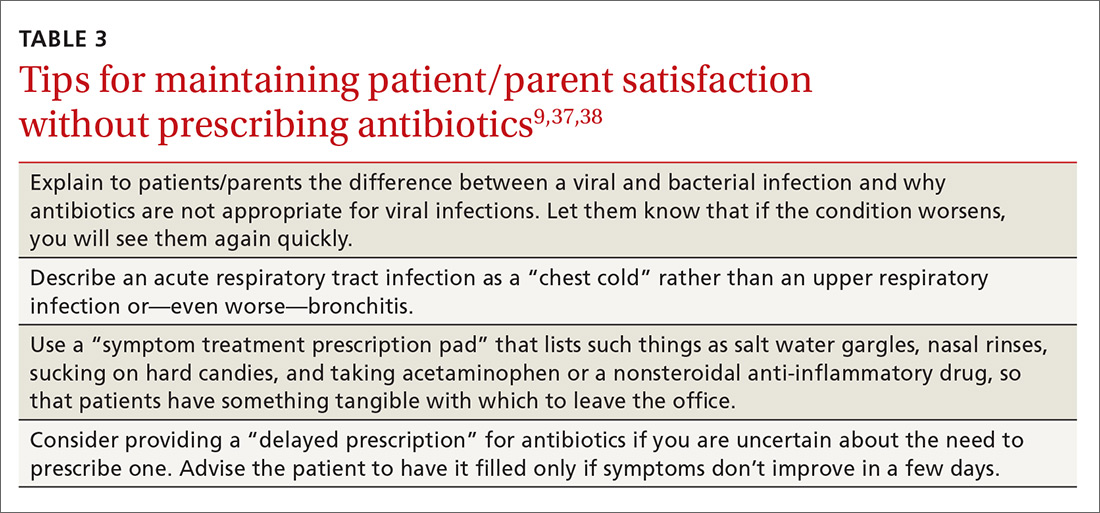
A more recent study found that higher antibiotic prescribing practices in Britain were associated with modestly higher patient satisfaction ratings.39 The authors of this study noted, however, that reduced antibiotic prescribing may be a proxy for other practice patterns that affected satisfaction ratings.
Reducing antibiotic prescribing reduces resistance
There is also strong evidence that when physicians decrease antibiotic prescribing, antimicrobial resistance follows suit. One of the earlier landmark studies to demonstrate this was a Finnish study published in 1997.40 The authors found that a reduction of macrolide antibiotic consumption in Finland led to a reduction in streptococci macrolide resistance from 16.5% to 8.6%.40
Since then, multiple studies have demonstrated similar results for both respiratory and urinary tract infections.41,42 A 2017 meta-analysis analyzing 32 studies found that antibiotic stewardship programs reduced the incidence of infections and colonization with multidrug-resistant Gram-negative bacteria (51% reduction), extended-spectrum beta-lactamase–producing Gram-negative bacteria (48%), and methicillin-resistant Staphylococcus aureus (37%). There was also a reduction in the incidence of C. difficile infections (32%).43
CORRESPONDENCE
David C. Fiore, MD, Department of Family and Community Medicine, University of Nevada, Reno School of Medicine, Brigham Bldg, MS 316, Reno, NV 89557; [email protected].
1. Bailey LC, Forrest CB, Zhang P, et al. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168:1063-1069.
2. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
3. Gleckman RA, Czachor JS. Antibiotic side effects. Semin Respir Crit Care Med. 2000;21:53-60.
4. Jernberg C, Löfmark S, Edlund C, et al. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156:3216-3223.
5. Logan AC, Jacka FN, Craig JM, et al. The microbiome and mental health: looking back, moving forward with lessons from allergic diseases. Clin Psychopharmacol Neurosci. 2016;14:131-147.
6. Marra F, Marra CA, Richardson K, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics. 2009;123:1003-1010.
7. Harris AM, Hicks LA, Qaseem A, for the High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164:425-434.
8. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
9. Centers for Disease Control and Prevention. Antibiotic prescribing and use. Available at: http://www.cdc.gov/getsmart/. Accessed October 23, 2017.
10. The White House. National action plan for combating antibiotic-resistant bacteria. March 2015:1-63. Available at: https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed October 23, 2017.
11. World Health Organization. Global action plan on antimicrobial resistance. 2015. Available at: http://www.who.int/drugresistance/global_action_plan/en/. Accessed October 23, 2017.
12. Barlam TF, Soria-Saucedo R, Cabral HJ, et al. Unnecessary antibiotics for acute respiratory tract infections: association with care setting and patient demographics. Open Forum Infect Dis. 2016;3:1-7.
13. Hersh AL, Shapiro DJ, Pavia AT, et al. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128:1053-1061.
14. Chow AW, Benninger MS, Brook I, et al. Executive summary: IDSA Clinical Practice Guideline for Acute Bacterial Rhinosinusitis in Children and Adults. Clin Infect Dis. 2012;54:1041-1045.
15. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 Suppl):S1-S39.
16. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279-1282.
17. Shapiro DJ, Hicks LA, Pavia AT, et al. Antibiotic prescribing for adults in ambulatory care in the USA, 2007-09. J Antimicrob Chemother. 2014;69:234-240.
18. Bergmark RW, Sedaghat AR. Antibiotic prescription for acute rhinosinusitis: emergency departments versus primary care providers. Laryngoscope. 2016;(November):1-6.
19. Hersh AL, Fleming-Dutra KE, Shapiro DJ, et al. Frequency of first-line antibiotic selection among US ambulatory care visits for otitis media, sinusitis, and pharyngitis. JAMA Intern Med. 2016;176:1870-1872.
20. Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60:1308-1316.
21. Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8:39.
22. Lessa FC, Gould CV, McDonald CL. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(Suppl 2):S65-S70.
23. Zhang S, Palazuelos-Munoz S, Balsells EM, et al. Cost of hospital management of Clostridium difficile infection in United States—a meta-analysis and modelling study. BMC Infect Dis. 2016;16:447.
24. Pérez-Santiago J, Gianella S, Massanella M, et al. Gut lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27:1921-1931.
25. Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39-50.
26. Bravo JA, Julio-Pieper M, Forsythe P, et al. Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol. 2012;12:667-672.
27. Clemente JC, Ursell LK, Parfrey LW, et al. The impact of the gut microbiota on human health: An integrative view. Cell. 2012;148:1258-1270.
28. Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369-1378.
29. Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305-312.
30. Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014;38:1-12.
31. Riiser A. The human microbiome, asthma, and allergy. Allergy, Asthma, and Clinical Immunology. 2015;11:35.
32. Md Rezal RS, Hassali MA, Alrasheedy AA, et al. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: a systematic review of the literature. Expert Rev Anti Infect Ther. 2015;13:665-680.
33. Dempsey PP, Businger AC, Whaley LE, et al. Primary care clinicians’ perceptions about antibiotic prescribing for acute bronchitis: a qualitative study. BMC Fam Pract. 2014;15:194.
34. Gonzales R, Steiner JF, Lum A, et al. Decreasing antibiotic use in ambulatory practice. JAMA. 1999;281:1512-1519.
35. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315:562-570.
36. Perz JF, Craig AS, Coffey CS, et al. Changes in antibiotic prescribing for children after a community-wide campaign. JAMA. 2002;287:3103-3109.
37. Belongia EA, Sullivan BJ, Chyou PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001;108:575-583.
38. Mangione-Smith R, McGlynn EA, Elliott MN, et al. Parent expectations for antibiotics, physician-parent communication, and satisfaction. Arch Pediatr Adolesc Med. 2001;155:800-806.
39. Ashworth M, White P, Jongsma H,et al. Antibiotic prescribing and patient satisfaction in primary care in England: cross-sectional analysis of national patient survey data and prescribing data. Br J Gen Pract. 2016;66:e40-e46.
40. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med. 1997;337:441-446.
41. Guillemot D, Varon E, Bernède C, et al. Reduction of antibiotic use in the community reduces the rate of colonization with penicillin g–nonsusceptible Streptococcus pneumoniae. Clin Infect Dis. 2005;41:930-938.
42. Butler CC, Dunstan F, Heginbothom M, et al. Containing antibiotic resistance: decreased antibiotic-resistant coliform urinary tract infections with reduction in antibiotic prescribing by general practices. Br J Gen Pract. 2007;57:785-792.
43. Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:990-1001.
Despite universal agreement that antibiotic overprescribing is a problem, the practice continues to vex us. Antibiotic use—whether appropriate or not—has been linked to rising rates of antimicrobial resistance, disruption of the gut microbiome leading to Clostridium difficile infections, allergic reactions, and increased health care costs (TABLE 11-6). And yet, physicians continue to overprescribe this class of medication.

A 2016 Centers for Disease Control and Prevention (CDC) report estimates that at least 30% of antibiotics prescribed in US outpatient settings are unnecessary.7 Another report cites a slightly higher figure across a variety of health care settings.8 Pair these findings with the fact that there are currently few new drugs in development to target resistant bacteria, and you have the potential for a post-antibiotic era in which common infections could become lethal.7
In 2003, the CDC launched its “Get Smart: Know When Antibiotics Work” program, focused on decreasing inappropriate antibiotic use in the outpatient setting.9 In 2014, the White House released the National Action Plan for Combating Antibiotic-Resistant Bacteria with a goal of decreasing inappropriate outpatient antibiotic use by 50% and inappropriate inpatient use by 20% by 2020.10 And, on an international level, the World Health Organization (WHO) developed a 5-year strategic framework in 2015 for implementing its Global Action Plan on Antimicrobial Resistance.11
Family practitioners are on the front lines of this battle. Here’s what we can do now.
[polldaddy:9885811]
When and where are antibiotics most often inappropriately prescribed?
The diagnosis leading to the most frequent inappropriate prescribing of antibiotics is acute respiratory tract infection (ARTI), which includes bronchitis, otitis media, pharyngitis, sinusitis, tonsillitis, the common cold, and pneumonia. Up to 40% of antibiotic prescriptions for these conditions are unnecessary.8,12 Bronchitis is the most common ARTI diagnosis associated with inappropriate antibiotic prescriptions, while sinusitis, suppurative otitis media, and pharyngitis are the diagnoses associated with the lion’s share of all (appropriate and inappropriate) antibiotic prescriptions within the ARTI category.8,9,12,13 There are national clinical guidelines delineating when antibiotic treatment is appropriate for these conditions.14-16
With respect to setting, studies have presented conflicting results as to whether there is a difference between antibiotic prescribing in office-based vs emergency department (ED) settings. Here is a sample of some of the literature to date:
- One study found a higher rate of antibiotic prescribing during ED visits (21%) than office visits (9%), despite the fact that between 2007 and 2009, more antibiotic prescriptions were written for adults in primary care offices than in either outpatient hospital clinics or EDs.17
- A cross-sectional study focused on the frequency with which antibiotics were prescribed for uncomplicated acute rhinosinusitis. Researchers analyzed data from 2005 to 2010 National Ambulatory Medical Care Surveys (NAMCS) and National Hospital Ambulatory Medical Care Surveys (NHAMCS) and found that more than half of the patients received prescriptions for antibiotics, but that there was no overall difference in antibiotic prescriptions between primary care and ED presentation.18
- A retrospective analysis that examined antibiotic prescribing found that between 2006 and 2010, outpatient hospital practices (56%) and community-practice offices (60%) prescribed more antibiotics for ARTIs than EDs (51%).12
Stick to narrow-spectrum agents when possible
Using broad-spectrum antibiotics, such as quinolones or imipenem, first line, contributes more to the problem of antibiotic resistance than does prescribing narrow-spectrum antibiotics such as amoxicillin, cephalexin, or trimethoprim-sulfamethoxazole.7 Yet between 2007 and 2009, broad-spectrum agents were prescribed for 61% of outpatient adult visits in which patients received an antibiotic prescription.17 Quinolones (25%), macrolides (20%), and aminopenicillins (12%) were most commonly prescribed, and antibiotic prescriptions were most often written for respiratory conditions, such as bronchitis, for which we now know antibiotics are rarely indicated.17
Between 2006 and 2008, pediatric patients who received antibiotic prescriptions were given broad-spectrum agents 50% of the time, of which macrolides were the class most commonly prescribed.13
More recently, researchers examined the frequency with which physicians prescribe narrow-spectrum, first-line antibiotics for otitis media, sinusitis, and pharyngitis using 2010 to 2011 NAMCS/NHAMCS data. They found that physicians used first-line agents recommended by professional guidelines 52% of the time, although it was estimated that they would have been appropriate in 80% of cases; pediatric patients were more likely to receive appropriate first-line antibiotics than adult patients.19 Macrolides, especially azithromycin, were the most common non–first-line antibiotics prescribed.19,20 The bottom line is that when antibiotics are indicated for upper respiratory infections (otitis media, sinusitis, and pharyngitis), physicians should prescribe a narrow-spectrum antibiotic first.
Antibiotic overprescribing affects the gut and beyond
The human intestinal microbiome is composed of a diverse array of bacteria, viruses, and parasites.21 The main functions of the gut microbiome include interacting with the immune system and participating in biochemical reactions in the gut, such as absorption of fat-soluble vitamins and the production of vitamin K.
As we know, antibiotics decrease the diversity of gut bacteria, which, in turn, can cause less efficient nutrient extraction, as well as a vulnerability to enteric infections.21 It is well known, for example, that the bacterial gut microbiome can either inhibit or promote diarrheal illnesses such as those caused by C. difficile. C. difficile infection (CDI) is now the most common health care-related infection, accounting for approximately a half million health care facility infections a year.22 CDI extends hospital stays an average of almost 10 days and is estimated to cost the health care system $6.3 billion annually.23
Antibiotics can also eliminate antibiotic-susceptible organisms, allowing resistant organisms to proliferate.4 They also promote the transmission of genes for antibiotic resistance between gut bacteria.4
Beyond the gut
Less well known is that gut bacteria can promote or inhibit extraintestinal infections.
Gut bacteria and HIV. In early human immunodeficiency virus (HIV) infections, for example, gut populations of Lactobacillus and Bifidobacteria are reduced, and the gut barrier becomes compromised.24 Increasing translocation of bacterial products is associated with HIV disease progression. Preservation of Lactobacillus populations in the gut is associated with markers predictive of better HIV outcomes, including a higher CD4 count, a lower viral load, and less evidence of gut microbial translocation.24 This underscores the importance of maintaining a healthy gut flora in patients with HIV, using such steps as avoiding unnecessary antibiotics.
Gut bacteria and stress, depression. Antibiotics directly induce the expression of key genes that affect the stress response.25 While causative studies are lacking, there is a growing body of evidence suggesting that the gut microbiome is involved in 2-way communication with the brain and can affect, and be affected by, stress and depression.21,26-30 Diseases and conditions that seem to have a putative connection to a disordered microbiome (dysbiosis) include depression, anxiety, Crohn’s disease, type 2 diabetes, and obesity.
Gut bacteria and childhood obesity. Repeated use of broader-spectrum antibiotics in children <24 months of age increases the risk of developing childhood obesity.1,6 One theory for the association is that the effects of broad-spectrum antibiotics on the intestinal flora of young children may alter long-term energy homeostasis resulting in a higher risk for obesity.1
Gut bacteria and asthma. Studies demonstrate differences in the gut microbiome of asthmatic and nonasthmatic patients. These differences affect the activities of helper T-cell subsets (Th1 and Th2), which in turn affect the development of immune tolerance.31
Although additional studies are needed to confirm these findings, the evidence collected thus far should make us all pause before prescribing drugs that can alter our microbiome in complex and only partially understood ways.

What can we do right now?
The issues created by the inappropriate prescribing of antibiotics have been known for decades, and multiple attempts have been made to find solutions and implement change. Although some small successes have occurred, little overall progress has been made in reducing antibiotic prescribing in the general population. A historical review of why physicians prescribe antibiotics inappropriately and the interventions that have successfully reduced this prescribing may prove valuable as we continue to look for new, effective answers.
Why do we overprescribe antibiotics? A 2015 systematic literature review found that patient demand, pharmaceutical company marketing activities, limited up-to-date information sources, and physician fear of losing their patients are major reasons physicians cite for prescribing antibiotics.32
In a separate study that explored antibiotic prescribing habits for acute bronchitis,33 clinicians cited “patient demand” as the major reason for prescribing antibiotics. Respondents also reported that “other physicians were responsible for inappropriate antibiotic prescribing.”33
Strategies that work
Some early intervention programs directed at reducing antibiotic prescribing demonstrated success (TABLE 2).34-36 One example comes from a 1996 to 1998 study of 4 primary care practices.34 Researchers evaluated the impact of a multidimensional intervention effort targeted at clinicians and patients and aimed at lowering the use of antimicrobial agents for acute uncomplicated bronchitis in adults. It incorporated a number of elements, including office-based and household patient educational materials, and a clinician intervention involving education, practice profiling, and academic detailing. Physicians in this program reduced their rates of antibiotic prescribing for uncomplicated bronchitis from 74% to 48%.34

Employing EMRs. A more recent study focused on using electronic medical records (EMRs) and communications to modify physician antibiotic prescribing.35 By sending physicians monthly emails comparing their prescribing patterns to peers and “typical top performers,” inappropriate antibiotic prescriptions for ARTIs went from 19.9% to 3.7%.35
In another effort, the same researchers modified physicians’ EMRs to detect when potentially inappropriate antibiotics were prescribed. The system then prompted the physician to provide an “antibiotic justification note,” which remained visible in the patient’s chart. This approach, which encouraged physicians to follow prescribing guidelines by taking advantage of their concerns about their reputations, produced a 77% reduction in antibiotic prescribing.35
Focusing on the public. Studies have also examined the effectiveness of educating the public about when antibiotics are not likely to be helpful and of the harms of unnecessary antibiotics. Studies conducted in Tennessee and Wisconsin that combined prescriber and community education about unnecessary antibiotics for children found that the intervention reduced antibiotic prescribing in both locations by about 19% compared with about a 9% reduction in the control groups.36,37
Does prescribing antibiotics affect patient satisfaction?
The results are mixed as to whether prescribing antibiotics affects patient satisfaction. Two studies in the early 2000s found that both patients and parents reported higher satisfaction with physicians who explained why antibiotics were not indicated vs physicians who simply prescribed them, and that such explanations do not need to take a lot of time.37,38 (See TABLE 39,37,38 for patient care tips.)

A more recent study found that higher antibiotic prescribing practices in Britain were associated with modestly higher patient satisfaction ratings.39 The authors of this study noted, however, that reduced antibiotic prescribing may be a proxy for other practice patterns that affected satisfaction ratings.
Reducing antibiotic prescribing reduces resistance
There is also strong evidence that when physicians decrease antibiotic prescribing, antimicrobial resistance follows suit. One of the earlier landmark studies to demonstrate this was a Finnish study published in 1997.40 The authors found that a reduction of macrolide antibiotic consumption in Finland led to a reduction in streptococci macrolide resistance from 16.5% to 8.6%.40
Since then, multiple studies have demonstrated similar results for both respiratory and urinary tract infections.41,42 A 2017 meta-analysis analyzing 32 studies found that antibiotic stewardship programs reduced the incidence of infections and colonization with multidrug-resistant Gram-negative bacteria (51% reduction), extended-spectrum beta-lactamase–producing Gram-negative bacteria (48%), and methicillin-resistant Staphylococcus aureus (37%). There was also a reduction in the incidence of C. difficile infections (32%).43
CORRESPONDENCE
David C. Fiore, MD, Department of Family and Community Medicine, University of Nevada, Reno School of Medicine, Brigham Bldg, MS 316, Reno, NV 89557; [email protected].
Despite universal agreement that antibiotic overprescribing is a problem, the practice continues to vex us. Antibiotic use—whether appropriate or not—has been linked to rising rates of antimicrobial resistance, disruption of the gut microbiome leading to Clostridium difficile infections, allergic reactions, and increased health care costs (TABLE 11-6). And yet, physicians continue to overprescribe this class of medication.

A 2016 Centers for Disease Control and Prevention (CDC) report estimates that at least 30% of antibiotics prescribed in US outpatient settings are unnecessary.7 Another report cites a slightly higher figure across a variety of health care settings.8 Pair these findings with the fact that there are currently few new drugs in development to target resistant bacteria, and you have the potential for a post-antibiotic era in which common infections could become lethal.7
In 2003, the CDC launched its “Get Smart: Know When Antibiotics Work” program, focused on decreasing inappropriate antibiotic use in the outpatient setting.9 In 2014, the White House released the National Action Plan for Combating Antibiotic-Resistant Bacteria with a goal of decreasing inappropriate outpatient antibiotic use by 50% and inappropriate inpatient use by 20% by 2020.10 And, on an international level, the World Health Organization (WHO) developed a 5-year strategic framework in 2015 for implementing its Global Action Plan on Antimicrobial Resistance.11
Family practitioners are on the front lines of this battle. Here’s what we can do now.
[polldaddy:9885811]
When and where are antibiotics most often inappropriately prescribed?
The diagnosis leading to the most frequent inappropriate prescribing of antibiotics is acute respiratory tract infection (ARTI), which includes bronchitis, otitis media, pharyngitis, sinusitis, tonsillitis, the common cold, and pneumonia. Up to 40% of antibiotic prescriptions for these conditions are unnecessary.8,12 Bronchitis is the most common ARTI diagnosis associated with inappropriate antibiotic prescriptions, while sinusitis, suppurative otitis media, and pharyngitis are the diagnoses associated with the lion’s share of all (appropriate and inappropriate) antibiotic prescriptions within the ARTI category.8,9,12,13 There are national clinical guidelines delineating when antibiotic treatment is appropriate for these conditions.14-16
With respect to setting, studies have presented conflicting results as to whether there is a difference between antibiotic prescribing in office-based vs emergency department (ED) settings. Here is a sample of some of the literature to date:
- One study found a higher rate of antibiotic prescribing during ED visits (21%) than office visits (9%), despite the fact that between 2007 and 2009, more antibiotic prescriptions were written for adults in primary care offices than in either outpatient hospital clinics or EDs.17
- A cross-sectional study focused on the frequency with which antibiotics were prescribed for uncomplicated acute rhinosinusitis. Researchers analyzed data from 2005 to 2010 National Ambulatory Medical Care Surveys (NAMCS) and National Hospital Ambulatory Medical Care Surveys (NHAMCS) and found that more than half of the patients received prescriptions for antibiotics, but that there was no overall difference in antibiotic prescriptions between primary care and ED presentation.18
- A retrospective analysis that examined antibiotic prescribing found that between 2006 and 2010, outpatient hospital practices (56%) and community-practice offices (60%) prescribed more antibiotics for ARTIs than EDs (51%).12
Stick to narrow-spectrum agents when possible
Using broad-spectrum antibiotics, such as quinolones or imipenem, first line, contributes more to the problem of antibiotic resistance than does prescribing narrow-spectrum antibiotics such as amoxicillin, cephalexin, or trimethoprim-sulfamethoxazole.7 Yet between 2007 and 2009, broad-spectrum agents were prescribed for 61% of outpatient adult visits in which patients received an antibiotic prescription.17 Quinolones (25%), macrolides (20%), and aminopenicillins (12%) were most commonly prescribed, and antibiotic prescriptions were most often written for respiratory conditions, such as bronchitis, for which we now know antibiotics are rarely indicated.17
Between 2006 and 2008, pediatric patients who received antibiotic prescriptions were given broad-spectrum agents 50% of the time, of which macrolides were the class most commonly prescribed.13
More recently, researchers examined the frequency with which physicians prescribe narrow-spectrum, first-line antibiotics for otitis media, sinusitis, and pharyngitis using 2010 to 2011 NAMCS/NHAMCS data. They found that physicians used first-line agents recommended by professional guidelines 52% of the time, although it was estimated that they would have been appropriate in 80% of cases; pediatric patients were more likely to receive appropriate first-line antibiotics than adult patients.19 Macrolides, especially azithromycin, were the most common non–first-line antibiotics prescribed.19,20 The bottom line is that when antibiotics are indicated for upper respiratory infections (otitis media, sinusitis, and pharyngitis), physicians should prescribe a narrow-spectrum antibiotic first.
Antibiotic overprescribing affects the gut and beyond
The human intestinal microbiome is composed of a diverse array of bacteria, viruses, and parasites.21 The main functions of the gut microbiome include interacting with the immune system and participating in biochemical reactions in the gut, such as absorption of fat-soluble vitamins and the production of vitamin K.
As we know, antibiotics decrease the diversity of gut bacteria, which, in turn, can cause less efficient nutrient extraction, as well as a vulnerability to enteric infections.21 It is well known, for example, that the bacterial gut microbiome can either inhibit or promote diarrheal illnesses such as those caused by C. difficile. C. difficile infection (CDI) is now the most common health care-related infection, accounting for approximately a half million health care facility infections a year.22 CDI extends hospital stays an average of almost 10 days and is estimated to cost the health care system $6.3 billion annually.23
Antibiotics can also eliminate antibiotic-susceptible organisms, allowing resistant organisms to proliferate.4 They also promote the transmission of genes for antibiotic resistance between gut bacteria.4
Beyond the gut
Less well known is that gut bacteria can promote or inhibit extraintestinal infections.
Gut bacteria and HIV. In early human immunodeficiency virus (HIV) infections, for example, gut populations of Lactobacillus and Bifidobacteria are reduced, and the gut barrier becomes compromised.24 Increasing translocation of bacterial products is associated with HIV disease progression. Preservation of Lactobacillus populations in the gut is associated with markers predictive of better HIV outcomes, including a higher CD4 count, a lower viral load, and less evidence of gut microbial translocation.24 This underscores the importance of maintaining a healthy gut flora in patients with HIV, using such steps as avoiding unnecessary antibiotics.
Gut bacteria and stress, depression. Antibiotics directly induce the expression of key genes that affect the stress response.25 While causative studies are lacking, there is a growing body of evidence suggesting that the gut microbiome is involved in 2-way communication with the brain and can affect, and be affected by, stress and depression.21,26-30 Diseases and conditions that seem to have a putative connection to a disordered microbiome (dysbiosis) include depression, anxiety, Crohn’s disease, type 2 diabetes, and obesity.
Gut bacteria and childhood obesity. Repeated use of broader-spectrum antibiotics in children <24 months of age increases the risk of developing childhood obesity.1,6 One theory for the association is that the effects of broad-spectrum antibiotics on the intestinal flora of young children may alter long-term energy homeostasis resulting in a higher risk for obesity.1
Gut bacteria and asthma. Studies demonstrate differences in the gut microbiome of asthmatic and nonasthmatic patients. These differences affect the activities of helper T-cell subsets (Th1 and Th2), which in turn affect the development of immune tolerance.31
Although additional studies are needed to confirm these findings, the evidence collected thus far should make us all pause before prescribing drugs that can alter our microbiome in complex and only partially understood ways.

What can we do right now?
The issues created by the inappropriate prescribing of antibiotics have been known for decades, and multiple attempts have been made to find solutions and implement change. Although some small successes have occurred, little overall progress has been made in reducing antibiotic prescribing in the general population. A historical review of why physicians prescribe antibiotics inappropriately and the interventions that have successfully reduced this prescribing may prove valuable as we continue to look for new, effective answers.
Why do we overprescribe antibiotics? A 2015 systematic literature review found that patient demand, pharmaceutical company marketing activities, limited up-to-date information sources, and physician fear of losing their patients are major reasons physicians cite for prescribing antibiotics.32
In a separate study that explored antibiotic prescribing habits for acute bronchitis,33 clinicians cited “patient demand” as the major reason for prescribing antibiotics. Respondents also reported that “other physicians were responsible for inappropriate antibiotic prescribing.”33
Strategies that work
Some early intervention programs directed at reducing antibiotic prescribing demonstrated success (TABLE 2).34-36 One example comes from a 1996 to 1998 study of 4 primary care practices.34 Researchers evaluated the impact of a multidimensional intervention effort targeted at clinicians and patients and aimed at lowering the use of antimicrobial agents for acute uncomplicated bronchitis in adults. It incorporated a number of elements, including office-based and household patient educational materials, and a clinician intervention involving education, practice profiling, and academic detailing. Physicians in this program reduced their rates of antibiotic prescribing for uncomplicated bronchitis from 74% to 48%.34

Employing EMRs. A more recent study focused on using electronic medical records (EMRs) and communications to modify physician antibiotic prescribing.35 By sending physicians monthly emails comparing their prescribing patterns to peers and “typical top performers,” inappropriate antibiotic prescriptions for ARTIs went from 19.9% to 3.7%.35
In another effort, the same researchers modified physicians’ EMRs to detect when potentially inappropriate antibiotics were prescribed. The system then prompted the physician to provide an “antibiotic justification note,” which remained visible in the patient’s chart. This approach, which encouraged physicians to follow prescribing guidelines by taking advantage of their concerns about their reputations, produced a 77% reduction in antibiotic prescribing.35
Focusing on the public. Studies have also examined the effectiveness of educating the public about when antibiotics are not likely to be helpful and of the harms of unnecessary antibiotics. Studies conducted in Tennessee and Wisconsin that combined prescriber and community education about unnecessary antibiotics for children found that the intervention reduced antibiotic prescribing in both locations by about 19% compared with about a 9% reduction in the control groups.36,37
Does prescribing antibiotics affect patient satisfaction?
The results are mixed as to whether prescribing antibiotics affects patient satisfaction. Two studies in the early 2000s found that both patients and parents reported higher satisfaction with physicians who explained why antibiotics were not indicated vs physicians who simply prescribed them, and that such explanations do not need to take a lot of time.37,38 (See TABLE 39,37,38 for patient care tips.)

A more recent study found that higher antibiotic prescribing practices in Britain were associated with modestly higher patient satisfaction ratings.39 The authors of this study noted, however, that reduced antibiotic prescribing may be a proxy for other practice patterns that affected satisfaction ratings.
Reducing antibiotic prescribing reduces resistance
There is also strong evidence that when physicians decrease antibiotic prescribing, antimicrobial resistance follows suit. One of the earlier landmark studies to demonstrate this was a Finnish study published in 1997.40 The authors found that a reduction of macrolide antibiotic consumption in Finland led to a reduction in streptococci macrolide resistance from 16.5% to 8.6%.40
Since then, multiple studies have demonstrated similar results for both respiratory and urinary tract infections.41,42 A 2017 meta-analysis analyzing 32 studies found that antibiotic stewardship programs reduced the incidence of infections and colonization with multidrug-resistant Gram-negative bacteria (51% reduction), extended-spectrum beta-lactamase–producing Gram-negative bacteria (48%), and methicillin-resistant Staphylococcus aureus (37%). There was also a reduction in the incidence of C. difficile infections (32%).43
CORRESPONDENCE
David C. Fiore, MD, Department of Family and Community Medicine, University of Nevada, Reno School of Medicine, Brigham Bldg, MS 316, Reno, NV 89557; [email protected].
1. Bailey LC, Forrest CB, Zhang P, et al. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168:1063-1069.
2. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
3. Gleckman RA, Czachor JS. Antibiotic side effects. Semin Respir Crit Care Med. 2000;21:53-60.
4. Jernberg C, Löfmark S, Edlund C, et al. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156:3216-3223.
5. Logan AC, Jacka FN, Craig JM, et al. The microbiome and mental health: looking back, moving forward with lessons from allergic diseases. Clin Psychopharmacol Neurosci. 2016;14:131-147.
6. Marra F, Marra CA, Richardson K, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics. 2009;123:1003-1010.
7. Harris AM, Hicks LA, Qaseem A, for the High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164:425-434.
8. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
9. Centers for Disease Control and Prevention. Antibiotic prescribing and use. Available at: http://www.cdc.gov/getsmart/. Accessed October 23, 2017.
10. The White House. National action plan for combating antibiotic-resistant bacteria. March 2015:1-63. Available at: https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed October 23, 2017.
11. World Health Organization. Global action plan on antimicrobial resistance. 2015. Available at: http://www.who.int/drugresistance/global_action_plan/en/. Accessed October 23, 2017.
12. Barlam TF, Soria-Saucedo R, Cabral HJ, et al. Unnecessary antibiotics for acute respiratory tract infections: association with care setting and patient demographics. Open Forum Infect Dis. 2016;3:1-7.
13. Hersh AL, Shapiro DJ, Pavia AT, et al. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128:1053-1061.
14. Chow AW, Benninger MS, Brook I, et al. Executive summary: IDSA Clinical Practice Guideline for Acute Bacterial Rhinosinusitis in Children and Adults. Clin Infect Dis. 2012;54:1041-1045.
15. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 Suppl):S1-S39.
16. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279-1282.
17. Shapiro DJ, Hicks LA, Pavia AT, et al. Antibiotic prescribing for adults in ambulatory care in the USA, 2007-09. J Antimicrob Chemother. 2014;69:234-240.
18. Bergmark RW, Sedaghat AR. Antibiotic prescription for acute rhinosinusitis: emergency departments versus primary care providers. Laryngoscope. 2016;(November):1-6.
19. Hersh AL, Fleming-Dutra KE, Shapiro DJ, et al. Frequency of first-line antibiotic selection among US ambulatory care visits for otitis media, sinusitis, and pharyngitis. JAMA Intern Med. 2016;176:1870-1872.
20. Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60:1308-1316.
21. Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8:39.
22. Lessa FC, Gould CV, McDonald CL. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(Suppl 2):S65-S70.
23. Zhang S, Palazuelos-Munoz S, Balsells EM, et al. Cost of hospital management of Clostridium difficile infection in United States—a meta-analysis and modelling study. BMC Infect Dis. 2016;16:447.
24. Pérez-Santiago J, Gianella S, Massanella M, et al. Gut lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27:1921-1931.
25. Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39-50.
26. Bravo JA, Julio-Pieper M, Forsythe P, et al. Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol. 2012;12:667-672.
27. Clemente JC, Ursell LK, Parfrey LW, et al. The impact of the gut microbiota on human health: An integrative view. Cell. 2012;148:1258-1270.
28. Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369-1378.
29. Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305-312.
30. Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014;38:1-12.
31. Riiser A. The human microbiome, asthma, and allergy. Allergy, Asthma, and Clinical Immunology. 2015;11:35.
32. Md Rezal RS, Hassali MA, Alrasheedy AA, et al. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: a systematic review of the literature. Expert Rev Anti Infect Ther. 2015;13:665-680.
33. Dempsey PP, Businger AC, Whaley LE, et al. Primary care clinicians’ perceptions about antibiotic prescribing for acute bronchitis: a qualitative study. BMC Fam Pract. 2014;15:194.
34. Gonzales R, Steiner JF, Lum A, et al. Decreasing antibiotic use in ambulatory practice. JAMA. 1999;281:1512-1519.
35. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315:562-570.
36. Perz JF, Craig AS, Coffey CS, et al. Changes in antibiotic prescribing for children after a community-wide campaign. JAMA. 2002;287:3103-3109.
37. Belongia EA, Sullivan BJ, Chyou PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001;108:575-583.
38. Mangione-Smith R, McGlynn EA, Elliott MN, et al. Parent expectations for antibiotics, physician-parent communication, and satisfaction. Arch Pediatr Adolesc Med. 2001;155:800-806.
39. Ashworth M, White P, Jongsma H,et al. Antibiotic prescribing and patient satisfaction in primary care in England: cross-sectional analysis of national patient survey data and prescribing data. Br J Gen Pract. 2016;66:e40-e46.
40. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med. 1997;337:441-446.
41. Guillemot D, Varon E, Bernède C, et al. Reduction of antibiotic use in the community reduces the rate of colonization with penicillin g–nonsusceptible Streptococcus pneumoniae. Clin Infect Dis. 2005;41:930-938.
42. Butler CC, Dunstan F, Heginbothom M, et al. Containing antibiotic resistance: decreased antibiotic-resistant coliform urinary tract infections with reduction in antibiotic prescribing by general practices. Br J Gen Pract. 2007;57:785-792.
43. Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:990-1001.
1. Bailey LC, Forrest CB, Zhang P, et al. Association of antibiotics in infancy with early childhood obesity. JAMA Pediatr. 2014;168:1063-1069.
2. Costelloe C, Metcalfe C, Lovering A, et al. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096.
3. Gleckman RA, Czachor JS. Antibiotic side effects. Semin Respir Crit Care Med. 2000;21:53-60.
4. Jernberg C, Löfmark S, Edlund C, et al. Long-term impacts of antibiotic exposure on the human intestinal microbiota. Microbiology. 2010;156:3216-3223.
5. Logan AC, Jacka FN, Craig JM, et al. The microbiome and mental health: looking back, moving forward with lessons from allergic diseases. Clin Psychopharmacol Neurosci. 2016;14:131-147.
6. Marra F, Marra CA, Richardson K, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics. 2009;123:1003-1010.
7. Harris AM, Hicks LA, Qaseem A, for the High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med. 2016;164:425-434.
8. Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315:1864-1873.
9. Centers for Disease Control and Prevention. Antibiotic prescribing and use. Available at: http://www.cdc.gov/getsmart/. Accessed October 23, 2017.
10. The White House. National action plan for combating antibiotic-resistant bacteria. March 2015:1-63. Available at: https://obamawhitehouse.archives.gov/sites/default/files/docs/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed October 23, 2017.
11. World Health Organization. Global action plan on antimicrobial resistance. 2015. Available at: http://www.who.int/drugresistance/global_action_plan/en/. Accessed October 23, 2017.
12. Barlam TF, Soria-Saucedo R, Cabral HJ, et al. Unnecessary antibiotics for acute respiratory tract infections: association with care setting and patient demographics. Open Forum Infect Dis. 2016;3:1-7.
13. Hersh AL, Shapiro DJ, Pavia AT, et al. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128:1053-1061.
14. Chow AW, Benninger MS, Brook I, et al. Executive summary: IDSA Clinical Practice Guideline for Acute Bacterial Rhinosinusitis in Children and Adults. Clin Infect Dis. 2012;54:1041-1045.
15. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152(2 Suppl):S1-S39.
16. Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55:1279-1282.
17. Shapiro DJ, Hicks LA, Pavia AT, et al. Antibiotic prescribing for adults in ambulatory care in the USA, 2007-09. J Antimicrob Chemother. 2014;69:234-240.
18. Bergmark RW, Sedaghat AR. Antibiotic prescription for acute rhinosinusitis: emergency departments versus primary care providers. Laryngoscope. 2016;(November):1-6.
19. Hersh AL, Fleming-Dutra KE, Shapiro DJ, et al. Frequency of first-line antibiotic selection among US ambulatory care visits for otitis media, sinusitis, and pharyngitis. JAMA Intern Med. 2016;176:1870-1872.
20. Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60:1308-1316.
21. Langdon A, Crook N, Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8:39.
22. Lessa FC, Gould CV, McDonald CL. Current status of Clostridium difficile infection epidemiology. Clin Infect Dis. 2012;55(Suppl 2):S65-S70.
23. Zhang S, Palazuelos-Munoz S, Balsells EM, et al. Cost of hospital management of Clostridium difficile infection in United States—a meta-analysis and modelling study. BMC Infect Dis. 2016;16:447.
24. Pérez-Santiago J, Gianella S, Massanella M, et al. Gut lactobacillales are associated with higher CD4 and less microbial translocation during HIV infection. AIDS. 2013;27:1921-1931.
25. Maurice CF, Haiser HJ, Turnbaugh PJ. Xenobiotics shape the physiology and gene expression of the active human gut microbiome. Cell. 2013;152:39-50.
26. Bravo JA, Julio-Pieper M, Forsythe P, et al. Communication between gastrointestinal bacteria and the nervous system. Curr Opin Pharmacol. 2012;12:667-672.
27. Clemente JC, Ursell LK, Parfrey LW, et al. The impact of the gut microbiota on human health: An integrative view. Cell. 2012;148:1258-1270.
28. Dinan TG, Cryan JF. Regulation of the stress response by the gut microbiota: implications for psychoneuroendocrinology. Psychoneuroendocrinology. 2012;37:1369-1378.
29. Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36:305-312.
30. Wang Y, Kasper LH. The role of microbiome in central nervous system disorders. Brain Behav Immun. 2014;38:1-12.
31. Riiser A. The human microbiome, asthma, and allergy. Allergy, Asthma, and Clinical Immunology. 2015;11:35.
32. Md Rezal RS, Hassali MA, Alrasheedy AA, et al. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: a systematic review of the literature. Expert Rev Anti Infect Ther. 2015;13:665-680.
33. Dempsey PP, Businger AC, Whaley LE, et al. Primary care clinicians’ perceptions about antibiotic prescribing for acute bronchitis: a qualitative study. BMC Fam Pract. 2014;15:194.
34. Gonzales R, Steiner JF, Lum A, et al. Decreasing antibiotic use in ambulatory practice. JAMA. 1999;281:1512-1519.
35. Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315:562-570.
36. Perz JF, Craig AS, Coffey CS, et al. Changes in antibiotic prescribing for children after a community-wide campaign. JAMA. 2002;287:3103-3109.
37. Belongia EA, Sullivan BJ, Chyou PH, et al. A community intervention trial to promote judicious antibiotic use and reduce penicillin-resistant Streptococcus pneumoniae carriage in children. Pediatrics. 2001;108:575-583.
38. Mangione-Smith R, McGlynn EA, Elliott MN, et al. Parent expectations for antibiotics, physician-parent communication, and satisfaction. Arch Pediatr Adolesc Med. 2001;155:800-806.
39. Ashworth M, White P, Jongsma H,et al. Antibiotic prescribing and patient satisfaction in primary care in England: cross-sectional analysis of national patient survey data and prescribing data. Br J Gen Pract. 2016;66:e40-e46.
40. Seppälä H, Klaukka T, Vuopio-Varkila J, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med. 1997;337:441-446.
41. Guillemot D, Varon E, Bernède C, et al. Reduction of antibiotic use in the community reduces the rate of colonization with penicillin g–nonsusceptible Streptococcus pneumoniae. Clin Infect Dis. 2005;41:930-938.
42. Butler CC, Dunstan F, Heginbothom M, et al. Containing antibiotic resistance: decreased antibiotic-resistant coliform urinary tract infections with reduction in antibiotic prescribing by general practices. Br J Gen Pract. 2007;57:785-792.
43. Baur D, Gladstone BP, Burkert F, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17:990-1001.
PRACTICE RECOMMENDATIONS
› Explain to patients the rationale for not prescribing antibiotics when they are not indicated. A
› Advocate for health care system electronic medical record systems designed to limit antibiotic prescribing to only appropriate cases. A
› Provide patients and your community with educational materials to increase understanding of the risks of antibiotic overprescribing. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The benefits of physician-pharmacist collaboration
Over the past decade, physician-pharmacist collaborative practices have gained traction in primary care as a way to implement team-based-care models. And there is evidence pointing to the effectiveness of this multidisciplinary heath care team approach, in which pharmacists are typically responsible for such things as obtaining medication histories, identifying barriers to adherence, and adjusting medication regimens.
Several studies have shown the significant impact that physician-pharmacist collaborative management (PPCM) can have on blood pressure (BP) control among patients with hypertension (HTN).1-8 Additionally, PPCM may have positive effects on HbA1c reduction and diabetes control,9-11 suggesting that benefits may extend to other chronic diseases, too.
In the review that follows, we’ll detail the impact that PPCM can have on patient care, health-care utilization, and cost effectiveness. (For a look at PPCM “in action,” see the sidebar below.) We’ll also review the challenges of implementing this model that, at present, is mostly found in academically-affiliated clinics and large health systems.
SIDEBAR
The physician-pharmacist collaborative care model in actionFor patients with chronic diseases such as hypertension and diabetes, pharmacists can be invaluable members of multidisciplinary health care teams by providing direct consultation to optimize pharmacotherapy. Although their particular role and responsibilities can vary widely from one primary care setting to the next, the following describes the general workflow of a physician-pharmacist collaborative care model in action.
The patient, 60-year-old Isabel B, arrives for an appointment for pharmacotherapy management of her hypertension. After checking in, a registered nurse (RN), medical assistant (MA), or the pharmacist obtains her vital signs, height, and weight prior to rooming. Additionally, any necessary point-of-care lab tests are obtained at this time.
Once the patient is roomed, the pharmacist collects a thorough medication history from Ms. B, verifying and updating her current medication list, confirming the dose and frequency of each medication, and gathering information regarding adverse effects and barriers to adherence. The pharmacist may also review current laboratory results and vital signs to assess the appropriateness and therapeutic efficacy of the current drug therapy regimen.
Depending upon the collaborative practice plan in place, one of the following steps may occur:
A. The pharmacist makes a change to Ms. B's medication regimen and orders any necessary laboratory tests for monitoring. A progress note is forwarded to Ms. B's primary care provider (PCP) to inform him/her of the changes made to the regimen and the follow-up interval.
B. The pharmacist presents pharmacotherapy recommendations to the attending physician or Ms. B's PCP. The therapeutic and monitoring plans are discussed and approved as a team at the time of Ms. B's visit.
C. The pharmacist sends a message to Ms. B's PCP regarding information discovered during the interview and provides recommendations for a treatment plan based on the visit. The PCP reviews the recommendations, and can either 1) send approval to the pharmacist through a message or 2) implement the appropriate drug therapy changes at Ms. B's next visit.
In Cases A and B, the pharmacist then reviews the final pharmacotherapy plan with Ms. B, discusses the medication and monitoring parameters, answers any questions related to the new treatment regimen, and schedules a follow-up visit. In Case C, the pharmacist may still provide medication counseling and answer questions related to drug therapy during the visit; however, review of the final pharmacotherapy plan may be done over the telephone after approval by the PCP. Alternatively, a follow-up appointment with Ms. B's PCP can be scheduled shortly after the visit with the pharmacist to discuss any recommended drug therapy changes.
PPCM impacts chronic diseases
The current literature is rife with studies investigating the impact of PPCM on chronic diseases in the primary care setting.1-12 Although no specific guidelines on implementing PPCM exist, these studies utilized similar interventions that provided pharmacists with the ability to manage medication therapy under the supervision of a physician. A number of these studies incorporated collaborative practice plans to delineate the specific duties performed by physicians and pharmacists.2,6,8,10,11 Responsibilities for pharmacists often included assessing vital signs, reviewing laboratory parameters and ordering appropriate tests, providing patient education, screening for drug interactions, identifying barriers to medication adherence, and adjusting medication regimens. The TABLE1-12 provides a summary of studies investigating the impact of PPCM in the primary care setting.
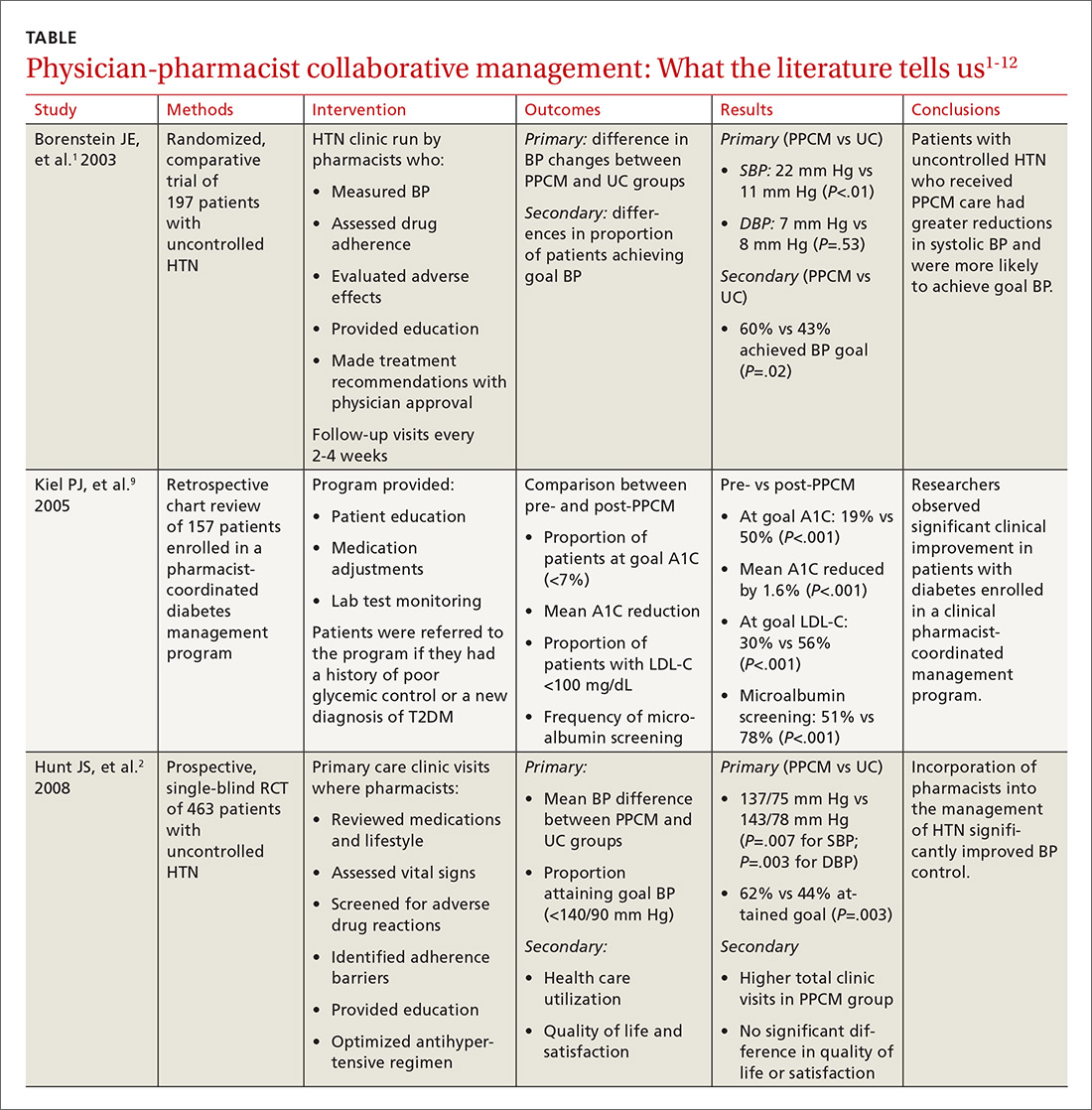
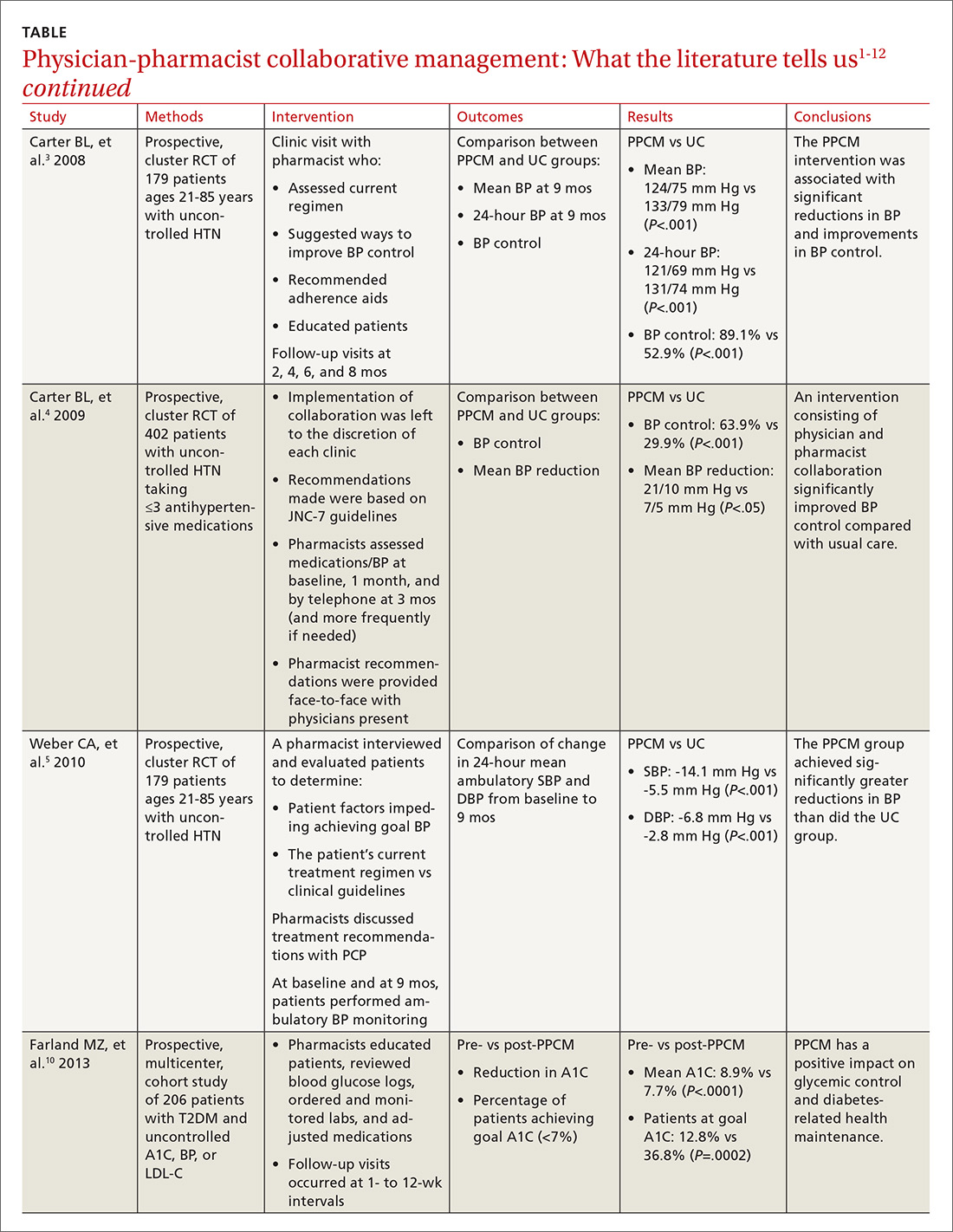
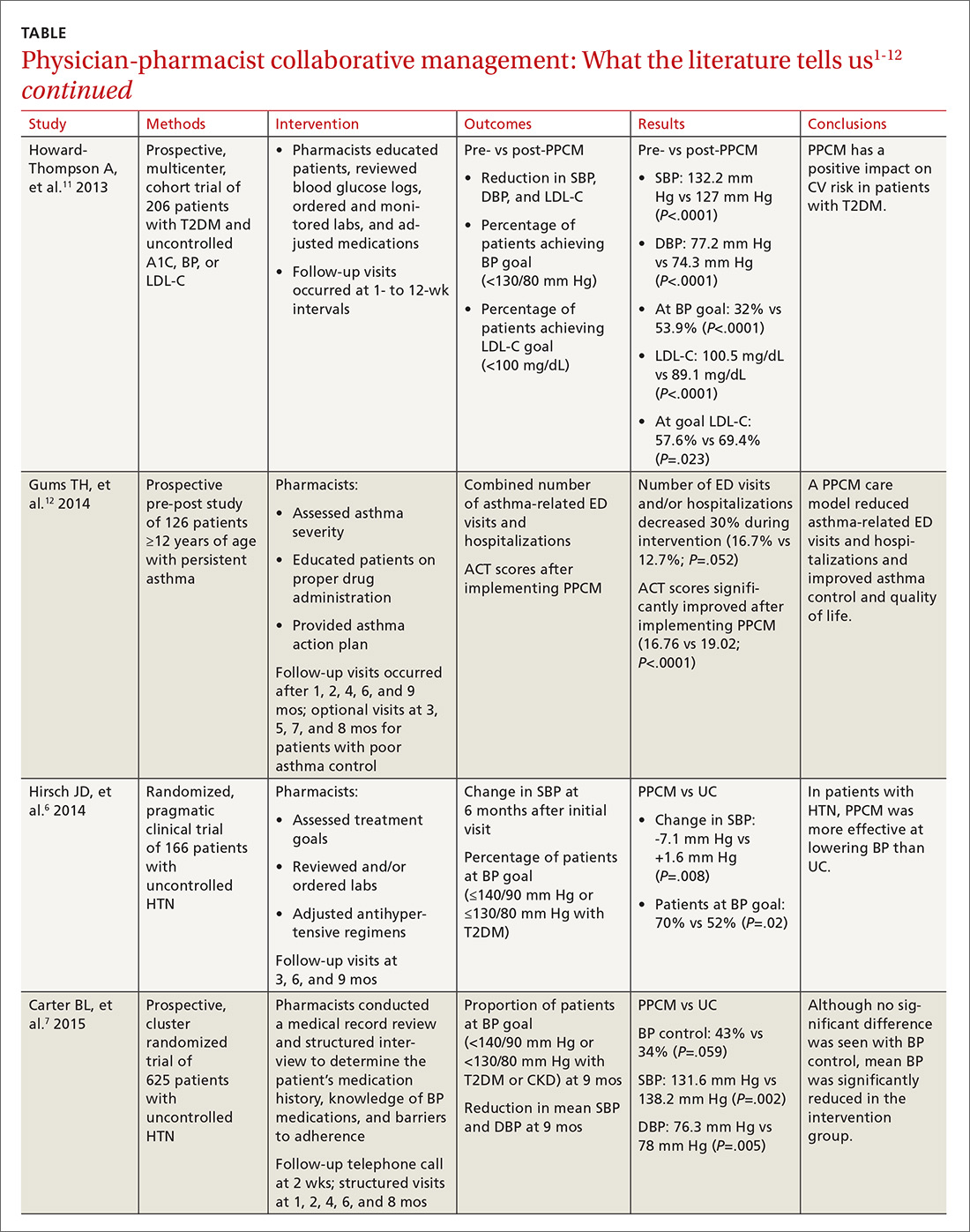
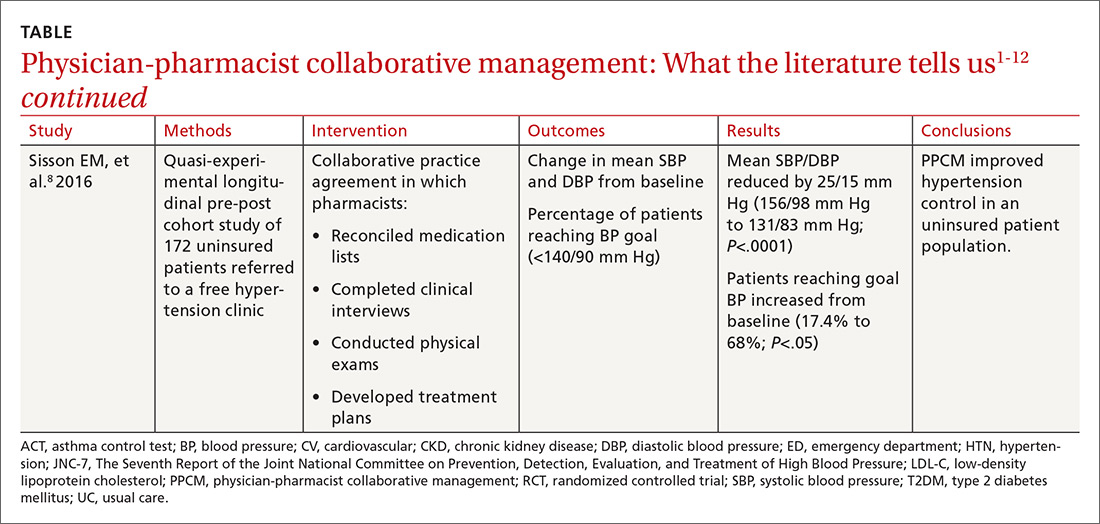
PPCM leads to greater BP reductions, improved BP control
The majority of research surrounding PPCM has focused on uncontrolled HTN.1-8 Patients in many of these studies saw a pharmacist in a specialized HTN clinic, where the multidisciplinary staff performed a thorough evaluation of the patient’s current hypertensive management. The pharmacists in these PPCM programs closely monitored patients and made adjustments to antihypertensive regimens as necessary. Systolic and diastolic BP reductions in the intervention groups ranged from 14 to 36 mm Hg and 7 to 15 mm Hg, respectively.1-5,7,8 The percentage of patients with BP control at the end of the studies ranged from 43% to 89%.1,3,4,6,7
In a prospective, cluster-randomized trial performed at 32 primary care offices in 15 states, researchers assigned 625 patients with uncontrolled HTN to receive physician-pharmacist collaborative care or usual care with primary care provider management.7 As part of the PPCM intervention, clinical pharmacists conducted a thorough medical record review and a structured interview of the patients. During the interview, the clinical pharmacists reviewed the patient’s medication history, assessed the patient’s knowledge of BP medications, and addressed any barriers to adherence. In collaboration with the physician, the pharmacists developed a care plan with recommendations for optimizing the drug regimen. After the baseline visit, the pharmacists conducted structured face-to-face interviews with patients at 1, 2, 4, 6, and 8 months, with additional visits scheduled if BP was still uncontrolled.
At 9 months, patients in the PPCM group had significantly greater reductions in BP than those in the control group, and BP control was achieved in 43% of the PPCM group vs 34% of the control group. This study corroborates results from previous (similar) studies investigating the impact of PPCM on patients with uncontrolled HTN.1-6
PPCM helps patients reduce their HbA1c levels
Researchers have also studied the impact of PPCM strategies on the management of diabetes mellitus.9-11 In one retrospective study of 157 patients, implementation of a pharmacy-coordinated diabetes (any type) management program significantly improved HbA1c and increased the percentage of patients reaching their HbA1c goal.9 Furthermore, researchers observed improvements in low-density lipoprotein cholesterol (LDL-C) levels and an increased number of patients obtaining a microalbumin screening after initiation of the program.
A more recent prospective, multicenter cohort study of 206 patients with uncontrolled type 2 diabetes had similar results.10 In collaboration with the primary care physician (PCP), clinical pharmacists provided medication therapy management through adjustment of antihyperglycemic, antihypertensive, or lipid-lowering medications. Additional interventions provided by the pharmacists included reviewing blood glucose logs, ordering and monitoring laboratory tests, performing sensory foot examinations, and providing patient education.
Implementation of PPCM reduced the average HbA1c by 1.2% and increased the percentage of patients achieving an HbA1c <7% by about 24%. The researchers also observed improvements in BP and LDL-C levels in this patient population.11
Asthma and beyond
Future studies may well show that the benefits of PPCM extend to the management of other chronic diseases. One prospective, pre-post study of 126 patients with asthma found that the number of emergency department (ED) visits and/or hospitalizations decreased 30% during 9 months with a PPCM intervention and then returned to levels similar to baseline once the intervention ceased.12 Other potential disease areas that have been studied, or are being studied, include chronic obstructive pulmonary disease, chronic kidney disease, dyslipidemia, and congestive heart failure.13
Benefits derive from altered health care utilization
Researchers attribute much of the benefit observed with PPCM to the increased—albeit different—health-care utilization among the patients in the intervention groups. In general, patients participating in PPCM have an increased total number of visits, but more of those visits are with pharmacists and fewer are with physicians; they also are prescribed more medications, but don’t necessarily take more pills per day.1,2,5 In the end, patients have been found to achieve significantly better disease control without compromising quality of life or satisfaction.2
Some studies have found that continued pharmacist involvement may be necessary to sustain the benefits achieved.6 However, other studies have suggested that the benefits are maintained even after discontinuation of the pharmacist intervention.14,15 Thus, further research is necessary to determine which patients may benefit most from ongoing involvement with a pharmacist.
How cost-effective is the PPCM model?
Implementing a PPCM model in a primary care setting often hinges upon whether the intervention will be cost-effective. Several studies have reported the cost-effectiveness of clinical pharmacists in the management of HTN.1,16,17
Borenstein and colleagues found significantly lower provider visit costs per patient in the PPCM group ($160) compared with the usual care group ($195), a difference that the authors attributed to a decreased number of visits to PCPs and an increased number of lower cost visits with pharmacists in the PPCM group.1 However, the difference could have been affected by the arbitrary measurement of physician-pharmacist collaboration time in the study.
Overcoming implementation challenges
Implementation of pharmacist collaboration within primary care medicine may pose a challenge, as the requirements and resources vary widely among primary care settings. Health-system administrators, for example, may need to reorganize the clinic structure and budget resources in order to overcome some of the obstacles to implementing a PPCM model.
Experts have reported several strategies that help in establishing PPCM within primary care clinics,18 including proactively identifying patients who may benefit from pharmacist intervention, requiring appropriate training and credentialing of pharmacists, and establishing a set schedule for pharmacists to interview patients. Clinics would also be well served to model interventions outlined in the studies mentioned in this article and provide adequate time for pharmacists to perform structured activities, including review of medication history, assessment of current disease state control, and adjustment of medication therapy regimens. And, of course, given the diversity of primary care settings, administrators will need to identify the specific PPCM strategies that best complement their respective collaborative practice plans and environments.
The lack of well-defined reimbursement models for pharmacy services has presented a challenge for generating revenue and effectively implementing PPCM within many primary care settings. Currently, the Centers for Medicare and Medicaid Services and third-party payers do not recognize pharmacists as independent providers, creating a barrier for obtaining reimbursement for clinical pharmacy services. Typically, pharmacists have charged for clinic visits under a consultant physician through the “incident to” billing model, with the option to bill at higher levels if the patient was seen jointly with the physician.
Can this model benefit the underserved?
A prospective, cluster-randomized clinic study has shown pharmacist intervention to reduce racial and socioeconomic disparities in the treatment of elevated BP.19 This study is the first to show that a team-care model can overcome inequalities arising from low income, low patient education status, and little or no insurance to produce the same health care benefit as in those with higher socioeconomic and educational status. This type of collaborative care model may be particularly beneficial when incorporated within a PCMH catering to underserved populations.20
However, sparse data currently exist regarding the benefits of the PPCM model within a PCMH, despite the fact that integration of this type of collaborative model is expected to contribute positively to patient care.21
Physician acceptance of pharmacist involvement is mixed
While physician acceptance of pharmacist recommendations is generally high, at least one study indicated that some health-care professionals in patient-care teams are reluctant to incorporate pharmacists into a PCMH. Reasons include difficulty in coordination of care with pharmacy services and limited knowledge by other professionals of pharmacists’ training.22
Centralization can combat a lack of resources
As noted earlier, primary care offices that implement PPCM models are mostly academically affiliated or are part of large health systems. Many private primary care offices lack the resources to employ a pharmacist in their office. As an alternative, prospective clinical trials are looking at a centralized, Web-based cardiovascular risk service managed by pharmacists.23,24 This service’s primary objective is to improve adherence to metric-based outcomes developed as part of The Guideline Advantage quality improvement program put forth by the American Cancer Society, American Diabetes Association, and the American Heart and Stroke Associations. (See http://www.guidelineadvantage.org/TGA/ for more information.)
Researchers hope to prove that a centralized, pharmacist-run, clinical service can meet metric-driven outcomes that many primary care offices are now being required to meet in order to receive compensation from insurance companies. One of these studies is specifically looking at rural private offices that lack many of the resources that many large academic offices possess.23 The study is ongoing and results are expected sometime in 2018.
CORRESPONDENCE
John G. Gums, PharmD, College of Pharmacy, University of Florida, 1225 Center Drive, HPNP 4332, Gainesville, FL 32601; [email protected].
1. Borenstein JE, Graber G, Saltiel E, et al. Physician-pharmacist comanagement of hypertension: a randomized, comparative trial. Pharmacotherapy. 2003;23:209-216.
2. Hunt JS, Siemienczuk J, Pape G, et al. A randomized controlled trial of team-based care: impact of physician-pharmacist collaboration on uncontrolled hypertension. J Gen Intern Med. 2008;23:1966-1972.
3. Carter BL, Bergus GR, Dawson JD, et al. A cluster randomized trial to evaluate physician/pharmacist collaboration to improve blood pressure control. J Clin Hypertens (Greenwich). 2008;10:260-271.
4. Carter BL, Ardery G, Dawson JD, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009;169:1996-2002.
5. Weber CA, Ernst ME, Sezate GS, et al. Pharmacist-physician comanagement of hypertension and reduction in 24-hour ambulatory blood pressures. Arch Intern Med. 2010;170:1634-1639.
6. Hirsch JD, Steers N, Adler DS, et al. Primary care-based, pharmacist-physician collaborative medication-therapy management of hypertension: a randomized, pragmatic trial. Clin Ther. 2014;36:1244-1254.
7. Carter BL, Coffey CS, Ardery G, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8:235-243.
8. Sisson EM, Dixon DL, Kildow DC, et al. Effectiveness of a pharmacist-physician team-based collaboration to improve long-term blood pressure control at an inner-city safety-net clinic. Pharmacotherapy. 2016;36:342-347.
9. Kiel PJ, McCord AD. Pharmacist impact on clinical outcomes in a diabetes disease management program via collaborative practice. Ann Pharmacother. 2005;39:1828-1832.
10. Farland MZ, Byrd DC, McFarland MS, et al. Pharmacist-physician collaboration for diabetes care: the diabetes initiative program. Ann Pharmacother. 2013;47:781-789.
11. Howard-Thompson A, Farland MZ, Byrd DC, et al. Pharmacist-physician collaboration for diabetes care: cardiovascular outcomes. Ann Pharmacother. 2013;47:1471-1477.
12. Gums TH, Carter BL, Milavetz G, et al. Physician-pharmacist collaborative management of asthma in primary care. Pharmacotherapy. 2014;34:1033-1042.
13. Greer N, Bolduc J, Geurkink E, et al. VA Evidence-based Synthesis Program Reports. Pharmacist-Led Chronic Disease Management: A Systematic Review of Effectiveness and Harms Compared to Usual Care. Washington (DC): Department of Veterans Affairs (US); 2015.
14. Wentzlaff DM, Carter BL, Ardery G, et al. Sustained blood pressure control following discontinuation of a pharmacist intervention. J Clin Hypertens (Greenwich). 2011;13:431-437.
15. Carter BL, Vander Weg MW, Parker CP, et al. Sustained blood pressure control following discontinuation of a pharmacist intervention for veterans. J Clin Hypertens (Greenwich). 2015;17:701-708.
16. Kulchaitanaroaj P, Brooks JM, Ardery G, et al. Incremental costs associated with physician and pharmacist collaboration to improve blood pressure control. Pharmacotherapy. 2012;32:772-780.
17. Polgreen LA, Han J, Carter BL, et al. Cost effectiveness of a physician-pharmacist collaboration intervention to improve blood pressure control. Hypertension. 2015;66:1145-1151.
18. Carter BL. Primary care physician-pharmacist collaborative care model: strategies for implementation. Pharmacotherapy. 2016;36:363-373.
19. Anderegg MD, Gums TH, Uribe L, et al. Physician-pharmacist collaborative management: narrowing the socioeconomic blood pressure gap. Hypertension. 2016;68:1314-1320.
20. Moczygemba LR, Goode JV, Gatewood SBS, et al. Integration of collaborative medication therapy management in a safety net patient-centered medical home. J Am Pharm Assoc (2003). 2011;51:167-172.
21. Scott MA, Hitch B, Ray L, et al. Integration of pharmacists into a patient-centered medical home. J Am Pharm Assoc (2003). 2011;51:161-166.
22. Patterson BJ, Solimeo SL, Stewart KR, et al. Perceptions of pharmacists’ integration into patient-centered medical home teams. Res Social Adm Pharm. 2015;11:85-95.
23. Carter BL, Levy BT, Gryzlak B, et al. A centralized cardiovascular risk service to improve guideline adherence in private primary care offices. Contemp Clin Trials. 2015;43:25-32.
24. Carter BL, Coffey CS, Chrischilles EA, et al. A cluster-randomized trial of a centralized clinical pharmacy cardiovascular risk service to improve guideline adherence. Pharmacotherapy. 2015;35:653-662.
Over the past decade, physician-pharmacist collaborative practices have gained traction in primary care as a way to implement team-based-care models. And there is evidence pointing to the effectiveness of this multidisciplinary heath care team approach, in which pharmacists are typically responsible for such things as obtaining medication histories, identifying barriers to adherence, and adjusting medication regimens.
Several studies have shown the significant impact that physician-pharmacist collaborative management (PPCM) can have on blood pressure (BP) control among patients with hypertension (HTN).1-8 Additionally, PPCM may have positive effects on HbA1c reduction and diabetes control,9-11 suggesting that benefits may extend to other chronic diseases, too.
In the review that follows, we’ll detail the impact that PPCM can have on patient care, health-care utilization, and cost effectiveness. (For a look at PPCM “in action,” see the sidebar below.) We’ll also review the challenges of implementing this model that, at present, is mostly found in academically-affiliated clinics and large health systems.
SIDEBAR
The physician-pharmacist collaborative care model in actionFor patients with chronic diseases such as hypertension and diabetes, pharmacists can be invaluable members of multidisciplinary health care teams by providing direct consultation to optimize pharmacotherapy. Although their particular role and responsibilities can vary widely from one primary care setting to the next, the following describes the general workflow of a physician-pharmacist collaborative care model in action.
The patient, 60-year-old Isabel B, arrives for an appointment for pharmacotherapy management of her hypertension. After checking in, a registered nurse (RN), medical assistant (MA), or the pharmacist obtains her vital signs, height, and weight prior to rooming. Additionally, any necessary point-of-care lab tests are obtained at this time.
Once the patient is roomed, the pharmacist collects a thorough medication history from Ms. B, verifying and updating her current medication list, confirming the dose and frequency of each medication, and gathering information regarding adverse effects and barriers to adherence. The pharmacist may also review current laboratory results and vital signs to assess the appropriateness and therapeutic efficacy of the current drug therapy regimen.
Depending upon the collaborative practice plan in place, one of the following steps may occur:
A. The pharmacist makes a change to Ms. B's medication regimen and orders any necessary laboratory tests for monitoring. A progress note is forwarded to Ms. B's primary care provider (PCP) to inform him/her of the changes made to the regimen and the follow-up interval.
B. The pharmacist presents pharmacotherapy recommendations to the attending physician or Ms. B's PCP. The therapeutic and monitoring plans are discussed and approved as a team at the time of Ms. B's visit.
C. The pharmacist sends a message to Ms. B's PCP regarding information discovered during the interview and provides recommendations for a treatment plan based on the visit. The PCP reviews the recommendations, and can either 1) send approval to the pharmacist through a message or 2) implement the appropriate drug therapy changes at Ms. B's next visit.
In Cases A and B, the pharmacist then reviews the final pharmacotherapy plan with Ms. B, discusses the medication and monitoring parameters, answers any questions related to the new treatment regimen, and schedules a follow-up visit. In Case C, the pharmacist may still provide medication counseling and answer questions related to drug therapy during the visit; however, review of the final pharmacotherapy plan may be done over the telephone after approval by the PCP. Alternatively, a follow-up appointment with Ms. B's PCP can be scheduled shortly after the visit with the pharmacist to discuss any recommended drug therapy changes.
PPCM impacts chronic diseases
The current literature is rife with studies investigating the impact of PPCM on chronic diseases in the primary care setting.1-12 Although no specific guidelines on implementing PPCM exist, these studies utilized similar interventions that provided pharmacists with the ability to manage medication therapy under the supervision of a physician. A number of these studies incorporated collaborative practice plans to delineate the specific duties performed by physicians and pharmacists.2,6,8,10,11 Responsibilities for pharmacists often included assessing vital signs, reviewing laboratory parameters and ordering appropriate tests, providing patient education, screening for drug interactions, identifying barriers to medication adherence, and adjusting medication regimens. The TABLE1-12 provides a summary of studies investigating the impact of PPCM in the primary care setting.




PPCM leads to greater BP reductions, improved BP control
The majority of research surrounding PPCM has focused on uncontrolled HTN.1-8 Patients in many of these studies saw a pharmacist in a specialized HTN clinic, where the multidisciplinary staff performed a thorough evaluation of the patient’s current hypertensive management. The pharmacists in these PPCM programs closely monitored patients and made adjustments to antihypertensive regimens as necessary. Systolic and diastolic BP reductions in the intervention groups ranged from 14 to 36 mm Hg and 7 to 15 mm Hg, respectively.1-5,7,8 The percentage of patients with BP control at the end of the studies ranged from 43% to 89%.1,3,4,6,7
In a prospective, cluster-randomized trial performed at 32 primary care offices in 15 states, researchers assigned 625 patients with uncontrolled HTN to receive physician-pharmacist collaborative care or usual care with primary care provider management.7 As part of the PPCM intervention, clinical pharmacists conducted a thorough medical record review and a structured interview of the patients. During the interview, the clinical pharmacists reviewed the patient’s medication history, assessed the patient’s knowledge of BP medications, and addressed any barriers to adherence. In collaboration with the physician, the pharmacists developed a care plan with recommendations for optimizing the drug regimen. After the baseline visit, the pharmacists conducted structured face-to-face interviews with patients at 1, 2, 4, 6, and 8 months, with additional visits scheduled if BP was still uncontrolled.
At 9 months, patients in the PPCM group had significantly greater reductions in BP than those in the control group, and BP control was achieved in 43% of the PPCM group vs 34% of the control group. This study corroborates results from previous (similar) studies investigating the impact of PPCM on patients with uncontrolled HTN.1-6
PPCM helps patients reduce their HbA1c levels
Researchers have also studied the impact of PPCM strategies on the management of diabetes mellitus.9-11 In one retrospective study of 157 patients, implementation of a pharmacy-coordinated diabetes (any type) management program significantly improved HbA1c and increased the percentage of patients reaching their HbA1c goal.9 Furthermore, researchers observed improvements in low-density lipoprotein cholesterol (LDL-C) levels and an increased number of patients obtaining a microalbumin screening after initiation of the program.
A more recent prospective, multicenter cohort study of 206 patients with uncontrolled type 2 diabetes had similar results.10 In collaboration with the primary care physician (PCP), clinical pharmacists provided medication therapy management through adjustment of antihyperglycemic, antihypertensive, or lipid-lowering medications. Additional interventions provided by the pharmacists included reviewing blood glucose logs, ordering and monitoring laboratory tests, performing sensory foot examinations, and providing patient education.
Implementation of PPCM reduced the average HbA1c by 1.2% and increased the percentage of patients achieving an HbA1c <7% by about 24%. The researchers also observed improvements in BP and LDL-C levels in this patient population.11
Asthma and beyond
Future studies may well show that the benefits of PPCM extend to the management of other chronic diseases. One prospective, pre-post study of 126 patients with asthma found that the number of emergency department (ED) visits and/or hospitalizations decreased 30% during 9 months with a PPCM intervention and then returned to levels similar to baseline once the intervention ceased.12 Other potential disease areas that have been studied, or are being studied, include chronic obstructive pulmonary disease, chronic kidney disease, dyslipidemia, and congestive heart failure.13
Benefits derive from altered health care utilization
Researchers attribute much of the benefit observed with PPCM to the increased—albeit different—health-care utilization among the patients in the intervention groups. In general, patients participating in PPCM have an increased total number of visits, but more of those visits are with pharmacists and fewer are with physicians; they also are prescribed more medications, but don’t necessarily take more pills per day.1,2,5 In the end, patients have been found to achieve significantly better disease control without compromising quality of life or satisfaction.2
Some studies have found that continued pharmacist involvement may be necessary to sustain the benefits achieved.6 However, other studies have suggested that the benefits are maintained even after discontinuation of the pharmacist intervention.14,15 Thus, further research is necessary to determine which patients may benefit most from ongoing involvement with a pharmacist.
How cost-effective is the PPCM model?
Implementing a PPCM model in a primary care setting often hinges upon whether the intervention will be cost-effective. Several studies have reported the cost-effectiveness of clinical pharmacists in the management of HTN.1,16,17
Borenstein and colleagues found significantly lower provider visit costs per patient in the PPCM group ($160) compared with the usual care group ($195), a difference that the authors attributed to a decreased number of visits to PCPs and an increased number of lower cost visits with pharmacists in the PPCM group.1 However, the difference could have been affected by the arbitrary measurement of physician-pharmacist collaboration time in the study.
Overcoming implementation challenges
Implementation of pharmacist collaboration within primary care medicine may pose a challenge, as the requirements and resources vary widely among primary care settings. Health-system administrators, for example, may need to reorganize the clinic structure and budget resources in order to overcome some of the obstacles to implementing a PPCM model.
Experts have reported several strategies that help in establishing PPCM within primary care clinics,18 including proactively identifying patients who may benefit from pharmacist intervention, requiring appropriate training and credentialing of pharmacists, and establishing a set schedule for pharmacists to interview patients. Clinics would also be well served to model interventions outlined in the studies mentioned in this article and provide adequate time for pharmacists to perform structured activities, including review of medication history, assessment of current disease state control, and adjustment of medication therapy regimens. And, of course, given the diversity of primary care settings, administrators will need to identify the specific PPCM strategies that best complement their respective collaborative practice plans and environments.
The lack of well-defined reimbursement models for pharmacy services has presented a challenge for generating revenue and effectively implementing PPCM within many primary care settings. Currently, the Centers for Medicare and Medicaid Services and third-party payers do not recognize pharmacists as independent providers, creating a barrier for obtaining reimbursement for clinical pharmacy services. Typically, pharmacists have charged for clinic visits under a consultant physician through the “incident to” billing model, with the option to bill at higher levels if the patient was seen jointly with the physician.
Can this model benefit the underserved?
A prospective, cluster-randomized clinic study has shown pharmacist intervention to reduce racial and socioeconomic disparities in the treatment of elevated BP.19 This study is the first to show that a team-care model can overcome inequalities arising from low income, low patient education status, and little or no insurance to produce the same health care benefit as in those with higher socioeconomic and educational status. This type of collaborative care model may be particularly beneficial when incorporated within a PCMH catering to underserved populations.20
However, sparse data currently exist regarding the benefits of the PPCM model within a PCMH, despite the fact that integration of this type of collaborative model is expected to contribute positively to patient care.21
Physician acceptance of pharmacist involvement is mixed
While physician acceptance of pharmacist recommendations is generally high, at least one study indicated that some health-care professionals in patient-care teams are reluctant to incorporate pharmacists into a PCMH. Reasons include difficulty in coordination of care with pharmacy services and limited knowledge by other professionals of pharmacists’ training.22
Centralization can combat a lack of resources
As noted earlier, primary care offices that implement PPCM models are mostly academically affiliated or are part of large health systems. Many private primary care offices lack the resources to employ a pharmacist in their office. As an alternative, prospective clinical trials are looking at a centralized, Web-based cardiovascular risk service managed by pharmacists.23,24 This service’s primary objective is to improve adherence to metric-based outcomes developed as part of The Guideline Advantage quality improvement program put forth by the American Cancer Society, American Diabetes Association, and the American Heart and Stroke Associations. (See http://www.guidelineadvantage.org/TGA/ for more information.)
Researchers hope to prove that a centralized, pharmacist-run, clinical service can meet metric-driven outcomes that many primary care offices are now being required to meet in order to receive compensation from insurance companies. One of these studies is specifically looking at rural private offices that lack many of the resources that many large academic offices possess.23 The study is ongoing and results are expected sometime in 2018.
CORRESPONDENCE
John G. Gums, PharmD, College of Pharmacy, University of Florida, 1225 Center Drive, HPNP 4332, Gainesville, FL 32601; [email protected].
Over the past decade, physician-pharmacist collaborative practices have gained traction in primary care as a way to implement team-based-care models. And there is evidence pointing to the effectiveness of this multidisciplinary heath care team approach, in which pharmacists are typically responsible for such things as obtaining medication histories, identifying barriers to adherence, and adjusting medication regimens.
Several studies have shown the significant impact that physician-pharmacist collaborative management (PPCM) can have on blood pressure (BP) control among patients with hypertension (HTN).1-8 Additionally, PPCM may have positive effects on HbA1c reduction and diabetes control,9-11 suggesting that benefits may extend to other chronic diseases, too.
In the review that follows, we’ll detail the impact that PPCM can have on patient care, health-care utilization, and cost effectiveness. (For a look at PPCM “in action,” see the sidebar below.) We’ll also review the challenges of implementing this model that, at present, is mostly found in academically-affiliated clinics and large health systems.
SIDEBAR
The physician-pharmacist collaborative care model in actionFor patients with chronic diseases such as hypertension and diabetes, pharmacists can be invaluable members of multidisciplinary health care teams by providing direct consultation to optimize pharmacotherapy. Although their particular role and responsibilities can vary widely from one primary care setting to the next, the following describes the general workflow of a physician-pharmacist collaborative care model in action.
The patient, 60-year-old Isabel B, arrives for an appointment for pharmacotherapy management of her hypertension. After checking in, a registered nurse (RN), medical assistant (MA), or the pharmacist obtains her vital signs, height, and weight prior to rooming. Additionally, any necessary point-of-care lab tests are obtained at this time.
Once the patient is roomed, the pharmacist collects a thorough medication history from Ms. B, verifying and updating her current medication list, confirming the dose and frequency of each medication, and gathering information regarding adverse effects and barriers to adherence. The pharmacist may also review current laboratory results and vital signs to assess the appropriateness and therapeutic efficacy of the current drug therapy regimen.
Depending upon the collaborative practice plan in place, one of the following steps may occur:
A. The pharmacist makes a change to Ms. B's medication regimen and orders any necessary laboratory tests for monitoring. A progress note is forwarded to Ms. B's primary care provider (PCP) to inform him/her of the changes made to the regimen and the follow-up interval.
B. The pharmacist presents pharmacotherapy recommendations to the attending physician or Ms. B's PCP. The therapeutic and monitoring plans are discussed and approved as a team at the time of Ms. B's visit.
C. The pharmacist sends a message to Ms. B's PCP regarding information discovered during the interview and provides recommendations for a treatment plan based on the visit. The PCP reviews the recommendations, and can either 1) send approval to the pharmacist through a message or 2) implement the appropriate drug therapy changes at Ms. B's next visit.
In Cases A and B, the pharmacist then reviews the final pharmacotherapy plan with Ms. B, discusses the medication and monitoring parameters, answers any questions related to the new treatment regimen, and schedules a follow-up visit. In Case C, the pharmacist may still provide medication counseling and answer questions related to drug therapy during the visit; however, review of the final pharmacotherapy plan may be done over the telephone after approval by the PCP. Alternatively, a follow-up appointment with Ms. B's PCP can be scheduled shortly after the visit with the pharmacist to discuss any recommended drug therapy changes.
PPCM impacts chronic diseases
The current literature is rife with studies investigating the impact of PPCM on chronic diseases in the primary care setting.1-12 Although no specific guidelines on implementing PPCM exist, these studies utilized similar interventions that provided pharmacists with the ability to manage medication therapy under the supervision of a physician. A number of these studies incorporated collaborative practice plans to delineate the specific duties performed by physicians and pharmacists.2,6,8,10,11 Responsibilities for pharmacists often included assessing vital signs, reviewing laboratory parameters and ordering appropriate tests, providing patient education, screening for drug interactions, identifying barriers to medication adherence, and adjusting medication regimens. The TABLE1-12 provides a summary of studies investigating the impact of PPCM in the primary care setting.




PPCM leads to greater BP reductions, improved BP control
The majority of research surrounding PPCM has focused on uncontrolled HTN.1-8 Patients in many of these studies saw a pharmacist in a specialized HTN clinic, where the multidisciplinary staff performed a thorough evaluation of the patient’s current hypertensive management. The pharmacists in these PPCM programs closely monitored patients and made adjustments to antihypertensive regimens as necessary. Systolic and diastolic BP reductions in the intervention groups ranged from 14 to 36 mm Hg and 7 to 15 mm Hg, respectively.1-5,7,8 The percentage of patients with BP control at the end of the studies ranged from 43% to 89%.1,3,4,6,7
In a prospective, cluster-randomized trial performed at 32 primary care offices in 15 states, researchers assigned 625 patients with uncontrolled HTN to receive physician-pharmacist collaborative care or usual care with primary care provider management.7 As part of the PPCM intervention, clinical pharmacists conducted a thorough medical record review and a structured interview of the patients. During the interview, the clinical pharmacists reviewed the patient’s medication history, assessed the patient’s knowledge of BP medications, and addressed any barriers to adherence. In collaboration with the physician, the pharmacists developed a care plan with recommendations for optimizing the drug regimen. After the baseline visit, the pharmacists conducted structured face-to-face interviews with patients at 1, 2, 4, 6, and 8 months, with additional visits scheduled if BP was still uncontrolled.
At 9 months, patients in the PPCM group had significantly greater reductions in BP than those in the control group, and BP control was achieved in 43% of the PPCM group vs 34% of the control group. This study corroborates results from previous (similar) studies investigating the impact of PPCM on patients with uncontrolled HTN.1-6
PPCM helps patients reduce their HbA1c levels
Researchers have also studied the impact of PPCM strategies on the management of diabetes mellitus.9-11 In one retrospective study of 157 patients, implementation of a pharmacy-coordinated diabetes (any type) management program significantly improved HbA1c and increased the percentage of patients reaching their HbA1c goal.9 Furthermore, researchers observed improvements in low-density lipoprotein cholesterol (LDL-C) levels and an increased number of patients obtaining a microalbumin screening after initiation of the program.
A more recent prospective, multicenter cohort study of 206 patients with uncontrolled type 2 diabetes had similar results.10 In collaboration with the primary care physician (PCP), clinical pharmacists provided medication therapy management through adjustment of antihyperglycemic, antihypertensive, or lipid-lowering medications. Additional interventions provided by the pharmacists included reviewing blood glucose logs, ordering and monitoring laboratory tests, performing sensory foot examinations, and providing patient education.
Implementation of PPCM reduced the average HbA1c by 1.2% and increased the percentage of patients achieving an HbA1c <7% by about 24%. The researchers also observed improvements in BP and LDL-C levels in this patient population.11
Asthma and beyond
Future studies may well show that the benefits of PPCM extend to the management of other chronic diseases. One prospective, pre-post study of 126 patients with asthma found that the number of emergency department (ED) visits and/or hospitalizations decreased 30% during 9 months with a PPCM intervention and then returned to levels similar to baseline once the intervention ceased.12 Other potential disease areas that have been studied, or are being studied, include chronic obstructive pulmonary disease, chronic kidney disease, dyslipidemia, and congestive heart failure.13
Benefits derive from altered health care utilization
Researchers attribute much of the benefit observed with PPCM to the increased—albeit different—health-care utilization among the patients in the intervention groups. In general, patients participating in PPCM have an increased total number of visits, but more of those visits are with pharmacists and fewer are with physicians; they also are prescribed more medications, but don’t necessarily take more pills per day.1,2,5 In the end, patients have been found to achieve significantly better disease control without compromising quality of life or satisfaction.2
Some studies have found that continued pharmacist involvement may be necessary to sustain the benefits achieved.6 However, other studies have suggested that the benefits are maintained even after discontinuation of the pharmacist intervention.14,15 Thus, further research is necessary to determine which patients may benefit most from ongoing involvement with a pharmacist.
How cost-effective is the PPCM model?
Implementing a PPCM model in a primary care setting often hinges upon whether the intervention will be cost-effective. Several studies have reported the cost-effectiveness of clinical pharmacists in the management of HTN.1,16,17
Borenstein and colleagues found significantly lower provider visit costs per patient in the PPCM group ($160) compared with the usual care group ($195), a difference that the authors attributed to a decreased number of visits to PCPs and an increased number of lower cost visits with pharmacists in the PPCM group.1 However, the difference could have been affected by the arbitrary measurement of physician-pharmacist collaboration time in the study.
Overcoming implementation challenges
Implementation of pharmacist collaboration within primary care medicine may pose a challenge, as the requirements and resources vary widely among primary care settings. Health-system administrators, for example, may need to reorganize the clinic structure and budget resources in order to overcome some of the obstacles to implementing a PPCM model.
Experts have reported several strategies that help in establishing PPCM within primary care clinics,18 including proactively identifying patients who may benefit from pharmacist intervention, requiring appropriate training and credentialing of pharmacists, and establishing a set schedule for pharmacists to interview patients. Clinics would also be well served to model interventions outlined in the studies mentioned in this article and provide adequate time for pharmacists to perform structured activities, including review of medication history, assessment of current disease state control, and adjustment of medication therapy regimens. And, of course, given the diversity of primary care settings, administrators will need to identify the specific PPCM strategies that best complement their respective collaborative practice plans and environments.
The lack of well-defined reimbursement models for pharmacy services has presented a challenge for generating revenue and effectively implementing PPCM within many primary care settings. Currently, the Centers for Medicare and Medicaid Services and third-party payers do not recognize pharmacists as independent providers, creating a barrier for obtaining reimbursement for clinical pharmacy services. Typically, pharmacists have charged for clinic visits under a consultant physician through the “incident to” billing model, with the option to bill at higher levels if the patient was seen jointly with the physician.
Can this model benefit the underserved?
A prospective, cluster-randomized clinic study has shown pharmacist intervention to reduce racial and socioeconomic disparities in the treatment of elevated BP.19 This study is the first to show that a team-care model can overcome inequalities arising from low income, low patient education status, and little or no insurance to produce the same health care benefit as in those with higher socioeconomic and educational status. This type of collaborative care model may be particularly beneficial when incorporated within a PCMH catering to underserved populations.20
However, sparse data currently exist regarding the benefits of the PPCM model within a PCMH, despite the fact that integration of this type of collaborative model is expected to contribute positively to patient care.21
Physician acceptance of pharmacist involvement is mixed
While physician acceptance of pharmacist recommendations is generally high, at least one study indicated that some health-care professionals in patient-care teams are reluctant to incorporate pharmacists into a PCMH. Reasons include difficulty in coordination of care with pharmacy services and limited knowledge by other professionals of pharmacists’ training.22
Centralization can combat a lack of resources
As noted earlier, primary care offices that implement PPCM models are mostly academically affiliated or are part of large health systems. Many private primary care offices lack the resources to employ a pharmacist in their office. As an alternative, prospective clinical trials are looking at a centralized, Web-based cardiovascular risk service managed by pharmacists.23,24 This service’s primary objective is to improve adherence to metric-based outcomes developed as part of The Guideline Advantage quality improvement program put forth by the American Cancer Society, American Diabetes Association, and the American Heart and Stroke Associations. (See http://www.guidelineadvantage.org/TGA/ for more information.)
Researchers hope to prove that a centralized, pharmacist-run, clinical service can meet metric-driven outcomes that many primary care offices are now being required to meet in order to receive compensation from insurance companies. One of these studies is specifically looking at rural private offices that lack many of the resources that many large academic offices possess.23 The study is ongoing and results are expected sometime in 2018.
CORRESPONDENCE
John G. Gums, PharmD, College of Pharmacy, University of Florida, 1225 Center Drive, HPNP 4332, Gainesville, FL 32601; [email protected].
1. Borenstein JE, Graber G, Saltiel E, et al. Physician-pharmacist comanagement of hypertension: a randomized, comparative trial. Pharmacotherapy. 2003;23:209-216.
2. Hunt JS, Siemienczuk J, Pape G, et al. A randomized controlled trial of team-based care: impact of physician-pharmacist collaboration on uncontrolled hypertension. J Gen Intern Med. 2008;23:1966-1972.
3. Carter BL, Bergus GR, Dawson JD, et al. A cluster randomized trial to evaluate physician/pharmacist collaboration to improve blood pressure control. J Clin Hypertens (Greenwich). 2008;10:260-271.
4. Carter BL, Ardery G, Dawson JD, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009;169:1996-2002.
5. Weber CA, Ernst ME, Sezate GS, et al. Pharmacist-physician comanagement of hypertension and reduction in 24-hour ambulatory blood pressures. Arch Intern Med. 2010;170:1634-1639.
6. Hirsch JD, Steers N, Adler DS, et al. Primary care-based, pharmacist-physician collaborative medication-therapy management of hypertension: a randomized, pragmatic trial. Clin Ther. 2014;36:1244-1254.
7. Carter BL, Coffey CS, Ardery G, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8:235-243.
8. Sisson EM, Dixon DL, Kildow DC, et al. Effectiveness of a pharmacist-physician team-based collaboration to improve long-term blood pressure control at an inner-city safety-net clinic. Pharmacotherapy. 2016;36:342-347.
9. Kiel PJ, McCord AD. Pharmacist impact on clinical outcomes in a diabetes disease management program via collaborative practice. Ann Pharmacother. 2005;39:1828-1832.
10. Farland MZ, Byrd DC, McFarland MS, et al. Pharmacist-physician collaboration for diabetes care: the diabetes initiative program. Ann Pharmacother. 2013;47:781-789.
11. Howard-Thompson A, Farland MZ, Byrd DC, et al. Pharmacist-physician collaboration for diabetes care: cardiovascular outcomes. Ann Pharmacother. 2013;47:1471-1477.
12. Gums TH, Carter BL, Milavetz G, et al. Physician-pharmacist collaborative management of asthma in primary care. Pharmacotherapy. 2014;34:1033-1042.
13. Greer N, Bolduc J, Geurkink E, et al. VA Evidence-based Synthesis Program Reports. Pharmacist-Led Chronic Disease Management: A Systematic Review of Effectiveness and Harms Compared to Usual Care. Washington (DC): Department of Veterans Affairs (US); 2015.
14. Wentzlaff DM, Carter BL, Ardery G, et al. Sustained blood pressure control following discontinuation of a pharmacist intervention. J Clin Hypertens (Greenwich). 2011;13:431-437.
15. Carter BL, Vander Weg MW, Parker CP, et al. Sustained blood pressure control following discontinuation of a pharmacist intervention for veterans. J Clin Hypertens (Greenwich). 2015;17:701-708.
16. Kulchaitanaroaj P, Brooks JM, Ardery G, et al. Incremental costs associated with physician and pharmacist collaboration to improve blood pressure control. Pharmacotherapy. 2012;32:772-780.
17. Polgreen LA, Han J, Carter BL, et al. Cost effectiveness of a physician-pharmacist collaboration intervention to improve blood pressure control. Hypertension. 2015;66:1145-1151.
18. Carter BL. Primary care physician-pharmacist collaborative care model: strategies for implementation. Pharmacotherapy. 2016;36:363-373.
19. Anderegg MD, Gums TH, Uribe L, et al. Physician-pharmacist collaborative management: narrowing the socioeconomic blood pressure gap. Hypertension. 2016;68:1314-1320.
20. Moczygemba LR, Goode JV, Gatewood SBS, et al. Integration of collaborative medication therapy management in a safety net patient-centered medical home. J Am Pharm Assoc (2003). 2011;51:167-172.
21. Scott MA, Hitch B, Ray L, et al. Integration of pharmacists into a patient-centered medical home. J Am Pharm Assoc (2003). 2011;51:161-166.
22. Patterson BJ, Solimeo SL, Stewart KR, et al. Perceptions of pharmacists’ integration into patient-centered medical home teams. Res Social Adm Pharm. 2015;11:85-95.
23. Carter BL, Levy BT, Gryzlak B, et al. A centralized cardiovascular risk service to improve guideline adherence in private primary care offices. Contemp Clin Trials. 2015;43:25-32.
24. Carter BL, Coffey CS, Chrischilles EA, et al. A cluster-randomized trial of a centralized clinical pharmacy cardiovascular risk service to improve guideline adherence. Pharmacotherapy. 2015;35:653-662.
1. Borenstein JE, Graber G, Saltiel E, et al. Physician-pharmacist comanagement of hypertension: a randomized, comparative trial. Pharmacotherapy. 2003;23:209-216.
2. Hunt JS, Siemienczuk J, Pape G, et al. A randomized controlled trial of team-based care: impact of physician-pharmacist collaboration on uncontrolled hypertension. J Gen Intern Med. 2008;23:1966-1972.
3. Carter BL, Bergus GR, Dawson JD, et al. A cluster randomized trial to evaluate physician/pharmacist collaboration to improve blood pressure control. J Clin Hypertens (Greenwich). 2008;10:260-271.
4. Carter BL, Ardery G, Dawson JD, et al. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009;169:1996-2002.
5. Weber CA, Ernst ME, Sezate GS, et al. Pharmacist-physician comanagement of hypertension and reduction in 24-hour ambulatory blood pressures. Arch Intern Med. 2010;170:1634-1639.
6. Hirsch JD, Steers N, Adler DS, et al. Primary care-based, pharmacist-physician collaborative medication-therapy management of hypertension: a randomized, pragmatic trial. Clin Ther. 2014;36:1244-1254.
7. Carter BL, Coffey CS, Ardery G, et al. Cluster-randomized trial of a physician/pharmacist collaborative model to improve blood pressure control. Circ Cardiovasc Qual Outcomes. 2015;8:235-243.
8. Sisson EM, Dixon DL, Kildow DC, et al. Effectiveness of a pharmacist-physician team-based collaboration to improve long-term blood pressure control at an inner-city safety-net clinic. Pharmacotherapy. 2016;36:342-347.
9. Kiel PJ, McCord AD. Pharmacist impact on clinical outcomes in a diabetes disease management program via collaborative practice. Ann Pharmacother. 2005;39:1828-1832.
10. Farland MZ, Byrd DC, McFarland MS, et al. Pharmacist-physician collaboration for diabetes care: the diabetes initiative program. Ann Pharmacother. 2013;47:781-789.
11. Howard-Thompson A, Farland MZ, Byrd DC, et al. Pharmacist-physician collaboration for diabetes care: cardiovascular outcomes. Ann Pharmacother. 2013;47:1471-1477.
12. Gums TH, Carter BL, Milavetz G, et al. Physician-pharmacist collaborative management of asthma in primary care. Pharmacotherapy. 2014;34:1033-1042.
13. Greer N, Bolduc J, Geurkink E, et al. VA Evidence-based Synthesis Program Reports. Pharmacist-Led Chronic Disease Management: A Systematic Review of Effectiveness and Harms Compared to Usual Care. Washington (DC): Department of Veterans Affairs (US); 2015.
14. Wentzlaff DM, Carter BL, Ardery G, et al. Sustained blood pressure control following discontinuation of a pharmacist intervention. J Clin Hypertens (Greenwich). 2011;13:431-437.
15. Carter BL, Vander Weg MW, Parker CP, et al. Sustained blood pressure control following discontinuation of a pharmacist intervention for veterans. J Clin Hypertens (Greenwich). 2015;17:701-708.
16. Kulchaitanaroaj P, Brooks JM, Ardery G, et al. Incremental costs associated with physician and pharmacist collaboration to improve blood pressure control. Pharmacotherapy. 2012;32:772-780.
17. Polgreen LA, Han J, Carter BL, et al. Cost effectiveness of a physician-pharmacist collaboration intervention to improve blood pressure control. Hypertension. 2015;66:1145-1151.
18. Carter BL. Primary care physician-pharmacist collaborative care model: strategies for implementation. Pharmacotherapy. 2016;36:363-373.
19. Anderegg MD, Gums TH, Uribe L, et al. Physician-pharmacist collaborative management: narrowing the socioeconomic blood pressure gap. Hypertension. 2016;68:1314-1320.
20. Moczygemba LR, Goode JV, Gatewood SBS, et al. Integration of collaborative medication therapy management in a safety net patient-centered medical home. J Am Pharm Assoc (2003). 2011;51:167-172.
21. Scott MA, Hitch B, Ray L, et al. Integration of pharmacists into a patient-centered medical home. J Am Pharm Assoc (2003). 2011;51:161-166.
22. Patterson BJ, Solimeo SL, Stewart KR, et al. Perceptions of pharmacists’ integration into patient-centered medical home teams. Res Social Adm Pharm. 2015;11:85-95.
23. Carter BL, Levy BT, Gryzlak B, et al. A centralized cardiovascular risk service to improve guideline adherence in private primary care offices. Contemp Clin Trials. 2015;43:25-32.
24. Carter BL, Coffey CS, Chrischilles EA, et al. A cluster-randomized trial of a centralized clinical pharmacy cardiovascular risk service to improve guideline adherence. Pharmacotherapy. 2015;35:653-662.
PRACTICE RECOMMENDATIONS
› Consider physician-pharmacist collaboration as a way by which to improve the management of your patients with hypertension and diabetes. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Best uses of osteopathic manipulation
Interest in osteopathy continues to rise in this country. Currently, more than 20% of medical students in the United States are training to be osteopathic physicians.1 In addition, the 2007 National Health Interview Survey found that spinal manipulation was among the most common complementary and alternative medicine (CAM) therapies used; with 8.6% of US adults reporting that they used it within the previous 12 months.2
With the growing number of DOs and the high utilization of osteopathic manipulative treatment (OMT), it is important for all physicians to understand the role OMT can play in the treatment of conditions ranging from low back pain to irritable bowel syndrome so that patients may be offered, or referred for, the treatment when appropriate.
To clarify when OMT may be most beneficial, we performed a literature review. Our findings are summarized here. But first, a word about osteopathic medicine and what OMT entails.
Osteopathic physicians view the body as a whole
According to the American Osteopathic Association, “the osteopathic philosophy of medicine sees an interrelated unity in all systems of the body, with each working with the other to heal in times of illness."3 This “whole-person approach to medicine” focuses on looking beyond symptoms alone to understand how lifestyle and environmental factors impact well-being.
As part of their education, DOs receive special training in the musculoskeletal system and in OMT. OMT is the process by which DOs use their hands to diagnose illness and injury and then mobilize a patient’s joints and soft tissues using techniques that include muscle activation, stretching, joint articulation, and gentle pressure to encourage the body’s natural tendency to heal itself.
These patients with low back pain will likely benefit
In the past, studies with small sample sizes, blinding issues, differing controls, and subjective outcome measurements have marred research efforts to demonstrate the effectiveness of OMT. More recently, researchers have attempted to minimize these issues, particularly when evaluating the efficacy of OMT for low back pain.
In addition to increasing sample size, studies have compared OMT to usual care, to sham manipulation, and more recently to other manual modalities including ultrasound to equalize the subjective effects of interventions.4 With improved study designs, there has been increased awareness of the effectiveness of spinal manipulation by organizations that develop guidelines for the care of patients with low back pain. The most recent clinical practice guideline from the American College of Physicians includes spinal manipulation as a treatment modality that should be considered by clinicians for patients who have acute, subacute, or chronic low back pain.5
Chronic nonspecific low back pain. Looking at OMT vs other interventions for chronic nonspecific low back pain, a 2014 meta-analysis found moderate quality evidence for clinically relevant effects of OMT on low back pain and function. In 6 studies that evaluated 769 patients with chronic nonspecific low back pain, there was a significant difference in pain—equivalent to a 1.5-point improvement (mean difference [MD]= -14.93; 95% confidence interval [CI], -25.18
Acute and chronic nonspecific low back pain. Similarly, in the same 2014 meta-analysis, 1141 participants with acute and chronic nonspecific low back pain in 10 studies had the equivalent of 1.3 points more pain relief with OMT compared with controls (MD= -12.91; 95% CI, -20.00 to -5.82). The authors used the standardized mean difference (SMD), which is the difference in means divided by the standard deviation, to interpret the magnitude of difference in function between participants who received OMT and those in the control groups. Further, 1046 participants with acute and chronic nonspecific low back pain in 9 studies had a small improvement in functional status using the Roland-Morris Disability Questionnaire (RMDQ) or Oswestry-Disability Index (SMD= -0.36; 95% CI, -0.58 to -0.14).6
A 2005 meta-analysis that evaluated 6 randomized controlled trials (RCTs) involving 549 patients with low back pain found that 318 patients who received OMT had significantly less low back pain compared with 231 controls (effect size= -0.30; 95% CI, -0.47 to -0.13; P=.001).7 Although significant, an effect size of this magnitude is characterized as small.8
Other benefits of OMT include increased patient satisfaction, fewer meds
A randomized double-blind, sham-controlled study involving 455 patients with chronic low back pain compared outcomes of OMT to sham OMT applied in 6 treatment sessions over 8 weeks.9 Intention-to-treat analysis was performed to measure moderate and substantial improvements in low back pain at Week 12 (≥30% and ≥50% pain reductions from baseline, respectively). Based on the Cochrane Back Review Group criteria for effect sizes, response ratios were calculated to determine if the differences seen were considered clinically relevant.10
Patients receiving OMT were more likely to achieve moderate (response ratio=1.38; 95% CI, 1.16-1.64; P<.001) and substantial (response ratio=1.41; 95% CI, 1.13-1.76; P=.002) improvements in low back pain at Week 12. The calculated number needed to treat (NNT) for moderate and significant improvement in pain at 12 weeks was 6 and 7, respectively. In addition, patients in the OMT group were more likely to be very satisfied with their care (P<.001) with an NNT of 5, and used fewer medications than did patients in the sham group during the 12 weeks of the study (use ratio=0.66; 95% CI, 0.43-1.00; P=.048; NNT=15).9
Pregnant women may benefit from OMT in the third trimester
A 2013 RCT involving 144 patients randomized to OMT, sham ultrasound, or usual obstetric care found that 68 patients (47%) experienced back-specific dysfunction during their third trimester of pregnancy (defined by a ≥2-point increase in the RMDQ).11
OMT reduced the risk of back-specific dysfunction by 40% vs the ultrasound group (relative risk [RR]=0.6; 95% CI, 0.3-1; P=.046) and 60% vs the usual obstetric care group (RR=0.4; 95% CI, 0.2-0.7; P<.001). The corresponding NNTs were 5.1 (95% CI, 2.7-282.2) for the OMT group vs the ultrasound group and 2.5 (95% CI, 1.8-4.9) vs the usual care group. The outcomes of this study were not conclusive because the initial RMDQ score was 1.8 points worse for the OMT group than for the usual care group.11
Subsequently, the PROMOTE (Pregnancy Research on Osteopathic Manipulation Optimizing Treatment Effects) study involving 400 patients demonstrated that a standard OMT protocol was effective for decreasing pain and function deterioration compared with usual obstetric care.12 However, results from the OMT group did not differ significantly from those of the ultrasound group, which were labeled as subtherapeutic in the study.12
The most recent Cochrane Review on low back pain in pregnancy noted that there was moderate quality evidence (due to study design limitations or imprecision) that OMT significantly reduced low back pain and function disability.13
OMT for other conditions? The evidence is limited
To date, studies on conditions other than low back pain have not demonstrated the same robust improvements in design as have those concerning low back pain (ie, larger sample sizes, comparisons to usual care and other treatments, etc.), and available data are not sufficiently significant to compel a change in clinical practice. Despite this, patients seek out, and receive, OMT as an alternative or adjunctive treatment for many conditions other than low back pain,2 and family physicians should be aware of the current evidence for OMT in those conditions.
OMT for acute neck pain: A comparison with ketorolac
Researchers randomized 58 patients presenting to 3 emergency departments with neck pain of less than 3 weeks’ duration to receive either OMT or 30 mg IM ketorolac.14 OMT techniques were provided at the discretion of the physician based on patient needs. Patients rated their pain intensity on an 11-point numerical scale at the time of presentation and one hour after treatment. Patients receiving ketorolac or OMT had significant reductions in pain intensity with improvements of 1.7 +/- 1.6 (95% CI, 1.1-2.3; P<.001) and 2.8 +/- 1.7 (95% CI, 2.1-3.4; P<.001), respectively.
Although the pain reduction changes were statistically significant in both groups, the improvements were small enough to question if they were functionally significant. Compared to those receiving ketorolac, those receiving OMT reported a significantly greater decrease in their pain intensity (2.8 vs 1.7; 95% CI, 0.2-1.9; P=.02), but it’s worth noting that the dose of ketorolac was half the recommended dose for moderate or severe pain.14
Patients may have more headache-free days with OMT
To assess the use of OMT to treat chronic migraine, researchers conducted a prospective, single-blind RCT in which 105 chronic migraine sufferers (average of 22.5 migraine days/month) were split into 3 treatment groups: OMT plus medications, sham OMT plus medications, and medications alone.15
OMT led to fewer days with migraines compared with the medication group (MD= -21.06; 95% CI, -23.19 to -18.92; P<.001) and sham OMT group (MD= -17.43; 95% CI, -19.57 to -15.29; P<.001), resulting in less functional disability (P<.001).15 Caution should be taken in interpreting the results of this small trial, however, as an effect of this size has not been replicated in other studies.
A small (N=29) single-blind RCT looked at progressive muscular relaxation with and without OMT for the treatment of tension headache. Patients who completed relaxation exercises plus 3 sessions of OMT experienced significantly more headache-free days (1.79 vs 0.21; P=.016).16 Despite this finding, headache intensity and headache diary ratings were not different between the 2 groups in this study.
Postoperative OMT may decrease length of stay
In a retrospective study evaluating the effect of OMT on postoperative outcomes in 55 patients who underwent gastrointestinal surgery, a total of 17 patients who received a single OMT session within 48 hours of surgery had a mean time to flatus of 3.1 days compared with 4.7 days in the usual care control group (P=.035).17 The mean length of stay was 6.1 days in the OMT group and 11.5 days in the non-OMT group (P=.006).
Major limitations of this study include that it was retrospective in design and that only 17 of 55 patients had OMT performed, indicating a possible selection bias.
Pneumonia: OMT may reduce LOS and duration of antibiotic usage
The Multicenter Osteopathic Pneumonia Study in the Elderly (MOPSE), a double-blind RCT, looked at 406 patients ≥50 years hospitalized with pneumonia. Researchers randomized the group to receive either conventional care (CC; antibiotic treatment only), OMT and antibiotic therapy, or light-touch sham therapy with antibiotics.18 The researchers found no significant differences between the groups for any outcomes in the intention-to-treat analysis.
In results obtained from the per protocol analysis, however, the median length of stay for those in the OMT group was 3.5 days, compared with 4.5 days for those in the CC group (95% CI, 3.2-4.0; P=.01). Multiple comparisons also indicated a reduction in mean duration of intravenous antibiotic use of 3 days in the OMT group (95% CI, 2.7-3.5) vs 3.5 days in the CC group (95% CI, 3.2-3.9). The treatment end-points of either death or respiratory failure occurred significantly less frequently in the OMT group compared with the CC group (P=.006).18
A Cochrane review of RCTs assessing the efficacy of adjunctive techniques compared with conventional therapy for patients with pneumonia revealed a reduction in hospital stay of 2 days (95% CI, -3.5 to -0.6) for patients who received OMT and positive expiratory pressure vs those who received neither intervention.19 Additionally, the duration of IV antibiotics and total duration of all (IV and oral) antibiotic treatment required in those treated adjunctively with OMT was shorter (MD for IV antibiotics= -2.1 days; 95% CI, -3.4 to -0.9 and MD for all antibiotics= -1.9 days; 95% CI, -3.1 to -0.7).19 The review was notable for a small sample size, with only 79 patients assessed.
OMT may improve IBS symptoms
A crossover study of 31 patients that compared visceral manipulation and sacral articulation OMT with sham therapy for the treatment of irritable bowel syndrome (IBS) demonstrated that OMT significantly decreased self-reported diarrhea (P=.016), abdominal distention (P=.043), abdominal pain (P=.013), and rectal sensitivity (P<.001), but did not significantly affect constipation.20
In another study, researchers randomized 30 patients with IBS in a 2:1 distribution to OMT vs sham treatment.21 OMT included abdominal visceral techniques and direct and indirect spine techniques. All of the patients received 2 treatment sessions, and the researchers evaluated them at 7 and 28 days. At 7 days, both groups demonstrated a significant reduction in IBS symptoms, although the OMT group had significantly greater improvement (P=.01). At 28 days, however, neither group showed a significant reduction in symptoms.21
The lack of a control group (in the first study due to the crossover design), small sample sizes, and self-reported symptoms are major limitations to applying these studies to IBS treatment recommendations.
CORRESPONDENCE
Andrew H. Slattengren, DO, Broadway Family Medicine Clinic, 1020 West Broadway Avenue, Minneapolis, MN 55411; [email protected].
1. American Association of Colleges of Osteopathic Medicine. What is osteopathic medicine? Available at: https://www.aacom.org/become-a-doctor/about-om. Accessed July 10, 2017.
2. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1-23. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19361005. Accessed November 10, 2015.
3. American Osteopathic Association. What is osteopathic medicine? Available at: http://www.osteopathic.org/osteopathic-health/Pages/what-is-osteopathic-medicine.aspx. Accessed November 17, 2017.
4. Licciardone JC, Russo DP. Blinding Protocols, Treatment Credibility, and Expectancy: Methodologic Issues in Clinical Trials of Osteopathic Manipulative Treatment. J Am Osteopath Assoc. 2006;106:457-463.
5. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166:514-530.
6. Franke H, Franke JD, Fryer G. Osteopathic manipulative treatment for nonspecific low back pain: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2014;15:286.
7. Licciardone JC, Brimhall AK, King LN. Osteopathic manipulative treatment for low back pain: a systematic review and meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2005;6:43.
8. Cohen J. Statistical Power Analysis for the Behavioral Sciences.82nd ed. Hillsdale NJ: Lawrence Erlbaum Associates; 1988.
9. Licciardone JC, Minotti DE, Gatchel RJ, et al. Osteopathic manual treatment and ultrasound therapy for chronic low back pain: a randomized controlled trial. Ann Fam Med. 2013;11:122-129.
10. Furlan AD, Pennick V, Bombardier C, et al, Editorial Board, Cochrane Back Review Group. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976). 2009;34:1929-1941.
11. Licciardone JC, Aryal S. Prevention of progressive back-specific dysfunction during pregnancy: an assessment of osteopathic manual treatment based on Cochrane Back Review Group criteria. J Am Osteopath Assoc. 2013;113:728-736.
12. Hensel KL, Buchanan S, Brown SK, et al. Pregnancy Research on Osteopathic Manipulation Optimizing Treatment Effects: the PROMOTE study. Am J Obstet Gynecol. 2015;212:108.e1-e9.
13. Pennick V, Liddle SD. Interventions for preventing and treating pelvic and back pain in pregnancy. Cochrane Database Syst Rev. 2013;8:CD001139.
14. McReynolds TM, Sheridan BJ. Intramuscular ketorolac versus osteopathic manipulative treatment in the management of acute neck pain in the emergency department: a randomized clinical trial. J Am Osteopath Assoc. 2005;105:57-68.
15. Cerritelli F, Ginevri L, Messi G, et al. Clinical effectiveness of osteopathic treatment in chronic migraine: 3-armed randomized controlled trial. Complement Ther Med. 2015;23:149-156.
16. Anderson RE, Seniscal C. A comparison of selected osteopathic treatment and relaxation for tension-type headaches. Headache. 2006;46:1273-1280.
17. Baltazar GA, Betler MP, Akella K, et al. Effect of osteopathic manipulative treatment on incidence of postoperative ileus and hospital length of stay in general surgical patients. J Am Osteopath Assoc. 2013;113:204-209.
18. Noll DR, Degenhardt BF, Morley TF, et al. Efficacy of osteopathic manipulation as an adjunctive treatment for hospitalized patients with pneumonia: a randomized controlled trial. Osteopath Med Prim Care. 2010;4:2.
19. Yang M, Yan Y, Yin X, et al. Chest physiotherapy for pneumonia in adults. Cochrane Database Syst Rev. 2013;2:CD006338.
20. Attali TV, Bouchoucha M, Benamouzig R. Treatment of refractory irritable bowel syndrome with visceral osteopathy: short-term and long-term results of a randomized trial. J Dig Dis. 2013;14:654-661.
21. Florance BM, Frin G, Dainese R, et al. Osteopathy improves the severity of irritable bowel syndrome: a pilot randomized sham-controlled study. Eur J Gastroenterol Hepatol. 2012;24:944-949.
Interest in osteopathy continues to rise in this country. Currently, more than 20% of medical students in the United States are training to be osteopathic physicians.1 In addition, the 2007 National Health Interview Survey found that spinal manipulation was among the most common complementary and alternative medicine (CAM) therapies used; with 8.6% of US adults reporting that they used it within the previous 12 months.2
With the growing number of DOs and the high utilization of osteopathic manipulative treatment (OMT), it is important for all physicians to understand the role OMT can play in the treatment of conditions ranging from low back pain to irritable bowel syndrome so that patients may be offered, or referred for, the treatment when appropriate.
To clarify when OMT may be most beneficial, we performed a literature review. Our findings are summarized here. But first, a word about osteopathic medicine and what OMT entails.
Osteopathic physicians view the body as a whole
According to the American Osteopathic Association, “the osteopathic philosophy of medicine sees an interrelated unity in all systems of the body, with each working with the other to heal in times of illness."3 This “whole-person approach to medicine” focuses on looking beyond symptoms alone to understand how lifestyle and environmental factors impact well-being.
As part of their education, DOs receive special training in the musculoskeletal system and in OMT. OMT is the process by which DOs use their hands to diagnose illness and injury and then mobilize a patient’s joints and soft tissues using techniques that include muscle activation, stretching, joint articulation, and gentle pressure to encourage the body’s natural tendency to heal itself.
These patients with low back pain will likely benefit
In the past, studies with small sample sizes, blinding issues, differing controls, and subjective outcome measurements have marred research efforts to demonstrate the effectiveness of OMT. More recently, researchers have attempted to minimize these issues, particularly when evaluating the efficacy of OMT for low back pain.
In addition to increasing sample size, studies have compared OMT to usual care, to sham manipulation, and more recently to other manual modalities including ultrasound to equalize the subjective effects of interventions.4 With improved study designs, there has been increased awareness of the effectiveness of spinal manipulation by organizations that develop guidelines for the care of patients with low back pain. The most recent clinical practice guideline from the American College of Physicians includes spinal manipulation as a treatment modality that should be considered by clinicians for patients who have acute, subacute, or chronic low back pain.5
Chronic nonspecific low back pain. Looking at OMT vs other interventions for chronic nonspecific low back pain, a 2014 meta-analysis found moderate quality evidence for clinically relevant effects of OMT on low back pain and function. In 6 studies that evaluated 769 patients with chronic nonspecific low back pain, there was a significant difference in pain—equivalent to a 1.5-point improvement (mean difference [MD]= -14.93; 95% confidence interval [CI], -25.18
Acute and chronic nonspecific low back pain. Similarly, in the same 2014 meta-analysis, 1141 participants with acute and chronic nonspecific low back pain in 10 studies had the equivalent of 1.3 points more pain relief with OMT compared with controls (MD= -12.91; 95% CI, -20.00 to -5.82). The authors used the standardized mean difference (SMD), which is the difference in means divided by the standard deviation, to interpret the magnitude of difference in function between participants who received OMT and those in the control groups. Further, 1046 participants with acute and chronic nonspecific low back pain in 9 studies had a small improvement in functional status using the Roland-Morris Disability Questionnaire (RMDQ) or Oswestry-Disability Index (SMD= -0.36; 95% CI, -0.58 to -0.14).6
A 2005 meta-analysis that evaluated 6 randomized controlled trials (RCTs) involving 549 patients with low back pain found that 318 patients who received OMT had significantly less low back pain compared with 231 controls (effect size= -0.30; 95% CI, -0.47 to -0.13; P=.001).7 Although significant, an effect size of this magnitude is characterized as small.8
Other benefits of OMT include increased patient satisfaction, fewer meds
A randomized double-blind, sham-controlled study involving 455 patients with chronic low back pain compared outcomes of OMT to sham OMT applied in 6 treatment sessions over 8 weeks.9 Intention-to-treat analysis was performed to measure moderate and substantial improvements in low back pain at Week 12 (≥30% and ≥50% pain reductions from baseline, respectively). Based on the Cochrane Back Review Group criteria for effect sizes, response ratios were calculated to determine if the differences seen were considered clinically relevant.10
Patients receiving OMT were more likely to achieve moderate (response ratio=1.38; 95% CI, 1.16-1.64; P<.001) and substantial (response ratio=1.41; 95% CI, 1.13-1.76; P=.002) improvements in low back pain at Week 12. The calculated number needed to treat (NNT) for moderate and significant improvement in pain at 12 weeks was 6 and 7, respectively. In addition, patients in the OMT group were more likely to be very satisfied with their care (P<.001) with an NNT of 5, and used fewer medications than did patients in the sham group during the 12 weeks of the study (use ratio=0.66; 95% CI, 0.43-1.00; P=.048; NNT=15).9
Pregnant women may benefit from OMT in the third trimester
A 2013 RCT involving 144 patients randomized to OMT, sham ultrasound, or usual obstetric care found that 68 patients (47%) experienced back-specific dysfunction during their third trimester of pregnancy (defined by a ≥2-point increase in the RMDQ).11
OMT reduced the risk of back-specific dysfunction by 40% vs the ultrasound group (relative risk [RR]=0.6; 95% CI, 0.3-1; P=.046) and 60% vs the usual obstetric care group (RR=0.4; 95% CI, 0.2-0.7; P<.001). The corresponding NNTs were 5.1 (95% CI, 2.7-282.2) for the OMT group vs the ultrasound group and 2.5 (95% CI, 1.8-4.9) vs the usual care group. The outcomes of this study were not conclusive because the initial RMDQ score was 1.8 points worse for the OMT group than for the usual care group.11
Subsequently, the PROMOTE (Pregnancy Research on Osteopathic Manipulation Optimizing Treatment Effects) study involving 400 patients demonstrated that a standard OMT protocol was effective for decreasing pain and function deterioration compared with usual obstetric care.12 However, results from the OMT group did not differ significantly from those of the ultrasound group, which were labeled as subtherapeutic in the study.12
The most recent Cochrane Review on low back pain in pregnancy noted that there was moderate quality evidence (due to study design limitations or imprecision) that OMT significantly reduced low back pain and function disability.13
OMT for other conditions? The evidence is limited
To date, studies on conditions other than low back pain have not demonstrated the same robust improvements in design as have those concerning low back pain (ie, larger sample sizes, comparisons to usual care and other treatments, etc.), and available data are not sufficiently significant to compel a change in clinical practice. Despite this, patients seek out, and receive, OMT as an alternative or adjunctive treatment for many conditions other than low back pain,2 and family physicians should be aware of the current evidence for OMT in those conditions.
OMT for acute neck pain: A comparison with ketorolac
Researchers randomized 58 patients presenting to 3 emergency departments with neck pain of less than 3 weeks’ duration to receive either OMT or 30 mg IM ketorolac.14 OMT techniques were provided at the discretion of the physician based on patient needs. Patients rated their pain intensity on an 11-point numerical scale at the time of presentation and one hour after treatment. Patients receiving ketorolac or OMT had significant reductions in pain intensity with improvements of 1.7 +/- 1.6 (95% CI, 1.1-2.3; P<.001) and 2.8 +/- 1.7 (95% CI, 2.1-3.4; P<.001), respectively.
Although the pain reduction changes were statistically significant in both groups, the improvements were small enough to question if they were functionally significant. Compared to those receiving ketorolac, those receiving OMT reported a significantly greater decrease in their pain intensity (2.8 vs 1.7; 95% CI, 0.2-1.9; P=.02), but it’s worth noting that the dose of ketorolac was half the recommended dose for moderate or severe pain.14
Patients may have more headache-free days with OMT
To assess the use of OMT to treat chronic migraine, researchers conducted a prospective, single-blind RCT in which 105 chronic migraine sufferers (average of 22.5 migraine days/month) were split into 3 treatment groups: OMT plus medications, sham OMT plus medications, and medications alone.15
OMT led to fewer days with migraines compared with the medication group (MD= -21.06; 95% CI, -23.19 to -18.92; P<.001) and sham OMT group (MD= -17.43; 95% CI, -19.57 to -15.29; P<.001), resulting in less functional disability (P<.001).15 Caution should be taken in interpreting the results of this small trial, however, as an effect of this size has not been replicated in other studies.
A small (N=29) single-blind RCT looked at progressive muscular relaxation with and without OMT for the treatment of tension headache. Patients who completed relaxation exercises plus 3 sessions of OMT experienced significantly more headache-free days (1.79 vs 0.21; P=.016).16 Despite this finding, headache intensity and headache diary ratings were not different between the 2 groups in this study.
Postoperative OMT may decrease length of stay
In a retrospective study evaluating the effect of OMT on postoperative outcomes in 55 patients who underwent gastrointestinal surgery, a total of 17 patients who received a single OMT session within 48 hours of surgery had a mean time to flatus of 3.1 days compared with 4.7 days in the usual care control group (P=.035).17 The mean length of stay was 6.1 days in the OMT group and 11.5 days in the non-OMT group (P=.006).
Major limitations of this study include that it was retrospective in design and that only 17 of 55 patients had OMT performed, indicating a possible selection bias.
Pneumonia: OMT may reduce LOS and duration of antibiotic usage
The Multicenter Osteopathic Pneumonia Study in the Elderly (MOPSE), a double-blind RCT, looked at 406 patients ≥50 years hospitalized with pneumonia. Researchers randomized the group to receive either conventional care (CC; antibiotic treatment only), OMT and antibiotic therapy, or light-touch sham therapy with antibiotics.18 The researchers found no significant differences between the groups for any outcomes in the intention-to-treat analysis.
In results obtained from the per protocol analysis, however, the median length of stay for those in the OMT group was 3.5 days, compared with 4.5 days for those in the CC group (95% CI, 3.2-4.0; P=.01). Multiple comparisons also indicated a reduction in mean duration of intravenous antibiotic use of 3 days in the OMT group (95% CI, 2.7-3.5) vs 3.5 days in the CC group (95% CI, 3.2-3.9). The treatment end-points of either death or respiratory failure occurred significantly less frequently in the OMT group compared with the CC group (P=.006).18
A Cochrane review of RCTs assessing the efficacy of adjunctive techniques compared with conventional therapy for patients with pneumonia revealed a reduction in hospital stay of 2 days (95% CI, -3.5 to -0.6) for patients who received OMT and positive expiratory pressure vs those who received neither intervention.19 Additionally, the duration of IV antibiotics and total duration of all (IV and oral) antibiotic treatment required in those treated adjunctively with OMT was shorter (MD for IV antibiotics= -2.1 days; 95% CI, -3.4 to -0.9 and MD for all antibiotics= -1.9 days; 95% CI, -3.1 to -0.7).19 The review was notable for a small sample size, with only 79 patients assessed.
OMT may improve IBS symptoms
A crossover study of 31 patients that compared visceral manipulation and sacral articulation OMT with sham therapy for the treatment of irritable bowel syndrome (IBS) demonstrated that OMT significantly decreased self-reported diarrhea (P=.016), abdominal distention (P=.043), abdominal pain (P=.013), and rectal sensitivity (P<.001), but did not significantly affect constipation.20
In another study, researchers randomized 30 patients with IBS in a 2:1 distribution to OMT vs sham treatment.21 OMT included abdominal visceral techniques and direct and indirect spine techniques. All of the patients received 2 treatment sessions, and the researchers evaluated them at 7 and 28 days. At 7 days, both groups demonstrated a significant reduction in IBS symptoms, although the OMT group had significantly greater improvement (P=.01). At 28 days, however, neither group showed a significant reduction in symptoms.21
The lack of a control group (in the first study due to the crossover design), small sample sizes, and self-reported symptoms are major limitations to applying these studies to IBS treatment recommendations.
CORRESPONDENCE
Andrew H. Slattengren, DO, Broadway Family Medicine Clinic, 1020 West Broadway Avenue, Minneapolis, MN 55411; [email protected].
Interest in osteopathy continues to rise in this country. Currently, more than 20% of medical students in the United States are training to be osteopathic physicians.1 In addition, the 2007 National Health Interview Survey found that spinal manipulation was among the most common complementary and alternative medicine (CAM) therapies used; with 8.6% of US adults reporting that they used it within the previous 12 months.2
With the growing number of DOs and the high utilization of osteopathic manipulative treatment (OMT), it is important for all physicians to understand the role OMT can play in the treatment of conditions ranging from low back pain to irritable bowel syndrome so that patients may be offered, or referred for, the treatment when appropriate.
To clarify when OMT may be most beneficial, we performed a literature review. Our findings are summarized here. But first, a word about osteopathic medicine and what OMT entails.
Osteopathic physicians view the body as a whole
According to the American Osteopathic Association, “the osteopathic philosophy of medicine sees an interrelated unity in all systems of the body, with each working with the other to heal in times of illness."3 This “whole-person approach to medicine” focuses on looking beyond symptoms alone to understand how lifestyle and environmental factors impact well-being.
As part of their education, DOs receive special training in the musculoskeletal system and in OMT. OMT is the process by which DOs use their hands to diagnose illness and injury and then mobilize a patient’s joints and soft tissues using techniques that include muscle activation, stretching, joint articulation, and gentle pressure to encourage the body’s natural tendency to heal itself.
These patients with low back pain will likely benefit
In the past, studies with small sample sizes, blinding issues, differing controls, and subjective outcome measurements have marred research efforts to demonstrate the effectiveness of OMT. More recently, researchers have attempted to minimize these issues, particularly when evaluating the efficacy of OMT for low back pain.
In addition to increasing sample size, studies have compared OMT to usual care, to sham manipulation, and more recently to other manual modalities including ultrasound to equalize the subjective effects of interventions.4 With improved study designs, there has been increased awareness of the effectiveness of spinal manipulation by organizations that develop guidelines for the care of patients with low back pain. The most recent clinical practice guideline from the American College of Physicians includes spinal manipulation as a treatment modality that should be considered by clinicians for patients who have acute, subacute, or chronic low back pain.5
Chronic nonspecific low back pain. Looking at OMT vs other interventions for chronic nonspecific low back pain, a 2014 meta-analysis found moderate quality evidence for clinically relevant effects of OMT on low back pain and function. In 6 studies that evaluated 769 patients with chronic nonspecific low back pain, there was a significant difference in pain—equivalent to a 1.5-point improvement (mean difference [MD]= -14.93; 95% confidence interval [CI], -25.18
Acute and chronic nonspecific low back pain. Similarly, in the same 2014 meta-analysis, 1141 participants with acute and chronic nonspecific low back pain in 10 studies had the equivalent of 1.3 points more pain relief with OMT compared with controls (MD= -12.91; 95% CI, -20.00 to -5.82). The authors used the standardized mean difference (SMD), which is the difference in means divided by the standard deviation, to interpret the magnitude of difference in function between participants who received OMT and those in the control groups. Further, 1046 participants with acute and chronic nonspecific low back pain in 9 studies had a small improvement in functional status using the Roland-Morris Disability Questionnaire (RMDQ) or Oswestry-Disability Index (SMD= -0.36; 95% CI, -0.58 to -0.14).6
A 2005 meta-analysis that evaluated 6 randomized controlled trials (RCTs) involving 549 patients with low back pain found that 318 patients who received OMT had significantly less low back pain compared with 231 controls (effect size= -0.30; 95% CI, -0.47 to -0.13; P=.001).7 Although significant, an effect size of this magnitude is characterized as small.8
Other benefits of OMT include increased patient satisfaction, fewer meds
A randomized double-blind, sham-controlled study involving 455 patients with chronic low back pain compared outcomes of OMT to sham OMT applied in 6 treatment sessions over 8 weeks.9 Intention-to-treat analysis was performed to measure moderate and substantial improvements in low back pain at Week 12 (≥30% and ≥50% pain reductions from baseline, respectively). Based on the Cochrane Back Review Group criteria for effect sizes, response ratios were calculated to determine if the differences seen were considered clinically relevant.10
Patients receiving OMT were more likely to achieve moderate (response ratio=1.38; 95% CI, 1.16-1.64; P<.001) and substantial (response ratio=1.41; 95% CI, 1.13-1.76; P=.002) improvements in low back pain at Week 12. The calculated number needed to treat (NNT) for moderate and significant improvement in pain at 12 weeks was 6 and 7, respectively. In addition, patients in the OMT group were more likely to be very satisfied with their care (P<.001) with an NNT of 5, and used fewer medications than did patients in the sham group during the 12 weeks of the study (use ratio=0.66; 95% CI, 0.43-1.00; P=.048; NNT=15).9
Pregnant women may benefit from OMT in the third trimester
A 2013 RCT involving 144 patients randomized to OMT, sham ultrasound, or usual obstetric care found that 68 patients (47%) experienced back-specific dysfunction during their third trimester of pregnancy (defined by a ≥2-point increase in the RMDQ).11
OMT reduced the risk of back-specific dysfunction by 40% vs the ultrasound group (relative risk [RR]=0.6; 95% CI, 0.3-1; P=.046) and 60% vs the usual obstetric care group (RR=0.4; 95% CI, 0.2-0.7; P<.001). The corresponding NNTs were 5.1 (95% CI, 2.7-282.2) for the OMT group vs the ultrasound group and 2.5 (95% CI, 1.8-4.9) vs the usual care group. The outcomes of this study were not conclusive because the initial RMDQ score was 1.8 points worse for the OMT group than for the usual care group.11
Subsequently, the PROMOTE (Pregnancy Research on Osteopathic Manipulation Optimizing Treatment Effects) study involving 400 patients demonstrated that a standard OMT protocol was effective for decreasing pain and function deterioration compared with usual obstetric care.12 However, results from the OMT group did not differ significantly from those of the ultrasound group, which were labeled as subtherapeutic in the study.12
The most recent Cochrane Review on low back pain in pregnancy noted that there was moderate quality evidence (due to study design limitations or imprecision) that OMT significantly reduced low back pain and function disability.13
OMT for other conditions? The evidence is limited
To date, studies on conditions other than low back pain have not demonstrated the same robust improvements in design as have those concerning low back pain (ie, larger sample sizes, comparisons to usual care and other treatments, etc.), and available data are not sufficiently significant to compel a change in clinical practice. Despite this, patients seek out, and receive, OMT as an alternative or adjunctive treatment for many conditions other than low back pain,2 and family physicians should be aware of the current evidence for OMT in those conditions.
OMT for acute neck pain: A comparison with ketorolac
Researchers randomized 58 patients presenting to 3 emergency departments with neck pain of less than 3 weeks’ duration to receive either OMT or 30 mg IM ketorolac.14 OMT techniques were provided at the discretion of the physician based on patient needs. Patients rated their pain intensity on an 11-point numerical scale at the time of presentation and one hour after treatment. Patients receiving ketorolac or OMT had significant reductions in pain intensity with improvements of 1.7 +/- 1.6 (95% CI, 1.1-2.3; P<.001) and 2.8 +/- 1.7 (95% CI, 2.1-3.4; P<.001), respectively.
Although the pain reduction changes were statistically significant in both groups, the improvements were small enough to question if they were functionally significant. Compared to those receiving ketorolac, those receiving OMT reported a significantly greater decrease in their pain intensity (2.8 vs 1.7; 95% CI, 0.2-1.9; P=.02), but it’s worth noting that the dose of ketorolac was half the recommended dose for moderate or severe pain.14
Patients may have more headache-free days with OMT
To assess the use of OMT to treat chronic migraine, researchers conducted a prospective, single-blind RCT in which 105 chronic migraine sufferers (average of 22.5 migraine days/month) were split into 3 treatment groups: OMT plus medications, sham OMT plus medications, and medications alone.15
OMT led to fewer days with migraines compared with the medication group (MD= -21.06; 95% CI, -23.19 to -18.92; P<.001) and sham OMT group (MD= -17.43; 95% CI, -19.57 to -15.29; P<.001), resulting in less functional disability (P<.001).15 Caution should be taken in interpreting the results of this small trial, however, as an effect of this size has not been replicated in other studies.
A small (N=29) single-blind RCT looked at progressive muscular relaxation with and without OMT for the treatment of tension headache. Patients who completed relaxation exercises plus 3 sessions of OMT experienced significantly more headache-free days (1.79 vs 0.21; P=.016).16 Despite this finding, headache intensity and headache diary ratings were not different between the 2 groups in this study.
Postoperative OMT may decrease length of stay
In a retrospective study evaluating the effect of OMT on postoperative outcomes in 55 patients who underwent gastrointestinal surgery, a total of 17 patients who received a single OMT session within 48 hours of surgery had a mean time to flatus of 3.1 days compared with 4.7 days in the usual care control group (P=.035).17 The mean length of stay was 6.1 days in the OMT group and 11.5 days in the non-OMT group (P=.006).
Major limitations of this study include that it was retrospective in design and that only 17 of 55 patients had OMT performed, indicating a possible selection bias.
Pneumonia: OMT may reduce LOS and duration of antibiotic usage
The Multicenter Osteopathic Pneumonia Study in the Elderly (MOPSE), a double-blind RCT, looked at 406 patients ≥50 years hospitalized with pneumonia. Researchers randomized the group to receive either conventional care (CC; antibiotic treatment only), OMT and antibiotic therapy, or light-touch sham therapy with antibiotics.18 The researchers found no significant differences between the groups for any outcomes in the intention-to-treat analysis.
In results obtained from the per protocol analysis, however, the median length of stay for those in the OMT group was 3.5 days, compared with 4.5 days for those in the CC group (95% CI, 3.2-4.0; P=.01). Multiple comparisons also indicated a reduction in mean duration of intravenous antibiotic use of 3 days in the OMT group (95% CI, 2.7-3.5) vs 3.5 days in the CC group (95% CI, 3.2-3.9). The treatment end-points of either death or respiratory failure occurred significantly less frequently in the OMT group compared with the CC group (P=.006).18
A Cochrane review of RCTs assessing the efficacy of adjunctive techniques compared with conventional therapy for patients with pneumonia revealed a reduction in hospital stay of 2 days (95% CI, -3.5 to -0.6) for patients who received OMT and positive expiratory pressure vs those who received neither intervention.19 Additionally, the duration of IV antibiotics and total duration of all (IV and oral) antibiotic treatment required in those treated adjunctively with OMT was shorter (MD for IV antibiotics= -2.1 days; 95% CI, -3.4 to -0.9 and MD for all antibiotics= -1.9 days; 95% CI, -3.1 to -0.7).19 The review was notable for a small sample size, with only 79 patients assessed.
OMT may improve IBS symptoms
A crossover study of 31 patients that compared visceral manipulation and sacral articulation OMT with sham therapy for the treatment of irritable bowel syndrome (IBS) demonstrated that OMT significantly decreased self-reported diarrhea (P=.016), abdominal distention (P=.043), abdominal pain (P=.013), and rectal sensitivity (P<.001), but did not significantly affect constipation.20
In another study, researchers randomized 30 patients with IBS in a 2:1 distribution to OMT vs sham treatment.21 OMT included abdominal visceral techniques and direct and indirect spine techniques. All of the patients received 2 treatment sessions, and the researchers evaluated them at 7 and 28 days. At 7 days, both groups demonstrated a significant reduction in IBS symptoms, although the OMT group had significantly greater improvement (P=.01). At 28 days, however, neither group showed a significant reduction in symptoms.21
The lack of a control group (in the first study due to the crossover design), small sample sizes, and self-reported symptoms are major limitations to applying these studies to IBS treatment recommendations.
CORRESPONDENCE
Andrew H. Slattengren, DO, Broadway Family Medicine Clinic, 1020 West Broadway Avenue, Minneapolis, MN 55411; [email protected].
1. American Association of Colleges of Osteopathic Medicine. What is osteopathic medicine? Available at: https://www.aacom.org/become-a-doctor/about-om. Accessed July 10, 2017.
2. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1-23. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19361005. Accessed November 10, 2015.
3. American Osteopathic Association. What is osteopathic medicine? Available at: http://www.osteopathic.org/osteopathic-health/Pages/what-is-osteopathic-medicine.aspx. Accessed November 17, 2017.
4. Licciardone JC, Russo DP. Blinding Protocols, Treatment Credibility, and Expectancy: Methodologic Issues in Clinical Trials of Osteopathic Manipulative Treatment. J Am Osteopath Assoc. 2006;106:457-463.
5. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166:514-530.
6. Franke H, Franke JD, Fryer G. Osteopathic manipulative treatment for nonspecific low back pain: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2014;15:286.
7. Licciardone JC, Brimhall AK, King LN. Osteopathic manipulative treatment for low back pain: a systematic review and meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2005;6:43.
8. Cohen J. Statistical Power Analysis for the Behavioral Sciences.82nd ed. Hillsdale NJ: Lawrence Erlbaum Associates; 1988.
9. Licciardone JC, Minotti DE, Gatchel RJ, et al. Osteopathic manual treatment and ultrasound therapy for chronic low back pain: a randomized controlled trial. Ann Fam Med. 2013;11:122-129.
10. Furlan AD, Pennick V, Bombardier C, et al, Editorial Board, Cochrane Back Review Group. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976). 2009;34:1929-1941.
11. Licciardone JC, Aryal S. Prevention of progressive back-specific dysfunction during pregnancy: an assessment of osteopathic manual treatment based on Cochrane Back Review Group criteria. J Am Osteopath Assoc. 2013;113:728-736.
12. Hensel KL, Buchanan S, Brown SK, et al. Pregnancy Research on Osteopathic Manipulation Optimizing Treatment Effects: the PROMOTE study. Am J Obstet Gynecol. 2015;212:108.e1-e9.
13. Pennick V, Liddle SD. Interventions for preventing and treating pelvic and back pain in pregnancy. Cochrane Database Syst Rev. 2013;8:CD001139.
14. McReynolds TM, Sheridan BJ. Intramuscular ketorolac versus osteopathic manipulative treatment in the management of acute neck pain in the emergency department: a randomized clinical trial. J Am Osteopath Assoc. 2005;105:57-68.
15. Cerritelli F, Ginevri L, Messi G, et al. Clinical effectiveness of osteopathic treatment in chronic migraine: 3-armed randomized controlled trial. Complement Ther Med. 2015;23:149-156.
16. Anderson RE, Seniscal C. A comparison of selected osteopathic treatment and relaxation for tension-type headaches. Headache. 2006;46:1273-1280.
17. Baltazar GA, Betler MP, Akella K, et al. Effect of osteopathic manipulative treatment on incidence of postoperative ileus and hospital length of stay in general surgical patients. J Am Osteopath Assoc. 2013;113:204-209.
18. Noll DR, Degenhardt BF, Morley TF, et al. Efficacy of osteopathic manipulation as an adjunctive treatment for hospitalized patients with pneumonia: a randomized controlled trial. Osteopath Med Prim Care. 2010;4:2.
19. Yang M, Yan Y, Yin X, et al. Chest physiotherapy for pneumonia in adults. Cochrane Database Syst Rev. 2013;2:CD006338.
20. Attali TV, Bouchoucha M, Benamouzig R. Treatment of refractory irritable bowel syndrome with visceral osteopathy: short-term and long-term results of a randomized trial. J Dig Dis. 2013;14:654-661.
21. Florance BM, Frin G, Dainese R, et al. Osteopathy improves the severity of irritable bowel syndrome: a pilot randomized sham-controlled study. Eur J Gastroenterol Hepatol. 2012;24:944-949.
1. American Association of Colleges of Osteopathic Medicine. What is osteopathic medicine? Available at: https://www.aacom.org/become-a-doctor/about-om. Accessed July 10, 2017.
2. Barnes PM, Bloom B, Nahin RL. Complementary and alternative medicine use among adults and children: United States, 2007. Natl Health Stat Report. 2008;12:1-23. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19361005. Accessed November 10, 2015.
3. American Osteopathic Association. What is osteopathic medicine? Available at: http://www.osteopathic.org/osteopathic-health/Pages/what-is-osteopathic-medicine.aspx. Accessed November 17, 2017.
4. Licciardone JC, Russo DP. Blinding Protocols, Treatment Credibility, and Expectancy: Methodologic Issues in Clinical Trials of Osteopathic Manipulative Treatment. J Am Osteopath Assoc. 2006;106:457-463.
5. Qaseem A, Wilt TJ, McLean RM, et al. Noninvasive Treatments for Acute, Subacute, and Chronic Low Back Pain: A Clinical Practice Guideline From the American College of Physicians. Ann Intern Med. 2017;166:514-530.
6. Franke H, Franke JD, Fryer G. Osteopathic manipulative treatment for nonspecific low back pain: a systematic review and meta-analysis. BMC Musculoskelet Disord. 2014;15:286.
7. Licciardone JC, Brimhall AK, King LN. Osteopathic manipulative treatment for low back pain: a systematic review and meta-analysis of randomized controlled trials. BMC Musculoskelet Disord. 2005;6:43.
8. Cohen J. Statistical Power Analysis for the Behavioral Sciences.82nd ed. Hillsdale NJ: Lawrence Erlbaum Associates; 1988.
9. Licciardone JC, Minotti DE, Gatchel RJ, et al. Osteopathic manual treatment and ultrasound therapy for chronic low back pain: a randomized controlled trial. Ann Fam Med. 2013;11:122-129.
10. Furlan AD, Pennick V, Bombardier C, et al, Editorial Board, Cochrane Back Review Group. 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976). 2009;34:1929-1941.
11. Licciardone JC, Aryal S. Prevention of progressive back-specific dysfunction during pregnancy: an assessment of osteopathic manual treatment based on Cochrane Back Review Group criteria. J Am Osteopath Assoc. 2013;113:728-736.
12. Hensel KL, Buchanan S, Brown SK, et al. Pregnancy Research on Osteopathic Manipulation Optimizing Treatment Effects: the PROMOTE study. Am J Obstet Gynecol. 2015;212:108.e1-e9.
13. Pennick V, Liddle SD. Interventions for preventing and treating pelvic and back pain in pregnancy. Cochrane Database Syst Rev. 2013;8:CD001139.
14. McReynolds TM, Sheridan BJ. Intramuscular ketorolac versus osteopathic manipulative treatment in the management of acute neck pain in the emergency department: a randomized clinical trial. J Am Osteopath Assoc. 2005;105:57-68.
15. Cerritelli F, Ginevri L, Messi G, et al. Clinical effectiveness of osteopathic treatment in chronic migraine: 3-armed randomized controlled trial. Complement Ther Med. 2015;23:149-156.
16. Anderson RE, Seniscal C. A comparison of selected osteopathic treatment and relaxation for tension-type headaches. Headache. 2006;46:1273-1280.
17. Baltazar GA, Betler MP, Akella K, et al. Effect of osteopathic manipulative treatment on incidence of postoperative ileus and hospital length of stay in general surgical patients. J Am Osteopath Assoc. 2013;113:204-209.
18. Noll DR, Degenhardt BF, Morley TF, et al. Efficacy of osteopathic manipulation as an adjunctive treatment for hospitalized patients with pneumonia: a randomized controlled trial. Osteopath Med Prim Care. 2010;4:2.
19. Yang M, Yan Y, Yin X, et al. Chest physiotherapy for pneumonia in adults. Cochrane Database Syst Rev. 2013;2:CD006338.
20. Attali TV, Bouchoucha M, Benamouzig R. Treatment of refractory irritable bowel syndrome with visceral osteopathy: short-term and long-term results of a randomized trial. J Dig Dis. 2013;14:654-661.
21. Florance BM, Frin G, Dainese R, et al. Osteopathy improves the severity of irritable bowel syndrome: a pilot randomized sham-controlled study. Eur J Gastroenterol Hepatol. 2012;24:944-949.
PRACTICE RECOMMENDATIONS
› Recommend osteopathic manipulative treatment to your patients with low back pain, as those who receive OMT have decreased pain, improved function, and use less medication. B
› Consider OMT as an adjunctive modality to decrease back-specific dysfunction in the third trimester of pregnancy. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
The evaluation and management of female sexual dysfunction
The care of women with female sexual disorders has made great strides since Masters and Johnson first began their study in 1957. In 2000, the Sexual Function Health Council of the American Foundation for Urologic Disease defined the classification system for female sexual dysfunction, which was eventually published and officially defined in the Diagnostic and Statistical Manual of Mental Disorders-IV-TR.1 There are now definitions for sexual desire disorders, sexual arousal disorders, orgasmic disorder, and sexual pain disorders.
Female sexual dysfunction (FSD) has complex physiologic and psychological components that require a detailed screening, history, and physical examination. Our goal in this review is to provide family physicians with insights and practical advice to help screen, diagnose, and treat female sexual dysfunction, which can have a profound impact on patients’ most intimate relationships.
Understanding the types of female sexual dysfunction
Most women consider sexual health an important part of their overall health.2 Factors that can disrupt normal sexual function include aging, socioeconomics, and other medical comorbidities. FSD is common in women throughout their lives and refers to various sexual dysfunctions including diminished arousal, problems achieving orgasm, dyspareunia, and low desire. Its prevalence is reported as high as 20% to 43%.3,4
The World Health Organization and the US Surgeon General have released statements encouraging health care providers to address sexual health during a patient’s annual visits.5 Unfortunately, despite this call to action, many patients and providers are initially hesitant to discuss these problems.6
The Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5) provides the definition and diagnostic guidelines for the different components of FSD. Its classification of sexual disorders was simplified and published in May 2013.7 There are now only 3 female dysfunctions as opposed to 5 in DSM-IV.
- Female hypoactive desire dysfunction and female arousal dysfunction were merged into a single syndrome labeled female sexual interest/arousal disorder.
- The formerly separate dyspareunia (painful intercourse) and vaginismus are now called genitopelvic pain/penetration disorder.
- Female orgasmic disorder remains as a category and is unchanged.
To qualify as a dysfunction, the problem must be present more than 75% of the time, for more than 6 months, causing significant distress, and must not be explained by a nonsexual mental disorder, relationship distress, substance abuse, or a medical condition.
Substance- or medication-induced sexual dysfunction falls under “Other Dysfunctions” and is defined as a clinically significant disturbance in sexual function that is predominant in the clinical picture. The criteria for substance- and medication-induced sexual dysfunction are unchanged and include neither the 75% nor the 6-month requirement. The diagnosis of sexual dysfunction due to a general medical condition and sexual aversion disorder are absent from the DSM-5.7
A common symptom. Female sexual disorders can be caused by several complex physiologic and psychological factors. A common symptom among many women is dyspareunia. It is seen more often in postmenopausal women, and its prevalence ranges from 8% to 22%.8 Pain on vaginal entry usually indicates vaginal atrophy, vaginal dermatitis, or provoked vestibulodynia. Pain on deep penetration could be caused by endometriosis, interstitial cystitis, or uterine leiomyomas.9
The physical examination will reproduce the pain when the vulva or vagina is touched with a cotton swab or when you insert a finger into the vagina. The differential diagnosis is listed in the TABLE.9-11
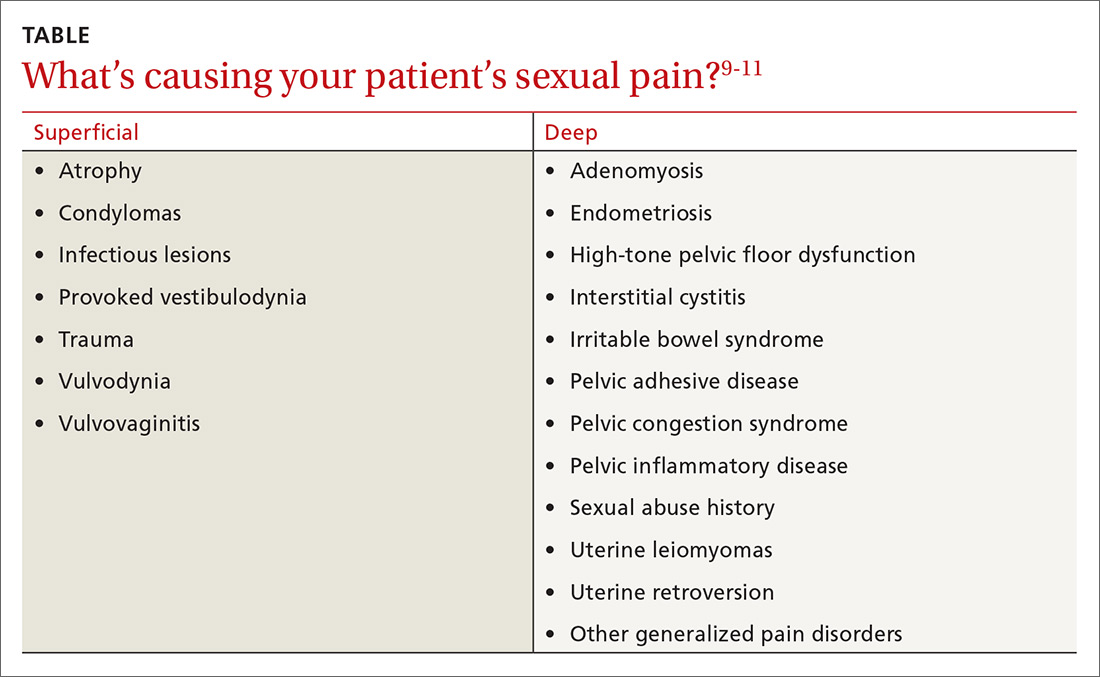
Evaluating the patient
Initially, many patients and providers may hesitate to discuss sexual dysfunction, but the annual exam is a good opportunity to broach the topic of sexual health.
Screening and history
Clinicians can screen all patients, regardless of age, with the help of a validated sex questionnaire or during a routine review of systems. There are many validated screening tools available. A simple, integrated screening tool to use is the Brief Sexual Symptom Checklist for Women (BSSC-W), created by the International Consultation in Sexual Medicine.12 Although recommended by the American Congress of Obstetricians and Gynecologists,9 the BSSC-W is not validated. The questionnaire includes 4 questions that ascertain personal information regarding an individual’s overall sexual function satisfaction, the problem causing dysfunction, how bothersome the symptoms are, and if the patient is interested in discussing it with her provider.12
It’s important to obtain a detailed obstetric and gynecologic history that includes any sexually transmitted diseases, sexual abuse, urinary and bowel complaints, or surgeries. In addition, you’ll want to differentiate between various types of dysfunctions. A thorough physical examination, including an external and internal pelvic exam, can help to rule out other causes of sexual dysfunction.
General examination: What to look for
The external pelvic examination begins with visual inspection of the vulva, labia majora, and labia minora. Often, this is best accomplished gently with a gloved hand and a cotton swab. This inspection may reveal changes in pubic hair distribution, vulvar skin disorders, lesions, masses, cracks, or fissures. Inspection may also reveal redness and pain typical of vestibulitis, a flattening and pallor of the labia that suggests estrogen deficiency, or pelvic organ prolapse.
The internal pelvic examination begins with a manual evaluation of the muscles of the pelvic floor, uterus, bladder, urethra, anus, and adnexa. Make careful note of any unusual tenderness or pelvic masses. Pelvic floor muscles (PFMs) should voluntarily contract and relax and are not normally tender to palpation. Pelvic organ prolapse and/or hypermobility of the bladder may indicate a weakening of the endopelvic fascia and may cause sexual pain. The size and flexion of the uterus, tenderness in the vaginal fornix possibly indicating endometriosis, and adnexal fullness and/or masses should be identified and evaluated.
Neurologic exam of the pelvis will involve evaluation of sensory and motor function of both lower extremities and include a screening lumbosacral neurologic examination. Lumbosacral examination includes assessment of PFM strength, anal sphincter resting tone, voluntary anal contraction, and perineal sensation. If abnormalities are noted in the screening assessment, a complete comprehensive neurologic examination should be performed.
It’s important to assess pelvic floor muscle strength
Sexual function is associated with normal PFM function.13,14 The PFMs, particularly the pubococcygeus and iliococcygeus, are responsible for involuntary contractions during orgasm.13 Orgasm has been considered a reflex, which is preceded by increased blood flow to the genital organs, tumescence of the vulva and vagina, increased secretions during sexual arousal, and increased tension and contractions of the PFMs.15
Lowenstein et al found that women with strong or moderate PFM contractions scored significantly higher on both orgasm and arousal domains of the female sexual function index (FSFI) compared with women with weak PFM contractions.16 Orgasm and arousal functions may be associated with PFM strength, with a positive association between pelvic floor strength and sexual activity and function.17,18
The function and dysfunction of the PFMs have been characterized as normal, overactive (high tone), underactive (low tone), and non-functioning.
- Normal PFMs are those that can voluntarily and involuntary contract and relax.19,20
- Overactive (high-tone) muscles are those that do not relax and possibly contract during times of relaxation for micturition or defecation. This type of dysfunction can lead to voiding dysfunction, defecatory dysfunction, and dyspareunia.19
- Underactive, or low-tone, PFMs cannot contract voluntarily. This can be associated with urinary and anal incontinence and pelvic organ prolapse.
- Nonfunctioning muscles are completely inactive.19
How to assess. There are several ways to assess PFM tone and strength.20 The first is intravaginal or intrarectal digital palpation, which can be performed when the patient is in a supine or standing position. This examination evaluates PFM tone, squeeze pressure during contraction, symmetry, and relaxation. However, there is no validated scale to quantify PFM strength. Contractions can be further divided into voluntary and involuntary.19
During the examination, the physician should ask the patient to contract as much as she can to evaluate the maximum strength and sustained contraction for endurance. This measurement can be done with digital palpation or with pressure manometry or dynamometry.
Examination can be focused on the levator ani, piriformis, and internal obturator muscles bilaterally and rated by the patient’s reactions. Pelvic muscle tenderness, which can be highly prevalent in women with chronic pelvic pain, is associated with higher degrees of dyspareunia.21 Digital evaluation of the pelvic floor musculature varies in scale, number of fingers used, and parameters evaluated. Lukban et al has described a zero
Effective treatment includes multiple options
Lifestyle modifications can help
Lifestyle changes may help improve sexual function. These modifications include physical activity, healthy diet, nutrition counseling, and adequate sleep.23,24
Identifying medical conditions such as depression and anxiety will help delineate differential diagnoses of sexual dysfunction. Cardiovascular diseases may contribute to arousal disorder as a result of atherosclerosis of the vessels supplying the vagina and clitoris. Neurologic diseases such as multiple sclerosis and diabetes can affect sexual dysfunction by impairing arousal and orgasm. Identification of concurrent comorbidities and implementation of lifestyle changes will help improve overall health and may improve sexual function.25
In addition, Herati et al26 found food sensitivities to grapefruit juice, spicy foods, alcohol, and caffeine were more prevalent in patients with interstitial cystitis and chronic pelvic pain. Avoiding irritants such as soap and other detergents in the perineal region may help decrease dysfunction.27 Finally, foods high in oxalate and other acidic items may cause bladder pain and worsening symptoms of vulvodynia.28
Topical therapies worth considering
Lubricants and moisturizers may help women with dyspareunia or symptoms of vaginal atrophy.
Zestra, for instance, which is applied to the vulva prior to sexual activity, has been proven more effective than placebo for improving desire and arousal.29
Neogyn is a non-hormonal cream containing cutaneous lysate and has been shown to improve vulvar pain in women with vulvodynia. A double-blind placebo-controlled randomized crossover trial followed 30 patients over 3 months and found a significant reduction in pain during sexual activity and a significant reduction in erythema.30
Alprostadil is a prostaglandin E1 analogue that increases genital vasodilation when applied topically and is currently undergoing investigational trials.31,32 Patients can also choose from many over-the-counter lubricants that contain water-based, oil-based, or silicone-based ingredients.
Don’t overlook physical therapy
Manual therapies, including the transvaginal technique, are used for female sexual dysfunction that results from a variety of causes, including high-tone pelvic floor dysfunction. The transvaginal technique can identify myofascial pain; treatment involves internal release of the PFMs and external trigger point identification and alleviation.
One pilot study, which involved transvaginal Thiele massage twice a week for 5 weeks on 21 symptomatic women with IC and high-tone pelvic floor dysfunction found it decreased hyptertonicity of the pelvic floor and generated statistically significant improvement in the Symptom and Problem Indexes of the O’Leary-Sant Questionnaire, Likert Visual Analogue Scales for urgency and pain, and the Physical and Mental Component Summary from the SF-12 Quality-of-Life Scale.33 Transvaginal physical therapy is also an effective treatment for myofascial pelvic pain.34
Biofeedback, which can be used in combination with pelvic floor physical therapy, teaches the patient to control the PFMs by visualizing the activity to achieve conscious control over contraction of the pelvic floor and ceasing the cycle of spasm.35 Ger et al36 investigated patients with levator spasm and found biofeedback decreased pain; relief was rated as good or excellent at 15-month follow-up in 6 out of 14 patients (43%).
Home devices such as Eros Therapy, an FDA-approved, nonpharmacologic battery-operated device, provide vacuum suction to the clitoris with vibratory sensation. Eros Therapy has been shown to increase blood flow to the clitoris, vagina, and pelvic floor and increase sensation, orgasm, lubrication, and satisfaction.37
Vaginal dilators allow increasing lengths and girths designed to treat vaginal and pelvic floor pain.38 In our practice, we encourage pelvic muscle strengthening tools in the form of kegal trainers and other insertion devices that may improve PFM coordination and strength.
Pharmacotherapy has its place
The treatment of FSD may require a multimodal systematic approach targeting genito-pelvic pain. But before the best options can be found, it is important to first establish the cause of the pain. Several drug formulations have been effectively used including hormonal and non-hormonal options.
Conjugated estrogens are FDA approved for the treatment of dyspareunia, which can contribute to decreased desire. Systemic estrogen in oral form, transdermal preparations, and topical formulations may increase sexual desire and arousal and decrease dyspareunia.39 Even synthetic steroid compounds such as tibolone may improve sexual function, although it is not FDA approved for that purpose.40
Ospemifene (Osphena) is a selective estrogen receptor modulator that acts as an estrogen agonist in select tissues, including vaginal epithelium. It is FDA approved for dyspareunia in postmenopausal women.41,42 A daily dose of 60 mg is effective and safe with minimal adverse effects.42 Studies suggest that testosterone, although not FDA approved in the United States for this purpose, improves sexual desire, pleasure, orgasm, and arousal satisfaction.39 The hormone has not gained FDA approval because of concerns about long-term safety and efficacy.42
Non-hormonal drugs including flibanserin (Addyi), a well-tolerated serotonin receptor 1A agonist, 2A antagonist shown to improve sexual desire, increase the number of satisfying sexual events, and reduce distress associated with low sexual desire when compared with placebo.43 The FDA has approved flibanserin as the first treatment targeted for women with hypoactive sexual desire disorder (HSDD). It can, however, cause severe hypotension and syncope, is not well tolerated with alcohol, and is contraindicated in patients who take strong CYP3A4 inhibitors, such as fluconazole, verapamil, and erythromycin, or who have liver impairment.
Buproprion, a mild dopamine and norepinephrine reuptake inhibitor and acetylcholine receptor antagonist, has been shown to improve desire in women with and without depression. Although it is FDA approved for major depressive disorder, it is not approved for female sexual dysfunction and is still under investigation.
Tricyclic antidepressants such as nortriptyline and amitriptyline may be effective in treating neuropathic pain. Starting doses of both amitriptyline and nortriptyline are 10 mg/d and can be increased to a maximum of 100 mg/d.44 Tricyclic antidepressants are still under investigation for the treatment of FSD.
Muscle relaxants in oral and topical compounded form are used to treat increased pelvic floor tension and spasticity. Cyclobenzaprine and tizanidine are FDA-approved muscle relaxants indicated for muscle spasticity.
Cyclobenzaprine, at a starting dose of 10 mg, can be taken up to 3 times a day for pelvic floor tension. Tizanidine is a centrally active alpha 2 agonist that’s superior to placebo in treating high-tone pelvic floor dysfunction.44
Other medications include benzodiazepines such as oral clonazepam and intra-vaginal diazepam, although they are not FDA approved for high-tone pelvic floor dysfunction. Rogalski et al reviewed 26 patients who received vaginal diazepam for bladder pain, sexual pain, and levator hypertonus.45 They found subjective and sexual pain improvement assessed on FSFI and the visual analog pain scale. PFM tone significantly improved during resting, squeezing, and relaxation phases. Multimodal therapy can be used for muscle spasticity and high-tone pelvic floor dysfunction.
Trigger point and Botox injections
Although drug therapy has its place in the management of sexual dysfunction, other modalities that involve trigger point injections or botulinum toxin injections to the PFMs may prove helpful for patients with high-tone pelvic floor dysfunction.
A prospective study investigated the role of trigger point injections in 18 women with levator ani muscle spasm with a mixture of 0.25% bupivacaine in 10 mL, 2% lidocaine in 10 mL, and 40 mg of triamcinolone in 1 mL combined and used for injection of 5 mL per trigger point.46 Three months after injections, 13 of the 18 women improved, resulting in a success rate of 72%. Trigger point injections can be applied externally or transvaginally.
OnabotulinumtoxinA (Botox) has also been tested for relief of levator ani muscle spasm. Botox is FDA approved for upper and lower limb spasticity but is not approved for pelvic floor spasticity or tension. It may reduce pressure in the PFMs and may be useful in women with high-tone pelvic floor dysfunction.47
In a prospective 6-month pilot study, 28 patients with pelvic pain who failed conservative treatment received up to 300 U Botox into the pelvic floor.11 The study, which used needle electromyography guidance and a transperineal approach, found that the dyspareunia visual analog scale improved significantly at Weeks 12 and 24. Keep in mind, however, that onabotulinumtoxinA should be reserved for patients who fail conventional treatments.47,48
Addressing psychological issues
Sex therapy is a traditional approach that aims to improve individual or couples’ sexual experiences and help reduce anxiety related to sex.42 Cognitive behavioral sex therapy includes traditional sex therapy components but puts greater emphasis on modifying thought patterns that interfere with intimacy and sex.42
Mindfulness-based cognitive-behavioral treatments have shown promise for sexual desire problems. It is an ancient eastern practice with Buddhist roots. This therapy is a nonjudgmental, present-moment awareness comprised of self-regulation of attention and accepting orientation to the present.49 Although there is little evidence from prospective studies, it may benefit women with sexual dysfunction after intervention with sex therapy and cognitive behavioral therapy.
Female sexual dysfunction is common and affects women of all ages. It can negatively impact a women’s quality of life and overall well-being. The etiology of FSD is complex, and treatments are based on the causes of the dysfunction. Difficult cases warrant referral to a specialist in sexual health and female pelvic medicine. Future prospective trials, randomized controlled trials, the use of validated questionnaires, and meta-analyses will continue to move us forward as we find better ways to understand, identify, and treat female sexual dysfunction.
CORRESPONDENCE
Melissa L. Dawson, DO, MS, Department of OB/GYN, Drexel University College of Medicine, 207 N Broad St. 4th Floor, Philadelphia, PA 19107; [email protected].
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Washington, DC; 1994.
2. Shifren, JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
3. Lewis RW, Fugl-Meyer KS, Bosch R, et al., Epidemiology/risk factors of sexual dysfunction. J Sex Med. 2004;1:35-39.
4. Laumann E, Paik A, Rosen RC. Sexual dysfunction in the United States prevalence and predictors. JAMA. 1999;281:537-544.
5. Office of the Surgeon General. The Surgeon General’s Call to Action to Promote Sexual Health and Responsible Sexual Behavior, Rockville, MD; 2001.
6. Pauls RN, Kleeman SD, Segal JL, et al. Practice patterns of physician members of the American Urogynecologic Society regarding female sexual dysfunction: results of a national survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:460-467.
7. American Psychiatric Association. Sexual Dysfunction. In: Diagnostic and Statistical Manual of Mental Disorders (5thed). Washington, DC; 2013.
8. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009. 113:1124-1136.
9. ACOG Practice Bulletin No. 119: Female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007.
10. Clayton AH, Hamilton DV. Female sexual dysfunction. Psychiatr Clin North Am. 2017;40:267-284.
11. Morrissey D, El-Khawand D, Ginzburg N, et al. Botulinum Toxin A injections into pelvic floor muscles under electromyographic guidance for women with refractory high-tone pelvic floor dysfunction: a 6-month prospective pilot study. Female Pelvic Med Reconstr Surg. 2015;21:277-282.
12. Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 Pt 2):337-348.
13. Kegel, A. Sexual functions of the pubococcygeus muscle. West J Surg Obstet Gynecol. 1952;60:521-524.
14. Shafik A. The Role of the levator ani muscle in evacuation, sexual performance and pelvic floor disorders. Int Urogynecol J. 2000;11:361-376.
15. Kinsey A, Pomeroy WB, Martin CE, et al. Sexual behavior in the human female. W. B. Saunders:Philadelphia, PA; 1998.
16. Lowenstein L, Gruenwald, Gartman I, et al. Can stronger pelvic muscle floor improve sexual function? Int Urogynecol J. 2010;21:553-556.
17. Kanter G, Rogers RG, Pauls RN, et al. A strong pelvic floor is associated with higher rates of sexual activity in women with pelvic floor disorders. Int Urogynecol J. 2015;26:991-996.
18. Wehbe SA, Kellogg-Spadt S, Whitmore K. Urogenital complaints and female sexual dysfunction. Part 2. J Sex Med. 2010;7:2304-2317.
19. Messelink B, Benson T, Berghmans B, et al. Standardization of terminology of pelvic floor muscle function and dysfunction: report from the pelvic floor clinical assessment group of the International Continence Society. Neurourol Urodyn. 2005;24:374-380.
20. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4-20.
21. Montenegro ML, Mateus-Vasconcelos EC, Rosa e Silva JC et al. Importance of pelvic muscle tenderness evaluation in women with chronic pelvic pain. Pain Med. 2010;11:224-228.
22. Lukban JC, Whitmore KE. Pelvic floor muscle re-education treatment of the overactive bladder and painful bladder syndrome. Clin Obstet Gynecol. 2002;45:273-285.
23. Kalmbach DA, Arnedt JT, Pillai V, et al. The impact of sleep on female sexual response and behavior: a pilot study. J Sex Med. 2015;12:1221-1232.
24. Aversa A, Bruzziches R, Francomano D, et al. Weight loss by multidisciplinary intervention improves endothelial and sexual function in obese fertile women. J Sex Med. 2013;10:1024-1033.
25. Pauls RN, Kleeman SD, Karram MM. Female sexual dysfunction: principles of diagnosis and therapy. Obstet Gynecol Surv. 2005;60:196-205.
26. Herati AS, Shorter B, Tai J, et al. Differences in food sensitivities between female interstitial cystitis/painful bladder syndrome (IC/PBS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) patients. J Urol. 2009;181(4)(Suppl):22.
27. Farrell J, Cacchioni T, The medicalization of women’s sexual pain. J Sex Res. 2012;49:328-336.
28. De Andres J, Sanchis-Lopez NM, Asensio-Samper JM, et al. Vulvodynia—an evidence-based literature review and proposed treatment algorithm. Pain Pract. 2016;16:204-236.
29. Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014:11:642-652.
30. Donders GG, Bellen G. Cream with cutaneous fibroblast lysate for the treatment of provoked vestibulodynia: a double-blind randomized placebo-controlled crossover study. J Low Genit Tract Dis. 2012;16:427-436.
31. Belkin ZR, Krapf JM, Goldstein AT. Drugs in early clinical development for the treatment of female sexual dysfunction. Expert Opin Investig Drugs. 2015;24:159-167.
32. Islam A, Mitchel J, Rosen R, et al. Topical alprostadil in the treatment of female sexual arousal disorder: a pilot study. J Sex Marital Ther. 2001;27:531-540.
33. Oyama IA, Rejba A, Lukban JC, et al. Modified Thiele massage as therapeutic intervention for female patients with interstitial cystitis and high-tone pelvic floor dysfunction. Urology. 2004;64:862-865.
34. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
35. Wehbe SA, Fariello JY, Whitmore K. Minimally invasive therapies for chronic pelvic pain syndrome. Curr Urol Rep. 2010;11:276-285.
36. Ger GC, Wexner SD, Jorge JM, et al. Evaluation and treatment of chronic intractable rectal pain—a frustrating endeavor. Dis Colon Rectum. 1993;36:139-145.
37. Billups KL, Berman L, Berman J, et al. A new non-pharmacological vacuum therapy for female sexual dysfunction. J Sex Marital Ther. 2001;27:435-441.
38. Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;9:Cd007291.
39. Goldstein I. Current management strategies of the postmenopausal patient with sexual health problems. J Sex Med. 2007;4(Suppl 3):235-253.
40. Modelska K, Cummings S. Female sexual dysfunction in postmenopausal women: systematic review of placebo-controlled trials. Am J Obstet Gynecol. 2003;188:286-293.
41. Constantine G, Graham S, Portman DJ, et al. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric. 2015;18:226-232.
42. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125:477-486.
43. Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014; 21:633-640.
44. Curtis Nickel J, Baranowski AP, Pontari M, et al. Management of men diagnosed with chronic prostatitis/chronic pelvic pain syndrome who have failed traditional management. Rev Urol. 2007;9:63-72.
45. Rogalski MJ, Kellogg-Spadt S, Hoffmann AR, et al. Retrospective chart review of vaginal diazepam suppository use in high-tone pelvic floor dysfunction. Int Urogynecol J. 2010:21:895-899.
46. Langford CF, Udvari Nagy S, Ghoniem GM. Levator ani trigger point injections: an underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26:59-62.
47. Abbott JA, Jarvis SK, Lyons SD, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006.108:915-923.
48. Kamanli A, Kaya A, Ardicoglu O, et al. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int. 2005;25:604-611.
49. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol. 2012;125:320-325.
The care of women with female sexual disorders has made great strides since Masters and Johnson first began their study in 1957. In 2000, the Sexual Function Health Council of the American Foundation for Urologic Disease defined the classification system for female sexual dysfunction, which was eventually published and officially defined in the Diagnostic and Statistical Manual of Mental Disorders-IV-TR.1 There are now definitions for sexual desire disorders, sexual arousal disorders, orgasmic disorder, and sexual pain disorders.
Female sexual dysfunction (FSD) has complex physiologic and psychological components that require a detailed screening, history, and physical examination. Our goal in this review is to provide family physicians with insights and practical advice to help screen, diagnose, and treat female sexual dysfunction, which can have a profound impact on patients’ most intimate relationships.
Understanding the types of female sexual dysfunction
Most women consider sexual health an important part of their overall health.2 Factors that can disrupt normal sexual function include aging, socioeconomics, and other medical comorbidities. FSD is common in women throughout their lives and refers to various sexual dysfunctions including diminished arousal, problems achieving orgasm, dyspareunia, and low desire. Its prevalence is reported as high as 20% to 43%.3,4
The World Health Organization and the US Surgeon General have released statements encouraging health care providers to address sexual health during a patient’s annual visits.5 Unfortunately, despite this call to action, many patients and providers are initially hesitant to discuss these problems.6
The Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5) provides the definition and diagnostic guidelines for the different components of FSD. Its classification of sexual disorders was simplified and published in May 2013.7 There are now only 3 female dysfunctions as opposed to 5 in DSM-IV.
- Female hypoactive desire dysfunction and female arousal dysfunction were merged into a single syndrome labeled female sexual interest/arousal disorder.
- The formerly separate dyspareunia (painful intercourse) and vaginismus are now called genitopelvic pain/penetration disorder.
- Female orgasmic disorder remains as a category and is unchanged.
To qualify as a dysfunction, the problem must be present more than 75% of the time, for more than 6 months, causing significant distress, and must not be explained by a nonsexual mental disorder, relationship distress, substance abuse, or a medical condition.
Substance- or medication-induced sexual dysfunction falls under “Other Dysfunctions” and is defined as a clinically significant disturbance in sexual function that is predominant in the clinical picture. The criteria for substance- and medication-induced sexual dysfunction are unchanged and include neither the 75% nor the 6-month requirement. The diagnosis of sexual dysfunction due to a general medical condition and sexual aversion disorder are absent from the DSM-5.7
A common symptom. Female sexual disorders can be caused by several complex physiologic and psychological factors. A common symptom among many women is dyspareunia. It is seen more often in postmenopausal women, and its prevalence ranges from 8% to 22%.8 Pain on vaginal entry usually indicates vaginal atrophy, vaginal dermatitis, or provoked vestibulodynia. Pain on deep penetration could be caused by endometriosis, interstitial cystitis, or uterine leiomyomas.9
The physical examination will reproduce the pain when the vulva or vagina is touched with a cotton swab or when you insert a finger into the vagina. The differential diagnosis is listed in the TABLE.9-11

Evaluating the patient
Initially, many patients and providers may hesitate to discuss sexual dysfunction, but the annual exam is a good opportunity to broach the topic of sexual health.
Screening and history
Clinicians can screen all patients, regardless of age, with the help of a validated sex questionnaire or during a routine review of systems. There are many validated screening tools available. A simple, integrated screening tool to use is the Brief Sexual Symptom Checklist for Women (BSSC-W), created by the International Consultation in Sexual Medicine.12 Although recommended by the American Congress of Obstetricians and Gynecologists,9 the BSSC-W is not validated. The questionnaire includes 4 questions that ascertain personal information regarding an individual’s overall sexual function satisfaction, the problem causing dysfunction, how bothersome the symptoms are, and if the patient is interested in discussing it with her provider.12
It’s important to obtain a detailed obstetric and gynecologic history that includes any sexually transmitted diseases, sexual abuse, urinary and bowel complaints, or surgeries. In addition, you’ll want to differentiate between various types of dysfunctions. A thorough physical examination, including an external and internal pelvic exam, can help to rule out other causes of sexual dysfunction.
General examination: What to look for
The external pelvic examination begins with visual inspection of the vulva, labia majora, and labia minora. Often, this is best accomplished gently with a gloved hand and a cotton swab. This inspection may reveal changes in pubic hair distribution, vulvar skin disorders, lesions, masses, cracks, or fissures. Inspection may also reveal redness and pain typical of vestibulitis, a flattening and pallor of the labia that suggests estrogen deficiency, or pelvic organ prolapse.
The internal pelvic examination begins with a manual evaluation of the muscles of the pelvic floor, uterus, bladder, urethra, anus, and adnexa. Make careful note of any unusual tenderness or pelvic masses. Pelvic floor muscles (PFMs) should voluntarily contract and relax and are not normally tender to palpation. Pelvic organ prolapse and/or hypermobility of the bladder may indicate a weakening of the endopelvic fascia and may cause sexual pain. The size and flexion of the uterus, tenderness in the vaginal fornix possibly indicating endometriosis, and adnexal fullness and/or masses should be identified and evaluated.
Neurologic exam of the pelvis will involve evaluation of sensory and motor function of both lower extremities and include a screening lumbosacral neurologic examination. Lumbosacral examination includes assessment of PFM strength, anal sphincter resting tone, voluntary anal contraction, and perineal sensation. If abnormalities are noted in the screening assessment, a complete comprehensive neurologic examination should be performed.
It’s important to assess pelvic floor muscle strength
Sexual function is associated with normal PFM function.13,14 The PFMs, particularly the pubococcygeus and iliococcygeus, are responsible for involuntary contractions during orgasm.13 Orgasm has been considered a reflex, which is preceded by increased blood flow to the genital organs, tumescence of the vulva and vagina, increased secretions during sexual arousal, and increased tension and contractions of the PFMs.15
Lowenstein et al found that women with strong or moderate PFM contractions scored significantly higher on both orgasm and arousal domains of the female sexual function index (FSFI) compared with women with weak PFM contractions.16 Orgasm and arousal functions may be associated with PFM strength, with a positive association between pelvic floor strength and sexual activity and function.17,18
The function and dysfunction of the PFMs have been characterized as normal, overactive (high tone), underactive (low tone), and non-functioning.
- Normal PFMs are those that can voluntarily and involuntary contract and relax.19,20
- Overactive (high-tone) muscles are those that do not relax and possibly contract during times of relaxation for micturition or defecation. This type of dysfunction can lead to voiding dysfunction, defecatory dysfunction, and dyspareunia.19
- Underactive, or low-tone, PFMs cannot contract voluntarily. This can be associated with urinary and anal incontinence and pelvic organ prolapse.
- Nonfunctioning muscles are completely inactive.19
How to assess. There are several ways to assess PFM tone and strength.20 The first is intravaginal or intrarectal digital palpation, which can be performed when the patient is in a supine or standing position. This examination evaluates PFM tone, squeeze pressure during contraction, symmetry, and relaxation. However, there is no validated scale to quantify PFM strength. Contractions can be further divided into voluntary and involuntary.19
During the examination, the physician should ask the patient to contract as much as she can to evaluate the maximum strength and sustained contraction for endurance. This measurement can be done with digital palpation or with pressure manometry or dynamometry.
Examination can be focused on the levator ani, piriformis, and internal obturator muscles bilaterally and rated by the patient’s reactions. Pelvic muscle tenderness, which can be highly prevalent in women with chronic pelvic pain, is associated with higher degrees of dyspareunia.21 Digital evaluation of the pelvic floor musculature varies in scale, number of fingers used, and parameters evaluated. Lukban et al has described a zero
Effective treatment includes multiple options
Lifestyle modifications can help
Lifestyle changes may help improve sexual function. These modifications include physical activity, healthy diet, nutrition counseling, and adequate sleep.23,24
Identifying medical conditions such as depression and anxiety will help delineate differential diagnoses of sexual dysfunction. Cardiovascular diseases may contribute to arousal disorder as a result of atherosclerosis of the vessels supplying the vagina and clitoris. Neurologic diseases such as multiple sclerosis and diabetes can affect sexual dysfunction by impairing arousal and orgasm. Identification of concurrent comorbidities and implementation of lifestyle changes will help improve overall health and may improve sexual function.25
In addition, Herati et al26 found food sensitivities to grapefruit juice, spicy foods, alcohol, and caffeine were more prevalent in patients with interstitial cystitis and chronic pelvic pain. Avoiding irritants such as soap and other detergents in the perineal region may help decrease dysfunction.27 Finally, foods high in oxalate and other acidic items may cause bladder pain and worsening symptoms of vulvodynia.28
Topical therapies worth considering
Lubricants and moisturizers may help women with dyspareunia or symptoms of vaginal atrophy.
Zestra, for instance, which is applied to the vulva prior to sexual activity, has been proven more effective than placebo for improving desire and arousal.29
Neogyn is a non-hormonal cream containing cutaneous lysate and has been shown to improve vulvar pain in women with vulvodynia. A double-blind placebo-controlled randomized crossover trial followed 30 patients over 3 months and found a significant reduction in pain during sexual activity and a significant reduction in erythema.30
Alprostadil is a prostaglandin E1 analogue that increases genital vasodilation when applied topically and is currently undergoing investigational trials.31,32 Patients can also choose from many over-the-counter lubricants that contain water-based, oil-based, or silicone-based ingredients.
Don’t overlook physical therapy
Manual therapies, including the transvaginal technique, are used for female sexual dysfunction that results from a variety of causes, including high-tone pelvic floor dysfunction. The transvaginal technique can identify myofascial pain; treatment involves internal release of the PFMs and external trigger point identification and alleviation.
One pilot study, which involved transvaginal Thiele massage twice a week for 5 weeks on 21 symptomatic women with IC and high-tone pelvic floor dysfunction found it decreased hyptertonicity of the pelvic floor and generated statistically significant improvement in the Symptom and Problem Indexes of the O’Leary-Sant Questionnaire, Likert Visual Analogue Scales for urgency and pain, and the Physical and Mental Component Summary from the SF-12 Quality-of-Life Scale.33 Transvaginal physical therapy is also an effective treatment for myofascial pelvic pain.34
Biofeedback, which can be used in combination with pelvic floor physical therapy, teaches the patient to control the PFMs by visualizing the activity to achieve conscious control over contraction of the pelvic floor and ceasing the cycle of spasm.35 Ger et al36 investigated patients with levator spasm and found biofeedback decreased pain; relief was rated as good or excellent at 15-month follow-up in 6 out of 14 patients (43%).
Home devices such as Eros Therapy, an FDA-approved, nonpharmacologic battery-operated device, provide vacuum suction to the clitoris with vibratory sensation. Eros Therapy has been shown to increase blood flow to the clitoris, vagina, and pelvic floor and increase sensation, orgasm, lubrication, and satisfaction.37
Vaginal dilators allow increasing lengths and girths designed to treat vaginal and pelvic floor pain.38 In our practice, we encourage pelvic muscle strengthening tools in the form of kegal trainers and other insertion devices that may improve PFM coordination and strength.
Pharmacotherapy has its place
The treatment of FSD may require a multimodal systematic approach targeting genito-pelvic pain. But before the best options can be found, it is important to first establish the cause of the pain. Several drug formulations have been effectively used including hormonal and non-hormonal options.
Conjugated estrogens are FDA approved for the treatment of dyspareunia, which can contribute to decreased desire. Systemic estrogen in oral form, transdermal preparations, and topical formulations may increase sexual desire and arousal and decrease dyspareunia.39 Even synthetic steroid compounds such as tibolone may improve sexual function, although it is not FDA approved for that purpose.40
Ospemifene (Osphena) is a selective estrogen receptor modulator that acts as an estrogen agonist in select tissues, including vaginal epithelium. It is FDA approved for dyspareunia in postmenopausal women.41,42 A daily dose of 60 mg is effective and safe with minimal adverse effects.42 Studies suggest that testosterone, although not FDA approved in the United States for this purpose, improves sexual desire, pleasure, orgasm, and arousal satisfaction.39 The hormone has not gained FDA approval because of concerns about long-term safety and efficacy.42
Non-hormonal drugs including flibanserin (Addyi), a well-tolerated serotonin receptor 1A agonist, 2A antagonist shown to improve sexual desire, increase the number of satisfying sexual events, and reduce distress associated with low sexual desire when compared with placebo.43 The FDA has approved flibanserin as the first treatment targeted for women with hypoactive sexual desire disorder (HSDD). It can, however, cause severe hypotension and syncope, is not well tolerated with alcohol, and is contraindicated in patients who take strong CYP3A4 inhibitors, such as fluconazole, verapamil, and erythromycin, or who have liver impairment.
Buproprion, a mild dopamine and norepinephrine reuptake inhibitor and acetylcholine receptor antagonist, has been shown to improve desire in women with and without depression. Although it is FDA approved for major depressive disorder, it is not approved for female sexual dysfunction and is still under investigation.
Tricyclic antidepressants such as nortriptyline and amitriptyline may be effective in treating neuropathic pain. Starting doses of both amitriptyline and nortriptyline are 10 mg/d and can be increased to a maximum of 100 mg/d.44 Tricyclic antidepressants are still under investigation for the treatment of FSD.
Muscle relaxants in oral and topical compounded form are used to treat increased pelvic floor tension and spasticity. Cyclobenzaprine and tizanidine are FDA-approved muscle relaxants indicated for muscle spasticity.
Cyclobenzaprine, at a starting dose of 10 mg, can be taken up to 3 times a day for pelvic floor tension. Tizanidine is a centrally active alpha 2 agonist that’s superior to placebo in treating high-tone pelvic floor dysfunction.44
Other medications include benzodiazepines such as oral clonazepam and intra-vaginal diazepam, although they are not FDA approved for high-tone pelvic floor dysfunction. Rogalski et al reviewed 26 patients who received vaginal diazepam for bladder pain, sexual pain, and levator hypertonus.45 They found subjective and sexual pain improvement assessed on FSFI and the visual analog pain scale. PFM tone significantly improved during resting, squeezing, and relaxation phases. Multimodal therapy can be used for muscle spasticity and high-tone pelvic floor dysfunction.
Trigger point and Botox injections
Although drug therapy has its place in the management of sexual dysfunction, other modalities that involve trigger point injections or botulinum toxin injections to the PFMs may prove helpful for patients with high-tone pelvic floor dysfunction.
A prospective study investigated the role of trigger point injections in 18 women with levator ani muscle spasm with a mixture of 0.25% bupivacaine in 10 mL, 2% lidocaine in 10 mL, and 40 mg of triamcinolone in 1 mL combined and used for injection of 5 mL per trigger point.46 Three months after injections, 13 of the 18 women improved, resulting in a success rate of 72%. Trigger point injections can be applied externally or transvaginally.
OnabotulinumtoxinA (Botox) has also been tested for relief of levator ani muscle spasm. Botox is FDA approved for upper and lower limb spasticity but is not approved for pelvic floor spasticity or tension. It may reduce pressure in the PFMs and may be useful in women with high-tone pelvic floor dysfunction.47
In a prospective 6-month pilot study, 28 patients with pelvic pain who failed conservative treatment received up to 300 U Botox into the pelvic floor.11 The study, which used needle electromyography guidance and a transperineal approach, found that the dyspareunia visual analog scale improved significantly at Weeks 12 and 24. Keep in mind, however, that onabotulinumtoxinA should be reserved for patients who fail conventional treatments.47,48
Addressing psychological issues
Sex therapy is a traditional approach that aims to improve individual or couples’ sexual experiences and help reduce anxiety related to sex.42 Cognitive behavioral sex therapy includes traditional sex therapy components but puts greater emphasis on modifying thought patterns that interfere with intimacy and sex.42
Mindfulness-based cognitive-behavioral treatments have shown promise for sexual desire problems. It is an ancient eastern practice with Buddhist roots. This therapy is a nonjudgmental, present-moment awareness comprised of self-regulation of attention and accepting orientation to the present.49 Although there is little evidence from prospective studies, it may benefit women with sexual dysfunction after intervention with sex therapy and cognitive behavioral therapy.
Female sexual dysfunction is common and affects women of all ages. It can negatively impact a women’s quality of life and overall well-being. The etiology of FSD is complex, and treatments are based on the causes of the dysfunction. Difficult cases warrant referral to a specialist in sexual health and female pelvic medicine. Future prospective trials, randomized controlled trials, the use of validated questionnaires, and meta-analyses will continue to move us forward as we find better ways to understand, identify, and treat female sexual dysfunction.
CORRESPONDENCE
Melissa L. Dawson, DO, MS, Department of OB/GYN, Drexel University College of Medicine, 207 N Broad St. 4th Floor, Philadelphia, PA 19107; [email protected].
The care of women with female sexual disorders has made great strides since Masters and Johnson first began their study in 1957. In 2000, the Sexual Function Health Council of the American Foundation for Urologic Disease defined the classification system for female sexual dysfunction, which was eventually published and officially defined in the Diagnostic and Statistical Manual of Mental Disorders-IV-TR.1 There are now definitions for sexual desire disorders, sexual arousal disorders, orgasmic disorder, and sexual pain disorders.
Female sexual dysfunction (FSD) has complex physiologic and psychological components that require a detailed screening, history, and physical examination. Our goal in this review is to provide family physicians with insights and practical advice to help screen, diagnose, and treat female sexual dysfunction, which can have a profound impact on patients’ most intimate relationships.
Understanding the types of female sexual dysfunction
Most women consider sexual health an important part of their overall health.2 Factors that can disrupt normal sexual function include aging, socioeconomics, and other medical comorbidities. FSD is common in women throughout their lives and refers to various sexual dysfunctions including diminished arousal, problems achieving orgasm, dyspareunia, and low desire. Its prevalence is reported as high as 20% to 43%.3,4
The World Health Organization and the US Surgeon General have released statements encouraging health care providers to address sexual health during a patient’s annual visits.5 Unfortunately, despite this call to action, many patients and providers are initially hesitant to discuss these problems.6
The Diagnostic and Statistical Manual of Mental Disorders, Fifth edition (DSM-5) provides the definition and diagnostic guidelines for the different components of FSD. Its classification of sexual disorders was simplified and published in May 2013.7 There are now only 3 female dysfunctions as opposed to 5 in DSM-IV.
- Female hypoactive desire dysfunction and female arousal dysfunction were merged into a single syndrome labeled female sexual interest/arousal disorder.
- The formerly separate dyspareunia (painful intercourse) and vaginismus are now called genitopelvic pain/penetration disorder.
- Female orgasmic disorder remains as a category and is unchanged.
To qualify as a dysfunction, the problem must be present more than 75% of the time, for more than 6 months, causing significant distress, and must not be explained by a nonsexual mental disorder, relationship distress, substance abuse, or a medical condition.
Substance- or medication-induced sexual dysfunction falls under “Other Dysfunctions” and is defined as a clinically significant disturbance in sexual function that is predominant in the clinical picture. The criteria for substance- and medication-induced sexual dysfunction are unchanged and include neither the 75% nor the 6-month requirement. The diagnosis of sexual dysfunction due to a general medical condition and sexual aversion disorder are absent from the DSM-5.7
A common symptom. Female sexual disorders can be caused by several complex physiologic and psychological factors. A common symptom among many women is dyspareunia. It is seen more often in postmenopausal women, and its prevalence ranges from 8% to 22%.8 Pain on vaginal entry usually indicates vaginal atrophy, vaginal dermatitis, or provoked vestibulodynia. Pain on deep penetration could be caused by endometriosis, interstitial cystitis, or uterine leiomyomas.9
The physical examination will reproduce the pain when the vulva or vagina is touched with a cotton swab or when you insert a finger into the vagina. The differential diagnosis is listed in the TABLE.9-11

Evaluating the patient
Initially, many patients and providers may hesitate to discuss sexual dysfunction, but the annual exam is a good opportunity to broach the topic of sexual health.
Screening and history
Clinicians can screen all patients, regardless of age, with the help of a validated sex questionnaire or during a routine review of systems. There are many validated screening tools available. A simple, integrated screening tool to use is the Brief Sexual Symptom Checklist for Women (BSSC-W), created by the International Consultation in Sexual Medicine.12 Although recommended by the American Congress of Obstetricians and Gynecologists,9 the BSSC-W is not validated. The questionnaire includes 4 questions that ascertain personal information regarding an individual’s overall sexual function satisfaction, the problem causing dysfunction, how bothersome the symptoms are, and if the patient is interested in discussing it with her provider.12
It’s important to obtain a detailed obstetric and gynecologic history that includes any sexually transmitted diseases, sexual abuse, urinary and bowel complaints, or surgeries. In addition, you’ll want to differentiate between various types of dysfunctions. A thorough physical examination, including an external and internal pelvic exam, can help to rule out other causes of sexual dysfunction.
General examination: What to look for
The external pelvic examination begins with visual inspection of the vulva, labia majora, and labia minora. Often, this is best accomplished gently with a gloved hand and a cotton swab. This inspection may reveal changes in pubic hair distribution, vulvar skin disorders, lesions, masses, cracks, or fissures. Inspection may also reveal redness and pain typical of vestibulitis, a flattening and pallor of the labia that suggests estrogen deficiency, or pelvic organ prolapse.
The internal pelvic examination begins with a manual evaluation of the muscles of the pelvic floor, uterus, bladder, urethra, anus, and adnexa. Make careful note of any unusual tenderness or pelvic masses. Pelvic floor muscles (PFMs) should voluntarily contract and relax and are not normally tender to palpation. Pelvic organ prolapse and/or hypermobility of the bladder may indicate a weakening of the endopelvic fascia and may cause sexual pain. The size and flexion of the uterus, tenderness in the vaginal fornix possibly indicating endometriosis, and adnexal fullness and/or masses should be identified and evaluated.
Neurologic exam of the pelvis will involve evaluation of sensory and motor function of both lower extremities and include a screening lumbosacral neurologic examination. Lumbosacral examination includes assessment of PFM strength, anal sphincter resting tone, voluntary anal contraction, and perineal sensation. If abnormalities are noted in the screening assessment, a complete comprehensive neurologic examination should be performed.
It’s important to assess pelvic floor muscle strength
Sexual function is associated with normal PFM function.13,14 The PFMs, particularly the pubococcygeus and iliococcygeus, are responsible for involuntary contractions during orgasm.13 Orgasm has been considered a reflex, which is preceded by increased blood flow to the genital organs, tumescence of the vulva and vagina, increased secretions during sexual arousal, and increased tension and contractions of the PFMs.15
Lowenstein et al found that women with strong or moderate PFM contractions scored significantly higher on both orgasm and arousal domains of the female sexual function index (FSFI) compared with women with weak PFM contractions.16 Orgasm and arousal functions may be associated with PFM strength, with a positive association between pelvic floor strength and sexual activity and function.17,18
The function and dysfunction of the PFMs have been characterized as normal, overactive (high tone), underactive (low tone), and non-functioning.
- Normal PFMs are those that can voluntarily and involuntary contract and relax.19,20
- Overactive (high-tone) muscles are those that do not relax and possibly contract during times of relaxation for micturition or defecation. This type of dysfunction can lead to voiding dysfunction, defecatory dysfunction, and dyspareunia.19
- Underactive, or low-tone, PFMs cannot contract voluntarily. This can be associated with urinary and anal incontinence and pelvic organ prolapse.
- Nonfunctioning muscles are completely inactive.19
How to assess. There are several ways to assess PFM tone and strength.20 The first is intravaginal or intrarectal digital palpation, which can be performed when the patient is in a supine or standing position. This examination evaluates PFM tone, squeeze pressure during contraction, symmetry, and relaxation. However, there is no validated scale to quantify PFM strength. Contractions can be further divided into voluntary and involuntary.19
During the examination, the physician should ask the patient to contract as much as she can to evaluate the maximum strength and sustained contraction for endurance. This measurement can be done with digital palpation or with pressure manometry or dynamometry.
Examination can be focused on the levator ani, piriformis, and internal obturator muscles bilaterally and rated by the patient’s reactions. Pelvic muscle tenderness, which can be highly prevalent in women with chronic pelvic pain, is associated with higher degrees of dyspareunia.21 Digital evaluation of the pelvic floor musculature varies in scale, number of fingers used, and parameters evaluated. Lukban et al has described a zero
Effective treatment includes multiple options
Lifestyle modifications can help
Lifestyle changes may help improve sexual function. These modifications include physical activity, healthy diet, nutrition counseling, and adequate sleep.23,24
Identifying medical conditions such as depression and anxiety will help delineate differential diagnoses of sexual dysfunction. Cardiovascular diseases may contribute to arousal disorder as a result of atherosclerosis of the vessels supplying the vagina and clitoris. Neurologic diseases such as multiple sclerosis and diabetes can affect sexual dysfunction by impairing arousal and orgasm. Identification of concurrent comorbidities and implementation of lifestyle changes will help improve overall health and may improve sexual function.25
In addition, Herati et al26 found food sensitivities to grapefruit juice, spicy foods, alcohol, and caffeine were more prevalent in patients with interstitial cystitis and chronic pelvic pain. Avoiding irritants such as soap and other detergents in the perineal region may help decrease dysfunction.27 Finally, foods high in oxalate and other acidic items may cause bladder pain and worsening symptoms of vulvodynia.28
Topical therapies worth considering
Lubricants and moisturizers may help women with dyspareunia or symptoms of vaginal atrophy.
Zestra, for instance, which is applied to the vulva prior to sexual activity, has been proven more effective than placebo for improving desire and arousal.29
Neogyn is a non-hormonal cream containing cutaneous lysate and has been shown to improve vulvar pain in women with vulvodynia. A double-blind placebo-controlled randomized crossover trial followed 30 patients over 3 months and found a significant reduction in pain during sexual activity and a significant reduction in erythema.30
Alprostadil is a prostaglandin E1 analogue that increases genital vasodilation when applied topically and is currently undergoing investigational trials.31,32 Patients can also choose from many over-the-counter lubricants that contain water-based, oil-based, or silicone-based ingredients.
Don’t overlook physical therapy
Manual therapies, including the transvaginal technique, are used for female sexual dysfunction that results from a variety of causes, including high-tone pelvic floor dysfunction. The transvaginal technique can identify myofascial pain; treatment involves internal release of the PFMs and external trigger point identification and alleviation.
One pilot study, which involved transvaginal Thiele massage twice a week for 5 weeks on 21 symptomatic women with IC and high-tone pelvic floor dysfunction found it decreased hyptertonicity of the pelvic floor and generated statistically significant improvement in the Symptom and Problem Indexes of the O’Leary-Sant Questionnaire, Likert Visual Analogue Scales for urgency and pain, and the Physical and Mental Component Summary from the SF-12 Quality-of-Life Scale.33 Transvaginal physical therapy is also an effective treatment for myofascial pelvic pain.34
Biofeedback, which can be used in combination with pelvic floor physical therapy, teaches the patient to control the PFMs by visualizing the activity to achieve conscious control over contraction of the pelvic floor and ceasing the cycle of spasm.35 Ger et al36 investigated patients with levator spasm and found biofeedback decreased pain; relief was rated as good or excellent at 15-month follow-up in 6 out of 14 patients (43%).
Home devices such as Eros Therapy, an FDA-approved, nonpharmacologic battery-operated device, provide vacuum suction to the clitoris with vibratory sensation. Eros Therapy has been shown to increase blood flow to the clitoris, vagina, and pelvic floor and increase sensation, orgasm, lubrication, and satisfaction.37
Vaginal dilators allow increasing lengths and girths designed to treat vaginal and pelvic floor pain.38 In our practice, we encourage pelvic muscle strengthening tools in the form of kegal trainers and other insertion devices that may improve PFM coordination and strength.
Pharmacotherapy has its place
The treatment of FSD may require a multimodal systematic approach targeting genito-pelvic pain. But before the best options can be found, it is important to first establish the cause of the pain. Several drug formulations have been effectively used including hormonal and non-hormonal options.
Conjugated estrogens are FDA approved for the treatment of dyspareunia, which can contribute to decreased desire. Systemic estrogen in oral form, transdermal preparations, and topical formulations may increase sexual desire and arousal and decrease dyspareunia.39 Even synthetic steroid compounds such as tibolone may improve sexual function, although it is not FDA approved for that purpose.40
Ospemifene (Osphena) is a selective estrogen receptor modulator that acts as an estrogen agonist in select tissues, including vaginal epithelium. It is FDA approved for dyspareunia in postmenopausal women.41,42 A daily dose of 60 mg is effective and safe with minimal adverse effects.42 Studies suggest that testosterone, although not FDA approved in the United States for this purpose, improves sexual desire, pleasure, orgasm, and arousal satisfaction.39 The hormone has not gained FDA approval because of concerns about long-term safety and efficacy.42
Non-hormonal drugs including flibanserin (Addyi), a well-tolerated serotonin receptor 1A agonist, 2A antagonist shown to improve sexual desire, increase the number of satisfying sexual events, and reduce distress associated with low sexual desire when compared with placebo.43 The FDA has approved flibanserin as the first treatment targeted for women with hypoactive sexual desire disorder (HSDD). It can, however, cause severe hypotension and syncope, is not well tolerated with alcohol, and is contraindicated in patients who take strong CYP3A4 inhibitors, such as fluconazole, verapamil, and erythromycin, or who have liver impairment.
Buproprion, a mild dopamine and norepinephrine reuptake inhibitor and acetylcholine receptor antagonist, has been shown to improve desire in women with and without depression. Although it is FDA approved for major depressive disorder, it is not approved for female sexual dysfunction and is still under investigation.
Tricyclic antidepressants such as nortriptyline and amitriptyline may be effective in treating neuropathic pain. Starting doses of both amitriptyline and nortriptyline are 10 mg/d and can be increased to a maximum of 100 mg/d.44 Tricyclic antidepressants are still under investigation for the treatment of FSD.
Muscle relaxants in oral and topical compounded form are used to treat increased pelvic floor tension and spasticity. Cyclobenzaprine and tizanidine are FDA-approved muscle relaxants indicated for muscle spasticity.
Cyclobenzaprine, at a starting dose of 10 mg, can be taken up to 3 times a day for pelvic floor tension. Tizanidine is a centrally active alpha 2 agonist that’s superior to placebo in treating high-tone pelvic floor dysfunction.44
Other medications include benzodiazepines such as oral clonazepam and intra-vaginal diazepam, although they are not FDA approved for high-tone pelvic floor dysfunction. Rogalski et al reviewed 26 patients who received vaginal diazepam for bladder pain, sexual pain, and levator hypertonus.45 They found subjective and sexual pain improvement assessed on FSFI and the visual analog pain scale. PFM tone significantly improved during resting, squeezing, and relaxation phases. Multimodal therapy can be used for muscle spasticity and high-tone pelvic floor dysfunction.
Trigger point and Botox injections
Although drug therapy has its place in the management of sexual dysfunction, other modalities that involve trigger point injections or botulinum toxin injections to the PFMs may prove helpful for patients with high-tone pelvic floor dysfunction.
A prospective study investigated the role of trigger point injections in 18 women with levator ani muscle spasm with a mixture of 0.25% bupivacaine in 10 mL, 2% lidocaine in 10 mL, and 40 mg of triamcinolone in 1 mL combined and used for injection of 5 mL per trigger point.46 Three months after injections, 13 of the 18 women improved, resulting in a success rate of 72%. Trigger point injections can be applied externally or transvaginally.
OnabotulinumtoxinA (Botox) has also been tested for relief of levator ani muscle spasm. Botox is FDA approved for upper and lower limb spasticity but is not approved for pelvic floor spasticity or tension. It may reduce pressure in the PFMs and may be useful in women with high-tone pelvic floor dysfunction.47
In a prospective 6-month pilot study, 28 patients with pelvic pain who failed conservative treatment received up to 300 U Botox into the pelvic floor.11 The study, which used needle electromyography guidance and a transperineal approach, found that the dyspareunia visual analog scale improved significantly at Weeks 12 and 24. Keep in mind, however, that onabotulinumtoxinA should be reserved for patients who fail conventional treatments.47,48
Addressing psychological issues
Sex therapy is a traditional approach that aims to improve individual or couples’ sexual experiences and help reduce anxiety related to sex.42 Cognitive behavioral sex therapy includes traditional sex therapy components but puts greater emphasis on modifying thought patterns that interfere with intimacy and sex.42
Mindfulness-based cognitive-behavioral treatments have shown promise for sexual desire problems. It is an ancient eastern practice with Buddhist roots. This therapy is a nonjudgmental, present-moment awareness comprised of self-regulation of attention and accepting orientation to the present.49 Although there is little evidence from prospective studies, it may benefit women with sexual dysfunction after intervention with sex therapy and cognitive behavioral therapy.
Female sexual dysfunction is common and affects women of all ages. It can negatively impact a women’s quality of life and overall well-being. The etiology of FSD is complex, and treatments are based on the causes of the dysfunction. Difficult cases warrant referral to a specialist in sexual health and female pelvic medicine. Future prospective trials, randomized controlled trials, the use of validated questionnaires, and meta-analyses will continue to move us forward as we find better ways to understand, identify, and treat female sexual dysfunction.
CORRESPONDENCE
Melissa L. Dawson, DO, MS, Department of OB/GYN, Drexel University College of Medicine, 207 N Broad St. 4th Floor, Philadelphia, PA 19107; [email protected].
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Washington, DC; 1994.
2. Shifren, JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
3. Lewis RW, Fugl-Meyer KS, Bosch R, et al., Epidemiology/risk factors of sexual dysfunction. J Sex Med. 2004;1:35-39.
4. Laumann E, Paik A, Rosen RC. Sexual dysfunction in the United States prevalence and predictors. JAMA. 1999;281:537-544.
5. Office of the Surgeon General. The Surgeon General’s Call to Action to Promote Sexual Health and Responsible Sexual Behavior, Rockville, MD; 2001.
6. Pauls RN, Kleeman SD, Segal JL, et al. Practice patterns of physician members of the American Urogynecologic Society regarding female sexual dysfunction: results of a national survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:460-467.
7. American Psychiatric Association. Sexual Dysfunction. In: Diagnostic and Statistical Manual of Mental Disorders (5thed). Washington, DC; 2013.
8. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009. 113:1124-1136.
9. ACOG Practice Bulletin No. 119: Female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007.
10. Clayton AH, Hamilton DV. Female sexual dysfunction. Psychiatr Clin North Am. 2017;40:267-284.
11. Morrissey D, El-Khawand D, Ginzburg N, et al. Botulinum Toxin A injections into pelvic floor muscles under electromyographic guidance for women with refractory high-tone pelvic floor dysfunction: a 6-month prospective pilot study. Female Pelvic Med Reconstr Surg. 2015;21:277-282.
12. Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 Pt 2):337-348.
13. Kegel, A. Sexual functions of the pubococcygeus muscle. West J Surg Obstet Gynecol. 1952;60:521-524.
14. Shafik A. The Role of the levator ani muscle in evacuation, sexual performance and pelvic floor disorders. Int Urogynecol J. 2000;11:361-376.
15. Kinsey A, Pomeroy WB, Martin CE, et al. Sexual behavior in the human female. W. B. Saunders:Philadelphia, PA; 1998.
16. Lowenstein L, Gruenwald, Gartman I, et al. Can stronger pelvic muscle floor improve sexual function? Int Urogynecol J. 2010;21:553-556.
17. Kanter G, Rogers RG, Pauls RN, et al. A strong pelvic floor is associated with higher rates of sexual activity in women with pelvic floor disorders. Int Urogynecol J. 2015;26:991-996.
18. Wehbe SA, Kellogg-Spadt S, Whitmore K. Urogenital complaints and female sexual dysfunction. Part 2. J Sex Med. 2010;7:2304-2317.
19. Messelink B, Benson T, Berghmans B, et al. Standardization of terminology of pelvic floor muscle function and dysfunction: report from the pelvic floor clinical assessment group of the International Continence Society. Neurourol Urodyn. 2005;24:374-380.
20. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4-20.
21. Montenegro ML, Mateus-Vasconcelos EC, Rosa e Silva JC et al. Importance of pelvic muscle tenderness evaluation in women with chronic pelvic pain. Pain Med. 2010;11:224-228.
22. Lukban JC, Whitmore KE. Pelvic floor muscle re-education treatment of the overactive bladder and painful bladder syndrome. Clin Obstet Gynecol. 2002;45:273-285.
23. Kalmbach DA, Arnedt JT, Pillai V, et al. The impact of sleep on female sexual response and behavior: a pilot study. J Sex Med. 2015;12:1221-1232.
24. Aversa A, Bruzziches R, Francomano D, et al. Weight loss by multidisciplinary intervention improves endothelial and sexual function in obese fertile women. J Sex Med. 2013;10:1024-1033.
25. Pauls RN, Kleeman SD, Karram MM. Female sexual dysfunction: principles of diagnosis and therapy. Obstet Gynecol Surv. 2005;60:196-205.
26. Herati AS, Shorter B, Tai J, et al. Differences in food sensitivities between female interstitial cystitis/painful bladder syndrome (IC/PBS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) patients. J Urol. 2009;181(4)(Suppl):22.
27. Farrell J, Cacchioni T, The medicalization of women’s sexual pain. J Sex Res. 2012;49:328-336.
28. De Andres J, Sanchis-Lopez NM, Asensio-Samper JM, et al. Vulvodynia—an evidence-based literature review and proposed treatment algorithm. Pain Pract. 2016;16:204-236.
29. Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014:11:642-652.
30. Donders GG, Bellen G. Cream with cutaneous fibroblast lysate for the treatment of provoked vestibulodynia: a double-blind randomized placebo-controlled crossover study. J Low Genit Tract Dis. 2012;16:427-436.
31. Belkin ZR, Krapf JM, Goldstein AT. Drugs in early clinical development for the treatment of female sexual dysfunction. Expert Opin Investig Drugs. 2015;24:159-167.
32. Islam A, Mitchel J, Rosen R, et al. Topical alprostadil in the treatment of female sexual arousal disorder: a pilot study. J Sex Marital Ther. 2001;27:531-540.
33. Oyama IA, Rejba A, Lukban JC, et al. Modified Thiele massage as therapeutic intervention for female patients with interstitial cystitis and high-tone pelvic floor dysfunction. Urology. 2004;64:862-865.
34. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
35. Wehbe SA, Fariello JY, Whitmore K. Minimally invasive therapies for chronic pelvic pain syndrome. Curr Urol Rep. 2010;11:276-285.
36. Ger GC, Wexner SD, Jorge JM, et al. Evaluation and treatment of chronic intractable rectal pain—a frustrating endeavor. Dis Colon Rectum. 1993;36:139-145.
37. Billups KL, Berman L, Berman J, et al. A new non-pharmacological vacuum therapy for female sexual dysfunction. J Sex Marital Ther. 2001;27:435-441.
38. Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;9:Cd007291.
39. Goldstein I. Current management strategies of the postmenopausal patient with sexual health problems. J Sex Med. 2007;4(Suppl 3):235-253.
40. Modelska K, Cummings S. Female sexual dysfunction in postmenopausal women: systematic review of placebo-controlled trials. Am J Obstet Gynecol. 2003;188:286-293.
41. Constantine G, Graham S, Portman DJ, et al. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric. 2015;18:226-232.
42. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125:477-486.
43. Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014; 21:633-640.
44. Curtis Nickel J, Baranowski AP, Pontari M, et al. Management of men diagnosed with chronic prostatitis/chronic pelvic pain syndrome who have failed traditional management. Rev Urol. 2007;9:63-72.
45. Rogalski MJ, Kellogg-Spadt S, Hoffmann AR, et al. Retrospective chart review of vaginal diazepam suppository use in high-tone pelvic floor dysfunction. Int Urogynecol J. 2010:21:895-899.
46. Langford CF, Udvari Nagy S, Ghoniem GM. Levator ani trigger point injections: an underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26:59-62.
47. Abbott JA, Jarvis SK, Lyons SD, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006.108:915-923.
48. Kamanli A, Kaya A, Ardicoglu O, et al. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int. 2005;25:604-611.
49. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol. 2012;125:320-325.
1. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (4th ed, text revision). Washington, DC; 1994.
2. Shifren, JL, Monz BU, Russo PA, et al. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112:970-978.
3. Lewis RW, Fugl-Meyer KS, Bosch R, et al., Epidemiology/risk factors of sexual dysfunction. J Sex Med. 2004;1:35-39.
4. Laumann E, Paik A, Rosen RC. Sexual dysfunction in the United States prevalence and predictors. JAMA. 1999;281:537-544.
5. Office of the Surgeon General. The Surgeon General’s Call to Action to Promote Sexual Health and Responsible Sexual Behavior, Rockville, MD; 2001.
6. Pauls RN, Kleeman SD, Segal JL, et al. Practice patterns of physician members of the American Urogynecologic Society regarding female sexual dysfunction: results of a national survey. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:460-467.
7. American Psychiatric Association. Sexual Dysfunction. In: Diagnostic and Statistical Manual of Mental Disorders (5thed). Washington, DC; 2013.
8. Steege JF, Zolnoun DA. Evaluation and treatment of dyspareunia. Obstet Gynecol. 2009. 113:1124-1136.
9. ACOG Practice Bulletin No. 119: Female sexual dysfunction. Obstet Gynecol. 2011;117:996-1007.
10. Clayton AH, Hamilton DV. Female sexual dysfunction. Psychiatr Clin North Am. 2017;40:267-284.
11. Morrissey D, El-Khawand D, Ginzburg N, et al. Botulinum Toxin A injections into pelvic floor muscles under electromyographic guidance for women with refractory high-tone pelvic floor dysfunction: a 6-month prospective pilot study. Female Pelvic Med Reconstr Surg. 2015;21:277-282.
12. Hatzichristou D, Rosen RC, Derogatis LR, et al. Recommendations for the clinical evaluation of men and women with sexual dysfunction. J Sex Med. 2010;7(1 Pt 2):337-348.
13. Kegel, A. Sexual functions of the pubococcygeus muscle. West J Surg Obstet Gynecol. 1952;60:521-524.
14. Shafik A. The Role of the levator ani muscle in evacuation, sexual performance and pelvic floor disorders. Int Urogynecol J. 2000;11:361-376.
15. Kinsey A, Pomeroy WB, Martin CE, et al. Sexual behavior in the human female. W. B. Saunders:Philadelphia, PA; 1998.
16. Lowenstein L, Gruenwald, Gartman I, et al. Can stronger pelvic muscle floor improve sexual function? Int Urogynecol J. 2010;21:553-556.
17. Kanter G, Rogers RG, Pauls RN, et al. A strong pelvic floor is associated with higher rates of sexual activity in women with pelvic floor disorders. Int Urogynecol J. 2015;26:991-996.
18. Wehbe SA, Kellogg-Spadt S, Whitmore K. Urogenital complaints and female sexual dysfunction. Part 2. J Sex Med. 2010;7:2304-2317.
19. Messelink B, Benson T, Berghmans B, et al. Standardization of terminology of pelvic floor muscle function and dysfunction: report from the pelvic floor clinical assessment group of the International Continence Society. Neurourol Urodyn. 2005;24:374-380.
20. Haylen BT, de Ridder D, Freeman RM, et al. An International Urogynecological Association (IUGA)/International Continence Society (ICS) joint report on the terminology for female pelvic floor dysfunction. Neurourol Urodyn. 2010;29:4-20.
21. Montenegro ML, Mateus-Vasconcelos EC, Rosa e Silva JC et al. Importance of pelvic muscle tenderness evaluation in women with chronic pelvic pain. Pain Med. 2010;11:224-228.
22. Lukban JC, Whitmore KE. Pelvic floor muscle re-education treatment of the overactive bladder and painful bladder syndrome. Clin Obstet Gynecol. 2002;45:273-285.
23. Kalmbach DA, Arnedt JT, Pillai V, et al. The impact of sleep on female sexual response and behavior: a pilot study. J Sex Med. 2015;12:1221-1232.
24. Aversa A, Bruzziches R, Francomano D, et al. Weight loss by multidisciplinary intervention improves endothelial and sexual function in obese fertile women. J Sex Med. 2013;10:1024-1033.
25. Pauls RN, Kleeman SD, Karram MM. Female sexual dysfunction: principles of diagnosis and therapy. Obstet Gynecol Surv. 2005;60:196-205.
26. Herati AS, Shorter B, Tai J, et al. Differences in food sensitivities between female interstitial cystitis/painful bladder syndrome (IC/PBS) and chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) patients. J Urol. 2009;181(4)(Suppl):22.
27. Farrell J, Cacchioni T, The medicalization of women’s sexual pain. J Sex Res. 2012;49:328-336.
28. De Andres J, Sanchis-Lopez NM, Asensio-Samper JM, et al. Vulvodynia—an evidence-based literature review and proposed treatment algorithm. Pain Pract. 2016;16:204-236.
29. Herbenick D, Reece M, Schick V, et al. Women’s use and perceptions of commercial lubricants: prevalence and characteristics in a nationally representative sample of American adults. J Sex Med. 2014:11:642-652.
30. Donders GG, Bellen G. Cream with cutaneous fibroblast lysate for the treatment of provoked vestibulodynia: a double-blind randomized placebo-controlled crossover study. J Low Genit Tract Dis. 2012;16:427-436.
31. Belkin ZR, Krapf JM, Goldstein AT. Drugs in early clinical development for the treatment of female sexual dysfunction. Expert Opin Investig Drugs. 2015;24:159-167.
32. Islam A, Mitchel J, Rosen R, et al. Topical alprostadil in the treatment of female sexual arousal disorder: a pilot study. J Sex Marital Ther. 2001;27:531-540.
33. Oyama IA, Rejba A, Lukban JC, et al. Modified Thiele massage as therapeutic intervention for female patients with interstitial cystitis and high-tone pelvic floor dysfunction. Urology. 2004;64:862-865.
34. Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58:504-510.
35. Wehbe SA, Fariello JY, Whitmore K. Minimally invasive therapies for chronic pelvic pain syndrome. Curr Urol Rep. 2010;11:276-285.
36. Ger GC, Wexner SD, Jorge JM, et al. Evaluation and treatment of chronic intractable rectal pain—a frustrating endeavor. Dis Colon Rectum. 1993;36:139-145.
37. Billups KL, Berman L, Berman J, et al. A new non-pharmacological vacuum therapy for female sexual dysfunction. J Sex Marital Ther. 2001;27:435-441.
38. Miles T, Johnson N. Vaginal dilator therapy for women receiving pelvic radiotherapy. Cochrane Database Syst Rev. 2014;9:Cd007291.
39. Goldstein I. Current management strategies of the postmenopausal patient with sexual health problems. J Sex Med. 2007;4(Suppl 3):235-253.
40. Modelska K, Cummings S. Female sexual dysfunction in postmenopausal women: systematic review of placebo-controlled trials. Am J Obstet Gynecol. 2003;188:286-293.
41. Constantine G, Graham S, Portman DJ, et al. Female sexual function improved with ospemifene in postmenopausal women with vulvar and vaginal atrophy: results of a randomized, placebo-controlled trial. Climacteric. 2015;18:226-232.
42. Kingsberg SA, Woodard T. Female sexual dysfunction: focus on low desire. Obstet Gynecol. 2015;125:477-486.
43. Simon JA, Kingsberg SA, Shumel B, et al. Efficacy and safety of flibanserin in postmenopausal women with hypoactive sexual desire disorder: results of the SNOWDROP trial. Menopause. 2014; 21:633-640.
44. Curtis Nickel J, Baranowski AP, Pontari M, et al. Management of men diagnosed with chronic prostatitis/chronic pelvic pain syndrome who have failed traditional management. Rev Urol. 2007;9:63-72.
45. Rogalski MJ, Kellogg-Spadt S, Hoffmann AR, et al. Retrospective chart review of vaginal diazepam suppository use in high-tone pelvic floor dysfunction. Int Urogynecol J. 2010:21:895-899.
46. Langford CF, Udvari Nagy S, Ghoniem GM. Levator ani trigger point injections: an underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26:59-62.
47. Abbott JA, Jarvis SK, Lyons SD, et al. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006.108:915-923.
48. Kamanli A, Kaya A, Ardicoglu O, et al. Comparison of lidocaine injection, botulinum toxin injection, and dry needling to trigger points in myofascial pain syndrome. Rheumatol Int. 2005;25:604-611.
49. Brotto LA, Erskine Y, Carey M, et al. A brief mindfulness-based cognitive behavioral intervention improves sexual functioning versus wait-list control in women treated for gynecologic cancer. Gynecol Oncol. 2012;125:320-325.
PRACTICE RECOMMENDATIONS
› Obtain a detailed history and evaluate obstetric, gynecologic, sexually transmitted disease, sexual abuse, urinary and bowel complaint, and surgical history in women of all ages. B
› Consider a variety of lifestyle and pharmacologic approaches, as well as biofeedback in combination with pelvic floor physical therapy, to address your female patient’s sexual dysfunction. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
What’s causing my older patient’s cognitive decline?
A 68-year-old woman with a history of well-controlled hypertension and diabetes presents to the office for routine follow-up. She says she has adhered to her current medications, and her blood pressure and hemoglobin A1c remain at goal. At the close of the visit, she mentions that she is worried she may be developing dementia. She says she has been having difficulty finding the right word in conversation and needs to write things down more than she used to.
What might be causing this patient’s changes in cognition?
In primary care settings, when patients complain of memory loss, there is a 20% to 30% chance they will be found to have mild cognitive impairment (MCI) or some level of dementia.1 Given the significant consequences of dementia, it’s important to maximize opportunities to distinguish those with age-related changes in cognition or reversible causes of memory loss from those who have irreversible pathologic changes.
Age-related changes in cognition
Changes in cognition associated with aging vary considerably among individuals and across domains of cognition. By their 7th decade, most people experience a decline in processing speed and working memory.2 However, some individuals retain excellent function into their 80s and perform as well as younger adults.3
Changes long thought to be due to brain senescence may, in fact, be related to the effects of age-related medical conditions on the brain’s function.4 Consistent with this theory is the observation that cognitive changes tend to occur earlier in individuals with cardiovascular disease, diabetes, and cancer.2 What constitutes a normal change depends on an individual’s baseline cognitive function, educational background, medical comorbidities, and the potential impact of sensory impairment on performance.

General cognitive trends with aging. Awareness of normal changes in an aging population is useful when assessing patients concerned about their memory. In general, an individual’s ability to maintain attention to a single task is preserved into late life. Ability to perform tasks requiring divided attention or attention-switching tends to decline.3 This has implications for driving, given the need to constantly switch one’s attention in response to the environment and the ability to sort relevant from irrelevant information.
Remote memory, semantic memory (factual information), and procedural memory (knowledge of skills and procedures) tend to remain intact with aging.4 Short-term memory (simple maintenance of information over a short period of time) shows little change with aging. However, working memory, which requires the manipulation of information in short-term memory, declines.
A simple demonstration of this is that performance on digit span testing tends to remain preserved (7±2), but digit span backwards declines. Holding digits in mind in the order they are received can be achieved through rehearsal. But to reverse the order requires reorganization of the information, and this ability declines with aging.3
Prospective memory (remembering to do things in the future) often requires increased dependence on external aids, such as a to-do list.3 The capacity to learn and recall new information declines. Even when given repeated opportunity to practice, older adults demonstrate a slower learning curve and lower total amount learned.4 Therefore, it becomes easier relying on well-learned cognitive processes such as cooking a familiar meal or relying on previously used principles for decision making.2
Language comprehension and vocabulary remain stable over time. However, difficulty with spontaneous word finding—the “tip-of-the-tongue” phenomenon—tends to increase. In contrast to the dysnomia related to dementia, the word-finding difficulties with normal aging typically improve with cues, indicating that the problem is in retrieval of information rather than storage. Verbal fluency, the rate at which words from a single category can be produced, shows decline. This is particularly true in tests of semantic verbal fluency (name all the animals you can think of); phonemic fluency (words that start with a certain letter) tends to be preserved.4
Some studies using neurocognitive testing have suggested a decline in executive functioning. But, in general, aging has little impact on “real world” executive functions that are required for planning and executing tasks.4 On the whole, cognitive changes related to aging typically do not interfere with an individual’s ability to function independently.
Mild cognitive impairment/mild neurocognitive disorder
Originally conceived as a precursor to Alzheimer’s dementia,5 mild cognitive impairment (MCI) is a diagnosis that has evolved to describe a heterogeneous syndrome of abnormal cognition characterized by:6-8
- a suspected change in cognition expressed by the patient, an acquaintance who knows the patient well, or a clinician;
- objectively measured impairment in one or more cognitive domains beyond what would be expected based on an individual’s age and educational background;
- preservation of functional abilities; and
- a lack of findings that would fulfill criteria for dementia.
In the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM V), this concept is identified as mild neurocognitive disorder, with the additional caveats that an individual’s cognitive deficits do not occur exclusively in the context of delirium and are not better explained by another mental disorder such as depression or schizophrenia.9
An accurate assessment of cognitive change is best measured against the individual’s baseline, which may necessitate the report of a reliable acquaintance. An assessment of functional abilities is also critical. Mild problems in performing complex functions (bill paying, shopping, etc) could be present and still allow a patient to meet the criteria for MCI. An individual may take more time, be less efficient, or make more errors than before; however, independence with minimal aid or assistance is preserved. It
MCI can be divided into 4 subtypes depending upon the cognitive domains affected (complex attention, executive function, learning and memory, language, visuospatial, social cognition):
- Amnestic MCI single domain, if only memory is affected.
- Amnestic MCI multiple domain, if memory and any other cognitive domains are affected.
- Non-amnestic MCI single domain, if any other cognitive domain aside from memory is the only one affected.
- Non-amnestic MCI multiple domain, if multiple domains other than memory are affected.
These distinctions may provide clues to the underlying cause of dysfunction and provide prognostic information regarding the risk of progression to dementia.6,7
Prevalence estimates for MCI vary widely due to differences in definitions used and populations studied. The best estimate is 5% to 10% prevalence among those ages 65 to 69 years old, and 12% to 25% among those ages 80 to 84.10 Similarly, estimates of the rate of progression to dementia vary. Among MCI populations identified through referral sources such as memory centers, the rate of progression to dementia has been 10% to 15% per year.11 In epidemiologic studies of general populations, the rate has been 6% to 10% per year.11 The rate of development of dementia among normal subjects is 1% to 2% per year.5
Dementia/major neurocognitive disorder
The primary feature distinguishing MCI/mild neurocognitive disorder from dementia or major neurocognitive disorder is a patient’s functional status. The core clinical criteria for all-cause dementia are cognitive or neurobehavioral symptoms that: 12
- interfere with work or usual daily function,
- represent a change from the prior baseline function,
- are not explained by delirium or a psychiatric illness, and
- include detectable impairment in 2 cognitive domains.
Criteria outlined in the DSM-V for major neurocognitive disorder are essentially the same but describe the functional change criteria as cognitive changes that “interfere with independence in everyday activities.”9 The DSM-V elaborates: “at a minimum, requiring assistance with complex instrumental activities of daily living such as paying bills or managing medications.”
Assessing functional status accurately in clinical practice typically requires the assistance of a collateral informant who knows the patient well. The Informant Questionnaire on Cognitive Decline in the Elderly (https://www.alz.org/documents_custom/shortiqcode_english.pdf) is one validated assessment tool that can be used for this purpose.13 With this self-administered form, the informant answers 16 questions regarding changes in the patient’s performance of different activities over the 10 years prior. Alternatively, a structured interview based on indices of activities of daily living (ADLs) and instrumental activities of daily living (IADLs) as listed in TABLE 1 can be employed.14,15
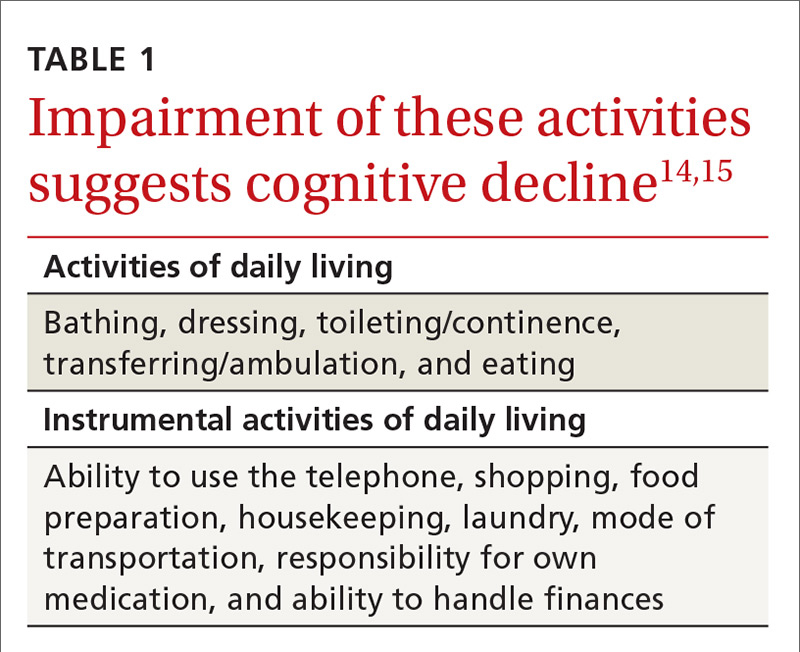
Review of the various causes of dementia is beyond the scope of this article, but a list of common diagnoses is presented in TABLE 2.
Dementia syndrome of depression (pseudodementia)
Elderly patients with depression commonly complain of memory impairment, and this interaction between depression and dementia has been investigated for decades. The term “pseudodementia” has been used since 1961 to describe signs of dementia in a patient with any psychiatric illness,16 but it has since been refined to apply solely to depression. The prevalence of depression among older adults varies depending on the population studied and how depression is defined. Approximately 2% to 3% of community-dwelling elders meet criteria for major depression, with 10% to 30% showing some symptoms of depression.17,18
Twenty percent to 40% of elderly patients diagnosed with depression will have evidence of cognitive impairment.19-21 Most improve with antidepressive treatment, though evidence of cognitive impairment may continue for some.19
A broad range of cognitive deficits have been associated with depression. Most consistently described are deficits in processing speed,22-25 attention,26-28 and executive function.22,25-29 Memory deficits can be apparent with tests of delayed recall, but recognition (the ability to identify items from a list) generally is preserved.26,28-30
Distinguishing between pseudodementia and true dementia can be challenging. An increased severity of deficits, particularly with delayed recall, is more indicative of dementia.31 Additionally, on clock drawing tasks, individuals with depression perform more comparably to controls than do those with true dementia.32
A 2013 meta-analysis reported a significant association of late-life depression with subsequent development of dementia, with an odds ratio (OR) of 1.85. The risk of subsequently developing vascular dementia (OR=2.52) was significantly higher than that for Alzheimer’s disease (OR=1.65). Individuals with evidence of reversible cognitive impairment at the time of diagnosis of depression seem to be particularly vulnerable, with dementia developing in 43% to 71%, compared with rates of 12% to 18% among elders diagnosed with depression but lacking signs of cognitive impairment.20,21
Other causes of reversible dementia
A meta-analysis performed in 1988 found that 11% of cases of dementia were reversible.33 However, an update using the same methodology in 2003 revealed the number had dropped to less than 1%.34 In the latest meta-analysis, one of the authors’ leading hypotheses for the dramatic decline in apparent prevalence was a significant shift in the study population from the inpatient to outpatient setting. In studies of community-based populations used in the re-analysis, the reported prevalence of reversibility was near zero.34
Metabolic abnormalities—most often B12 deficiency and hypothyroidism—are commonly cited as potential causes of dementia. Four systematic reviews, including one conducted by the Cochrane Collaborative, concluded there is a lack of evidence that treating low vitamin B12 in individuals with dementia improves cognition.35,36 There is some evidence, though, of a time-limited window for successful treatment within 12 months of the onset of symptoms.37,38 A study reviewing causes of dementia in nearly 3000 individuals found one case of reversible dementia attributable to hypothyroidism.39 A subsequent review reached similar conclusions about the lack of data to support the notion that treatment of hypothyroidism reverses dementia.40
Similarly, imaging for cerebral tumors, subdural hematomas, or normal-pressure hydrocephalus rarely identifies these as a cause of dementia.41 This is particularly true of unselected community-based populations, as there are typically signs or symptoms suggesting an intracranial pathology.
Numerous medications have been implicated in causing acute confusional states, and there is some evidence for their role in chronic confusion (TABLE 3).42,43 In my experience, many who experience adverse effects on cognition with medications will also have an underlying neurodegenerative process, and symptoms do not completely resolve with withdrawal of the offending agent.
Further assessment of the patient yielded a score of 29/30 on the Montreal Cognitive Assessment* and a zero on the Patient Health Questionnaire-2. Careful review of her daily function revealed no significant deficits in ADL or IADL performance, and her daughter confirmed that she had not observed any significant decline in her mother’s function. There was no significant family history of dementia. The patient was reassured that her cognitive changes were normal and age related.
Unfortunately, few data support specific interventions to reduce this patient’s risk of developing dementia. She was commended for keeping her blood pressure and blood sugar levels under control, thereby reducing her risk of vascular disease.
She and her daughter were directed to the Alzheimer’s Association Web site (alz.org) as a resource for information about signs and symptoms to watch for and for caregiving resources, should they be needed. She was briefly counseled to eliminate distractions to improve her ability to complete tasks and improve recall along with rehearsing or writing down information that she wished to retain.
Finally, she was counseled to remain physically, cognitively, and socially active as these are factors generally associated with healthy aging, have some evidence to support efficacy in reducing the risk of cognitive decline,44,45 and are unlikely to be of harm.
*The Montreal Cognitive Assessment is a validated office-based tool for the evaluation of cognitive impairment that is highly sensitive for the detection of mild cognitive impairment.
CORRESPONDENCE
Ian M. Deutchki, MD, Professor of Family Medicine and Geriatrics, University of Rochester Medical Center, 777 S. Clinton Avenue, Rochester, NY 14620; [email protected].
1. Mitchell AJ. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. Int J Geriatr Psychiatry. 2008;23:1191-1202.
2. Burnette V, Howell T. Cognitive changes in aging. In: Capezuti EA, Malone ML, Katz PR, et al, eds. The Encyclopedia of Elder Care. New York, NY, USA: Springer Publishing Company; 2013.
3. Glisky EL. Changes in cognitive function in human aging. In: Riddle DR, ed. Brain Aging: Models, Methods, and Mechanisms. Boca Raton, FL: Taylor & Francis Group, LLC; 2007:4-20.
4. Craft S, Cholerton B, Reger M. Cognitive changes associated with normal and pathological aging. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, et al, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:751-766.
5. Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303-308.
6. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183-194.
7. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240-246.
8. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270-279.
9. Neurocognitive disorders. In: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
10. Ward A. Arrighi HM, Michels S, et al. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 2012;8:14-21.
11. Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447-1455.
12. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263-269.
13. Jorm AF. A short form of the informant questionnaire on cognitive decline in the elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145-153.
14. Katz S, Downs TD, Cash HR, et al. Progress in development of the index of ADL. Gerontologist. 1970;10:20-30.
15. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179-186.
16. Kiloh LG. Pseudo-dementia. Acta Psychiatr Scand. 1961;37:336-351.
17. Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry. 1999;174:307-311.
18. Birrer RB, Vemuri SP. Depression in later life: a diagnostic and therapeutic challenge. Am Fam Physician. 2004;69:2375-2382.
19. Butters MA, Becker JT, Nebes RD, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157:1949-1954.
20. Alexopoulos GS, Meyers BS, Young RC, et al. The course of geriatric depression with “reversible dementia”: a controlled study. Am J Psychiatry. 1993;150:1693-1699.
21. Saez-Fonseca JA, Lee L, Walker Z. Long-term outcome of depressive pseudodementia in the elderly. J Affect Disord. 2007;101:123-129.
22. Dillon C, Allegri RF, Serrano CM, et al. Late- versus early-onset geriatric depression in a memory research center. Neuropsychiatr Dis Treat. 2009;5:517-526.
23. Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159:1119-1126.
24. Shimada H, Park H, Makizako H, et al. Depressive symptoms and cognitive performance in older adults. J Psychiatr Res. 2014;57:149-156.
25. Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587-595.
26. Dillon C, Machnicki G, Serrano CM, et al. Clinical manifestations of geriatric depression in a memory clinic: toward a proposed subtyping of geriatric depression. J Affect Disord. 2011;134:177-187.
27. Rapp MA, Dahlman K, Sano M, et al. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry. 2005;162:691-698.
28. Zihl J, Reppermund S, Thum S, et al. Neuropsychological profiles in MCI and in depression: differential cognitive dysfunction patterns or similar final common pathway disorder? J Psychiatr Res. 2010;44:647-654.
29. Dillon C, Tartaglini MF, Stefani D, et al. Geriatric depression and its relation with cognitive impairment and dementia. Arch Gerontol Geriatr. 2014;59:450-456.
30. Wright SL, Persad C. Distinguishing between depression and dementia in older persons: neuropsychological and neuropathological correlates. J Geriatr Psychiatry Neurol. 2007;20:189-198.
31. Visser PJ, Verhey FR, Ponds RW, et al. Distinction between preclinical Alzheimer’s disease and depression. J Am Geriatr Soc. 2000;48:479-484.
32. Bodner T, Delazer M, Kemmler G, et al. Clock drawing, clock reading, clock setting, and judgment of clock faces in elderly people with dementia and depression. J Am Geriatr Soc. 2004;52:1146-1150.
33. Clarfield AM. The reversible dementias: do they reverse? Ann Intern Med. 1988;109:476-486.
34. Clarfield AM. The decreasing prevalence of reversible dementias: an updated meta-analysis. Arch Intern Med. 2003;163:2219-2229.
35. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326.
36. Health Quality Ontario. Vitamin B12 and cognitive function: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13:1-45.
37. Abyad A. Prevalence of vitamin B12 deficiency among demented patients and cognitive recovery with cobalamin replacement. J Nutr Health Aging. 2002;6:254-260.
38. Martin DC, Francis J, Protetch J, et al. Time dependency of cognitive recovery with cobalamin replacement: Report of a pilot study. J Am Geriatr Soc. 1992;40:168-172.
39. Clarnette RM, Patterson CJ. Hypothyroidism: does treatment cure dementia? J Geriatr Psychiatry Neurol. 1994;7:23-27.
40. Dugbartey AT. Neurocognitive aspects of hypothyroidism. Arch Intern Med. 1998;158:1413-1418.
41. Alexander EM, Wagner EH, Buchner DM, et al. Do surgical brain lesions present as isolated dementia? A population-based study. J Am Geriatr Soc. 1995;43:138-143.
42. Moore AR, O’Keeffe ST. Drug-induced cognitive impairment in the elderly. Drugs Aging. 1999;15:15-28.
43. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American geriatrics society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
44. Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66:1210-1215.
45. Shatenstein B, Barberger-Gateau P, Mecocci P. Prevention of age-related cognitive decline: which strategies, when, and for whom? J Alzheimers Dis. 2015;48:35-53.
A 68-year-old woman with a history of well-controlled hypertension and diabetes presents to the office for routine follow-up. She says she has adhered to her current medications, and her blood pressure and hemoglobin A1c remain at goal. At the close of the visit, she mentions that she is worried she may be developing dementia. She says she has been having difficulty finding the right word in conversation and needs to write things down more than she used to.
What might be causing this patient’s changes in cognition?
In primary care settings, when patients complain of memory loss, there is a 20% to 30% chance they will be found to have mild cognitive impairment (MCI) or some level of dementia.1 Given the significant consequences of dementia, it’s important to maximize opportunities to distinguish those with age-related changes in cognition or reversible causes of memory loss from those who have irreversible pathologic changes.
Age-related changes in cognition
Changes in cognition associated with aging vary considerably among individuals and across domains of cognition. By their 7th decade, most people experience a decline in processing speed and working memory.2 However, some individuals retain excellent function into their 80s and perform as well as younger adults.3
Changes long thought to be due to brain senescence may, in fact, be related to the effects of age-related medical conditions on the brain’s function.4 Consistent with this theory is the observation that cognitive changes tend to occur earlier in individuals with cardiovascular disease, diabetes, and cancer.2 What constitutes a normal change depends on an individual’s baseline cognitive function, educational background, medical comorbidities, and the potential impact of sensory impairment on performance.

General cognitive trends with aging. Awareness of normal changes in an aging population is useful when assessing patients concerned about their memory. In general, an individual’s ability to maintain attention to a single task is preserved into late life. Ability to perform tasks requiring divided attention or attention-switching tends to decline.3 This has implications for driving, given the need to constantly switch one’s attention in response to the environment and the ability to sort relevant from irrelevant information.
Remote memory, semantic memory (factual information), and procedural memory (knowledge of skills and procedures) tend to remain intact with aging.4 Short-term memory (simple maintenance of information over a short period of time) shows little change with aging. However, working memory, which requires the manipulation of information in short-term memory, declines.
A simple demonstration of this is that performance on digit span testing tends to remain preserved (7±2), but digit span backwards declines. Holding digits in mind in the order they are received can be achieved through rehearsal. But to reverse the order requires reorganization of the information, and this ability declines with aging.3
Prospective memory (remembering to do things in the future) often requires increased dependence on external aids, such as a to-do list.3 The capacity to learn and recall new information declines. Even when given repeated opportunity to practice, older adults demonstrate a slower learning curve and lower total amount learned.4 Therefore, it becomes easier relying on well-learned cognitive processes such as cooking a familiar meal or relying on previously used principles for decision making.2
Language comprehension and vocabulary remain stable over time. However, difficulty with spontaneous word finding—the “tip-of-the-tongue” phenomenon—tends to increase. In contrast to the dysnomia related to dementia, the word-finding difficulties with normal aging typically improve with cues, indicating that the problem is in retrieval of information rather than storage. Verbal fluency, the rate at which words from a single category can be produced, shows decline. This is particularly true in tests of semantic verbal fluency (name all the animals you can think of); phonemic fluency (words that start with a certain letter) tends to be preserved.4
Some studies using neurocognitive testing have suggested a decline in executive functioning. But, in general, aging has little impact on “real world” executive functions that are required for planning and executing tasks.4 On the whole, cognitive changes related to aging typically do not interfere with an individual’s ability to function independently.
Mild cognitive impairment/mild neurocognitive disorder
Originally conceived as a precursor to Alzheimer’s dementia,5 mild cognitive impairment (MCI) is a diagnosis that has evolved to describe a heterogeneous syndrome of abnormal cognition characterized by:6-8
- a suspected change in cognition expressed by the patient, an acquaintance who knows the patient well, or a clinician;
- objectively measured impairment in one or more cognitive domains beyond what would be expected based on an individual’s age and educational background;
- preservation of functional abilities; and
- a lack of findings that would fulfill criteria for dementia.
In the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM V), this concept is identified as mild neurocognitive disorder, with the additional caveats that an individual’s cognitive deficits do not occur exclusively in the context of delirium and are not better explained by another mental disorder such as depression or schizophrenia.9
An accurate assessment of cognitive change is best measured against the individual’s baseline, which may necessitate the report of a reliable acquaintance. An assessment of functional abilities is also critical. Mild problems in performing complex functions (bill paying, shopping, etc) could be present and still allow a patient to meet the criteria for MCI. An individual may take more time, be less efficient, or make more errors than before; however, independence with minimal aid or assistance is preserved. It
MCI can be divided into 4 subtypes depending upon the cognitive domains affected (complex attention, executive function, learning and memory, language, visuospatial, social cognition):
- Amnestic MCI single domain, if only memory is affected.
- Amnestic MCI multiple domain, if memory and any other cognitive domains are affected.
- Non-amnestic MCI single domain, if any other cognitive domain aside from memory is the only one affected.
- Non-amnestic MCI multiple domain, if multiple domains other than memory are affected.
These distinctions may provide clues to the underlying cause of dysfunction and provide prognostic information regarding the risk of progression to dementia.6,7
Prevalence estimates for MCI vary widely due to differences in definitions used and populations studied. The best estimate is 5% to 10% prevalence among those ages 65 to 69 years old, and 12% to 25% among those ages 80 to 84.10 Similarly, estimates of the rate of progression to dementia vary. Among MCI populations identified through referral sources such as memory centers, the rate of progression to dementia has been 10% to 15% per year.11 In epidemiologic studies of general populations, the rate has been 6% to 10% per year.11 The rate of development of dementia among normal subjects is 1% to 2% per year.5
Dementia/major neurocognitive disorder
The primary feature distinguishing MCI/mild neurocognitive disorder from dementia or major neurocognitive disorder is a patient’s functional status. The core clinical criteria for all-cause dementia are cognitive or neurobehavioral symptoms that: 12
- interfere with work or usual daily function,
- represent a change from the prior baseline function,
- are not explained by delirium or a psychiatric illness, and
- include detectable impairment in 2 cognitive domains.
Criteria outlined in the DSM-V for major neurocognitive disorder are essentially the same but describe the functional change criteria as cognitive changes that “interfere with independence in everyday activities.”9 The DSM-V elaborates: “at a minimum, requiring assistance with complex instrumental activities of daily living such as paying bills or managing medications.”
Assessing functional status accurately in clinical practice typically requires the assistance of a collateral informant who knows the patient well. The Informant Questionnaire on Cognitive Decline in the Elderly (https://www.alz.org/documents_custom/shortiqcode_english.pdf) is one validated assessment tool that can be used for this purpose.13 With this self-administered form, the informant answers 16 questions regarding changes in the patient’s performance of different activities over the 10 years prior. Alternatively, a structured interview based on indices of activities of daily living (ADLs) and instrumental activities of daily living (IADLs) as listed in TABLE 1 can be employed.14,15

Review of the various causes of dementia is beyond the scope of this article, but a list of common diagnoses is presented in TABLE 2.

Dementia syndrome of depression (pseudodementia)
Elderly patients with depression commonly complain of memory impairment, and this interaction between depression and dementia has been investigated for decades. The term “pseudodementia” has been used since 1961 to describe signs of dementia in a patient with any psychiatric illness,16 but it has since been refined to apply solely to depression. The prevalence of depression among older adults varies depending on the population studied and how depression is defined. Approximately 2% to 3% of community-dwelling elders meet criteria for major depression, with 10% to 30% showing some symptoms of depression.17,18
Twenty percent to 40% of elderly patients diagnosed with depression will have evidence of cognitive impairment.19-21 Most improve with antidepressive treatment, though evidence of cognitive impairment may continue for some.19
A broad range of cognitive deficits have been associated with depression. Most consistently described are deficits in processing speed,22-25 attention,26-28 and executive function.22,25-29 Memory deficits can be apparent with tests of delayed recall, but recognition (the ability to identify items from a list) generally is preserved.26,28-30
Distinguishing between pseudodementia and true dementia can be challenging. An increased severity of deficits, particularly with delayed recall, is more indicative of dementia.31 Additionally, on clock drawing tasks, individuals with depression perform more comparably to controls than do those with true dementia.32
A 2013 meta-analysis reported a significant association of late-life depression with subsequent development of dementia, with an odds ratio (OR) of 1.85. The risk of subsequently developing vascular dementia (OR=2.52) was significantly higher than that for Alzheimer’s disease (OR=1.65). Individuals with evidence of reversible cognitive impairment at the time of diagnosis of depression seem to be particularly vulnerable, with dementia developing in 43% to 71%, compared with rates of 12% to 18% among elders diagnosed with depression but lacking signs of cognitive impairment.20,21
Other causes of reversible dementia
A meta-analysis performed in 1988 found that 11% of cases of dementia were reversible.33 However, an update using the same methodology in 2003 revealed the number had dropped to less than 1%.34 In the latest meta-analysis, one of the authors’ leading hypotheses for the dramatic decline in apparent prevalence was a significant shift in the study population from the inpatient to outpatient setting. In studies of community-based populations used in the re-analysis, the reported prevalence of reversibility was near zero.34
Metabolic abnormalities—most often B12 deficiency and hypothyroidism—are commonly cited as potential causes of dementia. Four systematic reviews, including one conducted by the Cochrane Collaborative, concluded there is a lack of evidence that treating low vitamin B12 in individuals with dementia improves cognition.35,36 There is some evidence, though, of a time-limited window for successful treatment within 12 months of the onset of symptoms.37,38 A study reviewing causes of dementia in nearly 3000 individuals found one case of reversible dementia attributable to hypothyroidism.39 A subsequent review reached similar conclusions about the lack of data to support the notion that treatment of hypothyroidism reverses dementia.40
Similarly, imaging for cerebral tumors, subdural hematomas, or normal-pressure hydrocephalus rarely identifies these as a cause of dementia.41 This is particularly true of unselected community-based populations, as there are typically signs or symptoms suggesting an intracranial pathology.
Numerous medications have been implicated in causing acute confusional states, and there is some evidence for their role in chronic confusion (TABLE 3).42,43 In my experience, many who experience adverse effects on cognition with medications will also have an underlying neurodegenerative process, and symptoms do not completely resolve with withdrawal of the offending agent.

Further assessment of the patient yielded a score of 29/30 on the Montreal Cognitive Assessment* and a zero on the Patient Health Questionnaire-2. Careful review of her daily function revealed no significant deficits in ADL or IADL performance, and her daughter confirmed that she had not observed any significant decline in her mother’s function. There was no significant family history of dementia. The patient was reassured that her cognitive changes were normal and age related.
Unfortunately, few data support specific interventions to reduce this patient’s risk of developing dementia. She was commended for keeping her blood pressure and blood sugar levels under control, thereby reducing her risk of vascular disease.
She and her daughter were directed to the Alzheimer’s Association Web site (alz.org) as a resource for information about signs and symptoms to watch for and for caregiving resources, should they be needed. She was briefly counseled to eliminate distractions to improve her ability to complete tasks and improve recall along with rehearsing or writing down information that she wished to retain.
Finally, she was counseled to remain physically, cognitively, and socially active as these are factors generally associated with healthy aging, have some evidence to support efficacy in reducing the risk of cognitive decline,44,45 and are unlikely to be of harm.
*The Montreal Cognitive Assessment is a validated office-based tool for the evaluation of cognitive impairment that is highly sensitive for the detection of mild cognitive impairment.
CORRESPONDENCE
Ian M. Deutchki, MD, Professor of Family Medicine and Geriatrics, University of Rochester Medical Center, 777 S. Clinton Avenue, Rochester, NY 14620; [email protected].
A 68-year-old woman with a history of well-controlled hypertension and diabetes presents to the office for routine follow-up. She says she has adhered to her current medications, and her blood pressure and hemoglobin A1c remain at goal. At the close of the visit, she mentions that she is worried she may be developing dementia. She says she has been having difficulty finding the right word in conversation and needs to write things down more than she used to.
What might be causing this patient’s changes in cognition?
In primary care settings, when patients complain of memory loss, there is a 20% to 30% chance they will be found to have mild cognitive impairment (MCI) or some level of dementia.1 Given the significant consequences of dementia, it’s important to maximize opportunities to distinguish those with age-related changes in cognition or reversible causes of memory loss from those who have irreversible pathologic changes.
Age-related changes in cognition
Changes in cognition associated with aging vary considerably among individuals and across domains of cognition. By their 7th decade, most people experience a decline in processing speed and working memory.2 However, some individuals retain excellent function into their 80s and perform as well as younger adults.3
Changes long thought to be due to brain senescence may, in fact, be related to the effects of age-related medical conditions on the brain’s function.4 Consistent with this theory is the observation that cognitive changes tend to occur earlier in individuals with cardiovascular disease, diabetes, and cancer.2 What constitutes a normal change depends on an individual’s baseline cognitive function, educational background, medical comorbidities, and the potential impact of sensory impairment on performance.

General cognitive trends with aging. Awareness of normal changes in an aging population is useful when assessing patients concerned about their memory. In general, an individual’s ability to maintain attention to a single task is preserved into late life. Ability to perform tasks requiring divided attention or attention-switching tends to decline.3 This has implications for driving, given the need to constantly switch one’s attention in response to the environment and the ability to sort relevant from irrelevant information.
Remote memory, semantic memory (factual information), and procedural memory (knowledge of skills and procedures) tend to remain intact with aging.4 Short-term memory (simple maintenance of information over a short period of time) shows little change with aging. However, working memory, which requires the manipulation of information in short-term memory, declines.
A simple demonstration of this is that performance on digit span testing tends to remain preserved (7±2), but digit span backwards declines. Holding digits in mind in the order they are received can be achieved through rehearsal. But to reverse the order requires reorganization of the information, and this ability declines with aging.3
Prospective memory (remembering to do things in the future) often requires increased dependence on external aids, such as a to-do list.3 The capacity to learn and recall new information declines. Even when given repeated opportunity to practice, older adults demonstrate a slower learning curve and lower total amount learned.4 Therefore, it becomes easier relying on well-learned cognitive processes such as cooking a familiar meal or relying on previously used principles for decision making.2
Language comprehension and vocabulary remain stable over time. However, difficulty with spontaneous word finding—the “tip-of-the-tongue” phenomenon—tends to increase. In contrast to the dysnomia related to dementia, the word-finding difficulties with normal aging typically improve with cues, indicating that the problem is in retrieval of information rather than storage. Verbal fluency, the rate at which words from a single category can be produced, shows decline. This is particularly true in tests of semantic verbal fluency (name all the animals you can think of); phonemic fluency (words that start with a certain letter) tends to be preserved.4
Some studies using neurocognitive testing have suggested a decline in executive functioning. But, in general, aging has little impact on “real world” executive functions that are required for planning and executing tasks.4 On the whole, cognitive changes related to aging typically do not interfere with an individual’s ability to function independently.
Mild cognitive impairment/mild neurocognitive disorder
Originally conceived as a precursor to Alzheimer’s dementia,5 mild cognitive impairment (MCI) is a diagnosis that has evolved to describe a heterogeneous syndrome of abnormal cognition characterized by:6-8
- a suspected change in cognition expressed by the patient, an acquaintance who knows the patient well, or a clinician;
- objectively measured impairment in one or more cognitive domains beyond what would be expected based on an individual’s age and educational background;
- preservation of functional abilities; and
- a lack of findings that would fulfill criteria for dementia.
In the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM V), this concept is identified as mild neurocognitive disorder, with the additional caveats that an individual’s cognitive deficits do not occur exclusively in the context of delirium and are not better explained by another mental disorder such as depression or schizophrenia.9
An accurate assessment of cognitive change is best measured against the individual’s baseline, which may necessitate the report of a reliable acquaintance. An assessment of functional abilities is also critical. Mild problems in performing complex functions (bill paying, shopping, etc) could be present and still allow a patient to meet the criteria for MCI. An individual may take more time, be less efficient, or make more errors than before; however, independence with minimal aid or assistance is preserved. It
MCI can be divided into 4 subtypes depending upon the cognitive domains affected (complex attention, executive function, learning and memory, language, visuospatial, social cognition):
- Amnestic MCI single domain, if only memory is affected.
- Amnestic MCI multiple domain, if memory and any other cognitive domains are affected.
- Non-amnestic MCI single domain, if any other cognitive domain aside from memory is the only one affected.
- Non-amnestic MCI multiple domain, if multiple domains other than memory are affected.
These distinctions may provide clues to the underlying cause of dysfunction and provide prognostic information regarding the risk of progression to dementia.6,7
Prevalence estimates for MCI vary widely due to differences in definitions used and populations studied. The best estimate is 5% to 10% prevalence among those ages 65 to 69 years old, and 12% to 25% among those ages 80 to 84.10 Similarly, estimates of the rate of progression to dementia vary. Among MCI populations identified through referral sources such as memory centers, the rate of progression to dementia has been 10% to 15% per year.11 In epidemiologic studies of general populations, the rate has been 6% to 10% per year.11 The rate of development of dementia among normal subjects is 1% to 2% per year.5
Dementia/major neurocognitive disorder
The primary feature distinguishing MCI/mild neurocognitive disorder from dementia or major neurocognitive disorder is a patient’s functional status. The core clinical criteria for all-cause dementia are cognitive or neurobehavioral symptoms that: 12
- interfere with work or usual daily function,
- represent a change from the prior baseline function,
- are not explained by delirium or a psychiatric illness, and
- include detectable impairment in 2 cognitive domains.
Criteria outlined in the DSM-V for major neurocognitive disorder are essentially the same but describe the functional change criteria as cognitive changes that “interfere with independence in everyday activities.”9 The DSM-V elaborates: “at a minimum, requiring assistance with complex instrumental activities of daily living such as paying bills or managing medications.”
Assessing functional status accurately in clinical practice typically requires the assistance of a collateral informant who knows the patient well. The Informant Questionnaire on Cognitive Decline in the Elderly (https://www.alz.org/documents_custom/shortiqcode_english.pdf) is one validated assessment tool that can be used for this purpose.13 With this self-administered form, the informant answers 16 questions regarding changes in the patient’s performance of different activities over the 10 years prior. Alternatively, a structured interview based on indices of activities of daily living (ADLs) and instrumental activities of daily living (IADLs) as listed in TABLE 1 can be employed.14,15

Review of the various causes of dementia is beyond the scope of this article, but a list of common diagnoses is presented in TABLE 2.

Dementia syndrome of depression (pseudodementia)
Elderly patients with depression commonly complain of memory impairment, and this interaction between depression and dementia has been investigated for decades. The term “pseudodementia” has been used since 1961 to describe signs of dementia in a patient with any psychiatric illness,16 but it has since been refined to apply solely to depression. The prevalence of depression among older adults varies depending on the population studied and how depression is defined. Approximately 2% to 3% of community-dwelling elders meet criteria for major depression, with 10% to 30% showing some symptoms of depression.17,18
Twenty percent to 40% of elderly patients diagnosed with depression will have evidence of cognitive impairment.19-21 Most improve with antidepressive treatment, though evidence of cognitive impairment may continue for some.19
A broad range of cognitive deficits have been associated with depression. Most consistently described are deficits in processing speed,22-25 attention,26-28 and executive function.22,25-29 Memory deficits can be apparent with tests of delayed recall, but recognition (the ability to identify items from a list) generally is preserved.26,28-30
Distinguishing between pseudodementia and true dementia can be challenging. An increased severity of deficits, particularly with delayed recall, is more indicative of dementia.31 Additionally, on clock drawing tasks, individuals with depression perform more comparably to controls than do those with true dementia.32
A 2013 meta-analysis reported a significant association of late-life depression with subsequent development of dementia, with an odds ratio (OR) of 1.85. The risk of subsequently developing vascular dementia (OR=2.52) was significantly higher than that for Alzheimer’s disease (OR=1.65). Individuals with evidence of reversible cognitive impairment at the time of diagnosis of depression seem to be particularly vulnerable, with dementia developing in 43% to 71%, compared with rates of 12% to 18% among elders diagnosed with depression but lacking signs of cognitive impairment.20,21
Other causes of reversible dementia
A meta-analysis performed in 1988 found that 11% of cases of dementia were reversible.33 However, an update using the same methodology in 2003 revealed the number had dropped to less than 1%.34 In the latest meta-analysis, one of the authors’ leading hypotheses for the dramatic decline in apparent prevalence was a significant shift in the study population from the inpatient to outpatient setting. In studies of community-based populations used in the re-analysis, the reported prevalence of reversibility was near zero.34
Metabolic abnormalities—most often B12 deficiency and hypothyroidism—are commonly cited as potential causes of dementia. Four systematic reviews, including one conducted by the Cochrane Collaborative, concluded there is a lack of evidence that treating low vitamin B12 in individuals with dementia improves cognition.35,36 There is some evidence, though, of a time-limited window for successful treatment within 12 months of the onset of symptoms.37,38 A study reviewing causes of dementia in nearly 3000 individuals found one case of reversible dementia attributable to hypothyroidism.39 A subsequent review reached similar conclusions about the lack of data to support the notion that treatment of hypothyroidism reverses dementia.40
Similarly, imaging for cerebral tumors, subdural hematomas, or normal-pressure hydrocephalus rarely identifies these as a cause of dementia.41 This is particularly true of unselected community-based populations, as there are typically signs or symptoms suggesting an intracranial pathology.
Numerous medications have been implicated in causing acute confusional states, and there is some evidence for their role in chronic confusion (TABLE 3).42,43 In my experience, many who experience adverse effects on cognition with medications will also have an underlying neurodegenerative process, and symptoms do not completely resolve with withdrawal of the offending agent.

Further assessment of the patient yielded a score of 29/30 on the Montreal Cognitive Assessment* and a zero on the Patient Health Questionnaire-2. Careful review of her daily function revealed no significant deficits in ADL or IADL performance, and her daughter confirmed that she had not observed any significant decline in her mother’s function. There was no significant family history of dementia. The patient was reassured that her cognitive changes were normal and age related.
Unfortunately, few data support specific interventions to reduce this patient’s risk of developing dementia. She was commended for keeping her blood pressure and blood sugar levels under control, thereby reducing her risk of vascular disease.
She and her daughter were directed to the Alzheimer’s Association Web site (alz.org) as a resource for information about signs and symptoms to watch for and for caregiving resources, should they be needed. She was briefly counseled to eliminate distractions to improve her ability to complete tasks and improve recall along with rehearsing or writing down information that she wished to retain.
Finally, she was counseled to remain physically, cognitively, and socially active as these are factors generally associated with healthy aging, have some evidence to support efficacy in reducing the risk of cognitive decline,44,45 and are unlikely to be of harm.
*The Montreal Cognitive Assessment is a validated office-based tool for the evaluation of cognitive impairment that is highly sensitive for the detection of mild cognitive impairment.
CORRESPONDENCE
Ian M. Deutchki, MD, Professor of Family Medicine and Geriatrics, University of Rochester Medical Center, 777 S. Clinton Avenue, Rochester, NY 14620; [email protected].
1. Mitchell AJ. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. Int J Geriatr Psychiatry. 2008;23:1191-1202.
2. Burnette V, Howell T. Cognitive changes in aging. In: Capezuti EA, Malone ML, Katz PR, et al, eds. The Encyclopedia of Elder Care. New York, NY, USA: Springer Publishing Company; 2013.
3. Glisky EL. Changes in cognitive function in human aging. In: Riddle DR, ed. Brain Aging: Models, Methods, and Mechanisms. Boca Raton, FL: Taylor & Francis Group, LLC; 2007:4-20.
4. Craft S, Cholerton B, Reger M. Cognitive changes associated with normal and pathological aging. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, et al, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:751-766.
5. Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303-308.
6. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183-194.
7. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240-246.
8. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270-279.
9. Neurocognitive disorders. In: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
10. Ward A. Arrighi HM, Michels S, et al. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 2012;8:14-21.
11. Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447-1455.
12. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263-269.
13. Jorm AF. A short form of the informant questionnaire on cognitive decline in the elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145-153.
14. Katz S, Downs TD, Cash HR, et al. Progress in development of the index of ADL. Gerontologist. 1970;10:20-30.
15. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179-186.
16. Kiloh LG. Pseudo-dementia. Acta Psychiatr Scand. 1961;37:336-351.
17. Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry. 1999;174:307-311.
18. Birrer RB, Vemuri SP. Depression in later life: a diagnostic and therapeutic challenge. Am Fam Physician. 2004;69:2375-2382.
19. Butters MA, Becker JT, Nebes RD, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157:1949-1954.
20. Alexopoulos GS, Meyers BS, Young RC, et al. The course of geriatric depression with “reversible dementia”: a controlled study. Am J Psychiatry. 1993;150:1693-1699.
21. Saez-Fonseca JA, Lee L, Walker Z. Long-term outcome of depressive pseudodementia in the elderly. J Affect Disord. 2007;101:123-129.
22. Dillon C, Allegri RF, Serrano CM, et al. Late- versus early-onset geriatric depression in a memory research center. Neuropsychiatr Dis Treat. 2009;5:517-526.
23. Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159:1119-1126.
24. Shimada H, Park H, Makizako H, et al. Depressive symptoms and cognitive performance in older adults. J Psychiatr Res. 2014;57:149-156.
25. Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587-595.
26. Dillon C, Machnicki G, Serrano CM, et al. Clinical manifestations of geriatric depression in a memory clinic: toward a proposed subtyping of geriatric depression. J Affect Disord. 2011;134:177-187.
27. Rapp MA, Dahlman K, Sano M, et al. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry. 2005;162:691-698.
28. Zihl J, Reppermund S, Thum S, et al. Neuropsychological profiles in MCI and in depression: differential cognitive dysfunction patterns or similar final common pathway disorder? J Psychiatr Res. 2010;44:647-654.
29. Dillon C, Tartaglini MF, Stefani D, et al. Geriatric depression and its relation with cognitive impairment and dementia. Arch Gerontol Geriatr. 2014;59:450-456.
30. Wright SL, Persad C. Distinguishing between depression and dementia in older persons: neuropsychological and neuropathological correlates. J Geriatr Psychiatry Neurol. 2007;20:189-198.
31. Visser PJ, Verhey FR, Ponds RW, et al. Distinction between preclinical Alzheimer’s disease and depression. J Am Geriatr Soc. 2000;48:479-484.
32. Bodner T, Delazer M, Kemmler G, et al. Clock drawing, clock reading, clock setting, and judgment of clock faces in elderly people with dementia and depression. J Am Geriatr Soc. 2004;52:1146-1150.
33. Clarfield AM. The reversible dementias: do they reverse? Ann Intern Med. 1988;109:476-486.
34. Clarfield AM. The decreasing prevalence of reversible dementias: an updated meta-analysis. Arch Intern Med. 2003;163:2219-2229.
35. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326.
36. Health Quality Ontario. Vitamin B12 and cognitive function: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13:1-45.
37. Abyad A. Prevalence of vitamin B12 deficiency among demented patients and cognitive recovery with cobalamin replacement. J Nutr Health Aging. 2002;6:254-260.
38. Martin DC, Francis J, Protetch J, et al. Time dependency of cognitive recovery with cobalamin replacement: Report of a pilot study. J Am Geriatr Soc. 1992;40:168-172.
39. Clarnette RM, Patterson CJ. Hypothyroidism: does treatment cure dementia? J Geriatr Psychiatry Neurol. 1994;7:23-27.
40. Dugbartey AT. Neurocognitive aspects of hypothyroidism. Arch Intern Med. 1998;158:1413-1418.
41. Alexander EM, Wagner EH, Buchner DM, et al. Do surgical brain lesions present as isolated dementia? A population-based study. J Am Geriatr Soc. 1995;43:138-143.
42. Moore AR, O’Keeffe ST. Drug-induced cognitive impairment in the elderly. Drugs Aging. 1999;15:15-28.
43. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American geriatrics society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
44. Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66:1210-1215.
45. Shatenstein B, Barberger-Gateau P, Mecocci P. Prevention of age-related cognitive decline: which strategies, when, and for whom? J Alzheimers Dis. 2015;48:35-53.
1. Mitchell AJ. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. Int J Geriatr Psychiatry. 2008;23:1191-1202.
2. Burnette V, Howell T. Cognitive changes in aging. In: Capezuti EA, Malone ML, Katz PR, et al, eds. The Encyclopedia of Elder Care. New York, NY, USA: Springer Publishing Company; 2013.
3. Glisky EL. Changes in cognitive function in human aging. In: Riddle DR, ed. Brain Aging: Models, Methods, and Mechanisms. Boca Raton, FL: Taylor & Francis Group, LLC; 2007:4-20.
4. Craft S, Cholerton B, Reger M. Cognitive changes associated with normal and pathological aging. In: Halter JB, Ouslander JG, Tinetti ME, Studenski S, et al, eds. Hazzard’s Geriatric Medicine and Gerontology. 6th ed. New York, NY: McGraw-Hill; 2009:751-766.
5. Petersen RC, Smith GE, Waring SC, et al. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303-308.
6. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183-194.
7. Winblad B, Palmer K, Kivipelto M, et al. Mild cognitive impairment—beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256:240-246.
8. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:270-279.
9. Neurocognitive disorders. In: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
10. Ward A. Arrighi HM, Michels S, et al. Mild cognitive impairment: disparity of incidence and prevalence estimates. Alzheimers Dement. 2012;8:14-21.
11. Petersen RC, Roberts RO, Knopman DS, et al. Mild cognitive impairment: ten years later. Arch Neurol. 2009;66:1447-1455.
12. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7:263-269.
13. Jorm AF. A short form of the informant questionnaire on cognitive decline in the elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145-153.
14. Katz S, Downs TD, Cash HR, et al. Progress in development of the index of ADL. Gerontologist. 1970;10:20-30.
15. Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179-186.
16. Kiloh LG. Pseudo-dementia. Acta Psychiatr Scand. 1961;37:336-351.
17. Beekman AT, Copeland JR, Prince MJ. Review of community prevalence of depression in later life. Br J Psychiatry. 1999;174:307-311.
18. Birrer RB, Vemuri SP. Depression in later life: a diagnostic and therapeutic challenge. Am Fam Physician. 2004;69:2375-2382.
19. Butters MA, Becker JT, Nebes RD, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157:1949-1954.
20. Alexopoulos GS, Meyers BS, Young RC, et al. The course of geriatric depression with “reversible dementia”: a controlled study. Am J Psychiatry. 1993;150:1693-1699.
21. Saez-Fonseca JA, Lee L, Walker Z. Long-term outcome of depressive pseudodementia in the elderly. J Affect Disord. 2007;101:123-129.
22. Dillon C, Allegri RF, Serrano CM, et al. Late- versus early-onset geriatric depression in a memory research center. Neuropsychiatr Dis Treat. 2009;5:517-526.
23. Lockwood KA, Alexopoulos GS, van Gorp WG. Executive dysfunction in geriatric depression. Am J Psychiatry. 2002;159:1119-1126.
24. Shimada H, Park H, Makizako H, et al. Depressive symptoms and cognitive performance in older adults. J Psychiatr Res. 2014;57:149-156.
25. Butters MA, Whyte EM, Nebes RD, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587-595.
26. Dillon C, Machnicki G, Serrano CM, et al. Clinical manifestations of geriatric depression in a memory clinic: toward a proposed subtyping of geriatric depression. J Affect Disord. 2011;134:177-187.
27. Rapp MA, Dahlman K, Sano M, et al. Neuropsychological differences between late-onset and recurrent geriatric major depression. Am J Psychiatry. 2005;162:691-698.
28. Zihl J, Reppermund S, Thum S, et al. Neuropsychological profiles in MCI and in depression: differential cognitive dysfunction patterns or similar final common pathway disorder? J Psychiatr Res. 2010;44:647-654.
29. Dillon C, Tartaglini MF, Stefani D, et al. Geriatric depression and its relation with cognitive impairment and dementia. Arch Gerontol Geriatr. 2014;59:450-456.
30. Wright SL, Persad C. Distinguishing between depression and dementia in older persons: neuropsychological and neuropathological correlates. J Geriatr Psychiatry Neurol. 2007;20:189-198.
31. Visser PJ, Verhey FR, Ponds RW, et al. Distinction between preclinical Alzheimer’s disease and depression. J Am Geriatr Soc. 2000;48:479-484.
32. Bodner T, Delazer M, Kemmler G, et al. Clock drawing, clock reading, clock setting, and judgment of clock faces in elderly people with dementia and depression. J Am Geriatr Soc. 2004;52:1146-1150.
33. Clarfield AM. The reversible dementias: do they reverse? Ann Intern Med. 1988;109:476-486.
34. Clarfield AM. The decreasing prevalence of reversible dementias: an updated meta-analysis. Arch Intern Med. 2003;163:2219-2229.
35. Malouf R, Areosa Sastre A. Vitamin B12 for cognition. Cochrane Database Syst Rev. 2003;(3):CD004326.
36. Health Quality Ontario. Vitamin B12 and cognitive function: an evidence-based analysis. Ont Health Technol Assess Ser. 2013;13:1-45.
37. Abyad A. Prevalence of vitamin B12 deficiency among demented patients and cognitive recovery with cobalamin replacement. J Nutr Health Aging. 2002;6:254-260.
38. Martin DC, Francis J, Protetch J, et al. Time dependency of cognitive recovery with cobalamin replacement: Report of a pilot study. J Am Geriatr Soc. 1992;40:168-172.
39. Clarnette RM, Patterson CJ. Hypothyroidism: does treatment cure dementia? J Geriatr Psychiatry Neurol. 1994;7:23-27.
40. Dugbartey AT. Neurocognitive aspects of hypothyroidism. Arch Intern Med. 1998;158:1413-1418.
41. Alexander EM, Wagner EH, Buchner DM, et al. Do surgical brain lesions present as isolated dementia? A population-based study. J Am Geriatr Soc. 1995;43:138-143.
42. Moore AR, O’Keeffe ST. Drug-induced cognitive impairment in the elderly. Drugs Aging. 1999;15:15-28.
43. American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American geriatrics society 2015 updated beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2015;63:2227-2246.
44. Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66:1210-1215.
45. Shatenstein B, Barberger-Gateau P, Mecocci P. Prevention of age-related cognitive decline: which strategies, when, and for whom? J Alzheimers Dis. 2015;48:35-53.
From The Journal of Family Practice | 2017;66(11):670-676.
PRACTICE RECOMMENDATIONS
› Evaluate cognitive domain involvement in cases of suspected mild cognitive impairment; findings could suggest an underlying cause and indicate risk of progression to dementia. C
› Consider the severity of a cognitive deficit (eg, delayed recall) when depression is diagnosed; a marked deficit is usually more indicative of true dementia than pseudodementia. C
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series